User login
D-RVd for frontline myeloma looks robust in GRIFFIN trial update
ORLANDO – While the benefit of daratumumab added to lenalidomide, bortezomib, and dexamethasone (D-RVd) continues to improve with longer follow-up of the GRIFFIN trial, even early adopters may want to wait for additional data before declaring the combination a first-line standard for transplant-eligible multiple myeloma, according to an investigator on the trial.
D-RVd has significantly improved both response rates and depth of response, compared with RVd alone, Peter M. Voorhees, MD, of Levine Cancer Institute, Atrium Health, Charlotte, N.C., reported at the annual meeting of the American Society of Hematology.
Additionally, rates of response and minimal residual disease (MRD) negativity with D-RVd have increased with longer follow-up beyond posttransplant consolidation, in the ongoing randomized phase 2 trial, Dr. Voorhees said.
“Those of you that are early adopters have good ammunition based on this result, but I would argue that we do need to confirm that the increased MRD-negative rate that we’re seeing translates into a sustained improvement in MRD negativity,” said Dr. Voorhees while presenting the updated results.
Most importantly, it needs to be confirmed that improved depth of response with D-RVd translates into an improvement in progression-free survival, not only in GRIFFIN, he said, but in PERSEUS, a large, randomized European phase 3 trial of subcutaneous daratumumab plus RVd versus RVd alone.
In the GRIFFIN trial, a total of 207 patients with transplant-eligible newly diagnosed multiple myeloma were randomized to intravenous daratumumab plus RVd versus RVd alone, with a primary endpoint of stringent complete response (sCR) by the end of consolidation.
Primary findings, presented in September at the 17th International Myeloma Workshop (IMW) meeting in Boston, indicated an sCR of 42.4% for D-RVd versus 32.0% for RVd at a median follow-up of 13.5 months, a difference that Dr. Voorhees said was statistically significant as defined by the protocol (1-sided P = .068), with an odds ratio of 1.57 (95% confidence interval, 0.87-2.82) in favor of the D-RVd arm.
With longer follow-up data, which Dr. Voorhees reported at ASH, the responses have “deepened over time” in both arms of the study, though he said the daratumumab arm continues to perform better. The sCR with 22.1 months of follow-up was 62.6% for D-RVd versus 45.4% for RVd.
The rates of MRD negativity at this clinical cutoff were 51.0% versus 20.4% for the D-RVd and RVd arms, respectively (P less than .0001), while the 24-month PFS rates were 95.8% for D-RVd and 89.8% for RVd. “Suffice it to say that both groups of patients are doing incredibly well at 2 years,” Dr. Voorhees said.
Rates of grade 3 and 4 neutropenia and thrombocytopenia were higher in the D-RVd arm, and there were more infections, though this was largely driven by an increased incidence of grade 1 or 2 upper respiratory tract infections, according to Dr. Voorhees.
Daratumumab did not impact time to engraftment, with a median CD34+ cell yield of 8.2 x 106 cells/kg for D-RVd and 9.4 x 106 cells/kg for RVd, a difference that Dr. Voorhees said was “not of clinical significance.”
Dr. Voorhees reported disclosures related to Takeda, Oncopeptides, Novartis, GSK, Janssen, Celgene, BMS, Adaptive Biotechnologies, Amgen, and TeneBio.
SOURCE: Voorhees PM et al. ASH 2019, Abstract 691.
ORLANDO – While the benefit of daratumumab added to lenalidomide, bortezomib, and dexamethasone (D-RVd) continues to improve with longer follow-up of the GRIFFIN trial, even early adopters may want to wait for additional data before declaring the combination a first-line standard for transplant-eligible multiple myeloma, according to an investigator on the trial.
D-RVd has significantly improved both response rates and depth of response, compared with RVd alone, Peter M. Voorhees, MD, of Levine Cancer Institute, Atrium Health, Charlotte, N.C., reported at the annual meeting of the American Society of Hematology.
Additionally, rates of response and minimal residual disease (MRD) negativity with D-RVd have increased with longer follow-up beyond posttransplant consolidation, in the ongoing randomized phase 2 trial, Dr. Voorhees said.
“Those of you that are early adopters have good ammunition based on this result, but I would argue that we do need to confirm that the increased MRD-negative rate that we’re seeing translates into a sustained improvement in MRD negativity,” said Dr. Voorhees while presenting the updated results.
Most importantly, it needs to be confirmed that improved depth of response with D-RVd translates into an improvement in progression-free survival, not only in GRIFFIN, he said, but in PERSEUS, a large, randomized European phase 3 trial of subcutaneous daratumumab plus RVd versus RVd alone.
In the GRIFFIN trial, a total of 207 patients with transplant-eligible newly diagnosed multiple myeloma were randomized to intravenous daratumumab plus RVd versus RVd alone, with a primary endpoint of stringent complete response (sCR) by the end of consolidation.
Primary findings, presented in September at the 17th International Myeloma Workshop (IMW) meeting in Boston, indicated an sCR of 42.4% for D-RVd versus 32.0% for RVd at a median follow-up of 13.5 months, a difference that Dr. Voorhees said was statistically significant as defined by the protocol (1-sided P = .068), with an odds ratio of 1.57 (95% confidence interval, 0.87-2.82) in favor of the D-RVd arm.
With longer follow-up data, which Dr. Voorhees reported at ASH, the responses have “deepened over time” in both arms of the study, though he said the daratumumab arm continues to perform better. The sCR with 22.1 months of follow-up was 62.6% for D-RVd versus 45.4% for RVd.
The rates of MRD negativity at this clinical cutoff were 51.0% versus 20.4% for the D-RVd and RVd arms, respectively (P less than .0001), while the 24-month PFS rates were 95.8% for D-RVd and 89.8% for RVd. “Suffice it to say that both groups of patients are doing incredibly well at 2 years,” Dr. Voorhees said.
Rates of grade 3 and 4 neutropenia and thrombocytopenia were higher in the D-RVd arm, and there were more infections, though this was largely driven by an increased incidence of grade 1 or 2 upper respiratory tract infections, according to Dr. Voorhees.
Daratumumab did not impact time to engraftment, with a median CD34+ cell yield of 8.2 x 106 cells/kg for D-RVd and 9.4 x 106 cells/kg for RVd, a difference that Dr. Voorhees said was “not of clinical significance.”
Dr. Voorhees reported disclosures related to Takeda, Oncopeptides, Novartis, GSK, Janssen, Celgene, BMS, Adaptive Biotechnologies, Amgen, and TeneBio.
SOURCE: Voorhees PM et al. ASH 2019, Abstract 691.
ORLANDO – While the benefit of daratumumab added to lenalidomide, bortezomib, and dexamethasone (D-RVd) continues to improve with longer follow-up of the GRIFFIN trial, even early adopters may want to wait for additional data before declaring the combination a first-line standard for transplant-eligible multiple myeloma, according to an investigator on the trial.
D-RVd has significantly improved both response rates and depth of response, compared with RVd alone, Peter M. Voorhees, MD, of Levine Cancer Institute, Atrium Health, Charlotte, N.C., reported at the annual meeting of the American Society of Hematology.
Additionally, rates of response and minimal residual disease (MRD) negativity with D-RVd have increased with longer follow-up beyond posttransplant consolidation, in the ongoing randomized phase 2 trial, Dr. Voorhees said.
“Those of you that are early adopters have good ammunition based on this result, but I would argue that we do need to confirm that the increased MRD-negative rate that we’re seeing translates into a sustained improvement in MRD negativity,” said Dr. Voorhees while presenting the updated results.
Most importantly, it needs to be confirmed that improved depth of response with D-RVd translates into an improvement in progression-free survival, not only in GRIFFIN, he said, but in PERSEUS, a large, randomized European phase 3 trial of subcutaneous daratumumab plus RVd versus RVd alone.
In the GRIFFIN trial, a total of 207 patients with transplant-eligible newly diagnosed multiple myeloma were randomized to intravenous daratumumab plus RVd versus RVd alone, with a primary endpoint of stringent complete response (sCR) by the end of consolidation.
Primary findings, presented in September at the 17th International Myeloma Workshop (IMW) meeting in Boston, indicated an sCR of 42.4% for D-RVd versus 32.0% for RVd at a median follow-up of 13.5 months, a difference that Dr. Voorhees said was statistically significant as defined by the protocol (1-sided P = .068), with an odds ratio of 1.57 (95% confidence interval, 0.87-2.82) in favor of the D-RVd arm.
With longer follow-up data, which Dr. Voorhees reported at ASH, the responses have “deepened over time” in both arms of the study, though he said the daratumumab arm continues to perform better. The sCR with 22.1 months of follow-up was 62.6% for D-RVd versus 45.4% for RVd.
The rates of MRD negativity at this clinical cutoff were 51.0% versus 20.4% for the D-RVd and RVd arms, respectively (P less than .0001), while the 24-month PFS rates were 95.8% for D-RVd and 89.8% for RVd. “Suffice it to say that both groups of patients are doing incredibly well at 2 years,” Dr. Voorhees said.
Rates of grade 3 and 4 neutropenia and thrombocytopenia were higher in the D-RVd arm, and there were more infections, though this was largely driven by an increased incidence of grade 1 or 2 upper respiratory tract infections, according to Dr. Voorhees.
Daratumumab did not impact time to engraftment, with a median CD34+ cell yield of 8.2 x 106 cells/kg for D-RVd and 9.4 x 106 cells/kg for RVd, a difference that Dr. Voorhees said was “not of clinical significance.”
Dr. Voorhees reported disclosures related to Takeda, Oncopeptides, Novartis, GSK, Janssen, Celgene, BMS, Adaptive Biotechnologies, Amgen, and TeneBio.
SOURCE: Voorhees PM et al. ASH 2019, Abstract 691.
REPORTING FROM ASH 2019
Zanubrutinib achieved high response rate in del(17p) CLL cohort
ORLANDO – Zanubrutinib has produced a high overall response rate in one the largest cohorts of patients with treatment-naive 17p-deletion chronic lymphocytic leukemia (CLL) studied to date.
An overall response rate of nearly 93% was seen in this 109-patient, high-risk cohort, enrolled as part of the phase 3 SEQUOIA study (BGB-3111-304), said Constantine S. Tam, MBBS, MD, of St. Vincent’s Hospital and Peter MacCallum Cancer Centre in Melbourne.
Tolerability of zanubrutinib was essentially consistent with previous reports of the agent as used in other B-cell malignancies, Dr. Tam said in an oral presentation of the results at the annual meeting of the American Society of Hematology.
Deletion of chromosome 17p13.1, or del(17p), is a marker of poor prognosis and poor response to chemotherapy in patients with CLL or small lymphocytic lymphoma (SLL). For patients with del(17p) CLL, the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become a standard of care, Dr. Tam said.
Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK occupancy and minimize off-target inhibition of TEC and epidermal growth factor receptor kinases. “What this effectively means is that we are able to dose this drug at levels much higher than that achievable with ibrutinib, and not get intolerable side effects,” Dr. Tam said.
Zanubrutinib has been approved in the United States for previously treated mantle cell lymphoma, and generated durable responses among CLL/SLL patients with or without del(17p) in a phase 1/2 study, according to Dr. Tam.
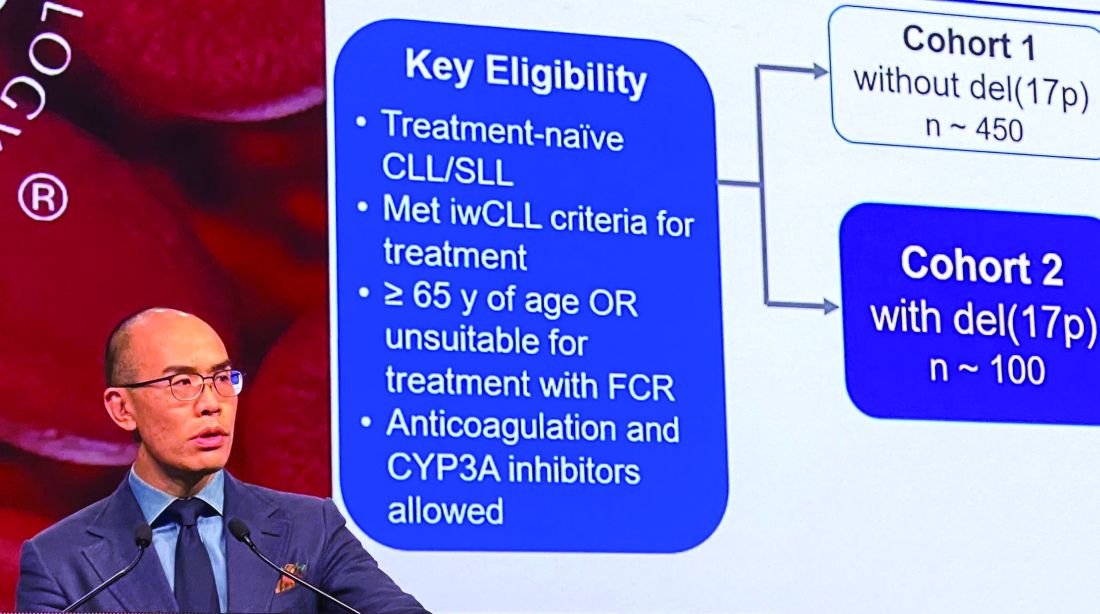
In the present study, which exclusively enrolled patients with del(17p) CLL/SLL, patients received 160 mg twice daily of zanubrutinib, Dr. Tam said. Out of 109 patients enrolled, 10 (9.2%) had SLL. All patients were aged at least 65 years or were deemed unsuitable for treatment with the combination of fludarabine, cyclophosphamide, and rituximab.
Of 109 patients enrolled, 104 received on-study treatment. The median age was 70 years, Dr. Tam reported, and a number of patients had other high-risk markers beyond del(17p), including unmutated IgVH status in 61.5% of patients.
With a median follow-up of 10 months, the overall response rate was 92.7%, including 1.9% complete responses and 78.9% partial responses. “Only one patient had primary progressive disease after starting this drug,” Dr. Tam said.
Time to response was rapid, according to the investigator, at about 2.8 months; after 6 months, 95% of responders remained in response.
Further analysis showed that the response rate was consistent across subgroups. “There was not a single group that did not respond with a high response rate, including poor prognostic groups,” Dr. Tam said.
Most adverse events were grade 1-2 in severity, and the most common events included confusion and upper respiratory tract infection. The only common grade 3 event, according to Dr. Tam, was neutropenia. Rates of grade 3 major bleeding were low, he said, and the rate of grade 3 atrial fibrillation was 0.9%. One patient died due to pneumonia.
The ongoing SEQUOIA study, designed to compare zanubrutinib to the combination of bendamustine and rituximab in patients with previously untreated CLL or SLL, is sponsored by BeiGene. Dr. Tam reported disclosures related to Novartis, Pharmacyclics, AbbVie, BeiGene, Janssen, and Roche.
SOURCE: Tam C et al. ASH 2019, Abstract 499.
ORLANDO – Zanubrutinib has produced a high overall response rate in one the largest cohorts of patients with treatment-naive 17p-deletion chronic lymphocytic leukemia (CLL) studied to date.
An overall response rate of nearly 93% was seen in this 109-patient, high-risk cohort, enrolled as part of the phase 3 SEQUOIA study (BGB-3111-304), said Constantine S. Tam, MBBS, MD, of St. Vincent’s Hospital and Peter MacCallum Cancer Centre in Melbourne.
Tolerability of zanubrutinib was essentially consistent with previous reports of the agent as used in other B-cell malignancies, Dr. Tam said in an oral presentation of the results at the annual meeting of the American Society of Hematology.
Deletion of chromosome 17p13.1, or del(17p), is a marker of poor prognosis and poor response to chemotherapy in patients with CLL or small lymphocytic lymphoma (SLL). For patients with del(17p) CLL, the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become a standard of care, Dr. Tam said.
Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK occupancy and minimize off-target inhibition of TEC and epidermal growth factor receptor kinases. “What this effectively means is that we are able to dose this drug at levels much higher than that achievable with ibrutinib, and not get intolerable side effects,” Dr. Tam said.
Zanubrutinib has been approved in the United States for previously treated mantle cell lymphoma, and generated durable responses among CLL/SLL patients with or without del(17p) in a phase 1/2 study, according to Dr. Tam.
In the present study, which exclusively enrolled patients with del(17p) CLL/SLL, patients received 160 mg twice daily of zanubrutinib, Dr. Tam said. Out of 109 patients enrolled, 10 (9.2%) had SLL. All patients were aged at least 65 years or were deemed unsuitable for treatment with the combination of fludarabine, cyclophosphamide, and rituximab.
Of 109 patients enrolled, 104 received on-study treatment. The median age was 70 years, Dr. Tam reported, and a number of patients had other high-risk markers beyond del(17p), including unmutated IgVH status in 61.5% of patients.
With a median follow-up of 10 months, the overall response rate was 92.7%, including 1.9% complete responses and 78.9% partial responses. “Only one patient had primary progressive disease after starting this drug,” Dr. Tam said.
Time to response was rapid, according to the investigator, at about 2.8 months; after 6 months, 95% of responders remained in response.
Further analysis showed that the response rate was consistent across subgroups. “There was not a single group that did not respond with a high response rate, including poor prognostic groups,” Dr. Tam said.
Most adverse events were grade 1-2 in severity, and the most common events included confusion and upper respiratory tract infection. The only common grade 3 event, according to Dr. Tam, was neutropenia. Rates of grade 3 major bleeding were low, he said, and the rate of grade 3 atrial fibrillation was 0.9%. One patient died due to pneumonia.
The ongoing SEQUOIA study, designed to compare zanubrutinib to the combination of bendamustine and rituximab in patients with previously untreated CLL or SLL, is sponsored by BeiGene. Dr. Tam reported disclosures related to Novartis, Pharmacyclics, AbbVie, BeiGene, Janssen, and Roche.
SOURCE: Tam C et al. ASH 2019, Abstract 499.
ORLANDO – Zanubrutinib has produced a high overall response rate in one the largest cohorts of patients with treatment-naive 17p-deletion chronic lymphocytic leukemia (CLL) studied to date.
An overall response rate of nearly 93% was seen in this 109-patient, high-risk cohort, enrolled as part of the phase 3 SEQUOIA study (BGB-3111-304), said Constantine S. Tam, MBBS, MD, of St. Vincent’s Hospital and Peter MacCallum Cancer Centre in Melbourne.
Tolerability of zanubrutinib was essentially consistent with previous reports of the agent as used in other B-cell malignancies, Dr. Tam said in an oral presentation of the results at the annual meeting of the American Society of Hematology.
Deletion of chromosome 17p13.1, or del(17p), is a marker of poor prognosis and poor response to chemotherapy in patients with CLL or small lymphocytic lymphoma (SLL). For patients with del(17p) CLL, the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become a standard of care, Dr. Tam said.
Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK occupancy and minimize off-target inhibition of TEC and epidermal growth factor receptor kinases. “What this effectively means is that we are able to dose this drug at levels much higher than that achievable with ibrutinib, and not get intolerable side effects,” Dr. Tam said.
Zanubrutinib has been approved in the United States for previously treated mantle cell lymphoma, and generated durable responses among CLL/SLL patients with or without del(17p) in a phase 1/2 study, according to Dr. Tam.
In the present study, which exclusively enrolled patients with del(17p) CLL/SLL, patients received 160 mg twice daily of zanubrutinib, Dr. Tam said. Out of 109 patients enrolled, 10 (9.2%) had SLL. All patients were aged at least 65 years or were deemed unsuitable for treatment with the combination of fludarabine, cyclophosphamide, and rituximab.
Of 109 patients enrolled, 104 received on-study treatment. The median age was 70 years, Dr. Tam reported, and a number of patients had other high-risk markers beyond del(17p), including unmutated IgVH status in 61.5% of patients.
With a median follow-up of 10 months, the overall response rate was 92.7%, including 1.9% complete responses and 78.9% partial responses. “Only one patient had primary progressive disease after starting this drug,” Dr. Tam said.
Time to response was rapid, according to the investigator, at about 2.8 months; after 6 months, 95% of responders remained in response.
Further analysis showed that the response rate was consistent across subgroups. “There was not a single group that did not respond with a high response rate, including poor prognostic groups,” Dr. Tam said.
Most adverse events were grade 1-2 in severity, and the most common events included confusion and upper respiratory tract infection. The only common grade 3 event, according to Dr. Tam, was neutropenia. Rates of grade 3 major bleeding were low, he said, and the rate of grade 3 atrial fibrillation was 0.9%. One patient died due to pneumonia.
The ongoing SEQUOIA study, designed to compare zanubrutinib to the combination of bendamustine and rituximab in patients with previously untreated CLL or SLL, is sponsored by BeiGene. Dr. Tam reported disclosures related to Novartis, Pharmacyclics, AbbVie, BeiGene, Janssen, and Roche.
SOURCE: Tam C et al. ASH 2019, Abstract 499.
REPORTING FROM ASH 2019
Some MCL patients can safely stop venetoclax-ibrutinib, study suggests
ORLANDO – Updated trial results have revealed durable responses with venetoclax and ibrutinib in patients with mantle cell lymphoma (MCL), allowing some patients to stop treatment.
Five of 24 patients were able to stop treatment after achieving minimal residual disease (MRD)-negative complete responses (CRs). Four of these patients remain in CR at up to 18 months off treatment, although one patient ultimately progressed and died.
“Treatment cessation was feasible for patients in MRD-negative complete responses, raising the prospect of limited-duration, targeted-agent therapy in the management of relapsed/refractory mantle cell lymphoma,” said Sasanka M. Handunnetti, MBBS, of Peter MacCallum Cancer Centre in Melbourne. Dr. Handunnetti presented these results, from the AIM trial, at the annual meeting of the American Society of Hematology.
The phase 2 trial enrolled 24 patients. At baseline, patients had a median age of 68 years (range, 47-81 years), and 88% were men. One patient was treatment-naive, but the rest had relapsed/refractory MCL. These patients had received a median of two prior therapies (range, 1-6).
The patients received venetoclax at 400 mg daily and ibrutinib at 560 mg daily.
In the primary analysis, the CR rate was 62% at week 16 and 71% overall, according to positron-emission tomography/computed tomography. MRD negativity was achieved by 67% of patients according to flow cytometry and 38% according to allele-specific oligonucleotide polymerase chain reaction (N Engl J Med. 2018 Mar 29;378[13]:1211-23).
Response and survival
For the current analysis, the median follow up was 37.5 months (range, 1.4-45.3 months). The median duration of response has not been reached, the median progression-free survival is 29 months, and the median overall survival is 32 months.
Thirteen patients have died, 8 of them due to progressive disease. The remaining 11 patients are still alive, and 9 of them are still in CR. One patient is still in partial response, and one has not responded but remains on ibrutinib and venetoclax.
Dr. Handunnetti pointed out that 12 patients had TP53 aberrations, and 8 of them died, but 4 remain alive and in CR. All four patients with SMARCA4 aberrations died.
Treatment status
Five patients are still receiving treatment with ibrutinib and venetoclax, and one patient is receiving only venetoclax. One patient went off study treatment due to a diagnosis of myelodysplastic syndrome, but that patient’s MCL is still in CR.
Five patients were able to stop treatment after achieving MRD-negative CR and were placed under “stringent surveillance,” Dr. Handunnetti said.
One of the five patients who stopped treatment progressed at 7 months and died. The remaining four patients are still alive and in CR at 6 months, 13 months, 17 months, and 18 months off treatment.
Safety update
Within the first 56 weeks of treatment, 15 patients required dose adjustments. Twelve patients required an adjustment to ibrutinib, seven to venetoclax, and four to both drugs. After 56 weeks, there were no dose adjustments.
Two patients developed therapy-related myelodysplastic syndrome. One patient had previously received FCR (fludarabine, cyclophosphamide, and rituximab) and BR (bendamustine and rituximab). The other patient had received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).
This investigator-initiated trial was funded by Janssen and Abbvie. Dr. Handunnetti reported relationships with Abbvie and Gilead.
SOURCE: Handunnetti S et al. ASH 2019. Abstract 756.
ORLANDO – Updated trial results have revealed durable responses with venetoclax and ibrutinib in patients with mantle cell lymphoma (MCL), allowing some patients to stop treatment.
Five of 24 patients were able to stop treatment after achieving minimal residual disease (MRD)-negative complete responses (CRs). Four of these patients remain in CR at up to 18 months off treatment, although one patient ultimately progressed and died.
“Treatment cessation was feasible for patients in MRD-negative complete responses, raising the prospect of limited-duration, targeted-agent therapy in the management of relapsed/refractory mantle cell lymphoma,” said Sasanka M. Handunnetti, MBBS, of Peter MacCallum Cancer Centre in Melbourne. Dr. Handunnetti presented these results, from the AIM trial, at the annual meeting of the American Society of Hematology.
The phase 2 trial enrolled 24 patients. At baseline, patients had a median age of 68 years (range, 47-81 years), and 88% were men. One patient was treatment-naive, but the rest had relapsed/refractory MCL. These patients had received a median of two prior therapies (range, 1-6).
The patients received venetoclax at 400 mg daily and ibrutinib at 560 mg daily.
In the primary analysis, the CR rate was 62% at week 16 and 71% overall, according to positron-emission tomography/computed tomography. MRD negativity was achieved by 67% of patients according to flow cytometry and 38% according to allele-specific oligonucleotide polymerase chain reaction (N Engl J Med. 2018 Mar 29;378[13]:1211-23).
Response and survival
For the current analysis, the median follow up was 37.5 months (range, 1.4-45.3 months). The median duration of response has not been reached, the median progression-free survival is 29 months, and the median overall survival is 32 months.
Thirteen patients have died, 8 of them due to progressive disease. The remaining 11 patients are still alive, and 9 of them are still in CR. One patient is still in partial response, and one has not responded but remains on ibrutinib and venetoclax.
Dr. Handunnetti pointed out that 12 patients had TP53 aberrations, and 8 of them died, but 4 remain alive and in CR. All four patients with SMARCA4 aberrations died.
Treatment status
Five patients are still receiving treatment with ibrutinib and venetoclax, and one patient is receiving only venetoclax. One patient went off study treatment due to a diagnosis of myelodysplastic syndrome, but that patient’s MCL is still in CR.
Five patients were able to stop treatment after achieving MRD-negative CR and were placed under “stringent surveillance,” Dr. Handunnetti said.
One of the five patients who stopped treatment progressed at 7 months and died. The remaining four patients are still alive and in CR at 6 months, 13 months, 17 months, and 18 months off treatment.
Safety update
Within the first 56 weeks of treatment, 15 patients required dose adjustments. Twelve patients required an adjustment to ibrutinib, seven to venetoclax, and four to both drugs. After 56 weeks, there were no dose adjustments.
Two patients developed therapy-related myelodysplastic syndrome. One patient had previously received FCR (fludarabine, cyclophosphamide, and rituximab) and BR (bendamustine and rituximab). The other patient had received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).
This investigator-initiated trial was funded by Janssen and Abbvie. Dr. Handunnetti reported relationships with Abbvie and Gilead.
SOURCE: Handunnetti S et al. ASH 2019. Abstract 756.
ORLANDO – Updated trial results have revealed durable responses with venetoclax and ibrutinib in patients with mantle cell lymphoma (MCL), allowing some patients to stop treatment.
Five of 24 patients were able to stop treatment after achieving minimal residual disease (MRD)-negative complete responses (CRs). Four of these patients remain in CR at up to 18 months off treatment, although one patient ultimately progressed and died.
“Treatment cessation was feasible for patients in MRD-negative complete responses, raising the prospect of limited-duration, targeted-agent therapy in the management of relapsed/refractory mantle cell lymphoma,” said Sasanka M. Handunnetti, MBBS, of Peter MacCallum Cancer Centre in Melbourne. Dr. Handunnetti presented these results, from the AIM trial, at the annual meeting of the American Society of Hematology.
The phase 2 trial enrolled 24 patients. At baseline, patients had a median age of 68 years (range, 47-81 years), and 88% were men. One patient was treatment-naive, but the rest had relapsed/refractory MCL. These patients had received a median of two prior therapies (range, 1-6).
The patients received venetoclax at 400 mg daily and ibrutinib at 560 mg daily.
In the primary analysis, the CR rate was 62% at week 16 and 71% overall, according to positron-emission tomography/computed tomography. MRD negativity was achieved by 67% of patients according to flow cytometry and 38% according to allele-specific oligonucleotide polymerase chain reaction (N Engl J Med. 2018 Mar 29;378[13]:1211-23).
Response and survival
For the current analysis, the median follow up was 37.5 months (range, 1.4-45.3 months). The median duration of response has not been reached, the median progression-free survival is 29 months, and the median overall survival is 32 months.
Thirteen patients have died, 8 of them due to progressive disease. The remaining 11 patients are still alive, and 9 of them are still in CR. One patient is still in partial response, and one has not responded but remains on ibrutinib and venetoclax.
Dr. Handunnetti pointed out that 12 patients had TP53 aberrations, and 8 of them died, but 4 remain alive and in CR. All four patients with SMARCA4 aberrations died.
Treatment status
Five patients are still receiving treatment with ibrutinib and venetoclax, and one patient is receiving only venetoclax. One patient went off study treatment due to a diagnosis of myelodysplastic syndrome, but that patient’s MCL is still in CR.
Five patients were able to stop treatment after achieving MRD-negative CR and were placed under “stringent surveillance,” Dr. Handunnetti said.
One of the five patients who stopped treatment progressed at 7 months and died. The remaining four patients are still alive and in CR at 6 months, 13 months, 17 months, and 18 months off treatment.
Safety update
Within the first 56 weeks of treatment, 15 patients required dose adjustments. Twelve patients required an adjustment to ibrutinib, seven to venetoclax, and four to both drugs. After 56 weeks, there were no dose adjustments.
Two patients developed therapy-related myelodysplastic syndrome. One patient had previously received FCR (fludarabine, cyclophosphamide, and rituximab) and BR (bendamustine and rituximab). The other patient had received R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).
This investigator-initiated trial was funded by Janssen and Abbvie. Dr. Handunnetti reported relationships with Abbvie and Gilead.
SOURCE: Handunnetti S et al. ASH 2019. Abstract 756.
REPORTING FROM ASH 2019
BCL11A-directed gene therapy advances in sickle cell disease
ORLANDO – A gene therapy approach that targets a major repressor of fetal hemoglobin appears to be acceptably safe and to mitigate the pathology of sickle cell disease among the five patients infused so far, an investigator reported at the annual meeting of the American Society of Hematology.
Knocking down BCL11A using a lentiviral vector-based approach resulted in effective induction of fetal hemoglobin and significant attenuation of the sickling phenotype, with no vector-related adverse events, investigator Erica B. Esrick, MD, of Children’s Hospital Boston, said during the meeting’s late-breaking abstracts session.
The single-center pilot and feasibility study, originally designed to include a total of seven patients, now has an expanded enrollment goal of 15 patients, and a multicenter phase 2/3 study is planned that will enroll a larger group of patients with sickle cell disease, according to Dr. Esrick.
BCL11A represents a promising target in sickle cell disease because of its regulation of the fetal-adult hemoglobin switch at the gamma-globin locus, investigators said in their late-breaking study abstract.
Dr. Esrick described BCH-BB694, a lentiviral vector encoding a BCL11A-targeting small hairpin RNA embedded in a microRNA scaffold (shmiR). “The advantage of this approach is that it harnesses the physiologic switch machinery, simultaneously increasing fetal hemoglobin and decreasing sickle hemoglobin, thus maintaining the alpha to beta globin ratio in the cell,” she said.
The results of the pilot study of the shmiR vector approach, although preliminary and in need of longer follow-up, contribute to a larger body of research showing that multiple gene therapy approaches hold promise in this disease, said Robert Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins School of Medicine, Baltimore.
“The exciting thing is that there are now multiple ways of going at this previously incurable disease,” Dr. Brodsky, who was not involved in the research, said during a press conference.
Development of the gene therapy described by Dr. Esrick involves mobilization of the patient’s peripheral stem cells using plerixafor, followed by selection of CD34+ cells that were transduced with the shmiR lentiviral vector, followed by infusion of gene modified cells into the patient after a busulfan conditioning regimen.
“In our treated patients, we’ve seen a consistent and substantial induction in fetal hemoglobin,” Dr. Esrick said, noting that the longest follow-up to date for the five treated patients is now 18 months.
The patients, who range in age from 12 to 26 years, are producing and maintaining very high numbers of F cells, or erythrocytes with measurable fetal hemoglobin, she said.
Total fetal hemoglobin has increased and remained stable at between 23% and 43% for the five patients, who are producing “stably high” average amounts of fetal hemoglobin per F cell, at 10 to 16 picograms of fetal hemoglobin per cell, while 37% to 62% of the F cells’ total hemoglobin is fetal hemoglobin, she added.
Following gene therapy, treated patients have had no instances of vaso-occlusive pain crises, respiratory events, or neurologic events. No patients have required transfusion, except one with severe underlying vascular disease for whom post–gene therapy transfusions were planned, she said.
Validated assays at the single-cell level are needed to better understand the effect of this gene therapy and eventually compare it to other therapeutic approaches in sickle cell disease, according to Dr. Esrick.
“We’re collaborating with several colleagues on exploratory assays to accomplish this,” she said, adding that the work is ongoing.
Dr. Esrick reported having no disclosures. Her coauthors reported disclosures related to Alerion Biosciences, Novartis, Orchard Therapeutics, Roche, AstraZeneca, and bluebird bio, among others.
SOURCE: Esrick EB et al. ASH 2019. Abstract LBA-5.
ORLANDO – A gene therapy approach that targets a major repressor of fetal hemoglobin appears to be acceptably safe and to mitigate the pathology of sickle cell disease among the five patients infused so far, an investigator reported at the annual meeting of the American Society of Hematology.
Knocking down BCL11A using a lentiviral vector-based approach resulted in effective induction of fetal hemoglobin and significant attenuation of the sickling phenotype, with no vector-related adverse events, investigator Erica B. Esrick, MD, of Children’s Hospital Boston, said during the meeting’s late-breaking abstracts session.
The single-center pilot and feasibility study, originally designed to include a total of seven patients, now has an expanded enrollment goal of 15 patients, and a multicenter phase 2/3 study is planned that will enroll a larger group of patients with sickle cell disease, according to Dr. Esrick.
BCL11A represents a promising target in sickle cell disease because of its regulation of the fetal-adult hemoglobin switch at the gamma-globin locus, investigators said in their late-breaking study abstract.
Dr. Esrick described BCH-BB694, a lentiviral vector encoding a BCL11A-targeting small hairpin RNA embedded in a microRNA scaffold (shmiR). “The advantage of this approach is that it harnesses the physiologic switch machinery, simultaneously increasing fetal hemoglobin and decreasing sickle hemoglobin, thus maintaining the alpha to beta globin ratio in the cell,” she said.
The results of the pilot study of the shmiR vector approach, although preliminary and in need of longer follow-up, contribute to a larger body of research showing that multiple gene therapy approaches hold promise in this disease, said Robert Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins School of Medicine, Baltimore.
“The exciting thing is that there are now multiple ways of going at this previously incurable disease,” Dr. Brodsky, who was not involved in the research, said during a press conference.
Development of the gene therapy described by Dr. Esrick involves mobilization of the patient’s peripheral stem cells using plerixafor, followed by selection of CD34+ cells that were transduced with the shmiR lentiviral vector, followed by infusion of gene modified cells into the patient after a busulfan conditioning regimen.
“In our treated patients, we’ve seen a consistent and substantial induction in fetal hemoglobin,” Dr. Esrick said, noting that the longest follow-up to date for the five treated patients is now 18 months.
The patients, who range in age from 12 to 26 years, are producing and maintaining very high numbers of F cells, or erythrocytes with measurable fetal hemoglobin, she said.
Total fetal hemoglobin has increased and remained stable at between 23% and 43% for the five patients, who are producing “stably high” average amounts of fetal hemoglobin per F cell, at 10 to 16 picograms of fetal hemoglobin per cell, while 37% to 62% of the F cells’ total hemoglobin is fetal hemoglobin, she added.
Following gene therapy, treated patients have had no instances of vaso-occlusive pain crises, respiratory events, or neurologic events. No patients have required transfusion, except one with severe underlying vascular disease for whom post–gene therapy transfusions were planned, she said.
Validated assays at the single-cell level are needed to better understand the effect of this gene therapy and eventually compare it to other therapeutic approaches in sickle cell disease, according to Dr. Esrick.
“We’re collaborating with several colleagues on exploratory assays to accomplish this,” she said, adding that the work is ongoing.
Dr. Esrick reported having no disclosures. Her coauthors reported disclosures related to Alerion Biosciences, Novartis, Orchard Therapeutics, Roche, AstraZeneca, and bluebird bio, among others.
SOURCE: Esrick EB et al. ASH 2019. Abstract LBA-5.
ORLANDO – A gene therapy approach that targets a major repressor of fetal hemoglobin appears to be acceptably safe and to mitigate the pathology of sickle cell disease among the five patients infused so far, an investigator reported at the annual meeting of the American Society of Hematology.
Knocking down BCL11A using a lentiviral vector-based approach resulted in effective induction of fetal hemoglobin and significant attenuation of the sickling phenotype, with no vector-related adverse events, investigator Erica B. Esrick, MD, of Children’s Hospital Boston, said during the meeting’s late-breaking abstracts session.
The single-center pilot and feasibility study, originally designed to include a total of seven patients, now has an expanded enrollment goal of 15 patients, and a multicenter phase 2/3 study is planned that will enroll a larger group of patients with sickle cell disease, according to Dr. Esrick.
BCL11A represents a promising target in sickle cell disease because of its regulation of the fetal-adult hemoglobin switch at the gamma-globin locus, investigators said in their late-breaking study abstract.
Dr. Esrick described BCH-BB694, a lentiviral vector encoding a BCL11A-targeting small hairpin RNA embedded in a microRNA scaffold (shmiR). “The advantage of this approach is that it harnesses the physiologic switch machinery, simultaneously increasing fetal hemoglobin and decreasing sickle hemoglobin, thus maintaining the alpha to beta globin ratio in the cell,” she said.
The results of the pilot study of the shmiR vector approach, although preliminary and in need of longer follow-up, contribute to a larger body of research showing that multiple gene therapy approaches hold promise in this disease, said Robert Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins School of Medicine, Baltimore.
“The exciting thing is that there are now multiple ways of going at this previously incurable disease,” Dr. Brodsky, who was not involved in the research, said during a press conference.
Development of the gene therapy described by Dr. Esrick involves mobilization of the patient’s peripheral stem cells using plerixafor, followed by selection of CD34+ cells that were transduced with the shmiR lentiviral vector, followed by infusion of gene modified cells into the patient after a busulfan conditioning regimen.
“In our treated patients, we’ve seen a consistent and substantial induction in fetal hemoglobin,” Dr. Esrick said, noting that the longest follow-up to date for the five treated patients is now 18 months.
The patients, who range in age from 12 to 26 years, are producing and maintaining very high numbers of F cells, or erythrocytes with measurable fetal hemoglobin, she said.
Total fetal hemoglobin has increased and remained stable at between 23% and 43% for the five patients, who are producing “stably high” average amounts of fetal hemoglobin per F cell, at 10 to 16 picograms of fetal hemoglobin per cell, while 37% to 62% of the F cells’ total hemoglobin is fetal hemoglobin, she added.
Following gene therapy, treated patients have had no instances of vaso-occlusive pain crises, respiratory events, or neurologic events. No patients have required transfusion, except one with severe underlying vascular disease for whom post–gene therapy transfusions were planned, she said.
Validated assays at the single-cell level are needed to better understand the effect of this gene therapy and eventually compare it to other therapeutic approaches in sickle cell disease, according to Dr. Esrick.
“We’re collaborating with several colleagues on exploratory assays to accomplish this,” she said, adding that the work is ongoing.
Dr. Esrick reported having no disclosures. Her coauthors reported disclosures related to Alerion Biosciences, Novartis, Orchard Therapeutics, Roche, AstraZeneca, and bluebird bio, among others.
SOURCE: Esrick EB et al. ASH 2019. Abstract LBA-5.
REPORTING FROM ASH 2019
KTE-X19 produces highest response rate in MCL subgroup
ORLANDO – KTE-X19, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, demonstrated unprecedented efficacy in the ZUMA-2 trial, according to an investigator involved in the study.
KTE-X19 produced a 93% overall response rate in patients with relapsed/refractory mantle cell lymphoma (MCL). This is the highest reported response rate in patients who have failed treatment with a Bruton’s tyrosine kinase (BTK) inhibitor, said Michael L. Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Dr. Wang presented results from ZUMA-2 at the annual meeting of the American Society of Hematology.
“Patients with relapsed/refractory MCL have very poor outcomes,” Dr. Wang noted. “In patients who progress after BTK inhibition therapy, the overall response rate is only between 25% and 42%, and the overall survival is only between 6 and 10 months. Few patients proceed to allogeneic transplantation.”
The phase 2 ZUMA-2 trial was designed to test KTE-X19 in these patients. KTE-X19 is an anti-CD19 CAR T-cell therapy containing a CD3-zeta T-cell activation domain and a CD28 signaling domain. KTE-X19 is distinct from axicabtagene ciloleucel (KTE-C19) because the manufacturing process for KTE-X19 removes circulating tumor cells.
The trial enrolled 74 patients, and 68 of them received KTE-X19. Manufacturing failed for three patients, two patients died of progressive disease before they could receive KTE-X19, and one patient was found to be ineligible for treatment.
The 68 patients had a median age of 65 years (range, 38-79 years), and 84% were men. A majority of patients (85%) had stage IV disease and classical (59%) or blastoid (25%) morphology. Most patients (69%) had a Ki-67 proliferation index of 50% or greater, and most (56%) were intermediate- or high-risk according to the Mantle Cell Lymphoma International Prognostic Index (MIPI).
Patients had received a median of three prior therapies (range, one to five). All had been treated with a BTK inhibitor, with 85% receiving ibrutinib, 24% receiving acalabrutinib, and 9% receiving both. Most patients (68%) were refractory to BTK inhibition, and 32% relapsed on or after BTK inhibitor therapy.
In this study, patients could receive bridging therapy to keep their disease stable while KTE-X19 was being manufactured. There were 25 patients who received bridging therapy, which consisted of ibrutinib (n = 14), acalabrutinib (n = 5), dexamethasone (n = 12), and/or methylprednisolone (n = 2). Six patients received both BTK inhibitors and steroids.
All patients received conditioning with fludarabine and cyclophosphamide, followed by a single infusion of KTE-X19 at 2x106.
Efficacy
Sixty patients were evaluable for efficacy, and the median follow-up was 12.3 months (range, 7.0-32.3 months).
The overall response rate was 93%, with 67% of patients achieving a complete response and 27% achieving a partial response. Three percent of patients had stable disease, and 3% had progressive disease.
“The overall response rate was consistent across key subgroups, without any statistical difference,” Dr. Wang said. “This includes Ki-67, MIPI, and prior use of either steroids or bridging therapy.”
The median time to response was 1.0 month, and the median time to complete response was 3.0 months. Responses deepened over time, with 35% of patients converting from a partial response to a complete response, and 5% converting from stable disease to complete response.
The median duration of response has not been reached. At last follow-up, 57% of all patients and 78% of complete responders were still in response.
The median progression-free and overall survival have not been reached. At 12 months, the progression-free survival rate was 61%, and the overall survival rate was 83%.
Safety
All 68 patients were evaluable for safety. The most common adverse events were pyrexia (94%), neutropenia (87%), thrombocytopenia (74%), anemia (68%), and hypotension (51%).
Grade 3/4 adverse events included pyrexia (13%), neutropenia (85%), thrombocytopenia (51%), anemia (50%), hypotension (22%), hypoxia (21%), hypophosphatemia (22%), fatigue (1%), and headache (1%).
There were two grade 5 treatment-related adverse events – organizing pneumonia on day 37 and staphylococcal bacteremia on day 134.
Cytokine release syndrome (CRS) occurred in 91% of patients, with 15% experiencing grade 3 or higher CRS. Patients were treated with tocilizumab or corticosteroids, and all CRS events resolved.
Neurologic adverse events occurred in 63% of patients, with grade 3 or higher events occurring in 31%. Neurologic events were treated with tocilizumab or corticosteroids, and 86% of neurologic events resolved.
This trial was sponsored by Kite, a Gilead company. Dr. Wang reported financial relationships with Kite and other companies.
SOURCE: Wang M et al. ASH 2019. Abstract 754.
ORLANDO – KTE-X19, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, demonstrated unprecedented efficacy in the ZUMA-2 trial, according to an investigator involved in the study.
KTE-X19 produced a 93% overall response rate in patients with relapsed/refractory mantle cell lymphoma (MCL). This is the highest reported response rate in patients who have failed treatment with a Bruton’s tyrosine kinase (BTK) inhibitor, said Michael L. Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Dr. Wang presented results from ZUMA-2 at the annual meeting of the American Society of Hematology.
“Patients with relapsed/refractory MCL have very poor outcomes,” Dr. Wang noted. “In patients who progress after BTK inhibition therapy, the overall response rate is only between 25% and 42%, and the overall survival is only between 6 and 10 months. Few patients proceed to allogeneic transplantation.”
The phase 2 ZUMA-2 trial was designed to test KTE-X19 in these patients. KTE-X19 is an anti-CD19 CAR T-cell therapy containing a CD3-zeta T-cell activation domain and a CD28 signaling domain. KTE-X19 is distinct from axicabtagene ciloleucel (KTE-C19) because the manufacturing process for KTE-X19 removes circulating tumor cells.
The trial enrolled 74 patients, and 68 of them received KTE-X19. Manufacturing failed for three patients, two patients died of progressive disease before they could receive KTE-X19, and one patient was found to be ineligible for treatment.
The 68 patients had a median age of 65 years (range, 38-79 years), and 84% were men. A majority of patients (85%) had stage IV disease and classical (59%) or blastoid (25%) morphology. Most patients (69%) had a Ki-67 proliferation index of 50% or greater, and most (56%) were intermediate- or high-risk according to the Mantle Cell Lymphoma International Prognostic Index (MIPI).
Patients had received a median of three prior therapies (range, one to five). All had been treated with a BTK inhibitor, with 85% receiving ibrutinib, 24% receiving acalabrutinib, and 9% receiving both. Most patients (68%) were refractory to BTK inhibition, and 32% relapsed on or after BTK inhibitor therapy.
In this study, patients could receive bridging therapy to keep their disease stable while KTE-X19 was being manufactured. There were 25 patients who received bridging therapy, which consisted of ibrutinib (n = 14), acalabrutinib (n = 5), dexamethasone (n = 12), and/or methylprednisolone (n = 2). Six patients received both BTK inhibitors and steroids.
All patients received conditioning with fludarabine and cyclophosphamide, followed by a single infusion of KTE-X19 at 2x106.
Efficacy
Sixty patients were evaluable for efficacy, and the median follow-up was 12.3 months (range, 7.0-32.3 months).
The overall response rate was 93%, with 67% of patients achieving a complete response and 27% achieving a partial response. Three percent of patients had stable disease, and 3% had progressive disease.
“The overall response rate was consistent across key subgroups, without any statistical difference,” Dr. Wang said. “This includes Ki-67, MIPI, and prior use of either steroids or bridging therapy.”
The median time to response was 1.0 month, and the median time to complete response was 3.0 months. Responses deepened over time, with 35% of patients converting from a partial response to a complete response, and 5% converting from stable disease to complete response.
The median duration of response has not been reached. At last follow-up, 57% of all patients and 78% of complete responders were still in response.
The median progression-free and overall survival have not been reached. At 12 months, the progression-free survival rate was 61%, and the overall survival rate was 83%.
Safety
All 68 patients were evaluable for safety. The most common adverse events were pyrexia (94%), neutropenia (87%), thrombocytopenia (74%), anemia (68%), and hypotension (51%).
Grade 3/4 adverse events included pyrexia (13%), neutropenia (85%), thrombocytopenia (51%), anemia (50%), hypotension (22%), hypoxia (21%), hypophosphatemia (22%), fatigue (1%), and headache (1%).
There were two grade 5 treatment-related adverse events – organizing pneumonia on day 37 and staphylococcal bacteremia on day 134.
Cytokine release syndrome (CRS) occurred in 91% of patients, with 15% experiencing grade 3 or higher CRS. Patients were treated with tocilizumab or corticosteroids, and all CRS events resolved.
Neurologic adverse events occurred in 63% of patients, with grade 3 or higher events occurring in 31%. Neurologic events were treated with tocilizumab or corticosteroids, and 86% of neurologic events resolved.
This trial was sponsored by Kite, a Gilead company. Dr. Wang reported financial relationships with Kite and other companies.
SOURCE: Wang M et al. ASH 2019. Abstract 754.
ORLANDO – KTE-X19, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, demonstrated unprecedented efficacy in the ZUMA-2 trial, according to an investigator involved in the study.
KTE-X19 produced a 93% overall response rate in patients with relapsed/refractory mantle cell lymphoma (MCL). This is the highest reported response rate in patients who have failed treatment with a Bruton’s tyrosine kinase (BTK) inhibitor, said Michael L. Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Dr. Wang presented results from ZUMA-2 at the annual meeting of the American Society of Hematology.
“Patients with relapsed/refractory MCL have very poor outcomes,” Dr. Wang noted. “In patients who progress after BTK inhibition therapy, the overall response rate is only between 25% and 42%, and the overall survival is only between 6 and 10 months. Few patients proceed to allogeneic transplantation.”
The phase 2 ZUMA-2 trial was designed to test KTE-X19 in these patients. KTE-X19 is an anti-CD19 CAR T-cell therapy containing a CD3-zeta T-cell activation domain and a CD28 signaling domain. KTE-X19 is distinct from axicabtagene ciloleucel (KTE-C19) because the manufacturing process for KTE-X19 removes circulating tumor cells.
The trial enrolled 74 patients, and 68 of them received KTE-X19. Manufacturing failed for three patients, two patients died of progressive disease before they could receive KTE-X19, and one patient was found to be ineligible for treatment.
The 68 patients had a median age of 65 years (range, 38-79 years), and 84% were men. A majority of patients (85%) had stage IV disease and classical (59%) or blastoid (25%) morphology. Most patients (69%) had a Ki-67 proliferation index of 50% or greater, and most (56%) were intermediate- or high-risk according to the Mantle Cell Lymphoma International Prognostic Index (MIPI).
Patients had received a median of three prior therapies (range, one to five). All had been treated with a BTK inhibitor, with 85% receiving ibrutinib, 24% receiving acalabrutinib, and 9% receiving both. Most patients (68%) were refractory to BTK inhibition, and 32% relapsed on or after BTK inhibitor therapy.
In this study, patients could receive bridging therapy to keep their disease stable while KTE-X19 was being manufactured. There were 25 patients who received bridging therapy, which consisted of ibrutinib (n = 14), acalabrutinib (n = 5), dexamethasone (n = 12), and/or methylprednisolone (n = 2). Six patients received both BTK inhibitors and steroids.
All patients received conditioning with fludarabine and cyclophosphamide, followed by a single infusion of KTE-X19 at 2x106.
Efficacy
Sixty patients were evaluable for efficacy, and the median follow-up was 12.3 months (range, 7.0-32.3 months).
The overall response rate was 93%, with 67% of patients achieving a complete response and 27% achieving a partial response. Three percent of patients had stable disease, and 3% had progressive disease.
“The overall response rate was consistent across key subgroups, without any statistical difference,” Dr. Wang said. “This includes Ki-67, MIPI, and prior use of either steroids or bridging therapy.”
The median time to response was 1.0 month, and the median time to complete response was 3.0 months. Responses deepened over time, with 35% of patients converting from a partial response to a complete response, and 5% converting from stable disease to complete response.
The median duration of response has not been reached. At last follow-up, 57% of all patients and 78% of complete responders were still in response.
The median progression-free and overall survival have not been reached. At 12 months, the progression-free survival rate was 61%, and the overall survival rate was 83%.
Safety
All 68 patients were evaluable for safety. The most common adverse events were pyrexia (94%), neutropenia (87%), thrombocytopenia (74%), anemia (68%), and hypotension (51%).
Grade 3/4 adverse events included pyrexia (13%), neutropenia (85%), thrombocytopenia (51%), anemia (50%), hypotension (22%), hypoxia (21%), hypophosphatemia (22%), fatigue (1%), and headache (1%).
There were two grade 5 treatment-related adverse events – organizing pneumonia on day 37 and staphylococcal bacteremia on day 134.
Cytokine release syndrome (CRS) occurred in 91% of patients, with 15% experiencing grade 3 or higher CRS. Patients were treated with tocilizumab or corticosteroids, and all CRS events resolved.
Neurologic adverse events occurred in 63% of patients, with grade 3 or higher events occurring in 31%. Neurologic events were treated with tocilizumab or corticosteroids, and 86% of neurologic events resolved.
This trial was sponsored by Kite, a Gilead company. Dr. Wang reported financial relationships with Kite and other companies.
SOURCE: Wang M et al. ASH 2019. Abstract 754.
REPORTING FROM ASH 2019
ASH releases guidelines on managing cardiopulmonary and kidney disease in SCD
ORLANDO – It is good practice to consult with a pulmonary hypertension (PH) expert before referring a patient with sickle cell disease (SCD) for right-heart catheterization or PH evaluation, according to new American Society of Hematology guidelines for the screening and management of cardiopulmonary and kidney disease in patients with SCD.
That “Good Practice” recommendation is one of several included in the evidence-based guidelines published Dec. 10 in Blood Advances and highlighted during a Special Education Session at the annual ASH meeting.
The guidelines provide 10 main recommendations intended to “support patients, clinicians, and other health care professionals in their decisions about screening, diagnosis, and management of cardiopulmonary and renal complications of SCD,” wrote Robert I. Liem, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago and colleagues.
The recommendations, agreed upon by a multidisciplinary guideline panel, relate to screening, diagnosis, and management of PH, pulmonary arterial hypertension (PAH), hypertension, proteinuria and chronic kidney disease, and venous thromboembolism (VTE). Most are “conditional,” as opposed to “strong,” because of a paucity of direct, high-quality outcomes data, and they are accompanied by the Good Practice Statements, descriptive remarks and caveats based on the available data, as well as suggestions for future research.
At the special ASH session, Ankit A. Desai, MD, highlighted some of the recommendations and discussed considerations for their practical application.
The Good Practice Statement on consulting a specialist before referring a patient for PH relates specifically to Recommendations 2a and 2b on the management of abnormal echocardiography, explained Dr. Desai of Indiana University, Indianapolis.
For asymptomatic children and adults with SCD and an isolated peak tricuspid regurgitant jet velocity (TRJV) of at least 2.5-2.9 m/s on echocardiography, the panel recommends against right-heart catheterization (Recommendation 2a, conditional), he said.
For children and adults with SCD and a peak TRJV of at least 2.5 m/s who also have a reduced 6-minute walk distance (6MWD) and/or elevated N-terminal proB-type natriuretic peptide (NT-proBNP), the panel supports right-heart catheterization (Recommendation 2b, conditional).
Dr. Desai noted that the 2.5 m/s threshold was found to be suboptimal when used as the sole criteria for right-heart catheterization. Using that threshold alone is associated with “moderate to large” harms, such as starting inappropriate PH-specific therapies and/or performing unnecessary right-heart catheterization. However, when used in combination with 6MWD, the predictive capacity improved significantly, and the risk for potential harm was low, he explained.
Another Good Practice Statement included in the guidelines, and relevant to these recommendations on managing abnormal echocardiography, addresses the importance of basing decisions about the need for right-heart catheterization on echocardiograms obtained at steady state rather than during acute illness, such as during hospitalization for pain or acute chest syndrome.
This is in part because of technical factors, Dr. Desai said.
“We know that repeating [echocardiography] is something that should be considered in patients because ... results vary – sometimes quite a bit – from study to study,” he said.
As for the cutoff values for 6MWD and NT-proBNP, “a decent amount of literature” suggests that less than 333 m and less than 160 pg/ml, respectively, are good thresholds, he said.
“Importantly, this should all be taken in the context of good clinical judgment ... along with discussion with a PH expert,” he added.
The full guidelines are available, along with additional ASH guidelines on immune thrombocytopenia and prevention of venous thromboembolism in surgical hospitalized patients, at the ASH publications website.
Of note, the SCD guidelines on cardiopulmonary disease and kidney disease are one of five sets of SCD guidelines that have been in development; these are the first of those to be published. The remaining four sets of guidelines will address pain, cerebrovascular complications, transfusion, and hematopoietic stem cell transplant. All will be published in Blood Advances, and according to Dr. Liem, the transfusion medicine guidelines have been accepted and should be published in January 2020, followed by those for cerebrovascular complications. Publication of the pain and transplant guidelines are anticipated later in 2020.
Dr. Liem and Dr. Desai reported having no conflicts of interest.
ORLANDO – It is good practice to consult with a pulmonary hypertension (PH) expert before referring a patient with sickle cell disease (SCD) for right-heart catheterization or PH evaluation, according to new American Society of Hematology guidelines for the screening and management of cardiopulmonary and kidney disease in patients with SCD.
That “Good Practice” recommendation is one of several included in the evidence-based guidelines published Dec. 10 in Blood Advances and highlighted during a Special Education Session at the annual ASH meeting.
The guidelines provide 10 main recommendations intended to “support patients, clinicians, and other health care professionals in their decisions about screening, diagnosis, and management of cardiopulmonary and renal complications of SCD,” wrote Robert I. Liem, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago and colleagues.
The recommendations, agreed upon by a multidisciplinary guideline panel, relate to screening, diagnosis, and management of PH, pulmonary arterial hypertension (PAH), hypertension, proteinuria and chronic kidney disease, and venous thromboembolism (VTE). Most are “conditional,” as opposed to “strong,” because of a paucity of direct, high-quality outcomes data, and they are accompanied by the Good Practice Statements, descriptive remarks and caveats based on the available data, as well as suggestions for future research.
At the special ASH session, Ankit A. Desai, MD, highlighted some of the recommendations and discussed considerations for their practical application.
The Good Practice Statement on consulting a specialist before referring a patient for PH relates specifically to Recommendations 2a and 2b on the management of abnormal echocardiography, explained Dr. Desai of Indiana University, Indianapolis.
For asymptomatic children and adults with SCD and an isolated peak tricuspid regurgitant jet velocity (TRJV) of at least 2.5-2.9 m/s on echocardiography, the panel recommends against right-heart catheterization (Recommendation 2a, conditional), he said.
For children and adults with SCD and a peak TRJV of at least 2.5 m/s who also have a reduced 6-minute walk distance (6MWD) and/or elevated N-terminal proB-type natriuretic peptide (NT-proBNP), the panel supports right-heart catheterization (Recommendation 2b, conditional).
Dr. Desai noted that the 2.5 m/s threshold was found to be suboptimal when used as the sole criteria for right-heart catheterization. Using that threshold alone is associated with “moderate to large” harms, such as starting inappropriate PH-specific therapies and/or performing unnecessary right-heart catheterization. However, when used in combination with 6MWD, the predictive capacity improved significantly, and the risk for potential harm was low, he explained.
Another Good Practice Statement included in the guidelines, and relevant to these recommendations on managing abnormal echocardiography, addresses the importance of basing decisions about the need for right-heart catheterization on echocardiograms obtained at steady state rather than during acute illness, such as during hospitalization for pain or acute chest syndrome.
This is in part because of technical factors, Dr. Desai said.
“We know that repeating [echocardiography] is something that should be considered in patients because ... results vary – sometimes quite a bit – from study to study,” he said.
As for the cutoff values for 6MWD and NT-proBNP, “a decent amount of literature” suggests that less than 333 m and less than 160 pg/ml, respectively, are good thresholds, he said.
“Importantly, this should all be taken in the context of good clinical judgment ... along with discussion with a PH expert,” he added.
The full guidelines are available, along with additional ASH guidelines on immune thrombocytopenia and prevention of venous thromboembolism in surgical hospitalized patients, at the ASH publications website.
Of note, the SCD guidelines on cardiopulmonary disease and kidney disease are one of five sets of SCD guidelines that have been in development; these are the first of those to be published. The remaining four sets of guidelines will address pain, cerebrovascular complications, transfusion, and hematopoietic stem cell transplant. All will be published in Blood Advances, and according to Dr. Liem, the transfusion medicine guidelines have been accepted and should be published in January 2020, followed by those for cerebrovascular complications. Publication of the pain and transplant guidelines are anticipated later in 2020.
Dr. Liem and Dr. Desai reported having no conflicts of interest.
ORLANDO – It is good practice to consult with a pulmonary hypertension (PH) expert before referring a patient with sickle cell disease (SCD) for right-heart catheterization or PH evaluation, according to new American Society of Hematology guidelines for the screening and management of cardiopulmonary and kidney disease in patients with SCD.
That “Good Practice” recommendation is one of several included in the evidence-based guidelines published Dec. 10 in Blood Advances and highlighted during a Special Education Session at the annual ASH meeting.
The guidelines provide 10 main recommendations intended to “support patients, clinicians, and other health care professionals in their decisions about screening, diagnosis, and management of cardiopulmonary and renal complications of SCD,” wrote Robert I. Liem, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago and colleagues.
The recommendations, agreed upon by a multidisciplinary guideline panel, relate to screening, diagnosis, and management of PH, pulmonary arterial hypertension (PAH), hypertension, proteinuria and chronic kidney disease, and venous thromboembolism (VTE). Most are “conditional,” as opposed to “strong,” because of a paucity of direct, high-quality outcomes data, and they are accompanied by the Good Practice Statements, descriptive remarks and caveats based on the available data, as well as suggestions for future research.
At the special ASH session, Ankit A. Desai, MD, highlighted some of the recommendations and discussed considerations for their practical application.
The Good Practice Statement on consulting a specialist before referring a patient for PH relates specifically to Recommendations 2a and 2b on the management of abnormal echocardiography, explained Dr. Desai of Indiana University, Indianapolis.
For asymptomatic children and adults with SCD and an isolated peak tricuspid regurgitant jet velocity (TRJV) of at least 2.5-2.9 m/s on echocardiography, the panel recommends against right-heart catheterization (Recommendation 2a, conditional), he said.
For children and adults with SCD and a peak TRJV of at least 2.5 m/s who also have a reduced 6-minute walk distance (6MWD) and/or elevated N-terminal proB-type natriuretic peptide (NT-proBNP), the panel supports right-heart catheterization (Recommendation 2b, conditional).
Dr. Desai noted that the 2.5 m/s threshold was found to be suboptimal when used as the sole criteria for right-heart catheterization. Using that threshold alone is associated with “moderate to large” harms, such as starting inappropriate PH-specific therapies and/or performing unnecessary right-heart catheterization. However, when used in combination with 6MWD, the predictive capacity improved significantly, and the risk for potential harm was low, he explained.
Another Good Practice Statement included in the guidelines, and relevant to these recommendations on managing abnormal echocardiography, addresses the importance of basing decisions about the need for right-heart catheterization on echocardiograms obtained at steady state rather than during acute illness, such as during hospitalization for pain or acute chest syndrome.
This is in part because of technical factors, Dr. Desai said.
“We know that repeating [echocardiography] is something that should be considered in patients because ... results vary – sometimes quite a bit – from study to study,” he said.
As for the cutoff values for 6MWD and NT-proBNP, “a decent amount of literature” suggests that less than 333 m and less than 160 pg/ml, respectively, are good thresholds, he said.
“Importantly, this should all be taken in the context of good clinical judgment ... along with discussion with a PH expert,” he added.
The full guidelines are available, along with additional ASH guidelines on immune thrombocytopenia and prevention of venous thromboembolism in surgical hospitalized patients, at the ASH publications website.
Of note, the SCD guidelines on cardiopulmonary disease and kidney disease are one of five sets of SCD guidelines that have been in development; these are the first of those to be published. The remaining four sets of guidelines will address pain, cerebrovascular complications, transfusion, and hematopoietic stem cell transplant. All will be published in Blood Advances, and according to Dr. Liem, the transfusion medicine guidelines have been accepted and should be published in January 2020, followed by those for cerebrovascular complications. Publication of the pain and transplant guidelines are anticipated later in 2020.
Dr. Liem and Dr. Desai reported having no conflicts of interest.
EXPERT ANALYSIS FROM ASH 2019
Blinatumomab instead of chemo in young patients with relapsed ALL
ORLANDO – In young patients who experience relapse after chemotherapy for B-cell acute lymphoblastic leukemia (B-ALL), the novel agent blinatumomab (Blincyto, Amgen) can be used instead of intensive chemotherapy to try to achieve a second remission, experts say.
In fact, blinatumomab should be the new standard of care in these patients because it yielded better overall survival, was less toxic, and allowed more patients to proceed to transplant, said Robert A. Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins University, Baltimore.
Dr. Brodsky was commenting on new data presented in a late-breaking abstract (LBA1) at the annual meeting of the American Society of Hematology, for which he holds the role of secretary.
These results are “truly practice changing,” he told journalists at a press briefing.
Cure rates for B-ALL in children and adolescents and young adults (AYAs) are high, but for the small group of patients who experience relapse (about 15%), the prognosis is poor.
When relapse occurs in these patients, “it’s a real problem,” Dr. Brodsky explained. “At that point, the major emphasis is trying to get them back into full remission and get them to a transplant,” he continued, “but it’s very hard to get these patients back into remission.”
The standard treatment approach for these patients includes intensive chemotherapy. In the new study, this was compared to monotherapy with blinatumomab, which is described as a bispecific T-cell engager antibody.
The results were presented by Patrick A. Brown, MD, from the division of pediatric oncology at the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University.
The Children’s Oncology Group Study AALL1331 trial was conducted in 208 children and AYA patients with B-ALL after a first relapse. Median follow-up was 1.4 years.
Blinatumomab was superior in achieving both disease-free survival (59.3 plus or minus 5.4% at 2 years vs. 41% plus or minus 6.2% at 2 years with chemo; P = .05) and overall survival (79.4 plus or minus 4.5% at 2 years vs. 59.2 plus or minus 6% at 2 years with chemo; P = .05).
In addition, more patients who received blinatumomab subsequently underwent transplant (79% vs. 45% with chemo; P less than .0001).
The drug was also better tolerated than chemotherapy, causing fewer and less severe toxicities, including fewer cases of grade 3+ infection, sepsis, and mucositis.
Dr. Brown concluded that, for children and AYA patients with high- or intermediate-risk first relapse of B-ALL, blinatumomab is superior to standard chemotherapy as postreinduction consolidation prior to transplant, resulting in fewer and less severe toxicities, higher rates of minimum residual disease response, greater likelihood of proceeding to hematopoietic stem cell transplant, and improved disease-free and overall survival.
Dr. Brown noted that blinatumomab already has conditional approval from the Food and Drug Administration for use in relapsed ALL in both adults and children, but that approval was based on clinical trial data in adults. This is now the definitive trial in children and AYAs, and it should support full approval for this indication, he said.
Dr. Brown has relationships with Novartis, Servier, and Jazz. Many coauthors also have relationships with pharmaceutical companies. Dr. Brodsky has relationships with Achillion, Alexion, and UpToDate.
A version of this story originally appeared on Medscape.com.
ORLANDO – In young patients who experience relapse after chemotherapy for B-cell acute lymphoblastic leukemia (B-ALL), the novel agent blinatumomab (Blincyto, Amgen) can be used instead of intensive chemotherapy to try to achieve a second remission, experts say.
In fact, blinatumomab should be the new standard of care in these patients because it yielded better overall survival, was less toxic, and allowed more patients to proceed to transplant, said Robert A. Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins University, Baltimore.
Dr. Brodsky was commenting on new data presented in a late-breaking abstract (LBA1) at the annual meeting of the American Society of Hematology, for which he holds the role of secretary.
These results are “truly practice changing,” he told journalists at a press briefing.
Cure rates for B-ALL in children and adolescents and young adults (AYAs) are high, but for the small group of patients who experience relapse (about 15%), the prognosis is poor.
When relapse occurs in these patients, “it’s a real problem,” Dr. Brodsky explained. “At that point, the major emphasis is trying to get them back into full remission and get them to a transplant,” he continued, “but it’s very hard to get these patients back into remission.”
The standard treatment approach for these patients includes intensive chemotherapy. In the new study, this was compared to monotherapy with blinatumomab, which is described as a bispecific T-cell engager antibody.
The results were presented by Patrick A. Brown, MD, from the division of pediatric oncology at the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University.
The Children’s Oncology Group Study AALL1331 trial was conducted in 208 children and AYA patients with B-ALL after a first relapse. Median follow-up was 1.4 years.
Blinatumomab was superior in achieving both disease-free survival (59.3 plus or minus 5.4% at 2 years vs. 41% plus or minus 6.2% at 2 years with chemo; P = .05) and overall survival (79.4 plus or minus 4.5% at 2 years vs. 59.2 plus or minus 6% at 2 years with chemo; P = .05).
In addition, more patients who received blinatumomab subsequently underwent transplant (79% vs. 45% with chemo; P less than .0001).
The drug was also better tolerated than chemotherapy, causing fewer and less severe toxicities, including fewer cases of grade 3+ infection, sepsis, and mucositis.
Dr. Brown concluded that, for children and AYA patients with high- or intermediate-risk first relapse of B-ALL, blinatumomab is superior to standard chemotherapy as postreinduction consolidation prior to transplant, resulting in fewer and less severe toxicities, higher rates of minimum residual disease response, greater likelihood of proceeding to hematopoietic stem cell transplant, and improved disease-free and overall survival.
Dr. Brown noted that blinatumomab already has conditional approval from the Food and Drug Administration for use in relapsed ALL in both adults and children, but that approval was based on clinical trial data in adults. This is now the definitive trial in children and AYAs, and it should support full approval for this indication, he said.
Dr. Brown has relationships with Novartis, Servier, and Jazz. Many coauthors also have relationships with pharmaceutical companies. Dr. Brodsky has relationships with Achillion, Alexion, and UpToDate.
A version of this story originally appeared on Medscape.com.
ORLANDO – In young patients who experience relapse after chemotherapy for B-cell acute lymphoblastic leukemia (B-ALL), the novel agent blinatumomab (Blincyto, Amgen) can be used instead of intensive chemotherapy to try to achieve a second remission, experts say.
In fact, blinatumomab should be the new standard of care in these patients because it yielded better overall survival, was less toxic, and allowed more patients to proceed to transplant, said Robert A. Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins University, Baltimore.
Dr. Brodsky was commenting on new data presented in a late-breaking abstract (LBA1) at the annual meeting of the American Society of Hematology, for which he holds the role of secretary.
These results are “truly practice changing,” he told journalists at a press briefing.
Cure rates for B-ALL in children and adolescents and young adults (AYAs) are high, but for the small group of patients who experience relapse (about 15%), the prognosis is poor.
When relapse occurs in these patients, “it’s a real problem,” Dr. Brodsky explained. “At that point, the major emphasis is trying to get them back into full remission and get them to a transplant,” he continued, “but it’s very hard to get these patients back into remission.”
The standard treatment approach for these patients includes intensive chemotherapy. In the new study, this was compared to monotherapy with blinatumomab, which is described as a bispecific T-cell engager antibody.
The results were presented by Patrick A. Brown, MD, from the division of pediatric oncology at the Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University.
The Children’s Oncology Group Study AALL1331 trial was conducted in 208 children and AYA patients with B-ALL after a first relapse. Median follow-up was 1.4 years.
Blinatumomab was superior in achieving both disease-free survival (59.3 plus or minus 5.4% at 2 years vs. 41% plus or minus 6.2% at 2 years with chemo; P = .05) and overall survival (79.4 plus or minus 4.5% at 2 years vs. 59.2 plus or minus 6% at 2 years with chemo; P = .05).
In addition, more patients who received blinatumomab subsequently underwent transplant (79% vs. 45% with chemo; P less than .0001).
The drug was also better tolerated than chemotherapy, causing fewer and less severe toxicities, including fewer cases of grade 3+ infection, sepsis, and mucositis.
Dr. Brown concluded that, for children and AYA patients with high- or intermediate-risk first relapse of B-ALL, blinatumomab is superior to standard chemotherapy as postreinduction consolidation prior to transplant, resulting in fewer and less severe toxicities, higher rates of minimum residual disease response, greater likelihood of proceeding to hematopoietic stem cell transplant, and improved disease-free and overall survival.
Dr. Brown noted that blinatumomab already has conditional approval from the Food and Drug Administration for use in relapsed ALL in both adults and children, but that approval was based on clinical trial data in adults. This is now the definitive trial in children and AYAs, and it should support full approval for this indication, he said.
Dr. Brown has relationships with Novartis, Servier, and Jazz. Many coauthors also have relationships with pharmaceutical companies. Dr. Brodsky has relationships with Achillion, Alexion, and UpToDate.
A version of this story originally appeared on Medscape.com.
‘Real-world’ data show CAR T therapies are cost effective
ORLANDO – Chimeric antigen receptor (CAR) T-cell therapy has been hailed as a major advance and a game changer, but their cost has redefined the meaning of “expensive.”
However, new “real-world” data now suggests that CAR T-cell therapy may actually be cost effective, as it may lower other expenses related to the illness.
When used in a population of older adults with non-Hodgkin lymphoma, these new data show that CAR T-cell therapy cut related expenditures, compared with health care costs prior to receiving this treatment.
“CAR T therapy was associated with fewer hospitalizations, shorter time in the hospital, fewer ED visits, and lower total health care costs,” said lead study author Karl M. Kilgore, PhD, of Avalere Health in Washington, D.C.
He presented the findings at the 2019 annual meeting of the American Society of Hematology (abstract 793).
CAR T-cell therapies were approved in the United States in 2017. First came tisagenlecleucel (Kymriah, Novartis), for the treatment of pediatric and young adult patients with acute lymphoblastic leukemia, with a price tag of $475,000. Closely following it was axicabtagene ciloleucel (Yescarta, Kite/Gliead), indicated for adult patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma who are ineligible for autologous stem cell transplant, with a price tag of $373,000.
The formidable price tags sent shock waves through the blood cancers community, which is struggling to incorporate this novel approach because of the remarkable responses that have been seen.
Real-world experience
In the current study, Dr. Kilgore and colleagues evaluated the demographic and clinical characteristics of Medicare patients who received CAR T therapy (axicabtagene ciloleucel or tisagenlecleucel) and then compared health care utilization, costs, and outcomes pre– and post–CAR T therapy.
“The goal of this study was to look at the real use of CAR T-cell therapy and real-world data on the use of these therapies,” said Dr. Kilgore. “And to look at health care utilization.”
Data was obtained from the Centers for Medicare & Medicaid Services 100% Medicare Fee-for-Service Part A and B claims data, and patients were included in the study if they had been diagnosed with lymphoma and received CAR T therapy between Oct. 1, 2017, and Sept. 30, 2018.
A total of 177 patients met all of the inclusion criteria and were included in the analysis.
The average age was 70 years, more than half (58.8%) were male, and they were primarily white (87.6%). Nearly all patients (91.5%) had a primary diagnosis of diffuse large B-cell lymphoma (DLBCL), as well as multiple comorbidities with 74.6% having a Charlson Comorbidity Index score of 3 or less. Fewer than 5% of patients had undergone a previous autologous stem cell transplant, and 51% had one or more comorbidities that would have disqualified them from participating in CAR T clinical trials (for example, renal failure, heart failure, recent history of deep vein thrombosis/pulmonary embolism).
Just over half of participants (52%) had been treated with intravenous chemotherapy in the 6 months prior to receiving CAR T-cell therapy, and 60% received outpatient lymphodepletion.
During their index episode of care for CAR T infusion, the patients spent a median of 16 days (interquartile range, 10) in the hospital during and 45.5% required ICU care after infusion. In the 6-month period prior to CAR T-cell therapy (preindex), 51.2% had been hospitalized at least once, and almost 20% had three or more periods of hospitalization. Of that group, 27.1% were readmitted during the postindex period.
Among patients who required hospitalization, the median length of stay preindex was 7 days and 5 days post index. The number of ED visits was also lower in the post versus preindex (15.8% vs. 29.9%).
“Patients spent 17% less time in the hospital 6 months after CAR T-cell therapy than before,” he said.
While there were no deaths during the postindex period, a small percentage (less than 5%) were admitted to hospice care. It is unclear if any patients received chemotherapy during the 100-day postindex period, which would suggest disease progression. However, the authors note that claims for the period might be lagging behind for some patients.
Dr. Kilgore pointed out that half of the patients had one or more chronic conditions that, in some cases, would have excluded them from clinical trials. “But 73% remain alive at 6 months,” he said. “We have data that goes out to 21 months, and over 50% are still alive at almost 2 years.”
As for cost, the median total health care costs during the preindex period were $51,999 (mean, 58,820; standard deviation, 45,701) and $14,014 post index (mean, 23,738; SD, 29,698). This extrapolates into $9,749 pre- versus $7,121 post index for each patient per month, which is a 27% decrease in expenditures.
Dr. Kilgore explained that the total paid amounts for CAR T from all sources (Medicare and patient) varied significantly, depending on whether patients were treated in a clinical trial and whether the hospital was reimbursed under standard Medicare prospective payment system for inpatient facilities or through the PPS-exempt payment system.
Impressive survival
Commenting on the study, Sarah Rutherford, MD, a hematologist at Weill Cornell Medical College, New York, and New York–Presbyterian, believes that the key takeaway from this study is that the majority of participants – who were older and sicker than many enrolled on CAR T-cell clinical trials – did quite well.
“Diffuse large B-cell lymphoma is a disease that usually causes people to die quickly if they are refractory to multiple lines of therapy, so the 6-month survival of 75% in this patient population is impressive,” she said. “A large proportion of these patients are likely to have died had they not received CAR T-cell therapy.”
Dr. Rutherford noted that the study authors analyzed the costs associated with patients in the pre– and post–CAR T-cell setting, finding that health care costs were lower following CAR T-cell therapy in this Medicare patient subset, compared with costs prior to the therapy.
“I think CAR T-cell use is certainly justified given the lack of efficacious therapies in relapsed and refractory DLBCL patients, and this study indicates that there may be a financial benefit as well, though the actual costs associated with CAR T-cell therapy were not included in the analysis,” she told Medscape Medical News.
Also weighing in on the study, James Essell, MD, from the U.S. Oncology Network, pointed out that CAR T-cell therapy is changing the paradigm of treatment. For example, refractory lymphoma has a life expectancy of about 6 months. “But with newer data we’re seeing about 50% of patients alive at 3 years after CAR T-cell therapy and we’re thinking that will equate into a cure,” he said.
Dr. Essell explained that, in the past, the scenario would be 6 months of chemotherapy, relapse, other chemotherapy, relapse, and then CAR T, but several clinical trials are now looking at giving CAR T at first relapse. “Instead of waiting until they’ve had a transplant, which is going to be about $100,000 at least, they are going to be randomized between autologous transplant and CAR T up front,” he said.
“We are also doing clinical trials through the network for patients who are not candidates for an autologous transplant,” Dr. Essell continued. “They will go straight to CAR-T therapy and that will really increase the cure rate because these are people who weren’t eligible for any curative therapy – and now they are being given a chance.”
Whether CAR T-cell use will expand to broader populations will depend on the results of the randomized trials that are ongoing now, he added.
“That’s our hypotheses right now, but they are being studied in a wide range of hematologic cancers, and in addition, there is a lot of research in solid tumors,” Dr. Essell said. “I don’t think you’d see this mass amount of research and dollars being poured into it if people didn’t think it was going to be a game changer.”
Dr. Kilgore has disclosed research funding from Kite Pharma. Several of the other coauthors have disclosed relationships with industry, which are noted in the abstract. Dr. Essell has disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
ORLANDO – Chimeric antigen receptor (CAR) T-cell therapy has been hailed as a major advance and a game changer, but their cost has redefined the meaning of “expensive.”
However, new “real-world” data now suggests that CAR T-cell therapy may actually be cost effective, as it may lower other expenses related to the illness.
When used in a population of older adults with non-Hodgkin lymphoma, these new data show that CAR T-cell therapy cut related expenditures, compared with health care costs prior to receiving this treatment.
“CAR T therapy was associated with fewer hospitalizations, shorter time in the hospital, fewer ED visits, and lower total health care costs,” said lead study author Karl M. Kilgore, PhD, of Avalere Health in Washington, D.C.
He presented the findings at the 2019 annual meeting of the American Society of Hematology (abstract 793).
CAR T-cell therapies were approved in the United States in 2017. First came tisagenlecleucel (Kymriah, Novartis), for the treatment of pediatric and young adult patients with acute lymphoblastic leukemia, with a price tag of $475,000. Closely following it was axicabtagene ciloleucel (Yescarta, Kite/Gliead), indicated for adult patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma who are ineligible for autologous stem cell transplant, with a price tag of $373,000.
The formidable price tags sent shock waves through the blood cancers community, which is struggling to incorporate this novel approach because of the remarkable responses that have been seen.
Real-world experience
In the current study, Dr. Kilgore and colleagues evaluated the demographic and clinical characteristics of Medicare patients who received CAR T therapy (axicabtagene ciloleucel or tisagenlecleucel) and then compared health care utilization, costs, and outcomes pre– and post–CAR T therapy.
“The goal of this study was to look at the real use of CAR T-cell therapy and real-world data on the use of these therapies,” said Dr. Kilgore. “And to look at health care utilization.”
Data was obtained from the Centers for Medicare & Medicaid Services 100% Medicare Fee-for-Service Part A and B claims data, and patients were included in the study if they had been diagnosed with lymphoma and received CAR T therapy between Oct. 1, 2017, and Sept. 30, 2018.
A total of 177 patients met all of the inclusion criteria and were included in the analysis.
The average age was 70 years, more than half (58.8%) were male, and they were primarily white (87.6%). Nearly all patients (91.5%) had a primary diagnosis of diffuse large B-cell lymphoma (DLBCL), as well as multiple comorbidities with 74.6% having a Charlson Comorbidity Index score of 3 or less. Fewer than 5% of patients had undergone a previous autologous stem cell transplant, and 51% had one or more comorbidities that would have disqualified them from participating in CAR T clinical trials (for example, renal failure, heart failure, recent history of deep vein thrombosis/pulmonary embolism).
Just over half of participants (52%) had been treated with intravenous chemotherapy in the 6 months prior to receiving CAR T-cell therapy, and 60% received outpatient lymphodepletion.
During their index episode of care for CAR T infusion, the patients spent a median of 16 days (interquartile range, 10) in the hospital during and 45.5% required ICU care after infusion. In the 6-month period prior to CAR T-cell therapy (preindex), 51.2% had been hospitalized at least once, and almost 20% had three or more periods of hospitalization. Of that group, 27.1% were readmitted during the postindex period.
Among patients who required hospitalization, the median length of stay preindex was 7 days and 5 days post index. The number of ED visits was also lower in the post versus preindex (15.8% vs. 29.9%).
“Patients spent 17% less time in the hospital 6 months after CAR T-cell therapy than before,” he said.
While there were no deaths during the postindex period, a small percentage (less than 5%) were admitted to hospice care. It is unclear if any patients received chemotherapy during the 100-day postindex period, which would suggest disease progression. However, the authors note that claims for the period might be lagging behind for some patients.
Dr. Kilgore pointed out that half of the patients had one or more chronic conditions that, in some cases, would have excluded them from clinical trials. “But 73% remain alive at 6 months,” he said. “We have data that goes out to 21 months, and over 50% are still alive at almost 2 years.”
As for cost, the median total health care costs during the preindex period were $51,999 (mean, 58,820; standard deviation, 45,701) and $14,014 post index (mean, 23,738; SD, 29,698). This extrapolates into $9,749 pre- versus $7,121 post index for each patient per month, which is a 27% decrease in expenditures.
Dr. Kilgore explained that the total paid amounts for CAR T from all sources (Medicare and patient) varied significantly, depending on whether patients were treated in a clinical trial and whether the hospital was reimbursed under standard Medicare prospective payment system for inpatient facilities or through the PPS-exempt payment system.
Impressive survival
Commenting on the study, Sarah Rutherford, MD, a hematologist at Weill Cornell Medical College, New York, and New York–Presbyterian, believes that the key takeaway from this study is that the majority of participants – who were older and sicker than many enrolled on CAR T-cell clinical trials – did quite well.
“Diffuse large B-cell lymphoma is a disease that usually causes people to die quickly if they are refractory to multiple lines of therapy, so the 6-month survival of 75% in this patient population is impressive,” she said. “A large proportion of these patients are likely to have died had they not received CAR T-cell therapy.”
Dr. Rutherford noted that the study authors analyzed the costs associated with patients in the pre– and post–CAR T-cell setting, finding that health care costs were lower following CAR T-cell therapy in this Medicare patient subset, compared with costs prior to the therapy.
“I think CAR T-cell use is certainly justified given the lack of efficacious therapies in relapsed and refractory DLBCL patients, and this study indicates that there may be a financial benefit as well, though the actual costs associated with CAR T-cell therapy were not included in the analysis,” she told Medscape Medical News.
Also weighing in on the study, James Essell, MD, from the U.S. Oncology Network, pointed out that CAR T-cell therapy is changing the paradigm of treatment. For example, refractory lymphoma has a life expectancy of about 6 months. “But with newer data we’re seeing about 50% of patients alive at 3 years after CAR T-cell therapy and we’re thinking that will equate into a cure,” he said.
Dr. Essell explained that, in the past, the scenario would be 6 months of chemotherapy, relapse, other chemotherapy, relapse, and then CAR T, but several clinical trials are now looking at giving CAR T at first relapse. “Instead of waiting until they’ve had a transplant, which is going to be about $100,000 at least, they are going to be randomized between autologous transplant and CAR T up front,” he said.
“We are also doing clinical trials through the network for patients who are not candidates for an autologous transplant,” Dr. Essell continued. “They will go straight to CAR-T therapy and that will really increase the cure rate because these are people who weren’t eligible for any curative therapy – and now they are being given a chance.”
Whether CAR T-cell use will expand to broader populations will depend on the results of the randomized trials that are ongoing now, he added.
“That’s our hypotheses right now, but they are being studied in a wide range of hematologic cancers, and in addition, there is a lot of research in solid tumors,” Dr. Essell said. “I don’t think you’d see this mass amount of research and dollars being poured into it if people didn’t think it was going to be a game changer.”
Dr. Kilgore has disclosed research funding from Kite Pharma. Several of the other coauthors have disclosed relationships with industry, which are noted in the abstract. Dr. Essell has disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
ORLANDO – Chimeric antigen receptor (CAR) T-cell therapy has been hailed as a major advance and a game changer, but their cost has redefined the meaning of “expensive.”
However, new “real-world” data now suggests that CAR T-cell therapy may actually be cost effective, as it may lower other expenses related to the illness.
When used in a population of older adults with non-Hodgkin lymphoma, these new data show that CAR T-cell therapy cut related expenditures, compared with health care costs prior to receiving this treatment.
“CAR T therapy was associated with fewer hospitalizations, shorter time in the hospital, fewer ED visits, and lower total health care costs,” said lead study author Karl M. Kilgore, PhD, of Avalere Health in Washington, D.C.
He presented the findings at the 2019 annual meeting of the American Society of Hematology (abstract 793).
CAR T-cell therapies were approved in the United States in 2017. First came tisagenlecleucel (Kymriah, Novartis), for the treatment of pediatric and young adult patients with acute lymphoblastic leukemia, with a price tag of $475,000. Closely following it was axicabtagene ciloleucel (Yescarta, Kite/Gliead), indicated for adult patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma who are ineligible for autologous stem cell transplant, with a price tag of $373,000.
The formidable price tags sent shock waves through the blood cancers community, which is struggling to incorporate this novel approach because of the remarkable responses that have been seen.
Real-world experience
In the current study, Dr. Kilgore and colleagues evaluated the demographic and clinical characteristics of Medicare patients who received CAR T therapy (axicabtagene ciloleucel or tisagenlecleucel) and then compared health care utilization, costs, and outcomes pre– and post–CAR T therapy.
“The goal of this study was to look at the real use of CAR T-cell therapy and real-world data on the use of these therapies,” said Dr. Kilgore. “And to look at health care utilization.”
Data was obtained from the Centers for Medicare & Medicaid Services 100% Medicare Fee-for-Service Part A and B claims data, and patients were included in the study if they had been diagnosed with lymphoma and received CAR T therapy between Oct. 1, 2017, and Sept. 30, 2018.
A total of 177 patients met all of the inclusion criteria and were included in the analysis.
The average age was 70 years, more than half (58.8%) were male, and they were primarily white (87.6%). Nearly all patients (91.5%) had a primary diagnosis of diffuse large B-cell lymphoma (DLBCL), as well as multiple comorbidities with 74.6% having a Charlson Comorbidity Index score of 3 or less. Fewer than 5% of patients had undergone a previous autologous stem cell transplant, and 51% had one or more comorbidities that would have disqualified them from participating in CAR T clinical trials (for example, renal failure, heart failure, recent history of deep vein thrombosis/pulmonary embolism).
Just over half of participants (52%) had been treated with intravenous chemotherapy in the 6 months prior to receiving CAR T-cell therapy, and 60% received outpatient lymphodepletion.
During their index episode of care for CAR T infusion, the patients spent a median of 16 days (interquartile range, 10) in the hospital during and 45.5% required ICU care after infusion. In the 6-month period prior to CAR T-cell therapy (preindex), 51.2% had been hospitalized at least once, and almost 20% had three or more periods of hospitalization. Of that group, 27.1% were readmitted during the postindex period.
Among patients who required hospitalization, the median length of stay preindex was 7 days and 5 days post index. The number of ED visits was also lower in the post versus preindex (15.8% vs. 29.9%).
“Patients spent 17% less time in the hospital 6 months after CAR T-cell therapy than before,” he said.
While there were no deaths during the postindex period, a small percentage (less than 5%) were admitted to hospice care. It is unclear if any patients received chemotherapy during the 100-day postindex period, which would suggest disease progression. However, the authors note that claims for the period might be lagging behind for some patients.
Dr. Kilgore pointed out that half of the patients had one or more chronic conditions that, in some cases, would have excluded them from clinical trials. “But 73% remain alive at 6 months,” he said. “We have data that goes out to 21 months, and over 50% are still alive at almost 2 years.”
As for cost, the median total health care costs during the preindex period were $51,999 (mean, 58,820; standard deviation, 45,701) and $14,014 post index (mean, 23,738; SD, 29,698). This extrapolates into $9,749 pre- versus $7,121 post index for each patient per month, which is a 27% decrease in expenditures.
Dr. Kilgore explained that the total paid amounts for CAR T from all sources (Medicare and patient) varied significantly, depending on whether patients were treated in a clinical trial and whether the hospital was reimbursed under standard Medicare prospective payment system for inpatient facilities or through the PPS-exempt payment system.
Impressive survival
Commenting on the study, Sarah Rutherford, MD, a hematologist at Weill Cornell Medical College, New York, and New York–Presbyterian, believes that the key takeaway from this study is that the majority of participants – who were older and sicker than many enrolled on CAR T-cell clinical trials – did quite well.
“Diffuse large B-cell lymphoma is a disease that usually causes people to die quickly if they are refractory to multiple lines of therapy, so the 6-month survival of 75% in this patient population is impressive,” she said. “A large proportion of these patients are likely to have died had they not received CAR T-cell therapy.”
Dr. Rutherford noted that the study authors analyzed the costs associated with patients in the pre– and post–CAR T-cell setting, finding that health care costs were lower following CAR T-cell therapy in this Medicare patient subset, compared with costs prior to the therapy.
“I think CAR T-cell use is certainly justified given the lack of efficacious therapies in relapsed and refractory DLBCL patients, and this study indicates that there may be a financial benefit as well, though the actual costs associated with CAR T-cell therapy were not included in the analysis,” she told Medscape Medical News.
Also weighing in on the study, James Essell, MD, from the U.S. Oncology Network, pointed out that CAR T-cell therapy is changing the paradigm of treatment. For example, refractory lymphoma has a life expectancy of about 6 months. “But with newer data we’re seeing about 50% of patients alive at 3 years after CAR T-cell therapy and we’re thinking that will equate into a cure,” he said.
Dr. Essell explained that, in the past, the scenario would be 6 months of chemotherapy, relapse, other chemotherapy, relapse, and then CAR T, but several clinical trials are now looking at giving CAR T at first relapse. “Instead of waiting until they’ve had a transplant, which is going to be about $100,000 at least, they are going to be randomized between autologous transplant and CAR T up front,” he said.
“We are also doing clinical trials through the network for patients who are not candidates for an autologous transplant,” Dr. Essell continued. “They will go straight to CAR-T therapy and that will really increase the cure rate because these are people who weren’t eligible for any curative therapy – and now they are being given a chance.”
Whether CAR T-cell use will expand to broader populations will depend on the results of the randomized trials that are ongoing now, he added.
“That’s our hypotheses right now, but they are being studied in a wide range of hematologic cancers, and in addition, there is a lot of research in solid tumors,” Dr. Essell said. “I don’t think you’d see this mass amount of research and dollars being poured into it if people didn’t think it was going to be a game changer.”
Dr. Kilgore has disclosed research funding from Kite Pharma. Several of the other coauthors have disclosed relationships with industry, which are noted in the abstract. Dr. Essell has disclosed no relevant financial relationships.
A version of this story originally appeared on Medscape.com.
An off-the-shelf drug to rival CAR T cells: ‘very exciting’
ORLANDO – An investigational drug that can achieve the same results as complex cell therapy is creating a buzz at the American Society of Hematology (ASH) meeting.
For the last few years, attention at this meeting has focused on the chimeric antigen receptor (CAR) T cells, mainly “because of their incredible efficacy,” commented ASH Secretary Robert A. Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins University, Baltimore.
But new results with an off-the-shelf product are “very exciting,” he said, because the drug can be given immediately and appears to achieve similar results.
The new product is mosunetuzumab (Genentech/Roche), a bispecific antibody that targets both CD3 (on the surface of T cells) and CD20 (on the surface of B cells). It works by redirecting T cells to engage and eliminate malignant B cells.
“The concept here is that this monoclonal antibody engages T cells and directs their cytotoxicity against B cells – it’s basically an antibody using the patient’s own T cells to do what a CAR T cell would do,” Dr. Brodsky explained.
However, unlike CAR T cells, which are prepared for each individual patient in a complex process that involves genetic engineering that can take several weeks, mosunetuzumab is an off-the-shelf product that can be given to patients immediately (by intravenous infusion).
This is important, commented Dr. Brodsky, because very-poor-prognosis patients can deteriorate rapidly, and some may not survive while the CAR T cells are being made.
Clinical trial results
Clinical results come from a phase 1/1b trial (known as GO29781) conducted in 270 patients with poor-prognosis refractory/relapsed non-Hodgkin’s lymphoma. These patients had previously been treated with a median of three therapies; in addition, 30 patients (11%) were resistant to or had relapsed after an initial response to CAR T-cell therapy, and 77 patients (29%) had progressed after a stem cell transplant.
“These patients had no available therapy that would be expected to improve survival,” noted lead author Stephen J. Schuster, MD, of Abramson Cancer Center at the University of Pennsylvania in Philadelphia.
All patients received mosunetuzumab with an initial treatment of eight cycles. Patients who achieved complete remission (CR) stopped therapy, while patients who had a partial response or had stable disease, continued treatment for 17 cycles.
Two-thirds of patients (n = 180; 67%) had aggressive lymphomas, mainly diffuse large B-cell lymphoma (DLBCL; n = 117), while 85 patients (31%) had indolent disease, mainly follicular lymphoma (FL; n = 82). Objective responses were seen in 46 of 124 patients (37%) with aggressive lymphomas, and 24 (19%) of these patients achieved a CR.
Among patients with indolent lymphoma, objective responses were seen in 42 of 67 patients (63%), and 29 of 67 (43%) had CR.
The complete remissions appear to be long lasting, Dr. Schuster commented. With a median follow-up of 6 months since achieving CR, 17 of 24 patients (71%) with aggressive lymphoma and 24 of 29 patients (83%) with indolent lymphomas remained free of disease.
“Some patients have remained in remission without additional therapy for more than a year,” he commented.
In the subgroup of 30 patients who had previously received CAR T-cell therapy, the objective response rate was 38.9%, and CR was achieved in 4 patients (22%). These rates are similar to what was seen in patients with aggressive lymphoma who had not previously received CAR T-cell therapy, Dr. Schuster commented.
He also noted that in some of these patients, molecular testing showed that the previously administered CAR T cells increased in number. This suggests that, in addition to its ability to kill cancerous B cells, mosunetuzumab may also help augment the effect of the prior CAR T-cell treatment.
Dr. Schuster also highlighted the results of repeat treatment with mosunetuzumab. Patients who achieved CR stopped treatment – but if they relapsed, they were treated again, and the responses seen on this repeat treatment were similar to those seen with initial treatment. “This is not seen with the CAR T cells,” he noted.
Adverse events with mosunetuzumab were similar to those seen with CAR T cells, he noted, namely cytokine release syndrome, which was mostly mild and seen in 29% of patients, and neurologic toxicity, which was moderately severe in 4% patients.
Overall, the results show that “mosunetuzumab generates long-lasting responses with a very tolerable safety profile in patients with B-cell non-Hodgkin lymphomas for whom multiple prior treatments have failed and whose prognosis is poor. Of particular interest, we are seeing durable complete remissions in patients whose lymphomas progressed after CAR T,” Dr. Schuster commented in a statement.
Approached for comment, Peter Martin MD, chief of the Lymphoma Program at Weill Cornell Medicine, New York, and New York-Presbyterian, said he was excited to see these new data. “It’s good news any time we find something with the potential to save lives.”
“The more options that we have to offer to people with lymphoma the better,” he told Medscape Medical News. “There will always be scenarios where one approach might be better than another. I think there is a good chance that bispecific antibodies will have fairly broad approval in previously treated DLBCL. In many centers, it may be that bispecific antibodies are used most frequently post–CAR T cells, while in other areas people who aren’t candidates for CAR T cells or can’t receive them for whatever reason [could benefit from this new approach].”
Laurie Sehn, MD, MPH, medical oncologist at the University of British Columbia in Vancouver, Canada, and chair of the Lymphoma Tumour Group, as well as an associate editor of ASH journal Blood, also commented for Medscape Medical News.
She agreed that the new data are exciting and noted that this abstract was chosen for the plenary session. She thought the data in the 30 patients who had already been treated with CAR T cells was interesting. “This is a patient population with no other options that offer durable benefit, and mosunetuzumab clearly has clinical activity, with encouraging responses.”
Dr. Sehn also noted that toxicity seen with the drug was “far less” than has been seen with CAR T cells, and the risk of high-grade cytokine release syndrome and neurological toxicity is “very low.”
There are several other new products that are using this bispecific technology, she noted. One example is Regeneron’s REGN1979, a bispecific antibody targeting CD20 and CD3, which is also being investigated in a clinical trial in relapsed/refractory B-cell non-Hodgkin’s lymphoma, including in patients who were previously treated with CAR T cells (abstract 762).
How would it be used clinically?
In response to a question from Medscape Medical News, Dr. Schuster suggested that initial use of mosunetuzumab would be in patients who have already tried CAR T-cell therapy and had either not responded or relapsed – in lymphoma, this is about two-thirds of patients who are treated with this approach. This group of patients represents an unmet medical need, and this indication may be the quickest route to approval, he suggested.
Gary Schiller, MD, from UCLA Health, who moderated the press briefing agreed, and said this would be the quickest route to market because it would need only a phase 2 clinical trial in this specific patient population. But this would likely be only the first use for this product, and then it could be expanded to a broader patient population, he added.
Another use would for mosunetuzumab would be to enhance CAR T-cell responses by redirecting the CAR T cells to other antigens without doing any additional gene editing, Dr. Schuster commented. The idea here is to “revive” previously administered CAR T cells that have stopped working, Dr. Schiller added.
This is a chemotherapy-free approach, Dr. Schuster emphasized. “In patients who have not had a lot of chemotherapy, you can see an increase in T cells,” he commented.
Mosunetuzumab “stimulates and invigorates T cells,” and it could be useful as a pretreatment or a bridge to CAR T-cell therapy, he said.
So the product could be used before CAR T-cell therapy, and equally it could be used after CAR T-cell therapy because it could boost responses in both cases.
“Larger, randomized trials are needed to further confirm these promising data and determine whether the treatment benefit of mosunetuzumab is enhanced when it is used earlier in the course of lymphoma therapy or in combination with other agents,” he added.
Genentech says that mosunetuzumab and another bispecific antibody, CD20-TCB, are being evaluated in a robust clinical development program, both as a monotherapies and in combination with other therapies, in both aggressive and indolent non-Hodgkin’s lymphoma.
Dr. Schuster reported relationships with Celgene, Genentech, Merck, Pharmacyclics, Acerta, AbbVie, Gilead, Nordic Nanovector, Pfizer, AstraZeneca, Loxo Oncology, and Novartis. Coauthors also have multiple disclosures, and several are employees of Genentech and Roche. Dr. Sehn consults with several pharmaceutics companies, including Verastem, Roche/Genentech, Morphosys, Takeda, Janssen, Lundbeck, Amgen, Teva, and AbbVie.
A version of this story originally appeared on Medscape.com.
ORLANDO – An investigational drug that can achieve the same results as complex cell therapy is creating a buzz at the American Society of Hematology (ASH) meeting.
For the last few years, attention at this meeting has focused on the chimeric antigen receptor (CAR) T cells, mainly “because of their incredible efficacy,” commented ASH Secretary Robert A. Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins University, Baltimore.
But new results with an off-the-shelf product are “very exciting,” he said, because the drug can be given immediately and appears to achieve similar results.
The new product is mosunetuzumab (Genentech/Roche), a bispecific antibody that targets both CD3 (on the surface of T cells) and CD20 (on the surface of B cells). It works by redirecting T cells to engage and eliminate malignant B cells.
“The concept here is that this monoclonal antibody engages T cells and directs their cytotoxicity against B cells – it’s basically an antibody using the patient’s own T cells to do what a CAR T cell would do,” Dr. Brodsky explained.
However, unlike CAR T cells, which are prepared for each individual patient in a complex process that involves genetic engineering that can take several weeks, mosunetuzumab is an off-the-shelf product that can be given to patients immediately (by intravenous infusion).
This is important, commented Dr. Brodsky, because very-poor-prognosis patients can deteriorate rapidly, and some may not survive while the CAR T cells are being made.
Clinical trial results
Clinical results come from a phase 1/1b trial (known as GO29781) conducted in 270 patients with poor-prognosis refractory/relapsed non-Hodgkin’s lymphoma. These patients had previously been treated with a median of three therapies; in addition, 30 patients (11%) were resistant to or had relapsed after an initial response to CAR T-cell therapy, and 77 patients (29%) had progressed after a stem cell transplant.
“These patients had no available therapy that would be expected to improve survival,” noted lead author Stephen J. Schuster, MD, of Abramson Cancer Center at the University of Pennsylvania in Philadelphia.
All patients received mosunetuzumab with an initial treatment of eight cycles. Patients who achieved complete remission (CR) stopped therapy, while patients who had a partial response or had stable disease, continued treatment for 17 cycles.
Two-thirds of patients (n = 180; 67%) had aggressive lymphomas, mainly diffuse large B-cell lymphoma (DLBCL; n = 117), while 85 patients (31%) had indolent disease, mainly follicular lymphoma (FL; n = 82). Objective responses were seen in 46 of 124 patients (37%) with aggressive lymphomas, and 24 (19%) of these patients achieved a CR.
Among patients with indolent lymphoma, objective responses were seen in 42 of 67 patients (63%), and 29 of 67 (43%) had CR.
The complete remissions appear to be long lasting, Dr. Schuster commented. With a median follow-up of 6 months since achieving CR, 17 of 24 patients (71%) with aggressive lymphoma and 24 of 29 patients (83%) with indolent lymphomas remained free of disease.
“Some patients have remained in remission without additional therapy for more than a year,” he commented.
In the subgroup of 30 patients who had previously received CAR T-cell therapy, the objective response rate was 38.9%, and CR was achieved in 4 patients (22%). These rates are similar to what was seen in patients with aggressive lymphoma who had not previously received CAR T-cell therapy, Dr. Schuster commented.
He also noted that in some of these patients, molecular testing showed that the previously administered CAR T cells increased in number. This suggests that, in addition to its ability to kill cancerous B cells, mosunetuzumab may also help augment the effect of the prior CAR T-cell treatment.
Dr. Schuster also highlighted the results of repeat treatment with mosunetuzumab. Patients who achieved CR stopped treatment – but if they relapsed, they were treated again, and the responses seen on this repeat treatment were similar to those seen with initial treatment. “This is not seen with the CAR T cells,” he noted.
Adverse events with mosunetuzumab were similar to those seen with CAR T cells, he noted, namely cytokine release syndrome, which was mostly mild and seen in 29% of patients, and neurologic toxicity, which was moderately severe in 4% patients.
Overall, the results show that “mosunetuzumab generates long-lasting responses with a very tolerable safety profile in patients with B-cell non-Hodgkin lymphomas for whom multiple prior treatments have failed and whose prognosis is poor. Of particular interest, we are seeing durable complete remissions in patients whose lymphomas progressed after CAR T,” Dr. Schuster commented in a statement.
Approached for comment, Peter Martin MD, chief of the Lymphoma Program at Weill Cornell Medicine, New York, and New York-Presbyterian, said he was excited to see these new data. “It’s good news any time we find something with the potential to save lives.”
“The more options that we have to offer to people with lymphoma the better,” he told Medscape Medical News. “There will always be scenarios where one approach might be better than another. I think there is a good chance that bispecific antibodies will have fairly broad approval in previously treated DLBCL. In many centers, it may be that bispecific antibodies are used most frequently post–CAR T cells, while in other areas people who aren’t candidates for CAR T cells or can’t receive them for whatever reason [could benefit from this new approach].”
Laurie Sehn, MD, MPH, medical oncologist at the University of British Columbia in Vancouver, Canada, and chair of the Lymphoma Tumour Group, as well as an associate editor of ASH journal Blood, also commented for Medscape Medical News.
She agreed that the new data are exciting and noted that this abstract was chosen for the plenary session. She thought the data in the 30 patients who had already been treated with CAR T cells was interesting. “This is a patient population with no other options that offer durable benefit, and mosunetuzumab clearly has clinical activity, with encouraging responses.”
Dr. Sehn also noted that toxicity seen with the drug was “far less” than has been seen with CAR T cells, and the risk of high-grade cytokine release syndrome and neurological toxicity is “very low.”
There are several other new products that are using this bispecific technology, she noted. One example is Regeneron’s REGN1979, a bispecific antibody targeting CD20 and CD3, which is also being investigated in a clinical trial in relapsed/refractory B-cell non-Hodgkin’s lymphoma, including in patients who were previously treated with CAR T cells (abstract 762).
How would it be used clinically?
In response to a question from Medscape Medical News, Dr. Schuster suggested that initial use of mosunetuzumab would be in patients who have already tried CAR T-cell therapy and had either not responded or relapsed – in lymphoma, this is about two-thirds of patients who are treated with this approach. This group of patients represents an unmet medical need, and this indication may be the quickest route to approval, he suggested.
Gary Schiller, MD, from UCLA Health, who moderated the press briefing agreed, and said this would be the quickest route to market because it would need only a phase 2 clinical trial in this specific patient population. But this would likely be only the first use for this product, and then it could be expanded to a broader patient population, he added.
Another use would for mosunetuzumab would be to enhance CAR T-cell responses by redirecting the CAR T cells to other antigens without doing any additional gene editing, Dr. Schuster commented. The idea here is to “revive” previously administered CAR T cells that have stopped working, Dr. Schiller added.
This is a chemotherapy-free approach, Dr. Schuster emphasized. “In patients who have not had a lot of chemotherapy, you can see an increase in T cells,” he commented.
Mosunetuzumab “stimulates and invigorates T cells,” and it could be useful as a pretreatment or a bridge to CAR T-cell therapy, he said.
So the product could be used before CAR T-cell therapy, and equally it could be used after CAR T-cell therapy because it could boost responses in both cases.
“Larger, randomized trials are needed to further confirm these promising data and determine whether the treatment benefit of mosunetuzumab is enhanced when it is used earlier in the course of lymphoma therapy or in combination with other agents,” he added.
Genentech says that mosunetuzumab and another bispecific antibody, CD20-TCB, are being evaluated in a robust clinical development program, both as a monotherapies and in combination with other therapies, in both aggressive and indolent non-Hodgkin’s lymphoma.
Dr. Schuster reported relationships with Celgene, Genentech, Merck, Pharmacyclics, Acerta, AbbVie, Gilead, Nordic Nanovector, Pfizer, AstraZeneca, Loxo Oncology, and Novartis. Coauthors also have multiple disclosures, and several are employees of Genentech and Roche. Dr. Sehn consults with several pharmaceutics companies, including Verastem, Roche/Genentech, Morphosys, Takeda, Janssen, Lundbeck, Amgen, Teva, and AbbVie.
A version of this story originally appeared on Medscape.com.
ORLANDO – An investigational drug that can achieve the same results as complex cell therapy is creating a buzz at the American Society of Hematology (ASH) meeting.
For the last few years, attention at this meeting has focused on the chimeric antigen receptor (CAR) T cells, mainly “because of their incredible efficacy,” commented ASH Secretary Robert A. Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins University, Baltimore.
But new results with an off-the-shelf product are “very exciting,” he said, because the drug can be given immediately and appears to achieve similar results.
The new product is mosunetuzumab (Genentech/Roche), a bispecific antibody that targets both CD3 (on the surface of T cells) and CD20 (on the surface of B cells). It works by redirecting T cells to engage and eliminate malignant B cells.
“The concept here is that this monoclonal antibody engages T cells and directs their cytotoxicity against B cells – it’s basically an antibody using the patient’s own T cells to do what a CAR T cell would do,” Dr. Brodsky explained.
However, unlike CAR T cells, which are prepared for each individual patient in a complex process that involves genetic engineering that can take several weeks, mosunetuzumab is an off-the-shelf product that can be given to patients immediately (by intravenous infusion).
This is important, commented Dr. Brodsky, because very-poor-prognosis patients can deteriorate rapidly, and some may not survive while the CAR T cells are being made.
Clinical trial results
Clinical results come from a phase 1/1b trial (known as GO29781) conducted in 270 patients with poor-prognosis refractory/relapsed non-Hodgkin’s lymphoma. These patients had previously been treated with a median of three therapies; in addition, 30 patients (11%) were resistant to or had relapsed after an initial response to CAR T-cell therapy, and 77 patients (29%) had progressed after a stem cell transplant.
“These patients had no available therapy that would be expected to improve survival,” noted lead author Stephen J. Schuster, MD, of Abramson Cancer Center at the University of Pennsylvania in Philadelphia.
All patients received mosunetuzumab with an initial treatment of eight cycles. Patients who achieved complete remission (CR) stopped therapy, while patients who had a partial response or had stable disease, continued treatment for 17 cycles.
Two-thirds of patients (n = 180; 67%) had aggressive lymphomas, mainly diffuse large B-cell lymphoma (DLBCL; n = 117), while 85 patients (31%) had indolent disease, mainly follicular lymphoma (FL; n = 82). Objective responses were seen in 46 of 124 patients (37%) with aggressive lymphomas, and 24 (19%) of these patients achieved a CR.
Among patients with indolent lymphoma, objective responses were seen in 42 of 67 patients (63%), and 29 of 67 (43%) had CR.
The complete remissions appear to be long lasting, Dr. Schuster commented. With a median follow-up of 6 months since achieving CR, 17 of 24 patients (71%) with aggressive lymphoma and 24 of 29 patients (83%) with indolent lymphomas remained free of disease.
“Some patients have remained in remission without additional therapy for more than a year,” he commented.
In the subgroup of 30 patients who had previously received CAR T-cell therapy, the objective response rate was 38.9%, and CR was achieved in 4 patients (22%). These rates are similar to what was seen in patients with aggressive lymphoma who had not previously received CAR T-cell therapy, Dr. Schuster commented.
He also noted that in some of these patients, molecular testing showed that the previously administered CAR T cells increased in number. This suggests that, in addition to its ability to kill cancerous B cells, mosunetuzumab may also help augment the effect of the prior CAR T-cell treatment.
Dr. Schuster also highlighted the results of repeat treatment with mosunetuzumab. Patients who achieved CR stopped treatment – but if they relapsed, they were treated again, and the responses seen on this repeat treatment were similar to those seen with initial treatment. “This is not seen with the CAR T cells,” he noted.
Adverse events with mosunetuzumab were similar to those seen with CAR T cells, he noted, namely cytokine release syndrome, which was mostly mild and seen in 29% of patients, and neurologic toxicity, which was moderately severe in 4% patients.
Overall, the results show that “mosunetuzumab generates long-lasting responses with a very tolerable safety profile in patients with B-cell non-Hodgkin lymphomas for whom multiple prior treatments have failed and whose prognosis is poor. Of particular interest, we are seeing durable complete remissions in patients whose lymphomas progressed after CAR T,” Dr. Schuster commented in a statement.
Approached for comment, Peter Martin MD, chief of the Lymphoma Program at Weill Cornell Medicine, New York, and New York-Presbyterian, said he was excited to see these new data. “It’s good news any time we find something with the potential to save lives.”
“The more options that we have to offer to people with lymphoma the better,” he told Medscape Medical News. “There will always be scenarios where one approach might be better than another. I think there is a good chance that bispecific antibodies will have fairly broad approval in previously treated DLBCL. In many centers, it may be that bispecific antibodies are used most frequently post–CAR T cells, while in other areas people who aren’t candidates for CAR T cells or can’t receive them for whatever reason [could benefit from this new approach].”
Laurie Sehn, MD, MPH, medical oncologist at the University of British Columbia in Vancouver, Canada, and chair of the Lymphoma Tumour Group, as well as an associate editor of ASH journal Blood, also commented for Medscape Medical News.
She agreed that the new data are exciting and noted that this abstract was chosen for the plenary session. She thought the data in the 30 patients who had already been treated with CAR T cells was interesting. “This is a patient population with no other options that offer durable benefit, and mosunetuzumab clearly has clinical activity, with encouraging responses.”
Dr. Sehn also noted that toxicity seen with the drug was “far less” than has been seen with CAR T cells, and the risk of high-grade cytokine release syndrome and neurological toxicity is “very low.”
There are several other new products that are using this bispecific technology, she noted. One example is Regeneron’s REGN1979, a bispecific antibody targeting CD20 and CD3, which is also being investigated in a clinical trial in relapsed/refractory B-cell non-Hodgkin’s lymphoma, including in patients who were previously treated with CAR T cells (abstract 762).
How would it be used clinically?
In response to a question from Medscape Medical News, Dr. Schuster suggested that initial use of mosunetuzumab would be in patients who have already tried CAR T-cell therapy and had either not responded or relapsed – in lymphoma, this is about two-thirds of patients who are treated with this approach. This group of patients represents an unmet medical need, and this indication may be the quickest route to approval, he suggested.
Gary Schiller, MD, from UCLA Health, who moderated the press briefing agreed, and said this would be the quickest route to market because it would need only a phase 2 clinical trial in this specific patient population. But this would likely be only the first use for this product, and then it could be expanded to a broader patient population, he added.
Another use would for mosunetuzumab would be to enhance CAR T-cell responses by redirecting the CAR T cells to other antigens without doing any additional gene editing, Dr. Schuster commented. The idea here is to “revive” previously administered CAR T cells that have stopped working, Dr. Schiller added.
This is a chemotherapy-free approach, Dr. Schuster emphasized. “In patients who have not had a lot of chemotherapy, you can see an increase in T cells,” he commented.
Mosunetuzumab “stimulates and invigorates T cells,” and it could be useful as a pretreatment or a bridge to CAR T-cell therapy, he said.
So the product could be used before CAR T-cell therapy, and equally it could be used after CAR T-cell therapy because it could boost responses in both cases.
“Larger, randomized trials are needed to further confirm these promising data and determine whether the treatment benefit of mosunetuzumab is enhanced when it is used earlier in the course of lymphoma therapy or in combination with other agents,” he added.
Genentech says that mosunetuzumab and another bispecific antibody, CD20-TCB, are being evaluated in a robust clinical development program, both as a monotherapies and in combination with other therapies, in both aggressive and indolent non-Hodgkin’s lymphoma.
Dr. Schuster reported relationships with Celgene, Genentech, Merck, Pharmacyclics, Acerta, AbbVie, Gilead, Nordic Nanovector, Pfizer, AstraZeneca, Loxo Oncology, and Novartis. Coauthors also have multiple disclosures, and several are employees of Genentech and Roche. Dr. Sehn consults with several pharmaceutics companies, including Verastem, Roche/Genentech, Morphosys, Takeda, Janssen, Lundbeck, Amgen, Teva, and AbbVie.
A version of this story originally appeared on Medscape.com.
Oral azacitidine: First maintenance therapy for AML
ORLANDO – For the first time, there is a maintenance therapy for patients with acute myeloid leukemia (AML) in remission that can improve overall survival – a new oral formulation of an old drug, azacitidine, known as CC-486 (Celgene).
“Oral azacitidine represents a new therapeutic standard for patients with AML in remission,” said lead author Andrew H. Wei, MBBS, PhD, from the Alfred Hospital in Melbourne.
“It’s not too hard to get these patients into remission,” commented another expert. “The problem comes in keeping them in remission.”
Dr. Wei noted that standard treatment with intensive induction chemotherapy for AML induces complete remission (CR) in 60%-80% of patients aged 60 years or younger and in 40%-60% of patients aged 60 years or older.
However, the majority of patients who attain complete remission (CR) will eventually relapse, and relapse is the primary obstacle to long-term survival, he said.
Despite various attempts, there has been no success over the past 30 years in defining maintenance treatment for these patients, Dr. Wei said.
The new results suggest that oral azacitidine could be an effective maintenance therapy.
Dr. Wei presented the results at the 2019 annual meeting of the American Society of Hematology. They come from the QUAZAR AML-001 study, conducted in 472 patients with poor-risk AML in first remission.
The results show that CC-486 significantly improved outcomes, compared with placebo plus best supportive care, in terms of median overall survival (24.7 vs. 14.8 months) and median relapse-free survival (10.2 vs. 4.8 months).
The trial was funded by Celgene, which said it will be submitting the data for regulatory approval for the new oral formulation of azacitidine, CC-486.
Experts predict new standard of care
Experts approached for comment agreed that maintenance oral azacitidine will become the new standard of care for patients with AML in first remission.
“Unlike therapy for acute lymphoblastic leukemia, maintenance therapy has not been part of the treatment algorithm for AML patients in first remission,” Harry P. Erba, MD, PhD, director of the leukemia program at the Duke Cancer Institute, Durham, N.C., told Medscape Medical News.
He explained that trials for maintenance after first remission in AML have failed. Recently, Dr. Erba noted, the HOVON97 trial with injectable azacitidine demonstrated improvement in relapse-free survival, compared with observation for older AML patients achieving remission after induction therapy. “However, there was no improvement in overall survival,” he said.
“Remission in AML is short lived,” Dr. Erba said. Oral azacitidine represents the first maintenance therapy in AML that has shown both significant and clinically meaningful improvements in overall and relapse-free survival and will represent a new standard of care for patients with AML in remission, Dr. Erba said. “Maintenance oral azacitidine will be practice changing,” he predicted.
HOVON97 was a small study of injectable azacitidine used as maintenance therapy for 12 months, but it was slow to accrue and did not meet its accrual target.
“In HOVON97, at 12 months, only one third of patients received less than the 12 cycles of therapy,” Dr. Wei said. He explained that, with injectable azacitidine, patients have to come into the hospital/clinic for 7 days a month, 84 days a year. Oral azacitidine is more convenient as patients do not have to come into the clinic, he said.
Dr. Wei pointed out that about 40 patients in the QUAZAR study, which started in 2013, are still on maintenance therapy, with one patient now having received 80 cycles of therapy (approximately 7 years). “Long-term maintenance therapy with azacitidine is possible,” he said.
Another expert was also impressed by the new results. “This is an important clinical trial that addresses an unmet need in AML care,” said John Mascarenhas, MD, director of the Adult Leukemia Program and leader of clinical investigation within the myeloproliferative disorders program at the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai, New York.
“Older patients can often receive induction chemotherapy but frequently do not ultimately do well, as the disease relapses and survival is limited,” he explained.
“This large, randomized, double-blind, controlled study of intermediate- or poor-risk AML patients over the age of 55 years supports the use of maintenance oral azacitidine after initial remission to extend overall and relapse-free survival in older AML patients not eligible for transplant,” Dr. Mascarenhas said.
“This is still not a curative approach,” Dr. Wei said, but added that it prolongs relapse-free survival for older patients while maintaining a quality of life for as long as possible.
Study details
The QUAZAR phase 3 study enrolled patients with poor- or intermediate-risk cytogenetics who had an Eastern Cooperative Oncology Group performance status less than or equal to 3 and who had achieved CR or CR with incomplete count recovery (CRi) after induction therapy with or without consolidation therapy. In addition, patients were not candidates for stem cell transplants.
Patients had predominantly de novo AML (89%). Other baseline characteristics of note:
- 85% of patients had intermediate-risk and 15% had poor-risk cytogenetics
- 79% achieved CR and 21% achieved CRi after induction therapy
- 78% received at least one cycle of consolidation therapy
- 43% of patients had minimal residual disease (MRD)–positive disease
Patients were randomized to receive oral azacitidine 200 mg daily on days 1-14 of a repeat 28-day cycle (n = 278) or matching placebo (n = 274). Treatment was continued indefinitely until blast count was more than 15% or patients experienced unacceptable toxicity or went on to transplant.
At a median follow-up of over 41.2 months (3 years, 5 months), median overall survival was significantly longer for patients receiving oral azacitidine at 24.7 months versus 14.8 months for placebo (P less than .0009; hazard ratio, 0.69).
Relapse-free survival was also significantly prolonged, to 10.2 months for patients on oral azacitidine versus 4.8 months for placebo (HR, 0.65; P less than .0001).
Patients on oral azacitidine reported more grade 1-2 gastrointestinal adverse events, such as nausea (65% vs. 24% on placebo), vomiting (60% vs. 10%), and diarrhea (50% vs 22%), as well as more cytopenia. The most common grade 3-4 adverse events were neutropenia (41% with oral azacitidine vs. 24% on placebo), thrombocytopenia (23% vs. 22%), and anemia (14% vs. 13%).
Although Dr. Erba supported the use of oral azacitidine as maintenance therapy, he pointed out that it was hard to convince patients, especially older ones, to continue on maintenance therapy indefinitely. “The toxicities of continuing on a drug indefinitely are real issues,” he said, explaining that most elderly patients cannot cope with even grade 1-2 nausea, diarrhea, and vomiting over the long term.
But he noted that, regardless of the higher incidence of some adverse events with oral azacitidine, the health-related quality of life of patients on oral azacitidine was similar to those on placebo.
Awaiting longer follow-up
Both experts said that longer-term follow-up is needed.
“We need a longer follow-up to see how the curves plateau,” Dr. Erba said. He would also like to see a comparative analysis of the data in patients who are MRD negative versus those who are MRD positive.
“The final results of this study, including the impact of measurable residual disease on outcome in this setting, will potentially have practice-changing implications,” said Dr. Mascarenhas.
At the press conference, Dr. Wei pointed out that, based on the data from QUAZAR, oral azacitidine is likely to be evaluated in the frontline setting of AML. “The elderly make up about two-thirds of all AML patients, and oral azacitidine will be a better option than 7 days per month for chemotherapy treatment in the clinic,” he said. “Oral azacitidine in the future may also be the backbone for other combinations.”
The study was funded by Celgene.
Dr. Wei receives honoraria from AbbVie, Macrogenics, Pfizer, Astellas, Janssen, Servier, Celgene, Amgen, AstraZeneca, Novartis, and Genentech; is on the board of directors or serves on the advisory committees for AbbVie, Macrogenics, Pfizer, Astellas, Servier, Celgene, Amgen, Novartis, and Genentech; and receives research funding from AbbVie, Servier, Celgene, Amgen, AstraZeneca, and Novartis. As a former employee of the Walter and Eliza Hall Institute, Dr. Wei receives a fraction of its royalty stream related to venetoclax.
A partial list of Dr. Erba’s conflict of interest includes consulting with Agios, Novartis, Daiichi Sankyo, MacroGenics, Jazz Pharmaceuticals, Seattle Genetics, GlycoMimetics, Amgen, Pfizer, Celgene, AbbVie, Covance, Immunogen, Astellas Pharma, Incyte; being on the speakers bureau or receiving lecture fees from Agios, Novartis, MacroGenics, Jazz Pharmaceuticals, Celgene; receiving research funding from Novartis, Daiichi Sankyo, MacroGenics, GlycoMimetics, Celgene; being on the data and safety monitoring board of GlycoMimetics; and chairing independent review boards for several trials across several companies.
A version of this story originally appeared on Medscape.com.
ORLANDO – For the first time, there is a maintenance therapy for patients with acute myeloid leukemia (AML) in remission that can improve overall survival – a new oral formulation of an old drug, azacitidine, known as CC-486 (Celgene).
“Oral azacitidine represents a new therapeutic standard for patients with AML in remission,” said lead author Andrew H. Wei, MBBS, PhD, from the Alfred Hospital in Melbourne.
“It’s not too hard to get these patients into remission,” commented another expert. “The problem comes in keeping them in remission.”
Dr. Wei noted that standard treatment with intensive induction chemotherapy for AML induces complete remission (CR) in 60%-80% of patients aged 60 years or younger and in 40%-60% of patients aged 60 years or older.
However, the majority of patients who attain complete remission (CR) will eventually relapse, and relapse is the primary obstacle to long-term survival, he said.
Despite various attempts, there has been no success over the past 30 years in defining maintenance treatment for these patients, Dr. Wei said.
The new results suggest that oral azacitidine could be an effective maintenance therapy.
Dr. Wei presented the results at the 2019 annual meeting of the American Society of Hematology. They come from the QUAZAR AML-001 study, conducted in 472 patients with poor-risk AML in first remission.
The results show that CC-486 significantly improved outcomes, compared with placebo plus best supportive care, in terms of median overall survival (24.7 vs. 14.8 months) and median relapse-free survival (10.2 vs. 4.8 months).
The trial was funded by Celgene, which said it will be submitting the data for regulatory approval for the new oral formulation of azacitidine, CC-486.
Experts predict new standard of care
Experts approached for comment agreed that maintenance oral azacitidine will become the new standard of care for patients with AML in first remission.
“Unlike therapy for acute lymphoblastic leukemia, maintenance therapy has not been part of the treatment algorithm for AML patients in first remission,” Harry P. Erba, MD, PhD, director of the leukemia program at the Duke Cancer Institute, Durham, N.C., told Medscape Medical News.
He explained that trials for maintenance after first remission in AML have failed. Recently, Dr. Erba noted, the HOVON97 trial with injectable azacitidine demonstrated improvement in relapse-free survival, compared with observation for older AML patients achieving remission after induction therapy. “However, there was no improvement in overall survival,” he said.
“Remission in AML is short lived,” Dr. Erba said. Oral azacitidine represents the first maintenance therapy in AML that has shown both significant and clinically meaningful improvements in overall and relapse-free survival and will represent a new standard of care for patients with AML in remission, Dr. Erba said. “Maintenance oral azacitidine will be practice changing,” he predicted.
HOVON97 was a small study of injectable azacitidine used as maintenance therapy for 12 months, but it was slow to accrue and did not meet its accrual target.
“In HOVON97, at 12 months, only one third of patients received less than the 12 cycles of therapy,” Dr. Wei said. He explained that, with injectable azacitidine, patients have to come into the hospital/clinic for 7 days a month, 84 days a year. Oral azacitidine is more convenient as patients do not have to come into the clinic, he said.
Dr. Wei pointed out that about 40 patients in the QUAZAR study, which started in 2013, are still on maintenance therapy, with one patient now having received 80 cycles of therapy (approximately 7 years). “Long-term maintenance therapy with azacitidine is possible,” he said.
Another expert was also impressed by the new results. “This is an important clinical trial that addresses an unmet need in AML care,” said John Mascarenhas, MD, director of the Adult Leukemia Program and leader of clinical investigation within the myeloproliferative disorders program at the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai, New York.
“Older patients can often receive induction chemotherapy but frequently do not ultimately do well, as the disease relapses and survival is limited,” he explained.
“This large, randomized, double-blind, controlled study of intermediate- or poor-risk AML patients over the age of 55 years supports the use of maintenance oral azacitidine after initial remission to extend overall and relapse-free survival in older AML patients not eligible for transplant,” Dr. Mascarenhas said.
“This is still not a curative approach,” Dr. Wei said, but added that it prolongs relapse-free survival for older patients while maintaining a quality of life for as long as possible.
Study details
The QUAZAR phase 3 study enrolled patients with poor- or intermediate-risk cytogenetics who had an Eastern Cooperative Oncology Group performance status less than or equal to 3 and who had achieved CR or CR with incomplete count recovery (CRi) after induction therapy with or without consolidation therapy. In addition, patients were not candidates for stem cell transplants.
Patients had predominantly de novo AML (89%). Other baseline characteristics of note:
- 85% of patients had intermediate-risk and 15% had poor-risk cytogenetics
- 79% achieved CR and 21% achieved CRi after induction therapy
- 78% received at least one cycle of consolidation therapy
- 43% of patients had minimal residual disease (MRD)–positive disease
Patients were randomized to receive oral azacitidine 200 mg daily on days 1-14 of a repeat 28-day cycle (n = 278) or matching placebo (n = 274). Treatment was continued indefinitely until blast count was more than 15% or patients experienced unacceptable toxicity or went on to transplant.
At a median follow-up of over 41.2 months (3 years, 5 months), median overall survival was significantly longer for patients receiving oral azacitidine at 24.7 months versus 14.8 months for placebo (P less than .0009; hazard ratio, 0.69).
Relapse-free survival was also significantly prolonged, to 10.2 months for patients on oral azacitidine versus 4.8 months for placebo (HR, 0.65; P less than .0001).
Patients on oral azacitidine reported more grade 1-2 gastrointestinal adverse events, such as nausea (65% vs. 24% on placebo), vomiting (60% vs. 10%), and diarrhea (50% vs 22%), as well as more cytopenia. The most common grade 3-4 adverse events were neutropenia (41% with oral azacitidine vs. 24% on placebo), thrombocytopenia (23% vs. 22%), and anemia (14% vs. 13%).
Although Dr. Erba supported the use of oral azacitidine as maintenance therapy, he pointed out that it was hard to convince patients, especially older ones, to continue on maintenance therapy indefinitely. “The toxicities of continuing on a drug indefinitely are real issues,” he said, explaining that most elderly patients cannot cope with even grade 1-2 nausea, diarrhea, and vomiting over the long term.
But he noted that, regardless of the higher incidence of some adverse events with oral azacitidine, the health-related quality of life of patients on oral azacitidine was similar to those on placebo.
Awaiting longer follow-up
Both experts said that longer-term follow-up is needed.
“We need a longer follow-up to see how the curves plateau,” Dr. Erba said. He would also like to see a comparative analysis of the data in patients who are MRD negative versus those who are MRD positive.
“The final results of this study, including the impact of measurable residual disease on outcome in this setting, will potentially have practice-changing implications,” said Dr. Mascarenhas.
At the press conference, Dr. Wei pointed out that, based on the data from QUAZAR, oral azacitidine is likely to be evaluated in the frontline setting of AML. “The elderly make up about two-thirds of all AML patients, and oral azacitidine will be a better option than 7 days per month for chemotherapy treatment in the clinic,” he said. “Oral azacitidine in the future may also be the backbone for other combinations.”
The study was funded by Celgene.
Dr. Wei receives honoraria from AbbVie, Macrogenics, Pfizer, Astellas, Janssen, Servier, Celgene, Amgen, AstraZeneca, Novartis, and Genentech; is on the board of directors or serves on the advisory committees for AbbVie, Macrogenics, Pfizer, Astellas, Servier, Celgene, Amgen, Novartis, and Genentech; and receives research funding from AbbVie, Servier, Celgene, Amgen, AstraZeneca, and Novartis. As a former employee of the Walter and Eliza Hall Institute, Dr. Wei receives a fraction of its royalty stream related to venetoclax.
A partial list of Dr. Erba’s conflict of interest includes consulting with Agios, Novartis, Daiichi Sankyo, MacroGenics, Jazz Pharmaceuticals, Seattle Genetics, GlycoMimetics, Amgen, Pfizer, Celgene, AbbVie, Covance, Immunogen, Astellas Pharma, Incyte; being on the speakers bureau or receiving lecture fees from Agios, Novartis, MacroGenics, Jazz Pharmaceuticals, Celgene; receiving research funding from Novartis, Daiichi Sankyo, MacroGenics, GlycoMimetics, Celgene; being on the data and safety monitoring board of GlycoMimetics; and chairing independent review boards for several trials across several companies.
A version of this story originally appeared on Medscape.com.
ORLANDO – For the first time, there is a maintenance therapy for patients with acute myeloid leukemia (AML) in remission that can improve overall survival – a new oral formulation of an old drug, azacitidine, known as CC-486 (Celgene).
“Oral azacitidine represents a new therapeutic standard for patients with AML in remission,” said lead author Andrew H. Wei, MBBS, PhD, from the Alfred Hospital in Melbourne.
“It’s not too hard to get these patients into remission,” commented another expert. “The problem comes in keeping them in remission.”
Dr. Wei noted that standard treatment with intensive induction chemotherapy for AML induces complete remission (CR) in 60%-80% of patients aged 60 years or younger and in 40%-60% of patients aged 60 years or older.
However, the majority of patients who attain complete remission (CR) will eventually relapse, and relapse is the primary obstacle to long-term survival, he said.
Despite various attempts, there has been no success over the past 30 years in defining maintenance treatment for these patients, Dr. Wei said.
The new results suggest that oral azacitidine could be an effective maintenance therapy.
Dr. Wei presented the results at the 2019 annual meeting of the American Society of Hematology. They come from the QUAZAR AML-001 study, conducted in 472 patients with poor-risk AML in first remission.
The results show that CC-486 significantly improved outcomes, compared with placebo plus best supportive care, in terms of median overall survival (24.7 vs. 14.8 months) and median relapse-free survival (10.2 vs. 4.8 months).
The trial was funded by Celgene, which said it will be submitting the data for regulatory approval for the new oral formulation of azacitidine, CC-486.
Experts predict new standard of care
Experts approached for comment agreed that maintenance oral azacitidine will become the new standard of care for patients with AML in first remission.
“Unlike therapy for acute lymphoblastic leukemia, maintenance therapy has not been part of the treatment algorithm for AML patients in first remission,” Harry P. Erba, MD, PhD, director of the leukemia program at the Duke Cancer Institute, Durham, N.C., told Medscape Medical News.
He explained that trials for maintenance after first remission in AML have failed. Recently, Dr. Erba noted, the HOVON97 trial with injectable azacitidine demonstrated improvement in relapse-free survival, compared with observation for older AML patients achieving remission after induction therapy. “However, there was no improvement in overall survival,” he said.
“Remission in AML is short lived,” Dr. Erba said. Oral azacitidine represents the first maintenance therapy in AML that has shown both significant and clinically meaningful improvements in overall and relapse-free survival and will represent a new standard of care for patients with AML in remission, Dr. Erba said. “Maintenance oral azacitidine will be practice changing,” he predicted.
HOVON97 was a small study of injectable azacitidine used as maintenance therapy for 12 months, but it was slow to accrue and did not meet its accrual target.
“In HOVON97, at 12 months, only one third of patients received less than the 12 cycles of therapy,” Dr. Wei said. He explained that, with injectable azacitidine, patients have to come into the hospital/clinic for 7 days a month, 84 days a year. Oral azacitidine is more convenient as patients do not have to come into the clinic, he said.
Dr. Wei pointed out that about 40 patients in the QUAZAR study, which started in 2013, are still on maintenance therapy, with one patient now having received 80 cycles of therapy (approximately 7 years). “Long-term maintenance therapy with azacitidine is possible,” he said.
Another expert was also impressed by the new results. “This is an important clinical trial that addresses an unmet need in AML care,” said John Mascarenhas, MD, director of the Adult Leukemia Program and leader of clinical investigation within the myeloproliferative disorders program at the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai, New York.
“Older patients can often receive induction chemotherapy but frequently do not ultimately do well, as the disease relapses and survival is limited,” he explained.
“This large, randomized, double-blind, controlled study of intermediate- or poor-risk AML patients over the age of 55 years supports the use of maintenance oral azacitidine after initial remission to extend overall and relapse-free survival in older AML patients not eligible for transplant,” Dr. Mascarenhas said.
“This is still not a curative approach,” Dr. Wei said, but added that it prolongs relapse-free survival for older patients while maintaining a quality of life for as long as possible.
Study details
The QUAZAR phase 3 study enrolled patients with poor- or intermediate-risk cytogenetics who had an Eastern Cooperative Oncology Group performance status less than or equal to 3 and who had achieved CR or CR with incomplete count recovery (CRi) after induction therapy with or without consolidation therapy. In addition, patients were not candidates for stem cell transplants.
Patients had predominantly de novo AML (89%). Other baseline characteristics of note:
- 85% of patients had intermediate-risk and 15% had poor-risk cytogenetics
- 79% achieved CR and 21% achieved CRi after induction therapy
- 78% received at least one cycle of consolidation therapy
- 43% of patients had minimal residual disease (MRD)–positive disease
Patients were randomized to receive oral azacitidine 200 mg daily on days 1-14 of a repeat 28-day cycle (n = 278) or matching placebo (n = 274). Treatment was continued indefinitely until blast count was more than 15% or patients experienced unacceptable toxicity or went on to transplant.
At a median follow-up of over 41.2 months (3 years, 5 months), median overall survival was significantly longer for patients receiving oral azacitidine at 24.7 months versus 14.8 months for placebo (P less than .0009; hazard ratio, 0.69).
Relapse-free survival was also significantly prolonged, to 10.2 months for patients on oral azacitidine versus 4.8 months for placebo (HR, 0.65; P less than .0001).
Patients on oral azacitidine reported more grade 1-2 gastrointestinal adverse events, such as nausea (65% vs. 24% on placebo), vomiting (60% vs. 10%), and diarrhea (50% vs 22%), as well as more cytopenia. The most common grade 3-4 adverse events were neutropenia (41% with oral azacitidine vs. 24% on placebo), thrombocytopenia (23% vs. 22%), and anemia (14% vs. 13%).
Although Dr. Erba supported the use of oral azacitidine as maintenance therapy, he pointed out that it was hard to convince patients, especially older ones, to continue on maintenance therapy indefinitely. “The toxicities of continuing on a drug indefinitely are real issues,” he said, explaining that most elderly patients cannot cope with even grade 1-2 nausea, diarrhea, and vomiting over the long term.
But he noted that, regardless of the higher incidence of some adverse events with oral azacitidine, the health-related quality of life of patients on oral azacitidine was similar to those on placebo.
Awaiting longer follow-up
Both experts said that longer-term follow-up is needed.
“We need a longer follow-up to see how the curves plateau,” Dr. Erba said. He would also like to see a comparative analysis of the data in patients who are MRD negative versus those who are MRD positive.
“The final results of this study, including the impact of measurable residual disease on outcome in this setting, will potentially have practice-changing implications,” said Dr. Mascarenhas.
At the press conference, Dr. Wei pointed out that, based on the data from QUAZAR, oral azacitidine is likely to be evaluated in the frontline setting of AML. “The elderly make up about two-thirds of all AML patients, and oral azacitidine will be a better option than 7 days per month for chemotherapy treatment in the clinic,” he said. “Oral azacitidine in the future may also be the backbone for other combinations.”
The study was funded by Celgene.
Dr. Wei receives honoraria from AbbVie, Macrogenics, Pfizer, Astellas, Janssen, Servier, Celgene, Amgen, AstraZeneca, Novartis, and Genentech; is on the board of directors or serves on the advisory committees for AbbVie, Macrogenics, Pfizer, Astellas, Servier, Celgene, Amgen, Novartis, and Genentech; and receives research funding from AbbVie, Servier, Celgene, Amgen, AstraZeneca, and Novartis. As a former employee of the Walter and Eliza Hall Institute, Dr. Wei receives a fraction of its royalty stream related to venetoclax.
A partial list of Dr. Erba’s conflict of interest includes consulting with Agios, Novartis, Daiichi Sankyo, MacroGenics, Jazz Pharmaceuticals, Seattle Genetics, GlycoMimetics, Amgen, Pfizer, Celgene, AbbVie, Covance, Immunogen, Astellas Pharma, Incyte; being on the speakers bureau or receiving lecture fees from Agios, Novartis, MacroGenics, Jazz Pharmaceuticals, Celgene; receiving research funding from Novartis, Daiichi Sankyo, MacroGenics, GlycoMimetics, Celgene; being on the data and safety monitoring board of GlycoMimetics; and chairing independent review boards for several trials across several companies.
A version of this story originally appeared on Medscape.com.