User login
Bempedoic acid cuts CV events in statin-intolerant patients: CLEAR Outcomes
A new approach to lowering cholesterol with the use of bempedoic acid (Nexletol, Esperion) brought about a significant reduction in cardiovascular events in patients intolerant to statins in the large phase 3, placebo-controlled CLEAR Outcomes trial.
The drug lowered LDL cholesterol by 21% in the study and reduced the composite primary endpoint, including cardiovascular death, MI, stroke, or coronary revascularization, by 13%; MI was reduced by 23% and coronary revascularization, by 19%.
The drug was also well tolerated in the mixed population of primary and secondary prevention patients unable or unwilling to take statins.
“These findings establish bempedoic acid as an effective approach to reduce major cardiovascular events in statin-intolerant patients,” study chair, Steven E. Nissen, MD, of the Cleveland Clinic concluded.
Dr. Nissen presented the CLEAR Outcomes trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The study was simultaneously published online in the New England Journal of Medicine. Top-line results were previously reported in December 2022.
Dr. Nissen pointed out that, while in the current study bempedoic acid was studied as monotherapy, he believes the drug will mainly be used in clinical practice in combination with ezetimibe, a combination shown to reduce LDL by 38%. “I think this is how it will be used in clinical practice. So, we can get an almost 40% LDL reduction – that’s about the same as 40 mg simvastatin or 20 mg atorvastatin – without giving a statin. And I think that’s where I see the potential of this therapy,” he said.
Dr. Nissen described statin intolerance as “a vexing problem” that prevents many patients from achieving LDL cholesterol levels associated with cardiovascular benefits.
He explained that bempedoic acid, an adenosine triphosphate citrate lyase inhibitor, inhibits hepatic cholesterol synthesis upstream of hydroxymethylglutaryl coenzyme A reductase, the enzyme inhibited by statins. Bempedoic acid is a prodrug activated in the liver, but not in peripheral tissues, resulting in a low incidence of muscle-related adverse events. Although bempedoic acid is approved for lowering LDL cholesterol, this is the first trial to assess its effects on cardiovascular outcomes.
CLEAR Outcomes
The CLEAR Outcomes trial included 13,970 patients (48% women) from 32 countries who were unable or unwilling to take statins owing to unacceptable adverse effects and who had, or were at high risk for, cardiovascular disease. They were randomly assigned to oral bempedoic acid, 180 mg daily, or placebo.
The mean LDL cholesterol level at baseline was 139 mg/dL in both groups, and after 6 months, the reduction in the level was greater with bempedoic acid than with placebo by 29.2 mg/dL (a 21.1% reduction).
The drug was also associated with a 22% reduction in high-sensitivity C-reactive protein.
After a median duration of follow-up of 40.6 months, the incidence of a primary endpoint (cardiovascular death, MI, stroke, or coronary revascularization) was significantly lower (by 13%) with bempedoic acid than with placebo (11.7% vs. 13.3%; hazard ratio, 0.87; P = .004).
The absolute risk reduction was 1.6 percentage points, and the number needed to treat for 40 months to prevent one event was 63.
The secondary composite endpoint of cardiovascular death/stroke/MI was reduced by 15% (8.2% vs. 9.5%; HR, 0.85; P = .006). Fatal or nonfatal MI was reduced by 23% (3.7% vs. 4.8%; HR, 0.77; P = .002), and coronary revascularization was reduced by 19% (6.2% vs. 7.6%; HR, 0.81; P = .001).
Bempedoic acid had no significant effects on fatal or nonfatal stroke, death from cardiovascular causes, and death from any cause.
Subgroup analysis showed similar results across all groups and no difference in treatment effect between men and women.
Adverse events were reported by 25% of patients in both groups, with adverse events leading to discontinuation reported by 10.8% of the bempedoic acid group and 10.4% of the placebo group.
Muscle disorders were reported in 15.0% of the bempedoic acid group versus 15.4% of the placebo group. And there was also no difference in new cases of diabetes (16.1% vs. 17.1%).
Bempedoic acid was associated with small increases in the incidence of gout (3.1% vs. 2.1%) and cholelithiasis (2.2% vs. 1.2%), and also small increases in serum creatinine, uric acid, and hepatic enzyme levels.
In the NEJM article, the authors pointed out that the concept of statin intolerance remains controversial. Some recent studies suggested that reported adverse effects represent an anticipation of harm, often described as the “nocebo” effect.
“Whether real or perceived, statin intolerance remains a vexing clinical problem that can prevent patients who are guideline eligible for statin treatment from reaching LDL cholesterol levels associated with clinical benefits. Accordingly, alternative nonstatin therapies are needed to manage the LDL cholesterol level in these patients,” they wrote.
“Management of patients unable or unwilling to take statins represents a challenging and frustrating clinical issue. Regardless whether this problem represents the ‘nocebo’ effect or actual intolerance, these high-risk patients need effective alternative therapies,” Dr. Nissen concluded. “The CLEAR Outcomes trial provides a sound rationale for use of bempedoic acid to reduce major adverse cardiovascular outcomes in patients intolerant to statins.”
‘Compelling findings’
Discussing the trial at the ACC late-breaking clinical trial session, Michelle O’Donoghue, MD, Brigham and Women’s Hospital, Boston, noted that this is the largest trial to date in statin-intolerant patients.
She pointed out that although the issue of statin intolerance remains controversial, adherence to statins is often not good, so this is an important patient population to study.
She said it was “quite remarkable” that 48% of the study were women, adding: “There is still much that we need to understand about why women appear to be less willing or able to tolerate statin therapy.”
Dr. O’Donoghue concluded that the study showed “compelling findings,” and the event reduction was in line with what would be expected from the LDL cholesterol reduction, further supporting the LDL cholesterol hypothesis.
She added: “Bempedoic acid is an important addition to our arsenal of nonstatin LDL-lowering therapies. And while it was overall well tolerated, it did not get a complete free pass, as there were some modest safety concerns.”
In an editorial accompanying the NEJM publication, John Alexander, MD, Duke Clinical Research Institute, Durham, N.C., wrote: “The compelling results of the CLEAR Outcomes trial will and should increase the use of bempedoic acid in patients with established atherosclerotic vascular disease and in those at high risk for vascular disease who are unable or unwilling to take statins.”
He warned, however, that it is premature to consider bempedoic acid as an alternative to statins. “Given the overwhelming evidence of the vascular benefits of statins, clinicians should continue their efforts to prescribe them at the maximum tolerated doses for appropriate patients, including those who may have discontinued statins because of presumed side effects.”.
Dr. Alexander also pointed out that although bempedoic acid also reduces the LDL cholesterol level in patients taking statins, the clinical benefits of bempedoic acid added to standard statin therapy are unknown.
On the observation that bempedoic acid had no observed effect on mortality, he noted that “Many individual trials of statins have also not shown an effect of the agent on mortality; it was only through the meta-analysis of multiple clinical trials that the effects of statins on mortality became clear.”
“Bempedoic acid has now entered the list of evidence-based alternatives to statins for primary and secondary prevention in patients at high cardiovascular risk,” Dr. Alexander concluded. “The benefits of bempedoic acid are now clearer, and it is now our responsibility to translate this information into better primary and secondary prevention for more at-risk patients, who will, as a result, benefit from fewer cardiovascular events.”
In a second editorial, John F. Keaney Jr., MD, Brigham and Women’s Hospital, said the lack of a clear association between bempedoic acid and muscle disorders, new-onset diabetes, or worsening hyperglycemia is “welcome news” for statin-intolerant patients.
But he cautioned that “these data must be interpreted cautiously, because bempedoic acid, when combined with a statin, appears to enhance the occurrence of muscle symptoms. Moreover, bempedoic acid has its own reported side effects, including tendon rupture, increased uric acid levels, gout, and reduced glomerular filtration rate, which are not seen with statin use.”
In terms of drug interactions, Dr. Keaney noted that bempedoic acid can increase the circulating levels of simvastatin and pravastatin, so it should not be used in patients who are receiving these agents at doses above 20 mg and 40 mg, respectively. Similarly, bempedoic acid should not be used with fibrates other than fenofibrate because of concerns regarding cholelithiasis.
“Available data clearly indicate that bempedoic acid can be used as an adjunct to statin and nonstatin therapies (except as noted above) to produce an additional 16%-26% reduction in the LDL cholesterol level,” he added. “However, it is not yet clear to what extent adjunctive bempedoic acid will further reduce the risk of cardiovascular events.”
The CLEAR Outcomes trial was supported by Esperion Therapeutics. Dr. Nissen reported receiving grants from AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Esperion, Novartis, and Silence Pharmaceuticals and consultancies with Amgen and Glenmark Pharmaceuticals.
A version of this article first appeared on Medscape.com.
A new approach to lowering cholesterol with the use of bempedoic acid (Nexletol, Esperion) brought about a significant reduction in cardiovascular events in patients intolerant to statins in the large phase 3, placebo-controlled CLEAR Outcomes trial.
The drug lowered LDL cholesterol by 21% in the study and reduced the composite primary endpoint, including cardiovascular death, MI, stroke, or coronary revascularization, by 13%; MI was reduced by 23% and coronary revascularization, by 19%.
The drug was also well tolerated in the mixed population of primary and secondary prevention patients unable or unwilling to take statins.
“These findings establish bempedoic acid as an effective approach to reduce major cardiovascular events in statin-intolerant patients,” study chair, Steven E. Nissen, MD, of the Cleveland Clinic concluded.
Dr. Nissen presented the CLEAR Outcomes trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The study was simultaneously published online in the New England Journal of Medicine. Top-line results were previously reported in December 2022.
Dr. Nissen pointed out that, while in the current study bempedoic acid was studied as monotherapy, he believes the drug will mainly be used in clinical practice in combination with ezetimibe, a combination shown to reduce LDL by 38%. “I think this is how it will be used in clinical practice. So, we can get an almost 40% LDL reduction – that’s about the same as 40 mg simvastatin or 20 mg atorvastatin – without giving a statin. And I think that’s where I see the potential of this therapy,” he said.
Dr. Nissen described statin intolerance as “a vexing problem” that prevents many patients from achieving LDL cholesterol levels associated with cardiovascular benefits.
He explained that bempedoic acid, an adenosine triphosphate citrate lyase inhibitor, inhibits hepatic cholesterol synthesis upstream of hydroxymethylglutaryl coenzyme A reductase, the enzyme inhibited by statins. Bempedoic acid is a prodrug activated in the liver, but not in peripheral tissues, resulting in a low incidence of muscle-related adverse events. Although bempedoic acid is approved for lowering LDL cholesterol, this is the first trial to assess its effects on cardiovascular outcomes.
CLEAR Outcomes
The CLEAR Outcomes trial included 13,970 patients (48% women) from 32 countries who were unable or unwilling to take statins owing to unacceptable adverse effects and who had, or were at high risk for, cardiovascular disease. They were randomly assigned to oral bempedoic acid, 180 mg daily, or placebo.
The mean LDL cholesterol level at baseline was 139 mg/dL in both groups, and after 6 months, the reduction in the level was greater with bempedoic acid than with placebo by 29.2 mg/dL (a 21.1% reduction).
The drug was also associated with a 22% reduction in high-sensitivity C-reactive protein.
After a median duration of follow-up of 40.6 months, the incidence of a primary endpoint (cardiovascular death, MI, stroke, or coronary revascularization) was significantly lower (by 13%) with bempedoic acid than with placebo (11.7% vs. 13.3%; hazard ratio, 0.87; P = .004).
The absolute risk reduction was 1.6 percentage points, and the number needed to treat for 40 months to prevent one event was 63.
The secondary composite endpoint of cardiovascular death/stroke/MI was reduced by 15% (8.2% vs. 9.5%; HR, 0.85; P = .006). Fatal or nonfatal MI was reduced by 23% (3.7% vs. 4.8%; HR, 0.77; P = .002), and coronary revascularization was reduced by 19% (6.2% vs. 7.6%; HR, 0.81; P = .001).
Bempedoic acid had no significant effects on fatal or nonfatal stroke, death from cardiovascular causes, and death from any cause.
Subgroup analysis showed similar results across all groups and no difference in treatment effect between men and women.
Adverse events were reported by 25% of patients in both groups, with adverse events leading to discontinuation reported by 10.8% of the bempedoic acid group and 10.4% of the placebo group.
Muscle disorders were reported in 15.0% of the bempedoic acid group versus 15.4% of the placebo group. And there was also no difference in new cases of diabetes (16.1% vs. 17.1%).
Bempedoic acid was associated with small increases in the incidence of gout (3.1% vs. 2.1%) and cholelithiasis (2.2% vs. 1.2%), and also small increases in serum creatinine, uric acid, and hepatic enzyme levels.
In the NEJM article, the authors pointed out that the concept of statin intolerance remains controversial. Some recent studies suggested that reported adverse effects represent an anticipation of harm, often described as the “nocebo” effect.
“Whether real or perceived, statin intolerance remains a vexing clinical problem that can prevent patients who are guideline eligible for statin treatment from reaching LDL cholesterol levels associated with clinical benefits. Accordingly, alternative nonstatin therapies are needed to manage the LDL cholesterol level in these patients,” they wrote.
“Management of patients unable or unwilling to take statins represents a challenging and frustrating clinical issue. Regardless whether this problem represents the ‘nocebo’ effect or actual intolerance, these high-risk patients need effective alternative therapies,” Dr. Nissen concluded. “The CLEAR Outcomes trial provides a sound rationale for use of bempedoic acid to reduce major adverse cardiovascular outcomes in patients intolerant to statins.”
‘Compelling findings’
Discussing the trial at the ACC late-breaking clinical trial session, Michelle O’Donoghue, MD, Brigham and Women’s Hospital, Boston, noted that this is the largest trial to date in statin-intolerant patients.
She pointed out that although the issue of statin intolerance remains controversial, adherence to statins is often not good, so this is an important patient population to study.
She said it was “quite remarkable” that 48% of the study were women, adding: “There is still much that we need to understand about why women appear to be less willing or able to tolerate statin therapy.”
Dr. O’Donoghue concluded that the study showed “compelling findings,” and the event reduction was in line with what would be expected from the LDL cholesterol reduction, further supporting the LDL cholesterol hypothesis.
She added: “Bempedoic acid is an important addition to our arsenal of nonstatin LDL-lowering therapies. And while it was overall well tolerated, it did not get a complete free pass, as there were some modest safety concerns.”
In an editorial accompanying the NEJM publication, John Alexander, MD, Duke Clinical Research Institute, Durham, N.C., wrote: “The compelling results of the CLEAR Outcomes trial will and should increase the use of bempedoic acid in patients with established atherosclerotic vascular disease and in those at high risk for vascular disease who are unable or unwilling to take statins.”
He warned, however, that it is premature to consider bempedoic acid as an alternative to statins. “Given the overwhelming evidence of the vascular benefits of statins, clinicians should continue their efforts to prescribe them at the maximum tolerated doses for appropriate patients, including those who may have discontinued statins because of presumed side effects.”.
Dr. Alexander also pointed out that although bempedoic acid also reduces the LDL cholesterol level in patients taking statins, the clinical benefits of bempedoic acid added to standard statin therapy are unknown.
On the observation that bempedoic acid had no observed effect on mortality, he noted that “Many individual trials of statins have also not shown an effect of the agent on mortality; it was only through the meta-analysis of multiple clinical trials that the effects of statins on mortality became clear.”
“Bempedoic acid has now entered the list of evidence-based alternatives to statins for primary and secondary prevention in patients at high cardiovascular risk,” Dr. Alexander concluded. “The benefits of bempedoic acid are now clearer, and it is now our responsibility to translate this information into better primary and secondary prevention for more at-risk patients, who will, as a result, benefit from fewer cardiovascular events.”
In a second editorial, John F. Keaney Jr., MD, Brigham and Women’s Hospital, said the lack of a clear association between bempedoic acid and muscle disorders, new-onset diabetes, or worsening hyperglycemia is “welcome news” for statin-intolerant patients.
But he cautioned that “these data must be interpreted cautiously, because bempedoic acid, when combined with a statin, appears to enhance the occurrence of muscle symptoms. Moreover, bempedoic acid has its own reported side effects, including tendon rupture, increased uric acid levels, gout, and reduced glomerular filtration rate, which are not seen with statin use.”
In terms of drug interactions, Dr. Keaney noted that bempedoic acid can increase the circulating levels of simvastatin and pravastatin, so it should not be used in patients who are receiving these agents at doses above 20 mg and 40 mg, respectively. Similarly, bempedoic acid should not be used with fibrates other than fenofibrate because of concerns regarding cholelithiasis.
“Available data clearly indicate that bempedoic acid can be used as an adjunct to statin and nonstatin therapies (except as noted above) to produce an additional 16%-26% reduction in the LDL cholesterol level,” he added. “However, it is not yet clear to what extent adjunctive bempedoic acid will further reduce the risk of cardiovascular events.”
The CLEAR Outcomes trial was supported by Esperion Therapeutics. Dr. Nissen reported receiving grants from AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Esperion, Novartis, and Silence Pharmaceuticals and consultancies with Amgen and Glenmark Pharmaceuticals.
A version of this article first appeared on Medscape.com.
A new approach to lowering cholesterol with the use of bempedoic acid (Nexletol, Esperion) brought about a significant reduction in cardiovascular events in patients intolerant to statins in the large phase 3, placebo-controlled CLEAR Outcomes trial.
The drug lowered LDL cholesterol by 21% in the study and reduced the composite primary endpoint, including cardiovascular death, MI, stroke, or coronary revascularization, by 13%; MI was reduced by 23% and coronary revascularization, by 19%.
The drug was also well tolerated in the mixed population of primary and secondary prevention patients unable or unwilling to take statins.
“These findings establish bempedoic acid as an effective approach to reduce major cardiovascular events in statin-intolerant patients,” study chair, Steven E. Nissen, MD, of the Cleveland Clinic concluded.
Dr. Nissen presented the CLEAR Outcomes trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The study was simultaneously published online in the New England Journal of Medicine. Top-line results were previously reported in December 2022.
Dr. Nissen pointed out that, while in the current study bempedoic acid was studied as monotherapy, he believes the drug will mainly be used in clinical practice in combination with ezetimibe, a combination shown to reduce LDL by 38%. “I think this is how it will be used in clinical practice. So, we can get an almost 40% LDL reduction – that’s about the same as 40 mg simvastatin or 20 mg atorvastatin – without giving a statin. And I think that’s where I see the potential of this therapy,” he said.
Dr. Nissen described statin intolerance as “a vexing problem” that prevents many patients from achieving LDL cholesterol levels associated with cardiovascular benefits.
He explained that bempedoic acid, an adenosine triphosphate citrate lyase inhibitor, inhibits hepatic cholesterol synthesis upstream of hydroxymethylglutaryl coenzyme A reductase, the enzyme inhibited by statins. Bempedoic acid is a prodrug activated in the liver, but not in peripheral tissues, resulting in a low incidence of muscle-related adverse events. Although bempedoic acid is approved for lowering LDL cholesterol, this is the first trial to assess its effects on cardiovascular outcomes.
CLEAR Outcomes
The CLEAR Outcomes trial included 13,970 patients (48% women) from 32 countries who were unable or unwilling to take statins owing to unacceptable adverse effects and who had, or were at high risk for, cardiovascular disease. They were randomly assigned to oral bempedoic acid, 180 mg daily, or placebo.
The mean LDL cholesterol level at baseline was 139 mg/dL in both groups, and after 6 months, the reduction in the level was greater with bempedoic acid than with placebo by 29.2 mg/dL (a 21.1% reduction).
The drug was also associated with a 22% reduction in high-sensitivity C-reactive protein.
After a median duration of follow-up of 40.6 months, the incidence of a primary endpoint (cardiovascular death, MI, stroke, or coronary revascularization) was significantly lower (by 13%) with bempedoic acid than with placebo (11.7% vs. 13.3%; hazard ratio, 0.87; P = .004).
The absolute risk reduction was 1.6 percentage points, and the number needed to treat for 40 months to prevent one event was 63.
The secondary composite endpoint of cardiovascular death/stroke/MI was reduced by 15% (8.2% vs. 9.5%; HR, 0.85; P = .006). Fatal or nonfatal MI was reduced by 23% (3.7% vs. 4.8%; HR, 0.77; P = .002), and coronary revascularization was reduced by 19% (6.2% vs. 7.6%; HR, 0.81; P = .001).
Bempedoic acid had no significant effects on fatal or nonfatal stroke, death from cardiovascular causes, and death from any cause.
Subgroup analysis showed similar results across all groups and no difference in treatment effect between men and women.
Adverse events were reported by 25% of patients in both groups, with adverse events leading to discontinuation reported by 10.8% of the bempedoic acid group and 10.4% of the placebo group.
Muscle disorders were reported in 15.0% of the bempedoic acid group versus 15.4% of the placebo group. And there was also no difference in new cases of diabetes (16.1% vs. 17.1%).
Bempedoic acid was associated with small increases in the incidence of gout (3.1% vs. 2.1%) and cholelithiasis (2.2% vs. 1.2%), and also small increases in serum creatinine, uric acid, and hepatic enzyme levels.
In the NEJM article, the authors pointed out that the concept of statin intolerance remains controversial. Some recent studies suggested that reported adverse effects represent an anticipation of harm, often described as the “nocebo” effect.
“Whether real or perceived, statin intolerance remains a vexing clinical problem that can prevent patients who are guideline eligible for statin treatment from reaching LDL cholesterol levels associated with clinical benefits. Accordingly, alternative nonstatin therapies are needed to manage the LDL cholesterol level in these patients,” they wrote.
“Management of patients unable or unwilling to take statins represents a challenging and frustrating clinical issue. Regardless whether this problem represents the ‘nocebo’ effect or actual intolerance, these high-risk patients need effective alternative therapies,” Dr. Nissen concluded. “The CLEAR Outcomes trial provides a sound rationale for use of bempedoic acid to reduce major adverse cardiovascular outcomes in patients intolerant to statins.”
‘Compelling findings’
Discussing the trial at the ACC late-breaking clinical trial session, Michelle O’Donoghue, MD, Brigham and Women’s Hospital, Boston, noted that this is the largest trial to date in statin-intolerant patients.
She pointed out that although the issue of statin intolerance remains controversial, adherence to statins is often not good, so this is an important patient population to study.
She said it was “quite remarkable” that 48% of the study were women, adding: “There is still much that we need to understand about why women appear to be less willing or able to tolerate statin therapy.”
Dr. O’Donoghue concluded that the study showed “compelling findings,” and the event reduction was in line with what would be expected from the LDL cholesterol reduction, further supporting the LDL cholesterol hypothesis.
She added: “Bempedoic acid is an important addition to our arsenal of nonstatin LDL-lowering therapies. And while it was overall well tolerated, it did not get a complete free pass, as there were some modest safety concerns.”
In an editorial accompanying the NEJM publication, John Alexander, MD, Duke Clinical Research Institute, Durham, N.C., wrote: “The compelling results of the CLEAR Outcomes trial will and should increase the use of bempedoic acid in patients with established atherosclerotic vascular disease and in those at high risk for vascular disease who are unable or unwilling to take statins.”
He warned, however, that it is premature to consider bempedoic acid as an alternative to statins. “Given the overwhelming evidence of the vascular benefits of statins, clinicians should continue their efforts to prescribe them at the maximum tolerated doses for appropriate patients, including those who may have discontinued statins because of presumed side effects.”.
Dr. Alexander also pointed out that although bempedoic acid also reduces the LDL cholesterol level in patients taking statins, the clinical benefits of bempedoic acid added to standard statin therapy are unknown.
On the observation that bempedoic acid had no observed effect on mortality, he noted that “Many individual trials of statins have also not shown an effect of the agent on mortality; it was only through the meta-analysis of multiple clinical trials that the effects of statins on mortality became clear.”
“Bempedoic acid has now entered the list of evidence-based alternatives to statins for primary and secondary prevention in patients at high cardiovascular risk,” Dr. Alexander concluded. “The benefits of bempedoic acid are now clearer, and it is now our responsibility to translate this information into better primary and secondary prevention for more at-risk patients, who will, as a result, benefit from fewer cardiovascular events.”
In a second editorial, John F. Keaney Jr., MD, Brigham and Women’s Hospital, said the lack of a clear association between bempedoic acid and muscle disorders, new-onset diabetes, or worsening hyperglycemia is “welcome news” for statin-intolerant patients.
But he cautioned that “these data must be interpreted cautiously, because bempedoic acid, when combined with a statin, appears to enhance the occurrence of muscle symptoms. Moreover, bempedoic acid has its own reported side effects, including tendon rupture, increased uric acid levels, gout, and reduced glomerular filtration rate, which are not seen with statin use.”
In terms of drug interactions, Dr. Keaney noted that bempedoic acid can increase the circulating levels of simvastatin and pravastatin, so it should not be used in patients who are receiving these agents at doses above 20 mg and 40 mg, respectively. Similarly, bempedoic acid should not be used with fibrates other than fenofibrate because of concerns regarding cholelithiasis.
“Available data clearly indicate that bempedoic acid can be used as an adjunct to statin and nonstatin therapies (except as noted above) to produce an additional 16%-26% reduction in the LDL cholesterol level,” he added. “However, it is not yet clear to what extent adjunctive bempedoic acid will further reduce the risk of cardiovascular events.”
The CLEAR Outcomes trial was supported by Esperion Therapeutics. Dr. Nissen reported receiving grants from AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Esperion, Novartis, and Silence Pharmaceuticals and consultancies with Amgen and Glenmark Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Transcatheter tricuspid valve repair effective and safe for regurgitation
NEW ORLEANS – In the first pivotal randomized, controlled trial of a transcatheter device for the repair of severe tricuspid regurgitation, a large reduction in valve dysfunction was associated with substantial improvement in quality of life (QOL) persisting out of 1 year of follow-up, according to results of the TRILUMINATE trial.
Based on the low procedural risks of the repair, the principal investigator, Paul Sorajja, MD, called the results “very clinically meaningful” as he presented the results at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Conducted at 65 centers in the United States, Canada, and North America, TRILUMINATE evaluated a transcatheter end-to-end (TEER) repair performed with the TriClip G4 Delivery System (Abbott). The study included two cohorts, both of which will be followed for 5 years. One included patients with very severe tricuspid regurgitation enrolled in a single arm. Data on this cohort is expected later in 2023.
In the randomized portion of the study, 350 patients enrolled with severe tricuspid regurgitation underwent TEER with a clipping device and then were followed on the guideline-directed therapy (GDMT) for heart failure they were receiving at baseline. The control group was managed on GDMT alone.
The primary composite endpoint at 1 year was a composite of death from any cause and/or tricuspid valve surgery, hospitalization for heart failure, and quality of life as measured with the Kansas City Cardiomyopathy questionnaire (KCCQ).
Benefit driven by quality of life
For the primary endpoint, the win ratio, a statistical calculation of those who did relative to those who did not benefit, was 1.48, signifying a 48% advantage (P = .02). This was driven almost entirely by the KCCQ endpoint. There was no significant difference death and/or tricuspid valve surgery, which occurred in about 10% of both groups (P = .75) or heart failure hospitalization, which was occurred in slightly more patients randomized to repair (14.9% vs. 12.1%; P = .41).
For KCCQ, the mean increase at 1 year was 12.3 points in the repair group versus 0.6 points (P < .001) in the control group. With an increase of 5-10 points typically considered to be clinically meaningful, the advantage of repair over GDMT at the threshold of 15 points or greater was highly statistically significant (49.7% vs. 26.4%; P < .0001).
This advantage was attributed to control of regurgitation. The proportion achieving moderate or less regurgitation sustained at 1 year was 87% in the repair group versus 4.8% in the GDMT group (P < .0001).
When assessed independent of treatment, KCCQ benefits at 1 year increased in a stepwise fashion as severity of regurgitation was reduced, climbing from 2 points if there was no improvement to 6 points with one grade in improvement and then to 18 points with at least a two-grade improvement.
For regurgitation, “the repair was extremely effective,” said Dr. Sorajja of Allina Health Minneapolis Heart Institute at Abbott Northwestern Hospital, Minneapolis. He added that the degree of regurgitation control in the TRILUMINATE trial “is the highest ever reported.” With previous trials with other transcatheter devices in development, the improvement so far has been on the order of 70%-80%.
For enrollment in TRILUMINATE, patients were required to have at least an intermediate risk of morbidity or mortality from tricuspid valve surgery. Exclusion criteria included a left ventricular ejection fraction (LVEF) less than 20% and severe pulmonary hypertension.
More than 70% of patients had the highest (torrential) or second highest (massive) category of regurgitation on a five-level scale by echocardiography. Almost all the remaining were at the third level (severe).
Of those enrolled, the average age was roughly 78 years. About 55% were women. Nearly 60% were in New York Heart Association class III or IV heart failure and most had significant comorbidities, including hypertension (> 80%), atrial fibrillation (about 90%), and renal disease (35%). Patients with diabetes (16%), chronic obstructive pulmonary disease (10%), and liver disease (7.5%) were represented in lower numbers.
Surgery is not necessarily an option
All enrolled patients were considered to be at intermediate or greater risk for mortality with surgical replacement of the tricuspid valve, but Dr. Sorajja pointed out that surgery, which involves valve replacement, is not necessarily an alternative to valve repair. Even in fit patients, the high morbidity, mortality, and extended hospital stay associated with surgical valve replacement makes this procedure unattractive.
In this trial, most patients who underwent the transcatheter procedure were discharged within a day. The safety was excellent, Dr. Sorajja said. Only three patients (1.7%) had a major adverse event. This included two cases of new-onset renal failure and one cardiovascular death. There were no cases of endocarditis requiring surgery or any other type of nonelective cardiovascular surgery, including for any device-related issue.
In the sick population enrolled, Dr. Sorajja characterized the number of adverse events over 1 year as “very low.”
These results are important, according to Kendra Grubb, MD, surgical director of the Structural Heart and Valve Center, Emory University, Atlanta. While she expressed surprise that there was no signal of benefit on hard endpoints at 1 year, she emphasized that “these patients feel terrible,” and they are frustrating to manage because surgery is often contraindicated or impractical.
“Finally, we have something for this group,” she said, noting that the mortality from valve replacement surgery even among patients who are fit enough for surgery to be considered is about 10%.
Ajay Kirtane, MD, director of the Cardiac Catheterization Laboratories at Columbia University, New York, was more circumspect. He agreed that the improvement in QOL was encouraging, but cautioned that QOL is a particularly soft outcome in a nonrandomized trial in which patients may feel better just knowing that there regurgitation has been controlled. He found the lack of benefit on hard outcomes not just surprising but “disappointing.”
Still, he agreed the improvement in QOL is potentially meaningful for a procedure that appears to be relatively safe.
Dr. Sorajja reported financial relationships with Boston Scientific, Edwards Lifesciences, Foldax. 4C Medical, Gore Medtronic, Phillips, Siemens, Shifamed, Vdyne, xDot, and Abbott Structural, which provided funding for this trial. Dr. Grubb reported financial relationships with Abbott Vascular, Ancora Heart, Bioventrix, Boston Scientific, Edwards Lifesciences, 4C Medical, JenaValve, and Medtronic. Dr. Kirtane reported financial relationships with Abbott Vascular, Amgen, Boston Scientific, Chiesi, Medtronic, Opsens, Phillips, ReCor, Regeneron, and Zoll.
NEW ORLEANS – In the first pivotal randomized, controlled trial of a transcatheter device for the repair of severe tricuspid regurgitation, a large reduction in valve dysfunction was associated with substantial improvement in quality of life (QOL) persisting out of 1 year of follow-up, according to results of the TRILUMINATE trial.
Based on the low procedural risks of the repair, the principal investigator, Paul Sorajja, MD, called the results “very clinically meaningful” as he presented the results at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Conducted at 65 centers in the United States, Canada, and North America, TRILUMINATE evaluated a transcatheter end-to-end (TEER) repair performed with the TriClip G4 Delivery System (Abbott). The study included two cohorts, both of which will be followed for 5 years. One included patients with very severe tricuspid regurgitation enrolled in a single arm. Data on this cohort is expected later in 2023.
In the randomized portion of the study, 350 patients enrolled with severe tricuspid regurgitation underwent TEER with a clipping device and then were followed on the guideline-directed therapy (GDMT) for heart failure they were receiving at baseline. The control group was managed on GDMT alone.
The primary composite endpoint at 1 year was a composite of death from any cause and/or tricuspid valve surgery, hospitalization for heart failure, and quality of life as measured with the Kansas City Cardiomyopathy questionnaire (KCCQ).
Benefit driven by quality of life
For the primary endpoint, the win ratio, a statistical calculation of those who did relative to those who did not benefit, was 1.48, signifying a 48% advantage (P = .02). This was driven almost entirely by the KCCQ endpoint. There was no significant difference death and/or tricuspid valve surgery, which occurred in about 10% of both groups (P = .75) or heart failure hospitalization, which was occurred in slightly more patients randomized to repair (14.9% vs. 12.1%; P = .41).
For KCCQ, the mean increase at 1 year was 12.3 points in the repair group versus 0.6 points (P < .001) in the control group. With an increase of 5-10 points typically considered to be clinically meaningful, the advantage of repair over GDMT at the threshold of 15 points or greater was highly statistically significant (49.7% vs. 26.4%; P < .0001).
This advantage was attributed to control of regurgitation. The proportion achieving moderate or less regurgitation sustained at 1 year was 87% in the repair group versus 4.8% in the GDMT group (P < .0001).
When assessed independent of treatment, KCCQ benefits at 1 year increased in a stepwise fashion as severity of regurgitation was reduced, climbing from 2 points if there was no improvement to 6 points with one grade in improvement and then to 18 points with at least a two-grade improvement.
For regurgitation, “the repair was extremely effective,” said Dr. Sorajja of Allina Health Minneapolis Heart Institute at Abbott Northwestern Hospital, Minneapolis. He added that the degree of regurgitation control in the TRILUMINATE trial “is the highest ever reported.” With previous trials with other transcatheter devices in development, the improvement so far has been on the order of 70%-80%.
For enrollment in TRILUMINATE, patients were required to have at least an intermediate risk of morbidity or mortality from tricuspid valve surgery. Exclusion criteria included a left ventricular ejection fraction (LVEF) less than 20% and severe pulmonary hypertension.
More than 70% of patients had the highest (torrential) or second highest (massive) category of regurgitation on a five-level scale by echocardiography. Almost all the remaining were at the third level (severe).
Of those enrolled, the average age was roughly 78 years. About 55% were women. Nearly 60% were in New York Heart Association class III or IV heart failure and most had significant comorbidities, including hypertension (> 80%), atrial fibrillation (about 90%), and renal disease (35%). Patients with diabetes (16%), chronic obstructive pulmonary disease (10%), and liver disease (7.5%) were represented in lower numbers.
Surgery is not necessarily an option
All enrolled patients were considered to be at intermediate or greater risk for mortality with surgical replacement of the tricuspid valve, but Dr. Sorajja pointed out that surgery, which involves valve replacement, is not necessarily an alternative to valve repair. Even in fit patients, the high morbidity, mortality, and extended hospital stay associated with surgical valve replacement makes this procedure unattractive.
In this trial, most patients who underwent the transcatheter procedure were discharged within a day. The safety was excellent, Dr. Sorajja said. Only three patients (1.7%) had a major adverse event. This included two cases of new-onset renal failure and one cardiovascular death. There were no cases of endocarditis requiring surgery or any other type of nonelective cardiovascular surgery, including for any device-related issue.
In the sick population enrolled, Dr. Sorajja characterized the number of adverse events over 1 year as “very low.”
These results are important, according to Kendra Grubb, MD, surgical director of the Structural Heart and Valve Center, Emory University, Atlanta. While she expressed surprise that there was no signal of benefit on hard endpoints at 1 year, she emphasized that “these patients feel terrible,” and they are frustrating to manage because surgery is often contraindicated or impractical.
“Finally, we have something for this group,” she said, noting that the mortality from valve replacement surgery even among patients who are fit enough for surgery to be considered is about 10%.
Ajay Kirtane, MD, director of the Cardiac Catheterization Laboratories at Columbia University, New York, was more circumspect. He agreed that the improvement in QOL was encouraging, but cautioned that QOL is a particularly soft outcome in a nonrandomized trial in which patients may feel better just knowing that there regurgitation has been controlled. He found the lack of benefit on hard outcomes not just surprising but “disappointing.”
Still, he agreed the improvement in QOL is potentially meaningful for a procedure that appears to be relatively safe.
Dr. Sorajja reported financial relationships with Boston Scientific, Edwards Lifesciences, Foldax. 4C Medical, Gore Medtronic, Phillips, Siemens, Shifamed, Vdyne, xDot, and Abbott Structural, which provided funding for this trial. Dr. Grubb reported financial relationships with Abbott Vascular, Ancora Heart, Bioventrix, Boston Scientific, Edwards Lifesciences, 4C Medical, JenaValve, and Medtronic. Dr. Kirtane reported financial relationships with Abbott Vascular, Amgen, Boston Scientific, Chiesi, Medtronic, Opsens, Phillips, ReCor, Regeneron, and Zoll.
NEW ORLEANS – In the first pivotal randomized, controlled trial of a transcatheter device for the repair of severe tricuspid regurgitation, a large reduction in valve dysfunction was associated with substantial improvement in quality of life (QOL) persisting out of 1 year of follow-up, according to results of the TRILUMINATE trial.
Based on the low procedural risks of the repair, the principal investigator, Paul Sorajja, MD, called the results “very clinically meaningful” as he presented the results at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
Conducted at 65 centers in the United States, Canada, and North America, TRILUMINATE evaluated a transcatheter end-to-end (TEER) repair performed with the TriClip G4 Delivery System (Abbott). The study included two cohorts, both of which will be followed for 5 years. One included patients with very severe tricuspid regurgitation enrolled in a single arm. Data on this cohort is expected later in 2023.
In the randomized portion of the study, 350 patients enrolled with severe tricuspid regurgitation underwent TEER with a clipping device and then were followed on the guideline-directed therapy (GDMT) for heart failure they were receiving at baseline. The control group was managed on GDMT alone.
The primary composite endpoint at 1 year was a composite of death from any cause and/or tricuspid valve surgery, hospitalization for heart failure, and quality of life as measured with the Kansas City Cardiomyopathy questionnaire (KCCQ).
Benefit driven by quality of life
For the primary endpoint, the win ratio, a statistical calculation of those who did relative to those who did not benefit, was 1.48, signifying a 48% advantage (P = .02). This was driven almost entirely by the KCCQ endpoint. There was no significant difference death and/or tricuspid valve surgery, which occurred in about 10% of both groups (P = .75) or heart failure hospitalization, which was occurred in slightly more patients randomized to repair (14.9% vs. 12.1%; P = .41).
For KCCQ, the mean increase at 1 year was 12.3 points in the repair group versus 0.6 points (P < .001) in the control group. With an increase of 5-10 points typically considered to be clinically meaningful, the advantage of repair over GDMT at the threshold of 15 points or greater was highly statistically significant (49.7% vs. 26.4%; P < .0001).
This advantage was attributed to control of regurgitation. The proportion achieving moderate or less regurgitation sustained at 1 year was 87% in the repair group versus 4.8% in the GDMT group (P < .0001).
When assessed independent of treatment, KCCQ benefits at 1 year increased in a stepwise fashion as severity of regurgitation was reduced, climbing from 2 points if there was no improvement to 6 points with one grade in improvement and then to 18 points with at least a two-grade improvement.
For regurgitation, “the repair was extremely effective,” said Dr. Sorajja of Allina Health Minneapolis Heart Institute at Abbott Northwestern Hospital, Minneapolis. He added that the degree of regurgitation control in the TRILUMINATE trial “is the highest ever reported.” With previous trials with other transcatheter devices in development, the improvement so far has been on the order of 70%-80%.
For enrollment in TRILUMINATE, patients were required to have at least an intermediate risk of morbidity or mortality from tricuspid valve surgery. Exclusion criteria included a left ventricular ejection fraction (LVEF) less than 20% and severe pulmonary hypertension.
More than 70% of patients had the highest (torrential) or second highest (massive) category of regurgitation on a five-level scale by echocardiography. Almost all the remaining were at the third level (severe).
Of those enrolled, the average age was roughly 78 years. About 55% were women. Nearly 60% were in New York Heart Association class III or IV heart failure and most had significant comorbidities, including hypertension (> 80%), atrial fibrillation (about 90%), and renal disease (35%). Patients with diabetes (16%), chronic obstructive pulmonary disease (10%), and liver disease (7.5%) were represented in lower numbers.
Surgery is not necessarily an option
All enrolled patients were considered to be at intermediate or greater risk for mortality with surgical replacement of the tricuspid valve, but Dr. Sorajja pointed out that surgery, which involves valve replacement, is not necessarily an alternative to valve repair. Even in fit patients, the high morbidity, mortality, and extended hospital stay associated with surgical valve replacement makes this procedure unattractive.
In this trial, most patients who underwent the transcatheter procedure were discharged within a day. The safety was excellent, Dr. Sorajja said. Only three patients (1.7%) had a major adverse event. This included two cases of new-onset renal failure and one cardiovascular death. There were no cases of endocarditis requiring surgery or any other type of nonelective cardiovascular surgery, including for any device-related issue.
In the sick population enrolled, Dr. Sorajja characterized the number of adverse events over 1 year as “very low.”
These results are important, according to Kendra Grubb, MD, surgical director of the Structural Heart and Valve Center, Emory University, Atlanta. While she expressed surprise that there was no signal of benefit on hard endpoints at 1 year, she emphasized that “these patients feel terrible,” and they are frustrating to manage because surgery is often contraindicated or impractical.
“Finally, we have something for this group,” she said, noting that the mortality from valve replacement surgery even among patients who are fit enough for surgery to be considered is about 10%.
Ajay Kirtane, MD, director of the Cardiac Catheterization Laboratories at Columbia University, New York, was more circumspect. He agreed that the improvement in QOL was encouraging, but cautioned that QOL is a particularly soft outcome in a nonrandomized trial in which patients may feel better just knowing that there regurgitation has been controlled. He found the lack of benefit on hard outcomes not just surprising but “disappointing.”
Still, he agreed the improvement in QOL is potentially meaningful for a procedure that appears to be relatively safe.
Dr. Sorajja reported financial relationships with Boston Scientific, Edwards Lifesciences, Foldax. 4C Medical, Gore Medtronic, Phillips, Siemens, Shifamed, Vdyne, xDot, and Abbott Structural, which provided funding for this trial. Dr. Grubb reported financial relationships with Abbott Vascular, Ancora Heart, Bioventrix, Boston Scientific, Edwards Lifesciences, 4C Medical, JenaValve, and Medtronic. Dr. Kirtane reported financial relationships with Abbott Vascular, Amgen, Boston Scientific, Chiesi, Medtronic, Opsens, Phillips, ReCor, Regeneron, and Zoll.
AT ACC 2023
Frequent cannabis use tied to coronary artery disease
In the first part, in an observational study, daily cannabis use was associated with 34% higher odds for CAD, compared with never-users, in a large population-based U.S. cohort. Less frequent use was not associated with increased odds for CAD.
In the second part, people with a genetic susceptibility to cannabis use disorder or severe cannabis dependency had an increased risk for CAD, compared with other people.
Ishan Paranjpe, MD, the study’s lead author, reported these results in a press briefing and will present the study at the upcoming joint scientific sessions of the American College of Cardiology and the World Heart Federation 2023.
“A couple of takeaway points are that daily cannabis use, but not less frequent cannabis use, was associated with CAD” in the large population-based cohort, said Dr. Paranjpe, a resident physician at Stanford (Calif.) University, during the press conference.
“This analysis was adjusted for several possible confounders including age, sex at birth, [body mass index (BMI)], race, education, cigarette use, hypertension, high cholesterol, and diabetes,” he noted, and even after accounting for these risk factors, the association with heart disease remained.
“And the next thing, using Mendelian randomization, we sort of implied that there might be a causal relationship between cannabis and heart disease. Importantly this effect is independent of alcohol and cigarette use.
“The notion that cannabis is completely benign is probably wrong, and there might be certain risk of certain cardiovascular effects of cannabis we should be more on the lookout for,” Dr. Paranjpe said in an interview.
“Our main conclusion was that prevalent CAD is associated with cannabis consumption,” he added. “Other mechanistic work published in Cell has also shown that cannabis causes vascular inflammation that may lead to CAD.
“Thus, there is growing evidence from both laboratory and population studies that cannabis consumption may be harmful for cardiovascular health,” he said. “However, we still need more work on whether it affects the risk of incident cardiovascular events (i.e., stroke, heart attack) in patient[s] with existing CAD.”
ASCVD risk
Invited to comment, Robert L. Page II, PharmD, chair of the writing group for the American Heart Association’s scientific statement Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, published in 2020, said, “This adds to our hypothesis that if you are using marijuana over a longer period, greater exposure, you’re going to see an increase in the risk” for atherosclerotic cardiovascular disease (ASCVD).
“We’re seeing this increased risk for ASCVD in young adults between ages 18 to 40 – people who think that they’re invincible,” Dr. Page, a professor at the University of Colorado at Denver, Aurora, who was not involved with this research, told this news organization in an interview.
“The bottom line is that the risk that they are seeing is what has also been documented in other observational studies, and it adds fuel to the fire. We need to be paying close attention to this,” he said.
“Primary care [clinicians], cardiologists, need to address this, particularly in younger adults – because that’s where you’re seeing the highest amount of use.”
‘All of Us’ observational study
In the first part of the study, the researchers analyzed data from the “All of Us” cohort comprising adults age 18 and older from 340 inpatient and outpatient sites across the United States.
They identified 57,958 individuals who replied to a questionnaire asking about cannabis use (medicinal or recreational and whether it was edible or used by smoking or vaping) over the past 3 months.
There were 39,678 never-users, 8,749 who used it once or twice, 2,075 who used it monthly, 2,720 who used it weekly, and 4,736 who used it daily.
Of these, 3,506 individuals had CAD, based on medical records.
Only daily users had a significantly higher risk for CAD, compared with never-users (odds ratio, 1.34; P = .001) after adjusting for age, sex, hypertension, hyperlipidemia, type 2 diabetes, BMI, education, insurance status, and cigarette use.
The median age for daily users was 41, whereas the median age for never-users was 59.
GWAS analyses
The researchers then performed a Mendelian randomization analysis based on genome-wide association studies (GWAS) of cannabis use disorder and of CAD.
“Cannabis use disorder is a psychiatric diagnosis of severe cannabis dependency, equivalent to ‘alcohol use disorder’ for alcohol consumption,” Dr. Paranjpe explained. “The exact definition involves frequent use leading to significant dependence (but does not specify how often it is used).”
The GWAS data for cannabis use disorder came from a recent meta-analysis of three cohorts: the Psychiatric Genomics Consortium Substance Use Disorders working group, iPSYCH, and deCODE.
The GWAS statistics for CAD were obtained from the CARDIoGRAMplusC4D Consortium.
Cannabis use disorder was associated with significantly increased odds for CAD (OR, 1.05; P = .001), which remained after adjusting for both cigarette and alcohol use (OR, 1.04).
A version of this article first appeared on Medscape.com.
In the first part, in an observational study, daily cannabis use was associated with 34% higher odds for CAD, compared with never-users, in a large population-based U.S. cohort. Less frequent use was not associated with increased odds for CAD.
In the second part, people with a genetic susceptibility to cannabis use disorder or severe cannabis dependency had an increased risk for CAD, compared with other people.
Ishan Paranjpe, MD, the study’s lead author, reported these results in a press briefing and will present the study at the upcoming joint scientific sessions of the American College of Cardiology and the World Heart Federation 2023.
“A couple of takeaway points are that daily cannabis use, but not less frequent cannabis use, was associated with CAD” in the large population-based cohort, said Dr. Paranjpe, a resident physician at Stanford (Calif.) University, during the press conference.
“This analysis was adjusted for several possible confounders including age, sex at birth, [body mass index (BMI)], race, education, cigarette use, hypertension, high cholesterol, and diabetes,” he noted, and even after accounting for these risk factors, the association with heart disease remained.
“And the next thing, using Mendelian randomization, we sort of implied that there might be a causal relationship between cannabis and heart disease. Importantly this effect is independent of alcohol and cigarette use.
“The notion that cannabis is completely benign is probably wrong, and there might be certain risk of certain cardiovascular effects of cannabis we should be more on the lookout for,” Dr. Paranjpe said in an interview.
“Our main conclusion was that prevalent CAD is associated with cannabis consumption,” he added. “Other mechanistic work published in Cell has also shown that cannabis causes vascular inflammation that may lead to CAD.
“Thus, there is growing evidence from both laboratory and population studies that cannabis consumption may be harmful for cardiovascular health,” he said. “However, we still need more work on whether it affects the risk of incident cardiovascular events (i.e., stroke, heart attack) in patient[s] with existing CAD.”
ASCVD risk
Invited to comment, Robert L. Page II, PharmD, chair of the writing group for the American Heart Association’s scientific statement Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, published in 2020, said, “This adds to our hypothesis that if you are using marijuana over a longer period, greater exposure, you’re going to see an increase in the risk” for atherosclerotic cardiovascular disease (ASCVD).
“We’re seeing this increased risk for ASCVD in young adults between ages 18 to 40 – people who think that they’re invincible,” Dr. Page, a professor at the University of Colorado at Denver, Aurora, who was not involved with this research, told this news organization in an interview.
“The bottom line is that the risk that they are seeing is what has also been documented in other observational studies, and it adds fuel to the fire. We need to be paying close attention to this,” he said.
“Primary care [clinicians], cardiologists, need to address this, particularly in younger adults – because that’s where you’re seeing the highest amount of use.”
‘All of Us’ observational study
In the first part of the study, the researchers analyzed data from the “All of Us” cohort comprising adults age 18 and older from 340 inpatient and outpatient sites across the United States.
They identified 57,958 individuals who replied to a questionnaire asking about cannabis use (medicinal or recreational and whether it was edible or used by smoking or vaping) over the past 3 months.
There were 39,678 never-users, 8,749 who used it once or twice, 2,075 who used it monthly, 2,720 who used it weekly, and 4,736 who used it daily.
Of these, 3,506 individuals had CAD, based on medical records.
Only daily users had a significantly higher risk for CAD, compared with never-users (odds ratio, 1.34; P = .001) after adjusting for age, sex, hypertension, hyperlipidemia, type 2 diabetes, BMI, education, insurance status, and cigarette use.
The median age for daily users was 41, whereas the median age for never-users was 59.
GWAS analyses
The researchers then performed a Mendelian randomization analysis based on genome-wide association studies (GWAS) of cannabis use disorder and of CAD.
“Cannabis use disorder is a psychiatric diagnosis of severe cannabis dependency, equivalent to ‘alcohol use disorder’ for alcohol consumption,” Dr. Paranjpe explained. “The exact definition involves frequent use leading to significant dependence (but does not specify how often it is used).”
The GWAS data for cannabis use disorder came from a recent meta-analysis of three cohorts: the Psychiatric Genomics Consortium Substance Use Disorders working group, iPSYCH, and deCODE.
The GWAS statistics for CAD were obtained from the CARDIoGRAMplusC4D Consortium.
Cannabis use disorder was associated with significantly increased odds for CAD (OR, 1.05; P = .001), which remained after adjusting for both cigarette and alcohol use (OR, 1.04).
A version of this article first appeared on Medscape.com.
In the first part, in an observational study, daily cannabis use was associated with 34% higher odds for CAD, compared with never-users, in a large population-based U.S. cohort. Less frequent use was not associated with increased odds for CAD.
In the second part, people with a genetic susceptibility to cannabis use disorder or severe cannabis dependency had an increased risk for CAD, compared with other people.
Ishan Paranjpe, MD, the study’s lead author, reported these results in a press briefing and will present the study at the upcoming joint scientific sessions of the American College of Cardiology and the World Heart Federation 2023.
“A couple of takeaway points are that daily cannabis use, but not less frequent cannabis use, was associated with CAD” in the large population-based cohort, said Dr. Paranjpe, a resident physician at Stanford (Calif.) University, during the press conference.
“This analysis was adjusted for several possible confounders including age, sex at birth, [body mass index (BMI)], race, education, cigarette use, hypertension, high cholesterol, and diabetes,” he noted, and even after accounting for these risk factors, the association with heart disease remained.
“And the next thing, using Mendelian randomization, we sort of implied that there might be a causal relationship between cannabis and heart disease. Importantly this effect is independent of alcohol and cigarette use.
“The notion that cannabis is completely benign is probably wrong, and there might be certain risk of certain cardiovascular effects of cannabis we should be more on the lookout for,” Dr. Paranjpe said in an interview.
“Our main conclusion was that prevalent CAD is associated with cannabis consumption,” he added. “Other mechanistic work published in Cell has also shown that cannabis causes vascular inflammation that may lead to CAD.
“Thus, there is growing evidence from both laboratory and population studies that cannabis consumption may be harmful for cardiovascular health,” he said. “However, we still need more work on whether it affects the risk of incident cardiovascular events (i.e., stroke, heart attack) in patient[s] with existing CAD.”
ASCVD risk
Invited to comment, Robert L. Page II, PharmD, chair of the writing group for the American Heart Association’s scientific statement Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, published in 2020, said, “This adds to our hypothesis that if you are using marijuana over a longer period, greater exposure, you’re going to see an increase in the risk” for atherosclerotic cardiovascular disease (ASCVD).
“We’re seeing this increased risk for ASCVD in young adults between ages 18 to 40 – people who think that they’re invincible,” Dr. Page, a professor at the University of Colorado at Denver, Aurora, who was not involved with this research, told this news organization in an interview.
“The bottom line is that the risk that they are seeing is what has also been documented in other observational studies, and it adds fuel to the fire. We need to be paying close attention to this,” he said.
“Primary care [clinicians], cardiologists, need to address this, particularly in younger adults – because that’s where you’re seeing the highest amount of use.”
‘All of Us’ observational study
In the first part of the study, the researchers analyzed data from the “All of Us” cohort comprising adults age 18 and older from 340 inpatient and outpatient sites across the United States.
They identified 57,958 individuals who replied to a questionnaire asking about cannabis use (medicinal or recreational and whether it was edible or used by smoking or vaping) over the past 3 months.
There were 39,678 never-users, 8,749 who used it once or twice, 2,075 who used it monthly, 2,720 who used it weekly, and 4,736 who used it daily.
Of these, 3,506 individuals had CAD, based on medical records.
Only daily users had a significantly higher risk for CAD, compared with never-users (odds ratio, 1.34; P = .001) after adjusting for age, sex, hypertension, hyperlipidemia, type 2 diabetes, BMI, education, insurance status, and cigarette use.
The median age for daily users was 41, whereas the median age for never-users was 59.
GWAS analyses
The researchers then performed a Mendelian randomization analysis based on genome-wide association studies (GWAS) of cannabis use disorder and of CAD.
“Cannabis use disorder is a psychiatric diagnosis of severe cannabis dependency, equivalent to ‘alcohol use disorder’ for alcohol consumption,” Dr. Paranjpe explained. “The exact definition involves frequent use leading to significant dependence (but does not specify how often it is used).”
The GWAS data for cannabis use disorder came from a recent meta-analysis of three cohorts: the Psychiatric Genomics Consortium Substance Use Disorders working group, iPSYCH, and deCODE.
The GWAS statistics for CAD were obtained from the CARDIoGRAMplusC4D Consortium.
Cannabis use disorder was associated with significantly increased odds for CAD (OR, 1.05; P = .001), which remained after adjusting for both cigarette and alcohol use (OR, 1.04).
A version of this article first appeared on Medscape.com.
FROM ACC 2023
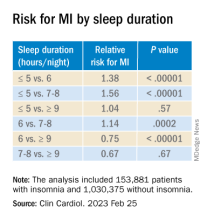
Insomnia, short sleep linked to greater risk for MI
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Therapy app cut A1c, drug intensification in T2D
An investigational smartphone app that delivers cognitive behavioral therapy (CBT) to people with type 2 diabetes led to a significant 10 percentage point cut in the incidence of antihyperglycemic-drug intensification during 6 months’ follow-up, when compared with a control phone app, in the CBT app’s pivotal trial with 669 randomized patients.
Previously reported results from this trial, called BT-001, showed that people randomized to use the CBT app had a significant average 0.4 percentage point reduction in hemoglobin A1c, compared with controls, after 90 days for the trial’s primary endpoint, and a significant 0.29 percentage point reduction in A1c, compared with controls, after 180 days.
The new finding, that these incremental drops in A1c occurred while the control patients also received significantly more intensification of their antihyperglycemic medication, provides further evidence for the efficacy of the CBT app, said Marc P. Bonaca, MD, in a press conference organized by the American College of Cardiology in advance of its upcoming joint scientific sessions.
The CBT app “significantly reduced A1c despite less intensification of antihyperglycemic therapy,” noted Dr. Bonaca, a vascular medicine specialist and executive director of CPC Clinical Research, an academic research organization created by and affiliated with the University of Colorado at Denver, Aurora.
Based on positive safety and efficacy findings from the primary-endpoint phase of the BT-001 trial, reported in Diabetes Care, the company developing the CBT app, Better Therapeutics, said in a statement that the U.S. Food and Drug Administration accepted the company’s application for de novo classification and marketing approval of the app, also called BT-001. If the agency grants this classification and marketing approval, the company plans to sell the app on a prescription basis for use by people with type 2 diabetes.
CBT app gives patients problem-solving skills
CBT gives people with type 2 diabetes a way to better understand their unhelpful behaviors and motivations and teaches them problem-solving skills. Providing this counseling via an app addresses the challenge of making the intervention scalable to a broad range of patients, Dr. Bonaca explained.
“Clinicians are frustrated by trying to produce behavioral change” in patients. The BT-001 app “provides a new avenue to treatment,” an approach that clinicians have been “very receptive” to using “once they understand the mechanism,” Dr. Bonaca said during the press conference. “The effect at 90 days was very similar to what a drug would do. It’s not just drugs any more” for treating people with type 2 diabetes, he declared.
“CBT is an empirically supported psychotherapy for a variety of emotional disorders, and it has been adapted to target specific emotional distress in the context of chronic illness,” commented Amit Shapira, PhD, a clinical psychologist at the Joslin Diabetes Center in Boston who has not been involved in the BT-001 studies. A CBT protocol designed for diabetes, CBT for Adherence and Depression “has been shown to have a positive impact on depression symptoms and glycemic control in adults with type 2 diabetes,” Dr. Shapira noted in an interview.
“Once a physician explains this [CBT] app and patients understand how to use it, then patients will be happy to use it,” commented Julia Grapsa, MD, PhD, a cardiologist at St. Thomas Hospital in London, who moderated the press conference. “We may see an explosion of apps like this one, designed to help better control” other chronic disorders, such as elevated blood pressure or abnormal lipid levels, Dr. Grapsa predicted. “I’m very optimistic that these apps have a great future in health care.”
Forty percent relative cut in new antihyperglycemic drug use
The BT-001 study randomized 669 adults with smartphone access and type 2 diabetes at any of six U.S. sites. The enrolled patients had type 2 diabetes for an average of 11 years, and an A1c of 7%-10.9% with an average level of 8.2%. Participants had to be on a stable medication regimen for at least 3 months but not using prandial insulin, and their treatment regimens could undergo adjustment during the trial. At baseline, each subject was on an average of 2.1 antihyperglycemic medications, including 90% on metformin and 42% on a sulfonylurea.
The new results reported by Dr. Bonaca showed that, during follow-up, people using the app had a 14.4% rate of antihyperglycemic drug intensification compared with a 24.4% rate among the controls, a roughly 40% relative decrease in new antihyperglycemic medication use. In addition, among those using insulin at baseline, 3.8% of controls increased their insulin dose, compared with 1.5% of those using the CBT app, while insulin doses decreased in 0.9% of the control subjects and in 2.2% of those using the BT-001 app.
Further study findings, first reported by Dr. Bonaca at the American Heart Association scientific sessions in late 2022, also showed a clear dose-response pattern for the CBT app: the more CBT lessons a person completed, the greater their reduction in A1c over 180 days of app use. People who used the app fewer than 10 times had an average reduction from baseline in their A1c of less than 0.1 percentage points. Among those who used the app 10-20 times (a subgroup with roughly one-third of the people randomized to app use), average A1c reduction increased to about 0.4 percentage points, and among those who used the app more than 20 times (also about one-third of the intervention group), the average A1c reduction from baseline was about 0.6 percentage points.
“It would be interesting to learn more about the adults who engaged with the app” and had a higher use rate “to provide more targeted care” with the app to people who match the profiles of those who were more likely to use the app during the trial, said Dr. Shapira.
This “clear” dose-response relationship “was one of the most exciting findings. It helps validate the mechanism,” Dr. Bonaca said during the press conference. “We’re now modeling which patients were the most engaged” with using the app, and “looking at ways to increase app engagement.”
Better Therapeutics also announced, in December 2022, results from a separate, uncontrolled study of a similar CBT app in 19 people with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. The findings showed that use of the tested app linked with an average 16% drop from baseline in liver fat content as measured by MRI, as well as other improvements in markers of hepatic function. The company said in a statement that based on these findings it planned to apply for breakthrough-device designation with the FDA for use of a liver-specific CBT app in people with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis.
The BT-001 trial was sponsored by Better Therapeutics, the company developing the app. CPC Clinical Research receives research and consulting funding from numerous companies. Dr. Bonaca has been a consultant to Audentes, and is a stockholder of Medtronic and Pfizer. Dr. Shapira and Dr. Grapsa had no disclosures.
An investigational smartphone app that delivers cognitive behavioral therapy (CBT) to people with type 2 diabetes led to a significant 10 percentage point cut in the incidence of antihyperglycemic-drug intensification during 6 months’ follow-up, when compared with a control phone app, in the CBT app’s pivotal trial with 669 randomized patients.
Previously reported results from this trial, called BT-001, showed that people randomized to use the CBT app had a significant average 0.4 percentage point reduction in hemoglobin A1c, compared with controls, after 90 days for the trial’s primary endpoint, and a significant 0.29 percentage point reduction in A1c, compared with controls, after 180 days.
The new finding, that these incremental drops in A1c occurred while the control patients also received significantly more intensification of their antihyperglycemic medication, provides further evidence for the efficacy of the CBT app, said Marc P. Bonaca, MD, in a press conference organized by the American College of Cardiology in advance of its upcoming joint scientific sessions.
The CBT app “significantly reduced A1c despite less intensification of antihyperglycemic therapy,” noted Dr. Bonaca, a vascular medicine specialist and executive director of CPC Clinical Research, an academic research organization created by and affiliated with the University of Colorado at Denver, Aurora.
Based on positive safety and efficacy findings from the primary-endpoint phase of the BT-001 trial, reported in Diabetes Care, the company developing the CBT app, Better Therapeutics, said in a statement that the U.S. Food and Drug Administration accepted the company’s application for de novo classification and marketing approval of the app, also called BT-001. If the agency grants this classification and marketing approval, the company plans to sell the app on a prescription basis for use by people with type 2 diabetes.
CBT app gives patients problem-solving skills
CBT gives people with type 2 diabetes a way to better understand their unhelpful behaviors and motivations and teaches them problem-solving skills. Providing this counseling via an app addresses the challenge of making the intervention scalable to a broad range of patients, Dr. Bonaca explained.
“Clinicians are frustrated by trying to produce behavioral change” in patients. The BT-001 app “provides a new avenue to treatment,” an approach that clinicians have been “very receptive” to using “once they understand the mechanism,” Dr. Bonaca said during the press conference. “The effect at 90 days was very similar to what a drug would do. It’s not just drugs any more” for treating people with type 2 diabetes, he declared.
“CBT is an empirically supported psychotherapy for a variety of emotional disorders, and it has been adapted to target specific emotional distress in the context of chronic illness,” commented Amit Shapira, PhD, a clinical psychologist at the Joslin Diabetes Center in Boston who has not been involved in the BT-001 studies. A CBT protocol designed for diabetes, CBT for Adherence and Depression “has been shown to have a positive impact on depression symptoms and glycemic control in adults with type 2 diabetes,” Dr. Shapira noted in an interview.
“Once a physician explains this [CBT] app and patients understand how to use it, then patients will be happy to use it,” commented Julia Grapsa, MD, PhD, a cardiologist at St. Thomas Hospital in London, who moderated the press conference. “We may see an explosion of apps like this one, designed to help better control” other chronic disorders, such as elevated blood pressure or abnormal lipid levels, Dr. Grapsa predicted. “I’m very optimistic that these apps have a great future in health care.”
Forty percent relative cut in new antihyperglycemic drug use
The BT-001 study randomized 669 adults with smartphone access and type 2 diabetes at any of six U.S. sites. The enrolled patients had type 2 diabetes for an average of 11 years, and an A1c of 7%-10.9% with an average level of 8.2%. Participants had to be on a stable medication regimen for at least 3 months but not using prandial insulin, and their treatment regimens could undergo adjustment during the trial. At baseline, each subject was on an average of 2.1 antihyperglycemic medications, including 90% on metformin and 42% on a sulfonylurea.
The new results reported by Dr. Bonaca showed that, during follow-up, people using the app had a 14.4% rate of antihyperglycemic drug intensification compared with a 24.4% rate among the controls, a roughly 40% relative decrease in new antihyperglycemic medication use. In addition, among those using insulin at baseline, 3.8% of controls increased their insulin dose, compared with 1.5% of those using the CBT app, while insulin doses decreased in 0.9% of the control subjects and in 2.2% of those using the BT-001 app.
Further study findings, first reported by Dr. Bonaca at the American Heart Association scientific sessions in late 2022, also showed a clear dose-response pattern for the CBT app: the more CBT lessons a person completed, the greater their reduction in A1c over 180 days of app use. People who used the app fewer than 10 times had an average reduction from baseline in their A1c of less than 0.1 percentage points. Among those who used the app 10-20 times (a subgroup with roughly one-third of the people randomized to app use), average A1c reduction increased to about 0.4 percentage points, and among those who used the app more than 20 times (also about one-third of the intervention group), the average A1c reduction from baseline was about 0.6 percentage points.
“It would be interesting to learn more about the adults who engaged with the app” and had a higher use rate “to provide more targeted care” with the app to people who match the profiles of those who were more likely to use the app during the trial, said Dr. Shapira.
This “clear” dose-response relationship “was one of the most exciting findings. It helps validate the mechanism,” Dr. Bonaca said during the press conference. “We’re now modeling which patients were the most engaged” with using the app, and “looking at ways to increase app engagement.”
Better Therapeutics also announced, in December 2022, results from a separate, uncontrolled study of a similar CBT app in 19 people with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. The findings showed that use of the tested app linked with an average 16% drop from baseline in liver fat content as measured by MRI, as well as other improvements in markers of hepatic function. The company said in a statement that based on these findings it planned to apply for breakthrough-device designation with the FDA for use of a liver-specific CBT app in people with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis.
The BT-001 trial was sponsored by Better Therapeutics, the company developing the app. CPC Clinical Research receives research and consulting funding from numerous companies. Dr. Bonaca has been a consultant to Audentes, and is a stockholder of Medtronic and Pfizer. Dr. Shapira and Dr. Grapsa had no disclosures.
An investigational smartphone app that delivers cognitive behavioral therapy (CBT) to people with type 2 diabetes led to a significant 10 percentage point cut in the incidence of antihyperglycemic-drug intensification during 6 months’ follow-up, when compared with a control phone app, in the CBT app’s pivotal trial with 669 randomized patients.
Previously reported results from this trial, called BT-001, showed that people randomized to use the CBT app had a significant average 0.4 percentage point reduction in hemoglobin A1c, compared with controls, after 90 days for the trial’s primary endpoint, and a significant 0.29 percentage point reduction in A1c, compared with controls, after 180 days.
The new finding, that these incremental drops in A1c occurred while the control patients also received significantly more intensification of their antihyperglycemic medication, provides further evidence for the efficacy of the CBT app, said Marc P. Bonaca, MD, in a press conference organized by the American College of Cardiology in advance of its upcoming joint scientific sessions.
The CBT app “significantly reduced A1c despite less intensification of antihyperglycemic therapy,” noted Dr. Bonaca, a vascular medicine specialist and executive director of CPC Clinical Research, an academic research organization created by and affiliated with the University of Colorado at Denver, Aurora.
Based on positive safety and efficacy findings from the primary-endpoint phase of the BT-001 trial, reported in Diabetes Care, the company developing the CBT app, Better Therapeutics, said in a statement that the U.S. Food and Drug Administration accepted the company’s application for de novo classification and marketing approval of the app, also called BT-001. If the agency grants this classification and marketing approval, the company plans to sell the app on a prescription basis for use by people with type 2 diabetes.
CBT app gives patients problem-solving skills
CBT gives people with type 2 diabetes a way to better understand their unhelpful behaviors and motivations and teaches them problem-solving skills. Providing this counseling via an app addresses the challenge of making the intervention scalable to a broad range of patients, Dr. Bonaca explained.
“Clinicians are frustrated by trying to produce behavioral change” in patients. The BT-001 app “provides a new avenue to treatment,” an approach that clinicians have been “very receptive” to using “once they understand the mechanism,” Dr. Bonaca said during the press conference. “The effect at 90 days was very similar to what a drug would do. It’s not just drugs any more” for treating people with type 2 diabetes, he declared.
“CBT is an empirically supported psychotherapy for a variety of emotional disorders, and it has been adapted to target specific emotional distress in the context of chronic illness,” commented Amit Shapira, PhD, a clinical psychologist at the Joslin Diabetes Center in Boston who has not been involved in the BT-001 studies. A CBT protocol designed for diabetes, CBT for Adherence and Depression “has been shown to have a positive impact on depression symptoms and glycemic control in adults with type 2 diabetes,” Dr. Shapira noted in an interview.
“Once a physician explains this [CBT] app and patients understand how to use it, then patients will be happy to use it,” commented Julia Grapsa, MD, PhD, a cardiologist at St. Thomas Hospital in London, who moderated the press conference. “We may see an explosion of apps like this one, designed to help better control” other chronic disorders, such as elevated blood pressure or abnormal lipid levels, Dr. Grapsa predicted. “I’m very optimistic that these apps have a great future in health care.”
Forty percent relative cut in new antihyperglycemic drug use
The BT-001 study randomized 669 adults with smartphone access and type 2 diabetes at any of six U.S. sites. The enrolled patients had type 2 diabetes for an average of 11 years, and an A1c of 7%-10.9% with an average level of 8.2%. Participants had to be on a stable medication regimen for at least 3 months but not using prandial insulin, and their treatment regimens could undergo adjustment during the trial. At baseline, each subject was on an average of 2.1 antihyperglycemic medications, including 90% on metformin and 42% on a sulfonylurea.
The new results reported by Dr. Bonaca showed that, during follow-up, people using the app had a 14.4% rate of antihyperglycemic drug intensification compared with a 24.4% rate among the controls, a roughly 40% relative decrease in new antihyperglycemic medication use. In addition, among those using insulin at baseline, 3.8% of controls increased their insulin dose, compared with 1.5% of those using the CBT app, while insulin doses decreased in 0.9% of the control subjects and in 2.2% of those using the BT-001 app.
Further study findings, first reported by Dr. Bonaca at the American Heart Association scientific sessions in late 2022, also showed a clear dose-response pattern for the CBT app: the more CBT lessons a person completed, the greater their reduction in A1c over 180 days of app use. People who used the app fewer than 10 times had an average reduction from baseline in their A1c of less than 0.1 percentage points. Among those who used the app 10-20 times (a subgroup with roughly one-third of the people randomized to app use), average A1c reduction increased to about 0.4 percentage points, and among those who used the app more than 20 times (also about one-third of the intervention group), the average A1c reduction from baseline was about 0.6 percentage points.
“It would be interesting to learn more about the adults who engaged with the app” and had a higher use rate “to provide more targeted care” with the app to people who match the profiles of those who were more likely to use the app during the trial, said Dr. Shapira.
This “clear” dose-response relationship “was one of the most exciting findings. It helps validate the mechanism,” Dr. Bonaca said during the press conference. “We’re now modeling which patients were the most engaged” with using the app, and “looking at ways to increase app engagement.”
Better Therapeutics also announced, in December 2022, results from a separate, uncontrolled study of a similar CBT app in 19 people with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. The findings showed that use of the tested app linked with an average 16% drop from baseline in liver fat content as measured by MRI, as well as other improvements in markers of hepatic function. The company said in a statement that based on these findings it planned to apply for breakthrough-device designation with the FDA for use of a liver-specific CBT app in people with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis.
The BT-001 trial was sponsored by Better Therapeutics, the company developing the app. CPC Clinical Research receives research and consulting funding from numerous companies. Dr. Bonaca has been a consultant to Audentes, and is a stockholder of Medtronic and Pfizer. Dr. Shapira and Dr. Grapsa had no disclosures.
FROM ACC 2023