User login
Prepare for deluge of JAK inhibitors for RA
MAUI, HAWAII – As it grows increasingly likely that oral Janus kinase inhibitors will constitute a major development in the treatment of rheumatoid arthritis, with a bevy of these agents becoming available for that indication, rheumatologists are asking questions about the coming revolution. Like, when should these agents be used? What are the major safety and efficacy differences, if any, within the class? How clinically relevant is JAK selectivity? And which JAK inhibitor is the best choice?
on these and other related questions at the 2019 Rheumatology Winter Clinical Symposium.
These issues take on growing relevance for clinicians and their RA patients because two oral small molecule JAK inhibitors – tofacitinib (Xeljanz) and baricitinib (Olumiant) – are already approved for RA, and three more – upadacitinib, filgotinib, and peficitinib – are on the horizon. Indeed, AbbVie has already filed for marketing approval of once-daily upadacitinib for RA on the basis of an impressive development program featuring six phase 3 trials, with a priority review decision from the Food and Drug Administration anticipated this fall. Filgotinib is the focus of three phase 3 studies, one of which is viewed as a home run, with the other two yet to report results. Peficitinib is backed by two positive phase 3 trials, although its manufacturer will at least initially seek marketing approval only in Japan and South Korea. And numerous other JAK inhibitors are in development for a variety of indications.
When should a JAK inhibitor be used?
That’s easy, according to Roy M. Fleischmann, MD: If the cost proves comparable, it makes sense to turn to a JAK inhibitor ahead of a tumor necrosis factor inhibitor or other biologic.
He noted that in the double-blind, phase 3 SELECT-COMPARE head-to-head comparison of upadacitinib at 15 mg/day, adalimumab (Humira) at 40 mg every other week, versus placebo, all on top of background methotrexate, upadacitinib proved superior to the market-leading tumor necrosis factor inhibitor in terms of both the American College of Rheumatology–defined 20% level of response (ACR 20) and 28-joint Disease Activity Score based on C-reactive protein (DAS28-CRP).
“The results were very dramatic,” noted Dr. Fleischmann, who presented the SELECT-COMPARE findings at the 2018 annual meeting of the American College of Rheumatology.
Moreover, other major trials have shown that baricitinib at 4 mg/day was superior in efficacy to adalimumab, and tofacitinib and peficitinib were “at least equal” to anti-TNF therapy, he added.
“These numbers are clinically meaningful – not so much for the difference in ACR 20, but in the depth of response: the ACR 50 and 70, the CDAI. I think these drugs are better than adalimumab,” declared Dr. Fleischmann, codirector of the division of rheumatology at Texas Health Presbyterian Medical Center, Dallas.
Mark Genovese, MD, concurred.
“I think that for most patients who don’t have a lot of other comorbidities, they would certainly prefer to take a pill over a shot. And if you have a drug that’s more effective than the standard of care and it comes at a reasonable price point – and ‘reasonable’ is in the eye of the beholder – but if I can get access to it on the formulary, I’d have no qualms about putting them on a JAK inhibitor before I’d move to a TNF inhibitor,” said Dr. Genovese, professor of medicine and director of the rheumatology clinic at Stanford (Calif.) University.
Upadacitinib elicited a better response at 30 mg than at 15 mg once daily in the phase 3 program; however, both speakers indicated they’d be happy with access to the 15-mg dose, should the FDA go that route, since it has a better safety profile.
Dr. Genovese was principal investigator in the previously reported multicenter FINCH2 trial of filgotinib at 100 or 200 mg/day in RA patients with a prior inadequate response to one or more biologic disease-modifying antirheumatic drugs.
“Impressive results in a refractory population,” he said. “I don’t see a big difference in safety between 100 and 200 mg, so I’d opt for the 200 because it worked really well in patients who had refractory disease.”
Other advantages of JAK inhibitors
Speed of onset is another advantage in addition to oral administration and efficacy greater than or equivalent to anti-TNF therapy, according to Dr. Genovese.
“As a class, JAK inhibitors have a faster onset than methotrexate in terms of improvement in disease activity and pain. So in a few weeks you can have a sense of whether folks are going to be responders,” the rheumatologist said.
Does JAK isoform selectivity really make a difference in terms of efficacy and safety?
It’s doubtful, the rheumatologists agreed. All of these oral small molecules target JAK1, and that’s what’s key.
Tofacitinib is relatively selective for JAK1 and JAK3, baricitinib for JAK1 and JAK2, upadacitinib and fibotinib for JAK1, and peficitinib is a pan-JAK inhibitor.
What are the safety concerns with this class of medications?
The risk of herpes zoster is higher than with TNF inhibitors, reinforcing the importance of varicella vaccination in JAK inhibitor candidates. Anemia occurs in a small percentage of patients. As for the risk of venous thromboembolism as a potential side effect of JAK inhibitors, a topic of great concern to the FDA, Dr. Fleischmann dismissed it as vastly overblown.
“I think VTEs are an RA effect. You see it with all the drugs, including methotrexate,” he said.
Idiosyncratic self-limited increases in creatine kinase have been seen in 2%-4% of patients on JAK inhibitors in pretty much all of the clinical trials. “I’m not aware of any cases of myositis, though,” Dr. Fleischmann noted.
As for the teratogenicity potential of JAK inhibitors, Dr. Genovese said that, as is true for most medications, it hasn’t been well studied.
“We don’t know. But I would not choose to use a JAK inhibitor in a woman who is going to conceive, has conceived, or is breastfeeding. I just don’t think that would be a good decision,” according to the rheumatologist.
Which JAK inhibitor is the best choice for treatment of RA?
It’s impossible to say because of the hazards in trying to draw meaningful conclusions from cross-study comparisons, the experts agreed.
“It’s a challenge. I think at the end of the day there will probably be one agent that looks like it might be best in class predicated on having the most number of indications, and that will probably become a preferred agent. The question is, does that happen before tofacitinib goes generic? And I don’t know the answer to that,” Dr. Genovese said.
Notably, upadacitinib is the subject of a plethora of ongoing phase 3 trials in atopic dermatitis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. It is also in earlier-phase investigation for the treatment of ankylosing spondylitis.
Do we really need all these JAK inhibitors?
“How many TNF inhibitors do you need?” Dr. Genovese retorted. “I think the reality is there’s probably a finite number and additional members add to the class, but there probably will always be one or two that are going to be best in class.”
Both rheumatologists indicated they serve as consultants to more than a dozen pharmaceutical companies and receive research grants from numerous firms.
MAUI, HAWAII – As it grows increasingly likely that oral Janus kinase inhibitors will constitute a major development in the treatment of rheumatoid arthritis, with a bevy of these agents becoming available for that indication, rheumatologists are asking questions about the coming revolution. Like, when should these agents be used? What are the major safety and efficacy differences, if any, within the class? How clinically relevant is JAK selectivity? And which JAK inhibitor is the best choice?
on these and other related questions at the 2019 Rheumatology Winter Clinical Symposium.
These issues take on growing relevance for clinicians and their RA patients because two oral small molecule JAK inhibitors – tofacitinib (Xeljanz) and baricitinib (Olumiant) – are already approved for RA, and three more – upadacitinib, filgotinib, and peficitinib – are on the horizon. Indeed, AbbVie has already filed for marketing approval of once-daily upadacitinib for RA on the basis of an impressive development program featuring six phase 3 trials, with a priority review decision from the Food and Drug Administration anticipated this fall. Filgotinib is the focus of three phase 3 studies, one of which is viewed as a home run, with the other two yet to report results. Peficitinib is backed by two positive phase 3 trials, although its manufacturer will at least initially seek marketing approval only in Japan and South Korea. And numerous other JAK inhibitors are in development for a variety of indications.
When should a JAK inhibitor be used?
That’s easy, according to Roy M. Fleischmann, MD: If the cost proves comparable, it makes sense to turn to a JAK inhibitor ahead of a tumor necrosis factor inhibitor or other biologic.
He noted that in the double-blind, phase 3 SELECT-COMPARE head-to-head comparison of upadacitinib at 15 mg/day, adalimumab (Humira) at 40 mg every other week, versus placebo, all on top of background methotrexate, upadacitinib proved superior to the market-leading tumor necrosis factor inhibitor in terms of both the American College of Rheumatology–defined 20% level of response (ACR 20) and 28-joint Disease Activity Score based on C-reactive protein (DAS28-CRP).
“The results were very dramatic,” noted Dr. Fleischmann, who presented the SELECT-COMPARE findings at the 2018 annual meeting of the American College of Rheumatology.
Moreover, other major trials have shown that baricitinib at 4 mg/day was superior in efficacy to adalimumab, and tofacitinib and peficitinib were “at least equal” to anti-TNF therapy, he added.
“These numbers are clinically meaningful – not so much for the difference in ACR 20, but in the depth of response: the ACR 50 and 70, the CDAI. I think these drugs are better than adalimumab,” declared Dr. Fleischmann, codirector of the division of rheumatology at Texas Health Presbyterian Medical Center, Dallas.
Mark Genovese, MD, concurred.
“I think that for most patients who don’t have a lot of other comorbidities, they would certainly prefer to take a pill over a shot. And if you have a drug that’s more effective than the standard of care and it comes at a reasonable price point – and ‘reasonable’ is in the eye of the beholder – but if I can get access to it on the formulary, I’d have no qualms about putting them on a JAK inhibitor before I’d move to a TNF inhibitor,” said Dr. Genovese, professor of medicine and director of the rheumatology clinic at Stanford (Calif.) University.
Upadacitinib elicited a better response at 30 mg than at 15 mg once daily in the phase 3 program; however, both speakers indicated they’d be happy with access to the 15-mg dose, should the FDA go that route, since it has a better safety profile.
Dr. Genovese was principal investigator in the previously reported multicenter FINCH2 trial of filgotinib at 100 or 200 mg/day in RA patients with a prior inadequate response to one or more biologic disease-modifying antirheumatic drugs.
“Impressive results in a refractory population,” he said. “I don’t see a big difference in safety between 100 and 200 mg, so I’d opt for the 200 because it worked really well in patients who had refractory disease.”
Other advantages of JAK inhibitors
Speed of onset is another advantage in addition to oral administration and efficacy greater than or equivalent to anti-TNF therapy, according to Dr. Genovese.
“As a class, JAK inhibitors have a faster onset than methotrexate in terms of improvement in disease activity and pain. So in a few weeks you can have a sense of whether folks are going to be responders,” the rheumatologist said.
Does JAK isoform selectivity really make a difference in terms of efficacy and safety?
It’s doubtful, the rheumatologists agreed. All of these oral small molecules target JAK1, and that’s what’s key.
Tofacitinib is relatively selective for JAK1 and JAK3, baricitinib for JAK1 and JAK2, upadacitinib and fibotinib for JAK1, and peficitinib is a pan-JAK inhibitor.
What are the safety concerns with this class of medications?
The risk of herpes zoster is higher than with TNF inhibitors, reinforcing the importance of varicella vaccination in JAK inhibitor candidates. Anemia occurs in a small percentage of patients. As for the risk of venous thromboembolism as a potential side effect of JAK inhibitors, a topic of great concern to the FDA, Dr. Fleischmann dismissed it as vastly overblown.
“I think VTEs are an RA effect. You see it with all the drugs, including methotrexate,” he said.
Idiosyncratic self-limited increases in creatine kinase have been seen in 2%-4% of patients on JAK inhibitors in pretty much all of the clinical trials. “I’m not aware of any cases of myositis, though,” Dr. Fleischmann noted.
As for the teratogenicity potential of JAK inhibitors, Dr. Genovese said that, as is true for most medications, it hasn’t been well studied.
“We don’t know. But I would not choose to use a JAK inhibitor in a woman who is going to conceive, has conceived, or is breastfeeding. I just don’t think that would be a good decision,” according to the rheumatologist.
Which JAK inhibitor is the best choice for treatment of RA?
It’s impossible to say because of the hazards in trying to draw meaningful conclusions from cross-study comparisons, the experts agreed.
“It’s a challenge. I think at the end of the day there will probably be one agent that looks like it might be best in class predicated on having the most number of indications, and that will probably become a preferred agent. The question is, does that happen before tofacitinib goes generic? And I don’t know the answer to that,” Dr. Genovese said.
Notably, upadacitinib is the subject of a plethora of ongoing phase 3 trials in atopic dermatitis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. It is also in earlier-phase investigation for the treatment of ankylosing spondylitis.
Do we really need all these JAK inhibitors?
“How many TNF inhibitors do you need?” Dr. Genovese retorted. “I think the reality is there’s probably a finite number and additional members add to the class, but there probably will always be one or two that are going to be best in class.”
Both rheumatologists indicated they serve as consultants to more than a dozen pharmaceutical companies and receive research grants from numerous firms.
MAUI, HAWAII – As it grows increasingly likely that oral Janus kinase inhibitors will constitute a major development in the treatment of rheumatoid arthritis, with a bevy of these agents becoming available for that indication, rheumatologists are asking questions about the coming revolution. Like, when should these agents be used? What are the major safety and efficacy differences, if any, within the class? How clinically relevant is JAK selectivity? And which JAK inhibitor is the best choice?
on these and other related questions at the 2019 Rheumatology Winter Clinical Symposium.
These issues take on growing relevance for clinicians and their RA patients because two oral small molecule JAK inhibitors – tofacitinib (Xeljanz) and baricitinib (Olumiant) – are already approved for RA, and three more – upadacitinib, filgotinib, and peficitinib – are on the horizon. Indeed, AbbVie has already filed for marketing approval of once-daily upadacitinib for RA on the basis of an impressive development program featuring six phase 3 trials, with a priority review decision from the Food and Drug Administration anticipated this fall. Filgotinib is the focus of three phase 3 studies, one of which is viewed as a home run, with the other two yet to report results. Peficitinib is backed by two positive phase 3 trials, although its manufacturer will at least initially seek marketing approval only in Japan and South Korea. And numerous other JAK inhibitors are in development for a variety of indications.
When should a JAK inhibitor be used?
That’s easy, according to Roy M. Fleischmann, MD: If the cost proves comparable, it makes sense to turn to a JAK inhibitor ahead of a tumor necrosis factor inhibitor or other biologic.
He noted that in the double-blind, phase 3 SELECT-COMPARE head-to-head comparison of upadacitinib at 15 mg/day, adalimumab (Humira) at 40 mg every other week, versus placebo, all on top of background methotrexate, upadacitinib proved superior to the market-leading tumor necrosis factor inhibitor in terms of both the American College of Rheumatology–defined 20% level of response (ACR 20) and 28-joint Disease Activity Score based on C-reactive protein (DAS28-CRP).
“The results were very dramatic,” noted Dr. Fleischmann, who presented the SELECT-COMPARE findings at the 2018 annual meeting of the American College of Rheumatology.
Moreover, other major trials have shown that baricitinib at 4 mg/day was superior in efficacy to adalimumab, and tofacitinib and peficitinib were “at least equal” to anti-TNF therapy, he added.
“These numbers are clinically meaningful – not so much for the difference in ACR 20, but in the depth of response: the ACR 50 and 70, the CDAI. I think these drugs are better than adalimumab,” declared Dr. Fleischmann, codirector of the division of rheumatology at Texas Health Presbyterian Medical Center, Dallas.
Mark Genovese, MD, concurred.
“I think that for most patients who don’t have a lot of other comorbidities, they would certainly prefer to take a pill over a shot. And if you have a drug that’s more effective than the standard of care and it comes at a reasonable price point – and ‘reasonable’ is in the eye of the beholder – but if I can get access to it on the formulary, I’d have no qualms about putting them on a JAK inhibitor before I’d move to a TNF inhibitor,” said Dr. Genovese, professor of medicine and director of the rheumatology clinic at Stanford (Calif.) University.
Upadacitinib elicited a better response at 30 mg than at 15 mg once daily in the phase 3 program; however, both speakers indicated they’d be happy with access to the 15-mg dose, should the FDA go that route, since it has a better safety profile.
Dr. Genovese was principal investigator in the previously reported multicenter FINCH2 trial of filgotinib at 100 or 200 mg/day in RA patients with a prior inadequate response to one or more biologic disease-modifying antirheumatic drugs.
“Impressive results in a refractory population,” he said. “I don’t see a big difference in safety between 100 and 200 mg, so I’d opt for the 200 because it worked really well in patients who had refractory disease.”
Other advantages of JAK inhibitors
Speed of onset is another advantage in addition to oral administration and efficacy greater than or equivalent to anti-TNF therapy, according to Dr. Genovese.
“As a class, JAK inhibitors have a faster onset than methotrexate in terms of improvement in disease activity and pain. So in a few weeks you can have a sense of whether folks are going to be responders,” the rheumatologist said.
Does JAK isoform selectivity really make a difference in terms of efficacy and safety?
It’s doubtful, the rheumatologists agreed. All of these oral small molecules target JAK1, and that’s what’s key.
Tofacitinib is relatively selective for JAK1 and JAK3, baricitinib for JAK1 and JAK2, upadacitinib and fibotinib for JAK1, and peficitinib is a pan-JAK inhibitor.
What are the safety concerns with this class of medications?
The risk of herpes zoster is higher than with TNF inhibitors, reinforcing the importance of varicella vaccination in JAK inhibitor candidates. Anemia occurs in a small percentage of patients. As for the risk of venous thromboembolism as a potential side effect of JAK inhibitors, a topic of great concern to the FDA, Dr. Fleischmann dismissed it as vastly overblown.
“I think VTEs are an RA effect. You see it with all the drugs, including methotrexate,” he said.
Idiosyncratic self-limited increases in creatine kinase have been seen in 2%-4% of patients on JAK inhibitors in pretty much all of the clinical trials. “I’m not aware of any cases of myositis, though,” Dr. Fleischmann noted.
As for the teratogenicity potential of JAK inhibitors, Dr. Genovese said that, as is true for most medications, it hasn’t been well studied.
“We don’t know. But I would not choose to use a JAK inhibitor in a woman who is going to conceive, has conceived, or is breastfeeding. I just don’t think that would be a good decision,” according to the rheumatologist.
Which JAK inhibitor is the best choice for treatment of RA?
It’s impossible to say because of the hazards in trying to draw meaningful conclusions from cross-study comparisons, the experts agreed.
“It’s a challenge. I think at the end of the day there will probably be one agent that looks like it might be best in class predicated on having the most number of indications, and that will probably become a preferred agent. The question is, does that happen before tofacitinib goes generic? And I don’t know the answer to that,” Dr. Genovese said.
Notably, upadacitinib is the subject of a plethora of ongoing phase 3 trials in atopic dermatitis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. It is also in earlier-phase investigation for the treatment of ankylosing spondylitis.
Do we really need all these JAK inhibitors?
“How many TNF inhibitors do you need?” Dr. Genovese retorted. “I think the reality is there’s probably a finite number and additional members add to the class, but there probably will always be one or two that are going to be best in class.”
Both rheumatologists indicated they serve as consultants to more than a dozen pharmaceutical companies and receive research grants from numerous firms.
REPORTING FROM RWCS 2019
Interosseous tendon inflammation is common prior to RA
MAUI, HAWAII – Inflammation of the hand interosseous tendons found on MRI is a novel target in efforts to preempt the development and progression of rheumatoid arthritis, Paul Emery, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He and his coinvestigators have previously shown there is a high prevalence of interosseous tendon inflammation in the hands of patients with established RA, but now they’ve demonstrated that this phenomenon also occurs in anti–cyclic citrullinated peptide (CCP)–positive individuals at increased risk for RA, even before onset of clinical synovitis.
This finding is consistent with the notion that, even though RA is classically considered a disease of the synovial joints, the joint involvement is a relatively late phenomenon in the disease development process and extracapsular structures may be important early targets of RA-related inflammation. Indeed, the MRI finding of tenosynovitis of the wrist and finger flexor tendons is known to be the strongest predictor of progression to arthritis in patients with recent-onset arthralgia or other musculoskeletal symptoms but no clinical synovitis, according to Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
Because the interosseous muscles of the hands play a critical role in hand function – pianists and other musicians not infrequently present to rheumatologists with overuse injuries of the muscles and their tendons – Dr. Emery and his coworkers decided to take a comprehensive look at interosseous tendon inflammation across the full spectrum of RA and pre-RA. They conducted a retrospective study of clinical and hand MRI data on 93 CCP-positive patients who presented with new-onset musculoskeletal symptoms but no clinical synovitis; 47 patients with early RA, all of whom were disease-modifying antirheumatic drug–naive; 28 patients with late RA as defined by at least 1 year of symptoms, anti-CCP and/or rheumatoid factor positivity, a Disease Activity Score in 28 joints (DAS28) of 3.2 or more, plus a history of exposure to one or more DMARDs at the time of their hand imaging; and 20 healthy controls.
The key finding is that the proportion of subjects with MRI evidence of interosseous tendon inflammation rose along the advancing RA continuum. It was present in 19% of the CCP-positive patients without clinical synovitis; 49% of the DMARD-naive early RA group; 57% of the late RA group; and in none of the healthy controls. Moreover, the number of inflamed interosseous tendons per patient also increased with RA progression.
A total of 12% of 507 nontender metacarpophalangeal joints showed MRI evidence of interosseous tendon inflammation, as did 28% of 141 tender ones (Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2018-214331).
As part of the study, Dr. Emery and coinvestigators performed cadaveric dissections that demonstrated that the interosseous tendons don’t possess a tendon sheath and don’t directly communicate with the joint capsule.
A prospective study is warranted in order to confirm the observed association between interosseous tendon inflammation and clinical and subclinical synovitis and to establish the predictive value of hand MRI as a harbinger of RA, he noted.
Dr. Emery reported having no financial conflicts regarding his presentation.
MAUI, HAWAII – Inflammation of the hand interosseous tendons found on MRI is a novel target in efforts to preempt the development and progression of rheumatoid arthritis, Paul Emery, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He and his coinvestigators have previously shown there is a high prevalence of interosseous tendon inflammation in the hands of patients with established RA, but now they’ve demonstrated that this phenomenon also occurs in anti–cyclic citrullinated peptide (CCP)–positive individuals at increased risk for RA, even before onset of clinical synovitis.
This finding is consistent with the notion that, even though RA is classically considered a disease of the synovial joints, the joint involvement is a relatively late phenomenon in the disease development process and extracapsular structures may be important early targets of RA-related inflammation. Indeed, the MRI finding of tenosynovitis of the wrist and finger flexor tendons is known to be the strongest predictor of progression to arthritis in patients with recent-onset arthralgia or other musculoskeletal symptoms but no clinical synovitis, according to Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
Because the interosseous muscles of the hands play a critical role in hand function – pianists and other musicians not infrequently present to rheumatologists with overuse injuries of the muscles and their tendons – Dr. Emery and his coworkers decided to take a comprehensive look at interosseous tendon inflammation across the full spectrum of RA and pre-RA. They conducted a retrospective study of clinical and hand MRI data on 93 CCP-positive patients who presented with new-onset musculoskeletal symptoms but no clinical synovitis; 47 patients with early RA, all of whom were disease-modifying antirheumatic drug–naive; 28 patients with late RA as defined by at least 1 year of symptoms, anti-CCP and/or rheumatoid factor positivity, a Disease Activity Score in 28 joints (DAS28) of 3.2 or more, plus a history of exposure to one or more DMARDs at the time of their hand imaging; and 20 healthy controls.
The key finding is that the proportion of subjects with MRI evidence of interosseous tendon inflammation rose along the advancing RA continuum. It was present in 19% of the CCP-positive patients without clinical synovitis; 49% of the DMARD-naive early RA group; 57% of the late RA group; and in none of the healthy controls. Moreover, the number of inflamed interosseous tendons per patient also increased with RA progression.
A total of 12% of 507 nontender metacarpophalangeal joints showed MRI evidence of interosseous tendon inflammation, as did 28% of 141 tender ones (Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2018-214331).
As part of the study, Dr. Emery and coinvestigators performed cadaveric dissections that demonstrated that the interosseous tendons don’t possess a tendon sheath and don’t directly communicate with the joint capsule.
A prospective study is warranted in order to confirm the observed association between interosseous tendon inflammation and clinical and subclinical synovitis and to establish the predictive value of hand MRI as a harbinger of RA, he noted.
Dr. Emery reported having no financial conflicts regarding his presentation.
MAUI, HAWAII – Inflammation of the hand interosseous tendons found on MRI is a novel target in efforts to preempt the development and progression of rheumatoid arthritis, Paul Emery, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He and his coinvestigators have previously shown there is a high prevalence of interosseous tendon inflammation in the hands of patients with established RA, but now they’ve demonstrated that this phenomenon also occurs in anti–cyclic citrullinated peptide (CCP)–positive individuals at increased risk for RA, even before onset of clinical synovitis.
This finding is consistent with the notion that, even though RA is classically considered a disease of the synovial joints, the joint involvement is a relatively late phenomenon in the disease development process and extracapsular structures may be important early targets of RA-related inflammation. Indeed, the MRI finding of tenosynovitis of the wrist and finger flexor tendons is known to be the strongest predictor of progression to arthritis in patients with recent-onset arthralgia or other musculoskeletal symptoms but no clinical synovitis, according to Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center.
Because the interosseous muscles of the hands play a critical role in hand function – pianists and other musicians not infrequently present to rheumatologists with overuse injuries of the muscles and their tendons – Dr. Emery and his coworkers decided to take a comprehensive look at interosseous tendon inflammation across the full spectrum of RA and pre-RA. They conducted a retrospective study of clinical and hand MRI data on 93 CCP-positive patients who presented with new-onset musculoskeletal symptoms but no clinical synovitis; 47 patients with early RA, all of whom were disease-modifying antirheumatic drug–naive; 28 patients with late RA as defined by at least 1 year of symptoms, anti-CCP and/or rheumatoid factor positivity, a Disease Activity Score in 28 joints (DAS28) of 3.2 or more, plus a history of exposure to one or more DMARDs at the time of their hand imaging; and 20 healthy controls.
The key finding is that the proportion of subjects with MRI evidence of interosseous tendon inflammation rose along the advancing RA continuum. It was present in 19% of the CCP-positive patients without clinical synovitis; 49% of the DMARD-naive early RA group; 57% of the late RA group; and in none of the healthy controls. Moreover, the number of inflamed interosseous tendons per patient also increased with RA progression.
A total of 12% of 507 nontender metacarpophalangeal joints showed MRI evidence of interosseous tendon inflammation, as did 28% of 141 tender ones (Ann Rheum Dis. 2019 Mar 23. doi: 10.1136/annrheumdis-2018-214331).
As part of the study, Dr. Emery and coinvestigators performed cadaveric dissections that demonstrated that the interosseous tendons don’t possess a tendon sheath and don’t directly communicate with the joint capsule.
A prospective study is warranted in order to confirm the observed association between interosseous tendon inflammation and clinical and subclinical synovitis and to establish the predictive value of hand MRI as a harbinger of RA, he noted.
Dr. Emery reported having no financial conflicts regarding his presentation.
REPORTING FROM RWCS 2019
Weight loss improves psoriatic arthritis
MAUI, HAWAII – Serious weight loss brings big improvement in psoriatic arthritis in obese patients, at least short term, according to a Swedish, single-arm, prospective, proof-of-concept study.
A dose-response effect was evident: the greater the lost poundage, the bigger the improvement across multiple dimensions of psoriatic arthritis.
The short-term efficacy was eye-catching, especially in view of the well-recognized increased prevalence of obesity in psoriatic arthritis patients. But the jury is still out as to the long-term impact of this nonpharmacologic therapy, Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He has spoken with the Swedish investigators and was happy to learn they’re continuing to follow study participants long term.
“That’s going to be the key, right? Because if you do this for 12 weeks, like every other fad crash diet, and then you let the weight go right back on again, you haven’t really accomplished anything. I think the key will be what happens at a year,” according to Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
The study included 46 obese psoriatic arthritis patients who signed on for a structured, medically supervised very-low-energy diet lasting 12-16 weeks, depending upon their baseline obesity level. The commercially available liquid diet (Cambridge Weight Plan Limited) is a type of therapy widely prescribed by Swedish physicians, clocking in at a mere 640 kcal/day.
“I don’t know about you, but I ate that at breakfast this morning,” quipped symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
Following completion of the strict very-low-energy diet, patients were gradually reintroduced to a less-draconian, solid-food, energy-restricted diet, to be followed through the 12-month mark. The full 12-month protocol was supervised by staff in the obesity unit at Sahlgrenska University Hospital in Gothenburg, Sweden. The 12-month results will be presented at the annual European Congress of Rheumatology in Madrid.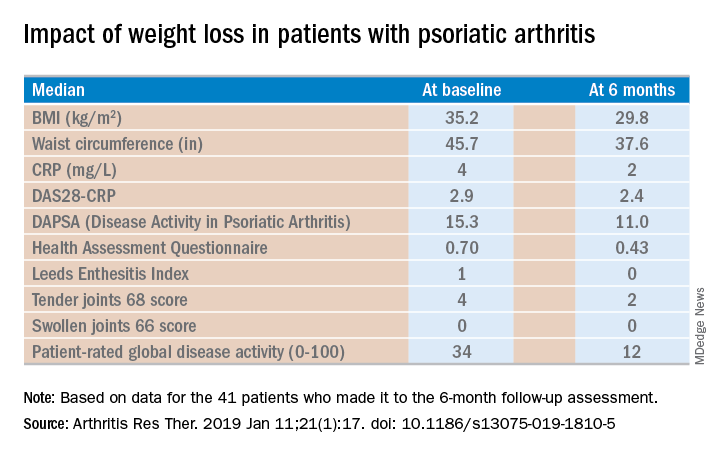
Of the 46 starters, 41 made it to the 6-month follow-up assessment. At that point they’d lost a median of 18.2 kg, or 18.6% of their baseline body weight. Their body mass index had dropped from an average of 35.2 to 29.8 kg/m2. And their psoriatic arthritis had improved significantly. For example, their median Disease Activity Score using 28 joint counts based upon C-reactive protein (DAS28-CRP) decreased from 2.9 at baseline to 2.4 at 6 months, with ACR 20, -50, and -70 responses of 51.2%, 34.1%, and 7.3% while disease-directed medications were held constant (Arthritis Res Ther. 2019 Jan 11;21[1]:17. doi: 10.1186/s13075-019-1810-5).
The investigators reported the very-low-energy diet phase was generally well tolerated. A total of 34 of the 41 patients deemed it “easier or much easier” than expected, prompting Dr. Ruderman to comment: “Because they thought it was going to be awful.”
Dr. Ruderman and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Serious weight loss brings big improvement in psoriatic arthritis in obese patients, at least short term, according to a Swedish, single-arm, prospective, proof-of-concept study.
A dose-response effect was evident: the greater the lost poundage, the bigger the improvement across multiple dimensions of psoriatic arthritis.
The short-term efficacy was eye-catching, especially in view of the well-recognized increased prevalence of obesity in psoriatic arthritis patients. But the jury is still out as to the long-term impact of this nonpharmacologic therapy, Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He has spoken with the Swedish investigators and was happy to learn they’re continuing to follow study participants long term.
“That’s going to be the key, right? Because if you do this for 12 weeks, like every other fad crash diet, and then you let the weight go right back on again, you haven’t really accomplished anything. I think the key will be what happens at a year,” according to Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
The study included 46 obese psoriatic arthritis patients who signed on for a structured, medically supervised very-low-energy diet lasting 12-16 weeks, depending upon their baseline obesity level. The commercially available liquid diet (Cambridge Weight Plan Limited) is a type of therapy widely prescribed by Swedish physicians, clocking in at a mere 640 kcal/day.
“I don’t know about you, but I ate that at breakfast this morning,” quipped symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
Following completion of the strict very-low-energy diet, patients were gradually reintroduced to a less-draconian, solid-food, energy-restricted diet, to be followed through the 12-month mark. The full 12-month protocol was supervised by staff in the obesity unit at Sahlgrenska University Hospital in Gothenburg, Sweden. The 12-month results will be presented at the annual European Congress of Rheumatology in Madrid.
Of the 46 starters, 41 made it to the 6-month follow-up assessment. At that point they’d lost a median of 18.2 kg, or 18.6% of their baseline body weight. Their body mass index had dropped from an average of 35.2 to 29.8 kg/m2. And their psoriatic arthritis had improved significantly. For example, their median Disease Activity Score using 28 joint counts based upon C-reactive protein (DAS28-CRP) decreased from 2.9 at baseline to 2.4 at 6 months, with ACR 20, -50, and -70 responses of 51.2%, 34.1%, and 7.3% while disease-directed medications were held constant (Arthritis Res Ther. 2019 Jan 11;21[1]:17. doi: 10.1186/s13075-019-1810-5).
The investigators reported the very-low-energy diet phase was generally well tolerated. A total of 34 of the 41 patients deemed it “easier or much easier” than expected, prompting Dr. Ruderman to comment: “Because they thought it was going to be awful.”
Dr. Ruderman and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Serious weight loss brings big improvement in psoriatic arthritis in obese patients, at least short term, according to a Swedish, single-arm, prospective, proof-of-concept study.
A dose-response effect was evident: the greater the lost poundage, the bigger the improvement across multiple dimensions of psoriatic arthritis.
The short-term efficacy was eye-catching, especially in view of the well-recognized increased prevalence of obesity in psoriatic arthritis patients. But the jury is still out as to the long-term impact of this nonpharmacologic therapy, Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He has spoken with the Swedish investigators and was happy to learn they’re continuing to follow study participants long term.
“That’s going to be the key, right? Because if you do this for 12 weeks, like every other fad crash diet, and then you let the weight go right back on again, you haven’t really accomplished anything. I think the key will be what happens at a year,” according to Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
The study included 46 obese psoriatic arthritis patients who signed on for a structured, medically supervised very-low-energy diet lasting 12-16 weeks, depending upon their baseline obesity level. The commercially available liquid diet (Cambridge Weight Plan Limited) is a type of therapy widely prescribed by Swedish physicians, clocking in at a mere 640 kcal/day.
“I don’t know about you, but I ate that at breakfast this morning,” quipped symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
Following completion of the strict very-low-energy diet, patients were gradually reintroduced to a less-draconian, solid-food, energy-restricted diet, to be followed through the 12-month mark. The full 12-month protocol was supervised by staff in the obesity unit at Sahlgrenska University Hospital in Gothenburg, Sweden. The 12-month results will be presented at the annual European Congress of Rheumatology in Madrid.
Of the 46 starters, 41 made it to the 6-month follow-up assessment. At that point they’d lost a median of 18.2 kg, or 18.6% of their baseline body weight. Their body mass index had dropped from an average of 35.2 to 29.8 kg/m2. And their psoriatic arthritis had improved significantly. For example, their median Disease Activity Score using 28 joint counts based upon C-reactive protein (DAS28-CRP) decreased from 2.9 at baseline to 2.4 at 6 months, with ACR 20, -50, and -70 responses of 51.2%, 34.1%, and 7.3% while disease-directed medications were held constant (Arthritis Res Ther. 2019 Jan 11;21[1]:17. doi: 10.1186/s13075-019-1810-5).
The investigators reported the very-low-energy diet phase was generally well tolerated. A total of 34 of the 41 patients deemed it “easier or much easier” than expected, prompting Dr. Ruderman to comment: “Because they thought it was going to be awful.”
Dr. Ruderman and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
Methotrexate pneumonitis called ‘super rare’
MAUI, HAWAII – The incidence of methotrexate pneumonitis has been reported as ranging from 3.5% to 7.6% among patients taking the disease-modifying antirheumatic drug. It’s an estimate that Aryeh Fischer, MD, counters with a one-word response: “Nonsense!”
“There’s just no way that methotrexate is causing that much lung disease,” he declared at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Fischer, a rheumatologist with joint appointments to the divisions of rheumatology and pulmonary sciences and critical care medicine at the University of Colorado at Denver, Aurora, noted that his opinion is considered controversial in the pulmonology world.
“I’m not allowed to talk about methotrexate at lung conferences. They stop you at the gate. They’re convinced in lung circles that methotrexate is the worst drug known to mankind,” he said.
“My take home on methotrexate lung toxicity is this: I would just say, yes, it can occur, but it’s super rare and most often we’re not really sure that it was methotrexate pneumonitis. The diagnosis is not definitive, it’s exclusionary. We know that patients with interstitial lung disease of all types get acute exacerbations, and in idiopathic pulmonary fibrosis it’s actually the leading cause of mortality,” the rheumatologist said.
He highlighted a meta-analysis of 22 randomized, double-blind clinical trials published in 1990-2013 of methotrexate versus placebo or active comparators in 8,584 RA patients. The Irish investigators of that meta-analysis found that methotrexate was associated with a small albeit statistically significant 10% increase in the risk of all adverse respiratory events and an 11% increase in the risk of respiratory infection. However, patients on methotrexate were not at increased risk of mortality because of lung disease. And not a single case of methotrexate pneumonitis was reported after 2002 (Arthritis Rheumatol. 2014 Apr;66[4]:803-12).
Methotrexate pneumonitis is not dose dependent, nor is it related to treatment duration.
“Just because your patient has been on methotrexate for years does not mean they won’t get methotrexate lung toxicity,” he cautioned. “But this is not a chronic fibrotic interstitial lung disease, this is an acute onset of peripheral infiltrates and ground glass opacifications on chest imaging.”
Bronchoalveolar lavage classically shows a hypersensitivity pneumonitis with lymphocytosis. Transbronchial or surgical lung biopsy may show an organizing pneumonia or airway-based nonnecrotizing granulomas, again indicative of a hypersensitivity reaction.
Because the diagnostic picture is so often cloudy, Dr. Fischer generally tries to avoid methotrexate in patients with moderate or severe interstitial lung disease. “I have the luxury of avoiding it because we have so many great arthritis drugs these days,” he noted.
“That being said, the notion that we’re going to stop methotrexate in an 80-year-old who’s been on it for years and has mild bibasilar fibrotic interstitial lung disease so that her lung doc can sleep better at night is not very helpful for our patients. If the patient is doing well on methotrexate and the interstitial lung disease is mild, I continue [the methotrexate],” Dr. Fischer said.
He reported receiving research grants from Boehringer Ingelheim and Corbus Pharmaceuticals and serving as a consultant to Boehringer Ingelheim and other pharmaceutical companies.
MAUI, HAWAII – The incidence of methotrexate pneumonitis has been reported as ranging from 3.5% to 7.6% among patients taking the disease-modifying antirheumatic drug. It’s an estimate that Aryeh Fischer, MD, counters with a one-word response: “Nonsense!”
“There’s just no way that methotrexate is causing that much lung disease,” he declared at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Fischer, a rheumatologist with joint appointments to the divisions of rheumatology and pulmonary sciences and critical care medicine at the University of Colorado at Denver, Aurora, noted that his opinion is considered controversial in the pulmonology world.
“I’m not allowed to talk about methotrexate at lung conferences. They stop you at the gate. They’re convinced in lung circles that methotrexate is the worst drug known to mankind,” he said.
“My take home on methotrexate lung toxicity is this: I would just say, yes, it can occur, but it’s super rare and most often we’re not really sure that it was methotrexate pneumonitis. The diagnosis is not definitive, it’s exclusionary. We know that patients with interstitial lung disease of all types get acute exacerbations, and in idiopathic pulmonary fibrosis it’s actually the leading cause of mortality,” the rheumatologist said.
He highlighted a meta-analysis of 22 randomized, double-blind clinical trials published in 1990-2013 of methotrexate versus placebo or active comparators in 8,584 RA patients. The Irish investigators of that meta-analysis found that methotrexate was associated with a small albeit statistically significant 10% increase in the risk of all adverse respiratory events and an 11% increase in the risk of respiratory infection. However, patients on methotrexate were not at increased risk of mortality because of lung disease. And not a single case of methotrexate pneumonitis was reported after 2002 (Arthritis Rheumatol. 2014 Apr;66[4]:803-12).
Methotrexate pneumonitis is not dose dependent, nor is it related to treatment duration.
“Just because your patient has been on methotrexate for years does not mean they won’t get methotrexate lung toxicity,” he cautioned. “But this is not a chronic fibrotic interstitial lung disease, this is an acute onset of peripheral infiltrates and ground glass opacifications on chest imaging.”
Bronchoalveolar lavage classically shows a hypersensitivity pneumonitis with lymphocytosis. Transbronchial or surgical lung biopsy may show an organizing pneumonia or airway-based nonnecrotizing granulomas, again indicative of a hypersensitivity reaction.
Because the diagnostic picture is so often cloudy, Dr. Fischer generally tries to avoid methotrexate in patients with moderate or severe interstitial lung disease. “I have the luxury of avoiding it because we have so many great arthritis drugs these days,” he noted.
“That being said, the notion that we’re going to stop methotrexate in an 80-year-old who’s been on it for years and has mild bibasilar fibrotic interstitial lung disease so that her lung doc can sleep better at night is not very helpful for our patients. If the patient is doing well on methotrexate and the interstitial lung disease is mild, I continue [the methotrexate],” Dr. Fischer said.
He reported receiving research grants from Boehringer Ingelheim and Corbus Pharmaceuticals and serving as a consultant to Boehringer Ingelheim and other pharmaceutical companies.
MAUI, HAWAII – The incidence of methotrexate pneumonitis has been reported as ranging from 3.5% to 7.6% among patients taking the disease-modifying antirheumatic drug. It’s an estimate that Aryeh Fischer, MD, counters with a one-word response: “Nonsense!”
“There’s just no way that methotrexate is causing that much lung disease,” he declared at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Fischer, a rheumatologist with joint appointments to the divisions of rheumatology and pulmonary sciences and critical care medicine at the University of Colorado at Denver, Aurora, noted that his opinion is considered controversial in the pulmonology world.
“I’m not allowed to talk about methotrexate at lung conferences. They stop you at the gate. They’re convinced in lung circles that methotrexate is the worst drug known to mankind,” he said.
“My take home on methotrexate lung toxicity is this: I would just say, yes, it can occur, but it’s super rare and most often we’re not really sure that it was methotrexate pneumonitis. The diagnosis is not definitive, it’s exclusionary. We know that patients with interstitial lung disease of all types get acute exacerbations, and in idiopathic pulmonary fibrosis it’s actually the leading cause of mortality,” the rheumatologist said.
He highlighted a meta-analysis of 22 randomized, double-blind clinical trials published in 1990-2013 of methotrexate versus placebo or active comparators in 8,584 RA patients. The Irish investigators of that meta-analysis found that methotrexate was associated with a small albeit statistically significant 10% increase in the risk of all adverse respiratory events and an 11% increase in the risk of respiratory infection. However, patients on methotrexate were not at increased risk of mortality because of lung disease. And not a single case of methotrexate pneumonitis was reported after 2002 (Arthritis Rheumatol. 2014 Apr;66[4]:803-12).
Methotrexate pneumonitis is not dose dependent, nor is it related to treatment duration.
“Just because your patient has been on methotrexate for years does not mean they won’t get methotrexate lung toxicity,” he cautioned. “But this is not a chronic fibrotic interstitial lung disease, this is an acute onset of peripheral infiltrates and ground glass opacifications on chest imaging.”
Bronchoalveolar lavage classically shows a hypersensitivity pneumonitis with lymphocytosis. Transbronchial or surgical lung biopsy may show an organizing pneumonia or airway-based nonnecrotizing granulomas, again indicative of a hypersensitivity reaction.
Because the diagnostic picture is so often cloudy, Dr. Fischer generally tries to avoid methotrexate in patients with moderate or severe interstitial lung disease. “I have the luxury of avoiding it because we have so many great arthritis drugs these days,” he noted.
“That being said, the notion that we’re going to stop methotrexate in an 80-year-old who’s been on it for years and has mild bibasilar fibrotic interstitial lung disease so that her lung doc can sleep better at night is not very helpful for our patients. If the patient is doing well on methotrexate and the interstitial lung disease is mild, I continue [the methotrexate],” Dr. Fischer said.
He reported receiving research grants from Boehringer Ingelheim and Corbus Pharmaceuticals and serving as a consultant to Boehringer Ingelheim and other pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2019
Surprise! MTX proves effective in psoriatic arthritis
MAUI, HAWAII – The first-ever, double-blind, randomized, controlled clinical trial evidence demonstrating that methotrexate indeed has therapeutic efficacy in psoriatic arthritis has come at an awkward time – on the heels of a basically negative Cochrane Collaboration systematic review as well as the latest American College of Rheumatology/National Psoriasis Foundation guidelines for treatment of psoriatic arthritis, which recommend anti–tumor necrosis factor therapy as first line, ahead of methotrexate.
The timing of the release of the SEAM-PsA randomized trial results was such that neither the Cochrane group nor the ACR/NPF guideline committee was able to consider the new, potentially game-changing study findings.
“I look at SEAM-PsA and have to say, methotrexate does seem to be an effective therapy. I think it calls into question the new guidelines, which were developed before the data were out. Now you look at this and have to ask, can you really say you should use a TNF inhibitor before methotrexate based on these results? I don’t know,” Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He also shared other problems he has with the new guidelines, which he considers seriously flawed.
The Cochrane Collaboration Systematic Review
The Cochrane group cast a net for all randomized, controlled clinical trials of methotrexate versus placebo or another disease-modifying antirheumatic drug (DMARD). They found eight, which they judged to be of poor quality. Their conclusion: “Low-quality evidence suggests that low-dose (15 mg or less) oral methotrexate might be slightly more effective than placebo when taken for 6 months; however, we are uncertain if it is more harmful” (Cochrane Database Syst Rev. 2019 Jan 18;1:CD012722. doi: 10.1002/14651858.CD012722.pub2).
“The new Cochrane Review concludes methotrexate doesn’t seem to work that well,” observed symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
“That’s because it’s based on published data, and there’s been very little of that,” said Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago.
“I think most people assume, based on clinical experience, that it does work well. It’s all we had for years and years and years,” he added.
That is, until SEAM-PsA.
SEAM-PsA
SEAM-PsA randomized 851 DMARD- and biologic-naive patients with a median 0.6-year duration of psoriatic arthritis to one of three treatment arms for 48 weeks: once-weekly etanercept at 50 mg plus oral methotrexate at 20 mg, etanercept plus oral placebo, or methotrexate plus injectable placebo.
This is a study that will reshape clinical practice for many rheumatologists, according to Dr. Kavanaugh. The hypothesis was that in psoriatic arthritis, just as has been shown to be the case in rheumatoid arthritis, the combination of a TNF inhibitor plus methotrexate would have greater efficacy than either agent alone. But the study brought a couple of major surprises.
“Methotrexate didn’t do so badly,” Dr. Kavanaugh observed. “And the combination did nothing. I would have bet that the combination would have shown methotrexate had a synergistic effect with the TNF inhibitor, especially for x-ray changes. But the combination didn’t do any better than etanercept alone.”
Make no mistake: Etanercept monotherapy significantly outperformed methotrexate monotherapy for the primary endpoint, the ACR 20 response at week 24, by a margin of 60.9% versus 50.7%. Dr. Ruderman deemed that methotrexate response rate to be quite respectable, although he bemoaned the absence of a double-placebo comparator arm. And the key secondary endpoint, the minimal disease activity response rate at week 24, was also significantly better with etanercept, at 35.9% compared with 22.9%. Moreover, both etanercept arms showed significantly less radiographic progression than with methotrexate alone.
However, that was it. There were no significant differences between etanercept and methotrexate in other secondary endpoints, including the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC), the Disease Activity in PSoriatic Arthritis (DAPSA) score, the Leeds Dactylitis Instrument (LDI), and quality of life as assessed by the 36-item Short Form Health Survey total score.
“Methotrexate showed generally good efficacy across multiple domains,” the investigators concluded (Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851).
“Another intriguing thing to come out of this study for me were the enthesitis and dactylitis results. My clinical experience suggested methotrexate wasn’t so great for that, but this study suggests that’s not true,” Dr. Ruderman said.
“There are a couple of key take-home points from this study,” according to Dr. Kavanaugh. “One is that the combination is not synergistic. When you start a rheumatoid arthritis patient on methotrexate, you try to keep him on methotrexate when you add a TNF inhibitor. This study would say there doesn’t seem like there’s a reason to do that in your psoriatic arthritis patient. And the second message is that methotrexate seems to work.”
New ACR/NPF psoriatic arthritis guidelines under fire
“The new guidelines are fuzzy, aren’t they?” Dr. Kavanaugh said in lobbing the topic over to Dr. Ruderman.
“Where do we start?” he replied, shaking his head. “These are evidence-based guidelines in an area in which there was virtually no evidence.”
Indeed, the guidelines committee proudly employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which forces committee members to issue “conditional” recommendations when there’s not enough evidence to make a “strong” recommendation.
“You’re not allowed to say, ‘We don’t know, there’s not enough evidence to make a choice,’ ” Dr. Ruderman said. “The problem with these guidelines is virtually everything in it is a conditional recommendation except ‘stop smoking,’ which was a strong recommendation.
“A conditional recommendation is pretty much a fancy term for expert opinion. It’s basically everybody in the room saying, ‘This is what we think.’ And that makes guidelines challenging because as a rheumatologist, you’re an expert. The people in the room have perhaps looked at the data more carefully than you’ve drilled down into the studies, but ultimately they’ve taken care of these patients and you’ve taken care of these patients, so why is their opinion better than your opinion, if it’s an informed opinion?”
His other critique of the 28-page guidelines is they don’t include the reasoning behind the conditional recommendations.
“If the conditional recommendation is, ‘In this situation, a TNF inhibitor is preferred over an IL-17 inhibitor,’ that would be great if they had also said, ‘This is why we thought that.’ But that’s not in the paper,” Dr. Ruderman said.
He and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – The first-ever, double-blind, randomized, controlled clinical trial evidence demonstrating that methotrexate indeed has therapeutic efficacy in psoriatic arthritis has come at an awkward time – on the heels of a basically negative Cochrane Collaboration systematic review as well as the latest American College of Rheumatology/National Psoriasis Foundation guidelines for treatment of psoriatic arthritis, which recommend anti–tumor necrosis factor therapy as first line, ahead of methotrexate.
The timing of the release of the SEAM-PsA randomized trial results was such that neither the Cochrane group nor the ACR/NPF guideline committee was able to consider the new, potentially game-changing study findings.
“I look at SEAM-PsA and have to say, methotrexate does seem to be an effective therapy. I think it calls into question the new guidelines, which were developed before the data were out. Now you look at this and have to ask, can you really say you should use a TNF inhibitor before methotrexate based on these results? I don’t know,” Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He also shared other problems he has with the new guidelines, which he considers seriously flawed.
The Cochrane Collaboration Systematic Review
The Cochrane group cast a net for all randomized, controlled clinical trials of methotrexate versus placebo or another disease-modifying antirheumatic drug (DMARD). They found eight, which they judged to be of poor quality. Their conclusion: “Low-quality evidence suggests that low-dose (15 mg or less) oral methotrexate might be slightly more effective than placebo when taken for 6 months; however, we are uncertain if it is more harmful” (Cochrane Database Syst Rev. 2019 Jan 18;1:CD012722. doi: 10.1002/14651858.CD012722.pub2).
“The new Cochrane Review concludes methotrexate doesn’t seem to work that well,” observed symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
“That’s because it’s based on published data, and there’s been very little of that,” said Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago.
“I think most people assume, based on clinical experience, that it does work well. It’s all we had for years and years and years,” he added.
That is, until SEAM-PsA.
SEAM-PsA
SEAM-PsA randomized 851 DMARD- and biologic-naive patients with a median 0.6-year duration of psoriatic arthritis to one of three treatment arms for 48 weeks: once-weekly etanercept at 50 mg plus oral methotrexate at 20 mg, etanercept plus oral placebo, or methotrexate plus injectable placebo.
This is a study that will reshape clinical practice for many rheumatologists, according to Dr. Kavanaugh. The hypothesis was that in psoriatic arthritis, just as has been shown to be the case in rheumatoid arthritis, the combination of a TNF inhibitor plus methotrexate would have greater efficacy than either agent alone. But the study brought a couple of major surprises.
“Methotrexate didn’t do so badly,” Dr. Kavanaugh observed. “And the combination did nothing. I would have bet that the combination would have shown methotrexate had a synergistic effect with the TNF inhibitor, especially for x-ray changes. But the combination didn’t do any better than etanercept alone.”
Make no mistake: Etanercept monotherapy significantly outperformed methotrexate monotherapy for the primary endpoint, the ACR 20 response at week 24, by a margin of 60.9% versus 50.7%. Dr. Ruderman deemed that methotrexate response rate to be quite respectable, although he bemoaned the absence of a double-placebo comparator arm. And the key secondary endpoint, the minimal disease activity response rate at week 24, was also significantly better with etanercept, at 35.9% compared with 22.9%. Moreover, both etanercept arms showed significantly less radiographic progression than with methotrexate alone.
However, that was it. There were no significant differences between etanercept and methotrexate in other secondary endpoints, including the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC), the Disease Activity in PSoriatic Arthritis (DAPSA) score, the Leeds Dactylitis Instrument (LDI), and quality of life as assessed by the 36-item Short Form Health Survey total score.
“Methotrexate showed generally good efficacy across multiple domains,” the investigators concluded (Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851).
“Another intriguing thing to come out of this study for me were the enthesitis and dactylitis results. My clinical experience suggested methotrexate wasn’t so great for that, but this study suggests that’s not true,” Dr. Ruderman said.
“There are a couple of key take-home points from this study,” according to Dr. Kavanaugh. “One is that the combination is not synergistic. When you start a rheumatoid arthritis patient on methotrexate, you try to keep him on methotrexate when you add a TNF inhibitor. This study would say there doesn’t seem like there’s a reason to do that in your psoriatic arthritis patient. And the second message is that methotrexate seems to work.”
New ACR/NPF psoriatic arthritis guidelines under fire
“The new guidelines are fuzzy, aren’t they?” Dr. Kavanaugh said in lobbing the topic over to Dr. Ruderman.
“Where do we start?” he replied, shaking his head. “These are evidence-based guidelines in an area in which there was virtually no evidence.”
Indeed, the guidelines committee proudly employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which forces committee members to issue “conditional” recommendations when there’s not enough evidence to make a “strong” recommendation.
“You’re not allowed to say, ‘We don’t know, there’s not enough evidence to make a choice,’ ” Dr. Ruderman said. “The problem with these guidelines is virtually everything in it is a conditional recommendation except ‘stop smoking,’ which was a strong recommendation.
“A conditional recommendation is pretty much a fancy term for expert opinion. It’s basically everybody in the room saying, ‘This is what we think.’ And that makes guidelines challenging because as a rheumatologist, you’re an expert. The people in the room have perhaps looked at the data more carefully than you’ve drilled down into the studies, but ultimately they’ve taken care of these patients and you’ve taken care of these patients, so why is their opinion better than your opinion, if it’s an informed opinion?”
His other critique of the 28-page guidelines is they don’t include the reasoning behind the conditional recommendations.
“If the conditional recommendation is, ‘In this situation, a TNF inhibitor is preferred over an IL-17 inhibitor,’ that would be great if they had also said, ‘This is why we thought that.’ But that’s not in the paper,” Dr. Ruderman said.
He and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – The first-ever, double-blind, randomized, controlled clinical trial evidence demonstrating that methotrexate indeed has therapeutic efficacy in psoriatic arthritis has come at an awkward time – on the heels of a basically negative Cochrane Collaboration systematic review as well as the latest American College of Rheumatology/National Psoriasis Foundation guidelines for treatment of psoriatic arthritis, which recommend anti–tumor necrosis factor therapy as first line, ahead of methotrexate.
The timing of the release of the SEAM-PsA randomized trial results was such that neither the Cochrane group nor the ACR/NPF guideline committee was able to consider the new, potentially game-changing study findings.
“I look at SEAM-PsA and have to say, methotrexate does seem to be an effective therapy. I think it calls into question the new guidelines, which were developed before the data were out. Now you look at this and have to ask, can you really say you should use a TNF inhibitor before methotrexate based on these results? I don’t know,” Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He also shared other problems he has with the new guidelines, which he considers seriously flawed.
The Cochrane Collaboration Systematic Review
The Cochrane group cast a net for all randomized, controlled clinical trials of methotrexate versus placebo or another disease-modifying antirheumatic drug (DMARD). They found eight, which they judged to be of poor quality. Their conclusion: “Low-quality evidence suggests that low-dose (15 mg or less) oral methotrexate might be slightly more effective than placebo when taken for 6 months; however, we are uncertain if it is more harmful” (Cochrane Database Syst Rev. 2019 Jan 18;1:CD012722. doi: 10.1002/14651858.CD012722.pub2).
“The new Cochrane Review concludes methotrexate doesn’t seem to work that well,” observed symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
“That’s because it’s based on published data, and there’s been very little of that,” said Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago.
“I think most people assume, based on clinical experience, that it does work well. It’s all we had for years and years and years,” he added.
That is, until SEAM-PsA.
SEAM-PsA
SEAM-PsA randomized 851 DMARD- and biologic-naive patients with a median 0.6-year duration of psoriatic arthritis to one of three treatment arms for 48 weeks: once-weekly etanercept at 50 mg plus oral methotrexate at 20 mg, etanercept plus oral placebo, or methotrexate plus injectable placebo.
This is a study that will reshape clinical practice for many rheumatologists, according to Dr. Kavanaugh. The hypothesis was that in psoriatic arthritis, just as has been shown to be the case in rheumatoid arthritis, the combination of a TNF inhibitor plus methotrexate would have greater efficacy than either agent alone. But the study brought a couple of major surprises.
“Methotrexate didn’t do so badly,” Dr. Kavanaugh observed. “And the combination did nothing. I would have bet that the combination would have shown methotrexate had a synergistic effect with the TNF inhibitor, especially for x-ray changes. But the combination didn’t do any better than etanercept alone.”
Make no mistake: Etanercept monotherapy significantly outperformed methotrexate monotherapy for the primary endpoint, the ACR 20 response at week 24, by a margin of 60.9% versus 50.7%. Dr. Ruderman deemed that methotrexate response rate to be quite respectable, although he bemoaned the absence of a double-placebo comparator arm. And the key secondary endpoint, the minimal disease activity response rate at week 24, was also significantly better with etanercept, at 35.9% compared with 22.9%. Moreover, both etanercept arms showed significantly less radiographic progression than with methotrexate alone.
However, that was it. There were no significant differences between etanercept and methotrexate in other secondary endpoints, including the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC), the Disease Activity in PSoriatic Arthritis (DAPSA) score, the Leeds Dactylitis Instrument (LDI), and quality of life as assessed by the 36-item Short Form Health Survey total score.
“Methotrexate showed generally good efficacy across multiple domains,” the investigators concluded (Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851).
“Another intriguing thing to come out of this study for me were the enthesitis and dactylitis results. My clinical experience suggested methotrexate wasn’t so great for that, but this study suggests that’s not true,” Dr. Ruderman said.
“There are a couple of key take-home points from this study,” according to Dr. Kavanaugh. “One is that the combination is not synergistic. When you start a rheumatoid arthritis patient on methotrexate, you try to keep him on methotrexate when you add a TNF inhibitor. This study would say there doesn’t seem like there’s a reason to do that in your psoriatic arthritis patient. And the second message is that methotrexate seems to work.”
New ACR/NPF psoriatic arthritis guidelines under fire
“The new guidelines are fuzzy, aren’t they?” Dr. Kavanaugh said in lobbing the topic over to Dr. Ruderman.
“Where do we start?” he replied, shaking his head. “These are evidence-based guidelines in an area in which there was virtually no evidence.”
Indeed, the guidelines committee proudly employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which forces committee members to issue “conditional” recommendations when there’s not enough evidence to make a “strong” recommendation.
“You’re not allowed to say, ‘We don’t know, there’s not enough evidence to make a choice,’ ” Dr. Ruderman said. “The problem with these guidelines is virtually everything in it is a conditional recommendation except ‘stop smoking,’ which was a strong recommendation.
“A conditional recommendation is pretty much a fancy term for expert opinion. It’s basically everybody in the room saying, ‘This is what we think.’ And that makes guidelines challenging because as a rheumatologist, you’re an expert. The people in the room have perhaps looked at the data more carefully than you’ve drilled down into the studies, but ultimately they’ve taken care of these patients and you’ve taken care of these patients, so why is their opinion better than your opinion, if it’s an informed opinion?”
His other critique of the 28-page guidelines is they don’t include the reasoning behind the conditional recommendations.
“If the conditional recommendation is, ‘In this situation, a TNF inhibitor is preferred over an IL-17 inhibitor,’ that would be great if they had also said, ‘This is why we thought that.’ But that’s not in the paper,” Dr. Ruderman said.
He and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
Lymphoma rate in RA patients is falling
MAUI, HAWAII – The incidence of lymphoma in patients with RA appears to have been dropping during the past 2 decades – and for rheumatologists, that’s news you can use.

“I think this is encouraging data about where we’re headed with therapy. And it’s encouraging data for your patients, that maybe more effective therapies can lead to a lower risk of cancer,” John J. Cush, MD, commented at the 2019 Rheumatology Winter Clinical Symposium.
“Patients are always worried about cancer,” observed symposium director Arthur Kavanaugh, MD. “I think this is very useful data to bring to a discussion with patients.”
The study they highlighted was presented at the 2018 annual meeting of the American College of Rheumatology by Namrata Singh, MD, of the University of Iowa, Iowa City and coinvestigators from Veterans Affairs medical centers around the country. They analyzed the incidence of lymphomas as well as all-site cancers in 50,870 men with RA in the national VA health care system during 2001-2015 and compared the rates with the background rates in the general U.S. population as captured in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The key finding: While the standardized incidence ratio for the development of lymphoma in the RA patients during 2001-2005 was 190% greater than in the SEER population, the SIR dropped to 1.6 in 2006-2010 and stayed low in 2011-2015.
“These are the only data I’m aware of that say maybe lymphomas are becoming less frequent among RA patients,” said Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Historically, RA has been associated with roughly a 100% increased risk of lymphoma. The source of the increased risk has been a matter of controversy: Is it the result of immunostimulation triggered by high RA disease activity, or a side effect of the drugs employed in treatment of the disease? The clear implication of the VA study is that it’s all about disease activity.
“The lymphoma rate is higher early in the use of our new therapies, in 2001-2005, because the patients who went on TNF [tumor necrosis factor] inhibitors then had the most disease activity. But with time, patients are getting those treatments earlier. Does this [lower lymphoma rate] reflect a change in the practice of rheumatology? I think it does,” according to Dr. Cush, professor of medicine and rheumatology at Baylor University Medical Center, Dallas.
Dr. Kavanaugh agreed. “Now, if we’re treating early and treating to target, we should see less lymphomas than we did back in the day.”
The rate of cancers at all sites in the VA RA patients has been going down as well, with the SIR dropping from 1.8 in 2001-2005 to close to 1, the background rate in the general population.
“What’s great about this study is this is a large data set. You really can’t compare an RA population on and off treatment. The right comparison is to a normal population – and SEER accounts for something like 14% of the U.S. population,” Dr. Cush said.
Previous support for the notion that the increased lymphoma risk associated with RA was a function of disease activity came from a Swedish study of 378 RA patients in the prebiologic era who developed lymphoma and a matched cohort of 378 others without lymphoma. The investigators found that patients with moderate overall RA disease activity were at a 700% increased risk of lymphoma, compared with those with low overall disease activity, and that patients with high RA disease activity were at a 6,900% increased risk (Arthritis Rheum. 2006 Mar;54[3]:692-701). But that was a cross-sectional study, whereas the VA study examined trends over time.
The VA RA cohort had a mean age of 64 years. About 60% were current or ex-smokers, 65% were positive for rheumatoid factor, and 62% were positive for anticyclic citrullinated peptide.
Dr. Kavanaugh said that, because of the potential for referral bias in the VA study, he’s eager to see the findings reproduced in another data set.
Both Dr. Cush and Dr. Kavanaugh reported serving as a consultant to and/or receiving research funding from numerous pharmaceutical companies.
MAUI, HAWAII – The incidence of lymphoma in patients with RA appears to have been dropping during the past 2 decades – and for rheumatologists, that’s news you can use.

“I think this is encouraging data about where we’re headed with therapy. And it’s encouraging data for your patients, that maybe more effective therapies can lead to a lower risk of cancer,” John J. Cush, MD, commented at the 2019 Rheumatology Winter Clinical Symposium.
“Patients are always worried about cancer,” observed symposium director Arthur Kavanaugh, MD. “I think this is very useful data to bring to a discussion with patients.”
The study they highlighted was presented at the 2018 annual meeting of the American College of Rheumatology by Namrata Singh, MD, of the University of Iowa, Iowa City and coinvestigators from Veterans Affairs medical centers around the country. They analyzed the incidence of lymphomas as well as all-site cancers in 50,870 men with RA in the national VA health care system during 2001-2015 and compared the rates with the background rates in the general U.S. population as captured in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The key finding: While the standardized incidence ratio for the development of lymphoma in the RA patients during 2001-2005 was 190% greater than in the SEER population, the SIR dropped to 1.6 in 2006-2010 and stayed low in 2011-2015.
“These are the only data I’m aware of that say maybe lymphomas are becoming less frequent among RA patients,” said Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Historically, RA has been associated with roughly a 100% increased risk of lymphoma. The source of the increased risk has been a matter of controversy: Is it the result of immunostimulation triggered by high RA disease activity, or a side effect of the drugs employed in treatment of the disease? The clear implication of the VA study is that it’s all about disease activity.
“The lymphoma rate is higher early in the use of our new therapies, in 2001-2005, because the patients who went on TNF [tumor necrosis factor] inhibitors then had the most disease activity. But with time, patients are getting those treatments earlier. Does this [lower lymphoma rate] reflect a change in the practice of rheumatology? I think it does,” according to Dr. Cush, professor of medicine and rheumatology at Baylor University Medical Center, Dallas.
Dr. Kavanaugh agreed. “Now, if we’re treating early and treating to target, we should see less lymphomas than we did back in the day.”
The rate of cancers at all sites in the VA RA patients has been going down as well, with the SIR dropping from 1.8 in 2001-2005 to close to 1, the background rate in the general population.
“What’s great about this study is this is a large data set. You really can’t compare an RA population on and off treatment. The right comparison is to a normal population – and SEER accounts for something like 14% of the U.S. population,” Dr. Cush said.
Previous support for the notion that the increased lymphoma risk associated with RA was a function of disease activity came from a Swedish study of 378 RA patients in the prebiologic era who developed lymphoma and a matched cohort of 378 others without lymphoma. The investigators found that patients with moderate overall RA disease activity were at a 700% increased risk of lymphoma, compared with those with low overall disease activity, and that patients with high RA disease activity were at a 6,900% increased risk (Arthritis Rheum. 2006 Mar;54[3]:692-701). But that was a cross-sectional study, whereas the VA study examined trends over time.
The VA RA cohort had a mean age of 64 years. About 60% were current or ex-smokers, 65% were positive for rheumatoid factor, and 62% were positive for anticyclic citrullinated peptide.
Dr. Kavanaugh said that, because of the potential for referral bias in the VA study, he’s eager to see the findings reproduced in another data set.
Both Dr. Cush and Dr. Kavanaugh reported serving as a consultant to and/or receiving research funding from numerous pharmaceutical companies.
MAUI, HAWAII – The incidence of lymphoma in patients with RA appears to have been dropping during the past 2 decades – and for rheumatologists, that’s news you can use.

“I think this is encouraging data about where we’re headed with therapy. And it’s encouraging data for your patients, that maybe more effective therapies can lead to a lower risk of cancer,” John J. Cush, MD, commented at the 2019 Rheumatology Winter Clinical Symposium.
“Patients are always worried about cancer,” observed symposium director Arthur Kavanaugh, MD. “I think this is very useful data to bring to a discussion with patients.”
The study they highlighted was presented at the 2018 annual meeting of the American College of Rheumatology by Namrata Singh, MD, of the University of Iowa, Iowa City and coinvestigators from Veterans Affairs medical centers around the country. They analyzed the incidence of lymphomas as well as all-site cancers in 50,870 men with RA in the national VA health care system during 2001-2015 and compared the rates with the background rates in the general U.S. population as captured in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The key finding: While the standardized incidence ratio for the development of lymphoma in the RA patients during 2001-2005 was 190% greater than in the SEER population, the SIR dropped to 1.6 in 2006-2010 and stayed low in 2011-2015.
“These are the only data I’m aware of that say maybe lymphomas are becoming less frequent among RA patients,” said Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Historically, RA has been associated with roughly a 100% increased risk of lymphoma. The source of the increased risk has been a matter of controversy: Is it the result of immunostimulation triggered by high RA disease activity, or a side effect of the drugs employed in treatment of the disease? The clear implication of the VA study is that it’s all about disease activity.
“The lymphoma rate is higher early in the use of our new therapies, in 2001-2005, because the patients who went on TNF [tumor necrosis factor] inhibitors then had the most disease activity. But with time, patients are getting those treatments earlier. Does this [lower lymphoma rate] reflect a change in the practice of rheumatology? I think it does,” according to Dr. Cush, professor of medicine and rheumatology at Baylor University Medical Center, Dallas.
Dr. Kavanaugh agreed. “Now, if we’re treating early and treating to target, we should see less lymphomas than we did back in the day.”
The rate of cancers at all sites in the VA RA patients has been going down as well, with the SIR dropping from 1.8 in 2001-2005 to close to 1, the background rate in the general population.
“What’s great about this study is this is a large data set. You really can’t compare an RA population on and off treatment. The right comparison is to a normal population – and SEER accounts for something like 14% of the U.S. population,” Dr. Cush said.
Previous support for the notion that the increased lymphoma risk associated with RA was a function of disease activity came from a Swedish study of 378 RA patients in the prebiologic era who developed lymphoma and a matched cohort of 378 others without lymphoma. The investigators found that patients with moderate overall RA disease activity were at a 700% increased risk of lymphoma, compared with those with low overall disease activity, and that patients with high RA disease activity were at a 6,900% increased risk (Arthritis Rheum. 2006 Mar;54[3]:692-701). But that was a cross-sectional study, whereas the VA study examined trends over time.
The VA RA cohort had a mean age of 64 years. About 60% were current or ex-smokers, 65% were positive for rheumatoid factor, and 62% were positive for anticyclic citrullinated peptide.
Dr. Kavanaugh said that, because of the potential for referral bias in the VA study, he’s eager to see the findings reproduced in another data set.
Both Dr. Cush and Dr. Kavanaugh reported serving as a consultant to and/or receiving research funding from numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
Bronchiolitis is a feared complication of connective tissue disease
MAUI, HAWAII – who develop shortness of breath and cough or a precipitous drop in their forced expiratory volume on pulmonary function testing, Aryeh Fischer, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
“This is an underappreciated – and I think among the most potentially devastating – of the lung diseases we as rheumatologists will encounter in our patients,” said Dr. Fischer, a rheumatologist at the University of Colorado at Denver, Aurora, with a special interest in autoimmune lung disease.
“If you’re seeing patients with rheumatoid arthritis, SLE, or Sjögren’s and they’ve got bad asthma they can’t get under control, you’ve got to think about bronchiolitis because I can tell you your lung doc quite often is not thinking about this,” he added.
Bronchiolitis involves inflammation, narrowing, or obliteration of the small airways. The diagnosis is often missed because of the false sense of reassurance provided by the normal chest x-ray and regular CT findings, which are a feature of the disease.
“This is really important: You have to get a high-resolution CT that includes expiratory images, because that’s the only way you’re going to be able to tell if your patient has small airways disease,” he explained. “You must, must, must do an expiratory CT.”
A normal expiratory CT image should be gray, since the lungs are empty. Air is black on CT, so large areas of black intermixed with gray on an expiratory CT – a finding known as mosaicism – indicate air trapping due to small airways disease, Dr. Fischer noted.
Surgical lung biopsy will yield a pathologic report documenting isolated constrictive, follicular, and/or lymphocytic bronchiolitis. However, the terminology can be confusing: What pathologists describe as constrictive bronchiolitis is called obliterative by pulmonologists and radiologists.
Pulmonary function testing shows an obstructive defect. The diffusing capacity of the lungs for carbon monoxide (DLCO) is fairly normal, the forced expiratory volume in 1 second (FEV1) is sharply reduced, and the forced vital capacity (FVC) is near normal, with a resultant abnormally low FEV1/FVC ratio. A patient with bronchiolitis may or may not have a response to bronchodilators.
“I tell you, I’ve seen a bunch of these patients. They typically have a precipitous drop in their FEV1 and then stay stable at a very low level of lung function without much opportunity for improvement,” Dr. Fischer said. “Stability equals success in these patients. It’s really unusual to see much improvement.”
In theory, patients with follicular or lymphocytic bronchiolitis have an ongoing inflammatory process that should be amenable to rheumatologic ministrations. But there is no convincing evidence of treatment efficacy to date. And in obliterative bronchiolitis, marked by airway scarring, there is no reason to think anti-inflammatory therapies should be helpful. Anecdotally, Dr. Fischer said, he has seen immunosuppression help patients with obliterative bronchiolitis.
“Actually, the only proven therapy is lung transplantation,” he said.
He recommended that his fellow rheumatologists periodically use office spirometry to check the FEV1 in their patients with rheumatoid arthritis, Sjögren’s, or SLE, the forms of connective tissue disease most often associated with bronchiolitis. Compared with all the other testing rheumatologists routinely order in their patients, having them blow into a tube is a simple enough matter.
“We really don’t have anything to impact the natural history, but I like the notion of not being surprised. What are you going to do with that [abnormal] FEV1 data? I have no idea. But maybe it’s better to know earlier,” he said.
Dr. Fischer reported having no financial conflicts of interest regarding his presentation.
MAUI, HAWAII – who develop shortness of breath and cough or a precipitous drop in their forced expiratory volume on pulmonary function testing, Aryeh Fischer, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
“This is an underappreciated – and I think among the most potentially devastating – of the lung diseases we as rheumatologists will encounter in our patients,” said Dr. Fischer, a rheumatologist at the University of Colorado at Denver, Aurora, with a special interest in autoimmune lung disease.
“If you’re seeing patients with rheumatoid arthritis, SLE, or Sjögren’s and they’ve got bad asthma they can’t get under control, you’ve got to think about bronchiolitis because I can tell you your lung doc quite often is not thinking about this,” he added.
Bronchiolitis involves inflammation, narrowing, or obliteration of the small airways. The diagnosis is often missed because of the false sense of reassurance provided by the normal chest x-ray and regular CT findings, which are a feature of the disease.
“This is really important: You have to get a high-resolution CT that includes expiratory images, because that’s the only way you’re going to be able to tell if your patient has small airways disease,” he explained. “You must, must, must do an expiratory CT.”
A normal expiratory CT image should be gray, since the lungs are empty. Air is black on CT, so large areas of black intermixed with gray on an expiratory CT – a finding known as mosaicism – indicate air trapping due to small airways disease, Dr. Fischer noted.
Surgical lung biopsy will yield a pathologic report documenting isolated constrictive, follicular, and/or lymphocytic bronchiolitis. However, the terminology can be confusing: What pathologists describe as constrictive bronchiolitis is called obliterative by pulmonologists and radiologists.
Pulmonary function testing shows an obstructive defect. The diffusing capacity of the lungs for carbon monoxide (DLCO) is fairly normal, the forced expiratory volume in 1 second (FEV1) is sharply reduced, and the forced vital capacity (FVC) is near normal, with a resultant abnormally low FEV1/FVC ratio. A patient with bronchiolitis may or may not have a response to bronchodilators.
“I tell you, I’ve seen a bunch of these patients. They typically have a precipitous drop in their FEV1 and then stay stable at a very low level of lung function without much opportunity for improvement,” Dr. Fischer said. “Stability equals success in these patients. It’s really unusual to see much improvement.”
In theory, patients with follicular or lymphocytic bronchiolitis have an ongoing inflammatory process that should be amenable to rheumatologic ministrations. But there is no convincing evidence of treatment efficacy to date. And in obliterative bronchiolitis, marked by airway scarring, there is no reason to think anti-inflammatory therapies should be helpful. Anecdotally, Dr. Fischer said, he has seen immunosuppression help patients with obliterative bronchiolitis.
“Actually, the only proven therapy is lung transplantation,” he said.
He recommended that his fellow rheumatologists periodically use office spirometry to check the FEV1 in their patients with rheumatoid arthritis, Sjögren’s, or SLE, the forms of connective tissue disease most often associated with bronchiolitis. Compared with all the other testing rheumatologists routinely order in their patients, having them blow into a tube is a simple enough matter.
“We really don’t have anything to impact the natural history, but I like the notion of not being surprised. What are you going to do with that [abnormal] FEV1 data? I have no idea. But maybe it’s better to know earlier,” he said.
Dr. Fischer reported having no financial conflicts of interest regarding his presentation.
MAUI, HAWAII – who develop shortness of breath and cough or a precipitous drop in their forced expiratory volume on pulmonary function testing, Aryeh Fischer, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
“This is an underappreciated – and I think among the most potentially devastating – of the lung diseases we as rheumatologists will encounter in our patients,” said Dr. Fischer, a rheumatologist at the University of Colorado at Denver, Aurora, with a special interest in autoimmune lung disease.
“If you’re seeing patients with rheumatoid arthritis, SLE, or Sjögren’s and they’ve got bad asthma they can’t get under control, you’ve got to think about bronchiolitis because I can tell you your lung doc quite often is not thinking about this,” he added.
Bronchiolitis involves inflammation, narrowing, or obliteration of the small airways. The diagnosis is often missed because of the false sense of reassurance provided by the normal chest x-ray and regular CT findings, which are a feature of the disease.
“This is really important: You have to get a high-resolution CT that includes expiratory images, because that’s the only way you’re going to be able to tell if your patient has small airways disease,” he explained. “You must, must, must do an expiratory CT.”
A normal expiratory CT image should be gray, since the lungs are empty. Air is black on CT, so large areas of black intermixed with gray on an expiratory CT – a finding known as mosaicism – indicate air trapping due to small airways disease, Dr. Fischer noted.
Surgical lung biopsy will yield a pathologic report documenting isolated constrictive, follicular, and/or lymphocytic bronchiolitis. However, the terminology can be confusing: What pathologists describe as constrictive bronchiolitis is called obliterative by pulmonologists and radiologists.
Pulmonary function testing shows an obstructive defect. The diffusing capacity of the lungs for carbon monoxide (DLCO) is fairly normal, the forced expiratory volume in 1 second (FEV1) is sharply reduced, and the forced vital capacity (FVC) is near normal, with a resultant abnormally low FEV1/FVC ratio. A patient with bronchiolitis may or may not have a response to bronchodilators.
“I tell you, I’ve seen a bunch of these patients. They typically have a precipitous drop in their FEV1 and then stay stable at a very low level of lung function without much opportunity for improvement,” Dr. Fischer said. “Stability equals success in these patients. It’s really unusual to see much improvement.”
In theory, patients with follicular or lymphocytic bronchiolitis have an ongoing inflammatory process that should be amenable to rheumatologic ministrations. But there is no convincing evidence of treatment efficacy to date. And in obliterative bronchiolitis, marked by airway scarring, there is no reason to think anti-inflammatory therapies should be helpful. Anecdotally, Dr. Fischer said, he has seen immunosuppression help patients with obliterative bronchiolitis.
“Actually, the only proven therapy is lung transplantation,” he said.
He recommended that his fellow rheumatologists periodically use office spirometry to check the FEV1 in their patients with rheumatoid arthritis, Sjögren’s, or SLE, the forms of connective tissue disease most often associated with bronchiolitis. Compared with all the other testing rheumatologists routinely order in their patients, having them blow into a tube is a simple enough matter.
“We really don’t have anything to impact the natural history, but I like the notion of not being surprised. What are you going to do with that [abnormal] FEV1 data? I have no idea. But maybe it’s better to know earlier,” he said.
Dr. Fischer reported having no financial conflicts of interest regarding his presentation.
REPORTING FROM RWCS 2019
Tackling the challenges of pediatric localized scleroderma
MAUI, HAWAII – One of the most important steps to take when a child has received a biopsy-confirmed diagnosis of localized scleroderma is to sit down with the family and explain the rationale for the aggressive therapies to come, Anne M. Stevens, MD, PhD, said at the 2019 Rheumatology Winter Clinical Symposium.
It can be a tough sell at first, especially when a child has only a small red streak on the nose and perhaps a subtle linear lesion on the forehead or scalp. But the family has to come to understand that this is a serious, chronic, progressive fibrotic disease.
“Talk about what a big impact this disease can have on growth of a limb and the normal life of a child because of the cosmetic appearance. Explain that the length of treatment course is based on the long-term outcomes and quality of life. This discussion is usually sufficient” to convince people to give their children “these pretty serious medications,” said Dr. Stevens, professor of pediatrics and head of the division of pediatric rheumatology at the University of Washington, Seattle.
“The treatment goal is to control inflammation and prevent damage in these patients, who we like to catch very early, when it’s a subtle lesion,” she added.
The biggest problem
The biggest contributors to poor quality of life in patients with juvenile localized scleroderma are the extracutaneous manifestations, which occur in up to 50% of cases. Joint pain occurs in roughly 20% of patients, joint contractures due to fibrosis of skin and/or tendons in 30%, and myalgia with or without myositis in 15%. Muscle atrophy due to the deep component of the scleroderma can occur. Moreover, growth problems – especially leg or arm length discrepancies – happen in about 20% of patients in prospective studies. These growth problems may not be obvious until a child enters a growth spurt, at which point there is a limited ability to achieve improvement. That’s why Dr. Stevens recommends that every child with localized scleroderma should get a full joint exam at every visit, with measurement and photos of lesions and recording of all erythematous, violaceous, and waxy-hued areas. And if there are lesions on the head, annual eye exams are warranted.
The prevalence of juvenile localized scleroderma in the United States is about 3 per 100,000, with a mean age of onset of 8.2 years. That makes it 100-fold more common than pediatric systemic sclerosis.
The treatment ladder
There are no Food and Drug Administration–approved medications for localized scleroderma in children. It’s all off label. That being said, there is strong consensus among members of the Childhood Arthritis and Rheumatology Research Alliance that the first-line therapy is methotrexate at 15 mg/m2 or a maximum of 20 mg/week plus intravenous corticosteroids weaned over the course of 3-6 months. This is the treatment regimen with the best supporting evidence of safety and efficacy, including a single Italian randomized, double-blind, placebo-controlled clinical trial (Arthritis Rheum. 2011 Jul;63[7]:1998-2006) and an accompanying long-term, open-label follow-up study (J Am Acad Dermatol. 2012 Dec;67[6]:1151-6).
All of the other treatments she uses for juvenile localized scleroderma – mycophenolate mofetil (CellCept), abatacept (Orencia), tocilizumab (Actemra), and occasionally others – are backed only by a smattering of small case series. However, given the serious potential trajectory of this disease, that modest evidence base has been sufficient for her to receive insurance coverage approval of these agents.
In the randomized trial of first-line methotrexate, 48 of 65 patients treated with methotrexate plus steroid (74%) were responders. And among those 48 responders, 35 (73%) maintained a clinical remission for a mean of 25 months off-drug, while another 13 (27%) were in clinical remission on methotrexate. Twenty-eight patients developed side effects that were generally mild; no one required treatment discontinuation. At the 5-year mark, after an average of an initial 2 years on methotrexate, half of the patients were in a sustained clinical remission, which Dr. Stevens deemed “pretty good” considering the well established and manageable safety profile of the drug.
If a patient fails to respond to methotrexate plus corticosteroids within a few months or later experiences disease progression, Dr. Stevens’ second-line therapy is mycophenolate mofetil in conjunction with corticosteroids. Its use in arresting juvenile localized scleroderma is supported by two favorable published case series, the largest of which includes 10 patients (Rheumatology [Oxford]. 2009 Nov;48[11]:1410-3).
Dr. Stevens’ third-line therapy is intravenous abatacept at 10 mg/kg monthly along with intravenous methylprednisolone at 500 mg/week. There are five published case series, the most recent and largest of which included 13 adult patients, two of whom had en coup de sabre lesions (Acta Derm Venereol. 2018 Apr 16;98[4]:465-6). The biologic also shows promise in patients with advanced severe disease with deep tissue involvement (Semin Arthritis Rheum. 2017 Jun;46[6]:775-81). And abatacept has a plausible mechanism of action in localized scleroderma: French investigators have shown it induces regression of skin fibrosis in a mouse model of the disease (Ann Rheum Dis. 2016 Dec;75[12]:2142-9).
Her fourth-line strategy is the anti-interleukin-6 agent tocilizumab, again in conjunction with corticosteroids. In a translational study, tocilizumab has been shown to normalize dermal fibroblasts and collagen in patients with systemic sclerosis (Ann Rheum Dis. 2018 Sep;77[9]:1362-71). And there have been two promising small retrospective case series as well. A more definitive clinical trial is planned.
Dr. Stevens said that when starting a biologic agent in a child with localized scleroderma, she routinely adds methotrexate until the disease is under control.
Drugs supported by case reports and worth considering on an individual basis as a last resort are hydroxychloroquine, azathioprine, cyclosporine, and imatinib mesylate (Gleevec).
For mild, superficial lesions that don’t cross joints, ultraviolet light A phototherapy is a therapeutic option. It displayed significant benefit in a systematic review and meta-analysis of 19 studies comparing it to methotrexate, although the results with methotrexate were deemed superior (Semin Arthritis Rheum. 2018 Dec;48[3]:495-503).
The pros and cons of getting a baseline brain MRI
Children with localized scleroderma have increased rates of severe headache, peripheral neuropathy, complex partial seizures, and stroke. So it had been Dr. Stevens’ routine practice to obtain an initial brain MRI at the time of diagnosis. Of late, though, she has reconsidered that practice.
“The problem is that some patients with abnormal MRI lesions have no CNS disease at all, and there are also a fair number of patients with a normal MRI who have CNS symptoms. So in our practice we’re pulling back on doing screening MRIs because we don’t know what to do with the findings, and it just makes everybody worried,” she said.
However, if a child with localized scleroderma develops headaches, seizures, neuropathies, or other CNS symptoms, then by all means get an MRI, and if it shows findings such as brain atrophy, white matter lesions, calcifications, or leptomeningeal enhancement, consider treatment, she added.
Dr. Stevens reported receiving research funding from Kineta and Seattle Genetics.
MAUI, HAWAII – One of the most important steps to take when a child has received a biopsy-confirmed diagnosis of localized scleroderma is to sit down with the family and explain the rationale for the aggressive therapies to come, Anne M. Stevens, MD, PhD, said at the 2019 Rheumatology Winter Clinical Symposium.
It can be a tough sell at first, especially when a child has only a small red streak on the nose and perhaps a subtle linear lesion on the forehead or scalp. But the family has to come to understand that this is a serious, chronic, progressive fibrotic disease.
“Talk about what a big impact this disease can have on growth of a limb and the normal life of a child because of the cosmetic appearance. Explain that the length of treatment course is based on the long-term outcomes and quality of life. This discussion is usually sufficient” to convince people to give their children “these pretty serious medications,” said Dr. Stevens, professor of pediatrics and head of the division of pediatric rheumatology at the University of Washington, Seattle.
“The treatment goal is to control inflammation and prevent damage in these patients, who we like to catch very early, when it’s a subtle lesion,” she added.
The biggest problem
The biggest contributors to poor quality of life in patients with juvenile localized scleroderma are the extracutaneous manifestations, which occur in up to 50% of cases. Joint pain occurs in roughly 20% of patients, joint contractures due to fibrosis of skin and/or tendons in 30%, and myalgia with or without myositis in 15%. Muscle atrophy due to the deep component of the scleroderma can occur. Moreover, growth problems – especially leg or arm length discrepancies – happen in about 20% of patients in prospective studies. These growth problems may not be obvious until a child enters a growth spurt, at which point there is a limited ability to achieve improvement. That’s why Dr. Stevens recommends that every child with localized scleroderma should get a full joint exam at every visit, with measurement and photos of lesions and recording of all erythematous, violaceous, and waxy-hued areas. And if there are lesions on the head, annual eye exams are warranted.
The prevalence of juvenile localized scleroderma in the United States is about 3 per 100,000, with a mean age of onset of 8.2 years. That makes it 100-fold more common than pediatric systemic sclerosis.
The treatment ladder
There are no Food and Drug Administration–approved medications for localized scleroderma in children. It’s all off label. That being said, there is strong consensus among members of the Childhood Arthritis and Rheumatology Research Alliance that the first-line therapy is methotrexate at 15 mg/m2 or a maximum of 20 mg/week plus intravenous corticosteroids weaned over the course of 3-6 months. This is the treatment regimen with the best supporting evidence of safety and efficacy, including a single Italian randomized, double-blind, placebo-controlled clinical trial (Arthritis Rheum. 2011 Jul;63[7]:1998-2006) and an accompanying long-term, open-label follow-up study (J Am Acad Dermatol. 2012 Dec;67[6]:1151-6).
All of the other treatments she uses for juvenile localized scleroderma – mycophenolate mofetil (CellCept), abatacept (Orencia), tocilizumab (Actemra), and occasionally others – are backed only by a smattering of small case series. However, given the serious potential trajectory of this disease, that modest evidence base has been sufficient for her to receive insurance coverage approval of these agents.
In the randomized trial of first-line methotrexate, 48 of 65 patients treated with methotrexate plus steroid (74%) were responders. And among those 48 responders, 35 (73%) maintained a clinical remission for a mean of 25 months off-drug, while another 13 (27%) were in clinical remission on methotrexate. Twenty-eight patients developed side effects that were generally mild; no one required treatment discontinuation. At the 5-year mark, after an average of an initial 2 years on methotrexate, half of the patients were in a sustained clinical remission, which Dr. Stevens deemed “pretty good” considering the well established and manageable safety profile of the drug.
If a patient fails to respond to methotrexate plus corticosteroids within a few months or later experiences disease progression, Dr. Stevens’ second-line therapy is mycophenolate mofetil in conjunction with corticosteroids. Its use in arresting juvenile localized scleroderma is supported by two favorable published case series, the largest of which includes 10 patients (Rheumatology [Oxford]. 2009 Nov;48[11]:1410-3).
Dr. Stevens’ third-line therapy is intravenous abatacept at 10 mg/kg monthly along with intravenous methylprednisolone at 500 mg/week. There are five published case series, the most recent and largest of which included 13 adult patients, two of whom had en coup de sabre lesions (Acta Derm Venereol. 2018 Apr 16;98[4]:465-6). The biologic also shows promise in patients with advanced severe disease with deep tissue involvement (Semin Arthritis Rheum. 2017 Jun;46[6]:775-81). And abatacept has a plausible mechanism of action in localized scleroderma: French investigators have shown it induces regression of skin fibrosis in a mouse model of the disease (Ann Rheum Dis. 2016 Dec;75[12]:2142-9).
Her fourth-line strategy is the anti-interleukin-6 agent tocilizumab, again in conjunction with corticosteroids. In a translational study, tocilizumab has been shown to normalize dermal fibroblasts and collagen in patients with systemic sclerosis (Ann Rheum Dis. 2018 Sep;77[9]:1362-71). And there have been two promising small retrospective case series as well. A more definitive clinical trial is planned.
Dr. Stevens said that when starting a biologic agent in a child with localized scleroderma, she routinely adds methotrexate until the disease is under control.
Drugs supported by case reports and worth considering on an individual basis as a last resort are hydroxychloroquine, azathioprine, cyclosporine, and imatinib mesylate (Gleevec).
For mild, superficial lesions that don’t cross joints, ultraviolet light A phototherapy is a therapeutic option. It displayed significant benefit in a systematic review and meta-analysis of 19 studies comparing it to methotrexate, although the results with methotrexate were deemed superior (Semin Arthritis Rheum. 2018 Dec;48[3]:495-503).
The pros and cons of getting a baseline brain MRI
Children with localized scleroderma have increased rates of severe headache, peripheral neuropathy, complex partial seizures, and stroke. So it had been Dr. Stevens’ routine practice to obtain an initial brain MRI at the time of diagnosis. Of late, though, she has reconsidered that practice.
“The problem is that some patients with abnormal MRI lesions have no CNS disease at all, and there are also a fair number of patients with a normal MRI who have CNS symptoms. So in our practice we’re pulling back on doing screening MRIs because we don’t know what to do with the findings, and it just makes everybody worried,” she said.
However, if a child with localized scleroderma develops headaches, seizures, neuropathies, or other CNS symptoms, then by all means get an MRI, and if it shows findings such as brain atrophy, white matter lesions, calcifications, or leptomeningeal enhancement, consider treatment, she added.
Dr. Stevens reported receiving research funding from Kineta and Seattle Genetics.
MAUI, HAWAII – One of the most important steps to take when a child has received a biopsy-confirmed diagnosis of localized scleroderma is to sit down with the family and explain the rationale for the aggressive therapies to come, Anne M. Stevens, MD, PhD, said at the 2019 Rheumatology Winter Clinical Symposium.
It can be a tough sell at first, especially when a child has only a small red streak on the nose and perhaps a subtle linear lesion on the forehead or scalp. But the family has to come to understand that this is a serious, chronic, progressive fibrotic disease.
“Talk about what a big impact this disease can have on growth of a limb and the normal life of a child because of the cosmetic appearance. Explain that the length of treatment course is based on the long-term outcomes and quality of life. This discussion is usually sufficient” to convince people to give their children “these pretty serious medications,” said Dr. Stevens, professor of pediatrics and head of the division of pediatric rheumatology at the University of Washington, Seattle.
“The treatment goal is to control inflammation and prevent damage in these patients, who we like to catch very early, when it’s a subtle lesion,” she added.
The biggest problem
The biggest contributors to poor quality of life in patients with juvenile localized scleroderma are the extracutaneous manifestations, which occur in up to 50% of cases. Joint pain occurs in roughly 20% of patients, joint contractures due to fibrosis of skin and/or tendons in 30%, and myalgia with or without myositis in 15%. Muscle atrophy due to the deep component of the scleroderma can occur. Moreover, growth problems – especially leg or arm length discrepancies – happen in about 20% of patients in prospective studies. These growth problems may not be obvious until a child enters a growth spurt, at which point there is a limited ability to achieve improvement. That’s why Dr. Stevens recommends that every child with localized scleroderma should get a full joint exam at every visit, with measurement and photos of lesions and recording of all erythematous, violaceous, and waxy-hued areas. And if there are lesions on the head, annual eye exams are warranted.
The prevalence of juvenile localized scleroderma in the United States is about 3 per 100,000, with a mean age of onset of 8.2 years. That makes it 100-fold more common than pediatric systemic sclerosis.
The treatment ladder
There are no Food and Drug Administration–approved medications for localized scleroderma in children. It’s all off label. That being said, there is strong consensus among members of the Childhood Arthritis and Rheumatology Research Alliance that the first-line therapy is methotrexate at 15 mg/m2 or a maximum of 20 mg/week plus intravenous corticosteroids weaned over the course of 3-6 months. This is the treatment regimen with the best supporting evidence of safety and efficacy, including a single Italian randomized, double-blind, placebo-controlled clinical trial (Arthritis Rheum. 2011 Jul;63[7]:1998-2006) and an accompanying long-term, open-label follow-up study (J Am Acad Dermatol. 2012 Dec;67[6]:1151-6).
All of the other treatments she uses for juvenile localized scleroderma – mycophenolate mofetil (CellCept), abatacept (Orencia), tocilizumab (Actemra), and occasionally others – are backed only by a smattering of small case series. However, given the serious potential trajectory of this disease, that modest evidence base has been sufficient for her to receive insurance coverage approval of these agents.
In the randomized trial of first-line methotrexate, 48 of 65 patients treated with methotrexate plus steroid (74%) were responders. And among those 48 responders, 35 (73%) maintained a clinical remission for a mean of 25 months off-drug, while another 13 (27%) were in clinical remission on methotrexate. Twenty-eight patients developed side effects that were generally mild; no one required treatment discontinuation. At the 5-year mark, after an average of an initial 2 years on methotrexate, half of the patients were in a sustained clinical remission, which Dr. Stevens deemed “pretty good” considering the well established and manageable safety profile of the drug.
If a patient fails to respond to methotrexate plus corticosteroids within a few months or later experiences disease progression, Dr. Stevens’ second-line therapy is mycophenolate mofetil in conjunction with corticosteroids. Its use in arresting juvenile localized scleroderma is supported by two favorable published case series, the largest of which includes 10 patients (Rheumatology [Oxford]. 2009 Nov;48[11]:1410-3).
Dr. Stevens’ third-line therapy is intravenous abatacept at 10 mg/kg monthly along with intravenous methylprednisolone at 500 mg/week. There are five published case series, the most recent and largest of which included 13 adult patients, two of whom had en coup de sabre lesions (Acta Derm Venereol. 2018 Apr 16;98[4]:465-6). The biologic also shows promise in patients with advanced severe disease with deep tissue involvement (Semin Arthritis Rheum. 2017 Jun;46[6]:775-81). And abatacept has a plausible mechanism of action in localized scleroderma: French investigators have shown it induces regression of skin fibrosis in a mouse model of the disease (Ann Rheum Dis. 2016 Dec;75[12]:2142-9).
Her fourth-line strategy is the anti-interleukin-6 agent tocilizumab, again in conjunction with corticosteroids. In a translational study, tocilizumab has been shown to normalize dermal fibroblasts and collagen in patients with systemic sclerosis (Ann Rheum Dis. 2018 Sep;77[9]:1362-71). And there have been two promising small retrospective case series as well. A more definitive clinical trial is planned.
Dr. Stevens said that when starting a biologic agent in a child with localized scleroderma, she routinely adds methotrexate until the disease is under control.
Drugs supported by case reports and worth considering on an individual basis as a last resort are hydroxychloroquine, azathioprine, cyclosporine, and imatinib mesylate (Gleevec).
For mild, superficial lesions that don’t cross joints, ultraviolet light A phototherapy is a therapeutic option. It displayed significant benefit in a systematic review and meta-analysis of 19 studies comparing it to methotrexate, although the results with methotrexate were deemed superior (Semin Arthritis Rheum. 2018 Dec;48[3]:495-503).
The pros and cons of getting a baseline brain MRI
Children with localized scleroderma have increased rates of severe headache, peripheral neuropathy, complex partial seizures, and stroke. So it had been Dr. Stevens’ routine practice to obtain an initial brain MRI at the time of diagnosis. Of late, though, she has reconsidered that practice.
“The problem is that some patients with abnormal MRI lesions have no CNS disease at all, and there are also a fair number of patients with a normal MRI who have CNS symptoms. So in our practice we’re pulling back on doing screening MRIs because we don’t know what to do with the findings, and it just makes everybody worried,” she said.
However, if a child with localized scleroderma develops headaches, seizures, neuropathies, or other CNS symptoms, then by all means get an MRI, and if it shows findings such as brain atrophy, white matter lesions, calcifications, or leptomeningeal enhancement, consider treatment, she added.
Dr. Stevens reported receiving research funding from Kineta and Seattle Genetics.
REPORTING FROM RWCS 2019
Biologics boost work outcomes in axial spondyloarthritis
MAUI, HAWAII – Biologic therapy improves work-related outcomes in patients with axial spondyloarthritis, according to a report from the British Society for Rheumatology Biologics Register.
“This gets to the issue of cost/benefit. But with benefit you have to look at the big picture. These are expensive drugs, but if these expensive drugs have societal benefits by keeping people at work, you have to throw that into the equation when you think about the value proposition of these agents,” Eric M. Ruderman, MD, observed in highlighting the British study at the 2019 Rheumatology Winter Clinical Symposium.
In drawing attention to this and other developments during the past year in the field of axial spondyloarthritis (SpA) outside the realm of pharmacologic randomized trials, he and copanelist Arthur Kavanaugh, MD, highlighted trends in diagnostic imaging for the disorder, where MRI’s stock may be going down while color Doppler ultrasound’s is rising, as well as a novel online tool designed to get individuals with a high probability of SpA into a rheumatologist’s office without years of bouncing around between other types of health care providers.
Biologics boost work performance
The British Society for Rheumatology Biologics Register study included 577 patients at 83 centers in Great Britain who met Assessment of SpondyloArthritis International Society criteria for radiographic or nonradiographic SpA, all of whom were employed and biologic-naive when they enrolled in the registry (Ann Rheum Dis. 2018 Nov;77[11]:1578-84). Upon enrollment, 28% of them were placed on adalimumab (Humira), etanercept (Enbrel), or certolizumab pegol (Cimzia) based upon physician recommendation. Work outcomes at the start and end of the first year in the registry were compared between SpA patients on biologic therapy or not using the validated Work Productivity and Activity Impairment Index, a patient self-report measure.
After propensity score adjustment to account for between-group differences, SpA patients on biologic therapy demonstrated a 9.4% reduction in presenteeism – that is, on-site work underperformance and productivity loss – compared with those not on a biologic. The group on biologics also averaged a 13.9% greater improvement from baseline in overall work impairment than did patients not on a biologic and a 19.2% greater improvement in overall activity impairment, which encompasses leisure activities. This works out to more than half a day of additional full productivity per week 12 months after starting on a biologic.
The investigators decided to confirm their findings by conducting what they believe to be the first-ever meta-analysis to quantify the impact of biologic therapy for SpA on work participation. The meta-analysis included five studies with 1,109 participants. The results: Biologic therapy was associated with significantly greater improvements in presenteeism, overall work impairment, and overall activity impairment, as in the British registry study, but was also no significant impact on work absenteeism, just as was the case in the registry study. The investigators noted that presenteeism is a much bigger problem than absenteeism in patients with SpA. They hypothesized that absenteeism is a relatively late-stage development in work impairment that isn’t reversible by biologic therapy alone.
“This is superimportant data,” commented Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Pharmacoeconomic analyses typically rely upon quality-of-life metrics and express cost/benefit in terms of QALYs, or quality-adjusted life-years, gained by utilization of a therapy. That’s a measure of particular importance from a payer’s perspective, but QALYs typically don’t incorporate work outcome data and other aspects of the wider societal costs and benefits of a therapy since they aren’t addressed in short-term, randomized, controlled trials.
“Work data are a more realistic way to do this: actual data on people getting back to their jobs,” the rheumatologist said.
Online accrual of likely SpA patients
The average delay between symptom onset and diagnosis of SpA is 7-9 years. Dr. Ruderman was favorably impressed by the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study of an outside-the-box online self-referral tool presented at the 2018 annual meeting of the American College of Rheumatology.
The German investigators placed advertisements in subways directing interested riders with back pain to a website where they completed what the rheumatologists called the Berlin referral tool. If they indicated they had experienced chronic back pain for more than 3 months with onset before age 45 and had at least one additional clue of SpA – inflammatory back pain symptoms, a good response to NSAIDs, psoriasis, inflammatory bowel disease, uveitis, a positive family history for SpA, an elevated C-reactive protein, HLA-B27 positivity, or peripheral symptoms suggestive of arthritis and/or enthesitis – they got an appointment with a rheumatologist straightaway.
“How do you get these people with back pain and potentially axial spondyloarthritis to see us? We’ve all seen patients stuck for years with orthopedists and physiatrists and chiropractors, and they finally get to you and you figure out what they have in a couple minutes and start them on effective therapy. This is an online tool that may pick up axial spondyloarthritis patients not identified by primary care,” explained Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
The study included 362 patients evaluated for suspected SpA by participating rheumatologists. Half made it to the rheumatologist by way of physician referral after experiencing back pain for a mean duration of 6.5 years; the other half came via the Berlin referral tool. A total of 39.2% of patients in the physician-referral group and 19.3% in the self-referral group were ultimately diagnosed with SpA.
“It’s not 100%. You’d never expect it to be. But I think all of us would say if you get five people and one of them turns out to have the real deal, it’s worth it to have this kind of method available to get people into your office and away from the four MRIs and the epidural steroid injections and potentially even the surgery before they get to you,” Dr. Ruderman commented.
Dr. Kavanaugh noted with approval that women accounted for 44% of the referrals from physicians and 57% of those who were self-referred.
“This is a way to get female patients, where you don’t suspect axial spondyloarthritis as much – and you don’t find it if you don’t suspect it. Any way to get a real patient into your office to offer them appropriate therapy is great,” he said.
MRI is no gold standard for SpA diagnosis
Dr. Ruderman drew attention to the MASH study, a Danish cross-sectional study of the effectiveness of MRI imaging of the sacroiliac joints in differentiating patients with SpA from other individuals who engage in hard physical work. The study, presented at the 2018 European Congress of Rheumatology, featured blinded reading of the MRIs of 204 participants, all aged 45 years or less. The study population, not all of whom had back pain for at least 2 months, included 41 patients known to have SpA as well as 23 distance runners, 26 room cleaners, 46 women who had given birth within the past year, 25 people with a herniated lumbar disc, and 29 healthy men.
The key finding was that while mean Spondyloarthritis Research Consortium of Canada sacroiliac joint MRI scores for inflammation, fatty deposition, and erosions were higher in the SpA group, many of the same changes were present to a lesser degree in the others.
“The takeaway is this is a clinical diagnosis and you can’t make the diagnosis just based on the imaging, regardless of what the radiologist is reporting. You have to put it in context,” the rheumatologist said.
“This adds to a growing body of evidence that says MRI is not the gold standard for diagnosing axial spondyloarthritis,” Dr. Kavanaugh added. “In other studies, you see those kinds of changes in active military, snowboarders, hockey players. So like with every diagnostic test, we have to wrestle with the fact that the more sensitive it is, the less specific it is, and vice versa.”
What about color Doppler ultrasound?
Argentinian rheumatologists used color Doppler ultrasound to look for sacroiliitis in 198 joints evaluated in 99 consecutive patients with inflammatory back pain and suspected SpA without a definitive diagnosis. All participants also had an MRI scan and clinical evaluation as well. At the joint level, ultrasound had a sensitivity of 60% and specificity of 93% for diagnosis of sacroiliitis. For diagnosis of SpA, the positive predictive value was 79% and the negative predictive value was 59% (J Rheumatol. 2018 Dec 15. doi: 10.3899/jrheum.180550).
“I don’t think this suggests that ultrasound replaces MRI, but MRI is a more expensive test and harder to get, and if you could get some information with an ultrasound done properly in the office it might be an interesting way to identify those patients who truly have axial spondyloarthritis and inflammatory sacroiliitis. That specificity of 93% is pretty good,” Dr. Ruderman noted.
“What about doing this: If it’s positive then you don’t need the MRI and maybe you do an injection at that time, but if it’s negative you do the MRI?” Dr. Kavanaugh asked.
Orrin M. Troum, MD, a pioneer in the use of extremity MRI in the United States for evaluation of patients with inflammatory peripheral arthritis, had reservations.
“Availability and cost are important, but one of the distinctions between MRI and ultrasound is that you can’t see bone marrow edema. I think that’s one of the classic features of MRI that’s important here,” according to Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles.
Dr. Kavanaugh asked Paul Emery, MD, a renowned authority on the use of ultrasound in rheumatology, for his thoughts.
“We don’t use ultrasound for sacroiliitis. It’s too unreliable,” said Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center. “It’s such a big decision to start a biologic for an ankylosing spondyloarthritis patient that none of our people who use ultrasound rely on it.”
Dr. Ruderman and Dr. Kavanaugh reported receiving research funding from and serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Biologic therapy improves work-related outcomes in patients with axial spondyloarthritis, according to a report from the British Society for Rheumatology Biologics Register.
“This gets to the issue of cost/benefit. But with benefit you have to look at the big picture. These are expensive drugs, but if these expensive drugs have societal benefits by keeping people at work, you have to throw that into the equation when you think about the value proposition of these agents,” Eric M. Ruderman, MD, observed in highlighting the British study at the 2019 Rheumatology Winter Clinical Symposium.
In drawing attention to this and other developments during the past year in the field of axial spondyloarthritis (SpA) outside the realm of pharmacologic randomized trials, he and copanelist Arthur Kavanaugh, MD, highlighted trends in diagnostic imaging for the disorder, where MRI’s stock may be going down while color Doppler ultrasound’s is rising, as well as a novel online tool designed to get individuals with a high probability of SpA into a rheumatologist’s office without years of bouncing around between other types of health care providers.
Biologics boost work performance
The British Society for Rheumatology Biologics Register study included 577 patients at 83 centers in Great Britain who met Assessment of SpondyloArthritis International Society criteria for radiographic or nonradiographic SpA, all of whom were employed and biologic-naive when they enrolled in the registry (Ann Rheum Dis. 2018 Nov;77[11]:1578-84). Upon enrollment, 28% of them were placed on adalimumab (Humira), etanercept (Enbrel), or certolizumab pegol (Cimzia) based upon physician recommendation. Work outcomes at the start and end of the first year in the registry were compared between SpA patients on biologic therapy or not using the validated Work Productivity and Activity Impairment Index, a patient self-report measure.
After propensity score adjustment to account for between-group differences, SpA patients on biologic therapy demonstrated a 9.4% reduction in presenteeism – that is, on-site work underperformance and productivity loss – compared with those not on a biologic. The group on biologics also averaged a 13.9% greater improvement from baseline in overall work impairment than did patients not on a biologic and a 19.2% greater improvement in overall activity impairment, which encompasses leisure activities. This works out to more than half a day of additional full productivity per week 12 months after starting on a biologic.
The investigators decided to confirm their findings by conducting what they believe to be the first-ever meta-analysis to quantify the impact of biologic therapy for SpA on work participation. The meta-analysis included five studies with 1,109 participants. The results: Biologic therapy was associated with significantly greater improvements in presenteeism, overall work impairment, and overall activity impairment, as in the British registry study, but was also no significant impact on work absenteeism, just as was the case in the registry study. The investigators noted that presenteeism is a much bigger problem than absenteeism in patients with SpA. They hypothesized that absenteeism is a relatively late-stage development in work impairment that isn’t reversible by biologic therapy alone.
“This is superimportant data,” commented Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Pharmacoeconomic analyses typically rely upon quality-of-life metrics and express cost/benefit in terms of QALYs, or quality-adjusted life-years, gained by utilization of a therapy. That’s a measure of particular importance from a payer’s perspective, but QALYs typically don’t incorporate work outcome data and other aspects of the wider societal costs and benefits of a therapy since they aren’t addressed in short-term, randomized, controlled trials.
“Work data are a more realistic way to do this: actual data on people getting back to their jobs,” the rheumatologist said.
Online accrual of likely SpA patients
The average delay between symptom onset and diagnosis of SpA is 7-9 years. Dr. Ruderman was favorably impressed by the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study of an outside-the-box online self-referral tool presented at the 2018 annual meeting of the American College of Rheumatology.
The German investigators placed advertisements in subways directing interested riders with back pain to a website where they completed what the rheumatologists called the Berlin referral tool. If they indicated they had experienced chronic back pain for more than 3 months with onset before age 45 and had at least one additional clue of SpA – inflammatory back pain symptoms, a good response to NSAIDs, psoriasis, inflammatory bowel disease, uveitis, a positive family history for SpA, an elevated C-reactive protein, HLA-B27 positivity, or peripheral symptoms suggestive of arthritis and/or enthesitis – they got an appointment with a rheumatologist straightaway.
“How do you get these people with back pain and potentially axial spondyloarthritis to see us? We’ve all seen patients stuck for years with orthopedists and physiatrists and chiropractors, and they finally get to you and you figure out what they have in a couple minutes and start them on effective therapy. This is an online tool that may pick up axial spondyloarthritis patients not identified by primary care,” explained Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
The study included 362 patients evaluated for suspected SpA by participating rheumatologists. Half made it to the rheumatologist by way of physician referral after experiencing back pain for a mean duration of 6.5 years; the other half came via the Berlin referral tool. A total of 39.2% of patients in the physician-referral group and 19.3% in the self-referral group were ultimately diagnosed with SpA.
“It’s not 100%. You’d never expect it to be. But I think all of us would say if you get five people and one of them turns out to have the real deal, it’s worth it to have this kind of method available to get people into your office and away from the four MRIs and the epidural steroid injections and potentially even the surgery before they get to you,” Dr. Ruderman commented.
Dr. Kavanaugh noted with approval that women accounted for 44% of the referrals from physicians and 57% of those who were self-referred.
“This is a way to get female patients, where you don’t suspect axial spondyloarthritis as much – and you don’t find it if you don’t suspect it. Any way to get a real patient into your office to offer them appropriate therapy is great,” he said.
MRI is no gold standard for SpA diagnosis
Dr. Ruderman drew attention to the MASH study, a Danish cross-sectional study of the effectiveness of MRI imaging of the sacroiliac joints in differentiating patients with SpA from other individuals who engage in hard physical work. The study, presented at the 2018 European Congress of Rheumatology, featured blinded reading of the MRIs of 204 participants, all aged 45 years or less. The study population, not all of whom had back pain for at least 2 months, included 41 patients known to have SpA as well as 23 distance runners, 26 room cleaners, 46 women who had given birth within the past year, 25 people with a herniated lumbar disc, and 29 healthy men.
The key finding was that while mean Spondyloarthritis Research Consortium of Canada sacroiliac joint MRI scores for inflammation, fatty deposition, and erosions were higher in the SpA group, many of the same changes were present to a lesser degree in the others.
“The takeaway is this is a clinical diagnosis and you can’t make the diagnosis just based on the imaging, regardless of what the radiologist is reporting. You have to put it in context,” the rheumatologist said.
“This adds to a growing body of evidence that says MRI is not the gold standard for diagnosing axial spondyloarthritis,” Dr. Kavanaugh added. “In other studies, you see those kinds of changes in active military, snowboarders, hockey players. So like with every diagnostic test, we have to wrestle with the fact that the more sensitive it is, the less specific it is, and vice versa.”
What about color Doppler ultrasound?
Argentinian rheumatologists used color Doppler ultrasound to look for sacroiliitis in 198 joints evaluated in 99 consecutive patients with inflammatory back pain and suspected SpA without a definitive diagnosis. All participants also had an MRI scan and clinical evaluation as well. At the joint level, ultrasound had a sensitivity of 60% and specificity of 93% for diagnosis of sacroiliitis. For diagnosis of SpA, the positive predictive value was 79% and the negative predictive value was 59% (J Rheumatol. 2018 Dec 15. doi: 10.3899/jrheum.180550).
“I don’t think this suggests that ultrasound replaces MRI, but MRI is a more expensive test and harder to get, and if you could get some information with an ultrasound done properly in the office it might be an interesting way to identify those patients who truly have axial spondyloarthritis and inflammatory sacroiliitis. That specificity of 93% is pretty good,” Dr. Ruderman noted.
“What about doing this: If it’s positive then you don’t need the MRI and maybe you do an injection at that time, but if it’s negative you do the MRI?” Dr. Kavanaugh asked.
Orrin M. Troum, MD, a pioneer in the use of extremity MRI in the United States for evaluation of patients with inflammatory peripheral arthritis, had reservations.
“Availability and cost are important, but one of the distinctions between MRI and ultrasound is that you can’t see bone marrow edema. I think that’s one of the classic features of MRI that’s important here,” according to Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles.
Dr. Kavanaugh asked Paul Emery, MD, a renowned authority on the use of ultrasound in rheumatology, for his thoughts.
“We don’t use ultrasound for sacroiliitis. It’s too unreliable,” said Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center. “It’s such a big decision to start a biologic for an ankylosing spondyloarthritis patient that none of our people who use ultrasound rely on it.”
Dr. Ruderman and Dr. Kavanaugh reported receiving research funding from and serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Biologic therapy improves work-related outcomes in patients with axial spondyloarthritis, according to a report from the British Society for Rheumatology Biologics Register.
“This gets to the issue of cost/benefit. But with benefit you have to look at the big picture. These are expensive drugs, but if these expensive drugs have societal benefits by keeping people at work, you have to throw that into the equation when you think about the value proposition of these agents,” Eric M. Ruderman, MD, observed in highlighting the British study at the 2019 Rheumatology Winter Clinical Symposium.
In drawing attention to this and other developments during the past year in the field of axial spondyloarthritis (SpA) outside the realm of pharmacologic randomized trials, he and copanelist Arthur Kavanaugh, MD, highlighted trends in diagnostic imaging for the disorder, where MRI’s stock may be going down while color Doppler ultrasound’s is rising, as well as a novel online tool designed to get individuals with a high probability of SpA into a rheumatologist’s office without years of bouncing around between other types of health care providers.
Biologics boost work performance
The British Society for Rheumatology Biologics Register study included 577 patients at 83 centers in Great Britain who met Assessment of SpondyloArthritis International Society criteria for radiographic or nonradiographic SpA, all of whom were employed and biologic-naive when they enrolled in the registry (Ann Rheum Dis. 2018 Nov;77[11]:1578-84). Upon enrollment, 28% of them were placed on adalimumab (Humira), etanercept (Enbrel), or certolizumab pegol (Cimzia) based upon physician recommendation. Work outcomes at the start and end of the first year in the registry were compared between SpA patients on biologic therapy or not using the validated Work Productivity and Activity Impairment Index, a patient self-report measure.
After propensity score adjustment to account for between-group differences, SpA patients on biologic therapy demonstrated a 9.4% reduction in presenteeism – that is, on-site work underperformance and productivity loss – compared with those not on a biologic. The group on biologics also averaged a 13.9% greater improvement from baseline in overall work impairment than did patients not on a biologic and a 19.2% greater improvement in overall activity impairment, which encompasses leisure activities. This works out to more than half a day of additional full productivity per week 12 months after starting on a biologic.
The investigators decided to confirm their findings by conducting what they believe to be the first-ever meta-analysis to quantify the impact of biologic therapy for SpA on work participation. The meta-analysis included five studies with 1,109 participants. The results: Biologic therapy was associated with significantly greater improvements in presenteeism, overall work impairment, and overall activity impairment, as in the British registry study, but was also no significant impact on work absenteeism, just as was the case in the registry study. The investigators noted that presenteeism is a much bigger problem than absenteeism in patients with SpA. They hypothesized that absenteeism is a relatively late-stage development in work impairment that isn’t reversible by biologic therapy alone.
“This is superimportant data,” commented Dr. Kavanaugh, professor of medicine at the University of California, San Diego.
Pharmacoeconomic analyses typically rely upon quality-of-life metrics and express cost/benefit in terms of QALYs, or quality-adjusted life-years, gained by utilization of a therapy. That’s a measure of particular importance from a payer’s perspective, but QALYs typically don’t incorporate work outcome data and other aspects of the wider societal costs and benefits of a therapy since they aren’t addressed in short-term, randomized, controlled trials.
“Work data are a more realistic way to do this: actual data on people getting back to their jobs,” the rheumatologist said.
Online accrual of likely SpA patients
The average delay between symptom onset and diagnosis of SpA is 7-9 years. Dr. Ruderman was favorably impressed by the Identification of the Optimal Referral Strategy for Early Diagnosis of Axial Spondyloarthritis (OptiRef) study of an outside-the-box online self-referral tool presented at the 2018 annual meeting of the American College of Rheumatology.
The German investigators placed advertisements in subways directing interested riders with back pain to a website where they completed what the rheumatologists called the Berlin referral tool. If they indicated they had experienced chronic back pain for more than 3 months with onset before age 45 and had at least one additional clue of SpA – inflammatory back pain symptoms, a good response to NSAIDs, psoriasis, inflammatory bowel disease, uveitis, a positive family history for SpA, an elevated C-reactive protein, HLA-B27 positivity, or peripheral symptoms suggestive of arthritis and/or enthesitis – they got an appointment with a rheumatologist straightaway.
“How do you get these people with back pain and potentially axial spondyloarthritis to see us? We’ve all seen patients stuck for years with orthopedists and physiatrists and chiropractors, and they finally get to you and you figure out what they have in a couple minutes and start them on effective therapy. This is an online tool that may pick up axial spondyloarthritis patients not identified by primary care,” explained Dr. Ruderman, professor of medicine at Northwestern University in Chicago.
The study included 362 patients evaluated for suspected SpA by participating rheumatologists. Half made it to the rheumatologist by way of physician referral after experiencing back pain for a mean duration of 6.5 years; the other half came via the Berlin referral tool. A total of 39.2% of patients in the physician-referral group and 19.3% in the self-referral group were ultimately diagnosed with SpA.
“It’s not 100%. You’d never expect it to be. But I think all of us would say if you get five people and one of them turns out to have the real deal, it’s worth it to have this kind of method available to get people into your office and away from the four MRIs and the epidural steroid injections and potentially even the surgery before they get to you,” Dr. Ruderman commented.
Dr. Kavanaugh noted with approval that women accounted for 44% of the referrals from physicians and 57% of those who were self-referred.
“This is a way to get female patients, where you don’t suspect axial spondyloarthritis as much – and you don’t find it if you don’t suspect it. Any way to get a real patient into your office to offer them appropriate therapy is great,” he said.
MRI is no gold standard for SpA diagnosis
Dr. Ruderman drew attention to the MASH study, a Danish cross-sectional study of the effectiveness of MRI imaging of the sacroiliac joints in differentiating patients with SpA from other individuals who engage in hard physical work. The study, presented at the 2018 European Congress of Rheumatology, featured blinded reading of the MRIs of 204 participants, all aged 45 years or less. The study population, not all of whom had back pain for at least 2 months, included 41 patients known to have SpA as well as 23 distance runners, 26 room cleaners, 46 women who had given birth within the past year, 25 people with a herniated lumbar disc, and 29 healthy men.
The key finding was that while mean Spondyloarthritis Research Consortium of Canada sacroiliac joint MRI scores for inflammation, fatty deposition, and erosions were higher in the SpA group, many of the same changes were present to a lesser degree in the others.
“The takeaway is this is a clinical diagnosis and you can’t make the diagnosis just based on the imaging, regardless of what the radiologist is reporting. You have to put it in context,” the rheumatologist said.
“This adds to a growing body of evidence that says MRI is not the gold standard for diagnosing axial spondyloarthritis,” Dr. Kavanaugh added. “In other studies, you see those kinds of changes in active military, snowboarders, hockey players. So like with every diagnostic test, we have to wrestle with the fact that the more sensitive it is, the less specific it is, and vice versa.”
What about color Doppler ultrasound?
Argentinian rheumatologists used color Doppler ultrasound to look for sacroiliitis in 198 joints evaluated in 99 consecutive patients with inflammatory back pain and suspected SpA without a definitive diagnosis. All participants also had an MRI scan and clinical evaluation as well. At the joint level, ultrasound had a sensitivity of 60% and specificity of 93% for diagnosis of sacroiliitis. For diagnosis of SpA, the positive predictive value was 79% and the negative predictive value was 59% (J Rheumatol. 2018 Dec 15. doi: 10.3899/jrheum.180550).
“I don’t think this suggests that ultrasound replaces MRI, but MRI is a more expensive test and harder to get, and if you could get some information with an ultrasound done properly in the office it might be an interesting way to identify those patients who truly have axial spondyloarthritis and inflammatory sacroiliitis. That specificity of 93% is pretty good,” Dr. Ruderman noted.
“What about doing this: If it’s positive then you don’t need the MRI and maybe you do an injection at that time, but if it’s negative you do the MRI?” Dr. Kavanaugh asked.
Orrin M. Troum, MD, a pioneer in the use of extremity MRI in the United States for evaluation of patients with inflammatory peripheral arthritis, had reservations.
“Availability and cost are important, but one of the distinctions between MRI and ultrasound is that you can’t see bone marrow edema. I think that’s one of the classic features of MRI that’s important here,” according to Dr. Troum, a rheumatologist at the University of Southern California, Los Angeles.
Dr. Kavanaugh asked Paul Emery, MD, a renowned authority on the use of ultrasound in rheumatology, for his thoughts.
“We don’t use ultrasound for sacroiliitis. It’s too unreliable,” said Dr. Emery, professor of rheumatology and director of the University of Leeds (England) Musculoskeletal Biomedical Research Center. “It’s such a big decision to start a biologic for an ankylosing spondyloarthritis patient that none of our people who use ultrasound rely on it.”
Dr. Ruderman and Dr. Kavanaugh reported receiving research funding from and serving as consultants to numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
Industry-funded rheumatology RCTs are higher quality
MAUI, HAWAII – Industry-funded randomized, controlled clinical trials published in the three top-rated rheumatology journals during the past 20 years are of significantly higher overall quality than the nonindustry-funded ones, Michael Putman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Putman, a second-year rheumatology fellow at Northwestern University, Chicago, analyzed all randomized, controlled trials (RCTs) of pharmacotherapy featuring a comparator – either placebo or an active agent – published in 1998, 2008, and 2018 in Annals of the Rheumatic Diseases, Rheumatology, and Arthritis & Rheumatology.
His main takeaway: “Rheumatologic interventions seem to work pretty well. The mean absolute risk reduction in the trials is 17.5%, so the average number of patients who need to be treated with a rheumatologic intervention is about five. This is why it’s such a great specialty to be a part of: A lot of our patients get better.”
He created an RCT quality rating scale that captured the strength of study design, methodology, and findings based upon whether a randomized trial used a double-blind design; identified a prespecified primary outcome; and featured patient-reported outcomes, power calculations, sensitivity analysis, adjustment for multiple hypotheses, and intention-to-treat analysis. He then applied the rating scale to the 85 published RCTs in the three study years.
Of note, 84% of the trials published in 2018 were industry funded, up from 74% in 2008 and 1998.
“Industry funds the vast majority of studies. Industry studies are significantly more likely to be appropriately double blinded, report patient-reported outcome measures, use intention to treat, and they have a higher overall quality,” according to Dr. Putman.
Indeed, the industry-funded studies averaged a 66% score on his quality grading scale, compared with 45% for nonindustry-funded studies.
Utilization of most of the quality metrics remained stable over time. The exceptions: Incorporation of intent-to-treat analysis increased from 58% in 1998 to 87% in 2018, and sensitivity analysis was employed in just 5% of the trials published in 1998, compared with 37% in 2008 and 26% in 2018.
The most important change over the past 2 decades, in his view, has been the shrinking proportion of RCTs featuring an active-drug, head-to-head comparator arm. In 1998, 42% of studies featured that design; for example, comparing methotrexate to sulfasalazine. By 2018, that figure had dropped to just 13%.
“Most of our trials today compare an active compound, such an interleukin-17 inhibitor, to a placebo. I think that’s a big change in how we do things,” Dr. Putman observed. “With 84% of our studies being funded by industry, the incentives in medicine right now don’t support active comparator research. It’s harder to show a difference between two things that work than it is to show a difference between something and nothing.”
However, he’d welcome a revival of head-to-head active comparator trials.
“I’d really love to have that happen,” he said. “We have basic questions we haven’t answered yet about a lot of our basic drugs: Like in myositis, should you start with Imuran [azathioprine], CellCept [mycophenolate mofetil], or methotrexate?”
Another striking change over time has been the dwindling proportion of published trials with a statistically significant finding for the primary outcome: 79% in 1998, 46% in 2008, and 36% last year. Dr. Putman suspects the explanation lies in the steady improvement in the effectiveness of standard background therapy for many conditions, which makes it tougher to show a striking difference between the add-on study drug and add-on placebo.
“We’re a victim of our own success,” he commented.
In any event, many key secondary outcomes in the RCTs were positive, even when the primary endpoint wasn’t, according to Dr. Putman, and there was a notable dearth of completely negative clinical RCTs published in the three top journals.
“The more cynical interpretation is there’s an incredible amount of publication bias, where we’re only publishing studies that show an effect and the journals or investigators are censoring the ones that don’t. The more charitable explanation, which is probably also true, is that by the time you get to putting on an RCT you kind of think, ‘This thing works.’ You’re not testing random stuff, so your pretest probability of a drug being effective when it enters into an RCT is probably shifted toward effectiveness,” Dr. Putman speculated.
He reported having no financial conflicts regarding his study.
MAUI, HAWAII – Industry-funded randomized, controlled clinical trials published in the three top-rated rheumatology journals during the past 20 years are of significantly higher overall quality than the nonindustry-funded ones, Michael Putman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Putman, a second-year rheumatology fellow at Northwestern University, Chicago, analyzed all randomized, controlled trials (RCTs) of pharmacotherapy featuring a comparator – either placebo or an active agent – published in 1998, 2008, and 2018 in Annals of the Rheumatic Diseases, Rheumatology, and Arthritis & Rheumatology.
His main takeaway: “Rheumatologic interventions seem to work pretty well. The mean absolute risk reduction in the trials is 17.5%, so the average number of patients who need to be treated with a rheumatologic intervention is about five. This is why it’s such a great specialty to be a part of: A lot of our patients get better.”
He created an RCT quality rating scale that captured the strength of study design, methodology, and findings based upon whether a randomized trial used a double-blind design; identified a prespecified primary outcome; and featured patient-reported outcomes, power calculations, sensitivity analysis, adjustment for multiple hypotheses, and intention-to-treat analysis. He then applied the rating scale to the 85 published RCTs in the three study years.
Of note, 84% of the trials published in 2018 were industry funded, up from 74% in 2008 and 1998.
“Industry funds the vast majority of studies. Industry studies are significantly more likely to be appropriately double blinded, report patient-reported outcome measures, use intention to treat, and they have a higher overall quality,” according to Dr. Putman.
Indeed, the industry-funded studies averaged a 66% score on his quality grading scale, compared with 45% for nonindustry-funded studies.
Utilization of most of the quality metrics remained stable over time. The exceptions: Incorporation of intent-to-treat analysis increased from 58% in 1998 to 87% in 2018, and sensitivity analysis was employed in just 5% of the trials published in 1998, compared with 37% in 2008 and 26% in 2018.
The most important change over the past 2 decades, in his view, has been the shrinking proportion of RCTs featuring an active-drug, head-to-head comparator arm. In 1998, 42% of studies featured that design; for example, comparing methotrexate to sulfasalazine. By 2018, that figure had dropped to just 13%.
“Most of our trials today compare an active compound, such an interleukin-17 inhibitor, to a placebo. I think that’s a big change in how we do things,” Dr. Putman observed. “With 84% of our studies being funded by industry, the incentives in medicine right now don’t support active comparator research. It’s harder to show a difference between two things that work than it is to show a difference between something and nothing.”
However, he’d welcome a revival of head-to-head active comparator trials.
“I’d really love to have that happen,” he said. “We have basic questions we haven’t answered yet about a lot of our basic drugs: Like in myositis, should you start with Imuran [azathioprine], CellCept [mycophenolate mofetil], or methotrexate?”
Another striking change over time has been the dwindling proportion of published trials with a statistically significant finding for the primary outcome: 79% in 1998, 46% in 2008, and 36% last year. Dr. Putman suspects the explanation lies in the steady improvement in the effectiveness of standard background therapy for many conditions, which makes it tougher to show a striking difference between the add-on study drug and add-on placebo.
“We’re a victim of our own success,” he commented.
In any event, many key secondary outcomes in the RCTs were positive, even when the primary endpoint wasn’t, according to Dr. Putman, and there was a notable dearth of completely negative clinical RCTs published in the three top journals.
“The more cynical interpretation is there’s an incredible amount of publication bias, where we’re only publishing studies that show an effect and the journals or investigators are censoring the ones that don’t. The more charitable explanation, which is probably also true, is that by the time you get to putting on an RCT you kind of think, ‘This thing works.’ You’re not testing random stuff, so your pretest probability of a drug being effective when it enters into an RCT is probably shifted toward effectiveness,” Dr. Putman speculated.
He reported having no financial conflicts regarding his study.
MAUI, HAWAII – Industry-funded randomized, controlled clinical trials published in the three top-rated rheumatology journals during the past 20 years are of significantly higher overall quality than the nonindustry-funded ones, Michael Putman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Putman, a second-year rheumatology fellow at Northwestern University, Chicago, analyzed all randomized, controlled trials (RCTs) of pharmacotherapy featuring a comparator – either placebo or an active agent – published in 1998, 2008, and 2018 in Annals of the Rheumatic Diseases, Rheumatology, and Arthritis & Rheumatology.
His main takeaway: “Rheumatologic interventions seem to work pretty well. The mean absolute risk reduction in the trials is 17.5%, so the average number of patients who need to be treated with a rheumatologic intervention is about five. This is why it’s such a great specialty to be a part of: A lot of our patients get better.”
He created an RCT quality rating scale that captured the strength of study design, methodology, and findings based upon whether a randomized trial used a double-blind design; identified a prespecified primary outcome; and featured patient-reported outcomes, power calculations, sensitivity analysis, adjustment for multiple hypotheses, and intention-to-treat analysis. He then applied the rating scale to the 85 published RCTs in the three study years.
Of note, 84% of the trials published in 2018 were industry funded, up from 74% in 2008 and 1998.
“Industry funds the vast majority of studies. Industry studies are significantly more likely to be appropriately double blinded, report patient-reported outcome measures, use intention to treat, and they have a higher overall quality,” according to Dr. Putman.
Indeed, the industry-funded studies averaged a 66% score on his quality grading scale, compared with 45% for nonindustry-funded studies.
Utilization of most of the quality metrics remained stable over time. The exceptions: Incorporation of intent-to-treat analysis increased from 58% in 1998 to 87% in 2018, and sensitivity analysis was employed in just 5% of the trials published in 1998, compared with 37% in 2008 and 26% in 2018.
The most important change over the past 2 decades, in his view, has been the shrinking proportion of RCTs featuring an active-drug, head-to-head comparator arm. In 1998, 42% of studies featured that design; for example, comparing methotrexate to sulfasalazine. By 2018, that figure had dropped to just 13%.
“Most of our trials today compare an active compound, such an interleukin-17 inhibitor, to a placebo. I think that’s a big change in how we do things,” Dr. Putman observed. “With 84% of our studies being funded by industry, the incentives in medicine right now don’t support active comparator research. It’s harder to show a difference between two things that work than it is to show a difference between something and nothing.”
However, he’d welcome a revival of head-to-head active comparator trials.
“I’d really love to have that happen,” he said. “We have basic questions we haven’t answered yet about a lot of our basic drugs: Like in myositis, should you start with Imuran [azathioprine], CellCept [mycophenolate mofetil], or methotrexate?”
Another striking change over time has been the dwindling proportion of published trials with a statistically significant finding for the primary outcome: 79% in 1998, 46% in 2008, and 36% last year. Dr. Putman suspects the explanation lies in the steady improvement in the effectiveness of standard background therapy for many conditions, which makes it tougher to show a striking difference between the add-on study drug and add-on placebo.
“We’re a victim of our own success,” he commented.
In any event, many key secondary outcomes in the RCTs were positive, even when the primary endpoint wasn’t, according to Dr. Putman, and there was a notable dearth of completely negative clinical RCTs published in the three top journals.
“The more cynical interpretation is there’s an incredible amount of publication bias, where we’re only publishing studies that show an effect and the journals or investigators are censoring the ones that don’t. The more charitable explanation, which is probably also true, is that by the time you get to putting on an RCT you kind of think, ‘This thing works.’ You’re not testing random stuff, so your pretest probability of a drug being effective when it enters into an RCT is probably shifted toward effectiveness,” Dr. Putman speculated.
He reported having no financial conflicts regarding his study.
REPORTING FROM RWCS 2019



















