User login
Multiple Sclerosis Hub
‘Real-world evidence’ used to compare agents for relapsing-remitting MS
SAN DIEGO – Delayed-release dimethyl fumarate did not show any differences versus fingolimod in relapse rate over a 1-year, “real-world” study of patients with relapsing-remitting multiple sclerosis, but a significantly greater proportion of patients taking delayed-release dimethyl fumarate achieved relapse-free status and a lower annualized relapsed rate, compared with patients on glatiramer acetate
“There is a need for real-world data that compares the effectiveness of the growing number of MS [multiple sclerosis] treatment options,” Christophe Hotermans, MD, vice president of Global Medical Therapeutic Areas at Boston-based Biogen, said in an interview during ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “These results were consistent with previous analyses of efficacy of these treatments in people with relapsing-remitting MS, which showed no significant differences in efficacy between delayed-release dimethyl fumarate [DMF, Tecfidera] versus fingolimod [FTY, Gilenya] and greater efficacy with dimethyl fumarate, compared with glatiramer acetate [GA].”
The findings come from EFFECT (Observational Study to Characterize Real-world Clinical Outcomes With Relapsing-remitting Multiple Sclerosis), a multicenter, international, retrospective, single-time-point medical record review study comparing the effectiveness of DMF vs. other disease-modifying therapies, including FTY and GA in patients with relapsing-remitting MS.
Endpoints included the Kaplan-Meier estimated proportion of patients who relapsed at 12 months and annualized relapse rate. Baseline covariates were used in estimating propensity scores. The data were divided into four strata using quartiles of propensity scores. After assessing for balance in baseline covariates between the treatment groups, Kaplan-Meier estimates of relapse and estimates of treatment effects were pooled across the four strata.
At the meeting, Jinny Min, PharmD, a medical postdoctoral research fellow at Biogen, reported results from 816 DMF patients, 781 FTY patients, and 1,042 GA patients. In the trimmed analysis set, the estimated proportion of DMF and FTY patients who relapsed at 12 months after treatment initiation was 12% vs. 13%, respectively (hazard ratio, 1.07, P = .693; the adjusted rate ratio for annualized relapse was 1.09, P = .617). In the analysis of DMF vs. GA patients, the estimated proportion of DMF patients that relapsed at 12 months was 12% vs. 21%, respectively (HR, 0.71), which represented a significant decrease of 29% (P less than .02). The adjusted rate ratio for annualized relapse was 0.69, representing a significant decrease of 31% (P less than .01).
“We hope that these data help health care providers and people living with MS as they consider their treatment options,” Dr. Hotermans said. “The limitations of this study are similar to those that would be present in other retrospective studies that utilize real-world data. However, we worked to mitigate many of those limitations through a propensity-score estimation approach to adjust for confounders. An additional limitation that is inherent to the study design (retrospective chart review) is that patients’ medical history, MS disease, treatment history, and relapse history were limited to the information available in the medical records.”
The study was supported by Biogen, which markets DMF. Dr. Hotermans and Dr. Min are employees of the company.
SOURCE: Min J et al. ACTRIMS Forum 2018, Abstract P016.
SAN DIEGO – Delayed-release dimethyl fumarate did not show any differences versus fingolimod in relapse rate over a 1-year, “real-world” study of patients with relapsing-remitting multiple sclerosis, but a significantly greater proportion of patients taking delayed-release dimethyl fumarate achieved relapse-free status and a lower annualized relapsed rate, compared with patients on glatiramer acetate
“There is a need for real-world data that compares the effectiveness of the growing number of MS [multiple sclerosis] treatment options,” Christophe Hotermans, MD, vice president of Global Medical Therapeutic Areas at Boston-based Biogen, said in an interview during ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “These results were consistent with previous analyses of efficacy of these treatments in people with relapsing-remitting MS, which showed no significant differences in efficacy between delayed-release dimethyl fumarate [DMF, Tecfidera] versus fingolimod [FTY, Gilenya] and greater efficacy with dimethyl fumarate, compared with glatiramer acetate [GA].”
The findings come from EFFECT (Observational Study to Characterize Real-world Clinical Outcomes With Relapsing-remitting Multiple Sclerosis), a multicenter, international, retrospective, single-time-point medical record review study comparing the effectiveness of DMF vs. other disease-modifying therapies, including FTY and GA in patients with relapsing-remitting MS.
Endpoints included the Kaplan-Meier estimated proportion of patients who relapsed at 12 months and annualized relapse rate. Baseline covariates were used in estimating propensity scores. The data were divided into four strata using quartiles of propensity scores. After assessing for balance in baseline covariates between the treatment groups, Kaplan-Meier estimates of relapse and estimates of treatment effects were pooled across the four strata.
At the meeting, Jinny Min, PharmD, a medical postdoctoral research fellow at Biogen, reported results from 816 DMF patients, 781 FTY patients, and 1,042 GA patients. In the trimmed analysis set, the estimated proportion of DMF and FTY patients who relapsed at 12 months after treatment initiation was 12% vs. 13%, respectively (hazard ratio, 1.07, P = .693; the adjusted rate ratio for annualized relapse was 1.09, P = .617). In the analysis of DMF vs. GA patients, the estimated proportion of DMF patients that relapsed at 12 months was 12% vs. 21%, respectively (HR, 0.71), which represented a significant decrease of 29% (P less than .02). The adjusted rate ratio for annualized relapse was 0.69, representing a significant decrease of 31% (P less than .01).
“We hope that these data help health care providers and people living with MS as they consider their treatment options,” Dr. Hotermans said. “The limitations of this study are similar to those that would be present in other retrospective studies that utilize real-world data. However, we worked to mitigate many of those limitations through a propensity-score estimation approach to adjust for confounders. An additional limitation that is inherent to the study design (retrospective chart review) is that patients’ medical history, MS disease, treatment history, and relapse history were limited to the information available in the medical records.”
The study was supported by Biogen, which markets DMF. Dr. Hotermans and Dr. Min are employees of the company.
SOURCE: Min J et al. ACTRIMS Forum 2018, Abstract P016.
SAN DIEGO – Delayed-release dimethyl fumarate did not show any differences versus fingolimod in relapse rate over a 1-year, “real-world” study of patients with relapsing-remitting multiple sclerosis, but a significantly greater proportion of patients taking delayed-release dimethyl fumarate achieved relapse-free status and a lower annualized relapsed rate, compared with patients on glatiramer acetate
“There is a need for real-world data that compares the effectiveness of the growing number of MS [multiple sclerosis] treatment options,” Christophe Hotermans, MD, vice president of Global Medical Therapeutic Areas at Boston-based Biogen, said in an interview during ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “These results were consistent with previous analyses of efficacy of these treatments in people with relapsing-remitting MS, which showed no significant differences in efficacy between delayed-release dimethyl fumarate [DMF, Tecfidera] versus fingolimod [FTY, Gilenya] and greater efficacy with dimethyl fumarate, compared with glatiramer acetate [GA].”
The findings come from EFFECT (Observational Study to Characterize Real-world Clinical Outcomes With Relapsing-remitting Multiple Sclerosis), a multicenter, international, retrospective, single-time-point medical record review study comparing the effectiveness of DMF vs. other disease-modifying therapies, including FTY and GA in patients with relapsing-remitting MS.
Endpoints included the Kaplan-Meier estimated proportion of patients who relapsed at 12 months and annualized relapse rate. Baseline covariates were used in estimating propensity scores. The data were divided into four strata using quartiles of propensity scores. After assessing for balance in baseline covariates between the treatment groups, Kaplan-Meier estimates of relapse and estimates of treatment effects were pooled across the four strata.
At the meeting, Jinny Min, PharmD, a medical postdoctoral research fellow at Biogen, reported results from 816 DMF patients, 781 FTY patients, and 1,042 GA patients. In the trimmed analysis set, the estimated proportion of DMF and FTY patients who relapsed at 12 months after treatment initiation was 12% vs. 13%, respectively (hazard ratio, 1.07, P = .693; the adjusted rate ratio for annualized relapse was 1.09, P = .617). In the analysis of DMF vs. GA patients, the estimated proportion of DMF patients that relapsed at 12 months was 12% vs. 21%, respectively (HR, 0.71), which represented a significant decrease of 29% (P less than .02). The adjusted rate ratio for annualized relapse was 0.69, representing a significant decrease of 31% (P less than .01).
“We hope that these data help health care providers and people living with MS as they consider their treatment options,” Dr. Hotermans said. “The limitations of this study are similar to those that would be present in other retrospective studies that utilize real-world data. However, we worked to mitigate many of those limitations through a propensity-score estimation approach to adjust for confounders. An additional limitation that is inherent to the study design (retrospective chart review) is that patients’ medical history, MS disease, treatment history, and relapse history were limited to the information available in the medical records.”
The study was supported by Biogen, which markets DMF. Dr. Hotermans and Dr. Min are employees of the company.
SOURCE: Min J et al. ACTRIMS Forum 2018, Abstract P016.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point:
Major finding: Delayed-release dimethyl fumarate had a 29% lower risk of relapse during the 12-month period vs. glatiramer acetate.
Study details: Results from a multicenter study of 816 delayed-release dimethyl fumarate patients, 781 fingolimod patients, and 1,042 glatiramer acetate patients with relapsing-remitting MS.
Disclosures: The study was supported by Biogen, which markets delayed-release dimethyl fumarate. Dr. Hotermans and Dr. Min are employees of the company.
Source: Min J et al. ACTRIMS Forum 2018, Abstract P016.
Trial of clozapine, risperidone halted in MS
SAN DIEGO – New Zealand researchers halted a small trial that was testing the use of the antipsychotics clozapine and risperidone to treat progressive multiple sclerosis because significant side effects caused participants to withdraw.
The adverse events appeared even though the doses were much smaller than those routinely given to patients with psychiatric illnesses. “The neurologists realized it was in the participants’ best interest to stop,” said study lead author Anne Camille La Flamme, PhD, of Victoria University of Wellington (New Zealand). “Adverse events included dizziness, muscle weakness, and falls.”
The researchers launched the study – a blinded, randomized, placebo-controlled trial – to learn whether the two antipsychotic drugs, also known by the brand names Clozaril and Risperdal, have potential as treatments for progressive multiple sclerosis.
Previous in-vitro research had linked the drugs to anti-inflammatory effects in the central nervous system, Dr. La Flamme said, and researchers believed that the progressive form of MS might be especially vulnerable to their effects because of high immune system involvement.
The researchers planned to randomly assign three groups of 12 patients per arm to placebo, clozapine, and risperidone.
For clozapine, “the doses were very low, much lower than you’d expect for psychiatric use,” Dr. La Flamme said. A typical dose for psychiatric disorders is about 350 mg/day, she said, and the trial aimed to use 100-150 mg/day with an eye toward preventing dose-dependent side effects.
As for risperidone, a typical dose is about 4 mg/day, and the trial began at 2 mg/day and would increase to 3.5 mg/day, she said.
Three subjects in the clozapine group had to withdraw within 2 weeks when their doses had only reached an average of 35 mg/day. Two of three in the risperidone group withdrew within 4 months.
In light of the adverse effects, “it was deemed not wise to continue,” Dr. La Flamme said.
The placebo group, meanwhile, completed the trial at 178 days and had adverse effects that were more indicative of MS, she said.
What happened? One possibility is that disability from MS made the adverse events more evident, Dr. La Flamme said. Another possible explanation is that the underlying MS physiology changed the targets of the medications, she said.
“We have no conclusive evidence that would suggest one over the other,” she said. “But a lot of the evidence supports the idea that it’s a change in the physiology, that something about those pathways has been altered.”
It’s clear, she said, that the doses of the drugs in the trial were not appropriate. However, a big question remains: “We do not know whether these medicines are effective at reducing neuroinflammation.”
It’s possible, she said, that a “whisper of a dose” could still be effective. “It may get back to how these agents metabolize and become an active form.”
The study was funded by New Zealand’s Ministry of Business, Innovation and Employment. Dr. La Flamme disclosed that the study team has a patent for repurposing of clozapine and risperidone to treat MS.
SOURCE: La Flamme A et al. ACTRIMS Forum 2018, abstract P031.
SAN DIEGO – New Zealand researchers halted a small trial that was testing the use of the antipsychotics clozapine and risperidone to treat progressive multiple sclerosis because significant side effects caused participants to withdraw.
The adverse events appeared even though the doses were much smaller than those routinely given to patients with psychiatric illnesses. “The neurologists realized it was in the participants’ best interest to stop,” said study lead author Anne Camille La Flamme, PhD, of Victoria University of Wellington (New Zealand). “Adverse events included dizziness, muscle weakness, and falls.”
The researchers launched the study – a blinded, randomized, placebo-controlled trial – to learn whether the two antipsychotic drugs, also known by the brand names Clozaril and Risperdal, have potential as treatments for progressive multiple sclerosis.
Previous in-vitro research had linked the drugs to anti-inflammatory effects in the central nervous system, Dr. La Flamme said, and researchers believed that the progressive form of MS might be especially vulnerable to their effects because of high immune system involvement.
The researchers planned to randomly assign three groups of 12 patients per arm to placebo, clozapine, and risperidone.
For clozapine, “the doses were very low, much lower than you’d expect for psychiatric use,” Dr. La Flamme said. A typical dose for psychiatric disorders is about 350 mg/day, she said, and the trial aimed to use 100-150 mg/day with an eye toward preventing dose-dependent side effects.
As for risperidone, a typical dose is about 4 mg/day, and the trial began at 2 mg/day and would increase to 3.5 mg/day, she said.
Three subjects in the clozapine group had to withdraw within 2 weeks when their doses had only reached an average of 35 mg/day. Two of three in the risperidone group withdrew within 4 months.
In light of the adverse effects, “it was deemed not wise to continue,” Dr. La Flamme said.
The placebo group, meanwhile, completed the trial at 178 days and had adverse effects that were more indicative of MS, she said.
What happened? One possibility is that disability from MS made the adverse events more evident, Dr. La Flamme said. Another possible explanation is that the underlying MS physiology changed the targets of the medications, she said.
“We have no conclusive evidence that would suggest one over the other,” she said. “But a lot of the evidence supports the idea that it’s a change in the physiology, that something about those pathways has been altered.”
It’s clear, she said, that the doses of the drugs in the trial were not appropriate. However, a big question remains: “We do not know whether these medicines are effective at reducing neuroinflammation.”
It’s possible, she said, that a “whisper of a dose” could still be effective. “It may get back to how these agents metabolize and become an active form.”
The study was funded by New Zealand’s Ministry of Business, Innovation and Employment. Dr. La Flamme disclosed that the study team has a patent for repurposing of clozapine and risperidone to treat MS.
SOURCE: La Flamme A et al. ACTRIMS Forum 2018, abstract P031.
SAN DIEGO – New Zealand researchers halted a small trial that was testing the use of the antipsychotics clozapine and risperidone to treat progressive multiple sclerosis because significant side effects caused participants to withdraw.
The adverse events appeared even though the doses were much smaller than those routinely given to patients with psychiatric illnesses. “The neurologists realized it was in the participants’ best interest to stop,” said study lead author Anne Camille La Flamme, PhD, of Victoria University of Wellington (New Zealand). “Adverse events included dizziness, muscle weakness, and falls.”
The researchers launched the study – a blinded, randomized, placebo-controlled trial – to learn whether the two antipsychotic drugs, also known by the brand names Clozaril and Risperdal, have potential as treatments for progressive multiple sclerosis.
Previous in-vitro research had linked the drugs to anti-inflammatory effects in the central nervous system, Dr. La Flamme said, and researchers believed that the progressive form of MS might be especially vulnerable to their effects because of high immune system involvement.
The researchers planned to randomly assign three groups of 12 patients per arm to placebo, clozapine, and risperidone.
For clozapine, “the doses were very low, much lower than you’d expect for psychiatric use,” Dr. La Flamme said. A typical dose for psychiatric disorders is about 350 mg/day, she said, and the trial aimed to use 100-150 mg/day with an eye toward preventing dose-dependent side effects.
As for risperidone, a typical dose is about 4 mg/day, and the trial began at 2 mg/day and would increase to 3.5 mg/day, she said.
Three subjects in the clozapine group had to withdraw within 2 weeks when their doses had only reached an average of 35 mg/day. Two of three in the risperidone group withdrew within 4 months.
In light of the adverse effects, “it was deemed not wise to continue,” Dr. La Flamme said.
The placebo group, meanwhile, completed the trial at 178 days and had adverse effects that were more indicative of MS, she said.
What happened? One possibility is that disability from MS made the adverse events more evident, Dr. La Flamme said. Another possible explanation is that the underlying MS physiology changed the targets of the medications, she said.
“We have no conclusive evidence that would suggest one over the other,” she said. “But a lot of the evidence supports the idea that it’s a change in the physiology, that something about those pathways has been altered.”
It’s clear, she said, that the doses of the drugs in the trial were not appropriate. However, a big question remains: “We do not know whether these medicines are effective at reducing neuroinflammation.”
It’s possible, she said, that a “whisper of a dose” could still be effective. “It may get back to how these agents metabolize and become an active form.”
The study was funded by New Zealand’s Ministry of Business, Innovation and Employment. Dr. La Flamme disclosed that the study team has a patent for repurposing of clozapine and risperidone to treat MS.
SOURCE: La Flamme A et al. ACTRIMS Forum 2018, abstract P031.
REPORTING FROM ACTRIMS Forum 2018
MAGNIMS and McDonald Criteria Have Similar Accuracy
The 2016 Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) criteria have accuracy similar to that of the 2010 McDonald criteria in predicting the development of clinically definite multiple sclerosis (MS), according to a retrospective study.
“Among the different modifications proposed, our results support removal of the distinction between symptomatic and asymptomatic lesions, which simplifies the clinical use of MRI criteria, and suggest that further consideration be given to increasing the number of lesions needed to define periventricular involvement from one to three, because this might slightly increase specificity,” said Massimo Filippi, MD, of the neuroimaging research unit in the division of neuroscience at San Raffaele Scientific Institute at Vita-Salute San Raffaele University in Milan, and colleagues. The report was published online ahead of print December 21, 2017, in Lancet Neurology. “Further effort is still needed to improve cortical lesion assessment, and more studies should be done to evaluate the effect of including optic nerve assessment as an additional dissemination in space [DIS] criterion.”
An Effort to Draft Revisions
In an effort to guide revisions of MS diagnostic criteria, Dr. Filippi and other members of the MAGNIMS network compared the performance of the 2010 McDonald and 2016 MAGNIMS criteria for MS in a cohort of 368 patients with clinically isolated syndrome (CIS) who were screened between June 16, 1995, and January 27, 2017. They used a time-dependent receiver operating characteristic curve analysis to evaluate MRI criteria performance for DIS, dissemination in time (DIT), and DIS plus DIT. Changes to the DIS definition contained in the 2016 MAGNIMS criteria included removal of the distinction between symptomatic and asymptomatic lesions, increasing the number of lesions needed to define periventricular involvement to three, combining cortical and juxtacortical lesions, and inclusion of optic nerve evaluation. For DIT, removal of the distinction between symptomatic and asymptomatic lesions was suggested.
Dr. Filippi and his coauthors at eight centers reported that 189 (51%) of the 368 patients had developed clinically definite MS by the last evaluation, which occurred at a median of 50 months from baseline. At 36 months, DIS alone showed high sensitivity in the 2010 McDonald and 2016 MAGNIMS criteria (91% vs 93%, respectively), similar specificity (33% vs 32%), and similar area under the curve values (AUC, 0.62 vs 0.63). Inclusion of symptomatic lesions did not alter performance. The researchers also found that requiring three periventricular lesions reduced sensitivity to 85% and increased specificity to 40%, but did not affect AUC values (AUC, 0.63). When optic nerve evaluation was included, sensitivity was similar (92%), while specificity decreased to 26%, and AUC declined to 0.59.
The 2016 MAGNIMS and 2010 McDonald criteria achieved similar sensitivity, specificity, and AUC values when compared on the performance of DIT criteria and DIS plus DIT criteria.
“For both sets of criteria, specificity was lower than that of previous studies that evaluated the diagnostic performance of the 2010 McDonald criteria,” the authors wrote. “Several factors could help explain our findings, including the different follow-up durations, the statistical methods (eg, using a time-to-event analysis in our study), and the effect of treatment, which might have delayed or prevented the occurrence of the second attack during the study period.” They acknowledged certain limitations of the study, including its retrospective design and the fact that patients were recruited in highly specialized centers, which may have resulted in the selection of patients at higher risk of conversion to clinically definite MS.
MRI Abnormalities May Not Indicate MS
As MS diagnosis evolves, revisions to existing diagnostic criteria have increased sensitivity, thus helping clinicians establish earlier diagnoses. Although the study by Dr. Filippi et al showed that for both sets of MRI criteria, sensitivity was greater than specificity for predicting clinically definite MS, the modest specificity is cause for concern, said Anne H. Cross, MD, Head of the Neuroimmunology (MS) Section, and Robert N. Naismith, MD, Associate Professor of Neurology, both at Washington University in St. Louis, in an accompanying editorial. They cited one study that emphasized the importance of not misdiagnosing other CNS diseases as MS. “In that study at four academic medical centers, 110 people seen over a period of less than 1.5 years were found to have been misdiagnosed,” said Drs. Cross and Naismith. “[Seventy percent] of the 110 individuals had received disease-modifying therapy, and 31% had unnecessary morbidity. Leading factors contributing to erroneous diagnosis in the study included overreliance on MRI abnormalities in patients with nonspecific neurologic symptoms.”
Vascular and other diseases can cause MRI abnormalities that could meet the 2016 MAGNIMS recommendations or the 2010 and 2017 McDonald MRI criteria, the authors continued. For example, patients with monophasic inflammatory and infectious diseases might have gadolinium-enhancing lesions that meet the 2017 McDonald criteria for DIT, which require only the simultaneous presence of gadolinium-enhancing and gadolinium-negative lesions in the proper locations. For patients with an atypical presentation who meet the 2010 and 2017 McDonald or 2016 MAGNIMS recommendations, clinicians should weigh all of the observed imaging features (including the number of periventricular lesions, along with lesion size, shape, and location) to improve diagnostic specificity and help to limit misdiagnoses, they concluded.
—Doug Brunk
Suggested Reading
Cross AH, Naismith RT. Refining the use of MRI to predict multiple sclerosis. Lancet Neurol. 2017 Dec 21 [Epub ahead of print].
Filippi M, Preziosa P, Meani A, et al. Prediction of a multiple sclerosis diagnosis in patients with clinically isolated syndrome using the 2016 MAGNIMS and 2010 McDonald criteria: a retrospective study. Lancet Neurol. 2017 Dec 21 [Epub ahead of print].
The 2016 Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) criteria have accuracy similar to that of the 2010 McDonald criteria in predicting the development of clinically definite multiple sclerosis (MS), according to a retrospective study.
“Among the different modifications proposed, our results support removal of the distinction between symptomatic and asymptomatic lesions, which simplifies the clinical use of MRI criteria, and suggest that further consideration be given to increasing the number of lesions needed to define periventricular involvement from one to three, because this might slightly increase specificity,” said Massimo Filippi, MD, of the neuroimaging research unit in the division of neuroscience at San Raffaele Scientific Institute at Vita-Salute San Raffaele University in Milan, and colleagues. The report was published online ahead of print December 21, 2017, in Lancet Neurology. “Further effort is still needed to improve cortical lesion assessment, and more studies should be done to evaluate the effect of including optic nerve assessment as an additional dissemination in space [DIS] criterion.”
An Effort to Draft Revisions
In an effort to guide revisions of MS diagnostic criteria, Dr. Filippi and other members of the MAGNIMS network compared the performance of the 2010 McDonald and 2016 MAGNIMS criteria for MS in a cohort of 368 patients with clinically isolated syndrome (CIS) who were screened between June 16, 1995, and January 27, 2017. They used a time-dependent receiver operating characteristic curve analysis to evaluate MRI criteria performance for DIS, dissemination in time (DIT), and DIS plus DIT. Changes to the DIS definition contained in the 2016 MAGNIMS criteria included removal of the distinction between symptomatic and asymptomatic lesions, increasing the number of lesions needed to define periventricular involvement to three, combining cortical and juxtacortical lesions, and inclusion of optic nerve evaluation. For DIT, removal of the distinction between symptomatic and asymptomatic lesions was suggested.
Dr. Filippi and his coauthors at eight centers reported that 189 (51%) of the 368 patients had developed clinically definite MS by the last evaluation, which occurred at a median of 50 months from baseline. At 36 months, DIS alone showed high sensitivity in the 2010 McDonald and 2016 MAGNIMS criteria (91% vs 93%, respectively), similar specificity (33% vs 32%), and similar area under the curve values (AUC, 0.62 vs 0.63). Inclusion of symptomatic lesions did not alter performance. The researchers also found that requiring three periventricular lesions reduced sensitivity to 85% and increased specificity to 40%, but did not affect AUC values (AUC, 0.63). When optic nerve evaluation was included, sensitivity was similar (92%), while specificity decreased to 26%, and AUC declined to 0.59.
The 2016 MAGNIMS and 2010 McDonald criteria achieved similar sensitivity, specificity, and AUC values when compared on the performance of DIT criteria and DIS plus DIT criteria.
“For both sets of criteria, specificity was lower than that of previous studies that evaluated the diagnostic performance of the 2010 McDonald criteria,” the authors wrote. “Several factors could help explain our findings, including the different follow-up durations, the statistical methods (eg, using a time-to-event analysis in our study), and the effect of treatment, which might have delayed or prevented the occurrence of the second attack during the study period.” They acknowledged certain limitations of the study, including its retrospective design and the fact that patients were recruited in highly specialized centers, which may have resulted in the selection of patients at higher risk of conversion to clinically definite MS.
MRI Abnormalities May Not Indicate MS
As MS diagnosis evolves, revisions to existing diagnostic criteria have increased sensitivity, thus helping clinicians establish earlier diagnoses. Although the study by Dr. Filippi et al showed that for both sets of MRI criteria, sensitivity was greater than specificity for predicting clinically definite MS, the modest specificity is cause for concern, said Anne H. Cross, MD, Head of the Neuroimmunology (MS) Section, and Robert N. Naismith, MD, Associate Professor of Neurology, both at Washington University in St. Louis, in an accompanying editorial. They cited one study that emphasized the importance of not misdiagnosing other CNS diseases as MS. “In that study at four academic medical centers, 110 people seen over a period of less than 1.5 years were found to have been misdiagnosed,” said Drs. Cross and Naismith. “[Seventy percent] of the 110 individuals had received disease-modifying therapy, and 31% had unnecessary morbidity. Leading factors contributing to erroneous diagnosis in the study included overreliance on MRI abnormalities in patients with nonspecific neurologic symptoms.”
Vascular and other diseases can cause MRI abnormalities that could meet the 2016 MAGNIMS recommendations or the 2010 and 2017 McDonald MRI criteria, the authors continued. For example, patients with monophasic inflammatory and infectious diseases might have gadolinium-enhancing lesions that meet the 2017 McDonald criteria for DIT, which require only the simultaneous presence of gadolinium-enhancing and gadolinium-negative lesions in the proper locations. For patients with an atypical presentation who meet the 2010 and 2017 McDonald or 2016 MAGNIMS recommendations, clinicians should weigh all of the observed imaging features (including the number of periventricular lesions, along with lesion size, shape, and location) to improve diagnostic specificity and help to limit misdiagnoses, they concluded.
—Doug Brunk
Suggested Reading
Cross AH, Naismith RT. Refining the use of MRI to predict multiple sclerosis. Lancet Neurol. 2017 Dec 21 [Epub ahead of print].
Filippi M, Preziosa P, Meani A, et al. Prediction of a multiple sclerosis diagnosis in patients with clinically isolated syndrome using the 2016 MAGNIMS and 2010 McDonald criteria: a retrospective study. Lancet Neurol. 2017 Dec 21 [Epub ahead of print].
The 2016 Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) criteria have accuracy similar to that of the 2010 McDonald criteria in predicting the development of clinically definite multiple sclerosis (MS), according to a retrospective study.
“Among the different modifications proposed, our results support removal of the distinction between symptomatic and asymptomatic lesions, which simplifies the clinical use of MRI criteria, and suggest that further consideration be given to increasing the number of lesions needed to define periventricular involvement from one to three, because this might slightly increase specificity,” said Massimo Filippi, MD, of the neuroimaging research unit in the division of neuroscience at San Raffaele Scientific Institute at Vita-Salute San Raffaele University in Milan, and colleagues. The report was published online ahead of print December 21, 2017, in Lancet Neurology. “Further effort is still needed to improve cortical lesion assessment, and more studies should be done to evaluate the effect of including optic nerve assessment as an additional dissemination in space [DIS] criterion.”
An Effort to Draft Revisions
In an effort to guide revisions of MS diagnostic criteria, Dr. Filippi and other members of the MAGNIMS network compared the performance of the 2010 McDonald and 2016 MAGNIMS criteria for MS in a cohort of 368 patients with clinically isolated syndrome (CIS) who were screened between June 16, 1995, and January 27, 2017. They used a time-dependent receiver operating characteristic curve analysis to evaluate MRI criteria performance for DIS, dissemination in time (DIT), and DIS plus DIT. Changes to the DIS definition contained in the 2016 MAGNIMS criteria included removal of the distinction between symptomatic and asymptomatic lesions, increasing the number of lesions needed to define periventricular involvement to three, combining cortical and juxtacortical lesions, and inclusion of optic nerve evaluation. For DIT, removal of the distinction between symptomatic and asymptomatic lesions was suggested.
Dr. Filippi and his coauthors at eight centers reported that 189 (51%) of the 368 patients had developed clinically definite MS by the last evaluation, which occurred at a median of 50 months from baseline. At 36 months, DIS alone showed high sensitivity in the 2010 McDonald and 2016 MAGNIMS criteria (91% vs 93%, respectively), similar specificity (33% vs 32%), and similar area under the curve values (AUC, 0.62 vs 0.63). Inclusion of symptomatic lesions did not alter performance. The researchers also found that requiring three periventricular lesions reduced sensitivity to 85% and increased specificity to 40%, but did not affect AUC values (AUC, 0.63). When optic nerve evaluation was included, sensitivity was similar (92%), while specificity decreased to 26%, and AUC declined to 0.59.
The 2016 MAGNIMS and 2010 McDonald criteria achieved similar sensitivity, specificity, and AUC values when compared on the performance of DIT criteria and DIS plus DIT criteria.
“For both sets of criteria, specificity was lower than that of previous studies that evaluated the diagnostic performance of the 2010 McDonald criteria,” the authors wrote. “Several factors could help explain our findings, including the different follow-up durations, the statistical methods (eg, using a time-to-event analysis in our study), and the effect of treatment, which might have delayed or prevented the occurrence of the second attack during the study period.” They acknowledged certain limitations of the study, including its retrospective design and the fact that patients were recruited in highly specialized centers, which may have resulted in the selection of patients at higher risk of conversion to clinically definite MS.
MRI Abnormalities May Not Indicate MS
As MS diagnosis evolves, revisions to existing diagnostic criteria have increased sensitivity, thus helping clinicians establish earlier diagnoses. Although the study by Dr. Filippi et al showed that for both sets of MRI criteria, sensitivity was greater than specificity for predicting clinically definite MS, the modest specificity is cause for concern, said Anne H. Cross, MD, Head of the Neuroimmunology (MS) Section, and Robert N. Naismith, MD, Associate Professor of Neurology, both at Washington University in St. Louis, in an accompanying editorial. They cited one study that emphasized the importance of not misdiagnosing other CNS diseases as MS. “In that study at four academic medical centers, 110 people seen over a period of less than 1.5 years were found to have been misdiagnosed,” said Drs. Cross and Naismith. “[Seventy percent] of the 110 individuals had received disease-modifying therapy, and 31% had unnecessary morbidity. Leading factors contributing to erroneous diagnosis in the study included overreliance on MRI abnormalities in patients with nonspecific neurologic symptoms.”
Vascular and other diseases can cause MRI abnormalities that could meet the 2016 MAGNIMS recommendations or the 2010 and 2017 McDonald MRI criteria, the authors continued. For example, patients with monophasic inflammatory and infectious diseases might have gadolinium-enhancing lesions that meet the 2017 McDonald criteria for DIT, which require only the simultaneous presence of gadolinium-enhancing and gadolinium-negative lesions in the proper locations. For patients with an atypical presentation who meet the 2010 and 2017 McDonald or 2016 MAGNIMS recommendations, clinicians should weigh all of the observed imaging features (including the number of periventricular lesions, along with lesion size, shape, and location) to improve diagnostic specificity and help to limit misdiagnoses, they concluded.
—Doug Brunk
Suggested Reading
Cross AH, Naismith RT. Refining the use of MRI to predict multiple sclerosis. Lancet Neurol. 2017 Dec 21 [Epub ahead of print].
Filippi M, Preziosa P, Meani A, et al. Prediction of a multiple sclerosis diagnosis in patients with clinically isolated syndrome using the 2016 MAGNIMS and 2010 McDonald criteria: a retrospective study. Lancet Neurol. 2017 Dec 21 [Epub ahead of print].
Rituximab May Provide Greater Benefits Than Other First-Line MS Therapies
Rituximab is associated with a lower drug discontinuation rate than all other commonly prescribed disease-modifying treatments (DMTs) used as initial therapy for relapsing-remitting multiple sclerosis (MS), according to a retrospective study of patient data from a Swedish MS registry.
In addition, relapse rates were lower with rituximab (Rituxan) than they were with injectable DMTs and dimethyl fumarate (Tecfidera), according to the study, which was published online ahead of print January 8 in JAMA Neurology.
The results suggest that rituximab “can be considered an option” for treatment-naive patients with relapsing-remitting MS, according to Mathias Granqvist, MD, a graduate student in the Department of Clinical Neuroscience at the Karolinska Institute in Stockholm, and his coauthors.
Anti-CD20 agents such as rituximab are “likely to become an additional treatment option” for relapsing-remitting MS, and off-label use of rituximab for this indication has “increased considerably” in Sweden in recent years, the investigators said. Relapsing-remitting MS is not an approved indication for rituximab in the United States, but in Sweden “there is no difference in reimbursement policy ... because all DMTs are covered by the national health insurance, including off-label medications.”
The emergence of new DMTs for relapsing-remitting MS has changed the treatment landscape recently, although in real-world practice, there is a lack of “detailed knowledge about how to tailor therapy,” the authors said. They noted that the majority of patients discontinue traditional first-line treatment with injectable DMTs (ie, interferon beta and glatiramer acetate) within two years, suggesting a need for better treatment options.
To evaluate the real-world effectiveness of rituximab in this setting, Dr. Granqvist and his colleagues selected patient registry data for two Swedish counties that included 494 participants who received a diagnosis of relapsing-remitting MS between January 1, 2012, and October 31, 2015.
The largest subset of patients (215) received injectable DMTs, while 120 received rituximab, 86 received dimethyl fumarate, 50 received natalizumab (Tysabri), 17 received fingolimod (Gilenya), and six received other treatments, according to the authors.
The proportion of patients who stayed on treatment was significantly higher for rituximab versus all other DMTs. Compared with rituximab, the hazard ratios for drug discontinuation, after adjusting for covariates and propensity score, were 11.4 for injectable DMTs, 15.1 for dimethyl fumarate, 5.9 for fingolimod, and 11.3 for natalizumab.
Rituximab-treated patients also had lower rates of clinical relapse, neuroradiologic disease activity, and adverse events, compared with patients who received injectable DMTs or dimethyl fumarate, according to the investigators.
In comparison with fingolimod and natalizumab, rituximab was associated with lower relapse rates and fewer gadolinium-enhancing lesions, but those differences did not reach statistical significance in all analyses.
The study was funded by the Swedish Medical Research Council, among other sources. Study authors reported conflicts of interest related to Biogen, Novartis, and Genzyme.
—Andrew D. Bowser
Suggested Reading
Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018 Jan 8 [Epub ahead of print].
Rituximab is associated with a lower drug discontinuation rate than all other commonly prescribed disease-modifying treatments (DMTs) used as initial therapy for relapsing-remitting multiple sclerosis (MS), according to a retrospective study of patient data from a Swedish MS registry.
In addition, relapse rates were lower with rituximab (Rituxan) than they were with injectable DMTs and dimethyl fumarate (Tecfidera), according to the study, which was published online ahead of print January 8 in JAMA Neurology.
The results suggest that rituximab “can be considered an option” for treatment-naive patients with relapsing-remitting MS, according to Mathias Granqvist, MD, a graduate student in the Department of Clinical Neuroscience at the Karolinska Institute in Stockholm, and his coauthors.
Anti-CD20 agents such as rituximab are “likely to become an additional treatment option” for relapsing-remitting MS, and off-label use of rituximab for this indication has “increased considerably” in Sweden in recent years, the investigators said. Relapsing-remitting MS is not an approved indication for rituximab in the United States, but in Sweden “there is no difference in reimbursement policy ... because all DMTs are covered by the national health insurance, including off-label medications.”
The emergence of new DMTs for relapsing-remitting MS has changed the treatment landscape recently, although in real-world practice, there is a lack of “detailed knowledge about how to tailor therapy,” the authors said. They noted that the majority of patients discontinue traditional first-line treatment with injectable DMTs (ie, interferon beta and glatiramer acetate) within two years, suggesting a need for better treatment options.
To evaluate the real-world effectiveness of rituximab in this setting, Dr. Granqvist and his colleagues selected patient registry data for two Swedish counties that included 494 participants who received a diagnosis of relapsing-remitting MS between January 1, 2012, and October 31, 2015.
The largest subset of patients (215) received injectable DMTs, while 120 received rituximab, 86 received dimethyl fumarate, 50 received natalizumab (Tysabri), 17 received fingolimod (Gilenya), and six received other treatments, according to the authors.
The proportion of patients who stayed on treatment was significantly higher for rituximab versus all other DMTs. Compared with rituximab, the hazard ratios for drug discontinuation, after adjusting for covariates and propensity score, were 11.4 for injectable DMTs, 15.1 for dimethyl fumarate, 5.9 for fingolimod, and 11.3 for natalizumab.
Rituximab-treated patients also had lower rates of clinical relapse, neuroradiologic disease activity, and adverse events, compared with patients who received injectable DMTs or dimethyl fumarate, according to the investigators.
In comparison with fingolimod and natalizumab, rituximab was associated with lower relapse rates and fewer gadolinium-enhancing lesions, but those differences did not reach statistical significance in all analyses.
The study was funded by the Swedish Medical Research Council, among other sources. Study authors reported conflicts of interest related to Biogen, Novartis, and Genzyme.
—Andrew D. Bowser
Suggested Reading
Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018 Jan 8 [Epub ahead of print].
Rituximab is associated with a lower drug discontinuation rate than all other commonly prescribed disease-modifying treatments (DMTs) used as initial therapy for relapsing-remitting multiple sclerosis (MS), according to a retrospective study of patient data from a Swedish MS registry.
In addition, relapse rates were lower with rituximab (Rituxan) than they were with injectable DMTs and dimethyl fumarate (Tecfidera), according to the study, which was published online ahead of print January 8 in JAMA Neurology.
The results suggest that rituximab “can be considered an option” for treatment-naive patients with relapsing-remitting MS, according to Mathias Granqvist, MD, a graduate student in the Department of Clinical Neuroscience at the Karolinska Institute in Stockholm, and his coauthors.
Anti-CD20 agents such as rituximab are “likely to become an additional treatment option” for relapsing-remitting MS, and off-label use of rituximab for this indication has “increased considerably” in Sweden in recent years, the investigators said. Relapsing-remitting MS is not an approved indication for rituximab in the United States, but in Sweden “there is no difference in reimbursement policy ... because all DMTs are covered by the national health insurance, including off-label medications.”
The emergence of new DMTs for relapsing-remitting MS has changed the treatment landscape recently, although in real-world practice, there is a lack of “detailed knowledge about how to tailor therapy,” the authors said. They noted that the majority of patients discontinue traditional first-line treatment with injectable DMTs (ie, interferon beta and glatiramer acetate) within two years, suggesting a need for better treatment options.
To evaluate the real-world effectiveness of rituximab in this setting, Dr. Granqvist and his colleagues selected patient registry data for two Swedish counties that included 494 participants who received a diagnosis of relapsing-remitting MS between January 1, 2012, and October 31, 2015.
The largest subset of patients (215) received injectable DMTs, while 120 received rituximab, 86 received dimethyl fumarate, 50 received natalizumab (Tysabri), 17 received fingolimod (Gilenya), and six received other treatments, according to the authors.
The proportion of patients who stayed on treatment was significantly higher for rituximab versus all other DMTs. Compared with rituximab, the hazard ratios for drug discontinuation, after adjusting for covariates and propensity score, were 11.4 for injectable DMTs, 15.1 for dimethyl fumarate, 5.9 for fingolimod, and 11.3 for natalizumab.
Rituximab-treated patients also had lower rates of clinical relapse, neuroradiologic disease activity, and adverse events, compared with patients who received injectable DMTs or dimethyl fumarate, according to the investigators.
In comparison with fingolimod and natalizumab, rituximab was associated with lower relapse rates and fewer gadolinium-enhancing lesions, but those differences did not reach statistical significance in all analyses.
The study was funded by the Swedish Medical Research Council, among other sources. Study authors reported conflicts of interest related to Biogen, Novartis, and Genzyme.
—Andrew D. Bowser
Suggested Reading
Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018 Jan 8 [Epub ahead of print].
MS: Past, Present, and Future
Stuart D. Cook, MD, and Abdul Rahman Alchaki
Dr. Cook is the Ruth Dunietz Kushner and Michael Jay Serwitz Professor of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark. Dr. Alchaki is a resident in the Deptartment of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark.
Disclosure: Stuart Cook has received honoraria for lectures from Bayer HealthCare and Merck Serono. He has served as a consultant for Merck Serono, Bayer HealthCare, Teva, Novartis, Sanofi-Aventis, Biogen Idec, and Actinobac Biomed. He has served on steering committees for the BEYOND and CLARITY Studies and as a member of Advisory Boards for Merck Serono, Bayer HealthCare, Teva, Biogen Idec, Sanofi Aventis, and Actinobac Biomed.
The Initial Years (1838 to 1930s)
The earliest recognition of MS clinical features and pathology was attributed to Jean-Martin Charcot, Robert Carswell, and Jean Cruveilhier in Europe from 1838 to 1868. Beyond those early descriptions, relatively few MS breakthroughs occurred until the 1930s, when Thomas Rivers discovered experimental autoimmune encephalomyelitis (EAE), a demyelinating disease, in animals. His insightful concepts were widely cited and ultimately contributed to undestanding of the immune mechanisms of MS and acute disseminated encephalomyelitis (ADEM).
Advances in Diagnosis (1965 to 1992)
In 1965, Schumacher et al provided the essential clinical criteria for MS diagnosis. Poser et al refined these criteria in 1983. In 2001, McDonald et al added neuroimaging, CSF analysis, and evoked potentials to further complement MS clinical diagnosis. For the first time, the disease could generally be recognized.
Early Treatments
Various treatments for MS were tried over the years, without great success. However, in 1953, a small descriptive trial by Miller and Gibbons reported clinical benefits in patients using intramuscular (IM) adrenocorticotropic hormone (ACTH) for MS and disseminated encephalomyelitis. This was followed in 1970 by a Cooperative Study of IM ACTH versus placebo by Rose et al, which resulted in ACTH, and subsequently oral corticosteroids, being widely used to treat MS, particularly for acute exacerbations of the disease. However, robust evidence of long-term steroids remain limited, even to the present.
High-Dose Steroids
By 1980, the initial descriptive treatment of high-dose intravenous (IV) steroids for demyelinating diseases, including MS and transverse myelitis, by Dowling et al resulted in rapid clinical improvement in some patients. This result was ultimately confirmed by others. High-dose IV steroids became the gold standard for acute attacks, particularly those aggressive in nature. In the mid 1980s, work by Troiano et al, as well as others, showed that the rapid use of high-dose IV as well as oral steroids showed similar effects, with reduction or elimination of CT contrast-enhancing lesions within as few as eight hours, while lower doses or alternative-day treatments were less effective. In addition, descriptive studies of immune modulatory and immunosuppressive drugs, as well as small randomized studies, were published. These agents did not receive FDA approval.
The Golden Age of Therapy (1993 to 2018)
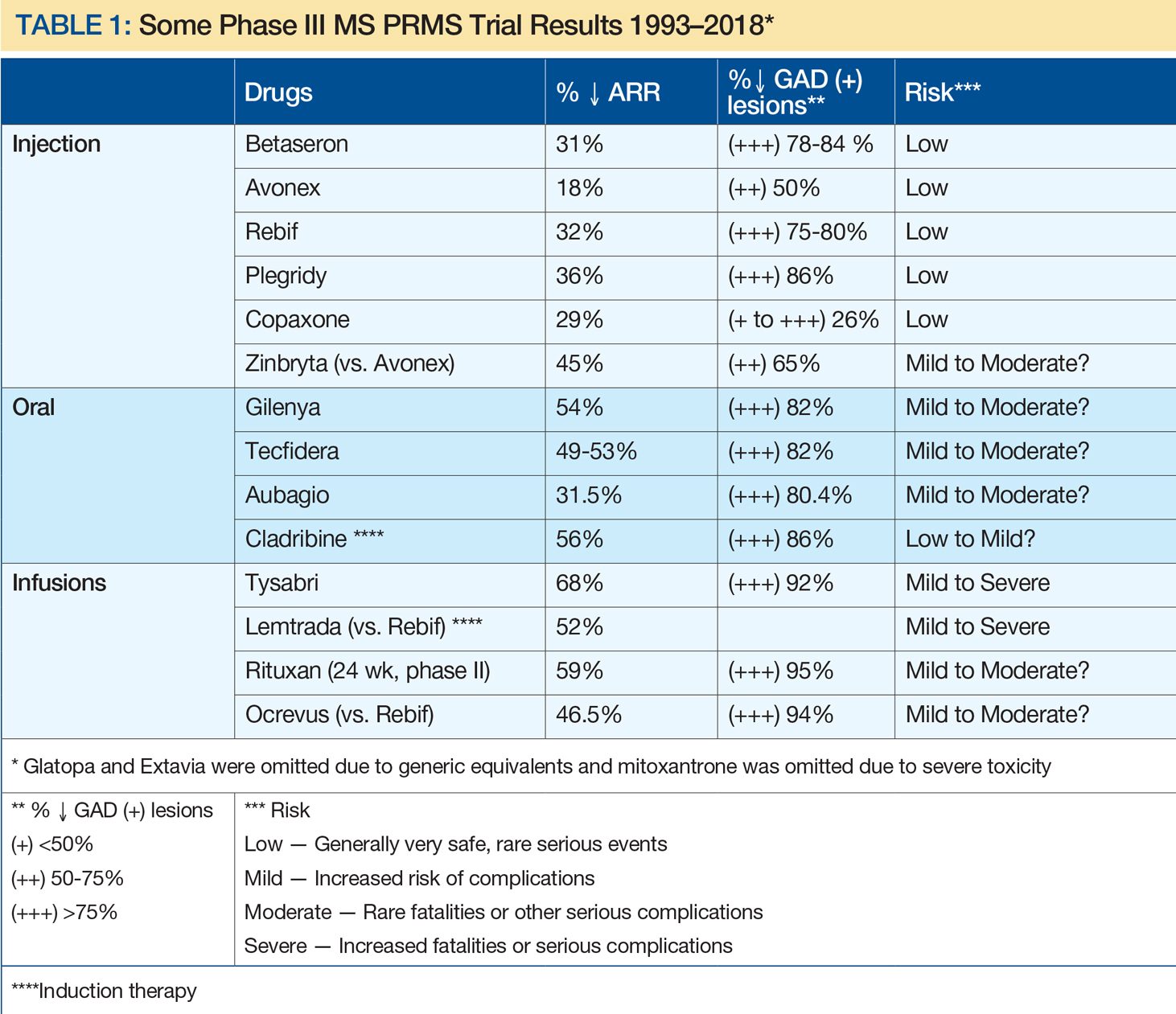
A remarkable era in MS prognosis and treatment began with immunomodulation injections of Betaseron (INFβ-1b), Avonex (INFβ-1a), and Copaxone (glatiramer acetate). This can be attributed, at least in part, to advances in molecular biology, genetics, and neuroimaging, and support by corporate, private, and public funding. Since the initial FDA approval of INFβ-1b, 15 MS therapies have become clinically available, including eight injectables, three orals, and four infusion treatments (see Table 1). In addition, two other drugs have been FDA approved for uses other than MS: rituximab (approved for lymphoma) and cladribine (for hairy cell leukemia), with the latter now approved by the European Medicines Agency for MS. Table 1 depicts characteristics of these therapies approved by US or European agencies (or for other disorders increasingly used off label for MS) in an attempt to compare annual relapse rates (ARR) and decreases in the percent of gadolinium-enhancing MS lesions versus placebo. This information was chosen because ARR has been uniformly selected and defined for such trials, while percent decrease of gadolinium-enhancing lesions on MRI has been the most sensitive barometer available for assessing acute clinical activity. As a result, risk-benefit considerations have been critical in evaluating these drug treatments, with efficacy improving greatly over time, whereas risks have been more variable.
Disease Categories
In 1996, Lublin and Reingold provided a new classification, not specifically for the diagnosis of MS, but rather for the clinical course of the disease. Initially, there were four categories—relapsing-remitting MS, secondary progressive MS, primary progressive MS, and progressive-relapsing MS—that were universally identified. These were thought to be relatively distinct clinical categories, but over time it became clear that the classification did not fully distinguish MS disease activity within these categories. For that reason, it was subsequently recommended, by Lincoln et al in 2009 and Cook et al in 2012, to include MRI, a vastly more sensitive modality, as well as clinical data in assessing disease activity.
On another note, MS and neuromyelitis optica (NMO), although having similar features, were clearly identified as different diseases by Lennon et al in 2004. Differences in pathology, clinical characteristics, immunology, and therapy separate the two disorders.
MRI in MS
Work by Young et al in 1981 established the central role of MRI brain imaging in MS diagnosis and therapeutic considerations. Since then it has become ubiquitous.
An example of a sensitive and highly productive MRI protocol is the BECOME study of MS and clinically isolated syndrome by Cadavid et al from 2009 to 2017. In this study, IFNβ-1b was compared with glatiramer acetate treatment. Cadavid et al used a 3T scanner with triple-dose gadolinium, performed monthly for as long as 24 consecutive months. This unique study brought about a virtual gold mine of valuable research and clinical information. This included proof that gadolinium-enhancing lesions persisted for six months or more, evidence of a 30:1 ratio of new MRI brain lesions to clinical activity, and documentation that 96% of T2 lesions and black holes derive from prior gadolinium-enhancing lesions. It was further noted that 80% to 90% of acute black holes disappeared with treatment and 75% to 80% of patients taking IFNβ-1b or glatiramer acetate had new MRI lesions despite continuing treatment. Perhaps most interestingly, monthly MRIs could predict relapse and disability in a relatively small number of patients, depending upon the frequency and activity of MRI lesions. In 2017, Brown et al documented that magnetization transfer ratio recovery in MS brain lesions occurred more significantly with glatiramer acetate than with IFNβ-1b, whereas more chronic black hole lesions were found with glatiramer acetate. Also in 2017, Maranzano et al found evidence of acute inflammatory leukocortical lesions, which were not as well recognized previously.
In summary, it has become increasingly clear that MRI is the most sen
The Future of MS
While it is not yet a curable disease, there is growing evidence that MS prognosis has improved and will continue to improve. This is based on incremental decreases in acute MS exacerbations, progressive disability, and MRI lesion activity, as well as a combination of the three—no evidence of disease activity (NEDA).
Not only are drug therapies becoming more effective, but patients and physicians now have many more treatment options to carefully consider with regard to efficacy, side effect profiles, treatment frequency, route of administration, cost, and quality of life. Newer drugs with different mechanisms of action such as cladribine, now approved in Europe, fulfill most of these beneficial criteria (see Giovannoni et al, 2010). More promising MS treatments, including long-acting induction therapies, are still being evaluated. As with other complex diseases, multiple therapies are likely to be used as well.
In summary, compared with the time before 1993, MS will be much less likely to be a progressive disease, and quality of life will be much improved. In my opinion, patients will be less fearful about their prognosis than ever before, and with appropriate evaluations and treatments, we may realize that disabling MS will be far less common.
Suggested Reading
Brown JW, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140(2):387-398.
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983.
Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5-7, 2010. Int J MS Care. 2012;14(3):105-114.
Dowling PC, Bosch VV, Cook SD. Possible beneficial effect of high-dose intravenous steroid therapy in acute demyelinating disease and transverse myelitis. Neurology. 1980;30(7 Pt 2):33-36.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112.
Lincoln JA, Cadavid D, Pollard J, et al. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66(3):412-414.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911.
Maranzano J, Rudko DA, Nakamura K, et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714-721.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
Miller HG, Gibbons JL. Acute disseminated encephalomyelitis and acute disseminated sclerosis; results of treatment with A.C.T.H. Br Med J. 1953;2(4850):1345-1348.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20(5):1-59.
Troiano R, Hafstein M, Ruderman M, et al. Effect of high-dose intravenous steroid administration on contrast-enhancing computed tomographic scan lesions in multiple sclerosis. Ann Neurol. 1984;15(3):257-263.
Troiano RA, Hafstein MP, Zito G, et al. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. J Neurol Sci. 1985;70(1):67-72.
Young IR, Hall AS, Pallis A, et al. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. 1981;2(8255):1063-1066.
Stuart D. Cook, MD, and Abdul Rahman Alchaki
Dr. Cook is the Ruth Dunietz Kushner and Michael Jay Serwitz Professor of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark. Dr. Alchaki is a resident in the Deptartment of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark.
Disclosure: Stuart Cook has received honoraria for lectures from Bayer HealthCare and Merck Serono. He has served as a consultant for Merck Serono, Bayer HealthCare, Teva, Novartis, Sanofi-Aventis, Biogen Idec, and Actinobac Biomed. He has served on steering committees for the BEYOND and CLARITY Studies and as a member of Advisory Boards for Merck Serono, Bayer HealthCare, Teva, Biogen Idec, Sanofi Aventis, and Actinobac Biomed.
The Initial Years (1838 to 1930s)
The earliest recognition of MS clinical features and pathology was attributed to Jean-Martin Charcot, Robert Carswell, and Jean Cruveilhier in Europe from 1838 to 1868. Beyond those early descriptions, relatively few MS breakthroughs occurred until the 1930s, when Thomas Rivers discovered experimental autoimmune encephalomyelitis (EAE), a demyelinating disease, in animals. His insightful concepts were widely cited and ultimately contributed to undestanding of the immune mechanisms of MS and acute disseminated encephalomyelitis (ADEM).
Advances in Diagnosis (1965 to 1992)
In 1965, Schumacher et al provided the essential clinical criteria for MS diagnosis. Poser et al refined these criteria in 1983. In 2001, McDonald et al added neuroimaging, CSF analysis, and evoked potentials to further complement MS clinical diagnosis. For the first time, the disease could generally be recognized.
Early Treatments
Various treatments for MS were tried over the years, without great success. However, in 1953, a small descriptive trial by Miller and Gibbons reported clinical benefits in patients using intramuscular (IM) adrenocorticotropic hormone (ACTH) for MS and disseminated encephalomyelitis. This was followed in 1970 by a Cooperative Study of IM ACTH versus placebo by Rose et al, which resulted in ACTH, and subsequently oral corticosteroids, being widely used to treat MS, particularly for acute exacerbations of the disease. However, robust evidence of long-term steroids remain limited, even to the present.
High-Dose Steroids
By 1980, the initial descriptive treatment of high-dose intravenous (IV) steroids for demyelinating diseases, including MS and transverse myelitis, by Dowling et al resulted in rapid clinical improvement in some patients. This result was ultimately confirmed by others. High-dose IV steroids became the gold standard for acute attacks, particularly those aggressive in nature. In the mid 1980s, work by Troiano et al, as well as others, showed that the rapid use of high-dose IV as well as oral steroids showed similar effects, with reduction or elimination of CT contrast-enhancing lesions within as few as eight hours, while lower doses or alternative-day treatments were less effective. In addition, descriptive studies of immune modulatory and immunosuppressive drugs, as well as small randomized studies, were published. These agents did not receive FDA approval.
The Golden Age of Therapy (1993 to 2018)
A remarkable era in MS prognosis and treatment began with immunomodulation injections of Betaseron (INFβ-1b), Avonex (INFβ-1a), and Copaxone (glatiramer acetate). This can be attributed, at least in part, to advances in molecular biology, genetics, and neuroimaging, and support by corporate, private, and public funding. Since the initial FDA approval of INFβ-1b, 15 MS therapies have become clinically available, including eight injectables, three orals, and four infusion treatments (see Table 1). In addition, two other drugs have been FDA approved for uses other than MS: rituximab (approved for lymphoma) and cladribine (for hairy cell leukemia), with the latter now approved by the European Medicines Agency for MS. Table 1 depicts characteristics of these therapies approved by US or European agencies (or for other disorders increasingly used off label for MS) in an attempt to compare annual relapse rates (ARR) and decreases in the percent of gadolinium-enhancing MS lesions versus placebo. This information was chosen because ARR has been uniformly selected and defined for such trials, while percent decrease of gadolinium-enhancing lesions on MRI has been the most sensitive barometer available for assessing acute clinical activity. As a result, risk-benefit considerations have been critical in evaluating these drug treatments, with efficacy improving greatly over time, whereas risks have been more variable.
Disease Categories
In 1996, Lublin and Reingold provided a new classification, not specifically for the diagnosis of MS, but rather for the clinical course of the disease. Initially, there were four categories—relapsing-remitting MS, secondary progressive MS, primary progressive MS, and progressive-relapsing MS—that were universally identified. These were thought to be relatively distinct clinical categories, but over time it became clear that the classification did not fully distinguish MS disease activity within these categories. For that reason, it was subsequently recommended, by Lincoln et al in 2009 and Cook et al in 2012, to include MRI, a vastly more sensitive modality, as well as clinical data in assessing disease activity.
On another note, MS and neuromyelitis optica (NMO), although having similar features, were clearly identified as different diseases by Lennon et al in 2004. Differences in pathology, clinical characteristics, immunology, and therapy separate the two disorders.
MRI in MS
Work by Young et al in 1981 established the central role of MRI brain imaging in MS diagnosis and therapeutic considerations. Since then it has become ubiquitous.
An example of a sensitive and highly productive MRI protocol is the BECOME study of MS and clinically isolated syndrome by Cadavid et al from 2009 to 2017. In this study, IFNβ-1b was compared with glatiramer acetate treatment. Cadavid et al used a 3T scanner with triple-dose gadolinium, performed monthly for as long as 24 consecutive months. This unique study brought about a virtual gold mine of valuable research and clinical information. This included proof that gadolinium-enhancing lesions persisted for six months or more, evidence of a 30:1 ratio of new MRI brain lesions to clinical activity, and documentation that 96% of T2 lesions and black holes derive from prior gadolinium-enhancing lesions. It was further noted that 80% to 90% of acute black holes disappeared with treatment and 75% to 80% of patients taking IFNβ-1b or glatiramer acetate had new MRI lesions despite continuing treatment. Perhaps most interestingly, monthly MRIs could predict relapse and disability in a relatively small number of patients, depending upon the frequency and activity of MRI lesions. In 2017, Brown et al documented that magnetization transfer ratio recovery in MS brain lesions occurred more significantly with glatiramer acetate than with IFNβ-1b, whereas more chronic black hole lesions were found with glatiramer acetate. Also in 2017, Maranzano et al found evidence of acute inflammatory leukocortical lesions, which were not as well recognized previously.
In summary, it has become increasingly clear that MRI is the most sen
The Future of MS
While it is not yet a curable disease, there is growing evidence that MS prognosis has improved and will continue to improve. This is based on incremental decreases in acute MS exacerbations, progressive disability, and MRI lesion activity, as well as a combination of the three—no evidence of disease activity (NEDA).
Not only are drug therapies becoming more effective, but patients and physicians now have many more treatment options to carefully consider with regard to efficacy, side effect profiles, treatment frequency, route of administration, cost, and quality of life. Newer drugs with different mechanisms of action such as cladribine, now approved in Europe, fulfill most of these beneficial criteria (see Giovannoni et al, 2010). More promising MS treatments, including long-acting induction therapies, are still being evaluated. As with other complex diseases, multiple therapies are likely to be used as well.
In summary, compared with the time before 1993, MS will be much less likely to be a progressive disease, and quality of life will be much improved. In my opinion, patients will be less fearful about their prognosis than ever before, and with appropriate evaluations and treatments, we may realize that disabling MS will be far less common.

Suggested Reading
Brown JW, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140(2):387-398.
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983.
Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5-7, 2010. Int J MS Care. 2012;14(3):105-114.
Dowling PC, Bosch VV, Cook SD. Possible beneficial effect of high-dose intravenous steroid therapy in acute demyelinating disease and transverse myelitis. Neurology. 1980;30(7 Pt 2):33-36.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112.
Lincoln JA, Cadavid D, Pollard J, et al. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66(3):412-414.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911.
Maranzano J, Rudko DA, Nakamura K, et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714-721.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
Miller HG, Gibbons JL. Acute disseminated encephalomyelitis and acute disseminated sclerosis; results of treatment with A.C.T.H. Br Med J. 1953;2(4850):1345-1348.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20(5):1-59.
Troiano R, Hafstein M, Ruderman M, et al. Effect of high-dose intravenous steroid administration on contrast-enhancing computed tomographic scan lesions in multiple sclerosis. Ann Neurol. 1984;15(3):257-263.
Troiano RA, Hafstein MP, Zito G, et al. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. J Neurol Sci. 1985;70(1):67-72.
Young IR, Hall AS, Pallis A, et al. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. 1981;2(8255):1063-1066.
Stuart D. Cook, MD, and Abdul Rahman Alchaki
Dr. Cook is the Ruth Dunietz Kushner and Michael Jay Serwitz Professor of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark. Dr. Alchaki is a resident in the Deptartment of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark.
Disclosure: Stuart Cook has received honoraria for lectures from Bayer HealthCare and Merck Serono. He has served as a consultant for Merck Serono, Bayer HealthCare, Teva, Novartis, Sanofi-Aventis, Biogen Idec, and Actinobac Biomed. He has served on steering committees for the BEYOND and CLARITY Studies and as a member of Advisory Boards for Merck Serono, Bayer HealthCare, Teva, Biogen Idec, Sanofi Aventis, and Actinobac Biomed.
The Initial Years (1838 to 1930s)
The earliest recognition of MS clinical features and pathology was attributed to Jean-Martin Charcot, Robert Carswell, and Jean Cruveilhier in Europe from 1838 to 1868. Beyond those early descriptions, relatively few MS breakthroughs occurred until the 1930s, when Thomas Rivers discovered experimental autoimmune encephalomyelitis (EAE), a demyelinating disease, in animals. His insightful concepts were widely cited and ultimately contributed to undestanding of the immune mechanisms of MS and acute disseminated encephalomyelitis (ADEM).
Advances in Diagnosis (1965 to 1992)
In 1965, Schumacher et al provided the essential clinical criteria for MS diagnosis. Poser et al refined these criteria in 1983. In 2001, McDonald et al added neuroimaging, CSF analysis, and evoked potentials to further complement MS clinical diagnosis. For the first time, the disease could generally be recognized.
Early Treatments
Various treatments for MS were tried over the years, without great success. However, in 1953, a small descriptive trial by Miller and Gibbons reported clinical benefits in patients using intramuscular (IM) adrenocorticotropic hormone (ACTH) for MS and disseminated encephalomyelitis. This was followed in 1970 by a Cooperative Study of IM ACTH versus placebo by Rose et al, which resulted in ACTH, and subsequently oral corticosteroids, being widely used to treat MS, particularly for acute exacerbations of the disease. However, robust evidence of long-term steroids remain limited, even to the present.
High-Dose Steroids
By 1980, the initial descriptive treatment of high-dose intravenous (IV) steroids for demyelinating diseases, including MS and transverse myelitis, by Dowling et al resulted in rapid clinical improvement in some patients. This result was ultimately confirmed by others. High-dose IV steroids became the gold standard for acute attacks, particularly those aggressive in nature. In the mid 1980s, work by Troiano et al, as well as others, showed that the rapid use of high-dose IV as well as oral steroids showed similar effects, with reduction or elimination of CT contrast-enhancing lesions within as few as eight hours, while lower doses or alternative-day treatments were less effective. In addition, descriptive studies of immune modulatory and immunosuppressive drugs, as well as small randomized studies, were published. These agents did not receive FDA approval.
The Golden Age of Therapy (1993 to 2018)
A remarkable era in MS prognosis and treatment began with immunomodulation injections of Betaseron (INFβ-1b), Avonex (INFβ-1a), and Copaxone (glatiramer acetate). This can be attributed, at least in part, to advances in molecular biology, genetics, and neuroimaging, and support by corporate, private, and public funding. Since the initial FDA approval of INFβ-1b, 15 MS therapies have become clinically available, including eight injectables, three orals, and four infusion treatments (see Table 1). In addition, two other drugs have been FDA approved for uses other than MS: rituximab (approved for lymphoma) and cladribine (for hairy cell leukemia), with the latter now approved by the European Medicines Agency for MS. Table 1 depicts characteristics of these therapies approved by US or European agencies (or for other disorders increasingly used off label for MS) in an attempt to compare annual relapse rates (ARR) and decreases in the percent of gadolinium-enhancing MS lesions versus placebo. This information was chosen because ARR has been uniformly selected and defined for such trials, while percent decrease of gadolinium-enhancing lesions on MRI has been the most sensitive barometer available for assessing acute clinical activity. As a result, risk-benefit considerations have been critical in evaluating these drug treatments, with efficacy improving greatly over time, whereas risks have been more variable.
Disease Categories
In 1996, Lublin and Reingold provided a new classification, not specifically for the diagnosis of MS, but rather for the clinical course of the disease. Initially, there were four categories—relapsing-remitting MS, secondary progressive MS, primary progressive MS, and progressive-relapsing MS—that were universally identified. These were thought to be relatively distinct clinical categories, but over time it became clear that the classification did not fully distinguish MS disease activity within these categories. For that reason, it was subsequently recommended, by Lincoln et al in 2009 and Cook et al in 2012, to include MRI, a vastly more sensitive modality, as well as clinical data in assessing disease activity.
On another note, MS and neuromyelitis optica (NMO), although having similar features, were clearly identified as different diseases by Lennon et al in 2004. Differences in pathology, clinical characteristics, immunology, and therapy separate the two disorders.
MRI in MS
Work by Young et al in 1981 established the central role of MRI brain imaging in MS diagnosis and therapeutic considerations. Since then it has become ubiquitous.
An example of a sensitive and highly productive MRI protocol is the BECOME study of MS and clinically isolated syndrome by Cadavid et al from 2009 to 2017. In this study, IFNβ-1b was compared with glatiramer acetate treatment. Cadavid et al used a 3T scanner with triple-dose gadolinium, performed monthly for as long as 24 consecutive months. This unique study brought about a virtual gold mine of valuable research and clinical information. This included proof that gadolinium-enhancing lesions persisted for six months or more, evidence of a 30:1 ratio of new MRI brain lesions to clinical activity, and documentation that 96% of T2 lesions and black holes derive from prior gadolinium-enhancing lesions. It was further noted that 80% to 90% of acute black holes disappeared with treatment and 75% to 80% of patients taking IFNβ-1b or glatiramer acetate had new MRI lesions despite continuing treatment. Perhaps most interestingly, monthly MRIs could predict relapse and disability in a relatively small number of patients, depending upon the frequency and activity of MRI lesions. In 2017, Brown et al documented that magnetization transfer ratio recovery in MS brain lesions occurred more significantly with glatiramer acetate than with IFNβ-1b, whereas more chronic black hole lesions were found with glatiramer acetate. Also in 2017, Maranzano et al found evidence of acute inflammatory leukocortical lesions, which were not as well recognized previously.
In summary, it has become increasingly clear that MRI is the most sen
The Future of MS
While it is not yet a curable disease, there is growing evidence that MS prognosis has improved and will continue to improve. This is based on incremental decreases in acute MS exacerbations, progressive disability, and MRI lesion activity, as well as a combination of the three—no evidence of disease activity (NEDA).
Not only are drug therapies becoming more effective, but patients and physicians now have many more treatment options to carefully consider with regard to efficacy, side effect profiles, treatment frequency, route of administration, cost, and quality of life. Newer drugs with different mechanisms of action such as cladribine, now approved in Europe, fulfill most of these beneficial criteria (see Giovannoni et al, 2010). More promising MS treatments, including long-acting induction therapies, are still being evaluated. As with other complex diseases, multiple therapies are likely to be used as well.
In summary, compared with the time before 1993, MS will be much less likely to be a progressive disease, and quality of life will be much improved. In my opinion, patients will be less fearful about their prognosis than ever before, and with appropriate evaluations and treatments, we may realize that disabling MS will be far less common.

Suggested Reading
Brown JW, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140(2):387-398.
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983.
Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5-7, 2010. Int J MS Care. 2012;14(3):105-114.
Dowling PC, Bosch VV, Cook SD. Possible beneficial effect of high-dose intravenous steroid therapy in acute demyelinating disease and transverse myelitis. Neurology. 1980;30(7 Pt 2):33-36.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112.
Lincoln JA, Cadavid D, Pollard J, et al. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66(3):412-414.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911.
Maranzano J, Rudko DA, Nakamura K, et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714-721.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
Miller HG, Gibbons JL. Acute disseminated encephalomyelitis and acute disseminated sclerosis; results of treatment with A.C.T.H. Br Med J. 1953;2(4850):1345-1348.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20(5):1-59.
Troiano R, Hafstein M, Ruderman M, et al. Effect of high-dose intravenous steroid administration on contrast-enhancing computed tomographic scan lesions in multiple sclerosis. Ann Neurol. 1984;15(3):257-263.
Troiano RA, Hafstein MP, Zito G, et al. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. J Neurol Sci. 1985;70(1):67-72.
Young IR, Hall AS, Pallis A, et al. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. 1981;2(8255):1063-1066.
VIDEO: Managing the alemtuzumab paradox
SAN DIEGO – A paradox of treating people with the monoclonal antibody alemtuzumab is that the agent can be an effective therapy for many people living with multiple sclerosis, but in some patients is associated with the development of other autoimmune diseases.
“I counsel patients with multiple sclerosis that this is a high risk, high gain drug,” Alasdair Coles, MD, of the University of Cambridge (England), said at the meeting, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Patients can make it safer through strict compliance with the drug’s risk monitoring program. But without patient compliance, alemtuzumab becomes a dangerous drug, he said in a video interview.
Knowing in advance which patients are at an elevated risk for subsequent autoimmune diseases has been difficult to predict. But researchers are getting closer, Dr. Coles said. In the future, measuring a serum factor, potentially interleukin 21, could produce a pretreatment risk assessment for each individual.
SAN DIEGO – A paradox of treating people with the monoclonal antibody alemtuzumab is that the agent can be an effective therapy for many people living with multiple sclerosis, but in some patients is associated with the development of other autoimmune diseases.
“I counsel patients with multiple sclerosis that this is a high risk, high gain drug,” Alasdair Coles, MD, of the University of Cambridge (England), said at the meeting, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Patients can make it safer through strict compliance with the drug’s risk monitoring program. But without patient compliance, alemtuzumab becomes a dangerous drug, he said in a video interview.
Knowing in advance which patients are at an elevated risk for subsequent autoimmune diseases has been difficult to predict. But researchers are getting closer, Dr. Coles said. In the future, measuring a serum factor, potentially interleukin 21, could produce a pretreatment risk assessment for each individual.
SAN DIEGO – A paradox of treating people with the monoclonal antibody alemtuzumab is that the agent can be an effective therapy for many people living with multiple sclerosis, but in some patients is associated with the development of other autoimmune diseases.
“I counsel patients with multiple sclerosis that this is a high risk, high gain drug,” Alasdair Coles, MD, of the University of Cambridge (England), said at the meeting, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Patients can make it safer through strict compliance with the drug’s risk monitoring program. But without patient compliance, alemtuzumab becomes a dangerous drug, he said in a video interview.
Knowing in advance which patients are at an elevated risk for subsequent autoimmune diseases has been difficult to predict. But researchers are getting closer, Dr. Coles said. In the future, measuring a serum factor, potentially interleukin 21, could produce a pretreatment risk assessment for each individual.
REPORTING FROM ACTRIMS FORUM 2018
Third course of alemtuzumab can improve MS outcomes
SAN DIEGO – Approximately 30% of people with active multiple sclerosis who initially responded well to two courses of alemtuzumab in the CARE-MS II trial experience relapse or MRI activity over time. But investigators set out to determine whether retreatment with a subsequent course of alemtuzumab is worthwhile.
“What we found is, after the third course, they continued to do well again – at this point for an average of another 3-4 years,” Ann D. Bass, MD, said in an interview at the meeting, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“The take-home message is if they do need to be retreated after those two courses, it doesn’t [necessarily] mean they are a treatment failure. Give them another chance and see if they will do well with a third course, which is what they did,” said Dr. Bass, of the Neurology Center of San Antonio.
Through year 6 of an extension study with 393 of the original CARE-MS II trial participants, 45% received one or more additional courses of alemtuzumab. Participants were required to wait 12 months or more after completion of the two initial courses of therapy. This figure includes 30% who received a third course of alemtuzumab, 12% who received a fourth course, and 2% and 1% who received a fifth or sixth course, respectively.
“The average time until they needed a third course was 2.5 years after the second course; so they didn’t need it right away,” Dr. Bass said. “The majority, even at 6 years, did not need to have a third course.”
When patients did require a subsequent course, “about half needed it because of clinical relapse; one-quarter needed it because of MRI relapse; and about one-quarter needed it because of both,” Dr. Bass said during a poster presentation.
In terms of effectiveness, the annual relapse rate significantly decreased following a third course of alemtuzumab, from 0.85 in the 12 months prior to the third course to 0.20 in the 12 months after (P less than .0001). In these patients, the annual relapse rate remained low, at 0.17, up to 3 years later.
Investigators also tracked disability using the Expanded Disability Status Scale. They found that more than two-thirds, 68%, maintained stable or had improved scores after administration of a third alemtuzumab course. In addition, the percentage of patients with confirmed disability improvement increased from 4.4% in the 12 months prior to a third course to 14.4% in the year following pretreatment.
Retreatment was at the patient’s discretion. “The patients have the right to say ‘No, I’m doing great. I don’t want to be retreated’ or ‘I want to explore other options’ for whatever reason,” Dr. Bass said. “That’s rare though; most patients actually say yes.”
To qualify for retreatment based on MRI findings, patients had to have at least two lesions – one enlarging and one enhancing, two enlarging, or two new.
Only patients who opted for a subsequent course of alemtuzumab were included in the current analysis; those who chose a different disease-modifying therapy were excluded.
“Many achieve clinical and MRI remission. I never say cure – you don’t want to say that word,” Dr. Bass said.
Sanofi and Bayer HealthCare Pharmaceuticals supported the study. Dr. Bass reported that she is a principal investigator, speaker, and member of the advisory board for Sanofi Genzyme.
SOURCE: Bass A et al. ACTRIMS Forum 2018 Poster P035.
SAN DIEGO – Approximately 30% of people with active multiple sclerosis who initially responded well to two courses of alemtuzumab in the CARE-MS II trial experience relapse or MRI activity over time. But investigators set out to determine whether retreatment with a subsequent course of alemtuzumab is worthwhile.
“What we found is, after the third course, they continued to do well again – at this point for an average of another 3-4 years,” Ann D. Bass, MD, said in an interview at the meeting, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“The take-home message is if they do need to be retreated after those two courses, it doesn’t [necessarily] mean they are a treatment failure. Give them another chance and see if they will do well with a third course, which is what they did,” said Dr. Bass, of the Neurology Center of San Antonio.
Through year 6 of an extension study with 393 of the original CARE-MS II trial participants, 45% received one or more additional courses of alemtuzumab. Participants were required to wait 12 months or more after completion of the two initial courses of therapy. This figure includes 30% who received a third course of alemtuzumab, 12% who received a fourth course, and 2% and 1% who received a fifth or sixth course, respectively.
“The average time until they needed a third course was 2.5 years after the second course; so they didn’t need it right away,” Dr. Bass said. “The majority, even at 6 years, did not need to have a third course.”
When patients did require a subsequent course, “about half needed it because of clinical relapse; one-quarter needed it because of MRI relapse; and about one-quarter needed it because of both,” Dr. Bass said during a poster presentation.
In terms of effectiveness, the annual relapse rate significantly decreased following a third course of alemtuzumab, from 0.85 in the 12 months prior to the third course to 0.20 in the 12 months after (P less than .0001). In these patients, the annual relapse rate remained low, at 0.17, up to 3 years later.
Investigators also tracked disability using the Expanded Disability Status Scale. They found that more than two-thirds, 68%, maintained stable or had improved scores after administration of a third alemtuzumab course. In addition, the percentage of patients with confirmed disability improvement increased from 4.4% in the 12 months prior to a third course to 14.4% in the year following pretreatment.
Retreatment was at the patient’s discretion. “The patients have the right to say ‘No, I’m doing great. I don’t want to be retreated’ or ‘I want to explore other options’ for whatever reason,” Dr. Bass said. “That’s rare though; most patients actually say yes.”
To qualify for retreatment based on MRI findings, patients had to have at least two lesions – one enlarging and one enhancing, two enlarging, or two new.
Only patients who opted for a subsequent course of alemtuzumab were included in the current analysis; those who chose a different disease-modifying therapy were excluded.
“Many achieve clinical and MRI remission. I never say cure – you don’t want to say that word,” Dr. Bass said.
Sanofi and Bayer HealthCare Pharmaceuticals supported the study. Dr. Bass reported that she is a principal investigator, speaker, and member of the advisory board for Sanofi Genzyme.
SOURCE: Bass A et al. ACTRIMS Forum 2018 Poster P035.
SAN DIEGO – Approximately 30% of people with active multiple sclerosis who initially responded well to two courses of alemtuzumab in the CARE-MS II trial experience relapse or MRI activity over time. But investigators set out to determine whether retreatment with a subsequent course of alemtuzumab is worthwhile.
“What we found is, after the third course, they continued to do well again – at this point for an average of another 3-4 years,” Ann D. Bass, MD, said in an interview at the meeting, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“The take-home message is if they do need to be retreated after those two courses, it doesn’t [necessarily] mean they are a treatment failure. Give them another chance and see if they will do well with a third course, which is what they did,” said Dr. Bass, of the Neurology Center of San Antonio.
Through year 6 of an extension study with 393 of the original CARE-MS II trial participants, 45% received one or more additional courses of alemtuzumab. Participants were required to wait 12 months or more after completion of the two initial courses of therapy. This figure includes 30% who received a third course of alemtuzumab, 12% who received a fourth course, and 2% and 1% who received a fifth or sixth course, respectively.
“The average time until they needed a third course was 2.5 years after the second course; so they didn’t need it right away,” Dr. Bass said. “The majority, even at 6 years, did not need to have a third course.”
When patients did require a subsequent course, “about half needed it because of clinical relapse; one-quarter needed it because of MRI relapse; and about one-quarter needed it because of both,” Dr. Bass said during a poster presentation.
In terms of effectiveness, the annual relapse rate significantly decreased following a third course of alemtuzumab, from 0.85 in the 12 months prior to the third course to 0.20 in the 12 months after (P less than .0001). In these patients, the annual relapse rate remained low, at 0.17, up to 3 years later.
Investigators also tracked disability using the Expanded Disability Status Scale. They found that more than two-thirds, 68%, maintained stable or had improved scores after administration of a third alemtuzumab course. In addition, the percentage of patients with confirmed disability improvement increased from 4.4% in the 12 months prior to a third course to 14.4% in the year following pretreatment.
Retreatment was at the patient’s discretion. “The patients have the right to say ‘No, I’m doing great. I don’t want to be retreated’ or ‘I want to explore other options’ for whatever reason,” Dr. Bass said. “That’s rare though; most patients actually say yes.”
To qualify for retreatment based on MRI findings, patients had to have at least two lesions – one enlarging and one enhancing, two enlarging, or two new.
Only patients who opted for a subsequent course of alemtuzumab were included in the current analysis; those who chose a different disease-modifying therapy were excluded.
“Many achieve clinical and MRI remission. I never say cure – you don’t want to say that word,” Dr. Bass said.
Sanofi and Bayer HealthCare Pharmaceuticals supported the study. Dr. Bass reported that she is a principal investigator, speaker, and member of the advisory board for Sanofi Genzyme.
SOURCE: Bass A et al. ACTRIMS Forum 2018 Poster P035.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point:
Major finding: The annual relapse rate significantly decreased following a third course of alemtuzumab, from 0.85 in the 12 months prior to the third course to 0.20 in the 12 months after (P less than .0001).
Study details: An extension study of the CARE-MS II trial involving 393 of the original study participants.
Disclosures: Sanofi and Bayer HealthCare Pharmaceuticals supported the study. Dr. Bass reported that she is a principal investigator, speaker, and member of the advisory board for Sanofi Genzyme.
Source: Bass A et al. ACTRIMS Forum 2018 Poster P035.
Switching RRMS patients to daclizumab beta appears safe
SAN DIEGO – Switching relapsing-remitting multiple sclerosis patients from glatiramer acetate to daclizumab beta resulted in no increase in the adverse event profile and was associated with superior efficacy, a post hoc analysis of data from the DECIDE study showed.
“There is always a challenge in transitioning patients from one therapeutic agent to another, with concerns for adequate efficacy to justify the switch, and heightened risks of toxicity or adverse events,” lead study author Stanley L. Cohan, MD, PhD, said in an interview prior to the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “Daclizumab is not a first-line or platform therapeutic agent, but has clearly superior efficacy to first-line medication, and, based upon the current data presented, safety and efficacy are not adversely influenced by prior treatment history with a first-line agent.”
In the phase 3 DECIDE trial, daclizumab beta 150 mg demonstrated greater efficacy versus intramuscular (IM) interferon (IFN) beta-1a 30 mcg on several clinical, radiographic, and patient-centered outcomes in patients with relapsing-remitting multiple sclerosis (N Engl J Med 2015; 373:1418-28). The purpose of the current study was to examine the efficacy and safety of daclizumab beta vs. IM IFN beta-1a in the subgroup of RRMS patients treated with glatiramer acetate (GA) as their only previous disease-modifying therapy (DMT) before entering DECIDE.
Dr. Cohan, a neurologist who directs the Providence MS Center at the Providence Brain and Spine Institute in Portland, Ore., and his associates reported data from 42 of 922 (5%) IM IFN beta-1a and 50 of 919 (5%) daclizumab beta patients who had received treatment with GA only prior to DECIDE. Baseline characteristics were balanced between treatment groups, including duration of prior treatment with GA, reasons for discontinuing GA, and time between GA discontinuation and start of treatment in DECIDE. The annualized relapse rate was 42% lower in patients treated with daclizumab beta vs. IM IFN beta-1a (rate ratio of 0.58). Daclizumab beta also reduced risk of relapse by 53% (hazard ratio of 0.47; P = .048) and the mean number of new or newly-enlarging T2-hyperintense lesions at week 96 by 58% (lesion mean ratio, 0.42; P = .021) vs. IM IFN beta-1a.
In patients treated with GA only before DECIDE, 98% of IM IFN beta-1a patients and 94% of daclizumab beta patients reported any adverse event (AE). In all, 2% of IM IFN beta-1a patients and 16% of daclizumab beta patients had a serious AE (excluding MS relapse), and 10% of IM IFN beta-1a and 12% of daclizumab beta patients discontinued treatment because of an AE (excluding MS relapse).
The incidence of elevations of alanine aminotransferase or aspartate aminotransferase three times the upper limit of normal or greater was 10% in the IM IFN beta-1a group and 8% in the daclizumab beta, while the ALT and AST elevations were greater than five times the ULN in 0% and 4% of patients, respectively.
Serious adverse events were reported in eight participants in the daclizumab beta group (including abortion induced, ankle fracture, anal fistula, anxiety, appendicitis perforated, convulsion, pelvic abscess, inguinal hernia, abnormal cervix smear), and one participant in the IM IFN beta-1a group (ligament rupture).
“This post hoc analysis demonstrates that in switching from glatiramer to daclizumab there was no unanticipated or increase in the AE profile, and that a switch from glatiramer to daclizumab was associated with superior efficacy, again in line with overall efficacy observed for daclizumab in this study,” Dr. Cohan said.
He acknowledged certain limitations of the study, including the post hoc nature of the analysis. “The small glatiramer cohort size, and the large percentage of former glatiramer patients who entered DECIDE because of lack of glatiramer efficacy may have introduced a selection bias which would magnify the seeming efficacy of daclizumab, and interferon-beta,” he added.
Dr. Cohan reported that he receives research support from Biogen, Novartis, Roche, Sanofi, and Mallinckrodt, and speaking honoraria from Acorda, Biogen, Roche, and Sanofi. He has served on advisory boards for Biogen, Sanofi, and Novartis.
SOURCE: Cohan et al. ACTRIMS Forum 2018, Poster 42.
SAN DIEGO – Switching relapsing-remitting multiple sclerosis patients from glatiramer acetate to daclizumab beta resulted in no increase in the adverse event profile and was associated with superior efficacy, a post hoc analysis of data from the DECIDE study showed.
“There is always a challenge in transitioning patients from one therapeutic agent to another, with concerns for adequate efficacy to justify the switch, and heightened risks of toxicity or adverse events,” lead study author Stanley L. Cohan, MD, PhD, said in an interview prior to the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “Daclizumab is not a first-line or platform therapeutic agent, but has clearly superior efficacy to first-line medication, and, based upon the current data presented, safety and efficacy are not adversely influenced by prior treatment history with a first-line agent.”
In the phase 3 DECIDE trial, daclizumab beta 150 mg demonstrated greater efficacy versus intramuscular (IM) interferon (IFN) beta-1a 30 mcg on several clinical, radiographic, and patient-centered outcomes in patients with relapsing-remitting multiple sclerosis (N Engl J Med 2015; 373:1418-28). The purpose of the current study was to examine the efficacy and safety of daclizumab beta vs. IM IFN beta-1a in the subgroup of RRMS patients treated with glatiramer acetate (GA) as their only previous disease-modifying therapy (DMT) before entering DECIDE.
Dr. Cohan, a neurologist who directs the Providence MS Center at the Providence Brain and Spine Institute in Portland, Ore., and his associates reported data from 42 of 922 (5%) IM IFN beta-1a and 50 of 919 (5%) daclizumab beta patients who had received treatment with GA only prior to DECIDE. Baseline characteristics were balanced between treatment groups, including duration of prior treatment with GA, reasons for discontinuing GA, and time between GA discontinuation and start of treatment in DECIDE. The annualized relapse rate was 42% lower in patients treated with daclizumab beta vs. IM IFN beta-1a (rate ratio of 0.58). Daclizumab beta also reduced risk of relapse by 53% (hazard ratio of 0.47; P = .048) and the mean number of new or newly-enlarging T2-hyperintense lesions at week 96 by 58% (lesion mean ratio, 0.42; P = .021) vs. IM IFN beta-1a.
In patients treated with GA only before DECIDE, 98% of IM IFN beta-1a patients and 94% of daclizumab beta patients reported any adverse event (AE). In all, 2% of IM IFN beta-1a patients and 16% of daclizumab beta patients had a serious AE (excluding MS relapse), and 10% of IM IFN beta-1a and 12% of daclizumab beta patients discontinued treatment because of an AE (excluding MS relapse).
The incidence of elevations of alanine aminotransferase or aspartate aminotransferase three times the upper limit of normal or greater was 10% in the IM IFN beta-1a group and 8% in the daclizumab beta, while the ALT and AST elevations were greater than five times the ULN in 0% and 4% of patients, respectively.
Serious adverse events were reported in eight participants in the daclizumab beta group (including abortion induced, ankle fracture, anal fistula, anxiety, appendicitis perforated, convulsion, pelvic abscess, inguinal hernia, abnormal cervix smear), and one participant in the IM IFN beta-1a group (ligament rupture).
“This post hoc analysis demonstrates that in switching from glatiramer to daclizumab there was no unanticipated or increase in the AE profile, and that a switch from glatiramer to daclizumab was associated with superior efficacy, again in line with overall efficacy observed for daclizumab in this study,” Dr. Cohan said.
He acknowledged certain limitations of the study, including the post hoc nature of the analysis. “The small glatiramer cohort size, and the large percentage of former glatiramer patients who entered DECIDE because of lack of glatiramer efficacy may have introduced a selection bias which would magnify the seeming efficacy of daclizumab, and interferon-beta,” he added.
Dr. Cohan reported that he receives research support from Biogen, Novartis, Roche, Sanofi, and Mallinckrodt, and speaking honoraria from Acorda, Biogen, Roche, and Sanofi. He has served on advisory boards for Biogen, Sanofi, and Novartis.
SOURCE: Cohan et al. ACTRIMS Forum 2018, Poster 42.
SAN DIEGO – Switching relapsing-remitting multiple sclerosis patients from glatiramer acetate to daclizumab beta resulted in no increase in the adverse event profile and was associated with superior efficacy, a post hoc analysis of data from the DECIDE study showed.
“There is always a challenge in transitioning patients from one therapeutic agent to another, with concerns for adequate efficacy to justify the switch, and heightened risks of toxicity or adverse events,” lead study author Stanley L. Cohan, MD, PhD, said in an interview prior to the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “Daclizumab is not a first-line or platform therapeutic agent, but has clearly superior efficacy to first-line medication, and, based upon the current data presented, safety and efficacy are not adversely influenced by prior treatment history with a first-line agent.”
In the phase 3 DECIDE trial, daclizumab beta 150 mg demonstrated greater efficacy versus intramuscular (IM) interferon (IFN) beta-1a 30 mcg on several clinical, radiographic, and patient-centered outcomes in patients with relapsing-remitting multiple sclerosis (N Engl J Med 2015; 373:1418-28). The purpose of the current study was to examine the efficacy and safety of daclizumab beta vs. IM IFN beta-1a in the subgroup of RRMS patients treated with glatiramer acetate (GA) as their only previous disease-modifying therapy (DMT) before entering DECIDE.
Dr. Cohan, a neurologist who directs the Providence MS Center at the Providence Brain and Spine Institute in Portland, Ore., and his associates reported data from 42 of 922 (5%) IM IFN beta-1a and 50 of 919 (5%) daclizumab beta patients who had received treatment with GA only prior to DECIDE. Baseline characteristics were balanced between treatment groups, including duration of prior treatment with GA, reasons for discontinuing GA, and time between GA discontinuation and start of treatment in DECIDE. The annualized relapse rate was 42% lower in patients treated with daclizumab beta vs. IM IFN beta-1a (rate ratio of 0.58). Daclizumab beta also reduced risk of relapse by 53% (hazard ratio of 0.47; P = .048) and the mean number of new or newly-enlarging T2-hyperintense lesions at week 96 by 58% (lesion mean ratio, 0.42; P = .021) vs. IM IFN beta-1a.
In patients treated with GA only before DECIDE, 98% of IM IFN beta-1a patients and 94% of daclizumab beta patients reported any adverse event (AE). In all, 2% of IM IFN beta-1a patients and 16% of daclizumab beta patients had a serious AE (excluding MS relapse), and 10% of IM IFN beta-1a and 12% of daclizumab beta patients discontinued treatment because of an AE (excluding MS relapse).
The incidence of elevations of alanine aminotransferase or aspartate aminotransferase three times the upper limit of normal or greater was 10% in the IM IFN beta-1a group and 8% in the daclizumab beta, while the ALT and AST elevations were greater than five times the ULN in 0% and 4% of patients, respectively.
Serious adverse events were reported in eight participants in the daclizumab beta group (including abortion induced, ankle fracture, anal fistula, anxiety, appendicitis perforated, convulsion, pelvic abscess, inguinal hernia, abnormal cervix smear), and one participant in the IM IFN beta-1a group (ligament rupture).
“This post hoc analysis demonstrates that in switching from glatiramer to daclizumab there was no unanticipated or increase in the AE profile, and that a switch from glatiramer to daclizumab was associated with superior efficacy, again in line with overall efficacy observed for daclizumab in this study,” Dr. Cohan said.
He acknowledged certain limitations of the study, including the post hoc nature of the analysis. “The small glatiramer cohort size, and the large percentage of former glatiramer patients who entered DECIDE because of lack of glatiramer efficacy may have introduced a selection bias which would magnify the seeming efficacy of daclizumab, and interferon-beta,” he added.
Dr. Cohan reported that he receives research support from Biogen, Novartis, Roche, Sanofi, and Mallinckrodt, and speaking honoraria from Acorda, Biogen, Roche, and Sanofi. He has served on advisory boards for Biogen, Sanofi, and Novartis.
SOURCE: Cohan et al. ACTRIMS Forum 2018, Poster 42.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point:
Major finding: The annualized relapse rate was 42% lower in patients treated with daclizumab beta vs. IM IFN beta-1a (rate ratio of 0.58).
Study details: A post hoc analysis of 42 of 922 (5%) IM IFN beta-1a and 50 of 919 (5%) daclizumab beta patients who had received treatment with glatiramer acetate only prior to the DECIDE trial.
Disclosures: Dr. Cohan reported that he receives research support from Biogen, Novartis, Roche, Sanofi, and Mallinckrodt, and speaking honoraria from Acorda, Biogen, Roche, and Sanofi. He has served on advisory boards for Biogen, Sanofi, and Novartis.
Source: Cohan S et al. ACTRIMS Forum 2018 Poster 42.
VIDEO: Oral ozanimod shows promise for relapsing MS
SAN DIEGO – A pair of phase 3 studies offer promising results regarding the safety and efficacy of ozanimod, an experimental immunomodulator, in the treatment of relapsing multiple sclerosis (RMS).
The medication targets sphingosine 1-phosphate 1 and 5 receptors. Industry-funded researchers tested it in two studies against interferon beta-1a.
One study, called SUNBEAM, tested once-daily oral ozanimod (1 mg or 0.5 mg with 7-day dose escalation) against interferon beta-1a (via 30 mcg weekly intramuscular injection) for at least 12 months in 1,346 patients with RMS. The annualized relapse rate, the primary endpoint, was lower in the ozanimod groups versus interferon. For the 1-mg dose, it was 0.181 (P less than .0001), and for 0.5-mg dose, 0.241 (P = .0013).
The number of serious treatment-emergent adverse events in the three groups was low, ranging from 2.5% to 3.5%.
The other study, called RADIANCE, was a similar trial that lasted 24 months. In it, the rate of serious treatment-emergent adverse events in the three groups were similar, ranging from 6.4% to 7.1%.
Ozanimod offers “an excellent therapeutic benefit for patients and a very clean safety profile,” said Bruce Cree, MD, PhD, clinical research director at the University of California, San Francisco, Multiple Sclerosis Center. He is an author on both studies and spoke in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
He said the ozanimod should be especially useful as a first-line treatment for MS. The drug is currently being evaluated by the Food and Drug Administration for an RMS indication, and it is also being developed for Crohn’s disease and ulcerative colitis, he said.
The study was funded by Receptos, a wholly owned subsidiary of Celgene. Dr. Cree reported that he has been a consultant to AbbVie, Biogen, EMD Serono, Genzyme, Novartis, and Shire.
SOURCE: Cree B et al. ACTRIMS Forum 2018, abstract P030, and Comi G et al. ACTRIMS Forum 2018, abstract P023
SAN DIEGO – A pair of phase 3 studies offer promising results regarding the safety and efficacy of ozanimod, an experimental immunomodulator, in the treatment of relapsing multiple sclerosis (RMS).
The medication targets sphingosine 1-phosphate 1 and 5 receptors. Industry-funded researchers tested it in two studies against interferon beta-1a.
One study, called SUNBEAM, tested once-daily oral ozanimod (1 mg or 0.5 mg with 7-day dose escalation) against interferon beta-1a (via 30 mcg weekly intramuscular injection) for at least 12 months in 1,346 patients with RMS. The annualized relapse rate, the primary endpoint, was lower in the ozanimod groups versus interferon. For the 1-mg dose, it was 0.181 (P less than .0001), and for 0.5-mg dose, 0.241 (P = .0013).
The number of serious treatment-emergent adverse events in the three groups was low, ranging from 2.5% to 3.5%.
The other study, called RADIANCE, was a similar trial that lasted 24 months. In it, the rate of serious treatment-emergent adverse events in the three groups were similar, ranging from 6.4% to 7.1%.
Ozanimod offers “an excellent therapeutic benefit for patients and a very clean safety profile,” said Bruce Cree, MD, PhD, clinical research director at the University of California, San Francisco, Multiple Sclerosis Center. He is an author on both studies and spoke in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
He said the ozanimod should be especially useful as a first-line treatment for MS. The drug is currently being evaluated by the Food and Drug Administration for an RMS indication, and it is also being developed for Crohn’s disease and ulcerative colitis, he said.
The study was funded by Receptos, a wholly owned subsidiary of Celgene. Dr. Cree reported that he has been a consultant to AbbVie, Biogen, EMD Serono, Genzyme, Novartis, and Shire.
SOURCE: Cree B et al. ACTRIMS Forum 2018, abstract P030, and Comi G et al. ACTRIMS Forum 2018, abstract P023
SAN DIEGO – A pair of phase 3 studies offer promising results regarding the safety and efficacy of ozanimod, an experimental immunomodulator, in the treatment of relapsing multiple sclerosis (RMS).
The medication targets sphingosine 1-phosphate 1 and 5 receptors. Industry-funded researchers tested it in two studies against interferon beta-1a.
One study, called SUNBEAM, tested once-daily oral ozanimod (1 mg or 0.5 mg with 7-day dose escalation) against interferon beta-1a (via 30 mcg weekly intramuscular injection) for at least 12 months in 1,346 patients with RMS. The annualized relapse rate, the primary endpoint, was lower in the ozanimod groups versus interferon. For the 1-mg dose, it was 0.181 (P less than .0001), and for 0.5-mg dose, 0.241 (P = .0013).
The number of serious treatment-emergent adverse events in the three groups was low, ranging from 2.5% to 3.5%.
The other study, called RADIANCE, was a similar trial that lasted 24 months. In it, the rate of serious treatment-emergent adverse events in the three groups were similar, ranging from 6.4% to 7.1%.
Ozanimod offers “an excellent therapeutic benefit for patients and a very clean safety profile,” said Bruce Cree, MD, PhD, clinical research director at the University of California, San Francisco, Multiple Sclerosis Center. He is an author on both studies and spoke in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
He said the ozanimod should be especially useful as a first-line treatment for MS. The drug is currently being evaluated by the Food and Drug Administration for an RMS indication, and it is also being developed for Crohn’s disease and ulcerative colitis, he said.
The study was funded by Receptos, a wholly owned subsidiary of Celgene. Dr. Cree reported that he has been a consultant to AbbVie, Biogen, EMD Serono, Genzyme, Novartis, and Shire.
SOURCE: Cree B et al. ACTRIMS Forum 2018, abstract P030, and Comi G et al. ACTRIMS Forum 2018, abstract P023
REPORTING FROM ACTRIMS FORUM 2018
VIDEO: Efficacy of DMTs decreases with age
San Diego – , and high-efficacy drugs do a better job of inhibiting MS disability compared with low-efficacy drugs only in patients younger than 40.5 years.
Those are the key conclusions from a meta-analysis of the age-dependent efficacy of MS treatments that was published in the November 2017 issue of Frontiers in Neurology. In a video interview, Ann Marie Weideman, lead study author, discussed highlights from the meta-analysis at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The meta-analysis drew from more than 28,000 individuals with MS participating in 38 trials of 13 categories of immunomodulatory drugs.
Ms. Weideman is an IRTA Fellow at the National Institute of Neurological Disorders and Stroke, Bethesda, Md. She reported that study coauthor Bibiana Bielekova, MD, is coinventor of several patents related to daclizumab.
San Diego – , and high-efficacy drugs do a better job of inhibiting MS disability compared with low-efficacy drugs only in patients younger than 40.5 years.
Those are the key conclusions from a meta-analysis of the age-dependent efficacy of MS treatments that was published in the November 2017 issue of Frontiers in Neurology. In a video interview, Ann Marie Weideman, lead study author, discussed highlights from the meta-analysis at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The meta-analysis drew from more than 28,000 individuals with MS participating in 38 trials of 13 categories of immunomodulatory drugs.
Ms. Weideman is an IRTA Fellow at the National Institute of Neurological Disorders and Stroke, Bethesda, Md. She reported that study coauthor Bibiana Bielekova, MD, is coinventor of several patents related to daclizumab.
San Diego – , and high-efficacy drugs do a better job of inhibiting MS disability compared with low-efficacy drugs only in patients younger than 40.5 years.
Those are the key conclusions from a meta-analysis of the age-dependent efficacy of MS treatments that was published in the November 2017 issue of Frontiers in Neurology. In a video interview, Ann Marie Weideman, lead study author, discussed highlights from the meta-analysis at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. The meta-analysis drew from more than 28,000 individuals with MS participating in 38 trials of 13 categories of immunomodulatory drugs.
Ms. Weideman is an IRTA Fellow at the National Institute of Neurological Disorders and Stroke, Bethesda, Md. She reported that study coauthor Bibiana Bielekova, MD, is coinventor of several patents related to daclizumab.
REPORTING FROM ACTRIMS FORUM 2018












