User login
Multiple Sclerosis Hub
ECTRIMS and EAN Publish Recommendations for Treating MS
The European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN) have published a guideline to offer up-to-date, evidence-based recommendations for the treatment of adult patients with MS. The guideline is intended to fill a perceived need for a comprehensive document that includes information about recently approved MS therapies and helps clinicians and patients resolve difficulties in everyday clinical practice. It was published in the February issue of Multiple Sclerosis.
Authors Addressed 10 Questions
Xavier Montalban, MD, PhD, Chair and Director of the Department of Neurology and Neuroimmunology at Vall d’Hebron University Hospital in Barcelona, and colleagues agreed to investigate 10 questions related to treatment efficacy, response criteria, strategies to address suboptimal response and safety concerns, and treatment of MS in pregnancy. They developed the guideline following the recommendations of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group, along with EAN recommendations for writing a neurologic management guideline. Literature searches relied upon databases such as the Cochrane Central Register of Controlled Trials, Excerpta Medica, and Medical Literature Analysis and Retrieval System Online.
The authors evaluated data for all disease-modifying treatments (DMTs) approved by the European Medicines Agency at the time of publication. They did not consider combination therapies, complementary or alternative medicine, or symptomatic treatment. Although focused on Europe, the guideline does not account for regulatory or organizational issues specific to any European country.
Strong Recommendations
Dr. Montalban and colleagues agreed upon 21 recommendations and consensus statements. The recommendations were categorized as strong or weak, according to the quality of evidence and the risk–benefit balance. The authors formulated consensus statements on questions for which evidence was insufficient to support a formal recommendation.
The first of the guideline’s three strong recommendations is that neurologists offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS. The second is to offer early treatment with DMTs to patients with active relapsing-remitting MS, as defined by clinical relapses or MRI activity. The authors defined active lesions as contrast-enhancing lesions or new or unequivocally enlarging T2 lesions assessed at least annually. This recommendation also applies to patients with CIS who fulfill current diagnostic criteria for MS. The third strong recommendation is to offer a more efficacious drug to patients treated with interferon or glatiramer acetate who show evidence of disease activity.
Weak Recommendations
Nine of the guideline’s recommendations are categorized as weak. For example, neurologists should consider treating patients with active secondary progressive MS with interferon beta-1a or -1b, taking into account the treatments’ “dubious efficacy,” as well as their safety and tolerability, according to the authors. Neurologists should consider mitoxantrone, ocrelizumab, and cladribine for this population. The authors recommend considering ocrelizumab as a treatment for patients with primary progressive MS.
As a way of monitoring treatment response, the authors recommend that neurologists consider combining MRI with clinical measures when evaluating disease evolution in treated patients. When making treatment decisions in the event of safety concerns, neurologists should consider the possibility that disease activity may resume or rebound if treatment is stopped, particularly with natalizumab, said Dr. Montalban and colleagues. Continuation of DMT treatment should be considered for patients who are clinically stable, who have stable MRI, and who have no problems with safety or tolerability, according to the guideline.
“For women planning a pregnancy, if there is a high risk of disease reactivation, consider using interferon or glatiramer acetate until pregnancy is confirmed,” said the authors. “In some very specific (active) cases, continuing this treatment during pregnancy could also be considered.” Delaying pregnancy is advisable for women with persistent high disease activity. If such a woman decides to become pregnant or has an unplanned pregnancy, neurologists may consider treatment with natalizumab throughout pregnancy after a full discussion with the patient of the potential implications. “Treatment with alemtuzumab could be an alternative therapeutic option for planned pregnancy in very active cases, provided that a four-month interval is strictly observed from the latest infusion until conception,” said Dr. Montalban and colleagues.
Consensus Statements
Several of the guideline’s consensus statements relate to the monitoring of treatment response. For patients treated with DMTs, the authors recommend performing a standardized reference brain MRI within six months of treatment onset, and comparing it with a subsequent brain MRI performed 12 months after starting treatment. The measurement of new or unequivocally enlarging T2 lesions is the preferred MRI method of gauging treatment response, supplemented by gadolinium-enhancing lesions. To monitor treatment safety, the authors recommend performing a standardized reference brain MRI every year in patients at low risk for progressive multifocal leukoencephalopathy (PML), and every three to six months in patients at high risk for PML.
If the neurologist and patient have decided to switch therapies, they should consider the patient’s characteristics and comorbidities, drug safety profiles, and disease severity when choosing a new DMT. If treatment of a highly efficacious drug is stopped because of safety or efficacy problems, the neurologist should consider starting another highly efficacious drug, according to the guideline. Disease activity, the drug’s half-life and biologic activity, and the potential for resumed disease activity or rebound should be considered when choosing a new therapy, said Dr. Montalban and colleagues.
—Erik Greb
Suggested Reading
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96-120.
The European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN) have published a guideline to offer up-to-date, evidence-based recommendations for the treatment of adult patients with MS. The guideline is intended to fill a perceived need for a comprehensive document that includes information about recently approved MS therapies and helps clinicians and patients resolve difficulties in everyday clinical practice. It was published in the February issue of Multiple Sclerosis.
Authors Addressed 10 Questions
Xavier Montalban, MD, PhD, Chair and Director of the Department of Neurology and Neuroimmunology at Vall d’Hebron University Hospital in Barcelona, and colleagues agreed to investigate 10 questions related to treatment efficacy, response criteria, strategies to address suboptimal response and safety concerns, and treatment of MS in pregnancy. They developed the guideline following the recommendations of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group, along with EAN recommendations for writing a neurologic management guideline. Literature searches relied upon databases such as the Cochrane Central Register of Controlled Trials, Excerpta Medica, and Medical Literature Analysis and Retrieval System Online.
The authors evaluated data for all disease-modifying treatments (DMTs) approved by the European Medicines Agency at the time of publication. They did not consider combination therapies, complementary or alternative medicine, or symptomatic treatment. Although focused on Europe, the guideline does not account for regulatory or organizational issues specific to any European country.
Strong Recommendations
Dr. Montalban and colleagues agreed upon 21 recommendations and consensus statements. The recommendations were categorized as strong or weak, according to the quality of evidence and the risk–benefit balance. The authors formulated consensus statements on questions for which evidence was insufficient to support a formal recommendation.
The first of the guideline’s three strong recommendations is that neurologists offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS. The second is to offer early treatment with DMTs to patients with active relapsing-remitting MS, as defined by clinical relapses or MRI activity. The authors defined active lesions as contrast-enhancing lesions or new or unequivocally enlarging T2 lesions assessed at least annually. This recommendation also applies to patients with CIS who fulfill current diagnostic criteria for MS. The third strong recommendation is to offer a more efficacious drug to patients treated with interferon or glatiramer acetate who show evidence of disease activity.
Weak Recommendations
Nine of the guideline’s recommendations are categorized as weak. For example, neurologists should consider treating patients with active secondary progressive MS with interferon beta-1a or -1b, taking into account the treatments’ “dubious efficacy,” as well as their safety and tolerability, according to the authors. Neurologists should consider mitoxantrone, ocrelizumab, and cladribine for this population. The authors recommend considering ocrelizumab as a treatment for patients with primary progressive MS.
As a way of monitoring treatment response, the authors recommend that neurologists consider combining MRI with clinical measures when evaluating disease evolution in treated patients. When making treatment decisions in the event of safety concerns, neurologists should consider the possibility that disease activity may resume or rebound if treatment is stopped, particularly with natalizumab, said Dr. Montalban and colleagues. Continuation of DMT treatment should be considered for patients who are clinically stable, who have stable MRI, and who have no problems with safety or tolerability, according to the guideline.
“For women planning a pregnancy, if there is a high risk of disease reactivation, consider using interferon or glatiramer acetate until pregnancy is confirmed,” said the authors. “In some very specific (active) cases, continuing this treatment during pregnancy could also be considered.” Delaying pregnancy is advisable for women with persistent high disease activity. If such a woman decides to become pregnant or has an unplanned pregnancy, neurologists may consider treatment with natalizumab throughout pregnancy after a full discussion with the patient of the potential implications. “Treatment with alemtuzumab could be an alternative therapeutic option for planned pregnancy in very active cases, provided that a four-month interval is strictly observed from the latest infusion until conception,” said Dr. Montalban and colleagues.
Consensus Statements
Several of the guideline’s consensus statements relate to the monitoring of treatment response. For patients treated with DMTs, the authors recommend performing a standardized reference brain MRI within six months of treatment onset, and comparing it with a subsequent brain MRI performed 12 months after starting treatment. The measurement of new or unequivocally enlarging T2 lesions is the preferred MRI method of gauging treatment response, supplemented by gadolinium-enhancing lesions. To monitor treatment safety, the authors recommend performing a standardized reference brain MRI every year in patients at low risk for progressive multifocal leukoencephalopathy (PML), and every three to six months in patients at high risk for PML.
If the neurologist and patient have decided to switch therapies, they should consider the patient’s characteristics and comorbidities, drug safety profiles, and disease severity when choosing a new DMT. If treatment of a highly efficacious drug is stopped because of safety or efficacy problems, the neurologist should consider starting another highly efficacious drug, according to the guideline. Disease activity, the drug’s half-life and biologic activity, and the potential for resumed disease activity or rebound should be considered when choosing a new therapy, said Dr. Montalban and colleagues.
—Erik Greb
Suggested Reading
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96-120.
The European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN) have published a guideline to offer up-to-date, evidence-based recommendations for the treatment of adult patients with MS. The guideline is intended to fill a perceived need for a comprehensive document that includes information about recently approved MS therapies and helps clinicians and patients resolve difficulties in everyday clinical practice. It was published in the February issue of Multiple Sclerosis.
Authors Addressed 10 Questions
Xavier Montalban, MD, PhD, Chair and Director of the Department of Neurology and Neuroimmunology at Vall d’Hebron University Hospital in Barcelona, and colleagues agreed to investigate 10 questions related to treatment efficacy, response criteria, strategies to address suboptimal response and safety concerns, and treatment of MS in pregnancy. They developed the guideline following the recommendations of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group, along with EAN recommendations for writing a neurologic management guideline. Literature searches relied upon databases such as the Cochrane Central Register of Controlled Trials, Excerpta Medica, and Medical Literature Analysis and Retrieval System Online.
The authors evaluated data for all disease-modifying treatments (DMTs) approved by the European Medicines Agency at the time of publication. They did not consider combination therapies, complementary or alternative medicine, or symptomatic treatment. Although focused on Europe, the guideline does not account for regulatory or organizational issues specific to any European country.
Strong Recommendations
Dr. Montalban and colleagues agreed upon 21 recommendations and consensus statements. The recommendations were categorized as strong or weak, according to the quality of evidence and the risk–benefit balance. The authors formulated consensus statements on questions for which evidence was insufficient to support a formal recommendation.
The first of the guideline’s three strong recommendations is that neurologists offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS. The second is to offer early treatment with DMTs to patients with active relapsing-remitting MS, as defined by clinical relapses or MRI activity. The authors defined active lesions as contrast-enhancing lesions or new or unequivocally enlarging T2 lesions assessed at least annually. This recommendation also applies to patients with CIS who fulfill current diagnostic criteria for MS. The third strong recommendation is to offer a more efficacious drug to patients treated with interferon or glatiramer acetate who show evidence of disease activity.
Weak Recommendations
Nine of the guideline’s recommendations are categorized as weak. For example, neurologists should consider treating patients with active secondary progressive MS with interferon beta-1a or -1b, taking into account the treatments’ “dubious efficacy,” as well as their safety and tolerability, according to the authors. Neurologists should consider mitoxantrone, ocrelizumab, and cladribine for this population. The authors recommend considering ocrelizumab as a treatment for patients with primary progressive MS.
As a way of monitoring treatment response, the authors recommend that neurologists consider combining MRI with clinical measures when evaluating disease evolution in treated patients. When making treatment decisions in the event of safety concerns, neurologists should consider the possibility that disease activity may resume or rebound if treatment is stopped, particularly with natalizumab, said Dr. Montalban and colleagues. Continuation of DMT treatment should be considered for patients who are clinically stable, who have stable MRI, and who have no problems with safety or tolerability, according to the guideline.
“For women planning a pregnancy, if there is a high risk of disease reactivation, consider using interferon or glatiramer acetate until pregnancy is confirmed,” said the authors. “In some very specific (active) cases, continuing this treatment during pregnancy could also be considered.” Delaying pregnancy is advisable for women with persistent high disease activity. If such a woman decides to become pregnant or has an unplanned pregnancy, neurologists may consider treatment with natalizumab throughout pregnancy after a full discussion with the patient of the potential implications. “Treatment with alemtuzumab could be an alternative therapeutic option for planned pregnancy in very active cases, provided that a four-month interval is strictly observed from the latest infusion until conception,” said Dr. Montalban and colleagues.
Consensus Statements
Several of the guideline’s consensus statements relate to the monitoring of treatment response. For patients treated with DMTs, the authors recommend performing a standardized reference brain MRI within six months of treatment onset, and comparing it with a subsequent brain MRI performed 12 months after starting treatment. The measurement of new or unequivocally enlarging T2 lesions is the preferred MRI method of gauging treatment response, supplemented by gadolinium-enhancing lesions. To monitor treatment safety, the authors recommend performing a standardized reference brain MRI every year in patients at low risk for progressive multifocal leukoencephalopathy (PML), and every three to six months in patients at high risk for PML.
If the neurologist and patient have decided to switch therapies, they should consider the patient’s characteristics and comorbidities, drug safety profiles, and disease severity when choosing a new DMT. If treatment of a highly efficacious drug is stopped because of safety or efficacy problems, the neurologist should consider starting another highly efficacious drug, according to the guideline. Disease activity, the drug’s half-life and biologic activity, and the potential for resumed disease activity or rebound should be considered when choosing a new therapy, said Dr. Montalban and colleagues.
—Erik Greb
Suggested Reading
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96-120.
Fingolimod switch from an injectable linked to improved outcomes in relapsing MS
SAN DIEGO – People with relapsing multiple sclerosis who switched from an injectable disease-modifying therapy to fingolimod after the randomized phase of the PREFERMS trial experienced improved annualized relapse rates, exposure-adjusted percentage brain volume loss, and satisfaction, compared with study participants who did not switch.
Importantly, there were no differences between switchers who were treatment-naive or previously treated with one injectable therapy class at baseline in the phase 4, active-controlled, open-label, and multicenter PREFERMS trial, according to the researchers.
Of the 875 patients with relapsing multiple sclerosis, 254 or 58% of those initially randomized to an injectable disease-modifying therapy (iDMT) switched to fingolimod (Gilenya) during the 12-month study. Participants in the trial were permitted to switch therapy once after at least 3 months, or sooner if warranted for safety or efficacy reasons.
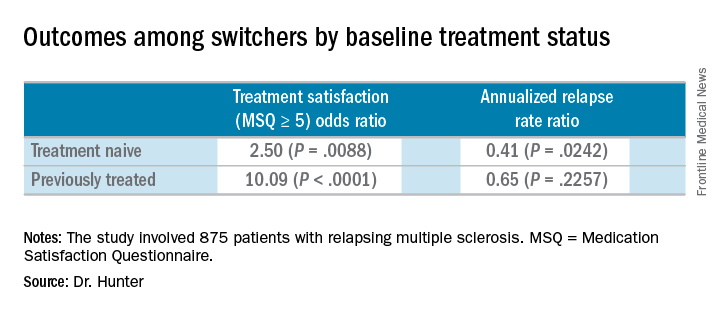
Compared with the end the randomization phase, patients who switched from an iDMT to fingolimod were more satisfied, defined as a Medication Satisfaction Questionnaire score of 5 or greater (odds ratio, 5.65; P less than .001) at 12 months. At the same time, their annualized relapse rate decreased (ARR ratio, 0.53; P = .0158) and their exposure-adjusted brain volume loss decreased from 0.874% to 0.463%.
The benefit of these switches was observed regardless of previous treatment status, Dr. Hunter said during a poster presentation at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
At the start of the PREFERMS trial, patients were randomized 1:1 to fingolimod 0.5 mg/day or a preselected iDMT. Just more than half, 54%, were treatment-naive and 46% had previous interferon or glatiramer acetate treatment.
“Generally, because most of these people were switched, and we’re looking at people who were treatment-naive or previously treated, when you look at brain volume loss, it’s very low on fingolimod versus high while they’re on injectable therapies,” Dr. Hunter said.
Dr. Hunter and his colleagues reported similar relapse rates and brain volume loss outcomes among the 254 patients who switched to fingolimod mid-study according to baseline treatment history. The investigators noted, however, that these post hoc analyses and P values are intended for hypothesis generation only.
In the 99 treatment-naive switchers, brain volume loss was 0.54% at the end of the study, compared with 0.84% at the end of the randomization period. Among the 155 previously treated switchers, brain volume loss also decreased, from 0.90% at the end of randomization to 0.42% at study end.
Dr. Hunter disclosed that he is an investigator for Novartis, the sponsor of the study.
SOURCE: Hunter S et al. ACTRIMS Forum 2018 Abstract P019.
SAN DIEGO – People with relapsing multiple sclerosis who switched from an injectable disease-modifying therapy to fingolimod after the randomized phase of the PREFERMS trial experienced improved annualized relapse rates, exposure-adjusted percentage brain volume loss, and satisfaction, compared with study participants who did not switch.
Importantly, there were no differences between switchers who were treatment-naive or previously treated with one injectable therapy class at baseline in the phase 4, active-controlled, open-label, and multicenter PREFERMS trial, according to the researchers.
Of the 875 patients with relapsing multiple sclerosis, 254 or 58% of those initially randomized to an injectable disease-modifying therapy (iDMT) switched to fingolimod (Gilenya) during the 12-month study. Participants in the trial were permitted to switch therapy once after at least 3 months, or sooner if warranted for safety or efficacy reasons.
Compared with the end the randomization phase, patients who switched from an iDMT to fingolimod were more satisfied, defined as a Medication Satisfaction Questionnaire score of 5 or greater (odds ratio, 5.65; P less than .001) at 12 months. At the same time, their annualized relapse rate decreased (ARR ratio, 0.53; P = .0158) and their exposure-adjusted brain volume loss decreased from 0.874% to 0.463%.
The benefit of these switches was observed regardless of previous treatment status, Dr. Hunter said during a poster presentation at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.

At the start of the PREFERMS trial, patients were randomized 1:1 to fingolimod 0.5 mg/day or a preselected iDMT. Just more than half, 54%, were treatment-naive and 46% had previous interferon or glatiramer acetate treatment.
“Generally, because most of these people were switched, and we’re looking at people who were treatment-naive or previously treated, when you look at brain volume loss, it’s very low on fingolimod versus high while they’re on injectable therapies,” Dr. Hunter said.
Dr. Hunter and his colleagues reported similar relapse rates and brain volume loss outcomes among the 254 patients who switched to fingolimod mid-study according to baseline treatment history. The investigators noted, however, that these post hoc analyses and P values are intended for hypothesis generation only.
In the 99 treatment-naive switchers, brain volume loss was 0.54% at the end of the study, compared with 0.84% at the end of the randomization period. Among the 155 previously treated switchers, brain volume loss also decreased, from 0.90% at the end of randomization to 0.42% at study end.
Dr. Hunter disclosed that he is an investigator for Novartis, the sponsor of the study.
SOURCE: Hunter S et al. ACTRIMS Forum 2018 Abstract P019.
SAN DIEGO – People with relapsing multiple sclerosis who switched from an injectable disease-modifying therapy to fingolimod after the randomized phase of the PREFERMS trial experienced improved annualized relapse rates, exposure-adjusted percentage brain volume loss, and satisfaction, compared with study participants who did not switch.
Importantly, there were no differences between switchers who were treatment-naive or previously treated with one injectable therapy class at baseline in the phase 4, active-controlled, open-label, and multicenter PREFERMS trial, according to the researchers.
Of the 875 patients with relapsing multiple sclerosis, 254 or 58% of those initially randomized to an injectable disease-modifying therapy (iDMT) switched to fingolimod (Gilenya) during the 12-month study. Participants in the trial were permitted to switch therapy once after at least 3 months, or sooner if warranted for safety or efficacy reasons.
Compared with the end the randomization phase, patients who switched from an iDMT to fingolimod were more satisfied, defined as a Medication Satisfaction Questionnaire score of 5 or greater (odds ratio, 5.65; P less than .001) at 12 months. At the same time, their annualized relapse rate decreased (ARR ratio, 0.53; P = .0158) and their exposure-adjusted brain volume loss decreased from 0.874% to 0.463%.
The benefit of these switches was observed regardless of previous treatment status, Dr. Hunter said during a poster presentation at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.

At the start of the PREFERMS trial, patients were randomized 1:1 to fingolimod 0.5 mg/day or a preselected iDMT. Just more than half, 54%, were treatment-naive and 46% had previous interferon or glatiramer acetate treatment.
“Generally, because most of these people were switched, and we’re looking at people who were treatment-naive or previously treated, when you look at brain volume loss, it’s very low on fingolimod versus high while they’re on injectable therapies,” Dr. Hunter said.
Dr. Hunter and his colleagues reported similar relapse rates and brain volume loss outcomes among the 254 patients who switched to fingolimod mid-study according to baseline treatment history. The investigators noted, however, that these post hoc analyses and P values are intended for hypothesis generation only.
In the 99 treatment-naive switchers, brain volume loss was 0.54% at the end of the study, compared with 0.84% at the end of the randomization period. Among the 155 previously treated switchers, brain volume loss also decreased, from 0.90% at the end of randomization to 0.42% at study end.
Dr. Hunter disclosed that he is an investigator for Novartis, the sponsor of the study.
SOURCE: Hunter S et al. ACTRIMS Forum 2018 Abstract P019.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point:
Major finding: Patients who switched from an injectable disease-modifying therapy to fingolimod were more satisfied, defined as a Medication Satisfaction Questionnaire score of 5 or greater (odds ratio, 5.65; P less than .001).
Study details: Post hoc analyses of the extension study of the PREFERMS trial.
Disclosures: Dr. Hunter disclosed that he is an investigator for Novartis, the sponsor of the study.
Source: Hunter S et al. ACTRIMS Forum 2018 Abstract P019.
Extended-interval dosing of natalizumab linked to lower risk of PML
SAN DIEGO – A large industry-funded study of the multiple sclerosis drug natalizumab suggests that physicians can dramatically lower the likelihood of progressive multifocal leukoencephalopathy in patients at higher risk of the condition by increasing the interval between doses.
Previous research has suggested that natalizumab (Tysabri) doesn’t lose efficacy when given less frequently.
Dr. Zhovtis Ryerson presented the study findings at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
As a 2014 report explains, natalizumab is a “highly effective disease-modifying therapy for the treatment of relapsing forms of multiple sclerosis,” but the risk of progressive multifocal leukoencephalopathy (PML) “has largely contributed to it being relegated to a second-line position” (Ther Adv Chronic Dis. 2014 Mar;5[2]:62-8).
Patients previously exposed to John Cunningham (JC) virus are at higher risk of PML, a rare viral brain disease that can be severely disabling or deadly. A 2017 report estimated the combined cumulative PML probability at 2.7% (with previous immunosuppressant use) and 1.7% (no previous immunosuppressant use) over 6 years in patients with signs of exposure to the JC virus (Lancet Neurol. 2017 Nov;16[11]:925-33). According to Dr. Zhovtis Ryerson, natalizumab manufacturer Biogen reported in December 2017 that it has confirmed 756 cases of PML in natalizumab-treated patients, all except for 3 in MS patients.
Doctors are wary in the at-risk patient population. “In general,” she said, “clinicians treating patients who are JC virus positive, and therefore have a risk for PML, may not utilize the drug or may take the patient off it after 2 years, which is when the risk goes up.”
Some physicians have experimented with longer intervals between treatments of 300 mg given intravenously, administering it every 5-8 weeks instead of every 4 weeks, with an eye on not extending the interval for too long “because MS disease activity returns after 12 weeks of withholding therapy,” Dr. Zhovtis Ryerson said.
In 2016, Dr. Zhovtis Ryerson and colleagues reported in a retrospective analysis that natalizumab dosing intervals of up to 8 weeks, 5 days didn’t affect the drug’s efficacy (J Neurol Neurosurg Psychiatry. 2016 Aug;87[8]:885-9).
For the primary analyses in the new study – whether dosing history in the last 18 months of natalizumab treatment affects PML – researchers tracked 1,988 patients who took the drug via an extended interval schedule and 13,132 who took it via a standard interval. The patients were tracked in the mandated TOUCH Prescribing Program; all participants showed signs of exposure to the JC virus.
The two groups were similar at about 68% female, an average age at first infusion of about 43, and a median number of about 48 natalizumab infusions.
For patients without previous immunosuppressant use who had received 49-60 doses, the researchers estimated the incidence of PML per 1,000 patients as 1.23 in the extended-interval group and 3.96 in the standard-interval group. The numbers were slightly higher for those who received 61-72 doses. At 48 or fewer doses, there were no PML cases in patients on extended-interval dosing.
“The data showed highly significant risk reductions of up to 94%,” Dr. Zhovtis Ryerson said.
Moving forward, “in collaboration with Biogen, we have more sensitivity analysis to be done to assure that our conclusions on this are correct,” she said. “Furthermore, more evidence of efficacy of extended-interval dosing natalizumab is needed, and we hope to move forward with a prospective, randomized, controlled trial to give clinicians the highest level of evidence we can.”
The study was funded by Biogen. Dr. Zhovtis Ryerson reported personal compensation from Teva, speaker/advisory board activities for Biogen, and research support from Biogen. Seven other authors reported being Biogen employees. Other authors reported no disclosures or various disclosures, including connections to Biogen.
SOURCE: Zhovtis R et al. ACTRIMS Forum 2018 Abstract LB250.
SAN DIEGO – A large industry-funded study of the multiple sclerosis drug natalizumab suggests that physicians can dramatically lower the likelihood of progressive multifocal leukoencephalopathy in patients at higher risk of the condition by increasing the interval between doses.
Previous research has suggested that natalizumab (Tysabri) doesn’t lose efficacy when given less frequently.
Dr. Zhovtis Ryerson presented the study findings at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
As a 2014 report explains, natalizumab is a “highly effective disease-modifying therapy for the treatment of relapsing forms of multiple sclerosis,” but the risk of progressive multifocal leukoencephalopathy (PML) “has largely contributed to it being relegated to a second-line position” (Ther Adv Chronic Dis. 2014 Mar;5[2]:62-8).
Patients previously exposed to John Cunningham (JC) virus are at higher risk of PML, a rare viral brain disease that can be severely disabling or deadly. A 2017 report estimated the combined cumulative PML probability at 2.7% (with previous immunosuppressant use) and 1.7% (no previous immunosuppressant use) over 6 years in patients with signs of exposure to the JC virus (Lancet Neurol. 2017 Nov;16[11]:925-33). According to Dr. Zhovtis Ryerson, natalizumab manufacturer Biogen reported in December 2017 that it has confirmed 756 cases of PML in natalizumab-treated patients, all except for 3 in MS patients.
Doctors are wary in the at-risk patient population. “In general,” she said, “clinicians treating patients who are JC virus positive, and therefore have a risk for PML, may not utilize the drug or may take the patient off it after 2 years, which is when the risk goes up.”
Some physicians have experimented with longer intervals between treatments of 300 mg given intravenously, administering it every 5-8 weeks instead of every 4 weeks, with an eye on not extending the interval for too long “because MS disease activity returns after 12 weeks of withholding therapy,” Dr. Zhovtis Ryerson said.
In 2016, Dr. Zhovtis Ryerson and colleagues reported in a retrospective analysis that natalizumab dosing intervals of up to 8 weeks, 5 days didn’t affect the drug’s efficacy (J Neurol Neurosurg Psychiatry. 2016 Aug;87[8]:885-9).
For the primary analyses in the new study – whether dosing history in the last 18 months of natalizumab treatment affects PML – researchers tracked 1,988 patients who took the drug via an extended interval schedule and 13,132 who took it via a standard interval. The patients were tracked in the mandated TOUCH Prescribing Program; all participants showed signs of exposure to the JC virus.
The two groups were similar at about 68% female, an average age at first infusion of about 43, and a median number of about 48 natalizumab infusions.
For patients without previous immunosuppressant use who had received 49-60 doses, the researchers estimated the incidence of PML per 1,000 patients as 1.23 in the extended-interval group and 3.96 in the standard-interval group. The numbers were slightly higher for those who received 61-72 doses. At 48 or fewer doses, there were no PML cases in patients on extended-interval dosing.
“The data showed highly significant risk reductions of up to 94%,” Dr. Zhovtis Ryerson said.
Moving forward, “in collaboration with Biogen, we have more sensitivity analysis to be done to assure that our conclusions on this are correct,” she said. “Furthermore, more evidence of efficacy of extended-interval dosing natalizumab is needed, and we hope to move forward with a prospective, randomized, controlled trial to give clinicians the highest level of evidence we can.”
The study was funded by Biogen. Dr. Zhovtis Ryerson reported personal compensation from Teva, speaker/advisory board activities for Biogen, and research support from Biogen. Seven other authors reported being Biogen employees. Other authors reported no disclosures or various disclosures, including connections to Biogen.
SOURCE: Zhovtis R et al. ACTRIMS Forum 2018 Abstract LB250.
SAN DIEGO – A large industry-funded study of the multiple sclerosis drug natalizumab suggests that physicians can dramatically lower the likelihood of progressive multifocal leukoencephalopathy in patients at higher risk of the condition by increasing the interval between doses.
Previous research has suggested that natalizumab (Tysabri) doesn’t lose efficacy when given less frequently.
Dr. Zhovtis Ryerson presented the study findings at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
As a 2014 report explains, natalizumab is a “highly effective disease-modifying therapy for the treatment of relapsing forms of multiple sclerosis,” but the risk of progressive multifocal leukoencephalopathy (PML) “has largely contributed to it being relegated to a second-line position” (Ther Adv Chronic Dis. 2014 Mar;5[2]:62-8).
Patients previously exposed to John Cunningham (JC) virus are at higher risk of PML, a rare viral brain disease that can be severely disabling or deadly. A 2017 report estimated the combined cumulative PML probability at 2.7% (with previous immunosuppressant use) and 1.7% (no previous immunosuppressant use) over 6 years in patients with signs of exposure to the JC virus (Lancet Neurol. 2017 Nov;16[11]:925-33). According to Dr. Zhovtis Ryerson, natalizumab manufacturer Biogen reported in December 2017 that it has confirmed 756 cases of PML in natalizumab-treated patients, all except for 3 in MS patients.
Doctors are wary in the at-risk patient population. “In general,” she said, “clinicians treating patients who are JC virus positive, and therefore have a risk for PML, may not utilize the drug or may take the patient off it after 2 years, which is when the risk goes up.”
Some physicians have experimented with longer intervals between treatments of 300 mg given intravenously, administering it every 5-8 weeks instead of every 4 weeks, with an eye on not extending the interval for too long “because MS disease activity returns after 12 weeks of withholding therapy,” Dr. Zhovtis Ryerson said.
In 2016, Dr. Zhovtis Ryerson and colleagues reported in a retrospective analysis that natalizumab dosing intervals of up to 8 weeks, 5 days didn’t affect the drug’s efficacy (J Neurol Neurosurg Psychiatry. 2016 Aug;87[8]:885-9).
For the primary analyses in the new study – whether dosing history in the last 18 months of natalizumab treatment affects PML – researchers tracked 1,988 patients who took the drug via an extended interval schedule and 13,132 who took it via a standard interval. The patients were tracked in the mandated TOUCH Prescribing Program; all participants showed signs of exposure to the JC virus.
The two groups were similar at about 68% female, an average age at first infusion of about 43, and a median number of about 48 natalizumab infusions.
For patients without previous immunosuppressant use who had received 49-60 doses, the researchers estimated the incidence of PML per 1,000 patients as 1.23 in the extended-interval group and 3.96 in the standard-interval group. The numbers were slightly higher for those who received 61-72 doses. At 48 or fewer doses, there were no PML cases in patients on extended-interval dosing.
“The data showed highly significant risk reductions of up to 94%,” Dr. Zhovtis Ryerson said.
Moving forward, “in collaboration with Biogen, we have more sensitivity analysis to be done to assure that our conclusions on this are correct,” she said. “Furthermore, more evidence of efficacy of extended-interval dosing natalizumab is needed, and we hope to move forward with a prospective, randomized, controlled trial to give clinicians the highest level of evidence we can.”
The study was funded by Biogen. Dr. Zhovtis Ryerson reported personal compensation from Teva, speaker/advisory board activities for Biogen, and research support from Biogen. Seven other authors reported being Biogen employees. Other authors reported no disclosures or various disclosures, including connections to Biogen.
SOURCE: Zhovtis R et al. ACTRIMS Forum 2018 Abstract LB250.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point: , compared with standard-interval dosing in patients who showed signs of exposure to the JC virus.
Major finding: Estimated incidence of PML per 1,000 patients was 1.23 in an extended-interval group and 3.96 in a standard-interval group among those who had received 49-60 natalizumab doses.
Study details: 18-month analysis of multiple sclerosis patients on natalizumab who showed signs of exposure to the JC virus: 1,988 on extended-interval dosing and 13,132 on standard-interval dosing.
Disclosures: The study was funded by Biogen, and multiple study authors report being employees of Biogen or having other relationships to the company.
Source: Zhovtis R et al. ACTRIMS Forum 2018, Abstract LB250.
Is MS caused by one-two punch of pinworm and Epstein-Barr virus?
SAN DIEGO – What causes multiple sclerosis (MS)? A team of Scottish researchers offers a new theory that it’s triggered in part by a one-two punch of infection with pinworm – a common condition in the United States, especially among children – and the Epstein-Barr virus (EBV).
The theory identifies pinworm as the prime suspect to be the “missing link” that explains why EBV and MS are so tightly connected, said Patrick Kearns, MBChB, a graduate student at Harvard T.H. Chan School of Public Health in Boston.
Dr. Kearns is the lead author of two reports about the possible role of pinworm that were presented at ACTRIMS Forum 2018, which is held by the Americas Committee for Treatment and Research in Multiple Sclerosis. He spoke in an interview.
Dr. Kearns and his colleagues focused on a well-known cluster of MS cases that began to appear in the Faroe Islands – a Danish possession in the North Atlantic – during World War II. The cases began to appear after British troops occupied the islands.
“Many of the occupation soldiers were from the Scottish Highlands, where the MS prevalence is quite high: 90 cases per 100,000, comparable to the northern U.S.,” according to a National MS Society summary about MS clusters.
In a theory that spawned controversy, the late neurologist John Kurtzke, MD, speculated that the British soldiers brought a transmissible agent to the islands, which triggered MS cases.
Could the agent be EBV alone? The authors of the new studies don’t think so, although they note that EBV is “robustly linked” to MS. Indeed, a 2012 meta-analysis reported that the virus “appears to be present in 100% of MS patients,” based on studies considered to be the strongest (Mult Scler. 2013 Feb;19[2]:162-6).
The authors of the new reports note that, while EBV infection “appears necessary,” it is “clearly not sufficient” to cause the disease on its own.
“Certainly almost everyone gets EBV eventually,” Dr. Kearns said. “So mere presence of the virus is certainly not sufficient for causing the disease. But it seems still to be necessary, and timing of infection might be everything.”
So what’s the missing piece of the puzzle?
Dr. Kearns began to think it might be the lowly pinworm after helping a colleague by analyzing data from appendicitis samples in children. He noted that uninflamed samples often had pinworms in them, but the inflamed samples often didn’t, which suggested that “the rate of pinworms in normal appendices must be very high in the healthy pediatric population at any given time.”
More data confirmed this to be true, and medical literature told Dr. Kearns that pinworms were common in high latitudes – places where people often are especially prone to MS.
“Most remarkably, they are known to have very little migration and stay spatially stable in populations over long periods of time,” Dr. Kearns said, “and typically everyone in an affected population will encounter them because their eggs are transmitted in household dust.”
And, he said, “they are known to be common in soldiers who live in military accommodation.”
According to the Centers for Disease Control and Prevention, pinworm prevalence can be as high as 50% in at-risk groups – children, caregivers of infected children, and people who live in institutions. Pinworms, which are spread through ingestion, are often asymptomatic but may cause anal itching and trigger bacterial infections.
The researchers suggest that pinworm infection comes first, followed by EBV infection. This makes sense because “late EBV infection in the form of infectious mononucleosis is known to be a risk factor for MS,” Dr. Kearns said.
The one-two punch of pinworm and then EBV is a plausible theory “because EBV lives in memory B cells, which are known to be important in MS and could be specific for the previous exposure to pinworm,” Dr. Kearns said. “However, this is very speculative and some researchers will argue this is very unlikely to be the case. But I think there is a chance it could explain some of the epidemiology, so I’m keen to try and test the theory if I can.”
What’s next? Dr. Kearns wants to explore data from Scotland in search of areas of high and low MS incidence that could offer insight into environmental triggers.
He added that the development of a serological blood test to prove a history of pinworm infection would be “the most effective way to prove or disprove this theory.”
“I have approached an investigator who has a track record of doing this for other infections and have been encouraged that he thinks that it would be achievable,” he said. “But this will definitely take time and funding.”
No specific funding was reported. The study authors reported no relevant disclosures.
SOURCE: Kearns P et al. ACTRIMS Forum 2018, Abstracts LB257 and LB264.
SAN DIEGO – What causes multiple sclerosis (MS)? A team of Scottish researchers offers a new theory that it’s triggered in part by a one-two punch of infection with pinworm – a common condition in the United States, especially among children – and the Epstein-Barr virus (EBV).
The theory identifies pinworm as the prime suspect to be the “missing link” that explains why EBV and MS are so tightly connected, said Patrick Kearns, MBChB, a graduate student at Harvard T.H. Chan School of Public Health in Boston.
Dr. Kearns is the lead author of two reports about the possible role of pinworm that were presented at ACTRIMS Forum 2018, which is held by the Americas Committee for Treatment and Research in Multiple Sclerosis. He spoke in an interview.
Dr. Kearns and his colleagues focused on a well-known cluster of MS cases that began to appear in the Faroe Islands – a Danish possession in the North Atlantic – during World War II. The cases began to appear after British troops occupied the islands.
“Many of the occupation soldiers were from the Scottish Highlands, where the MS prevalence is quite high: 90 cases per 100,000, comparable to the northern U.S.,” according to a National MS Society summary about MS clusters.
In a theory that spawned controversy, the late neurologist John Kurtzke, MD, speculated that the British soldiers brought a transmissible agent to the islands, which triggered MS cases.
Could the agent be EBV alone? The authors of the new studies don’t think so, although they note that EBV is “robustly linked” to MS. Indeed, a 2012 meta-analysis reported that the virus “appears to be present in 100% of MS patients,” based on studies considered to be the strongest (Mult Scler. 2013 Feb;19[2]:162-6).
The authors of the new reports note that, while EBV infection “appears necessary,” it is “clearly not sufficient” to cause the disease on its own.
“Certainly almost everyone gets EBV eventually,” Dr. Kearns said. “So mere presence of the virus is certainly not sufficient for causing the disease. But it seems still to be necessary, and timing of infection might be everything.”
So what’s the missing piece of the puzzle?
Dr. Kearns began to think it might be the lowly pinworm after helping a colleague by analyzing data from appendicitis samples in children. He noted that uninflamed samples often had pinworms in them, but the inflamed samples often didn’t, which suggested that “the rate of pinworms in normal appendices must be very high in the healthy pediatric population at any given time.”
More data confirmed this to be true, and medical literature told Dr. Kearns that pinworms were common in high latitudes – places where people often are especially prone to MS.
“Most remarkably, they are known to have very little migration and stay spatially stable in populations over long periods of time,” Dr. Kearns said, “and typically everyone in an affected population will encounter them because their eggs are transmitted in household dust.”
And, he said, “they are known to be common in soldiers who live in military accommodation.”
According to the Centers for Disease Control and Prevention, pinworm prevalence can be as high as 50% in at-risk groups – children, caregivers of infected children, and people who live in institutions. Pinworms, which are spread through ingestion, are often asymptomatic but may cause anal itching and trigger bacterial infections.
The researchers suggest that pinworm infection comes first, followed by EBV infection. This makes sense because “late EBV infection in the form of infectious mononucleosis is known to be a risk factor for MS,” Dr. Kearns said.
The one-two punch of pinworm and then EBV is a plausible theory “because EBV lives in memory B cells, which are known to be important in MS and could be specific for the previous exposure to pinworm,” Dr. Kearns said. “However, this is very speculative and some researchers will argue this is very unlikely to be the case. But I think there is a chance it could explain some of the epidemiology, so I’m keen to try and test the theory if I can.”
What’s next? Dr. Kearns wants to explore data from Scotland in search of areas of high and low MS incidence that could offer insight into environmental triggers.
He added that the development of a serological blood test to prove a history of pinworm infection would be “the most effective way to prove or disprove this theory.”
“I have approached an investigator who has a track record of doing this for other infections and have been encouraged that he thinks that it would be achievable,” he said. “But this will definitely take time and funding.”
No specific funding was reported. The study authors reported no relevant disclosures.
SOURCE: Kearns P et al. ACTRIMS Forum 2018, Abstracts LB257 and LB264.
SAN DIEGO – What causes multiple sclerosis (MS)? A team of Scottish researchers offers a new theory that it’s triggered in part by a one-two punch of infection with pinworm – a common condition in the United States, especially among children – and the Epstein-Barr virus (EBV).
The theory identifies pinworm as the prime suspect to be the “missing link” that explains why EBV and MS are so tightly connected, said Patrick Kearns, MBChB, a graduate student at Harvard T.H. Chan School of Public Health in Boston.
Dr. Kearns is the lead author of two reports about the possible role of pinworm that were presented at ACTRIMS Forum 2018, which is held by the Americas Committee for Treatment and Research in Multiple Sclerosis. He spoke in an interview.
Dr. Kearns and his colleagues focused on a well-known cluster of MS cases that began to appear in the Faroe Islands – a Danish possession in the North Atlantic – during World War II. The cases began to appear after British troops occupied the islands.
“Many of the occupation soldiers were from the Scottish Highlands, where the MS prevalence is quite high: 90 cases per 100,000, comparable to the northern U.S.,” according to a National MS Society summary about MS clusters.
In a theory that spawned controversy, the late neurologist John Kurtzke, MD, speculated that the British soldiers brought a transmissible agent to the islands, which triggered MS cases.
Could the agent be EBV alone? The authors of the new studies don’t think so, although they note that EBV is “robustly linked” to MS. Indeed, a 2012 meta-analysis reported that the virus “appears to be present in 100% of MS patients,” based on studies considered to be the strongest (Mult Scler. 2013 Feb;19[2]:162-6).
The authors of the new reports note that, while EBV infection “appears necessary,” it is “clearly not sufficient” to cause the disease on its own.
“Certainly almost everyone gets EBV eventually,” Dr. Kearns said. “So mere presence of the virus is certainly not sufficient for causing the disease. But it seems still to be necessary, and timing of infection might be everything.”
So what’s the missing piece of the puzzle?
Dr. Kearns began to think it might be the lowly pinworm after helping a colleague by analyzing data from appendicitis samples in children. He noted that uninflamed samples often had pinworms in them, but the inflamed samples often didn’t, which suggested that “the rate of pinworms in normal appendices must be very high in the healthy pediatric population at any given time.”
More data confirmed this to be true, and medical literature told Dr. Kearns that pinworms were common in high latitudes – places where people often are especially prone to MS.
“Most remarkably, they are known to have very little migration and stay spatially stable in populations over long periods of time,” Dr. Kearns said, “and typically everyone in an affected population will encounter them because their eggs are transmitted in household dust.”
And, he said, “they are known to be common in soldiers who live in military accommodation.”
According to the Centers for Disease Control and Prevention, pinworm prevalence can be as high as 50% in at-risk groups – children, caregivers of infected children, and people who live in institutions. Pinworms, which are spread through ingestion, are often asymptomatic but may cause anal itching and trigger bacterial infections.
The researchers suggest that pinworm infection comes first, followed by EBV infection. This makes sense because “late EBV infection in the form of infectious mononucleosis is known to be a risk factor for MS,” Dr. Kearns said.
The one-two punch of pinworm and then EBV is a plausible theory “because EBV lives in memory B cells, which are known to be important in MS and could be specific for the previous exposure to pinworm,” Dr. Kearns said. “However, this is very speculative and some researchers will argue this is very unlikely to be the case. But I think there is a chance it could explain some of the epidemiology, so I’m keen to try and test the theory if I can.”
What’s next? Dr. Kearns wants to explore data from Scotland in search of areas of high and low MS incidence that could offer insight into environmental triggers.
He added that the development of a serological blood test to prove a history of pinworm infection would be “the most effective way to prove or disprove this theory.”
“I have approached an investigator who has a track record of doing this for other infections and have been encouraged that he thinks that it would be achievable,” he said. “But this will definitely take time and funding.”
No specific funding was reported. The study authors reported no relevant disclosures.
SOURCE: Kearns P et al. ACTRIMS Forum 2018, Abstracts LB257 and LB264.
REPORTING FROM ACTRIMS FORUM 2018
Do Neurologists Adequately Screen Patients With MS for Cognitive Impairment and Depression?
SAN DIEGO—Current practice related to screening patients with multiple sclerosis (MS) for cognitive impairment and depression may need improvement, according to a study presented at the ACTRIMS 2018 Forum. Not all patients undergo screening for these comorbidities, and patients who undergo screening are not necessarily those most likely to have cognitive impairment or depression, said the researchers. In addition, the vast majority of clinicians who perform this screening do not use validated tools.
“Education on validated screening tools, development of more accessible tools, and the sharing of best practices by MS experts may all help address these barriers,” said Guy J. Buckle, MD, MPH, Director of Neuroimaging Research at Shepherd Center in Atlanta, and colleagues. “Assisting MS clinicians in cognitive and depression screening and early referral and treatment may all positively impact outcomes for patients with MS.”
Study Included Chart Review and Survey
The National MS Society reported that as much as half of patients with MS may have cognitive impairment. A meta-analysis by Foley et al suggested that 35% of patients with MS met the criteria for clinically significant depression. Yet not all patients with MS undergo screening for these comorbidities.
Dr. Buckle and colleagues examined neurologists’ current screening practices, perceptions about screening, and barriers to screening. The investigators reviewed charts for 300 patients with MS who were seen at two large specialty clinics (150 charts per clinic). Eligible patients were 18 or older and had two or more visits during the study period. The investigators also conducted informal email interviews with 13 MS specialists recognized as leaders in research and treatment.
Dr. Buckle’s group sought to determine the extent to which screening for cognitive impairment and depression are documented in practice, as well as the extent to which clinicians use validated tools for this screening. In addition, the researchers solicited neurologists’ perceptions of the clinical value of formal screening, along with barriers to using screening tools.
Rate of Screening May Have Been Suboptimal
Participants’ median age was 52, and 76% of participants were female. Average time since diagnosis was 13 years. Fifteen patients had had one or more relapse in the previous 24 months.
Screening for cognitive impairment was documented for 52% of patients in the previous 12 months, and cognitive impairment was documented in 37% of those screened. About 2% of clinicians used a validated tool for screening, and 28% of patients were referred for more specialized care.
Screening for depression was documented for 63% of patients in the previous 12 months, and depression was documented in 42% of those screened. About 4% of clinicians used a validated tool for screening, and 25% of patients were referred for specialized care.
About 48% of patients younger than 65 were screened for cognitive impairment, compared with 73% of patients age 65 or older. Cognitive impairment was diagnosed in 69% of patients younger than 65 and in 78% of patients 65 or older. Patients younger than 65 were less likely to be screened for depression than those 65 or older (60% vs 73%), but more likely to be diagnosed with depression (71% vs 54%).
White patients were more likely to be screened for cognitive impairment than black patients (57% vs 52%), but less likely to be diagnosed with cognitive impairment (68% vs 82%). Similarly, white patients were more likely to be screened for depression (71% vs 53%), but less likely to be diagnosed with depression (65% vs 75%).
Patients who were employed were more likely to undergo cognitive screening than unemployed, retired, or disabled patients, but the latter were more likely to be diagnosed with cognitive impairment. “The analyses support previous findings suggesting links between cognitive dysfunction, patient age, and physical disability,” said Dr. Buckle and colleagues.
Specialists Cited Time as a Barrier
About 54% of the MS specialists surveyed reported that they used validated tools to assess cognitive impairment. Among the tools they named were the Symbol Digit Modalities Test, the Mini-Mental State Examination, the California Verbal Learning Test II, and the Brief International Cognitive Assessment for MS. Among the reasons the specialists gave for not using validated tools were time constraints, lack of qualified staff for administration, lack of integration of the tests into electronic medical records, and lack of reimbursement.
Approximately 46% of the MS specialists reported that they used validated tools to assess depression. The tools that they reported using included the Patient Health Questionnaire-9, the Beck Depression Inventory-II, CNS Vital Signs, and routine questions about appetite, weight loss, sleep, sexual activity, and suicidal ideation. The specialists cited time constraints, lack of compensation, cost, and inability to document results in the electronic medical record as reasons for not using validated tools.
“Integration of formal tools in clinical practice may support clinicians in appropriate and consistent identification of patient populations with these conditions,” said the investigators.
Study May Have Limited Generalizability
The study results may not accurately reflect current practice throughout the country. “The small sample size and relatively low proportions of racial minorities may limit the ability to generalize these results and necessitate large-scale studies,” said Dr. Buckle and colleagues. “Future research should examine healthcare disparities in MS and underlying contributors (eg, bias and perceptions) that hinder the use of formal screening tools in MS patients.”
—Erik Greb
Suggested Reading
Benedict RH, Cox D, Thompson LL, et al. Reliable screening for neuropsychological impairment in multiple sclerosis. Mult
Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the Symbol digit Modalities Test. Mult Scler. 2007;13(1):52-57.
SAN DIEGO—Current practice related to screening patients with multiple sclerosis (MS) for cognitive impairment and depression may need improvement, according to a study presented at the ACTRIMS 2018 Forum. Not all patients undergo screening for these comorbidities, and patients who undergo screening are not necessarily those most likely to have cognitive impairment or depression, said the researchers. In addition, the vast majority of clinicians who perform this screening do not use validated tools.
“Education on validated screening tools, development of more accessible tools, and the sharing of best practices by MS experts may all help address these barriers,” said Guy J. Buckle, MD, MPH, Director of Neuroimaging Research at Shepherd Center in Atlanta, and colleagues. “Assisting MS clinicians in cognitive and depression screening and early referral and treatment may all positively impact outcomes for patients with MS.”
Study Included Chart Review and Survey
The National MS Society reported that as much as half of patients with MS may have cognitive impairment. A meta-analysis by Foley et al suggested that 35% of patients with MS met the criteria for clinically significant depression. Yet not all patients with MS undergo screening for these comorbidities.
Dr. Buckle and colleagues examined neurologists’ current screening practices, perceptions about screening, and barriers to screening. The investigators reviewed charts for 300 patients with MS who were seen at two large specialty clinics (150 charts per clinic). Eligible patients were 18 or older and had two or more visits during the study period. The investigators also conducted informal email interviews with 13 MS specialists recognized as leaders in research and treatment.
Dr. Buckle’s group sought to determine the extent to which screening for cognitive impairment and depression are documented in practice, as well as the extent to which clinicians use validated tools for this screening. In addition, the researchers solicited neurologists’ perceptions of the clinical value of formal screening, along with barriers to using screening tools.
Rate of Screening May Have Been Suboptimal
Participants’ median age was 52, and 76% of participants were female. Average time since diagnosis was 13 years. Fifteen patients had had one or more relapse in the previous 24 months.
Screening for cognitive impairment was documented for 52% of patients in the previous 12 months, and cognitive impairment was documented in 37% of those screened. About 2% of clinicians used a validated tool for screening, and 28% of patients were referred for more specialized care.
Screening for depression was documented for 63% of patients in the previous 12 months, and depression was documented in 42% of those screened. About 4% of clinicians used a validated tool for screening, and 25% of patients were referred for specialized care.
About 48% of patients younger than 65 were screened for cognitive impairment, compared with 73% of patients age 65 or older. Cognitive impairment was diagnosed in 69% of patients younger than 65 and in 78% of patients 65 or older. Patients younger than 65 were less likely to be screened for depression than those 65 or older (60% vs 73%), but more likely to be diagnosed with depression (71% vs 54%).
White patients were more likely to be screened for cognitive impairment than black patients (57% vs 52%), but less likely to be diagnosed with cognitive impairment (68% vs 82%). Similarly, white patients were more likely to be screened for depression (71% vs 53%), but less likely to be diagnosed with depression (65% vs 75%).
Patients who were employed were more likely to undergo cognitive screening than unemployed, retired, or disabled patients, but the latter were more likely to be diagnosed with cognitive impairment. “The analyses support previous findings suggesting links between cognitive dysfunction, patient age, and physical disability,” said Dr. Buckle and colleagues.
Specialists Cited Time as a Barrier
About 54% of the MS specialists surveyed reported that they used validated tools to assess cognitive impairment. Among the tools they named were the Symbol Digit Modalities Test, the Mini-Mental State Examination, the California Verbal Learning Test II, and the Brief International Cognitive Assessment for MS. Among the reasons the specialists gave for not using validated tools were time constraints, lack of qualified staff for administration, lack of integration of the tests into electronic medical records, and lack of reimbursement.
Approximately 46% of the MS specialists reported that they used validated tools to assess depression. The tools that they reported using included the Patient Health Questionnaire-9, the Beck Depression Inventory-II, CNS Vital Signs, and routine questions about appetite, weight loss, sleep, sexual activity, and suicidal ideation. The specialists cited time constraints, lack of compensation, cost, and inability to document results in the electronic medical record as reasons for not using validated tools.
“Integration of formal tools in clinical practice may support clinicians in appropriate and consistent identification of patient populations with these conditions,” said the investigators.
Study May Have Limited Generalizability
The study results may not accurately reflect current practice throughout the country. “The small sample size and relatively low proportions of racial minorities may limit the ability to generalize these results and necessitate large-scale studies,” said Dr. Buckle and colleagues. “Future research should examine healthcare disparities in MS and underlying contributors (eg, bias and perceptions) that hinder the use of formal screening tools in MS patients.”
—Erik Greb
Suggested Reading
Benedict RH, Cox D, Thompson LL, et al. Reliable screening for neuropsychological impairment in multiple sclerosis. Mult
Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the Symbol digit Modalities Test. Mult Scler. 2007;13(1):52-57.
SAN DIEGO—Current practice related to screening patients with multiple sclerosis (MS) for cognitive impairment and depression may need improvement, according to a study presented at the ACTRIMS 2018 Forum. Not all patients undergo screening for these comorbidities, and patients who undergo screening are not necessarily those most likely to have cognitive impairment or depression, said the researchers. In addition, the vast majority of clinicians who perform this screening do not use validated tools.
“Education on validated screening tools, development of more accessible tools, and the sharing of best practices by MS experts may all help address these barriers,” said Guy J. Buckle, MD, MPH, Director of Neuroimaging Research at Shepherd Center in Atlanta, and colleagues. “Assisting MS clinicians in cognitive and depression screening and early referral and treatment may all positively impact outcomes for patients with MS.”
Study Included Chart Review and Survey
The National MS Society reported that as much as half of patients with MS may have cognitive impairment. A meta-analysis by Foley et al suggested that 35% of patients with MS met the criteria for clinically significant depression. Yet not all patients with MS undergo screening for these comorbidities.
Dr. Buckle and colleagues examined neurologists’ current screening practices, perceptions about screening, and barriers to screening. The investigators reviewed charts for 300 patients with MS who were seen at two large specialty clinics (150 charts per clinic). Eligible patients were 18 or older and had two or more visits during the study period. The investigators also conducted informal email interviews with 13 MS specialists recognized as leaders in research and treatment.
Dr. Buckle’s group sought to determine the extent to which screening for cognitive impairment and depression are documented in practice, as well as the extent to which clinicians use validated tools for this screening. In addition, the researchers solicited neurologists’ perceptions of the clinical value of formal screening, along with barriers to using screening tools.
Rate of Screening May Have Been Suboptimal
Participants’ median age was 52, and 76% of participants were female. Average time since diagnosis was 13 years. Fifteen patients had had one or more relapse in the previous 24 months.
Screening for cognitive impairment was documented for 52% of patients in the previous 12 months, and cognitive impairment was documented in 37% of those screened. About 2% of clinicians used a validated tool for screening, and 28% of patients were referred for more specialized care.
Screening for depression was documented for 63% of patients in the previous 12 months, and depression was documented in 42% of those screened. About 4% of clinicians used a validated tool for screening, and 25% of patients were referred for specialized care.
About 48% of patients younger than 65 were screened for cognitive impairment, compared with 73% of patients age 65 or older. Cognitive impairment was diagnosed in 69% of patients younger than 65 and in 78% of patients 65 or older. Patients younger than 65 were less likely to be screened for depression than those 65 or older (60% vs 73%), but more likely to be diagnosed with depression (71% vs 54%).
White patients were more likely to be screened for cognitive impairment than black patients (57% vs 52%), but less likely to be diagnosed with cognitive impairment (68% vs 82%). Similarly, white patients were more likely to be screened for depression (71% vs 53%), but less likely to be diagnosed with depression (65% vs 75%).
Patients who were employed were more likely to undergo cognitive screening than unemployed, retired, or disabled patients, but the latter were more likely to be diagnosed with cognitive impairment. “The analyses support previous findings suggesting links between cognitive dysfunction, patient age, and physical disability,” said Dr. Buckle and colleagues.
Specialists Cited Time as a Barrier
About 54% of the MS specialists surveyed reported that they used validated tools to assess cognitive impairment. Among the tools they named were the Symbol Digit Modalities Test, the Mini-Mental State Examination, the California Verbal Learning Test II, and the Brief International Cognitive Assessment for MS. Among the reasons the specialists gave for not using validated tools were time constraints, lack of qualified staff for administration, lack of integration of the tests into electronic medical records, and lack of reimbursement.
Approximately 46% of the MS specialists reported that they used validated tools to assess depression. The tools that they reported using included the Patient Health Questionnaire-9, the Beck Depression Inventory-II, CNS Vital Signs, and routine questions about appetite, weight loss, sleep, sexual activity, and suicidal ideation. The specialists cited time constraints, lack of compensation, cost, and inability to document results in the electronic medical record as reasons for not using validated tools.
“Integration of formal tools in clinical practice may support clinicians in appropriate and consistent identification of patient populations with these conditions,” said the investigators.
Study May Have Limited Generalizability
The study results may not accurately reflect current practice throughout the country. “The small sample size and relatively low proportions of racial minorities may limit the ability to generalize these results and necessitate large-scale studies,” said Dr. Buckle and colleagues. “Future research should examine healthcare disparities in MS and underlying contributors (eg, bias and perceptions) that hinder the use of formal screening tools in MS patients.”
—Erik Greb
Suggested Reading
Benedict RH, Cox D, Thompson LL, et al. Reliable screening for neuropsychological impairment in multiple sclerosis. Mult
Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the Symbol digit Modalities Test. Mult Scler. 2007;13(1):52-57.
Study identifies characteristics that may constitute the MS prodrome
SAN DIEGO – Compared with controls, patients who developed MS were more frequently admitted to the hospital or visited a physician for problems related to the nervous system, sensory organs, musculoskeletal system, and genitourinary system in the 5 years prior to MS onset, a multicenter matched cohort study found.
“People are seeking help for different conditions that are most likely related to their MS,” one of the study authors, Elaine Kingwell, PhD, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “It suggests there could be opportunity to recognize and possibly diagnose and treat MS earlier.”
In a previously published study (Lancet Neurol. 2017;16[6]:445-51) led by Helen Tremlett, PhD, and first author Jose Wijnands, PhD, the team found increased health care utilization in people with MS across all health sectors – physician, hospital, and pharmacy (prescriptions filled).
For the current study, the team set out to identify early signs and symptoms that could define the MS prodrome. “We wanted to know why people went to the hospital, why people went to their physician, what kind of drugs they were prescribed, and what kind of specialists they saw,” explained Dr. Kingwell, an epidemiologist in the department of neurology at the University of British Columbia, Vancouver.
The researchers used health administrative and MS-specific data from four Canadian provinces to conduct a multicenter matched cohort study. Individuals were required to be in the province from 5 years pre-index date, measured as either the first demyelinating event in health administrative data or MS onset as determined by the treating neurologist, until the MS cases’ third demyelinating claim or diagnosis date. The potential for a prodromal period was examined in the 5 years pre-index date, and outcomes of interest were number of physician and hospital encounters per ICD-10 chapter, number of physician encounters per physician specialty, and percentage of people with one or more filled prescription per drug class.
The researchers used rate ratios and 95% confidence intervals to compare the rates of physician and hospital encounters between MS cases and controls and the proportion test to compare the percentage of people with one or more filled prescriptions between MS cases and controls. They used random-effects meta-analyses to pool results across Canadian provinces.
The population consisted of 13,951 MS patients and 66,940 controls derived from a health administration cohort and 3,202 MS patients and 16,006 controls derived from MS clinics. Compared with controls, in the 5 years before the first demyelinating claim or symptom onset, cases had more physician and hospital encounters for the nervous system (rate ratio = 1.70-4.75), sensory organs (RR = 1.40-2.28), musculoskeletal system (RR = 1.19-1.70), and genitourinary system (RR = 1.17-1.59), Dr. Kingwell and her associates reported.
Cases also had more encounters with psychiatrists (RR = 1.48-1.66) and urologists (RR = 1.49-1.80), and a higher proportion of filled prescriptions for hormonal preparations and drugs related to the musculoskeletal or genitourinary systems (ranging from 1.1 to 1.5 times higher; P less than .02 for all associations). “While we did not examine each individual drug, we did group drugs by their therapeutic class,” Dr. Wijnands noted in an interview after the meeting. “As for the increased number of visits to psychiatrists, it is intriguing. We don’t necessarily know all the reasons why. It opens up a lot of questions for people to follow up on.”
In contrast, MS cases had fewer pregnancy-related encounters, compared with controls (RR = 0.78-0.88).
Dr. Wijnands acknowledged certain limitations of the study, including its reliance on administrative data to measure the prodromal period. “When we described the prodrome itself, we relied on ICD codes from the physician and hospital data as well as prescriptions filled. Issues or problems for which individuals do not seek medical attention, for instance, would not be captured in our study,” she said.
The study was funded by the National MS Society. Dr. Wijnands receives salary support from the Michael Smith Foundation for Health Research /The Koehle Family Foundation, coauthor Ruth Ann Marrie, MD, PhD, holds the Waugh Family Chair in Multiple Sclerosis, and Dr. Tremlett is funded by the Canada Research Chair Program. Dr. Kingwell reported having no financial disclosures.
[email protected]
SOURCE: Kingwell E et al. ACTRIMS Forum 2018, late-breaking poster 254.
SAN DIEGO – Compared with controls, patients who developed MS were more frequently admitted to the hospital or visited a physician for problems related to the nervous system, sensory organs, musculoskeletal system, and genitourinary system in the 5 years prior to MS onset, a multicenter matched cohort study found.
“People are seeking help for different conditions that are most likely related to their MS,” one of the study authors, Elaine Kingwell, PhD, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “It suggests there could be opportunity to recognize and possibly diagnose and treat MS earlier.”
In a previously published study (Lancet Neurol. 2017;16[6]:445-51) led by Helen Tremlett, PhD, and first author Jose Wijnands, PhD, the team found increased health care utilization in people with MS across all health sectors – physician, hospital, and pharmacy (prescriptions filled).
For the current study, the team set out to identify early signs and symptoms that could define the MS prodrome. “We wanted to know why people went to the hospital, why people went to their physician, what kind of drugs they were prescribed, and what kind of specialists they saw,” explained Dr. Kingwell, an epidemiologist in the department of neurology at the University of British Columbia, Vancouver.
The researchers used health administrative and MS-specific data from four Canadian provinces to conduct a multicenter matched cohort study. Individuals were required to be in the province from 5 years pre-index date, measured as either the first demyelinating event in health administrative data or MS onset as determined by the treating neurologist, until the MS cases’ third demyelinating claim or diagnosis date. The potential for a prodromal period was examined in the 5 years pre-index date, and outcomes of interest were number of physician and hospital encounters per ICD-10 chapter, number of physician encounters per physician specialty, and percentage of people with one or more filled prescription per drug class.
The researchers used rate ratios and 95% confidence intervals to compare the rates of physician and hospital encounters between MS cases and controls and the proportion test to compare the percentage of people with one or more filled prescriptions between MS cases and controls. They used random-effects meta-analyses to pool results across Canadian provinces.
The population consisted of 13,951 MS patients and 66,940 controls derived from a health administration cohort and 3,202 MS patients and 16,006 controls derived from MS clinics. Compared with controls, in the 5 years before the first demyelinating claim or symptom onset, cases had more physician and hospital encounters for the nervous system (rate ratio = 1.70-4.75), sensory organs (RR = 1.40-2.28), musculoskeletal system (RR = 1.19-1.70), and genitourinary system (RR = 1.17-1.59), Dr. Kingwell and her associates reported.
Cases also had more encounters with psychiatrists (RR = 1.48-1.66) and urologists (RR = 1.49-1.80), and a higher proportion of filled prescriptions for hormonal preparations and drugs related to the musculoskeletal or genitourinary systems (ranging from 1.1 to 1.5 times higher; P less than .02 for all associations). “While we did not examine each individual drug, we did group drugs by their therapeutic class,” Dr. Wijnands noted in an interview after the meeting. “As for the increased number of visits to psychiatrists, it is intriguing. We don’t necessarily know all the reasons why. It opens up a lot of questions for people to follow up on.”
In contrast, MS cases had fewer pregnancy-related encounters, compared with controls (RR = 0.78-0.88).
Dr. Wijnands acknowledged certain limitations of the study, including its reliance on administrative data to measure the prodromal period. “When we described the prodrome itself, we relied on ICD codes from the physician and hospital data as well as prescriptions filled. Issues or problems for which individuals do not seek medical attention, for instance, would not be captured in our study,” she said.
The study was funded by the National MS Society. Dr. Wijnands receives salary support from the Michael Smith Foundation for Health Research /The Koehle Family Foundation, coauthor Ruth Ann Marrie, MD, PhD, holds the Waugh Family Chair in Multiple Sclerosis, and Dr. Tremlett is funded by the Canada Research Chair Program. Dr. Kingwell reported having no financial disclosures.
[email protected]
SOURCE: Kingwell E et al. ACTRIMS Forum 2018, late-breaking poster 254.
SAN DIEGO – Compared with controls, patients who developed MS were more frequently admitted to the hospital or visited a physician for problems related to the nervous system, sensory organs, musculoskeletal system, and genitourinary system in the 5 years prior to MS onset, a multicenter matched cohort study found.
“People are seeking help for different conditions that are most likely related to their MS,” one of the study authors, Elaine Kingwell, PhD, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “It suggests there could be opportunity to recognize and possibly diagnose and treat MS earlier.”
In a previously published study (Lancet Neurol. 2017;16[6]:445-51) led by Helen Tremlett, PhD, and first author Jose Wijnands, PhD, the team found increased health care utilization in people with MS across all health sectors – physician, hospital, and pharmacy (prescriptions filled).
For the current study, the team set out to identify early signs and symptoms that could define the MS prodrome. “We wanted to know why people went to the hospital, why people went to their physician, what kind of drugs they were prescribed, and what kind of specialists they saw,” explained Dr. Kingwell, an epidemiologist in the department of neurology at the University of British Columbia, Vancouver.
The researchers used health administrative and MS-specific data from four Canadian provinces to conduct a multicenter matched cohort study. Individuals were required to be in the province from 5 years pre-index date, measured as either the first demyelinating event in health administrative data or MS onset as determined by the treating neurologist, until the MS cases’ third demyelinating claim or diagnosis date. The potential for a prodromal period was examined in the 5 years pre-index date, and outcomes of interest were number of physician and hospital encounters per ICD-10 chapter, number of physician encounters per physician specialty, and percentage of people with one or more filled prescription per drug class.
The researchers used rate ratios and 95% confidence intervals to compare the rates of physician and hospital encounters between MS cases and controls and the proportion test to compare the percentage of people with one or more filled prescriptions between MS cases and controls. They used random-effects meta-analyses to pool results across Canadian provinces.
The population consisted of 13,951 MS patients and 66,940 controls derived from a health administration cohort and 3,202 MS patients and 16,006 controls derived from MS clinics. Compared with controls, in the 5 years before the first demyelinating claim or symptom onset, cases had more physician and hospital encounters for the nervous system (rate ratio = 1.70-4.75), sensory organs (RR = 1.40-2.28), musculoskeletal system (RR = 1.19-1.70), and genitourinary system (RR = 1.17-1.59), Dr. Kingwell and her associates reported.
Cases also had more encounters with psychiatrists (RR = 1.48-1.66) and urologists (RR = 1.49-1.80), and a higher proportion of filled prescriptions for hormonal preparations and drugs related to the musculoskeletal or genitourinary systems (ranging from 1.1 to 1.5 times higher; P less than .02 for all associations). “While we did not examine each individual drug, we did group drugs by their therapeutic class,” Dr. Wijnands noted in an interview after the meeting. “As for the increased number of visits to psychiatrists, it is intriguing. We don’t necessarily know all the reasons why. It opens up a lot of questions for people to follow up on.”
In contrast, MS cases had fewer pregnancy-related encounters, compared with controls (RR = 0.78-0.88).
Dr. Wijnands acknowledged certain limitations of the study, including its reliance on administrative data to measure the prodromal period. “When we described the prodrome itself, we relied on ICD codes from the physician and hospital data as well as prescriptions filled. Issues or problems for which individuals do not seek medical attention, for instance, would not be captured in our study,” she said.
The study was funded by the National MS Society. Dr. Wijnands receives salary support from the Michael Smith Foundation for Health Research /The Koehle Family Foundation, coauthor Ruth Ann Marrie, MD, PhD, holds the Waugh Family Chair in Multiple Sclerosis, and Dr. Tremlett is funded by the Canada Research Chair Program. Dr. Kingwell reported having no financial disclosures.
[email protected]
SOURCE: Kingwell E et al. ACTRIMS Forum 2018, late-breaking poster 254.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point: Several phenotypic characteristics may constitute the MS prodrome.
Major finding: Compared with controls, in the 5 years before the first demyelinating claim or symptom onset, MS cases had more physician and hospital encounters for the nervous system (rate ratio = 1.70-4.75), sensory organs (RR = 1.40-2.28), and musculoskeletal system (RR = 1.19-1.70).
Study details: A multicenter matched cohort study of 13,951 MS patients and 66,940 controls derived from a health administration cohort and 3,202 MS patients and 16,006 controls derived from MS clinics.
Disclosures: The study was funded by the National MS Society. Dr. Wijnands receives salary support from the Michael Smith Foundation for Health Research /The Koehle Family Foundation, coauthor Ruth Ann Marrie, MD, PhD, holds the Waugh Family Chair in Multiple Sclerosis, and Dr. Tremlett is funded by the Canada Research Chair Program. Dr. Kingwell reported having no financial disclosures.
Source: Kingwell E et al. ACTRIMS Forum 2018, late-breaking poster 254.
Physicians often bypass cognition, depression screening in MS
SAN DIEGO – A new study finds that physicians at two . Physicians who did perform screening hardly ever used validated tools and often didn’t refer appropriate patients for higher-level care.
In addition, researchers interviewed 13 leading MS specialists from coast to coast and “found that about half reported not using formal screening tools to assess cognitive impairment and depression,” said study coauthor Tamar Sapir, PhD, chief scientific officer with Prime Education, a firm based in Fort Lauderdale, Fla., that provides a variety of health-related services such as training and research.
The study findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Three of the study authors spoke in interviews.
The researchers sought to understand how frequently MS patients are screened for cognitive problems and depression.
“Cognitive impairment is experienced by approximately half of patients with multiple sclerosis, yet many are never screened or treated, which can impact their daily activities, their ability to work, and overall quality of life,” Dr. Sapir said.
Depression, meanwhile, is believed to be much more common in patients with MS than in the general population, with one recent meta-analysis of 58 studies finding that the average prevalence was 31%. Other research suggests depression is underdiagnosed and undertreated in this population (J Neurol Sci. 2017 Jan 15;372:331-41; ISRN Neurology. 2012, Article ID 427102. doi: 10.5402/2012/427102).
For the current study, researchers tracked 300 patients at two unidentified MS clinics via their charts over a 2-year period from 2014 to 2016. Their median age was 52 years, 76% were women, and 15 had experienced at least one relapse within the previous 24 months.
“Screening for cognitive impairment and depression was documented for only 52% and 63% of MS patients, respectively, and only about a quarter of patients diagnosed with these conditions were referred to a higher level of care,” said lead author Guy J. Buckle, MD, MPH, of the Andrew C. Carlos MS Institute at Shepherd Center in Atlanta.
Among all 300 patients, just 2% and 4% were screened using a validated tool for cognitive impairment and depression, respectively.
The screening often turned up evidence of the conditions: Physicians saw signs of cognitive impairment in 69% and 78% of those screened aged under 65 years and aged 65 and older, respectively, and they detected depression in 71% and 54% of those screened in those two age groups, respectively.
Researchers also noted several disparities. “Cognitive screening was conducted more frequently in older, employed, or white patients, while the presence of cognitive impairment was documented more often in black, nonworking, and those on Medicare or Medicaid,” Dr. Buckle said. “Depression screening was performed most frequently in older or white patients, yet depression was more common in younger, nonworking patients and those on Medicare/Medicaid.”
In another part of their study, researchers surveyed 13 unidentified “national leaders” in MS research and treatment. Just seven said they used validated tools to screen for cognitive impairment and six said they used them to screen for depression.
“We hear from MS specialists that they want to be measuring for cognition but don’t know how to efficiently work it into their routine, how to approach the patient, and what tools to use,” said study coauthor Derrick S. Robertson, MD, of the University of South Florida, Tampa. “In addition, there is no one tool that is accepted in the MS treatment community.”
MS specialists who didn’t use the screening tests also pointed to factors like lack of reimbursement and lack of integration into electronic medical records. “Doubt very much that neurologists have time to use any of these tests,” one respondent said, referring to cognitive impairment screening.
What’s next? “There are several new exciting developments in clinical trials demonstrating efficacy of disease-modifying therapies in maintaining or improving cognition in patients with relapsing MS,” Dr. Robertson said. “This highlights the urgent need to overcome barriers to use of formal cognitive screening tools in clinical practice to identify patients who need a higher level of care, and perhaps even a change in treatment with the ultimate goal to improve quality of life and overall outcomes.”
Genentech funded the study through an educational grant. Dr. Sapir and three other study authors reported no relevant disclosures. Dr. Buckle and Dr. Robertson reported multiple disclosures, including principle investigator and advisory board/panel member work.
SOURCE: Buckle GJ et al. ACTRIMS Forum 2018, abstract No. P161.
SAN DIEGO – A new study finds that physicians at two . Physicians who did perform screening hardly ever used validated tools and often didn’t refer appropriate patients for higher-level care.
In addition, researchers interviewed 13 leading MS specialists from coast to coast and “found that about half reported not using formal screening tools to assess cognitive impairment and depression,” said study coauthor Tamar Sapir, PhD, chief scientific officer with Prime Education, a firm based in Fort Lauderdale, Fla., that provides a variety of health-related services such as training and research.
The study findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Three of the study authors spoke in interviews.
The researchers sought to understand how frequently MS patients are screened for cognitive problems and depression.
“Cognitive impairment is experienced by approximately half of patients with multiple sclerosis, yet many are never screened or treated, which can impact their daily activities, their ability to work, and overall quality of life,” Dr. Sapir said.
Depression, meanwhile, is believed to be much more common in patients with MS than in the general population, with one recent meta-analysis of 58 studies finding that the average prevalence was 31%. Other research suggests depression is underdiagnosed and undertreated in this population (J Neurol Sci. 2017 Jan 15;372:331-41; ISRN Neurology. 2012, Article ID 427102. doi: 10.5402/2012/427102).
For the current study, researchers tracked 300 patients at two unidentified MS clinics via their charts over a 2-year period from 2014 to 2016. Their median age was 52 years, 76% were women, and 15 had experienced at least one relapse within the previous 24 months.
“Screening for cognitive impairment and depression was documented for only 52% and 63% of MS patients, respectively, and only about a quarter of patients diagnosed with these conditions were referred to a higher level of care,” said lead author Guy J. Buckle, MD, MPH, of the Andrew C. Carlos MS Institute at Shepherd Center in Atlanta.
Among all 300 patients, just 2% and 4% were screened using a validated tool for cognitive impairment and depression, respectively.
The screening often turned up evidence of the conditions: Physicians saw signs of cognitive impairment in 69% and 78% of those screened aged under 65 years and aged 65 and older, respectively, and they detected depression in 71% and 54% of those screened in those two age groups, respectively.
Researchers also noted several disparities. “Cognitive screening was conducted more frequently in older, employed, or white patients, while the presence of cognitive impairment was documented more often in black, nonworking, and those on Medicare or Medicaid,” Dr. Buckle said. “Depression screening was performed most frequently in older or white patients, yet depression was more common in younger, nonworking patients and those on Medicare/Medicaid.”
In another part of their study, researchers surveyed 13 unidentified “national leaders” in MS research and treatment. Just seven said they used validated tools to screen for cognitive impairment and six said they used them to screen for depression.
“We hear from MS specialists that they want to be measuring for cognition but don’t know how to efficiently work it into their routine, how to approach the patient, and what tools to use,” said study coauthor Derrick S. Robertson, MD, of the University of South Florida, Tampa. “In addition, there is no one tool that is accepted in the MS treatment community.”
MS specialists who didn’t use the screening tests also pointed to factors like lack of reimbursement and lack of integration into electronic medical records. “Doubt very much that neurologists have time to use any of these tests,” one respondent said, referring to cognitive impairment screening.
What’s next? “There are several new exciting developments in clinical trials demonstrating efficacy of disease-modifying therapies in maintaining or improving cognition in patients with relapsing MS,” Dr. Robertson said. “This highlights the urgent need to overcome barriers to use of formal cognitive screening tools in clinical practice to identify patients who need a higher level of care, and perhaps even a change in treatment with the ultimate goal to improve quality of life and overall outcomes.”
Genentech funded the study through an educational grant. Dr. Sapir and three other study authors reported no relevant disclosures. Dr. Buckle and Dr. Robertson reported multiple disclosures, including principle investigator and advisory board/panel member work.
SOURCE: Buckle GJ et al. ACTRIMS Forum 2018, abstract No. P161.
SAN DIEGO – A new study finds that physicians at two . Physicians who did perform screening hardly ever used validated tools and often didn’t refer appropriate patients for higher-level care.
In addition, researchers interviewed 13 leading MS specialists from coast to coast and “found that about half reported not using formal screening tools to assess cognitive impairment and depression,” said study coauthor Tamar Sapir, PhD, chief scientific officer with Prime Education, a firm based in Fort Lauderdale, Fla., that provides a variety of health-related services such as training and research.
The study findings were presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Three of the study authors spoke in interviews.
The researchers sought to understand how frequently MS patients are screened for cognitive problems and depression.
“Cognitive impairment is experienced by approximately half of patients with multiple sclerosis, yet many are never screened or treated, which can impact their daily activities, their ability to work, and overall quality of life,” Dr. Sapir said.
Depression, meanwhile, is believed to be much more common in patients with MS than in the general population, with one recent meta-analysis of 58 studies finding that the average prevalence was 31%. Other research suggests depression is underdiagnosed and undertreated in this population (J Neurol Sci. 2017 Jan 15;372:331-41; ISRN Neurology. 2012, Article ID 427102. doi: 10.5402/2012/427102).
For the current study, researchers tracked 300 patients at two unidentified MS clinics via their charts over a 2-year period from 2014 to 2016. Their median age was 52 years, 76% were women, and 15 had experienced at least one relapse within the previous 24 months.
“Screening for cognitive impairment and depression was documented for only 52% and 63% of MS patients, respectively, and only about a quarter of patients diagnosed with these conditions were referred to a higher level of care,” said lead author Guy J. Buckle, MD, MPH, of the Andrew C. Carlos MS Institute at Shepherd Center in Atlanta.
Among all 300 patients, just 2% and 4% were screened using a validated tool for cognitive impairment and depression, respectively.
The screening often turned up evidence of the conditions: Physicians saw signs of cognitive impairment in 69% and 78% of those screened aged under 65 years and aged 65 and older, respectively, and they detected depression in 71% and 54% of those screened in those two age groups, respectively.
Researchers also noted several disparities. “Cognitive screening was conducted more frequently in older, employed, or white patients, while the presence of cognitive impairment was documented more often in black, nonworking, and those on Medicare or Medicaid,” Dr. Buckle said. “Depression screening was performed most frequently in older or white patients, yet depression was more common in younger, nonworking patients and those on Medicare/Medicaid.”
In another part of their study, researchers surveyed 13 unidentified “national leaders” in MS research and treatment. Just seven said they used validated tools to screen for cognitive impairment and six said they used them to screen for depression.
“We hear from MS specialists that they want to be measuring for cognition but don’t know how to efficiently work it into their routine, how to approach the patient, and what tools to use,” said study coauthor Derrick S. Robertson, MD, of the University of South Florida, Tampa. “In addition, there is no one tool that is accepted in the MS treatment community.”
MS specialists who didn’t use the screening tests also pointed to factors like lack of reimbursement and lack of integration into electronic medical records. “Doubt very much that neurologists have time to use any of these tests,” one respondent said, referring to cognitive impairment screening.
What’s next? “There are several new exciting developments in clinical trials demonstrating efficacy of disease-modifying therapies in maintaining or improving cognition in patients with relapsing MS,” Dr. Robertson said. “This highlights the urgent need to overcome barriers to use of formal cognitive screening tools in clinical practice to identify patients who need a higher level of care, and perhaps even a change in treatment with the ultimate goal to improve quality of life and overall outcomes.”
Genentech funded the study through an educational grant. Dr. Sapir and three other study authors reported no relevant disclosures. Dr. Buckle and Dr. Robertson reported multiple disclosures, including principle investigator and advisory board/panel member work.
SOURCE: Buckle GJ et al. ACTRIMS Forum 2018, abstract No. P161.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point: Screening often turns up signs of trouble, but many MS patients are not screened annually for depression and cognitive impairment.
Major finding: 52% and 63% of patients with MS were screened for cognitive impairment and depression, respectively, over a 1-year period. Study details: 2-year analysis of medical records from two MS clinics in the Southeast.
Disclosures: Genentech funded the study through an educational grant. Some of the study authors reported various disclosures.
Source: Buckle GJ et al. ACTRIMS Forum 2018, abstract No. P161.
VIDEO: Teriflunomide and dimethyl fumarate are comparable in relapsing-remitting MS
SAN DIEGO – New industry-funded research finds that
“The fundamental conclusion is that there’s little to separate them. If you look at efficacy, safety, and MRI scanning, there were no differences between the two medications that matter. ... Both drugs can be used with equal confidence,” said Stanley Cohan, MD, PhD, medical director of Providence MS Center in Portland, Ore.
Dr. Cohan spoke in a video interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The researchers analyzed retrospective data collected in the phase 4 Teri-RADAR study, which tracked two groups of 50 patients each at MS centers in the United States as they took 14 mg of teriflunomide (Aubagio) daily or 240 mg of delayed-release dimethyl fumarate (Tecfidera) twice daily.
In both groups, participants had an average age of about 49 years, about three-quarters were women, and about 93% were white. Twenty-two percent of those in the teriflunomide group had relapses in the 18 months before the index date, compared with 10% of the dimethyl fumarate group.
The study – funded by Sanofi, maker of teriflunomide – followed up with patients at a mean of 15-16 months.
Mean annualized percentage of brain volume change (PBVC) was lower in teriflunomide-treated patients, compared with those treated with dimethyl fumarate (–0.2% vs. –0.5%; P = .02). Also, fewer teriflunomide-treated patients showed signs of annualized PBVC above a mean annualized brain volume loss rate threshold of 0.4% (25.9% vs. 58.6%; P = .01).
As for adverse effects, rashes affected none of the teriflunomide group but 28% of the dimethyl fumarate group. The teriflunomide group experienced skin reactions (12%) and diarrhea (16%), while these adverse events were not reported in the dimethyl fumarate group.
The study authors reported a variety of disclosures, and several are employees of Sanofi. Dr. Cohan reported a variety of disclosures, including multiple relationships with Sanofi.
SAN DIEGO – New industry-funded research finds that
“The fundamental conclusion is that there’s little to separate them. If you look at efficacy, safety, and MRI scanning, there were no differences between the two medications that matter. ... Both drugs can be used with equal confidence,” said Stanley Cohan, MD, PhD, medical director of Providence MS Center in Portland, Ore.
Dr. Cohan spoke in a video interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The researchers analyzed retrospective data collected in the phase 4 Teri-RADAR study, which tracked two groups of 50 patients each at MS centers in the United States as they took 14 mg of teriflunomide (Aubagio) daily or 240 mg of delayed-release dimethyl fumarate (Tecfidera) twice daily.
In both groups, participants had an average age of about 49 years, about three-quarters were women, and about 93% were white. Twenty-two percent of those in the teriflunomide group had relapses in the 18 months before the index date, compared with 10% of the dimethyl fumarate group.
The study – funded by Sanofi, maker of teriflunomide – followed up with patients at a mean of 15-16 months.
Mean annualized percentage of brain volume change (PBVC) was lower in teriflunomide-treated patients, compared with those treated with dimethyl fumarate (–0.2% vs. –0.5%; P = .02). Also, fewer teriflunomide-treated patients showed signs of annualized PBVC above a mean annualized brain volume loss rate threshold of 0.4% (25.9% vs. 58.6%; P = .01).
As for adverse effects, rashes affected none of the teriflunomide group but 28% of the dimethyl fumarate group. The teriflunomide group experienced skin reactions (12%) and diarrhea (16%), while these adverse events were not reported in the dimethyl fumarate group.
The study authors reported a variety of disclosures, and several are employees of Sanofi. Dr. Cohan reported a variety of disclosures, including multiple relationships with Sanofi.
SAN DIEGO – New industry-funded research finds that
“The fundamental conclusion is that there’s little to separate them. If you look at efficacy, safety, and MRI scanning, there were no differences between the two medications that matter. ... Both drugs can be used with equal confidence,” said Stanley Cohan, MD, PhD, medical director of Providence MS Center in Portland, Ore.
Dr. Cohan spoke in a video interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The researchers analyzed retrospective data collected in the phase 4 Teri-RADAR study, which tracked two groups of 50 patients each at MS centers in the United States as they took 14 mg of teriflunomide (Aubagio) daily or 240 mg of delayed-release dimethyl fumarate (Tecfidera) twice daily.
In both groups, participants had an average age of about 49 years, about three-quarters were women, and about 93% were white. Twenty-two percent of those in the teriflunomide group had relapses in the 18 months before the index date, compared with 10% of the dimethyl fumarate group.
The study – funded by Sanofi, maker of teriflunomide – followed up with patients at a mean of 15-16 months.
Mean annualized percentage of brain volume change (PBVC) was lower in teriflunomide-treated patients, compared with those treated with dimethyl fumarate (–0.2% vs. –0.5%; P = .02). Also, fewer teriflunomide-treated patients showed signs of annualized PBVC above a mean annualized brain volume loss rate threshold of 0.4% (25.9% vs. 58.6%; P = .01).
As for adverse effects, rashes affected none of the teriflunomide group but 28% of the dimethyl fumarate group. The teriflunomide group experienced skin reactions (12%) and diarrhea (16%), while these adverse events were not reported in the dimethyl fumarate group.
The study authors reported a variety of disclosures, and several are employees of Sanofi. Dr. Cohan reported a variety of disclosures, including multiple relationships with Sanofi.
REPORTING FROM ACTRIMS Forum 2018
Adil Harroud, MD
VIDEO: Advanced practice providers take on many roles in MS care
SAN DIEGO – A new survey suggests that advanced practice providers who care for multiple sclerosis patients are highly satisfied with their jobs, which encompass a wide range of responsibilities.
Researchers received survey responses from 215 nurse practitioners and 395 physician assistants who answered Web questionnaires in 2016 and 2017. Of those who care for multiple sclerosis (MS) patients, 92.5% and 77.8% respectively said they provide at least 9 of 11 services, such as direct care, supportive services, and care coordination.
“Nurse practitioners in particular are providing lots of different services from diagnosis to education to symptom management,” said Michael T. Halpern, MD, PhD, MPH, of Temple University, Philadelphia. “Physician assistants are also providing a diverse range of MS services, but not as frequently as nurse practitioners.”
Dr. Halpern was the presenting author of the study reporting the survey results. He spoke in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis, where the study findings were presented.
Advanced practice providers also reported high levels of job satisfaction. About 80% in both groups reported being very or extremely satisfied with their careers and with their colleagues; 90% of nurse practitioners reported being very or extremely satisfied by their relationships with patients, as did 86% of physician assistants.
The providers “appear to really enjoy working with individuals with MS,” Dr. Halpern said. But he cautioned that there’s a need for additional training for these providers; some respondents said their lack of knowledge was a hindrance to care.
The study was funded by the National Multiple Sclerosis Society. Dr. Halpern reported no relevant disclosures.
SAN DIEGO – A new survey suggests that advanced practice providers who care for multiple sclerosis patients are highly satisfied with their jobs, which encompass a wide range of responsibilities.
Researchers received survey responses from 215 nurse practitioners and 395 physician assistants who answered Web questionnaires in 2016 and 2017. Of those who care for multiple sclerosis (MS) patients, 92.5% and 77.8% respectively said they provide at least 9 of 11 services, such as direct care, supportive services, and care coordination.
“Nurse practitioners in particular are providing lots of different services from diagnosis to education to symptom management,” said Michael T. Halpern, MD, PhD, MPH, of Temple University, Philadelphia. “Physician assistants are also providing a diverse range of MS services, but not as frequently as nurse practitioners.”
Dr. Halpern was the presenting author of the study reporting the survey results. He spoke in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis, where the study findings were presented.
Advanced practice providers also reported high levels of job satisfaction. About 80% in both groups reported being very or extremely satisfied with their careers and with their colleagues; 90% of nurse practitioners reported being very or extremely satisfied by their relationships with patients, as did 86% of physician assistants.
The providers “appear to really enjoy working with individuals with MS,” Dr. Halpern said. But he cautioned that there’s a need for additional training for these providers; some respondents said their lack of knowledge was a hindrance to care.
The study was funded by the National Multiple Sclerosis Society. Dr. Halpern reported no relevant disclosures.
SAN DIEGO – A new survey suggests that advanced practice providers who care for multiple sclerosis patients are highly satisfied with their jobs, which encompass a wide range of responsibilities.
Researchers received survey responses from 215 nurse practitioners and 395 physician assistants who answered Web questionnaires in 2016 and 2017. Of those who care for multiple sclerosis (MS) patients, 92.5% and 77.8% respectively said they provide at least 9 of 11 services, such as direct care, supportive services, and care coordination.
“Nurse practitioners in particular are providing lots of different services from diagnosis to education to symptom management,” said Michael T. Halpern, MD, PhD, MPH, of Temple University, Philadelphia. “Physician assistants are also providing a diverse range of MS services, but not as frequently as nurse practitioners.”
Dr. Halpern was the presenting author of the study reporting the survey results. He spoke in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis, where the study findings were presented.
Advanced practice providers also reported high levels of job satisfaction. About 80% in both groups reported being very or extremely satisfied with their careers and with their colleagues; 90% of nurse practitioners reported being very or extremely satisfied by their relationships with patients, as did 86% of physician assistants.
The providers “appear to really enjoy working with individuals with MS,” Dr. Halpern said. But he cautioned that there’s a need for additional training for these providers; some respondents said their lack of knowledge was a hindrance to care.
The study was funded by the National Multiple Sclerosis Society. Dr. Halpern reported no relevant disclosures.
REPORTING FROM ACTRIMS FORUM 2018