User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
COVID-detecting dogs pilot first airport program
If she identifies a specific scent, she’ll let her handler know simply by sitting down. When this good girl sits, that means Cobra has detected an olfactory signal of the coronavirus, the virus that causes COVID-19.
Cobra, a Belgian Malinois, is one of two canines – her partner is One Betta, a Dutch shepherd – working this checkpoint at Miami International. They are part of a pilot program with the Global Forensic and Justice Center at Florida International University, using the detection dogs as a quick screen for people who have COVID-19.
Their detection rate is high, at more than 98%, and the program has been such a success that it’s being extended for another month at the airport.
If these two dogs continue to accurately detect COVID-19, they and other canines with similar training could be deployed to other places with lots of people coming and going at once, including other airports or even schools. In fact, COVID-sniffing dogs are in use in some university classrooms already.
But building up a big brigade of live animals as disease detectors involves some thorny issues, including where the animals retire once their careers are complete.
“When COVID first arose, we said let’s see if we can train these two dogs on either the virus or the odor of COVID-19,” says Kenneth Furton, PhD, a professor of chemistry and biochemistry, provost, and executive vice president at Florida International University.
His team had completed a study with what he calls “medical detector dogs,” animals that might be able to detect the odor of someone having a seizure. That led them to see how well the animals could detect other kinds of disorders.
Training a dog to sniff out specific odors starts with getting them to understand the task in general. Dr. Furton says that the animals first are trained to grasp that their job is to detect one odor among many. Once the dogs grasp that, they can be trained on just about any specific odor.
In fact, in addition to detecting seizures, dogs reportedly have been able to identify diabetes and even some cancers, such as ovarian cancer.
Dr. Furton says he’s not aware of any previous use of dogs to screen for infectious disease. That may simply be because nothing recently has struck with the global ferocity of COVID, driving humans to turn to their best friends for help.
Cobra and One Betta got their start learning to identify the presence of laurel wilt, a fungus that attacks avocado trees and kills them, costing Florida growers millions. With that expertise under their collars, the two dogs need only a few weeks to get good at detecting other smells assigned to them.
Training the dogs, safely
To train Cobra and One Betta on COVID-19 odors, Dr. Furton’s team first acquired mask samples from people hospitalized with COVID and people who did not have the disease. In battling the viruses, people produce certain chemicals that they exhale every time they breathe. When Dr. Furton and his colleagues compared the exhaled components trapped in the masks, they found differences between masks from people with COVID and those without.
Having confirmed that exhalations can be COVID-specific, the research team trained four dogs – Cobra, One Betta, Hubble, and Max – to detect masks from people with COVID among an assortment of mask choices. Before this step, though, the researchers made sure that any trace of active virus was destroyed by ultraviolet light so that the dogs would not be infected.
Each time the dogs accurately selected a mask from a COVID patient, their reward was access to a favorite toy: A red ball to chew on. Although all four dogs performed very well, yes, they did, Cobra and One Betta showed the most accuracy, outperforming their training colleagues. From their training scores, Cobra ranked first, with 99.45% accuracy. Despite her name, says Dr. Furton, One Betta was “not one better,” coming in second at 98.1%, which is still quite high.
Both dogs are good at their airport screening duties. If one of them sits after sniffing a mask at the checkpoint, the next step is for the mask owner to be tested.
From Aug. 23 to Sept. 8, the two canines screened 1,093 people during 8 working days, alerting on only one case, according to Greg Chin, communications director for the Miami-Dade Aviation Department. That person had tested positive for COVID 2 weeks earlier and was returning to work after quarantine, and their rapid test after the dog alerted was negative.
Dr. Furton says that there are some reports of dogs also alerting before tests can show a positive result, suggesting the dogs’ odor detection can be more precise. They hope to expand their study to see how tight the window of dog-based detection is.
For now, the detector dogs are doing so well that the program has been extended for 30 more days, Mr. Chin says.
As promising as this seems, using dogs for screening carries some logistical and ethical tangles. Training a canine army to deploy for high-volume detection points means that once the work is done, a whole lot of dogs will need a safe place to retire. In addition, the initial training takes several months, says Dr. Furton, whereas if a device were developed for screening, manufacturing could likely be ramped up quickly to meet demand.
The dogs might not need to retire right away, though.
“We envision that they could be redeployed to another type of detection for another infectious disease” if the need arises, Dr. Furton says. But in the end, when working with dogs, he says, there is “a moral connection that you don’t have to deal with using instruments.”
Although the pilot screening at Miami International is the first airport test, the dogs have also done this work in other venues, including at a state emergency operations center in Florida and in some university classrooms, says Dr. Furton.
A version of this article first appeared on WebMD.com.
If she identifies a specific scent, she’ll let her handler know simply by sitting down. When this good girl sits, that means Cobra has detected an olfactory signal of the coronavirus, the virus that causes COVID-19.
Cobra, a Belgian Malinois, is one of two canines – her partner is One Betta, a Dutch shepherd – working this checkpoint at Miami International. They are part of a pilot program with the Global Forensic and Justice Center at Florida International University, using the detection dogs as a quick screen for people who have COVID-19.
Their detection rate is high, at more than 98%, and the program has been such a success that it’s being extended for another month at the airport.
If these two dogs continue to accurately detect COVID-19, they and other canines with similar training could be deployed to other places with lots of people coming and going at once, including other airports or even schools. In fact, COVID-sniffing dogs are in use in some university classrooms already.
But building up a big brigade of live animals as disease detectors involves some thorny issues, including where the animals retire once their careers are complete.
“When COVID first arose, we said let’s see if we can train these two dogs on either the virus or the odor of COVID-19,” says Kenneth Furton, PhD, a professor of chemistry and biochemistry, provost, and executive vice president at Florida International University.
His team had completed a study with what he calls “medical detector dogs,” animals that might be able to detect the odor of someone having a seizure. That led them to see how well the animals could detect other kinds of disorders.
Training a dog to sniff out specific odors starts with getting them to understand the task in general. Dr. Furton says that the animals first are trained to grasp that their job is to detect one odor among many. Once the dogs grasp that, they can be trained on just about any specific odor.
In fact, in addition to detecting seizures, dogs reportedly have been able to identify diabetes and even some cancers, such as ovarian cancer.
Dr. Furton says he’s not aware of any previous use of dogs to screen for infectious disease. That may simply be because nothing recently has struck with the global ferocity of COVID, driving humans to turn to their best friends for help.
Cobra and One Betta got their start learning to identify the presence of laurel wilt, a fungus that attacks avocado trees and kills them, costing Florida growers millions. With that expertise under their collars, the two dogs need only a few weeks to get good at detecting other smells assigned to them.
Training the dogs, safely
To train Cobra and One Betta on COVID-19 odors, Dr. Furton’s team first acquired mask samples from people hospitalized with COVID and people who did not have the disease. In battling the viruses, people produce certain chemicals that they exhale every time they breathe. When Dr. Furton and his colleagues compared the exhaled components trapped in the masks, they found differences between masks from people with COVID and those without.
Having confirmed that exhalations can be COVID-specific, the research team trained four dogs – Cobra, One Betta, Hubble, and Max – to detect masks from people with COVID among an assortment of mask choices. Before this step, though, the researchers made sure that any trace of active virus was destroyed by ultraviolet light so that the dogs would not be infected.
Each time the dogs accurately selected a mask from a COVID patient, their reward was access to a favorite toy: A red ball to chew on. Although all four dogs performed very well, yes, they did, Cobra and One Betta showed the most accuracy, outperforming their training colleagues. From their training scores, Cobra ranked first, with 99.45% accuracy. Despite her name, says Dr. Furton, One Betta was “not one better,” coming in second at 98.1%, which is still quite high.
Both dogs are good at their airport screening duties. If one of them sits after sniffing a mask at the checkpoint, the next step is for the mask owner to be tested.
From Aug. 23 to Sept. 8, the two canines screened 1,093 people during 8 working days, alerting on only one case, according to Greg Chin, communications director for the Miami-Dade Aviation Department. That person had tested positive for COVID 2 weeks earlier and was returning to work after quarantine, and their rapid test after the dog alerted was negative.
Dr. Furton says that there are some reports of dogs also alerting before tests can show a positive result, suggesting the dogs’ odor detection can be more precise. They hope to expand their study to see how tight the window of dog-based detection is.
For now, the detector dogs are doing so well that the program has been extended for 30 more days, Mr. Chin says.
As promising as this seems, using dogs for screening carries some logistical and ethical tangles. Training a canine army to deploy for high-volume detection points means that once the work is done, a whole lot of dogs will need a safe place to retire. In addition, the initial training takes several months, says Dr. Furton, whereas if a device were developed for screening, manufacturing could likely be ramped up quickly to meet demand.
The dogs might not need to retire right away, though.
“We envision that they could be redeployed to another type of detection for another infectious disease” if the need arises, Dr. Furton says. But in the end, when working with dogs, he says, there is “a moral connection that you don’t have to deal with using instruments.”
Although the pilot screening at Miami International is the first airport test, the dogs have also done this work in other venues, including at a state emergency operations center in Florida and in some university classrooms, says Dr. Furton.
A version of this article first appeared on WebMD.com.
If she identifies a specific scent, she’ll let her handler know simply by sitting down. When this good girl sits, that means Cobra has detected an olfactory signal of the coronavirus, the virus that causes COVID-19.
Cobra, a Belgian Malinois, is one of two canines – her partner is One Betta, a Dutch shepherd – working this checkpoint at Miami International. They are part of a pilot program with the Global Forensic and Justice Center at Florida International University, using the detection dogs as a quick screen for people who have COVID-19.
Their detection rate is high, at more than 98%, and the program has been such a success that it’s being extended for another month at the airport.
If these two dogs continue to accurately detect COVID-19, they and other canines with similar training could be deployed to other places with lots of people coming and going at once, including other airports or even schools. In fact, COVID-sniffing dogs are in use in some university classrooms already.
But building up a big brigade of live animals as disease detectors involves some thorny issues, including where the animals retire once their careers are complete.
“When COVID first arose, we said let’s see if we can train these two dogs on either the virus or the odor of COVID-19,” says Kenneth Furton, PhD, a professor of chemistry and biochemistry, provost, and executive vice president at Florida International University.
His team had completed a study with what he calls “medical detector dogs,” animals that might be able to detect the odor of someone having a seizure. That led them to see how well the animals could detect other kinds of disorders.
Training a dog to sniff out specific odors starts with getting them to understand the task in general. Dr. Furton says that the animals first are trained to grasp that their job is to detect one odor among many. Once the dogs grasp that, they can be trained on just about any specific odor.
In fact, in addition to detecting seizures, dogs reportedly have been able to identify diabetes and even some cancers, such as ovarian cancer.
Dr. Furton says he’s not aware of any previous use of dogs to screen for infectious disease. That may simply be because nothing recently has struck with the global ferocity of COVID, driving humans to turn to their best friends for help.
Cobra and One Betta got their start learning to identify the presence of laurel wilt, a fungus that attacks avocado trees and kills them, costing Florida growers millions. With that expertise under their collars, the two dogs need only a few weeks to get good at detecting other smells assigned to them.
Training the dogs, safely
To train Cobra and One Betta on COVID-19 odors, Dr. Furton’s team first acquired mask samples from people hospitalized with COVID and people who did not have the disease. In battling the viruses, people produce certain chemicals that they exhale every time they breathe. When Dr. Furton and his colleagues compared the exhaled components trapped in the masks, they found differences between masks from people with COVID and those without.
Having confirmed that exhalations can be COVID-specific, the research team trained four dogs – Cobra, One Betta, Hubble, and Max – to detect masks from people with COVID among an assortment of mask choices. Before this step, though, the researchers made sure that any trace of active virus was destroyed by ultraviolet light so that the dogs would not be infected.
Each time the dogs accurately selected a mask from a COVID patient, their reward was access to a favorite toy: A red ball to chew on. Although all four dogs performed very well, yes, they did, Cobra and One Betta showed the most accuracy, outperforming their training colleagues. From their training scores, Cobra ranked first, with 99.45% accuracy. Despite her name, says Dr. Furton, One Betta was “not one better,” coming in second at 98.1%, which is still quite high.
Both dogs are good at their airport screening duties. If one of them sits after sniffing a mask at the checkpoint, the next step is for the mask owner to be tested.
From Aug. 23 to Sept. 8, the two canines screened 1,093 people during 8 working days, alerting on only one case, according to Greg Chin, communications director for the Miami-Dade Aviation Department. That person had tested positive for COVID 2 weeks earlier and was returning to work after quarantine, and their rapid test after the dog alerted was negative.
Dr. Furton says that there are some reports of dogs also alerting before tests can show a positive result, suggesting the dogs’ odor detection can be more precise. They hope to expand their study to see how tight the window of dog-based detection is.
For now, the detector dogs are doing so well that the program has been extended for 30 more days, Mr. Chin says.
As promising as this seems, using dogs for screening carries some logistical and ethical tangles. Training a canine army to deploy for high-volume detection points means that once the work is done, a whole lot of dogs will need a safe place to retire. In addition, the initial training takes several months, says Dr. Furton, whereas if a device were developed for screening, manufacturing could likely be ramped up quickly to meet demand.
The dogs might not need to retire right away, though.
“We envision that they could be redeployed to another type of detection for another infectious disease” if the need arises, Dr. Furton says. But in the end, when working with dogs, he says, there is “a moral connection that you don’t have to deal with using instruments.”
Although the pilot screening at Miami International is the first airport test, the dogs have also done this work in other venues, including at a state emergency operations center in Florida and in some university classrooms, says Dr. Furton.
A version of this article first appeared on WebMD.com.
Surge in new-onset tics in adults tied to COVID-19 stress
, new research suggests.
Results from a large, single-center study show several cases of tic-like movements and vocalizations with abrupt onset among older adolescents and adults during the pandemic. None had a previous diagnosis of a tic disorder. Among 10 patients, two were diagnosed with a purely functional movement disorder, four with an organic tic disorder, and four with both.
“Within our movement disorders clinic specifically ... we’ve been seeing an increased number of patients with an almost explosive onset of these tic-like movements and vocalizations later in life than what is typically seen with organic tic disorders and Tourette syndrome, which is typically in school-aged children,” said study investigator Caroline Olvera, MD, Rush University Medical Center, Chicago.
“Abrupt onset of symptoms can be seen in patients with tic disorders, although this is typically quoted as less than 10%, or even 5% is more characteristic of functional neurological disorders in general and also with psychogenic tics,” she added.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Anxiety, other psychiatric conditions
Tic disorders typically start in childhood. However, the researchers observed an increase in the number of patients with abrupt onset of tic-like movements and vocalizations later in life, which is more often characteristic of functional neurological disorders.
To examine the profile, associated conditions, and risk factors in this population, the investigators conducted a thorough chart review of patients attending movement disorder clinics between March 2020, when the COVID pandemic was officially declared, and March 2021.
Patients with acute onset of tics were identified using the International Classification of Diseases codes for behavioral tics, tic vocalizations, and Tourette syndrome.
The charts were then narrowed down to patients with no previous diagnosis of these conditions. Most patients were videotaped for assessment by the rest of the movement disorder neurologists in the practice. Since the end of the study inclusion period in March 2021, Dr. Olvera estimates that the clinic experienced a doubling or tripling of the number of similar patients.
In the study cohort of 10 patients, the median age at presentation was 19 years (range, 15-41 years), nine were female, the gender of the other one was unknown, and the duration of tics was 8 weeks (range, 1-24 weeks) by the time they were first seen in the clinic. Four patients reported having COVID infection before tic onset.
All exhibited motor tics and nine had vocal tics. Two were diagnosed with a purely functional neurologic disorder, four with only an organic tic disorder, and four with organic tics with a functional overlay.
“All patients, including those with organic tic disorders, had a history of anxiety and also reported worsening anxiety in the setting of the COVID pandemic,” Dr. Olvera said.
The majority of patients were on a psychotropic medication prior to coming to the clinic, and these were primarily for anxiety and depression. Three patients had a history of suicidality, often very severe and leading to hospitalization, she noted.
“In terms of our conclusions from the project, we feel that this phenotype of acute explosive onset of tic-like movements and vocalizations in this older population of adults, compared with typical organic tic disorders and Tourette syndrome, appears novel to the pandemic,” she said.
She cautioned that functional and organic tics share many characteristics and therefore may be difficult to differentiate.
COVID stress
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said the research highlights how clinicians’ understanding of particular diseases can be challenged during extraordinary events such as COVID-19 and the heightened stress it causes.
“I’m not surprised that these [disorders] might have had a spike during a stressful time as COVID,” he said.
Patients are “really scared and really anxious, they’re afraid to die, and they’re afraid that their life will be over. So they might express their psychological difficulty, their discomfort, with these calls for help that look like tics. But they’re not what we consider physiological or organic things,” he added.
Dr. Tagliati added that he doesn’t believe rapid tic onset in adults is not a complication of the coronavirus infection, but rather a consequence of psychological pressure brought on by the pandemic.
Treating underlying anxiety may be a useful approach, possibly with the support of psychiatrists, which in many cases is enough to relieve the conditions and overcome the symptoms, he noted.
However, at other times, it’s not that simple, he added. Sometimes patients “fall through the cracks between neurology and psychiatry,” Dr. Tagliati said.
Dr. Olvera and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large, single-center study show several cases of tic-like movements and vocalizations with abrupt onset among older adolescents and adults during the pandemic. None had a previous diagnosis of a tic disorder. Among 10 patients, two were diagnosed with a purely functional movement disorder, four with an organic tic disorder, and four with both.
“Within our movement disorders clinic specifically ... we’ve been seeing an increased number of patients with an almost explosive onset of these tic-like movements and vocalizations later in life than what is typically seen with organic tic disorders and Tourette syndrome, which is typically in school-aged children,” said study investigator Caroline Olvera, MD, Rush University Medical Center, Chicago.
“Abrupt onset of symptoms can be seen in patients with tic disorders, although this is typically quoted as less than 10%, or even 5% is more characteristic of functional neurological disorders in general and also with psychogenic tics,” she added.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Anxiety, other psychiatric conditions
Tic disorders typically start in childhood. However, the researchers observed an increase in the number of patients with abrupt onset of tic-like movements and vocalizations later in life, which is more often characteristic of functional neurological disorders.
To examine the profile, associated conditions, and risk factors in this population, the investigators conducted a thorough chart review of patients attending movement disorder clinics between March 2020, when the COVID pandemic was officially declared, and March 2021.
Patients with acute onset of tics were identified using the International Classification of Diseases codes for behavioral tics, tic vocalizations, and Tourette syndrome.
The charts were then narrowed down to patients with no previous diagnosis of these conditions. Most patients were videotaped for assessment by the rest of the movement disorder neurologists in the practice. Since the end of the study inclusion period in March 2021, Dr. Olvera estimates that the clinic experienced a doubling or tripling of the number of similar patients.
In the study cohort of 10 patients, the median age at presentation was 19 years (range, 15-41 years), nine were female, the gender of the other one was unknown, and the duration of tics was 8 weeks (range, 1-24 weeks) by the time they were first seen in the clinic. Four patients reported having COVID infection before tic onset.
All exhibited motor tics and nine had vocal tics. Two were diagnosed with a purely functional neurologic disorder, four with only an organic tic disorder, and four with organic tics with a functional overlay.
“All patients, including those with organic tic disorders, had a history of anxiety and also reported worsening anxiety in the setting of the COVID pandemic,” Dr. Olvera said.
The majority of patients were on a psychotropic medication prior to coming to the clinic, and these were primarily for anxiety and depression. Three patients had a history of suicidality, often very severe and leading to hospitalization, she noted.
“In terms of our conclusions from the project, we feel that this phenotype of acute explosive onset of tic-like movements and vocalizations in this older population of adults, compared with typical organic tic disorders and Tourette syndrome, appears novel to the pandemic,” she said.
She cautioned that functional and organic tics share many characteristics and therefore may be difficult to differentiate.
COVID stress
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said the research highlights how clinicians’ understanding of particular diseases can be challenged during extraordinary events such as COVID-19 and the heightened stress it causes.
“I’m not surprised that these [disorders] might have had a spike during a stressful time as COVID,” he said.
Patients are “really scared and really anxious, they’re afraid to die, and they’re afraid that their life will be over. So they might express their psychological difficulty, their discomfort, with these calls for help that look like tics. But they’re not what we consider physiological or organic things,” he added.
Dr. Tagliati added that he doesn’t believe rapid tic onset in adults is not a complication of the coronavirus infection, but rather a consequence of psychological pressure brought on by the pandemic.
Treating underlying anxiety may be a useful approach, possibly with the support of psychiatrists, which in many cases is enough to relieve the conditions and overcome the symptoms, he noted.
However, at other times, it’s not that simple, he added. Sometimes patients “fall through the cracks between neurology and psychiatry,” Dr. Tagliati said.
Dr. Olvera and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large, single-center study show several cases of tic-like movements and vocalizations with abrupt onset among older adolescents and adults during the pandemic. None had a previous diagnosis of a tic disorder. Among 10 patients, two were diagnosed with a purely functional movement disorder, four with an organic tic disorder, and four with both.
“Within our movement disorders clinic specifically ... we’ve been seeing an increased number of patients with an almost explosive onset of these tic-like movements and vocalizations later in life than what is typically seen with organic tic disorders and Tourette syndrome, which is typically in school-aged children,” said study investigator Caroline Olvera, MD, Rush University Medical Center, Chicago.
“Abrupt onset of symptoms can be seen in patients with tic disorders, although this is typically quoted as less than 10%, or even 5% is more characteristic of functional neurological disorders in general and also with psychogenic tics,” she added.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Anxiety, other psychiatric conditions
Tic disorders typically start in childhood. However, the researchers observed an increase in the number of patients with abrupt onset of tic-like movements and vocalizations later in life, which is more often characteristic of functional neurological disorders.
To examine the profile, associated conditions, and risk factors in this population, the investigators conducted a thorough chart review of patients attending movement disorder clinics between March 2020, when the COVID pandemic was officially declared, and March 2021.
Patients with acute onset of tics were identified using the International Classification of Diseases codes for behavioral tics, tic vocalizations, and Tourette syndrome.
The charts were then narrowed down to patients with no previous diagnosis of these conditions. Most patients were videotaped for assessment by the rest of the movement disorder neurologists in the practice. Since the end of the study inclusion period in March 2021, Dr. Olvera estimates that the clinic experienced a doubling or tripling of the number of similar patients.
In the study cohort of 10 patients, the median age at presentation was 19 years (range, 15-41 years), nine were female, the gender of the other one was unknown, and the duration of tics was 8 weeks (range, 1-24 weeks) by the time they were first seen in the clinic. Four patients reported having COVID infection before tic onset.
All exhibited motor tics and nine had vocal tics. Two were diagnosed with a purely functional neurologic disorder, four with only an organic tic disorder, and four with organic tics with a functional overlay.
“All patients, including those with organic tic disorders, had a history of anxiety and also reported worsening anxiety in the setting of the COVID pandemic,” Dr. Olvera said.
The majority of patients were on a psychotropic medication prior to coming to the clinic, and these were primarily for anxiety and depression. Three patients had a history of suicidality, often very severe and leading to hospitalization, she noted.
“In terms of our conclusions from the project, we feel that this phenotype of acute explosive onset of tic-like movements and vocalizations in this older population of adults, compared with typical organic tic disorders and Tourette syndrome, appears novel to the pandemic,” she said.
She cautioned that functional and organic tics share many characteristics and therefore may be difficult to differentiate.
COVID stress
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said the research highlights how clinicians’ understanding of particular diseases can be challenged during extraordinary events such as COVID-19 and the heightened stress it causes.
“I’m not surprised that these [disorders] might have had a spike during a stressful time as COVID,” he said.
Patients are “really scared and really anxious, they’re afraid to die, and they’re afraid that their life will be over. So they might express their psychological difficulty, their discomfort, with these calls for help that look like tics. But they’re not what we consider physiological or organic things,” he added.
Dr. Tagliati added that he doesn’t believe rapid tic onset in adults is not a complication of the coronavirus infection, but rather a consequence of psychological pressure brought on by the pandemic.
Treating underlying anxiety may be a useful approach, possibly with the support of psychiatrists, which in many cases is enough to relieve the conditions and overcome the symptoms, he noted.
However, at other times, it’s not that simple, he added. Sometimes patients “fall through the cracks between neurology and psychiatry,” Dr. Tagliati said.
Dr. Olvera and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Doctor who claimed masks hurt health loses license
Steven Arthur LaTulippe’s advice to patients about face masking amounted to “gross negligence” in the practice of medicine and was grounds for discipline, the medical board said in a report.
Mr. LaTulippe, who had a family practice in Dallas, was fined $10,000, Insider reported. The board also said he’d overprescribed opioids for some patients.
The medical board report said Mr. LaTulippe and his wife, who ran the clinic with him, didn’t wear face masks while treating patients from March to December 2020.
Mr. LaTulippe told elderly and pediatric patients that mask wearing could hurt their health by exacerbating COPD and asthma and could contribute to heart attacks and other medical problems, the report said.
“Licensee asserts masks are likely to harm patients by increasing the body’s carbon dioxide content through rebreathing of gas trapped behind a mask,” the report said.
The report noted that “the amount of carbon dioxide rebreathed within a mask is trivial and would easily be expelled by an increase in minute ventilation so small it would not be noticed.”
The report said Mr. LaTulippe told patients they didn’t have to wear a mask in the clinic unless they were “acutely ill,” “coughing,” or “congested,” even though the Centers for Disease Control and Prevention and the Oregon governor had recommended masks be worn to prevent the spread of the virus.
Before coming into the office, patients weren’t asked if they’d had recent contact with anybody who was infected or showed COVID symptoms, the report said.
The medical board first suspended his license in September. He said he would not change his conduct concerning face masks.
“Licensee has confirmed that he will refuse to abide by the state’s COVID-19 protocols in the future as well, affirming that in a choice between losing his medical license versus wearing a mask in his clinic and requiring his patients and staff to wear a mask in his clinic, he will, ‘choose to sacrifice my medical license with no hesitation’ ” the medical board’s report said.
Mr. LaTulippe told the medical board that he was “a strong asset to the public in educating them on the real facts about this pandemic” and that “at least 98% of my patients were so extremely thankful that I did not wear a mask or demand wearing a mask in my clinic.”
The medical board found Mr. LaTulippe engaged in 8 instances of unprofessional or dishonorable conduct, 22 instances of negligence in the practice of medicine, and 5 instances of gross negligence in the practice of medicine.
A version of this article first appeared on WebMD.com.
Steven Arthur LaTulippe’s advice to patients about face masking amounted to “gross negligence” in the practice of medicine and was grounds for discipline, the medical board said in a report.
Mr. LaTulippe, who had a family practice in Dallas, was fined $10,000, Insider reported. The board also said he’d overprescribed opioids for some patients.
The medical board report said Mr. LaTulippe and his wife, who ran the clinic with him, didn’t wear face masks while treating patients from March to December 2020.
Mr. LaTulippe told elderly and pediatric patients that mask wearing could hurt their health by exacerbating COPD and asthma and could contribute to heart attacks and other medical problems, the report said.
“Licensee asserts masks are likely to harm patients by increasing the body’s carbon dioxide content through rebreathing of gas trapped behind a mask,” the report said.
The report noted that “the amount of carbon dioxide rebreathed within a mask is trivial and would easily be expelled by an increase in minute ventilation so small it would not be noticed.”
The report said Mr. LaTulippe told patients they didn’t have to wear a mask in the clinic unless they were “acutely ill,” “coughing,” or “congested,” even though the Centers for Disease Control and Prevention and the Oregon governor had recommended masks be worn to prevent the spread of the virus.
Before coming into the office, patients weren’t asked if they’d had recent contact with anybody who was infected or showed COVID symptoms, the report said.
The medical board first suspended his license in September. He said he would not change his conduct concerning face masks.
“Licensee has confirmed that he will refuse to abide by the state’s COVID-19 protocols in the future as well, affirming that in a choice between losing his medical license versus wearing a mask in his clinic and requiring his patients and staff to wear a mask in his clinic, he will, ‘choose to sacrifice my medical license with no hesitation’ ” the medical board’s report said.
Mr. LaTulippe told the medical board that he was “a strong asset to the public in educating them on the real facts about this pandemic” and that “at least 98% of my patients were so extremely thankful that I did not wear a mask or demand wearing a mask in my clinic.”
The medical board found Mr. LaTulippe engaged in 8 instances of unprofessional or dishonorable conduct, 22 instances of negligence in the practice of medicine, and 5 instances of gross negligence in the practice of medicine.
A version of this article first appeared on WebMD.com.
Steven Arthur LaTulippe’s advice to patients about face masking amounted to “gross negligence” in the practice of medicine and was grounds for discipline, the medical board said in a report.
Mr. LaTulippe, who had a family practice in Dallas, was fined $10,000, Insider reported. The board also said he’d overprescribed opioids for some patients.
The medical board report said Mr. LaTulippe and his wife, who ran the clinic with him, didn’t wear face masks while treating patients from March to December 2020.
Mr. LaTulippe told elderly and pediatric patients that mask wearing could hurt their health by exacerbating COPD and asthma and could contribute to heart attacks and other medical problems, the report said.
“Licensee asserts masks are likely to harm patients by increasing the body’s carbon dioxide content through rebreathing of gas trapped behind a mask,” the report said.
The report noted that “the amount of carbon dioxide rebreathed within a mask is trivial and would easily be expelled by an increase in minute ventilation so small it would not be noticed.”
The report said Mr. LaTulippe told patients they didn’t have to wear a mask in the clinic unless they were “acutely ill,” “coughing,” or “congested,” even though the Centers for Disease Control and Prevention and the Oregon governor had recommended masks be worn to prevent the spread of the virus.
Before coming into the office, patients weren’t asked if they’d had recent contact with anybody who was infected or showed COVID symptoms, the report said.
The medical board first suspended his license in September. He said he would not change his conduct concerning face masks.
“Licensee has confirmed that he will refuse to abide by the state’s COVID-19 protocols in the future as well, affirming that in a choice between losing his medical license versus wearing a mask in his clinic and requiring his patients and staff to wear a mask in his clinic, he will, ‘choose to sacrifice my medical license with no hesitation’ ” the medical board’s report said.
Mr. LaTulippe told the medical board that he was “a strong asset to the public in educating them on the real facts about this pandemic” and that “at least 98% of my patients were so extremely thankful that I did not wear a mask or demand wearing a mask in my clinic.”
The medical board found Mr. LaTulippe engaged in 8 instances of unprofessional or dishonorable conduct, 22 instances of negligence in the practice of medicine, and 5 instances of gross negligence in the practice of medicine.
A version of this article first appeared on WebMD.com.
Aloha
In July, 2021, JAMA published a study about physicians’ sartorial habits, basically saying that people prefer doctors to dress “professionally.” Even today the white coat still carries some weight.
And I still don’t care.
Today, like every workday since June 2006, I put on my standard patient-seeing attire: shorts, sneakers, and a Hawaiian shirt. The only significant change has been the addition of a face mask since March 2020.
I have no plans to change anytime between now and retirement. I live in Phoenix, the hottest major city in the U.S., and have no desire to be uncomfortable because someone doesn’t think I look professional. It’s even become, albeit unintentionally, a trademark of sorts.
Now, as always, I let my patients be the judge. If someone isn’t happy with my appearance, or feels it makes me less competent, they certainly have the right to feel that way. There are plenty of other neurologists here who dress to higher standards (though jackets and ties, outside of the Mayo Clinic down the road, are getting pretty hard to find).
This is one of the things I like about having a small solo practice. I can be who I am, not who some administrator or dress code specialist says I have to be.
I do my best for my patients, and those who know me are aware that my complete lack of fashion sense doesn’t represent (I hope) an equal lack of medical care. Most of them seem to come back, so I guess I’m doing something right.
But it brings up the question of what should a doctor look like? In a world of changing demographics the stereotype of a neatly-dressed middle-aged white male certainly isn’t it anymore.
Nor should there be. Medicine should be open to all with the drive, brains, and talent who want to follow to path of Hippocrates. Maybe I’m naive, but I still see this as a calling more than a job. Judging someone’s medical competence solely on their sex, race, appearance, or fashion sense is foolhardy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In July, 2021, JAMA published a study about physicians’ sartorial habits, basically saying that people prefer doctors to dress “professionally.” Even today the white coat still carries some weight.
And I still don’t care.
Today, like every workday since June 2006, I put on my standard patient-seeing attire: shorts, sneakers, and a Hawaiian shirt. The only significant change has been the addition of a face mask since March 2020.
I have no plans to change anytime between now and retirement. I live in Phoenix, the hottest major city in the U.S., and have no desire to be uncomfortable because someone doesn’t think I look professional. It’s even become, albeit unintentionally, a trademark of sorts.
Now, as always, I let my patients be the judge. If someone isn’t happy with my appearance, or feels it makes me less competent, they certainly have the right to feel that way. There are plenty of other neurologists here who dress to higher standards (though jackets and ties, outside of the Mayo Clinic down the road, are getting pretty hard to find).
This is one of the things I like about having a small solo practice. I can be who I am, not who some administrator or dress code specialist says I have to be.
I do my best for my patients, and those who know me are aware that my complete lack of fashion sense doesn’t represent (I hope) an equal lack of medical care. Most of them seem to come back, so I guess I’m doing something right.
But it brings up the question of what should a doctor look like? In a world of changing demographics the stereotype of a neatly-dressed middle-aged white male certainly isn’t it anymore.
Nor should there be. Medicine should be open to all with the drive, brains, and talent who want to follow to path of Hippocrates. Maybe I’m naive, but I still see this as a calling more than a job. Judging someone’s medical competence solely on their sex, race, appearance, or fashion sense is foolhardy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In July, 2021, JAMA published a study about physicians’ sartorial habits, basically saying that people prefer doctors to dress “professionally.” Even today the white coat still carries some weight.
And I still don’t care.
Today, like every workday since June 2006, I put on my standard patient-seeing attire: shorts, sneakers, and a Hawaiian shirt. The only significant change has been the addition of a face mask since March 2020.
I have no plans to change anytime between now and retirement. I live in Phoenix, the hottest major city in the U.S., and have no desire to be uncomfortable because someone doesn’t think I look professional. It’s even become, albeit unintentionally, a trademark of sorts.
Now, as always, I let my patients be the judge. If someone isn’t happy with my appearance, or feels it makes me less competent, they certainly have the right to feel that way. There are plenty of other neurologists here who dress to higher standards (though jackets and ties, outside of the Mayo Clinic down the road, are getting pretty hard to find).
This is one of the things I like about having a small solo practice. I can be who I am, not who some administrator or dress code specialist says I have to be.
I do my best for my patients, and those who know me are aware that my complete lack of fashion sense doesn’t represent (I hope) an equal lack of medical care. Most of them seem to come back, so I guess I’m doing something right.
But it brings up the question of what should a doctor look like? In a world of changing demographics the stereotype of a neatly-dressed middle-aged white male certainly isn’t it anymore.
Nor should there be. Medicine should be open to all with the drive, brains, and talent who want to follow to path of Hippocrates. Maybe I’m naive, but I still see this as a calling more than a job. Judging someone’s medical competence solely on their sex, race, appearance, or fashion sense is foolhardy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
COVID-19 claims more than 675,000 U.S. lives, surpassing the 1918 flu
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
Sublingual film well tolerated for Parkinson ‘off’ episodes
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.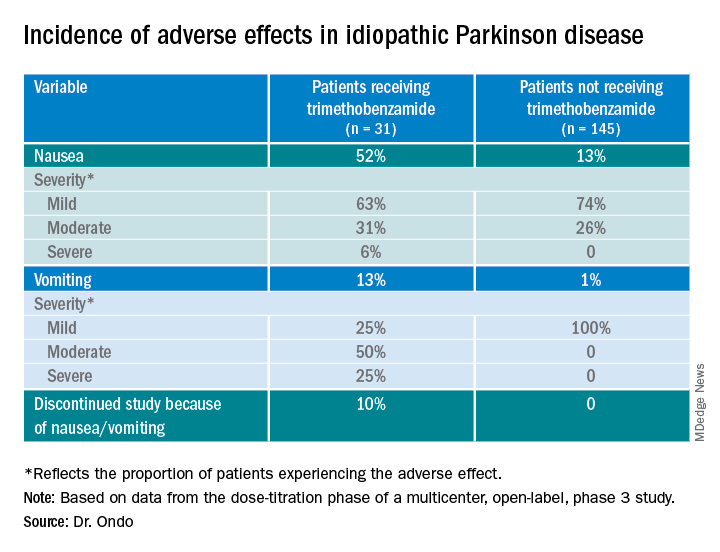
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Friedreich’s ataxia treatment shows extended benefit
according to results of a clinical trial presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders.
The study, labeled the Delayed-Start Study, is an extension study of the two-part MOXIE phase 2 trial of omaveloxolone.
“This study shows two things,” said David Lynch, MD, PhD, of Children’s Hospital of Philadelphia. “It doesn’t matter when you started omaveloxolone for you to see a benefit; and that the benefit that the active group saw in the first part of the study was maintained as they went into the delayed-start part. So in fact omaveloxolone does modify the long-term behavior of the disease.”
Friedreich’s ataxia only affects about 22,000 people worldwide, and children typically present between the ages of 5 and 15, Dr. Lynch said.
The extension study included 73 patients who completed either of the first two parts of the MOXIe trial. The MOXIe trial randomized patients on a 3:1 basis to either omaveloxolone 2.5-300 mg or placebo for 12 weeks in the first part. The second part was a double-blind trial of 103 patients randomized on a 1:1 basis to 150 mg omaveloxolone or placebo for 48 weeks. Participants had a baseline modified Friedreich’s ataxia scale (mFARS) of 20-80 and were aged 16-40 years.
Patients in the extension study did not have severe pes cavus. The extension study was a 72-week evaluation of patients who were in either the treatment or placebo groups in the first two parts. There was a 4-week off-treatment period between the end of MOXIe part 2 and the beginning of the extension study, in which all patients received omaveloxolone.
At the end of the placebo-controlled study, patients taking omaveloxolone showed a –2.18-point (±0.96) difference in improvement in mFARS score (P = .027), compared with the placebo group, which was preserved at the end of the delayed-start period, with a –2.92-point (±2.13) improvement (P = .179), Dr. Lynch said.
In the extension study, former placebo patients who went on omaveloxolone had annualized mFARS slopes similar to the previously treated patients – 0.29 (±0.68) and 0.17 (±0.61), respectively (P = .85) – from weeks 48 to 144, Dr. Lynch said.
“This study showed that, when analyzed in a delayed-start fashion, it does not matter when you start omaveloxolone to see a benefit: Each cohort benefited almost equally once they started the drug,” Dr. Lynch said in an interview. “Also, in both groups, once they started omaveloxolone, they changed slower than people in natural history studies.”
A clinically meaningful difference?
Reached for comment, Massimo Pandolfo, MD, a neurologist at McGill University, Montreal, noted that the Delayed-Start Study included only patients without pes cavus, an indication that the patients had less severe disease. “It would be important to see how overall patients with Friedreich’s ataxia would have responded to the medication without this kind of selection,” Dr. Pandolfo said in an interview.
He also noted that the seemingly modest improvement in mFARS score could be an issue. “It’s a very difficult question: What is a clinically meaningful difference in this kind of rating scale? I would argue that probably 2 points is not a huge difference by itself, but it may be meaningful and one indicator of that is that if it was accompanied by also a significant difference in activities of daily living scale.”
In any event, Dr. Pandolfo said this is the first medication for Friedreich’s ataxia that has “survived” a randomized clinical trial.
Dr. Lynch said the study sponsor, Reata, may prepare a new drug application for omaveloxolone in patients ages 16 and older. “That would leave a need for investigation in younger FA patients.”
Dr. Lynch disclosed that his institution receives a grant from trial sponsor Reata to conduct the MOXIe trial. Dr. Pandolfo reports financial relationships with Design Therapeutics, Exicure and Voyager Therapeutics.
according to results of a clinical trial presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders.
The study, labeled the Delayed-Start Study, is an extension study of the two-part MOXIE phase 2 trial of omaveloxolone.
“This study shows two things,” said David Lynch, MD, PhD, of Children’s Hospital of Philadelphia. “It doesn’t matter when you started omaveloxolone for you to see a benefit; and that the benefit that the active group saw in the first part of the study was maintained as they went into the delayed-start part. So in fact omaveloxolone does modify the long-term behavior of the disease.”
Friedreich’s ataxia only affects about 22,000 people worldwide, and children typically present between the ages of 5 and 15, Dr. Lynch said.
The extension study included 73 patients who completed either of the first two parts of the MOXIe trial. The MOXIe trial randomized patients on a 3:1 basis to either omaveloxolone 2.5-300 mg or placebo for 12 weeks in the first part. The second part was a double-blind trial of 103 patients randomized on a 1:1 basis to 150 mg omaveloxolone or placebo for 48 weeks. Participants had a baseline modified Friedreich’s ataxia scale (mFARS) of 20-80 and were aged 16-40 years.
Patients in the extension study did not have severe pes cavus. The extension study was a 72-week evaluation of patients who were in either the treatment or placebo groups in the first two parts. There was a 4-week off-treatment period between the end of MOXIe part 2 and the beginning of the extension study, in which all patients received omaveloxolone.
At the end of the placebo-controlled study, patients taking omaveloxolone showed a –2.18-point (±0.96) difference in improvement in mFARS score (P = .027), compared with the placebo group, which was preserved at the end of the delayed-start period, with a –2.92-point (±2.13) improvement (P = .179), Dr. Lynch said.
In the extension study, former placebo patients who went on omaveloxolone had annualized mFARS slopes similar to the previously treated patients – 0.29 (±0.68) and 0.17 (±0.61), respectively (P = .85) – from weeks 48 to 144, Dr. Lynch said.
“This study showed that, when analyzed in a delayed-start fashion, it does not matter when you start omaveloxolone to see a benefit: Each cohort benefited almost equally once they started the drug,” Dr. Lynch said in an interview. “Also, in both groups, once they started omaveloxolone, they changed slower than people in natural history studies.”
A clinically meaningful difference?
Reached for comment, Massimo Pandolfo, MD, a neurologist at McGill University, Montreal, noted that the Delayed-Start Study included only patients without pes cavus, an indication that the patients had less severe disease. “It would be important to see how overall patients with Friedreich’s ataxia would have responded to the medication without this kind of selection,” Dr. Pandolfo said in an interview.
He also noted that the seemingly modest improvement in mFARS score could be an issue. “It’s a very difficult question: What is a clinically meaningful difference in this kind of rating scale? I would argue that probably 2 points is not a huge difference by itself, but it may be meaningful and one indicator of that is that if it was accompanied by also a significant difference in activities of daily living scale.”
In any event, Dr. Pandolfo said this is the first medication for Friedreich’s ataxia that has “survived” a randomized clinical trial.
Dr. Lynch said the study sponsor, Reata, may prepare a new drug application for omaveloxolone in patients ages 16 and older. “That would leave a need for investigation in younger FA patients.”
Dr. Lynch disclosed that his institution receives a grant from trial sponsor Reata to conduct the MOXIe trial. Dr. Pandolfo reports financial relationships with Design Therapeutics, Exicure and Voyager Therapeutics.
according to results of a clinical trial presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders.
The study, labeled the Delayed-Start Study, is an extension study of the two-part MOXIE phase 2 trial of omaveloxolone.
“This study shows two things,” said David Lynch, MD, PhD, of Children’s Hospital of Philadelphia. “It doesn’t matter when you started omaveloxolone for you to see a benefit; and that the benefit that the active group saw in the first part of the study was maintained as they went into the delayed-start part. So in fact omaveloxolone does modify the long-term behavior of the disease.”
Friedreich’s ataxia only affects about 22,000 people worldwide, and children typically present between the ages of 5 and 15, Dr. Lynch said.
The extension study included 73 patients who completed either of the first two parts of the MOXIe trial. The MOXIe trial randomized patients on a 3:1 basis to either omaveloxolone 2.5-300 mg or placebo for 12 weeks in the first part. The second part was a double-blind trial of 103 patients randomized on a 1:1 basis to 150 mg omaveloxolone or placebo for 48 weeks. Participants had a baseline modified Friedreich’s ataxia scale (mFARS) of 20-80 and were aged 16-40 years.
Patients in the extension study did not have severe pes cavus. The extension study was a 72-week evaluation of patients who were in either the treatment or placebo groups in the first two parts. There was a 4-week off-treatment period between the end of MOXIe part 2 and the beginning of the extension study, in which all patients received omaveloxolone.
At the end of the placebo-controlled study, patients taking omaveloxolone showed a –2.18-point (±0.96) difference in improvement in mFARS score (P = .027), compared with the placebo group, which was preserved at the end of the delayed-start period, with a –2.92-point (±2.13) improvement (P = .179), Dr. Lynch said.
In the extension study, former placebo patients who went on omaveloxolone had annualized mFARS slopes similar to the previously treated patients – 0.29 (±0.68) and 0.17 (±0.61), respectively (P = .85) – from weeks 48 to 144, Dr. Lynch said.
“This study showed that, when analyzed in a delayed-start fashion, it does not matter when you start omaveloxolone to see a benefit: Each cohort benefited almost equally once they started the drug,” Dr. Lynch said in an interview. “Also, in both groups, once they started omaveloxolone, they changed slower than people in natural history studies.”
A clinically meaningful difference?
Reached for comment, Massimo Pandolfo, MD, a neurologist at McGill University, Montreal, noted that the Delayed-Start Study included only patients without pes cavus, an indication that the patients had less severe disease. “It would be important to see how overall patients with Friedreich’s ataxia would have responded to the medication without this kind of selection,” Dr. Pandolfo said in an interview.
He also noted that the seemingly modest improvement in mFARS score could be an issue. “It’s a very difficult question: What is a clinically meaningful difference in this kind of rating scale? I would argue that probably 2 points is not a huge difference by itself, but it may be meaningful and one indicator of that is that if it was accompanied by also a significant difference in activities of daily living scale.”
In any event, Dr. Pandolfo said this is the first medication for Friedreich’s ataxia that has “survived” a randomized clinical trial.
Dr. Lynch said the study sponsor, Reata, may prepare a new drug application for omaveloxolone in patients ages 16 and older. “That would leave a need for investigation in younger FA patients.”
Dr. Lynch disclosed that his institution receives a grant from trial sponsor Reata to conduct the MOXIe trial. Dr. Pandolfo reports financial relationships with Design Therapeutics, Exicure and Voyager Therapeutics.
FROM MDS VIRTUAL CONGRESS 2021
Survey identifies clinicians’ unease with genetic testing
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
FROM MDS VIRTUAL CONGRESS 2021
Guideline gives weak support to trying oral medical cannabis for chronic pain
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
FROM THE BMJ
Moderna vaccine more effective than Pfizer and J&J
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.







