User login
The Official Newspaper of the American Association for Thoracic Surgery
LDL levels below 10 mg/dL shown safe, effective
BARCELONA – The maxim that lower is better for LDL cholesterol continues to hold true, even at jaw-droppingly low levels of less than 10 mg/dL in a new analysis of data from the FOURIER trial.
The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial was the pivotal efficacy and safety study for the proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitor evolocumab (Repatha) and enrolled patients with atherosclerotic cardiovascular disease and LDL cholesterol levels of at least 70 mg/dL (N Engl J Med. 2017 May 4;376[18]:1713-22).

After a median follow-up of 26 months, the incidence of the study’s primary endpoint (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) dropped by a statistically significant 15% in patients with an achieved LDL cholesterol of 20-49 mg/dL, compared with patients whose 4-week LDL cholesterol was at or above 100 mg/dL (primarily patients randomized to the study’s control arm), by 24% in all patients with LDL cholesterol less than 20 mg/dL, and by 31% in the 2% of patients whose LDL cholesterol levels fell below 10 mg/dL.
These strikingly improved event rates at the lowest levels of LDL cholesterol occurred with no signal of excess adverse events, Robert P. Giugliano, MD, said at the annual congress of the European Society of Cardiology.
In contrast, the 13% of patents whose achieved LDL cholesterol was 50-69 mg/dL had an event rate just 6% below the referent group of 100 mg/dL or more, a nonsignificant difference. Existing cholesterol management guidelines that set LDL cholesterol targets for secondary prevention have used a level below 70 mg/dL as the target, such as the European Society of Cardiology’s 2016 guidelines (Eur Heart J. 2016 Oct 14;37[39]:2999-3058).
“The data suggest that we should target considerably lower LDL cholesterol than is currently recommended for our patients with atherosclerotic cardiovascular disease,” said Dr. Giugliano, a cardiologist at Brigham and Women’s Hospital in Boston.
“Lowest is best with LDL. You don’t need a lot of LDL in the serum for normal human function,” he noted during the discussion of his report.
While FOURIER’s event curve continued to drop as LDL cholesterol fell below 10 mg/dL, the study’s wide-ranging safety assessment showed no signal of harm at the lowest levels. This “gives us some reassurance it’s safe,” he said in an interview. “We saw benefit that continued down to the lowest LDL levels, so it’s hard to pick a LDL target. I no longer feel comfortable treating my patients to just less than 70 mg/dL. I’m not sure what is the optimal LDL target, but I think it needs to be lower than that.”
To achieve such ultralow LDL levels, most patients need treatment with a PCSK9 inhibitor plus at least one and perhaps two additional cholesterol-lowering drugs, a statin and ezetimibe, he noted.
The FOURIER analyses Dr. Giugliano reported included data on the incidence during the study of 10 specific types of adverse events: noncardiovascular death, serious adverse events, adverse events leading to study discontinuation, and new onset of diabetes, cancer, cataract, neurocognitive deficit, significant liver enzyme increase, significant creatine kinase increase, and hemorrhagic stroke. The incidence of each of these was similar among the patients in five study subgroups based on achieved levels of LDL cholesterol: less than 20 mg/dL, 20-49 mg/dL, 50-69 mg/dL, 70-99 mg/dL, and 100 mg/dL or higher. In addition, the rates of both serious adverse events and adverse events leading to study discontinuation was roughly the same in the subgroup of patients with an achieved LDL cholesterol of less than 10 mg/dL as in those with an achieved LDL of at least 100 mg/dL.
Concurrently with Dr. Giugliano’s report, the results also appeared in an online article (Lancet. 2017 Aug 28. doi: 10.1016/S0140-6736[17]32290-0).
FOURIER was funded by Amgen, the company that markets evolocumab (Repatha). Dr. Giugliano has been a consultant to and has received research funding from Amgen, and he has also been a consultant to Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Merck, and Pfizer.
[email protected]
On Twitter @mitchelzoler
BARCELONA – The maxim that lower is better for LDL cholesterol continues to hold true, even at jaw-droppingly low levels of less than 10 mg/dL in a new analysis of data from the FOURIER trial.
The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial was the pivotal efficacy and safety study for the proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitor evolocumab (Repatha) and enrolled patients with atherosclerotic cardiovascular disease and LDL cholesterol levels of at least 70 mg/dL (N Engl J Med. 2017 May 4;376[18]:1713-22).

After a median follow-up of 26 months, the incidence of the study’s primary endpoint (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) dropped by a statistically significant 15% in patients with an achieved LDL cholesterol of 20-49 mg/dL, compared with patients whose 4-week LDL cholesterol was at or above 100 mg/dL (primarily patients randomized to the study’s control arm), by 24% in all patients with LDL cholesterol less than 20 mg/dL, and by 31% in the 2% of patients whose LDL cholesterol levels fell below 10 mg/dL.
These strikingly improved event rates at the lowest levels of LDL cholesterol occurred with no signal of excess adverse events, Robert P. Giugliano, MD, said at the annual congress of the European Society of Cardiology.
In contrast, the 13% of patents whose achieved LDL cholesterol was 50-69 mg/dL had an event rate just 6% below the referent group of 100 mg/dL or more, a nonsignificant difference. Existing cholesterol management guidelines that set LDL cholesterol targets for secondary prevention have used a level below 70 mg/dL as the target, such as the European Society of Cardiology’s 2016 guidelines (Eur Heart J. 2016 Oct 14;37[39]:2999-3058).
“The data suggest that we should target considerably lower LDL cholesterol than is currently recommended for our patients with atherosclerotic cardiovascular disease,” said Dr. Giugliano, a cardiologist at Brigham and Women’s Hospital in Boston.
“Lowest is best with LDL. You don’t need a lot of LDL in the serum for normal human function,” he noted during the discussion of his report.
While FOURIER’s event curve continued to drop as LDL cholesterol fell below 10 mg/dL, the study’s wide-ranging safety assessment showed no signal of harm at the lowest levels. This “gives us some reassurance it’s safe,” he said in an interview. “We saw benefit that continued down to the lowest LDL levels, so it’s hard to pick a LDL target. I no longer feel comfortable treating my patients to just less than 70 mg/dL. I’m not sure what is the optimal LDL target, but I think it needs to be lower than that.”
To achieve such ultralow LDL levels, most patients need treatment with a PCSK9 inhibitor plus at least one and perhaps two additional cholesterol-lowering drugs, a statin and ezetimibe, he noted.
The FOURIER analyses Dr. Giugliano reported included data on the incidence during the study of 10 specific types of adverse events: noncardiovascular death, serious adverse events, adverse events leading to study discontinuation, and new onset of diabetes, cancer, cataract, neurocognitive deficit, significant liver enzyme increase, significant creatine kinase increase, and hemorrhagic stroke. The incidence of each of these was similar among the patients in five study subgroups based on achieved levels of LDL cholesterol: less than 20 mg/dL, 20-49 mg/dL, 50-69 mg/dL, 70-99 mg/dL, and 100 mg/dL or higher. In addition, the rates of both serious adverse events and adverse events leading to study discontinuation was roughly the same in the subgroup of patients with an achieved LDL cholesterol of less than 10 mg/dL as in those with an achieved LDL of at least 100 mg/dL.
Concurrently with Dr. Giugliano’s report, the results also appeared in an online article (Lancet. 2017 Aug 28. doi: 10.1016/S0140-6736[17]32290-0).
FOURIER was funded by Amgen, the company that markets evolocumab (Repatha). Dr. Giugliano has been a consultant to and has received research funding from Amgen, and he has also been a consultant to Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Merck, and Pfizer.
[email protected]
On Twitter @mitchelzoler
BARCELONA – The maxim that lower is better for LDL cholesterol continues to hold true, even at jaw-droppingly low levels of less than 10 mg/dL in a new analysis of data from the FOURIER trial.
The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial was the pivotal efficacy and safety study for the proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitor evolocumab (Repatha) and enrolled patients with atherosclerotic cardiovascular disease and LDL cholesterol levels of at least 70 mg/dL (N Engl J Med. 2017 May 4;376[18]:1713-22).

After a median follow-up of 26 months, the incidence of the study’s primary endpoint (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) dropped by a statistically significant 15% in patients with an achieved LDL cholesterol of 20-49 mg/dL, compared with patients whose 4-week LDL cholesterol was at or above 100 mg/dL (primarily patients randomized to the study’s control arm), by 24% in all patients with LDL cholesterol less than 20 mg/dL, and by 31% in the 2% of patients whose LDL cholesterol levels fell below 10 mg/dL.
These strikingly improved event rates at the lowest levels of LDL cholesterol occurred with no signal of excess adverse events, Robert P. Giugliano, MD, said at the annual congress of the European Society of Cardiology.
In contrast, the 13% of patents whose achieved LDL cholesterol was 50-69 mg/dL had an event rate just 6% below the referent group of 100 mg/dL or more, a nonsignificant difference. Existing cholesterol management guidelines that set LDL cholesterol targets for secondary prevention have used a level below 70 mg/dL as the target, such as the European Society of Cardiology’s 2016 guidelines (Eur Heart J. 2016 Oct 14;37[39]:2999-3058).
“The data suggest that we should target considerably lower LDL cholesterol than is currently recommended for our patients with atherosclerotic cardiovascular disease,” said Dr. Giugliano, a cardiologist at Brigham and Women’s Hospital in Boston.
“Lowest is best with LDL. You don’t need a lot of LDL in the serum for normal human function,” he noted during the discussion of his report.
While FOURIER’s event curve continued to drop as LDL cholesterol fell below 10 mg/dL, the study’s wide-ranging safety assessment showed no signal of harm at the lowest levels. This “gives us some reassurance it’s safe,” he said in an interview. “We saw benefit that continued down to the lowest LDL levels, so it’s hard to pick a LDL target. I no longer feel comfortable treating my patients to just less than 70 mg/dL. I’m not sure what is the optimal LDL target, but I think it needs to be lower than that.”
To achieve such ultralow LDL levels, most patients need treatment with a PCSK9 inhibitor plus at least one and perhaps two additional cholesterol-lowering drugs, a statin and ezetimibe, he noted.
The FOURIER analyses Dr. Giugliano reported included data on the incidence during the study of 10 specific types of adverse events: noncardiovascular death, serious adverse events, adverse events leading to study discontinuation, and new onset of diabetes, cancer, cataract, neurocognitive deficit, significant liver enzyme increase, significant creatine kinase increase, and hemorrhagic stroke. The incidence of each of these was similar among the patients in five study subgroups based on achieved levels of LDL cholesterol: less than 20 mg/dL, 20-49 mg/dL, 50-69 mg/dL, 70-99 mg/dL, and 100 mg/dL or higher. In addition, the rates of both serious adverse events and adverse events leading to study discontinuation was roughly the same in the subgroup of patients with an achieved LDL cholesterol of less than 10 mg/dL as in those with an achieved LDL of at least 100 mg/dL.
Concurrently with Dr. Giugliano’s report, the results also appeared in an online article (Lancet. 2017 Aug 28. doi: 10.1016/S0140-6736[17]32290-0).
FOURIER was funded by Amgen, the company that markets evolocumab (Repatha). Dr. Giugliano has been a consultant to and has received research funding from Amgen, and he has also been a consultant to Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Merck, and Pfizer.
[email protected]
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: Patients with an achieved LDL of less than 10 mg/dL had an event rate 31% below patients with an LDL at or above 100 mg/dL.
Data source: FOURIER, an international multicenter trial with 27,564 patients.
Disclosures: FOURIER was funded by Amgen, the company that markets evolocumab (Repatha). Dr. Giugliano has been a consultant to and has received research funding from Amgen, and he has also been a consultant to Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Merck, and Pfizer.
Lifesaving future seen for electronic cigarettes
A switch from cigarettes to e-cigarettes has the potential to prevent almost 90,000 premature deaths in the United States in the year 2026, according to a study examining e-cigarette substitution scenarios.
The investigators’ “optimistic scenario” – in which new smokers use e-cigarettes instead of cigarettes, smoking prevalence falls to 5% over a 10-year period, and e-cigarettes have a 5% excess risk over regular cigarettes – projects 380,832 premature deaths from smoking in the year 2026. Under a “status quo scenario,” which projected current cigarette initiation and cessation rates and did not include e-cigarettes or other tobacco products, there would be 470,743 deaths, reported David T. Levy, PhD, and his associates (Tob Control. 2017 Oct 2. doi: 10.1136/tobaccocontrol-2017-053759).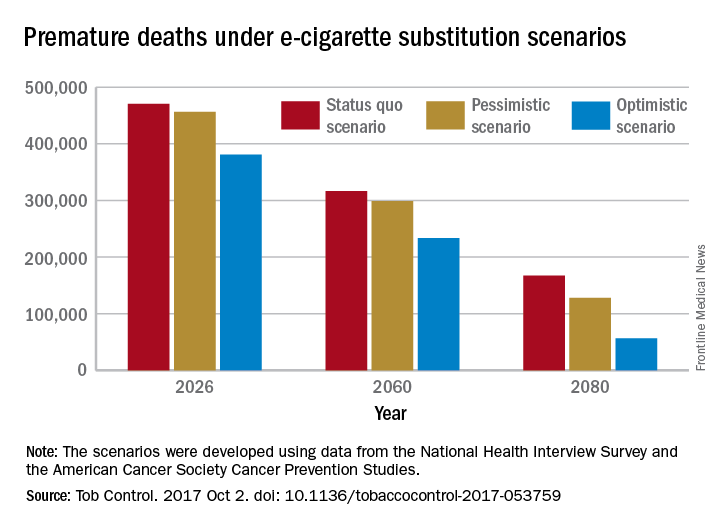
Further projections suggest that the optimistic scenario could result in almost 6.6 million fewer premature deaths and 86.7 million years of life gained by the year 2100, compared with the status quo scenario, while the pessimistic scenario would prevent 1.6 million deaths and add an extra 20.8 million years of life, they noted.
Since “a strategy of replacing cigarette by e-cigarette use can yield substantial gains, even with conservative assumptions about related risks … an endgame scenario for cigarettes might well be within reach, if new technologies for delivering nicotine with substantially less harm, but sufficient satisfaction, are harnessed with sufficient passion and political will to aggressively phase out tobacco cigarettes,” Dr. Levy and his associates wrote.
The study was funded by grants from the National Institute on Drug Abuse and the National Cancer Institute. One investigator received a research grant from Pfizer and served as an advisory board member to Johnson & Johnson, which manufactures smoking cessation medications. No other conflicts of interest were declared.
A switch from cigarettes to e-cigarettes has the potential to prevent almost 90,000 premature deaths in the United States in the year 2026, according to a study examining e-cigarette substitution scenarios.
The investigators’ “optimistic scenario” – in which new smokers use e-cigarettes instead of cigarettes, smoking prevalence falls to 5% over a 10-year period, and e-cigarettes have a 5% excess risk over regular cigarettes – projects 380,832 premature deaths from smoking in the year 2026. Under a “status quo scenario,” which projected current cigarette initiation and cessation rates and did not include e-cigarettes or other tobacco products, there would be 470,743 deaths, reported David T. Levy, PhD, and his associates (Tob Control. 2017 Oct 2. doi: 10.1136/tobaccocontrol-2017-053759).
Further projections suggest that the optimistic scenario could result in almost 6.6 million fewer premature deaths and 86.7 million years of life gained by the year 2100, compared with the status quo scenario, while the pessimistic scenario would prevent 1.6 million deaths and add an extra 20.8 million years of life, they noted.
Since “a strategy of replacing cigarette by e-cigarette use can yield substantial gains, even with conservative assumptions about related risks … an endgame scenario for cigarettes might well be within reach, if new technologies for delivering nicotine with substantially less harm, but sufficient satisfaction, are harnessed with sufficient passion and political will to aggressively phase out tobacco cigarettes,” Dr. Levy and his associates wrote.
The study was funded by grants from the National Institute on Drug Abuse and the National Cancer Institute. One investigator received a research grant from Pfizer and served as an advisory board member to Johnson & Johnson, which manufactures smoking cessation medications. No other conflicts of interest were declared.
A switch from cigarettes to e-cigarettes has the potential to prevent almost 90,000 premature deaths in the United States in the year 2026, according to a study examining e-cigarette substitution scenarios.
The investigators’ “optimistic scenario” – in which new smokers use e-cigarettes instead of cigarettes, smoking prevalence falls to 5% over a 10-year period, and e-cigarettes have a 5% excess risk over regular cigarettes – projects 380,832 premature deaths from smoking in the year 2026. Under a “status quo scenario,” which projected current cigarette initiation and cessation rates and did not include e-cigarettes or other tobacco products, there would be 470,743 deaths, reported David T. Levy, PhD, and his associates (Tob Control. 2017 Oct 2. doi: 10.1136/tobaccocontrol-2017-053759).
Further projections suggest that the optimistic scenario could result in almost 6.6 million fewer premature deaths and 86.7 million years of life gained by the year 2100, compared with the status quo scenario, while the pessimistic scenario would prevent 1.6 million deaths and add an extra 20.8 million years of life, they noted.
Since “a strategy of replacing cigarette by e-cigarette use can yield substantial gains, even with conservative assumptions about related risks … an endgame scenario for cigarettes might well be within reach, if new technologies for delivering nicotine with substantially less harm, but sufficient satisfaction, are harnessed with sufficient passion and political will to aggressively phase out tobacco cigarettes,” Dr. Levy and his associates wrote.
The study was funded by grants from the National Institute on Drug Abuse and the National Cancer Institute. One investigator received a research grant from Pfizer and served as an advisory board member to Johnson & Johnson, which manufactures smoking cessation medications. No other conflicts of interest were declared.
FROM TOBACCO CONTROL
How to apply SPRINT findings to elderly patients
SAN FRANCISCO – The benefit of lowering blood pressure exceeded the potential for harm, even among the most frail elderly, in SPRINT, but it’s important to remember who was excluded from the trial when using the findings in the clinic, according to Mark Supiano, MD.
SPRINT (Systolic Blood Pressure Intervention Trial) excluded people with histories of stroke, diabetes, heart failure, and chronic kidney disease with a markedly reduced glomerular filtration rate. People living in nursing homes, assisted living centers, and those with prevalent dementia were also excluded, as were individuals with a standing systolic pressure below 110 mm Hg (N Engl J Med. 2015 Nov 26;373:2103-16).
Even with those exclusions, however, the 2,636 patients in SPRINT who were 75 years and older “were not a super healthy group of older people,” Dr. Supiano said at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
They were at high risk for cardiovascular disease (CVD), with a median 10-year Framingham risk score of almost 25%. More than a quarter had gait speeds below 0.8 m/sec, and almost a third were classified as frail. Many had mild cognitive impairment at baseline.
In the United States, Dr. Supiano and his colleagues estimate that there are almost 6 million similar people 75 years or older with hypertension who would likely achieve the same benefits from hypertension control as elderly subjects in the trial. “As a geriatrician, there are very few things that I can offer patients 75 years and older that will have a profound improvement in their overall mortality.” Blood pressure control is one of them, said Dr. Supiano, chief of geriatrics at the University of Utah, Salt Lake City, and a SPRINT investigator.
In SPRINT, intensive treatment to systolic pressure below 120 mm Hg showed greater benefit for patients 75 years and older than it did for younger patients, even among the frail, with a 34% reduction in fatal and nonfatal CVD events versus patients treated to below 140 mm Hg, and a 33% lower rate of death from any cause.
It should be no surprise that older patients had greater benefit from tighter control, because elderly patients have “a greater CVD risk. There’s more bang for the buck” with blood pressure lowering in an older population. “Overall, benefits exceed the potential for harm, even among the frailest older patients,” Dr. Supiano said.
“A systemic target of less than 140 mm Hg is, I believe, appropriate for most healthy people age 60 and older. A benefit-based systemic target of less than 120 mm Hg may be appropriate for those at higher CVD risk.” Among patients 60-75 years old, that would include those with a Framingham score above 15%. Among patients older than age 75 with an elevated CVD risk, treatment to below 120 mm Hg makes sense if it aligns with patient’s goals of care, Dr. Supiano said.
The 120–mm Hg target in SPRINT was associated with a greater incidence of some transient side effects in the elderly, including hypotension, syncope, acute kidney injury, and electrolyte imbalance, but not a higher risk of serious adverse events or injurious falls.
There were concerns raised at the joint sessions about the effect of blood pressure lowering on the cognitive function of older people. Dr. Supiano noted that the cognitive outcomes in SPRINT, as well as outcomes in patients with chronic kidney disease, have not yet been released, but are expected soon.
Dr. Supiano had no relevant disclosures.
SAN FRANCISCO – The benefit of lowering blood pressure exceeded the potential for harm, even among the most frail elderly, in SPRINT, but it’s important to remember who was excluded from the trial when using the findings in the clinic, according to Mark Supiano, MD.
SPRINT (Systolic Blood Pressure Intervention Trial) excluded people with histories of stroke, diabetes, heart failure, and chronic kidney disease with a markedly reduced glomerular filtration rate. People living in nursing homes, assisted living centers, and those with prevalent dementia were also excluded, as were individuals with a standing systolic pressure below 110 mm Hg (N Engl J Med. 2015 Nov 26;373:2103-16).
Even with those exclusions, however, the 2,636 patients in SPRINT who were 75 years and older “were not a super healthy group of older people,” Dr. Supiano said at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
They were at high risk for cardiovascular disease (CVD), with a median 10-year Framingham risk score of almost 25%. More than a quarter had gait speeds below 0.8 m/sec, and almost a third were classified as frail. Many had mild cognitive impairment at baseline.
In the United States, Dr. Supiano and his colleagues estimate that there are almost 6 million similar people 75 years or older with hypertension who would likely achieve the same benefits from hypertension control as elderly subjects in the trial. “As a geriatrician, there are very few things that I can offer patients 75 years and older that will have a profound improvement in their overall mortality.” Blood pressure control is one of them, said Dr. Supiano, chief of geriatrics at the University of Utah, Salt Lake City, and a SPRINT investigator.
In SPRINT, intensive treatment to systolic pressure below 120 mm Hg showed greater benefit for patients 75 years and older than it did for younger patients, even among the frail, with a 34% reduction in fatal and nonfatal CVD events versus patients treated to below 140 mm Hg, and a 33% lower rate of death from any cause.
It should be no surprise that older patients had greater benefit from tighter control, because elderly patients have “a greater CVD risk. There’s more bang for the buck” with blood pressure lowering in an older population. “Overall, benefits exceed the potential for harm, even among the frailest older patients,” Dr. Supiano said.
“A systemic target of less than 140 mm Hg is, I believe, appropriate for most healthy people age 60 and older. A benefit-based systemic target of less than 120 mm Hg may be appropriate for those at higher CVD risk.” Among patients 60-75 years old, that would include those with a Framingham score above 15%. Among patients older than age 75 with an elevated CVD risk, treatment to below 120 mm Hg makes sense if it aligns with patient’s goals of care, Dr. Supiano said.
The 120–mm Hg target in SPRINT was associated with a greater incidence of some transient side effects in the elderly, including hypotension, syncope, acute kidney injury, and electrolyte imbalance, but not a higher risk of serious adverse events or injurious falls.
There were concerns raised at the joint sessions about the effect of blood pressure lowering on the cognitive function of older people. Dr. Supiano noted that the cognitive outcomes in SPRINT, as well as outcomes in patients with chronic kidney disease, have not yet been released, but are expected soon.
Dr. Supiano had no relevant disclosures.
SAN FRANCISCO – The benefit of lowering blood pressure exceeded the potential for harm, even among the most frail elderly, in SPRINT, but it’s important to remember who was excluded from the trial when using the findings in the clinic, according to Mark Supiano, MD.
SPRINT (Systolic Blood Pressure Intervention Trial) excluded people with histories of stroke, diabetes, heart failure, and chronic kidney disease with a markedly reduced glomerular filtration rate. People living in nursing homes, assisted living centers, and those with prevalent dementia were also excluded, as were individuals with a standing systolic pressure below 110 mm Hg (N Engl J Med. 2015 Nov 26;373:2103-16).
Even with those exclusions, however, the 2,636 patients in SPRINT who were 75 years and older “were not a super healthy group of older people,” Dr. Supiano said at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
They were at high risk for cardiovascular disease (CVD), with a median 10-year Framingham risk score of almost 25%. More than a quarter had gait speeds below 0.8 m/sec, and almost a third were classified as frail. Many had mild cognitive impairment at baseline.
In the United States, Dr. Supiano and his colleagues estimate that there are almost 6 million similar people 75 years or older with hypertension who would likely achieve the same benefits from hypertension control as elderly subjects in the trial. “As a geriatrician, there are very few things that I can offer patients 75 years and older that will have a profound improvement in their overall mortality.” Blood pressure control is one of them, said Dr. Supiano, chief of geriatrics at the University of Utah, Salt Lake City, and a SPRINT investigator.
In SPRINT, intensive treatment to systolic pressure below 120 mm Hg showed greater benefit for patients 75 years and older than it did for younger patients, even among the frail, with a 34% reduction in fatal and nonfatal CVD events versus patients treated to below 140 mm Hg, and a 33% lower rate of death from any cause.
It should be no surprise that older patients had greater benefit from tighter control, because elderly patients have “a greater CVD risk. There’s more bang for the buck” with blood pressure lowering in an older population. “Overall, benefits exceed the potential for harm, even among the frailest older patients,” Dr. Supiano said.
“A systemic target of less than 140 mm Hg is, I believe, appropriate for most healthy people age 60 and older. A benefit-based systemic target of less than 120 mm Hg may be appropriate for those at higher CVD risk.” Among patients 60-75 years old, that would include those with a Framingham score above 15%. Among patients older than age 75 with an elevated CVD risk, treatment to below 120 mm Hg makes sense if it aligns with patient’s goals of care, Dr. Supiano said.
The 120–mm Hg target in SPRINT was associated with a greater incidence of some transient side effects in the elderly, including hypotension, syncope, acute kidney injury, and electrolyte imbalance, but not a higher risk of serious adverse events or injurious falls.
There were concerns raised at the joint sessions about the effect of blood pressure lowering on the cognitive function of older people. Dr. Supiano noted that the cognitive outcomes in SPRINT, as well as outcomes in patients with chronic kidney disease, have not yet been released, but are expected soon.
Dr. Supiano had no relevant disclosures.
EXPERT ANALYSIS FROM JOINT HYPERTENSION 2017
FDA approves higher dose brigatinib tablet for advanced ALK+ NSCLC
The Food and Drug Administration has approved 180 mg brigatinib (Alunbrig) tablets for treatment of anaplastic lymphoma kinase–positive (ALK+) metastatic non–small cell lung cancer (NSCLC), expanding on previously available 30-mg tablets.*
“With the approval of a 180-mg tablet, Alunbrig has become the only ALK inhibitor available as a one tablet per day dose that can be taken with or without food,” Ryan Cohlhepp, PharmD, vice president, U.S. Commercial, at Takeda Oncology said in a press release.
Approval of the regimen was based on objective response in the ongoing, two-arm, open-label, multicenter phase 2 ALTA trial, which enrolled 222 patients with metastatic or locally advanced ALK+ NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily or 180 mg once daily following a 7-day lead-in at 90 mg once daily. Of those in the 180-mg arm, 53% had an objective response, compared with 48% in the 90-mg arm.
Adverse reactions occurred in 40% of the patients in the 180-mg arm, compared with 38% in the 90-mg arm. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. Fatal adverse reactions occurred in 3.7% of the patients, caused by pneumonia (two patients), sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis and urosepsis (one patient each).
The ALTA trial is ongoing, and updated results will be presented at the World Conference on Lung Cancer in Yokohama, Japan, on Oct. 15-18, the company said in the press release.
*Correction, 10/4/17: An earlier version of this article misstated the previously available tablet sizes.
The Food and Drug Administration has approved 180 mg brigatinib (Alunbrig) tablets for treatment of anaplastic lymphoma kinase–positive (ALK+) metastatic non–small cell lung cancer (NSCLC), expanding on previously available 30-mg tablets.*
“With the approval of a 180-mg tablet, Alunbrig has become the only ALK inhibitor available as a one tablet per day dose that can be taken with or without food,” Ryan Cohlhepp, PharmD, vice president, U.S. Commercial, at Takeda Oncology said in a press release.
Approval of the regimen was based on objective response in the ongoing, two-arm, open-label, multicenter phase 2 ALTA trial, which enrolled 222 patients with metastatic or locally advanced ALK+ NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily or 180 mg once daily following a 7-day lead-in at 90 mg once daily. Of those in the 180-mg arm, 53% had an objective response, compared with 48% in the 90-mg arm.
Adverse reactions occurred in 40% of the patients in the 180-mg arm, compared with 38% in the 90-mg arm. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. Fatal adverse reactions occurred in 3.7% of the patients, caused by pneumonia (two patients), sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis and urosepsis (one patient each).
The ALTA trial is ongoing, and updated results will be presented at the World Conference on Lung Cancer in Yokohama, Japan, on Oct. 15-18, the company said in the press release.
*Correction, 10/4/17: An earlier version of this article misstated the previously available tablet sizes.
The Food and Drug Administration has approved 180 mg brigatinib (Alunbrig) tablets for treatment of anaplastic lymphoma kinase–positive (ALK+) metastatic non–small cell lung cancer (NSCLC), expanding on previously available 30-mg tablets.*
“With the approval of a 180-mg tablet, Alunbrig has become the only ALK inhibitor available as a one tablet per day dose that can be taken with or without food,” Ryan Cohlhepp, PharmD, vice president, U.S. Commercial, at Takeda Oncology said in a press release.
Approval of the regimen was based on objective response in the ongoing, two-arm, open-label, multicenter phase 2 ALTA trial, which enrolled 222 patients with metastatic or locally advanced ALK+ NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily or 180 mg once daily following a 7-day lead-in at 90 mg once daily. Of those in the 180-mg arm, 53% had an objective response, compared with 48% in the 90-mg arm.
Adverse reactions occurred in 40% of the patients in the 180-mg arm, compared with 38% in the 90-mg arm. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. Fatal adverse reactions occurred in 3.7% of the patients, caused by pneumonia (two patients), sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis and urosepsis (one patient each).
The ALTA trial is ongoing, and updated results will be presented at the World Conference on Lung Cancer in Yokohama, Japan, on Oct. 15-18, the company said in the press release.
*Correction, 10/4/17: An earlier version of this article misstated the previously available tablet sizes.
HCA is the country’s highest-volume health system
HCA of Nashville, Tenn., had more discharges in 2016 than any other health system in the United States, according to the Agency for Healthcare Research and Quality.
HCA’s 192 hospitals recorded over 1.7 million discharges last year, more than twice as many as Ascension Health in St. Louis, which discharged almost 860,000 patients from its 136 hospitals. Tennessee is also the home of the nation’s third-largest health system, Community Health Systems of Franklin, which totaled just over 805,000 discharges in 2016. Tenet Healthcare Corporation in Dallas was fourth with 752,000 discharges, and Trinity Health in Livonia, Mich., was fifth with 737,000 discharges, the AHRQ said in its Compendium of U.S. Health Systems, 2016.
HCA of Nashville, Tenn., had more discharges in 2016 than any other health system in the United States, according to the Agency for Healthcare Research and Quality.
HCA’s 192 hospitals recorded over 1.7 million discharges last year, more than twice as many as Ascension Health in St. Louis, which discharged almost 860,000 patients from its 136 hospitals. Tennessee is also the home of the nation’s third-largest health system, Community Health Systems of Franklin, which totaled just over 805,000 discharges in 2016. Tenet Healthcare Corporation in Dallas was fourth with 752,000 discharges, and Trinity Health in Livonia, Mich., was fifth with 737,000 discharges, the AHRQ said in its Compendium of U.S. Health Systems, 2016.
HCA of Nashville, Tenn., had more discharges in 2016 than any other health system in the United States, according to the Agency for Healthcare Research and Quality.
HCA’s 192 hospitals recorded over 1.7 million discharges last year, more than twice as many as Ascension Health in St. Louis, which discharged almost 860,000 patients from its 136 hospitals. Tennessee is also the home of the nation’s third-largest health system, Community Health Systems of Franklin, which totaled just over 805,000 discharges in 2016. Tenet Healthcare Corporation in Dallas was fourth with 752,000 discharges, and Trinity Health in Livonia, Mich., was fifth with 737,000 discharges, the AHRQ said in its Compendium of U.S. Health Systems, 2016.
LVAD use soars in elderly Americans
DALLAS – The percentage of left ventricular assist devices placed in U.S. heart failure patients at least 75 years of age jumped sharply during 2003-2014, and concurrently the short-term survival of these patients improved dramatically, according to data collected by the National Inpatient Sample.
During the 12-year period examined, the percentage of left-ventricular assist devices (LVADs) placed in U.S. heart failure patients aged 75 years and older rose from 3% of all LVADs in 2003 to 11% in 2014, Aniket S. Rali, MD, said at the annual scientific meeting of the Heart Failure Society of America.
The U.S. national numbers also showed that throughout the period studied, elderly U.S. patients who received an LVAD were increasingly sicker, with steadily increasing numbers of patients with a Charlson Comorbidity Index score of four or greater. Despite this, in-hospital mortality rates of elderly patients receiving an LVAD plummeted, dropping from 61% of elderly LVAD recipients in 2003 to 18% in 2014. During the same time, the percentage of elderly patients with a Charlson Comorbidity Index score greater than four doubled from 33% in 2003 to 66% in 2014, said Dr. Rali, a cardiologist at the University of Kansas Medical Center in Kansas City.
“If the Charlson Comorbidity Index score is increasing but in-hospital mortality is decreasing, then increased LVAD use is not a bad trend,” Dr. Rali said in an interview. He hopes that future analysis of longitudinal data from patients could identify clinical factors that link with better patient survival and help target LVAD placement to the patients who stand to gain the most benefit.
“We may be able to give these elderly patients not just longer life but improved quality of life” by a more informed targeting of LVADs, he suggested. “I think these numbers will help convince people that all is not lost,” he noted, for elderly heart failure patients who receive an LVAD as destination therapy. Patients at least 75 years old are not eligible for heart transplantation, so when these patients receive an LVAD it is, by definition, destination therapy.
The data also showed a marked sex disparity in LVAD use, with LVAD placement in men at least 75 years old rising from 1.4/1,000 patients in 2003 to 2.78/1,000 patients in 2014. In contrast, among women these rates rose from 0.8/1,000 patients in 2003 to 1.36/1,000 patients in 2014.
The average age for elderly U.S. LVAD recipients for the entire 12-year period studied was 77.6 years among a total of 2,090 recipients. For all 21,323 U.S. LVAD recipients during 2003-2014 the average age was 51.5 years old.
[email protected]
On Twitter @mitchelzoler
DALLAS – The percentage of left ventricular assist devices placed in U.S. heart failure patients at least 75 years of age jumped sharply during 2003-2014, and concurrently the short-term survival of these patients improved dramatically, according to data collected by the National Inpatient Sample.
During the 12-year period examined, the percentage of left-ventricular assist devices (LVADs) placed in U.S. heart failure patients aged 75 years and older rose from 3% of all LVADs in 2003 to 11% in 2014, Aniket S. Rali, MD, said at the annual scientific meeting of the Heart Failure Society of America.
The U.S. national numbers also showed that throughout the period studied, elderly U.S. patients who received an LVAD were increasingly sicker, with steadily increasing numbers of patients with a Charlson Comorbidity Index score of four or greater. Despite this, in-hospital mortality rates of elderly patients receiving an LVAD plummeted, dropping from 61% of elderly LVAD recipients in 2003 to 18% in 2014. During the same time, the percentage of elderly patients with a Charlson Comorbidity Index score greater than four doubled from 33% in 2003 to 66% in 2014, said Dr. Rali, a cardiologist at the University of Kansas Medical Center in Kansas City.
“If the Charlson Comorbidity Index score is increasing but in-hospital mortality is decreasing, then increased LVAD use is not a bad trend,” Dr. Rali said in an interview. He hopes that future analysis of longitudinal data from patients could identify clinical factors that link with better patient survival and help target LVAD placement to the patients who stand to gain the most benefit.
“We may be able to give these elderly patients not just longer life but improved quality of life” by a more informed targeting of LVADs, he suggested. “I think these numbers will help convince people that all is not lost,” he noted, for elderly heart failure patients who receive an LVAD as destination therapy. Patients at least 75 years old are not eligible for heart transplantation, so when these patients receive an LVAD it is, by definition, destination therapy.
The data also showed a marked sex disparity in LVAD use, with LVAD placement in men at least 75 years old rising from 1.4/1,000 patients in 2003 to 2.78/1,000 patients in 2014. In contrast, among women these rates rose from 0.8/1,000 patients in 2003 to 1.36/1,000 patients in 2014.
The average age for elderly U.S. LVAD recipients for the entire 12-year period studied was 77.6 years among a total of 2,090 recipients. For all 21,323 U.S. LVAD recipients during 2003-2014 the average age was 51.5 years old.
[email protected]
On Twitter @mitchelzoler
DALLAS – The percentage of left ventricular assist devices placed in U.S. heart failure patients at least 75 years of age jumped sharply during 2003-2014, and concurrently the short-term survival of these patients improved dramatically, according to data collected by the National Inpatient Sample.
During the 12-year period examined, the percentage of left-ventricular assist devices (LVADs) placed in U.S. heart failure patients aged 75 years and older rose from 3% of all LVADs in 2003 to 11% in 2014, Aniket S. Rali, MD, said at the annual scientific meeting of the Heart Failure Society of America.
The U.S. national numbers also showed that throughout the period studied, elderly U.S. patients who received an LVAD were increasingly sicker, with steadily increasing numbers of patients with a Charlson Comorbidity Index score of four or greater. Despite this, in-hospital mortality rates of elderly patients receiving an LVAD plummeted, dropping from 61% of elderly LVAD recipients in 2003 to 18% in 2014. During the same time, the percentage of elderly patients with a Charlson Comorbidity Index score greater than four doubled from 33% in 2003 to 66% in 2014, said Dr. Rali, a cardiologist at the University of Kansas Medical Center in Kansas City.
“If the Charlson Comorbidity Index score is increasing but in-hospital mortality is decreasing, then increased LVAD use is not a bad trend,” Dr. Rali said in an interview. He hopes that future analysis of longitudinal data from patients could identify clinical factors that link with better patient survival and help target LVAD placement to the patients who stand to gain the most benefit.
“We may be able to give these elderly patients not just longer life but improved quality of life” by a more informed targeting of LVADs, he suggested. “I think these numbers will help convince people that all is not lost,” he noted, for elderly heart failure patients who receive an LVAD as destination therapy. Patients at least 75 years old are not eligible for heart transplantation, so when these patients receive an LVAD it is, by definition, destination therapy.
The data also showed a marked sex disparity in LVAD use, with LVAD placement in men at least 75 years old rising from 1.4/1,000 patients in 2003 to 2.78/1,000 patients in 2014. In contrast, among women these rates rose from 0.8/1,000 patients in 2003 to 1.36/1,000 patients in 2014.
The average age for elderly U.S. LVAD recipients for the entire 12-year period studied was 77.6 years among a total of 2,090 recipients. For all 21,323 U.S. LVAD recipients during 2003-2014 the average age was 51.5 years old.
[email protected]
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point:
Major finding: Elderly U.S. patients receiving an LVAD rose from 3% of all LVADs placed in 2003 to 11% in 2014.
Data source: The U.S. National Inpatient Survey during 2003-2014.
Disclosures: Dr. Rali had no disclosures.
Call for Abstracts and Videos
You are invited to submit abstracts and videos for consideration at the AATS Aortic Symposium, which will take place on April 26-27 in New York. The deadline for submissions is Monday, December 18, 2017 at 11:59 p.m. Easter Time.
For more information, visit: www.aats.org/aortic.
You are invited to submit abstracts and videos for consideration at the AATS Aortic Symposium, which will take place on April 26-27 in New York. The deadline for submissions is Monday, December 18, 2017 at 11:59 p.m. Easter Time.
For more information, visit: www.aats.org/aortic.
You are invited to submit abstracts and videos for consideration at the AATS Aortic Symposium, which will take place on April 26-27 in New York. The deadline for submissions is Monday, December 18, 2017 at 11:59 p.m. Easter Time.
For more information, visit: www.aats.org/aortic.
What the Experts are Saying about the 2017 AATS International Cardiovascular Symposium
December 8-9, 2017
Renaissance São Paulo Hotel
São Paulo, Brazil
Join others who are interested in preventing, diagnosing and treating heart valve disease at the 2017 AATS International Cardiovascular Symposium. A faculty of international leaders will be discussing state-of the-art information on devices, long-term results and surgical techniques. The program is available in English, Spanish and Portuguese and the meeting will have translations available.
Several faculty members have shared the why surgeons should attend the Symposium. Miguel Sousa-Uva from the Hospital Santa Cruz in Lisbon, Portugal says, “The AATS International Cardiovascular Symposium in São Paulo, Brazil includes a wide-ranging program focusing on today’s hot topics and is a fantastic opportunity to meet experts, share ideas and learn from each other to improve patients’ outcomes.”
Vinod H. Thourani of Medstar Washington Hospital Center, Georgetown University in Washington, DC shared this: “The meeting in Brazil this December is extremely exciting; the world will come together to share ideas, techniques, and management skills in adult patients with cardiac surgery disease processes.”
Program Directors
Joseph S. Coselli
Walter J. Gomes
Marc R. Moon
Thoralf M. Sundt, III
To see the program, register or learn more about the faculty visit: aats.org/ics
December 8-9, 2017
Renaissance São Paulo Hotel
São Paulo, Brazil
Join others who are interested in preventing, diagnosing and treating heart valve disease at the 2017 AATS International Cardiovascular Symposium. A faculty of international leaders will be discussing state-of the-art information on devices, long-term results and surgical techniques. The program is available in English, Spanish and Portuguese and the meeting will have translations available.
Several faculty members have shared the why surgeons should attend the Symposium. Miguel Sousa-Uva from the Hospital Santa Cruz in Lisbon, Portugal says, “The AATS International Cardiovascular Symposium in São Paulo, Brazil includes a wide-ranging program focusing on today’s hot topics and is a fantastic opportunity to meet experts, share ideas and learn from each other to improve patients’ outcomes.”
Vinod H. Thourani of Medstar Washington Hospital Center, Georgetown University in Washington, DC shared this: “The meeting in Brazil this December is extremely exciting; the world will come together to share ideas, techniques, and management skills in adult patients with cardiac surgery disease processes.”
Program Directors
Joseph S. Coselli
Walter J. Gomes
Marc R. Moon
Thoralf M. Sundt, III
To see the program, register or learn more about the faculty visit: aats.org/ics
December 8-9, 2017
Renaissance São Paulo Hotel
São Paulo, Brazil
Join others who are interested in preventing, diagnosing and treating heart valve disease at the 2017 AATS International Cardiovascular Symposium. A faculty of international leaders will be discussing state-of the-art information on devices, long-term results and surgical techniques. The program is available in English, Spanish and Portuguese and the meeting will have translations available.
Several faculty members have shared the why surgeons should attend the Symposium. Miguel Sousa-Uva from the Hospital Santa Cruz in Lisbon, Portugal says, “The AATS International Cardiovascular Symposium in São Paulo, Brazil includes a wide-ranging program focusing on today’s hot topics and is a fantastic opportunity to meet experts, share ideas and learn from each other to improve patients’ outcomes.”
Vinod H. Thourani of Medstar Washington Hospital Center, Georgetown University in Washington, DC shared this: “The meeting in Brazil this December is extremely exciting; the world will come together to share ideas, techniques, and management skills in adult patients with cardiac surgery disease processes.”
Program Directors
Joseph S. Coselli
Walter J. Gomes
Marc R. Moon
Thoralf M. Sundt, III
To see the program, register or learn more about the faculty visit: aats.org/ics
Attend the AATS Surgical Treatment of Arrhythmias and Rhythm Disorders 2017
November 17-18, 2017
Nobu Eden Roc Hotel
Miami Beach, FL USA
Surgeons and electrophysiologists looking for a comprehensive, unbiased review of the surgical treatment of cardiac arrhythmias and rhythm disorders can attend the newest AATS meeting, the Surgical Treatment of Arrhythmias and Rhythm Disorders. Covering basic underlying mechanisms, cardiac recording and mapping techniques, clinical electrophysiology, operative techniques, cardiac monitoring and appropriate follow-up, the interactive forum is designed to support the exchange of ideas between surgeons and electrophysiologists.
Attendees will be able to go to a wide variety of sessions, including:
- Concomitant Ablation – How I Do It and Why
- Hybrid Ablation and Other Alternative Approaches for Lone Atrial Fibrillation
- Lead Extraction: Modern Techniques in Management of Complications
- Management of the Left Atrial Appendage
- Mechanisms of Atrial Fibrillation
- Special Issues and Controversies in Surgical Ablation
- Surgical Ablation Guidelines
- Surgical Treatment of Arrhythmias: The Basics
- Surgical Treatment of Lone Atrial Fibrillation: How I Do It
- Ventricular Tachycardia Ablation
Program Directors
Ralph J. Damiano, Jr.
A. Marc Gillinov
Program Committee
Niv Ad
Vinay Badhwar
Manuel Castella
James L. Cox
Mark LaMeir
Patrick M. McCarthy
Takashi Nitta
Harold G. Roberts
Richard Schuessler
To view the preliminary program, go to: aats.org/stars
November 17-18, 2017
Nobu Eden Roc Hotel
Miami Beach, FL USA
Surgeons and electrophysiologists looking for a comprehensive, unbiased review of the surgical treatment of cardiac arrhythmias and rhythm disorders can attend the newest AATS meeting, the Surgical Treatment of Arrhythmias and Rhythm Disorders. Covering basic underlying mechanisms, cardiac recording and mapping techniques, clinical electrophysiology, operative techniques, cardiac monitoring and appropriate follow-up, the interactive forum is designed to support the exchange of ideas between surgeons and electrophysiologists.
Attendees will be able to go to a wide variety of sessions, including:
- Concomitant Ablation – How I Do It and Why
- Hybrid Ablation and Other Alternative Approaches for Lone Atrial Fibrillation
- Lead Extraction: Modern Techniques in Management of Complications
- Management of the Left Atrial Appendage
- Mechanisms of Atrial Fibrillation
- Special Issues and Controversies in Surgical Ablation
- Surgical Ablation Guidelines
- Surgical Treatment of Arrhythmias: The Basics
- Surgical Treatment of Lone Atrial Fibrillation: How I Do It
- Ventricular Tachycardia Ablation
Program Directors
Ralph J. Damiano, Jr.
A. Marc Gillinov
Program Committee
Niv Ad
Vinay Badhwar
Manuel Castella
James L. Cox
Mark LaMeir
Patrick M. McCarthy
Takashi Nitta
Harold G. Roberts
Richard Schuessler
To view the preliminary program, go to: aats.org/stars
November 17-18, 2017
Nobu Eden Roc Hotel
Miami Beach, FL USA
Surgeons and electrophysiologists looking for a comprehensive, unbiased review of the surgical treatment of cardiac arrhythmias and rhythm disorders can attend the newest AATS meeting, the Surgical Treatment of Arrhythmias and Rhythm Disorders. Covering basic underlying mechanisms, cardiac recording and mapping techniques, clinical electrophysiology, operative techniques, cardiac monitoring and appropriate follow-up, the interactive forum is designed to support the exchange of ideas between surgeons and electrophysiologists.
Attendees will be able to go to a wide variety of sessions, including:
- Concomitant Ablation – How I Do It and Why
- Hybrid Ablation and Other Alternative Approaches for Lone Atrial Fibrillation
- Lead Extraction: Modern Techniques in Management of Complications
- Management of the Left Atrial Appendage
- Mechanisms of Atrial Fibrillation
- Special Issues and Controversies in Surgical Ablation
- Surgical Ablation Guidelines
- Surgical Treatment of Arrhythmias: The Basics
- Surgical Treatment of Lone Atrial Fibrillation: How I Do It
- Ventricular Tachycardia Ablation
Program Directors
Ralph J. Damiano, Jr.
A. Marc Gillinov
Program Committee
Niv Ad
Vinay Badhwar
Manuel Castella
James L. Cox
Mark LaMeir
Patrick M. McCarthy
Takashi Nitta
Harold G. Roberts
Richard Schuessler
To view the preliminary program, go to: aats.org/stars
Focus on the Impact of Technology at the AATS Focus on Thoracic Surgery: Mastering Surgical Innovation 2017
October 27-28, 2017
Encore at Wynn Las Vegas
Las Vegas, Nevada, USA
Spend two days in Las Vegas learning about the technological advances that are changing the approaches to benign and malignant esophageal disease, and lung cancer and related diseases. AATS Focus on Thoracic Surgery: Mastering Surgical Innovation 2017 features the most elite experts in the field presenting the latest information on new and emerging technologies in diagnostic and therapeutic techniques, minimally invasive surgery and robotic surgeries. The meeting’s interactive format will enable attendees to incorporate technologies into their own practices, as well as see how surgical innovation can impact patient outcomes and excellence in patient care.
One session that attendees won’t want to miss is the debate on “The Optimal Route for the Introduction of New Technologies in Surgery.” Shaf Keshavjee from Toronto General Hospital will look at the “Academic Pathway” while Robert J. Cerfolio of the University of Alabama at Birmingham will present the “Industry Driven Pathway”
Program Directors
G. Alexander Patterson
David S. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Traves D. Crabtree
Register and reserve housing at: aats.org/focus
October 27-28, 2017
Encore at Wynn Las Vegas
Las Vegas, Nevada, USA
Spend two days in Las Vegas learning about the technological advances that are changing the approaches to benign and malignant esophageal disease, and lung cancer and related diseases. AATS Focus on Thoracic Surgery: Mastering Surgical Innovation 2017 features the most elite experts in the field presenting the latest information on new and emerging technologies in diagnostic and therapeutic techniques, minimally invasive surgery and robotic surgeries. The meeting’s interactive format will enable attendees to incorporate technologies into their own practices, as well as see how surgical innovation can impact patient outcomes and excellence in patient care.
One session that attendees won’t want to miss is the debate on “The Optimal Route for the Introduction of New Technologies in Surgery.” Shaf Keshavjee from Toronto General Hospital will look at the “Academic Pathway” while Robert J. Cerfolio of the University of Alabama at Birmingham will present the “Industry Driven Pathway”
Program Directors
G. Alexander Patterson
David S. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Traves D. Crabtree
Register and reserve housing at: aats.org/focus
October 27-28, 2017
Encore at Wynn Las Vegas
Las Vegas, Nevada, USA
Spend two days in Las Vegas learning about the technological advances that are changing the approaches to benign and malignant esophageal disease, and lung cancer and related diseases. AATS Focus on Thoracic Surgery: Mastering Surgical Innovation 2017 features the most elite experts in the field presenting the latest information on new and emerging technologies in diagnostic and therapeutic techniques, minimally invasive surgery and robotic surgeries. The meeting’s interactive format will enable attendees to incorporate technologies into their own practices, as well as see how surgical innovation can impact patient outcomes and excellence in patient care.
One session that attendees won’t want to miss is the debate on “The Optimal Route for the Introduction of New Technologies in Surgery.” Shaf Keshavjee from Toronto General Hospital will look at the “Academic Pathway” while Robert J. Cerfolio of the University of Alabama at Birmingham will present the “Industry Driven Pathway”
Program Directors
G. Alexander Patterson
David S. Sugarbaker
Program Committee
Thomas A. D’Amico
Shaf Keshavjee
James D. Luketich
Bryan F. Meyers
Scott J. Swanson
Traves D. Crabtree
Register and reserve housing at: aats.org/focus



