User login
The Official Newspaper of the American Association for Thoracic Surgery
Novel drug-eluting coronary stent looks good in DESSOLVE III
PARIS – A unique drug-eluting coronary stent showed positive interim results in the DESSOLVE III trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
In DESSOLVE III, 1,398 patients undergoing percutaneous coronary intervention were randomized to the widely used Xience everolimus-eluting stent or to MiStent, a novel thin-strut stent with a polymer coating that is quickly absorbed after delivering microcrystalline sirolimus into the vessel wall for prolonged release at a near-linear rate.
At an interim analysis at 12 months of follow-up, the primary endpoint – a composite of cardiac death, target vessel MI, and clinically indicated target lesion revascularization – had occurred in 5.8% of the MiStent group and 6.5% of Xience recipients in the study, which was designed as a noninferiority trial, reported Robbert J. de Winter, MD, professor of clinical cardiology at the Academic Medical Center in Amsterdam.
“The data support the hypothesis that long-term cytostatic inhibition of early neointima could prevent the late neointimal growth seen at medium and long term with conventional drug-eluting stents,” he said.
MiStent is designed to overcome a shortcoming of conventional drug-eluting stents: namely, their durable polymer coating sticks around after the cytostatic drug is finished being released. It is believed that this residual polymer, which may not disappear for 6-9 months, induces inflammation in the vessel wall, eventually leading to intimal growth, restenosis, and new atherosclerosis.
“The unique feature of the MiStent is that the polymer is bioabsorbable and is fully absorbed at 3 months, whereas the drug is present in the vessel wall out to 9 months, well after the coating has disappeared. So in theory you would expect that any inflammatory response caused by the polymer coating will be counteracted by the drug. This was seen in animal models. And in the DESSOLVE I and II studies, angiographic follow-up showed that late luminal loss was flat after 6 months, in contrast to other drug-eluting stents, which show accrual of late neointimal growth after 6 months to a year,” according to the cardiologist.
DESSOLVE III is planned as a 3-year study. Already by 6 months the curves for target lesion revascularization started to separate, with a 12-month rate of 2.6% in the MiStent group versus 3.8% for Xience. And while this difference is not yet statistically significant, Dr. de Winter and his coinvestigators expect that by 3 years the separation will have grown to the point that the difference becomes statistically significant and clinically meaningful.
The MiStent platform is composed of a cobalt/chromium alloy. The stent strut thickness is only 64 microns, in contrast to 81 microns for the Xience stent. Thinner stent struts have previously been shown to be less injurious to the vessel wall.
DESSOLVE III is an all-comers trial conducted at 20 sites in four European countries. Participants had to have a reference vessel diameter of 2.5-3.5 mm. Roughly 60% of patients had an acute coronary syndrome as their indication for PCI. The study population included, among others, patients with left main coronary artery lesions, restenotic lesions, and failed saphenous vein grafts. Dual-antiplatelet therapy was given for 6 months in patients with stable angina and 12 months for those with ACS, in accordance with European Society of Cardiology guidelines.
Discussant Robert A. Byrne, MD, of the German Heart Center in Munich, declared: “For me, this is a potentially interesting device. It’s the only device where we have a drug elution that’s more prolonged than the polymer, and this does offer the potential for later benefit.”
Dr. Byrne was a member of a European Commission–backed task force that developed European regulatory guidance for the evaluation of new coronary stents. “This MiStent trial program ticks off a lot of boxes: We had a successful first human use study, then we had a modest-size angiographic endpoint study where the late lumen loss looked good, and now we have a clinical endpoint study. This is what we want to see in the regulatory space.”
Another discussant, Chaim Lotan, MD, pronounced the MiStent “definitely another good stent on the shelf.”
It’s impossible to say whether the excellent 1-year outcomes seen with MiStent in DESSOLVE III were due to the prolonged-release microcrystalline sirolimus, the ultrathin stent struts, or both. In any case, the major adverse cardiovascular event rates seen in DESSOLVE III are so low by historical standards that it will become extremely difficult to show superiority for one contemporary drug-eluting stent over another, predicted Dr. Lotan of Hadassah Medical Center in Jerusalem.
Dr. de Winter concurred.
“I think we can now say that the benchmark for present day drug-eluting stents is a target lesion failure rate of about 6% at 12 months and a stent thrombosis rate below 1% at 12 months. It’s going to be increasingly more difficult to improve on that,” he said.
The MiStent, manufactured by Micell Technologies, is commercially available in Europe but investigational in the United States.
DESSOLVE III was sponsored by the European Cardiovascular Research Institute and supported by grants from Micell Technologies and Stentys.
Dr. de Winter reported receiving research grants from OrbusNeich, Abbott Vascular, AstraZeneca, Stentys, and Tryton.
PARIS – A unique drug-eluting coronary stent showed positive interim results in the DESSOLVE III trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
In DESSOLVE III, 1,398 patients undergoing percutaneous coronary intervention were randomized to the widely used Xience everolimus-eluting stent or to MiStent, a novel thin-strut stent with a polymer coating that is quickly absorbed after delivering microcrystalline sirolimus into the vessel wall for prolonged release at a near-linear rate.
At an interim analysis at 12 months of follow-up, the primary endpoint – a composite of cardiac death, target vessel MI, and clinically indicated target lesion revascularization – had occurred in 5.8% of the MiStent group and 6.5% of Xience recipients in the study, which was designed as a noninferiority trial, reported Robbert J. de Winter, MD, professor of clinical cardiology at the Academic Medical Center in Amsterdam.
“The data support the hypothesis that long-term cytostatic inhibition of early neointima could prevent the late neointimal growth seen at medium and long term with conventional drug-eluting stents,” he said.
MiStent is designed to overcome a shortcoming of conventional drug-eluting stents: namely, their durable polymer coating sticks around after the cytostatic drug is finished being released. It is believed that this residual polymer, which may not disappear for 6-9 months, induces inflammation in the vessel wall, eventually leading to intimal growth, restenosis, and new atherosclerosis.
“The unique feature of the MiStent is that the polymer is bioabsorbable and is fully absorbed at 3 months, whereas the drug is present in the vessel wall out to 9 months, well after the coating has disappeared. So in theory you would expect that any inflammatory response caused by the polymer coating will be counteracted by the drug. This was seen in animal models. And in the DESSOLVE I and II studies, angiographic follow-up showed that late luminal loss was flat after 6 months, in contrast to other drug-eluting stents, which show accrual of late neointimal growth after 6 months to a year,” according to the cardiologist.
DESSOLVE III is planned as a 3-year study. Already by 6 months the curves for target lesion revascularization started to separate, with a 12-month rate of 2.6% in the MiStent group versus 3.8% for Xience. And while this difference is not yet statistically significant, Dr. de Winter and his coinvestigators expect that by 3 years the separation will have grown to the point that the difference becomes statistically significant and clinically meaningful.
The MiStent platform is composed of a cobalt/chromium alloy. The stent strut thickness is only 64 microns, in contrast to 81 microns for the Xience stent. Thinner stent struts have previously been shown to be less injurious to the vessel wall.
DESSOLVE III is an all-comers trial conducted at 20 sites in four European countries. Participants had to have a reference vessel diameter of 2.5-3.5 mm. Roughly 60% of patients had an acute coronary syndrome as their indication for PCI. The study population included, among others, patients with left main coronary artery lesions, restenotic lesions, and failed saphenous vein grafts. Dual-antiplatelet therapy was given for 6 months in patients with stable angina and 12 months for those with ACS, in accordance with European Society of Cardiology guidelines.
Discussant Robert A. Byrne, MD, of the German Heart Center in Munich, declared: “For me, this is a potentially interesting device. It’s the only device where we have a drug elution that’s more prolonged than the polymer, and this does offer the potential for later benefit.”
Dr. Byrne was a member of a European Commission–backed task force that developed European regulatory guidance for the evaluation of new coronary stents. “This MiStent trial program ticks off a lot of boxes: We had a successful first human use study, then we had a modest-size angiographic endpoint study where the late lumen loss looked good, and now we have a clinical endpoint study. This is what we want to see in the regulatory space.”
Another discussant, Chaim Lotan, MD, pronounced the MiStent “definitely another good stent on the shelf.”
It’s impossible to say whether the excellent 1-year outcomes seen with MiStent in DESSOLVE III were due to the prolonged-release microcrystalline sirolimus, the ultrathin stent struts, or both. In any case, the major adverse cardiovascular event rates seen in DESSOLVE III are so low by historical standards that it will become extremely difficult to show superiority for one contemporary drug-eluting stent over another, predicted Dr. Lotan of Hadassah Medical Center in Jerusalem.
Dr. de Winter concurred.
“I think we can now say that the benchmark for present day drug-eluting stents is a target lesion failure rate of about 6% at 12 months and a stent thrombosis rate below 1% at 12 months. It’s going to be increasingly more difficult to improve on that,” he said.
The MiStent, manufactured by Micell Technologies, is commercially available in Europe but investigational in the United States.
DESSOLVE III was sponsored by the European Cardiovascular Research Institute and supported by grants from Micell Technologies and Stentys.
Dr. de Winter reported receiving research grants from OrbusNeich, Abbott Vascular, AstraZeneca, Stentys, and Tryton.
PARIS – A unique drug-eluting coronary stent showed positive interim results in the DESSOLVE III trial presented at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
In DESSOLVE III, 1,398 patients undergoing percutaneous coronary intervention were randomized to the widely used Xience everolimus-eluting stent or to MiStent, a novel thin-strut stent with a polymer coating that is quickly absorbed after delivering microcrystalline sirolimus into the vessel wall for prolonged release at a near-linear rate.
At an interim analysis at 12 months of follow-up, the primary endpoint – a composite of cardiac death, target vessel MI, and clinically indicated target lesion revascularization – had occurred in 5.8% of the MiStent group and 6.5% of Xience recipients in the study, which was designed as a noninferiority trial, reported Robbert J. de Winter, MD, professor of clinical cardiology at the Academic Medical Center in Amsterdam.
“The data support the hypothesis that long-term cytostatic inhibition of early neointima could prevent the late neointimal growth seen at medium and long term with conventional drug-eluting stents,” he said.
MiStent is designed to overcome a shortcoming of conventional drug-eluting stents: namely, their durable polymer coating sticks around after the cytostatic drug is finished being released. It is believed that this residual polymer, which may not disappear for 6-9 months, induces inflammation in the vessel wall, eventually leading to intimal growth, restenosis, and new atherosclerosis.
“The unique feature of the MiStent is that the polymer is bioabsorbable and is fully absorbed at 3 months, whereas the drug is present in the vessel wall out to 9 months, well after the coating has disappeared. So in theory you would expect that any inflammatory response caused by the polymer coating will be counteracted by the drug. This was seen in animal models. And in the DESSOLVE I and II studies, angiographic follow-up showed that late luminal loss was flat after 6 months, in contrast to other drug-eluting stents, which show accrual of late neointimal growth after 6 months to a year,” according to the cardiologist.
DESSOLVE III is planned as a 3-year study. Already by 6 months the curves for target lesion revascularization started to separate, with a 12-month rate of 2.6% in the MiStent group versus 3.8% for Xience. And while this difference is not yet statistically significant, Dr. de Winter and his coinvestigators expect that by 3 years the separation will have grown to the point that the difference becomes statistically significant and clinically meaningful.
The MiStent platform is composed of a cobalt/chromium alloy. The stent strut thickness is only 64 microns, in contrast to 81 microns for the Xience stent. Thinner stent struts have previously been shown to be less injurious to the vessel wall.
DESSOLVE III is an all-comers trial conducted at 20 sites in four European countries. Participants had to have a reference vessel diameter of 2.5-3.5 mm. Roughly 60% of patients had an acute coronary syndrome as their indication for PCI. The study population included, among others, patients with left main coronary artery lesions, restenotic lesions, and failed saphenous vein grafts. Dual-antiplatelet therapy was given for 6 months in patients with stable angina and 12 months for those with ACS, in accordance with European Society of Cardiology guidelines.
Discussant Robert A. Byrne, MD, of the German Heart Center in Munich, declared: “For me, this is a potentially interesting device. It’s the only device where we have a drug elution that’s more prolonged than the polymer, and this does offer the potential for later benefit.”
Dr. Byrne was a member of a European Commission–backed task force that developed European regulatory guidance for the evaluation of new coronary stents. “This MiStent trial program ticks off a lot of boxes: We had a successful first human use study, then we had a modest-size angiographic endpoint study where the late lumen loss looked good, and now we have a clinical endpoint study. This is what we want to see in the regulatory space.”
Another discussant, Chaim Lotan, MD, pronounced the MiStent “definitely another good stent on the shelf.”
It’s impossible to say whether the excellent 1-year outcomes seen with MiStent in DESSOLVE III were due to the prolonged-release microcrystalline sirolimus, the ultrathin stent struts, or both. In any case, the major adverse cardiovascular event rates seen in DESSOLVE III are so low by historical standards that it will become extremely difficult to show superiority for one contemporary drug-eluting stent over another, predicted Dr. Lotan of Hadassah Medical Center in Jerusalem.
Dr. de Winter concurred.
“I think we can now say that the benchmark for present day drug-eluting stents is a target lesion failure rate of about 6% at 12 months and a stent thrombosis rate below 1% at 12 months. It’s going to be increasingly more difficult to improve on that,” he said.
The MiStent, manufactured by Micell Technologies, is commercially available in Europe but investigational in the United States.
DESSOLVE III was sponsored by the European Cardiovascular Research Institute and supported by grants from Micell Technologies and Stentys.
Dr. de Winter reported receiving research grants from OrbusNeich, Abbott Vascular, AstraZeneca, Stentys, and Tryton.
AT EUROPCR
Key clinical point:
Major finding: At an interim 12-month analysis, the composite rate of cardiac death, target vessel MI, and clinically indicated target lesion revascularization was 6.5% in recipients of a Xience everolimus-eluting coronary stent and 5.8% in those randomized to the novel MiStent.
Data source: DESSOLVE III, a prospective, randomized, international trial that randomized 1,398 real-world-type all-comers undergoing PCI to the Xience device or the MiStent.
Disclosures: DESSOLVE III was sponsored by the European Cardiovascular Research Institute and supported by grants from Micell Technologies and Stentys. The presenter reported receiving research grants from both companies.
Tips and tricks for appealing an audit
CHICAGO – The question is not if a physician will face a Medicare or Medicaid billing audit, but when, according to Abby Pendleton, a New York–based health law attorney. That’s why it pays to know how to handle an audit before one probe disrupts your practice. At a recent American Bar Association meeting, Ms. Pendleton and H. Rusty Comley, a Jackson, Mississippi–based health law attorney, offered answers to top audit questions and provided guidance on how physicians can successfully appeal an audit.
When should you appeal?
There are a number of factors to consider when deciding whether to appeal audit findings. For starters, consider the cost of the payback amount and the basis of the findings.
“In other words, the provider would spend more money and time to appeal the audit than to pay the audit, and the issue or mistake is not likely repeated in past or future claims,” it might make sense to just pay, he said.
If the basis of the findings stem from an interpretation of a local coverage decision that the physician disagrees with, he or she may also want to appeal, Ms. Pendleton added.
“If you don’t fight it, there’s an argument that, ‘Well, guess what? You had that issue going back 6 years for all these other claims, and now we get into the [Medicare] 60 day overpayment identification [rule],’ ” she said at the meeting. “If a physician is [not] aware of payments they’re not entitled to, even if they think they were right on the front end, but they later become aware, they have 60 days to refund it or its a false claim. Those are considerations that really need to be looked at.”
What should you expect from an appeal?
Expect to go through more than one appeals process step to succeed. There are five stages to the appeals process .
“At the redetermination stage, I don’t see a whole lot of movement in terms of great success at that first stage,” Ms. Pendleton said. “So, don’t think, ‘If we get to that first level of appeal, we’re expecting to win.’ If you look at the statistics, it’s not really that realistic.”
Although a provider has 120 days to file an appeal, it’s smarter to file within the first 30 days, Ms. Pendleton advised. If an appeal is filed within 30 days, the government cannot recoup it’s demand from a doctor’s current Medicare payments.
Expect a lengthy time frame for a final outcome. Under federal law, once an appeal gets to the administrative law judge (ALJ) stage (the third stage), the appellant should receive a hearing decision within 90 days. However, because of heavy case backlogs, physicians typically don’t get a hearing for 3 years, Ms. Pendleton said.
“The problem is, your MAC [Medicare administrative contractor] can start taking your money after the [second] stage,” she said. “If it’s a huge dollar amount, you’re probably going to have to enter into a payment plan [with the government]. You will eventually get your money back, if you win, three to four years later.”
Note that physicians generally experience a higher degree of success at the ALJ stage, so it may be worth continuing the appeal through this stage, she noted.
Overall, more than one-third of audit findings are reversed in providers’ favor during the appeals process. Of 170,482 Medicare appeal decisions in 2015, 37% were made in favor of the health provider, an increase from 23% in 2014, according to 2015 Medicare data and 2014 reports.
The cost to appeal varies significantly between Medicaid and Medicare and depends largely on the complexity of the audit, Mr. Comley said. A Medicaid audit appeal, through an ALJ hearing and written appeal to a court, may cost between $20,000 to $60,000 depending on the circumstances, he said. By contrast, a Medicare appeal resolved in the first stage of appeal may only cost a few thousand dollars for a relatively simple audit.
“Of course, the costs will rise at each level of the Medicare appeal process, especially in the third stage involving the ALJ telephonic hearing, but, in most cases, the Medicare appeal costs will still be below a similar Medicaid appeal,” he said.
What strategies can help you win?
Consider reaching out to your congressional representative or senators, Mr. Comley advised. Particularly if the issue involves a medical treatment decision or a medically necessary determination, it may be helpful to copy “your favorite Congressman or senator’s office” on correspondence with the MAC. Clearly state your argument against the findings and how/why the medical decision was made. Legislators will often get involved and could help your appeal, Mr. Comley said.
Further, don’t just review the claims that auditors denied. Also evaluate the claims they have approved in the past, he added.
“In almost every case I’ve been involved in, they’ll approve claims that, on the other hand, they deny,” Mr. Comley said. “Under most legal standards, that’s a good way to win – it’s called arbitrary and capricious.”
Find the best experts to back your case, Ms. Pendleton advised. Consider including expert opinions in written responses to the government that support the services provided and/or have medical experts ready to testify during hearings. If the government based its findings on statistics or cited statistics in its review, involve a statistical expert who can argue against the government’s conclusion.
If the case is significant enough, consider skipping steps in the appeals process to get the case before a federal court sooner. Appellants can escalate their appeal through the process at nearly every stage if the government fails to respond within a timely manner. At the second stage, for example, if the qualified independent contractor does not issue a decision within 60 days, an appellant generally has the right to escalate the case to an administrative law judge. If the ALJ does not issue a decision within 90 days, the appeal can generally be escalated to the Appeals Council level, and, if the council does not issue a decision within 90 days, appellants can seek judicial review.
It may be worth it to have your day in court sooner, Ms. Pendleton said.
“It might be an option for providers if you have a large audit with a lot at stake,” she said. “Escalate it through. Get it to federal court and argue it.”
The 5 steps of the Medicare appeals process
There are five stages of the Medicare audit appeals process, according to the Centers for Medicare & Medicaid Services. They include:
1. Redetermination by the Fiscal Intermediary. A redetermination is an examination of a claim by a Medicare administrative contractor (MAC) separate from the personnel who made the initial claim determination. The appellant has 120 days from the date of initial claim determination receipt to file an appeal.
2. Reconsideration by a Qualified Independent Contractor (QIC). A QIC is an independent contractor who didn’t take part in the level 1 decision. The QIC will review the request for a reconsideration and make a decision. An appellant must file a request for reconsideration within 180 days of Medicare redetermination notice or remittance advice receipt.
3. Administrative Law Judge (ALJ) hearing. Appellants present their case to an ALJ who will review the facts of the appeal and listen to testimony before making a decision. An ALJ hearing is usually held by phone or video conference. Appellants can ask the ALJ to make a decision without a hearing. The ALJ may also issue a decision without holding a hearing if evidence in the record supports a decision that’s fully in the appellant’s favor.
4. Medicare Appeals Council review. If you disagree with the ALJ decision or wish to escalate the appeal because the ALJ ruling time frame has passed, a request for a Medicare Appeals Council review can be made. A request for a Medicare Appeals Council review must be made within 60 days of receipt of the ALJ’s decision or after the ALJ ruling time frame expires.
5. Judicial review in U.S. District Court. A party may file an action in federal district court within 60 calendar days after the date receiving notice of the Medicare Appeals Council’s decision or after a council notice that it is not able to reach a decision. To get a judicial review in federal district court, the case amount must meet a minimum dollar amount ($1,560 in 2017).
Each state has its own Medicaid appeals process. Contact your state’s Medicaid office to find out how to appeal a Medicaid audit finding.
[email protected]
On Twitter @legal_med
CHICAGO – The question is not if a physician will face a Medicare or Medicaid billing audit, but when, according to Abby Pendleton, a New York–based health law attorney. That’s why it pays to know how to handle an audit before one probe disrupts your practice. At a recent American Bar Association meeting, Ms. Pendleton and H. Rusty Comley, a Jackson, Mississippi–based health law attorney, offered answers to top audit questions and provided guidance on how physicians can successfully appeal an audit.
When should you appeal?
There are a number of factors to consider when deciding whether to appeal audit findings. For starters, consider the cost of the payback amount and the basis of the findings.
“In other words, the provider would spend more money and time to appeal the audit than to pay the audit, and the issue or mistake is not likely repeated in past or future claims,” it might make sense to just pay, he said.
If the basis of the findings stem from an interpretation of a local coverage decision that the physician disagrees with, he or she may also want to appeal, Ms. Pendleton added.
“If you don’t fight it, there’s an argument that, ‘Well, guess what? You had that issue going back 6 years for all these other claims, and now we get into the [Medicare] 60 day overpayment identification [rule],’ ” she said at the meeting. “If a physician is [not] aware of payments they’re not entitled to, even if they think they were right on the front end, but they later become aware, they have 60 days to refund it or its a false claim. Those are considerations that really need to be looked at.”
What should you expect from an appeal?
Expect to go through more than one appeals process step to succeed. There are five stages to the appeals process .
“At the redetermination stage, I don’t see a whole lot of movement in terms of great success at that first stage,” Ms. Pendleton said. “So, don’t think, ‘If we get to that first level of appeal, we’re expecting to win.’ If you look at the statistics, it’s not really that realistic.”
Although a provider has 120 days to file an appeal, it’s smarter to file within the first 30 days, Ms. Pendleton advised. If an appeal is filed within 30 days, the government cannot recoup it’s demand from a doctor’s current Medicare payments.
Expect a lengthy time frame for a final outcome. Under federal law, once an appeal gets to the administrative law judge (ALJ) stage (the third stage), the appellant should receive a hearing decision within 90 days. However, because of heavy case backlogs, physicians typically don’t get a hearing for 3 years, Ms. Pendleton said.
“The problem is, your MAC [Medicare administrative contractor] can start taking your money after the [second] stage,” she said. “If it’s a huge dollar amount, you’re probably going to have to enter into a payment plan [with the government]. You will eventually get your money back, if you win, three to four years later.”
Note that physicians generally experience a higher degree of success at the ALJ stage, so it may be worth continuing the appeal through this stage, she noted.
Overall, more than one-third of audit findings are reversed in providers’ favor during the appeals process. Of 170,482 Medicare appeal decisions in 2015, 37% were made in favor of the health provider, an increase from 23% in 2014, according to 2015 Medicare data and 2014 reports.
The cost to appeal varies significantly between Medicaid and Medicare and depends largely on the complexity of the audit, Mr. Comley said. A Medicaid audit appeal, through an ALJ hearing and written appeal to a court, may cost between $20,000 to $60,000 depending on the circumstances, he said. By contrast, a Medicare appeal resolved in the first stage of appeal may only cost a few thousand dollars for a relatively simple audit.
“Of course, the costs will rise at each level of the Medicare appeal process, especially in the third stage involving the ALJ telephonic hearing, but, in most cases, the Medicare appeal costs will still be below a similar Medicaid appeal,” he said.
What strategies can help you win?
Consider reaching out to your congressional representative or senators, Mr. Comley advised. Particularly if the issue involves a medical treatment decision or a medically necessary determination, it may be helpful to copy “your favorite Congressman or senator’s office” on correspondence with the MAC. Clearly state your argument against the findings and how/why the medical decision was made. Legislators will often get involved and could help your appeal, Mr. Comley said.
Further, don’t just review the claims that auditors denied. Also evaluate the claims they have approved in the past, he added.
“In almost every case I’ve been involved in, they’ll approve claims that, on the other hand, they deny,” Mr. Comley said. “Under most legal standards, that’s a good way to win – it’s called arbitrary and capricious.”
Find the best experts to back your case, Ms. Pendleton advised. Consider including expert opinions in written responses to the government that support the services provided and/or have medical experts ready to testify during hearings. If the government based its findings on statistics or cited statistics in its review, involve a statistical expert who can argue against the government’s conclusion.
If the case is significant enough, consider skipping steps in the appeals process to get the case before a federal court sooner. Appellants can escalate their appeal through the process at nearly every stage if the government fails to respond within a timely manner. At the second stage, for example, if the qualified independent contractor does not issue a decision within 60 days, an appellant generally has the right to escalate the case to an administrative law judge. If the ALJ does not issue a decision within 90 days, the appeal can generally be escalated to the Appeals Council level, and, if the council does not issue a decision within 90 days, appellants can seek judicial review.
It may be worth it to have your day in court sooner, Ms. Pendleton said.
“It might be an option for providers if you have a large audit with a lot at stake,” she said. “Escalate it through. Get it to federal court and argue it.”
The 5 steps of the Medicare appeals process
There are five stages of the Medicare audit appeals process, according to the Centers for Medicare & Medicaid Services. They include:
1. Redetermination by the Fiscal Intermediary. A redetermination is an examination of a claim by a Medicare administrative contractor (MAC) separate from the personnel who made the initial claim determination. The appellant has 120 days from the date of initial claim determination receipt to file an appeal.
2. Reconsideration by a Qualified Independent Contractor (QIC). A QIC is an independent contractor who didn’t take part in the level 1 decision. The QIC will review the request for a reconsideration and make a decision. An appellant must file a request for reconsideration within 180 days of Medicare redetermination notice or remittance advice receipt.
3. Administrative Law Judge (ALJ) hearing. Appellants present their case to an ALJ who will review the facts of the appeal and listen to testimony before making a decision. An ALJ hearing is usually held by phone or video conference. Appellants can ask the ALJ to make a decision without a hearing. The ALJ may also issue a decision without holding a hearing if evidence in the record supports a decision that’s fully in the appellant’s favor.
4. Medicare Appeals Council review. If you disagree with the ALJ decision or wish to escalate the appeal because the ALJ ruling time frame has passed, a request for a Medicare Appeals Council review can be made. A request for a Medicare Appeals Council review must be made within 60 days of receipt of the ALJ’s decision or after the ALJ ruling time frame expires.
5. Judicial review in U.S. District Court. A party may file an action in federal district court within 60 calendar days after the date receiving notice of the Medicare Appeals Council’s decision or after a council notice that it is not able to reach a decision. To get a judicial review in federal district court, the case amount must meet a minimum dollar amount ($1,560 in 2017).
Each state has its own Medicaid appeals process. Contact your state’s Medicaid office to find out how to appeal a Medicaid audit finding.
[email protected]
On Twitter @legal_med
CHICAGO – The question is not if a physician will face a Medicare or Medicaid billing audit, but when, according to Abby Pendleton, a New York–based health law attorney. That’s why it pays to know how to handle an audit before one probe disrupts your practice. At a recent American Bar Association meeting, Ms. Pendleton and H. Rusty Comley, a Jackson, Mississippi–based health law attorney, offered answers to top audit questions and provided guidance on how physicians can successfully appeal an audit.
When should you appeal?
There are a number of factors to consider when deciding whether to appeal audit findings. For starters, consider the cost of the payback amount and the basis of the findings.
“In other words, the provider would spend more money and time to appeal the audit than to pay the audit, and the issue or mistake is not likely repeated in past or future claims,” it might make sense to just pay, he said.
If the basis of the findings stem from an interpretation of a local coverage decision that the physician disagrees with, he or she may also want to appeal, Ms. Pendleton added.
“If you don’t fight it, there’s an argument that, ‘Well, guess what? You had that issue going back 6 years for all these other claims, and now we get into the [Medicare] 60 day overpayment identification [rule],’ ” she said at the meeting. “If a physician is [not] aware of payments they’re not entitled to, even if they think they were right on the front end, but they later become aware, they have 60 days to refund it or its a false claim. Those are considerations that really need to be looked at.”
What should you expect from an appeal?
Expect to go through more than one appeals process step to succeed. There are five stages to the appeals process .
“At the redetermination stage, I don’t see a whole lot of movement in terms of great success at that first stage,” Ms. Pendleton said. “So, don’t think, ‘If we get to that first level of appeal, we’re expecting to win.’ If you look at the statistics, it’s not really that realistic.”
Although a provider has 120 days to file an appeal, it’s smarter to file within the first 30 days, Ms. Pendleton advised. If an appeal is filed within 30 days, the government cannot recoup it’s demand from a doctor’s current Medicare payments.
Expect a lengthy time frame for a final outcome. Under federal law, once an appeal gets to the administrative law judge (ALJ) stage (the third stage), the appellant should receive a hearing decision within 90 days. However, because of heavy case backlogs, physicians typically don’t get a hearing for 3 years, Ms. Pendleton said.
“The problem is, your MAC [Medicare administrative contractor] can start taking your money after the [second] stage,” she said. “If it’s a huge dollar amount, you’re probably going to have to enter into a payment plan [with the government]. You will eventually get your money back, if you win, three to four years later.”
Note that physicians generally experience a higher degree of success at the ALJ stage, so it may be worth continuing the appeal through this stage, she noted.
Overall, more than one-third of audit findings are reversed in providers’ favor during the appeals process. Of 170,482 Medicare appeal decisions in 2015, 37% were made in favor of the health provider, an increase from 23% in 2014, according to 2015 Medicare data and 2014 reports.
The cost to appeal varies significantly between Medicaid and Medicare and depends largely on the complexity of the audit, Mr. Comley said. A Medicaid audit appeal, through an ALJ hearing and written appeal to a court, may cost between $20,000 to $60,000 depending on the circumstances, he said. By contrast, a Medicare appeal resolved in the first stage of appeal may only cost a few thousand dollars for a relatively simple audit.
“Of course, the costs will rise at each level of the Medicare appeal process, especially in the third stage involving the ALJ telephonic hearing, but, in most cases, the Medicare appeal costs will still be below a similar Medicaid appeal,” he said.
What strategies can help you win?
Consider reaching out to your congressional representative or senators, Mr. Comley advised. Particularly if the issue involves a medical treatment decision or a medically necessary determination, it may be helpful to copy “your favorite Congressman or senator’s office” on correspondence with the MAC. Clearly state your argument against the findings and how/why the medical decision was made. Legislators will often get involved and could help your appeal, Mr. Comley said.
Further, don’t just review the claims that auditors denied. Also evaluate the claims they have approved in the past, he added.
“In almost every case I’ve been involved in, they’ll approve claims that, on the other hand, they deny,” Mr. Comley said. “Under most legal standards, that’s a good way to win – it’s called arbitrary and capricious.”
Find the best experts to back your case, Ms. Pendleton advised. Consider including expert opinions in written responses to the government that support the services provided and/or have medical experts ready to testify during hearings. If the government based its findings on statistics or cited statistics in its review, involve a statistical expert who can argue against the government’s conclusion.
If the case is significant enough, consider skipping steps in the appeals process to get the case before a federal court sooner. Appellants can escalate their appeal through the process at nearly every stage if the government fails to respond within a timely manner. At the second stage, for example, if the qualified independent contractor does not issue a decision within 60 days, an appellant generally has the right to escalate the case to an administrative law judge. If the ALJ does not issue a decision within 90 days, the appeal can generally be escalated to the Appeals Council level, and, if the council does not issue a decision within 90 days, appellants can seek judicial review.
It may be worth it to have your day in court sooner, Ms. Pendleton said.
“It might be an option for providers if you have a large audit with a lot at stake,” she said. “Escalate it through. Get it to federal court and argue it.”
The 5 steps of the Medicare appeals process
There are five stages of the Medicare audit appeals process, according to the Centers for Medicare & Medicaid Services. They include:
1. Redetermination by the Fiscal Intermediary. A redetermination is an examination of a claim by a Medicare administrative contractor (MAC) separate from the personnel who made the initial claim determination. The appellant has 120 days from the date of initial claim determination receipt to file an appeal.
2. Reconsideration by a Qualified Independent Contractor (QIC). A QIC is an independent contractor who didn’t take part in the level 1 decision. The QIC will review the request for a reconsideration and make a decision. An appellant must file a request for reconsideration within 180 days of Medicare redetermination notice or remittance advice receipt.
3. Administrative Law Judge (ALJ) hearing. Appellants present their case to an ALJ who will review the facts of the appeal and listen to testimony before making a decision. An ALJ hearing is usually held by phone or video conference. Appellants can ask the ALJ to make a decision without a hearing. The ALJ may also issue a decision without holding a hearing if evidence in the record supports a decision that’s fully in the appellant’s favor.
4. Medicare Appeals Council review. If you disagree with the ALJ decision or wish to escalate the appeal because the ALJ ruling time frame has passed, a request for a Medicare Appeals Council review can be made. A request for a Medicare Appeals Council review must be made within 60 days of receipt of the ALJ’s decision or after the ALJ ruling time frame expires.
5. Judicial review in U.S. District Court. A party may file an action in federal district court within 60 calendar days after the date receiving notice of the Medicare Appeals Council’s decision or after a council notice that it is not able to reach a decision. To get a judicial review in federal district court, the case amount must meet a minimum dollar amount ($1,560 in 2017).
Each state has its own Medicaid appeals process. Contact your state’s Medicaid office to find out how to appeal a Medicaid audit finding.
[email protected]
On Twitter @legal_med
AT THE PHYSICIAN LEGAL ISSUES CONFERENCE
Potential new role for FFR
Paris – Fractional flow reserve is under study for a potential major application: guidance on percutaneous coronary intervention (PCI) optimization immediately after stent placement, Roberto Diletti, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At the end of the procedure, FFR measurement using a novel monorail optical pressure sensor catheter revealed that 43% of the lesions had a suboptimal FFR value of 0.90 or less.
“Just this year, two meta-analyses showed that a cutoff of 0.90 is important to define a group of patients at high risk for major adverse cardiovascular events and revascularization,” noted Dr. Diletti of Erasmus University Medical Center in Rotterdam, the Netherlands.
Using the older, more conservative cutoff of an FFR of 0.85 or less, 20% of the lesions would potentially benefit from further action to optimize the physiologic result, most often in the form of additional expansion of the stent.
“We are used to thinking of FFR as a tool to understand whether a lesion has to be treated or not. Now we can also start thinking about FFR as a tool to guide PCI optimization,” the cardiologist said.
Optimization wasn’t actually performed in this observational registry. That will be the focus of FFR-REACT, a planned randomized trial investigating the clinical impact of intravascular ultrasound (IVUS)-directed FFR optimization of PCI.
In a per-patient analysis, 48% of FFR-SEARCH participants had a poststent FFR of 0.90 or less in one or more treated lesions. Another 22% had a postprocedure FFR of 0.85 or less in at least one treated lesion, while 8.9% had an FFR of 0.80, which is below the threshold for ischemia.
The primary endpoint in the ongoing FFR-SEARCH study is the 2-year composite rate of major adverse cardiovascular events, defined as MI, any revascularization, or all-cause mortality. Only the 30-day MACE rate was available at the time of Dr. Diletti’s presentation in Paris. The rate was 1.5% in patients with a postprocedure FFR greater than 0.90, 2.0% in those with an FFR of 0.86-0.90, 2.6% with an FFR of 0.81-0.85, and 2.8% with a poststent FFR of 0.80 or less. While those early between-group differences weren’t statistically significant, the trend is encouraging, he noted.
Postprocedure FFR measurement took an average of 5 minutes. The procedure was simple and safe, according to Dr. Diletti. There were no complications related to the use of the Navvus MicroCatheter technology. He explained that the device profile is comparable to a 0.022-inch diameter at the lesion site. Wire access to the vessel was maintained throughout. The rapid-exchange monorail microcatheter was inserted over the previously used standard 0.014-inch coronary guidewire. The optical pressure sensor was positioned roughly 20 mm distal to the distal stent edge. Manual pullback with measurements obtained at various locations in the vicinity of the stented lesion was repeated as necessary in order to identify where optimization, if appropriate, should be focused.
Operators were unable to crossover the microcatheter in 3.5% of cases, mostly because of vessel tortuosity or calcification.
Audience members commented that many of the low FFRs after stenting may reflect diffuse coronary disease, which can be corrected only by placing numerous additional stents, creating its own problems. Dr. Diletti offered reassurance on that score. He explained that in an IVUS substudy of FFR-SEARCH, an unstented physiologically important focal lesion or stent underexpansion was identified in 86% of the cases of low FFR.
“That means you can do something about it. In the other 14% of cases, in my opinion, you cannot do a lot because of very diffuse disease distally,” he said.
He reported having no financial conflicts of interest in connection with the study, supported by ACIST Medical Systems. The Navvus MicroCatheter is approved by both the Food and Drug Administration and the European regulatory agency.
Paris – Fractional flow reserve is under study for a potential major application: guidance on percutaneous coronary intervention (PCI) optimization immediately after stent placement, Roberto Diletti, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At the end of the procedure, FFR measurement using a novel monorail optical pressure sensor catheter revealed that 43% of the lesions had a suboptimal FFR value of 0.90 or less.
“Just this year, two meta-analyses showed that a cutoff of 0.90 is important to define a group of patients at high risk for major adverse cardiovascular events and revascularization,” noted Dr. Diletti of Erasmus University Medical Center in Rotterdam, the Netherlands.
Using the older, more conservative cutoff of an FFR of 0.85 or less, 20% of the lesions would potentially benefit from further action to optimize the physiologic result, most often in the form of additional expansion of the stent.
“We are used to thinking of FFR as a tool to understand whether a lesion has to be treated or not. Now we can also start thinking about FFR as a tool to guide PCI optimization,” the cardiologist said.
Optimization wasn’t actually performed in this observational registry. That will be the focus of FFR-REACT, a planned randomized trial investigating the clinical impact of intravascular ultrasound (IVUS)-directed FFR optimization of PCI.
In a per-patient analysis, 48% of FFR-SEARCH participants had a poststent FFR of 0.90 or less in one or more treated lesions. Another 22% had a postprocedure FFR of 0.85 or less in at least one treated lesion, while 8.9% had an FFR of 0.80, which is below the threshold for ischemia.
The primary endpoint in the ongoing FFR-SEARCH study is the 2-year composite rate of major adverse cardiovascular events, defined as MI, any revascularization, or all-cause mortality. Only the 30-day MACE rate was available at the time of Dr. Diletti’s presentation in Paris. The rate was 1.5% in patients with a postprocedure FFR greater than 0.90, 2.0% in those with an FFR of 0.86-0.90, 2.6% with an FFR of 0.81-0.85, and 2.8% with a poststent FFR of 0.80 or less. While those early between-group differences weren’t statistically significant, the trend is encouraging, he noted.
Postprocedure FFR measurement took an average of 5 minutes. The procedure was simple and safe, according to Dr. Diletti. There were no complications related to the use of the Navvus MicroCatheter technology. He explained that the device profile is comparable to a 0.022-inch diameter at the lesion site. Wire access to the vessel was maintained throughout. The rapid-exchange monorail microcatheter was inserted over the previously used standard 0.014-inch coronary guidewire. The optical pressure sensor was positioned roughly 20 mm distal to the distal stent edge. Manual pullback with measurements obtained at various locations in the vicinity of the stented lesion was repeated as necessary in order to identify where optimization, if appropriate, should be focused.
Operators were unable to crossover the microcatheter in 3.5% of cases, mostly because of vessel tortuosity or calcification.
Audience members commented that many of the low FFRs after stenting may reflect diffuse coronary disease, which can be corrected only by placing numerous additional stents, creating its own problems. Dr. Diletti offered reassurance on that score. He explained that in an IVUS substudy of FFR-SEARCH, an unstented physiologically important focal lesion or stent underexpansion was identified in 86% of the cases of low FFR.
“That means you can do something about it. In the other 14% of cases, in my opinion, you cannot do a lot because of very diffuse disease distally,” he said.
He reported having no financial conflicts of interest in connection with the study, supported by ACIST Medical Systems. The Navvus MicroCatheter is approved by both the Food and Drug Administration and the European regulatory agency.
Paris – Fractional flow reserve is under study for a potential major application: guidance on percutaneous coronary intervention (PCI) optimization immediately after stent placement, Roberto Diletti, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At the end of the procedure, FFR measurement using a novel monorail optical pressure sensor catheter revealed that 43% of the lesions had a suboptimal FFR value of 0.90 or less.
“Just this year, two meta-analyses showed that a cutoff of 0.90 is important to define a group of patients at high risk for major adverse cardiovascular events and revascularization,” noted Dr. Diletti of Erasmus University Medical Center in Rotterdam, the Netherlands.
Using the older, more conservative cutoff of an FFR of 0.85 or less, 20% of the lesions would potentially benefit from further action to optimize the physiologic result, most often in the form of additional expansion of the stent.
“We are used to thinking of FFR as a tool to understand whether a lesion has to be treated or not. Now we can also start thinking about FFR as a tool to guide PCI optimization,” the cardiologist said.
Optimization wasn’t actually performed in this observational registry. That will be the focus of FFR-REACT, a planned randomized trial investigating the clinical impact of intravascular ultrasound (IVUS)-directed FFR optimization of PCI.
In a per-patient analysis, 48% of FFR-SEARCH participants had a poststent FFR of 0.90 or less in one or more treated lesions. Another 22% had a postprocedure FFR of 0.85 or less in at least one treated lesion, while 8.9% had an FFR of 0.80, which is below the threshold for ischemia.
The primary endpoint in the ongoing FFR-SEARCH study is the 2-year composite rate of major adverse cardiovascular events, defined as MI, any revascularization, or all-cause mortality. Only the 30-day MACE rate was available at the time of Dr. Diletti’s presentation in Paris. The rate was 1.5% in patients with a postprocedure FFR greater than 0.90, 2.0% in those with an FFR of 0.86-0.90, 2.6% with an FFR of 0.81-0.85, and 2.8% with a poststent FFR of 0.80 or less. While those early between-group differences weren’t statistically significant, the trend is encouraging, he noted.
Postprocedure FFR measurement took an average of 5 minutes. The procedure was simple and safe, according to Dr. Diletti. There were no complications related to the use of the Navvus MicroCatheter technology. He explained that the device profile is comparable to a 0.022-inch diameter at the lesion site. Wire access to the vessel was maintained throughout. The rapid-exchange monorail microcatheter was inserted over the previously used standard 0.014-inch coronary guidewire. The optical pressure sensor was positioned roughly 20 mm distal to the distal stent edge. Manual pullback with measurements obtained at various locations in the vicinity of the stented lesion was repeated as necessary in order to identify where optimization, if appropriate, should be focused.
Operators were unable to crossover the microcatheter in 3.5% of cases, mostly because of vessel tortuosity or calcification.
Audience members commented that many of the low FFRs after stenting may reflect diffuse coronary disease, which can be corrected only by placing numerous additional stents, creating its own problems. Dr. Diletti offered reassurance on that score. He explained that in an IVUS substudy of FFR-SEARCH, an unstented physiologically important focal lesion or stent underexpansion was identified in 86% of the cases of low FFR.
“That means you can do something about it. In the other 14% of cases, in my opinion, you cannot do a lot because of very diffuse disease distally,” he said.
He reported having no financial conflicts of interest in connection with the study, supported by ACIST Medical Systems. The Navvus MicroCatheter is approved by both the Food and Drug Administration and the European regulatory agency.
EXPERT ANALYSIS FROM EuroPCR
First Senate vote to repeal and replace ACA fails
Senate Republicans’ first attempt to repeal and replace the Affordable Care Act failed July 25 as nine Republican senators crossed the aisle to vote against a measure that had no chance of passing.
The process started with a dramatic appearance by Sen. John McCain (R-Ariz.), recovering from surgery to diagnose glioblastoma, who returned to Washington to cast a key vote that would allow debate to move forward. The Senate split 50-50 on that vote, with Sen. Susan Collins (R-Maine) and Sen. Lisa Murkowski (R-Alaska) crossing the aisle to vote with all the chamber’s Democrats against the motion to proceed. Vice President Mike Pence cast the tie-breaking vote in favor of beginning debate.
However, the first amendment voted on would have replaced that language with the most recent version of the Senate GOP repeal-and-replace plan, the Better Care Reconciliation Act, with two additional provisions. One was from Sen. Ted Cruz (R-Tex.) and would allow insurers to offer more limited health insurance plans along side plans that cover the ACA’s essential benefits package. A second provision from Sen. Rob Portman (R-Ohio) would have added an additional $100 billion to the state stability fund to help low-income individuals whose Medicaid coverage was repealed.
The amendment was dead on arrival, as it would have needed 60 votes to pass. Throughout the repeal-and-replace effort, the 48 Senate Democrats have been firm in voting against any action. While much of the repeal-and-replace effort to date has relied on the budget reconciliation process, which requires a simple majority for passage, this legislation did not qualify and needed a supermajority of 60 votes for passage.
The nine GOP senators voting against the amendment included Collins, Murkowski, Bob Corker (Tenn.), Tom Cotton (Ark.), Lindsey Graham (S.C.), Dena Heller (Nev.), Mike Lee (Utah), Jerry Moran (Kan.), and Rand Paul (Ky.).
Earlier in the day, just after voting in favor of action on this legislation, Sen. McCain pleaded with his colleagues to return to regular order and write a bill that has bipartisan support. He criticized congressional Democrats for forcing the ACA through without bipartisan support 7 years ago and congressional Republicans for doing the exact same thing now with their repeal-and-replace efforts.
“Why don’t we try the old way of legislating in the Senate, the way our rules and customs encourage us to act,” Sen. McCain asked. “Let the Health, Education, Labor, and Pensions Committee under Chairman Alexander and Ranking Member Murray hold hearings, try to report a bill out of committee with contributions from both sides. Then bring it to the floor for amendment and debate and see if we can pass something that will be imperfect, full of compromises, and not very pleasing to implacable partisans on either side but that might provide workable solutions to problems Americans are struggling with today.”
Senate Republicans’ first attempt to repeal and replace the Affordable Care Act failed July 25 as nine Republican senators crossed the aisle to vote against a measure that had no chance of passing.
The process started with a dramatic appearance by Sen. John McCain (R-Ariz.), recovering from surgery to diagnose glioblastoma, who returned to Washington to cast a key vote that would allow debate to move forward. The Senate split 50-50 on that vote, with Sen. Susan Collins (R-Maine) and Sen. Lisa Murkowski (R-Alaska) crossing the aisle to vote with all the chamber’s Democrats against the motion to proceed. Vice President Mike Pence cast the tie-breaking vote in favor of beginning debate.
However, the first amendment voted on would have replaced that language with the most recent version of the Senate GOP repeal-and-replace plan, the Better Care Reconciliation Act, with two additional provisions. One was from Sen. Ted Cruz (R-Tex.) and would allow insurers to offer more limited health insurance plans along side plans that cover the ACA’s essential benefits package. A second provision from Sen. Rob Portman (R-Ohio) would have added an additional $100 billion to the state stability fund to help low-income individuals whose Medicaid coverage was repealed.
The amendment was dead on arrival, as it would have needed 60 votes to pass. Throughout the repeal-and-replace effort, the 48 Senate Democrats have been firm in voting against any action. While much of the repeal-and-replace effort to date has relied on the budget reconciliation process, which requires a simple majority for passage, this legislation did not qualify and needed a supermajority of 60 votes for passage.
The nine GOP senators voting against the amendment included Collins, Murkowski, Bob Corker (Tenn.), Tom Cotton (Ark.), Lindsey Graham (S.C.), Dena Heller (Nev.), Mike Lee (Utah), Jerry Moran (Kan.), and Rand Paul (Ky.).
Earlier in the day, just after voting in favor of action on this legislation, Sen. McCain pleaded with his colleagues to return to regular order and write a bill that has bipartisan support. He criticized congressional Democrats for forcing the ACA through without bipartisan support 7 years ago and congressional Republicans for doing the exact same thing now with their repeal-and-replace efforts.
“Why don’t we try the old way of legislating in the Senate, the way our rules and customs encourage us to act,” Sen. McCain asked. “Let the Health, Education, Labor, and Pensions Committee under Chairman Alexander and Ranking Member Murray hold hearings, try to report a bill out of committee with contributions from both sides. Then bring it to the floor for amendment and debate and see if we can pass something that will be imperfect, full of compromises, and not very pleasing to implacable partisans on either side but that might provide workable solutions to problems Americans are struggling with today.”
Senate Republicans’ first attempt to repeal and replace the Affordable Care Act failed July 25 as nine Republican senators crossed the aisle to vote against a measure that had no chance of passing.
The process started with a dramatic appearance by Sen. John McCain (R-Ariz.), recovering from surgery to diagnose glioblastoma, who returned to Washington to cast a key vote that would allow debate to move forward. The Senate split 50-50 on that vote, with Sen. Susan Collins (R-Maine) and Sen. Lisa Murkowski (R-Alaska) crossing the aisle to vote with all the chamber’s Democrats against the motion to proceed. Vice President Mike Pence cast the tie-breaking vote in favor of beginning debate.
However, the first amendment voted on would have replaced that language with the most recent version of the Senate GOP repeal-and-replace plan, the Better Care Reconciliation Act, with two additional provisions. One was from Sen. Ted Cruz (R-Tex.) and would allow insurers to offer more limited health insurance plans along side plans that cover the ACA’s essential benefits package. A second provision from Sen. Rob Portman (R-Ohio) would have added an additional $100 billion to the state stability fund to help low-income individuals whose Medicaid coverage was repealed.
The amendment was dead on arrival, as it would have needed 60 votes to pass. Throughout the repeal-and-replace effort, the 48 Senate Democrats have been firm in voting against any action. While much of the repeal-and-replace effort to date has relied on the budget reconciliation process, which requires a simple majority for passage, this legislation did not qualify and needed a supermajority of 60 votes for passage.
The nine GOP senators voting against the amendment included Collins, Murkowski, Bob Corker (Tenn.), Tom Cotton (Ark.), Lindsey Graham (S.C.), Dena Heller (Nev.), Mike Lee (Utah), Jerry Moran (Kan.), and Rand Paul (Ky.).
Earlier in the day, just after voting in favor of action on this legislation, Sen. McCain pleaded with his colleagues to return to regular order and write a bill that has bipartisan support. He criticized congressional Democrats for forcing the ACA through without bipartisan support 7 years ago and congressional Republicans for doing the exact same thing now with their repeal-and-replace efforts.
“Why don’t we try the old way of legislating in the Senate, the way our rules and customs encourage us to act,” Sen. McCain asked. “Let the Health, Education, Labor, and Pensions Committee under Chairman Alexander and Ranking Member Murray hold hearings, try to report a bill out of committee with contributions from both sides. Then bring it to the floor for amendment and debate and see if we can pass something that will be imperfect, full of compromises, and not very pleasing to implacable partisans on either side but that might provide workable solutions to problems Americans are struggling with today.”
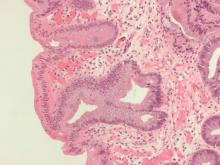
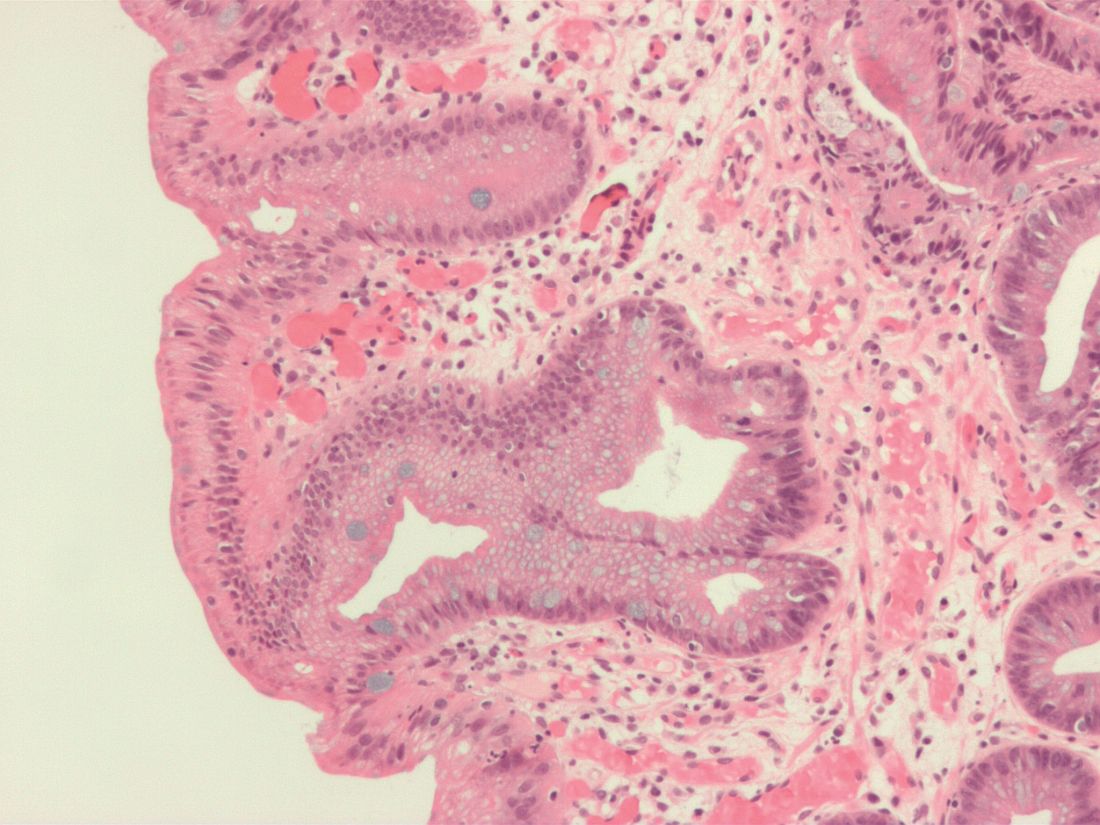
New cryoballoon treatment eradicated esophageal squamous cell neoplasias
For the first time, endoscopists have used focal cryoballoon ablation to eradicate early esophageal squamous cell neoplasias, including high-grade lesions in treatment-experienced patients.
A single session of treatment with the novel, portable nitrous oxide system (C2 Therapeutics, Redwood City, Calif.) produced complete pathologic and endoscopic responses at 3 months in 8 of 10 patients, reported Marcia Irene Canto, MD, of Johns Hopkins University in Baltimore, with her associates. Two to five sessions produced complete responses in the other two patients, and all patients were disease-free at their last visit (median follow-up, 10.7 months; interquartile range, 4-14 months). The study was published online July 15 in Gastrointestinal Endoscopy (2017. doi: 10.1016/j.gie.2017.07.013).
Standard treatments for esophageal squamous cell neoplasia have drawbacks. Submucosal dissection can cause bleeding, perforation, and stenosis, and radiofrequency ablation (RFA) is associated with stricture. To test an alternative, the researchers used a portable, hand-held, battery-powered cryoballoon ablation system that successfully eradicated Barrett’s esophagus lesions in two prior studies. The machine contains liquid nitrous oxide, which turns to gas when released into a balloon catheter in the endoscope. The gas inflates the balloon, enabling the endoscopist to see through it to the neoplastic mucosa, which is pretreated with Lugol chromoendoscopy. The endoscopist then cryoablates the target lesion across the balloon wall. Unlike cryospray, the entire dose of cryogen hits the lesion, the investigators noted. Also, the gas is automatically evacuated without the need for suctioning or decompression, and the treatment process does not require heavy gas tanks or repeated freeze-thaw cycles.
In this study, all 10 patients achieved complete endoscopic and pathologic responses at 3 months. Seven patients were followed for 1 year, and none showed biopsy evidence of neoplastic progression. In contrast, a prospective trial of circumferential radiofrequency ablation without endoscopic mucosal resection or endoscopic submucosal dissection reported a 12-month rate of complete response of 84%, the researchers noted (Endoscopy. 2015;47:398-408). There were no serious treatment-related adverse events. Pain was usually self-limited, although one patient required 2 days of narcotics. Two patients developed symptomatic strictures 6-8 weeks after treatment that resolved with balloon dilation. Both of these patients had undergone extensive multifocal ablation. “Circumferential treatment of ESCN [esophageal squamous cell neoplasia] remains problematic for all endoscopic ablative therapies, with a high stricture rate of 20% in our small pilot study of cryoablation and 20%-23% with RFA,” the investigators wrote. “The risk for postablation stricture appears to be related to a long length of ESCN. Optimizing the cryogen dose, technique, and decrease in Lugol’s solution concentration might alter the incidence of postablation strictures for ablative treatments.”
Study participants were 55-90 years old, and six were female. Four were treatment naive, and six had progressed despite endoscopic mucosal resection, RFA, spray cryotherapy, argon plasma coagulation, or multiple therapies. Seven patients had high-grade lesions, two had low-grade lesions, and one had early esophageal squamous cell carcinoma. Most lesions were distally located, but two were in the middle esophagus and one was proximal. The largest lesion measured 4 cm and the maximum length of all lesions in a single patient was 10 cm. Patients did not have visible esophageal masses or evidence of advanced or metastatic disease.
“Comparative effectiveness trials might eventually determine the role of cryoablation in the treatment of early ESCN,” the researchers concluded. An open-label, nonrandomized clinical trial of the balloon cryotherapy device is underway in China (NCT02605759). The article did not list funding sources or disclosures.
For the first time, endoscopists have used focal cryoballoon ablation to eradicate early esophageal squamous cell neoplasias, including high-grade lesions in treatment-experienced patients.
A single session of treatment with the novel, portable nitrous oxide system (C2 Therapeutics, Redwood City, Calif.) produced complete pathologic and endoscopic responses at 3 months in 8 of 10 patients, reported Marcia Irene Canto, MD, of Johns Hopkins University in Baltimore, with her associates. Two to five sessions produced complete responses in the other two patients, and all patients were disease-free at their last visit (median follow-up, 10.7 months; interquartile range, 4-14 months). The study was published online July 15 in Gastrointestinal Endoscopy (2017. doi: 10.1016/j.gie.2017.07.013).
Standard treatments for esophageal squamous cell neoplasia have drawbacks. Submucosal dissection can cause bleeding, perforation, and stenosis, and radiofrequency ablation (RFA) is associated with stricture. To test an alternative, the researchers used a portable, hand-held, battery-powered cryoballoon ablation system that successfully eradicated Barrett’s esophagus lesions in two prior studies. The machine contains liquid nitrous oxide, which turns to gas when released into a balloon catheter in the endoscope. The gas inflates the balloon, enabling the endoscopist to see through it to the neoplastic mucosa, which is pretreated with Lugol chromoendoscopy. The endoscopist then cryoablates the target lesion across the balloon wall. Unlike cryospray, the entire dose of cryogen hits the lesion, the investigators noted. Also, the gas is automatically evacuated without the need for suctioning or decompression, and the treatment process does not require heavy gas tanks or repeated freeze-thaw cycles.
In this study, all 10 patients achieved complete endoscopic and pathologic responses at 3 months. Seven patients were followed for 1 year, and none showed biopsy evidence of neoplastic progression. In contrast, a prospective trial of circumferential radiofrequency ablation without endoscopic mucosal resection or endoscopic submucosal dissection reported a 12-month rate of complete response of 84%, the researchers noted (Endoscopy. 2015;47:398-408). There were no serious treatment-related adverse events. Pain was usually self-limited, although one patient required 2 days of narcotics. Two patients developed symptomatic strictures 6-8 weeks after treatment that resolved with balloon dilation. Both of these patients had undergone extensive multifocal ablation. “Circumferential treatment of ESCN [esophageal squamous cell neoplasia] remains problematic for all endoscopic ablative therapies, with a high stricture rate of 20% in our small pilot study of cryoablation and 20%-23% with RFA,” the investigators wrote. “The risk for postablation stricture appears to be related to a long length of ESCN. Optimizing the cryogen dose, technique, and decrease in Lugol’s solution concentration might alter the incidence of postablation strictures for ablative treatments.”
Study participants were 55-90 years old, and six were female. Four were treatment naive, and six had progressed despite endoscopic mucosal resection, RFA, spray cryotherapy, argon plasma coagulation, or multiple therapies. Seven patients had high-grade lesions, two had low-grade lesions, and one had early esophageal squamous cell carcinoma. Most lesions were distally located, but two were in the middle esophagus and one was proximal. The largest lesion measured 4 cm and the maximum length of all lesions in a single patient was 10 cm. Patients did not have visible esophageal masses or evidence of advanced or metastatic disease.
“Comparative effectiveness trials might eventually determine the role of cryoablation in the treatment of early ESCN,” the researchers concluded. An open-label, nonrandomized clinical trial of the balloon cryotherapy device is underway in China (NCT02605759). The article did not list funding sources or disclosures.
For the first time, endoscopists have used focal cryoballoon ablation to eradicate early esophageal squamous cell neoplasias, including high-grade lesions in treatment-experienced patients.
A single session of treatment with the novel, portable nitrous oxide system (C2 Therapeutics, Redwood City, Calif.) produced complete pathologic and endoscopic responses at 3 months in 8 of 10 patients, reported Marcia Irene Canto, MD, of Johns Hopkins University in Baltimore, with her associates. Two to five sessions produced complete responses in the other two patients, and all patients were disease-free at their last visit (median follow-up, 10.7 months; interquartile range, 4-14 months). The study was published online July 15 in Gastrointestinal Endoscopy (2017. doi: 10.1016/j.gie.2017.07.013).
Standard treatments for esophageal squamous cell neoplasia have drawbacks. Submucosal dissection can cause bleeding, perforation, and stenosis, and radiofrequency ablation (RFA) is associated with stricture. To test an alternative, the researchers used a portable, hand-held, battery-powered cryoballoon ablation system that successfully eradicated Barrett’s esophagus lesions in two prior studies. The machine contains liquid nitrous oxide, which turns to gas when released into a balloon catheter in the endoscope. The gas inflates the balloon, enabling the endoscopist to see through it to the neoplastic mucosa, which is pretreated with Lugol chromoendoscopy. The endoscopist then cryoablates the target lesion across the balloon wall. Unlike cryospray, the entire dose of cryogen hits the lesion, the investigators noted. Also, the gas is automatically evacuated without the need for suctioning or decompression, and the treatment process does not require heavy gas tanks or repeated freeze-thaw cycles.
In this study, all 10 patients achieved complete endoscopic and pathologic responses at 3 months. Seven patients were followed for 1 year, and none showed biopsy evidence of neoplastic progression. In contrast, a prospective trial of circumferential radiofrequency ablation without endoscopic mucosal resection or endoscopic submucosal dissection reported a 12-month rate of complete response of 84%, the researchers noted (Endoscopy. 2015;47:398-408). There were no serious treatment-related adverse events. Pain was usually self-limited, although one patient required 2 days of narcotics. Two patients developed symptomatic strictures 6-8 weeks after treatment that resolved with balloon dilation. Both of these patients had undergone extensive multifocal ablation. “Circumferential treatment of ESCN [esophageal squamous cell neoplasia] remains problematic for all endoscopic ablative therapies, with a high stricture rate of 20% in our small pilot study of cryoablation and 20%-23% with RFA,” the investigators wrote. “The risk for postablation stricture appears to be related to a long length of ESCN. Optimizing the cryogen dose, technique, and decrease in Lugol’s solution concentration might alter the incidence of postablation strictures for ablative treatments.”
Study participants were 55-90 years old, and six were female. Four were treatment naive, and six had progressed despite endoscopic mucosal resection, RFA, spray cryotherapy, argon plasma coagulation, or multiple therapies. Seven patients had high-grade lesions, two had low-grade lesions, and one had early esophageal squamous cell carcinoma. Most lesions were distally located, but two were in the middle esophagus and one was proximal. The largest lesion measured 4 cm and the maximum length of all lesions in a single patient was 10 cm. Patients did not have visible esophageal masses or evidence of advanced or metastatic disease.
“Comparative effectiveness trials might eventually determine the role of cryoablation in the treatment of early ESCN,” the researchers concluded. An open-label, nonrandomized clinical trial of the balloon cryotherapy device is underway in China (NCT02605759). The article did not list funding sources or disclosures.
FROM GIE
Key clinical point: In a first-in-kind study, endoscopists used focal cryoballoon ablation to eradicate early esophageal squamous cell neoplasias.
Major finding: All 10 patients had a complete response at 3 months. All remained disease free at last follow-up (median, 10.7 months).
Data source: A multicenter study of 10 patients with early high-grade or low-grade esophageal squamous cell neoplasias or early squamous cell carcinomas.
Disclosures: The article did not list funding sources or disclosures.
Genetic predisposition to hypercalcemia linked to CAD, MI
in a large mendelian randomization study published online July 25 in JAMA.
Each 0.5-mg rise in genetically predicted serum calcium concentration increased the odds of coronary artery disease (CAD) and myocardial infarction by about 25%, reported Susanna C. Larsson, Ph.D., of Karolinska Institutet in Stockholm, Sweden, and her associates. It remains unclear whether short- or medium-term calcium supplementation also increases the risk of these outcomes, they added.
Observational studies have linked high serum calcium with cardiovascular disease, but such studies are subject to confounding, the researchers noted. Randomized trials indicate that calcium supplementation might contribute to MI, but the trials are not designed to quantify long-term risks. Therefore, the investigators evaluated a proxy for lifelong hypercalcemia – six single nucleotide polymorphisms (SNPs) that have been linked to high serum calcium, but not to other CAD risk factors such as type 2 diabetes, fasting glucose and insulin levels, body mass index, waist-to-hip ratio, major lipids, or hypertension (JAMA. 2017 Jul 25;318[4]:371-80. doi: 10.1001/jama.2017.8981).
To examine how these SNPs affect the risk of CAD and MI, the researchers analyzed summary statistics for 184,305 individuals from a meta-analysis of CAD genome-wide association studies (Nat Genet. 2015;47:1121-30), including 60,801 cases (of whom about 70% also had MI) and 123,504 controls.
Together, these six SNPs explained about 0.8% of variations in serum calcium levels. Each 0.5-mg/dL (about one standard deviation) increase in genetically predicted serum calcium level significantly increased the risk of CAD (odds ratio, 1.25; 95% confidence interval, 1.08-1.45; P = .003) and MI (OR, 1.24; 95% CI, 1.05-1.46; P = .009). The genetic variant rs1801725 exerted the greatest effect on serum calcium levels, the investigators noted. This SNP affects the CASR gene, which encodes a calcium-sensing receptor that “plays a key role in calcium homeostasis.” However, four of the other five variants also had odds ratios above 1.0, and three had odds ratios above 1.25. A sensitivity analysis that excluded the CASR variant generated an identical odds ratio, although the confidence interval was wider. Studies of other risk factors for CAD have yielded odds ratios between 1.3 (triglyceride levels) and 1.7 (LDL cholesterol levels), the researchers noted.
A link between calcium supplementation and MI remains debatable. However, supplementation can lead to hypercalcemia and greater formation of insoluble calciprotein particles, the investigators said. Coronary artery disease might result from downstream effects on vascular calcification, vascular cells, blood coagulation pathways, or gene expression, but such mechanisms need more study, they added.
This analysis included men and women from the United States, Canada, the United Kingdom, Germany, Sweden, Ireland, the Netherlands, Finland, Iceland, Italy, Estonia, Lebanon, China, Korea, India, Pakistan and Greece. Participants tended to be men in their 50s and 60s, but more than half of studies lacked data on age and sex. Nearly all participants were of white European ancestry.
Karolinska Institutet supported Dr. Larsson. The investigators reported having no relevant conflicts of interest.
in a large mendelian randomization study published online July 25 in JAMA.
Each 0.5-mg rise in genetically predicted serum calcium concentration increased the odds of coronary artery disease (CAD) and myocardial infarction by about 25%, reported Susanna C. Larsson, Ph.D., of Karolinska Institutet in Stockholm, Sweden, and her associates. It remains unclear whether short- or medium-term calcium supplementation also increases the risk of these outcomes, they added.
Observational studies have linked high serum calcium with cardiovascular disease, but such studies are subject to confounding, the researchers noted. Randomized trials indicate that calcium supplementation might contribute to MI, but the trials are not designed to quantify long-term risks. Therefore, the investigators evaluated a proxy for lifelong hypercalcemia – six single nucleotide polymorphisms (SNPs) that have been linked to high serum calcium, but not to other CAD risk factors such as type 2 diabetes, fasting glucose and insulin levels, body mass index, waist-to-hip ratio, major lipids, or hypertension (JAMA. 2017 Jul 25;318[4]:371-80. doi: 10.1001/jama.2017.8981).
To examine how these SNPs affect the risk of CAD and MI, the researchers analyzed summary statistics for 184,305 individuals from a meta-analysis of CAD genome-wide association studies (Nat Genet. 2015;47:1121-30), including 60,801 cases (of whom about 70% also had MI) and 123,504 controls.
Together, these six SNPs explained about 0.8% of variations in serum calcium levels. Each 0.5-mg/dL (about one standard deviation) increase in genetically predicted serum calcium level significantly increased the risk of CAD (odds ratio, 1.25; 95% confidence interval, 1.08-1.45; P = .003) and MI (OR, 1.24; 95% CI, 1.05-1.46; P = .009). The genetic variant rs1801725 exerted the greatest effect on serum calcium levels, the investigators noted. This SNP affects the CASR gene, which encodes a calcium-sensing receptor that “plays a key role in calcium homeostasis.” However, four of the other five variants also had odds ratios above 1.0, and three had odds ratios above 1.25. A sensitivity analysis that excluded the CASR variant generated an identical odds ratio, although the confidence interval was wider. Studies of other risk factors for CAD have yielded odds ratios between 1.3 (triglyceride levels) and 1.7 (LDL cholesterol levels), the researchers noted.
A link between calcium supplementation and MI remains debatable. However, supplementation can lead to hypercalcemia and greater formation of insoluble calciprotein particles, the investigators said. Coronary artery disease might result from downstream effects on vascular calcification, vascular cells, blood coagulation pathways, or gene expression, but such mechanisms need more study, they added.
This analysis included men and women from the United States, Canada, the United Kingdom, Germany, Sweden, Ireland, the Netherlands, Finland, Iceland, Italy, Estonia, Lebanon, China, Korea, India, Pakistan and Greece. Participants tended to be men in their 50s and 60s, but more than half of studies lacked data on age and sex. Nearly all participants were of white European ancestry.
Karolinska Institutet supported Dr. Larsson. The investigators reported having no relevant conflicts of interest.
in a large mendelian randomization study published online July 25 in JAMA.
Each 0.5-mg rise in genetically predicted serum calcium concentration increased the odds of coronary artery disease (CAD) and myocardial infarction by about 25%, reported Susanna C. Larsson, Ph.D., of Karolinska Institutet in Stockholm, Sweden, and her associates. It remains unclear whether short- or medium-term calcium supplementation also increases the risk of these outcomes, they added.
Observational studies have linked high serum calcium with cardiovascular disease, but such studies are subject to confounding, the researchers noted. Randomized trials indicate that calcium supplementation might contribute to MI, but the trials are not designed to quantify long-term risks. Therefore, the investigators evaluated a proxy for lifelong hypercalcemia – six single nucleotide polymorphisms (SNPs) that have been linked to high serum calcium, but not to other CAD risk factors such as type 2 diabetes, fasting glucose and insulin levels, body mass index, waist-to-hip ratio, major lipids, or hypertension (JAMA. 2017 Jul 25;318[4]:371-80. doi: 10.1001/jama.2017.8981).
To examine how these SNPs affect the risk of CAD and MI, the researchers analyzed summary statistics for 184,305 individuals from a meta-analysis of CAD genome-wide association studies (Nat Genet. 2015;47:1121-30), including 60,801 cases (of whom about 70% also had MI) and 123,504 controls.
Together, these six SNPs explained about 0.8% of variations in serum calcium levels. Each 0.5-mg/dL (about one standard deviation) increase in genetically predicted serum calcium level significantly increased the risk of CAD (odds ratio, 1.25; 95% confidence interval, 1.08-1.45; P = .003) and MI (OR, 1.24; 95% CI, 1.05-1.46; P = .009). The genetic variant rs1801725 exerted the greatest effect on serum calcium levels, the investigators noted. This SNP affects the CASR gene, which encodes a calcium-sensing receptor that “plays a key role in calcium homeostasis.” However, four of the other five variants also had odds ratios above 1.0, and three had odds ratios above 1.25. A sensitivity analysis that excluded the CASR variant generated an identical odds ratio, although the confidence interval was wider. Studies of other risk factors for CAD have yielded odds ratios between 1.3 (triglyceride levels) and 1.7 (LDL cholesterol levels), the researchers noted.
A link between calcium supplementation and MI remains debatable. However, supplementation can lead to hypercalcemia and greater formation of insoluble calciprotein particles, the investigators said. Coronary artery disease might result from downstream effects on vascular calcification, vascular cells, blood coagulation pathways, or gene expression, but such mechanisms need more study, they added.
This analysis included men and women from the United States, Canada, the United Kingdom, Germany, Sweden, Ireland, the Netherlands, Finland, Iceland, Italy, Estonia, Lebanon, China, Korea, India, Pakistan and Greece. Participants tended to be men in their 50s and 60s, but more than half of studies lacked data on age and sex. Nearly all participants were of white European ancestry.
Karolinska Institutet supported Dr. Larsson. The investigators reported having no relevant conflicts of interest.
FROM JAMA
Key clinical point: Genetic predisposition to higher serum calcium levels was significantly associated with coronary artery disease and myocardial infarction.
Major finding: Each 0.5-mg per dL rise in serum calcium increased the odds of these outcomes by about 25% (odds ratios, 1.25 and 1.24, respectively).
Data source: A mendelian randomization study of 60,801 cases of coronary artery disease, 123,504 controls, and six single nucleotide polymorphisms linked to serum calcium but not to other risk factors for coronary artery disease.
Disclosures: Karolinska Institutet supported Dr. Larsson. The investigators reported having no relevant conflicts of interest.
Senate parliamentarian upends GOP hopes for health bill
The official rules keeper in the Senate tossed a bucket of cold water July 21 on the Senate Republican health bill by advising that major parts of the bill cannot be passed with a simple majority, but rather would require 60 votes. Republicans hold only 52 seats in the Senate.
Senate Parliamentarian Elizabeth MacDonough said that a super-majority is needed for the temporary defunding of Planned Parenthood, abortion coverage restrictions to health plans purchased with tax credits, and the requirement that people with breaks in coverage wait 6 months before they can purchase new plans.
The list was released by Democrats on the Senate Budget Committee and later confirmed by a spokesman for the committee Republicans. It is the result of what is called the “Byrd Bath,” a process in which the parliamentarian hears arguments from Democrats and Republicans and then advises on which provisions comply with the Byrd Rule. That rule requires that only matters directly pertaining to the federal budget are included. The rule is named for former Senate Majority Leader Robert Byrd (D-W.Va.), who first wrote it.
Senate Republicans were quick to point out that the document is “guidance” that they can use to try to rewrite impermissible language. The guidance “will help inform action on the legislation going forward,” said a spokesman for Senate Budget Committee Chairman Mike Enzi (R-Wyo.).
Among the other provisions that the parliamentarian has advised should require 60 votes are ones that would eliminate Medicaid requirements to provide 10 “essential health benefits.” Also on the list is a provision to repeal a requirement that insurers spend a minimum amount of each premium dollar on direct medical services, rather than administration or profits.
The determination also pertains to a part of the bill that would continue payments for “cost-sharing subsidies” to insurers for 2 more years. Those subsidies help lower-income people afford out-of-pocket costs such as deductibles. The parliamentarian said that duplicated existing law.
Ms. MacDonough also said that a provision in the House version of the bill that pertains directly to New York violates the Byrd Rule. That measure would change the way the state collects money for Medicaid. That could suggest efforts by Senate Majority Leader Mitch McConnell (R-Ky.) to offer state-specific changes to gain support for the bill might meet the same fate.
Minority Leader Chuck Schumer (D-N.Y.) said that decision could have “the greatest effect on Republicans’ ability to pass this bill.” He predicted it would “tie the majority leader’s hands as he tries to win over reluctant Republicans.”
Some of the provisions that didn’t pass muster with Ms. MacDonough were key to getting the bill through the House. And if they are dropped, it might make it difficult for the House to approve a final version of the bill.
Not all the decisions went the Democrats’ way. Ms. MacDonough found that only a simple majority is needed for language allowing states to impose work requirements for Medicaid recipients. She also said that a provision that will ban abortions if the services are paid through a new fund provided to states would be allowed. That’s because that fund will be governed by existing rules that already ban abortion in most cases.
A few provisions remain under review, according to the list. Those include allowing states to waive a long list of insurance protections, including the ACA’s essential health benefits and preexisting coverage guarantees. Also still under review is language allowing small businesses to pool together to purchase insurance as well as a provision changing requirements related to how much more insurers can charge older adults.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
The official rules keeper in the Senate tossed a bucket of cold water July 21 on the Senate Republican health bill by advising that major parts of the bill cannot be passed with a simple majority, but rather would require 60 votes. Republicans hold only 52 seats in the Senate.
Senate Parliamentarian Elizabeth MacDonough said that a super-majority is needed for the temporary defunding of Planned Parenthood, abortion coverage restrictions to health plans purchased with tax credits, and the requirement that people with breaks in coverage wait 6 months before they can purchase new plans.
The list was released by Democrats on the Senate Budget Committee and later confirmed by a spokesman for the committee Republicans. It is the result of what is called the “Byrd Bath,” a process in which the parliamentarian hears arguments from Democrats and Republicans and then advises on which provisions comply with the Byrd Rule. That rule requires that only matters directly pertaining to the federal budget are included. The rule is named for former Senate Majority Leader Robert Byrd (D-W.Va.), who first wrote it.
Senate Republicans were quick to point out that the document is “guidance” that they can use to try to rewrite impermissible language. The guidance “will help inform action on the legislation going forward,” said a spokesman for Senate Budget Committee Chairman Mike Enzi (R-Wyo.).
Among the other provisions that the parliamentarian has advised should require 60 votes are ones that would eliminate Medicaid requirements to provide 10 “essential health benefits.” Also on the list is a provision to repeal a requirement that insurers spend a minimum amount of each premium dollar on direct medical services, rather than administration or profits.
The determination also pertains to a part of the bill that would continue payments for “cost-sharing subsidies” to insurers for 2 more years. Those subsidies help lower-income people afford out-of-pocket costs such as deductibles. The parliamentarian said that duplicated existing law.
Ms. MacDonough also said that a provision in the House version of the bill that pertains directly to New York violates the Byrd Rule. That measure would change the way the state collects money for Medicaid. That could suggest efforts by Senate Majority Leader Mitch McConnell (R-Ky.) to offer state-specific changes to gain support for the bill might meet the same fate.
Minority Leader Chuck Schumer (D-N.Y.) said that decision could have “the greatest effect on Republicans’ ability to pass this bill.” He predicted it would “tie the majority leader’s hands as he tries to win over reluctant Republicans.”
Some of the provisions that didn’t pass muster with Ms. MacDonough were key to getting the bill through the House. And if they are dropped, it might make it difficult for the House to approve a final version of the bill.
Not all the decisions went the Democrats’ way. Ms. MacDonough found that only a simple majority is needed for language allowing states to impose work requirements for Medicaid recipients. She also said that a provision that will ban abortions if the services are paid through a new fund provided to states would be allowed. That’s because that fund will be governed by existing rules that already ban abortion in most cases.
A few provisions remain under review, according to the list. Those include allowing states to waive a long list of insurance protections, including the ACA’s essential health benefits and preexisting coverage guarantees. Also still under review is language allowing small businesses to pool together to purchase insurance as well as a provision changing requirements related to how much more insurers can charge older adults.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
The official rules keeper in the Senate tossed a bucket of cold water July 21 on the Senate Republican health bill by advising that major parts of the bill cannot be passed with a simple majority, but rather would require 60 votes. Republicans hold only 52 seats in the Senate.
Senate Parliamentarian Elizabeth MacDonough said that a super-majority is needed for the temporary defunding of Planned Parenthood, abortion coverage restrictions to health plans purchased with tax credits, and the requirement that people with breaks in coverage wait 6 months before they can purchase new plans.
The list was released by Democrats on the Senate Budget Committee and later confirmed by a spokesman for the committee Republicans. It is the result of what is called the “Byrd Bath,” a process in which the parliamentarian hears arguments from Democrats and Republicans and then advises on which provisions comply with the Byrd Rule. That rule requires that only matters directly pertaining to the federal budget are included. The rule is named for former Senate Majority Leader Robert Byrd (D-W.Va.), who first wrote it.
Senate Republicans were quick to point out that the document is “guidance” that they can use to try to rewrite impermissible language. The guidance “will help inform action on the legislation going forward,” said a spokesman for Senate Budget Committee Chairman Mike Enzi (R-Wyo.).
Among the other provisions that the parliamentarian has advised should require 60 votes are ones that would eliminate Medicaid requirements to provide 10 “essential health benefits.” Also on the list is a provision to repeal a requirement that insurers spend a minimum amount of each premium dollar on direct medical services, rather than administration or profits.
The determination also pertains to a part of the bill that would continue payments for “cost-sharing subsidies” to insurers for 2 more years. Those subsidies help lower-income people afford out-of-pocket costs such as deductibles. The parliamentarian said that duplicated existing law.
Ms. MacDonough also said that a provision in the House version of the bill that pertains directly to New York violates the Byrd Rule. That measure would change the way the state collects money for Medicaid. That could suggest efforts by Senate Majority Leader Mitch McConnell (R-Ky.) to offer state-specific changes to gain support for the bill might meet the same fate.
Minority Leader Chuck Schumer (D-N.Y.) said that decision could have “the greatest effect on Republicans’ ability to pass this bill.” He predicted it would “tie the majority leader’s hands as he tries to win over reluctant Republicans.”
Some of the provisions that didn’t pass muster with Ms. MacDonough were key to getting the bill through the House. And if they are dropped, it might make it difficult for the House to approve a final version of the bill.
Not all the decisions went the Democrats’ way. Ms. MacDonough found that only a simple majority is needed for language allowing states to impose work requirements for Medicaid recipients. She also said that a provision that will ban abortions if the services are paid through a new fund provided to states would be allowed. That’s because that fund will be governed by existing rules that already ban abortion in most cases.
A few provisions remain under review, according to the list. Those include allowing states to waive a long list of insurance protections, including the ACA’s essential health benefits and preexisting coverage guarantees. Also still under review is language allowing small businesses to pool together to purchase insurance as well as a provision changing requirements related to how much more insurers can charge older adults.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
5 ways White House can use its muscle to undercut Obamacare
President Donald Trump has vowed to “let Obamacare fail,” after legislative efforts to undo the Affordable Care Act have stalled.
He and congressional Republicans have repeatedly portrayed the Affordable Care Act insurance marketplaces, also known as exchanges, as being in a “death spiral.” But independent analyses have concluded that such spontaneous disintegration isn’t happening.
In a number of ways, the Trump administration’s policies are pushing Obamacare into the vortex.
Reports from Standard & Poor’s, the Congressional Budget Office and the Kaiser Family Foundation all suggest that the exchanges – where people can shop for coverage, often with the help of a government subsidy – are stabilizing. (Kaiser Health News is an editorially independent program of the foundation.)
“The administration has a lot of power to undermine the markets and make them dysfunctional,” said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms, who specializes in private insurance markets.
Here’s a look at five ways the White House is already working to weaken the health law, and what that means for consumers.
‘Cost-sharing reductions’
Under the ACA, when someone’s income falls between 100% and 250% of the federal poverty level – up to about $29,000 for an individual or around $61,000 for a family of four – marketplace carriers must offer a plan with “cost-sharing reductions” (CSRs) that reduce consumers’ out-of-pocket expenses.
Reducing cost-sharing – generally copayments and deductibles – makes plans more expensive for the insurers. The Obama administration used its rule-making power to set up direct payments to carriers to help offset this burden. The Trump White House has inherited that responsibility but also has the power to end the payment program.
The nonpartisan Congressional Budget Office estimated CSR subsidies in 2017 would total about $7 billion. Without that money, analysts say, more insurers might choose to exit, limiting options for consumers, and letting the insurers who remain charge higher prices.
Trump has been committedly noncommittal, publicly indicating he would like to halt the subsidies, but so far – on a month-to-month basis – letting them continue.
The uncertainty makes insurance companies skittish about participating, analysts noted. It’s also one reason some plans say they have had to increase their rates, noted Charles Gaba, a Michigan-based blogger who tracks ACA sign-ups. For instance: When filing plans for the 2018 marketplace, carriers on average raised premiums by about 34% – with about 20 points stemming from CSR uncertainty, Mr. Gaba said, based on an analysis of 21 states’ initial rate filings. Dropping the subsidies altogether would be even more damaging.
Weaken the mandate
The White House has already signaled it does not want to enforce the individual mandate – the health law’s requirement that all people have coverage. And administration officials have repeated that position.
Meanwhile, in January, it issued an executive order that encouraged U.S. agencies to grant exemptions and waive or defer health law provisions that could put financial strain on companies or individuals – which could also be applied to the individual mandate.
For 2016 tax returns, though, the Internal Revenue Service continued to impose a financial penalty on people who didn’t have health insurance and who didn’t qualify for an exemption.
But enforcement may be waning. This year, the IRS was supposed to reject tax returns if people didn’t indicate whether they had coverage, flagging them for a potential penalty. Instead, it continued processing them, citing Trump’s executive order.
If the IRS has already processed any tax refunds for consumers, then they “don’t have much leverage” when attempting to collect the mandate fee, said Timothy Jost, emeritus law professor at Washington and Lee University in Virginia and an expert on health reform.
Enforcement of the mandate, economists note, is crucial to ensuring that enough healthy people buy coverage to balance the costs of sicker beneficiaries.
But even with the mandate in effect, the efforts to defang it bring confusion.
“A lot of people believe the Trump administration is not enforcing it,” Mr. Jost said.
As a result, healthy people may become less likely to buy insurance, even as sick ones continue seeking it. That means higher prices, and a shakier pool.
“If they don’t think they’re going to get healthy people in the risk pool, they’re going to increase their rates further to protect themselves,” Mr. Jost said. “And as they raise their rates further to protect themselves, people … start to drop out.”
Thus, the president’s position on the mandate is leaving insurance carriers and commissioners “apprehensive,” noted Mike Kreidler, Washington state’s insurance commissioner.
A bare market
Skittishness on the part of insurers could lead them to drop out of some marketplaces, leaving consumers in some areas with few or no choices. Those “bare markets” are possible under even stable circumstances – and preventing them requires active federal involvement.
Under the Obama administration, high-level officials were “on the phone daily with insurance company executives … trying to get them to participate,” Ms. Corlette said. “It was very much an all-hands-on-deck, ‘we’re going to make it work for you guys’ kind of communication.”
And so far Trump’s Department of Health and Human Services doesn’t appear to be emphasizing this kind of essential outreach, both Ms. Corlette and Mr. Jost suggested. A few months ago, Mr. Kreidler agreed, HHS staffers appeared interested in helping states fill their bare counties – but that support has since dwindled.
“This may be sort of under the radar, but it can have real, lasting effects” for consumer choice, Ms. Corlette said.
All quiet on the enrollment front
The administration could further undermine the marketplace by dropping outreach to consumers. It’s already a shorter enrollment period this year – spanning 6 weeks instead of 3 months, from Nov. 1 to Dec. 15 – though that change was already slated to eventually take effect.
That shorter period means people may miss the memo on signing up – or at least need an extra push, Ms. Corlette said. And that’s another way the administration could undermine the marketplaces: simply choosing not to advertise them.
Last sign-up season, HHS stopped open enrollment advertising in January, pulling ads a few days before the period ended. Enrollment dropped compared with previous years, Mr. Jost and Mr. Gaba noted, with young, healthy people being more likely not to buy coverage.
The administration also just stopped funding federal contractors that supported efforts by community groups and other organizations in some of the nation’s largest cities to sign up people.
Dropping advertising, shortening open enrollment, or simply scaling back on technical maintenance for the marketplace website could all have significant impact, Ms. Corlette said. People who are sick and need insurance will likely seek it out, but those who are healthier – for whom health insurance is a less pressing priority – could miss the boat.
Again, Mr. Jost said, that affects insurer participation.
“Insurance is a product that needs to be sold,” he said. “If the insurers believe they’re not going to get any help at all in marketing their product,” he added, fewer will want to enter the marketplace.
Word of (bad) mouth
HHS has taken an active role in criticizing the health law – pushing press releases and videos that argue it has helped more than hurt. That strategy could do a lot of harm, experts said.
If consumers keep hearing the law is failing, Mr. Jost noted, some will ultimately believe it, buying coverage only if they need it and thereby skewing the insurance risk pool.
Perceived hostility also has an effect on insurers, steering them away from marketplace participation.
“When you undermine confidence in the marketplace, you don’t need a Ph.D. in economics to know it’s not good long term,” Ms. Corlette said.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
President Donald Trump has vowed to “let Obamacare fail,” after legislative efforts to undo the Affordable Care Act have stalled.
He and congressional Republicans have repeatedly portrayed the Affordable Care Act insurance marketplaces, also known as exchanges, as being in a “death spiral.” But independent analyses have concluded that such spontaneous disintegration isn’t happening.
In a number of ways, the Trump administration’s policies are pushing Obamacare into the vortex.
Reports from Standard & Poor’s, the Congressional Budget Office and the Kaiser Family Foundation all suggest that the exchanges – where people can shop for coverage, often with the help of a government subsidy – are stabilizing. (Kaiser Health News is an editorially independent program of the foundation.)
“The administration has a lot of power to undermine the markets and make them dysfunctional,” said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms, who specializes in private insurance markets.
Here’s a look at five ways the White House is already working to weaken the health law, and what that means for consumers.
‘Cost-sharing reductions’
Under the ACA, when someone’s income falls between 100% and 250% of the federal poverty level – up to about $29,000 for an individual or around $61,000 for a family of four – marketplace carriers must offer a plan with “cost-sharing reductions” (CSRs) that reduce consumers’ out-of-pocket expenses.
Reducing cost-sharing – generally copayments and deductibles – makes plans more expensive for the insurers. The Obama administration used its rule-making power to set up direct payments to carriers to help offset this burden. The Trump White House has inherited that responsibility but also has the power to end the payment program.
The nonpartisan Congressional Budget Office estimated CSR subsidies in 2017 would total about $7 billion. Without that money, analysts say, more insurers might choose to exit, limiting options for consumers, and letting the insurers who remain charge higher prices.
Trump has been committedly noncommittal, publicly indicating he would like to halt the subsidies, but so far – on a month-to-month basis – letting them continue.
The uncertainty makes insurance companies skittish about participating, analysts noted. It’s also one reason some plans say they have had to increase their rates, noted Charles Gaba, a Michigan-based blogger who tracks ACA sign-ups. For instance: When filing plans for the 2018 marketplace, carriers on average raised premiums by about 34% – with about 20 points stemming from CSR uncertainty, Mr. Gaba said, based on an analysis of 21 states’ initial rate filings. Dropping the subsidies altogether would be even more damaging.
Weaken the mandate
The White House has already signaled it does not want to enforce the individual mandate – the health law’s requirement that all people have coverage. And administration officials have repeated that position.
Meanwhile, in January, it issued an executive order that encouraged U.S. agencies to grant exemptions and waive or defer health law provisions that could put financial strain on companies or individuals – which could also be applied to the individual mandate.
For 2016 tax returns, though, the Internal Revenue Service continued to impose a financial penalty on people who didn’t have health insurance and who didn’t qualify for an exemption.
But enforcement may be waning. This year, the IRS was supposed to reject tax returns if people didn’t indicate whether they had coverage, flagging them for a potential penalty. Instead, it continued processing them, citing Trump’s executive order.
If the IRS has already processed any tax refunds for consumers, then they “don’t have much leverage” when attempting to collect the mandate fee, said Timothy Jost, emeritus law professor at Washington and Lee University in Virginia and an expert on health reform.
Enforcement of the mandate, economists note, is crucial to ensuring that enough healthy people buy coverage to balance the costs of sicker beneficiaries.
But even with the mandate in effect, the efforts to defang it bring confusion.
“A lot of people believe the Trump administration is not enforcing it,” Mr. Jost said.
As a result, healthy people may become less likely to buy insurance, even as sick ones continue seeking it. That means higher prices, and a shakier pool.
“If they don’t think they’re going to get healthy people in the risk pool, they’re going to increase their rates further to protect themselves,” Mr. Jost said. “And as they raise their rates further to protect themselves, people … start to drop out.”
Thus, the president’s position on the mandate is leaving insurance carriers and commissioners “apprehensive,” noted Mike Kreidler, Washington state’s insurance commissioner.
A bare market
Skittishness on the part of insurers could lead them to drop out of some marketplaces, leaving consumers in some areas with few or no choices. Those “bare markets” are possible under even stable circumstances – and preventing them requires active federal involvement.
Under the Obama administration, high-level officials were “on the phone daily with insurance company executives … trying to get them to participate,” Ms. Corlette said. “It was very much an all-hands-on-deck, ‘we’re going to make it work for you guys’ kind of communication.”
And so far Trump’s Department of Health and Human Services doesn’t appear to be emphasizing this kind of essential outreach, both Ms. Corlette and Mr. Jost suggested. A few months ago, Mr. Kreidler agreed, HHS staffers appeared interested in helping states fill their bare counties – but that support has since dwindled.
“This may be sort of under the radar, but it can have real, lasting effects” for consumer choice, Ms. Corlette said.
All quiet on the enrollment front
The administration could further undermine the marketplace by dropping outreach to consumers. It’s already a shorter enrollment period this year – spanning 6 weeks instead of 3 months, from Nov. 1 to Dec. 15 – though that change was already slated to eventually take effect.
That shorter period means people may miss the memo on signing up – or at least need an extra push, Ms. Corlette said. And that’s another way the administration could undermine the marketplaces: simply choosing not to advertise them.
Last sign-up season, HHS stopped open enrollment advertising in January, pulling ads a few days before the period ended. Enrollment dropped compared with previous years, Mr. Jost and Mr. Gaba noted, with young, healthy people being more likely not to buy coverage.
The administration also just stopped funding federal contractors that supported efforts by community groups and other organizations in some of the nation’s largest cities to sign up people.
Dropping advertising, shortening open enrollment, or simply scaling back on technical maintenance for the marketplace website could all have significant impact, Ms. Corlette said. People who are sick and need insurance will likely seek it out, but those who are healthier – for whom health insurance is a less pressing priority – could miss the boat.
Again, Mr. Jost said, that affects insurer participation.
“Insurance is a product that needs to be sold,” he said. “If the insurers believe they’re not going to get any help at all in marketing their product,” he added, fewer will want to enter the marketplace.
Word of (bad) mouth
HHS has taken an active role in criticizing the health law – pushing press releases and videos that argue it has helped more than hurt. That strategy could do a lot of harm, experts said.
If consumers keep hearing the law is failing, Mr. Jost noted, some will ultimately believe it, buying coverage only if they need it and thereby skewing the insurance risk pool.
Perceived hostility also has an effect on insurers, steering them away from marketplace participation.
“When you undermine confidence in the marketplace, you don’t need a Ph.D. in economics to know it’s not good long term,” Ms. Corlette said.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
President Donald Trump has vowed to “let Obamacare fail,” after legislative efforts to undo the Affordable Care Act have stalled.
He and congressional Republicans have repeatedly portrayed the Affordable Care Act insurance marketplaces, also known as exchanges, as being in a “death spiral.” But independent analyses have concluded that such spontaneous disintegration isn’t happening.
In a number of ways, the Trump administration’s policies are pushing Obamacare into the vortex.
Reports from Standard & Poor’s, the Congressional Budget Office and the Kaiser Family Foundation all suggest that the exchanges – where people can shop for coverage, often with the help of a government subsidy – are stabilizing. (Kaiser Health News is an editorially independent program of the foundation.)
“The administration has a lot of power to undermine the markets and make them dysfunctional,” said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms, who specializes in private insurance markets.
Here’s a look at five ways the White House is already working to weaken the health law, and what that means for consumers.
‘Cost-sharing reductions’
Under the ACA, when someone’s income falls between 100% and 250% of the federal poverty level – up to about $29,000 for an individual or around $61,000 for a family of four – marketplace carriers must offer a plan with “cost-sharing reductions” (CSRs) that reduce consumers’ out-of-pocket expenses.
Reducing cost-sharing – generally copayments and deductibles – makes plans more expensive for the insurers. The Obama administration used its rule-making power to set up direct payments to carriers to help offset this burden. The Trump White House has inherited that responsibility but also has the power to end the payment program.
The nonpartisan Congressional Budget Office estimated CSR subsidies in 2017 would total about $7 billion. Without that money, analysts say, more insurers might choose to exit, limiting options for consumers, and letting the insurers who remain charge higher prices.
Trump has been committedly noncommittal, publicly indicating he would like to halt the subsidies, but so far – on a month-to-month basis – letting them continue.
The uncertainty makes insurance companies skittish about participating, analysts noted. It’s also one reason some plans say they have had to increase their rates, noted Charles Gaba, a Michigan-based blogger who tracks ACA sign-ups. For instance: When filing plans for the 2018 marketplace, carriers on average raised premiums by about 34% – with about 20 points stemming from CSR uncertainty, Mr. Gaba said, based on an analysis of 21 states’ initial rate filings. Dropping the subsidies altogether would be even more damaging.
Weaken the mandate
The White House has already signaled it does not want to enforce the individual mandate – the health law’s requirement that all people have coverage. And administration officials have repeated that position.
Meanwhile, in January, it issued an executive order that encouraged U.S. agencies to grant exemptions and waive or defer health law provisions that could put financial strain on companies or individuals – which could also be applied to the individual mandate.
For 2016 tax returns, though, the Internal Revenue Service continued to impose a financial penalty on people who didn’t have health insurance and who didn’t qualify for an exemption.
But enforcement may be waning. This year, the IRS was supposed to reject tax returns if people didn’t indicate whether they had coverage, flagging them for a potential penalty. Instead, it continued processing them, citing Trump’s executive order.
If the IRS has already processed any tax refunds for consumers, then they “don’t have much leverage” when attempting to collect the mandate fee, said Timothy Jost, emeritus law professor at Washington and Lee University in Virginia and an expert on health reform.
Enforcement of the mandate, economists note, is crucial to ensuring that enough healthy people buy coverage to balance the costs of sicker beneficiaries.
But even with the mandate in effect, the efforts to defang it bring confusion.
“A lot of people believe the Trump administration is not enforcing it,” Mr. Jost said.
As a result, healthy people may become less likely to buy insurance, even as sick ones continue seeking it. That means higher prices, and a shakier pool.
“If they don’t think they’re going to get healthy people in the risk pool, they’re going to increase their rates further to protect themselves,” Mr. Jost said. “And as they raise their rates further to protect themselves, people … start to drop out.”
Thus, the president’s position on the mandate is leaving insurance carriers and commissioners “apprehensive,” noted Mike Kreidler, Washington state’s insurance commissioner.
A bare market
Skittishness on the part of insurers could lead them to drop out of some marketplaces, leaving consumers in some areas with few or no choices. Those “bare markets” are possible under even stable circumstances – and preventing them requires active federal involvement.
Under the Obama administration, high-level officials were “on the phone daily with insurance company executives … trying to get them to participate,” Ms. Corlette said. “It was very much an all-hands-on-deck, ‘we’re going to make it work for you guys’ kind of communication.”
And so far Trump’s Department of Health and Human Services doesn’t appear to be emphasizing this kind of essential outreach, both Ms. Corlette and Mr. Jost suggested. A few months ago, Mr. Kreidler agreed, HHS staffers appeared interested in helping states fill their bare counties – but that support has since dwindled.
“This may be sort of under the radar, but it can have real, lasting effects” for consumer choice, Ms. Corlette said.
All quiet on the enrollment front
The administration could further undermine the marketplace by dropping outreach to consumers. It’s already a shorter enrollment period this year – spanning 6 weeks instead of 3 months, from Nov. 1 to Dec. 15 – though that change was already slated to eventually take effect.
That shorter period means people may miss the memo on signing up – or at least need an extra push, Ms. Corlette said. And that’s another way the administration could undermine the marketplaces: simply choosing not to advertise them.
Last sign-up season, HHS stopped open enrollment advertising in January, pulling ads a few days before the period ended. Enrollment dropped compared with previous years, Mr. Jost and Mr. Gaba noted, with young, healthy people being more likely not to buy coverage.
The administration also just stopped funding federal contractors that supported efforts by community groups and other organizations in some of the nation’s largest cities to sign up people.
Dropping advertising, shortening open enrollment, or simply scaling back on technical maintenance for the marketplace website could all have significant impact, Ms. Corlette said. People who are sick and need insurance will likely seek it out, but those who are healthier – for whom health insurance is a less pressing priority – could miss the boat.
Again, Mr. Jost said, that affects insurer participation.
“Insurance is a product that needs to be sold,” he said. “If the insurers believe they’re not going to get any help at all in marketing their product,” he added, fewer will want to enter the marketplace.
Word of (bad) mouth
HHS has taken an active role in criticizing the health law – pushing press releases and videos that argue it has helped more than hurt. That strategy could do a lot of harm, experts said.
If consumers keep hearing the law is failing, Mr. Jost noted, some will ultimately believe it, buying coverage only if they need it and thereby skewing the insurance risk pool.
Perceived hostility also has an effect on insurers, steering them away from marketplace participation.
“When you undermine confidence in the marketplace, you don’t need a Ph.D. in economics to know it’s not good long term,” Ms. Corlette said.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Physician compensation growing but at a slightly slower pace
Physicians working in large multispecialty groups saw their compensation increase in 2016, albeit at a slower pace than in 2015, according to survey results reported by AMGA.
The 2017 Medical Group Compensation and Productivity Survey shows that the overall weighted average increase in physician compensation for the calendar year 2016 was 2.9%, slightly lower than the 3.1% increase seen in 2015. Doctors in more than three-quarters (77%) of specialties saw increases in 2016.
Opthalmologic surgery saw the largest compensation increase at 7.7%, followed by cardiothoracic surgery (7.0%), hematology and medical oncology (6.7%), allergy/immunology (5.9%) and pulmonary disease (5.6%). Emergency medicine saw a decrease in compensation of 2.0% in 2016 after experiencing a 9.6% increase in 2015.
Value-based payment is beginning to factor into the growth in payment by specialty. Overall, about 8% of compensation is being linked to value-based pay, and that number is expected to rise, with some medical practice groups linking 15% or more of compensation to value-based metrics.
“In almost all of the groups that I have worked with in the last few years on [compensation] design, that has been one of the drivers of decision to do the compensation redesign is to allocate to the value-based metrics,” Wayne Hartley, vice president of AMGA Consulting, said in an interview. 
AMGA said that the data covers responses from 269 medical groups covering more than 102,000 providers and is representative of large multispecialty groups and integrated health systems that average 380 providers per group.
Physicians working in large multispecialty groups saw their compensation increase in 2016, albeit at a slower pace than in 2015, according to survey results reported by AMGA.
The 2017 Medical Group Compensation and Productivity Survey shows that the overall weighted average increase in physician compensation for the calendar year 2016 was 2.9%, slightly lower than the 3.1% increase seen in 2015. Doctors in more than three-quarters (77%) of specialties saw increases in 2016.
Opthalmologic surgery saw the largest compensation increase at 7.7%, followed by cardiothoracic surgery (7.0%), hematology and medical oncology (6.7%), allergy/immunology (5.9%) and pulmonary disease (5.6%). Emergency medicine saw a decrease in compensation of 2.0% in 2016 after experiencing a 9.6% increase in 2015.
Value-based payment is beginning to factor into the growth in payment by specialty. Overall, about 8% of compensation is being linked to value-based pay, and that number is expected to rise, with some medical practice groups linking 15% or more of compensation to value-based metrics.
“In almost all of the groups that I have worked with in the last few years on [compensation] design, that has been one of the drivers of decision to do the compensation redesign is to allocate to the value-based metrics,” Wayne Hartley, vice president of AMGA Consulting, said in an interview. 
AMGA said that the data covers responses from 269 medical groups covering more than 102,000 providers and is representative of large multispecialty groups and integrated health systems that average 380 providers per group.
Physicians working in large multispecialty groups saw their compensation increase in 2016, albeit at a slower pace than in 2015, according to survey results reported by AMGA.
The 2017 Medical Group Compensation and Productivity Survey shows that the overall weighted average increase in physician compensation for the calendar year 2016 was 2.9%, slightly lower than the 3.1% increase seen in 2015. Doctors in more than three-quarters (77%) of specialties saw increases in 2016.
Opthalmologic surgery saw the largest compensation increase at 7.7%, followed by cardiothoracic surgery (7.0%), hematology and medical oncology (6.7%), allergy/immunology (5.9%) and pulmonary disease (5.6%). Emergency medicine saw a decrease in compensation of 2.0% in 2016 after experiencing a 9.6% increase in 2015.
Value-based payment is beginning to factor into the growth in payment by specialty. Overall, about 8% of compensation is being linked to value-based pay, and that number is expected to rise, with some medical practice groups linking 15% or more of compensation to value-based metrics.
“In almost all of the groups that I have worked with in the last few years on [compensation] design, that has been one of the drivers of decision to do the compensation redesign is to allocate to the value-based metrics,” Wayne Hartley, vice president of AMGA Consulting, said in an interview. 
AMGA said that the data covers responses from 269 medical groups covering more than 102,000 providers and is representative of large multispecialty groups and integrated health systems that average 380 providers per group.
Blocking a lipoprotein lipase inhibitor improves lipid profiles
Two different approaches to shutting down the function of a human liver protein that inhibits lipoprotein lipase showed preliminary evidence of safely producing favorable lipid changes in healthy volunteers in two separate, phase 1 studies.
These findings, coupled with promising observational data from people who carry loss-of-function mutations in the gene for this protein, angiopoietin-like 3 (ANGPTL3), have raised hopes that interventions that interfere with the function of the ANGPTL3 protein may provide new and effective ways to improve lipid levels and cut the incidence of cardiovascular disease events.
“There is now a growing body of epidemiologic, genetic, and genomewide association studies supporting the hypothesis that lowering levels of ANGPTL3 in plasma by inhibiting hepatic ANGPTL3 synthesis will be beneficial in terms of reducing plasma levels of atherogenic apolipoprotein B and in improving metabolic measures associated with dyslipidemia,” wrote Mark J. Graham and his associates in a recently published article (N Engl J Med. 2017 Jul 20;377[3]:222-32).
The phase 1 study results reported by this group came from 44 healthy adults aged 18-65 years who received varying doses of a commercially developed antisense drug, ANGPTL3-LRX, as either single or serial subcutaneous injections. ANGPTL3-LRX is an oligonucleotide designed to inhibit production of messenger RNA for the ANGPTL3 protein.
Six people received the highest ANGPTL3-LRX dosage administered, 60 mg given as a weekly injection for 6 weeks, and after this regimen they showed an average 50% cut in triglycerides levels, compared with baseline, and an average 33% drop in their low LDL cholesterol levels from baseline. None of the 33 people treated with ANGPTL3-LRX in the trial had a documented serious adverse event, and the only treatment dropout was a patient who was lost to follow-up during the treatment phase, reported Mr. Graham, a researcher at Ionis Pharmaceuticals, the sponsor of the study and the company developing the antisense drug, and his associates.
The second phase 1 study examined a different way to block ANGPTL3 activity, with a human monoclonal antibody to this protein. The study involved 83 healthy adults aged 18-65 years with fasting triglyceride levels of 150-450 mg/dL and fasting LDL cholesterol levels of at least 100 mg/dL. Each participant received a single subcutaneous injection or intravenous dose of the antibody, evinacumab, at varying amounts or placebo. The maximum observed lipid changes seen was a drop in triglycerides of 76% and a fall in LDL cholesterol by 23%. Treatment also produced a maximum drop in HDL cholesterol of 18%. No person left the study because of an adverse event. The most common adverse event was headache, in seven people (11% of evinacumab recipients), reported Frederick E. Dewey, MD, a researcher at Regeneron Pharmaceuticals, the company developing evinacumab, and his associates (N Engl J Med. 2017 Jul 20;377[3]:211-21).
The Regeneron report also included results from population studies they ran. They reported performing genome sequencing on specimens from 58,335 adults enrolled in the DiscovEHR study, and identified 13 distinct loss-of-function variants in the ANGPTL3 genes that occurred individually in a small number of these people.
They then ran analyses of lipid levels and coronary artery disease prevalence rates in people who carry one of these 13 loss-of-function genetic signatures in one of their ANGTPL3 genes. Among 45,226 of the people in DiscovEHR those with a variant had on average a 27% lower triglyceride level, a 9% lower LDL cholesterol level, and a 4% lower HDL cholesterol level than noncarriers, after adjustment for covariates. Analysis of coronary artery disease prevalence showed that, after adjusting for age, sex, and ancestry, carrying a loss-of-function variant was linked with a statistically significant 41% lower prevalence of all coronary artery disease, and a 34% lower prevalence of myocardial infarction that fell short of statistical significance.
A second population study looked at links between loss-of-function variants and coronary artery disease in more than 130,000 Danish people. This showed a nonsignificant 37% lower prevalence of coronary artery disease in people with a variant. Two different types of meta-analyses of the data from both the DiscovEHR and Danish studies showed coronary disease rates reduced by either 31% or 39% in variant carriers depending on which meta-analysis approach the researchers used, both statistically significant reductions.
[email protected]
On Twitter @mitchelzoler
The findings from these two studies together with other recent study results open a new therapeutic window for reducing elevated levels of triglyceride-rich lipoproteins by activating lipoprotein lipase.
The findings also suggest that inhibition of the ANGPTL3 gene or protein is potentially an effective way to treat patients with familial hypercholesterolemia because of a deficiency in the receptor for low density lipoprotein. It is likely that lowering triglyceride levels with an agent that boosts the activity of lipoprotein lipase will result in a different spectrum of benefits and adverse effects, compared with agents that boost the number of LDL receptors.
New approaches to boost lipoprotein lipase activity, such as inhibiting ANGPTL3 function as was done in these two reports, represents a fresh frontier for treatment of hypertriglyceridemia and coronary artery disease.
Alan R. Tall, MD , professor of medicine at Columbia University, New York, made these comments in an editorial ( N Engl J Med. 2017 Jul 20;377[3]:280-3 ).
The findings from these two studies together with other recent study results open a new therapeutic window for reducing elevated levels of triglyceride-rich lipoproteins by activating lipoprotein lipase.
The findings also suggest that inhibition of the ANGPTL3 gene or protein is potentially an effective way to treat patients with familial hypercholesterolemia because of a deficiency in the receptor for low density lipoprotein. It is likely that lowering triglyceride levels with an agent that boosts the activity of lipoprotein lipase will result in a different spectrum of benefits and adverse effects, compared with agents that boost the number of LDL receptors.
New approaches to boost lipoprotein lipase activity, such as inhibiting ANGPTL3 function as was done in these two reports, represents a fresh frontier for treatment of hypertriglyceridemia and coronary artery disease.
Alan R. Tall, MD , professor of medicine at Columbia University, New York, made these comments in an editorial ( N Engl J Med. 2017 Jul 20;377[3]:280-3 ).
The findings from these two studies together with other recent study results open a new therapeutic window for reducing elevated levels of triglyceride-rich lipoproteins by activating lipoprotein lipase.
The findings also suggest that inhibition of the ANGPTL3 gene or protein is potentially an effective way to treat patients with familial hypercholesterolemia because of a deficiency in the receptor for low density lipoprotein. It is likely that lowering triglyceride levels with an agent that boosts the activity of lipoprotein lipase will result in a different spectrum of benefits and adverse effects, compared with agents that boost the number of LDL receptors.
New approaches to boost lipoprotein lipase activity, such as inhibiting ANGPTL3 function as was done in these two reports, represents a fresh frontier for treatment of hypertriglyceridemia and coronary artery disease.
Alan R. Tall, MD , professor of medicine at Columbia University, New York, made these comments in an editorial ( N Engl J Med. 2017 Jul 20;377[3]:280-3 ).
Two different approaches to shutting down the function of a human liver protein that inhibits lipoprotein lipase showed preliminary evidence of safely producing favorable lipid changes in healthy volunteers in two separate, phase 1 studies.
These findings, coupled with promising observational data from people who carry loss-of-function mutations in the gene for this protein, angiopoietin-like 3 (ANGPTL3), have raised hopes that interventions that interfere with the function of the ANGPTL3 protein may provide new and effective ways to improve lipid levels and cut the incidence of cardiovascular disease events.
“There is now a growing body of epidemiologic, genetic, and genomewide association studies supporting the hypothesis that lowering levels of ANGPTL3 in plasma by inhibiting hepatic ANGPTL3 synthesis will be beneficial in terms of reducing plasma levels of atherogenic apolipoprotein B and in improving metabolic measures associated with dyslipidemia,” wrote Mark J. Graham and his associates in a recently published article (N Engl J Med. 2017 Jul 20;377[3]:222-32).
The phase 1 study results reported by this group came from 44 healthy adults aged 18-65 years who received varying doses of a commercially developed antisense drug, ANGPTL3-LRX, as either single or serial subcutaneous injections. ANGPTL3-LRX is an oligonucleotide designed to inhibit production of messenger RNA for the ANGPTL3 protein.
Six people received the highest ANGPTL3-LRX dosage administered, 60 mg given as a weekly injection for 6 weeks, and after this regimen they showed an average 50% cut in triglycerides levels, compared with baseline, and an average 33% drop in their low LDL cholesterol levels from baseline. None of the 33 people treated with ANGPTL3-LRX in the trial had a documented serious adverse event, and the only treatment dropout was a patient who was lost to follow-up during the treatment phase, reported Mr. Graham, a researcher at Ionis Pharmaceuticals, the sponsor of the study and the company developing the antisense drug, and his associates.
The second phase 1 study examined a different way to block ANGPTL3 activity, with a human monoclonal antibody to this protein. The study involved 83 healthy adults aged 18-65 years with fasting triglyceride levels of 150-450 mg/dL and fasting LDL cholesterol levels of at least 100 mg/dL. Each participant received a single subcutaneous injection or intravenous dose of the antibody, evinacumab, at varying amounts or placebo. The maximum observed lipid changes seen was a drop in triglycerides of 76% and a fall in LDL cholesterol by 23%. Treatment also produced a maximum drop in HDL cholesterol of 18%. No person left the study because of an adverse event. The most common adverse event was headache, in seven people (11% of evinacumab recipients), reported Frederick E. Dewey, MD, a researcher at Regeneron Pharmaceuticals, the company developing evinacumab, and his associates (N Engl J Med. 2017 Jul 20;377[3]:211-21).
The Regeneron report also included results from population studies they ran. They reported performing genome sequencing on specimens from 58,335 adults enrolled in the DiscovEHR study, and identified 13 distinct loss-of-function variants in the ANGPTL3 genes that occurred individually in a small number of these people.
They then ran analyses of lipid levels and coronary artery disease prevalence rates in people who carry one of these 13 loss-of-function genetic signatures in one of their ANGTPL3 genes. Among 45,226 of the people in DiscovEHR those with a variant had on average a 27% lower triglyceride level, a 9% lower LDL cholesterol level, and a 4% lower HDL cholesterol level than noncarriers, after adjustment for covariates. Analysis of coronary artery disease prevalence showed that, after adjusting for age, sex, and ancestry, carrying a loss-of-function variant was linked with a statistically significant 41% lower prevalence of all coronary artery disease, and a 34% lower prevalence of myocardial infarction that fell short of statistical significance.
A second population study looked at links between loss-of-function variants and coronary artery disease in more than 130,000 Danish people. This showed a nonsignificant 37% lower prevalence of coronary artery disease in people with a variant. Two different types of meta-analyses of the data from both the DiscovEHR and Danish studies showed coronary disease rates reduced by either 31% or 39% in variant carriers depending on which meta-analysis approach the researchers used, both statistically significant reductions.
[email protected]
On Twitter @mitchelzoler
Two different approaches to shutting down the function of a human liver protein that inhibits lipoprotein lipase showed preliminary evidence of safely producing favorable lipid changes in healthy volunteers in two separate, phase 1 studies.
These findings, coupled with promising observational data from people who carry loss-of-function mutations in the gene for this protein, angiopoietin-like 3 (ANGPTL3), have raised hopes that interventions that interfere with the function of the ANGPTL3 protein may provide new and effective ways to improve lipid levels and cut the incidence of cardiovascular disease events.
“There is now a growing body of epidemiologic, genetic, and genomewide association studies supporting the hypothesis that lowering levels of ANGPTL3 in plasma by inhibiting hepatic ANGPTL3 synthesis will be beneficial in terms of reducing plasma levels of atherogenic apolipoprotein B and in improving metabolic measures associated with dyslipidemia,” wrote Mark J. Graham and his associates in a recently published article (N Engl J Med. 2017 Jul 20;377[3]:222-32).
The phase 1 study results reported by this group came from 44 healthy adults aged 18-65 years who received varying doses of a commercially developed antisense drug, ANGPTL3-LRX, as either single or serial subcutaneous injections. ANGPTL3-LRX is an oligonucleotide designed to inhibit production of messenger RNA for the ANGPTL3 protein.
Six people received the highest ANGPTL3-LRX dosage administered, 60 mg given as a weekly injection for 6 weeks, and after this regimen they showed an average 50% cut in triglycerides levels, compared with baseline, and an average 33% drop in their low LDL cholesterol levels from baseline. None of the 33 people treated with ANGPTL3-LRX in the trial had a documented serious adverse event, and the only treatment dropout was a patient who was lost to follow-up during the treatment phase, reported Mr. Graham, a researcher at Ionis Pharmaceuticals, the sponsor of the study and the company developing the antisense drug, and his associates.
The second phase 1 study examined a different way to block ANGPTL3 activity, with a human monoclonal antibody to this protein. The study involved 83 healthy adults aged 18-65 years with fasting triglyceride levels of 150-450 mg/dL and fasting LDL cholesterol levels of at least 100 mg/dL. Each participant received a single subcutaneous injection or intravenous dose of the antibody, evinacumab, at varying amounts or placebo. The maximum observed lipid changes seen was a drop in triglycerides of 76% and a fall in LDL cholesterol by 23%. Treatment also produced a maximum drop in HDL cholesterol of 18%. No person left the study because of an adverse event. The most common adverse event was headache, in seven people (11% of evinacumab recipients), reported Frederick E. Dewey, MD, a researcher at Regeneron Pharmaceuticals, the company developing evinacumab, and his associates (N Engl J Med. 2017 Jul 20;377[3]:211-21).
The Regeneron report also included results from population studies they ran. They reported performing genome sequencing on specimens from 58,335 adults enrolled in the DiscovEHR study, and identified 13 distinct loss-of-function variants in the ANGPTL3 genes that occurred individually in a small number of these people.
They then ran analyses of lipid levels and coronary artery disease prevalence rates in people who carry one of these 13 loss-of-function genetic signatures in one of their ANGTPL3 genes. Among 45,226 of the people in DiscovEHR those with a variant had on average a 27% lower triglyceride level, a 9% lower LDL cholesterol level, and a 4% lower HDL cholesterol level than noncarriers, after adjustment for covariates. Analysis of coronary artery disease prevalence showed that, after adjusting for age, sex, and ancestry, carrying a loss-of-function variant was linked with a statistically significant 41% lower prevalence of all coronary artery disease, and a 34% lower prevalence of myocardial infarction that fell short of statistical significance.
A second population study looked at links between loss-of-function variants and coronary artery disease in more than 130,000 Danish people. This showed a nonsignificant 37% lower prevalence of coronary artery disease in people with a variant. Two different types of meta-analyses of the data from both the DiscovEHR and Danish studies showed coronary disease rates reduced by either 31% or 39% in variant carriers depending on which meta-analysis approach the researchers used, both statistically significant reductions.
[email protected]
On Twitter @mitchelzoler
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Serial doses of the antisense oligonucleotide ANGPTL3-LRX produced an average 50% cut in triglycerides and 33% cut in LDL cholesterol.
Data source: The ANGPTL3-LRX phase 1 trial enrolled 44 healthy adults. The evinacumab phase 1 trial enrolled 83 healthy adults.
Disclosures: The ANGPTL3-LRX study was funded by Ionis. Mr. Graham is an employee of Ionis. The evinacumab study was funded by Regeneron. Dr. Dewey is an employee of Regeneron.