User login
The Official Newspaper of the American Association for Thoracic Surgery
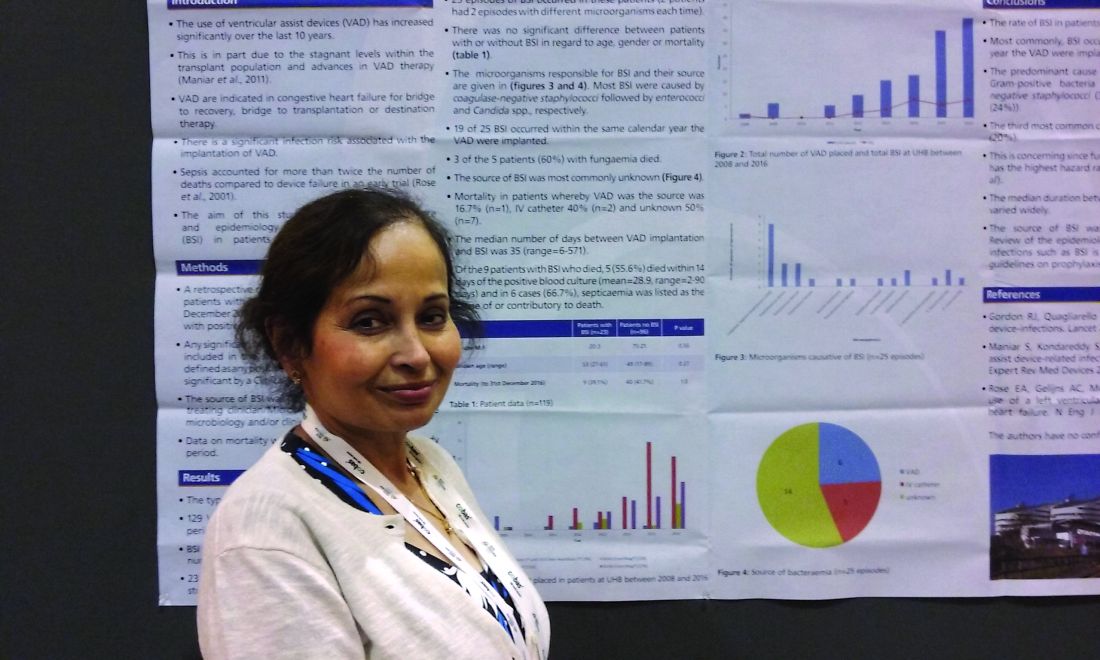
Ventricular assist devices linked to sepsis
NEW ORLEANS – Back in 2008, there was only one case.
Since then, however, the number of patients with ventricular assist devices who developed sepsis while being treated in the cardiac unit at Queen Elizabeth Hospital in Birmingham, England, appeared to be noticeably growing. So, investigators launched a study to confirm their suspicions and to learn more about the underlying causes.
“Bloodstream infection is a serious infection, so I thought, ‘Let’s see what’s happening,’ ” explained Ira Das, MD, a consultant microbiologist at Queen Elizabeth Hospital.
Coagulase-negative staphylococci were the most common cause, present in 32% of the 25 cases. Sepsis was caused by Enterococcus faecium in 12%, Candida parapsilosis in 8%, and Staphylococcus aureus in 2%. Another 4% were either Enterococcus faecalis, Serratia marcescens, Pseudomonas aeruginosa, C. guilliermondii, or C. orthopsilosis. The remaining 16% of bloodstream infections were polymicrobial.
Less certain was the source of these infections.
“In the majority of cases, we didn’t know where it was coming from,” Dr. Das said at the annual meeting of the American Society for Microbiology. In 6 of the 25 cases, VAD was confirmed to be the focus of infection, either through imaging or because a failing component of the explanted device was examined later. An intravascular catheter was the source in another 5 patients, and in 14 cases, the source remained a mystery.
“Some of these infections just might have been hard to see,” Dr. Das said. “If the infection is inside the device, it’s not always easy to visualize.”
The study supports earlier findings from a review article that points to a significant infection risk associated with the implantation of VADs (Expert Rev Med Devices. 2011 Sep;8[5]:627-34). That article’s authors noted, “Despite recent improvements in outcomes, device-related infections remain a significant complication of LVAD [left ventricular assist device] therapy.”
In a previous study of people with end-stage heart failure, other investigators noted that, “despite the substantial survival benefit, the morbidity and mortality associated with the use of the left ventricular assist device were considerable. In particular, infection and mechanical failure of the device were major factors in the 2-year survival rate of only 23%” (N Engl J Med. 2001 Nov 15;345[20]:1435-43).
Similarly, in the current study, mortality was higher among those with sepsis and a VAD. Mortality was 39% – including eight patients who died with a VAD in situ and one following cardiac transplantation. However, Dr. Das cautioned, “It’s a small number, and there are other factors that could have contributed. They all go on anticoagulants so they have bleeding tendencies, and many of the patients are in the ICU with multiorgan failure.”
Infection prevention remains paramount to minimize mortality and other adverse events associated with a patient’s having a VAD. “We have to make sure that infection control procedures and our treatments are up to the optimal standard,” Dr. Das said. “It’s not easy to remove the device.”
Of the 129 VADs implanted, 68 were long-term LVADs, 11 were short-term LVADs, 15 were right ventricular devices, and 35 were biventricular devices.
The study is ongoing. The data presented at the meeting were collected up until December 2016.
“Since then, I’ve seen two more cases, and – very interestingly – one was Haemophilus influenzae,” Dr. Das said. “The patient was on the device, he was at home, and he came in with bacteremia.” Again, the source of infection proved elusive. “With H. influenzae, you would think it was coming from his chest, but the chest x-ray was normal.”
The second case, a patient with a coagulase-negative staphylococci bloodstream infection, was scheduled for a PET scan at the time of Dr. Das’ presentation to try to identify the source of infection.
Dr. Das had no relevant disclosures.
Modern technology saves our patients' lives, but there is always another side to the coin. Reports that LVAD devices are associated with a high incidence of bloodstream infections is important for future clinical practice. The fact that the causes and risk factors for these infections are unknown make this phenomena one of high interest.
Modern technology saves our patients' lives, but there is always another side to the coin. Reports that LVAD devices are associated with a high incidence of bloodstream infections is important for future clinical practice. The fact that the causes and risk factors for these infections are unknown make this phenomena one of high interest.
Modern technology saves our patients' lives, but there is always another side to the coin. Reports that LVAD devices are associated with a high incidence of bloodstream infections is important for future clinical practice. The fact that the causes and risk factors for these infections are unknown make this phenomena one of high interest.
NEW ORLEANS – Back in 2008, there was only one case.
Since then, however, the number of patients with ventricular assist devices who developed sepsis while being treated in the cardiac unit at Queen Elizabeth Hospital in Birmingham, England, appeared to be noticeably growing. So, investigators launched a study to confirm their suspicions and to learn more about the underlying causes.
“Bloodstream infection is a serious infection, so I thought, ‘Let’s see what’s happening,’ ” explained Ira Das, MD, a consultant microbiologist at Queen Elizabeth Hospital.
Coagulase-negative staphylococci were the most common cause, present in 32% of the 25 cases. Sepsis was caused by Enterococcus faecium in 12%, Candida parapsilosis in 8%, and Staphylococcus aureus in 2%. Another 4% were either Enterococcus faecalis, Serratia marcescens, Pseudomonas aeruginosa, C. guilliermondii, or C. orthopsilosis. The remaining 16% of bloodstream infections were polymicrobial.
Less certain was the source of these infections.
“In the majority of cases, we didn’t know where it was coming from,” Dr. Das said at the annual meeting of the American Society for Microbiology. In 6 of the 25 cases, VAD was confirmed to be the focus of infection, either through imaging or because a failing component of the explanted device was examined later. An intravascular catheter was the source in another 5 patients, and in 14 cases, the source remained a mystery.
“Some of these infections just might have been hard to see,” Dr. Das said. “If the infection is inside the device, it’s not always easy to visualize.”
The study supports earlier findings from a review article that points to a significant infection risk associated with the implantation of VADs (Expert Rev Med Devices. 2011 Sep;8[5]:627-34). That article’s authors noted, “Despite recent improvements in outcomes, device-related infections remain a significant complication of LVAD [left ventricular assist device] therapy.”
In a previous study of people with end-stage heart failure, other investigators noted that, “despite the substantial survival benefit, the morbidity and mortality associated with the use of the left ventricular assist device were considerable. In particular, infection and mechanical failure of the device were major factors in the 2-year survival rate of only 23%” (N Engl J Med. 2001 Nov 15;345[20]:1435-43).
Similarly, in the current study, mortality was higher among those with sepsis and a VAD. Mortality was 39% – including eight patients who died with a VAD in situ and one following cardiac transplantation. However, Dr. Das cautioned, “It’s a small number, and there are other factors that could have contributed. They all go on anticoagulants so they have bleeding tendencies, and many of the patients are in the ICU with multiorgan failure.”
Infection prevention remains paramount to minimize mortality and other adverse events associated with a patient’s having a VAD. “We have to make sure that infection control procedures and our treatments are up to the optimal standard,” Dr. Das said. “It’s not easy to remove the device.”
Of the 129 VADs implanted, 68 were long-term LVADs, 11 were short-term LVADs, 15 were right ventricular devices, and 35 were biventricular devices.
The study is ongoing. The data presented at the meeting were collected up until December 2016.
“Since then, I’ve seen two more cases, and – very interestingly – one was Haemophilus influenzae,” Dr. Das said. “The patient was on the device, he was at home, and he came in with bacteremia.” Again, the source of infection proved elusive. “With H. influenzae, you would think it was coming from his chest, but the chest x-ray was normal.”
The second case, a patient with a coagulase-negative staphylococci bloodstream infection, was scheduled for a PET scan at the time of Dr. Das’ presentation to try to identify the source of infection.
Dr. Das had no relevant disclosures.
NEW ORLEANS – Back in 2008, there was only one case.
Since then, however, the number of patients with ventricular assist devices who developed sepsis while being treated in the cardiac unit at Queen Elizabeth Hospital in Birmingham, England, appeared to be noticeably growing. So, investigators launched a study to confirm their suspicions and to learn more about the underlying causes.
“Bloodstream infection is a serious infection, so I thought, ‘Let’s see what’s happening,’ ” explained Ira Das, MD, a consultant microbiologist at Queen Elizabeth Hospital.
Coagulase-negative staphylococci were the most common cause, present in 32% of the 25 cases. Sepsis was caused by Enterococcus faecium in 12%, Candida parapsilosis in 8%, and Staphylococcus aureus in 2%. Another 4% were either Enterococcus faecalis, Serratia marcescens, Pseudomonas aeruginosa, C. guilliermondii, or C. orthopsilosis. The remaining 16% of bloodstream infections were polymicrobial.
Less certain was the source of these infections.
“In the majority of cases, we didn’t know where it was coming from,” Dr. Das said at the annual meeting of the American Society for Microbiology. In 6 of the 25 cases, VAD was confirmed to be the focus of infection, either through imaging or because a failing component of the explanted device was examined later. An intravascular catheter was the source in another 5 patients, and in 14 cases, the source remained a mystery.
“Some of these infections just might have been hard to see,” Dr. Das said. “If the infection is inside the device, it’s not always easy to visualize.”
The study supports earlier findings from a review article that points to a significant infection risk associated with the implantation of VADs (Expert Rev Med Devices. 2011 Sep;8[5]:627-34). That article’s authors noted, “Despite recent improvements in outcomes, device-related infections remain a significant complication of LVAD [left ventricular assist device] therapy.”
In a previous study of people with end-stage heart failure, other investigators noted that, “despite the substantial survival benefit, the morbidity and mortality associated with the use of the left ventricular assist device were considerable. In particular, infection and mechanical failure of the device were major factors in the 2-year survival rate of only 23%” (N Engl J Med. 2001 Nov 15;345[20]:1435-43).
Similarly, in the current study, mortality was higher among those with sepsis and a VAD. Mortality was 39% – including eight patients who died with a VAD in situ and one following cardiac transplantation. However, Dr. Das cautioned, “It’s a small number, and there are other factors that could have contributed. They all go on anticoagulants so they have bleeding tendencies, and many of the patients are in the ICU with multiorgan failure.”
Infection prevention remains paramount to minimize mortality and other adverse events associated with a patient’s having a VAD. “We have to make sure that infection control procedures and our treatments are up to the optimal standard,” Dr. Das said. “It’s not easy to remove the device.”
Of the 129 VADs implanted, 68 were long-term LVADs, 11 were short-term LVADs, 15 were right ventricular devices, and 35 were biventricular devices.
The study is ongoing. The data presented at the meeting were collected up until December 2016.
“Since then, I’ve seen two more cases, and – very interestingly – one was Haemophilus influenzae,” Dr. Das said. “The patient was on the device, he was at home, and he came in with bacteremia.” Again, the source of infection proved elusive. “With H. influenzae, you would think it was coming from his chest, but the chest x-ray was normal.”
The second case, a patient with a coagulase-negative staphylococci bloodstream infection, was scheduled for a PET scan at the time of Dr. Das’ presentation to try to identify the source of infection.
Dr. Das had no relevant disclosures.
AT ASM MICROBE 2017
Key clinical point: There may be a significant rate of bloodstream infections among people with a ventricular assist device.
Major finding: A total of 20% of the 118 people with a VAD had a bloodstream infection.
Data source: A retrospective study of 129 ventricular assist devices placed in 118 people between 2008 and 2016.
Disclosures: Dr. Das had no relevant disclosures.
Factory contamination seen as likely source of postop endocarditis outbreak
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point:
Major finding: Cardiac surgery–related patient isolates were all classified into the same group, in which all, except one, formed a distinct subgroup of Mycobacterium chimaera, which also comprised most isolates from LivaNova HCUs, and one from the equipment production site.
Data source: Phylogenetic analysis based on whole-genome sequencing of 250 M. chimaera isolates obtained from cardiac surgery patients, hospitals, and other sources.
Disclosures: Partly funded by the EU Horizon 2020 program and several German, Swiss, and U.K. infectious disease–related NGOs. The authors reported having no disclosures.
Recovery: Where TAVR gains advantage over SAVR
A post hoc analysis of the first randomized clinical to show the superiority of an interventional technique for aortic valve repair over surgery in terms of postoperative death has found the period of 30 days to 4 months after the procedure to be the most perilous for surgery patients, when their risk of death was almost twice that of interventional patients, likely because surgery patients were more vulnerable to complications and were less likely to go home after the procedure.
“This mortality difference was largely driven by higher rates of technical failure, surgical complications, and lack of recovery following surgery,” said Vincent A. Gaudiani, MD, of El Camino Hospital, Mountain View, Calif., and his coauthors (J Thorac Cardiovasc Surg. 2017;153:1293-99). The analysis investigated causes and timing of death in the CoreValve US Pivotal High-Risk Trial, a randomized, high-risk trial of the CoreValve self-expanding bioprosthesis (Medtronic). The trial favored transcatheter aortic valve replacement (TAVR) over surgical aortic valve replacement (SAVR).
The post hoc analysis evaluated all-cause mortality through the first year based on three time periods: early, up to 30 days; recovery, 31-120 days; and late, 121-365 days. Death rates for the two procedures were similar in the early and late postoperative periods, but deviated significantly in the recovery period: 4% for TAVR vs. 7.9% for SAVR (P = .25). SAVR patients were more likely affected by the overall influence of physical stress associated with surgery, the study found, whereas rates of technical failure and complications were similar between the two groups. “This suggests that early TAVR results can improve with technical refinements and that high-risk surgical patients will benefit from reducing complications,” wrote Dr. Gaudiani and his coauthors.
They noted the CoreValve trial findings, in terms of the survival differences between TAVR and SAVR, are significant because previous trials that compared TAVR and SAVR, including the Placement of Aortic Transcatheter Valves A trial (Lancet. 2015;385:2477-84), showed equivalent survival between the two procedures at up to 5 years. “This unique finding is provocative and the reason for this survival difference is important to understanding TAVR and SAVR and improving both therapies,” said Dr. Gaudiani and his coauthors.
While SAVR patients had a higher overall death rate in the recovery period, TAVR patients had a larger proportion of cardiovascular deaths – 12 of 15 (80%) vs. 16 of 27 (59.3%) for SAVR. The leading noncardiovascular cause of death in the SAVR group was sepsis (six), followed by malignancy (one), chronic obstructive pulmonary disease (one) and other (three). “Although these deaths were adjudicated as noncardiovascular by the CEC [clinical events committee], our review showed that some of these patients had never really recovered from the initial procedure,” the researchers wrote.
In the early period, death rates were 3.3% for TAVR and 4.5% for SAVR, a nonsignificant difference. TAVR patients who died had higher rates of peripheral vascular disease and recent falls; SAVR patients who died were more likely to have had a pacemaker. In the late period, the death rates were 7.5% for TAVR and 7.7% for SAVR, and the researchers also found no significant difference in the number of cardiovascular deaths (4.4% and 4.2%, respectively). “Hierarchical causes of death were primarily due to other reasons deemed unrelated to the initial aortic valve replacement,” noted Dr. Gaudiani and his coauthors.
However, the study also found that TAVR patients were significantly more likely to go home after hospital discharge rather than to a rehabilitation facility or another hospital – 66.9% vs. 39.7% (P less than .001).
In the SAVR group, five cardiovascular deaths in the recovery period occurred because the operation failed to correct aortic stenosis – all related to placement of a valve too small for the patient. “Placing a valve appropriately sized to the patient should be a priority for surgeons if we are to improve our outcomes,” the researchers noted. “Most other deaths were the result of patients’ inability to cope with the physical trauma of surgery.”
Dr. Gaudiani disclosed that he is a consultant and paid instructor for Medtronic, St. Jude Medical, and Edwards Lifesciences. Coauthors disclosed relationships with Edwards Lifesciences, Terumo, Gore Medical, Medtronic, Boston Scientific, and other device companies.
In his invited discussion, Craig R. Smith, MD, of New York, noted that comparisons “are odious” and that comparing clinical trials requires caution. (J Thorac Cardiovasc Surg. 2017;153:1300-1) He also acknowledged that surgeons would hope for evidence that the findings of the CoreValve US Pivotal High-Risk Trial were somehow wrong.
Dr. Smith raised a question about the CoreValve trial, which was designed to enroll high-risk patients, “but actually enrolled at the upper end of the intermediate risk range with a Society of Thoracic Surgeons (STS) score of 7.4 versus 11.3 in the high-risk PARTNER 1.” However, he noted that it would not be fair to consider the self-expanding TAVR trial intermediate risk, because the intermediate risk PARTNER 2 trial had an STS score of 5.8. And while outcomes for SAVR in the CoreValve trial were within the expected variable of less than 1 using the STS Predicted Risk for Mortality, the “bulge” of deaths in the recovery phase raises “a whiff of concern.”
Dr. Smith said that the early technical mortalities with TAVR in the trial are already disappearing with experience. He also noted that Dr. Gaudiani and his coauthors pointed out the frequency of failure to repair and failure to recover. “Whether competing against TAVR in a randomized trial or operating on TAVR in eligible patients in the future, as the authors have emphasized, it behooves us to correct the problem as completely as possible and take the best possible care of our patients afterward,” Dr. Smith said. He also noted the difference in discharge rates home “illustrates a very significant advantage of TAVR.”
Dr. Smith disclosed he has received reimbursement for expenses in his leadership role in the Placement of Aortic Transcatheter Valves (PARTNER) trials.
In his invited discussion, Craig R. Smith, MD, of New York, noted that comparisons “are odious” and that comparing clinical trials requires caution. (J Thorac Cardiovasc Surg. 2017;153:1300-1) He also acknowledged that surgeons would hope for evidence that the findings of the CoreValve US Pivotal High-Risk Trial were somehow wrong.
Dr. Smith raised a question about the CoreValve trial, which was designed to enroll high-risk patients, “but actually enrolled at the upper end of the intermediate risk range with a Society of Thoracic Surgeons (STS) score of 7.4 versus 11.3 in the high-risk PARTNER 1.” However, he noted that it would not be fair to consider the self-expanding TAVR trial intermediate risk, because the intermediate risk PARTNER 2 trial had an STS score of 5.8. And while outcomes for SAVR in the CoreValve trial were within the expected variable of less than 1 using the STS Predicted Risk for Mortality, the “bulge” of deaths in the recovery phase raises “a whiff of concern.”
Dr. Smith said that the early technical mortalities with TAVR in the trial are already disappearing with experience. He also noted that Dr. Gaudiani and his coauthors pointed out the frequency of failure to repair and failure to recover. “Whether competing against TAVR in a randomized trial or operating on TAVR in eligible patients in the future, as the authors have emphasized, it behooves us to correct the problem as completely as possible and take the best possible care of our patients afterward,” Dr. Smith said. He also noted the difference in discharge rates home “illustrates a very significant advantage of TAVR.”
Dr. Smith disclosed he has received reimbursement for expenses in his leadership role in the Placement of Aortic Transcatheter Valves (PARTNER) trials.
In his invited discussion, Craig R. Smith, MD, of New York, noted that comparisons “are odious” and that comparing clinical trials requires caution. (J Thorac Cardiovasc Surg. 2017;153:1300-1) He also acknowledged that surgeons would hope for evidence that the findings of the CoreValve US Pivotal High-Risk Trial were somehow wrong.
Dr. Smith raised a question about the CoreValve trial, which was designed to enroll high-risk patients, “but actually enrolled at the upper end of the intermediate risk range with a Society of Thoracic Surgeons (STS) score of 7.4 versus 11.3 in the high-risk PARTNER 1.” However, he noted that it would not be fair to consider the self-expanding TAVR trial intermediate risk, because the intermediate risk PARTNER 2 trial had an STS score of 5.8. And while outcomes for SAVR in the CoreValve trial were within the expected variable of less than 1 using the STS Predicted Risk for Mortality, the “bulge” of deaths in the recovery phase raises “a whiff of concern.”
Dr. Smith said that the early technical mortalities with TAVR in the trial are already disappearing with experience. He also noted that Dr. Gaudiani and his coauthors pointed out the frequency of failure to repair and failure to recover. “Whether competing against TAVR in a randomized trial or operating on TAVR in eligible patients in the future, as the authors have emphasized, it behooves us to correct the problem as completely as possible and take the best possible care of our patients afterward,” Dr. Smith said. He also noted the difference in discharge rates home “illustrates a very significant advantage of TAVR.”
Dr. Smith disclosed he has received reimbursement for expenses in his leadership role in the Placement of Aortic Transcatheter Valves (PARTNER) trials.
A post hoc analysis of the first randomized clinical to show the superiority of an interventional technique for aortic valve repair over surgery in terms of postoperative death has found the period of 30 days to 4 months after the procedure to be the most perilous for surgery patients, when their risk of death was almost twice that of interventional patients, likely because surgery patients were more vulnerable to complications and were less likely to go home after the procedure.
“This mortality difference was largely driven by higher rates of technical failure, surgical complications, and lack of recovery following surgery,” said Vincent A. Gaudiani, MD, of El Camino Hospital, Mountain View, Calif., and his coauthors (J Thorac Cardiovasc Surg. 2017;153:1293-99). The analysis investigated causes and timing of death in the CoreValve US Pivotal High-Risk Trial, a randomized, high-risk trial of the CoreValve self-expanding bioprosthesis (Medtronic). The trial favored transcatheter aortic valve replacement (TAVR) over surgical aortic valve replacement (SAVR).
The post hoc analysis evaluated all-cause mortality through the first year based on three time periods: early, up to 30 days; recovery, 31-120 days; and late, 121-365 days. Death rates for the two procedures were similar in the early and late postoperative periods, but deviated significantly in the recovery period: 4% for TAVR vs. 7.9% for SAVR (P = .25). SAVR patients were more likely affected by the overall influence of physical stress associated with surgery, the study found, whereas rates of technical failure and complications were similar between the two groups. “This suggests that early TAVR results can improve with technical refinements and that high-risk surgical patients will benefit from reducing complications,” wrote Dr. Gaudiani and his coauthors.
They noted the CoreValve trial findings, in terms of the survival differences between TAVR and SAVR, are significant because previous trials that compared TAVR and SAVR, including the Placement of Aortic Transcatheter Valves A trial (Lancet. 2015;385:2477-84), showed equivalent survival between the two procedures at up to 5 years. “This unique finding is provocative and the reason for this survival difference is important to understanding TAVR and SAVR and improving both therapies,” said Dr. Gaudiani and his coauthors.
While SAVR patients had a higher overall death rate in the recovery period, TAVR patients had a larger proportion of cardiovascular deaths – 12 of 15 (80%) vs. 16 of 27 (59.3%) for SAVR. The leading noncardiovascular cause of death in the SAVR group was sepsis (six), followed by malignancy (one), chronic obstructive pulmonary disease (one) and other (three). “Although these deaths were adjudicated as noncardiovascular by the CEC [clinical events committee], our review showed that some of these patients had never really recovered from the initial procedure,” the researchers wrote.
In the early period, death rates were 3.3% for TAVR and 4.5% for SAVR, a nonsignificant difference. TAVR patients who died had higher rates of peripheral vascular disease and recent falls; SAVR patients who died were more likely to have had a pacemaker. In the late period, the death rates were 7.5% for TAVR and 7.7% for SAVR, and the researchers also found no significant difference in the number of cardiovascular deaths (4.4% and 4.2%, respectively). “Hierarchical causes of death were primarily due to other reasons deemed unrelated to the initial aortic valve replacement,” noted Dr. Gaudiani and his coauthors.
However, the study also found that TAVR patients were significantly more likely to go home after hospital discharge rather than to a rehabilitation facility or another hospital – 66.9% vs. 39.7% (P less than .001).
In the SAVR group, five cardiovascular deaths in the recovery period occurred because the operation failed to correct aortic stenosis – all related to placement of a valve too small for the patient. “Placing a valve appropriately sized to the patient should be a priority for surgeons if we are to improve our outcomes,” the researchers noted. “Most other deaths were the result of patients’ inability to cope with the physical trauma of surgery.”
Dr. Gaudiani disclosed that he is a consultant and paid instructor for Medtronic, St. Jude Medical, and Edwards Lifesciences. Coauthors disclosed relationships with Edwards Lifesciences, Terumo, Gore Medical, Medtronic, Boston Scientific, and other device companies.
A post hoc analysis of the first randomized clinical to show the superiority of an interventional technique for aortic valve repair over surgery in terms of postoperative death has found the period of 30 days to 4 months after the procedure to be the most perilous for surgery patients, when their risk of death was almost twice that of interventional patients, likely because surgery patients were more vulnerable to complications and were less likely to go home after the procedure.
“This mortality difference was largely driven by higher rates of technical failure, surgical complications, and lack of recovery following surgery,” said Vincent A. Gaudiani, MD, of El Camino Hospital, Mountain View, Calif., and his coauthors (J Thorac Cardiovasc Surg. 2017;153:1293-99). The analysis investigated causes and timing of death in the CoreValve US Pivotal High-Risk Trial, a randomized, high-risk trial of the CoreValve self-expanding bioprosthesis (Medtronic). The trial favored transcatheter aortic valve replacement (TAVR) over surgical aortic valve replacement (SAVR).
The post hoc analysis evaluated all-cause mortality through the first year based on three time periods: early, up to 30 days; recovery, 31-120 days; and late, 121-365 days. Death rates for the two procedures were similar in the early and late postoperative periods, but deviated significantly in the recovery period: 4% for TAVR vs. 7.9% for SAVR (P = .25). SAVR patients were more likely affected by the overall influence of physical stress associated with surgery, the study found, whereas rates of technical failure and complications were similar between the two groups. “This suggests that early TAVR results can improve with technical refinements and that high-risk surgical patients will benefit from reducing complications,” wrote Dr. Gaudiani and his coauthors.
They noted the CoreValve trial findings, in terms of the survival differences between TAVR and SAVR, are significant because previous trials that compared TAVR and SAVR, including the Placement of Aortic Transcatheter Valves A trial (Lancet. 2015;385:2477-84), showed equivalent survival between the two procedures at up to 5 years. “This unique finding is provocative and the reason for this survival difference is important to understanding TAVR and SAVR and improving both therapies,” said Dr. Gaudiani and his coauthors.
While SAVR patients had a higher overall death rate in the recovery period, TAVR patients had a larger proportion of cardiovascular deaths – 12 of 15 (80%) vs. 16 of 27 (59.3%) for SAVR. The leading noncardiovascular cause of death in the SAVR group was sepsis (six), followed by malignancy (one), chronic obstructive pulmonary disease (one) and other (three). “Although these deaths were adjudicated as noncardiovascular by the CEC [clinical events committee], our review showed that some of these patients had never really recovered from the initial procedure,” the researchers wrote.
In the early period, death rates were 3.3% for TAVR and 4.5% for SAVR, a nonsignificant difference. TAVR patients who died had higher rates of peripheral vascular disease and recent falls; SAVR patients who died were more likely to have had a pacemaker. In the late period, the death rates were 7.5% for TAVR and 7.7% for SAVR, and the researchers also found no significant difference in the number of cardiovascular deaths (4.4% and 4.2%, respectively). “Hierarchical causes of death were primarily due to other reasons deemed unrelated to the initial aortic valve replacement,” noted Dr. Gaudiani and his coauthors.
However, the study also found that TAVR patients were significantly more likely to go home after hospital discharge rather than to a rehabilitation facility or another hospital – 66.9% vs. 39.7% (P less than .001).
In the SAVR group, five cardiovascular deaths in the recovery period occurred because the operation failed to correct aortic stenosis – all related to placement of a valve too small for the patient. “Placing a valve appropriately sized to the patient should be a priority for surgeons if we are to improve our outcomes,” the researchers noted. “Most other deaths were the result of patients’ inability to cope with the physical trauma of surgery.”
Dr. Gaudiani disclosed that he is a consultant and paid instructor for Medtronic, St. Jude Medical, and Edwards Lifesciences. Coauthors disclosed relationships with Edwards Lifesciences, Terumo, Gore Medical, Medtronic, Boston Scientific, and other device companies.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Differences in survival between transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) in high-risk patients appear to be driven primarily by issues with disease repair and patient recovery from SAVR.
Major finding: During the recovery period (31-120 days) the death rate was 4% for TAVR vs. 7.9% for SAVR (P = .025).
Data source: Post hoc analysis of CoreValve US Pivotal High-Risk Trial involving 750 patients and 45 sites with outcomes reported through 3 years.
Disclosures: Dr. Gaudiani disclosed that he is a consultant and paid instructor for Medtronic, St. Jude Medical, and Edwards Lifesciences. Coauthors disclosed relationships with Edwards Lifesciences, Terumo, Gore Medical, Medtronic, Boston Scientific, and other device companies.
GOP health reform dead for now
Senate Republicans are scrambling to come up with another plan now that at least four member of their caucus have said that they would vote against moving forward with debate on the Better Care Reconciliation Act.
Support for the bill, which included dramatic Medicaid cuts and stripped many coverage provisions of the Affordable Care Act, was lacking after revisions were announced on July 13. At that time, conservative Sen. Ran Paul (R-Ky.) and moderate Susan Collins (R-Maine) voiced their opposition for different ideological reasons. They were joined by Sen. Mike Lee (R-Utah) and Sen. Jerry Moran (R-Kan.), who also declined to support the bill. Senate GOP leadership, with a slim 52-48 majority, could only afford to lose two votes (Vice President Mike Pence would have been the tie-breaking vote).
No new timeline has been revealed for the next steps.
“The health reform debate is by no means over,” David Barbe, MD, president of the American Medical Association, said in a statement. “Congress must begin a collaborative process that produces a bipartisan solution. ... Near-term action is needed to stabilize the individual/nongroup health insurance marketplace. In the long term, stakeholders and policymakers need to address the unsustainable trends in health care costs while achieving meaningful, affordable coverage for all Americans.”
Senate Republicans are scrambling to come up with another plan now that at least four member of their caucus have said that they would vote against moving forward with debate on the Better Care Reconciliation Act.
Support for the bill, which included dramatic Medicaid cuts and stripped many coverage provisions of the Affordable Care Act, was lacking after revisions were announced on July 13. At that time, conservative Sen. Ran Paul (R-Ky.) and moderate Susan Collins (R-Maine) voiced their opposition for different ideological reasons. They were joined by Sen. Mike Lee (R-Utah) and Sen. Jerry Moran (R-Kan.), who also declined to support the bill. Senate GOP leadership, with a slim 52-48 majority, could only afford to lose two votes (Vice President Mike Pence would have been the tie-breaking vote).
No new timeline has been revealed for the next steps.
“The health reform debate is by no means over,” David Barbe, MD, president of the American Medical Association, said in a statement. “Congress must begin a collaborative process that produces a bipartisan solution. ... Near-term action is needed to stabilize the individual/nongroup health insurance marketplace. In the long term, stakeholders and policymakers need to address the unsustainable trends in health care costs while achieving meaningful, affordable coverage for all Americans.”
Senate Republicans are scrambling to come up with another plan now that at least four member of their caucus have said that they would vote against moving forward with debate on the Better Care Reconciliation Act.
Support for the bill, which included dramatic Medicaid cuts and stripped many coverage provisions of the Affordable Care Act, was lacking after revisions were announced on July 13. At that time, conservative Sen. Ran Paul (R-Ky.) and moderate Susan Collins (R-Maine) voiced their opposition for different ideological reasons. They were joined by Sen. Mike Lee (R-Utah) and Sen. Jerry Moran (R-Kan.), who also declined to support the bill. Senate GOP leadership, with a slim 52-48 majority, could only afford to lose two votes (Vice President Mike Pence would have been the tie-breaking vote).
No new timeline has been revealed for the next steps.
“The health reform debate is by no means over,” David Barbe, MD, president of the American Medical Association, said in a statement. “Congress must begin a collaborative process that produces a bipartisan solution. ... Near-term action is needed to stabilize the individual/nongroup health insurance marketplace. In the long term, stakeholders and policymakers need to address the unsustainable trends in health care costs while achieving meaningful, affordable coverage for all Americans.”
New trial shows thymectomy benefits myasthenia gravis
The effectiveness of thymectomy as a cure for myasthenia gravis has long been debated, but the publication of Myasthenia Gravis Thymectomy Treatment (MGTX) trial results, showing that thymectomy improved outcomes over 3 years in patients with nonthymomatous myasthenia gravis, has gone a long way toward settling the debate, Joshua R. Sonett, MD, and his coauthors noted in a feature expert opinion (J Thorac Cardiovasc Surg. 2017;154:306-9).
The MGTX trial randomized patients with nonthymomatous MG into two treatment groups: medical therapy alone or thymectomy with medical therapy (N Engl J Med. 2016;375:511-22). For uniformity, the study mandated one type of thymectomy, an extended transsternal approach. The study was 12 years in the making, with 6 years of patient accrual followed by 3 years of surveillance, Dr. Sonett and his coauthors noted.
Those markers include an average quantitative myasthenia score of 6.15 for the thymectomy group vs. 8.99 for the medical therapy group (P less than .0001); a lower dose of prednisone to attain improved neurologic status (44 mg vs. 60 mg; P less than .001); time-weighted average score on the Myasthenia Gravis Activities of Daily Living scale (2.24 vs. 3.41; P = .008); azathioprine use (17% vs. 48%; P less than .001); percentage of patients who had minimal-manifestation status at month 36 (67% vs. 47%; P = .03); and hospitalization for myasthenia-related symptoms (9% vs. 37%). “Interestingly,” the researchers wrote, “despite these quantitative results, no difference was seen in the quality of life measured surveys.”
An ancillary study, Bio-MGTX, was performed simultaneously to investigate pathologic and serum markers. “Many questions still need to be answered in regard to the role of thymectomy in MG,” Dr. Sonett and his coauthors maintained. They include an analysis of radiologic predictors of success with thymectomy, and the role of thymectomy in seronegative MG, ocular MG and elderly patients.
“Future studies may be directed at achieving a more rapid and consistent time to a complete symptom response,” they said.
The MGTX trial does support the use of high-dose prednisone induction combined with thymectomy to achieve higher complete early remission rates, but Bio-MGTX data may help to refine induction protocols. “The debate will likely continue in regard to widespread adoption of extended transsternal maximal thymectomy,” the researchers wrote. “What was categorically measured in this trial was the effect of maximal thymectomy, as sternotomy offers no particular independent therapeutic benefit.”
The structure of the MGTX trial despite its small cohort (126) “enabled the medical and surgical community to definitively answer an important question,” they noted. Nonetheless, further investigation of the role of thymectomy in MG is “sorely needed.”
Patients may need up to 3 years to achieve an optimal response, and complete cure in a shorter time frame should be the goal for each patient. Multimodal therapy should be the basis of MG treatment. “Continued progress in the management of MG will require diligent, multidisciplinary teams designing and completing prospective studies like the MGTX,” the researchers wrote.
Dr. Sonett and his coauthors had no financial relationships to disclose. The MGTX trial was funded by the U.S. National Institute of Neurological Disorders and Stroke. There was no commercial support for the trial.
In the MGTX trial, patients in the thymectomy group still needed a high average dose of prednisone, and the rates of remission may decrease over time, Michael K. Hsin, MD, of Queen Mary Hospital, Hong Kong, wrote in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:310-1). But he added that the trial did finally answer in a positive manner whether thymectomy could serve a beneficial role.
He also noted that the MGTX trial left at least four questions unanswered:
• The long-term effect of thymectomy on MG status with regard to future relapse.
• The role of surgery in the era of advances in medical treatment, including azathioprine to reduce the prednisone dose and emergence of stem-cell transplantation.
• The extent to which MGTX findings can be applied to acetylcholine receptor-negative pediatric patients.
• Whether alternative techniques to extended transsternal thymectomy can achieve comparable results.
Dr. Hsin had no financial relationships to disclose.
In the MGTX trial, patients in the thymectomy group still needed a high average dose of prednisone, and the rates of remission may decrease over time, Michael K. Hsin, MD, of Queen Mary Hospital, Hong Kong, wrote in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:310-1). But he added that the trial did finally answer in a positive manner whether thymectomy could serve a beneficial role.
He also noted that the MGTX trial left at least four questions unanswered:
• The long-term effect of thymectomy on MG status with regard to future relapse.
• The role of surgery in the era of advances in medical treatment, including azathioprine to reduce the prednisone dose and emergence of stem-cell transplantation.
• The extent to which MGTX findings can be applied to acetylcholine receptor-negative pediatric patients.
• Whether alternative techniques to extended transsternal thymectomy can achieve comparable results.
Dr. Hsin had no financial relationships to disclose.
In the MGTX trial, patients in the thymectomy group still needed a high average dose of prednisone, and the rates of remission may decrease over time, Michael K. Hsin, MD, of Queen Mary Hospital, Hong Kong, wrote in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:310-1). But he added that the trial did finally answer in a positive manner whether thymectomy could serve a beneficial role.
He also noted that the MGTX trial left at least four questions unanswered:
• The long-term effect of thymectomy on MG status with regard to future relapse.
• The role of surgery in the era of advances in medical treatment, including azathioprine to reduce the prednisone dose and emergence of stem-cell transplantation.
• The extent to which MGTX findings can be applied to acetylcholine receptor-negative pediatric patients.
• Whether alternative techniques to extended transsternal thymectomy can achieve comparable results.
Dr. Hsin had no financial relationships to disclose.
The effectiveness of thymectomy as a cure for myasthenia gravis has long been debated, but the publication of Myasthenia Gravis Thymectomy Treatment (MGTX) trial results, showing that thymectomy improved outcomes over 3 years in patients with nonthymomatous myasthenia gravis, has gone a long way toward settling the debate, Joshua R. Sonett, MD, and his coauthors noted in a feature expert opinion (J Thorac Cardiovasc Surg. 2017;154:306-9).
The MGTX trial randomized patients with nonthymomatous MG into two treatment groups: medical therapy alone or thymectomy with medical therapy (N Engl J Med. 2016;375:511-22). For uniformity, the study mandated one type of thymectomy, an extended transsternal approach. The study was 12 years in the making, with 6 years of patient accrual followed by 3 years of surveillance, Dr. Sonett and his coauthors noted.
Those markers include an average quantitative myasthenia score of 6.15 for the thymectomy group vs. 8.99 for the medical therapy group (P less than .0001); a lower dose of prednisone to attain improved neurologic status (44 mg vs. 60 mg; P less than .001); time-weighted average score on the Myasthenia Gravis Activities of Daily Living scale (2.24 vs. 3.41; P = .008); azathioprine use (17% vs. 48%; P less than .001); percentage of patients who had minimal-manifestation status at month 36 (67% vs. 47%; P = .03); and hospitalization for myasthenia-related symptoms (9% vs. 37%). “Interestingly,” the researchers wrote, “despite these quantitative results, no difference was seen in the quality of life measured surveys.”
An ancillary study, Bio-MGTX, was performed simultaneously to investigate pathologic and serum markers. “Many questions still need to be answered in regard to the role of thymectomy in MG,” Dr. Sonett and his coauthors maintained. They include an analysis of radiologic predictors of success with thymectomy, and the role of thymectomy in seronegative MG, ocular MG and elderly patients.
“Future studies may be directed at achieving a more rapid and consistent time to a complete symptom response,” they said.
The MGTX trial does support the use of high-dose prednisone induction combined with thymectomy to achieve higher complete early remission rates, but Bio-MGTX data may help to refine induction protocols. “The debate will likely continue in regard to widespread adoption of extended transsternal maximal thymectomy,” the researchers wrote. “What was categorically measured in this trial was the effect of maximal thymectomy, as sternotomy offers no particular independent therapeutic benefit.”
The structure of the MGTX trial despite its small cohort (126) “enabled the medical and surgical community to definitively answer an important question,” they noted. Nonetheless, further investigation of the role of thymectomy in MG is “sorely needed.”
Patients may need up to 3 years to achieve an optimal response, and complete cure in a shorter time frame should be the goal for each patient. Multimodal therapy should be the basis of MG treatment. “Continued progress in the management of MG will require diligent, multidisciplinary teams designing and completing prospective studies like the MGTX,” the researchers wrote.
Dr. Sonett and his coauthors had no financial relationships to disclose. The MGTX trial was funded by the U.S. National Institute of Neurological Disorders and Stroke. There was no commercial support for the trial.
The effectiveness of thymectomy as a cure for myasthenia gravis has long been debated, but the publication of Myasthenia Gravis Thymectomy Treatment (MGTX) trial results, showing that thymectomy improved outcomes over 3 years in patients with nonthymomatous myasthenia gravis, has gone a long way toward settling the debate, Joshua R. Sonett, MD, and his coauthors noted in a feature expert opinion (J Thorac Cardiovasc Surg. 2017;154:306-9).
The MGTX trial randomized patients with nonthymomatous MG into two treatment groups: medical therapy alone or thymectomy with medical therapy (N Engl J Med. 2016;375:511-22). For uniformity, the study mandated one type of thymectomy, an extended transsternal approach. The study was 12 years in the making, with 6 years of patient accrual followed by 3 years of surveillance, Dr. Sonett and his coauthors noted.
Those markers include an average quantitative myasthenia score of 6.15 for the thymectomy group vs. 8.99 for the medical therapy group (P less than .0001); a lower dose of prednisone to attain improved neurologic status (44 mg vs. 60 mg; P less than .001); time-weighted average score on the Myasthenia Gravis Activities of Daily Living scale (2.24 vs. 3.41; P = .008); azathioprine use (17% vs. 48%; P less than .001); percentage of patients who had minimal-manifestation status at month 36 (67% vs. 47%; P = .03); and hospitalization for myasthenia-related symptoms (9% vs. 37%). “Interestingly,” the researchers wrote, “despite these quantitative results, no difference was seen in the quality of life measured surveys.”
An ancillary study, Bio-MGTX, was performed simultaneously to investigate pathologic and serum markers. “Many questions still need to be answered in regard to the role of thymectomy in MG,” Dr. Sonett and his coauthors maintained. They include an analysis of radiologic predictors of success with thymectomy, and the role of thymectomy in seronegative MG, ocular MG and elderly patients.
“Future studies may be directed at achieving a more rapid and consistent time to a complete symptom response,” they said.
The MGTX trial does support the use of high-dose prednisone induction combined with thymectomy to achieve higher complete early remission rates, but Bio-MGTX data may help to refine induction protocols. “The debate will likely continue in regard to widespread adoption of extended transsternal maximal thymectomy,” the researchers wrote. “What was categorically measured in this trial was the effect of maximal thymectomy, as sternotomy offers no particular independent therapeutic benefit.”
The structure of the MGTX trial despite its small cohort (126) “enabled the medical and surgical community to definitively answer an important question,” they noted. Nonetheless, further investigation of the role of thymectomy in MG is “sorely needed.”
Patients may need up to 3 years to achieve an optimal response, and complete cure in a shorter time frame should be the goal for each patient. Multimodal therapy should be the basis of MG treatment. “Continued progress in the management of MG will require diligent, multidisciplinary teams designing and completing prospective studies like the MGTX,” the researchers wrote.
Dr. Sonett and his coauthors had no financial relationships to disclose. The MGTX trial was funded by the U.S. National Institute of Neurological Disorders and Stroke. There was no commercial support for the trial.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: A recently published prospective randomized trial provides definitive evidence that thymectomy significantly improves outcomes of patients with myasthenia gravis.
Major finding: Patients who underwent thymectomy had an average quantitative myasthenia score of 6.15 vs. 8.99 for the medical therapy group, a significant difference.
Data source: Myasthenia Gravis Thymectomy Trial, a prospective trial of 126 patients randomized to thymectomy with medical therapy or medical therapy alone.
Disclosures: Dr. Sonett and his coauthors had no financial relationships to disclose. The MGTX trial was funded by the U.S. National Institute of Neurological Disorders and Stroke. There was no commercial support for the trial.
Pulmonary metastasectomy may be useful for soft-tissue sarcoma spread
The rate of soft-tissue sarcoma has nearly doubled over the past two decades, and up to 50% of patients with tissue sarcoma develop lung metastasis. A single-center study of 539 patients who had treatment for soft-tissue sarcoma has revealed disease and treatment characteristics that may aid patient selection and help predict overall and disease-free survival after diagnosis and treatment.
“Histologic subtype and size of the primary tumor were significantly associated with overall survival,” said lead author Neel P. Chudgar, MD, and his coauthors in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2017;154:319-30).
“Patients who underwent pulmonary metastasectomy [PM] for pleomorphic sarcoma/malignant fibrous histocytoma had the shortest median overall survival (23.6 months), whereas those who underwent PM for leiomyosarcoma had a median overall survival of 42 months,” he said.
The study subjects had pulmonary metastasectomies at Memorial Sloan Kettering Cancer Center, New York, during September 1991–June 2014. The median overall survival was 33.2 months, and median disease-free survival was 6.8 months for the entire cohort.
Among the disease characteristics associated with a lower hazard ratio of death shown by multivariable analyses were leiomyosarcoma histologic subtype (HR, 0.57), primary tumor size of 10 cm or less (HR, 1.00 vs. HR, 1.37 for those greater than 10 cm), increasing time from primary tumor resection to development of metastases (HR, 0.4 at less than 24 months vs. 1.0 at less than 6 months), solitary lung metastasis (HR, 1.0 vs. 1.8 for one year or more), and minimally invasive resection (HR, 0.71), all of which were statistically significant differences. Disease-free interval of more than one year and one pulmonary metastasis were significantly associated with lower hazard of disease recurrence.
Of patients, 70% had pulmonary metastasectomy as their primary treatment. The remainder had induction chemotherapy. In addition, 71% had open procedures over the 23-year study period, but minimally invasive operations became more common with time, increasing more than fourfold from the first half of the study period, vs. the last. They accounted for more than half of all procedures in the last five years of the study.
With regard to tumor type, fibrosarcoma was associated with longest median overall survival (65.2 months). Dr. Chudgar and his colleagues noted that 43% of these patients had low-grade primary tumors. Patients with low-grade tumors of all types had a median overall survival of 71.8 months, vs. 30.8 months for those with high-grade tumors.
“Our results indicate that therapeutic-intent pulmonary metastasectomy for soft-tissue sarcoma can be associated with prolonged survival,” Dr. Chudgar and his coauthors said. “The median survivals in our study are comparable with those in previous studies.” However, their analysis went beyond previous studies because they identified positive prognostic factors.
Dr. Chudgar and his coauthors acknowledge that various studies have drawn conflicting conclusions about the validity of histologic subtype as a prognostic factor, but their study differs from previous studies because it is a single-center cohort, “which increases the power to potentially identify significant differences, and we focused on soft-tissue sarcoma exclusively to enhance the homogeneity of the study population.”
Nonetheless, the researchers noted some limitations of their study, namely their collective analysis of the various soft-tissue sarcoma subtypes and the lack of a control group. Soft tissue sarcoma, because of its heterogeneous nature, challenges the adoption of precision medicine for this cancer type, but, until clinicians better understand the underlying mechanism of metastasis in these tumor types, Dr. Chudgar and his coauthors said, pulmonary metastasectomy “remains the best available treatment for soft tissue sarcoma pulmonary metastases.”
Dr. Chudgar and his coauthors had no financial relationships to disclose.
The findings that surgery for pulmonary metastases achieves “relatively good median survival” that Dr. Chudgar and coauthors reported are “especially impressive when considering that more than 25% of these patients with metastatic cancer had five or more pulmonary lesions,” said Mark F. Berry, MD, MHS, of Stanford University in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:117-8).
Dr. Berry also said, however, that surgeons still must consider these results cautiously for several reasons. One, the study is retrospective and uncontrolled. Two, the study does not address whether the researchers selected healthy patients “with favorable disease characteristics” for pulmonary metastasectomy. “The sobering reality is that most patients still had recurrence relatively soon after complete pulmonary resection,” Dr. Berry said.
The study does support the current practice of pulmonary metastasectomy, which many patients may prefer for its invasive nature, compared with systemic chemotherapy treatment, he said. “Overall, surgeons can use this study to aid patient selection [and] to support the clinical decision to pursue resection of soft-tissue sarcoma pulmonary metastases for patients judged to be appropriate surgical candidates,” Dr. Berry concluded.
Dr. Berry had reported no financial disclosures.
The findings that surgery for pulmonary metastases achieves “relatively good median survival” that Dr. Chudgar and coauthors reported are “especially impressive when considering that more than 25% of these patients with metastatic cancer had five or more pulmonary lesions,” said Mark F. Berry, MD, MHS, of Stanford University in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:117-8).
Dr. Berry also said, however, that surgeons still must consider these results cautiously for several reasons. One, the study is retrospective and uncontrolled. Two, the study does not address whether the researchers selected healthy patients “with favorable disease characteristics” for pulmonary metastasectomy. “The sobering reality is that most patients still had recurrence relatively soon after complete pulmonary resection,” Dr. Berry said.
The study does support the current practice of pulmonary metastasectomy, which many patients may prefer for its invasive nature, compared with systemic chemotherapy treatment, he said. “Overall, surgeons can use this study to aid patient selection [and] to support the clinical decision to pursue resection of soft-tissue sarcoma pulmonary metastases for patients judged to be appropriate surgical candidates,” Dr. Berry concluded.
Dr. Berry had reported no financial disclosures.
The findings that surgery for pulmonary metastases achieves “relatively good median survival” that Dr. Chudgar and coauthors reported are “especially impressive when considering that more than 25% of these patients with metastatic cancer had five or more pulmonary lesions,” said Mark F. Berry, MD, MHS, of Stanford University in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:117-8).
Dr. Berry also said, however, that surgeons still must consider these results cautiously for several reasons. One, the study is retrospective and uncontrolled. Two, the study does not address whether the researchers selected healthy patients “with favorable disease characteristics” for pulmonary metastasectomy. “The sobering reality is that most patients still had recurrence relatively soon after complete pulmonary resection,” Dr. Berry said.
The study does support the current practice of pulmonary metastasectomy, which many patients may prefer for its invasive nature, compared with systemic chemotherapy treatment, he said. “Overall, surgeons can use this study to aid patient selection [and] to support the clinical decision to pursue resection of soft-tissue sarcoma pulmonary metastases for patients judged to be appropriate surgical candidates,” Dr. Berry concluded.
Dr. Berry had reported no financial disclosures.
The rate of soft-tissue sarcoma has nearly doubled over the past two decades, and up to 50% of patients with tissue sarcoma develop lung metastasis. A single-center study of 539 patients who had treatment for soft-tissue sarcoma has revealed disease and treatment characteristics that may aid patient selection and help predict overall and disease-free survival after diagnosis and treatment.
“Histologic subtype and size of the primary tumor were significantly associated with overall survival,” said lead author Neel P. Chudgar, MD, and his coauthors in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2017;154:319-30).
“Patients who underwent pulmonary metastasectomy [PM] for pleomorphic sarcoma/malignant fibrous histocytoma had the shortest median overall survival (23.6 months), whereas those who underwent PM for leiomyosarcoma had a median overall survival of 42 months,” he said.
The study subjects had pulmonary metastasectomies at Memorial Sloan Kettering Cancer Center, New York, during September 1991–June 2014. The median overall survival was 33.2 months, and median disease-free survival was 6.8 months for the entire cohort.
Among the disease characteristics associated with a lower hazard ratio of death shown by multivariable analyses were leiomyosarcoma histologic subtype (HR, 0.57), primary tumor size of 10 cm or less (HR, 1.00 vs. HR, 1.37 for those greater than 10 cm), increasing time from primary tumor resection to development of metastases (HR, 0.4 at less than 24 months vs. 1.0 at less than 6 months), solitary lung metastasis (HR, 1.0 vs. 1.8 for one year or more), and minimally invasive resection (HR, 0.71), all of which were statistically significant differences. Disease-free interval of more than one year and one pulmonary metastasis were significantly associated with lower hazard of disease recurrence.
Of patients, 70% had pulmonary metastasectomy as their primary treatment. The remainder had induction chemotherapy. In addition, 71% had open procedures over the 23-year study period, but minimally invasive operations became more common with time, increasing more than fourfold from the first half of the study period, vs. the last. They accounted for more than half of all procedures in the last five years of the study.
With regard to tumor type, fibrosarcoma was associated with longest median overall survival (65.2 months). Dr. Chudgar and his colleagues noted that 43% of these patients had low-grade primary tumors. Patients with low-grade tumors of all types had a median overall survival of 71.8 months, vs. 30.8 months for those with high-grade tumors.
“Our results indicate that therapeutic-intent pulmonary metastasectomy for soft-tissue sarcoma can be associated with prolonged survival,” Dr. Chudgar and his coauthors said. “The median survivals in our study are comparable with those in previous studies.” However, their analysis went beyond previous studies because they identified positive prognostic factors.
Dr. Chudgar and his coauthors acknowledge that various studies have drawn conflicting conclusions about the validity of histologic subtype as a prognostic factor, but their study differs from previous studies because it is a single-center cohort, “which increases the power to potentially identify significant differences, and we focused on soft-tissue sarcoma exclusively to enhance the homogeneity of the study population.”
Nonetheless, the researchers noted some limitations of their study, namely their collective analysis of the various soft-tissue sarcoma subtypes and the lack of a control group. Soft tissue sarcoma, because of its heterogeneous nature, challenges the adoption of precision medicine for this cancer type, but, until clinicians better understand the underlying mechanism of metastasis in these tumor types, Dr. Chudgar and his coauthors said, pulmonary metastasectomy “remains the best available treatment for soft tissue sarcoma pulmonary metastases.”
Dr. Chudgar and his coauthors had no financial relationships to disclose.
The rate of soft-tissue sarcoma has nearly doubled over the past two decades, and up to 50% of patients with tissue sarcoma develop lung metastasis. A single-center study of 539 patients who had treatment for soft-tissue sarcoma has revealed disease and treatment characteristics that may aid patient selection and help predict overall and disease-free survival after diagnosis and treatment.
“Histologic subtype and size of the primary tumor were significantly associated with overall survival,” said lead author Neel P. Chudgar, MD, and his coauthors in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2017;154:319-30).
“Patients who underwent pulmonary metastasectomy [PM] for pleomorphic sarcoma/malignant fibrous histocytoma had the shortest median overall survival (23.6 months), whereas those who underwent PM for leiomyosarcoma had a median overall survival of 42 months,” he said.
The study subjects had pulmonary metastasectomies at Memorial Sloan Kettering Cancer Center, New York, during September 1991–June 2014. The median overall survival was 33.2 months, and median disease-free survival was 6.8 months for the entire cohort.
Among the disease characteristics associated with a lower hazard ratio of death shown by multivariable analyses were leiomyosarcoma histologic subtype (HR, 0.57), primary tumor size of 10 cm or less (HR, 1.00 vs. HR, 1.37 for those greater than 10 cm), increasing time from primary tumor resection to development of metastases (HR, 0.4 at less than 24 months vs. 1.0 at less than 6 months), solitary lung metastasis (HR, 1.0 vs. 1.8 for one year or more), and minimally invasive resection (HR, 0.71), all of which were statistically significant differences. Disease-free interval of more than one year and one pulmonary metastasis were significantly associated with lower hazard of disease recurrence.
Of patients, 70% had pulmonary metastasectomy as their primary treatment. The remainder had induction chemotherapy. In addition, 71% had open procedures over the 23-year study period, but minimally invasive operations became more common with time, increasing more than fourfold from the first half of the study period, vs. the last. They accounted for more than half of all procedures in the last five years of the study.
With regard to tumor type, fibrosarcoma was associated with longest median overall survival (65.2 months). Dr. Chudgar and his colleagues noted that 43% of these patients had low-grade primary tumors. Patients with low-grade tumors of all types had a median overall survival of 71.8 months, vs. 30.8 months for those with high-grade tumors.
“Our results indicate that therapeutic-intent pulmonary metastasectomy for soft-tissue sarcoma can be associated with prolonged survival,” Dr. Chudgar and his coauthors said. “The median survivals in our study are comparable with those in previous studies.” However, their analysis went beyond previous studies because they identified positive prognostic factors.
Dr. Chudgar and his coauthors acknowledge that various studies have drawn conflicting conclusions about the validity of histologic subtype as a prognostic factor, but their study differs from previous studies because it is a single-center cohort, “which increases the power to potentially identify significant differences, and we focused on soft-tissue sarcoma exclusively to enhance the homogeneity of the study population.”
Nonetheless, the researchers noted some limitations of their study, namely their collective analysis of the various soft-tissue sarcoma subtypes and the lack of a control group. Soft tissue sarcoma, because of its heterogeneous nature, challenges the adoption of precision medicine for this cancer type, but, until clinicians better understand the underlying mechanism of metastasis in these tumor types, Dr. Chudgar and his coauthors said, pulmonary metastasectomy “remains the best available treatment for soft tissue sarcoma pulmonary metastases.”
Dr. Chudgar and his coauthors had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Characteristics that determine survival in patients with sarcomatous pulmonary metastases are tumor subtype and size, number of and time to metastases, and minimally invasive surgery.
Major finding: Patients with leiomyosarcoma histologic subtype tumor had a hazard ratio of 0.57 (P = .001), and those with a primary tumor size of 10 cm or less had an HR of 1, vs. an HR of 1.37 for those greater than 10 cm (P = .006)
Data source: A single-institution study of 539 patients who had pulmonary mastectomy for metastatic soft tissue sarcoma from September 1991 to June 2014.
Disclosures: Dr. Chudgar and his coauthors had no financial relationships to disclose.
Immune signature shows good prognostic performance in early-stage NSCLC
A new tumor immune-related gene signature may help take the guesswork out of prognostication in patients with early-stage non–small cell lung cancer (NSCLC), according to a retrospective cohort study.
“Various components of the immune system have been shown to be a determining factor during cancer initiation and progression,” note the investigators, who were led by Bailiang Li, PhD, of Stanford (Calif.) University. “Recent immunotherapies targeting specific immune checkpoints such as programmed death 1 or programmed death ligand 1 have demonstrated a remarkable, durable response in NSCLC. Certain histopathologic patterns, such as intratumoral infiltration by cytotoxic lymphocytes, have also been associated with better prognoses in several cancer types, including NSCLC.”
For the study, the investigators developed and validated an immune-related gene signature using frozen tumors from 2,414 patients with stage I or II nonsquamous NSCLC from 19 public cohorts who underwent resection with negative margins and did not receive any neoadjuvant or adjuvant therapy.
The new signature contained 25 gene pairs consisting of 40 unique immune-related genes, Dr. Li and associates report (JAMA Oncol. 2017 Jul 6. doi: 10.1001/jamaoncol.2017.1609).
Processes such as chemotaxis were enriched among the included genes.
The signature significantly stratified patients into groups that have high and low risks of death during follow-up, both across and within subsets with stage I, IA, IB, or II disease. Relative to counterparts falling into the signature-defined low-risk group, those falling into the signature-defined high-risk group had roughly twice the risk of death after adjustment for clinical and pathologic characteristics, with a hazard ratio range of 1.72 (P less than .001) to 2.36 (P less than .001).
Accuracy of the immune signature exceeded that of two commercialized gene signatures for estimating survival in similar validation cohorts (mean concordance index [C-index], 0.64 vs 0.53 and 0.61).
Moreover, the combination of the immune signature with clinical factors outperformed the signature alone (mean C-index, 0.70 vs 0.63) and another commercialized clinical-molecular combination signature (mean C-index, 0.68 vs 0.65).
“The proposed immune-related gene pair–based signature is a promising prognostic biomarker in nonsquamous NSCLC, including early-stage disease,” concluded the investigators. “Prospective studies are needed to further validate its analytical accuracy for estimating prognoses and to test its clinical utility in individualized management of nonsquamous NSCLC.”
M. Patricia Rivera, MD, FCCP, comments: As lung cancer screening implementation increases, it is expected that the prevalence of early-stage non–small cell lung cancer (NSCLC) will increase.
Currently, adjuvant therapy is reserved for patients with tumors greater than 4 cm or those with N1 disease. Having reliable biomarkers to identify patients at a high risk for recurrence after surgical resection is a significant clinical advantage in order to guide adjuvant therapy. The clinical-immune signature described in this study is an exciting and promising biomarker for estimating overall survival in NSCLC.
M. Patricia Rivera, MD, FCCP, comments: As lung cancer screening implementation increases, it is expected that the prevalence of early-stage non–small cell lung cancer (NSCLC) will increase.
Currently, adjuvant therapy is reserved for patients with tumors greater than 4 cm or those with N1 disease. Having reliable biomarkers to identify patients at a high risk for recurrence after surgical resection is a significant clinical advantage in order to guide adjuvant therapy. The clinical-immune signature described in this study is an exciting and promising biomarker for estimating overall survival in NSCLC.
M. Patricia Rivera, MD, FCCP, comments: As lung cancer screening implementation increases, it is expected that the prevalence of early-stage non–small cell lung cancer (NSCLC) will increase.
Currently, adjuvant therapy is reserved for patients with tumors greater than 4 cm or those with N1 disease. Having reliable biomarkers to identify patients at a high risk for recurrence after surgical resection is a significant clinical advantage in order to guide adjuvant therapy. The clinical-immune signature described in this study is an exciting and promising biomarker for estimating overall survival in NSCLC.
A new tumor immune-related gene signature may help take the guesswork out of prognostication in patients with early-stage non–small cell lung cancer (NSCLC), according to a retrospective cohort study.
“Various components of the immune system have been shown to be a determining factor during cancer initiation and progression,” note the investigators, who were led by Bailiang Li, PhD, of Stanford (Calif.) University. “Recent immunotherapies targeting specific immune checkpoints such as programmed death 1 or programmed death ligand 1 have demonstrated a remarkable, durable response in NSCLC. Certain histopathologic patterns, such as intratumoral infiltration by cytotoxic lymphocytes, have also been associated with better prognoses in several cancer types, including NSCLC.”
For the study, the investigators developed and validated an immune-related gene signature using frozen tumors from 2,414 patients with stage I or II nonsquamous NSCLC from 19 public cohorts who underwent resection with negative margins and did not receive any neoadjuvant or adjuvant therapy.
The new signature contained 25 gene pairs consisting of 40 unique immune-related genes, Dr. Li and associates report (JAMA Oncol. 2017 Jul 6. doi: 10.1001/jamaoncol.2017.1609).
Processes such as chemotaxis were enriched among the included genes.
The signature significantly stratified patients into groups that have high and low risks of death during follow-up, both across and within subsets with stage I, IA, IB, or II disease. Relative to counterparts falling into the signature-defined low-risk group, those falling into the signature-defined high-risk group had roughly twice the risk of death after adjustment for clinical and pathologic characteristics, with a hazard ratio range of 1.72 (P less than .001) to 2.36 (P less than .001).
Accuracy of the immune signature exceeded that of two commercialized gene signatures for estimating survival in similar validation cohorts (mean concordance index [C-index], 0.64 vs 0.53 and 0.61).
Moreover, the combination of the immune signature with clinical factors outperformed the signature alone (mean C-index, 0.70 vs 0.63) and another commercialized clinical-molecular combination signature (mean C-index, 0.68 vs 0.65).
“The proposed immune-related gene pair–based signature is a promising prognostic biomarker in nonsquamous NSCLC, including early-stage disease,” concluded the investigators. “Prospective studies are needed to further validate its analytical accuracy for estimating prognoses and to test its clinical utility in individualized management of nonsquamous NSCLC.”
A new tumor immune-related gene signature may help take the guesswork out of prognostication in patients with early-stage non–small cell lung cancer (NSCLC), according to a retrospective cohort study.
“Various components of the immune system have been shown to be a determining factor during cancer initiation and progression,” note the investigators, who were led by Bailiang Li, PhD, of Stanford (Calif.) University. “Recent immunotherapies targeting specific immune checkpoints such as programmed death 1 or programmed death ligand 1 have demonstrated a remarkable, durable response in NSCLC. Certain histopathologic patterns, such as intratumoral infiltration by cytotoxic lymphocytes, have also been associated with better prognoses in several cancer types, including NSCLC.”
For the study, the investigators developed and validated an immune-related gene signature using frozen tumors from 2,414 patients with stage I or II nonsquamous NSCLC from 19 public cohorts who underwent resection with negative margins and did not receive any neoadjuvant or adjuvant therapy.
The new signature contained 25 gene pairs consisting of 40 unique immune-related genes, Dr. Li and associates report (JAMA Oncol. 2017 Jul 6. doi: 10.1001/jamaoncol.2017.1609).
Processes such as chemotaxis were enriched among the included genes.
The signature significantly stratified patients into groups that have high and low risks of death during follow-up, both across and within subsets with stage I, IA, IB, or II disease. Relative to counterparts falling into the signature-defined low-risk group, those falling into the signature-defined high-risk group had roughly twice the risk of death after adjustment for clinical and pathologic characteristics, with a hazard ratio range of 1.72 (P less than .001) to 2.36 (P less than .001).
Accuracy of the immune signature exceeded that of two commercialized gene signatures for estimating survival in similar validation cohorts (mean concordance index [C-index], 0.64 vs 0.53 and 0.61).
Moreover, the combination of the immune signature with clinical factors outperformed the signature alone (mean C-index, 0.70 vs 0.63) and another commercialized clinical-molecular combination signature (mean C-index, 0.68 vs 0.65).
“The proposed immune-related gene pair–based signature is a promising prognostic biomarker in nonsquamous NSCLC, including early-stage disease,” concluded the investigators. “Prospective studies are needed to further validate its analytical accuracy for estimating prognoses and to test its clinical utility in individualized management of nonsquamous NSCLC.”
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: Compared with peers in the signature-defined low-risk group, patients in the signature-defined high-risk group had roughly twice the adjusted risk of death (hazard ratio range, 1.72-2.36).
Data source: A retrospective cohort study using frozen tumors from 2,414 patients with stage I or II nonsquamous NSCLC who underwent complete resection and did not receive adjuvant or neoadjuvant therapy.
Disclosures: The investigators reported that they had no relevant disclosures. The study was supported in part by the National Institutes of Health.
Appeals court strikes down Wisconsin medical malpractice cap
An appeals court has struck down Wisconsin’s medical malpractice cap for noneconomic damages, ruling that the $750,000 limit is unconstitutional.
In a July opinion, a panel of the First District Court of Appeals wrote that the medical liability cap denies equal protection for the most severely injured patients who are awarded damages exceeding the limit. The decision reinstates a $16.5 million noneconomic damages verdict awarded to a Wisconsin women who lost her limbs after a delayed sepsis diagnosis, according to court documents.
The Wisconsin Medical Society expressed its disappointment at the decision, warning that the ruling could destabilize Wisconsin’s medical liability environment and endanger patients’ access to high-quality, affordable care.
“This decision endangers the long-term solvency of the Injured Patients and Families Compensation Fund and its ability to adequately compensate patients and incentivizes attorneys to file questionable cases in hopes of astronomical jury awards seen in other states without caps,” Noel Deep, MD, president of the Wisconsin Medical Society, said in a statement. “We look forward to further opportunities to explain the importance of the cap to the stability of Wisconsin’s medical liability environment and its benefits for all Wisconsin patients as this case progresses.”
The Wisconsin Association for Justice, an association for the plaintiffs’ bar, praised the cap’s elimination.
“The Court of Appeals, in a concise and well-reasoned opinion, took a brave step toward ensuring that injured patients in Wisconsin can achieve justice by utilizing their constitutional right to a civil jury trial,” Association President Benjamin S. Wagner said in a statement. “The data the court relied upon speaks for itself. The imposition of damage caps against a person like Ascaris Mayo has no rational relationship to controlling medical costs, nor does the verdict impose a financial hardship on the Injured Patients and Families Compensation Fund. To the contrary, the court’s decision ensures that the fund meets its dual statutory obligations to provide excess insurance to medical professionals and provide compensation to injured patients and families.”
The ruling results from a medical malpractice lawsuit filed by patient Ascaris Mayo and her family against Infinity Health Care in Milwaukee, an emergency physician, a physician assistant, ProAssurance Wisconsin Insurance, and the Wisconsin Injured Patients and Families Compensation Fund. The suit claimed that, during a visit to the emergency department at Columbia St. Mary’s Hospital in Milwaukee for abdominal pain and a high fever, health providers failed to diagnosis and treat Ms. Mayo for sepsis. The sepsis led to organ failure, dry gangrene, and, eventually, amputation of her extremities, according to court documents.
A jury awarded Ms. Mayo and her husband $9 million in economic damages and $16.5 million in noneconomic damages. After the verdict, representatives for the Wisconsin Injured Patients and Families Compensation Fund moved to reduce the noneconomic damages award to $750,000 per the state’s cap. The Mayos challenged the reduction, arguing that the cap violated their constitutional rights. A circuit court concluded that the cap was not facially unconstitutional and that it was unconstitutional as applied to the Mayos because it violated the Mayos’ rights to equal protection and due process. Both the Mayos and representatives for the compensation fund appealed.
In their decision, the appeals panel shot down the reasoning used by Wisconsin’s Legislature to enact the noneconomic damages cap, raising doubts that the limit reduces defensive medicine by physicians and prevents a doctor shortage in the state.
“The record before us does not support a finding that the legislative objectives articulated in [the statute] are promoted in any way because the amount of the noneconomic damages cap is $750,000,” the judges wrote. “Data demonstrate that many states with no caps on noneconomic damages actually have higher physician retention rates than Wisconsin. [In addition,] the record before us shows that the ability to accurately measure the financial impact of ‘defensive medicine’ practices has not improved. ... Indeed, data suggest that the existence of noneconomic damages caps may actually increase the risk to patient safety.”
The case is expected to be appealed to the Wisconsin Supreme Court.
[email protected]
On Twitter @legal_med
An appeals court has struck down Wisconsin’s medical malpractice cap for noneconomic damages, ruling that the $750,000 limit is unconstitutional.
In a July opinion, a panel of the First District Court of Appeals wrote that the medical liability cap denies equal protection for the most severely injured patients who are awarded damages exceeding the limit. The decision reinstates a $16.5 million noneconomic damages verdict awarded to a Wisconsin women who lost her limbs after a delayed sepsis diagnosis, according to court documents.
The Wisconsin Medical Society expressed its disappointment at the decision, warning that the ruling could destabilize Wisconsin’s medical liability environment and endanger patients’ access to high-quality, affordable care.
“This decision endangers the long-term solvency of the Injured Patients and Families Compensation Fund and its ability to adequately compensate patients and incentivizes attorneys to file questionable cases in hopes of astronomical jury awards seen in other states without caps,” Noel Deep, MD, president of the Wisconsin Medical Society, said in a statement. “We look forward to further opportunities to explain the importance of the cap to the stability of Wisconsin’s medical liability environment and its benefits for all Wisconsin patients as this case progresses.”
The Wisconsin Association for Justice, an association for the plaintiffs’ bar, praised the cap’s elimination.
“The Court of Appeals, in a concise and well-reasoned opinion, took a brave step toward ensuring that injured patients in Wisconsin can achieve justice by utilizing their constitutional right to a civil jury trial,” Association President Benjamin S. Wagner said in a statement. “The data the court relied upon speaks for itself. The imposition of damage caps against a person like Ascaris Mayo has no rational relationship to controlling medical costs, nor does the verdict impose a financial hardship on the Injured Patients and Families Compensation Fund. To the contrary, the court’s decision ensures that the fund meets its dual statutory obligations to provide excess insurance to medical professionals and provide compensation to injured patients and families.”
The ruling results from a medical malpractice lawsuit filed by patient Ascaris Mayo and her family against Infinity Health Care in Milwaukee, an emergency physician, a physician assistant, ProAssurance Wisconsin Insurance, and the Wisconsin Injured Patients and Families Compensation Fund. The suit claimed that, during a visit to the emergency department at Columbia St. Mary’s Hospital in Milwaukee for abdominal pain and a high fever, health providers failed to diagnosis and treat Ms. Mayo for sepsis. The sepsis led to organ failure, dry gangrene, and, eventually, amputation of her extremities, according to court documents.
A jury awarded Ms. Mayo and her husband $9 million in economic damages and $16.5 million in noneconomic damages. After the verdict, representatives for the Wisconsin Injured Patients and Families Compensation Fund moved to reduce the noneconomic damages award to $750,000 per the state’s cap. The Mayos challenged the reduction, arguing that the cap violated their constitutional rights. A circuit court concluded that the cap was not facially unconstitutional and that it was unconstitutional as applied to the Mayos because it violated the Mayos’ rights to equal protection and due process. Both the Mayos and representatives for the compensation fund appealed.
In their decision, the appeals panel shot down the reasoning used by Wisconsin’s Legislature to enact the noneconomic damages cap, raising doubts that the limit reduces defensive medicine by physicians and prevents a doctor shortage in the state.
“The record before us does not support a finding that the legislative objectives articulated in [the statute] are promoted in any way because the amount of the noneconomic damages cap is $750,000,” the judges wrote. “Data demonstrate that many states with no caps on noneconomic damages actually have higher physician retention rates than Wisconsin. [In addition,] the record before us shows that the ability to accurately measure the financial impact of ‘defensive medicine’ practices has not improved. ... Indeed, data suggest that the existence of noneconomic damages caps may actually increase the risk to patient safety.”
The case is expected to be appealed to the Wisconsin Supreme Court.
[email protected]
On Twitter @legal_med
An appeals court has struck down Wisconsin’s medical malpractice cap for noneconomic damages, ruling that the $750,000 limit is unconstitutional.
In a July opinion, a panel of the First District Court of Appeals wrote that the medical liability cap denies equal protection for the most severely injured patients who are awarded damages exceeding the limit. The decision reinstates a $16.5 million noneconomic damages verdict awarded to a Wisconsin women who lost her limbs after a delayed sepsis diagnosis, according to court documents.
The Wisconsin Medical Society expressed its disappointment at the decision, warning that the ruling could destabilize Wisconsin’s medical liability environment and endanger patients’ access to high-quality, affordable care.
“This decision endangers the long-term solvency of the Injured Patients and Families Compensation Fund and its ability to adequately compensate patients and incentivizes attorneys to file questionable cases in hopes of astronomical jury awards seen in other states without caps,” Noel Deep, MD, president of the Wisconsin Medical Society, said in a statement. “We look forward to further opportunities to explain the importance of the cap to the stability of Wisconsin’s medical liability environment and its benefits for all Wisconsin patients as this case progresses.”
The Wisconsin Association for Justice, an association for the plaintiffs’ bar, praised the cap’s elimination.
“The Court of Appeals, in a concise and well-reasoned opinion, took a brave step toward ensuring that injured patients in Wisconsin can achieve justice by utilizing their constitutional right to a civil jury trial,” Association President Benjamin S. Wagner said in a statement. “The data the court relied upon speaks for itself. The imposition of damage caps against a person like Ascaris Mayo has no rational relationship to controlling medical costs, nor does the verdict impose a financial hardship on the Injured Patients and Families Compensation Fund. To the contrary, the court’s decision ensures that the fund meets its dual statutory obligations to provide excess insurance to medical professionals and provide compensation to injured patients and families.”
The ruling results from a medical malpractice lawsuit filed by patient Ascaris Mayo and her family against Infinity Health Care in Milwaukee, an emergency physician, a physician assistant, ProAssurance Wisconsin Insurance, and the Wisconsin Injured Patients and Families Compensation Fund. The suit claimed that, during a visit to the emergency department at Columbia St. Mary’s Hospital in Milwaukee for abdominal pain and a high fever, health providers failed to diagnosis and treat Ms. Mayo for sepsis. The sepsis led to organ failure, dry gangrene, and, eventually, amputation of her extremities, according to court documents.
A jury awarded Ms. Mayo and her husband $9 million in economic damages and $16.5 million in noneconomic damages. After the verdict, representatives for the Wisconsin Injured Patients and Families Compensation Fund moved to reduce the noneconomic damages award to $750,000 per the state’s cap. The Mayos challenged the reduction, arguing that the cap violated their constitutional rights. A circuit court concluded that the cap was not facially unconstitutional and that it was unconstitutional as applied to the Mayos because it violated the Mayos’ rights to equal protection and due process. Both the Mayos and representatives for the compensation fund appealed.
In their decision, the appeals panel shot down the reasoning used by Wisconsin’s Legislature to enact the noneconomic damages cap, raising doubts that the limit reduces defensive medicine by physicians and prevents a doctor shortage in the state.
“The record before us does not support a finding that the legislative objectives articulated in [the statute] are promoted in any way because the amount of the noneconomic damages cap is $750,000,” the judges wrote. “Data demonstrate that many states with no caps on noneconomic damages actually have higher physician retention rates than Wisconsin. [In addition,] the record before us shows that the ability to accurately measure the financial impact of ‘defensive medicine’ practices has not improved. ... Indeed, data suggest that the existence of noneconomic damages caps may actually increase the risk to patient safety.”
The case is expected to be appealed to the Wisconsin Supreme Court.
[email protected]
On Twitter @legal_med
CABG with arterial grafts provides excellent outcomes for CTO
PARIS – Eighty-eight percent of chronic total occlusions (CTOs) in a large series of patients undergoing coronary artery bypass graft surgery were successfully bypassed using arterial conduits with durable patency.
“Bypass graft surgery using arterial grafts is an acceptable modality of treatment for patients with CTOs and perhaps can be a benchmark against which PCI [percutaneous coronary intervention] for CTOs should be measured,” Teresa May Kieser, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We have previously shown in another paper that arterial grafting mitigates the adverse effect of incomplete revascularization,” said Dr. Kieser, a cardiothoracic surgeon at the University of Calgary (Alt.).
In recent years, treatment of CTOs has increasingly drawn the attention of interventional cardiologists. Dr. Kieser presented what she thinks is the first study of coronary artery bypass graft (CABG) surgery for management of CTOs. It included 1,333 consecutive CABG patients with a total of 3,906 bypasses, a whopping 98% of which were arterial grafts, with a mean of 2.9 grafts per patient. Eleven percent of the CABGs were done emergently, 48% urgently, and 41% electively.
The key epidemiologic finding to emerge from the study is that CTOs are quite common in CABG patients. In this series, 47% of CABG patients had a mean of 1.35 chronically occluded coronary arteries.
Of 843 CTOs in three major territories, 88% were able to be bypassed. All of the 246 CTOs in the left anterior descending coronary artery were able to be bypassed, as were 84% of 415 CTOs in the right coronary artery and 85% in the circumflex system.
The CTO group as a whole had significantly greater impairment of left ventricular function. Thirty-seven percent of them had an ejection fraction of 30%-50%, compared with 22% of the non-CTO patients. The 10% prevalence of an left ventricular ejection fraction (LVEF) below 30% in the CTO group was twice that of the non-CTO group. The CTO group was also significantly more likely to undergo incomplete revascularization, by a margin of 21% versus 5.7%.
Operative mortality was 3.7% overall and just 0.55% in the elective CABG patients. In a multivariate logistic regression analysis controlled for surgical urgency, incomplete revascularization, and EuroSCORE risk, operative mortality didn’t differ significantly between the CTO and non-CTO groups.
However, in the presence of CTOs, incomplete revascularization was associated with an 11.6% operative mortality, compared with a 2.8% rate in fully revascularized CTO patients.
A total of 110 patients with bypassed CTOs underwent symptom-driven follow-up coronary angiography at a median of 3.6 years after CABG. Reassuringly, CTO graft patency was noted in 95% of the LAD grafts, 92% of the right coronary artery grafts, and 79% of the circumflex grafts.
Dr. Kieser’s audience of interventional cardiologists was clearly bowled over by her results, not only the high rate of successful surgical bypass of CTOs, but also by her use of arterial grafts 98% of the time.
“This is my personal practice,” she explained. “I just believe in arterial grafting so much. They perform best in CTO arteries because of their lack of competitive flow.”
Session chair Oliver Gämperli, MD, of University Hospital Zurich, commented, “We are very concerned about patency rates, and you showed us fantastic patency rates. This is much better than what we’re used to with saphenous vein grafts. I think we need to talk to our surgeons and try to get them to do more arterial grafts of CTOs.”
It’s worth noting that in the CTO subgroup from the landmark randomized SYNTAX trial, the complete revascularization rate was only about 50% in the PCI group, compared with nearly 65% in the CABG group, he added.
Asked why a cardiac surgeon wouldn’t bypass a CTO, Dr. Kieser rattled off several technical reasons, including a vessel size of less than 1 mm, diffuse disease, extensive scar, or an inaccessible location. But that’s not the whole story, she added. She has heard surgical colleagues say, “The patient doesn’t need that artery, he’s learned to live without it.” That burns her up.
“Patients need every artery in the heart, and the one with a CTO is the best one for an arterial graft because it almost cannot fail, especially to the left anterior descending artery. I think we have to change the mentality of the surgeons to ‘If it can be done, it should be done,’ ” Dr. Kieser said.
She reported having no financial conflicts of interest regarding her study.
PARIS – Eighty-eight percent of chronic total occlusions (CTOs) in a large series of patients undergoing coronary artery bypass graft surgery were successfully bypassed using arterial conduits with durable patency.
“Bypass graft surgery using arterial grafts is an acceptable modality of treatment for patients with CTOs and perhaps can be a benchmark against which PCI [percutaneous coronary intervention] for CTOs should be measured,” Teresa May Kieser, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We have previously shown in another paper that arterial grafting mitigates the adverse effect of incomplete revascularization,” said Dr. Kieser, a cardiothoracic surgeon at the University of Calgary (Alt.).
In recent years, treatment of CTOs has increasingly drawn the attention of interventional cardiologists. Dr. Kieser presented what she thinks is the first study of coronary artery bypass graft (CABG) surgery for management of CTOs. It included 1,333 consecutive CABG patients with a total of 3,906 bypasses, a whopping 98% of which were arterial grafts, with a mean of 2.9 grafts per patient. Eleven percent of the CABGs were done emergently, 48% urgently, and 41% electively.
The key epidemiologic finding to emerge from the study is that CTOs are quite common in CABG patients. In this series, 47% of CABG patients had a mean of 1.35 chronically occluded coronary arteries.
Of 843 CTOs in three major territories, 88% were able to be bypassed. All of the 246 CTOs in the left anterior descending coronary artery were able to be bypassed, as were 84% of 415 CTOs in the right coronary artery and 85% in the circumflex system.
The CTO group as a whole had significantly greater impairment of left ventricular function. Thirty-seven percent of them had an ejection fraction of 30%-50%, compared with 22% of the non-CTO patients. The 10% prevalence of an left ventricular ejection fraction (LVEF) below 30% in the CTO group was twice that of the non-CTO group. The CTO group was also significantly more likely to undergo incomplete revascularization, by a margin of 21% versus 5.7%.
Operative mortality was 3.7% overall and just 0.55% in the elective CABG patients. In a multivariate logistic regression analysis controlled for surgical urgency, incomplete revascularization, and EuroSCORE risk, operative mortality didn’t differ significantly between the CTO and non-CTO groups.
However, in the presence of CTOs, incomplete revascularization was associated with an 11.6% operative mortality, compared with a 2.8% rate in fully revascularized CTO patients.
A total of 110 patients with bypassed CTOs underwent symptom-driven follow-up coronary angiography at a median of 3.6 years after CABG. Reassuringly, CTO graft patency was noted in 95% of the LAD grafts, 92% of the right coronary artery grafts, and 79% of the circumflex grafts.
Dr. Kieser’s audience of interventional cardiologists was clearly bowled over by her results, not only the high rate of successful surgical bypass of CTOs, but also by her use of arterial grafts 98% of the time.
“This is my personal practice,” she explained. “I just believe in arterial grafting so much. They perform best in CTO arteries because of their lack of competitive flow.”
Session chair Oliver Gämperli, MD, of University Hospital Zurich, commented, “We are very concerned about patency rates, and you showed us fantastic patency rates. This is much better than what we’re used to with saphenous vein grafts. I think we need to talk to our surgeons and try to get them to do more arterial grafts of CTOs.”
It’s worth noting that in the CTO subgroup from the landmark randomized SYNTAX trial, the complete revascularization rate was only about 50% in the PCI group, compared with nearly 65% in the CABG group, he added.
Asked why a cardiac surgeon wouldn’t bypass a CTO, Dr. Kieser rattled off several technical reasons, including a vessel size of less than 1 mm, diffuse disease, extensive scar, or an inaccessible location. But that’s not the whole story, she added. She has heard surgical colleagues say, “The patient doesn’t need that artery, he’s learned to live without it.” That burns her up.
“Patients need every artery in the heart, and the one with a CTO is the best one for an arterial graft because it almost cannot fail, especially to the left anterior descending artery. I think we have to change the mentality of the surgeons to ‘If it can be done, it should be done,’ ” Dr. Kieser said.
She reported having no financial conflicts of interest regarding her study.
PARIS – Eighty-eight percent of chronic total occlusions (CTOs) in a large series of patients undergoing coronary artery bypass graft surgery were successfully bypassed using arterial conduits with durable patency.
“Bypass graft surgery using arterial grafts is an acceptable modality of treatment for patients with CTOs and perhaps can be a benchmark against which PCI [percutaneous coronary intervention] for CTOs should be measured,” Teresa May Kieser, MD, said at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
“We have previously shown in another paper that arterial grafting mitigates the adverse effect of incomplete revascularization,” said Dr. Kieser, a cardiothoracic surgeon at the University of Calgary (Alt.).
In recent years, treatment of CTOs has increasingly drawn the attention of interventional cardiologists. Dr. Kieser presented what she thinks is the first study of coronary artery bypass graft (CABG) surgery for management of CTOs. It included 1,333 consecutive CABG patients with a total of 3,906 bypasses, a whopping 98% of which were arterial grafts, with a mean of 2.9 grafts per patient. Eleven percent of the CABGs were done emergently, 48% urgently, and 41% electively.
The key epidemiologic finding to emerge from the study is that CTOs are quite common in CABG patients. In this series, 47% of CABG patients had a mean of 1.35 chronically occluded coronary arteries.
Of 843 CTOs in three major territories, 88% were able to be bypassed. All of the 246 CTOs in the left anterior descending coronary artery were able to be bypassed, as were 84% of 415 CTOs in the right coronary artery and 85% in the circumflex system.
The CTO group as a whole had significantly greater impairment of left ventricular function. Thirty-seven percent of them had an ejection fraction of 30%-50%, compared with 22% of the non-CTO patients. The 10% prevalence of an left ventricular ejection fraction (LVEF) below 30% in the CTO group was twice that of the non-CTO group. The CTO group was also significantly more likely to undergo incomplete revascularization, by a margin of 21% versus 5.7%.
Operative mortality was 3.7% overall and just 0.55% in the elective CABG patients. In a multivariate logistic regression analysis controlled for surgical urgency, incomplete revascularization, and EuroSCORE risk, operative mortality didn’t differ significantly between the CTO and non-CTO groups.
However, in the presence of CTOs, incomplete revascularization was associated with an 11.6% operative mortality, compared with a 2.8% rate in fully revascularized CTO patients.
A total of 110 patients with bypassed CTOs underwent symptom-driven follow-up coronary angiography at a median of 3.6 years after CABG. Reassuringly, CTO graft patency was noted in 95% of the LAD grafts, 92% of the right coronary artery grafts, and 79% of the circumflex grafts.
Dr. Kieser’s audience of interventional cardiologists was clearly bowled over by her results, not only the high rate of successful surgical bypass of CTOs, but also by her use of arterial grafts 98% of the time.
“This is my personal practice,” she explained. “I just believe in arterial grafting so much. They perform best in CTO arteries because of their lack of competitive flow.”
Session chair Oliver Gämperli, MD, of University Hospital Zurich, commented, “We are very concerned about patency rates, and you showed us fantastic patency rates. This is much better than what we’re used to with saphenous vein grafts. I think we need to talk to our surgeons and try to get them to do more arterial grafts of CTOs.”
It’s worth noting that in the CTO subgroup from the landmark randomized SYNTAX trial, the complete revascularization rate was only about 50% in the PCI group, compared with nearly 65% in the CABG group, he added.
Asked why a cardiac surgeon wouldn’t bypass a CTO, Dr. Kieser rattled off several technical reasons, including a vessel size of less than 1 mm, diffuse disease, extensive scar, or an inaccessible location. But that’s not the whole story, she added. She has heard surgical colleagues say, “The patient doesn’t need that artery, he’s learned to live without it.” That burns her up.
“Patients need every artery in the heart, and the one with a CTO is the best one for an arterial graft because it almost cannot fail, especially to the left anterior descending artery. I think we have to change the mentality of the surgeons to ‘If it can be done, it should be done,’ ” Dr. Kieser said.
She reported having no financial conflicts of interest regarding her study.
AT EUROPCR
Key clinical point:
Major finding: Chronic total occlusions were present in 47% of 1,333 consecutive CABG patients, and 88% of the CTOs were successfully bypassed using arterial conduits.
Data source: A retrospective observational study of 1,333 consecutive CABG patients, 47% of whom had one or more chronic total occlusions.
Disclosures: The study presenter reported having no financial conflicts.
Plasma biomarker distinguishes ARDS, acute heart failure
WASHINGTON – Plasma levels of an interleukin-33 receptor that’s involved in inflammation regulation appeared able to discriminate between acute respiratory distress syndrome and acute decompensated heart failure in an analysis with 72 patients.
In a second study, high plasma levels of the same interleukin-33 receptor, known as soluble suppressor of tumorgenicity 2 (sST2), identified acute respiratory distress syndrome (ARDS) patients who were sicker and more responsive to conservative fluid management, Sean D. Levy, MD, said at an international conference of the American Thoracic Society.
In order to assess the ability of sST2 to reliably distinguish patients with ARDS from those with acute decompensated heart failure, he and his associates selected 72 patients seen at the Massachusetts General Hospital in Boston with an initial diagnosis of acute decompensated heart failure accompanied by bilateral lung infiltrates and acute hypoxemia respiratory failure requiring endotracheal intubation and mechanical ventilation. The investigators measured the sST2 level in a plasma specimen from each patient. In addition, after each patient either left the hospital or died, their case underwent review by two critical care physicians who retrospectively rediagnosed the patients as either having ARDS or acute decompensated heart failure. This divided the cohort into 30 patients with ARDS and 42 with true acute heart failure. The two subgroups matched up fairly closely for most clinical measures and comorbidities, but APACHE III (Acute Physiology and Chronic Health Evaluation III) scores averaged significantly higher in the ARDS patients.
The plasma levels of sST2 showed a dramatic split between the two subgroups. The 30 patients retrospectively diagnosed with ARDS had an average level of 386 ng/mL with an interquartile range of 318-611 ng/mL. The 42 acute decompensated heart failure patients averaged a sST2 level of 148 ng/mL, with an interquartile range of 84-225 ng/mL. The area under the receiver operator curve for discriminating between ARDS and acute heart failure using a cutpoint of 271 mg/mL was 0.86, showing “good” discrimination, Dr. Levy said. This cutpoint had a sensitivity of 83% and specificity of 88% for correctly distinguishing between ARDS and acute heart failure.
In a second analysis, Dr. Levy and his associates looked at the ability of sST2 levels to separate out patients with acute lung injury who had a more robust response to either the conservative or liberal fluid-management strategies tested in the Fluid and Catheter Treatment Trial (FACTT), run by the National Heart, Lung, and Blood Institute’s ARDS Clinical Trials Network. The primary outcome of FACTT was death from any cause 60 days after entry, and this showed no significant difference between conservative (restricted fluids and increased urine output) and liberal (the reverse) fluid management strategies in acute lung injury patients (N Engl J Med. 2006 Jun 15;354[14]:2564-75). From among the 1,001 patients enrolled in FACTT, 826 had specimens available for measuring sST2 (Crit Care Med. 2013 Nov;41[11]:2521-31),
The researchers applied the sST2 cut point they derived in the first analysis to the FACTT cohort and identified 133 (16%) patients with a low sST2 level and 693 (84%) with a high level. The patients with high sST2 were sicker, with significantly higher APACHE III scores, worse acidemia, and worse renal function.
Patients with high sST2 levels had a significant increase in ventilator-free days on conservative fluid management, compared with liberal management, while the two management strategies produced virtually identical results in the patients with low levels of sST2. Patients with high sST2 also had a significantly quicker time to extubation on a conservative strategy compared with the liberal strategy, and again this correlation did not exist among patients with low sST2. However, as in the overall trial a conservative strategy had no discernible impact on 60-day mortality, compared with the liberal strategy, even in the subgroup with high sST2.
Dr. Levy had no disclosures.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – Plasma levels of an interleukin-33 receptor that’s involved in inflammation regulation appeared able to discriminate between acute respiratory distress syndrome and acute decompensated heart failure in an analysis with 72 patients.
In a second study, high plasma levels of the same interleukin-33 receptor, known as soluble suppressor of tumorgenicity 2 (sST2), identified acute respiratory distress syndrome (ARDS) patients who were sicker and more responsive to conservative fluid management, Sean D. Levy, MD, said at an international conference of the American Thoracic Society.
In order to assess the ability of sST2 to reliably distinguish patients with ARDS from those with acute decompensated heart failure, he and his associates selected 72 patients seen at the Massachusetts General Hospital in Boston with an initial diagnosis of acute decompensated heart failure accompanied by bilateral lung infiltrates and acute hypoxemia respiratory failure requiring endotracheal intubation and mechanical ventilation. The investigators measured the sST2 level in a plasma specimen from each patient. In addition, after each patient either left the hospital or died, their case underwent review by two critical care physicians who retrospectively rediagnosed the patients as either having ARDS or acute decompensated heart failure. This divided the cohort into 30 patients with ARDS and 42 with true acute heart failure. The two subgroups matched up fairly closely for most clinical measures and comorbidities, but APACHE III (Acute Physiology and Chronic Health Evaluation III) scores averaged significantly higher in the ARDS patients.
The plasma levels of sST2 showed a dramatic split between the two subgroups. The 30 patients retrospectively diagnosed with ARDS had an average level of 386 ng/mL with an interquartile range of 318-611 ng/mL. The 42 acute decompensated heart failure patients averaged a sST2 level of 148 ng/mL, with an interquartile range of 84-225 ng/mL. The area under the receiver operator curve for discriminating between ARDS and acute heart failure using a cutpoint of 271 mg/mL was 0.86, showing “good” discrimination, Dr. Levy said. This cutpoint had a sensitivity of 83% and specificity of 88% for correctly distinguishing between ARDS and acute heart failure.
In a second analysis, Dr. Levy and his associates looked at the ability of sST2 levels to separate out patients with acute lung injury who had a more robust response to either the conservative or liberal fluid-management strategies tested in the Fluid and Catheter Treatment Trial (FACTT), run by the National Heart, Lung, and Blood Institute’s ARDS Clinical Trials Network. The primary outcome of FACTT was death from any cause 60 days after entry, and this showed no significant difference between conservative (restricted fluids and increased urine output) and liberal (the reverse) fluid management strategies in acute lung injury patients (N Engl J Med. 2006 Jun 15;354[14]:2564-75). From among the 1,001 patients enrolled in FACTT, 826 had specimens available for measuring sST2 (Crit Care Med. 2013 Nov;41[11]:2521-31),
The researchers applied the sST2 cut point they derived in the first analysis to the FACTT cohort and identified 133 (16%) patients with a low sST2 level and 693 (84%) with a high level. The patients with high sST2 were sicker, with significantly higher APACHE III scores, worse acidemia, and worse renal function.
Patients with high sST2 levels had a significant increase in ventilator-free days on conservative fluid management, compared with liberal management, while the two management strategies produced virtually identical results in the patients with low levels of sST2. Patients with high sST2 also had a significantly quicker time to extubation on a conservative strategy compared with the liberal strategy, and again this correlation did not exist among patients with low sST2. However, as in the overall trial a conservative strategy had no discernible impact on 60-day mortality, compared with the liberal strategy, even in the subgroup with high sST2.
Dr. Levy had no disclosures.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – Plasma levels of an interleukin-33 receptor that’s involved in inflammation regulation appeared able to discriminate between acute respiratory distress syndrome and acute decompensated heart failure in an analysis with 72 patients.
In a second study, high plasma levels of the same interleukin-33 receptor, known as soluble suppressor of tumorgenicity 2 (sST2), identified acute respiratory distress syndrome (ARDS) patients who were sicker and more responsive to conservative fluid management, Sean D. Levy, MD, said at an international conference of the American Thoracic Society.
In order to assess the ability of sST2 to reliably distinguish patients with ARDS from those with acute decompensated heart failure, he and his associates selected 72 patients seen at the Massachusetts General Hospital in Boston with an initial diagnosis of acute decompensated heart failure accompanied by bilateral lung infiltrates and acute hypoxemia respiratory failure requiring endotracheal intubation and mechanical ventilation. The investigators measured the sST2 level in a plasma specimen from each patient. In addition, after each patient either left the hospital or died, their case underwent review by two critical care physicians who retrospectively rediagnosed the patients as either having ARDS or acute decompensated heart failure. This divided the cohort into 30 patients with ARDS and 42 with true acute heart failure. The two subgroups matched up fairly closely for most clinical measures and comorbidities, but APACHE III (Acute Physiology and Chronic Health Evaluation III) scores averaged significantly higher in the ARDS patients.
The plasma levels of sST2 showed a dramatic split between the two subgroups. The 30 patients retrospectively diagnosed with ARDS had an average level of 386 ng/mL with an interquartile range of 318-611 ng/mL. The 42 acute decompensated heart failure patients averaged a sST2 level of 148 ng/mL, with an interquartile range of 84-225 ng/mL. The area under the receiver operator curve for discriminating between ARDS and acute heart failure using a cutpoint of 271 mg/mL was 0.86, showing “good” discrimination, Dr. Levy said. This cutpoint had a sensitivity of 83% and specificity of 88% for correctly distinguishing between ARDS and acute heart failure.
In a second analysis, Dr. Levy and his associates looked at the ability of sST2 levels to separate out patients with acute lung injury who had a more robust response to either the conservative or liberal fluid-management strategies tested in the Fluid and Catheter Treatment Trial (FACTT), run by the National Heart, Lung, and Blood Institute’s ARDS Clinical Trials Network. The primary outcome of FACTT was death from any cause 60 days after entry, and this showed no significant difference between conservative (restricted fluids and increased urine output) and liberal (the reverse) fluid management strategies in acute lung injury patients (N Engl J Med. 2006 Jun 15;354[14]:2564-75). From among the 1,001 patients enrolled in FACTT, 826 had specimens available for measuring sST2 (Crit Care Med. 2013 Nov;41[11]:2521-31),
The researchers applied the sST2 cut point they derived in the first analysis to the FACTT cohort and identified 133 (16%) patients with a low sST2 level and 693 (84%) with a high level. The patients with high sST2 were sicker, with significantly higher APACHE III scores, worse acidemia, and worse renal function.
Patients with high sST2 levels had a significant increase in ventilator-free days on conservative fluid management, compared with liberal management, while the two management strategies produced virtually identical results in the patients with low levels of sST2. Patients with high sST2 also had a significantly quicker time to extubation on a conservative strategy compared with the liberal strategy, and again this correlation did not exist among patients with low sST2. However, as in the overall trial a conservative strategy had no discernible impact on 60-day mortality, compared with the liberal strategy, even in the subgroup with high sST2.
Dr. Levy had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: An sST2 cutpoint of 271 ng/mL discriminated between ARDS and acute heart failure with 83% sensitivity and 88% specificity.
Data source: Review of 72 patients admitted for acute decompensated heart failure at one U.S. center.
Disclosures: Dr. Levy had no disclosures.