User login
More evidence links asthma severity to age of onset
A recently published multinational cohort study may be the largest to date that’s found the age of asthma onset is an integral factor in defining the severity of disease and the frequency of comorbidities.
“It’s very simple to ask your patient: ‘Did you have asthma as a child? When did your asthma start?’ ” coauthor Guy Brusselle, MD, a professor at the University of Ghent (Belgium), said in an interview. “You do not need expensive investigations, CT scans or proteomics or genomics; just two simple questions.”
The retrospective cohort study, published in the Journal of Allergy and Clinical Immunology: In Practice, combined national electronic health records databases from five different countries – the United Kingdom, Spain, Italy, the Netherlands, and Denmark – that included 586,436 adult asthma patients. The study divided the patients into three subtypes: childhood-onset asthma, meaning a diagnosis before age 18 (n = 81,691); adult-onset disease, defined as a diagnosis between ages 18 and 40 (n = 218,184); and late onset, defined as a diagnosis made after age 40 (n = 286,561).
Dr. Brusselle said the study found stark differences in characteristics between the three subtypes, including an increasing risk for women with later age of onset. Across the five databases, females comprised approximately 45% of those with childhood-onset asthma, but about 60% of those with later-onset disease, Dr. Brusselle said.
As for characteristics of asthma, 7.2% of the cohort (n = 42,611) had severe asthma, but the proportion was highest in late-onset asthma, 10% versus 5% in adult onset and 3% in childhood onset. The percentage of uncontrolled asthma followed a similar trend: 8%, 6%, and 0.4% in the respective treatment groups.
The most common comorbidities were atopic disorders (31%) and overweight/obesity (50%). The prevalence of atopic disorders was highest in the childhood-onset group, 45% versus 35%, and 25% in the adult-onset and late-onset patients. However, the trend for overweight/obesity was reversed: 30%, 43%, and 61%, respectively.
“The larger differences were when late-onset asthma was compared to adult-onset asthma with respect to comorbidities,” Dr. Brusselle said. “The late-onset asthma patients more frequently had nasal polyposis.” These patients typically lose their sense of smell, as in COVID-19. However, in nasal polyposis the loss is chronic rather than transient.
Pulmonologists should be attuned to the prevalence of overweight/obesity in the late-onset group, Dr. Brusselle said. “We know that obesity is an important risk factor for diabetes, and then obesity is also associated with gastroesophageal reflux – and we know that gastroesophageal reflux is a risk factor for asthma exacerbations.”
Smaller studies have arrived at the same conclusions regarding the relationships between asthma severity and age of onset, Dr. Brusselle said. What’s notable about this study is its size and the consistency of findings across different national databases.
“In childhood onset you need to watch for different allergies – atopic dermatitis and allergic rhinitis – but in late-onset asthma look for obesity, diabetes and reflux disease, and nasal polyposis,” he said.
Sally E. Wenzel, MD, professor at the University of Pittsburgh and director of the Asthma and Environmental Lung Health Institute at the University of Pittsburgh Medical Center, concurred that the size of this study makes it noteworthy.
“It’s certainly far and away the largest study of its kind that’s ever been done, and it’s multinational,” she said in an interview. “Just doing a study like this with thousands and thousands of patients is a step in the right direction. That’s probably what’s very unique about it, to bring all of these clinical cohorts as it were together and to look at what is the relationship of the age of onset.”
She also said the study is unique in how it delineates the groups by age of onset.
“In addition to this concept that there’s a difference in asthma by the age that you got diagnosed with it, I think it’s also important to just remember that when any physician, be they a specialist or nonspecialist, sees a patient with asthma, they should ask them when did their symptoms develop,” she said. “These are really simple questions that don’t take any sophisticated training and don’t take any sophisticated instruments to measure, but they can be really helpful.”
GlaxoSmithKline supplied a grant for the study. Dr. Brusselle disclosed relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Sanofi, and Teva. A study coauthor is an employee of GSK. Dr. Wenzel reported no disclosures.
A version of this article first appeared on Medscape.com.
A recently published multinational cohort study may be the largest to date that’s found the age of asthma onset is an integral factor in defining the severity of disease and the frequency of comorbidities.
“It’s very simple to ask your patient: ‘Did you have asthma as a child? When did your asthma start?’ ” coauthor Guy Brusselle, MD, a professor at the University of Ghent (Belgium), said in an interview. “You do not need expensive investigations, CT scans or proteomics or genomics; just two simple questions.”
The retrospective cohort study, published in the Journal of Allergy and Clinical Immunology: In Practice, combined national electronic health records databases from five different countries – the United Kingdom, Spain, Italy, the Netherlands, and Denmark – that included 586,436 adult asthma patients. The study divided the patients into three subtypes: childhood-onset asthma, meaning a diagnosis before age 18 (n = 81,691); adult-onset disease, defined as a diagnosis between ages 18 and 40 (n = 218,184); and late onset, defined as a diagnosis made after age 40 (n = 286,561).
Dr. Brusselle said the study found stark differences in characteristics between the three subtypes, including an increasing risk for women with later age of onset. Across the five databases, females comprised approximately 45% of those with childhood-onset asthma, but about 60% of those with later-onset disease, Dr. Brusselle said.
As for characteristics of asthma, 7.2% of the cohort (n = 42,611) had severe asthma, but the proportion was highest in late-onset asthma, 10% versus 5% in adult onset and 3% in childhood onset. The percentage of uncontrolled asthma followed a similar trend: 8%, 6%, and 0.4% in the respective treatment groups.
The most common comorbidities were atopic disorders (31%) and overweight/obesity (50%). The prevalence of atopic disorders was highest in the childhood-onset group, 45% versus 35%, and 25% in the adult-onset and late-onset patients. However, the trend for overweight/obesity was reversed: 30%, 43%, and 61%, respectively.
“The larger differences were when late-onset asthma was compared to adult-onset asthma with respect to comorbidities,” Dr. Brusselle said. “The late-onset asthma patients more frequently had nasal polyposis.” These patients typically lose their sense of smell, as in COVID-19. However, in nasal polyposis the loss is chronic rather than transient.
Pulmonologists should be attuned to the prevalence of overweight/obesity in the late-onset group, Dr. Brusselle said. “We know that obesity is an important risk factor for diabetes, and then obesity is also associated with gastroesophageal reflux – and we know that gastroesophageal reflux is a risk factor for asthma exacerbations.”
Smaller studies have arrived at the same conclusions regarding the relationships between asthma severity and age of onset, Dr. Brusselle said. What’s notable about this study is its size and the consistency of findings across different national databases.
“In childhood onset you need to watch for different allergies – atopic dermatitis and allergic rhinitis – but in late-onset asthma look for obesity, diabetes and reflux disease, and nasal polyposis,” he said.
Sally E. Wenzel, MD, professor at the University of Pittsburgh and director of the Asthma and Environmental Lung Health Institute at the University of Pittsburgh Medical Center, concurred that the size of this study makes it noteworthy.
“It’s certainly far and away the largest study of its kind that’s ever been done, and it’s multinational,” she said in an interview. “Just doing a study like this with thousands and thousands of patients is a step in the right direction. That’s probably what’s very unique about it, to bring all of these clinical cohorts as it were together and to look at what is the relationship of the age of onset.”
She also said the study is unique in how it delineates the groups by age of onset.
“In addition to this concept that there’s a difference in asthma by the age that you got diagnosed with it, I think it’s also important to just remember that when any physician, be they a specialist or nonspecialist, sees a patient with asthma, they should ask them when did their symptoms develop,” she said. “These are really simple questions that don’t take any sophisticated training and don’t take any sophisticated instruments to measure, but they can be really helpful.”
GlaxoSmithKline supplied a grant for the study. Dr. Brusselle disclosed relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Sanofi, and Teva. A study coauthor is an employee of GSK. Dr. Wenzel reported no disclosures.
A version of this article first appeared on Medscape.com.
A recently published multinational cohort study may be the largest to date that’s found the age of asthma onset is an integral factor in defining the severity of disease and the frequency of comorbidities.
“It’s very simple to ask your patient: ‘Did you have asthma as a child? When did your asthma start?’ ” coauthor Guy Brusselle, MD, a professor at the University of Ghent (Belgium), said in an interview. “You do not need expensive investigations, CT scans or proteomics or genomics; just two simple questions.”
The retrospective cohort study, published in the Journal of Allergy and Clinical Immunology: In Practice, combined national electronic health records databases from five different countries – the United Kingdom, Spain, Italy, the Netherlands, and Denmark – that included 586,436 adult asthma patients. The study divided the patients into three subtypes: childhood-onset asthma, meaning a diagnosis before age 18 (n = 81,691); adult-onset disease, defined as a diagnosis between ages 18 and 40 (n = 218,184); and late onset, defined as a diagnosis made after age 40 (n = 286,561).
Dr. Brusselle said the study found stark differences in characteristics between the three subtypes, including an increasing risk for women with later age of onset. Across the five databases, females comprised approximately 45% of those with childhood-onset asthma, but about 60% of those with later-onset disease, Dr. Brusselle said.
As for characteristics of asthma, 7.2% of the cohort (n = 42,611) had severe asthma, but the proportion was highest in late-onset asthma, 10% versus 5% in adult onset and 3% in childhood onset. The percentage of uncontrolled asthma followed a similar trend: 8%, 6%, and 0.4% in the respective treatment groups.
The most common comorbidities were atopic disorders (31%) and overweight/obesity (50%). The prevalence of atopic disorders was highest in the childhood-onset group, 45% versus 35%, and 25% in the adult-onset and late-onset patients. However, the trend for overweight/obesity was reversed: 30%, 43%, and 61%, respectively.
“The larger differences were when late-onset asthma was compared to adult-onset asthma with respect to comorbidities,” Dr. Brusselle said. “The late-onset asthma patients more frequently had nasal polyposis.” These patients typically lose their sense of smell, as in COVID-19. However, in nasal polyposis the loss is chronic rather than transient.
Pulmonologists should be attuned to the prevalence of overweight/obesity in the late-onset group, Dr. Brusselle said. “We know that obesity is an important risk factor for diabetes, and then obesity is also associated with gastroesophageal reflux – and we know that gastroesophageal reflux is a risk factor for asthma exacerbations.”
Smaller studies have arrived at the same conclusions regarding the relationships between asthma severity and age of onset, Dr. Brusselle said. What’s notable about this study is its size and the consistency of findings across different national databases.
“In childhood onset you need to watch for different allergies – atopic dermatitis and allergic rhinitis – but in late-onset asthma look for obesity, diabetes and reflux disease, and nasal polyposis,” he said.
Sally E. Wenzel, MD, professor at the University of Pittsburgh and director of the Asthma and Environmental Lung Health Institute at the University of Pittsburgh Medical Center, concurred that the size of this study makes it noteworthy.
“It’s certainly far and away the largest study of its kind that’s ever been done, and it’s multinational,” she said in an interview. “Just doing a study like this with thousands and thousands of patients is a step in the right direction. That’s probably what’s very unique about it, to bring all of these clinical cohorts as it were together and to look at what is the relationship of the age of onset.”
She also said the study is unique in how it delineates the groups by age of onset.
“In addition to this concept that there’s a difference in asthma by the age that you got diagnosed with it, I think it’s also important to just remember that when any physician, be they a specialist or nonspecialist, sees a patient with asthma, they should ask them when did their symptoms develop,” she said. “These are really simple questions that don’t take any sophisticated training and don’t take any sophisticated instruments to measure, but they can be really helpful.”
GlaxoSmithKline supplied a grant for the study. Dr. Brusselle disclosed relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Sanofi, and Teva. A study coauthor is an employee of GSK. Dr. Wenzel reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Topical treatment for EB recommended for approval in the EU
A topical (EMA’s) Committee for Medicinal Products for Human Use.
“The benefit of Filsuvez is its ability to promote healing of EB partial thickness wounds,” the EMA said in an announcement on April 22. “It is thought to work by modulating inflammatory mediators and stimulating keratinocyte differentiation and migration, thereby promoting wound health and closure,” the statement adds.
The recommended indication for the product – developed by Amryt Pharmaceuticals DAC and currently designated as an orphan drug – is for the treatment of partial-thickness wounds associated with dystrophic and junctional EB in patients aged 6 months and older. The recommendation for approval came after the EMA sought and received external advice from independent physicians treating EB and from patients with the rare disease.
The most common side effects, according to the EMA announcement, are wound complications, application site reactions, wound infections, pruritus, and hypersensitivity reactions.
In February 2022, the Food and Drug Administration declined to approve the company’s new drug application as it was presented and asked the company to submit additional evidence of effectiveness for Oleogel-S10 in EB, the company announced at that time. The statement noted that the company was committed to working with the FDA to identify "the most expeditious pathway towards a potential approval.”
The company’s pivotal phase 3 trial enrolled 223 patients with EB, including 156 pediatric patients. The patients variously had three types of EB. The trial has two components: A 3-month, double-blind, randomized controlled phase, which has been completed, and an ongoing 24-month open-label, single-arm phase. The trial is being performed at 58 sites in 28 countries.
Results from the randomized controlled phase, reported in 2020, include a statistically significant increase in the proportion of patients achieving complete closure of an EB target wound within 45 days: 41.3% in the Oleogel-S10 group and 28.9% in the control group (P = .013). (Target wounds measured 10 cm² to 50 cm² and were present for at least 21 days but less than 9 months.) The safety profile of the treatment gel was acceptable and was well tolerated, compared with the control gel, according to Amryt’s press release. The results were presented at the European Academy of Dermatology and Venereology Congress in October 2020.
Data from a 12-month interim analysis of the follow-up phase were presented at the annual meeting of the American Academy of Dermatology in March 2022. Results showed further reductions in total body surface area percentage wounding to 5.4% among (from 7.4% at the end of the double-blind period and 12.1% at the beginning of the study) among the patients who continued treatment and who underwent assessment, according to a company press release. Treatment was well tolerated, and no new safety signals were identified, the release said.
A decision by the European Commission is expected within the next 2 months.
A version of this article first appeared on Medscape.com.
A topical (EMA’s) Committee for Medicinal Products for Human Use.
“The benefit of Filsuvez is its ability to promote healing of EB partial thickness wounds,” the EMA said in an announcement on April 22. “It is thought to work by modulating inflammatory mediators and stimulating keratinocyte differentiation and migration, thereby promoting wound health and closure,” the statement adds.
The recommended indication for the product – developed by Amryt Pharmaceuticals DAC and currently designated as an orphan drug – is for the treatment of partial-thickness wounds associated with dystrophic and junctional EB in patients aged 6 months and older. The recommendation for approval came after the EMA sought and received external advice from independent physicians treating EB and from patients with the rare disease.
The most common side effects, according to the EMA announcement, are wound complications, application site reactions, wound infections, pruritus, and hypersensitivity reactions.
In February 2022, the Food and Drug Administration declined to approve the company’s new drug application as it was presented and asked the company to submit additional evidence of effectiveness for Oleogel-S10 in EB, the company announced at that time. The statement noted that the company was committed to working with the FDA to identify "the most expeditious pathway towards a potential approval.”
The company’s pivotal phase 3 trial enrolled 223 patients with EB, including 156 pediatric patients. The patients variously had three types of EB. The trial has two components: A 3-month, double-blind, randomized controlled phase, which has been completed, and an ongoing 24-month open-label, single-arm phase. The trial is being performed at 58 sites in 28 countries.
Results from the randomized controlled phase, reported in 2020, include a statistically significant increase in the proportion of patients achieving complete closure of an EB target wound within 45 days: 41.3% in the Oleogel-S10 group and 28.9% in the control group (P = .013). (Target wounds measured 10 cm² to 50 cm² and were present for at least 21 days but less than 9 months.) The safety profile of the treatment gel was acceptable and was well tolerated, compared with the control gel, according to Amryt’s press release. The results were presented at the European Academy of Dermatology and Venereology Congress in October 2020.
Data from a 12-month interim analysis of the follow-up phase were presented at the annual meeting of the American Academy of Dermatology in March 2022. Results showed further reductions in total body surface area percentage wounding to 5.4% among (from 7.4% at the end of the double-blind period and 12.1% at the beginning of the study) among the patients who continued treatment and who underwent assessment, according to a company press release. Treatment was well tolerated, and no new safety signals were identified, the release said.
A decision by the European Commission is expected within the next 2 months.
A version of this article first appeared on Medscape.com.
A topical (EMA’s) Committee for Medicinal Products for Human Use.
“The benefit of Filsuvez is its ability to promote healing of EB partial thickness wounds,” the EMA said in an announcement on April 22. “It is thought to work by modulating inflammatory mediators and stimulating keratinocyte differentiation and migration, thereby promoting wound health and closure,” the statement adds.
The recommended indication for the product – developed by Amryt Pharmaceuticals DAC and currently designated as an orphan drug – is for the treatment of partial-thickness wounds associated with dystrophic and junctional EB in patients aged 6 months and older. The recommendation for approval came after the EMA sought and received external advice from independent physicians treating EB and from patients with the rare disease.
The most common side effects, according to the EMA announcement, are wound complications, application site reactions, wound infections, pruritus, and hypersensitivity reactions.
In February 2022, the Food and Drug Administration declined to approve the company’s new drug application as it was presented and asked the company to submit additional evidence of effectiveness for Oleogel-S10 in EB, the company announced at that time. The statement noted that the company was committed to working with the FDA to identify "the most expeditious pathway towards a potential approval.”
The company’s pivotal phase 3 trial enrolled 223 patients with EB, including 156 pediatric patients. The patients variously had three types of EB. The trial has two components: A 3-month, double-blind, randomized controlled phase, which has been completed, and an ongoing 24-month open-label, single-arm phase. The trial is being performed at 58 sites in 28 countries.
Results from the randomized controlled phase, reported in 2020, include a statistically significant increase in the proportion of patients achieving complete closure of an EB target wound within 45 days: 41.3% in the Oleogel-S10 group and 28.9% in the control group (P = .013). (Target wounds measured 10 cm² to 50 cm² and were present for at least 21 days but less than 9 months.) The safety profile of the treatment gel was acceptable and was well tolerated, compared with the control gel, according to Amryt’s press release. The results were presented at the European Academy of Dermatology and Venereology Congress in October 2020.
Data from a 12-month interim analysis of the follow-up phase were presented at the annual meeting of the American Academy of Dermatology in March 2022. Results showed further reductions in total body surface area percentage wounding to 5.4% among (from 7.4% at the end of the double-blind period and 12.1% at the beginning of the study) among the patients who continued treatment and who underwent assessment, according to a company press release. Treatment was well tolerated, and no new safety signals were identified, the release said.
A decision by the European Commission is expected within the next 2 months.
A version of this article first appeared on Medscape.com.
Which solid organ transplant recipients face the highest risk of skin cancer?
BOSTON – .
White patients who meet these criteria should be screening within 2 years after transplant, while Black patients should be screened within 5 years after transplant, Ally-Khan Somani, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Dr. Somani, director of dermatologic surgery and the division of cutaneous oncology at Indiana University, Indianapolis, based his remarks on consensus screening guidelines assembled from three rounds of Delphi method surveys with 47 dermatologists and 37 transplant physicians, with the goal of establishing skin cancer screening recommendations for SOTRs. Among the dermatologists surveyed, 45% were Mohs surgeons and 55% were general dermatologists.
The panel recommended that the transplant team should perform risk assessment for SOTRs to risk stratify patients for skin cancer screening (high risk vs. low risk). They also proposed that dermatologists perform skin cancer screening by full-body skin examinations, and that SOTRs with a history of skin cancer should continue with routine skin cancer surveillance as recommended by their dermatologists.
Those at low risk for skin cancer include abdominal organ recipients, SOTR age of younger than 50 at time of transplant, and female gender. The guidelines recommend that White, Asian, and Hispanic patients who meet those criteria should be screened within 5 years after transplant, while no consensus was reached for Black patients who meet those criteria.
Based on posttransplant skin cancer incidence rates, risk is increased among males, Whites, thoracic organ recipients, and being age 50 or older, Dr. Somani said. “At our institution, we make sure there’s a good connection between our transplant teams and dermatologists. We recommend rapid referral for suspicious lesions and we educate patients and screen them within 1 year of transplant, or sooner for high-risk patients. Surveillance is increased to every 3 or 4 months for patients with a history of multiple or high-risk cancers or sooner, followed by routine surveillance as recommended by the patient’s dermatologist.”
To risk stratify patients on the development of their first skin cancer post transplantation, researchers developed the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator (SUNTRAC), a prediction tool with a freely available app. Data for the tool were drawn from the Transplant Skin Cancer Network study, a 5-year analysis of 6,340 adult recipients of a first solid organ transplant at 26 transplant centers in the United States. It generates a risk score for SOTRs (low, medium, high, or very high), which informs transplant care providers of a patient’s risk of skin cancer.
Dr. Somani disclosed that he has received grants and funding from Castle Biosciences. He is an adviser to Cook Biotech and a consultant to Sanara MedTech.
BOSTON – .
White patients who meet these criteria should be screening within 2 years after transplant, while Black patients should be screened within 5 years after transplant, Ally-Khan Somani, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Dr. Somani, director of dermatologic surgery and the division of cutaneous oncology at Indiana University, Indianapolis, based his remarks on consensus screening guidelines assembled from three rounds of Delphi method surveys with 47 dermatologists and 37 transplant physicians, with the goal of establishing skin cancer screening recommendations for SOTRs. Among the dermatologists surveyed, 45% were Mohs surgeons and 55% were general dermatologists.
The panel recommended that the transplant team should perform risk assessment for SOTRs to risk stratify patients for skin cancer screening (high risk vs. low risk). They also proposed that dermatologists perform skin cancer screening by full-body skin examinations, and that SOTRs with a history of skin cancer should continue with routine skin cancer surveillance as recommended by their dermatologists.
Those at low risk for skin cancer include abdominal organ recipients, SOTR age of younger than 50 at time of transplant, and female gender. The guidelines recommend that White, Asian, and Hispanic patients who meet those criteria should be screened within 5 years after transplant, while no consensus was reached for Black patients who meet those criteria.
Based on posttransplant skin cancer incidence rates, risk is increased among males, Whites, thoracic organ recipients, and being age 50 or older, Dr. Somani said. “At our institution, we make sure there’s a good connection between our transplant teams and dermatologists. We recommend rapid referral for suspicious lesions and we educate patients and screen them within 1 year of transplant, or sooner for high-risk patients. Surveillance is increased to every 3 or 4 months for patients with a history of multiple or high-risk cancers or sooner, followed by routine surveillance as recommended by the patient’s dermatologist.”
To risk stratify patients on the development of their first skin cancer post transplantation, researchers developed the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator (SUNTRAC), a prediction tool with a freely available app. Data for the tool were drawn from the Transplant Skin Cancer Network study, a 5-year analysis of 6,340 adult recipients of a first solid organ transplant at 26 transplant centers in the United States. It generates a risk score for SOTRs (low, medium, high, or very high), which informs transplant care providers of a patient’s risk of skin cancer.
Dr. Somani disclosed that he has received grants and funding from Castle Biosciences. He is an adviser to Cook Biotech and a consultant to Sanara MedTech.
BOSTON – .
White patients who meet these criteria should be screening within 2 years after transplant, while Black patients should be screened within 5 years after transplant, Ally-Khan Somani, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
Dr. Somani, director of dermatologic surgery and the division of cutaneous oncology at Indiana University, Indianapolis, based his remarks on consensus screening guidelines assembled from three rounds of Delphi method surveys with 47 dermatologists and 37 transplant physicians, with the goal of establishing skin cancer screening recommendations for SOTRs. Among the dermatologists surveyed, 45% were Mohs surgeons and 55% were general dermatologists.
The panel recommended that the transplant team should perform risk assessment for SOTRs to risk stratify patients for skin cancer screening (high risk vs. low risk). They also proposed that dermatologists perform skin cancer screening by full-body skin examinations, and that SOTRs with a history of skin cancer should continue with routine skin cancer surveillance as recommended by their dermatologists.
Those at low risk for skin cancer include abdominal organ recipients, SOTR age of younger than 50 at time of transplant, and female gender. The guidelines recommend that White, Asian, and Hispanic patients who meet those criteria should be screened within 5 years after transplant, while no consensus was reached for Black patients who meet those criteria.
Based on posttransplant skin cancer incidence rates, risk is increased among males, Whites, thoracic organ recipients, and being age 50 or older, Dr. Somani said. “At our institution, we make sure there’s a good connection between our transplant teams and dermatologists. We recommend rapid referral for suspicious lesions and we educate patients and screen them within 1 year of transplant, or sooner for high-risk patients. Surveillance is increased to every 3 or 4 months for patients with a history of multiple or high-risk cancers or sooner, followed by routine surveillance as recommended by the patient’s dermatologist.”
To risk stratify patients on the development of their first skin cancer post transplantation, researchers developed the Skin and Ultraviolet Neoplasia Transplant Risk Assessment Calculator (SUNTRAC), a prediction tool with a freely available app. Data for the tool were drawn from the Transplant Skin Cancer Network study, a 5-year analysis of 6,340 adult recipients of a first solid organ transplant at 26 transplant centers in the United States. It generates a risk score for SOTRs (low, medium, high, or very high), which informs transplant care providers of a patient’s risk of skin cancer.
Dr. Somani disclosed that he has received grants and funding from Castle Biosciences. He is an adviser to Cook Biotech and a consultant to Sanara MedTech.
AT AAD 22
Almost 60% of U.S. population has been infected by COVID-19: CDC
The percentage of Americans who have been infected with COVID-19 jumped from 34% in December 2021 to 58% in February 2022, a new study from the Centers for Disease Control and Prevention reveals.
This is the first time the seroprevalence of prior infection is more than 50% in the American population.
“I definitely expected that we were going to see an increase continue ... but I didn’t expect it to increase quite this much. But we follow the data ... and this is what the evidence is showing us,” lead study researcher Kristie E. N. Clarke, MD, said during a CDC media briefing April 26.
Researchers found that presence of antinucleocapsid (anti-N) antibodies from prior infection varied by age. The rate varied from as high as 75% in children and teenagers 17 years and younger to 33% in those 65 and older, for example.
The study showed that the anti-N antibodies were more common in age groups with the lowest vaccination numbers.
Combined with up-to-date CDC data on deaths, hospitalizations, and cases, the study provides a clearer picture of where we are now and where we might be headed in terms of the pandemic.
Vaccination still valuable
The fact that nearly 60% of Americans have antibodies from prior infection is not a reason to think people with a history of COVID-19 should skip vaccination, said CDC director Rochelle P. Walensky, MD.
“I can’t underscore enough that those with detectable antibodies from previous infection, we encourage them to still get vaccinated,” Dr. Walensky said.
“We do know that reinfections happen,” she said, “so that’s important in terms of thinking forward.”
The CDC continues to encourage all Americans to stay up to date with their COVID-19 vaccinations, said Dr. Clarke, colead for the CDC’s COVID-19 Epidemiology and Surveillance Taskforce Seroprevalence Team. “Having infection-induced antibodies does not necessarily mean you are protected against future infections.”
The study, published in the CDC’s Morbidity and Mortality Weekly Report (MMWR), did not evaluate antibody protection from COVID-19 vaccination.
It should also be noted that the study looked at presence or absence of anti-N antibodies, and not whether certain levels were linked to less or more protection.
Where are we now?
Dr. Walensky used the media briefing as an opportunity to share current COVID-19 numbers.
“Overall, we can continue to have some mixed trends. Deaths, fortunately, are continuing to trend downward with a 7-day average of about 300 per day, which represents an estimated 18% decline from the prior week,” she said.
Hospital admissions also remain low, at about 1,500 per day. “But we should note that for the second week in a row, they are slowly trending upwards,” Dr. Walensky said. There was an increase of about 9% at press time compared with the prior week.
Cases remain “comparatively low” to even where we were a month ago, at 44,000 per day,” Dr. Walensky said. “Although this too represents an increase of about 25% in the past week.”
Dr. Walensky noted that positive test numbers are not as reliable a metric as they were before the growth in use of rapid home tests. But it’s not the only measure. “We continue to believe that our PCR testing data, especially when we corroborate it with information from our other surveillance systems – like wastewater surveillance and emergency department surveillance – provide us a reliable picture of the trajectory of COVID-19 across our country.”
She recommended that people continue to consult the CDC’s COVID-19 county tracker to monitor local levels of COVID-19.
Dr. Walensky also shared recent findings from genomic sequencing that continue to show the predominance of the Omicron variant. “Essentially a hundred percent of what we’re finding now is Omicron,” she said. In terms of individual variants, the Omicron BA.1 variant is about 3% of circulating virus, the BA.2 variant is about 68%, and BA.2.12.1 makes up about 35%.
“We’re just starting to learn about the impact of BA2.121,” Dr. Walensky said. “It appears it might have a transmission advantage of about 25% over the BA2 subvariant.”
A version of this article first appeared on Medscape.com.
The percentage of Americans who have been infected with COVID-19 jumped from 34% in December 2021 to 58% in February 2022, a new study from the Centers for Disease Control and Prevention reveals.
This is the first time the seroprevalence of prior infection is more than 50% in the American population.
“I definitely expected that we were going to see an increase continue ... but I didn’t expect it to increase quite this much. But we follow the data ... and this is what the evidence is showing us,” lead study researcher Kristie E. N. Clarke, MD, said during a CDC media briefing April 26.
Researchers found that presence of antinucleocapsid (anti-N) antibodies from prior infection varied by age. The rate varied from as high as 75% in children and teenagers 17 years and younger to 33% in those 65 and older, for example.
The study showed that the anti-N antibodies were more common in age groups with the lowest vaccination numbers.
Combined with up-to-date CDC data on deaths, hospitalizations, and cases, the study provides a clearer picture of where we are now and where we might be headed in terms of the pandemic.
Vaccination still valuable
The fact that nearly 60% of Americans have antibodies from prior infection is not a reason to think people with a history of COVID-19 should skip vaccination, said CDC director Rochelle P. Walensky, MD.
“I can’t underscore enough that those with detectable antibodies from previous infection, we encourage them to still get vaccinated,” Dr. Walensky said.
“We do know that reinfections happen,” she said, “so that’s important in terms of thinking forward.”
The CDC continues to encourage all Americans to stay up to date with their COVID-19 vaccinations, said Dr. Clarke, colead for the CDC’s COVID-19 Epidemiology and Surveillance Taskforce Seroprevalence Team. “Having infection-induced antibodies does not necessarily mean you are protected against future infections.”
The study, published in the CDC’s Morbidity and Mortality Weekly Report (MMWR), did not evaluate antibody protection from COVID-19 vaccination.
It should also be noted that the study looked at presence or absence of anti-N antibodies, and not whether certain levels were linked to less or more protection.
Where are we now?
Dr. Walensky used the media briefing as an opportunity to share current COVID-19 numbers.
“Overall, we can continue to have some mixed trends. Deaths, fortunately, are continuing to trend downward with a 7-day average of about 300 per day, which represents an estimated 18% decline from the prior week,” she said.
Hospital admissions also remain low, at about 1,500 per day. “But we should note that for the second week in a row, they are slowly trending upwards,” Dr. Walensky said. There was an increase of about 9% at press time compared with the prior week.
Cases remain “comparatively low” to even where we were a month ago, at 44,000 per day,” Dr. Walensky said. “Although this too represents an increase of about 25% in the past week.”
Dr. Walensky noted that positive test numbers are not as reliable a metric as they were before the growth in use of rapid home tests. But it’s not the only measure. “We continue to believe that our PCR testing data, especially when we corroborate it with information from our other surveillance systems – like wastewater surveillance and emergency department surveillance – provide us a reliable picture of the trajectory of COVID-19 across our country.”
She recommended that people continue to consult the CDC’s COVID-19 county tracker to monitor local levels of COVID-19.
Dr. Walensky also shared recent findings from genomic sequencing that continue to show the predominance of the Omicron variant. “Essentially a hundred percent of what we’re finding now is Omicron,” she said. In terms of individual variants, the Omicron BA.1 variant is about 3% of circulating virus, the BA.2 variant is about 68%, and BA.2.12.1 makes up about 35%.
“We’re just starting to learn about the impact of BA2.121,” Dr. Walensky said. “It appears it might have a transmission advantage of about 25% over the BA2 subvariant.”
A version of this article first appeared on Medscape.com.
The percentage of Americans who have been infected with COVID-19 jumped from 34% in December 2021 to 58% in February 2022, a new study from the Centers for Disease Control and Prevention reveals.
This is the first time the seroprevalence of prior infection is more than 50% in the American population.
“I definitely expected that we were going to see an increase continue ... but I didn’t expect it to increase quite this much. But we follow the data ... and this is what the evidence is showing us,” lead study researcher Kristie E. N. Clarke, MD, said during a CDC media briefing April 26.
Researchers found that presence of antinucleocapsid (anti-N) antibodies from prior infection varied by age. The rate varied from as high as 75% in children and teenagers 17 years and younger to 33% in those 65 and older, for example.
The study showed that the anti-N antibodies were more common in age groups with the lowest vaccination numbers.
Combined with up-to-date CDC data on deaths, hospitalizations, and cases, the study provides a clearer picture of where we are now and where we might be headed in terms of the pandemic.
Vaccination still valuable
The fact that nearly 60% of Americans have antibodies from prior infection is not a reason to think people with a history of COVID-19 should skip vaccination, said CDC director Rochelle P. Walensky, MD.
“I can’t underscore enough that those with detectable antibodies from previous infection, we encourage them to still get vaccinated,” Dr. Walensky said.
“We do know that reinfections happen,” she said, “so that’s important in terms of thinking forward.”
The CDC continues to encourage all Americans to stay up to date with their COVID-19 vaccinations, said Dr. Clarke, colead for the CDC’s COVID-19 Epidemiology and Surveillance Taskforce Seroprevalence Team. “Having infection-induced antibodies does not necessarily mean you are protected against future infections.”
The study, published in the CDC’s Morbidity and Mortality Weekly Report (MMWR), did not evaluate antibody protection from COVID-19 vaccination.
It should also be noted that the study looked at presence or absence of anti-N antibodies, and not whether certain levels were linked to less or more protection.
Where are we now?
Dr. Walensky used the media briefing as an opportunity to share current COVID-19 numbers.
“Overall, we can continue to have some mixed trends. Deaths, fortunately, are continuing to trend downward with a 7-day average of about 300 per day, which represents an estimated 18% decline from the prior week,” she said.
Hospital admissions also remain low, at about 1,500 per day. “But we should note that for the second week in a row, they are slowly trending upwards,” Dr. Walensky said. There was an increase of about 9% at press time compared with the prior week.
Cases remain “comparatively low” to even where we were a month ago, at 44,000 per day,” Dr. Walensky said. “Although this too represents an increase of about 25% in the past week.”
Dr. Walensky noted that positive test numbers are not as reliable a metric as they were before the growth in use of rapid home tests. But it’s not the only measure. “We continue to believe that our PCR testing data, especially when we corroborate it with information from our other surveillance systems – like wastewater surveillance and emergency department surveillance – provide us a reliable picture of the trajectory of COVID-19 across our country.”
She recommended that people continue to consult the CDC’s COVID-19 county tracker to monitor local levels of COVID-19.
Dr. Walensky also shared recent findings from genomic sequencing that continue to show the predominance of the Omicron variant. “Essentially a hundred percent of what we’re finding now is Omicron,” she said. In terms of individual variants, the Omicron BA.1 variant is about 3% of circulating virus, the BA.2 variant is about 68%, and BA.2.12.1 makes up about 35%.
“We’re just starting to learn about the impact of BA2.121,” Dr. Walensky said. “It appears it might have a transmission advantage of about 25% over the BA2 subvariant.”
A version of this article first appeared on Medscape.com.
FROM MMWR
Erythematous Plaque on the Groin and Buttocks
The Diagnosis: Pseudomonas Pyoderma
A skin swab confirmed the presence of a ciprofloxacinsusceptible Pseudomonas aeruginosa strain. Our patient received oral ciprofloxacin 500 mg twice daily for 10 days with remarkable clinical improvement. The remaining skin lesion was successfully treated with more frequent diaper changes and the use of topical corticosteroids and emollients.
The topographical location, cutaneous morphology, clinical context, and sometimes the type of exudate are fundamental for the diagnosis of eruptions in intertriginous areas. Cutaneous Candida infections are common in these locations. They classically present as markedly erythematous plaques that occasionally are erosive, accompanied by satellite papules and pustules.1 Tinea cruris is a dermatophyte infection of the groin, proximal medial thighs, perineum, and buttocks. It usually presents as an erythematous patch that spreads centrifugally with partial central clearing and a slightly elevated, scaly border. Although candidiasis was higher on the differential, it was less likely, as our patient had a concomitant exudate inconsistent with Candida infections. Also, the lack of response to antifungal agents made hypotheses of fungal infections improbable.1
Inverse psoriasis is a variant of psoriasis identified by the development of well-demarcated, nonscaly, shiny plaques on body folds.2 Psoriasis is a chronic disease with several other cutaneous manifestations, such as nail and scalp involvement, as well as erythematous scaly plaques on the extensor surfaces of the limbs. The absence of a history of psoriasis, lack of other cutaneous manifestations, and no response to topical corticosteroids made the diagnosis of inverse psoriasis unlikely in our patient.
Erythrasma is a common superficial cutaneous infection caused by Corynebacterium minutissimum, a grampositive bacillus. It typically presents as an intertriginous eruption characterized by small erythematous to brown patches or thin plaques with fine scaling and sharp borders.3 Erythrasma displays a coral red fluorescence on Wood lamp examination that can be useful in the distinction from other causes of intertrigo.1 Although this examination had not been performed in our patient, the striking exudate made erythrasma less likely, and the culture performed on skin swab material would help to rule out this diagnosis.
Pseudomonas aeruginosa is a gram-negative strict aerobic bacillus of ubiquitous distribution with a preference for humid environments.4,5 Pseudomonas aeruginosa infections were first reported in the 19th century by physicians who noticed a peculiar odorous condition that caused a blue-green discoloration on bandages. This coloration explains the species name aeruginosa which is derived from the Latin word for copper rust.4 It comes from several water-soluble pigments produced by this microorganism, the most prevalent of which are pyocyanin and pyoverdine. Pyocyanin has a greenish-blue color and is nonfluorescent, while pyoverdine is green-yellowish and fluoresces under Wood light.5 Other pigments, such as pyorubin and pyomelanin, can be produced by some Pseudomonas strains.4
Pseudomonas aeruginosa has become one of the main pathogens involved in hospital-acquired infections,6 especially in immunocompromised patients.6,7 It is a frequent cause of respiratory infections in patients with cystic fibrosis, as it is present in the airways of up to 70% of these patients in adulthood.7 Also, due to a variety of adaptive mechanisms with the development of resistance to a range of antibiotics, P aeruginosa has become a worldwide public health problem and is involved in several life-threatening nosocomial infections.7,8
Cutaneous P aeruginosa infections range from superficial to deep tissue involvement and can affect both immunocompromised and immunocompetent individuals.9 They are classified as primary when they originate directly from the skin or secondary when they occur in the context of bacteremia. Primary infections mostly are mild and often are seen in healthy individuals; they usually occur by inoculation and predominate in moist areas where skin breakdown is frequent. Secondary infections typically affect immunocompromised individuals and portend a poor prognosis.5,9
Denominated as Pseudomonas pyoderma, the superficial skin infection by P aeruginosa is described as a condition where the epidermis has a moth-eaten appearance with macerated or eroded borders.10 A blue-greenish exudate and a grape juice odor often are present. This infection usually occurs as a complication of several skin conditions such as tinea pedis, eczema, burns, wounds, and ulcers.5,10
We believe that our patient developed Pseudomonas pyoderma as a complication of diaper dermatitis. His extended hospital stay with the use of different antibiotic regimens for the treatment of several infectious complications may have contributed to the development of infection by P aeruginosa.11 Despite its great clinical relevance, there are few studies in the literature on primary skin infections caused by P aeruginosa, and clinical descriptions with images are rare. Our patient had a nonspecific noneczematous dermatitis, and the projections on the periphery of the lesion resembled the moth-eaten appearance of the classic description of Pseudomonas pyoderma.5,10 The presence of a greenish exudate should promptly raise suspicion for this entity. We believe that the presentation of this case can illustrate this finding and help physicians to recognize this infection.
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Micali G, Verzi AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019; 12:953-959.
- Somerville DA. Erythrasma in normal young adults. J Med Microbiol. 1970;3:57-64.
- D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol 2. 8th ed. Elsevier; 2015:2518-2531.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999;38:419-431.
- Young LS, Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972;3:291-347.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: device-associated module. Am J Infect Control. 2020;48:423-432.
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011;12:157-169.
- Hall JH, Callaway JL, Tindall JP, et al. Pseudomonas aeruginosa in dermatology. Arch Dermatol. 1968;97:312-324.
- Merchant S, Proudfoot EM, Quadri HN, et al. Risk factors for Pseudomonas aeruginosa infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J Glob Antimicrob Resist. 2018;14:33-44.
The Diagnosis: Pseudomonas Pyoderma
A skin swab confirmed the presence of a ciprofloxacinsusceptible Pseudomonas aeruginosa strain. Our patient received oral ciprofloxacin 500 mg twice daily for 10 days with remarkable clinical improvement. The remaining skin lesion was successfully treated with more frequent diaper changes and the use of topical corticosteroids and emollients.
The topographical location, cutaneous morphology, clinical context, and sometimes the type of exudate are fundamental for the diagnosis of eruptions in intertriginous areas. Cutaneous Candida infections are common in these locations. They classically present as markedly erythematous plaques that occasionally are erosive, accompanied by satellite papules and pustules.1 Tinea cruris is a dermatophyte infection of the groin, proximal medial thighs, perineum, and buttocks. It usually presents as an erythematous patch that spreads centrifugally with partial central clearing and a slightly elevated, scaly border. Although candidiasis was higher on the differential, it was less likely, as our patient had a concomitant exudate inconsistent with Candida infections. Also, the lack of response to antifungal agents made hypotheses of fungal infections improbable.1
Inverse psoriasis is a variant of psoriasis identified by the development of well-demarcated, nonscaly, shiny plaques on body folds.2 Psoriasis is a chronic disease with several other cutaneous manifestations, such as nail and scalp involvement, as well as erythematous scaly plaques on the extensor surfaces of the limbs. The absence of a history of psoriasis, lack of other cutaneous manifestations, and no response to topical corticosteroids made the diagnosis of inverse psoriasis unlikely in our patient.
Erythrasma is a common superficial cutaneous infection caused by Corynebacterium minutissimum, a grampositive bacillus. It typically presents as an intertriginous eruption characterized by small erythematous to brown patches or thin plaques with fine scaling and sharp borders.3 Erythrasma displays a coral red fluorescence on Wood lamp examination that can be useful in the distinction from other causes of intertrigo.1 Although this examination had not been performed in our patient, the striking exudate made erythrasma less likely, and the culture performed on skin swab material would help to rule out this diagnosis.
Pseudomonas aeruginosa is a gram-negative strict aerobic bacillus of ubiquitous distribution with a preference for humid environments.4,5 Pseudomonas aeruginosa infections were first reported in the 19th century by physicians who noticed a peculiar odorous condition that caused a blue-green discoloration on bandages. This coloration explains the species name aeruginosa which is derived from the Latin word for copper rust.4 It comes from several water-soluble pigments produced by this microorganism, the most prevalent of which are pyocyanin and pyoverdine. Pyocyanin has a greenish-blue color and is nonfluorescent, while pyoverdine is green-yellowish and fluoresces under Wood light.5 Other pigments, such as pyorubin and pyomelanin, can be produced by some Pseudomonas strains.4
Pseudomonas aeruginosa has become one of the main pathogens involved in hospital-acquired infections,6 especially in immunocompromised patients.6,7 It is a frequent cause of respiratory infections in patients with cystic fibrosis, as it is present in the airways of up to 70% of these patients in adulthood.7 Also, due to a variety of adaptive mechanisms with the development of resistance to a range of antibiotics, P aeruginosa has become a worldwide public health problem and is involved in several life-threatening nosocomial infections.7,8
Cutaneous P aeruginosa infections range from superficial to deep tissue involvement and can affect both immunocompromised and immunocompetent individuals.9 They are classified as primary when they originate directly from the skin or secondary when they occur in the context of bacteremia. Primary infections mostly are mild and often are seen in healthy individuals; they usually occur by inoculation and predominate in moist areas where skin breakdown is frequent. Secondary infections typically affect immunocompromised individuals and portend a poor prognosis.5,9
Denominated as Pseudomonas pyoderma, the superficial skin infection by P aeruginosa is described as a condition where the epidermis has a moth-eaten appearance with macerated or eroded borders.10 A blue-greenish exudate and a grape juice odor often are present. This infection usually occurs as a complication of several skin conditions such as tinea pedis, eczema, burns, wounds, and ulcers.5,10
We believe that our patient developed Pseudomonas pyoderma as a complication of diaper dermatitis. His extended hospital stay with the use of different antibiotic regimens for the treatment of several infectious complications may have contributed to the development of infection by P aeruginosa.11 Despite its great clinical relevance, there are few studies in the literature on primary skin infections caused by P aeruginosa, and clinical descriptions with images are rare. Our patient had a nonspecific noneczematous dermatitis, and the projections on the periphery of the lesion resembled the moth-eaten appearance of the classic description of Pseudomonas pyoderma.5,10 The presence of a greenish exudate should promptly raise suspicion for this entity. We believe that the presentation of this case can illustrate this finding and help physicians to recognize this infection.
The Diagnosis: Pseudomonas Pyoderma
A skin swab confirmed the presence of a ciprofloxacinsusceptible Pseudomonas aeruginosa strain. Our patient received oral ciprofloxacin 500 mg twice daily for 10 days with remarkable clinical improvement. The remaining skin lesion was successfully treated with more frequent diaper changes and the use of topical corticosteroids and emollients.
The topographical location, cutaneous morphology, clinical context, and sometimes the type of exudate are fundamental for the diagnosis of eruptions in intertriginous areas. Cutaneous Candida infections are common in these locations. They classically present as markedly erythematous plaques that occasionally are erosive, accompanied by satellite papules and pustules.1 Tinea cruris is a dermatophyte infection of the groin, proximal medial thighs, perineum, and buttocks. It usually presents as an erythematous patch that spreads centrifugally with partial central clearing and a slightly elevated, scaly border. Although candidiasis was higher on the differential, it was less likely, as our patient had a concomitant exudate inconsistent with Candida infections. Also, the lack of response to antifungal agents made hypotheses of fungal infections improbable.1
Inverse psoriasis is a variant of psoriasis identified by the development of well-demarcated, nonscaly, shiny plaques on body folds.2 Psoriasis is a chronic disease with several other cutaneous manifestations, such as nail and scalp involvement, as well as erythematous scaly plaques on the extensor surfaces of the limbs. The absence of a history of psoriasis, lack of other cutaneous manifestations, and no response to topical corticosteroids made the diagnosis of inverse psoriasis unlikely in our patient.
Erythrasma is a common superficial cutaneous infection caused by Corynebacterium minutissimum, a grampositive bacillus. It typically presents as an intertriginous eruption characterized by small erythematous to brown patches or thin plaques with fine scaling and sharp borders.3 Erythrasma displays a coral red fluorescence on Wood lamp examination that can be useful in the distinction from other causes of intertrigo.1 Although this examination had not been performed in our patient, the striking exudate made erythrasma less likely, and the culture performed on skin swab material would help to rule out this diagnosis.
Pseudomonas aeruginosa is a gram-negative strict aerobic bacillus of ubiquitous distribution with a preference for humid environments.4,5 Pseudomonas aeruginosa infections were first reported in the 19th century by physicians who noticed a peculiar odorous condition that caused a blue-green discoloration on bandages. This coloration explains the species name aeruginosa which is derived from the Latin word for copper rust.4 It comes from several water-soluble pigments produced by this microorganism, the most prevalent of which are pyocyanin and pyoverdine. Pyocyanin has a greenish-blue color and is nonfluorescent, while pyoverdine is green-yellowish and fluoresces under Wood light.5 Other pigments, such as pyorubin and pyomelanin, can be produced by some Pseudomonas strains.4
Pseudomonas aeruginosa has become one of the main pathogens involved in hospital-acquired infections,6 especially in immunocompromised patients.6,7 It is a frequent cause of respiratory infections in patients with cystic fibrosis, as it is present in the airways of up to 70% of these patients in adulthood.7 Also, due to a variety of adaptive mechanisms with the development of resistance to a range of antibiotics, P aeruginosa has become a worldwide public health problem and is involved in several life-threatening nosocomial infections.7,8
Cutaneous P aeruginosa infections range from superficial to deep tissue involvement and can affect both immunocompromised and immunocompetent individuals.9 They are classified as primary when they originate directly from the skin or secondary when they occur in the context of bacteremia. Primary infections mostly are mild and often are seen in healthy individuals; they usually occur by inoculation and predominate in moist areas where skin breakdown is frequent. Secondary infections typically affect immunocompromised individuals and portend a poor prognosis.5,9
Denominated as Pseudomonas pyoderma, the superficial skin infection by P aeruginosa is described as a condition where the epidermis has a moth-eaten appearance with macerated or eroded borders.10 A blue-greenish exudate and a grape juice odor often are present. This infection usually occurs as a complication of several skin conditions such as tinea pedis, eczema, burns, wounds, and ulcers.5,10
We believe that our patient developed Pseudomonas pyoderma as a complication of diaper dermatitis. His extended hospital stay with the use of different antibiotic regimens for the treatment of several infectious complications may have contributed to the development of infection by P aeruginosa.11 Despite its great clinical relevance, there are few studies in the literature on primary skin infections caused by P aeruginosa, and clinical descriptions with images are rare. Our patient had a nonspecific noneczematous dermatitis, and the projections on the periphery of the lesion resembled the moth-eaten appearance of the classic description of Pseudomonas pyoderma.5,10 The presence of a greenish exudate should promptly raise suspicion for this entity. We believe that the presentation of this case can illustrate this finding and help physicians to recognize this infection.
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Micali G, Verzi AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019; 12:953-959.
- Somerville DA. Erythrasma in normal young adults. J Med Microbiol. 1970;3:57-64.
- D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol 2. 8th ed. Elsevier; 2015:2518-2531.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999;38:419-431.
- Young LS, Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972;3:291-347.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: device-associated module. Am J Infect Control. 2020;48:423-432.
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011;12:157-169.
- Hall JH, Callaway JL, Tindall JP, et al. Pseudomonas aeruginosa in dermatology. Arch Dermatol. 1968;97:312-324.
- Merchant S, Proudfoot EM, Quadri HN, et al. Risk factors for Pseudomonas aeruginosa infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J Glob Antimicrob Resist. 2018;14:33-44.
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Micali G, Verzi AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019; 12:953-959.
- Somerville DA. Erythrasma in normal young adults. J Med Microbiol. 1970;3:57-64.
- D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol 2. 8th ed. Elsevier; 2015:2518-2531.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999;38:419-431.
- Young LS, Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972;3:291-347.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: device-associated module. Am J Infect Control. 2020;48:423-432.
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011;12:157-169.
- Hall JH, Callaway JL, Tindall JP, et al. Pseudomonas aeruginosa in dermatology. Arch Dermatol. 1968;97:312-324.
- Merchant S, Proudfoot EM, Quadri HN, et al. Risk factors for Pseudomonas aeruginosa infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J Glob Antimicrob Resist. 2018;14:33-44.
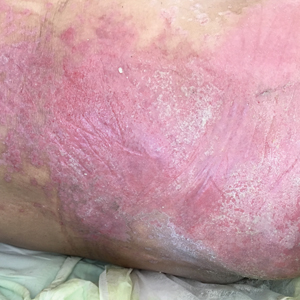
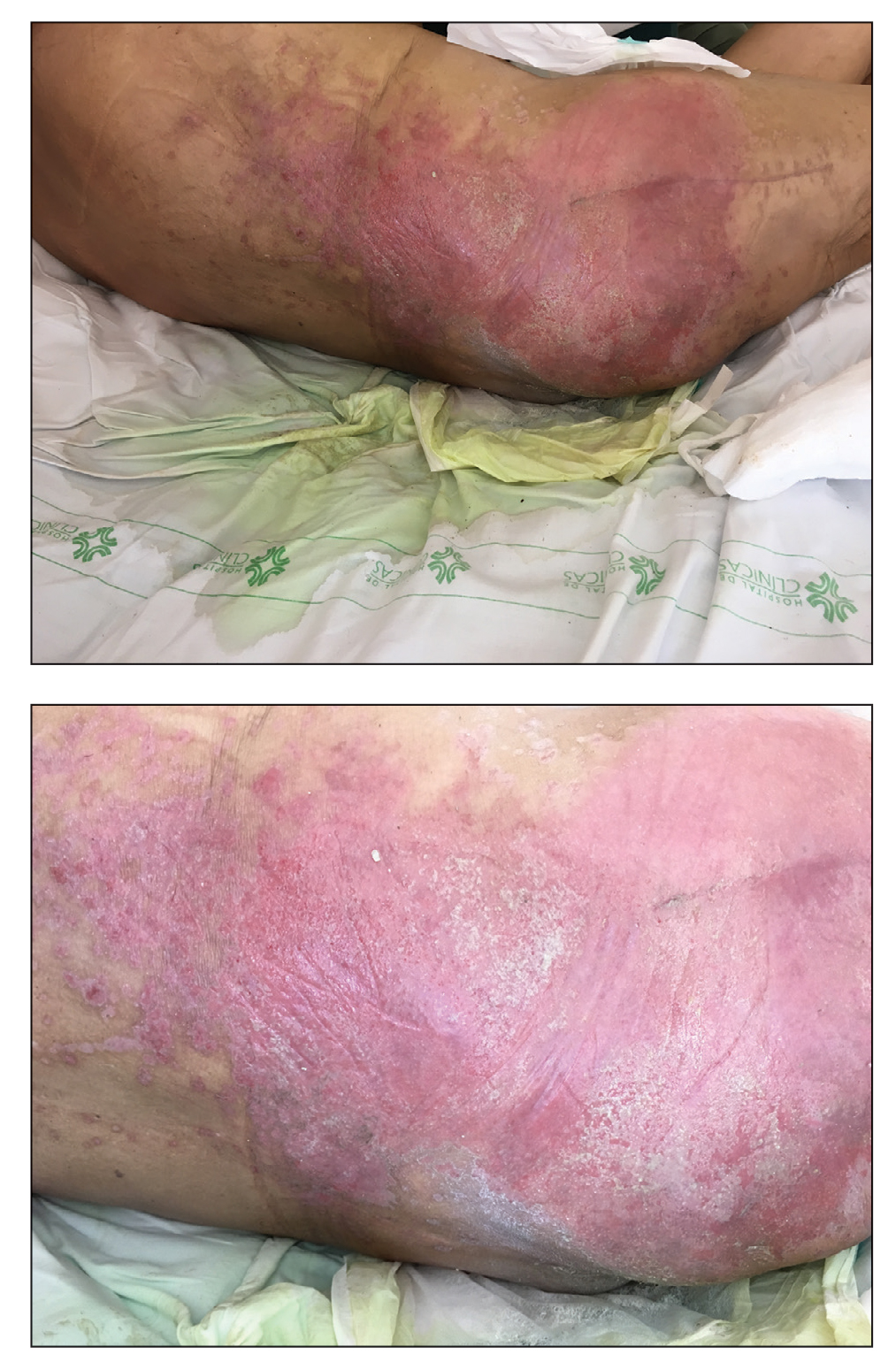
A 68-year-old man presented with an extensive erythematous plaque of 3 weeks’ duration that started in the groin and spread to the buttocks. It was associated with pruritus and a burning sensation. He was admitted to the palliative care unit 1 year prior for the management of terminal lung cancer. Despite the use of topical corticosteroids and antifungals, the lesions gradually worsened with dissemination to the back. Physical examination revealed an erythematous macerated plaque that extended from the buttocks and groin region to the scapular area (top). Its borders had an eroded appearance with projections compatible with radial spread (bottom). A greenish exudate soaked the diaper and sheets. No other cutaneous lesions were noted.
Pfizer recalls more quinapril because of potential carcinogen
, the company announced.
The Accupril recall comes one month after Pfizer recalled six lots of Accuretic (Quinapril HCI/hydrochlorathiazide) tablets for the same problem.
Accupril is indicated for the treatment of hypertension and management of heart failure when added to conventional therapy, including diuretics and/or digitalis.
To date, Pfizer is not aware of any reports of adverse events related to the Accupril recall, and the company believes the benefit/risk profile remains positive based on currently available data.
“Although long-term ingestion of N-nitroso-quinapril may be associated with a potential increased cancer risk in humans, there is no immediate risk to patients taking this medication,” the company said April 22 in a news release.
Patients currently taking the recalled products are asked to consult with their doctor about alternative treatment options.
The recalled Accupril tablets were sold in 90-count bottles distributed nationwide to wholesalers and distributors in the United States and Puerto Rico from December 2019 to April 2022.
National drug codes (NDC), lot numbers, and expiration dates are listed in the company announcement posted on the Food and Drug Administration’s website.
Patients who are taking this product should consult with their health care provider or pharmacy to determine if they have the affected product. Those with the affected tablets should contact claims management firm Sedgwick by phone at 888-345-0481 Monday through Friday from 8 AM to 5 PM ET for instructions on how to return the product and obtain reimbursement.
Healthcare providers with questions regarding the recall can contact Pfizer by telephone at 800-438-1985, option 3, Monday through Friday from 8 AM to 9 PM ET.
Adverse reactions or quality problems related to this recall should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
, the company announced.
The Accupril recall comes one month after Pfizer recalled six lots of Accuretic (Quinapril HCI/hydrochlorathiazide) tablets for the same problem.
Accupril is indicated for the treatment of hypertension and management of heart failure when added to conventional therapy, including diuretics and/or digitalis.
To date, Pfizer is not aware of any reports of adverse events related to the Accupril recall, and the company believes the benefit/risk profile remains positive based on currently available data.
“Although long-term ingestion of N-nitroso-quinapril may be associated with a potential increased cancer risk in humans, there is no immediate risk to patients taking this medication,” the company said April 22 in a news release.
Patients currently taking the recalled products are asked to consult with their doctor about alternative treatment options.
The recalled Accupril tablets were sold in 90-count bottles distributed nationwide to wholesalers and distributors in the United States and Puerto Rico from December 2019 to April 2022.
National drug codes (NDC), lot numbers, and expiration dates are listed in the company announcement posted on the Food and Drug Administration’s website.
Patients who are taking this product should consult with their health care provider or pharmacy to determine if they have the affected product. Those with the affected tablets should contact claims management firm Sedgwick by phone at 888-345-0481 Monday through Friday from 8 AM to 5 PM ET for instructions on how to return the product and obtain reimbursement.
Healthcare providers with questions regarding the recall can contact Pfizer by telephone at 800-438-1985, option 3, Monday through Friday from 8 AM to 9 PM ET.
Adverse reactions or quality problems related to this recall should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
, the company announced.
The Accupril recall comes one month after Pfizer recalled six lots of Accuretic (Quinapril HCI/hydrochlorathiazide) tablets for the same problem.
Accupril is indicated for the treatment of hypertension and management of heart failure when added to conventional therapy, including diuretics and/or digitalis.
To date, Pfizer is not aware of any reports of adverse events related to the Accupril recall, and the company believes the benefit/risk profile remains positive based on currently available data.
“Although long-term ingestion of N-nitroso-quinapril may be associated with a potential increased cancer risk in humans, there is no immediate risk to patients taking this medication,” the company said April 22 in a news release.
Patients currently taking the recalled products are asked to consult with their doctor about alternative treatment options.
The recalled Accupril tablets were sold in 90-count bottles distributed nationwide to wholesalers and distributors in the United States and Puerto Rico from December 2019 to April 2022.
National drug codes (NDC), lot numbers, and expiration dates are listed in the company announcement posted on the Food and Drug Administration’s website.
Patients who are taking this product should consult with their health care provider or pharmacy to determine if they have the affected product. Those with the affected tablets should contact claims management firm Sedgwick by phone at 888-345-0481 Monday through Friday from 8 AM to 5 PM ET for instructions on how to return the product and obtain reimbursement.
Healthcare providers with questions regarding the recall can contact Pfizer by telephone at 800-438-1985, option 3, Monday through Friday from 8 AM to 9 PM ET.
Adverse reactions or quality problems related to this recall should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Liquid biopsy a valuable tool for detecting, monitoring HCC
Liquid biopsy using circulating tumor (ctDNA) detection and profiling is a valuable tool for clinicians in monitoring hepatocellular carcinoma (HCC), particularly in monitoring progression, researchers wrote in a recent review.
Details of the review, led by co–first authors Xueying Lyu and Yu-Man Tsui, both of the department of pathology and State Key Laboratory of Liver Research at the University of Hong Kong, were published in Cellular and Molecular Gastroenterology and Hepatology.
Because there are few treatment options for advanced-stage liver cancer, scientists are searching for noninvasive ways to detect liver cancer before is progresses. Liver resection is the primary treatment for HCC, but the recurrence rate is high. Early detection increases the ability to identify relevant molecular-targeted drugs and helps predict patient response.
There is growing interest in noninvasive circulating cell-free DNA (cfDNA) as well as in ctDNA – both are part of promising strategies to test circulating DNA in the bloodstream. Together with other circulating biomarkers, they are called liquid biopsy.
HCC can be detected noninvasively by detecting plasma ctDNA released from dying cancer cells. Detection depends on determining whether the circulating tumor DNA has the same molecular alterations as its tumor source. cfDNA contains genomic DNA from different tumor clones or tumors from different sites within a patient to help real-time monitoring of tumor progression.
Barriers to widespread clinical use of liquid biopsy include lack of standardization of the collection process. Procedures differ across health systems on how much blood should be collected, which tubes should be used for collection and how samples should be stored and shipped. The study authors suggested that “specialized tubes can be used for blood sample collection to reduce the chance of white blood cell rupture and genomic DNA contamination from the damaged white blood cells.”
Further research is needed
The study findings indicated that some aspects of liquid biopsy with cfDNA/ctDNA still need further exploration. For example, the effects of tumor vascularization, tumor aggressiveness, metabolic activity, and cell death mechanism on the dynamics of ctDNA in the bloodstream need to be identified.
It’s not yet clear how cfDNA is released into the bloodstream. Actively released cfDNA from the tumor may convey a different message from cfDNA released passively from dying cells upon treatment. The first represents treatment-resistant cells/subclones while the second represents treatment-responsive cells/subclones. Moreover, it is difficult to detect ctDNA mutation in early stage cancers that have lower tumor burden.
The investigators wrote: “The contributions of cfDNA from apoptosis, necrosis, autophagic cell death, and active release at different time points during disease progression, treatment response, and resistance appearance are poorly understood and will affect interpretation of the clinical observation in cfDNA assays.” A lower limit of detection needs to be determined and a standard curve set so that researchers can quantify the allelic frequencies of the mutants in cfDNA and avoid false-negative detection.
They urged establishing external quality assurance to verify laboratory performance, the proficiency in the cfDNA diagnostic test, and interpretation of results to identify errors in sampling, procedures, and decision making. Legal liability and cost effectiveness of using plasma cfDNA in treatment decisions also need to be considered.
The researchers wrote that, to better understand how ctDNA/cfDNA can be used to complement precision medicine in liver cancer, large multicenter cohorts and long-term follow-up are needed to compare ctDNA-guided decision-making against standard treatment without guidance from ctDNA profiling.
The authors disclosed having no conflicts of interest.
Detection and characterization of circulating tumor DNA (ctDNA) is one of the major forms of liquid biopsy. Because ctDNA can reflect molecular features of cancer tissues, it is considered an ideal alternative to tissue biopsy. Furthermore, it can overcome the limitation of tumor tissue biopsies such as bleeding, needle tract seeding, and sampling error.
Currently, several large biomarker trials of ctDNA for early HCC detection are underway. Once its accuracy is established in phase 3-4 biomarker studies, the role of ctDNA in the context of the existing surveillance program should be further defined. As the combination of ctDNA and other orthogonal circulating biomarkers was shown to enhance the performance, future research should explore biomarker panels that include ctDNA and other promising markers to maximize performance. Predictive biomarkers for treatment response is an unmet need in HCC. Investigating the role of a specific ctDNA marker panel as a predictor of immunotherapy responsiveness would be of great interest and is under active investigation.
Ju Dong Yang, MD, is with the Karsh Division of Digestive and Liver Diseases in the department of medicine, with the Comprehensive Transplant Center, and with the Samuel Oschin Comprehensive Cancer Institute at Cedars Sinai Medical Center, Los Angeles. He disclosed providing consulting services for Exact Sciences and Exelixis and Eisai.
Detection and characterization of circulating tumor DNA (ctDNA) is one of the major forms of liquid biopsy. Because ctDNA can reflect molecular features of cancer tissues, it is considered an ideal alternative to tissue biopsy. Furthermore, it can overcome the limitation of tumor tissue biopsies such as bleeding, needle tract seeding, and sampling error.
Currently, several large biomarker trials of ctDNA for early HCC detection are underway. Once its accuracy is established in phase 3-4 biomarker studies, the role of ctDNA in the context of the existing surveillance program should be further defined. As the combination of ctDNA and other orthogonal circulating biomarkers was shown to enhance the performance, future research should explore biomarker panels that include ctDNA and other promising markers to maximize performance. Predictive biomarkers for treatment response is an unmet need in HCC. Investigating the role of a specific ctDNA marker panel as a predictor of immunotherapy responsiveness would be of great interest and is under active investigation.
Ju Dong Yang, MD, is with the Karsh Division of Digestive and Liver Diseases in the department of medicine, with the Comprehensive Transplant Center, and with the Samuel Oschin Comprehensive Cancer Institute at Cedars Sinai Medical Center, Los Angeles. He disclosed providing consulting services for Exact Sciences and Exelixis and Eisai.
Detection and characterization of circulating tumor DNA (ctDNA) is one of the major forms of liquid biopsy. Because ctDNA can reflect molecular features of cancer tissues, it is considered an ideal alternative to tissue biopsy. Furthermore, it can overcome the limitation of tumor tissue biopsies such as bleeding, needle tract seeding, and sampling error.
Currently, several large biomarker trials of ctDNA for early HCC detection are underway. Once its accuracy is established in phase 3-4 biomarker studies, the role of ctDNA in the context of the existing surveillance program should be further defined. As the combination of ctDNA and other orthogonal circulating biomarkers was shown to enhance the performance, future research should explore biomarker panels that include ctDNA and other promising markers to maximize performance. Predictive biomarkers for treatment response is an unmet need in HCC. Investigating the role of a specific ctDNA marker panel as a predictor of immunotherapy responsiveness would be of great interest and is under active investigation.
Ju Dong Yang, MD, is with the Karsh Division of Digestive and Liver Diseases in the department of medicine, with the Comprehensive Transplant Center, and with the Samuel Oschin Comprehensive Cancer Institute at Cedars Sinai Medical Center, Los Angeles. He disclosed providing consulting services for Exact Sciences and Exelixis and Eisai.
Liquid biopsy using circulating tumor (ctDNA) detection and profiling is a valuable tool for clinicians in monitoring hepatocellular carcinoma (HCC), particularly in monitoring progression, researchers wrote in a recent review.
Details of the review, led by co–first authors Xueying Lyu and Yu-Man Tsui, both of the department of pathology and State Key Laboratory of Liver Research at the University of Hong Kong, were published in Cellular and Molecular Gastroenterology and Hepatology.
Because there are few treatment options for advanced-stage liver cancer, scientists are searching for noninvasive ways to detect liver cancer before is progresses. Liver resection is the primary treatment for HCC, but the recurrence rate is high. Early detection increases the ability to identify relevant molecular-targeted drugs and helps predict patient response.
There is growing interest in noninvasive circulating cell-free DNA (cfDNA) as well as in ctDNA – both are part of promising strategies to test circulating DNA in the bloodstream. Together with other circulating biomarkers, they are called liquid biopsy.
HCC can be detected noninvasively by detecting plasma ctDNA released from dying cancer cells. Detection depends on determining whether the circulating tumor DNA has the same molecular alterations as its tumor source. cfDNA contains genomic DNA from different tumor clones or tumors from different sites within a patient to help real-time monitoring of tumor progression.
Barriers to widespread clinical use of liquid biopsy include lack of standardization of the collection process. Procedures differ across health systems on how much blood should be collected, which tubes should be used for collection and how samples should be stored and shipped. The study authors suggested that “specialized tubes can be used for blood sample collection to reduce the chance of white blood cell rupture and genomic DNA contamination from the damaged white blood cells.”
Further research is needed
The study findings indicated that some aspects of liquid biopsy with cfDNA/ctDNA still need further exploration. For example, the effects of tumor vascularization, tumor aggressiveness, metabolic activity, and cell death mechanism on the dynamics of ctDNA in the bloodstream need to be identified.
It’s not yet clear how cfDNA is released into the bloodstream. Actively released cfDNA from the tumor may convey a different message from cfDNA released passively from dying cells upon treatment. The first represents treatment-resistant cells/subclones while the second represents treatment-responsive cells/subclones. Moreover, it is difficult to detect ctDNA mutation in early stage cancers that have lower tumor burden.
The investigators wrote: “The contributions of cfDNA from apoptosis, necrosis, autophagic cell death, and active release at different time points during disease progression, treatment response, and resistance appearance are poorly understood and will affect interpretation of the clinical observation in cfDNA assays.” A lower limit of detection needs to be determined and a standard curve set so that researchers can quantify the allelic frequencies of the mutants in cfDNA and avoid false-negative detection.
They urged establishing external quality assurance to verify laboratory performance, the proficiency in the cfDNA diagnostic test, and interpretation of results to identify errors in sampling, procedures, and decision making. Legal liability and cost effectiveness of using plasma cfDNA in treatment decisions also need to be considered.
The researchers wrote that, to better understand how ctDNA/cfDNA can be used to complement precision medicine in liver cancer, large multicenter cohorts and long-term follow-up are needed to compare ctDNA-guided decision-making against standard treatment without guidance from ctDNA profiling.
The authors disclosed having no conflicts of interest.
Liquid biopsy using circulating tumor (ctDNA) detection and profiling is a valuable tool for clinicians in monitoring hepatocellular carcinoma (HCC), particularly in monitoring progression, researchers wrote in a recent review.
Details of the review, led by co–first authors Xueying Lyu and Yu-Man Tsui, both of the department of pathology and State Key Laboratory of Liver Research at the University of Hong Kong, were published in Cellular and Molecular Gastroenterology and Hepatology.
Because there are few treatment options for advanced-stage liver cancer, scientists are searching for noninvasive ways to detect liver cancer before is progresses. Liver resection is the primary treatment for HCC, but the recurrence rate is high. Early detection increases the ability to identify relevant molecular-targeted drugs and helps predict patient response.
There is growing interest in noninvasive circulating cell-free DNA (cfDNA) as well as in ctDNA – both are part of promising strategies to test circulating DNA in the bloodstream. Together with other circulating biomarkers, they are called liquid biopsy.
HCC can be detected noninvasively by detecting plasma ctDNA released from dying cancer cells. Detection depends on determining whether the circulating tumor DNA has the same molecular alterations as its tumor source. cfDNA contains genomic DNA from different tumor clones or tumors from different sites within a patient to help real-time monitoring of tumor progression.
Barriers to widespread clinical use of liquid biopsy include lack of standardization of the collection process. Procedures differ across health systems on how much blood should be collected, which tubes should be used for collection and how samples should be stored and shipped. The study authors suggested that “specialized tubes can be used for blood sample collection to reduce the chance of white blood cell rupture and genomic DNA contamination from the damaged white blood cells.”
Further research is needed
The study findings indicated that some aspects of liquid biopsy with cfDNA/ctDNA still need further exploration. For example, the effects of tumor vascularization, tumor aggressiveness, metabolic activity, and cell death mechanism on the dynamics of ctDNA in the bloodstream need to be identified.
It’s not yet clear how cfDNA is released into the bloodstream. Actively released cfDNA from the tumor may convey a different message from cfDNA released passively from dying cells upon treatment. The first represents treatment-resistant cells/subclones while the second represents treatment-responsive cells/subclones. Moreover, it is difficult to detect ctDNA mutation in early stage cancers that have lower tumor burden.
The investigators wrote: “The contributions of cfDNA from apoptosis, necrosis, autophagic cell death, and active release at different time points during disease progression, treatment response, and resistance appearance are poorly understood and will affect interpretation of the clinical observation in cfDNA assays.” A lower limit of detection needs to be determined and a standard curve set so that researchers can quantify the allelic frequencies of the mutants in cfDNA and avoid false-negative detection.
They urged establishing external quality assurance to verify laboratory performance, the proficiency in the cfDNA diagnostic test, and interpretation of results to identify errors in sampling, procedures, and decision making. Legal liability and cost effectiveness of using plasma cfDNA in treatment decisions also need to be considered.
The researchers wrote that, to better understand how ctDNA/cfDNA can be used to complement precision medicine in liver cancer, large multicenter cohorts and long-term follow-up are needed to compare ctDNA-guided decision-making against standard treatment without guidance from ctDNA profiling.
The authors disclosed having no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Lowering BP according to newest guidance would cut CV events
Using the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) guideline target of systolic blood pressure (BP) < 120 mm Hg, 66% of adults with chronic kidney disease (CKD) would be eligible for BP lowering, according to a study from Korea.
This represents an added > 10% of patients compared with two earlier guidelines, and these patients have a high risk of cardiovascular disease (CVD), Hyeok-Hee Lee, MD, Yonsei University College of Medicine, Seoul, South Korea, and colleagues reported.
The study was published online in the Journal of the American College of Cardiology.
“New candidates for BP-lowering treatment per the 2021 KDIGO guideline account for a substantial proportion of the total CKD population and bear significantly high CVD risk,” the researchers concluded.
“Undoubtedly, a multipronged approach will be required to address the swelling number of people needing more intense treatment, especially against a background of falling rates of BP control in the general community,” Alexander G. Logan, MD, of Mount Sinai Hospital, Toronto, and the University of Toronto, wrote in an accompanying editorial.
“Let’s not forget hypertension is the number one killer today,” Valentin Fuster, MD, of Icahn School of Medicine at Mount Sinai, New York, who is editor-in-chief of the Journal of the American College of Cardiology, stressed in a podcast that accompanied the article.
“Only 50% of individuals know of their blood pressure, and from this, less than half are properly treated,” he said.
“Today the details of knowing blood pressure levels appear to dominate over the huge ignorance of not knowing about blood pressure at all. Let’s think more and more about this reality,” he urged.
Three guidelines, two study objectives
The researchers compared three guidelines:
- The 2021 KDIGO guidelines, with a target systolic BP of < 120 mm Hg (largely based on the SPRINT trial).
- The 2012 KDIGO guidelines, with a target BP of ≤ 130/80 mm Hg for patients with albuminuria and ≤ 140/90 mm Hg for patients without albuminuria.
- The 2017 American College of Cardiology/American Heart Association (ACC/AHA) BP guideline target of < 130/80 mm Hg.
The study had two objectives:
- To examine the proportions of concordance and discordance between the three guidelines among adults with CKD based on cross-sectional data from the Korea National Health and Nutrition Examination Survey (KNHANES).
- To evaluate the association of each concordance/discordance group with cardiovascular outcomes of patients in the Korean National Health Insurance Service (NHIS) database.
For the first objective, the researchers identified 1,939 adults with CKD from the 2011-2014 survey cycles of KNHANES. Patients were a median age of 59 and 51% were men.
Comparison of the KDIGO 2021 versus 2012 BP targets showed that 50% of patients had BP above both targets; 16% had BP above the KDIGO 2021 target only; 4% had BP above the KDIGO 2012 target only; and 30% had BP control within both targets.
Comparison of the KDIGO 2021 versus 2017 ACC/AHA BP targets showed that 55% of patients had BP above both targets; 11% had BP above the KDIGO 2021 target only; 5% had BP above the 2017 ACC/AHA target only; and 29% had BP control within both targets.
For the second objective, using the NHIS database, researchers identified 412,167 adults with CKD who had routine health examinations during 2009 and 2010. The patients were a median age of 65 and 44% were men.
During a median follow-up of 10 years, the patients had 37,912 incident CVD events, defined as the first hospitalization for myocardial infarction, stroke, or heart failure, or death from CVD.
The adjusted risk of a composite CVD event was higher in patients with BP above the 2021 KDIGO target only (HR, 1.28) or above both the 2012 and 2021 KDIGO targets (HR, 1.52), compared to patients who had BP within both targets.
The adjusted risk of a composite CVD event was also higher in patients with BP above the 2021 KDIGO target only (HR, 1.18) or above both the 2021 KDIGO target and the 2017 ACC/AHA target (HR, 1.41), compared with patients who had BP within both targets.
Editorialist highlights three study aspects
Dr. Fuster noted three main points made by Dr. Logan.
First, the KDIGO 2021 guideline is based on office blood pressure, measured according to the procedure used in the 2017 ACC/AHA guideline. However, the SPRINT ambulatory BP ancillary study found that daytime ambulatory systolic BP was 6.8 mm Hg higher in the < 120 mm Hg group than clinic systolic BP that was measured with an automated BP device, mostly without study personnel.
Second, Dr. Logan noted that “not surprisingly, the investigators showed that the weighted proportion of adults with CKD eligible for BP lowering was highest (66.1%) according to 2021 KDIGO guideline,” compared with the two earlier guidelines.
The findings by Dr. Lee and colleagues align with those of a study that used data from the 2015-2018 U.S. NHANES to estimate the proportion of U.S. adults with CKD eligible for BP lowering according to the 2021 KDIGO guidelines, Dr. Logan added. The study found that 69% of U.S. adults (roughly 24.5 million) should correct their BP.
Third, the study in Korea showed a small percentage of patients (3%-5% of the total) had elevated diastolic BP but controlled systolic BP (< 120 mm Hg) with no increased risk of CVD compared to a reference group of patients with well-controlled BP.
“There is a paucity of evidence examining the relationship between diastolic hypertension and outcomes independently from systolic BP level in CKD patients,” Dr. Logan wrote. Similarly, Dr. Lee and colleagues identified this as an area for further research.
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea. The authors and editorialist have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Using the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) guideline target of systolic blood pressure (BP) < 120 mm Hg, 66% of adults with chronic kidney disease (CKD) would be eligible for BP lowering, according to a study from Korea.
This represents an added > 10% of patients compared with two earlier guidelines, and these patients have a high risk of cardiovascular disease (CVD), Hyeok-Hee Lee, MD, Yonsei University College of Medicine, Seoul, South Korea, and colleagues reported.
The study was published online in the Journal of the American College of Cardiology.
“New candidates for BP-lowering treatment per the 2021 KDIGO guideline account for a substantial proportion of the total CKD population and bear significantly high CVD risk,” the researchers concluded.
“Undoubtedly, a multipronged approach will be required to address the swelling number of people needing more intense treatment, especially against a background of falling rates of BP control in the general community,” Alexander G. Logan, MD, of Mount Sinai Hospital, Toronto, and the University of Toronto, wrote in an accompanying editorial.
“Let’s not forget hypertension is the number one killer today,” Valentin Fuster, MD, of Icahn School of Medicine at Mount Sinai, New York, who is editor-in-chief of the Journal of the American College of Cardiology, stressed in a podcast that accompanied the article.
“Only 50% of individuals know of their blood pressure, and from this, less than half are properly treated,” he said.
“Today the details of knowing blood pressure levels appear to dominate over the huge ignorance of not knowing about blood pressure at all. Let’s think more and more about this reality,” he urged.
Three guidelines, two study objectives
The researchers compared three guidelines:
- The 2021 KDIGO guidelines, with a target systolic BP of < 120 mm Hg (largely based on the SPRINT trial).
- The 2012 KDIGO guidelines, with a target BP of ≤ 130/80 mm Hg for patients with albuminuria and ≤ 140/90 mm Hg for patients without albuminuria.
- The 2017 American College of Cardiology/American Heart Association (ACC/AHA) BP guideline target of < 130/80 mm Hg.
The study had two objectives:
- To examine the proportions of concordance and discordance between the three guidelines among adults with CKD based on cross-sectional data from the Korea National Health and Nutrition Examination Survey (KNHANES).
- To evaluate the association of each concordance/discordance group with cardiovascular outcomes of patients in the Korean National Health Insurance Service (NHIS) database.
For the first objective, the researchers identified 1,939 adults with CKD from the 2011-2014 survey cycles of KNHANES. Patients were a median age of 59 and 51% were men.
Comparison of the KDIGO 2021 versus 2012 BP targets showed that 50% of patients had BP above both targets; 16% had BP above the KDIGO 2021 target only; 4% had BP above the KDIGO 2012 target only; and 30% had BP control within both targets.
Comparison of the KDIGO 2021 versus 2017 ACC/AHA BP targets showed that 55% of patients had BP above both targets; 11% had BP above the KDIGO 2021 target only; 5% had BP above the 2017 ACC/AHA target only; and 29% had BP control within both targets.
For the second objective, using the NHIS database, researchers identified 412,167 adults with CKD who had routine health examinations during 2009 and 2010. The patients were a median age of 65 and 44% were men.
During a median follow-up of 10 years, the patients had 37,912 incident CVD events, defined as the first hospitalization for myocardial infarction, stroke, or heart failure, or death from CVD.
The adjusted risk of a composite CVD event was higher in patients with BP above the 2021 KDIGO target only (HR, 1.28) or above both the 2012 and 2021 KDIGO targets (HR, 1.52), compared to patients who had BP within both targets.
The adjusted risk of a composite CVD event was also higher in patients with BP above the 2021 KDIGO target only (HR, 1.18) or above both the 2021 KDIGO target and the 2017 ACC/AHA target (HR, 1.41), compared with patients who had BP within both targets.
Editorialist highlights three study aspects
Dr. Fuster noted three main points made by Dr. Logan.
First, the KDIGO 2021 guideline is based on office blood pressure, measured according to the procedure used in the 2017 ACC/AHA guideline. However, the SPRINT ambulatory BP ancillary study found that daytime ambulatory systolic BP was 6.8 mm Hg higher in the < 120 mm Hg group than clinic systolic BP that was measured with an automated BP device, mostly without study personnel.
Second, Dr. Logan noted that “not surprisingly, the investigators showed that the weighted proportion of adults with CKD eligible for BP lowering was highest (66.1%) according to 2021 KDIGO guideline,” compared with the two earlier guidelines.
The findings by Dr. Lee and colleagues align with those of a study that used data from the 2015-2018 U.S. NHANES to estimate the proportion of U.S. adults with CKD eligible for BP lowering according to the 2021 KDIGO guidelines, Dr. Logan added. The study found that 69% of U.S. adults (roughly 24.5 million) should correct their BP.
Third, the study in Korea showed a small percentage of patients (3%-5% of the total) had elevated diastolic BP but controlled systolic BP (< 120 mm Hg) with no increased risk of CVD compared to a reference group of patients with well-controlled BP.
“There is a paucity of evidence examining the relationship between diastolic hypertension and outcomes independently from systolic BP level in CKD patients,” Dr. Logan wrote. Similarly, Dr. Lee and colleagues identified this as an area for further research.
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea. The authors and editorialist have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Using the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) guideline target of systolic blood pressure (BP) < 120 mm Hg, 66% of adults with chronic kidney disease (CKD) would be eligible for BP lowering, according to a study from Korea.
This represents an added > 10% of patients compared with two earlier guidelines, and these patients have a high risk of cardiovascular disease (CVD), Hyeok-Hee Lee, MD, Yonsei University College of Medicine, Seoul, South Korea, and colleagues reported.
The study was published online in the Journal of the American College of Cardiology.
“New candidates for BP-lowering treatment per the 2021 KDIGO guideline account for a substantial proportion of the total CKD population and bear significantly high CVD risk,” the researchers concluded.
“Undoubtedly, a multipronged approach will be required to address the swelling number of people needing more intense treatment, especially against a background of falling rates of BP control in the general community,” Alexander G. Logan, MD, of Mount Sinai Hospital, Toronto, and the University of Toronto, wrote in an accompanying editorial.
“Let’s not forget hypertension is the number one killer today,” Valentin Fuster, MD, of Icahn School of Medicine at Mount Sinai, New York, who is editor-in-chief of the Journal of the American College of Cardiology, stressed in a podcast that accompanied the article.
“Only 50% of individuals know of their blood pressure, and from this, less than half are properly treated,” he said.
“Today the details of knowing blood pressure levels appear to dominate over the huge ignorance of not knowing about blood pressure at all. Let’s think more and more about this reality,” he urged.
Three guidelines, two study objectives
The researchers compared three guidelines:
- The 2021 KDIGO guidelines, with a target systolic BP of < 120 mm Hg (largely based on the SPRINT trial).
- The 2012 KDIGO guidelines, with a target BP of ≤ 130/80 mm Hg for patients with albuminuria and ≤ 140/90 mm Hg for patients without albuminuria.
- The 2017 American College of Cardiology/American Heart Association (ACC/AHA) BP guideline target of < 130/80 mm Hg.
The study had two objectives:
- To examine the proportions of concordance and discordance between the three guidelines among adults with CKD based on cross-sectional data from the Korea National Health and Nutrition Examination Survey (KNHANES).
- To evaluate the association of each concordance/discordance group with cardiovascular outcomes of patients in the Korean National Health Insurance Service (NHIS) database.
For the first objective, the researchers identified 1,939 adults with CKD from the 2011-2014 survey cycles of KNHANES. Patients were a median age of 59 and 51% were men.
Comparison of the KDIGO 2021 versus 2012 BP targets showed that 50% of patients had BP above both targets; 16% had BP above the KDIGO 2021 target only; 4% had BP above the KDIGO 2012 target only; and 30% had BP control within both targets.
Comparison of the KDIGO 2021 versus 2017 ACC/AHA BP targets showed that 55% of patients had BP above both targets; 11% had BP above the KDIGO 2021 target only; 5% had BP above the 2017 ACC/AHA target only; and 29% had BP control within both targets.
For the second objective, using the NHIS database, researchers identified 412,167 adults with CKD who had routine health examinations during 2009 and 2010. The patients were a median age of 65 and 44% were men.
During a median follow-up of 10 years, the patients had 37,912 incident CVD events, defined as the first hospitalization for myocardial infarction, stroke, or heart failure, or death from CVD.
The adjusted risk of a composite CVD event was higher in patients with BP above the 2021 KDIGO target only (HR, 1.28) or above both the 2012 and 2021 KDIGO targets (HR, 1.52), compared to patients who had BP within both targets.
The adjusted risk of a composite CVD event was also higher in patients with BP above the 2021 KDIGO target only (HR, 1.18) or above both the 2021 KDIGO target and the 2017 ACC/AHA target (HR, 1.41), compared with patients who had BP within both targets.
Editorialist highlights three study aspects
Dr. Fuster noted three main points made by Dr. Logan.
First, the KDIGO 2021 guideline is based on office blood pressure, measured according to the procedure used in the 2017 ACC/AHA guideline. However, the SPRINT ambulatory BP ancillary study found that daytime ambulatory systolic BP was 6.8 mm Hg higher in the < 120 mm Hg group than clinic systolic BP that was measured with an automated BP device, mostly without study personnel.
Second, Dr. Logan noted that “not surprisingly, the investigators showed that the weighted proportion of adults with CKD eligible for BP lowering was highest (66.1%) according to 2021 KDIGO guideline,” compared with the two earlier guidelines.
The findings by Dr. Lee and colleagues align with those of a study that used data from the 2015-2018 U.S. NHANES to estimate the proportion of U.S. adults with CKD eligible for BP lowering according to the 2021 KDIGO guidelines, Dr. Logan added. The study found that 69% of U.S. adults (roughly 24.5 million) should correct their BP.
Third, the study in Korea showed a small percentage of patients (3%-5% of the total) had elevated diastolic BP but controlled systolic BP (< 120 mm Hg) with no increased risk of CVD compared to a reference group of patients with well-controlled BP.
“There is a paucity of evidence examining the relationship between diastolic hypertension and outcomes independently from systolic BP level in CKD patients,” Dr. Logan wrote. Similarly, Dr. Lee and colleagues identified this as an area for further research.
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea. The authors and editorialist have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
EU approves new blood and lung cancer drugs
The European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion for the two products at its April meeting.
New drug for certain lung cancer patients
Capmatinib is a selective, reversible inhibitor of MET tyrosine kinase and is indicated for the treatment of patients with advanced NSCLC harboring alterations leading to mesenchymal-epithelial transition factor gene exon 14 (METex14) skipping. Patients must have already been treated with immunotherapy and/or platinum-based chemotherapy.
The product is approved in the United States, and the Food and Drug Administration noted that it is the first approved treatment for NSCLC with MET exon 14-skipping mutations.
The FDA granted the drug an accelerated approval based on overall response rate and response duration in the GEOMETRY mono-1 trial, which included a cohort of previously treated and treatment-naive patients. The overall response rate was 68% in the treatment-naive patients and 41% in the previously treated patients. The median duration of response was 12.6 months and 9.7 months.
The most common side effects were peripheral edema, nausea, fatigue, vomiting, dyspnea, and decreased appetite.
Conditional approval for lymphoma
Mosunetuzumab is an investigational bispecific antibody targeting CD20 and CD3, and redirects T cells to engage and eliminate malignant B cells.
The CHMP recommended a conditional approval for this drug for use as monotherapy for the treatment of adult patients with relapsed or refractory follicular lymphoma who have received at least two prior systemic therapies.
Mosunetuzumab was reviewed under EMA’s accelerated access program, which usually takes 150 evaluation days as opposed to 210, and it was designated as an orphan medicine during its development.
The EMA stated that the benefits of this product are the high proportion of patients with a complete response and the durability of the treatment response.
As previously reported by this news organization, results from a phase 2 expansion study showed that when used as monotherapy, it induced high response rates and long-duration responses in patients with heavily pretreated, relapsed, or refractory follicular lymphoma.
At a median follow-up of 18.3 months, 54 of 90 patients (60%) had a complete response, and 18 (20%) had a partial response after treatment with mosunetuzumab.
The most common reported side effects were cytokine release syndrome, neutropenia, pyrexia (fever), hypophosphatemia, and headache.
Mosunetuzumab is awaiting FDA approval in the United States.
A conditional marketing authorization from CHMP is granted to products that meet an unmet medical need, and when the benefit to public health of immediate availability outweighs the risk inherent in the fact that additional data are still required. The marketing authorization holder is expected to provide comprehensive clinical data at a later stage.
Detailed recommendations for the use of both products will be described in the summary of product characteristics (SmPC), which will be published in the European public assessment report (EPAR) and made available in all official European Union languages after the marketing authorization has been granted by the European Commission.
A version of this article first appeared on Medscape.com.
The European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion for the two products at its April meeting.
New drug for certain lung cancer patients
Capmatinib is a selective, reversible inhibitor of MET tyrosine kinase and is indicated for the treatment of patients with advanced NSCLC harboring alterations leading to mesenchymal-epithelial transition factor gene exon 14 (METex14) skipping. Patients must have already been treated with immunotherapy and/or platinum-based chemotherapy.
The product is approved in the United States, and the Food and Drug Administration noted that it is the first approved treatment for NSCLC with MET exon 14-skipping mutations.
The FDA granted the drug an accelerated approval based on overall response rate and response duration in the GEOMETRY mono-1 trial, which included a cohort of previously treated and treatment-naive patients. The overall response rate was 68% in the treatment-naive patients and 41% in the previously treated patients. The median duration of response was 12.6 months and 9.7 months.
The most common side effects were peripheral edema, nausea, fatigue, vomiting, dyspnea, and decreased appetite.
Conditional approval for lymphoma
Mosunetuzumab is an investigational bispecific antibody targeting CD20 and CD3, and redirects T cells to engage and eliminate malignant B cells.
The CHMP recommended a conditional approval for this drug for use as monotherapy for the treatment of adult patients with relapsed or refractory follicular lymphoma who have received at least two prior systemic therapies.
Mosunetuzumab was reviewed under EMA’s accelerated access program, which usually takes 150 evaluation days as opposed to 210, and it was designated as an orphan medicine during its development.
The EMA stated that the benefits of this product are the high proportion of patients with a complete response and the durability of the treatment response.
As previously reported by this news organization, results from a phase 2 expansion study showed that when used as monotherapy, it induced high response rates and long-duration responses in patients with heavily pretreated, relapsed, or refractory follicular lymphoma.
At a median follow-up of 18.3 months, 54 of 90 patients (60%) had a complete response, and 18 (20%) had a partial response after treatment with mosunetuzumab.
The most common reported side effects were cytokine release syndrome, neutropenia, pyrexia (fever), hypophosphatemia, and headache.
Mosunetuzumab is awaiting FDA approval in the United States.
A conditional marketing authorization from CHMP is granted to products that meet an unmet medical need, and when the benefit to public health of immediate availability outweighs the risk inherent in the fact that additional data are still required. The marketing authorization holder is expected to provide comprehensive clinical data at a later stage.
Detailed recommendations for the use of both products will be described in the summary of product characteristics (SmPC), which will be published in the European public assessment report (EPAR) and made available in all official European Union languages after the marketing authorization has been granted by the European Commission.
A version of this article first appeared on Medscape.com.
The European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion for the two products at its April meeting.
New drug for certain lung cancer patients
Capmatinib is a selective, reversible inhibitor of MET tyrosine kinase and is indicated for the treatment of patients with advanced NSCLC harboring alterations leading to mesenchymal-epithelial transition factor gene exon 14 (METex14) skipping. Patients must have already been treated with immunotherapy and/or platinum-based chemotherapy.
The product is approved in the United States, and the Food and Drug Administration noted that it is the first approved treatment for NSCLC with MET exon 14-skipping mutations.
The FDA granted the drug an accelerated approval based on overall response rate and response duration in the GEOMETRY mono-1 trial, which included a cohort of previously treated and treatment-naive patients. The overall response rate was 68% in the treatment-naive patients and 41% in the previously treated patients. The median duration of response was 12.6 months and 9.7 months.
The most common side effects were peripheral edema, nausea, fatigue, vomiting, dyspnea, and decreased appetite.
Conditional approval for lymphoma
Mosunetuzumab is an investigational bispecific antibody targeting CD20 and CD3, and redirects T cells to engage and eliminate malignant B cells.
The CHMP recommended a conditional approval for this drug for use as monotherapy for the treatment of adult patients with relapsed or refractory follicular lymphoma who have received at least two prior systemic therapies.
Mosunetuzumab was reviewed under EMA’s accelerated access program, which usually takes 150 evaluation days as opposed to 210, and it was designated as an orphan medicine during its development.
The EMA stated that the benefits of this product are the high proportion of patients with a complete response and the durability of the treatment response.
As previously reported by this news organization, results from a phase 2 expansion study showed that when used as monotherapy, it induced high response rates and long-duration responses in patients with heavily pretreated, relapsed, or refractory follicular lymphoma.
At a median follow-up of 18.3 months, 54 of 90 patients (60%) had a complete response, and 18 (20%) had a partial response after treatment with mosunetuzumab.
The most common reported side effects were cytokine release syndrome, neutropenia, pyrexia (fever), hypophosphatemia, and headache.
Mosunetuzumab is awaiting FDA approval in the United States.
A conditional marketing authorization from CHMP is granted to products that meet an unmet medical need, and when the benefit to public health of immediate availability outweighs the risk inherent in the fact that additional data are still required. The marketing authorization holder is expected to provide comprehensive clinical data at a later stage.
Detailed recommendations for the use of both products will be described in the summary of product characteristics (SmPC), which will be published in the European public assessment report (EPAR) and made available in all official European Union languages after the marketing authorization has been granted by the European Commission.
A version of this article first appeared on Medscape.com.
How do we distinguish between viral and bacterial meningitis?
Bacteria and viruses are the leading causes of community-acquired meningitis. Bacterial meningitis is associated with high morbidity and mortality, and prompt treatment with appropriate antibiotics is essential to optimize outcomes. Early diagnosis is therefore crucial for selecting patients who need antibiotics. On the other hand, the course of viral meningitis is generally benign, and there is usually no specific antimicrobial treatment required. Distinguishing between viral and bacterial causes of meningitis can be challenging; therefore, many patients receive empiric antibiotic treatment.
Etiology

Among the etiologic agents of viral meningitis, the nonpolio enteroviruses (Echovirus 30, 11, 9, 6, 7, 18, 16, 71, 25; Coxsackie B2, A9, B1, B3, B4) are the most common, responsible for more than 85% of cases. Other viruses potentially responsible for meningitis include the herpes simplex virus (HSV), primarily type 2, and flavivirus (such as the Dengue virus).
Clinical presentation
The clinical presentation of bacterial meningitis is more severe than that of viral meningitis. The classic clinical triad of bacterial meningitis consists of fever, neck stiffness, and altered mental status. Only 41% of cases present with these three symptoms, however. Other clinical characteristics include severe headaches, decreased level of consciousness, nausea, vomiting, seizures, focal neurologic signs, and skin rash.
Viral meningitis is usually not associated with a decreased level of consciousness or significant decline in overall health status. The most frequently reported symptoms are unusual headaches, fever, nausea, vomiting, sensitivity to light, and neck stiffness. Patients may also present with skin changes and lymphadenopathy, and, depending on etiology, genital ulcers.
Diagnosis
The diagnosis of bacterial meningitis is based on clinical symptoms, blood panels (blood count, inflammation markers, cultures), and cerebrospinal fluid (CSF) cultures. Gram staining and latex agglutination may lead to false-negative results, and cultures may take a few days to provide a definitive result. Therefore, empiric antibiotic treatment is often started until the etiology can be determined.
A spinal tap must always be performed, preferably after a scan is taken, to rule out the risk of herniation. After CSF samples have been collected, they must undergo complete analysis, including cytological, biochemical, and microbiological evaluation, using conventional and molecular testing methods, when available.
Cytological and biochemical analyses of CSF may be helpful, as findings may indicate a higher probability of either bacterial or viral etiology.
CSF samples collected from patients with acute bacterial meningitis present characteristic neutrophilic pleocytosis (cell count usually ranging from hundreds to a few thousand, with >80% polymorphonuclear cells). In some cases of L. monocytogenes meningitis (from 25% to 30%), a lymphocytic predominance may occur. Normally, glucose is low (CSF glucose-to-blood-glucose ratio of ≤0.4 or <40 mg/dL), protein is very high (>200 mg/dL), and the CSF lactate level is high (≥31.53 mg/dL).
In viral meningitis, the white blood cell count is generally 10-300 cells/mm3. Although glucose levels are normal in most cases, they may be below normal limits in lymphocytic choriomeningitis virus (LCMV), HSV, mumps virus, and poliovirus meningitis. Protein levels tend to be slightly elevated, but they may still be within the reference range.
A recent study investigated which of the cytological or biochemical markers best correlate with the definite etiologic diagnosis. This study, in which CSF samples were collected and analyzed from 2013 to 2017, considered cases of bacterial or viral meningitis confirmed via microbiological evaluation or polymerase chain reaction (PCR). CSF lactate was the best single CSF parameter, and CSF lactate above 30 mg/dL virtually excludes the possibility of a viral etiology.
Etiologic determination
Despite the major contribution of globally analyzing CSF and secondary parameters, particularly CSF lactate, the precise etiologic definition is of great importance in cases of acute meningitis. Such precise definition is not simple, as identification of the causative microorganism is often difficult. Moreover, there are limits to conventional microbiological methods. Bacterioscopy is poorly sensitive, and although bacterial cultures are more sensitive, they can delay diagnosis because of the time it takes for the bacteria to grow in culture media.
Targeted molecular detection methods are usually more sensitive than conventional microbiological methods. Panel-based molecular tests identify multiple pathogens in a single test. In 2015, the U.S. Food and Drug Administration authorized the first commercial multiplex detection system for infectious causes of community-acquired meningitis and encephalitis. This test, the BioFire FilmArray system, detects 14 bacterial, viral, and fungal pathogens in a turnaround time of about 1 hour, including S. pneumoniae, N. meningitidis, H. influenzae, S. agalactiae (i.e., group B Streptococcus), E. coli (serotype K1), L. monocytogenes, HSV-1, HSV-2, varicella-zoster virus (VZV), cytomegalovirus (CMV), human herpesvirus 6 (HHV-6), human parechovirus (HPeV), and Cryptococcus neoformans/gattii.
A meta-analysis of eight precise diagnostic studies evaluating the BioFire FilmArray system showed a high sensitivity of 90% (95% confidence interval, 86%-93%) and specificity of 97% (95% CI, 94%-99%). The FilmArray ME panel can halve the time to microbiological result, allowing for earlier discontinuation of antimicrobial agents and hospital discharge in cases of viral meningitis.
Conclusion
Acute community-acquired meningitis is usually the result of viral or bacterial infections. Given the low specificity of clinical symptoms and, very often, of the general laboratory panel findings, many patients are empirically treated with antibiotics. High-sensitivity and -specificity molecular techniques allow for rapid identification of the bacterial etiology (which requires antibiotic therapy) or the viral etiology of meningitis. The latter can be managed only with symptom-specific medications and does not usually require extended hospitalization. Therefore, these new techniques can improve the quality of care for these patients with viral meningitis.
A version of this article first appeared on Medscape.com.
Bacteria and viruses are the leading causes of community-acquired meningitis. Bacterial meningitis is associated with high morbidity and mortality, and prompt treatment with appropriate antibiotics is essential to optimize outcomes. Early diagnosis is therefore crucial for selecting patients who need antibiotics. On the other hand, the course of viral meningitis is generally benign, and there is usually no specific antimicrobial treatment required. Distinguishing between viral and bacterial causes of meningitis can be challenging; therefore, many patients receive empiric antibiotic treatment.
Etiology

Among the etiologic agents of viral meningitis, the nonpolio enteroviruses (Echovirus 30, 11, 9, 6, 7, 18, 16, 71, 25; Coxsackie B2, A9, B1, B3, B4) are the most common, responsible for more than 85% of cases. Other viruses potentially responsible for meningitis include the herpes simplex virus (HSV), primarily type 2, and flavivirus (such as the Dengue virus).
Clinical presentation
The clinical presentation of bacterial meningitis is more severe than that of viral meningitis. The classic clinical triad of bacterial meningitis consists of fever, neck stiffness, and altered mental status. Only 41% of cases present with these three symptoms, however. Other clinical characteristics include severe headaches, decreased level of consciousness, nausea, vomiting, seizures, focal neurologic signs, and skin rash.
Viral meningitis is usually not associated with a decreased level of consciousness or significant decline in overall health status. The most frequently reported symptoms are unusual headaches, fever, nausea, vomiting, sensitivity to light, and neck stiffness. Patients may also present with skin changes and lymphadenopathy, and, depending on etiology, genital ulcers.
Diagnosis
The diagnosis of bacterial meningitis is based on clinical symptoms, blood panels (blood count, inflammation markers, cultures), and cerebrospinal fluid (CSF) cultures. Gram staining and latex agglutination may lead to false-negative results, and cultures may take a few days to provide a definitive result. Therefore, empiric antibiotic treatment is often started until the etiology can be determined.
A spinal tap must always be performed, preferably after a scan is taken, to rule out the risk of herniation. After CSF samples have been collected, they must undergo complete analysis, including cytological, biochemical, and microbiological evaluation, using conventional and molecular testing methods, when available.
Cytological and biochemical analyses of CSF may be helpful, as findings may indicate a higher probability of either bacterial or viral etiology.
CSF samples collected from patients with acute bacterial meningitis present characteristic neutrophilic pleocytosis (cell count usually ranging from hundreds to a few thousand, with >80% polymorphonuclear cells). In some cases of L. monocytogenes meningitis (from 25% to 30%), a lymphocytic predominance may occur. Normally, glucose is low (CSF glucose-to-blood-glucose ratio of ≤0.4 or <40 mg/dL), protein is very high (>200 mg/dL), and the CSF lactate level is high (≥31.53 mg/dL).
In viral meningitis, the white blood cell count is generally 10-300 cells/mm3. Although glucose levels are normal in most cases, they may be below normal limits in lymphocytic choriomeningitis virus (LCMV), HSV, mumps virus, and poliovirus meningitis. Protein levels tend to be slightly elevated, but they may still be within the reference range.
A recent study investigated which of the cytological or biochemical markers best correlate with the definite etiologic diagnosis. This study, in which CSF samples were collected and analyzed from 2013 to 2017, considered cases of bacterial or viral meningitis confirmed via microbiological evaluation or polymerase chain reaction (PCR). CSF lactate was the best single CSF parameter, and CSF lactate above 30 mg/dL virtually excludes the possibility of a viral etiology.
Etiologic determination
Despite the major contribution of globally analyzing CSF and secondary parameters, particularly CSF lactate, the precise etiologic definition is of great importance in cases of acute meningitis. Such precise definition is not simple, as identification of the causative microorganism is often difficult. Moreover, there are limits to conventional microbiological methods. Bacterioscopy is poorly sensitive, and although bacterial cultures are more sensitive, they can delay diagnosis because of the time it takes for the bacteria to grow in culture media.
Targeted molecular detection methods are usually more sensitive than conventional microbiological methods. Panel-based molecular tests identify multiple pathogens in a single test. In 2015, the U.S. Food and Drug Administration authorized the first commercial multiplex detection system for infectious causes of community-acquired meningitis and encephalitis. This test, the BioFire FilmArray system, detects 14 bacterial, viral, and fungal pathogens in a turnaround time of about 1 hour, including S. pneumoniae, N. meningitidis, H. influenzae, S. agalactiae (i.e., group B Streptococcus), E. coli (serotype K1), L. monocytogenes, HSV-1, HSV-2, varicella-zoster virus (VZV), cytomegalovirus (CMV), human herpesvirus 6 (HHV-6), human parechovirus (HPeV), and Cryptococcus neoformans/gattii.
A meta-analysis of eight precise diagnostic studies evaluating the BioFire FilmArray system showed a high sensitivity of 90% (95% confidence interval, 86%-93%) and specificity of 97% (95% CI, 94%-99%). The FilmArray ME panel can halve the time to microbiological result, allowing for earlier discontinuation of antimicrobial agents and hospital discharge in cases of viral meningitis.
Conclusion
Acute community-acquired meningitis is usually the result of viral or bacterial infections. Given the low specificity of clinical symptoms and, very often, of the general laboratory panel findings, many patients are empirically treated with antibiotics. High-sensitivity and -specificity molecular techniques allow for rapid identification of the bacterial etiology (which requires antibiotic therapy) or the viral etiology of meningitis. The latter can be managed only with symptom-specific medications and does not usually require extended hospitalization. Therefore, these new techniques can improve the quality of care for these patients with viral meningitis.
A version of this article first appeared on Medscape.com.
Bacteria and viruses are the leading causes of community-acquired meningitis. Bacterial meningitis is associated with high morbidity and mortality, and prompt treatment with appropriate antibiotics is essential to optimize outcomes. Early diagnosis is therefore crucial for selecting patients who need antibiotics. On the other hand, the course of viral meningitis is generally benign, and there is usually no specific antimicrobial treatment required. Distinguishing between viral and bacterial causes of meningitis can be challenging; therefore, many patients receive empiric antibiotic treatment.
Etiology

Among the etiologic agents of viral meningitis, the nonpolio enteroviruses (Echovirus 30, 11, 9, 6, 7, 18, 16, 71, 25; Coxsackie B2, A9, B1, B3, B4) are the most common, responsible for more than 85% of cases. Other viruses potentially responsible for meningitis include the herpes simplex virus (HSV), primarily type 2, and flavivirus (such as the Dengue virus).
Clinical presentation
The clinical presentation of bacterial meningitis is more severe than that of viral meningitis. The classic clinical triad of bacterial meningitis consists of fever, neck stiffness, and altered mental status. Only 41% of cases present with these three symptoms, however. Other clinical characteristics include severe headaches, decreased level of consciousness, nausea, vomiting, seizures, focal neurologic signs, and skin rash.
Viral meningitis is usually not associated with a decreased level of consciousness or significant decline in overall health status. The most frequently reported symptoms are unusual headaches, fever, nausea, vomiting, sensitivity to light, and neck stiffness. Patients may also present with skin changes and lymphadenopathy, and, depending on etiology, genital ulcers.
Diagnosis
The diagnosis of bacterial meningitis is based on clinical symptoms, blood panels (blood count, inflammation markers, cultures), and cerebrospinal fluid (CSF) cultures. Gram staining and latex agglutination may lead to false-negative results, and cultures may take a few days to provide a definitive result. Therefore, empiric antibiotic treatment is often started until the etiology can be determined.
A spinal tap must always be performed, preferably after a scan is taken, to rule out the risk of herniation. After CSF samples have been collected, they must undergo complete analysis, including cytological, biochemical, and microbiological evaluation, using conventional and molecular testing methods, when available.
Cytological and biochemical analyses of CSF may be helpful, as findings may indicate a higher probability of either bacterial or viral etiology.
CSF samples collected from patients with acute bacterial meningitis present characteristic neutrophilic pleocytosis (cell count usually ranging from hundreds to a few thousand, with >80% polymorphonuclear cells). In some cases of L. monocytogenes meningitis (from 25% to 30%), a lymphocytic predominance may occur. Normally, glucose is low (CSF glucose-to-blood-glucose ratio of ≤0.4 or <40 mg/dL), protein is very high (>200 mg/dL), and the CSF lactate level is high (≥31.53 mg/dL).
In viral meningitis, the white blood cell count is generally 10-300 cells/mm3. Although glucose levels are normal in most cases, they may be below normal limits in lymphocytic choriomeningitis virus (LCMV), HSV, mumps virus, and poliovirus meningitis. Protein levels tend to be slightly elevated, but they may still be within the reference range.
A recent study investigated which of the cytological or biochemical markers best correlate with the definite etiologic diagnosis. This study, in which CSF samples were collected and analyzed from 2013 to 2017, considered cases of bacterial or viral meningitis confirmed via microbiological evaluation or polymerase chain reaction (PCR). CSF lactate was the best single CSF parameter, and CSF lactate above 30 mg/dL virtually excludes the possibility of a viral etiology.
Etiologic determination
Despite the major contribution of globally analyzing CSF and secondary parameters, particularly CSF lactate, the precise etiologic definition is of great importance in cases of acute meningitis. Such precise definition is not simple, as identification of the causative microorganism is often difficult. Moreover, there are limits to conventional microbiological methods. Bacterioscopy is poorly sensitive, and although bacterial cultures are more sensitive, they can delay diagnosis because of the time it takes for the bacteria to grow in culture media.
Targeted molecular detection methods are usually more sensitive than conventional microbiological methods. Panel-based molecular tests identify multiple pathogens in a single test. In 2015, the U.S. Food and Drug Administration authorized the first commercial multiplex detection system for infectious causes of community-acquired meningitis and encephalitis. This test, the BioFire FilmArray system, detects 14 bacterial, viral, and fungal pathogens in a turnaround time of about 1 hour, including S. pneumoniae, N. meningitidis, H. influenzae, S. agalactiae (i.e., group B Streptococcus), E. coli (serotype K1), L. monocytogenes, HSV-1, HSV-2, varicella-zoster virus (VZV), cytomegalovirus (CMV), human herpesvirus 6 (HHV-6), human parechovirus (HPeV), and Cryptococcus neoformans/gattii.
A meta-analysis of eight precise diagnostic studies evaluating the BioFire FilmArray system showed a high sensitivity of 90% (95% confidence interval, 86%-93%) and specificity of 97% (95% CI, 94%-99%). The FilmArray ME panel can halve the time to microbiological result, allowing for earlier discontinuation of antimicrobial agents and hospital discharge in cases of viral meningitis.
Conclusion
Acute community-acquired meningitis is usually the result of viral or bacterial infections. Given the low specificity of clinical symptoms and, very often, of the general laboratory panel findings, many patients are empirically treated with antibiotics. High-sensitivity and -specificity molecular techniques allow for rapid identification of the bacterial etiology (which requires antibiotic therapy) or the viral etiology of meningitis. The latter can be managed only with symptom-specific medications and does not usually require extended hospitalization. Therefore, these new techniques can improve the quality of care for these patients with viral meningitis.
A version of this article first appeared on Medscape.com.