User login
Bringing HCC Patients Hope Through Trials, Advanced Treatments
For Reena Salgia, MD, the most rewarding part about working with patients with hepatocellular carcinoma is being there for their entire journey, thanks to advancements in treatment. “It brings a smile to my face just to think about it,” says Dr. Salgia, medical director of Henry Ford Health’s Liver Cancer Clinic in Detroit.
Hepatocellular carcinoma accounts for 80% of all liver cancer. When she first entered the field, Dr. Salgia often heard that survival rates 5 years after diagnosis were less than 10%. Over the last decade however, “I’ve seen an expansion in the procedural options that we offer these patients. We have an array of options both surgically as well as procedurally,” she said.
Especially over the last three to four years, “we’ve seen meaningful responses for patients with medications that we previously didn’t have in our toolbox. That’s really been exciting, along with continued involvement in clinical trials and being able to offer patients a number of different approaches to their care of liver cancer,” said Dr. Salgia.
A regular attendee and presenter at national GI meetings, Dr. Salgia participated in AGA’s Women’s Executive Leadership Conference in 2023. Her academic resume includes a long list of clinical trials to assess treatments for patients at different stages of hepatocellular carcinoma.
In an interview, she discussed the highlights of her career as a researcher and mentor of fellows, and how she guides and supports her transplant patients.
What drove you to pursue the field of hepatology and transplant hepatology?
I came across this field during my fourth year of medical school. I didn’t know anything about hepatology when I reached that stage and had the opportunity to do an elective. I just fell in love with the specialty. I liked the complex pathophysiology of liver disease, the long-term follow-up and care of patients. It appealed to the type of science that I had enjoyed back in college.
As I went into my GI fellowship training, I got to learn more about the field of transplant medicine. For instance, how you can take these patients who are incredibly ill, really at a very vulnerable point of their illness, and then offer them great hope and see their lives turn around afterwards. When I had the opportunity to see patients go from end stage liver disease to such significant improvement in their quality of life, and restoring their physical functioning beyond what we would’ve ever imagined when they were ill, it reaffirmed my interest in both hepatology as well as in transplant medicine.
How do you help those patients waiting on transplant lists for a liver?
We are intimately involved in their care all the way through their journey with liver disease, up until the time of physically getting the liver transplant, which is performed by our colleagues in transplant surgery. From the time they are transplanted, we are involved in their inpatient and outpatient post-transplant care. We’ve helped to get them on the transplant list with the work of the multidisciplinary team. If there are opportunities to help them understand their position on the list or obtaining exceptions—though that is done in a very objective fashion through the regulatory system—we help to guide them through that journey.
You’ve worked on many studies that involve treatments for hepatocellular carcinoma. Can you highlight a paper that yielded clinically significant benefits?
What really stands out the most to me was our site’s involvement in the IMbrave150 trial, which was published in 2020. This multicenter study made a big difference in the outcomes and treatments for patients, as it brought the adoption of first-line immunotherapy (atezolizumab plus bevacizumab) for patients with advanced hepatocellular carcinoma. I remember vividly the patients we had the opportunity to enroll in that trial – some who we continue to care for today. This stands out as one of the trials that I was involved in that had a lasting impact.
What were the clinical endpoints and key results of that trial?
The endpoint was to see an improvement in overall survival utilizing immunotherapy, compared with the prior standard of care then available, oral therapy. The results led to the adoption and FDA approval of immunotherapy in the first line setting for advanced unresectable hepatocellular carcinoma patients.
What are some of the highlights of serving as director of Henry Ford’s fellowship program?
Education is my passion. I went into medical training feeling that at some point I would love to blend in teaching in a formal role. Becoming program director of the gastroenterology and hepatology fellowship at Henry Ford in 2018 was one of the most meaningful things that I’ve had the opportunity to do in my career. I get to see trainees who are at a very impressionable point of their journey go on to become gastroenterologists and then launch into their first job and really develop in this field. Seeing them come in day one, not knowing how to hold a scope or do a procedure on a patient of this nature, then quickly evolve over the first year and grow over three years to achieve this specialty training [is rewarding]. I’ve learned a lot from the fellows along the way. I think of them as an extension of my family. We have 15 fellows currently in our program and we’ll be growing this summer. So that’s really been a highlight of my career thus far.
What fears did you have to push past to get to where you are in your career?
I think that there have been a few. One is certainly the fear of making the wrong choice with your first career opportunity. I did choose to leave my comfort zone from where I had done my training. I met that with some fear, but also excitement for new opportunities of personal and professional growth.
Another fear is: Am I going to be able to be ambitious in this field? Can I pursue research, become a program director, and do things that my role models and mentors were able to achieve? There’s also the fear of being able to balance a busy work life with a busy home life and figuring out how to do both well and minimize the guilt on both sides. I have a family with two girls. They are definitely a top priority.
What teacher or mentor had the greatest impact on you?
Helen Te, MD, a hepatologist at the University of Chicago. When I was a medical student there, I had the opportunity to work with her and saw her passion for this field. She really had so much enthusiasm for teaching and was a big part of why I started to fall in love with liver disease.
Karen Kim, MD, now the dean of Penn State College of Medicine, was one of my assigned mentors as a medical student. She helped me explore the fields where there were opportunities for residency and helped me make the decision to go into internal medicine, which often is a key deciding point for medical students. She was also a very influential teacher. The other individual who stands out is my fellowship program director, Hari Sree Conjeevaram, MD, MSc, at University of Michigan Health. He exhibited the qualities as an educator and program director that helped me recognize that education was something that I wanted to pursue in a formal fashion once I moved on in my career.
Describe how you would spend a free Saturday afternoon.
Likely taking a hike or go to a park with my family, enjoying the outdoors and spending time with them.
Lightning Round
If you weren’t a gastroenterologist, what would you be?
Philanthropist
Favorite city in U.S. besides the one you live in?
Chicago
Place you most want to travel?
New Zealand
Favorite breakfast?
Avocado toast
Favorite ice cream flavor?
Cookies and cream
How many cups of coffee do you drink per day?
Two…or more
Cat person or dog person?
Dog
Texting or talking?
Talk
Favorite season?
Autumn
Favorite type of music?
Pop
Favorite movie genre?
Action
For Reena Salgia, MD, the most rewarding part about working with patients with hepatocellular carcinoma is being there for their entire journey, thanks to advancements in treatment. “It brings a smile to my face just to think about it,” says Dr. Salgia, medical director of Henry Ford Health’s Liver Cancer Clinic in Detroit.
Hepatocellular carcinoma accounts for 80% of all liver cancer. When she first entered the field, Dr. Salgia often heard that survival rates 5 years after diagnosis were less than 10%. Over the last decade however, “I’ve seen an expansion in the procedural options that we offer these patients. We have an array of options both surgically as well as procedurally,” she said.
Especially over the last three to four years, “we’ve seen meaningful responses for patients with medications that we previously didn’t have in our toolbox. That’s really been exciting, along with continued involvement in clinical trials and being able to offer patients a number of different approaches to their care of liver cancer,” said Dr. Salgia.
A regular attendee and presenter at national GI meetings, Dr. Salgia participated in AGA’s Women’s Executive Leadership Conference in 2023. Her academic resume includes a long list of clinical trials to assess treatments for patients at different stages of hepatocellular carcinoma.
In an interview, she discussed the highlights of her career as a researcher and mentor of fellows, and how she guides and supports her transplant patients.
What drove you to pursue the field of hepatology and transplant hepatology?
I came across this field during my fourth year of medical school. I didn’t know anything about hepatology when I reached that stage and had the opportunity to do an elective. I just fell in love with the specialty. I liked the complex pathophysiology of liver disease, the long-term follow-up and care of patients. It appealed to the type of science that I had enjoyed back in college.
As I went into my GI fellowship training, I got to learn more about the field of transplant medicine. For instance, how you can take these patients who are incredibly ill, really at a very vulnerable point of their illness, and then offer them great hope and see their lives turn around afterwards. When I had the opportunity to see patients go from end stage liver disease to such significant improvement in their quality of life, and restoring their physical functioning beyond what we would’ve ever imagined when they were ill, it reaffirmed my interest in both hepatology as well as in transplant medicine.
How do you help those patients waiting on transplant lists for a liver?
We are intimately involved in their care all the way through their journey with liver disease, up until the time of physically getting the liver transplant, which is performed by our colleagues in transplant surgery. From the time they are transplanted, we are involved in their inpatient and outpatient post-transplant care. We’ve helped to get them on the transplant list with the work of the multidisciplinary team. If there are opportunities to help them understand their position on the list or obtaining exceptions—though that is done in a very objective fashion through the regulatory system—we help to guide them through that journey.
You’ve worked on many studies that involve treatments for hepatocellular carcinoma. Can you highlight a paper that yielded clinically significant benefits?
What really stands out the most to me was our site’s involvement in the IMbrave150 trial, which was published in 2020. This multicenter study made a big difference in the outcomes and treatments for patients, as it brought the adoption of first-line immunotherapy (atezolizumab plus bevacizumab) for patients with advanced hepatocellular carcinoma. I remember vividly the patients we had the opportunity to enroll in that trial – some who we continue to care for today. This stands out as one of the trials that I was involved in that had a lasting impact.
What were the clinical endpoints and key results of that trial?
The endpoint was to see an improvement in overall survival utilizing immunotherapy, compared with the prior standard of care then available, oral therapy. The results led to the adoption and FDA approval of immunotherapy in the first line setting for advanced unresectable hepatocellular carcinoma patients.
What are some of the highlights of serving as director of Henry Ford’s fellowship program?
Education is my passion. I went into medical training feeling that at some point I would love to blend in teaching in a formal role. Becoming program director of the gastroenterology and hepatology fellowship at Henry Ford in 2018 was one of the most meaningful things that I’ve had the opportunity to do in my career. I get to see trainees who are at a very impressionable point of their journey go on to become gastroenterologists and then launch into their first job and really develop in this field. Seeing them come in day one, not knowing how to hold a scope or do a procedure on a patient of this nature, then quickly evolve over the first year and grow over three years to achieve this specialty training [is rewarding]. I’ve learned a lot from the fellows along the way. I think of them as an extension of my family. We have 15 fellows currently in our program and we’ll be growing this summer. So that’s really been a highlight of my career thus far.
What fears did you have to push past to get to where you are in your career?
I think that there have been a few. One is certainly the fear of making the wrong choice with your first career opportunity. I did choose to leave my comfort zone from where I had done my training. I met that with some fear, but also excitement for new opportunities of personal and professional growth.
Another fear is: Am I going to be able to be ambitious in this field? Can I pursue research, become a program director, and do things that my role models and mentors were able to achieve? There’s also the fear of being able to balance a busy work life with a busy home life and figuring out how to do both well and minimize the guilt on both sides. I have a family with two girls. They are definitely a top priority.
What teacher or mentor had the greatest impact on you?
Helen Te, MD, a hepatologist at the University of Chicago. When I was a medical student there, I had the opportunity to work with her and saw her passion for this field. She really had so much enthusiasm for teaching and was a big part of why I started to fall in love with liver disease.
Karen Kim, MD, now the dean of Penn State College of Medicine, was one of my assigned mentors as a medical student. She helped me explore the fields where there were opportunities for residency and helped me make the decision to go into internal medicine, which often is a key deciding point for medical students. She was also a very influential teacher. The other individual who stands out is my fellowship program director, Hari Sree Conjeevaram, MD, MSc, at University of Michigan Health. He exhibited the qualities as an educator and program director that helped me recognize that education was something that I wanted to pursue in a formal fashion once I moved on in my career.
Describe how you would spend a free Saturday afternoon.
Likely taking a hike or go to a park with my family, enjoying the outdoors and spending time with them.
Lightning Round
If you weren’t a gastroenterologist, what would you be?
Philanthropist
Favorite city in U.S. besides the one you live in?
Chicago
Place you most want to travel?
New Zealand
Favorite breakfast?
Avocado toast
Favorite ice cream flavor?
Cookies and cream
How many cups of coffee do you drink per day?
Two…or more
Cat person or dog person?
Dog
Texting or talking?
Talk
Favorite season?
Autumn
Favorite type of music?
Pop
Favorite movie genre?
Action
For Reena Salgia, MD, the most rewarding part about working with patients with hepatocellular carcinoma is being there for their entire journey, thanks to advancements in treatment. “It brings a smile to my face just to think about it,” says Dr. Salgia, medical director of Henry Ford Health’s Liver Cancer Clinic in Detroit.
Hepatocellular carcinoma accounts for 80% of all liver cancer. When she first entered the field, Dr. Salgia often heard that survival rates 5 years after diagnosis were less than 10%. Over the last decade however, “I’ve seen an expansion in the procedural options that we offer these patients. We have an array of options both surgically as well as procedurally,” she said.
Especially over the last three to four years, “we’ve seen meaningful responses for patients with medications that we previously didn’t have in our toolbox. That’s really been exciting, along with continued involvement in clinical trials and being able to offer patients a number of different approaches to their care of liver cancer,” said Dr. Salgia.
A regular attendee and presenter at national GI meetings, Dr. Salgia participated in AGA’s Women’s Executive Leadership Conference in 2023. Her academic resume includes a long list of clinical trials to assess treatments for patients at different stages of hepatocellular carcinoma.
In an interview, she discussed the highlights of her career as a researcher and mentor of fellows, and how she guides and supports her transplant patients.
What drove you to pursue the field of hepatology and transplant hepatology?
I came across this field during my fourth year of medical school. I didn’t know anything about hepatology when I reached that stage and had the opportunity to do an elective. I just fell in love with the specialty. I liked the complex pathophysiology of liver disease, the long-term follow-up and care of patients. It appealed to the type of science that I had enjoyed back in college.
As I went into my GI fellowship training, I got to learn more about the field of transplant medicine. For instance, how you can take these patients who are incredibly ill, really at a very vulnerable point of their illness, and then offer them great hope and see their lives turn around afterwards. When I had the opportunity to see patients go from end stage liver disease to such significant improvement in their quality of life, and restoring their physical functioning beyond what we would’ve ever imagined when they were ill, it reaffirmed my interest in both hepatology as well as in transplant medicine.
How do you help those patients waiting on transplant lists for a liver?
We are intimately involved in their care all the way through their journey with liver disease, up until the time of physically getting the liver transplant, which is performed by our colleagues in transplant surgery. From the time they are transplanted, we are involved in their inpatient and outpatient post-transplant care. We’ve helped to get them on the transplant list with the work of the multidisciplinary team. If there are opportunities to help them understand their position on the list or obtaining exceptions—though that is done in a very objective fashion through the regulatory system—we help to guide them through that journey.
You’ve worked on many studies that involve treatments for hepatocellular carcinoma. Can you highlight a paper that yielded clinically significant benefits?
What really stands out the most to me was our site’s involvement in the IMbrave150 trial, which was published in 2020. This multicenter study made a big difference in the outcomes and treatments for patients, as it brought the adoption of first-line immunotherapy (atezolizumab plus bevacizumab) for patients with advanced hepatocellular carcinoma. I remember vividly the patients we had the opportunity to enroll in that trial – some who we continue to care for today. This stands out as one of the trials that I was involved in that had a lasting impact.
What were the clinical endpoints and key results of that trial?
The endpoint was to see an improvement in overall survival utilizing immunotherapy, compared with the prior standard of care then available, oral therapy. The results led to the adoption and FDA approval of immunotherapy in the first line setting for advanced unresectable hepatocellular carcinoma patients.
What are some of the highlights of serving as director of Henry Ford’s fellowship program?
Education is my passion. I went into medical training feeling that at some point I would love to blend in teaching in a formal role. Becoming program director of the gastroenterology and hepatology fellowship at Henry Ford in 2018 was one of the most meaningful things that I’ve had the opportunity to do in my career. I get to see trainees who are at a very impressionable point of their journey go on to become gastroenterologists and then launch into their first job and really develop in this field. Seeing them come in day one, not knowing how to hold a scope or do a procedure on a patient of this nature, then quickly evolve over the first year and grow over three years to achieve this specialty training [is rewarding]. I’ve learned a lot from the fellows along the way. I think of them as an extension of my family. We have 15 fellows currently in our program and we’ll be growing this summer. So that’s really been a highlight of my career thus far.
What fears did you have to push past to get to where you are in your career?
I think that there have been a few. One is certainly the fear of making the wrong choice with your first career opportunity. I did choose to leave my comfort zone from where I had done my training. I met that with some fear, but also excitement for new opportunities of personal and professional growth.
Another fear is: Am I going to be able to be ambitious in this field? Can I pursue research, become a program director, and do things that my role models and mentors were able to achieve? There’s also the fear of being able to balance a busy work life with a busy home life and figuring out how to do both well and minimize the guilt on both sides. I have a family with two girls. They are definitely a top priority.
What teacher or mentor had the greatest impact on you?
Helen Te, MD, a hepatologist at the University of Chicago. When I was a medical student there, I had the opportunity to work with her and saw her passion for this field. She really had so much enthusiasm for teaching and was a big part of why I started to fall in love with liver disease.
Karen Kim, MD, now the dean of Penn State College of Medicine, was one of my assigned mentors as a medical student. She helped me explore the fields where there were opportunities for residency and helped me make the decision to go into internal medicine, which often is a key deciding point for medical students. She was also a very influential teacher. The other individual who stands out is my fellowship program director, Hari Sree Conjeevaram, MD, MSc, at University of Michigan Health. He exhibited the qualities as an educator and program director that helped me recognize that education was something that I wanted to pursue in a formal fashion once I moved on in my career.
Describe how you would spend a free Saturday afternoon.
Likely taking a hike or go to a park with my family, enjoying the outdoors and spending time with them.
Lightning Round
If you weren’t a gastroenterologist, what would you be?
Philanthropist
Favorite city in U.S. besides the one you live in?
Chicago
Place you most want to travel?
New Zealand
Favorite breakfast?
Avocado toast
Favorite ice cream flavor?
Cookies and cream
How many cups of coffee do you drink per day?
Two…or more
Cat person or dog person?
Dog
Texting or talking?
Talk
Favorite season?
Autumn
Favorite type of music?
Pop
Favorite movie genre?
Action

Cancer Data Trends 2025
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
In this issue:
- Access, Race, and "Colon Age": Improving CRC Screening
- Lung Cancer: Mortality Trends in Veterans and New Treatments
- Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
- Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
- HCC Updates: Quality Care Framework and Risk Stratification Data
- Rising Kidney Cancer Cases and Emerging Treatments for Veterans
- Advances in Blood Cancer Care for Veterans
- AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
- Brain Cancer: Epidemiology, TBI, and New Treatments
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
In this issue:
- Access, Race, and "Colon Age": Improving CRC Screening
- Lung Cancer: Mortality Trends in Veterans and New Treatments
- Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
- Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
- HCC Updates: Quality Care Framework and Risk Stratification Data
- Rising Kidney Cancer Cases and Emerging Treatments for Veterans
- Advances in Blood Cancer Care for Veterans
- AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
- Brain Cancer: Epidemiology, TBI, and New Treatments
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
In this issue:
- Access, Race, and "Colon Age": Improving CRC Screening
- Lung Cancer: Mortality Trends in Veterans and New Treatments
- Racial Disparities, Germline Testing, and Improved Overall Survival in Prostate Cancer
- Breast and Uterine Cancer: Screening Guidelines, Genetic Testing, and Mortality Trends
- HCC Updates: Quality Care Framework and Risk Stratification Data
- Rising Kidney Cancer Cases and Emerging Treatments for Veterans
- Advances in Blood Cancer Care for Veterans
- AI-Based Risk Stratification for Oropharyngeal Carcinomas: AIROC
- Brain Cancer: Epidemiology, TBI, and New Treatments
Prevention and Risk-Based Surveillance Key to Curbing HCC
BERLIN — according to a joint statement from United European Gastroenterology (UEG) and the German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS).
The statement calls on EU and national policymakers to embed a twofold approach into healthcare systems that combines surveillance and prevention, rather than relying on voluntary participation. It also encourages stronger prevention measures, such as improved food labeling and restrictions on marketing unhealthy foods to children. The statement — which was also endorsed by the European Association for the Study of the Liver (EASL) — was presented at UEG Week 2025 .
“Curing HCC in early stages rather than treating the disease in a palliative setting should be the goal for all liver doctors and carers, and this is certainly the goal for patients,” said Thomas Seufferlein, MD, professor of gastroenterology at Ulm University, Germany, and one of the members of the DGVS who initiated the statement.
“We have to take HCC screening seriously which means setting up a structured, nationwide, well-documented, and evaluated program for HCC screening in Germany,” he said in an interview.
HCC is mainly curable in the early stages by local ablation, resection, or liver transplantation, “so early diagnosis is of the utmost importance for improving survival,” added Patrick Michl, MD, gastroenterologist, University of Heidelberg, Germany, DGVS member and co-initiator of the statement.
Risk-Stratified HCC Surveillance
In the face of rising rates worldwide, the UEG/DGVS call on policymakers to recognize liver cancer as a preventable and growing public health priority and to implement structured surveillance programs guided by risk thresholds. In particular, they support the recent policy statement from EASL recommending risk-based screening.
EASL’s key recommendations include:
- Targeted surveillance for individuals with an annual HCC risk exceeding 1.5%, where it is both clinically beneficial and cost-effective
- Risk scoring tools such as the age-male-albumin-bilirubin-platelets score that incorporates age, sex, platelet count, albumin, and bilirubin, to stratify patients by HCC risk, including those without established cirrhosis
- Enhanced surveillance for very high-risk groups, where MRI-based surveillance may be warranted despite higher costs, given its superior sensitivity for early-stage disease
- A de-escalation in low-risk individuals
- Patients with an annual HCC risk < 0.5% may be safely spared surveillance, avoiding unnecessary interventions
Evidence from France, Italy, and the UK showed that structured surveillance in high-risk groups is both clinically beneficial and cost-effective. National models in France have demonstrated higher curative treatment rates and fewer costly late-stage cases with structured surveillance. In the UK, health technology assessments indicate targeted surveillance is an efficient use of National Health Services resources, particularly when uptake is optimized. Italian models show that earlier diagnosis in well-defined high-risk groups can offset downstream treatment costs.
Seufferlein noted that Germany needs a “structured program to be implemented and there is currently little public awareness regarding this surveillance strategy.” However, he added there is a structured hepatitis B vaccination program in Germany, which has been successful. “Studies show that the inclusion of hep B vaccination in infancy and childhood has led to good uptake among young age groups.”
Germany, however, has yet to conduct national studies. “Prospective data on HCC surveillance benefits in Germany are lacking,” said Michl, “but multi-country models incorporating Germany’s cost structures suggest similar benefits would accrue if there were greater adherence to guideline-based recommendations and if publicly funded screening programs were implemented.”
Current recommendations in Germany for surveillance are based on evidence-based guidelines of the DGVS with stronger (‘should’) or weaker (‘may’) evidence-based recommendations. For example, patients with chronic hepatitis B virus infection should be offered regular surveillance once their platelet age gender–hepatitis B risk score is ≥ 10. In patients with advanced fibrosis because of chronic hepatitis C virus infection, regular surveillance should also be offered.
Barriers to Screening Uptake
HCC remains one of the most lethal cancers in Europe, largely because it is often diagnosed too late. Underdiagnosis of chronic liver disease, limited access to imaging, and reimbursement gaps prevent timely intervention.
Maria Buti, MD, consultant hepatologist, Hospital Vall d’Hebron, Barcelona, Spain, who was not involved in drafting the statement, remarked that “Patients with liver cirrhosis, or with advanced fibrosis, and also some high-risk noncirrhotic patients such as those with hepatitis B, clearly benefit from surveillance. Surveillance can change life expectancy and also reduce morbidity.”
However, structural barriers continue to impede uptake. “It is not always easy to identify patients with liver cirrhosis because the majority are completely asymptomatic in the early stages,” she said.
Even when risk factors are identified, adherence to 6-monthly surveillance remains patchy. “Sometimes physicians forget to request ultrasounds, or patients don’t understand the importance of it because they feel well,” Buti told GI & Hepatology News.
Expanded Training and Public Health Measures
The joint statement also advocates for expanded physician training in nutrition and hepatology, equitable access to diagnostic tools including MRI, and EU-wide nutrition labeling systems such as Nutri-Score.
The authors also called for strengthened public health measures to tackle obesity, alcohol misuse, and hepatitis transmission, and fiscal and regulatory measures such as taxation of obesogenic foods, and reducing the cost burden of healthier foods.
“If we decrease the percentage of people with liver cirrhosis through prevention, fewer people will need surveillance,” Buti stated.
Seufferlein, Michl, and Buti all declared no relevant disclosures. All three experts are members of the UEG Public Affairs Group.
A version of this article appeared on Medscape.com.
BERLIN — according to a joint statement from United European Gastroenterology (UEG) and the German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS).
The statement calls on EU and national policymakers to embed a twofold approach into healthcare systems that combines surveillance and prevention, rather than relying on voluntary participation. It also encourages stronger prevention measures, such as improved food labeling and restrictions on marketing unhealthy foods to children. The statement — which was also endorsed by the European Association for the Study of the Liver (EASL) — was presented at UEG Week 2025 .
“Curing HCC in early stages rather than treating the disease in a palliative setting should be the goal for all liver doctors and carers, and this is certainly the goal for patients,” said Thomas Seufferlein, MD, professor of gastroenterology at Ulm University, Germany, and one of the members of the DGVS who initiated the statement.
“We have to take HCC screening seriously which means setting up a structured, nationwide, well-documented, and evaluated program for HCC screening in Germany,” he said in an interview.
HCC is mainly curable in the early stages by local ablation, resection, or liver transplantation, “so early diagnosis is of the utmost importance for improving survival,” added Patrick Michl, MD, gastroenterologist, University of Heidelberg, Germany, DGVS member and co-initiator of the statement.
Risk-Stratified HCC Surveillance
In the face of rising rates worldwide, the UEG/DGVS call on policymakers to recognize liver cancer as a preventable and growing public health priority and to implement structured surveillance programs guided by risk thresholds. In particular, they support the recent policy statement from EASL recommending risk-based screening.
EASL’s key recommendations include:
- Targeted surveillance for individuals with an annual HCC risk exceeding 1.5%, where it is both clinically beneficial and cost-effective
- Risk scoring tools such as the age-male-albumin-bilirubin-platelets score that incorporates age, sex, platelet count, albumin, and bilirubin, to stratify patients by HCC risk, including those without established cirrhosis
- Enhanced surveillance for very high-risk groups, where MRI-based surveillance may be warranted despite higher costs, given its superior sensitivity for early-stage disease
- A de-escalation in low-risk individuals
- Patients with an annual HCC risk < 0.5% may be safely spared surveillance, avoiding unnecessary interventions
Evidence from France, Italy, and the UK showed that structured surveillance in high-risk groups is both clinically beneficial and cost-effective. National models in France have demonstrated higher curative treatment rates and fewer costly late-stage cases with structured surveillance. In the UK, health technology assessments indicate targeted surveillance is an efficient use of National Health Services resources, particularly when uptake is optimized. Italian models show that earlier diagnosis in well-defined high-risk groups can offset downstream treatment costs.
Seufferlein noted that Germany needs a “structured program to be implemented and there is currently little public awareness regarding this surveillance strategy.” However, he added there is a structured hepatitis B vaccination program in Germany, which has been successful. “Studies show that the inclusion of hep B vaccination in infancy and childhood has led to good uptake among young age groups.”
Germany, however, has yet to conduct national studies. “Prospective data on HCC surveillance benefits in Germany are lacking,” said Michl, “but multi-country models incorporating Germany’s cost structures suggest similar benefits would accrue if there were greater adherence to guideline-based recommendations and if publicly funded screening programs were implemented.”
Current recommendations in Germany for surveillance are based on evidence-based guidelines of the DGVS with stronger (‘should’) or weaker (‘may’) evidence-based recommendations. For example, patients with chronic hepatitis B virus infection should be offered regular surveillance once their platelet age gender–hepatitis B risk score is ≥ 10. In patients with advanced fibrosis because of chronic hepatitis C virus infection, regular surveillance should also be offered.
Barriers to Screening Uptake
HCC remains one of the most lethal cancers in Europe, largely because it is often diagnosed too late. Underdiagnosis of chronic liver disease, limited access to imaging, and reimbursement gaps prevent timely intervention.
Maria Buti, MD, consultant hepatologist, Hospital Vall d’Hebron, Barcelona, Spain, who was not involved in drafting the statement, remarked that “Patients with liver cirrhosis, or with advanced fibrosis, and also some high-risk noncirrhotic patients such as those with hepatitis B, clearly benefit from surveillance. Surveillance can change life expectancy and also reduce morbidity.”
However, structural barriers continue to impede uptake. “It is not always easy to identify patients with liver cirrhosis because the majority are completely asymptomatic in the early stages,” she said.
Even when risk factors are identified, adherence to 6-monthly surveillance remains patchy. “Sometimes physicians forget to request ultrasounds, or patients don’t understand the importance of it because they feel well,” Buti told GI & Hepatology News.
Expanded Training and Public Health Measures
The joint statement also advocates for expanded physician training in nutrition and hepatology, equitable access to diagnostic tools including MRI, and EU-wide nutrition labeling systems such as Nutri-Score.
The authors also called for strengthened public health measures to tackle obesity, alcohol misuse, and hepatitis transmission, and fiscal and regulatory measures such as taxation of obesogenic foods, and reducing the cost burden of healthier foods.
“If we decrease the percentage of people with liver cirrhosis through prevention, fewer people will need surveillance,” Buti stated.
Seufferlein, Michl, and Buti all declared no relevant disclosures. All three experts are members of the UEG Public Affairs Group.
A version of this article appeared on Medscape.com.
BERLIN — according to a joint statement from United European Gastroenterology (UEG) and the German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS).
The statement calls on EU and national policymakers to embed a twofold approach into healthcare systems that combines surveillance and prevention, rather than relying on voluntary participation. It also encourages stronger prevention measures, such as improved food labeling and restrictions on marketing unhealthy foods to children. The statement — which was also endorsed by the European Association for the Study of the Liver (EASL) — was presented at UEG Week 2025 .
“Curing HCC in early stages rather than treating the disease in a palliative setting should be the goal for all liver doctors and carers, and this is certainly the goal for patients,” said Thomas Seufferlein, MD, professor of gastroenterology at Ulm University, Germany, and one of the members of the DGVS who initiated the statement.
“We have to take HCC screening seriously which means setting up a structured, nationwide, well-documented, and evaluated program for HCC screening in Germany,” he said in an interview.
HCC is mainly curable in the early stages by local ablation, resection, or liver transplantation, “so early diagnosis is of the utmost importance for improving survival,” added Patrick Michl, MD, gastroenterologist, University of Heidelberg, Germany, DGVS member and co-initiator of the statement.
Risk-Stratified HCC Surveillance
In the face of rising rates worldwide, the UEG/DGVS call on policymakers to recognize liver cancer as a preventable and growing public health priority and to implement structured surveillance programs guided by risk thresholds. In particular, they support the recent policy statement from EASL recommending risk-based screening.
EASL’s key recommendations include:
- Targeted surveillance for individuals with an annual HCC risk exceeding 1.5%, where it is both clinically beneficial and cost-effective
- Risk scoring tools such as the age-male-albumin-bilirubin-platelets score that incorporates age, sex, platelet count, albumin, and bilirubin, to stratify patients by HCC risk, including those without established cirrhosis
- Enhanced surveillance for very high-risk groups, where MRI-based surveillance may be warranted despite higher costs, given its superior sensitivity for early-stage disease
- A de-escalation in low-risk individuals
- Patients with an annual HCC risk < 0.5% may be safely spared surveillance, avoiding unnecessary interventions
Evidence from France, Italy, and the UK showed that structured surveillance in high-risk groups is both clinically beneficial and cost-effective. National models in France have demonstrated higher curative treatment rates and fewer costly late-stage cases with structured surveillance. In the UK, health technology assessments indicate targeted surveillance is an efficient use of National Health Services resources, particularly when uptake is optimized. Italian models show that earlier diagnosis in well-defined high-risk groups can offset downstream treatment costs.
Seufferlein noted that Germany needs a “structured program to be implemented and there is currently little public awareness regarding this surveillance strategy.” However, he added there is a structured hepatitis B vaccination program in Germany, which has been successful. “Studies show that the inclusion of hep B vaccination in infancy and childhood has led to good uptake among young age groups.”
Germany, however, has yet to conduct national studies. “Prospective data on HCC surveillance benefits in Germany are lacking,” said Michl, “but multi-country models incorporating Germany’s cost structures suggest similar benefits would accrue if there were greater adherence to guideline-based recommendations and if publicly funded screening programs were implemented.”
Current recommendations in Germany for surveillance are based on evidence-based guidelines of the DGVS with stronger (‘should’) or weaker (‘may’) evidence-based recommendations. For example, patients with chronic hepatitis B virus infection should be offered regular surveillance once their platelet age gender–hepatitis B risk score is ≥ 10. In patients with advanced fibrosis because of chronic hepatitis C virus infection, regular surveillance should also be offered.
Barriers to Screening Uptake
HCC remains one of the most lethal cancers in Europe, largely because it is often diagnosed too late. Underdiagnosis of chronic liver disease, limited access to imaging, and reimbursement gaps prevent timely intervention.
Maria Buti, MD, consultant hepatologist, Hospital Vall d’Hebron, Barcelona, Spain, who was not involved in drafting the statement, remarked that “Patients with liver cirrhosis, or with advanced fibrosis, and also some high-risk noncirrhotic patients such as those with hepatitis B, clearly benefit from surveillance. Surveillance can change life expectancy and also reduce morbidity.”
However, structural barriers continue to impede uptake. “It is not always easy to identify patients with liver cirrhosis because the majority are completely asymptomatic in the early stages,” she said.
Even when risk factors are identified, adherence to 6-monthly surveillance remains patchy. “Sometimes physicians forget to request ultrasounds, or patients don’t understand the importance of it because they feel well,” Buti told GI & Hepatology News.
Expanded Training and Public Health Measures
The joint statement also advocates for expanded physician training in nutrition and hepatology, equitable access to diagnostic tools including MRI, and EU-wide nutrition labeling systems such as Nutri-Score.
The authors also called for strengthened public health measures to tackle obesity, alcohol misuse, and hepatitis transmission, and fiscal and regulatory measures such as taxation of obesogenic foods, and reducing the cost burden of healthier foods.
“If we decrease the percentage of people with liver cirrhosis through prevention, fewer people will need surveillance,” Buti stated.
Seufferlein, Michl, and Buti all declared no relevant disclosures. All three experts are members of the UEG Public Affairs Group.
A version of this article appeared on Medscape.com.
FROM UEG WEEK 2025
Analysis of the Frequency of level 1 OncoKB Genomic Alterations in Veterans With Various Solid Organ Malignancies
Purpose
The aim of this study is to quantify the frequency of Memorial Sloan Kettering (MSK) Precision Oncology Knowledge Base (OncoKB) Level 1 genetic alterations in Veterans with various solid organ malignancies and evaluate the clinical benefit and impact of testing on treatment of these patients.
Background
The VA National Precision Oncology Program (NPOP) facilitates comprehensive genomic profiling (CGP) testing of Veterans with advanced cancer. While CGP is increasingly utilized and routinely ordered in patients with advanced solid organ malignancies, the clinical utility and value has not been proven in certain cancers. We present data from 5,979 patients with head and neck (H&N), pancreatic, hepatocellular (HCC), esophageal and kidney cancers who underwent CGP.
Methods
Our cohort consists of Veterans that received CGP testing to identify somatic variants between 1/1/2019 and 4/2/2025. Identified variants and biomarkers were formatted for use with oncoKB-annotator, a publicly available tool to annotate genomic variants with FDA approved drug recommendations stored as Level 1 annotations in OncoKB, and prescribed drugs were extracted from the Veteran Health Administration’s (VHA) Corporate Data Warehouse (CDW). Cancers were grouped by MSK’s OncoTree codes, and summary counts of Veterans tested, Veterans recommended, Veterans prescribed recommended FDA approved drugs were determined. Percentages were calculated using the total number of Veterans tested as the denominator.
Results
Level 1 OncoKB alterations were infrequent in H&N (0.94%), kidney (0.45%), HCC(0.28%), and pancreatic adenocarcinomas (1%). The frequency of Level 1 alterations in esophageal adenocarcinomas (EAC) was 20%. Approximately 98% of the Level 1 alterations in EAC patients were HER2 positivity or MSI-High status, which can be determined by other diagnostic methodologies such as IHC. The remaining 2% of EAC patients with level 1 alterations had BRAF V600E or NTRK rearrangements.
Conclusions
The incidence of level 1 genetic variants in H&N, kidney, HCC and pancreatic adenocarcinoma is very low and would very uncommonly result in clinical benefit. Although there is an expanding number of precision oncology-based therapies available, the proportion of patients with the aforementioned solid organ malignancies who benefitted from CGP was low, suggesting CGP has minimal impact on the treatment of Veterans with these malignancies.
Purpose
The aim of this study is to quantify the frequency of Memorial Sloan Kettering (MSK) Precision Oncology Knowledge Base (OncoKB) Level 1 genetic alterations in Veterans with various solid organ malignancies and evaluate the clinical benefit and impact of testing on treatment of these patients.
Background
The VA National Precision Oncology Program (NPOP) facilitates comprehensive genomic profiling (CGP) testing of Veterans with advanced cancer. While CGP is increasingly utilized and routinely ordered in patients with advanced solid organ malignancies, the clinical utility and value has not been proven in certain cancers. We present data from 5,979 patients with head and neck (H&N), pancreatic, hepatocellular (HCC), esophageal and kidney cancers who underwent CGP.
Methods
Our cohort consists of Veterans that received CGP testing to identify somatic variants between 1/1/2019 and 4/2/2025. Identified variants and biomarkers were formatted for use with oncoKB-annotator, a publicly available tool to annotate genomic variants with FDA approved drug recommendations stored as Level 1 annotations in OncoKB, and prescribed drugs were extracted from the Veteran Health Administration’s (VHA) Corporate Data Warehouse (CDW). Cancers were grouped by MSK’s OncoTree codes, and summary counts of Veterans tested, Veterans recommended, Veterans prescribed recommended FDA approved drugs were determined. Percentages were calculated using the total number of Veterans tested as the denominator.
Results
Level 1 OncoKB alterations were infrequent in H&N (0.94%), kidney (0.45%), HCC(0.28%), and pancreatic adenocarcinomas (1%). The frequency of Level 1 alterations in esophageal adenocarcinomas (EAC) was 20%. Approximately 98% of the Level 1 alterations in EAC patients were HER2 positivity or MSI-High status, which can be determined by other diagnostic methodologies such as IHC. The remaining 2% of EAC patients with level 1 alterations had BRAF V600E or NTRK rearrangements.
Conclusions
The incidence of level 1 genetic variants in H&N, kidney, HCC and pancreatic adenocarcinoma is very low and would very uncommonly result in clinical benefit. Although there is an expanding number of precision oncology-based therapies available, the proportion of patients with the aforementioned solid organ malignancies who benefitted from CGP was low, suggesting CGP has minimal impact on the treatment of Veterans with these malignancies.
Purpose
The aim of this study is to quantify the frequency of Memorial Sloan Kettering (MSK) Precision Oncology Knowledge Base (OncoKB) Level 1 genetic alterations in Veterans with various solid organ malignancies and evaluate the clinical benefit and impact of testing on treatment of these patients.
Background
The VA National Precision Oncology Program (NPOP) facilitates comprehensive genomic profiling (CGP) testing of Veterans with advanced cancer. While CGP is increasingly utilized and routinely ordered in patients with advanced solid organ malignancies, the clinical utility and value has not been proven in certain cancers. We present data from 5,979 patients with head and neck (H&N), pancreatic, hepatocellular (HCC), esophageal and kidney cancers who underwent CGP.
Methods
Our cohort consists of Veterans that received CGP testing to identify somatic variants between 1/1/2019 and 4/2/2025. Identified variants and biomarkers were formatted for use with oncoKB-annotator, a publicly available tool to annotate genomic variants with FDA approved drug recommendations stored as Level 1 annotations in OncoKB, and prescribed drugs were extracted from the Veteran Health Administration’s (VHA) Corporate Data Warehouse (CDW). Cancers were grouped by MSK’s OncoTree codes, and summary counts of Veterans tested, Veterans recommended, Veterans prescribed recommended FDA approved drugs were determined. Percentages were calculated using the total number of Veterans tested as the denominator.
Results
Level 1 OncoKB alterations were infrequent in H&N (0.94%), kidney (0.45%), HCC(0.28%), and pancreatic adenocarcinomas (1%). The frequency of Level 1 alterations in esophageal adenocarcinomas (EAC) was 20%. Approximately 98% of the Level 1 alterations in EAC patients were HER2 positivity or MSI-High status, which can be determined by other diagnostic methodologies such as IHC. The remaining 2% of EAC patients with level 1 alterations had BRAF V600E or NTRK rearrangements.
Conclusions
The incidence of level 1 genetic variants in H&N, kidney, HCC and pancreatic adenocarcinoma is very low and would very uncommonly result in clinical benefit. Although there is an expanding number of precision oncology-based therapies available, the proportion of patients with the aforementioned solid organ malignancies who benefitted from CGP was low, suggesting CGP has minimal impact on the treatment of Veterans with these malignancies.
Journal Highlights: January-April 2025
Esophagus/Motility
Carlson DA, et al. A Standardized Approach to Performing and Interpreting Functional Lumen Imaging Probe Panometry for Esophageal Motility Disorders: The Dallas Consensus. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.234.
Parkman HP, et al; NIDDK Gastroparesis Clinical Research Consortium. Characterization of Patients with Symptoms of Gastroparesis Having Frequent Emergency Department Visits and Hospitalizations. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.01.033.
Dellon ES, et al. Long-term Safety and Efficacy of Budesonide Oral Suspension for Eosinophilic Esophagitis: A 4-Year, Phase 3, Open-Label Study. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.12.024.
Small Bowel
Hård Af Segerstad EM, et al; TEDDY Study Group. Early Dietary Fiber Intake Reduces Celiac Disease Risk in Genetically Prone Children: Insights From the TEDDY Study. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.241.
Colon
Shaukat A, et al. AGA Clinical Practice Update on Current Role of Blood Tests for Colorectal Cancer Screening: Commentary. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.04.003.
Bergman D, et al. Cholecystectomy is a Risk Factor for Microscopic Colitis: A Nationwide Population-based Matched Case Control Study. Clin Gastroenterol Hepatol. 2025 Mar. doi: 10.1016/j.cgh.2024.12.032.
Inflammatory Bowel Disease
Ben-Horin S, et al; Israeli IBD Research Nucleus (IIRN). Capsule Endoscopy-Guided Proactive Treat-to-Target Versus Continued Standard Care in Patients With Quiescent Crohn’s Disease: A Randomized Controlled Trial. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.031.
Pancreas
Guilabert L, et al; ERICA Consortium. Impact of Fluid Therapy in the Emergency Department in Acute Pancreatitis: a posthoc analysis of the WATERFALL Trial. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.01.038.
Hepatology
Rhee H, et al. Noncontrast Magnetic Resonance Imaging vs Ultrasonography for Hepatocellular Carcinoma Surveillance: A Randomized, Single-Center Trial. Gastroenterology. 2025 Jan. doi: 10.1053/j.gastro.2024.12.035.
Kronsten VT, et al. Hepatic Encephalopathy: When Lactulose and Rifaximin Are Not Working. Gastroenterology. 2025 Jan. doi: 10.1053/j.gastro.2025.01.010.
Edelson JC, et al. Accuracy and Safety of Endoscopic Ultrasound–Guided Liver Biopsy in Patients with Metabolic Dysfunction–Associated Liver Disease. Tech Innov Gastrointest Endosc. 2025 Apr. doi: 10.1016/j.tige.2025.250918.
Miscellaneous
Martin J, et al. Practical and Impactful Tips for Private Industry Collaborations with Gastroenterology Practices. Clin Gastroenterol Hepatol. 2025 Mar. doi: 10.1016/j.cgh.2025.01.021.
Tejada, Natalia et al. Glucagon-like Peptide-1 Receptor Agonists Are Not Associated With Increased Incidence of Pneumonia After Endoscopic Procedures. Tech Innov Gastrointest Endosc. 2025 Apr. doi: 10.1016/j.tige.2025.250925.
Lazaridis KN, et al. Microplastics and Nanoplastics and the Digestive System. Gastro Hep Adv. 2025 May. doi: 10.1016/j.gastha.2025.100694.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Carlson DA, et al. A Standardized Approach to Performing and Interpreting Functional Lumen Imaging Probe Panometry for Esophageal Motility Disorders: The Dallas Consensus. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.234.
Parkman HP, et al; NIDDK Gastroparesis Clinical Research Consortium. Characterization of Patients with Symptoms of Gastroparesis Having Frequent Emergency Department Visits and Hospitalizations. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.01.033.
Dellon ES, et al. Long-term Safety and Efficacy of Budesonide Oral Suspension for Eosinophilic Esophagitis: A 4-Year, Phase 3, Open-Label Study. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.12.024.
Small Bowel
Hård Af Segerstad EM, et al; TEDDY Study Group. Early Dietary Fiber Intake Reduces Celiac Disease Risk in Genetically Prone Children: Insights From the TEDDY Study. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.241.
Colon
Shaukat A, et al. AGA Clinical Practice Update on Current Role of Blood Tests for Colorectal Cancer Screening: Commentary. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.04.003.
Bergman D, et al. Cholecystectomy is a Risk Factor for Microscopic Colitis: A Nationwide Population-based Matched Case Control Study. Clin Gastroenterol Hepatol. 2025 Mar. doi: 10.1016/j.cgh.2024.12.032.
Inflammatory Bowel Disease
Ben-Horin S, et al; Israeli IBD Research Nucleus (IIRN). Capsule Endoscopy-Guided Proactive Treat-to-Target Versus Continued Standard Care in Patients With Quiescent Crohn’s Disease: A Randomized Controlled Trial. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.031.
Pancreas
Guilabert L, et al; ERICA Consortium. Impact of Fluid Therapy in the Emergency Department in Acute Pancreatitis: a posthoc analysis of the WATERFALL Trial. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.01.038.
Hepatology
Rhee H, et al. Noncontrast Magnetic Resonance Imaging vs Ultrasonography for Hepatocellular Carcinoma Surveillance: A Randomized, Single-Center Trial. Gastroenterology. 2025 Jan. doi: 10.1053/j.gastro.2024.12.035.
Kronsten VT, et al. Hepatic Encephalopathy: When Lactulose and Rifaximin Are Not Working. Gastroenterology. 2025 Jan. doi: 10.1053/j.gastro.2025.01.010.
Edelson JC, et al. Accuracy and Safety of Endoscopic Ultrasound–Guided Liver Biopsy in Patients with Metabolic Dysfunction–Associated Liver Disease. Tech Innov Gastrointest Endosc. 2025 Apr. doi: 10.1016/j.tige.2025.250918.
Miscellaneous
Martin J, et al. Practical and Impactful Tips for Private Industry Collaborations with Gastroenterology Practices. Clin Gastroenterol Hepatol. 2025 Mar. doi: 10.1016/j.cgh.2025.01.021.
Tejada, Natalia et al. Glucagon-like Peptide-1 Receptor Agonists Are Not Associated With Increased Incidence of Pneumonia After Endoscopic Procedures. Tech Innov Gastrointest Endosc. 2025 Apr. doi: 10.1016/j.tige.2025.250925.
Lazaridis KN, et al. Microplastics and Nanoplastics and the Digestive System. Gastro Hep Adv. 2025 May. doi: 10.1016/j.gastha.2025.100694.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Carlson DA, et al. A Standardized Approach to Performing and Interpreting Functional Lumen Imaging Probe Panometry for Esophageal Motility Disorders: The Dallas Consensus. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.234.
Parkman HP, et al; NIDDK Gastroparesis Clinical Research Consortium. Characterization of Patients with Symptoms of Gastroparesis Having Frequent Emergency Department Visits and Hospitalizations. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.01.033.
Dellon ES, et al. Long-term Safety and Efficacy of Budesonide Oral Suspension for Eosinophilic Esophagitis: A 4-Year, Phase 3, Open-Label Study. Clin Gastroenterol Hepatol. 2025 Feb. doi: 10.1016/j.cgh.2024.12.024.
Small Bowel
Hård Af Segerstad EM, et al; TEDDY Study Group. Early Dietary Fiber Intake Reduces Celiac Disease Risk in Genetically Prone Children: Insights From the TEDDY Study. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.241.
Colon
Shaukat A, et al. AGA Clinical Practice Update on Current Role of Blood Tests for Colorectal Cancer Screening: Commentary. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.04.003.
Bergman D, et al. Cholecystectomy is a Risk Factor for Microscopic Colitis: A Nationwide Population-based Matched Case Control Study. Clin Gastroenterol Hepatol. 2025 Mar. doi: 10.1016/j.cgh.2024.12.032.
Inflammatory Bowel Disease
Ben-Horin S, et al; Israeli IBD Research Nucleus (IIRN). Capsule Endoscopy-Guided Proactive Treat-to-Target Versus Continued Standard Care in Patients With Quiescent Crohn’s Disease: A Randomized Controlled Trial. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.031.
Pancreas
Guilabert L, et al; ERICA Consortium. Impact of Fluid Therapy in the Emergency Department in Acute Pancreatitis: a posthoc analysis of the WATERFALL Trial. Clin Gastroenterol Hepatol. 2025 Apr. doi: 10.1016/j.cgh.2025.01.038.
Hepatology
Rhee H, et al. Noncontrast Magnetic Resonance Imaging vs Ultrasonography for Hepatocellular Carcinoma Surveillance: A Randomized, Single-Center Trial. Gastroenterology. 2025 Jan. doi: 10.1053/j.gastro.2024.12.035.
Kronsten VT, et al. Hepatic Encephalopathy: When Lactulose and Rifaximin Are Not Working. Gastroenterology. 2025 Jan. doi: 10.1053/j.gastro.2025.01.010.
Edelson JC, et al. Accuracy and Safety of Endoscopic Ultrasound–Guided Liver Biopsy in Patients with Metabolic Dysfunction–Associated Liver Disease. Tech Innov Gastrointest Endosc. 2025 Apr. doi: 10.1016/j.tige.2025.250918.
Miscellaneous
Martin J, et al. Practical and Impactful Tips for Private Industry Collaborations with Gastroenterology Practices. Clin Gastroenterol Hepatol. 2025 Mar. doi: 10.1016/j.cgh.2025.01.021.
Tejada, Natalia et al. Glucagon-like Peptide-1 Receptor Agonists Are Not Associated With Increased Incidence of Pneumonia After Endoscopic Procedures. Tech Innov Gastrointest Endosc. 2025 Apr. doi: 10.1016/j.tige.2025.250925.
Lazaridis KN, et al. Microplastics and Nanoplastics and the Digestive System. Gastro Hep Adv. 2025 May. doi: 10.1016/j.gastha.2025.100694.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
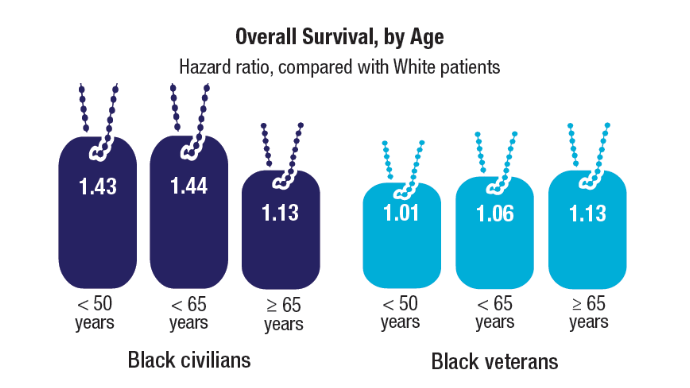
HCC Updates: Quality Care Framework and Risk Stratification Data
HCC Updates: Quality Care Framework and Risk Stratification Data
Click here to view more from Cancer Data Trends 2025.
1. Rogal SS, Taddei TH, Monto A, et al. Hepatocellular Carcinoma Diagnosis and Management in 2021: A National Veterans Affairs Quality Improvement Project. Clin Gastroenterol Hepatol. 2024 Feb;22(2):324-338. doi:10.1016/j.cgh.2023.07.002
2. John BV, Dang Y, Kaplan DE, et al. Liver Stiffness Measurement and Risk Prediction of Hepatocellular Carcinoma After HCV Eradication in Veterans With Cirrhosis. Clin Gastroenterol Hepatol. 2024 Apr;22(4):778-788.e7. doi:10.1016/j.cgh.2023.11.020
Click here to view more from Cancer Data Trends 2025.
Click here to view more from Cancer Data Trends 2025.
1. Rogal SS, Taddei TH, Monto A, et al. Hepatocellular Carcinoma Diagnosis and Management in 2021: A National Veterans Affairs Quality Improvement Project. Clin Gastroenterol Hepatol. 2024 Feb;22(2):324-338. doi:10.1016/j.cgh.2023.07.002
2. John BV, Dang Y, Kaplan DE, et al. Liver Stiffness Measurement and Risk Prediction of Hepatocellular Carcinoma After HCV Eradication in Veterans With Cirrhosis. Clin Gastroenterol Hepatol. 2024 Apr;22(4):778-788.e7. doi:10.1016/j.cgh.2023.11.020
1. Rogal SS, Taddei TH, Monto A, et al. Hepatocellular Carcinoma Diagnosis and Management in 2021: A National Veterans Affairs Quality Improvement Project. Clin Gastroenterol Hepatol. 2024 Feb;22(2):324-338. doi:10.1016/j.cgh.2023.07.002
2. John BV, Dang Y, Kaplan DE, et al. Liver Stiffness Measurement and Risk Prediction of Hepatocellular Carcinoma After HCV Eradication in Veterans With Cirrhosis. Clin Gastroenterol Hepatol. 2024 Apr;22(4):778-788.e7. doi:10.1016/j.cgh.2023.11.020
HCC Updates: Quality Care Framework and Risk Stratification Data
HCC Updates: Quality Care Framework and Risk Stratification Data
Infrequent HDV Testing Raises Concern for Worse Liver Outcomes
—according to new findings.
The low testing rate suggests limited awareness of HDV-associated risks in patients with CHB, and underscores the need for earlier testing and diagnosis, lead author Robert J. Wong, MD, of Stanford University School of Medicine, Stanford, California, and colleagues, reported.
“Data among US populations are lacking to describe the epidemiology and long-term outcomes of patients with CHB and concurrent HDV infection,” the investigators wrote in Gastro Hep Advances (2025 Oct. doi: 10.1016/j.gastha.2024.10.015).
Prior studies have found that only 6% to 19% of patients with CHB get tested for HDV, and among those tested, the prevalence is relatively low—between 2% and 4.6%. Although relatively uncommon, HDV carries a substantial clinical and economic burden, Dr. Wong and colleagues noted, highlighting the importance of clinical awareness and accurate epidemiologic data.
The present study analyzed data from the Veterans Affairs (VA) Corporate Data Warehouse between 2010 and 2023. Adults with CHB were identified based on laboratory-confirmed markers and ICD-9/10 codes. HDV testing (anti-HDV antibody and HDV RNA) was assessed, and predictors of testing were evaluated using multivariable logistic regression.
To examine liver-related outcomes, patients who tested positive for HDV were propensity score–matched 1:2 with CHB patients who tested negative. Matching accounted for age, sex, race/ethnicity, HBeAg status, antiviral treatment, HCV and HIV coinfection, diabetes, and alcohol use. Patients with cirrhosis or hepatocellular carcinoma (HCC) at base-line were excluded. Incidence of cirrhosis, hepatic decompensation, and HCC was estimated using competing risks Nelson-Aalen methods.
Among 27,548 veterans with CHB, only 16.1% underwent HDV testing. Of those tested, 3.25% were HDV positive. Testing rates were higher among patients who were HBeAg positive, on antiviral therapy, or identified as Asian or Pacific Islander.
Conversely, testing was significantly less common among patients with high-risk alcohol use, past or current drug use, cirrhosis at diagnosis, or HCV coinfection. In contrast, HIV coinfection was associated with increased odds of being tested.
Among those tested, HDV positivity was more likely in patients with HCV coinfection, cirrhosis, or a history of drug use. On multivariable analysis, these factors were independent predictors of HDV positivity.
In the matched cohort of 71 HDV-positive patients and 140 HDV-negative controls, the incidence of cirrhosis was more than 3-fold higher in HDV-positive patients (4.39 vs 1.30 per 100,000 person-years; P less than .01), and hepatic decompensation was over 5 times more common (2.18 vs 0.41 per 100,000 person-years; P = .01). There was also a non-significant trend toward increased HCC risk in the HDV group.
“These findings align with existing studies and confirm that among a predominantly non-Asian US cohort of CHB patients, presence of concurrent HDV is associated with more severe liver disease progression,” the investigators wrote. “These observations, taken together with the low rates of HDV testing overall and particularly among high-risk individuals, emphasizes the need for greater awareness and novel strategies on how to improve HDV testing and diagnosis, particularly given that novel HDV therapies are on the near horizon.”
The study was supported by Gilead. The investigators disclosed additional relationships with Exact Sciences, GSK, Novo Nordisk, and others.
Hepatitis D virus (HDV) is an RNA “sub-virus” that infects patients with co-existing hepatitis B virus (HBV) infections. HDV infection currently affects approximately 15-20 million people worldwide but is an orphan disease in the United States with fewer than 100,000 individuals infected today.
Those with HDV have a 70% lifetime risk of hepatocellular carcinoma (HCC), cirrhosis, liver failure, death, or liver transplant. But there are no current treatments in the US that are Food and Drug Administration (FDA)-approved for the treatment of HDV, and only one therapy in the European Union with full approval by the European Medicines Agency.
Despite HDV severity and limited treatment options, screening for HDV remains severely inadequate, often only testing those individuals at high risk sequentially. HDV screening, would benefit from a revamped approach that automatically reflexes testing when individuals are diagnosed with HBV if positive for hepatitis B surface antigen (HBsAg+), then proceeds to anti-HDV antibody total testing, and then double reflexed to HDV-RNA polymerase chain reaction (PCR) quantitation. This is especially true in the Veterans Administration (VA)’s hospitals and clinics, where Wong and colleagues found very low rates of HDV testing among a national cohort of US Veterans with chronic HBV.
This study highlights the importance of timely HDV testing using reflex tools to improve diagnosis and HDV treatment, reducing long-term risks of liver-related morbidity and mortality.
Robert G. Gish, MD, AGAF, is principal at Robert G Gish Consultants LLC, clinical professor of medicine at Loma Linda University, Loma Linda, Calif., and medical director of the Hepatitis B Foundation. His complete list of disclosures can be found at www.robertgish.com/about.
Hepatitis D virus (HDV) is an RNA “sub-virus” that infects patients with co-existing hepatitis B virus (HBV) infections. HDV infection currently affects approximately 15-20 million people worldwide but is an orphan disease in the United States with fewer than 100,000 individuals infected today.
Those with HDV have a 70% lifetime risk of hepatocellular carcinoma (HCC), cirrhosis, liver failure, death, or liver transplant. But there are no current treatments in the US that are Food and Drug Administration (FDA)-approved for the treatment of HDV, and only one therapy in the European Union with full approval by the European Medicines Agency.
Despite HDV severity and limited treatment options, screening for HDV remains severely inadequate, often only testing those individuals at high risk sequentially. HDV screening, would benefit from a revamped approach that automatically reflexes testing when individuals are diagnosed with HBV if positive for hepatitis B surface antigen (HBsAg+), then proceeds to anti-HDV antibody total testing, and then double reflexed to HDV-RNA polymerase chain reaction (PCR) quantitation. This is especially true in the Veterans Administration (VA)’s hospitals and clinics, where Wong and colleagues found very low rates of HDV testing among a national cohort of US Veterans with chronic HBV.
This study highlights the importance of timely HDV testing using reflex tools to improve diagnosis and HDV treatment, reducing long-term risks of liver-related morbidity and mortality.
Robert G. Gish, MD, AGAF, is principal at Robert G Gish Consultants LLC, clinical professor of medicine at Loma Linda University, Loma Linda, Calif., and medical director of the Hepatitis B Foundation. His complete list of disclosures can be found at www.robertgish.com/about.
Hepatitis D virus (HDV) is an RNA “sub-virus” that infects patients with co-existing hepatitis B virus (HBV) infections. HDV infection currently affects approximately 15-20 million people worldwide but is an orphan disease in the United States with fewer than 100,000 individuals infected today.
Those with HDV have a 70% lifetime risk of hepatocellular carcinoma (HCC), cirrhosis, liver failure, death, or liver transplant. But there are no current treatments in the US that are Food and Drug Administration (FDA)-approved for the treatment of HDV, and only one therapy in the European Union with full approval by the European Medicines Agency.
Despite HDV severity and limited treatment options, screening for HDV remains severely inadequate, often only testing those individuals at high risk sequentially. HDV screening, would benefit from a revamped approach that automatically reflexes testing when individuals are diagnosed with HBV if positive for hepatitis B surface antigen (HBsAg+), then proceeds to anti-HDV antibody total testing, and then double reflexed to HDV-RNA polymerase chain reaction (PCR) quantitation. This is especially true in the Veterans Administration (VA)’s hospitals and clinics, where Wong and colleagues found very low rates of HDV testing among a national cohort of US Veterans with chronic HBV.
This study highlights the importance of timely HDV testing using reflex tools to improve diagnosis and HDV treatment, reducing long-term risks of liver-related morbidity and mortality.
Robert G. Gish, MD, AGAF, is principal at Robert G Gish Consultants LLC, clinical professor of medicine at Loma Linda University, Loma Linda, Calif., and medical director of the Hepatitis B Foundation. His complete list of disclosures can be found at www.robertgish.com/about.
—according to new findings.
The low testing rate suggests limited awareness of HDV-associated risks in patients with CHB, and underscores the need for earlier testing and diagnosis, lead author Robert J. Wong, MD, of Stanford University School of Medicine, Stanford, California, and colleagues, reported.
“Data among US populations are lacking to describe the epidemiology and long-term outcomes of patients with CHB and concurrent HDV infection,” the investigators wrote in Gastro Hep Advances (2025 Oct. doi: 10.1016/j.gastha.2024.10.015).
Prior studies have found that only 6% to 19% of patients with CHB get tested for HDV, and among those tested, the prevalence is relatively low—between 2% and 4.6%. Although relatively uncommon, HDV carries a substantial clinical and economic burden, Dr. Wong and colleagues noted, highlighting the importance of clinical awareness and accurate epidemiologic data.
The present study analyzed data from the Veterans Affairs (VA) Corporate Data Warehouse between 2010 and 2023. Adults with CHB were identified based on laboratory-confirmed markers and ICD-9/10 codes. HDV testing (anti-HDV antibody and HDV RNA) was assessed, and predictors of testing were evaluated using multivariable logistic regression.
To examine liver-related outcomes, patients who tested positive for HDV were propensity score–matched 1:2 with CHB patients who tested negative. Matching accounted for age, sex, race/ethnicity, HBeAg status, antiviral treatment, HCV and HIV coinfection, diabetes, and alcohol use. Patients with cirrhosis or hepatocellular carcinoma (HCC) at base-line were excluded. Incidence of cirrhosis, hepatic decompensation, and HCC was estimated using competing risks Nelson-Aalen methods.
Among 27,548 veterans with CHB, only 16.1% underwent HDV testing. Of those tested, 3.25% were HDV positive. Testing rates were higher among patients who were HBeAg positive, on antiviral therapy, or identified as Asian or Pacific Islander.
Conversely, testing was significantly less common among patients with high-risk alcohol use, past or current drug use, cirrhosis at diagnosis, or HCV coinfection. In contrast, HIV coinfection was associated with increased odds of being tested.
Among those tested, HDV positivity was more likely in patients with HCV coinfection, cirrhosis, or a history of drug use. On multivariable analysis, these factors were independent predictors of HDV positivity.
In the matched cohort of 71 HDV-positive patients and 140 HDV-negative controls, the incidence of cirrhosis was more than 3-fold higher in HDV-positive patients (4.39 vs 1.30 per 100,000 person-years; P less than .01), and hepatic decompensation was over 5 times more common (2.18 vs 0.41 per 100,000 person-years; P = .01). There was also a non-significant trend toward increased HCC risk in the HDV group.
“These findings align with existing studies and confirm that among a predominantly non-Asian US cohort of CHB patients, presence of concurrent HDV is associated with more severe liver disease progression,” the investigators wrote. “These observations, taken together with the low rates of HDV testing overall and particularly among high-risk individuals, emphasizes the need for greater awareness and novel strategies on how to improve HDV testing and diagnosis, particularly given that novel HDV therapies are on the near horizon.”
The study was supported by Gilead. The investigators disclosed additional relationships with Exact Sciences, GSK, Novo Nordisk, and others.
—according to new findings.
The low testing rate suggests limited awareness of HDV-associated risks in patients with CHB, and underscores the need for earlier testing and diagnosis, lead author Robert J. Wong, MD, of Stanford University School of Medicine, Stanford, California, and colleagues, reported.
“Data among US populations are lacking to describe the epidemiology and long-term outcomes of patients with CHB and concurrent HDV infection,” the investigators wrote in Gastro Hep Advances (2025 Oct. doi: 10.1016/j.gastha.2024.10.015).
Prior studies have found that only 6% to 19% of patients with CHB get tested for HDV, and among those tested, the prevalence is relatively low—between 2% and 4.6%. Although relatively uncommon, HDV carries a substantial clinical and economic burden, Dr. Wong and colleagues noted, highlighting the importance of clinical awareness and accurate epidemiologic data.
The present study analyzed data from the Veterans Affairs (VA) Corporate Data Warehouse between 2010 and 2023. Adults with CHB were identified based on laboratory-confirmed markers and ICD-9/10 codes. HDV testing (anti-HDV antibody and HDV RNA) was assessed, and predictors of testing were evaluated using multivariable logistic regression.
To examine liver-related outcomes, patients who tested positive for HDV were propensity score–matched 1:2 with CHB patients who tested negative. Matching accounted for age, sex, race/ethnicity, HBeAg status, antiviral treatment, HCV and HIV coinfection, diabetes, and alcohol use. Patients with cirrhosis or hepatocellular carcinoma (HCC) at base-line were excluded. Incidence of cirrhosis, hepatic decompensation, and HCC was estimated using competing risks Nelson-Aalen methods.
Among 27,548 veterans with CHB, only 16.1% underwent HDV testing. Of those tested, 3.25% were HDV positive. Testing rates were higher among patients who were HBeAg positive, on antiviral therapy, or identified as Asian or Pacific Islander.
Conversely, testing was significantly less common among patients with high-risk alcohol use, past or current drug use, cirrhosis at diagnosis, or HCV coinfection. In contrast, HIV coinfection was associated with increased odds of being tested.
Among those tested, HDV positivity was more likely in patients with HCV coinfection, cirrhosis, or a history of drug use. On multivariable analysis, these factors were independent predictors of HDV positivity.
In the matched cohort of 71 HDV-positive patients and 140 HDV-negative controls, the incidence of cirrhosis was more than 3-fold higher in HDV-positive patients (4.39 vs 1.30 per 100,000 person-years; P less than .01), and hepatic decompensation was over 5 times more common (2.18 vs 0.41 per 100,000 person-years; P = .01). There was also a non-significant trend toward increased HCC risk in the HDV group.
“These findings align with existing studies and confirm that among a predominantly non-Asian US cohort of CHB patients, presence of concurrent HDV is associated with more severe liver disease progression,” the investigators wrote. “These observations, taken together with the low rates of HDV testing overall and particularly among high-risk individuals, emphasizes the need for greater awareness and novel strategies on how to improve HDV testing and diagnosis, particularly given that novel HDV therapies are on the near horizon.”
The study was supported by Gilead. The investigators disclosed additional relationships with Exact Sciences, GSK, Novo Nordisk, and others.
FROM GASTRO HEP ADVANCES
Could Statins Prevent Hepatocellular Carcinoma?
, emerging research, including several large cohort studies, suggested.
The most recent study, published in JAMA Internal Medicine, showed a lower incidence of hepatic decompensation among statin users in a registry for adults aged 40 years or older with baseline chronic liver disease.
“Our findings support the idea that statins may offer benefits beyond lipid-lowering in patients with [chronic liver disease], and clinicians may be more confident in prescribing statins when indicated,” even in these patients, said corresponding Co-author Raymond T. Chung, MD, gastroenterology investigator at Mass General Research Institute, Boston, in an interview.
“While prior studies have suggested an association between statin use and reduced hepatocellular carcinoma risk, our study aimed to build on that evidence by using a large, real-world, hospital-based cohort inclusive of all etiologies of chronic liver disease,” Chung told GI & Hepatology News.
Chung, along with Jonggi Choi, MD, of the University of Ulsan College of Medicine, Seoul, South Korea, and colleagues, reviewed data from the Research Patient Data Registry from 2000 to 2023 for 16,501 participantsaged 40 years or older with baseline chronic liver disease and baseline Fibrosis-4 (FIB-4) scores ≥ 1.3.
The study population had a mean age of 59.7 years, and 40.9% were women. The researchers divided the population into statin users (n = 3610) and nonusers (n = 12,891). Statin use was defined as a cumulative defined daily dose ≥ 30 mg.
The primary outcome was the cumulative incidence of hepatocellular carcinoma and hepatic decompensation.
At 10 years follow-up, statin users showed a significantly reduced incidence of hepatocellular carcinoma vs nonusers (3.8% vs 8.0%; P < .001) as well as a significantly reduced incidence of hepatic decompensation (10.6% vs 19.5%; P < .001).
Incorporating FIB-4 scores, a surrogate marker for liver fibrosis, also showed that statin users were less likely to experience fibrosis progression, offering a potential mechanism of action for the observed reduction in adverse liver outcomes, Chung told GI & Hepatology News.
“Similar trends have been observed in prior observational studies, but our findings now support a real effect of statin use on fibrosis progression,” he said. “However, what strengthened our study was that the association remained consistent across multiple subgroups and sensitivity analyses.”
Another study published in Clinical Gastroenterology and Hepatology showed a reduced risk of developing severe liver disease in a Swedish cohort of noncirrhotic adults with chronic liver disease who used statins (n = 3862) compared with control patients with chronic liver disease (matched 1:1) and who did not use statins (hazard ratio [HR], 0.60).
In that study, Rajani Sharma, MD, and colleagues found a protective association in both prefibrosis and fibrosis stages at diagnosis, and statin use was associated with reduced rates of progression to both cirrhosis and hepatocellular carcinoma (HR, 0.62 and 0.44, respectively).
Exciting and Necessary Research
The research by Choi and colleagues is “exciting,” said Bubu Banini, MD, PhD, an assistant professor in digestive diseases at Yale School of Medicine, New Haven, Connecticut, in an interview.
Liver cancer prevalence has risen over the past few decades in the United States and worldwide, and the 5-year overall survival rate of liver cancer is less than 20%, Banini told GI & Hepatology News.
Clinicians often withhold statins out of fear of liver injury in persons with chronic liver disease; however, a takeaway from this study is that for persons with chronic liver disease who have indications for statin use, the medication should not be withheld, she said.
Of course, prospective studies are needed to replicate the results, Banini added.
The study findings were limited by several factors, including the inability to adjust for all potential confounding variables, lack of data on post-index treatments, and the use of wide, cumulative, defined daily dose categories to ensure statistical power, the researchers noted.
“Moving forward, randomized controlled trials are essential to establish a causal relationship and clarify the molecular and clinical pathways through which statins exert hepatoprotective effects,” Chung added.
Randomized controlled trials are also needed to determine whether statins can actually reduce the risk for hepatocellular carcinoma and hepatic decompensation in patients with chronic liver disease, and cost-effectiveness analyses may be essential for translating this evidence into clinical guidelines, he added.
Statins and HCC Risk in the General Population
A large cohort study, published in JAMA Network Open by Mara Sophie Vell, PhD, and colleagues, showed an association between reduced risk for hepatocellular carcinoma and statin use in the general population and in those at increased risk for liver disease.
The study, which included data for individuals aged 37-73 years from the UK Biobank, found a 15% reduced risk for new-onset liver disease and a 28% reduced risk for liver-related death among regular statin users than among nonusers (HR, 0.85 and 0.72, respectively).
In addition, regular statin users showed a 74% reduced risk (P = .003) of developing hepatocellular carcinoma compared with those not using statins. The researchers identified a particular impact on liver disease risk reduction among men, individuals with diabetes, and patients with high levels of liver scarring at baseline based on the FIB-4 index.
A meta-analysis of 24 studies, previously published in the journal Cancers, showed a significant reduction of 46% in hepatocellular carcinoma risk among statins users compared with nonusers.
The researchers found this risk reduction was significant in subgroups of patients with diabetes, liver cirrhosis, and those on antiviral therapy, and they suggested that the antiangiogenic, immunomodulatory, antiproliferative, and antifibrotic properties of statins may contribute to their potential to reduce tumor growth or hepatocellular carcinoma development.
The meta-analysis authors noted that although most studies have reported a low risk for statin-induced hepatotoxicity, clinicians should proceed with caution in some patients with existing cirrhosis.
“If the patients are diagnosed with decompensated cirrhosis, then statins should be prescribed with caution at low doses,” they wrote.
Advocating statin use solely for chemoprevention may be premature based on observational data, Chung told GI & Hepatology News.
“However, in patients with [chronic liver disease] who already meet indications for statin therapy, the potential added benefit of reducing liver-related complications strengthens the rationale for their use,” he said. Future randomized clinical trials will be key to defining the risk-benefit profile in this context.
The study by Choi and colleagues was supported by the National Institutes of Health.
The study by Sharma and colleagues was supported by the Karolinska Institutet, Stockholm, Sweden, and the Columbia University Irving Medical Center, New York City; researchers were supported by grants from the Swedish Research Council, Center for Innovative Medicine, the Swedish Cancer Society, and the National Institutes of Health.
The study by Vell and colleagues had no outside funding.
The study by Mohaimenul Islam and colleagues was supported by the Ministry of Education and Ministry of Science and Technology, Taiwan.
Chung and Banini had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
, emerging research, including several large cohort studies, suggested.
The most recent study, published in JAMA Internal Medicine, showed a lower incidence of hepatic decompensation among statin users in a registry for adults aged 40 years or older with baseline chronic liver disease.
“Our findings support the idea that statins may offer benefits beyond lipid-lowering in patients with [chronic liver disease], and clinicians may be more confident in prescribing statins when indicated,” even in these patients, said corresponding Co-author Raymond T. Chung, MD, gastroenterology investigator at Mass General Research Institute, Boston, in an interview.
“While prior studies have suggested an association between statin use and reduced hepatocellular carcinoma risk, our study aimed to build on that evidence by using a large, real-world, hospital-based cohort inclusive of all etiologies of chronic liver disease,” Chung told GI & Hepatology News.
Chung, along with Jonggi Choi, MD, of the University of Ulsan College of Medicine, Seoul, South Korea, and colleagues, reviewed data from the Research Patient Data Registry from 2000 to 2023 for 16,501 participantsaged 40 years or older with baseline chronic liver disease and baseline Fibrosis-4 (FIB-4) scores ≥ 1.3.
The study population had a mean age of 59.7 years, and 40.9% were women. The researchers divided the population into statin users (n = 3610) and nonusers (n = 12,891). Statin use was defined as a cumulative defined daily dose ≥ 30 mg.
The primary outcome was the cumulative incidence of hepatocellular carcinoma and hepatic decompensation.
At 10 years follow-up, statin users showed a significantly reduced incidence of hepatocellular carcinoma vs nonusers (3.8% vs 8.0%; P < .001) as well as a significantly reduced incidence of hepatic decompensation (10.6% vs 19.5%; P < .001).
Incorporating FIB-4 scores, a surrogate marker for liver fibrosis, also showed that statin users were less likely to experience fibrosis progression, offering a potential mechanism of action for the observed reduction in adverse liver outcomes, Chung told GI & Hepatology News.
“Similar trends have been observed in prior observational studies, but our findings now support a real effect of statin use on fibrosis progression,” he said. “However, what strengthened our study was that the association remained consistent across multiple subgroups and sensitivity analyses.”
Another study published in Clinical Gastroenterology and Hepatology showed a reduced risk of developing severe liver disease in a Swedish cohort of noncirrhotic adults with chronic liver disease who used statins (n = 3862) compared with control patients with chronic liver disease (matched 1:1) and who did not use statins (hazard ratio [HR], 0.60).
In that study, Rajani Sharma, MD, and colleagues found a protective association in both prefibrosis and fibrosis stages at diagnosis, and statin use was associated with reduced rates of progression to both cirrhosis and hepatocellular carcinoma (HR, 0.62 and 0.44, respectively).
Exciting and Necessary Research
The research by Choi and colleagues is “exciting,” said Bubu Banini, MD, PhD, an assistant professor in digestive diseases at Yale School of Medicine, New Haven, Connecticut, in an interview.
Liver cancer prevalence has risen over the past few decades in the United States and worldwide, and the 5-year overall survival rate of liver cancer is less than 20%, Banini told GI & Hepatology News.
Clinicians often withhold statins out of fear of liver injury in persons with chronic liver disease; however, a takeaway from this study is that for persons with chronic liver disease who have indications for statin use, the medication should not be withheld, she said.
Of course, prospective studies are needed to replicate the results, Banini added.
The study findings were limited by several factors, including the inability to adjust for all potential confounding variables, lack of data on post-index treatments, and the use of wide, cumulative, defined daily dose categories to ensure statistical power, the researchers noted.
“Moving forward, randomized controlled trials are essential to establish a causal relationship and clarify the molecular and clinical pathways through which statins exert hepatoprotective effects,” Chung added.
Randomized controlled trials are also needed to determine whether statins can actually reduce the risk for hepatocellular carcinoma and hepatic decompensation in patients with chronic liver disease, and cost-effectiveness analyses may be essential for translating this evidence into clinical guidelines, he added.
Statins and HCC Risk in the General Population
A large cohort study, published in JAMA Network Open by Mara Sophie Vell, PhD, and colleagues, showed an association between reduced risk for hepatocellular carcinoma and statin use in the general population and in those at increased risk for liver disease.
The study, which included data for individuals aged 37-73 years from the UK Biobank, found a 15% reduced risk for new-onset liver disease and a 28% reduced risk for liver-related death among regular statin users than among nonusers (HR, 0.85 and 0.72, respectively).
In addition, regular statin users showed a 74% reduced risk (P = .003) of developing hepatocellular carcinoma compared with those not using statins. The researchers identified a particular impact on liver disease risk reduction among men, individuals with diabetes, and patients with high levels of liver scarring at baseline based on the FIB-4 index.
A meta-analysis of 24 studies, previously published in the journal Cancers, showed a significant reduction of 46% in hepatocellular carcinoma risk among statins users compared with nonusers.
The researchers found this risk reduction was significant in subgroups of patients with diabetes, liver cirrhosis, and those on antiviral therapy, and they suggested that the antiangiogenic, immunomodulatory, antiproliferative, and antifibrotic properties of statins may contribute to their potential to reduce tumor growth or hepatocellular carcinoma development.
The meta-analysis authors noted that although most studies have reported a low risk for statin-induced hepatotoxicity, clinicians should proceed with caution in some patients with existing cirrhosis.
“If the patients are diagnosed with decompensated cirrhosis, then statins should be prescribed with caution at low doses,” they wrote.
Advocating statin use solely for chemoprevention may be premature based on observational data, Chung told GI & Hepatology News.
“However, in patients with [chronic liver disease] who already meet indications for statin therapy, the potential added benefit of reducing liver-related complications strengthens the rationale for their use,” he said. Future randomized clinical trials will be key to defining the risk-benefit profile in this context.
The study by Choi and colleagues was supported by the National Institutes of Health.
The study by Sharma and colleagues was supported by the Karolinska Institutet, Stockholm, Sweden, and the Columbia University Irving Medical Center, New York City; researchers were supported by grants from the Swedish Research Council, Center for Innovative Medicine, the Swedish Cancer Society, and the National Institutes of Health.
The study by Vell and colleagues had no outside funding.
The study by Mohaimenul Islam and colleagues was supported by the Ministry of Education and Ministry of Science and Technology, Taiwan.
Chung and Banini had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
, emerging research, including several large cohort studies, suggested.
The most recent study, published in JAMA Internal Medicine, showed a lower incidence of hepatic decompensation among statin users in a registry for adults aged 40 years or older with baseline chronic liver disease.
“Our findings support the idea that statins may offer benefits beyond lipid-lowering in patients with [chronic liver disease], and clinicians may be more confident in prescribing statins when indicated,” even in these patients, said corresponding Co-author Raymond T. Chung, MD, gastroenterology investigator at Mass General Research Institute, Boston, in an interview.
“While prior studies have suggested an association between statin use and reduced hepatocellular carcinoma risk, our study aimed to build on that evidence by using a large, real-world, hospital-based cohort inclusive of all etiologies of chronic liver disease,” Chung told GI & Hepatology News.
Chung, along with Jonggi Choi, MD, of the University of Ulsan College of Medicine, Seoul, South Korea, and colleagues, reviewed data from the Research Patient Data Registry from 2000 to 2023 for 16,501 participantsaged 40 years or older with baseline chronic liver disease and baseline Fibrosis-4 (FIB-4) scores ≥ 1.3.
The study population had a mean age of 59.7 years, and 40.9% were women. The researchers divided the population into statin users (n = 3610) and nonusers (n = 12,891). Statin use was defined as a cumulative defined daily dose ≥ 30 mg.
The primary outcome was the cumulative incidence of hepatocellular carcinoma and hepatic decompensation.
At 10 years follow-up, statin users showed a significantly reduced incidence of hepatocellular carcinoma vs nonusers (3.8% vs 8.0%; P < .001) as well as a significantly reduced incidence of hepatic decompensation (10.6% vs 19.5%; P < .001).
Incorporating FIB-4 scores, a surrogate marker for liver fibrosis, also showed that statin users were less likely to experience fibrosis progression, offering a potential mechanism of action for the observed reduction in adverse liver outcomes, Chung told GI & Hepatology News.
“Similar trends have been observed in prior observational studies, but our findings now support a real effect of statin use on fibrosis progression,” he said. “However, what strengthened our study was that the association remained consistent across multiple subgroups and sensitivity analyses.”
Another study published in Clinical Gastroenterology and Hepatology showed a reduced risk of developing severe liver disease in a Swedish cohort of noncirrhotic adults with chronic liver disease who used statins (n = 3862) compared with control patients with chronic liver disease (matched 1:1) and who did not use statins (hazard ratio [HR], 0.60).
In that study, Rajani Sharma, MD, and colleagues found a protective association in both prefibrosis and fibrosis stages at diagnosis, and statin use was associated with reduced rates of progression to both cirrhosis and hepatocellular carcinoma (HR, 0.62 and 0.44, respectively).
Exciting and Necessary Research
The research by Choi and colleagues is “exciting,” said Bubu Banini, MD, PhD, an assistant professor in digestive diseases at Yale School of Medicine, New Haven, Connecticut, in an interview.
Liver cancer prevalence has risen over the past few decades in the United States and worldwide, and the 5-year overall survival rate of liver cancer is less than 20%, Banini told GI & Hepatology News.
Clinicians often withhold statins out of fear of liver injury in persons with chronic liver disease; however, a takeaway from this study is that for persons with chronic liver disease who have indications for statin use, the medication should not be withheld, she said.
Of course, prospective studies are needed to replicate the results, Banini added.
The study findings were limited by several factors, including the inability to adjust for all potential confounding variables, lack of data on post-index treatments, and the use of wide, cumulative, defined daily dose categories to ensure statistical power, the researchers noted.
“Moving forward, randomized controlled trials are essential to establish a causal relationship and clarify the molecular and clinical pathways through which statins exert hepatoprotective effects,” Chung added.
Randomized controlled trials are also needed to determine whether statins can actually reduce the risk for hepatocellular carcinoma and hepatic decompensation in patients with chronic liver disease, and cost-effectiveness analyses may be essential for translating this evidence into clinical guidelines, he added.
Statins and HCC Risk in the General Population
A large cohort study, published in JAMA Network Open by Mara Sophie Vell, PhD, and colleagues, showed an association between reduced risk for hepatocellular carcinoma and statin use in the general population and in those at increased risk for liver disease.
The study, which included data for individuals aged 37-73 years from the UK Biobank, found a 15% reduced risk for new-onset liver disease and a 28% reduced risk for liver-related death among regular statin users than among nonusers (HR, 0.85 and 0.72, respectively).
In addition, regular statin users showed a 74% reduced risk (P = .003) of developing hepatocellular carcinoma compared with those not using statins. The researchers identified a particular impact on liver disease risk reduction among men, individuals with diabetes, and patients with high levels of liver scarring at baseline based on the FIB-4 index.
A meta-analysis of 24 studies, previously published in the journal Cancers, showed a significant reduction of 46% in hepatocellular carcinoma risk among statins users compared with nonusers.
The researchers found this risk reduction was significant in subgroups of patients with diabetes, liver cirrhosis, and those on antiviral therapy, and they suggested that the antiangiogenic, immunomodulatory, antiproliferative, and antifibrotic properties of statins may contribute to their potential to reduce tumor growth or hepatocellular carcinoma development.
The meta-analysis authors noted that although most studies have reported a low risk for statin-induced hepatotoxicity, clinicians should proceed with caution in some patients with existing cirrhosis.
“If the patients are diagnosed with decompensated cirrhosis, then statins should be prescribed with caution at low doses,” they wrote.
Advocating statin use solely for chemoprevention may be premature based on observational data, Chung told GI & Hepatology News.
“However, in patients with [chronic liver disease] who already meet indications for statin therapy, the potential added benefit of reducing liver-related complications strengthens the rationale for their use,” he said. Future randomized clinical trials will be key to defining the risk-benefit profile in this context.
The study by Choi and colleagues was supported by the National Institutes of Health.
The study by Sharma and colleagues was supported by the Karolinska Institutet, Stockholm, Sweden, and the Columbia University Irving Medical Center, New York City; researchers were supported by grants from the Swedish Research Council, Center for Innovative Medicine, the Swedish Cancer Society, and the National Institutes of Health.
The study by Vell and colleagues had no outside funding.
The study by Mohaimenul Islam and colleagues was supported by the Ministry of Education and Ministry of Science and Technology, Taiwan.
Chung and Banini had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
New Risk Score Might Improve HCC Surveillance Among Cirrhosis Patients
, based to a recent phase 3 biomarker validation study.
The Prognostic Liver Secretome Signature with Alpha-Fetoprotein plus Age, Male Sex, Albumin-Bilirubin, and Platelets (PAaM) score integrates both molecular and clinical variables to effectively classify cirrhosis patients by their risk of developing HCC, potentially sparing low-risk patients from unnecessary surveillance, lead author Naoto Fujiwara, MD, PhD, of the University of Texas Southwestern Medical Center, Dallas, and colleagues reported.
“Hepatocellular carcinoma risk stratification is an urgent unmet need for cost-effective screening and early detection in patients with cirrhosis,” the investigators wrote in Gastroenterology. “This study represents the largest and first phase 3 biomarker validation study that establishes an integrative molecular/clinical score, PAaM, for HCC risk stratification.”
The PAaM score combines an 8-protein prognostic liver secretome signature with traditional clinical variables, including alpha-fetoprotein (AFP) levels, age, sex, albumin-bilirubin levels, and platelet counts. The score stratifies patients into high-, intermediate-, and low-risk categories.
The PAaM score was validated using 2 independent prospective cohorts in the United States: the statewide Texas Hepatocellular Carcinoma Consortium (THCCC) and the nationwide Hepatocellular Carcinoma Early Detection Strategy (HEDS). Across both cohorts, 3,484 patients with cirrhosis were followed over time to assess the development of HCC.
In the Texas cohort, comprising 2,156 patients with cirrhosis, PAaM classified 19% of patients as high risk, 42% as intermediate risk, and 39% as low risk. The annual incidence of HCC was significantly different across these groups, with high-risk patients experiencing a 5.3% incidence rate, versus 2.7% for intermediate-risk patients and 0.6% for low-risk patients (P less than .001). Compared with those in the low-risk group, high-risk patients had sub-distribution hazard ratio (sHR) of 7.51 for developing HCC, while intermediate-risk patients had an sHR of 4.20.
In the nationwide HEDS cohort, which included 1,328 patients, PAaM similarly stratified 15% of participants as high risk, 41% as intermediate risk, and 44% as low risk. Annual HCC incidence rates were 6.2%, 1.8%, and 0.8% for high-, intermediate-, and low-risk patients, respectively (P less than .001). Among these patients, sub-distribution hazard ratios for HCC were 6.54 for high-risk patients and 1.77 for intermediate-risk patients, again underscoring the tool’s potential to identify individuals at elevated risk of developing HCC.
The PAaM score outperformed existing models like the aMAP score and the PLSec-AFP molecular marker alone, with consistent superiority across a diverse range of cirrhosis etiologies, including metabolic dysfunction–associated steatotic liver disease (MASLD), alcohol-associated liver disease (ALD), and cured hepatitis C virus (HCV) infection.
Based on these findings, high-risk patients might benefit from more intensive screening strategies, Fujiwara and colleagues suggested, while intermediate-risk patients could continue with semi-annual ultrasound-based screening. Of note, low-risk patients—comprising about 40% of the study population—could potentially avoid frequent screenings, thus reducing healthcare costs and minimizing unnecessary interventions.
“This represents a significant step toward the clinical translation of an individual risk-based HCC screening strategy to improve early HCC detection and reduce HCC mortality,” the investigators concluded.This study was supported by various the National Cancer Institute, Veterans Affairs, the Japan Society for the Promotion of Science, and others. The investigators disclosed additional relationships with Boston Scientific, Sirtex, Bayer, and others.
Nancy S. Reau, MD, AGAF, of RUSH University in Chicago, highlighted both the promise and challenges of the PAaM score for HCC risk stratification, emphasizing that current liver cancer screening strategies remain inadequate, with only about 25% of patients receiving guideline-recommended surveillance.
“An easy-to-apply cost effective tool could significantly improve screening strategies, which should lead to earlier identification of liver cancer—at a time when curative treatment options are available,” Reau said.
PAaM, however, may be impractical for routine use.
“A tool that classifies people into 3 different screening strategies and requires longitudinal applications and re-classification could add complexity,” she explained, predicting that “clinicians aren’t going to use it correctly.
Reau was particularly concerned about the need for repeated assessments over time.
“People change,” she said. “A low-risk categorization by PAaM at the age of 40 may no longer be relevant at 50 or 60 as liver disease progresses.”
Although the tool is “exciting,” Reau suggested that it is also “premature” until appropriate reclassification intervals are understood.
She also noted that some patients still develop HCC despite being considered low risk, including cases of HCC that develop in non-cirrhotic HCV infection or MASLD.
Beyond the above clinical considerations, Dr. Reau pointed out several barriers to implementing PAaM in routine practice, starting with the under-recognition of cirrhosis. Even if patients are identified, ensuring both clinicians and patients adhere to screening recommendations remains a challenge.
Finally, financial considerations may pose obstacles.
“If some payers cover the tool and others do not, it will be very difficult to implement,” Dr. Reau concluded.
Reau reported no conflicts of interest.
Nancy S. Reau, MD, AGAF, of RUSH University in Chicago, highlighted both the promise and challenges of the PAaM score for HCC risk stratification, emphasizing that current liver cancer screening strategies remain inadequate, with only about 25% of patients receiving guideline-recommended surveillance.
“An easy-to-apply cost effective tool could significantly improve screening strategies, which should lead to earlier identification of liver cancer—at a time when curative treatment options are available,” Reau said.
PAaM, however, may be impractical for routine use.
“A tool that classifies people into 3 different screening strategies and requires longitudinal applications and re-classification could add complexity,” she explained, predicting that “clinicians aren’t going to use it correctly.
Reau was particularly concerned about the need for repeated assessments over time.
“People change,” she said. “A low-risk categorization by PAaM at the age of 40 may no longer be relevant at 50 or 60 as liver disease progresses.”
Although the tool is “exciting,” Reau suggested that it is also “premature” until appropriate reclassification intervals are understood.
She also noted that some patients still develop HCC despite being considered low risk, including cases of HCC that develop in non-cirrhotic HCV infection or MASLD.
Beyond the above clinical considerations, Dr. Reau pointed out several barriers to implementing PAaM in routine practice, starting with the under-recognition of cirrhosis. Even if patients are identified, ensuring both clinicians and patients adhere to screening recommendations remains a challenge.
Finally, financial considerations may pose obstacles.
“If some payers cover the tool and others do not, it will be very difficult to implement,” Dr. Reau concluded.
Reau reported no conflicts of interest.
Nancy S. Reau, MD, AGAF, of RUSH University in Chicago, highlighted both the promise and challenges of the PAaM score for HCC risk stratification, emphasizing that current liver cancer screening strategies remain inadequate, with only about 25% of patients receiving guideline-recommended surveillance.
“An easy-to-apply cost effective tool could significantly improve screening strategies, which should lead to earlier identification of liver cancer—at a time when curative treatment options are available,” Reau said.
PAaM, however, may be impractical for routine use.
“A tool that classifies people into 3 different screening strategies and requires longitudinal applications and re-classification could add complexity,” she explained, predicting that “clinicians aren’t going to use it correctly.
Reau was particularly concerned about the need for repeated assessments over time.
“People change,” she said. “A low-risk categorization by PAaM at the age of 40 may no longer be relevant at 50 or 60 as liver disease progresses.”
Although the tool is “exciting,” Reau suggested that it is also “premature” until appropriate reclassification intervals are understood.
She also noted that some patients still develop HCC despite being considered low risk, including cases of HCC that develop in non-cirrhotic HCV infection or MASLD.
Beyond the above clinical considerations, Dr. Reau pointed out several barriers to implementing PAaM in routine practice, starting with the under-recognition of cirrhosis. Even if patients are identified, ensuring both clinicians and patients adhere to screening recommendations remains a challenge.
Finally, financial considerations may pose obstacles.
“If some payers cover the tool and others do not, it will be very difficult to implement,” Dr. Reau concluded.
Reau reported no conflicts of interest.
, based to a recent phase 3 biomarker validation study.
The Prognostic Liver Secretome Signature with Alpha-Fetoprotein plus Age, Male Sex, Albumin-Bilirubin, and Platelets (PAaM) score integrates both molecular and clinical variables to effectively classify cirrhosis patients by their risk of developing HCC, potentially sparing low-risk patients from unnecessary surveillance, lead author Naoto Fujiwara, MD, PhD, of the University of Texas Southwestern Medical Center, Dallas, and colleagues reported.
“Hepatocellular carcinoma risk stratification is an urgent unmet need for cost-effective screening and early detection in patients with cirrhosis,” the investigators wrote in Gastroenterology. “This study represents the largest and first phase 3 biomarker validation study that establishes an integrative molecular/clinical score, PAaM, for HCC risk stratification.”
The PAaM score combines an 8-protein prognostic liver secretome signature with traditional clinical variables, including alpha-fetoprotein (AFP) levels, age, sex, albumin-bilirubin levels, and platelet counts. The score stratifies patients into high-, intermediate-, and low-risk categories.
The PAaM score was validated using 2 independent prospective cohorts in the United States: the statewide Texas Hepatocellular Carcinoma Consortium (THCCC) and the nationwide Hepatocellular Carcinoma Early Detection Strategy (HEDS). Across both cohorts, 3,484 patients with cirrhosis were followed over time to assess the development of HCC.
In the Texas cohort, comprising 2,156 patients with cirrhosis, PAaM classified 19% of patients as high risk, 42% as intermediate risk, and 39% as low risk. The annual incidence of HCC was significantly different across these groups, with high-risk patients experiencing a 5.3% incidence rate, versus 2.7% for intermediate-risk patients and 0.6% for low-risk patients (P less than .001). Compared with those in the low-risk group, high-risk patients had sub-distribution hazard ratio (sHR) of 7.51 for developing HCC, while intermediate-risk patients had an sHR of 4.20.
In the nationwide HEDS cohort, which included 1,328 patients, PAaM similarly stratified 15% of participants as high risk, 41% as intermediate risk, and 44% as low risk. Annual HCC incidence rates were 6.2%, 1.8%, and 0.8% for high-, intermediate-, and low-risk patients, respectively (P less than .001). Among these patients, sub-distribution hazard ratios for HCC were 6.54 for high-risk patients and 1.77 for intermediate-risk patients, again underscoring the tool’s potential to identify individuals at elevated risk of developing HCC.
The PAaM score outperformed existing models like the aMAP score and the PLSec-AFP molecular marker alone, with consistent superiority across a diverse range of cirrhosis etiologies, including metabolic dysfunction–associated steatotic liver disease (MASLD), alcohol-associated liver disease (ALD), and cured hepatitis C virus (HCV) infection.
Based on these findings, high-risk patients might benefit from more intensive screening strategies, Fujiwara and colleagues suggested, while intermediate-risk patients could continue with semi-annual ultrasound-based screening. Of note, low-risk patients—comprising about 40% of the study population—could potentially avoid frequent screenings, thus reducing healthcare costs and minimizing unnecessary interventions.
“This represents a significant step toward the clinical translation of an individual risk-based HCC screening strategy to improve early HCC detection and reduce HCC mortality,” the investigators concluded.This study was supported by various the National Cancer Institute, Veterans Affairs, the Japan Society for the Promotion of Science, and others. The investigators disclosed additional relationships with Boston Scientific, Sirtex, Bayer, and others.
, based to a recent phase 3 biomarker validation study.
The Prognostic Liver Secretome Signature with Alpha-Fetoprotein plus Age, Male Sex, Albumin-Bilirubin, and Platelets (PAaM) score integrates both molecular and clinical variables to effectively classify cirrhosis patients by their risk of developing HCC, potentially sparing low-risk patients from unnecessary surveillance, lead author Naoto Fujiwara, MD, PhD, of the University of Texas Southwestern Medical Center, Dallas, and colleagues reported.
“Hepatocellular carcinoma risk stratification is an urgent unmet need for cost-effective screening and early detection in patients with cirrhosis,” the investigators wrote in Gastroenterology. “This study represents the largest and first phase 3 biomarker validation study that establishes an integrative molecular/clinical score, PAaM, for HCC risk stratification.”
The PAaM score combines an 8-protein prognostic liver secretome signature with traditional clinical variables, including alpha-fetoprotein (AFP) levels, age, sex, albumin-bilirubin levels, and platelet counts. The score stratifies patients into high-, intermediate-, and low-risk categories.
The PAaM score was validated using 2 independent prospective cohorts in the United States: the statewide Texas Hepatocellular Carcinoma Consortium (THCCC) and the nationwide Hepatocellular Carcinoma Early Detection Strategy (HEDS). Across both cohorts, 3,484 patients with cirrhosis were followed over time to assess the development of HCC.
In the Texas cohort, comprising 2,156 patients with cirrhosis, PAaM classified 19% of patients as high risk, 42% as intermediate risk, and 39% as low risk. The annual incidence of HCC was significantly different across these groups, with high-risk patients experiencing a 5.3% incidence rate, versus 2.7% for intermediate-risk patients and 0.6% for low-risk patients (P less than .001). Compared with those in the low-risk group, high-risk patients had sub-distribution hazard ratio (sHR) of 7.51 for developing HCC, while intermediate-risk patients had an sHR of 4.20.
In the nationwide HEDS cohort, which included 1,328 patients, PAaM similarly stratified 15% of participants as high risk, 41% as intermediate risk, and 44% as low risk. Annual HCC incidence rates were 6.2%, 1.8%, and 0.8% for high-, intermediate-, and low-risk patients, respectively (P less than .001). Among these patients, sub-distribution hazard ratios for HCC were 6.54 for high-risk patients and 1.77 for intermediate-risk patients, again underscoring the tool’s potential to identify individuals at elevated risk of developing HCC.
The PAaM score outperformed existing models like the aMAP score and the PLSec-AFP molecular marker alone, with consistent superiority across a diverse range of cirrhosis etiologies, including metabolic dysfunction–associated steatotic liver disease (MASLD), alcohol-associated liver disease (ALD), and cured hepatitis C virus (HCV) infection.
Based on these findings, high-risk patients might benefit from more intensive screening strategies, Fujiwara and colleagues suggested, while intermediate-risk patients could continue with semi-annual ultrasound-based screening. Of note, low-risk patients—comprising about 40% of the study population—could potentially avoid frequent screenings, thus reducing healthcare costs and minimizing unnecessary interventions.
“This represents a significant step toward the clinical translation of an individual risk-based HCC screening strategy to improve early HCC detection and reduce HCC mortality,” the investigators concluded.This study was supported by various the National Cancer Institute, Veterans Affairs, the Japan Society for the Promotion of Science, and others. The investigators disclosed additional relationships with Boston Scientific, Sirtex, Bayer, and others.
FROM GASTROENTEROLOGY
New Model Estimates Hepatocellular Carcinoma Risk in Patients With Chronic Hepatitis B
The model, called Revised REACH-B or reREACH-B, stems from cohort studies in Hong Kong, South Korea, and Taiwan, and looks at the nonlinear parabolic association between serum hepatitis B virus (HBV) DNA levels and HCC risk.
“Current clinical practice guidelines don’t advocate antiviral treatment for patients with CHB who don’t show elevated alanine aminotransferase (ALT) levels, even in those with high HBV viral loads,” said coauthor Young-Suk Lim, MD, PhD, professor of gastroenterology at the University of Ulsan College of Medicine and Asan Medical Center in Seoul, South Korea.
“This stance is rooted in the notion that patients in the immune-tolerant phase are at very low risk for developing HCC,” Lim said. “However, the immune-tolerant phase includes patients with HBV DNA levels who face the highest risk for HCC, and many patients with moderate HBV viremia fall into an undefined gray zone.”
The study was published in Annals of Internal Medicine.
Validating reREACH-B
During a course of CHB, HBV viral loads and HCC risks evolve over time because of viral replication and host immune responses, Lim explained. Most patients typically move to seroclearance and an “inactive hepatitis” phase, but about 10%-20% can progress to a “reactivation” phase, where HBV DNA levels and ALT levels increase, which can increase HCC risk as well.
In a previous cohort study in Taiwan, a prognostic model called Risk Estimation for HCC in CHB — or REACH-B — found the risk for HCC increases tenfold with increasing levels of HBV DNA up to 5 log10IU/mL in noncirrhotic patients with CHB, regardless of ALT levels. Another cohort study in South Korea found a nonlinear parabolic association between HCC risk and HBV DNA levels up to 9 log10 IU/mL, with the highest risks found for moderate HBV DNA levels around 6 log10 IU/mL.
In this study, Lim and colleagues developed a prognostic model to integrate the nonlinear relationship and validated it externally, as well as compared it with the previous REACH-B model. The Revised REACH-B model incorporates six variables: age, sex, platelet count, HBV DNA level, ALT, and hepatitis B e-antigen (HBeAg).
The study included 14,378 treatment-naive, noncirrhotic adults with CHB and serum ALT levels < two times the upper limit of normal for at least 1 year and serum hepatitis B surface antigen for at least 6 months. The internal validation cohort included 6,949 patients from Asan Medical Center, and the external validation cohort included 7,429 patients from previous studies in Hong Kong, South Korea, and Taiwan.
Among the Asan cohort, the mean age was 45 years, 29.9% were HBeAg positive, median HBV DNA levels were 3.1 log10 IU/mL, and the median ALT level was 25 U/L. In the external cohort, the mean age was 46 years, 21% were HBeAg positive, median HBV DNA levels were 3.4 log10 IU/mL, and the median ALT level was 20 U/L.
In the Asan cohort, 435 patients (6.3%) developed HCC during a median follow-up of 10 years. The annual HCC incidence rate was 0.63 per 100 person-years, and the estimated cumulative probability of developing HCC at 10 years was 6.4%.
In the external cohort, 467 patients (6.3%) developed HCC during a median follow-up of 12 years. The annual HCC incidence rate was 0.42 per 100 person-years, and the estimated cumulative probability of developing HCC at 10 years was 3.1%.
Overall, the association between HBV viral load and HCC risk was linear in the HBeAg-negative groups and inverse in the HBeAg-positive groups, with the association between HBV viral load and HCC risk showing a nonlinear parabolic pattern.
Across both cohorts, patients with HBV DNA levels between 5 and 6 log10 IU/mL had the highest risk for HCC in both the HBeAg-negative and HBeAg-positive groups, which was more than eight times higher than those HBV DNA levels ≤ 3 log10 IU/mL.
For internal validation, the Revised REACH-B model had a c-statistic of 0.844 and 5-year area under the curve of 0.864. For external validation across the three external cohorts, the reREACH-B had c-statistics of 0.804, 0.808, and 0.813, and 5-year area under the curve of 0.839, 0.860, and 0.865.
In addition, the revised model yielded a greater positive net benefit than the REACH-B model in the threshold probability range between 0% and 18%.
“These analyses indicate the reREACH-B model can be a valuable tool in clinical practice, aiding in timely management decisions,” Lim said.
Considering Prognostic Models
This study highlights the importance of recognizing that the association between HBV DNA viral load and HCC risk isn’t linear, said Norah Terrault, MD, chief of Gastroenterology and Hepatology at the Keck School of Medicine at the University of Southern California, Los Angeles.
“In contrast to most chronic liver diseases where liver cancer develops only among those with advanced fibrosis/cirrhosis, people with chronic hepatitis B are at risk prior to the development of cirrhosis,” she said. “Risk prediction scores for HCC can be a useful means of identifying those without cirrhosis who should be enrolled in HCC surveillance programs.”
For instance, patients with HBV DNA levels < 3 log10 IU/mL or > 8 log10 IU/mL don’t have an increased risk, Terrault noted. However, the highest risk group appears to be around 5-6 log10 IU/mL.
“Future risk prediction models should acknowledge that relationship in modeling HCC risk,” she said. “The re-REACH-B provides modest improvement over the REACH-B, but further validation of this score in more diverse cohorts is essential.”
The study received financial support from the Korean government and grants from the Patient-Centered Clinical Research Coordinating Center of the National Evidence-based Healthcare Collaborating Agency and the National R&D Program for Cancer Control through the National Cancer Center, which is funded by Korea’s Ministry of Health and Welfare. Lim and Terrault reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The model, called Revised REACH-B or reREACH-B, stems from cohort studies in Hong Kong, South Korea, and Taiwan, and looks at the nonlinear parabolic association between serum hepatitis B virus (HBV) DNA levels and HCC risk.
“Current clinical practice guidelines don’t advocate antiviral treatment for patients with CHB who don’t show elevated alanine aminotransferase (ALT) levels, even in those with high HBV viral loads,” said coauthor Young-Suk Lim, MD, PhD, professor of gastroenterology at the University of Ulsan College of Medicine and Asan Medical Center in Seoul, South Korea.
“This stance is rooted in the notion that patients in the immune-tolerant phase are at very low risk for developing HCC,” Lim said. “However, the immune-tolerant phase includes patients with HBV DNA levels who face the highest risk for HCC, and many patients with moderate HBV viremia fall into an undefined gray zone.”
The study was published in Annals of Internal Medicine.
Validating reREACH-B
During a course of CHB, HBV viral loads and HCC risks evolve over time because of viral replication and host immune responses, Lim explained. Most patients typically move to seroclearance and an “inactive hepatitis” phase, but about 10%-20% can progress to a “reactivation” phase, where HBV DNA levels and ALT levels increase, which can increase HCC risk as well.
In a previous cohort study in Taiwan, a prognostic model called Risk Estimation for HCC in CHB — or REACH-B — found the risk for HCC increases tenfold with increasing levels of HBV DNA up to 5 log10IU/mL in noncirrhotic patients with CHB, regardless of ALT levels. Another cohort study in South Korea found a nonlinear parabolic association between HCC risk and HBV DNA levels up to 9 log10 IU/mL, with the highest risks found for moderate HBV DNA levels around 6 log10 IU/mL.
In this study, Lim and colleagues developed a prognostic model to integrate the nonlinear relationship and validated it externally, as well as compared it with the previous REACH-B model. The Revised REACH-B model incorporates six variables: age, sex, platelet count, HBV DNA level, ALT, and hepatitis B e-antigen (HBeAg).
The study included 14,378 treatment-naive, noncirrhotic adults with CHB and serum ALT levels < two times the upper limit of normal for at least 1 year and serum hepatitis B surface antigen for at least 6 months. The internal validation cohort included 6,949 patients from Asan Medical Center, and the external validation cohort included 7,429 patients from previous studies in Hong Kong, South Korea, and Taiwan.
Among the Asan cohort, the mean age was 45 years, 29.9% were HBeAg positive, median HBV DNA levels were 3.1 log10 IU/mL, and the median ALT level was 25 U/L. In the external cohort, the mean age was 46 years, 21% were HBeAg positive, median HBV DNA levels were 3.4 log10 IU/mL, and the median ALT level was 20 U/L.
In the Asan cohort, 435 patients (6.3%) developed HCC during a median follow-up of 10 years. The annual HCC incidence rate was 0.63 per 100 person-years, and the estimated cumulative probability of developing HCC at 10 years was 6.4%.
In the external cohort, 467 patients (6.3%) developed HCC during a median follow-up of 12 years. The annual HCC incidence rate was 0.42 per 100 person-years, and the estimated cumulative probability of developing HCC at 10 years was 3.1%.
Overall, the association between HBV viral load and HCC risk was linear in the HBeAg-negative groups and inverse in the HBeAg-positive groups, with the association between HBV viral load and HCC risk showing a nonlinear parabolic pattern.
Across both cohorts, patients with HBV DNA levels between 5 and 6 log10 IU/mL had the highest risk for HCC in both the HBeAg-negative and HBeAg-positive groups, which was more than eight times higher than those HBV DNA levels ≤ 3 log10 IU/mL.
For internal validation, the Revised REACH-B model had a c-statistic of 0.844 and 5-year area under the curve of 0.864. For external validation across the three external cohorts, the reREACH-B had c-statistics of 0.804, 0.808, and 0.813, and 5-year area under the curve of 0.839, 0.860, and 0.865.
In addition, the revised model yielded a greater positive net benefit than the REACH-B model in the threshold probability range between 0% and 18%.
“These analyses indicate the reREACH-B model can be a valuable tool in clinical practice, aiding in timely management decisions,” Lim said.
Considering Prognostic Models
This study highlights the importance of recognizing that the association between HBV DNA viral load and HCC risk isn’t linear, said Norah Terrault, MD, chief of Gastroenterology and Hepatology at the Keck School of Medicine at the University of Southern California, Los Angeles.
“In contrast to most chronic liver diseases where liver cancer develops only among those with advanced fibrosis/cirrhosis, people with chronic hepatitis B are at risk prior to the development of cirrhosis,” she said. “Risk prediction scores for HCC can be a useful means of identifying those without cirrhosis who should be enrolled in HCC surveillance programs.”
For instance, patients with HBV DNA levels < 3 log10 IU/mL or > 8 log10 IU/mL don’t have an increased risk, Terrault noted. However, the highest risk group appears to be around 5-6 log10 IU/mL.
“Future risk prediction models should acknowledge that relationship in modeling HCC risk,” she said. “The re-REACH-B provides modest improvement over the REACH-B, but further validation of this score in more diverse cohorts is essential.”
The study received financial support from the Korean government and grants from the Patient-Centered Clinical Research Coordinating Center of the National Evidence-based Healthcare Collaborating Agency and the National R&D Program for Cancer Control through the National Cancer Center, which is funded by Korea’s Ministry of Health and Welfare. Lim and Terrault reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The model, called Revised REACH-B or reREACH-B, stems from cohort studies in Hong Kong, South Korea, and Taiwan, and looks at the nonlinear parabolic association between serum hepatitis B virus (HBV) DNA levels and HCC risk.
“Current clinical practice guidelines don’t advocate antiviral treatment for patients with CHB who don’t show elevated alanine aminotransferase (ALT) levels, even in those with high HBV viral loads,” said coauthor Young-Suk Lim, MD, PhD, professor of gastroenterology at the University of Ulsan College of Medicine and Asan Medical Center in Seoul, South Korea.
“This stance is rooted in the notion that patients in the immune-tolerant phase are at very low risk for developing HCC,” Lim said. “However, the immune-tolerant phase includes patients with HBV DNA levels who face the highest risk for HCC, and many patients with moderate HBV viremia fall into an undefined gray zone.”
The study was published in Annals of Internal Medicine.
Validating reREACH-B
During a course of CHB, HBV viral loads and HCC risks evolve over time because of viral replication and host immune responses, Lim explained. Most patients typically move to seroclearance and an “inactive hepatitis” phase, but about 10%-20% can progress to a “reactivation” phase, where HBV DNA levels and ALT levels increase, which can increase HCC risk as well.
In a previous cohort study in Taiwan, a prognostic model called Risk Estimation for HCC in CHB — or REACH-B — found the risk for HCC increases tenfold with increasing levels of HBV DNA up to 5 log10IU/mL in noncirrhotic patients with CHB, regardless of ALT levels. Another cohort study in South Korea found a nonlinear parabolic association between HCC risk and HBV DNA levels up to 9 log10 IU/mL, with the highest risks found for moderate HBV DNA levels around 6 log10 IU/mL.
In this study, Lim and colleagues developed a prognostic model to integrate the nonlinear relationship and validated it externally, as well as compared it with the previous REACH-B model. The Revised REACH-B model incorporates six variables: age, sex, platelet count, HBV DNA level, ALT, and hepatitis B e-antigen (HBeAg).
The study included 14,378 treatment-naive, noncirrhotic adults with CHB and serum ALT levels < two times the upper limit of normal for at least 1 year and serum hepatitis B surface antigen for at least 6 months. The internal validation cohort included 6,949 patients from Asan Medical Center, and the external validation cohort included 7,429 patients from previous studies in Hong Kong, South Korea, and Taiwan.
Among the Asan cohort, the mean age was 45 years, 29.9% were HBeAg positive, median HBV DNA levels were 3.1 log10 IU/mL, and the median ALT level was 25 U/L. In the external cohort, the mean age was 46 years, 21% were HBeAg positive, median HBV DNA levels were 3.4 log10 IU/mL, and the median ALT level was 20 U/L.
In the Asan cohort, 435 patients (6.3%) developed HCC during a median follow-up of 10 years. The annual HCC incidence rate was 0.63 per 100 person-years, and the estimated cumulative probability of developing HCC at 10 years was 6.4%.
In the external cohort, 467 patients (6.3%) developed HCC during a median follow-up of 12 years. The annual HCC incidence rate was 0.42 per 100 person-years, and the estimated cumulative probability of developing HCC at 10 years was 3.1%.
Overall, the association between HBV viral load and HCC risk was linear in the HBeAg-negative groups and inverse in the HBeAg-positive groups, with the association between HBV viral load and HCC risk showing a nonlinear parabolic pattern.
Across both cohorts, patients with HBV DNA levels between 5 and 6 log10 IU/mL had the highest risk for HCC in both the HBeAg-negative and HBeAg-positive groups, which was more than eight times higher than those HBV DNA levels ≤ 3 log10 IU/mL.
For internal validation, the Revised REACH-B model had a c-statistic of 0.844 and 5-year area under the curve of 0.864. For external validation across the three external cohorts, the reREACH-B had c-statistics of 0.804, 0.808, and 0.813, and 5-year area under the curve of 0.839, 0.860, and 0.865.
In addition, the revised model yielded a greater positive net benefit than the REACH-B model in the threshold probability range between 0% and 18%.
“These analyses indicate the reREACH-B model can be a valuable tool in clinical practice, aiding in timely management decisions,” Lim said.
Considering Prognostic Models
This study highlights the importance of recognizing that the association between HBV DNA viral load and HCC risk isn’t linear, said Norah Terrault, MD, chief of Gastroenterology and Hepatology at the Keck School of Medicine at the University of Southern California, Los Angeles.
“In contrast to most chronic liver diseases where liver cancer develops only among those with advanced fibrosis/cirrhosis, people with chronic hepatitis B are at risk prior to the development of cirrhosis,” she said. “Risk prediction scores for HCC can be a useful means of identifying those without cirrhosis who should be enrolled in HCC surveillance programs.”
For instance, patients with HBV DNA levels < 3 log10 IU/mL or > 8 log10 IU/mL don’t have an increased risk, Terrault noted. However, the highest risk group appears to be around 5-6 log10 IU/mL.
“Future risk prediction models should acknowledge that relationship in modeling HCC risk,” she said. “The re-REACH-B provides modest improvement over the REACH-B, but further validation of this score in more diverse cohorts is essential.”
The study received financial support from the Korean government and grants from the Patient-Centered Clinical Research Coordinating Center of the National Evidence-based Healthcare Collaborating Agency and the National R&D Program for Cancer Control through the National Cancer Center, which is funded by Korea’s Ministry of Health and Welfare. Lim and Terrault reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE