User login
Hospital readmission remains common for teens with nonfatal drug overdose
Approximately 1 in 5 adolescents hospitalized for nonfatal drug overdoses were readmitted within 6 months, based on data from more than 12,000 individuals.
Previous studies suggest that many adolescents fail to receive timely treatment for addiction after a nonfatal overdose, but the rates of hospital readmission in this population have not been examined, according to Julie Gaither, PhD, of Yale University, New Haven, Conn.
In a study presented at the annual meeting of the Pediatric Academic Societies, Dr. Gaither and her colleague, John M. Leventhal, MD, also of Yale University, used data from the 2016 Nationwide Readmissions Database to examine incidence and recurrent hospitalizations for nonfatal drug overdoses in adolescents. The study population included 12,952 patients aged 11-21 years who were admitted to a hospital after a nonfatal drug overdose in 2016. Of these, 15% were younger than 15 years, and 52.1% were females.
Overall, 76.2% of the overdoses involved opioids; 77.9% involved a prescription opioid, 15.3% involved heroin, and 7.9% involved fentanyl.
Across all drug overdoses, the majority (86.5%) were attributed to accidental intent and 11.8% were attributed to self-harm. Notably, females were nearly four times more likely than males to attempt suicide (odds ratio, 3.57). After the initial hospitalization, 79.3% of the patients were discharged home, and 11.5% went to a short-term care facility.
The 6-month hospital readmission rate was 21.4%. Of the patients readmitted for any cause, 18.2% of readmissions were for recurrent overdoses, and 92.1% of these were attributed to opioids.
The median cost of the initial hospital admission was $23,705 (ranging from $11,902 to $54,682) and the median cost of the first readmission was $25,416 (ranging from $13,905 to $48,810). In 42.1% of all hospitalizations, Medicaid was the primary payer.
The study findings were limited by the relatively high number of Medicaid patients, which may limit generalizability, but is strengthened by the large sample size.
The findings highlight not only the need for prevention efforts to limit opioid use among adolescents, but also “speak to the need for timely evidenced-based addiction treatment and appropriate follow-up care for teens following hospitalization for a nonfatal drug overdose,” the researchers wrote in their abstract.
Potential for postpandemic surge in drug use
Interestingly, some recent research has shown a decline in teens’ substance use during the pandemic, Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“However, as the world begins ‘opening up’ again, I suspect rates of drug use will rise – especially with the significant burden of mental health issues adolescents have struggled with during the last few years,” said Dr. Curran, who was not involved with the current study.
“Sadly, I am not surprised by this study’s findings. Too often, teens with substance abuse issues are not connected to effective, evidenced-based treatment, and for those who are, the wait list can be long,” she said.
“Teens who are misusing drugs – either to get high or to attempt suicide – who are admitted for nonfatal overdose have a high rate of readmission for recurrent drug overdose,” Dr. Curran said. “This high rate of readmission has serious social and financial implications,” she added. “This study is part of a growing body of literature that supports the importance of getting adolescents into effective, evidence-based substance abuse treatment, such as medication-assisted treatment in opioid abuse. However, we also should be advocating for improved funding for and access to these treatments for all individuals.”
The study received no outside funding. Dr. Gaither had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Approximately 1 in 5 adolescents hospitalized for nonfatal drug overdoses were readmitted within 6 months, based on data from more than 12,000 individuals.
Previous studies suggest that many adolescents fail to receive timely treatment for addiction after a nonfatal overdose, but the rates of hospital readmission in this population have not been examined, according to Julie Gaither, PhD, of Yale University, New Haven, Conn.
In a study presented at the annual meeting of the Pediatric Academic Societies, Dr. Gaither and her colleague, John M. Leventhal, MD, also of Yale University, used data from the 2016 Nationwide Readmissions Database to examine incidence and recurrent hospitalizations for nonfatal drug overdoses in adolescents. The study population included 12,952 patients aged 11-21 years who were admitted to a hospital after a nonfatal drug overdose in 2016. Of these, 15% were younger than 15 years, and 52.1% were females.
Overall, 76.2% of the overdoses involved opioids; 77.9% involved a prescription opioid, 15.3% involved heroin, and 7.9% involved fentanyl.
Across all drug overdoses, the majority (86.5%) were attributed to accidental intent and 11.8% were attributed to self-harm. Notably, females were nearly four times more likely than males to attempt suicide (odds ratio, 3.57). After the initial hospitalization, 79.3% of the patients were discharged home, and 11.5% went to a short-term care facility.
The 6-month hospital readmission rate was 21.4%. Of the patients readmitted for any cause, 18.2% of readmissions were for recurrent overdoses, and 92.1% of these were attributed to opioids.
The median cost of the initial hospital admission was $23,705 (ranging from $11,902 to $54,682) and the median cost of the first readmission was $25,416 (ranging from $13,905 to $48,810). In 42.1% of all hospitalizations, Medicaid was the primary payer.
The study findings were limited by the relatively high number of Medicaid patients, which may limit generalizability, but is strengthened by the large sample size.
The findings highlight not only the need for prevention efforts to limit opioid use among adolescents, but also “speak to the need for timely evidenced-based addiction treatment and appropriate follow-up care for teens following hospitalization for a nonfatal drug overdose,” the researchers wrote in their abstract.
Potential for postpandemic surge in drug use
Interestingly, some recent research has shown a decline in teens’ substance use during the pandemic, Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“However, as the world begins ‘opening up’ again, I suspect rates of drug use will rise – especially with the significant burden of mental health issues adolescents have struggled with during the last few years,” said Dr. Curran, who was not involved with the current study.
“Sadly, I am not surprised by this study’s findings. Too often, teens with substance abuse issues are not connected to effective, evidenced-based treatment, and for those who are, the wait list can be long,” she said.
“Teens who are misusing drugs – either to get high or to attempt suicide – who are admitted for nonfatal overdose have a high rate of readmission for recurrent drug overdose,” Dr. Curran said. “This high rate of readmission has serious social and financial implications,” she added. “This study is part of a growing body of literature that supports the importance of getting adolescents into effective, evidence-based substance abuse treatment, such as medication-assisted treatment in opioid abuse. However, we also should be advocating for improved funding for and access to these treatments for all individuals.”
The study received no outside funding. Dr. Gaither had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Approximately 1 in 5 adolescents hospitalized for nonfatal drug overdoses were readmitted within 6 months, based on data from more than 12,000 individuals.
Previous studies suggest that many adolescents fail to receive timely treatment for addiction after a nonfatal overdose, but the rates of hospital readmission in this population have not been examined, according to Julie Gaither, PhD, of Yale University, New Haven, Conn.
In a study presented at the annual meeting of the Pediatric Academic Societies, Dr. Gaither and her colleague, John M. Leventhal, MD, also of Yale University, used data from the 2016 Nationwide Readmissions Database to examine incidence and recurrent hospitalizations for nonfatal drug overdoses in adolescents. The study population included 12,952 patients aged 11-21 years who were admitted to a hospital after a nonfatal drug overdose in 2016. Of these, 15% were younger than 15 years, and 52.1% were females.
Overall, 76.2% of the overdoses involved opioids; 77.9% involved a prescription opioid, 15.3% involved heroin, and 7.9% involved fentanyl.
Across all drug overdoses, the majority (86.5%) were attributed to accidental intent and 11.8% were attributed to self-harm. Notably, females were nearly four times more likely than males to attempt suicide (odds ratio, 3.57). After the initial hospitalization, 79.3% of the patients were discharged home, and 11.5% went to a short-term care facility.
The 6-month hospital readmission rate was 21.4%. Of the patients readmitted for any cause, 18.2% of readmissions were for recurrent overdoses, and 92.1% of these were attributed to opioids.
The median cost of the initial hospital admission was $23,705 (ranging from $11,902 to $54,682) and the median cost of the first readmission was $25,416 (ranging from $13,905 to $48,810). In 42.1% of all hospitalizations, Medicaid was the primary payer.
The study findings were limited by the relatively high number of Medicaid patients, which may limit generalizability, but is strengthened by the large sample size.
The findings highlight not only the need for prevention efforts to limit opioid use among adolescents, but also “speak to the need for timely evidenced-based addiction treatment and appropriate follow-up care for teens following hospitalization for a nonfatal drug overdose,” the researchers wrote in their abstract.
Potential for postpandemic surge in drug use
Interestingly, some recent research has shown a decline in teens’ substance use during the pandemic, Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview.
“However, as the world begins ‘opening up’ again, I suspect rates of drug use will rise – especially with the significant burden of mental health issues adolescents have struggled with during the last few years,” said Dr. Curran, who was not involved with the current study.
“Sadly, I am not surprised by this study’s findings. Too often, teens with substance abuse issues are not connected to effective, evidenced-based treatment, and for those who are, the wait list can be long,” she said.
“Teens who are misusing drugs – either to get high or to attempt suicide – who are admitted for nonfatal overdose have a high rate of readmission for recurrent drug overdose,” Dr. Curran said. “This high rate of readmission has serious social and financial implications,” she added. “This study is part of a growing body of literature that supports the importance of getting adolescents into effective, evidence-based substance abuse treatment, such as medication-assisted treatment in opioid abuse. However, we also should be advocating for improved funding for and access to these treatments for all individuals.”
The study received no outside funding. Dr. Gaither had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM PAS 2022
Nap length linked to cognitive changes
No wonder we feel worse after naps
Some of us have hectic schedules that may make a nap feel more necessary. It’s common knowledge that naps shouldn’t be too long – maybe 20 minutes or so – but if you frequently take 3-hour naps and wake up thinking you’re late for school even though you’re 47 and have your PhD, this LOTME is for you.
Studies have shown that there is a link between napping during the day and Alzheimer’s/cognitive decline, but now we’ve got a double whammy for you: Longer and more frequent napping is linked to worse cognition after a year, and in turn, those with cognitive decline and Alzheimer’s are known to nap longer and more frequently during the day.
“We now know that the pathology related to cognitive decline can cause other changes in function,” he said. “It’s really a multisystem disorder, also including difficulty sleeping, changes in movement, changes in body composition, depression symptoms, behavioral changes, etc.,” coauthor Aron Buchman, MD, said in a statement from Rush University Medical Center.
The investigators monitored 1,400 patients over the course of 14 years with wrist bracelets that recorded when a person was not active during the day and considered that a nap.
At the beginning of the study, 75% of the study subjects had no cognitive impairment, 19.5% had some cognitive impairment, and approximately 4% had Alzheimer’s. Napping during the day only increased about 11 minutes a year for those with no signs of cognitive impairment, but those who showed significantly more signs of cognitive decline doubled their nap time and those actually diagnosed with Alzheimer’s tripled theirs.
The investigators did not imply that napping causes Alzheimer’s, but they noted that people who are older and nap more than an hour a day are 40% more likely to be at risk. It is something to consider and monitor.
Sometimes, after all, a nap seems like the best idea ever, but more often than not we wake up feeling 10 times worse. Our bodies may be giving us a heads up.
Pokemon Go away depression
The summer of 2016 was a great time if you happened to be a fan of Pokemon. Which is quite a lot of people. For almost 20 years millions have enjoyed the games and animated series, but Pokemon Go brought the thrill of catching Pokemon to life in a whole new way. For the first time, you could go out into the world and pretend you were a real Pokemon trainer, and everywhere you went, there would be others like you.
The ability to chase after Pikachu and Charizard in real life (well, augmented reality, but close enough) seemed to bring people a lot of joy, but seemed is never good enough for science. Can’t have anecdotes, we need data! So researchers at the London School of Economics and Political Science conducted a study into how Pokemon Go affected local Internet search rates of depression as the game was released slowly around the world.
Through analyzing Google Trend data of words like “depression,” “anxiety,” and “stress,” the researchers found that the release of Pokemon Go was significantly associated with a noticeable, though short-term, drop in depression-related Internet searches. Location-based augmented reality games may alleviate symptoms of mild depression, the researchers said, as they encourage physical activity, face-to-face socialization, and exposure to nature, though they added that simply going outside is likely not enough to combat clinical cases of severe depression.
Still, augmented reality games represent a viable target for public health investment, since they’re easy to use and inexpensive to make. That said, we’re not sure we want the FDA or CDC making a new Pokemon Go game. They’d probably end up filling the streets with Mr. Mime. And no one would leave their house for that.
And now a word from our sponsor
How many times has this happened to you? You need to repair a jet engine, inspect a nuclear reactor cooling system, AND perform bowel surgery, but you can’t carry around all the heavy, old-fashioned tools needed for those jobs.
Well, we’ve got one tool that can do it all! And that tool is a snake. No, it’s a robot.
It’s both! It’s the COntinuum roBot for Remote Applications. COBRA is the robot that looks like a snake! A snake that’s 5 meters long but only as thick as a pencil (about 9 mm in diameter). A robot with “extraordinary manoeuvrability and responsiveness due to … a compliant-joint structure and multiple continuous sections that enable it to bend at around 90 degrees,” according to the team at the University of Nottingham (England) that developed it.
COBRA comes equipped with a stereovision camera and a miniature cutting tool to perform complex industrial repair, but other devices can be interchanged for possible medical use.
COBRA and its joystick-like controller were designed to be easy to use. Dr. Oladejo Olaleye, the ear, nose, and throat and robotic surgeon at University Hospitals of Leicester who is directing its surgical development, was able to use COBRA on a dummy after just 5 minutes of training. He called it “the future of diagnostic endoscopy and therapeutic surgery.”
Don’t be the last aircraft engineer/nuclear technician/surgeon on your block to have this ultraslender, ultramaneuverable reptilian repair robot. Get your COBRA now! Operators are standing by.
Disclaimer: Robot is still under development and not yet on sale.
Rule, (worm) Britannia!
As long as there have been people, there have been parasitic worms living in their guts. Helminth infection is a continuing and largely ignored crisis in poor, tropical nations, though worm-based diseases have been basically eliminated from wealthier countries.
This wasn’t always the case, however, as a study published in PLOS Neglected Tropical Diseases (now there’s a specific topic) has found. The researchers detail the glorious history of helminth infestation in the United Kingdom from the Victorian era all the way back to prehistory, scouring hundreds of skeletons found in 17 sites across the country for eggs, which can remain intact for thousands of years.
The researchers found that two eras in particular had very high rates of infection. Unsurprisingly, the late medieval era was one of them, but the other is less obvious. The Romans were famous for their hygiene, their baths, and their plumbing, but maybe they also should be famous for the abundance of worms in their bellies. That doesn’t make sense at first: Shouldn’t good hygiene lower infection? The benefits of a good sewer system, however, are lessened when the waste containing said infectious organisms is used to fertilize crops. Recycling is generally a good thing, but less so when you’re recycling parasitic worms.
Curiously, of the three sites from the industrial age, only the one in London had high levels of worm infestation. Considering how dirty and cramped 19th-century British cities were, one might expect disease to run rampant (tuberculosis certainly did), but the sites in Oxford and Birmingham were almost devoid of worms. The researchers theorized that this was because of access to clean well water. Or maybe worms just have a thing for London. [Editor’s note: It’s probably not that.]
No wonder we feel worse after naps
Some of us have hectic schedules that may make a nap feel more necessary. It’s common knowledge that naps shouldn’t be too long – maybe 20 minutes or so – but if you frequently take 3-hour naps and wake up thinking you’re late for school even though you’re 47 and have your PhD, this LOTME is for you.
Studies have shown that there is a link between napping during the day and Alzheimer’s/cognitive decline, but now we’ve got a double whammy for you: Longer and more frequent napping is linked to worse cognition after a year, and in turn, those with cognitive decline and Alzheimer’s are known to nap longer and more frequently during the day.
“We now know that the pathology related to cognitive decline can cause other changes in function,” he said. “It’s really a multisystem disorder, also including difficulty sleeping, changes in movement, changes in body composition, depression symptoms, behavioral changes, etc.,” coauthor Aron Buchman, MD, said in a statement from Rush University Medical Center.
The investigators monitored 1,400 patients over the course of 14 years with wrist bracelets that recorded when a person was not active during the day and considered that a nap.
At the beginning of the study, 75% of the study subjects had no cognitive impairment, 19.5% had some cognitive impairment, and approximately 4% had Alzheimer’s. Napping during the day only increased about 11 minutes a year for those with no signs of cognitive impairment, but those who showed significantly more signs of cognitive decline doubled their nap time and those actually diagnosed with Alzheimer’s tripled theirs.
The investigators did not imply that napping causes Alzheimer’s, but they noted that people who are older and nap more than an hour a day are 40% more likely to be at risk. It is something to consider and monitor.
Sometimes, after all, a nap seems like the best idea ever, but more often than not we wake up feeling 10 times worse. Our bodies may be giving us a heads up.
Pokemon Go away depression
The summer of 2016 was a great time if you happened to be a fan of Pokemon. Which is quite a lot of people. For almost 20 years millions have enjoyed the games and animated series, but Pokemon Go brought the thrill of catching Pokemon to life in a whole new way. For the first time, you could go out into the world and pretend you were a real Pokemon trainer, and everywhere you went, there would be others like you.
The ability to chase after Pikachu and Charizard in real life (well, augmented reality, but close enough) seemed to bring people a lot of joy, but seemed is never good enough for science. Can’t have anecdotes, we need data! So researchers at the London School of Economics and Political Science conducted a study into how Pokemon Go affected local Internet search rates of depression as the game was released slowly around the world.
Through analyzing Google Trend data of words like “depression,” “anxiety,” and “stress,” the researchers found that the release of Pokemon Go was significantly associated with a noticeable, though short-term, drop in depression-related Internet searches. Location-based augmented reality games may alleviate symptoms of mild depression, the researchers said, as they encourage physical activity, face-to-face socialization, and exposure to nature, though they added that simply going outside is likely not enough to combat clinical cases of severe depression.
Still, augmented reality games represent a viable target for public health investment, since they’re easy to use and inexpensive to make. That said, we’re not sure we want the FDA or CDC making a new Pokemon Go game. They’d probably end up filling the streets with Mr. Mime. And no one would leave their house for that.
And now a word from our sponsor
How many times has this happened to you? You need to repair a jet engine, inspect a nuclear reactor cooling system, AND perform bowel surgery, but you can’t carry around all the heavy, old-fashioned tools needed for those jobs.
Well, we’ve got one tool that can do it all! And that tool is a snake. No, it’s a robot.
It’s both! It’s the COntinuum roBot for Remote Applications. COBRA is the robot that looks like a snake! A snake that’s 5 meters long but only as thick as a pencil (about 9 mm in diameter). A robot with “extraordinary manoeuvrability and responsiveness due to … a compliant-joint structure and multiple continuous sections that enable it to bend at around 90 degrees,” according to the team at the University of Nottingham (England) that developed it.
COBRA comes equipped with a stereovision camera and a miniature cutting tool to perform complex industrial repair, but other devices can be interchanged for possible medical use.
COBRA and its joystick-like controller were designed to be easy to use. Dr. Oladejo Olaleye, the ear, nose, and throat and robotic surgeon at University Hospitals of Leicester who is directing its surgical development, was able to use COBRA on a dummy after just 5 minutes of training. He called it “the future of diagnostic endoscopy and therapeutic surgery.”
Don’t be the last aircraft engineer/nuclear technician/surgeon on your block to have this ultraslender, ultramaneuverable reptilian repair robot. Get your COBRA now! Operators are standing by.
Disclaimer: Robot is still under development and not yet on sale.
Rule, (worm) Britannia!
As long as there have been people, there have been parasitic worms living in their guts. Helminth infection is a continuing and largely ignored crisis in poor, tropical nations, though worm-based diseases have been basically eliminated from wealthier countries.
This wasn’t always the case, however, as a study published in PLOS Neglected Tropical Diseases (now there’s a specific topic) has found. The researchers detail the glorious history of helminth infestation in the United Kingdom from the Victorian era all the way back to prehistory, scouring hundreds of skeletons found in 17 sites across the country for eggs, which can remain intact for thousands of years.
The researchers found that two eras in particular had very high rates of infection. Unsurprisingly, the late medieval era was one of them, but the other is less obvious. The Romans were famous for their hygiene, their baths, and their plumbing, but maybe they also should be famous for the abundance of worms in their bellies. That doesn’t make sense at first: Shouldn’t good hygiene lower infection? The benefits of a good sewer system, however, are lessened when the waste containing said infectious organisms is used to fertilize crops. Recycling is generally a good thing, but less so when you’re recycling parasitic worms.
Curiously, of the three sites from the industrial age, only the one in London had high levels of worm infestation. Considering how dirty and cramped 19th-century British cities were, one might expect disease to run rampant (tuberculosis certainly did), but the sites in Oxford and Birmingham were almost devoid of worms. The researchers theorized that this was because of access to clean well water. Or maybe worms just have a thing for London. [Editor’s note: It’s probably not that.]
No wonder we feel worse after naps
Some of us have hectic schedules that may make a nap feel more necessary. It’s common knowledge that naps shouldn’t be too long – maybe 20 minutes or so – but if you frequently take 3-hour naps and wake up thinking you’re late for school even though you’re 47 and have your PhD, this LOTME is for you.
Studies have shown that there is a link between napping during the day and Alzheimer’s/cognitive decline, but now we’ve got a double whammy for you: Longer and more frequent napping is linked to worse cognition after a year, and in turn, those with cognitive decline and Alzheimer’s are known to nap longer and more frequently during the day.
“We now know that the pathology related to cognitive decline can cause other changes in function,” he said. “It’s really a multisystem disorder, also including difficulty sleeping, changes in movement, changes in body composition, depression symptoms, behavioral changes, etc.,” coauthor Aron Buchman, MD, said in a statement from Rush University Medical Center.
The investigators monitored 1,400 patients over the course of 14 years with wrist bracelets that recorded when a person was not active during the day and considered that a nap.
At the beginning of the study, 75% of the study subjects had no cognitive impairment, 19.5% had some cognitive impairment, and approximately 4% had Alzheimer’s. Napping during the day only increased about 11 minutes a year for those with no signs of cognitive impairment, but those who showed significantly more signs of cognitive decline doubled their nap time and those actually diagnosed with Alzheimer’s tripled theirs.
The investigators did not imply that napping causes Alzheimer’s, but they noted that people who are older and nap more than an hour a day are 40% more likely to be at risk. It is something to consider and monitor.
Sometimes, after all, a nap seems like the best idea ever, but more often than not we wake up feeling 10 times worse. Our bodies may be giving us a heads up.
Pokemon Go away depression
The summer of 2016 was a great time if you happened to be a fan of Pokemon. Which is quite a lot of people. For almost 20 years millions have enjoyed the games and animated series, but Pokemon Go brought the thrill of catching Pokemon to life in a whole new way. For the first time, you could go out into the world and pretend you were a real Pokemon trainer, and everywhere you went, there would be others like you.
The ability to chase after Pikachu and Charizard in real life (well, augmented reality, but close enough) seemed to bring people a lot of joy, but seemed is never good enough for science. Can’t have anecdotes, we need data! So researchers at the London School of Economics and Political Science conducted a study into how Pokemon Go affected local Internet search rates of depression as the game was released slowly around the world.
Through analyzing Google Trend data of words like “depression,” “anxiety,” and “stress,” the researchers found that the release of Pokemon Go was significantly associated with a noticeable, though short-term, drop in depression-related Internet searches. Location-based augmented reality games may alleviate symptoms of mild depression, the researchers said, as they encourage physical activity, face-to-face socialization, and exposure to nature, though they added that simply going outside is likely not enough to combat clinical cases of severe depression.
Still, augmented reality games represent a viable target for public health investment, since they’re easy to use and inexpensive to make. That said, we’re not sure we want the FDA or CDC making a new Pokemon Go game. They’d probably end up filling the streets with Mr. Mime. And no one would leave their house for that.
And now a word from our sponsor
How many times has this happened to you? You need to repair a jet engine, inspect a nuclear reactor cooling system, AND perform bowel surgery, but you can’t carry around all the heavy, old-fashioned tools needed for those jobs.
Well, we’ve got one tool that can do it all! And that tool is a snake. No, it’s a robot.
It’s both! It’s the COntinuum roBot for Remote Applications. COBRA is the robot that looks like a snake! A snake that’s 5 meters long but only as thick as a pencil (about 9 mm in diameter). A robot with “extraordinary manoeuvrability and responsiveness due to … a compliant-joint structure and multiple continuous sections that enable it to bend at around 90 degrees,” according to the team at the University of Nottingham (England) that developed it.
COBRA comes equipped with a stereovision camera and a miniature cutting tool to perform complex industrial repair, but other devices can be interchanged for possible medical use.
COBRA and its joystick-like controller were designed to be easy to use. Dr. Oladejo Olaleye, the ear, nose, and throat and robotic surgeon at University Hospitals of Leicester who is directing its surgical development, was able to use COBRA on a dummy after just 5 minutes of training. He called it “the future of diagnostic endoscopy and therapeutic surgery.”
Don’t be the last aircraft engineer/nuclear technician/surgeon on your block to have this ultraslender, ultramaneuverable reptilian repair robot. Get your COBRA now! Operators are standing by.
Disclaimer: Robot is still under development and not yet on sale.
Rule, (worm) Britannia!
As long as there have been people, there have been parasitic worms living in their guts. Helminth infection is a continuing and largely ignored crisis in poor, tropical nations, though worm-based diseases have been basically eliminated from wealthier countries.
This wasn’t always the case, however, as a study published in PLOS Neglected Tropical Diseases (now there’s a specific topic) has found. The researchers detail the glorious history of helminth infestation in the United Kingdom from the Victorian era all the way back to prehistory, scouring hundreds of skeletons found in 17 sites across the country for eggs, which can remain intact for thousands of years.
The researchers found that two eras in particular had very high rates of infection. Unsurprisingly, the late medieval era was one of them, but the other is less obvious. The Romans were famous for their hygiene, their baths, and their plumbing, but maybe they also should be famous for the abundance of worms in their bellies. That doesn’t make sense at first: Shouldn’t good hygiene lower infection? The benefits of a good sewer system, however, are lessened when the waste containing said infectious organisms is used to fertilize crops. Recycling is generally a good thing, but less so when you’re recycling parasitic worms.
Curiously, of the three sites from the industrial age, only the one in London had high levels of worm infestation. Considering how dirty and cramped 19th-century British cities were, one might expect disease to run rampant (tuberculosis certainly did), but the sites in Oxford and Birmingham were almost devoid of worms. The researchers theorized that this was because of access to clean well water. Or maybe worms just have a thing for London. [Editor’s note: It’s probably not that.]
Facial Follicular Spicules: A Rare Cutaneous Presentation of Trichodysplasia Spinulosa
To the Editor:
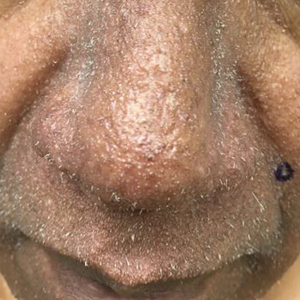
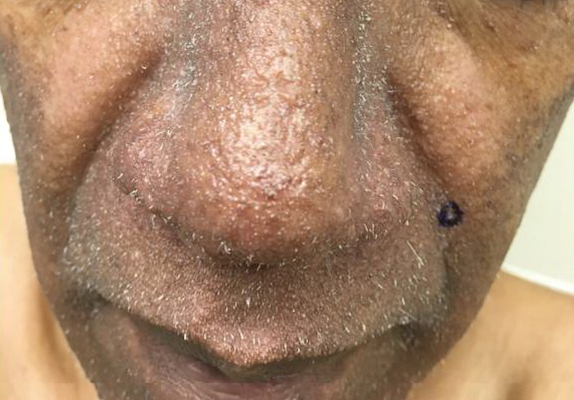
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.
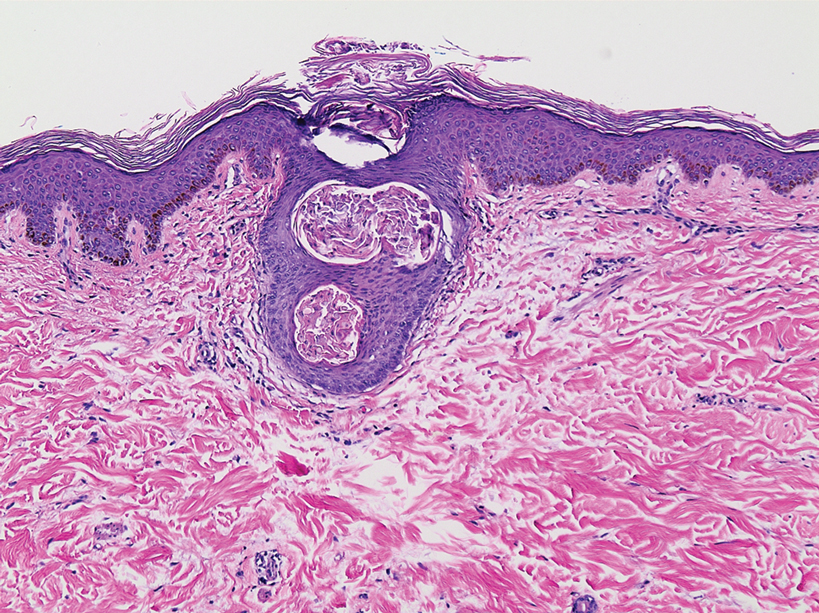
A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.
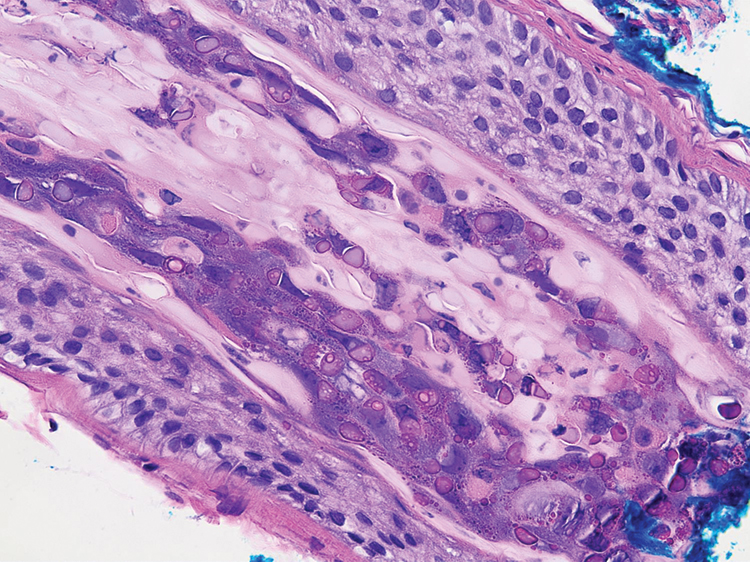
Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.
Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5
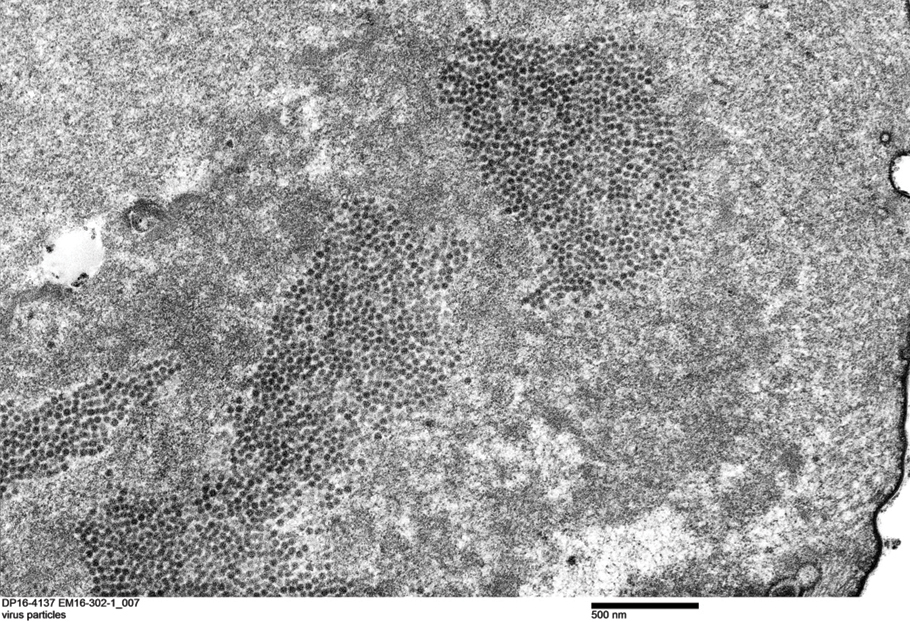
Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.

Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.

A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.

Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.

Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5

Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.

Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.

A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.

Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.

Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5

Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.

Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
Practice Points
- Trichodysplasia spinulosa (TS) is a rare skin disease caused by primary TS-associated polyomavirus (TSPyV) infecting follicular keratinocytes in immunocompromised patients.
- Trichodysplasia spinulosa typically presents with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes.
- The viral protein can be verified through immunohistochemistry TSPyV major capsid protein VP1 staining or can be visualized on transmission electron microscopy.
- Follicular spicules of multiple myeloma should be ruled out before initiating treatment with cidofovir gel 1% for TS.
NB-UVB phototherapy plays a key role in psoriasis treatment, expert says
BOSTON – In 2012, about 50% of patients receiving phototherapy at Brigham and Women’s Hospital in Boston were being treated for psoriasis. A decade later, that proportion has dropped to 20%.
Several factors have contributed to this trend, namely, the development of biologics, the COVID-19 pandemic, “and the rise of home phototherapy options,” Elizabeth A. Buzney, MD, codirector of the phototherapy center at Brigham and Women’s department of dermatology, said at the annual meeting of the American Academy of Dermatology. In her clinical opinion, phototherapy plays an essential role in the treatment of psoriasis.
“It is medically and financially responsible to review the option of phototherapy with every psoriasis patient,” Dr. Buzney said. “Many patients are not medical or financial candidates for biologic/apremilast therapy, or just would prefer nonsystemic therapy.”
In one meta-analysis, the proportion of patients achieving Psoriasis Area and Severity Index (PASI) 75 with NB-UVB therapy was 70% after 20-40 sessions, just below the efficacy of newer biologics – but better than ustekinumab and adalimumab.
“Phototherapy is not so far out of range as you might think it is,” she said, noting that other studies of NB-UVB therapy show PASI 75 responses of 62% and PASI 90 responses of 40%.
Phototherapy can also be an appealing option because biologics aren’t the best option for all patients with psoriasis. They are expensive for the health care system and potentially for patients, require initial and potentially continued lab testing and monitoring, and require injections, “which some patients don’t like,” said Dr. Buzney, who is also vice-chair of clinical affairs at the Brigham and Women’s Hospital department of dermatology. “There’s an infrequent risk of serious infection and there is risk in patients with HIV, TB, and hepatitis that you have to address. There is also concern for the impact of biologics on patients with a recent cancer.”
On the other hand, few contraindications to NB-UVB exist. According to joint American Academy of Dermatology-National Psoriasis Foundation guidelines on the management and treatment of psoriasis with phototherapy, published in 2019, NB-UVB therapy is only contraindicated in patients with xeroderma pigmentosa and other photosensitive disorders. Concurrent use of cyclosporine and NB-UVB treatment is also contraindicated because of the calculated increase in risk of skin cancer, extrapolated from data on risk with cyclosporine and PUVA (psoralen and ultraviolet A therapy).
The guidelines state that NB-UVB can be used with caution in lupus patients with no history of photosensitivity and who are SS-A negative, as well as patients with a history of melanoma or multiple nonmelanoma skin cancers, a history of recurrent oral herpes simplex virus infection, a history of arsenic intake, prior exposure to ionizing radiation, and those taking photosensitizing medications (since NB-UVB lamps emit “negligible” UVA).
It’s also safe to use during pregnancy and in children. “It’s safe and effective for the right patient,” Dr. Buzney said, discussing how phototherapy can be modified to accommodate children. “You can consider a slower dose-increased regimen. Will children keep the eye protection on? That’s a tricky one. How are you going to manage their anxiety during treatment and involve their family?”
Subgroups of patients who demonstrate a better response to NB-UVB treatment include those with guttate psoriasis, compared with plaque psoriasis, nonsmokers, those with a lower BMI, those with a higher baseline PASI, and those who demonstrate a faster trajectory of clinical response over the first 2-3 weeks of treatment.
Why would one not use phototherapy for psoriasis? “Cost and convenience,” Dr. Buzney said. “There is lost time/revenue to commute to treatment, which may involve multiple times per week. Coming to a public space when COVID-19 is still lingering is another concern, as are the out-of-pocket costs for copays and parking.”
For these reasons, she considers home phototherapy as a transformative option for many patients. Home phototherapy booths provide a safe and effective way to use NB-UVB phototherapy while minimizing copays and commuting costs. The one-time price tag of home NB-UVB booths runs between $5,000 and $7,000, but that is “much less expensive than the biologics,” which can cost $40,000-$50,000 per year, she said.
A small cross-sectional study of office- versus home-based NB-UVB in patients with vitiligo found a cost savings for home-based NB-UVB after 3 months.
One of the challenges with home phototherapy is the lack of long-term studies on patient use. In a small study Dr. Buzney conducted of 30 patients who were prescribed home phototherapy in the last 5 years, 65% practiced (or had practiced) conservative dosing, 83% had continued care with a dermatologist, 19% reported sunburns (5 mild and 1 severe), and 50% had discontinued the therapy at the time of survey because of a perceived lack of efficacy and inconvenience. But 30% of those who had stopped had done so within one month of getting their home booth.
“This tells me that we have to educate our patients better about what expectations should be and make sure they understand how to use their booths,” she said. “Home phototherapy has changed my practice, but not everyone is a candidate for it. Some patients are not dependable. Others are unable to understand instructions.”
Cost to purchase a NB-UVB booth is also an issue, she noted. “Typically, a percentage of cost is covered by insurance, but it’s problematic to purchase a booth if patients don’t know it’s going to work for them or not. Then you have college students who don’t have the space in their apartment or dorm room for a booth.”
Dr. Buzney reported having no relevant financial conflicts.
BOSTON – In 2012, about 50% of patients receiving phototherapy at Brigham and Women’s Hospital in Boston were being treated for psoriasis. A decade later, that proportion has dropped to 20%.
Several factors have contributed to this trend, namely, the development of biologics, the COVID-19 pandemic, “and the rise of home phototherapy options,” Elizabeth A. Buzney, MD, codirector of the phototherapy center at Brigham and Women’s department of dermatology, said at the annual meeting of the American Academy of Dermatology. In her clinical opinion, phototherapy plays an essential role in the treatment of psoriasis.
“It is medically and financially responsible to review the option of phototherapy with every psoriasis patient,” Dr. Buzney said. “Many patients are not medical or financial candidates for biologic/apremilast therapy, or just would prefer nonsystemic therapy.”
In one meta-analysis, the proportion of patients achieving Psoriasis Area and Severity Index (PASI) 75 with NB-UVB therapy was 70% after 20-40 sessions, just below the efficacy of newer biologics – but better than ustekinumab and adalimumab.
“Phototherapy is not so far out of range as you might think it is,” she said, noting that other studies of NB-UVB therapy show PASI 75 responses of 62% and PASI 90 responses of 40%.
Phototherapy can also be an appealing option because biologics aren’t the best option for all patients with psoriasis. They are expensive for the health care system and potentially for patients, require initial and potentially continued lab testing and monitoring, and require injections, “which some patients don’t like,” said Dr. Buzney, who is also vice-chair of clinical affairs at the Brigham and Women’s Hospital department of dermatology. “There’s an infrequent risk of serious infection and there is risk in patients with HIV, TB, and hepatitis that you have to address. There is also concern for the impact of biologics on patients with a recent cancer.”
On the other hand, few contraindications to NB-UVB exist. According to joint American Academy of Dermatology-National Psoriasis Foundation guidelines on the management and treatment of psoriasis with phototherapy, published in 2019, NB-UVB therapy is only contraindicated in patients with xeroderma pigmentosa and other photosensitive disorders. Concurrent use of cyclosporine and NB-UVB treatment is also contraindicated because of the calculated increase in risk of skin cancer, extrapolated from data on risk with cyclosporine and PUVA (psoralen and ultraviolet A therapy).
The guidelines state that NB-UVB can be used with caution in lupus patients with no history of photosensitivity and who are SS-A negative, as well as patients with a history of melanoma or multiple nonmelanoma skin cancers, a history of recurrent oral herpes simplex virus infection, a history of arsenic intake, prior exposure to ionizing radiation, and those taking photosensitizing medications (since NB-UVB lamps emit “negligible” UVA).
It’s also safe to use during pregnancy and in children. “It’s safe and effective for the right patient,” Dr. Buzney said, discussing how phototherapy can be modified to accommodate children. “You can consider a slower dose-increased regimen. Will children keep the eye protection on? That’s a tricky one. How are you going to manage their anxiety during treatment and involve their family?”
Subgroups of patients who demonstrate a better response to NB-UVB treatment include those with guttate psoriasis, compared with plaque psoriasis, nonsmokers, those with a lower BMI, those with a higher baseline PASI, and those who demonstrate a faster trajectory of clinical response over the first 2-3 weeks of treatment.
Why would one not use phototherapy for psoriasis? “Cost and convenience,” Dr. Buzney said. “There is lost time/revenue to commute to treatment, which may involve multiple times per week. Coming to a public space when COVID-19 is still lingering is another concern, as are the out-of-pocket costs for copays and parking.”
For these reasons, she considers home phototherapy as a transformative option for many patients. Home phototherapy booths provide a safe and effective way to use NB-UVB phototherapy while minimizing copays and commuting costs. The one-time price tag of home NB-UVB booths runs between $5,000 and $7,000, but that is “much less expensive than the biologics,” which can cost $40,000-$50,000 per year, she said.
A small cross-sectional study of office- versus home-based NB-UVB in patients with vitiligo found a cost savings for home-based NB-UVB after 3 months.
One of the challenges with home phototherapy is the lack of long-term studies on patient use. In a small study Dr. Buzney conducted of 30 patients who were prescribed home phototherapy in the last 5 years, 65% practiced (or had practiced) conservative dosing, 83% had continued care with a dermatologist, 19% reported sunburns (5 mild and 1 severe), and 50% had discontinued the therapy at the time of survey because of a perceived lack of efficacy and inconvenience. But 30% of those who had stopped had done so within one month of getting their home booth.
“This tells me that we have to educate our patients better about what expectations should be and make sure they understand how to use their booths,” she said. “Home phototherapy has changed my practice, but not everyone is a candidate for it. Some patients are not dependable. Others are unable to understand instructions.”
Cost to purchase a NB-UVB booth is also an issue, she noted. “Typically, a percentage of cost is covered by insurance, but it’s problematic to purchase a booth if patients don’t know it’s going to work for them or not. Then you have college students who don’t have the space in their apartment or dorm room for a booth.”
Dr. Buzney reported having no relevant financial conflicts.
BOSTON – In 2012, about 50% of patients receiving phototherapy at Brigham and Women’s Hospital in Boston were being treated for psoriasis. A decade later, that proportion has dropped to 20%.
Several factors have contributed to this trend, namely, the development of biologics, the COVID-19 pandemic, “and the rise of home phototherapy options,” Elizabeth A. Buzney, MD, codirector of the phototherapy center at Brigham and Women’s department of dermatology, said at the annual meeting of the American Academy of Dermatology. In her clinical opinion, phototherapy plays an essential role in the treatment of psoriasis.
“It is medically and financially responsible to review the option of phototherapy with every psoriasis patient,” Dr. Buzney said. “Many patients are not medical or financial candidates for biologic/apremilast therapy, or just would prefer nonsystemic therapy.”
In one meta-analysis, the proportion of patients achieving Psoriasis Area and Severity Index (PASI) 75 with NB-UVB therapy was 70% after 20-40 sessions, just below the efficacy of newer biologics – but better than ustekinumab and adalimumab.
“Phototherapy is not so far out of range as you might think it is,” she said, noting that other studies of NB-UVB therapy show PASI 75 responses of 62% and PASI 90 responses of 40%.
Phototherapy can also be an appealing option because biologics aren’t the best option for all patients with psoriasis. They are expensive for the health care system and potentially for patients, require initial and potentially continued lab testing and monitoring, and require injections, “which some patients don’t like,” said Dr. Buzney, who is also vice-chair of clinical affairs at the Brigham and Women’s Hospital department of dermatology. “There’s an infrequent risk of serious infection and there is risk in patients with HIV, TB, and hepatitis that you have to address. There is also concern for the impact of biologics on patients with a recent cancer.”
On the other hand, few contraindications to NB-UVB exist. According to joint American Academy of Dermatology-National Psoriasis Foundation guidelines on the management and treatment of psoriasis with phototherapy, published in 2019, NB-UVB therapy is only contraindicated in patients with xeroderma pigmentosa and other photosensitive disorders. Concurrent use of cyclosporine and NB-UVB treatment is also contraindicated because of the calculated increase in risk of skin cancer, extrapolated from data on risk with cyclosporine and PUVA (psoralen and ultraviolet A therapy).
The guidelines state that NB-UVB can be used with caution in lupus patients with no history of photosensitivity and who are SS-A negative, as well as patients with a history of melanoma or multiple nonmelanoma skin cancers, a history of recurrent oral herpes simplex virus infection, a history of arsenic intake, prior exposure to ionizing radiation, and those taking photosensitizing medications (since NB-UVB lamps emit “negligible” UVA).
It’s also safe to use during pregnancy and in children. “It’s safe and effective for the right patient,” Dr. Buzney said, discussing how phototherapy can be modified to accommodate children. “You can consider a slower dose-increased regimen. Will children keep the eye protection on? That’s a tricky one. How are you going to manage their anxiety during treatment and involve their family?”
Subgroups of patients who demonstrate a better response to NB-UVB treatment include those with guttate psoriasis, compared with plaque psoriasis, nonsmokers, those with a lower BMI, those with a higher baseline PASI, and those who demonstrate a faster trajectory of clinical response over the first 2-3 weeks of treatment.
Why would one not use phototherapy for psoriasis? “Cost and convenience,” Dr. Buzney said. “There is lost time/revenue to commute to treatment, which may involve multiple times per week. Coming to a public space when COVID-19 is still lingering is another concern, as are the out-of-pocket costs for copays and parking.”
For these reasons, she considers home phototherapy as a transformative option for many patients. Home phototherapy booths provide a safe and effective way to use NB-UVB phototherapy while minimizing copays and commuting costs. The one-time price tag of home NB-UVB booths runs between $5,000 and $7,000, but that is “much less expensive than the biologics,” which can cost $40,000-$50,000 per year, she said.
A small cross-sectional study of office- versus home-based NB-UVB in patients with vitiligo found a cost savings for home-based NB-UVB after 3 months.
One of the challenges with home phototherapy is the lack of long-term studies on patient use. In a small study Dr. Buzney conducted of 30 patients who were prescribed home phototherapy in the last 5 years, 65% practiced (or had practiced) conservative dosing, 83% had continued care with a dermatologist, 19% reported sunburns (5 mild and 1 severe), and 50% had discontinued the therapy at the time of survey because of a perceived lack of efficacy and inconvenience. But 30% of those who had stopped had done so within one month of getting their home booth.
“This tells me that we have to educate our patients better about what expectations should be and make sure they understand how to use their booths,” she said. “Home phototherapy has changed my practice, but not everyone is a candidate for it. Some patients are not dependable. Others are unable to understand instructions.”
Cost to purchase a NB-UVB booth is also an issue, she noted. “Typically, a percentage of cost is covered by insurance, but it’s problematic to purchase a booth if patients don’t know it’s going to work for them or not. Then you have college students who don’t have the space in their apartment or dorm room for a booth.”
Dr. Buzney reported having no relevant financial conflicts.
AT AAD 22
IBD risk ‘uncertain’ in biologic-treated AxSpA patients
Considerable uncertainty surrounds whether people with axial spondyloarthritis (axSpA) who are treated with biologic drugs have an increased risk for developing inflammatory bowel disease (IBD) that is higher than if they receive other treatments, according to data reported at the annual meeting of the British Society for Rheumatology.
“We noticed two patterns,” Gary Macfarlane, MD, PhD, Dsc, of the University of Aberdeen (Scotland) said in presenting findings from an analysis of the British Society for Rheumatology Biologics Register for Ankylosing Spondylitis (BSRBR-AS) and a meta-analysis of available studies.
There was a “large excess risk in observational studies associated with biologic therapies, which was not replicated in RCTs [randomized, controlled trials],” he said, “and trials under extensions suggested a small absolute increased risk associated with etanercept and with [interleukin]-17 [inhibitors], although again with considerable uncertainty.”
While these data make it difficult to draw any firm conclusions, “we should be reassured that the patient groups receiving these specific biologics in routine clinical care have not demonstrated an excess risk of IBD,” Dr. Macfarlane told delegates at the meeting.
Addressing clinical questions
IBD is a known extra-articular manifestation of axSpA, with an estimated prevalence of about 7%, according to a 2015 meta-analysis of 69 studies involving more than 30,000 patients.
The idea that people being treated with biologics may be at higher risk for developing IBD than those taking other treatments was suggested by the results of a large (n = 80,326) Danish study in which patients who were treated with an anti–tumor necrosis factor (TNF)–alpha medication were found to be more likely to develop de novo ulcerative colitis or Crohn’s disease than were patients who did not receive biologics.
Notably, the risk for IBD seemed higher with etanercept than with other anti–TNF-alpha agents, such as infliximab and adalimumab.
The aim of the analyses that Dr. Macfarlane presented was therefore to see if there was a difference in IBD risk among patients treated with biologic agents versus other agents, and if etanercept really did pose a greater cause for concern.
“The reason that we are asking this question is that a clinician called us up and asked us if we had any data on it,” Dr. Macfarlane said. “I think that’s really important to say that one of the things the registers are designed for are to answer questions that clinicians may have.”
Looking for new-onset IBD
Although no longer recruiting patients, the BSRBR-AS provides a wealth of data on the real-life management of patients with axSpA who were or were not taking a biologic. Patients were recruited into the register between 2012 and 2017, with follow-up until 2018. Data analyses are still ongoing and expected to continue for another couple of years.
The current analysis of data from the BSRBR-AS included patients who did not already have IBD at enrollment into the register, and patients who had been treated with a biologic could have been treated only with a single agent. Of just over 1,800 eligible patients, 793 had been treated with a biologic and 1,058 had been given nonbiologic treatment.
As expected, there were some differences between the two groups of patients studied, with biologic-treated patients having a younger age than non–biologic-treated patients. Those who took a biologic also had higher disease activity, inflammatory scores, and rates of psoriasis, enthesitis, and peripheral joint involvement.
Incidence rates for new-onset IBD per 1,000 person-years of treatment were calculated as 17 (95% confidence interval, 10.7-25.8) for patients taking a biologic and 5.1 (95% CI, 2.7-8.7) for those not taking a biologic, giving an incidence rate difference of 11.9 (95% CI, 4.3-19.6).
There was some observed differences in the incidence of new-onset IBD associated with specific agents. Etanercept did not have a higher rate (13.9/1,000 patient-years; 95% CI, 5.1-30.3) than did other agents. But in comparison, the incidence of new-onset IBD for adalimumab was 20.4 (95% CI, 11.7-33.1) and zero for other anti-TNF agents such as certolizumab pegol and infliximab, although the duration of exposure to these drugs was much lower.
The IRDs for etanercept versus nonbiologic treatment and versus other anti-TNFs were 8.8 (95% CI, –2.7 to 20.3) and -6.4 (95% CI, –21.3 to 8.5), but with “considerable uncertainty” because the confidence intervals were very wide.
Uncertainty not helped by meta-analysis
“Given the uncertainty associated with the results from BSRBR-AS, we decided to undertake a meta-analysis to try to accumulate other data that could help us answer this question,” Dr. Macfarlane explained.
However, this didn’t really help clarify things because combining BSRBR-AS data with the results of a couple of observational studies suggested that the odds of of IBD doubled with any biologic treatment versus no biologic treatment (odds ratio, 2.19), and a 2.5-fold higher likelihood considering etanercept versus no biologic treatment, but no difference was seen comparing etanercept to other anti-TNF agents (OR, 0.93).
When the meta-analysis was restricted to RCTs, the rate of IBD per 1,000 person-years was 3.43 for placebo, 5.64 for all biologics, 8.14 for etanercept, 2.35 for other anti-TNFs, and 7.02 for IL-17 inhibitors.
For extensions of RCTs, IBD rates per 1,000 person-years of follow-up were 2.91 for etanercept, 0.83 for other anti-TNFs, 3.61 for IL-17 inhibitors, and 2.79 for all biologics.
“There was only a small difference in IBD incidence between the biologic therapy and the placebo groups” in the RCTs and associated studies, Dr. Macfarlane said, adding that “there was a small excess incidence associated with etanercept, compared to other anti-TNF agents, and [for] IL-17 therapy, compared to nonetanercept, anti–TNF-alpha therapies.”
Of course, the different study designs and durations of exposure to the various treatments raises significant methodological issues.
“Randomized, controlled trials should provide the highest quality evidence as a result of their design and randomizing patients to treatment,” Dr. Macfarlane said. “However, their relatively short follow-up, as well as their restrictive eligibility criteria, may work against finding a difference in IBD incidence if it were to exist.”
Observational studies are very valuable in the data they can provide but are also beset with problems, such as surveillance bias and confounding by indication.
The higher risk of IBD that was observed in observational studies could be an issue with study design, or perhaps, “in routine clinical practice, rheumatologists are taking on board factors that we have not measured, that are negating any slight increased risk,” Dr. Macfarlane said.
Session chair Nicola Goodson, MBChB, PhD, of Liverpool (England) University NHS Foundation Trust, commented: “I think that could well be a very reasonable explanation, because I think as a clinician, you do tend to channel drugs away from some people and channel drugs towards others.
However, Dr. Goodson noted that there was “a glimmer” of signal coming from the RCTs.
“Methodologically, that is what you would have to take as the most robust evidence,” Dr. Macfarlane said, “but even with all the evidence available, it’s still very hard for us to quantify; that has enormous uncertainty.”
Dr. Macfarlane and Dr. Goodson had no relevant conflicts of interest to disclose. The BSRBR-AS is supported by the BSR, which receives funds to support the registry from Pfizer, AbbVie, and UCB.
Considerable uncertainty surrounds whether people with axial spondyloarthritis (axSpA) who are treated with biologic drugs have an increased risk for developing inflammatory bowel disease (IBD) that is higher than if they receive other treatments, according to data reported at the annual meeting of the British Society for Rheumatology.
“We noticed two patterns,” Gary Macfarlane, MD, PhD, Dsc, of the University of Aberdeen (Scotland) said in presenting findings from an analysis of the British Society for Rheumatology Biologics Register for Ankylosing Spondylitis (BSRBR-AS) and a meta-analysis of available studies.
There was a “large excess risk in observational studies associated with biologic therapies, which was not replicated in RCTs [randomized, controlled trials],” he said, “and trials under extensions suggested a small absolute increased risk associated with etanercept and with [interleukin]-17 [inhibitors], although again with considerable uncertainty.”
While these data make it difficult to draw any firm conclusions, “we should be reassured that the patient groups receiving these specific biologics in routine clinical care have not demonstrated an excess risk of IBD,” Dr. Macfarlane told delegates at the meeting.
Addressing clinical questions
IBD is a known extra-articular manifestation of axSpA, with an estimated prevalence of about 7%, according to a 2015 meta-analysis of 69 studies involving more than 30,000 patients.
The idea that people being treated with biologics may be at higher risk for developing IBD than those taking other treatments was suggested by the results of a large (n = 80,326) Danish study in which patients who were treated with an anti–tumor necrosis factor (TNF)–alpha medication were found to be more likely to develop de novo ulcerative colitis or Crohn’s disease than were patients who did not receive biologics.
Notably, the risk for IBD seemed higher with etanercept than with other anti–TNF-alpha agents, such as infliximab and adalimumab.
The aim of the analyses that Dr. Macfarlane presented was therefore to see if there was a difference in IBD risk among patients treated with biologic agents versus other agents, and if etanercept really did pose a greater cause for concern.
“The reason that we are asking this question is that a clinician called us up and asked us if we had any data on it,” Dr. Macfarlane said. “I think that’s really important to say that one of the things the registers are designed for are to answer questions that clinicians may have.”
Looking for new-onset IBD
Although no longer recruiting patients, the BSRBR-AS provides a wealth of data on the real-life management of patients with axSpA who were or were not taking a biologic. Patients were recruited into the register between 2012 and 2017, with follow-up until 2018. Data analyses are still ongoing and expected to continue for another couple of years.
The current analysis of data from the BSRBR-AS included patients who did not already have IBD at enrollment into the register, and patients who had been treated with a biologic could have been treated only with a single agent. Of just over 1,800 eligible patients, 793 had been treated with a biologic and 1,058 had been given nonbiologic treatment.
As expected, there were some differences between the two groups of patients studied, with biologic-treated patients having a younger age than non–biologic-treated patients. Those who took a biologic also had higher disease activity, inflammatory scores, and rates of psoriasis, enthesitis, and peripheral joint involvement.
Incidence rates for new-onset IBD per 1,000 person-years of treatment were calculated as 17 (95% confidence interval, 10.7-25.8) for patients taking a biologic and 5.1 (95% CI, 2.7-8.7) for those not taking a biologic, giving an incidence rate difference of 11.9 (95% CI, 4.3-19.6).
There was some observed differences in the incidence of new-onset IBD associated with specific agents. Etanercept did not have a higher rate (13.9/1,000 patient-years; 95% CI, 5.1-30.3) than did other agents. But in comparison, the incidence of new-onset IBD for adalimumab was 20.4 (95% CI, 11.7-33.1) and zero for other anti-TNF agents such as certolizumab pegol and infliximab, although the duration of exposure to these drugs was much lower.
The IRDs for etanercept versus nonbiologic treatment and versus other anti-TNFs were 8.8 (95% CI, –2.7 to 20.3) and -6.4 (95% CI, –21.3 to 8.5), but with “considerable uncertainty” because the confidence intervals were very wide.
Uncertainty not helped by meta-analysis
“Given the uncertainty associated with the results from BSRBR-AS, we decided to undertake a meta-analysis to try to accumulate other data that could help us answer this question,” Dr. Macfarlane explained.
However, this didn’t really help clarify things because combining BSRBR-AS data with the results of a couple of observational studies suggested that the odds of of IBD doubled with any biologic treatment versus no biologic treatment (odds ratio, 2.19), and a 2.5-fold higher likelihood considering etanercept versus no biologic treatment, but no difference was seen comparing etanercept to other anti-TNF agents (OR, 0.93).
When the meta-analysis was restricted to RCTs, the rate of IBD per 1,000 person-years was 3.43 for placebo, 5.64 for all biologics, 8.14 for etanercept, 2.35 for other anti-TNFs, and 7.02 for IL-17 inhibitors.
For extensions of RCTs, IBD rates per 1,000 person-years of follow-up were 2.91 for etanercept, 0.83 for other anti-TNFs, 3.61 for IL-17 inhibitors, and 2.79 for all biologics.
“There was only a small difference in IBD incidence between the biologic therapy and the placebo groups” in the RCTs and associated studies, Dr. Macfarlane said, adding that “there was a small excess incidence associated with etanercept, compared to other anti-TNF agents, and [for] IL-17 therapy, compared to nonetanercept, anti–TNF-alpha therapies.”
Of course, the different study designs and durations of exposure to the various treatments raises significant methodological issues.
“Randomized, controlled trials should provide the highest quality evidence as a result of their design and randomizing patients to treatment,” Dr. Macfarlane said. “However, their relatively short follow-up, as well as their restrictive eligibility criteria, may work against finding a difference in IBD incidence if it were to exist.”
Observational studies are very valuable in the data they can provide but are also beset with problems, such as surveillance bias and confounding by indication.
The higher risk of IBD that was observed in observational studies could be an issue with study design, or perhaps, “in routine clinical practice, rheumatologists are taking on board factors that we have not measured, that are negating any slight increased risk,” Dr. Macfarlane said.
Session chair Nicola Goodson, MBChB, PhD, of Liverpool (England) University NHS Foundation Trust, commented: “I think that could well be a very reasonable explanation, because I think as a clinician, you do tend to channel drugs away from some people and channel drugs towards others.
However, Dr. Goodson noted that there was “a glimmer” of signal coming from the RCTs.
“Methodologically, that is what you would have to take as the most robust evidence,” Dr. Macfarlane said, “but even with all the evidence available, it’s still very hard for us to quantify; that has enormous uncertainty.”
Dr. Macfarlane and Dr. Goodson had no relevant conflicts of interest to disclose. The BSRBR-AS is supported by the BSR, which receives funds to support the registry from Pfizer, AbbVie, and UCB.
Considerable uncertainty surrounds whether people with axial spondyloarthritis (axSpA) who are treated with biologic drugs have an increased risk for developing inflammatory bowel disease (IBD) that is higher than if they receive other treatments, according to data reported at the annual meeting of the British Society for Rheumatology.
“We noticed two patterns,” Gary Macfarlane, MD, PhD, Dsc, of the University of Aberdeen (Scotland) said in presenting findings from an analysis of the British Society for Rheumatology Biologics Register for Ankylosing Spondylitis (BSRBR-AS) and a meta-analysis of available studies.
There was a “large excess risk in observational studies associated with biologic therapies, which was not replicated in RCTs [randomized, controlled trials],” he said, “and trials under extensions suggested a small absolute increased risk associated with etanercept and with [interleukin]-17 [inhibitors], although again with considerable uncertainty.”
While these data make it difficult to draw any firm conclusions, “we should be reassured that the patient groups receiving these specific biologics in routine clinical care have not demonstrated an excess risk of IBD,” Dr. Macfarlane told delegates at the meeting.
Addressing clinical questions
IBD is a known extra-articular manifestation of axSpA, with an estimated prevalence of about 7%, according to a 2015 meta-analysis of 69 studies involving more than 30,000 patients.
The idea that people being treated with biologics may be at higher risk for developing IBD than those taking other treatments was suggested by the results of a large (n = 80,326) Danish study in which patients who were treated with an anti–tumor necrosis factor (TNF)–alpha medication were found to be more likely to develop de novo ulcerative colitis or Crohn’s disease than were patients who did not receive biologics.
Notably, the risk for IBD seemed higher with etanercept than with other anti–TNF-alpha agents, such as infliximab and adalimumab.
The aim of the analyses that Dr. Macfarlane presented was therefore to see if there was a difference in IBD risk among patients treated with biologic agents versus other agents, and if etanercept really did pose a greater cause for concern.
“The reason that we are asking this question is that a clinician called us up and asked us if we had any data on it,” Dr. Macfarlane said. “I think that’s really important to say that one of the things the registers are designed for are to answer questions that clinicians may have.”
Looking for new-onset IBD
Although no longer recruiting patients, the BSRBR-AS provides a wealth of data on the real-life management of patients with axSpA who were or were not taking a biologic. Patients were recruited into the register between 2012 and 2017, with follow-up until 2018. Data analyses are still ongoing and expected to continue for another couple of years.
The current analysis of data from the BSRBR-AS included patients who did not already have IBD at enrollment into the register, and patients who had been treated with a biologic could have been treated only with a single agent. Of just over 1,800 eligible patients, 793 had been treated with a biologic and 1,058 had been given nonbiologic treatment.
As expected, there were some differences between the two groups of patients studied, with biologic-treated patients having a younger age than non–biologic-treated patients. Those who took a biologic also had higher disease activity, inflammatory scores, and rates of psoriasis, enthesitis, and peripheral joint involvement.
Incidence rates for new-onset IBD per 1,000 person-years of treatment were calculated as 17 (95% confidence interval, 10.7-25.8) for patients taking a biologic and 5.1 (95% CI, 2.7-8.7) for those not taking a biologic, giving an incidence rate difference of 11.9 (95% CI, 4.3-19.6).
There was some observed differences in the incidence of new-onset IBD associated with specific agents. Etanercept did not have a higher rate (13.9/1,000 patient-years; 95% CI, 5.1-30.3) than did other agents. But in comparison, the incidence of new-onset IBD for adalimumab was 20.4 (95% CI, 11.7-33.1) and zero for other anti-TNF agents such as certolizumab pegol and infliximab, although the duration of exposure to these drugs was much lower.
The IRDs for etanercept versus nonbiologic treatment and versus other anti-TNFs were 8.8 (95% CI, –2.7 to 20.3) and -6.4 (95% CI, –21.3 to 8.5), but with “considerable uncertainty” because the confidence intervals were very wide.
Uncertainty not helped by meta-analysis
“Given the uncertainty associated with the results from BSRBR-AS, we decided to undertake a meta-analysis to try to accumulate other data that could help us answer this question,” Dr. Macfarlane explained.
However, this didn’t really help clarify things because combining BSRBR-AS data with the results of a couple of observational studies suggested that the odds of of IBD doubled with any biologic treatment versus no biologic treatment (odds ratio, 2.19), and a 2.5-fold higher likelihood considering etanercept versus no biologic treatment, but no difference was seen comparing etanercept to other anti-TNF agents (OR, 0.93).
When the meta-analysis was restricted to RCTs, the rate of IBD per 1,000 person-years was 3.43 for placebo, 5.64 for all biologics, 8.14 for etanercept, 2.35 for other anti-TNFs, and 7.02 for IL-17 inhibitors.
For extensions of RCTs, IBD rates per 1,000 person-years of follow-up were 2.91 for etanercept, 0.83 for other anti-TNFs, 3.61 for IL-17 inhibitors, and 2.79 for all biologics.
“There was only a small difference in IBD incidence between the biologic therapy and the placebo groups” in the RCTs and associated studies, Dr. Macfarlane said, adding that “there was a small excess incidence associated with etanercept, compared to other anti-TNF agents, and [for] IL-17 therapy, compared to nonetanercept, anti–TNF-alpha therapies.”
Of course, the different study designs and durations of exposure to the various treatments raises significant methodological issues.
“Randomized, controlled trials should provide the highest quality evidence as a result of their design and randomizing patients to treatment,” Dr. Macfarlane said. “However, their relatively short follow-up, as well as their restrictive eligibility criteria, may work against finding a difference in IBD incidence if it were to exist.”
Observational studies are very valuable in the data they can provide but are also beset with problems, such as surveillance bias and confounding by indication.
The higher risk of IBD that was observed in observational studies could be an issue with study design, or perhaps, “in routine clinical practice, rheumatologists are taking on board factors that we have not measured, that are negating any slight increased risk,” Dr. Macfarlane said.
Session chair Nicola Goodson, MBChB, PhD, of Liverpool (England) University NHS Foundation Trust, commented: “I think that could well be a very reasonable explanation, because I think as a clinician, you do tend to channel drugs away from some people and channel drugs towards others.
However, Dr. Goodson noted that there was “a glimmer” of signal coming from the RCTs.
“Methodologically, that is what you would have to take as the most robust evidence,” Dr. Macfarlane said, “but even with all the evidence available, it’s still very hard for us to quantify; that has enormous uncertainty.”
Dr. Macfarlane and Dr. Goodson had no relevant conflicts of interest to disclose. The BSRBR-AS is supported by the BSR, which receives funds to support the registry from Pfizer, AbbVie, and UCB.
FROM BSR 2022
Unilateral eye irritation
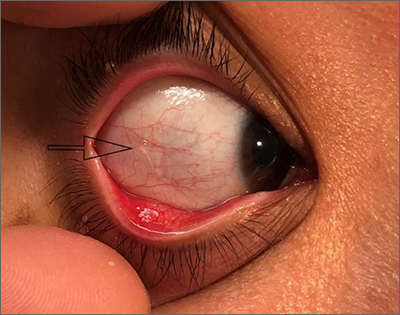
Physical examination revealed an irregularly shaped conjunctival cyst on the lateral (temporal) field of the right eye. (This diagnosis is usually made based on the clinical examination alone.)
Primary care physicians encounter patients with a variety of eye conditions; pruritis and foreign body sensation are among the most common complaints.1 While viral or allergic conjunctivitis is often to blame for “itchy eyes,” the cause can also be a conjunctival mass.
Conjunctival masses can be divided into 2 groups: solid tumors or cysts.2 Conjunctival cysts form due to trauma, infection, or inflammation that disrupts the conjunctival epithelium. They can be congenital or acquired (more common) and are rarely caused by over-the-counter eye drops.2,3 The differential diagnosis for a conjunctival cyst includes conjunctival bleb, pinguecula, pterygium, pyogenic granuloma, and tumors of the conjunctiva. An external eye exam plus a slit-lamp examination can help confirm the diagnosis.
Small, asymptomatic conjunctival cysts will mostly resolve on their own and can be managed conservatively with lubricating eye drops.3 When inflammation surrounds the cyst, short-term use of a mild topical corticosteroid is reasonable.2 Simple needle aspiration can be performed but may lead to recurrence of the cyst. Lesions larger than 15 mm, or those that have grown or changed, should be evaluated by an ophthalmologist for biopsy and further management.2,3
After a discussion of the benefits and risks of different approaches, this patient decided on conservative management. Supportive care with lubricating eye drops was started. At her 1-month follow-up, all symptoms had resolved.
Photos courtesy of Morteza Khodaee, MD, MPH. Text courtesy of Amy S. Li, MD, Department of Internal Medicine, Jennifer Cogburn, MD, and Morteza Khodaee, MD, MPH, Department of Family Medicine, University of Colorado School of Medicine, Denver
1. Pflipsen M, Massaquoi M, Wolf S. Evaluation of the painful eye. Am Fam Physician. 2016 Jun 15;93:991-998.
2. Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Indian J Ophthalmol. 2019;67:1930-1948. doi: 10.4103/ijo.IJO_2040_19
3. Olivier JF. Common conjunctival lesions. S Afr J CPD. 2013;31:134-137.

Physical examination revealed an irregularly shaped conjunctival cyst on the lateral (temporal) field of the right eye. (This diagnosis is usually made based on the clinical examination alone.)
Primary care physicians encounter patients with a variety of eye conditions; pruritis and foreign body sensation are among the most common complaints.1 While viral or allergic conjunctivitis is often to blame for “itchy eyes,” the cause can also be a conjunctival mass.
Conjunctival masses can be divided into 2 groups: solid tumors or cysts.2 Conjunctival cysts form due to trauma, infection, or inflammation that disrupts the conjunctival epithelium. They can be congenital or acquired (more common) and are rarely caused by over-the-counter eye drops.2,3 The differential diagnosis for a conjunctival cyst includes conjunctival bleb, pinguecula, pterygium, pyogenic granuloma, and tumors of the conjunctiva. An external eye exam plus a slit-lamp examination can help confirm the diagnosis.
Small, asymptomatic conjunctival cysts will mostly resolve on their own and can be managed conservatively with lubricating eye drops.3 When inflammation surrounds the cyst, short-term use of a mild topical corticosteroid is reasonable.2 Simple needle aspiration can be performed but may lead to recurrence of the cyst. Lesions larger than 15 mm, or those that have grown or changed, should be evaluated by an ophthalmologist for biopsy and further management.2,3
After a discussion of the benefits and risks of different approaches, this patient decided on conservative management. Supportive care with lubricating eye drops was started. At her 1-month follow-up, all symptoms had resolved.
Photos courtesy of Morteza Khodaee, MD, MPH. Text courtesy of Amy S. Li, MD, Department of Internal Medicine, Jennifer Cogburn, MD, and Morteza Khodaee, MD, MPH, Department of Family Medicine, University of Colorado School of Medicine, Denver

Physical examination revealed an irregularly shaped conjunctival cyst on the lateral (temporal) field of the right eye. (This diagnosis is usually made based on the clinical examination alone.)
Primary care physicians encounter patients with a variety of eye conditions; pruritis and foreign body sensation are among the most common complaints.1 While viral or allergic conjunctivitis is often to blame for “itchy eyes,” the cause can also be a conjunctival mass.
Conjunctival masses can be divided into 2 groups: solid tumors or cysts.2 Conjunctival cysts form due to trauma, infection, or inflammation that disrupts the conjunctival epithelium. They can be congenital or acquired (more common) and are rarely caused by over-the-counter eye drops.2,3 The differential diagnosis for a conjunctival cyst includes conjunctival bleb, pinguecula, pterygium, pyogenic granuloma, and tumors of the conjunctiva. An external eye exam plus a slit-lamp examination can help confirm the diagnosis.
Small, asymptomatic conjunctival cysts will mostly resolve on their own and can be managed conservatively with lubricating eye drops.3 When inflammation surrounds the cyst, short-term use of a mild topical corticosteroid is reasonable.2 Simple needle aspiration can be performed but may lead to recurrence of the cyst. Lesions larger than 15 mm, or those that have grown or changed, should be evaluated by an ophthalmologist for biopsy and further management.2,3
After a discussion of the benefits and risks of different approaches, this patient decided on conservative management. Supportive care with lubricating eye drops was started. At her 1-month follow-up, all symptoms had resolved.
Photos courtesy of Morteza Khodaee, MD, MPH. Text courtesy of Amy S. Li, MD, Department of Internal Medicine, Jennifer Cogburn, MD, and Morteza Khodaee, MD, MPH, Department of Family Medicine, University of Colorado School of Medicine, Denver
1. Pflipsen M, Massaquoi M, Wolf S. Evaluation of the painful eye. Am Fam Physician. 2016 Jun 15;93:991-998.
2. Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Indian J Ophthalmol. 2019;67:1930-1948. doi: 10.4103/ijo.IJO_2040_19
3. Olivier JF. Common conjunctival lesions. S Afr J CPD. 2013;31:134-137.
1. Pflipsen M, Massaquoi M, Wolf S. Evaluation of the painful eye. Am Fam Physician. 2016 Jun 15;93:991-998.
2. Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Indian J Ophthalmol. 2019;67:1930-1948. doi: 10.4103/ijo.IJO_2040_19
3. Olivier JF. Common conjunctival lesions. S Afr J CPD. 2013;31:134-137.
Highlights in Relapsing-Remitting Multiple Sclerosis From AAN 2022
Dr Aaron Miller, from the Icahn School of Medicine at Mount Sinai in New York, discusses updates in relapsing-remitting multiple sclerosis (RRMS) presented at the 2022 American Academy of Neurology (AAN) Annual Meeting.
Dr Miller reports on compelling outcomes linking adherence to an MS-prescribed Mediterranean diet with increased thalamic volume and an enhanced predictability of functional composite scoring.
Next, Dr Miller turns to DMTs and discusses two abstracts looking at results of ublituximab in regard to expanded disability status scale (EDSS) scores and no evidence of disease activity (NEDA) status, and a third comparing ublituximab with teriflunomide.
Finally, Dr Miller presents promising data on Bruton tyrosine kinase inhibitors tolebrutinib and evobrutinib, and the latest results of the NOVA study on the monoclonal antibody natalizumab.
--
Professor, Department of Neurology; Medical Director, Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Academic Medical Center, Icahn School of Medicine at Mount Sinai, New York, NY
Aaron Miller, MD, has disclosed no relevant financial relationships.
Dr Aaron Miller, from the Icahn School of Medicine at Mount Sinai in New York, discusses updates in relapsing-remitting multiple sclerosis (RRMS) presented at the 2022 American Academy of Neurology (AAN) Annual Meeting.
Dr Miller reports on compelling outcomes linking adherence to an MS-prescribed Mediterranean diet with increased thalamic volume and an enhanced predictability of functional composite scoring.
Next, Dr Miller turns to DMTs and discusses two abstracts looking at results of ublituximab in regard to expanded disability status scale (EDSS) scores and no evidence of disease activity (NEDA) status, and a third comparing ublituximab with teriflunomide.
Finally, Dr Miller presents promising data on Bruton tyrosine kinase inhibitors tolebrutinib and evobrutinib, and the latest results of the NOVA study on the monoclonal antibody natalizumab.
--
Professor, Department of Neurology; Medical Director, Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Academic Medical Center, Icahn School of Medicine at Mount Sinai, New York, NY
Aaron Miller, MD, has disclosed no relevant financial relationships.
Dr Aaron Miller, from the Icahn School of Medicine at Mount Sinai in New York, discusses updates in relapsing-remitting multiple sclerosis (RRMS) presented at the 2022 American Academy of Neurology (AAN) Annual Meeting.
Dr Miller reports on compelling outcomes linking adherence to an MS-prescribed Mediterranean diet with increased thalamic volume and an enhanced predictability of functional composite scoring.
Next, Dr Miller turns to DMTs and discusses two abstracts looking at results of ublituximab in regard to expanded disability status scale (EDSS) scores and no evidence of disease activity (NEDA) status, and a third comparing ublituximab with teriflunomide.
Finally, Dr Miller presents promising data on Bruton tyrosine kinase inhibitors tolebrutinib and evobrutinib, and the latest results of the NOVA study on the monoclonal antibody natalizumab.
--
Professor, Department of Neurology; Medical Director, Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Academic Medical Center, Icahn School of Medicine at Mount Sinai, New York, NY
Aaron Miller, MD, has disclosed no relevant financial relationships.

Multimodal imaging of DIP-joint and SEC can help distinguish PsA from psoriasis or OA
Key clinical point: Ultrasound (US), magnetic resonance imaging (MRI), and X-ray could differentiate psoriatic arthritis (PsA) from psoriasis and hand osteoarthritis (OA) based on the degree of structural involvement in the distal interphalangeal (DIP)-joint and synovio-entheseal complex (SEC).
Major finding: US-detected new bone formation (NBF; risk ratio [RR] 0.52; P < .001) and DIP-joint synovial hypertrophy (RR 0.66; P = .005) along with PsA MRI scores (all P < .001) were associated with a lower risk for PsA vs. OA. Patients with PsA vs. psoriasis had a higher prevalence of X-ray entheseal change (mean difference 0.42; P = .024) and a higher trend toward US-detected NBF and erosions.
Study details: This prospective, cross-sectional study included 50 patients with DIP-joint PsA and nail involvement, 12 patients with psoriasis and nail involvement, and 13 patients with erosive and nonerosive OA.
Disclosures: The study was funded by Novartis, The Oak Foundation, and others. Some authors declared receiving speaker/consulting fees, research grants, or honoraria or serving as members of advisory board or consultants for several sources.
Source: Guldberg-Møller J et al. Multimodal imaging of the distal interphalangeal-joint synovio-entheseal complex in psoriatic arthritis (MIDAS): A cross-sectional study on the diagnostic accuracy of different imaging modalities comparing psoriatic arthritis to psoriasis and osteoarthritis. RMD Open. 2022;8:e002109 (Mar 28). Doi: 10.1136/rmdopen-2021-002109
Key clinical point: Ultrasound (US), magnetic resonance imaging (MRI), and X-ray could differentiate psoriatic arthritis (PsA) from psoriasis and hand osteoarthritis (OA) based on the degree of structural involvement in the distal interphalangeal (DIP)-joint and synovio-entheseal complex (SEC).
Major finding: US-detected new bone formation (NBF; risk ratio [RR] 0.52; P < .001) and DIP-joint synovial hypertrophy (RR 0.66; P = .005) along with PsA MRI scores (all P < .001) were associated with a lower risk for PsA vs. OA. Patients with PsA vs. psoriasis had a higher prevalence of X-ray entheseal change (mean difference 0.42; P = .024) and a higher trend toward US-detected NBF and erosions.
Study details: This prospective, cross-sectional study included 50 patients with DIP-joint PsA and nail involvement, 12 patients with psoriasis and nail involvement, and 13 patients with erosive and nonerosive OA.
Disclosures: The study was funded by Novartis, The Oak Foundation, and others. Some authors declared receiving speaker/consulting fees, research grants, or honoraria or serving as members of advisory board or consultants for several sources.
Source: Guldberg-Møller J et al. Multimodal imaging of the distal interphalangeal-joint synovio-entheseal complex in psoriatic arthritis (MIDAS): A cross-sectional study on the diagnostic accuracy of different imaging modalities comparing psoriatic arthritis to psoriasis and osteoarthritis. RMD Open. 2022;8:e002109 (Mar 28). Doi: 10.1136/rmdopen-2021-002109
Key clinical point: Ultrasound (US), magnetic resonance imaging (MRI), and X-ray could differentiate psoriatic arthritis (PsA) from psoriasis and hand osteoarthritis (OA) based on the degree of structural involvement in the distal interphalangeal (DIP)-joint and synovio-entheseal complex (SEC).
Major finding: US-detected new bone formation (NBF; risk ratio [RR] 0.52; P < .001) and DIP-joint synovial hypertrophy (RR 0.66; P = .005) along with PsA MRI scores (all P < .001) were associated with a lower risk for PsA vs. OA. Patients with PsA vs. psoriasis had a higher prevalence of X-ray entheseal change (mean difference 0.42; P = .024) and a higher trend toward US-detected NBF and erosions.
Study details: This prospective, cross-sectional study included 50 patients with DIP-joint PsA and nail involvement, 12 patients with psoriasis and nail involvement, and 13 patients with erosive and nonerosive OA.
Disclosures: The study was funded by Novartis, The Oak Foundation, and others. Some authors declared receiving speaker/consulting fees, research grants, or honoraria or serving as members of advisory board or consultants for several sources.
Source: Guldberg-Møller J et al. Multimodal imaging of the distal interphalangeal-joint synovio-entheseal complex in psoriatic arthritis (MIDAS): A cross-sectional study on the diagnostic accuracy of different imaging modalities comparing psoriatic arthritis to psoriasis and osteoarthritis. RMD Open. 2022;8:e002109 (Mar 28). Doi: 10.1136/rmdopen-2021-002109
Will you have cardiac arrest? New tech may predict if and when
Deaths from COVID-19 may have caught more attention lately, but heart disease remains the leading cause of death in the United States.
More than 300,000 Americans will die this year of sudden cardiac arrest (also called sudden cardiac death, or SCD), when the heart abruptly stops working.
These events happen suddenly and often without warning, making them nearly impossible to predict. But that may be changing, thanks to 3D imaging and artificial intelligence (AI) technology under study at Johns Hopkins University, Baltimore.
There, researchers are working to create more accurate and personalized models of the heart – and not just any heart, your heart, if you have heart disease.
“Right now, a clinician can only say whether a patient is at risk or not at risk for sudden death,” says Dan Popescu, PhD, a Johns Hopkins research scientist and first author of a new study on AI’s ability to predict sudden cardiac arrest. “With this new technology, you can have much more nuanced predictions of probability of an event over time.”
Put another way: With AI, clinicians may be able not only to predict if someone is at risk for sudden cardiac arrest, but also when it is most likely to happen. They can do this using a much clearer and more personalized look at the electrical “wiring” of your heart.
Your heart, the conductor
Your heart isn’t just a metronome responsible for keeping a steady stream of blood pumping to tissues with every beat. It’s also a conductor through which vital energy flows.
To make the heart beat, electrical impulses flow from the top to the bottom of the organ. Healthy heart cells relay this electricity seamlessly. But in a heart damaged by inflammation or a past heart attack, scar tissue will block the energy flow.
When an electrical impulse encounters a scarred area, the signal can become erratic, disrupting the set top-to-bottom path and causing irregular heartbeats (arrhythmias), which increase someone’s danger of sudden cardiac death.
Seeing the heart in 3D
Today’s tests offer some insights into the heart’s makeup. For example, MRI scans can reveal damaged areas. PET scans can show inflammation. And EKGs can record the heart’s electrical signals from beat to beat.
But all these technologies offer only a snapshot, showing heart health at a moment in time. They can’t predict the future. That’s why scientists at Johns Hopkins are going further to develop 3D digital replicas of a person’s heart, known as computational heart models.
Computational models are computer-simulated replicas that combine mathematics, physics, and computer science. These models have been around for a long time and are used in many fields, ranging from manufacturing to economics.
In heart medicine, these models are populated with digital “cells,” which imitate living cells and can be programmed with different electrical properties, depending on whether they are healthy or diseased.
“Currently available imaging and testing (MRIs, PETs, EKGs) give some representation of the scarring, but you cannot translate that to what is going to happen over time,” says Natalia Trayanova, PhD, of the Johns Hopkins department of biomedical engineering.
“With computational heart models, we create a dynamic digital image of the heart. We can then give the digital image an electrical stimulus and assess how the heart is able to respond. Then you can better predict what is going to happen.”
The computerized 3D models also mean better, more accurate treatment for heart conditions.
For example, a common treatment for a type of arrhythmia known as atrial fibrillation is ablation, or burning some heart tissue. Ablation stops the erratic electrical impulses causing the arrhythmia, but it can also damage otherwise healthy heart cells.
A personalized computational heart model could allow doctors to see more accurately what areas should and shouldn’t be treated for a specific patient.
Using deep learning AI to predict health outcomes
Dr. Trayanova’s colleague Dr. Popescu is applying deep learning and AI to do more with computerized heart models to predict the future.
In a recent paper in Nature Cardiovascular Research, the research team showed their algorithm assessed the health of 269 patients and was able to predict the chance of sudden cardiac arrest up to 10 years in advance.
“This is really the first time ever, as far as we know, where deep learning technology has been proven to analyze scarring of the heart in a successful way,” Dr. Popescu says.
Dr. Popescu and Dr. Trayanova say the AI algorithm gathers information from the 3D computational heart models with patient data like MRIs, ethnicity, age, lifestyle, and other clinical information. Analyzing all these data can produce accurate and consistent estimates about how long patients might live if they are at risk for sudden death.
“You can’t afford to be wrong. If you are wrong, you can actually impact a patient’s quality of life dramatically,” Dr. Popescu says. “Having clinicians use this technology in the decision-making process will provide confidence in a better diagnosis and prognosis.”
While the current study was specifically about patients with a particular type of heart disease, Dr. Popescu says his algorithm can also be trained to assess other health conditions.
So when might you see this being used outside of a research study? Dr. Trayanova predicts 3D imaging of heart models could be available in 2 years, but first the technique must be tested in more clinical trials – some of which are happening right now.
Adding AI to the heart models will require more studies and Food and Drug Administration approval, so the timeline is less clear. But perhaps the biggest hurdle is that after approval the technologies would need to be adopted and used by clinicians and caregivers.
“The much harder question to answer is, ‘When will doctors be perfectly comfortable with AI tools?’ And I don’t know the answer,” Dr. Popescu says. “How to use AI as an aid in the decision-making process is something that’s not currently taught.”
A version of this article first appeared on WebMD.com.
Deaths from COVID-19 may have caught more attention lately, but heart disease remains the leading cause of death in the United States.
More than 300,000 Americans will die this year of sudden cardiac arrest (also called sudden cardiac death, or SCD), when the heart abruptly stops working.
These events happen suddenly and often without warning, making them nearly impossible to predict. But that may be changing, thanks to 3D imaging and artificial intelligence (AI) technology under study at Johns Hopkins University, Baltimore.
There, researchers are working to create more accurate and personalized models of the heart – and not just any heart, your heart, if you have heart disease.
“Right now, a clinician can only say whether a patient is at risk or not at risk for sudden death,” says Dan Popescu, PhD, a Johns Hopkins research scientist and first author of a new study on AI’s ability to predict sudden cardiac arrest. “With this new technology, you can have much more nuanced predictions of probability of an event over time.”
Put another way: With AI, clinicians may be able not only to predict if someone is at risk for sudden cardiac arrest, but also when it is most likely to happen. They can do this using a much clearer and more personalized look at the electrical “wiring” of your heart.
Your heart, the conductor
Your heart isn’t just a metronome responsible for keeping a steady stream of blood pumping to tissues with every beat. It’s also a conductor through which vital energy flows.
To make the heart beat, electrical impulses flow from the top to the bottom of the organ. Healthy heart cells relay this electricity seamlessly. But in a heart damaged by inflammation or a past heart attack, scar tissue will block the energy flow.
When an electrical impulse encounters a scarred area, the signal can become erratic, disrupting the set top-to-bottom path and causing irregular heartbeats (arrhythmias), which increase someone’s danger of sudden cardiac death.
Seeing the heart in 3D
Today’s tests offer some insights into the heart’s makeup. For example, MRI scans can reveal damaged areas. PET scans can show inflammation. And EKGs can record the heart’s electrical signals from beat to beat.
But all these technologies offer only a snapshot, showing heart health at a moment in time. They can’t predict the future. That’s why scientists at Johns Hopkins are going further to develop 3D digital replicas of a person’s heart, known as computational heart models.
Computational models are computer-simulated replicas that combine mathematics, physics, and computer science. These models have been around for a long time and are used in many fields, ranging from manufacturing to economics.
In heart medicine, these models are populated with digital “cells,” which imitate living cells and can be programmed with different electrical properties, depending on whether they are healthy or diseased.
“Currently available imaging and testing (MRIs, PETs, EKGs) give some representation of the scarring, but you cannot translate that to what is going to happen over time,” says Natalia Trayanova, PhD, of the Johns Hopkins department of biomedical engineering.
“With computational heart models, we create a dynamic digital image of the heart. We can then give the digital image an electrical stimulus and assess how the heart is able to respond. Then you can better predict what is going to happen.”
The computerized 3D models also mean better, more accurate treatment for heart conditions.
For example, a common treatment for a type of arrhythmia known as atrial fibrillation is ablation, or burning some heart tissue. Ablation stops the erratic electrical impulses causing the arrhythmia, but it can also damage otherwise healthy heart cells.
A personalized computational heart model could allow doctors to see more accurately what areas should and shouldn’t be treated for a specific patient.
Using deep learning AI to predict health outcomes
Dr. Trayanova’s colleague Dr. Popescu is applying deep learning and AI to do more with computerized heart models to predict the future.
In a recent paper in Nature Cardiovascular Research, the research team showed their algorithm assessed the health of 269 patients and was able to predict the chance of sudden cardiac arrest up to 10 years in advance.
“This is really the first time ever, as far as we know, where deep learning technology has been proven to analyze scarring of the heart in a successful way,” Dr. Popescu says.
Dr. Popescu and Dr. Trayanova say the AI algorithm gathers information from the 3D computational heart models with patient data like MRIs, ethnicity, age, lifestyle, and other clinical information. Analyzing all these data can produce accurate and consistent estimates about how long patients might live if they are at risk for sudden death.
“You can’t afford to be wrong. If you are wrong, you can actually impact a patient’s quality of life dramatically,” Dr. Popescu says. “Having clinicians use this technology in the decision-making process will provide confidence in a better diagnosis and prognosis.”
While the current study was specifically about patients with a particular type of heart disease, Dr. Popescu says his algorithm can also be trained to assess other health conditions.
So when might you see this being used outside of a research study? Dr. Trayanova predicts 3D imaging of heart models could be available in 2 years, but first the technique must be tested in more clinical trials – some of which are happening right now.
Adding AI to the heart models will require more studies and Food and Drug Administration approval, so the timeline is less clear. But perhaps the biggest hurdle is that after approval the technologies would need to be adopted and used by clinicians and caregivers.
“The much harder question to answer is, ‘When will doctors be perfectly comfortable with AI tools?’ And I don’t know the answer,” Dr. Popescu says. “How to use AI as an aid in the decision-making process is something that’s not currently taught.”
A version of this article first appeared on WebMD.com.
Deaths from COVID-19 may have caught more attention lately, but heart disease remains the leading cause of death in the United States.
More than 300,000 Americans will die this year of sudden cardiac arrest (also called sudden cardiac death, or SCD), when the heart abruptly stops working.
These events happen suddenly and often without warning, making them nearly impossible to predict. But that may be changing, thanks to 3D imaging and artificial intelligence (AI) technology under study at Johns Hopkins University, Baltimore.
There, researchers are working to create more accurate and personalized models of the heart – and not just any heart, your heart, if you have heart disease.
“Right now, a clinician can only say whether a patient is at risk or not at risk for sudden death,” says Dan Popescu, PhD, a Johns Hopkins research scientist and first author of a new study on AI’s ability to predict sudden cardiac arrest. “With this new technology, you can have much more nuanced predictions of probability of an event over time.”
Put another way: With AI, clinicians may be able not only to predict if someone is at risk for sudden cardiac arrest, but also when it is most likely to happen. They can do this using a much clearer and more personalized look at the electrical “wiring” of your heart.
Your heart, the conductor
Your heart isn’t just a metronome responsible for keeping a steady stream of blood pumping to tissues with every beat. It’s also a conductor through which vital energy flows.
To make the heart beat, electrical impulses flow from the top to the bottom of the organ. Healthy heart cells relay this electricity seamlessly. But in a heart damaged by inflammation or a past heart attack, scar tissue will block the energy flow.
When an electrical impulse encounters a scarred area, the signal can become erratic, disrupting the set top-to-bottom path and causing irregular heartbeats (arrhythmias), which increase someone’s danger of sudden cardiac death.
Seeing the heart in 3D
Today’s tests offer some insights into the heart’s makeup. For example, MRI scans can reveal damaged areas. PET scans can show inflammation. And EKGs can record the heart’s electrical signals from beat to beat.
But all these technologies offer only a snapshot, showing heart health at a moment in time. They can’t predict the future. That’s why scientists at Johns Hopkins are going further to develop 3D digital replicas of a person’s heart, known as computational heart models.
Computational models are computer-simulated replicas that combine mathematics, physics, and computer science. These models have been around for a long time and are used in many fields, ranging from manufacturing to economics.
In heart medicine, these models are populated with digital “cells,” which imitate living cells and can be programmed with different electrical properties, depending on whether they are healthy or diseased.
“Currently available imaging and testing (MRIs, PETs, EKGs) give some representation of the scarring, but you cannot translate that to what is going to happen over time,” says Natalia Trayanova, PhD, of the Johns Hopkins department of biomedical engineering.
“With computational heart models, we create a dynamic digital image of the heart. We can then give the digital image an electrical stimulus and assess how the heart is able to respond. Then you can better predict what is going to happen.”
The computerized 3D models also mean better, more accurate treatment for heart conditions.
For example, a common treatment for a type of arrhythmia known as atrial fibrillation is ablation, or burning some heart tissue. Ablation stops the erratic electrical impulses causing the arrhythmia, but it can also damage otherwise healthy heart cells.
A personalized computational heart model could allow doctors to see more accurately what areas should and shouldn’t be treated for a specific patient.
Using deep learning AI to predict health outcomes
Dr. Trayanova’s colleague Dr. Popescu is applying deep learning and AI to do more with computerized heart models to predict the future.
In a recent paper in Nature Cardiovascular Research, the research team showed their algorithm assessed the health of 269 patients and was able to predict the chance of sudden cardiac arrest up to 10 years in advance.
“This is really the first time ever, as far as we know, where deep learning technology has been proven to analyze scarring of the heart in a successful way,” Dr. Popescu says.
Dr. Popescu and Dr. Trayanova say the AI algorithm gathers information from the 3D computational heart models with patient data like MRIs, ethnicity, age, lifestyle, and other clinical information. Analyzing all these data can produce accurate and consistent estimates about how long patients might live if they are at risk for sudden death.
“You can’t afford to be wrong. If you are wrong, you can actually impact a patient’s quality of life dramatically,” Dr. Popescu says. “Having clinicians use this technology in the decision-making process will provide confidence in a better diagnosis and prognosis.”
While the current study was specifically about patients with a particular type of heart disease, Dr. Popescu says his algorithm can also be trained to assess other health conditions.
So when might you see this being used outside of a research study? Dr. Trayanova predicts 3D imaging of heart models could be available in 2 years, but first the technique must be tested in more clinical trials – some of which are happening right now.
Adding AI to the heart models will require more studies and Food and Drug Administration approval, so the timeline is less clear. But perhaps the biggest hurdle is that after approval the technologies would need to be adopted and used by clinicians and caregivers.
“The much harder question to answer is, ‘When will doctors be perfectly comfortable with AI tools?’ And I don’t know the answer,” Dr. Popescu says. “How to use AI as an aid in the decision-making process is something that’s not currently taught.”
A version of this article first appeared on WebMD.com.
What to expect when you’re expecting ... a preemie
The prospect of having a premature infant can be highly stressful. But a new study found that providing pregnant patients hospitalized for preterm labor with detailed information about what to expect with an early birth significantly reduced their anxiety about the process.
The study found that both printed handouts and a tablet app were associated with a 50% reduction in anxiety and appeared to be equally effective, although the handouts are likely easier to use in the high-stress environment of neonatal intensive care facilities, according to the researchers, who presented the findings April 25 at the annual meeting of the Pediatric Academic Societies.
“When patients get admitted for preterm labor a neonatologist comes to talk to parents about outcomes, short- and long-term, like bleeding in the baby’s brain and the possible need to have surgeries,” said Nicole Rau, MD, assistant professor of clinical pediatrics at University of Illinois at Peoria, who led the study. “Then parents are asked to make decisions during a high-stress time while they’re still processing everything. Everyone agrees that’s really not ideal.”
About 1 in 10 babies in the United States are born prematurely – or before 37 weeks of gestation – each year. That adds up to about 500,000 per year. Many spend days or weeks in neonatal intensive care units – watched from a distance by their anxious parents desperate for answers and reassurance. Potential complications for infants born prematurely include heart issues, trouble breathing, brain bleeds, and difficulty controlling their body temperature.
The American Academy of Pediatrics and the National Institute of Child Health and Human Development have warned that birth parents at risk for premature delivery may not be adequately prepared for what to expect. According to the groups, although clinicians may counsel these patients on admission to the hospital, factors such as stress, pain, and maternal medication can make the message difficult to comprehend.
For the study, Dr. Rau and her colleagues divided patients at the Medical College of Wisconsin and Children’s Hospital of Wisconsin who were hospitalized between 22 and 33 weeks of pregnancy into two groups: Some received a handout on preterm labor, and some were given a bedside tablet with an app called Preemie Prep for Parents.
Seventy-six women were randomized in gestational age blocks of 22-24 weeks and 25-33 weeks. After some opted not to complete the study, 59 participants remained – 32 of whom received handouts, and 27 who had access to tablets.
After distributing the materials, Dr. Rau’s group gave patients a questionnaire asking about delivery resuscitation, short-term problems, long-term problems, treatments, length of stay, and miscellaneous questions about their care. The two groups performed similarly – the tablet group’s median score was 20/30, and the handout group’s median score was 22/30.
Using the State-Trait Anxiety Inventory, researchers found both groups experienced a 50% reduction in anxiety after learning more from their respective materials.
Dr. Rau said she and her colleagues expected patients with access to the app would perform better based on cognition studies that have shown multimedia tools are more effective than tools that use visual or audio information but not both. However, both groups seemed to benefit comparably, which she said may reflect underuse of the app.
What was clear, though, is that patients absorbed more information and felt better prepared when they received it in ways beyond verbal communication.
“Well-written, parent-friendly information is a great tool to supplement counseling,” Dr. Rau told this news organization.
Because preterm labor is a relatively common occurrence, expectant parents should be well-prepared with proper information, said Erika Werner, MD, chair of obstetrics & gynecology at Tufts Medical Center, Boston, who was not involved in the study.
“Preterm labor is something that’s way more common than people think,” Dr. Werner told this news organization. “As long as it’s coming from a trusted source, additional information is a good thing. Knowing in advance some of the things that might be different from what you expect is always important. The more that we as providers have time to educate patients about potential risks, the better the outcomes will be.”
The authors reported no relevant financial conflicts of interest. The study was supported by grants from Children’s Research Institute and AMAG Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The prospect of having a premature infant can be highly stressful. But a new study found that providing pregnant patients hospitalized for preterm labor with detailed information about what to expect with an early birth significantly reduced their anxiety about the process.
The study found that both printed handouts and a tablet app were associated with a 50% reduction in anxiety and appeared to be equally effective, although the handouts are likely easier to use in the high-stress environment of neonatal intensive care facilities, according to the researchers, who presented the findings April 25 at the annual meeting of the Pediatric Academic Societies.
“When patients get admitted for preterm labor a neonatologist comes to talk to parents about outcomes, short- and long-term, like bleeding in the baby’s brain and the possible need to have surgeries,” said Nicole Rau, MD, assistant professor of clinical pediatrics at University of Illinois at Peoria, who led the study. “Then parents are asked to make decisions during a high-stress time while they’re still processing everything. Everyone agrees that’s really not ideal.”
About 1 in 10 babies in the United States are born prematurely – or before 37 weeks of gestation – each year. That adds up to about 500,000 per year. Many spend days or weeks in neonatal intensive care units – watched from a distance by their anxious parents desperate for answers and reassurance. Potential complications for infants born prematurely include heart issues, trouble breathing, brain bleeds, and difficulty controlling their body temperature.
The American Academy of Pediatrics and the National Institute of Child Health and Human Development have warned that birth parents at risk for premature delivery may not be adequately prepared for what to expect. According to the groups, although clinicians may counsel these patients on admission to the hospital, factors such as stress, pain, and maternal medication can make the message difficult to comprehend.
For the study, Dr. Rau and her colleagues divided patients at the Medical College of Wisconsin and Children’s Hospital of Wisconsin who were hospitalized between 22 and 33 weeks of pregnancy into two groups: Some received a handout on preterm labor, and some were given a bedside tablet with an app called Preemie Prep for Parents.
Seventy-six women were randomized in gestational age blocks of 22-24 weeks and 25-33 weeks. After some opted not to complete the study, 59 participants remained – 32 of whom received handouts, and 27 who had access to tablets.
After distributing the materials, Dr. Rau’s group gave patients a questionnaire asking about delivery resuscitation, short-term problems, long-term problems, treatments, length of stay, and miscellaneous questions about their care. The two groups performed similarly – the tablet group’s median score was 20/30, and the handout group’s median score was 22/30.
Using the State-Trait Anxiety Inventory, researchers found both groups experienced a 50% reduction in anxiety after learning more from their respective materials.
Dr. Rau said she and her colleagues expected patients with access to the app would perform better based on cognition studies that have shown multimedia tools are more effective than tools that use visual or audio information but not both. However, both groups seemed to benefit comparably, which she said may reflect underuse of the app.
What was clear, though, is that patients absorbed more information and felt better prepared when they received it in ways beyond verbal communication.
“Well-written, parent-friendly information is a great tool to supplement counseling,” Dr. Rau told this news organization.
Because preterm labor is a relatively common occurrence, expectant parents should be well-prepared with proper information, said Erika Werner, MD, chair of obstetrics & gynecology at Tufts Medical Center, Boston, who was not involved in the study.
“Preterm labor is something that’s way more common than people think,” Dr. Werner told this news organization. “As long as it’s coming from a trusted source, additional information is a good thing. Knowing in advance some of the things that might be different from what you expect is always important. The more that we as providers have time to educate patients about potential risks, the better the outcomes will be.”
The authors reported no relevant financial conflicts of interest. The study was supported by grants from Children’s Research Institute and AMAG Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The prospect of having a premature infant can be highly stressful. But a new study found that providing pregnant patients hospitalized for preterm labor with detailed information about what to expect with an early birth significantly reduced their anxiety about the process.
The study found that both printed handouts and a tablet app were associated with a 50% reduction in anxiety and appeared to be equally effective, although the handouts are likely easier to use in the high-stress environment of neonatal intensive care facilities, according to the researchers, who presented the findings April 25 at the annual meeting of the Pediatric Academic Societies.
“When patients get admitted for preterm labor a neonatologist comes to talk to parents about outcomes, short- and long-term, like bleeding in the baby’s brain and the possible need to have surgeries,” said Nicole Rau, MD, assistant professor of clinical pediatrics at University of Illinois at Peoria, who led the study. “Then parents are asked to make decisions during a high-stress time while they’re still processing everything. Everyone agrees that’s really not ideal.”
About 1 in 10 babies in the United States are born prematurely – or before 37 weeks of gestation – each year. That adds up to about 500,000 per year. Many spend days or weeks in neonatal intensive care units – watched from a distance by their anxious parents desperate for answers and reassurance. Potential complications for infants born prematurely include heart issues, trouble breathing, brain bleeds, and difficulty controlling their body temperature.
The American Academy of Pediatrics and the National Institute of Child Health and Human Development have warned that birth parents at risk for premature delivery may not be adequately prepared for what to expect. According to the groups, although clinicians may counsel these patients on admission to the hospital, factors such as stress, pain, and maternal medication can make the message difficult to comprehend.
For the study, Dr. Rau and her colleagues divided patients at the Medical College of Wisconsin and Children’s Hospital of Wisconsin who were hospitalized between 22 and 33 weeks of pregnancy into two groups: Some received a handout on preterm labor, and some were given a bedside tablet with an app called Preemie Prep for Parents.
Seventy-six women were randomized in gestational age blocks of 22-24 weeks and 25-33 weeks. After some opted not to complete the study, 59 participants remained – 32 of whom received handouts, and 27 who had access to tablets.
After distributing the materials, Dr. Rau’s group gave patients a questionnaire asking about delivery resuscitation, short-term problems, long-term problems, treatments, length of stay, and miscellaneous questions about their care. The two groups performed similarly – the tablet group’s median score was 20/30, and the handout group’s median score was 22/30.
Using the State-Trait Anxiety Inventory, researchers found both groups experienced a 50% reduction in anxiety after learning more from their respective materials.
Dr. Rau said she and her colleagues expected patients with access to the app would perform better based on cognition studies that have shown multimedia tools are more effective than tools that use visual or audio information but not both. However, both groups seemed to benefit comparably, which she said may reflect underuse of the app.
What was clear, though, is that patients absorbed more information and felt better prepared when they received it in ways beyond verbal communication.
“Well-written, parent-friendly information is a great tool to supplement counseling,” Dr. Rau told this news organization.
Because preterm labor is a relatively common occurrence, expectant parents should be well-prepared with proper information, said Erika Werner, MD, chair of obstetrics & gynecology at Tufts Medical Center, Boston, who was not involved in the study.
“Preterm labor is something that’s way more common than people think,” Dr. Werner told this news organization. “As long as it’s coming from a trusted source, additional information is a good thing. Knowing in advance some of the things that might be different from what you expect is always important. The more that we as providers have time to educate patients about potential risks, the better the outcomes will be.”
The authors reported no relevant financial conflicts of interest. The study was supported by grants from Children’s Research Institute and AMAG Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM PAS 2022