User login
Can aspirin prolong survival in patients with NSCLC?
(NSCLC), according to a new study from Taiwan.
The analysis, published online Nov. 22 in BMC Cancer , adds another data point to a small and inconsistent evidence base.
“Despite the need for future prospective randomized clinical trials, aspirin may be considered as an additional treatment for inoperable NSCLC patients,” Ming-Szu Hung, MD, of Chang-Gung University, Taoyuan City, and colleagues write.
The current literature suggests that the over-the-counter medication may help ward off various types of cancer, including lung cancer, but the various study findings do not always align. For lung-cancer survival, in particular, a few observational studies have found increased survival among aspirin users while others have not.
To help bring clarity to the literature, Dr. Hung’s team examined data from Taiwan’s National Health Insurance Research Database on more than 38,000 patients diagnosed with NSCLC between 2000 and 2012, almost 5,000 of whom were taking aspirin at the time of diagnosis.
The researchers found that aspirin users survived for a median of 1.73 years, compared with 1.30 years for nonusers. Taking the drug was associated with longer overall survival in time-varying covariate analysis (hazard ratio, 0.83; 95% CI, 0.80-0.86). This finding was confirmed in a propensity-score analysis of 4,932 matched pairs (HR, 0.79; 95% CI, 0.75-0.83).
“These results warrant further randomized clinical trials to evaluate the actual role of aspirin in the treatment of NSCLC patients,” the researchers conclude.
But Úna McMenamin, PhD, a cancer epidemiologist at Queen’s University Belfast, Ireland, was not convinced by the study’s methods.
While she praised its large size and use of population-based health registers, she expressed concern about the potential for reverse causation, “as it is unclear whether authors lagged the aspirin exposure in the cohort of lung cancer patients.”
There is evidence that common medications such as aspirin may be withdrawn from patients who are thought to be near the end of their life, Dr. McMenamin told this news organization. When not factored into the statistical analysis, aspirin may appear “to be spuriously associated with a reduced risk of death when, in fact, no association may be present.”
Previous studies of aspirin use in lung cancer patients that have included a lag, such as one Dr. McMenamin and colleagues conducted in 2015, have found no evidence of a protective effect.
That is why, according to Dr. McMenamin, “additional population-based studies, in diverse populations, are required to investigate the association between aspirin use and survival outcomes in lung-cancer patients to determine whether randomized controlled trials are warranted in this patient group.”
In addition, she noted, “any potential benefit of aspirin in lung cancer patients needs to be balanced against known adverse events associated with prolonged aspirin use, such as gastrointestinal bleeding.”
Dr. Hung did not reply to requests for comment.
The study had no funding, and the researchers report no conflicts of interest.
A version of this article first appeared on Medscape.com.
(NSCLC), according to a new study from Taiwan.
The analysis, published online Nov. 22 in BMC Cancer , adds another data point to a small and inconsistent evidence base.
“Despite the need for future prospective randomized clinical trials, aspirin may be considered as an additional treatment for inoperable NSCLC patients,” Ming-Szu Hung, MD, of Chang-Gung University, Taoyuan City, and colleagues write.
The current literature suggests that the over-the-counter medication may help ward off various types of cancer, including lung cancer, but the various study findings do not always align. For lung-cancer survival, in particular, a few observational studies have found increased survival among aspirin users while others have not.
To help bring clarity to the literature, Dr. Hung’s team examined data from Taiwan’s National Health Insurance Research Database on more than 38,000 patients diagnosed with NSCLC between 2000 and 2012, almost 5,000 of whom were taking aspirin at the time of diagnosis.
The researchers found that aspirin users survived for a median of 1.73 years, compared with 1.30 years for nonusers. Taking the drug was associated with longer overall survival in time-varying covariate analysis (hazard ratio, 0.83; 95% CI, 0.80-0.86). This finding was confirmed in a propensity-score analysis of 4,932 matched pairs (HR, 0.79; 95% CI, 0.75-0.83).
“These results warrant further randomized clinical trials to evaluate the actual role of aspirin in the treatment of NSCLC patients,” the researchers conclude.
But Úna McMenamin, PhD, a cancer epidemiologist at Queen’s University Belfast, Ireland, was not convinced by the study’s methods.
While she praised its large size and use of population-based health registers, she expressed concern about the potential for reverse causation, “as it is unclear whether authors lagged the aspirin exposure in the cohort of lung cancer patients.”
There is evidence that common medications such as aspirin may be withdrawn from patients who are thought to be near the end of their life, Dr. McMenamin told this news organization. When not factored into the statistical analysis, aspirin may appear “to be spuriously associated with a reduced risk of death when, in fact, no association may be present.”
Previous studies of aspirin use in lung cancer patients that have included a lag, such as one Dr. McMenamin and colleagues conducted in 2015, have found no evidence of a protective effect.
That is why, according to Dr. McMenamin, “additional population-based studies, in diverse populations, are required to investigate the association between aspirin use and survival outcomes in lung-cancer patients to determine whether randomized controlled trials are warranted in this patient group.”
In addition, she noted, “any potential benefit of aspirin in lung cancer patients needs to be balanced against known adverse events associated with prolonged aspirin use, such as gastrointestinal bleeding.”
Dr. Hung did not reply to requests for comment.
The study had no funding, and the researchers report no conflicts of interest.
A version of this article first appeared on Medscape.com.
(NSCLC), according to a new study from Taiwan.
The analysis, published online Nov. 22 in BMC Cancer , adds another data point to a small and inconsistent evidence base.
“Despite the need for future prospective randomized clinical trials, aspirin may be considered as an additional treatment for inoperable NSCLC patients,” Ming-Szu Hung, MD, of Chang-Gung University, Taoyuan City, and colleagues write.
The current literature suggests that the over-the-counter medication may help ward off various types of cancer, including lung cancer, but the various study findings do not always align. For lung-cancer survival, in particular, a few observational studies have found increased survival among aspirin users while others have not.
To help bring clarity to the literature, Dr. Hung’s team examined data from Taiwan’s National Health Insurance Research Database on more than 38,000 patients diagnosed with NSCLC between 2000 and 2012, almost 5,000 of whom were taking aspirin at the time of diagnosis.
The researchers found that aspirin users survived for a median of 1.73 years, compared with 1.30 years for nonusers. Taking the drug was associated with longer overall survival in time-varying covariate analysis (hazard ratio, 0.83; 95% CI, 0.80-0.86). This finding was confirmed in a propensity-score analysis of 4,932 matched pairs (HR, 0.79; 95% CI, 0.75-0.83).
“These results warrant further randomized clinical trials to evaluate the actual role of aspirin in the treatment of NSCLC patients,” the researchers conclude.
But Úna McMenamin, PhD, a cancer epidemiologist at Queen’s University Belfast, Ireland, was not convinced by the study’s methods.
While she praised its large size and use of population-based health registers, she expressed concern about the potential for reverse causation, “as it is unclear whether authors lagged the aspirin exposure in the cohort of lung cancer patients.”
There is evidence that common medications such as aspirin may be withdrawn from patients who are thought to be near the end of their life, Dr. McMenamin told this news organization. When not factored into the statistical analysis, aspirin may appear “to be spuriously associated with a reduced risk of death when, in fact, no association may be present.”
Previous studies of aspirin use in lung cancer patients that have included a lag, such as one Dr. McMenamin and colleagues conducted in 2015, have found no evidence of a protective effect.
That is why, according to Dr. McMenamin, “additional population-based studies, in diverse populations, are required to investigate the association between aspirin use and survival outcomes in lung-cancer patients to determine whether randomized controlled trials are warranted in this patient group.”
In addition, she noted, “any potential benefit of aspirin in lung cancer patients needs to be balanced against known adverse events associated with prolonged aspirin use, such as gastrointestinal bleeding.”
Dr. Hung did not reply to requests for comment.
The study had no funding, and the researchers report no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM BMC CANCER
Moderna warns of material drop in vaccine efficacy against Omicron
“There is no world, I think, where [the effectiveness] is the same level … we had with Delta,” Stephane Bancel told the Financial Times .
“I think it’s going to be a material drop,” he said. “I just don’t know how much, because we need to wait for the data. But all the scientists I’ve talked to … are like, ‘This is not going to be good.’”
Vaccine companies are now studying whether the new Omicron variant could evade the current shots. Some data is expected in about 2 weeks.
Mr. Bancel said that if a new vaccine is needed, it could take several months to produce at scale. He estimated that Moderna could make billions of vaccine doses in 2022.
“[Moderna] and Pfizer cannot get a billion doses next week. The math doesn’t work,” he said. “But could we get the billion doses out by the summer? Sure.”
The news caused some panic on Nov. 30, prompting financial markets to fall sharply, according to Reuters. But the markets recovered after European officials gave a more reassuring outlook.
“Even if the new variant becomes more widespread, the vaccines we have will continue to provide protection,” Emer Cooke, executive director of the European Medicines Agency, told the European Parliament.
Mr. Cooke said the agency could approve new vaccines that target the Omicron variant within 3 to 4 months, if needed. Moderna and Pfizer have announced they are beginning to tailor a shot to address the Omicron variant in case the data shows they are necessary.
Also on Nov. 30, the European Centre for Disease Prevention and Control announced that 42 Omicron cases had been identified in 10 European Union countries, according to Reuters.
The cases were mild or had no symptoms, although they were found in younger people who may have mild or no symptoms anyway.
“For the assessment of whether [Omicron] escapes immunity, we still have to wait until investigations in the laboratories with [blood samples] from people who have recovered have been carried out,” Andrea Ammon, MD, chair of the agency, said during an online conference.
The University of Oxford, which developed a COVID-19 vaccine with AstraZeneca, said Nov. 30 that there’s no evidence that vaccines won’t prevent severe disease from the Omicron variant, according to Reuters.
“Despite the appearance of new variants over the past year, vaccines have continued to provide very high levels of protection against severe disease and there is no evidence so far that Omicron is any different,” the university said in a statement. “However, we have the necessary tools and processes in place for rapid development of an updated COVID-19 vaccine if it should be necessary.”
A version of this article first appeared on WebMD.com.
“There is no world, I think, where [the effectiveness] is the same level … we had with Delta,” Stephane Bancel told the Financial Times .
“I think it’s going to be a material drop,” he said. “I just don’t know how much, because we need to wait for the data. But all the scientists I’ve talked to … are like, ‘This is not going to be good.’”
Vaccine companies are now studying whether the new Omicron variant could evade the current shots. Some data is expected in about 2 weeks.
Mr. Bancel said that if a new vaccine is needed, it could take several months to produce at scale. He estimated that Moderna could make billions of vaccine doses in 2022.
“[Moderna] and Pfizer cannot get a billion doses next week. The math doesn’t work,” he said. “But could we get the billion doses out by the summer? Sure.”
The news caused some panic on Nov. 30, prompting financial markets to fall sharply, according to Reuters. But the markets recovered after European officials gave a more reassuring outlook.
“Even if the new variant becomes more widespread, the vaccines we have will continue to provide protection,” Emer Cooke, executive director of the European Medicines Agency, told the European Parliament.
Mr. Cooke said the agency could approve new vaccines that target the Omicron variant within 3 to 4 months, if needed. Moderna and Pfizer have announced they are beginning to tailor a shot to address the Omicron variant in case the data shows they are necessary.
Also on Nov. 30, the European Centre for Disease Prevention and Control announced that 42 Omicron cases had been identified in 10 European Union countries, according to Reuters.
The cases were mild or had no symptoms, although they were found in younger people who may have mild or no symptoms anyway.
“For the assessment of whether [Omicron] escapes immunity, we still have to wait until investigations in the laboratories with [blood samples] from people who have recovered have been carried out,” Andrea Ammon, MD, chair of the agency, said during an online conference.
The University of Oxford, which developed a COVID-19 vaccine with AstraZeneca, said Nov. 30 that there’s no evidence that vaccines won’t prevent severe disease from the Omicron variant, according to Reuters.
“Despite the appearance of new variants over the past year, vaccines have continued to provide very high levels of protection against severe disease and there is no evidence so far that Omicron is any different,” the university said in a statement. “However, we have the necessary tools and processes in place for rapid development of an updated COVID-19 vaccine if it should be necessary.”
A version of this article first appeared on WebMD.com.
“There is no world, I think, where [the effectiveness] is the same level … we had with Delta,” Stephane Bancel told the Financial Times .
“I think it’s going to be a material drop,” he said. “I just don’t know how much, because we need to wait for the data. But all the scientists I’ve talked to … are like, ‘This is not going to be good.’”
Vaccine companies are now studying whether the new Omicron variant could evade the current shots. Some data is expected in about 2 weeks.
Mr. Bancel said that if a new vaccine is needed, it could take several months to produce at scale. He estimated that Moderna could make billions of vaccine doses in 2022.
“[Moderna] and Pfizer cannot get a billion doses next week. The math doesn’t work,” he said. “But could we get the billion doses out by the summer? Sure.”
The news caused some panic on Nov. 30, prompting financial markets to fall sharply, according to Reuters. But the markets recovered after European officials gave a more reassuring outlook.
“Even if the new variant becomes more widespread, the vaccines we have will continue to provide protection,” Emer Cooke, executive director of the European Medicines Agency, told the European Parliament.
Mr. Cooke said the agency could approve new vaccines that target the Omicron variant within 3 to 4 months, if needed. Moderna and Pfizer have announced they are beginning to tailor a shot to address the Omicron variant in case the data shows they are necessary.
Also on Nov. 30, the European Centre for Disease Prevention and Control announced that 42 Omicron cases had been identified in 10 European Union countries, according to Reuters.
The cases were mild or had no symptoms, although they were found in younger people who may have mild or no symptoms anyway.
“For the assessment of whether [Omicron] escapes immunity, we still have to wait until investigations in the laboratories with [blood samples] from people who have recovered have been carried out,” Andrea Ammon, MD, chair of the agency, said during an online conference.
The University of Oxford, which developed a COVID-19 vaccine with AstraZeneca, said Nov. 30 that there’s no evidence that vaccines won’t prevent severe disease from the Omicron variant, according to Reuters.
“Despite the appearance of new variants over the past year, vaccines have continued to provide very high levels of protection against severe disease and there is no evidence so far that Omicron is any different,” the university said in a statement. “However, we have the necessary tools and processes in place for rapid development of an updated COVID-19 vaccine if it should be necessary.”
A version of this article first appeared on WebMD.com.
Children and COVID: New cases, vaccinations both decline
States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
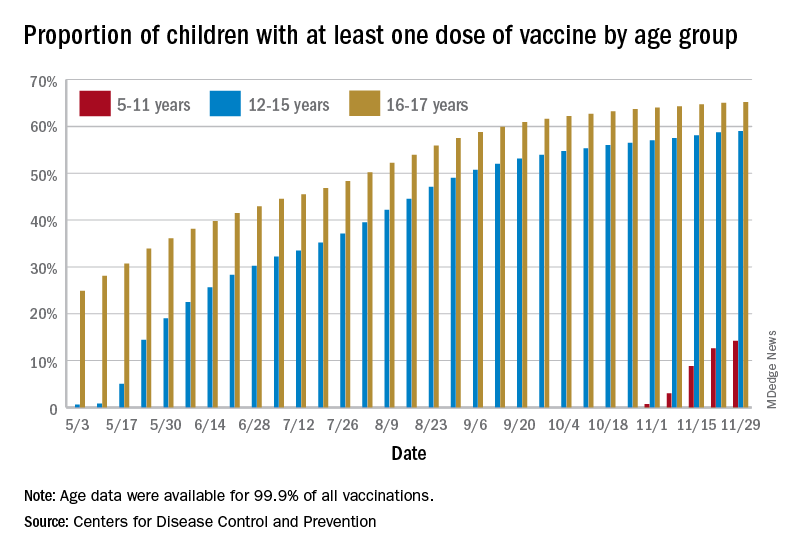
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.

States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.

States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.
What’s hot at the world’s premiere breast cancer meeting
Dr. Arteaga, the meeting’s codirector, said the first-ever hybrid symposium will take place virtually from Dec. 7 to 10 as well as in person. Online availability appears to be a boon to attendance, with a record 9,325 registrants for the 2020 symposium, held only virtually because of the COVID-19 pandemic.
The meeting will have an app available, which can be accessed by searching “San Antonio Breast Cancer Symposium” (Google Play for Android, Apple for iOS) and downloading, or by going to www.core-apps.com/dl/sabcs from a desktop computer.
Dr. Arteaga provided a sneak peek of the most exciting research being presented at the upcoming meeting.
On the horizon for advanced breast cancer
A “very important” study of an investigational oral agent employed in heavily pretreated postmenopausal women with estrogen receptor–positive (ER+) advanced breast cancer headlines the meeting.
This international, multicenter trial could have “practice-changing implications,” Dr. Arteaga said in an interview.
The phase 3 EMERALD trial (abstract GS2-02) pits elacestrant, a selective estrogen receptor degrader (SERD), against standard endocrine therapy (fulvestrant or an aromatase inhibitor) in patients with metastatic breast cancer whose disease has progressed after treatment with at least one endocrine therapy and a CDK4/6 inhibitor.
The trial is important because many patients with breast cancer have estrogen receptor mutations, which are a “major mechanism of [drug] resistance” and thus progression on earlier therapy, Dr. Arteaga said.
Elacestrant is in good company among a plethora of oral SERDs under investigation in advanced breast cancer; however, currently, fulvestrant – which requires an intramuscular injection in the buttocks every month – is the only approved SERD.
“There’s plenty of preclinical data that suggest that these drugs [SERDs] may have activity against these mutant forms of the receptor, which occur in up to 40% of patients with advanced ER+ breast cancer,” he explained.
Researchers will present data on two primary outcome measures from the phase 3 trial: progression-free survival (PFS) based on mutations of the estrogen receptor 1 gene (ESR1-mut) and PFS in all subjects regardless of ESR1 status.
In addition to the EMERALD trial, PADA-1 (abstract GS3-05) is another important randomized, phase 3 trial focused on treating estrogen receptor mutations in patients with metastatic disease, said Dr. Arteaga.
The trial has enrolled patients with ER+ metastatic breast cancer who received an aromatase inhibitor (letrozole, anastrozole, or exemestane) and the CDK4/6 inhibitor palbociclib as first-line therapy.
In step 1 of the trial, approximately 1,000 patients were screened for circulating blood ESR1 mutation detection at regular intervals while being treated with palbociclib and an aromatase inhibitor in a continuous scheme until tumor progression or ESR1 mutation detection.
In step 2, up to 200 patients with a rising circulating ESR1 mutation and no tumor progression were randomized 1:1 to no change in therapy until tumor progression or to receive palbociclib plus fulvestrant until tumor progression.
The trial examines the safety and efficacy of “a clinical conundrum that we face” in this setting: whether or not to switch treatment from an aromatase inhibitor to fulvestrant while continuing a CDK4/6 inhibitor at the sign of mutation detection, Dr. Arteaga explained.
Refining who gets the ‘kitchen sink’
Dr. Arteaga highlighted two trials focused on the immune checkpoint inhibitor pembrolizumab.
The phase 3 KEYNOTE-522 study led to the approval of neoadjuvant pembrolizumab plus chemotherapy for early-stage triple-negative breast cancer (TNBC) in July 2021. At this year’s SABCS, researchers will present new data from KEYNOTE-522 (abstract GS1-01), representing final results from the trial’s event-free survival (EFS) outcome.
Previously, investigators reported a statistically significant and clinically meaningful improvement in EFS. These data suggest “that deploying immunotherapy early before surgery ... may be curative in some patients,” Dr. Arteaga said. The new data will allow the “robustness and consistency” of the earlier findings to be assessed.
But, he added, this is a “tough” treatment, which includes five drugs. “It’s the kitchen sink, and not everybody needs the kitchen sink. It’s important to refine these findings. Some patients may not need pembrolizumab, but some do.”
The second trial exploring pembrolizumab – KEYNOTE-355 (abstract GS1-02) – mirrors KEYNOTE-522 but in patients with previously untreated locally recurrent inoperable or metastatic TNBC whose tumors expressed PD-L1.
Previously, investigators reported that pembrolizumab combined with chemotherapy showed statistically significant improvements in overall survival and PFS compared to placebo plus chemotherapy. At the 2021 SABCS, researchers will provide final study results, including outcomes in subgroups of patients by additional combined positive score cutoffs.
Metformin trial: ‘This is it’
Dr. Arteaga highlighted CCTGMA.32 (abstract GS1-08), a phase 3 randomized, placebo-controlled adjuvant trial of the diabetes drug metformin versus placebo in early breast cancer. Results of the primary efficacy analysis of the trial will be presented at the meeting.
The Canadian-led study seeks to determine if metformin can decrease breast cancer cell growth and work with cancer therapies to prevent disease recurrence. The study design calls for patients to take twice-daily oral metformin or placebo pills for up to 5 years in the absence of disease progression.
The primary outcome of the 3,500-plus patient trial is invasive disease-free survival in hormone receptor (ER and PgR) negative and positive (ER and/or PgR) subgroups.
“Metformin has actually been associated with improved survival [in breast cancer] in patients on chemotherapy. But we don’t know exactly how,” he said. “There’s never been a head-to-head comparison in the adjuvant setting [before]. This is it.”
TKI for breast cancer with brain mets
The SABCS codirector spotlighted an updated overall survival analysis of the randomized phase 3 PHOEBE trial (abstract GS3-02).
Previous research confirmed the superiority of pyrotinib, a novel TKI targeting HER1, HER2, and HER4, over lapatinib when given in combination with capecitabine in HER2-positive metastatic breast cancer.
In the United States, the lapatinib-capecitabine combination is “mostly used” in patients with HER2 metastatic disease and brain metastases who also undergo stereotactic radiation, Dr. Arteaga said.
This use has continued despite groundbreaking results from the HER2CLIMB trial, featuring the TKI tucatinib, he said.
As reported last year, adding tucatinib to trastuzumab and capecitabine in patients with HER2-positive breast cancer and brain metastases increased median overall survival from 12 months to 18.1 months. The results were called the first of their kind at that time.
The pyrotinib study may matter to American clinicians because pyrotinib is used mostly in China, not the United States, and this analysis suggests that pyrotinib could be part of the armamentarium in the United States, alongside tucatinib.
TKIs are like Coke and Pepsi, Dr. Arteaga said: “Similar but not identical.” Therefore, it is worth taking a look at the new study, he said. “There may be some benefit in having more than one [TKI] in the therapeutic armamentarium.”
Dr. Arteaga receives or has received grant support from Pfizer and Lilly and serves or has served in a scientific advisory role with Novartis, Lilly, TAIHO Oncology, Daiichi Sankyo, Merck, AstraZeneca, OrigiMed, Immunomedics, ARVINAS, Sanofi, Athenex, and the Susan G. Komen Foundation. He also holds minor stock options from Provista.
A version of this article first appeared on Medscape.com.
Dr. Arteaga, the meeting’s codirector, said the first-ever hybrid symposium will take place virtually from Dec. 7 to 10 as well as in person. Online availability appears to be a boon to attendance, with a record 9,325 registrants for the 2020 symposium, held only virtually because of the COVID-19 pandemic.
The meeting will have an app available, which can be accessed by searching “San Antonio Breast Cancer Symposium” (Google Play for Android, Apple for iOS) and downloading, or by going to www.core-apps.com/dl/sabcs from a desktop computer.
Dr. Arteaga provided a sneak peek of the most exciting research being presented at the upcoming meeting.
On the horizon for advanced breast cancer
A “very important” study of an investigational oral agent employed in heavily pretreated postmenopausal women with estrogen receptor–positive (ER+) advanced breast cancer headlines the meeting.
This international, multicenter trial could have “practice-changing implications,” Dr. Arteaga said in an interview.
The phase 3 EMERALD trial (abstract GS2-02) pits elacestrant, a selective estrogen receptor degrader (SERD), against standard endocrine therapy (fulvestrant or an aromatase inhibitor) in patients with metastatic breast cancer whose disease has progressed after treatment with at least one endocrine therapy and a CDK4/6 inhibitor.
The trial is important because many patients with breast cancer have estrogen receptor mutations, which are a “major mechanism of [drug] resistance” and thus progression on earlier therapy, Dr. Arteaga said.
Elacestrant is in good company among a plethora of oral SERDs under investigation in advanced breast cancer; however, currently, fulvestrant – which requires an intramuscular injection in the buttocks every month – is the only approved SERD.
“There’s plenty of preclinical data that suggest that these drugs [SERDs] may have activity against these mutant forms of the receptor, which occur in up to 40% of patients with advanced ER+ breast cancer,” he explained.
Researchers will present data on two primary outcome measures from the phase 3 trial: progression-free survival (PFS) based on mutations of the estrogen receptor 1 gene (ESR1-mut) and PFS in all subjects regardless of ESR1 status.
In addition to the EMERALD trial, PADA-1 (abstract GS3-05) is another important randomized, phase 3 trial focused on treating estrogen receptor mutations in patients with metastatic disease, said Dr. Arteaga.
The trial has enrolled patients with ER+ metastatic breast cancer who received an aromatase inhibitor (letrozole, anastrozole, or exemestane) and the CDK4/6 inhibitor palbociclib as first-line therapy.
In step 1 of the trial, approximately 1,000 patients were screened for circulating blood ESR1 mutation detection at regular intervals while being treated with palbociclib and an aromatase inhibitor in a continuous scheme until tumor progression or ESR1 mutation detection.
In step 2, up to 200 patients with a rising circulating ESR1 mutation and no tumor progression were randomized 1:1 to no change in therapy until tumor progression or to receive palbociclib plus fulvestrant until tumor progression.
The trial examines the safety and efficacy of “a clinical conundrum that we face” in this setting: whether or not to switch treatment from an aromatase inhibitor to fulvestrant while continuing a CDK4/6 inhibitor at the sign of mutation detection, Dr. Arteaga explained.
Refining who gets the ‘kitchen sink’
Dr. Arteaga highlighted two trials focused on the immune checkpoint inhibitor pembrolizumab.
The phase 3 KEYNOTE-522 study led to the approval of neoadjuvant pembrolizumab plus chemotherapy for early-stage triple-negative breast cancer (TNBC) in July 2021. At this year’s SABCS, researchers will present new data from KEYNOTE-522 (abstract GS1-01), representing final results from the trial’s event-free survival (EFS) outcome.
Previously, investigators reported a statistically significant and clinically meaningful improvement in EFS. These data suggest “that deploying immunotherapy early before surgery ... may be curative in some patients,” Dr. Arteaga said. The new data will allow the “robustness and consistency” of the earlier findings to be assessed.
But, he added, this is a “tough” treatment, which includes five drugs. “It’s the kitchen sink, and not everybody needs the kitchen sink. It’s important to refine these findings. Some patients may not need pembrolizumab, but some do.”
The second trial exploring pembrolizumab – KEYNOTE-355 (abstract GS1-02) – mirrors KEYNOTE-522 but in patients with previously untreated locally recurrent inoperable or metastatic TNBC whose tumors expressed PD-L1.
Previously, investigators reported that pembrolizumab combined with chemotherapy showed statistically significant improvements in overall survival and PFS compared to placebo plus chemotherapy. At the 2021 SABCS, researchers will provide final study results, including outcomes in subgroups of patients by additional combined positive score cutoffs.
Metformin trial: ‘This is it’
Dr. Arteaga highlighted CCTGMA.32 (abstract GS1-08), a phase 3 randomized, placebo-controlled adjuvant trial of the diabetes drug metformin versus placebo in early breast cancer. Results of the primary efficacy analysis of the trial will be presented at the meeting.
The Canadian-led study seeks to determine if metformin can decrease breast cancer cell growth and work with cancer therapies to prevent disease recurrence. The study design calls for patients to take twice-daily oral metformin or placebo pills for up to 5 years in the absence of disease progression.
The primary outcome of the 3,500-plus patient trial is invasive disease-free survival in hormone receptor (ER and PgR) negative and positive (ER and/or PgR) subgroups.
“Metformin has actually been associated with improved survival [in breast cancer] in patients on chemotherapy. But we don’t know exactly how,” he said. “There’s never been a head-to-head comparison in the adjuvant setting [before]. This is it.”
TKI for breast cancer with brain mets
The SABCS codirector spotlighted an updated overall survival analysis of the randomized phase 3 PHOEBE trial (abstract GS3-02).
Previous research confirmed the superiority of pyrotinib, a novel TKI targeting HER1, HER2, and HER4, over lapatinib when given in combination with capecitabine in HER2-positive metastatic breast cancer.
In the United States, the lapatinib-capecitabine combination is “mostly used” in patients with HER2 metastatic disease and brain metastases who also undergo stereotactic radiation, Dr. Arteaga said.
This use has continued despite groundbreaking results from the HER2CLIMB trial, featuring the TKI tucatinib, he said.
As reported last year, adding tucatinib to trastuzumab and capecitabine in patients with HER2-positive breast cancer and brain metastases increased median overall survival from 12 months to 18.1 months. The results were called the first of their kind at that time.
The pyrotinib study may matter to American clinicians because pyrotinib is used mostly in China, not the United States, and this analysis suggests that pyrotinib could be part of the armamentarium in the United States, alongside tucatinib.
TKIs are like Coke and Pepsi, Dr. Arteaga said: “Similar but not identical.” Therefore, it is worth taking a look at the new study, he said. “There may be some benefit in having more than one [TKI] in the therapeutic armamentarium.”
Dr. Arteaga receives or has received grant support from Pfizer and Lilly and serves or has served in a scientific advisory role with Novartis, Lilly, TAIHO Oncology, Daiichi Sankyo, Merck, AstraZeneca, OrigiMed, Immunomedics, ARVINAS, Sanofi, Athenex, and the Susan G. Komen Foundation. He also holds minor stock options from Provista.
A version of this article first appeared on Medscape.com.
Dr. Arteaga, the meeting’s codirector, said the first-ever hybrid symposium will take place virtually from Dec. 7 to 10 as well as in person. Online availability appears to be a boon to attendance, with a record 9,325 registrants for the 2020 symposium, held only virtually because of the COVID-19 pandemic.
The meeting will have an app available, which can be accessed by searching “San Antonio Breast Cancer Symposium” (Google Play for Android, Apple for iOS) and downloading, or by going to www.core-apps.com/dl/sabcs from a desktop computer.
Dr. Arteaga provided a sneak peek of the most exciting research being presented at the upcoming meeting.
On the horizon for advanced breast cancer
A “very important” study of an investigational oral agent employed in heavily pretreated postmenopausal women with estrogen receptor–positive (ER+) advanced breast cancer headlines the meeting.
This international, multicenter trial could have “practice-changing implications,” Dr. Arteaga said in an interview.
The phase 3 EMERALD trial (abstract GS2-02) pits elacestrant, a selective estrogen receptor degrader (SERD), against standard endocrine therapy (fulvestrant or an aromatase inhibitor) in patients with metastatic breast cancer whose disease has progressed after treatment with at least one endocrine therapy and a CDK4/6 inhibitor.
The trial is important because many patients with breast cancer have estrogen receptor mutations, which are a “major mechanism of [drug] resistance” and thus progression on earlier therapy, Dr. Arteaga said.
Elacestrant is in good company among a plethora of oral SERDs under investigation in advanced breast cancer; however, currently, fulvestrant – which requires an intramuscular injection in the buttocks every month – is the only approved SERD.
“There’s plenty of preclinical data that suggest that these drugs [SERDs] may have activity against these mutant forms of the receptor, which occur in up to 40% of patients with advanced ER+ breast cancer,” he explained.
Researchers will present data on two primary outcome measures from the phase 3 trial: progression-free survival (PFS) based on mutations of the estrogen receptor 1 gene (ESR1-mut) and PFS in all subjects regardless of ESR1 status.
In addition to the EMERALD trial, PADA-1 (abstract GS3-05) is another important randomized, phase 3 trial focused on treating estrogen receptor mutations in patients with metastatic disease, said Dr. Arteaga.
The trial has enrolled patients with ER+ metastatic breast cancer who received an aromatase inhibitor (letrozole, anastrozole, or exemestane) and the CDK4/6 inhibitor palbociclib as first-line therapy.
In step 1 of the trial, approximately 1,000 patients were screened for circulating blood ESR1 mutation detection at regular intervals while being treated with palbociclib and an aromatase inhibitor in a continuous scheme until tumor progression or ESR1 mutation detection.
In step 2, up to 200 patients with a rising circulating ESR1 mutation and no tumor progression were randomized 1:1 to no change in therapy until tumor progression or to receive palbociclib plus fulvestrant until tumor progression.
The trial examines the safety and efficacy of “a clinical conundrum that we face” in this setting: whether or not to switch treatment from an aromatase inhibitor to fulvestrant while continuing a CDK4/6 inhibitor at the sign of mutation detection, Dr. Arteaga explained.
Refining who gets the ‘kitchen sink’
Dr. Arteaga highlighted two trials focused on the immune checkpoint inhibitor pembrolizumab.
The phase 3 KEYNOTE-522 study led to the approval of neoadjuvant pembrolizumab plus chemotherapy for early-stage triple-negative breast cancer (TNBC) in July 2021. At this year’s SABCS, researchers will present new data from KEYNOTE-522 (abstract GS1-01), representing final results from the trial’s event-free survival (EFS) outcome.
Previously, investigators reported a statistically significant and clinically meaningful improvement in EFS. These data suggest “that deploying immunotherapy early before surgery ... may be curative in some patients,” Dr. Arteaga said. The new data will allow the “robustness and consistency” of the earlier findings to be assessed.
But, he added, this is a “tough” treatment, which includes five drugs. “It’s the kitchen sink, and not everybody needs the kitchen sink. It’s important to refine these findings. Some patients may not need pembrolizumab, but some do.”
The second trial exploring pembrolizumab – KEYNOTE-355 (abstract GS1-02) – mirrors KEYNOTE-522 but in patients with previously untreated locally recurrent inoperable or metastatic TNBC whose tumors expressed PD-L1.
Previously, investigators reported that pembrolizumab combined with chemotherapy showed statistically significant improvements in overall survival and PFS compared to placebo plus chemotherapy. At the 2021 SABCS, researchers will provide final study results, including outcomes in subgroups of patients by additional combined positive score cutoffs.
Metformin trial: ‘This is it’
Dr. Arteaga highlighted CCTGMA.32 (abstract GS1-08), a phase 3 randomized, placebo-controlled adjuvant trial of the diabetes drug metformin versus placebo in early breast cancer. Results of the primary efficacy analysis of the trial will be presented at the meeting.
The Canadian-led study seeks to determine if metformin can decrease breast cancer cell growth and work with cancer therapies to prevent disease recurrence. The study design calls for patients to take twice-daily oral metformin or placebo pills for up to 5 years in the absence of disease progression.
The primary outcome of the 3,500-plus patient trial is invasive disease-free survival in hormone receptor (ER and PgR) negative and positive (ER and/or PgR) subgroups.
“Metformin has actually been associated with improved survival [in breast cancer] in patients on chemotherapy. But we don’t know exactly how,” he said. “There’s never been a head-to-head comparison in the adjuvant setting [before]. This is it.”
TKI for breast cancer with brain mets
The SABCS codirector spotlighted an updated overall survival analysis of the randomized phase 3 PHOEBE trial (abstract GS3-02).
Previous research confirmed the superiority of pyrotinib, a novel TKI targeting HER1, HER2, and HER4, over lapatinib when given in combination with capecitabine in HER2-positive metastatic breast cancer.
In the United States, the lapatinib-capecitabine combination is “mostly used” in patients with HER2 metastatic disease and brain metastases who also undergo stereotactic radiation, Dr. Arteaga said.
This use has continued despite groundbreaking results from the HER2CLIMB trial, featuring the TKI tucatinib, he said.
As reported last year, adding tucatinib to trastuzumab and capecitabine in patients with HER2-positive breast cancer and brain metastases increased median overall survival from 12 months to 18.1 months. The results were called the first of their kind at that time.
The pyrotinib study may matter to American clinicians because pyrotinib is used mostly in China, not the United States, and this analysis suggests that pyrotinib could be part of the armamentarium in the United States, alongside tucatinib.
TKIs are like Coke and Pepsi, Dr. Arteaga said: “Similar but not identical.” Therefore, it is worth taking a look at the new study, he said. “There may be some benefit in having more than one [TKI] in the therapeutic armamentarium.”
Dr. Arteaga receives or has received grant support from Pfizer and Lilly and serves or has served in a scientific advisory role with Novartis, Lilly, TAIHO Oncology, Daiichi Sankyo, Merck, AstraZeneca, OrigiMed, Immunomedics, ARVINAS, Sanofi, Athenex, and the Susan G. Komen Foundation. He also holds minor stock options from Provista.
A version of this article first appeared on Medscape.com.
FROM SABCS 2021
Association of height, BMI, and AD in young children may be transient
The , according to a large cohort study published online in JAMA Dermatology.
“The potential for ‘catch up’ in height for children with atopic dermatitis observed in our study may be explained with resolution of atopic dermatitis or successful treatment,” write senior author Aaron M. Drucker, MD, ScM, from the division of dermatology, University of Toronto, and Women’s College Hospital in Toronto, and colleagues. They postulated that, while the association between AD and shorter height is “is likely multifactorial,” it may be driven in part by sleep loss caused by AD, or corticosteroid treatment of AD, both of which can result in growth retardation and subsequent increased BMI.
The researchers used data from TARGet Kids!, a prospective, longitudinal cohort study designed to study multiple health conditions in children from general pediatric and family practices across Toronto. Their study included 10,611 children for whom there was data on height, weight, BMI, and standardized z scores, which account for age and sex differences in anthropometric characteristics. Clinically relevant covariates that were collected included child age, sex, birth weight, history of asthma, family income, maternal and paternal ethnicity, and maternal height and BMI.
The mean age of the children in the study at cohort entry was 23 months, and they were followed for a median of 28.5 months, during which time they had a median of two visits. At baseline, 947 (8.9%) children had parent-reported AD, with this number rising to 1,834 (17.3%) during follow-up.
After adjusting for covariates, AD was associated with lower mean z-height (P < .001), higher mean z-BMI (P = .008), but lower mean z-weight (P < .001), compared with children without AD. Using World Health Organization growth tables, the researchers estimated that “children with atopic dermatitis were, on average, approximately 0.5 cm shorter at age 2 years and 0.6 cm shorter at age 5 years than children without atopic dermatitis” after adjusting for covariates. They also estimated that children with AD were “on average, approximately 0.2 more BMI units at age 2 years” than children without AD. The associations between AD and height diminished by age 14 years, as did the association between AD and BMI by age 5.5 years.
“Given that we found children with atopic dermatitis to be somewhat less heavy, as measured by z-weight, than children without atopic dermatitis and that this association did not attenuate with age, it is possible that our findings for BMI, and perhaps those of previous studies, are explained mainly by differences in height,” the authors write. “This distinction has obvious clinical importance – rather than a focus on obesity and obesogenic behaviors being problematic in children with atopic dermatitis, research might be better directed at understanding the association between atopic dermatitis and initially shorter stature.”
Asked to comment on the study results, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, told this news organization he would have preferred using the wording “in addition to focusing on obesity,” rather than “focus on obesity.”
“We should not ignore diet and sedentary activity as important factors,” he said, pointing to another recent study that found higher rates of eating disorders associated with AD.
Dr. Silverberg said that he was not familiar enough with the cohort sample to comment on how representative it is of the Canadian population, or on how generalizable the results are to other regions and populations. Generalizability, he added, “is an important issue, as we previously found regional differences with respect to the association between AD and obesity.”
In addition, he noted that in the study AD was defined as an “ever history” of disease rather than “in the past year or currently,” so, even though it is a longitudinal study, “it is really looking at how AD at any point in patients’ lives is related to weight or stature,” he explained. But, he added, “many cases of childhood AD ‘burn out’ or become milder/clear as the children get older. So, if the AD clears, then one would expect to see attenuation of associations as the children get older. However, this doesn’t tell us about how persistent AD into later childhood or adolescence is related to height or weight.”
Previous studies found that short stature and obesity were particularly associated with moderate – and even more to severe – atopic dermatitis, Dr. Silverberg said. It is likely that most patients in this primary care cohort had mild disease, he noted, so the effect sizes are likely diluted by mostly mild disease “and not relevant to the more persistent and severe AD patients encountered in the dermatology practice setting.”
The study was supported by the department of medicine, Women’s College Hospital, and the Canadian Institutes of Health Research.
One author reported receiving compensation from the British Journal of Dermatology, the American Academy of Dermatology, and the National Eczema Association and has served as a paid consultant for the Canadian Agency for Drugs and Technologies in Health outside the submitted work. No other disclosures were reported. Dr. Silverberg has disclosed no relevant financial relationships.
Commentary by Robert Sidbury, MD, MPH
Among the more puzzling “associations” to emerge in recent literature has been the association between atopic dermatitis (AD) and obesity. I see many children with severe AD every day and my gestalt “association” is a thinner, shorter child rather than an overweight one. Dr. Drucker and colleagues’ data has helped me understand this dissonance. Children with AD do in fact, on average, weigh less but they are also shorter, possibly explaining their higher body mass index (BMI). More important, these findings are transient, with height differences dissipating by 14 years of age, and BMI differences by kindergarten. This information should train providers’ sights on optimal AD treatment and optimal nutritional and lifestyle support without undue concern for obesity or obesogenic behaviors.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
The , according to a large cohort study published online in JAMA Dermatology.
“The potential for ‘catch up’ in height for children with atopic dermatitis observed in our study may be explained with resolution of atopic dermatitis or successful treatment,” write senior author Aaron M. Drucker, MD, ScM, from the division of dermatology, University of Toronto, and Women’s College Hospital in Toronto, and colleagues. They postulated that, while the association between AD and shorter height is “is likely multifactorial,” it may be driven in part by sleep loss caused by AD, or corticosteroid treatment of AD, both of which can result in growth retardation and subsequent increased BMI.
The researchers used data from TARGet Kids!, a prospective, longitudinal cohort study designed to study multiple health conditions in children from general pediatric and family practices across Toronto. Their study included 10,611 children for whom there was data on height, weight, BMI, and standardized z scores, which account for age and sex differences in anthropometric characteristics. Clinically relevant covariates that were collected included child age, sex, birth weight, history of asthma, family income, maternal and paternal ethnicity, and maternal height and BMI.
The mean age of the children in the study at cohort entry was 23 months, and they were followed for a median of 28.5 months, during which time they had a median of two visits. At baseline, 947 (8.9%) children had parent-reported AD, with this number rising to 1,834 (17.3%) during follow-up.
After adjusting for covariates, AD was associated with lower mean z-height (P < .001), higher mean z-BMI (P = .008), but lower mean z-weight (P < .001), compared with children without AD. Using World Health Organization growth tables, the researchers estimated that “children with atopic dermatitis were, on average, approximately 0.5 cm shorter at age 2 years and 0.6 cm shorter at age 5 years than children without atopic dermatitis” after adjusting for covariates. They also estimated that children with AD were “on average, approximately 0.2 more BMI units at age 2 years” than children without AD. The associations between AD and height diminished by age 14 years, as did the association between AD and BMI by age 5.5 years.
“Given that we found children with atopic dermatitis to be somewhat less heavy, as measured by z-weight, than children without atopic dermatitis and that this association did not attenuate with age, it is possible that our findings for BMI, and perhaps those of previous studies, are explained mainly by differences in height,” the authors write. “This distinction has obvious clinical importance – rather than a focus on obesity and obesogenic behaviors being problematic in children with atopic dermatitis, research might be better directed at understanding the association between atopic dermatitis and initially shorter stature.”
Asked to comment on the study results, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, told this news organization he would have preferred using the wording “in addition to focusing on obesity,” rather than “focus on obesity.”
“We should not ignore diet and sedentary activity as important factors,” he said, pointing to another recent study that found higher rates of eating disorders associated with AD.
Dr. Silverberg said that he was not familiar enough with the cohort sample to comment on how representative it is of the Canadian population, or on how generalizable the results are to other regions and populations. Generalizability, he added, “is an important issue, as we previously found regional differences with respect to the association between AD and obesity.”
In addition, he noted that in the study AD was defined as an “ever history” of disease rather than “in the past year or currently,” so, even though it is a longitudinal study, “it is really looking at how AD at any point in patients’ lives is related to weight or stature,” he explained. But, he added, “many cases of childhood AD ‘burn out’ or become milder/clear as the children get older. So, if the AD clears, then one would expect to see attenuation of associations as the children get older. However, this doesn’t tell us about how persistent AD into later childhood or adolescence is related to height or weight.”
Previous studies found that short stature and obesity were particularly associated with moderate – and even more to severe – atopic dermatitis, Dr. Silverberg said. It is likely that most patients in this primary care cohort had mild disease, he noted, so the effect sizes are likely diluted by mostly mild disease “and not relevant to the more persistent and severe AD patients encountered in the dermatology practice setting.”
The study was supported by the department of medicine, Women’s College Hospital, and the Canadian Institutes of Health Research.
One author reported receiving compensation from the British Journal of Dermatology, the American Academy of Dermatology, and the National Eczema Association and has served as a paid consultant for the Canadian Agency for Drugs and Technologies in Health outside the submitted work. No other disclosures were reported. Dr. Silverberg has disclosed no relevant financial relationships.
Commentary by Robert Sidbury, MD, MPH
Among the more puzzling “associations” to emerge in recent literature has been the association between atopic dermatitis (AD) and obesity. I see many children with severe AD every day and my gestalt “association” is a thinner, shorter child rather than an overweight one. Dr. Drucker and colleagues’ data has helped me understand this dissonance. Children with AD do in fact, on average, weigh less but they are also shorter, possibly explaining their higher body mass index (BMI). More important, these findings are transient, with height differences dissipating by 14 years of age, and BMI differences by kindergarten. This information should train providers’ sights on optimal AD treatment and optimal nutritional and lifestyle support without undue concern for obesity or obesogenic behaviors.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
The , according to a large cohort study published online in JAMA Dermatology.
“The potential for ‘catch up’ in height for children with atopic dermatitis observed in our study may be explained with resolution of atopic dermatitis or successful treatment,” write senior author Aaron M. Drucker, MD, ScM, from the division of dermatology, University of Toronto, and Women’s College Hospital in Toronto, and colleagues. They postulated that, while the association between AD and shorter height is “is likely multifactorial,” it may be driven in part by sleep loss caused by AD, or corticosteroid treatment of AD, both of which can result in growth retardation and subsequent increased BMI.
The researchers used data from TARGet Kids!, a prospective, longitudinal cohort study designed to study multiple health conditions in children from general pediatric and family practices across Toronto. Their study included 10,611 children for whom there was data on height, weight, BMI, and standardized z scores, which account for age and sex differences in anthropometric characteristics. Clinically relevant covariates that were collected included child age, sex, birth weight, history of asthma, family income, maternal and paternal ethnicity, and maternal height and BMI.
The mean age of the children in the study at cohort entry was 23 months, and they were followed for a median of 28.5 months, during which time they had a median of two visits. At baseline, 947 (8.9%) children had parent-reported AD, with this number rising to 1,834 (17.3%) during follow-up.
After adjusting for covariates, AD was associated with lower mean z-height (P < .001), higher mean z-BMI (P = .008), but lower mean z-weight (P < .001), compared with children without AD. Using World Health Organization growth tables, the researchers estimated that “children with atopic dermatitis were, on average, approximately 0.5 cm shorter at age 2 years and 0.6 cm shorter at age 5 years than children without atopic dermatitis” after adjusting for covariates. They also estimated that children with AD were “on average, approximately 0.2 more BMI units at age 2 years” than children without AD. The associations between AD and height diminished by age 14 years, as did the association between AD and BMI by age 5.5 years.
“Given that we found children with atopic dermatitis to be somewhat less heavy, as measured by z-weight, than children without atopic dermatitis and that this association did not attenuate with age, it is possible that our findings for BMI, and perhaps those of previous studies, are explained mainly by differences in height,” the authors write. “This distinction has obvious clinical importance – rather than a focus on obesity and obesogenic behaviors being problematic in children with atopic dermatitis, research might be better directed at understanding the association between atopic dermatitis and initially shorter stature.”
Asked to comment on the study results, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, told this news organization he would have preferred using the wording “in addition to focusing on obesity,” rather than “focus on obesity.”
“We should not ignore diet and sedentary activity as important factors,” he said, pointing to another recent study that found higher rates of eating disorders associated with AD.
Dr. Silverberg said that he was not familiar enough with the cohort sample to comment on how representative it is of the Canadian population, or on how generalizable the results are to other regions and populations. Generalizability, he added, “is an important issue, as we previously found regional differences with respect to the association between AD and obesity.”
In addition, he noted that in the study AD was defined as an “ever history” of disease rather than “in the past year or currently,” so, even though it is a longitudinal study, “it is really looking at how AD at any point in patients’ lives is related to weight or stature,” he explained. But, he added, “many cases of childhood AD ‘burn out’ or become milder/clear as the children get older. So, if the AD clears, then one would expect to see attenuation of associations as the children get older. However, this doesn’t tell us about how persistent AD into later childhood or adolescence is related to height or weight.”
Previous studies found that short stature and obesity were particularly associated with moderate – and even more to severe – atopic dermatitis, Dr. Silverberg said. It is likely that most patients in this primary care cohort had mild disease, he noted, so the effect sizes are likely diluted by mostly mild disease “and not relevant to the more persistent and severe AD patients encountered in the dermatology practice setting.”
The study was supported by the department of medicine, Women’s College Hospital, and the Canadian Institutes of Health Research.
One author reported receiving compensation from the British Journal of Dermatology, the American Academy of Dermatology, and the National Eczema Association and has served as a paid consultant for the Canadian Agency for Drugs and Technologies in Health outside the submitted work. No other disclosures were reported. Dr. Silverberg has disclosed no relevant financial relationships.
Commentary by Robert Sidbury, MD, MPH
Among the more puzzling “associations” to emerge in recent literature has been the association between atopic dermatitis (AD) and obesity. I see many children with severe AD every day and my gestalt “association” is a thinner, shorter child rather than an overweight one. Dr. Drucker and colleagues’ data has helped me understand this dissonance. Children with AD do in fact, on average, weigh less but they are also shorter, possibly explaining their higher body mass index (BMI). More important, these findings are transient, with height differences dissipating by 14 years of age, and BMI differences by kindergarten. This information should train providers’ sights on optimal AD treatment and optimal nutritional and lifestyle support without undue concern for obesity or obesogenic behaviors.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM JAMA DERMATOLOGY
After doc uproar, NCCN reverses prostate cancer guidance
After making a controversial change in September to a long-standing recommendation about the use of active surveillance in men with prostate cancer, the National Comprehensive Cancer Network (NCCN) has reversed course and reinstated its original advice, with a slight tweak.
The influential cancer organization, which is best known for its guidelines, now recommends that “most” men with low-risk prostate cancer be offered active surveillance as the lone “preferred” initial treatment option. This advice aligns closely with the group’s initial recommendation, published over a decade ago.
But controversy erupted in late September when the NCCN suddenly changed its tune on active surveillance, recommending that men with low-risk disease be managed with either active surveillance, radiation therapy, or surgery, with equal weight given to all three.
The new advice angered physicians who support the concept of active surveillance, which aims to avoid or delay treatment — and potentially life-changing side effects — until signs of disease progression.
The NCCN listened to the complaints.
On Nov. 30, the group largely reverted back to the original recommendation. In updated guidelines,
In an email sent to this news organization, Edward Schaeffer, MD, PhD, chair of the prostate cancer treatment panel, said that “the NCCN Prostate Cancer Panel recently convened” and “extensively revised the Principles of Active Surveillance and Observation.”
Dr. Schaeffer, who is from the Lurie Comprehensive Cancer Center of Northwestern University in Chicago, cited the heterogeneity across the low-risk disease group. Factors associated with an increased probability of near-term grade reclassification from low risk to higher risk include high PSA density, a high number of positive cores (≥ 3), high genomic risk (from tissue-based molecular tumor analysis), and/or a known BRCA2 germline mutation, he added.
Urologists cheered the NCCN’s reversal on Twitter.
“Big news! NCCN guidelines updated again — active surveillance is ‘preferred for most patients’ with low risk prostate cancer and life expectancy >=10 years,” tweeted Stacy Loeb of NYU Langone in New York City.
“Very exciting if true,” tweeted Matthew Cooperberg, MD, of University of California San Francisco, who was one of the most vocal critics of the NCCN’s change in September, calling that move a “step backward” that would likely lead to overtreatment.
A version of this article first appeared on Medscape.com.
After making a controversial change in September to a long-standing recommendation about the use of active surveillance in men with prostate cancer, the National Comprehensive Cancer Network (NCCN) has reversed course and reinstated its original advice, with a slight tweak.
The influential cancer organization, which is best known for its guidelines, now recommends that “most” men with low-risk prostate cancer be offered active surveillance as the lone “preferred” initial treatment option. This advice aligns closely with the group’s initial recommendation, published over a decade ago.
But controversy erupted in late September when the NCCN suddenly changed its tune on active surveillance, recommending that men with low-risk disease be managed with either active surveillance, radiation therapy, or surgery, with equal weight given to all three.
The new advice angered physicians who support the concept of active surveillance, which aims to avoid or delay treatment — and potentially life-changing side effects — until signs of disease progression.
The NCCN listened to the complaints.
On Nov. 30, the group largely reverted back to the original recommendation. In updated guidelines,
In an email sent to this news organization, Edward Schaeffer, MD, PhD, chair of the prostate cancer treatment panel, said that “the NCCN Prostate Cancer Panel recently convened” and “extensively revised the Principles of Active Surveillance and Observation.”
Dr. Schaeffer, who is from the Lurie Comprehensive Cancer Center of Northwestern University in Chicago, cited the heterogeneity across the low-risk disease group. Factors associated with an increased probability of near-term grade reclassification from low risk to higher risk include high PSA density, a high number of positive cores (≥ 3), high genomic risk (from tissue-based molecular tumor analysis), and/or a known BRCA2 germline mutation, he added.
Urologists cheered the NCCN’s reversal on Twitter.
“Big news! NCCN guidelines updated again — active surveillance is ‘preferred for most patients’ with low risk prostate cancer and life expectancy >=10 years,” tweeted Stacy Loeb of NYU Langone in New York City.
“Very exciting if true,” tweeted Matthew Cooperberg, MD, of University of California San Francisco, who was one of the most vocal critics of the NCCN’s change in September, calling that move a “step backward” that would likely lead to overtreatment.
A version of this article first appeared on Medscape.com.
After making a controversial change in September to a long-standing recommendation about the use of active surveillance in men with prostate cancer, the National Comprehensive Cancer Network (NCCN) has reversed course and reinstated its original advice, with a slight tweak.
The influential cancer organization, which is best known for its guidelines, now recommends that “most” men with low-risk prostate cancer be offered active surveillance as the lone “preferred” initial treatment option. This advice aligns closely with the group’s initial recommendation, published over a decade ago.
But controversy erupted in late September when the NCCN suddenly changed its tune on active surveillance, recommending that men with low-risk disease be managed with either active surveillance, radiation therapy, or surgery, with equal weight given to all three.
The new advice angered physicians who support the concept of active surveillance, which aims to avoid or delay treatment — and potentially life-changing side effects — until signs of disease progression.
The NCCN listened to the complaints.
On Nov. 30, the group largely reverted back to the original recommendation. In updated guidelines,
In an email sent to this news organization, Edward Schaeffer, MD, PhD, chair of the prostate cancer treatment panel, said that “the NCCN Prostate Cancer Panel recently convened” and “extensively revised the Principles of Active Surveillance and Observation.”
Dr. Schaeffer, who is from the Lurie Comprehensive Cancer Center of Northwestern University in Chicago, cited the heterogeneity across the low-risk disease group. Factors associated with an increased probability of near-term grade reclassification from low risk to higher risk include high PSA density, a high number of positive cores (≥ 3), high genomic risk (from tissue-based molecular tumor analysis), and/or a known BRCA2 germline mutation, he added.
Urologists cheered the NCCN’s reversal on Twitter.
“Big news! NCCN guidelines updated again — active surveillance is ‘preferred for most patients’ with low risk prostate cancer and life expectancy >=10 years,” tweeted Stacy Loeb of NYU Langone in New York City.
“Very exciting if true,” tweeted Matthew Cooperberg, MD, of University of California San Francisco, who was one of the most vocal critics of the NCCN’s change in September, calling that move a “step backward” that would likely lead to overtreatment.
A version of this article first appeared on Medscape.com.
ESD vs. cEMR: Rates of complete remission in Barrett’s compared
Treatment with endoscopic submucosal dissection (ESD) is associated with higher rates of complete remission of dysplasia at 2 years, compared with cap-assisted endoscopic mucosal resection (cEMR) in patients with Barrett’s esophagus with dysplasia or early-stage intramucosal esophageal adenocarcinoma (EAC), according to study findings.
Despite the seeming advantage of ESD over cEMR, the study found similar rates of complete remission of intestinal metaplasia (CRIM) between the treatment groups at 2 years.
The study authors explained that ESD, a recent development in endoscopic resection, allows for en bloc resection of larger lesions in dysplastic Barrett’s and EAC and features less diagnostic uncertainty, compared with cEMR. Findings from the study highlight the importance of this newer technique but also emphasize the utility of both treatments. “In expert hands both sets of procedures appear to be safe and well tolerated,” wrote study authors Don Codipilly, MD, of the Mayo Clinic in Rochester, Minn., and colleagues in Clinical Gastroenterology and Hepatology.
Given the lack of comparative data on the long-term outcomes of cEMR versus ESD in patients with neoplasia associated with Barrett’s esophagus, Dr. Codipilly and colleagues examined histologic outcomes in a prospectively maintained database of 537 patients who underwent endoscopic eradication therapy for Barrett’s esophagus or EAC at the Mayo Clinic between 2006 and 2020. Only patients who had undergone either cEMR (n = 456) or ESD (n = 81) followed by endoscopic ablation were included in the analysis.
The primary endpoint of the study was the rate and time to complete remission of dysplasia (CRD), which was defined by the absence of dysplasia on biopsy from the gastroesophageal junction and tubular esophagus during at least one surveillance endoscopy. Researchers also examined the rates of complications, such as clinically significant intraprocedural or postprocedural bleeding that required hospitalization, perforation, receipt of red blood cells within 30 days of the initial procedure, and stricture formation that required dilation within 120 days of the index procedure.
Patients in the ESD group had a longer mean length of resected specimens (23.9 vs. 10.9 mm; P < .01) as well as higher rates of en bloc (97.5% vs. 41.9%; P < .01) and R0 resection (58% vs. 20.2%; P < .01). Patients were generally balanced on other basic baseline demographics, including age, sex distribution, and smoking status.
Over a median 11.2-year follow-up period, a total of 420 patients in the cEMR group achieved CRD. In the ESD group, 48 patients achieved CRD over a median 1.4-year follow-up period. The 2-year cumulative probability of CRD was lower in patients who received cEMR versus those who received ESD (75.8% vs. 85.6%, respectively). In a univariate analysis, the odds of achieving CRD were lower in cEMR versus ESD (hazard ratio, 0.41; 95% CI, 0.31-0.54; P < .01).
According to multivariate analysis, two independent predictors of CRD included ESD (hazard ratio, 2.38; P <.01) and shorter Barrett’s segment length (HR, 1.11; P < .01).
The investigators also assessed whether advancements made in cEMR technique have contributed to the findings in an analysis of patients who underwent cEMR (n = 48) with ESD (n = 80) from 2015 to 2019. In this analysis, the researchers found that the odds of CRD were lower than that of ESD (HR, 0.67; 95% CI, 0.45-0.99). Additionally, higher odds of achieving CRD in the cEMR group were observed in years between 2013 and 2019 (n = 129), compared with years 2006-2012 (n = 112) (HR, 2.09; 95% CI, 1.59-2.75; P < .01).
Demographic and clinical variables were incorporated into a Cox proportional hazard model to identify factors associated with decreased odds of CRD. This analysis found that decreased odds of CRD were associated with longer Barrett’s esophagus segment length (HR, 0.90; P <.01) and treatment with cEMR versus ESD (HR, 0.42; P < .01).
Over median follow-up periods of 7.8 years in the cEMR group and 1.1 years in the ESD group, approximately 78.5% and 40.7% of patients, respectively, achieved CRIM. While those in the ESD group achieved CRIM earlier, the cumulative probabilities of CRIM were similar by 2 years (59.3% vs. 50.6%; HR, 0.74; 95% CI, 0.52-1.07; P = .11). Shorter Barrett’s esophagus segment was the only independent predictor of CRIM (HR, 1.16; P < .01).
The researchers noted that the study population may have included patients with more severe disease than that in the general population, which may limit the generalizability of the findings. Additionally, the lack of a randomized design was cited as an additional study limitation.
In spite of their findings, the researchers explained that “continued monitoring for additional outcomes such as recurrence are required for further elucidation of the optimal role of these procedures in the management of” neoplasia associated with Barrett’s esophagus.”
The study was funded by the National Cancer Institute and the Freeman Foundation. The researchers reported no conflicts of interest with any pharmaceutical companies.
When compared with cap-assisted EMR (cEMR), endoscopic submucosal dissection (ESD) of visible abnormalities within a Barrett’s segment leads to higher R0 resection rates in patients with Barrett’s related neoplasia. However, its superiority over cEMR with regards to clinical and histological outcomes has remained in question. The current study by Codipilly and colleagues attempts to address this issue by comparing histologic outcomes of cEMR versus ESD in dysplastic Barrett’s.
Since therapies that achieve CRIM, rather than primarily CRD, decrease risk of recurrence, the current study suggests ESD is not superior to cEMR in preventing recurrence for Barrett’s related neoplasia, and either technique may be employed based on local expertise. However, ESD is more effective for achieving CRD and may be preferable for lesions greater than 15 mm or lesions where superficial submucosal invasion is suspected and providing an accurate histopathologic specimen would help direct appropriate oncologic therapy. Further, long-term randomized clinical trials are needed to address differences in recurrence between both treatment modalities.
Salmaan Jawaid, MD, is an assistant professor of medicine in interventional endoscopy at Baylor College of Medicine, Houston. He has no relevant conflicts of interest.
When compared with cap-assisted EMR (cEMR), endoscopic submucosal dissection (ESD) of visible abnormalities within a Barrett’s segment leads to higher R0 resection rates in patients with Barrett’s related neoplasia. However, its superiority over cEMR with regards to clinical and histological outcomes has remained in question. The current study by Codipilly and colleagues attempts to address this issue by comparing histologic outcomes of cEMR versus ESD in dysplastic Barrett’s.
Since therapies that achieve CRIM, rather than primarily CRD, decrease risk of recurrence, the current study suggests ESD is not superior to cEMR in preventing recurrence for Barrett’s related neoplasia, and either technique may be employed based on local expertise. However, ESD is more effective for achieving CRD and may be preferable for lesions greater than 15 mm or lesions where superficial submucosal invasion is suspected and providing an accurate histopathologic specimen would help direct appropriate oncologic therapy. Further, long-term randomized clinical trials are needed to address differences in recurrence between both treatment modalities.
Salmaan Jawaid, MD, is an assistant professor of medicine in interventional endoscopy at Baylor College of Medicine, Houston. He has no relevant conflicts of interest.
When compared with cap-assisted EMR (cEMR), endoscopic submucosal dissection (ESD) of visible abnormalities within a Barrett’s segment leads to higher R0 resection rates in patients with Barrett’s related neoplasia. However, its superiority over cEMR with regards to clinical and histological outcomes has remained in question. The current study by Codipilly and colleagues attempts to address this issue by comparing histologic outcomes of cEMR versus ESD in dysplastic Barrett’s.
Since therapies that achieve CRIM, rather than primarily CRD, decrease risk of recurrence, the current study suggests ESD is not superior to cEMR in preventing recurrence for Barrett’s related neoplasia, and either technique may be employed based on local expertise. However, ESD is more effective for achieving CRD and may be preferable for lesions greater than 15 mm or lesions where superficial submucosal invasion is suspected and providing an accurate histopathologic specimen would help direct appropriate oncologic therapy. Further, long-term randomized clinical trials are needed to address differences in recurrence between both treatment modalities.
Salmaan Jawaid, MD, is an assistant professor of medicine in interventional endoscopy at Baylor College of Medicine, Houston. He has no relevant conflicts of interest.
Treatment with endoscopic submucosal dissection (ESD) is associated with higher rates of complete remission of dysplasia at 2 years, compared with cap-assisted endoscopic mucosal resection (cEMR) in patients with Barrett’s esophagus with dysplasia or early-stage intramucosal esophageal adenocarcinoma (EAC), according to study findings.
Despite the seeming advantage of ESD over cEMR, the study found similar rates of complete remission of intestinal metaplasia (CRIM) between the treatment groups at 2 years.
The study authors explained that ESD, a recent development in endoscopic resection, allows for en bloc resection of larger lesions in dysplastic Barrett’s and EAC and features less diagnostic uncertainty, compared with cEMR. Findings from the study highlight the importance of this newer technique but also emphasize the utility of both treatments. “In expert hands both sets of procedures appear to be safe and well tolerated,” wrote study authors Don Codipilly, MD, of the Mayo Clinic in Rochester, Minn., and colleagues in Clinical Gastroenterology and Hepatology.
Given the lack of comparative data on the long-term outcomes of cEMR versus ESD in patients with neoplasia associated with Barrett’s esophagus, Dr. Codipilly and colleagues examined histologic outcomes in a prospectively maintained database of 537 patients who underwent endoscopic eradication therapy for Barrett’s esophagus or EAC at the Mayo Clinic between 2006 and 2020. Only patients who had undergone either cEMR (n = 456) or ESD (n = 81) followed by endoscopic ablation were included in the analysis.
The primary endpoint of the study was the rate and time to complete remission of dysplasia (CRD), which was defined by the absence of dysplasia on biopsy from the gastroesophageal junction and tubular esophagus during at least one surveillance endoscopy. Researchers also examined the rates of complications, such as clinically significant intraprocedural or postprocedural bleeding that required hospitalization, perforation, receipt of red blood cells within 30 days of the initial procedure, and stricture formation that required dilation within 120 days of the index procedure.
Patients in the ESD group had a longer mean length of resected specimens (23.9 vs. 10.9 mm; P < .01) as well as higher rates of en bloc (97.5% vs. 41.9%; P < .01) and R0 resection (58% vs. 20.2%; P < .01). Patients were generally balanced on other basic baseline demographics, including age, sex distribution, and smoking status.
Over a median 11.2-year follow-up period, a total of 420 patients in the cEMR group achieved CRD. In the ESD group, 48 patients achieved CRD over a median 1.4-year follow-up period. The 2-year cumulative probability of CRD was lower in patients who received cEMR versus those who received ESD (75.8% vs. 85.6%, respectively). In a univariate analysis, the odds of achieving CRD were lower in cEMR versus ESD (hazard ratio, 0.41; 95% CI, 0.31-0.54; P < .01).
According to multivariate analysis, two independent predictors of CRD included ESD (hazard ratio, 2.38; P <.01) and shorter Barrett’s segment length (HR, 1.11; P < .01).
The investigators also assessed whether advancements made in cEMR technique have contributed to the findings in an analysis of patients who underwent cEMR (n = 48) with ESD (n = 80) from 2015 to 2019. In this analysis, the researchers found that the odds of CRD were lower than that of ESD (HR, 0.67; 95% CI, 0.45-0.99). Additionally, higher odds of achieving CRD in the cEMR group were observed in years between 2013 and 2019 (n = 129), compared with years 2006-2012 (n = 112) (HR, 2.09; 95% CI, 1.59-2.75; P < .01).
Demographic and clinical variables were incorporated into a Cox proportional hazard model to identify factors associated with decreased odds of CRD. This analysis found that decreased odds of CRD were associated with longer Barrett’s esophagus segment length (HR, 0.90; P <.01) and treatment with cEMR versus ESD (HR, 0.42; P < .01).
Over median follow-up periods of 7.8 years in the cEMR group and 1.1 years in the ESD group, approximately 78.5% and 40.7% of patients, respectively, achieved CRIM. While those in the ESD group achieved CRIM earlier, the cumulative probabilities of CRIM were similar by 2 years (59.3% vs. 50.6%; HR, 0.74; 95% CI, 0.52-1.07; P = .11). Shorter Barrett’s esophagus segment was the only independent predictor of CRIM (HR, 1.16; P < .01).
The researchers noted that the study population may have included patients with more severe disease than that in the general population, which may limit the generalizability of the findings. Additionally, the lack of a randomized design was cited as an additional study limitation.
In spite of their findings, the researchers explained that “continued monitoring for additional outcomes such as recurrence are required for further elucidation of the optimal role of these procedures in the management of” neoplasia associated with Barrett’s esophagus.”
The study was funded by the National Cancer Institute and the Freeman Foundation. The researchers reported no conflicts of interest with any pharmaceutical companies.
Treatment with endoscopic submucosal dissection (ESD) is associated with higher rates of complete remission of dysplasia at 2 years, compared with cap-assisted endoscopic mucosal resection (cEMR) in patients with Barrett’s esophagus with dysplasia or early-stage intramucosal esophageal adenocarcinoma (EAC), according to study findings.
Despite the seeming advantage of ESD over cEMR, the study found similar rates of complete remission of intestinal metaplasia (CRIM) between the treatment groups at 2 years.
The study authors explained that ESD, a recent development in endoscopic resection, allows for en bloc resection of larger lesions in dysplastic Barrett’s and EAC and features less diagnostic uncertainty, compared with cEMR. Findings from the study highlight the importance of this newer technique but also emphasize the utility of both treatments. “In expert hands both sets of procedures appear to be safe and well tolerated,” wrote study authors Don Codipilly, MD, of the Mayo Clinic in Rochester, Minn., and colleagues in Clinical Gastroenterology and Hepatology.
Given the lack of comparative data on the long-term outcomes of cEMR versus ESD in patients with neoplasia associated with Barrett’s esophagus, Dr. Codipilly and colleagues examined histologic outcomes in a prospectively maintained database of 537 patients who underwent endoscopic eradication therapy for Barrett’s esophagus or EAC at the Mayo Clinic between 2006 and 2020. Only patients who had undergone either cEMR (n = 456) or ESD (n = 81) followed by endoscopic ablation were included in the analysis.
The primary endpoint of the study was the rate and time to complete remission of dysplasia (CRD), which was defined by the absence of dysplasia on biopsy from the gastroesophageal junction and tubular esophagus during at least one surveillance endoscopy. Researchers also examined the rates of complications, such as clinically significant intraprocedural or postprocedural bleeding that required hospitalization, perforation, receipt of red blood cells within 30 days of the initial procedure, and stricture formation that required dilation within 120 days of the index procedure.
Patients in the ESD group had a longer mean length of resected specimens (23.9 vs. 10.9 mm; P < .01) as well as higher rates of en bloc (97.5% vs. 41.9%; P < .01) and R0 resection (58% vs. 20.2%; P < .01). Patients were generally balanced on other basic baseline demographics, including age, sex distribution, and smoking status.
Over a median 11.2-year follow-up period, a total of 420 patients in the cEMR group achieved CRD. In the ESD group, 48 patients achieved CRD over a median 1.4-year follow-up period. The 2-year cumulative probability of CRD was lower in patients who received cEMR versus those who received ESD (75.8% vs. 85.6%, respectively). In a univariate analysis, the odds of achieving CRD were lower in cEMR versus ESD (hazard ratio, 0.41; 95% CI, 0.31-0.54; P < .01).
According to multivariate analysis, two independent predictors of CRD included ESD (hazard ratio, 2.38; P <.01) and shorter Barrett’s segment length (HR, 1.11; P < .01).
The investigators also assessed whether advancements made in cEMR technique have contributed to the findings in an analysis of patients who underwent cEMR (n = 48) with ESD (n = 80) from 2015 to 2019. In this analysis, the researchers found that the odds of CRD were lower than that of ESD (HR, 0.67; 95% CI, 0.45-0.99). Additionally, higher odds of achieving CRD in the cEMR group were observed in years between 2013 and 2019 (n = 129), compared with years 2006-2012 (n = 112) (HR, 2.09; 95% CI, 1.59-2.75; P < .01).
Demographic and clinical variables were incorporated into a Cox proportional hazard model to identify factors associated with decreased odds of CRD. This analysis found that decreased odds of CRD were associated with longer Barrett’s esophagus segment length (HR, 0.90; P <.01) and treatment with cEMR versus ESD (HR, 0.42; P < .01).
Over median follow-up periods of 7.8 years in the cEMR group and 1.1 years in the ESD group, approximately 78.5% and 40.7% of patients, respectively, achieved CRIM. While those in the ESD group achieved CRIM earlier, the cumulative probabilities of CRIM were similar by 2 years (59.3% vs. 50.6%; HR, 0.74; 95% CI, 0.52-1.07; P = .11). Shorter Barrett’s esophagus segment was the only independent predictor of CRIM (HR, 1.16; P < .01).
The researchers noted that the study population may have included patients with more severe disease than that in the general population, which may limit the generalizability of the findings. Additionally, the lack of a randomized design was cited as an additional study limitation.
In spite of their findings, the researchers explained that “continued monitoring for additional outcomes such as recurrence are required for further elucidation of the optimal role of these procedures in the management of” neoplasia associated with Barrett’s esophagus.”
The study was funded by the National Cancer Institute and the Freeman Foundation. The researchers reported no conflicts of interest with any pharmaceutical companies.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
A Starter Guide to Immunofluorescence Testing in Dermatology
Direct immunofluorescence (DIF) is the go-to diagnostic test when evaluating vesiculobullous eruptions, connective tissue disease, and vasculitis. This specialized test allows visualization of autoantibodies and their reaction products in the epidermis and dermis (skin) and epithelium and subepithelium (mucosa). Indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA) are additional tests that can help in the diagnosis of autoimmune blistering disease. In the blistering autoimmune diseases, the autoantibodies target components in skin and mucous membranes that are essential for cell-cell and cell-matrix adhesion causing separation within or beneath the epidermis, depending on where the target components are located. This article is intended to serve as a helpful primer for immunofluorescence testing in dermatology, with an overview of the tests available as well as pragmatic tips for optimal biopsy sites and specimen transport.
Direct Immunofluorescence
Immunofluorescence techniques date back to 1941 when Albert Coons, an American physician, pathologist, and immunologist, fluorescently labelled antibodies to visualize pneumococcal antigens in infected tissues.1-3 In dermatology, similar methodology was used to visualize the deposition of immunoglobulins and complement in the skin of patients with systemic lupus erythematosus in 1963.4 Basement membrane zone antibodies were first visualized via DIF in bullous pemphigoid in 1967.5 This elegant test utilizes specific antibodies labeled with fluorophores that are then incubated with the patient’s tissue, ultimately forming antibody-antigen conjugates that can be visualized with a fluorescent microscope. Antibodies usually include IgG, IgM, IgA, fibrinogen, and C3. Some institutions also evaluate for IgG4.
Transport medium is critical for proper evaluation of tissues using DIF. Inappropriate storage of tissue can degrade the antigen and confuse the interpretation of specimens. An acceptable medium for DIF includes Michel transport medium, which allows tissue to be stored for days while being transported at ambient temperature without loss of signal.6,7 Zeus medium also can be used and is more readily available. Alternatively, biopsy tissue can be snap frozen using liquid nitrogen. Specimens also may be stored on saline gauze but should be analyzed within 24 to 48 hours.8 Most importantly, do not place the specimen in formalin; even a brief soak in formalin can greatly alter results, especially when trying to diagnose pemphigus.9 Proper transport conditions are critical to prevent autolysis, mitigate putrefaction, and preserve morphology while maintaining antigenicity.10
Indirect Immunofluorescence
Indirect immunofluorescence can be helpful for detecting antibodies circulating in patient serum. Indirect immunofluorescence can be used to help diagnose pemphigoid, pemphigus, epidermolysis bullosa acquisita, bullous lupus erythematosus, and dermatitis herpetiformis. Serum testing also can be a helpful alternative when obtaining tissue is difficult, such as in children.
Indirect immunofluorescence is a 2-part technique that takes a bit longer to assay than DIF.11 The first step involves incubating prepared tissue substrates with patient serum. Unlabeled antibodies in the patient serum are allowed to bind to antigens in the substrate tissue for about 30 minutes. Doubling dilutions of patient serum can be performed to titer antibody levels. The second step uses fluorescein-labeled antihuman antibodies to recognize the antigen-antibody conjugates. Normal whole tissues (eg, monkey esophagus for pemphigus vulgaris, rat bladder for paraneoplastic pemphigus, salt-split normal human skin substrate for pemphigoid and epidermolysis bullosa) are the usual substrates for testing.11,12 Again, this test requires serum and should be collected in a red-top tube or serum-separator tube. Usually, a minimum of 0.5 mL is required for testing, but check with your preferred immunodermatology send-out laboratory before collecting.13
Indirect immunofluorescence usually involves an initial screening panel using 1 or 2 tissue substrates followed by individual antigen-specific assays that correspond to the clinical suspicion and IIF screening results.11 Salt-split skin is used to localize basement membrane zone autoantibodies to either the epidermal (roof) or dermal (floor) side. Although many dermatopathology laboratories offer DIF testing, IIF is more specialized and may be a send-out test at your institution.
Enzyme-linked Immunosorbent Assays
Another tool in the immunodermatology armamentarium is ELISA. Commercial ELISA systems are available for the detection of autoantibodies against bullous pemphigoid (BP) antigen 180, BP230, type VII collagen, desmoglein (Dsg) 1, Dsg3, and envoplakin.11 This test allows semiquantitative measurement of antibody levels and thus can be used to monitor response to treatment or identify relapse and treatment failure.11 For example, in BP, significantly increased baseline anti-BP180 IgG levels correlate with 1-year mortality rates (P=.001) and relapse rates (P=.041).14,15 Numerous additional studies support the observation that monitoring anti-BP180 as a potential marker of disease relapse can be helpful.16,17 In pemphigus, the presence or increase of autoantibodies at remission, either anti-Dsg3 or anti-Dsg1, may be a useful tool in predicting disease relapse.18 It is important for physicians to be aware of this to be able to offer guidance on prognosis.
Where Should I Biopsy?
Knowing where to biopsy can be confusing when beginning residency. But the short answer is, it depends. Let your clinical suspicion guide your specimen site. The Figure provides a quick reference for which location will give you the highest yield for a specific diagnosis.

A few cardinal rules should guide which site is biopsied. Avoid obtaining specimens from the lower extremities as much as possible, as this site has been linked with false-negative results, especially in bullous pemphigoid.19,20 As a dependent area prone to stasis, this site gets a lot of abuse and inflammatory changes secondary to everyday insults that can theoretically alter DIF findings, especially fibrinogen deposition.
Although tissue sent for hematoxylin and eosin staining should be lesional, biopsy for DIF ideally should not contain a new or active blister, ulcer, erosion, or bulla. Immunoreactants are more likely to be degraded in these areas, and DIF may be falsely negative.21
It is worthwhile to briefly discuss the definitions of the terms perilesional and nonlesional. Perilesional skin most frequently refers to skin adjacent to a bulla or vesicle. This skin can be erythematous/inflamed or appear normal. When obtaining tissue for a diagnosis of blistering disease, the general recommendation is to obtain the biopsy from lesional nonbullous skin or perilesional uninvolved skin within 1 cm of the bulla.22-24 The only exception to this is dermatitis herpetiformis, which is best diagnosed on tissue obtained from normal-appearing perilesional skin within 1 cm of an active lesion.25 Additionally, if your patient has oral disease, the recommendation is to obtain the biopsy from nonlesional buccal mucosa, especially if there is desquamative gingivitis.26,27
The ideal biopsy size is 4 or 5 mm. If considering both DIF and histopathology, it is best to procure 2 separate specimens. One larger biopsy can be carefully bisected in 2 but often is subject to more handling artifacts, which can affect findings. In the case of 1 biopsy bisected into 2 specimens, the punch should be at least 6 mm. Shave biopsies also can be performed as long as they extend into the reticular dermis.23
For vasculitis, biopsies for DIF should be taken from lesions that are less than 24 hours old for highest yield, as the level of tissue immunoreactants tends to decline over time.28 This guideline does differ from hematoxylin and eosin specimens sent for evaluation of vasculitis, which ideally should be lesional tissue over 72 hours old. When evaluating for lupus (including subacute cutaneous lupus, discoid lupus, and systemic lupus), DIF is more likely to be positive in well-established, active lesions.
Which Test Should I Order?
The answer to this question depends, but the use of all 3 tests has a specificity close to 100% when evaluating for autoantibody-associated diseases.23 For autoimmune blistering disease, DIF is considered the diagnostic standard. The sensitivity of DIF for diagnosing BP is in the range of 82% to 90.5%, while specificity is 98%.29-31 Other autoimmune blistering diseases, such as pemphigus or dermatitis herpetiformis, have even higher sensitivities and specificities. Direct immunofluorescence often is used as a screening test, but false negatives do occur.32,33 Although rare, false positives also can occur, especially in cases of infection, and should be suspected when there is a lack of clinicopathologic correlation.34 If DIF is negative but clinical suspicion remains high, IIF should be ordered to directly evaluate a patient’s serum for autoantibodies.
In acute cutaneous lupus, subacute cutaneous lupus, and discoid lupus, DIF of active lesions may be helpful if histopathologic examination of a cutaneous lupus erythematosus lesion is nondiagnostic. However, histopathologic examination of formalin-fixed tissue remains the standard for these diagnoses. In vasculitis, while DIF is not used for diagnosis, it is useful to evaluate for IgA deposition. This is important in adults, as IgA deposition has been associated with a greater risk for developing end-stage renal disease.35
Final Thoughts
This is an overview of the tests available for diagnosing autoimmune blistering diseases. Residents should keep in mind that these tests are just one part of the puzzle when it comes to diagnosing these diseases. Results of DIF, IIF, and ELISA testing should be considered in conjunction with patient history and physical examination as well as histopathologic examination of lesional tissue when evaluating for dermatologic diseases with autoantibodies.
- Arthur G. Albert Coons: harnessing the power of the antibody. Lancet Respir Med. 2016;4:181-182.
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47:200-202.
- Coons AH, Creech HJ, Jones RN, et al. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 1942;45:159-170.
- Burnham TK, Neblett TR, Fine G. The application of the fluorescent antibody technic to the investigation of lupus erythematosus and various dermatoses. J Invest Dermatol. 1963;41:451-456.
- Jordon RE, Beutner EH, Witebsky E, et al. Basement zone antibodies in bullous pemphigoid. JAMA. 1967;200:751-756.
- Vaughan Jones SA, Salas J, McGrath JA, et al. A retrospective analysis of tissue-fixed immunoreactants from skin biopsies maintained in Michel’s medium. Dermatology. 1994;189(suppl 1):131-132.
- Kim RH, Brinster NK. Practical direct immunofluorescence. Am J Dermatopathol. 2020;42:75-85.
- Vodegel RM, de Jong MC, Meijer HJ, et al. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10.
- Arbesman J, Grover R, Helm TN, et al. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol. 2011;65:106-111.
- Im K, Mareninov S, Diaz MFP, et al. An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299-311.
- Saschenbrecker S, Karl I, Komorowski L, et al. Serological diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489.
- Immunobullous disease panel, epithelial. ARUP Laboratories website. Accessed November 22, 2021. https://ltd.aruplab.com/Tests/Pub/3001409
- Monshi B, Gulz L, Piringer B, et al. Anti-BP180 autoantibody levels at diagnosis correlate with 1-year mortality rates in patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2020;34:1583-1589.
- Koga H, Teye K, Ishii N, et al. High index values of enzyme-linked immunosorbent assay for BP180 at baseline predict relapse in patients with bullous pemphigoid. Front Med (Lausanne). 2018;5:139.
- Fichel F, Barbe C, Joly P, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014;150:25-33.
- Cai SC, Lim YL, Li W, et al. Anti-BP180 NC16A IgG titres as an indicator of disease activity and outcome in Asian patients with bullous pemphigoid. Ann Acad Med Singap. 2015;44:119-126.
- Genovese G, Maronese CA, Casazza G, et al. Clinical and serological predictors of relapse in pemphigus: a study of 143 patients [published online July 20, 2021]. Clin Exp Dermatol. doi:10.1111/ced.14854
- Weigand DA. Effect of anatomic region on immunofluorescence diagnosis of bullous pemphigoid. J Am Acad Dermatol. 1985;12(2, pt 1):274-278.
- Weigand DA, Clements MK. Direct immunofluorescence in bullous pemphigoid: effects of extent and location of lesions. J Am Acad Dermatol. 1989;20:437-440.
- Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803-822; quiz 822-824.
- Sladden C, Kirchhof MG, Crawford RI. Biopsy location for direct immunofluorescence in patients with suspected bullous pemphigoid impacts probability of a positive test result. J Cutan Med Surg. 2014;18:392-396.
- Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol. 2016;74:1-16; quiz 17-18.
- Seishima M, Izumi T, Kitajima Y. Antibody to bullous pemphigoid antigen 1 binds to the antigen at perilesional but not uninvolved skin, in localized bullous pemphigoid. Eur J Dermatol. 1999;9:39-42.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Kamaguchi M, Iwata H, Ujiie I, et al. Direct immunofluorescence using non-lesional buccal mucosa in mucous membrane pemphigoid. Front Med (Lausanne). 2018;5:20.
- Carey B, Joshi S, Abdelghani A, et al. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol. 2020;182:747-753.
- Kulthanan K, Pinkaew S, Jiamton S, et al. Cutaneous leukocytoclastic vasculitis: the yield of direct immunofluorescence study. J Med Assoc Thai. 2004;87:531-535.
- Chaidemenos GC, Maltezos E, Chrysomallis F, et al. Value of routine diagnostic criteria of bullous pemphigoid. Int J Dermatol. 1998;37:206-210.
- Mysorekar VV, Sumathy TK, Shyam Prasad AL. Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. 2015;6:172-180.
- Fudge JG, Crawford RI. Bullous pemphigoid: a 10-year study of discordant results on direct immunofluorescence. J Cutan Med Surg. 2018;22:472-475.
- Sárdy M, Kostaki D, Varga R, et al. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748-753.
- Buch AC, Kumar H, Panicker N, et al. A cross-sectional study of direct immunofluorescence in the diagnosis of immunobullous dermatoses. Indian J Dermatol. 2014;59:364-368.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
- Cao R, Lau S, Tan V, et al. Adult Henoch-Schönlein purpura: clinical and histopathological predictors of systemic disease and profound renal disease. Indian J Dermatol Venereol Leprol. 2017;83:577-582.
Direct immunofluorescence (DIF) is the go-to diagnostic test when evaluating vesiculobullous eruptions, connective tissue disease, and vasculitis. This specialized test allows visualization of autoantibodies and their reaction products in the epidermis and dermis (skin) and epithelium and subepithelium (mucosa). Indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA) are additional tests that can help in the diagnosis of autoimmune blistering disease. In the blistering autoimmune diseases, the autoantibodies target components in skin and mucous membranes that are essential for cell-cell and cell-matrix adhesion causing separation within or beneath the epidermis, depending on where the target components are located. This article is intended to serve as a helpful primer for immunofluorescence testing in dermatology, with an overview of the tests available as well as pragmatic tips for optimal biopsy sites and specimen transport.
Direct Immunofluorescence
Immunofluorescence techniques date back to 1941 when Albert Coons, an American physician, pathologist, and immunologist, fluorescently labelled antibodies to visualize pneumococcal antigens in infected tissues.1-3 In dermatology, similar methodology was used to visualize the deposition of immunoglobulins and complement in the skin of patients with systemic lupus erythematosus in 1963.4 Basement membrane zone antibodies were first visualized via DIF in bullous pemphigoid in 1967.5 This elegant test utilizes specific antibodies labeled with fluorophores that are then incubated with the patient’s tissue, ultimately forming antibody-antigen conjugates that can be visualized with a fluorescent microscope. Antibodies usually include IgG, IgM, IgA, fibrinogen, and C3. Some institutions also evaluate for IgG4.
Transport medium is critical for proper evaluation of tissues using DIF. Inappropriate storage of tissue can degrade the antigen and confuse the interpretation of specimens. An acceptable medium for DIF includes Michel transport medium, which allows tissue to be stored for days while being transported at ambient temperature without loss of signal.6,7 Zeus medium also can be used and is more readily available. Alternatively, biopsy tissue can be snap frozen using liquid nitrogen. Specimens also may be stored on saline gauze but should be analyzed within 24 to 48 hours.8 Most importantly, do not place the specimen in formalin; even a brief soak in formalin can greatly alter results, especially when trying to diagnose pemphigus.9 Proper transport conditions are critical to prevent autolysis, mitigate putrefaction, and preserve morphology while maintaining antigenicity.10
Indirect Immunofluorescence
Indirect immunofluorescence can be helpful for detecting antibodies circulating in patient serum. Indirect immunofluorescence can be used to help diagnose pemphigoid, pemphigus, epidermolysis bullosa acquisita, bullous lupus erythematosus, and dermatitis herpetiformis. Serum testing also can be a helpful alternative when obtaining tissue is difficult, such as in children.
Indirect immunofluorescence is a 2-part technique that takes a bit longer to assay than DIF.11 The first step involves incubating prepared tissue substrates with patient serum. Unlabeled antibodies in the patient serum are allowed to bind to antigens in the substrate tissue for about 30 minutes. Doubling dilutions of patient serum can be performed to titer antibody levels. The second step uses fluorescein-labeled antihuman antibodies to recognize the antigen-antibody conjugates. Normal whole tissues (eg, monkey esophagus for pemphigus vulgaris, rat bladder for paraneoplastic pemphigus, salt-split normal human skin substrate for pemphigoid and epidermolysis bullosa) are the usual substrates for testing.11,12 Again, this test requires serum and should be collected in a red-top tube or serum-separator tube. Usually, a minimum of 0.5 mL is required for testing, but check with your preferred immunodermatology send-out laboratory before collecting.13
Indirect immunofluorescence usually involves an initial screening panel using 1 or 2 tissue substrates followed by individual antigen-specific assays that correspond to the clinical suspicion and IIF screening results.11 Salt-split skin is used to localize basement membrane zone autoantibodies to either the epidermal (roof) or dermal (floor) side. Although many dermatopathology laboratories offer DIF testing, IIF is more specialized and may be a send-out test at your institution.
Enzyme-linked Immunosorbent Assays
Another tool in the immunodermatology armamentarium is ELISA. Commercial ELISA systems are available for the detection of autoantibodies against bullous pemphigoid (BP) antigen 180, BP230, type VII collagen, desmoglein (Dsg) 1, Dsg3, and envoplakin.11 This test allows semiquantitative measurement of antibody levels and thus can be used to monitor response to treatment or identify relapse and treatment failure.11 For example, in BP, significantly increased baseline anti-BP180 IgG levels correlate with 1-year mortality rates (P=.001) and relapse rates (P=.041).14,15 Numerous additional studies support the observation that monitoring anti-BP180 as a potential marker of disease relapse can be helpful.16,17 In pemphigus, the presence or increase of autoantibodies at remission, either anti-Dsg3 or anti-Dsg1, may be a useful tool in predicting disease relapse.18 It is important for physicians to be aware of this to be able to offer guidance on prognosis.
Where Should I Biopsy?
Knowing where to biopsy can be confusing when beginning residency. But the short answer is, it depends. Let your clinical suspicion guide your specimen site. The Figure provides a quick reference for which location will give you the highest yield for a specific diagnosis.

A few cardinal rules should guide which site is biopsied. Avoid obtaining specimens from the lower extremities as much as possible, as this site has been linked with false-negative results, especially in bullous pemphigoid.19,20 As a dependent area prone to stasis, this site gets a lot of abuse and inflammatory changes secondary to everyday insults that can theoretically alter DIF findings, especially fibrinogen deposition.
Although tissue sent for hematoxylin and eosin staining should be lesional, biopsy for DIF ideally should not contain a new or active blister, ulcer, erosion, or bulla. Immunoreactants are more likely to be degraded in these areas, and DIF may be falsely negative.21
It is worthwhile to briefly discuss the definitions of the terms perilesional and nonlesional. Perilesional skin most frequently refers to skin adjacent to a bulla or vesicle. This skin can be erythematous/inflamed or appear normal. When obtaining tissue for a diagnosis of blistering disease, the general recommendation is to obtain the biopsy from lesional nonbullous skin or perilesional uninvolved skin within 1 cm of the bulla.22-24 The only exception to this is dermatitis herpetiformis, which is best diagnosed on tissue obtained from normal-appearing perilesional skin within 1 cm of an active lesion.25 Additionally, if your patient has oral disease, the recommendation is to obtain the biopsy from nonlesional buccal mucosa, especially if there is desquamative gingivitis.26,27
The ideal biopsy size is 4 or 5 mm. If considering both DIF and histopathology, it is best to procure 2 separate specimens. One larger biopsy can be carefully bisected in 2 but often is subject to more handling artifacts, which can affect findings. In the case of 1 biopsy bisected into 2 specimens, the punch should be at least 6 mm. Shave biopsies also can be performed as long as they extend into the reticular dermis.23
For vasculitis, biopsies for DIF should be taken from lesions that are less than 24 hours old for highest yield, as the level of tissue immunoreactants tends to decline over time.28 This guideline does differ from hematoxylin and eosin specimens sent for evaluation of vasculitis, which ideally should be lesional tissue over 72 hours old. When evaluating for lupus (including subacute cutaneous lupus, discoid lupus, and systemic lupus), DIF is more likely to be positive in well-established, active lesions.
Which Test Should I Order?
The answer to this question depends, but the use of all 3 tests has a specificity close to 100% when evaluating for autoantibody-associated diseases.23 For autoimmune blistering disease, DIF is considered the diagnostic standard. The sensitivity of DIF for diagnosing BP is in the range of 82% to 90.5%, while specificity is 98%.29-31 Other autoimmune blistering diseases, such as pemphigus or dermatitis herpetiformis, have even higher sensitivities and specificities. Direct immunofluorescence often is used as a screening test, but false negatives do occur.32,33 Although rare, false positives also can occur, especially in cases of infection, and should be suspected when there is a lack of clinicopathologic correlation.34 If DIF is negative but clinical suspicion remains high, IIF should be ordered to directly evaluate a patient’s serum for autoantibodies.
In acute cutaneous lupus, subacute cutaneous lupus, and discoid lupus, DIF of active lesions may be helpful if histopathologic examination of a cutaneous lupus erythematosus lesion is nondiagnostic. However, histopathologic examination of formalin-fixed tissue remains the standard for these diagnoses. In vasculitis, while DIF is not used for diagnosis, it is useful to evaluate for IgA deposition. This is important in adults, as IgA deposition has been associated with a greater risk for developing end-stage renal disease.35
Final Thoughts
This is an overview of the tests available for diagnosing autoimmune blistering diseases. Residents should keep in mind that these tests are just one part of the puzzle when it comes to diagnosing these diseases. Results of DIF, IIF, and ELISA testing should be considered in conjunction with patient history and physical examination as well as histopathologic examination of lesional tissue when evaluating for dermatologic diseases with autoantibodies.
Direct immunofluorescence (DIF) is the go-to diagnostic test when evaluating vesiculobullous eruptions, connective tissue disease, and vasculitis. This specialized test allows visualization of autoantibodies and their reaction products in the epidermis and dermis (skin) and epithelium and subepithelium (mucosa). Indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA) are additional tests that can help in the diagnosis of autoimmune blistering disease. In the blistering autoimmune diseases, the autoantibodies target components in skin and mucous membranes that are essential for cell-cell and cell-matrix adhesion causing separation within or beneath the epidermis, depending on where the target components are located. This article is intended to serve as a helpful primer for immunofluorescence testing in dermatology, with an overview of the tests available as well as pragmatic tips for optimal biopsy sites and specimen transport.
Direct Immunofluorescence
Immunofluorescence techniques date back to 1941 when Albert Coons, an American physician, pathologist, and immunologist, fluorescently labelled antibodies to visualize pneumococcal antigens in infected tissues.1-3 In dermatology, similar methodology was used to visualize the deposition of immunoglobulins and complement in the skin of patients with systemic lupus erythematosus in 1963.4 Basement membrane zone antibodies were first visualized via DIF in bullous pemphigoid in 1967.5 This elegant test utilizes specific antibodies labeled with fluorophores that are then incubated with the patient’s tissue, ultimately forming antibody-antigen conjugates that can be visualized with a fluorescent microscope. Antibodies usually include IgG, IgM, IgA, fibrinogen, and C3. Some institutions also evaluate for IgG4.
Transport medium is critical for proper evaluation of tissues using DIF. Inappropriate storage of tissue can degrade the antigen and confuse the interpretation of specimens. An acceptable medium for DIF includes Michel transport medium, which allows tissue to be stored for days while being transported at ambient temperature without loss of signal.6,7 Zeus medium also can be used and is more readily available. Alternatively, biopsy tissue can be snap frozen using liquid nitrogen. Specimens also may be stored on saline gauze but should be analyzed within 24 to 48 hours.8 Most importantly, do not place the specimen in formalin; even a brief soak in formalin can greatly alter results, especially when trying to diagnose pemphigus.9 Proper transport conditions are critical to prevent autolysis, mitigate putrefaction, and preserve morphology while maintaining antigenicity.10
Indirect Immunofluorescence
Indirect immunofluorescence can be helpful for detecting antibodies circulating in patient serum. Indirect immunofluorescence can be used to help diagnose pemphigoid, pemphigus, epidermolysis bullosa acquisita, bullous lupus erythematosus, and dermatitis herpetiformis. Serum testing also can be a helpful alternative when obtaining tissue is difficult, such as in children.
Indirect immunofluorescence is a 2-part technique that takes a bit longer to assay than DIF.11 The first step involves incubating prepared tissue substrates with patient serum. Unlabeled antibodies in the patient serum are allowed to bind to antigens in the substrate tissue for about 30 minutes. Doubling dilutions of patient serum can be performed to titer antibody levels. The second step uses fluorescein-labeled antihuman antibodies to recognize the antigen-antibody conjugates. Normal whole tissues (eg, monkey esophagus for pemphigus vulgaris, rat bladder for paraneoplastic pemphigus, salt-split normal human skin substrate for pemphigoid and epidermolysis bullosa) are the usual substrates for testing.11,12 Again, this test requires serum and should be collected in a red-top tube or serum-separator tube. Usually, a minimum of 0.5 mL is required for testing, but check with your preferred immunodermatology send-out laboratory before collecting.13
Indirect immunofluorescence usually involves an initial screening panel using 1 or 2 tissue substrates followed by individual antigen-specific assays that correspond to the clinical suspicion and IIF screening results.11 Salt-split skin is used to localize basement membrane zone autoantibodies to either the epidermal (roof) or dermal (floor) side. Although many dermatopathology laboratories offer DIF testing, IIF is more specialized and may be a send-out test at your institution.
Enzyme-linked Immunosorbent Assays
Another tool in the immunodermatology armamentarium is ELISA. Commercial ELISA systems are available for the detection of autoantibodies against bullous pemphigoid (BP) antigen 180, BP230, type VII collagen, desmoglein (Dsg) 1, Dsg3, and envoplakin.11 This test allows semiquantitative measurement of antibody levels and thus can be used to monitor response to treatment or identify relapse and treatment failure.11 For example, in BP, significantly increased baseline anti-BP180 IgG levels correlate with 1-year mortality rates (P=.001) and relapse rates (P=.041).14,15 Numerous additional studies support the observation that monitoring anti-BP180 as a potential marker of disease relapse can be helpful.16,17 In pemphigus, the presence or increase of autoantibodies at remission, either anti-Dsg3 or anti-Dsg1, may be a useful tool in predicting disease relapse.18 It is important for physicians to be aware of this to be able to offer guidance on prognosis.
Where Should I Biopsy?
Knowing where to biopsy can be confusing when beginning residency. But the short answer is, it depends. Let your clinical suspicion guide your specimen site. The Figure provides a quick reference for which location will give you the highest yield for a specific diagnosis.

A few cardinal rules should guide which site is biopsied. Avoid obtaining specimens from the lower extremities as much as possible, as this site has been linked with false-negative results, especially in bullous pemphigoid.19,20 As a dependent area prone to stasis, this site gets a lot of abuse and inflammatory changes secondary to everyday insults that can theoretically alter DIF findings, especially fibrinogen deposition.
Although tissue sent for hematoxylin and eosin staining should be lesional, biopsy for DIF ideally should not contain a new or active blister, ulcer, erosion, or bulla. Immunoreactants are more likely to be degraded in these areas, and DIF may be falsely negative.21
It is worthwhile to briefly discuss the definitions of the terms perilesional and nonlesional. Perilesional skin most frequently refers to skin adjacent to a bulla or vesicle. This skin can be erythematous/inflamed or appear normal. When obtaining tissue for a diagnosis of blistering disease, the general recommendation is to obtain the biopsy from lesional nonbullous skin or perilesional uninvolved skin within 1 cm of the bulla.22-24 The only exception to this is dermatitis herpetiformis, which is best diagnosed on tissue obtained from normal-appearing perilesional skin within 1 cm of an active lesion.25 Additionally, if your patient has oral disease, the recommendation is to obtain the biopsy from nonlesional buccal mucosa, especially if there is desquamative gingivitis.26,27
The ideal biopsy size is 4 or 5 mm. If considering both DIF and histopathology, it is best to procure 2 separate specimens. One larger biopsy can be carefully bisected in 2 but often is subject to more handling artifacts, which can affect findings. In the case of 1 biopsy bisected into 2 specimens, the punch should be at least 6 mm. Shave biopsies also can be performed as long as they extend into the reticular dermis.23
For vasculitis, biopsies for DIF should be taken from lesions that are less than 24 hours old for highest yield, as the level of tissue immunoreactants tends to decline over time.28 This guideline does differ from hematoxylin and eosin specimens sent for evaluation of vasculitis, which ideally should be lesional tissue over 72 hours old. When evaluating for lupus (including subacute cutaneous lupus, discoid lupus, and systemic lupus), DIF is more likely to be positive in well-established, active lesions.
Which Test Should I Order?
The answer to this question depends, but the use of all 3 tests has a specificity close to 100% when evaluating for autoantibody-associated diseases.23 For autoimmune blistering disease, DIF is considered the diagnostic standard. The sensitivity of DIF for diagnosing BP is in the range of 82% to 90.5%, while specificity is 98%.29-31 Other autoimmune blistering diseases, such as pemphigus or dermatitis herpetiformis, have even higher sensitivities and specificities. Direct immunofluorescence often is used as a screening test, but false negatives do occur.32,33 Although rare, false positives also can occur, especially in cases of infection, and should be suspected when there is a lack of clinicopathologic correlation.34 If DIF is negative but clinical suspicion remains high, IIF should be ordered to directly evaluate a patient’s serum for autoantibodies.
In acute cutaneous lupus, subacute cutaneous lupus, and discoid lupus, DIF of active lesions may be helpful if histopathologic examination of a cutaneous lupus erythematosus lesion is nondiagnostic. However, histopathologic examination of formalin-fixed tissue remains the standard for these diagnoses. In vasculitis, while DIF is not used for diagnosis, it is useful to evaluate for IgA deposition. This is important in adults, as IgA deposition has been associated with a greater risk for developing end-stage renal disease.35
Final Thoughts
This is an overview of the tests available for diagnosing autoimmune blistering diseases. Residents should keep in mind that these tests are just one part of the puzzle when it comes to diagnosing these diseases. Results of DIF, IIF, and ELISA testing should be considered in conjunction with patient history and physical examination as well as histopathologic examination of lesional tissue when evaluating for dermatologic diseases with autoantibodies.
- Arthur G. Albert Coons: harnessing the power of the antibody. Lancet Respir Med. 2016;4:181-182.
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47:200-202.
- Coons AH, Creech HJ, Jones RN, et al. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 1942;45:159-170.
- Burnham TK, Neblett TR, Fine G. The application of the fluorescent antibody technic to the investigation of lupus erythematosus and various dermatoses. J Invest Dermatol. 1963;41:451-456.
- Jordon RE, Beutner EH, Witebsky E, et al. Basement zone antibodies in bullous pemphigoid. JAMA. 1967;200:751-756.
- Vaughan Jones SA, Salas J, McGrath JA, et al. A retrospective analysis of tissue-fixed immunoreactants from skin biopsies maintained in Michel’s medium. Dermatology. 1994;189(suppl 1):131-132.
- Kim RH, Brinster NK. Practical direct immunofluorescence. Am J Dermatopathol. 2020;42:75-85.
- Vodegel RM, de Jong MC, Meijer HJ, et al. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10.
- Arbesman J, Grover R, Helm TN, et al. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol. 2011;65:106-111.
- Im K, Mareninov S, Diaz MFP, et al. An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299-311.
- Saschenbrecker S, Karl I, Komorowski L, et al. Serological diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489.
- Immunobullous disease panel, epithelial. ARUP Laboratories website. Accessed November 22, 2021. https://ltd.aruplab.com/Tests/Pub/3001409
- Monshi B, Gulz L, Piringer B, et al. Anti-BP180 autoantibody levels at diagnosis correlate with 1-year mortality rates in patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2020;34:1583-1589.
- Koga H, Teye K, Ishii N, et al. High index values of enzyme-linked immunosorbent assay for BP180 at baseline predict relapse in patients with bullous pemphigoid. Front Med (Lausanne). 2018;5:139.
- Fichel F, Barbe C, Joly P, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014;150:25-33.
- Cai SC, Lim YL, Li W, et al. Anti-BP180 NC16A IgG titres as an indicator of disease activity and outcome in Asian patients with bullous pemphigoid. Ann Acad Med Singap. 2015;44:119-126.
- Genovese G, Maronese CA, Casazza G, et al. Clinical and serological predictors of relapse in pemphigus: a study of 143 patients [published online July 20, 2021]. Clin Exp Dermatol. doi:10.1111/ced.14854
- Weigand DA. Effect of anatomic region on immunofluorescence diagnosis of bullous pemphigoid. J Am Acad Dermatol. 1985;12(2, pt 1):274-278.
- Weigand DA, Clements MK. Direct immunofluorescence in bullous pemphigoid: effects of extent and location of lesions. J Am Acad Dermatol. 1989;20:437-440.
- Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803-822; quiz 822-824.
- Sladden C, Kirchhof MG, Crawford RI. Biopsy location for direct immunofluorescence in patients with suspected bullous pemphigoid impacts probability of a positive test result. J Cutan Med Surg. 2014;18:392-396.
- Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol. 2016;74:1-16; quiz 17-18.
- Seishima M, Izumi T, Kitajima Y. Antibody to bullous pemphigoid antigen 1 binds to the antigen at perilesional but not uninvolved skin, in localized bullous pemphigoid. Eur J Dermatol. 1999;9:39-42.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Kamaguchi M, Iwata H, Ujiie I, et al. Direct immunofluorescence using non-lesional buccal mucosa in mucous membrane pemphigoid. Front Med (Lausanne). 2018;5:20.
- Carey B, Joshi S, Abdelghani A, et al. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol. 2020;182:747-753.
- Kulthanan K, Pinkaew S, Jiamton S, et al. Cutaneous leukocytoclastic vasculitis: the yield of direct immunofluorescence study. J Med Assoc Thai. 2004;87:531-535.
- Chaidemenos GC, Maltezos E, Chrysomallis F, et al. Value of routine diagnostic criteria of bullous pemphigoid. Int J Dermatol. 1998;37:206-210.
- Mysorekar VV, Sumathy TK, Shyam Prasad AL. Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. 2015;6:172-180.
- Fudge JG, Crawford RI. Bullous pemphigoid: a 10-year study of discordant results on direct immunofluorescence. J Cutan Med Surg. 2018;22:472-475.
- Sárdy M, Kostaki D, Varga R, et al. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748-753.
- Buch AC, Kumar H, Panicker N, et al. A cross-sectional study of direct immunofluorescence in the diagnosis of immunobullous dermatoses. Indian J Dermatol. 2014;59:364-368.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
- Cao R, Lau S, Tan V, et al. Adult Henoch-Schönlein purpura: clinical and histopathological predictors of systemic disease and profound renal disease. Indian J Dermatol Venereol Leprol. 2017;83:577-582.
- Arthur G. Albert Coons: harnessing the power of the antibody. Lancet Respir Med. 2016;4:181-182.
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Proc Soc Exp Biol Med. 1941;47:200-202.
- Coons AH, Creech HJ, Jones RN, et al. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol. 1942;45:159-170.
- Burnham TK, Neblett TR, Fine G. The application of the fluorescent antibody technic to the investigation of lupus erythematosus and various dermatoses. J Invest Dermatol. 1963;41:451-456.
- Jordon RE, Beutner EH, Witebsky E, et al. Basement zone antibodies in bullous pemphigoid. JAMA. 1967;200:751-756.
- Vaughan Jones SA, Salas J, McGrath JA, et al. A retrospective analysis of tissue-fixed immunoreactants from skin biopsies maintained in Michel’s medium. Dermatology. 1994;189(suppl 1):131-132.
- Kim RH, Brinster NK. Practical direct immunofluorescence. Am J Dermatopathol. 2020;42:75-85.
- Vodegel RM, de Jong MC, Meijer HJ, et al. Enhanced diagnostic immunofluorescence using biopsies transported in saline. BMC Dermatol. 2004;4:10.
- Arbesman J, Grover R, Helm TN, et al. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol. 2011;65:106-111.
- Im K, Mareninov S, Diaz MFP, et al. An introduction to performing immunofluorescence staining. Methods Mol Biol. 2019;1897:299-311.
- Saschenbrecker S, Karl I, Komorowski L, et al. Serological diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489.
- Immunobullous disease panel, epithelial. ARUP Laboratories website. Accessed November 22, 2021. https://ltd.aruplab.com/Tests/Pub/3001409
- Monshi B, Gulz L, Piringer B, et al. Anti-BP180 autoantibody levels at diagnosis correlate with 1-year mortality rates in patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2020;34:1583-1589.
- Koga H, Teye K, Ishii N, et al. High index values of enzyme-linked immunosorbent assay for BP180 at baseline predict relapse in patients with bullous pemphigoid. Front Med (Lausanne). 2018;5:139.
- Fichel F, Barbe C, Joly P, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014;150:25-33.
- Cai SC, Lim YL, Li W, et al. Anti-BP180 NC16A IgG titres as an indicator of disease activity and outcome in Asian patients with bullous pemphigoid. Ann Acad Med Singap. 2015;44:119-126.
- Genovese G, Maronese CA, Casazza G, et al. Clinical and serological predictors of relapse in pemphigus: a study of 143 patients [published online July 20, 2021]. Clin Exp Dermatol. doi:10.1111/ced.14854
- Weigand DA. Effect of anatomic region on immunofluorescence diagnosis of bullous pemphigoid. J Am Acad Dermatol. 1985;12(2, pt 1):274-278.
- Weigand DA, Clements MK. Direct immunofluorescence in bullous pemphigoid: effects of extent and location of lesions. J Am Acad Dermatol. 1989;20:437-440.
- Mutasim DF, Adams BB. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45:803-822; quiz 822-824.
- Sladden C, Kirchhof MG, Crawford RI. Biopsy location for direct immunofluorescence in patients with suspected bullous pemphigoid impacts probability of a positive test result. J Cutan Med Surg. 2014;18:392-396.
- Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol. 2016;74:1-16; quiz 17-18.
- Seishima M, Izumi T, Kitajima Y. Antibody to bullous pemphigoid antigen 1 binds to the antigen at perilesional but not uninvolved skin, in localized bullous pemphigoid. Eur J Dermatol. 1999;9:39-42.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Kamaguchi M, Iwata H, Ujiie I, et al. Direct immunofluorescence using non-lesional buccal mucosa in mucous membrane pemphigoid. Front Med (Lausanne). 2018;5:20.
- Carey B, Joshi S, Abdelghani A, et al. The optimal oral biopsy site for diagnosis of mucous membrane pemphigoid and pemphigus vulgaris. Br J Dermatol. 2020;182:747-753.
- Kulthanan K, Pinkaew S, Jiamton S, et al. Cutaneous leukocytoclastic vasculitis: the yield of direct immunofluorescence study. J Med Assoc Thai. 2004;87:531-535.
- Chaidemenos GC, Maltezos E, Chrysomallis F, et al. Value of routine diagnostic criteria of bullous pemphigoid. Int J Dermatol. 1998;37:206-210.
- Mysorekar VV, Sumathy TK, Shyam Prasad AL. Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. 2015;6:172-180.
- Fudge JG, Crawford RI. Bullous pemphigoid: a 10-year study of discordant results on direct immunofluorescence. J Cutan Med Surg. 2018;22:472-475.
- Sárdy M, Kostaki D, Varga R, et al. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748-753.
- Buch AC, Kumar H, Panicker N, et al. A cross-sectional study of direct immunofluorescence in the diagnosis of immunobullous dermatoses. Indian J Dermatol. 2014;59:364-368.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
- Cao R, Lau S, Tan V, et al. Adult Henoch-Schönlein purpura: clinical and histopathological predictors of systemic disease and profound renal disease. Indian J Dermatol Venereol Leprol. 2017;83:577-582.
Resident Pearl
- Direct immunofluorescence, indirect immunofluorescence, and enzyme-linked immunosorbent assay are important tests for residents to have in their diagnostic tool box, especially when evaluating patients with blistering diseases.
COVID-19 vaccines: Lower serologic response among IBD, rheumatic diseases
Patients with immune-mediated inflammatory diseases (IMIDs), such as inflammatory bowel disease and rheumatic conditions, have a reduced serologic response to a two-dose vaccination regimen with mRNA COVID-19 vaccines, according to the findings of a meta-analysis.
“These results suggest that IMID patients receiving mRNA vaccines should complete the vaccine series without delay and support the strategy of providing a third dose of the vaccine,” wrote study authors Atsushi Sakuraba, MD, of the University of Chicago Medicine, and colleagues in Gastroenterology.
During the COVID-19 pandemic, concerns were raised about the susceptibility of patients with pre-existing conditions to infection with the novel coronavirus, the authors noted. Likewise, ongoing concerns have centered on the risk of worse COVID-19–related outcomes among patients with IMIDs who are treated with immunosuppressive agents.
Since the onset of the pandemic, several registries have been established to gauge the incidence and prognosis of COVID-19 in patients with IMID, including the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE)–Inflammatory Bowel Disease (IBD) registry and the COVID-19 Global Rheumatology Alliance 75 (C19-GRA), which includes patients with rheumatic diseases.
Authorization of COVID-19 mRNA vaccines provided hope that the COVID-19 pandemic could soon come to an end given the overwhelming safety and efficacy data supporting the use of these vaccines for preventing hospitalization and death. Despite these data, little is known regarding the efficacy of mRNA COVID-19 vaccines in patients with IMIDs and/or patients treated with immunosuppressive therapies, as these patients were excluded from the regulatory vaccine studies.
The study by Dr. Sakuraba and colleagues was a meta-analysis of 25 observational studies that reported serologic response rates to COVID-19 vaccination in a pooled cohort of 5,360 patients with IMIDs. Data regarding the reference population, medications, vaccination, and proportion of patients who achieved a serologic response were extracted from the observational studies and included in the meta-analysis.
In the analyzed studies, serologic response was evaluated separately after one or two vaccine doses. The researchers also examined the post-vaccine serologic response rate in patients with IMIDs versus controls without IMIDs.
A total of 23 studies used the BNT162b2 or mRNA-1273 vaccines, while 3 studies reported that 50% to 75.9% of patients received the AZD1222 vaccine. Some studies also included patients who received other COVID-19 vaccines, including CoronaVac, BBV152, and Ad26.COV2.S.
While 6 studies assessed serologic response to COVID-19 after just 1 dose, 20 studies assessed the post-vaccination serologic response following 2 doses. In most cases, researchers evaluated serologic response at 2 to 3 weeks after the first dose. After the second vaccine dose, most studies examined serologic response at 1 to 3 weeks.
The serologic response after 1 dose of the mRNA vaccines was 73.2% (95% CI 65.7-79.5). In a multivariate meta-regression analysis, the researchers found that a significantly greater proportion of patients with IMIDs who took anti-tumor necrosis factor (anti-TNF) therapies had a lower serologic response rate (coefficient, –2.60; 95% CI –4.49 to –0.72; P =.0069). The investigators indicated this “likely contributed to the difference in serologic response rates and overall heterogeneity.”
Studies with patients with IBD reported a lower serologic response rate compared with studies that included patients with rheumatoid arthritis (49.2% vs. 65.0%, respectively), which the investigators explained was likely reflective of the increased use of anti-TNF agents in patients with IBD.
After 2 doses of the mRNA vaccines, the pooled serologic response was 83.4% (95% CI, 76.8%-88.4%). Multivariate meta-regression found that a significantly greater proportion of patients who took anti-CD20 treatments had a lower serologic response (coefficient, -6.08; 95% CI -9.40 to -2.76; P <.001). The investigators found that older age was significantly associated with lower serologic response after 2 doses (coefficient, -0.044; 95% CI -0.083 to -0.0050; P =.027).
For the non-mRNA COVID-19 vaccines, the rates of serologic response after 2 doses were 93.5% with AZD1222, 22.9% with CoronaVac, and 55.6% with BBV152.
Compared with controls without IMIDs, those with IMIDs were significantly less likely to achieve a serologic response following 2 mRNA vaccine doses (odds ratio, 0.086; 95% CI 0.036-0.206; P <.001). The investigators noted that there were not enough studies to examine and compare serologic response rates to adenoviral or inactivated vaccines between patients and controls.
In terms of limitations, the researchers wrote that additional studies examining humoral and cellular immunity to COVID-19 vaccines are needed to determine vaccine efficacy and durability in patients with IMIDs. Additionally, there is a need for studies with larger patient populations to determine serologic response to COVID-19 vaccines in the broader IMID population.
The researchers reported no funding for the study and no relevant conflicts of interest with the pharmaceutical industry.
Messenger RNA vaccines against COVID-19 play a certain role in controlling the pandemic. There has been no clear evidence about the efficacy of vaccination against various vaccine-preventable diseases in patients with IMIDs including IBD, but this global pandemic has led to huge progress in this field. This study by Sakuraba et al. helps us to interpret such information by putting 25 recent studies together. Unfortunately but not unexpectedly, patients with IMIDs were shown to have a lower serologic response to the vaccine, especially if they were treated with anti-TNF therapy. However, this study was incapable of showing the influence of other immunosuppressive therapies such as steroids, antimetabolites, and biologics. It is also still unclear whether their antibody titer would decrease sooner than that in the general population.
Large-scale registries of IBD patients suggest that their disease itself is not a risk for severe COVID-19; however, lower effectiveness of vaccination may result in a serious disadvantage in this patient population, compared with others. Therefore, results from this study strongly suggest that it is critical for patients with IBD not only to complete the regular two-dose vaccination but also to consider the booster shot to maintain immunity for the upcoming months. Further studies are needed to optimize the vaccination strategy specifically in this patient population.
Taku Kobayashi, MD, PhD, is the associate professor and vice director of the Center for Advanced IBD Research and Treatment and codirector of department of gastroenterology, Kitasato University Kitasato Institute Hospital, Tokyo. He has received lecture and advisory fees from Janssen, Pfizer, and Takeda.
Messenger RNA vaccines against COVID-19 play a certain role in controlling the pandemic. There has been no clear evidence about the efficacy of vaccination against various vaccine-preventable diseases in patients with IMIDs including IBD, but this global pandemic has led to huge progress in this field. This study by Sakuraba et al. helps us to interpret such information by putting 25 recent studies together. Unfortunately but not unexpectedly, patients with IMIDs were shown to have a lower serologic response to the vaccine, especially if they were treated with anti-TNF therapy. However, this study was incapable of showing the influence of other immunosuppressive therapies such as steroids, antimetabolites, and biologics. It is also still unclear whether their antibody titer would decrease sooner than that in the general population.
Large-scale registries of IBD patients suggest that their disease itself is not a risk for severe COVID-19; however, lower effectiveness of vaccination may result in a serious disadvantage in this patient population, compared with others. Therefore, results from this study strongly suggest that it is critical for patients with IBD not only to complete the regular two-dose vaccination but also to consider the booster shot to maintain immunity for the upcoming months. Further studies are needed to optimize the vaccination strategy specifically in this patient population.
Taku Kobayashi, MD, PhD, is the associate professor and vice director of the Center for Advanced IBD Research and Treatment and codirector of department of gastroenterology, Kitasato University Kitasato Institute Hospital, Tokyo. He has received lecture and advisory fees from Janssen, Pfizer, and Takeda.
Messenger RNA vaccines against COVID-19 play a certain role in controlling the pandemic. There has been no clear evidence about the efficacy of vaccination against various vaccine-preventable diseases in patients with IMIDs including IBD, but this global pandemic has led to huge progress in this field. This study by Sakuraba et al. helps us to interpret such information by putting 25 recent studies together. Unfortunately but not unexpectedly, patients with IMIDs were shown to have a lower serologic response to the vaccine, especially if they were treated with anti-TNF therapy. However, this study was incapable of showing the influence of other immunosuppressive therapies such as steroids, antimetabolites, and biologics. It is also still unclear whether their antibody titer would decrease sooner than that in the general population.
Large-scale registries of IBD patients suggest that their disease itself is not a risk for severe COVID-19; however, lower effectiveness of vaccination may result in a serious disadvantage in this patient population, compared with others. Therefore, results from this study strongly suggest that it is critical for patients with IBD not only to complete the regular two-dose vaccination but also to consider the booster shot to maintain immunity for the upcoming months. Further studies are needed to optimize the vaccination strategy specifically in this patient population.
Taku Kobayashi, MD, PhD, is the associate professor and vice director of the Center for Advanced IBD Research and Treatment and codirector of department of gastroenterology, Kitasato University Kitasato Institute Hospital, Tokyo. He has received lecture and advisory fees from Janssen, Pfizer, and Takeda.
Patients with immune-mediated inflammatory diseases (IMIDs), such as inflammatory bowel disease and rheumatic conditions, have a reduced serologic response to a two-dose vaccination regimen with mRNA COVID-19 vaccines, according to the findings of a meta-analysis.
“These results suggest that IMID patients receiving mRNA vaccines should complete the vaccine series without delay and support the strategy of providing a third dose of the vaccine,” wrote study authors Atsushi Sakuraba, MD, of the University of Chicago Medicine, and colleagues in Gastroenterology.
During the COVID-19 pandemic, concerns were raised about the susceptibility of patients with pre-existing conditions to infection with the novel coronavirus, the authors noted. Likewise, ongoing concerns have centered on the risk of worse COVID-19–related outcomes among patients with IMIDs who are treated with immunosuppressive agents.
Since the onset of the pandemic, several registries have been established to gauge the incidence and prognosis of COVID-19 in patients with IMID, including the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE)–Inflammatory Bowel Disease (IBD) registry and the COVID-19 Global Rheumatology Alliance 75 (C19-GRA), which includes patients with rheumatic diseases.
Authorization of COVID-19 mRNA vaccines provided hope that the COVID-19 pandemic could soon come to an end given the overwhelming safety and efficacy data supporting the use of these vaccines for preventing hospitalization and death. Despite these data, little is known regarding the efficacy of mRNA COVID-19 vaccines in patients with IMIDs and/or patients treated with immunosuppressive therapies, as these patients were excluded from the regulatory vaccine studies.
The study by Dr. Sakuraba and colleagues was a meta-analysis of 25 observational studies that reported serologic response rates to COVID-19 vaccination in a pooled cohort of 5,360 patients with IMIDs. Data regarding the reference population, medications, vaccination, and proportion of patients who achieved a serologic response were extracted from the observational studies and included in the meta-analysis.
In the analyzed studies, serologic response was evaluated separately after one or two vaccine doses. The researchers also examined the post-vaccine serologic response rate in patients with IMIDs versus controls without IMIDs.
A total of 23 studies used the BNT162b2 or mRNA-1273 vaccines, while 3 studies reported that 50% to 75.9% of patients received the AZD1222 vaccine. Some studies also included patients who received other COVID-19 vaccines, including CoronaVac, BBV152, and Ad26.COV2.S.
While 6 studies assessed serologic response to COVID-19 after just 1 dose, 20 studies assessed the post-vaccination serologic response following 2 doses. In most cases, researchers evaluated serologic response at 2 to 3 weeks after the first dose. After the second vaccine dose, most studies examined serologic response at 1 to 3 weeks.
The serologic response after 1 dose of the mRNA vaccines was 73.2% (95% CI 65.7-79.5). In a multivariate meta-regression analysis, the researchers found that a significantly greater proportion of patients with IMIDs who took anti-tumor necrosis factor (anti-TNF) therapies had a lower serologic response rate (coefficient, –2.60; 95% CI –4.49 to –0.72; P =.0069). The investigators indicated this “likely contributed to the difference in serologic response rates and overall heterogeneity.”
Studies with patients with IBD reported a lower serologic response rate compared with studies that included patients with rheumatoid arthritis (49.2% vs. 65.0%, respectively), which the investigators explained was likely reflective of the increased use of anti-TNF agents in patients with IBD.
After 2 doses of the mRNA vaccines, the pooled serologic response was 83.4% (95% CI, 76.8%-88.4%). Multivariate meta-regression found that a significantly greater proportion of patients who took anti-CD20 treatments had a lower serologic response (coefficient, -6.08; 95% CI -9.40 to -2.76; P <.001). The investigators found that older age was significantly associated with lower serologic response after 2 doses (coefficient, -0.044; 95% CI -0.083 to -0.0050; P =.027).
For the non-mRNA COVID-19 vaccines, the rates of serologic response after 2 doses were 93.5% with AZD1222, 22.9% with CoronaVac, and 55.6% with BBV152.
Compared with controls without IMIDs, those with IMIDs were significantly less likely to achieve a serologic response following 2 mRNA vaccine doses (odds ratio, 0.086; 95% CI 0.036-0.206; P <.001). The investigators noted that there were not enough studies to examine and compare serologic response rates to adenoviral or inactivated vaccines between patients and controls.
In terms of limitations, the researchers wrote that additional studies examining humoral and cellular immunity to COVID-19 vaccines are needed to determine vaccine efficacy and durability in patients with IMIDs. Additionally, there is a need for studies with larger patient populations to determine serologic response to COVID-19 vaccines in the broader IMID population.
The researchers reported no funding for the study and no relevant conflicts of interest with the pharmaceutical industry.
Patients with immune-mediated inflammatory diseases (IMIDs), such as inflammatory bowel disease and rheumatic conditions, have a reduced serologic response to a two-dose vaccination regimen with mRNA COVID-19 vaccines, according to the findings of a meta-analysis.
“These results suggest that IMID patients receiving mRNA vaccines should complete the vaccine series without delay and support the strategy of providing a third dose of the vaccine,” wrote study authors Atsushi Sakuraba, MD, of the University of Chicago Medicine, and colleagues in Gastroenterology.
During the COVID-19 pandemic, concerns were raised about the susceptibility of patients with pre-existing conditions to infection with the novel coronavirus, the authors noted. Likewise, ongoing concerns have centered on the risk of worse COVID-19–related outcomes among patients with IMIDs who are treated with immunosuppressive agents.
Since the onset of the pandemic, several registries have been established to gauge the incidence and prognosis of COVID-19 in patients with IMID, including the Surveillance Epidemiology of Coronavirus Under Research Exclusion (SECURE)–Inflammatory Bowel Disease (IBD) registry and the COVID-19 Global Rheumatology Alliance 75 (C19-GRA), which includes patients with rheumatic diseases.
Authorization of COVID-19 mRNA vaccines provided hope that the COVID-19 pandemic could soon come to an end given the overwhelming safety and efficacy data supporting the use of these vaccines for preventing hospitalization and death. Despite these data, little is known regarding the efficacy of mRNA COVID-19 vaccines in patients with IMIDs and/or patients treated with immunosuppressive therapies, as these patients were excluded from the regulatory vaccine studies.
The study by Dr. Sakuraba and colleagues was a meta-analysis of 25 observational studies that reported serologic response rates to COVID-19 vaccination in a pooled cohort of 5,360 patients with IMIDs. Data regarding the reference population, medications, vaccination, and proportion of patients who achieved a serologic response were extracted from the observational studies and included in the meta-analysis.
In the analyzed studies, serologic response was evaluated separately after one or two vaccine doses. The researchers also examined the post-vaccine serologic response rate in patients with IMIDs versus controls without IMIDs.
A total of 23 studies used the BNT162b2 or mRNA-1273 vaccines, while 3 studies reported that 50% to 75.9% of patients received the AZD1222 vaccine. Some studies also included patients who received other COVID-19 vaccines, including CoronaVac, BBV152, and Ad26.COV2.S.
While 6 studies assessed serologic response to COVID-19 after just 1 dose, 20 studies assessed the post-vaccination serologic response following 2 doses. In most cases, researchers evaluated serologic response at 2 to 3 weeks after the first dose. After the second vaccine dose, most studies examined serologic response at 1 to 3 weeks.
The serologic response after 1 dose of the mRNA vaccines was 73.2% (95% CI 65.7-79.5). In a multivariate meta-regression analysis, the researchers found that a significantly greater proportion of patients with IMIDs who took anti-tumor necrosis factor (anti-TNF) therapies had a lower serologic response rate (coefficient, –2.60; 95% CI –4.49 to –0.72; P =.0069). The investigators indicated this “likely contributed to the difference in serologic response rates and overall heterogeneity.”
Studies with patients with IBD reported a lower serologic response rate compared with studies that included patients with rheumatoid arthritis (49.2% vs. 65.0%, respectively), which the investigators explained was likely reflective of the increased use of anti-TNF agents in patients with IBD.
After 2 doses of the mRNA vaccines, the pooled serologic response was 83.4% (95% CI, 76.8%-88.4%). Multivariate meta-regression found that a significantly greater proportion of patients who took anti-CD20 treatments had a lower serologic response (coefficient, -6.08; 95% CI -9.40 to -2.76; P <.001). The investigators found that older age was significantly associated with lower serologic response after 2 doses (coefficient, -0.044; 95% CI -0.083 to -0.0050; P =.027).
For the non-mRNA COVID-19 vaccines, the rates of serologic response after 2 doses were 93.5% with AZD1222, 22.9% with CoronaVac, and 55.6% with BBV152.
Compared with controls without IMIDs, those with IMIDs were significantly less likely to achieve a serologic response following 2 mRNA vaccine doses (odds ratio, 0.086; 95% CI 0.036-0.206; P <.001). The investigators noted that there were not enough studies to examine and compare serologic response rates to adenoviral or inactivated vaccines between patients and controls.
In terms of limitations, the researchers wrote that additional studies examining humoral and cellular immunity to COVID-19 vaccines are needed to determine vaccine efficacy and durability in patients with IMIDs. Additionally, there is a need for studies with larger patient populations to determine serologic response to COVID-19 vaccines in the broader IMID population.
The researchers reported no funding for the study and no relevant conflicts of interest with the pharmaceutical industry.
FROM GASTROENTEROLOGY
Human CRP protects against acetaminophen-induced liver injury in mice
While often linked to deleterious outcomes in certain disease states, the hepatocyte-produced inflammatory marker C-reactive protein (CRP) may be a checkpoint that protects against acetaminophen-induced acute liver injury, according to research findings.
Based on the study findings, researchers believe long-term suppression of CRP function or expression may increase an individual’s susceptibility to acetaminophen-induced liver injury. In contrast, CRP “could be exploited as a promising therapeutic approach to treat hepatotoxicity caused by drug overdose” wrote study authors Hai-Yun Li, MD, of the Xi’an Jiaotong University in Shaanxi, China, and colleagues in Cellular and Molecular Gastroenterology and Hepatology.
According to Dr. Li and colleagues, a major cause of acute liver failure is acetaminophen-induced liver injury, but despite this risk, very few treatment options for this condition exist. The only approved treatment for this complication is N-acetyl cysteine (NAC).
Although CRP represents a marker for inflammation following tissue injury, a study from 2020 and one from 2018 suggest the protein regulates complement activation and may modulate responses of immune cells. The authors of the current study noted that few studies have explored what roles complement activation and modulated immune cell responses via CRP play in acetaminophen-induced acute liver injury.
To further elucidate the role of CRP in this setting, Dr. Li and researchers assessed the mechanisms of CRP action both in vitro as well as in CRP mice with Fcy receptor 2B knockout. The researchers suggested CRP may modulate immune cell responses via these receptors. Additionally, the investigators assessed CRP action in mice with C3 knockout, given previous studies suggesting C3 knockout may alleviate acetaminophen-induced liver injury in mice. The researchers also investigated hepatic expression of CRP mutants that were defective in complement interaction. Finally, the researchers sought to understand the therapeutic potential of the inflammatory marker by performing intraperitoneal administration of human CRP at 2 or 6 hours after induction of acetaminophen-induced acute liver injury in wild-type mice.
Injection of 300 mg/kg acetaminophen over 24 hours led to overt liver injury in wild-type mice, which was characterized by increased levels of circulating alanine transaminase and aspartate transaminase as well as massive necrosis of hepatocytes. The researchers noted that these manifestations were exacerbated significantly in the CRP knockout mice.
The intravenous administration of human CRP in the mice with the drug-induced liver injury rescued defects caused by mouse CRP knockout. Additionally, human CRP administration alleviated acetaminophen-induced acute liver injury in the wild-type mice. The researchers wrote that these findings demonstrate that endogenous and human CRP “are both protective,” at least in mouse models of acetaminophen-induced liver injury.
In a second experiment, the researchers examined the mechanisms involved in CRP protection in early phases of drug-induced liver injury. Based on the experiment, the researchers found that the knockout of an inhibitory Fcy receptor mediating the anti-inflammatory activities of CRP demonstrated only “marginal effects” on the protection of the protein in acetaminophen-induced liver injury. Overall, the investigators suggested that the inflammatory marker does not likely act via the cellular Fcy receptor 2B to inhibit early phases of acetaminophen-induced hepatocyte injury. Rather, the investigators explained that CRP may act via factor H, which is recruited by CRP in regulating complement activation, to inhibit overactivation of complement on injured hepatocytes. Ultimately, the researchers explained, this results in suppression of the late phase amplification of inflammation that is mediated by neutrophils’ C3a-dependent actions.
Finally, the researchers found that intraperitoneal administration of human CRP at 2.5 mg/kg in wild-type mice at 2 hours following induction of acetaminophen-induced liver injury led to “markedly reduced liver injury,” with an efficacy that was similar to that of 500 mg/kg N-acetylcysteine, the only available treatment approved for acetaminophen-induced liver injury.
The researchers noted that N-acetylcysteine is only effective during the early phases of the acetaminophen-induced liver injury and loses effectiveness at 6 hours following injury. In contrast, human CRP in this study was still highly effective at this time point. “Given that people can tolerate high levels of circulating CRP, the administration of this protein might be a promising option to treat [acetaminophen-induced liver injury] with minimal side effects,” the researchers wrote.
The study was funded by the National Natural Science Foundation of China. The researchers reported no conflicts of interest with any pharmaceutical companies.
This article was updated on Sep. 20, 2022.
Acetaminophen is one of the most widely used pain relievers in the world. Acetaminophen use is considered safe at therapeutic doses; however it is a dose-dependent hepatotoxin, and acetaminophen overdose is one of the leading causes of acute liver failure (ALF) in industrialized countries. Despite intensive efforts, the mechanisms involved in acetaminophen hepatotoxicity are not fully understood, which has hampered the availability of effective therapy for acetaminophen hepatotoxicity.
In Cellular and Molecular Gastroenterology and Hepatology, Li et al. uncovered a crucial role of C-reactive protein in acetaminophen-mediated ALF. Despite its well recognized role as an acute-phase protein in inflammation, CRP also regulates complement activation and hence the modulation of immune cell responses and the generation of anaphylotoxins via specific receptors. With use of models of genetic deletion of CRP in rats and mice, Li et al. demonstrate a protective role for CRP in acetaminophen-induced ALF by regulating the late phase of acetaminophen-induced liver failure via complement overactivation through antagonism of C3aR that prevented neutrophil recruitment.
From a clinically relevant perspective, the protective effect of CRP was more effective than the currently used therapeutic approach of giving N-acetylcysteine (NAC) to patients after acetaminophen hepatotoxicity. The superiority of CRP vs. NAC is related to the limited period for NAC administration after acetaminophen overdose, while the administration of CRP was effective even when given several hours after acetaminophen dosage, consistent with its ability to target the late phase of events involved in acetaminophen hepatotoxicity. Therefore, these findings identify CRP as a promising approach for acetaminophen hepatotoxicity with significant therapeutic advantage, compared with NAC treatment, which may change the paradigm of management of acetaminophen-induced liver failure.
Jose C. Fernandez-Checa, PhD, is a professor at the Spanish National Research Council at the Institute of Biomedical Research of Barcelona, investigator of the Institute of Biomedical Research August Pi i Sunyer, group leader of the Center for Biomedical Network Research on Hepatic and Digestive Diseases, and visiting professor at the department of medicine University of Southern California, Los Angeles. He has no relevant conflicts of interest.
Acetaminophen is one of the most widely used pain relievers in the world. Acetaminophen use is considered safe at therapeutic doses; however it is a dose-dependent hepatotoxin, and acetaminophen overdose is one of the leading causes of acute liver failure (ALF) in industrialized countries. Despite intensive efforts, the mechanisms involved in acetaminophen hepatotoxicity are not fully understood, which has hampered the availability of effective therapy for acetaminophen hepatotoxicity.
In Cellular and Molecular Gastroenterology and Hepatology, Li et al. uncovered a crucial role of C-reactive protein in acetaminophen-mediated ALF. Despite its well recognized role as an acute-phase protein in inflammation, CRP also regulates complement activation and hence the modulation of immune cell responses and the generation of anaphylotoxins via specific receptors. With use of models of genetic deletion of CRP in rats and mice, Li et al. demonstrate a protective role for CRP in acetaminophen-induced ALF by regulating the late phase of acetaminophen-induced liver failure via complement overactivation through antagonism of C3aR that prevented neutrophil recruitment.
From a clinically relevant perspective, the protective effect of CRP was more effective than the currently used therapeutic approach of giving N-acetylcysteine (NAC) to patients after acetaminophen hepatotoxicity. The superiority of CRP vs. NAC is related to the limited period for NAC administration after acetaminophen overdose, while the administration of CRP was effective even when given several hours after acetaminophen dosage, consistent with its ability to target the late phase of events involved in acetaminophen hepatotoxicity. Therefore, these findings identify CRP as a promising approach for acetaminophen hepatotoxicity with significant therapeutic advantage, compared with NAC treatment, which may change the paradigm of management of acetaminophen-induced liver failure.
Jose C. Fernandez-Checa, PhD, is a professor at the Spanish National Research Council at the Institute of Biomedical Research of Barcelona, investigator of the Institute of Biomedical Research August Pi i Sunyer, group leader of the Center for Biomedical Network Research on Hepatic and Digestive Diseases, and visiting professor at the department of medicine University of Southern California, Los Angeles. He has no relevant conflicts of interest.
Acetaminophen is one of the most widely used pain relievers in the world. Acetaminophen use is considered safe at therapeutic doses; however it is a dose-dependent hepatotoxin, and acetaminophen overdose is one of the leading causes of acute liver failure (ALF) in industrialized countries. Despite intensive efforts, the mechanisms involved in acetaminophen hepatotoxicity are not fully understood, which has hampered the availability of effective therapy for acetaminophen hepatotoxicity.
In Cellular and Molecular Gastroenterology and Hepatology, Li et al. uncovered a crucial role of C-reactive protein in acetaminophen-mediated ALF. Despite its well recognized role as an acute-phase protein in inflammation, CRP also regulates complement activation and hence the modulation of immune cell responses and the generation of anaphylotoxins via specific receptors. With use of models of genetic deletion of CRP in rats and mice, Li et al. demonstrate a protective role for CRP in acetaminophen-induced ALF by regulating the late phase of acetaminophen-induced liver failure via complement overactivation through antagonism of C3aR that prevented neutrophil recruitment.
From a clinically relevant perspective, the protective effect of CRP was more effective than the currently used therapeutic approach of giving N-acetylcysteine (NAC) to patients after acetaminophen hepatotoxicity. The superiority of CRP vs. NAC is related to the limited period for NAC administration after acetaminophen overdose, while the administration of CRP was effective even when given several hours after acetaminophen dosage, consistent with its ability to target the late phase of events involved in acetaminophen hepatotoxicity. Therefore, these findings identify CRP as a promising approach for acetaminophen hepatotoxicity with significant therapeutic advantage, compared with NAC treatment, which may change the paradigm of management of acetaminophen-induced liver failure.
Jose C. Fernandez-Checa, PhD, is a professor at the Spanish National Research Council at the Institute of Biomedical Research of Barcelona, investigator of the Institute of Biomedical Research August Pi i Sunyer, group leader of the Center for Biomedical Network Research on Hepatic and Digestive Diseases, and visiting professor at the department of medicine University of Southern California, Los Angeles. He has no relevant conflicts of interest.
While often linked to deleterious outcomes in certain disease states, the hepatocyte-produced inflammatory marker C-reactive protein (CRP) may be a checkpoint that protects against acetaminophen-induced acute liver injury, according to research findings.
Based on the study findings, researchers believe long-term suppression of CRP function or expression may increase an individual’s susceptibility to acetaminophen-induced liver injury. In contrast, CRP “could be exploited as a promising therapeutic approach to treat hepatotoxicity caused by drug overdose” wrote study authors Hai-Yun Li, MD, of the Xi’an Jiaotong University in Shaanxi, China, and colleagues in Cellular and Molecular Gastroenterology and Hepatology.
According to Dr. Li and colleagues, a major cause of acute liver failure is acetaminophen-induced liver injury, but despite this risk, very few treatment options for this condition exist. The only approved treatment for this complication is N-acetyl cysteine (NAC).
Although CRP represents a marker for inflammation following tissue injury, a study from 2020 and one from 2018 suggest the protein regulates complement activation and may modulate responses of immune cells. The authors of the current study noted that few studies have explored what roles complement activation and modulated immune cell responses via CRP play in acetaminophen-induced acute liver injury.
To further elucidate the role of CRP in this setting, Dr. Li and researchers assessed the mechanisms of CRP action both in vitro as well as in CRP mice with Fcy receptor 2B knockout. The researchers suggested CRP may modulate immune cell responses via these receptors. Additionally, the investigators assessed CRP action in mice with C3 knockout, given previous studies suggesting C3 knockout may alleviate acetaminophen-induced liver injury in mice. The researchers also investigated hepatic expression of CRP mutants that were defective in complement interaction. Finally, the researchers sought to understand the therapeutic potential of the inflammatory marker by performing intraperitoneal administration of human CRP at 2 or 6 hours after induction of acetaminophen-induced acute liver injury in wild-type mice.
Injection of 300 mg/kg acetaminophen over 24 hours led to overt liver injury in wild-type mice, which was characterized by increased levels of circulating alanine transaminase and aspartate transaminase as well as massive necrosis of hepatocytes. The researchers noted that these manifestations were exacerbated significantly in the CRP knockout mice.
The intravenous administration of human CRP in the mice with the drug-induced liver injury rescued defects caused by mouse CRP knockout. Additionally, human CRP administration alleviated acetaminophen-induced acute liver injury in the wild-type mice. The researchers wrote that these findings demonstrate that endogenous and human CRP “are both protective,” at least in mouse models of acetaminophen-induced liver injury.
In a second experiment, the researchers examined the mechanisms involved in CRP protection in early phases of drug-induced liver injury. Based on the experiment, the researchers found that the knockout of an inhibitory Fcy receptor mediating the anti-inflammatory activities of CRP demonstrated only “marginal effects” on the protection of the protein in acetaminophen-induced liver injury. Overall, the investigators suggested that the inflammatory marker does not likely act via the cellular Fcy receptor 2B to inhibit early phases of acetaminophen-induced hepatocyte injury. Rather, the investigators explained that CRP may act via factor H, which is recruited by CRP in regulating complement activation, to inhibit overactivation of complement on injured hepatocytes. Ultimately, the researchers explained, this results in suppression of the late phase amplification of inflammation that is mediated by neutrophils’ C3a-dependent actions.
Finally, the researchers found that intraperitoneal administration of human CRP at 2.5 mg/kg in wild-type mice at 2 hours following induction of acetaminophen-induced liver injury led to “markedly reduced liver injury,” with an efficacy that was similar to that of 500 mg/kg N-acetylcysteine, the only available treatment approved for acetaminophen-induced liver injury.
The researchers noted that N-acetylcysteine is only effective during the early phases of the acetaminophen-induced liver injury and loses effectiveness at 6 hours following injury. In contrast, human CRP in this study was still highly effective at this time point. “Given that people can tolerate high levels of circulating CRP, the administration of this protein might be a promising option to treat [acetaminophen-induced liver injury] with minimal side effects,” the researchers wrote.
The study was funded by the National Natural Science Foundation of China. The researchers reported no conflicts of interest with any pharmaceutical companies.
This article was updated on Sep. 20, 2022.
While often linked to deleterious outcomes in certain disease states, the hepatocyte-produced inflammatory marker C-reactive protein (CRP) may be a checkpoint that protects against acetaminophen-induced acute liver injury, according to research findings.
Based on the study findings, researchers believe long-term suppression of CRP function or expression may increase an individual’s susceptibility to acetaminophen-induced liver injury. In contrast, CRP “could be exploited as a promising therapeutic approach to treat hepatotoxicity caused by drug overdose” wrote study authors Hai-Yun Li, MD, of the Xi’an Jiaotong University in Shaanxi, China, and colleagues in Cellular and Molecular Gastroenterology and Hepatology.
According to Dr. Li and colleagues, a major cause of acute liver failure is acetaminophen-induced liver injury, but despite this risk, very few treatment options for this condition exist. The only approved treatment for this complication is N-acetyl cysteine (NAC).
Although CRP represents a marker for inflammation following tissue injury, a study from 2020 and one from 2018 suggest the protein regulates complement activation and may modulate responses of immune cells. The authors of the current study noted that few studies have explored what roles complement activation and modulated immune cell responses via CRP play in acetaminophen-induced acute liver injury.
To further elucidate the role of CRP in this setting, Dr. Li and researchers assessed the mechanisms of CRP action both in vitro as well as in CRP mice with Fcy receptor 2B knockout. The researchers suggested CRP may modulate immune cell responses via these receptors. Additionally, the investigators assessed CRP action in mice with C3 knockout, given previous studies suggesting C3 knockout may alleviate acetaminophen-induced liver injury in mice. The researchers also investigated hepatic expression of CRP mutants that were defective in complement interaction. Finally, the researchers sought to understand the therapeutic potential of the inflammatory marker by performing intraperitoneal administration of human CRP at 2 or 6 hours after induction of acetaminophen-induced acute liver injury in wild-type mice.
Injection of 300 mg/kg acetaminophen over 24 hours led to overt liver injury in wild-type mice, which was characterized by increased levels of circulating alanine transaminase and aspartate transaminase as well as massive necrosis of hepatocytes. The researchers noted that these manifestations were exacerbated significantly in the CRP knockout mice.
The intravenous administration of human CRP in the mice with the drug-induced liver injury rescued defects caused by mouse CRP knockout. Additionally, human CRP administration alleviated acetaminophen-induced acute liver injury in the wild-type mice. The researchers wrote that these findings demonstrate that endogenous and human CRP “are both protective,” at least in mouse models of acetaminophen-induced liver injury.
In a second experiment, the researchers examined the mechanisms involved in CRP protection in early phases of drug-induced liver injury. Based on the experiment, the researchers found that the knockout of an inhibitory Fcy receptor mediating the anti-inflammatory activities of CRP demonstrated only “marginal effects” on the protection of the protein in acetaminophen-induced liver injury. Overall, the investigators suggested that the inflammatory marker does not likely act via the cellular Fcy receptor 2B to inhibit early phases of acetaminophen-induced hepatocyte injury. Rather, the investigators explained that CRP may act via factor H, which is recruited by CRP in regulating complement activation, to inhibit overactivation of complement on injured hepatocytes. Ultimately, the researchers explained, this results in suppression of the late phase amplification of inflammation that is mediated by neutrophils’ C3a-dependent actions.
Finally, the researchers found that intraperitoneal administration of human CRP at 2.5 mg/kg in wild-type mice at 2 hours following induction of acetaminophen-induced liver injury led to “markedly reduced liver injury,” with an efficacy that was similar to that of 500 mg/kg N-acetylcysteine, the only available treatment approved for acetaminophen-induced liver injury.
The researchers noted that N-acetylcysteine is only effective during the early phases of the acetaminophen-induced liver injury and loses effectiveness at 6 hours following injury. In contrast, human CRP in this study was still highly effective at this time point. “Given that people can tolerate high levels of circulating CRP, the administration of this protein might be a promising option to treat [acetaminophen-induced liver injury] with minimal side effects,” the researchers wrote.
The study was funded by the National Natural Science Foundation of China. The researchers reported no conflicts of interest with any pharmaceutical companies.
This article was updated on Sep. 20, 2022.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY