User login
For older adults, smelling the roses may be more difficult
Young and old alike are affected – more than 80%-90% of those diagnosed with the virus, according to some estimates. While most people recover in a few months, 16% take half a year or longer to do so, research has found. According to new estimates, up to 1.6 million Americans have chronic olfactory dysfunction due to COVID-19.
Seniors are especially vulnerable, experts suggest. “We know that many older adults have a compromised sense of smell to begin with. Add to that the insult of COVID, and it made these problems worse,” said Dr. Jayant Pinto, professor of surgery and a specialist in sinus and nasal diseases at the University of Chicago Medical Center.
Recent data highlight the interaction between COVID-19, advanced age, and loss of smell. When Italian researchers evaluated 101 patients who’d been hospitalized for mild to moderate COVID-19, 50 showed objective signs of smell impairment 6 months later. Those 65 or older were nearly twice as likely to be impaired; those 75 or older were more than 2½ times as likely.
Most people aren’t aware of the extent to which smell can be diminished in later life. More than half of 65- to 80-year-olds have some degree of smell loss, or olfactory dysfunction, as it’s known in the scientific literature. That rises to as high as 80% for those even older. People affected often report concerns about safety, less enjoyment eating, and an impaired quality of life.
But because the ability to detect, identify, and discriminate among odors declines gradually, most older adults – up to 75% of those with some degree of olfactory dysfunction – don’t realize they’re affected.
A host of factors are believed to contribute to age-related smell loss, including a reduction in the number of olfactory sensory neurons in the nose, which are essential for detecting odors; changes in stem cells that replenish these neurons every few months; atrophy of the processing center for smell in the brain, called the olfactory bulb; and the shrinkage of brain centers closely connected with the olfactory bulb, such as the hippocampus, a region central to learning and memory.
Also, environmental toxic substances such as air pollution play a part, research shows. “Olfactory neurons in your nose are basically little pieces of your brain hanging out in the outside world,” and exposure to them over time damages those neurons and the tissues that support them, explained Pamela Dalton, PhD, a principal investigator at the Monell Chemical Senses Center, a smell and taste research institute in Philadelphia.
Still, the complex workings of the olfactory system have not been mapped in detail yet, and much remains unknown, said Dr. Sandeep Robert Datta, professor of neurobiology at Harvard Medical School, Boston.
“We tend to think of our sense of smell as primarily aesthetic,” he said. “What’s very clear is that it’s far more important. The olfactory system plays a key role in maintaining our emotional well-being and connecting us with the world.”
Dr. Datta experienced this after having a bone marrow transplant followed by chemotherapy years ago. Unable to smell or taste food, he said, he felt “very disoriented” in his environment.
Common consequences of smell loss include a loss of appetite (without smell, taste is deeply compromised), difficulty monitoring personal hygiene, depression, and an inability to detect noxious fumes. In older adults, this can lead to weight loss, malnutrition, frailty, inadequate personal care, and accidents caused by gas leaks or fires.
Jerome Pisano, 75, of Bloomington, Ill., has been living with smell loss for 5 years. Repeated tests and consultations with physicians haven’t pinpointed a reason for this ailment, and sometimes he feels “hopeless,” he admitted.
Before he became smell-impaired, Mr. Pisano was certified as a wine specialist. He has an 800-bottle wine cellar. “I can’t appreciate that as much as I’d like. I miss the smell of cut grass. Flowers. My wife’s cooking,” he said. “It certainly does decrease my quality of life.”
Smell loss is also associated in various research studies with a higher risk of death for older adults. One study, authored by Dr. Pinto and colleagues, found that older adults with olfactory dysfunction were nearly three times as likely to die over a period of 5 years as were seniors whose sense of smell remained intact.
“Our sense of smell signals how our nervous system is doing and how well our brain is doing overall,” Dr. Pinto said. According to a review published earlier this year, 90% of people with early-stage Parkinson’s disease and more than 80% of people with Alzheimer’s disease have olfactory dysfunction – a symptom that can precede other symptoms by many years.
There is no treatment for smell loss associated with neurological illness or head trauma, but if someone has persistent sinus problems or allergies that cause congestion, an over-the-counter antihistamine or nasal steroid spray can help. Usually, smell returns in a few weeks.
For smell loss following a viral infection, the picture is less clear. It’s not known, yet, which viruses are associated with olfactory dysfunction, why they damage smell, and what trajectory recovery takes. COVID-19 may help shine a light on this since it has inspired a wave of research on olfaction loss around the world.
“What characteristics make people more vulnerable to a persistent loss of smell after a virus? We don’t know that, but I think we will because that research is underway and we’ve never had a cohort [of people with smell loss] this large to study,” said Dr. Dalton, of the Monell center.
Some experts recommend smell training, noting evidence of efficacy and no indication of harm. This involves sniffing four distinct scents (often eucalyptus, lemon, rose, and cloves) twice a day for 30 seconds each, usually for 4 weeks. Sometimes the practice is combined with pictures of the items being smelled, a form of visual reinforcement.
The theory is that “practice, practice, practice” will stimulate the olfactory system, said Charles Greer, PhD, professor of neurosurgery and neuroscience at Yale University, New Haven, Conn. Although scientific support isn’t well established, he said, he often recommends that people who think their smell is declining “get a shelf full of spices and smell them on a regular basis.”
Richard Doty, PhD, director of the University of Pennsylvania’s Smell and Taste Center, remains skeptical. He’s writing a review of smell training and notes that 20%-30% of people with viral infections and smell loss recover in a relatively short time, whether or not they pursue this therapy.
“The main thing we recommend is avoid polluted environments and get your full complement of vitamins,” since several vitamins play an important role in maintaining the olfactory system, he said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Young and old alike are affected – more than 80%-90% of those diagnosed with the virus, according to some estimates. While most people recover in a few months, 16% take half a year or longer to do so, research has found. According to new estimates, up to 1.6 million Americans have chronic olfactory dysfunction due to COVID-19.
Seniors are especially vulnerable, experts suggest. “We know that many older adults have a compromised sense of smell to begin with. Add to that the insult of COVID, and it made these problems worse,” said Dr. Jayant Pinto, professor of surgery and a specialist in sinus and nasal diseases at the University of Chicago Medical Center.
Recent data highlight the interaction between COVID-19, advanced age, and loss of smell. When Italian researchers evaluated 101 patients who’d been hospitalized for mild to moderate COVID-19, 50 showed objective signs of smell impairment 6 months later. Those 65 or older were nearly twice as likely to be impaired; those 75 or older were more than 2½ times as likely.
Most people aren’t aware of the extent to which smell can be diminished in later life. More than half of 65- to 80-year-olds have some degree of smell loss, or olfactory dysfunction, as it’s known in the scientific literature. That rises to as high as 80% for those even older. People affected often report concerns about safety, less enjoyment eating, and an impaired quality of life.
But because the ability to detect, identify, and discriminate among odors declines gradually, most older adults – up to 75% of those with some degree of olfactory dysfunction – don’t realize they’re affected.
A host of factors are believed to contribute to age-related smell loss, including a reduction in the number of olfactory sensory neurons in the nose, which are essential for detecting odors; changes in stem cells that replenish these neurons every few months; atrophy of the processing center for smell in the brain, called the olfactory bulb; and the shrinkage of brain centers closely connected with the olfactory bulb, such as the hippocampus, a region central to learning and memory.
Also, environmental toxic substances such as air pollution play a part, research shows. “Olfactory neurons in your nose are basically little pieces of your brain hanging out in the outside world,” and exposure to them over time damages those neurons and the tissues that support them, explained Pamela Dalton, PhD, a principal investigator at the Monell Chemical Senses Center, a smell and taste research institute in Philadelphia.
Still, the complex workings of the olfactory system have not been mapped in detail yet, and much remains unknown, said Dr. Sandeep Robert Datta, professor of neurobiology at Harvard Medical School, Boston.
“We tend to think of our sense of smell as primarily aesthetic,” he said. “What’s very clear is that it’s far more important. The olfactory system plays a key role in maintaining our emotional well-being and connecting us with the world.”
Dr. Datta experienced this after having a bone marrow transplant followed by chemotherapy years ago. Unable to smell or taste food, he said, he felt “very disoriented” in his environment.
Common consequences of smell loss include a loss of appetite (without smell, taste is deeply compromised), difficulty monitoring personal hygiene, depression, and an inability to detect noxious fumes. In older adults, this can lead to weight loss, malnutrition, frailty, inadequate personal care, and accidents caused by gas leaks or fires.
Jerome Pisano, 75, of Bloomington, Ill., has been living with smell loss for 5 years. Repeated tests and consultations with physicians haven’t pinpointed a reason for this ailment, and sometimes he feels “hopeless,” he admitted.
Before he became smell-impaired, Mr. Pisano was certified as a wine specialist. He has an 800-bottle wine cellar. “I can’t appreciate that as much as I’d like. I miss the smell of cut grass. Flowers. My wife’s cooking,” he said. “It certainly does decrease my quality of life.”
Smell loss is also associated in various research studies with a higher risk of death for older adults. One study, authored by Dr. Pinto and colleagues, found that older adults with olfactory dysfunction were nearly three times as likely to die over a period of 5 years as were seniors whose sense of smell remained intact.
“Our sense of smell signals how our nervous system is doing and how well our brain is doing overall,” Dr. Pinto said. According to a review published earlier this year, 90% of people with early-stage Parkinson’s disease and more than 80% of people with Alzheimer’s disease have olfactory dysfunction – a symptom that can precede other symptoms by many years.
There is no treatment for smell loss associated with neurological illness or head trauma, but if someone has persistent sinus problems or allergies that cause congestion, an over-the-counter antihistamine or nasal steroid spray can help. Usually, smell returns in a few weeks.
For smell loss following a viral infection, the picture is less clear. It’s not known, yet, which viruses are associated with olfactory dysfunction, why they damage smell, and what trajectory recovery takes. COVID-19 may help shine a light on this since it has inspired a wave of research on olfaction loss around the world.
“What characteristics make people more vulnerable to a persistent loss of smell after a virus? We don’t know that, but I think we will because that research is underway and we’ve never had a cohort [of people with smell loss] this large to study,” said Dr. Dalton, of the Monell center.
Some experts recommend smell training, noting evidence of efficacy and no indication of harm. This involves sniffing four distinct scents (often eucalyptus, lemon, rose, and cloves) twice a day for 30 seconds each, usually for 4 weeks. Sometimes the practice is combined with pictures of the items being smelled, a form of visual reinforcement.
The theory is that “practice, practice, practice” will stimulate the olfactory system, said Charles Greer, PhD, professor of neurosurgery and neuroscience at Yale University, New Haven, Conn. Although scientific support isn’t well established, he said, he often recommends that people who think their smell is declining “get a shelf full of spices and smell them on a regular basis.”
Richard Doty, PhD, director of the University of Pennsylvania’s Smell and Taste Center, remains skeptical. He’s writing a review of smell training and notes that 20%-30% of people with viral infections and smell loss recover in a relatively short time, whether or not they pursue this therapy.
“The main thing we recommend is avoid polluted environments and get your full complement of vitamins,” since several vitamins play an important role in maintaining the olfactory system, he said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Young and old alike are affected – more than 80%-90% of those diagnosed with the virus, according to some estimates. While most people recover in a few months, 16% take half a year or longer to do so, research has found. According to new estimates, up to 1.6 million Americans have chronic olfactory dysfunction due to COVID-19.
Seniors are especially vulnerable, experts suggest. “We know that many older adults have a compromised sense of smell to begin with. Add to that the insult of COVID, and it made these problems worse,” said Dr. Jayant Pinto, professor of surgery and a specialist in sinus and nasal diseases at the University of Chicago Medical Center.
Recent data highlight the interaction between COVID-19, advanced age, and loss of smell. When Italian researchers evaluated 101 patients who’d been hospitalized for mild to moderate COVID-19, 50 showed objective signs of smell impairment 6 months later. Those 65 or older were nearly twice as likely to be impaired; those 75 or older were more than 2½ times as likely.
Most people aren’t aware of the extent to which smell can be diminished in later life. More than half of 65- to 80-year-olds have some degree of smell loss, or olfactory dysfunction, as it’s known in the scientific literature. That rises to as high as 80% for those even older. People affected often report concerns about safety, less enjoyment eating, and an impaired quality of life.
But because the ability to detect, identify, and discriminate among odors declines gradually, most older adults – up to 75% of those with some degree of olfactory dysfunction – don’t realize they’re affected.
A host of factors are believed to contribute to age-related smell loss, including a reduction in the number of olfactory sensory neurons in the nose, which are essential for detecting odors; changes in stem cells that replenish these neurons every few months; atrophy of the processing center for smell in the brain, called the olfactory bulb; and the shrinkage of brain centers closely connected with the olfactory bulb, such as the hippocampus, a region central to learning and memory.
Also, environmental toxic substances such as air pollution play a part, research shows. “Olfactory neurons in your nose are basically little pieces of your brain hanging out in the outside world,” and exposure to them over time damages those neurons and the tissues that support them, explained Pamela Dalton, PhD, a principal investigator at the Monell Chemical Senses Center, a smell and taste research institute in Philadelphia.
Still, the complex workings of the olfactory system have not been mapped in detail yet, and much remains unknown, said Dr. Sandeep Robert Datta, professor of neurobiology at Harvard Medical School, Boston.
“We tend to think of our sense of smell as primarily aesthetic,” he said. “What’s very clear is that it’s far more important. The olfactory system plays a key role in maintaining our emotional well-being and connecting us with the world.”
Dr. Datta experienced this after having a bone marrow transplant followed by chemotherapy years ago. Unable to smell or taste food, he said, he felt “very disoriented” in his environment.
Common consequences of smell loss include a loss of appetite (without smell, taste is deeply compromised), difficulty monitoring personal hygiene, depression, and an inability to detect noxious fumes. In older adults, this can lead to weight loss, malnutrition, frailty, inadequate personal care, and accidents caused by gas leaks or fires.
Jerome Pisano, 75, of Bloomington, Ill., has been living with smell loss for 5 years. Repeated tests and consultations with physicians haven’t pinpointed a reason for this ailment, and sometimes he feels “hopeless,” he admitted.
Before he became smell-impaired, Mr. Pisano was certified as a wine specialist. He has an 800-bottle wine cellar. “I can’t appreciate that as much as I’d like. I miss the smell of cut grass. Flowers. My wife’s cooking,” he said. “It certainly does decrease my quality of life.”
Smell loss is also associated in various research studies with a higher risk of death for older adults. One study, authored by Dr. Pinto and colleagues, found that older adults with olfactory dysfunction were nearly three times as likely to die over a period of 5 years as were seniors whose sense of smell remained intact.
“Our sense of smell signals how our nervous system is doing and how well our brain is doing overall,” Dr. Pinto said. According to a review published earlier this year, 90% of people with early-stage Parkinson’s disease and more than 80% of people with Alzheimer’s disease have olfactory dysfunction – a symptom that can precede other symptoms by many years.
There is no treatment for smell loss associated with neurological illness or head trauma, but if someone has persistent sinus problems or allergies that cause congestion, an over-the-counter antihistamine or nasal steroid spray can help. Usually, smell returns in a few weeks.
For smell loss following a viral infection, the picture is less clear. It’s not known, yet, which viruses are associated with olfactory dysfunction, why they damage smell, and what trajectory recovery takes. COVID-19 may help shine a light on this since it has inspired a wave of research on olfaction loss around the world.
“What characteristics make people more vulnerable to a persistent loss of smell after a virus? We don’t know that, but I think we will because that research is underway and we’ve never had a cohort [of people with smell loss] this large to study,” said Dr. Dalton, of the Monell center.
Some experts recommend smell training, noting evidence of efficacy and no indication of harm. This involves sniffing four distinct scents (often eucalyptus, lemon, rose, and cloves) twice a day for 30 seconds each, usually for 4 weeks. Sometimes the practice is combined with pictures of the items being smelled, a form of visual reinforcement.
The theory is that “practice, practice, practice” will stimulate the olfactory system, said Charles Greer, PhD, professor of neurosurgery and neuroscience at Yale University, New Haven, Conn. Although scientific support isn’t well established, he said, he often recommends that people who think their smell is declining “get a shelf full of spices and smell them on a regular basis.”
Richard Doty, PhD, director of the University of Pennsylvania’s Smell and Taste Center, remains skeptical. He’s writing a review of smell training and notes that 20%-30% of people with viral infections and smell loss recover in a relatively short time, whether or not they pursue this therapy.
“The main thing we recommend is avoid polluted environments and get your full complement of vitamins,” since several vitamins play an important role in maintaining the olfactory system, he said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Clinical Edge Journal Scan Commentary: Uterine Fibroids December 2021
A recent study by Lee et al in the Journal of Obstetrics and Gynaecology Research evaluated the feasibility of robotic single-port myomectomy (RSPM) using the da Vinci SP surgical system. In this prospective observational study, 61 women with symptomatic fibroids underwent RSPM. In women with less than 7 resected uterine fibroids (maximal diameter < 10 cm) as well as those with at least 7 resected fibroids (maximal diameter of resected fibroids ≥10 cm), there was no conversion to single-port laparoscopic myomectomy, multiport laparoscopic myomectomy, or laparotomy. Reported complications were minor and included fever, transient ileus and blood transfusion in 15 patients. The authors proposed that robotic single-port myomectomy could solve many of the ergonomic problems associated with single-port laparoscopic myomectomy.
When performing myomectomy during C-section, is there a method that is advantageous? This question was evaluated by Karaca SY et al in European Journal of Obstetrics and Gynecology and Reproductive Biology who compared transendometrial myomectomy with conventional myomectomy. Overall, 41 patients underwent transendometrial myomectomy, and 52 patients underwent conventional myomectomy, with all patients having one single anterior intramural fibroid removed. The mean duration of surgery (50.5 minutes vs 63.6 minutes; P = .001) was lower in the transendometrial group versus the conventional myomectomy group. Additionally, patients who underwent transendometrial myomectomy (0.58 ± 0.61) had significantly lower adhesion scores in their subsequent pregnancy compared to patients who underwent conventional myomectomy (1,76 ± 1,1) (P = 0.001). Length of hospital stay, procedure-related hemoglobin difference, blood transfusion requirement, and postoperative fever were similar in both groups.
A recent study by Lee et al in the Journal of Obstetrics and Gynaecology Research evaluated the feasibility of robotic single-port myomectomy (RSPM) using the da Vinci SP surgical system. In this prospective observational study, 61 women with symptomatic fibroids underwent RSPM. In women with less than 7 resected uterine fibroids (maximal diameter < 10 cm) as well as those with at least 7 resected fibroids (maximal diameter of resected fibroids ≥10 cm), there was no conversion to single-port laparoscopic myomectomy, multiport laparoscopic myomectomy, or laparotomy. Reported complications were minor and included fever, transient ileus and blood transfusion in 15 patients. The authors proposed that robotic single-port myomectomy could solve many of the ergonomic problems associated with single-port laparoscopic myomectomy.
When performing myomectomy during C-section, is there a method that is advantageous? This question was evaluated by Karaca SY et al in European Journal of Obstetrics and Gynecology and Reproductive Biology who compared transendometrial myomectomy with conventional myomectomy. Overall, 41 patients underwent transendometrial myomectomy, and 52 patients underwent conventional myomectomy, with all patients having one single anterior intramural fibroid removed. The mean duration of surgery (50.5 minutes vs 63.6 minutes; P = .001) was lower in the transendometrial group versus the conventional myomectomy group. Additionally, patients who underwent transendometrial myomectomy (0.58 ± 0.61) had significantly lower adhesion scores in their subsequent pregnancy compared to patients who underwent conventional myomectomy (1,76 ± 1,1) (P = 0.001). Length of hospital stay, procedure-related hemoglobin difference, blood transfusion requirement, and postoperative fever were similar in both groups.
A recent study by Lee et al in the Journal of Obstetrics and Gynaecology Research evaluated the feasibility of robotic single-port myomectomy (RSPM) using the da Vinci SP surgical system. In this prospective observational study, 61 women with symptomatic fibroids underwent RSPM. In women with less than 7 resected uterine fibroids (maximal diameter < 10 cm) as well as those with at least 7 resected fibroids (maximal diameter of resected fibroids ≥10 cm), there was no conversion to single-port laparoscopic myomectomy, multiport laparoscopic myomectomy, or laparotomy. Reported complications were minor and included fever, transient ileus and blood transfusion in 15 patients. The authors proposed that robotic single-port myomectomy could solve many of the ergonomic problems associated with single-port laparoscopic myomectomy.
When performing myomectomy during C-section, is there a method that is advantageous? This question was evaluated by Karaca SY et al in European Journal of Obstetrics and Gynecology and Reproductive Biology who compared transendometrial myomectomy with conventional myomectomy. Overall, 41 patients underwent transendometrial myomectomy, and 52 patients underwent conventional myomectomy, with all patients having one single anterior intramural fibroid removed. The mean duration of surgery (50.5 minutes vs 63.6 minutes; P = .001) was lower in the transendometrial group versus the conventional myomectomy group. Additionally, patients who underwent transendometrial myomectomy (0.58 ± 0.61) had significantly lower adhesion scores in their subsequent pregnancy compared to patients who underwent conventional myomectomy (1,76 ± 1,1) (P = 0.001). Length of hospital stay, procedure-related hemoglobin difference, blood transfusion requirement, and postoperative fever were similar in both groups.
A Treatment Option for Patients with Relapsed/Refractory AML
Over the past 5 years, the prognosis for patients with acute myeloid leukemia (AML) has changed markedly, thanks to the development and approval of several therapeutic agents.1 This supplement to Federal Practitioner reviews a once-daily oral formulation for relapsed/refractory patients with AML.
Click here to read the supplement.
11/21 HE-US-2100483
1. Bohl SR, Bullinger L, Rücker FG. New targeted agents in acute myeloid leukemia: new hope on the rise. Int J Mol Sci. 2019;20(8):1983.
Over the past 5 years, the prognosis for patients with acute myeloid leukemia (AML) has changed markedly, thanks to the development and approval of several therapeutic agents.1 This supplement to Federal Practitioner reviews a once-daily oral formulation for relapsed/refractory patients with AML.
Click here to read the supplement.
11/21 HE-US-2100483
1. Bohl SR, Bullinger L, Rücker FG. New targeted agents in acute myeloid leukemia: new hope on the rise. Int J Mol Sci. 2019;20(8):1983.
Over the past 5 years, the prognosis for patients with acute myeloid leukemia (AML) has changed markedly, thanks to the development and approval of several therapeutic agents.1 This supplement to Federal Practitioner reviews a once-daily oral formulation for relapsed/refractory patients with AML.
Click here to read the supplement.
11/21 HE-US-2100483
1. Bohl SR, Bullinger L, Rücker FG. New targeted agents in acute myeloid leukemia: new hope on the rise. Int J Mol Sci. 2019;20(8):1983.
Hospitalist movers and shakers – December 2021
Narine Sargsyan, MD, recently was named the 2021 Alton Memorial Hospital (Alton, Ill.) Chairman’s Award winner. Serving as BJC Medical Group’s hospitalist medical director and hospital department chief of medicine, Dr. Sargsyan won the award based on the nominations of her fellow physicians.
The Chairman’s Award goes to an Alton Memorial staff member acknowledged for contributions to the facility and the community, including promotion and execution of outstanding customer service. Dr. Sargsyan has been a point person for Alton’s treatment of patients during the COVID-19 pandemic, recruiting new hospitalists to treat hospital inpatients. She also served on a committee selecting the inaugural resident class for the Southern Illinois University School of Medicine’s Family Residency program.
Alice Tang, DO, recently was named chief medical officer at Sentara Northern Virginia Medical Center (Woodbridge, Va.). The former medical director at Sentara Lake Ridge Hospital also directed the stroke program at Sentara Northern Virginia Medical Center, so she is familiar with her new facility.
The hospital medicine veteran specialized in emergency medicine and earned her health care MBA from George Washington University. Dr. Tang said her goal as CMO is to enhance the care environment while simultaneously raising the level of the care given by Sentara providers.
Faisal Keen, MD, has been named 2021 Physician of the Year at Sarasota Memorial Hospital’s Sarasota (Fla.) campus. The award winner was selected by a panel of SMH physician leaders.
Dr. Keen has been a hospitalist at SMH Sarasota for the past 6 years.
In presenting Dr. Keen with the award, the staff paid particular compliment to the care he provided to the facility’s hundreds of COVID-19 patients throughout the pandemic. At one point during the surge, Dr. Keen worked 30 shifts during a single month. Among the praises he received during the award presentation were those for his efforts in hurricane preparedness and helping physicians at SMH utilize technology in their patient care.
Jeffrey Crowder, MSPA, PA-C, recently became the first physician assistant to be named chief of hospitalist service at Maine Veterans Affairs Medical Center (Augusta, Me.). He is the first PA to hold the position at any Maine VA hospital. Mr. Crowder held the role in an acting position for the previous year, helping Maine VA Augusta navigate the COVID-19 pandemic.
Mr. Crowder will oversee 13 physicians and 9 PAs in providing care to Maine’s veterans. Included in the facility are intensive care and medical surgery units. Mr. Crowder’s group is responsible for part-time coverage at the 60-bed Togus Community Living Center.
Southeast Iowa Regional Medical Center (West Burlington, Iowa) has expanded its hospitalist program, adding the service to its Fort Madison campus. The health system’s hospitalist program was initiated at SEIRMC’s West Burlington campus back in 2010. That facility now includes 12 full-time and five part-time hospitalist physicians.
OB Hospitalist Group (Greenville, S.C.) has been acquired by Kohlberg & Company LLC (Mount Kisco, N.Y.), giving the nation’s largest dedicated obstetric hospitalist provider a new stakeholder. OBHG hopes to expand its services, which already include 200 hospital partners across 34 states.
OBHG’s network of providers includes more than 1,100 clinicians, with sites normally featuring an OB emergency department with a practicing ob.gyn. on site around the clock. Kohlberg & Company was founded in 1987 and has organized nine private equity funds, raising $12 billion of equity capital.
Narine Sargsyan, MD, recently was named the 2021 Alton Memorial Hospital (Alton, Ill.) Chairman’s Award winner. Serving as BJC Medical Group’s hospitalist medical director and hospital department chief of medicine, Dr. Sargsyan won the award based on the nominations of her fellow physicians.
The Chairman’s Award goes to an Alton Memorial staff member acknowledged for contributions to the facility and the community, including promotion and execution of outstanding customer service. Dr. Sargsyan has been a point person for Alton’s treatment of patients during the COVID-19 pandemic, recruiting new hospitalists to treat hospital inpatients. She also served on a committee selecting the inaugural resident class for the Southern Illinois University School of Medicine’s Family Residency program.
Alice Tang, DO, recently was named chief medical officer at Sentara Northern Virginia Medical Center (Woodbridge, Va.). The former medical director at Sentara Lake Ridge Hospital also directed the stroke program at Sentara Northern Virginia Medical Center, so she is familiar with her new facility.
The hospital medicine veteran specialized in emergency medicine and earned her health care MBA from George Washington University. Dr. Tang said her goal as CMO is to enhance the care environment while simultaneously raising the level of the care given by Sentara providers.
Faisal Keen, MD, has been named 2021 Physician of the Year at Sarasota Memorial Hospital’s Sarasota (Fla.) campus. The award winner was selected by a panel of SMH physician leaders.
Dr. Keen has been a hospitalist at SMH Sarasota for the past 6 years.
In presenting Dr. Keen with the award, the staff paid particular compliment to the care he provided to the facility’s hundreds of COVID-19 patients throughout the pandemic. At one point during the surge, Dr. Keen worked 30 shifts during a single month. Among the praises he received during the award presentation were those for his efforts in hurricane preparedness and helping physicians at SMH utilize technology in their patient care.
Jeffrey Crowder, MSPA, PA-C, recently became the first physician assistant to be named chief of hospitalist service at Maine Veterans Affairs Medical Center (Augusta, Me.). He is the first PA to hold the position at any Maine VA hospital. Mr. Crowder held the role in an acting position for the previous year, helping Maine VA Augusta navigate the COVID-19 pandemic.
Mr. Crowder will oversee 13 physicians and 9 PAs in providing care to Maine’s veterans. Included in the facility are intensive care and medical surgery units. Mr. Crowder’s group is responsible for part-time coverage at the 60-bed Togus Community Living Center.
Southeast Iowa Regional Medical Center (West Burlington, Iowa) has expanded its hospitalist program, adding the service to its Fort Madison campus. The health system’s hospitalist program was initiated at SEIRMC’s West Burlington campus back in 2010. That facility now includes 12 full-time and five part-time hospitalist physicians.
OB Hospitalist Group (Greenville, S.C.) has been acquired by Kohlberg & Company LLC (Mount Kisco, N.Y.), giving the nation’s largest dedicated obstetric hospitalist provider a new stakeholder. OBHG hopes to expand its services, which already include 200 hospital partners across 34 states.
OBHG’s network of providers includes more than 1,100 clinicians, with sites normally featuring an OB emergency department with a practicing ob.gyn. on site around the clock. Kohlberg & Company was founded in 1987 and has organized nine private equity funds, raising $12 billion of equity capital.
Narine Sargsyan, MD, recently was named the 2021 Alton Memorial Hospital (Alton, Ill.) Chairman’s Award winner. Serving as BJC Medical Group’s hospitalist medical director and hospital department chief of medicine, Dr. Sargsyan won the award based on the nominations of her fellow physicians.
The Chairman’s Award goes to an Alton Memorial staff member acknowledged for contributions to the facility and the community, including promotion and execution of outstanding customer service. Dr. Sargsyan has been a point person for Alton’s treatment of patients during the COVID-19 pandemic, recruiting new hospitalists to treat hospital inpatients. She also served on a committee selecting the inaugural resident class for the Southern Illinois University School of Medicine’s Family Residency program.
Alice Tang, DO, recently was named chief medical officer at Sentara Northern Virginia Medical Center (Woodbridge, Va.). The former medical director at Sentara Lake Ridge Hospital also directed the stroke program at Sentara Northern Virginia Medical Center, so she is familiar with her new facility.
The hospital medicine veteran specialized in emergency medicine and earned her health care MBA from George Washington University. Dr. Tang said her goal as CMO is to enhance the care environment while simultaneously raising the level of the care given by Sentara providers.
Faisal Keen, MD, has been named 2021 Physician of the Year at Sarasota Memorial Hospital’s Sarasota (Fla.) campus. The award winner was selected by a panel of SMH physician leaders.
Dr. Keen has been a hospitalist at SMH Sarasota for the past 6 years.
In presenting Dr. Keen with the award, the staff paid particular compliment to the care he provided to the facility’s hundreds of COVID-19 patients throughout the pandemic. At one point during the surge, Dr. Keen worked 30 shifts during a single month. Among the praises he received during the award presentation were those for his efforts in hurricane preparedness and helping physicians at SMH utilize technology in their patient care.
Jeffrey Crowder, MSPA, PA-C, recently became the first physician assistant to be named chief of hospitalist service at Maine Veterans Affairs Medical Center (Augusta, Me.). He is the first PA to hold the position at any Maine VA hospital. Mr. Crowder held the role in an acting position for the previous year, helping Maine VA Augusta navigate the COVID-19 pandemic.
Mr. Crowder will oversee 13 physicians and 9 PAs in providing care to Maine’s veterans. Included in the facility are intensive care and medical surgery units. Mr. Crowder’s group is responsible for part-time coverage at the 60-bed Togus Community Living Center.
Southeast Iowa Regional Medical Center (West Burlington, Iowa) has expanded its hospitalist program, adding the service to its Fort Madison campus. The health system’s hospitalist program was initiated at SEIRMC’s West Burlington campus back in 2010. That facility now includes 12 full-time and five part-time hospitalist physicians.
OB Hospitalist Group (Greenville, S.C.) has been acquired by Kohlberg & Company LLC (Mount Kisco, N.Y.), giving the nation’s largest dedicated obstetric hospitalist provider a new stakeholder. OBHG hopes to expand its services, which already include 200 hospital partners across 34 states.
OBHG’s network of providers includes more than 1,100 clinicians, with sites normally featuring an OB emergency department with a practicing ob.gyn. on site around the clock. Kohlberg & Company was founded in 1987 and has organized nine private equity funds, raising $12 billion of equity capital.
Supreme Court receptive to case that could overturn Roe v. Wade
The justices heard from lawyers arguing for and against a 2018 Mississippi law that, with few exceptions, bans abortion after 15 weeks, claiming that a fetus is viable outside the womb at that age. The Supreme Court’s 1973 Roe v. Wade decision and legal rulings in the decades since, including the 1992 decision in Planned Parenthood v. Casey, have said that abortion should be available to the point of viability – established as about 23 weeks.
The court also ruled in Casey that state laws could not present an “undue burden” on a woman’s ability to obtain an abortion.
The Mississippi attorney general did not initially seek to overturn Roe and Casey, but later argued in Dobbs v. Jackson Women’s Health Organization that both cases were erroneously decided and should be completely thrown out.
“It is an egregiously wrong decision that has inflicted tremendous damage on our country and will continue to do so and take innumerable human lives unless and until this court overrules it,” said Scott G. Stewart, Mississippi’s solicitor general.
When it accepted the Mississippi case, the Supreme Court did not agree to weigh in on overturning Roe or Casey, but the justices’ leanings were evident during the hearing, and it is possible they would throw out those landmark cases.
Justice Clarence Thomas asked repeatedly for the law’s challengers to point out where the right to an abortion was written in the Constitution, as did Justice Samuel Alito.
“If we were talking about the Second Amendment, I know exactly what we’re talking about, if we’re talking about the Fourth Amendment, I know what we’re talking about, because it’s written, it’s there,” said Justice Thomas. “What specifically is the right here that we’re talking about?” he asked U.S. Solicitor General Elizabeth Prelogar.
She said the right to abortion was embedded in the 14th amendment’s guarantee of the pursuit of liberty.
“If this Court renounces the liberty interest recognized in Roe and reaffirmed in Casey, it would be an unprecedented contraction of individual rights,” and a departure from court doctrine of upholding precedent, known as stare decisis, she said.
Chief Justice John Roberts seemed to be against throwing out either of the landmark abortion cases, but instead wanted to focus on whether the 15 weeks was a reasonable time point. But he seemed to be alone in honing-in on that issue.
“Roberts seem desperate for some limiting principle that isn’t reversing Roe, and none of the other conservative justices are biting,” tweeted Mary Ziegler, a historian who has written about abortion.
But justices Neil Gorsuch, Amy Coney Barrett, and Brett Kavanaugh all appeared to be receptive to the idea that the prior precedent set by Roe and Casey could be overturned.
Neil Katyal, the former U.S. acting solicitor general and a Supreme Court lawyer, tweeted during the arguments that he saw “nothing so far sympathetic to the challengers. And a lot that has been very hostile.”
He cautioned that questions during oral arguments “often are just trying to understand a lawyer’s position,” adding, “But the tea leaves here are ominous.”
If Roe v. Wade is overturned, 22 states have laws already on the books that could be used to restrict abortion, according to the Guttmacher Institute. Almost all abortions would be banned in 12 states that have so-called “trigger” laws: Arkansas, Idaho, Kentucky, Louisiana, Mississippi, Missouri, North Dakota, Oklahoma, South Dakota, Tennessee, Texas, and Utah.
Seventeen states have abortion restrictions that have been unenforced or blocked by courts that would go back into effect if Roe is nullified. An additional seven states have laws that intend to restrict abortion in the absence of Roe and four states have passed constitutional amendments to specifically not protect the right to abortion.
Guttmacher reports that 15 states and the District of Columbia have passed laws that protect the right to abortion.
Jackson Women’s Health – the state’s sole abortion provider – sued to block the Mississippi law soon after it passed. A federal judge ruled against the state and that decision was upheld by the U.S. Fifth Circuit Court of Appeals, which also issued a permanent injunction against the law. The Supreme Court in May 2021 agreed to take Mississippi’s appeal.
Earlier in November, the Supreme Court heard arguments in two cases challenging a restrictive Texas law, Whole Woman’s Health v. Jackson and U.S. v. Texas. The justices seemed receptive to the idea that the law, SB 8, was unconstitutional. But the court did not grant a request by the Biden administration to halt the law while the challenges made their way through the courts.
A version of this article first appeared on WebMD.com.
The justices heard from lawyers arguing for and against a 2018 Mississippi law that, with few exceptions, bans abortion after 15 weeks, claiming that a fetus is viable outside the womb at that age. The Supreme Court’s 1973 Roe v. Wade decision and legal rulings in the decades since, including the 1992 decision in Planned Parenthood v. Casey, have said that abortion should be available to the point of viability – established as about 23 weeks.
The court also ruled in Casey that state laws could not present an “undue burden” on a woman’s ability to obtain an abortion.
The Mississippi attorney general did not initially seek to overturn Roe and Casey, but later argued in Dobbs v. Jackson Women’s Health Organization that both cases were erroneously decided and should be completely thrown out.
“It is an egregiously wrong decision that has inflicted tremendous damage on our country and will continue to do so and take innumerable human lives unless and until this court overrules it,” said Scott G. Stewart, Mississippi’s solicitor general.
When it accepted the Mississippi case, the Supreme Court did not agree to weigh in on overturning Roe or Casey, but the justices’ leanings were evident during the hearing, and it is possible they would throw out those landmark cases.
Justice Clarence Thomas asked repeatedly for the law’s challengers to point out where the right to an abortion was written in the Constitution, as did Justice Samuel Alito.
“If we were talking about the Second Amendment, I know exactly what we’re talking about, if we’re talking about the Fourth Amendment, I know what we’re talking about, because it’s written, it’s there,” said Justice Thomas. “What specifically is the right here that we’re talking about?” he asked U.S. Solicitor General Elizabeth Prelogar.
She said the right to abortion was embedded in the 14th amendment’s guarantee of the pursuit of liberty.
“If this Court renounces the liberty interest recognized in Roe and reaffirmed in Casey, it would be an unprecedented contraction of individual rights,” and a departure from court doctrine of upholding precedent, known as stare decisis, she said.
Chief Justice John Roberts seemed to be against throwing out either of the landmark abortion cases, but instead wanted to focus on whether the 15 weeks was a reasonable time point. But he seemed to be alone in honing-in on that issue.
“Roberts seem desperate for some limiting principle that isn’t reversing Roe, and none of the other conservative justices are biting,” tweeted Mary Ziegler, a historian who has written about abortion.
But justices Neil Gorsuch, Amy Coney Barrett, and Brett Kavanaugh all appeared to be receptive to the idea that the prior precedent set by Roe and Casey could be overturned.
Neil Katyal, the former U.S. acting solicitor general and a Supreme Court lawyer, tweeted during the arguments that he saw “nothing so far sympathetic to the challengers. And a lot that has been very hostile.”
He cautioned that questions during oral arguments “often are just trying to understand a lawyer’s position,” adding, “But the tea leaves here are ominous.”
If Roe v. Wade is overturned, 22 states have laws already on the books that could be used to restrict abortion, according to the Guttmacher Institute. Almost all abortions would be banned in 12 states that have so-called “trigger” laws: Arkansas, Idaho, Kentucky, Louisiana, Mississippi, Missouri, North Dakota, Oklahoma, South Dakota, Tennessee, Texas, and Utah.
Seventeen states have abortion restrictions that have been unenforced or blocked by courts that would go back into effect if Roe is nullified. An additional seven states have laws that intend to restrict abortion in the absence of Roe and four states have passed constitutional amendments to specifically not protect the right to abortion.
Guttmacher reports that 15 states and the District of Columbia have passed laws that protect the right to abortion.
Jackson Women’s Health – the state’s sole abortion provider – sued to block the Mississippi law soon after it passed. A federal judge ruled against the state and that decision was upheld by the U.S. Fifth Circuit Court of Appeals, which also issued a permanent injunction against the law. The Supreme Court in May 2021 agreed to take Mississippi’s appeal.
Earlier in November, the Supreme Court heard arguments in two cases challenging a restrictive Texas law, Whole Woman’s Health v. Jackson and U.S. v. Texas. The justices seemed receptive to the idea that the law, SB 8, was unconstitutional. But the court did not grant a request by the Biden administration to halt the law while the challenges made their way through the courts.
A version of this article first appeared on WebMD.com.
The justices heard from lawyers arguing for and against a 2018 Mississippi law that, with few exceptions, bans abortion after 15 weeks, claiming that a fetus is viable outside the womb at that age. The Supreme Court’s 1973 Roe v. Wade decision and legal rulings in the decades since, including the 1992 decision in Planned Parenthood v. Casey, have said that abortion should be available to the point of viability – established as about 23 weeks.
The court also ruled in Casey that state laws could not present an “undue burden” on a woman’s ability to obtain an abortion.
The Mississippi attorney general did not initially seek to overturn Roe and Casey, but later argued in Dobbs v. Jackson Women’s Health Organization that both cases were erroneously decided and should be completely thrown out.
“It is an egregiously wrong decision that has inflicted tremendous damage on our country and will continue to do so and take innumerable human lives unless and until this court overrules it,” said Scott G. Stewart, Mississippi’s solicitor general.
When it accepted the Mississippi case, the Supreme Court did not agree to weigh in on overturning Roe or Casey, but the justices’ leanings were evident during the hearing, and it is possible they would throw out those landmark cases.
Justice Clarence Thomas asked repeatedly for the law’s challengers to point out where the right to an abortion was written in the Constitution, as did Justice Samuel Alito.
“If we were talking about the Second Amendment, I know exactly what we’re talking about, if we’re talking about the Fourth Amendment, I know what we’re talking about, because it’s written, it’s there,” said Justice Thomas. “What specifically is the right here that we’re talking about?” he asked U.S. Solicitor General Elizabeth Prelogar.
She said the right to abortion was embedded in the 14th amendment’s guarantee of the pursuit of liberty.
“If this Court renounces the liberty interest recognized in Roe and reaffirmed in Casey, it would be an unprecedented contraction of individual rights,” and a departure from court doctrine of upholding precedent, known as stare decisis, she said.
Chief Justice John Roberts seemed to be against throwing out either of the landmark abortion cases, but instead wanted to focus on whether the 15 weeks was a reasonable time point. But he seemed to be alone in honing-in on that issue.
“Roberts seem desperate for some limiting principle that isn’t reversing Roe, and none of the other conservative justices are biting,” tweeted Mary Ziegler, a historian who has written about abortion.
But justices Neil Gorsuch, Amy Coney Barrett, and Brett Kavanaugh all appeared to be receptive to the idea that the prior precedent set by Roe and Casey could be overturned.
Neil Katyal, the former U.S. acting solicitor general and a Supreme Court lawyer, tweeted during the arguments that he saw “nothing so far sympathetic to the challengers. And a lot that has been very hostile.”
He cautioned that questions during oral arguments “often are just trying to understand a lawyer’s position,” adding, “But the tea leaves here are ominous.”
If Roe v. Wade is overturned, 22 states have laws already on the books that could be used to restrict abortion, according to the Guttmacher Institute. Almost all abortions would be banned in 12 states that have so-called “trigger” laws: Arkansas, Idaho, Kentucky, Louisiana, Mississippi, Missouri, North Dakota, Oklahoma, South Dakota, Tennessee, Texas, and Utah.
Seventeen states have abortion restrictions that have been unenforced or blocked by courts that would go back into effect if Roe is nullified. An additional seven states have laws that intend to restrict abortion in the absence of Roe and four states have passed constitutional amendments to specifically not protect the right to abortion.
Guttmacher reports that 15 states and the District of Columbia have passed laws that protect the right to abortion.
Jackson Women’s Health – the state’s sole abortion provider – sued to block the Mississippi law soon after it passed. A federal judge ruled against the state and that decision was upheld by the U.S. Fifth Circuit Court of Appeals, which also issued a permanent injunction against the law. The Supreme Court in May 2021 agreed to take Mississippi’s appeal.
Earlier in November, the Supreme Court heard arguments in two cases challenging a restrictive Texas law, Whole Woman’s Health v. Jackson and U.S. v. Texas. The justices seemed receptive to the idea that the law, SB 8, was unconstitutional. But the court did not grant a request by the Biden administration to halt the law while the challenges made their way through the courts.
A version of this article first appeared on WebMD.com.
Acute Severe Urticaria From Minocycline
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.
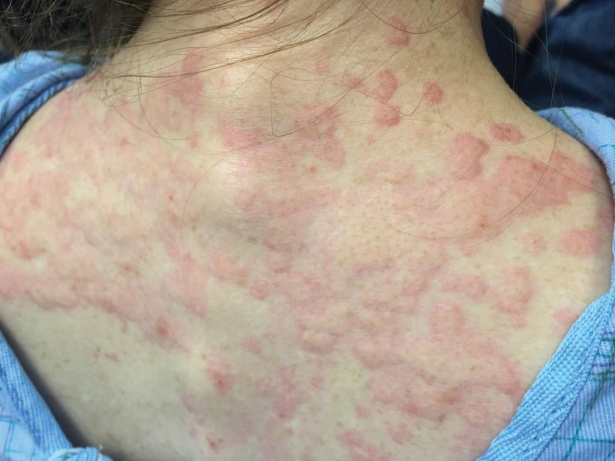
The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
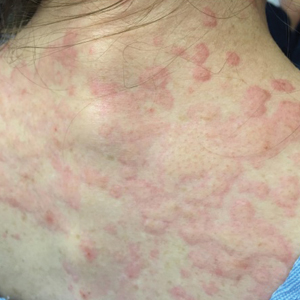
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.

The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
To the Editor:
Minocycline is a commonly prescribed semisynthetic tetracycline derivative used for long-term treatment of acne vulgaris.1 Given the continued popularity of minocycline and other tetracyclines in treating acne, more adverse side effects are being reported. We report a patient who experienced acute severe urticaria with angioedema from minocycline.
A 35-year-old woman with a history of acne vulgaris presented to the emergency department with urticaria and associated angioedema. Fifteen days after starting minocycline, she awoke with diffuse hives sparing only the abdomen that resolved with diphenhydramine. Later that day, she developed generalized pruritus, hives, and lip swelling. She received intravenous methylprednisolone, diphenhydramine, and famotidine in the emergency department. She returned to the emergency department the next day due to facial and lip swelling, diffuse urticaria that was most pronounced on the arms, and throat irritation. Intramuscular epinephrine was administered first followed by methylprednisolone, famotidine, and cetirizine. She was discharged and advised to start daily prednisone 50 mg and cetirizine 20 mg every evening.
She returned to the emergency department the following morning due to worsening generalized urticaria and angioedema of the lips. She denied any associated respiratory, joint, or gastrointestinal tract symptoms. She had several urticarial plaques on the scalp, face, and body (Figure), only sparing the abdomen. Her hives were erythematous, raised, pruritic, and blanching. There was no residual purpura, ecchymosis, or hyperpigmentation associated with the urticaria, and each lesion was present for less than 24 hours. There was no swelling on examination. Additionally, she was afebrile. The C4 level was 18 mg/dL (reference range, 15–45 mg/dL). She did not develop eosinophilia (absolute eosinophil count, 0/µL [reference range, 50–500/µL]), lymphocytosis (absolute lymphocyte count, 1300/µL [reference range, 1000–4800/µL]), or abnormal liver or renal function. She was hospitalized for 3 days with severe urticaria and required 7 days of prednisone 40 to 50 mg, fexofenadine 360 mg, and cetirizine 20 mg. A viral infection was considered as a possible etiology; however, she had no supporting signs or symptoms of an upper respiratory illness or other viral illness.

The patient’s minocycline use was considered the most likely etiology, as an oral contraceptive was the only other medication. She was labelled allergic to minocycline and discharged with intramuscular epinephrine. She was evaluated in the outpatient allergy immunology clinic 9 days later, and all her symptoms had resolved. Due to the severity of our patient’s reaction and the possibility of further severe reactions, an oral challenge was not carried out. Our patient was not interested in pursuing any further minocycline or other tetracycline-based therapy for her acne. She also was not interested in pursuing any minocycline skin-prick testing or oral challenge. One limitation to this case is our patient declining a confirmatory drug challenge; however, given the severity of the symptoms, the physicians involved agreed the patient's safety outweighed the benefits of confirmatory testing.
A PubMed search of articles indexed for MEDLINE and a Google Scholar search using the terms minocycline, drug hypersensitivity, urticaria, anaphylaxis, minocycline allergy, and angioedema yielded only 16 articles and correspondences. Reported adverse effects of minocycline included drug-induced lupus erythematosus, vasculitis, nausea, photosensitivity, and DRESS-like (drug reaction with eosinophilia and systemic symptoms syndrome) conditions. Three case reports of anaphylaxis/anaphylactoid reactions have been published,2-4 but cases of urticaria attributable to minocycline appear to be exceedingly rare.2,3 Reports of serum sickness in patients aged 15 to 62 years were rare. Women were noted to experience a higher frequency of adverse effects compared to men.5 Symptoms typically presented 3 to 28 days after initiation of minocycline. Data currently suggest that the pathogenesis of hypersensitivity reactions to minocycline remains unknown6; however, one hypothesis is that minocycline or its metabolites act as a superantigen, resulting in lymphocyte overactivation and massive cytokine release.7
Minocycline generally is well tolerated by patients. Physicians should be aware that minocycline is a possible causative agent of allergic drug reactions. Our patient’s presentation of severe acute urticaria with angioedema of the face and lips is a rarity.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
- Levenson T, Masood D, Patterson R. Minocycline-induced serum sickness. Allergy Asthma Proc. 1996;17:79-81.
- Okano M, Imai S. Anaphylactoid symptoms due to oral minocycline. Acta Derm Venereol. 1996;76:164.
- Jang JW, Bae Y-J, Kim YG, et al. A case of anaphylaxis to oral minocycline. J Korean Med Sci. 2010;25:1233.
- Nakamura R, Tanaka A, Kinoshita H, et al. Minocycline-induced anaphylaxis mediated by antigen-specific immunoglobulin E [published online November 9, 2021]. J Dermatol. doi:10.1111/1346-8138.16228
- MacNeil M, Haase DA, Tremaine R, et al. Fever, lymphadenopathy, eosinophilia, lymphocytosis, hepatitis, and dermatitis: a severe adverse reaction to minocycline. J Am Acad Dermatol. 1997;36:347-350.
- DePaz S, Perez A, Gomez M, et al. Severe hypersensitivity reaction to minocycline. J Invest Allergol Clin Immunol. 1999;9:403-404.
- Somech R, Arav-Boger R, Assia A, et al. Complications of minocycline therapy for acne vulgaris: case reports and review of the literature. Pediatr Dermatol. 1999;16:469-472.
Practice Points
- Minocycline is a commonly prescribed long-term treatment for acne vulgaris.
- Minocycline-induced acute urticaria and anaphylaxis are rare adverse events.
First Omicron variant case identified in U.S.
He or she was fully vaccinated against COVID-19 and experienced only “mild symptoms that are improving,” officials with the Centers for Disease Control and Prevention said.
The patient, who was not named in the CDC’s announcement of the first U.S. case of the Omicron variant Dec. 1, is self-quarantining.
“All close contacts have been contacted and have tested negative,” officials said.
The announcement comes as no surprise to many as the Omicron variant, first identified in South Africa, has been reported in countries around the world in recent days. Hong Kong, the United Kingdom, and Germany each reported this variant, as have Italy and the Netherlands. Over the weekend, the first North American cases were identified in Canada.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, announced over the weekend that this newest variant was likely already in the United States, telling ABC’s This Week its appearance here was “inevitable.”
Similar to previous variants, this new strain likely started circulating in the United States before scientists could do genetic tests to confirm its presence.
The World Health Organization named Omicron a “variant of concern” on Nov. 26, even though much remains unknown about how well it spreads, how severe it can be, and how it may resist vaccines. In the meantime, the United States enacted travel bans from multiple South African countries.
It remains to be seen if Omicron will follow the pattern of the Delta variant, which was first identified in the United States in May and became the dominant strain by July. It’s also possible it will follow the path taken by the Mu variant. Mu emerged in March and April to much concern, only to fizzle out by September because it was unable to compete with the Delta variant.
A version of this article first appeared on WebMD.com.
He or she was fully vaccinated against COVID-19 and experienced only “mild symptoms that are improving,” officials with the Centers for Disease Control and Prevention said.
The patient, who was not named in the CDC’s announcement of the first U.S. case of the Omicron variant Dec. 1, is self-quarantining.
“All close contacts have been contacted and have tested negative,” officials said.
The announcement comes as no surprise to many as the Omicron variant, first identified in South Africa, has been reported in countries around the world in recent days. Hong Kong, the United Kingdom, and Germany each reported this variant, as have Italy and the Netherlands. Over the weekend, the first North American cases were identified in Canada.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, announced over the weekend that this newest variant was likely already in the United States, telling ABC’s This Week its appearance here was “inevitable.”
Similar to previous variants, this new strain likely started circulating in the United States before scientists could do genetic tests to confirm its presence.
The World Health Organization named Omicron a “variant of concern” on Nov. 26, even though much remains unknown about how well it spreads, how severe it can be, and how it may resist vaccines. In the meantime, the United States enacted travel bans from multiple South African countries.
It remains to be seen if Omicron will follow the pattern of the Delta variant, which was first identified in the United States in May and became the dominant strain by July. It’s also possible it will follow the path taken by the Mu variant. Mu emerged in March and April to much concern, only to fizzle out by September because it was unable to compete with the Delta variant.
A version of this article first appeared on WebMD.com.
He or she was fully vaccinated against COVID-19 and experienced only “mild symptoms that are improving,” officials with the Centers for Disease Control and Prevention said.
The patient, who was not named in the CDC’s announcement of the first U.S. case of the Omicron variant Dec. 1, is self-quarantining.
“All close contacts have been contacted and have tested negative,” officials said.
The announcement comes as no surprise to many as the Omicron variant, first identified in South Africa, has been reported in countries around the world in recent days. Hong Kong, the United Kingdom, and Germany each reported this variant, as have Italy and the Netherlands. Over the weekend, the first North American cases were identified in Canada.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, announced over the weekend that this newest variant was likely already in the United States, telling ABC’s This Week its appearance here was “inevitable.”
Similar to previous variants, this new strain likely started circulating in the United States before scientists could do genetic tests to confirm its presence.
The World Health Organization named Omicron a “variant of concern” on Nov. 26, even though much remains unknown about how well it spreads, how severe it can be, and how it may resist vaccines. In the meantime, the United States enacted travel bans from multiple South African countries.
It remains to be seen if Omicron will follow the pattern of the Delta variant, which was first identified in the United States in May and became the dominant strain by July. It’s also possible it will follow the path taken by the Mu variant. Mu emerged in March and April to much concern, only to fizzle out by September because it was unable to compete with the Delta variant.
A version of this article first appeared on WebMD.com.
Bedside frailty assessment can determine when CPR will be nonbeneficial
Background: Although average survival after in-hospital cardiac arrest is 17%-20%, many clinicians feel that survival is lower in older patients or patients with multiple comorbidities. The Clinical Frailty Scale (CFS) is a simple bedside visual tool that encapsulates patients’ mobility and functional status, with a score greater than 4 indicating frailty. How this measure of frailty correlates with outcomes after in-hospital cardiac arrest is unknown.
Study design: Retrospective review.
Setting: Tertiary referral center in England.
Synopsis: The study included patients over 60 years old who received CPR between May 2017 and December 2018. CFS was retroactively applied based on available chart data. The patients’ median age was 77 years old, and 71% were male. The initial cardiac rhythm was nonshockable in 82% of cases, and overall in-hospital mortality was 86%. Frailty was independently associated with increased mortality when controlling for age, comorbidities, and rhythm. No frail patients survived to hospital discharge, while 26% of patients with CFS greater than 4 survived. Although patients with a shockable rhythm had a better chance of survival overall, compared with those with a nonshockable rhythm (92% vs. 23%, P less than .001), 15% of frail patients had a shockable rhythm, and none survived to discharge. Limitations of the study include relatively small sample size and the possibility of confounding variables, such as comorbid conditions.
Bottom line: When adjusted for age and rhythm, no frail patients older than 60 who received CPR for cardiac arrest survived to hospital discharge. Clinicians should discuss the limited chance of survival and potential burdens of resuscitation with frail patients and their families to avoid inappropriate CPR at the end of life.
Citation: Ibitoye SE et al. Frailty status predicts futility of cardiopulmonary resuscitation in older adults. Age Ageing. 2020 Jun 5;[e-pub]. doi: doi.org/10.1093/ageing/afaa104.
Dr. Chokshi is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Although average survival after in-hospital cardiac arrest is 17%-20%, many clinicians feel that survival is lower in older patients or patients with multiple comorbidities. The Clinical Frailty Scale (CFS) is a simple bedside visual tool that encapsulates patients’ mobility and functional status, with a score greater than 4 indicating frailty. How this measure of frailty correlates with outcomes after in-hospital cardiac arrest is unknown.
Study design: Retrospective review.
Setting: Tertiary referral center in England.
Synopsis: The study included patients over 60 years old who received CPR between May 2017 and December 2018. CFS was retroactively applied based on available chart data. The patients’ median age was 77 years old, and 71% were male. The initial cardiac rhythm was nonshockable in 82% of cases, and overall in-hospital mortality was 86%. Frailty was independently associated with increased mortality when controlling for age, comorbidities, and rhythm. No frail patients survived to hospital discharge, while 26% of patients with CFS greater than 4 survived. Although patients with a shockable rhythm had a better chance of survival overall, compared with those with a nonshockable rhythm (92% vs. 23%, P less than .001), 15% of frail patients had a shockable rhythm, and none survived to discharge. Limitations of the study include relatively small sample size and the possibility of confounding variables, such as comorbid conditions.
Bottom line: When adjusted for age and rhythm, no frail patients older than 60 who received CPR for cardiac arrest survived to hospital discharge. Clinicians should discuss the limited chance of survival and potential burdens of resuscitation with frail patients and their families to avoid inappropriate CPR at the end of life.
Citation: Ibitoye SE et al. Frailty status predicts futility of cardiopulmonary resuscitation in older adults. Age Ageing. 2020 Jun 5;[e-pub]. doi: doi.org/10.1093/ageing/afaa104.
Dr. Chokshi is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Although average survival after in-hospital cardiac arrest is 17%-20%, many clinicians feel that survival is lower in older patients or patients with multiple comorbidities. The Clinical Frailty Scale (CFS) is a simple bedside visual tool that encapsulates patients’ mobility and functional status, with a score greater than 4 indicating frailty. How this measure of frailty correlates with outcomes after in-hospital cardiac arrest is unknown.
Study design: Retrospective review.
Setting: Tertiary referral center in England.
Synopsis: The study included patients over 60 years old who received CPR between May 2017 and December 2018. CFS was retroactively applied based on available chart data. The patients’ median age was 77 years old, and 71% were male. The initial cardiac rhythm was nonshockable in 82% of cases, and overall in-hospital mortality was 86%. Frailty was independently associated with increased mortality when controlling for age, comorbidities, and rhythm. No frail patients survived to hospital discharge, while 26% of patients with CFS greater than 4 survived. Although patients with a shockable rhythm had a better chance of survival overall, compared with those with a nonshockable rhythm (92% vs. 23%, P less than .001), 15% of frail patients had a shockable rhythm, and none survived to discharge. Limitations of the study include relatively small sample size and the possibility of confounding variables, such as comorbid conditions.
Bottom line: When adjusted for age and rhythm, no frail patients older than 60 who received CPR for cardiac arrest survived to hospital discharge. Clinicians should discuss the limited chance of survival and potential burdens of resuscitation with frail patients and their families to avoid inappropriate CPR at the end of life.
Citation: Ibitoye SE et al. Frailty status predicts futility of cardiopulmonary resuscitation in older adults. Age Ageing. 2020 Jun 5;[e-pub]. doi: doi.org/10.1093/ageing/afaa104.
Dr. Chokshi is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
IUDs may increase background enhancement on breast MRI
Intrauterine contraceptive devices (IUDs) have been linked to increased background enhancement on breast MRI, according to research presented at the Radiological Society of North America 2021 annual meeting.
About 10.4% of women 15-49 years of age who use contraception have an IUD or contraceptive implant, according to the Centers for Disease Control and Prevention. Unlike oral or transdermal hormonal contraceptives and hormone replacement therapy, levonorgestrel-releasing IUDs release a small amount of the hormone directly into the uterus and are thought to have a much more localized effect, Luisa Huck, MD, the lead author of the study, said in an interview.
But women with IUDs have long reported adverse effects associated with other hormonal medication. “In the past, some women reported depression, headaches, sleep disorders, and panic attacks,” noted Dr. Huck, a radiology resident at RWTH Aachen University in Germany.
Christiane Kuhl, MD, chief of the department of radiology at RWTH Aachen University and senior author of the research, had also observed that women with hormonal IUDs often have increased background parenchymal enhancement (BPE) on contrast-enhanced MRI. BPE “has been established as a sensitive marker of hormonal stimulation of breast,” the study authors wrote, and previous studies have shown that women using hormonal medications have higher BPE on breast MRIs.
To better understand whether IUDs can increase BPE, Dr. Huck and colleagues used the hospital database to search for premenopausal women who had undergone breast MRIs for screening between January 2014 and July 2020. To be included, women had to have had at least two scans: one with and one without an IUD in place, with the scan conducted at least 4 weeks after IUD placement or removal. All women in the study had no history of breast cancer or hormone or antihormone intake.
The study involved 48 women with an average age of 45 years and a median of 27 months between the two scans. Forty-six of the women had the Mirena levonorgestrel-releasing IUD and two had the Jaydess IUD. To account for hormone variations between patients, the researchers used each patient as their own reference point. To control for age-related effects, 25 women had their first MRI without an IUD and their second scan with an IUD in place. The second group of 23 women underwent their first MRI with an IUD and had it removed before the second scan.
Hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging.
For 23 women in the study, background enhancement was higher on scans with the IUD than without (P < .001). For 24 women, there was no change in BPE with or without an IUD, and one woman had lower BPE with an IUD than without.
“It is very interesting and relevant to practice to consider that the presence of an intrauterine device would have potential impact on the enhancement we see in the breast on MRI imaging,” Samantha Heller, MD, PhD, associate professor of radiology at New York University, said in an interview.
However, the study used BPE as a measure for hormonal shifts, and “hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging,” she noted. BPE on MRI can fluctuate, so testing actual hormone levels in patients with elevated BPE could be helpful to identify hormonal shifts, she added. It is also important to understand why half of the women in the study showed no variation in BPE, she said.
The study findings are not very surprising, considering that it is known that low levels of progesterone from IUDs circulate in the blood stream, Frances Casey, MD, MPH, associate professor in the department of obstetrics and gynecology at Virginia Commonwealth University in Richmond, said in an interview. They do not suggest that there should be any changes to IUD guidelines, she added.
However, “the study findings raise the question as to whether IUD status should be documented as a matter of course prior to performing breast MRI,” said Dr. Heller. “It is standard to document the timing of a woman’s menstrual cycle, as well as to note any hormone suppression or replacement therapy. This is in part so that the radiologist may understand the etiology of any observed variation in background enhancement,” she explained.
Although increased enhancement on MRI has sometimes been linked to higher chances of recommendations for additional imaging or biopsies, she noted, “more work would be needed to understand the impact – if any – of an IUD on breast MRI recommendations due to enhancement changes.”
Dr. Huck, Dr. Heller, and Dr. Casey disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intrauterine contraceptive devices (IUDs) have been linked to increased background enhancement on breast MRI, according to research presented at the Radiological Society of North America 2021 annual meeting.
About 10.4% of women 15-49 years of age who use contraception have an IUD or contraceptive implant, according to the Centers for Disease Control and Prevention. Unlike oral or transdermal hormonal contraceptives and hormone replacement therapy, levonorgestrel-releasing IUDs release a small amount of the hormone directly into the uterus and are thought to have a much more localized effect, Luisa Huck, MD, the lead author of the study, said in an interview.
But women with IUDs have long reported adverse effects associated with other hormonal medication. “In the past, some women reported depression, headaches, sleep disorders, and panic attacks,” noted Dr. Huck, a radiology resident at RWTH Aachen University in Germany.
Christiane Kuhl, MD, chief of the department of radiology at RWTH Aachen University and senior author of the research, had also observed that women with hormonal IUDs often have increased background parenchymal enhancement (BPE) on contrast-enhanced MRI. BPE “has been established as a sensitive marker of hormonal stimulation of breast,” the study authors wrote, and previous studies have shown that women using hormonal medications have higher BPE on breast MRIs.
To better understand whether IUDs can increase BPE, Dr. Huck and colleagues used the hospital database to search for premenopausal women who had undergone breast MRIs for screening between January 2014 and July 2020. To be included, women had to have had at least two scans: one with and one without an IUD in place, with the scan conducted at least 4 weeks after IUD placement or removal. All women in the study had no history of breast cancer or hormone or antihormone intake.
The study involved 48 women with an average age of 45 years and a median of 27 months between the two scans. Forty-six of the women had the Mirena levonorgestrel-releasing IUD and two had the Jaydess IUD. To account for hormone variations between patients, the researchers used each patient as their own reference point. To control for age-related effects, 25 women had their first MRI without an IUD and their second scan with an IUD in place. The second group of 23 women underwent their first MRI with an IUD and had it removed before the second scan.
Hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging.
For 23 women in the study, background enhancement was higher on scans with the IUD than without (P < .001). For 24 women, there was no change in BPE with or without an IUD, and one woman had lower BPE with an IUD than without.
“It is very interesting and relevant to practice to consider that the presence of an intrauterine device would have potential impact on the enhancement we see in the breast on MRI imaging,” Samantha Heller, MD, PhD, associate professor of radiology at New York University, said in an interview.
However, the study used BPE as a measure for hormonal shifts, and “hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging,” she noted. BPE on MRI can fluctuate, so testing actual hormone levels in patients with elevated BPE could be helpful to identify hormonal shifts, she added. It is also important to understand why half of the women in the study showed no variation in BPE, she said.
The study findings are not very surprising, considering that it is known that low levels of progesterone from IUDs circulate in the blood stream, Frances Casey, MD, MPH, associate professor in the department of obstetrics and gynecology at Virginia Commonwealth University in Richmond, said in an interview. They do not suggest that there should be any changes to IUD guidelines, she added.
However, “the study findings raise the question as to whether IUD status should be documented as a matter of course prior to performing breast MRI,” said Dr. Heller. “It is standard to document the timing of a woman’s menstrual cycle, as well as to note any hormone suppression or replacement therapy. This is in part so that the radiologist may understand the etiology of any observed variation in background enhancement,” she explained.
Although increased enhancement on MRI has sometimes been linked to higher chances of recommendations for additional imaging or biopsies, she noted, “more work would be needed to understand the impact – if any – of an IUD on breast MRI recommendations due to enhancement changes.”
Dr. Huck, Dr. Heller, and Dr. Casey disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intrauterine contraceptive devices (IUDs) have been linked to increased background enhancement on breast MRI, according to research presented at the Radiological Society of North America 2021 annual meeting.
About 10.4% of women 15-49 years of age who use contraception have an IUD or contraceptive implant, according to the Centers for Disease Control and Prevention. Unlike oral or transdermal hormonal contraceptives and hormone replacement therapy, levonorgestrel-releasing IUDs release a small amount of the hormone directly into the uterus and are thought to have a much more localized effect, Luisa Huck, MD, the lead author of the study, said in an interview.
But women with IUDs have long reported adverse effects associated with other hormonal medication. “In the past, some women reported depression, headaches, sleep disorders, and panic attacks,” noted Dr. Huck, a radiology resident at RWTH Aachen University in Germany.
Christiane Kuhl, MD, chief of the department of radiology at RWTH Aachen University and senior author of the research, had also observed that women with hormonal IUDs often have increased background parenchymal enhancement (BPE) on contrast-enhanced MRI. BPE “has been established as a sensitive marker of hormonal stimulation of breast,” the study authors wrote, and previous studies have shown that women using hormonal medications have higher BPE on breast MRIs.
To better understand whether IUDs can increase BPE, Dr. Huck and colleagues used the hospital database to search for premenopausal women who had undergone breast MRIs for screening between January 2014 and July 2020. To be included, women had to have had at least two scans: one with and one without an IUD in place, with the scan conducted at least 4 weeks after IUD placement or removal. All women in the study had no history of breast cancer or hormone or antihormone intake.
The study involved 48 women with an average age of 45 years and a median of 27 months between the two scans. Forty-six of the women had the Mirena levonorgestrel-releasing IUD and two had the Jaydess IUD. To account for hormone variations between patients, the researchers used each patient as their own reference point. To control for age-related effects, 25 women had their first MRI without an IUD and their second scan with an IUD in place. The second group of 23 women underwent their first MRI with an IUD and had it removed before the second scan.
Hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging.
For 23 women in the study, background enhancement was higher on scans with the IUD than without (P < .001). For 24 women, there was no change in BPE with or without an IUD, and one woman had lower BPE with an IUD than without.
“It is very interesting and relevant to practice to consider that the presence of an intrauterine device would have potential impact on the enhancement we see in the breast on MRI imaging,” Samantha Heller, MD, PhD, associate professor of radiology at New York University, said in an interview.
However, the study used BPE as a measure for hormonal shifts, and “hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging,” she noted. BPE on MRI can fluctuate, so testing actual hormone levels in patients with elevated BPE could be helpful to identify hormonal shifts, she added. It is also important to understand why half of the women in the study showed no variation in BPE, she said.
The study findings are not very surprising, considering that it is known that low levels of progesterone from IUDs circulate in the blood stream, Frances Casey, MD, MPH, associate professor in the department of obstetrics and gynecology at Virginia Commonwealth University in Richmond, said in an interview. They do not suggest that there should be any changes to IUD guidelines, she added.
However, “the study findings raise the question as to whether IUD status should be documented as a matter of course prior to performing breast MRI,” said Dr. Heller. “It is standard to document the timing of a woman’s menstrual cycle, as well as to note any hormone suppression or replacement therapy. This is in part so that the radiologist may understand the etiology of any observed variation in background enhancement,” she explained.
Although increased enhancement on MRI has sometimes been linked to higher chances of recommendations for additional imaging or biopsies, she noted, “more work would be needed to understand the impact – if any – of an IUD on breast MRI recommendations due to enhancement changes.”
Dr. Huck, Dr. Heller, and Dr. Casey disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.