User login
Smaller inguinal hernias linked to more postoperative pain
according to a study published in Annals of Surgery.
Researchers used data from the European Herniamed registry to look at 1-year outcomes in 57,999 male patients who underwent primary unilateral inguinal hernia repair.
While patients with larger hernias showed significantly longer operation times and had a significantly larger body mass index, those with smaller hernias had significantly higher rates of pain at rest or on exertion, and chronic pain requiring treatment.
Individuals with smaller hernias (EHS I) had a 35% higher odds of pain at rest, compared with those with medium-sized hernias (EHS II), and 84% higher odds, compared with individuals with large hernias (EHS III). Smaller hernias were also associated with a 100% higher odds of pain on exertion and greater than 100% higher odds of chronic postoperative pain requiring treatment, compared with large hernias.
“CPIP [chronic postoperative inguinal pain] has become one of the most important surgical quality parameters after elective inguinal hernia repair with significant consequences affecting patient productivity, employment, and quality of life,” wrote Henry Hoffmann, MD, of the clinic for visceral surgery at the University Hospital Basel in Switzerland, and his coauthors. “With our findings we contribute to the important discussion of prevention, risk factors, and treatment of CPIP identifying smaller inguinal hernias [EHS I-II] as a new independent, patient-related risk factor for the development of CPIP.”
Noting that an association between smaller hernias and increased postoperative pain seems counterintuitive, the authors suggested that patients’ expectations of outcomes may be higher in those with smaller hernias than in patients with larger hernias. While larger hernias are likely more bothersome rather than painful or uncomfortable, smaller hernias may be associated more with pain and discomfort. As such, patients with smaller hernias may have higher hopes for relief from surgery, and therefore may be more likely to experience disappointment.
“Based on a cognitive information processing model it has been suggested that a greater discrepancy between expected and actual pain after surgery leads to significant postoperative distress,” the authors wrote.
The study also found patients aged younger than 55 years were also at greater risk of pain at rest, pain on exertion, and chronic pain requiring treatment.
Overall, around half the patients in the study underwent transabdominal preperitoneal repair, 35.6% underwent totally extraperitoneal repair, 10.1% had Lichtenstein repair, and 3.8% had Shouldice repair.
Lichtenstein was associated with more postoperative pain at rest and on exertion, compared with other surgical methods, and there was a trend toward an increased odds for developing pain requiring treatment in patients with smaller hernias.
The Herniamed Registry is supported by Johnson & Johnson, Karl Storz, pfm medical Cologne, Dahlhausen Cologne, B. Braun Tuttlingen, MenkeMed, and Bard. No conflicts of interest were reported.
SOURCE: Hoffmann H et al. Ann Surg. 2018 Oct 10. doi: 10.1097/SLA.0000000000003065.
according to a study published in Annals of Surgery.
Researchers used data from the European Herniamed registry to look at 1-year outcomes in 57,999 male patients who underwent primary unilateral inguinal hernia repair.
While patients with larger hernias showed significantly longer operation times and had a significantly larger body mass index, those with smaller hernias had significantly higher rates of pain at rest or on exertion, and chronic pain requiring treatment.
Individuals with smaller hernias (EHS I) had a 35% higher odds of pain at rest, compared with those with medium-sized hernias (EHS II), and 84% higher odds, compared with individuals with large hernias (EHS III). Smaller hernias were also associated with a 100% higher odds of pain on exertion and greater than 100% higher odds of chronic postoperative pain requiring treatment, compared with large hernias.
“CPIP [chronic postoperative inguinal pain] has become one of the most important surgical quality parameters after elective inguinal hernia repair with significant consequences affecting patient productivity, employment, and quality of life,” wrote Henry Hoffmann, MD, of the clinic for visceral surgery at the University Hospital Basel in Switzerland, and his coauthors. “With our findings we contribute to the important discussion of prevention, risk factors, and treatment of CPIP identifying smaller inguinal hernias [EHS I-II] as a new independent, patient-related risk factor for the development of CPIP.”
Noting that an association between smaller hernias and increased postoperative pain seems counterintuitive, the authors suggested that patients’ expectations of outcomes may be higher in those with smaller hernias than in patients with larger hernias. While larger hernias are likely more bothersome rather than painful or uncomfortable, smaller hernias may be associated more with pain and discomfort. As such, patients with smaller hernias may have higher hopes for relief from surgery, and therefore may be more likely to experience disappointment.
“Based on a cognitive information processing model it has been suggested that a greater discrepancy between expected and actual pain after surgery leads to significant postoperative distress,” the authors wrote.
The study also found patients aged younger than 55 years were also at greater risk of pain at rest, pain on exertion, and chronic pain requiring treatment.
Overall, around half the patients in the study underwent transabdominal preperitoneal repair, 35.6% underwent totally extraperitoneal repair, 10.1% had Lichtenstein repair, and 3.8% had Shouldice repair.
Lichtenstein was associated with more postoperative pain at rest and on exertion, compared with other surgical methods, and there was a trend toward an increased odds for developing pain requiring treatment in patients with smaller hernias.
The Herniamed Registry is supported by Johnson & Johnson, Karl Storz, pfm medical Cologne, Dahlhausen Cologne, B. Braun Tuttlingen, MenkeMed, and Bard. No conflicts of interest were reported.
SOURCE: Hoffmann H et al. Ann Surg. 2018 Oct 10. doi: 10.1097/SLA.0000000000003065.
according to a study published in Annals of Surgery.
Researchers used data from the European Herniamed registry to look at 1-year outcomes in 57,999 male patients who underwent primary unilateral inguinal hernia repair.
While patients with larger hernias showed significantly longer operation times and had a significantly larger body mass index, those with smaller hernias had significantly higher rates of pain at rest or on exertion, and chronic pain requiring treatment.
Individuals with smaller hernias (EHS I) had a 35% higher odds of pain at rest, compared with those with medium-sized hernias (EHS II), and 84% higher odds, compared with individuals with large hernias (EHS III). Smaller hernias were also associated with a 100% higher odds of pain on exertion and greater than 100% higher odds of chronic postoperative pain requiring treatment, compared with large hernias.
“CPIP [chronic postoperative inguinal pain] has become one of the most important surgical quality parameters after elective inguinal hernia repair with significant consequences affecting patient productivity, employment, and quality of life,” wrote Henry Hoffmann, MD, of the clinic for visceral surgery at the University Hospital Basel in Switzerland, and his coauthors. “With our findings we contribute to the important discussion of prevention, risk factors, and treatment of CPIP identifying smaller inguinal hernias [EHS I-II] as a new independent, patient-related risk factor for the development of CPIP.”
Noting that an association between smaller hernias and increased postoperative pain seems counterintuitive, the authors suggested that patients’ expectations of outcomes may be higher in those with smaller hernias than in patients with larger hernias. While larger hernias are likely more bothersome rather than painful or uncomfortable, smaller hernias may be associated more with pain and discomfort. As such, patients with smaller hernias may have higher hopes for relief from surgery, and therefore may be more likely to experience disappointment.
“Based on a cognitive information processing model it has been suggested that a greater discrepancy between expected and actual pain after surgery leads to significant postoperative distress,” the authors wrote.
The study also found patients aged younger than 55 years were also at greater risk of pain at rest, pain on exertion, and chronic pain requiring treatment.
Overall, around half the patients in the study underwent transabdominal preperitoneal repair, 35.6% underwent totally extraperitoneal repair, 10.1% had Lichtenstein repair, and 3.8% had Shouldice repair.
Lichtenstein was associated with more postoperative pain at rest and on exertion, compared with other surgical methods, and there was a trend toward an increased odds for developing pain requiring treatment in patients with smaller hernias.
The Herniamed Registry is supported by Johnson & Johnson, Karl Storz, pfm medical Cologne, Dahlhausen Cologne, B. Braun Tuttlingen, MenkeMed, and Bard. No conflicts of interest were reported.
SOURCE: Hoffmann H et al. Ann Surg. 2018 Oct 10. doi: 10.1097/SLA.0000000000003065.
FROM ANNALS OF SURGERY
Key clinical point: Smaller inguinal hernias associated with more postoperative pain than larger hernias.
Major finding: Smaller hernias are associated with a 100% higher odds of pain on exertion after surgery than those with large hernias.
Study details: An analysis of registry data from 57,999 patients who underwent hernia repair.
Disclosures: The Herniamed registry is supported by Johnson & Johnson, Karl Storz, pfm medical Cologne, Dahlhausen Cologne, B. Braun Tuttlingen, MenkeMed, and Bard. No conflicts of interest were reported.
Source: Hoffmann H et al. Ann Surg. 2018 Oct 10. doi: 10.1097/SLA.0000000000003065.
Rheumatologists underscreened RA patients for hyperlipidemia
Patients with rheumatoid arthritis had low rates of screening for primary hyperlipidemia, but patients who visited both a rheumatologist and a nonrheumatology clinician for follow-up were more likely to be screened, according to research published in Arthritis Care & Research.
Iris Navarro-Millán, MD, MSPH, from Cornell University, New York, and her colleagues performed a retrospective study of roughly 244,000 participants with private insurance, Medicare, or Medicaid who had rheumatoid arthritis (13,000 patients), diabetes (63,000), both rheumatoid arthritis (RA) and diabetes (1,000), and neither RA nor diabetes (168,000) during 2006-2010. Patients were between the ages of 41 years and 85 years. There were similar rates of hypertension among the RA patients (40%) and patients with neither RA nor diabetes (39%), as well as among the diabetes patients (79%) and the both patients with both RA and diabetes (70%).
The researchers found 37% of RA patients, 60% of diabetes patients, 55% of patients with both RA and diabetes, and 41% of patients with neither RA nor diabetes received primary lipid screening over 2 years’ follow-up. For RA-only patients, 22% saw a rheumatologist only, while 56% saw both a rheumatologist and then a nonrheumatology clinician at 2-years’ follow-up. Patients who visited a rheumatologist and a nonrheumatology clinician for follow-up were 55% more likely to receive primary lipid screening, and RA patients who visited a nonrheumatology clinician only were 22% more likely to receive screening than RA patients who saw only a rheumatologist.
Dr. Navarro-Millán and her colleagues said European RA patients may be more likely to receive screening for hyperlipidemia because their health care systems follow European League Against Rheumatism (EULAR) recommendations for management of cardiovascular disease (CVD) risk, while many rheumatologists based in the United States are “still reluctant to take responsibility to assess and mitigate (if needed) CVD risk for patients with RA.”
“Measures to achieve this goal must be implemented and may include defining specific roles for rheumatologists, nonrheumatology practitioners, and patients to determine who should be responsible for hyperlipidemia screening and treatment for patients with RA,” Dr. Navarro-Millán and her colleagues wrote.
Limitations to the study include lack of information on uninsured patients, lack of some clinical and demographic data, and potential misclassification of some clinicians who may have provided rheumatology-specific care but may have been in primary care settings.
This study is funded by grants and contracts from the National Institutes of Health and the Department of Health and Human Services. The authors report no relevant financial disclosures.
SOURCE: Navarro-Millán I et al. Arthritis Care Res. 2018. doi: 10.1002/acr.23810.
Patients with rheumatoid arthritis had low rates of screening for primary hyperlipidemia, but patients who visited both a rheumatologist and a nonrheumatology clinician for follow-up were more likely to be screened, according to research published in Arthritis Care & Research.
Iris Navarro-Millán, MD, MSPH, from Cornell University, New York, and her colleagues performed a retrospective study of roughly 244,000 participants with private insurance, Medicare, or Medicaid who had rheumatoid arthritis (13,000 patients), diabetes (63,000), both rheumatoid arthritis (RA) and diabetes (1,000), and neither RA nor diabetes (168,000) during 2006-2010. Patients were between the ages of 41 years and 85 years. There were similar rates of hypertension among the RA patients (40%) and patients with neither RA nor diabetes (39%), as well as among the diabetes patients (79%) and the both patients with both RA and diabetes (70%).
The researchers found 37% of RA patients, 60% of diabetes patients, 55% of patients with both RA and diabetes, and 41% of patients with neither RA nor diabetes received primary lipid screening over 2 years’ follow-up. For RA-only patients, 22% saw a rheumatologist only, while 56% saw both a rheumatologist and then a nonrheumatology clinician at 2-years’ follow-up. Patients who visited a rheumatologist and a nonrheumatology clinician for follow-up were 55% more likely to receive primary lipid screening, and RA patients who visited a nonrheumatology clinician only were 22% more likely to receive screening than RA patients who saw only a rheumatologist.
Dr. Navarro-Millán and her colleagues said European RA patients may be more likely to receive screening for hyperlipidemia because their health care systems follow European League Against Rheumatism (EULAR) recommendations for management of cardiovascular disease (CVD) risk, while many rheumatologists based in the United States are “still reluctant to take responsibility to assess and mitigate (if needed) CVD risk for patients with RA.”
“Measures to achieve this goal must be implemented and may include defining specific roles for rheumatologists, nonrheumatology practitioners, and patients to determine who should be responsible for hyperlipidemia screening and treatment for patients with RA,” Dr. Navarro-Millán and her colleagues wrote.
Limitations to the study include lack of information on uninsured patients, lack of some clinical and demographic data, and potential misclassification of some clinicians who may have provided rheumatology-specific care but may have been in primary care settings.
This study is funded by grants and contracts from the National Institutes of Health and the Department of Health and Human Services. The authors report no relevant financial disclosures.
SOURCE: Navarro-Millán I et al. Arthritis Care Res. 2018. doi: 10.1002/acr.23810.
Patients with rheumatoid arthritis had low rates of screening for primary hyperlipidemia, but patients who visited both a rheumatologist and a nonrheumatology clinician for follow-up were more likely to be screened, according to research published in Arthritis Care & Research.
Iris Navarro-Millán, MD, MSPH, from Cornell University, New York, and her colleagues performed a retrospective study of roughly 244,000 participants with private insurance, Medicare, or Medicaid who had rheumatoid arthritis (13,000 patients), diabetes (63,000), both rheumatoid arthritis (RA) and diabetes (1,000), and neither RA nor diabetes (168,000) during 2006-2010. Patients were between the ages of 41 years and 85 years. There were similar rates of hypertension among the RA patients (40%) and patients with neither RA nor diabetes (39%), as well as among the diabetes patients (79%) and the both patients with both RA and diabetes (70%).
The researchers found 37% of RA patients, 60% of diabetes patients, 55% of patients with both RA and diabetes, and 41% of patients with neither RA nor diabetes received primary lipid screening over 2 years’ follow-up. For RA-only patients, 22% saw a rheumatologist only, while 56% saw both a rheumatologist and then a nonrheumatology clinician at 2-years’ follow-up. Patients who visited a rheumatologist and a nonrheumatology clinician for follow-up were 55% more likely to receive primary lipid screening, and RA patients who visited a nonrheumatology clinician only were 22% more likely to receive screening than RA patients who saw only a rheumatologist.
Dr. Navarro-Millán and her colleagues said European RA patients may be more likely to receive screening for hyperlipidemia because their health care systems follow European League Against Rheumatism (EULAR) recommendations for management of cardiovascular disease (CVD) risk, while many rheumatologists based in the United States are “still reluctant to take responsibility to assess and mitigate (if needed) CVD risk for patients with RA.”
“Measures to achieve this goal must be implemented and may include defining specific roles for rheumatologists, nonrheumatology practitioners, and patients to determine who should be responsible for hyperlipidemia screening and treatment for patients with RA,” Dr. Navarro-Millán and her colleagues wrote.
Limitations to the study include lack of information on uninsured patients, lack of some clinical and demographic data, and potential misclassification of some clinicians who may have provided rheumatology-specific care but may have been in primary care settings.
This study is funded by grants and contracts from the National Institutes of Health and the Department of Health and Human Services. The authors report no relevant financial disclosures.
SOURCE: Navarro-Millán I et al. Arthritis Care Res. 2018. doi: 10.1002/acr.23810.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Patients with rheumatoid arthritis who saw only a rheumatologist were less likely to receive primary lipid screening than those who saw both a rheumatologist and then a clinician at 2-year follow-up.
Major finding: 37% of RA patients, 60% of diabetes patients, 55% of patients with both RA and diabetes, and 41% of patients with neither RA nor diabetes received primary lipid screening over 2-year follow-up.
Study details: A retrospective study of claims data from 243,909 participants aged 41-85 years in private and public health plans during 2006-2010.
Disclosures: This study is funded by grants and contracts from the National Institutes of Health and the Department of Health and Human Services. The authors report no relevant financial disclosures.
Source: Navarro-Millán I et al. Arthritis Care Res. 2018. doi: 10.1002/acr.23810.
Rapid recovery pathway for pediatric PSF/AIS patients
An important alternative amidst the opioid crisis
Clinical question
In pediatric postoperative spinal fusion/adolescent idiopathic scoliosis patients, do alternatives to traditional opioid-based analgesic pain regimens lead to improved clinical outcomes?
Background
Traditional care for pediatric postoperative spinal fusion (PSF) patients has included late mobilization most often because of significant pain that requires significant opioid administration. This has led to side effects of heavy opioid use, primarily nausea/vomiting and sleepiness.
In the United States, prescribers have become more aware of the pitfalls of opioid use given that more people now die of opioid misuse than breast cancer. An approach of multimodal analgesia with early mobilization has been shown to have decreased length of stay (LOS) and improve patient satisfaction, but data on clinical outcomes have been lacking.
Study design
Single-center quality improvement (QI) project.
Setting
Urban, 527-bed, quaternary care, free-standing children’s hospital.
Synopsis
Based on the recognition that multiple “standards” of care were utilized in the postoperative management of PSF patients, a QI project was undertaken. The primary outcome measured was functional recovery, as measured by average LOS and pain scores at the first 6:00 am after surgery then on postoperative days 1, 2, and 3.
Process measures were: use of multimodal agents (gabapentin and ketorolac) and discontinuation of patient-controlled analgesia (PCA) before postoperative day 3. Balancing measures were 30-day readmissions or ED revisit. Patients were divided into three groups by analyzing outcomes in three consecutive time periods: conventional management (n = 134), transition period (n = 104), and rapid recovery pathway (n = 84). In the conventional management time period, patients received intraoperative methadone and postoperative morphine/hydromorphone PCA. During the transition period, plan-do-study-act (PDSA) cycles with ketorolac and gabapentin pilots were instituted and assessed. Finally, a rapid recovery pathway (RRP) was designed and published as a web-based algorithm. Standardized entry order sets were developed to maintain compliance and consistency among health care professionals, and a transition period was allowed to reach the highest possible percentage of patients adhering to multimodal analgesia regimen.
Adherence to the multimodal regimen led to 90% of patients receiving ketorolac on postoperative day 1, 100% receiving gabapentin on night of surgery, 86% off of IV PCA by postoperative day 3, and 100% order set adherence after full implementation of the RRP. LOS decreased from 5.7 to 4 days after RRP implementation. Pain scores also showed significant improvement on postoperative day 0 (average pain score, 3.8 vs. 4.9) and postoperative day 1 (3.8 vs. 5). Balancing measures of 30-day readmissions or ED visits after discharge was 2.9% and rose to 3.6% after full implementation.
Bottom line
Multimodal analgesia – including preoperative gabapentin and acetaminophen, intraoperative methadone and acetaminophen, and postoperative PCA diazepam, gabapentin, acetaminophen, and ketorolac – results in decreased length of stay and improved self-reported daily pain scores.
Citation
Muhly WT et al. Rapid recovery pathway after spinal fusion for idiopathic scoliosis. Pediatrics. 2016 Apr;137(4):e20151568.
Dr. Giordano is a pediatric neurosurgery hospitalist and assistant professor in pediatrics at Columbia University Irving Medical Center in New York.
An important alternative amidst the opioid crisis
An important alternative amidst the opioid crisis
Clinical question
In pediatric postoperative spinal fusion/adolescent idiopathic scoliosis patients, do alternatives to traditional opioid-based analgesic pain regimens lead to improved clinical outcomes?
Background
Traditional care for pediatric postoperative spinal fusion (PSF) patients has included late mobilization most often because of significant pain that requires significant opioid administration. This has led to side effects of heavy opioid use, primarily nausea/vomiting and sleepiness.
In the United States, prescribers have become more aware of the pitfalls of opioid use given that more people now die of opioid misuse than breast cancer. An approach of multimodal analgesia with early mobilization has been shown to have decreased length of stay (LOS) and improve patient satisfaction, but data on clinical outcomes have been lacking.
Study design
Single-center quality improvement (QI) project.
Setting
Urban, 527-bed, quaternary care, free-standing children’s hospital.
Synopsis
Based on the recognition that multiple “standards” of care were utilized in the postoperative management of PSF patients, a QI project was undertaken. The primary outcome measured was functional recovery, as measured by average LOS and pain scores at the first 6:00 am after surgery then on postoperative days 1, 2, and 3.
Process measures were: use of multimodal agents (gabapentin and ketorolac) and discontinuation of patient-controlled analgesia (PCA) before postoperative day 3. Balancing measures were 30-day readmissions or ED revisit. Patients were divided into three groups by analyzing outcomes in three consecutive time periods: conventional management (n = 134), transition period (n = 104), and rapid recovery pathway (n = 84). In the conventional management time period, patients received intraoperative methadone and postoperative morphine/hydromorphone PCA. During the transition period, plan-do-study-act (PDSA) cycles with ketorolac and gabapentin pilots were instituted and assessed. Finally, a rapid recovery pathway (RRP) was designed and published as a web-based algorithm. Standardized entry order sets were developed to maintain compliance and consistency among health care professionals, and a transition period was allowed to reach the highest possible percentage of patients adhering to multimodal analgesia regimen.
Adherence to the multimodal regimen led to 90% of patients receiving ketorolac on postoperative day 1, 100% receiving gabapentin on night of surgery, 86% off of IV PCA by postoperative day 3, and 100% order set adherence after full implementation of the RRP. LOS decreased from 5.7 to 4 days after RRP implementation. Pain scores also showed significant improvement on postoperative day 0 (average pain score, 3.8 vs. 4.9) and postoperative day 1 (3.8 vs. 5). Balancing measures of 30-day readmissions or ED visits after discharge was 2.9% and rose to 3.6% after full implementation.
Bottom line
Multimodal analgesia – including preoperative gabapentin and acetaminophen, intraoperative methadone and acetaminophen, and postoperative PCA diazepam, gabapentin, acetaminophen, and ketorolac – results in decreased length of stay and improved self-reported daily pain scores.
Citation
Muhly WT et al. Rapid recovery pathway after spinal fusion for idiopathic scoliosis. Pediatrics. 2016 Apr;137(4):e20151568.
Dr. Giordano is a pediatric neurosurgery hospitalist and assistant professor in pediatrics at Columbia University Irving Medical Center in New York.
Clinical question
In pediatric postoperative spinal fusion/adolescent idiopathic scoliosis patients, do alternatives to traditional opioid-based analgesic pain regimens lead to improved clinical outcomes?
Background
Traditional care for pediatric postoperative spinal fusion (PSF) patients has included late mobilization most often because of significant pain that requires significant opioid administration. This has led to side effects of heavy opioid use, primarily nausea/vomiting and sleepiness.
In the United States, prescribers have become more aware of the pitfalls of opioid use given that more people now die of opioid misuse than breast cancer. An approach of multimodal analgesia with early mobilization has been shown to have decreased length of stay (LOS) and improve patient satisfaction, but data on clinical outcomes have been lacking.
Study design
Single-center quality improvement (QI) project.
Setting
Urban, 527-bed, quaternary care, free-standing children’s hospital.
Synopsis
Based on the recognition that multiple “standards” of care were utilized in the postoperative management of PSF patients, a QI project was undertaken. The primary outcome measured was functional recovery, as measured by average LOS and pain scores at the first 6:00 am after surgery then on postoperative days 1, 2, and 3.
Process measures were: use of multimodal agents (gabapentin and ketorolac) and discontinuation of patient-controlled analgesia (PCA) before postoperative day 3. Balancing measures were 30-day readmissions or ED revisit. Patients were divided into three groups by analyzing outcomes in three consecutive time periods: conventional management (n = 134), transition period (n = 104), and rapid recovery pathway (n = 84). In the conventional management time period, patients received intraoperative methadone and postoperative morphine/hydromorphone PCA. During the transition period, plan-do-study-act (PDSA) cycles with ketorolac and gabapentin pilots were instituted and assessed. Finally, a rapid recovery pathway (RRP) was designed and published as a web-based algorithm. Standardized entry order sets were developed to maintain compliance and consistency among health care professionals, and a transition period was allowed to reach the highest possible percentage of patients adhering to multimodal analgesia regimen.
Adherence to the multimodal regimen led to 90% of patients receiving ketorolac on postoperative day 1, 100% receiving gabapentin on night of surgery, 86% off of IV PCA by postoperative day 3, and 100% order set adherence after full implementation of the RRP. LOS decreased from 5.7 to 4 days after RRP implementation. Pain scores also showed significant improvement on postoperative day 0 (average pain score, 3.8 vs. 4.9) and postoperative day 1 (3.8 vs. 5). Balancing measures of 30-day readmissions or ED visits after discharge was 2.9% and rose to 3.6% after full implementation.
Bottom line
Multimodal analgesia – including preoperative gabapentin and acetaminophen, intraoperative methadone and acetaminophen, and postoperative PCA diazepam, gabapentin, acetaminophen, and ketorolac – results in decreased length of stay and improved self-reported daily pain scores.
Citation
Muhly WT et al. Rapid recovery pathway after spinal fusion for idiopathic scoliosis. Pediatrics. 2016 Apr;137(4):e20151568.
Dr. Giordano is a pediatric neurosurgery hospitalist and assistant professor in pediatrics at Columbia University Irving Medical Center in New York.
Staying up to date on screening may cut risk of death from CRC
according to the results of a large retrospective case-control study.
Source: American Gastroenterological Association
The findings signify “potentially modifiable” screening failures in a population known for relatively high uptake of colorectal cancer screening, wrote Chyke A. Doubeni, MD, MPH, of the University of Pennsylvania, Philadelphia, and his associates in Gastroenterology. Strikingly, 76% of patients who died from colorectal cancer were not current on screening versus 55% of cancer-free patients, they said. Being up to date on screening decreased the odds of dying from colorectal cancer by 62% (odds ratio, 0.38; 95% confidence interval, 0.33-0.44), even after adjustment for race, ethnicity, socioeconomic status, comorbidities, and frequency of contact with primary care providers, they added.
Colonoscopy, sigmoidoscopy, and fecal testing are effective and recommended screening techniques that help prevent deaths from colorectal cancer. Therefore, most such deaths are thought to result from “breakdowns in the screening process,” the researchers wrote. However, interval cancers and missed lesions also play a role, and no prior study has examined detailed screening histories and their association with colorectal cancer mortality.
Accordingly, the researchers reviewed medical records and registry data for 1,750 enrollees in the Kaiser Permanente Northern and Southern California systems who died from colorectal cancer during 2002-2012 and were part of the health plan for at least 5 years before their cancer diagnosis. They compared these patients with 3,486 cancer-free controls matched by age, sex, study site, and numbers of years enrolled in the health plan. Patients were considered up to date on screening if they were screened at intervals recommended by the 2008 multisociety colorectal cancer screening guidelines – that is, if they had received a colonoscopy within 10 years of colorectal cancer diagnosis or sigmoidoscopy or barium enema within 5 years of it. For fecal testing, the investigators used a 2-year interval based on its efficacy in clinical trials.
Among patients who died from colorectal cancer, only 24% were up to date on screening versus 45% of cancer-free-patients, the investigators determined. Furthermore, 68% of patients who died from colorectal cancer were never screened or were not screened at appropriate intervals, compared with 53% of cancer-free patients.
Additionally, while 8% of colorectal cancer deaths occurred in patients who had not followed up on abnormal screening results, only 2% of controls who had received abnormal screening results had failed to follow up.
“In two health systems with high rates of screening, we observed that most patients dying from colorectal cancer had potentially modifiable failures of the screening process,” the researchers concluded. “This study suggests that, even in settings with high screening uptake, access to and timely uptake of screening, regular rescreening, appropriate use of testing given patient characteristics, completion of timely diagnostic testing when screening is positive, and improving the effectiveness of screening tests, particularly for right colon cancer, remain important areas of focus for further decreasing colorectal cancer deaths.”
The National Institutes of Health funded the work. The investigators reported having no conflicts of interest except that one coinvestigator is editor in chief of the journal Gastroenterology.
SOURCE: Doubeni CA et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.09.040.
Screening for colorectal cancer (CRC) is a major success story – one of only two cancers (the other being cervical cancer) with an A recommendation for screening from the U.S. Preventive Services Task Force. Multiple randomized trials for two CRC screening modalities, stool-based tests and sigmoidoscopy, have shown significant reductions in CRC incidence and mortality.
Within this context, Doubeni et al. examined the association of CRC screening with death from CRC in a real-world HMO setting. Their study is notable for several reasons. First, it showed a highly protective effect on CRC mortality of being up to date with screening (odds ratio, 0.38; 95% confidence interval, 0.33-0.44). Second, it examined CRC screening as a process, with various steps of that process related to CRC mortality. Finally, methodologically, the study’s utilization of electronic medical records and cancer registry linkages highlights the importance of integrated data systems in the efficient performance of epidemiologic research.
Of note, screening was primarily stool-based tests (fecal occult blood test/fecal immunochemical test ) and sigmoidoscopy, in contrast to most of the U.S., where colonoscopy is predominant. Randomized trials of these modalities show mortality reductions of 15%-20% (FOBT/FIT) and 25%-30% (sigmoidoscopy), respectively. Therefore, some of the reported effect is likely due to selection bias, with healthier persons more likely to choose screening.
It would be of interest to see similar studies performed in a colonoscopy-predominant screening setting and with the effect on CRC incidence as well as mortality examined.
Paul F. Pinsky, PhD, chief of the Early Detection Research Branch, National Cancer Institute, Bethesda, MD. He has no conflicts of interest.
Screening for colorectal cancer (CRC) is a major success story – one of only two cancers (the other being cervical cancer) with an A recommendation for screening from the U.S. Preventive Services Task Force. Multiple randomized trials for two CRC screening modalities, stool-based tests and sigmoidoscopy, have shown significant reductions in CRC incidence and mortality.
Within this context, Doubeni et al. examined the association of CRC screening with death from CRC in a real-world HMO setting. Their study is notable for several reasons. First, it showed a highly protective effect on CRC mortality of being up to date with screening (odds ratio, 0.38; 95% confidence interval, 0.33-0.44). Second, it examined CRC screening as a process, with various steps of that process related to CRC mortality. Finally, methodologically, the study’s utilization of electronic medical records and cancer registry linkages highlights the importance of integrated data systems in the efficient performance of epidemiologic research.
Of note, screening was primarily stool-based tests (fecal occult blood test/fecal immunochemical test ) and sigmoidoscopy, in contrast to most of the U.S., where colonoscopy is predominant. Randomized trials of these modalities show mortality reductions of 15%-20% (FOBT/FIT) and 25%-30% (sigmoidoscopy), respectively. Therefore, some of the reported effect is likely due to selection bias, with healthier persons more likely to choose screening.
It would be of interest to see similar studies performed in a colonoscopy-predominant screening setting and with the effect on CRC incidence as well as mortality examined.
Paul F. Pinsky, PhD, chief of the Early Detection Research Branch, National Cancer Institute, Bethesda, MD. He has no conflicts of interest.
Screening for colorectal cancer (CRC) is a major success story – one of only two cancers (the other being cervical cancer) with an A recommendation for screening from the U.S. Preventive Services Task Force. Multiple randomized trials for two CRC screening modalities, stool-based tests and sigmoidoscopy, have shown significant reductions in CRC incidence and mortality.
Within this context, Doubeni et al. examined the association of CRC screening with death from CRC in a real-world HMO setting. Their study is notable for several reasons. First, it showed a highly protective effect on CRC mortality of being up to date with screening (odds ratio, 0.38; 95% confidence interval, 0.33-0.44). Second, it examined CRC screening as a process, with various steps of that process related to CRC mortality. Finally, methodologically, the study’s utilization of electronic medical records and cancer registry linkages highlights the importance of integrated data systems in the efficient performance of epidemiologic research.
Of note, screening was primarily stool-based tests (fecal occult blood test/fecal immunochemical test ) and sigmoidoscopy, in contrast to most of the U.S., where colonoscopy is predominant. Randomized trials of these modalities show mortality reductions of 15%-20% (FOBT/FIT) and 25%-30% (sigmoidoscopy), respectively. Therefore, some of the reported effect is likely due to selection bias, with healthier persons more likely to choose screening.
It would be of interest to see similar studies performed in a colonoscopy-predominant screening setting and with the effect on CRC incidence as well as mortality examined.
Paul F. Pinsky, PhD, chief of the Early Detection Research Branch, National Cancer Institute, Bethesda, MD. He has no conflicts of interest.
according to the results of a large retrospective case-control study.
Source: American Gastroenterological Association
The findings signify “potentially modifiable” screening failures in a population known for relatively high uptake of colorectal cancer screening, wrote Chyke A. Doubeni, MD, MPH, of the University of Pennsylvania, Philadelphia, and his associates in Gastroenterology. Strikingly, 76% of patients who died from colorectal cancer were not current on screening versus 55% of cancer-free patients, they said. Being up to date on screening decreased the odds of dying from colorectal cancer by 62% (odds ratio, 0.38; 95% confidence interval, 0.33-0.44), even after adjustment for race, ethnicity, socioeconomic status, comorbidities, and frequency of contact with primary care providers, they added.
Colonoscopy, sigmoidoscopy, and fecal testing are effective and recommended screening techniques that help prevent deaths from colorectal cancer. Therefore, most such deaths are thought to result from “breakdowns in the screening process,” the researchers wrote. However, interval cancers and missed lesions also play a role, and no prior study has examined detailed screening histories and their association with colorectal cancer mortality.
Accordingly, the researchers reviewed medical records and registry data for 1,750 enrollees in the Kaiser Permanente Northern and Southern California systems who died from colorectal cancer during 2002-2012 and were part of the health plan for at least 5 years before their cancer diagnosis. They compared these patients with 3,486 cancer-free controls matched by age, sex, study site, and numbers of years enrolled in the health plan. Patients were considered up to date on screening if they were screened at intervals recommended by the 2008 multisociety colorectal cancer screening guidelines – that is, if they had received a colonoscopy within 10 years of colorectal cancer diagnosis or sigmoidoscopy or barium enema within 5 years of it. For fecal testing, the investigators used a 2-year interval based on its efficacy in clinical trials.
Among patients who died from colorectal cancer, only 24% were up to date on screening versus 45% of cancer-free-patients, the investigators determined. Furthermore, 68% of patients who died from colorectal cancer were never screened or were not screened at appropriate intervals, compared with 53% of cancer-free patients.
Additionally, while 8% of colorectal cancer deaths occurred in patients who had not followed up on abnormal screening results, only 2% of controls who had received abnormal screening results had failed to follow up.
“In two health systems with high rates of screening, we observed that most patients dying from colorectal cancer had potentially modifiable failures of the screening process,” the researchers concluded. “This study suggests that, even in settings with high screening uptake, access to and timely uptake of screening, regular rescreening, appropriate use of testing given patient characteristics, completion of timely diagnostic testing when screening is positive, and improving the effectiveness of screening tests, particularly for right colon cancer, remain important areas of focus for further decreasing colorectal cancer deaths.”
The National Institutes of Health funded the work. The investigators reported having no conflicts of interest except that one coinvestigator is editor in chief of the journal Gastroenterology.
SOURCE: Doubeni CA et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.09.040.
according to the results of a large retrospective case-control study.
Source: American Gastroenterological Association
The findings signify “potentially modifiable” screening failures in a population known for relatively high uptake of colorectal cancer screening, wrote Chyke A. Doubeni, MD, MPH, of the University of Pennsylvania, Philadelphia, and his associates in Gastroenterology. Strikingly, 76% of patients who died from colorectal cancer were not current on screening versus 55% of cancer-free patients, they said. Being up to date on screening decreased the odds of dying from colorectal cancer by 62% (odds ratio, 0.38; 95% confidence interval, 0.33-0.44), even after adjustment for race, ethnicity, socioeconomic status, comorbidities, and frequency of contact with primary care providers, they added.
Colonoscopy, sigmoidoscopy, and fecal testing are effective and recommended screening techniques that help prevent deaths from colorectal cancer. Therefore, most such deaths are thought to result from “breakdowns in the screening process,” the researchers wrote. However, interval cancers and missed lesions also play a role, and no prior study has examined detailed screening histories and their association with colorectal cancer mortality.
Accordingly, the researchers reviewed medical records and registry data for 1,750 enrollees in the Kaiser Permanente Northern and Southern California systems who died from colorectal cancer during 2002-2012 and were part of the health plan for at least 5 years before their cancer diagnosis. They compared these patients with 3,486 cancer-free controls matched by age, sex, study site, and numbers of years enrolled in the health plan. Patients were considered up to date on screening if they were screened at intervals recommended by the 2008 multisociety colorectal cancer screening guidelines – that is, if they had received a colonoscopy within 10 years of colorectal cancer diagnosis or sigmoidoscopy or barium enema within 5 years of it. For fecal testing, the investigators used a 2-year interval based on its efficacy in clinical trials.
Among patients who died from colorectal cancer, only 24% were up to date on screening versus 45% of cancer-free-patients, the investigators determined. Furthermore, 68% of patients who died from colorectal cancer were never screened or were not screened at appropriate intervals, compared with 53% of cancer-free patients.
Additionally, while 8% of colorectal cancer deaths occurred in patients who had not followed up on abnormal screening results, only 2% of controls who had received abnormal screening results had failed to follow up.
“In two health systems with high rates of screening, we observed that most patients dying from colorectal cancer had potentially modifiable failures of the screening process,” the researchers concluded. “This study suggests that, even in settings with high screening uptake, access to and timely uptake of screening, regular rescreening, appropriate use of testing given patient characteristics, completion of timely diagnostic testing when screening is positive, and improving the effectiveness of screening tests, particularly for right colon cancer, remain important areas of focus for further decreasing colorectal cancer deaths.”
The National Institutes of Health funded the work. The investigators reported having no conflicts of interest except that one coinvestigator is editor in chief of the journal Gastroenterology.
SOURCE: Doubeni CA et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.09.040.
FROM GASTROENTEROLOGY
Key clinical point: Being up to date on screening was associated with a significant reduction in the risk of dying from colon cancer.
Major finding: Being up to date on screening decreased the odds of dying from colorectal cancer by 62% (odds ratio, 0.38; 95% confidence interval, 0.33-0.44).
Study details: Retrospective cohort study of 1,750 patients who died from colorectal cancer during 2002-2012 and 3,486 matched controls.
Disclosures: The National Institutes of Health funded the work. The investigators reported having no conflicts of interest except that one coinvestigator is editor in chief of Gastroenterology.
Source: Doubeni CA et al. Gastroenterology. 2018 Sep 27. doi: 10.1053/j.gastro.2018.09.040.
Expert highlights rare causes of stroke to keep in mind
ATLANTA – According to Peter Berlit, MD, clinicians should .
Other factors include combination of ischemic and hemorrhagic stroke, exclusive involvement of intracranial vessels, systemic signs, and lab tests indicating inflammation.
At the annual meeting of the American Neurological Association, Dr. Berlit, secretary general of the German Society of Neurology in Berlin, discussed the diagnosis and management of rare causes of stroke.
Giant cell arteritis (GCA)
One of the rare causes of stroke, GCA can be diagnosed when three of five criteria are met: being 50 years of age or older, having a newly developed headache, tenderness of the superficial temporal artery, elevated sedimentation rate of at least 50 mm per hour, and GCA in a biopsy specimen from the temporal artery.
“What we fear most is sudden blindness due to involvement of arteries serving the eyes, which appears in up to 30% of GCA patients,” said Dr. Berlit, who formerly chaired the department of neurology at Alfried Krupp Hospital, Essen, Germany. “Stroke occurs in approximately 2% of GCA patients, so it’s a lot rarer.” GCA can also be diagnosed by ultrasound. One meta-analysis of 23 studies using halo, stenosis, and occlusion as ultrasound criteria found a sensitivity of 87% and a specificity of 96% (Ann Intern Med. 2005;142[5]:359-69). “You can also use 3-Tesla MRI with the use of contrast agent, which shows inflammation of the temporal artery, but also other large vessels including the aortic arch,” he said. “The treatment of GCA has changed since the end of 2017 and involves starting with prednisolone 1 mg/kg body weight.” After a dose of 30 mg for 4 weeks, reduce the dose by 2.5 mg every 2 weeks. After reaching the dose of 15 mg daily, reduce by 1 mg per month. “The recommended steroid-sparing treatment is subcutaneous tocilizumab at a dose of 162 mg weekly or every other week, combined with a prednisone taper for a minimum of 26 weeks,” he said. Supportive therapies include pantoprazole 20 mg, aspirin 100 mg, calcium, vitamin D, and bisphosphonates.
Primary angiitis of the central nervous system (PACNS)
Next, Dr. Berlit discussed diagnostic criteria for PACNS, an acquired neurological deficit unexplained after complete evaluation. “You should have a diagnostic cerebral angiogram or biopsy demonstrating vasculitis,” he said. “There should be no evidence of systemic vasculitis or any other conditions that could mimic the angiogram findings. Usually you have abnormal CSF findings, including pleocytosis and protein elevation, and a biopsy demonstrating vasculitis.”
MRI studies in suspected vasculitis include fluid-attenuated inversion recovery (FLAIR), diffusion imaging with apparent diffusion coefficient (ADC) maps, gradient ECHO, MR angiography, and contrast-enhanced imaging. “These usually show multifocal lesions of different ages, and hemorrhages occur in about 10% of lesions,” Dr. Berlit said. “Leptomeningeal enhancement is an indicator of good treatment response.”
A brain and leptomeningeal biopsy demonstrating the angiitis remains the preferred method for diagnosis of PACNS. “Open biopsies out of recent MRI lesions are especially diagnostic,” he said. “If there are no lesions accessible for surgery in noneloquent brain areas, a biopsy from the right frontal lobe is recommended.” The histologic findings of PACNS consist of granulomatous inflammation, fibrinoid necrosis of vessel walls, or exclusively lymphocytic cellular infiltrates. “The treatment of choice in PACNS is the combination of steroids and cyclophosphamide pulse therapy,” he said. “There are also data showing that rituximab or methotrexate might be treatment options. With a relapse rate of 25% and a reduced survival rate, a close follow-up of suspected PACNS is mandatory.”
Reversible cerebral vasoconstriction syndrome (RCVS)
Another rare cause of stroke is RCVS, which typically presents as thunderclap headaches with or without neurologic symptoms. MRI may be normal, but symmetric border zone infarctions and small subarachnoid hemorrhages are possible. Catheter, CT, or MR angiography show segmental arterial vasoconstriction. “You always have to exclude cerebral aneurysm,” Dr. Berlit said. “There is reversibility of RCVS within 3 months.” RCVS is often associated with a long list of drugs, including phenylpropanolamine, Methergine (methylergonovine), bromocriptine, lisuride, SSRIs, triptans, isometheptene, tacrolimus, cyclophosphamide, erythropoietin, intravenous immunoglobulins, erythrocyte concentrates, nasal sprays, cocaine, ecstasy, amphetamines, cannabis, and LSD. “After stopping responsible medications, treatment involves a course of nimodipine,” he said.
Moyamoya disease (MMD)
Dr. Berlit closed his presentation by discussing MMD, a rare occlusive cerebrovascular disorder characterized by progressive stenosis or occlusion of the intracranial portion of the internal carotid artery and proximal cerebral arteries with an extensive network of fine collaterals. “This is an idiopathic vasculopathy with remarkable regional and racial differences worldwide; it’s most frequently found in Asians, especially in Japan and Korea,” he said. “In Europe, there is about one-tenth the incidence, compared with that of Japan. In Asian MMD, about 15% of cases follow an autosomal dominant inheritance. The collaterals in MMD present histologically as a thin media, a fragmented elastic laminae, and the formation of microaneurysms. There is no inflammation.”
MMD diagnostic criteria include stenosis or occlusion of the terminal portion of the internal carotid artery and at the proximal portion of the anterior and middle cerebral arteries. Abnormal vascular networks are present in the basal ganglia and angiographic findings present bilaterally. Cases with unilateral angiographic findings are considered probable. Clinicians should exclude the following conditions: arteriosclerosis, autoimmune disease, brain neoplasm, history of cranial irradiation, Down syndrome, head trauma, neurofibromatosis, and meningitis. “If the angiographic pattern is resembled by one of these conditions, this is called moyamoya syndrome,” Dr. Berlit noted. “MMD is a progressive disorder. Within a few months you can see occlusion of the middle cerebral artery and the anterior cerebral artery, so you have to treat these patients.”
In patients who are white, MMD presents with lower rates of hemorrhage, but in Asians, microbleeds occur in up to 44% of patients and hemorrhages in up to 65% patients. “Both subarachnoidal and intracerebral hemorrhages occur, especially in connection with pregnancy and delivery,” he said. “The risk of both cerebral ischemia and hemorrhagic complications increases with stages of MMD.”
Direct or indirect intracranial bypass surgery is recommended in stages 3 or more, and has been shown to significantly reduce the 5-year stroke risk. To date, Dr. Berlit and his associates have treated 86 hemispheres in 56 patients. The average age of the patients was 42 years, 70% were female, and the average follow-up was 39 months. All intracranial bypasses were open on follow-up, and a decrease of the typical moyamoya vessels was observed in 81% of patients.
Dr. Berlit reported having no financial disclosures.
ATLANTA – According to Peter Berlit, MD, clinicians should .
Other factors include combination of ischemic and hemorrhagic stroke, exclusive involvement of intracranial vessels, systemic signs, and lab tests indicating inflammation.
At the annual meeting of the American Neurological Association, Dr. Berlit, secretary general of the German Society of Neurology in Berlin, discussed the diagnosis and management of rare causes of stroke.
Giant cell arteritis (GCA)
One of the rare causes of stroke, GCA can be diagnosed when three of five criteria are met: being 50 years of age or older, having a newly developed headache, tenderness of the superficial temporal artery, elevated sedimentation rate of at least 50 mm per hour, and GCA in a biopsy specimen from the temporal artery.
“What we fear most is sudden blindness due to involvement of arteries serving the eyes, which appears in up to 30% of GCA patients,” said Dr. Berlit, who formerly chaired the department of neurology at Alfried Krupp Hospital, Essen, Germany. “Stroke occurs in approximately 2% of GCA patients, so it’s a lot rarer.” GCA can also be diagnosed by ultrasound. One meta-analysis of 23 studies using halo, stenosis, and occlusion as ultrasound criteria found a sensitivity of 87% and a specificity of 96% (Ann Intern Med. 2005;142[5]:359-69). “You can also use 3-Tesla MRI with the use of contrast agent, which shows inflammation of the temporal artery, but also other large vessels including the aortic arch,” he said. “The treatment of GCA has changed since the end of 2017 and involves starting with prednisolone 1 mg/kg body weight.” After a dose of 30 mg for 4 weeks, reduce the dose by 2.5 mg every 2 weeks. After reaching the dose of 15 mg daily, reduce by 1 mg per month. “The recommended steroid-sparing treatment is subcutaneous tocilizumab at a dose of 162 mg weekly or every other week, combined with a prednisone taper for a minimum of 26 weeks,” he said. Supportive therapies include pantoprazole 20 mg, aspirin 100 mg, calcium, vitamin D, and bisphosphonates.
Primary angiitis of the central nervous system (PACNS)
Next, Dr. Berlit discussed diagnostic criteria for PACNS, an acquired neurological deficit unexplained after complete evaluation. “You should have a diagnostic cerebral angiogram or biopsy demonstrating vasculitis,” he said. “There should be no evidence of systemic vasculitis or any other conditions that could mimic the angiogram findings. Usually you have abnormal CSF findings, including pleocytosis and protein elevation, and a biopsy demonstrating vasculitis.”
MRI studies in suspected vasculitis include fluid-attenuated inversion recovery (FLAIR), diffusion imaging with apparent diffusion coefficient (ADC) maps, gradient ECHO, MR angiography, and contrast-enhanced imaging. “These usually show multifocal lesions of different ages, and hemorrhages occur in about 10% of lesions,” Dr. Berlit said. “Leptomeningeal enhancement is an indicator of good treatment response.”
A brain and leptomeningeal biopsy demonstrating the angiitis remains the preferred method for diagnosis of PACNS. “Open biopsies out of recent MRI lesions are especially diagnostic,” he said. “If there are no lesions accessible for surgery in noneloquent brain areas, a biopsy from the right frontal lobe is recommended.” The histologic findings of PACNS consist of granulomatous inflammation, fibrinoid necrosis of vessel walls, or exclusively lymphocytic cellular infiltrates. “The treatment of choice in PACNS is the combination of steroids and cyclophosphamide pulse therapy,” he said. “There are also data showing that rituximab or methotrexate might be treatment options. With a relapse rate of 25% and a reduced survival rate, a close follow-up of suspected PACNS is mandatory.”
Reversible cerebral vasoconstriction syndrome (RCVS)
Another rare cause of stroke is RCVS, which typically presents as thunderclap headaches with or without neurologic symptoms. MRI may be normal, but symmetric border zone infarctions and small subarachnoid hemorrhages are possible. Catheter, CT, or MR angiography show segmental arterial vasoconstriction. “You always have to exclude cerebral aneurysm,” Dr. Berlit said. “There is reversibility of RCVS within 3 months.” RCVS is often associated with a long list of drugs, including phenylpropanolamine, Methergine (methylergonovine), bromocriptine, lisuride, SSRIs, triptans, isometheptene, tacrolimus, cyclophosphamide, erythropoietin, intravenous immunoglobulins, erythrocyte concentrates, nasal sprays, cocaine, ecstasy, amphetamines, cannabis, and LSD. “After stopping responsible medications, treatment involves a course of nimodipine,” he said.
Moyamoya disease (MMD)
Dr. Berlit closed his presentation by discussing MMD, a rare occlusive cerebrovascular disorder characterized by progressive stenosis or occlusion of the intracranial portion of the internal carotid artery and proximal cerebral arteries with an extensive network of fine collaterals. “This is an idiopathic vasculopathy with remarkable regional and racial differences worldwide; it’s most frequently found in Asians, especially in Japan and Korea,” he said. “In Europe, there is about one-tenth the incidence, compared with that of Japan. In Asian MMD, about 15% of cases follow an autosomal dominant inheritance. The collaterals in MMD present histologically as a thin media, a fragmented elastic laminae, and the formation of microaneurysms. There is no inflammation.”
MMD diagnostic criteria include stenosis or occlusion of the terminal portion of the internal carotid artery and at the proximal portion of the anterior and middle cerebral arteries. Abnormal vascular networks are present in the basal ganglia and angiographic findings present bilaterally. Cases with unilateral angiographic findings are considered probable. Clinicians should exclude the following conditions: arteriosclerosis, autoimmune disease, brain neoplasm, history of cranial irradiation, Down syndrome, head trauma, neurofibromatosis, and meningitis. “If the angiographic pattern is resembled by one of these conditions, this is called moyamoya syndrome,” Dr. Berlit noted. “MMD is a progressive disorder. Within a few months you can see occlusion of the middle cerebral artery and the anterior cerebral artery, so you have to treat these patients.”
In patients who are white, MMD presents with lower rates of hemorrhage, but in Asians, microbleeds occur in up to 44% of patients and hemorrhages in up to 65% patients. “Both subarachnoidal and intracerebral hemorrhages occur, especially in connection with pregnancy and delivery,” he said. “The risk of both cerebral ischemia and hemorrhagic complications increases with stages of MMD.”
Direct or indirect intracranial bypass surgery is recommended in stages 3 or more, and has been shown to significantly reduce the 5-year stroke risk. To date, Dr. Berlit and his associates have treated 86 hemispheres in 56 patients. The average age of the patients was 42 years, 70% were female, and the average follow-up was 39 months. All intracranial bypasses were open on follow-up, and a decrease of the typical moyamoya vessels was observed in 81% of patients.
Dr. Berlit reported having no financial disclosures.
ATLANTA – According to Peter Berlit, MD, clinicians should .
Other factors include combination of ischemic and hemorrhagic stroke, exclusive involvement of intracranial vessels, systemic signs, and lab tests indicating inflammation.
At the annual meeting of the American Neurological Association, Dr. Berlit, secretary general of the German Society of Neurology in Berlin, discussed the diagnosis and management of rare causes of stroke.
Giant cell arteritis (GCA)
One of the rare causes of stroke, GCA can be diagnosed when three of five criteria are met: being 50 years of age or older, having a newly developed headache, tenderness of the superficial temporal artery, elevated sedimentation rate of at least 50 mm per hour, and GCA in a biopsy specimen from the temporal artery.
“What we fear most is sudden blindness due to involvement of arteries serving the eyes, which appears in up to 30% of GCA patients,” said Dr. Berlit, who formerly chaired the department of neurology at Alfried Krupp Hospital, Essen, Germany. “Stroke occurs in approximately 2% of GCA patients, so it’s a lot rarer.” GCA can also be diagnosed by ultrasound. One meta-analysis of 23 studies using halo, stenosis, and occlusion as ultrasound criteria found a sensitivity of 87% and a specificity of 96% (Ann Intern Med. 2005;142[5]:359-69). “You can also use 3-Tesla MRI with the use of contrast agent, which shows inflammation of the temporal artery, but also other large vessels including the aortic arch,” he said. “The treatment of GCA has changed since the end of 2017 and involves starting with prednisolone 1 mg/kg body weight.” After a dose of 30 mg for 4 weeks, reduce the dose by 2.5 mg every 2 weeks. After reaching the dose of 15 mg daily, reduce by 1 mg per month. “The recommended steroid-sparing treatment is subcutaneous tocilizumab at a dose of 162 mg weekly or every other week, combined with a prednisone taper for a minimum of 26 weeks,” he said. Supportive therapies include pantoprazole 20 mg, aspirin 100 mg, calcium, vitamin D, and bisphosphonates.
Primary angiitis of the central nervous system (PACNS)
Next, Dr. Berlit discussed diagnostic criteria for PACNS, an acquired neurological deficit unexplained after complete evaluation. “You should have a diagnostic cerebral angiogram or biopsy demonstrating vasculitis,” he said. “There should be no evidence of systemic vasculitis or any other conditions that could mimic the angiogram findings. Usually you have abnormal CSF findings, including pleocytosis and protein elevation, and a biopsy demonstrating vasculitis.”
MRI studies in suspected vasculitis include fluid-attenuated inversion recovery (FLAIR), diffusion imaging with apparent diffusion coefficient (ADC) maps, gradient ECHO, MR angiography, and contrast-enhanced imaging. “These usually show multifocal lesions of different ages, and hemorrhages occur in about 10% of lesions,” Dr. Berlit said. “Leptomeningeal enhancement is an indicator of good treatment response.”
A brain and leptomeningeal biopsy demonstrating the angiitis remains the preferred method for diagnosis of PACNS. “Open biopsies out of recent MRI lesions are especially diagnostic,” he said. “If there are no lesions accessible for surgery in noneloquent brain areas, a biopsy from the right frontal lobe is recommended.” The histologic findings of PACNS consist of granulomatous inflammation, fibrinoid necrosis of vessel walls, or exclusively lymphocytic cellular infiltrates. “The treatment of choice in PACNS is the combination of steroids and cyclophosphamide pulse therapy,” he said. “There are also data showing that rituximab or methotrexate might be treatment options. With a relapse rate of 25% and a reduced survival rate, a close follow-up of suspected PACNS is mandatory.”
Reversible cerebral vasoconstriction syndrome (RCVS)
Another rare cause of stroke is RCVS, which typically presents as thunderclap headaches with or without neurologic symptoms. MRI may be normal, but symmetric border zone infarctions and small subarachnoid hemorrhages are possible. Catheter, CT, or MR angiography show segmental arterial vasoconstriction. “You always have to exclude cerebral aneurysm,” Dr. Berlit said. “There is reversibility of RCVS within 3 months.” RCVS is often associated with a long list of drugs, including phenylpropanolamine, Methergine (methylergonovine), bromocriptine, lisuride, SSRIs, triptans, isometheptene, tacrolimus, cyclophosphamide, erythropoietin, intravenous immunoglobulins, erythrocyte concentrates, nasal sprays, cocaine, ecstasy, amphetamines, cannabis, and LSD. “After stopping responsible medications, treatment involves a course of nimodipine,” he said.
Moyamoya disease (MMD)
Dr. Berlit closed his presentation by discussing MMD, a rare occlusive cerebrovascular disorder characterized by progressive stenosis or occlusion of the intracranial portion of the internal carotid artery and proximal cerebral arteries with an extensive network of fine collaterals. “This is an idiopathic vasculopathy with remarkable regional and racial differences worldwide; it’s most frequently found in Asians, especially in Japan and Korea,” he said. “In Europe, there is about one-tenth the incidence, compared with that of Japan. In Asian MMD, about 15% of cases follow an autosomal dominant inheritance. The collaterals in MMD present histologically as a thin media, a fragmented elastic laminae, and the formation of microaneurysms. There is no inflammation.”
MMD diagnostic criteria include stenosis or occlusion of the terminal portion of the internal carotid artery and at the proximal portion of the anterior and middle cerebral arteries. Abnormal vascular networks are present in the basal ganglia and angiographic findings present bilaterally. Cases with unilateral angiographic findings are considered probable. Clinicians should exclude the following conditions: arteriosclerosis, autoimmune disease, brain neoplasm, history of cranial irradiation, Down syndrome, head trauma, neurofibromatosis, and meningitis. “If the angiographic pattern is resembled by one of these conditions, this is called moyamoya syndrome,” Dr. Berlit noted. “MMD is a progressive disorder. Within a few months you can see occlusion of the middle cerebral artery and the anterior cerebral artery, so you have to treat these patients.”
In patients who are white, MMD presents with lower rates of hemorrhage, but in Asians, microbleeds occur in up to 44% of patients and hemorrhages in up to 65% patients. “Both subarachnoidal and intracerebral hemorrhages occur, especially in connection with pregnancy and delivery,” he said. “The risk of both cerebral ischemia and hemorrhagic complications increases with stages of MMD.”
Direct or indirect intracranial bypass surgery is recommended in stages 3 or more, and has been shown to significantly reduce the 5-year stroke risk. To date, Dr. Berlit and his associates have treated 86 hemispheres in 56 patients. The average age of the patients was 42 years, 70% were female, and the average follow-up was 39 months. All intracranial bypasses were open on follow-up, and a decrease of the typical moyamoya vessels was observed in 81% of patients.
Dr. Berlit reported having no financial disclosures.
EXPERT ANALYSIS FROM ANA 2018
Health Care Barriers and Quality of Life in Central Centrifugal Cicatricial Alopecia Patients
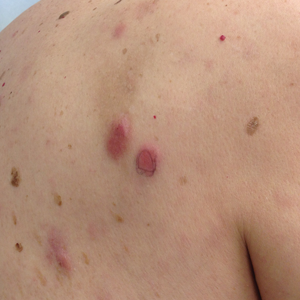
The etiology of central centrifugal cicatricial alopecia (CCCA), a clinical and histological pattern of hair loss on the central scalp, has been well studied. This disease is chronic and progressive, with extensive follicular destruction and eventual burnout.1,2 Central centrifugal cicatricial alopecia is most commonly seen in patients of African descent and has been shown to be 1 of the 5 most common dermatologic diagnoses in black patients.3,4 The top 5 dermatologic diagnoses within this population include acne vulgaris (28.4%), dyschromia (19.9%), eczema (9.1%), alopecia (8.3%), and seborrheic dermatitis (6.7%).4 The incidence rate of CCCA is estimated to be 5.6%.3,5 Most patients are women, with onset between the second and fourth decades of life.6
Central centrifugal cicatricial alopecia treatment efficacy is inversely correlated with disease duration. The primary goal of treatment is to prevent progression. Efforts are made to stimulate regrowth in areas that are not permanently scarred. When patients present with a substantial amount of scarring hair loss, dermatologists often are limited in their ability to achieve a cosmetically acceptable pattern of growth. Generally, hair is connected to a sense of self-worth in black women, and any type of hair loss has been shown to lead to frustration and decreased self-esteem.7 A 1994 study showed that 75% (44/58) of women with androgenetic alopecia had decreased self-esteem and 50% (29/58) had social challenges.8
The purpose of this pilot study was to determine the personal, historical, logistical, or environmental factors that preclude women from obtaining medical care for CCCA and to investigate how CCCA affects quality of life (QOL) and psychological well-being.
Methods
The investigators designed a survey study of adult, English-speaking, black women diagnosed with CCCA at the Northwestern University Department of Dermatology (Chicago, Illinois) between 2011 and 2017. Patients were selected from the electronic data warehouse compiled by the Department of Dermatology and were included if they fulfilled the following criteria: evaluated in the dermatology department between September 1, 2011, and September 30, 2017, by any faculty physician; diagnosed with CCCA; and aged 18 years or older. Patients were excluded if they did not speak English, as interpreters were not available. All patients who fulfilled the inclusion criteria provided signed informed consent prior to participation. All surveys were disseminated in the office or via telephone from fall 2016 to spring 2017 and took 10 to 15 minutes to complete. The research was approved by the authors’ institutional review board (IRB ID STU00203449).
Survey Instrument
The
Data Analysis
Analyses were completed using data analysis software JMP Pro 13 from SAS and a Microsoft Excel spreadsheet. Continuous data were presented as mean, SD, median, minimum, and maximum. Categorical data were presented as counts and percentages. Nine QOL items were aggregated into a self-esteem category (questions 30–38).
Cronbach α, a statistical measure of internal consistency and how closely related items are in a group, was used to evaluate internal consistency reliability; values of 0.70 or greater indicate acceptable reliability.
Results
Of 501 individuals contacted, 34 completed the survey (7% completion rate). Nonrespondents included 7 who refused to participate and 460 who could not be contacted. All respondents self-identified as black women. Median age at time of survey administration was 46 years (range, 28–79 years); median age at CCCA diagnosis was 42 years (range, 15–73 years). Respondents did not significantly differ in age from nonrespondents (P=.46). The majority of respondents had an associate’s degree, bachelor’s degree, or advanced degree of education (master of arts, doctor of medicine, doctor of jurisprudence, doctor of philosophy); however, 8 women reported completing some college, 1 reported completing high school, and 1 reported no schooling. Three respondents had no health insurance.
Initial Hair Loss Discovery
The majority of respondents (22/34 [65%]) were first to notice their hair loss, while 5 (15%) reported hairstylists as the initial observers. Twelve respondents (35%) initially went to a physician to learn why they were losing hair; 6 (18%) instead utilized hairstylists or the Internet. Fifteen women (44%) waited more than 1 month up to 6 months after noticing hair loss before seeing a physician instead of going immediately within a 4-week period, and 16 (47%) waited 1 year or more.
Nondermatologist Consultation
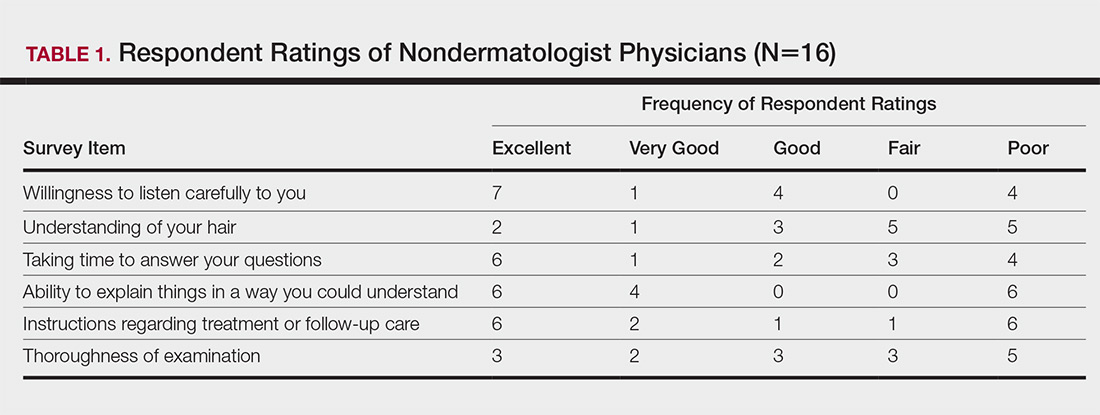
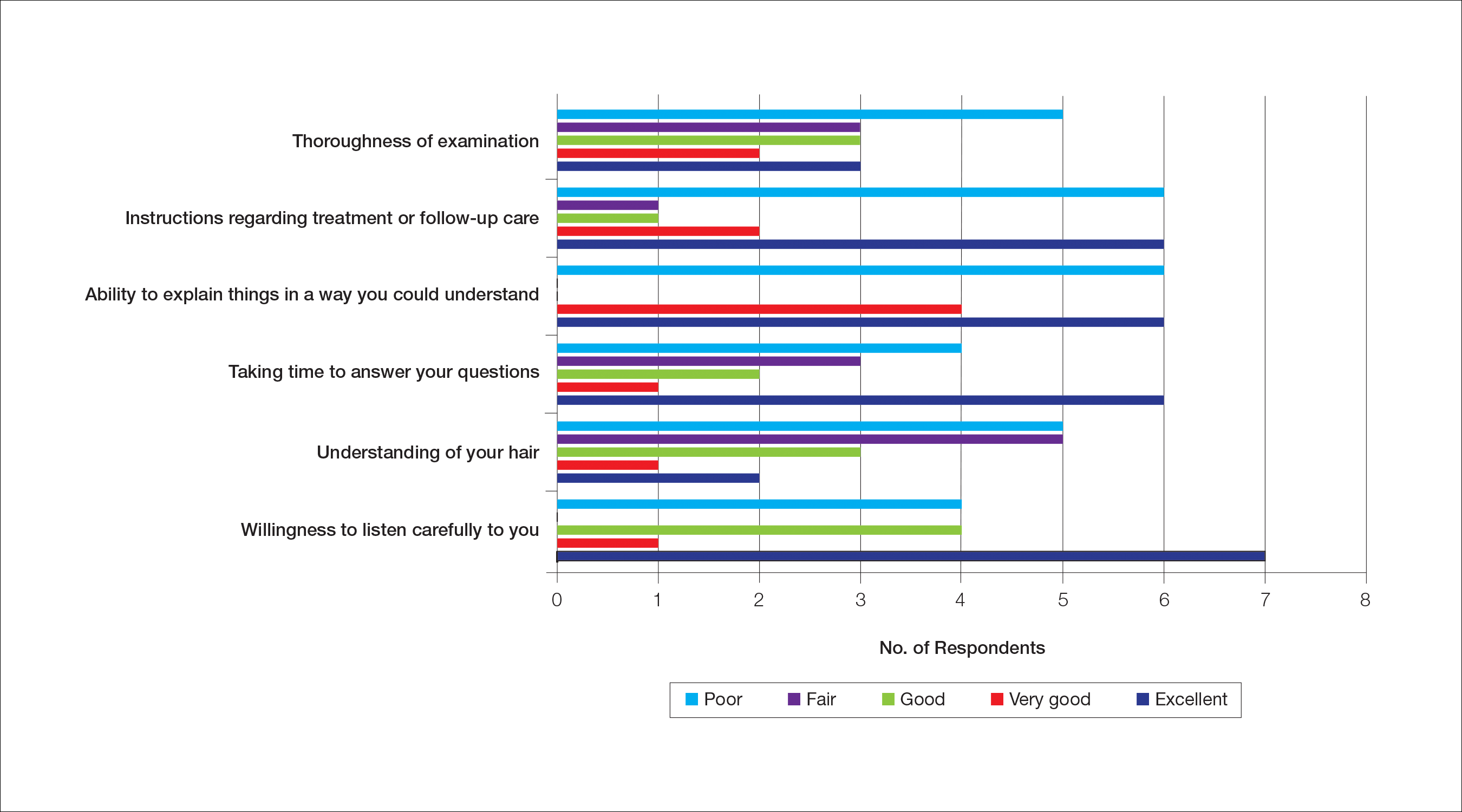
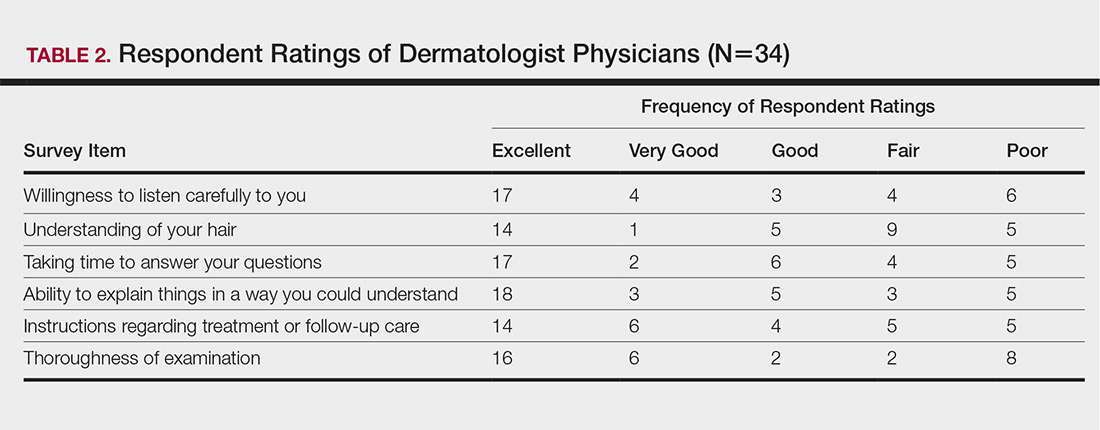
Almost half (16/34 [47%]) of the women went to a nondermatologist physician regarding their hair loss; of them, half (8/16 [50%]) reported their physician did not examine the scalp, 3 (19%) reported their physician offered a biopsy, and none of them reported that their physician diagnosed them with CCCA. The median patient rating of their nondermatologist physician interactions was good (3 on a 5-point scale). Table 1 and Figure 1 show responses to individual items.
Dermatologist Consultation
All 34 respondents presented to a dermatologist. The majority of respondents (22/34 [65%]) saw either 1 or 2 dermatologists for their hair loss. Three (9%) reported their dermatologist did not examine their scalp. Twelve respondents (35%) reported their dermatologist did not offer a biopsy. Twenty-one respondents (62%) reported a CCCA diagnosis from the first dermatologist they saw. Twenty-three respondents (68%) were diagnosed by dermatologists with expertise in hair disorders. Sixteen (47%) were diagnosed by dermatologists within a skin-of-color center. Fourteen (41%) initial dermatology consultations were race concordant.
The median patient rating of their dermatologist interactions was excellent (5 on a 5-point scale). Table 2 and Figure 2 show responses to individual items. Respondents saw an average of 3 different providers, both dermatologists and otherwise.
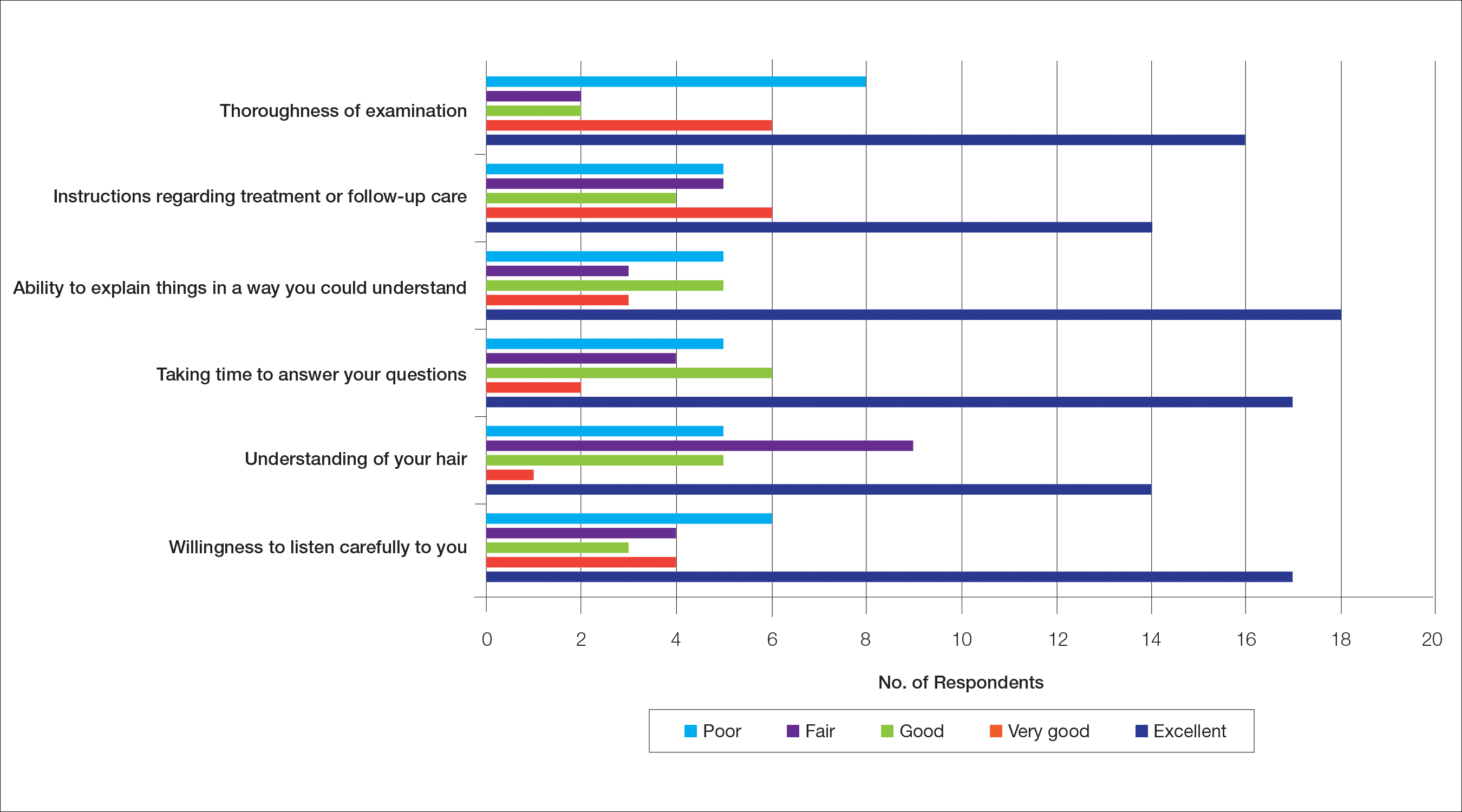
Waiting to See a Dermatologist
Nearly all respondents (31/34 [91%]) recommended that other women with hair loss immediately go see a dermatologist.
Barriers to Care
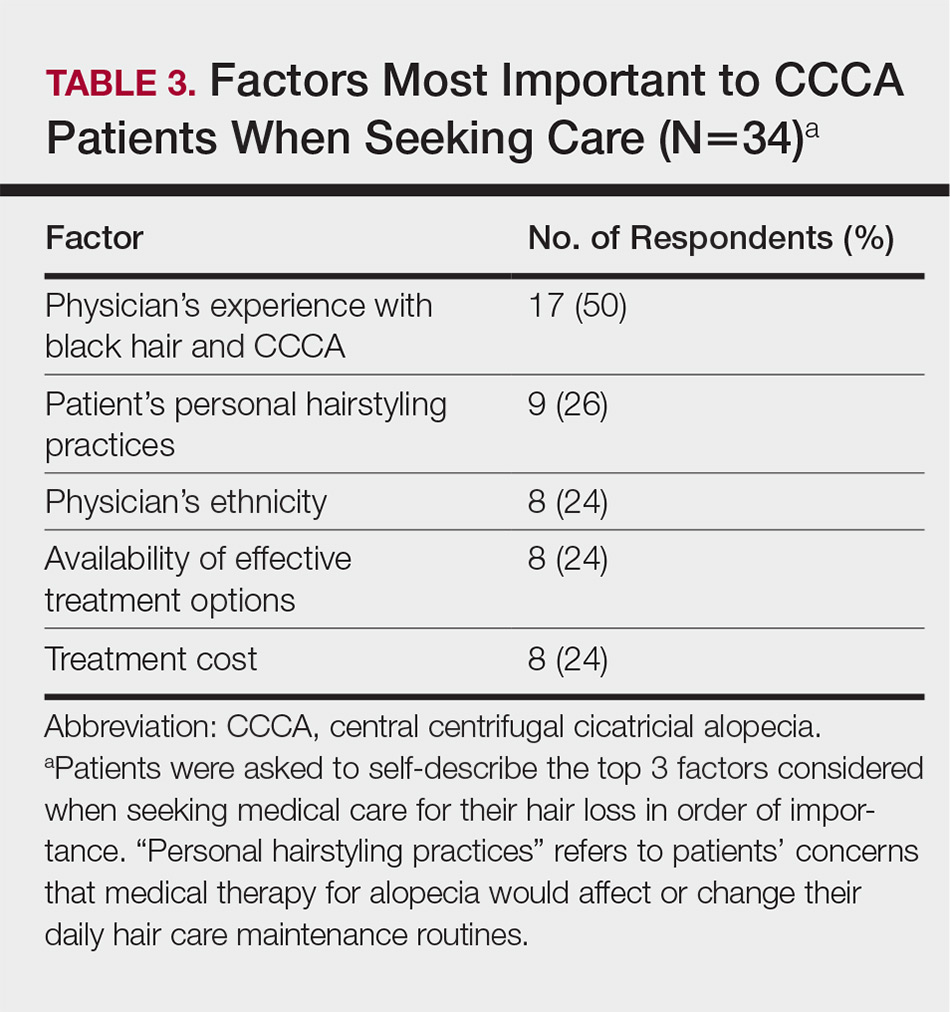
The top 5 factors reported as most important when initially seeking care included the physician’s experience with black hair and CCCA, the patient’s personal hairstyling practices, the physician’s ethnicity, availability of effective treatment options, and treatment cost. Table 3 shows frequency counts for these freely reported factors.
Quality of Life
The median score on 9 aggregated self-esteem items was 4 on a 5-point scale, representing an agree response to statements such as “I feel embarrassed, self-conscious, or frustrated about my hair loss” (28/34 [82%]) and “My hair loss bothers me” (28/34 [82%])(Table 4). Cronbach α for self-esteem survey items was 0.7826.

For the nonaggregated items, many respondents strongly disagreed with statements pertaining to activities of daily living, including “I take care of where I sit or stand at social gatherings due to my hair loss” (18/34 [53%]), “My hair loss makes it difficult for me to go to the grocery store” (29/34 [85%]), “My hair loss makes it difficult for me to attend faith-based activities” (30/34 [88%]), “My hair loss makes it difficult for me to exercise” (23/34 [68%]), “My hair loss makes it difficult for me to go to work and/or school” (24/34 [71%]), “My hair loss makes it difficult for me to go out with a significant other” (24/34 [71%]), “My hair loss makes it difficult for me to spend time with family” (27/34 [79%]), and “My hair loss makes it difficult for me to go to a hairstylist” (16/34 [47%]).
Comment
The majority of respondents were first to discover their hair loss. Harbingers of CCCA hair loss include paresthesia, tenderness, and itch,6 symptoms that are hard to ignore. Unfortunately, many patients notice hair thinning years after the scarring process has begun and a notable amount of hair has already been lost.6,9
Fifteen percent of respondents learned about their hair loss from their hairstylist. Women of African descent often maintain hairstyles that require frequent interactions with a hair care professional.7,10 As a result, hairstylists are at the forefront of early alopecia detection and are a valued resource in the black community. Open dialogue between dermatologists and hair care professionals could funnel women with hair loss into treatment before extensive damage.
Fifteen women (44%) recalled a waiting period of several months before seeking medical assistance, and 16 (47%) reported waiting 1 year or more. However, 91% of respondents indicated that women with hair loss should immediately see a physician for evaluation, thus patient experiences underscore the importance of early treatment. In our experience, many patients wait years before presenting to a physician. Some work with their hairstylists first to address the issue, while others do not realize how notable the loss has become. Some have a negative experience with one provider or are told there is nothing that can be done and then wait many years to see a second provider. Proper education of patients, physicians, and hairstylists is important in the identification and prompt treatment of this condition.
It is perhaps to be expected that patients rated interactions with dermatologists as excellent and very good more frequently than interactions with nondermatologists, which may be due to an absence of thorough hair evaluation with nondermatologists. Respondents reported that only half of nondermatologist providers actually examined their scalp during an initial encounter. However, both physician groups had the lowest frequencies of excellent and very good ratings on “understanding of your hair” (Tables 1 and 2). Patients with hair loss seek immediate answers, and often it is the specialist that can give them a firm diagnosis as opposed to a primary care provider. The fact that dermatologists and nondermatologists alike scored poorly on patient-perceived understanding of CCCA indicates an area for improvement within patient-physician interactions and physician knowledge.
The top 5 factors important to respondents when obtaining medical care included the physician’s experience with black hair and CCCA, the patient’s personal hairstyling practices, the physician’s ethnicity, availability of effective treatment options, and treatment cost. Patients with CCCA seeing dermatologists may discern a lack of experience with ethnic hair that leads patients to doubt their physicians’ ability to provide adequate care and decreased shared decision-making.11,12 These patient perceptions are not unfounded; a 2008 study showed that dermatology residents are not uniformly trained in diseases pertaining to patients with skin of color.13 Thus, incorporation of education on skin of color in dermatology training programs is critical.
Finally, hair loss patients often have concerns regarding how medical therapeutics could adversely affect personal hair care regimens, including washing and hairstyling practices. Current research demonstrates that patients consider treatment effectiveness and ability to be integrated into daily routines after establishing medical care.14 The present study shows that some CCCA patients contemplate how well a therapy will work before seeking medical care, demonstrating that patients continue to have these concerns after establishing medical care. Consideration of treatment effectiveness is important for both patients and providers, as there is minimal evidence behind current CCCA management practices. The ability for treatments to be easily integrated into daily hair care habits is important to maintain patient compliance.
Participants’ median self-esteem scores indicate the effect of CCCA on morale and self-perception. Items scrutinizing this construct had acceptable internal consistency reliability. It is interesting to note that activities of daily living were not impacted by hair loss. Examination of self-esteem is important in the alopecia population because the effect of hair loss on mental status is well documented.15-17 Low self-esteem has been reported as a prospective risk factor for clinical depression.18-20 In black patients, clinical depression rates surpass those of Hispanics and non-Hispanic white individuals.21 Dermatologists must consider the psychological status of all patients, particularly populations at risk for severe disease.
Limitations of this study include the small (34 participants) and mostly highly educated sample size, limited survey validity, and potential patient bias. Because many patients changed their address and/or telephone number in the time between CCCA diagnosis and the present study, we were left with a small pilot study, which minimizes the impact of our findings. Furthermore, our survey was created by a single expert’s opinion and modeling from preexisting alopecia questionnaires16; full validity procedures analyzing face, content, and criterion validity were not undertaken. Finally, the majority of respondents were patients of one of the study’s authors (S.S.L.P.), which could influence survey responses. The fact that some providers were hair experts and some were race concordant with their patients also could potentially affect the responses received, which was not analyzed in the present study. Future studies with more respondents from multiple providers would help clarify our preliminary findings.
Conclusion
Analysis of barriers to care and QOL in patients with skin of color is an essential addition to dermatologic discourse. Alopecia is particularly important to investigate, as prior research has found it to be one of the top 5 diagnoses made in patients with skin of color.3,4 Alopecia has been shown to negatively affect QOL.15,22,23 This study, although limited by small sample size, suggests CCCA also is a contributor to self-esteem challenges, similar to other forms of hair loss. Patient-physician interactions and personal hairstyling practices are prominent barriers to care for CCCA patients, demonstrating the need for quality education on skin of color and cultural competency in dermatology residencies across the country.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181.
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668.
- Gathers RC, Mahan MG. African american women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Van Der Donk J, Hunfeld JA, Passchier J, et al. Quality of life and maladjustment associated with hair loss in women with alopecia androgenetica. Social Sci Med. 1994;38:159-163.
- Sperling LC, Sau P. The follicular degeneration syndrome in black patients. ‘hot comb alopecia’ revisited and revised. Arch Dermatol. 1992;128:68-74.
- Gathers RC, Jankowski M, Eide M, et al. Hair grooming practices and central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2009;60:574-578.
- Harvey VM, Ozoemena U, Paul J, et al. Patient-provider communication, concordance, and ratings of care in dermatology: results of a cross-sectional study. Dermatol Online J. 2016;22. pii: 13030/qt06j6p7gh.
- Laveist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav. 2002;43:296-306.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Suchonwanit P, Hector CE, Bin Saif GA, et al. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:E338-E343.
- Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15:137-139.
- Fabbrocini G, Panariello L, De Vita V, et al. Quality of life in alopecia areata: a disease-specific questionnaire. J Eur Acad Dermatol Venereol. 2013;27:E276-E281.
- Ramos PM, Miot HA. Female pattern hair loss: a clinical and pathophysiological review. An Bras Dermatol. 2015;90:529-543.
- Sowislo JF, Orth U. Does low self-esteem predict depression and anxiety? a meta-analysis of longitudinal studies. Psychol Bull. 2013;139:213-240.
- Steiger AE, Allemand M, Robins RW, et al. Low and decreasing self-esteem during adolescence predict adult depression two decades later. J Pers Soc Psychol. 2014;106:325-338.
- Wegener I, Geiser F, Alfter S, et al. Changes of explicitly and implicitly measured self-esteem in the treatment of major depression: evidence for implicit self-esteem compensation. Compr Psychiatry. 2015;58:57-67.
- Pratt LAB, Brody DJ. Depression in the U.S. Household Population, 2009-2012. Hyattsville, MD: National Center for Health Statistics; 2014. NCHS Data Brief, No. 172. https://www.cdc.gov/nchs/data/databriefs/db172.pdf. Published December 2014. Accessed November 19, 2018.
- Schmidt S, Fischer TW, Chren MM, et al. Strategies of coping and quality of life in women with alopecia. Br J Dermatol. 2001;144:1038-1043.
- Hunt N, McHale S. The psychological impact of alopecia. Br Med J. 2005;331:951-953.
The etiology of central centrifugal cicatricial alopecia (CCCA), a clinical and histological pattern of hair loss on the central scalp, has been well studied. This disease is chronic and progressive, with extensive follicular destruction and eventual burnout.1,2 Central centrifugal cicatricial alopecia is most commonly seen in patients of African descent and has been shown to be 1 of the 5 most common dermatologic diagnoses in black patients.3,4 The top 5 dermatologic diagnoses within this population include acne vulgaris (28.4%), dyschromia (19.9%), eczema (9.1%), alopecia (8.3%), and seborrheic dermatitis (6.7%).4 The incidence rate of CCCA is estimated to be 5.6%.3,5 Most patients are women, with onset between the second and fourth decades of life.6
Central centrifugal cicatricial alopecia treatment efficacy is inversely correlated with disease duration. The primary goal of treatment is to prevent progression. Efforts are made to stimulate regrowth in areas that are not permanently scarred. When patients present with a substantial amount of scarring hair loss, dermatologists often are limited in their ability to achieve a cosmetically acceptable pattern of growth. Generally, hair is connected to a sense of self-worth in black women, and any type of hair loss has been shown to lead to frustration and decreased self-esteem.7 A 1994 study showed that 75% (44/58) of women with androgenetic alopecia had decreased self-esteem and 50% (29/58) had social challenges.8
The purpose of this pilot study was to determine the personal, historical, logistical, or environmental factors that preclude women from obtaining medical care for CCCA and to investigate how CCCA affects quality of life (QOL) and psychological well-being.
Methods
The investigators designed a survey study of adult, English-speaking, black women diagnosed with CCCA at the Northwestern University Department of Dermatology (Chicago, Illinois) between 2011 and 2017. Patients were selected from the electronic data warehouse compiled by the Department of Dermatology and were included if they fulfilled the following criteria: evaluated in the dermatology department between September 1, 2011, and September 30, 2017, by any faculty physician; diagnosed with CCCA; and aged 18 years or older. Patients were excluded if they did not speak English, as interpreters were not available. All patients who fulfilled the inclusion criteria provided signed informed consent prior to participation. All surveys were disseminated in the office or via telephone from fall 2016 to spring 2017 and took 10 to 15 minutes to complete. The research was approved by the authors’ institutional review board (IRB ID STU00203449).
Survey Instrument
The
Data Analysis
Analyses were completed using data analysis software JMP Pro 13 from SAS and a Microsoft Excel spreadsheet. Continuous data were presented as mean, SD, median, minimum, and maximum. Categorical data were presented as counts and percentages. Nine QOL items were aggregated into a self-esteem category (questions 30–38).
Cronbach α, a statistical measure of internal consistency and how closely related items are in a group, was used to evaluate internal consistency reliability; values of 0.70 or greater indicate acceptable reliability.
Results
Of 501 individuals contacted, 34 completed the survey (7% completion rate). Nonrespondents included 7 who refused to participate and 460 who could not be contacted. All respondents self-identified as black women. Median age at time of survey administration was 46 years (range, 28–79 years); median age at CCCA diagnosis was 42 years (range, 15–73 years). Respondents did not significantly differ in age from nonrespondents (P=.46). The majority of respondents had an associate’s degree, bachelor’s degree, or advanced degree of education (master of arts, doctor of medicine, doctor of jurisprudence, doctor of philosophy); however, 8 women reported completing some college, 1 reported completing high school, and 1 reported no schooling. Three respondents had no health insurance.
Initial Hair Loss Discovery
The majority of respondents (22/34 [65%]) were first to notice their hair loss, while 5 (15%) reported hairstylists as the initial observers. Twelve respondents (35%) initially went to a physician to learn why they were losing hair; 6 (18%) instead utilized hairstylists or the Internet. Fifteen women (44%) waited more than 1 month up to 6 months after noticing hair loss before seeing a physician instead of going immediately within a 4-week period, and 16 (47%) waited 1 year or more.
Nondermatologist Consultation
Almost half (16/34 [47%]) of the women went to a nondermatologist physician regarding their hair loss; of them, half (8/16 [50%]) reported their physician did not examine the scalp, 3 (19%) reported their physician offered a biopsy, and none of them reported that their physician diagnosed them with CCCA. The median patient rating of their nondermatologist physician interactions was good (3 on a 5-point scale). Table 1 and Figure 1 show responses to individual items.


Dermatologist Consultation
All 34 respondents presented to a dermatologist. The majority of respondents (22/34 [65%]) saw either 1 or 2 dermatologists for their hair loss. Three (9%) reported their dermatologist did not examine their scalp. Twelve respondents (35%) reported their dermatologist did not offer a biopsy. Twenty-one respondents (62%) reported a CCCA diagnosis from the first dermatologist they saw. Twenty-three respondents (68%) were diagnosed by dermatologists with expertise in hair disorders. Sixteen (47%) were diagnosed by dermatologists within a skin-of-color center. Fourteen (41%) initial dermatology consultations were race concordant.
The median patient rating of their dermatologist interactions was excellent (5 on a 5-point scale). Table 2 and Figure 2 show responses to individual items. Respondents saw an average of 3 different providers, both dermatologists and otherwise.


Waiting to See a Dermatologist
Nearly all respondents (31/34 [91%]) recommended that other women with hair loss immediately go see a dermatologist.
Barriers to Care
The top 5 factors reported as most important when initially seeking care included the physician’s experience with black hair and CCCA, the patient’s personal hairstyling practices, the physician’s ethnicity, availability of effective treatment options, and treatment cost. Table 3 shows frequency counts for these freely reported factors.

Quality of Life
The median score on 9 aggregated self-esteem items was 4 on a 5-point scale, representing an agree response to statements such as “I feel embarrassed, self-conscious, or frustrated about my hair loss” (28/34 [82%]) and “My hair loss bothers me” (28/34 [82%])(Table 4). Cronbach α for self-esteem survey items was 0.7826.

For the nonaggregated items, many respondents strongly disagreed with statements pertaining to activities of daily living, including “I take care of where I sit or stand at social gatherings due to my hair loss” (18/34 [53%]), “My hair loss makes it difficult for me to go to the grocery store” (29/34 [85%]), “My hair loss makes it difficult for me to attend faith-based activities” (30/34 [88%]), “My hair loss makes it difficult for me to exercise” (23/34 [68%]), “My hair loss makes it difficult for me to go to work and/or school” (24/34 [71%]), “My hair loss makes it difficult for me to go out with a significant other” (24/34 [71%]), “My hair loss makes it difficult for me to spend time with family” (27/34 [79%]), and “My hair loss makes it difficult for me to go to a hairstylist” (16/34 [47%]).
Comment
The majority of respondents were first to discover their hair loss. Harbingers of CCCA hair loss include paresthesia, tenderness, and itch,6 symptoms that are hard to ignore. Unfortunately, many patients notice hair thinning years after the scarring process has begun and a notable amount of hair has already been lost.6,9
Fifteen percent of respondents learned about their hair loss from their hairstylist. Women of African descent often maintain hairstyles that require frequent interactions with a hair care professional.7,10 As a result, hairstylists are at the forefront of early alopecia detection and are a valued resource in the black community. Open dialogue between dermatologists and hair care professionals could funnel women with hair loss into treatment before extensive damage.
Fifteen women (44%) recalled a waiting period of several months before seeking medical assistance, and 16 (47%) reported waiting 1 year or more. However, 91% of respondents indicated that women with hair loss should immediately see a physician for evaluation, thus patient experiences underscore the importance of early treatment. In our experience, many patients wait years before presenting to a physician. Some work with their hairstylists first to address the issue, while others do not realize how notable the loss has become. Some have a negative experience with one provider or are told there is nothing that can be done and then wait many years to see a second provider. Proper education of patients, physicians, and hairstylists is important in the identification and prompt treatment of this condition.
It is perhaps to be expected that patients rated interactions with dermatologists as excellent and very good more frequently than interactions with nondermatologists, which may be due to an absence of thorough hair evaluation with nondermatologists. Respondents reported that only half of nondermatologist providers actually examined their scalp during an initial encounter. However, both physician groups had the lowest frequencies of excellent and very good ratings on “understanding of your hair” (Tables 1 and 2). Patients with hair loss seek immediate answers, and often it is the specialist that can give them a firm diagnosis as opposed to a primary care provider. The fact that dermatologists and nondermatologists alike scored poorly on patient-perceived understanding of CCCA indicates an area for improvement within patient-physician interactions and physician knowledge.
The top 5 factors important to respondents when obtaining medical care included the physician’s experience with black hair and CCCA, the patient’s personal hairstyling practices, the physician’s ethnicity, availability of effective treatment options, and treatment cost. Patients with CCCA seeing dermatologists may discern a lack of experience with ethnic hair that leads patients to doubt their physicians’ ability to provide adequate care and decreased shared decision-making.11,12 These patient perceptions are not unfounded; a 2008 study showed that dermatology residents are not uniformly trained in diseases pertaining to patients with skin of color.13 Thus, incorporation of education on skin of color in dermatology training programs is critical.
Finally, hair loss patients often have concerns regarding how medical therapeutics could adversely affect personal hair care regimens, including washing and hairstyling practices. Current research demonstrates that patients consider treatment effectiveness and ability to be integrated into daily routines after establishing medical care.14 The present study shows that some CCCA patients contemplate how well a therapy will work before seeking medical care, demonstrating that patients continue to have these concerns after establishing medical care. Consideration of treatment effectiveness is important for both patients and providers, as there is minimal evidence behind current CCCA management practices. The ability for treatments to be easily integrated into daily hair care habits is important to maintain patient compliance.
Participants’ median self-esteem scores indicate the effect of CCCA on morale and self-perception. Items scrutinizing this construct had acceptable internal consistency reliability. It is interesting to note that activities of daily living were not impacted by hair loss. Examination of self-esteem is important in the alopecia population because the effect of hair loss on mental status is well documented.15-17 Low self-esteem has been reported as a prospective risk factor for clinical depression.18-20 In black patients, clinical depression rates surpass those of Hispanics and non-Hispanic white individuals.21 Dermatologists must consider the psychological status of all patients, particularly populations at risk for severe disease.
Limitations of this study include the small (34 participants) and mostly highly educated sample size, limited survey validity, and potential patient bias. Because many patients changed their address and/or telephone number in the time between CCCA diagnosis and the present study, we were left with a small pilot study, which minimizes the impact of our findings. Furthermore, our survey was created by a single expert’s opinion and modeling from preexisting alopecia questionnaires16; full validity procedures analyzing face, content, and criterion validity were not undertaken. Finally, the majority of respondents were patients of one of the study’s authors (S.S.L.P.), which could influence survey responses. The fact that some providers were hair experts and some were race concordant with their patients also could potentially affect the responses received, which was not analyzed in the present study. Future studies with more respondents from multiple providers would help clarify our preliminary findings.
Conclusion
Analysis of barriers to care and QOL in patients with skin of color is an essential addition to dermatologic discourse. Alopecia is particularly important to investigate, as prior research has found it to be one of the top 5 diagnoses made in patients with skin of color.3,4 Alopecia has been shown to negatively affect QOL.15,22,23 This study, although limited by small sample size, suggests CCCA also is a contributor to self-esteem challenges, similar to other forms of hair loss. Patient-physician interactions and personal hairstyling practices are prominent barriers to care for CCCA patients, demonstrating the need for quality education on skin of color and cultural competency in dermatology residencies across the country.
The etiology of central centrifugal cicatricial alopecia (CCCA), a clinical and histological pattern of hair loss on the central scalp, has been well studied. This disease is chronic and progressive, with extensive follicular destruction and eventual burnout.1,2 Central centrifugal cicatricial alopecia is most commonly seen in patients of African descent and has been shown to be 1 of the 5 most common dermatologic diagnoses in black patients.3,4 The top 5 dermatologic diagnoses within this population include acne vulgaris (28.4%), dyschromia (19.9%), eczema (9.1%), alopecia (8.3%), and seborrheic dermatitis (6.7%).4 The incidence rate of CCCA is estimated to be 5.6%.3,5 Most patients are women, with onset between the second and fourth decades of life.6
Central centrifugal cicatricial alopecia treatment efficacy is inversely correlated with disease duration. The primary goal of treatment is to prevent progression. Efforts are made to stimulate regrowth in areas that are not permanently scarred. When patients present with a substantial amount of scarring hair loss, dermatologists often are limited in their ability to achieve a cosmetically acceptable pattern of growth. Generally, hair is connected to a sense of self-worth in black women, and any type of hair loss has been shown to lead to frustration and decreased self-esteem.7 A 1994 study showed that 75% (44/58) of women with androgenetic alopecia had decreased self-esteem and 50% (29/58) had social challenges.8
The purpose of this pilot study was to determine the personal, historical, logistical, or environmental factors that preclude women from obtaining medical care for CCCA and to investigate how CCCA affects quality of life (QOL) and psychological well-being.
Methods
The investigators designed a survey study of adult, English-speaking, black women diagnosed with CCCA at the Northwestern University Department of Dermatology (Chicago, Illinois) between 2011 and 2017. Patients were selected from the electronic data warehouse compiled by the Department of Dermatology and were included if they fulfilled the following criteria: evaluated in the dermatology department between September 1, 2011, and September 30, 2017, by any faculty physician; diagnosed with CCCA; and aged 18 years or older. Patients were excluded if they did not speak English, as interpreters were not available. All patients who fulfilled the inclusion criteria provided signed informed consent prior to participation. All surveys were disseminated in the office or via telephone from fall 2016 to spring 2017 and took 10 to 15 minutes to complete. The research was approved by the authors’ institutional review board (IRB ID STU00203449).
Survey Instrument
The
Data Analysis
Analyses were completed using data analysis software JMP Pro 13 from SAS and a Microsoft Excel spreadsheet. Continuous data were presented as mean, SD, median, minimum, and maximum. Categorical data were presented as counts and percentages. Nine QOL items were aggregated into a self-esteem category (questions 30–38).
Cronbach α, a statistical measure of internal consistency and how closely related items are in a group, was used to evaluate internal consistency reliability; values of 0.70 or greater indicate acceptable reliability.
Results
Of 501 individuals contacted, 34 completed the survey (7% completion rate). Nonrespondents included 7 who refused to participate and 460 who could not be contacted. All respondents self-identified as black women. Median age at time of survey administration was 46 years (range, 28–79 years); median age at CCCA diagnosis was 42 years (range, 15–73 years). Respondents did not significantly differ in age from nonrespondents (P=.46). The majority of respondents had an associate’s degree, bachelor’s degree, or advanced degree of education (master of arts, doctor of medicine, doctor of jurisprudence, doctor of philosophy); however, 8 women reported completing some college, 1 reported completing high school, and 1 reported no schooling. Three respondents had no health insurance.
Initial Hair Loss Discovery
The majority of respondents (22/34 [65%]) were first to notice their hair loss, while 5 (15%) reported hairstylists as the initial observers. Twelve respondents (35%) initially went to a physician to learn why they were losing hair; 6 (18%) instead utilized hairstylists or the Internet. Fifteen women (44%) waited more than 1 month up to 6 months after noticing hair loss before seeing a physician instead of going immediately within a 4-week period, and 16 (47%) waited 1 year or more.
Nondermatologist Consultation
Almost half (16/34 [47%]) of the women went to a nondermatologist physician regarding their hair loss; of them, half (8/16 [50%]) reported their physician did not examine the scalp, 3 (19%) reported their physician offered a biopsy, and none of them reported that their physician diagnosed them with CCCA. The median patient rating of their nondermatologist physician interactions was good (3 on a 5-point scale). Table 1 and Figure 1 show responses to individual items.


Dermatologist Consultation
All 34 respondents presented to a dermatologist. The majority of respondents (22/34 [65%]) saw either 1 or 2 dermatologists for their hair loss. Three (9%) reported their dermatologist did not examine their scalp. Twelve respondents (35%) reported their dermatologist did not offer a biopsy. Twenty-one respondents (62%) reported a CCCA diagnosis from the first dermatologist they saw. Twenty-three respondents (68%) were diagnosed by dermatologists with expertise in hair disorders. Sixteen (47%) were diagnosed by dermatologists within a skin-of-color center. Fourteen (41%) initial dermatology consultations were race concordant.
The median patient rating of their dermatologist interactions was excellent (5 on a 5-point scale). Table 2 and Figure 2 show responses to individual items. Respondents saw an average of 3 different providers, both dermatologists and otherwise.


Waiting to See a Dermatologist
Nearly all respondents (31/34 [91%]) recommended that other women with hair loss immediately go see a dermatologist.
Barriers to Care
The top 5 factors reported as most important when initially seeking care included the physician’s experience with black hair and CCCA, the patient’s personal hairstyling practices, the physician’s ethnicity, availability of effective treatment options, and treatment cost. Table 3 shows frequency counts for these freely reported factors.

Quality of Life
The median score on 9 aggregated self-esteem items was 4 on a 5-point scale, representing an agree response to statements such as “I feel embarrassed, self-conscious, or frustrated about my hair loss” (28/34 [82%]) and “My hair loss bothers me” (28/34 [82%])(Table 4). Cronbach α for self-esteem survey items was 0.7826.

For the nonaggregated items, many respondents strongly disagreed with statements pertaining to activities of daily living, including “I take care of where I sit or stand at social gatherings due to my hair loss” (18/34 [53%]), “My hair loss makes it difficult for me to go to the grocery store” (29/34 [85%]), “My hair loss makes it difficult for me to attend faith-based activities” (30/34 [88%]), “My hair loss makes it difficult for me to exercise” (23/34 [68%]), “My hair loss makes it difficult for me to go to work and/or school” (24/34 [71%]), “My hair loss makes it difficult for me to go out with a significant other” (24/34 [71%]), “My hair loss makes it difficult for me to spend time with family” (27/34 [79%]), and “My hair loss makes it difficult for me to go to a hairstylist” (16/34 [47%]).
Comment
The majority of respondents were first to discover their hair loss. Harbingers of CCCA hair loss include paresthesia, tenderness, and itch,6 symptoms that are hard to ignore. Unfortunately, many patients notice hair thinning years after the scarring process has begun and a notable amount of hair has already been lost.6,9
Fifteen percent of respondents learned about their hair loss from their hairstylist. Women of African descent often maintain hairstyles that require frequent interactions with a hair care professional.7,10 As a result, hairstylists are at the forefront of early alopecia detection and are a valued resource in the black community. Open dialogue between dermatologists and hair care professionals could funnel women with hair loss into treatment before extensive damage.
Fifteen women (44%) recalled a waiting period of several months before seeking medical assistance, and 16 (47%) reported waiting 1 year or more. However, 91% of respondents indicated that women with hair loss should immediately see a physician for evaluation, thus patient experiences underscore the importance of early treatment. In our experience, many patients wait years before presenting to a physician. Some work with their hairstylists first to address the issue, while others do not realize how notable the loss has become. Some have a negative experience with one provider or are told there is nothing that can be done and then wait many years to see a second provider. Proper education of patients, physicians, and hairstylists is important in the identification and prompt treatment of this condition.
It is perhaps to be expected that patients rated interactions with dermatologists as excellent and very good more frequently than interactions with nondermatologists, which may be due to an absence of thorough hair evaluation with nondermatologists. Respondents reported that only half of nondermatologist providers actually examined their scalp during an initial encounter. However, both physician groups had the lowest frequencies of excellent and very good ratings on “understanding of your hair” (Tables 1 and 2). Patients with hair loss seek immediate answers, and often it is the specialist that can give them a firm diagnosis as opposed to a primary care provider. The fact that dermatologists and nondermatologists alike scored poorly on patient-perceived understanding of CCCA indicates an area for improvement within patient-physician interactions and physician knowledge.
The top 5 factors important to respondents when obtaining medical care included the physician’s experience with black hair and CCCA, the patient’s personal hairstyling practices, the physician’s ethnicity, availability of effective treatment options, and treatment cost. Patients with CCCA seeing dermatologists may discern a lack of experience with ethnic hair that leads patients to doubt their physicians’ ability to provide adequate care and decreased shared decision-making.11,12 These patient perceptions are not unfounded; a 2008 study showed that dermatology residents are not uniformly trained in diseases pertaining to patients with skin of color.13 Thus, incorporation of education on skin of color in dermatology training programs is critical.
Finally, hair loss patients often have concerns regarding how medical therapeutics could adversely affect personal hair care regimens, including washing and hairstyling practices. Current research demonstrates that patients consider treatment effectiveness and ability to be integrated into daily routines after establishing medical care.14 The present study shows that some CCCA patients contemplate how well a therapy will work before seeking medical care, demonstrating that patients continue to have these concerns after establishing medical care. Consideration of treatment effectiveness is important for both patients and providers, as there is minimal evidence behind current CCCA management practices. The ability for treatments to be easily integrated into daily hair care habits is important to maintain patient compliance.
Participants’ median self-esteem scores indicate the effect of CCCA on morale and self-perception. Items scrutinizing this construct had acceptable internal consistency reliability. It is interesting to note that activities of daily living were not impacted by hair loss. Examination of self-esteem is important in the alopecia population because the effect of hair loss on mental status is well documented.15-17 Low self-esteem has been reported as a prospective risk factor for clinical depression.18-20 In black patients, clinical depression rates surpass those of Hispanics and non-Hispanic white individuals.21 Dermatologists must consider the psychological status of all patients, particularly populations at risk for severe disease.
Limitations of this study include the small (34 participants) and mostly highly educated sample size, limited survey validity, and potential patient bias. Because many patients changed their address and/or telephone number in the time between CCCA diagnosis and the present study, we were left with a small pilot study, which minimizes the impact of our findings. Furthermore, our survey was created by a single expert’s opinion and modeling from preexisting alopecia questionnaires16; full validity procedures analyzing face, content, and criterion validity were not undertaken. Finally, the majority of respondents were patients of one of the study’s authors (S.S.L.P.), which could influence survey responses. The fact that some providers were hair experts and some were race concordant with their patients also could potentially affect the responses received, which was not analyzed in the present study. Future studies with more respondents from multiple providers would help clarify our preliminary findings.
Conclusion
Analysis of barriers to care and QOL in patients with skin of color is an essential addition to dermatologic discourse. Alopecia is particularly important to investigate, as prior research has found it to be one of the top 5 diagnoses made in patients with skin of color.3,4 Alopecia has been shown to negatively affect QOL.15,22,23 This study, although limited by small sample size, suggests CCCA also is a contributor to self-esteem challenges, similar to other forms of hair loss. Patient-physician interactions and personal hairstyling practices are prominent barriers to care for CCCA patients, demonstrating the need for quality education on skin of color and cultural competency in dermatology residencies across the country.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181.
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668.
- Gathers RC, Mahan MG. African american women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Van Der Donk J, Hunfeld JA, Passchier J, et al. Quality of life and maladjustment associated with hair loss in women with alopecia androgenetica. Social Sci Med. 1994;38:159-163.
- Sperling LC, Sau P. The follicular degeneration syndrome in black patients. ‘hot comb alopecia’ revisited and revised. Arch Dermatol. 1992;128:68-74.
- Gathers RC, Jankowski M, Eide M, et al. Hair grooming practices and central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2009;60:574-578.
- Harvey VM, Ozoemena U, Paul J, et al. Patient-provider communication, concordance, and ratings of care in dermatology: results of a cross-sectional study. Dermatol Online J. 2016;22. pii: 13030/qt06j6p7gh.
- Laveist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav. 2002;43:296-306.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Suchonwanit P, Hector CE, Bin Saif GA, et al. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:E338-E343.
- Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15:137-139.
- Fabbrocini G, Panariello L, De Vita V, et al. Quality of life in alopecia areata: a disease-specific questionnaire. J Eur Acad Dermatol Venereol. 2013;27:E276-E281.
- Ramos PM, Miot HA. Female pattern hair loss: a clinical and pathophysiological review. An Bras Dermatol. 2015;90:529-543.
- Sowislo JF, Orth U. Does low self-esteem predict depression and anxiety? a meta-analysis of longitudinal studies. Psychol Bull. 2013;139:213-240.
- Steiger AE, Allemand M, Robins RW, et al. Low and decreasing self-esteem during adolescence predict adult depression two decades later. J Pers Soc Psychol. 2014;106:325-338.
- Wegener I, Geiser F, Alfter S, et al. Changes of explicitly and implicitly measured self-esteem in the treatment of major depression: evidence for implicit self-esteem compensation. Compr Psychiatry. 2015;58:57-67.
- Pratt LAB, Brody DJ. Depression in the U.S. Household Population, 2009-2012. Hyattsville, MD: National Center for Health Statistics; 2014. NCHS Data Brief, No. 172. https://www.cdc.gov/nchs/data/databriefs/db172.pdf. Published December 2014. Accessed November 19, 2018.
- Schmidt S, Fischer TW, Chren MM, et al. Strategies of coping and quality of life in women with alopecia. Br J Dermatol. 2001;144:1038-1043.
- Hunt N, McHale S. The psychological impact of alopecia. Br Med J. 2005;331:951-953.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181.
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668.
- Gathers RC, Mahan MG. African american women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Van Der Donk J, Hunfeld JA, Passchier J, et al. Quality of life and maladjustment associated with hair loss in women with alopecia androgenetica. Social Sci Med. 1994;38:159-163.
- Sperling LC, Sau P. The follicular degeneration syndrome in black patients. ‘hot comb alopecia’ revisited and revised. Arch Dermatol. 1992;128:68-74.
- Gathers RC, Jankowski M, Eide M, et al. Hair grooming practices and central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2009;60:574-578.
- Harvey VM, Ozoemena U, Paul J, et al. Patient-provider communication, concordance, and ratings of care in dermatology: results of a cross-sectional study. Dermatol Online J. 2016;22. pii: 13030/qt06j6p7gh.
- Laveist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav. 2002;43:296-306.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Suchonwanit P, Hector CE, Bin Saif GA, et al. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:E338-E343.
- Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15:137-139.
- Fabbrocini G, Panariello L, De Vita V, et al. Quality of life in alopecia areata: a disease-specific questionnaire. J Eur Acad Dermatol Venereol. 2013;27:E276-E281.
- Ramos PM, Miot HA. Female pattern hair loss: a clinical and pathophysiological review. An Bras Dermatol. 2015;90:529-543.
- Sowislo JF, Orth U. Does low self-esteem predict depression and anxiety? a meta-analysis of longitudinal studies. Psychol Bull. 2013;139:213-240.
- Steiger AE, Allemand M, Robins RW, et al. Low and decreasing self-esteem during adolescence predict adult depression two decades later. J Pers Soc Psychol. 2014;106:325-338.
- Wegener I, Geiser F, Alfter S, et al. Changes of explicitly and implicitly measured self-esteem in the treatment of major depression: evidence for implicit self-esteem compensation. Compr Psychiatry. 2015;58:57-67.
- Pratt LAB, Brody DJ. Depression in the U.S. Household Population, 2009-2012. Hyattsville, MD: National Center for Health Statistics; 2014. NCHS Data Brief, No. 172. https://www.cdc.gov/nchs/data/databriefs/db172.pdf. Published December 2014. Accessed November 19, 2018.
- Schmidt S, Fischer TW, Chren MM, et al. Strategies of coping and quality of life in women with alopecia. Br J Dermatol. 2001;144:1038-1043.
- Hunt N, McHale S. The psychological impact of alopecia. Br Med J. 2005;331:951-953.
Practice Points
- Central centrifugal cicatricial alopecia (CCCA) presents a unique set of challenges for both patients and providers.
- Lack of physician experience with black hair/CCCA and the potential impact of care on personal hairstyling practices are 2 barriers to care for many patients with this disease.
- There is a need for improved patient-provider communication strategies, quality education on hair in skin of color patients, and cultural competency training in dermatology residencies across the country.
Nerve growth factor antibody cuts OA pain with low AEs
CHICAGO – The nerve growth factor antibody tanezumab met its primary efficacy endpoints for improving pain and physical function in patients with knee or hip OA while also showing a low incidence of the drug’s most concerning adverse effect in the first results from a phase 3 study of a drug in this new class.
The placebo-controlled, multicenter study enrolled 696 U.S. OA patients with moderate to severe knee or hip pain and showed that two subcutaneous injections of the humanized antibody tanezumab spaced 8 weeks apart led to statistically significant improvements relative to placebo for pain, physical function, and patient global assessment of OA, Thomas J. Schnitzer, MD, said at the annual meeting of the American College of Rheumatology.
The primary efficacy measurements occurred 8 weeks following the second subcutaneous injection. The study included two active treatment arms, and all the efficacy measures responses showed consistent, “modest” improvements in the patients who received a 2.5 mg injection followed by a 5 mg injection, compared with those who received two 2.5 mg injections.
For the safety analysis the researchers followed patients out to 24 weeks after they received their final injection. The main adverse event of interest was rapidly progressive OA (RPOA), which occurred in five patients who received two 2.5-mg dosages and in one patient who received the 2.5 mg followed by 5 mg regimen; no RPOA occurred among placebo patients. The 1.3% incidence of RPOA among all 494 patients who received tanezumab “aligned with expectations based on the risk mitigation procedures used,” said Dr. Schnitzer, a rheumatologist and professor of medicine, anesthesiology, and physical medicine and rehabilitation at Northwestern University, Chicago. No patient developed primary osteonecrosis.
Two patients who developed RPOA then underwent total joint replacement. The overall rate of total joint replacement was 4 among placebo patients, all involving knees, and 24 among patients treated with tanezumab, including 12 knee replacements and 12 hip replacements. Blinded adjudication determined that 26 of the total 28 joint replacements resulted from “normal” OA progression.
The rates of all adverse events, serious adverse events, and adverse events leading to treatment discontinuation were low and similar in the three treatment arms.
Earlier clinical studies of tanezumab had signaled a problem with RPOA (Arthritis Rheumatol. 2016 Feb;68[2]:382-91), which led the Food and Drug Administration to order a temporary halt to clinical testing of all nerve growth factor antagonists in 2010 that the agency then lifted in 2015 (Clin Exp Rheumatol. 2017 Sept-Oct;35[suppl 107]:85-7). In 2017, the FDA gave clinical development of tanezumab “fast-track” status. The results that Dr. Schnitzer reported represent the first outcomes from several phase 3 studies that the companies developing tanezumab are now running and that collectively include about 7,000 total patients, according to a written statement from the developing companies. The companies released an initial statement about the current results in July 2018.
The study reported by Dr. Schnitzer enrolled patients with OA of the knee or hip at several U.S. sites. For the primary endpoint of mean change from baseline in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score, the results at 16 weeks were –2.6 units in the placebo patients and –3.4 units among patients who received the higher dosage. For the primary outcome of mean change in WOMAC physical function score, the results were –2.6 in the placebo arm and –3.5 in patients on the higher dosage. For the primary outcome of change in patient’s global assessment of their OA, the results were –0.65 in the placebo patients and –0.90 in those on the highest dosage. All three between-group differences were statistically significant.
A key secondary outcome was the percentage of patients having at least a 50% reduction in their WOMAC pain score, which occurred in 38% of the placebo patients and in 57% on the higher tanezumab dosage, a statistically significant difference, Dr. Schnitzer reported. A final efficacy finding was the percentage of patients who had a 50% or greater pain reduction at week 16 who did not have a pain response at week 8, a parameter that could reflect incremental benefit from the larger, second tanezumab dose. This outcome occurred in 19% of placebo patients and in 22% of patients who received two 2.5-mg doses of tanezumab; among those who received a 5-mg dose after the first 2.5-mg dose, the rate was 33%.
The study was sponsored by Eli Lilly and Pfizer, the companies jointly developing tanezumab. Dr. Schnitzer has been a consultant to and has received research support from Eli Lilly and Pfizer. He has also been a consultant to AbbVie, Aptinyx, Genzyme, Regeneron, and Vertex Pharmaceuticals, and has received research support from Grünenthal and Radius Health.
SOURCE: Schnitzer TJ et al. Arthritis Rheumatol. 2018;70(Suppl 10) Abstract L20.
CHICAGO – The nerve growth factor antibody tanezumab met its primary efficacy endpoints for improving pain and physical function in patients with knee or hip OA while also showing a low incidence of the drug’s most concerning adverse effect in the first results from a phase 3 study of a drug in this new class.
The placebo-controlled, multicenter study enrolled 696 U.S. OA patients with moderate to severe knee or hip pain and showed that two subcutaneous injections of the humanized antibody tanezumab spaced 8 weeks apart led to statistically significant improvements relative to placebo for pain, physical function, and patient global assessment of OA, Thomas J. Schnitzer, MD, said at the annual meeting of the American College of Rheumatology.
The primary efficacy measurements occurred 8 weeks following the second subcutaneous injection. The study included two active treatment arms, and all the efficacy measures responses showed consistent, “modest” improvements in the patients who received a 2.5 mg injection followed by a 5 mg injection, compared with those who received two 2.5 mg injections.
For the safety analysis the researchers followed patients out to 24 weeks after they received their final injection. The main adverse event of interest was rapidly progressive OA (RPOA), which occurred in five patients who received two 2.5-mg dosages and in one patient who received the 2.5 mg followed by 5 mg regimen; no RPOA occurred among placebo patients. The 1.3% incidence of RPOA among all 494 patients who received tanezumab “aligned with expectations based on the risk mitigation procedures used,” said Dr. Schnitzer, a rheumatologist and professor of medicine, anesthesiology, and physical medicine and rehabilitation at Northwestern University, Chicago. No patient developed primary osteonecrosis.
Two patients who developed RPOA then underwent total joint replacement. The overall rate of total joint replacement was 4 among placebo patients, all involving knees, and 24 among patients treated with tanezumab, including 12 knee replacements and 12 hip replacements. Blinded adjudication determined that 26 of the total 28 joint replacements resulted from “normal” OA progression.
The rates of all adverse events, serious adverse events, and adverse events leading to treatment discontinuation were low and similar in the three treatment arms.
Earlier clinical studies of tanezumab had signaled a problem with RPOA (Arthritis Rheumatol. 2016 Feb;68[2]:382-91), which led the Food and Drug Administration to order a temporary halt to clinical testing of all nerve growth factor antagonists in 2010 that the agency then lifted in 2015 (Clin Exp Rheumatol. 2017 Sept-Oct;35[suppl 107]:85-7). In 2017, the FDA gave clinical development of tanezumab “fast-track” status. The results that Dr. Schnitzer reported represent the first outcomes from several phase 3 studies that the companies developing tanezumab are now running and that collectively include about 7,000 total patients, according to a written statement from the developing companies. The companies released an initial statement about the current results in July 2018.
The study reported by Dr. Schnitzer enrolled patients with OA of the knee or hip at several U.S. sites. For the primary endpoint of mean change from baseline in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score, the results at 16 weeks were –2.6 units in the placebo patients and –3.4 units among patients who received the higher dosage. For the primary outcome of mean change in WOMAC physical function score, the results were –2.6 in the placebo arm and –3.5 in patients on the higher dosage. For the primary outcome of change in patient’s global assessment of their OA, the results were –0.65 in the placebo patients and –0.90 in those on the highest dosage. All three between-group differences were statistically significant.
A key secondary outcome was the percentage of patients having at least a 50% reduction in their WOMAC pain score, which occurred in 38% of the placebo patients and in 57% on the higher tanezumab dosage, a statistically significant difference, Dr. Schnitzer reported. A final efficacy finding was the percentage of patients who had a 50% or greater pain reduction at week 16 who did not have a pain response at week 8, a parameter that could reflect incremental benefit from the larger, second tanezumab dose. This outcome occurred in 19% of placebo patients and in 22% of patients who received two 2.5-mg doses of tanezumab; among those who received a 5-mg dose after the first 2.5-mg dose, the rate was 33%.
The study was sponsored by Eli Lilly and Pfizer, the companies jointly developing tanezumab. Dr. Schnitzer has been a consultant to and has received research support from Eli Lilly and Pfizer. He has also been a consultant to AbbVie, Aptinyx, Genzyme, Regeneron, and Vertex Pharmaceuticals, and has received research support from Grünenthal and Radius Health.
SOURCE: Schnitzer TJ et al. Arthritis Rheumatol. 2018;70(Suppl 10) Abstract L20.
CHICAGO – The nerve growth factor antibody tanezumab met its primary efficacy endpoints for improving pain and physical function in patients with knee or hip OA while also showing a low incidence of the drug’s most concerning adverse effect in the first results from a phase 3 study of a drug in this new class.
The placebo-controlled, multicenter study enrolled 696 U.S. OA patients with moderate to severe knee or hip pain and showed that two subcutaneous injections of the humanized antibody tanezumab spaced 8 weeks apart led to statistically significant improvements relative to placebo for pain, physical function, and patient global assessment of OA, Thomas J. Schnitzer, MD, said at the annual meeting of the American College of Rheumatology.
The primary efficacy measurements occurred 8 weeks following the second subcutaneous injection. The study included two active treatment arms, and all the efficacy measures responses showed consistent, “modest” improvements in the patients who received a 2.5 mg injection followed by a 5 mg injection, compared with those who received two 2.5 mg injections.
For the safety analysis the researchers followed patients out to 24 weeks after they received their final injection. The main adverse event of interest was rapidly progressive OA (RPOA), which occurred in five patients who received two 2.5-mg dosages and in one patient who received the 2.5 mg followed by 5 mg regimen; no RPOA occurred among placebo patients. The 1.3% incidence of RPOA among all 494 patients who received tanezumab “aligned with expectations based on the risk mitigation procedures used,” said Dr. Schnitzer, a rheumatologist and professor of medicine, anesthesiology, and physical medicine and rehabilitation at Northwestern University, Chicago. No patient developed primary osteonecrosis.
Two patients who developed RPOA then underwent total joint replacement. The overall rate of total joint replacement was 4 among placebo patients, all involving knees, and 24 among patients treated with tanezumab, including 12 knee replacements and 12 hip replacements. Blinded adjudication determined that 26 of the total 28 joint replacements resulted from “normal” OA progression.
The rates of all adverse events, serious adverse events, and adverse events leading to treatment discontinuation were low and similar in the three treatment arms.
Earlier clinical studies of tanezumab had signaled a problem with RPOA (Arthritis Rheumatol. 2016 Feb;68[2]:382-91), which led the Food and Drug Administration to order a temporary halt to clinical testing of all nerve growth factor antagonists in 2010 that the agency then lifted in 2015 (Clin Exp Rheumatol. 2017 Sept-Oct;35[suppl 107]:85-7). In 2017, the FDA gave clinical development of tanezumab “fast-track” status. The results that Dr. Schnitzer reported represent the first outcomes from several phase 3 studies that the companies developing tanezumab are now running and that collectively include about 7,000 total patients, according to a written statement from the developing companies. The companies released an initial statement about the current results in July 2018.
The study reported by Dr. Schnitzer enrolled patients with OA of the knee or hip at several U.S. sites. For the primary endpoint of mean change from baseline in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score, the results at 16 weeks were –2.6 units in the placebo patients and –3.4 units among patients who received the higher dosage. For the primary outcome of mean change in WOMAC physical function score, the results were –2.6 in the placebo arm and –3.5 in patients on the higher dosage. For the primary outcome of change in patient’s global assessment of their OA, the results were –0.65 in the placebo patients and –0.90 in those on the highest dosage. All three between-group differences were statistically significant.
A key secondary outcome was the percentage of patients having at least a 50% reduction in their WOMAC pain score, which occurred in 38% of the placebo patients and in 57% on the higher tanezumab dosage, a statistically significant difference, Dr. Schnitzer reported. A final efficacy finding was the percentage of patients who had a 50% or greater pain reduction at week 16 who did not have a pain response at week 8, a parameter that could reflect incremental benefit from the larger, second tanezumab dose. This outcome occurred in 19% of placebo patients and in 22% of patients who received two 2.5-mg doses of tanezumab; among those who received a 5-mg dose after the first 2.5-mg dose, the rate was 33%.
The study was sponsored by Eli Lilly and Pfizer, the companies jointly developing tanezumab. Dr. Schnitzer has been a consultant to and has received research support from Eli Lilly and Pfizer. He has also been a consultant to AbbVie, Aptinyx, Genzyme, Regeneron, and Vertex Pharmaceuticals, and has received research support from Grünenthal and Radius Health.
SOURCE: Schnitzer TJ et al. Arthritis Rheumatol. 2018;70(Suppl 10) Abstract L20.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The Western Ontario and McMaster Universities Osteoarthritis Index pain score fell by an average –3.4 points with maximum tanezumab treatment and by –2.6 points with placebo.
Study details: A multicenter, randomized study with 696 patients with moderate to severe OA of the knee or hip and moderate to severe pain.
Disclosures: The study was sponsored by Eli Lilly and Pfizer, the companies jointly developing tanezumab. Dr. Schnitzer has been a consultant to and has received research support from Eli Lilly and Pfizer. He has also been a consultant to AbbVie, Aptinyx, Genzyme, Regeneron, and Vertex Pharmaceuticals, and has received research support from Grünenthal and Radius Health.
Source: Schnitzer TJ et al. Arthritis Rheumatol. 2018;70(suppl 10) Abstract L20.
Product News: 12 2018
Altreno Lotion Now Available for Acne Vulgaris
Ortho Dermatologics launches Altreno (tretinoin) Lotion 0.05% for the treatment of acne vulgaris in patients 9 years and older. It was approved by the US Food and Drug Administration in August 2018, providing patients with the efficacy of tretinoin and the tolerability of a lotion formulation containing hyaluronic acid, glycerin, and collagen to help hydrate and moisturize the skin. For more information, visit www.ortho-dermatologics.com.
Bryhali Approved for Plaque Psoriasis in Adults
Ortho Dermatologics announces US Food and Drug Administration approval of Bryhali (halobetasol propionate) Lotion 0.01% for the treatment of plaque psoriasis in adults. Bryhali is a corticosteroid in a novel vehicle lotion with safety established for dosing up to 8 weeks, offering patients a longer duration of use than other topical steroids. For more information, visit www.bryhali.com.
CoolSculpting Cleared for the Submandibular Area
Zeltiq Aesthetics, Inc, an Allergan affiliate, announces US Food and Drug Administration (FDA) clearance of CoolSculpting to treat the submandibular area. The FDA clearance also was expanded to include patients with a body mass index of up to 46.2 when treating the submental and submandibular areas. CoolSculpting is a nonsurgical treatment that works by gently cooling targeted fat cells in the body to induce natural controlled elimination of fat cells without affecting surrounding tissue. CoolSculpting also is cleared for treatment of visible fat bulges on the thighs, abdomen, and flanks. For more information, visit www.coolsculpting.com.
Glytone Age-Defying Vitamin C+E Serum Reduces Signs of Aging
Pierre Fabre Dermo-Cosmetique introduces Glytone Age-Defying Vitamin C+E Serum, a layering serum with time-released, high concentrations of stabilized vitamins C and E combined with red tea flavonoids to deliver antioxidant protection and antiaging benefits. It is indicated for premature aging caused by environmental damage and oxidative stress as well as postprocedure relief. For more information, visit www.glytone-usa.com.
Hyrimoz Biosimilar Approved for Psoriasis
Sandoz, a Novartis Division, announces US Food and Drug Administration (FDA) approval of Hyrimoz (adalimumab-adaz), a biosimilar indicated for the treatment of psoriatic arthritis and plaque psoriasis, as well as rheumatoid arthritis, juvenile idiopathic arthritis in patients 4 years and older, ankylosing spondylitis, adult Crohn disease, and ulcerative colitis. Hyrimoz is the third FDA-approved biosimilar from Sandoz. For more information, visit www.sandoz.com.
Libtayo Approved for SCC
Regeneron Pharmaceuticals, Inc, and sanofi-aventis US LLC, announce US Food and Drug Administration (FDA) approval of Libtayo (cemiplimab-rwlc) for the treatment of patients with metastatic cutaneous squamous cell carcinoma (SCC) or locally advanced cutaneous SCC who are not candidates for curative surgery or radiation. Libtayo is a monoclonal antibody targeting the programmed death receptor 1.
Restylane Lyft Now Approved for Hand Rejuvenation
Nestlé Skin Health announces US Food and Drug Administration approval of Restylane Lyft with Lidocaine, a hyaluronic acid dermal filler, for the correction of age-related volume loss in the back of the hands for patients older than 21 years. Restylane Lyft with Lidocaine also is indicated for correction of moderate to severe facial wrinkles and folds such as the nasolabial folds, cheek augmentation, and age-related midface contour deficiencies. For more information, visit www.RestylaneUSA.com.
Xepi Launches for Impetigo
Cutanea Life Sciences launches Xepi (ozenoxacin) Cream 1%, a quinolone antimicrobial, for the treatment of impetigo in adult and pediatric patients 2 months or older. Xepi is applied twice daily for 5 days and has been shown to be active against most isolates of Staphylococcus aureus (including methicillin-resistant isolates) and Streptococcus pyogenes, both in vitro and in clinical infections. For more information, visit www.XepiCream.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Altreno Lotion Now Available for Acne Vulgaris
Ortho Dermatologics launches Altreno (tretinoin) Lotion 0.05% for the treatment of acne vulgaris in patients 9 years and older. It was approved by the US Food and Drug Administration in August 2018, providing patients with the efficacy of tretinoin and the tolerability of a lotion formulation containing hyaluronic acid, glycerin, and collagen to help hydrate and moisturize the skin. For more information, visit www.ortho-dermatologics.com.
Bryhali Approved for Plaque Psoriasis in Adults
Ortho Dermatologics announces US Food and Drug Administration approval of Bryhali (halobetasol propionate) Lotion 0.01% for the treatment of plaque psoriasis in adults. Bryhali is a corticosteroid in a novel vehicle lotion with safety established for dosing up to 8 weeks, offering patients a longer duration of use than other topical steroids. For more information, visit www.bryhali.com.
CoolSculpting Cleared for the Submandibular Area
Zeltiq Aesthetics, Inc, an Allergan affiliate, announces US Food and Drug Administration (FDA) clearance of CoolSculpting to treat the submandibular area. The FDA clearance also was expanded to include patients with a body mass index of up to 46.2 when treating the submental and submandibular areas. CoolSculpting is a nonsurgical treatment that works by gently cooling targeted fat cells in the body to induce natural controlled elimination of fat cells without affecting surrounding tissue. CoolSculpting also is cleared for treatment of visible fat bulges on the thighs, abdomen, and flanks. For more information, visit www.coolsculpting.com.
Glytone Age-Defying Vitamin C+E Serum Reduces Signs of Aging
Pierre Fabre Dermo-Cosmetique introduces Glytone Age-Defying Vitamin C+E Serum, a layering serum with time-released, high concentrations of stabilized vitamins C and E combined with red tea flavonoids to deliver antioxidant protection and antiaging benefits. It is indicated for premature aging caused by environmental damage and oxidative stress as well as postprocedure relief. For more information, visit www.glytone-usa.com.
Hyrimoz Biosimilar Approved for Psoriasis
Sandoz, a Novartis Division, announces US Food and Drug Administration (FDA) approval of Hyrimoz (adalimumab-adaz), a biosimilar indicated for the treatment of psoriatic arthritis and plaque psoriasis, as well as rheumatoid arthritis, juvenile idiopathic arthritis in patients 4 years and older, ankylosing spondylitis, adult Crohn disease, and ulcerative colitis. Hyrimoz is the third FDA-approved biosimilar from Sandoz. For more information, visit www.sandoz.com.
Libtayo Approved for SCC
Regeneron Pharmaceuticals, Inc, and sanofi-aventis US LLC, announce US Food and Drug Administration (FDA) approval of Libtayo (cemiplimab-rwlc) for the treatment of patients with metastatic cutaneous squamous cell carcinoma (SCC) or locally advanced cutaneous SCC who are not candidates for curative surgery or radiation. Libtayo is a monoclonal antibody targeting the programmed death receptor 1.
Restylane Lyft Now Approved for Hand Rejuvenation
Nestlé Skin Health announces US Food and Drug Administration approval of Restylane Lyft with Lidocaine, a hyaluronic acid dermal filler, for the correction of age-related volume loss in the back of the hands for patients older than 21 years. Restylane Lyft with Lidocaine also is indicated for correction of moderate to severe facial wrinkles and folds such as the nasolabial folds, cheek augmentation, and age-related midface contour deficiencies. For more information, visit www.RestylaneUSA.com.
Xepi Launches for Impetigo
Cutanea Life Sciences launches Xepi (ozenoxacin) Cream 1%, a quinolone antimicrobial, for the treatment of impetigo in adult and pediatric patients 2 months or older. Xepi is applied twice daily for 5 days and has been shown to be active against most isolates of Staphylococcus aureus (including methicillin-resistant isolates) and Streptococcus pyogenes, both in vitro and in clinical infections. For more information, visit www.XepiCream.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Altreno Lotion Now Available for Acne Vulgaris
Ortho Dermatologics launches Altreno (tretinoin) Lotion 0.05% for the treatment of acne vulgaris in patients 9 years and older. It was approved by the US Food and Drug Administration in August 2018, providing patients with the efficacy of tretinoin and the tolerability of a lotion formulation containing hyaluronic acid, glycerin, and collagen to help hydrate and moisturize the skin. For more information, visit www.ortho-dermatologics.com.
Bryhali Approved for Plaque Psoriasis in Adults
Ortho Dermatologics announces US Food and Drug Administration approval of Bryhali (halobetasol propionate) Lotion 0.01% for the treatment of plaque psoriasis in adults. Bryhali is a corticosteroid in a novel vehicle lotion with safety established for dosing up to 8 weeks, offering patients a longer duration of use than other topical steroids. For more information, visit www.bryhali.com.
CoolSculpting Cleared for the Submandibular Area
Zeltiq Aesthetics, Inc, an Allergan affiliate, announces US Food and Drug Administration (FDA) clearance of CoolSculpting to treat the submandibular area. The FDA clearance also was expanded to include patients with a body mass index of up to 46.2 when treating the submental and submandibular areas. CoolSculpting is a nonsurgical treatment that works by gently cooling targeted fat cells in the body to induce natural controlled elimination of fat cells without affecting surrounding tissue. CoolSculpting also is cleared for treatment of visible fat bulges on the thighs, abdomen, and flanks. For more information, visit www.coolsculpting.com.
Glytone Age-Defying Vitamin C+E Serum Reduces Signs of Aging
Pierre Fabre Dermo-Cosmetique introduces Glytone Age-Defying Vitamin C+E Serum, a layering serum with time-released, high concentrations of stabilized vitamins C and E combined with red tea flavonoids to deliver antioxidant protection and antiaging benefits. It is indicated for premature aging caused by environmental damage and oxidative stress as well as postprocedure relief. For more information, visit www.glytone-usa.com.
Hyrimoz Biosimilar Approved for Psoriasis
Sandoz, a Novartis Division, announces US Food and Drug Administration (FDA) approval of Hyrimoz (adalimumab-adaz), a biosimilar indicated for the treatment of psoriatic arthritis and plaque psoriasis, as well as rheumatoid arthritis, juvenile idiopathic arthritis in patients 4 years and older, ankylosing spondylitis, adult Crohn disease, and ulcerative colitis. Hyrimoz is the third FDA-approved biosimilar from Sandoz. For more information, visit www.sandoz.com.
Libtayo Approved for SCC
Regeneron Pharmaceuticals, Inc, and sanofi-aventis US LLC, announce US Food and Drug Administration (FDA) approval of Libtayo (cemiplimab-rwlc) for the treatment of patients with metastatic cutaneous squamous cell carcinoma (SCC) or locally advanced cutaneous SCC who are not candidates for curative surgery or radiation. Libtayo is a monoclonal antibody targeting the programmed death receptor 1.
Restylane Lyft Now Approved for Hand Rejuvenation
Nestlé Skin Health announces US Food and Drug Administration approval of Restylane Lyft with Lidocaine, a hyaluronic acid dermal filler, for the correction of age-related volume loss in the back of the hands for patients older than 21 years. Restylane Lyft with Lidocaine also is indicated for correction of moderate to severe facial wrinkles and folds such as the nasolabial folds, cheek augmentation, and age-related midface contour deficiencies. For more information, visit www.RestylaneUSA.com.
Xepi Launches for Impetigo
Cutanea Life Sciences launches Xepi (ozenoxacin) Cream 1%, a quinolone antimicrobial, for the treatment of impetigo in adult and pediatric patients 2 months or older. Xepi is applied twice daily for 5 days and has been shown to be active against most isolates of Staphylococcus aureus (including methicillin-resistant isolates) and Streptococcus pyogenes, both in vitro and in clinical infections. For more information, visit www.XepiCream.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Primary Cutaneous Epstein-Barr Virus–Positive Diffuse Large B-Cell Lymphoma: A Rare and Aggressive Cutaneous Lymphoma
Cutaneous B-cell lymphomas represent a group of lymphomas derived from B lymphocytes in various stages of differentiation. The skin can be the site of primary or secondary involvement of any of the B-cell lymphomas. Primary cutaneous B-cell lymphomas present in the skin without evidence of extracutaneous disease at the time of diagnosis.1 The World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues recognizes 5 distinct primary cutaneous B-cell lymphoma subtypes: primary cutaneous follicle center lymphoma; primary cutaneous marginal zone lymphoma; primary cutaneous diffuse large B-cell lymphoma (DLBCL), leg type; DLBCL, not otherwise specified; and intravascular DLBCL.1-3 The DLBCL, not otherwise specified, category includes less common provisional entities with insufficient evidence to be recognized as distinct diseases. Epstein-Barr virus (EBV)–positive DLBCL is a rare subtype in this group.4
This article reviews the different clinicopathologic subtypes of primary cutaneous B-cell lymphoma. It also serves to help dermatologists recognize primary cutaneous EBV-positive DLBCL as a rare and aggressive form of this disease.
Case Report
An 84-year-old white man presented with a pruritic eruption on the arms, legs, back, neck, and face of 5 months’ duration. His medical history was notable for prostate cancer that was successfully treated with radiation therapy 6 years prior. The patient denied any constitutional symptoms such as fever, chills, night sweats, or weight loss, and review of systems was negative. The patient was taking prednisone, which alleviated the pruritus, but the lesions persisted.
Physical examination revealed multiple pink to erythematous papules and subcutaneous nodules involving the face, neck, back, arms, and legs (Figure 1). No scale, crust, or ulceration was present. Palpation of the cervical, supraclavicular, axillary, and inguinal lymph nodes was negative for lymphadenopathy.
Punch biopsies of representative lesions on the upper back and right arm revealed diffuse and nodular infiltrates of large atypical lymphoid cells with scattered centroblasts and immunoblasts (Figures 2 and 3). Immunohistochemical staining demonstrated CD79, MUM-1, and EBV-encoded RNA positivity among the neoplastic cells. The Ki-67 proliferative index was greater than 90%. The neoplastic cells were negative for CD5, CD10, CD20, CD21, CD30, CD56, CD123, CD138, PAX5, C-MYC, BCL-2, BCL-6, cyclin D1, TCL-1A, and terminal deoxynucleotidyl transferase
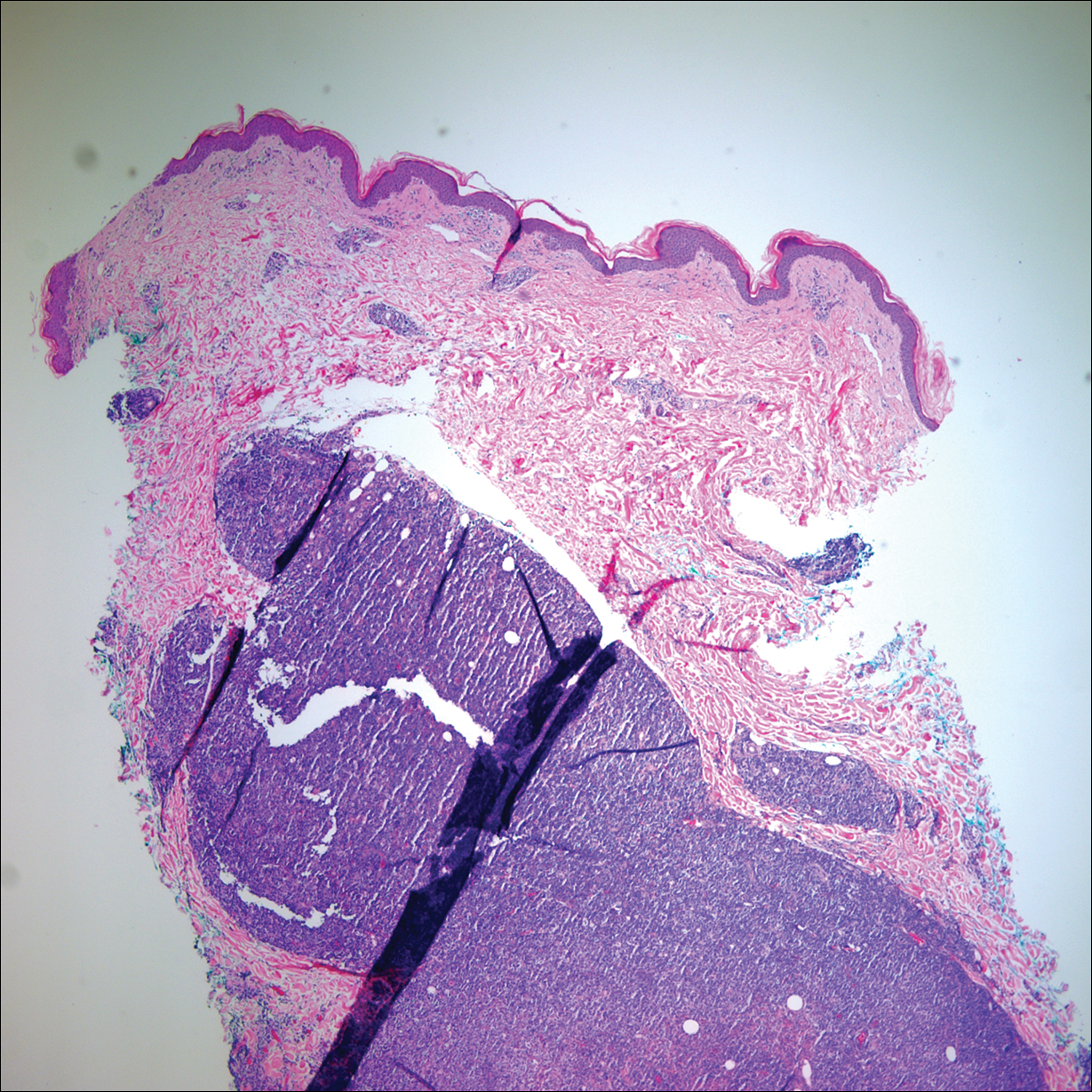
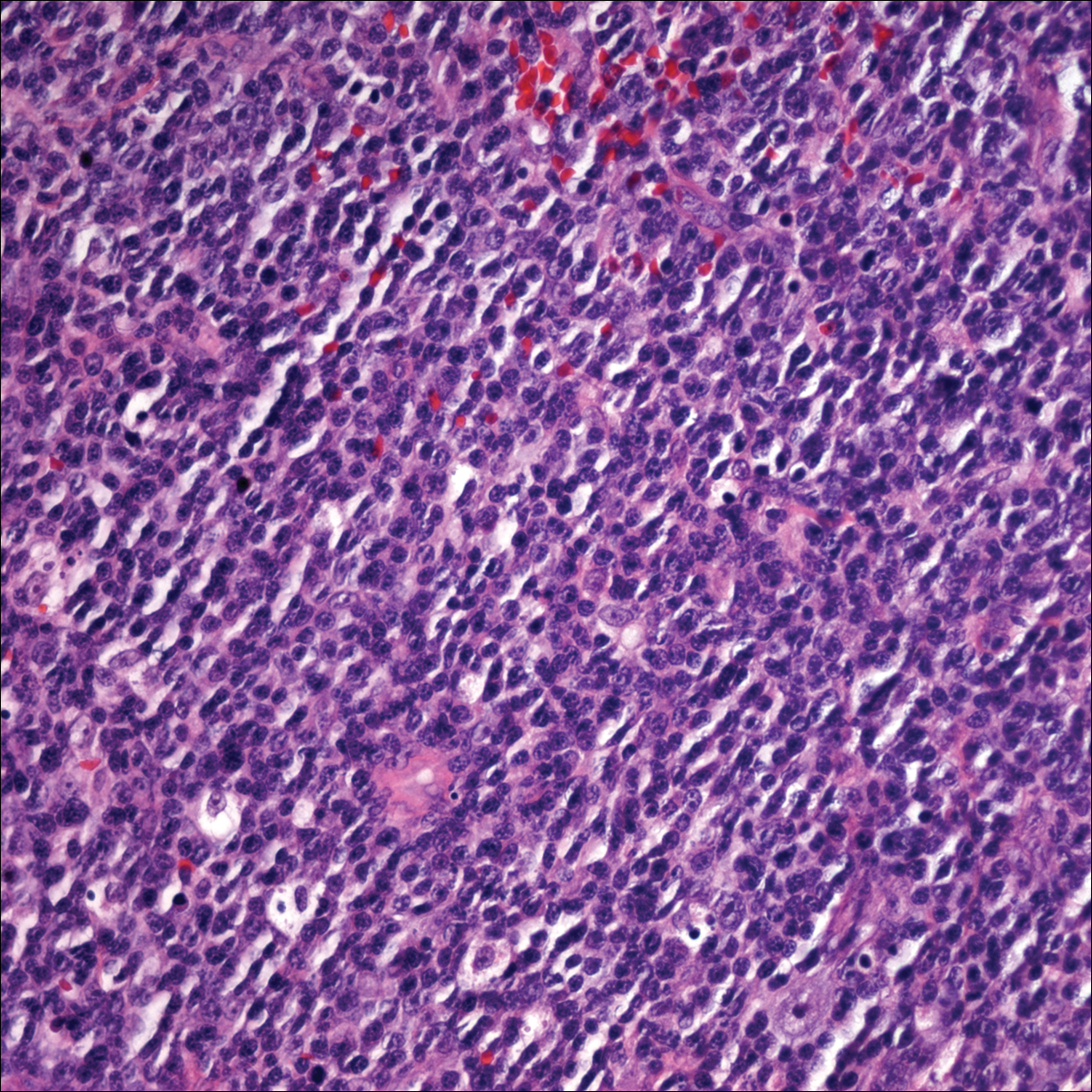
A peripheral blood smear did not show evidence of a B-cell lymphoproliferative process. A bone marrow biopsy was performed and did not show evidence of B-cell lymphoid neoplasia but did show reactive lymphoid aggregates composed of CD4+ and CD10+ T cells. Peripheral blood T-cell rearrangement and JAK2 were negative.
Based on clinical and histologic findings, the patient was diagnosed with primary cutaneous EBV-positive DLBCL. The patient was started on CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy for treatment of this aggressive cutaneous lymphoma, which initially resulted in clinical improvement of the lesions and complete involution of the subcutaneous nodules. After the sixth cycle of CHOP, he developed faintly erythematous indurated papules on the upper arms, chest, and back. Biopsy confirmed recurrence of the EBV-positive cutaneous lymphoma, and he started salvage chemotherapy with gemcitabine, oxaliplatin, and rituximab every 2 weeks; however, 4 months later (9 months after the initial presentation) he died from complications of the disease.
Comment
Etiology
Epstein-Barr virus–positive DLBCL, also called EBV-positive DLBCL of the elderly, was initially described in 2003 by Oyama et al5 and was included as a provisional entity in the 2008 World Health Organization classification system as a rare subtype of the DLBCL, not otherwise specified, category.2 It is defined as an EBV-positive monoclonal large B-cell proliferation that occurs in immunocompetent patients older than 50 years.6 Epstein-Barr virus is a human herpesvirus that demonstrates tropism for lymphocytes and survives in human hosts by establishing latency in B cells. Under normal immune conditions, the proliferation of EBV-infected B cells is prevented by cytotoxic T cells.7 It is important to recognize that patients with EBV-positive DLBCL do not have a known immunodeficiency state; therefore, it has been postulated that EBV-positive DLBCL might be caused by age-related senescence of the immune system.4,8
Epidemiology and Clinical Features
Epstein-Barr virus–positive DLBCL is more common in Asian countries than in Western countries, and there is a slight male predominance.6 A majority of patients present with extranodal disease at the time of diagnosis, and the skin is the most common extranodal site of involvement.6,9 Rare cases of primary cutaneous involvement also have been described.7,9,10 Cutaneous manifestations include erythematous papules and subcutaneous nodules. Other sites of extranodal involvement include the lungs, oral cavity, pharynx, gastrointestinal tract, and bone marrow.8,9 However, EBV-positive DLBCL is an aggressive lymphoma and prognosis is poor irrespective of the primary site of involvement.
Histopathology
Two morphologic subtypes can be seen on histology. The polymorphic pattern is characterized by a broad range of B-cell maturation with admixed reactive cells (eg, lymphocytes, histiocytes, plasma cells). The monomorphic or large-cell pattern is characterized by monotonous sheets of large transformed B cells.4,11 Many cases show both histologic patterns, and these morphologic variants do not impart any clinical or prognostic significance. Regardless of the histologic subtype, the neoplastic cells express pan B-cell antigens (eg, CD19, CD20, CD79a, PAX5), as well as MUM-1, BCL-2, and EBV-encoded RNA.4 Cases with plasmablastic features, as in our patient, may show weak or absent CD20 staining.12 Detection of EBV by in situ hybridization is required for the diagnosis.
Diagnosis
Workup for a suspected cutaneous lymphoma should include a complete history and physical examination; laboratory studies; and relevant imaging evaluation such as computed tomography of the chest, abdomen, and pelvis with or without whole-body positron emission tomography. A bone marrow biopsy and aspirate also should be performed in all cutaneous lymphomas with intermediate to aggressive clinical behavior. Accurate staging evaluation is integral to confirm the absence of extracutaneous involvement and to provide prognostic and anatomic information for the appropriate selection of treatment.13
Prognosis and Management
Primary cutaneous lymphomas tend to have different clinical behaviors and prognoses compared to histologically similar systemic lymphomas; therefore, different therapeutic strategies are warranted.14 Epstein-Barr virus–positive DLBCL has an aggressive clinical course with a median survival of 2 years.8 Patients with EBV-positive DLBCL have a poorer overall survival and treatment response when compared to patients with EBV-negative DLBCLs.4 Primary cutaneous B-cell lymphomas with indolent behavior, such as primary cutaneous marginal zone lymphoma and primary cutaneous follicle center lymphoma, can be treated with surgical excision, radiation therapy, or observation.15 No standard treatment exists for EBV-positive DLBCL, but R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), which is the standard treatment of primary cutaneous DLBCL, leg type, may provide a survival benefit.13,15 Further studies are required to determine optimal treatment strategies.
Conclusion
Although rare, EBV-positive DLBCL is an important entity to consider when evaluating a patient with a suspected primary cutaneous lymphoma. Workup to rule out an underlying systemic lymphoma with relevant laboratory evaluation, imaging studies, and bone marrow biopsy is critical. Prognosis is poor and treatment is difficult, as standard treatment protocols have yet to be determined.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nakmura S, Jaffe ES, Swerdlow SH. EBV positive diffuse large B-cell lymphoma of the elderly. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2008:243-244.
- Kempf W, Sander CA. Classification of cutaneous lymphomas—an update. Histopathology. 2010;56:57-70.
- Castillo JJ, Beltran BE, Miranda RN, et al. Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly: what we know so far. Oncologist. 2011;16:87-96.
- Oyama T, Ichimura K, Suzuki R, et al. Senile EBV+ B-cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol. 2003;27:16-26.
- Ok CY, Papathomas TG, Medeiros LJ, et al. EBV-positive diffuse large B-cell lymphoma of the elderly. Blood. 2013;122:328-340.
- Tokuda Y, Fukushima M, Nakazawa K, et al. A case of primary Epstein-Barr virus-associated cutaneous diffuse large B-cell lymphoma unassociated with iatrogenic or endogenous immune dysregulation. J Cutan Pathol. 2008;35:666-671.
- Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007;13:5124-5132.
- Eminger LA, Hall LD, Hesterman KS, et al. Epstein-Barr virus: dermatologic associations and implications. J Am Acad Dermatol. 2015;72:21-34.
- Martin B, Whittaker S, Morris S, et al. A case of primary cutaneous senile EBV-related diffuse large B-cell lymphoma. Am J Dermatopathol. 2010;32:190-193.
- Gibson SE, Hsi ED. Epstein-Barr virus-positive B-cell lymphoma of the elderly at a United States tertiary medical center: an uncommon aggressive lymphoma with a nongerminal center B-cell phenotype. Hum Pathol. 2009;40:653-661.
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125:2323-2330.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-329.e13; quiz 341-342.
- Suárez AL, Querfeld C, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part II. therapy and future directions. J Am Acad Dermatol. 2013;69:343.e1-343.e11; quiz 355-356.
Cutaneous B-cell lymphomas represent a group of lymphomas derived from B lymphocytes in various stages of differentiation. The skin can be the site of primary or secondary involvement of any of the B-cell lymphomas. Primary cutaneous B-cell lymphomas present in the skin without evidence of extracutaneous disease at the time of diagnosis.1 The World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues recognizes 5 distinct primary cutaneous B-cell lymphoma subtypes: primary cutaneous follicle center lymphoma; primary cutaneous marginal zone lymphoma; primary cutaneous diffuse large B-cell lymphoma (DLBCL), leg type; DLBCL, not otherwise specified; and intravascular DLBCL.1-3 The DLBCL, not otherwise specified, category includes less common provisional entities with insufficient evidence to be recognized as distinct diseases. Epstein-Barr virus (EBV)–positive DLBCL is a rare subtype in this group.4
This article reviews the different clinicopathologic subtypes of primary cutaneous B-cell lymphoma. It also serves to help dermatologists recognize primary cutaneous EBV-positive DLBCL as a rare and aggressive form of this disease.
Case Report
An 84-year-old white man presented with a pruritic eruption on the arms, legs, back, neck, and face of 5 months’ duration. His medical history was notable for prostate cancer that was successfully treated with radiation therapy 6 years prior. The patient denied any constitutional symptoms such as fever, chills, night sweats, or weight loss, and review of systems was negative. The patient was taking prednisone, which alleviated the pruritus, but the lesions persisted.
Physical examination revealed multiple pink to erythematous papules and subcutaneous nodules involving the face, neck, back, arms, and legs (Figure 1). No scale, crust, or ulceration was present. Palpation of the cervical, supraclavicular, axillary, and inguinal lymph nodes was negative for lymphadenopathy.

Punch biopsies of representative lesions on the upper back and right arm revealed diffuse and nodular infiltrates of large atypical lymphoid cells with scattered centroblasts and immunoblasts (Figures 2 and 3). Immunohistochemical staining demonstrated CD79, MUM-1, and EBV-encoded RNA positivity among the neoplastic cells. The Ki-67 proliferative index was greater than 90%. The neoplastic cells were negative for CD5, CD10, CD20, CD21, CD30, CD56, CD123, CD138, PAX5, C-MYC, BCL-2, BCL-6, cyclin D1, TCL-1A, and terminal deoxynucleotidyl transferase


A peripheral blood smear did not show evidence of a B-cell lymphoproliferative process. A bone marrow biopsy was performed and did not show evidence of B-cell lymphoid neoplasia but did show reactive lymphoid aggregates composed of CD4+ and CD10+ T cells. Peripheral blood T-cell rearrangement and JAK2 were negative.
Based on clinical and histologic findings, the patient was diagnosed with primary cutaneous EBV-positive DLBCL. The patient was started on CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy for treatment of this aggressive cutaneous lymphoma, which initially resulted in clinical improvement of the lesions and complete involution of the subcutaneous nodules. After the sixth cycle of CHOP, he developed faintly erythematous indurated papules on the upper arms, chest, and back. Biopsy confirmed recurrence of the EBV-positive cutaneous lymphoma, and he started salvage chemotherapy with gemcitabine, oxaliplatin, and rituximab every 2 weeks; however, 4 months later (9 months after the initial presentation) he died from complications of the disease.
Comment
Etiology
Epstein-Barr virus–positive DLBCL, also called EBV-positive DLBCL of the elderly, was initially described in 2003 by Oyama et al5 and was included as a provisional entity in the 2008 World Health Organization classification system as a rare subtype of the DLBCL, not otherwise specified, category.2 It is defined as an EBV-positive monoclonal large B-cell proliferation that occurs in immunocompetent patients older than 50 years.6 Epstein-Barr virus is a human herpesvirus that demonstrates tropism for lymphocytes and survives in human hosts by establishing latency in B cells. Under normal immune conditions, the proliferation of EBV-infected B cells is prevented by cytotoxic T cells.7 It is important to recognize that patients with EBV-positive DLBCL do not have a known immunodeficiency state; therefore, it has been postulated that EBV-positive DLBCL might be caused by age-related senescence of the immune system.4,8
Epidemiology and Clinical Features
Epstein-Barr virus–positive DLBCL is more common in Asian countries than in Western countries, and there is a slight male predominance.6 A majority of patients present with extranodal disease at the time of diagnosis, and the skin is the most common extranodal site of involvement.6,9 Rare cases of primary cutaneous involvement also have been described.7,9,10 Cutaneous manifestations include erythematous papules and subcutaneous nodules. Other sites of extranodal involvement include the lungs, oral cavity, pharynx, gastrointestinal tract, and bone marrow.8,9 However, EBV-positive DLBCL is an aggressive lymphoma and prognosis is poor irrespective of the primary site of involvement.
Histopathology
Two morphologic subtypes can be seen on histology. The polymorphic pattern is characterized by a broad range of B-cell maturation with admixed reactive cells (eg, lymphocytes, histiocytes, plasma cells). The monomorphic or large-cell pattern is characterized by monotonous sheets of large transformed B cells.4,11 Many cases show both histologic patterns, and these morphologic variants do not impart any clinical or prognostic significance. Regardless of the histologic subtype, the neoplastic cells express pan B-cell antigens (eg, CD19, CD20, CD79a, PAX5), as well as MUM-1, BCL-2, and EBV-encoded RNA.4 Cases with plasmablastic features, as in our patient, may show weak or absent CD20 staining.12 Detection of EBV by in situ hybridization is required for the diagnosis.
Diagnosis
Workup for a suspected cutaneous lymphoma should include a complete history and physical examination; laboratory studies; and relevant imaging evaluation such as computed tomography of the chest, abdomen, and pelvis with or without whole-body positron emission tomography. A bone marrow biopsy and aspirate also should be performed in all cutaneous lymphomas with intermediate to aggressive clinical behavior. Accurate staging evaluation is integral to confirm the absence of extracutaneous involvement and to provide prognostic and anatomic information for the appropriate selection of treatment.13
Prognosis and Management
Primary cutaneous lymphomas tend to have different clinical behaviors and prognoses compared to histologically similar systemic lymphomas; therefore, different therapeutic strategies are warranted.14 Epstein-Barr virus–positive DLBCL has an aggressive clinical course with a median survival of 2 years.8 Patients with EBV-positive DLBCL have a poorer overall survival and treatment response when compared to patients with EBV-negative DLBCLs.4 Primary cutaneous B-cell lymphomas with indolent behavior, such as primary cutaneous marginal zone lymphoma and primary cutaneous follicle center lymphoma, can be treated with surgical excision, radiation therapy, or observation.15 No standard treatment exists for EBV-positive DLBCL, but R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), which is the standard treatment of primary cutaneous DLBCL, leg type, may provide a survival benefit.13,15 Further studies are required to determine optimal treatment strategies.
Conclusion
Although rare, EBV-positive DLBCL is an important entity to consider when evaluating a patient with a suspected primary cutaneous lymphoma. Workup to rule out an underlying systemic lymphoma with relevant laboratory evaluation, imaging studies, and bone marrow biopsy is critical. Prognosis is poor and treatment is difficult, as standard treatment protocols have yet to be determined.
Cutaneous B-cell lymphomas represent a group of lymphomas derived from B lymphocytes in various stages of differentiation. The skin can be the site of primary or secondary involvement of any of the B-cell lymphomas. Primary cutaneous B-cell lymphomas present in the skin without evidence of extracutaneous disease at the time of diagnosis.1 The World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues recognizes 5 distinct primary cutaneous B-cell lymphoma subtypes: primary cutaneous follicle center lymphoma; primary cutaneous marginal zone lymphoma; primary cutaneous diffuse large B-cell lymphoma (DLBCL), leg type; DLBCL, not otherwise specified; and intravascular DLBCL.1-3 The DLBCL, not otherwise specified, category includes less common provisional entities with insufficient evidence to be recognized as distinct diseases. Epstein-Barr virus (EBV)–positive DLBCL is a rare subtype in this group.4
This article reviews the different clinicopathologic subtypes of primary cutaneous B-cell lymphoma. It also serves to help dermatologists recognize primary cutaneous EBV-positive DLBCL as a rare and aggressive form of this disease.
Case Report
An 84-year-old white man presented with a pruritic eruption on the arms, legs, back, neck, and face of 5 months’ duration. His medical history was notable for prostate cancer that was successfully treated with radiation therapy 6 years prior. The patient denied any constitutional symptoms such as fever, chills, night sweats, or weight loss, and review of systems was negative. The patient was taking prednisone, which alleviated the pruritus, but the lesions persisted.
Physical examination revealed multiple pink to erythematous papules and subcutaneous nodules involving the face, neck, back, arms, and legs (Figure 1). No scale, crust, or ulceration was present. Palpation of the cervical, supraclavicular, axillary, and inguinal lymph nodes was negative for lymphadenopathy.

Punch biopsies of representative lesions on the upper back and right arm revealed diffuse and nodular infiltrates of large atypical lymphoid cells with scattered centroblasts and immunoblasts (Figures 2 and 3). Immunohistochemical staining demonstrated CD79, MUM-1, and EBV-encoded RNA positivity among the neoplastic cells. The Ki-67 proliferative index was greater than 90%. The neoplastic cells were negative for CD5, CD10, CD20, CD21, CD30, CD56, CD123, CD138, PAX5, C-MYC, BCL-2, BCL-6, cyclin D1, TCL-1A, and terminal deoxynucleotidyl transferase


A peripheral blood smear did not show evidence of a B-cell lymphoproliferative process. A bone marrow biopsy was performed and did not show evidence of B-cell lymphoid neoplasia but did show reactive lymphoid aggregates composed of CD4+ and CD10+ T cells. Peripheral blood T-cell rearrangement and JAK2 were negative.
Based on clinical and histologic findings, the patient was diagnosed with primary cutaneous EBV-positive DLBCL. The patient was started on CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy for treatment of this aggressive cutaneous lymphoma, which initially resulted in clinical improvement of the lesions and complete involution of the subcutaneous nodules. After the sixth cycle of CHOP, he developed faintly erythematous indurated papules on the upper arms, chest, and back. Biopsy confirmed recurrence of the EBV-positive cutaneous lymphoma, and he started salvage chemotherapy with gemcitabine, oxaliplatin, and rituximab every 2 weeks; however, 4 months later (9 months after the initial presentation) he died from complications of the disease.
Comment
Etiology
Epstein-Barr virus–positive DLBCL, also called EBV-positive DLBCL of the elderly, was initially described in 2003 by Oyama et al5 and was included as a provisional entity in the 2008 World Health Organization classification system as a rare subtype of the DLBCL, not otherwise specified, category.2 It is defined as an EBV-positive monoclonal large B-cell proliferation that occurs in immunocompetent patients older than 50 years.6 Epstein-Barr virus is a human herpesvirus that demonstrates tropism for lymphocytes and survives in human hosts by establishing latency in B cells. Under normal immune conditions, the proliferation of EBV-infected B cells is prevented by cytotoxic T cells.7 It is important to recognize that patients with EBV-positive DLBCL do not have a known immunodeficiency state; therefore, it has been postulated that EBV-positive DLBCL might be caused by age-related senescence of the immune system.4,8
Epidemiology and Clinical Features
Epstein-Barr virus–positive DLBCL is more common in Asian countries than in Western countries, and there is a slight male predominance.6 A majority of patients present with extranodal disease at the time of diagnosis, and the skin is the most common extranodal site of involvement.6,9 Rare cases of primary cutaneous involvement also have been described.7,9,10 Cutaneous manifestations include erythematous papules and subcutaneous nodules. Other sites of extranodal involvement include the lungs, oral cavity, pharynx, gastrointestinal tract, and bone marrow.8,9 However, EBV-positive DLBCL is an aggressive lymphoma and prognosis is poor irrespective of the primary site of involvement.
Histopathology
Two morphologic subtypes can be seen on histology. The polymorphic pattern is characterized by a broad range of B-cell maturation with admixed reactive cells (eg, lymphocytes, histiocytes, plasma cells). The monomorphic or large-cell pattern is characterized by monotonous sheets of large transformed B cells.4,11 Many cases show both histologic patterns, and these morphologic variants do not impart any clinical or prognostic significance. Regardless of the histologic subtype, the neoplastic cells express pan B-cell antigens (eg, CD19, CD20, CD79a, PAX5), as well as MUM-1, BCL-2, and EBV-encoded RNA.4 Cases with plasmablastic features, as in our patient, may show weak or absent CD20 staining.12 Detection of EBV by in situ hybridization is required for the diagnosis.
Diagnosis
Workup for a suspected cutaneous lymphoma should include a complete history and physical examination; laboratory studies; and relevant imaging evaluation such as computed tomography of the chest, abdomen, and pelvis with or without whole-body positron emission tomography. A bone marrow biopsy and aspirate also should be performed in all cutaneous lymphomas with intermediate to aggressive clinical behavior. Accurate staging evaluation is integral to confirm the absence of extracutaneous involvement and to provide prognostic and anatomic information for the appropriate selection of treatment.13
Prognosis and Management
Primary cutaneous lymphomas tend to have different clinical behaviors and prognoses compared to histologically similar systemic lymphomas; therefore, different therapeutic strategies are warranted.14 Epstein-Barr virus–positive DLBCL has an aggressive clinical course with a median survival of 2 years.8 Patients with EBV-positive DLBCL have a poorer overall survival and treatment response when compared to patients with EBV-negative DLBCLs.4 Primary cutaneous B-cell lymphomas with indolent behavior, such as primary cutaneous marginal zone lymphoma and primary cutaneous follicle center lymphoma, can be treated with surgical excision, radiation therapy, or observation.15 No standard treatment exists for EBV-positive DLBCL, but R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), which is the standard treatment of primary cutaneous DLBCL, leg type, may provide a survival benefit.13,15 Further studies are required to determine optimal treatment strategies.
Conclusion
Although rare, EBV-positive DLBCL is an important entity to consider when evaluating a patient with a suspected primary cutaneous lymphoma. Workup to rule out an underlying systemic lymphoma with relevant laboratory evaluation, imaging studies, and bone marrow biopsy is critical. Prognosis is poor and treatment is difficult, as standard treatment protocols have yet to be determined.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nakmura S, Jaffe ES, Swerdlow SH. EBV positive diffuse large B-cell lymphoma of the elderly. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2008:243-244.
- Kempf W, Sander CA. Classification of cutaneous lymphomas—an update. Histopathology. 2010;56:57-70.
- Castillo JJ, Beltran BE, Miranda RN, et al. Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly: what we know so far. Oncologist. 2011;16:87-96.
- Oyama T, Ichimura K, Suzuki R, et al. Senile EBV+ B-cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol. 2003;27:16-26.
- Ok CY, Papathomas TG, Medeiros LJ, et al. EBV-positive diffuse large B-cell lymphoma of the elderly. Blood. 2013;122:328-340.
- Tokuda Y, Fukushima M, Nakazawa K, et al. A case of primary Epstein-Barr virus-associated cutaneous diffuse large B-cell lymphoma unassociated with iatrogenic or endogenous immune dysregulation. J Cutan Pathol. 2008;35:666-671.
- Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007;13:5124-5132.
- Eminger LA, Hall LD, Hesterman KS, et al. Epstein-Barr virus: dermatologic associations and implications. J Am Acad Dermatol. 2015;72:21-34.
- Martin B, Whittaker S, Morris S, et al. A case of primary cutaneous senile EBV-related diffuse large B-cell lymphoma. Am J Dermatopathol. 2010;32:190-193.
- Gibson SE, Hsi ED. Epstein-Barr virus-positive B-cell lymphoma of the elderly at a United States tertiary medical center: an uncommon aggressive lymphoma with a nongerminal center B-cell phenotype. Hum Pathol. 2009;40:653-661.
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125:2323-2330.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-329.e13; quiz 341-342.
- Suárez AL, Querfeld C, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part II. therapy and future directions. J Am Acad Dermatol. 2013;69:343.e1-343.e11; quiz 355-356.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nakmura S, Jaffe ES, Swerdlow SH. EBV positive diffuse large B-cell lymphoma of the elderly. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2008:243-244.
- Kempf W, Sander CA. Classification of cutaneous lymphomas—an update. Histopathology. 2010;56:57-70.
- Castillo JJ, Beltran BE, Miranda RN, et al. Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly: what we know so far. Oncologist. 2011;16:87-96.
- Oyama T, Ichimura K, Suzuki R, et al. Senile EBV+ B-cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol. 2003;27:16-26.
- Ok CY, Papathomas TG, Medeiros LJ, et al. EBV-positive diffuse large B-cell lymphoma of the elderly. Blood. 2013;122:328-340.
- Tokuda Y, Fukushima M, Nakazawa K, et al. A case of primary Epstein-Barr virus-associated cutaneous diffuse large B-cell lymphoma unassociated with iatrogenic or endogenous immune dysregulation. J Cutan Pathol. 2008;35:666-671.
- Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007;13:5124-5132.
- Eminger LA, Hall LD, Hesterman KS, et al. Epstein-Barr virus: dermatologic associations and implications. J Am Acad Dermatol. 2015;72:21-34.
- Martin B, Whittaker S, Morris S, et al. A case of primary cutaneous senile EBV-related diffuse large B-cell lymphoma. Am J Dermatopathol. 2010;32:190-193.
- Gibson SE, Hsi ED. Epstein-Barr virus-positive B-cell lymphoma of the elderly at a United States tertiary medical center: an uncommon aggressive lymphoma with a nongerminal center B-cell phenotype. Hum Pathol. 2009;40:653-661.
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125:2323-2330.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-329.e13; quiz 341-342.
- Suárez AL, Querfeld C, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part II. therapy and future directions. J Am Acad Dermatol. 2013;69:343.e1-343.e11; quiz 355-356.
Practice Points
- Primary cutaneous lymphomas are malignant lymphomas confined to the skin.
- Complete staging workup is necessary to rule out secondary involvement of the skin from a nodal lymphoma.
- Epstein-Barr virus-positive diffuse large B-cell lymphoma is a rare and aggressive primary cutaneous lymphoma.
Automobile Injury: A Common Familiar Risk for Presenting and Comparing Risks in Dermatology
Numerous highly efficacious treatment modalities exist in dermatology, yet patients may be highly wary of their possible adverse events, even when those risks are rare.1,2 Such fears can lead to poor medication adherence and treatment refusal. A key determinant in successful patient-provider care is to effectively communicate risk. The communication of risk is hampered by the lack of any common currency for comparing risks. The development of a standardized unit of risk could help facilitate risk comparisons, allowing physicians and patients to put risk levels into better perspective.
One easily relatable event is the risk of injury in an automobile crash. Driving, whether to the dermatology clinic for a monitoring visit or to the supermarket for weekly groceries, is associated with risk of injury and death. The risk of automobile-related injury warranting a visit to the emergency department could provide a comparator that physicians can use to give patients a more objective sense of treatment risks or to introduce the justification of a monitoring visit. The objective of this study was to develop a standard risk unit based on the lifetime risk (LTR) of automobile injury and to compare this unit of risk to various risks of dermatologic treatments.
Methods
Literature Review
We first identified common risks in dermatology that would be illustrative and then identified keywords. PubMed searches for articles indexed for MEDLINE from November 1996 to February 2017 were performed combining the following terms: (relative risk, odds ratio, lifetime risk) and (isotretinoin, IBD; melanoma, SCC, transplantation; indoor tanning, BCC, SCC; transplant and SCC; biologics and tuberculosis; hydroxychloroquine retinal toxicity; psoriasis and psoriatic arthritis). An additional search was performed in June 2018 including the term blindness and injectable fillers. Our search combined these terms in numerous ways. Results were focused on meta-analyses and observational studies.
The references of relevant studies were included. Articles not focused on meta-analyses but rather on observational studies were individually analyzed for quality and bias using the 9-point Newcastle-Ottawa Scale, with a score of 7 or more as a cutoff for inclusion.
Determination of Risk Comparators
Data from the 2016 National Safety Council’s Injury Facts report were searched for nonmedical-related risk comparators, such as the risk of death by dog attack, by lightning, and by fire or smoke.3 Data from the 2015 US Department of Transportation Traffic Safety Facts were searched for relatable risk comparators, such as the LTR of automobile death and injury.4
Definitions
Automobile injury was defined as an injury warranting a visit to the emergency department.5 Automobile was defined as a road vehicle with 4 wheels and powered by an internal combustion engine or electric motor.6 This definition excluded light trucks, large trucks, and motorcycles.
LTR Calculation
Lifetime risk was used as the comparative measure. Lifetime risk is a type of absolute risk that depicts the probability that a specific disease or event will occur in an individual’s lifespan. The LTRof developing a disease or adverse event due to a dermatologic therapy or interventionwas denoted as LTRadverse event and calculated by the following equation7,8:

In this equation, LTRgeneral population is the LTR of developing the disease or adverse event without being subject to the therapy or intervention, and RRintervention is the relative risk (RR) from previously published RR data (relating to the development of the disease in question or an adverse event of the intervention). The use of equation (1) holds true only when the absolute risk of developing the disease or adverse event (LTRgeneral population) is low.7 Although the calculation of an LTR using a constant lifetime RR may require major approximations, studies evaluating the variation of RR over time are sparse.7,9 The Newcastle-Ottawa Scale was used to control such variance; only high-quality, nonrandomized studies were included. Although the use of residual LTR would be preferable, as LTR depends on age, such epidemiological data do not exist for complex diseases.
When not available, the LTRgeneral population was calculated from the rate of disease (cases per 100,000 individuals per year) multiplied by the average lifespan of an American (78.8 years)10:
When an odds ratio (OR) was presented, its conversion to RR followed11:
In this equation, RC is the absolute risk in the unexposed group. If the prevalence of the disease was considered low, the rare disease assumption was implemented as the following11,12:

The use of this approximation overestimates the LTR of an event. From a patient perspective, this approach is conservative. If prior LTR values were available, such as the LTR of automobile injury, automobile death, or other intervention, they were used without the need for calculation.
Unit Comparator
The LTRs of all adverse events were normalized to a unit comparator, using the LTR of an automobile injury as reference point, denoted as 1 risk unit (RU):

This equation allows for quick comparison of the magnitude of LTRs between events. Events with an RU less than 1 are less likely to occur than the risk of automobile injury; events with an RU greater than 1 are more likely than the risk of automobile injury. All RR, LTR, and unit comparators were presented as a single pooled estimate of their respective upper-limit CIs. The use of the upper-limit CI conservatively overestimates the LTR of an event.
Results
Ten dermatologic interventions were identified as illustrative, to be presented alongside the risk of automobile injury and death. The LTR of automobile injury was 32%, defined as 1.0 RU. The LTR of automobile death was 0.89% (1/36 RU).
Two events had LTRs roughly similar to automobile injury: development of a subsequent basal cell carcinoma within 3 years (1.4 RU) and development of a squamous cell carcinoma (SCC) secondary to indoor tanning (1.6 RU). Development of SCC following organ transplantation (34 RU) was considerably more likely than automobile injury. All other identified events had lower RUs than automobile injury (Table). Three events with small RUs included tuberculosis development with a tumor necrosis factor α inhibitor (1/32 RU), Crohn disease development with isotretinoin (1/41 RU), and blindness following facial hyaluronic acid injection (1/80 RU). The LTR of death by dog attack (1/42,436 RU) and death by lightning strike (1/36,542 RU) also had small RUs.
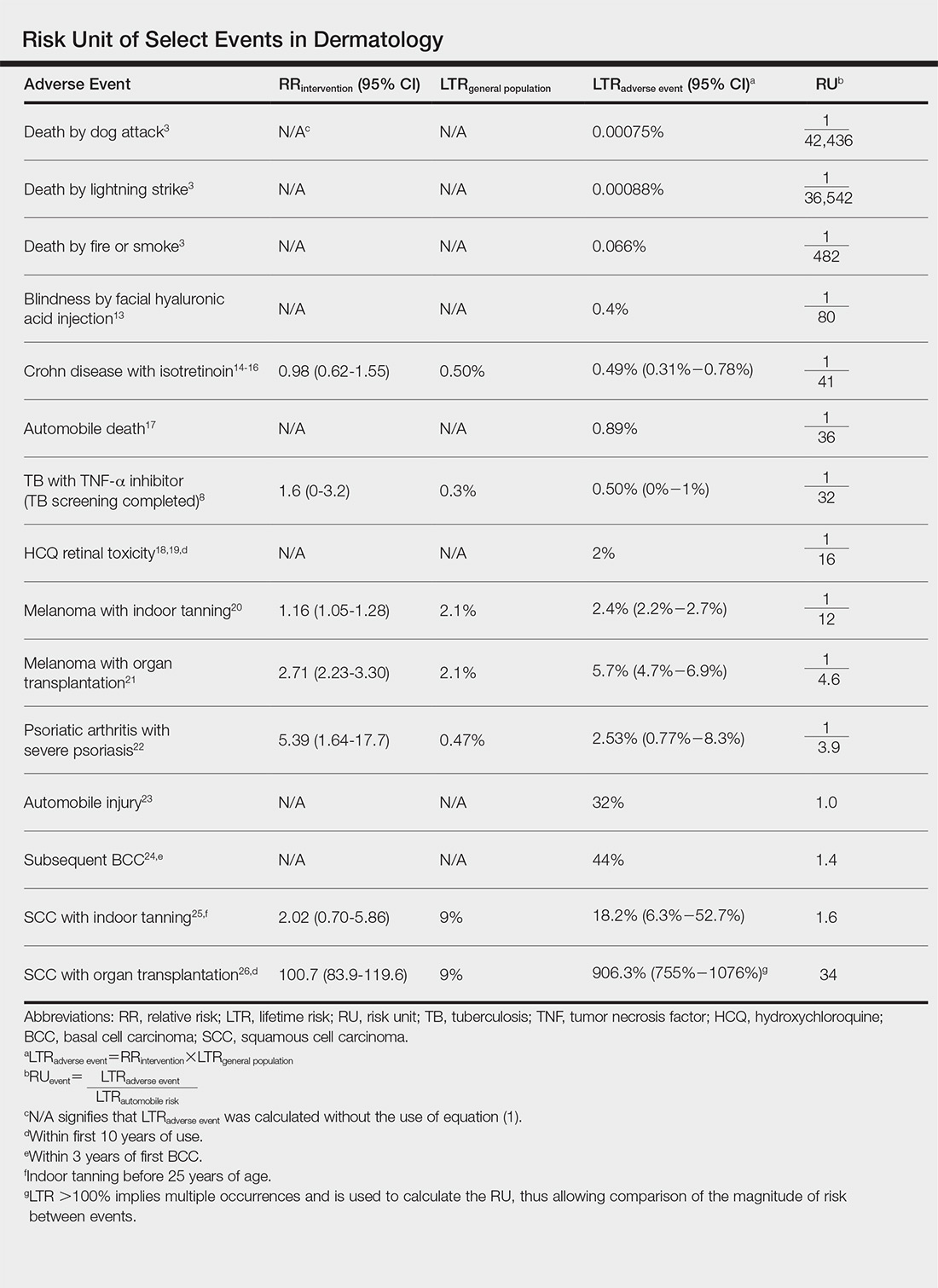
The unit comparators from the Table were adapted into graphic form to depict risk relative to the risk of automobile injury (Figure).
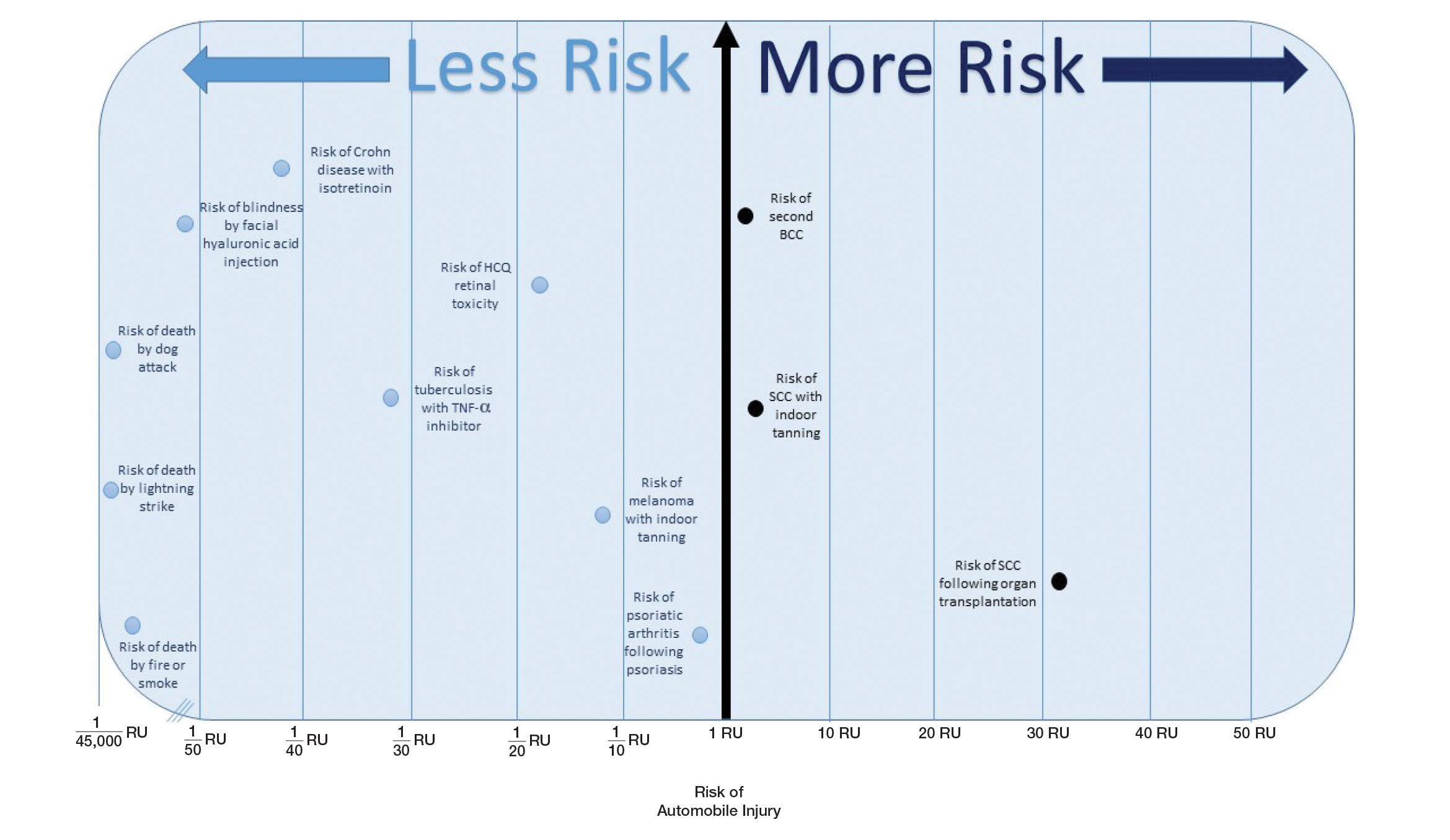
Comment
Numerous interventions in dermatology offer much less risk of an adverse event than the LTR of automobile injury. However, this concept of risk includes only the likelihood of development of an event, not the severity of the measured event, as our numerical and visual tool objectively captures the related risks using an RU comparator. Such use of a standardized RU demonstrates the essence of risk; “zero risk” does not exist, and each intervention or treatment, albeit how small, must be justified in concordance with other types of risk, such as the automobile.
The development of adverse events secondary to dermatologic intervention or therapy, for which monitoring visits are utilized, were used as important comparators to the risk of automobile injury. The continuous practice of monitoring visits may increase patient’s fears regarding possible adverse events secondary to therapy. Hydroxychloroquine retinal toxicity (1/16 RU) and psoriatic arthritis development following severe psoriasis (1/3.9 RU) were less likely to occur than automobile injury. The development of abnormal blood counts or blood tests secondary to therapy or intervention could not be formatted into an RU. The use of equation (1) for the calculation of LTRadverse eventholds true only when the absolute risk of developing the adverse event in the general population—in this case, abnormal blood counts or blood tests—is low.7
Although the unit comparator allows for the comparison of different dermatologic risk, a limitation of the RU model and its visual tool are a dependence on RR, a value that changes following publication of new studies. A solution was the use of a single pooled estimate to represent the upper-limit CIs of LTR. This practice overestimates risk. As with RR, new automobile injury rates are published annually.10 In the last 5 years, the LTR of automobile injury has stayed relatively constant: between 32% and 33%.4 Although the RU calculations and Figure included a wide variety of interventions in dermatology, select clinical situations were not included. It is beyond the scope of this article to systematically review all risk in dermatology but rather introduce the concept of the RU founded on automobile-associated risks. With the introduction of a methodical framework, the reader is invited to calculate RUs pertinent to their clinical interests.
Any intervention or treatment in dermatology is accompanied by risk. The use of a unit comparator using an easily relatable event—the LTR of automobile injury—allows the patient to easily compare risk and internally justify the practice of monitoring visits. Inclusion of a visual tool, such as the Figure, might alleviate many irrational fears that accompany some of the highly effective treatments and interventions used in dermatology and thus lead to better patient outcomes.
Acknowledgment
We thank Taranjeet Singh, MS (Dunn, North Carolina), for her comments on an earlier version of the manuscript.
- Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making. 2003;23:511-517.
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014;15:165-180.
- National Safety Council. Odds of dying. Injury Facts website. http://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/. Accessed November 4, 2018.
- National Center for Statistics and Analysis (NCSA) motor vehicle traffic crash data resource page. National Highway Traffic Safety Administration website. https://crashstats.nhtsa.dot.gov/#/. Accessed November 4, 2018.
- CDC report shows motor vehicle crash injuries are frequent and costly. Centers for Disease Control and Prevention website. http://www.cdc.gov/media/releases/2014/p1007-crash-injuries.html. Published October 7, 2014. Accessed November 4, 2018.
- Automobile. Business Dictionary website. http://www.businessdictionary.com/definition/automobile.html. Accessed November 4, 2018.
- Dupont WD, Plummer WD Jr. Understanding the relationship between relative and absolute risk. Cancer. 1996;77:2193-2199.
- Kaminska E, Patel I, Dabade TS, et al. Comparing the lifetime risks of TNF-alpha inhibitor use to common benchmarks of risk. J Dermatolog Treat. 2011;24:101-106.
- Dupont WD. Converting relative risks to absolute risks: a graphical approach. Stat Med. 1989;8:641-651.
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1-122.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration website. http://handbook.cochrane.org. Updated March 2011. Accessed November 15, 2018.
- Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142:761-764.
- Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207-214.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424-1429.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
- Lee SY, Jamal MM, Nguyen ET, et al. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:210-216.
- Injury Facts, 2017. Itasca, IL: National Safety Council; 2017.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-1394.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-1460.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847-857.e1-18.
- Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta Derm Venereol. 2015;95:923-927.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- National Highway Traffic Safety Administration (NHTSA). Traffic Safety Facts 2015. Washington, DC: US Department of Transportation; 2015.
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:E5909.
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-519.
Numerous highly efficacious treatment modalities exist in dermatology, yet patients may be highly wary of their possible adverse events, even when those risks are rare.1,2 Such fears can lead to poor medication adherence and treatment refusal. A key determinant in successful patient-provider care is to effectively communicate risk. The communication of risk is hampered by the lack of any common currency for comparing risks. The development of a standardized unit of risk could help facilitate risk comparisons, allowing physicians and patients to put risk levels into better perspective.
One easily relatable event is the risk of injury in an automobile crash. Driving, whether to the dermatology clinic for a monitoring visit or to the supermarket for weekly groceries, is associated with risk of injury and death. The risk of automobile-related injury warranting a visit to the emergency department could provide a comparator that physicians can use to give patients a more objective sense of treatment risks or to introduce the justification of a monitoring visit. The objective of this study was to develop a standard risk unit based on the lifetime risk (LTR) of automobile injury and to compare this unit of risk to various risks of dermatologic treatments.
Methods
Literature Review
We first identified common risks in dermatology that would be illustrative and then identified keywords. PubMed searches for articles indexed for MEDLINE from November 1996 to February 2017 were performed combining the following terms: (relative risk, odds ratio, lifetime risk) and (isotretinoin, IBD; melanoma, SCC, transplantation; indoor tanning, BCC, SCC; transplant and SCC; biologics and tuberculosis; hydroxychloroquine retinal toxicity; psoriasis and psoriatic arthritis). An additional search was performed in June 2018 including the term blindness and injectable fillers. Our search combined these terms in numerous ways. Results were focused on meta-analyses and observational studies.
The references of relevant studies were included. Articles not focused on meta-analyses but rather on observational studies were individually analyzed for quality and bias using the 9-point Newcastle-Ottawa Scale, with a score of 7 or more as a cutoff for inclusion.
Determination of Risk Comparators
Data from the 2016 National Safety Council’s Injury Facts report were searched for nonmedical-related risk comparators, such as the risk of death by dog attack, by lightning, and by fire or smoke.3 Data from the 2015 US Department of Transportation Traffic Safety Facts were searched for relatable risk comparators, such as the LTR of automobile death and injury.4
Definitions
Automobile injury was defined as an injury warranting a visit to the emergency department.5 Automobile was defined as a road vehicle with 4 wheels and powered by an internal combustion engine or electric motor.6 This definition excluded light trucks, large trucks, and motorcycles.
LTR Calculation
Lifetime risk was used as the comparative measure. Lifetime risk is a type of absolute risk that depicts the probability that a specific disease or event will occur in an individual’s lifespan. The LTRof developing a disease or adverse event due to a dermatologic therapy or interventionwas denoted as LTRadverse event and calculated by the following equation7,8:

In this equation, LTRgeneral population is the LTR of developing the disease or adverse event without being subject to the therapy or intervention, and RRintervention is the relative risk (RR) from previously published RR data (relating to the development of the disease in question or an adverse event of the intervention). The use of equation (1) holds true only when the absolute risk of developing the disease or adverse event (LTRgeneral population) is low.7 Although the calculation of an LTR using a constant lifetime RR may require major approximations, studies evaluating the variation of RR over time are sparse.7,9 The Newcastle-Ottawa Scale was used to control such variance; only high-quality, nonrandomized studies were included. Although the use of residual LTR would be preferable, as LTR depends on age, such epidemiological data do not exist for complex diseases.
When not available, the LTRgeneral population was calculated from the rate of disease (cases per 100,000 individuals per year) multiplied by the average lifespan of an American (78.8 years)10:
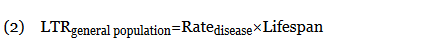
When an odds ratio (OR) was presented, its conversion to RR followed11:
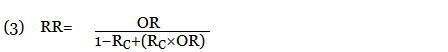
In this equation, RC is the absolute risk in the unexposed group. If the prevalence of the disease was considered low, the rare disease assumption was implemented as the following11,12:

The use of this approximation overestimates the LTR of an event. From a patient perspective, this approach is conservative. If prior LTR values were available, such as the LTR of automobile injury, automobile death, or other intervention, they were used without the need for calculation.
Unit Comparator
The LTRs of all adverse events were normalized to a unit comparator, using the LTR of an automobile injury as reference point, denoted as 1 risk unit (RU):

This equation allows for quick comparison of the magnitude of LTRs between events. Events with an RU less than 1 are less likely to occur than the risk of automobile injury; events with an RU greater than 1 are more likely than the risk of automobile injury. All RR, LTR, and unit comparators were presented as a single pooled estimate of their respective upper-limit CIs. The use of the upper-limit CI conservatively overestimates the LTR of an event.
Results
Ten dermatologic interventions were identified as illustrative, to be presented alongside the risk of automobile injury and death. The LTR of automobile injury was 32%, defined as 1.0 RU. The LTR of automobile death was 0.89% (1/36 RU).
Two events had LTRs roughly similar to automobile injury: development of a subsequent basal cell carcinoma within 3 years (1.4 RU) and development of a squamous cell carcinoma (SCC) secondary to indoor tanning (1.6 RU). Development of SCC following organ transplantation (34 RU) was considerably more likely than automobile injury. All other identified events had lower RUs than automobile injury (Table). Three events with small RUs included tuberculosis development with a tumor necrosis factor α inhibitor (1/32 RU), Crohn disease development with isotretinoin (1/41 RU), and blindness following facial hyaluronic acid injection (1/80 RU). The LTR of death by dog attack (1/42,436 RU) and death by lightning strike (1/36,542 RU) also had small RUs.

The unit comparators from the Table were adapted into graphic form to depict risk relative to the risk of automobile injury (Figure).

Comment
Numerous interventions in dermatology offer much less risk of an adverse event than the LTR of automobile injury. However, this concept of risk includes only the likelihood of development of an event, not the severity of the measured event, as our numerical and visual tool objectively captures the related risks using an RU comparator. Such use of a standardized RU demonstrates the essence of risk; “zero risk” does not exist, and each intervention or treatment, albeit how small, must be justified in concordance with other types of risk, such as the automobile.
The development of adverse events secondary to dermatologic intervention or therapy, for which monitoring visits are utilized, were used as important comparators to the risk of automobile injury. The continuous practice of monitoring visits may increase patient’s fears regarding possible adverse events secondary to therapy. Hydroxychloroquine retinal toxicity (1/16 RU) and psoriatic arthritis development following severe psoriasis (1/3.9 RU) were less likely to occur than automobile injury. The development of abnormal blood counts or blood tests secondary to therapy or intervention could not be formatted into an RU. The use of equation (1) for the calculation of LTRadverse eventholds true only when the absolute risk of developing the adverse event in the general population—in this case, abnormal blood counts or blood tests—is low.7
Although the unit comparator allows for the comparison of different dermatologic risk, a limitation of the RU model and its visual tool are a dependence on RR, a value that changes following publication of new studies. A solution was the use of a single pooled estimate to represent the upper-limit CIs of LTR. This practice overestimates risk. As with RR, new automobile injury rates are published annually.10 In the last 5 years, the LTR of automobile injury has stayed relatively constant: between 32% and 33%.4 Although the RU calculations and Figure included a wide variety of interventions in dermatology, select clinical situations were not included. It is beyond the scope of this article to systematically review all risk in dermatology but rather introduce the concept of the RU founded on automobile-associated risks. With the introduction of a methodical framework, the reader is invited to calculate RUs pertinent to their clinical interests.
Any intervention or treatment in dermatology is accompanied by risk. The use of a unit comparator using an easily relatable event—the LTR of automobile injury—allows the patient to easily compare risk and internally justify the practice of monitoring visits. Inclusion of a visual tool, such as the Figure, might alleviate many irrational fears that accompany some of the highly effective treatments and interventions used in dermatology and thus lead to better patient outcomes.
Acknowledgment
We thank Taranjeet Singh, MS (Dunn, North Carolina), for her comments on an earlier version of the manuscript.
Numerous highly efficacious treatment modalities exist in dermatology, yet patients may be highly wary of their possible adverse events, even when those risks are rare.1,2 Such fears can lead to poor medication adherence and treatment refusal. A key determinant in successful patient-provider care is to effectively communicate risk. The communication of risk is hampered by the lack of any common currency for comparing risks. The development of a standardized unit of risk could help facilitate risk comparisons, allowing physicians and patients to put risk levels into better perspective.
One easily relatable event is the risk of injury in an automobile crash. Driving, whether to the dermatology clinic for a monitoring visit or to the supermarket for weekly groceries, is associated with risk of injury and death. The risk of automobile-related injury warranting a visit to the emergency department could provide a comparator that physicians can use to give patients a more objective sense of treatment risks or to introduce the justification of a monitoring visit. The objective of this study was to develop a standard risk unit based on the lifetime risk (LTR) of automobile injury and to compare this unit of risk to various risks of dermatologic treatments.
Methods
Literature Review
We first identified common risks in dermatology that would be illustrative and then identified keywords. PubMed searches for articles indexed for MEDLINE from November 1996 to February 2017 were performed combining the following terms: (relative risk, odds ratio, lifetime risk) and (isotretinoin, IBD; melanoma, SCC, transplantation; indoor tanning, BCC, SCC; transplant and SCC; biologics and tuberculosis; hydroxychloroquine retinal toxicity; psoriasis and psoriatic arthritis). An additional search was performed in June 2018 including the term blindness and injectable fillers. Our search combined these terms in numerous ways. Results were focused on meta-analyses and observational studies.
The references of relevant studies were included. Articles not focused on meta-analyses but rather on observational studies were individually analyzed for quality and bias using the 9-point Newcastle-Ottawa Scale, with a score of 7 or more as a cutoff for inclusion.
Determination of Risk Comparators
Data from the 2016 National Safety Council’s Injury Facts report were searched for nonmedical-related risk comparators, such as the risk of death by dog attack, by lightning, and by fire or smoke.3 Data from the 2015 US Department of Transportation Traffic Safety Facts were searched for relatable risk comparators, such as the LTR of automobile death and injury.4
Definitions
Automobile injury was defined as an injury warranting a visit to the emergency department.5 Automobile was defined as a road vehicle with 4 wheels and powered by an internal combustion engine or electric motor.6 This definition excluded light trucks, large trucks, and motorcycles.
LTR Calculation
Lifetime risk was used as the comparative measure. Lifetime risk is a type of absolute risk that depicts the probability that a specific disease or event will occur in an individual’s lifespan. The LTRof developing a disease or adverse event due to a dermatologic therapy or interventionwas denoted as LTRadverse event and calculated by the following equation7,8:

In this equation, LTRgeneral population is the LTR of developing the disease or adverse event without being subject to the therapy or intervention, and RRintervention is the relative risk (RR) from previously published RR data (relating to the development of the disease in question or an adverse event of the intervention). The use of equation (1) holds true only when the absolute risk of developing the disease or adverse event (LTRgeneral population) is low.7 Although the calculation of an LTR using a constant lifetime RR may require major approximations, studies evaluating the variation of RR over time are sparse.7,9 The Newcastle-Ottawa Scale was used to control such variance; only high-quality, nonrandomized studies were included. Although the use of residual LTR would be preferable, as LTR depends on age, such epidemiological data do not exist for complex diseases.
When not available, the LTRgeneral population was calculated from the rate of disease (cases per 100,000 individuals per year) multiplied by the average lifespan of an American (78.8 years)10:
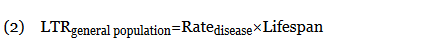
When an odds ratio (OR) was presented, its conversion to RR followed11:
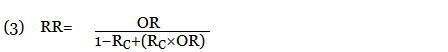
In this equation, RC is the absolute risk in the unexposed group. If the prevalence of the disease was considered low, the rare disease assumption was implemented as the following11,12:

The use of this approximation overestimates the LTR of an event. From a patient perspective, this approach is conservative. If prior LTR values were available, such as the LTR of automobile injury, automobile death, or other intervention, they were used without the need for calculation.
Unit Comparator
The LTRs of all adverse events were normalized to a unit comparator, using the LTR of an automobile injury as reference point, denoted as 1 risk unit (RU):

This equation allows for quick comparison of the magnitude of LTRs between events. Events with an RU less than 1 are less likely to occur than the risk of automobile injury; events with an RU greater than 1 are more likely than the risk of automobile injury. All RR, LTR, and unit comparators were presented as a single pooled estimate of their respective upper-limit CIs. The use of the upper-limit CI conservatively overestimates the LTR of an event.
Results
Ten dermatologic interventions were identified as illustrative, to be presented alongside the risk of automobile injury and death. The LTR of automobile injury was 32%, defined as 1.0 RU. The LTR of automobile death was 0.89% (1/36 RU).
Two events had LTRs roughly similar to automobile injury: development of a subsequent basal cell carcinoma within 3 years (1.4 RU) and development of a squamous cell carcinoma (SCC) secondary to indoor tanning (1.6 RU). Development of SCC following organ transplantation (34 RU) was considerably more likely than automobile injury. All other identified events had lower RUs than automobile injury (Table). Three events with small RUs included tuberculosis development with a tumor necrosis factor α inhibitor (1/32 RU), Crohn disease development with isotretinoin (1/41 RU), and blindness following facial hyaluronic acid injection (1/80 RU). The LTR of death by dog attack (1/42,436 RU) and death by lightning strike (1/36,542 RU) also had small RUs.

The unit comparators from the Table were adapted into graphic form to depict risk relative to the risk of automobile injury (Figure).

Comment
Numerous interventions in dermatology offer much less risk of an adverse event than the LTR of automobile injury. However, this concept of risk includes only the likelihood of development of an event, not the severity of the measured event, as our numerical and visual tool objectively captures the related risks using an RU comparator. Such use of a standardized RU demonstrates the essence of risk; “zero risk” does not exist, and each intervention or treatment, albeit how small, must be justified in concordance with other types of risk, such as the automobile.
The development of adverse events secondary to dermatologic intervention or therapy, for which monitoring visits are utilized, were used as important comparators to the risk of automobile injury. The continuous practice of monitoring visits may increase patient’s fears regarding possible adverse events secondary to therapy. Hydroxychloroquine retinal toxicity (1/16 RU) and psoriatic arthritis development following severe psoriasis (1/3.9 RU) were less likely to occur than automobile injury. The development of abnormal blood counts or blood tests secondary to therapy or intervention could not be formatted into an RU. The use of equation (1) for the calculation of LTRadverse eventholds true only when the absolute risk of developing the adverse event in the general population—in this case, abnormal blood counts or blood tests—is low.7
Although the unit comparator allows for the comparison of different dermatologic risk, a limitation of the RU model and its visual tool are a dependence on RR, a value that changes following publication of new studies. A solution was the use of a single pooled estimate to represent the upper-limit CIs of LTR. This practice overestimates risk. As with RR, new automobile injury rates are published annually.10 In the last 5 years, the LTR of automobile injury has stayed relatively constant: between 32% and 33%.4 Although the RU calculations and Figure included a wide variety of interventions in dermatology, select clinical situations were not included. It is beyond the scope of this article to systematically review all risk in dermatology but rather introduce the concept of the RU founded on automobile-associated risks. With the introduction of a methodical framework, the reader is invited to calculate RUs pertinent to their clinical interests.
Any intervention or treatment in dermatology is accompanied by risk. The use of a unit comparator using an easily relatable event—the LTR of automobile injury—allows the patient to easily compare risk and internally justify the practice of monitoring visits. Inclusion of a visual tool, such as the Figure, might alleviate many irrational fears that accompany some of the highly effective treatments and interventions used in dermatology and thus lead to better patient outcomes.
Acknowledgment
We thank Taranjeet Singh, MS (Dunn, North Carolina), for her comments on an earlier version of the manuscript.
- Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making. 2003;23:511-517.
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014;15:165-180.
- National Safety Council. Odds of dying. Injury Facts website. http://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/. Accessed November 4, 2018.
- National Center for Statistics and Analysis (NCSA) motor vehicle traffic crash data resource page. National Highway Traffic Safety Administration website. https://crashstats.nhtsa.dot.gov/#/. Accessed November 4, 2018.
- CDC report shows motor vehicle crash injuries are frequent and costly. Centers for Disease Control and Prevention website. http://www.cdc.gov/media/releases/2014/p1007-crash-injuries.html. Published October 7, 2014. Accessed November 4, 2018.
- Automobile. Business Dictionary website. http://www.businessdictionary.com/definition/automobile.html. Accessed November 4, 2018.
- Dupont WD, Plummer WD Jr. Understanding the relationship between relative and absolute risk. Cancer. 1996;77:2193-2199.
- Kaminska E, Patel I, Dabade TS, et al. Comparing the lifetime risks of TNF-alpha inhibitor use to common benchmarks of risk. J Dermatolog Treat. 2011;24:101-106.
- Dupont WD. Converting relative risks to absolute risks: a graphical approach. Stat Med. 1989;8:641-651.
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1-122.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration website. http://handbook.cochrane.org. Updated March 2011. Accessed November 15, 2018.
- Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142:761-764.
- Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207-214.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424-1429.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
- Lee SY, Jamal MM, Nguyen ET, et al. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:210-216.
- Injury Facts, 2017. Itasca, IL: National Safety Council; 2017.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-1394.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-1460.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847-857.e1-18.
- Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta Derm Venereol. 2015;95:923-927.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- National Highway Traffic Safety Administration (NHTSA). Traffic Safety Facts 2015. Washington, DC: US Department of Transportation; 2015.
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:E5909.
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-519.
- Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making. 2003;23:511-517.
- Sandoval LF, Pierce A, Feldman SR. Systemic therapies for psoriasis: an evidence-based update. Am J Clin Dermatol. 2014;15:165-180.
- National Safety Council. Odds of dying. Injury Facts website. http://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/. Accessed November 4, 2018.
- National Center for Statistics and Analysis (NCSA) motor vehicle traffic crash data resource page. National Highway Traffic Safety Administration website. https://crashstats.nhtsa.dot.gov/#/. Accessed November 4, 2018.
- CDC report shows motor vehicle crash injuries are frequent and costly. Centers for Disease Control and Prevention website. http://www.cdc.gov/media/releases/2014/p1007-crash-injuries.html. Published October 7, 2014. Accessed November 4, 2018.
- Automobile. Business Dictionary website. http://www.businessdictionary.com/definition/automobile.html. Accessed November 4, 2018.
- Dupont WD, Plummer WD Jr. Understanding the relationship between relative and absolute risk. Cancer. 1996;77:2193-2199.
- Kaminska E, Patel I, Dabade TS, et al. Comparing the lifetime risks of TNF-alpha inhibitor use to common benchmarks of risk. J Dermatolog Treat. 2011;24:101-106.
- Dupont WD. Converting relative risks to absolute risks: a graphical approach. Stat Med. 1989;8:641-651.
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1-122.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration website. http://handbook.cochrane.org. Updated March 2011. Accessed November 15, 2018.
- Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142:761-764.
- Rayess HM, Svider PF, Hanba C, et al. A cross-sectional analysis of adverse events and litigation for injectable fillers. JAMA Facial Plast Surg. 2018;20:207-214.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424-1429.
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
- Lee SY, Jamal MM, Nguyen ET, et al. Does exposure to isotretinoin increase the risk for the development of inflammatory bowel disease? A meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:210-216.
- Injury Facts, 2017. Itasca, IL: National Safety Council; 2017.
- Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386-1394.
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453-1460.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: systematic review and meta-analysis. J Am Acad Dermatol. 2014;70:847-857.e1-18.
- Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta Derm Venereol. 2015;95:923-927.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- National Highway Traffic Safety Administration (NHTSA). Traffic Safety Facts 2015. Washington, DC: US Department of Transportation; 2015.
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524-1530.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:E5909.
- Lindelöf B, Sigurgeirsson B, Gäbel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513-519.
Practice Points
- Using common identifiable risks may help patients put the risk of certain dermatologic interventions into perspective.
- Numerous interventions in dermatology offer much less risk of an adverse event than the lifetime risk of automobile injury.