User login
Expert discusses risks of biosimilars in rheumatology
SAN DIEGO – If you perceive that the use of biosimilars for rheumatic diseases hasn’t gained the traction one would expect, you’re not alone. In the opinion of J. Eugene Huffstutter, MD, the expected risks of biosimilar products have clinicians concerned, especially when it comes to immunologic characteristics.
“We’ve all had patients on biologics for years: Everything’s fine, but then the patient has an infusion reaction,” Dr. Huffstutter said at the annual meeting of the American College of Rheumatology. “I’ve had it happen with the first infusion of a biologic and I’ve had it with the 20th infusion of that same biologic product. It’s impossible to predict.”
Other unforeseen risks of biosimilars include the impact on pharmacovigilance, manufacturing, and politics. “What if our government says we want to use this agent or that agent? Or, what if it advises everyone to use the bio-originator? Then there are coverage challenges,” he said. “How are the insurance companies going to look at this? Then there are corporate impacts. The companies that make biosimilars have invested a tremendous amount of resources to bring these to market. We have a huge market in the U.S., but the uptake has been very slow. So are they going to say, ‘We’re going to take our resources and do something else?’ ”
Practice protocols for using biosimilars vary depending on your practice environment. For example, if biosimilars are administered by a freestanding practice, “you have to depend a lot on your state laws in terms of what the pharmacist is able to do,” said Dr. Huffstutter, who is also the past president of the Tennessee Rheumatology Society. “What are the patient support programs available for that biosimilar compared with bio-originator? What do the insurance contracts say in terms of can you get this filled or not? What’s the formulary status? How well do physicians in your community accept biosimilars? More importantly, how do your patients feel about biosimilars? I think they’ve been left out of this whole discussion.”
Dr. Huffstutter disclosed that he has received research support from GlaxoSmithKline, Pfizer, and UCB. He is also a member of the speakers bureau and/or is an adviser to Janssen, Lilly, Pfizer, Genentech, Novartis, Regeneron, and UCB.
SAN DIEGO – If you perceive that the use of biosimilars for rheumatic diseases hasn’t gained the traction one would expect, you’re not alone. In the opinion of J. Eugene Huffstutter, MD, the expected risks of biosimilar products have clinicians concerned, especially when it comes to immunologic characteristics.
“We’ve all had patients on biologics for years: Everything’s fine, but then the patient has an infusion reaction,” Dr. Huffstutter said at the annual meeting of the American College of Rheumatology. “I’ve had it happen with the first infusion of a biologic and I’ve had it with the 20th infusion of that same biologic product. It’s impossible to predict.”
Other unforeseen risks of biosimilars include the impact on pharmacovigilance, manufacturing, and politics. “What if our government says we want to use this agent or that agent? Or, what if it advises everyone to use the bio-originator? Then there are coverage challenges,” he said. “How are the insurance companies going to look at this? Then there are corporate impacts. The companies that make biosimilars have invested a tremendous amount of resources to bring these to market. We have a huge market in the U.S., but the uptake has been very slow. So are they going to say, ‘We’re going to take our resources and do something else?’ ”
Practice protocols for using biosimilars vary depending on your practice environment. For example, if biosimilars are administered by a freestanding practice, “you have to depend a lot on your state laws in terms of what the pharmacist is able to do,” said Dr. Huffstutter, who is also the past president of the Tennessee Rheumatology Society. “What are the patient support programs available for that biosimilar compared with bio-originator? What do the insurance contracts say in terms of can you get this filled or not? What’s the formulary status? How well do physicians in your community accept biosimilars? More importantly, how do your patients feel about biosimilars? I think they’ve been left out of this whole discussion.”
Dr. Huffstutter disclosed that he has received research support from GlaxoSmithKline, Pfizer, and UCB. He is also a member of the speakers bureau and/or is an adviser to Janssen, Lilly, Pfizer, Genentech, Novartis, Regeneron, and UCB.
SAN DIEGO – If you perceive that the use of biosimilars for rheumatic diseases hasn’t gained the traction one would expect, you’re not alone. In the opinion of J. Eugene Huffstutter, MD, the expected risks of biosimilar products have clinicians concerned, especially when it comes to immunologic characteristics.
“We’ve all had patients on biologics for years: Everything’s fine, but then the patient has an infusion reaction,” Dr. Huffstutter said at the annual meeting of the American College of Rheumatology. “I’ve had it happen with the first infusion of a biologic and I’ve had it with the 20th infusion of that same biologic product. It’s impossible to predict.”
Other unforeseen risks of biosimilars include the impact on pharmacovigilance, manufacturing, and politics. “What if our government says we want to use this agent or that agent? Or, what if it advises everyone to use the bio-originator? Then there are coverage challenges,” he said. “How are the insurance companies going to look at this? Then there are corporate impacts. The companies that make biosimilars have invested a tremendous amount of resources to bring these to market. We have a huge market in the U.S., but the uptake has been very slow. So are they going to say, ‘We’re going to take our resources and do something else?’ ”
Practice protocols for using biosimilars vary depending on your practice environment. For example, if biosimilars are administered by a freestanding practice, “you have to depend a lot on your state laws in terms of what the pharmacist is able to do,” said Dr. Huffstutter, who is also the past president of the Tennessee Rheumatology Society. “What are the patient support programs available for that biosimilar compared with bio-originator? What do the insurance contracts say in terms of can you get this filled or not? What’s the formulary status? How well do physicians in your community accept biosimilars? More importantly, how do your patients feel about biosimilars? I think they’ve been left out of this whole discussion.”
Dr. Huffstutter disclosed that he has received research support from GlaxoSmithKline, Pfizer, and UCB. He is also a member of the speakers bureau and/or is an adviser to Janssen, Lilly, Pfizer, Genentech, Novartis, Regeneron, and UCB.
EXPERT ANALYSIS FROM ACR 2017
VA, Kaiser lauded for hypertension control
At a time when the Department of Veterans Affairs is criticized for the care it delivers, and when some also see it threatened by privatization, it was refreshing to hear the VA praised for the quality of its hypertension care, a model for success in a new era of reduced blood pressure treatment targets and revised hypertension guidelines that classify millions more Americans as having hypertension.
“In systems of care, like the VA and Kaiser Permanente Northern California, we are doing much better with hypertension control, reaching control rates greater than 90%,” Paul Whelton, MD, said in November during a talk at the American Heart Association scientific sessions in Anaheim, Calif. In a separate report at the same meeting, Dr. Whelton, a professor of public health at Tulane University in New Orleans, first presented the new hypertension diagnosis and management guidelines, produced by the American College of Cardiology/American Heart Association panel that he chaired (J Am Coll Cardiol. 2017 Nov 13. doi: 10.1016/j.jacc.2017.11.006).
He again stressed that the VA and Kaiser are doing “remarkably well” when it came to controlling hypertension in the vast majority of their patients.
That assessment seems especially appropriate for Kaiser Permanente Northern California, Oakland, Calif. Data from an audit of Kaiser’s hypertension registry showed that during 2000-2013 the percentage of patients with hypertension at their goal blood pressure rose from 44% in 2000 to 90% in 2013 (J Clin Hypertension. 2016 April;18[4]:260-1). The two Kaiser researchers who reported these findings attributed the rise in control rates to a hypertension treatment program that Kaiser Permanente Northern California put into practice starting in 2000.
Current success in the VA Health System is harder to pin down and put in the Kaiser ballpark. The most up-to-date audit I could find was a 2012 report from a team of VA researchers who reviewed control rates of more than half a million hypertensive veterans at 15 VA medical centers during 2000-2010. They found that, during that 11-year period, the percentage of hypertensive patients with their blood pressure controlled to their target level had risen from 46% in 2000 to 76% in 2010 (Circulation. 2012 May 22;125[20]:2462-68).
While this 76% rate of control in 2010 is short of the 90% rate in Kaiser in 2013, it’s still not shabby. To put the 76% control rate in perspective, consider data reported at the AHA meeting from TargetBP, a national program begun in late 2015 to aid all U.S. health care programs in improving their hypertension control rates: This data showed that, among 310 participating programs that filed 2016 control-rate data with TargetBP, the average control rate was 66%. Specifically, of those 310 reporting programs, 191 (62%) had control rates that exceeded 70%, with an average control rate among these more successful programs of 76%.
As-yet-unpublished data collected by the VA show that other centers in the system beyond those 15 included in the study discussed above have also recently reached a similar control level of about 75%, said Vasilios Papademetriou, MD, a professor of medicine at Georgetown University and the director of the Interventional Hypertension and Vascular Medicine Program at the VA Medical Center in Washington. Plus, certain VA centers are now up to an 85% control rate, he added in an interview. “Blood pressure control rates have been exceptionally good in the VA medical system,” he declared. Dr. Papademetriou attributed the rising control rates to a concerted hypertension program the VA instituted starting in the early 2000s.
“The VA has had some physicians who have championed this issue, and they have built computer-based systems to identify patients with uncontrolled hypertension, and then they plug these patients into their care algorithms,” commented Donald M. Lloyd-Jones, MD, a professor and chairman of preventive medicine at Northwestern University in Chicago. “Often, when there are champions, things change,” he noted.
William C. Cushman, MD, a hypertension specialist who is chief of preventive medicine for the VA Medical Center in Memphis and professor of preventive medicine at the University of Tennessee, also in Memphis, highlighted several steps the VA took that have helped fuel the program’s success in controlling blood pressure.
Dr. Cushman couldn’t resist adding that this successful approach to hypertension management is now threatened by potential changes to the VA system that could take some patients out of the existing program and move them to privatized medical care. “If that happens, patients will not get the same comprehensive care” that until now has produced such high rates of hypertension control, he warned.
[email protected]
On Twitter @mitchelzoler
At a time when the Department of Veterans Affairs is criticized for the care it delivers, and when some also see it threatened by privatization, it was refreshing to hear the VA praised for the quality of its hypertension care, a model for success in a new era of reduced blood pressure treatment targets and revised hypertension guidelines that classify millions more Americans as having hypertension.
“In systems of care, like the VA and Kaiser Permanente Northern California, we are doing much better with hypertension control, reaching control rates greater than 90%,” Paul Whelton, MD, said in November during a talk at the American Heart Association scientific sessions in Anaheim, Calif. In a separate report at the same meeting, Dr. Whelton, a professor of public health at Tulane University in New Orleans, first presented the new hypertension diagnosis and management guidelines, produced by the American College of Cardiology/American Heart Association panel that he chaired (J Am Coll Cardiol. 2017 Nov 13. doi: 10.1016/j.jacc.2017.11.006).
He again stressed that the VA and Kaiser are doing “remarkably well” when it came to controlling hypertension in the vast majority of their patients.
That assessment seems especially appropriate for Kaiser Permanente Northern California, Oakland, Calif. Data from an audit of Kaiser’s hypertension registry showed that during 2000-2013 the percentage of patients with hypertension at their goal blood pressure rose from 44% in 2000 to 90% in 2013 (J Clin Hypertension. 2016 April;18[4]:260-1). The two Kaiser researchers who reported these findings attributed the rise in control rates to a hypertension treatment program that Kaiser Permanente Northern California put into practice starting in 2000.
Current success in the VA Health System is harder to pin down and put in the Kaiser ballpark. The most up-to-date audit I could find was a 2012 report from a team of VA researchers who reviewed control rates of more than half a million hypertensive veterans at 15 VA medical centers during 2000-2010. They found that, during that 11-year period, the percentage of hypertensive patients with their blood pressure controlled to their target level had risen from 46% in 2000 to 76% in 2010 (Circulation. 2012 May 22;125[20]:2462-68).
While this 76% rate of control in 2010 is short of the 90% rate in Kaiser in 2013, it’s still not shabby. To put the 76% control rate in perspective, consider data reported at the AHA meeting from TargetBP, a national program begun in late 2015 to aid all U.S. health care programs in improving their hypertension control rates: This data showed that, among 310 participating programs that filed 2016 control-rate data with TargetBP, the average control rate was 66%. Specifically, of those 310 reporting programs, 191 (62%) had control rates that exceeded 70%, with an average control rate among these more successful programs of 76%.
As-yet-unpublished data collected by the VA show that other centers in the system beyond those 15 included in the study discussed above have also recently reached a similar control level of about 75%, said Vasilios Papademetriou, MD, a professor of medicine at Georgetown University and the director of the Interventional Hypertension and Vascular Medicine Program at the VA Medical Center in Washington. Plus, certain VA centers are now up to an 85% control rate, he added in an interview. “Blood pressure control rates have been exceptionally good in the VA medical system,” he declared. Dr. Papademetriou attributed the rising control rates to a concerted hypertension program the VA instituted starting in the early 2000s.
“The VA has had some physicians who have championed this issue, and they have built computer-based systems to identify patients with uncontrolled hypertension, and then they plug these patients into their care algorithms,” commented Donald M. Lloyd-Jones, MD, a professor and chairman of preventive medicine at Northwestern University in Chicago. “Often, when there are champions, things change,” he noted.
William C. Cushman, MD, a hypertension specialist who is chief of preventive medicine for the VA Medical Center in Memphis and professor of preventive medicine at the University of Tennessee, also in Memphis, highlighted several steps the VA took that have helped fuel the program’s success in controlling blood pressure.
Dr. Cushman couldn’t resist adding that this successful approach to hypertension management is now threatened by potential changes to the VA system that could take some patients out of the existing program and move them to privatized medical care. “If that happens, patients will not get the same comprehensive care” that until now has produced such high rates of hypertension control, he warned.
[email protected]
On Twitter @mitchelzoler
At a time when the Department of Veterans Affairs is criticized for the care it delivers, and when some also see it threatened by privatization, it was refreshing to hear the VA praised for the quality of its hypertension care, a model for success in a new era of reduced blood pressure treatment targets and revised hypertension guidelines that classify millions more Americans as having hypertension.
“In systems of care, like the VA and Kaiser Permanente Northern California, we are doing much better with hypertension control, reaching control rates greater than 90%,” Paul Whelton, MD, said in November during a talk at the American Heart Association scientific sessions in Anaheim, Calif. In a separate report at the same meeting, Dr. Whelton, a professor of public health at Tulane University in New Orleans, first presented the new hypertension diagnosis and management guidelines, produced by the American College of Cardiology/American Heart Association panel that he chaired (J Am Coll Cardiol. 2017 Nov 13. doi: 10.1016/j.jacc.2017.11.006).
He again stressed that the VA and Kaiser are doing “remarkably well” when it came to controlling hypertension in the vast majority of their patients.
That assessment seems especially appropriate for Kaiser Permanente Northern California, Oakland, Calif. Data from an audit of Kaiser’s hypertension registry showed that during 2000-2013 the percentage of patients with hypertension at their goal blood pressure rose from 44% in 2000 to 90% in 2013 (J Clin Hypertension. 2016 April;18[4]:260-1). The two Kaiser researchers who reported these findings attributed the rise in control rates to a hypertension treatment program that Kaiser Permanente Northern California put into practice starting in 2000.
Current success in the VA Health System is harder to pin down and put in the Kaiser ballpark. The most up-to-date audit I could find was a 2012 report from a team of VA researchers who reviewed control rates of more than half a million hypertensive veterans at 15 VA medical centers during 2000-2010. They found that, during that 11-year period, the percentage of hypertensive patients with their blood pressure controlled to their target level had risen from 46% in 2000 to 76% in 2010 (Circulation. 2012 May 22;125[20]:2462-68).
While this 76% rate of control in 2010 is short of the 90% rate in Kaiser in 2013, it’s still not shabby. To put the 76% control rate in perspective, consider data reported at the AHA meeting from TargetBP, a national program begun in late 2015 to aid all U.S. health care programs in improving their hypertension control rates: This data showed that, among 310 participating programs that filed 2016 control-rate data with TargetBP, the average control rate was 66%. Specifically, of those 310 reporting programs, 191 (62%) had control rates that exceeded 70%, with an average control rate among these more successful programs of 76%.
As-yet-unpublished data collected by the VA show that other centers in the system beyond those 15 included in the study discussed above have also recently reached a similar control level of about 75%, said Vasilios Papademetriou, MD, a professor of medicine at Georgetown University and the director of the Interventional Hypertension and Vascular Medicine Program at the VA Medical Center in Washington. Plus, certain VA centers are now up to an 85% control rate, he added in an interview. “Blood pressure control rates have been exceptionally good in the VA medical system,” he declared. Dr. Papademetriou attributed the rising control rates to a concerted hypertension program the VA instituted starting in the early 2000s.
“The VA has had some physicians who have championed this issue, and they have built computer-based systems to identify patients with uncontrolled hypertension, and then they plug these patients into their care algorithms,” commented Donald M. Lloyd-Jones, MD, a professor and chairman of preventive medicine at Northwestern University in Chicago. “Often, when there are champions, things change,” he noted.
William C. Cushman, MD, a hypertension specialist who is chief of preventive medicine for the VA Medical Center in Memphis and professor of preventive medicine at the University of Tennessee, also in Memphis, highlighted several steps the VA took that have helped fuel the program’s success in controlling blood pressure.
Dr. Cushman couldn’t resist adding that this successful approach to hypertension management is now threatened by potential changes to the VA system that could take some patients out of the existing program and move them to privatized medical care. “If that happens, patients will not get the same comprehensive care” that until now has produced such high rates of hypertension control, he warned.
[email protected]
On Twitter @mitchelzoler
ADHD and insomnia appear intertwined
PARIS – Converging evidence suggests that attention-deficit/hyperactivity disorder and sleep difficulties share a common underlying etiology involving circadian rhythm disturbance, J.J. Sandra Kooij, MD, PhD, declared at the annual congress of the European College of Neuropsychopharmacology.
“If you review the evidence, it looks more and more like ADHD and sleeplessness are two sides of the same physiological and mental coin,” said Dr. Kooij, a psychiatrist at VU University Medical Center, Amsterdam, and chair of the European Network Adult ADHD.
Since this study remains ongoing, Dr. Kooij focused instead on the evidence suggesting that ADHD and sleep problems have a shared etiology.
Multiple studies have shown that roughly 75% of children and adults with ADHD have sleep-onset insomnia. It takes them longer to fall asleep, and they have a shorter than normal sleep duration because they have to get up in the morning for school or work. Dr. Kooij and her colleagues have shown that in adult ADHD patients with delayed sleep onset syndrome, their evening dim light melatonin onset, change in core body temperature, and other physiologic harbingers of sleep are delayed by an average of 1.5 hours (J Sleep Res. 2013 Dec;22[6]:607-16).
“My ADHD patients sleep a mean of 5-6 hours per night on a chronic basis, versus 7-8 hours in normal individuals. It leads to daytime sleepiness and dysfunction due to inattentiveness and social problems,” the psychiatrist said.
Other investigators have demonstrated that the prevalence of ADHD varies across the United States and that geographic differences in solar intensity explain 34%-57% of this variance in ADHD rates. The investigators postulated that the ADHD-preventive effect of high solar irradiation might be tied to improvement in circadian clock disturbances (Biol Psychiatry. 2013 Oct 15;74[8]:585-90).
In a study of 2,090 adult participants in The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues showed that ADHD, depression, anxiety, and circadian rhythm sleep problems are fellow travelers.
The prevalence of sleep duration of fewer than 6 hours per night was 15% in subjects with high ADHD symptoms and a lifetime history of an anxiety and/or depression diagnosis, 5% in those with lifetime anxiety/depression but no ADHD, and 4% in healthy controls. Delayed sleep phase syndrome was present in 16% of individuals with ADHD and a history of depression and/or anxiety, 8% in those with a lifetime history of anxiety/depression without ADHD, and 5% of healthy controls. The take-home message: Circadian rhythm sleep disorders in patients with ADHD are not necessarily attributable to comorbid anxiety and/or depression (J Affect Disord. 2016 Aug;200:74-81).
Seasonal affective disorder is commonly comorbid with ADHD. In another analysis from The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues determined that the prevalence of probable seasonal affective disorder using the Seasonal Pattern Assessment Questionnaire was 9.9% in participants with clinically significant ADHD symptoms, compared with 3.3% in the non-ADHD subjects. Self-reported delayed sleep onset was extremely common in participants with ADHD as well as in those with probable seasonal affective disorder (J Psychiat Res. 2016 Oct;81:87-94).
Patients with ADHD have an increased prevalence of obesity. Their chronic short sleep pushes them toward an unstable eating pattern in which they skip breakfast, then engage in binge eating later in the day. The hope is that treating the circadian rhythm disruption associated with ADHD will prevent obesity in this population (J Psychosom Res. 2015 Nov;79[5]:443-50).
“If you mess up sleep, you mess up the body: bowel movements, blood pressure, body temperature, the leptin/ghrelin ratio, reaction time, coordination. That’s why I call my patients chronically jet-lagged,” Dr. Kooij said.
She reported having no financial conflicts regarding her presentation.
PARIS – Converging evidence suggests that attention-deficit/hyperactivity disorder and sleep difficulties share a common underlying etiology involving circadian rhythm disturbance, J.J. Sandra Kooij, MD, PhD, declared at the annual congress of the European College of Neuropsychopharmacology.
“If you review the evidence, it looks more and more like ADHD and sleeplessness are two sides of the same physiological and mental coin,” said Dr. Kooij, a psychiatrist at VU University Medical Center, Amsterdam, and chair of the European Network Adult ADHD.
Since this study remains ongoing, Dr. Kooij focused instead on the evidence suggesting that ADHD and sleep problems have a shared etiology.
Multiple studies have shown that roughly 75% of children and adults with ADHD have sleep-onset insomnia. It takes them longer to fall asleep, and they have a shorter than normal sleep duration because they have to get up in the morning for school or work. Dr. Kooij and her colleagues have shown that in adult ADHD patients with delayed sleep onset syndrome, their evening dim light melatonin onset, change in core body temperature, and other physiologic harbingers of sleep are delayed by an average of 1.5 hours (J Sleep Res. 2013 Dec;22[6]:607-16).
“My ADHD patients sleep a mean of 5-6 hours per night on a chronic basis, versus 7-8 hours in normal individuals. It leads to daytime sleepiness and dysfunction due to inattentiveness and social problems,” the psychiatrist said.
Other investigators have demonstrated that the prevalence of ADHD varies across the United States and that geographic differences in solar intensity explain 34%-57% of this variance in ADHD rates. The investigators postulated that the ADHD-preventive effect of high solar irradiation might be tied to improvement in circadian clock disturbances (Biol Psychiatry. 2013 Oct 15;74[8]:585-90).
In a study of 2,090 adult participants in The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues showed that ADHD, depression, anxiety, and circadian rhythm sleep problems are fellow travelers.
The prevalence of sleep duration of fewer than 6 hours per night was 15% in subjects with high ADHD symptoms and a lifetime history of an anxiety and/or depression diagnosis, 5% in those with lifetime anxiety/depression but no ADHD, and 4% in healthy controls. Delayed sleep phase syndrome was present in 16% of individuals with ADHD and a history of depression and/or anxiety, 8% in those with a lifetime history of anxiety/depression without ADHD, and 5% of healthy controls. The take-home message: Circadian rhythm sleep disorders in patients with ADHD are not necessarily attributable to comorbid anxiety and/or depression (J Affect Disord. 2016 Aug;200:74-81).
Seasonal affective disorder is commonly comorbid with ADHD. In another analysis from The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues determined that the prevalence of probable seasonal affective disorder using the Seasonal Pattern Assessment Questionnaire was 9.9% in participants with clinically significant ADHD symptoms, compared with 3.3% in the non-ADHD subjects. Self-reported delayed sleep onset was extremely common in participants with ADHD as well as in those with probable seasonal affective disorder (J Psychiat Res. 2016 Oct;81:87-94).
Patients with ADHD have an increased prevalence of obesity. Their chronic short sleep pushes them toward an unstable eating pattern in which they skip breakfast, then engage in binge eating later in the day. The hope is that treating the circadian rhythm disruption associated with ADHD will prevent obesity in this population (J Psychosom Res. 2015 Nov;79[5]:443-50).
“If you mess up sleep, you mess up the body: bowel movements, blood pressure, body temperature, the leptin/ghrelin ratio, reaction time, coordination. That’s why I call my patients chronically jet-lagged,” Dr. Kooij said.
She reported having no financial conflicts regarding her presentation.
PARIS – Converging evidence suggests that attention-deficit/hyperactivity disorder and sleep difficulties share a common underlying etiology involving circadian rhythm disturbance, J.J. Sandra Kooij, MD, PhD, declared at the annual congress of the European College of Neuropsychopharmacology.
“If you review the evidence, it looks more and more like ADHD and sleeplessness are two sides of the same physiological and mental coin,” said Dr. Kooij, a psychiatrist at VU University Medical Center, Amsterdam, and chair of the European Network Adult ADHD.
Since this study remains ongoing, Dr. Kooij focused instead on the evidence suggesting that ADHD and sleep problems have a shared etiology.
Multiple studies have shown that roughly 75% of children and adults with ADHD have sleep-onset insomnia. It takes them longer to fall asleep, and they have a shorter than normal sleep duration because they have to get up in the morning for school or work. Dr. Kooij and her colleagues have shown that in adult ADHD patients with delayed sleep onset syndrome, their evening dim light melatonin onset, change in core body temperature, and other physiologic harbingers of sleep are delayed by an average of 1.5 hours (J Sleep Res. 2013 Dec;22[6]:607-16).
“My ADHD patients sleep a mean of 5-6 hours per night on a chronic basis, versus 7-8 hours in normal individuals. It leads to daytime sleepiness and dysfunction due to inattentiveness and social problems,” the psychiatrist said.
Other investigators have demonstrated that the prevalence of ADHD varies across the United States and that geographic differences in solar intensity explain 34%-57% of this variance in ADHD rates. The investigators postulated that the ADHD-preventive effect of high solar irradiation might be tied to improvement in circadian clock disturbances (Biol Psychiatry. 2013 Oct 15;74[8]:585-90).
In a study of 2,090 adult participants in The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues showed that ADHD, depression, anxiety, and circadian rhythm sleep problems are fellow travelers.
The prevalence of sleep duration of fewer than 6 hours per night was 15% in subjects with high ADHD symptoms and a lifetime history of an anxiety and/or depression diagnosis, 5% in those with lifetime anxiety/depression but no ADHD, and 4% in healthy controls. Delayed sleep phase syndrome was present in 16% of individuals with ADHD and a history of depression and/or anxiety, 8% in those with a lifetime history of anxiety/depression without ADHD, and 5% of healthy controls. The take-home message: Circadian rhythm sleep disorders in patients with ADHD are not necessarily attributable to comorbid anxiety and/or depression (J Affect Disord. 2016 Aug;200:74-81).
Seasonal affective disorder is commonly comorbid with ADHD. In another analysis from The Netherlands Study of Depression and Anxiety, Dr. Kooij and her colleagues determined that the prevalence of probable seasonal affective disorder using the Seasonal Pattern Assessment Questionnaire was 9.9% in participants with clinically significant ADHD symptoms, compared with 3.3% in the non-ADHD subjects. Self-reported delayed sleep onset was extremely common in participants with ADHD as well as in those with probable seasonal affective disorder (J Psychiat Res. 2016 Oct;81:87-94).
Patients with ADHD have an increased prevalence of obesity. Their chronic short sleep pushes them toward an unstable eating pattern in which they skip breakfast, then engage in binge eating later in the day. The hope is that treating the circadian rhythm disruption associated with ADHD will prevent obesity in this population (J Psychosom Res. 2015 Nov;79[5]:443-50).
“If you mess up sleep, you mess up the body: bowel movements, blood pressure, body temperature, the leptin/ghrelin ratio, reaction time, coordination. That’s why I call my patients chronically jet-lagged,” Dr. Kooij said.
She reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
ASCT or novel therapies in early relapsing follicular lymphoma?
NEW YORK – For patients with newly diagnosed follicular lymphoma and other indolent non-Hodgkin lymphoma, the combination of bendamustine (Treanda) and rituximab is associated with significantly better progression-free survival (PFS) and longer time-to-next treatment than is rituximab plus CHOP chemotherapy, results of the BRIGHT study indicate.
But That was the question taken on in a debate at an international congress on hematologic malignancies by Carla Casulo, MD of the James P. Wilmot Cancer Institute at the University of Rochester (N.Y.), and Brian K. Link, MD, of the University of Iowa Hospitals & Clinics in Iowa City.
Dr. Casulo: ASCT
“Follicular lymphoma with a short remission duration has been established as a poor prognostic marker for survival, and the optimal therapy for these patients is really not known,” Dr. Casulo said.
“Of course, [novel] therapies can be considered, I think, for the appropriate patient, and hopefully in the context of a clinical study,” she added.
To lay out her argument for ASCT, Dr. Casulo pointed to four studies suggesting that about 20% of patients with follicular lymphoma will experience disease progression within 24 months of chemoimmunotherapy. Similar patterns of progression at 24 months were seen with R-CHOP in the SWOG S0016 trial, with both bendamustine-rituximab and R-CHOP in the StiL Study, with lenalidomide (Revlimid) and rituximab in a phase 3 clinical trial, and with one of three rituximab-based immunochemotherapy regimens in the PRIMA trial.
The results from these trials suggest that “there is an inherent biology to this population that relapses early, regardless of what induction strategy is used. However, what’s not known, until now, is whether early relapse implies poor survival in this disease,” she said.
To examine this question Dr. Casulo and her colleagues performed an analysis of time to progression among patients with newly-diagnosed follicular lymphoma treated with R-CHOP who were enrolled in the National LymphoCare Study (NLCS). “What we found was that there were very poor outcomes associated with early-relapsing follicular lymphoma,” she said.
Overall survival (OS) at 8 years was 50% for patients with disease progression within 24 months of therapy, compared with 90% for patients who did not have early progression, a finding that was validated in a cohort of patients from the University of Iowa and the Mayo Clinic in which 8-year OS for early progressors was 34%, compared with 90% for other patients. The results held up when the researchers controlled for Follicular Lymphoma International Prognostic Index scores and in patients treated with rituximab and the cyclophosphamide, vincristine, and prednisone regimen rather than CHOP, Dr. Casulo noted.
“So, given these findings, how does one navigate the treatment landscape for patients with early relapsing follicular lymphoma? The reality is that there is really no standard of care or best approach,” she said.
“Ultimately, the goal of therapy, at least in my opinion, should be overcoming the chemoresistance that’s inherent to this biology, and establishing durable disease control, and there are a couple of strategies that might be able to achieve that,” she added.
There have been only two clinical trials of ASCT in patients with relapsed follicular lymphoma.
In the CUP trial, initiated prior to the introduction of rituximab, 89 patients with relapsed follicular lymphoma were treated with three cycles of CHOP, and those with a response were then randomized to either purged or unpurged ASCT, or to three additional cycles of CHOP. Four-year OS in this study was 70% for patients who underwent ASCT vs. 50% for those who received six cycles of CHOP.
In the EBMT LYM1 trial, 280 rituximab-naive patients with relapsed follicular lymphoma after a partial or complete remission were randomized to rituximab-purged or unpurged ASCT, followed by randomization to observation or rituximab maintenance. In this trial, the 10-year OS with ASCT ranged from 68% to 73%.
A Spanish registry study presented in a poster session at the 14th International Conference on Malignant Lymphoma in Lugano, Italy, showed long-term efficacy of ASCT in relapsed follicular lymphoma, with plateaus in both PFS and OS about 9 years after transplant for both rituximab-exposed and rituximab-naive patients, “suggesting that perhaps a subset of patients with relapsed follicular lymphoma can be cured with this approach,” Dr. Casulo said.
Similarly, a trial from the German Low Grade Lymphoma Studies group, presented at the 2016 American Society of Hematology annual meeting, showed 5-year OS of 77% with ASCT vs. 46% for patients who did not receive a transplant.
Dr. Casulo and her colleagues collaborated with investigators at the Center for International Blood and Marrow Transplant Research and the NLCS to see whether ASCT can improve OS compared with no transplant in patients with early-relapsed follicular lymphoma. They found that patients who received ASCT within 1 year of therapy failure had a 5-year OS of 73%, compared with 60% for those who did not receive ASCT (P = .02).
She acknowledged that toxicities associated with ASCT are a concern, pointing to a 2007 study looking at long-term follow-up of myeloablative ASCT for follicular lymphoma at the time of second or subsequent remission. The investigators found that rates of myelodysplasia were as high as 20% at 10 years, especially among patients who had undergone total body irradiation, a practice that has since fallen out of favor.
A separate study led by Matt Kalaycio, MD, of the Cleveland Clinic, showed that more lines of prior therapy (4-6 vs. 1-3) and radiation were both risk factors for subsequent myelodysplastic syndrome and acute myeloid leukemia.
“I hope we have demonstrated that autologous transplant can have durable response in these patients, with possibly a cure in a subset; but, ultimately, I think strategies that combine novel agents and autologous transplant in a clinical trial are the way to go to improve outcomes,” she said.
Dr. Link: Novel agents
“I actually happen to agree with very much of what Carla had to share, but I do have a couple of caveats,” Dr. Link said.
“The focus of this discussion is on patients with high-risk follicular lymphoma, as Carla very carefully defined in her analysis of the NLCS. These are the early progressors,” he said.
He cited data from the University of Iowa/Mayo Clinic series, validated in a cohort of patients from Lyon, France, showing that high-risk patients with early progression after immunochemotherapy had “especially poor outcomes.” In contrast, patients who were not early progressors fared quite well.
“It suggested that with agents that were available as of 2015, if you’re not an early progressor, your survival at least matches, or essentially matches with statistical power, that of the expected age- and gender-matched populations. So, novel agents are not required necessarily nor are clinical trials necessarily required for people who have good early outcomes,” Dr. Link said.
The best snapshot of current practice for high-risk patients comes from unpublished data from the NLCS showing that after a median follow-up of 8 years, 889 of 2,652 patients had received a second line of therapy, with the choice of agents or approaches generally similar between early progressors and others.
Early progressors were slightly less likely to receive rituximab monotherapy (30% vs. 36%) or an investigational agent (4.4% vs. 5.5%), whereas they were slightly more likely to receive an anthracycline (18% vs. 13%) or to undergo ASCT (3.5% vs. 1.1%).
In the treatment of patients with high-risk follicular lymphoma, a novel agent can be considered as one that was either not available or had not been used in follicular lymphoma when the previously mentioned survival data were generated, including immunomodulators such as thalidomide analogues, targeted kinase inhibitors, new anti-CD20 antibodies such as obinutuzumab (Gazyva), and immune checkpoint inhibitors.
For example, in Alliance 50803, a phase 2 trial in patients with previously untreated stage II-IV follicular lymphoma, the combination of lenalidomide (Revlimid) and rituximab was associated with a 95% overall response rate (ORR), including 72% complete response, and 5-year PFS rate of 70%, comparable to trials with rituximab plus bendamustine, CHOP, or cyclophosphamide-vincristine-prednisone, Dr. Link said.
In the phase 2 GALEN study, the combination of lenalidomide and obinutuzumab was associated with an ORR of 74% among 86 patients with relapsed/refractory follicular lymphoma, with a 1-year PFS rate of 76%.
An analysis of responses by time to relapse in GALEN showed that the ORR among 24 patients with disease progression within 24 months was 70.8%, including 33.3% complete or unconfirmed complete responses by the 1999 International Working Group criteria, and 66.7% with 54.2% complete or unconfirmed complete responses by the 2007 criteria.
Idelalisib, an inhibitor of the delta isoform of phosphatidylinositol 3-kinase (PI3K), was granted accelerated approval in 2014 for treatment of patients with follicular lymphoma after two or more prior lines of therapy, but toxicities associated with this agent caused the drug maker Gilead to dial back its development of this agent.
“But idelalisib is not the only PI3 kinase inhibitor on the block,” Dr. Link said, noting that more than a dozen similar agents are currently in development.
In clinical trials, PI3 kinase inhibitors have been associated with ORRs of about 60% in patients who experience early disease progression on other therapies, “suggesting an uncoupling between the paradigm that says that early progressors are going to have a less effective outcome than late progressors, perhaps, with targeted therapies.
The best evidence for the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) comes from the DAWN study, a phase 2 trial in patients with follicular lymphoma refractory to immunochemotherapy. The drug showed some biologic activity, but only a 21% ORR.
Dr. Link noted that the S1608 trial, currently recruiting patients, may give clinicians a better idea of which novel agent is most effective. The phase 2 trial is enrolling patients with early-progressing or refractory follicular lymphoma who will be randomized to receive obinutuzumab with either the investigational PI3 kinase inhibitor TGR-1202, lenalidomide, or CHOP chemotherapy.
“High-risk follicular lymphoma is a bad hombre,” Dr. Link said. “If we want to be any smarter as a society 10 years from now, we should incorporate clinical trials with novel therapies as standard operating practice into this setting of high-risk follicular lymphoma.”
Dr. Casulo reported serving on the speakers bureau for Gilead. Dr. Link reported serving as a consultant to AbbVie, Celgene, Genentech, and Gilead.
NEW YORK – For patients with newly diagnosed follicular lymphoma and other indolent non-Hodgkin lymphoma, the combination of bendamustine (Treanda) and rituximab is associated with significantly better progression-free survival (PFS) and longer time-to-next treatment than is rituximab plus CHOP chemotherapy, results of the BRIGHT study indicate.
But That was the question taken on in a debate at an international congress on hematologic malignancies by Carla Casulo, MD of the James P. Wilmot Cancer Institute at the University of Rochester (N.Y.), and Brian K. Link, MD, of the University of Iowa Hospitals & Clinics in Iowa City.
Dr. Casulo: ASCT
“Follicular lymphoma with a short remission duration has been established as a poor prognostic marker for survival, and the optimal therapy for these patients is really not known,” Dr. Casulo said.
“Of course, [novel] therapies can be considered, I think, for the appropriate patient, and hopefully in the context of a clinical study,” she added.
To lay out her argument for ASCT, Dr. Casulo pointed to four studies suggesting that about 20% of patients with follicular lymphoma will experience disease progression within 24 months of chemoimmunotherapy. Similar patterns of progression at 24 months were seen with R-CHOP in the SWOG S0016 trial, with both bendamustine-rituximab and R-CHOP in the StiL Study, with lenalidomide (Revlimid) and rituximab in a phase 3 clinical trial, and with one of three rituximab-based immunochemotherapy regimens in the PRIMA trial.
The results from these trials suggest that “there is an inherent biology to this population that relapses early, regardless of what induction strategy is used. However, what’s not known, until now, is whether early relapse implies poor survival in this disease,” she said.
To examine this question Dr. Casulo and her colleagues performed an analysis of time to progression among patients with newly-diagnosed follicular lymphoma treated with R-CHOP who were enrolled in the National LymphoCare Study (NLCS). “What we found was that there were very poor outcomes associated with early-relapsing follicular lymphoma,” she said.
Overall survival (OS) at 8 years was 50% for patients with disease progression within 24 months of therapy, compared with 90% for patients who did not have early progression, a finding that was validated in a cohort of patients from the University of Iowa and the Mayo Clinic in which 8-year OS for early progressors was 34%, compared with 90% for other patients. The results held up when the researchers controlled for Follicular Lymphoma International Prognostic Index scores and in patients treated with rituximab and the cyclophosphamide, vincristine, and prednisone regimen rather than CHOP, Dr. Casulo noted.
“So, given these findings, how does one navigate the treatment landscape for patients with early relapsing follicular lymphoma? The reality is that there is really no standard of care or best approach,” she said.
“Ultimately, the goal of therapy, at least in my opinion, should be overcoming the chemoresistance that’s inherent to this biology, and establishing durable disease control, and there are a couple of strategies that might be able to achieve that,” she added.
There have been only two clinical trials of ASCT in patients with relapsed follicular lymphoma.
In the CUP trial, initiated prior to the introduction of rituximab, 89 patients with relapsed follicular lymphoma were treated with three cycles of CHOP, and those with a response were then randomized to either purged or unpurged ASCT, or to three additional cycles of CHOP. Four-year OS in this study was 70% for patients who underwent ASCT vs. 50% for those who received six cycles of CHOP.
In the EBMT LYM1 trial, 280 rituximab-naive patients with relapsed follicular lymphoma after a partial or complete remission were randomized to rituximab-purged or unpurged ASCT, followed by randomization to observation or rituximab maintenance. In this trial, the 10-year OS with ASCT ranged from 68% to 73%.
A Spanish registry study presented in a poster session at the 14th International Conference on Malignant Lymphoma in Lugano, Italy, showed long-term efficacy of ASCT in relapsed follicular lymphoma, with plateaus in both PFS and OS about 9 years after transplant for both rituximab-exposed and rituximab-naive patients, “suggesting that perhaps a subset of patients with relapsed follicular lymphoma can be cured with this approach,” Dr. Casulo said.
Similarly, a trial from the German Low Grade Lymphoma Studies group, presented at the 2016 American Society of Hematology annual meeting, showed 5-year OS of 77% with ASCT vs. 46% for patients who did not receive a transplant.
Dr. Casulo and her colleagues collaborated with investigators at the Center for International Blood and Marrow Transplant Research and the NLCS to see whether ASCT can improve OS compared with no transplant in patients with early-relapsed follicular lymphoma. They found that patients who received ASCT within 1 year of therapy failure had a 5-year OS of 73%, compared with 60% for those who did not receive ASCT (P = .02).
She acknowledged that toxicities associated with ASCT are a concern, pointing to a 2007 study looking at long-term follow-up of myeloablative ASCT for follicular lymphoma at the time of second or subsequent remission. The investigators found that rates of myelodysplasia were as high as 20% at 10 years, especially among patients who had undergone total body irradiation, a practice that has since fallen out of favor.
A separate study led by Matt Kalaycio, MD, of the Cleveland Clinic, showed that more lines of prior therapy (4-6 vs. 1-3) and radiation were both risk factors for subsequent myelodysplastic syndrome and acute myeloid leukemia.
“I hope we have demonstrated that autologous transplant can have durable response in these patients, with possibly a cure in a subset; but, ultimately, I think strategies that combine novel agents and autologous transplant in a clinical trial are the way to go to improve outcomes,” she said.
Dr. Link: Novel agents
“I actually happen to agree with very much of what Carla had to share, but I do have a couple of caveats,” Dr. Link said.
“The focus of this discussion is on patients with high-risk follicular lymphoma, as Carla very carefully defined in her analysis of the NLCS. These are the early progressors,” he said.
He cited data from the University of Iowa/Mayo Clinic series, validated in a cohort of patients from Lyon, France, showing that high-risk patients with early progression after immunochemotherapy had “especially poor outcomes.” In contrast, patients who were not early progressors fared quite well.
“It suggested that with agents that were available as of 2015, if you’re not an early progressor, your survival at least matches, or essentially matches with statistical power, that of the expected age- and gender-matched populations. So, novel agents are not required necessarily nor are clinical trials necessarily required for people who have good early outcomes,” Dr. Link said.
The best snapshot of current practice for high-risk patients comes from unpublished data from the NLCS showing that after a median follow-up of 8 years, 889 of 2,652 patients had received a second line of therapy, with the choice of agents or approaches generally similar between early progressors and others.
Early progressors were slightly less likely to receive rituximab monotherapy (30% vs. 36%) or an investigational agent (4.4% vs. 5.5%), whereas they were slightly more likely to receive an anthracycline (18% vs. 13%) or to undergo ASCT (3.5% vs. 1.1%).
In the treatment of patients with high-risk follicular lymphoma, a novel agent can be considered as one that was either not available or had not been used in follicular lymphoma when the previously mentioned survival data were generated, including immunomodulators such as thalidomide analogues, targeted kinase inhibitors, new anti-CD20 antibodies such as obinutuzumab (Gazyva), and immune checkpoint inhibitors.
For example, in Alliance 50803, a phase 2 trial in patients with previously untreated stage II-IV follicular lymphoma, the combination of lenalidomide (Revlimid) and rituximab was associated with a 95% overall response rate (ORR), including 72% complete response, and 5-year PFS rate of 70%, comparable to trials with rituximab plus bendamustine, CHOP, or cyclophosphamide-vincristine-prednisone, Dr. Link said.
In the phase 2 GALEN study, the combination of lenalidomide and obinutuzumab was associated with an ORR of 74% among 86 patients with relapsed/refractory follicular lymphoma, with a 1-year PFS rate of 76%.
An analysis of responses by time to relapse in GALEN showed that the ORR among 24 patients with disease progression within 24 months was 70.8%, including 33.3% complete or unconfirmed complete responses by the 1999 International Working Group criteria, and 66.7% with 54.2% complete or unconfirmed complete responses by the 2007 criteria.
Idelalisib, an inhibitor of the delta isoform of phosphatidylinositol 3-kinase (PI3K), was granted accelerated approval in 2014 for treatment of patients with follicular lymphoma after two or more prior lines of therapy, but toxicities associated with this agent caused the drug maker Gilead to dial back its development of this agent.
“But idelalisib is not the only PI3 kinase inhibitor on the block,” Dr. Link said, noting that more than a dozen similar agents are currently in development.
In clinical trials, PI3 kinase inhibitors have been associated with ORRs of about 60% in patients who experience early disease progression on other therapies, “suggesting an uncoupling between the paradigm that says that early progressors are going to have a less effective outcome than late progressors, perhaps, with targeted therapies.
The best evidence for the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) comes from the DAWN study, a phase 2 trial in patients with follicular lymphoma refractory to immunochemotherapy. The drug showed some biologic activity, but only a 21% ORR.
Dr. Link noted that the S1608 trial, currently recruiting patients, may give clinicians a better idea of which novel agent is most effective. The phase 2 trial is enrolling patients with early-progressing or refractory follicular lymphoma who will be randomized to receive obinutuzumab with either the investigational PI3 kinase inhibitor TGR-1202, lenalidomide, or CHOP chemotherapy.
“High-risk follicular lymphoma is a bad hombre,” Dr. Link said. “If we want to be any smarter as a society 10 years from now, we should incorporate clinical trials with novel therapies as standard operating practice into this setting of high-risk follicular lymphoma.”
Dr. Casulo reported serving on the speakers bureau for Gilead. Dr. Link reported serving as a consultant to AbbVie, Celgene, Genentech, and Gilead.
NEW YORK – For patients with newly diagnosed follicular lymphoma and other indolent non-Hodgkin lymphoma, the combination of bendamustine (Treanda) and rituximab is associated with significantly better progression-free survival (PFS) and longer time-to-next treatment than is rituximab plus CHOP chemotherapy, results of the BRIGHT study indicate.
But That was the question taken on in a debate at an international congress on hematologic malignancies by Carla Casulo, MD of the James P. Wilmot Cancer Institute at the University of Rochester (N.Y.), and Brian K. Link, MD, of the University of Iowa Hospitals & Clinics in Iowa City.
Dr. Casulo: ASCT
“Follicular lymphoma with a short remission duration has been established as a poor prognostic marker for survival, and the optimal therapy for these patients is really not known,” Dr. Casulo said.
“Of course, [novel] therapies can be considered, I think, for the appropriate patient, and hopefully in the context of a clinical study,” she added.
To lay out her argument for ASCT, Dr. Casulo pointed to four studies suggesting that about 20% of patients with follicular lymphoma will experience disease progression within 24 months of chemoimmunotherapy. Similar patterns of progression at 24 months were seen with R-CHOP in the SWOG S0016 trial, with both bendamustine-rituximab and R-CHOP in the StiL Study, with lenalidomide (Revlimid) and rituximab in a phase 3 clinical trial, and with one of three rituximab-based immunochemotherapy regimens in the PRIMA trial.
The results from these trials suggest that “there is an inherent biology to this population that relapses early, regardless of what induction strategy is used. However, what’s not known, until now, is whether early relapse implies poor survival in this disease,” she said.
To examine this question Dr. Casulo and her colleagues performed an analysis of time to progression among patients with newly-diagnosed follicular lymphoma treated with R-CHOP who were enrolled in the National LymphoCare Study (NLCS). “What we found was that there were very poor outcomes associated with early-relapsing follicular lymphoma,” she said.
Overall survival (OS) at 8 years was 50% for patients with disease progression within 24 months of therapy, compared with 90% for patients who did not have early progression, a finding that was validated in a cohort of patients from the University of Iowa and the Mayo Clinic in which 8-year OS for early progressors was 34%, compared with 90% for other patients. The results held up when the researchers controlled for Follicular Lymphoma International Prognostic Index scores and in patients treated with rituximab and the cyclophosphamide, vincristine, and prednisone regimen rather than CHOP, Dr. Casulo noted.
“So, given these findings, how does one navigate the treatment landscape for patients with early relapsing follicular lymphoma? The reality is that there is really no standard of care or best approach,” she said.
“Ultimately, the goal of therapy, at least in my opinion, should be overcoming the chemoresistance that’s inherent to this biology, and establishing durable disease control, and there are a couple of strategies that might be able to achieve that,” she added.
There have been only two clinical trials of ASCT in patients with relapsed follicular lymphoma.
In the CUP trial, initiated prior to the introduction of rituximab, 89 patients with relapsed follicular lymphoma were treated with three cycles of CHOP, and those with a response were then randomized to either purged or unpurged ASCT, or to three additional cycles of CHOP. Four-year OS in this study was 70% for patients who underwent ASCT vs. 50% for those who received six cycles of CHOP.
In the EBMT LYM1 trial, 280 rituximab-naive patients with relapsed follicular lymphoma after a partial or complete remission were randomized to rituximab-purged or unpurged ASCT, followed by randomization to observation or rituximab maintenance. In this trial, the 10-year OS with ASCT ranged from 68% to 73%.
A Spanish registry study presented in a poster session at the 14th International Conference on Malignant Lymphoma in Lugano, Italy, showed long-term efficacy of ASCT in relapsed follicular lymphoma, with plateaus in both PFS and OS about 9 years after transplant for both rituximab-exposed and rituximab-naive patients, “suggesting that perhaps a subset of patients with relapsed follicular lymphoma can be cured with this approach,” Dr. Casulo said.
Similarly, a trial from the German Low Grade Lymphoma Studies group, presented at the 2016 American Society of Hematology annual meeting, showed 5-year OS of 77% with ASCT vs. 46% for patients who did not receive a transplant.
Dr. Casulo and her colleagues collaborated with investigators at the Center for International Blood and Marrow Transplant Research and the NLCS to see whether ASCT can improve OS compared with no transplant in patients with early-relapsed follicular lymphoma. They found that patients who received ASCT within 1 year of therapy failure had a 5-year OS of 73%, compared with 60% for those who did not receive ASCT (P = .02).
She acknowledged that toxicities associated with ASCT are a concern, pointing to a 2007 study looking at long-term follow-up of myeloablative ASCT for follicular lymphoma at the time of second or subsequent remission. The investigators found that rates of myelodysplasia were as high as 20% at 10 years, especially among patients who had undergone total body irradiation, a practice that has since fallen out of favor.
A separate study led by Matt Kalaycio, MD, of the Cleveland Clinic, showed that more lines of prior therapy (4-6 vs. 1-3) and radiation were both risk factors for subsequent myelodysplastic syndrome and acute myeloid leukemia.
“I hope we have demonstrated that autologous transplant can have durable response in these patients, with possibly a cure in a subset; but, ultimately, I think strategies that combine novel agents and autologous transplant in a clinical trial are the way to go to improve outcomes,” she said.
Dr. Link: Novel agents
“I actually happen to agree with very much of what Carla had to share, but I do have a couple of caveats,” Dr. Link said.
“The focus of this discussion is on patients with high-risk follicular lymphoma, as Carla very carefully defined in her analysis of the NLCS. These are the early progressors,” he said.
He cited data from the University of Iowa/Mayo Clinic series, validated in a cohort of patients from Lyon, France, showing that high-risk patients with early progression after immunochemotherapy had “especially poor outcomes.” In contrast, patients who were not early progressors fared quite well.
“It suggested that with agents that were available as of 2015, if you’re not an early progressor, your survival at least matches, or essentially matches with statistical power, that of the expected age- and gender-matched populations. So, novel agents are not required necessarily nor are clinical trials necessarily required for people who have good early outcomes,” Dr. Link said.
The best snapshot of current practice for high-risk patients comes from unpublished data from the NLCS showing that after a median follow-up of 8 years, 889 of 2,652 patients had received a second line of therapy, with the choice of agents or approaches generally similar between early progressors and others.
Early progressors were slightly less likely to receive rituximab monotherapy (30% vs. 36%) or an investigational agent (4.4% vs. 5.5%), whereas they were slightly more likely to receive an anthracycline (18% vs. 13%) or to undergo ASCT (3.5% vs. 1.1%).
In the treatment of patients with high-risk follicular lymphoma, a novel agent can be considered as one that was either not available or had not been used in follicular lymphoma when the previously mentioned survival data were generated, including immunomodulators such as thalidomide analogues, targeted kinase inhibitors, new anti-CD20 antibodies such as obinutuzumab (Gazyva), and immune checkpoint inhibitors.
For example, in Alliance 50803, a phase 2 trial in patients with previously untreated stage II-IV follicular lymphoma, the combination of lenalidomide (Revlimid) and rituximab was associated with a 95% overall response rate (ORR), including 72% complete response, and 5-year PFS rate of 70%, comparable to trials with rituximab plus bendamustine, CHOP, or cyclophosphamide-vincristine-prednisone, Dr. Link said.
In the phase 2 GALEN study, the combination of lenalidomide and obinutuzumab was associated with an ORR of 74% among 86 patients with relapsed/refractory follicular lymphoma, with a 1-year PFS rate of 76%.
An analysis of responses by time to relapse in GALEN showed that the ORR among 24 patients with disease progression within 24 months was 70.8%, including 33.3% complete or unconfirmed complete responses by the 1999 International Working Group criteria, and 66.7% with 54.2% complete or unconfirmed complete responses by the 2007 criteria.
Idelalisib, an inhibitor of the delta isoform of phosphatidylinositol 3-kinase (PI3K), was granted accelerated approval in 2014 for treatment of patients with follicular lymphoma after two or more prior lines of therapy, but toxicities associated with this agent caused the drug maker Gilead to dial back its development of this agent.
“But idelalisib is not the only PI3 kinase inhibitor on the block,” Dr. Link said, noting that more than a dozen similar agents are currently in development.
In clinical trials, PI3 kinase inhibitors have been associated with ORRs of about 60% in patients who experience early disease progression on other therapies, “suggesting an uncoupling between the paradigm that says that early progressors are going to have a less effective outcome than late progressors, perhaps, with targeted therapies.
The best evidence for the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) comes from the DAWN study, a phase 2 trial in patients with follicular lymphoma refractory to immunochemotherapy. The drug showed some biologic activity, but only a 21% ORR.
Dr. Link noted that the S1608 trial, currently recruiting patients, may give clinicians a better idea of which novel agent is most effective. The phase 2 trial is enrolling patients with early-progressing or refractory follicular lymphoma who will be randomized to receive obinutuzumab with either the investigational PI3 kinase inhibitor TGR-1202, lenalidomide, or CHOP chemotherapy.
“High-risk follicular lymphoma is a bad hombre,” Dr. Link said. “If we want to be any smarter as a society 10 years from now, we should incorporate clinical trials with novel therapies as standard operating practice into this setting of high-risk follicular lymphoma.”
Dr. Casulo reported serving on the speakers bureau for Gilead. Dr. Link reported serving as a consultant to AbbVie, Celgene, Genentech, and Gilead.
EXPERT ANALYSIS AT LYMPHOMA & MYELOMA
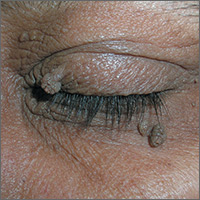
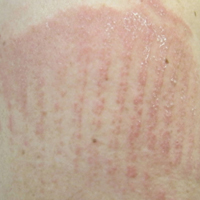
Consider different T. capitis presentations in children with hair loss
Categorizing hair loss in children depends on many factors, but it is important to rule out an infectious etiology as early as possible, according to Sheila Fallon Friedlander, MD.
“What can Tinea capitis look like? Anything,” she said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Although T. capitis most often presents in children aged 3-7 years as a pattern of localized hair loss, often with scaling, sometimes with nodules, other possibilities include pustules, boggy masses, and diffuse hair loss, said Dr. Friedlander, professor of pediatrics and dermatology at the University of California, San Diego.
Sometimes the hair loss may be so subtle that families come in complaining of “dandruff” rather than hair loss, she noted. Evaluating the patient for the presence of cervical or occipital lymph nodes is crucial; big nodes are usually a tip-off that infection is present.
Clinicians treating T. capitis should ask about family pets, advised Dr. Friedlander, adding that city dwellers’ conditions may be more likely caused by Trichophyton tonsurans, T. violaceum, or Trichophyton soudanense. Also consider immigration status and family history when evaluating T. capitis, and use a Wood’s lamp for diagnosis if one is available, she advised. M. canis will fluoresce and T. tonsurans will not, she pointed out.
Other strategies to evaluate the condition include KOH, culture, polymerase chain reaction, and trichoscopy.
The optimal treatment plan for T. capitis depends on the source, Dr. Friedlander explained. If M. canis is the cause, “griseofulvin is the drug of choice,” along with a twice-weekly sporicidal shampoo, she said.
Other treatment options include terbinafine, itraconazole, and fluconazole, and each have their pros and cons, she said. Terbinafine – which persists for months in the skin, nails, and hair – is the least expensive, and is her first choice for infections caused by T. tonsurans.
Itraconazole is available as a liquid, but costs more, causes diarrhea, and comes with a boxed warning about the potential for cardiac complications; fluconazole is the most expensive, but may be used in infants, she added.
Other high-risk groups for T. capitis include female caretakers of high-risk individuals, such as “grandma”; wrestlers; and Buddhist monks, she said. “Short hair, sharing combs, and unclean barbers” contributed to a documented increased risk of T. capitis according to a recently published study of 60 Buddhist monks whose average age was 11.6 years, she added. (Pediatr Dermatol. 2017 May;34[3]:371-3).
Dr. Friedlander had no relevant financial conflicts to disclose.
SDEF and this news organization are owned by Frontline Medical Communications.
Categorizing hair loss in children depends on many factors, but it is important to rule out an infectious etiology as early as possible, according to Sheila Fallon Friedlander, MD.
“What can Tinea capitis look like? Anything,” she said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Although T. capitis most often presents in children aged 3-7 years as a pattern of localized hair loss, often with scaling, sometimes with nodules, other possibilities include pustules, boggy masses, and diffuse hair loss, said Dr. Friedlander, professor of pediatrics and dermatology at the University of California, San Diego.
Sometimes the hair loss may be so subtle that families come in complaining of “dandruff” rather than hair loss, she noted. Evaluating the patient for the presence of cervical or occipital lymph nodes is crucial; big nodes are usually a tip-off that infection is present.
Clinicians treating T. capitis should ask about family pets, advised Dr. Friedlander, adding that city dwellers’ conditions may be more likely caused by Trichophyton tonsurans, T. violaceum, or Trichophyton soudanense. Also consider immigration status and family history when evaluating T. capitis, and use a Wood’s lamp for diagnosis if one is available, she advised. M. canis will fluoresce and T. tonsurans will not, she pointed out.
Other strategies to evaluate the condition include KOH, culture, polymerase chain reaction, and trichoscopy.
The optimal treatment plan for T. capitis depends on the source, Dr. Friedlander explained. If M. canis is the cause, “griseofulvin is the drug of choice,” along with a twice-weekly sporicidal shampoo, she said.
Other treatment options include terbinafine, itraconazole, and fluconazole, and each have their pros and cons, she said. Terbinafine – which persists for months in the skin, nails, and hair – is the least expensive, and is her first choice for infections caused by T. tonsurans.
Itraconazole is available as a liquid, but costs more, causes diarrhea, and comes with a boxed warning about the potential for cardiac complications; fluconazole is the most expensive, but may be used in infants, she added.
Other high-risk groups for T. capitis include female caretakers of high-risk individuals, such as “grandma”; wrestlers; and Buddhist monks, she said. “Short hair, sharing combs, and unclean barbers” contributed to a documented increased risk of T. capitis according to a recently published study of 60 Buddhist monks whose average age was 11.6 years, she added. (Pediatr Dermatol. 2017 May;34[3]:371-3).
Dr. Friedlander had no relevant financial conflicts to disclose.
SDEF and this news organization are owned by Frontline Medical Communications.
Categorizing hair loss in children depends on many factors, but it is important to rule out an infectious etiology as early as possible, according to Sheila Fallon Friedlander, MD.
“What can Tinea capitis look like? Anything,” she said in a presentation at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
Although T. capitis most often presents in children aged 3-7 years as a pattern of localized hair loss, often with scaling, sometimes with nodules, other possibilities include pustules, boggy masses, and diffuse hair loss, said Dr. Friedlander, professor of pediatrics and dermatology at the University of California, San Diego.
Sometimes the hair loss may be so subtle that families come in complaining of “dandruff” rather than hair loss, she noted. Evaluating the patient for the presence of cervical or occipital lymph nodes is crucial; big nodes are usually a tip-off that infection is present.
Clinicians treating T. capitis should ask about family pets, advised Dr. Friedlander, adding that city dwellers’ conditions may be more likely caused by Trichophyton tonsurans, T. violaceum, or Trichophyton soudanense. Also consider immigration status and family history when evaluating T. capitis, and use a Wood’s lamp for diagnosis if one is available, she advised. M. canis will fluoresce and T. tonsurans will not, she pointed out.
Other strategies to evaluate the condition include KOH, culture, polymerase chain reaction, and trichoscopy.
The optimal treatment plan for T. capitis depends on the source, Dr. Friedlander explained. If M. canis is the cause, “griseofulvin is the drug of choice,” along with a twice-weekly sporicidal shampoo, she said.
Other treatment options include terbinafine, itraconazole, and fluconazole, and each have their pros and cons, she said. Terbinafine – which persists for months in the skin, nails, and hair – is the least expensive, and is her first choice for infections caused by T. tonsurans.
Itraconazole is available as a liquid, but costs more, causes diarrhea, and comes with a boxed warning about the potential for cardiac complications; fluconazole is the most expensive, but may be used in infants, she added.
Other high-risk groups for T. capitis include female caretakers of high-risk individuals, such as “grandma”; wrestlers; and Buddhist monks, she said. “Short hair, sharing combs, and unclean barbers” contributed to a documented increased risk of T. capitis according to a recently published study of 60 Buddhist monks whose average age was 11.6 years, she added. (Pediatr Dermatol. 2017 May;34[3]:371-3).
Dr. Friedlander had no relevant financial conflicts to disclose.
SDEF and this news organization are owned by Frontline Medical Communications.
FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Inclusion valued by advanced practice providers
Editor’s note: Each month, the Society of Hospital Medicine puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Log on to www.hospitalmedicine.org/getinvolved for more information on how you can lend your expertise to help SHM improve the care of hospitalized patients.
This month, The Hospitalist spotlights Lorraine Britting, ANP, SFHM, clinical director of advanced practice in cardiology medicine at Beth Israel Deaconess Medical Center, Boston. Ms. Britting has been an SHM member for over 10 years, has served on various SHM committees, and was one of the first nurse practitioners to earn the Senior Fellow in Hospital Medicine designation.
How did you become a hospital medicine nurse practitioner, and when did you join SHM?
I was a nurse working in a CCU and MICU for 19 years when I graduated from a master’s program as a nurse practitioner (NP) in adult care. I thought I was going to work in the outpatient side after graduation, but my experience was much more suited to hospital medicine.
My first job in 2004 was as a hospitalist in a very small community hospital affiliated with Beth Israel Deaconess Medical Center. I was the first NP to work as an inpatient provider there, which was challenging, but I had the opportunity to wear many hats and be involved with numerous quality initiatives that helped me grow as a provider and a leader. I was working as the clinical manager of three hospitalist programs under the director by the time I left. I now work in inpatient cardiology and am the director of advanced practice providers (APPs) for cardiology medicine. I joined SHM in 2005 when it was a small but rapidly growing society, and I started work on the NP/PA Committee. I was also involved in the Hospital Quality and Patient Safety Committee for 6 years and worked as a peer reviewer for the Journal of Hospital Medicine.
Describe your role on the Membership Committee. What is the committee currently working on?
I am finishing my 3rd year on the committee. In the last few months, we have been focusing on member engagement. We have collected information on why members choose to join SHM and what deters potential members from joining SHM and we are developing strategies to build and retain our membership. The Membership Committee also reviews Fellows applications and discusses modifications of requirements each year.
As an NP, I have unique insight into motivations for why other APPs would join SHM and which membership benefits are most valuable. I find that many APPs join SHM because they feel that SHM treats them as equals, not junior members, as in some other physician organizations.
What does the Senior Fellow in Hospital Medicine designation mean to you?
I am grateful that SHM allows all members to be a part of the Fellows program, and I was honored to be one of the first NPs to become a Senior Fellow. Many medical societies allow APPs to join but do not offer the opportunity to become Fellows.
The Senior Fellowship application was a rigorous process and required experience in multiple areas, including quality projects, hospital committees, SHM Annual Conference attendance, and other clinical and nonclinical work that advances the profession.
As a nurse practitioner, which SHM resources do you find most valuable?
As a specialist NP, it’s easy for me to be current in cardiology but harder to keep current in general medicine. I find the clinical information very helpful to keep me up to date on hospital medicine. The Journal of Hospital Medicine and The Hospitalist are must reads, and the Annual Conference is, of course, very informative. I also enjoy the conversations on the Hospital Medicine Exchange and feel that the Choosing Wisely campaign is an excellent contribution to the goal of cost containment in everyday practice.
One of the best features of SHM is that I can meet other clinicians from around the country and around the world who have innovations or novel ideas that I can bring back to my institution.
What advice do you have for nurse practitioners as their role in hospital medicine continues to evolve?
I say to my staff that they should always say yes. Yes to continuing education, yes to opportunities for growth and advancement, yes to promotions, yes to research, etc. Careers develop in nonlinear ways, and you have to follow the opportunities as they come.
Ms. Steele is the marketing communications specialist at the Society of Hospital Medicine.
Editor’s note: Each month, the Society of Hospital Medicine puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Log on to www.hospitalmedicine.org/getinvolved for more information on how you can lend your expertise to help SHM improve the care of hospitalized patients.
This month, The Hospitalist spotlights Lorraine Britting, ANP, SFHM, clinical director of advanced practice in cardiology medicine at Beth Israel Deaconess Medical Center, Boston. Ms. Britting has been an SHM member for over 10 years, has served on various SHM committees, and was one of the first nurse practitioners to earn the Senior Fellow in Hospital Medicine designation.
How did you become a hospital medicine nurse practitioner, and when did you join SHM?
I was a nurse working in a CCU and MICU for 19 years when I graduated from a master’s program as a nurse practitioner (NP) in adult care. I thought I was going to work in the outpatient side after graduation, but my experience was much more suited to hospital medicine.
My first job in 2004 was as a hospitalist in a very small community hospital affiliated with Beth Israel Deaconess Medical Center. I was the first NP to work as an inpatient provider there, which was challenging, but I had the opportunity to wear many hats and be involved with numerous quality initiatives that helped me grow as a provider and a leader. I was working as the clinical manager of three hospitalist programs under the director by the time I left. I now work in inpatient cardiology and am the director of advanced practice providers (APPs) for cardiology medicine. I joined SHM in 2005 when it was a small but rapidly growing society, and I started work on the NP/PA Committee. I was also involved in the Hospital Quality and Patient Safety Committee for 6 years and worked as a peer reviewer for the Journal of Hospital Medicine.
Describe your role on the Membership Committee. What is the committee currently working on?
I am finishing my 3rd year on the committee. In the last few months, we have been focusing on member engagement. We have collected information on why members choose to join SHM and what deters potential members from joining SHM and we are developing strategies to build and retain our membership. The Membership Committee also reviews Fellows applications and discusses modifications of requirements each year.
As an NP, I have unique insight into motivations for why other APPs would join SHM and which membership benefits are most valuable. I find that many APPs join SHM because they feel that SHM treats them as equals, not junior members, as in some other physician organizations.
What does the Senior Fellow in Hospital Medicine designation mean to you?
I am grateful that SHM allows all members to be a part of the Fellows program, and I was honored to be one of the first NPs to become a Senior Fellow. Many medical societies allow APPs to join but do not offer the opportunity to become Fellows.
The Senior Fellowship application was a rigorous process and required experience in multiple areas, including quality projects, hospital committees, SHM Annual Conference attendance, and other clinical and nonclinical work that advances the profession.
As a nurse practitioner, which SHM resources do you find most valuable?
As a specialist NP, it’s easy for me to be current in cardiology but harder to keep current in general medicine. I find the clinical information very helpful to keep me up to date on hospital medicine. The Journal of Hospital Medicine and The Hospitalist are must reads, and the Annual Conference is, of course, very informative. I also enjoy the conversations on the Hospital Medicine Exchange and feel that the Choosing Wisely campaign is an excellent contribution to the goal of cost containment in everyday practice.
One of the best features of SHM is that I can meet other clinicians from around the country and around the world who have innovations or novel ideas that I can bring back to my institution.
What advice do you have for nurse practitioners as their role in hospital medicine continues to evolve?
I say to my staff that they should always say yes. Yes to continuing education, yes to opportunities for growth and advancement, yes to promotions, yes to research, etc. Careers develop in nonlinear ways, and you have to follow the opportunities as they come.
Ms. Steele is the marketing communications specialist at the Society of Hospital Medicine.
Editor’s note: Each month, the Society of Hospital Medicine puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Log on to www.hospitalmedicine.org/getinvolved for more information on how you can lend your expertise to help SHM improve the care of hospitalized patients.
This month, The Hospitalist spotlights Lorraine Britting, ANP, SFHM, clinical director of advanced practice in cardiology medicine at Beth Israel Deaconess Medical Center, Boston. Ms. Britting has been an SHM member for over 10 years, has served on various SHM committees, and was one of the first nurse practitioners to earn the Senior Fellow in Hospital Medicine designation.
How did you become a hospital medicine nurse practitioner, and when did you join SHM?
I was a nurse working in a CCU and MICU for 19 years when I graduated from a master’s program as a nurse practitioner (NP) in adult care. I thought I was going to work in the outpatient side after graduation, but my experience was much more suited to hospital medicine.
My first job in 2004 was as a hospitalist in a very small community hospital affiliated with Beth Israel Deaconess Medical Center. I was the first NP to work as an inpatient provider there, which was challenging, but I had the opportunity to wear many hats and be involved with numerous quality initiatives that helped me grow as a provider and a leader. I was working as the clinical manager of three hospitalist programs under the director by the time I left. I now work in inpatient cardiology and am the director of advanced practice providers (APPs) for cardiology medicine. I joined SHM in 2005 when it was a small but rapidly growing society, and I started work on the NP/PA Committee. I was also involved in the Hospital Quality and Patient Safety Committee for 6 years and worked as a peer reviewer for the Journal of Hospital Medicine.
Describe your role on the Membership Committee. What is the committee currently working on?
I am finishing my 3rd year on the committee. In the last few months, we have been focusing on member engagement. We have collected information on why members choose to join SHM and what deters potential members from joining SHM and we are developing strategies to build and retain our membership. The Membership Committee also reviews Fellows applications and discusses modifications of requirements each year.
As an NP, I have unique insight into motivations for why other APPs would join SHM and which membership benefits are most valuable. I find that many APPs join SHM because they feel that SHM treats them as equals, not junior members, as in some other physician organizations.
What does the Senior Fellow in Hospital Medicine designation mean to you?
I am grateful that SHM allows all members to be a part of the Fellows program, and I was honored to be one of the first NPs to become a Senior Fellow. Many medical societies allow APPs to join but do not offer the opportunity to become Fellows.
The Senior Fellowship application was a rigorous process and required experience in multiple areas, including quality projects, hospital committees, SHM Annual Conference attendance, and other clinical and nonclinical work that advances the profession.
As a nurse practitioner, which SHM resources do you find most valuable?
As a specialist NP, it’s easy for me to be current in cardiology but harder to keep current in general medicine. I find the clinical information very helpful to keep me up to date on hospital medicine. The Journal of Hospital Medicine and The Hospitalist are must reads, and the Annual Conference is, of course, very informative. I also enjoy the conversations on the Hospital Medicine Exchange and feel that the Choosing Wisely campaign is an excellent contribution to the goal of cost containment in everyday practice.
One of the best features of SHM is that I can meet other clinicians from around the country and around the world who have innovations or novel ideas that I can bring back to my institution.
What advice do you have for nurse practitioners as their role in hospital medicine continues to evolve?
I say to my staff that they should always say yes. Yes to continuing education, yes to opportunities for growth and advancement, yes to promotions, yes to research, etc. Careers develop in nonlinear ways, and you have to follow the opportunities as they come.
Ms. Steele is the marketing communications specialist at the Society of Hospital Medicine.
Growths on eyelids
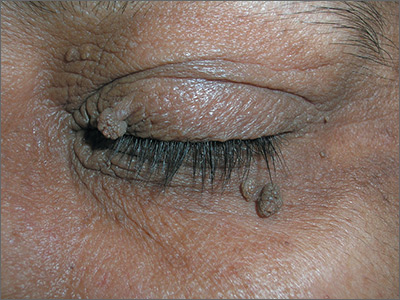
The FP diagnosed skin tags (acrochordons) on her eyelids and explained that they were not dangerous. Removal options include cryotherapy, snip excisions, and electrosurgery.
The safest method of cryotherapy in a case like this is to use Cryo Tweezers. (See a video on how to perform cryosurgery here.) Using a cryo-spray in this area could result in the treatment getting into the eye.
Cryo Tweezers can apply cold to the skin tags without risking damage to the eye. The Cryo Tweezers are made cold by dipping them into liquid nitrogen. Then, each skin tag is grasped while gently pulling it away from the orbit. The skin tag is held between the 2 ends of the tweezer until it turns white. This method is very safe and causes very little discomfort to the patient.
Snip excisions on the eyelid are challenging for a number of reasons, including the fact that aluminum chloride or other chemical hemostatic agents are not safe for the eye. Electrosurgery can be performed with a loop electrode but only after the skin tags are numbed by injecting a local anesthetic into the eyelids. While it is possible for this to be performed safely, it is more challenging than using the Cryo Tweezers, which do not require a local anesthetic.
The patient in this case chose Cryo Tweezers for treatment and tolerated it well. The FP also documented that the patient believed the skin tags were affecting her vision in an effort to increase the likelihood that her insurance would cover the procedure. At a follow-up appointment 2 months later for the patient’s diabetes, the skin tags on her eyelids had resolved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith AM. Skin tags. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 922-925.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed skin tags (acrochordons) on her eyelids and explained that they were not dangerous. Removal options include cryotherapy, snip excisions, and electrosurgery.
The safest method of cryotherapy in a case like this is to use Cryo Tweezers. (See a video on how to perform cryosurgery here.) Using a cryo-spray in this area could result in the treatment getting into the eye.
Cryo Tweezers can apply cold to the skin tags without risking damage to the eye. The Cryo Tweezers are made cold by dipping them into liquid nitrogen. Then, each skin tag is grasped while gently pulling it away from the orbit. The skin tag is held between the 2 ends of the tweezer until it turns white. This method is very safe and causes very little discomfort to the patient.
Snip excisions on the eyelid are challenging for a number of reasons, including the fact that aluminum chloride or other chemical hemostatic agents are not safe for the eye. Electrosurgery can be performed with a loop electrode but only after the skin tags are numbed by injecting a local anesthetic into the eyelids. While it is possible for this to be performed safely, it is more challenging than using the Cryo Tweezers, which do not require a local anesthetic.
The patient in this case chose Cryo Tweezers for treatment and tolerated it well. The FP also documented that the patient believed the skin tags were affecting her vision in an effort to increase the likelihood that her insurance would cover the procedure. At a follow-up appointment 2 months later for the patient’s diabetes, the skin tags on her eyelids had resolved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith AM. Skin tags. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 922-925.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed skin tags (acrochordons) on her eyelids and explained that they were not dangerous. Removal options include cryotherapy, snip excisions, and electrosurgery.
The safest method of cryotherapy in a case like this is to use Cryo Tweezers. (See a video on how to perform cryosurgery here.) Using a cryo-spray in this area could result in the treatment getting into the eye.
Cryo Tweezers can apply cold to the skin tags without risking damage to the eye. The Cryo Tweezers are made cold by dipping them into liquid nitrogen. Then, each skin tag is grasped while gently pulling it away from the orbit. The skin tag is held between the 2 ends of the tweezer until it turns white. This method is very safe and causes very little discomfort to the patient.
Snip excisions on the eyelid are challenging for a number of reasons, including the fact that aluminum chloride or other chemical hemostatic agents are not safe for the eye. Electrosurgery can be performed with a loop electrode but only after the skin tags are numbed by injecting a local anesthetic into the eyelids. While it is possible for this to be performed safely, it is more challenging than using the Cryo Tweezers, which do not require a local anesthetic.
The patient in this case chose Cryo Tweezers for treatment and tolerated it well. The FP also documented that the patient believed the skin tags were affecting her vision in an effort to increase the likelihood that her insurance would cover the procedure. At a follow-up appointment 2 months later for the patient’s diabetes, the skin tags on her eyelids had resolved.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith AM. Skin tags. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 922-925.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Over-the-counter Topical Musculoskeletal Pain Relievers Used With a Heat Source: A Dangerous Combination
To the Editor:
The combination of menthol and methyl salicylate found in a variety of over-the-counter (OTC) creams in conjunction with a heat source such as a heating pad used for musculoskeletal symptoms can be a dire combination due to increased systemic absorption with associated toxicity and localized effects ranging from contact dermatitis or irritation to burn or necrosis.1-6 We present a case of localized burn due a combination of topical methyl salicylate and heating pad use. We also discuss 2 commonly encountered side effects in the literature—localized burns and systemic toxicity associated with percutaneous absorption—and provide specific considerations related to the geriatric and pediatric populations.
A 62-year-old woman with a history of eczematous dermatitis and osteoarthritis with pain of the left shoulder presented to the dermatology clinic with painful skin-related changes on the left arm of 1 week’s duration. She was prescribed acetaminophen and ibuprofen. However, she self-medicated the left shoulder pain with 2 OTC products containing topical menthol and/or methyl salicylate in combination with a heating pad and likely fell asleep with this combination therapy applied. She noticed the burn the next morning. On examination, the left arm exhibited a geometric, irregularly shaped, erythematous, scaly plaque with a sharp transverse linear demarcation proximally and numerous erythematous linear scaly plaques oriented in an axial orientation with less-defined borders distally (Figure). The patient was diagnosed with burn secondary to combination of topical methyl salicylate and heating pad use. The patient was advised to discontinue the topical medication and to use caution with the heating pad in the future. She was prescribed pramoxine-hydrocortisone lotion to be applied to the affected area twice daily up to 5 days weekly until resolution. Subsequent evaluations revealed progressive improvement with only mild postinflammatory hyperpigmentation noted at 6 months after the burn.
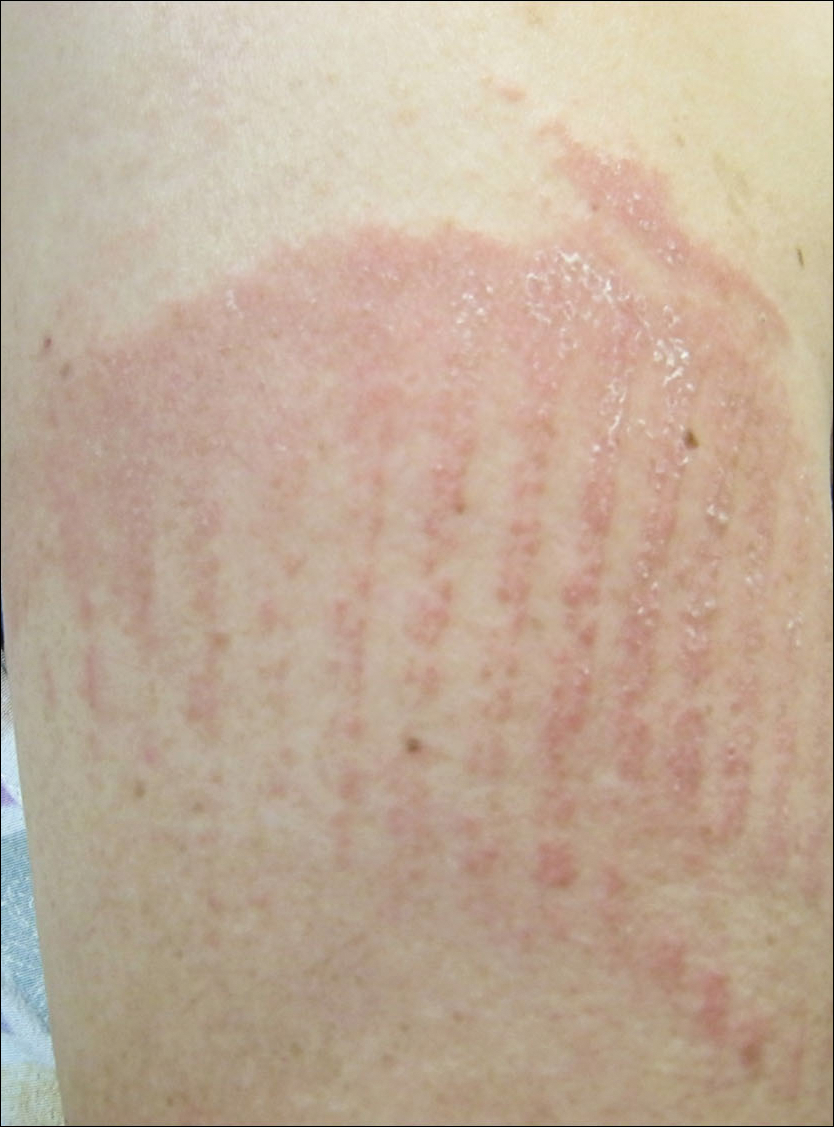
The US Food and Drug Administration (FDA) released statements in 2012 regarding concern for burns related to use of OTC musculoskeletal pain relievers, with 43 cases of burns reported due to methyl salicylate and menthol from 2004 to 2010. Most of the second- and third-degree burns occurred following topical applications of products containing either menthol monotherapy or a combination of methyl salicylate and menthol.1,2 In 2006, the FDA had already ordered 5 firms to stop compounding topical pain relief formulations containing these ingredients, with concerns that it puts patients at increased risk because the compounded formulations had not received FDA approval.3 Despite package warnings, patients may not be aware of the concerning side effects and risks associated with use of OTC creams, especially in combination with occlusion or heating pad use. Our case highlights the importance of ongoing patient education and physician counseling when encountering patients with arthritis or musculoskeletal pain who may often try various OTC self-treatments for pain relief.7
In 2012, the FDA reports stated that the cases of mild to serious burns were associated with methyl salicylate and menthol usage, in some cases 24 hours after first usage. Typically, these effects occur when concentrations are more than either 3% menthol alone or a combination of more than 3% menthol and more than 10% methyl salicylate.1,2 In our case, the patient had been using 2 different OTC products that may have contained as much as 11% menthol and/or 30% methyl salicylate. Electronic resources are available that disclose safety instructions including not to occlude the site, not to use on wounds, and not to be used in conjunction with a heating pad.8,9 Skin breakdown and vasodilation are more likely to occur in a setting of heat and occlusion, which allows for more absorption and localized side effects.4,10 Localized reactions may range from contact dermatitis4 to muscle necrosis.5
The most noteworthy case of localized destruction described a 62-year-old man who had applied topical methyl salicylate and menthol to the forearms, calves, and thighs, then intermittently used a heating pad for 15 to 20 minutes (total duration).5 He subsequently developed erythema and numerous 7.62- to 10.16-cm bullae, which was thought to be consistent with contact dermatitis. Three days later, he was found to have full-thickness cutaneous, fascial, and muscle necrosis in a linear pattern. He was hospitalized for approximately 1 year and treated with extensive debridement and a skin graft. His serum creatinine level increased from 0.7 mg per 100 mL to 2.7 mg per 100 mL (reference range, 0.6–1.2 mg/dL) with evidence of toxic nephrosis and persistent interstitial nephritis, demonstrating the severity of localized destruction that may result when combining these products with direct heat and potential subsequent systemic consequences of this combination.5
The systemic absorption of OTC formulations also has been studied. Morra et al10 studied 12 volunteers (6 women, 6 men) who applied either 5 g of methyl salicylate ointment 12.5% twice daily for 4 days to an area on the thigh (approximately equal to 567 mg salicylate) or trolamine cream 10% twice for 1 day. The participants underwent a break for 7 days and then switched to the alternate treatment. They found that 0.31 to 0.91 mg/L methyl salicylate was detected in the serum 1 hour after applying the ointment consisting of methyl salicylate, and 2 to 6 mg/L methyl salicylate was detected on day 4. Therapeutic serum salicylate levels are 150 to 300 mg/L. They found that approximately 22% of the methyl salicylate also was found in urine samples on day 4. Although these figures may appear small, this study was prompted when a 62-year-old man presented to the emergency department with symptoms of salicylate toxicity and a serum concentration of 518 mg/L from twice-daily use of an OTC formulation containing methyl salicylate over the course of multiple weeks.10 Additionally, those who have aspirin hypersensitivity should be cautious when using such products due to the risk for reported angioedema.4
Providers must exercise extreme caution while caring for geriatric patients, especially if patients are taking warfarin. The combined effects of warfarin and methyl salicylate have previously caused cutaneous purpura, gastrointestinal bleeding, and elevated international normalized ratio values.4,10 Older individuals also have increased skin fragility, allowing microtraumatic insult to easily develop. This fragility, along with an overall decreased intactness of the skin barrier, may lead to increased skin absorption. Furthermore, the addition of applying any heat source places the geriatric patient at greater risk for adverse events.10
In considering the limits of age, the pediatric population also has been studied regarding salicylate toxicity. Most commonly, oral ingestion has caused fatalities, as oil of wintergreen has been cited as extremely dangerous for children if swallowed; doses as small as a teaspoon (5 mL: 7000 mg salicylate) have resulted in fatalities.4,6 Although the consumption of a large amount of a cream- or ointment-based product is unlikely due to the consistency of the medication,6 the thought does merit consideration in the inquisitive toddler age group. For a 15-kg toddler, 150 mg/kg of aspirin or 2250 mg of aspirin, is considered the toxic level, which upon conversion to methyl salicylate levels using a 1.4 factor equates to 1607 mg of methyl salicylate to reach toxicity.6 If using a product with methyl salicylate 30% composition, 1 g of the product contains 300 mg of methyl salicylate; therefore if the toddler consumed approximately 5.3 g of the product (1607 mg methyl salicylate [toxic level] divided by 300 mg methyl salicylate per 1 g of product), he/she would reach toxic levels.6,11 To put this into perspective, a 2-oz tube contains 57 g (approximately 10 times the toxic dose) of the product.8 Thus, although there is less concern overall for consumption of cream- or ointment-based methyl salicylate, there still is potential for harm if a small child were to ingest such a product containing higher percentages of methyl salicylate.6
There also have been reports of pediatric toxicity related to percutaneous absorption, even leading to pediatric fatality.4,6 In particular, there was a case of a young boy hospitalized with ichthyosis who received escalating doses of percutaneous salicylate, which resulted in toxicity; when therapy was discontinued, he experienced full recovery.12 In 2007, a 17-year-old adolescent girl died from methyl salicylate toxicity after numerous applications of salicylate-containing products in conjunction with medicated pads.7
Although the FDA has drawn attention and encouraged caution with use of OTC topical musculoskeletal pain relievers, the importance of ensuring patients are fully aware of potential burns, permanent skin or muscle damage, and even death if used inappropriately cannot be overstated. The FDA consumer health information website has 2 patient-directed handouts2,3 that may be useful to post in patient waiting areas to increase overall understanding of the risks associated with OTC products containing methyl salicylate and menthol ingredients. Fortunately, our patient suffered only mild postinflammatory hyperpigmentation without substantial sustained consequences.
- US Food and Drug Administration. FDA Drug Safety Communication: rare cases of serious burns with the use of over-the-counter topical muscle and joint pain relievers. http://www.fda.gov/Drugs/DrugSafety/ucm318858.htm. Published September 13, 2012. Updated February 11, 2016. Accessed October 31, 2017.
- US Food and Drug Administration. Topical pain relievers may cause burns. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm318674.htm. Published September 13, 2012. Updated November 5, 2015. Accessed October 31, 2017.
- US Food and Drug Administration. Use caution with over-the-counter creams, ointments. http://www.fda.gov/forconsumers/consumerupdates/ucm049367.htm. Updated October 17, 2017. Accessed October 31, 2017.
- Chan TY. Potential dangers from topical preparations containing methyl salicylate. Hum Exp Toxicol. 1996;15:747-750.
- Heng MC. Local necrosis and interstitial nephritis due to topical methyl salicylate and menthol. Cutis. 1987;39:442-444.
- Davis JE. Are one or two dangerous? methyl salicylate exposure in toddlers. J Emerg Med. 2007;32:63-69.
- Associated Press. Sports cream warnings urged after teen’s death: track star’s overdose points to risks of popular muscle salve. NBC News. http://www.nbcnews.com/id/19208195. Updated June 13, 2007. Accessed October 31, 2017.
- Ultra Strength Bengay Cream. Bengay website. http://www.bengay.com/bengay-ultra-strength-cream. Accessed November 1, 2017.
- Tiger Balm Arthritis Rub. Tiger Balm website. http://www.tigerbalm.com/us/pages/tb_product?product_id=6. Accessed November 1, 2017.
- Morra P, Bartle WR, Walker SE, et al. Serum concentrations of salicylic acid following topically applied salicylate derivatives. Ann Pharmacother. 1996;9:935-940.
- US National Library of Medicine. Bengay Ultra Strength non greasy pain relieving- camphor (synthetic), menthol, and methyl salicylate cream. Daily Med website. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5aa265f8-ab45-47b2-b5ab-d4df54daed01. Updated November 3, 2016. Accessed November 1, 2017.
- Aspinall JB, Goel KM. Salicylate poisoning in dermatological therapy. Br Med J. 1978;2:1373.
To the Editor:
The combination of menthol and methyl salicylate found in a variety of over-the-counter (OTC) creams in conjunction with a heat source such as a heating pad used for musculoskeletal symptoms can be a dire combination due to increased systemic absorption with associated toxicity and localized effects ranging from contact dermatitis or irritation to burn or necrosis.1-6 We present a case of localized burn due a combination of topical methyl salicylate and heating pad use. We also discuss 2 commonly encountered side effects in the literature—localized burns and systemic toxicity associated with percutaneous absorption—and provide specific considerations related to the geriatric and pediatric populations.
A 62-year-old woman with a history of eczematous dermatitis and osteoarthritis with pain of the left shoulder presented to the dermatology clinic with painful skin-related changes on the left arm of 1 week’s duration. She was prescribed acetaminophen and ibuprofen. However, she self-medicated the left shoulder pain with 2 OTC products containing topical menthol and/or methyl salicylate in combination with a heating pad and likely fell asleep with this combination therapy applied. She noticed the burn the next morning. On examination, the left arm exhibited a geometric, irregularly shaped, erythematous, scaly plaque with a sharp transverse linear demarcation proximally and numerous erythematous linear scaly plaques oriented in an axial orientation with less-defined borders distally (Figure). The patient was diagnosed with burn secondary to combination of topical methyl salicylate and heating pad use. The patient was advised to discontinue the topical medication and to use caution with the heating pad in the future. She was prescribed pramoxine-hydrocortisone lotion to be applied to the affected area twice daily up to 5 days weekly until resolution. Subsequent evaluations revealed progressive improvement with only mild postinflammatory hyperpigmentation noted at 6 months after the burn.

The US Food and Drug Administration (FDA) released statements in 2012 regarding concern for burns related to use of OTC musculoskeletal pain relievers, with 43 cases of burns reported due to methyl salicylate and menthol from 2004 to 2010. Most of the second- and third-degree burns occurred following topical applications of products containing either menthol monotherapy or a combination of methyl salicylate and menthol.1,2 In 2006, the FDA had already ordered 5 firms to stop compounding topical pain relief formulations containing these ingredients, with concerns that it puts patients at increased risk because the compounded formulations had not received FDA approval.3 Despite package warnings, patients may not be aware of the concerning side effects and risks associated with use of OTC creams, especially in combination with occlusion or heating pad use. Our case highlights the importance of ongoing patient education and physician counseling when encountering patients with arthritis or musculoskeletal pain who may often try various OTC self-treatments for pain relief.7
In 2012, the FDA reports stated that the cases of mild to serious burns were associated with methyl salicylate and menthol usage, in some cases 24 hours after first usage. Typically, these effects occur when concentrations are more than either 3% menthol alone or a combination of more than 3% menthol and more than 10% methyl salicylate.1,2 In our case, the patient had been using 2 different OTC products that may have contained as much as 11% menthol and/or 30% methyl salicylate. Electronic resources are available that disclose safety instructions including not to occlude the site, not to use on wounds, and not to be used in conjunction with a heating pad.8,9 Skin breakdown and vasodilation are more likely to occur in a setting of heat and occlusion, which allows for more absorption and localized side effects.4,10 Localized reactions may range from contact dermatitis4 to muscle necrosis.5
The most noteworthy case of localized destruction described a 62-year-old man who had applied topical methyl salicylate and menthol to the forearms, calves, and thighs, then intermittently used a heating pad for 15 to 20 minutes (total duration).5 He subsequently developed erythema and numerous 7.62- to 10.16-cm bullae, which was thought to be consistent with contact dermatitis. Three days later, he was found to have full-thickness cutaneous, fascial, and muscle necrosis in a linear pattern. He was hospitalized for approximately 1 year and treated with extensive debridement and a skin graft. His serum creatinine level increased from 0.7 mg per 100 mL to 2.7 mg per 100 mL (reference range, 0.6–1.2 mg/dL) with evidence of toxic nephrosis and persistent interstitial nephritis, demonstrating the severity of localized destruction that may result when combining these products with direct heat and potential subsequent systemic consequences of this combination.5
The systemic absorption of OTC formulations also has been studied. Morra et al10 studied 12 volunteers (6 women, 6 men) who applied either 5 g of methyl salicylate ointment 12.5% twice daily for 4 days to an area on the thigh (approximately equal to 567 mg salicylate) or trolamine cream 10% twice for 1 day. The participants underwent a break for 7 days and then switched to the alternate treatment. They found that 0.31 to 0.91 mg/L methyl salicylate was detected in the serum 1 hour after applying the ointment consisting of methyl salicylate, and 2 to 6 mg/L methyl salicylate was detected on day 4. Therapeutic serum salicylate levels are 150 to 300 mg/L. They found that approximately 22% of the methyl salicylate also was found in urine samples on day 4. Although these figures may appear small, this study was prompted when a 62-year-old man presented to the emergency department with symptoms of salicylate toxicity and a serum concentration of 518 mg/L from twice-daily use of an OTC formulation containing methyl salicylate over the course of multiple weeks.10 Additionally, those who have aspirin hypersensitivity should be cautious when using such products due to the risk for reported angioedema.4
Providers must exercise extreme caution while caring for geriatric patients, especially if patients are taking warfarin. The combined effects of warfarin and methyl salicylate have previously caused cutaneous purpura, gastrointestinal bleeding, and elevated international normalized ratio values.4,10 Older individuals also have increased skin fragility, allowing microtraumatic insult to easily develop. This fragility, along with an overall decreased intactness of the skin barrier, may lead to increased skin absorption. Furthermore, the addition of applying any heat source places the geriatric patient at greater risk for adverse events.10
In considering the limits of age, the pediatric population also has been studied regarding salicylate toxicity. Most commonly, oral ingestion has caused fatalities, as oil of wintergreen has been cited as extremely dangerous for children if swallowed; doses as small as a teaspoon (5 mL: 7000 mg salicylate) have resulted in fatalities.4,6 Although the consumption of a large amount of a cream- or ointment-based product is unlikely due to the consistency of the medication,6 the thought does merit consideration in the inquisitive toddler age group. For a 15-kg toddler, 150 mg/kg of aspirin or 2250 mg of aspirin, is considered the toxic level, which upon conversion to methyl salicylate levels using a 1.4 factor equates to 1607 mg of methyl salicylate to reach toxicity.6 If using a product with methyl salicylate 30% composition, 1 g of the product contains 300 mg of methyl salicylate; therefore if the toddler consumed approximately 5.3 g of the product (1607 mg methyl salicylate [toxic level] divided by 300 mg methyl salicylate per 1 g of product), he/she would reach toxic levels.6,11 To put this into perspective, a 2-oz tube contains 57 g (approximately 10 times the toxic dose) of the product.8 Thus, although there is less concern overall for consumption of cream- or ointment-based methyl salicylate, there still is potential for harm if a small child were to ingest such a product containing higher percentages of methyl salicylate.6
There also have been reports of pediatric toxicity related to percutaneous absorption, even leading to pediatric fatality.4,6 In particular, there was a case of a young boy hospitalized with ichthyosis who received escalating doses of percutaneous salicylate, which resulted in toxicity; when therapy was discontinued, he experienced full recovery.12 In 2007, a 17-year-old adolescent girl died from methyl salicylate toxicity after numerous applications of salicylate-containing products in conjunction with medicated pads.7
Although the FDA has drawn attention and encouraged caution with use of OTC topical musculoskeletal pain relievers, the importance of ensuring patients are fully aware of potential burns, permanent skin or muscle damage, and even death if used inappropriately cannot be overstated. The FDA consumer health information website has 2 patient-directed handouts2,3 that may be useful to post in patient waiting areas to increase overall understanding of the risks associated with OTC products containing methyl salicylate and menthol ingredients. Fortunately, our patient suffered only mild postinflammatory hyperpigmentation without substantial sustained consequences.
To the Editor:
The combination of menthol and methyl salicylate found in a variety of over-the-counter (OTC) creams in conjunction with a heat source such as a heating pad used for musculoskeletal symptoms can be a dire combination due to increased systemic absorption with associated toxicity and localized effects ranging from contact dermatitis or irritation to burn or necrosis.1-6 We present a case of localized burn due a combination of topical methyl salicylate and heating pad use. We also discuss 2 commonly encountered side effects in the literature—localized burns and systemic toxicity associated with percutaneous absorption—and provide specific considerations related to the geriatric and pediatric populations.
A 62-year-old woman with a history of eczematous dermatitis and osteoarthritis with pain of the left shoulder presented to the dermatology clinic with painful skin-related changes on the left arm of 1 week’s duration. She was prescribed acetaminophen and ibuprofen. However, she self-medicated the left shoulder pain with 2 OTC products containing topical menthol and/or methyl salicylate in combination with a heating pad and likely fell asleep with this combination therapy applied. She noticed the burn the next morning. On examination, the left arm exhibited a geometric, irregularly shaped, erythematous, scaly plaque with a sharp transverse linear demarcation proximally and numerous erythematous linear scaly plaques oriented in an axial orientation with less-defined borders distally (Figure). The patient was diagnosed with burn secondary to combination of topical methyl salicylate and heating pad use. The patient was advised to discontinue the topical medication and to use caution with the heating pad in the future. She was prescribed pramoxine-hydrocortisone lotion to be applied to the affected area twice daily up to 5 days weekly until resolution. Subsequent evaluations revealed progressive improvement with only mild postinflammatory hyperpigmentation noted at 6 months after the burn.

The US Food and Drug Administration (FDA) released statements in 2012 regarding concern for burns related to use of OTC musculoskeletal pain relievers, with 43 cases of burns reported due to methyl salicylate and menthol from 2004 to 2010. Most of the second- and third-degree burns occurred following topical applications of products containing either menthol monotherapy or a combination of methyl salicylate and menthol.1,2 In 2006, the FDA had already ordered 5 firms to stop compounding topical pain relief formulations containing these ingredients, with concerns that it puts patients at increased risk because the compounded formulations had not received FDA approval.3 Despite package warnings, patients may not be aware of the concerning side effects and risks associated with use of OTC creams, especially in combination with occlusion or heating pad use. Our case highlights the importance of ongoing patient education and physician counseling when encountering patients with arthritis or musculoskeletal pain who may often try various OTC self-treatments for pain relief.7
In 2012, the FDA reports stated that the cases of mild to serious burns were associated with methyl salicylate and menthol usage, in some cases 24 hours after first usage. Typically, these effects occur when concentrations are more than either 3% menthol alone or a combination of more than 3% menthol and more than 10% methyl salicylate.1,2 In our case, the patient had been using 2 different OTC products that may have contained as much as 11% menthol and/or 30% methyl salicylate. Electronic resources are available that disclose safety instructions including not to occlude the site, not to use on wounds, and not to be used in conjunction with a heating pad.8,9 Skin breakdown and vasodilation are more likely to occur in a setting of heat and occlusion, which allows for more absorption and localized side effects.4,10 Localized reactions may range from contact dermatitis4 to muscle necrosis.5
The most noteworthy case of localized destruction described a 62-year-old man who had applied topical methyl salicylate and menthol to the forearms, calves, and thighs, then intermittently used a heating pad for 15 to 20 minutes (total duration).5 He subsequently developed erythema and numerous 7.62- to 10.16-cm bullae, which was thought to be consistent with contact dermatitis. Three days later, he was found to have full-thickness cutaneous, fascial, and muscle necrosis in a linear pattern. He was hospitalized for approximately 1 year and treated with extensive debridement and a skin graft. His serum creatinine level increased from 0.7 mg per 100 mL to 2.7 mg per 100 mL (reference range, 0.6–1.2 mg/dL) with evidence of toxic nephrosis and persistent interstitial nephritis, demonstrating the severity of localized destruction that may result when combining these products with direct heat and potential subsequent systemic consequences of this combination.5
The systemic absorption of OTC formulations also has been studied. Morra et al10 studied 12 volunteers (6 women, 6 men) who applied either 5 g of methyl salicylate ointment 12.5% twice daily for 4 days to an area on the thigh (approximately equal to 567 mg salicylate) or trolamine cream 10% twice for 1 day. The participants underwent a break for 7 days and then switched to the alternate treatment. They found that 0.31 to 0.91 mg/L methyl salicylate was detected in the serum 1 hour after applying the ointment consisting of methyl salicylate, and 2 to 6 mg/L methyl salicylate was detected on day 4. Therapeutic serum salicylate levels are 150 to 300 mg/L. They found that approximately 22% of the methyl salicylate also was found in urine samples on day 4. Although these figures may appear small, this study was prompted when a 62-year-old man presented to the emergency department with symptoms of salicylate toxicity and a serum concentration of 518 mg/L from twice-daily use of an OTC formulation containing methyl salicylate over the course of multiple weeks.10 Additionally, those who have aspirin hypersensitivity should be cautious when using such products due to the risk for reported angioedema.4
Providers must exercise extreme caution while caring for geriatric patients, especially if patients are taking warfarin. The combined effects of warfarin and methyl salicylate have previously caused cutaneous purpura, gastrointestinal bleeding, and elevated international normalized ratio values.4,10 Older individuals also have increased skin fragility, allowing microtraumatic insult to easily develop. This fragility, along with an overall decreased intactness of the skin barrier, may lead to increased skin absorption. Furthermore, the addition of applying any heat source places the geriatric patient at greater risk for adverse events.10
In considering the limits of age, the pediatric population also has been studied regarding salicylate toxicity. Most commonly, oral ingestion has caused fatalities, as oil of wintergreen has been cited as extremely dangerous for children if swallowed; doses as small as a teaspoon (5 mL: 7000 mg salicylate) have resulted in fatalities.4,6 Although the consumption of a large amount of a cream- or ointment-based product is unlikely due to the consistency of the medication,6 the thought does merit consideration in the inquisitive toddler age group. For a 15-kg toddler, 150 mg/kg of aspirin or 2250 mg of aspirin, is considered the toxic level, which upon conversion to methyl salicylate levels using a 1.4 factor equates to 1607 mg of methyl salicylate to reach toxicity.6 If using a product with methyl salicylate 30% composition, 1 g of the product contains 300 mg of methyl salicylate; therefore if the toddler consumed approximately 5.3 g of the product (1607 mg methyl salicylate [toxic level] divided by 300 mg methyl salicylate per 1 g of product), he/she would reach toxic levels.6,11 To put this into perspective, a 2-oz tube contains 57 g (approximately 10 times the toxic dose) of the product.8 Thus, although there is less concern overall for consumption of cream- or ointment-based methyl salicylate, there still is potential for harm if a small child were to ingest such a product containing higher percentages of methyl salicylate.6
There also have been reports of pediatric toxicity related to percutaneous absorption, even leading to pediatric fatality.4,6 In particular, there was a case of a young boy hospitalized with ichthyosis who received escalating doses of percutaneous salicylate, which resulted in toxicity; when therapy was discontinued, he experienced full recovery.12 In 2007, a 17-year-old adolescent girl died from methyl salicylate toxicity after numerous applications of salicylate-containing products in conjunction with medicated pads.7
Although the FDA has drawn attention and encouraged caution with use of OTC topical musculoskeletal pain relievers, the importance of ensuring patients are fully aware of potential burns, permanent skin or muscle damage, and even death if used inappropriately cannot be overstated. The FDA consumer health information website has 2 patient-directed handouts2,3 that may be useful to post in patient waiting areas to increase overall understanding of the risks associated with OTC products containing methyl salicylate and menthol ingredients. Fortunately, our patient suffered only mild postinflammatory hyperpigmentation without substantial sustained consequences.
- US Food and Drug Administration. FDA Drug Safety Communication: rare cases of serious burns with the use of over-the-counter topical muscle and joint pain relievers. http://www.fda.gov/Drugs/DrugSafety/ucm318858.htm. Published September 13, 2012. Updated February 11, 2016. Accessed October 31, 2017.
- US Food and Drug Administration. Topical pain relievers may cause burns. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm318674.htm. Published September 13, 2012. Updated November 5, 2015. Accessed October 31, 2017.
- US Food and Drug Administration. Use caution with over-the-counter creams, ointments. http://www.fda.gov/forconsumers/consumerupdates/ucm049367.htm. Updated October 17, 2017. Accessed October 31, 2017.
- Chan TY. Potential dangers from topical preparations containing methyl salicylate. Hum Exp Toxicol. 1996;15:747-750.
- Heng MC. Local necrosis and interstitial nephritis due to topical methyl salicylate and menthol. Cutis. 1987;39:442-444.
- Davis JE. Are one or two dangerous? methyl salicylate exposure in toddlers. J Emerg Med. 2007;32:63-69.
- Associated Press. Sports cream warnings urged after teen’s death: track star’s overdose points to risks of popular muscle salve. NBC News. http://www.nbcnews.com/id/19208195. Updated June 13, 2007. Accessed October 31, 2017.
- Ultra Strength Bengay Cream. Bengay website. http://www.bengay.com/bengay-ultra-strength-cream. Accessed November 1, 2017.
- Tiger Balm Arthritis Rub. Tiger Balm website. http://www.tigerbalm.com/us/pages/tb_product?product_id=6. Accessed November 1, 2017.
- Morra P, Bartle WR, Walker SE, et al. Serum concentrations of salicylic acid following topically applied salicylate derivatives. Ann Pharmacother. 1996;9:935-940.
- US National Library of Medicine. Bengay Ultra Strength non greasy pain relieving- camphor (synthetic), menthol, and methyl salicylate cream. Daily Med website. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5aa265f8-ab45-47b2-b5ab-d4df54daed01. Updated November 3, 2016. Accessed November 1, 2017.
- Aspinall JB, Goel KM. Salicylate poisoning in dermatological therapy. Br Med J. 1978;2:1373.
- US Food and Drug Administration. FDA Drug Safety Communication: rare cases of serious burns with the use of over-the-counter topical muscle and joint pain relievers. http://www.fda.gov/Drugs/DrugSafety/ucm318858.htm. Published September 13, 2012. Updated February 11, 2016. Accessed October 31, 2017.
- US Food and Drug Administration. Topical pain relievers may cause burns. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm318674.htm. Published September 13, 2012. Updated November 5, 2015. Accessed October 31, 2017.
- US Food and Drug Administration. Use caution with over-the-counter creams, ointments. http://www.fda.gov/forconsumers/consumerupdates/ucm049367.htm. Updated October 17, 2017. Accessed October 31, 2017.
- Chan TY. Potential dangers from topical preparations containing methyl salicylate. Hum Exp Toxicol. 1996;15:747-750.
- Heng MC. Local necrosis and interstitial nephritis due to topical methyl salicylate and menthol. Cutis. 1987;39:442-444.
- Davis JE. Are one or two dangerous? methyl salicylate exposure in toddlers. J Emerg Med. 2007;32:63-69.
- Associated Press. Sports cream warnings urged after teen’s death: track star’s overdose points to risks of popular muscle salve. NBC News. http://www.nbcnews.com/id/19208195. Updated June 13, 2007. Accessed October 31, 2017.
- Ultra Strength Bengay Cream. Bengay website. http://www.bengay.com/bengay-ultra-strength-cream. Accessed November 1, 2017.
- Tiger Balm Arthritis Rub. Tiger Balm website. http://www.tigerbalm.com/us/pages/tb_product?product_id=6. Accessed November 1, 2017.
- Morra P, Bartle WR, Walker SE, et al. Serum concentrations of salicylic acid following topically applied salicylate derivatives. Ann Pharmacother. 1996;9:935-940.
- US National Library of Medicine. Bengay Ultra Strength non greasy pain relieving- camphor (synthetic), menthol, and methyl salicylate cream. Daily Med website. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5aa265f8-ab45-47b2-b5ab-d4df54daed01. Updated November 3, 2016. Accessed November 1, 2017.
- Aspinall JB, Goel KM. Salicylate poisoning in dermatological therapy. Br Med J. 1978;2:1373.
Practice Points
- Recognize the potential complication of burn from use of over-the-counter (OTC) musculoskeletal relievers in combination with a heat source.
- Screen for OTC product use as well as device application when evaluating an atypically patterned cutaneous eruption.
- Recognize potential toxicity associated with both topical application and accidental ingestion in the pediatric population.
- Physicians should become familiar with resources available, including patient handouts that describe risks associated with use of OTC musculoskeletal relievers containing methyl salicylate and menthol ingredients.
Pediatricians urge focus on human trafficking victims
For pediatricians to better help victims of human trafficking, it is critical that there be improvements in research, medical education, advocacy, and community collaborations, according to a policy statement issued by the American Academy of Pediatrics (AAP) Committee on Child Abuse and Neglect, Section on International Child Health.
The full effects of human trafficking are still very much hidden in shadows, leading the AAP to add human trafficking education to its list of top 10 priorities at the 2014 AAP Annual Leadership Forum.
The policy statement is the latest step in the AAP’s effort to involve medical professionals more heavily in combating child sex and labor trafficking, which the AAP asserts is a critical role for pediatricians. Even with the limited information available, the United Nations Office of Drugs and Crime 2016 report found 33% of the 40,000 trafficking victims reported were children, an indication of the vulnerability of younger populations.
“We wanted to give not just background information on trafficking, but we also wanted to give some guidance for academy members in regard to how we can increase awareness of human trafficking, how we can start to implement it into medical education for medical trainees and practicing pediatricians,” Nia Bodrick, MD, MPH, coauthor of the statement and a member of the AAP Section on International Child Health, said in an interview. “Due to the very narrow amount of evidence-based research that is available on this issue, there is a call to look more deeply into the topic.”
Currently, many health care professionals may be more exposed to child trafficking than they think because of a lack knowledge on how to recognize it. Victims can pass unnoticed.
“Most victims and survivors of human trafficking have had some sort of interaction with the health care setting at some point before they escape their situation,” said Dr. Bodrick. “So whether we as pediatricians realize it or not, we are more than likely interacting with young people who are in these situations.”
There are not enough data to list definitive characteristics of trafficking victims; however, there are certain signs associated with those who have been identified as victims so far. While history of neglect, homelessness, substance abuse, mental disorder, and identifying as a member of the LGBTQ community are more common among trafficking victims, many doctors are not equipped to handle confronting patients about the possibility of sex or labor trafficking, nor do they know what resources are available.
The AAP emphasized a need to expand the current scope of knowledge medical professionals have regarding such victims to create better, evidence-based care programs and protective policies.
An influx of information would help pediatricians understand the prevalence of trafficking, the extent of physical and emotional harm victims experience, and the effectiveness of psychological and mental health interventions, according to the statement (Pediatrics. 2017 November. doi: 10.1542/peds.2017-3138).
It also may help shed light on male trafficking victims, which Dr. Bodrick and coauthor Jordan Greenbaum, MD, of the Stephanie V. Blank Center for Safe and Healthy Children at Children’s Healthcare of Atlanta, assert are highly underrepresented.
Separating human trafficking from other types of violence and exploitation in the International Classification of Diseases codes may improve research initiatives, according to Dr. Bodrick.
There is also a need to delve deeper into the social determinants of health to better predict what makes victims more vulnerable.
While improving the number of studies on trafficking is essential, the results are more long term, which means in the mean time other actions can, and should, be made, according to the statement.
One of the best things pediatricians can do is gather as much knowledge on the topic as they can, according to Dr. Bodrick.
“For the average pediatrician, one of the first things one could do is educate oneself on the topic,” she explained. “Look for CME associated with your local AAP chapter or even on a national level online or even if you just have a couple hours for lunch, to become aware of what’s happening.”
The AAP also is encouraging physicians to advocate for legislative policies that will help improve victim care and to create partnerships within the community.
“In DC, in our local AAP chapter, we have opportunities to go to the Hill and advocate for bills, and while not everyone can do that, there are always local legislators that pediatricians can push to do more,” said Dr. Bodrick. “If you have a local paper, you could write an op-ed about the topic, you could educate your local schools about the topic by talking to the school board, or you could even engage in interviews on a local radio or television station.”
While many states have laws protecting victims of sex trafficking from being prosecuted for performing illegal acts, many have not passed such legislation.
Pediatricians can see what protections their states have through the National Conference of State Legislatures’ web page, as well as review current human trafficking laws.
Dr. Greenbaum and Dr. Bodrick reported no relevant financial disclosures.
[email protected]
On Twitter@eaztweets
This statement does a good job bringing the health aspects of human trafficking into the open. Human trafficking has become a significant issue in the United States, and this document serves as a way to open the door for discussion on how pediatricians can become a part of the solution.
Armed with a clearer idea of the issues of child trafficking, pediatricians should take this document as a call to increase their – and their colleagues’ – awareness on this issue. Whether that involves a deeper focus on the social environmental determinants of health or political advocacy, education is key to developing a better role as a pediatrician as we work together to help those who have experienced trafficking first hand.
Francis Rushton Jr., MD, practiced pediatrics in Beaufort, S.C. for 32 years. He also is a member of the Pediatric News editorial advisory board.
This statement does a good job bringing the health aspects of human trafficking into the open. Human trafficking has become a significant issue in the United States, and this document serves as a way to open the door for discussion on how pediatricians can become a part of the solution.
Armed with a clearer idea of the issues of child trafficking, pediatricians should take this document as a call to increase their – and their colleagues’ – awareness on this issue. Whether that involves a deeper focus on the social environmental determinants of health or political advocacy, education is key to developing a better role as a pediatrician as we work together to help those who have experienced trafficking first hand.
Francis Rushton Jr., MD, practiced pediatrics in Beaufort, S.C. for 32 years. He also is a member of the Pediatric News editorial advisory board.
This statement does a good job bringing the health aspects of human trafficking into the open. Human trafficking has become a significant issue in the United States, and this document serves as a way to open the door for discussion on how pediatricians can become a part of the solution.
Armed with a clearer idea of the issues of child trafficking, pediatricians should take this document as a call to increase their – and their colleagues’ – awareness on this issue. Whether that involves a deeper focus on the social environmental determinants of health or political advocacy, education is key to developing a better role as a pediatrician as we work together to help those who have experienced trafficking first hand.
Francis Rushton Jr., MD, practiced pediatrics in Beaufort, S.C. for 32 years. He also is a member of the Pediatric News editorial advisory board.
For pediatricians to better help victims of human trafficking, it is critical that there be improvements in research, medical education, advocacy, and community collaborations, according to a policy statement issued by the American Academy of Pediatrics (AAP) Committee on Child Abuse and Neglect, Section on International Child Health.
The full effects of human trafficking are still very much hidden in shadows, leading the AAP to add human trafficking education to its list of top 10 priorities at the 2014 AAP Annual Leadership Forum.
The policy statement is the latest step in the AAP’s effort to involve medical professionals more heavily in combating child sex and labor trafficking, which the AAP asserts is a critical role for pediatricians. Even with the limited information available, the United Nations Office of Drugs and Crime 2016 report found 33% of the 40,000 trafficking victims reported were children, an indication of the vulnerability of younger populations.
“We wanted to give not just background information on trafficking, but we also wanted to give some guidance for academy members in regard to how we can increase awareness of human trafficking, how we can start to implement it into medical education for medical trainees and practicing pediatricians,” Nia Bodrick, MD, MPH, coauthor of the statement and a member of the AAP Section on International Child Health, said in an interview. “Due to the very narrow amount of evidence-based research that is available on this issue, there is a call to look more deeply into the topic.”
Currently, many health care professionals may be more exposed to child trafficking than they think because of a lack knowledge on how to recognize it. Victims can pass unnoticed.
“Most victims and survivors of human trafficking have had some sort of interaction with the health care setting at some point before they escape their situation,” said Dr. Bodrick. “So whether we as pediatricians realize it or not, we are more than likely interacting with young people who are in these situations.”
There are not enough data to list definitive characteristics of trafficking victims; however, there are certain signs associated with those who have been identified as victims so far. While history of neglect, homelessness, substance abuse, mental disorder, and identifying as a member of the LGBTQ community are more common among trafficking victims, many doctors are not equipped to handle confronting patients about the possibility of sex or labor trafficking, nor do they know what resources are available.
The AAP emphasized a need to expand the current scope of knowledge medical professionals have regarding such victims to create better, evidence-based care programs and protective policies.
An influx of information would help pediatricians understand the prevalence of trafficking, the extent of physical and emotional harm victims experience, and the effectiveness of psychological and mental health interventions, according to the statement (Pediatrics. 2017 November. doi: 10.1542/peds.2017-3138).
It also may help shed light on male trafficking victims, which Dr. Bodrick and coauthor Jordan Greenbaum, MD, of the Stephanie V. Blank Center for Safe and Healthy Children at Children’s Healthcare of Atlanta, assert are highly underrepresented.
Separating human trafficking from other types of violence and exploitation in the International Classification of Diseases codes may improve research initiatives, according to Dr. Bodrick.
There is also a need to delve deeper into the social determinants of health to better predict what makes victims more vulnerable.
While improving the number of studies on trafficking is essential, the results are more long term, which means in the mean time other actions can, and should, be made, according to the statement.
One of the best things pediatricians can do is gather as much knowledge on the topic as they can, according to Dr. Bodrick.
“For the average pediatrician, one of the first things one could do is educate oneself on the topic,” she explained. “Look for CME associated with your local AAP chapter or even on a national level online or even if you just have a couple hours for lunch, to become aware of what’s happening.”
The AAP also is encouraging physicians to advocate for legislative policies that will help improve victim care and to create partnerships within the community.
“In DC, in our local AAP chapter, we have opportunities to go to the Hill and advocate for bills, and while not everyone can do that, there are always local legislators that pediatricians can push to do more,” said Dr. Bodrick. “If you have a local paper, you could write an op-ed about the topic, you could educate your local schools about the topic by talking to the school board, or you could even engage in interviews on a local radio or television station.”
While many states have laws protecting victims of sex trafficking from being prosecuted for performing illegal acts, many have not passed such legislation.
Pediatricians can see what protections their states have through the National Conference of State Legislatures’ web page, as well as review current human trafficking laws.
Dr. Greenbaum and Dr. Bodrick reported no relevant financial disclosures.
[email protected]
On Twitter@eaztweets
For pediatricians to better help victims of human trafficking, it is critical that there be improvements in research, medical education, advocacy, and community collaborations, according to a policy statement issued by the American Academy of Pediatrics (AAP) Committee on Child Abuse and Neglect, Section on International Child Health.
The full effects of human trafficking are still very much hidden in shadows, leading the AAP to add human trafficking education to its list of top 10 priorities at the 2014 AAP Annual Leadership Forum.
The policy statement is the latest step in the AAP’s effort to involve medical professionals more heavily in combating child sex and labor trafficking, which the AAP asserts is a critical role for pediatricians. Even with the limited information available, the United Nations Office of Drugs and Crime 2016 report found 33% of the 40,000 trafficking victims reported were children, an indication of the vulnerability of younger populations.
“We wanted to give not just background information on trafficking, but we also wanted to give some guidance for academy members in regard to how we can increase awareness of human trafficking, how we can start to implement it into medical education for medical trainees and practicing pediatricians,” Nia Bodrick, MD, MPH, coauthor of the statement and a member of the AAP Section on International Child Health, said in an interview. “Due to the very narrow amount of evidence-based research that is available on this issue, there is a call to look more deeply into the topic.”
Currently, many health care professionals may be more exposed to child trafficking than they think because of a lack knowledge on how to recognize it. Victims can pass unnoticed.
“Most victims and survivors of human trafficking have had some sort of interaction with the health care setting at some point before they escape their situation,” said Dr. Bodrick. “So whether we as pediatricians realize it or not, we are more than likely interacting with young people who are in these situations.”
There are not enough data to list definitive characteristics of trafficking victims; however, there are certain signs associated with those who have been identified as victims so far. While history of neglect, homelessness, substance abuse, mental disorder, and identifying as a member of the LGBTQ community are more common among trafficking victims, many doctors are not equipped to handle confronting patients about the possibility of sex or labor trafficking, nor do they know what resources are available.
The AAP emphasized a need to expand the current scope of knowledge medical professionals have regarding such victims to create better, evidence-based care programs and protective policies.
An influx of information would help pediatricians understand the prevalence of trafficking, the extent of physical and emotional harm victims experience, and the effectiveness of psychological and mental health interventions, according to the statement (Pediatrics. 2017 November. doi: 10.1542/peds.2017-3138).
It also may help shed light on male trafficking victims, which Dr. Bodrick and coauthor Jordan Greenbaum, MD, of the Stephanie V. Blank Center for Safe and Healthy Children at Children’s Healthcare of Atlanta, assert are highly underrepresented.
Separating human trafficking from other types of violence and exploitation in the International Classification of Diseases codes may improve research initiatives, according to Dr. Bodrick.
There is also a need to delve deeper into the social determinants of health to better predict what makes victims more vulnerable.
While improving the number of studies on trafficking is essential, the results are more long term, which means in the mean time other actions can, and should, be made, according to the statement.
One of the best things pediatricians can do is gather as much knowledge on the topic as they can, according to Dr. Bodrick.
“For the average pediatrician, one of the first things one could do is educate oneself on the topic,” she explained. “Look for CME associated with your local AAP chapter or even on a national level online or even if you just have a couple hours for lunch, to become aware of what’s happening.”
The AAP also is encouraging physicians to advocate for legislative policies that will help improve victim care and to create partnerships within the community.
“In DC, in our local AAP chapter, we have opportunities to go to the Hill and advocate for bills, and while not everyone can do that, there are always local legislators that pediatricians can push to do more,” said Dr. Bodrick. “If you have a local paper, you could write an op-ed about the topic, you could educate your local schools about the topic by talking to the school board, or you could even engage in interviews on a local radio or television station.”
While many states have laws protecting victims of sex trafficking from being prosecuted for performing illegal acts, many have not passed such legislation.
Pediatricians can see what protections their states have through the National Conference of State Legislatures’ web page, as well as review current human trafficking laws.
Dr. Greenbaum and Dr. Bodrick reported no relevant financial disclosures.
[email protected]
On Twitter@eaztweets
FROM PEDIATRICS
Cellular Versus Acellular Grafts for Diabetic Foot Ulcers: Altering the Protocol to Improve Recruitment to a Comparative Efficacy Trial
Chronic diabetic foot ulcers (DFUs) remain a serious therapeutic challenge worldwide.1-2 Patients with DFUs are at higher risk for infections, which may lead to limb loss.1-5 In fact, 1 in 6 patients with DFUs will undergo an amputation.6 The long-term consequences of DFUs are numerous and can severely affect patients’ quality of life, including loss of productivity.7 The current standard of care for DFUs consists of debridement of the necrotic tissue, application of a moist dressing, and use of an off-loading device that protects the wound from pressure or trauma related to ambulation and other acts of daily living.4-6,8 Unfortunately, studies have shown that the best standard of care (SOC) only heals 30% of DFUs after 20 weeks of therapy.9 With the estimated cost per episode of care approaching $40,000, DFUs remain a costly and important problem.10
The altered extracellular matrix (ECM) in DFUs has been a target for the development of new therapeutic devices that provide a new matrix that is either devoid of cells or can be enriched with fibroblasts.8,11 These bioengineered skin substitutes stimulate the growth of new vessels and generate cytokines essential for tissue repair. In 2013, Lev-Tov et al12 published this study protocol (Dermagraft Oasis Longitudinal Comparative Efficacy [DOLCE] trial) to compare the effectiveness of 2 advanced wound care devices, specifically to evaluate the clinical efficacy of a cellular matrix versus an acellular matrix, which we have amended. The cellular matrix used in the study is a dermal substitute composed of viable newborn foreskin fibroblasts seeded onto a bioabsorbable polyglactin mesh on which fibroblasts generate an ECM.13,14 It is supplied frozen and requires specific thawing steps prior to application. The recommended regimen for treatment of DFUs for this cellular matrix is 8 weekly applications.13,14 In 2016, the cost of the product was reported as $1411 per 5.0×7.5-cm sheet.15 The acellular matrix product used in the study is a bioabsorbable ECM that is derived from porcine small intestinal submucosa.16,17 It is stored at room temperature and has a long shelf life, with a current price of $112.6 for a 3.0×3.5-cm single-layer fenestrated sheet ($1126.60 per box of 10 sheets). The industry-supported randomized controlled trials for each of these devices have reported a 20% added benefit in the rate of wound closure at week 12 compared to SOC.14,17
This article provides the interim report of the trial (registered at www.clinicaltrials.gov with the identifier NCT01450943) described in the published protocol and initiated in 2011,12 focusing on elements that required modification during the trial’s duration.
Methods
Study Protocol
The clinical trial was approved by the Veterans’ Affairs Institutional Research and Development Committee and their institutional review board. This study was funded by the Veteran’s Administration Merit Award (#10554640), which was awarded to 2 of the investigators (S.E.D. and R.R.I.). Eligible veterans were recruited from all 7 sites of the VA Northern California Healthcare System. This trial is a randomized, single-blinded, 3-armed, controlled clinical equivalence trial comparing the effectiveness of an SOC treatment, cellular ECM, and acellular ECM.
Study Products
The SOC dressing applied in the clinical trial included a sterile antimicrobial gel, a nonadherent dressing, and gauze.12 The SOC dressing also was used as a secondary dressing for the active treatment arms. Bacitracin antibiotic ointment was used as an alternative for patients with allergy to iodine.12
Randomization
The inclusion and exclusion criteria were previously outlined.12 After a 2-week screening phase to exclude rapid healers, patients were randomized into a treatment arm and entered the active phase for 12 weeks.
Primary and Secondary Outcomes
The primary outcome was complete wound closure by week 12.12 Complete healing was defined as full reepithelialization with no drainage or dressing requirement. The secondary outcomes included healing at 28 weeks, rate of healing, ulcer recurrence at week 20, association of wound healing with ulcer characteristics or patients’ characteristic, incidence of adverse events, and cost-effectiveness of each treatment compared to the SOC arm.12
Statistical Analysis
To detect a 25% difference in the incidence of ulcer closure between the 2 study groups and the SOC group, the estimation of the sample size was based on 80% power with a significance level of 0.05. Specifically, it was expected that 50% of the cellular and acellular matrix groups and 25% of the SOC group would reach complete wound closure. The protocol indicated that 57 participants would be enrolled in each arm (total of 171 participants). Lev-Tov et al12 discussed the statistical analysis in more detail.
Results
Study Protocol Amendments
Given the number of diabetic patients in the US veteran population, we anticipated that there would be enough participants meeting the inclusion and exclusion criteria; however, because of the difficulty with recruitment, the initial study criteria were modified. The study was initially designed to incorporate DFUs with a minimum size of 1.0 cm2.12
Another limiting criterion was the percentage of total hemoglobin level for hemoglobin A1C (HbA1C). The study was originally established to include participants with an HbA1C level of 10% of total hemoglobin or below.12 Unfortunately, the majority of the potential participants had values substantially higher, and thus could not be enrolled in the trial, requiring another amendment to the study protocol in 2014, which was approved to include patients with an HbA1C level less than 12% of total hemoglobin. This change contributed considerably to the noted increase in enrollment rates in 2015, which almost doubled relative to enrollment under the original exclusion criteria (Figure).
The study has screened more than 600 patients. Among them, 137 were assessed for eligibility; 71 were excluded for various reasons, including screen failure (eg, decrease in wound size by >40% during the 2-week screening phase), loss to follow-up, and adverse events. Sixty-six participants reached the primary outcome at week 12, while 55 participants completed the study (19 in the SOC group; 18 in the cellular matrix group; 18 in the acellular matrix group).
We have stopped enrolling patients from all sites and the community, as we have reached our target enrollment.
Comment
One of the challenges of clinical trials is the recruitment of an adequate number of participants within an appropriate time frame, which is explained by Lasagna’s Law,18 a well-described phenomenon whereby the investigator overestimates the number of potential participants available to meet the inclusion criteria. This so-called funnel-effect was partly encountered in our selection process. A review of the veteran population with DFUs seemed to be more than adequate to fulfill the sample size; however, some important participant-related factors also played a substantial role.
In addition, the Veterans’ Affairs network centralizes health information, making it readily available to all providers participating in their care. As a result, patients with diabetes mellitus typically are seen by a primary care physician along with an endocrinologist, a diabetic nurse, and/or a dietician. Despite the collaboration with an interdisciplinary team, the glycemic control of the participants remains an issue along with other psychosocial factors that are deterrents in patient compliance. As a result, patients with poorly controlled diabetes and an HbA1C level above 10% (and less than 12%) of total hemoglobin who were initially excluded from the study were reincluded after modifying the inclusion criteria. Some patients were interested in joining the study, but physical limitations (eg, impaired mobility) prompted their decision not to join the trial, even though they met all the inclusion criteria.
As far as research-related factors that could affect participation, it is notable that most of the patients were retired; thus, the interventions did not cause additional burden of taking time off from work or loss of productivity. Although randomization could be a deterrent in many clinical trials, the majority of patients were willing to participate without demanding to be assigned to a particular treatment group.
There are many factors that are intertwined and can lead to enrollment and/or attrition rates. It was critical for our team to make some adjustment without compromising the controlled nature of a randomized trial.
Acknowledgment
The authors wish to acknowledge Huong Le, DPM, MPH, who was the coauthor of the study protocol.
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743.
- Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(suppl 1):S3-S6.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228.
- Vuorisalo S, Venermo M, Lepäntalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50:275-291.
- Meijer JW, Trip J, Jaegers SM, et al. Quality of life in patients with diabetic foot ulcers. Disabil Rehabil. 2001;23:336-340.
- Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. a meta-analysis. Diabetes Care. 1999;22:692-695.
- Cavanagh P, Attinger C, Abbas Z, et al. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev. 2012;2(suppl 1):107-111.
- Panuncialman J, Falanga V. The science of wound bed preparation. Surg Clin North Am. 2009;89:611-626.
- Lev-Tov H, Li CS, Dahle S, et al. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials. 2013;14:8.
- Gentzkow GD, Iwasaki SD, Hershon KS, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350-354.
- Marston WA, Hanft J, Norwood P, et al; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701-1705.
- 2016 Dermagraft® Medicare Product and Related Procedure Payment. http://www.dermagraft.com/wp-content/uploads/sites/1/Dermagraft_Hotsheet%202016%20Q1%20HOSPITAL_FINAL.pdf. Accessed November 23, 2017.
- Oasis® Wound Matrix. http://www.oasiswoundmatrix.com/aboutowm. Accessed November 23, 2017.
- Niezgoda JA, Van Gils CC, Frykberg RG, et al. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5, pt 1):258-266.
- Torgerson JS, Arlinger K, Käppi M, et al. Principles for enhanced recruitment of subjects in a large clinical trial. the XENDOS (XENical in the prevention of Diabetes in Obese Subjects) study experience. Controlled Clin Trials. 2001;22:515-525.
Chronic diabetic foot ulcers (DFUs) remain a serious therapeutic challenge worldwide.1-2 Patients with DFUs are at higher risk for infections, which may lead to limb loss.1-5 In fact, 1 in 6 patients with DFUs will undergo an amputation.6 The long-term consequences of DFUs are numerous and can severely affect patients’ quality of life, including loss of productivity.7 The current standard of care for DFUs consists of debridement of the necrotic tissue, application of a moist dressing, and use of an off-loading device that protects the wound from pressure or trauma related to ambulation and other acts of daily living.4-6,8 Unfortunately, studies have shown that the best standard of care (SOC) only heals 30% of DFUs after 20 weeks of therapy.9 With the estimated cost per episode of care approaching $40,000, DFUs remain a costly and important problem.10
The altered extracellular matrix (ECM) in DFUs has been a target for the development of new therapeutic devices that provide a new matrix that is either devoid of cells or can be enriched with fibroblasts.8,11 These bioengineered skin substitutes stimulate the growth of new vessels and generate cytokines essential for tissue repair. In 2013, Lev-Tov et al12 published this study protocol (Dermagraft Oasis Longitudinal Comparative Efficacy [DOLCE] trial) to compare the effectiveness of 2 advanced wound care devices, specifically to evaluate the clinical efficacy of a cellular matrix versus an acellular matrix, which we have amended. The cellular matrix used in the study is a dermal substitute composed of viable newborn foreskin fibroblasts seeded onto a bioabsorbable polyglactin mesh on which fibroblasts generate an ECM.13,14 It is supplied frozen and requires specific thawing steps prior to application. The recommended regimen for treatment of DFUs for this cellular matrix is 8 weekly applications.13,14 In 2016, the cost of the product was reported as $1411 per 5.0×7.5-cm sheet.15 The acellular matrix product used in the study is a bioabsorbable ECM that is derived from porcine small intestinal submucosa.16,17 It is stored at room temperature and has a long shelf life, with a current price of $112.6 for a 3.0×3.5-cm single-layer fenestrated sheet ($1126.60 per box of 10 sheets). The industry-supported randomized controlled trials for each of these devices have reported a 20% added benefit in the rate of wound closure at week 12 compared to SOC.14,17
This article provides the interim report of the trial (registered at www.clinicaltrials.gov with the identifier NCT01450943) described in the published protocol and initiated in 2011,12 focusing on elements that required modification during the trial’s duration.
Methods
Study Protocol
The clinical trial was approved by the Veterans’ Affairs Institutional Research and Development Committee and their institutional review board. This study was funded by the Veteran’s Administration Merit Award (#10554640), which was awarded to 2 of the investigators (S.E.D. and R.R.I.). Eligible veterans were recruited from all 7 sites of the VA Northern California Healthcare System. This trial is a randomized, single-blinded, 3-armed, controlled clinical equivalence trial comparing the effectiveness of an SOC treatment, cellular ECM, and acellular ECM.
Study Products
The SOC dressing applied in the clinical trial included a sterile antimicrobial gel, a nonadherent dressing, and gauze.12 The SOC dressing also was used as a secondary dressing for the active treatment arms. Bacitracin antibiotic ointment was used as an alternative for patients with allergy to iodine.12
Randomization
The inclusion and exclusion criteria were previously outlined.12 After a 2-week screening phase to exclude rapid healers, patients were randomized into a treatment arm and entered the active phase for 12 weeks.
Primary and Secondary Outcomes
The primary outcome was complete wound closure by week 12.12 Complete healing was defined as full reepithelialization with no drainage or dressing requirement. The secondary outcomes included healing at 28 weeks, rate of healing, ulcer recurrence at week 20, association of wound healing with ulcer characteristics or patients’ characteristic, incidence of adverse events, and cost-effectiveness of each treatment compared to the SOC arm.12
Statistical Analysis
To detect a 25% difference in the incidence of ulcer closure between the 2 study groups and the SOC group, the estimation of the sample size was based on 80% power with a significance level of 0.05. Specifically, it was expected that 50% of the cellular and acellular matrix groups and 25% of the SOC group would reach complete wound closure. The protocol indicated that 57 participants would be enrolled in each arm (total of 171 participants). Lev-Tov et al12 discussed the statistical analysis in more detail.
Results
Study Protocol Amendments
Given the number of diabetic patients in the US veteran population, we anticipated that there would be enough participants meeting the inclusion and exclusion criteria; however, because of the difficulty with recruitment, the initial study criteria were modified. The study was initially designed to incorporate DFUs with a minimum size of 1.0 cm2.12
Another limiting criterion was the percentage of total hemoglobin level for hemoglobin A1C (HbA1C). The study was originally established to include participants with an HbA1C level of 10% of total hemoglobin or below.12 Unfortunately, the majority of the potential participants had values substantially higher, and thus could not be enrolled in the trial, requiring another amendment to the study protocol in 2014, which was approved to include patients with an HbA1C level less than 12% of total hemoglobin. This change contributed considerably to the noted increase in enrollment rates in 2015, which almost doubled relative to enrollment under the original exclusion criteria (Figure).

The study has screened more than 600 patients. Among them, 137 were assessed for eligibility; 71 were excluded for various reasons, including screen failure (eg, decrease in wound size by >40% during the 2-week screening phase), loss to follow-up, and adverse events. Sixty-six participants reached the primary outcome at week 12, while 55 participants completed the study (19 in the SOC group; 18 in the cellular matrix group; 18 in the acellular matrix group).
We have stopped enrolling patients from all sites and the community, as we have reached our target enrollment.
Comment
One of the challenges of clinical trials is the recruitment of an adequate number of participants within an appropriate time frame, which is explained by Lasagna’s Law,18 a well-described phenomenon whereby the investigator overestimates the number of potential participants available to meet the inclusion criteria. This so-called funnel-effect was partly encountered in our selection process. A review of the veteran population with DFUs seemed to be more than adequate to fulfill the sample size; however, some important participant-related factors also played a substantial role.
In addition, the Veterans’ Affairs network centralizes health information, making it readily available to all providers participating in their care. As a result, patients with diabetes mellitus typically are seen by a primary care physician along with an endocrinologist, a diabetic nurse, and/or a dietician. Despite the collaboration with an interdisciplinary team, the glycemic control of the participants remains an issue along with other psychosocial factors that are deterrents in patient compliance. As a result, patients with poorly controlled diabetes and an HbA1C level above 10% (and less than 12%) of total hemoglobin who were initially excluded from the study were reincluded after modifying the inclusion criteria. Some patients were interested in joining the study, but physical limitations (eg, impaired mobility) prompted their decision not to join the trial, even though they met all the inclusion criteria.
As far as research-related factors that could affect participation, it is notable that most of the patients were retired; thus, the interventions did not cause additional burden of taking time off from work or loss of productivity. Although randomization could be a deterrent in many clinical trials, the majority of patients were willing to participate without demanding to be assigned to a particular treatment group.
There are many factors that are intertwined and can lead to enrollment and/or attrition rates. It was critical for our team to make some adjustment without compromising the controlled nature of a randomized trial.
Acknowledgment
The authors wish to acknowledge Huong Le, DPM, MPH, who was the coauthor of the study protocol.
Chronic diabetic foot ulcers (DFUs) remain a serious therapeutic challenge worldwide.1-2 Patients with DFUs are at higher risk for infections, which may lead to limb loss.1-5 In fact, 1 in 6 patients with DFUs will undergo an amputation.6 The long-term consequences of DFUs are numerous and can severely affect patients’ quality of life, including loss of productivity.7 The current standard of care for DFUs consists of debridement of the necrotic tissue, application of a moist dressing, and use of an off-loading device that protects the wound from pressure or trauma related to ambulation and other acts of daily living.4-6,8 Unfortunately, studies have shown that the best standard of care (SOC) only heals 30% of DFUs after 20 weeks of therapy.9 With the estimated cost per episode of care approaching $40,000, DFUs remain a costly and important problem.10
The altered extracellular matrix (ECM) in DFUs has been a target for the development of new therapeutic devices that provide a new matrix that is either devoid of cells or can be enriched with fibroblasts.8,11 These bioengineered skin substitutes stimulate the growth of new vessels and generate cytokines essential for tissue repair. In 2013, Lev-Tov et al12 published this study protocol (Dermagraft Oasis Longitudinal Comparative Efficacy [DOLCE] trial) to compare the effectiveness of 2 advanced wound care devices, specifically to evaluate the clinical efficacy of a cellular matrix versus an acellular matrix, which we have amended. The cellular matrix used in the study is a dermal substitute composed of viable newborn foreskin fibroblasts seeded onto a bioabsorbable polyglactin mesh on which fibroblasts generate an ECM.13,14 It is supplied frozen and requires specific thawing steps prior to application. The recommended regimen for treatment of DFUs for this cellular matrix is 8 weekly applications.13,14 In 2016, the cost of the product was reported as $1411 per 5.0×7.5-cm sheet.15 The acellular matrix product used in the study is a bioabsorbable ECM that is derived from porcine small intestinal submucosa.16,17 It is stored at room temperature and has a long shelf life, with a current price of $112.6 for a 3.0×3.5-cm single-layer fenestrated sheet ($1126.60 per box of 10 sheets). The industry-supported randomized controlled trials for each of these devices have reported a 20% added benefit in the rate of wound closure at week 12 compared to SOC.14,17
This article provides the interim report of the trial (registered at www.clinicaltrials.gov with the identifier NCT01450943) described in the published protocol and initiated in 2011,12 focusing on elements that required modification during the trial’s duration.
Methods
Study Protocol
The clinical trial was approved by the Veterans’ Affairs Institutional Research and Development Committee and their institutional review board. This study was funded by the Veteran’s Administration Merit Award (#10554640), which was awarded to 2 of the investigators (S.E.D. and R.R.I.). Eligible veterans were recruited from all 7 sites of the VA Northern California Healthcare System. This trial is a randomized, single-blinded, 3-armed, controlled clinical equivalence trial comparing the effectiveness of an SOC treatment, cellular ECM, and acellular ECM.
Study Products
The SOC dressing applied in the clinical trial included a sterile antimicrobial gel, a nonadherent dressing, and gauze.12 The SOC dressing also was used as a secondary dressing for the active treatment arms. Bacitracin antibiotic ointment was used as an alternative for patients with allergy to iodine.12
Randomization
The inclusion and exclusion criteria were previously outlined.12 After a 2-week screening phase to exclude rapid healers, patients were randomized into a treatment arm and entered the active phase for 12 weeks.
Primary and Secondary Outcomes
The primary outcome was complete wound closure by week 12.12 Complete healing was defined as full reepithelialization with no drainage or dressing requirement. The secondary outcomes included healing at 28 weeks, rate of healing, ulcer recurrence at week 20, association of wound healing with ulcer characteristics or patients’ characteristic, incidence of adverse events, and cost-effectiveness of each treatment compared to the SOC arm.12
Statistical Analysis
To detect a 25% difference in the incidence of ulcer closure between the 2 study groups and the SOC group, the estimation of the sample size was based on 80% power with a significance level of 0.05. Specifically, it was expected that 50% of the cellular and acellular matrix groups and 25% of the SOC group would reach complete wound closure. The protocol indicated that 57 participants would be enrolled in each arm (total of 171 participants). Lev-Tov et al12 discussed the statistical analysis in more detail.
Results
Study Protocol Amendments
Given the number of diabetic patients in the US veteran population, we anticipated that there would be enough participants meeting the inclusion and exclusion criteria; however, because of the difficulty with recruitment, the initial study criteria were modified. The study was initially designed to incorporate DFUs with a minimum size of 1.0 cm2.12
Another limiting criterion was the percentage of total hemoglobin level for hemoglobin A1C (HbA1C). The study was originally established to include participants with an HbA1C level of 10% of total hemoglobin or below.12 Unfortunately, the majority of the potential participants had values substantially higher, and thus could not be enrolled in the trial, requiring another amendment to the study protocol in 2014, which was approved to include patients with an HbA1C level less than 12% of total hemoglobin. This change contributed considerably to the noted increase in enrollment rates in 2015, which almost doubled relative to enrollment under the original exclusion criteria (Figure).

The study has screened more than 600 patients. Among them, 137 were assessed for eligibility; 71 were excluded for various reasons, including screen failure (eg, decrease in wound size by >40% during the 2-week screening phase), loss to follow-up, and adverse events. Sixty-six participants reached the primary outcome at week 12, while 55 participants completed the study (19 in the SOC group; 18 in the cellular matrix group; 18 in the acellular matrix group).
We have stopped enrolling patients from all sites and the community, as we have reached our target enrollment.
Comment
One of the challenges of clinical trials is the recruitment of an adequate number of participants within an appropriate time frame, which is explained by Lasagna’s Law,18 a well-described phenomenon whereby the investigator overestimates the number of potential participants available to meet the inclusion criteria. This so-called funnel-effect was partly encountered in our selection process. A review of the veteran population with DFUs seemed to be more than adequate to fulfill the sample size; however, some important participant-related factors also played a substantial role.
In addition, the Veterans’ Affairs network centralizes health information, making it readily available to all providers participating in their care. As a result, patients with diabetes mellitus typically are seen by a primary care physician along with an endocrinologist, a diabetic nurse, and/or a dietician. Despite the collaboration with an interdisciplinary team, the glycemic control of the participants remains an issue along with other psychosocial factors that are deterrents in patient compliance. As a result, patients with poorly controlled diabetes and an HbA1C level above 10% (and less than 12%) of total hemoglobin who were initially excluded from the study were reincluded after modifying the inclusion criteria. Some patients were interested in joining the study, but physical limitations (eg, impaired mobility) prompted their decision not to join the trial, even though they met all the inclusion criteria.
As far as research-related factors that could affect participation, it is notable that most of the patients were retired; thus, the interventions did not cause additional burden of taking time off from work or loss of productivity. Although randomization could be a deterrent in many clinical trials, the majority of patients were willing to participate without demanding to be assigned to a particular treatment group.
There are many factors that are intertwined and can lead to enrollment and/or attrition rates. It was critical for our team to make some adjustment without compromising the controlled nature of a randomized trial.
Acknowledgment
The authors wish to acknowledge Huong Le, DPM, MPH, who was the coauthor of the study protocol.
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743.
- Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(suppl 1):S3-S6.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228.
- Vuorisalo S, Venermo M, Lepäntalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50:275-291.
- Meijer JW, Trip J, Jaegers SM, et al. Quality of life in patients with diabetic foot ulcers. Disabil Rehabil. 2001;23:336-340.
- Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. a meta-analysis. Diabetes Care. 1999;22:692-695.
- Cavanagh P, Attinger C, Abbas Z, et al. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev. 2012;2(suppl 1):107-111.
- Panuncialman J, Falanga V. The science of wound bed preparation. Surg Clin North Am. 2009;89:611-626.
- Lev-Tov H, Li CS, Dahle S, et al. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials. 2013;14:8.
- Gentzkow GD, Iwasaki SD, Hershon KS, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350-354.
- Marston WA, Hanft J, Norwood P, et al; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701-1705.
- 2016 Dermagraft® Medicare Product and Related Procedure Payment. http://www.dermagraft.com/wp-content/uploads/sites/1/Dermagraft_Hotsheet%202016%20Q1%20HOSPITAL_FINAL.pdf. Accessed November 23, 2017.
- Oasis® Wound Matrix. http://www.oasiswoundmatrix.com/aboutowm. Accessed November 23, 2017.
- Niezgoda JA, Van Gils CC, Frykberg RG, et al. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5, pt 1):258-266.
- Torgerson JS, Arlinger K, Käppi M, et al. Principles for enhanced recruitment of subjects in a large clinical trial. the XENDOS (XENical in the prevention of Diabetes in Obese Subjects) study experience. Controlled Clin Trials. 2001;22:515-525.
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743.
- Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(suppl 1):S3-S6.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228.
- Vuorisalo S, Venermo M, Lepäntalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50:275-291.
- Meijer JW, Trip J, Jaegers SM, et al. Quality of life in patients with diabetic foot ulcers. Disabil Rehabil. 2001;23:336-340.
- Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. a meta-analysis. Diabetes Care. 1999;22:692-695.
- Cavanagh P, Attinger C, Abbas Z, et al. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev. 2012;2(suppl 1):107-111.
- Panuncialman J, Falanga V. The science of wound bed preparation. Surg Clin North Am. 2009;89:611-626.
- Lev-Tov H, Li CS, Dahle S, et al. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials. 2013;14:8.
- Gentzkow GD, Iwasaki SD, Hershon KS, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350-354.
- Marston WA, Hanft J, Norwood P, et al; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701-1705.
- 2016 Dermagraft® Medicare Product and Related Procedure Payment. http://www.dermagraft.com/wp-content/uploads/sites/1/Dermagraft_Hotsheet%202016%20Q1%20HOSPITAL_FINAL.pdf. Accessed November 23, 2017.
- Oasis® Wound Matrix. http://www.oasiswoundmatrix.com/aboutowm. Accessed November 23, 2017.
- Niezgoda JA, Van Gils CC, Frykberg RG, et al. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5, pt 1):258-266.
- Torgerson JS, Arlinger K, Käppi M, et al. Principles for enhanced recruitment of subjects in a large clinical trial. the XENDOS (XENical in the prevention of Diabetes in Obese Subjects) study experience. Controlled Clin Trials. 2001;22:515-525.
Resident Pearl
- Deciding on the appropriate wound care regimen for diabetic foot ulcers is difficult given the vast amount of wound products on the market. This head-to-head clinical trial compared the use of an expensive cellular matrix and an inexpensive acellular matrix relative to the standard of care. We hope that this study will help to guide therapy based on cost-effectiveness of wound adjuncts without compromising patient care.