User login
Fremanezumab may reduce chronic migraine frequency
Subcutaneous injections of fremanezumab, a humanized monoclonal antibody, reduced headache frequency, compared with placebo, in patients with chronic migraine, according to results of a randomized, double-blind, placebo-controlled trial.
Beneficial effects of fremanezumab were seen within 4 weeks of the initial treatment, according to Stephen D. Silberstein, MD, director of the Jefferson Headache Center at Thomas Jefferson University, Philadelphia, and his coauthors.
“Expert opinion has been that patients with chronic migraine should receive preventive treatment,” they wrote. “However, these treatments may be underused, not adhered to, associated with side effects, or ineffective.”
Fremanezumab targets calcitonin gene-related peptide, which is “involved in central and peripheral pathophysiological events of migraine,” according to the investigators.
The trial comprised 1,130 patients with chronic migraine, defined as occurring at least 8 days per month and headache of any severity at least 15 days per month. They were randomly assigned to receive fremanezumab on a planned quarterly regimen (a single dose at baseline, followed by placebo injections at weeks 4 and 8), fremanezumab monthly (a single dose at baseline, followed by lower doses at weeks 4 and 8), or matching placebo.
For the 12-week period after the first dose, the average number of headache days per month dropped by 4.3 from a baseline mean of 13.2 in the group of patients receiving treatment quarterly, and by 4.6 from a baseline of 12.8 in patients on monthly treatment, compared with a reduction of only 2.5 from a baseline of 13.3 in the placebo-treated patient group (P less than .001 for both fremanezumab groups vs. placebo).
Migraine days also declined significantly more among patients receiving quarterly and monthly fremanezumab by 12 weeks (from a mean of 16.2 to 11.3 and from 16.0 to 11.0, respectively) when compared with placebo (from 16.4 to 13.2; P less than .001 for both comparisons).
The number of patients experiencing a reduction of at least 50% in average number of headache days was higher in both fremanezumab groups at 38% for the quarterly dosing and 41% for monthly dosing, compared with placebo at 18% (P less than .001 for comparisons of fremanezumab to placebo).
Injection site pain was the most common adverse event in the trial, occurring in 30% and 26% of the fremanezumab quarterly and monthly groups, respectively, and 28% of the placebo group, according to the reported data.
Serious adverse events occurred in 1% of patients in the quarterly treatment group, 2% of the monthly group, and 2% of the placebo group, the data showed.
An ongoing extension of the trial will provide “further insights” on the safety and efficacy of treating chronic migraine with fremanezumab over a longer term, the investigators said.
Teva Pharmaceuticals funded the study. Dr. Silberstein and some of his coauthors reported receiving consulting fees from Teva and others. Many coauthors were employees of Teva.
Subcutaneous injections of fremanezumab, a humanized monoclonal antibody, reduced headache frequency, compared with placebo, in patients with chronic migraine, according to results of a randomized, double-blind, placebo-controlled trial.
Beneficial effects of fremanezumab were seen within 4 weeks of the initial treatment, according to Stephen D. Silberstein, MD, director of the Jefferson Headache Center at Thomas Jefferson University, Philadelphia, and his coauthors.
“Expert opinion has been that patients with chronic migraine should receive preventive treatment,” they wrote. “However, these treatments may be underused, not adhered to, associated with side effects, or ineffective.”
Fremanezumab targets calcitonin gene-related peptide, which is “involved in central and peripheral pathophysiological events of migraine,” according to the investigators.
The trial comprised 1,130 patients with chronic migraine, defined as occurring at least 8 days per month and headache of any severity at least 15 days per month. They were randomly assigned to receive fremanezumab on a planned quarterly regimen (a single dose at baseline, followed by placebo injections at weeks 4 and 8), fremanezumab monthly (a single dose at baseline, followed by lower doses at weeks 4 and 8), or matching placebo.
For the 12-week period after the first dose, the average number of headache days per month dropped by 4.3 from a baseline mean of 13.2 in the group of patients receiving treatment quarterly, and by 4.6 from a baseline of 12.8 in patients on monthly treatment, compared with a reduction of only 2.5 from a baseline of 13.3 in the placebo-treated patient group (P less than .001 for both fremanezumab groups vs. placebo).
Migraine days also declined significantly more among patients receiving quarterly and monthly fremanezumab by 12 weeks (from a mean of 16.2 to 11.3 and from 16.0 to 11.0, respectively) when compared with placebo (from 16.4 to 13.2; P less than .001 for both comparisons).
The number of patients experiencing a reduction of at least 50% in average number of headache days was higher in both fremanezumab groups at 38% for the quarterly dosing and 41% for monthly dosing, compared with placebo at 18% (P less than .001 for comparisons of fremanezumab to placebo).
Injection site pain was the most common adverse event in the trial, occurring in 30% and 26% of the fremanezumab quarterly and monthly groups, respectively, and 28% of the placebo group, according to the reported data.
Serious adverse events occurred in 1% of patients in the quarterly treatment group, 2% of the monthly group, and 2% of the placebo group, the data showed.
An ongoing extension of the trial will provide “further insights” on the safety and efficacy of treating chronic migraine with fremanezumab over a longer term, the investigators said.
Teva Pharmaceuticals funded the study. Dr. Silberstein and some of his coauthors reported receiving consulting fees from Teva and others. Many coauthors were employees of Teva.
Subcutaneous injections of fremanezumab, a humanized monoclonal antibody, reduced headache frequency, compared with placebo, in patients with chronic migraine, according to results of a randomized, double-blind, placebo-controlled trial.
Beneficial effects of fremanezumab were seen within 4 weeks of the initial treatment, according to Stephen D. Silberstein, MD, director of the Jefferson Headache Center at Thomas Jefferson University, Philadelphia, and his coauthors.
“Expert opinion has been that patients with chronic migraine should receive preventive treatment,” they wrote. “However, these treatments may be underused, not adhered to, associated with side effects, or ineffective.”
Fremanezumab targets calcitonin gene-related peptide, which is “involved in central and peripheral pathophysiological events of migraine,” according to the investigators.
The trial comprised 1,130 patients with chronic migraine, defined as occurring at least 8 days per month and headache of any severity at least 15 days per month. They were randomly assigned to receive fremanezumab on a planned quarterly regimen (a single dose at baseline, followed by placebo injections at weeks 4 and 8), fremanezumab monthly (a single dose at baseline, followed by lower doses at weeks 4 and 8), or matching placebo.
For the 12-week period after the first dose, the average number of headache days per month dropped by 4.3 from a baseline mean of 13.2 in the group of patients receiving treatment quarterly, and by 4.6 from a baseline of 12.8 in patients on monthly treatment, compared with a reduction of only 2.5 from a baseline of 13.3 in the placebo-treated patient group (P less than .001 for both fremanezumab groups vs. placebo).
Migraine days also declined significantly more among patients receiving quarterly and monthly fremanezumab by 12 weeks (from a mean of 16.2 to 11.3 and from 16.0 to 11.0, respectively) when compared with placebo (from 16.4 to 13.2; P less than .001 for both comparisons).
The number of patients experiencing a reduction of at least 50% in average number of headache days was higher in both fremanezumab groups at 38% for the quarterly dosing and 41% for monthly dosing, compared with placebo at 18% (P less than .001 for comparisons of fremanezumab to placebo).
Injection site pain was the most common adverse event in the trial, occurring in 30% and 26% of the fremanezumab quarterly and monthly groups, respectively, and 28% of the placebo group, according to the reported data.
Serious adverse events occurred in 1% of patients in the quarterly treatment group, 2% of the monthly group, and 2% of the placebo group, the data showed.
An ongoing extension of the trial will provide “further insights” on the safety and efficacy of treating chronic migraine with fremanezumab over a longer term, the investigators said.
Teva Pharmaceuticals funded the study. Dr. Silberstein and some of his coauthors reported receiving consulting fees from Teva and others. Many coauthors were employees of Teva.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The average number of headache days was reduced by 4.3 and 4.6 for fremanezumab quarterly and monthly, respectively, compared with 2.5 for placebo (P less than .001 for both comparisons of fremanezumab to placebo).
Data source: A randomized, double-blind, placebo-controlled, parallel-group study of 1,130 patients with chronic migraine who received 12 weeks of treatment.
Disclosures: Teva Pharmaceuticals funded the study. Dr. Silberstein and some of his coauthors reported receiving consulting fees from Teva and others. Many coauthors were employees of Teva.
Vasopressin stimulates red blood cell production
The hormone vasopressin, well known for its antidiuretic effects, also appears to stimulate proliferation and differentiation of red blood cell (RBC) precursors, results of a series of preclinical investigations suggest.
Treating anemic mice with an arginine vasopressin (AVP) receptor agonist increased hematocrit and reticulocyte counts significantly, compared with controls, according to the results published in Science Translational Medicine (2017 Nov 29;9:eaao1632).
That finding could have implications for the development of new treatments designed to stimulate RBC production after bleeding, chemotherapy, or drug toxicity, according to the investigators.
“Currently, EPO is the only agent that is used clinically to stimulate erythropoiesis, but there are patients who do not respond to EPO or who cannot take the drug because it stimulates tumor growth,” the investigators wrote. “AVP appears to be an EPO-independent, fast-acting agent that increases RBC numbers after anemia.”
Dr. Mayer and his colleagues initially asked whether AVP might play a role in RBC production after observing that patients with central diabetes insipidus (CDI), who lack the antidiuretic hormone, are frequently anemic. A review of patient records from an NIH database revealed that 60% of CDI patients were anemic despite treatment with desmopressin.
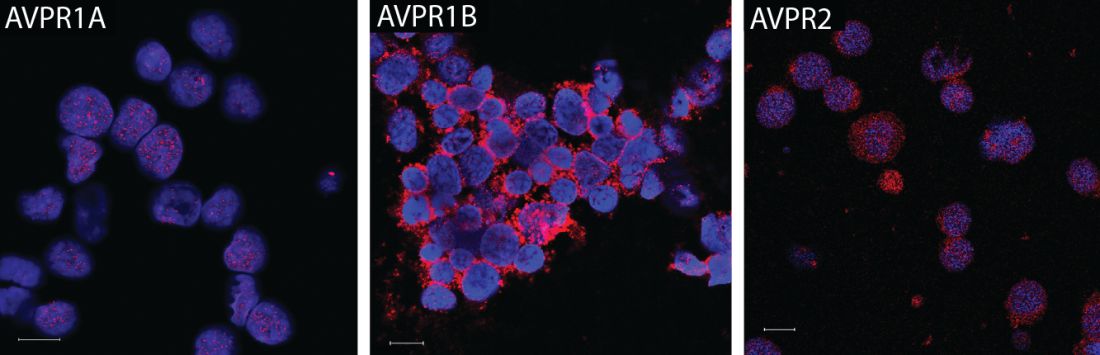
They subsequently found that all three AVP receptor subtypes are expressed in human and mouse hematopoietic stem and progenitor cells. In particular, the AVPR1B subtype appeared to play the most important role in regulating erythropoiesis.
Accordingly, they tested the ability of both AVP and a AVPR1B-specific agonist to stimulate production of RBCs in mice that had anemia induced by bleeding or irradiation. They found significant improvements in both hematocrit and reticulocyte numbers as early as 2 days after treatment started.
Subsequent experiments were designed to determine whether the effect of AVP on RBC production was caused by EPO release. In fact, the effects of AVP occurred “long before an effect of EPO was observed,” investigators wrote.
The research was supported by the NIH. Some of the study authors are listed as inventors on a patent application held by the U.S. Department of Health and Human Services covering methods for modulating erythropoiesis with arginine vasopressin receptor 1b molecules.
The hormone vasopressin, well known for its antidiuretic effects, also appears to stimulate proliferation and differentiation of red blood cell (RBC) precursors, results of a series of preclinical investigations suggest.
Treating anemic mice with an arginine vasopressin (AVP) receptor agonist increased hematocrit and reticulocyte counts significantly, compared with controls, according to the results published in Science Translational Medicine (2017 Nov 29;9:eaao1632).

That finding could have implications for the development of new treatments designed to stimulate RBC production after bleeding, chemotherapy, or drug toxicity, according to the investigators.
“Currently, EPO is the only agent that is used clinically to stimulate erythropoiesis, but there are patients who do not respond to EPO or who cannot take the drug because it stimulates tumor growth,” the investigators wrote. “AVP appears to be an EPO-independent, fast-acting agent that increases RBC numbers after anemia.”
Dr. Mayer and his colleagues initially asked whether AVP might play a role in RBC production after observing that patients with central diabetes insipidus (CDI), who lack the antidiuretic hormone, are frequently anemic. A review of patient records from an NIH database revealed that 60% of CDI patients were anemic despite treatment with desmopressin.
They subsequently found that all three AVP receptor subtypes are expressed in human and mouse hematopoietic stem and progenitor cells. In particular, the AVPR1B subtype appeared to play the most important role in regulating erythropoiesis.
Accordingly, they tested the ability of both AVP and a AVPR1B-specific agonist to stimulate production of RBCs in mice that had anemia induced by bleeding or irradiation. They found significant improvements in both hematocrit and reticulocyte numbers as early as 2 days after treatment started.
Subsequent experiments were designed to determine whether the effect of AVP on RBC production was caused by EPO release. In fact, the effects of AVP occurred “long before an effect of EPO was observed,” investigators wrote.
The research was supported by the NIH. Some of the study authors are listed as inventors on a patent application held by the U.S. Department of Health and Human Services covering methods for modulating erythropoiesis with arginine vasopressin receptor 1b molecules.
The hormone vasopressin, well known for its antidiuretic effects, also appears to stimulate proliferation and differentiation of red blood cell (RBC) precursors, results of a series of preclinical investigations suggest.
Treating anemic mice with an arginine vasopressin (AVP) receptor agonist increased hematocrit and reticulocyte counts significantly, compared with controls, according to the results published in Science Translational Medicine (2017 Nov 29;9:eaao1632).

That finding could have implications for the development of new treatments designed to stimulate RBC production after bleeding, chemotherapy, or drug toxicity, according to the investigators.
“Currently, EPO is the only agent that is used clinically to stimulate erythropoiesis, but there are patients who do not respond to EPO or who cannot take the drug because it stimulates tumor growth,” the investigators wrote. “AVP appears to be an EPO-independent, fast-acting agent that increases RBC numbers after anemia.”
Dr. Mayer and his colleagues initially asked whether AVP might play a role in RBC production after observing that patients with central diabetes insipidus (CDI), who lack the antidiuretic hormone, are frequently anemic. A review of patient records from an NIH database revealed that 60% of CDI patients were anemic despite treatment with desmopressin.
They subsequently found that all three AVP receptor subtypes are expressed in human and mouse hematopoietic stem and progenitor cells. In particular, the AVPR1B subtype appeared to play the most important role in regulating erythropoiesis.
Accordingly, they tested the ability of both AVP and a AVPR1B-specific agonist to stimulate production of RBCs in mice that had anemia induced by bleeding or irradiation. They found significant improvements in both hematocrit and reticulocyte numbers as early as 2 days after treatment started.
Subsequent experiments were designed to determine whether the effect of AVP on RBC production was caused by EPO release. In fact, the effects of AVP occurred “long before an effect of EPO was observed,” investigators wrote.
The research was supported by the NIH. Some of the study authors are listed as inventors on a patent application held by the U.S. Department of Health and Human Services covering methods for modulating erythropoiesis with arginine vasopressin receptor 1b molecules.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:
Major finding: In anemic mice, treatment with a vasopressin or a vasopressin receptor agonist significantly increased hematocrit and reticulocyte number vs. controls.
Data source: A series of in vitro and in vivo experiments, plus a retrospective review of anemia incidence data in patients with central diabetes insipidus.
Disclosures: The research was supported by the National Institutes of Health. Some of the study authors are listed as inventors on a patent application held by the U.S. Department of Health and Human Services covering methods for modulating erythropoiesis with arginine vasopressin receptor 1b molecules.
Revised McDonald Criteria Allow Substitution for Dissemination in Time
PARIS—Recommended revisions to the 2010 McDonald diagnostic criteria for multiple sclerosis (MS) include changes that are intended to enable neurologists to diagnose MS sooner in patients with a high likelihood of the disease. One addition allows the use of CSF-specific oligoclonal bands in lieu of demonstration of dissemination in time to make a diagnosis of MS in patients with a clinically isolated syndrome and demonstration of dissemination in space clinically or by MRI. Symptomatic and cortical lesions also may satisfy diagnostic criteria, according to the recommendations. In addition, the revised criteria include guidance to reduce the risk of misdiagnosing MS.
Jeffrey A. Cohen, MD, a neurologist with the Cleveland Clinic’s Mellen Center for MS Treatment and Research, presented the 2017 proposed revisions at the Seventh Joint ECTRIMS–ACTRIMS Meeting.
Dr. Cohen and Alan J. Thompson, MD, consultant neurologist at the National Hospital for Neurology and Neurosurgery in London, cochaired the International Panel on Diagnosis of MS, which drafted the new recommendations. The panel convened in November 2016 and May 2017. The meetings were organized under the International Advisory Committee on Clinical Trials in MS and supported by the US National MS Society and the European Committee for Treatment and Research in MS (ECTRIMS). The panel’s recommendations have been submitted for publication and are in the late stages of revision, Dr. Cohen said.
Facilitate Diagnosis
New data regarding the utility of MRI, CSF, and other tests in the diagnostic process motivated neurologists to reconvene the panel. In addition, “there has been increasing recognition of the continued frequency and potential consequences of misdiagnosis of MS,” Dr. Cohen said.
“We felt that the 2010 McDonald criteria overall performed well,” he said. “We did not anticipate making major changes to the criteria. But we sought to simplify and clarify some of the components of the 2010 criteria. We wanted to facilitate the ability to make the diagnosis of MS in patients who had a high likelihood of the disease but were not currently diagnosable by the 2010 criteria. We wanted to preserve the specificity of the 2010 criteria but promote their appropriate application … to reduce the risk of misdiagnosis.” Finally, the panel “wanted to ensure that any proposed changes did not weaken the existing criteria and were supported by reasonable evidence,” he said. Dr. Cohen highlighted five of the panel’s key revisions.
Recommended Changes
First and probably most controversially, “we propose that in a patient with a typical clinically isolated syndrome, and with fulfillment by either clinical or MRI criteria for dissemination in space, that the presence of CSF-specific oligoclonal bands now allows for diagnosis of MS,” Dr. Cohen said. “It does not represent demonstration of dissemination in time per se, but it allows substitution for demonstration of dissemination in time.”
A second recommendation is that symptomatic and asymptomatic MRI lesions can be considered in the determination of dissemination in space and time. In the 2010 criteria, a symptomatic lesion in a patient with a brainstem or spinal cord syndrome could not be included as MRI evidence of dissemination in time and space.
Third, in addition to juxtacortical lesions, cortical lesions can demonstrate dissemination in space. Neurologists’ ability to detect purely cortical MRI
Fourth, the criteria for primary progressive MS now allow the inclusion of symptomatic and cortical lesions as evidence of the disease. These criteria otherwise have not changed.
Finally, the panel recommends that neurologists determine a provisional disease course, as specified by Lublin et al, at the time of diagnosis and then periodically reevaluate the provisional course based on accumulated evidence.
Avoiding Misdiagnosis
“Much of our discussion started with the issue of misdiagnosis and differential diagnosis in MS,” Dr. Cohen said. “The potential differential diagnosis of MS is quite broad, and misdiagnosis remains an issue even today with advancements in MRI and other testing.” Solomon et al found that neuromyelitis optica spectrum disorders (NMOSDs) were the disorders most commonly misdiagnosed as MS. Physicians also misdiagnosed common conditions like migraine as MS. “Misdiagnosis may have harmful consequences, including inappropriate institution of disease therapy,” Dr. Cohen said.
Neurologists now recognize that aquaporin 4–related NMOSD is a distinct disorder from MS. “However, if you think back to the time that the 2010 criteria were developed, the relationship between NMOSD and MS was not quite as clear. Substantial data have been published since that time,” Dr. Cohen said. “We agreed that the McDonald criteria and the formal criteria for NMOSD largely distinguish the two diseases. However, there may be cases in which there is some uncertainty. Our recommendation is that … the possibility of NMOSD should be considered in all patients being evaluated for MS,” and any patient with features suggesting NMOSD should undergo aquaporin 4 testing.
In general, neurologists should recognize that the McDonald criteria originally were developed to make the diagnosis of MS in patients who have a high likelihood of the disease, not to differentiate MS from other disorders, he said. “Historical events being taken to represent a prior attack should be interpreted with caution if there is no corroborating objective evidence,” Dr. Cohen said. “In cases in which the diagnosis of MS is uncertain, further testing should be pursued. And in some cases, a clinician may want to postpone making a diagnosis pending the accumulation of sufficient data.” CSF testing and spinal MRI are not required to make the diagnosis of MS, but there should be a low threshold for obtaining them.
While data generally support the validity of the 2010 McDonald criteria in geographically diverse populations, children, and older individuals, neurologists should address potentially relevant alternative diagnoses that may be more common in these and other populations (eg, patients with comorbidities), such as infections and nutritional deficiencies.
MAGNIMS Proposal
Several proposals generated discussion during the panel meetings but were not adopted, primarily because the evidence did not justify changing the current criteria. For instance, the 2016 MAGNIMS MRI criteria propose that the number of acquired periventricular lesions be increased from one to three to provide additional specificity. “We reviewed those data … and felt that the modest increase in specificity did not justify making the change,” Dr. Cohen said. The panel also discussed incorporating optic nerve involvement into the criteria, but this proposal was not included in the update.
“The 2017 revisions further refine the well-established McDonald criteria,” Dr. Cohen said. “The appropriate application of the criteria is critical to avoid misdiagnosis. Fundamentally, MS remains a clinical diagnosis. … It requires rigorous synthesis of clinical, imaging, and laboratory data by a clinician with expertise in MS.”
Making a Diagnosis Sooner
Neurologists have been diagnosing MS in patients sooner. At the same time, misdiagnosing other conditions as MS can have profound consequences, including the potentially serious side effects of disease-modifying therapy, said Jeremy Chataway, PhD, consultant neurologist at the National Hospital for Neurology and Neurosurgery, London, in a lecture about the application of the proposed criteria. He described cases in which the 2017 proposed criteria—by allowing the inclusion of a symptomatic spinal cord lesion, or by substituting CSF-specific oligoclonal bands in place of dissemination in time—would have allowed neurologists to diagnose MS sooner. In one case, a patient might have received a diagnosis of MS about two years earlier.
Sometimes a diagnosis of MS “is obvious,” Dr. Chataway said. “Sometimes it is hard, even with advanced MRI. You can see the gradation that is required … to get us to the correct diagnosis.”
—Jake Remaly
Suggested Reading
Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302.
Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology. 2016;87(13):1393-1399.
PARIS—Recommended revisions to the 2010 McDonald diagnostic criteria for multiple sclerosis (MS) include changes that are intended to enable neurologists to diagnose MS sooner in patients with a high likelihood of the disease. One addition allows the use of CSF-specific oligoclonal bands in lieu of demonstration of dissemination in time to make a diagnosis of MS in patients with a clinically isolated syndrome and demonstration of dissemination in space clinically or by MRI. Symptomatic and cortical lesions also may satisfy diagnostic criteria, according to the recommendations. In addition, the revised criteria include guidance to reduce the risk of misdiagnosing MS.
Jeffrey A. Cohen, MD, a neurologist with the Cleveland Clinic’s Mellen Center for MS Treatment and Research, presented the 2017 proposed revisions at the Seventh Joint ECTRIMS–ACTRIMS Meeting.
Dr. Cohen and Alan J. Thompson, MD, consultant neurologist at the National Hospital for Neurology and Neurosurgery in London, cochaired the International Panel on Diagnosis of MS, which drafted the new recommendations. The panel convened in November 2016 and May 2017. The meetings were organized under the International Advisory Committee on Clinical Trials in MS and supported by the US National MS Society and the European Committee for Treatment and Research in MS (ECTRIMS). The panel’s recommendations have been submitted for publication and are in the late stages of revision, Dr. Cohen said.
Facilitate Diagnosis
New data regarding the utility of MRI, CSF, and other tests in the diagnostic process motivated neurologists to reconvene the panel. In addition, “there has been increasing recognition of the continued frequency and potential consequences of misdiagnosis of MS,” Dr. Cohen said.
“We felt that the 2010 McDonald criteria overall performed well,” he said. “We did not anticipate making major changes to the criteria. But we sought to simplify and clarify some of the components of the 2010 criteria. We wanted to facilitate the ability to make the diagnosis of MS in patients who had a high likelihood of the disease but were not currently diagnosable by the 2010 criteria. We wanted to preserve the specificity of the 2010 criteria but promote their appropriate application … to reduce the risk of misdiagnosis.” Finally, the panel “wanted to ensure that any proposed changes did not weaken the existing criteria and were supported by reasonable evidence,” he said. Dr. Cohen highlighted five of the panel’s key revisions.
Recommended Changes
First and probably most controversially, “we propose that in a patient with a typical clinically isolated syndrome, and with fulfillment by either clinical or MRI criteria for dissemination in space, that the presence of CSF-specific oligoclonal bands now allows for diagnosis of MS,” Dr. Cohen said. “It does not represent demonstration of dissemination in time per se, but it allows substitution for demonstration of dissemination in time.”
A second recommendation is that symptomatic and asymptomatic MRI lesions can be considered in the determination of dissemination in space and time. In the 2010 criteria, a symptomatic lesion in a patient with a brainstem or spinal cord syndrome could not be included as MRI evidence of dissemination in time and space.
Third, in addition to juxtacortical lesions, cortical lesions can demonstrate dissemination in space. Neurologists’ ability to detect purely cortical MRI
Fourth, the criteria for primary progressive MS now allow the inclusion of symptomatic and cortical lesions as evidence of the disease. These criteria otherwise have not changed.
Finally, the panel recommends that neurologists determine a provisional disease course, as specified by Lublin et al, at the time of diagnosis and then periodically reevaluate the provisional course based on accumulated evidence.
Avoiding Misdiagnosis
“Much of our discussion started with the issue of misdiagnosis and differential diagnosis in MS,” Dr. Cohen said. “The potential differential diagnosis of MS is quite broad, and misdiagnosis remains an issue even today with advancements in MRI and other testing.” Solomon et al found that neuromyelitis optica spectrum disorders (NMOSDs) were the disorders most commonly misdiagnosed as MS. Physicians also misdiagnosed common conditions like migraine as MS. “Misdiagnosis may have harmful consequences, including inappropriate institution of disease therapy,” Dr. Cohen said.
Neurologists now recognize that aquaporin 4–related NMOSD is a distinct disorder from MS. “However, if you think back to the time that the 2010 criteria were developed, the relationship between NMOSD and MS was not quite as clear. Substantial data have been published since that time,” Dr. Cohen said. “We agreed that the McDonald criteria and the formal criteria for NMOSD largely distinguish the two diseases. However, there may be cases in which there is some uncertainty. Our recommendation is that … the possibility of NMOSD should be considered in all patients being evaluated for MS,” and any patient with features suggesting NMOSD should undergo aquaporin 4 testing.
In general, neurologists should recognize that the McDonald criteria originally were developed to make the diagnosis of MS in patients who have a high likelihood of the disease, not to differentiate MS from other disorders, he said. “Historical events being taken to represent a prior attack should be interpreted with caution if there is no corroborating objective evidence,” Dr. Cohen said. “In cases in which the diagnosis of MS is uncertain, further testing should be pursued. And in some cases, a clinician may want to postpone making a diagnosis pending the accumulation of sufficient data.” CSF testing and spinal MRI are not required to make the diagnosis of MS, but there should be a low threshold for obtaining them.
While data generally support the validity of the 2010 McDonald criteria in geographically diverse populations, children, and older individuals, neurologists should address potentially relevant alternative diagnoses that may be more common in these and other populations (eg, patients with comorbidities), such as infections and nutritional deficiencies.
MAGNIMS Proposal
Several proposals generated discussion during the panel meetings but were not adopted, primarily because the evidence did not justify changing the current criteria. For instance, the 2016 MAGNIMS MRI criteria propose that the number of acquired periventricular lesions be increased from one to three to provide additional specificity. “We reviewed those data … and felt that the modest increase in specificity did not justify making the change,” Dr. Cohen said. The panel also discussed incorporating optic nerve involvement into the criteria, but this proposal was not included in the update.
“The 2017 revisions further refine the well-established McDonald criteria,” Dr. Cohen said. “The appropriate application of the criteria is critical to avoid misdiagnosis. Fundamentally, MS remains a clinical diagnosis. … It requires rigorous synthesis of clinical, imaging, and laboratory data by a clinician with expertise in MS.”
Making a Diagnosis Sooner
Neurologists have been diagnosing MS in patients sooner. At the same time, misdiagnosing other conditions as MS can have profound consequences, including the potentially serious side effects of disease-modifying therapy, said Jeremy Chataway, PhD, consultant neurologist at the National Hospital for Neurology and Neurosurgery, London, in a lecture about the application of the proposed criteria. He described cases in which the 2017 proposed criteria—by allowing the inclusion of a symptomatic spinal cord lesion, or by substituting CSF-specific oligoclonal bands in place of dissemination in time—would have allowed neurologists to diagnose MS sooner. In one case, a patient might have received a diagnosis of MS about two years earlier.
Sometimes a diagnosis of MS “is obvious,” Dr. Chataway said. “Sometimes it is hard, even with advanced MRI. You can see the gradation that is required … to get us to the correct diagnosis.”
—Jake Remaly
Suggested Reading
Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302.
Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology. 2016;87(13):1393-1399.
PARIS—Recommended revisions to the 2010 McDonald diagnostic criteria for multiple sclerosis (MS) include changes that are intended to enable neurologists to diagnose MS sooner in patients with a high likelihood of the disease. One addition allows the use of CSF-specific oligoclonal bands in lieu of demonstration of dissemination in time to make a diagnosis of MS in patients with a clinically isolated syndrome and demonstration of dissemination in space clinically or by MRI. Symptomatic and cortical lesions also may satisfy diagnostic criteria, according to the recommendations. In addition, the revised criteria include guidance to reduce the risk of misdiagnosing MS.
Jeffrey A. Cohen, MD, a neurologist with the Cleveland Clinic’s Mellen Center for MS Treatment and Research, presented the 2017 proposed revisions at the Seventh Joint ECTRIMS–ACTRIMS Meeting.
Dr. Cohen and Alan J. Thompson, MD, consultant neurologist at the National Hospital for Neurology and Neurosurgery in London, cochaired the International Panel on Diagnosis of MS, which drafted the new recommendations. The panel convened in November 2016 and May 2017. The meetings were organized under the International Advisory Committee on Clinical Trials in MS and supported by the US National MS Society and the European Committee for Treatment and Research in MS (ECTRIMS). The panel’s recommendations have been submitted for publication and are in the late stages of revision, Dr. Cohen said.
Facilitate Diagnosis
New data regarding the utility of MRI, CSF, and other tests in the diagnostic process motivated neurologists to reconvene the panel. In addition, “there has been increasing recognition of the continued frequency and potential consequences of misdiagnosis of MS,” Dr. Cohen said.
“We felt that the 2010 McDonald criteria overall performed well,” he said. “We did not anticipate making major changes to the criteria. But we sought to simplify and clarify some of the components of the 2010 criteria. We wanted to facilitate the ability to make the diagnosis of MS in patients who had a high likelihood of the disease but were not currently diagnosable by the 2010 criteria. We wanted to preserve the specificity of the 2010 criteria but promote their appropriate application … to reduce the risk of misdiagnosis.” Finally, the panel “wanted to ensure that any proposed changes did not weaken the existing criteria and were supported by reasonable evidence,” he said. Dr. Cohen highlighted five of the panel’s key revisions.
Recommended Changes
First and probably most controversially, “we propose that in a patient with a typical clinically isolated syndrome, and with fulfillment by either clinical or MRI criteria for dissemination in space, that the presence of CSF-specific oligoclonal bands now allows for diagnosis of MS,” Dr. Cohen said. “It does not represent demonstration of dissemination in time per se, but it allows substitution for demonstration of dissemination in time.”
A second recommendation is that symptomatic and asymptomatic MRI lesions can be considered in the determination of dissemination in space and time. In the 2010 criteria, a symptomatic lesion in a patient with a brainstem or spinal cord syndrome could not be included as MRI evidence of dissemination in time and space.
Third, in addition to juxtacortical lesions, cortical lesions can demonstrate dissemination in space. Neurologists’ ability to detect purely cortical MRI
Fourth, the criteria for primary progressive MS now allow the inclusion of symptomatic and cortical lesions as evidence of the disease. These criteria otherwise have not changed.
Finally, the panel recommends that neurologists determine a provisional disease course, as specified by Lublin et al, at the time of diagnosis and then periodically reevaluate the provisional course based on accumulated evidence.
Avoiding Misdiagnosis
“Much of our discussion started with the issue of misdiagnosis and differential diagnosis in MS,” Dr. Cohen said. “The potential differential diagnosis of MS is quite broad, and misdiagnosis remains an issue even today with advancements in MRI and other testing.” Solomon et al found that neuromyelitis optica spectrum disorders (NMOSDs) were the disorders most commonly misdiagnosed as MS. Physicians also misdiagnosed common conditions like migraine as MS. “Misdiagnosis may have harmful consequences, including inappropriate institution of disease therapy,” Dr. Cohen said.
Neurologists now recognize that aquaporin 4–related NMOSD is a distinct disorder from MS. “However, if you think back to the time that the 2010 criteria were developed, the relationship between NMOSD and MS was not quite as clear. Substantial data have been published since that time,” Dr. Cohen said. “We agreed that the McDonald criteria and the formal criteria for NMOSD largely distinguish the two diseases. However, there may be cases in which there is some uncertainty. Our recommendation is that … the possibility of NMOSD should be considered in all patients being evaluated for MS,” and any patient with features suggesting NMOSD should undergo aquaporin 4 testing.
In general, neurologists should recognize that the McDonald criteria originally were developed to make the diagnosis of MS in patients who have a high likelihood of the disease, not to differentiate MS from other disorders, he said. “Historical events being taken to represent a prior attack should be interpreted with caution if there is no corroborating objective evidence,” Dr. Cohen said. “In cases in which the diagnosis of MS is uncertain, further testing should be pursued. And in some cases, a clinician may want to postpone making a diagnosis pending the accumulation of sufficient data.” CSF testing and spinal MRI are not required to make the diagnosis of MS, but there should be a low threshold for obtaining them.
While data generally support the validity of the 2010 McDonald criteria in geographically diverse populations, children, and older individuals, neurologists should address potentially relevant alternative diagnoses that may be more common in these and other populations (eg, patients with comorbidities), such as infections and nutritional deficiencies.
MAGNIMS Proposal
Several proposals generated discussion during the panel meetings but were not adopted, primarily because the evidence did not justify changing the current criteria. For instance, the 2016 MAGNIMS MRI criteria propose that the number of acquired periventricular lesions be increased from one to three to provide additional specificity. “We reviewed those data … and felt that the modest increase in specificity did not justify making the change,” Dr. Cohen said. The panel also discussed incorporating optic nerve involvement into the criteria, but this proposal was not included in the update.
“The 2017 revisions further refine the well-established McDonald criteria,” Dr. Cohen said. “The appropriate application of the criteria is critical to avoid misdiagnosis. Fundamentally, MS remains a clinical diagnosis. … It requires rigorous synthesis of clinical, imaging, and laboratory data by a clinician with expertise in MS.”
Making a Diagnosis Sooner
Neurologists have been diagnosing MS in patients sooner. At the same time, misdiagnosing other conditions as MS can have profound consequences, including the potentially serious side effects of disease-modifying therapy, said Jeremy Chataway, PhD, consultant neurologist at the National Hospital for Neurology and Neurosurgery, London, in a lecture about the application of the proposed criteria. He described cases in which the 2017 proposed criteria—by allowing the inclusion of a symptomatic spinal cord lesion, or by substituting CSF-specific oligoclonal bands in place of dissemination in time—would have allowed neurologists to diagnose MS sooner. In one case, a patient might have received a diagnosis of MS about two years earlier.
Sometimes a diagnosis of MS “is obvious,” Dr. Chataway said. “Sometimes it is hard, even with advanced MRI. You can see the gradation that is required … to get us to the correct diagnosis.”
—Jake Remaly
Suggested Reading
Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302.
Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology. 2016;87(13):1393-1399.
‘Tea with Freud’: Engaging, authentic, but nonanalytic
If I traveled back in time to meet with a 60-year-old Sigmund Freud, the first thing I would say to him is: “Stop smoking, and get out of Austria!”
That was my thought as I read “Tea with Freud: An Imaginary Conversation about How Psychotherapy Really Works” (Dog Ear Publishing, 2016), in which the author, psychiatrist Steven B. Sandler, MD, holds a series of imaginary meetings with Freud to discuss the evolution of psychoanalysis into Sandler’s preferred mode of short-term dynamic psychotherapy (STDP) and to present case material for Freud’s supervision.
The chapters in “Tea with Freud” alternate between the imagined meetings with Freud and Sandler’s clinical work, presented from what I assume are transcripts of videotaped sessions with some disguises and composites to protect patients’ privacy. These clinical vignettes bring the reader into the nitty-gritty of the treatment room, which may be highly instructive for a lay person – particularly one who has never been in therapy.
At the same time, the book has the potential to be quite misleading. This would not be the case if Sandler were simply trying to introduce the reader to STDP. Instead, he attempts to convince the reader, and apparently himself, that the therapy he practices is a modern rendition of psychoanalysis because it tries to access the patient’s unacceptable, unconscious feelings; encourages her to “remember with emotion” or “experience” her feelings; and leads to some sort of cathartic resolution and improvement in symptoms and outlook.
While, “Aha!” moments and cathartic abreaction were characteristic of very early analyses, modern psychoanalysis is about slow but permanent change in character structure. The unwritten message in the book is that Freud’s true heirs practice psychotherapy as Sandler does. He does not seem to consider the significance of the many psychoanalysts, myself included, practicing psychoanalysis today.
Sandler uses a (mercifully) attenuated Davanloo technique to provoke patients into dramatic enactments. He is highly directive, with statements like, “We don’t solve any particular problem if we jump around all over.” I wonder how he can possibly learn about his patients when he begins with a foregone conclusion about where they should be headed.
His treatments are very brief. During his first session with a patient named Carla, he deduces that she is suffering from unresolved anger related to childhood trauma and manifesting it in chronic anxiety with angry outbursts. He then proceeds to “cure” her in five sessions.
Sandler wonders why some of his patients relapse and decides it is because they have not explored their “positive memories” in treatment, as though memories were univalent.
And he talks way too much.
All of this is decidedly un-analytic, which, again, would not matter if he were only trying to demonstrate STDP in action. Nonanalytic psychotherapies are entitled to be nonanalytic. Sandler has Freud point out precisely these analytic errors, so he must be aware that he is making them. And, yet, he stubbornly maintains his position that his work is analytic. What a waste of time travel it would be to meet with Freud only to reinforce one’s own opinions.
“Tea with Freud” is a way for Sandler to promote STDP and his theories about “positive memories” using an established authority, Freud, to validate them. This makes the book disappointing, but fortunately, there is something more to it. I kept wondering why it was so important to the author to seek out Freud’s – that is, his father’s – approval for his work. The book never answers that question. But in his attempts to understand his motives, Sandler, who is very adept at describing his own thoughts and feelings, becomes a model for the awareness of internal states and the effects of unconscious processes. Perhaps this is the most important lesson in “Tea with Freud.”
Dr. Twersky-Kengmana is a psychiatrist and psychoanalyst in private practice in New York.
If I traveled back in time to meet with a 60-year-old Sigmund Freud, the first thing I would say to him is: “Stop smoking, and get out of Austria!”
That was my thought as I read “Tea with Freud: An Imaginary Conversation about How Psychotherapy Really Works” (Dog Ear Publishing, 2016), in which the author, psychiatrist Steven B. Sandler, MD, holds a series of imaginary meetings with Freud to discuss the evolution of psychoanalysis into Sandler’s preferred mode of short-term dynamic psychotherapy (STDP) and to present case material for Freud’s supervision.
The chapters in “Tea with Freud” alternate between the imagined meetings with Freud and Sandler’s clinical work, presented from what I assume are transcripts of videotaped sessions with some disguises and composites to protect patients’ privacy. These clinical vignettes bring the reader into the nitty-gritty of the treatment room, which may be highly instructive for a lay person – particularly one who has never been in therapy.
At the same time, the book has the potential to be quite misleading. This would not be the case if Sandler were simply trying to introduce the reader to STDP. Instead, he attempts to convince the reader, and apparently himself, that the therapy he practices is a modern rendition of psychoanalysis because it tries to access the patient’s unacceptable, unconscious feelings; encourages her to “remember with emotion” or “experience” her feelings; and leads to some sort of cathartic resolution and improvement in symptoms and outlook.
While, “Aha!” moments and cathartic abreaction were characteristic of very early analyses, modern psychoanalysis is about slow but permanent change in character structure. The unwritten message in the book is that Freud’s true heirs practice psychotherapy as Sandler does. He does not seem to consider the significance of the many psychoanalysts, myself included, practicing psychoanalysis today.
Sandler uses a (mercifully) attenuated Davanloo technique to provoke patients into dramatic enactments. He is highly directive, with statements like, “We don’t solve any particular problem if we jump around all over.” I wonder how he can possibly learn about his patients when he begins with a foregone conclusion about where they should be headed.
His treatments are very brief. During his first session with a patient named Carla, he deduces that she is suffering from unresolved anger related to childhood trauma and manifesting it in chronic anxiety with angry outbursts. He then proceeds to “cure” her in five sessions.
Sandler wonders why some of his patients relapse and decides it is because they have not explored their “positive memories” in treatment, as though memories were univalent.
And he talks way too much.
All of this is decidedly un-analytic, which, again, would not matter if he were only trying to demonstrate STDP in action. Nonanalytic psychotherapies are entitled to be nonanalytic. Sandler has Freud point out precisely these analytic errors, so he must be aware that he is making them. And, yet, he stubbornly maintains his position that his work is analytic. What a waste of time travel it would be to meet with Freud only to reinforce one’s own opinions.
“Tea with Freud” is a way for Sandler to promote STDP and his theories about “positive memories” using an established authority, Freud, to validate them. This makes the book disappointing, but fortunately, there is something more to it. I kept wondering why it was so important to the author to seek out Freud’s – that is, his father’s – approval for his work. The book never answers that question. But in his attempts to understand his motives, Sandler, who is very adept at describing his own thoughts and feelings, becomes a model for the awareness of internal states and the effects of unconscious processes. Perhaps this is the most important lesson in “Tea with Freud.”
Dr. Twersky-Kengmana is a psychiatrist and psychoanalyst in private practice in New York.
If I traveled back in time to meet with a 60-year-old Sigmund Freud, the first thing I would say to him is: “Stop smoking, and get out of Austria!”
That was my thought as I read “Tea with Freud: An Imaginary Conversation about How Psychotherapy Really Works” (Dog Ear Publishing, 2016), in which the author, psychiatrist Steven B. Sandler, MD, holds a series of imaginary meetings with Freud to discuss the evolution of psychoanalysis into Sandler’s preferred mode of short-term dynamic psychotherapy (STDP) and to present case material for Freud’s supervision.
The chapters in “Tea with Freud” alternate between the imagined meetings with Freud and Sandler’s clinical work, presented from what I assume are transcripts of videotaped sessions with some disguises and composites to protect patients’ privacy. These clinical vignettes bring the reader into the nitty-gritty of the treatment room, which may be highly instructive for a lay person – particularly one who has never been in therapy.
At the same time, the book has the potential to be quite misleading. This would not be the case if Sandler were simply trying to introduce the reader to STDP. Instead, he attempts to convince the reader, and apparently himself, that the therapy he practices is a modern rendition of psychoanalysis because it tries to access the patient’s unacceptable, unconscious feelings; encourages her to “remember with emotion” or “experience” her feelings; and leads to some sort of cathartic resolution and improvement in symptoms and outlook.
While, “Aha!” moments and cathartic abreaction were characteristic of very early analyses, modern psychoanalysis is about slow but permanent change in character structure. The unwritten message in the book is that Freud’s true heirs practice psychotherapy as Sandler does. He does not seem to consider the significance of the many psychoanalysts, myself included, practicing psychoanalysis today.
Sandler uses a (mercifully) attenuated Davanloo technique to provoke patients into dramatic enactments. He is highly directive, with statements like, “We don’t solve any particular problem if we jump around all over.” I wonder how he can possibly learn about his patients when he begins with a foregone conclusion about where they should be headed.
His treatments are very brief. During his first session with a patient named Carla, he deduces that she is suffering from unresolved anger related to childhood trauma and manifesting it in chronic anxiety with angry outbursts. He then proceeds to “cure” her in five sessions.
Sandler wonders why some of his patients relapse and decides it is because they have not explored their “positive memories” in treatment, as though memories were univalent.
And he talks way too much.
All of this is decidedly un-analytic, which, again, would not matter if he were only trying to demonstrate STDP in action. Nonanalytic psychotherapies are entitled to be nonanalytic. Sandler has Freud point out precisely these analytic errors, so he must be aware that he is making them. And, yet, he stubbornly maintains his position that his work is analytic. What a waste of time travel it would be to meet with Freud only to reinforce one’s own opinions.
“Tea with Freud” is a way for Sandler to promote STDP and his theories about “positive memories” using an established authority, Freud, to validate them. This makes the book disappointing, but fortunately, there is something more to it. I kept wondering why it was so important to the author to seek out Freud’s – that is, his father’s – approval for his work. The book never answers that question. But in his attempts to understand his motives, Sandler, who is very adept at describing his own thoughts and feelings, becomes a model for the awareness of internal states and the effects of unconscious processes. Perhaps this is the most important lesson in “Tea with Freud.”
Dr. Twersky-Kengmana is a psychiatrist and psychoanalyst in private practice in New York.
Accounting 101: Basics you need to know
Physicians practice medicine and communicate within the world of medical language, yet there is a lack of awareness and understanding by many health care professionals of the universal language of business, which is accounting. Just as Latin provides the basic framework for medically related terminology, accounting is the standard language used to convey financial information to both internal and external stakeholders.
Accounting principles are important to physicians at any level. Whether you are starting out in private practice, running a clinical department, or working as an executive in a health care system, most decisions are based on the interpretation of financial data using accounting principles. Accounting standards in the United States are developed by the Financial Accounting Standard Board (FASB) and established as a set of principles and guidelines called Generally Accepted Accounting Principles (GAAP).1–3
Accrual- vs cash-based accounting
There are 2 approaches to recording financial transactions: accrual- and cash-based accounting methods. The main difference between them is in the timing of the recorded financial transactions (when revenue and expenses are recognized on the accounting books). Under GAAP, the matching principle, which is one of the most basic and utilized guidelines of accounting, specifies that accrual accounting be used. In the United States, most businesses (publically traded companies and moderate- to large-sized companies) use accrual accounting, while some individual and smaller businesses, including health care services such as physician practices, use the cash method.1–4
Accrual-based accounting
Accrual-based accounting specifies that revenues are recorded when they are earned, and expenses are recorded when they occur. A health care business may earn revenue for services on one day, but the cash may not be received or recorded on the accounting books for several weeks or months and at an amount less than billed.
Accrual-based accounting provides a more accurate representation of a business’ financial performance, since it uses the principle in which expenses are matched to revenues in the same time period. This enables a more precise representation of true financial performance during a given time frame.1–4
Cash-based accounting
Cash-based accounting is the easiest method to understand and implement because financial transactions are recorded in the accounting books when money is received or spent without the need for complex accounting techniques or integration of accounts receivable or payable.
Despite the ease of use and simplicity in tracking cash flow, this method can be deceiving because revenue may be received or expenses may need to be paid at times that are not consistent with when the revenue has been earned or expenses incurred. This can result in misleading information on the business’ health and the accuracy of tracking financial performance over time, since revenue and expenses for a particular transaction may occur at different times.1–4
Which accounting process to choose?
Even though accrual-based accounting may provide a more accurate financial representation of a business’ performance, many smaller businesses, including physician practices, prefer to use cash-based accounting. In addition, many health care businesses are eligible to use cash-based accounting per Internal Revenue Service (IRS) rules by qualifying for the Gross Receipts Test and being a qualified Personal Service Corporation (PSC):
- The Gross Receipts Test states that if the average annual gross receipts of the business are less than $5 million, the business can use the cash-based accounting method.
- If at least 95% of a business activity involves performing health care services, and at least 95% of the business is owned by employees performing health care services, then the business qualifies as a PSC that may use the cash-based accounting method.
Many physician practices qualify to use cash-based accounting, which reduces the complexity of following accrual-based accounting rules and simplifies overall cash-flow management.5
Read about insurance, capital equipment depreciation, more
CASE New practice opens
Practice A opens its practice on January 1. The practice borrows $20,000 from the bank to purchase hysteroscopic equipment for office-based tubal sterilizations and an additional $50,000 for an ultrasound machine. Both loans have a 5% annual interest rate amortized over 5 years. The practice leases office space and pays rent 2 months in advance at $8,000 ($4,000 per month). On January 1, the practice pays a $1,200 premium for annual property and liability insurance and $12,500 for the first quarter payment for professional liability insurance ($50,000 annually, paid quarterly). Other costs the practice pays in January include: utilities, $400; EHR licensing, $300; technical support, $200; and salaries, $10,000.
The practice purchases 4 sets of sterilization spring devices at $1,500 each ($6,000) to have in stock. One hysteroscopic sterilization procedure is performed on a patient in January using 1 device. The practice is reimbursed $2,500 for the procedure.
In January, the practice bills $150,000 in charges, but after insurance contractual adjustments, January’s revenue is $50,000. Actual cash payments from billings received are $10,000 in January, $30,000 in February, and $10,000 in March.
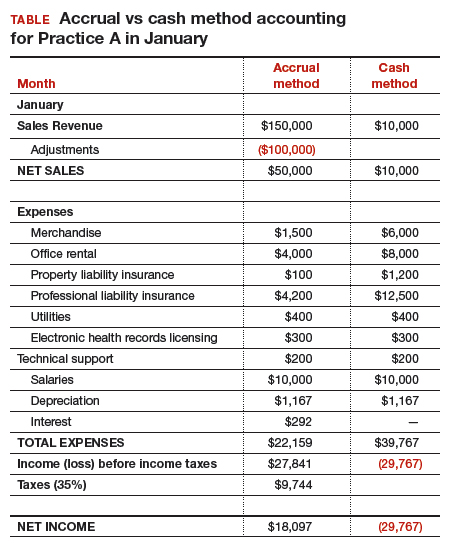
At first glance, there is a noticeable difference on the sales or recognition of revenues based on the type of accounting (TABLE). With the accrual method, because the billing charges are submitted in January when the services were provided (minus the insurance contractual adjustments), the $50,000 revenue is immediately counted and recognized, even though the practice only received $10,000 cash for those billings during January. While the benefit to accrual accounting is the timely recognition of the revenue when the service was provided, the downside is that much of those billings might actually be paid over 90 days, and some of those billings may go unpaid by the insurance company or the patients, which would require adjustments in later months.
The cash-based method is simpler to understand because the cash received for the month is recognized as the revenue, regardless of the amount charged that month.
Merchandise. In the accrual method, the cost of merchandise sold (the hysteroscopic sterilization implants) is recognized as an expense when the revenue is generated from its sale. In this case, the date that the patient has the hysteroscopic in-office sterilization procedure is when the revenue and the expense of the implant are recognized.
In a cash-based accounting method, the $6,000 cost of the implants is recognized at the time of purchase in January.
Lease. In this scenario, even though 2 months of lease for the office were paid, the accrual method only recognizes the January payment; the second payment is recognized in February. In the cash method, because both months were paid in January, the total expense of $8,000 is recognized in January.
Property liability insurance. The property liability insurance payment is required at the start of the year. In accrual accounting, this expense is divided over 12 months, while in the cash method, the expense is counted at the time the payment is made.
Professional liability insurance. The professional liability insurance expense of $50,000 per year is made in quarterly payments, so for the accrual method, the annual amount would be distributed over 12 months at $4,200 per month. With the cash method, it would be paid—and recognized as an expense—quarterly at $12,500, starting in January.
Capital equipment depreciation. Capital medical equipment (hysteroscopy and ultrasound) can be depreciated using a straight-line 5-year depreciation. A total $70,000 worth of equipment divided by 5 years is $14,000 per year, depreciated over 5 years. One-twelfth of $14,000 equals $1,167, which is recorded as a January depreciation expense. Because the Internal Revenue Code requires capital assets to be depreciated, even for cash-basis taxpayers, the common practice is to record depreciation expense for both cash- and accrual-basis income accounting.6
Interest on loans. A loan’s principal payment will not be included on the income statement. The principal payment, a reduction of a liability (loans payable), is reported on the balance sheet. Only the interest portion of a loan payment is reported on the income statement (interest expense). In accrual accounting, the accrued interest on the loan payment for the year is $3,500 ($292 for January). For the cash-basis method, because the interest is paid annually at year-end, interest will not be expensed until December.
Taxes. The IRS states that, “Individuals, including sole proprietors, partners, and S corporation shareholders, generally have to make estimated tax payments if they expect to owe tax of $1,000 or more when their return is filed. Corporations generally have to make estimated tax payments if they expect to owe tax of $500 or more when their return is filed.”7
Assuming 35% tax liability, the accrual method would create a tax liability of $9,744 on a profit of $27,841. With the cash method, there would be no tax liability because there was no net profit.
Other expenses. The utilities, EHR licensing, tech support, and salaries are expensed the same way for both methods.
Net income. The resulting final net income is vastly different for the month of January depending on the accounting method utilized. The accrual method results in a net income of $18,097, while the cash-basis method results in a net loss of $29,767. Over the course of the year, these imbalances are likely to even out.
Related article:
Business law critical to your practice
Choosing an accounting method
Depending on the accounting method, a practice’s performance and profit will seem very different. The type of accounting method chosen will depend on what goals the owners want to achieve.
The accrual method provides a more accurate picture of business flow and performance and will be less subject to monthly variations due to large purchases or variations in expenses. If the practice chooses this method using an income statement, it should also employ a cash-flow statement.
The cash method of accounting will give a convenient and practical summary of the practice’s cash flow.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- About the FASB. Financial Accounting Standards Board website. http://www.fasb.org/jsp/FASB/PageSectionPage&cid=1176154526495. Accessed November 7, 2017.
- What is the difference between accrual accounting and cash accounting? Investopedia. https://www.investopedia.com/ask/answers/121514/what-difference-between-accrual-accounting-and-cash-accounting.asp. Accessed November 7, 2017.
- Accounting Basics (Explanation). Part 2: Income Statement. Accounting Coach. https://www.accountingcoach.com/accounting-basics/explanation/2. Accessed November 7, 2017.
- Stickney C, Weil R. Financial Accounting: An Introduction to Concepts, Methods, and Uses. 11th ed. Nashville, TN: Southwestern College Publishing Group; 2006:97-110.
- Internal Revenue Service. Publication 538 (12/2016), Accounting Periods and Methods. https://www.irs.gov/publications/p538#en_US_201612_publink1000270634. Revised December 2016. Accessed November 7, 2017.
- Klinefelter D, McCorkle D, Klose S. Financial Management: Cash vs. Accrual Accounting. Risk Management. AgriLife Extension. Texas A&M System. http://agrilife.org/agecoext/files/2013/10/rm5-16.pdf. Published 2013. Accessed November 7, 2017.
- Internal Revenue Service. Small Business and Self-Employed Tax Center: Estimated Taxes. https://www.irs.gov/businesses/small-businesses-self-employed/estimated-taxes. Updated November 2, 2017. Accessed November 7, 2017.
Physicians practice medicine and communicate within the world of medical language, yet there is a lack of awareness and understanding by many health care professionals of the universal language of business, which is accounting. Just as Latin provides the basic framework for medically related terminology, accounting is the standard language used to convey financial information to both internal and external stakeholders.
Accounting principles are important to physicians at any level. Whether you are starting out in private practice, running a clinical department, or working as an executive in a health care system, most decisions are based on the interpretation of financial data using accounting principles. Accounting standards in the United States are developed by the Financial Accounting Standard Board (FASB) and established as a set of principles and guidelines called Generally Accepted Accounting Principles (GAAP).1–3
Accrual- vs cash-based accounting
There are 2 approaches to recording financial transactions: accrual- and cash-based accounting methods. The main difference between them is in the timing of the recorded financial transactions (when revenue and expenses are recognized on the accounting books). Under GAAP, the matching principle, which is one of the most basic and utilized guidelines of accounting, specifies that accrual accounting be used. In the United States, most businesses (publically traded companies and moderate- to large-sized companies) use accrual accounting, while some individual and smaller businesses, including health care services such as physician practices, use the cash method.1–4
Accrual-based accounting
Accrual-based accounting specifies that revenues are recorded when they are earned, and expenses are recorded when they occur. A health care business may earn revenue for services on one day, but the cash may not be received or recorded on the accounting books for several weeks or months and at an amount less than billed.
Accrual-based accounting provides a more accurate representation of a business’ financial performance, since it uses the principle in which expenses are matched to revenues in the same time period. This enables a more precise representation of true financial performance during a given time frame.1–4
Cash-based accounting
Cash-based accounting is the easiest method to understand and implement because financial transactions are recorded in the accounting books when money is received or spent without the need for complex accounting techniques or integration of accounts receivable or payable.
Despite the ease of use and simplicity in tracking cash flow, this method can be deceiving because revenue may be received or expenses may need to be paid at times that are not consistent with when the revenue has been earned or expenses incurred. This can result in misleading information on the business’ health and the accuracy of tracking financial performance over time, since revenue and expenses for a particular transaction may occur at different times.1–4
Which accounting process to choose?
Even though accrual-based accounting may provide a more accurate financial representation of a business’ performance, many smaller businesses, including physician practices, prefer to use cash-based accounting. In addition, many health care businesses are eligible to use cash-based accounting per Internal Revenue Service (IRS) rules by qualifying for the Gross Receipts Test and being a qualified Personal Service Corporation (PSC):
- The Gross Receipts Test states that if the average annual gross receipts of the business are less than $5 million, the business can use the cash-based accounting method.
- If at least 95% of a business activity involves performing health care services, and at least 95% of the business is owned by employees performing health care services, then the business qualifies as a PSC that may use the cash-based accounting method.
Many physician practices qualify to use cash-based accounting, which reduces the complexity of following accrual-based accounting rules and simplifies overall cash-flow management.5
Read about insurance, capital equipment depreciation, more
CASE New practice opens
Practice A opens its practice on January 1. The practice borrows $20,000 from the bank to purchase hysteroscopic equipment for office-based tubal sterilizations and an additional $50,000 for an ultrasound machine. Both loans have a 5% annual interest rate amortized over 5 years. The practice leases office space and pays rent 2 months in advance at $8,000 ($4,000 per month). On January 1, the practice pays a $1,200 premium for annual property and liability insurance and $12,500 for the first quarter payment for professional liability insurance ($50,000 annually, paid quarterly). Other costs the practice pays in January include: utilities, $400; EHR licensing, $300; technical support, $200; and salaries, $10,000.
The practice purchases 4 sets of sterilization spring devices at $1,500 each ($6,000) to have in stock. One hysteroscopic sterilization procedure is performed on a patient in January using 1 device. The practice is reimbursed $2,500 for the procedure.
In January, the practice bills $150,000 in charges, but after insurance contractual adjustments, January’s revenue is $50,000. Actual cash payments from billings received are $10,000 in January, $30,000 in February, and $10,000 in March.
At first glance, there is a noticeable difference on the sales or recognition of revenues based on the type of accounting (TABLE). With the accrual method, because the billing charges are submitted in January when the services were provided (minus the insurance contractual adjustments), the $50,000 revenue is immediately counted and recognized, even though the practice only received $10,000 cash for those billings during January. While the benefit to accrual accounting is the timely recognition of the revenue when the service was provided, the downside is that much of those billings might actually be paid over 90 days, and some of those billings may go unpaid by the insurance company or the patients, which would require adjustments in later months.

The cash-based method is simpler to understand because the cash received for the month is recognized as the revenue, regardless of the amount charged that month.
Merchandise. In the accrual method, the cost of merchandise sold (the hysteroscopic sterilization implants) is recognized as an expense when the revenue is generated from its sale. In this case, the date that the patient has the hysteroscopic in-office sterilization procedure is when the revenue and the expense of the implant are recognized.
In a cash-based accounting method, the $6,000 cost of the implants is recognized at the time of purchase in January.
Lease. In this scenario, even though 2 months of lease for the office were paid, the accrual method only recognizes the January payment; the second payment is recognized in February. In the cash method, because both months were paid in January, the total expense of $8,000 is recognized in January.
Property liability insurance. The property liability insurance payment is required at the start of the year. In accrual accounting, this expense is divided over 12 months, while in the cash method, the expense is counted at the time the payment is made.
Professional liability insurance. The professional liability insurance expense of $50,000 per year is made in quarterly payments, so for the accrual method, the annual amount would be distributed over 12 months at $4,200 per month. With the cash method, it would be paid—and recognized as an expense—quarterly at $12,500, starting in January.
Capital equipment depreciation. Capital medical equipment (hysteroscopy and ultrasound) can be depreciated using a straight-line 5-year depreciation. A total $70,000 worth of equipment divided by 5 years is $14,000 per year, depreciated over 5 years. One-twelfth of $14,000 equals $1,167, which is recorded as a January depreciation expense. Because the Internal Revenue Code requires capital assets to be depreciated, even for cash-basis taxpayers, the common practice is to record depreciation expense for both cash- and accrual-basis income accounting.6
Interest on loans. A loan’s principal payment will not be included on the income statement. The principal payment, a reduction of a liability (loans payable), is reported on the balance sheet. Only the interest portion of a loan payment is reported on the income statement (interest expense). In accrual accounting, the accrued interest on the loan payment for the year is $3,500 ($292 for January). For the cash-basis method, because the interest is paid annually at year-end, interest will not be expensed until December.
Taxes. The IRS states that, “Individuals, including sole proprietors, partners, and S corporation shareholders, generally have to make estimated tax payments if they expect to owe tax of $1,000 or more when their return is filed. Corporations generally have to make estimated tax payments if they expect to owe tax of $500 or more when their return is filed.”7
Assuming 35% tax liability, the accrual method would create a tax liability of $9,744 on a profit of $27,841. With the cash method, there would be no tax liability because there was no net profit.
Other expenses. The utilities, EHR licensing, tech support, and salaries are expensed the same way for both methods.
Net income. The resulting final net income is vastly different for the month of January depending on the accounting method utilized. The accrual method results in a net income of $18,097, while the cash-basis method results in a net loss of $29,767. Over the course of the year, these imbalances are likely to even out.
Related article:
Business law critical to your practice
Choosing an accounting method
Depending on the accounting method, a practice’s performance and profit will seem very different. The type of accounting method chosen will depend on what goals the owners want to achieve.
The accrual method provides a more accurate picture of business flow and performance and will be less subject to monthly variations due to large purchases or variations in expenses. If the practice chooses this method using an income statement, it should also employ a cash-flow statement.
The cash method of accounting will give a convenient and practical summary of the practice’s cash flow.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Physicians practice medicine and communicate within the world of medical language, yet there is a lack of awareness and understanding by many health care professionals of the universal language of business, which is accounting. Just as Latin provides the basic framework for medically related terminology, accounting is the standard language used to convey financial information to both internal and external stakeholders.
Accounting principles are important to physicians at any level. Whether you are starting out in private practice, running a clinical department, or working as an executive in a health care system, most decisions are based on the interpretation of financial data using accounting principles. Accounting standards in the United States are developed by the Financial Accounting Standard Board (FASB) and established as a set of principles and guidelines called Generally Accepted Accounting Principles (GAAP).1–3
Accrual- vs cash-based accounting
There are 2 approaches to recording financial transactions: accrual- and cash-based accounting methods. The main difference between them is in the timing of the recorded financial transactions (when revenue and expenses are recognized on the accounting books). Under GAAP, the matching principle, which is one of the most basic and utilized guidelines of accounting, specifies that accrual accounting be used. In the United States, most businesses (publically traded companies and moderate- to large-sized companies) use accrual accounting, while some individual and smaller businesses, including health care services such as physician practices, use the cash method.1–4
Accrual-based accounting
Accrual-based accounting specifies that revenues are recorded when they are earned, and expenses are recorded when they occur. A health care business may earn revenue for services on one day, but the cash may not be received or recorded on the accounting books for several weeks or months and at an amount less than billed.
Accrual-based accounting provides a more accurate representation of a business’ financial performance, since it uses the principle in which expenses are matched to revenues in the same time period. This enables a more precise representation of true financial performance during a given time frame.1–4
Cash-based accounting
Cash-based accounting is the easiest method to understand and implement because financial transactions are recorded in the accounting books when money is received or spent without the need for complex accounting techniques or integration of accounts receivable or payable.
Despite the ease of use and simplicity in tracking cash flow, this method can be deceiving because revenue may be received or expenses may need to be paid at times that are not consistent with when the revenue has been earned or expenses incurred. This can result in misleading information on the business’ health and the accuracy of tracking financial performance over time, since revenue and expenses for a particular transaction may occur at different times.1–4
Which accounting process to choose?
Even though accrual-based accounting may provide a more accurate financial representation of a business’ performance, many smaller businesses, including physician practices, prefer to use cash-based accounting. In addition, many health care businesses are eligible to use cash-based accounting per Internal Revenue Service (IRS) rules by qualifying for the Gross Receipts Test and being a qualified Personal Service Corporation (PSC):
- The Gross Receipts Test states that if the average annual gross receipts of the business are less than $5 million, the business can use the cash-based accounting method.
- If at least 95% of a business activity involves performing health care services, and at least 95% of the business is owned by employees performing health care services, then the business qualifies as a PSC that may use the cash-based accounting method.
Many physician practices qualify to use cash-based accounting, which reduces the complexity of following accrual-based accounting rules and simplifies overall cash-flow management.5
Read about insurance, capital equipment depreciation, more
CASE New practice opens
Practice A opens its practice on January 1. The practice borrows $20,000 from the bank to purchase hysteroscopic equipment for office-based tubal sterilizations and an additional $50,000 for an ultrasound machine. Both loans have a 5% annual interest rate amortized over 5 years. The practice leases office space and pays rent 2 months in advance at $8,000 ($4,000 per month). On January 1, the practice pays a $1,200 premium for annual property and liability insurance and $12,500 for the first quarter payment for professional liability insurance ($50,000 annually, paid quarterly). Other costs the practice pays in January include: utilities, $400; EHR licensing, $300; technical support, $200; and salaries, $10,000.
The practice purchases 4 sets of sterilization spring devices at $1,500 each ($6,000) to have in stock. One hysteroscopic sterilization procedure is performed on a patient in January using 1 device. The practice is reimbursed $2,500 for the procedure.
In January, the practice bills $150,000 in charges, but after insurance contractual adjustments, January’s revenue is $50,000. Actual cash payments from billings received are $10,000 in January, $30,000 in February, and $10,000 in March.
At first glance, there is a noticeable difference on the sales or recognition of revenues based on the type of accounting (TABLE). With the accrual method, because the billing charges are submitted in January when the services were provided (minus the insurance contractual adjustments), the $50,000 revenue is immediately counted and recognized, even though the practice only received $10,000 cash for those billings during January. While the benefit to accrual accounting is the timely recognition of the revenue when the service was provided, the downside is that much of those billings might actually be paid over 90 days, and some of those billings may go unpaid by the insurance company or the patients, which would require adjustments in later months.

The cash-based method is simpler to understand because the cash received for the month is recognized as the revenue, regardless of the amount charged that month.
Merchandise. In the accrual method, the cost of merchandise sold (the hysteroscopic sterilization implants) is recognized as an expense when the revenue is generated from its sale. In this case, the date that the patient has the hysteroscopic in-office sterilization procedure is when the revenue and the expense of the implant are recognized.
In a cash-based accounting method, the $6,000 cost of the implants is recognized at the time of purchase in January.
Lease. In this scenario, even though 2 months of lease for the office were paid, the accrual method only recognizes the January payment; the second payment is recognized in February. In the cash method, because both months were paid in January, the total expense of $8,000 is recognized in January.
Property liability insurance. The property liability insurance payment is required at the start of the year. In accrual accounting, this expense is divided over 12 months, while in the cash method, the expense is counted at the time the payment is made.
Professional liability insurance. The professional liability insurance expense of $50,000 per year is made in quarterly payments, so for the accrual method, the annual amount would be distributed over 12 months at $4,200 per month. With the cash method, it would be paid—and recognized as an expense—quarterly at $12,500, starting in January.
Capital equipment depreciation. Capital medical equipment (hysteroscopy and ultrasound) can be depreciated using a straight-line 5-year depreciation. A total $70,000 worth of equipment divided by 5 years is $14,000 per year, depreciated over 5 years. One-twelfth of $14,000 equals $1,167, which is recorded as a January depreciation expense. Because the Internal Revenue Code requires capital assets to be depreciated, even for cash-basis taxpayers, the common practice is to record depreciation expense for both cash- and accrual-basis income accounting.6
Interest on loans. A loan’s principal payment will not be included on the income statement. The principal payment, a reduction of a liability (loans payable), is reported on the balance sheet. Only the interest portion of a loan payment is reported on the income statement (interest expense). In accrual accounting, the accrued interest on the loan payment for the year is $3,500 ($292 for January). For the cash-basis method, because the interest is paid annually at year-end, interest will not be expensed until December.
Taxes. The IRS states that, “Individuals, including sole proprietors, partners, and S corporation shareholders, generally have to make estimated tax payments if they expect to owe tax of $1,000 or more when their return is filed. Corporations generally have to make estimated tax payments if they expect to owe tax of $500 or more when their return is filed.”7
Assuming 35% tax liability, the accrual method would create a tax liability of $9,744 on a profit of $27,841. With the cash method, there would be no tax liability because there was no net profit.
Other expenses. The utilities, EHR licensing, tech support, and salaries are expensed the same way for both methods.
Net income. The resulting final net income is vastly different for the month of January depending on the accounting method utilized. The accrual method results in a net income of $18,097, while the cash-basis method results in a net loss of $29,767. Over the course of the year, these imbalances are likely to even out.
Related article:
Business law critical to your practice
Choosing an accounting method
Depending on the accounting method, a practice’s performance and profit will seem very different. The type of accounting method chosen will depend on what goals the owners want to achieve.
The accrual method provides a more accurate picture of business flow and performance and will be less subject to monthly variations due to large purchases or variations in expenses. If the practice chooses this method using an income statement, it should also employ a cash-flow statement.
The cash method of accounting will give a convenient and practical summary of the practice’s cash flow.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- About the FASB. Financial Accounting Standards Board website. http://www.fasb.org/jsp/FASB/PageSectionPage&cid=1176154526495. Accessed November 7, 2017.
- What is the difference between accrual accounting and cash accounting? Investopedia. https://www.investopedia.com/ask/answers/121514/what-difference-between-accrual-accounting-and-cash-accounting.asp. Accessed November 7, 2017.
- Accounting Basics (Explanation). Part 2: Income Statement. Accounting Coach. https://www.accountingcoach.com/accounting-basics/explanation/2. Accessed November 7, 2017.
- Stickney C, Weil R. Financial Accounting: An Introduction to Concepts, Methods, and Uses. 11th ed. Nashville, TN: Southwestern College Publishing Group; 2006:97-110.
- Internal Revenue Service. Publication 538 (12/2016), Accounting Periods and Methods. https://www.irs.gov/publications/p538#en_US_201612_publink1000270634. Revised December 2016. Accessed November 7, 2017.
- Klinefelter D, McCorkle D, Klose S. Financial Management: Cash vs. Accrual Accounting. Risk Management. AgriLife Extension. Texas A&M System. http://agrilife.org/agecoext/files/2013/10/rm5-16.pdf. Published 2013. Accessed November 7, 2017.
- Internal Revenue Service. Small Business and Self-Employed Tax Center: Estimated Taxes. https://www.irs.gov/businesses/small-businesses-self-employed/estimated-taxes. Updated November 2, 2017. Accessed November 7, 2017.
- About the FASB. Financial Accounting Standards Board website. http://www.fasb.org/jsp/FASB/PageSectionPage&cid=1176154526495. Accessed November 7, 2017.
- What is the difference between accrual accounting and cash accounting? Investopedia. https://www.investopedia.com/ask/answers/121514/what-difference-between-accrual-accounting-and-cash-accounting.asp. Accessed November 7, 2017.
- Accounting Basics (Explanation). Part 2: Income Statement. Accounting Coach. https://www.accountingcoach.com/accounting-basics/explanation/2. Accessed November 7, 2017.
- Stickney C, Weil R. Financial Accounting: An Introduction to Concepts, Methods, and Uses. 11th ed. Nashville, TN: Southwestern College Publishing Group; 2006:97-110.
- Internal Revenue Service. Publication 538 (12/2016), Accounting Periods and Methods. https://www.irs.gov/publications/p538#en_US_201612_publink1000270634. Revised December 2016. Accessed November 7, 2017.
- Klinefelter D, McCorkle D, Klose S. Financial Management: Cash vs. Accrual Accounting. Risk Management. AgriLife Extension. Texas A&M System. http://agrilife.org/agecoext/files/2013/10/rm5-16.pdf. Published 2013. Accessed November 7, 2017.
- Internal Revenue Service. Small Business and Self-Employed Tax Center: Estimated Taxes. https://www.irs.gov/businesses/small-businesses-self-employed/estimated-taxes. Updated November 2, 2017. Accessed November 7, 2017.
Inhaled loxapine quells agitation in personality disorders
PARIS – The inhaled powder formulation of loxapine appears to be a safe and effective treatment for acute agitation in patients with personality disorders, Diego R. Mendez Mareque, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Inhaled loxapine is approved for the treatment of acute agitation associated with schizophrenia or bipolar I disorder. Its use in patients with borderline personality and other personality disorders is off label. But this inhaled typical antipsychotic shows promise in filling an unmet need for a rapid-acting, minimally invasive treatment for acute agitation in patients with personality disorders, a very common scenario in psychiatric wards and emergency departments, and one that can quickly escalate to aggression and violence, noted Dr. Mendez Mareque of the Galician Health Service in Ferrol, Spain.
He presented a prospective, longitudinal, observational pilot study of 14 patients with personality disorders treated with a single 10-mg dose of inhaled loxapine while experiencing acute agitation in a psychiatric emergency department or psychiatric ward. Their mean baseline score on the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) was 20.8 out of a possible 35 points. The scale assesses five domains: excitement, tension, uncooperativeness, hostility, and poor impulse control.
Within 10 minutes after administration of inhaled loxapine by a health care professional, 11 patients showed a significant drop on the PANSS-EC. They were calm, nonsedated, and ready for assessment. Within 20 minutes, their PANSS-EC scores were reduced by roughly half, compared with baseline.
Three patients were nonresponders. They received rescue treatment with oral or injectable antipsychotics and benzodiazepines.
None of the 14 patients had a history of airway disease, and none experienced bronchospasm, a known possible side effect of inhaled loxapine, the psychiatrist noted.
The results of this Spanish observational study confirm the benefits of inhaled loxapine for treating agitation in patients with borderline personality disorder previously described in a German case series (J Clin Psychopharmacol. 2015 Dec;35[6]:741-3).
Dr. Mendez Mareque reported having no financial conflicts of interest regarding his study.
PARIS – The inhaled powder formulation of loxapine appears to be a safe and effective treatment for acute agitation in patients with personality disorders, Diego R. Mendez Mareque, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Inhaled loxapine is approved for the treatment of acute agitation associated with schizophrenia or bipolar I disorder. Its use in patients with borderline personality and other personality disorders is off label. But this inhaled typical antipsychotic shows promise in filling an unmet need for a rapid-acting, minimally invasive treatment for acute agitation in patients with personality disorders, a very common scenario in psychiatric wards and emergency departments, and one that can quickly escalate to aggression and violence, noted Dr. Mendez Mareque of the Galician Health Service in Ferrol, Spain.
He presented a prospective, longitudinal, observational pilot study of 14 patients with personality disorders treated with a single 10-mg dose of inhaled loxapine while experiencing acute agitation in a psychiatric emergency department or psychiatric ward. Their mean baseline score on the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) was 20.8 out of a possible 35 points. The scale assesses five domains: excitement, tension, uncooperativeness, hostility, and poor impulse control.
Within 10 minutes after administration of inhaled loxapine by a health care professional, 11 patients showed a significant drop on the PANSS-EC. They were calm, nonsedated, and ready for assessment. Within 20 minutes, their PANSS-EC scores were reduced by roughly half, compared with baseline.
Three patients were nonresponders. They received rescue treatment with oral or injectable antipsychotics and benzodiazepines.
None of the 14 patients had a history of airway disease, and none experienced bronchospasm, a known possible side effect of inhaled loxapine, the psychiatrist noted.
The results of this Spanish observational study confirm the benefits of inhaled loxapine for treating agitation in patients with borderline personality disorder previously described in a German case series (J Clin Psychopharmacol. 2015 Dec;35[6]:741-3).
Dr. Mendez Mareque reported having no financial conflicts of interest regarding his study.
PARIS – The inhaled powder formulation of loxapine appears to be a safe and effective treatment for acute agitation in patients with personality disorders, Diego R. Mendez Mareque, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
Inhaled loxapine is approved for the treatment of acute agitation associated with schizophrenia or bipolar I disorder. Its use in patients with borderline personality and other personality disorders is off label. But this inhaled typical antipsychotic shows promise in filling an unmet need for a rapid-acting, minimally invasive treatment for acute agitation in patients with personality disorders, a very common scenario in psychiatric wards and emergency departments, and one that can quickly escalate to aggression and violence, noted Dr. Mendez Mareque of the Galician Health Service in Ferrol, Spain.
He presented a prospective, longitudinal, observational pilot study of 14 patients with personality disorders treated with a single 10-mg dose of inhaled loxapine while experiencing acute agitation in a psychiatric emergency department or psychiatric ward. Their mean baseline score on the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) was 20.8 out of a possible 35 points. The scale assesses five domains: excitement, tension, uncooperativeness, hostility, and poor impulse control.
Within 10 minutes after administration of inhaled loxapine by a health care professional, 11 patients showed a significant drop on the PANSS-EC. They were calm, nonsedated, and ready for assessment. Within 20 minutes, their PANSS-EC scores were reduced by roughly half, compared with baseline.
Three patients were nonresponders. They received rescue treatment with oral or injectable antipsychotics and benzodiazepines.
None of the 14 patients had a history of airway disease, and none experienced bronchospasm, a known possible side effect of inhaled loxapine, the psychiatrist noted.
The results of this Spanish observational study confirm the benefits of inhaled loxapine for treating agitation in patients with borderline personality disorder previously described in a German case series (J Clin Psychopharmacol. 2015 Dec;35[6]:741-3).
Dr. Mendez Mareque reported having no financial conflicts of interest regarding his study.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: Eleven of 14 acutely agitated patients with various personality disorders were calm, nonsedated, and ready for assessment within 10 minutes after a single dose of inhaled loxapine.
Data source: A prospective observational pilot study of inhaled loxapine in 14 acutely agitated patients with personality disorders.
Disclosures: The study presenter reported having no financial conflicts of interest.
What Are the Mechanisms of SUDEP?
KANSAS CITY, MO—Brain, cardiac, and respiratory dysfunctions may cause sudden unexpected death in epilepsy (SUDEP), according to an overview presented at the 46th Annual Meeting of the Child Neurology Society.
“In all likelihood, like the rest of medicine, [SUDEP] is not going to be as simple as one thing or the other. It will likely be a combination of multiple organs and susceptibilities and likely heterogeneous etiologies,” said Jeffrey Buchhalter, MD, PhD, Professor at the University of Calgary Cumming School of Medicine in Alberta.
SUDEP research publications have increased exponentially over the last two decades. Although the exact cause of SUDEP is unknown, understanding potential mechanisms and biomarkers may help researchers develop preventive strategies, said Dr. Buchhalter.
Brain Mechanisms
Studies have suggested that postictal generalized EEG suppression (PGES) is a risk factor and perhaps a biomarker for SUDEP. Lhatoo et al found that PGES lasting more than 80 seconds quadruples the risk of SUDEP. In addition, the postictal period is longer in adults than in children, said Dr. Buchhalter.
Freitas et al studied semiologic and EEG differences between generalized tonic-clonic seizures (GTCS) of adults and children. The researchers analyzed video-EEG data of 105 GTCS events in 61 consecutive patients (12 children, 23 seizure events; 49 adults, 82 seizure events) who were recruited from an epilepsy monitoring unit. They concluded that prolonged seizure phases and prolonged PGES duration might be electroclinical markers of SUDEP risk.
Seyal et al
A study by Walczak et al has indicated that one to three GTCS per year doubled the SUDEP risk, and more than three GTCS per year increase the risk of SUDEP eight times. “At face value, these numbers are powerful because so many of us see kids and adults who have a lot of GTCS,” said Dr. Buchhalter.
Cardiac and Respiratory Mechanisms
Seizure-related tachycardia and postictal ST-segment changes are common, and bradycardia and asystole have been observed in epilepsy units, said Dr. Buchhalter. Nevertheless, “phenomena associated with seizures are not always associated with SUDEP,” he said.
Central and obstructive apneas, desaturation, and hyperventilation may occur with generalized or focal seizures, and respiratory dysfunction may be a cause of SUDEP. In the MORTEMUS study, Ryvlin et al found that SUDEP in epilepsy monitoring units primarily follows an early postictal, centrally mediated, severe alteration of respiratory and cardiac function. Researchers examined data about patients’ respiration and cardiac function to determine when patients stopped breathing and when their hearts stopped beating. “In each instance, the lungs stopped first,” said Dr. Buchhalter. Despite the study’s ascertainment bias, MORTEMUS “is the best and only large series of this kind of data that has been presented,” he added.
The Role of Genetics
Bagnall et al searched for genetic risk factors in SUDEP cases. They performed an exome-based analysis of rare variants by collecting clinical information from 61 definite and probable SUDEP cases.
The researchers identified de novo mutations, previously reported pathogenic mutations, or candidate pathogenic variants in 46% of SUDEP cases. They concluded that a sizable proportion of SUDEP cases has clinically relevant mutations in cardiac arrhythmia and epilepsy genes and that understanding the genetic components of SUDEP can help to inform cascade testing of at-risk family members.
“I think this is a very hopeful set of experiments. This is the kind of [research] that can go from the lab to the bedside,” Dr. Buchhalter concluded.
—Erica Tricarico
Suggested Reading
Bagnall RD, Crompton DE, Petrovski S, et al. Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol. 2016;79(4):522-534.
Freitas J, Kaur G, Fernandez GB, et al. Age-specific periictal electroclinical features of generalized tonic-clonic seizures and potential risk of sudden unexpected death in epilepsy (SUDEP). Epilepsy Behav. 2013;29(2):289-294.
Goldman AM. Mechanisms of sudden unexplained death in epilepsy. Curr Opin Neurol. 2015;28(2):166-174.
Lhatoo SD, Faulkner HJ, Dembny K, et al. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol. 2010;68(6):787-796.
Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966-977.
Scorza FA, de Carmo AC, Scorza CA, Fiorini AC. SUDEP: A steep increase in publication since its definition. Epilepsy Behav. 2017;72:195-197.
Seyal M, Bateman LM, Li CS. Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia. 2013;54(2):377-382.
Shankar R, Cox D, Jalihal V, et al. Sudden unexpected death in epilepsy (SUDEP): Development of a safety checklist. Seizure. 2013;22(10):812-817.
KANSAS CITY, MO—Brain, cardiac, and respiratory dysfunctions may cause sudden unexpected death in epilepsy (SUDEP), according to an overview presented at the 46th Annual Meeting of the Child Neurology Society.
“In all likelihood, like the rest of medicine, [SUDEP] is not going to be as simple as one thing or the other. It will likely be a combination of multiple organs and susceptibilities and likely heterogeneous etiologies,” said Jeffrey Buchhalter, MD, PhD, Professor at the University of Calgary Cumming School of Medicine in Alberta.
SUDEP research publications have increased exponentially over the last two decades. Although the exact cause of SUDEP is unknown, understanding potential mechanisms and biomarkers may help researchers develop preventive strategies, said Dr. Buchhalter.
Brain Mechanisms
Studies have suggested that postictal generalized EEG suppression (PGES) is a risk factor and perhaps a biomarker for SUDEP. Lhatoo et al found that PGES lasting more than 80 seconds quadruples the risk of SUDEP. In addition, the postictal period is longer in adults than in children, said Dr. Buchhalter.
Freitas et al studied semiologic and EEG differences between generalized tonic-clonic seizures (GTCS) of adults and children. The researchers analyzed video-EEG data of 105 GTCS events in 61 consecutive patients (12 children, 23 seizure events; 49 adults, 82 seizure events) who were recruited from an epilepsy monitoring unit. They concluded that prolonged seizure phases and prolonged PGES duration might be electroclinical markers of SUDEP risk.
Seyal et al
A study by Walczak et al has indicated that one to three GTCS per year doubled the SUDEP risk, and more than three GTCS per year increase the risk of SUDEP eight times. “At face value, these numbers are powerful because so many of us see kids and adults who have a lot of GTCS,” said Dr. Buchhalter.
Cardiac and Respiratory Mechanisms
Seizure-related tachycardia and postictal ST-segment changes are common, and bradycardia and asystole have been observed in epilepsy units, said Dr. Buchhalter. Nevertheless, “phenomena associated with seizures are not always associated with SUDEP,” he said.
Central and obstructive apneas, desaturation, and hyperventilation may occur with generalized or focal seizures, and respiratory dysfunction may be a cause of SUDEP. In the MORTEMUS study, Ryvlin et al found that SUDEP in epilepsy monitoring units primarily follows an early postictal, centrally mediated, severe alteration of respiratory and cardiac function. Researchers examined data about patients’ respiration and cardiac function to determine when patients stopped breathing and when their hearts stopped beating. “In each instance, the lungs stopped first,” said Dr. Buchhalter. Despite the study’s ascertainment bias, MORTEMUS “is the best and only large series of this kind of data that has been presented,” he added.
The Role of Genetics
Bagnall et al searched for genetic risk factors in SUDEP cases. They performed an exome-based analysis of rare variants by collecting clinical information from 61 definite and probable SUDEP cases.
The researchers identified de novo mutations, previously reported pathogenic mutations, or candidate pathogenic variants in 46% of SUDEP cases. They concluded that a sizable proportion of SUDEP cases has clinically relevant mutations in cardiac arrhythmia and epilepsy genes and that understanding the genetic components of SUDEP can help to inform cascade testing of at-risk family members.
“I think this is a very hopeful set of experiments. This is the kind of [research] that can go from the lab to the bedside,” Dr. Buchhalter concluded.
—Erica Tricarico
Suggested Reading
Bagnall RD, Crompton DE, Petrovski S, et al. Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol. 2016;79(4):522-534.
Freitas J, Kaur G, Fernandez GB, et al. Age-specific periictal electroclinical features of generalized tonic-clonic seizures and potential risk of sudden unexpected death in epilepsy (SUDEP). Epilepsy Behav. 2013;29(2):289-294.
Goldman AM. Mechanisms of sudden unexplained death in epilepsy. Curr Opin Neurol. 2015;28(2):166-174.
Lhatoo SD, Faulkner HJ, Dembny K, et al. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol. 2010;68(6):787-796.
Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966-977.
Scorza FA, de Carmo AC, Scorza CA, Fiorini AC. SUDEP: A steep increase in publication since its definition. Epilepsy Behav. 2017;72:195-197.
Seyal M, Bateman LM, Li CS. Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia. 2013;54(2):377-382.
Shankar R, Cox D, Jalihal V, et al. Sudden unexpected death in epilepsy (SUDEP): Development of a safety checklist. Seizure. 2013;22(10):812-817.
KANSAS CITY, MO—Brain, cardiac, and respiratory dysfunctions may cause sudden unexpected death in epilepsy (SUDEP), according to an overview presented at the 46th Annual Meeting of the Child Neurology Society.
“In all likelihood, like the rest of medicine, [SUDEP] is not going to be as simple as one thing or the other. It will likely be a combination of multiple organs and susceptibilities and likely heterogeneous etiologies,” said Jeffrey Buchhalter, MD, PhD, Professor at the University of Calgary Cumming School of Medicine in Alberta.
SUDEP research publications have increased exponentially over the last two decades. Although the exact cause of SUDEP is unknown, understanding potential mechanisms and biomarkers may help researchers develop preventive strategies, said Dr. Buchhalter.
Brain Mechanisms
Studies have suggested that postictal generalized EEG suppression (PGES) is a risk factor and perhaps a biomarker for SUDEP. Lhatoo et al found that PGES lasting more than 80 seconds quadruples the risk of SUDEP. In addition, the postictal period is longer in adults than in children, said Dr. Buchhalter.
Freitas et al studied semiologic and EEG differences between generalized tonic-clonic seizures (GTCS) of adults and children. The researchers analyzed video-EEG data of 105 GTCS events in 61 consecutive patients (12 children, 23 seizure events; 49 adults, 82 seizure events) who were recruited from an epilepsy monitoring unit. They concluded that prolonged seizure phases and prolonged PGES duration might be electroclinical markers of SUDEP risk.
Seyal et al
A study by Walczak et al has indicated that one to three GTCS per year doubled the SUDEP risk, and more than three GTCS per year increase the risk of SUDEP eight times. “At face value, these numbers are powerful because so many of us see kids and adults who have a lot of GTCS,” said Dr. Buchhalter.
Cardiac and Respiratory Mechanisms
Seizure-related tachycardia and postictal ST-segment changes are common, and bradycardia and asystole have been observed in epilepsy units, said Dr. Buchhalter. Nevertheless, “phenomena associated with seizures are not always associated with SUDEP,” he said.
Central and obstructive apneas, desaturation, and hyperventilation may occur with generalized or focal seizures, and respiratory dysfunction may be a cause of SUDEP. In the MORTEMUS study, Ryvlin et al found that SUDEP in epilepsy monitoring units primarily follows an early postictal, centrally mediated, severe alteration of respiratory and cardiac function. Researchers examined data about patients’ respiration and cardiac function to determine when patients stopped breathing and when their hearts stopped beating. “In each instance, the lungs stopped first,” said Dr. Buchhalter. Despite the study’s ascertainment bias, MORTEMUS “is the best and only large series of this kind of data that has been presented,” he added.
The Role of Genetics
Bagnall et al searched for genetic risk factors in SUDEP cases. They performed an exome-based analysis of rare variants by collecting clinical information from 61 definite and probable SUDEP cases.
The researchers identified de novo mutations, previously reported pathogenic mutations, or candidate pathogenic variants in 46% of SUDEP cases. They concluded that a sizable proportion of SUDEP cases has clinically relevant mutations in cardiac arrhythmia and epilepsy genes and that understanding the genetic components of SUDEP can help to inform cascade testing of at-risk family members.
“I think this is a very hopeful set of experiments. This is the kind of [research] that can go from the lab to the bedside,” Dr. Buchhalter concluded.
—Erica Tricarico
Suggested Reading
Bagnall RD, Crompton DE, Petrovski S, et al. Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol. 2016;79(4):522-534.
Freitas J, Kaur G, Fernandez GB, et al. Age-specific periictal electroclinical features of generalized tonic-clonic seizures and potential risk of sudden unexpected death in epilepsy (SUDEP). Epilepsy Behav. 2013;29(2):289-294.
Goldman AM. Mechanisms of sudden unexplained death in epilepsy. Curr Opin Neurol. 2015;28(2):166-174.
Lhatoo SD, Faulkner HJ, Dembny K, et al. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol. 2010;68(6):787-796.
Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12(10):966-977.
Scorza FA, de Carmo AC, Scorza CA, Fiorini AC. SUDEP: A steep increase in publication since its definition. Epilepsy Behav. 2017;72:195-197.
Seyal M, Bateman LM, Li CS. Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia. 2013;54(2):377-382.
Shankar R, Cox D, Jalihal V, et al. Sudden unexpected death in epilepsy (SUDEP): Development of a safety checklist. Seizure. 2013;22(10):812-817.
Fecal microbiota transplants by oral capsule noninferior to colonoscopy
Fecal microbiota transplantation (FMT) using oral capsules as the delivery method has been shown to be noninferior to delivery using colonoscopy for the treatment of Clostridium difficile infection, but with a significantly lower price tag.
In an unblended noninferiority trial, published in the Nov. 28 issue of JAMA, 116 adults with at least three documented episodes of C. difficile infection were randomized to either 360 mL of fecal slurry delivered to the cecum via colonoscopy or to 40 capsules of processed fecal microbiota swallowed under direct observation.
Dina Kao, MD, of the department of medicine at the University of Alberta, Edmonton, and coauthors commented that the response rate with the capsules was higher than that seen in other studies of fecal microbiota capsules, which they suggested may partly be due to the larger amount of donor stool used in the study: 80-100 g, compared with 17 g and 25 g used in other studies.
“The higher efficacy observed in this study suggests a dose-dependent response to FMT, and a benefit of bowel lavage prior to FMT, because residual vancomycin was detected up to 8 days despite its discontinuation,” they wrote.
Both treatment modalities achieved similar quality of life improvements. Both groups reported major improvements in domains including physical and emotional health, physical and social functioning, and general health, with no significant differences between the two arms of the study.
The cost per treatment in the colonoscopy group was $874 per patient, compared with $308 per patient in the capsule group.
“Although colonoscopy delivery is more invasive, resource intensive, costly, and inconvenient for patients, it has the advantage of identifying alternative diagnoses,” the authors wrote. “Conversely, when FMT is given by oral capsules, it can be administered in an office setting, which could substantially reduce cost and wait time.”
Both groups also showed significantly improved gut microbiota diversity, which approached that of the donor just 1 week after administration of the treatment.
While 30% of patients characterized FMTs as “unpleasant, gross, or disgusting,” 79% of participants said the unpleasantness was the same or less than anticipated, and 97% said they would undergo the same treatment by the same delivery method again if needed.
However, significantly more patients in the capsule group described their experience as “not at all unpleasant,” compared with the colonoscopy group (66% vs. 44%; 95% CI, 3%-40%; P = .01).
There were no colonic perforations seen in the colonoscopy group, and no infectious complications relating to the treatment in either group. One patient in each group died of underlying cardiopulmonary illness that was unrelated to the treatment, and the rate of minor adverse events was 5.4% in the capsule group and 12.5% in the colonoscopy group.
The authors acknowledged that the lack of a placebo group in the study meant they were not able to measure the size of the effect of fecal microbiota transplantation by either route. One earlier trial had also shown a placebo response rate of 45%.
The study was funded by Alberta Health Services and the University of Alberta Hospital Foundation. Four authors declared grants and other funding from the study funder and the pharmaceutical industry.
Clostridium difficile infection costs the U.S. health care system an estimated $1.5 billion each year, with 450,000 cases reported annually, 20% of which involve a recurrence of the infection. Fecal microbiota transplantation is increasingly being used as a treatment, but more widespread adoption is limited partly by the logistical difficulties of delivery.
This study offers encouraging data on delivery of fecal microbiota transplants via capsule, which may reduce barriers to adoption of this treatment; however, there are still some questions to be answered about the treatment’s efficacy, such as the timing of delivery and the relative importance of stool components.
There are also other approaches that should be considered in future research on C. difficile infection, including the use of vancomycin tapers with and without “chasers” of fidaxomicin/rifaximin, the use of defined microbial communities, and the use of sterile, fecal-derived products, which may even supplant standard fecal microbial transplants in the future.
Krishna Rao, MD, Vincent B. Young, MD, PhD, and Preeti N. Malani, MD, are with the division of infectious diseases in the department of internal medicine at the University of Michigan, Ann Arbor. These comments are taken from an accompanying editorial (JAMA. 2017 Nov 28;381:1979-80. doi: 10.1001/jama.2017.17969). Dr. Young reported consulting fees from Vedanta, Merck, and Finch Therapeutics and grants from MedImmune. No other disclosures were reported.
Clostridium difficile infection costs the U.S. health care system an estimated $1.5 billion each year, with 450,000 cases reported annually, 20% of which involve a recurrence of the infection. Fecal microbiota transplantation is increasingly being used as a treatment, but more widespread adoption is limited partly by the logistical difficulties of delivery.
This study offers encouraging data on delivery of fecal microbiota transplants via capsule, which may reduce barriers to adoption of this treatment; however, there are still some questions to be answered about the treatment’s efficacy, such as the timing of delivery and the relative importance of stool components.
There are also other approaches that should be considered in future research on C. difficile infection, including the use of vancomycin tapers with and without “chasers” of fidaxomicin/rifaximin, the use of defined microbial communities, and the use of sterile, fecal-derived products, which may even supplant standard fecal microbial transplants in the future.
Krishna Rao, MD, Vincent B. Young, MD, PhD, and Preeti N. Malani, MD, are with the division of infectious diseases in the department of internal medicine at the University of Michigan, Ann Arbor. These comments are taken from an accompanying editorial (JAMA. 2017 Nov 28;381:1979-80. doi: 10.1001/jama.2017.17969). Dr. Young reported consulting fees from Vedanta, Merck, and Finch Therapeutics and grants from MedImmune. No other disclosures were reported.
Clostridium difficile infection costs the U.S. health care system an estimated $1.5 billion each year, with 450,000 cases reported annually, 20% of which involve a recurrence of the infection. Fecal microbiota transplantation is increasingly being used as a treatment, but more widespread adoption is limited partly by the logistical difficulties of delivery.
This study offers encouraging data on delivery of fecal microbiota transplants via capsule, which may reduce barriers to adoption of this treatment; however, there are still some questions to be answered about the treatment’s efficacy, such as the timing of delivery and the relative importance of stool components.
There are also other approaches that should be considered in future research on C. difficile infection, including the use of vancomycin tapers with and without “chasers” of fidaxomicin/rifaximin, the use of defined microbial communities, and the use of sterile, fecal-derived products, which may even supplant standard fecal microbial transplants in the future.
Krishna Rao, MD, Vincent B. Young, MD, PhD, and Preeti N. Malani, MD, are with the division of infectious diseases in the department of internal medicine at the University of Michigan, Ann Arbor. These comments are taken from an accompanying editorial (JAMA. 2017 Nov 28;381:1979-80. doi: 10.1001/jama.2017.17969). Dr. Young reported consulting fees from Vedanta, Merck, and Finch Therapeutics and grants from MedImmune. No other disclosures were reported.
Fecal microbiota transplantation (FMT) using oral capsules as the delivery method has been shown to be noninferior to delivery using colonoscopy for the treatment of Clostridium difficile infection, but with a significantly lower price tag.
In an unblended noninferiority trial, published in the Nov. 28 issue of JAMA, 116 adults with at least three documented episodes of C. difficile infection were randomized to either 360 mL of fecal slurry delivered to the cecum via colonoscopy or to 40 capsules of processed fecal microbiota swallowed under direct observation.
Dina Kao, MD, of the department of medicine at the University of Alberta, Edmonton, and coauthors commented that the response rate with the capsules was higher than that seen in other studies of fecal microbiota capsules, which they suggested may partly be due to the larger amount of donor stool used in the study: 80-100 g, compared with 17 g and 25 g used in other studies.
“The higher efficacy observed in this study suggests a dose-dependent response to FMT, and a benefit of bowel lavage prior to FMT, because residual vancomycin was detected up to 8 days despite its discontinuation,” they wrote.
Both treatment modalities achieved similar quality of life improvements. Both groups reported major improvements in domains including physical and emotional health, physical and social functioning, and general health, with no significant differences between the two arms of the study.
The cost per treatment in the colonoscopy group was $874 per patient, compared with $308 per patient in the capsule group.
“Although colonoscopy delivery is more invasive, resource intensive, costly, and inconvenient for patients, it has the advantage of identifying alternative diagnoses,” the authors wrote. “Conversely, when FMT is given by oral capsules, it can be administered in an office setting, which could substantially reduce cost and wait time.”
Both groups also showed significantly improved gut microbiota diversity, which approached that of the donor just 1 week after administration of the treatment.
While 30% of patients characterized FMTs as “unpleasant, gross, or disgusting,” 79% of participants said the unpleasantness was the same or less than anticipated, and 97% said they would undergo the same treatment by the same delivery method again if needed.
However, significantly more patients in the capsule group described their experience as “not at all unpleasant,” compared with the colonoscopy group (66% vs. 44%; 95% CI, 3%-40%; P = .01).
There were no colonic perforations seen in the colonoscopy group, and no infectious complications relating to the treatment in either group. One patient in each group died of underlying cardiopulmonary illness that was unrelated to the treatment, and the rate of minor adverse events was 5.4% in the capsule group and 12.5% in the colonoscopy group.
The authors acknowledged that the lack of a placebo group in the study meant they were not able to measure the size of the effect of fecal microbiota transplantation by either route. One earlier trial had also shown a placebo response rate of 45%.
The study was funded by Alberta Health Services and the University of Alberta Hospital Foundation. Four authors declared grants and other funding from the study funder and the pharmaceutical industry.
Fecal microbiota transplantation (FMT) using oral capsules as the delivery method has been shown to be noninferior to delivery using colonoscopy for the treatment of Clostridium difficile infection, but with a significantly lower price tag.
In an unblended noninferiority trial, published in the Nov. 28 issue of JAMA, 116 adults with at least three documented episodes of C. difficile infection were randomized to either 360 mL of fecal slurry delivered to the cecum via colonoscopy or to 40 capsules of processed fecal microbiota swallowed under direct observation.
Dina Kao, MD, of the department of medicine at the University of Alberta, Edmonton, and coauthors commented that the response rate with the capsules was higher than that seen in other studies of fecal microbiota capsules, which they suggested may partly be due to the larger amount of donor stool used in the study: 80-100 g, compared with 17 g and 25 g used in other studies.
“The higher efficacy observed in this study suggests a dose-dependent response to FMT, and a benefit of bowel lavage prior to FMT, because residual vancomycin was detected up to 8 days despite its discontinuation,” they wrote.
Both treatment modalities achieved similar quality of life improvements. Both groups reported major improvements in domains including physical and emotional health, physical and social functioning, and general health, with no significant differences between the two arms of the study.
The cost per treatment in the colonoscopy group was $874 per patient, compared with $308 per patient in the capsule group.
“Although colonoscopy delivery is more invasive, resource intensive, costly, and inconvenient for patients, it has the advantage of identifying alternative diagnoses,” the authors wrote. “Conversely, when FMT is given by oral capsules, it can be administered in an office setting, which could substantially reduce cost and wait time.”
Both groups also showed significantly improved gut microbiota diversity, which approached that of the donor just 1 week after administration of the treatment.
While 30% of patients characterized FMTs as “unpleasant, gross, or disgusting,” 79% of participants said the unpleasantness was the same or less than anticipated, and 97% said they would undergo the same treatment by the same delivery method again if needed.
However, significantly more patients in the capsule group described their experience as “not at all unpleasant,” compared with the colonoscopy group (66% vs. 44%; 95% CI, 3%-40%; P = .01).
There were no colonic perforations seen in the colonoscopy group, and no infectious complications relating to the treatment in either group. One patient in each group died of underlying cardiopulmonary illness that was unrelated to the treatment, and the rate of minor adverse events was 5.4% in the capsule group and 12.5% in the colonoscopy group.
The authors acknowledged that the lack of a placebo group in the study meant they were not able to measure the size of the effect of fecal microbiota transplantation by either route. One earlier trial had also shown a placebo response rate of 45%.
The study was funded by Alberta Health Services and the University of Alberta Hospital Foundation. Four authors declared grants and other funding from the study funder and the pharmaceutical industry.
FROM JAMA
Key clinical point: Delivering fecal microbiota transplants using oral capsules is noninferior to delivery via colonoscopy in the treatment of Clostridium difficile infection.
Major finding: The rates of resolution of recurrent C. difficile infection with fecal microbiota transplants are similar for delivery via oral capsule or via colonoscopy.
Data source: A randomized, unblended noninferiority trial in 116 adults with recurrent C. difficile infection.
Disclosures: The study was funded by Alberta Health Services and the University of Alberta Hospital Foundation. Four authors declared grants and other funding from the study funder and the pharmaceutical industry.
How Can Neurologists Treat Psychogenic Nonepileptic Seizures?
KANSAS CITY, MO—Many neurologists see, but few treat, patients with psychogenic nonepileptic seizures (PNES) and other conversion disorders, said W. Curt LaFrance Jr, MD, MPH, at the 46th Annual Meeting of the Child Neurology Society.
PNES may be associated with stigma in the minds of neurologists and may require a shift in perspective. Once a diagnosis is made by a neurologist or epileptologist, a patient with PNES is usually referred to a psychiatrist, he said. “Many times, however, those patients are lost, falling between the gaps at the borderlands of neurology and psychiatry.”
Because treatments for PNES, including cognitive behavioral therapy (CBT)-informed psychotherapy, have reduced seizures in randomized clinical trials, neurologists can tell patients that there are ways to help them, said Dr. LaFrance. Dr. LaFrance is a dually-boarded neurologist and psychiatrist and is Director of Neuropsychiatry and Behavioral Neurology at Rhode Island Hospital and Associate Professor of Psychiatry and Neurology at the Warren Alpert Medical School of Brown University in Providence.
Diagnosing Conversion Disorder
PNES is a common, disabling, and costly disorder. Patients may see numerous providers and try numerous medications over time. Neurologists’ ability to distinguish between epileptic and nonepileptic seizures is essential. In addition, DSM-5 allows clinicians to diagnose conversion disorder based on the new criteria, which include documenting the presence of non-neuroanatomic signs.
Once neurologists have confirmed that a patient has PNES, they should not hedge their diagnosis or unnecessarily treat the patient with antiepileptic drugs (AEDs) if the patient has lone PNES. AEDs do not treat nonepileptic seizures, and neurologists may safely withdraw AEDs in patients with confirmed nonepileptic seizures who do not have an indication for an AED. “Once the diagnosis is made, in therapy, we then begin doing the hard work of getting to what lies underneath,” Dr. LaFrance said. “The conversion seizure or the psychogenic tremor is just the tip of the iceberg.”
Many patients have comorbid depression, anxiety, or personality disorders, and there is a high prevalence of abuse and trauma among children and adults with PNES. Effective treatment of PNES requires understanding the patient’s social context, and these developmental factors are “part of the history taking that we are responsible for in our exam as neurologists,” Dr. LaFrance said.
Wyllie et al found that about 80% of children with PNES were seizure-free at three years, compared with 40% of adults with PNES. In a study by Yadav et al, a third of young patients with PNES had resolution of symptoms by six months, while a third remained symptomatic at two years. Approaches to treatment for PNES have included conventional CBT, group and individual therapy, social interventions, physical and occupational therapy, medication, and treatment by neurologists and psychiatrists.
Therapeutic Trials
Dr. LaFrance and colleagues in 2010 published results from a pilot trial of an SSRI for the treatment of PNES. Patients ages 18 to 65 with video-EEG–confirmed PNES received sertraline or placebo over 12 weeks. Among the 33 patients included in an intent-to-treat analysis, those who received sertraline had a 45% reduction in seizure rate from baseline to final visit, compared with an 8% increase among patients in the placebo group. The study gave preliminary data for addressing comorbidities with PNES but was not powered to gauge the efficacy of an SSRI for PNES.
In a separate study published in 2009, Dr. LaFrance and colleagues evaluated the effect of CBT-informed psychotherapy in patients with PNES. Researchers treated participants at 12 weekly sessions using a manualized therapy. The treatment workbook had been modified from one originally developed as a psychotherapeutic intervention for aura identification and behavioral interventions to reduce epileptic seizures. In the open-label clinical trial, CBT-informed psychotherapy significantly reduced seizures, depression, and anxiety, and improved quality of life. Seizures initially increased, however, before decreasing during the therapy, which may reflect some of the psychological issues that patients are addressing, Dr. LaFrance said. Patients who had a reduction in seizures at the end of the pilot study maintained the reduction at one year.
This treatment approach is based on a theoretical fear-avoidance model in which patients have an injury or traumatic event and then develop PNES. They catastrophize, fear the next seizure, become hypervigilant to somatic cues, and avoid external environments. These factors create a “pattern of disuse, disability, and depression, and it becomes a vicious cycle,” Dr. LaFrance said. The goal of treatment is to give patients tools to enter a “virtuous cycle of confronting the fear and moving into recovery.”
A Multisite Study
The researchers then combined aspects of the pharmacologic and CBT trials in a 2014 multisite pilot randomized clinical trial that included the following four treatment arms: CBT-informed psychotherapy, medication (ie, flexible-dose sertraline hydrochloride), CBT-informed psychotherapy plus medication, and standard medical care (ie, biweekly assessments with a treating neurologist). Significant within-group seizure reduction occurred in the two arms receiving CBT-informed psychotherapy—a 51.4% weekly reduction with CBT-informed psychotherapy alone, and a 59.3% weekly reduction with CBT-informed psychotherapy plus sertral
The research team is now examining neurocircuitry mechanisms of seizures with a recently funded multisite study of fMRI before and after treatment in patients with epilepsy or with PNES. Elements of the one-hour, CBT-informed psychotherapy sessions use different psychotherapeutic modalities, including motivational interviewing, interpersonal therapy, psychodynamic psychotherapy, distress tolerance, and psychoeducation about medications. Providers around the country, including neurologists, are being trained to deliver the intervention. “What we do is not rocket science. It is just good therapy,” Dr. LaFrance said.
—Jake Remaly
Suggested Reading
LaFrance WC Jr, Baird GL, Barry JJ, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry. 2014;71(9):997-1005.
LaFrance WC Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010;75(13):1166-1173.
LaFrance WC Jr, Miller IW, Ryan CE, et al. Cognitive behavioral therapy for psychogenic nonepileptic seizures. Epilepsy Behav. 2009;14(4):591-596.
LaFrance WC Jr, Reuber M, Goldstein LH. Management of psychogenic nonepileptic seizures. Epilepsia. 2013;54 Suppl 1:53-67.
LaFrance Jr WC, Wincze JP. Treating Nonepileptic Seizures: Therapist Guide. New York, NY: Oxford University Press; 2015.
Reiter JM, Andrews D, Reiter C, LaFrance Jr WC. Taking Control of Your Seizures: Workbook. New York, NY: Oxford University Press; 2015.
Wyllie E, Friedman D, Lüders H, et al. Outcome of psychogenic seizures in children and adolescents compared with adults. Neurology. 1991;41(5):742-744.
Yadav A, Agarwal R, Park J. Outcome of psychogenic nonepileptic seizures (PNES) in children: A 2-year follow-up study. Epilepsy Behav. 2015;53:168-173.
Veterans Health Administration. Veterans and Epilepsy: Basic Training: Psychogenic Non-Epileptic Seizures [video]. YouTube. https://youtu.be/NlX-yNTX86w. Published February 27, 2017.
KANSAS CITY, MO—Many neurologists see, but few treat, patients with psychogenic nonepileptic seizures (PNES) and other conversion disorders, said W. Curt LaFrance Jr, MD, MPH, at the 46th Annual Meeting of the Child Neurology Society.
PNES may be associated with stigma in the minds of neurologists and may require a shift in perspective. Once a diagnosis is made by a neurologist or epileptologist, a patient with PNES is usually referred to a psychiatrist, he said. “Many times, however, those patients are lost, falling between the gaps at the borderlands of neurology and psychiatry.”
Because treatments for PNES, including cognitive behavioral therapy (CBT)-informed psychotherapy, have reduced seizures in randomized clinical trials, neurologists can tell patients that there are ways to help them, said Dr. LaFrance. Dr. LaFrance is a dually-boarded neurologist and psychiatrist and is Director of Neuropsychiatry and Behavioral Neurology at Rhode Island Hospital and Associate Professor of Psychiatry and Neurology at the Warren Alpert Medical School of Brown University in Providence.
Diagnosing Conversion Disorder
PNES is a common, disabling, and costly disorder. Patients may see numerous providers and try numerous medications over time. Neurologists’ ability to distinguish between epileptic and nonepileptic seizures is essential. In addition, DSM-5 allows clinicians to diagnose conversion disorder based on the new criteria, which include documenting the presence of non-neuroanatomic signs.
Once neurologists have confirmed that a patient has PNES, they should not hedge their diagnosis or unnecessarily treat the patient with antiepileptic drugs (AEDs) if the patient has lone PNES. AEDs do not treat nonepileptic seizures, and neurologists may safely withdraw AEDs in patients with confirmed nonepileptic seizures who do not have an indication for an AED. “Once the diagnosis is made, in therapy, we then begin doing the hard work of getting to what lies underneath,” Dr. LaFrance said. “The conversion seizure or the psychogenic tremor is just the tip of the iceberg.”
Many patients have comorbid depression, anxiety, or personality disorders, and there is a high prevalence of abuse and trauma among children and adults with PNES. Effective treatment of PNES requires understanding the patient’s social context, and these developmental factors are “part of the history taking that we are responsible for in our exam as neurologists,” Dr. LaFrance said.
Wyllie et al found that about 80% of children with PNES were seizure-free at three years, compared with 40% of adults with PNES. In a study by Yadav et al, a third of young patients with PNES had resolution of symptoms by six months, while a third remained symptomatic at two years. Approaches to treatment for PNES have included conventional CBT, group and individual therapy, social interventions, physical and occupational therapy, medication, and treatment by neurologists and psychiatrists.
Therapeutic Trials
Dr. LaFrance and colleagues in 2010 published results from a pilot trial of an SSRI for the treatment of PNES. Patients ages 18 to 65 with video-EEG–confirmed PNES received sertraline or placebo over 12 weeks. Among the 33 patients included in an intent-to-treat analysis, those who received sertraline had a 45% reduction in seizure rate from baseline to final visit, compared with an 8% increase among patients in the placebo group. The study gave preliminary data for addressing comorbidities with PNES but was not powered to gauge the efficacy of an SSRI for PNES.
In a separate study published in 2009, Dr. LaFrance and colleagues evaluated the effect of CBT-informed psychotherapy in patients with PNES. Researchers treated participants at 12 weekly sessions using a manualized therapy. The treatment workbook had been modified from one originally developed as a psychotherapeutic intervention for aura identification and behavioral interventions to reduce epileptic seizures. In the open-label clinical trial, CBT-informed psychotherapy significantly reduced seizures, depression, and anxiety, and improved quality of life. Seizures initially increased, however, before decreasing during the therapy, which may reflect some of the psychological issues that patients are addressing, Dr. LaFrance said. Patients who had a reduction in seizures at the end of the pilot study maintained the reduction at one year.
This treatment approach is based on a theoretical fear-avoidance model in which patients have an injury or traumatic event and then develop PNES. They catastrophize, fear the next seizure, become hypervigilant to somatic cues, and avoid external environments. These factors create a “pattern of disuse, disability, and depression, and it becomes a vicious cycle,” Dr. LaFrance said. The goal of treatment is to give patients tools to enter a “virtuous cycle of confronting the fear and moving into recovery.”
A Multisite Study
The researchers then combined aspects of the pharmacologic and CBT trials in a 2014 multisite pilot randomized clinical trial that included the following four treatment arms: CBT-informed psychotherapy, medication (ie, flexible-dose sertraline hydrochloride), CBT-informed psychotherapy plus medication, and standard medical care (ie, biweekly assessments with a treating neurologist). Significant within-group seizure reduction occurred in the two arms receiving CBT-informed psychotherapy—a 51.4% weekly reduction with CBT-informed psychotherapy alone, and a 59.3% weekly reduction with CBT-informed psychotherapy plus sertral
The research team is now examining neurocircuitry mechanisms of seizures with a recently funded multisite study of fMRI before and after treatment in patients with epilepsy or with PNES. Elements of the one-hour, CBT-informed psychotherapy sessions use different psychotherapeutic modalities, including motivational interviewing, interpersonal therapy, psychodynamic psychotherapy, distress tolerance, and psychoeducation about medications. Providers around the country, including neurologists, are being trained to deliver the intervention. “What we do is not rocket science. It is just good therapy,” Dr. LaFrance said.
—Jake Remaly
Suggested Reading
LaFrance WC Jr, Baird GL, Barry JJ, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry. 2014;71(9):997-1005.
LaFrance WC Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010;75(13):1166-1173.
LaFrance WC Jr, Miller IW, Ryan CE, et al. Cognitive behavioral therapy for psychogenic nonepileptic seizures. Epilepsy Behav. 2009;14(4):591-596.
LaFrance WC Jr, Reuber M, Goldstein LH. Management of psychogenic nonepileptic seizures. Epilepsia. 2013;54 Suppl 1:53-67.
LaFrance Jr WC, Wincze JP. Treating Nonepileptic Seizures: Therapist Guide. New York, NY: Oxford University Press; 2015.
Reiter JM, Andrews D, Reiter C, LaFrance Jr WC. Taking Control of Your Seizures: Workbook. New York, NY: Oxford University Press; 2015.
Wyllie E, Friedman D, Lüders H, et al. Outcome of psychogenic seizures in children and adolescents compared with adults. Neurology. 1991;41(5):742-744.
Yadav A, Agarwal R, Park J. Outcome of psychogenic nonepileptic seizures (PNES) in children: A 2-year follow-up study. Epilepsy Behav. 2015;53:168-173.
Veterans Health Administration. Veterans and Epilepsy: Basic Training: Psychogenic Non-Epileptic Seizures [video]. YouTube. https://youtu.be/NlX-yNTX86w. Published February 27, 2017.
KANSAS CITY, MO—Many neurologists see, but few treat, patients with psychogenic nonepileptic seizures (PNES) and other conversion disorders, said W. Curt LaFrance Jr, MD, MPH, at the 46th Annual Meeting of the Child Neurology Society.
PNES may be associated with stigma in the minds of neurologists and may require a shift in perspective. Once a diagnosis is made by a neurologist or epileptologist, a patient with PNES is usually referred to a psychiatrist, he said. “Many times, however, those patients are lost, falling between the gaps at the borderlands of neurology and psychiatry.”
Because treatments for PNES, including cognitive behavioral therapy (CBT)-informed psychotherapy, have reduced seizures in randomized clinical trials, neurologists can tell patients that there are ways to help them, said Dr. LaFrance. Dr. LaFrance is a dually-boarded neurologist and psychiatrist and is Director of Neuropsychiatry and Behavioral Neurology at Rhode Island Hospital and Associate Professor of Psychiatry and Neurology at the Warren Alpert Medical School of Brown University in Providence.
Diagnosing Conversion Disorder
PNES is a common, disabling, and costly disorder. Patients may see numerous providers and try numerous medications over time. Neurologists’ ability to distinguish between epileptic and nonepileptic seizures is essential. In addition, DSM-5 allows clinicians to diagnose conversion disorder based on the new criteria, which include documenting the presence of non-neuroanatomic signs.
Once neurologists have confirmed that a patient has PNES, they should not hedge their diagnosis or unnecessarily treat the patient with antiepileptic drugs (AEDs) if the patient has lone PNES. AEDs do not treat nonepileptic seizures, and neurologists may safely withdraw AEDs in patients with confirmed nonepileptic seizures who do not have an indication for an AED. “Once the diagnosis is made, in therapy, we then begin doing the hard work of getting to what lies underneath,” Dr. LaFrance said. “The conversion seizure or the psychogenic tremor is just the tip of the iceberg.”
Many patients have comorbid depression, anxiety, or personality disorders, and there is a high prevalence of abuse and trauma among children and adults with PNES. Effective treatment of PNES requires understanding the patient’s social context, and these developmental factors are “part of the history taking that we are responsible for in our exam as neurologists,” Dr. LaFrance said.
Wyllie et al found that about 80% of children with PNES were seizure-free at three years, compared with 40% of adults with PNES. In a study by Yadav et al, a third of young patients with PNES had resolution of symptoms by six months, while a third remained symptomatic at two years. Approaches to treatment for PNES have included conventional CBT, group and individual therapy, social interventions, physical and occupational therapy, medication, and treatment by neurologists and psychiatrists.
Therapeutic Trials
Dr. LaFrance and colleagues in 2010 published results from a pilot trial of an SSRI for the treatment of PNES. Patients ages 18 to 65 with video-EEG–confirmed PNES received sertraline or placebo over 12 weeks. Among the 33 patients included in an intent-to-treat analysis, those who received sertraline had a 45% reduction in seizure rate from baseline to final visit, compared with an 8% increase among patients in the placebo group. The study gave preliminary data for addressing comorbidities with PNES but was not powered to gauge the efficacy of an SSRI for PNES.
In a separate study published in 2009, Dr. LaFrance and colleagues evaluated the effect of CBT-informed psychotherapy in patients with PNES. Researchers treated participants at 12 weekly sessions using a manualized therapy. The treatment workbook had been modified from one originally developed as a psychotherapeutic intervention for aura identification and behavioral interventions to reduce epileptic seizures. In the open-label clinical trial, CBT-informed psychotherapy significantly reduced seizures, depression, and anxiety, and improved quality of life. Seizures initially increased, however, before decreasing during the therapy, which may reflect some of the psychological issues that patients are addressing, Dr. LaFrance said. Patients who had a reduction in seizures at the end of the pilot study maintained the reduction at one year.
This treatment approach is based on a theoretical fear-avoidance model in which patients have an injury or traumatic event and then develop PNES. They catastrophize, fear the next seizure, become hypervigilant to somatic cues, and avoid external environments. These factors create a “pattern of disuse, disability, and depression, and it becomes a vicious cycle,” Dr. LaFrance said. The goal of treatment is to give patients tools to enter a “virtuous cycle of confronting the fear and moving into recovery.”
A Multisite Study
The researchers then combined aspects of the pharmacologic and CBT trials in a 2014 multisite pilot randomized clinical trial that included the following four treatment arms: CBT-informed psychotherapy, medication (ie, flexible-dose sertraline hydrochloride), CBT-informed psychotherapy plus medication, and standard medical care (ie, biweekly assessments with a treating neurologist). Significant within-group seizure reduction occurred in the two arms receiving CBT-informed psychotherapy—a 51.4% weekly reduction with CBT-informed psychotherapy alone, and a 59.3% weekly reduction with CBT-informed psychotherapy plus sertral
The research team is now examining neurocircuitry mechanisms of seizures with a recently funded multisite study of fMRI before and after treatment in patients with epilepsy or with PNES. Elements of the one-hour, CBT-informed psychotherapy sessions use different psychotherapeutic modalities, including motivational interviewing, interpersonal therapy, psychodynamic psychotherapy, distress tolerance, and psychoeducation about medications. Providers around the country, including neurologists, are being trained to deliver the intervention. “What we do is not rocket science. It is just good therapy,” Dr. LaFrance said.
—Jake Remaly
Suggested Reading
LaFrance WC Jr, Baird GL, Barry JJ, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry. 2014;71(9):997-1005.
LaFrance WC Jr, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology. 2010;75(13):1166-1173.
LaFrance WC Jr, Miller IW, Ryan CE, et al. Cognitive behavioral therapy for psychogenic nonepileptic seizures. Epilepsy Behav. 2009;14(4):591-596.
LaFrance WC Jr, Reuber M, Goldstein LH. Management of psychogenic nonepileptic seizures. Epilepsia. 2013;54 Suppl 1:53-67.
LaFrance Jr WC, Wincze JP. Treating Nonepileptic Seizures: Therapist Guide. New York, NY: Oxford University Press; 2015.
Reiter JM, Andrews D, Reiter C, LaFrance Jr WC. Taking Control of Your Seizures: Workbook. New York, NY: Oxford University Press; 2015.
Wyllie E, Friedman D, Lüders H, et al. Outcome of psychogenic seizures in children and adolescents compared with adults. Neurology. 1991;41(5):742-744.
Yadav A, Agarwal R, Park J. Outcome of psychogenic nonepileptic seizures (PNES) in children: A 2-year follow-up study. Epilepsy Behav. 2015;53:168-173.
Veterans Health Administration. Veterans and Epilepsy: Basic Training: Psychogenic Non-Epileptic Seizures [video]. YouTube. https://youtu.be/NlX-yNTX86w. Published February 27, 2017.
Defining quality in lung cancer surgery
Implementing quality initiatives and creating reporting mechanisms for lung cancer patients can lead to better outcomes, including overall survival. While barriers exist – namely the conflicting perspectives of providers, payers, hospitals, and patients – thoracic oncologic surgeons should seize the opportunity to establish robust quality and value metrics for lung cancer programs, said Whitney S. Brandt, MD, and her coauthors in an expert opinion in the Journal of Thoracic and Cardiovascular Surgery (2017;154:1397-403).
Dr. Brandt, a surgeon at Memorial Sloan Kettering Cancer Center in New York, and her coauthors examined the key elements of quality and value initiatives, categorizing them into preoperative, intraoperative, and postoperative components and primarily focusing on early stage lung cancer. The National Institutes of Health/National Cancer Center provided a grant for the authors’ work.
The preoperative evaluation should at least include CT imaging of the tumor and, for smokers, smoking cessation, said Dr. Brandt and her coauthors. All candidates for pulmonary lung resection should have spirometry and diffusion capacity tests; furthermore, both predicted postoperative forced expiratory volume in 1 second and diffusing capacity of the lungs for CO should be calculated. “Patients with a predicted postoperative value less than 40% for either measurement should be considered high risk for lobectomy and should be offered either sublobar resection or nonsurgical therapy,” they recommended.
Dr. Brandt and her colleagues also clarified preoperative management of patients with cardiac disease. Only patients with significant cardiac disease risk factors need to undergo cardiac testing before lung surgery, and patients with stable cardiac disease do not require revascularization beforehand.
For preoperative staging, the most comprehensive clinical guidelines come from the National Comprehensive Cancer Network, they stated. The guidelines recommend that all patients with a small cell lung cancer or stage II to IV non–small cell lung cancer (NSCLC) receive a brain MRI or – if that’s not available – a head CT with contrast to assess for brain metastasis.
Intraoperative quality measures take into account the surgical approach, including cost, resection and margins, and lymph node evaluation. With regard to surgical approach, trials have shown traditional video-assisted surgery (VATS) lobectomy results in shorter hospital stays and thereby lower costs, as well as fewer complications and deaths, than thoracotomy, said Dr. Brandt and her coauthors. But that cost advantage has not yet carried over to robotic-assisted VATS. That said, “robotic-assisted VATS remains a relatively new technology, and with time and increased robotic platform competition, costs will likely decrease.”
Dr. Brandt and her coauthors also noted that clinical trials support resection margins of 2 cm in patients having surgery for NSCLC and that adequate lymph node evaluation is a critical component of a lung cancer quality initiative. “Regardless of whether lymph nodes are sampled or dissected, we believe that systematic acquisition of mediastinal nodal tissue based on nodal station(s) is a useful quality metric, and, therefore, we recommend each program adopt a preferred approach and track adherence,” they said.
As for postoperative quality metrics, the most obvious are morbidity and mortality. “A quality program should track 30-day or in-hospital mortality, as well as 90-day mortality, following lung cancer resection.” Such metrics can serve as “starting points” for quality improvement initiatives. Length of stay has also emerged as an important metric because it is a surrogate of other metrics, such as patient comorbidities, age, and socioeconomic status. “Length-of-stay metrics likely need to be risk-stratified on the basis of these and other variables to be meaningful to a practicing surgeon,” Dr. Brandt and her coauthors said, adding that: “Studying the effectiveness of enhanced recovery after surgery programs in thoracic surgical oncology poses an opportunity for a well-designed trial.”
Two other key quality metrics for lung cancer programs that need further development were pointed out in the paper: hospital readmissions and tracking of adjuvant therapies. “Programmatic oncologic quality metrics to track appropriate and inappropriate referrals for adjuvant therapy and the number of patients who complete such therapy are important,” they said.
Another step programs should take: Participating in a national or regional database, as recommended by the Society of Thoracic Surgeons, and taking advantage of the “clear benefits to benchmarking your program to others.”
Dr. Brandt and her coauthors reported having no financial disclosures. The National Institutes of Health/National Cancer Center provided grant support.
Whitney S. Brandt, MD, and her coauthors pointed out the difficulty of finding a comprehensive quality metric because of the multitude of contributing indicators, said Alessandro Brunelli, MD, of St. James University Hospital in Leeds, England, in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:1404-5). But he added that two nonclinical indicators needed further consideration: patient perspectives and costs.
“Satisfaction with care depends on multiple subjective factors and is affected by different socioeconomic and cultural backgrounds,” Dr. Brunelli said. “There have been very few attempts to use patient satisfaction scales as a measure of quality in our specialty.” Residual quality of life after surgery is another key measure of patient perspective. “Long-term survival in fact cannot be assessed in isolation and without taking into consideration the actual quality of life of the cancer survivors,” he said. That information would help inform surgical decision-making.
To be meaningful as a quality metric, cost requires clinical risk adjustment, Dr. Brunelli wrote, and surgeons should take the lead here “to prevent misleading evaluations by third parties.” He added, “There have been few studies reporting on financial risk models in our specialty, and more research is needed in this field.”
Dr. Brunelli reported having no financial disclosures.
Whitney S. Brandt, MD, and her coauthors pointed out the difficulty of finding a comprehensive quality metric because of the multitude of contributing indicators, said Alessandro Brunelli, MD, of St. James University Hospital in Leeds, England, in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:1404-5). But he added that two nonclinical indicators needed further consideration: patient perspectives and costs.
“Satisfaction with care depends on multiple subjective factors and is affected by different socioeconomic and cultural backgrounds,” Dr. Brunelli said. “There have been very few attempts to use patient satisfaction scales as a measure of quality in our specialty.” Residual quality of life after surgery is another key measure of patient perspective. “Long-term survival in fact cannot be assessed in isolation and without taking into consideration the actual quality of life of the cancer survivors,” he said. That information would help inform surgical decision-making.
To be meaningful as a quality metric, cost requires clinical risk adjustment, Dr. Brunelli wrote, and surgeons should take the lead here “to prevent misleading evaluations by third parties.” He added, “There have been few studies reporting on financial risk models in our specialty, and more research is needed in this field.”
Dr. Brunelli reported having no financial disclosures.
Whitney S. Brandt, MD, and her coauthors pointed out the difficulty of finding a comprehensive quality metric because of the multitude of contributing indicators, said Alessandro Brunelli, MD, of St. James University Hospital in Leeds, England, in his invited commentary (J Thorac Cardiovasc Surg. 2017;154:1404-5). But he added that two nonclinical indicators needed further consideration: patient perspectives and costs.
“Satisfaction with care depends on multiple subjective factors and is affected by different socioeconomic and cultural backgrounds,” Dr. Brunelli said. “There have been very few attempts to use patient satisfaction scales as a measure of quality in our specialty.” Residual quality of life after surgery is another key measure of patient perspective. “Long-term survival in fact cannot be assessed in isolation and without taking into consideration the actual quality of life of the cancer survivors,” he said. That information would help inform surgical decision-making.
To be meaningful as a quality metric, cost requires clinical risk adjustment, Dr. Brunelli wrote, and surgeons should take the lead here “to prevent misleading evaluations by third parties.” He added, “There have been few studies reporting on financial risk models in our specialty, and more research is needed in this field.”
Dr. Brunelli reported having no financial disclosures.
Implementing quality initiatives and creating reporting mechanisms for lung cancer patients can lead to better outcomes, including overall survival. While barriers exist – namely the conflicting perspectives of providers, payers, hospitals, and patients – thoracic oncologic surgeons should seize the opportunity to establish robust quality and value metrics for lung cancer programs, said Whitney S. Brandt, MD, and her coauthors in an expert opinion in the Journal of Thoracic and Cardiovascular Surgery (2017;154:1397-403).
Dr. Brandt, a surgeon at Memorial Sloan Kettering Cancer Center in New York, and her coauthors examined the key elements of quality and value initiatives, categorizing them into preoperative, intraoperative, and postoperative components and primarily focusing on early stage lung cancer. The National Institutes of Health/National Cancer Center provided a grant for the authors’ work.
The preoperative evaluation should at least include CT imaging of the tumor and, for smokers, smoking cessation, said Dr. Brandt and her coauthors. All candidates for pulmonary lung resection should have spirometry and diffusion capacity tests; furthermore, both predicted postoperative forced expiratory volume in 1 second and diffusing capacity of the lungs for CO should be calculated. “Patients with a predicted postoperative value less than 40% for either measurement should be considered high risk for lobectomy and should be offered either sublobar resection or nonsurgical therapy,” they recommended.
Dr. Brandt and her colleagues also clarified preoperative management of patients with cardiac disease. Only patients with significant cardiac disease risk factors need to undergo cardiac testing before lung surgery, and patients with stable cardiac disease do not require revascularization beforehand.
For preoperative staging, the most comprehensive clinical guidelines come from the National Comprehensive Cancer Network, they stated. The guidelines recommend that all patients with a small cell lung cancer or stage II to IV non–small cell lung cancer (NSCLC) receive a brain MRI or – if that’s not available – a head CT with contrast to assess for brain metastasis.
Intraoperative quality measures take into account the surgical approach, including cost, resection and margins, and lymph node evaluation. With regard to surgical approach, trials have shown traditional video-assisted surgery (VATS) lobectomy results in shorter hospital stays and thereby lower costs, as well as fewer complications and deaths, than thoracotomy, said Dr. Brandt and her coauthors. But that cost advantage has not yet carried over to robotic-assisted VATS. That said, “robotic-assisted VATS remains a relatively new technology, and with time and increased robotic platform competition, costs will likely decrease.”
Dr. Brandt and her coauthors also noted that clinical trials support resection margins of 2 cm in patients having surgery for NSCLC and that adequate lymph node evaluation is a critical component of a lung cancer quality initiative. “Regardless of whether lymph nodes are sampled or dissected, we believe that systematic acquisition of mediastinal nodal tissue based on nodal station(s) is a useful quality metric, and, therefore, we recommend each program adopt a preferred approach and track adherence,” they said.
As for postoperative quality metrics, the most obvious are morbidity and mortality. “A quality program should track 30-day or in-hospital mortality, as well as 90-day mortality, following lung cancer resection.” Such metrics can serve as “starting points” for quality improvement initiatives. Length of stay has also emerged as an important metric because it is a surrogate of other metrics, such as patient comorbidities, age, and socioeconomic status. “Length-of-stay metrics likely need to be risk-stratified on the basis of these and other variables to be meaningful to a practicing surgeon,” Dr. Brandt and her coauthors said, adding that: “Studying the effectiveness of enhanced recovery after surgery programs in thoracic surgical oncology poses an opportunity for a well-designed trial.”
Two other key quality metrics for lung cancer programs that need further development were pointed out in the paper: hospital readmissions and tracking of adjuvant therapies. “Programmatic oncologic quality metrics to track appropriate and inappropriate referrals for adjuvant therapy and the number of patients who complete such therapy are important,” they said.
Another step programs should take: Participating in a national or regional database, as recommended by the Society of Thoracic Surgeons, and taking advantage of the “clear benefits to benchmarking your program to others.”
Dr. Brandt and her coauthors reported having no financial disclosures. The National Institutes of Health/National Cancer Center provided grant support.
Implementing quality initiatives and creating reporting mechanisms for lung cancer patients can lead to better outcomes, including overall survival. While barriers exist – namely the conflicting perspectives of providers, payers, hospitals, and patients – thoracic oncologic surgeons should seize the opportunity to establish robust quality and value metrics for lung cancer programs, said Whitney S. Brandt, MD, and her coauthors in an expert opinion in the Journal of Thoracic and Cardiovascular Surgery (2017;154:1397-403).
Dr. Brandt, a surgeon at Memorial Sloan Kettering Cancer Center in New York, and her coauthors examined the key elements of quality and value initiatives, categorizing them into preoperative, intraoperative, and postoperative components and primarily focusing on early stage lung cancer. The National Institutes of Health/National Cancer Center provided a grant for the authors’ work.
The preoperative evaluation should at least include CT imaging of the tumor and, for smokers, smoking cessation, said Dr. Brandt and her coauthors. All candidates for pulmonary lung resection should have spirometry and diffusion capacity tests; furthermore, both predicted postoperative forced expiratory volume in 1 second and diffusing capacity of the lungs for CO should be calculated. “Patients with a predicted postoperative value less than 40% for either measurement should be considered high risk for lobectomy and should be offered either sublobar resection or nonsurgical therapy,” they recommended.
Dr. Brandt and her colleagues also clarified preoperative management of patients with cardiac disease. Only patients with significant cardiac disease risk factors need to undergo cardiac testing before lung surgery, and patients with stable cardiac disease do not require revascularization beforehand.
For preoperative staging, the most comprehensive clinical guidelines come from the National Comprehensive Cancer Network, they stated. The guidelines recommend that all patients with a small cell lung cancer or stage II to IV non–small cell lung cancer (NSCLC) receive a brain MRI or – if that’s not available – a head CT with contrast to assess for brain metastasis.
Intraoperative quality measures take into account the surgical approach, including cost, resection and margins, and lymph node evaluation. With regard to surgical approach, trials have shown traditional video-assisted surgery (VATS) lobectomy results in shorter hospital stays and thereby lower costs, as well as fewer complications and deaths, than thoracotomy, said Dr. Brandt and her coauthors. But that cost advantage has not yet carried over to robotic-assisted VATS. That said, “robotic-assisted VATS remains a relatively new technology, and with time and increased robotic platform competition, costs will likely decrease.”
Dr. Brandt and her coauthors also noted that clinical trials support resection margins of 2 cm in patients having surgery for NSCLC and that adequate lymph node evaluation is a critical component of a lung cancer quality initiative. “Regardless of whether lymph nodes are sampled or dissected, we believe that systematic acquisition of mediastinal nodal tissue based on nodal station(s) is a useful quality metric, and, therefore, we recommend each program adopt a preferred approach and track adherence,” they said.
As for postoperative quality metrics, the most obvious are morbidity and mortality. “A quality program should track 30-day or in-hospital mortality, as well as 90-day mortality, following lung cancer resection.” Such metrics can serve as “starting points” for quality improvement initiatives. Length of stay has also emerged as an important metric because it is a surrogate of other metrics, such as patient comorbidities, age, and socioeconomic status. “Length-of-stay metrics likely need to be risk-stratified on the basis of these and other variables to be meaningful to a practicing surgeon,” Dr. Brandt and her coauthors said, adding that: “Studying the effectiveness of enhanced recovery after surgery programs in thoracic surgical oncology poses an opportunity for a well-designed trial.”
Two other key quality metrics for lung cancer programs that need further development were pointed out in the paper: hospital readmissions and tracking of adjuvant therapies. “Programmatic oncologic quality metrics to track appropriate and inappropriate referrals for adjuvant therapy and the number of patients who complete such therapy are important,” they said.
Another step programs should take: Participating in a national or regional database, as recommended by the Society of Thoracic Surgeons, and taking advantage of the “clear benefits to benchmarking your program to others.”
Dr. Brandt and her coauthors reported having no financial disclosures. The National Institutes of Health/National Cancer Center provided grant support.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Quality and value initiatives in lung cancer surgery are complex and multifaceted.
Major finding: Expert opinion identifies quality and value strategies for the preoperative, intraoperative, and postoperative stages.
Data source: Review of elements of quality and value for lung cancer surgery, including the Donabedian classification of structure, process and outcomes.
Disclosures: Dr. Brandt and co-authors reported having no financial disclosures. The National Institutes of Health/National Cancer Center provided grant support.