User login
U.S. study finds unexpectedly high prevalence of myasthenia gravis
PHOENIX – The prevalence is higher than what has been seen in other studies, which could represent a true difference in prevalence, or reflect limitations of the database.
Worldwide estimates suggest that myasthenia gravis affects 700,000 people globally, with incidence rates ranging between 6.3 and 29 per 1,000,000 person-years in Europe and a prevalence between 111.7 and 361 per 1,000,000. Data from Australia, Taiwan, and South Korea also show evidence of increased prevalence in recent years.
However, there is little data about the prevalence of myasthenia gravis in the United States, or about differences between racial groups, according to Bhaskar Roy, MBBS, who presented the study at the 2023 annual meeting of the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM). He noted that most studies are outdated, and the most recent study focused on ocular myasthenia gravis.
True incidence or artifact?
The finding is surprising and may be an artifact of the immature nature of the All of Us database, according to Srikanth Muppidi, MD, who asked about the limitation during the Q&A session following the talk. “The incidence of 0.13 is definitely higher than what we would think would be the true incidence of myasthenia gravis from [clinical experience]. It’s possible that our understanding of true incidence is wrong and this is the actual incidence. What I would like them to do, and I think they’re trying to do, is to look at this finding [and compare it with] other more mature databases and other regional databases. One of the current challenges of All of Us is that our patients are basically being recruited from some parts of the country, and the middle of the country has hardly any presence in the database, so it becomes really challenging to understand it,” Dr. Muppidi said in an interview.
However, Dr. Muppidi, who is a clinical professor of neurology at Stanford (Calif.) Medicine, noted that the All of Us database is still growing. When it has recruited more patients with a diverse population, “it [will be a] valuable source for rare diseases to try to understand true incidence of those diseases,” he said.
Understanding the true prevalence
Dr. Roy recognized the geographic limitations of the database. “Some states, particularly Massachusetts, New York, and California, had a lot of patients in the database, where there were no patients from many states,” said Dr. Roy, associate professor of neurology at Yale University, New Haven, Conn.
He said that the group is working with other databases, including UK Biobank. “The goal is to incorporate all of these databases together [to determine the true incidence],” said Dr. Roy.
It’s critical to understand the true prevalence of myasthenia gravis since new therapies are in development and coming to market. “I worry that myasthenia gravis might be considered less common than it truly is, and that will limit growth of the field if the feeling is that there are not that many [myasthenia gravis patients] in the country,” said Dr. Muppidi.
The study included data from 369,297 adult patients, using Systematized Nomenclature of Medicine (SNOMED) and International Classification of Diseases (ICD) codes to identify patients with myasthenia gravis. There were 479 cases of myasthenia gravis, for a prevalence of 0.13 (95% confidence interval [CI], 0.12-0.14). Of myasthenia gravis patients, 65% were female and the mean age was 64 years. The prevalence of myasthenia gravis in White individuals was 0.16 (95% CI, 0.15-0.18), of which 63% were female, and the mean age was 66 years. The prevalence among Black individuals was 0.078 (95% CI, 0.060-0.10), with 77% of the population female and a mean age of 58 years. The prevalence in Hispanics was 0.091 (95% CI, 0.070-0.12), with 80% female and a mean age of 58 years. Among Asians, the prevalence was 0.056 (95% CI, 0.025-0.12) and 57% were female, with a mean age of 58 years.
The researchers also looked at the EXPLORE-MG database drawn from Yale (n = 3,269,000), which showed a much lower overall myasthenia gravis prevalence of 0.019 (95% CI, 0.017-0.020), a female proportion of 46.8%, and a mean age of 56.6 years. Notably, EXPLORE-MG had a lower proportion of women and a younger population than All of Us.
The researchers compared data from All of Us with other databases for other conditions. The prevalence of ALS was the same as in other conditions, while diabetic neuropathy was significantly lower (2.7 versus 28.5-50 among diabetic patients) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) was higher (0.084 versus 0.028).
Dr. Muppidi has been on advisory boards for Alexion, Argenx, UBC, and Immunovant. Dr. Roy has consulted for Alexion, Takeda Pharmaceuticals, and Argenx and owns stock in Cabaletta Bio. He has received research support from Takeda, Abcuro, and Argenx.
PHOENIX – The prevalence is higher than what has been seen in other studies, which could represent a true difference in prevalence, or reflect limitations of the database.
Worldwide estimates suggest that myasthenia gravis affects 700,000 people globally, with incidence rates ranging between 6.3 and 29 per 1,000,000 person-years in Europe and a prevalence between 111.7 and 361 per 1,000,000. Data from Australia, Taiwan, and South Korea also show evidence of increased prevalence in recent years.
However, there is little data about the prevalence of myasthenia gravis in the United States, or about differences between racial groups, according to Bhaskar Roy, MBBS, who presented the study at the 2023 annual meeting of the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM). He noted that most studies are outdated, and the most recent study focused on ocular myasthenia gravis.
True incidence or artifact?
The finding is surprising and may be an artifact of the immature nature of the All of Us database, according to Srikanth Muppidi, MD, who asked about the limitation during the Q&A session following the talk. “The incidence of 0.13 is definitely higher than what we would think would be the true incidence of myasthenia gravis from [clinical experience]. It’s possible that our understanding of true incidence is wrong and this is the actual incidence. What I would like them to do, and I think they’re trying to do, is to look at this finding [and compare it with] other more mature databases and other regional databases. One of the current challenges of All of Us is that our patients are basically being recruited from some parts of the country, and the middle of the country has hardly any presence in the database, so it becomes really challenging to understand it,” Dr. Muppidi said in an interview.
However, Dr. Muppidi, who is a clinical professor of neurology at Stanford (Calif.) Medicine, noted that the All of Us database is still growing. When it has recruited more patients with a diverse population, “it [will be a] valuable source for rare diseases to try to understand true incidence of those diseases,” he said.
Understanding the true prevalence
Dr. Roy recognized the geographic limitations of the database. “Some states, particularly Massachusetts, New York, and California, had a lot of patients in the database, where there were no patients from many states,” said Dr. Roy, associate professor of neurology at Yale University, New Haven, Conn.
He said that the group is working with other databases, including UK Biobank. “The goal is to incorporate all of these databases together [to determine the true incidence],” said Dr. Roy.
It’s critical to understand the true prevalence of myasthenia gravis since new therapies are in development and coming to market. “I worry that myasthenia gravis might be considered less common than it truly is, and that will limit growth of the field if the feeling is that there are not that many [myasthenia gravis patients] in the country,” said Dr. Muppidi.
The study included data from 369,297 adult patients, using Systematized Nomenclature of Medicine (SNOMED) and International Classification of Diseases (ICD) codes to identify patients with myasthenia gravis. There were 479 cases of myasthenia gravis, for a prevalence of 0.13 (95% confidence interval [CI], 0.12-0.14). Of myasthenia gravis patients, 65% were female and the mean age was 64 years. The prevalence of myasthenia gravis in White individuals was 0.16 (95% CI, 0.15-0.18), of which 63% were female, and the mean age was 66 years. The prevalence among Black individuals was 0.078 (95% CI, 0.060-0.10), with 77% of the population female and a mean age of 58 years. The prevalence in Hispanics was 0.091 (95% CI, 0.070-0.12), with 80% female and a mean age of 58 years. Among Asians, the prevalence was 0.056 (95% CI, 0.025-0.12) and 57% were female, with a mean age of 58 years.
The researchers also looked at the EXPLORE-MG database drawn from Yale (n = 3,269,000), which showed a much lower overall myasthenia gravis prevalence of 0.019 (95% CI, 0.017-0.020), a female proportion of 46.8%, and a mean age of 56.6 years. Notably, EXPLORE-MG had a lower proportion of women and a younger population than All of Us.
The researchers compared data from All of Us with other databases for other conditions. The prevalence of ALS was the same as in other conditions, while diabetic neuropathy was significantly lower (2.7 versus 28.5-50 among diabetic patients) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) was higher (0.084 versus 0.028).
Dr. Muppidi has been on advisory boards for Alexion, Argenx, UBC, and Immunovant. Dr. Roy has consulted for Alexion, Takeda Pharmaceuticals, and Argenx and owns stock in Cabaletta Bio. He has received research support from Takeda, Abcuro, and Argenx.
PHOENIX – The prevalence is higher than what has been seen in other studies, which could represent a true difference in prevalence, or reflect limitations of the database.
Worldwide estimates suggest that myasthenia gravis affects 700,000 people globally, with incidence rates ranging between 6.3 and 29 per 1,000,000 person-years in Europe and a prevalence between 111.7 and 361 per 1,000,000. Data from Australia, Taiwan, and South Korea also show evidence of increased prevalence in recent years.
However, there is little data about the prevalence of myasthenia gravis in the United States, or about differences between racial groups, according to Bhaskar Roy, MBBS, who presented the study at the 2023 annual meeting of the American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM). He noted that most studies are outdated, and the most recent study focused on ocular myasthenia gravis.
True incidence or artifact?
The finding is surprising and may be an artifact of the immature nature of the All of Us database, according to Srikanth Muppidi, MD, who asked about the limitation during the Q&A session following the talk. “The incidence of 0.13 is definitely higher than what we would think would be the true incidence of myasthenia gravis from [clinical experience]. It’s possible that our understanding of true incidence is wrong and this is the actual incidence. What I would like them to do, and I think they’re trying to do, is to look at this finding [and compare it with] other more mature databases and other regional databases. One of the current challenges of All of Us is that our patients are basically being recruited from some parts of the country, and the middle of the country has hardly any presence in the database, so it becomes really challenging to understand it,” Dr. Muppidi said in an interview.
However, Dr. Muppidi, who is a clinical professor of neurology at Stanford (Calif.) Medicine, noted that the All of Us database is still growing. When it has recruited more patients with a diverse population, “it [will be a] valuable source for rare diseases to try to understand true incidence of those diseases,” he said.
Understanding the true prevalence
Dr. Roy recognized the geographic limitations of the database. “Some states, particularly Massachusetts, New York, and California, had a lot of patients in the database, where there were no patients from many states,” said Dr. Roy, associate professor of neurology at Yale University, New Haven, Conn.
He said that the group is working with other databases, including UK Biobank. “The goal is to incorporate all of these databases together [to determine the true incidence],” said Dr. Roy.
It’s critical to understand the true prevalence of myasthenia gravis since new therapies are in development and coming to market. “I worry that myasthenia gravis might be considered less common than it truly is, and that will limit growth of the field if the feeling is that there are not that many [myasthenia gravis patients] in the country,” said Dr. Muppidi.
The study included data from 369,297 adult patients, using Systematized Nomenclature of Medicine (SNOMED) and International Classification of Diseases (ICD) codes to identify patients with myasthenia gravis. There were 479 cases of myasthenia gravis, for a prevalence of 0.13 (95% confidence interval [CI], 0.12-0.14). Of myasthenia gravis patients, 65% were female and the mean age was 64 years. The prevalence of myasthenia gravis in White individuals was 0.16 (95% CI, 0.15-0.18), of which 63% were female, and the mean age was 66 years. The prevalence among Black individuals was 0.078 (95% CI, 0.060-0.10), with 77% of the population female and a mean age of 58 years. The prevalence in Hispanics was 0.091 (95% CI, 0.070-0.12), with 80% female and a mean age of 58 years. Among Asians, the prevalence was 0.056 (95% CI, 0.025-0.12) and 57% were female, with a mean age of 58 years.
The researchers also looked at the EXPLORE-MG database drawn from Yale (n = 3,269,000), which showed a much lower overall myasthenia gravis prevalence of 0.019 (95% CI, 0.017-0.020), a female proportion of 46.8%, and a mean age of 56.6 years. Notably, EXPLORE-MG had a lower proportion of women and a younger population than All of Us.
The researchers compared data from All of Us with other databases for other conditions. The prevalence of ALS was the same as in other conditions, while diabetic neuropathy was significantly lower (2.7 versus 28.5-50 among diabetic patients) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) was higher (0.084 versus 0.028).
Dr. Muppidi has been on advisory boards for Alexion, Argenx, UBC, and Immunovant. Dr. Roy has consulted for Alexion, Takeda Pharmaceuticals, and Argenx and owns stock in Cabaletta Bio. He has received research support from Takeda, Abcuro, and Argenx.
AT AANEM 2023
Topical ivermectin study sheds light on dysbiosis in rosacea
, according to a report presented at the recent European Academy of Dermatology and Venereology (EADV) 2023 Congress.
“This is the first hint that the host’s cutaneous microbiome plays a secondary role in the immunopathogenesis of rosacea,” said Bernard Homey, MD, director of the department of dermatology at University Hospital Düsseldorf in Germany.
“In rosacea, we are well aware of trigger factors such as stress, UV light, heat, cold, food, and alcohol,” he said. “We are also well aware that there is an increase in Demodex mites in the pilosebaceous unit.”
Research over the past decade has also started to look at the potential role of the skin microbiome in the disease process, but answers have remained “largely elusive,” Dr. Homey said.
Ivermectin helps, but how?
Ivermectin 1% cream (Soolantra) has been approved by the U.S. Food and Drug Administration since 2014 for the treatment of the inflammatory lesions that are characteristic of rosacea, but its mechanism of action is not clear.
Dr. Homey presented the results of a study of 61 patients designed to look at how ivermectin might be working in the treatment of people with rosacea and investigate if there was any relation to the skin microbiome and transcriptome of patients.
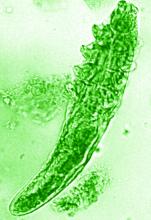
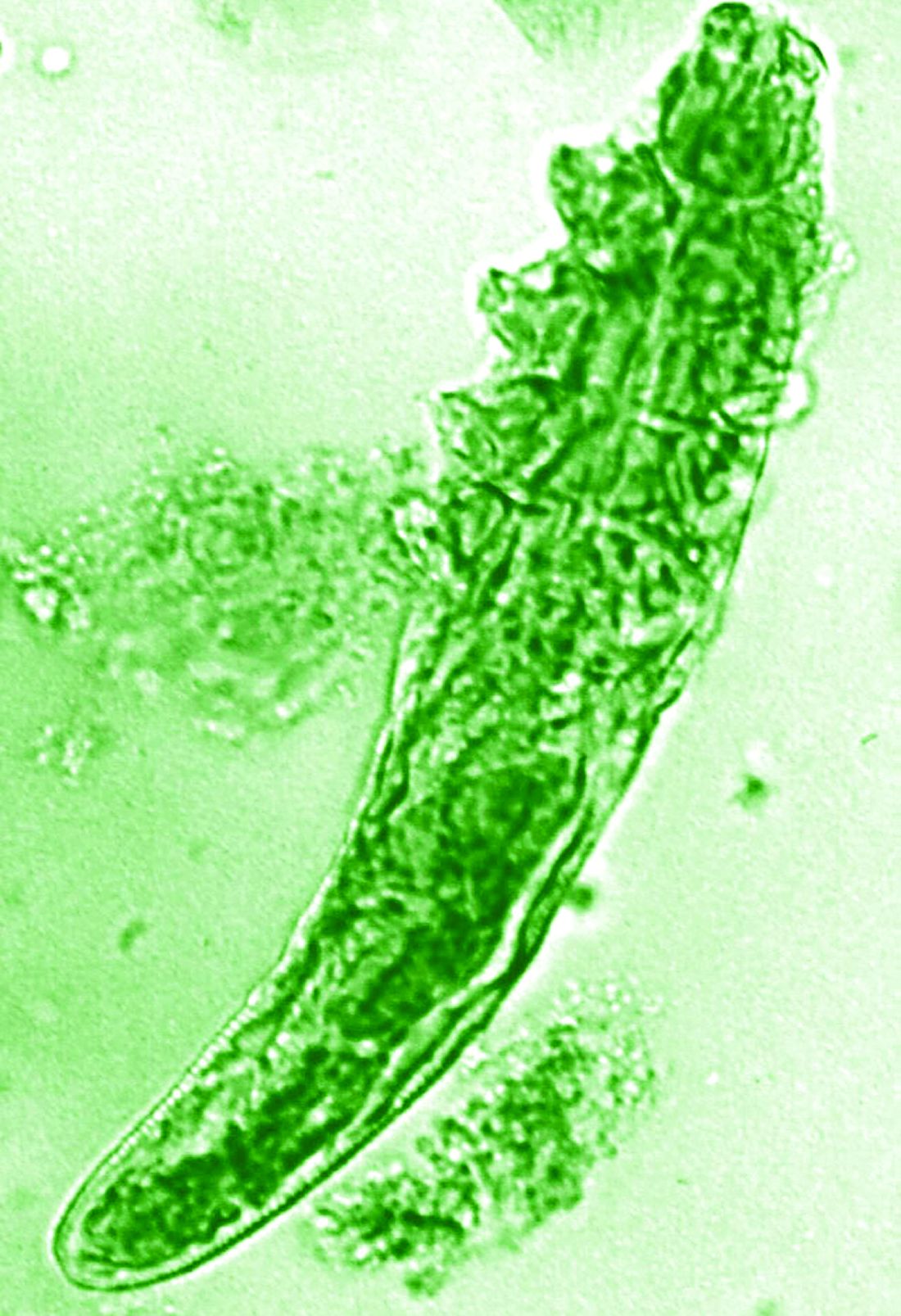
The trial included 41 individuals with papulopustular rosacea and 20 individuals who did not have rosacea. For all patients, surface skin biopsies were performed twice 30 days apart using cyanoacrylate glue; patients with rosacea were treated with topical ivermectin 1% between biopsies. Skin samples obtained at day 0 and day 30 were examined under the microscope, and Demodex counts (mites/cm2) of skin and RNA sequencing of the cutaneous microbiome were undertaken.
The mean age of the patients with rosacea was 54.9 years, and the mean Demodex counts before and after treatment were a respective 7.2 cm2 and 0.9 cm2.
Using the Investigator’s General Assessment to assess the severity of rosacea, Homey reported that 43.9% of patients with rosacea had a decrease in scores at day 30, indicating improvement.
In addition, topical ivermectin resulted in a marked or total decrease in Demodex mite density for 87.5% of patients (n = 24) who were identified as having the mites.
Skin microbiome changes seen
As a form of quality control, skin microbiome changes among the patients were compared with control patients using 16S rRNA sequencing.
“The taxa we find within the cutaneous niche of inflammatory lesions of rosacea patients are significantly different from healthy volunteers,” Dr. Homey said.
Cutibacterium species are predominant in healthy control persons but are not present when there is inflammation in patients with rosacea. Instead, staphylococcus species “take over the niche, similar to atopic dermatitis,” he noted.
Looking at how treatment with ivermectin influences the organisms, the decrease in C. acnes seen in patients with rosacea persisted despite treatment, and the abundance of Staphylococcus epidermidis, S. hominis, and S. capitis increased further. This suggests a possible protective or homeostatic role of C. acnes but a pathogenic role for staphylococci, explained Dr. Homey.
“Surprisingly, although inflammatory lesions decrease, patients get better, the cutaneous microbiome does not revert to homeostatic conditions during topical ivermectin treatment,” he observed.
There is, of course, variability among individuals.
Dr. Homey also reported that Snodgrassella alvi – a microorganism believed to reside in the gut of Demodex folliculorum mites – was found in the skin microbiome of patients with rosacea before but not after ivermectin treatment. This may mean that this microorganism could be partially triggering inflammation in rosacea patients.
Looking at the transcriptome of patients, Dr. Homey said that there was downregulation of distinct genes that might make for more favorable conditions for Demodex mites.
Moreover, insufficient upregulation of interleukin-17 pathways might be working together with barrier defects in the skin and metabolic changes to “pave the way” for colonization by S. epidermidis.
Pulling it together
Dr. Homey and associates conclude in their abstract that the findings “support that rosacea lesions are associated with dysbiosis.”
Although treatment with ivermectin did not normalize the skin’s microbiome, it was associated with a decrease in Demodex mite density and the reduction of microbes associated with Demodex.
Margarida Gonçalo, MD, PhD, professor of dermatology at the University of Coimbra in Portugal, who cochaired the late-breaking news session where the data were presented, asked whether healthy and affected skin in patients with rosacea had been compared, rather than comparing the skin of rosacea lesions with healthy control samples.
“No, we did not this, as this is methodologically a little bit more difficult,” Dr. Homey responded.
Also cochairing the session was Michel Gilliet, MD, chair of the department of dermatology at the University Hospital CHUV in Lausanne, Switzerland. He commented that these “data suggest that there’s an intimate link between Demodex and the skin microbiota and dysbiosis in in rosacea.”
Dr. Gilliet added: “You have a whole dysbiosis going on in rosacea, which is probably only dependent on these bacteria.”
It would be “very interesting,” as a “proof-of-concept” study, to look at whether depleting Demodex would also delete S. alvi, he suggested.
The study was funded by Galderma. Dr. Homey has acted as a consultant, speaker or investigator for many pharmaceutical companies including Galderma.
A version of this article first appeared on Medscape.com.
, according to a report presented at the recent European Academy of Dermatology and Venereology (EADV) 2023 Congress.
“This is the first hint that the host’s cutaneous microbiome plays a secondary role in the immunopathogenesis of rosacea,” said Bernard Homey, MD, director of the department of dermatology at University Hospital Düsseldorf in Germany.
“In rosacea, we are well aware of trigger factors such as stress, UV light, heat, cold, food, and alcohol,” he said. “We are also well aware that there is an increase in Demodex mites in the pilosebaceous unit.”
Research over the past decade has also started to look at the potential role of the skin microbiome in the disease process, but answers have remained “largely elusive,” Dr. Homey said.
Ivermectin helps, but how?
Ivermectin 1% cream (Soolantra) has been approved by the U.S. Food and Drug Administration since 2014 for the treatment of the inflammatory lesions that are characteristic of rosacea, but its mechanism of action is not clear.
Dr. Homey presented the results of a study of 61 patients designed to look at how ivermectin might be working in the treatment of people with rosacea and investigate if there was any relation to the skin microbiome and transcriptome of patients.
The trial included 41 individuals with papulopustular rosacea and 20 individuals who did not have rosacea. For all patients, surface skin biopsies were performed twice 30 days apart using cyanoacrylate glue; patients with rosacea were treated with topical ivermectin 1% between biopsies. Skin samples obtained at day 0 and day 30 were examined under the microscope, and Demodex counts (mites/cm2) of skin and RNA sequencing of the cutaneous microbiome were undertaken.
The mean age of the patients with rosacea was 54.9 years, and the mean Demodex counts before and after treatment were a respective 7.2 cm2 and 0.9 cm2.
Using the Investigator’s General Assessment to assess the severity of rosacea, Homey reported that 43.9% of patients with rosacea had a decrease in scores at day 30, indicating improvement.
In addition, topical ivermectin resulted in a marked or total decrease in Demodex mite density for 87.5% of patients (n = 24) who were identified as having the mites.
Skin microbiome changes seen
As a form of quality control, skin microbiome changes among the patients were compared with control patients using 16S rRNA sequencing.
“The taxa we find within the cutaneous niche of inflammatory lesions of rosacea patients are significantly different from healthy volunteers,” Dr. Homey said.
Cutibacterium species are predominant in healthy control persons but are not present when there is inflammation in patients with rosacea. Instead, staphylococcus species “take over the niche, similar to atopic dermatitis,” he noted.
Looking at how treatment with ivermectin influences the organisms, the decrease in C. acnes seen in patients with rosacea persisted despite treatment, and the abundance of Staphylococcus epidermidis, S. hominis, and S. capitis increased further. This suggests a possible protective or homeostatic role of C. acnes but a pathogenic role for staphylococci, explained Dr. Homey.
“Surprisingly, although inflammatory lesions decrease, patients get better, the cutaneous microbiome does not revert to homeostatic conditions during topical ivermectin treatment,” he observed.
There is, of course, variability among individuals.
Dr. Homey also reported that Snodgrassella alvi – a microorganism believed to reside in the gut of Demodex folliculorum mites – was found in the skin microbiome of patients with rosacea before but not after ivermectin treatment. This may mean that this microorganism could be partially triggering inflammation in rosacea patients.
Looking at the transcriptome of patients, Dr. Homey said that there was downregulation of distinct genes that might make for more favorable conditions for Demodex mites.
Moreover, insufficient upregulation of interleukin-17 pathways might be working together with barrier defects in the skin and metabolic changes to “pave the way” for colonization by S. epidermidis.
Pulling it together
Dr. Homey and associates conclude in their abstract that the findings “support that rosacea lesions are associated with dysbiosis.”
Although treatment with ivermectin did not normalize the skin’s microbiome, it was associated with a decrease in Demodex mite density and the reduction of microbes associated with Demodex.
Margarida Gonçalo, MD, PhD, professor of dermatology at the University of Coimbra in Portugal, who cochaired the late-breaking news session where the data were presented, asked whether healthy and affected skin in patients with rosacea had been compared, rather than comparing the skin of rosacea lesions with healthy control samples.
“No, we did not this, as this is methodologically a little bit more difficult,” Dr. Homey responded.
Also cochairing the session was Michel Gilliet, MD, chair of the department of dermatology at the University Hospital CHUV in Lausanne, Switzerland. He commented that these “data suggest that there’s an intimate link between Demodex and the skin microbiota and dysbiosis in in rosacea.”
Dr. Gilliet added: “You have a whole dysbiosis going on in rosacea, which is probably only dependent on these bacteria.”
It would be “very interesting,” as a “proof-of-concept” study, to look at whether depleting Demodex would also delete S. alvi, he suggested.
The study was funded by Galderma. Dr. Homey has acted as a consultant, speaker or investigator for many pharmaceutical companies including Galderma.
A version of this article first appeared on Medscape.com.
, according to a report presented at the recent European Academy of Dermatology and Venereology (EADV) 2023 Congress.
“This is the first hint that the host’s cutaneous microbiome plays a secondary role in the immunopathogenesis of rosacea,” said Bernard Homey, MD, director of the department of dermatology at University Hospital Düsseldorf in Germany.
“In rosacea, we are well aware of trigger factors such as stress, UV light, heat, cold, food, and alcohol,” he said. “We are also well aware that there is an increase in Demodex mites in the pilosebaceous unit.”
Research over the past decade has also started to look at the potential role of the skin microbiome in the disease process, but answers have remained “largely elusive,” Dr. Homey said.
Ivermectin helps, but how?
Ivermectin 1% cream (Soolantra) has been approved by the U.S. Food and Drug Administration since 2014 for the treatment of the inflammatory lesions that are characteristic of rosacea, but its mechanism of action is not clear.
Dr. Homey presented the results of a study of 61 patients designed to look at how ivermectin might be working in the treatment of people with rosacea and investigate if there was any relation to the skin microbiome and transcriptome of patients.
The trial included 41 individuals with papulopustular rosacea and 20 individuals who did not have rosacea. For all patients, surface skin biopsies were performed twice 30 days apart using cyanoacrylate glue; patients with rosacea were treated with topical ivermectin 1% between biopsies. Skin samples obtained at day 0 and day 30 were examined under the microscope, and Demodex counts (mites/cm2) of skin and RNA sequencing of the cutaneous microbiome were undertaken.
The mean age of the patients with rosacea was 54.9 years, and the mean Demodex counts before and after treatment were a respective 7.2 cm2 and 0.9 cm2.
Using the Investigator’s General Assessment to assess the severity of rosacea, Homey reported that 43.9% of patients with rosacea had a decrease in scores at day 30, indicating improvement.
In addition, topical ivermectin resulted in a marked or total decrease in Demodex mite density for 87.5% of patients (n = 24) who were identified as having the mites.
Skin microbiome changes seen
As a form of quality control, skin microbiome changes among the patients were compared with control patients using 16S rRNA sequencing.
“The taxa we find within the cutaneous niche of inflammatory lesions of rosacea patients are significantly different from healthy volunteers,” Dr. Homey said.
Cutibacterium species are predominant in healthy control persons but are not present when there is inflammation in patients with rosacea. Instead, staphylococcus species “take over the niche, similar to atopic dermatitis,” he noted.
Looking at how treatment with ivermectin influences the organisms, the decrease in C. acnes seen in patients with rosacea persisted despite treatment, and the abundance of Staphylococcus epidermidis, S. hominis, and S. capitis increased further. This suggests a possible protective or homeostatic role of C. acnes but a pathogenic role for staphylococci, explained Dr. Homey.
“Surprisingly, although inflammatory lesions decrease, patients get better, the cutaneous microbiome does not revert to homeostatic conditions during topical ivermectin treatment,” he observed.
There is, of course, variability among individuals.
Dr. Homey also reported that Snodgrassella alvi – a microorganism believed to reside in the gut of Demodex folliculorum mites – was found in the skin microbiome of patients with rosacea before but not after ivermectin treatment. This may mean that this microorganism could be partially triggering inflammation in rosacea patients.
Looking at the transcriptome of patients, Dr. Homey said that there was downregulation of distinct genes that might make for more favorable conditions for Demodex mites.
Moreover, insufficient upregulation of interleukin-17 pathways might be working together with barrier defects in the skin and metabolic changes to “pave the way” for colonization by S. epidermidis.
Pulling it together
Dr. Homey and associates conclude in their abstract that the findings “support that rosacea lesions are associated with dysbiosis.”
Although treatment with ivermectin did not normalize the skin’s microbiome, it was associated with a decrease in Demodex mite density and the reduction of microbes associated with Demodex.
Margarida Gonçalo, MD, PhD, professor of dermatology at the University of Coimbra in Portugal, who cochaired the late-breaking news session where the data were presented, asked whether healthy and affected skin in patients with rosacea had been compared, rather than comparing the skin of rosacea lesions with healthy control samples.
“No, we did not this, as this is methodologically a little bit more difficult,” Dr. Homey responded.
Also cochairing the session was Michel Gilliet, MD, chair of the department of dermatology at the University Hospital CHUV in Lausanne, Switzerland. He commented that these “data suggest that there’s an intimate link between Demodex and the skin microbiota and dysbiosis in in rosacea.”
Dr. Gilliet added: “You have a whole dysbiosis going on in rosacea, which is probably only dependent on these bacteria.”
It would be “very interesting,” as a “proof-of-concept” study, to look at whether depleting Demodex would also delete S. alvi, he suggested.
The study was funded by Galderma. Dr. Homey has acted as a consultant, speaker or investigator for many pharmaceutical companies including Galderma.
A version of this article first appeared on Medscape.com.
FROM EADV 2023
AGA provides leadership development for women in GI
As a part of AGA’s ongoing goal to support women in GI and advance gender equity in gastroenterology, we hosted nearly 60 women executives in GI for the inaugural Women’s Executive Leadership Conference held recently in Denver.
– academia, hospital systems, and private practice – for seminars on strengthening leadership skills and career progression, along with opportunities for networking and socializing with other women in the AGA Gastro Squad.

Women on the AGA governing board, including Kim E. Barrett, PhD, AGAF and Sheryl Pfeil, MD, AGAF, led sessions on how to best communicate as a leader and pathways to society leadership. In addition, other leaders such as Aja McCutchen, MD and Gyongyi Szabo, MD, PhD, shared their best practices for leadership and managing others.
Thank you to Fasiha Kanwal, MD, MSHS, and Aimee Lucas, MD, MS, cochairs of the AGA Women’s Executive Leadership Conference, for leading the weekend, and to everyone who contributed to a productive weekend. Stay tuned for more opportunities to engage with the AGA Gastro Squad.
As a part of AGA’s ongoing goal to support women in GI and advance gender equity in gastroenterology, we hosted nearly 60 women executives in GI for the inaugural Women’s Executive Leadership Conference held recently in Denver.
– academia, hospital systems, and private practice – for seminars on strengthening leadership skills and career progression, along with opportunities for networking and socializing with other women in the AGA Gastro Squad.

Women on the AGA governing board, including Kim E. Barrett, PhD, AGAF and Sheryl Pfeil, MD, AGAF, led sessions on how to best communicate as a leader and pathways to society leadership. In addition, other leaders such as Aja McCutchen, MD and Gyongyi Szabo, MD, PhD, shared their best practices for leadership and managing others.
Thank you to Fasiha Kanwal, MD, MSHS, and Aimee Lucas, MD, MS, cochairs of the AGA Women’s Executive Leadership Conference, for leading the weekend, and to everyone who contributed to a productive weekend. Stay tuned for more opportunities to engage with the AGA Gastro Squad.
As a part of AGA’s ongoing goal to support women in GI and advance gender equity in gastroenterology, we hosted nearly 60 women executives in GI for the inaugural Women’s Executive Leadership Conference held recently in Denver.
– academia, hospital systems, and private practice – for seminars on strengthening leadership skills and career progression, along with opportunities for networking and socializing with other women in the AGA Gastro Squad.

Women on the AGA governing board, including Kim E. Barrett, PhD, AGAF and Sheryl Pfeil, MD, AGAF, led sessions on how to best communicate as a leader and pathways to society leadership. In addition, other leaders such as Aja McCutchen, MD and Gyongyi Szabo, MD, PhD, shared their best practices for leadership and managing others.
Thank you to Fasiha Kanwal, MD, MSHS, and Aimee Lucas, MD, MS, cochairs of the AGA Women’s Executive Leadership Conference, for leading the weekend, and to everyone who contributed to a productive weekend. Stay tuned for more opportunities to engage with the AGA Gastro Squad.
A letter from Michael Camilleri, MD, DSc, AGAF, AGA Research Foundation chair and past AGA Institute president
And you understand the tremendous value of research to advance patient care.
We are in a time of major scientific breakthroughs; however, there is a growing gap in federal funding for research. Without gastroenterology and hepatology research, there would be no discoveries to develop new diagnostic and therapeutic approaches and to improve our understanding of the pathogenesis of digestive diseases.
The AGA Research Foundation funds promising GI investigators who don’t receive funding at crucial times in their early careers. The research of these talented individuals, while important to the field, could end prematurely if they are left unfunded. That’s something the fields of gastroenterology and hepatology can’t afford, and that’s why, as an AGA member, I’m making a year-end donation to the AGA Research Foundation. You can help fill the funding gap and protect the next generation of investigators by joining me in supporting the AGA Research Foundation through a personal year-end gift.
Gifts to the AGA Research Foundation this past year directly supported 71 investigators. Despite this success, close to 245 other promising research proposals were not funded.
We must continue to foster the careers of talented scientists and clinicians, and protect the GI research pipeline. A financial contribution to the AGA Research Foundation is the opportunity for you to help foster the careers of talented scientists and protect the GI research pipeline.
Help make a difference. You can make your tax-deductible donation online at www.gastro.org/donateonline; by phone at 301-222-4002; or, by mail:
AGA Research Foundation
4930 Del Ray Avenue
Bethesda, MD 20814
All gifts are tax-deductible to the fullest extent of U.S. law.
Thank you for your support and best wishes for a happy, healthy holiday season and prosperous New Year.
And you understand the tremendous value of research to advance patient care.
We are in a time of major scientific breakthroughs; however, there is a growing gap in federal funding for research. Without gastroenterology and hepatology research, there would be no discoveries to develop new diagnostic and therapeutic approaches and to improve our understanding of the pathogenesis of digestive diseases.
The AGA Research Foundation funds promising GI investigators who don’t receive funding at crucial times in their early careers. The research of these talented individuals, while important to the field, could end prematurely if they are left unfunded. That’s something the fields of gastroenterology and hepatology can’t afford, and that’s why, as an AGA member, I’m making a year-end donation to the AGA Research Foundation. You can help fill the funding gap and protect the next generation of investigators by joining me in supporting the AGA Research Foundation through a personal year-end gift.
Gifts to the AGA Research Foundation this past year directly supported 71 investigators. Despite this success, close to 245 other promising research proposals were not funded.
We must continue to foster the careers of talented scientists and clinicians, and protect the GI research pipeline. A financial contribution to the AGA Research Foundation is the opportunity for you to help foster the careers of talented scientists and protect the GI research pipeline.
Help make a difference. You can make your tax-deductible donation online at www.gastro.org/donateonline; by phone at 301-222-4002; or, by mail:
AGA Research Foundation
4930 Del Ray Avenue
Bethesda, MD 20814
All gifts are tax-deductible to the fullest extent of U.S. law.
Thank you for your support and best wishes for a happy, healthy holiday season and prosperous New Year.
And you understand the tremendous value of research to advance patient care.
We are in a time of major scientific breakthroughs; however, there is a growing gap in federal funding for research. Without gastroenterology and hepatology research, there would be no discoveries to develop new diagnostic and therapeutic approaches and to improve our understanding of the pathogenesis of digestive diseases.
The AGA Research Foundation funds promising GI investigators who don’t receive funding at crucial times in their early careers. The research of these talented individuals, while important to the field, could end prematurely if they are left unfunded. That’s something the fields of gastroenterology and hepatology can’t afford, and that’s why, as an AGA member, I’m making a year-end donation to the AGA Research Foundation. You can help fill the funding gap and protect the next generation of investigators by joining me in supporting the AGA Research Foundation through a personal year-end gift.
Gifts to the AGA Research Foundation this past year directly supported 71 investigators. Despite this success, close to 245 other promising research proposals were not funded.
We must continue to foster the careers of talented scientists and clinicians, and protect the GI research pipeline. A financial contribution to the AGA Research Foundation is the opportunity for you to help foster the careers of talented scientists and protect the GI research pipeline.
Help make a difference. You can make your tax-deductible donation online at www.gastro.org/donateonline; by phone at 301-222-4002; or, by mail:
AGA Research Foundation
4930 Del Ray Avenue
Bethesda, MD 20814
All gifts are tax-deductible to the fullest extent of U.S. law.
Thank you for your support and best wishes for a happy, healthy holiday season and prosperous New Year.
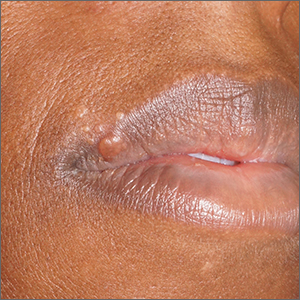
Papules on lip
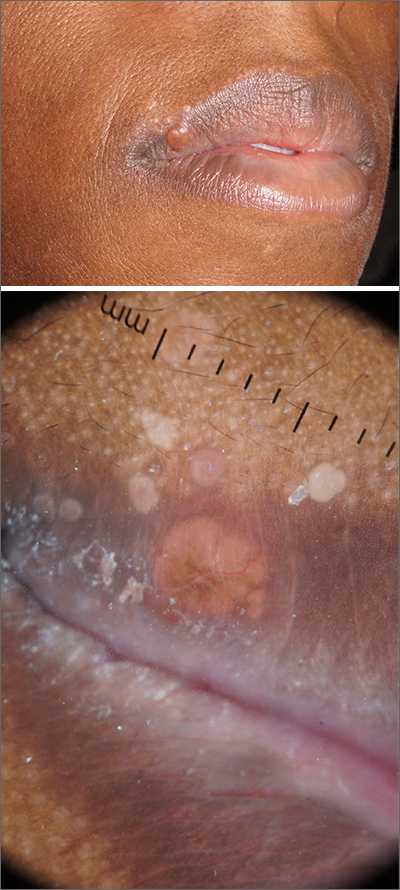
Pathology showed noncaseating granulomas consistent with cutaneous sarcoidosis. Based on these biopsy findings, a chest x-ray was ordered, and it confirmed a pulmonary sarcoid. A multidisciplinary work-up (including cardiac evaluation, continued rheumatologic care, and evaluation by Hematology) addressed this new finding.
Sarcoidosis is a multisystem inflammatory disorder characterized by the development of granulomas that can arise in any organ, but frequently involve the skin and lungs. Patients with cutaneous disease develop smooth skin lesions, including flesh-colored to pink or brown papules on the face. Genetic and environmental factors are both thought to contribute to the disease.
Race is a significant factor in the development of disease. Hispanic and Asian patients are significantly less likely to develop the disease compared to White or Black patients. In the Black Women’s Health Study, incidence in Black women was 71 per 100,000.1 Women are more likely to be affected than men.1
Many patients with sarcoidosis have a mild course, but for others the disease may progress on the skin or include pulmonary, renal, neurologic, or cardiac disease. Sometimes sarcoidosis is fatal. Recurrence can occur at any point later in life. Race influences disease severity as well as incidence, with hospitalization being 9 times as likely in Black patients compared with White patients.2 One recent study puts sarcoidosis mortality rates for Black women at 10 per million compared with 3 per million in Black men, and 1 per million in White women or men.3
Patients with disease limited to the skin may be treated with topical steroids such as clobetasol 0.05% cream or ointment or intralesional triamcinolone 10 mg/mL injected into affected lesions every 2 to 4 weeks. With pulmonary or other systemic disease, treatment may include various disease-modifying agents including prednisone, methotrexate, hydroxychloroquine, and TNF-alpha inhibitors. Because of the long-term adverse effects of systemic steroids, these agents are reserved for instances when pulmonary function is significantly impacted.
This patient had a reassuring cardiac and hematology work-up. Her pulmonary function was impacted sufficiently enough that her pulmonologist added a course of prednisone 10 mg daily tapered over 6 weeks. She had been on hydroxychloroquine 200 mg twice daily prior to the diagnosis of sarcoidosis for presumed mixed connective tissue disease and was continued on it for sarcoidosis after completing the prednisone taper. With these treatments, her facial lesions cleared and her breathing symptoms and fatigue improved. She remains under surveillance with a multidisciplinary team.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Cozier Y, Berman J, Palmer J, et al. Sarcoidosis in black women in the United States: data from the Black Women's Health Study. Chest. 2011;139:144-150. doi: 10.1378/chest.10-0413
2. Foreman MG, Mannino DM, Kamugisha L, et al. Hospitalization for patients with sarcoidosis: 1979-2000. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:124-129.
3. Mirsaeidi M, Machado R, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015; 147: 438-449. doi: 10.1378/chest.14-1120

Pathology showed noncaseating granulomas consistent with cutaneous sarcoidosis. Based on these biopsy findings, a chest x-ray was ordered, and it confirmed a pulmonary sarcoid. A multidisciplinary work-up (including cardiac evaluation, continued rheumatologic care, and evaluation by Hematology) addressed this new finding.
Sarcoidosis is a multisystem inflammatory disorder characterized by the development of granulomas that can arise in any organ, but frequently involve the skin and lungs. Patients with cutaneous disease develop smooth skin lesions, including flesh-colored to pink or brown papules on the face. Genetic and environmental factors are both thought to contribute to the disease.
Race is a significant factor in the development of disease. Hispanic and Asian patients are significantly less likely to develop the disease compared to White or Black patients. In the Black Women’s Health Study, incidence in Black women was 71 per 100,000.1 Women are more likely to be affected than men.1
Many patients with sarcoidosis have a mild course, but for others the disease may progress on the skin or include pulmonary, renal, neurologic, or cardiac disease. Sometimes sarcoidosis is fatal. Recurrence can occur at any point later in life. Race influences disease severity as well as incidence, with hospitalization being 9 times as likely in Black patients compared with White patients.2 One recent study puts sarcoidosis mortality rates for Black women at 10 per million compared with 3 per million in Black men, and 1 per million in White women or men.3
Patients with disease limited to the skin may be treated with topical steroids such as clobetasol 0.05% cream or ointment or intralesional triamcinolone 10 mg/mL injected into affected lesions every 2 to 4 weeks. With pulmonary or other systemic disease, treatment may include various disease-modifying agents including prednisone, methotrexate, hydroxychloroquine, and TNF-alpha inhibitors. Because of the long-term adverse effects of systemic steroids, these agents are reserved for instances when pulmonary function is significantly impacted.
This patient had a reassuring cardiac and hematology work-up. Her pulmonary function was impacted sufficiently enough that her pulmonologist added a course of prednisone 10 mg daily tapered over 6 weeks. She had been on hydroxychloroquine 200 mg twice daily prior to the diagnosis of sarcoidosis for presumed mixed connective tissue disease and was continued on it for sarcoidosis after completing the prednisone taper. With these treatments, her facial lesions cleared and her breathing symptoms and fatigue improved. She remains under surveillance with a multidisciplinary team.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Pathology showed noncaseating granulomas consistent with cutaneous sarcoidosis. Based on these biopsy findings, a chest x-ray was ordered, and it confirmed a pulmonary sarcoid. A multidisciplinary work-up (including cardiac evaluation, continued rheumatologic care, and evaluation by Hematology) addressed this new finding.
Sarcoidosis is a multisystem inflammatory disorder characterized by the development of granulomas that can arise in any organ, but frequently involve the skin and lungs. Patients with cutaneous disease develop smooth skin lesions, including flesh-colored to pink or brown papules on the face. Genetic and environmental factors are both thought to contribute to the disease.
Race is a significant factor in the development of disease. Hispanic and Asian patients are significantly less likely to develop the disease compared to White or Black patients. In the Black Women’s Health Study, incidence in Black women was 71 per 100,000.1 Women are more likely to be affected than men.1
Many patients with sarcoidosis have a mild course, but for others the disease may progress on the skin or include pulmonary, renal, neurologic, or cardiac disease. Sometimes sarcoidosis is fatal. Recurrence can occur at any point later in life. Race influences disease severity as well as incidence, with hospitalization being 9 times as likely in Black patients compared with White patients.2 One recent study puts sarcoidosis mortality rates for Black women at 10 per million compared with 3 per million in Black men, and 1 per million in White women or men.3
Patients with disease limited to the skin may be treated with topical steroids such as clobetasol 0.05% cream or ointment or intralesional triamcinolone 10 mg/mL injected into affected lesions every 2 to 4 weeks. With pulmonary or other systemic disease, treatment may include various disease-modifying agents including prednisone, methotrexate, hydroxychloroquine, and TNF-alpha inhibitors. Because of the long-term adverse effects of systemic steroids, these agents are reserved for instances when pulmonary function is significantly impacted.
This patient had a reassuring cardiac and hematology work-up. Her pulmonary function was impacted sufficiently enough that her pulmonologist added a course of prednisone 10 mg daily tapered over 6 weeks. She had been on hydroxychloroquine 200 mg twice daily prior to the diagnosis of sarcoidosis for presumed mixed connective tissue disease and was continued on it for sarcoidosis after completing the prednisone taper. With these treatments, her facial lesions cleared and her breathing symptoms and fatigue improved. She remains under surveillance with a multidisciplinary team.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Cozier Y, Berman J, Palmer J, et al. Sarcoidosis in black women in the United States: data from the Black Women's Health Study. Chest. 2011;139:144-150. doi: 10.1378/chest.10-0413
2. Foreman MG, Mannino DM, Kamugisha L, et al. Hospitalization for patients with sarcoidosis: 1979-2000. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:124-129.
3. Mirsaeidi M, Machado R, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015; 147: 438-449. doi: 10.1378/chest.14-1120
1. Cozier Y, Berman J, Palmer J, et al. Sarcoidosis in black women in the United States: data from the Black Women's Health Study. Chest. 2011;139:144-150. doi: 10.1378/chest.10-0413
2. Foreman MG, Mannino DM, Kamugisha L, et al. Hospitalization for patients with sarcoidosis: 1979-2000. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:124-129.
3. Mirsaeidi M, Machado R, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015; 147: 438-449. doi: 10.1378/chest.14-1120
Low vitamin D linked to paclitaxel-induced peripheral neuropathy
TOPLINE:
, suggesting that correcting levels before treatment might help prevent the condition.
METHODOLOGY:
- Past studies have suggested an association between vitamin D insufficiency and paclitaxel-induced peripheral neuropathy, a largely untreatable and sometimes permanent side effect of chemotherapy.
- To confirm the association, investigators reviewed data and samples from 1,191 women in the phase 3 SWOG S0221 trial, which compared weekly and biweekly paclitaxel regimens for early-stage breast cancer.
- Using serum samples collected at baseline, the team evaluated the relationship between insufficient vitamin D levels (20 ng/mL or less) before treatment and grade 3 or higher sensory chemotherapy-induced peripheral neuropathy.
TAKEAWAY:
- Overall, 33.3% of the women had insufficient vitamin D levels at baseline, and 16.4% developed grade 3 or worse sensory chemotherapy-induced peripheral neuropathy.
- The incidence of peripheral neuropathy of grade 3 or greater was higher among patients with pretreatment vitamin D insufficiency (20.7% vs. 14.2%; odds ratio, 1.57; P = .005).
- The association grew stronger after adjusting for age and paclitaxel schedule (adjusted OR, 1.65; P = .003), but not after adjusting for race (adjusted OR, 1.39; P = .066).
IN PRACTICE:
The study “confirms that patients with pretreatment vitamin D insufficiency have a higher incidence of [chemotherapy-induced peripheral neuropathy],” the authors concluded. These results also “suggest that vitamin D supplementation in patients with lower levels of vitamin D may reduce peripheral neuropathy, and particularly high-grade peripheral neuropathy, which would improve these patients’ long-term quality of life,” senior researcher Daniel L. Hertz, PharmD, PhD, University of Michigan College of Pharmacy, Ann Arbor, said in a press release.
SOURCE:
The study, led by Ciao-Sin Chen, PharmD, of the University of Michigan, Ann Arbor, was published in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The trial did not collect data on other peripheral neuropathy risk factors, including preexisting peripheral neuropathy and diabetes. The study included a limited number of non-White participants (16%); larger numbers are needed to elucidate a potential interplay between race, vitamin D, and chemotherapy-induced peripheral neuropathy. The researchers also did not collect data on grade 1 and 2 chemotherapy-induced peripheral neuropathy.
DISCLOSURES:
The study was funded by Amgen, the American Cancer Society, and others. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that correcting levels before treatment might help prevent the condition.
METHODOLOGY:
- Past studies have suggested an association between vitamin D insufficiency and paclitaxel-induced peripheral neuropathy, a largely untreatable and sometimes permanent side effect of chemotherapy.
- To confirm the association, investigators reviewed data and samples from 1,191 women in the phase 3 SWOG S0221 trial, which compared weekly and biweekly paclitaxel regimens for early-stage breast cancer.
- Using serum samples collected at baseline, the team evaluated the relationship between insufficient vitamin D levels (20 ng/mL or less) before treatment and grade 3 or higher sensory chemotherapy-induced peripheral neuropathy.
TAKEAWAY:
- Overall, 33.3% of the women had insufficient vitamin D levels at baseline, and 16.4% developed grade 3 or worse sensory chemotherapy-induced peripheral neuropathy.
- The incidence of peripheral neuropathy of grade 3 or greater was higher among patients with pretreatment vitamin D insufficiency (20.7% vs. 14.2%; odds ratio, 1.57; P = .005).
- The association grew stronger after adjusting for age and paclitaxel schedule (adjusted OR, 1.65; P = .003), but not after adjusting for race (adjusted OR, 1.39; P = .066).
IN PRACTICE:
The study “confirms that patients with pretreatment vitamin D insufficiency have a higher incidence of [chemotherapy-induced peripheral neuropathy],” the authors concluded. These results also “suggest that vitamin D supplementation in patients with lower levels of vitamin D may reduce peripheral neuropathy, and particularly high-grade peripheral neuropathy, which would improve these patients’ long-term quality of life,” senior researcher Daniel L. Hertz, PharmD, PhD, University of Michigan College of Pharmacy, Ann Arbor, said in a press release.
SOURCE:
The study, led by Ciao-Sin Chen, PharmD, of the University of Michigan, Ann Arbor, was published in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The trial did not collect data on other peripheral neuropathy risk factors, including preexisting peripheral neuropathy and diabetes. The study included a limited number of non-White participants (16%); larger numbers are needed to elucidate a potential interplay between race, vitamin D, and chemotherapy-induced peripheral neuropathy. The researchers also did not collect data on grade 1 and 2 chemotherapy-induced peripheral neuropathy.
DISCLOSURES:
The study was funded by Amgen, the American Cancer Society, and others. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that correcting levels before treatment might help prevent the condition.
METHODOLOGY:
- Past studies have suggested an association between vitamin D insufficiency and paclitaxel-induced peripheral neuropathy, a largely untreatable and sometimes permanent side effect of chemotherapy.
- To confirm the association, investigators reviewed data and samples from 1,191 women in the phase 3 SWOG S0221 trial, which compared weekly and biweekly paclitaxel regimens for early-stage breast cancer.
- Using serum samples collected at baseline, the team evaluated the relationship between insufficient vitamin D levels (20 ng/mL or less) before treatment and grade 3 or higher sensory chemotherapy-induced peripheral neuropathy.
TAKEAWAY:
- Overall, 33.3% of the women had insufficient vitamin D levels at baseline, and 16.4% developed grade 3 or worse sensory chemotherapy-induced peripheral neuropathy.
- The incidence of peripheral neuropathy of grade 3 or greater was higher among patients with pretreatment vitamin D insufficiency (20.7% vs. 14.2%; odds ratio, 1.57; P = .005).
- The association grew stronger after adjusting for age and paclitaxel schedule (adjusted OR, 1.65; P = .003), but not after adjusting for race (adjusted OR, 1.39; P = .066).
IN PRACTICE:
The study “confirms that patients with pretreatment vitamin D insufficiency have a higher incidence of [chemotherapy-induced peripheral neuropathy],” the authors concluded. These results also “suggest that vitamin D supplementation in patients with lower levels of vitamin D may reduce peripheral neuropathy, and particularly high-grade peripheral neuropathy, which would improve these patients’ long-term quality of life,” senior researcher Daniel L. Hertz, PharmD, PhD, University of Michigan College of Pharmacy, Ann Arbor, said in a press release.
SOURCE:
The study, led by Ciao-Sin Chen, PharmD, of the University of Michigan, Ann Arbor, was published in the Journal of the National Comprehensive Cancer Network.
LIMITATIONS:
The trial did not collect data on other peripheral neuropathy risk factors, including preexisting peripheral neuropathy and diabetes. The study included a limited number of non-White participants (16%); larger numbers are needed to elucidate a potential interplay between race, vitamin D, and chemotherapy-induced peripheral neuropathy. The researchers also did not collect data on grade 1 and 2 chemotherapy-induced peripheral neuropathy.
DISCLOSURES:
The study was funded by Amgen, the American Cancer Society, and others. The investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
RNA therapeutics will ‘change everything’ in epilepsy
Epilepsy affects over 50 million people worldwide, making it one of the most common neurologic disorders. Though current antiseizure medications can control seizures in two-thirds of patients, drug-resistant epilepsy remains a major challenge for the remaining one-third, as does the lack of disease-modifying therapies.
But RNA-based therapeutics offer new hope, and experts predict they could fill these gaps and revolutionize epilepsy treatment.
“Current medicines for epilepsy are barely scraping the surface of what could be targeted. RNA therapeutics is going to change everything. It opens up entirely new targets – virtually anything in our genome becomes ‘druggable,’ ” said David Henshall, PhD, Royal College of Surgeons Ireland, Dublin.
Edward Kaye, MD, a pediatric neurologist and CEO of Stoke Therapeutics, agrees. “RNA therapeutics open up possibilities that could not have been imagined when I started my career,” he said in an interview.
Dr. Kaye said.
Thank COVID?
Henrik Klitgaard, PhD, and Sakari Kauppinen, PhD, scientific co-founders of NEUmiRNA Therapeutics, noted that the success of messenger RNA (mRNA) vaccines to counter the COVID-19 pandemic has fueled interest in exploring the potential of RNA-based therapies as a new modality in epilepsy with improved therapeutic properties.
Dr. Klitgaard and Dr. Kauppinen recently co-authored a “critical review” on RNA therapies for epilepsy published online in Epilepsia.
Unlike current antiseizure medications, which only target ion channels and receptors, RNA therapeutics can directly intervene at the genetic level.
RNA drugs can be targeted toward noncoding RNAs, such as microRNAs, or toward mRNA. Targeting noncoding RNAs shows promise in sporadic, nongenetic epilepsies, and targeting of mRNAs shows promise in childhood monogenic epilepsies.
Preclinical studies have highlighted the potential of RNA therapies for treatment of epilepsy.
“At NEUmiRNA Therapeutics, we have successfully designed potent and selective RNA drugs for a novel disease target that enable unprecedented elimination of the drug resistance and chronic epilepsy in a preclinical model mimicking temporal lobe epilepsy,” said Dr. Klitgaard.
“Interestingly,” he said, “these experiments also showed a disappearance of symptoms for epilepsy that outlasted drug exposure, suggesting significant disease-modifying properties with a curative potential for epilepsy.”
Hope for Dravet syndrome
Currently, there is significant interest in development of antisense oligonucleotides (ASOs), particularly for Dravet syndrome, a rare genetic epileptic encephalopathy that begins in infancy and gives rise to seizures that don’t respond well to seizure medications.
Stoke Therapeutics is developing antisense therapies aimed at correcting mutations in sodium channel genes, which cause up to 80% of cases of Dravet syndrome.
The company recently reported positive safety and efficacy data from patients treated with STK-001, a proprietary ASO, in the two ongoing phase 1/2a studies (MONARCH and ADMIRAL) and the SWALLOWTAIL open-label extension study.
“These new data suggest clinical benefit for patients 2-18 years of age treated with multiple doses of STK-001. The observed reductions in convulsive seizure frequency as well as substantial improvements in cognition and behavior support the potential for disease modification in a highly refractory patient population,” the company said in a news release.
Dr. Kaye noted that the company anticipates reporting additional data in the first quarter of 2024 and expects to provide an update on phase 3 planning in the first half of 2024.
“Twenty-five years ago, when I was caring for patients in my clinic, half of epilepsy was considered idiopathic because we didn’t know the cause,” Dr. Kaye commented.
“Since then, thanks to an understanding of the genetics and more widely available access to genetic testing, we can determine the root cause of most of them. Today, I believe we are on the verge of a fundamental shift in how we approach the treatment of Dravet syndrome and, hopefully, other genetic epilepsies,” said Dr. Kaye.
“We are now finally getting to the point that we not only know the causes, but we are in a position to develop medicines that target those causes. We have seen this happen in other diseases like cystic fibrosis, and the time has come for genetic epilepsies,” he added.
A promising future
Dr. Henshall said that the ability to target the cause rather than just the symptoms of epilepsy “offers the promise of disease-modifying and potentially curative medicines in the future.”
And what’s exciting is that the time frame of developing RNA medicines may be “radically” different than it is for traditional small-drug development, he noted.
Take, for example, a case reported recently in the New England Journal of Medicine.
Researchers identified a novel mutation in a child with neuronal ceroid lipofuscinosis 7 (a form of Batten’s disease), a rare and fatal neurodegenerative disease. Identification of the mutation was followed by the development and use (within 1 year) of a tailored RNA drug to treat the patient.
One downside perhaps is that current RNA drugs for epilepsy are delivered intrathecally, which is different from oral administration of small-molecule drugs.
However, Dr. Kauppinen from NEUmiRNA Therapeutics noted that “advances in intrathecal delivery technologies [and] the frequent use of this route of administration in other diseases and IT administration only being required two to three times per year will certainly facilitate use of RNA medicines.”
“This will also eliminate the issue of drug adherence by ensuring full patient compliance to treatment,” Dr. Kauppinen said.
The review article on RNA therapies in epilepsy had no commercial funding. Dr. Henshall holds a patent and has filed intellectual property related to microRNA targeting therapies for epilepsy and has received funding for microRNA research from NEUmiRNA Therapeutics. Dr. Klitgaard and Dr. Kauppinen are cofounders of NEUmiRNA Therapeutics. Dr. Kaye is CEO of Stoke Therapeutics.
A version of this article first appeared on Medscape.com.
Epilepsy affects over 50 million people worldwide, making it one of the most common neurologic disorders. Though current antiseizure medications can control seizures in two-thirds of patients, drug-resistant epilepsy remains a major challenge for the remaining one-third, as does the lack of disease-modifying therapies.
But RNA-based therapeutics offer new hope, and experts predict they could fill these gaps and revolutionize epilepsy treatment.
“Current medicines for epilepsy are barely scraping the surface of what could be targeted. RNA therapeutics is going to change everything. It opens up entirely new targets – virtually anything in our genome becomes ‘druggable,’ ” said David Henshall, PhD, Royal College of Surgeons Ireland, Dublin.
Edward Kaye, MD, a pediatric neurologist and CEO of Stoke Therapeutics, agrees. “RNA therapeutics open up possibilities that could not have been imagined when I started my career,” he said in an interview.
Dr. Kaye said.
Thank COVID?
Henrik Klitgaard, PhD, and Sakari Kauppinen, PhD, scientific co-founders of NEUmiRNA Therapeutics, noted that the success of messenger RNA (mRNA) vaccines to counter the COVID-19 pandemic has fueled interest in exploring the potential of RNA-based therapies as a new modality in epilepsy with improved therapeutic properties.
Dr. Klitgaard and Dr. Kauppinen recently co-authored a “critical review” on RNA therapies for epilepsy published online in Epilepsia.
Unlike current antiseizure medications, which only target ion channels and receptors, RNA therapeutics can directly intervene at the genetic level.
RNA drugs can be targeted toward noncoding RNAs, such as microRNAs, or toward mRNA. Targeting noncoding RNAs shows promise in sporadic, nongenetic epilepsies, and targeting of mRNAs shows promise in childhood monogenic epilepsies.
Preclinical studies have highlighted the potential of RNA therapies for treatment of epilepsy.
“At NEUmiRNA Therapeutics, we have successfully designed potent and selective RNA drugs for a novel disease target that enable unprecedented elimination of the drug resistance and chronic epilepsy in a preclinical model mimicking temporal lobe epilepsy,” said Dr. Klitgaard.
“Interestingly,” he said, “these experiments also showed a disappearance of symptoms for epilepsy that outlasted drug exposure, suggesting significant disease-modifying properties with a curative potential for epilepsy.”
Hope for Dravet syndrome
Currently, there is significant interest in development of antisense oligonucleotides (ASOs), particularly for Dravet syndrome, a rare genetic epileptic encephalopathy that begins in infancy and gives rise to seizures that don’t respond well to seizure medications.
Stoke Therapeutics is developing antisense therapies aimed at correcting mutations in sodium channel genes, which cause up to 80% of cases of Dravet syndrome.
The company recently reported positive safety and efficacy data from patients treated with STK-001, a proprietary ASO, in the two ongoing phase 1/2a studies (MONARCH and ADMIRAL) and the SWALLOWTAIL open-label extension study.
“These new data suggest clinical benefit for patients 2-18 years of age treated with multiple doses of STK-001. The observed reductions in convulsive seizure frequency as well as substantial improvements in cognition and behavior support the potential for disease modification in a highly refractory patient population,” the company said in a news release.
Dr. Kaye noted that the company anticipates reporting additional data in the first quarter of 2024 and expects to provide an update on phase 3 planning in the first half of 2024.
“Twenty-five years ago, when I was caring for patients in my clinic, half of epilepsy was considered idiopathic because we didn’t know the cause,” Dr. Kaye commented.
“Since then, thanks to an understanding of the genetics and more widely available access to genetic testing, we can determine the root cause of most of them. Today, I believe we are on the verge of a fundamental shift in how we approach the treatment of Dravet syndrome and, hopefully, other genetic epilepsies,” said Dr. Kaye.
“We are now finally getting to the point that we not only know the causes, but we are in a position to develop medicines that target those causes. We have seen this happen in other diseases like cystic fibrosis, and the time has come for genetic epilepsies,” he added.
A promising future
Dr. Henshall said that the ability to target the cause rather than just the symptoms of epilepsy “offers the promise of disease-modifying and potentially curative medicines in the future.”
And what’s exciting is that the time frame of developing RNA medicines may be “radically” different than it is for traditional small-drug development, he noted.
Take, for example, a case reported recently in the New England Journal of Medicine.
Researchers identified a novel mutation in a child with neuronal ceroid lipofuscinosis 7 (a form of Batten’s disease), a rare and fatal neurodegenerative disease. Identification of the mutation was followed by the development and use (within 1 year) of a tailored RNA drug to treat the patient.
One downside perhaps is that current RNA drugs for epilepsy are delivered intrathecally, which is different from oral administration of small-molecule drugs.
However, Dr. Kauppinen from NEUmiRNA Therapeutics noted that “advances in intrathecal delivery technologies [and] the frequent use of this route of administration in other diseases and IT administration only being required two to three times per year will certainly facilitate use of RNA medicines.”
“This will also eliminate the issue of drug adherence by ensuring full patient compliance to treatment,” Dr. Kauppinen said.
The review article on RNA therapies in epilepsy had no commercial funding. Dr. Henshall holds a patent and has filed intellectual property related to microRNA targeting therapies for epilepsy and has received funding for microRNA research from NEUmiRNA Therapeutics. Dr. Klitgaard and Dr. Kauppinen are cofounders of NEUmiRNA Therapeutics. Dr. Kaye is CEO of Stoke Therapeutics.
A version of this article first appeared on Medscape.com.
Epilepsy affects over 50 million people worldwide, making it one of the most common neurologic disorders. Though current antiseizure medications can control seizures in two-thirds of patients, drug-resistant epilepsy remains a major challenge for the remaining one-third, as does the lack of disease-modifying therapies.
But RNA-based therapeutics offer new hope, and experts predict they could fill these gaps and revolutionize epilepsy treatment.
“Current medicines for epilepsy are barely scraping the surface of what could be targeted. RNA therapeutics is going to change everything. It opens up entirely new targets – virtually anything in our genome becomes ‘druggable,’ ” said David Henshall, PhD, Royal College of Surgeons Ireland, Dublin.
Edward Kaye, MD, a pediatric neurologist and CEO of Stoke Therapeutics, agrees. “RNA therapeutics open up possibilities that could not have been imagined when I started my career,” he said in an interview.
Dr. Kaye said.
Thank COVID?
Henrik Klitgaard, PhD, and Sakari Kauppinen, PhD, scientific co-founders of NEUmiRNA Therapeutics, noted that the success of messenger RNA (mRNA) vaccines to counter the COVID-19 pandemic has fueled interest in exploring the potential of RNA-based therapies as a new modality in epilepsy with improved therapeutic properties.
Dr. Klitgaard and Dr. Kauppinen recently co-authored a “critical review” on RNA therapies for epilepsy published online in Epilepsia.
Unlike current antiseizure medications, which only target ion channels and receptors, RNA therapeutics can directly intervene at the genetic level.
RNA drugs can be targeted toward noncoding RNAs, such as microRNAs, or toward mRNA. Targeting noncoding RNAs shows promise in sporadic, nongenetic epilepsies, and targeting of mRNAs shows promise in childhood monogenic epilepsies.
Preclinical studies have highlighted the potential of RNA therapies for treatment of epilepsy.
“At NEUmiRNA Therapeutics, we have successfully designed potent and selective RNA drugs for a novel disease target that enable unprecedented elimination of the drug resistance and chronic epilepsy in a preclinical model mimicking temporal lobe epilepsy,” said Dr. Klitgaard.
“Interestingly,” he said, “these experiments also showed a disappearance of symptoms for epilepsy that outlasted drug exposure, suggesting significant disease-modifying properties with a curative potential for epilepsy.”
Hope for Dravet syndrome
Currently, there is significant interest in development of antisense oligonucleotides (ASOs), particularly for Dravet syndrome, a rare genetic epileptic encephalopathy that begins in infancy and gives rise to seizures that don’t respond well to seizure medications.
Stoke Therapeutics is developing antisense therapies aimed at correcting mutations in sodium channel genes, which cause up to 80% of cases of Dravet syndrome.
The company recently reported positive safety and efficacy data from patients treated with STK-001, a proprietary ASO, in the two ongoing phase 1/2a studies (MONARCH and ADMIRAL) and the SWALLOWTAIL open-label extension study.
“These new data suggest clinical benefit for patients 2-18 years of age treated with multiple doses of STK-001. The observed reductions in convulsive seizure frequency as well as substantial improvements in cognition and behavior support the potential for disease modification in a highly refractory patient population,” the company said in a news release.
Dr. Kaye noted that the company anticipates reporting additional data in the first quarter of 2024 and expects to provide an update on phase 3 planning in the first half of 2024.
“Twenty-five years ago, when I was caring for patients in my clinic, half of epilepsy was considered idiopathic because we didn’t know the cause,” Dr. Kaye commented.
“Since then, thanks to an understanding of the genetics and more widely available access to genetic testing, we can determine the root cause of most of them. Today, I believe we are on the verge of a fundamental shift in how we approach the treatment of Dravet syndrome and, hopefully, other genetic epilepsies,” said Dr. Kaye.
“We are now finally getting to the point that we not only know the causes, but we are in a position to develop medicines that target those causes. We have seen this happen in other diseases like cystic fibrosis, and the time has come for genetic epilepsies,” he added.
A promising future
Dr. Henshall said that the ability to target the cause rather than just the symptoms of epilepsy “offers the promise of disease-modifying and potentially curative medicines in the future.”
And what’s exciting is that the time frame of developing RNA medicines may be “radically” different than it is for traditional small-drug development, he noted.
Take, for example, a case reported recently in the New England Journal of Medicine.
Researchers identified a novel mutation in a child with neuronal ceroid lipofuscinosis 7 (a form of Batten’s disease), a rare and fatal neurodegenerative disease. Identification of the mutation was followed by the development and use (within 1 year) of a tailored RNA drug to treat the patient.
One downside perhaps is that current RNA drugs for epilepsy are delivered intrathecally, which is different from oral administration of small-molecule drugs.
However, Dr. Kauppinen from NEUmiRNA Therapeutics noted that “advances in intrathecal delivery technologies [and] the frequent use of this route of administration in other diseases and IT administration only being required two to three times per year will certainly facilitate use of RNA medicines.”
“This will also eliminate the issue of drug adherence by ensuring full patient compliance to treatment,” Dr. Kauppinen said.
The review article on RNA therapies in epilepsy had no commercial funding. Dr. Henshall holds a patent and has filed intellectual property related to microRNA targeting therapies for epilepsy and has received funding for microRNA research from NEUmiRNA Therapeutics. Dr. Klitgaard and Dr. Kauppinen are cofounders of NEUmiRNA Therapeutics. Dr. Kaye is CEO of Stoke Therapeutics.
A version of this article first appeared on Medscape.com.
What not to prescribe to older adults and what to use instead
This transcript has been edited for clarity.
These criteria have been updated and revised approximately every 5 years since 1991 and serve to alert us to medications for which the risk-benefit ratio is not as good in older adults as in the rest of the population.
These are important criteria because medications are metabolized differently in older adults and have different effects compared with younger patients. For the sake of these criteria, older adults are 65 years of age or older. That said, we know that everyone from 65 to 100 is not the same. As people age, they develop more comorbidities, they become more frail, and they are more sensitive to the effects and side effects of drugs.
The guidance covers potentially inappropriate medications for older adults. The word “potentially” is important because this is guidance. As clinicians, we make decisions involving individuals. This guidance should be used with judgment, integrating the clinical context of the individual patient.
There is a lot in this guidance. I am going to try to cover what I feel are the most important points.
Aspirin. Since the risk for major bleeding increases with age, for primary prevention of atherosclerotic cardiovascular disease, the harm can be greater than the benefit in older adults, so aspirin should not be used for primary prevention. Aspirin remains indicated for secondary prevention in individuals with established cardiovascular disease.
Warfarin. For treatment of atrial fibrillation or venous thromboembolism (deep vein thrombosis or pulmonary embolism), warfarin should be avoided if possible. Warfarin has a higher risk for major bleeding, particularly intracranial bleeding, than direct oral anticoagulants (DOACs); therefore the latter are preferred. Rivaroxaban should be avoided, as it has a higher risk for major bleeding in older adults than the other DOACs. Apixaban is preferred over dabigatran. If a patient is well controlled on warfarin, you can consider continuing that treatment.
Antipsychotics. These include first- and second-generation antipsychotics such as aripiprazole, haloperidol, olanzapine, quetiapine, risperidone, and others. The guidance says to avoid these agents except for FDA-approved indications such as schizophrenia, bipolar disorder, and adjuvant treatment of depression. Use of these antipsychotics can increase risk for stroke, heart attack, and mortality. Essentially, the guidance says do not use these medications lightly for the treatment of agitated dementia. For those of us with older patients, this can get tricky because agitated dementia is a difficult issue for which there are no good effective medications. The Beers guidance recognizes this in saying that these medications should be avoided unless behavioral interventions have failed. So, there are times where you may need to use these medicines, but use them judiciously.
For patients with dementia, anticholinergics, antipsychotics, and benzodiazepines should be avoided if possible.
Benzodiazepines. Benzodiazepines should also be avoided because older adults have increased sensitivity to their effects due to slower metabolism and clearance of these medications, which can lead to a much longer half-life and higher serum level. In older adults, benzodiazepines increase the risk for cognitive impairment, delirium, falls, fractures, and even motor accidents. The same concerns affect the group of non-benzodiazepine sleeping medicines known as “Z-drugs.”
Nonsteroidal anti-inflammatory drugs (NSAIDs). Used frequently in our practices, NSAIDs are nevertheless on the list. As we think through the risk-benefit ratio of using NSAIDs in older adults, we often underappreciate the risks of these agents. Upper gastrointestinal ulcers with bleeding occur in approximately 1% of patients treated for 3-6 months with an NSAID and in 2%-4% of patients treated for a year. NSAIDs also increase the risk for renal impairment and cardiovascular disease.
Other medications to avoid (if possible). These include:
Sulfonylureas, due to a high risk for hypoglycemia. A short-acting sulfonylurea, such as glipizide, should be used if one is needed.
Proton pump inhibitors should not be used long-term if it can be avoided.
Digoxin should not be first-line treatment for atrial fibrillation or heart failure. Decreased renal clearance in older adults can lead to toxic levels of digoxin, particularly during acute illnesses. Avoid doses > 0.125 mg/day.
Nitrofurantoin should be avoided when the patient’s creatinine clearance is < 30 or for long-term suppressive therapy.
Avoid combining medications that have high anticholinergic side effects, such as scopolamine, diphenhydramine, oxybutynin, cyclobenzaprine, and others.
It is always important to understand the benefits and the risks of the drugs we prescribe. It is also important to remember that older adults are a particularly vulnerable population. The Beers criteria provide important guidance, which we can then use to make decisions about medicines for individual patients.
Dr. Skolnik is a professor in the department of family medicine at Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director in the department of family medicine at Abington (Pa.) Jefferson Health. He disclosed ties with AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GSK, Merck, Sanofi, Sanofi Pasteur, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
These criteria have been updated and revised approximately every 5 years since 1991 and serve to alert us to medications for which the risk-benefit ratio is not as good in older adults as in the rest of the population.
These are important criteria because medications are metabolized differently in older adults and have different effects compared with younger patients. For the sake of these criteria, older adults are 65 years of age or older. That said, we know that everyone from 65 to 100 is not the same. As people age, they develop more comorbidities, they become more frail, and they are more sensitive to the effects and side effects of drugs.
The guidance covers potentially inappropriate medications for older adults. The word “potentially” is important because this is guidance. As clinicians, we make decisions involving individuals. This guidance should be used with judgment, integrating the clinical context of the individual patient.
There is a lot in this guidance. I am going to try to cover what I feel are the most important points.
Aspirin. Since the risk for major bleeding increases with age, for primary prevention of atherosclerotic cardiovascular disease, the harm can be greater than the benefit in older adults, so aspirin should not be used for primary prevention. Aspirin remains indicated for secondary prevention in individuals with established cardiovascular disease.
Warfarin. For treatment of atrial fibrillation or venous thromboembolism (deep vein thrombosis or pulmonary embolism), warfarin should be avoided if possible. Warfarin has a higher risk for major bleeding, particularly intracranial bleeding, than direct oral anticoagulants (DOACs); therefore the latter are preferred. Rivaroxaban should be avoided, as it has a higher risk for major bleeding in older adults than the other DOACs. Apixaban is preferred over dabigatran. If a patient is well controlled on warfarin, you can consider continuing that treatment.
Antipsychotics. These include first- and second-generation antipsychotics such as aripiprazole, haloperidol, olanzapine, quetiapine, risperidone, and others. The guidance says to avoid these agents except for FDA-approved indications such as schizophrenia, bipolar disorder, and adjuvant treatment of depression. Use of these antipsychotics can increase risk for stroke, heart attack, and mortality. Essentially, the guidance says do not use these medications lightly for the treatment of agitated dementia. For those of us with older patients, this can get tricky because agitated dementia is a difficult issue for which there are no good effective medications. The Beers guidance recognizes this in saying that these medications should be avoided unless behavioral interventions have failed. So, there are times where you may need to use these medicines, but use them judiciously.
For patients with dementia, anticholinergics, antipsychotics, and benzodiazepines should be avoided if possible.
Benzodiazepines. Benzodiazepines should also be avoided because older adults have increased sensitivity to their effects due to slower metabolism and clearance of these medications, which can lead to a much longer half-life and higher serum level. In older adults, benzodiazepines increase the risk for cognitive impairment, delirium, falls, fractures, and even motor accidents. The same concerns affect the group of non-benzodiazepine sleeping medicines known as “Z-drugs.”
Nonsteroidal anti-inflammatory drugs (NSAIDs). Used frequently in our practices, NSAIDs are nevertheless on the list. As we think through the risk-benefit ratio of using NSAIDs in older adults, we often underappreciate the risks of these agents. Upper gastrointestinal ulcers with bleeding occur in approximately 1% of patients treated for 3-6 months with an NSAID and in 2%-4% of patients treated for a year. NSAIDs also increase the risk for renal impairment and cardiovascular disease.
Other medications to avoid (if possible). These include:
Sulfonylureas, due to a high risk for hypoglycemia. A short-acting sulfonylurea, such as glipizide, should be used if one is needed.
Proton pump inhibitors should not be used long-term if it can be avoided.
Digoxin should not be first-line treatment for atrial fibrillation or heart failure. Decreased renal clearance in older adults can lead to toxic levels of digoxin, particularly during acute illnesses. Avoid doses > 0.125 mg/day.
Nitrofurantoin should be avoided when the patient’s creatinine clearance is < 30 or for long-term suppressive therapy.
Avoid combining medications that have high anticholinergic side effects, such as scopolamine, diphenhydramine, oxybutynin, cyclobenzaprine, and others.
It is always important to understand the benefits and the risks of the drugs we prescribe. It is also important to remember that older adults are a particularly vulnerable population. The Beers criteria provide important guidance, which we can then use to make decisions about medicines for individual patients.
Dr. Skolnik is a professor in the department of family medicine at Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director in the department of family medicine at Abington (Pa.) Jefferson Health. He disclosed ties with AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GSK, Merck, Sanofi, Sanofi Pasteur, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
These criteria have been updated and revised approximately every 5 years since 1991 and serve to alert us to medications for which the risk-benefit ratio is not as good in older adults as in the rest of the population.
These are important criteria because medications are metabolized differently in older adults and have different effects compared with younger patients. For the sake of these criteria, older adults are 65 years of age or older. That said, we know that everyone from 65 to 100 is not the same. As people age, they develop more comorbidities, they become more frail, and they are more sensitive to the effects and side effects of drugs.
The guidance covers potentially inappropriate medications for older adults. The word “potentially” is important because this is guidance. As clinicians, we make decisions involving individuals. This guidance should be used with judgment, integrating the clinical context of the individual patient.
There is a lot in this guidance. I am going to try to cover what I feel are the most important points.
Aspirin. Since the risk for major bleeding increases with age, for primary prevention of atherosclerotic cardiovascular disease, the harm can be greater than the benefit in older adults, so aspirin should not be used for primary prevention. Aspirin remains indicated for secondary prevention in individuals with established cardiovascular disease.
Warfarin. For treatment of atrial fibrillation or venous thromboembolism (deep vein thrombosis or pulmonary embolism), warfarin should be avoided if possible. Warfarin has a higher risk for major bleeding, particularly intracranial bleeding, than direct oral anticoagulants (DOACs); therefore the latter are preferred. Rivaroxaban should be avoided, as it has a higher risk for major bleeding in older adults than the other DOACs. Apixaban is preferred over dabigatran. If a patient is well controlled on warfarin, you can consider continuing that treatment.
Antipsychotics. These include first- and second-generation antipsychotics such as aripiprazole, haloperidol, olanzapine, quetiapine, risperidone, and others. The guidance says to avoid these agents except for FDA-approved indications such as schizophrenia, bipolar disorder, and adjuvant treatment of depression. Use of these antipsychotics can increase risk for stroke, heart attack, and mortality. Essentially, the guidance says do not use these medications lightly for the treatment of agitated dementia. For those of us with older patients, this can get tricky because agitated dementia is a difficult issue for which there are no good effective medications. The Beers guidance recognizes this in saying that these medications should be avoided unless behavioral interventions have failed. So, there are times where you may need to use these medicines, but use them judiciously.
For patients with dementia, anticholinergics, antipsychotics, and benzodiazepines should be avoided if possible.
Benzodiazepines. Benzodiazepines should also be avoided because older adults have increased sensitivity to their effects due to slower metabolism and clearance of these medications, which can lead to a much longer half-life and higher serum level. In older adults, benzodiazepines increase the risk for cognitive impairment, delirium, falls, fractures, and even motor accidents. The same concerns affect the group of non-benzodiazepine sleeping medicines known as “Z-drugs.”
Nonsteroidal anti-inflammatory drugs (NSAIDs). Used frequently in our practices, NSAIDs are nevertheless on the list. As we think through the risk-benefit ratio of using NSAIDs in older adults, we often underappreciate the risks of these agents. Upper gastrointestinal ulcers with bleeding occur in approximately 1% of patients treated for 3-6 months with an NSAID and in 2%-4% of patients treated for a year. NSAIDs also increase the risk for renal impairment and cardiovascular disease.
Other medications to avoid (if possible). These include:
Sulfonylureas, due to a high risk for hypoglycemia. A short-acting sulfonylurea, such as glipizide, should be used if one is needed.
Proton pump inhibitors should not be used long-term if it can be avoided.
Digoxin should not be first-line treatment for atrial fibrillation or heart failure. Decreased renal clearance in older adults can lead to toxic levels of digoxin, particularly during acute illnesses. Avoid doses > 0.125 mg/day.
Nitrofurantoin should be avoided when the patient’s creatinine clearance is < 30 or for long-term suppressive therapy.
Avoid combining medications that have high anticholinergic side effects, such as scopolamine, diphenhydramine, oxybutynin, cyclobenzaprine, and others.
It is always important to understand the benefits and the risks of the drugs we prescribe. It is also important to remember that older adults are a particularly vulnerable population. The Beers criteria provide important guidance, which we can then use to make decisions about medicines for individual patients.
Dr. Skolnik is a professor in the department of family medicine at Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director in the department of family medicine at Abington (Pa.) Jefferson Health. He disclosed ties with AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GSK, Merck, Sanofi, Sanofi Pasteur, and Teva.
A version of this article appeared on Medscape.com.
Prior authorizations interfere with recommended cancer care
Of 178 respondents with a prior authorization (PA) experience, 39 (22%) did not receive the care recommended by their treatment team because of a PA requirement, and 123 (69%) experienced a delay in receiving the recommended care, Fumiko Chino, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues reported.
Reasons for not receiving recommended care included complete denial by the insurance company (26 of 39 patients), and a change in treatment plan because of initial denial (13 of 39 patients). Delays in receiving recommended care were 2 or more weeks for 90 of 123 patients, and 1 month or more in 40 of 123 patients.
Delays in receiving recommended care were associated with increased patient anxiety, a negative perception of the PA process, and patient administrative burden, the investigators noted.
The findings, which capture patient-based perspectives in the ongoing PA debacle, were reported online in JAMA Network Open.
“Prior authorization requires clinicians and patients to navigate a complex approval pathway. Resultant delays and denial can be particularly problematic for patients with cancer, who often need urgent treatment or symptom management,” the investigators explained. “Focusing on patient experiences with PA highlights a missing perspective in policy discussions and suggests another potential factor associated with eroding trust in the health care system.”
To assess the impact of PA, they conducted an anonymous survey using a convenience sample of patients with any cancer-related PA experience from July 1 to Oct. 6, 2022. Mean self-reported PA-related anxiety scores were 74.7 on a scale of 0-100, whereas usual anxiety scores were 37.5.
PA-related anxiety scores were significantly correlated with the length of treatment delay (P = .04), time spent on PA (P < .001), and overall PA experience (P < .001).
“Dealing with PA issues adds an extra layer of stress, which is known to increase anxiety and can worsen treatment-related and disease-related symptoms and adverse effects,” the investigators noted.
PA issues also eroded trust: 89% of respondents trusted their insurance company less, and 83% trusted the health care system less after a PA experience. Patient involvement in the PA process increased the likelihood of such distrust and of having a negative experience.
Of the 178 respondents, most were women (88%), non-Hispanic White individuals (84%), college graduates (84%), and young (18-39 years, 41%; 40-54 years, 33%). Most (67%) had to personally become involved in the PA process by calling their insurance or filing an appeal.
The investigators noted that “efforts to create national health policy solutions that streamline PA and make the process more transparent have been a major lobbying effort of large oncology societies,” and that bipartisan legislation to “establish regulations on the quality and timeliness of PA in the Medicare Advantage population” has stalled.
“In the meantime, the Centers for Medicare & Medicaid Services acted directly by issuing a final rule in April 2023 aimed at improving PA processes within the Medicare Advantage population by 2024,” they wrote, adding that “streamlining the PA process is key to optimizing the quality of care delivered and improving patients’ experience with cancer care.
“Policy interventions will be necessary to reform the PA process, as will advocacy efforts at the patient, clinician, and hospital level,” they concluded.
Chino reported funding through a National Institutes of Health/National Cancer Institute Cancer Center Support Grant.
Of 178 respondents with a prior authorization (PA) experience, 39 (22%) did not receive the care recommended by their treatment team because of a PA requirement, and 123 (69%) experienced a delay in receiving the recommended care, Fumiko Chino, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues reported.
Reasons for not receiving recommended care included complete denial by the insurance company (26 of 39 patients), and a change in treatment plan because of initial denial (13 of 39 patients). Delays in receiving recommended care were 2 or more weeks for 90 of 123 patients, and 1 month or more in 40 of 123 patients.
Delays in receiving recommended care were associated with increased patient anxiety, a negative perception of the PA process, and patient administrative burden, the investigators noted.
The findings, which capture patient-based perspectives in the ongoing PA debacle, were reported online in JAMA Network Open.
“Prior authorization requires clinicians and patients to navigate a complex approval pathway. Resultant delays and denial can be particularly problematic for patients with cancer, who often need urgent treatment or symptom management,” the investigators explained. “Focusing on patient experiences with PA highlights a missing perspective in policy discussions and suggests another potential factor associated with eroding trust in the health care system.”
To assess the impact of PA, they conducted an anonymous survey using a convenience sample of patients with any cancer-related PA experience from July 1 to Oct. 6, 2022. Mean self-reported PA-related anxiety scores were 74.7 on a scale of 0-100, whereas usual anxiety scores were 37.5.
PA-related anxiety scores were significantly correlated with the length of treatment delay (P = .04), time spent on PA (P < .001), and overall PA experience (P < .001).
“Dealing with PA issues adds an extra layer of stress, which is known to increase anxiety and can worsen treatment-related and disease-related symptoms and adverse effects,” the investigators noted.
PA issues also eroded trust: 89% of respondents trusted their insurance company less, and 83% trusted the health care system less after a PA experience. Patient involvement in the PA process increased the likelihood of such distrust and of having a negative experience.
Of the 178 respondents, most were women (88%), non-Hispanic White individuals (84%), college graduates (84%), and young (18-39 years, 41%; 40-54 years, 33%). Most (67%) had to personally become involved in the PA process by calling their insurance or filing an appeal.
The investigators noted that “efforts to create national health policy solutions that streamline PA and make the process more transparent have been a major lobbying effort of large oncology societies,” and that bipartisan legislation to “establish regulations on the quality and timeliness of PA in the Medicare Advantage population” has stalled.
“In the meantime, the Centers for Medicare & Medicaid Services acted directly by issuing a final rule in April 2023 aimed at improving PA processes within the Medicare Advantage population by 2024,” they wrote, adding that “streamlining the PA process is key to optimizing the quality of care delivered and improving patients’ experience with cancer care.
“Policy interventions will be necessary to reform the PA process, as will advocacy efforts at the patient, clinician, and hospital level,” they concluded.
Chino reported funding through a National Institutes of Health/National Cancer Institute Cancer Center Support Grant.
Of 178 respondents with a prior authorization (PA) experience, 39 (22%) did not receive the care recommended by their treatment team because of a PA requirement, and 123 (69%) experienced a delay in receiving the recommended care, Fumiko Chino, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues reported.
Reasons for not receiving recommended care included complete denial by the insurance company (26 of 39 patients), and a change in treatment plan because of initial denial (13 of 39 patients). Delays in receiving recommended care were 2 or more weeks for 90 of 123 patients, and 1 month or more in 40 of 123 patients.
Delays in receiving recommended care were associated with increased patient anxiety, a negative perception of the PA process, and patient administrative burden, the investigators noted.
The findings, which capture patient-based perspectives in the ongoing PA debacle, were reported online in JAMA Network Open.
“Prior authorization requires clinicians and patients to navigate a complex approval pathway. Resultant delays and denial can be particularly problematic for patients with cancer, who often need urgent treatment or symptom management,” the investigators explained. “Focusing on patient experiences with PA highlights a missing perspective in policy discussions and suggests another potential factor associated with eroding trust in the health care system.”
To assess the impact of PA, they conducted an anonymous survey using a convenience sample of patients with any cancer-related PA experience from July 1 to Oct. 6, 2022. Mean self-reported PA-related anxiety scores were 74.7 on a scale of 0-100, whereas usual anxiety scores were 37.5.
PA-related anxiety scores were significantly correlated with the length of treatment delay (P = .04), time spent on PA (P < .001), and overall PA experience (P < .001).
“Dealing with PA issues adds an extra layer of stress, which is known to increase anxiety and can worsen treatment-related and disease-related symptoms and adverse effects,” the investigators noted.
PA issues also eroded trust: 89% of respondents trusted their insurance company less, and 83% trusted the health care system less after a PA experience. Patient involvement in the PA process increased the likelihood of such distrust and of having a negative experience.
Of the 178 respondents, most were women (88%), non-Hispanic White individuals (84%), college graduates (84%), and young (18-39 years, 41%; 40-54 years, 33%). Most (67%) had to personally become involved in the PA process by calling their insurance or filing an appeal.
The investigators noted that “efforts to create national health policy solutions that streamline PA and make the process more transparent have been a major lobbying effort of large oncology societies,” and that bipartisan legislation to “establish regulations on the quality and timeliness of PA in the Medicare Advantage population” has stalled.
“In the meantime, the Centers for Medicare & Medicaid Services acted directly by issuing a final rule in April 2023 aimed at improving PA processes within the Medicare Advantage population by 2024,” they wrote, adding that “streamlining the PA process is key to optimizing the quality of care delivered and improving patients’ experience with cancer care.
“Policy interventions will be necessary to reform the PA process, as will advocacy efforts at the patient, clinician, and hospital level,” they concluded.
Chino reported funding through a National Institutes of Health/National Cancer Institute Cancer Center Support Grant.
FROM JAMA NETWORK OPEN
Children with sickle cell disease at risk for vision loss
Clinicians must monitor children with sickle cell disease for eye complications as much as they do for adults, a new research review suggests.
Earlier research indicated that older patients were more at risk for eye complications from sickle cell disease, but the new study found that a full third of young people aged 10-25 years with sickle cell disease had retinopathy, including nonproliferative retinopathy (33%) and proliferative retinopathy (6%), which can progress to vision loss.
Two patients experienced retinal detachment, while two suffered retinal artery occlusion. One patient with retinal artery occlusion lost their vision and had a final best-corrected visual acuity of 20/60, according to the researchers, who presented their findings at the annual meeting of the American Academy of Ophthalmology.
“Our data underscores the need for patients – including pediatric patients – with sickle cell disease to get routine ophthalmic screenings along with appropriate systemic and ophthalmic treatment,” Mary Ellen Hoehn, MD, a professor of ophthalmology at the University of Tennessee Health Science Center, Memphis, who led the research, said in a press release.
The review covered records for 652 patients with sickle cell disease aged 10-25 years (median age, 14 years), who underwent eye exams over a 12-year period.
Besides looking at rates of retinopathy, Dr. Hoehn’s group studied which treatments were most effective. They found that hydroxyurea and chronic transfusions best lowered retinopathy rates among all genotypes.
“We hope that people will use this information to better care for patients with sickle cell disease, and that more timely ophthalmic screen exams will be performed so that vision-threatening complications from this disease are prevented,” Dr. Hoehn said.
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians must monitor children with sickle cell disease for eye complications as much as they do for adults, a new research review suggests.
Earlier research indicated that older patients were more at risk for eye complications from sickle cell disease, but the new study found that a full third of young people aged 10-25 years with sickle cell disease had retinopathy, including nonproliferative retinopathy (33%) and proliferative retinopathy (6%), which can progress to vision loss.
Two patients experienced retinal detachment, while two suffered retinal artery occlusion. One patient with retinal artery occlusion lost their vision and had a final best-corrected visual acuity of 20/60, according to the researchers, who presented their findings at the annual meeting of the American Academy of Ophthalmology.
“Our data underscores the need for patients – including pediatric patients – with sickle cell disease to get routine ophthalmic screenings along with appropriate systemic and ophthalmic treatment,” Mary Ellen Hoehn, MD, a professor of ophthalmology at the University of Tennessee Health Science Center, Memphis, who led the research, said in a press release.
The review covered records for 652 patients with sickle cell disease aged 10-25 years (median age, 14 years), who underwent eye exams over a 12-year period.
Besides looking at rates of retinopathy, Dr. Hoehn’s group studied which treatments were most effective. They found that hydroxyurea and chronic transfusions best lowered retinopathy rates among all genotypes.
“We hope that people will use this information to better care for patients with sickle cell disease, and that more timely ophthalmic screen exams will be performed so that vision-threatening complications from this disease are prevented,” Dr. Hoehn said.
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians must monitor children with sickle cell disease for eye complications as much as they do for adults, a new research review suggests.
Earlier research indicated that older patients were more at risk for eye complications from sickle cell disease, but the new study found that a full third of young people aged 10-25 years with sickle cell disease had retinopathy, including nonproliferative retinopathy (33%) and proliferative retinopathy (6%), which can progress to vision loss.
Two patients experienced retinal detachment, while two suffered retinal artery occlusion. One patient with retinal artery occlusion lost their vision and had a final best-corrected visual acuity of 20/60, according to the researchers, who presented their findings at the annual meeting of the American Academy of Ophthalmology.
“Our data underscores the need for patients – including pediatric patients – with sickle cell disease to get routine ophthalmic screenings along with appropriate systemic and ophthalmic treatment,” Mary Ellen Hoehn, MD, a professor of ophthalmology at the University of Tennessee Health Science Center, Memphis, who led the research, said in a press release.
The review covered records for 652 patients with sickle cell disease aged 10-25 years (median age, 14 years), who underwent eye exams over a 12-year period.
Besides looking at rates of retinopathy, Dr. Hoehn’s group studied which treatments were most effective. They found that hydroxyurea and chronic transfusions best lowered retinopathy rates among all genotypes.
“We hope that people will use this information to better care for patients with sickle cell disease, and that more timely ophthalmic screen exams will be performed so that vision-threatening complications from this disease are prevented,” Dr. Hoehn said.
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAO 2023