User login
Not enough evidence for primary care to routinely conduct dental screenings
Routine screenings for signs of cavities and gum disease by primary care clinicians may not catch patients most at risk of these conditions, according to a statement by the U.S. Preventive Services Task Force (USPSTF) that was published in JAMA.
Suggesting ways to improve oral health also may fail to engage the patients who most need the message, the group said in its statement.
The task force is not suggesting that primary care providers stop all oral health screening of adults or that they never discuss ways to improve oral health. But the current evidence of the most effective oral health screenings or enhancement strategies in primary care settings received an “I” rating, for “Inconclusive.” The highest ranking a screening can receive is an “A” or “B,” which indicate that there is strong evidence for conducting a screening, while a “C” would indicate that clinicians could rarely provide a screening, and a “D” would indicate not to, given the current evidence.
Primary care clinicians should immediately refer any patients with apparent caries or gum disease to a dentist, the USPSTF noted. But what clinicians should do for patients who have no obvious oral health problems is up for debate.
“The ‘I’ is a note about where the evidence is at this point and then a call for more research to see if we can’t get some more clarity for next time,” said John Ruiz, PhD, professor of clinical psychology at the University of Arizona, Tucson, who is a member of the task force.
More than 90% of U.S. adults may have caries, including 26% with untreated caries that can cause serious infections or tooth loss. In addition, 42% of adults have some type of gum disease. More than two-thirds of Americans aged 65 or older have gum disease, and it is the leading cause of tooth loss in this population. People earning low incomes and those who do not have health insurance or who belong to a marginalized racial or ethnic group are at greater risk of the harms of caries and gum disease.
“Oral health care is important to overall health,” and any new research on oral health screening and enhancement efforts should be demographically representative of adults affected by these conditions, Dr. Ruiz said.
In an accompanying editorial, oral health researchers from the National Institutes of Health and the University of California, San Francisco, echoed the call for representative research and encouraged closer collaboration between primary care providers and dentists to promote oral health.
“Oral health screening and referral by medical primary care clinicians can help ensure that individuals get to the dental chair to receive needed interventions that can benefit both oral and potentially overall health,” the authors wrote. “Likewise, medical challenges and oral mucosal manifestations of chronic health conditions detected at a dental visit should result in medical referral, allowing prompt evaluation and treatment.”
Lack of data
The USPSTF defined oral health screenings for patients older than 18 who have no obvious signs of caries or gum disease as looking at a patient’s mouth during physical exams. Additionally, clinicians might use prediction models to identify patients at greater risk of facing these problems.
Strategies to improve oral health include providing encouragement to patients to reduce intake of refined sugar, to floss and brush effectively to reduce bacteria, and to use fluoride gels, fluoride varnishes, or other kinds of sealants to make caries harder to form.
A literature review found that there has been limited analysis of primary care clinicians performing these tasks. Perhaps unsurprisingly, more such studies about dentists existed, leaving an open field for dedicated studies about what primary care clinicians should do to optimize oral health with patients.
“Clinicians, in the absence of clear guidelines, should continue to use their best judgment,” Dr. Ruiz said.
One dentist interviewed said screening could be as simple as doctors asking patients how often they brush their teeth and giving patients a toothbrush as part of the office visit.
“It all comes down to, ‘Is the person brushing their teeth?’ ” said Jennifer Hartshorn, DDS, who specializes in community and preventive dentistry at the University of Iowa, Iowa City.
“By all means look in their mouth, ask how much they are brushing, and urge them to find a dental home if at all possible,” Dr. Hartshorn said, especially for patients who smoke or have conditions such as dry mouth, which can increase the risk of oral disease.
Dr. Ruiz and Dr. Hartshorn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Routine screenings for signs of cavities and gum disease by primary care clinicians may not catch patients most at risk of these conditions, according to a statement by the U.S. Preventive Services Task Force (USPSTF) that was published in JAMA.
Suggesting ways to improve oral health also may fail to engage the patients who most need the message, the group said in its statement.
The task force is not suggesting that primary care providers stop all oral health screening of adults or that they never discuss ways to improve oral health. But the current evidence of the most effective oral health screenings or enhancement strategies in primary care settings received an “I” rating, for “Inconclusive.” The highest ranking a screening can receive is an “A” or “B,” which indicate that there is strong evidence for conducting a screening, while a “C” would indicate that clinicians could rarely provide a screening, and a “D” would indicate not to, given the current evidence.
Primary care clinicians should immediately refer any patients with apparent caries or gum disease to a dentist, the USPSTF noted. But what clinicians should do for patients who have no obvious oral health problems is up for debate.
“The ‘I’ is a note about where the evidence is at this point and then a call for more research to see if we can’t get some more clarity for next time,” said John Ruiz, PhD, professor of clinical psychology at the University of Arizona, Tucson, who is a member of the task force.
More than 90% of U.S. adults may have caries, including 26% with untreated caries that can cause serious infections or tooth loss. In addition, 42% of adults have some type of gum disease. More than two-thirds of Americans aged 65 or older have gum disease, and it is the leading cause of tooth loss in this population. People earning low incomes and those who do not have health insurance or who belong to a marginalized racial or ethnic group are at greater risk of the harms of caries and gum disease.
“Oral health care is important to overall health,” and any new research on oral health screening and enhancement efforts should be demographically representative of adults affected by these conditions, Dr. Ruiz said.
In an accompanying editorial, oral health researchers from the National Institutes of Health and the University of California, San Francisco, echoed the call for representative research and encouraged closer collaboration between primary care providers and dentists to promote oral health.
“Oral health screening and referral by medical primary care clinicians can help ensure that individuals get to the dental chair to receive needed interventions that can benefit both oral and potentially overall health,” the authors wrote. “Likewise, medical challenges and oral mucosal manifestations of chronic health conditions detected at a dental visit should result in medical referral, allowing prompt evaluation and treatment.”
Lack of data
The USPSTF defined oral health screenings for patients older than 18 who have no obvious signs of caries or gum disease as looking at a patient’s mouth during physical exams. Additionally, clinicians might use prediction models to identify patients at greater risk of facing these problems.
Strategies to improve oral health include providing encouragement to patients to reduce intake of refined sugar, to floss and brush effectively to reduce bacteria, and to use fluoride gels, fluoride varnishes, or other kinds of sealants to make caries harder to form.
A literature review found that there has been limited analysis of primary care clinicians performing these tasks. Perhaps unsurprisingly, more such studies about dentists existed, leaving an open field for dedicated studies about what primary care clinicians should do to optimize oral health with patients.
“Clinicians, in the absence of clear guidelines, should continue to use their best judgment,” Dr. Ruiz said.
One dentist interviewed said screening could be as simple as doctors asking patients how often they brush their teeth and giving patients a toothbrush as part of the office visit.
“It all comes down to, ‘Is the person brushing their teeth?’ ” said Jennifer Hartshorn, DDS, who specializes in community and preventive dentistry at the University of Iowa, Iowa City.
“By all means look in their mouth, ask how much they are brushing, and urge them to find a dental home if at all possible,” Dr. Hartshorn said, especially for patients who smoke or have conditions such as dry mouth, which can increase the risk of oral disease.
Dr. Ruiz and Dr. Hartshorn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Routine screenings for signs of cavities and gum disease by primary care clinicians may not catch patients most at risk of these conditions, according to a statement by the U.S. Preventive Services Task Force (USPSTF) that was published in JAMA.
Suggesting ways to improve oral health also may fail to engage the patients who most need the message, the group said in its statement.
The task force is not suggesting that primary care providers stop all oral health screening of adults or that they never discuss ways to improve oral health. But the current evidence of the most effective oral health screenings or enhancement strategies in primary care settings received an “I” rating, for “Inconclusive.” The highest ranking a screening can receive is an “A” or “B,” which indicate that there is strong evidence for conducting a screening, while a “C” would indicate that clinicians could rarely provide a screening, and a “D” would indicate not to, given the current evidence.
Primary care clinicians should immediately refer any patients with apparent caries or gum disease to a dentist, the USPSTF noted. But what clinicians should do for patients who have no obvious oral health problems is up for debate.
“The ‘I’ is a note about where the evidence is at this point and then a call for more research to see if we can’t get some more clarity for next time,” said John Ruiz, PhD, professor of clinical psychology at the University of Arizona, Tucson, who is a member of the task force.
More than 90% of U.S. adults may have caries, including 26% with untreated caries that can cause serious infections or tooth loss. In addition, 42% of adults have some type of gum disease. More than two-thirds of Americans aged 65 or older have gum disease, and it is the leading cause of tooth loss in this population. People earning low incomes and those who do not have health insurance or who belong to a marginalized racial or ethnic group are at greater risk of the harms of caries and gum disease.
“Oral health care is important to overall health,” and any new research on oral health screening and enhancement efforts should be demographically representative of adults affected by these conditions, Dr. Ruiz said.
In an accompanying editorial, oral health researchers from the National Institutes of Health and the University of California, San Francisco, echoed the call for representative research and encouraged closer collaboration between primary care providers and dentists to promote oral health.
“Oral health screening and referral by medical primary care clinicians can help ensure that individuals get to the dental chair to receive needed interventions that can benefit both oral and potentially overall health,” the authors wrote. “Likewise, medical challenges and oral mucosal manifestations of chronic health conditions detected at a dental visit should result in medical referral, allowing prompt evaluation and treatment.”
Lack of data
The USPSTF defined oral health screenings for patients older than 18 who have no obvious signs of caries or gum disease as looking at a patient’s mouth during physical exams. Additionally, clinicians might use prediction models to identify patients at greater risk of facing these problems.
Strategies to improve oral health include providing encouragement to patients to reduce intake of refined sugar, to floss and brush effectively to reduce bacteria, and to use fluoride gels, fluoride varnishes, or other kinds of sealants to make caries harder to form.
A literature review found that there has been limited analysis of primary care clinicians performing these tasks. Perhaps unsurprisingly, more such studies about dentists existed, leaving an open field for dedicated studies about what primary care clinicians should do to optimize oral health with patients.
“Clinicians, in the absence of clear guidelines, should continue to use their best judgment,” Dr. Ruiz said.
One dentist interviewed said screening could be as simple as doctors asking patients how often they brush their teeth and giving patients a toothbrush as part of the office visit.
“It all comes down to, ‘Is the person brushing their teeth?’ ” said Jennifer Hartshorn, DDS, who specializes in community and preventive dentistry at the University of Iowa, Iowa City.
“By all means look in their mouth, ask how much they are brushing, and urge them to find a dental home if at all possible,” Dr. Hartshorn said, especially for patients who smoke or have conditions such as dry mouth, which can increase the risk of oral disease.
Dr. Ruiz and Dr. Hartshorn report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Report cards, additional observer improve adenoma detection rate
Although multimodal interventions like extra training with periodic feedback showed some signs of improving ADR, withdrawal time monitoring was not significantly associated with a better detection rate, reported Anshul Arora, MD, of Western University, London, Ont., and colleagues.
“Given the increased risk of postcolonoscopy colorectal cancer associated with low ADR, improving [this performance metric] has become a major focus for quality improvement,” the investigators wrote in Clinical Gastroenterology and Hepatology.
They noted that “numerous strategies” have been evaluated for this purpose, which may be sorted into three groups: endoscopy unit–level interventions (i.e., system changes), procedure-targeted interventions (i.e., technique changes), and technology-based interventions.
“Of these categories, endoscopy unit–level interventions are perhaps the easiest to implement widely because they generally require fewer changes in the technical aspect of how a colonoscopy is performed,” the investigators wrote. “Thus, the objective of this study was to conduct a systematic review and meta-analysis to identify endoscopy unit–level interventions aimed at improving ADRs and their effectiveness.”
To this end, Dr. Arora and colleagues analyzed data from 34 randomized controlled trials and observational studies involving 1,501 endoscopists and 371,041 procedures. They evaluated the relationship between ADR and implementation of four interventions: a performance report card, a multimodal intervention (e.g., training sessions with periodic feedback), presence of an additional observer, and withdrawal time monitoring.
Provision of report cards was associated with the greatest improvement in ADR, at 28% (odds ratio, 1.28; 95% confidence interval, 1.13-1.45; P less than .001), followed by presence of an additional observer, which bumped ADR by 25% (OR, 1.25; 95% CI, 1.09-1.43; P = .002). The impact of multimodal interventions was “borderline significant,” the investigators wrote, with an 18% improvement in ADR (OR, 1.18; 95% CI, 1.00-1.40; P = .05). In contrast, withdrawal time monitoring showed no significant benefit (OR, 1.35; 95% CI, 0.93-1.96; P = .11).
In their discussion, Dr. Arora and colleagues offered guidance on the use of report cards, which were associated with the greatest improvement in ADR.
“We found that benchmarking individual endoscopists against their peers was important for improving ADR performance because this was the common thread among all report card–based interventions,” they wrote. “In terms of the method of delivery for feedback, only one study used public reporting of colonoscopy quality indicators, whereas the rest delivered report cards privately to physicians. This suggests that confidential feedback did not impede self-improvement, which is desirable to avoid stigmatization of low ADR performers.”
The findings also suggest that additional observers can boost ADR without specialized training.
“[The benefit of an additional observer] may be explained by the presence of a second set of eyes to identify polyps or, more pragmatically, by the Hawthorne effect, whereby endoscopists may be more careful because they know someone else is watching the screen,” the investigators wrote. “Regardless, extra training for the observer does not seem to be necessary because the three RCTs [evaluating this intervention] all used endoscopy nurses who did not receive any additional polyp detection training. Thus, endoscopy unit nurses should be encouraged to speak up should they see a polyp the endoscopist missed.”
The investigators disclosed no conflicts of interest.
The effectiveness of colonoscopy to prevent colorectal cancer depends on the quality of the exam. Adenoma detection rate (ADR) is a validated quality indicator, associated with lower risk of postcolonoscopy colorectal cancer. There are multiple interventions that can improve endoscopists’ ADR, but it is unclear which ones are higher yield than others. This study summarizes the existing studies on various interventions and finds the largest increase in ADR with the use of physician report cards. This is not surprising, as report cards both provide measurement and are an intervention for improvement.
Interestingly the included studies mostly used individual confidential report cards, and demonstrated an improvement in ADR. Having a second set of eyes looking at the monitor was also associated with increase in ADR. Whether it’s the observer picking up missed polyps, or the endoscopist doing a more thorough exam because someone else is watching the screen, is unclear. This is the same principle that current computer assisted detection (CADe) devices help with. While having a second observer may not be practical or cost effective, and CADe is expensive, the take-away is that there are multiple ways to improve ADR, and at the very least every physician should be receiving report cards or feedback on their quality indicators and working towards achieving and exceeding the minimum benchmarks.
Aasma Shaukat, MD, MPH, is the Robert M. and Mary H. Glickman professor of medicine, New York University Grossman School of Medicine where she also holds a professorship in population health. She serves as director of outcomes research in the division of gastroenterology and hepatology, and codirector of Translational Research Education and Careers (TREC). She disclosed serving as an adviser for Motus-GI and Iterative Health.
The effectiveness of colonoscopy to prevent colorectal cancer depends on the quality of the exam. Adenoma detection rate (ADR) is a validated quality indicator, associated with lower risk of postcolonoscopy colorectal cancer. There are multiple interventions that can improve endoscopists’ ADR, but it is unclear which ones are higher yield than others. This study summarizes the existing studies on various interventions and finds the largest increase in ADR with the use of physician report cards. This is not surprising, as report cards both provide measurement and are an intervention for improvement.
Interestingly the included studies mostly used individual confidential report cards, and demonstrated an improvement in ADR. Having a second set of eyes looking at the monitor was also associated with increase in ADR. Whether it’s the observer picking up missed polyps, or the endoscopist doing a more thorough exam because someone else is watching the screen, is unclear. This is the same principle that current computer assisted detection (CADe) devices help with. While having a second observer may not be practical or cost effective, and CADe is expensive, the take-away is that there are multiple ways to improve ADR, and at the very least every physician should be receiving report cards or feedback on their quality indicators and working towards achieving and exceeding the minimum benchmarks.
Aasma Shaukat, MD, MPH, is the Robert M. and Mary H. Glickman professor of medicine, New York University Grossman School of Medicine where she also holds a professorship in population health. She serves as director of outcomes research in the division of gastroenterology and hepatology, and codirector of Translational Research Education and Careers (TREC). She disclosed serving as an adviser for Motus-GI and Iterative Health.
The effectiveness of colonoscopy to prevent colorectal cancer depends on the quality of the exam. Adenoma detection rate (ADR) is a validated quality indicator, associated with lower risk of postcolonoscopy colorectal cancer. There are multiple interventions that can improve endoscopists’ ADR, but it is unclear which ones are higher yield than others. This study summarizes the existing studies on various interventions and finds the largest increase in ADR with the use of physician report cards. This is not surprising, as report cards both provide measurement and are an intervention for improvement.
Interestingly the included studies mostly used individual confidential report cards, and demonstrated an improvement in ADR. Having a second set of eyes looking at the monitor was also associated with increase in ADR. Whether it’s the observer picking up missed polyps, or the endoscopist doing a more thorough exam because someone else is watching the screen, is unclear. This is the same principle that current computer assisted detection (CADe) devices help with. While having a second observer may not be practical or cost effective, and CADe is expensive, the take-away is that there are multiple ways to improve ADR, and at the very least every physician should be receiving report cards or feedback on their quality indicators and working towards achieving and exceeding the minimum benchmarks.
Aasma Shaukat, MD, MPH, is the Robert M. and Mary H. Glickman professor of medicine, New York University Grossman School of Medicine where she also holds a professorship in population health. She serves as director of outcomes research in the division of gastroenterology and hepatology, and codirector of Translational Research Education and Careers (TREC). She disclosed serving as an adviser for Motus-GI and Iterative Health.
Although multimodal interventions like extra training with periodic feedback showed some signs of improving ADR, withdrawal time monitoring was not significantly associated with a better detection rate, reported Anshul Arora, MD, of Western University, London, Ont., and colleagues.
“Given the increased risk of postcolonoscopy colorectal cancer associated with low ADR, improving [this performance metric] has become a major focus for quality improvement,” the investigators wrote in Clinical Gastroenterology and Hepatology.
They noted that “numerous strategies” have been evaluated for this purpose, which may be sorted into three groups: endoscopy unit–level interventions (i.e., system changes), procedure-targeted interventions (i.e., technique changes), and technology-based interventions.
“Of these categories, endoscopy unit–level interventions are perhaps the easiest to implement widely because they generally require fewer changes in the technical aspect of how a colonoscopy is performed,” the investigators wrote. “Thus, the objective of this study was to conduct a systematic review and meta-analysis to identify endoscopy unit–level interventions aimed at improving ADRs and their effectiveness.”
To this end, Dr. Arora and colleagues analyzed data from 34 randomized controlled trials and observational studies involving 1,501 endoscopists and 371,041 procedures. They evaluated the relationship between ADR and implementation of four interventions: a performance report card, a multimodal intervention (e.g., training sessions with periodic feedback), presence of an additional observer, and withdrawal time monitoring.
Provision of report cards was associated with the greatest improvement in ADR, at 28% (odds ratio, 1.28; 95% confidence interval, 1.13-1.45; P less than .001), followed by presence of an additional observer, which bumped ADR by 25% (OR, 1.25; 95% CI, 1.09-1.43; P = .002). The impact of multimodal interventions was “borderline significant,” the investigators wrote, with an 18% improvement in ADR (OR, 1.18; 95% CI, 1.00-1.40; P = .05). In contrast, withdrawal time monitoring showed no significant benefit (OR, 1.35; 95% CI, 0.93-1.96; P = .11).
In their discussion, Dr. Arora and colleagues offered guidance on the use of report cards, which were associated with the greatest improvement in ADR.
“We found that benchmarking individual endoscopists against their peers was important for improving ADR performance because this was the common thread among all report card–based interventions,” they wrote. “In terms of the method of delivery for feedback, only one study used public reporting of colonoscopy quality indicators, whereas the rest delivered report cards privately to physicians. This suggests that confidential feedback did not impede self-improvement, which is desirable to avoid stigmatization of low ADR performers.”
The findings also suggest that additional observers can boost ADR without specialized training.
“[The benefit of an additional observer] may be explained by the presence of a second set of eyes to identify polyps or, more pragmatically, by the Hawthorne effect, whereby endoscopists may be more careful because they know someone else is watching the screen,” the investigators wrote. “Regardless, extra training for the observer does not seem to be necessary because the three RCTs [evaluating this intervention] all used endoscopy nurses who did not receive any additional polyp detection training. Thus, endoscopy unit nurses should be encouraged to speak up should they see a polyp the endoscopist missed.”
The investigators disclosed no conflicts of interest.
Although multimodal interventions like extra training with periodic feedback showed some signs of improving ADR, withdrawal time monitoring was not significantly associated with a better detection rate, reported Anshul Arora, MD, of Western University, London, Ont., and colleagues.
“Given the increased risk of postcolonoscopy colorectal cancer associated with low ADR, improving [this performance metric] has become a major focus for quality improvement,” the investigators wrote in Clinical Gastroenterology and Hepatology.
They noted that “numerous strategies” have been evaluated for this purpose, which may be sorted into three groups: endoscopy unit–level interventions (i.e., system changes), procedure-targeted interventions (i.e., technique changes), and technology-based interventions.
“Of these categories, endoscopy unit–level interventions are perhaps the easiest to implement widely because they generally require fewer changes in the technical aspect of how a colonoscopy is performed,” the investigators wrote. “Thus, the objective of this study was to conduct a systematic review and meta-analysis to identify endoscopy unit–level interventions aimed at improving ADRs and their effectiveness.”
To this end, Dr. Arora and colleagues analyzed data from 34 randomized controlled trials and observational studies involving 1,501 endoscopists and 371,041 procedures. They evaluated the relationship between ADR and implementation of four interventions: a performance report card, a multimodal intervention (e.g., training sessions with periodic feedback), presence of an additional observer, and withdrawal time monitoring.
Provision of report cards was associated with the greatest improvement in ADR, at 28% (odds ratio, 1.28; 95% confidence interval, 1.13-1.45; P less than .001), followed by presence of an additional observer, which bumped ADR by 25% (OR, 1.25; 95% CI, 1.09-1.43; P = .002). The impact of multimodal interventions was “borderline significant,” the investigators wrote, with an 18% improvement in ADR (OR, 1.18; 95% CI, 1.00-1.40; P = .05). In contrast, withdrawal time monitoring showed no significant benefit (OR, 1.35; 95% CI, 0.93-1.96; P = .11).
In their discussion, Dr. Arora and colleagues offered guidance on the use of report cards, which were associated with the greatest improvement in ADR.
“We found that benchmarking individual endoscopists against their peers was important for improving ADR performance because this was the common thread among all report card–based interventions,” they wrote. “In terms of the method of delivery for feedback, only one study used public reporting of colonoscopy quality indicators, whereas the rest delivered report cards privately to physicians. This suggests that confidential feedback did not impede self-improvement, which is desirable to avoid stigmatization of low ADR performers.”
The findings also suggest that additional observers can boost ADR without specialized training.
“[The benefit of an additional observer] may be explained by the presence of a second set of eyes to identify polyps or, more pragmatically, by the Hawthorne effect, whereby endoscopists may be more careful because they know someone else is watching the screen,” the investigators wrote. “Regardless, extra training for the observer does not seem to be necessary because the three RCTs [evaluating this intervention] all used endoscopy nurses who did not receive any additional polyp detection training. Thus, endoscopy unit nurses should be encouraged to speak up should they see a polyp the endoscopist missed.”
The investigators disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Short aspirin therapy noninferior to DAPT for 1 year after PCI for ACS
SAN FRANCISCO – Stopping aspirin within 1 month of implanting a drug-eluting stent (DES) for acute coronary syndrome (ACS) followed by ticagrelor monotherapy was shown to be noninferior to 12 months of dual antiplatelet therapy (DAPT) in net adverse cardiovascular and bleeding events in the T-PASS trial.
of death, myocardial infarction, stent thrombosis, stroke, and major bleeding, primarily due to a significant reduction in bleeding events,” senior author Myeong-Ki Hong, MD, PhD, Yonsei University, Seoul, Korea, told attendees at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
“This study provides evidence that stopping aspirin within 1 month after implantation of drug-eluting stents for ticagrelor monotherapy is a reasonable alternative to 12-month DAPT as for adverse cardiovascular and bleeding events,” Dr. Hong concluded.
The study was published in Circulation ahead of print to coincide with the presentation.
Three months to 1 month
Previous trials (TICO and TWILIGHT) have shown that ticagrelor monotherapy after 3 months of DAPT can be safe and effectively prevent ischemic events after percutaneous coronary intervention (PCI) in ACS or high-risk PCI patients.
The current study aimed to investigate whether ticagrelor monotherapy after less than 1 month of DAPT was noninferior to 12 months of ticagrelor-based DAPT for preventing adverse cardiovascular and bleeding events in patients with ACS undergoing PCI with a DES implant.
T-PASS, carried out at 24 centers in Korea, enrolled ACS patients aged 19 years or older who received an ultrathin, bioresorbable polymer sirolimus-eluting stent (Orsiro, Biotronik). They were randomized 1:1 to ticagrelor monotherapy after less than 1 month of DAPT (n = 1,426) or to ticagrelor-based DAPT for 12 months (n = 1,424).
The primary outcome measure was net adverse clinical events (NACE) at 12 months, consisting of major bleeding plus major adverse cardiovascular events. All patients were included in the intention-to-treat analysis.
The study could enroll patients aged 19-80 years. It excluded anyone with active bleeding, at increased risk for bleeding, with anemia (hemoglobin ≤ 8 g/dL), platelets less than 100,000/mcL, need for oral anticoagulation therapy, current or potential pregnancy, or a life expectancy less than 1 year.
Baseline characteristics of the two groups were well balanced. The extended monotherapy and DAPT arms had an average age of 61 ± 10 years, were 84% and 83% male and had diabetes mellitus in 30% and 29%, respectively, with 74% of each group admitted via the emergency room. ST-elevation myocardial infarction occurred in 40% and 41% of patients in each group, respectively.
Results showed that stopping aspirin early was noninferior and possibly superior to 12 months of DAPT.
For the 12-month clinical outcome, fewer patients in the less than 1 month DAPT followed by ticagrelor monotherapy arm reached the primary clinical endpoint of NACE versus the ticagrelor-based 12-month DAPT arm, both in terms of noninferiority (P < .001) and superiority (P = .002). Similar results were found for the 1-month landmark analyses.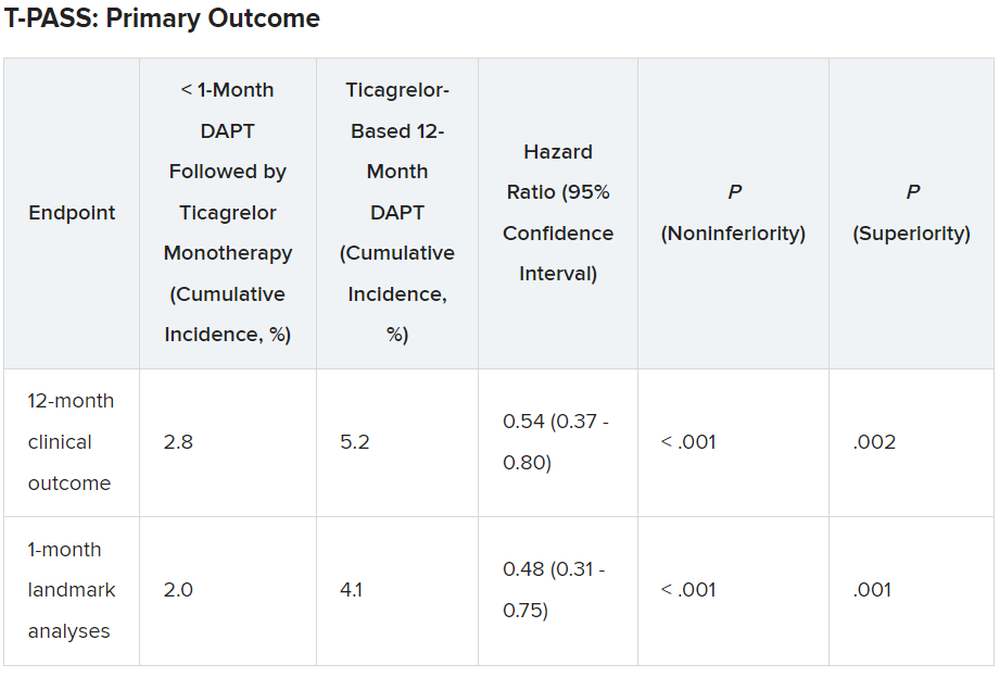
For both the 12-month clinical outcome and the 1-month landmark analyses, the curves for the two arms began to diverge at about 150 days, with the one for ticagrelor monotherapy essentially flattening out just after that and the one for the 12-month DAPT therapy continuing to rise out to the 1-year point.
In the less than 1 month DAPT arm, aspirin was stopped at a median of 16 days. Panelist Adnan Kastrati, MD, Deutsches Herzzentrum München, Technische Universität, Munich, Germany, asked Dr. Hong about the criteria for the point at which aspirin was stopped in the less than 1 month arm.
Dr. Hong replied: “Actually, we recommend less than 1 month, so therefore in some patients, it was the operator’s decision,” depending on risk factors for stopping or continuing aspirin. He said that in some patients it may be reasonable to stop aspirin even in 7-10 days. Fewer than 10% of patients in the less than 1 month arm continued on aspirin past 30 days, but a few continued on it to the 1-year point.
There was no difference between the less than 1 month DAPT followed by ticagrelor monotherapy arm and the 12-month DAPT arm in terms of major adverse cardiac and cerebrovascular events at 1 year (1.8% vs. 2.2%, respectively; hazard ratio, 0.84; 95% confidence interval, 0.50-1.41; log-rank, P = .51).
However, the 12-month DAPT arm showed a significantly greater incidence of major bleeding at 1 year: 3.4% versus 1.2% for less than 1 month aspirin arm (HR, 0.35; 95% CI, 0.20-0.61; log-rank, P < .001).
Dr. Hong said that a limitation of the study was that it was open label and not placebo controlled. However, an independent clinical event adjudication committee assessed all clinical outcomes.
Lead discussant Marco Valgimigli, MD, PhD, Cardiocentro Ticino Foundation, Lugano, Switzerland, noted that T-PASS is the fifth study to investigate ticagrelor monotherapy versus a DAPT, giving randomized data on almost 22,000 patients.
“T-PASS showed very consistently with the prior four studies that by dropping aspirin and continuation with ticagrelor therapy, compared with the standard DAPT regimen, is associated with no penalty ... and in fact leading to a very significant and clinically very convincing risk reduction, and I would like to underline major bleeding risk reduction,” he said, pointing out that this study comes from the same research group that carried out the TICO trial.
Dr. Hong has received institutional research grants from Samjin Pharmaceutical and Chong Kun Dang Pharmaceutical, and speaker’s fees from Medtronic and Edwards Lifesciences. Dr. Kastrati has disclosed no relevant financial relationships. Dr. Valgimigli has received grant support/research contracts from Terumo Medical and AstraZeneca; consultant fees/honoraria/speaker’s bureau for Terumo Medical Corporation, Bayer, Daiichi Sankyo/Eli Lilly, Amgen, Alvimedica, AstraZenca, Idorsia, Coreflow, Vifor, Bristol-Myers Squibb, and iVascular. The study was funded by Biotronik.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Stopping aspirin within 1 month of implanting a drug-eluting stent (DES) for acute coronary syndrome (ACS) followed by ticagrelor monotherapy was shown to be noninferior to 12 months of dual antiplatelet therapy (DAPT) in net adverse cardiovascular and bleeding events in the T-PASS trial.
of death, myocardial infarction, stent thrombosis, stroke, and major bleeding, primarily due to a significant reduction in bleeding events,” senior author Myeong-Ki Hong, MD, PhD, Yonsei University, Seoul, Korea, told attendees at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
“This study provides evidence that stopping aspirin within 1 month after implantation of drug-eluting stents for ticagrelor monotherapy is a reasonable alternative to 12-month DAPT as for adverse cardiovascular and bleeding events,” Dr. Hong concluded.
The study was published in Circulation ahead of print to coincide with the presentation.
Three months to 1 month
Previous trials (TICO and TWILIGHT) have shown that ticagrelor monotherapy after 3 months of DAPT can be safe and effectively prevent ischemic events after percutaneous coronary intervention (PCI) in ACS or high-risk PCI patients.
The current study aimed to investigate whether ticagrelor monotherapy after less than 1 month of DAPT was noninferior to 12 months of ticagrelor-based DAPT for preventing adverse cardiovascular and bleeding events in patients with ACS undergoing PCI with a DES implant.
T-PASS, carried out at 24 centers in Korea, enrolled ACS patients aged 19 years or older who received an ultrathin, bioresorbable polymer sirolimus-eluting stent (Orsiro, Biotronik). They were randomized 1:1 to ticagrelor monotherapy after less than 1 month of DAPT (n = 1,426) or to ticagrelor-based DAPT for 12 months (n = 1,424).
The primary outcome measure was net adverse clinical events (NACE) at 12 months, consisting of major bleeding plus major adverse cardiovascular events. All patients were included in the intention-to-treat analysis.
The study could enroll patients aged 19-80 years. It excluded anyone with active bleeding, at increased risk for bleeding, with anemia (hemoglobin ≤ 8 g/dL), platelets less than 100,000/mcL, need for oral anticoagulation therapy, current or potential pregnancy, or a life expectancy less than 1 year.
Baseline characteristics of the two groups were well balanced. The extended monotherapy and DAPT arms had an average age of 61 ± 10 years, were 84% and 83% male and had diabetes mellitus in 30% and 29%, respectively, with 74% of each group admitted via the emergency room. ST-elevation myocardial infarction occurred in 40% and 41% of patients in each group, respectively.
Results showed that stopping aspirin early was noninferior and possibly superior to 12 months of DAPT.
For the 12-month clinical outcome, fewer patients in the less than 1 month DAPT followed by ticagrelor monotherapy arm reached the primary clinical endpoint of NACE versus the ticagrelor-based 12-month DAPT arm, both in terms of noninferiority (P < .001) and superiority (P = .002). Similar results were found for the 1-month landmark analyses.
For both the 12-month clinical outcome and the 1-month landmark analyses, the curves for the two arms began to diverge at about 150 days, with the one for ticagrelor monotherapy essentially flattening out just after that and the one for the 12-month DAPT therapy continuing to rise out to the 1-year point.
In the less than 1 month DAPT arm, aspirin was stopped at a median of 16 days. Panelist Adnan Kastrati, MD, Deutsches Herzzentrum München, Technische Universität, Munich, Germany, asked Dr. Hong about the criteria for the point at which aspirin was stopped in the less than 1 month arm.
Dr. Hong replied: “Actually, we recommend less than 1 month, so therefore in some patients, it was the operator’s decision,” depending on risk factors for stopping or continuing aspirin. He said that in some patients it may be reasonable to stop aspirin even in 7-10 days. Fewer than 10% of patients in the less than 1 month arm continued on aspirin past 30 days, but a few continued on it to the 1-year point.
There was no difference between the less than 1 month DAPT followed by ticagrelor monotherapy arm and the 12-month DAPT arm in terms of major adverse cardiac and cerebrovascular events at 1 year (1.8% vs. 2.2%, respectively; hazard ratio, 0.84; 95% confidence interval, 0.50-1.41; log-rank, P = .51).
However, the 12-month DAPT arm showed a significantly greater incidence of major bleeding at 1 year: 3.4% versus 1.2% for less than 1 month aspirin arm (HR, 0.35; 95% CI, 0.20-0.61; log-rank, P < .001).
Dr. Hong said that a limitation of the study was that it was open label and not placebo controlled. However, an independent clinical event adjudication committee assessed all clinical outcomes.
Lead discussant Marco Valgimigli, MD, PhD, Cardiocentro Ticino Foundation, Lugano, Switzerland, noted that T-PASS is the fifth study to investigate ticagrelor monotherapy versus a DAPT, giving randomized data on almost 22,000 patients.
“T-PASS showed very consistently with the prior four studies that by dropping aspirin and continuation with ticagrelor therapy, compared with the standard DAPT regimen, is associated with no penalty ... and in fact leading to a very significant and clinically very convincing risk reduction, and I would like to underline major bleeding risk reduction,” he said, pointing out that this study comes from the same research group that carried out the TICO trial.
Dr. Hong has received institutional research grants from Samjin Pharmaceutical and Chong Kun Dang Pharmaceutical, and speaker’s fees from Medtronic and Edwards Lifesciences. Dr. Kastrati has disclosed no relevant financial relationships. Dr. Valgimigli has received grant support/research contracts from Terumo Medical and AstraZeneca; consultant fees/honoraria/speaker’s bureau for Terumo Medical Corporation, Bayer, Daiichi Sankyo/Eli Lilly, Amgen, Alvimedica, AstraZenca, Idorsia, Coreflow, Vifor, Bristol-Myers Squibb, and iVascular. The study was funded by Biotronik.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Stopping aspirin within 1 month of implanting a drug-eluting stent (DES) for acute coronary syndrome (ACS) followed by ticagrelor monotherapy was shown to be noninferior to 12 months of dual antiplatelet therapy (DAPT) in net adverse cardiovascular and bleeding events in the T-PASS trial.
of death, myocardial infarction, stent thrombosis, stroke, and major bleeding, primarily due to a significant reduction in bleeding events,” senior author Myeong-Ki Hong, MD, PhD, Yonsei University, Seoul, Korea, told attendees at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
“This study provides evidence that stopping aspirin within 1 month after implantation of drug-eluting stents for ticagrelor monotherapy is a reasonable alternative to 12-month DAPT as for adverse cardiovascular and bleeding events,” Dr. Hong concluded.
The study was published in Circulation ahead of print to coincide with the presentation.
Three months to 1 month
Previous trials (TICO and TWILIGHT) have shown that ticagrelor monotherapy after 3 months of DAPT can be safe and effectively prevent ischemic events after percutaneous coronary intervention (PCI) in ACS or high-risk PCI patients.
The current study aimed to investigate whether ticagrelor monotherapy after less than 1 month of DAPT was noninferior to 12 months of ticagrelor-based DAPT for preventing adverse cardiovascular and bleeding events in patients with ACS undergoing PCI with a DES implant.
T-PASS, carried out at 24 centers in Korea, enrolled ACS patients aged 19 years or older who received an ultrathin, bioresorbable polymer sirolimus-eluting stent (Orsiro, Biotronik). They were randomized 1:1 to ticagrelor monotherapy after less than 1 month of DAPT (n = 1,426) or to ticagrelor-based DAPT for 12 months (n = 1,424).
The primary outcome measure was net adverse clinical events (NACE) at 12 months, consisting of major bleeding plus major adverse cardiovascular events. All patients were included in the intention-to-treat analysis.
The study could enroll patients aged 19-80 years. It excluded anyone with active bleeding, at increased risk for bleeding, with anemia (hemoglobin ≤ 8 g/dL), platelets less than 100,000/mcL, need for oral anticoagulation therapy, current or potential pregnancy, or a life expectancy less than 1 year.
Baseline characteristics of the two groups were well balanced. The extended monotherapy and DAPT arms had an average age of 61 ± 10 years, were 84% and 83% male and had diabetes mellitus in 30% and 29%, respectively, with 74% of each group admitted via the emergency room. ST-elevation myocardial infarction occurred in 40% and 41% of patients in each group, respectively.
Results showed that stopping aspirin early was noninferior and possibly superior to 12 months of DAPT.
For the 12-month clinical outcome, fewer patients in the less than 1 month DAPT followed by ticagrelor monotherapy arm reached the primary clinical endpoint of NACE versus the ticagrelor-based 12-month DAPT arm, both in terms of noninferiority (P < .001) and superiority (P = .002). Similar results were found for the 1-month landmark analyses.
For both the 12-month clinical outcome and the 1-month landmark analyses, the curves for the two arms began to diverge at about 150 days, with the one for ticagrelor monotherapy essentially flattening out just after that and the one for the 12-month DAPT therapy continuing to rise out to the 1-year point.
In the less than 1 month DAPT arm, aspirin was stopped at a median of 16 days. Panelist Adnan Kastrati, MD, Deutsches Herzzentrum München, Technische Universität, Munich, Germany, asked Dr. Hong about the criteria for the point at which aspirin was stopped in the less than 1 month arm.
Dr. Hong replied: “Actually, we recommend less than 1 month, so therefore in some patients, it was the operator’s decision,” depending on risk factors for stopping or continuing aspirin. He said that in some patients it may be reasonable to stop aspirin even in 7-10 days. Fewer than 10% of patients in the less than 1 month arm continued on aspirin past 30 days, but a few continued on it to the 1-year point.
There was no difference between the less than 1 month DAPT followed by ticagrelor monotherapy arm and the 12-month DAPT arm in terms of major adverse cardiac and cerebrovascular events at 1 year (1.8% vs. 2.2%, respectively; hazard ratio, 0.84; 95% confidence interval, 0.50-1.41; log-rank, P = .51).
However, the 12-month DAPT arm showed a significantly greater incidence of major bleeding at 1 year: 3.4% versus 1.2% for less than 1 month aspirin arm (HR, 0.35; 95% CI, 0.20-0.61; log-rank, P < .001).
Dr. Hong said that a limitation of the study was that it was open label and not placebo controlled. However, an independent clinical event adjudication committee assessed all clinical outcomes.
Lead discussant Marco Valgimigli, MD, PhD, Cardiocentro Ticino Foundation, Lugano, Switzerland, noted that T-PASS is the fifth study to investigate ticagrelor monotherapy versus a DAPT, giving randomized data on almost 22,000 patients.
“T-PASS showed very consistently with the prior four studies that by dropping aspirin and continuation with ticagrelor therapy, compared with the standard DAPT regimen, is associated with no penalty ... and in fact leading to a very significant and clinically very convincing risk reduction, and I would like to underline major bleeding risk reduction,” he said, pointing out that this study comes from the same research group that carried out the TICO trial.
Dr. Hong has received institutional research grants from Samjin Pharmaceutical and Chong Kun Dang Pharmaceutical, and speaker’s fees from Medtronic and Edwards Lifesciences. Dr. Kastrati has disclosed no relevant financial relationships. Dr. Valgimigli has received grant support/research contracts from Terumo Medical and AstraZeneca; consultant fees/honoraria/speaker’s bureau for Terumo Medical Corporation, Bayer, Daiichi Sankyo/Eli Lilly, Amgen, Alvimedica, AstraZenca, Idorsia, Coreflow, Vifor, Bristol-Myers Squibb, and iVascular. The study was funded by Biotronik.
A version of this article first appeared on Medscape.com.
AT TCT 2023
Outcomes of PF ablation for AFib similar between sexes
TOPLINE:
results of a large registry study show.
METHODOLOGY:
- The study included all 1,568 patients (mean age 64.5 years and 35.3% women) in the MANIFEST-PF registry, which includes 24 European centers that began using PFA for treating AFib after regulatory approval in 2021.
- Researchers categorized patients by sex and evaluated them for clinical outcomes of PFA, including freedom from AFib and adverse events.
- All patients underwent pulmonary vein isolation (Farawave, Boston Scientific) and were followed up at 3, 6, and 12 months.
- The primary effectiveness outcome was freedom from atrial arrhythmia outside the 90-day blanking period lasting 30 seconds or longer.
- The primary safety outcome included the composite of acute (less than 7 days post-procedure) and chronic (more than 7 days post-procedure) major adverse events, including atrioesophageal fistula, symptomatic pulmonary vein stenosis, cardiac tamponade/perforation requiring intervention or surgery, stroke or systemic thromboembolism, persistent phrenic nerve injury, vascular access complications requiring surgery, coronary artery spasm, and death.
TAKEAWAY:
- There was no significant difference in 12-month recurrence of atrial arrhythmia between male and female patients (79.0% vs 76.3%; P = .28), with greater overall effectiveness in the paroxysmal AFib cohort (men, 82.5% vs women, 80.2%; P = .30) than in the persistent AF/long-standing persistent AFib cohort (men, 73.3% vs women, 67.3%; P = .40).
- Repeated ablation rates were similar between sexes (men, 8.3% vs women, 10.0%; P = .32).
- Among patients who underwent repeat ablation, pulmonary vein isolation durability was higher in female than in male patients (per vein, 82.6% vs 68.1%; P = .15 and per patient, 63.0% vs 37.8%; P = .005).
- Major adverse events occurred in 2.5% of women and 1.5% of men (P = .19), with such events mostly consisting of cardiac tamponade (women, 1.4% vs men, 1.0%; P = .46) and stroke (0.4% vs 0.4%, P > .99), and with no atrioesophageal fistulas or symptomatic pulmonary valve stenosis in either group.
IN PRACTICE:
“These results are important, as women are underrepresented in prior ablation studies and the results have been mixed with regards to both safety and effectiveness using conventional ablation strategies such as radiofrequency or cryoablation,” lead author Mohit Turagam, MD, associate professor of medicine (cardiology), Icahn School of Medicine at Mount Sinai, New York, NY, said in a press release.
In an accompanying commentary, Peter M. Kistler, MBBS, PhD, Department of Cardiology, Alfred Hospital, Melbourne, Victoria, Australia, and a colleague said that the study authors should be congratulated “for presenting much-needed data on sex-specific outcomes in catheter ablation,” which “reassuringly” suggest that success and safety for AFib ablation are comparable between the sexes, although the study does have “important limitations.”
SOURCE:
The study was conducted by Turagam and colleagues. It was published online in JAMA Cardiology.
LIMITATIONS:
Researchers can’t rule out the possibility that treatment selection and unmeasured confounders between sexes affected the validity of the study findings. The median number of follow-up 24-hour Holter monitors used for AFib monitoring was only two, which may have resulted in inaccurate estimates of AFib recurrence rates and treatment effectiveness.
DISCLOSURES:
The study was supported by Boston Scientific Corporation, the PFA device manufacturer. Turagam has no relevant conflicts of interest; see paper for disclosures of other study authors. The commentary authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
results of a large registry study show.
METHODOLOGY:
- The study included all 1,568 patients (mean age 64.5 years and 35.3% women) in the MANIFEST-PF registry, which includes 24 European centers that began using PFA for treating AFib after regulatory approval in 2021.
- Researchers categorized patients by sex and evaluated them for clinical outcomes of PFA, including freedom from AFib and adverse events.
- All patients underwent pulmonary vein isolation (Farawave, Boston Scientific) and were followed up at 3, 6, and 12 months.
- The primary effectiveness outcome was freedom from atrial arrhythmia outside the 90-day blanking period lasting 30 seconds or longer.
- The primary safety outcome included the composite of acute (less than 7 days post-procedure) and chronic (more than 7 days post-procedure) major adverse events, including atrioesophageal fistula, symptomatic pulmonary vein stenosis, cardiac tamponade/perforation requiring intervention or surgery, stroke or systemic thromboembolism, persistent phrenic nerve injury, vascular access complications requiring surgery, coronary artery spasm, and death.
TAKEAWAY:
- There was no significant difference in 12-month recurrence of atrial arrhythmia between male and female patients (79.0% vs 76.3%; P = .28), with greater overall effectiveness in the paroxysmal AFib cohort (men, 82.5% vs women, 80.2%; P = .30) than in the persistent AF/long-standing persistent AFib cohort (men, 73.3% vs women, 67.3%; P = .40).
- Repeated ablation rates were similar between sexes (men, 8.3% vs women, 10.0%; P = .32).
- Among patients who underwent repeat ablation, pulmonary vein isolation durability was higher in female than in male patients (per vein, 82.6% vs 68.1%; P = .15 and per patient, 63.0% vs 37.8%; P = .005).
- Major adverse events occurred in 2.5% of women and 1.5% of men (P = .19), with such events mostly consisting of cardiac tamponade (women, 1.4% vs men, 1.0%; P = .46) and stroke (0.4% vs 0.4%, P > .99), and with no atrioesophageal fistulas or symptomatic pulmonary valve stenosis in either group.
IN PRACTICE:
“These results are important, as women are underrepresented in prior ablation studies and the results have been mixed with regards to both safety and effectiveness using conventional ablation strategies such as radiofrequency or cryoablation,” lead author Mohit Turagam, MD, associate professor of medicine (cardiology), Icahn School of Medicine at Mount Sinai, New York, NY, said in a press release.
In an accompanying commentary, Peter M. Kistler, MBBS, PhD, Department of Cardiology, Alfred Hospital, Melbourne, Victoria, Australia, and a colleague said that the study authors should be congratulated “for presenting much-needed data on sex-specific outcomes in catheter ablation,” which “reassuringly” suggest that success and safety for AFib ablation are comparable between the sexes, although the study does have “important limitations.”
SOURCE:
The study was conducted by Turagam and colleagues. It was published online in JAMA Cardiology.
LIMITATIONS:
Researchers can’t rule out the possibility that treatment selection and unmeasured confounders between sexes affected the validity of the study findings. The median number of follow-up 24-hour Holter monitors used for AFib monitoring was only two, which may have resulted in inaccurate estimates of AFib recurrence rates and treatment effectiveness.
DISCLOSURES:
The study was supported by Boston Scientific Corporation, the PFA device manufacturer. Turagam has no relevant conflicts of interest; see paper for disclosures of other study authors. The commentary authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
results of a large registry study show.
METHODOLOGY:
- The study included all 1,568 patients (mean age 64.5 years and 35.3% women) in the MANIFEST-PF registry, which includes 24 European centers that began using PFA for treating AFib after regulatory approval in 2021.
- Researchers categorized patients by sex and evaluated them for clinical outcomes of PFA, including freedom from AFib and adverse events.
- All patients underwent pulmonary vein isolation (Farawave, Boston Scientific) and were followed up at 3, 6, and 12 months.
- The primary effectiveness outcome was freedom from atrial arrhythmia outside the 90-day blanking period lasting 30 seconds or longer.
- The primary safety outcome included the composite of acute (less than 7 days post-procedure) and chronic (more than 7 days post-procedure) major adverse events, including atrioesophageal fistula, symptomatic pulmonary vein stenosis, cardiac tamponade/perforation requiring intervention or surgery, stroke or systemic thromboembolism, persistent phrenic nerve injury, vascular access complications requiring surgery, coronary artery spasm, and death.
TAKEAWAY:
- There was no significant difference in 12-month recurrence of atrial arrhythmia between male and female patients (79.0% vs 76.3%; P = .28), with greater overall effectiveness in the paroxysmal AFib cohort (men, 82.5% vs women, 80.2%; P = .30) than in the persistent AF/long-standing persistent AFib cohort (men, 73.3% vs women, 67.3%; P = .40).
- Repeated ablation rates were similar between sexes (men, 8.3% vs women, 10.0%; P = .32).
- Among patients who underwent repeat ablation, pulmonary vein isolation durability was higher in female than in male patients (per vein, 82.6% vs 68.1%; P = .15 and per patient, 63.0% vs 37.8%; P = .005).
- Major adverse events occurred in 2.5% of women and 1.5% of men (P = .19), with such events mostly consisting of cardiac tamponade (women, 1.4% vs men, 1.0%; P = .46) and stroke (0.4% vs 0.4%, P > .99), and with no atrioesophageal fistulas or symptomatic pulmonary valve stenosis in either group.
IN PRACTICE:
“These results are important, as women are underrepresented in prior ablation studies and the results have been mixed with regards to both safety and effectiveness using conventional ablation strategies such as radiofrequency or cryoablation,” lead author Mohit Turagam, MD, associate professor of medicine (cardiology), Icahn School of Medicine at Mount Sinai, New York, NY, said in a press release.
In an accompanying commentary, Peter M. Kistler, MBBS, PhD, Department of Cardiology, Alfred Hospital, Melbourne, Victoria, Australia, and a colleague said that the study authors should be congratulated “for presenting much-needed data on sex-specific outcomes in catheter ablation,” which “reassuringly” suggest that success and safety for AFib ablation are comparable between the sexes, although the study does have “important limitations.”
SOURCE:
The study was conducted by Turagam and colleagues. It was published online in JAMA Cardiology.
LIMITATIONS:
Researchers can’t rule out the possibility that treatment selection and unmeasured confounders between sexes affected the validity of the study findings. The median number of follow-up 24-hour Holter monitors used for AFib monitoring was only two, which may have resulted in inaccurate estimates of AFib recurrence rates and treatment effectiveness.
DISCLOSURES:
The study was supported by Boston Scientific Corporation, the PFA device manufacturer. Turagam has no relevant conflicts of interest; see paper for disclosures of other study authors. The commentary authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Newer antiobesity meds lower the body’s defended fat mass
The current highly effective antiobesity medications approved for treating obesity (semaglutide), under review (tirzepatide), or in late-stage clinical trials “appear to lower the body’s target and defended fat mass [set point]” but do not permanently fix it at a lower point, Lee M. Kaplan, MD, PhD, explained in a lecture during the annual meeting of the Obesity Society.
It is very likely that patients with obesity will have to take these antiobesity medications “forever,” he said, “until we identify and can repair the cellular and molecular mechanisms that the body uses to regulate body fat mass throughout the life cycle and that are dysfunctional in obesity.”
“The body is able to regulate fat mass at multiple stages during development,” Dr. Kaplan, from Massachusetts General Hospital and Harvard Medical School, Boston, explained, “and when it doesn’t do it appropriately, that becomes the physiological basis of obesity.”
The loss of baby fat, as well as fat changes during puberty, menopause, aging, and, in particular, during and after pregnancy, “all occur without conscious or purposeful input,” he noted.
The body uses food intake and energy expenditure to reach and defend its intended fat mass, and there is an evolutionary benefit to doing this.
For example, people recovering from an acute illness can regain the lost fat and weight. A woman can support a pregnancy and lactation by increasing fat mass.
However, “the idea that [with antiobesity medications] we should be aiming for a fixed lower amount of fat is probably not a good idea.” Dr. Kaplan cautioned.
People need the flexibility to recover lost fat and weight after an acute illness or injury, and pregnant women need to gain an appropriate amount of body fat to support pregnancy and lactation.
Intermittent therapy: A practical strategy?
The long-term benefit of antiobesity medications requires continuous use, Dr. Kaplan noted. For example, in the STEP 1 trial of semaglutide in patients with obesity and without diabetes, when treatment was stopped at 68 weeks, average weight increased through 120 weeks, although it did not return to baseline levels.
Intermittent antiobesity therapy may be an effective, “very practical strategy” to maintain weight loss, which would also “address current challenges of high cost, limited drug availability, and inadequate access to care.”
“Until we have strategies for decreasing the cost of effective obesity treatment, and ensuring more equitable access to obesity care,” Dr. Kaplan said, “optimizing algorithms for the use of intermittent therapy may be an effective stopgap measure.”
Dr. Kaplan is or has recently been a paid consultant for Eli Lilly, Novo Nordisk, and multiple pharmaceutical companies developing antiobesity medications.
A version of this article appeared on Medscape.com.
The current highly effective antiobesity medications approved for treating obesity (semaglutide), under review (tirzepatide), or in late-stage clinical trials “appear to lower the body’s target and defended fat mass [set point]” but do not permanently fix it at a lower point, Lee M. Kaplan, MD, PhD, explained in a lecture during the annual meeting of the Obesity Society.
It is very likely that patients with obesity will have to take these antiobesity medications “forever,” he said, “until we identify and can repair the cellular and molecular mechanisms that the body uses to regulate body fat mass throughout the life cycle and that are dysfunctional in obesity.”
“The body is able to regulate fat mass at multiple stages during development,” Dr. Kaplan, from Massachusetts General Hospital and Harvard Medical School, Boston, explained, “and when it doesn’t do it appropriately, that becomes the physiological basis of obesity.”
The loss of baby fat, as well as fat changes during puberty, menopause, aging, and, in particular, during and after pregnancy, “all occur without conscious or purposeful input,” he noted.
The body uses food intake and energy expenditure to reach and defend its intended fat mass, and there is an evolutionary benefit to doing this.
For example, people recovering from an acute illness can regain the lost fat and weight. A woman can support a pregnancy and lactation by increasing fat mass.
However, “the idea that [with antiobesity medications] we should be aiming for a fixed lower amount of fat is probably not a good idea.” Dr. Kaplan cautioned.
People need the flexibility to recover lost fat and weight after an acute illness or injury, and pregnant women need to gain an appropriate amount of body fat to support pregnancy and lactation.
Intermittent therapy: A practical strategy?
The long-term benefit of antiobesity medications requires continuous use, Dr. Kaplan noted. For example, in the STEP 1 trial of semaglutide in patients with obesity and without diabetes, when treatment was stopped at 68 weeks, average weight increased through 120 weeks, although it did not return to baseline levels.
Intermittent antiobesity therapy may be an effective, “very practical strategy” to maintain weight loss, which would also “address current challenges of high cost, limited drug availability, and inadequate access to care.”
“Until we have strategies for decreasing the cost of effective obesity treatment, and ensuring more equitable access to obesity care,” Dr. Kaplan said, “optimizing algorithms for the use of intermittent therapy may be an effective stopgap measure.”
Dr. Kaplan is or has recently been a paid consultant for Eli Lilly, Novo Nordisk, and multiple pharmaceutical companies developing antiobesity medications.
A version of this article appeared on Medscape.com.
The current highly effective antiobesity medications approved for treating obesity (semaglutide), under review (tirzepatide), or in late-stage clinical trials “appear to lower the body’s target and defended fat mass [set point]” but do not permanently fix it at a lower point, Lee M. Kaplan, MD, PhD, explained in a lecture during the annual meeting of the Obesity Society.
It is very likely that patients with obesity will have to take these antiobesity medications “forever,” he said, “until we identify and can repair the cellular and molecular mechanisms that the body uses to regulate body fat mass throughout the life cycle and that are dysfunctional in obesity.”
“The body is able to regulate fat mass at multiple stages during development,” Dr. Kaplan, from Massachusetts General Hospital and Harvard Medical School, Boston, explained, “and when it doesn’t do it appropriately, that becomes the physiological basis of obesity.”
The loss of baby fat, as well as fat changes during puberty, menopause, aging, and, in particular, during and after pregnancy, “all occur without conscious or purposeful input,” he noted.
The body uses food intake and energy expenditure to reach and defend its intended fat mass, and there is an evolutionary benefit to doing this.
For example, people recovering from an acute illness can regain the lost fat and weight. A woman can support a pregnancy and lactation by increasing fat mass.
However, “the idea that [with antiobesity medications] we should be aiming for a fixed lower amount of fat is probably not a good idea.” Dr. Kaplan cautioned.
People need the flexibility to recover lost fat and weight after an acute illness or injury, and pregnant women need to gain an appropriate amount of body fat to support pregnancy and lactation.
Intermittent therapy: A practical strategy?
The long-term benefit of antiobesity medications requires continuous use, Dr. Kaplan noted. For example, in the STEP 1 trial of semaglutide in patients with obesity and without diabetes, when treatment was stopped at 68 weeks, average weight increased through 120 weeks, although it did not return to baseline levels.
Intermittent antiobesity therapy may be an effective, “very practical strategy” to maintain weight loss, which would also “address current challenges of high cost, limited drug availability, and inadequate access to care.”
“Until we have strategies for decreasing the cost of effective obesity treatment, and ensuring more equitable access to obesity care,” Dr. Kaplan said, “optimizing algorithms for the use of intermittent therapy may be an effective stopgap measure.”
Dr. Kaplan is or has recently been a paid consultant for Eli Lilly, Novo Nordisk, and multiple pharmaceutical companies developing antiobesity medications.
A version of this article appeared on Medscape.com.
FROM OBESITYWEEK® 2023
Essential oils: How safe? How effective?
Essential oils (EOs), which are concentrated plant-based oils, have become ubiquitous over the past decade. Given the far reach of EOs and their longtime use in traditional, complementary, alternative, and integrative medicine, it is imperative that clinicians have some knowledge of the potential benefits, risks, and overall efficacy.
Commonly used for aromatic benefits (aromatherapy), EOs are now also incorporated into a multitude of products promoting health and wellness. EOs are sold as individual products and can be a component in consumer goods such as cosmetics, body care/hygiene/beauty products, laundry detergents, insect repellents, over-the-counter medications, and food.
The review that follows presents the most current evidence available. With that said, it’s important to keep in mind some caveats that relate to this evidence. First, the studies cited tend to have a small sample size. Second, a majority of these studies were conducted in countries where there appears to be a significant culture of EO use, which could contribute to confirmation bias. Finally, in a number of the studies, there is concern for publication bias as well as a discrepancy between calculated statistical significance and actual clinical relevance.

What are essential oils?
EOs generally are made by extracting the oil from leaves, bark, flowers, seeds/fruit, rinds, and/or roots by steaming or pressing parts of a plant. It can take several pounds of plant material to produce a single bottle of EO, which usually contains ≥ 15 to 30 mL (.5 to 1 oz).1
Some commonly used EOs in the United States are lavender, peppermint, rose, clary sage, tea tree, eucalyptus, and citrus; however, there are approximately 300 EOs available.2 EOs are used most often via topical application, inhalation, or ingestion.
As with any botanical agent, EOs are complex substances often containing a multitude of chemical compounds.1 Because of the complex makeup of EOs, which often contain up to 100 volatile organic compounds, and their wide-ranging potential effects, applying the scientific method to study effectiveness poses a challenge that has limited their adoption in evidence-based practice.2
Availability and cost. EOs can be purchased at large retailers (eg, grocery stores, drug stores) and smaller health food stores, as well as on the Internet. Various EO vehicles, such as inhalers and topical creams, also can be purchased at these stores.
Continue to: The cost varies...
The cost varies enormously by manufacturer and type of plant used to make the EO. Common EOs such as peppermint and lavender oil generally cost $10 to $25, while rarer plant oils can cost $80 or more per bottle.
How safe are essential oils?
Patients may assume EOs are harmless because they are derived from natural plants and have been used medicinally for centuries. However, care must be taken with their use.
The safest way to use EOs is topically, although due to their highly concentrated nature, EOs should be diluted in an unscented neutral carrier oil such as coconut, jojoba, olive, or sweet almond.3 Ingestion of certain oils can cause hepatotoxicity, seizures, and even death.3 In fact, patients should speak with a knowledgeable physician before purchasing any oral EO capsules.
Whether used topically or ingested, all EOs carry risk for skin irritation and allergic reactions, and oral ingestion may result in some negative gastrointestinal (GI) adverse effects.4 A case report of 3 patients published in 2007 identified the potential for lavender and tea tree EOs to be endocrine disruptors.5
Inhalation of EOs may be harmful, as they emit many volatile organic compounds, some of which are considered potentially hazardous.6 At this time, there is insufficient evidence regarding inhaled EOs and their direct connection to respiratory health. It is reasonable to suggest, however, that the prolonged use of EOs and their use by patients who have lung conditions such as asthma or COPD should be avoided.7
Continue to: How are quality and purity assessed?
How are quality and purity assessed?
Like other dietary supplements, EOs are not regulated. No US regulatory agencies (eg, the US Food and Drug Administration [FDA] or Department of Agriculture [USDA]) certify or approve EOs for quality and purity. Bottles labeled with “QAI” for Quality Assurance International or “USDA Organic” will ensure the plant constituents used in the EO are from organic farming but do not attest to quality or purity.
Manufacturers commonly use marketing terms such as “therapeutic grade” or “pure” to sell products, but again, these terms do not reflect the product’s quality or purity. A labeled single EO may contain contaminants, alcohol, or additional ingredients.7 When choosing to use EOs, identifying reputable brands is essential; one resource is the independent testing organization ConsumerLab.com.
It is important to assess the manufacturer and read ingredient labels before purchasing an EO to understand what the product contains. Reputable companies will identify the plant ingredient, usually by the formal Latin binomial name, and explain the extraction process. A more certain way to assess the quality and purity of an EO is to ask the manufacturer to provide a certificate of analysis and gas chromatography/mass spectroscopy (GC/MS) data for the specific product. Some manufacturers offer GC/MS test results on their website Quality page.8 Others have detailed information on quality and testing, and GC/MS test reports can be obtained.9 Yet another manufacturer has test results on a product page matching reports to batch codes.10
Which conditions have evidence of benefit from essential oils?
EOs currently are being studied for treatment of many conditions—including pain, GI disorders, behavioral health disorders, and women’s health issues. The TABLE summarizes the conditions treated, outcomes, and practical applications of EOs.11-44
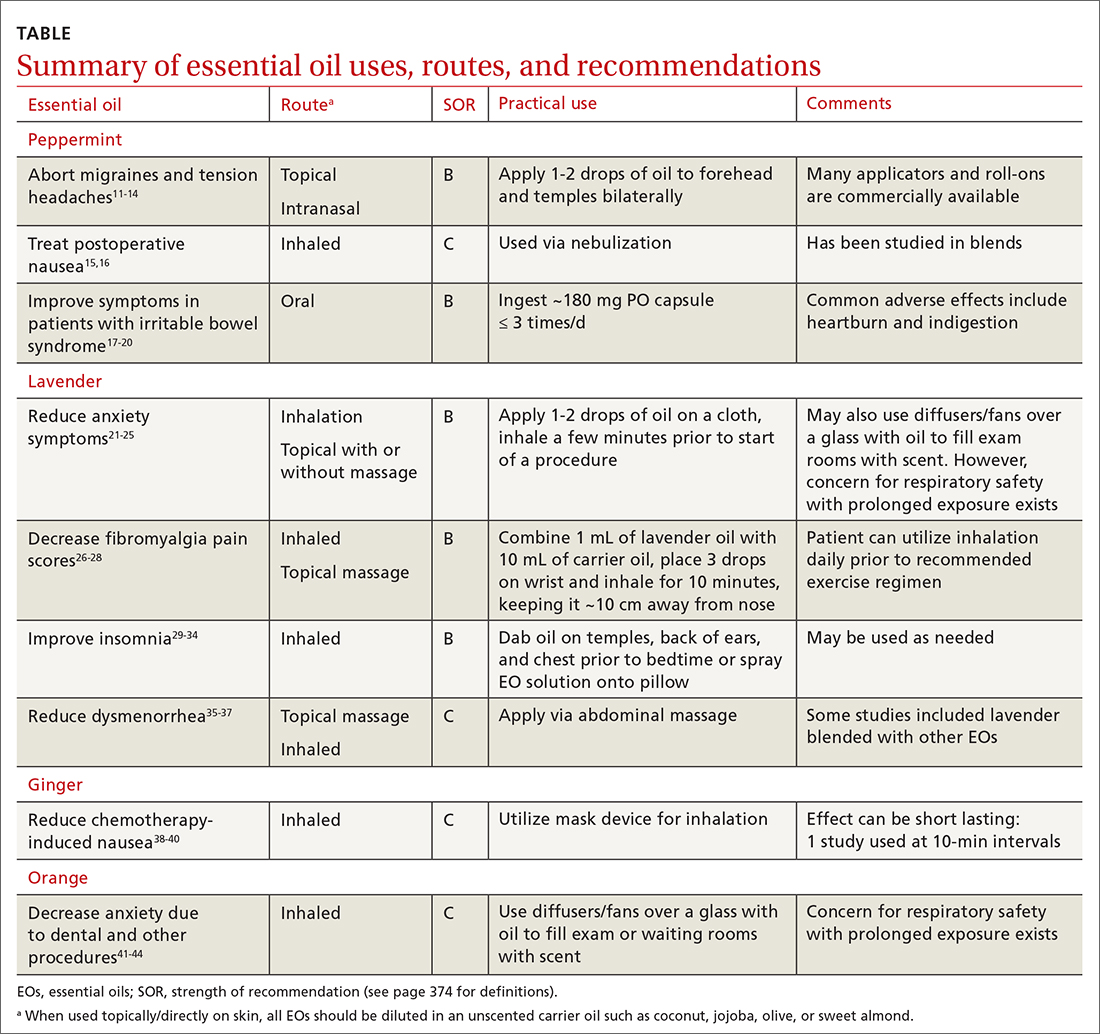
Pain
Headache. As an adjunct to available medications and procedures for headache treatment, EOs are one of the nonpharmacologic modalities that patients and clinicians have at their disposal for both migraine and tension-type headaches. A systematic review of 19 randomized controlled trials (RCTs) examining the effects of herbal ingredients for the acute treatment or prophylaxis of migraines found certain topically applied or inhaled EOs, such as peppermint and chamomile, to be effective for migraine pain alleviation; however, topically applied rose oil was not effective.11-13 Note: “topical application” in these studies implies application of the EO to ≥ 1 of the following areas: temples, forehead, behind ears, or above upper lip/below the nose.
Continue to: One RCT with 120 patients...
One RCT with 120 patients evaluated diluted intranasal peppermint oil and found that it reduced migraine intensity at similar rates to intranasal lidocaine.13 In this study, patients were randomized to receive one of the following: 4% lidocaine, 1.5% peppermint EO, or placebo. Two drops of the intranasal intervention were self-administered while the patient was in a supine position with their head suspended off the edge of the surface on which they were lying. They were instructed to stay in this position for at least 30 seconds after administration.
With regard to tension headache treatment, there is limited literature on the use of EOs. One study found that a preparation of peppermint oil applied topically to the temples and forehead of study participants resulted in significant analgesic effect.14
Fibromyalgia. Usual treatments for fibromyalgia include exercise, antidepressant and anticonvulsant medications, and stress management. Evidence also supports the use of inhaled and topically applied (with and without massage) lavender oil to improve symptoms.26 Positive effects may be related to the analgesic, anti-inflammatory, sleep-regulating, and anxiety-reducing effects of the major volatile compounds contained in lavender oil.
In one RCT with 42 patients with fibromyalgia, the use of inhaled lavender oil was shown to increase the perception of well-being (assessed on the validated SF-36 Health Survey Questionnaire) after 4 weeks.27 In this study, the patient applied 3 drops of an oil mixture, comprising 1 mL lavender EO and 10 mL of fixed neutral base oil, to the wrist and inhaled for 10 minutes before going to bed.
The use of a topical oil blend labeled “Oil 24” (containing camphor, rosemary, eucalyptus, peppermint, aloe vera, and lemon/orange) also has been shown to be more effective than placebo in managing fibromyalgia symptoms. A randomized controlled pilot study of 153 participants found that regular application of Oil 24 improved scores on pain scales and the Fibromyalgia Impact Questionnaire.28
Continue to: GI disorders
GI disorders
Irritable bowel syndrome. Peppermint oil relaxes GI smooth muscle, which has led to investigation of its use in irritable bowel syndrome (IBS) symptom amelioration.17 One meta-analysis including 12 RCTs with 835 patients with undifferentiated IBS found that orally ingested peppermint EO capsules reduced patient-reported symptoms of either abdominal pain or global symptoms.18
One study utilized the Total IBS Symptom Score to evaluate symptom reduction in patients with IBS-D (with diarrhea) and IBS-M (mixed) using 180-mg peppermint EO capsules ingested 3 times daily. There was a significant improvement in abdominal pain/discomfort, bloating/distension, pain at evacuation, and bowel urgency.19 A reduction in symptoms was observed after the first 24 hours of treatment and at the end of the 4-week treatment period.
In another study, among the 190 patients meeting Rome IV criteria for general (nonspecific) IBS who were treated with 182-mg peppermint EO capsules, no statistically significant reduction in overall symptom relief was found (based on outcome measures by the FDA and European Medicines Agency). However, in a secondary outcome analysis, peppermint oil produced greater improvements than placebo for the alleviation of abdominal pain, discomfort, and general IBS severity.20
Chemotherapy-induced nausea and vomiting. Patients with cancer undergoing chemotherapy often explore integrative medicine approaches, including aromatherapy, to ameliorate adverse effects and improve quality of life.38 A few small studies have shown potential for the use of inhaled ginger oil to reduce nausea and vomiting severity and improve health-related quality-of-life measures in these patients.
For example, a study with 60 participants found that inhaling ginger EO for 10 minutes was beneficial for reducing both nausea and vomiting.39 A single-blind, controlled, randomized crossover study of 60 patients with breast cancer receiving chemotherapy showed that ginger EO inhaled 3 times per day for 2 minutes at a time can decrease the severity of nausea but had no effect on vomiting. The same study showed that health-related quality of life improved with the ginger oil treatment.40
Continue to: Other EOs such as cardamom...
Other EOs such as cardamom and peppermint show promise as an adjunctive treatment for chemotherapy-induced nausea and vomiting as well.38
Postoperative nausea. A 2013 randomized trial of 303 patients examined the use of ginger EO, a blend of EOs (including ginger, spearmint, peppermint, and cardamom), and isopropyl alcohol. Both the single EO and EO blend significantly reduced the symptom of nausea. The number of antiemetic medications requested by patients receiving an EO also was significantly reduced compared to those receiving saline.15
The use of EOs to reduce nausea after cardiac operations was reviewed in an RCT of 60 surgical candidates using 10% peppermint oil via nebulization for 10 minutes.16 This technique was effective in reducing nausea during cardiac postoperative periods. Although the evidence for the use of EOs for postoperative nausea is not robust, it may be a useful and generally safe approach for this common issue.
Behavioral health
Insomnia. EOs have been used as a treatment for insomnia traditionally and in complementary, alternative, and integrative medicine. A 2014 systematic review of 15 quantitative studies, including 11 RCTs, evaluated the hypnotic effects of EOs through inhalation, finding the strongest evidence for lavender, jasmine, and peppermint oils.29 The majority of the studies in the systematic review used the Pittsburgh Sleep Quality Index (PSQI) to evaluate EO effectiveness. A more recent 2021 systematic review and meta-analysis that evaluated 34 RCTs found that inhalation of EOs, most notably lavender aromatherapy, is effective in improving sleep problems such as insomnia.30
Findings from multiple smaller RCTs were consistent with those of the aforementioned systematic reviews. For example, in a well-conducted parallel randomized double-blind placebo-controlled trial of 100 people using orally ingested lemon verbena, the authors concluded that this intervention can be a complementary therapy for improving sleep quality and reducing insomnia severity.31 Another RCT with 60 participants evaluated an inhaled EO blend (lemon, eucalyptus, tea tree, and peppermint) over 4 weeks and found lowered perceived stress and depression as well as better sleep quality, but no influence on objective physiologic data such as stress indices or immune states.32
Continue to: In a 2020 randomized crossover...
In a 2020 randomized crossover placebocontrolled trial of 37 participants with diabetes reporting insomnia, inhaled lavender improved sleep quality and quantity, quality of life, and mood but not physiologic or metabolic measures, such as fasting glucose.33 Findings were similar in a cohort of cardiac rehabilitation patients (n = 37) who were treated with either an inhaled combination of lavender, bergamot, and ylang ylang, or placebo; cotton balls infused with the intervention oil or placebo oil were placed at the patient’s bedside for 5 nights. Sleep quality of participants receiving intervention oil was significantly better than the sleep quality of participants receiving the placebo oil as measured by participant completion of the PSQI.34
Anxiety is a common disorder that can be managed with nonpharmacologic treatments such as yoga, deep breathing, meditation, and EO therapy.21,22 In a systematic review and meta-analysis, the inhaled and topical use (with or without massage) of lavender EO was shown to improve psychological and physical manifestations of anxiety.23 Lavender EO is purported to affect the parasympathetic nervous system via anxiolytic, sedative, analgesic, and anticonvulsant properties.24 One systematic review and meta-analysis evaluating the anxiolytic effect of both inhaled and topical lavender EO found improvement in several biomarkers and physiologic data including blood pressure, heart rate, and cortisol levels, as well as a reduction in self-reported levels of anxiety, compared with placebo.25
Anxiety related to dental procedures is another area of study for the use of EOs. Two RCTs demonstrate statistically significant improvement in anxiety-related physiologic markers such as heart rate, blood pressure, and salivary cortisol levels in children who inhaled lavender EO during dental procedures.41,42 In 1 of the RCTs, the intervention was described as 3 drops of 100% lavender EO applied to a cloth and inhaled over the course of 3 minutes.41 Additionally, 2 studies found that orange EO was beneficial for dental procedure–induced anxiety, reducing pulse rates, cortisol levels, and self-reported anxiety.43,44
Dementia-related behavioral disturbances. A small, poorly designed study examining 2 EO blends—rosemary with lemon and lavender with orange—found some potential for improving cognitive function, especially in patients with Alzheimer disease.45 A Cochrane review of 13 RCTs totaling 708 patients concluded that it is not certain from the available evidence that EO therapy benefits patients with dementia in long-term-care facilities and hospital wards.46 Given that reporting of adverse events in the trials was poor, it is not possible to make conclusions about the risk vs benefit of EO therapy in this population.
Women’s health
Dysmenorrhea.
Continue to: In a randomized, double-blind clinical trial...
In a randomized, double-blind clinical trial of 48 women, a cream-based blend of lavender, clary sage, and marjoram EO (used topically in a 2:1:1 ratio diluted in unscented cream at 3% concentration and applied daily via abdominal massage) reduced participants’ reported menstrual pain symptoms and duration of pain.36 In a meta-analysis of 6 studies, topical abdominal application of EO (mainly lavender with or without other oils) with massage showed superiority over massage with placebo oils in reducing menstrual pain.37 A reduction in pain, mood symptoms, and fatigue in women with premenstrual symptoms was seen in an RCT of 77 patients using 3 drops of inhaled lavender EO.47
Labor. There is limited evidence for the use of EOs during labor. In an RCT of 104 women, patient-selected diffused EOs, including lavender, rose geranium, citrus, or jasmine, were found to help lower pain scores during the latent and early active phase of labor. There were no differences in labor augmentation, length of labor, perinatal outcomes, or need for additional pain medication.48
Other uses
Antimicrobial support. Some common EOs that have demonstrated antimicrobial properties are oregano, thyme, clove, lavender, clary sage, garlic, and cinnamon.49,50 Topical lemongrass and tea tree EOs have shown some degree of efficacy as an alternative treatment for acne, decolonization of methicillin-resistant Staphylococcus aureus, and superficial fungal infections.51 Support for an oral mixture of EOs labeled Myrtol (containing eucalyptus, citrus myrtle, and lavender) for viral acute bronchitis and sinusitis was found in a review of 7 studies.52 More research needs to be done before clear recommendations can be made on the use of EOs as antimicrobials, but the current data are encouraging.
Insect repellent. Reviews of the insect-repellent properties of EOs have shown promise and are in the public’s interest due to increasing awareness of the potential health and environmental hazards of synthetic repellents.53 Individual compounds present in EOs such as citronella/lemongrass, basil, and eucalyptus species demonstrate high repellent activity.54 Since EOs require frequent reapplication for efficacy due to their highly volatile nature, scientists are currently developing a means to prolong their protection time through cream-based formulations.55
The bottom line
Because of the ubiquity of EOs, family physicians will undoubtedly be asked about them by patients, and it would be beneficial to feel comfortable discussing their most common uses. For most adult patients, the topical and periodic inhaled usage of EOs is generally safe.56
There is existing evidence of efficacy for a number of EOs, most strongly for lavender and peppermint. Future research into EOs should include higher-powered and higher-quality studies in order to provide more conclusive evidence regarding the continued use of EOs for many common conditions. More evidence-based information on dosing, application/use regimens, and safety in long-term use also will help providers better instruct patients on how to utilize EOs effectively and safely.
CORRESPONDENCE
Pooja Amy Shah, MD, Columbia University College of Physicians & Surgeons, 610 West 158th Street, New York, NY 10032; [email protected]
1. Butnariu M, Sarac I. Essential oils from plants. J Biotechnol Biomed Sci. 2018;1:35-43. doi: 10.14302/issn.2576-6694.jbbs-18-2489
2. Singh B, Sellam P, Majumder, J, et al. Floral essential oils : importance and uses for mankind. HortFlora Res Spectr. 2014;3:7-13. www.academia.edu/6707801/Floral_essential_oils_Importance_and_uses_for_mankind
3. Posadzki P, Alotaibi A, Ernst E. Adverse effects of aromatherapy: a systematic review of case reports and case series. Int J Risk Saf Med. 2012;24:147-161. doi: 10.3233/JRS-2012-0568
4. Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666. doi: 10.3390/molecules26030666
5. Henley DV, Lipson N, Korach KS, et al. Prepubertal gynecomastia linked to lavender and tea tree oils. N Engl J Med. 2007;356:479-485. doi: 10.1056/NEJMoa064725
6. Nematollahi N, Weinberg JL, Flattery J, et al. Volatile chemical emissions from essential oils with therapeutic claims. Air Qual Atmosphere Health. 2021;14:365-369. doi: 10.1007/s11869-020-00941-4
7. Balekian D, Long A. Essential oil diffusers and asthma. Published February 24, 2020. Accessed September 22, 2023. www.aaaai.org/Allergist-Resources/Ask-the-Expert/Answers/Old-Ask-the-Experts/oil-diffusers-asthma
8. Aura Cacia. Quality. Accessed September 22, 2023. www.auracacia.com/quality
9. Now. Essential oil identity & purity testing. Accessed September 22, 2023. www.nowfoods.com/quality-safety/essential-oil-identity-purity-testing
10. Aura Cacia. GCMS documents. Accessed September 22, 2023. www.auracacia.com/aura-cacia-gcms-documents
11. Lopresti AL, Smith SJ, Drummond PD. Herbal treatments for migraine: a systematic review of randomised-controlled studies. Phytother Res. 2020;34:2493-2517. doi: 10.1002/ptr.6701
12. Niazi M, Hashempur MH, Taghizadeh M, et al. Efficacy of topical Rose (Rosa damascena Mill.) oil for migraine headache: A randomized double-blinded placebo-controlled cross-over trial. Complement Ther Med. 2017;34:35-41. doi: 10.1016/j.ctim. 2017.07.009
13. Rafieian-Kopaei M, Hasanpour-Dehkordi A, Lorigooini Z, et al. Comparing the effect of intranasal lidocaine 4% with peppermint essential oil drop 1.5% on migraine attacks: a double-blind clinical trial. Int J Prev Med. 2019;10:121. doi: 10.4103/ijpvm.IJPVM_530_17
14. Göbel H, Fresenius J, Heinze A, et al. [Effectiveness of Oleum menthae piperitae and paracetamol in therapy of headache of the tension type]. Nervenarzt. 1996;67:672-681. doi: 10.1007/s001150050040
15. Hunt R, Dienemann J, Norton HJ, et al. Aromatherapy as treatment for postoperative nausea: a randomized trial. Anesth Analg. 2013;117:597-604. doi: 10.1213/ANE.0b013e31824a0b1c
16. Maghami M, Afazel MR, Azizi-Fini I, et al. The effect of aromatherapy with peppermint essential oil on nausea and vomiting after cardiac surgery: a randomized clinical trial. Complement Ther Clin Pract. 2020;40:101199. doi: 10.1016/j.ctcp.2020.101199
17. Hills JM, Aaronson PI. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology. 1991;101:55-65. doi: 10.1016/0016-5085(91)90459-x
18. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi: 10.1186/s12906-018-2409-0
19. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571. doi: 10.1007/s10620-015-3858-7
20. Weerts ZZRM, Masclee AAM, Witteman BJM, et al. Efficacy and safety of peppermint oil in a randomized, double-blind trial of patients with irritable bowel syndrome. Gastroenterology. 2020;158:123-136. doi: 10.1053/j.gastro.2019.08.026
21. Ma X, Yue ZQ, Gong ZQ, et al. The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Front Psychol. 2017;8:874. doi: 10.3389/fpsyg.2017.00874
22. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. Published July 7, 2011. doi: 10.4088/PCC.10r01068
23. Donelli D, Antonelli M, Bellinazzi C, et ala. Effects of lavender on anxiety: systematic review and meta-analysis. Phytomedicine Int J Phytother Phytopharm. 2019;65:153099. doi: 10.1016/j.phymed.2019.153099
24. Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:1-10. doi: 10.1155/2013/681304
25. Kang HJ, Nam ES, Lee Y, et al. How strong is the evidence for the anxiolytic efficacy of lavender? Systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res. 2019;13:295-305. doi: 10.1016/j.anr.2019.11.003
26. Barão Paixão VL, Freire de Carvalho J. Essential oil therapy in rheumatic diseases: a systematic review. Complement Ther Clin Pract. 2021;43:101391. doi: 10.1016/j.ctcp.2021.101391
27. Yasa Ozturk G, Bashan I. The effect of aromatherapy with lavender oil on the health-related quality of life in patients with fibromyalgia. J Food Qual. 2021;2021:1-5. doi: 10.1155/2021/9938630
28. Ko GD, Hum A, Traitses G, et al. Effects of topical O24 essential oils on patients with fibromyalgia syndrome: a randomized, placebo controlled pilot study. J Musculoskelet Pain. 2007;15:11-19. doi: 10.1300/J094v15n01_03
29. Lillehei AS, Halcon LL. A systematic review of the effect of inhaled essential oils on sleep. J Altern Complement Med. 2014;20:441-451. doi: 10.1089/acm.2013.0311
30. Cheong MJ, Kim S, Kim JS, et al. A systematic literature review and meta-analysis of the clinical effects of aroma inhalation therapy on sleep problems. Medicine (Baltimore). 2021;100:e24652. doi: 10.1097/MD.0000000000024652
31. Afrasiabian F, Mirabzadeh Ardakani M, Rahmani K, et al. Aloysia citriodora Paláu (lemon verbena) for insomnia patients: a randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Phytother Res PTR. 2019;33:350-359. doi: 10.1002/ptr.6228
32. Lee M, Lim S, Song JA, et al. The effects of aromatherapy essential oil inhalation on stress, sleep quality and immunity in healthy adults: randomized controlled trial. Eur J Integr Med. 2017;12:79-86. doi: 10.1016/j.eujim.2017.04.009
33. Nasiri Lari Z, Hajimonfarednejad M, Riasatian M, et al. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J Ethnopharmacol. 2020;251:112560. doi: 10.1016/j.jep.2020.112560
34. McDonnell B, Newcomb P. Trial of essential oils to improve sleep for patients in cardiac rehabilitation. J Altern Complement Med N Y N. 2019;25:1193-1199. doi: 10.1089/acm.2019.0222
35. Song JA, Lee MK, Min E, et al. Effects of aromatherapy on dysmenorrhea: a systematic review and meta-analysis. Int J Nurs Stud. 2018;84:1-11. doi: 10.1016/j.ijnurstu.2018.01.016
36. Ou MC, Hsu TF, Lai AC, et al. Pain relief assessment by aromatic essential oil massage on outpatients with primary dysmenorrhea: a randomized, double-blind clinical trial: PD pain relief by aromatic oil massage. J Obstet Gynaecol Res. 2012;38:817-822. doi: 10.1111/j.1447-0756.2011.01802.x
37. Sut N, Kahyaoglu-Sut H. Effect of aromatherapy massage on pain in primary dysmenorrhea: a meta-analysis. Complement Ther Clin Pract. 2017;27:5-10. doi: 10.1016/j.ctcp.2017.01.001
38. Keyhanmehr AS, Kolouri S, Heydarirad G, et al. Aromatherapy for the management of cancer complications: a narrative review. Complement Ther Clin Pract. 2018;31:175-180. doi: 10.1016/j.ctcp.2018.02.009
39. Sriningsih I, Elisa E, Lestari KP. Aromatherapy ginger use in patients with nausea & vomiting on post cervical cancer chemotherapy. KEMAS J Kesehat Masy. 2017;13:59-68. doi: 10.15294/kemas.v13i1.5367
40. Lua PL, Salihah N, Mazlan N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement Ther Med. 2015;23:396-404. doi: 10.1016/j.ctim.2015.03.009
41. Arslan I, Aydinoglu S, Karan NB. Can lavender oil inhalation help to overcome dental anxiety and pain in children? A randomized clinical trial. Eur J Pediatr. 2020;179:985-992. doi: 10.1007/s00431-020-03595-7
42. Ghaderi F, Solhjou N. The effects of lavender aromatherapy on stress and pain perception in children during dental treatment: a randomized clinical trial. Complement Ther Clin Pract. 2020;40:101182. doi: 10.1016/j.ctcp.2020.101182
43. Jafarzadeh M, Arman S, Pour FF. Effect of aromatherapy with orange essential oil on salivary cortisol and pulse rate in children during dental treatment: a randomized controlled clinical trial. Adv Biomed Res. 2013;2:10. doi: 10.4103/2277-9175.107968
44. Lehrner J, Eckersberger C, Walla P, et al. Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiol Behav. 2000;71:83-86. doi: 10.1016/S0031-9384(00)00308-5
45. Jimbo D, Kimura Y, Taniguchi M, et al. Effect of aromatherapy on patients with Alzheimer’s disease. Psychogeriatrics. 2009;9:173-179. doi: 10.1111/j.1479-8301.2009.00299.x
46. Ball EL, Owen-Booth B, Gray A, et al. Aromatherapy for dementia. Cochrane Database Syst Rev. 2020;(8). doi: 10.1002/14651858.CD003150.pub3
47. Uzunçakmak T, Ayaz Alkaya S. Effect of aromatherapy on coping with premenstrual syndrome: a randomized controlled trial. Complement Ther Med. 2018;36:63-67. doi: 10.1016/j.ctim.2017.11.022
48. Tanvisut R, Traisrisilp K, Tongsong T. Efficacy of aromatherapy for reducing pain during labor: a randomized controlled trial. Arch Gynecol Obstet. 2018;297:1145-1150. doi: 10.1007/s00404-018-4700-1
49. Ramsey JT, Shropshire BC, Nagy TR, et al. Essential oils and health. Yale J Biol Med. 2020;93:291-305.
50. Puškárová A, Bučková M, Kraková L, et al. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci Rep. 2017;7:8211. doi: 10.1038/s41598-017-08673-9
51. Deyno S, Mtewa AG, Abebe A, et al. Essential oils as topical anti-infective agents: a systematic review and meta-analysis. Complement Ther Med. 2019;47:102224. doi: 10.1016/j.ctim.2019.102224
52. Prall S, Bowles EJ, Bennett K, et al. Effects of essential oils on symptoms and course (duration and severity) of viral respiratory infections in humans: a rapid review. Adv Integr Med. 2020;7:218-221. doi: 10.1016/j.aimed.2020.07.005
53. Weeks JA, Guiney PD, Nikiforov AI. Assessment of the environmental fate and ecotoxicity of N,N-diethyl-m-toluamide (DEET). Integr Environ Assess Manag. 2012;8:120-134. doi: 10.1002/ieam.1246
54. Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol. 2010;101:372-378. doi: 10.1016/j.biortech.2009.07.048
55. Lee MY. Essential oils as repellents against arthropods. BioMed Res Int. 2018;2018:6860271. doi: 10.1155/2018/6860271
56. Göbel H, Heinze A, Heinze-Kuhn K, et al. [Peppermint oil in the acute treatment of tension-type headache]. Schmerz Berl Ger. 2016;30:295-310. doi: 10.1007/s00482-016-0109-6
Essential oils (EOs), which are concentrated plant-based oils, have become ubiquitous over the past decade. Given the far reach of EOs and their longtime use in traditional, complementary, alternative, and integrative medicine, it is imperative that clinicians have some knowledge of the potential benefits, risks, and overall efficacy.
Commonly used for aromatic benefits (aromatherapy), EOs are now also incorporated into a multitude of products promoting health and wellness. EOs are sold as individual products and can be a component in consumer goods such as cosmetics, body care/hygiene/beauty products, laundry detergents, insect repellents, over-the-counter medications, and food.
The review that follows presents the most current evidence available. With that said, it’s important to keep in mind some caveats that relate to this evidence. First, the studies cited tend to have a small sample size. Second, a majority of these studies were conducted in countries where there appears to be a significant culture of EO use, which could contribute to confirmation bias. Finally, in a number of the studies, there is concern for publication bias as well as a discrepancy between calculated statistical significance and actual clinical relevance.

What are essential oils?
EOs generally are made by extracting the oil from leaves, bark, flowers, seeds/fruit, rinds, and/or roots by steaming or pressing parts of a plant. It can take several pounds of plant material to produce a single bottle of EO, which usually contains ≥ 15 to 30 mL (.5 to 1 oz).1
Some commonly used EOs in the United States are lavender, peppermint, rose, clary sage, tea tree, eucalyptus, and citrus; however, there are approximately 300 EOs available.2 EOs are used most often via topical application, inhalation, or ingestion.
As with any botanical agent, EOs are complex substances often containing a multitude of chemical compounds.1 Because of the complex makeup of EOs, which often contain up to 100 volatile organic compounds, and their wide-ranging potential effects, applying the scientific method to study effectiveness poses a challenge that has limited their adoption in evidence-based practice.2
Availability and cost. EOs can be purchased at large retailers (eg, grocery stores, drug stores) and smaller health food stores, as well as on the Internet. Various EO vehicles, such as inhalers and topical creams, also can be purchased at these stores.
Continue to: The cost varies...
The cost varies enormously by manufacturer and type of plant used to make the EO. Common EOs such as peppermint and lavender oil generally cost $10 to $25, while rarer plant oils can cost $80 or more per bottle.
How safe are essential oils?
Patients may assume EOs are harmless because they are derived from natural plants and have been used medicinally for centuries. However, care must be taken with their use.
The safest way to use EOs is topically, although due to their highly concentrated nature, EOs should be diluted in an unscented neutral carrier oil such as coconut, jojoba, olive, or sweet almond.3 Ingestion of certain oils can cause hepatotoxicity, seizures, and even death.3 In fact, patients should speak with a knowledgeable physician before purchasing any oral EO capsules.
Whether used topically or ingested, all EOs carry risk for skin irritation and allergic reactions, and oral ingestion may result in some negative gastrointestinal (GI) adverse effects.4 A case report of 3 patients published in 2007 identified the potential for lavender and tea tree EOs to be endocrine disruptors.5
Inhalation of EOs may be harmful, as they emit many volatile organic compounds, some of which are considered potentially hazardous.6 At this time, there is insufficient evidence regarding inhaled EOs and their direct connection to respiratory health. It is reasonable to suggest, however, that the prolonged use of EOs and their use by patients who have lung conditions such as asthma or COPD should be avoided.7
Continue to: How are quality and purity assessed?
How are quality and purity assessed?
Like other dietary supplements, EOs are not regulated. No US regulatory agencies (eg, the US Food and Drug Administration [FDA] or Department of Agriculture [USDA]) certify or approve EOs for quality and purity. Bottles labeled with “QAI” for Quality Assurance International or “USDA Organic” will ensure the plant constituents used in the EO are from organic farming but do not attest to quality or purity.
Manufacturers commonly use marketing terms such as “therapeutic grade” or “pure” to sell products, but again, these terms do not reflect the product’s quality or purity. A labeled single EO may contain contaminants, alcohol, or additional ingredients.7 When choosing to use EOs, identifying reputable brands is essential; one resource is the independent testing organization ConsumerLab.com.
It is important to assess the manufacturer and read ingredient labels before purchasing an EO to understand what the product contains. Reputable companies will identify the plant ingredient, usually by the formal Latin binomial name, and explain the extraction process. A more certain way to assess the quality and purity of an EO is to ask the manufacturer to provide a certificate of analysis and gas chromatography/mass spectroscopy (GC/MS) data for the specific product. Some manufacturers offer GC/MS test results on their website Quality page.8 Others have detailed information on quality and testing, and GC/MS test reports can be obtained.9 Yet another manufacturer has test results on a product page matching reports to batch codes.10
Which conditions have evidence of benefit from essential oils?
EOs currently are being studied for treatment of many conditions—including pain, GI disorders, behavioral health disorders, and women’s health issues. The TABLE summarizes the conditions treated, outcomes, and practical applications of EOs.11-44

Pain
Headache. As an adjunct to available medications and procedures for headache treatment, EOs are one of the nonpharmacologic modalities that patients and clinicians have at their disposal for both migraine and tension-type headaches. A systematic review of 19 randomized controlled trials (RCTs) examining the effects of herbal ingredients for the acute treatment or prophylaxis of migraines found certain topically applied or inhaled EOs, such as peppermint and chamomile, to be effective for migraine pain alleviation; however, topically applied rose oil was not effective.11-13 Note: “topical application” in these studies implies application of the EO to ≥ 1 of the following areas: temples, forehead, behind ears, or above upper lip/below the nose.
Continue to: One RCT with 120 patients...
One RCT with 120 patients evaluated diluted intranasal peppermint oil and found that it reduced migraine intensity at similar rates to intranasal lidocaine.13 In this study, patients were randomized to receive one of the following: 4% lidocaine, 1.5% peppermint EO, or placebo. Two drops of the intranasal intervention were self-administered while the patient was in a supine position with their head suspended off the edge of the surface on which they were lying. They were instructed to stay in this position for at least 30 seconds after administration.
With regard to tension headache treatment, there is limited literature on the use of EOs. One study found that a preparation of peppermint oil applied topically to the temples and forehead of study participants resulted in significant analgesic effect.14
Fibromyalgia. Usual treatments for fibromyalgia include exercise, antidepressant and anticonvulsant medications, and stress management. Evidence also supports the use of inhaled and topically applied (with and without massage) lavender oil to improve symptoms.26 Positive effects may be related to the analgesic, anti-inflammatory, sleep-regulating, and anxiety-reducing effects of the major volatile compounds contained in lavender oil.
In one RCT with 42 patients with fibromyalgia, the use of inhaled lavender oil was shown to increase the perception of well-being (assessed on the validated SF-36 Health Survey Questionnaire) after 4 weeks.27 In this study, the patient applied 3 drops of an oil mixture, comprising 1 mL lavender EO and 10 mL of fixed neutral base oil, to the wrist and inhaled for 10 minutes before going to bed.
The use of a topical oil blend labeled “Oil 24” (containing camphor, rosemary, eucalyptus, peppermint, aloe vera, and lemon/orange) also has been shown to be more effective than placebo in managing fibromyalgia symptoms. A randomized controlled pilot study of 153 participants found that regular application of Oil 24 improved scores on pain scales and the Fibromyalgia Impact Questionnaire.28
Continue to: GI disorders
GI disorders
Irritable bowel syndrome. Peppermint oil relaxes GI smooth muscle, which has led to investigation of its use in irritable bowel syndrome (IBS) symptom amelioration.17 One meta-analysis including 12 RCTs with 835 patients with undifferentiated IBS found that orally ingested peppermint EO capsules reduced patient-reported symptoms of either abdominal pain or global symptoms.18
One study utilized the Total IBS Symptom Score to evaluate symptom reduction in patients with IBS-D (with diarrhea) and IBS-M (mixed) using 180-mg peppermint EO capsules ingested 3 times daily. There was a significant improvement in abdominal pain/discomfort, bloating/distension, pain at evacuation, and bowel urgency.19 A reduction in symptoms was observed after the first 24 hours of treatment and at the end of the 4-week treatment period.
In another study, among the 190 patients meeting Rome IV criteria for general (nonspecific) IBS who were treated with 182-mg peppermint EO capsules, no statistically significant reduction in overall symptom relief was found (based on outcome measures by the FDA and European Medicines Agency). However, in a secondary outcome analysis, peppermint oil produced greater improvements than placebo for the alleviation of abdominal pain, discomfort, and general IBS severity.20
Chemotherapy-induced nausea and vomiting. Patients with cancer undergoing chemotherapy often explore integrative medicine approaches, including aromatherapy, to ameliorate adverse effects and improve quality of life.38 A few small studies have shown potential for the use of inhaled ginger oil to reduce nausea and vomiting severity and improve health-related quality-of-life measures in these patients.
For example, a study with 60 participants found that inhaling ginger EO for 10 minutes was beneficial for reducing both nausea and vomiting.39 A single-blind, controlled, randomized crossover study of 60 patients with breast cancer receiving chemotherapy showed that ginger EO inhaled 3 times per day for 2 minutes at a time can decrease the severity of nausea but had no effect on vomiting. The same study showed that health-related quality of life improved with the ginger oil treatment.40
Continue to: Other EOs such as cardamom...
Other EOs such as cardamom and peppermint show promise as an adjunctive treatment for chemotherapy-induced nausea and vomiting as well.38
Postoperative nausea. A 2013 randomized trial of 303 patients examined the use of ginger EO, a blend of EOs (including ginger, spearmint, peppermint, and cardamom), and isopropyl alcohol. Both the single EO and EO blend significantly reduced the symptom of nausea. The number of antiemetic medications requested by patients receiving an EO also was significantly reduced compared to those receiving saline.15
The use of EOs to reduce nausea after cardiac operations was reviewed in an RCT of 60 surgical candidates using 10% peppermint oil via nebulization for 10 minutes.16 This technique was effective in reducing nausea during cardiac postoperative periods. Although the evidence for the use of EOs for postoperative nausea is not robust, it may be a useful and generally safe approach for this common issue.
Behavioral health
Insomnia. EOs have been used as a treatment for insomnia traditionally and in complementary, alternative, and integrative medicine. A 2014 systematic review of 15 quantitative studies, including 11 RCTs, evaluated the hypnotic effects of EOs through inhalation, finding the strongest evidence for lavender, jasmine, and peppermint oils.29 The majority of the studies in the systematic review used the Pittsburgh Sleep Quality Index (PSQI) to evaluate EO effectiveness. A more recent 2021 systematic review and meta-analysis that evaluated 34 RCTs found that inhalation of EOs, most notably lavender aromatherapy, is effective in improving sleep problems such as insomnia.30
Findings from multiple smaller RCTs were consistent with those of the aforementioned systematic reviews. For example, in a well-conducted parallel randomized double-blind placebo-controlled trial of 100 people using orally ingested lemon verbena, the authors concluded that this intervention can be a complementary therapy for improving sleep quality and reducing insomnia severity.31 Another RCT with 60 participants evaluated an inhaled EO blend (lemon, eucalyptus, tea tree, and peppermint) over 4 weeks and found lowered perceived stress and depression as well as better sleep quality, but no influence on objective physiologic data such as stress indices or immune states.32
Continue to: In a 2020 randomized crossover...
In a 2020 randomized crossover placebocontrolled trial of 37 participants with diabetes reporting insomnia, inhaled lavender improved sleep quality and quantity, quality of life, and mood but not physiologic or metabolic measures, such as fasting glucose.33 Findings were similar in a cohort of cardiac rehabilitation patients (n = 37) who were treated with either an inhaled combination of lavender, bergamot, and ylang ylang, or placebo; cotton balls infused with the intervention oil or placebo oil were placed at the patient’s bedside for 5 nights. Sleep quality of participants receiving intervention oil was significantly better than the sleep quality of participants receiving the placebo oil as measured by participant completion of the PSQI.34
Anxiety is a common disorder that can be managed with nonpharmacologic treatments such as yoga, deep breathing, meditation, and EO therapy.21,22 In a systematic review and meta-analysis, the inhaled and topical use (with or without massage) of lavender EO was shown to improve psychological and physical manifestations of anxiety.23 Lavender EO is purported to affect the parasympathetic nervous system via anxiolytic, sedative, analgesic, and anticonvulsant properties.24 One systematic review and meta-analysis evaluating the anxiolytic effect of both inhaled and topical lavender EO found improvement in several biomarkers and physiologic data including blood pressure, heart rate, and cortisol levels, as well as a reduction in self-reported levels of anxiety, compared with placebo.25
Anxiety related to dental procedures is another area of study for the use of EOs. Two RCTs demonstrate statistically significant improvement in anxiety-related physiologic markers such as heart rate, blood pressure, and salivary cortisol levels in children who inhaled lavender EO during dental procedures.41,42 In 1 of the RCTs, the intervention was described as 3 drops of 100% lavender EO applied to a cloth and inhaled over the course of 3 minutes.41 Additionally, 2 studies found that orange EO was beneficial for dental procedure–induced anxiety, reducing pulse rates, cortisol levels, and self-reported anxiety.43,44
Dementia-related behavioral disturbances. A small, poorly designed study examining 2 EO blends—rosemary with lemon and lavender with orange—found some potential for improving cognitive function, especially in patients with Alzheimer disease.45 A Cochrane review of 13 RCTs totaling 708 patients concluded that it is not certain from the available evidence that EO therapy benefits patients with dementia in long-term-care facilities and hospital wards.46 Given that reporting of adverse events in the trials was poor, it is not possible to make conclusions about the risk vs benefit of EO therapy in this population.
Women’s health
Dysmenorrhea.
Continue to: In a randomized, double-blind clinical trial...
In a randomized, double-blind clinical trial of 48 women, a cream-based blend of lavender, clary sage, and marjoram EO (used topically in a 2:1:1 ratio diluted in unscented cream at 3% concentration and applied daily via abdominal massage) reduced participants’ reported menstrual pain symptoms and duration of pain.36 In a meta-analysis of 6 studies, topical abdominal application of EO (mainly lavender with or without other oils) with massage showed superiority over massage with placebo oils in reducing menstrual pain.37 A reduction in pain, mood symptoms, and fatigue in women with premenstrual symptoms was seen in an RCT of 77 patients using 3 drops of inhaled lavender EO.47
Labor. There is limited evidence for the use of EOs during labor. In an RCT of 104 women, patient-selected diffused EOs, including lavender, rose geranium, citrus, or jasmine, were found to help lower pain scores during the latent and early active phase of labor. There were no differences in labor augmentation, length of labor, perinatal outcomes, or need for additional pain medication.48
Other uses
Antimicrobial support. Some common EOs that have demonstrated antimicrobial properties are oregano, thyme, clove, lavender, clary sage, garlic, and cinnamon.49,50 Topical lemongrass and tea tree EOs have shown some degree of efficacy as an alternative treatment for acne, decolonization of methicillin-resistant Staphylococcus aureus, and superficial fungal infections.51 Support for an oral mixture of EOs labeled Myrtol (containing eucalyptus, citrus myrtle, and lavender) for viral acute bronchitis and sinusitis was found in a review of 7 studies.52 More research needs to be done before clear recommendations can be made on the use of EOs as antimicrobials, but the current data are encouraging.
Insect repellent. Reviews of the insect-repellent properties of EOs have shown promise and are in the public’s interest due to increasing awareness of the potential health and environmental hazards of synthetic repellents.53 Individual compounds present in EOs such as citronella/lemongrass, basil, and eucalyptus species demonstrate high repellent activity.54 Since EOs require frequent reapplication for efficacy due to their highly volatile nature, scientists are currently developing a means to prolong their protection time through cream-based formulations.55
The bottom line
Because of the ubiquity of EOs, family physicians will undoubtedly be asked about them by patients, and it would be beneficial to feel comfortable discussing their most common uses. For most adult patients, the topical and periodic inhaled usage of EOs is generally safe.56
There is existing evidence of efficacy for a number of EOs, most strongly for lavender and peppermint. Future research into EOs should include higher-powered and higher-quality studies in order to provide more conclusive evidence regarding the continued use of EOs for many common conditions. More evidence-based information on dosing, application/use regimens, and safety in long-term use also will help providers better instruct patients on how to utilize EOs effectively and safely.
CORRESPONDENCE
Pooja Amy Shah, MD, Columbia University College of Physicians & Surgeons, 610 West 158th Street, New York, NY 10032; [email protected]
Essential oils (EOs), which are concentrated plant-based oils, have become ubiquitous over the past decade. Given the far reach of EOs and their longtime use in traditional, complementary, alternative, and integrative medicine, it is imperative that clinicians have some knowledge of the potential benefits, risks, and overall efficacy.
Commonly used for aromatic benefits (aromatherapy), EOs are now also incorporated into a multitude of products promoting health and wellness. EOs are sold as individual products and can be a component in consumer goods such as cosmetics, body care/hygiene/beauty products, laundry detergents, insect repellents, over-the-counter medications, and food.
The review that follows presents the most current evidence available. With that said, it’s important to keep in mind some caveats that relate to this evidence. First, the studies cited tend to have a small sample size. Second, a majority of these studies were conducted in countries where there appears to be a significant culture of EO use, which could contribute to confirmation bias. Finally, in a number of the studies, there is concern for publication bias as well as a discrepancy between calculated statistical significance and actual clinical relevance.

What are essential oils?
EOs generally are made by extracting the oil from leaves, bark, flowers, seeds/fruit, rinds, and/or roots by steaming or pressing parts of a plant. It can take several pounds of plant material to produce a single bottle of EO, which usually contains ≥ 15 to 30 mL (.5 to 1 oz).1
Some commonly used EOs in the United States are lavender, peppermint, rose, clary sage, tea tree, eucalyptus, and citrus; however, there are approximately 300 EOs available.2 EOs are used most often via topical application, inhalation, or ingestion.
As with any botanical agent, EOs are complex substances often containing a multitude of chemical compounds.1 Because of the complex makeup of EOs, which often contain up to 100 volatile organic compounds, and their wide-ranging potential effects, applying the scientific method to study effectiveness poses a challenge that has limited their adoption in evidence-based practice.2
Availability and cost. EOs can be purchased at large retailers (eg, grocery stores, drug stores) and smaller health food stores, as well as on the Internet. Various EO vehicles, such as inhalers and topical creams, also can be purchased at these stores.
Continue to: The cost varies...
The cost varies enormously by manufacturer and type of plant used to make the EO. Common EOs such as peppermint and lavender oil generally cost $10 to $25, while rarer plant oils can cost $80 or more per bottle.
How safe are essential oils?
Patients may assume EOs are harmless because they are derived from natural plants and have been used medicinally for centuries. However, care must be taken with their use.
The safest way to use EOs is topically, although due to their highly concentrated nature, EOs should be diluted in an unscented neutral carrier oil such as coconut, jojoba, olive, or sweet almond.3 Ingestion of certain oils can cause hepatotoxicity, seizures, and even death.3 In fact, patients should speak with a knowledgeable physician before purchasing any oral EO capsules.
Whether used topically or ingested, all EOs carry risk for skin irritation and allergic reactions, and oral ingestion may result in some negative gastrointestinal (GI) adverse effects.4 A case report of 3 patients published in 2007 identified the potential for lavender and tea tree EOs to be endocrine disruptors.5
Inhalation of EOs may be harmful, as they emit many volatile organic compounds, some of which are considered potentially hazardous.6 At this time, there is insufficient evidence regarding inhaled EOs and their direct connection to respiratory health. It is reasonable to suggest, however, that the prolonged use of EOs and their use by patients who have lung conditions such as asthma or COPD should be avoided.7
Continue to: How are quality and purity assessed?
How are quality and purity assessed?
Like other dietary supplements, EOs are not regulated. No US regulatory agencies (eg, the US Food and Drug Administration [FDA] or Department of Agriculture [USDA]) certify or approve EOs for quality and purity. Bottles labeled with “QAI” for Quality Assurance International or “USDA Organic” will ensure the plant constituents used in the EO are from organic farming but do not attest to quality or purity.
Manufacturers commonly use marketing terms such as “therapeutic grade” or “pure” to sell products, but again, these terms do not reflect the product’s quality or purity. A labeled single EO may contain contaminants, alcohol, or additional ingredients.7 When choosing to use EOs, identifying reputable brands is essential; one resource is the independent testing organization ConsumerLab.com.
It is important to assess the manufacturer and read ingredient labels before purchasing an EO to understand what the product contains. Reputable companies will identify the plant ingredient, usually by the formal Latin binomial name, and explain the extraction process. A more certain way to assess the quality and purity of an EO is to ask the manufacturer to provide a certificate of analysis and gas chromatography/mass spectroscopy (GC/MS) data for the specific product. Some manufacturers offer GC/MS test results on their website Quality page.8 Others have detailed information on quality and testing, and GC/MS test reports can be obtained.9 Yet another manufacturer has test results on a product page matching reports to batch codes.10
Which conditions have evidence of benefit from essential oils?
EOs currently are being studied for treatment of many conditions—including pain, GI disorders, behavioral health disorders, and women’s health issues. The TABLE summarizes the conditions treated, outcomes, and practical applications of EOs.11-44

Pain
Headache. As an adjunct to available medications and procedures for headache treatment, EOs are one of the nonpharmacologic modalities that patients and clinicians have at their disposal for both migraine and tension-type headaches. A systematic review of 19 randomized controlled trials (RCTs) examining the effects of herbal ingredients for the acute treatment or prophylaxis of migraines found certain topically applied or inhaled EOs, such as peppermint and chamomile, to be effective for migraine pain alleviation; however, topically applied rose oil was not effective.11-13 Note: “topical application” in these studies implies application of the EO to ≥ 1 of the following areas: temples, forehead, behind ears, or above upper lip/below the nose.
Continue to: One RCT with 120 patients...
One RCT with 120 patients evaluated diluted intranasal peppermint oil and found that it reduced migraine intensity at similar rates to intranasal lidocaine.13 In this study, patients were randomized to receive one of the following: 4% lidocaine, 1.5% peppermint EO, or placebo. Two drops of the intranasal intervention were self-administered while the patient was in a supine position with their head suspended off the edge of the surface on which they were lying. They were instructed to stay in this position for at least 30 seconds after administration.
With regard to tension headache treatment, there is limited literature on the use of EOs. One study found that a preparation of peppermint oil applied topically to the temples and forehead of study participants resulted in significant analgesic effect.14
Fibromyalgia. Usual treatments for fibromyalgia include exercise, antidepressant and anticonvulsant medications, and stress management. Evidence also supports the use of inhaled and topically applied (with and without massage) lavender oil to improve symptoms.26 Positive effects may be related to the analgesic, anti-inflammatory, sleep-regulating, and anxiety-reducing effects of the major volatile compounds contained in lavender oil.
In one RCT with 42 patients with fibromyalgia, the use of inhaled lavender oil was shown to increase the perception of well-being (assessed on the validated SF-36 Health Survey Questionnaire) after 4 weeks.27 In this study, the patient applied 3 drops of an oil mixture, comprising 1 mL lavender EO and 10 mL of fixed neutral base oil, to the wrist and inhaled for 10 minutes before going to bed.
The use of a topical oil blend labeled “Oil 24” (containing camphor, rosemary, eucalyptus, peppermint, aloe vera, and lemon/orange) also has been shown to be more effective than placebo in managing fibromyalgia symptoms. A randomized controlled pilot study of 153 participants found that regular application of Oil 24 improved scores on pain scales and the Fibromyalgia Impact Questionnaire.28
Continue to: GI disorders
GI disorders
Irritable bowel syndrome. Peppermint oil relaxes GI smooth muscle, which has led to investigation of its use in irritable bowel syndrome (IBS) symptom amelioration.17 One meta-analysis including 12 RCTs with 835 patients with undifferentiated IBS found that orally ingested peppermint EO capsules reduced patient-reported symptoms of either abdominal pain or global symptoms.18
One study utilized the Total IBS Symptom Score to evaluate symptom reduction in patients with IBS-D (with diarrhea) and IBS-M (mixed) using 180-mg peppermint EO capsules ingested 3 times daily. There was a significant improvement in abdominal pain/discomfort, bloating/distension, pain at evacuation, and bowel urgency.19 A reduction in symptoms was observed after the first 24 hours of treatment and at the end of the 4-week treatment period.
In another study, among the 190 patients meeting Rome IV criteria for general (nonspecific) IBS who were treated with 182-mg peppermint EO capsules, no statistically significant reduction in overall symptom relief was found (based on outcome measures by the FDA and European Medicines Agency). However, in a secondary outcome analysis, peppermint oil produced greater improvements than placebo for the alleviation of abdominal pain, discomfort, and general IBS severity.20
Chemotherapy-induced nausea and vomiting. Patients with cancer undergoing chemotherapy often explore integrative medicine approaches, including aromatherapy, to ameliorate adverse effects and improve quality of life.38 A few small studies have shown potential for the use of inhaled ginger oil to reduce nausea and vomiting severity and improve health-related quality-of-life measures in these patients.
For example, a study with 60 participants found that inhaling ginger EO for 10 minutes was beneficial for reducing both nausea and vomiting.39 A single-blind, controlled, randomized crossover study of 60 patients with breast cancer receiving chemotherapy showed that ginger EO inhaled 3 times per day for 2 minutes at a time can decrease the severity of nausea but had no effect on vomiting. The same study showed that health-related quality of life improved with the ginger oil treatment.40
Continue to: Other EOs such as cardamom...
Other EOs such as cardamom and peppermint show promise as an adjunctive treatment for chemotherapy-induced nausea and vomiting as well.38
Postoperative nausea. A 2013 randomized trial of 303 patients examined the use of ginger EO, a blend of EOs (including ginger, spearmint, peppermint, and cardamom), and isopropyl alcohol. Both the single EO and EO blend significantly reduced the symptom of nausea. The number of antiemetic medications requested by patients receiving an EO also was significantly reduced compared to those receiving saline.15
The use of EOs to reduce nausea after cardiac operations was reviewed in an RCT of 60 surgical candidates using 10% peppermint oil via nebulization for 10 minutes.16 This technique was effective in reducing nausea during cardiac postoperative periods. Although the evidence for the use of EOs for postoperative nausea is not robust, it may be a useful and generally safe approach for this common issue.
Behavioral health
Insomnia. EOs have been used as a treatment for insomnia traditionally and in complementary, alternative, and integrative medicine. A 2014 systematic review of 15 quantitative studies, including 11 RCTs, evaluated the hypnotic effects of EOs through inhalation, finding the strongest evidence for lavender, jasmine, and peppermint oils.29 The majority of the studies in the systematic review used the Pittsburgh Sleep Quality Index (PSQI) to evaluate EO effectiveness. A more recent 2021 systematic review and meta-analysis that evaluated 34 RCTs found that inhalation of EOs, most notably lavender aromatherapy, is effective in improving sleep problems such as insomnia.30
Findings from multiple smaller RCTs were consistent with those of the aforementioned systematic reviews. For example, in a well-conducted parallel randomized double-blind placebo-controlled trial of 100 people using orally ingested lemon verbena, the authors concluded that this intervention can be a complementary therapy for improving sleep quality and reducing insomnia severity.31 Another RCT with 60 participants evaluated an inhaled EO blend (lemon, eucalyptus, tea tree, and peppermint) over 4 weeks and found lowered perceived stress and depression as well as better sleep quality, but no influence on objective physiologic data such as stress indices or immune states.32
Continue to: In a 2020 randomized crossover...
In a 2020 randomized crossover placebocontrolled trial of 37 participants with diabetes reporting insomnia, inhaled lavender improved sleep quality and quantity, quality of life, and mood but not physiologic or metabolic measures, such as fasting glucose.33 Findings were similar in a cohort of cardiac rehabilitation patients (n = 37) who were treated with either an inhaled combination of lavender, bergamot, and ylang ylang, or placebo; cotton balls infused with the intervention oil or placebo oil were placed at the patient’s bedside for 5 nights. Sleep quality of participants receiving intervention oil was significantly better than the sleep quality of participants receiving the placebo oil as measured by participant completion of the PSQI.34
Anxiety is a common disorder that can be managed with nonpharmacologic treatments such as yoga, deep breathing, meditation, and EO therapy.21,22 In a systematic review and meta-analysis, the inhaled and topical use (with or without massage) of lavender EO was shown to improve psychological and physical manifestations of anxiety.23 Lavender EO is purported to affect the parasympathetic nervous system via anxiolytic, sedative, analgesic, and anticonvulsant properties.24 One systematic review and meta-analysis evaluating the anxiolytic effect of both inhaled and topical lavender EO found improvement in several biomarkers and physiologic data including blood pressure, heart rate, and cortisol levels, as well as a reduction in self-reported levels of anxiety, compared with placebo.25
Anxiety related to dental procedures is another area of study for the use of EOs. Two RCTs demonstrate statistically significant improvement in anxiety-related physiologic markers such as heart rate, blood pressure, and salivary cortisol levels in children who inhaled lavender EO during dental procedures.41,42 In 1 of the RCTs, the intervention was described as 3 drops of 100% lavender EO applied to a cloth and inhaled over the course of 3 minutes.41 Additionally, 2 studies found that orange EO was beneficial for dental procedure–induced anxiety, reducing pulse rates, cortisol levels, and self-reported anxiety.43,44
Dementia-related behavioral disturbances. A small, poorly designed study examining 2 EO blends—rosemary with lemon and lavender with orange—found some potential for improving cognitive function, especially in patients with Alzheimer disease.45 A Cochrane review of 13 RCTs totaling 708 patients concluded that it is not certain from the available evidence that EO therapy benefits patients with dementia in long-term-care facilities and hospital wards.46 Given that reporting of adverse events in the trials was poor, it is not possible to make conclusions about the risk vs benefit of EO therapy in this population.
Women’s health
Dysmenorrhea.
Continue to: In a randomized, double-blind clinical trial...
In a randomized, double-blind clinical trial of 48 women, a cream-based blend of lavender, clary sage, and marjoram EO (used topically in a 2:1:1 ratio diluted in unscented cream at 3% concentration and applied daily via abdominal massage) reduced participants’ reported menstrual pain symptoms and duration of pain.36 In a meta-analysis of 6 studies, topical abdominal application of EO (mainly lavender with or without other oils) with massage showed superiority over massage with placebo oils in reducing menstrual pain.37 A reduction in pain, mood symptoms, and fatigue in women with premenstrual symptoms was seen in an RCT of 77 patients using 3 drops of inhaled lavender EO.47
Labor. There is limited evidence for the use of EOs during labor. In an RCT of 104 women, patient-selected diffused EOs, including lavender, rose geranium, citrus, or jasmine, were found to help lower pain scores during the latent and early active phase of labor. There were no differences in labor augmentation, length of labor, perinatal outcomes, or need for additional pain medication.48
Other uses
Antimicrobial support. Some common EOs that have demonstrated antimicrobial properties are oregano, thyme, clove, lavender, clary sage, garlic, and cinnamon.49,50 Topical lemongrass and tea tree EOs have shown some degree of efficacy as an alternative treatment for acne, decolonization of methicillin-resistant Staphylococcus aureus, and superficial fungal infections.51 Support for an oral mixture of EOs labeled Myrtol (containing eucalyptus, citrus myrtle, and lavender) for viral acute bronchitis and sinusitis was found in a review of 7 studies.52 More research needs to be done before clear recommendations can be made on the use of EOs as antimicrobials, but the current data are encouraging.
Insect repellent. Reviews of the insect-repellent properties of EOs have shown promise and are in the public’s interest due to increasing awareness of the potential health and environmental hazards of synthetic repellents.53 Individual compounds present in EOs such as citronella/lemongrass, basil, and eucalyptus species demonstrate high repellent activity.54 Since EOs require frequent reapplication for efficacy due to their highly volatile nature, scientists are currently developing a means to prolong their protection time through cream-based formulations.55
The bottom line
Because of the ubiquity of EOs, family physicians will undoubtedly be asked about them by patients, and it would be beneficial to feel comfortable discussing their most common uses. For most adult patients, the topical and periodic inhaled usage of EOs is generally safe.56
There is existing evidence of efficacy for a number of EOs, most strongly for lavender and peppermint. Future research into EOs should include higher-powered and higher-quality studies in order to provide more conclusive evidence regarding the continued use of EOs for many common conditions. More evidence-based information on dosing, application/use regimens, and safety in long-term use also will help providers better instruct patients on how to utilize EOs effectively and safely.
CORRESPONDENCE
Pooja Amy Shah, MD, Columbia University College of Physicians & Surgeons, 610 West 158th Street, New York, NY 10032; [email protected]
1. Butnariu M, Sarac I. Essential oils from plants. J Biotechnol Biomed Sci. 2018;1:35-43. doi: 10.14302/issn.2576-6694.jbbs-18-2489
2. Singh B, Sellam P, Majumder, J, et al. Floral essential oils : importance and uses for mankind. HortFlora Res Spectr. 2014;3:7-13. www.academia.edu/6707801/Floral_essential_oils_Importance_and_uses_for_mankind
3. Posadzki P, Alotaibi A, Ernst E. Adverse effects of aromatherapy: a systematic review of case reports and case series. Int J Risk Saf Med. 2012;24:147-161. doi: 10.3233/JRS-2012-0568
4. Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666. doi: 10.3390/molecules26030666
5. Henley DV, Lipson N, Korach KS, et al. Prepubertal gynecomastia linked to lavender and tea tree oils. N Engl J Med. 2007;356:479-485. doi: 10.1056/NEJMoa064725
6. Nematollahi N, Weinberg JL, Flattery J, et al. Volatile chemical emissions from essential oils with therapeutic claims. Air Qual Atmosphere Health. 2021;14:365-369. doi: 10.1007/s11869-020-00941-4
7. Balekian D, Long A. Essential oil diffusers and asthma. Published February 24, 2020. Accessed September 22, 2023. www.aaaai.org/Allergist-Resources/Ask-the-Expert/Answers/Old-Ask-the-Experts/oil-diffusers-asthma
8. Aura Cacia. Quality. Accessed September 22, 2023. www.auracacia.com/quality
9. Now. Essential oil identity & purity testing. Accessed September 22, 2023. www.nowfoods.com/quality-safety/essential-oil-identity-purity-testing
10. Aura Cacia. GCMS documents. Accessed September 22, 2023. www.auracacia.com/aura-cacia-gcms-documents
11. Lopresti AL, Smith SJ, Drummond PD. Herbal treatments for migraine: a systematic review of randomised-controlled studies. Phytother Res. 2020;34:2493-2517. doi: 10.1002/ptr.6701
12. Niazi M, Hashempur MH, Taghizadeh M, et al. Efficacy of topical Rose (Rosa damascena Mill.) oil for migraine headache: A randomized double-blinded placebo-controlled cross-over trial. Complement Ther Med. 2017;34:35-41. doi: 10.1016/j.ctim. 2017.07.009
13. Rafieian-Kopaei M, Hasanpour-Dehkordi A, Lorigooini Z, et al. Comparing the effect of intranasal lidocaine 4% with peppermint essential oil drop 1.5% on migraine attacks: a double-blind clinical trial. Int J Prev Med. 2019;10:121. doi: 10.4103/ijpvm.IJPVM_530_17
14. Göbel H, Fresenius J, Heinze A, et al. [Effectiveness of Oleum menthae piperitae and paracetamol in therapy of headache of the tension type]. Nervenarzt. 1996;67:672-681. doi: 10.1007/s001150050040
15. Hunt R, Dienemann J, Norton HJ, et al. Aromatherapy as treatment for postoperative nausea: a randomized trial. Anesth Analg. 2013;117:597-604. doi: 10.1213/ANE.0b013e31824a0b1c
16. Maghami M, Afazel MR, Azizi-Fini I, et al. The effect of aromatherapy with peppermint essential oil on nausea and vomiting after cardiac surgery: a randomized clinical trial. Complement Ther Clin Pract. 2020;40:101199. doi: 10.1016/j.ctcp.2020.101199
17. Hills JM, Aaronson PI. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology. 1991;101:55-65. doi: 10.1016/0016-5085(91)90459-x
18. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi: 10.1186/s12906-018-2409-0
19. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571. doi: 10.1007/s10620-015-3858-7
20. Weerts ZZRM, Masclee AAM, Witteman BJM, et al. Efficacy and safety of peppermint oil in a randomized, double-blind trial of patients with irritable bowel syndrome. Gastroenterology. 2020;158:123-136. doi: 10.1053/j.gastro.2019.08.026
21. Ma X, Yue ZQ, Gong ZQ, et al. The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Front Psychol. 2017;8:874. doi: 10.3389/fpsyg.2017.00874
22. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. Published July 7, 2011. doi: 10.4088/PCC.10r01068
23. Donelli D, Antonelli M, Bellinazzi C, et ala. Effects of lavender on anxiety: systematic review and meta-analysis. Phytomedicine Int J Phytother Phytopharm. 2019;65:153099. doi: 10.1016/j.phymed.2019.153099
24. Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:1-10. doi: 10.1155/2013/681304
25. Kang HJ, Nam ES, Lee Y, et al. How strong is the evidence for the anxiolytic efficacy of lavender? Systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res. 2019;13:295-305. doi: 10.1016/j.anr.2019.11.003
26. Barão Paixão VL, Freire de Carvalho J. Essential oil therapy in rheumatic diseases: a systematic review. Complement Ther Clin Pract. 2021;43:101391. doi: 10.1016/j.ctcp.2021.101391
27. Yasa Ozturk G, Bashan I. The effect of aromatherapy with lavender oil on the health-related quality of life in patients with fibromyalgia. J Food Qual. 2021;2021:1-5. doi: 10.1155/2021/9938630
28. Ko GD, Hum A, Traitses G, et al. Effects of topical O24 essential oils on patients with fibromyalgia syndrome: a randomized, placebo controlled pilot study. J Musculoskelet Pain. 2007;15:11-19. doi: 10.1300/J094v15n01_03
29. Lillehei AS, Halcon LL. A systematic review of the effect of inhaled essential oils on sleep. J Altern Complement Med. 2014;20:441-451. doi: 10.1089/acm.2013.0311
30. Cheong MJ, Kim S, Kim JS, et al. A systematic literature review and meta-analysis of the clinical effects of aroma inhalation therapy on sleep problems. Medicine (Baltimore). 2021;100:e24652. doi: 10.1097/MD.0000000000024652
31. Afrasiabian F, Mirabzadeh Ardakani M, Rahmani K, et al. Aloysia citriodora Paláu (lemon verbena) for insomnia patients: a randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Phytother Res PTR. 2019;33:350-359. doi: 10.1002/ptr.6228
32. Lee M, Lim S, Song JA, et al. The effects of aromatherapy essential oil inhalation on stress, sleep quality and immunity in healthy adults: randomized controlled trial. Eur J Integr Med. 2017;12:79-86. doi: 10.1016/j.eujim.2017.04.009
33. Nasiri Lari Z, Hajimonfarednejad M, Riasatian M, et al. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J Ethnopharmacol. 2020;251:112560. doi: 10.1016/j.jep.2020.112560
34. McDonnell B, Newcomb P. Trial of essential oils to improve sleep for patients in cardiac rehabilitation. J Altern Complement Med N Y N. 2019;25:1193-1199. doi: 10.1089/acm.2019.0222
35. Song JA, Lee MK, Min E, et al. Effects of aromatherapy on dysmenorrhea: a systematic review and meta-analysis. Int J Nurs Stud. 2018;84:1-11. doi: 10.1016/j.ijnurstu.2018.01.016
36. Ou MC, Hsu TF, Lai AC, et al. Pain relief assessment by aromatic essential oil massage on outpatients with primary dysmenorrhea: a randomized, double-blind clinical trial: PD pain relief by aromatic oil massage. J Obstet Gynaecol Res. 2012;38:817-822. doi: 10.1111/j.1447-0756.2011.01802.x
37. Sut N, Kahyaoglu-Sut H. Effect of aromatherapy massage on pain in primary dysmenorrhea: a meta-analysis. Complement Ther Clin Pract. 2017;27:5-10. doi: 10.1016/j.ctcp.2017.01.001
38. Keyhanmehr AS, Kolouri S, Heydarirad G, et al. Aromatherapy for the management of cancer complications: a narrative review. Complement Ther Clin Pract. 2018;31:175-180. doi: 10.1016/j.ctcp.2018.02.009
39. Sriningsih I, Elisa E, Lestari KP. Aromatherapy ginger use in patients with nausea & vomiting on post cervical cancer chemotherapy. KEMAS J Kesehat Masy. 2017;13:59-68. doi: 10.15294/kemas.v13i1.5367
40. Lua PL, Salihah N, Mazlan N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement Ther Med. 2015;23:396-404. doi: 10.1016/j.ctim.2015.03.009
41. Arslan I, Aydinoglu S, Karan NB. Can lavender oil inhalation help to overcome dental anxiety and pain in children? A randomized clinical trial. Eur J Pediatr. 2020;179:985-992. doi: 10.1007/s00431-020-03595-7
42. Ghaderi F, Solhjou N. The effects of lavender aromatherapy on stress and pain perception in children during dental treatment: a randomized clinical trial. Complement Ther Clin Pract. 2020;40:101182. doi: 10.1016/j.ctcp.2020.101182
43. Jafarzadeh M, Arman S, Pour FF. Effect of aromatherapy with orange essential oil on salivary cortisol and pulse rate in children during dental treatment: a randomized controlled clinical trial. Adv Biomed Res. 2013;2:10. doi: 10.4103/2277-9175.107968
44. Lehrner J, Eckersberger C, Walla P, et al. Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiol Behav. 2000;71:83-86. doi: 10.1016/S0031-9384(00)00308-5
45. Jimbo D, Kimura Y, Taniguchi M, et al. Effect of aromatherapy on patients with Alzheimer’s disease. Psychogeriatrics. 2009;9:173-179. doi: 10.1111/j.1479-8301.2009.00299.x
46. Ball EL, Owen-Booth B, Gray A, et al. Aromatherapy for dementia. Cochrane Database Syst Rev. 2020;(8). doi: 10.1002/14651858.CD003150.pub3
47. Uzunçakmak T, Ayaz Alkaya S. Effect of aromatherapy on coping with premenstrual syndrome: a randomized controlled trial. Complement Ther Med. 2018;36:63-67. doi: 10.1016/j.ctim.2017.11.022
48. Tanvisut R, Traisrisilp K, Tongsong T. Efficacy of aromatherapy for reducing pain during labor: a randomized controlled trial. Arch Gynecol Obstet. 2018;297:1145-1150. doi: 10.1007/s00404-018-4700-1
49. Ramsey JT, Shropshire BC, Nagy TR, et al. Essential oils and health. Yale J Biol Med. 2020;93:291-305.
50. Puškárová A, Bučková M, Kraková L, et al. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci Rep. 2017;7:8211. doi: 10.1038/s41598-017-08673-9
51. Deyno S, Mtewa AG, Abebe A, et al. Essential oils as topical anti-infective agents: a systematic review and meta-analysis. Complement Ther Med. 2019;47:102224. doi: 10.1016/j.ctim.2019.102224
52. Prall S, Bowles EJ, Bennett K, et al. Effects of essential oils on symptoms and course (duration and severity) of viral respiratory infections in humans: a rapid review. Adv Integr Med. 2020;7:218-221. doi: 10.1016/j.aimed.2020.07.005
53. Weeks JA, Guiney PD, Nikiforov AI. Assessment of the environmental fate and ecotoxicity of N,N-diethyl-m-toluamide (DEET). Integr Environ Assess Manag. 2012;8:120-134. doi: 10.1002/ieam.1246
54. Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol. 2010;101:372-378. doi: 10.1016/j.biortech.2009.07.048
55. Lee MY. Essential oils as repellents against arthropods. BioMed Res Int. 2018;2018:6860271. doi: 10.1155/2018/6860271
56. Göbel H, Heinze A, Heinze-Kuhn K, et al. [Peppermint oil in the acute treatment of tension-type headache]. Schmerz Berl Ger. 2016;30:295-310. doi: 10.1007/s00482-016-0109-6
1. Butnariu M, Sarac I. Essential oils from plants. J Biotechnol Biomed Sci. 2018;1:35-43. doi: 10.14302/issn.2576-6694.jbbs-18-2489
2. Singh B, Sellam P, Majumder, J, et al. Floral essential oils : importance and uses for mankind. HortFlora Res Spectr. 2014;3:7-13. www.academia.edu/6707801/Floral_essential_oils_Importance_and_uses_for_mankind
3. Posadzki P, Alotaibi A, Ernst E. Adverse effects of aromatherapy: a systematic review of case reports and case series. Int J Risk Saf Med. 2012;24:147-161. doi: 10.3233/JRS-2012-0568
4. Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666. doi: 10.3390/molecules26030666
5. Henley DV, Lipson N, Korach KS, et al. Prepubertal gynecomastia linked to lavender and tea tree oils. N Engl J Med. 2007;356:479-485. doi: 10.1056/NEJMoa064725
6. Nematollahi N, Weinberg JL, Flattery J, et al. Volatile chemical emissions from essential oils with therapeutic claims. Air Qual Atmosphere Health. 2021;14:365-369. doi: 10.1007/s11869-020-00941-4
7. Balekian D, Long A. Essential oil diffusers and asthma. Published February 24, 2020. Accessed September 22, 2023. www.aaaai.org/Allergist-Resources/Ask-the-Expert/Answers/Old-Ask-the-Experts/oil-diffusers-asthma
8. Aura Cacia. Quality. Accessed September 22, 2023. www.auracacia.com/quality
9. Now. Essential oil identity & purity testing. Accessed September 22, 2023. www.nowfoods.com/quality-safety/essential-oil-identity-purity-testing
10. Aura Cacia. GCMS documents. Accessed September 22, 2023. www.auracacia.com/aura-cacia-gcms-documents
11. Lopresti AL, Smith SJ, Drummond PD. Herbal treatments for migraine: a systematic review of randomised-controlled studies. Phytother Res. 2020;34:2493-2517. doi: 10.1002/ptr.6701
12. Niazi M, Hashempur MH, Taghizadeh M, et al. Efficacy of topical Rose (Rosa damascena Mill.) oil for migraine headache: A randomized double-blinded placebo-controlled cross-over trial. Complement Ther Med. 2017;34:35-41. doi: 10.1016/j.ctim. 2017.07.009
13. Rafieian-Kopaei M, Hasanpour-Dehkordi A, Lorigooini Z, et al. Comparing the effect of intranasal lidocaine 4% with peppermint essential oil drop 1.5% on migraine attacks: a double-blind clinical trial. Int J Prev Med. 2019;10:121. doi: 10.4103/ijpvm.IJPVM_530_17
14. Göbel H, Fresenius J, Heinze A, et al. [Effectiveness of Oleum menthae piperitae and paracetamol in therapy of headache of the tension type]. Nervenarzt. 1996;67:672-681. doi: 10.1007/s001150050040
15. Hunt R, Dienemann J, Norton HJ, et al. Aromatherapy as treatment for postoperative nausea: a randomized trial. Anesth Analg. 2013;117:597-604. doi: 10.1213/ANE.0b013e31824a0b1c
16. Maghami M, Afazel MR, Azizi-Fini I, et al. The effect of aromatherapy with peppermint essential oil on nausea and vomiting after cardiac surgery: a randomized clinical trial. Complement Ther Clin Pract. 2020;40:101199. doi: 10.1016/j.ctcp.2020.101199
17. Hills JM, Aaronson PI. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology. 1991;101:55-65. doi: 10.1016/0016-5085(91)90459-x
18. Alammar N, Wang L, Saberi B, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19:21. doi: 10.1186/s12906-018-2409-0
19. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571. doi: 10.1007/s10620-015-3858-7
20. Weerts ZZRM, Masclee AAM, Witteman BJM, et al. Efficacy and safety of peppermint oil in a randomized, double-blind trial of patients with irritable bowel syndrome. Gastroenterology. 2020;158:123-136. doi: 10.1053/j.gastro.2019.08.026
21. Ma X, Yue ZQ, Gong ZQ, et al. The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Front Psychol. 2017;8:874. doi: 10.3389/fpsyg.2017.00874
22. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. Published July 7, 2011. doi: 10.4088/PCC.10r01068
23. Donelli D, Antonelli M, Bellinazzi C, et ala. Effects of lavender on anxiety: systematic review and meta-analysis. Phytomedicine Int J Phytother Phytopharm. 2019;65:153099. doi: 10.1016/j.phymed.2019.153099
24. Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:1-10. doi: 10.1155/2013/681304
25. Kang HJ, Nam ES, Lee Y, et al. How strong is the evidence for the anxiolytic efficacy of lavender? Systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res. 2019;13:295-305. doi: 10.1016/j.anr.2019.11.003
26. Barão Paixão VL, Freire de Carvalho J. Essential oil therapy in rheumatic diseases: a systematic review. Complement Ther Clin Pract. 2021;43:101391. doi: 10.1016/j.ctcp.2021.101391
27. Yasa Ozturk G, Bashan I. The effect of aromatherapy with lavender oil on the health-related quality of life in patients with fibromyalgia. J Food Qual. 2021;2021:1-5. doi: 10.1155/2021/9938630
28. Ko GD, Hum A, Traitses G, et al. Effects of topical O24 essential oils on patients with fibromyalgia syndrome: a randomized, placebo controlled pilot study. J Musculoskelet Pain. 2007;15:11-19. doi: 10.1300/J094v15n01_03
29. Lillehei AS, Halcon LL. A systematic review of the effect of inhaled essential oils on sleep. J Altern Complement Med. 2014;20:441-451. doi: 10.1089/acm.2013.0311
30. Cheong MJ, Kim S, Kim JS, et al. A systematic literature review and meta-analysis of the clinical effects of aroma inhalation therapy on sleep problems. Medicine (Baltimore). 2021;100:e24652. doi: 10.1097/MD.0000000000024652
31. Afrasiabian F, Mirabzadeh Ardakani M, Rahmani K, et al. Aloysia citriodora Paláu (lemon verbena) for insomnia patients: a randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Phytother Res PTR. 2019;33:350-359. doi: 10.1002/ptr.6228
32. Lee M, Lim S, Song JA, et al. The effects of aromatherapy essential oil inhalation on stress, sleep quality and immunity in healthy adults: randomized controlled trial. Eur J Integr Med. 2017;12:79-86. doi: 10.1016/j.eujim.2017.04.009
33. Nasiri Lari Z, Hajimonfarednejad M, Riasatian M, et al. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J Ethnopharmacol. 2020;251:112560. doi: 10.1016/j.jep.2020.112560
34. McDonnell B, Newcomb P. Trial of essential oils to improve sleep for patients in cardiac rehabilitation. J Altern Complement Med N Y N. 2019;25:1193-1199. doi: 10.1089/acm.2019.0222
35. Song JA, Lee MK, Min E, et al. Effects of aromatherapy on dysmenorrhea: a systematic review and meta-analysis. Int J Nurs Stud. 2018;84:1-11. doi: 10.1016/j.ijnurstu.2018.01.016
36. Ou MC, Hsu TF, Lai AC, et al. Pain relief assessment by aromatic essential oil massage on outpatients with primary dysmenorrhea: a randomized, double-blind clinical trial: PD pain relief by aromatic oil massage. J Obstet Gynaecol Res. 2012;38:817-822. doi: 10.1111/j.1447-0756.2011.01802.x
37. Sut N, Kahyaoglu-Sut H. Effect of aromatherapy massage on pain in primary dysmenorrhea: a meta-analysis. Complement Ther Clin Pract. 2017;27:5-10. doi: 10.1016/j.ctcp.2017.01.001
38. Keyhanmehr AS, Kolouri S, Heydarirad G, et al. Aromatherapy for the management of cancer complications: a narrative review. Complement Ther Clin Pract. 2018;31:175-180. doi: 10.1016/j.ctcp.2018.02.009
39. Sriningsih I, Elisa E, Lestari KP. Aromatherapy ginger use in patients with nausea & vomiting on post cervical cancer chemotherapy. KEMAS J Kesehat Masy. 2017;13:59-68. doi: 10.15294/kemas.v13i1.5367
40. Lua PL, Salihah N, Mazlan N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement Ther Med. 2015;23:396-404. doi: 10.1016/j.ctim.2015.03.009
41. Arslan I, Aydinoglu S, Karan NB. Can lavender oil inhalation help to overcome dental anxiety and pain in children? A randomized clinical trial. Eur J Pediatr. 2020;179:985-992. doi: 10.1007/s00431-020-03595-7
42. Ghaderi F, Solhjou N. The effects of lavender aromatherapy on stress and pain perception in children during dental treatment: a randomized clinical trial. Complement Ther Clin Pract. 2020;40:101182. doi: 10.1016/j.ctcp.2020.101182
43. Jafarzadeh M, Arman S, Pour FF. Effect of aromatherapy with orange essential oil on salivary cortisol and pulse rate in children during dental treatment: a randomized controlled clinical trial. Adv Biomed Res. 2013;2:10. doi: 10.4103/2277-9175.107968
44. Lehrner J, Eckersberger C, Walla P, et al. Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiol Behav. 2000;71:83-86. doi: 10.1016/S0031-9384(00)00308-5
45. Jimbo D, Kimura Y, Taniguchi M, et al. Effect of aromatherapy on patients with Alzheimer’s disease. Psychogeriatrics. 2009;9:173-179. doi: 10.1111/j.1479-8301.2009.00299.x
46. Ball EL, Owen-Booth B, Gray A, et al. Aromatherapy for dementia. Cochrane Database Syst Rev. 2020;(8). doi: 10.1002/14651858.CD003150.pub3
47. Uzunçakmak T, Ayaz Alkaya S. Effect of aromatherapy on coping with premenstrual syndrome: a randomized controlled trial. Complement Ther Med. 2018;36:63-67. doi: 10.1016/j.ctim.2017.11.022
48. Tanvisut R, Traisrisilp K, Tongsong T. Efficacy of aromatherapy for reducing pain during labor: a randomized controlled trial. Arch Gynecol Obstet. 2018;297:1145-1150. doi: 10.1007/s00404-018-4700-1
49. Ramsey JT, Shropshire BC, Nagy TR, et al. Essential oils and health. Yale J Biol Med. 2020;93:291-305.
50. Puškárová A, Bučková M, Kraková L, et al. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci Rep. 2017;7:8211. doi: 10.1038/s41598-017-08673-9
51. Deyno S, Mtewa AG, Abebe A, et al. Essential oils as topical anti-infective agents: a systematic review and meta-analysis. Complement Ther Med. 2019;47:102224. doi: 10.1016/j.ctim.2019.102224
52. Prall S, Bowles EJ, Bennett K, et al. Effects of essential oils on symptoms and course (duration and severity) of viral respiratory infections in humans: a rapid review. Adv Integr Med. 2020;7:218-221. doi: 10.1016/j.aimed.2020.07.005
53. Weeks JA, Guiney PD, Nikiforov AI. Assessment of the environmental fate and ecotoxicity of N,N-diethyl-m-toluamide (DEET). Integr Environ Assess Manag. 2012;8:120-134. doi: 10.1002/ieam.1246
54. Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol. 2010;101:372-378. doi: 10.1016/j.biortech.2009.07.048
55. Lee MY. Essential oils as repellents against arthropods. BioMed Res Int. 2018;2018:6860271. doi: 10.1155/2018/6860271
56. Göbel H, Heinze A, Heinze-Kuhn K, et al. [Peppermint oil in the acute treatment of tension-type headache]. Schmerz Berl Ger. 2016;30:295-310. doi: 10.1007/s00482-016-0109-6
PRACTICE RECOMMENDATIONS
› Utilize lavender essential oil as an adjunctive treatment for fibromyalgia, dysmenorrhea, anxiety, and insomnia symptoms. B
› Recommend peppermint essential oil as an adjunctive treatment for irritable bowel syndrome, chemotherapy-induced nausea, and headache. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Stroke patients benefit from neurologic music therapy
Neurologic music therapy (NMT), a specially designed intervention targeting movement, balance, and cognitive functioning, improves depressive symptoms and increases brain-derived neurotrophic factor (BDNF), early results of a small study suggest.
“We’re really happy with the results,” said lead study author psychotherapist Honey Bryant, a PhD candidate and research assistant at the Centre for Neuroscience Studies, Queen’s University, Kingston, Ont.
“We showed ”
The findings were presented at the virtual XXVI World Congress of Neurology.
Moving with music
With improved stroke survival rates and longer life expectancy, there’s an increasing need for effective post-stroke interventions for neurocognitive impairments and mood disorders, the authors noted.
NMT is an evidence-based treatment system that uses elements of music such as rhythm, melody, and tempo to treat various brain conditions. A trained NMT therapist uses standardized techniques to address goals in the areas of speech, movement, and cognition.
The intervention is not new – it’s been around for a few decades – but there are “minimal papers on NMT and nothing on stroke rehabilitation used in the way we did it,” said Ms. Bryant.
The study included 57 patients, mean age 75 years, receiving rehabilitation following a stroke who were randomly assigned to NMT or passive music listening.
In the NMT group, a music therapist asked participants to choose music beforehand and integrated this into each session.
“Each day was different,” said Ms. Bryant. “For example, if it involved motor movement, the music therapist would say, ‘When I sing this word, raise your arm up.’ For Johnny Cash’s ‘Ring of Fire,’ we made our arms into a circle.”
She explained that the rhythm and timing of the music can affect the motor system and other areas of the brain.
Those in the passive music group listened to a curated list of calming classical and relaxing spa music.
Both groups were offered five 45-minute sessions per week for 2 weeks.
Among other things, researchers used the Hospital Anxiety and Depression Scale (HADS), administered a semistructured interview, and collected blood samples to determine levels of cortisol and BDNF.
After the 2-week intervention, the researchers found participants in the NMT group had a significant mean decrease in depression.
They also had increased cortisol levels, which is not unexpected after a stroke, especially with increased anxiety linked to financial and other stressors, said Ms. Bryant, adding these levels should decrease with treatment.
Recipients of the NMT had significant increases in BDNF, a neurotrophin that plays an important role in neuronal survival and growth, but only in those who attended several consecutive sessions.
Increased plasticity
“We see greater increases in plasticity when the therapy is used intensively, meaning at least four treatments consecutively,” said Ms. Bryant. Participants in the NMT group also reported they “overall felt well,” she added.
She noted NMT can be tailored to individual deficit, “so you can make it solely for motor movement or you can make it solely for language.”
Next steps could include more closely targeting the music to individual preferences and investigating whether the benefits of the intervention extend to other types of brain injury, for example traumatic brain injury, which typically affects younger people, said Ms. Bryant.
“In this study, participants were older and there was an unknown; a lot of them were going back into the community but didn’t know if it was into a retirement home or long-term care.”
It’s unclear if the benefits are sustained after the intervention stops, she said.
There are also the issues of cost and accessibility; in Kingston, there are few music therapists certified in the area of NMT.
Ms. Bryant hopes NMT is eventually included in stroke rehabilitation. “Stroke therapy is typically very intensive on its own; you’re doing it every single day for about a month or 6 weeks,” she said. “It would be interesting to see whether we would see a shorter hospital stay if this is included in stroke rehab.”
Asked to comment, Michael H. Thaut, PhD, professor, faculty of music and faculty of medicine, and Canada research chair in music, neuroscience and health at the University of Toronto, said while these data are preliminary, “they do extend the benefits of NMT in stroke rehabilitation, especially measuring BDNF in addition to having behavioral data.”
However, it’s “unfortunate” the poster didn’t specify which cognitive intervention techniques were used in the study, said Dr. Thaut. “There are nine coded techniques in NMT, including for attention, memory, psychosocial function, and executive function.”
His own study, published in NeuroRehabilitation, focused on training for motor goals in stroke patients. It showed that NMT benefited cognitive functioning and affective responses.
The study was funded by a Queen’s University Research Initiation Grant. Ms. Bryant and Dr. Thaut have not disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
Neurologic music therapy (NMT), a specially designed intervention targeting movement, balance, and cognitive functioning, improves depressive symptoms and increases brain-derived neurotrophic factor (BDNF), early results of a small study suggest.
“We’re really happy with the results,” said lead study author psychotherapist Honey Bryant, a PhD candidate and research assistant at the Centre for Neuroscience Studies, Queen’s University, Kingston, Ont.
“We showed ”
The findings were presented at the virtual XXVI World Congress of Neurology.
Moving with music
With improved stroke survival rates and longer life expectancy, there’s an increasing need for effective post-stroke interventions for neurocognitive impairments and mood disorders, the authors noted.
NMT is an evidence-based treatment system that uses elements of music such as rhythm, melody, and tempo to treat various brain conditions. A trained NMT therapist uses standardized techniques to address goals in the areas of speech, movement, and cognition.
The intervention is not new – it’s been around for a few decades – but there are “minimal papers on NMT and nothing on stroke rehabilitation used in the way we did it,” said Ms. Bryant.
The study included 57 patients, mean age 75 years, receiving rehabilitation following a stroke who were randomly assigned to NMT or passive music listening.
In the NMT group, a music therapist asked participants to choose music beforehand and integrated this into each session.
“Each day was different,” said Ms. Bryant. “For example, if it involved motor movement, the music therapist would say, ‘When I sing this word, raise your arm up.’ For Johnny Cash’s ‘Ring of Fire,’ we made our arms into a circle.”
She explained that the rhythm and timing of the music can affect the motor system and other areas of the brain.
Those in the passive music group listened to a curated list of calming classical and relaxing spa music.
Both groups were offered five 45-minute sessions per week for 2 weeks.
Among other things, researchers used the Hospital Anxiety and Depression Scale (HADS), administered a semistructured interview, and collected blood samples to determine levels of cortisol and BDNF.
After the 2-week intervention, the researchers found participants in the NMT group had a significant mean decrease in depression.
They also had increased cortisol levels, which is not unexpected after a stroke, especially with increased anxiety linked to financial and other stressors, said Ms. Bryant, adding these levels should decrease with treatment.
Recipients of the NMT had significant increases in BDNF, a neurotrophin that plays an important role in neuronal survival and growth, but only in those who attended several consecutive sessions.
Increased plasticity
“We see greater increases in plasticity when the therapy is used intensively, meaning at least four treatments consecutively,” said Ms. Bryant. Participants in the NMT group also reported they “overall felt well,” she added.
She noted NMT can be tailored to individual deficit, “so you can make it solely for motor movement or you can make it solely for language.”
Next steps could include more closely targeting the music to individual preferences and investigating whether the benefits of the intervention extend to other types of brain injury, for example traumatic brain injury, which typically affects younger people, said Ms. Bryant.
“In this study, participants were older and there was an unknown; a lot of them were going back into the community but didn’t know if it was into a retirement home or long-term care.”
It’s unclear if the benefits are sustained after the intervention stops, she said.
There are also the issues of cost and accessibility; in Kingston, there are few music therapists certified in the area of NMT.
Ms. Bryant hopes NMT is eventually included in stroke rehabilitation. “Stroke therapy is typically very intensive on its own; you’re doing it every single day for about a month or 6 weeks,” she said. “It would be interesting to see whether we would see a shorter hospital stay if this is included in stroke rehab.”
Asked to comment, Michael H. Thaut, PhD, professor, faculty of music and faculty of medicine, and Canada research chair in music, neuroscience and health at the University of Toronto, said while these data are preliminary, “they do extend the benefits of NMT in stroke rehabilitation, especially measuring BDNF in addition to having behavioral data.”
However, it’s “unfortunate” the poster didn’t specify which cognitive intervention techniques were used in the study, said Dr. Thaut. “There are nine coded techniques in NMT, including for attention, memory, psychosocial function, and executive function.”
His own study, published in NeuroRehabilitation, focused on training for motor goals in stroke patients. It showed that NMT benefited cognitive functioning and affective responses.
The study was funded by a Queen’s University Research Initiation Grant. Ms. Bryant and Dr. Thaut have not disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
Neurologic music therapy (NMT), a specially designed intervention targeting movement, balance, and cognitive functioning, improves depressive symptoms and increases brain-derived neurotrophic factor (BDNF), early results of a small study suggest.
“We’re really happy with the results,” said lead study author psychotherapist Honey Bryant, a PhD candidate and research assistant at the Centre for Neuroscience Studies, Queen’s University, Kingston, Ont.
“We showed ”
The findings were presented at the virtual XXVI World Congress of Neurology.
Moving with music
With improved stroke survival rates and longer life expectancy, there’s an increasing need for effective post-stroke interventions for neurocognitive impairments and mood disorders, the authors noted.
NMT is an evidence-based treatment system that uses elements of music such as rhythm, melody, and tempo to treat various brain conditions. A trained NMT therapist uses standardized techniques to address goals in the areas of speech, movement, and cognition.
The intervention is not new – it’s been around for a few decades – but there are “minimal papers on NMT and nothing on stroke rehabilitation used in the way we did it,” said Ms. Bryant.
The study included 57 patients, mean age 75 years, receiving rehabilitation following a stroke who were randomly assigned to NMT or passive music listening.
In the NMT group, a music therapist asked participants to choose music beforehand and integrated this into each session.
“Each day was different,” said Ms. Bryant. “For example, if it involved motor movement, the music therapist would say, ‘When I sing this word, raise your arm up.’ For Johnny Cash’s ‘Ring of Fire,’ we made our arms into a circle.”
She explained that the rhythm and timing of the music can affect the motor system and other areas of the brain.
Those in the passive music group listened to a curated list of calming classical and relaxing spa music.
Both groups were offered five 45-minute sessions per week for 2 weeks.
Among other things, researchers used the Hospital Anxiety and Depression Scale (HADS), administered a semistructured interview, and collected blood samples to determine levels of cortisol and BDNF.
After the 2-week intervention, the researchers found participants in the NMT group had a significant mean decrease in depression.
They also had increased cortisol levels, which is not unexpected after a stroke, especially with increased anxiety linked to financial and other stressors, said Ms. Bryant, adding these levels should decrease with treatment.
Recipients of the NMT had significant increases in BDNF, a neurotrophin that plays an important role in neuronal survival and growth, but only in those who attended several consecutive sessions.
Increased plasticity
“We see greater increases in plasticity when the therapy is used intensively, meaning at least four treatments consecutively,” said Ms. Bryant. Participants in the NMT group also reported they “overall felt well,” she added.
She noted NMT can be tailored to individual deficit, “so you can make it solely for motor movement or you can make it solely for language.”
Next steps could include more closely targeting the music to individual preferences and investigating whether the benefits of the intervention extend to other types of brain injury, for example traumatic brain injury, which typically affects younger people, said Ms. Bryant.
“In this study, participants were older and there was an unknown; a lot of them were going back into the community but didn’t know if it was into a retirement home or long-term care.”
It’s unclear if the benefits are sustained after the intervention stops, she said.
There are also the issues of cost and accessibility; in Kingston, there are few music therapists certified in the area of NMT.
Ms. Bryant hopes NMT is eventually included in stroke rehabilitation. “Stroke therapy is typically very intensive on its own; you’re doing it every single day for about a month or 6 weeks,” she said. “It would be interesting to see whether we would see a shorter hospital stay if this is included in stroke rehab.”
Asked to comment, Michael H. Thaut, PhD, professor, faculty of music and faculty of medicine, and Canada research chair in music, neuroscience and health at the University of Toronto, said while these data are preliminary, “they do extend the benefits of NMT in stroke rehabilitation, especially measuring BDNF in addition to having behavioral data.”
However, it’s “unfortunate” the poster didn’t specify which cognitive intervention techniques were used in the study, said Dr. Thaut. “There are nine coded techniques in NMT, including for attention, memory, psychosocial function, and executive function.”
His own study, published in NeuroRehabilitation, focused on training for motor goals in stroke patients. It showed that NMT benefited cognitive functioning and affective responses.
The study was funded by a Queen’s University Research Initiation Grant. Ms. Bryant and Dr. Thaut have not disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM WCN 2023
AHA joins new cardiovascular certification group ABCVM
T to be known as the American Board of Cardiovascular Medicine (ABCVM).
The ABCVM would be independent of the American Board of Internal Medicine (ABIM), the current organization providing maintenance of certification for cardiologists along with 20 other internal medicine subspecialties. The ABIM’s maintenance of certification process has been widely criticized for many years and has been described as “needlessly burdensome and expensive.”
The AHA will be joining the American College of Cardiology (ACC), Heart Failure Society of America (HFSA), Heart Rhythm Society (HRS), and Society for Cardiovascular Angiography & Interventions (SCAI) in forming the ABCVM.
These four other societies issued a joint statement in September saying that they will apply to the American Board of Medical Specialties (ABMS) to request an independent cardiology board that follows a “new competency-based approach to continuous certification — one that harnesses the knowledge, skills, and attitudes required to sustain professional excellence and care for cardiovascular patients effectively.”
The new board requirements will “de-emphasize timed, high stakes performance exams in the continuous certification process and instead will focus on learning assessments to identify gaps in current knowledge or skills,” the statement noted.
At the time the September statement was issued, the AHA was said to be supportive of the move but was waiting for formal endorsement to join the effort by its board of directors.
That has now happened, with the AHA’s national board of directors voting to provide “full support” for the creation of the proposed ABCVM.
“We enthusiastically join with our colleagues in proposing a new professional certification body to accredit cardiovascular professionals called the American Board of Cardiovascular Medicine,” said the association’s volunteer president Joseph C. Wu, MD. “The new ABCVM will be independent of the ABIM and focus on the specific competency-based trainings and appropriate ongoing certifications that align with and strengthen skills for cardiovascular physicians and enhance quality of care for people with cardiovascular disease,” Wu said.
“The AHA joins the consortium to submit the application to the American Board of Medical Specialties (ABMS) requesting an independent medical board for cardiovascular medicine. The consortium’s robust proposal harnesses the knowledge, skills, and benchmarks appropriate for professional excellence and delivery of effective, high-quality cardiovascular care,” Wu added.
The leaders of the ABCVM will include professional representatives from the consortium of member organizations, with a specific focus on relevant education, trainings, and supports that recognize the increasing specialization in cardiology and the latest advances in the various subspecialties of cardiovascular medicine, the AHA notes in a statement.
Professional certification by ABIM is a condition of employment for physicians practicing in large hospitals or health systems. A dedicated certification board separate from ABIM will help to ensure that cardiovascular professionals are maintaining the expertise appropriate to high-quality care and improved outcomes for their patients, the AHA said.
A version of this article first appeared on Medscape.com.
T to be known as the American Board of Cardiovascular Medicine (ABCVM).
The ABCVM would be independent of the American Board of Internal Medicine (ABIM), the current organization providing maintenance of certification for cardiologists along with 20 other internal medicine subspecialties. The ABIM’s maintenance of certification process has been widely criticized for many years and has been described as “needlessly burdensome and expensive.”
The AHA will be joining the American College of Cardiology (ACC), Heart Failure Society of America (HFSA), Heart Rhythm Society (HRS), and Society for Cardiovascular Angiography & Interventions (SCAI) in forming the ABCVM.
These four other societies issued a joint statement in September saying that they will apply to the American Board of Medical Specialties (ABMS) to request an independent cardiology board that follows a “new competency-based approach to continuous certification — one that harnesses the knowledge, skills, and attitudes required to sustain professional excellence and care for cardiovascular patients effectively.”
The new board requirements will “de-emphasize timed, high stakes performance exams in the continuous certification process and instead will focus on learning assessments to identify gaps in current knowledge or skills,” the statement noted.
At the time the September statement was issued, the AHA was said to be supportive of the move but was waiting for formal endorsement to join the effort by its board of directors.
That has now happened, with the AHA’s national board of directors voting to provide “full support” for the creation of the proposed ABCVM.
“We enthusiastically join with our colleagues in proposing a new professional certification body to accredit cardiovascular professionals called the American Board of Cardiovascular Medicine,” said the association’s volunteer president Joseph C. Wu, MD. “The new ABCVM will be independent of the ABIM and focus on the specific competency-based trainings and appropriate ongoing certifications that align with and strengthen skills for cardiovascular physicians and enhance quality of care for people with cardiovascular disease,” Wu said.
“The AHA joins the consortium to submit the application to the American Board of Medical Specialties (ABMS) requesting an independent medical board for cardiovascular medicine. The consortium’s robust proposal harnesses the knowledge, skills, and benchmarks appropriate for professional excellence and delivery of effective, high-quality cardiovascular care,” Wu added.
The leaders of the ABCVM will include professional representatives from the consortium of member organizations, with a specific focus on relevant education, trainings, and supports that recognize the increasing specialization in cardiology and the latest advances in the various subspecialties of cardiovascular medicine, the AHA notes in a statement.
Professional certification by ABIM is a condition of employment for physicians practicing in large hospitals or health systems. A dedicated certification board separate from ABIM will help to ensure that cardiovascular professionals are maintaining the expertise appropriate to high-quality care and improved outcomes for their patients, the AHA said.
A version of this article first appeared on Medscape.com.
T to be known as the American Board of Cardiovascular Medicine (ABCVM).
The ABCVM would be independent of the American Board of Internal Medicine (ABIM), the current organization providing maintenance of certification for cardiologists along with 20 other internal medicine subspecialties. The ABIM’s maintenance of certification process has been widely criticized for many years and has been described as “needlessly burdensome and expensive.”
The AHA will be joining the American College of Cardiology (ACC), Heart Failure Society of America (HFSA), Heart Rhythm Society (HRS), and Society for Cardiovascular Angiography & Interventions (SCAI) in forming the ABCVM.
These four other societies issued a joint statement in September saying that they will apply to the American Board of Medical Specialties (ABMS) to request an independent cardiology board that follows a “new competency-based approach to continuous certification — one that harnesses the knowledge, skills, and attitudes required to sustain professional excellence and care for cardiovascular patients effectively.”
The new board requirements will “de-emphasize timed, high stakes performance exams in the continuous certification process and instead will focus on learning assessments to identify gaps in current knowledge or skills,” the statement noted.
At the time the September statement was issued, the AHA was said to be supportive of the move but was waiting for formal endorsement to join the effort by its board of directors.
That has now happened, with the AHA’s national board of directors voting to provide “full support” for the creation of the proposed ABCVM.
“We enthusiastically join with our colleagues in proposing a new professional certification body to accredit cardiovascular professionals called the American Board of Cardiovascular Medicine,” said the association’s volunteer president Joseph C. Wu, MD. “The new ABCVM will be independent of the ABIM and focus on the specific competency-based trainings and appropriate ongoing certifications that align with and strengthen skills for cardiovascular physicians and enhance quality of care for people with cardiovascular disease,” Wu said.
“The AHA joins the consortium to submit the application to the American Board of Medical Specialties (ABMS) requesting an independent medical board for cardiovascular medicine. The consortium’s robust proposal harnesses the knowledge, skills, and benchmarks appropriate for professional excellence and delivery of effective, high-quality cardiovascular care,” Wu added.
The leaders of the ABCVM will include professional representatives from the consortium of member organizations, with a specific focus on relevant education, trainings, and supports that recognize the increasing specialization in cardiology and the latest advances in the various subspecialties of cardiovascular medicine, the AHA notes in a statement.
Professional certification by ABIM is a condition of employment for physicians practicing in large hospitals or health systems. A dedicated certification board separate from ABIM will help to ensure that cardiovascular professionals are maintaining the expertise appropriate to high-quality care and improved outcomes for their patients, the AHA said.
A version of this article first appeared on Medscape.com.
Reimagining rehabilitation: In-home physical therapy gets a boost
As the aging population grows and telehealth expands in the wake of the COVID-19 pandemic, an emerging trend of in-home care is reshaping how patients access and receive physical therapy services.
Partnerships between hospitals and home health companies are increasing access to rehabilitation services not only for older adults but also for people in rural areas, those without reliable transportation, and patients with injuries that hinder their driving abilities.
“We find more and more that physical therapy at their home, instead of coming to an outpatient facility, is something more and more folks are requesting,” said Bill Benoit, MBA, chief operating officer of University Hospitals, Cleveland. “In this post-COVID environment, people are getting all different types of services in their home when they’re available, and this is one of them. The pandemic sped up the process of us moving away from the traditional brick and mortar hospital.”
UH recently announced a partnership with Luna Physical Therapy, a company founded in 2018 that provides home services. Luna has teamed up with more than two dozen other hospitals in the United States to offer home-based rehabilitation, according to the company.
The process for arranging in-home therapies through hospital-clinic partnerships is like any other inpatient or outpatient rehabilitation, Mr. Benoit said: A patient meets with a specialist or primary care practitioner, they discuss options, and eventually the clinician recommends physical therapy. The only difference here, he said, is rather than going to a separate facility or a hospital, the patient logs onto a mobile app that matches them with a physical therapist on the basis of their location, needs, and the times they are available.
The prescribing physician oversees the patient’s progress through notes provided by the therapist.
“For the primary care physician or surgeon, they’re not going to see much of a difference,” Mr. Benoit said. “This just adds to that list of options for patients.”
Safer, more productive PT
A study, published in the journal Family Practice, found that 76% of patients who are prescribed physical therapy do not initiate the services after it has been recommended.
Aside from the convenience and expanded accessibility for patients, the home therapy option can be more productive, said Denise Wagner, PT, DPT, a physical therapist with Johns Hopkins, Baltimore.
“Home is safer for many patients, but home is also more engaging and motivating,” she said. “Home health clinicians are experts in using whatever they find in the home environment as equipment; many people have stairs in their home, so we can use the rail as something to hold. If patient likes to walk their dog, we can use putting a leash on dog as balance activity.”
Therapy in the home setting helps physical therapists customize programs to fit each patient’s lifestyle, said Gira Shah, PT, a physical therapist with Providence Home Services in Seattle.
For example, patients generally want to know how to function within their own space – navigate their kitchens to make food or get in and out of their bathtubs. Staying in that space allows therapists to focus on those specific goals, Ms. Shah said. “It’s more of a functional therapy. The beauty of this [is that] as therapists we’re trying to assess, ‘what does the patient need to be independent?’ ”
The consulting firm McKinsey predicts that as much as $265 billion in health care services for Medicare recipients will be provided within the home by 2025.
The obvious question is: Why would hospitals partner with clinics rather than offer in-home services on their own?
The answer, like most things in health care, boils down to money.
The billing and documentation system that they use is more efficient than anything hospitals have, said John Brickley, PT, MA, vice president and physical therapist at MedStar Health, a health care system in Maryland and the Washington, D.C., area. MedStar and Luna announced a partnership last June.
“We would financially fall on our face if we tried to use our own billing systems; it would take too much time,” Mr. Brickley said. “Do we need them from a quality-of-care standpoint? No. They have the type of technology that’s not at our disposal.”
Patients should be aware of the difference between home-based PT and other health services for homebound patients, Mr. Brickley said. Medicare considers a patient homebound if they need the help of another person or medical equipment to leave their home or if their doctor believes their condition would worsen with greater mobility.
From the perspective of an insurance company, a home therapy session arranged by a hospital-clinic partnership is an ambulatory appointment and uses the same charging mechanism as most other visits. For a home health care visit, patients must qualify as homebound.
Home-based PT can be used for conditions including neurologic issues, bone and joint problems, balance, and fall deconditioning and prevention. But if a patient needs heavy equipment that cannot be transported, outpatient services are more practical.
That should be determined by the primary care practitioner or specialist evaluating each patient, said Palak Shah, PT, cofounder and head of clinical services at Luna.
“Primary care physicians play a huge role – that’s where patients express their initial concerns,” she said. “It’s up to them to make patients aware about all the options.”
A version of this article first appeared on Medscape.com.
As the aging population grows and telehealth expands in the wake of the COVID-19 pandemic, an emerging trend of in-home care is reshaping how patients access and receive physical therapy services.
Partnerships between hospitals and home health companies are increasing access to rehabilitation services not only for older adults but also for people in rural areas, those without reliable transportation, and patients with injuries that hinder their driving abilities.
“We find more and more that physical therapy at their home, instead of coming to an outpatient facility, is something more and more folks are requesting,” said Bill Benoit, MBA, chief operating officer of University Hospitals, Cleveland. “In this post-COVID environment, people are getting all different types of services in their home when they’re available, and this is one of them. The pandemic sped up the process of us moving away from the traditional brick and mortar hospital.”
UH recently announced a partnership with Luna Physical Therapy, a company founded in 2018 that provides home services. Luna has teamed up with more than two dozen other hospitals in the United States to offer home-based rehabilitation, according to the company.
The process for arranging in-home therapies through hospital-clinic partnerships is like any other inpatient or outpatient rehabilitation, Mr. Benoit said: A patient meets with a specialist or primary care practitioner, they discuss options, and eventually the clinician recommends physical therapy. The only difference here, he said, is rather than going to a separate facility or a hospital, the patient logs onto a mobile app that matches them with a physical therapist on the basis of their location, needs, and the times they are available.
The prescribing physician oversees the patient’s progress through notes provided by the therapist.
“For the primary care physician or surgeon, they’re not going to see much of a difference,” Mr. Benoit said. “This just adds to that list of options for patients.”
Safer, more productive PT
A study, published in the journal Family Practice, found that 76% of patients who are prescribed physical therapy do not initiate the services after it has been recommended.
Aside from the convenience and expanded accessibility for patients, the home therapy option can be more productive, said Denise Wagner, PT, DPT, a physical therapist with Johns Hopkins, Baltimore.
“Home is safer for many patients, but home is also more engaging and motivating,” she said. “Home health clinicians are experts in using whatever they find in the home environment as equipment; many people have stairs in their home, so we can use the rail as something to hold. If patient likes to walk their dog, we can use putting a leash on dog as balance activity.”
Therapy in the home setting helps physical therapists customize programs to fit each patient’s lifestyle, said Gira Shah, PT, a physical therapist with Providence Home Services in Seattle.
For example, patients generally want to know how to function within their own space – navigate their kitchens to make food or get in and out of their bathtubs. Staying in that space allows therapists to focus on those specific goals, Ms. Shah said. “It’s more of a functional therapy. The beauty of this [is that] as therapists we’re trying to assess, ‘what does the patient need to be independent?’ ”
The consulting firm McKinsey predicts that as much as $265 billion in health care services for Medicare recipients will be provided within the home by 2025.
The obvious question is: Why would hospitals partner with clinics rather than offer in-home services on their own?
The answer, like most things in health care, boils down to money.
The billing and documentation system that they use is more efficient than anything hospitals have, said John Brickley, PT, MA, vice president and physical therapist at MedStar Health, a health care system in Maryland and the Washington, D.C., area. MedStar and Luna announced a partnership last June.
“We would financially fall on our face if we tried to use our own billing systems; it would take too much time,” Mr. Brickley said. “Do we need them from a quality-of-care standpoint? No. They have the type of technology that’s not at our disposal.”
Patients should be aware of the difference between home-based PT and other health services for homebound patients, Mr. Brickley said. Medicare considers a patient homebound if they need the help of another person or medical equipment to leave their home or if their doctor believes their condition would worsen with greater mobility.
From the perspective of an insurance company, a home therapy session arranged by a hospital-clinic partnership is an ambulatory appointment and uses the same charging mechanism as most other visits. For a home health care visit, patients must qualify as homebound.
Home-based PT can be used for conditions including neurologic issues, bone and joint problems, balance, and fall deconditioning and prevention. But if a patient needs heavy equipment that cannot be transported, outpatient services are more practical.
That should be determined by the primary care practitioner or specialist evaluating each patient, said Palak Shah, PT, cofounder and head of clinical services at Luna.
“Primary care physicians play a huge role – that’s where patients express their initial concerns,” she said. “It’s up to them to make patients aware about all the options.”
A version of this article first appeared on Medscape.com.
As the aging population grows and telehealth expands in the wake of the COVID-19 pandemic, an emerging trend of in-home care is reshaping how patients access and receive physical therapy services.
Partnerships between hospitals and home health companies are increasing access to rehabilitation services not only for older adults but also for people in rural areas, those without reliable transportation, and patients with injuries that hinder their driving abilities.
“We find more and more that physical therapy at their home, instead of coming to an outpatient facility, is something more and more folks are requesting,” said Bill Benoit, MBA, chief operating officer of University Hospitals, Cleveland. “In this post-COVID environment, people are getting all different types of services in their home when they’re available, and this is one of them. The pandemic sped up the process of us moving away from the traditional brick and mortar hospital.”
UH recently announced a partnership with Luna Physical Therapy, a company founded in 2018 that provides home services. Luna has teamed up with more than two dozen other hospitals in the United States to offer home-based rehabilitation, according to the company.
The process for arranging in-home therapies through hospital-clinic partnerships is like any other inpatient or outpatient rehabilitation, Mr. Benoit said: A patient meets with a specialist or primary care practitioner, they discuss options, and eventually the clinician recommends physical therapy. The only difference here, he said, is rather than going to a separate facility or a hospital, the patient logs onto a mobile app that matches them with a physical therapist on the basis of their location, needs, and the times they are available.
The prescribing physician oversees the patient’s progress through notes provided by the therapist.
“For the primary care physician or surgeon, they’re not going to see much of a difference,” Mr. Benoit said. “This just adds to that list of options for patients.”
Safer, more productive PT
A study, published in the journal Family Practice, found that 76% of patients who are prescribed physical therapy do not initiate the services after it has been recommended.
Aside from the convenience and expanded accessibility for patients, the home therapy option can be more productive, said Denise Wagner, PT, DPT, a physical therapist with Johns Hopkins, Baltimore.
“Home is safer for many patients, but home is also more engaging and motivating,” she said. “Home health clinicians are experts in using whatever they find in the home environment as equipment; many people have stairs in their home, so we can use the rail as something to hold. If patient likes to walk their dog, we can use putting a leash on dog as balance activity.”
Therapy in the home setting helps physical therapists customize programs to fit each patient’s lifestyle, said Gira Shah, PT, a physical therapist with Providence Home Services in Seattle.
For example, patients generally want to know how to function within their own space – navigate their kitchens to make food or get in and out of their bathtubs. Staying in that space allows therapists to focus on those specific goals, Ms. Shah said. “It’s more of a functional therapy. The beauty of this [is that] as therapists we’re trying to assess, ‘what does the patient need to be independent?’ ”
The consulting firm McKinsey predicts that as much as $265 billion in health care services for Medicare recipients will be provided within the home by 2025.
The obvious question is: Why would hospitals partner with clinics rather than offer in-home services on their own?
The answer, like most things in health care, boils down to money.
The billing and documentation system that they use is more efficient than anything hospitals have, said John Brickley, PT, MA, vice president and physical therapist at MedStar Health, a health care system in Maryland and the Washington, D.C., area. MedStar and Luna announced a partnership last June.
“We would financially fall on our face if we tried to use our own billing systems; it would take too much time,” Mr. Brickley said. “Do we need them from a quality-of-care standpoint? No. They have the type of technology that’s not at our disposal.”
Patients should be aware of the difference between home-based PT and other health services for homebound patients, Mr. Brickley said. Medicare considers a patient homebound if they need the help of another person or medical equipment to leave their home or if their doctor believes their condition would worsen with greater mobility.
From the perspective of an insurance company, a home therapy session arranged by a hospital-clinic partnership is an ambulatory appointment and uses the same charging mechanism as most other visits. For a home health care visit, patients must qualify as homebound.
Home-based PT can be used for conditions including neurologic issues, bone and joint problems, balance, and fall deconditioning and prevention. But if a patient needs heavy equipment that cannot be transported, outpatient services are more practical.
That should be determined by the primary care practitioner or specialist evaluating each patient, said Palak Shah, PT, cofounder and head of clinical services at Luna.
“Primary care physicians play a huge role – that’s where patients express their initial concerns,” she said. “It’s up to them to make patients aware about all the options.”
A version of this article first appeared on Medscape.com.
Pustular Eruption on the Face
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
The Diagnosis: Eczema Herpeticum
The patient’s condition with worsening facial edema and notable pain prompted a bedside Tzanck smear using a sample from the base of a deroofed forehead vesicle. In addition, a swab of a deroofed lesion was sent for herpes simplex virus and varicella-zoster virus (VZV) polymerase chain reaction (PCR) testing. The Tzanck smear demonstrated ballooning multinucleated syncytial giant cells and eosinophilic inclusion bodies (Figure), which are characteristic of certain herpesviruses including herpes simplex virus and VZV. He was started on intravenous acyclovir while PCR results were pending; the PCR test later confirmed positivity for herpes simplex virus type 1. Treatment was transitioned to oral valacyclovir once the lesions started crusting over. Notable healing and epithelialization of the lesions occurred during his hospital stay, and he was discharged home 5 days after starting treatment. He was counseled on autoinoculation, advised that he was considered infectious until all lesions had crusted over, and encouraged to employ frequent handwashing. Complete resolution of eczema herpeticum (EH) was noted at 3-week follow-up.

Eczema herpeticum (also known as Kaposi varicelliform eruption) is a potentially life-threatening disseminated cutaneous infection caused by herpes simplex virus types 1 and 2 in patients with pre-existing skin disease.1 It typically presents as a complication of atopic dermatitis (AD) but also has been identified as a rare complication in other conditions that disrupt the normal skin barrier, including mycosis fungoides, pemphigus foliaceus, pemphigus vulgaris, Darier disease, pityriasis rubra pilaris, contact dermatitis, and seborrheic dermatitis.1-4
The pathogenesis of EH is multifactorial. Disruption of the stratum corneum; impaired natural killer cell function; early-onset, untreated, or severe AD; disrupted skin microbiota with skewed colonization by Staphylococcus aureus; immunosuppressive AD therapies such as calcineurin inhibitors; eosinophilia; and helper T cell (TH2) cytokine predominance all have been suggested to play a role in the development of EH.5-8
As seen in our patient, EH presents with a sudden eruption of painful or pruritic, grouped, monomorphic, domeshaped vesicles with background swelling and erythema typically on the head, neck, and trunk. Vesicles then progress to punched-out erosions with overlying hemorrhagic crusting that can coalesce to form large denuded areas susceptible to superinfection with bacteria.9 Other accompanying symptoms include high fever, chills, malaise, and lymphadenopathy. Associated inflammation, classically described as erythema, may be difficult to discern in patients with darker skin and appears as hyperpigmentation; therefore, identification of clusters of monomorphic vesicles in areas of pre-existing dermatitis is particularly important for clinical diagnosis in people with darker skin types.
Various tests are available to confirm diagnosis in ambiguous cases. Bedside Tzanck smears can be performed rapidly and are considered positive if characteristic multinucleated giant cells are noted; however, they do not differentiate between the various herpesviruses. Direct fluorescent antibody testing of scraped lesions and viral cultures of swabbed vesicular fluid are equally effective in distinguishing between herpes simplex virus type 1, herpes simplex virus type 2, and VZV; PCR confirms the diagnosis with high specificity and sensitivity.10
In our patient, the initial differential diagnosis included EH, acute generalized exanthematous pustulosis, allergic contact dermatitis, and Orthopoxvirus infection. The positive Tzanck smear reduced the likelihood of a nonviral etiology. Additionally, worsening of the rash despite discontinuation of medications and utilization of topical steroids argued against acute generalized exanthematous pustulosis and allergic contact dermatitis. The laboratory findings reduced the likelihood of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, and PCR findings ultimately ruled out Orthopoxvirus infections. Additional differential diagnoses for EH include dermatitis herpetiformis; primary VZV infection; hand, foot, and mouth disease; disseminated zoster infection; disseminated molluscum contagiosum; and eczema coxsackium.
Complications of EH include scarring; herpetic keratitis due to corneal infection, which if left untreated can progress to blindness; and rarely death due to multiorgan failure or septicemia.11 The traditional smallpox vaccine (ACAM2000) is contraindicated in patients with AD and EH, even when AD is in remission. These patients should avoid contact with recently vaccinated individuals.12 An alternative vaccine—Jynneos (Bavarian Nordic)—is available for these patients and their family members.13 Clinicians should be aware of this guideline, especially given the recent mpox (monkeypox) outbreaks.
Mild cases of EH are more common, may sometimes go unnoticed, and self-resolve in healthy patients. Severe cases may require systemic antiviral therapy. Acyclovir and its prodrug valacyclovir are standard treatments for EH. Alternatively, foscarnet or cidofovir can be used in the treatment of acyclovir-resistant thymidine kinase– deficient herpes simplex virus and other acyclovirresistant cases.14 Any secondary bacterial superinfections, usually due to staphylococcal or streptococcal bacteria, should be treated with antibiotics. A thorough ophthalmologic evaluation should be performed for patients with periocular involvement of EH. Empiric treatment should be started immediately, given a relative low toxicity of systemic antiviral therapy and high morbidity and mortality associated with untreated widespread EH.
It is important to maintain a high index of clinical suspicion for EH, especially in patients with pre-existing conditions such as AD who present with systemic symptoms and facial vesicles, pustules, or erosions to ensure prompt diagnosis and appropriate treatment.
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
- Baaniya B, Agrawal S. Kaposi varicelliform eruption in a patient with pemphigus vulgaris: a case report and review of the literature. Case Rep Dermatol Med. 2020;2020:6695342. doi:10.1155/2020/6695342
- Tayabali K, Pothiwalla H, Lowitt M. Eczema herpeticum in Darier’s disease: a topical storm. J Community Hosp Intern Med Perspect. 2019;9:347. doi:10.1080/20009666.2019.1650590
- Cavalié M, Giacchero D, Cardot-Leccia N, et al. Kaposi’s varicelliform eruption in a patient with pityriasis rubra pilaris (pityriasis rubra pilaris herpeticum). J Eur Acad Dermatol Venereol. 2013;27:1585-1586. doi:10.1111/JDV.12120
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314. doi:10.5021/ad.2009.21.3.311
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum— a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074-1079. doi:10.1111/JDV.16090
- Kawakami Y, Ando T, Lee J-R, et al. Defective natural killer cell activity in a mouse model of eczema herpeticum. J Allergy Clin Immunol. 2017;139:997-1006.e10. doi:10.1016/j.jaci.2016.06.034
- Beck L, Latchney L, Zaccaro D, et al. Biomarkers of disease severity and Th2 polarity are predictors of risk for eczema herpeticum. J Allergy Clin Immunol. 2008;121:S37-S37. doi:10.1016/j.jaci.2007.12.152
- Kim M, Jung M, Hong SP, et al. Topical calcineurin inhibitors compromise stratum corneum integrity, epidermal permeability and antimicrobial barrier function. Exp Dermatol. 2010; 19:501-510. doi:10.1111/J.1600-0625.2009.00941.X
- Karray M, Kwan E, Souissi A. Kaposi varicelliform eruption. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482432/
- Dominguez SR, Pretty K, Hengartner R, et al. Comparison of herpes simplex virus PCR with culture for virus detection in multisource surface swab specimens from neonates [published online September 25, 2018]. J Clin Microbiol. doi:10.1128/JCM.00632-18
- Feye F, De Halleux C, Gillet JB, et al. Exacerbation of atopic dermatitis in the emergency department. Eur J Emerg Med. 2004;11:49-52. doi:10.1097/00063110-200412000-00014
- Casey C, Vellozzi C, Mootrey GT, et al; Vaccinia Case Definition Development Working Group; Advisory Committee on Immunization Practices-Armed Forces Epidemiological Board Smallpox Vaccine Safety Working Group. Surveillance guidelines for smallpox vaccine (vaccinia) adverse reactions. MMWR Recomm Rep. 2006;55:1-16.
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585 /MMWR.MM7122E1
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459. doi:10.1128/AAC.00615-10
A 52-year-old man developed a sudden eruption of small pustules on background erythema and edema covering the forehead, nasal bridge, periorbital region, cheeks, and perioral region on day 3 of hospitalization in the intensive care unit for management of septic shock secondary to a complicated urinary tract infection. He had a medical history of benign prostatic hyperplasia, sarcoidosis, and atopic dermatitis. He initially presented to the emergency department with fever, chills, and dysuria of 2 days’ duration. Because he received ceftriaxone, vancomycin, ciprofloxacin, and tamsulosin while hospitalized for the infection, the primary medical team suspected a drug reaction and empirically started applying hydrocortisone cream 2.5%. The rash continued to spread over the ensuing day, prompting a dermatology consultation to rule out a drug eruption and to help guide further management. The patient was in substantial distress and pain. Physical examination revealed numerous discrete and confluent monomorphic pustules on background erythema with faint collarettes of scale covering most of the face. Substantial periorbital and facial edema forced the eyes closed. There was no mucous membrane involvement. A review of systems was negative for dyspnea and dysphagia, and the rash was not present elsewhere on the body. Ophthalmologic evaluation revealed no ocular involvement or vision changes. Laboratory studies demonstrated neutrophilia (17.27×109 cells/L [reference range, 2.0–6.9×109 cells/L]). The eosinophil count, blood urea nitrogen/creatinine, and liver function tests were within reference range.