User login
Knowing when enough is enough
“On which side of the bed did you get up this morning?” Obviously, your inquisitor assumes that to avoid clumsily crawling over your sleeping partner you always get up on the side with the table stacked with unread books.
You know as well as I do that you have just received a totally undisguised comment on your recent behavior that has been several shades less than cheery. You may have already sensed your own grumpiness. Do you have an explanation? Did the commute leave you with a case of unresolved road rage? Did you wake up feeling unrested? How often does that happen? Do you think you are getting enough sleep?
A few weeks ago I wrote a Letters From Maine column in which I shared a study suggesting that the regularity of an individual’s sleep pattern may, in many cases, be more important than his or her total number of hours slept. In that same column I wrote that sleep scientists don’t as yet have a good definition of sleep irregularity, nor can they give us any more than a broad range for the total number of hours a person needs to maintain wellness.
How do you determine whether you are getting enough sleep? Do you keep a chart of how many times you were asked which side of the bed you got up on in a week? Or is it how you feel in the morning? Is it when you instantly doze off any time you sit down in a quiet place?
Although many adults are clueless (or in denial) that they are sleep deprived, generally if you ask them and take a brief history they will tell you. On the other hand, determining when a child, particularly one who is preverbal, is sleep deprived is a bit more difficult. Asking the patient isn’t going to give you the answer. You must rely on parental observations. And, to some extent, this can be difficult because parents are, by definition, learning on the job. They may not realize the symptoms and behaviors they are seeing in their child are the result of sleep deficiency.
Over the last half century of observing children, I have developed a very low threshold for diagnosing sleep deprivation. Basically, any child who is cranky and not obviously sick is overtired until proven otherwise. For example, colic does not appear on my frequently used, or in fact ever used, list of diagnoses. Colicky is an adjective that I may use to describe some episodic pain or behavior, but colic as a working diagnosis? Never.
When presented with a child who has already been diagnosed with “colic” by its aunt or the lady next door, this is when the astute pediatrician must be at his or her best. If a thorough history, including sleep pattern, yields no obvious evidence of illness, the next step should be some sleep coaching. However, this is where the “until proven otherwise” thing becomes important, because not providing close follow-up and continuing to keep an open mind for the less likely coexisting conditions can be dangerous and certainly not in the patient’s best interest.
For the older child crankiness, temper tantrums, mood disorders and signs and symptoms often (some might say too often) associated with attention-deficit disorder should trigger an immediate investigation of sleep habits and appropriate advice. Less well-known conditions associated with sleep deprivation are migraine and nocturnal leg pains, often mislabeled as growing pains.
The physicians planning on using sleep as a therapeutic modality is going to quickly run into several challenges. First is convincing the parents, the patient, and the family that the condition is to a greater or lesser degree the result of sleep deprivation. Because sleep is still underappreciated as a component of wellness, this is often not an easy sell.
Second, everyone must accept that altering sleep patterns regardless of age is often not easy and will not be achieved in 1 night or 2. Keeping up the drumbeat of encouragement with close follow-up is critical. Parents must be continually reminded that sleep is being used as a medicine and the dose is not measured in hours. The improvement in symptoms will tell us when enough is enough.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
“On which side of the bed did you get up this morning?” Obviously, your inquisitor assumes that to avoid clumsily crawling over your sleeping partner you always get up on the side with the table stacked with unread books.
You know as well as I do that you have just received a totally undisguised comment on your recent behavior that has been several shades less than cheery. You may have already sensed your own grumpiness. Do you have an explanation? Did the commute leave you with a case of unresolved road rage? Did you wake up feeling unrested? How often does that happen? Do you think you are getting enough sleep?
A few weeks ago I wrote a Letters From Maine column in which I shared a study suggesting that the regularity of an individual’s sleep pattern may, in many cases, be more important than his or her total number of hours slept. In that same column I wrote that sleep scientists don’t as yet have a good definition of sleep irregularity, nor can they give us any more than a broad range for the total number of hours a person needs to maintain wellness.
How do you determine whether you are getting enough sleep? Do you keep a chart of how many times you were asked which side of the bed you got up on in a week? Or is it how you feel in the morning? Is it when you instantly doze off any time you sit down in a quiet place?
Although many adults are clueless (or in denial) that they are sleep deprived, generally if you ask them and take a brief history they will tell you. On the other hand, determining when a child, particularly one who is preverbal, is sleep deprived is a bit more difficult. Asking the patient isn’t going to give you the answer. You must rely on parental observations. And, to some extent, this can be difficult because parents are, by definition, learning on the job. They may not realize the symptoms and behaviors they are seeing in their child are the result of sleep deficiency.
Over the last half century of observing children, I have developed a very low threshold for diagnosing sleep deprivation. Basically, any child who is cranky and not obviously sick is overtired until proven otherwise. For example, colic does not appear on my frequently used, or in fact ever used, list of diagnoses. Colicky is an adjective that I may use to describe some episodic pain or behavior, but colic as a working diagnosis? Never.
When presented with a child who has already been diagnosed with “colic” by its aunt or the lady next door, this is when the astute pediatrician must be at his or her best. If a thorough history, including sleep pattern, yields no obvious evidence of illness, the next step should be some sleep coaching. However, this is where the “until proven otherwise” thing becomes important, because not providing close follow-up and continuing to keep an open mind for the less likely coexisting conditions can be dangerous and certainly not in the patient’s best interest.
For the older child crankiness, temper tantrums, mood disorders and signs and symptoms often (some might say too often) associated with attention-deficit disorder should trigger an immediate investigation of sleep habits and appropriate advice. Less well-known conditions associated with sleep deprivation are migraine and nocturnal leg pains, often mislabeled as growing pains.
The physicians planning on using sleep as a therapeutic modality is going to quickly run into several challenges. First is convincing the parents, the patient, and the family that the condition is to a greater or lesser degree the result of sleep deprivation. Because sleep is still underappreciated as a component of wellness, this is often not an easy sell.
Second, everyone must accept that altering sleep patterns regardless of age is often not easy and will not be achieved in 1 night or 2. Keeping up the drumbeat of encouragement with close follow-up is critical. Parents must be continually reminded that sleep is being used as a medicine and the dose is not measured in hours. The improvement in symptoms will tell us when enough is enough.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
“On which side of the bed did you get up this morning?” Obviously, your inquisitor assumes that to avoid clumsily crawling over your sleeping partner you always get up on the side with the table stacked with unread books.
You know as well as I do that you have just received a totally undisguised comment on your recent behavior that has been several shades less than cheery. You may have already sensed your own grumpiness. Do you have an explanation? Did the commute leave you with a case of unresolved road rage? Did you wake up feeling unrested? How often does that happen? Do you think you are getting enough sleep?
A few weeks ago I wrote a Letters From Maine column in which I shared a study suggesting that the regularity of an individual’s sleep pattern may, in many cases, be more important than his or her total number of hours slept. In that same column I wrote that sleep scientists don’t as yet have a good definition of sleep irregularity, nor can they give us any more than a broad range for the total number of hours a person needs to maintain wellness.
How do you determine whether you are getting enough sleep? Do you keep a chart of how many times you were asked which side of the bed you got up on in a week? Or is it how you feel in the morning? Is it when you instantly doze off any time you sit down in a quiet place?
Although many adults are clueless (or in denial) that they are sleep deprived, generally if you ask them and take a brief history they will tell you. On the other hand, determining when a child, particularly one who is preverbal, is sleep deprived is a bit more difficult. Asking the patient isn’t going to give you the answer. You must rely on parental observations. And, to some extent, this can be difficult because parents are, by definition, learning on the job. They may not realize the symptoms and behaviors they are seeing in their child are the result of sleep deficiency.
Over the last half century of observing children, I have developed a very low threshold for diagnosing sleep deprivation. Basically, any child who is cranky and not obviously sick is overtired until proven otherwise. For example, colic does not appear on my frequently used, or in fact ever used, list of diagnoses. Colicky is an adjective that I may use to describe some episodic pain or behavior, but colic as a working diagnosis? Never.
When presented with a child who has already been diagnosed with “colic” by its aunt or the lady next door, this is when the astute pediatrician must be at his or her best. If a thorough history, including sleep pattern, yields no obvious evidence of illness, the next step should be some sleep coaching. However, this is where the “until proven otherwise” thing becomes important, because not providing close follow-up and continuing to keep an open mind for the less likely coexisting conditions can be dangerous and certainly not in the patient’s best interest.
For the older child crankiness, temper tantrums, mood disorders and signs and symptoms often (some might say too often) associated with attention-deficit disorder should trigger an immediate investigation of sleep habits and appropriate advice. Less well-known conditions associated with sleep deprivation are migraine and nocturnal leg pains, often mislabeled as growing pains.
The physicians planning on using sleep as a therapeutic modality is going to quickly run into several challenges. First is convincing the parents, the patient, and the family that the condition is to a greater or lesser degree the result of sleep deprivation. Because sleep is still underappreciated as a component of wellness, this is often not an easy sell.
Second, everyone must accept that altering sleep patterns regardless of age is often not easy and will not be achieved in 1 night or 2. Keeping up the drumbeat of encouragement with close follow-up is critical. Parents must be continually reminded that sleep is being used as a medicine and the dose is not measured in hours. The improvement in symptoms will tell us when enough is enough.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Marijuana use dramatically increases risk of heart problems, stroke
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
FROM AHA 2023
Body dysmorphic disorder diagnosis guidelines completed in Europe
BERLIN – were outlined in a late-breaker presentation at the annual Congress of the European Academy of Dermatology and Venereology.
The development of guidelines for BDD, a disorder familiar to many clinical dermatologists, is intended as a practical tool, according to Maria-Angeliki Gkini, MD, who has appointments at both Bart’s Health NHS Trust in London and the 401 General Army Hospital in Athens.
“BDD is a relatively common disorder in which the patients are preoccupied with a perceived defect or defects,” Dr. Gkini explained. “This affects them so intensely that it affects their mental health and their quality of life.”
In the DSM-5, published by the American Psychiatric Association, BDD is specifically defined as a preoccupation with “one or more perceived defects or flaws in physical appearance that are not observable or appear slight to others.” But Dr. Gkini said that BDD can also develop as a comorbidity of dermatological disorders that are visible.
These patients are challenging because they are difficult to please, added Dr. Gkini, who said they commonly become involved in doctor shopping, leaving negative reviews on social media for the clinicians they have cycled through. The problem is that the defects they seek to resolve typically stem from distorted perceptions.
BDD is related to obsessive-compulsive disorder by the frequency with which patients pursue repetitive behaviors related to their preoccupation, such as intensive grooming, frequent trips to the mirror, or difficulty in focusing on topics other than their own appearance.
The process to develop the soon-to-be-published guidelines began with a literature search. Of the approximately 3,200 articles identified on BDD, only 10 involved randomized controlled trials. Moreover, even the quality of these trials was considered “low to very low” by the experts who reviewed them, Dr. Gkini said.
One explanation is that psychodermatology has only recently started to attract more research interest, and better studies are now underway, she noted.
However, because of the dearth of high quality evidence now available, the guideline development relied on a Delphi method to reach consensus based on expert opinion in discussion of the available data.
Consensus reached by 17 experts
Specifically, 17 experts, all of whom were members of the European Society for Dermatology and Psychiatry proceeded to systematically address a series of clinical questions and recommendations. Consensus was defined as at least 75% of the participants strongly agreeing or agreeing. Several rounds of discussion were often required.
Among the conclusions, the guidelines support uniform screening for BDD in all patients prior to cosmetic procedures. In identifying depression, anxiety, and distorted perceptions, simple tools, such as the Patient Health Questionnaire might be adequate for an initial evaluation, but Dr. Gkini also recommended routinely inquiring about suicidal ideation, which has been reported in up to 80% of individuals with BDD.
Other instruments for screening that can be considered include DSM-5 criteria for BDD and the Body Dysmorphic Disorder Questionnaire–Dermatology Version, which might be particularly useful and appropriate for dermatologists.
One of the reasons to screen for BDD is that these patients often convince themselves that some specific procedure is needed to resolve the source of their obsession. The goal of screening is to verify that it is the dermatologic concern, not an underlying psychiatric disorder that is driving their search for relief. The risk of dermatologic interventions is not only that expectations are not met, but the patient’s perception of a failed intervention “sometimes makes these worse,” Dr. Gkini explained.
Collaboration with psychiatrists recommended
The guidelines include suggestions for treatment of BDD. Of these, SSRIs are recommended at high relative doses, according to Dr. Gkini. Consistent with the consensus recommendation of collaborating with mental health specialists, she said that the recommendations acknowledge evidence of greater benefits when SSRIs are combined with psychotherapy.
Katharine A. Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, has been conducting BDD research for several years and has written numerous books and articles about this topic, including a review in the journal Focus. She cautioned that, because of a normal concern for appearance, BDD is easily missed by dermatologists.
“For BDD to be diagnosed, the preoccupation with a nonexistent or slight defect in appearance must cause clinically significant distress or impairment in functioning,” she said in an interview. “This is necessary to differentiate BDD from more normal and common appearance concerns that do not qualify for the diagnosis”
She specified that patients should be considered for cognitive-behavioral therapy rather than psychotherapy, a generic term that covers many forms of treatment. She said that most other types of psychotherapy “are probably not effective” for BDD.
Dr. Phillips highly endorsed the development of BDD guidelines for dermatologists because of the frequency with which physicians in this specialty encounter BDD – and believes that more attention to this diagnosis is needed.
“I recommend that dermatologists who have a patient with BDD collaborate with a psychiatrist in delivering care with an SSRI,” she said. “High doses of these medications are often needed to effectively treat BDD.”
Dr. Gkini reported financial relationships with AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO, Novartis, Sanofi, and Regenlab. Dr. Phillips reported no relevant financial relationships.
BERLIN – were outlined in a late-breaker presentation at the annual Congress of the European Academy of Dermatology and Venereology.
The development of guidelines for BDD, a disorder familiar to many clinical dermatologists, is intended as a practical tool, according to Maria-Angeliki Gkini, MD, who has appointments at both Bart’s Health NHS Trust in London and the 401 General Army Hospital in Athens.
“BDD is a relatively common disorder in which the patients are preoccupied with a perceived defect or defects,” Dr. Gkini explained. “This affects them so intensely that it affects their mental health and their quality of life.”
In the DSM-5, published by the American Psychiatric Association, BDD is specifically defined as a preoccupation with “one or more perceived defects or flaws in physical appearance that are not observable or appear slight to others.” But Dr. Gkini said that BDD can also develop as a comorbidity of dermatological disorders that are visible.
These patients are challenging because they are difficult to please, added Dr. Gkini, who said they commonly become involved in doctor shopping, leaving negative reviews on social media for the clinicians they have cycled through. The problem is that the defects they seek to resolve typically stem from distorted perceptions.
BDD is related to obsessive-compulsive disorder by the frequency with which patients pursue repetitive behaviors related to their preoccupation, such as intensive grooming, frequent trips to the mirror, or difficulty in focusing on topics other than their own appearance.
The process to develop the soon-to-be-published guidelines began with a literature search. Of the approximately 3,200 articles identified on BDD, only 10 involved randomized controlled trials. Moreover, even the quality of these trials was considered “low to very low” by the experts who reviewed them, Dr. Gkini said.
One explanation is that psychodermatology has only recently started to attract more research interest, and better studies are now underway, she noted.
However, because of the dearth of high quality evidence now available, the guideline development relied on a Delphi method to reach consensus based on expert opinion in discussion of the available data.
Consensus reached by 17 experts
Specifically, 17 experts, all of whom were members of the European Society for Dermatology and Psychiatry proceeded to systematically address a series of clinical questions and recommendations. Consensus was defined as at least 75% of the participants strongly agreeing or agreeing. Several rounds of discussion were often required.
Among the conclusions, the guidelines support uniform screening for BDD in all patients prior to cosmetic procedures. In identifying depression, anxiety, and distorted perceptions, simple tools, such as the Patient Health Questionnaire might be adequate for an initial evaluation, but Dr. Gkini also recommended routinely inquiring about suicidal ideation, which has been reported in up to 80% of individuals with BDD.
Other instruments for screening that can be considered include DSM-5 criteria for BDD and the Body Dysmorphic Disorder Questionnaire–Dermatology Version, which might be particularly useful and appropriate for dermatologists.
One of the reasons to screen for BDD is that these patients often convince themselves that some specific procedure is needed to resolve the source of their obsession. The goal of screening is to verify that it is the dermatologic concern, not an underlying psychiatric disorder that is driving their search for relief. The risk of dermatologic interventions is not only that expectations are not met, but the patient’s perception of a failed intervention “sometimes makes these worse,” Dr. Gkini explained.
Collaboration with psychiatrists recommended
The guidelines include suggestions for treatment of BDD. Of these, SSRIs are recommended at high relative doses, according to Dr. Gkini. Consistent with the consensus recommendation of collaborating with mental health specialists, she said that the recommendations acknowledge evidence of greater benefits when SSRIs are combined with psychotherapy.
Katharine A. Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, has been conducting BDD research for several years and has written numerous books and articles about this topic, including a review in the journal Focus. She cautioned that, because of a normal concern for appearance, BDD is easily missed by dermatologists.
“For BDD to be diagnosed, the preoccupation with a nonexistent or slight defect in appearance must cause clinically significant distress or impairment in functioning,” she said in an interview. “This is necessary to differentiate BDD from more normal and common appearance concerns that do not qualify for the diagnosis”
She specified that patients should be considered for cognitive-behavioral therapy rather than psychotherapy, a generic term that covers many forms of treatment. She said that most other types of psychotherapy “are probably not effective” for BDD.
Dr. Phillips highly endorsed the development of BDD guidelines for dermatologists because of the frequency with which physicians in this specialty encounter BDD – and believes that more attention to this diagnosis is needed.
“I recommend that dermatologists who have a patient with BDD collaborate with a psychiatrist in delivering care with an SSRI,” she said. “High doses of these medications are often needed to effectively treat BDD.”
Dr. Gkini reported financial relationships with AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO, Novartis, Sanofi, and Regenlab. Dr. Phillips reported no relevant financial relationships.
BERLIN – were outlined in a late-breaker presentation at the annual Congress of the European Academy of Dermatology and Venereology.
The development of guidelines for BDD, a disorder familiar to many clinical dermatologists, is intended as a practical tool, according to Maria-Angeliki Gkini, MD, who has appointments at both Bart’s Health NHS Trust in London and the 401 General Army Hospital in Athens.
“BDD is a relatively common disorder in which the patients are preoccupied with a perceived defect or defects,” Dr. Gkini explained. “This affects them so intensely that it affects their mental health and their quality of life.”
In the DSM-5, published by the American Psychiatric Association, BDD is specifically defined as a preoccupation with “one or more perceived defects or flaws in physical appearance that are not observable or appear slight to others.” But Dr. Gkini said that BDD can also develop as a comorbidity of dermatological disorders that are visible.
These patients are challenging because they are difficult to please, added Dr. Gkini, who said they commonly become involved in doctor shopping, leaving negative reviews on social media for the clinicians they have cycled through. The problem is that the defects they seek to resolve typically stem from distorted perceptions.
BDD is related to obsessive-compulsive disorder by the frequency with which patients pursue repetitive behaviors related to their preoccupation, such as intensive grooming, frequent trips to the mirror, or difficulty in focusing on topics other than their own appearance.
The process to develop the soon-to-be-published guidelines began with a literature search. Of the approximately 3,200 articles identified on BDD, only 10 involved randomized controlled trials. Moreover, even the quality of these trials was considered “low to very low” by the experts who reviewed them, Dr. Gkini said.
One explanation is that psychodermatology has only recently started to attract more research interest, and better studies are now underway, she noted.
However, because of the dearth of high quality evidence now available, the guideline development relied on a Delphi method to reach consensus based on expert opinion in discussion of the available data.
Consensus reached by 17 experts
Specifically, 17 experts, all of whom were members of the European Society for Dermatology and Psychiatry proceeded to systematically address a series of clinical questions and recommendations. Consensus was defined as at least 75% of the participants strongly agreeing or agreeing. Several rounds of discussion were often required.
Among the conclusions, the guidelines support uniform screening for BDD in all patients prior to cosmetic procedures. In identifying depression, anxiety, and distorted perceptions, simple tools, such as the Patient Health Questionnaire might be adequate for an initial evaluation, but Dr. Gkini also recommended routinely inquiring about suicidal ideation, which has been reported in up to 80% of individuals with BDD.
Other instruments for screening that can be considered include DSM-5 criteria for BDD and the Body Dysmorphic Disorder Questionnaire–Dermatology Version, which might be particularly useful and appropriate for dermatologists.
One of the reasons to screen for BDD is that these patients often convince themselves that some specific procedure is needed to resolve the source of their obsession. The goal of screening is to verify that it is the dermatologic concern, not an underlying psychiatric disorder that is driving their search for relief. The risk of dermatologic interventions is not only that expectations are not met, but the patient’s perception of a failed intervention “sometimes makes these worse,” Dr. Gkini explained.
Collaboration with psychiatrists recommended
The guidelines include suggestions for treatment of BDD. Of these, SSRIs are recommended at high relative doses, according to Dr. Gkini. Consistent with the consensus recommendation of collaborating with mental health specialists, she said that the recommendations acknowledge evidence of greater benefits when SSRIs are combined with psychotherapy.
Katharine A. Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, has been conducting BDD research for several years and has written numerous books and articles about this topic, including a review in the journal Focus. She cautioned that, because of a normal concern for appearance, BDD is easily missed by dermatologists.
“For BDD to be diagnosed, the preoccupation with a nonexistent or slight defect in appearance must cause clinically significant distress or impairment in functioning,” she said in an interview. “This is necessary to differentiate BDD from more normal and common appearance concerns that do not qualify for the diagnosis”
She specified that patients should be considered for cognitive-behavioral therapy rather than psychotherapy, a generic term that covers many forms of treatment. She said that most other types of psychotherapy “are probably not effective” for BDD.
Dr. Phillips highly endorsed the development of BDD guidelines for dermatologists because of the frequency with which physicians in this specialty encounter BDD – and believes that more attention to this diagnosis is needed.
“I recommend that dermatologists who have a patient with BDD collaborate with a psychiatrist in delivering care with an SSRI,” she said. “High doses of these medications are often needed to effectively treat BDD.”
Dr. Gkini reported financial relationships with AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO, Novartis, Sanofi, and Regenlab. Dr. Phillips reported no relevant financial relationships.
AT THE EADV CONGRESS
Discomfort in right breast
Breast cancer is the most common tumor type and second only to lung cancer as a cause of cancer death in women. Nearly 300,000 women (and 2800 men) will receive a new diagnosis of breast cancer in the United States in 2023. Despite improvements in treatment options, breast cancer still will lead to 43,700 deaths among women this year.
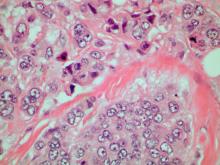
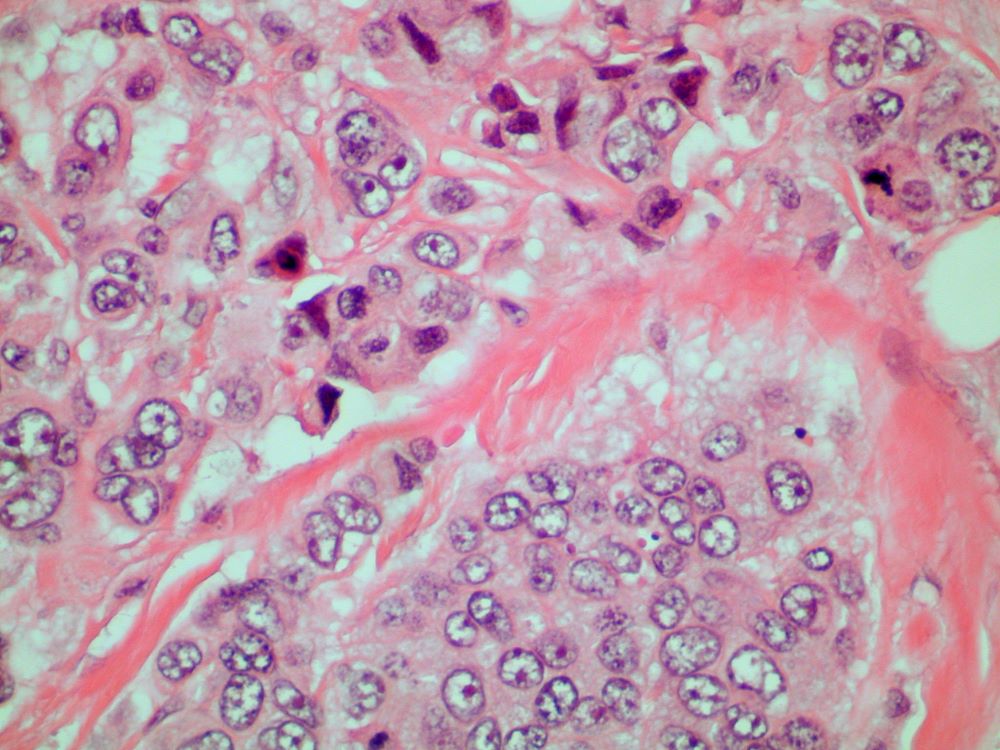
Breast tumors generally are either ductal or lobular in origin. Ductal carcinomas arise in the lining of the milk ducts and are the most commonly found tumor type in breast cancer. Almost 3 in 4 diagnosed breast cancers histologically are invasive ductal carcinomas, which have spread from the source into surrounding structures. It has no specific histologic indicators and is differentiated from ductal carcinoma in situ by its having spread outside the duct. The presence of lymph node involvement in this patient also confirms invasive ductal carcinoma without metastatic spread. Invasive lobular carcinomas occur much less frequently and have a different histologic appearance of smaller cells that appear to be arranged in linear groups.
Mammography involves low-dose radiation and is used in diagnosis and is the most widely used screening technique for breast cancer. As a screening tool, mammography may detect lesions 1-2 years before they become palpable on breast self-examination. While the incidence of breast cancer in women has slowly increased over the past 20 years, mortality has decreased largely as a result of improved awareness and uptake of screening mammography. Current American Cancer Society guidelines recommend continued mammography screenings at least every other year after age 55 and continued for as long as a woman has a life expectancy > 10 years. Screening or diagnosis using MRI is usually reserved for individuals at high risk for breast cancer or a history of breast cancer before age 50.
For all newly diagnosed primary invasive breast cancers, biomarker testing for estrogen and progesterone receptor expression and HER2 expression are part of the standard workup recommended by the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) to help guide treatment decisions. Biomarker testing showed that her tumor was ER+ and HER2-negative, the most common finding in breast cancer. The patient's history did not suggest a risk for BRCA or other familial cancer mutations, but molecular testing was done and was negative for BRCA1 and BRCA2. In postmenopausal women, ASCO and NCCN also recommend use of risk assessment tools, such as Oncotype DX, to determine whether chemotherapy will add benefit to systemic endocrine therapy. Postmenopausal patients with ER+/HER2-negative breast cancer, one to three positive nodes, and a score < 26 on the 21-gene Oncotype DX can safely forego cytotoxic chemotherapy and derive maximum survival benefit from hormonal therapy alone.
This patient was diagnosed with a primary tumor of approximately 25 mm, metastasis to two ipsilateral nodes, and no distant metastasis, or stage IIIA. Her risk recurrence score was 20, indicating that she could safely forego the rigors of cytotoxic chemotherapy. She underwent localized breast-conserving surgery and began adjuvant tamoxifen therapy (x 2 years) to be followed with an aromatase inhibitor (x 3 years).
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Breast cancer is the most common tumor type and second only to lung cancer as a cause of cancer death in women. Nearly 300,000 women (and 2800 men) will receive a new diagnosis of breast cancer in the United States in 2023. Despite improvements in treatment options, breast cancer still will lead to 43,700 deaths among women this year.
Breast tumors generally are either ductal or lobular in origin. Ductal carcinomas arise in the lining of the milk ducts and are the most commonly found tumor type in breast cancer. Almost 3 in 4 diagnosed breast cancers histologically are invasive ductal carcinomas, which have spread from the source into surrounding structures. It has no specific histologic indicators and is differentiated from ductal carcinoma in situ by its having spread outside the duct. The presence of lymph node involvement in this patient also confirms invasive ductal carcinoma without metastatic spread. Invasive lobular carcinomas occur much less frequently and have a different histologic appearance of smaller cells that appear to be arranged in linear groups.
Mammography involves low-dose radiation and is used in diagnosis and is the most widely used screening technique for breast cancer. As a screening tool, mammography may detect lesions 1-2 years before they become palpable on breast self-examination. While the incidence of breast cancer in women has slowly increased over the past 20 years, mortality has decreased largely as a result of improved awareness and uptake of screening mammography. Current American Cancer Society guidelines recommend continued mammography screenings at least every other year after age 55 and continued for as long as a woman has a life expectancy > 10 years. Screening or diagnosis using MRI is usually reserved for individuals at high risk for breast cancer or a history of breast cancer before age 50.
For all newly diagnosed primary invasive breast cancers, biomarker testing for estrogen and progesterone receptor expression and HER2 expression are part of the standard workup recommended by the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) to help guide treatment decisions. Biomarker testing showed that her tumor was ER+ and HER2-negative, the most common finding in breast cancer. The patient's history did not suggest a risk for BRCA or other familial cancer mutations, but molecular testing was done and was negative for BRCA1 and BRCA2. In postmenopausal women, ASCO and NCCN also recommend use of risk assessment tools, such as Oncotype DX, to determine whether chemotherapy will add benefit to systemic endocrine therapy. Postmenopausal patients with ER+/HER2-negative breast cancer, one to three positive nodes, and a score < 26 on the 21-gene Oncotype DX can safely forego cytotoxic chemotherapy and derive maximum survival benefit from hormonal therapy alone.
This patient was diagnosed with a primary tumor of approximately 25 mm, metastasis to two ipsilateral nodes, and no distant metastasis, or stage IIIA. Her risk recurrence score was 20, indicating that she could safely forego the rigors of cytotoxic chemotherapy. She underwent localized breast-conserving surgery and began adjuvant tamoxifen therapy (x 2 years) to be followed with an aromatase inhibitor (x 3 years).
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Breast cancer is the most common tumor type and second only to lung cancer as a cause of cancer death in women. Nearly 300,000 women (and 2800 men) will receive a new diagnosis of breast cancer in the United States in 2023. Despite improvements in treatment options, breast cancer still will lead to 43,700 deaths among women this year.
Breast tumors generally are either ductal or lobular in origin. Ductal carcinomas arise in the lining of the milk ducts and are the most commonly found tumor type in breast cancer. Almost 3 in 4 diagnosed breast cancers histologically are invasive ductal carcinomas, which have spread from the source into surrounding structures. It has no specific histologic indicators and is differentiated from ductal carcinoma in situ by its having spread outside the duct. The presence of lymph node involvement in this patient also confirms invasive ductal carcinoma without metastatic spread. Invasive lobular carcinomas occur much less frequently and have a different histologic appearance of smaller cells that appear to be arranged in linear groups.
Mammography involves low-dose radiation and is used in diagnosis and is the most widely used screening technique for breast cancer. As a screening tool, mammography may detect lesions 1-2 years before they become palpable on breast self-examination. While the incidence of breast cancer in women has slowly increased over the past 20 years, mortality has decreased largely as a result of improved awareness and uptake of screening mammography. Current American Cancer Society guidelines recommend continued mammography screenings at least every other year after age 55 and continued for as long as a woman has a life expectancy > 10 years. Screening or diagnosis using MRI is usually reserved for individuals at high risk for breast cancer or a history of breast cancer before age 50.
For all newly diagnosed primary invasive breast cancers, biomarker testing for estrogen and progesterone receptor expression and HER2 expression are part of the standard workup recommended by the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) to help guide treatment decisions. Biomarker testing showed that her tumor was ER+ and HER2-negative, the most common finding in breast cancer. The patient's history did not suggest a risk for BRCA or other familial cancer mutations, but molecular testing was done and was negative for BRCA1 and BRCA2. In postmenopausal women, ASCO and NCCN also recommend use of risk assessment tools, such as Oncotype DX, to determine whether chemotherapy will add benefit to systemic endocrine therapy. Postmenopausal patients with ER+/HER2-negative breast cancer, one to three positive nodes, and a score < 26 on the 21-gene Oncotype DX can safely forego cytotoxic chemotherapy and derive maximum survival benefit from hormonal therapy alone.
This patient was diagnosed with a primary tumor of approximately 25 mm, metastasis to two ipsilateral nodes, and no distant metastasis, or stage IIIA. Her risk recurrence score was 20, indicating that she could safely forego the rigors of cytotoxic chemotherapy. She underwent localized breast-conserving surgery and began adjuvant tamoxifen therapy (x 2 years) to be followed with an aromatase inhibitor (x 3 years).
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 70-year-old woman presents for an annual exam and reports development of a firm lump in her right breast. She remarks that she has had discomfort in the same area for "at least a year," but the lump has become noticeable with even a cursory self-exam over the past 2 months. She has no previous history of breast cancer, hypertension, type 2 diabetes, or other cardiometabolic disease. She had no previous abnormal findings on mammograms through age 65 but has not had one since. Her family history includes a grandmother who died of breast cancer at age 64 and her father who lived with prostate cancer for 20 years after diagnosis at age 60. The physical exam reveals a firm, palpable lump in the upper right quadrant of her right breast. The exam is otherwise normal for the patient's age, with minor osteoarthritis that she remarks has worsened over the past year. Mammography, image-guided biopsy, and biomarker and molecular testing are ordered. Lymph node testing reveals two involved nodes, and histology reveals the following (see image).
Second infection hikes long COVID risk: Expert Q&A
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
EMR prompt boosts albuminuria measurement in T2D
PHILADELPHIA – An electronic medical record alert to primary care physicians that their adult patients with type 2 diabetes were due for an albuminuria and renal-function check boosted screening for chronic kidney disease (CKD) by roughly half compared with the preintervention rate in a single U.S. academic health system.
“Screening rates for CKD more rapidly improved after implementation” of the EMR alert, said Maggy M. Spolnik, MD, at Kidney Week 2023, organized by the American Society of Nephrology.
“There was an immediate and ongoing effect over a year,” said Dr. Spolnik, a nephrologist at Indiana University in Indianapolis.
However, CKD screening rates in the primary care setting remain a challenge. In the study, the EMR alert produced a urine albumin-to-creatinine ratio (UACR) screening rate of about 26% of patient encounters, she reported. While this was significantly above the roughly 17% rate that had persisted for months before the intervention, it still fell short of the universal annual screening for adults with type 2 diabetes not previously diagnosed with CKD recommended by medical groups such as the American Diabetes Association and the Kidney Disease: Improving Global Outcomes organization. The U.S. Preventive Services Task Force’s assessment in 2012 concluded inadequate information existed at that time to make recommendations about CKD screening, but the group is now revisiting the issue.
‘Albuminuria is an earlier marker’ than eGFR
“Primary care physicians need to regularly monitor albuminuria in adults with type 2 diabetes,” commented Karen A. Griffin, MD, a nephrologist and professor at Loyola University in Maywood, Ill. “By the time you diagnose CKD based on reduced estimated glomerular filtration rate (eGFR), a patient has already lost more than half their renal function. Albuminuria is an earlier marker of a problem,” Dr. Griffin said in an interview.
Primary care physicians have been slow to adopt at least annual checks on both eGFR and the urinary albumin-to-creatinine ratio (UACR) in their adult patients with type 2 diabetes. Dr. Spolnik cited reasons such as the brief 15-minute consultation that primary care physicians have when seeing a patient, and an often confusing ordering menu that gives a UACR test various other names such as tests for microalbuminuria or macroalbuminuria.
To simplify ordering, the EMR prompt assessed in Dr. Spolnik’s study called the test “kidney screening” that automatically bundled an order for both eGFR calculation with UACR measurement. Another limitation is that UACR measurement requires a urine sample, which patients often find inconvenient to provide at the time of their examination.
The study run by Dr. Spolnik involved 10,744 adults with type 2 diabetes without an existing diagnosis of CKD seen in an outpatient, primary care visit to the UVA Health system centered in Charlottesville, Va. during April 2021–April 2022. A total of 23,419 encounters served as usual-care controls. The intervention period with active EMR alerts for kidney screening included 10,204 similar patients seen during April 2022–April 2023 in a total of 20,358 encounters. The patients averaged about 61-62 years old, and about 45% were men.
Bundling alerts into a single pop-up
The primary care clinicians who received the prompts were generally receptive to them, but they asked the researchers to bundle the UACR and eGFR measurement prompts along with any other alerts they received in the EMR into a single on-screen pop-up.
Dr. Spolnik acknowledged the need for further research and refinement to the prompt. For example, she wants to assess prompts for patients identified as having CKD that would promote best-practice management, including lifestyle and medical interventions. She also envisions expanding the prompts to also include other, related disorders such as hypertension.
But she and her colleagues were convinced enough by the results that they have not only continued the program at UVA Health but they also expanded it, starting in October 2023, to the academic primary care practice at Indiana University.
If the Indiana University trial confirms the efficacy seen in Virginia, the next step might be inclusion by Epic of the CKD screening alert as a routine option in the EMR software it distributes to its U.S. clients, Dr. Spolnik said in an interview.
Dr. Spolnik and Dr. Griffin had no disclosures.
PHILADELPHIA – An electronic medical record alert to primary care physicians that their adult patients with type 2 diabetes were due for an albuminuria and renal-function check boosted screening for chronic kidney disease (CKD) by roughly half compared with the preintervention rate in a single U.S. academic health system.
“Screening rates for CKD more rapidly improved after implementation” of the EMR alert, said Maggy M. Spolnik, MD, at Kidney Week 2023, organized by the American Society of Nephrology.
“There was an immediate and ongoing effect over a year,” said Dr. Spolnik, a nephrologist at Indiana University in Indianapolis.
However, CKD screening rates in the primary care setting remain a challenge. In the study, the EMR alert produced a urine albumin-to-creatinine ratio (UACR) screening rate of about 26% of patient encounters, she reported. While this was significantly above the roughly 17% rate that had persisted for months before the intervention, it still fell short of the universal annual screening for adults with type 2 diabetes not previously diagnosed with CKD recommended by medical groups such as the American Diabetes Association and the Kidney Disease: Improving Global Outcomes organization. The U.S. Preventive Services Task Force’s assessment in 2012 concluded inadequate information existed at that time to make recommendations about CKD screening, but the group is now revisiting the issue.
‘Albuminuria is an earlier marker’ than eGFR
“Primary care physicians need to regularly monitor albuminuria in adults with type 2 diabetes,” commented Karen A. Griffin, MD, a nephrologist and professor at Loyola University in Maywood, Ill. “By the time you diagnose CKD based on reduced estimated glomerular filtration rate (eGFR), a patient has already lost more than half their renal function. Albuminuria is an earlier marker of a problem,” Dr. Griffin said in an interview.
Primary care physicians have been slow to adopt at least annual checks on both eGFR and the urinary albumin-to-creatinine ratio (UACR) in their adult patients with type 2 diabetes. Dr. Spolnik cited reasons such as the brief 15-minute consultation that primary care physicians have when seeing a patient, and an often confusing ordering menu that gives a UACR test various other names such as tests for microalbuminuria or macroalbuminuria.
To simplify ordering, the EMR prompt assessed in Dr. Spolnik’s study called the test “kidney screening” that automatically bundled an order for both eGFR calculation with UACR measurement. Another limitation is that UACR measurement requires a urine sample, which patients often find inconvenient to provide at the time of their examination.
The study run by Dr. Spolnik involved 10,744 adults with type 2 diabetes without an existing diagnosis of CKD seen in an outpatient, primary care visit to the UVA Health system centered in Charlottesville, Va. during April 2021–April 2022. A total of 23,419 encounters served as usual-care controls. The intervention period with active EMR alerts for kidney screening included 10,204 similar patients seen during April 2022–April 2023 in a total of 20,358 encounters. The patients averaged about 61-62 years old, and about 45% were men.
Bundling alerts into a single pop-up
The primary care clinicians who received the prompts were generally receptive to them, but they asked the researchers to bundle the UACR and eGFR measurement prompts along with any other alerts they received in the EMR into a single on-screen pop-up.
Dr. Spolnik acknowledged the need for further research and refinement to the prompt. For example, she wants to assess prompts for patients identified as having CKD that would promote best-practice management, including lifestyle and medical interventions. She also envisions expanding the prompts to also include other, related disorders such as hypertension.
But she and her colleagues were convinced enough by the results that they have not only continued the program at UVA Health but they also expanded it, starting in October 2023, to the academic primary care practice at Indiana University.
If the Indiana University trial confirms the efficacy seen in Virginia, the next step might be inclusion by Epic of the CKD screening alert as a routine option in the EMR software it distributes to its U.S. clients, Dr. Spolnik said in an interview.
Dr. Spolnik and Dr. Griffin had no disclosures.
PHILADELPHIA – An electronic medical record alert to primary care physicians that their adult patients with type 2 diabetes were due for an albuminuria and renal-function check boosted screening for chronic kidney disease (CKD) by roughly half compared with the preintervention rate in a single U.S. academic health system.
“Screening rates for CKD more rapidly improved after implementation” of the EMR alert, said Maggy M. Spolnik, MD, at Kidney Week 2023, organized by the American Society of Nephrology.
“There was an immediate and ongoing effect over a year,” said Dr. Spolnik, a nephrologist at Indiana University in Indianapolis.
However, CKD screening rates in the primary care setting remain a challenge. In the study, the EMR alert produced a urine albumin-to-creatinine ratio (UACR) screening rate of about 26% of patient encounters, she reported. While this was significantly above the roughly 17% rate that had persisted for months before the intervention, it still fell short of the universal annual screening for adults with type 2 diabetes not previously diagnosed with CKD recommended by medical groups such as the American Diabetes Association and the Kidney Disease: Improving Global Outcomes organization. The U.S. Preventive Services Task Force’s assessment in 2012 concluded inadequate information existed at that time to make recommendations about CKD screening, but the group is now revisiting the issue.
‘Albuminuria is an earlier marker’ than eGFR
“Primary care physicians need to regularly monitor albuminuria in adults with type 2 diabetes,” commented Karen A. Griffin, MD, a nephrologist and professor at Loyola University in Maywood, Ill. “By the time you diagnose CKD based on reduced estimated glomerular filtration rate (eGFR), a patient has already lost more than half their renal function. Albuminuria is an earlier marker of a problem,” Dr. Griffin said in an interview.
Primary care physicians have been slow to adopt at least annual checks on both eGFR and the urinary albumin-to-creatinine ratio (UACR) in their adult patients with type 2 diabetes. Dr. Spolnik cited reasons such as the brief 15-minute consultation that primary care physicians have when seeing a patient, and an often confusing ordering menu that gives a UACR test various other names such as tests for microalbuminuria or macroalbuminuria.
To simplify ordering, the EMR prompt assessed in Dr. Spolnik’s study called the test “kidney screening” that automatically bundled an order for both eGFR calculation with UACR measurement. Another limitation is that UACR measurement requires a urine sample, which patients often find inconvenient to provide at the time of their examination.
The study run by Dr. Spolnik involved 10,744 adults with type 2 diabetes without an existing diagnosis of CKD seen in an outpatient, primary care visit to the UVA Health system centered in Charlottesville, Va. during April 2021–April 2022. A total of 23,419 encounters served as usual-care controls. The intervention period with active EMR alerts for kidney screening included 10,204 similar patients seen during April 2022–April 2023 in a total of 20,358 encounters. The patients averaged about 61-62 years old, and about 45% were men.
Bundling alerts into a single pop-up
The primary care clinicians who received the prompts were generally receptive to them, but they asked the researchers to bundle the UACR and eGFR measurement prompts along with any other alerts they received in the EMR into a single on-screen pop-up.
Dr. Spolnik acknowledged the need for further research and refinement to the prompt. For example, she wants to assess prompts for patients identified as having CKD that would promote best-practice management, including lifestyle and medical interventions. She also envisions expanding the prompts to also include other, related disorders such as hypertension.
But she and her colleagues were convinced enough by the results that they have not only continued the program at UVA Health but they also expanded it, starting in October 2023, to the academic primary care practice at Indiana University.
If the Indiana University trial confirms the efficacy seen in Virginia, the next step might be inclusion by Epic of the CKD screening alert as a routine option in the EMR software it distributes to its U.S. clients, Dr. Spolnik said in an interview.
Dr. Spolnik and Dr. Griffin had no disclosures.
REPORTING FROM KIDNEY WEEK 2023
Enhanced natural killer cell therapy shows promise in Alzheimer’s
BOSTON – .
SNK01, being developed by NKGen Biotech, is an autologous, nongenetically modified NK cell product that has enhanced cytotoxicity and activating receptor expression.
“When we give these enhanced natural killer cells intravenously, not only do they get into the brain, but we’ve shown, through CSF biomarker data, that they reduce both amyloid and tau proteins, dramatically reducing the neuroinflammation,” said Paul Song, MD, chief executive officer of NKGen Biotech.
“Remarkably,” in the first 6 months, 90% of patients with Alzheimer’s disease demonstrated improvement or maintained stable cognitive function, based on the Alzheimer’s Disease Composite Score (ADCOMS), suggesting that SNK01 may do more than simply slow disease progression, Dr. Song said.
The findings were presented at the 16th Clinical Trials on Alzheimer’s Disease (CTAD) conference.
Sound rationale
NK cells are an essential part of the innate immune system that can shape the adaptive response by eliminating activated (not resting) autologous CD4+ T cells. Weak or deficient NK cells have been found to correlate with various diseases, including autoimmune diseases, and emerging data suggest an autoimmune component to Alzheimer’s disease.
The phase 1 study evaluated the safety, tolerability, and exploratory efficacy of SNK01 given intravenously in escalating doses every 3 weeks (four treatments in total).
Participants included 10 patients with Alzheimer’s disease confirmed by imaging. Five had mild Alzheimer’s disease, three had moderate Alzheimer’s disease, and two had advanced Alzheimer’s disease, based on baseline Clinical Dementia Rating–Sum of Boxes (CDR-SB) scores. Median baseline CDR-SB score was 9 (range, 4-18).
Cognitive assessments included CDR-SB, Mini-Mental State Examination (MMSE), Alzheimer’s disease Assessment Scale-Cognitive Subscale (ADAS-Cog) and ADCOMS. CSF biomarker analyses were performed at baseline and at 1 and 12 weeks after the final dose (weeks 11 and 22, respectively).
NK cells were successfully activated and expanded in all 10 patients and no treatment-related adverse events were observed.
Based on the CSF biomarker data, SNK01 crossed the blood–brain barrier and reduced CSF amyloid-beta 42/40 and pTau181 levels and neuroinflammation, as measured by glial fibrillary acid protein (GFAP), and the effects appeared to persist 12 weeks after the final dose.
The exploratory efficacy data show that, 1 week after final dose (week 11), compared with baseline, 30% of patients showed clinical improvement on the composite ADCOMS and 60% had a stable ADCOMS score; 50%-70% of patients were stable or improved on the CDR-SB, ADAS-Cog and/or MMSE.
“One patient went from a MMSE score of 14, which is moderate dementia, to 22, which is mild cognitive impairment,” said Dr. Song.
At 12 weeks after the final dose (week 22), 44%-89% of patients remained stable or improved in all cognitive scores compared with week 11; and 50% of the patients with stable ADCOMS scores at week 11 remained stable.
Based on the positive phase 1 data, the Food and Drug Administration has approved a phase 1/2a study in patients with moderate Alzheimer’s disease. “The trial will use a much higher dose and a prolonged dosing regimen and we think we’ll see even more dramatic results with more sustained higher dosing,” Dr. Song said.
Down the road, it will be interesting to see how NK cell therapy could “complement” anti-amyloid and anti-tau therapies, he added.
Ideal treatment approach
Commenting on this research, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said NK cells have “natural abilities that could make them an ideal treatment approach for Alzheimer’s and similar neurodegenerative diseases.
“NK cells have properties that allow them to recognize and destroy diseased brain cells while leaving healthy cells intact, without causing excessive inflammation or autoimmune issues. It has historically been difficult to get immune cells to access the privileged immunological environment of the brain,” Dr. Lakhan explained.
“However, this line of early research shows that NK cells safely crosses the blood–brain barrier, infiltrates brain tissue, and may stave off Alzheimer’s disease as measured by biomarkers and clinical symptoms,” he noted.
“This emerging cell-based immunotherapy is highly-specific to the cells responsible for Alzheimer’s, avoids drug resistance, has long-lasting results, and has fewer side effects than drug counterparts,” Dr. Lakhan said.
The trial was sponsored by NKGen Biotech. Dr. Song and six coauthors are employees and shareholders in the company. Dr. Lakhan reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
BOSTON – .
SNK01, being developed by NKGen Biotech, is an autologous, nongenetically modified NK cell product that has enhanced cytotoxicity and activating receptor expression.
“When we give these enhanced natural killer cells intravenously, not only do they get into the brain, but we’ve shown, through CSF biomarker data, that they reduce both amyloid and tau proteins, dramatically reducing the neuroinflammation,” said Paul Song, MD, chief executive officer of NKGen Biotech.
“Remarkably,” in the first 6 months, 90% of patients with Alzheimer’s disease demonstrated improvement or maintained stable cognitive function, based on the Alzheimer’s Disease Composite Score (ADCOMS), suggesting that SNK01 may do more than simply slow disease progression, Dr. Song said.
The findings were presented at the 16th Clinical Trials on Alzheimer’s Disease (CTAD) conference.
Sound rationale
NK cells are an essential part of the innate immune system that can shape the adaptive response by eliminating activated (not resting) autologous CD4+ T cells. Weak or deficient NK cells have been found to correlate with various diseases, including autoimmune diseases, and emerging data suggest an autoimmune component to Alzheimer’s disease.
The phase 1 study evaluated the safety, tolerability, and exploratory efficacy of SNK01 given intravenously in escalating doses every 3 weeks (four treatments in total).
Participants included 10 patients with Alzheimer’s disease confirmed by imaging. Five had mild Alzheimer’s disease, three had moderate Alzheimer’s disease, and two had advanced Alzheimer’s disease, based on baseline Clinical Dementia Rating–Sum of Boxes (CDR-SB) scores. Median baseline CDR-SB score was 9 (range, 4-18).
Cognitive assessments included CDR-SB, Mini-Mental State Examination (MMSE), Alzheimer’s disease Assessment Scale-Cognitive Subscale (ADAS-Cog) and ADCOMS. CSF biomarker analyses were performed at baseline and at 1 and 12 weeks after the final dose (weeks 11 and 22, respectively).
NK cells were successfully activated and expanded in all 10 patients and no treatment-related adverse events were observed.
Based on the CSF biomarker data, SNK01 crossed the blood–brain barrier and reduced CSF amyloid-beta 42/40 and pTau181 levels and neuroinflammation, as measured by glial fibrillary acid protein (GFAP), and the effects appeared to persist 12 weeks after the final dose.
The exploratory efficacy data show that, 1 week after final dose (week 11), compared with baseline, 30% of patients showed clinical improvement on the composite ADCOMS and 60% had a stable ADCOMS score; 50%-70% of patients were stable or improved on the CDR-SB, ADAS-Cog and/or MMSE.
“One patient went from a MMSE score of 14, which is moderate dementia, to 22, which is mild cognitive impairment,” said Dr. Song.
At 12 weeks after the final dose (week 22), 44%-89% of patients remained stable or improved in all cognitive scores compared with week 11; and 50% of the patients with stable ADCOMS scores at week 11 remained stable.
Based on the positive phase 1 data, the Food and Drug Administration has approved a phase 1/2a study in patients with moderate Alzheimer’s disease. “The trial will use a much higher dose and a prolonged dosing regimen and we think we’ll see even more dramatic results with more sustained higher dosing,” Dr. Song said.
Down the road, it will be interesting to see how NK cell therapy could “complement” anti-amyloid and anti-tau therapies, he added.
Ideal treatment approach
Commenting on this research, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said NK cells have “natural abilities that could make them an ideal treatment approach for Alzheimer’s and similar neurodegenerative diseases.
“NK cells have properties that allow them to recognize and destroy diseased brain cells while leaving healthy cells intact, without causing excessive inflammation or autoimmune issues. It has historically been difficult to get immune cells to access the privileged immunological environment of the brain,” Dr. Lakhan explained.
“However, this line of early research shows that NK cells safely crosses the blood–brain barrier, infiltrates brain tissue, and may stave off Alzheimer’s disease as measured by biomarkers and clinical symptoms,” he noted.
“This emerging cell-based immunotherapy is highly-specific to the cells responsible for Alzheimer’s, avoids drug resistance, has long-lasting results, and has fewer side effects than drug counterparts,” Dr. Lakhan said.
The trial was sponsored by NKGen Biotech. Dr. Song and six coauthors are employees and shareholders in the company. Dr. Lakhan reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
BOSTON – .
SNK01, being developed by NKGen Biotech, is an autologous, nongenetically modified NK cell product that has enhanced cytotoxicity and activating receptor expression.
“When we give these enhanced natural killer cells intravenously, not only do they get into the brain, but we’ve shown, through CSF biomarker data, that they reduce both amyloid and tau proteins, dramatically reducing the neuroinflammation,” said Paul Song, MD, chief executive officer of NKGen Biotech.
“Remarkably,” in the first 6 months, 90% of patients with Alzheimer’s disease demonstrated improvement or maintained stable cognitive function, based on the Alzheimer’s Disease Composite Score (ADCOMS), suggesting that SNK01 may do more than simply slow disease progression, Dr. Song said.
The findings were presented at the 16th Clinical Trials on Alzheimer’s Disease (CTAD) conference.
Sound rationale
NK cells are an essential part of the innate immune system that can shape the adaptive response by eliminating activated (not resting) autologous CD4+ T cells. Weak or deficient NK cells have been found to correlate with various diseases, including autoimmune diseases, and emerging data suggest an autoimmune component to Alzheimer’s disease.
The phase 1 study evaluated the safety, tolerability, and exploratory efficacy of SNK01 given intravenously in escalating doses every 3 weeks (four treatments in total).
Participants included 10 patients with Alzheimer’s disease confirmed by imaging. Five had mild Alzheimer’s disease, three had moderate Alzheimer’s disease, and two had advanced Alzheimer’s disease, based on baseline Clinical Dementia Rating–Sum of Boxes (CDR-SB) scores. Median baseline CDR-SB score was 9 (range, 4-18).
Cognitive assessments included CDR-SB, Mini-Mental State Examination (MMSE), Alzheimer’s disease Assessment Scale-Cognitive Subscale (ADAS-Cog) and ADCOMS. CSF biomarker analyses were performed at baseline and at 1 and 12 weeks after the final dose (weeks 11 and 22, respectively).
NK cells were successfully activated and expanded in all 10 patients and no treatment-related adverse events were observed.
Based on the CSF biomarker data, SNK01 crossed the blood–brain barrier and reduced CSF amyloid-beta 42/40 and pTau181 levels and neuroinflammation, as measured by glial fibrillary acid protein (GFAP), and the effects appeared to persist 12 weeks after the final dose.
The exploratory efficacy data show that, 1 week after final dose (week 11), compared with baseline, 30% of patients showed clinical improvement on the composite ADCOMS and 60% had a stable ADCOMS score; 50%-70% of patients were stable or improved on the CDR-SB, ADAS-Cog and/or MMSE.
“One patient went from a MMSE score of 14, which is moderate dementia, to 22, which is mild cognitive impairment,” said Dr. Song.
At 12 weeks after the final dose (week 22), 44%-89% of patients remained stable or improved in all cognitive scores compared with week 11; and 50% of the patients with stable ADCOMS scores at week 11 remained stable.
Based on the positive phase 1 data, the Food and Drug Administration has approved a phase 1/2a study in patients with moderate Alzheimer’s disease. “The trial will use a much higher dose and a prolonged dosing regimen and we think we’ll see even more dramatic results with more sustained higher dosing,” Dr. Song said.
Down the road, it will be interesting to see how NK cell therapy could “complement” anti-amyloid and anti-tau therapies, he added.
Ideal treatment approach
Commenting on this research, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said NK cells have “natural abilities that could make them an ideal treatment approach for Alzheimer’s and similar neurodegenerative diseases.
“NK cells have properties that allow them to recognize and destroy diseased brain cells while leaving healthy cells intact, without causing excessive inflammation or autoimmune issues. It has historically been difficult to get immune cells to access the privileged immunological environment of the brain,” Dr. Lakhan explained.
“However, this line of early research shows that NK cells safely crosses the blood–brain barrier, infiltrates brain tissue, and may stave off Alzheimer’s disease as measured by biomarkers and clinical symptoms,” he noted.
“This emerging cell-based immunotherapy is highly-specific to the cells responsible for Alzheimer’s, avoids drug resistance, has long-lasting results, and has fewer side effects than drug counterparts,” Dr. Lakhan said.
The trial was sponsored by NKGen Biotech. Dr. Song and six coauthors are employees and shareholders in the company. Dr. Lakhan reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CTAD 2023
Medicare 2024 base rate cut triggers calls for pay overhaul
Physicians in 2024 can expect a 3.4% drop in the conversion factor that determines their base Medicare pay, according to federal officials, but they also will receive more money for primary care and treating complex conditions.
The Centers for Medicare & Medicaid Services on Nov. 2 released its 2024 final physician fee schedule, triggering renewed concerns from doctors’ groups, who protested CMS’ cuts when they were first previewed earlier in 2023.
The 2024 conversion factor, or base rate for clinician pay, will be $32.74, a decrease of $1.15, or 3.4%, from 2023’s level. The pay cuts come as costs of providing health care are expected to rise as much as 4.6% in 2024, the American Medical Association said.
The new rule follows a 2% payment reduction in 2023, AMA president Jesse M. Ehrenfeld, MD, MPH, said in a statement.
“This is a recipe for financial instability,” Dr. Ehrenfeld said. “Patients and physicians will wonder why such thin gruel is being served.”
The AMA is among the many physician groups pressing Congress to change its approach to paying clinicians and consider inflation rates in determining future payments.
Medicare already includes automatic inflation adjusters in other payment rules, such as the ones for care provided in hospitals. But Congress in 2015 eliminated this feature for the physician fee schedule when it passed the Medicare Access and CHIP Reauthorization Act.
A pending House bill, the bipartisan Strengthening Medicare for Patients and Providers Act (H.R.2474), would return to permanently including a broader inflation adjuster in the Medicare physician fee schedule.
“This long-overdue change would not only help provide greater stability within the Medicare payment system, but it would also help physicians’ practices – many of whom operate as small business owners – more effectively navigate the ever-changing economic factors that impact their practices, including rising medical costs, workforce and labor challenges, administrative burdens, office rental prices and more,” Larry Bucshon, MD (R-Ind.), Ami Bera, MD (D-Calif.), Raul Ruiz, MD (D-Calif.), and Mariannette Miller-Meeks, MD (R-Iowa), wrote in an opinion article in the newspaper The Hill.
Major changes to determining Medicare physician pay remain unlikely in 2023. Still, Congress has softened or blocked slated cuts in physician pay in recent years, passing temporary “doc fixes” as add-ons to spending packages.
E/M add-on payment
“We’re encouraged to see that CMS listened to our concerns and extended telehealth flexibilities as well as implemented the G2211 code, which will help Medicare beneficiaries and their physicians better manage complex and chronic rheumatic diseases,” said Douglas White, MD, PhD, president of the ACR.
A version of this article first appeared on Medscape.com.
Physicians in 2024 can expect a 3.4% drop in the conversion factor that determines their base Medicare pay, according to federal officials, but they also will receive more money for primary care and treating complex conditions.
The Centers for Medicare & Medicaid Services on Nov. 2 released its 2024 final physician fee schedule, triggering renewed concerns from doctors’ groups, who protested CMS’ cuts when they were first previewed earlier in 2023.
The 2024 conversion factor, or base rate for clinician pay, will be $32.74, a decrease of $1.15, or 3.4%, from 2023’s level. The pay cuts come as costs of providing health care are expected to rise as much as 4.6% in 2024, the American Medical Association said.
The new rule follows a 2% payment reduction in 2023, AMA president Jesse M. Ehrenfeld, MD, MPH, said in a statement.
“This is a recipe for financial instability,” Dr. Ehrenfeld said. “Patients and physicians will wonder why such thin gruel is being served.”
The AMA is among the many physician groups pressing Congress to change its approach to paying clinicians and consider inflation rates in determining future payments.
Medicare already includes automatic inflation adjusters in other payment rules, such as the ones for care provided in hospitals. But Congress in 2015 eliminated this feature for the physician fee schedule when it passed the Medicare Access and CHIP Reauthorization Act.
A pending House bill, the bipartisan Strengthening Medicare for Patients and Providers Act (H.R.2474), would return to permanently including a broader inflation adjuster in the Medicare physician fee schedule.
“This long-overdue change would not only help provide greater stability within the Medicare payment system, but it would also help physicians’ practices – many of whom operate as small business owners – more effectively navigate the ever-changing economic factors that impact their practices, including rising medical costs, workforce and labor challenges, administrative burdens, office rental prices and more,” Larry Bucshon, MD (R-Ind.), Ami Bera, MD (D-Calif.), Raul Ruiz, MD (D-Calif.), and Mariannette Miller-Meeks, MD (R-Iowa), wrote in an opinion article in the newspaper The Hill.
Major changes to determining Medicare physician pay remain unlikely in 2023. Still, Congress has softened or blocked slated cuts in physician pay in recent years, passing temporary “doc fixes” as add-ons to spending packages.
E/M add-on payment
“We’re encouraged to see that CMS listened to our concerns and extended telehealth flexibilities as well as implemented the G2211 code, which will help Medicare beneficiaries and their physicians better manage complex and chronic rheumatic diseases,” said Douglas White, MD, PhD, president of the ACR.
A version of this article first appeared on Medscape.com.
Physicians in 2024 can expect a 3.4% drop in the conversion factor that determines their base Medicare pay, according to federal officials, but they also will receive more money for primary care and treating complex conditions.
The Centers for Medicare & Medicaid Services on Nov. 2 released its 2024 final physician fee schedule, triggering renewed concerns from doctors’ groups, who protested CMS’ cuts when they were first previewed earlier in 2023.
The 2024 conversion factor, or base rate for clinician pay, will be $32.74, a decrease of $1.15, or 3.4%, from 2023’s level. The pay cuts come as costs of providing health care are expected to rise as much as 4.6% in 2024, the American Medical Association said.
The new rule follows a 2% payment reduction in 2023, AMA president Jesse M. Ehrenfeld, MD, MPH, said in a statement.
“This is a recipe for financial instability,” Dr. Ehrenfeld said. “Patients and physicians will wonder why such thin gruel is being served.”
The AMA is among the many physician groups pressing Congress to change its approach to paying clinicians and consider inflation rates in determining future payments.
Medicare already includes automatic inflation adjusters in other payment rules, such as the ones for care provided in hospitals. But Congress in 2015 eliminated this feature for the physician fee schedule when it passed the Medicare Access and CHIP Reauthorization Act.
A pending House bill, the bipartisan Strengthening Medicare for Patients and Providers Act (H.R.2474), would return to permanently including a broader inflation adjuster in the Medicare physician fee schedule.
“This long-overdue change would not only help provide greater stability within the Medicare payment system, but it would also help physicians’ practices – many of whom operate as small business owners – more effectively navigate the ever-changing economic factors that impact their practices, including rising medical costs, workforce and labor challenges, administrative burdens, office rental prices and more,” Larry Bucshon, MD (R-Ind.), Ami Bera, MD (D-Calif.), Raul Ruiz, MD (D-Calif.), and Mariannette Miller-Meeks, MD (R-Iowa), wrote in an opinion article in the newspaper The Hill.
Major changes to determining Medicare physician pay remain unlikely in 2023. Still, Congress has softened or blocked slated cuts in physician pay in recent years, passing temporary “doc fixes” as add-ons to spending packages.
E/M add-on payment
“We’re encouraged to see that CMS listened to our concerns and extended telehealth flexibilities as well as implemented the G2211 code, which will help Medicare beneficiaries and their physicians better manage complex and chronic rheumatic diseases,” said Douglas White, MD, PhD, president of the ACR.
A version of this article first appeared on Medscape.com.
Patient contact time vs. admin: Is your contract fair?
What’s in a day’s work? For doctors, it’s typically a mix of seeing patients and completing paperwork and follow-up. Often it extends well past the standard workday.
Dennis Hursh, JD, managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, describes one overwhelmed ob.gyn. who recently consulted him for this problem.
“My client had accepted a position in a group practice where his contract stated he would be working during normal office hours, Monday through Friday, from 8 a.m. to 5 p.m. – in other words, a 40-hour workweek,” Mr. Hursh said.
But the distressed physician discovered that actually, he was working almost twice as many hours. “He’d get to work early to do charting, then see patients during the 40 hours, perhaps grabbing a quick sandwich for a few minutes – and then stay after 5 [p.m.] for a few more hours when he’d work on charts or other administrative tasks. Then he’d get something to eat, work on more charts, then go to bed, get up in the morning, and repeat.”
Mr. Hursh summarized the client’s life: “Eating, sleeping, practicing clinical medicine, and doing nonclinical tasks.”
It turned out that the 40-hour workweek included in the contract referred to patient-facing hours, not to all of the ancillary tasks that are part of practicing medicine in this day and age. “Unfortunately, this is far from an isolated story,” said Mr. Hursh.
Be aware of what’s in the contract
“The first draft of many standard physician employment contracts often omits mention of patient contact hour requirements and rather uses vague verbiage such as ‘full-time’ employment or ‘1.0 FTE’ – or full-time equivalent – without defining that term,” said Mr. Hursh. Typically, the 40 hours exclude call coverage, but most physicians understand that and, at least at first glance, it all sounds very reasonable.
But once charting, hours on the phone, arguing with managed care companies, sending in prescriptions, administrative meetings, and other tasks are thrown in, the work hours expand dramatically. Moreover, if your employer doesn’t utilize hospitalists, you may be expected to “round” outside of the 40 hours, which can be particularly burdensome if the employer admits patients to multiple hospitals.
Amanda Hill, JD, owner of Hill Health Law based in Austin, Texas, told this news organization that this predicament isn’t unique to physicians. Exempt employees who don’t clock in and out are often expected to work overtime – that is, to “work as long as it takes to get the job done.” It can affect NPs, PAs, and many others in the health care space. But the number of tasks that fall upon a doctor’s shoulders and the fact that patients’ health and lives are at stake up the ante and make the situation far more difficult for doctors than for employees in other industries.
So it’s important to nail down precise terms in the contract and, if possible, negotiate for a more humane schedule by specifying how the working hours will be used.
“It’s true that a 1.0 FTE definition is too vague,” Ms. Hill said. “I’ve negotiated a lot of contracts where we nail down in writing that the in-office schedule equals 34 hours per week, so the physician is guaranteed an additional 6 hours for administrative time.”
Mr. Hursh usually asks for 32 hours of patient contact per week, which leaves 1 full day per week to catch up on basic administrative tasks. “It’s important for employers to recognize that seeing patients isn’t the only thing a doctor does and there’s a lot of work in addition to face-to-face time,” he said.
But he hasn’t always been successful. One physician client was seeking a workweek consisting of 36 patient contact hours, “which is 90% of the usual FTE of a 40-hour week,” said Mr. Hursh. “But the employer called it ‘part-time,’ as if the doctor were planning to be lying in the sun for the other 4 hours.”
The client decided to accept a 10% pay cut and 10% less vacation to guarantee that she had those extra hours for administrative tasks. “She’s probably working way more than 36 hours a week, but maybe closer to 50 or 60 instead of 70 or more,” he said.
Clarify call coverage
Call coverage is typically not included in the hours a physician is contracted to work on a weekly basis. “Most contracts have call, and it’s usually evenly distributed among parties in a practice, but call can expand if another doctor is out sick, for example,” said Ms. Hill.
Sometimes the language in the contract is vague regarding call coverage. “I ask, how many shifts per year is the doctor is expected to work? Then, I try to negotiate extra pay if more shifts arise,” she said. “The hospital or practice may not demand extra call because they don’t want to pay extra money to the physician.”
On the other hand, some physicians may be eager to take extra call if it means extra income.
Ms. Hill stated that one of her clients was being paid as a “part-time, 2-day-a-week provider” but was asked to be on call and take night and weekend work. When you added it all up, she was putting in almost 30 hours a week.
“This is abusive to a provider that works so hard for patients,” Ms. Hill said. “We have to protect them through the contract language, so they have something hard and fast to point to when their administrator pushes them too hard. Doctors should get value for their time.”
Ms. Hill and her client pushed for more money, and the employer gave in. “All we had to do was to point out how many hours she was actually working. She didn’t mind all the extra call, but she wanted to be compensated.” The doctor’s salary was hiked by $25,000.
Differences in specialties and settings
There are some specialties where it might be easier to have more defined hours, while other specialties are more challenging. Anu Murthy, Esq., an attorney and associate contract review specialist at Contract Diagnostics (a national firm that reviews physician contracts) told this news organization that the work of hospitalists, intensivists, and emergency department physicians, for example, is done in shifts, which tend to be fixed hours.
“They need to get their charting completed so that whoever takes over on the next shift has access to the most recent notes about the patient,” she said. By contrast, surgeons can’t always account for how long a given surgery will take. “It could be as long as 9 hours,” she said. Notes need to be written immediately for the sake of the patient’s postsurgical care.
Dermatologists tend to deal with fewer emergencies, compared with other specialists, and it’s easier for their patients to be slotted into an organized schedule. On the other hand, primary care doctors – internists, family practice physicians, and pediatricians – may be seeing 40-50 patients a day, one every 15 minutes.
Practice setting also makes a difference, said Ms. Murthy. Veterans Administration (VA) hospitals or government-run clinics tend to have more rigidly defined hours, compared with other settings, so if you’re in a VA hospital or government-run clinic, work-life balance tends to be better.
Physicians who work remotely via telehealth also tend to have a better work-life balance, compared with those who see patients in person, Ms. Murthy said. But the difference may be in not having to spend extra time commuting to work or interacting with others in the work environment, since some research has suggested that telehealth physicians may actually spend more time engaged in charting after hours, compared with their in-person counterparts.
Using scribes to maximize your time
Elliott Trotter, MD, is an emergency medicine physician, associate clinical professor of emergency medicine at Texas Christian University Medical Schools, and founder of the ScribeNest, a Texas-based company that trains health care scribes. He told this news organization that there are ways to maximize one’s time during shifts so that much of the charting can be accomplished during working hours.
“About 28 years ago, I realized that the documentation load for physicians was enormous and at that time I developed the Modern Scribe, using premed students for ‘elbow support’ to help with the workload by documenting the ED encounters in real time during the encounter so I wouldn’t have to do so later.”
Over the years, as EHRs have become more ubiquitous and onerous, the role of the scribe has “evolved from a luxury to a necessity,” said Dr. Trotter. The scribes can actually record the encounter directly into the EHR so that the physician doesn’t have to do so later and doesn’t have to look at a computer screen but can look at the patient during the encounter.
“This enhances communication and has been shown to improve patient care,” he said.
Dr. Trotter said he rarely, if ever, needs to do documentation after hours. “But one of my physician colleagues had over 500 charts in his in-basket on a regular basis, which was overwhelming and untenable.”
The use of AI in health care is rapidly growing. Tools to help hasten the process of taking notes through use of AI-generated summaries is something appealing to many doctors. Ms. Hill warned physicians to “be careful not to rely so heavily on AI that you trust it over your own words.” She noted that it can make mistakes, and the liability always remains with the clinician.
Creating time-efficient strategies
Wilfrid Noel Raby, PhD, MD, a psychiatrist in private practice in Teaneck, N.J., was formerly a psychiatrist in the substance abuse unit at Montefiore Hospital, New York. He told this news organization that he developed a system whereby he rarely had to take work home with him. “I was working only 20 hours a week, but I was usually able to do my charting during those hours, as well as seeing patients,” he said. “I scheduled my appointments and structured a little ‘buffer time’ between them so that I had time to document the first appointment before moving on to the next one.”
There were days when this wasn’t possible because there were too many patients who needed to be seen back-to-back. “So I developed my own template where I could take rapid, very standardized notes that fit into the format of the EHR and met those expectations.” Then, when he had finished seeing patients, he could quickly enter the content of his notes into the EHR. If necessary, he completed his charting on a different day.
Viwek Bisen, DO, assistant professor of psychiatry, Hackensack (N.J.) University Medical Center, is a psychiatrist in the emergency department. “My contract is based on a traditional 40-hour workweek, with 80% of my time allotted to seeing patients and 20% of my time allotted to administration.”
But the way his time actually plays out is that he’s seeing patients during about half of the 32 hours. “The rest of the time, I’m charting, speaking to family members of patients, writing notes, engaging in team meetings, and dealing with insurance companies.” Dr. Bisen has developed his own system of completing his notes while still in the hospital. “I’ve learned to be efficient and manage my time better, so I no longer have to take work home with me.”
“At the end of the day, doctors are people,” Ms. Hill said. “They are not machines. Maybe in residency and fellowship they may grind out impossible shifts with little sleep, but this pace isn’t tenable for an entire career.”
A version of this article first appeared on Medscape.com.
What’s in a day’s work? For doctors, it’s typically a mix of seeing patients and completing paperwork and follow-up. Often it extends well past the standard workday.
Dennis Hursh, JD, managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, describes one overwhelmed ob.gyn. who recently consulted him for this problem.
“My client had accepted a position in a group practice where his contract stated he would be working during normal office hours, Monday through Friday, from 8 a.m. to 5 p.m. – in other words, a 40-hour workweek,” Mr. Hursh said.
But the distressed physician discovered that actually, he was working almost twice as many hours. “He’d get to work early to do charting, then see patients during the 40 hours, perhaps grabbing a quick sandwich for a few minutes – and then stay after 5 [p.m.] for a few more hours when he’d work on charts or other administrative tasks. Then he’d get something to eat, work on more charts, then go to bed, get up in the morning, and repeat.”
Mr. Hursh summarized the client’s life: “Eating, sleeping, practicing clinical medicine, and doing nonclinical tasks.”
It turned out that the 40-hour workweek included in the contract referred to patient-facing hours, not to all of the ancillary tasks that are part of practicing medicine in this day and age. “Unfortunately, this is far from an isolated story,” said Mr. Hursh.
Be aware of what’s in the contract
“The first draft of many standard physician employment contracts often omits mention of patient contact hour requirements and rather uses vague verbiage such as ‘full-time’ employment or ‘1.0 FTE’ – or full-time equivalent – without defining that term,” said Mr. Hursh. Typically, the 40 hours exclude call coverage, but most physicians understand that and, at least at first glance, it all sounds very reasonable.
But once charting, hours on the phone, arguing with managed care companies, sending in prescriptions, administrative meetings, and other tasks are thrown in, the work hours expand dramatically. Moreover, if your employer doesn’t utilize hospitalists, you may be expected to “round” outside of the 40 hours, which can be particularly burdensome if the employer admits patients to multiple hospitals.
Amanda Hill, JD, owner of Hill Health Law based in Austin, Texas, told this news organization that this predicament isn’t unique to physicians. Exempt employees who don’t clock in and out are often expected to work overtime – that is, to “work as long as it takes to get the job done.” It can affect NPs, PAs, and many others in the health care space. But the number of tasks that fall upon a doctor’s shoulders and the fact that patients’ health and lives are at stake up the ante and make the situation far more difficult for doctors than for employees in other industries.
So it’s important to nail down precise terms in the contract and, if possible, negotiate for a more humane schedule by specifying how the working hours will be used.
“It’s true that a 1.0 FTE definition is too vague,” Ms. Hill said. “I’ve negotiated a lot of contracts where we nail down in writing that the in-office schedule equals 34 hours per week, so the physician is guaranteed an additional 6 hours for administrative time.”
Mr. Hursh usually asks for 32 hours of patient contact per week, which leaves 1 full day per week to catch up on basic administrative tasks. “It’s important for employers to recognize that seeing patients isn’t the only thing a doctor does and there’s a lot of work in addition to face-to-face time,” he said.
But he hasn’t always been successful. One physician client was seeking a workweek consisting of 36 patient contact hours, “which is 90% of the usual FTE of a 40-hour week,” said Mr. Hursh. “But the employer called it ‘part-time,’ as if the doctor were planning to be lying in the sun for the other 4 hours.”
The client decided to accept a 10% pay cut and 10% less vacation to guarantee that she had those extra hours for administrative tasks. “She’s probably working way more than 36 hours a week, but maybe closer to 50 or 60 instead of 70 or more,” he said.
Clarify call coverage
Call coverage is typically not included in the hours a physician is contracted to work on a weekly basis. “Most contracts have call, and it’s usually evenly distributed among parties in a practice, but call can expand if another doctor is out sick, for example,” said Ms. Hill.
Sometimes the language in the contract is vague regarding call coverage. “I ask, how many shifts per year is the doctor is expected to work? Then, I try to negotiate extra pay if more shifts arise,” she said. “The hospital or practice may not demand extra call because they don’t want to pay extra money to the physician.”
On the other hand, some physicians may be eager to take extra call if it means extra income.
Ms. Hill stated that one of her clients was being paid as a “part-time, 2-day-a-week provider” but was asked to be on call and take night and weekend work. When you added it all up, she was putting in almost 30 hours a week.
“This is abusive to a provider that works so hard for patients,” Ms. Hill said. “We have to protect them through the contract language, so they have something hard and fast to point to when their administrator pushes them too hard. Doctors should get value for their time.”
Ms. Hill and her client pushed for more money, and the employer gave in. “All we had to do was to point out how many hours she was actually working. She didn’t mind all the extra call, but she wanted to be compensated.” The doctor’s salary was hiked by $25,000.
Differences in specialties and settings
There are some specialties where it might be easier to have more defined hours, while other specialties are more challenging. Anu Murthy, Esq., an attorney and associate contract review specialist at Contract Diagnostics (a national firm that reviews physician contracts) told this news organization that the work of hospitalists, intensivists, and emergency department physicians, for example, is done in shifts, which tend to be fixed hours.
“They need to get their charting completed so that whoever takes over on the next shift has access to the most recent notes about the patient,” she said. By contrast, surgeons can’t always account for how long a given surgery will take. “It could be as long as 9 hours,” she said. Notes need to be written immediately for the sake of the patient’s postsurgical care.
Dermatologists tend to deal with fewer emergencies, compared with other specialists, and it’s easier for their patients to be slotted into an organized schedule. On the other hand, primary care doctors – internists, family practice physicians, and pediatricians – may be seeing 40-50 patients a day, one every 15 minutes.
Practice setting also makes a difference, said Ms. Murthy. Veterans Administration (VA) hospitals or government-run clinics tend to have more rigidly defined hours, compared with other settings, so if you’re in a VA hospital or government-run clinic, work-life balance tends to be better.
Physicians who work remotely via telehealth also tend to have a better work-life balance, compared with those who see patients in person, Ms. Murthy said. But the difference may be in not having to spend extra time commuting to work or interacting with others in the work environment, since some research has suggested that telehealth physicians may actually spend more time engaged in charting after hours, compared with their in-person counterparts.
Using scribes to maximize your time
Elliott Trotter, MD, is an emergency medicine physician, associate clinical professor of emergency medicine at Texas Christian University Medical Schools, and founder of the ScribeNest, a Texas-based company that trains health care scribes. He told this news organization that there are ways to maximize one’s time during shifts so that much of the charting can be accomplished during working hours.
“About 28 years ago, I realized that the documentation load for physicians was enormous and at that time I developed the Modern Scribe, using premed students for ‘elbow support’ to help with the workload by documenting the ED encounters in real time during the encounter so I wouldn’t have to do so later.”
Over the years, as EHRs have become more ubiquitous and onerous, the role of the scribe has “evolved from a luxury to a necessity,” said Dr. Trotter. The scribes can actually record the encounter directly into the EHR so that the physician doesn’t have to do so later and doesn’t have to look at a computer screen but can look at the patient during the encounter.
“This enhances communication and has been shown to improve patient care,” he said.
Dr. Trotter said he rarely, if ever, needs to do documentation after hours. “But one of my physician colleagues had over 500 charts in his in-basket on a regular basis, which was overwhelming and untenable.”
The use of AI in health care is rapidly growing. Tools to help hasten the process of taking notes through use of AI-generated summaries is something appealing to many doctors. Ms. Hill warned physicians to “be careful not to rely so heavily on AI that you trust it over your own words.” She noted that it can make mistakes, and the liability always remains with the clinician.
Creating time-efficient strategies
Wilfrid Noel Raby, PhD, MD, a psychiatrist in private practice in Teaneck, N.J., was formerly a psychiatrist in the substance abuse unit at Montefiore Hospital, New York. He told this news organization that he developed a system whereby he rarely had to take work home with him. “I was working only 20 hours a week, but I was usually able to do my charting during those hours, as well as seeing patients,” he said. “I scheduled my appointments and structured a little ‘buffer time’ between them so that I had time to document the first appointment before moving on to the next one.”
There were days when this wasn’t possible because there were too many patients who needed to be seen back-to-back. “So I developed my own template where I could take rapid, very standardized notes that fit into the format of the EHR and met those expectations.” Then, when he had finished seeing patients, he could quickly enter the content of his notes into the EHR. If necessary, he completed his charting on a different day.
Viwek Bisen, DO, assistant professor of psychiatry, Hackensack (N.J.) University Medical Center, is a psychiatrist in the emergency department. “My contract is based on a traditional 40-hour workweek, with 80% of my time allotted to seeing patients and 20% of my time allotted to administration.”
But the way his time actually plays out is that he’s seeing patients during about half of the 32 hours. “The rest of the time, I’m charting, speaking to family members of patients, writing notes, engaging in team meetings, and dealing with insurance companies.” Dr. Bisen has developed his own system of completing his notes while still in the hospital. “I’ve learned to be efficient and manage my time better, so I no longer have to take work home with me.”
“At the end of the day, doctors are people,” Ms. Hill said. “They are not machines. Maybe in residency and fellowship they may grind out impossible shifts with little sleep, but this pace isn’t tenable for an entire career.”
A version of this article first appeared on Medscape.com.
What’s in a day’s work? For doctors, it’s typically a mix of seeing patients and completing paperwork and follow-up. Often it extends well past the standard workday.
Dennis Hursh, JD, managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, describes one overwhelmed ob.gyn. who recently consulted him for this problem.
“My client had accepted a position in a group practice where his contract stated he would be working during normal office hours, Monday through Friday, from 8 a.m. to 5 p.m. – in other words, a 40-hour workweek,” Mr. Hursh said.
But the distressed physician discovered that actually, he was working almost twice as many hours. “He’d get to work early to do charting, then see patients during the 40 hours, perhaps grabbing a quick sandwich for a few minutes – and then stay after 5 [p.m.] for a few more hours when he’d work on charts or other administrative tasks. Then he’d get something to eat, work on more charts, then go to bed, get up in the morning, and repeat.”
Mr. Hursh summarized the client’s life: “Eating, sleeping, practicing clinical medicine, and doing nonclinical tasks.”
It turned out that the 40-hour workweek included in the contract referred to patient-facing hours, not to all of the ancillary tasks that are part of practicing medicine in this day and age. “Unfortunately, this is far from an isolated story,” said Mr. Hursh.
Be aware of what’s in the contract
“The first draft of many standard physician employment contracts often omits mention of patient contact hour requirements and rather uses vague verbiage such as ‘full-time’ employment or ‘1.0 FTE’ – or full-time equivalent – without defining that term,” said Mr. Hursh. Typically, the 40 hours exclude call coverage, but most physicians understand that and, at least at first glance, it all sounds very reasonable.
But once charting, hours on the phone, arguing with managed care companies, sending in prescriptions, administrative meetings, and other tasks are thrown in, the work hours expand dramatically. Moreover, if your employer doesn’t utilize hospitalists, you may be expected to “round” outside of the 40 hours, which can be particularly burdensome if the employer admits patients to multiple hospitals.
Amanda Hill, JD, owner of Hill Health Law based in Austin, Texas, told this news organization that this predicament isn’t unique to physicians. Exempt employees who don’t clock in and out are often expected to work overtime – that is, to “work as long as it takes to get the job done.” It can affect NPs, PAs, and many others in the health care space. But the number of tasks that fall upon a doctor’s shoulders and the fact that patients’ health and lives are at stake up the ante and make the situation far more difficult for doctors than for employees in other industries.
So it’s important to nail down precise terms in the contract and, if possible, negotiate for a more humane schedule by specifying how the working hours will be used.
“It’s true that a 1.0 FTE definition is too vague,” Ms. Hill said. “I’ve negotiated a lot of contracts where we nail down in writing that the in-office schedule equals 34 hours per week, so the physician is guaranteed an additional 6 hours for administrative time.”
Mr. Hursh usually asks for 32 hours of patient contact per week, which leaves 1 full day per week to catch up on basic administrative tasks. “It’s important for employers to recognize that seeing patients isn’t the only thing a doctor does and there’s a lot of work in addition to face-to-face time,” he said.
But he hasn’t always been successful. One physician client was seeking a workweek consisting of 36 patient contact hours, “which is 90% of the usual FTE of a 40-hour week,” said Mr. Hursh. “But the employer called it ‘part-time,’ as if the doctor were planning to be lying in the sun for the other 4 hours.”
The client decided to accept a 10% pay cut and 10% less vacation to guarantee that she had those extra hours for administrative tasks. “She’s probably working way more than 36 hours a week, but maybe closer to 50 or 60 instead of 70 or more,” he said.
Clarify call coverage
Call coverage is typically not included in the hours a physician is contracted to work on a weekly basis. “Most contracts have call, and it’s usually evenly distributed among parties in a practice, but call can expand if another doctor is out sick, for example,” said Ms. Hill.
Sometimes the language in the contract is vague regarding call coverage. “I ask, how many shifts per year is the doctor is expected to work? Then, I try to negotiate extra pay if more shifts arise,” she said. “The hospital or practice may not demand extra call because they don’t want to pay extra money to the physician.”
On the other hand, some physicians may be eager to take extra call if it means extra income.
Ms. Hill stated that one of her clients was being paid as a “part-time, 2-day-a-week provider” but was asked to be on call and take night and weekend work. When you added it all up, she was putting in almost 30 hours a week.
“This is abusive to a provider that works so hard for patients,” Ms. Hill said. “We have to protect them through the contract language, so they have something hard and fast to point to when their administrator pushes them too hard. Doctors should get value for their time.”
Ms. Hill and her client pushed for more money, and the employer gave in. “All we had to do was to point out how many hours she was actually working. She didn’t mind all the extra call, but she wanted to be compensated.” The doctor’s salary was hiked by $25,000.
Differences in specialties and settings
There are some specialties where it might be easier to have more defined hours, while other specialties are more challenging. Anu Murthy, Esq., an attorney and associate contract review specialist at Contract Diagnostics (a national firm that reviews physician contracts) told this news organization that the work of hospitalists, intensivists, and emergency department physicians, for example, is done in shifts, which tend to be fixed hours.
“They need to get their charting completed so that whoever takes over on the next shift has access to the most recent notes about the patient,” she said. By contrast, surgeons can’t always account for how long a given surgery will take. “It could be as long as 9 hours,” she said. Notes need to be written immediately for the sake of the patient’s postsurgical care.
Dermatologists tend to deal with fewer emergencies, compared with other specialists, and it’s easier for their patients to be slotted into an organized schedule. On the other hand, primary care doctors – internists, family practice physicians, and pediatricians – may be seeing 40-50 patients a day, one every 15 minutes.
Practice setting also makes a difference, said Ms. Murthy. Veterans Administration (VA) hospitals or government-run clinics tend to have more rigidly defined hours, compared with other settings, so if you’re in a VA hospital or government-run clinic, work-life balance tends to be better.
Physicians who work remotely via telehealth also tend to have a better work-life balance, compared with those who see patients in person, Ms. Murthy said. But the difference may be in not having to spend extra time commuting to work or interacting with others in the work environment, since some research has suggested that telehealth physicians may actually spend more time engaged in charting after hours, compared with their in-person counterparts.
Using scribes to maximize your time
Elliott Trotter, MD, is an emergency medicine physician, associate clinical professor of emergency medicine at Texas Christian University Medical Schools, and founder of the ScribeNest, a Texas-based company that trains health care scribes. He told this news organization that there are ways to maximize one’s time during shifts so that much of the charting can be accomplished during working hours.
“About 28 years ago, I realized that the documentation load for physicians was enormous and at that time I developed the Modern Scribe, using premed students for ‘elbow support’ to help with the workload by documenting the ED encounters in real time during the encounter so I wouldn’t have to do so later.”
Over the years, as EHRs have become more ubiquitous and onerous, the role of the scribe has “evolved from a luxury to a necessity,” said Dr. Trotter. The scribes can actually record the encounter directly into the EHR so that the physician doesn’t have to do so later and doesn’t have to look at a computer screen but can look at the patient during the encounter.
“This enhances communication and has been shown to improve patient care,” he said.
Dr. Trotter said he rarely, if ever, needs to do documentation after hours. “But one of my physician colleagues had over 500 charts in his in-basket on a regular basis, which was overwhelming and untenable.”
The use of AI in health care is rapidly growing. Tools to help hasten the process of taking notes through use of AI-generated summaries is something appealing to many doctors. Ms. Hill warned physicians to “be careful not to rely so heavily on AI that you trust it over your own words.” She noted that it can make mistakes, and the liability always remains with the clinician.
Creating time-efficient strategies
Wilfrid Noel Raby, PhD, MD, a psychiatrist in private practice in Teaneck, N.J., was formerly a psychiatrist in the substance abuse unit at Montefiore Hospital, New York. He told this news organization that he developed a system whereby he rarely had to take work home with him. “I was working only 20 hours a week, but I was usually able to do my charting during those hours, as well as seeing patients,” he said. “I scheduled my appointments and structured a little ‘buffer time’ between them so that I had time to document the first appointment before moving on to the next one.”
There were days when this wasn’t possible because there were too many patients who needed to be seen back-to-back. “So I developed my own template where I could take rapid, very standardized notes that fit into the format of the EHR and met those expectations.” Then, when he had finished seeing patients, he could quickly enter the content of his notes into the EHR. If necessary, he completed his charting on a different day.
Viwek Bisen, DO, assistant professor of psychiatry, Hackensack (N.J.) University Medical Center, is a psychiatrist in the emergency department. “My contract is based on a traditional 40-hour workweek, with 80% of my time allotted to seeing patients and 20% of my time allotted to administration.”
But the way his time actually plays out is that he’s seeing patients during about half of the 32 hours. “The rest of the time, I’m charting, speaking to family members of patients, writing notes, engaging in team meetings, and dealing with insurance companies.” Dr. Bisen has developed his own system of completing his notes while still in the hospital. “I’ve learned to be efficient and manage my time better, so I no longer have to take work home with me.”
“At the end of the day, doctors are people,” Ms. Hill said. “They are not machines. Maybe in residency and fellowship they may grind out impossible shifts with little sleep, but this pace isn’t tenable for an entire career.”
A version of this article first appeared on Medscape.com.
Gaps persist in awareness, treatment of high LDL cholesterol
TOPLINE:
The prevalence of elevated LDL cholesterol (LDL-C) has declined over the past 2 decades, but 1 in 17 Americans still have a level of 160-189 mg/dL, and 1 in 48 have a level of at least 190 mg/dL, new research shows. Among people with the higher LDL-C level, one in four are both unaware and untreated, the authors report.
METHODOLOGY:
- Using data on 23,667 adult participants in the National Health and Nutrition Examination Survey conducted from 1999 to 2020, researchers identified 1,851 (7.8%) with an LDL-C level of 160-189 mg/dL and 669 (2.8%) with an LDL-C level of at least 190 mg/dL.
- Individuals were classified as “unaware” if they had never had their LDL-C measured or had never been informed of having elevated LDL-C and as “untreated” if their medications didn’t include a statin, ezetimibe, a bile acid sequestrant, or a proprotein convertase subtilisin/kexin type 9 inhibitor.
- The authors compared the prevalence of “unaware” and “untreated” by age, sex, race and ethnicity, educational attainment, poverty index, and insurance status.
TAKEAWAY:
- During the study period, the age-adjusted prevalence of an LDL-C level of 160-189 mg/dL declined from 12.4% (95% confidence interval, 10.0%-15.3%), representing 21.5 million U.S. adults, to 6.1% (95% CI, 4.8%-7.6%), representing 14.0 million adults (P < .001).
- The age-adjusted prevalence of an LDL-C level of at least 190 mg/dL declined from 3.8% (95% CI, 2.8%-5.2%), representing 6.6 million adults, to 2.1% (95% CI, 1.4%-3.0%), representing 4.8 million adults (P = .001).
- Among those with an LDL-C level of 160-189 mg/dL, the proportion of who were unaware and untreated declined from 52.1% to 42.7%, and among those with an LDL-C level of at least 190 mg/dL, it declined from 40.8% to 26.8%.
- Being unaware and untreated was more common in younger adults, men, racial and ethnic minority groups, those with lower educational attainment, those with lower income, and those without health insurance.
IN PRACTICE:
The lack of awareness and treatment of high LDL-C uncovered by the study “may be due to difficulties accessing primary care, low rates of screening in primary care, lack of consensus on screening recommendations, insufficient emphasis on LDL-C as a quality measure, and hesitance to treat asymptomatic individuals,” the authors concluded.
SOURCE:
The research was led by Ahmed Sayed, MBBS, faculty of medicine, Ain Shams University, Cairo, Egypt. It was published online in JAMA Cardiology.
LIMITATIONS:
The analysis was limited by a small number of participants with LDL-C levels of at least 190 mg/dL, possible nonresponse bias, and dependency on participant recall of whether LDL-C was previously measured. The inclusion of pregnant women may have influenced LDL-C levels.
DISCLOSURES:
Dr. Sayed has no relevant conflict of interest. The disclosures of the other authors are listed in the original publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
The prevalence of elevated LDL cholesterol (LDL-C) has declined over the past 2 decades, but 1 in 17 Americans still have a level of 160-189 mg/dL, and 1 in 48 have a level of at least 190 mg/dL, new research shows. Among people with the higher LDL-C level, one in four are both unaware and untreated, the authors report.
METHODOLOGY:
- Using data on 23,667 adult participants in the National Health and Nutrition Examination Survey conducted from 1999 to 2020, researchers identified 1,851 (7.8%) with an LDL-C level of 160-189 mg/dL and 669 (2.8%) with an LDL-C level of at least 190 mg/dL.
- Individuals were classified as “unaware” if they had never had their LDL-C measured or had never been informed of having elevated LDL-C and as “untreated” if their medications didn’t include a statin, ezetimibe, a bile acid sequestrant, or a proprotein convertase subtilisin/kexin type 9 inhibitor.
- The authors compared the prevalence of “unaware” and “untreated” by age, sex, race and ethnicity, educational attainment, poverty index, and insurance status.
TAKEAWAY:
- During the study period, the age-adjusted prevalence of an LDL-C level of 160-189 mg/dL declined from 12.4% (95% confidence interval, 10.0%-15.3%), representing 21.5 million U.S. adults, to 6.1% (95% CI, 4.8%-7.6%), representing 14.0 million adults (P < .001).
- The age-adjusted prevalence of an LDL-C level of at least 190 mg/dL declined from 3.8% (95% CI, 2.8%-5.2%), representing 6.6 million adults, to 2.1% (95% CI, 1.4%-3.0%), representing 4.8 million adults (P = .001).
- Among those with an LDL-C level of 160-189 mg/dL, the proportion of who were unaware and untreated declined from 52.1% to 42.7%, and among those with an LDL-C level of at least 190 mg/dL, it declined from 40.8% to 26.8%.
- Being unaware and untreated was more common in younger adults, men, racial and ethnic minority groups, those with lower educational attainment, those with lower income, and those without health insurance.
IN PRACTICE:
The lack of awareness and treatment of high LDL-C uncovered by the study “may be due to difficulties accessing primary care, low rates of screening in primary care, lack of consensus on screening recommendations, insufficient emphasis on LDL-C as a quality measure, and hesitance to treat asymptomatic individuals,” the authors concluded.
SOURCE:
The research was led by Ahmed Sayed, MBBS, faculty of medicine, Ain Shams University, Cairo, Egypt. It was published online in JAMA Cardiology.
LIMITATIONS:
The analysis was limited by a small number of participants with LDL-C levels of at least 190 mg/dL, possible nonresponse bias, and dependency on participant recall of whether LDL-C was previously measured. The inclusion of pregnant women may have influenced LDL-C levels.
DISCLOSURES:
Dr. Sayed has no relevant conflict of interest. The disclosures of the other authors are listed in the original publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
The prevalence of elevated LDL cholesterol (LDL-C) has declined over the past 2 decades, but 1 in 17 Americans still have a level of 160-189 mg/dL, and 1 in 48 have a level of at least 190 mg/dL, new research shows. Among people with the higher LDL-C level, one in four are both unaware and untreated, the authors report.
METHODOLOGY:
- Using data on 23,667 adult participants in the National Health and Nutrition Examination Survey conducted from 1999 to 2020, researchers identified 1,851 (7.8%) with an LDL-C level of 160-189 mg/dL and 669 (2.8%) with an LDL-C level of at least 190 mg/dL.
- Individuals were classified as “unaware” if they had never had their LDL-C measured or had never been informed of having elevated LDL-C and as “untreated” if their medications didn’t include a statin, ezetimibe, a bile acid sequestrant, or a proprotein convertase subtilisin/kexin type 9 inhibitor.
- The authors compared the prevalence of “unaware” and “untreated” by age, sex, race and ethnicity, educational attainment, poverty index, and insurance status.
TAKEAWAY:
- During the study period, the age-adjusted prevalence of an LDL-C level of 160-189 mg/dL declined from 12.4% (95% confidence interval, 10.0%-15.3%), representing 21.5 million U.S. adults, to 6.1% (95% CI, 4.8%-7.6%), representing 14.0 million adults (P < .001).
- The age-adjusted prevalence of an LDL-C level of at least 190 mg/dL declined from 3.8% (95% CI, 2.8%-5.2%), representing 6.6 million adults, to 2.1% (95% CI, 1.4%-3.0%), representing 4.8 million adults (P = .001).
- Among those with an LDL-C level of 160-189 mg/dL, the proportion of who were unaware and untreated declined from 52.1% to 42.7%, and among those with an LDL-C level of at least 190 mg/dL, it declined from 40.8% to 26.8%.
- Being unaware and untreated was more common in younger adults, men, racial and ethnic minority groups, those with lower educational attainment, those with lower income, and those without health insurance.
IN PRACTICE:
The lack of awareness and treatment of high LDL-C uncovered by the study “may be due to difficulties accessing primary care, low rates of screening in primary care, lack of consensus on screening recommendations, insufficient emphasis on LDL-C as a quality measure, and hesitance to treat asymptomatic individuals,” the authors concluded.
SOURCE:
The research was led by Ahmed Sayed, MBBS, faculty of medicine, Ain Shams University, Cairo, Egypt. It was published online in JAMA Cardiology.
LIMITATIONS:
The analysis was limited by a small number of participants with LDL-C levels of at least 190 mg/dL, possible nonresponse bias, and dependency on participant recall of whether LDL-C was previously measured. The inclusion of pregnant women may have influenced LDL-C levels.
DISCLOSURES:
Dr. Sayed has no relevant conflict of interest. The disclosures of the other authors are listed in the original publication.
A version of this article first appeared on Medscape.com.