User login
Standing BP measures improve hypertension diagnosis
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- The study included 125 adults, mean age 49 years and 62% female, who were free of cardiovascular disease and had no previous history of hypertension.
- Researchers collected data on 24-hour ambulatory blood pressure monitoring (ABPM), and three BP measurements in the seated position, then three in the standing position.
- They assessed overall diagnostic accuracy of seated and standing BP using the area under the receiver operating characteristic (AUROC) curve and considered a Bayes factor (BF) of 3 or greater as significant.
- They defined the presence of hypertension (HTN) by the 2017 American College of Cardiology/American Heart Association and 2023 European Society of Hypertension HTN guidelines based on ABPM.
- Sensitivity and specificity of standing BP was determined using cutoffs derived from Youden index, while sensitivity and specificity of seated BP was determined using the cutoff of 130/80 mm Hg and by 140/90 mm Hg.
TAKEAWAY:
- The AUROC for standing office systolic blood pressure (SBP; 0.81; 0.71-0.92) was significantly higher than for seated office SBP (0.70; 0.49-0.91) in diagnosing HTN when defined as an average 24-hour SBP ≥ 125 mm Hg (BF = 11.8), and significantly higher for seated versus standing office diastolic blood pressure (DBP; 0.65; 0.49-0.82) in diagnosing HTN when defined as an average 24-hour DBP ≥ 75 mm Hg (BF = 4.9).
- The AUROCs for adding standing office BP to seated office BP improved the accuracy of detecting HTN, compared with seated office BP alone when HTN was defined as an average 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg, or when defined as an average 24-hour SBP/DBP ≥ 130/80 mm Hg or daytime SBP/DBP ≥ 135/85 mm Hg (all BFs > 3).
- Sensitivity of standing SBP was 71%, compared with 43% for seated SBP.
IN PRACTICE:
The “excellent diagnostic performance” for standing BP measures revealed by the study “highlights that standing office BP has acceptable discriminative capabilities in identifying the presence of hypertension in adults,” the authors write.
SOURCE:
The study was conducted by John M. Giacona, Hypertension Section, department of internal medicine, University of Texas Southwestern Medical Center, Dallas, and colleagues. It was published online in Scientific Reports.
LIMITATIONS:
As the study enrolled only adults free of comorbidities who were not taking antihypertensive medications, the results may not be applicable to other patients. The study design was retrospective, and the order of BP measurements was not randomized (standing BP measurements were obtained only after seated BP).
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- The study included 125 adults, mean age 49 years and 62% female, who were free of cardiovascular disease and had no previous history of hypertension.
- Researchers collected data on 24-hour ambulatory blood pressure monitoring (ABPM), and three BP measurements in the seated position, then three in the standing position.
- They assessed overall diagnostic accuracy of seated and standing BP using the area under the receiver operating characteristic (AUROC) curve and considered a Bayes factor (BF) of 3 or greater as significant.
- They defined the presence of hypertension (HTN) by the 2017 American College of Cardiology/American Heart Association and 2023 European Society of Hypertension HTN guidelines based on ABPM.
- Sensitivity and specificity of standing BP was determined using cutoffs derived from Youden index, while sensitivity and specificity of seated BP was determined using the cutoff of 130/80 mm Hg and by 140/90 mm Hg.
TAKEAWAY:
- The AUROC for standing office systolic blood pressure (SBP; 0.81; 0.71-0.92) was significantly higher than for seated office SBP (0.70; 0.49-0.91) in diagnosing HTN when defined as an average 24-hour SBP ≥ 125 mm Hg (BF = 11.8), and significantly higher for seated versus standing office diastolic blood pressure (DBP; 0.65; 0.49-0.82) in diagnosing HTN when defined as an average 24-hour DBP ≥ 75 mm Hg (BF = 4.9).
- The AUROCs for adding standing office BP to seated office BP improved the accuracy of detecting HTN, compared with seated office BP alone when HTN was defined as an average 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg, or when defined as an average 24-hour SBP/DBP ≥ 130/80 mm Hg or daytime SBP/DBP ≥ 135/85 mm Hg (all BFs > 3).
- Sensitivity of standing SBP was 71%, compared with 43% for seated SBP.
IN PRACTICE:
The “excellent diagnostic performance” for standing BP measures revealed by the study “highlights that standing office BP has acceptable discriminative capabilities in identifying the presence of hypertension in adults,” the authors write.
SOURCE:
The study was conducted by John M. Giacona, Hypertension Section, department of internal medicine, University of Texas Southwestern Medical Center, Dallas, and colleagues. It was published online in Scientific Reports.
LIMITATIONS:
As the study enrolled only adults free of comorbidities who were not taking antihypertensive medications, the results may not be applicable to other patients. The study design was retrospective, and the order of BP measurements was not randomized (standing BP measurements were obtained only after seated BP).
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- The study included 125 adults, mean age 49 years and 62% female, who were free of cardiovascular disease and had no previous history of hypertension.
- Researchers collected data on 24-hour ambulatory blood pressure monitoring (ABPM), and three BP measurements in the seated position, then three in the standing position.
- They assessed overall diagnostic accuracy of seated and standing BP using the area under the receiver operating characteristic (AUROC) curve and considered a Bayes factor (BF) of 3 or greater as significant.
- They defined the presence of hypertension (HTN) by the 2017 American College of Cardiology/American Heart Association and 2023 European Society of Hypertension HTN guidelines based on ABPM.
- Sensitivity and specificity of standing BP was determined using cutoffs derived from Youden index, while sensitivity and specificity of seated BP was determined using the cutoff of 130/80 mm Hg and by 140/90 mm Hg.
TAKEAWAY:
- The AUROC for standing office systolic blood pressure (SBP; 0.81; 0.71-0.92) was significantly higher than for seated office SBP (0.70; 0.49-0.91) in diagnosing HTN when defined as an average 24-hour SBP ≥ 125 mm Hg (BF = 11.8), and significantly higher for seated versus standing office diastolic blood pressure (DBP; 0.65; 0.49-0.82) in diagnosing HTN when defined as an average 24-hour DBP ≥ 75 mm Hg (BF = 4.9).
- The AUROCs for adding standing office BP to seated office BP improved the accuracy of detecting HTN, compared with seated office BP alone when HTN was defined as an average 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg, or when defined as an average 24-hour SBP/DBP ≥ 130/80 mm Hg or daytime SBP/DBP ≥ 135/85 mm Hg (all BFs > 3).
- Sensitivity of standing SBP was 71%, compared with 43% for seated SBP.
IN PRACTICE:
The “excellent diagnostic performance” for standing BP measures revealed by the study “highlights that standing office BP has acceptable discriminative capabilities in identifying the presence of hypertension in adults,” the authors write.
SOURCE:
The study was conducted by John M. Giacona, Hypertension Section, department of internal medicine, University of Texas Southwestern Medical Center, Dallas, and colleagues. It was published online in Scientific Reports.
LIMITATIONS:
As the study enrolled only adults free of comorbidities who were not taking antihypertensive medications, the results may not be applicable to other patients. The study design was retrospective, and the order of BP measurements was not randomized (standing BP measurements were obtained only after seated BP).
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FDA approves tirzepatide for treating obesity
Eli Lilly will market tirzepatide injections for weight management under the trade name Zepbound. It was approved in May 2022 for treating type 2 diabetes. The new indication is for adults with either obesity, defined as a body mass index of 30 kg/m2 or greater, or overweight, with a BMI of 27 or greater with at least one weight-related comorbidity, including hypertension, type 2 diabetes, or dyslipidemia.
“Obesity and overweight are serious conditions that can be associated with some of the leading causes of death, such as heart disease, stroke, and diabetes,” said John Sharretts, MD, director of the division of diabetes, lipid disorders, and obesity in the FDA’s Center for Drug Evaluation and Research. “In light of increasing rates of both obesity and overweight in the United States, today’s approval addresses an unmet medical need.”
A once-weekly injection, tirzepatide reduces appetite by activating two gut hormones, glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). The dosage is increased over 4-20 weeks to achieve a weekly dose target of 5 mg, 10 mg, or 15 mg maximum.
Efficacy was established in two pivotal randomized, double-blind, placebo-controlled trials of adults with obesity or overweight plus another condition. One trial measured weight reduction after 72 weeks in a total of 2,519 patients without diabetes who received either 5 mg, 10 mg or 15 mg of tirzepatide once weekly. Those who received the 15-mg dose achieved on average 18% of their initial body weight, compared with placebo.
The other pivotal trial enrolled a total of 938 patients with type 2 diabetes. These patients achieved an average weight loss of 12% with once-weekly tirzepatide compared to placebo.
Another trial, which was presented at the 2023 Obesity Week meeting and was published in Nature Medicine, showed clinically meaningful added weight loss for adults with obesity who did not have diabetes and who had already experienced weight loss of at least 5% after a 12-week intensive lifestyle intervention.
Another trial, which was reported at the 2023 annual meeting of the European Association for the Study of Diabetes, found that tirzepatide continued to produce “highly significant weight loss” when the drug was continued in a 1-year follow-up trial. Those who discontinued taking the drug regained some weight but not all.
Tirzepatide can cause gastrointestinal side effects, such as nausea, diarrhea, vomiting, constipation, and abdominal pain or discomfort. Site reactions, hypersensitivity, hair loss, burping, and gastrointestinal reflux disease have also been reported.
The medication should not be used by patients with a personal or family history of medullary thyroid cancer or by patients with multiple endocrine neoplasia syndrome type 2. It should also not be used in combination with Mounjaro or another GLP-1 receptor agonist. The safety and effectiveness of the coadministration of tirzepatide with other medications for weight management have not been established.
Zepbound should go to market in the United States by the end of 2023, with an anticipated monthly list price of $1,060, according to a news release from Eli Lilly.
A version of this article first appeared on Medscape.com.
Eli Lilly will market tirzepatide injections for weight management under the trade name Zepbound. It was approved in May 2022 for treating type 2 diabetes. The new indication is for adults with either obesity, defined as a body mass index of 30 kg/m2 or greater, or overweight, with a BMI of 27 or greater with at least one weight-related comorbidity, including hypertension, type 2 diabetes, or dyslipidemia.
“Obesity and overweight are serious conditions that can be associated with some of the leading causes of death, such as heart disease, stroke, and diabetes,” said John Sharretts, MD, director of the division of diabetes, lipid disorders, and obesity in the FDA’s Center for Drug Evaluation and Research. “In light of increasing rates of both obesity and overweight in the United States, today’s approval addresses an unmet medical need.”
A once-weekly injection, tirzepatide reduces appetite by activating two gut hormones, glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). The dosage is increased over 4-20 weeks to achieve a weekly dose target of 5 mg, 10 mg, or 15 mg maximum.
Efficacy was established in two pivotal randomized, double-blind, placebo-controlled trials of adults with obesity or overweight plus another condition. One trial measured weight reduction after 72 weeks in a total of 2,519 patients without diabetes who received either 5 mg, 10 mg or 15 mg of tirzepatide once weekly. Those who received the 15-mg dose achieved on average 18% of their initial body weight, compared with placebo.
The other pivotal trial enrolled a total of 938 patients with type 2 diabetes. These patients achieved an average weight loss of 12% with once-weekly tirzepatide compared to placebo.
Another trial, which was presented at the 2023 Obesity Week meeting and was published in Nature Medicine, showed clinically meaningful added weight loss for adults with obesity who did not have diabetes and who had already experienced weight loss of at least 5% after a 12-week intensive lifestyle intervention.
Another trial, which was reported at the 2023 annual meeting of the European Association for the Study of Diabetes, found that tirzepatide continued to produce “highly significant weight loss” when the drug was continued in a 1-year follow-up trial. Those who discontinued taking the drug regained some weight but not all.
Tirzepatide can cause gastrointestinal side effects, such as nausea, diarrhea, vomiting, constipation, and abdominal pain or discomfort. Site reactions, hypersensitivity, hair loss, burping, and gastrointestinal reflux disease have also been reported.
The medication should not be used by patients with a personal or family history of medullary thyroid cancer or by patients with multiple endocrine neoplasia syndrome type 2. It should also not be used in combination with Mounjaro or another GLP-1 receptor agonist. The safety and effectiveness of the coadministration of tirzepatide with other medications for weight management have not been established.
Zepbound should go to market in the United States by the end of 2023, with an anticipated monthly list price of $1,060, according to a news release from Eli Lilly.
A version of this article first appeared on Medscape.com.
Eli Lilly will market tirzepatide injections for weight management under the trade name Zepbound. It was approved in May 2022 for treating type 2 diabetes. The new indication is for adults with either obesity, defined as a body mass index of 30 kg/m2 or greater, or overweight, with a BMI of 27 or greater with at least one weight-related comorbidity, including hypertension, type 2 diabetes, or dyslipidemia.
“Obesity and overweight are serious conditions that can be associated with some of the leading causes of death, such as heart disease, stroke, and diabetes,” said John Sharretts, MD, director of the division of diabetes, lipid disorders, and obesity in the FDA’s Center for Drug Evaluation and Research. “In light of increasing rates of both obesity and overweight in the United States, today’s approval addresses an unmet medical need.”
A once-weekly injection, tirzepatide reduces appetite by activating two gut hormones, glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). The dosage is increased over 4-20 weeks to achieve a weekly dose target of 5 mg, 10 mg, or 15 mg maximum.
Efficacy was established in two pivotal randomized, double-blind, placebo-controlled trials of adults with obesity or overweight plus another condition. One trial measured weight reduction after 72 weeks in a total of 2,519 patients without diabetes who received either 5 mg, 10 mg or 15 mg of tirzepatide once weekly. Those who received the 15-mg dose achieved on average 18% of their initial body weight, compared with placebo.
The other pivotal trial enrolled a total of 938 patients with type 2 diabetes. These patients achieved an average weight loss of 12% with once-weekly tirzepatide compared to placebo.
Another trial, which was presented at the 2023 Obesity Week meeting and was published in Nature Medicine, showed clinically meaningful added weight loss for adults with obesity who did not have diabetes and who had already experienced weight loss of at least 5% after a 12-week intensive lifestyle intervention.
Another trial, which was reported at the 2023 annual meeting of the European Association for the Study of Diabetes, found that tirzepatide continued to produce “highly significant weight loss” when the drug was continued in a 1-year follow-up trial. Those who discontinued taking the drug regained some weight but not all.
Tirzepatide can cause gastrointestinal side effects, such as nausea, diarrhea, vomiting, constipation, and abdominal pain or discomfort. Site reactions, hypersensitivity, hair loss, burping, and gastrointestinal reflux disease have also been reported.
The medication should not be used by patients with a personal or family history of medullary thyroid cancer or by patients with multiple endocrine neoplasia syndrome type 2. It should also not be used in combination with Mounjaro or another GLP-1 receptor agonist. The safety and effectiveness of the coadministration of tirzepatide with other medications for weight management have not been established.
Zepbound should go to market in the United States by the end of 2023, with an anticipated monthly list price of $1,060, according to a news release from Eli Lilly.
A version of this article first appeared on Medscape.com.
Microsimulation model identifies 4-year window for pancreatic cancer screening
, based on a microsimulation model.
To seize this opportunity, however, a greater understanding of natural disease course is needed, along with more sensitive screening tools, reported Brechtje D. M. Koopmann, MD, of Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues.
Previous studies have suggested that the window of opportunity for pancreatic cancer screening may span decades, with estimates ranging from 12 to 50 years, the investigators wrote. Their report was published in Gastroenterology.
“Unfortunately, the poor results of pancreatic cancer screening do not align with this assumption, leaving unanswered whether this large window of opportunity truly exists,” they noted. “Microsimulation modeling, combined with available, if limited data, can provide new information on the natural disease course.”
For the present study, the investigators used the Microsimulation Screening Analysis (MISCAN) model, which has guided development of screening programs around the world for cervical, breast, and colorectal cancer. The model incorporates natural disease course, screening, and demographic data, then uses observable inputs such as precursor lesion prevalence and cancer incidence to estimate unobservable outcomes like stage durations and precursor lesion onset.
Dr. Koopmann and colleagues programmed this model with Dutch pancreatic cancer incidence data and findings from Japanese autopsy cases without pancreatic cancer.
First, the model offered insights into precursor lesion prevalence.
The estimated prevalence of any cystic lesion in the pancreas was 6.1% for individuals 50 years of age and 29.6% for those 80 years of age. Solid precursor lesions (PanINs) were estimated to be mainly multifocal (three or more lesions) in individuals older than 80 years. By this age, almost 12% had at least two PanINs. For those lesions that eventually became cancerous, the mean time since cyst onset was estimated to be 8.8 years, and mean time since PanIN onset was 9.0 years.
However, less than 10% of cystic and PanIN lesions progress to become cancers. PanIN lesions are not visible on imaging, and therefore current screening focuses on finding cystic precursor lesions, although these represent only about 10% of pancreatic cancers.
“Given the low pancreatic cancer progression risk of cysts, evaluation of the efficiency of current surveillance guidelines is necessary,” the investigators noted.
Screening should instead focus on identifying high-grade dysplastic lesions, they suggested. While these lesions may have a very low estimated prevalence, at just 0.3% among individuals 90 years of age, they present the greatest risk of pancreatic cancer.
For precursor cysts exhibiting HGD that progressed to pancreatic cancer, the mean interval between dysplasia and cancer was just 4 years. Among 13.7% of individuals, the interval was less than 1 year, suggesting an even shorter window of opportunity for screening.
Beyond this brief timeframe, low test sensitivity explains why screening efforts to date have fallen short, the investigators wrote.
Better tests are “urgently needed,” they added, while acknowledging the challenges inherent to this endeavor. Previous research has shown that precursor lesions in the pancreas are often less than 5 mm in diameter, making them extremely challenging to detect. An effective tool would need to identify solid precursor lesions (PanINs), and also need to simultaneously determine grade of dysplasia.
“Biomarkers could be the future in this matter,” the investigators suggested.
Dr. Koopmann and colleagues concluded by noting that more research is needed to characterize the pathophysiology of pancreatic cancer. On their part, “the current model will be validated, adjusted, and improved whenever new data from autopsy or prospective surveillance studies become available.”
The study was funded in part by Maag Lever Darm Stichting. The investigators disclosed no conflicts of interest.
We continue to search for a way to effectively screen for and prevent pancreatic cancer. Most pancreatic cancers come from pancreatic intraepithelial neoplasms (PanINs), which are essentially invisible on imaging. Pancreatic cysts are relatively common, and only a small number will progress to cancer. Screening via MRI or EUS can look for high-risk features of visible cysts or find early-stage cancers, but whom to screen, how often, and what to do with the results remains unclear. Many of the steps from development of the initial cyst or PanIN to the transformation to cancer cannot be observed, and as such this is a perfect application for disease modeling that allows us to fill in the gaps of what can be observed and estimate what we cannot see.
Mary Linton B. Peters, MD, MS, is a medical oncologist specializing in hepatic and pancreatobiliary cancers at Beth Israel Deaconess Medical Center, Boston, an assistant professor at Harvard Medical School, and a senior scientist at the Institute for Technology Assessment of Massachusetts General Hospital. She reports unrelated institutional research funding from NuCana and Helsinn.
We continue to search for a way to effectively screen for and prevent pancreatic cancer. Most pancreatic cancers come from pancreatic intraepithelial neoplasms (PanINs), which are essentially invisible on imaging. Pancreatic cysts are relatively common, and only a small number will progress to cancer. Screening via MRI or EUS can look for high-risk features of visible cysts or find early-stage cancers, but whom to screen, how often, and what to do with the results remains unclear. Many of the steps from development of the initial cyst or PanIN to the transformation to cancer cannot be observed, and as such this is a perfect application for disease modeling that allows us to fill in the gaps of what can be observed and estimate what we cannot see.
Mary Linton B. Peters, MD, MS, is a medical oncologist specializing in hepatic and pancreatobiliary cancers at Beth Israel Deaconess Medical Center, Boston, an assistant professor at Harvard Medical School, and a senior scientist at the Institute for Technology Assessment of Massachusetts General Hospital. She reports unrelated institutional research funding from NuCana and Helsinn.
We continue to search for a way to effectively screen for and prevent pancreatic cancer. Most pancreatic cancers come from pancreatic intraepithelial neoplasms (PanINs), which are essentially invisible on imaging. Pancreatic cysts are relatively common, and only a small number will progress to cancer. Screening via MRI or EUS can look for high-risk features of visible cysts or find early-stage cancers, but whom to screen, how often, and what to do with the results remains unclear. Many of the steps from development of the initial cyst or PanIN to the transformation to cancer cannot be observed, and as such this is a perfect application for disease modeling that allows us to fill in the gaps of what can be observed and estimate what we cannot see.
Mary Linton B. Peters, MD, MS, is a medical oncologist specializing in hepatic and pancreatobiliary cancers at Beth Israel Deaconess Medical Center, Boston, an assistant professor at Harvard Medical School, and a senior scientist at the Institute for Technology Assessment of Massachusetts General Hospital. She reports unrelated institutional research funding from NuCana and Helsinn.
, based on a microsimulation model.
To seize this opportunity, however, a greater understanding of natural disease course is needed, along with more sensitive screening tools, reported Brechtje D. M. Koopmann, MD, of Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues.
Previous studies have suggested that the window of opportunity for pancreatic cancer screening may span decades, with estimates ranging from 12 to 50 years, the investigators wrote. Their report was published in Gastroenterology.
“Unfortunately, the poor results of pancreatic cancer screening do not align with this assumption, leaving unanswered whether this large window of opportunity truly exists,” they noted. “Microsimulation modeling, combined with available, if limited data, can provide new information on the natural disease course.”
For the present study, the investigators used the Microsimulation Screening Analysis (MISCAN) model, which has guided development of screening programs around the world for cervical, breast, and colorectal cancer. The model incorporates natural disease course, screening, and demographic data, then uses observable inputs such as precursor lesion prevalence and cancer incidence to estimate unobservable outcomes like stage durations and precursor lesion onset.
Dr. Koopmann and colleagues programmed this model with Dutch pancreatic cancer incidence data and findings from Japanese autopsy cases without pancreatic cancer.
First, the model offered insights into precursor lesion prevalence.
The estimated prevalence of any cystic lesion in the pancreas was 6.1% for individuals 50 years of age and 29.6% for those 80 years of age. Solid precursor lesions (PanINs) were estimated to be mainly multifocal (three or more lesions) in individuals older than 80 years. By this age, almost 12% had at least two PanINs. For those lesions that eventually became cancerous, the mean time since cyst onset was estimated to be 8.8 years, and mean time since PanIN onset was 9.0 years.
However, less than 10% of cystic and PanIN lesions progress to become cancers. PanIN lesions are not visible on imaging, and therefore current screening focuses on finding cystic precursor lesions, although these represent only about 10% of pancreatic cancers.
“Given the low pancreatic cancer progression risk of cysts, evaluation of the efficiency of current surveillance guidelines is necessary,” the investigators noted.
Screening should instead focus on identifying high-grade dysplastic lesions, they suggested. While these lesions may have a very low estimated prevalence, at just 0.3% among individuals 90 years of age, they present the greatest risk of pancreatic cancer.
For precursor cysts exhibiting HGD that progressed to pancreatic cancer, the mean interval between dysplasia and cancer was just 4 years. Among 13.7% of individuals, the interval was less than 1 year, suggesting an even shorter window of opportunity for screening.
Beyond this brief timeframe, low test sensitivity explains why screening efforts to date have fallen short, the investigators wrote.
Better tests are “urgently needed,” they added, while acknowledging the challenges inherent to this endeavor. Previous research has shown that precursor lesions in the pancreas are often less than 5 mm in diameter, making them extremely challenging to detect. An effective tool would need to identify solid precursor lesions (PanINs), and also need to simultaneously determine grade of dysplasia.
“Biomarkers could be the future in this matter,” the investigators suggested.
Dr. Koopmann and colleagues concluded by noting that more research is needed to characterize the pathophysiology of pancreatic cancer. On their part, “the current model will be validated, adjusted, and improved whenever new data from autopsy or prospective surveillance studies become available.”
The study was funded in part by Maag Lever Darm Stichting. The investigators disclosed no conflicts of interest.
, based on a microsimulation model.
To seize this opportunity, however, a greater understanding of natural disease course is needed, along with more sensitive screening tools, reported Brechtje D. M. Koopmann, MD, of Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues.
Previous studies have suggested that the window of opportunity for pancreatic cancer screening may span decades, with estimates ranging from 12 to 50 years, the investigators wrote. Their report was published in Gastroenterology.
“Unfortunately, the poor results of pancreatic cancer screening do not align with this assumption, leaving unanswered whether this large window of opportunity truly exists,” they noted. “Microsimulation modeling, combined with available, if limited data, can provide new information on the natural disease course.”
For the present study, the investigators used the Microsimulation Screening Analysis (MISCAN) model, which has guided development of screening programs around the world for cervical, breast, and colorectal cancer. The model incorporates natural disease course, screening, and demographic data, then uses observable inputs such as precursor lesion prevalence and cancer incidence to estimate unobservable outcomes like stage durations and precursor lesion onset.
Dr. Koopmann and colleagues programmed this model with Dutch pancreatic cancer incidence data and findings from Japanese autopsy cases without pancreatic cancer.
First, the model offered insights into precursor lesion prevalence.
The estimated prevalence of any cystic lesion in the pancreas was 6.1% for individuals 50 years of age and 29.6% for those 80 years of age. Solid precursor lesions (PanINs) were estimated to be mainly multifocal (three or more lesions) in individuals older than 80 years. By this age, almost 12% had at least two PanINs. For those lesions that eventually became cancerous, the mean time since cyst onset was estimated to be 8.8 years, and mean time since PanIN onset was 9.0 years.
However, less than 10% of cystic and PanIN lesions progress to become cancers. PanIN lesions are not visible on imaging, and therefore current screening focuses on finding cystic precursor lesions, although these represent only about 10% of pancreatic cancers.
“Given the low pancreatic cancer progression risk of cysts, evaluation of the efficiency of current surveillance guidelines is necessary,” the investigators noted.
Screening should instead focus on identifying high-grade dysplastic lesions, they suggested. While these lesions may have a very low estimated prevalence, at just 0.3% among individuals 90 years of age, they present the greatest risk of pancreatic cancer.
For precursor cysts exhibiting HGD that progressed to pancreatic cancer, the mean interval between dysplasia and cancer was just 4 years. Among 13.7% of individuals, the interval was less than 1 year, suggesting an even shorter window of opportunity for screening.
Beyond this brief timeframe, low test sensitivity explains why screening efforts to date have fallen short, the investigators wrote.
Better tests are “urgently needed,” they added, while acknowledging the challenges inherent to this endeavor. Previous research has shown that precursor lesions in the pancreas are often less than 5 mm in diameter, making them extremely challenging to detect. An effective tool would need to identify solid precursor lesions (PanINs), and also need to simultaneously determine grade of dysplasia.
“Biomarkers could be the future in this matter,” the investigators suggested.
Dr. Koopmann and colleagues concluded by noting that more research is needed to characterize the pathophysiology of pancreatic cancer. On their part, “the current model will be validated, adjusted, and improved whenever new data from autopsy or prospective surveillance studies become available.”
The study was funded in part by Maag Lever Darm Stichting. The investigators disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
Alopecia Universalis Treated With Tofacitinib: The Role of JAK/STAT Inhibitors in Hair Regrowth
Alopecia areata (AA) is an autoimmune disease that immunopathogenetically is thought to be due to breakdown of the immune privilege of the proximal hair follicle during the anagen growth phase. Alopecia areata has been reported to have a lifetime prevalence of 1.7%.1 Recent studies have specifically identified cytotoxic CD8+ NKG2D+ T cells as being responsible for the activation of AA.2-4 Two interleukins—IL-2 and IL-15—have been implicated to be cytotoxic sensitizers allowing CD8+ T cells to secrete IFN-γ and recognize autoantigens via major histocompatibility complex class I.5,6 Janus kinases (JAKs) are enzymes that play major roles in many different molecular processes. Specifically, JAK1/3 has been determined to arbitrate IL-15 activation of receptors on CD8+ T cells.7 These cells then interact with CD4 T cells, mast cells, and other inflammatory cells to cause destruction of the hair follicle without damage to the keratinocyte and melanocyte stem cells, allowing for reversible yet relapsing hair loss.8
Treatment of AA is difficult, requiring patience and strict compliance while taking into account duration of disease, age at presentation, site involvement, patient expectations, cost and insurance coverage, prior therapies, and any comorbidities. At the time of this case, no US Food and Drug Administration–approved drug regimen existed for the treatment of AA, and, to date, no treatment is preventative.4 We present a case of a patient with alopecia universalis of 11 years’ duration that was refractory to intralesional triamcinolone, clobetasol, minoxidil, and UVB brush therapy yet was successfully treated with tofacitinib.
Case Report
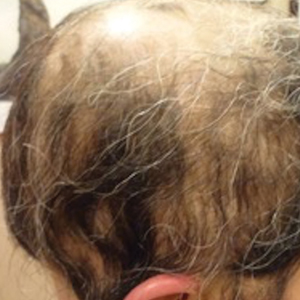
A 29-year-old otherwise-healthy woman presented to our clinic for treatment of alopecia universalis of 11 years’ duration that flared intermittently despite various treatments. Her medical history was unremarkable; however, she had a brother with alopecia universalis. She had no family history of any other autoimmune disorders. At the current presentation, the patient was known to have alopecia universalis with scant evidence of exclamation-point hairs on dermoscopy. Her treatment plan at this point consisted of intralesional triamcinolone to the active areas at 10 mg/mL every 4 weeks, plus clobetasol foam 0.05% at bedtime, minoxidil foam 5% at bedtime, and a UVB brush 3 times a week for 6 months before progressing to universalis type because of hair loss in the eyebrows and eyelashes. This treatment plan continued for 1 year with minimal improvement of the alopecia (Figure 1).
The patient was dissatisfied and wanted to discontinue therapy. Because these treatment options were exhausted with minimal benefit, the patient was then considered for treatment with tofacitinib. Baseline studies were performed, including purified protein derivative, complete blood cell count with differential, comprehensive metabolic panel, lipid profile, and liver function tests, all of which were within reference range. Insurance initially denied coverage of this therapy; a prior authorization was subsequently submitted and denied. A letter of medical necessity was then proposed, and approval for tofacitinib was finally granted. The patient was started on tofacitinib 5 mg twice daily and was monitored every 2 months with a complete blood cell count, comprehensive metabolic panel, lipid panels, and liver function tests. She had a platelet count of 112,000/μL (reference range, 150,000–450,000/μL) at baseline, and continued monitoring revealed a platelet count of 83,000 after 7 months of treatment. This platelet abnormality was evaluated by a hematologist and found to be within reference range; subsequent monitoring did not reveal any abnormalities.
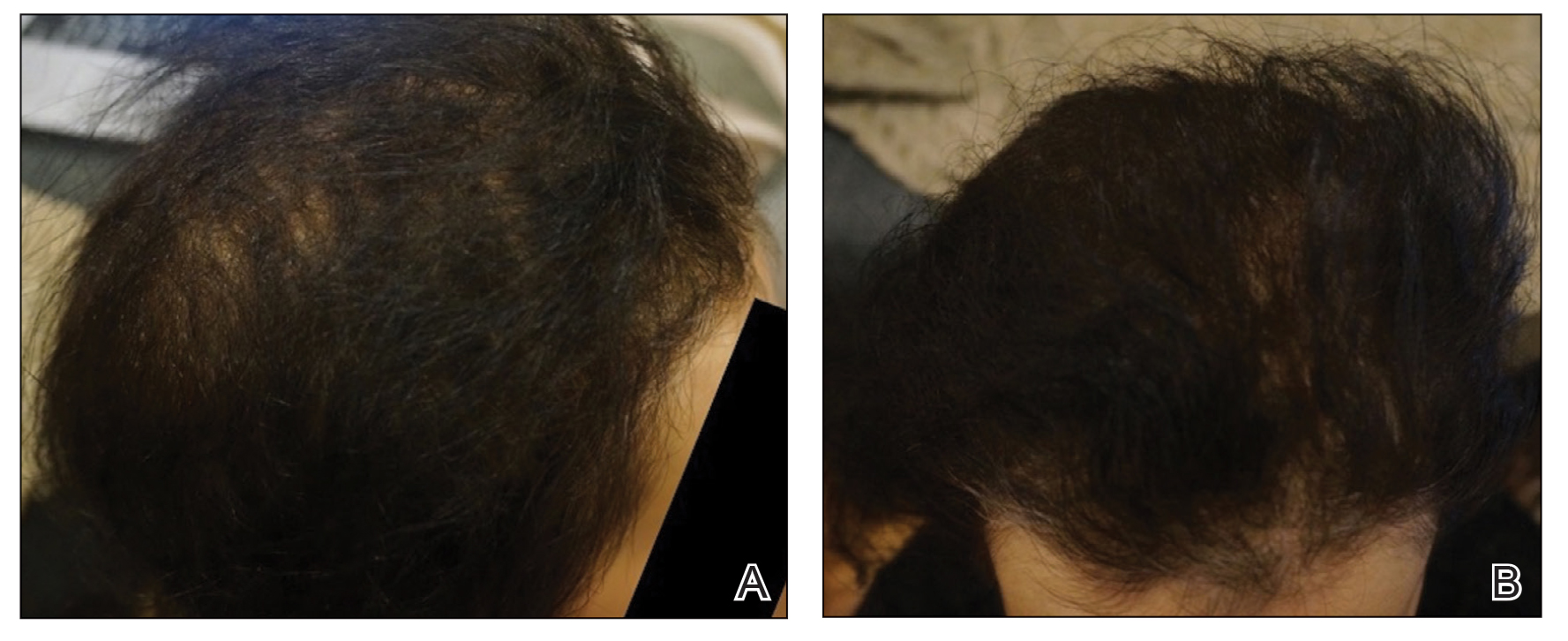
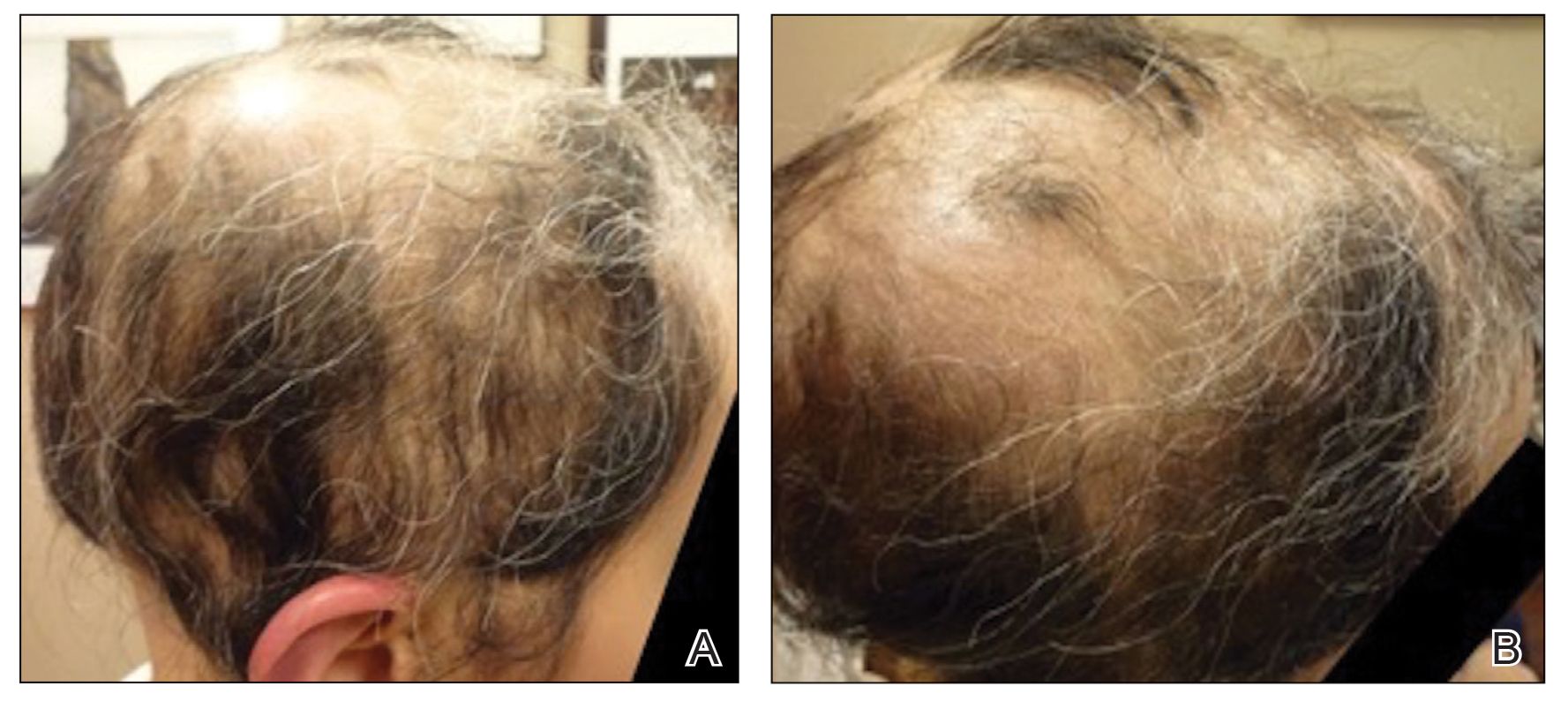
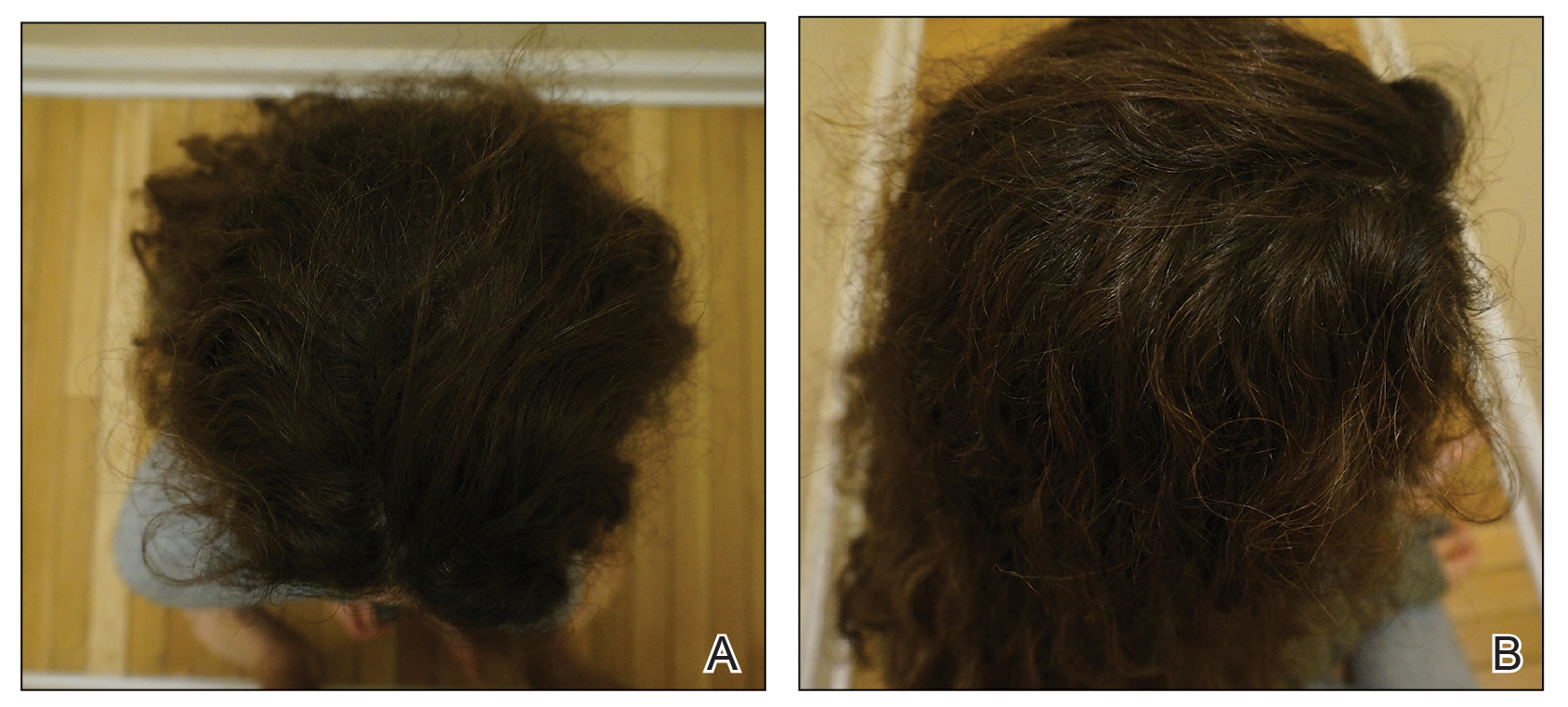
Initial hair growth on the scalp was diffuse with thin, white to light brown hairs in areas of hair loss at months 1 and 2, with progressive hair growth over months 3 to 7. Eyebrow hair growth was noted beginning at month 6. One year later, only hair regrowth occurred without any adverse events (Figure 2). After 5 years of treatment, the patient had a full head of thick hair (Figure 3). The tofacitinib dosage was 5 mg twice daily at initiation, and after 1 year increased to 10 mg twice daily. Her medical insurance subsequently changed and the regimen was adjusted to an 11-mg tablet and 5-mg tablet daily. She remained on this regimen with success.
Comment
Use of JAK Inhibitors—Reports and studies have shed light on the use and efficacy of JAK inhibitors in AA (Table).5-11 Tofacitinib is a selective JAK1/3 inhibitor that predominantly inhibits JAK3 but also inhibits JAK1, albeit to a lesser degree, which interferes with the JAK/STAT (signal transducer and activator of transcription) cascade responsible for the production, differentiation, and function of various B cells, T cells, and natural killer cells.2 Although it was developed for the management of allograft rejection, tofacitinib has made headway in rheumatology for treatment of patients with moderate to severe rheumatoid arthritis who are unable to take or are not responding to methotrexate.2 Since 2014, tofacitinib has been introduced to the therapeutic realm for AA but is not yet approved by the US Food and Drug Administration.3,4
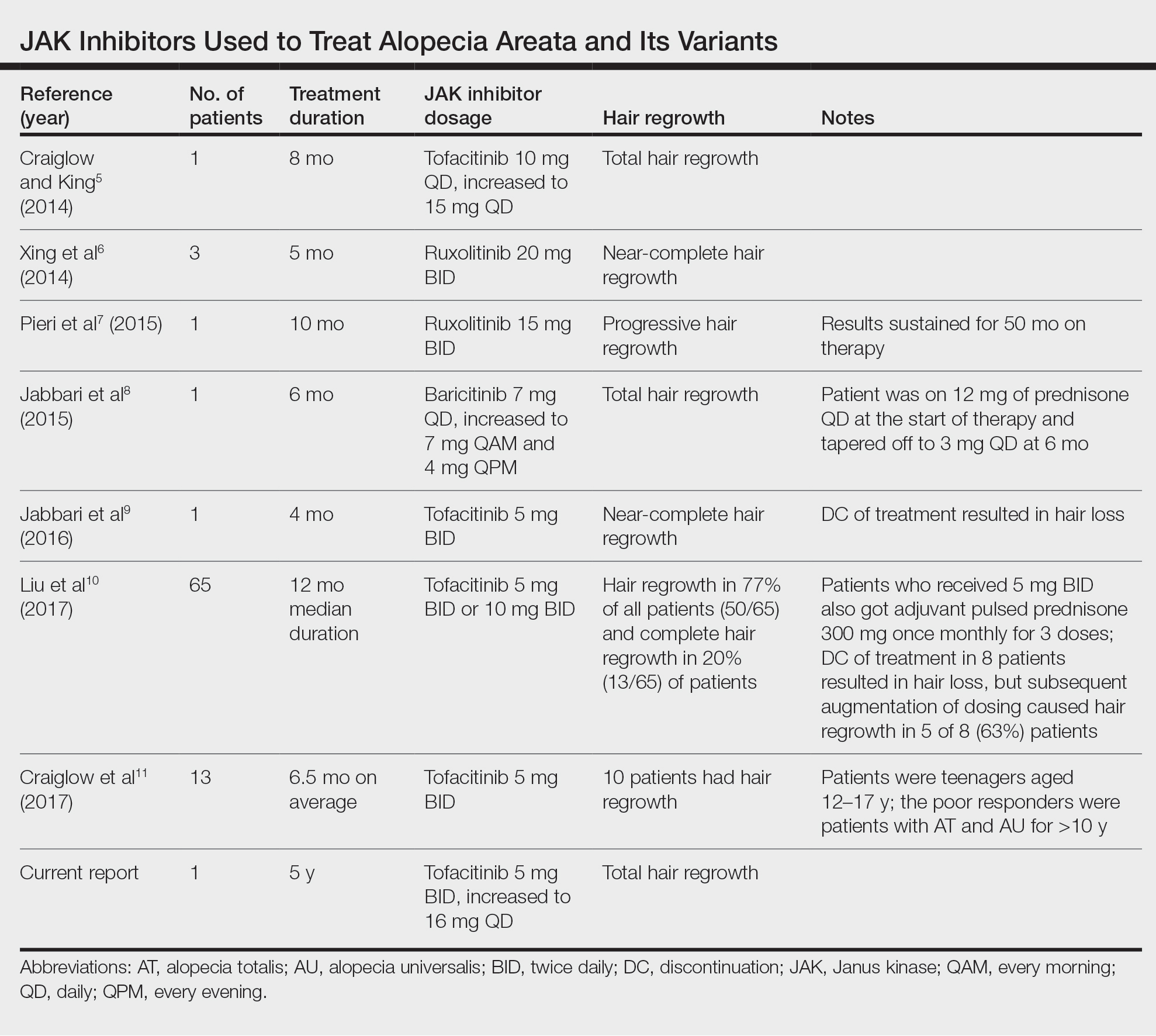
In 2014, Craiglow and King5 reported use of tofacitinib with dosages beginning at 10 mg/d and increasing to 15 mg/d in a patient with alopecia universalis and psoriasis. Total hair regrowth was noted after 8 months of therapy.5 Xing et al6 described 3 patients treated with ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, at an oral dose of 20 mg twice daily with near-complete hair regrowth after 5 months of treatment.6 Biopsies from lesions at baseline and after 3 months of therapy revealed a reduction in perifollicular T cells and in HLA class I and II expression in follicles.6 A patient in Italy with essential thrombocythemia and concurrent alopecia universalis was enrolled in a clinical trial with ruxolitinib and was treated with 15 mg twice daily. After 10 months of treatment, the patient had progressive hair regrowth that was sustained for more than 50 months of therapy.7 Baricitinib, a JAK1/2 inhibitor, was used in a 17-year-old adolescent boy to assess efficacy of the drug in
A recent retrospective study assessing response to tofacitinib in adults with AA (>40% hair loss), alopecia totalis, alopecia universalis, and stable or progressive diseases for at least 6 months determined a clinical response in 50 of 65 (77%) patients, with 13 patients exhibiting a complete response.10 Patients in this study were started on tofacitinib 5 mg twice daily with the addition of adjuvant pulsed prednisone (300 mg once monthly for 3 doses) with or without doubled dosing of tofacitinib if they had a halt in hair regrowth. This study demonstrated some benefit when pulsed prednisone was combined with the daily tofacitinib therapy. However, the study emphasized the importance of maintenance therapy, as 8 patients experienced hair loss with discontinuation after previously having hair regrowth; 5 (63%) of these patients experienced regrowth with augmentation of dosing or addition of adjuvant therapy.10
Another group of investigators assessed the efficacy of tofacitinib 5 mg in 13 adolescents aged 12 to 17 years, most with alopecia universalis (46% [6/13]); 10 of 13 (77%) patients responded to treatment with a mean duration of 6.5 months. The patients who had alopecia totalis and alopecia universalis for more than 10 years were poor responders to tofacitinib, and in fact, 1 of 13 (33%) patients in the study who did not respond to therapy had disease for 12 years.11 Therefore, starting tofacitinib either long-term or intermittently should be considered in children diagnosed early with severe AA, alopecia totalis, or alopecia universalis to prevent irreversible hair loss or progressive disease12,13; however, further data are required to assess efficacy and long-term benefits of this type of regimen.
Safety Profile—Widespread use of a medication is determined not only by its efficacy profile but also its safety profile. With any medication that exhibits immunosuppressive effects, adverse events must be considered and thoroughly discussed with patients and their primary care physicians. A prospective, open-label, single-arm trial examined the efficacy and safety of tofacitinib 5 mg twice daily in the treatment of AA and its more severe forms over 3 months.12 Of the 66 patients who completed the trial, 64% (42/66) exhibited a positive response to tofacitinib. Relapse was noted in 8.5 weeks after discontinuation of tofacitinib, reiterating the potential need for a maintenance regimen. In this study, 25.8% (17/66) of patients experienced infections as adverse events including (in decreasing order) upper respiratory tract infections, urinary tract infections, herpes zoster, conjunctivitis, bronchitis, mononucleosis, and paronychia. No reports of new or recurrent malignancy were noted. Other more constitutional adverse events were noted including headaches, abdominal pain, acne, diarrhea, fatigue, nausea, pruritus, hot flashes, cough, folliculitis, weight gain, dry eyes, and amenorrhea. One patient with a pre-existing liver condition experienced transaminitis that resolved with weight loss. There also were noted increases in low- and high-density lipoprotein levels.12 Our patient with baseline thrombocytopenia had mild drops in platelet count that subsequently stabilized and did not result in any bleeding abnormalities.
Duration of Therapy—Tofacitinib has demonstrated some preliminary success in the management of AA, but the appropriate duration of treatment requires further investigation. Our patient has been on tofacitinib for more than 5 years. She started at a total dosage of 10 mg/d, which increased to 16 mg/d. Initial dosing with maintenance regimens needs to be established for further widespread use to maximize benefit and minimize harm.
At what point do we decide to continue or stop treatment in patients who do not respond as expected or plateau? This is another critical question; our patient had periods of slowed growth and plateauing, but knowing the risks and benefits, she continued the medication and eventually experienced improved regrowth again.
Conclusion
Throughout the literature and in our patient, tofacitinib has demonstrated efficacy in treating AA. When other conventional therapies have failed, use of tofacitinib should be considered.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmstead County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Borazan NH, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2015:618-642.
- Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42-S44.
- Shapiro J. Dermatologic therapy: alopecia areata update. Dermatol Ther. 2011;24:301.
- Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988-2990.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82-83.
- Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EbioMedicine. 2015;2:351-355.
- Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25:642-643.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28.
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:E89776.
- Iorizzo M, Tosti A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin Emerg Drugs. 2018;23:77-81.
Alopecia areata (AA) is an autoimmune disease that immunopathogenetically is thought to be due to breakdown of the immune privilege of the proximal hair follicle during the anagen growth phase. Alopecia areata has been reported to have a lifetime prevalence of 1.7%.1 Recent studies have specifically identified cytotoxic CD8+ NKG2D+ T cells as being responsible for the activation of AA.2-4 Two interleukins—IL-2 and IL-15—have been implicated to be cytotoxic sensitizers allowing CD8+ T cells to secrete IFN-γ and recognize autoantigens via major histocompatibility complex class I.5,6 Janus kinases (JAKs) are enzymes that play major roles in many different molecular processes. Specifically, JAK1/3 has been determined to arbitrate IL-15 activation of receptors on CD8+ T cells.7 These cells then interact with CD4 T cells, mast cells, and other inflammatory cells to cause destruction of the hair follicle without damage to the keratinocyte and melanocyte stem cells, allowing for reversible yet relapsing hair loss.8
Treatment of AA is difficult, requiring patience and strict compliance while taking into account duration of disease, age at presentation, site involvement, patient expectations, cost and insurance coverage, prior therapies, and any comorbidities. At the time of this case, no US Food and Drug Administration–approved drug regimen existed for the treatment of AA, and, to date, no treatment is preventative.4 We present a case of a patient with alopecia universalis of 11 years’ duration that was refractory to intralesional triamcinolone, clobetasol, minoxidil, and UVB brush therapy yet was successfully treated with tofacitinib.
Case Report
A 29-year-old otherwise-healthy woman presented to our clinic for treatment of alopecia universalis of 11 years’ duration that flared intermittently despite various treatments. Her medical history was unremarkable; however, she had a brother with alopecia universalis. She had no family history of any other autoimmune disorders. At the current presentation, the patient was known to have alopecia universalis with scant evidence of exclamation-point hairs on dermoscopy. Her treatment plan at this point consisted of intralesional triamcinolone to the active areas at 10 mg/mL every 4 weeks, plus clobetasol foam 0.05% at bedtime, minoxidil foam 5% at bedtime, and a UVB brush 3 times a week for 6 months before progressing to universalis type because of hair loss in the eyebrows and eyelashes. This treatment plan continued for 1 year with minimal improvement of the alopecia (Figure 1).

The patient was dissatisfied and wanted to discontinue therapy. Because these treatment options were exhausted with minimal benefit, the patient was then considered for treatment with tofacitinib. Baseline studies were performed, including purified protein derivative, complete blood cell count with differential, comprehensive metabolic panel, lipid profile, and liver function tests, all of which were within reference range. Insurance initially denied coverage of this therapy; a prior authorization was subsequently submitted and denied. A letter of medical necessity was then proposed, and approval for tofacitinib was finally granted. The patient was started on tofacitinib 5 mg twice daily and was monitored every 2 months with a complete blood cell count, comprehensive metabolic panel, lipid panels, and liver function tests. She had a platelet count of 112,000/μL (reference range, 150,000–450,000/μL) at baseline, and continued monitoring revealed a platelet count of 83,000 after 7 months of treatment. This platelet abnormality was evaluated by a hematologist and found to be within reference range; subsequent monitoring did not reveal any abnormalities.

Initial hair growth on the scalp was diffuse with thin, white to light brown hairs in areas of hair loss at months 1 and 2, with progressive hair growth over months 3 to 7. Eyebrow hair growth was noted beginning at month 6. One year later, only hair regrowth occurred without any adverse events (Figure 2). After 5 years of treatment, the patient had a full head of thick hair (Figure 3). The tofacitinib dosage was 5 mg twice daily at initiation, and after 1 year increased to 10 mg twice daily. Her medical insurance subsequently changed and the regimen was adjusted to an 11-mg tablet and 5-mg tablet daily. She remained on this regimen with success.

Comment
Use of JAK Inhibitors—Reports and studies have shed light on the use and efficacy of JAK inhibitors in AA (Table).5-11 Tofacitinib is a selective JAK1/3 inhibitor that predominantly inhibits JAK3 but also inhibits JAK1, albeit to a lesser degree, which interferes with the JAK/STAT (signal transducer and activator of transcription) cascade responsible for the production, differentiation, and function of various B cells, T cells, and natural killer cells.2 Although it was developed for the management of allograft rejection, tofacitinib has made headway in rheumatology for treatment of patients with moderate to severe rheumatoid arthritis who are unable to take or are not responding to methotrexate.2 Since 2014, tofacitinib has been introduced to the therapeutic realm for AA but is not yet approved by the US Food and Drug Administration.3,4

In 2014, Craiglow and King5 reported use of tofacitinib with dosages beginning at 10 mg/d and increasing to 15 mg/d in a patient with alopecia universalis and psoriasis. Total hair regrowth was noted after 8 months of therapy.5 Xing et al6 described 3 patients treated with ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, at an oral dose of 20 mg twice daily with near-complete hair regrowth after 5 months of treatment.6 Biopsies from lesions at baseline and after 3 months of therapy revealed a reduction in perifollicular T cells and in HLA class I and II expression in follicles.6 A patient in Italy with essential thrombocythemia and concurrent alopecia universalis was enrolled in a clinical trial with ruxolitinib and was treated with 15 mg twice daily. After 10 months of treatment, the patient had progressive hair regrowth that was sustained for more than 50 months of therapy.7 Baricitinib, a JAK1/2 inhibitor, was used in a 17-year-old adolescent boy to assess efficacy of the drug in
A recent retrospective study assessing response to tofacitinib in adults with AA (>40% hair loss), alopecia totalis, alopecia universalis, and stable or progressive diseases for at least 6 months determined a clinical response in 50 of 65 (77%) patients, with 13 patients exhibiting a complete response.10 Patients in this study were started on tofacitinib 5 mg twice daily with the addition of adjuvant pulsed prednisone (300 mg once monthly for 3 doses) with or without doubled dosing of tofacitinib if they had a halt in hair regrowth. This study demonstrated some benefit when pulsed prednisone was combined with the daily tofacitinib therapy. However, the study emphasized the importance of maintenance therapy, as 8 patients experienced hair loss with discontinuation after previously having hair regrowth; 5 (63%) of these patients experienced regrowth with augmentation of dosing or addition of adjuvant therapy.10
Another group of investigators assessed the efficacy of tofacitinib 5 mg in 13 adolescents aged 12 to 17 years, most with alopecia universalis (46% [6/13]); 10 of 13 (77%) patients responded to treatment with a mean duration of 6.5 months. The patients who had alopecia totalis and alopecia universalis for more than 10 years were poor responders to tofacitinib, and in fact, 1 of 13 (33%) patients in the study who did not respond to therapy had disease for 12 years.11 Therefore, starting tofacitinib either long-term or intermittently should be considered in children diagnosed early with severe AA, alopecia totalis, or alopecia universalis to prevent irreversible hair loss or progressive disease12,13; however, further data are required to assess efficacy and long-term benefits of this type of regimen.
Safety Profile—Widespread use of a medication is determined not only by its efficacy profile but also its safety profile. With any medication that exhibits immunosuppressive effects, adverse events must be considered and thoroughly discussed with patients and their primary care physicians. A prospective, open-label, single-arm trial examined the efficacy and safety of tofacitinib 5 mg twice daily in the treatment of AA and its more severe forms over 3 months.12 Of the 66 patients who completed the trial, 64% (42/66) exhibited a positive response to tofacitinib. Relapse was noted in 8.5 weeks after discontinuation of tofacitinib, reiterating the potential need for a maintenance regimen. In this study, 25.8% (17/66) of patients experienced infections as adverse events including (in decreasing order) upper respiratory tract infections, urinary tract infections, herpes zoster, conjunctivitis, bronchitis, mononucleosis, and paronychia. No reports of new or recurrent malignancy were noted. Other more constitutional adverse events were noted including headaches, abdominal pain, acne, diarrhea, fatigue, nausea, pruritus, hot flashes, cough, folliculitis, weight gain, dry eyes, and amenorrhea. One patient with a pre-existing liver condition experienced transaminitis that resolved with weight loss. There also were noted increases in low- and high-density lipoprotein levels.12 Our patient with baseline thrombocytopenia had mild drops in platelet count that subsequently stabilized and did not result in any bleeding abnormalities.
Duration of Therapy—Tofacitinib has demonstrated some preliminary success in the management of AA, but the appropriate duration of treatment requires further investigation. Our patient has been on tofacitinib for more than 5 years. She started at a total dosage of 10 mg/d, which increased to 16 mg/d. Initial dosing with maintenance regimens needs to be established for further widespread use to maximize benefit and minimize harm.
At what point do we decide to continue or stop treatment in patients who do not respond as expected or plateau? This is another critical question; our patient had periods of slowed growth and plateauing, but knowing the risks and benefits, she continued the medication and eventually experienced improved regrowth again.
Conclusion
Throughout the literature and in our patient, tofacitinib has demonstrated efficacy in treating AA. When other conventional therapies have failed, use of tofacitinib should be considered.
Alopecia areata (AA) is an autoimmune disease that immunopathogenetically is thought to be due to breakdown of the immune privilege of the proximal hair follicle during the anagen growth phase. Alopecia areata has been reported to have a lifetime prevalence of 1.7%.1 Recent studies have specifically identified cytotoxic CD8+ NKG2D+ T cells as being responsible for the activation of AA.2-4 Two interleukins—IL-2 and IL-15—have been implicated to be cytotoxic sensitizers allowing CD8+ T cells to secrete IFN-γ and recognize autoantigens via major histocompatibility complex class I.5,6 Janus kinases (JAKs) are enzymes that play major roles in many different molecular processes. Specifically, JAK1/3 has been determined to arbitrate IL-15 activation of receptors on CD8+ T cells.7 These cells then interact with CD4 T cells, mast cells, and other inflammatory cells to cause destruction of the hair follicle without damage to the keratinocyte and melanocyte stem cells, allowing for reversible yet relapsing hair loss.8
Treatment of AA is difficult, requiring patience and strict compliance while taking into account duration of disease, age at presentation, site involvement, patient expectations, cost and insurance coverage, prior therapies, and any comorbidities. At the time of this case, no US Food and Drug Administration–approved drug regimen existed for the treatment of AA, and, to date, no treatment is preventative.4 We present a case of a patient with alopecia universalis of 11 years’ duration that was refractory to intralesional triamcinolone, clobetasol, minoxidil, and UVB brush therapy yet was successfully treated with tofacitinib.
Case Report
A 29-year-old otherwise-healthy woman presented to our clinic for treatment of alopecia universalis of 11 years’ duration that flared intermittently despite various treatments. Her medical history was unremarkable; however, she had a brother with alopecia universalis. She had no family history of any other autoimmune disorders. At the current presentation, the patient was known to have alopecia universalis with scant evidence of exclamation-point hairs on dermoscopy. Her treatment plan at this point consisted of intralesional triamcinolone to the active areas at 10 mg/mL every 4 weeks, plus clobetasol foam 0.05% at bedtime, minoxidil foam 5% at bedtime, and a UVB brush 3 times a week for 6 months before progressing to universalis type because of hair loss in the eyebrows and eyelashes. This treatment plan continued for 1 year with minimal improvement of the alopecia (Figure 1).

The patient was dissatisfied and wanted to discontinue therapy. Because these treatment options were exhausted with minimal benefit, the patient was then considered for treatment with tofacitinib. Baseline studies were performed, including purified protein derivative, complete blood cell count with differential, comprehensive metabolic panel, lipid profile, and liver function tests, all of which were within reference range. Insurance initially denied coverage of this therapy; a prior authorization was subsequently submitted and denied. A letter of medical necessity was then proposed, and approval for tofacitinib was finally granted. The patient was started on tofacitinib 5 mg twice daily and was monitored every 2 months with a complete blood cell count, comprehensive metabolic panel, lipid panels, and liver function tests. She had a platelet count of 112,000/μL (reference range, 150,000–450,000/μL) at baseline, and continued monitoring revealed a platelet count of 83,000 after 7 months of treatment. This platelet abnormality was evaluated by a hematologist and found to be within reference range; subsequent monitoring did not reveal any abnormalities.

Initial hair growth on the scalp was diffuse with thin, white to light brown hairs in areas of hair loss at months 1 and 2, with progressive hair growth over months 3 to 7. Eyebrow hair growth was noted beginning at month 6. One year later, only hair regrowth occurred without any adverse events (Figure 2). After 5 years of treatment, the patient had a full head of thick hair (Figure 3). The tofacitinib dosage was 5 mg twice daily at initiation, and after 1 year increased to 10 mg twice daily. Her medical insurance subsequently changed and the regimen was adjusted to an 11-mg tablet and 5-mg tablet daily. She remained on this regimen with success.

Comment
Use of JAK Inhibitors—Reports and studies have shed light on the use and efficacy of JAK inhibitors in AA (Table).5-11 Tofacitinib is a selective JAK1/3 inhibitor that predominantly inhibits JAK3 but also inhibits JAK1, albeit to a lesser degree, which interferes with the JAK/STAT (signal transducer and activator of transcription) cascade responsible for the production, differentiation, and function of various B cells, T cells, and natural killer cells.2 Although it was developed for the management of allograft rejection, tofacitinib has made headway in rheumatology for treatment of patients with moderate to severe rheumatoid arthritis who are unable to take or are not responding to methotrexate.2 Since 2014, tofacitinib has been introduced to the therapeutic realm for AA but is not yet approved by the US Food and Drug Administration.3,4

In 2014, Craiglow and King5 reported use of tofacitinib with dosages beginning at 10 mg/d and increasing to 15 mg/d in a patient with alopecia universalis and psoriasis. Total hair regrowth was noted after 8 months of therapy.5 Xing et al6 described 3 patients treated with ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, at an oral dose of 20 mg twice daily with near-complete hair regrowth after 5 months of treatment.6 Biopsies from lesions at baseline and after 3 months of therapy revealed a reduction in perifollicular T cells and in HLA class I and II expression in follicles.6 A patient in Italy with essential thrombocythemia and concurrent alopecia universalis was enrolled in a clinical trial with ruxolitinib and was treated with 15 mg twice daily. After 10 months of treatment, the patient had progressive hair regrowth that was sustained for more than 50 months of therapy.7 Baricitinib, a JAK1/2 inhibitor, was used in a 17-year-old adolescent boy to assess efficacy of the drug in
A recent retrospective study assessing response to tofacitinib in adults with AA (>40% hair loss), alopecia totalis, alopecia universalis, and stable or progressive diseases for at least 6 months determined a clinical response in 50 of 65 (77%) patients, with 13 patients exhibiting a complete response.10 Patients in this study were started on tofacitinib 5 mg twice daily with the addition of adjuvant pulsed prednisone (300 mg once monthly for 3 doses) with or without doubled dosing of tofacitinib if they had a halt in hair regrowth. This study demonstrated some benefit when pulsed prednisone was combined with the daily tofacitinib therapy. However, the study emphasized the importance of maintenance therapy, as 8 patients experienced hair loss with discontinuation after previously having hair regrowth; 5 (63%) of these patients experienced regrowth with augmentation of dosing or addition of adjuvant therapy.10
Another group of investigators assessed the efficacy of tofacitinib 5 mg in 13 adolescents aged 12 to 17 years, most with alopecia universalis (46% [6/13]); 10 of 13 (77%) patients responded to treatment with a mean duration of 6.5 months. The patients who had alopecia totalis and alopecia universalis for more than 10 years were poor responders to tofacitinib, and in fact, 1 of 13 (33%) patients in the study who did not respond to therapy had disease for 12 years.11 Therefore, starting tofacitinib either long-term or intermittently should be considered in children diagnosed early with severe AA, alopecia totalis, or alopecia universalis to prevent irreversible hair loss or progressive disease12,13; however, further data are required to assess efficacy and long-term benefits of this type of regimen.
Safety Profile—Widespread use of a medication is determined not only by its efficacy profile but also its safety profile. With any medication that exhibits immunosuppressive effects, adverse events must be considered and thoroughly discussed with patients and their primary care physicians. A prospective, open-label, single-arm trial examined the efficacy and safety of tofacitinib 5 mg twice daily in the treatment of AA and its more severe forms over 3 months.12 Of the 66 patients who completed the trial, 64% (42/66) exhibited a positive response to tofacitinib. Relapse was noted in 8.5 weeks after discontinuation of tofacitinib, reiterating the potential need for a maintenance regimen. In this study, 25.8% (17/66) of patients experienced infections as adverse events including (in decreasing order) upper respiratory tract infections, urinary tract infections, herpes zoster, conjunctivitis, bronchitis, mononucleosis, and paronychia. No reports of new or recurrent malignancy were noted. Other more constitutional adverse events were noted including headaches, abdominal pain, acne, diarrhea, fatigue, nausea, pruritus, hot flashes, cough, folliculitis, weight gain, dry eyes, and amenorrhea. One patient with a pre-existing liver condition experienced transaminitis that resolved with weight loss. There also were noted increases in low- and high-density lipoprotein levels.12 Our patient with baseline thrombocytopenia had mild drops in platelet count that subsequently stabilized and did not result in any bleeding abnormalities.
Duration of Therapy—Tofacitinib has demonstrated some preliminary success in the management of AA, but the appropriate duration of treatment requires further investigation. Our patient has been on tofacitinib for more than 5 years. She started at a total dosage of 10 mg/d, which increased to 16 mg/d. Initial dosing with maintenance regimens needs to be established for further widespread use to maximize benefit and minimize harm.
At what point do we decide to continue or stop treatment in patients who do not respond as expected or plateau? This is another critical question; our patient had periods of slowed growth and plateauing, but knowing the risks and benefits, she continued the medication and eventually experienced improved regrowth again.
Conclusion
Throughout the literature and in our patient, tofacitinib has demonstrated efficacy in treating AA. When other conventional therapies have failed, use of tofacitinib should be considered.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmstead County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Borazan NH, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2015:618-642.
- Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42-S44.
- Shapiro J. Dermatologic therapy: alopecia areata update. Dermatol Ther. 2011;24:301.
- Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988-2990.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82-83.
- Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EbioMedicine. 2015;2:351-355.
- Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25:642-643.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28.
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:E89776.
- Iorizzo M, Tosti A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin Emerg Drugs. 2018;23:77-81.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmstead County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Borazan NH, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2015:618-642.
- Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42-S44.
- Shapiro J. Dermatologic therapy: alopecia areata update. Dermatol Ther. 2011;24:301.
- Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988-2990.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82-83.
- Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EbioMedicine. 2015;2:351-355.
- Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25:642-643.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28.
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:E89776.
- Iorizzo M, Tosti A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin Emerg Drugs. 2018;23:77-81.
Practice Points
- Janus kinase inhibitors target one of the cellular pathogeneses of alopecia areata.
- Janus kinase inhibitors may be an option for patients who have exhausted other treatment modalities for alopecia.
Pediatric Primary Cutaneous Marginal Zone Lymphoma Treated With Doxycycline
Case Report
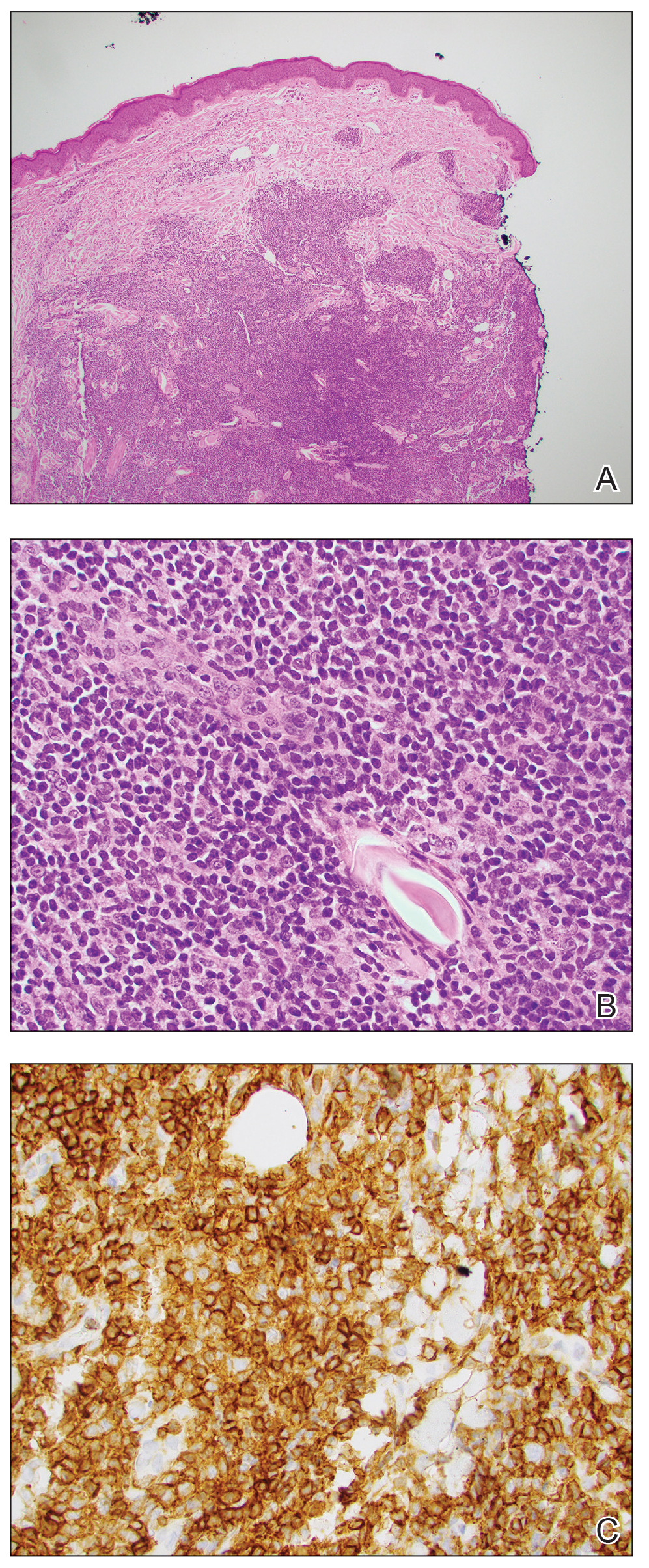
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.
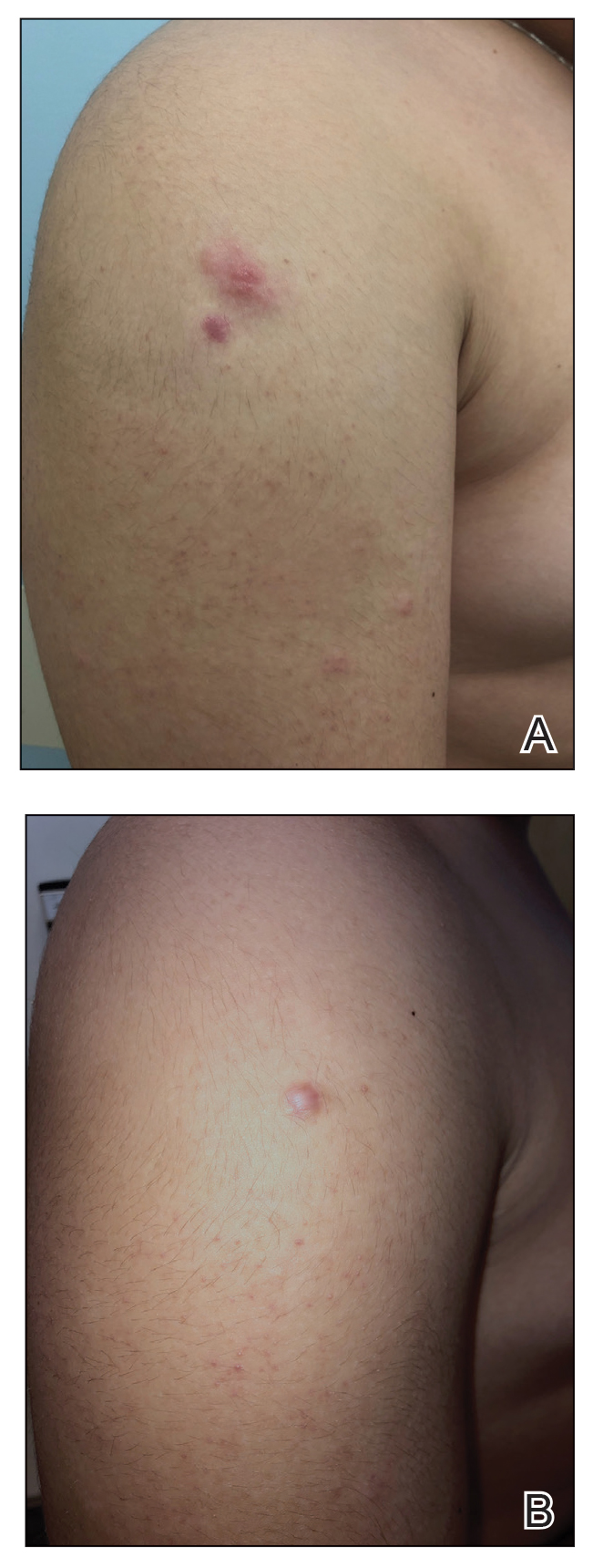
The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.
Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8 Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10 Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.

The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.

Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8 Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10 Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.

The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.

Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8 Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10 Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
Practice Points
- When skin biopsy reveals marginal zone lymphoma, laboratory workup should include a complete blood cell count, chemistry, and serum lactate dehydrogenase levels. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed.
- For patients with multifocal skin lesions, positive emission tomography with computed tomography is utilized to exclude systemic disease and assess lymph node involvement.
- Treatments for primary cutaneous marginal zone lymphoma include excision, topical steroids, intralesional steroids, intralesional rituximab, radiation therapy, and antibiotics.
- Doxycycline can be considered as a treatment option for pediatric patients with widespread cutaneous involvement.
Many preoperative EAC biopsies fail to predict true tumor grade, leading to unnecessary esophagectomy
Inaccurate preoperative biopsy findings could mean that patients who are candidates for endoscopic resection (ER) are unnecessarily undergoing esophagectomy, a procedure with greater risks of morbidity and mortality, reported Ravi S. Shah, MD, of Cleveland Clinic, and colleagues.
“It is unclear how accurate tumor differentiation on endoscopic biopsies is and if it can be used for clinical decision-making,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy. “Given that tumors may be considerably heterogeneous in gland formation, the limited amount of tissue obtained from endoscopic forceps biopsies may not be representative of the entire tumor for pathologic grading, which may result in discrepant tumor grading between biopsy and resection specimens.”
While previous studies have compared esophagogastroduodenoscopy-guided biopsy results with histological findings after surgical resection, scant evidence is available to compare biopsy findings with both surgically and endoscopically resected tissue.
Despite this potential knowledge gap, “many patients with poorly differentiated EAC on preresection biopsy do not undergo ER, with the belief that the final resection pathology would be noncurative,” the investigators noted.
To help clarify how congruent pre- and postoperative biopsies are for both resection methods, Dr. Shah and colleagues conducted a retrospective study involving 346 EAC lesions. Samples were drawn from 121 ERs and 225 esophagectomies performed at two tertiary referral centers. Preoperative and postoperative findings were compared for accuracy and for level of agreement via Gwet’s AC2 interrater analysis.
For all evaluable lesions, preoperative biopsy had an accuracy of 68%, with a “substantial” agreement coefficient (Gwet’s AC2, 0.70; P less than .001). Accuracy in the esophagectomy group was similar, at 72%, again with “substantial” agreement (Gwet’s AC2, 0.74; P less than .001). For the ER group, however, accuracy was just 56%, with a “moderate” level of agreement (Gwet’s AC2, 0.60; P less than .001).
“We speculate that the discrepancy of tumor differentiation on endoscopic forceps biopsies and resection specimens is due to nonrepresentative sampling of tumors to accurately determine the percentage of gland formation and thus tumor grade,” the investigators noted.
Further analysis showed that 22.7% of moderately differentiated tumors were upgraded to poorly differentiated upon final histology. Conversely, 19.6% of poorly differentiated tumors were downgraded to moderately differentiated. Downgrading was even more common among T1a tumors, 40% of which were changed from poorly to moderately differentiated between pre- and postprocedural histology.
These latter findings concerning downgrading are particularly relevant for clinical decision-making, the investigators noted, as patients with poorly differentiated EAC on preoperative biopsy are typically sent for esophagectomy – a more invasive and riskier procedure – out of concern that ER will be noncurative.
“If poor differentiation was the only high-risk feature, these patients may have unnecessarily undergone esophagectomy,” Dr. Shah and colleagues wrote. “Especially in marginal surgical candidates, staging ER should be considered in patients with early esophageal cancer with preoperative biopsies showing poorly differentiated cancer.”
The investigators disclosed relationships with Medtronic, Lucid Diagnostics, Lumendi, and others.
Inaccurate preoperative biopsy findings could mean that patients who are candidates for endoscopic resection (ER) are unnecessarily undergoing esophagectomy, a procedure with greater risks of morbidity and mortality, reported Ravi S. Shah, MD, of Cleveland Clinic, and colleagues.
“It is unclear how accurate tumor differentiation on endoscopic biopsies is and if it can be used for clinical decision-making,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy. “Given that tumors may be considerably heterogeneous in gland formation, the limited amount of tissue obtained from endoscopic forceps biopsies may not be representative of the entire tumor for pathologic grading, which may result in discrepant tumor grading between biopsy and resection specimens.”
While previous studies have compared esophagogastroduodenoscopy-guided biopsy results with histological findings after surgical resection, scant evidence is available to compare biopsy findings with both surgically and endoscopically resected tissue.
Despite this potential knowledge gap, “many patients with poorly differentiated EAC on preresection biopsy do not undergo ER, with the belief that the final resection pathology would be noncurative,” the investigators noted.
To help clarify how congruent pre- and postoperative biopsies are for both resection methods, Dr. Shah and colleagues conducted a retrospective study involving 346 EAC lesions. Samples were drawn from 121 ERs and 225 esophagectomies performed at two tertiary referral centers. Preoperative and postoperative findings were compared for accuracy and for level of agreement via Gwet’s AC2 interrater analysis.
For all evaluable lesions, preoperative biopsy had an accuracy of 68%, with a “substantial” agreement coefficient (Gwet’s AC2, 0.70; P less than .001). Accuracy in the esophagectomy group was similar, at 72%, again with “substantial” agreement (Gwet’s AC2, 0.74; P less than .001). For the ER group, however, accuracy was just 56%, with a “moderate” level of agreement (Gwet’s AC2, 0.60; P less than .001).
“We speculate that the discrepancy of tumor differentiation on endoscopic forceps biopsies and resection specimens is due to nonrepresentative sampling of tumors to accurately determine the percentage of gland formation and thus tumor grade,” the investigators noted.
Further analysis showed that 22.7% of moderately differentiated tumors were upgraded to poorly differentiated upon final histology. Conversely, 19.6% of poorly differentiated tumors were downgraded to moderately differentiated. Downgrading was even more common among T1a tumors, 40% of which were changed from poorly to moderately differentiated between pre- and postprocedural histology.
These latter findings concerning downgrading are particularly relevant for clinical decision-making, the investigators noted, as patients with poorly differentiated EAC on preoperative biopsy are typically sent for esophagectomy – a more invasive and riskier procedure – out of concern that ER will be noncurative.
“If poor differentiation was the only high-risk feature, these patients may have unnecessarily undergone esophagectomy,” Dr. Shah and colleagues wrote. “Especially in marginal surgical candidates, staging ER should be considered in patients with early esophageal cancer with preoperative biopsies showing poorly differentiated cancer.”
The investigators disclosed relationships with Medtronic, Lucid Diagnostics, Lumendi, and others.
Inaccurate preoperative biopsy findings could mean that patients who are candidates for endoscopic resection (ER) are unnecessarily undergoing esophagectomy, a procedure with greater risks of morbidity and mortality, reported Ravi S. Shah, MD, of Cleveland Clinic, and colleagues.
“It is unclear how accurate tumor differentiation on endoscopic biopsies is and if it can be used for clinical decision-making,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy. “Given that tumors may be considerably heterogeneous in gland formation, the limited amount of tissue obtained from endoscopic forceps biopsies may not be representative of the entire tumor for pathologic grading, which may result in discrepant tumor grading between biopsy and resection specimens.”
While previous studies have compared esophagogastroduodenoscopy-guided biopsy results with histological findings after surgical resection, scant evidence is available to compare biopsy findings with both surgically and endoscopically resected tissue.
Despite this potential knowledge gap, “many patients with poorly differentiated EAC on preresection biopsy do not undergo ER, with the belief that the final resection pathology would be noncurative,” the investigators noted.
To help clarify how congruent pre- and postoperative biopsies are for both resection methods, Dr. Shah and colleagues conducted a retrospective study involving 346 EAC lesions. Samples were drawn from 121 ERs and 225 esophagectomies performed at two tertiary referral centers. Preoperative and postoperative findings were compared for accuracy and for level of agreement via Gwet’s AC2 interrater analysis.
For all evaluable lesions, preoperative biopsy had an accuracy of 68%, with a “substantial” agreement coefficient (Gwet’s AC2, 0.70; P less than .001). Accuracy in the esophagectomy group was similar, at 72%, again with “substantial” agreement (Gwet’s AC2, 0.74; P less than .001). For the ER group, however, accuracy was just 56%, with a “moderate” level of agreement (Gwet’s AC2, 0.60; P less than .001).
“We speculate that the discrepancy of tumor differentiation on endoscopic forceps biopsies and resection specimens is due to nonrepresentative sampling of tumors to accurately determine the percentage of gland formation and thus tumor grade,” the investigators noted.
Further analysis showed that 22.7% of moderately differentiated tumors were upgraded to poorly differentiated upon final histology. Conversely, 19.6% of poorly differentiated tumors were downgraded to moderately differentiated. Downgrading was even more common among T1a tumors, 40% of which were changed from poorly to moderately differentiated between pre- and postprocedural histology.
These latter findings concerning downgrading are particularly relevant for clinical decision-making, the investigators noted, as patients with poorly differentiated EAC on preoperative biopsy are typically sent for esophagectomy – a more invasive and riskier procedure – out of concern that ER will be noncurative.
“If poor differentiation was the only high-risk feature, these patients may have unnecessarily undergone esophagectomy,” Dr. Shah and colleagues wrote. “Especially in marginal surgical candidates, staging ER should be considered in patients with early esophageal cancer with preoperative biopsies showing poorly differentiated cancer.”
The investigators disclosed relationships with Medtronic, Lucid Diagnostics, Lumendi, and others.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Review estimates acne risk with JAK inhibitor therapy
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
T-cell cancers: CAR T therapy to the rescue?
As Baylor College of Medicine’s Max Mamonkin, PhD, noted in a presentation, patients with conditions such as T-cell lymphoma and T-cell acute lymphoblastic leukemia (ALL) have limited treatment options and grim prognoses. “This is an area with huge unmet need,” he said. “They don’t have options that patients with B-cell malignancies have, like [CAR T-cell therapy] and bispecifics.”
One big challenge is that CAR-targeted antigens in T-cell blood cancers are shared by both normal and malignant T-cells, he said. That poses a risk during therapy that the engineered cells will target each other with “disastrous consequences.”
Research by his team and others have shown that gene editing can help the cells to stop engaging in “fratricide,” Dr. Mamonkin said.
The problem is “it’s much easier to do gene editing on the bench and much harder to translate it into the clinic,” especially in light of limitations posed by the Food and Drug administration, he said. “We started to think about alternative methods to get this approach to the clinic.”
One strategy is to use pharmacologic inhibition via the Bruton’s tyrosine kinase inhibitors ibrutinib and dasatinib to mute the tendency of CAR T toward self-destruction. When tested in mice, “the unedited cells not just persisted, they expanded with sustained anti-leukemic activity and significantly prolonged their lives even more than the knock-out [gene-edited] cells.”
The research has now moved to human subjects. In 2021, researchers at Texas Children’s Hospital and Houston Methodist Hospital launched a clinical trial to test CD7 CAR T-cell therapy with CD28 in 21 patients with CD7-positive T-cell lymphoma. The initial part of the transplant-enabling CRIMSON-NE study is expected to be completed by mid-2024, and patients will be followed for 15 years.
Early results show that CD7 CAR T-cells have persisted in the blood of patients over weeks and months, Dr. Mamonkin said. In eight patients, “we’re seeing good evidence of activity,” with two patients reaching complete remissions.
The findings suggest that CD7 can be targeted in T-cell malignancies, he said. What about CD5? A similar study known as MAGENTA is testing CD5 CAR T-cell therapy with CD28 in T-cell leukemia and lymphoma in 42 patients. The phase 1 trial began in 2017. It’s expected to be completed by 2024 and to track patients for 15 years.
Results so far have been positive with complete remission achieved in three of nine patients with T-cell lymphoma; two remained in remission for more than 4 years.
Results in T-cell ALL improved after researchers adjusted the manufacturing of the cells. As for durability in these patients, “we try to bridge them to transplantation as soon as possible.”
As for side effects overall, there wasn’t much immune effector cell-associated neurotoxicity syndrome, and the CD7 approach seems to be more inflammatory, he said.
The presentation didn’t address the potential cost of the therapies. CAR T-cell therapy can cost between $500,000 and $1 million. Medicare covers it, but Medicaid may not depending on the state, and insurers may refuse to pay for it.
Dr. Mamonkin disclosed ties with Allogene, Amgen, Fate, Galapagos, March Bio, and NKILT.
As Baylor College of Medicine’s Max Mamonkin, PhD, noted in a presentation, patients with conditions such as T-cell lymphoma and T-cell acute lymphoblastic leukemia (ALL) have limited treatment options and grim prognoses. “This is an area with huge unmet need,” he said. “They don’t have options that patients with B-cell malignancies have, like [CAR T-cell therapy] and bispecifics.”
One big challenge is that CAR-targeted antigens in T-cell blood cancers are shared by both normal and malignant T-cells, he said. That poses a risk during therapy that the engineered cells will target each other with “disastrous consequences.”
Research by his team and others have shown that gene editing can help the cells to stop engaging in “fratricide,” Dr. Mamonkin said.
The problem is “it’s much easier to do gene editing on the bench and much harder to translate it into the clinic,” especially in light of limitations posed by the Food and Drug administration, he said. “We started to think about alternative methods to get this approach to the clinic.”
One strategy is to use pharmacologic inhibition via the Bruton’s tyrosine kinase inhibitors ibrutinib and dasatinib to mute the tendency of CAR T toward self-destruction. When tested in mice, “the unedited cells not just persisted, they expanded with sustained anti-leukemic activity and significantly prolonged their lives even more than the knock-out [gene-edited] cells.”
The research has now moved to human subjects. In 2021, researchers at Texas Children’s Hospital and Houston Methodist Hospital launched a clinical trial to test CD7 CAR T-cell therapy with CD28 in 21 patients with CD7-positive T-cell lymphoma. The initial part of the transplant-enabling CRIMSON-NE study is expected to be completed by mid-2024, and patients will be followed for 15 years.
Early results show that CD7 CAR T-cells have persisted in the blood of patients over weeks and months, Dr. Mamonkin said. In eight patients, “we’re seeing good evidence of activity,” with two patients reaching complete remissions.
The findings suggest that CD7 can be targeted in T-cell malignancies, he said. What about CD5? A similar study known as MAGENTA is testing CD5 CAR T-cell therapy with CD28 in T-cell leukemia and lymphoma in 42 patients. The phase 1 trial began in 2017. It’s expected to be completed by 2024 and to track patients for 15 years.
Results so far have been positive with complete remission achieved in three of nine patients with T-cell lymphoma; two remained in remission for more than 4 years.
Results in T-cell ALL improved after researchers adjusted the manufacturing of the cells. As for durability in these patients, “we try to bridge them to transplantation as soon as possible.”
As for side effects overall, there wasn’t much immune effector cell-associated neurotoxicity syndrome, and the CD7 approach seems to be more inflammatory, he said.
The presentation didn’t address the potential cost of the therapies. CAR T-cell therapy can cost between $500,000 and $1 million. Medicare covers it, but Medicaid may not depending on the state, and insurers may refuse to pay for it.
Dr. Mamonkin disclosed ties with Allogene, Amgen, Fate, Galapagos, March Bio, and NKILT.
As Baylor College of Medicine’s Max Mamonkin, PhD, noted in a presentation, patients with conditions such as T-cell lymphoma and T-cell acute lymphoblastic leukemia (ALL) have limited treatment options and grim prognoses. “This is an area with huge unmet need,” he said. “They don’t have options that patients with B-cell malignancies have, like [CAR T-cell therapy] and bispecifics.”
One big challenge is that CAR-targeted antigens in T-cell blood cancers are shared by both normal and malignant T-cells, he said. That poses a risk during therapy that the engineered cells will target each other with “disastrous consequences.”
Research by his team and others have shown that gene editing can help the cells to stop engaging in “fratricide,” Dr. Mamonkin said.
The problem is “it’s much easier to do gene editing on the bench and much harder to translate it into the clinic,” especially in light of limitations posed by the Food and Drug administration, he said. “We started to think about alternative methods to get this approach to the clinic.”
One strategy is to use pharmacologic inhibition via the Bruton’s tyrosine kinase inhibitors ibrutinib and dasatinib to mute the tendency of CAR T toward self-destruction. When tested in mice, “the unedited cells not just persisted, they expanded with sustained anti-leukemic activity and significantly prolonged their lives even more than the knock-out [gene-edited] cells.”
The research has now moved to human subjects. In 2021, researchers at Texas Children’s Hospital and Houston Methodist Hospital launched a clinical trial to test CD7 CAR T-cell therapy with CD28 in 21 patients with CD7-positive T-cell lymphoma. The initial part of the transplant-enabling CRIMSON-NE study is expected to be completed by mid-2024, and patients will be followed for 15 years.
Early results show that CD7 CAR T-cells have persisted in the blood of patients over weeks and months, Dr. Mamonkin said. In eight patients, “we’re seeing good evidence of activity,” with two patients reaching complete remissions.
The findings suggest that CD7 can be targeted in T-cell malignancies, he said. What about CD5? A similar study known as MAGENTA is testing CD5 CAR T-cell therapy with CD28 in T-cell leukemia and lymphoma in 42 patients. The phase 1 trial began in 2017. It’s expected to be completed by 2024 and to track patients for 15 years.
Results so far have been positive with complete remission achieved in three of nine patients with T-cell lymphoma; two remained in remission for more than 4 years.
Results in T-cell ALL improved after researchers adjusted the manufacturing of the cells. As for durability in these patients, “we try to bridge them to transplantation as soon as possible.”
As for side effects overall, there wasn’t much immune effector cell-associated neurotoxicity syndrome, and the CD7 approach seems to be more inflammatory, he said.
The presentation didn’t address the potential cost of the therapies. CAR T-cell therapy can cost between $500,000 and $1 million. Medicare covers it, but Medicaid may not depending on the state, and insurers may refuse to pay for it.
Dr. Mamonkin disclosed ties with Allogene, Amgen, Fate, Galapagos, March Bio, and NKILT.
FROM SITC 2023
Case Q: How soon after taking emergency contraception can a patient begin hormonal contraception?
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have two evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding). Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In this 3-part series we review 3 clinical cases, existing evidence to guide management decisions, and our recommendations. In part 1, we focus on restarting hormonal contraception after ulipristal acetate administration. In parts 2 and 3, we will discuss removal of a nonpalpable contraceptive implant and the consideration of a levonorgestrel-releasing intrauterine device (LNG-IUD) for emergency contraception.
- After using ulipristal acetate for emergency contraception, advise patients to wait at least 5 days to initiate hormonal contraception and about the importance of abstaining or using a back-up method for another 7 days with the start of their hormonal contraceptive method
CASE Meeting emergency and follow-up contraception needs
A 27-year-old woman (G0) presents to you after having unprotected intercourse 4 days ago. She does not formally track her menstrual cycles and is unsure when her last menstrual period was. She is not using contraception but is interested in starting a method. After counseling, she elects to take a dose of oral ulipristal acetate (UPA; Ella) now for emergency contraception and would like to start a combined oral contraceptive (COC) pill moving forward.
How soon after taking UPA should you tell her to start the combined hormonal pill?
Effectiveness of hormonal contraception following UPA
UPA does not appear to decrease the efficacy of COCs when started around the same time. However, immediately starting a hormonal contraceptive can decrease the effectiveness of UPA, and as such, it is recommended to take UPA and then abstain or use a backup method for 7 days before initiating a hormonal contraceptive method.1 By obtaining some additional information from your patient and with the use of shared decision making, though, your patient may be able to start their contraceptive method earlier than 5 days after UPA.
What is UPA
UPA is a progesterone receptor modulator used for emergency contraception intenhded to prevent pregnancy after unprotected intercourse or contraceptive failure.3 It works by delaying follicular rupture at least 5 days, if taken before the peak of the luteinizing hormone (LH) surge. If taken after that timeframe, it does not work. Since UPA competes for the progesterone receptor, there is a concern that the effectiveness of UPA may be decreased if a progestin-containing form of contraception is started immediately after taking UPA, or vice versa.4 Several studies have now specifically looked at the interaction between UPA and progestin-containing contraceptives, including at how UPA is impacted by the contraceptive method, and conversely, how the contraceptive method is impacted by UPA.5-8
Data on types of hormonal contraception. Brache and colleagues demonstrated that UPA users who started a desogestrel progestin-only pill (DSG POP) the next day had higher rates of ovulation within 5 days of taking UPA (45%), compared with those who the next day started a placebo pill (3%).6 This type of progestin-only pill is not available in the United States.
A study by Edelman and colleagues demonstrated similar findings in those starting a COC pill containing estrogen and progestin. When taking a COC two days after UPA use, more participants had evidence of follicular rupture in less than 5 days.5 It should be noted that these studies focused on ovulation, which—while necessary for conception to occur—is a surrogate biomarker for pregnancy risk. Additional studies have looked at the impact of UPA on the COC and have not found that UPA impacts ovulation suppression of the COC with its initiation or use.8
Considering unprotected intercourse and UPA timing. Of course, the risk of pregnancy is reliant on cycle timing plus the presence of viable sperm in the reproductive tract. Sperm have been shown to only be viable in the reproductive tract for 5 days, which could result in fertilization and subsequent pregnancy. Longevity of an egg is much shorter, at 12 to 24 hours after ovulation. For this patient, her exposure was 4 days ago, but sperm are only viable for approximately 5 days—she could consider taking the UPA now and then starting a COC earlier than 5 days since she only needs an extra day or two of protection from the UPA from the sperm in her reproductive tract. Your patient’s involvement in this decision making is paramount, as only they can prioritize their desire to avoid pregnancy from their recent act of unprotected intercourse versus their immediate needs for starting their method of contraception. It is important that individuals abstain from sexual activity or use an additional back-up method during the first 7 days of starting their method of contraception.
Continue to: Counseling considerations for the case patient...
Counseling considerations for the case patient
For a patient planning to start or resume a hormonal contraceptive method after taking UPA, the waiting period recommended by the CDC (5 days) is most beneficial for patients who are uncertain about their menstrual cycle timing in relation to the act of unprotected intercourse that already occurred and need to prioritize maximum effectiveness of emergency contraception.
Patients with unsure cycle-sex timing planning to self-start or resume a short-term hormonal contraceptive method (eg, pills, patches, or rings), should be counseled to wait 5 days after the most recent act of unprotected sex, before taking their hormonal contraceptive method.7 Patients with unsure cycle-sex timing planning to use provider-dependent hormonal contraceptive methods (eg, those requiring a prescription, including a progestin-contraceptive implant or depot medroxyprogesterone acetate) should also be counseled to wait. Timing of levonorgestrel and copper intrauterine devices are addressed in part 3 of this series.
However, if your patient has a good understanding of their menstrual cycle, and the primary concern is exposure from subsequent sexual encounters and not the recent unprotected intercourse, it is advisable to provide UPA and immediately initiate a contraceptive method. One of the primary reasons for emergency contraception failure is that its effectiveness is limited to the most recent act of unprotected sexual intercourse and does not extend to subsequent acts throughout the month.
For these patients with sure cycle-sex timing who are planning to start or resume short-or long-term contraceptive methods, and whose primary concern is to prevent pregnancy risk from subsequent sexual encounters, immediately initiating a contraceptive method is advisable. For provider-dependent methods, we must weigh the risk of unintended pregnancy from the act of intercourse that already occurred (and the potential to increase that risk by initiating a method that could compromise UPA efficacy) versus the future risk of pregnancy if the patient cannot return for a contraception visit.7
In short, starting the contraceptive method at the time of UPA use can be considered after shared decision making with the patient and understanding what their primary concerns are.
Important point
Counsel on using backup barrier contraception after UPA
Oral emergency contraception only covers that one act of unprotected intercourse and does not continue to protect a patient from pregnancy for the rest of their cycle. When taken before ovulation, UPA works by delaying follicular development and rupture for at least 5 days. Patients who continue to have unprotected intercourse after taking UPA are at a high risk of an unintended pregnancy from this ‘stalled’ follicle that will eventually ovulate. Follicular maturation resumes after UPA’s effects wane, and the patient is primed for ovulation (and therefore unintended pregnancy) if ongoing unprotected intercourse occurs for the rest of their cycle.
Therefore, it is important to counsel patients on the need, if they do not desire a pregnancy, to abstain or start a method of contraception.
Final question
What about starting or resuming non–hormonal contraceptive methods?
Non-hormonal contraceptive methods can be started immediately with UPA use.1
CASE Resolved
After shared decision making, the patient decides to start using the COC pill. You prescribe her both UPA for emergency contraception and a combined hormonal contraceptive pill. Given her unsure cycle-sex timing, she expresses to you that her most important priority is preventing unintended pregnancy. You counsel her to set a reminder on her phone to start taking the pill 5 days from her most recent act of unprotected intercourse. You also counsel her to use a back-up barrier method of contraception for 7 days after starting her COC pill. ●
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Ella [package insert]. Charleston, SC; Afaxys, Inc. 2014.
- Salcedo J, Rodriguez MI, Curtis KM, et al. When can a woman resume or initiate contraception after taking emergency contraceptive pills? A systematic review. Contraception. 2013;87:602-604. https://doi.org/10.1016 /j.contraception.2012.08.013
- Edelman AB, Jensen JT, McCrimmon S, et al. Combined oral contraceptive interference with the ability of ulipristal acetate to delay ovulation: a prospective cohort study. Contraception. 2018;98:463-466. doi: 10.1016/j.contraception.2018.08.003
- Brache V, Cochon L, Duijkers IJM, et al. A prospective, randomized, pharmacodynamic study of quick-starting a desogestrel progestin-only pill following ulipristal acetate for emergency contraception. Hum Reprod Oxf Engl. 2015;30:2785-2793. https://doi.org/10.1093/humrep /dev241
- Cameron ST, Berger C, Michie L, et al. The effects on ovarian activity of ulipristal acetate when ‘quickstarting’ a combined oral contraceptive pill: a prospective, randomized, doubleblind parallel-arm, placebo-controlled study. Hum Reprod. 2015;30:1566-1572. doi: 10.1093/humrep/dev115
- Banh C, Rautenberg T, Diujkers I, et al. The effects on ovarian activity of delaying versus immediately restarting combined oral contraception after missing three pills and taking ulipristal acetate 30 mg. Contraception. 2020;102:145-151. doi: 10.1016/j.contraception.2020.05.013
- American Society for Emergency Contraception. Providing ongoing hormonal contraception after use of emergency contraceptive pills. September 2016. Accessed October 11, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj /https://www.americansocietyforec.org/_files/ugd/7f2e0b _ff1bc90bea204644ba28d1b0e6a6a6a8.pdf
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have two evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding). Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In this 3-part series we review 3 clinical cases, existing evidence to guide management decisions, and our recommendations. In part 1, we focus on restarting hormonal contraception after ulipristal acetate administration. In parts 2 and 3, we will discuss removal of a nonpalpable contraceptive implant and the consideration of a levonorgestrel-releasing intrauterine device (LNG-IUD) for emergency contraception.
- After using ulipristal acetate for emergency contraception, advise patients to wait at least 5 days to initiate hormonal contraception and about the importance of abstaining or using a back-up method for another 7 days with the start of their hormonal contraceptive method
CASE Meeting emergency and follow-up contraception needs
A 27-year-old woman (G0) presents to you after having unprotected intercourse 4 days ago. She does not formally track her menstrual cycles and is unsure when her last menstrual period was. She is not using contraception but is interested in starting a method. After counseling, she elects to take a dose of oral ulipristal acetate (UPA; Ella) now for emergency contraception and would like to start a combined oral contraceptive (COC) pill moving forward.
How soon after taking UPA should you tell her to start the combined hormonal pill?
Effectiveness of hormonal contraception following UPA
UPA does not appear to decrease the efficacy of COCs when started around the same time. However, immediately starting a hormonal contraceptive can decrease the effectiveness of UPA, and as such, it is recommended to take UPA and then abstain or use a backup method for 7 days before initiating a hormonal contraceptive method.1 By obtaining some additional information from your patient and with the use of shared decision making, though, your patient may be able to start their contraceptive method earlier than 5 days after UPA.
What is UPA
UPA is a progesterone receptor modulator used for emergency contraception intenhded to prevent pregnancy after unprotected intercourse or contraceptive failure.3 It works by delaying follicular rupture at least 5 days, if taken before the peak of the luteinizing hormone (LH) surge. If taken after that timeframe, it does not work. Since UPA competes for the progesterone receptor, there is a concern that the effectiveness of UPA may be decreased if a progestin-containing form of contraception is started immediately after taking UPA, or vice versa.4 Several studies have now specifically looked at the interaction between UPA and progestin-containing contraceptives, including at how UPA is impacted by the contraceptive method, and conversely, how the contraceptive method is impacted by UPA.5-8
Data on types of hormonal contraception. Brache and colleagues demonstrated that UPA users who started a desogestrel progestin-only pill (DSG POP) the next day had higher rates of ovulation within 5 days of taking UPA (45%), compared with those who the next day started a placebo pill (3%).6 This type of progestin-only pill is not available in the United States.
A study by Edelman and colleagues demonstrated similar findings in those starting a COC pill containing estrogen and progestin. When taking a COC two days after UPA use, more participants had evidence of follicular rupture in less than 5 days.5 It should be noted that these studies focused on ovulation, which—while necessary for conception to occur—is a surrogate biomarker for pregnancy risk. Additional studies have looked at the impact of UPA on the COC and have not found that UPA impacts ovulation suppression of the COC with its initiation or use.8
Considering unprotected intercourse and UPA timing. Of course, the risk of pregnancy is reliant on cycle timing plus the presence of viable sperm in the reproductive tract. Sperm have been shown to only be viable in the reproductive tract for 5 days, which could result in fertilization and subsequent pregnancy. Longevity of an egg is much shorter, at 12 to 24 hours after ovulation. For this patient, her exposure was 4 days ago, but sperm are only viable for approximately 5 days—she could consider taking the UPA now and then starting a COC earlier than 5 days since she only needs an extra day or two of protection from the UPA from the sperm in her reproductive tract. Your patient’s involvement in this decision making is paramount, as only they can prioritize their desire to avoid pregnancy from their recent act of unprotected intercourse versus their immediate needs for starting their method of contraception. It is important that individuals abstain from sexual activity or use an additional back-up method during the first 7 days of starting their method of contraception.
Continue to: Counseling considerations for the case patient...
Counseling considerations for the case patient
For a patient planning to start or resume a hormonal contraceptive method after taking UPA, the waiting period recommended by the CDC (5 days) is most beneficial for patients who are uncertain about their menstrual cycle timing in relation to the act of unprotected intercourse that already occurred and need to prioritize maximum effectiveness of emergency contraception.
Patients with unsure cycle-sex timing planning to self-start or resume a short-term hormonal contraceptive method (eg, pills, patches, or rings), should be counseled to wait 5 days after the most recent act of unprotected sex, before taking their hormonal contraceptive method.7 Patients with unsure cycle-sex timing planning to use provider-dependent hormonal contraceptive methods (eg, those requiring a prescription, including a progestin-contraceptive implant or depot medroxyprogesterone acetate) should also be counseled to wait. Timing of levonorgestrel and copper intrauterine devices are addressed in part 3 of this series.
However, if your patient has a good understanding of their menstrual cycle, and the primary concern is exposure from subsequent sexual encounters and not the recent unprotected intercourse, it is advisable to provide UPA and immediately initiate a contraceptive method. One of the primary reasons for emergency contraception failure is that its effectiveness is limited to the most recent act of unprotected sexual intercourse and does not extend to subsequent acts throughout the month.
For these patients with sure cycle-sex timing who are planning to start or resume short-or long-term contraceptive methods, and whose primary concern is to prevent pregnancy risk from subsequent sexual encounters, immediately initiating a contraceptive method is advisable. For provider-dependent methods, we must weigh the risk of unintended pregnancy from the act of intercourse that already occurred (and the potential to increase that risk by initiating a method that could compromise UPA efficacy) versus the future risk of pregnancy if the patient cannot return for a contraception visit.7
In short, starting the contraceptive method at the time of UPA use can be considered after shared decision making with the patient and understanding what their primary concerns are.
Important point
Counsel on using backup barrier contraception after UPA
Oral emergency contraception only covers that one act of unprotected intercourse and does not continue to protect a patient from pregnancy for the rest of their cycle. When taken before ovulation, UPA works by delaying follicular development and rupture for at least 5 days. Patients who continue to have unprotected intercourse after taking UPA are at a high risk of an unintended pregnancy from this ‘stalled’ follicle that will eventually ovulate. Follicular maturation resumes after UPA’s effects wane, and the patient is primed for ovulation (and therefore unintended pregnancy) if ongoing unprotected intercourse occurs for the rest of their cycle.
Therefore, it is important to counsel patients on the need, if they do not desire a pregnancy, to abstain or start a method of contraception.
Final question
What about starting or resuming non–hormonal contraceptive methods?
Non-hormonal contraceptive methods can be started immediately with UPA use.1
CASE Resolved
After shared decision making, the patient decides to start using the COC pill. You prescribe her both UPA for emergency contraception and a combined hormonal contraceptive pill. Given her unsure cycle-sex timing, she expresses to you that her most important priority is preventing unintended pregnancy. You counsel her to set a reminder on her phone to start taking the pill 5 days from her most recent act of unprotected intercourse. You also counsel her to use a back-up barrier method of contraception for 7 days after starting her COC pill. ●

Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have two evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding). Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In this 3-part series we review 3 clinical cases, existing evidence to guide management decisions, and our recommendations. In part 1, we focus on restarting hormonal contraception after ulipristal acetate administration. In parts 2 and 3, we will discuss removal of a nonpalpable contraceptive implant and the consideration of a levonorgestrel-releasing intrauterine device (LNG-IUD) for emergency contraception.
- After using ulipristal acetate for emergency contraception, advise patients to wait at least 5 days to initiate hormonal contraception and about the importance of abstaining or using a back-up method for another 7 days with the start of their hormonal contraceptive method
CASE Meeting emergency and follow-up contraception needs
A 27-year-old woman (G0) presents to you after having unprotected intercourse 4 days ago. She does not formally track her menstrual cycles and is unsure when her last menstrual period was. She is not using contraception but is interested in starting a method. After counseling, she elects to take a dose of oral ulipristal acetate (UPA; Ella) now for emergency contraception and would like to start a combined oral contraceptive (COC) pill moving forward.
How soon after taking UPA should you tell her to start the combined hormonal pill?
Effectiveness of hormonal contraception following UPA
UPA does not appear to decrease the efficacy of COCs when started around the same time. However, immediately starting a hormonal contraceptive can decrease the effectiveness of UPA, and as such, it is recommended to take UPA and then abstain or use a backup method for 7 days before initiating a hormonal contraceptive method.1 By obtaining some additional information from your patient and with the use of shared decision making, though, your patient may be able to start their contraceptive method earlier than 5 days after UPA.
What is UPA
UPA is a progesterone receptor modulator used for emergency contraception intenhded to prevent pregnancy after unprotected intercourse or contraceptive failure.3 It works by delaying follicular rupture at least 5 days, if taken before the peak of the luteinizing hormone (LH) surge. If taken after that timeframe, it does not work. Since UPA competes for the progesterone receptor, there is a concern that the effectiveness of UPA may be decreased if a progestin-containing form of contraception is started immediately after taking UPA, or vice versa.4 Several studies have now specifically looked at the interaction between UPA and progestin-containing contraceptives, including at how UPA is impacted by the contraceptive method, and conversely, how the contraceptive method is impacted by UPA.5-8
Data on types of hormonal contraception. Brache and colleagues demonstrated that UPA users who started a desogestrel progestin-only pill (DSG POP) the next day had higher rates of ovulation within 5 days of taking UPA (45%), compared with those who the next day started a placebo pill (3%).6 This type of progestin-only pill is not available in the United States.
A study by Edelman and colleagues demonstrated similar findings in those starting a COC pill containing estrogen and progestin. When taking a COC two days after UPA use, more participants had evidence of follicular rupture in less than 5 days.5 It should be noted that these studies focused on ovulation, which—while necessary for conception to occur—is a surrogate biomarker for pregnancy risk. Additional studies have looked at the impact of UPA on the COC and have not found that UPA impacts ovulation suppression of the COC with its initiation or use.8
Considering unprotected intercourse and UPA timing. Of course, the risk of pregnancy is reliant on cycle timing plus the presence of viable sperm in the reproductive tract. Sperm have been shown to only be viable in the reproductive tract for 5 days, which could result in fertilization and subsequent pregnancy. Longevity of an egg is much shorter, at 12 to 24 hours after ovulation. For this patient, her exposure was 4 days ago, but sperm are only viable for approximately 5 days—she could consider taking the UPA now and then starting a COC earlier than 5 days since she only needs an extra day or two of protection from the UPA from the sperm in her reproductive tract. Your patient’s involvement in this decision making is paramount, as only they can prioritize their desire to avoid pregnancy from their recent act of unprotected intercourse versus their immediate needs for starting their method of contraception. It is important that individuals abstain from sexual activity or use an additional back-up method during the first 7 days of starting their method of contraception.
Continue to: Counseling considerations for the case patient...
Counseling considerations for the case patient
For a patient planning to start or resume a hormonal contraceptive method after taking UPA, the waiting period recommended by the CDC (5 days) is most beneficial for patients who are uncertain about their menstrual cycle timing in relation to the act of unprotected intercourse that already occurred and need to prioritize maximum effectiveness of emergency contraception.
Patients with unsure cycle-sex timing planning to self-start or resume a short-term hormonal contraceptive method (eg, pills, patches, or rings), should be counseled to wait 5 days after the most recent act of unprotected sex, before taking their hormonal contraceptive method.7 Patients with unsure cycle-sex timing planning to use provider-dependent hormonal contraceptive methods (eg, those requiring a prescription, including a progestin-contraceptive implant or depot medroxyprogesterone acetate) should also be counseled to wait. Timing of levonorgestrel and copper intrauterine devices are addressed in part 3 of this series.
However, if your patient has a good understanding of their menstrual cycle, and the primary concern is exposure from subsequent sexual encounters and not the recent unprotected intercourse, it is advisable to provide UPA and immediately initiate a contraceptive method. One of the primary reasons for emergency contraception failure is that its effectiveness is limited to the most recent act of unprotected sexual intercourse and does not extend to subsequent acts throughout the month.
For these patients with sure cycle-sex timing who are planning to start or resume short-or long-term contraceptive methods, and whose primary concern is to prevent pregnancy risk from subsequent sexual encounters, immediately initiating a contraceptive method is advisable. For provider-dependent methods, we must weigh the risk of unintended pregnancy from the act of intercourse that already occurred (and the potential to increase that risk by initiating a method that could compromise UPA efficacy) versus the future risk of pregnancy if the patient cannot return for a contraception visit.7
In short, starting the contraceptive method at the time of UPA use can be considered after shared decision making with the patient and understanding what their primary concerns are.
Important point
Counsel on using backup barrier contraception after UPA
Oral emergency contraception only covers that one act of unprotected intercourse and does not continue to protect a patient from pregnancy for the rest of their cycle. When taken before ovulation, UPA works by delaying follicular development and rupture for at least 5 days. Patients who continue to have unprotected intercourse after taking UPA are at a high risk of an unintended pregnancy from this ‘stalled’ follicle that will eventually ovulate. Follicular maturation resumes after UPA’s effects wane, and the patient is primed for ovulation (and therefore unintended pregnancy) if ongoing unprotected intercourse occurs for the rest of their cycle.
Therefore, it is important to counsel patients on the need, if they do not desire a pregnancy, to abstain or start a method of contraception.
Final question
What about starting or resuming non–hormonal contraceptive methods?
Non-hormonal contraceptive methods can be started immediately with UPA use.1
CASE Resolved
After shared decision making, the patient decides to start using the COC pill. You prescribe her both UPA for emergency contraception and a combined hormonal contraceptive pill. Given her unsure cycle-sex timing, she expresses to you that her most important priority is preventing unintended pregnancy. You counsel her to set a reminder on her phone to start taking the pill 5 days from her most recent act of unprotected intercourse. You also counsel her to use a back-up barrier method of contraception for 7 days after starting her COC pill. ●

- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Ella [package insert]. Charleston, SC; Afaxys, Inc. 2014.
- Salcedo J, Rodriguez MI, Curtis KM, et al. When can a woman resume or initiate contraception after taking emergency contraceptive pills? A systematic review. Contraception. 2013;87:602-604. https://doi.org/10.1016 /j.contraception.2012.08.013
- Edelman AB, Jensen JT, McCrimmon S, et al. Combined oral contraceptive interference with the ability of ulipristal acetate to delay ovulation: a prospective cohort study. Contraception. 2018;98:463-466. doi: 10.1016/j.contraception.2018.08.003
- Brache V, Cochon L, Duijkers IJM, et al. A prospective, randomized, pharmacodynamic study of quick-starting a desogestrel progestin-only pill following ulipristal acetate for emergency contraception. Hum Reprod Oxf Engl. 2015;30:2785-2793. https://doi.org/10.1093/humrep /dev241
- Cameron ST, Berger C, Michie L, et al. The effects on ovarian activity of ulipristal acetate when ‘quickstarting’ a combined oral contraceptive pill: a prospective, randomized, doubleblind parallel-arm, placebo-controlled study. Hum Reprod. 2015;30:1566-1572. doi: 10.1093/humrep/dev115
- Banh C, Rautenberg T, Diujkers I, et al. The effects on ovarian activity of delaying versus immediately restarting combined oral contraception after missing three pills and taking ulipristal acetate 30 mg. Contraception. 2020;102:145-151. doi: 10.1016/j.contraception.2020.05.013
- American Society for Emergency Contraception. Providing ongoing hormonal contraception after use of emergency contraceptive pills. September 2016. Accessed October 11, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj /https://www.americansocietyforec.org/_files/ugd/7f2e0b _ff1bc90bea204644ba28d1b0e6a6a6a8.pdf
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Ella [package insert]. Charleston, SC; Afaxys, Inc. 2014.
- Salcedo J, Rodriguez MI, Curtis KM, et al. When can a woman resume or initiate contraception after taking emergency contraceptive pills? A systematic review. Contraception. 2013;87:602-604. https://doi.org/10.1016 /j.contraception.2012.08.013
- Edelman AB, Jensen JT, McCrimmon S, et al. Combined oral contraceptive interference with the ability of ulipristal acetate to delay ovulation: a prospective cohort study. Contraception. 2018;98:463-466. doi: 10.1016/j.contraception.2018.08.003
- Brache V, Cochon L, Duijkers IJM, et al. A prospective, randomized, pharmacodynamic study of quick-starting a desogestrel progestin-only pill following ulipristal acetate for emergency contraception. Hum Reprod Oxf Engl. 2015;30:2785-2793. https://doi.org/10.1093/humrep /dev241
- Cameron ST, Berger C, Michie L, et al. The effects on ovarian activity of ulipristal acetate when ‘quickstarting’ a combined oral contraceptive pill: a prospective, randomized, doubleblind parallel-arm, placebo-controlled study. Hum Reprod. 2015;30:1566-1572. doi: 10.1093/humrep/dev115
- Banh C, Rautenberg T, Diujkers I, et al. The effects on ovarian activity of delaying versus immediately restarting combined oral contraception after missing three pills and taking ulipristal acetate 30 mg. Contraception. 2020;102:145-151. doi: 10.1016/j.contraception.2020.05.013
- American Society for Emergency Contraception. Providing ongoing hormonal contraception after use of emergency contraceptive pills. September 2016. Accessed October 11, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj /https://www.americansocietyforec.org/_files/ugd/7f2e0b _ff1bc90bea204644ba28d1b0e6a6a6a8.pdf
RSV vaccination during pregnancy: Finally ready for prime time
A 28-year-old primigravid woman at 30 weeks’ gestation inquires about the new vaccine to protect her newborn baby against respiratory syncytial virus infection (RSV). Her neighbor’s daughter recently was hospitalized for the treatment of RSV, and she is understandably concerned about her own newborn. The patient is healthy, and she has never had any serious respiratory infection. She is taking no medications other than prenatal vitamins.
What advice should you give her?
If you decide to administer this vaccine, what is the appropriate timing of administration?
Are there any maternal or fetal safety concerns related to use of this vaccine in pregnancy?
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family. It is an enveloped, single-stranded RNA virus that is 150-300 nm in size. The virus codes for 10 virus-specific proteins. The 2 most important are the G protein, which enables the virus to attach to host cells, and the F protein, which facilitates the entry of the virus into the host cell by fusing the host and viral membranes. Two distinct subtypes exist: A and B. There is genetic variation within each subtype and between subtypes. These subtle genetic variations create the potential for reinfections, and hence, research has focused on development of a vaccine that covers both subtypes.1
RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age. In these children, RSV is one of the most prominent causes of death, with mortality particularly marked in low- and middle-resource countries as well as in children who were born premature and/or who are immunocompromised. RSV has its greatest impact during winter epidemics in temperate climates and during the rainy seasons in tropical climates. The virus rarely is encountered in the summer.1 Among young children, RSV primarily is transmitted via close contact with contaminated fingers or fomites and by self-inoculation of the conjunctiva or anterior nares. The incubation period of the infection is 4 to 6 days, and viral shedding may persist for 2 weeks or longer. Most patients gradually recover within 1 to 2 weeks.1 Adults who contract RSV usually have symptoms suggestive of a common cold; however, in older adults or those who have comorbidities, serious and potentially life-threatening lower respiratory tract infections may develop.
Recently, there have been 2 main approaches to the prevention and treatment of RSV in infants. One has been the development of monoclonal antibodies such as motavizumab, palivizumab, and nirsevimab. The other has been the development of a vaccine that could be administered to pregnant women and which could provide protection for the neonate in the early months of life.2,3
In late August 2023, the US Food and Drug Administration (FDA) announced the approval of a new bivalent RSV prefusion F vaccine (ABRYSVO, Pfizer) intended for administration to pregnant women.4 Of note, previous efforts to develop whole-virus vaccines either have been ineffective or have potentiated the disease in infants who became infected; development of an effective vaccine had eluded scientists and clinicians for nearly 50 years.2 Thus, the new vaccine that targets the F protein of the virus represents a major and welcomed breakthrough.
This article reviews the 3 most recent investigations that preceded the ultimate approval of this vaccine and discusses specific logistical issues related to vaccine administration.
Continue to: First step toward vaccine approval...
First step toward vaccine approval
Madhi and colleagues5 were among the first to conduct a large well-designed study to evaluate the effectiveness of maternal vaccination in preventing neonatal infection in the first few months of life. The authors enrolled more than 4,500 healthy pregnant women at 28 to 36 weeks of gestation and assigned them to receive either a single intramuscular dose of an RSV fusion (F) protein vaccine or placebo in a ratio of 2:1. The primary end point was a “medically significant lower respiratory tract infection” within the first 90 days of life. The percentage of infants who met the primary end point was low in both groups: 1.5% in the vaccine group and 2.4% in the placebo group (efficacy 39.4%). The efficacy of the vaccine in preventing lower respiratory tract infection with severe hypoxemia was 48.3% and 44.4% in preventing hospitalization. Although there were differences between the 2 groups, they did not meet the prespecified success criterion for efficacy. Vaccine recipients had more local injection site reactions (40.7% vs 9.9%); however, there was no difference in the frequency of other adverse effects.
Intermediate step: Continued assessment of vaccine safety and immunogenicity
The next important step in the development of the RSV vaccine was a study by Simoes et al,6 who conducted a phase 2b trial to determine the safety and immunogenicity of the RSVpreF vaccine. The authors randomly assigned pregnant women at 24 to 36 weeks of gestation to receive either 120 or 240 µg of RSVpreF vaccine or placebo. The key endpoints were the following: maternal and infant safety; the maternal-to-infant transplacental transfer ratio; and the presence of RSV A, B, and combined A/B neutralizing antibody in maternal serum and umbilical cord blood at delivery. The authors conducted a planned interim analysis that included 327 mothers who received the vaccine. The incidence of adverse effects was similar in mothers and infants in the vaccine compared with the placebo group. None of the adverse effects were judged to be serious. The transplacental neutralizing antibody transfer ratios ranged from 1.4 to 2.1 across a range of gestational ages. The vaccine elicited meaningful neutralizing titers of antibody in maternal serum even up to 7 weeks after immunization. The levels of neutralizing antibodies in umbilical cord blood did not vary substantially with respect to gestational age. A post hoc analysis showed that the transferred antibodies prevented medically-attended RSV-associated lower respiratory tract illnesses in the infants.
Final step: Convincing proof of efficacy
The most recent of the 3 studies, and the one that had the greatest impact in convincing the FDA to approve the vaccine, was the report by Kampmann and colleagues.7 The authors conducted a phase 3 prospective, randomized, double-blind trial in 18 different countries over 4 RSV seasons: 2 in the northern hemisphere and 2 in the southern hemisphere. They enrolled healthy pregnant women with singleton gestations at 24 to 36 weeks of gestation and assigned them in a 1:1 ratio to a single intramuscular injection of 120 µg of a bivalent RSV prefusion F protein-based (RSVpreF) vaccine or placebo. They excluded patients with any recognized risk factor for an adverse pregnancy outcome, including preterm labor. The 2 primary efficacy endpoints were a medically-attended severe RSV–lower respiratory tract infection and any medically attended RSV-associated lower respiratory tract illness in infants within 90, 120, 150, and 180 days after birth.
The efficacy of the vaccine in preventing severe lower respiratory tract illness within 90 days of delivery was 81.8% (99.5% confidence interval [CI], 40.6–96.3). The efficacy within 180 days of delivery was 69.4% (97.58% CI, 44.3–84.1). These differences reached the study’s pre-established statistical criteria for success. The overall rate of lower respiratory tract infections was not significantly different. The frequencies of adverse effects in mothers and infants were similar in the vaccine and placebo groups. In particular, the frequency of preterm delivery in the vaccine group was 0.8%, compared with 0.6% in the placebo group (P = NS).
In previous reports to the FDA,4 the frequency rate of preterm delivery in RSV vaccine recipients was slightly increased in vaccine recipients compared with patients who received placebo. The difference among the groups was too small to infer a causal relationship; however, as a condition of vaccine approval, the FDA has required Pfizer to conduct a postmarketing study to be certain that administration of the vaccine does not increase the risk for preterm delivery.
Practical details
The new vaccine is a bivalent recombinant vaccine that elicits a robust antibody response against the F (fusion) protein of the virus. In addition to the F antigen, the vaccine contains the following buffer ingredients: tromethamine, sucrose, mannitol, polysorbate, and sodium chloride.8 There are no preservatives in the vaccine.
The vaccine should be administered in a single, 0.5 mL, intramuscular injection at 32 to 36 weeks of gestation. Patients who are allergic to any of the components of the vaccine should not be vaccinated. Patients with a mild upper respiratory tract infection may receive the vaccine. Administration should be delayed in patients who are moderately to severely ill. The vaccine may be administered at the same time as other vaccines, such as influenza or Tdap.
The most common side effects of the vaccine are local injection site reactions, such as pain, redness, or swelling. Some patients may experience mild systemic manifestations, including fatigue, fever, headache, nausea, diarrhea, arthralgias, and myalgias. According to the Centers for Disease Control and Prevention, the approximate wholesale acquisition cost of the vaccine is $320 for 1 injection.
CASE Resolution
This patient is healthy and has no contraindication to the new RSV vaccine. According to the FDA, the optimal time for administration of the vaccine is 32 to 36 weeks of gestation. The patient should anticipate very few side effects following the vaccination, and the vaccine has approximately 80% efficacy in preventing severe lower respiratory tract infection in her neonate. ●
- RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age.
- In low- and middle-resource countries, RSV is a leading cause of infant death.
- In late August 2023, the FDA approved the first RSV vaccine that can be administered to pregnant women to provide protection for the infant in the first few months of life.
- The vaccine specifically targets the F protein of the virus, a protein which is essential for facilitating fusion between the viral and host cell membranes, resulting in penetration of the virus into the host cell.
- The vaccine should be administered as a single intramuscular injection at 32 to 36 weeks’ gestation.
- The vaccine is approximately 82% effective in preventing severe lower respiratory tract infection in infants within the first 6 months of life.
- To exercise an abundance of caution, because of a possible association between administration of the vaccine and an increased risk for preterm delivery, vaccination should be delayed until 36 weeks in patients clearly identified as at-risk for preterm delivery.
- Dolin R. Common viral respiratory infections. In, Isselbacher KJ, Braunwald E, Wilson JD, et al, eds. Harrison’s Principles of Internal Medicine. 13th ed. McGraw-Hill; 1994:805-806.
- Mazur N, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23:E2-E21.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- US Food and Drug Administration News Release. August 21, 2023. Accessed October 26, 2023. https://www.fda.gov/news -events/press-announcements/fda-approves-first-vaccine -pregnant-individuals-prevent-rsv-infants
- Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426-439.
- Simoes EAF, Center KJ, Tita ATN, et al. Prefusion F proteinbased respiratory syncytial virus immunization in pregnancy. N Eng J Med. 2022;386:1615-1626.
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451-1464.
- Centers for Disease Control and Prevention. Vaccine Information Statement. Respiratory Syncytial Virus (RSV) Vaccine VIS. October 19, 2023. Accessed October 26, 2023. https://www. cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
A 28-year-old primigravid woman at 30 weeks’ gestation inquires about the new vaccine to protect her newborn baby against respiratory syncytial virus infection (RSV). Her neighbor’s daughter recently was hospitalized for the treatment of RSV, and she is understandably concerned about her own newborn. The patient is healthy, and she has never had any serious respiratory infection. She is taking no medications other than prenatal vitamins.
What advice should you give her?
If you decide to administer this vaccine, what is the appropriate timing of administration?
Are there any maternal or fetal safety concerns related to use of this vaccine in pregnancy?
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family. It is an enveloped, single-stranded RNA virus that is 150-300 nm in size. The virus codes for 10 virus-specific proteins. The 2 most important are the G protein, which enables the virus to attach to host cells, and the F protein, which facilitates the entry of the virus into the host cell by fusing the host and viral membranes. Two distinct subtypes exist: A and B. There is genetic variation within each subtype and between subtypes. These subtle genetic variations create the potential for reinfections, and hence, research has focused on development of a vaccine that covers both subtypes.1
RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age. In these children, RSV is one of the most prominent causes of death, with mortality particularly marked in low- and middle-resource countries as well as in children who were born premature and/or who are immunocompromised. RSV has its greatest impact during winter epidemics in temperate climates and during the rainy seasons in tropical climates. The virus rarely is encountered in the summer.1 Among young children, RSV primarily is transmitted via close contact with contaminated fingers or fomites and by self-inoculation of the conjunctiva or anterior nares. The incubation period of the infection is 4 to 6 days, and viral shedding may persist for 2 weeks or longer. Most patients gradually recover within 1 to 2 weeks.1 Adults who contract RSV usually have symptoms suggestive of a common cold; however, in older adults or those who have comorbidities, serious and potentially life-threatening lower respiratory tract infections may develop.
Recently, there have been 2 main approaches to the prevention and treatment of RSV in infants. One has been the development of monoclonal antibodies such as motavizumab, palivizumab, and nirsevimab. The other has been the development of a vaccine that could be administered to pregnant women and which could provide protection for the neonate in the early months of life.2,3
In late August 2023, the US Food and Drug Administration (FDA) announced the approval of a new bivalent RSV prefusion F vaccine (ABRYSVO, Pfizer) intended for administration to pregnant women.4 Of note, previous efforts to develop whole-virus vaccines either have been ineffective or have potentiated the disease in infants who became infected; development of an effective vaccine had eluded scientists and clinicians for nearly 50 years.2 Thus, the new vaccine that targets the F protein of the virus represents a major and welcomed breakthrough.
This article reviews the 3 most recent investigations that preceded the ultimate approval of this vaccine and discusses specific logistical issues related to vaccine administration.
Continue to: First step toward vaccine approval...
First step toward vaccine approval
Madhi and colleagues5 were among the first to conduct a large well-designed study to evaluate the effectiveness of maternal vaccination in preventing neonatal infection in the first few months of life. The authors enrolled more than 4,500 healthy pregnant women at 28 to 36 weeks of gestation and assigned them to receive either a single intramuscular dose of an RSV fusion (F) protein vaccine or placebo in a ratio of 2:1. The primary end point was a “medically significant lower respiratory tract infection” within the first 90 days of life. The percentage of infants who met the primary end point was low in both groups: 1.5% in the vaccine group and 2.4% in the placebo group (efficacy 39.4%). The efficacy of the vaccine in preventing lower respiratory tract infection with severe hypoxemia was 48.3% and 44.4% in preventing hospitalization. Although there were differences between the 2 groups, they did not meet the prespecified success criterion for efficacy. Vaccine recipients had more local injection site reactions (40.7% vs 9.9%); however, there was no difference in the frequency of other adverse effects.
Intermediate step: Continued assessment of vaccine safety and immunogenicity
The next important step in the development of the RSV vaccine was a study by Simoes et al,6 who conducted a phase 2b trial to determine the safety and immunogenicity of the RSVpreF vaccine. The authors randomly assigned pregnant women at 24 to 36 weeks of gestation to receive either 120 or 240 µg of RSVpreF vaccine or placebo. The key endpoints were the following: maternal and infant safety; the maternal-to-infant transplacental transfer ratio; and the presence of RSV A, B, and combined A/B neutralizing antibody in maternal serum and umbilical cord blood at delivery. The authors conducted a planned interim analysis that included 327 mothers who received the vaccine. The incidence of adverse effects was similar in mothers and infants in the vaccine compared with the placebo group. None of the adverse effects were judged to be serious. The transplacental neutralizing antibody transfer ratios ranged from 1.4 to 2.1 across a range of gestational ages. The vaccine elicited meaningful neutralizing titers of antibody in maternal serum even up to 7 weeks after immunization. The levels of neutralizing antibodies in umbilical cord blood did not vary substantially with respect to gestational age. A post hoc analysis showed that the transferred antibodies prevented medically-attended RSV-associated lower respiratory tract illnesses in the infants.
Final step: Convincing proof of efficacy
The most recent of the 3 studies, and the one that had the greatest impact in convincing the FDA to approve the vaccine, was the report by Kampmann and colleagues.7 The authors conducted a phase 3 prospective, randomized, double-blind trial in 18 different countries over 4 RSV seasons: 2 in the northern hemisphere and 2 in the southern hemisphere. They enrolled healthy pregnant women with singleton gestations at 24 to 36 weeks of gestation and assigned them in a 1:1 ratio to a single intramuscular injection of 120 µg of a bivalent RSV prefusion F protein-based (RSVpreF) vaccine or placebo. They excluded patients with any recognized risk factor for an adverse pregnancy outcome, including preterm labor. The 2 primary efficacy endpoints were a medically-attended severe RSV–lower respiratory tract infection and any medically attended RSV-associated lower respiratory tract illness in infants within 90, 120, 150, and 180 days after birth.
The efficacy of the vaccine in preventing severe lower respiratory tract illness within 90 days of delivery was 81.8% (99.5% confidence interval [CI], 40.6–96.3). The efficacy within 180 days of delivery was 69.4% (97.58% CI, 44.3–84.1). These differences reached the study’s pre-established statistical criteria for success. The overall rate of lower respiratory tract infections was not significantly different. The frequencies of adverse effects in mothers and infants were similar in the vaccine and placebo groups. In particular, the frequency of preterm delivery in the vaccine group was 0.8%, compared with 0.6% in the placebo group (P = NS).
In previous reports to the FDA,4 the frequency rate of preterm delivery in RSV vaccine recipients was slightly increased in vaccine recipients compared with patients who received placebo. The difference among the groups was too small to infer a causal relationship; however, as a condition of vaccine approval, the FDA has required Pfizer to conduct a postmarketing study to be certain that administration of the vaccine does not increase the risk for preterm delivery.
Practical details
The new vaccine is a bivalent recombinant vaccine that elicits a robust antibody response against the F (fusion) protein of the virus. In addition to the F antigen, the vaccine contains the following buffer ingredients: tromethamine, sucrose, mannitol, polysorbate, and sodium chloride.8 There are no preservatives in the vaccine.
The vaccine should be administered in a single, 0.5 mL, intramuscular injection at 32 to 36 weeks of gestation. Patients who are allergic to any of the components of the vaccine should not be vaccinated. Patients with a mild upper respiratory tract infection may receive the vaccine. Administration should be delayed in patients who are moderately to severely ill. The vaccine may be administered at the same time as other vaccines, such as influenza or Tdap.
The most common side effects of the vaccine are local injection site reactions, such as pain, redness, or swelling. Some patients may experience mild systemic manifestations, including fatigue, fever, headache, nausea, diarrhea, arthralgias, and myalgias. According to the Centers for Disease Control and Prevention, the approximate wholesale acquisition cost of the vaccine is $320 for 1 injection.
CASE Resolution
This patient is healthy and has no contraindication to the new RSV vaccine. According to the FDA, the optimal time for administration of the vaccine is 32 to 36 weeks of gestation. The patient should anticipate very few side effects following the vaccination, and the vaccine has approximately 80% efficacy in preventing severe lower respiratory tract infection in her neonate. ●
- RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age.
- In low- and middle-resource countries, RSV is a leading cause of infant death.
- In late August 2023, the FDA approved the first RSV vaccine that can be administered to pregnant women to provide protection for the infant in the first few months of life.
- The vaccine specifically targets the F protein of the virus, a protein which is essential for facilitating fusion between the viral and host cell membranes, resulting in penetration of the virus into the host cell.
- The vaccine should be administered as a single intramuscular injection at 32 to 36 weeks’ gestation.
- The vaccine is approximately 82% effective in preventing severe lower respiratory tract infection in infants within the first 6 months of life.
- To exercise an abundance of caution, because of a possible association between administration of the vaccine and an increased risk for preterm delivery, vaccination should be delayed until 36 weeks in patients clearly identified as at-risk for preterm delivery.
A 28-year-old primigravid woman at 30 weeks’ gestation inquires about the new vaccine to protect her newborn baby against respiratory syncytial virus infection (RSV). Her neighbor’s daughter recently was hospitalized for the treatment of RSV, and she is understandably concerned about her own newborn. The patient is healthy, and she has never had any serious respiratory infection. She is taking no medications other than prenatal vitamins.
What advice should you give her?
If you decide to administer this vaccine, what is the appropriate timing of administration?
Are there any maternal or fetal safety concerns related to use of this vaccine in pregnancy?
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family. It is an enveloped, single-stranded RNA virus that is 150-300 nm in size. The virus codes for 10 virus-specific proteins. The 2 most important are the G protein, which enables the virus to attach to host cells, and the F protein, which facilitates the entry of the virus into the host cell by fusing the host and viral membranes. Two distinct subtypes exist: A and B. There is genetic variation within each subtype and between subtypes. These subtle genetic variations create the potential for reinfections, and hence, research has focused on development of a vaccine that covers both subtypes.1
RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age. In these children, RSV is one of the most prominent causes of death, with mortality particularly marked in low- and middle-resource countries as well as in children who were born premature and/or who are immunocompromised. RSV has its greatest impact during winter epidemics in temperate climates and during the rainy seasons in tropical climates. The virus rarely is encountered in the summer.1 Among young children, RSV primarily is transmitted via close contact with contaminated fingers or fomites and by self-inoculation of the conjunctiva or anterior nares. The incubation period of the infection is 4 to 6 days, and viral shedding may persist for 2 weeks or longer. Most patients gradually recover within 1 to 2 weeks.1 Adults who contract RSV usually have symptoms suggestive of a common cold; however, in older adults or those who have comorbidities, serious and potentially life-threatening lower respiratory tract infections may develop.
Recently, there have been 2 main approaches to the prevention and treatment of RSV in infants. One has been the development of monoclonal antibodies such as motavizumab, palivizumab, and nirsevimab. The other has been the development of a vaccine that could be administered to pregnant women and which could provide protection for the neonate in the early months of life.2,3
In late August 2023, the US Food and Drug Administration (FDA) announced the approval of a new bivalent RSV prefusion F vaccine (ABRYSVO, Pfizer) intended for administration to pregnant women.4 Of note, previous efforts to develop whole-virus vaccines either have been ineffective or have potentiated the disease in infants who became infected; development of an effective vaccine had eluded scientists and clinicians for nearly 50 years.2 Thus, the new vaccine that targets the F protein of the virus represents a major and welcomed breakthrough.
This article reviews the 3 most recent investigations that preceded the ultimate approval of this vaccine and discusses specific logistical issues related to vaccine administration.
Continue to: First step toward vaccine approval...
First step toward vaccine approval
Madhi and colleagues5 were among the first to conduct a large well-designed study to evaluate the effectiveness of maternal vaccination in preventing neonatal infection in the first few months of life. The authors enrolled more than 4,500 healthy pregnant women at 28 to 36 weeks of gestation and assigned them to receive either a single intramuscular dose of an RSV fusion (F) protein vaccine or placebo in a ratio of 2:1. The primary end point was a “medically significant lower respiratory tract infection” within the first 90 days of life. The percentage of infants who met the primary end point was low in both groups: 1.5% in the vaccine group and 2.4% in the placebo group (efficacy 39.4%). The efficacy of the vaccine in preventing lower respiratory tract infection with severe hypoxemia was 48.3% and 44.4% in preventing hospitalization. Although there were differences between the 2 groups, they did not meet the prespecified success criterion for efficacy. Vaccine recipients had more local injection site reactions (40.7% vs 9.9%); however, there was no difference in the frequency of other adverse effects.
Intermediate step: Continued assessment of vaccine safety and immunogenicity
The next important step in the development of the RSV vaccine was a study by Simoes et al,6 who conducted a phase 2b trial to determine the safety and immunogenicity of the RSVpreF vaccine. The authors randomly assigned pregnant women at 24 to 36 weeks of gestation to receive either 120 or 240 µg of RSVpreF vaccine or placebo. The key endpoints were the following: maternal and infant safety; the maternal-to-infant transplacental transfer ratio; and the presence of RSV A, B, and combined A/B neutralizing antibody in maternal serum and umbilical cord blood at delivery. The authors conducted a planned interim analysis that included 327 mothers who received the vaccine. The incidence of adverse effects was similar in mothers and infants in the vaccine compared with the placebo group. None of the adverse effects were judged to be serious. The transplacental neutralizing antibody transfer ratios ranged from 1.4 to 2.1 across a range of gestational ages. The vaccine elicited meaningful neutralizing titers of antibody in maternal serum even up to 7 weeks after immunization. The levels of neutralizing antibodies in umbilical cord blood did not vary substantially with respect to gestational age. A post hoc analysis showed that the transferred antibodies prevented medically-attended RSV-associated lower respiratory tract illnesses in the infants.
Final step: Convincing proof of efficacy
The most recent of the 3 studies, and the one that had the greatest impact in convincing the FDA to approve the vaccine, was the report by Kampmann and colleagues.7 The authors conducted a phase 3 prospective, randomized, double-blind trial in 18 different countries over 4 RSV seasons: 2 in the northern hemisphere and 2 in the southern hemisphere. They enrolled healthy pregnant women with singleton gestations at 24 to 36 weeks of gestation and assigned them in a 1:1 ratio to a single intramuscular injection of 120 µg of a bivalent RSV prefusion F protein-based (RSVpreF) vaccine or placebo. They excluded patients with any recognized risk factor for an adverse pregnancy outcome, including preterm labor. The 2 primary efficacy endpoints were a medically-attended severe RSV–lower respiratory tract infection and any medically attended RSV-associated lower respiratory tract illness in infants within 90, 120, 150, and 180 days after birth.
The efficacy of the vaccine in preventing severe lower respiratory tract illness within 90 days of delivery was 81.8% (99.5% confidence interval [CI], 40.6–96.3). The efficacy within 180 days of delivery was 69.4% (97.58% CI, 44.3–84.1). These differences reached the study’s pre-established statistical criteria for success. The overall rate of lower respiratory tract infections was not significantly different. The frequencies of adverse effects in mothers and infants were similar in the vaccine and placebo groups. In particular, the frequency of preterm delivery in the vaccine group was 0.8%, compared with 0.6% in the placebo group (P = NS).
In previous reports to the FDA,4 the frequency rate of preterm delivery in RSV vaccine recipients was slightly increased in vaccine recipients compared with patients who received placebo. The difference among the groups was too small to infer a causal relationship; however, as a condition of vaccine approval, the FDA has required Pfizer to conduct a postmarketing study to be certain that administration of the vaccine does not increase the risk for preterm delivery.
Practical details
The new vaccine is a bivalent recombinant vaccine that elicits a robust antibody response against the F (fusion) protein of the virus. In addition to the F antigen, the vaccine contains the following buffer ingredients: tromethamine, sucrose, mannitol, polysorbate, and sodium chloride.8 There are no preservatives in the vaccine.
The vaccine should be administered in a single, 0.5 mL, intramuscular injection at 32 to 36 weeks of gestation. Patients who are allergic to any of the components of the vaccine should not be vaccinated. Patients with a mild upper respiratory tract infection may receive the vaccine. Administration should be delayed in patients who are moderately to severely ill. The vaccine may be administered at the same time as other vaccines, such as influenza or Tdap.
The most common side effects of the vaccine are local injection site reactions, such as pain, redness, or swelling. Some patients may experience mild systemic manifestations, including fatigue, fever, headache, nausea, diarrhea, arthralgias, and myalgias. According to the Centers for Disease Control and Prevention, the approximate wholesale acquisition cost of the vaccine is $320 for 1 injection.
CASE Resolution
This patient is healthy and has no contraindication to the new RSV vaccine. According to the FDA, the optimal time for administration of the vaccine is 32 to 36 weeks of gestation. The patient should anticipate very few side effects following the vaccination, and the vaccine has approximately 80% efficacy in preventing severe lower respiratory tract infection in her neonate. ●
- RSV is the most common cause of acute lower respiratory tract infection in infants younger than 6 months of age.
- In low- and middle-resource countries, RSV is a leading cause of infant death.
- In late August 2023, the FDA approved the first RSV vaccine that can be administered to pregnant women to provide protection for the infant in the first few months of life.
- The vaccine specifically targets the F protein of the virus, a protein which is essential for facilitating fusion between the viral and host cell membranes, resulting in penetration of the virus into the host cell.
- The vaccine should be administered as a single intramuscular injection at 32 to 36 weeks’ gestation.
- The vaccine is approximately 82% effective in preventing severe lower respiratory tract infection in infants within the first 6 months of life.
- To exercise an abundance of caution, because of a possible association between administration of the vaccine and an increased risk for preterm delivery, vaccination should be delayed until 36 weeks in patients clearly identified as at-risk for preterm delivery.
- Dolin R. Common viral respiratory infections. In, Isselbacher KJ, Braunwald E, Wilson JD, et al, eds. Harrison’s Principles of Internal Medicine. 13th ed. McGraw-Hill; 1994:805-806.
- Mazur N, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23:E2-E21.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- US Food and Drug Administration News Release. August 21, 2023. Accessed October 26, 2023. https://www.fda.gov/news -events/press-announcements/fda-approves-first-vaccine -pregnant-individuals-prevent-rsv-infants
- Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426-439.
- Simoes EAF, Center KJ, Tita ATN, et al. Prefusion F proteinbased respiratory syncytial virus immunization in pregnancy. N Eng J Med. 2022;386:1615-1626.
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451-1464.
- Centers for Disease Control and Prevention. Vaccine Information Statement. Respiratory Syncytial Virus (RSV) Vaccine VIS. October 19, 2023. Accessed October 26, 2023. https://www. cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
- Dolin R. Common viral respiratory infections. In, Isselbacher KJ, Braunwald E, Wilson JD, et al, eds. Harrison’s Principles of Internal Medicine. 13th ed. McGraw-Hill; 1994:805-806.
- Mazur N, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23:E2-E21.
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837-846.
- US Food and Drug Administration News Release. August 21, 2023. Accessed October 26, 2023. https://www.fda.gov/news -events/press-announcements/fda-approves-first-vaccine -pregnant-individuals-prevent-rsv-infants
- Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426-439.
- Simoes EAF, Center KJ, Tita ATN, et al. Prefusion F proteinbased respiratory syncytial virus immunization in pregnancy. N Eng J Med. 2022;386:1615-1626.
- Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451-1464.
- Centers for Disease Control and Prevention. Vaccine Information Statement. Respiratory Syncytial Virus (RSV) Vaccine VIS. October 19, 2023. Accessed October 26, 2023. https://www. cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html