User login
Watchful waiting a suitable option for pediatric acute ITP
MONTREAL – Clinicians who manage the care of children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) have to decide whether to treat patients early and possibly increase risk for chronic ITP down the road or to leave well enough alone and treat only as needed.
Evidence from a retrospective study suggests that, for most patients, early treatment of acute ITP does not appear to increase the risk for chronic ITP in the future, reported Chelsea L. Grama, a fourth-year medical student, and her colleagues from Penn State Health Children’s Hospital in Hershey, Pennsylvania.
“It’s controversial whether to treat or not. Some people think steroids are the best way to go, some think IVIG [intravenous immunoglobulin] is the best way to go, and some think that no treatment could be the best option. There is really no consensus in the literature to say what the ideal treatment for ITP is,” she commented in an interview at the annual meeting of the American Society of Pediatric Hematology/Oncology.
That lack of consensus is the result of the fact that serious consequences of ITP, such as intracranial hemorrhage, are rare, making it difficult for investigators to compare clinical outcomes with various forms of treatment. It’s also unclear whether early interventions could lead to later chronic disease.
In hopes of answering this question, Ms. Grama and her coinvestigators, led by Andrew S. Freiberg, MD, took a retrospective look at data on 249 patients with ITP diagnosed from birth through age 21 from 1994 to 2011.
They looked at demographic variables, treatments received, and subsequent platelet counts. Outcomes included intracranial hemorrhage and long-term improvements in platelet counts. They defined chronic ITP as a platelet count below 140,000/mcL for at least 6 months following diagnosis.
Of the 249 patients, 126 (66 boys and 60 girls) progressed to chronic ITP. The incidence of chronic ITP among treated patients was 51.1% and, in untreated patients, 49.3%.
However, in two subgroups, there was a significant association between treatment and chronic ITP. Among girls from birth through age 3 years, 58% of those treated went on to chronic ITP, compared with 0% for untreated patients (P = .008). When the age cohort was expanded to girls younger than 6 years, a similar pattern emerged, with chronic ITP rates of 45% for treated patients, compared with 0% for untreated patients. There were only 44 total patients in this age and sex subgroup, however, making it difficult to draw strong inferences about a potential relationship between treatment and chronic ITP, Ms. Grama noted.
Only three patients had cerebral hemorrhage. Two had hemorrhage as their initial ITP presentation, and the third had bleeding despite being on treatment. The sample size was too small to correlate the outcome with treatment, the authors noted.
In multivariate analysis controlling for demographic, clinical, and treatment factors, only age and platelet count at presentation independently predicted chronic thrombocytopenia (P less than .0001 for each). Steroid therapy was the only independent predictor for acute ITP (P = .0001).
Although their data suggest that there could be a benefit to treating patients who first present with acute ITP at age 12 years or older, “I do think it’s reasonable to wait and watch these patients,” Ms. Grama said.
“The incidence of brain bleeds, which is the really scary complication, was so low in these patients that I think watchful waiting would be a better way to go, just monitoring patients very closely. Hopefully, they will resolve without having treatment,” she added.
The study was internally supported. The authors reported no relevant disclosures.
MONTREAL – Clinicians who manage the care of children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) have to decide whether to treat patients early and possibly increase risk for chronic ITP down the road or to leave well enough alone and treat only as needed.
Evidence from a retrospective study suggests that, for most patients, early treatment of acute ITP does not appear to increase the risk for chronic ITP in the future, reported Chelsea L. Grama, a fourth-year medical student, and her colleagues from Penn State Health Children’s Hospital in Hershey, Pennsylvania.
“It’s controversial whether to treat or not. Some people think steroids are the best way to go, some think IVIG [intravenous immunoglobulin] is the best way to go, and some think that no treatment could be the best option. There is really no consensus in the literature to say what the ideal treatment for ITP is,” she commented in an interview at the annual meeting of the American Society of Pediatric Hematology/Oncology.
That lack of consensus is the result of the fact that serious consequences of ITP, such as intracranial hemorrhage, are rare, making it difficult for investigators to compare clinical outcomes with various forms of treatment. It’s also unclear whether early interventions could lead to later chronic disease.
In hopes of answering this question, Ms. Grama and her coinvestigators, led by Andrew S. Freiberg, MD, took a retrospective look at data on 249 patients with ITP diagnosed from birth through age 21 from 1994 to 2011.
They looked at demographic variables, treatments received, and subsequent platelet counts. Outcomes included intracranial hemorrhage and long-term improvements in platelet counts. They defined chronic ITP as a platelet count below 140,000/mcL for at least 6 months following diagnosis.
Of the 249 patients, 126 (66 boys and 60 girls) progressed to chronic ITP. The incidence of chronic ITP among treated patients was 51.1% and, in untreated patients, 49.3%.
However, in two subgroups, there was a significant association between treatment and chronic ITP. Among girls from birth through age 3 years, 58% of those treated went on to chronic ITP, compared with 0% for untreated patients (P = .008). When the age cohort was expanded to girls younger than 6 years, a similar pattern emerged, with chronic ITP rates of 45% for treated patients, compared with 0% for untreated patients. There were only 44 total patients in this age and sex subgroup, however, making it difficult to draw strong inferences about a potential relationship between treatment and chronic ITP, Ms. Grama noted.
Only three patients had cerebral hemorrhage. Two had hemorrhage as their initial ITP presentation, and the third had bleeding despite being on treatment. The sample size was too small to correlate the outcome with treatment, the authors noted.
In multivariate analysis controlling for demographic, clinical, and treatment factors, only age and platelet count at presentation independently predicted chronic thrombocytopenia (P less than .0001 for each). Steroid therapy was the only independent predictor for acute ITP (P = .0001).
Although their data suggest that there could be a benefit to treating patients who first present with acute ITP at age 12 years or older, “I do think it’s reasonable to wait and watch these patients,” Ms. Grama said.
“The incidence of brain bleeds, which is the really scary complication, was so low in these patients that I think watchful waiting would be a better way to go, just monitoring patients very closely. Hopefully, they will resolve without having treatment,” she added.
The study was internally supported. The authors reported no relevant disclosures.
MONTREAL – Clinicians who manage the care of children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) have to decide whether to treat patients early and possibly increase risk for chronic ITP down the road or to leave well enough alone and treat only as needed.
Evidence from a retrospective study suggests that, for most patients, early treatment of acute ITP does not appear to increase the risk for chronic ITP in the future, reported Chelsea L. Grama, a fourth-year medical student, and her colleagues from Penn State Health Children’s Hospital in Hershey, Pennsylvania.
“It’s controversial whether to treat or not. Some people think steroids are the best way to go, some think IVIG [intravenous immunoglobulin] is the best way to go, and some think that no treatment could be the best option. There is really no consensus in the literature to say what the ideal treatment for ITP is,” she commented in an interview at the annual meeting of the American Society of Pediatric Hematology/Oncology.
That lack of consensus is the result of the fact that serious consequences of ITP, such as intracranial hemorrhage, are rare, making it difficult for investigators to compare clinical outcomes with various forms of treatment. It’s also unclear whether early interventions could lead to later chronic disease.
In hopes of answering this question, Ms. Grama and her coinvestigators, led by Andrew S. Freiberg, MD, took a retrospective look at data on 249 patients with ITP diagnosed from birth through age 21 from 1994 to 2011.
They looked at demographic variables, treatments received, and subsequent platelet counts. Outcomes included intracranial hemorrhage and long-term improvements in platelet counts. They defined chronic ITP as a platelet count below 140,000/mcL for at least 6 months following diagnosis.
Of the 249 patients, 126 (66 boys and 60 girls) progressed to chronic ITP. The incidence of chronic ITP among treated patients was 51.1% and, in untreated patients, 49.3%.
However, in two subgroups, there was a significant association between treatment and chronic ITP. Among girls from birth through age 3 years, 58% of those treated went on to chronic ITP, compared with 0% for untreated patients (P = .008). When the age cohort was expanded to girls younger than 6 years, a similar pattern emerged, with chronic ITP rates of 45% for treated patients, compared with 0% for untreated patients. There were only 44 total patients in this age and sex subgroup, however, making it difficult to draw strong inferences about a potential relationship between treatment and chronic ITP, Ms. Grama noted.
Only three patients had cerebral hemorrhage. Two had hemorrhage as their initial ITP presentation, and the third had bleeding despite being on treatment. The sample size was too small to correlate the outcome with treatment, the authors noted.
In multivariate analysis controlling for demographic, clinical, and treatment factors, only age and platelet count at presentation independently predicted chronic thrombocytopenia (P less than .0001 for each). Steroid therapy was the only independent predictor for acute ITP (P = .0001).
Although their data suggest that there could be a benefit to treating patients who first present with acute ITP at age 12 years or older, “I do think it’s reasonable to wait and watch these patients,” Ms. Grama said.
“The incidence of brain bleeds, which is the really scary complication, was so low in these patients that I think watchful waiting would be a better way to go, just monitoring patients very closely. Hopefully, they will resolve without having treatment,” she added.
The study was internally supported. The authors reported no relevant disclosures.
FROM ASPHO 2017
Key clinical point: Treatment of acute idiopathic thrombocytopenic purpura does not appear to increase risk of later chronic ITP in children older than 6 years.
Major finding: Girls younger than 6 years were the only group in which early treatment of acute ITP may have increased risk for chronic ITP.
Data source: Retrospective data review on 249 children and young adults with ITP.
Disclosures: The study was internally supported. The authors reported no relevant disclosures.
Presenters hand out tips for better handoffs
LAS VEGAS – When the resident in the handoff training video approached another resident, she first vented about her “really crazy day” and how she’d been hoping to “get out of here on time for once.” Then, she tore through a case like an auctioneer, leaving out important details. At the end, she got a page and, of course, didn’t finish the handoff.
In Wednesday’s Quality Track session, “Strategies for Implementing a Successful Handoff” – with lessons from SHM’s I-PASS mentored implementation project – some attendees were eager to point out all of the flaws in the handoff. They were, however, slower to point to the good. For instance, the resident in the video made the effort to meet face-to-face and asked whether her colleague had any questions.
The audience had learned an important lesson about how to foster better handoffs, said Glenn Rosenbluth, MD, director of quality and safety programs at the University of California, San Francisco. He noted that it can be difficult to find the positives when the negatives are so glaring.
“This is one of the hard parts about doing feedback,” he said.
It was just one of many lessons taught in the session, also led by Amy Starmer, MD, MPH, a lecturer on pediatrics at Harvard Medical School, and Courtney Edgar-Zarate, MD, a pediatric hospitalist at Arkansas Children’s Hospital who was one of the I-PASS site leaders.
I-PASS was a nine-site study in the United States and Canada that found that using a bundle of interventions while doing handoffs resulted in a 30% reduction in preventable adverse events, meaning less harm to patients. The hallmark is the “I-PASS” mnemonic. It stands for:
- Illness severity – describing the stability level of a patient.
- Patient summary, including general information, such as the events leading to admission.
- Action list – essentially a to-do list for the patient.
- Situation awareness and contingency planning, which involves having a plan for what might happen.
- Synthesis by the receiver, in which the recipient of the information summarizes what was heard and asks questions.
Beyond that, the I-PASS system involves an introductory workshop, simulation exercises, structured observation and feedback, among other elements, Dr. Starmer said.
“This intervention was certainly not just a five-letter mnemonic,” she said.
The I-PASS Mentored Implementation Program, a collaboration with SHM that is funded by the Agency for Healthcare Research and Quality, is an effort to help implement a similar program in 32 hospitals in the United States.
Dr. Edgar-Zarate outlined the steps that worked at her site to make their I-PASS project successful. She said that project managers have to establish institutional support; assess a given center’s needs; gauge where to begin by identifying the most vulnerable transition points; find providers who will champion the project; establish good communication, in part by incorporating I-PASS into previously scheduled meetings; and collect data as time goes on.
Dr. Starmer directed attendees to www.ipassstudygroup.com, where anyone can download the material for free.
“This mentored implementation process,” she said, “has really been a helpful vehicle for disseminating the curriculum and implementation across different areas.”
LAS VEGAS – When the resident in the handoff training video approached another resident, she first vented about her “really crazy day” and how she’d been hoping to “get out of here on time for once.” Then, she tore through a case like an auctioneer, leaving out important details. At the end, she got a page and, of course, didn’t finish the handoff.
In Wednesday’s Quality Track session, “Strategies for Implementing a Successful Handoff” – with lessons from SHM’s I-PASS mentored implementation project – some attendees were eager to point out all of the flaws in the handoff. They were, however, slower to point to the good. For instance, the resident in the video made the effort to meet face-to-face and asked whether her colleague had any questions.
The audience had learned an important lesson about how to foster better handoffs, said Glenn Rosenbluth, MD, director of quality and safety programs at the University of California, San Francisco. He noted that it can be difficult to find the positives when the negatives are so glaring.
“This is one of the hard parts about doing feedback,” he said.
It was just one of many lessons taught in the session, also led by Amy Starmer, MD, MPH, a lecturer on pediatrics at Harvard Medical School, and Courtney Edgar-Zarate, MD, a pediatric hospitalist at Arkansas Children’s Hospital who was one of the I-PASS site leaders.
I-PASS was a nine-site study in the United States and Canada that found that using a bundle of interventions while doing handoffs resulted in a 30% reduction in preventable adverse events, meaning less harm to patients. The hallmark is the “I-PASS” mnemonic. It stands for:
- Illness severity – describing the stability level of a patient.
- Patient summary, including general information, such as the events leading to admission.
- Action list – essentially a to-do list for the patient.
- Situation awareness and contingency planning, which involves having a plan for what might happen.
- Synthesis by the receiver, in which the recipient of the information summarizes what was heard and asks questions.
Beyond that, the I-PASS system involves an introductory workshop, simulation exercises, structured observation and feedback, among other elements, Dr. Starmer said.
“This intervention was certainly not just a five-letter mnemonic,” she said.
The I-PASS Mentored Implementation Program, a collaboration with SHM that is funded by the Agency for Healthcare Research and Quality, is an effort to help implement a similar program in 32 hospitals in the United States.
Dr. Edgar-Zarate outlined the steps that worked at her site to make their I-PASS project successful. She said that project managers have to establish institutional support; assess a given center’s needs; gauge where to begin by identifying the most vulnerable transition points; find providers who will champion the project; establish good communication, in part by incorporating I-PASS into previously scheduled meetings; and collect data as time goes on.
Dr. Starmer directed attendees to www.ipassstudygroup.com, where anyone can download the material for free.
“This mentored implementation process,” she said, “has really been a helpful vehicle for disseminating the curriculum and implementation across different areas.”
LAS VEGAS – When the resident in the handoff training video approached another resident, she first vented about her “really crazy day” and how she’d been hoping to “get out of here on time for once.” Then, she tore through a case like an auctioneer, leaving out important details. At the end, she got a page and, of course, didn’t finish the handoff.
In Wednesday’s Quality Track session, “Strategies for Implementing a Successful Handoff” – with lessons from SHM’s I-PASS mentored implementation project – some attendees were eager to point out all of the flaws in the handoff. They were, however, slower to point to the good. For instance, the resident in the video made the effort to meet face-to-face and asked whether her colleague had any questions.
The audience had learned an important lesson about how to foster better handoffs, said Glenn Rosenbluth, MD, director of quality and safety programs at the University of California, San Francisco. He noted that it can be difficult to find the positives when the negatives are so glaring.
“This is one of the hard parts about doing feedback,” he said.
It was just one of many lessons taught in the session, also led by Amy Starmer, MD, MPH, a lecturer on pediatrics at Harvard Medical School, and Courtney Edgar-Zarate, MD, a pediatric hospitalist at Arkansas Children’s Hospital who was one of the I-PASS site leaders.
I-PASS was a nine-site study in the United States and Canada that found that using a bundle of interventions while doing handoffs resulted in a 30% reduction in preventable adverse events, meaning less harm to patients. The hallmark is the “I-PASS” mnemonic. It stands for:
- Illness severity – describing the stability level of a patient.
- Patient summary, including general information, such as the events leading to admission.
- Action list – essentially a to-do list for the patient.
- Situation awareness and contingency planning, which involves having a plan for what might happen.
- Synthesis by the receiver, in which the recipient of the information summarizes what was heard and asks questions.
Beyond that, the I-PASS system involves an introductory workshop, simulation exercises, structured observation and feedback, among other elements, Dr. Starmer said.
“This intervention was certainly not just a five-letter mnemonic,” she said.
The I-PASS Mentored Implementation Program, a collaboration with SHM that is funded by the Agency for Healthcare Research and Quality, is an effort to help implement a similar program in 32 hospitals in the United States.
Dr. Edgar-Zarate outlined the steps that worked at her site to make their I-PASS project successful. She said that project managers have to establish institutional support; assess a given center’s needs; gauge where to begin by identifying the most vulnerable transition points; find providers who will champion the project; establish good communication, in part by incorporating I-PASS into previously scheduled meetings; and collect data as time goes on.
Dr. Starmer directed attendees to www.ipassstudygroup.com, where anyone can download the material for free.
“This mentored implementation process,” she said, “has really been a helpful vehicle for disseminating the curriculum and implementation across different areas.”
AT HM17
Cervical cancer incidence declines after age 85
Women should stop attending their cervical screening appointments at the recommended age of 65 and over only if they have an adequate prior screening history with negative results, researchers have warned.
“Messages about a ‘stopping age’ should emphasize the recommendation for an adequate screening history of previous negative tests before screening is discontinued, not just chronological age,” wrote the study authors. The report was published in the American Journal of Preventive Medicine (2017 May 1. doi: 10.1016/j.amepre.2017.02.024).
Leading professional organizations recommend “average-risk” women should be screened for cervical cancer between the ages of 21 and 65 years.
However, the authors, led by Mary C. White, ScD, of the Centers for Disease Control and Prevention, Atlanta, and her colleagues noted that data from 2013 showed that one-fifth of cervical cancer deaths occurred in women in the United States who were over the age of 65 years.
In the current study, the research team looked at data from the cross-sectional household National Health Interview Surveys from 2013 and 2015 to determine the number of women aged 41-70 years who had never had a Pap test or had not had one for 5 years or more.
After correcting for women who had undergone a hysterectomy, the researchers found that the incidence rate for cervical cancer increased with age until about 70 years and then declined after the age of 85.
Yet the proportion of women who had not been screened recently increased with age, from about 12% for women in their 40s to nearly 24% for women aged 66-70 years.
Efforts should be made to clarify misperceptions about the risk of cervical cancer among older women and health care providers, the investigators said.
“A recommended upper age limit for routine screening may lead women and providers to assume that cervical cancer is a younger woman’s disease,” they said.
Catch-up screening might be needed for underscreened women after the age of 65 years, although the physical and psychological issues associated with this should be taken into account, the study authors said.
“Age itself may need to be reconsidered, given high cervical cancer incidence rates after the age of 65 years, increases in life expectancy, and different human papillomavirus exposures by birth cohort,” they concluded.
Women should stop attending their cervical screening appointments at the recommended age of 65 and over only if they have an adequate prior screening history with negative results, researchers have warned.
“Messages about a ‘stopping age’ should emphasize the recommendation for an adequate screening history of previous negative tests before screening is discontinued, not just chronological age,” wrote the study authors. The report was published in the American Journal of Preventive Medicine (2017 May 1. doi: 10.1016/j.amepre.2017.02.024).
Leading professional organizations recommend “average-risk” women should be screened for cervical cancer between the ages of 21 and 65 years.
However, the authors, led by Mary C. White, ScD, of the Centers for Disease Control and Prevention, Atlanta, and her colleagues noted that data from 2013 showed that one-fifth of cervical cancer deaths occurred in women in the United States who were over the age of 65 years.
In the current study, the research team looked at data from the cross-sectional household National Health Interview Surveys from 2013 and 2015 to determine the number of women aged 41-70 years who had never had a Pap test or had not had one for 5 years or more.
After correcting for women who had undergone a hysterectomy, the researchers found that the incidence rate for cervical cancer increased with age until about 70 years and then declined after the age of 85.
Yet the proportion of women who had not been screened recently increased with age, from about 12% for women in their 40s to nearly 24% for women aged 66-70 years.
Efforts should be made to clarify misperceptions about the risk of cervical cancer among older women and health care providers, the investigators said.
“A recommended upper age limit for routine screening may lead women and providers to assume that cervical cancer is a younger woman’s disease,” they said.
Catch-up screening might be needed for underscreened women after the age of 65 years, although the physical and psychological issues associated with this should be taken into account, the study authors said.
“Age itself may need to be reconsidered, given high cervical cancer incidence rates after the age of 65 years, increases in life expectancy, and different human papillomavirus exposures by birth cohort,” they concluded.
Women should stop attending their cervical screening appointments at the recommended age of 65 and over only if they have an adequate prior screening history with negative results, researchers have warned.
“Messages about a ‘stopping age’ should emphasize the recommendation for an adequate screening history of previous negative tests before screening is discontinued, not just chronological age,” wrote the study authors. The report was published in the American Journal of Preventive Medicine (2017 May 1. doi: 10.1016/j.amepre.2017.02.024).
Leading professional organizations recommend “average-risk” women should be screened for cervical cancer between the ages of 21 and 65 years.
However, the authors, led by Mary C. White, ScD, of the Centers for Disease Control and Prevention, Atlanta, and her colleagues noted that data from 2013 showed that one-fifth of cervical cancer deaths occurred in women in the United States who were over the age of 65 years.
In the current study, the research team looked at data from the cross-sectional household National Health Interview Surveys from 2013 and 2015 to determine the number of women aged 41-70 years who had never had a Pap test or had not had one for 5 years or more.
After correcting for women who had undergone a hysterectomy, the researchers found that the incidence rate for cervical cancer increased with age until about 70 years and then declined after the age of 85.
Yet the proportion of women who had not been screened recently increased with age, from about 12% for women in their 40s to nearly 24% for women aged 66-70 years.
Efforts should be made to clarify misperceptions about the risk of cervical cancer among older women and health care providers, the investigators said.
“A recommended upper age limit for routine screening may lead women and providers to assume that cervical cancer is a younger woman’s disease,” they said.
Catch-up screening might be needed for underscreened women after the age of 65 years, although the physical and psychological issues associated with this should be taken into account, the study authors said.
“Age itself may need to be reconsidered, given high cervical cancer incidence rates after the age of 65 years, increases in life expectancy, and different human papillomavirus exposures by birth cohort,” they concluded.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Key clinical point: Women should stop cervical cancer screening at the recommended age of 65 only if they have an adequate prior history of negative tests.
Major finding: The incidence rate for cervical cancer increases with age until about 70 years and then declines after the age of 85. Yet data show the proportion of women who have not recently been screened for cervical cancer increases with age (12% for women in their 40s vs. 24% for women aged 66-70 years).
Data source: Analysis of data from the cross-sectional household National Health Interview Surveys from 2013 and 2015.
Disclosures: No relevant disclosures were reported by the authors.
Approach to Management of Giant Basal Cell Carcinomas
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
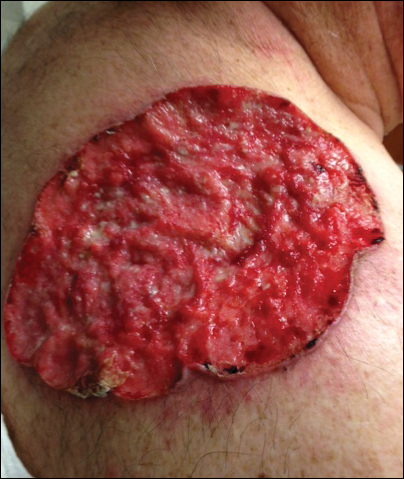
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
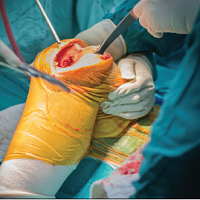
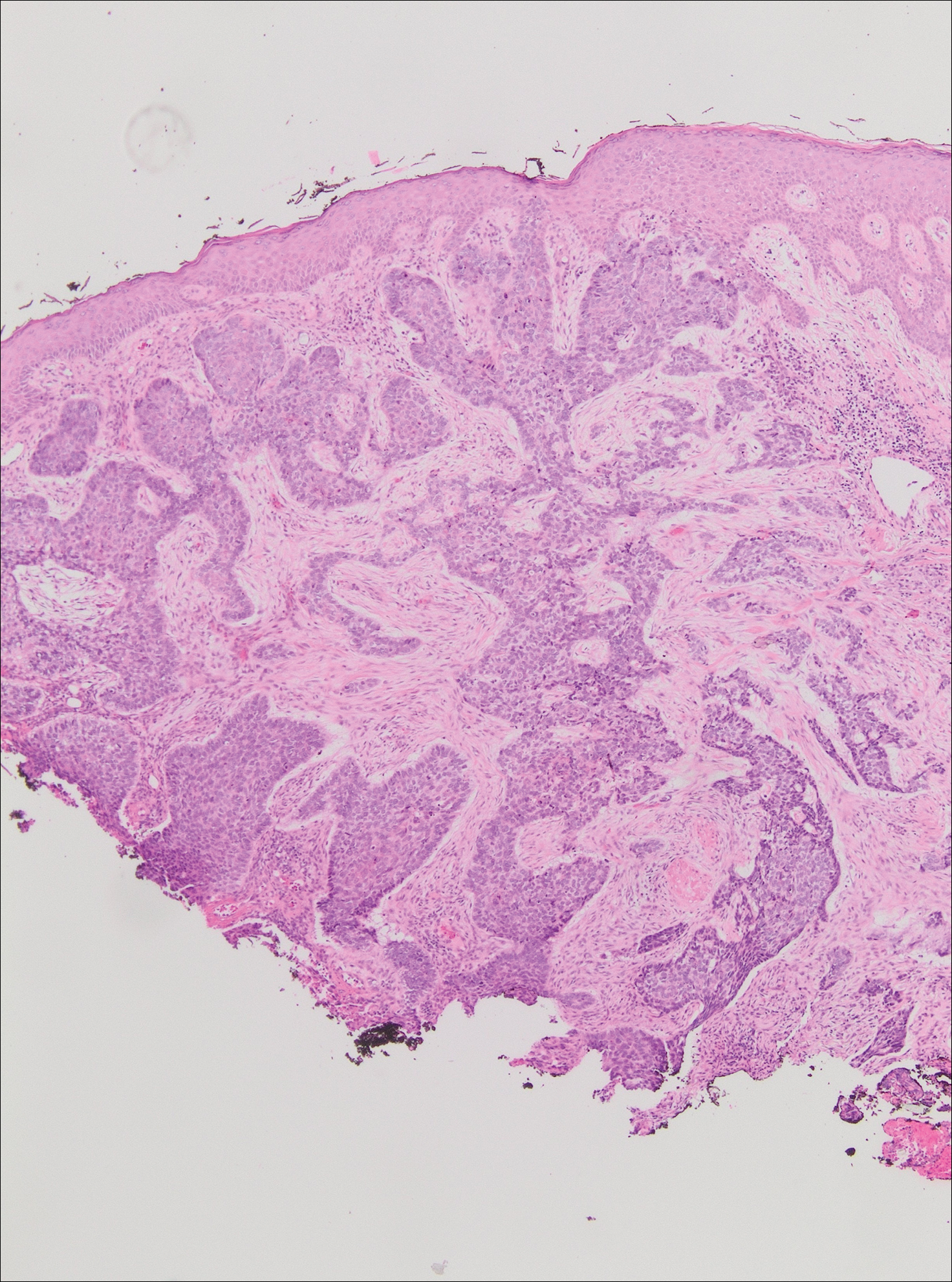
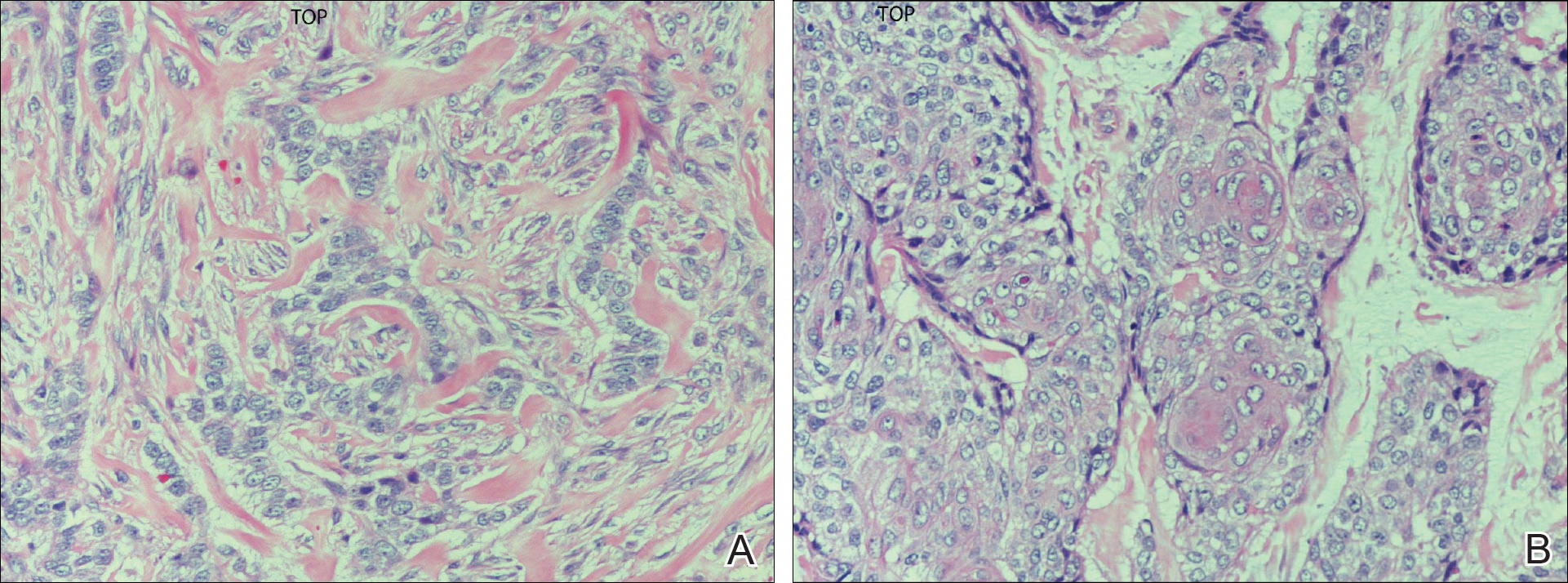
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.
The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.
The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
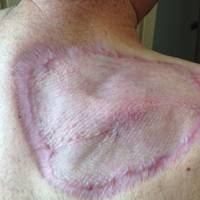
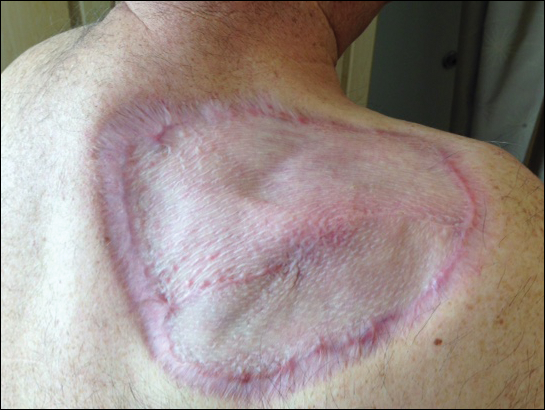
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.
Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.


The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.

The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.

Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.


The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.

The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.

Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
Practice Points
- Unusually large basal cell carcinomas (BCCs) present a therapeutic challenge.
- A number of therapeutic options exist. Wide excision with margin control and complex reconstruction remains an excellent treatment option for BCC.
Pamela Rist, ScD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Blood Loss and Need for Blood Transfusions in Total Knee and Total Hip Arthroplasty
The number of patients who undergo total knee arthroplasty (TKA) and total hip arthroplasty (THA) procedures has significantly increased over the past 2 to 3 decades. As life expectancy in the U.S. increases and medical advances allow patients with preexisting conditions to successfully undergo joint replacements, the demand for these procedures is expected to grow.1 In 2010, the CDC estimated that 719,000 patients underwent TKA procedures and 332,000 patients underwent THA procedures in the U.S.2 Kurtz and colleagues have projected that by 2030 the annual number of TKA procedures will increase to 3.5 million and the number of THA procedures will increase to 572,000.1
Although becoming more prevalent, these procedures are still associated with considerable intra- and postoperative blood loss that may lead to complications and blood transfusions.3 Previous studies have shown that perioperative anemia and red blood cell transfusions are associated with negative outcomes, including increased health care resource utilization; length of hospitalization; pulmonary, septic, wound, or thromboembolic complications; and mortality.4,5
In order to prevent excessive blood loss during TKA and THA procedures, antifibrinolytic agents, such as tranexamic acid, have been used. Tranexamic acid is a synthetic form of lysine that binds to the lysine-binding sites on plasminogen and slows the conversion of plasminogen to plasmin. This interaction inhibits fibrinolysis and theoretically decreases bleeding.6 Because tranexamic acid is an antifibrinolytic, its mechanism of action has raised concerns that it could increase the risk of clotting complications, such as venous thromboembolism and myocardial infarction.7
Several published meta-analyses and systematic reviews have shown that tranexamic acid reduces blood loss in patients undergoing orthopedic surgery. Despite positive results, many study authors have acknowledged limitations in their analyses, such as heterogeneity of study results, small trial size, and varied dosing strategies.3,7-12 There is no FDA-approved tranexamic acid dosing strategy for orthopedic procedures; therefore, its use in TKA and THA procedures is off label. This lack of guidance results in the medication being used at varied doses, timing of doses, and routes of administration with no clear dosing strategy showing the best outcomes.
Tranexamic acid was first used in TKA and THA surgical procedures at the Sioux Falls VA Health Care System (SFVAHCS) in South Dakota in October 2012. The dose used during these procedures was 1 g IV at first surgical incision and 1 g IV at incision closure. The objective of this study was to determine whether this tranexamic acid dosing strategy utilized at SFVAHCS safely improved outcomes related to blood loss.
Methods
A single-center retrospective chart review was performed on all patients who underwent TKA and THA procedures by 4 orthopedic surgeons between January 2010 and August 2015 at SFVAHCS. This study received approval by the local institutional review board and research and development committee in September 2015.
Patients were included in the study if they were aged ≥ 18 years and underwent primary unilateral TKA or THA procedures during the study time frame. Patients were excluded if they underwent bilateral or revision TKA or THA procedures, did not have recorded blood loss measurements during and/or after the procedure, or did not receive tranexamic acid between October 2012 and August 2015 at the standard dosing strategy utilized at SFVAHCS.
Patients who underwent surgery between January 2010 and October 2012 and did not receive tranexamic acid were included in the control groups. The treatment groups contained patients who underwent surgery between October 2012 and August 2015 and received tranexamic acid at the standard SFVAHCS dosing strategy. Patients in the control and treatment groups were divided and compared with patients who underwent the same type of surgery.
The primary endpoint of this study was total blood loss, which included intraoperative and postoperative blood loss. Intraoperative blood loss was measured by a suctioning device that the surgical physician’s assistant used to keep the surgical site clear of bodily fluids. The suctioning device collected blood as well as irrigation fluids used throughout the surgical procedure. The volume of irrigation fluids used during the procedure was subtracted from the total volume collected by the suctioning device to estimate the total blood volume lost during surgery. Sponges and other surgical materials that may have collected blood were not included in the intraoperative blood loss calculation. Postoperative blood loss was collected by a drain that was placed in the surgical site prior to incision closure. The drain collected postoperative blood loss until it was removed 1 day after surgery.
The secondary endpoints for the study were changes in hemoglobin (Hgb) and hematocrit (Hct) from before surgery to after surgery. These changes were calculated by subtracting the lowest measured postoperative Hgb or Hct level within 21 days postsurgery from the closest measured Hgb or Hct level obtained presurgery.
Follow-up appointments were routinely conducted 2 weeks after surgery, so the 21-day time frame would include any laboratory results drawn at these appointments. Other secondary endpoints included the number of patients receiving at least 1 blood transfusion during hospitalization and the number of patients experiencing clotting complications within 30 days of surgery. Postoperative and progress notes were reviewed by a single study investigator in order to record blood transfusions and clotting complications.
All patients who underwent TKA or THA procedures were instructed to stop taking antiplatelet agents 7 days prior to surgery and warfarin 5 days prior to surgery. If patients were determined to be at high risk for thromboembolic complications following warfarin discontinuation, therapeutic doses of low-molecular weight heparin or unfractionated heparin were used as a bridging therapy pre- and postsurgery. Enoxaparin 30 mg twice daily was started the day after surgery in all patients not previously on warfarin therapy prior to surgery to prevent clotting complications. If a patient was on warfarin therapy prior to the procedure but not considered to be at high risk of thromboembolic complications by the surgeon, warfarin was restarted after surgery, and enoxaparin 30 mg twice daily was used until therapeutic international normalized ratio values were obtained. If the patient had ongoing bleeding after the procedure or was determined to be at high risk for bleeding complications, the provider may have delayed anticoagulant use.
Some patients who underwent TKA or THA procedures during the study time frame received periarticular pain injections during surgery. These pain injections included a combination of ropivacaine 200 mg, ketorolac 30 mg, epinephrine 0.5 mg, and clonidine 0.08 mg and were compounded in a sterile mixture with normal saline. Several injections of this mixture were administered into the surgical site to reduce postoperative pain. These periarticular pain injections were first implemented into TKA and THA procedures in August 2012 and were used in patients at the surgeon’s discretion.
Baseline characteristics were analyzed using a chi-square test for categoric variables and an unpaired t test for continuous variables to determine whether any differences were present. Total blood loss, change in Hgb, and change in Hct were analyzed using an unpaired t test. Patients receiving at least 1 blood transfusion during hospitalization and patients experiencing a clotting complication were analyzed using a chi-square test. P values < .05 were considered to indicate statistical significance. Descriptive statistics were calculated using Microsoft Excel (Redmond, WA), and GraphPad Prism (La Jolla, CA) was used for all statistical analyses.
Results
Initially, a total of 443 TKA patients and 111 THA patients were reviewed. Of these patients, 418 TKA patients and 100 THA patients met the inclusion criteria. Due to the retrospective design of this study, not all of the baseline characteristics were equal between groups (Table 1). Most notably, the number of patients who received the periarticular pain injection and the distribution of surgeons performing the procedures were different between groups in both the TKA and THA procedures.
Baseline Hgb levels were not found to be different between groups in either type of procedure; however, the baseline Hct levels of patients undergoing TKA who received tranexamic acid were found to be statistically higher when compared with those who did not receive tranexamic acid. Other baseline characteristics with statistically higher values included average weight and BMI in patients who received tranexamic acid and underwent THA and serum creatinine in patients who did not receive tranexamic acid and underwent TKA.
In the primary analysis (Tables 2 and 3), the mean estimated total blood loss in TKA patients was lower in patients who received tranexamic acid than it was in the control group (339.4 mL vs 457.4 mL, P < .001). Patients who underwent THA receiving tranexamic acid similarly had significantly less total blood loss than that of the control group (419.7 mL vs 585.7 mL, P < .001). Consistent with previous studies, patients undergoing TKA procedures in the treatment group when compared to the control group, respectively, were likely to have more blood loss postoperatively (275.9 mL vs 399.7 mL) than intraoperatively (63.5 mL vs 57.7 mL) regardless of tranexamic acid administration.6 On the other hand, patients who had undergone THA were more likely to experience more intraoperative blood loss (281.6 mL in treatment group vs 328.9 mL in control group) than postoperative blood loss (138.1 mL in treatment group vs 256.8 mL in control group) regardless of tranexamic acid administration.
In the secondary analysis, the change between preoperative and postoperative Hgb and Hct had results consistent with the total blood loss results. Patients receiving tranexamic acid in TKA procedures had a lower decrease in Hgb compared with the control group (3.3 mg/dL vs 4.0 mg/dL, P < .001). Similarly, patients undergoing TKA who received tranexamic acid had a smaller decrease in Hct than that of the control group (9.4% vs 11.1%, P < .001). Consistent with the TKA procedure results, patients undergoing THA who received tranexamic acid had a smaller decrease in Hgb (3.6 mg/dL vs 4.7 mg/dL, P < .001) and Hct (10.5% vs 13.0%, P = .0012) than that of the control group.
Patients who did not receive tranexamic acid were more likely to require at least 1 blood transfusion than were patients who received tranexamic acid in TKA (14 patients vs 0 patients, P = .0005) and THA procedures (8 patients vs 2 patients, P = .019). Despite the theoretically increased likelihood of clotting complications with tranexamic acid, no significant differences were observed between the treatment and control groups in either TKA (0 vs 4, P = .065) or THA (1 vs 0, P = .363) procedures.
Discussion
Patients undergoing total joint arthroplasty are at risk for significant blood loss with a potential need for postoperative blood transfusions.6,13 The use of tranexamic acid during these procedures offers a possible solution to decrease blood loss and minimize blood transfusions. This study retrospectively evaluated whether tranexamic acid safely decreased blood loss and the need for blood transfusions at SFVAHCS with the dosing strategy of 1 g IV at first incision and 1 g IV at incision closure.
Patients who received tranexamic acid in TKA and THA procedures had significantly less blood loss than did patients who did not receive tranexamic acid. Patients receiving tranexamic acid also had a significantly lower change from preoperative to postoperative Hgb and Hct levels than did patients who did not receive tranexamic acid in TKA and THA procedures. In addition to decreasing blood loss, the tranexamic acid groups had significantly fewer patients who required blood transfusions than that of the control groups.
This reduction in blood transfusions should be considered clinically significant.
Even though baseline Hct levels were found to be significantly different between the tranexamic acid group and the control group for patients who underwent TKA, medical record documentation indicated that the determination whether postoperative blood transfusions were needed was based primarily on Hgb levels. Baseline Hgb levels were found not to be significantly different between the tranexamic acid and control groups for either TKA or THA procedures. This suggests that at baseline the tranexamic acid and control groups had the same risk of reaching the Hgb threshold where blood transfusions would be required.
There was a significant difference in the proportion of patients receiving the periarticular pain injection of ropivacaine, ketorolac, epinephrine, and clonidine between the groups who received tranexamic acid and those who did not. Originally, this baseline characteristic was theorized to be a major confounder in the primary and secondary analyses because epinephrine is a vasoconstrictor and ketorolac is a reversible antiplatelet agent. However, Vendittoli and colleagues showed that patients receiving these periarticular pain injections during surgery actually had greater total blood loss than did patients who did not receive the injections, although this comparison did not reach statistical significance.14 The Vendittoli and colleagues results suggest that the pain injections did not confound the primary and secondary analyses by aiding in the process of reducing blood loss in these procedures.
Limitations
There are several limitations for extrapolating the results from this study to the general population. Due to the retrospective study design, there was no way to actively control potentially confounding variables during the TKA and THA procedures. Surgeons and surgery teams likely had slightly different techniques and protocols during and after surgery. Several baseline characteristics were not equal between patients who received tranexamic acid and those who did not. Therefore, it is unknown whether these baseline characteristics affected the results of this study. Postoperative anticoagulant use was not recorded and may have differed between study groups, depending on the patient’s risk of thromboembolic complications; however, the drains that collected blood loss were removed prior to the first dose of enoxaparin, which was administered the day after surgery.
Another limitation is that the method of measuring blood loss during and after the procedure was imprecise. Blood not suctioned through the suctioning device during surgery or not collected in the drain after surgery was not measured and may have increased the total blood loss. Hemoglobin and Hct levels also are sensitive to intravascular volume changes. If a patient required more IV fluids during or after a procedure, the fluids may have lowered the Hgb and/or Hct levels by dilution.
Conclusion
This study suggests that using tranexamic acid at a dose of 1 g IV at first incision and 1 g IV at incision closure safely and effectively reduced blood loss and the need for transfusions in patients undergoing TKA and THA procedures at SFVAHCS. Further prospective studies are needed to compare different tranexamic dosing strategies to minimize blood loss during these procedures. ˜
Acknowledgments
This study is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System in South Dakota.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Published 2010. Accessed March 15, 2017.
3. Wei Z, Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med. 2015;25(3):151-162.
4. Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114(2):283-292.
5. Wu WC, Smith TS, Henderson WG, et al. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg. 2010;252(1):283-292.
6. Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86(4):561-565.
7. Gandhi R, Evans HMK, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184.
8. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
9. Yang ZG, Chen WP, Wu LE. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
10. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
11. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
12. Zhou XD, Tao LJ, Li J, Wu LD. Do we really need tranexamic acid in total hip arthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Surg. 2013;133(7):1017-1027.
13. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
14. Vendittoli PA, Makinen P, Drolet P, et al. A multimodal analgesia protocol for total knee arthroplasty: a randomized, controlled study. J Bone Joint Surg Am. 2006;88(2):282-289.
The number of patients who undergo total knee arthroplasty (TKA) and total hip arthroplasty (THA) procedures has significantly increased over the past 2 to 3 decades. As life expectancy in the U.S. increases and medical advances allow patients with preexisting conditions to successfully undergo joint replacements, the demand for these procedures is expected to grow.1 In 2010, the CDC estimated that 719,000 patients underwent TKA procedures and 332,000 patients underwent THA procedures in the U.S.2 Kurtz and colleagues have projected that by 2030 the annual number of TKA procedures will increase to 3.5 million and the number of THA procedures will increase to 572,000.1
Although becoming more prevalent, these procedures are still associated with considerable intra- and postoperative blood loss that may lead to complications and blood transfusions.3 Previous studies have shown that perioperative anemia and red blood cell transfusions are associated with negative outcomes, including increased health care resource utilization; length of hospitalization; pulmonary, septic, wound, or thromboembolic complications; and mortality.4,5
In order to prevent excessive blood loss during TKA and THA procedures, antifibrinolytic agents, such as tranexamic acid, have been used. Tranexamic acid is a synthetic form of lysine that binds to the lysine-binding sites on plasminogen and slows the conversion of plasminogen to plasmin. This interaction inhibits fibrinolysis and theoretically decreases bleeding.6 Because tranexamic acid is an antifibrinolytic, its mechanism of action has raised concerns that it could increase the risk of clotting complications, such as venous thromboembolism and myocardial infarction.7
Several published meta-analyses and systematic reviews have shown that tranexamic acid reduces blood loss in patients undergoing orthopedic surgery. Despite positive results, many study authors have acknowledged limitations in their analyses, such as heterogeneity of study results, small trial size, and varied dosing strategies.3,7-12 There is no FDA-approved tranexamic acid dosing strategy for orthopedic procedures; therefore, its use in TKA and THA procedures is off label. This lack of guidance results in the medication being used at varied doses, timing of doses, and routes of administration with no clear dosing strategy showing the best outcomes.
Tranexamic acid was first used in TKA and THA surgical procedures at the Sioux Falls VA Health Care System (SFVAHCS) in South Dakota in October 2012. The dose used during these procedures was 1 g IV at first surgical incision and 1 g IV at incision closure. The objective of this study was to determine whether this tranexamic acid dosing strategy utilized at SFVAHCS safely improved outcomes related to blood loss.
Methods
A single-center retrospective chart review was performed on all patients who underwent TKA and THA procedures by 4 orthopedic surgeons between January 2010 and August 2015 at SFVAHCS. This study received approval by the local institutional review board and research and development committee in September 2015.
Patients were included in the study if they were aged ≥ 18 years and underwent primary unilateral TKA or THA procedures during the study time frame. Patients were excluded if they underwent bilateral or revision TKA or THA procedures, did not have recorded blood loss measurements during and/or after the procedure, or did not receive tranexamic acid between October 2012 and August 2015 at the standard dosing strategy utilized at SFVAHCS.
Patients who underwent surgery between January 2010 and October 2012 and did not receive tranexamic acid were included in the control groups. The treatment groups contained patients who underwent surgery between October 2012 and August 2015 and received tranexamic acid at the standard SFVAHCS dosing strategy. Patients in the control and treatment groups were divided and compared with patients who underwent the same type of surgery.
The primary endpoint of this study was total blood loss, which included intraoperative and postoperative blood loss. Intraoperative blood loss was measured by a suctioning device that the surgical physician’s assistant used to keep the surgical site clear of bodily fluids. The suctioning device collected blood as well as irrigation fluids used throughout the surgical procedure. The volume of irrigation fluids used during the procedure was subtracted from the total volume collected by the suctioning device to estimate the total blood volume lost during surgery. Sponges and other surgical materials that may have collected blood were not included in the intraoperative blood loss calculation. Postoperative blood loss was collected by a drain that was placed in the surgical site prior to incision closure. The drain collected postoperative blood loss until it was removed 1 day after surgery.
The secondary endpoints for the study were changes in hemoglobin (Hgb) and hematocrit (Hct) from before surgery to after surgery. These changes were calculated by subtracting the lowest measured postoperative Hgb or Hct level within 21 days postsurgery from the closest measured Hgb or Hct level obtained presurgery.
Follow-up appointments were routinely conducted 2 weeks after surgery, so the 21-day time frame would include any laboratory results drawn at these appointments. Other secondary endpoints included the number of patients receiving at least 1 blood transfusion during hospitalization and the number of patients experiencing clotting complications within 30 days of surgery. Postoperative and progress notes were reviewed by a single study investigator in order to record blood transfusions and clotting complications.
All patients who underwent TKA or THA procedures were instructed to stop taking antiplatelet agents 7 days prior to surgery and warfarin 5 days prior to surgery. If patients were determined to be at high risk for thromboembolic complications following warfarin discontinuation, therapeutic doses of low-molecular weight heparin or unfractionated heparin were used as a bridging therapy pre- and postsurgery. Enoxaparin 30 mg twice daily was started the day after surgery in all patients not previously on warfarin therapy prior to surgery to prevent clotting complications. If a patient was on warfarin therapy prior to the procedure but not considered to be at high risk of thromboembolic complications by the surgeon, warfarin was restarted after surgery, and enoxaparin 30 mg twice daily was used until therapeutic international normalized ratio values were obtained. If the patient had ongoing bleeding after the procedure or was determined to be at high risk for bleeding complications, the provider may have delayed anticoagulant use.
Some patients who underwent TKA or THA procedures during the study time frame received periarticular pain injections during surgery. These pain injections included a combination of ropivacaine 200 mg, ketorolac 30 mg, epinephrine 0.5 mg, and clonidine 0.08 mg and were compounded in a sterile mixture with normal saline. Several injections of this mixture were administered into the surgical site to reduce postoperative pain. These periarticular pain injections were first implemented into TKA and THA procedures in August 2012 and were used in patients at the surgeon’s discretion.
Baseline characteristics were analyzed using a chi-square test for categoric variables and an unpaired t test for continuous variables to determine whether any differences were present. Total blood loss, change in Hgb, and change in Hct were analyzed using an unpaired t test. Patients receiving at least 1 blood transfusion during hospitalization and patients experiencing a clotting complication were analyzed using a chi-square test. P values < .05 were considered to indicate statistical significance. Descriptive statistics were calculated using Microsoft Excel (Redmond, WA), and GraphPad Prism (La Jolla, CA) was used for all statistical analyses.
Results
Initially, a total of 443 TKA patients and 111 THA patients were reviewed. Of these patients, 418 TKA patients and 100 THA patients met the inclusion criteria. Due to the retrospective design of this study, not all of the baseline characteristics were equal between groups (Table 1). Most notably, the number of patients who received the periarticular pain injection and the distribution of surgeons performing the procedures were different between groups in both the TKA and THA procedures.
Baseline Hgb levels were not found to be different between groups in either type of procedure; however, the baseline Hct levels of patients undergoing TKA who received tranexamic acid were found to be statistically higher when compared with those who did not receive tranexamic acid. Other baseline characteristics with statistically higher values included average weight and BMI in patients who received tranexamic acid and underwent THA and serum creatinine in patients who did not receive tranexamic acid and underwent TKA.
In the primary analysis (Tables 2 and 3), the mean estimated total blood loss in TKA patients was lower in patients who received tranexamic acid than it was in the control group (339.4 mL vs 457.4 mL, P < .001). Patients who underwent THA receiving tranexamic acid similarly had significantly less total blood loss than that of the control group (419.7 mL vs 585.7 mL, P < .001). Consistent with previous studies, patients undergoing TKA procedures in the treatment group when compared to the control group, respectively, were likely to have more blood loss postoperatively (275.9 mL vs 399.7 mL) than intraoperatively (63.5 mL vs 57.7 mL) regardless of tranexamic acid administration.6 On the other hand, patients who had undergone THA were more likely to experience more intraoperative blood loss (281.6 mL in treatment group vs 328.9 mL in control group) than postoperative blood loss (138.1 mL in treatment group vs 256.8 mL in control group) regardless of tranexamic acid administration.
In the secondary analysis, the change between preoperative and postoperative Hgb and Hct had results consistent with the total blood loss results. Patients receiving tranexamic acid in TKA procedures had a lower decrease in Hgb compared with the control group (3.3 mg/dL vs 4.0 mg/dL, P < .001). Similarly, patients undergoing TKA who received tranexamic acid had a smaller decrease in Hct than that of the control group (9.4% vs 11.1%, P < .001). Consistent with the TKA procedure results, patients undergoing THA who received tranexamic acid had a smaller decrease in Hgb (3.6 mg/dL vs 4.7 mg/dL, P < .001) and Hct (10.5% vs 13.0%, P = .0012) than that of the control group.
Patients who did not receive tranexamic acid were more likely to require at least 1 blood transfusion than were patients who received tranexamic acid in TKA (14 patients vs 0 patients, P = .0005) and THA procedures (8 patients vs 2 patients, P = .019). Despite the theoretically increased likelihood of clotting complications with tranexamic acid, no significant differences were observed between the treatment and control groups in either TKA (0 vs 4, P = .065) or THA (1 vs 0, P = .363) procedures.
Discussion
Patients undergoing total joint arthroplasty are at risk for significant blood loss with a potential need for postoperative blood transfusions.6,13 The use of tranexamic acid during these procedures offers a possible solution to decrease blood loss and minimize blood transfusions. This study retrospectively evaluated whether tranexamic acid safely decreased blood loss and the need for blood transfusions at SFVAHCS with the dosing strategy of 1 g IV at first incision and 1 g IV at incision closure.
Patients who received tranexamic acid in TKA and THA procedures had significantly less blood loss than did patients who did not receive tranexamic acid. Patients receiving tranexamic acid also had a significantly lower change from preoperative to postoperative Hgb and Hct levels than did patients who did not receive tranexamic acid in TKA and THA procedures. In addition to decreasing blood loss, the tranexamic acid groups had significantly fewer patients who required blood transfusions than that of the control groups.
This reduction in blood transfusions should be considered clinically significant.
Even though baseline Hct levels were found to be significantly different between the tranexamic acid group and the control group for patients who underwent TKA, medical record documentation indicated that the determination whether postoperative blood transfusions were needed was based primarily on Hgb levels. Baseline Hgb levels were found not to be significantly different between the tranexamic acid and control groups for either TKA or THA procedures. This suggests that at baseline the tranexamic acid and control groups had the same risk of reaching the Hgb threshold where blood transfusions would be required.
There was a significant difference in the proportion of patients receiving the periarticular pain injection of ropivacaine, ketorolac, epinephrine, and clonidine between the groups who received tranexamic acid and those who did not. Originally, this baseline characteristic was theorized to be a major confounder in the primary and secondary analyses because epinephrine is a vasoconstrictor and ketorolac is a reversible antiplatelet agent. However, Vendittoli and colleagues showed that patients receiving these periarticular pain injections during surgery actually had greater total blood loss than did patients who did not receive the injections, although this comparison did not reach statistical significance.14 The Vendittoli and colleagues results suggest that the pain injections did not confound the primary and secondary analyses by aiding in the process of reducing blood loss in these procedures.
Limitations
There are several limitations for extrapolating the results from this study to the general population. Due to the retrospective study design, there was no way to actively control potentially confounding variables during the TKA and THA procedures. Surgeons and surgery teams likely had slightly different techniques and protocols during and after surgery. Several baseline characteristics were not equal between patients who received tranexamic acid and those who did not. Therefore, it is unknown whether these baseline characteristics affected the results of this study. Postoperative anticoagulant use was not recorded and may have differed between study groups, depending on the patient’s risk of thromboembolic complications; however, the drains that collected blood loss were removed prior to the first dose of enoxaparin, which was administered the day after surgery.
Another limitation is that the method of measuring blood loss during and after the procedure was imprecise. Blood not suctioned through the suctioning device during surgery or not collected in the drain after surgery was not measured and may have increased the total blood loss. Hemoglobin and Hct levels also are sensitive to intravascular volume changes. If a patient required more IV fluids during or after a procedure, the fluids may have lowered the Hgb and/or Hct levels by dilution.
Conclusion
This study suggests that using tranexamic acid at a dose of 1 g IV at first incision and 1 g IV at incision closure safely and effectively reduced blood loss and the need for transfusions in patients undergoing TKA and THA procedures at SFVAHCS. Further prospective studies are needed to compare different tranexamic dosing strategies to minimize blood loss during these procedures. ˜
Acknowledgments
This study is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System in South Dakota.
The number of patients who undergo total knee arthroplasty (TKA) and total hip arthroplasty (THA) procedures has significantly increased over the past 2 to 3 decades. As life expectancy in the U.S. increases and medical advances allow patients with preexisting conditions to successfully undergo joint replacements, the demand for these procedures is expected to grow.1 In 2010, the CDC estimated that 719,000 patients underwent TKA procedures and 332,000 patients underwent THA procedures in the U.S.2 Kurtz and colleagues have projected that by 2030 the annual number of TKA procedures will increase to 3.5 million and the number of THA procedures will increase to 572,000.1
Although becoming more prevalent, these procedures are still associated with considerable intra- and postoperative blood loss that may lead to complications and blood transfusions.3 Previous studies have shown that perioperative anemia and red blood cell transfusions are associated with negative outcomes, including increased health care resource utilization; length of hospitalization; pulmonary, septic, wound, or thromboembolic complications; and mortality.4,5
In order to prevent excessive blood loss during TKA and THA procedures, antifibrinolytic agents, such as tranexamic acid, have been used. Tranexamic acid is a synthetic form of lysine that binds to the lysine-binding sites on plasminogen and slows the conversion of plasminogen to plasmin. This interaction inhibits fibrinolysis and theoretically decreases bleeding.6 Because tranexamic acid is an antifibrinolytic, its mechanism of action has raised concerns that it could increase the risk of clotting complications, such as venous thromboembolism and myocardial infarction.7
Several published meta-analyses and systematic reviews have shown that tranexamic acid reduces blood loss in patients undergoing orthopedic surgery. Despite positive results, many study authors have acknowledged limitations in their analyses, such as heterogeneity of study results, small trial size, and varied dosing strategies.3,7-12 There is no FDA-approved tranexamic acid dosing strategy for orthopedic procedures; therefore, its use in TKA and THA procedures is off label. This lack of guidance results in the medication being used at varied doses, timing of doses, and routes of administration with no clear dosing strategy showing the best outcomes.
Tranexamic acid was first used in TKA and THA surgical procedures at the Sioux Falls VA Health Care System (SFVAHCS) in South Dakota in October 2012. The dose used during these procedures was 1 g IV at first surgical incision and 1 g IV at incision closure. The objective of this study was to determine whether this tranexamic acid dosing strategy utilized at SFVAHCS safely improved outcomes related to blood loss.
Methods
A single-center retrospective chart review was performed on all patients who underwent TKA and THA procedures by 4 orthopedic surgeons between January 2010 and August 2015 at SFVAHCS. This study received approval by the local institutional review board and research and development committee in September 2015.
Patients were included in the study if they were aged ≥ 18 years and underwent primary unilateral TKA or THA procedures during the study time frame. Patients were excluded if they underwent bilateral or revision TKA or THA procedures, did not have recorded blood loss measurements during and/or after the procedure, or did not receive tranexamic acid between October 2012 and August 2015 at the standard dosing strategy utilized at SFVAHCS.
Patients who underwent surgery between January 2010 and October 2012 and did not receive tranexamic acid were included in the control groups. The treatment groups contained patients who underwent surgery between October 2012 and August 2015 and received tranexamic acid at the standard SFVAHCS dosing strategy. Patients in the control and treatment groups were divided and compared with patients who underwent the same type of surgery.
The primary endpoint of this study was total blood loss, which included intraoperative and postoperative blood loss. Intraoperative blood loss was measured by a suctioning device that the surgical physician’s assistant used to keep the surgical site clear of bodily fluids. The suctioning device collected blood as well as irrigation fluids used throughout the surgical procedure. The volume of irrigation fluids used during the procedure was subtracted from the total volume collected by the suctioning device to estimate the total blood volume lost during surgery. Sponges and other surgical materials that may have collected blood were not included in the intraoperative blood loss calculation. Postoperative blood loss was collected by a drain that was placed in the surgical site prior to incision closure. The drain collected postoperative blood loss until it was removed 1 day after surgery.
The secondary endpoints for the study were changes in hemoglobin (Hgb) and hematocrit (Hct) from before surgery to after surgery. These changes were calculated by subtracting the lowest measured postoperative Hgb or Hct level within 21 days postsurgery from the closest measured Hgb or Hct level obtained presurgery.
Follow-up appointments were routinely conducted 2 weeks after surgery, so the 21-day time frame would include any laboratory results drawn at these appointments. Other secondary endpoints included the number of patients receiving at least 1 blood transfusion during hospitalization and the number of patients experiencing clotting complications within 30 days of surgery. Postoperative and progress notes were reviewed by a single study investigator in order to record blood transfusions and clotting complications.
All patients who underwent TKA or THA procedures were instructed to stop taking antiplatelet agents 7 days prior to surgery and warfarin 5 days prior to surgery. If patients were determined to be at high risk for thromboembolic complications following warfarin discontinuation, therapeutic doses of low-molecular weight heparin or unfractionated heparin were used as a bridging therapy pre- and postsurgery. Enoxaparin 30 mg twice daily was started the day after surgery in all patients not previously on warfarin therapy prior to surgery to prevent clotting complications. If a patient was on warfarin therapy prior to the procedure but not considered to be at high risk of thromboembolic complications by the surgeon, warfarin was restarted after surgery, and enoxaparin 30 mg twice daily was used until therapeutic international normalized ratio values were obtained. If the patient had ongoing bleeding after the procedure or was determined to be at high risk for bleeding complications, the provider may have delayed anticoagulant use.
Some patients who underwent TKA or THA procedures during the study time frame received periarticular pain injections during surgery. These pain injections included a combination of ropivacaine 200 mg, ketorolac 30 mg, epinephrine 0.5 mg, and clonidine 0.08 mg and were compounded in a sterile mixture with normal saline. Several injections of this mixture were administered into the surgical site to reduce postoperative pain. These periarticular pain injections were first implemented into TKA and THA procedures in August 2012 and were used in patients at the surgeon’s discretion.
Baseline characteristics were analyzed using a chi-square test for categoric variables and an unpaired t test for continuous variables to determine whether any differences were present. Total blood loss, change in Hgb, and change in Hct were analyzed using an unpaired t test. Patients receiving at least 1 blood transfusion during hospitalization and patients experiencing a clotting complication were analyzed using a chi-square test. P values < .05 were considered to indicate statistical significance. Descriptive statistics were calculated using Microsoft Excel (Redmond, WA), and GraphPad Prism (La Jolla, CA) was used for all statistical analyses.
Results
Initially, a total of 443 TKA patients and 111 THA patients were reviewed. Of these patients, 418 TKA patients and 100 THA patients met the inclusion criteria. Due to the retrospective design of this study, not all of the baseline characteristics were equal between groups (Table 1). Most notably, the number of patients who received the periarticular pain injection and the distribution of surgeons performing the procedures were different between groups in both the TKA and THA procedures.
Baseline Hgb levels were not found to be different between groups in either type of procedure; however, the baseline Hct levels of patients undergoing TKA who received tranexamic acid were found to be statistically higher when compared with those who did not receive tranexamic acid. Other baseline characteristics with statistically higher values included average weight and BMI in patients who received tranexamic acid and underwent THA and serum creatinine in patients who did not receive tranexamic acid and underwent TKA.
In the primary analysis (Tables 2 and 3), the mean estimated total blood loss in TKA patients was lower in patients who received tranexamic acid than it was in the control group (339.4 mL vs 457.4 mL, P < .001). Patients who underwent THA receiving tranexamic acid similarly had significantly less total blood loss than that of the control group (419.7 mL vs 585.7 mL, P < .001). Consistent with previous studies, patients undergoing TKA procedures in the treatment group when compared to the control group, respectively, were likely to have more blood loss postoperatively (275.9 mL vs 399.7 mL) than intraoperatively (63.5 mL vs 57.7 mL) regardless of tranexamic acid administration.6 On the other hand, patients who had undergone THA were more likely to experience more intraoperative blood loss (281.6 mL in treatment group vs 328.9 mL in control group) than postoperative blood loss (138.1 mL in treatment group vs 256.8 mL in control group) regardless of tranexamic acid administration.
In the secondary analysis, the change between preoperative and postoperative Hgb and Hct had results consistent with the total blood loss results. Patients receiving tranexamic acid in TKA procedures had a lower decrease in Hgb compared with the control group (3.3 mg/dL vs 4.0 mg/dL, P < .001). Similarly, patients undergoing TKA who received tranexamic acid had a smaller decrease in Hct than that of the control group (9.4% vs 11.1%, P < .001). Consistent with the TKA procedure results, patients undergoing THA who received tranexamic acid had a smaller decrease in Hgb (3.6 mg/dL vs 4.7 mg/dL, P < .001) and Hct (10.5% vs 13.0%, P = .0012) than that of the control group.
Patients who did not receive tranexamic acid were more likely to require at least 1 blood transfusion than were patients who received tranexamic acid in TKA (14 patients vs 0 patients, P = .0005) and THA procedures (8 patients vs 2 patients, P = .019). Despite the theoretically increased likelihood of clotting complications with tranexamic acid, no significant differences were observed between the treatment and control groups in either TKA (0 vs 4, P = .065) or THA (1 vs 0, P = .363) procedures.
Discussion
Patients undergoing total joint arthroplasty are at risk for significant blood loss with a potential need for postoperative blood transfusions.6,13 The use of tranexamic acid during these procedures offers a possible solution to decrease blood loss and minimize blood transfusions. This study retrospectively evaluated whether tranexamic acid safely decreased blood loss and the need for blood transfusions at SFVAHCS with the dosing strategy of 1 g IV at first incision and 1 g IV at incision closure.
Patients who received tranexamic acid in TKA and THA procedures had significantly less blood loss than did patients who did not receive tranexamic acid. Patients receiving tranexamic acid also had a significantly lower change from preoperative to postoperative Hgb and Hct levels than did patients who did not receive tranexamic acid in TKA and THA procedures. In addition to decreasing blood loss, the tranexamic acid groups had significantly fewer patients who required blood transfusions than that of the control groups.
This reduction in blood transfusions should be considered clinically significant.
Even though baseline Hct levels were found to be significantly different between the tranexamic acid group and the control group for patients who underwent TKA, medical record documentation indicated that the determination whether postoperative blood transfusions were needed was based primarily on Hgb levels. Baseline Hgb levels were found not to be significantly different between the tranexamic acid and control groups for either TKA or THA procedures. This suggests that at baseline the tranexamic acid and control groups had the same risk of reaching the Hgb threshold where blood transfusions would be required.
There was a significant difference in the proportion of patients receiving the periarticular pain injection of ropivacaine, ketorolac, epinephrine, and clonidine between the groups who received tranexamic acid and those who did not. Originally, this baseline characteristic was theorized to be a major confounder in the primary and secondary analyses because epinephrine is a vasoconstrictor and ketorolac is a reversible antiplatelet agent. However, Vendittoli and colleagues showed that patients receiving these periarticular pain injections during surgery actually had greater total blood loss than did patients who did not receive the injections, although this comparison did not reach statistical significance.14 The Vendittoli and colleagues results suggest that the pain injections did not confound the primary and secondary analyses by aiding in the process of reducing blood loss in these procedures.
Limitations
There are several limitations for extrapolating the results from this study to the general population. Due to the retrospective study design, there was no way to actively control potentially confounding variables during the TKA and THA procedures. Surgeons and surgery teams likely had slightly different techniques and protocols during and after surgery. Several baseline characteristics were not equal between patients who received tranexamic acid and those who did not. Therefore, it is unknown whether these baseline characteristics affected the results of this study. Postoperative anticoagulant use was not recorded and may have differed between study groups, depending on the patient’s risk of thromboembolic complications; however, the drains that collected blood loss were removed prior to the first dose of enoxaparin, which was administered the day after surgery.
Another limitation is that the method of measuring blood loss during and after the procedure was imprecise. Blood not suctioned through the suctioning device during surgery or not collected in the drain after surgery was not measured and may have increased the total blood loss. Hemoglobin and Hct levels also are sensitive to intravascular volume changes. If a patient required more IV fluids during or after a procedure, the fluids may have lowered the Hgb and/or Hct levels by dilution.
Conclusion
This study suggests that using tranexamic acid at a dose of 1 g IV at first incision and 1 g IV at incision closure safely and effectively reduced blood loss and the need for transfusions in patients undergoing TKA and THA procedures at SFVAHCS. Further prospective studies are needed to compare different tranexamic dosing strategies to minimize blood loss during these procedures. ˜
Acknowledgments
This study is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System in South Dakota.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Published 2010. Accessed March 15, 2017.
3. Wei Z, Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med. 2015;25(3):151-162.
4. Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114(2):283-292.
5. Wu WC, Smith TS, Henderson WG, et al. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg. 2010;252(1):283-292.
6. Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86(4):561-565.
7. Gandhi R, Evans HMK, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184.
8. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
9. Yang ZG, Chen WP, Wu LE. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
10. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
11. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
12. Zhou XD, Tao LJ, Li J, Wu LD. Do we really need tranexamic acid in total hip arthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Surg. 2013;133(7):1017-1027.
13. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
14. Vendittoli PA, Makinen P, Drolet P, et al. A multimodal analgesia protocol for total knee arthroplasty: a randomized, controlled study. J Bone Joint Surg Am. 2006;88(2):282-289.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Published 2010. Accessed March 15, 2017.
3. Wei Z, Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med. 2015;25(3):151-162.
4. Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114(2):283-292.
5. Wu WC, Smith TS, Henderson WG, et al. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg. 2010;252(1):283-292.
6. Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86(4):561-565.
7. Gandhi R, Evans HMK, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184.
8. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
9. Yang ZG, Chen WP, Wu LE. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
10. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
11. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
12. Zhou XD, Tao LJ, Li J, Wu LD. Do we really need tranexamic acid in total hip arthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Surg. 2013;133(7):1017-1027.
13. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
14. Vendittoli PA, Makinen P, Drolet P, et al. A multimodal analgesia protocol for total knee arthroplasty: a randomized, controlled study. J Bone Joint Surg Am. 2006;88(2):282-289.
Barbara Jobst, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: NPs, PAs weigh common issues in hospitalist practice
Practicing at the top of your license, billing and reimbursement, recruiting and orientation – Those were some of the hot topics discussed by more than 50 attendees of HM17’s Special Interest Forum for nurse practitioners (NPs) and physician assistants (PAs).
“Every year, we are seeing more and more HM groups integrating NPs and PAs into their practice,” said forum moderator Emilie Thornhill, PA-C, a certified PA, who works for Oschner Health in New Orleans, La.
Ms. Thornhill emphasized that a common issue among attendees is restrictive HM policies in dictating the scope of practice for NP/PAs in hospitalist groups.
“That seems to be the thing that is holding us back the most,” she said. “SHM is really going to be the home for these individuals to find the resources they need to address these issues.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Practicing at the top of your license, billing and reimbursement, recruiting and orientation – Those were some of the hot topics discussed by more than 50 attendees of HM17’s Special Interest Forum for nurse practitioners (NPs) and physician assistants (PAs).
“Every year, we are seeing more and more HM groups integrating NPs and PAs into their practice,” said forum moderator Emilie Thornhill, PA-C, a certified PA, who works for Oschner Health in New Orleans, La.
Ms. Thornhill emphasized that a common issue among attendees is restrictive HM policies in dictating the scope of practice for NP/PAs in hospitalist groups.
“That seems to be the thing that is holding us back the most,” she said. “SHM is really going to be the home for these individuals to find the resources they need to address these issues.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Practicing at the top of your license, billing and reimbursement, recruiting and orientation – Those were some of the hot topics discussed by more than 50 attendees of HM17’s Special Interest Forum for nurse practitioners (NPs) and physician assistants (PAs).
“Every year, we are seeing more and more HM groups integrating NPs and PAs into their practice,” said forum moderator Emilie Thornhill, PA-C, a certified PA, who works for Oschner Health in New Orleans, La.
Ms. Thornhill emphasized that a common issue among attendees is restrictive HM policies in dictating the scope of practice for NP/PAs in hospitalist groups.
“That seems to be the thing that is holding us back the most,” she said. “SHM is really going to be the home for these individuals to find the resources they need to address these issues.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Connect: Community hospitalists brainstorm ways to be stronger as a group
Coping with disjointed administrative goals, demonstrating value to hospital leadership, and strengthening support networks for one another were hot-button topics during the Special Interest Group for Community Hospitalists at this year’s HM17.
A mix of hospitalists from rural, urban, and suburban facilities with an average 200-500 beds joined in the discussion, moderated by Stephen Behnke, MD, an internist and president of MedOne in Columbus, Ohio, and Jason Robertson, MD, an internist with HealthPartners in Bloomington, Minn.
Burnout was seen by several in the crowd of about two dozen physicians as being related in part to poor staffing and scheduling decisions at the administrative level, and not allocating clerical work to other staff, often forcing hospitalists to perform tasks not at the top of their license. One solution offered was to amortize the cost of physicians doing paperwork according to their salaries, and to bring those numbers to the attention of hospital leadership.
The group called on the Society of Hospital Medicine to create and disseminate evidence-based resources to help demonstrate their value to hospital administration. Many in the group expressed interest in learning how to communicate their value effectively to their respective C-suites to underscore the essential nature HM has to the core business. In an interview directly after the session, Dr. Behnke explained that hospital leaders often underfund HM programs, only to find that the decision ends up costing them more in the long run.
Lots of upset was vented by session attendees over patient discharge protocols that often resulted in higher lengths of stay or increased readmissions, which then reflected poorly on the hospitalist. The group agreed that since there was no one-size-fits-all approach to this, it would be helpful to start a listserv of community hospitalists in the SHM that was organized by hospital size, location, and types of staffing, so it would be easier to find solutions by connecting with others with similar concerns.
Many in the group also shared how their respective facilities promoted wellness through togetherness activities: staff retreats, movie nights, book clubs, group family outings, and forming alliances with hospitalists at other local hospitals. The general consensus was that this helped improve staff morale.
Coping with disjointed administrative goals, demonstrating value to hospital leadership, and strengthening support networks for one another were hot-button topics during the Special Interest Group for Community Hospitalists at this year’s HM17.
A mix of hospitalists from rural, urban, and suburban facilities with an average 200-500 beds joined in the discussion, moderated by Stephen Behnke, MD, an internist and president of MedOne in Columbus, Ohio, and Jason Robertson, MD, an internist with HealthPartners in Bloomington, Minn.
Burnout was seen by several in the crowd of about two dozen physicians as being related in part to poor staffing and scheduling decisions at the administrative level, and not allocating clerical work to other staff, often forcing hospitalists to perform tasks not at the top of their license. One solution offered was to amortize the cost of physicians doing paperwork according to their salaries, and to bring those numbers to the attention of hospital leadership.
The group called on the Society of Hospital Medicine to create and disseminate evidence-based resources to help demonstrate their value to hospital administration. Many in the group expressed interest in learning how to communicate their value effectively to their respective C-suites to underscore the essential nature HM has to the core business. In an interview directly after the session, Dr. Behnke explained that hospital leaders often underfund HM programs, only to find that the decision ends up costing them more in the long run.
Lots of upset was vented by session attendees over patient discharge protocols that often resulted in higher lengths of stay or increased readmissions, which then reflected poorly on the hospitalist. The group agreed that since there was no one-size-fits-all approach to this, it would be helpful to start a listserv of community hospitalists in the SHM that was organized by hospital size, location, and types of staffing, so it would be easier to find solutions by connecting with others with similar concerns.
Many in the group also shared how their respective facilities promoted wellness through togetherness activities: staff retreats, movie nights, book clubs, group family outings, and forming alliances with hospitalists at other local hospitals. The general consensus was that this helped improve staff morale.
Coping with disjointed administrative goals, demonstrating value to hospital leadership, and strengthening support networks for one another were hot-button topics during the Special Interest Group for Community Hospitalists at this year’s HM17.
A mix of hospitalists from rural, urban, and suburban facilities with an average 200-500 beds joined in the discussion, moderated by Stephen Behnke, MD, an internist and president of MedOne in Columbus, Ohio, and Jason Robertson, MD, an internist with HealthPartners in Bloomington, Minn.
Burnout was seen by several in the crowd of about two dozen physicians as being related in part to poor staffing and scheduling decisions at the administrative level, and not allocating clerical work to other staff, often forcing hospitalists to perform tasks not at the top of their license. One solution offered was to amortize the cost of physicians doing paperwork according to their salaries, and to bring those numbers to the attention of hospital leadership.
The group called on the Society of Hospital Medicine to create and disseminate evidence-based resources to help demonstrate their value to hospital administration. Many in the group expressed interest in learning how to communicate their value effectively to their respective C-suites to underscore the essential nature HM has to the core business. In an interview directly after the session, Dr. Behnke explained that hospital leaders often underfund HM programs, only to find that the decision ends up costing them more in the long run.
Lots of upset was vented by session attendees over patient discharge protocols that often resulted in higher lengths of stay or increased readmissions, which then reflected poorly on the hospitalist. The group agreed that since there was no one-size-fits-all approach to this, it would be helpful to start a listserv of community hospitalists in the SHM that was organized by hospital size, location, and types of staffing, so it would be easier to find solutions by connecting with others with similar concerns.
Many in the group also shared how their respective facilities promoted wellness through togetherness activities: staff retreats, movie nights, book clubs, group family outings, and forming alliances with hospitalists at other local hospitals. The general consensus was that this helped improve staff morale.
Handheld Reflectance Confocal Microscopy to Aid in the Management of Complex Facial Lentigo Maligna
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).
Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.
Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.
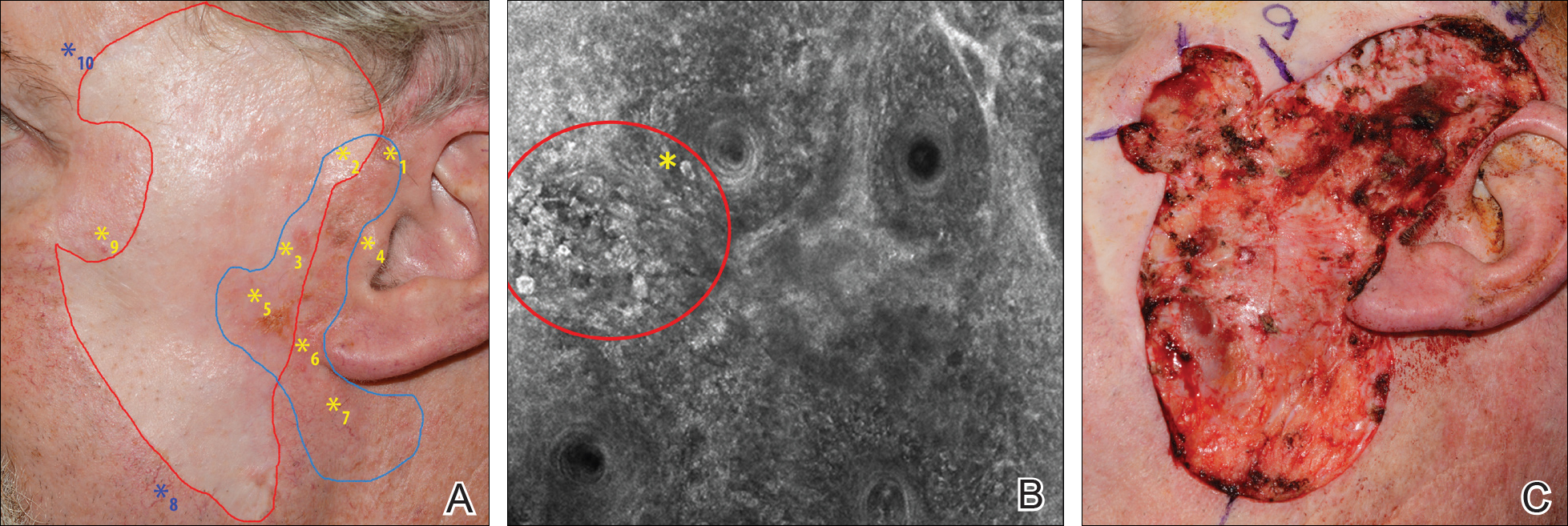
Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).

Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.

Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.

Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).

Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.

Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.

Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
Practice Points
- Diagnosis and management of lentigo maligna (LM) and LM melanoma (LMM) is challenging due to their ill-defined margins and location mainly on the head and neck.
- Handheld reflectance confocal microscopy (RCM) has high diagnostic accuracy for LM/LMM and can be used in curved locations to assess large lesions.
- Handheld RCM can be a versatile tool in pretreatment decision-making, intraoperative surgical mapping, and posttreatment monitoring of both surgical and nonsurgical therapies for complex facial LM/LMM.