User login
David Henry's JCSO podcast, March-April 2017
For the March-April issue of the Journal of Community and Supportive Oncology, the Editor in Chief, Dr David Henry, discusses two informative “how-to” articles, one on the implementation of a distress management program at an oncology hospital in Puerto Rico, the other on the prevention and treatment options for mTOR inhibitor-associated stomatitis. Dr Henry also shares his preferences for addressing health care reform, and he highlights a letter to the journal in response to the January-February issue Commentary on physician-assisted dying. Immunotherapies are at the fore again, this time with an insightful essay by Jane de Lartigue who writes that combination therapy is likely to be key in expanding the scope of immunotherapy into currently unresponsive patient populations, which raises questions about the optimal combinations and the timing and sequencing of combination immunotherapy. Three Original Reports span the clinical, supportive, and quality- and value-based care components of cancer care, with their respective foci on APF530 for nausea and vomiting prevention after cisplatin; patterns of care in whole-brain radiotherapy technique and delivery; and emergency department use by newly diagnosed cancer patients. As usual, there is a line-up of rare and challenging presentations in Case Reports on pulmonary sarcomatoid carcinoma presenting as a necrotizing cavitary lung lesions, palmoplantar exacerbation of psoriasis after nivolumab for lung cancer, primary cardiac prosthetic valve-associated lymphoma; and atraumatic splenic rupture as an initial presentation of chronic myelogenous leukemia.
Listen to the podcast below.
For the March-April issue of the Journal of Community and Supportive Oncology, the Editor in Chief, Dr David Henry, discusses two informative “how-to” articles, one on the implementation of a distress management program at an oncology hospital in Puerto Rico, the other on the prevention and treatment options for mTOR inhibitor-associated stomatitis. Dr Henry also shares his preferences for addressing health care reform, and he highlights a letter to the journal in response to the January-February issue Commentary on physician-assisted dying. Immunotherapies are at the fore again, this time with an insightful essay by Jane de Lartigue who writes that combination therapy is likely to be key in expanding the scope of immunotherapy into currently unresponsive patient populations, which raises questions about the optimal combinations and the timing and sequencing of combination immunotherapy. Three Original Reports span the clinical, supportive, and quality- and value-based care components of cancer care, with their respective foci on APF530 for nausea and vomiting prevention after cisplatin; patterns of care in whole-brain radiotherapy technique and delivery; and emergency department use by newly diagnosed cancer patients. As usual, there is a line-up of rare and challenging presentations in Case Reports on pulmonary sarcomatoid carcinoma presenting as a necrotizing cavitary lung lesions, palmoplantar exacerbation of psoriasis after nivolumab for lung cancer, primary cardiac prosthetic valve-associated lymphoma; and atraumatic splenic rupture as an initial presentation of chronic myelogenous leukemia.
Listen to the podcast below.
For the March-April issue of the Journal of Community and Supportive Oncology, the Editor in Chief, Dr David Henry, discusses two informative “how-to” articles, one on the implementation of a distress management program at an oncology hospital in Puerto Rico, the other on the prevention and treatment options for mTOR inhibitor-associated stomatitis. Dr Henry also shares his preferences for addressing health care reform, and he highlights a letter to the journal in response to the January-February issue Commentary on physician-assisted dying. Immunotherapies are at the fore again, this time with an insightful essay by Jane de Lartigue who writes that combination therapy is likely to be key in expanding the scope of immunotherapy into currently unresponsive patient populations, which raises questions about the optimal combinations and the timing and sequencing of combination immunotherapy. Three Original Reports span the clinical, supportive, and quality- and value-based care components of cancer care, with their respective foci on APF530 for nausea and vomiting prevention after cisplatin; patterns of care in whole-brain radiotherapy technique and delivery; and emergency department use by newly diagnosed cancer patients. As usual, there is a line-up of rare and challenging presentations in Case Reports on pulmonary sarcomatoid carcinoma presenting as a necrotizing cavitary lung lesions, palmoplantar exacerbation of psoriasis after nivolumab for lung cancer, primary cardiac prosthetic valve-associated lymphoma; and atraumatic splenic rupture as an initial presentation of chronic myelogenous leukemia.
Listen to the podcast below.
Increased IUD use suggests ‘Trump effect’
Patient visits for IUD prescriptions and insertions were up 21% between October 2016 and January 2017, compared with those in the same period a year earlier, according to athenahealth, a provider of EHR systems and point-of-care mobile applications.
The rise in IUD-related visits since the presidential election – the first time in 5 years that the volume of visits for IUD procedures and follow-ups has increased in both November and December – suggests a “Trump effect.” As the replacement of the Affordable Care Act is debated, “uncertainly swirls over whether any new law would mandate free insurance coverage without copays for birth control and contraception devices,” athenahealth said.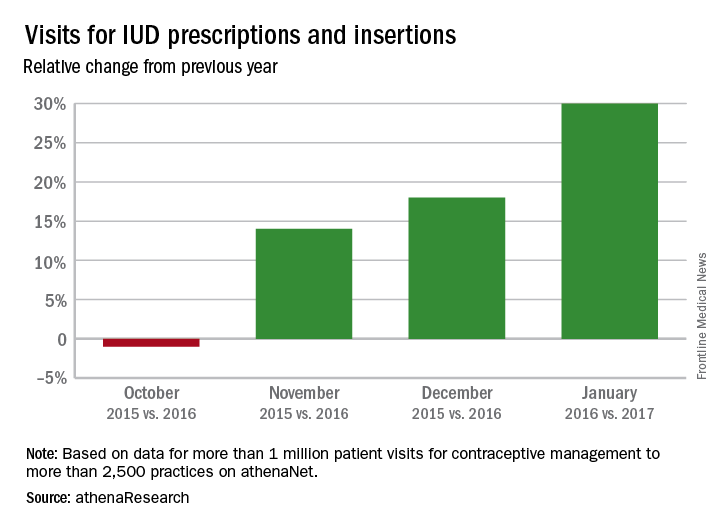
The athenahealth data include more than 1 million visits for IUD management to more than 2,500 practices using the company’s EHRs.
Patient visits for IUD prescriptions and insertions were up 21% between October 2016 and January 2017, compared with those in the same period a year earlier, according to athenahealth, a provider of EHR systems and point-of-care mobile applications.
The rise in IUD-related visits since the presidential election – the first time in 5 years that the volume of visits for IUD procedures and follow-ups has increased in both November and December – suggests a “Trump effect.” As the replacement of the Affordable Care Act is debated, “uncertainly swirls over whether any new law would mandate free insurance coverage without copays for birth control and contraception devices,” athenahealth said.
The athenahealth data include more than 1 million visits for IUD management to more than 2,500 practices using the company’s EHRs.
Patient visits for IUD prescriptions and insertions were up 21% between October 2016 and January 2017, compared with those in the same period a year earlier, according to athenahealth, a provider of EHR systems and point-of-care mobile applications.
The rise in IUD-related visits since the presidential election – the first time in 5 years that the volume of visits for IUD procedures and follow-ups has increased in both November and December – suggests a “Trump effect.” As the replacement of the Affordable Care Act is debated, “uncertainly swirls over whether any new law would mandate free insurance coverage without copays for birth control and contraception devices,” athenahealth said.
The athenahealth data include more than 1 million visits for IUD management to more than 2,500 practices using the company’s EHRs.
VIDEO: Striking improvement in PFS with olaparib for ovarian cancer
NATIONAL HARBOR, MD. – Maintenance therapy with the first-in-class PARP inhibitor olaparib was associated with a striking improvement in progression-free survival in patients with platinum-sensitive relapsed ovarian cancer and BRCA 1/2 mutation in the randomized, placebo-controlled phase III SOLO2 trial.
Compared with placebo, the tablet formulation of olaparib was associated with investigator-assessed PFS of 19.1 months in 196 patients, compared with 5.5 months in 99 patients who received placebo (hazard ratio, 0.30), Dr. Eric Pujade-Lauraine reported at the annual meeting of the Society of Gynecologic Oncology.
The SOLO2 results were both clinically meaningful and highly statistically significant, said Dr. Pujade-Lauraine of Hopital Hotel-Dieu, Paris.
Active treatment, which involved a twice-daily 300-mg oral dose of olaparib, was well tolerated; 75% of patients completed the study without dose reduction, he noted.
In this video interview, Dr. Pujade-Lauraine discussed the SOLO2 study and findings, which confirmed those of the phase II Study 19. Study 19 looked at olaparib in all-comers with platinum-sensitive relapsed ovarian cancer and involved a different formulation of the drug, which required that patients take 16 capsules each day to achieve a twice-daily dose of 400 mg. Patients with BRCA mutations were found in that study to derive the most benefit from olaparib.
The current findings are practice changing, Dr. Pujade-Lauraine said, concluding that “it is important to test BRCA, and if this test is positive, to offer olaparib in patients who are platinum sensitive.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NATIONAL HARBOR, MD. – Maintenance therapy with the first-in-class PARP inhibitor olaparib was associated with a striking improvement in progression-free survival in patients with platinum-sensitive relapsed ovarian cancer and BRCA 1/2 mutation in the randomized, placebo-controlled phase III SOLO2 trial.
Compared with placebo, the tablet formulation of olaparib was associated with investigator-assessed PFS of 19.1 months in 196 patients, compared with 5.5 months in 99 patients who received placebo (hazard ratio, 0.30), Dr. Eric Pujade-Lauraine reported at the annual meeting of the Society of Gynecologic Oncology.
The SOLO2 results were both clinically meaningful and highly statistically significant, said Dr. Pujade-Lauraine of Hopital Hotel-Dieu, Paris.
Active treatment, which involved a twice-daily 300-mg oral dose of olaparib, was well tolerated; 75% of patients completed the study without dose reduction, he noted.
In this video interview, Dr. Pujade-Lauraine discussed the SOLO2 study and findings, which confirmed those of the phase II Study 19. Study 19 looked at olaparib in all-comers with platinum-sensitive relapsed ovarian cancer and involved a different formulation of the drug, which required that patients take 16 capsules each day to achieve a twice-daily dose of 400 mg. Patients with BRCA mutations were found in that study to derive the most benefit from olaparib.
The current findings are practice changing, Dr. Pujade-Lauraine said, concluding that “it is important to test BRCA, and if this test is positive, to offer olaparib in patients who are platinum sensitive.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NATIONAL HARBOR, MD. – Maintenance therapy with the first-in-class PARP inhibitor olaparib was associated with a striking improvement in progression-free survival in patients with platinum-sensitive relapsed ovarian cancer and BRCA 1/2 mutation in the randomized, placebo-controlled phase III SOLO2 trial.
Compared with placebo, the tablet formulation of olaparib was associated with investigator-assessed PFS of 19.1 months in 196 patients, compared with 5.5 months in 99 patients who received placebo (hazard ratio, 0.30), Dr. Eric Pujade-Lauraine reported at the annual meeting of the Society of Gynecologic Oncology.
The SOLO2 results were both clinically meaningful and highly statistically significant, said Dr. Pujade-Lauraine of Hopital Hotel-Dieu, Paris.
Active treatment, which involved a twice-daily 300-mg oral dose of olaparib, was well tolerated; 75% of patients completed the study without dose reduction, he noted.
In this video interview, Dr. Pujade-Lauraine discussed the SOLO2 study and findings, which confirmed those of the phase II Study 19. Study 19 looked at olaparib in all-comers with platinum-sensitive relapsed ovarian cancer and involved a different formulation of the drug, which required that patients take 16 capsules each day to achieve a twice-daily dose of 400 mg. Patients with BRCA mutations were found in that study to derive the most benefit from olaparib.
The current findings are practice changing, Dr. Pujade-Lauraine said, concluding that “it is important to test BRCA, and if this test is positive, to offer olaparib in patients who are platinum sensitive.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ANNUAL MEETING ON WOMEN'S CANCER
VIDEO: Vaginal brachytherapy linked with better survival in rare uterine cancer
NATIONAL HARBOR, MD. – A large retrospective database study linked the use of vaginal brachytherapy and chemotherapy with improved survival among patients with early-stage uterine papillary serous carcinoma.
The findings offer a degree of clinical guidance on the adjuvant treatment of this relatively rare, aggressive histologic cancer subtype, Stephanie Cham, MD, said during a video interview at the annual meeting of the Society of Gynecologic Oncology.
Large dataset analyses can be especially helpful for exploring the treatment of rare diseases for which clinical trials can be infeasible, said Dr. Cham of Columbia University College of Physicians and Surgeons in New York. To evaluate chemotherapy, vaginal brachytherapy, and whole beam pelvic radiation therapy in stage I and stage II uterine papillary serous carcinoma, she and her associates analyzed the National Cancer Database.
Among 7,325 patients treated between 1998 and 2012, 38% of patients had received chemotherapy, 18% had received external beam radiation, and 20% received brachytherapy, Dr. Cham said. The use of chemotherapy rose significantly over time, regardless of stage (P less than .0001), as did the use of brachytherapy, while the use of external beam radiation decreased.
After the researchers controlled for numerous demographic and clinical variables, chemotherapy was associated with a statistically significant decrease in the risk of death overall (hazard ratio, 0.78; 95% confidence interval, 0.69 to 0.88) and in patients with stage IB (HR, 0.58; 95% CI, 0.44-0.77) or stage II cancer (HR, 0.74; 95% CI, 0.60-0.90). The use of brachytherapy also was associated with significantly improved survival overall (HR, 0.67), in stage IA cancer (HR, 0.67), and in stage II cancer (HR, 0.64).
The survival effect of brachytherapy held up in additional subgroup analyses, Dr. Cham explained. In contrast, external beam radiation therapy was not associated with improved survival overall or in any subgroup, she said. The results highlight the potential use of vaginal brachytherapy in early-stage uterine papillary serous carcinoma, she emphasized.
Dr. Cham cited no funding sources and reported having no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NATIONAL HARBOR, MD. – A large retrospective database study linked the use of vaginal brachytherapy and chemotherapy with improved survival among patients with early-stage uterine papillary serous carcinoma.
The findings offer a degree of clinical guidance on the adjuvant treatment of this relatively rare, aggressive histologic cancer subtype, Stephanie Cham, MD, said during a video interview at the annual meeting of the Society of Gynecologic Oncology.
Large dataset analyses can be especially helpful for exploring the treatment of rare diseases for which clinical trials can be infeasible, said Dr. Cham of Columbia University College of Physicians and Surgeons in New York. To evaluate chemotherapy, vaginal brachytherapy, and whole beam pelvic radiation therapy in stage I and stage II uterine papillary serous carcinoma, she and her associates analyzed the National Cancer Database.
Among 7,325 patients treated between 1998 and 2012, 38% of patients had received chemotherapy, 18% had received external beam radiation, and 20% received brachytherapy, Dr. Cham said. The use of chemotherapy rose significantly over time, regardless of stage (P less than .0001), as did the use of brachytherapy, while the use of external beam radiation decreased.
After the researchers controlled for numerous demographic and clinical variables, chemotherapy was associated with a statistically significant decrease in the risk of death overall (hazard ratio, 0.78; 95% confidence interval, 0.69 to 0.88) and in patients with stage IB (HR, 0.58; 95% CI, 0.44-0.77) or stage II cancer (HR, 0.74; 95% CI, 0.60-0.90). The use of brachytherapy also was associated with significantly improved survival overall (HR, 0.67), in stage IA cancer (HR, 0.67), and in stage II cancer (HR, 0.64).
The survival effect of brachytherapy held up in additional subgroup analyses, Dr. Cham explained. In contrast, external beam radiation therapy was not associated with improved survival overall or in any subgroup, she said. The results highlight the potential use of vaginal brachytherapy in early-stage uterine papillary serous carcinoma, she emphasized.
Dr. Cham cited no funding sources and reported having no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NATIONAL HARBOR, MD. – A large retrospective database study linked the use of vaginal brachytherapy and chemotherapy with improved survival among patients with early-stage uterine papillary serous carcinoma.
The findings offer a degree of clinical guidance on the adjuvant treatment of this relatively rare, aggressive histologic cancer subtype, Stephanie Cham, MD, said during a video interview at the annual meeting of the Society of Gynecologic Oncology.
Large dataset analyses can be especially helpful for exploring the treatment of rare diseases for which clinical trials can be infeasible, said Dr. Cham of Columbia University College of Physicians and Surgeons in New York. To evaluate chemotherapy, vaginal brachytherapy, and whole beam pelvic radiation therapy in stage I and stage II uterine papillary serous carcinoma, she and her associates analyzed the National Cancer Database.
Among 7,325 patients treated between 1998 and 2012, 38% of patients had received chemotherapy, 18% had received external beam radiation, and 20% received brachytherapy, Dr. Cham said. The use of chemotherapy rose significantly over time, regardless of stage (P less than .0001), as did the use of brachytherapy, while the use of external beam radiation decreased.
After the researchers controlled for numerous demographic and clinical variables, chemotherapy was associated with a statistically significant decrease in the risk of death overall (hazard ratio, 0.78; 95% confidence interval, 0.69 to 0.88) and in patients with stage IB (HR, 0.58; 95% CI, 0.44-0.77) or stage II cancer (HR, 0.74; 95% CI, 0.60-0.90). The use of brachytherapy also was associated with significantly improved survival overall (HR, 0.67), in stage IA cancer (HR, 0.67), and in stage II cancer (HR, 0.64).
The survival effect of brachytherapy held up in additional subgroup analyses, Dr. Cham explained. In contrast, external beam radiation therapy was not associated with improved survival overall or in any subgroup, she said. The results highlight the potential use of vaginal brachytherapy in early-stage uterine papillary serous carcinoma, she emphasized.
Dr. Cham cited no funding sources and reported having no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Chemotherapy and brachytherapy were independently associated with survival in early-stage uterine papillary serous carcinoma.
Major finding: Multivariable analyses linked chemotherapy with a statistically significant decrease in the risk of death overall (hazard ratio, 0.78) and in stage IB (HR, 0.58; 95% CI, 0.44 to 0.77) and stage II cancer (HR, 0.74; 95% CI, 0.60 to 0.90). The use of brachytherapy also was associated with significantly improved survival in the entire cohort (HR, 0.67), in stage IA cancer (HR, 0.67), and in stage II cancer (HR, 0.64).
Data source: A retrospective analysis of patients with stage I or II uterine papillary serous carcinoma from the National Cancer Database.
Disclosures: Dr. Cham cited no funding sources and reported having no conflicts of interest.
Adalimumab for psoriasis: Blocking TNF-alpha had no effect on vascular inflammation
ORLANDO – Vascular inflammation was no different in patients with moderate to severe psoriasis after 16 weeks of treatment with adalimumab than in untreated controls, according to a study that evaluated the impact of blocking tumor necrosis factor–alpha on vascular inflammation.
Further, there was a modest increase in vascular inflammation in carotid arteries after 52 weeks of treatment with the tumor necrosis factor (TNF) alpha-antagonist adalimumab, Robert Bissonnette, MD, president of Innovaderm Research, Montreal, reported in a late-breaking session at the annual meeting of the American Academy of Dermatology.
At 16 weeks, there were no significant differences in vascular inflammation between the treatment and control arms, based on the change from baseline in the vessel wall target to background ratio from the ascending aorta (the primary endpoint). In the carotid arteries at 16 weeks, differences in vascular inflammation between the adalimumab group and control group were also not significant.
At 52 weeks, there was no significant change in target to background ratio from the ascending aorta between baseline and the start of adalimumab, although in the carotid arteries, there was a modest increase in vascular inflammation.
Several previous studies have suggested that reducing inflammation in psoriasis can also reduce the risk of some cardiovascular events. As to why this study did not demonstrate a correlation between vascular inflammation and treatment, Dr. Bissonnette said during the question and answer portion of the presentation that “either the dose of adalimumab that is used for psoriasis has no impact on vascular inflammation or it may be possible that levels of interleukin-6, a key cytokine correlated with vascular inflammation, were very low in our study.”
Interleukin-6 typically is increased in patients with psoriatic arthritis, he explained, noting that in this study, only 7.5% of patients in the treatment group and about 10% of those in the control group had a history of psoriatic arthritis.
Additionally, at baseline, high-sensitivity C-reactive protein levels were significantly higher in controls: 5.32, compared with 2.72 in the treatment arm (P = .003).
Another possible reason for the results could be the molecule size of adalimumab, session comoderator Joel Gelfand, MD, noted in an interview. “The drug may not penetrate the aorta. These are large molecules. It also might be that [TNF–alpha] is not the main driver of aortic inflammation in people with psoriasis. Increasingly, we think of people with psoriasis as an IL-23 and IL-17 disease, so maybe that is part of it,” said Dr. Gelfand, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. He also directs the psoriasis and phototherapy treatment center at the university.
In addition to being presented at the meeting, the results were published in the Journal of Investigative Dermatology (2017 Feb 7. doi: 10.1016/j.jid.2017.02.977).
Adalimumab is marketed as Humira by Abbvie; a biosimilar version, adalimumab-atto (Amjevita), was approved in the United States in 2016.
Dr. Bissonnette’s disclosures included serving as an adviser and consultant to Abbvie, which sponsored the study, as well as relationships with other companies, including Amgen, Celgene, Janssen, and Novartis. Dr. Gelfand’s disclosures included serving as a consultant and investigator for Abbvie, and serving as an investigator, speaker, and/or consultant for other companies, including Eli Lilly, Janssen, and Novartis.
[email protected]
On Twitter @whitneymcknight
ORLANDO – Vascular inflammation was no different in patients with moderate to severe psoriasis after 16 weeks of treatment with adalimumab than in untreated controls, according to a study that evaluated the impact of blocking tumor necrosis factor–alpha on vascular inflammation.
Further, there was a modest increase in vascular inflammation in carotid arteries after 52 weeks of treatment with the tumor necrosis factor (TNF) alpha-antagonist adalimumab, Robert Bissonnette, MD, president of Innovaderm Research, Montreal, reported in a late-breaking session at the annual meeting of the American Academy of Dermatology.
At 16 weeks, there were no significant differences in vascular inflammation between the treatment and control arms, based on the change from baseline in the vessel wall target to background ratio from the ascending aorta (the primary endpoint). In the carotid arteries at 16 weeks, differences in vascular inflammation between the adalimumab group and control group were also not significant.
At 52 weeks, there was no significant change in target to background ratio from the ascending aorta between baseline and the start of adalimumab, although in the carotid arteries, there was a modest increase in vascular inflammation.
Several previous studies have suggested that reducing inflammation in psoriasis can also reduce the risk of some cardiovascular events. As to why this study did not demonstrate a correlation between vascular inflammation and treatment, Dr. Bissonnette said during the question and answer portion of the presentation that “either the dose of adalimumab that is used for psoriasis has no impact on vascular inflammation or it may be possible that levels of interleukin-6, a key cytokine correlated with vascular inflammation, were very low in our study.”
Interleukin-6 typically is increased in patients with psoriatic arthritis, he explained, noting that in this study, only 7.5% of patients in the treatment group and about 10% of those in the control group had a history of psoriatic arthritis.
Additionally, at baseline, high-sensitivity C-reactive protein levels were significantly higher in controls: 5.32, compared with 2.72 in the treatment arm (P = .003).
Another possible reason for the results could be the molecule size of adalimumab, session comoderator Joel Gelfand, MD, noted in an interview. “The drug may not penetrate the aorta. These are large molecules. It also might be that [TNF–alpha] is not the main driver of aortic inflammation in people with psoriasis. Increasingly, we think of people with psoriasis as an IL-23 and IL-17 disease, so maybe that is part of it,” said Dr. Gelfand, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. He also directs the psoriasis and phototherapy treatment center at the university.
In addition to being presented at the meeting, the results were published in the Journal of Investigative Dermatology (2017 Feb 7. doi: 10.1016/j.jid.2017.02.977).
Adalimumab is marketed as Humira by Abbvie; a biosimilar version, adalimumab-atto (Amjevita), was approved in the United States in 2016.
Dr. Bissonnette’s disclosures included serving as an adviser and consultant to Abbvie, which sponsored the study, as well as relationships with other companies, including Amgen, Celgene, Janssen, and Novartis. Dr. Gelfand’s disclosures included serving as a consultant and investigator for Abbvie, and serving as an investigator, speaker, and/or consultant for other companies, including Eli Lilly, Janssen, and Novartis.
[email protected]
On Twitter @whitneymcknight
ORLANDO – Vascular inflammation was no different in patients with moderate to severe psoriasis after 16 weeks of treatment with adalimumab than in untreated controls, according to a study that evaluated the impact of blocking tumor necrosis factor–alpha on vascular inflammation.
Further, there was a modest increase in vascular inflammation in carotid arteries after 52 weeks of treatment with the tumor necrosis factor (TNF) alpha-antagonist adalimumab, Robert Bissonnette, MD, president of Innovaderm Research, Montreal, reported in a late-breaking session at the annual meeting of the American Academy of Dermatology.
At 16 weeks, there were no significant differences in vascular inflammation between the treatment and control arms, based on the change from baseline in the vessel wall target to background ratio from the ascending aorta (the primary endpoint). In the carotid arteries at 16 weeks, differences in vascular inflammation between the adalimumab group and control group were also not significant.
At 52 weeks, there was no significant change in target to background ratio from the ascending aorta between baseline and the start of adalimumab, although in the carotid arteries, there was a modest increase in vascular inflammation.
Several previous studies have suggested that reducing inflammation in psoriasis can also reduce the risk of some cardiovascular events. As to why this study did not demonstrate a correlation between vascular inflammation and treatment, Dr. Bissonnette said during the question and answer portion of the presentation that “either the dose of adalimumab that is used for psoriasis has no impact on vascular inflammation or it may be possible that levels of interleukin-6, a key cytokine correlated with vascular inflammation, were very low in our study.”
Interleukin-6 typically is increased in patients with psoriatic arthritis, he explained, noting that in this study, only 7.5% of patients in the treatment group and about 10% of those in the control group had a history of psoriatic arthritis.
Additionally, at baseline, high-sensitivity C-reactive protein levels were significantly higher in controls: 5.32, compared with 2.72 in the treatment arm (P = .003).
Another possible reason for the results could be the molecule size of adalimumab, session comoderator Joel Gelfand, MD, noted in an interview. “The drug may not penetrate the aorta. These are large molecules. It also might be that [TNF–alpha] is not the main driver of aortic inflammation in people with psoriasis. Increasingly, we think of people with psoriasis as an IL-23 and IL-17 disease, so maybe that is part of it,” said Dr. Gelfand, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. He also directs the psoriasis and phototherapy treatment center at the university.
In addition to being presented at the meeting, the results were published in the Journal of Investigative Dermatology (2017 Feb 7. doi: 10.1016/j.jid.2017.02.977).
Adalimumab is marketed as Humira by Abbvie; a biosimilar version, adalimumab-atto (Amjevita), was approved in the United States in 2016.
Dr. Bissonnette’s disclosures included serving as an adviser and consultant to Abbvie, which sponsored the study, as well as relationships with other companies, including Amgen, Celgene, Janssen, and Novartis. Dr. Gelfand’s disclosures included serving as a consultant and investigator for Abbvie, and serving as an investigator, speaker, and/or consultant for other companies, including Eli Lilly, Janssen, and Novartis.
[email protected]
On Twitter @whitneymcknight
Key clinical point:
Major finding: At 16 weeks, there were no significant differences in aortic and carotid inflammation in the change from baseline in moderate psoriasis patients given adalimumab and controls.
Data source: A randomized, double-blind multicenter study that used PET-CT scans to evaluate the impact of treatment on vascular inflammation in 107 adults with moderate to severe psoriasis.
Disclosures: Dr. Bissonnette’s disclosures included serving as an adviser and consultant to Abbvie, which sponsored the study, as well as relationships with other companies, including Amgen, Celgene, Janssen, and Novartis. Dr. Gelfand’s disclosures included serving as a consultant and investigator for Abbvie, and serving as an investigator, speaker, and/or consultant for other companies, including Eli Lilly, Janssen, and Novartis.
Surveillance
A few weeks ago I received an email from a pediatrician thanking me for supporting her decision to quit work so that she could be home when her teenage son came home from school. She felt that by being home during her son’s adolescence, not only had she provided him a secure base but she also had helped protect him from a drug-dominated culture that permeated the community where they lived. While I hadn’t touched on it in my column, “Perfect Attendance” (Pediatric News, March 2017), this pediatrician’s experience highlights another benefit of a parental presence during those potentially stormy adolescent years.
In a recent article in the New York Times (“Teenagers Do Dumb Things, but There Are Ways to Limit Recklessness,” by Lisa Damour, March 8, 2017), Dr. Laurence Steinberg, a psychology professor at Temple University, is quoted as saying that “the context in which kids grow up must matter a great deal, and that recklessness isn’t the inevitable byproduct of the period’s biology.”
As writer Lisa Damour cogently states in her article, “For teenagers to find trouble, temptation must meet opportunity.”
Here in Brunswick, high school students finish their school day at 2:10 pm. If the student doesn’t play on a sports team and even if his or her home is at the end of the longest bus route, he or she is going to be home before 3 p.m. ... probably unsupervised. And stuff happens.
Although I may have been unsupervised, I was – or at least I believed that I was – always under constant surveillance. In the 1950s and 1960s, the population of Pleasantville, N.Y. was 5,000 and my mother had me convinced that she knew 4,000 of them. She recounted enough little things she had heard to make me believe that I was being watched by 8,000 eyes. She and the other mothers in town were masters of information sharing long before anyone had heard of networking.
These were not helicopter mothers hovering over every shady corner of our lives. They were simply concerned parents and fellow citizens going about their daily business who were not afraid to say something if they saw something. My mother’s apparent omniscience was a powerful deterrent to my adolescent recklessness. Only after I could afford to buy a car did I feel I could escape her surveillance network. And even then I wasn’t always sure.
The Internet has opened opportunities for mischief that are several orders of magnitude greater than the ones my friends and I sought to exploit in the 1950s and 1960s. However, parents today do have tools with which they can create a surveillance network to protect adolescents from their biologically predetermined urges. They simply need to have to courage to use them and not be afraid to say something if they see something.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A few weeks ago I received an email from a pediatrician thanking me for supporting her decision to quit work so that she could be home when her teenage son came home from school. She felt that by being home during her son’s adolescence, not only had she provided him a secure base but she also had helped protect him from a drug-dominated culture that permeated the community where they lived. While I hadn’t touched on it in my column, “Perfect Attendance” (Pediatric News, March 2017), this pediatrician’s experience highlights another benefit of a parental presence during those potentially stormy adolescent years.
In a recent article in the New York Times (“Teenagers Do Dumb Things, but There Are Ways to Limit Recklessness,” by Lisa Damour, March 8, 2017), Dr. Laurence Steinberg, a psychology professor at Temple University, is quoted as saying that “the context in which kids grow up must matter a great deal, and that recklessness isn’t the inevitable byproduct of the period’s biology.”
As writer Lisa Damour cogently states in her article, “For teenagers to find trouble, temptation must meet opportunity.”
Here in Brunswick, high school students finish their school day at 2:10 pm. If the student doesn’t play on a sports team and even if his or her home is at the end of the longest bus route, he or she is going to be home before 3 p.m. ... probably unsupervised. And stuff happens.
Although I may have been unsupervised, I was – or at least I believed that I was – always under constant surveillance. In the 1950s and 1960s, the population of Pleasantville, N.Y. was 5,000 and my mother had me convinced that she knew 4,000 of them. She recounted enough little things she had heard to make me believe that I was being watched by 8,000 eyes. She and the other mothers in town were masters of information sharing long before anyone had heard of networking.
These were not helicopter mothers hovering over every shady corner of our lives. They were simply concerned parents and fellow citizens going about their daily business who were not afraid to say something if they saw something. My mother’s apparent omniscience was a powerful deterrent to my adolescent recklessness. Only after I could afford to buy a car did I feel I could escape her surveillance network. And even then I wasn’t always sure.
The Internet has opened opportunities for mischief that are several orders of magnitude greater than the ones my friends and I sought to exploit in the 1950s and 1960s. However, parents today do have tools with which they can create a surveillance network to protect adolescents from their biologically predetermined urges. They simply need to have to courage to use them and not be afraid to say something if they see something.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A few weeks ago I received an email from a pediatrician thanking me for supporting her decision to quit work so that she could be home when her teenage son came home from school. She felt that by being home during her son’s adolescence, not only had she provided him a secure base but she also had helped protect him from a drug-dominated culture that permeated the community where they lived. While I hadn’t touched on it in my column, “Perfect Attendance” (Pediatric News, March 2017), this pediatrician’s experience highlights another benefit of a parental presence during those potentially stormy adolescent years.
In a recent article in the New York Times (“Teenagers Do Dumb Things, but There Are Ways to Limit Recklessness,” by Lisa Damour, March 8, 2017), Dr. Laurence Steinberg, a psychology professor at Temple University, is quoted as saying that “the context in which kids grow up must matter a great deal, and that recklessness isn’t the inevitable byproduct of the period’s biology.”
As writer Lisa Damour cogently states in her article, “For teenagers to find trouble, temptation must meet opportunity.”
Here in Brunswick, high school students finish their school day at 2:10 pm. If the student doesn’t play on a sports team and even if his or her home is at the end of the longest bus route, he or she is going to be home before 3 p.m. ... probably unsupervised. And stuff happens.
Although I may have been unsupervised, I was – or at least I believed that I was – always under constant surveillance. In the 1950s and 1960s, the population of Pleasantville, N.Y. was 5,000 and my mother had me convinced that she knew 4,000 of them. She recounted enough little things she had heard to make me believe that I was being watched by 8,000 eyes. She and the other mothers in town were masters of information sharing long before anyone had heard of networking.
These were not helicopter mothers hovering over every shady corner of our lives. They were simply concerned parents and fellow citizens going about their daily business who were not afraid to say something if they saw something. My mother’s apparent omniscience was a powerful deterrent to my adolescent recklessness. Only after I could afford to buy a car did I feel I could escape her surveillance network. And even then I wasn’t always sure.
The Internet has opened opportunities for mischief that are several orders of magnitude greater than the ones my friends and I sought to exploit in the 1950s and 1960s. However, parents today do have tools with which they can create a surveillance network to protect adolescents from their biologically predetermined urges. They simply need to have to courage to use them and not be afraid to say something if they see something.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Aortic repair in Loeys-Dietz syndrome requires close follow-up
The knowledge about Loeys-Dietz syndrome has evolved quickly since Hal Dietz, MD, and Bart Loeys, MD, at Johns Hopkins University, Baltimore, first reported on it in 2005.
Now, another team of Johns Hopkins investigators have reported that an aggressive approach with aortic root replacement coupled with valve-sparing whenever possible produces favorable results, but that clinicians must follow these patients closely with cardiovascular imaging.
“Growing experience with Loeys-Dietz syndrome has confirmed early impressions of its aggressive nature and proclivity toward aortic catastrophe,” Nishant D. Patel, MD, and his coauthors said in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:406-12). They reported on results of all 79 patients with Loeys-Dietz syndrome (LDS) who had cardiovascular surgery at Johns Hopkins. There were two (3%) deaths during surgery and eight (10%) late deaths. Patients with LDS are at risk for dissection early when the aortic root reaches 4 cm. Despite what they termed “favorable” outcomes of surgery, Dr. Patel and his coauthors acknowledged that reintervention rates for this population are high – 19 patients (24%) had subsequent operations. That suggests cardiac surgeons must closely monitor these patients. “Meticulous follow-up with cardiovascular surveillance imaging remains important for management, particularly as clinical LDS subtypes are characterized and more tailored treatment is developed,” Dr. Patel and his coauthors reported.
They advise echocardiography every 3 to 6 months for the first year after surgery and then every 6 to 12 months afterward. Full-body imaging should occur at least every 2 years. “In particular, patients with type B dissections should be monitored aggressively for aneurysm growth,” Dr. Patel and his coauthors said. They recommend imaging at seven to 14 days after dissection, then repeat imaging at 1, 3, 6, and 12 months, and then yearly thereafter.
They noted that four LDS subtypes have been identified. Although those with LDS1 and 2 subtypes are prone to aortic rupture at an earlier age and at smaller aortic diameters than other connective tissue disorders, the medical and surgical management for all subtypes are similar, Dr. Patel and his coauthors indicated.
“Certain congenital heart defects are more common among patients with LDS, compared with the normal population, including patent ductus arteriosus and mitral valve prolapse/insufficiency,” they said. Genotype is one factor that determines the need for surgery in LDS patients, Dr. Patel and his coauthors said. Others are growth rate, aortic valve function, family history, and severity of noncardiac phenotype.
The 79 patients in the study were divided almost evenly between gender, and the average age at first operation was 24.9 years; 38 were children younger than 18 years and 20 had a previous sternotomy. Aortic root replacement represented the predominant operation in the group, accounting for 65 operations (82.3%), of which 52 (80%) were valve-sparing procedures and the remainder were composite valve-graft procedures. The other procedures the researchers performed were nine aortic arch replacements (11.4%), three open thoracoabdominal repairs (3.8%) and two ascending aorta replacements (2.5%).
“Valve-sparing root replacement has become a safe and reliable option for appropriately selected younger patients with LDS,” Dr. Patel and his coauthors wrote. Five patients needed a second operation on the aortic valve or root; three of them had a Florida sleeve procedure. “Based on these initial outcomes with the Florida sleeve at our institution, we have abandoned this procedure in favor of conventional valve-sparing root replacement,” Dr. Patel and his coauthors stated.
Dr. Patel and his coauthors had no financial relationships to disclose.
A rare disease such as Loeys-Dietz syndrome (LDS) seems more common now in vascular surgery, as we are the specialty making decisions on disorders of the descending thoracic aorta. Understanding not only the clinical presentation with the aortic root involvement but the fact that these patients require close surveillance is the key message.
Often we are consulted regarding or admit a patient with an acute type B dissection who usually has a history of poorly controlled hypertension but, also, has a history of “aneurysms” or a family member who passed away at a young age. These clues should lead us to explore the “molecular diagnosis” as well to see if patients have a connective tissue disorder such as LDS or vascular Ehlers-Danlos syndrome (vEDS). Because many of these syndromes have overlapping symptoms, understanding the type of connective tissue disorder will allow us to appreciate the differences, especially in a syndrome such as LDS, which was first reported in 2005 and is associated with more cardiac and aortic root pathologies than vEDS.
At the University of Washington, my colleague Dr. Sherene Shalhub heads a vascular genetics clinic in which she examines patients with potential connective tissue disorders in an attempt to make the molecular diagnosis and identify surveillance protocols and study the effect of open and endovascular repairs in these patients. Pooling data on these patients from around the country and collaborating within, as well as outside of, our specialty is imperative to increasing our understanding of these disorders. I believe we are seeing a trend in which we crave studies such as Dr. Patel’s to increase our knowledge on these rare (but now more common) entities.
Niten Singh, MD, is the director of the Limb Preservation Service at the Regional Vascular Center at Harborview Medical Center, Seattle, and an Associate Editor of Vascular Specialist.
A rare disease such as Loeys-Dietz syndrome (LDS) seems more common now in vascular surgery, as we are the specialty making decisions on disorders of the descending thoracic aorta. Understanding not only the clinical presentation with the aortic root involvement but the fact that these patients require close surveillance is the key message.
Often we are consulted regarding or admit a patient with an acute type B dissection who usually has a history of poorly controlled hypertension but, also, has a history of “aneurysms” or a family member who passed away at a young age. These clues should lead us to explore the “molecular diagnosis” as well to see if patients have a connective tissue disorder such as LDS or vascular Ehlers-Danlos syndrome (vEDS). Because many of these syndromes have overlapping symptoms, understanding the type of connective tissue disorder will allow us to appreciate the differences, especially in a syndrome such as LDS, which was first reported in 2005 and is associated with more cardiac and aortic root pathologies than vEDS.
At the University of Washington, my colleague Dr. Sherene Shalhub heads a vascular genetics clinic in which she examines patients with potential connective tissue disorders in an attempt to make the molecular diagnosis and identify surveillance protocols and study the effect of open and endovascular repairs in these patients. Pooling data on these patients from around the country and collaborating within, as well as outside of, our specialty is imperative to increasing our understanding of these disorders. I believe we are seeing a trend in which we crave studies such as Dr. Patel’s to increase our knowledge on these rare (but now more common) entities.
Niten Singh, MD, is the director of the Limb Preservation Service at the Regional Vascular Center at Harborview Medical Center, Seattle, and an Associate Editor of Vascular Specialist.
A rare disease such as Loeys-Dietz syndrome (LDS) seems more common now in vascular surgery, as we are the specialty making decisions on disorders of the descending thoracic aorta. Understanding not only the clinical presentation with the aortic root involvement but the fact that these patients require close surveillance is the key message.
Often we are consulted regarding or admit a patient with an acute type B dissection who usually has a history of poorly controlled hypertension but, also, has a history of “aneurysms” or a family member who passed away at a young age. These clues should lead us to explore the “molecular diagnosis” as well to see if patients have a connective tissue disorder such as LDS or vascular Ehlers-Danlos syndrome (vEDS). Because many of these syndromes have overlapping symptoms, understanding the type of connective tissue disorder will allow us to appreciate the differences, especially in a syndrome such as LDS, which was first reported in 2005 and is associated with more cardiac and aortic root pathologies than vEDS.
At the University of Washington, my colleague Dr. Sherene Shalhub heads a vascular genetics clinic in which she examines patients with potential connective tissue disorders in an attempt to make the molecular diagnosis and identify surveillance protocols and study the effect of open and endovascular repairs in these patients. Pooling data on these patients from around the country and collaborating within, as well as outside of, our specialty is imperative to increasing our understanding of these disorders. I believe we are seeing a trend in which we crave studies such as Dr. Patel’s to increase our knowledge on these rare (but now more common) entities.
Niten Singh, MD, is the director of the Limb Preservation Service at the Regional Vascular Center at Harborview Medical Center, Seattle, and an Associate Editor of Vascular Specialist.
The knowledge about Loeys-Dietz syndrome has evolved quickly since Hal Dietz, MD, and Bart Loeys, MD, at Johns Hopkins University, Baltimore, first reported on it in 2005.
Now, another team of Johns Hopkins investigators have reported that an aggressive approach with aortic root replacement coupled with valve-sparing whenever possible produces favorable results, but that clinicians must follow these patients closely with cardiovascular imaging.
“Growing experience with Loeys-Dietz syndrome has confirmed early impressions of its aggressive nature and proclivity toward aortic catastrophe,” Nishant D. Patel, MD, and his coauthors said in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:406-12). They reported on results of all 79 patients with Loeys-Dietz syndrome (LDS) who had cardiovascular surgery at Johns Hopkins. There were two (3%) deaths during surgery and eight (10%) late deaths. Patients with LDS are at risk for dissection early when the aortic root reaches 4 cm. Despite what they termed “favorable” outcomes of surgery, Dr. Patel and his coauthors acknowledged that reintervention rates for this population are high – 19 patients (24%) had subsequent operations. That suggests cardiac surgeons must closely monitor these patients. “Meticulous follow-up with cardiovascular surveillance imaging remains important for management, particularly as clinical LDS subtypes are characterized and more tailored treatment is developed,” Dr. Patel and his coauthors reported.
They advise echocardiography every 3 to 6 months for the first year after surgery and then every 6 to 12 months afterward. Full-body imaging should occur at least every 2 years. “In particular, patients with type B dissections should be monitored aggressively for aneurysm growth,” Dr. Patel and his coauthors said. They recommend imaging at seven to 14 days after dissection, then repeat imaging at 1, 3, 6, and 12 months, and then yearly thereafter.
They noted that four LDS subtypes have been identified. Although those with LDS1 and 2 subtypes are prone to aortic rupture at an earlier age and at smaller aortic diameters than other connective tissue disorders, the medical and surgical management for all subtypes are similar, Dr. Patel and his coauthors indicated.
“Certain congenital heart defects are more common among patients with LDS, compared with the normal population, including patent ductus arteriosus and mitral valve prolapse/insufficiency,” they said. Genotype is one factor that determines the need for surgery in LDS patients, Dr. Patel and his coauthors said. Others are growth rate, aortic valve function, family history, and severity of noncardiac phenotype.
The 79 patients in the study were divided almost evenly between gender, and the average age at first operation was 24.9 years; 38 were children younger than 18 years and 20 had a previous sternotomy. Aortic root replacement represented the predominant operation in the group, accounting for 65 operations (82.3%), of which 52 (80%) were valve-sparing procedures and the remainder were composite valve-graft procedures. The other procedures the researchers performed were nine aortic arch replacements (11.4%), three open thoracoabdominal repairs (3.8%) and two ascending aorta replacements (2.5%).
“Valve-sparing root replacement has become a safe and reliable option for appropriately selected younger patients with LDS,” Dr. Patel and his coauthors wrote. Five patients needed a second operation on the aortic valve or root; three of them had a Florida sleeve procedure. “Based on these initial outcomes with the Florida sleeve at our institution, we have abandoned this procedure in favor of conventional valve-sparing root replacement,” Dr. Patel and his coauthors stated.
Dr. Patel and his coauthors had no financial relationships to disclose.
The knowledge about Loeys-Dietz syndrome has evolved quickly since Hal Dietz, MD, and Bart Loeys, MD, at Johns Hopkins University, Baltimore, first reported on it in 2005.
Now, another team of Johns Hopkins investigators have reported that an aggressive approach with aortic root replacement coupled with valve-sparing whenever possible produces favorable results, but that clinicians must follow these patients closely with cardiovascular imaging.
“Growing experience with Loeys-Dietz syndrome has confirmed early impressions of its aggressive nature and proclivity toward aortic catastrophe,” Nishant D. Patel, MD, and his coauthors said in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:406-12). They reported on results of all 79 patients with Loeys-Dietz syndrome (LDS) who had cardiovascular surgery at Johns Hopkins. There were two (3%) deaths during surgery and eight (10%) late deaths. Patients with LDS are at risk for dissection early when the aortic root reaches 4 cm. Despite what they termed “favorable” outcomes of surgery, Dr. Patel and his coauthors acknowledged that reintervention rates for this population are high – 19 patients (24%) had subsequent operations. That suggests cardiac surgeons must closely monitor these patients. “Meticulous follow-up with cardiovascular surveillance imaging remains important for management, particularly as clinical LDS subtypes are characterized and more tailored treatment is developed,” Dr. Patel and his coauthors reported.
They advise echocardiography every 3 to 6 months for the first year after surgery and then every 6 to 12 months afterward. Full-body imaging should occur at least every 2 years. “In particular, patients with type B dissections should be monitored aggressively for aneurysm growth,” Dr. Patel and his coauthors said. They recommend imaging at seven to 14 days after dissection, then repeat imaging at 1, 3, 6, and 12 months, and then yearly thereafter.
They noted that four LDS subtypes have been identified. Although those with LDS1 and 2 subtypes are prone to aortic rupture at an earlier age and at smaller aortic diameters than other connective tissue disorders, the medical and surgical management for all subtypes are similar, Dr. Patel and his coauthors indicated.
“Certain congenital heart defects are more common among patients with LDS, compared with the normal population, including patent ductus arteriosus and mitral valve prolapse/insufficiency,” they said. Genotype is one factor that determines the need for surgery in LDS patients, Dr. Patel and his coauthors said. Others are growth rate, aortic valve function, family history, and severity of noncardiac phenotype.
The 79 patients in the study were divided almost evenly between gender, and the average age at first operation was 24.9 years; 38 were children younger than 18 years and 20 had a previous sternotomy. Aortic root replacement represented the predominant operation in the group, accounting for 65 operations (82.3%), of which 52 (80%) were valve-sparing procedures and the remainder were composite valve-graft procedures. The other procedures the researchers performed were nine aortic arch replacements (11.4%), three open thoracoabdominal repairs (3.8%) and two ascending aorta replacements (2.5%).
“Valve-sparing root replacement has become a safe and reliable option for appropriately selected younger patients with LDS,” Dr. Patel and his coauthors wrote. Five patients needed a second operation on the aortic valve or root; three of them had a Florida sleeve procedure. “Based on these initial outcomes with the Florida sleeve at our institution, we have abandoned this procedure in favor of conventional valve-sparing root replacement,” Dr. Patel and his coauthors stated.
Dr. Patel and his coauthors had no financial relationships to disclose.
Key clinical point: Outcomes for aortic surgery in Loeys-Dietz syndrome are favorable, but reintervention rates are high.
Major finding: Patients require close postoperative follow-up with cardiovascular imaging.
Data source: Retrospective review of 79 patients who had cardiovascular surgery for LDS over 26 years at Johns Hopkins University.
VA Secretary Shulkin Calls for Expansion of Health Care to Less Than Honorably Discharged Veterans
Secretary of Veterans Affairs David J. Shulkin, MD, testified before the House Committee on Veterans’ Affairs, promising to tackle the epidemic of suicide and to continue the process to improve the Veterans Choice Act. Dr. Shulkin pledged to begin providing some mental health care service to veterans with other than honorable (OTH) discharges. “We know the rate of death by suicide among veterans who do not use VA care is increasing at a greater rate than veterans who use VA care,” Shulkin told the panel. “This is a national emergency that requires bold action. We must and we will do all that we can to help former service members who may be at risk. When we say even 1 veteran suicide is 1 too many, we mean it.”
Related: Senate, VA Agree—Veterans Choice Act Needs Fixing
The VA estimates that there are more than 500,000 former service members with OTH discharges. Previously, these veterans were not eligible for VA health benefits. As part of the proposal, former OTH service members would be able to seek treatment at a VA emergency department, Vet Center, or Veterans Crisis Line.
“I appreciate Secretary Shulkin taking steps to ensure veterans in crisis with OTH discharges have access to mental health services,” Phil Roe, MD (R-Tenn), chairman of the House Committee on Veterans’ Affairs, said in a written response. “With that said, this must be done in a fair, transparent way that ensures no veteran, especially those who have honorably served, are being skipped over for the care they need. I look forward to continuing this conversation with Secretary Shulkin.”
Related: "Call to Action" on Veteran Suicide Yields Policy Shifts
In addition, Dr. Shulkin revealed that 5.5 million appointments have been made through the Veterans Choice Act and only 5,000 use community care exclusively. The bulk of veterans access both Veterans Choice and VA health care services. The Choice Act is set to expire in less than 6 months on August 7, 2017, if it does not receive congressional reapproval.
To modernize and consolidating the community care portion of the Veterans Choice Act, Shulkin outlined 7 steps:
- High performance integrated network, including VA, other federal health care, and community providers;
- Increase choice for all veterans, starting with those with service-connected health needs;
- Help veterans get care closer to their homes;
- Optimize and coordinate veterans’ care with other insurance providers;
- Maintain affordability for lowest income veterans;
- Assist in care coordination for veterans with multiple care providers; and
- Apply industry standards for quality and affordability to the program.
Secretary of Veterans Affairs David J. Shulkin, MD, testified before the House Committee on Veterans’ Affairs, promising to tackle the epidemic of suicide and to continue the process to improve the Veterans Choice Act. Dr. Shulkin pledged to begin providing some mental health care service to veterans with other than honorable (OTH) discharges. “We know the rate of death by suicide among veterans who do not use VA care is increasing at a greater rate than veterans who use VA care,” Shulkin told the panel. “This is a national emergency that requires bold action. We must and we will do all that we can to help former service members who may be at risk. When we say even 1 veteran suicide is 1 too many, we mean it.”
Related: Senate, VA Agree—Veterans Choice Act Needs Fixing
The VA estimates that there are more than 500,000 former service members with OTH discharges. Previously, these veterans were not eligible for VA health benefits. As part of the proposal, former OTH service members would be able to seek treatment at a VA emergency department, Vet Center, or Veterans Crisis Line.
“I appreciate Secretary Shulkin taking steps to ensure veterans in crisis with OTH discharges have access to mental health services,” Phil Roe, MD (R-Tenn), chairman of the House Committee on Veterans’ Affairs, said in a written response. “With that said, this must be done in a fair, transparent way that ensures no veteran, especially those who have honorably served, are being skipped over for the care they need. I look forward to continuing this conversation with Secretary Shulkin.”
Related: "Call to Action" on Veteran Suicide Yields Policy Shifts
In addition, Dr. Shulkin revealed that 5.5 million appointments have been made through the Veterans Choice Act and only 5,000 use community care exclusively. The bulk of veterans access both Veterans Choice and VA health care services. The Choice Act is set to expire in less than 6 months on August 7, 2017, if it does not receive congressional reapproval.
To modernize and consolidating the community care portion of the Veterans Choice Act, Shulkin outlined 7 steps:
- High performance integrated network, including VA, other federal health care, and community providers;
- Increase choice for all veterans, starting with those with service-connected health needs;
- Help veterans get care closer to their homes;
- Optimize and coordinate veterans’ care with other insurance providers;
- Maintain affordability for lowest income veterans;
- Assist in care coordination for veterans with multiple care providers; and
- Apply industry standards for quality and affordability to the program.
Secretary of Veterans Affairs David J. Shulkin, MD, testified before the House Committee on Veterans’ Affairs, promising to tackle the epidemic of suicide and to continue the process to improve the Veterans Choice Act. Dr. Shulkin pledged to begin providing some mental health care service to veterans with other than honorable (OTH) discharges. “We know the rate of death by suicide among veterans who do not use VA care is increasing at a greater rate than veterans who use VA care,” Shulkin told the panel. “This is a national emergency that requires bold action. We must and we will do all that we can to help former service members who may be at risk. When we say even 1 veteran suicide is 1 too many, we mean it.”
Related: Senate, VA Agree—Veterans Choice Act Needs Fixing
The VA estimates that there are more than 500,000 former service members with OTH discharges. Previously, these veterans were not eligible for VA health benefits. As part of the proposal, former OTH service members would be able to seek treatment at a VA emergency department, Vet Center, or Veterans Crisis Line.
“I appreciate Secretary Shulkin taking steps to ensure veterans in crisis with OTH discharges have access to mental health services,” Phil Roe, MD (R-Tenn), chairman of the House Committee on Veterans’ Affairs, said in a written response. “With that said, this must be done in a fair, transparent way that ensures no veteran, especially those who have honorably served, are being skipped over for the care they need. I look forward to continuing this conversation with Secretary Shulkin.”
Related: "Call to Action" on Veteran Suicide Yields Policy Shifts
In addition, Dr. Shulkin revealed that 5.5 million appointments have been made through the Veterans Choice Act and only 5,000 use community care exclusively. The bulk of veterans access both Veterans Choice and VA health care services. The Choice Act is set to expire in less than 6 months on August 7, 2017, if it does not receive congressional reapproval.
To modernize and consolidating the community care portion of the Veterans Choice Act, Shulkin outlined 7 steps:
- High performance integrated network, including VA, other federal health care, and community providers;
- Increase choice for all veterans, starting with those with service-connected health needs;
- Help veterans get care closer to their homes;
- Optimize and coordinate veterans’ care with other insurance providers;
- Maintain affordability for lowest income veterans;
- Assist in care coordination for veterans with multiple care providers; and
- Apply industry standards for quality and affordability to the program.
When Grief Becomes a Syndrome
Some patients who experience long-term grief may be slipping through the health care net. With data collected in 2 National Institute of Mental Health-funded treatment studies, researchers used proposed criteria from DSM-5 to identify patients with a stress-response syndrome of “persistent impairing grief”—that is, persistent complex bereavement disorder (PCBD), prolonged grief disorder (BGD) and complicated grief (CG). They studied 2 groups of patients in university-based psychiatric research clinics: 240 grief-treatment seeking participants scored ≥ 30 on the Inventory of Complicated Grief (ICG), and 86 bereaved adults scored < 20 on the ICG.
The PCBD criteria diagnosed 70% of the first group, PGD criteria identified 59.6%, and CG criteria identified 99.6%. None of the 3 proposed criteria identified cases in the bereaved comparison group. Only the CG criteria produced rates of case identification sufficient to be of clinical utility, the researchers say.
Their findings are “virtually identical” with those of the community-based National Military Family Bereavement Study, the researchers say, in which all 3 criteria sets identified < 2% of the bereaved military family survey population that scored < 20 on the ICG.
There are treatments specific to grief, the researchers note. But as of yet there is no gold standard for diagnosing persistent impairing grief. The researchers say the solution could lie in using the CG criteria set and modifying decision rules for CBD or PGD criteria or developing a new group of symptoms and decision rules. However it’s done, the researchers conclude, they see a “pressing need” to establish criteria that can lead to correct diagnosis and targeted treatment.
Some patients who experience long-term grief may be slipping through the health care net. With data collected in 2 National Institute of Mental Health-funded treatment studies, researchers used proposed criteria from DSM-5 to identify patients with a stress-response syndrome of “persistent impairing grief”—that is, persistent complex bereavement disorder (PCBD), prolonged grief disorder (BGD) and complicated grief (CG). They studied 2 groups of patients in university-based psychiatric research clinics: 240 grief-treatment seeking participants scored ≥ 30 on the Inventory of Complicated Grief (ICG), and 86 bereaved adults scored < 20 on the ICG.
The PCBD criteria diagnosed 70% of the first group, PGD criteria identified 59.6%, and CG criteria identified 99.6%. None of the 3 proposed criteria identified cases in the bereaved comparison group. Only the CG criteria produced rates of case identification sufficient to be of clinical utility, the researchers say.
Their findings are “virtually identical” with those of the community-based National Military Family Bereavement Study, the researchers say, in which all 3 criteria sets identified < 2% of the bereaved military family survey population that scored < 20 on the ICG.
There are treatments specific to grief, the researchers note. But as of yet there is no gold standard for diagnosing persistent impairing grief. The researchers say the solution could lie in using the CG criteria set and modifying decision rules for CBD or PGD criteria or developing a new group of symptoms and decision rules. However it’s done, the researchers conclude, they see a “pressing need” to establish criteria that can lead to correct diagnosis and targeted treatment.
Some patients who experience long-term grief may be slipping through the health care net. With data collected in 2 National Institute of Mental Health-funded treatment studies, researchers used proposed criteria from DSM-5 to identify patients with a stress-response syndrome of “persistent impairing grief”—that is, persistent complex bereavement disorder (PCBD), prolonged grief disorder (BGD) and complicated grief (CG). They studied 2 groups of patients in university-based psychiatric research clinics: 240 grief-treatment seeking participants scored ≥ 30 on the Inventory of Complicated Grief (ICG), and 86 bereaved adults scored < 20 on the ICG.
The PCBD criteria diagnosed 70% of the first group, PGD criteria identified 59.6%, and CG criteria identified 99.6%. None of the 3 proposed criteria identified cases in the bereaved comparison group. Only the CG criteria produced rates of case identification sufficient to be of clinical utility, the researchers say.
Their findings are “virtually identical” with those of the community-based National Military Family Bereavement Study, the researchers say, in which all 3 criteria sets identified < 2% of the bereaved military family survey population that scored < 20 on the ICG.
There are treatments specific to grief, the researchers note. But as of yet there is no gold standard for diagnosing persistent impairing grief. The researchers say the solution could lie in using the CG criteria set and modifying decision rules for CBD or PGD criteria or developing a new group of symptoms and decision rules. However it’s done, the researchers conclude, they see a “pressing need” to establish criteria that can lead to correct diagnosis and targeted treatment.
Veterans don’t have higher risk of leukemia, lymphoma
People who have served in the Armed Forces do not have an increased risk of leukemia or lymphoma, according to research published in Cancer Epidemiology.
Researchers analyzed the long-term risks of developing leukemia, Hodgkin lymphoma (HL), and non-Hodgkin lymphoma (NHL) in veterans living in Scotland.
At a mean 30 years of follow-up, there were no significant differences in the risk of the aforementioned malignancies between veterans and non-veterans in Scotland.
This retrospective study included 56,205 veterans and 172,741 non-veterans.
The veterans’ earliest date of entering service was January 1960, and the latest date of leaving service was December 2012.
At a mean follow-up of 29.3 years, 294 (0.52%) veterans and 974 (0.56%) non-veterans were diagnosed with leukemia, HL, or NHL.
There were 125 (0.22%) cases of leukemia in veterans and 365 (0.21%) in non-veterans. There were 59 (0.10%) cases of HL in veterans and 182 (0.11%) in non-veterans. And there were 144 (0.26%) cases of NHL in veterans and 538 (0.31%) in non-veterans.
There was no significant difference in the risk of all 3 cancer types between the veterans and non-veterans. The unadjusted hazard ratio (HR) was 0.96 (P=0.541).
There were no significant differences in an adjusted analysis either. (The analysis was adjusted for regional deprivation, which takes into account information on income, employment, health, education, housing, crime, and access to services.)
The adjusted HR was 1.03 (P=0.773) for leukemias, 1.19 (P=0.272) for HL, and 0.86 (P=0.110) for NHL.
“This is an important study which provides reassurance that military service in the last 50 years does not increase people’s risk of leukemia overall,” said study author Beverly Bergman, PhD, of the University of Glasgow in the UK.
“The Armed Forces comply with all relevant health and safety legislation and regulations, and we can now see that their risk is no different from the general population.” ![]()
People who have served in the Armed Forces do not have an increased risk of leukemia or lymphoma, according to research published in Cancer Epidemiology.
Researchers analyzed the long-term risks of developing leukemia, Hodgkin lymphoma (HL), and non-Hodgkin lymphoma (NHL) in veterans living in Scotland.
At a mean 30 years of follow-up, there were no significant differences in the risk of the aforementioned malignancies between veterans and non-veterans in Scotland.
This retrospective study included 56,205 veterans and 172,741 non-veterans.
The veterans’ earliest date of entering service was January 1960, and the latest date of leaving service was December 2012.
At a mean follow-up of 29.3 years, 294 (0.52%) veterans and 974 (0.56%) non-veterans were diagnosed with leukemia, HL, or NHL.
There were 125 (0.22%) cases of leukemia in veterans and 365 (0.21%) in non-veterans. There were 59 (0.10%) cases of HL in veterans and 182 (0.11%) in non-veterans. And there were 144 (0.26%) cases of NHL in veterans and 538 (0.31%) in non-veterans.
There was no significant difference in the risk of all 3 cancer types between the veterans and non-veterans. The unadjusted hazard ratio (HR) was 0.96 (P=0.541).
There were no significant differences in an adjusted analysis either. (The analysis was adjusted for regional deprivation, which takes into account information on income, employment, health, education, housing, crime, and access to services.)
The adjusted HR was 1.03 (P=0.773) for leukemias, 1.19 (P=0.272) for HL, and 0.86 (P=0.110) for NHL.
“This is an important study which provides reassurance that military service in the last 50 years does not increase people’s risk of leukemia overall,” said study author Beverly Bergman, PhD, of the University of Glasgow in the UK.
“The Armed Forces comply with all relevant health and safety legislation and regulations, and we can now see that their risk is no different from the general population.” ![]()
People who have served in the Armed Forces do not have an increased risk of leukemia or lymphoma, according to research published in Cancer Epidemiology.
Researchers analyzed the long-term risks of developing leukemia, Hodgkin lymphoma (HL), and non-Hodgkin lymphoma (NHL) in veterans living in Scotland.
At a mean 30 years of follow-up, there were no significant differences in the risk of the aforementioned malignancies between veterans and non-veterans in Scotland.
This retrospective study included 56,205 veterans and 172,741 non-veterans.
The veterans’ earliest date of entering service was January 1960, and the latest date of leaving service was December 2012.
At a mean follow-up of 29.3 years, 294 (0.52%) veterans and 974 (0.56%) non-veterans were diagnosed with leukemia, HL, or NHL.
There were 125 (0.22%) cases of leukemia in veterans and 365 (0.21%) in non-veterans. There were 59 (0.10%) cases of HL in veterans and 182 (0.11%) in non-veterans. And there were 144 (0.26%) cases of NHL in veterans and 538 (0.31%) in non-veterans.
There was no significant difference in the risk of all 3 cancer types between the veterans and non-veterans. The unadjusted hazard ratio (HR) was 0.96 (P=0.541).
There were no significant differences in an adjusted analysis either. (The analysis was adjusted for regional deprivation, which takes into account information on income, employment, health, education, housing, crime, and access to services.)
The adjusted HR was 1.03 (P=0.773) for leukemias, 1.19 (P=0.272) for HL, and 0.86 (P=0.110) for NHL.
“This is an important study which provides reassurance that military service in the last 50 years does not increase people’s risk of leukemia overall,” said study author Beverly Bergman, PhD, of the University of Glasgow in the UK.
“The Armed Forces comply with all relevant health and safety legislation and regulations, and we can now see that their risk is no different from the general population.” ![]()








