User login
Quantifying Treatment Intensity
Healthcare spending exceeded $2.5 trillion in 2007, and payments to hospitals represented the largest portion of this spending (more than 30%), equaling the combined cost of physician services and prescription drugs.[1, 2] Researchers and policymakers have emphasized the need to improve the value of hospital care in the United States, but this has been challenging, in part because of the difficulty in identifying hospitals that have high resource utilization relative to their peers.[3, 4, 5, 6, 7, 8, 9, 10, 11]
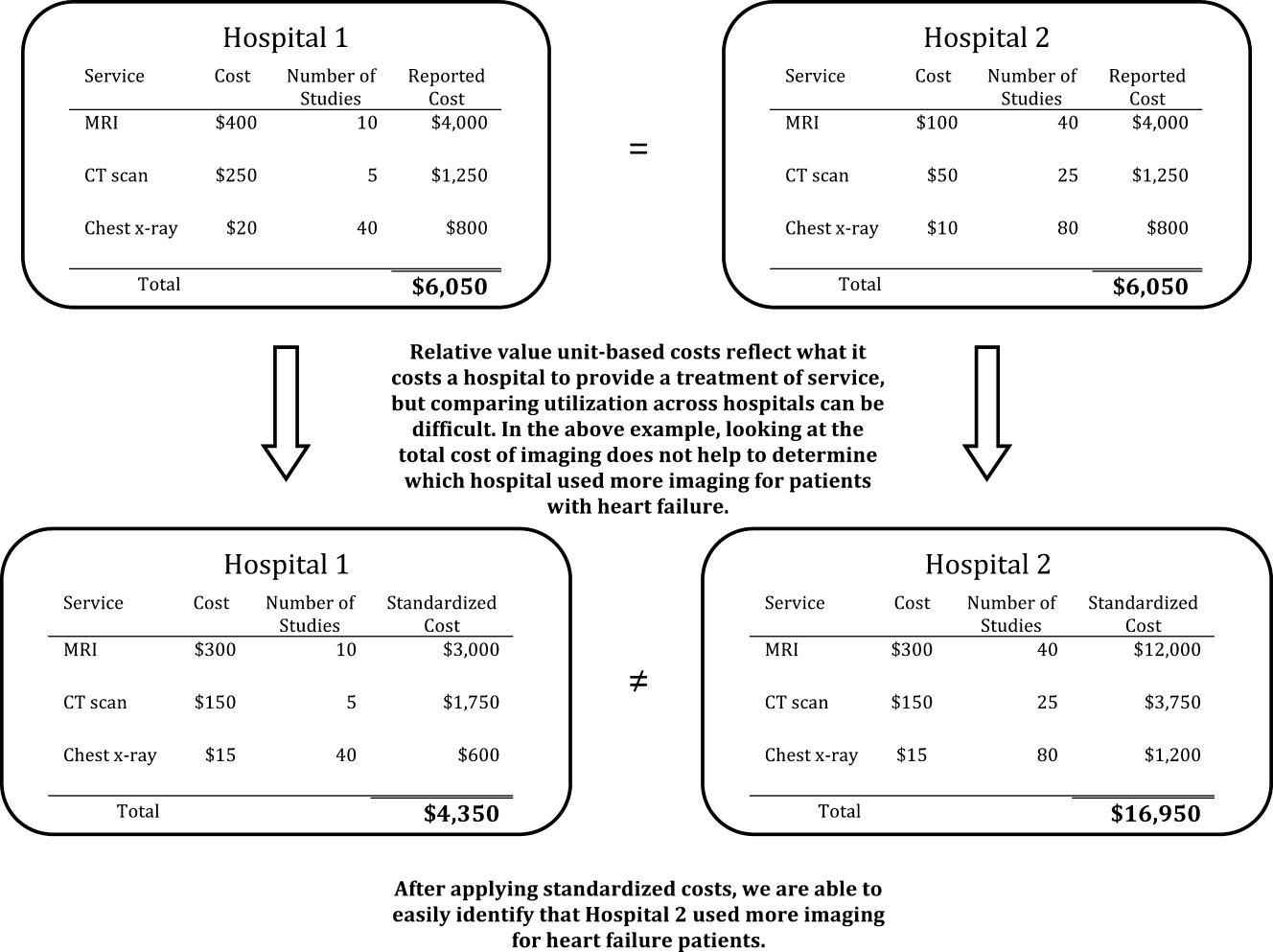
Most hospitals calculate their costs using internal accounting systems that determine resource utilization via relative value units (RVUs).[7, 8] RVU‐derived costs, also known as hospital reported costs, have proven to be an excellent method for quantifying what it costs a given hospital to provide a treatment, test, or procedure. However, RVU‐based costs are less useful for comparing resource utilization across hospitals because the cost to provide a treatment or service varies widely across hospitals. The cost of an item calculated using RVUs includes not just the item itself, but also a portion of the fixed costs of the hospital (overhead, labor, and infrastructure investments such as electronic records, new buildings, or expensive radiological or surgical equipment).[12] These costs vary by institution, patient population, region of the country, teaching status, and many other variables, making it difficult to identify resource utilization across hospitals.[13, 14]
Recently, a few claims‐based multi‐institutional datasets have begun incorporating item‐level RVU‐based costs derived directly from the cost accounting systems of participating institutions.[15] Such datasets allow researchers to compare reported costs of care from hospital to hospital, but because of the limitations we described above, they still cannot be used to answer the question: Which hospitals with higher costs of care are actually providing more treatments and services to patients?
To better facilitate the comparison of resource utilization patterns across hospitals, we standardized the unit costs of all treatments and services across hospitals by applying a single cost to every item across hospitals. This standardized cost allowed to compare utilization of that item (and the 15,000 other items in the database) across hospitals. We then compared estimates of resource utilization as measured by the 2 approaches: standardized and RVU‐based costs.
METHODS
Ethics Statement
All data were deidentified, by Premier, Inc., at both the hospital and patient level in accordance with the Health Insurance Portability and Accountability Act. The Yale University Human Investigation Committee reviewed the protocol for this study and determined that it is not considered to be human subjects research as defined by the Office of Human Research Protections.
Data Source
We conducted a cross‐sectional study using data from hospitals that participated in the database maintained by Premier Healthcare Informatics (Charlotte, NC) in the years 2009 to 2010. The Premier database is a voluntary, fee‐supported database created to measure quality and healthcare utilization.[3, 16, 17, 18] In 2010, it included detailed billing data from 500 hospitals in the United States, with more than 130 million cumulative hospital discharges. The detailed billing data includes all elements found in hospital claims derived from the uniform billing‐04 form, as well as an itemized, date‐stamped log of all items and services charged to the patient or insurer, such as medications, laboratory tests, and diagnostic and therapeutic services. The database includes approximately 15% of all US hospitalizations. Participating hospitals are similar to the composition of acute care hospitals nationwide. They represent all regions of the United States, and represent predominantly small‐ to mid‐sized nonteaching facilities that serve a largely urban population. The database also contains hospital reported costs at the item level as well as the total cost of the hospitalization. Approximately 75% of hospitals that participate submit RVU‐based costs taken from internal cost accounting systems. Because of our focus on comparing standardized costs to reported costs, we included only data from hospitals that use RVU‐based costs in this study.
Study Subjects
We included adult patients with a hospitalization recorded in the Premier database between January 1, 2009 and December 31, 2010, and a principal discharge diagnosis of heart failure (HF) (International Classification of Diseases, Ninth Revision, Clinical Modification codes: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx). We excluded transfers, patients assigned a pediatrician as the attending of record, and those who received a heart transplant or ventricular assist device during their stay. Because cost data are prone to extreme outliers, we excluded hospitalizations that were in the top 0.1% of length of stay, number of billing records, quantity of items billed, or total standardized cost. We also excluded hospitals that admitted fewer than 25 HF patients during the study period to reduce the possibility that a single high‐cost patient affected the hospital's cost profile.
Hospital Information
For each hospital included in the study, we recorded number of beds, teaching status, geographic region, and whether it served an urban or rural population.
Assignment of Standardized Costs
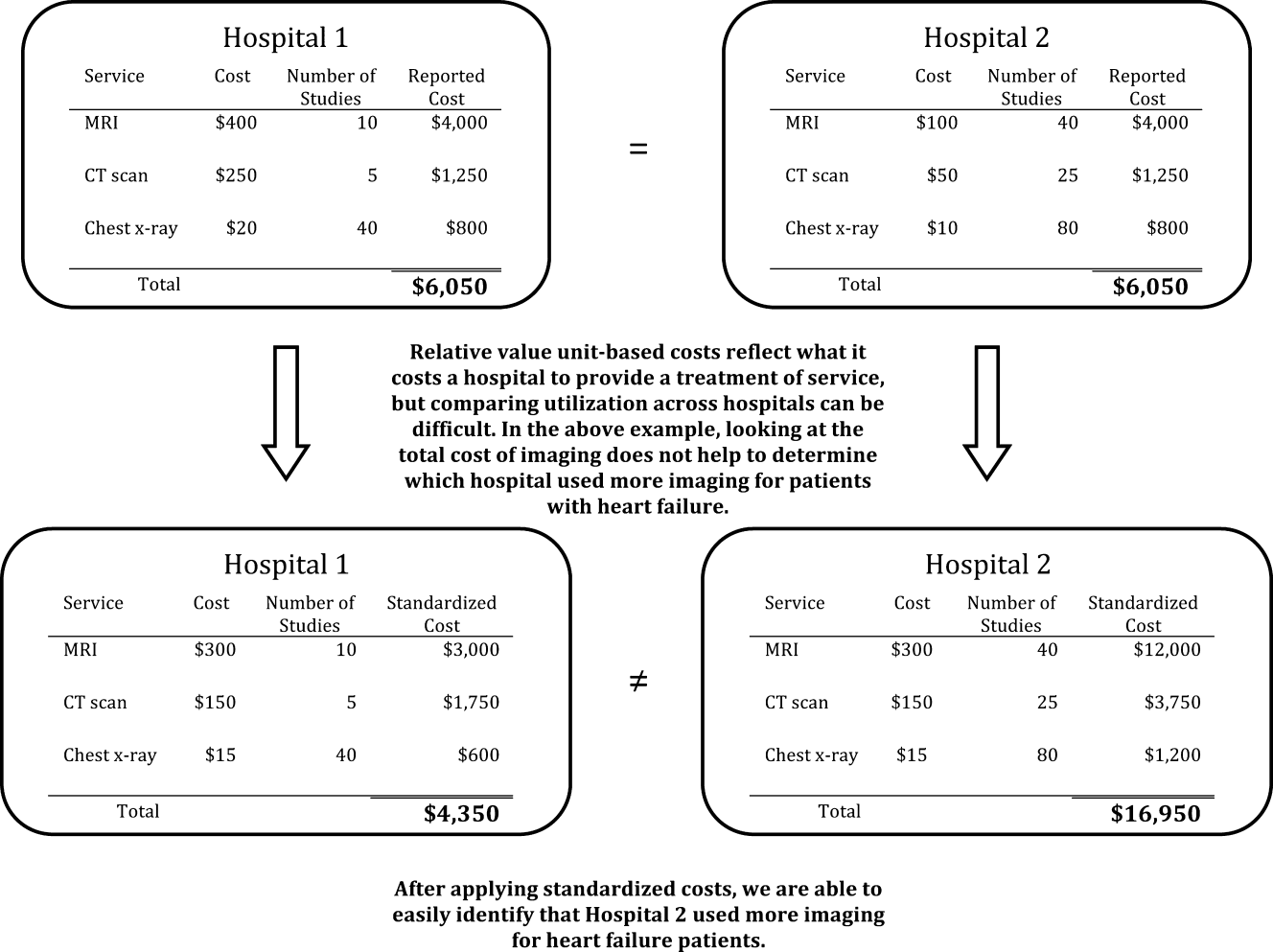
We defined reported cost as the RVU‐based cost per item in the database. We then calculated the median across hospitals for each item in the database and set this as the standardized unit cost of that item at every hospital (Figure 1). Once standardized costs were assigned at the item level, we summed the costs of all items assigned to each patient and calculated the standardized cost of a hospitalization per patient at each hospital.
Examination of Cost Variation
We compared the standardized and reported costs of hospitalizations using medians, interquartile ranges, and interquartile ratios (Q75/Q25). To examine whether standardized costs can reduce the noise due to differences in overhead and other fixed costs, we calculated, for each hospital, the coefficients of variation (CV) for per‐day reported and standardized costs and per‐hospitalization reported and standardized costs. We used the Fligner‐Killeen test to determine whether the variance of CVs was different for reported and standardized costs.[19]
Creation of Basket of Goods
Because there can be differences in the costs of items, the number and types of items administered during hospitalizations, 2 hospitals with similar reported costs for a hospitalization might deliver different quantities and combinations of treatments (Figure 1). We wished to demonstrate that there is variation in reported costs of items when the quantity and type of item is held constant, so we created a basket of items. We chose items that are commonly administered to patients with heart failure, but could have chosen any combination of items. The basket included a day of medical room and board, a day of intensive care unit (ICU) room and board, a single dose of ‐blocker, a single dose of angiotensin‐converting enzyme inhibitor, complete blood count, a B‐natriuretic peptide level, a chest radiograph, a chest computed tomography, and an echocardiogram. We then examined the range of hospitals' reported costs for this basket of goods using percentiles, medians, and interquartile ranges.
Reported to Standardized Cost Ratio
Next, we calculated standardized costs of hospitalizations for included hospitals and examined the relationship between hospitals' mean reported costs and mean standardized costs. This ratio could help diagnose the mechanism of high reported costs for a hospital, because high reported costs with low utilization would indicate high fixed costs, while high reported costs with high utilization would indicate greater use of tests and treatments. We assigned hospitals to strata based on reported costs greater than standardized costs by more than 25%, reported costs within 25% of standardized costs, and reported costs less than standardized costs by more than 25%. We examined the association between hospital characteristics and strata using a 2 test. All analyses were carried out using SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
The 234 hospitals included in the analysis contributed a total of 165,647 hospitalizations, with the number of hospitalizations ranging from 33 to 2,772 hospitalizations per hospital (see Supporting Table 1 in the online version of this article). Most were located in urban areas (84%), and many were in the southern United States (42%). The median hospital reported cost per hospitalization was $6,535, with an interquartile range of $5,541 to $7,454. The median standardized cost per hospitalization was $6,602, with a range of $5,866 to $7,386. The interquartile ratio (Q75/Q25) of the reported costs of a hospitalization was 1.35. After costs were standardized, the interquartile ratio fell to 1.26, indicating that variation decreased. We found that the median hospital reported cost per day was $1,651, with an IQR of $1,400 to $1,933 (ratio 1.38), whereas the median standardized cost per day was $1,640, with an IQR of $1,511 to $1,812 (ratio 1.20).
There were more than 15,000 items (eg, treatments, tests, and supplies) that received a standardized charge code in our cohort. These were divided into 11 summary departments and 40 standard departments (see Supporting Table 2 in the online version of this article). We observed a high level of variation in the reported costs of individual items: the reported costs of a day of room and board in an ICU ranged from $773 at hospitals at the 10th percentile to $2,471 at the 90th percentile (Table 1.). The standardized cost of a day of ICU room and board was $1,577. We also observed variation in the reported costs of items across item categories. Although a day of medical room and board showed a 3‐fold difference between the 10th and 90th percentile, we observed a more than 10‐fold difference in the reported cost of an echocardiogram, from $31 at the 10th percentile to $356 at the 90th percentile. After examining the hospital‐level cost for a basket of goods, we found variation in the reported costs for these items across hospitals, with a 10th percentile cost of $1,552 and a 90th percentile cost of $3,967.
| Reported Costs | 10th Percentile | 25th Percentile | 75th Percentile | 90th Percentile | Median (Standardized Cost) |
|---|---|---|---|---|---|
| |||||
| Item | |||||
| Day of medical | 490.03 | 586.41 | 889.95 | 1121.20 | 722.59 |
| Day of ICU | 773.01 | 1275.84 | 1994.81 | 2471.75 | 1577.93 |
| Complete blood count | 6.87 | 9.34 | 18.34 | 23.46 | 13.07 |
| B‐natriuretic peptide | 12.13 | 19.22 | 44.19 | 60.56 | 28.23 |
| Metoprolol | 0.20 | 0.68 | 2.67 | 3.74 | 1.66 |
| Lisinopril | 0.28 | 1.02 | 2.79 | 4.06 | 1.72 |
| Spironolactone | 0.22 | 0.53 | 2.68 | 3.83 | 1.63 |
| Furosemide | 1.27 | 2.45 | 5.73 | 8.12 | 3.82 |
| Chest x‐ray | 43.88 | 51.54 | 89.96 | 117.16 | 67.45 |
| Echocardiogram | 31.53 | 98.63 | 244.63 | 356.50 | 159.07 |
| Chest CT (w & w/o contrast) | 65.17 | 83.99 | 157.23 | 239.27 | 110.76 |
| Noninvasive positive pressure ventilation | 126.23 | 127.25 | 370.44 | 514.67 | 177.24 |
| Electrocardiogram | 12.08 | 18.77 | 42.74 | 64.94 | 29.78 |
| Total basket | 1552.50 | 2157.85 | 3417.34 | 3967.78 | 2710.49 |
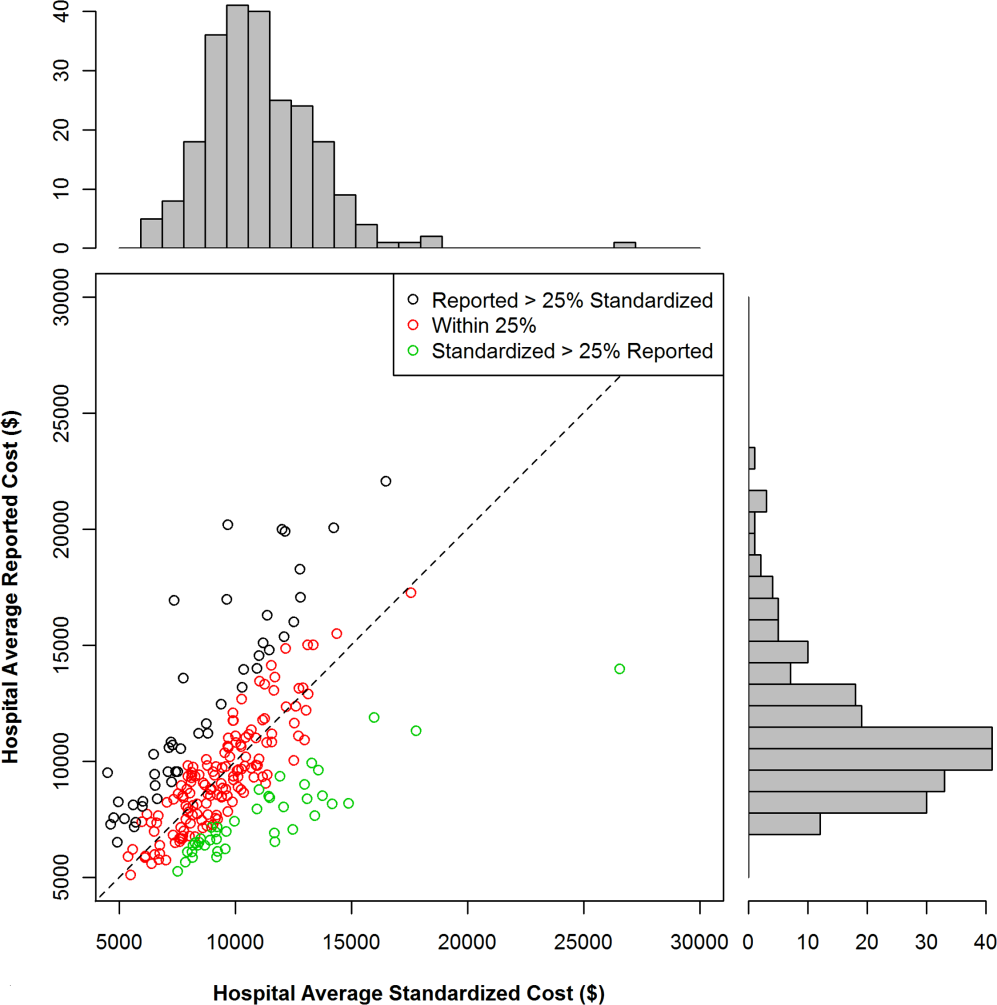
We found that 46 (20%) hospitals had reported costs of hospitalizations that were 25% greater than standardized costs (Figure 2). This group of hospitals had overestimated reported costs of utilization; 146 (62%) had reported costs within 25% of standardized costs, and 42 (17%) had reported costs that were 25% less than standardized costs (indicating that reported costs underestimated utilization). We examined the relationship between hospital characteristics and strata and found no significant association between the reported to standardized cost ratio and number of beds, teaching status, or urban location (Table 2). Hospitals in the Midwest and South were more likely to have a lower reported cost of hospitalizations, whereas hospitals in the West were more likely to have higher reported costs (P<0.001). When using the CV to compare reported costs to standardized costs, we found that per‐day standardized costs showed reduced variance (P=0.0238), but there was no significant difference in variance of the reported and standardized costs when examining the entire hospitalization (P=0.1423). At the level of the hospitalization, the Spearman correlation coefficient between reported and standardized cost was 0.89.
| Reported Greater Than Standardized by >25%, n (%) | Reported Within 25% (2‐tailed) of Standardized, n (%) | Reported Less Than Standardized by >25%, n (%) | P for 2 Test | |
|---|---|---|---|---|
| Total | 46 (19.7) | 146 (62.4) | 42 (17.0) | |
| No. of beds | 0.2313 | |||
| <200 | 19 (41.3) | 40 (27.4) | 12 (28.6) | |
| 200400 | 14 (30.4) | 67 (45.9) | 15 (35.7) | |
| >400 | 13 (28.3) | 39 (26.7) | 15 (35.7) | |
| Teaching | 0.8278 | |||
| Yes | 13 (28.3) | 45 (30.8) | 11 (26.2) | |
| No | 33 (71.7) | 101 (69.2) | 31 (73.8) | |
| Region | <0.0001 | |||
| Midwest | 7 (15.2) | 43 (29.5) | 19 (45.2) | |
| Northeast | 6 (13.0) | 18 (12.3) | 3 (7.1) | |
| South | 14 (30.4) | 64 (43.8) | 20 (47.6) | |
| West | 19 (41.3) | 21 (14.4) | 0 (0) | |
| Urban vs rural | 36 (78.3) | 128 (87.7) | 33 (78.6) | 0.1703 |
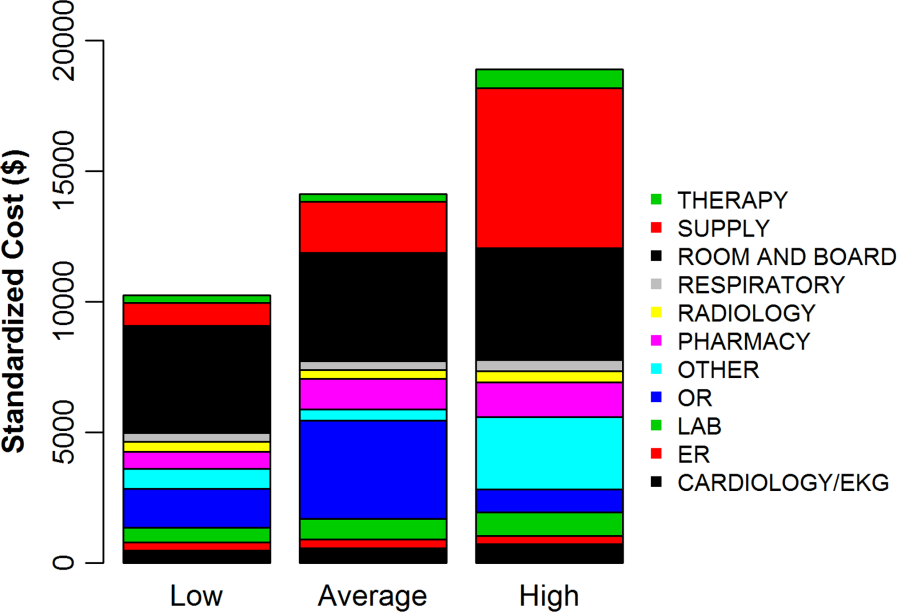
To better understand how hospitals can achieve high reported costs through different mechanisms, we more closely examined 3 hospitals with similar reported costs (Figure 3). These hospitals represented low, average, and high utilization according to their standardized costs, but had similar average per‐hospitalization reported costs: $11,643, $11,787, and $11,892, respectively. The corresponding standardized costs were $8,757, $11,169, and $15,978. The hospital with high utilization ($15,978 in standardized costs) was accounted for by increased use of supplies and other services. In contrast, the low‐ and average‐utilization hospitals had proportionally lower standardized costs across categories, with the greatest percentage of spending going toward room and board (includes nursing).
DISCUSSION
In a large national sample of hospitals, we observed variation in the reported costs for a uniform basket of goods, with a more than 2‐fold difference in cost between the 10th and 90th percentile hospitals. These findings suggest that reported costs have limited ability to reliably describe differences in utilization across hospitals. In contrast, when we applied standardized costs, the variance of per‐day costs decreased significantly, and the interquartile ratio of per‐day and hospitalization costs decreased as well, suggesting less variation in utilization across hospitals than would have been inferred from a comparison of reported costs. Applying a single, standard cost to all items can facilitate comparisons of utilization between hospitals (Figure 1). Standardized costs will give hospitals the potential to compare their utilization to their competitors and will facilitate research that examines the comparative effectiveness of high and low utilization in the management of medical and surgical conditions.
The reported to standardized cost ratio is another useful tool. It indicates whether the hospital's reported costs exaggerate its utilization relative to other hospitals. In this study, we found that a significant proportion of hospitals (20%) had reported costs that exceeded standardized costs by more than 25%. These hospitals have higher infrastructure, labor, or acquisition costs relative to their peers. To the extent that these hospitals might wish to lower the cost of care at their institution, they could focus on renegotiating purchasing or labor contracts, identifying areas where they may be overstaffed, or holding off on future infrastructure investments (Table 3).[14] In contrast, 17% of hospitals had reported costs that were 25% less than standardized costs. High‐cost hospitals in this group are therefore providing more treatments and testing to patients relative to their peers and could focus cost‐control efforts on reducing unnecessary utilization and duplicative testing.[20] Our examination of the hospital with high reported costs and very high utilization revealed a high percentage of supplies and other items, which is a category used primarily for nursing expenditures (Figure 3). Because the use of nursing services is directly related to days spent in the hospital, this hospital may wish to more closely examine specific strategies for reducing length of stay.
| High Reported Costs/High Standardized Costs | High Reported Costs/Low Standardized Costs | Low Reported Costs/High Standardized Costs | Low Reported Costs/Low Standardized Costs | |
|---|---|---|---|---|
| Utilization | High | Low | High | Low |
| Severity of illness | Likely to be higher | Likely to be lower | Likely to be higher | Likely to be lower |
| Practice style | Likely to be more intense | Likely to be less intense | Likely to be more intense | Likely to be less intense |
| Fixed costs | High or average | High | Low | Low |
| Infrastructure costs | Likely to be higher | Likely to be higher | Likely to be lower | Likely to be lower |
| Labor costs | Likely to be higher | Likely to be higher | Likely to be lower | Likely to be lower |
| Reported‐to‐standardized cost ratio | Close to 1 | >1 | <1 | Close to 1 |
| Causes of high costs | High utilization, high fixed costs, or both | High acquisition costs, high labor costs, or expensive infrastructure | High utilization | |
| Interventions to reduce costs | Work with clinicians to alter practice style, consider renegotiating cost of acquisitions, hold off on new infrastructure investments | Consider renegotiating cost of acquisitions, hold off on new infrastructure investments, consider reducing size of labor force | Work with clinicians to alter practice style | |
| Usefulness of reported‐ to‐standardized cost ratio | Less useful | More useful | More useful | Less useful |
We did not find a consistent association between the reported to standardized cost ratio and hospital characteristics. This is an important finding that contradicts prior work examining associations between hospital characteristics and costs for heart failure patients,[21] further indicating the complexity of the relationship between fixed costs and variable costs and the difficulty in adjusting reported costs to calculate utilization. For example, small hospitals may have higher acquisition costs and more supply chain difficulties, but they may also have less technology, lower overhead costs, and fewer specialists to order tests and procedures. Hospital characteristics, such as urban location and teaching status, are commonly used as adjustors in cost studies because hospitals in urban areas with teaching missions (which often provide care to low‐income populations) are assumed to have higher fixed costs,[3, 4, 5, 6] but the lack of a consistent relationship between these characteristics and the standardized cost ratio may indicate that using these factors as adjustors for cost may not be effective and could even obscure differences in utilization between hospitals. Notably, we did find an association between hospital region and the reported to standardized cost ratio, but we hesitate to draw conclusions from this finding because the Premier database is imbalanced in terms of regional representation, with fewer hospitals in the Midwest and West and the bulk of the hospitals in the South.
Although standardized costs have great potential, this method has limitations as well. Standardized costs can only be applied when detailed billing data with item‐level costs are available. This is because calculation of standardized costs requires taking the median of item costs and applying the median cost across the database, maintaining the integrity of the relative cost of items to one another. The relative cost of items is preserved (ie, magnetic resonance imaging still costs more than an aspirin), which maintains the general scheme of RVU‐based costs while removing the noise of varying RVU‐based costs across hospitals.[7] Application of an arbitrary item cost would result in the loss of this relative cost difference. Because item costs are not available in traditional administrative datasets, these datasets would not be amenable to this method. However, highly detailed billing data are now being shared by hundreds of hospitals in the Premier network and the University Health System Consortium. These data are widely available to investigators, meaning that the generalizability of this method will only improve over time. It was also a limitation of the study that we chose a limited basket of items common to patients with heart failure to describe the range of reported costs and to provide a standardized snapshot by which to compare hospitals. Because we only included a few items, we may have overestimated or underestimated the range of reported costs for such a basket.
Standardized costs are a novel method for comparing utilization across hospitals. Used properly, they will help identify high‐ and low‐intensity providers of hospital care.
- Health care costs–a primer. Kaiser Family Foundation Web site. Available at: http://www.kff.org/insurance/7670.cfm. Accessed July 20, 2012.
- . Explaining high health care spending in the United States: an international comparison of supply, utilization, prices, and quality. The Commonwealth Fund. 2012. Available at: http://www.commonwealthfund.org/Publications/Issue‐Briefs/2012/May/High‐Health‐Care‐Spending. aspx. Accessed on July 20, 2012.
- , , , , , . The relationship between hospital spending and mortality in patients with sepsis. Arch Intern Med. 2011;171(4):292–299.
- , , , . The elusive connection between health care spending and quality. Health Aff (Millwood). 2009;28(1):w119–w123.
- , , , . Hospital quality and intensity of spending: is there an association? Health Aff (Millwood). 2009;28(4):w566–w572.
- , , , , . Measuring efficiency: the association of hospital costs and quality of care. Health Aff (Millwood). 2009;28(3):897–906.
- , . Assigning resources to health care use for health services research: options and consequences. Med Care. 2009;47(7 suppl 1):S70–S75.
- , , , , . Health care costing: data, methods, current applications. Med Care. 2009;47(7 suppl 1):S1–S6.
- . Determination of VA health care costs. Med Care Res Rev. 2003;60(3 suppl):124S–141S.
- . An improved set of standards for finding cost for cost‐effectiveness analysis. Med Care. 2009;47(7 suppl 1):S82–S88.
- , , , et al. Comparison of approaches for estimating prevalence costs of care for cancer patients: what is the impact of data source? Med Care. 2009;47(7 suppl 1):S64–S69.
- . Principles involved in costing. Med J Aust. 1990;153Suppl:S10–S12.
- . Spending more through “cost control:” our obsessive quest to gut the hospital. Health Aff (Millwood). 1996;15(2):145–154.
- , , , et al. Distribution of variable vs. fixed costs of hospital care. JAMA. 1999;281(7):644–649.
- . Administrative and claims records as sources of health care cost data. Med Care. 2009;47(7 suppl 1):S51–S55.
- , , , , , . Perioperative beta‐blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349–361.
- , , , et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med. 2007;356(5):486–496.
- , , , et al. Procedure intensity and the cost of care. Circ Cardiovasc Qual Outcomes. 2012;5(3):308–313.
- , , . A comparative study of tests for homogeneity of variances, with applications to the outer continental shelf bidding data. Technometrics. 1981;23:351–361.
- , , . Beyond the efficiency index: finding a better way to reduce overuse and increase efficiency in physician care. Health Aff (Millwood). 2008;27(4):w250–w259.
- , , . The association between hospital volume and processes, outcomes, and costs of care for congestive heart failure. Ann Intern Med. 2011;154(2):94–102.
Healthcare spending exceeded $2.5 trillion in 2007, and payments to hospitals represented the largest portion of this spending (more than 30%), equaling the combined cost of physician services and prescription drugs.[1, 2] Researchers and policymakers have emphasized the need to improve the value of hospital care in the United States, but this has been challenging, in part because of the difficulty in identifying hospitals that have high resource utilization relative to their peers.[3, 4, 5, 6, 7, 8, 9, 10, 11]
Most hospitals calculate their costs using internal accounting systems that determine resource utilization via relative value units (RVUs).[7, 8] RVU‐derived costs, also known as hospital reported costs, have proven to be an excellent method for quantifying what it costs a given hospital to provide a treatment, test, or procedure. However, RVU‐based costs are less useful for comparing resource utilization across hospitals because the cost to provide a treatment or service varies widely across hospitals. The cost of an item calculated using RVUs includes not just the item itself, but also a portion of the fixed costs of the hospital (overhead, labor, and infrastructure investments such as electronic records, new buildings, or expensive radiological or surgical equipment).[12] These costs vary by institution, patient population, region of the country, teaching status, and many other variables, making it difficult to identify resource utilization across hospitals.[13, 14]
Recently, a few claims‐based multi‐institutional datasets have begun incorporating item‐level RVU‐based costs derived directly from the cost accounting systems of participating institutions.[15] Such datasets allow researchers to compare reported costs of care from hospital to hospital, but because of the limitations we described above, they still cannot be used to answer the question: Which hospitals with higher costs of care are actually providing more treatments and services to patients?
To better facilitate the comparison of resource utilization patterns across hospitals, we standardized the unit costs of all treatments and services across hospitals by applying a single cost to every item across hospitals. This standardized cost allowed to compare utilization of that item (and the 15,000 other items in the database) across hospitals. We then compared estimates of resource utilization as measured by the 2 approaches: standardized and RVU‐based costs.
METHODS
Ethics Statement
All data were deidentified, by Premier, Inc., at both the hospital and patient level in accordance with the Health Insurance Portability and Accountability Act. The Yale University Human Investigation Committee reviewed the protocol for this study and determined that it is not considered to be human subjects research as defined by the Office of Human Research Protections.
Data Source
We conducted a cross‐sectional study using data from hospitals that participated in the database maintained by Premier Healthcare Informatics (Charlotte, NC) in the years 2009 to 2010. The Premier database is a voluntary, fee‐supported database created to measure quality and healthcare utilization.[3, 16, 17, 18] In 2010, it included detailed billing data from 500 hospitals in the United States, with more than 130 million cumulative hospital discharges. The detailed billing data includes all elements found in hospital claims derived from the uniform billing‐04 form, as well as an itemized, date‐stamped log of all items and services charged to the patient or insurer, such as medications, laboratory tests, and diagnostic and therapeutic services. The database includes approximately 15% of all US hospitalizations. Participating hospitals are similar to the composition of acute care hospitals nationwide. They represent all regions of the United States, and represent predominantly small‐ to mid‐sized nonteaching facilities that serve a largely urban population. The database also contains hospital reported costs at the item level as well as the total cost of the hospitalization. Approximately 75% of hospitals that participate submit RVU‐based costs taken from internal cost accounting systems. Because of our focus on comparing standardized costs to reported costs, we included only data from hospitals that use RVU‐based costs in this study.
Study Subjects
We included adult patients with a hospitalization recorded in the Premier database between January 1, 2009 and December 31, 2010, and a principal discharge diagnosis of heart failure (HF) (International Classification of Diseases, Ninth Revision, Clinical Modification codes: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx). We excluded transfers, patients assigned a pediatrician as the attending of record, and those who received a heart transplant or ventricular assist device during their stay. Because cost data are prone to extreme outliers, we excluded hospitalizations that were in the top 0.1% of length of stay, number of billing records, quantity of items billed, or total standardized cost. We also excluded hospitals that admitted fewer than 25 HF patients during the study period to reduce the possibility that a single high‐cost patient affected the hospital's cost profile.
Hospital Information
For each hospital included in the study, we recorded number of beds, teaching status, geographic region, and whether it served an urban or rural population.
Assignment of Standardized Costs
We defined reported cost as the RVU‐based cost per item in the database. We then calculated the median across hospitals for each item in the database and set this as the standardized unit cost of that item at every hospital (Figure 1). Once standardized costs were assigned at the item level, we summed the costs of all items assigned to each patient and calculated the standardized cost of a hospitalization per patient at each hospital.

Examination of Cost Variation
We compared the standardized and reported costs of hospitalizations using medians, interquartile ranges, and interquartile ratios (Q75/Q25). To examine whether standardized costs can reduce the noise due to differences in overhead and other fixed costs, we calculated, for each hospital, the coefficients of variation (CV) for per‐day reported and standardized costs and per‐hospitalization reported and standardized costs. We used the Fligner‐Killeen test to determine whether the variance of CVs was different for reported and standardized costs.[19]
Creation of Basket of Goods
Because there can be differences in the costs of items, the number and types of items administered during hospitalizations, 2 hospitals with similar reported costs for a hospitalization might deliver different quantities and combinations of treatments (Figure 1). We wished to demonstrate that there is variation in reported costs of items when the quantity and type of item is held constant, so we created a basket of items. We chose items that are commonly administered to patients with heart failure, but could have chosen any combination of items. The basket included a day of medical room and board, a day of intensive care unit (ICU) room and board, a single dose of ‐blocker, a single dose of angiotensin‐converting enzyme inhibitor, complete blood count, a B‐natriuretic peptide level, a chest radiograph, a chest computed tomography, and an echocardiogram. We then examined the range of hospitals' reported costs for this basket of goods using percentiles, medians, and interquartile ranges.
Reported to Standardized Cost Ratio
Next, we calculated standardized costs of hospitalizations for included hospitals and examined the relationship between hospitals' mean reported costs and mean standardized costs. This ratio could help diagnose the mechanism of high reported costs for a hospital, because high reported costs with low utilization would indicate high fixed costs, while high reported costs with high utilization would indicate greater use of tests and treatments. We assigned hospitals to strata based on reported costs greater than standardized costs by more than 25%, reported costs within 25% of standardized costs, and reported costs less than standardized costs by more than 25%. We examined the association between hospital characteristics and strata using a 2 test. All analyses were carried out using SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
The 234 hospitals included in the analysis contributed a total of 165,647 hospitalizations, with the number of hospitalizations ranging from 33 to 2,772 hospitalizations per hospital (see Supporting Table 1 in the online version of this article). Most were located in urban areas (84%), and many were in the southern United States (42%). The median hospital reported cost per hospitalization was $6,535, with an interquartile range of $5,541 to $7,454. The median standardized cost per hospitalization was $6,602, with a range of $5,866 to $7,386. The interquartile ratio (Q75/Q25) of the reported costs of a hospitalization was 1.35. After costs were standardized, the interquartile ratio fell to 1.26, indicating that variation decreased. We found that the median hospital reported cost per day was $1,651, with an IQR of $1,400 to $1,933 (ratio 1.38), whereas the median standardized cost per day was $1,640, with an IQR of $1,511 to $1,812 (ratio 1.20).
There were more than 15,000 items (eg, treatments, tests, and supplies) that received a standardized charge code in our cohort. These were divided into 11 summary departments and 40 standard departments (see Supporting Table 2 in the online version of this article). We observed a high level of variation in the reported costs of individual items: the reported costs of a day of room and board in an ICU ranged from $773 at hospitals at the 10th percentile to $2,471 at the 90th percentile (Table 1.). The standardized cost of a day of ICU room and board was $1,577. We also observed variation in the reported costs of items across item categories. Although a day of medical room and board showed a 3‐fold difference between the 10th and 90th percentile, we observed a more than 10‐fold difference in the reported cost of an echocardiogram, from $31 at the 10th percentile to $356 at the 90th percentile. After examining the hospital‐level cost for a basket of goods, we found variation in the reported costs for these items across hospitals, with a 10th percentile cost of $1,552 and a 90th percentile cost of $3,967.
| Reported Costs | 10th Percentile | 25th Percentile | 75th Percentile | 90th Percentile | Median (Standardized Cost) |
|---|---|---|---|---|---|
| |||||
| Item | |||||
| Day of medical | 490.03 | 586.41 | 889.95 | 1121.20 | 722.59 |
| Day of ICU | 773.01 | 1275.84 | 1994.81 | 2471.75 | 1577.93 |
| Complete blood count | 6.87 | 9.34 | 18.34 | 23.46 | 13.07 |
| B‐natriuretic peptide | 12.13 | 19.22 | 44.19 | 60.56 | 28.23 |
| Metoprolol | 0.20 | 0.68 | 2.67 | 3.74 | 1.66 |
| Lisinopril | 0.28 | 1.02 | 2.79 | 4.06 | 1.72 |
| Spironolactone | 0.22 | 0.53 | 2.68 | 3.83 | 1.63 |
| Furosemide | 1.27 | 2.45 | 5.73 | 8.12 | 3.82 |
| Chest x‐ray | 43.88 | 51.54 | 89.96 | 117.16 | 67.45 |
| Echocardiogram | 31.53 | 98.63 | 244.63 | 356.50 | 159.07 |
| Chest CT (w & w/o contrast) | 65.17 | 83.99 | 157.23 | 239.27 | 110.76 |
| Noninvasive positive pressure ventilation | 126.23 | 127.25 | 370.44 | 514.67 | 177.24 |
| Electrocardiogram | 12.08 | 18.77 | 42.74 | 64.94 | 29.78 |
| Total basket | 1552.50 | 2157.85 | 3417.34 | 3967.78 | 2710.49 |
We found that 46 (20%) hospitals had reported costs of hospitalizations that were 25% greater than standardized costs (Figure 2). This group of hospitals had overestimated reported costs of utilization; 146 (62%) had reported costs within 25% of standardized costs, and 42 (17%) had reported costs that were 25% less than standardized costs (indicating that reported costs underestimated utilization). We examined the relationship between hospital characteristics and strata and found no significant association between the reported to standardized cost ratio and number of beds, teaching status, or urban location (Table 2). Hospitals in the Midwest and South were more likely to have a lower reported cost of hospitalizations, whereas hospitals in the West were more likely to have higher reported costs (P<0.001). When using the CV to compare reported costs to standardized costs, we found that per‐day standardized costs showed reduced variance (P=0.0238), but there was no significant difference in variance of the reported and standardized costs when examining the entire hospitalization (P=0.1423). At the level of the hospitalization, the Spearman correlation coefficient between reported and standardized cost was 0.89.

| Reported Greater Than Standardized by >25%, n (%) | Reported Within 25% (2‐tailed) of Standardized, n (%) | Reported Less Than Standardized by >25%, n (%) | P for 2 Test | |
|---|---|---|---|---|
| Total | 46 (19.7) | 146 (62.4) | 42 (17.0) | |
| No. of beds | 0.2313 | |||
| <200 | 19 (41.3) | 40 (27.4) | 12 (28.6) | |
| 200400 | 14 (30.4) | 67 (45.9) | 15 (35.7) | |
| >400 | 13 (28.3) | 39 (26.7) | 15 (35.7) | |
| Teaching | 0.8278 | |||
| Yes | 13 (28.3) | 45 (30.8) | 11 (26.2) | |
| No | 33 (71.7) | 101 (69.2) | 31 (73.8) | |
| Region | <0.0001 | |||
| Midwest | 7 (15.2) | 43 (29.5) | 19 (45.2) | |
| Northeast | 6 (13.0) | 18 (12.3) | 3 (7.1) | |
| South | 14 (30.4) | 64 (43.8) | 20 (47.6) | |
| West | 19 (41.3) | 21 (14.4) | 0 (0) | |
| Urban vs rural | 36 (78.3) | 128 (87.7) | 33 (78.6) | 0.1703 |
To better understand how hospitals can achieve high reported costs through different mechanisms, we more closely examined 3 hospitals with similar reported costs (Figure 3). These hospitals represented low, average, and high utilization according to their standardized costs, but had similar average per‐hospitalization reported costs: $11,643, $11,787, and $11,892, respectively. The corresponding standardized costs were $8,757, $11,169, and $15,978. The hospital with high utilization ($15,978 in standardized costs) was accounted for by increased use of supplies and other services. In contrast, the low‐ and average‐utilization hospitals had proportionally lower standardized costs across categories, with the greatest percentage of spending going toward room and board (includes nursing).

DISCUSSION
In a large national sample of hospitals, we observed variation in the reported costs for a uniform basket of goods, with a more than 2‐fold difference in cost between the 10th and 90th percentile hospitals. These findings suggest that reported costs have limited ability to reliably describe differences in utilization across hospitals. In contrast, when we applied standardized costs, the variance of per‐day costs decreased significantly, and the interquartile ratio of per‐day and hospitalization costs decreased as well, suggesting less variation in utilization across hospitals than would have been inferred from a comparison of reported costs. Applying a single, standard cost to all items can facilitate comparisons of utilization between hospitals (Figure 1). Standardized costs will give hospitals the potential to compare their utilization to their competitors and will facilitate research that examines the comparative effectiveness of high and low utilization in the management of medical and surgical conditions.
The reported to standardized cost ratio is another useful tool. It indicates whether the hospital's reported costs exaggerate its utilization relative to other hospitals. In this study, we found that a significant proportion of hospitals (20%) had reported costs that exceeded standardized costs by more than 25%. These hospitals have higher infrastructure, labor, or acquisition costs relative to their peers. To the extent that these hospitals might wish to lower the cost of care at their institution, they could focus on renegotiating purchasing or labor contracts, identifying areas where they may be overstaffed, or holding off on future infrastructure investments (Table 3).[14] In contrast, 17% of hospitals had reported costs that were 25% less than standardized costs. High‐cost hospitals in this group are therefore providing more treatments and testing to patients relative to their peers and could focus cost‐control efforts on reducing unnecessary utilization and duplicative testing.[20] Our examination of the hospital with high reported costs and very high utilization revealed a high percentage of supplies and other items, which is a category used primarily for nursing expenditures (Figure 3). Because the use of nursing services is directly related to days spent in the hospital, this hospital may wish to more closely examine specific strategies for reducing length of stay.
| High Reported Costs/High Standardized Costs | High Reported Costs/Low Standardized Costs | Low Reported Costs/High Standardized Costs | Low Reported Costs/Low Standardized Costs | |
|---|---|---|---|---|
| Utilization | High | Low | High | Low |
| Severity of illness | Likely to be higher | Likely to be lower | Likely to be higher | Likely to be lower |
| Practice style | Likely to be more intense | Likely to be less intense | Likely to be more intense | Likely to be less intense |
| Fixed costs | High or average | High | Low | Low |
| Infrastructure costs | Likely to be higher | Likely to be higher | Likely to be lower | Likely to be lower |
| Labor costs | Likely to be higher | Likely to be higher | Likely to be lower | Likely to be lower |
| Reported‐to‐standardized cost ratio | Close to 1 | >1 | <1 | Close to 1 |
| Causes of high costs | High utilization, high fixed costs, or both | High acquisition costs, high labor costs, or expensive infrastructure | High utilization | |
| Interventions to reduce costs | Work with clinicians to alter practice style, consider renegotiating cost of acquisitions, hold off on new infrastructure investments | Consider renegotiating cost of acquisitions, hold off on new infrastructure investments, consider reducing size of labor force | Work with clinicians to alter practice style | |
| Usefulness of reported‐ to‐standardized cost ratio | Less useful | More useful | More useful | Less useful |
We did not find a consistent association between the reported to standardized cost ratio and hospital characteristics. This is an important finding that contradicts prior work examining associations between hospital characteristics and costs for heart failure patients,[21] further indicating the complexity of the relationship between fixed costs and variable costs and the difficulty in adjusting reported costs to calculate utilization. For example, small hospitals may have higher acquisition costs and more supply chain difficulties, but they may also have less technology, lower overhead costs, and fewer specialists to order tests and procedures. Hospital characteristics, such as urban location and teaching status, are commonly used as adjustors in cost studies because hospitals in urban areas with teaching missions (which often provide care to low‐income populations) are assumed to have higher fixed costs,[3, 4, 5, 6] but the lack of a consistent relationship between these characteristics and the standardized cost ratio may indicate that using these factors as adjustors for cost may not be effective and could even obscure differences in utilization between hospitals. Notably, we did find an association between hospital region and the reported to standardized cost ratio, but we hesitate to draw conclusions from this finding because the Premier database is imbalanced in terms of regional representation, with fewer hospitals in the Midwest and West and the bulk of the hospitals in the South.
Although standardized costs have great potential, this method has limitations as well. Standardized costs can only be applied when detailed billing data with item‐level costs are available. This is because calculation of standardized costs requires taking the median of item costs and applying the median cost across the database, maintaining the integrity of the relative cost of items to one another. The relative cost of items is preserved (ie, magnetic resonance imaging still costs more than an aspirin), which maintains the general scheme of RVU‐based costs while removing the noise of varying RVU‐based costs across hospitals.[7] Application of an arbitrary item cost would result in the loss of this relative cost difference. Because item costs are not available in traditional administrative datasets, these datasets would not be amenable to this method. However, highly detailed billing data are now being shared by hundreds of hospitals in the Premier network and the University Health System Consortium. These data are widely available to investigators, meaning that the generalizability of this method will only improve over time. It was also a limitation of the study that we chose a limited basket of items common to patients with heart failure to describe the range of reported costs and to provide a standardized snapshot by which to compare hospitals. Because we only included a few items, we may have overestimated or underestimated the range of reported costs for such a basket.
Standardized costs are a novel method for comparing utilization across hospitals. Used properly, they will help identify high‐ and low‐intensity providers of hospital care.
Healthcare spending exceeded $2.5 trillion in 2007, and payments to hospitals represented the largest portion of this spending (more than 30%), equaling the combined cost of physician services and prescription drugs.[1, 2] Researchers and policymakers have emphasized the need to improve the value of hospital care in the United States, but this has been challenging, in part because of the difficulty in identifying hospitals that have high resource utilization relative to their peers.[3, 4, 5, 6, 7, 8, 9, 10, 11]
Most hospitals calculate their costs using internal accounting systems that determine resource utilization via relative value units (RVUs).[7, 8] RVU‐derived costs, also known as hospital reported costs, have proven to be an excellent method for quantifying what it costs a given hospital to provide a treatment, test, or procedure. However, RVU‐based costs are less useful for comparing resource utilization across hospitals because the cost to provide a treatment or service varies widely across hospitals. The cost of an item calculated using RVUs includes not just the item itself, but also a portion of the fixed costs of the hospital (overhead, labor, and infrastructure investments such as electronic records, new buildings, or expensive radiological or surgical equipment).[12] These costs vary by institution, patient population, region of the country, teaching status, and many other variables, making it difficult to identify resource utilization across hospitals.[13, 14]
Recently, a few claims‐based multi‐institutional datasets have begun incorporating item‐level RVU‐based costs derived directly from the cost accounting systems of participating institutions.[15] Such datasets allow researchers to compare reported costs of care from hospital to hospital, but because of the limitations we described above, they still cannot be used to answer the question: Which hospitals with higher costs of care are actually providing more treatments and services to patients?
To better facilitate the comparison of resource utilization patterns across hospitals, we standardized the unit costs of all treatments and services across hospitals by applying a single cost to every item across hospitals. This standardized cost allowed to compare utilization of that item (and the 15,000 other items in the database) across hospitals. We then compared estimates of resource utilization as measured by the 2 approaches: standardized and RVU‐based costs.
METHODS
Ethics Statement
All data were deidentified, by Premier, Inc., at both the hospital and patient level in accordance with the Health Insurance Portability and Accountability Act. The Yale University Human Investigation Committee reviewed the protocol for this study and determined that it is not considered to be human subjects research as defined by the Office of Human Research Protections.
Data Source
We conducted a cross‐sectional study using data from hospitals that participated in the database maintained by Premier Healthcare Informatics (Charlotte, NC) in the years 2009 to 2010. The Premier database is a voluntary, fee‐supported database created to measure quality and healthcare utilization.[3, 16, 17, 18] In 2010, it included detailed billing data from 500 hospitals in the United States, with more than 130 million cumulative hospital discharges. The detailed billing data includes all elements found in hospital claims derived from the uniform billing‐04 form, as well as an itemized, date‐stamped log of all items and services charged to the patient or insurer, such as medications, laboratory tests, and diagnostic and therapeutic services. The database includes approximately 15% of all US hospitalizations. Participating hospitals are similar to the composition of acute care hospitals nationwide. They represent all regions of the United States, and represent predominantly small‐ to mid‐sized nonteaching facilities that serve a largely urban population. The database also contains hospital reported costs at the item level as well as the total cost of the hospitalization. Approximately 75% of hospitals that participate submit RVU‐based costs taken from internal cost accounting systems. Because of our focus on comparing standardized costs to reported costs, we included only data from hospitals that use RVU‐based costs in this study.
Study Subjects
We included adult patients with a hospitalization recorded in the Premier database between January 1, 2009 and December 31, 2010, and a principal discharge diagnosis of heart failure (HF) (International Classification of Diseases, Ninth Revision, Clinical Modification codes: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx). We excluded transfers, patients assigned a pediatrician as the attending of record, and those who received a heart transplant or ventricular assist device during their stay. Because cost data are prone to extreme outliers, we excluded hospitalizations that were in the top 0.1% of length of stay, number of billing records, quantity of items billed, or total standardized cost. We also excluded hospitals that admitted fewer than 25 HF patients during the study period to reduce the possibility that a single high‐cost patient affected the hospital's cost profile.
Hospital Information
For each hospital included in the study, we recorded number of beds, teaching status, geographic region, and whether it served an urban or rural population.
Assignment of Standardized Costs
We defined reported cost as the RVU‐based cost per item in the database. We then calculated the median across hospitals for each item in the database and set this as the standardized unit cost of that item at every hospital (Figure 1). Once standardized costs were assigned at the item level, we summed the costs of all items assigned to each patient and calculated the standardized cost of a hospitalization per patient at each hospital.

Examination of Cost Variation
We compared the standardized and reported costs of hospitalizations using medians, interquartile ranges, and interquartile ratios (Q75/Q25). To examine whether standardized costs can reduce the noise due to differences in overhead and other fixed costs, we calculated, for each hospital, the coefficients of variation (CV) for per‐day reported and standardized costs and per‐hospitalization reported and standardized costs. We used the Fligner‐Killeen test to determine whether the variance of CVs was different for reported and standardized costs.[19]
Creation of Basket of Goods
Because there can be differences in the costs of items, the number and types of items administered during hospitalizations, 2 hospitals with similar reported costs for a hospitalization might deliver different quantities and combinations of treatments (Figure 1). We wished to demonstrate that there is variation in reported costs of items when the quantity and type of item is held constant, so we created a basket of items. We chose items that are commonly administered to patients with heart failure, but could have chosen any combination of items. The basket included a day of medical room and board, a day of intensive care unit (ICU) room and board, a single dose of ‐blocker, a single dose of angiotensin‐converting enzyme inhibitor, complete blood count, a B‐natriuretic peptide level, a chest radiograph, a chest computed tomography, and an echocardiogram. We then examined the range of hospitals' reported costs for this basket of goods using percentiles, medians, and interquartile ranges.
Reported to Standardized Cost Ratio
Next, we calculated standardized costs of hospitalizations for included hospitals and examined the relationship between hospitals' mean reported costs and mean standardized costs. This ratio could help diagnose the mechanism of high reported costs for a hospital, because high reported costs with low utilization would indicate high fixed costs, while high reported costs with high utilization would indicate greater use of tests and treatments. We assigned hospitals to strata based on reported costs greater than standardized costs by more than 25%, reported costs within 25% of standardized costs, and reported costs less than standardized costs by more than 25%. We examined the association between hospital characteristics and strata using a 2 test. All analyses were carried out using SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
The 234 hospitals included in the analysis contributed a total of 165,647 hospitalizations, with the number of hospitalizations ranging from 33 to 2,772 hospitalizations per hospital (see Supporting Table 1 in the online version of this article). Most were located in urban areas (84%), and many were in the southern United States (42%). The median hospital reported cost per hospitalization was $6,535, with an interquartile range of $5,541 to $7,454. The median standardized cost per hospitalization was $6,602, with a range of $5,866 to $7,386. The interquartile ratio (Q75/Q25) of the reported costs of a hospitalization was 1.35. After costs were standardized, the interquartile ratio fell to 1.26, indicating that variation decreased. We found that the median hospital reported cost per day was $1,651, with an IQR of $1,400 to $1,933 (ratio 1.38), whereas the median standardized cost per day was $1,640, with an IQR of $1,511 to $1,812 (ratio 1.20).
There were more than 15,000 items (eg, treatments, tests, and supplies) that received a standardized charge code in our cohort. These were divided into 11 summary departments and 40 standard departments (see Supporting Table 2 in the online version of this article). We observed a high level of variation in the reported costs of individual items: the reported costs of a day of room and board in an ICU ranged from $773 at hospitals at the 10th percentile to $2,471 at the 90th percentile (Table 1.). The standardized cost of a day of ICU room and board was $1,577. We also observed variation in the reported costs of items across item categories. Although a day of medical room and board showed a 3‐fold difference between the 10th and 90th percentile, we observed a more than 10‐fold difference in the reported cost of an echocardiogram, from $31 at the 10th percentile to $356 at the 90th percentile. After examining the hospital‐level cost for a basket of goods, we found variation in the reported costs for these items across hospitals, with a 10th percentile cost of $1,552 and a 90th percentile cost of $3,967.
| Reported Costs | 10th Percentile | 25th Percentile | 75th Percentile | 90th Percentile | Median (Standardized Cost) |
|---|---|---|---|---|---|
| |||||
| Item | |||||
| Day of medical | 490.03 | 586.41 | 889.95 | 1121.20 | 722.59 |
| Day of ICU | 773.01 | 1275.84 | 1994.81 | 2471.75 | 1577.93 |
| Complete blood count | 6.87 | 9.34 | 18.34 | 23.46 | 13.07 |
| B‐natriuretic peptide | 12.13 | 19.22 | 44.19 | 60.56 | 28.23 |
| Metoprolol | 0.20 | 0.68 | 2.67 | 3.74 | 1.66 |
| Lisinopril | 0.28 | 1.02 | 2.79 | 4.06 | 1.72 |
| Spironolactone | 0.22 | 0.53 | 2.68 | 3.83 | 1.63 |
| Furosemide | 1.27 | 2.45 | 5.73 | 8.12 | 3.82 |
| Chest x‐ray | 43.88 | 51.54 | 89.96 | 117.16 | 67.45 |
| Echocardiogram | 31.53 | 98.63 | 244.63 | 356.50 | 159.07 |
| Chest CT (w & w/o contrast) | 65.17 | 83.99 | 157.23 | 239.27 | 110.76 |
| Noninvasive positive pressure ventilation | 126.23 | 127.25 | 370.44 | 514.67 | 177.24 |
| Electrocardiogram | 12.08 | 18.77 | 42.74 | 64.94 | 29.78 |
| Total basket | 1552.50 | 2157.85 | 3417.34 | 3967.78 | 2710.49 |
We found that 46 (20%) hospitals had reported costs of hospitalizations that were 25% greater than standardized costs (Figure 2). This group of hospitals had overestimated reported costs of utilization; 146 (62%) had reported costs within 25% of standardized costs, and 42 (17%) had reported costs that were 25% less than standardized costs (indicating that reported costs underestimated utilization). We examined the relationship between hospital characteristics and strata and found no significant association between the reported to standardized cost ratio and number of beds, teaching status, or urban location (Table 2). Hospitals in the Midwest and South were more likely to have a lower reported cost of hospitalizations, whereas hospitals in the West were more likely to have higher reported costs (P<0.001). When using the CV to compare reported costs to standardized costs, we found that per‐day standardized costs showed reduced variance (P=0.0238), but there was no significant difference in variance of the reported and standardized costs when examining the entire hospitalization (P=0.1423). At the level of the hospitalization, the Spearman correlation coefficient between reported and standardized cost was 0.89.

| Reported Greater Than Standardized by >25%, n (%) | Reported Within 25% (2‐tailed) of Standardized, n (%) | Reported Less Than Standardized by >25%, n (%) | P for 2 Test | |
|---|---|---|---|---|
| Total | 46 (19.7) | 146 (62.4) | 42 (17.0) | |
| No. of beds | 0.2313 | |||
| <200 | 19 (41.3) | 40 (27.4) | 12 (28.6) | |
| 200400 | 14 (30.4) | 67 (45.9) | 15 (35.7) | |
| >400 | 13 (28.3) | 39 (26.7) | 15 (35.7) | |
| Teaching | 0.8278 | |||
| Yes | 13 (28.3) | 45 (30.8) | 11 (26.2) | |
| No | 33 (71.7) | 101 (69.2) | 31 (73.8) | |
| Region | <0.0001 | |||
| Midwest | 7 (15.2) | 43 (29.5) | 19 (45.2) | |
| Northeast | 6 (13.0) | 18 (12.3) | 3 (7.1) | |
| South | 14 (30.4) | 64 (43.8) | 20 (47.6) | |
| West | 19 (41.3) | 21 (14.4) | 0 (0) | |
| Urban vs rural | 36 (78.3) | 128 (87.7) | 33 (78.6) | 0.1703 |
To better understand how hospitals can achieve high reported costs through different mechanisms, we more closely examined 3 hospitals with similar reported costs (Figure 3). These hospitals represented low, average, and high utilization according to their standardized costs, but had similar average per‐hospitalization reported costs: $11,643, $11,787, and $11,892, respectively. The corresponding standardized costs were $8,757, $11,169, and $15,978. The hospital with high utilization ($15,978 in standardized costs) was accounted for by increased use of supplies and other services. In contrast, the low‐ and average‐utilization hospitals had proportionally lower standardized costs across categories, with the greatest percentage of spending going toward room and board (includes nursing).

DISCUSSION
In a large national sample of hospitals, we observed variation in the reported costs for a uniform basket of goods, with a more than 2‐fold difference in cost between the 10th and 90th percentile hospitals. These findings suggest that reported costs have limited ability to reliably describe differences in utilization across hospitals. In contrast, when we applied standardized costs, the variance of per‐day costs decreased significantly, and the interquartile ratio of per‐day and hospitalization costs decreased as well, suggesting less variation in utilization across hospitals than would have been inferred from a comparison of reported costs. Applying a single, standard cost to all items can facilitate comparisons of utilization between hospitals (Figure 1). Standardized costs will give hospitals the potential to compare their utilization to their competitors and will facilitate research that examines the comparative effectiveness of high and low utilization in the management of medical and surgical conditions.
The reported to standardized cost ratio is another useful tool. It indicates whether the hospital's reported costs exaggerate its utilization relative to other hospitals. In this study, we found that a significant proportion of hospitals (20%) had reported costs that exceeded standardized costs by more than 25%. These hospitals have higher infrastructure, labor, or acquisition costs relative to their peers. To the extent that these hospitals might wish to lower the cost of care at their institution, they could focus on renegotiating purchasing or labor contracts, identifying areas where they may be overstaffed, or holding off on future infrastructure investments (Table 3).[14] In contrast, 17% of hospitals had reported costs that were 25% less than standardized costs. High‐cost hospitals in this group are therefore providing more treatments and testing to patients relative to their peers and could focus cost‐control efforts on reducing unnecessary utilization and duplicative testing.[20] Our examination of the hospital with high reported costs and very high utilization revealed a high percentage of supplies and other items, which is a category used primarily for nursing expenditures (Figure 3). Because the use of nursing services is directly related to days spent in the hospital, this hospital may wish to more closely examine specific strategies for reducing length of stay.
| High Reported Costs/High Standardized Costs | High Reported Costs/Low Standardized Costs | Low Reported Costs/High Standardized Costs | Low Reported Costs/Low Standardized Costs | |
|---|---|---|---|---|
| Utilization | High | Low | High | Low |
| Severity of illness | Likely to be higher | Likely to be lower | Likely to be higher | Likely to be lower |
| Practice style | Likely to be more intense | Likely to be less intense | Likely to be more intense | Likely to be less intense |
| Fixed costs | High or average | High | Low | Low |
| Infrastructure costs | Likely to be higher | Likely to be higher | Likely to be lower | Likely to be lower |
| Labor costs | Likely to be higher | Likely to be higher | Likely to be lower | Likely to be lower |
| Reported‐to‐standardized cost ratio | Close to 1 | >1 | <1 | Close to 1 |
| Causes of high costs | High utilization, high fixed costs, or both | High acquisition costs, high labor costs, or expensive infrastructure | High utilization | |
| Interventions to reduce costs | Work with clinicians to alter practice style, consider renegotiating cost of acquisitions, hold off on new infrastructure investments | Consider renegotiating cost of acquisitions, hold off on new infrastructure investments, consider reducing size of labor force | Work with clinicians to alter practice style | |
| Usefulness of reported‐ to‐standardized cost ratio | Less useful | More useful | More useful | Less useful |
We did not find a consistent association between the reported to standardized cost ratio and hospital characteristics. This is an important finding that contradicts prior work examining associations between hospital characteristics and costs for heart failure patients,[21] further indicating the complexity of the relationship between fixed costs and variable costs and the difficulty in adjusting reported costs to calculate utilization. For example, small hospitals may have higher acquisition costs and more supply chain difficulties, but they may also have less technology, lower overhead costs, and fewer specialists to order tests and procedures. Hospital characteristics, such as urban location and teaching status, are commonly used as adjustors in cost studies because hospitals in urban areas with teaching missions (which often provide care to low‐income populations) are assumed to have higher fixed costs,[3, 4, 5, 6] but the lack of a consistent relationship between these characteristics and the standardized cost ratio may indicate that using these factors as adjustors for cost may not be effective and could even obscure differences in utilization between hospitals. Notably, we did find an association between hospital region and the reported to standardized cost ratio, but we hesitate to draw conclusions from this finding because the Premier database is imbalanced in terms of regional representation, with fewer hospitals in the Midwest and West and the bulk of the hospitals in the South.
Although standardized costs have great potential, this method has limitations as well. Standardized costs can only be applied when detailed billing data with item‐level costs are available. This is because calculation of standardized costs requires taking the median of item costs and applying the median cost across the database, maintaining the integrity of the relative cost of items to one another. The relative cost of items is preserved (ie, magnetic resonance imaging still costs more than an aspirin), which maintains the general scheme of RVU‐based costs while removing the noise of varying RVU‐based costs across hospitals.[7] Application of an arbitrary item cost would result in the loss of this relative cost difference. Because item costs are not available in traditional administrative datasets, these datasets would not be amenable to this method. However, highly detailed billing data are now being shared by hundreds of hospitals in the Premier network and the University Health System Consortium. These data are widely available to investigators, meaning that the generalizability of this method will only improve over time. It was also a limitation of the study that we chose a limited basket of items common to patients with heart failure to describe the range of reported costs and to provide a standardized snapshot by which to compare hospitals. Because we only included a few items, we may have overestimated or underestimated the range of reported costs for such a basket.
Standardized costs are a novel method for comparing utilization across hospitals. Used properly, they will help identify high‐ and low‐intensity providers of hospital care.
- Health care costs–a primer. Kaiser Family Foundation Web site. Available at: http://www.kff.org/insurance/7670.cfm. Accessed July 20, 2012.
- . Explaining high health care spending in the United States: an international comparison of supply, utilization, prices, and quality. The Commonwealth Fund. 2012. Available at: http://www.commonwealthfund.org/Publications/Issue‐Briefs/2012/May/High‐Health‐Care‐Spending. aspx. Accessed on July 20, 2012.
- , , , , , . The relationship between hospital spending and mortality in patients with sepsis. Arch Intern Med. 2011;171(4):292–299.
- , , , . The elusive connection between health care spending and quality. Health Aff (Millwood). 2009;28(1):w119–w123.
- , , , . Hospital quality and intensity of spending: is there an association? Health Aff (Millwood). 2009;28(4):w566–w572.
- , , , , . Measuring efficiency: the association of hospital costs and quality of care. Health Aff (Millwood). 2009;28(3):897–906.
- , . Assigning resources to health care use for health services research: options and consequences. Med Care. 2009;47(7 suppl 1):S70–S75.
- , , , , . Health care costing: data, methods, current applications. Med Care. 2009;47(7 suppl 1):S1–S6.
- . Determination of VA health care costs. Med Care Res Rev. 2003;60(3 suppl):124S–141S.
- . An improved set of standards for finding cost for cost‐effectiveness analysis. Med Care. 2009;47(7 suppl 1):S82–S88.
- , , , et al. Comparison of approaches for estimating prevalence costs of care for cancer patients: what is the impact of data source? Med Care. 2009;47(7 suppl 1):S64–S69.
- . Principles involved in costing. Med J Aust. 1990;153Suppl:S10–S12.
- . Spending more through “cost control:” our obsessive quest to gut the hospital. Health Aff (Millwood). 1996;15(2):145–154.
- , , , et al. Distribution of variable vs. fixed costs of hospital care. JAMA. 1999;281(7):644–649.
- . Administrative and claims records as sources of health care cost data. Med Care. 2009;47(7 suppl 1):S51–S55.
- , , , , , . Perioperative beta‐blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349–361.
- , , , et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med. 2007;356(5):486–496.
- , , , et al. Procedure intensity and the cost of care. Circ Cardiovasc Qual Outcomes. 2012;5(3):308–313.
- , , . A comparative study of tests for homogeneity of variances, with applications to the outer continental shelf bidding data. Technometrics. 1981;23:351–361.
- , , . Beyond the efficiency index: finding a better way to reduce overuse and increase efficiency in physician care. Health Aff (Millwood). 2008;27(4):w250–w259.
- , , . The association between hospital volume and processes, outcomes, and costs of care for congestive heart failure. Ann Intern Med. 2011;154(2):94–102.
- Health care costs–a primer. Kaiser Family Foundation Web site. Available at: http://www.kff.org/insurance/7670.cfm. Accessed July 20, 2012.
- . Explaining high health care spending in the United States: an international comparison of supply, utilization, prices, and quality. The Commonwealth Fund. 2012. Available at: http://www.commonwealthfund.org/Publications/Issue‐Briefs/2012/May/High‐Health‐Care‐Spending. aspx. Accessed on July 20, 2012.
- , , , , , . The relationship between hospital spending and mortality in patients with sepsis. Arch Intern Med. 2011;171(4):292–299.
- , , , . The elusive connection between health care spending and quality. Health Aff (Millwood). 2009;28(1):w119–w123.
- , , , . Hospital quality and intensity of spending: is there an association? Health Aff (Millwood). 2009;28(4):w566–w572.
- , , , , . Measuring efficiency: the association of hospital costs and quality of care. Health Aff (Millwood). 2009;28(3):897–906.
- , . Assigning resources to health care use for health services research: options and consequences. Med Care. 2009;47(7 suppl 1):S70–S75.
- , , , , . Health care costing: data, methods, current applications. Med Care. 2009;47(7 suppl 1):S1–S6.
- . Determination of VA health care costs. Med Care Res Rev. 2003;60(3 suppl):124S–141S.
- . An improved set of standards for finding cost for cost‐effectiveness analysis. Med Care. 2009;47(7 suppl 1):S82–S88.
- , , , et al. Comparison of approaches for estimating prevalence costs of care for cancer patients: what is the impact of data source? Med Care. 2009;47(7 suppl 1):S64–S69.
- . Principles involved in costing. Med J Aust. 1990;153Suppl:S10–S12.
- . Spending more through “cost control:” our obsessive quest to gut the hospital. Health Aff (Millwood). 1996;15(2):145–154.
- , , , et al. Distribution of variable vs. fixed costs of hospital care. JAMA. 1999;281(7):644–649.
- . Administrative and claims records as sources of health care cost data. Med Care. 2009;47(7 suppl 1):S51–S55.
- , , , , , . Perioperative beta‐blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349–361.
- , , , et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med. 2007;356(5):486–496.
- , , , et al. Procedure intensity and the cost of care. Circ Cardiovasc Qual Outcomes. 2012;5(3):308–313.
- , , . A comparative study of tests for homogeneity of variances, with applications to the outer continental shelf bidding data. Technometrics. 1981;23:351–361.
- , , . Beyond the efficiency index: finding a better way to reduce overuse and increase efficiency in physician care. Health Aff (Millwood). 2008;27(4):w250–w259.
- , , . The association between hospital volume and processes, outcomes, and costs of care for congestive heart failure. Ann Intern Med. 2011;154(2):94–102.
© 2013 Society of Hospital Medicine
Review of VTE Prophylaxis Strategies
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is estimated to affect 900,000 Americans each year and is a cause of significant morbidity and mortality with associated high healthcare costs.[1] Accordingly, the comparative effectiveness and safety of interventions for the prevention and treatment of VTE are among the national priorities for comparative effectiveness research.[2] Whereas we have evidence‐based guidelines for the prophylaxis of VTE in the general population, there are no guidelines informing the care of select patient populations. Select populations are those patients in whom there is decisional uncertainty about the optimal choice, timing, and dose of VTE prophylaxis. Not only do these patients have an increased risk of DVT and PE, but most are also at high risk of bleeding, the most important complication of VTE prophylaxis.[3, 4, 5, 6]
The objectives of this systematic review were to define the comparative effectiveness and safety of pharmacologic and mechanical strategies for VTE prevention in some of these select medical populations including obese patients, patients on concomitant antiplatelet therapy, patients with renal insufficiency, patients who are underweight, and patients with coagulopathy due to liver disease.
METHODS
The methods for this comparative effectiveness review (CER) follow the guidelines suggested in the Agency for Healthcare Research and Quality (AHRQ) Methods Guide for Effectiveness and Comparative Effectiveness Reviews.[7] The protocol was publically posted.[8]
Search Strategy
We searched MEDLINE, EMBASE, and SCOPUS through August 2011, CINAHL, International Pharmaceutical Abstracts,
Study Selection
We reviewed titles followed by abstracts to identify randomized controlled trials (RCTs) or observational studies with comparison groups reporting on the effectiveness or safety of VTE prevention in our populations. Two investigators independently reviewed abstracts, and we excluded the abstracts if both investigators agreed that the article met 1 or more of the exclusion criteria. We included only English‐language articles that evaluated the effectiveness of pharmacological or mechanical interventions that have been approved for clinical use in the United States. To be eligible, the studies must have addressed relevant key questions in the population of our interest. We resolved disagreements by consensus. We used DistillerSR (Evidence Partners Inc., Ottawa, Ontario, Canada), a Web‐based database management program to manage the review process. Two investigators assessed the risk of bias in each study independently, using the Downs and Black instrument for observational studies and trials.[10]
Data Synthesis
For each select population, we created detailed evidence tables containing the information abstracted from the eligible studies. After synthesizing the evidence, we graded the quantity, quality, and consistency of the best available evidence for each select population by adapting an evidence‐grading scheme recommended in the Methods Guide for Conducting Comparative Effectiveness Reviews.[7]
RESULTS
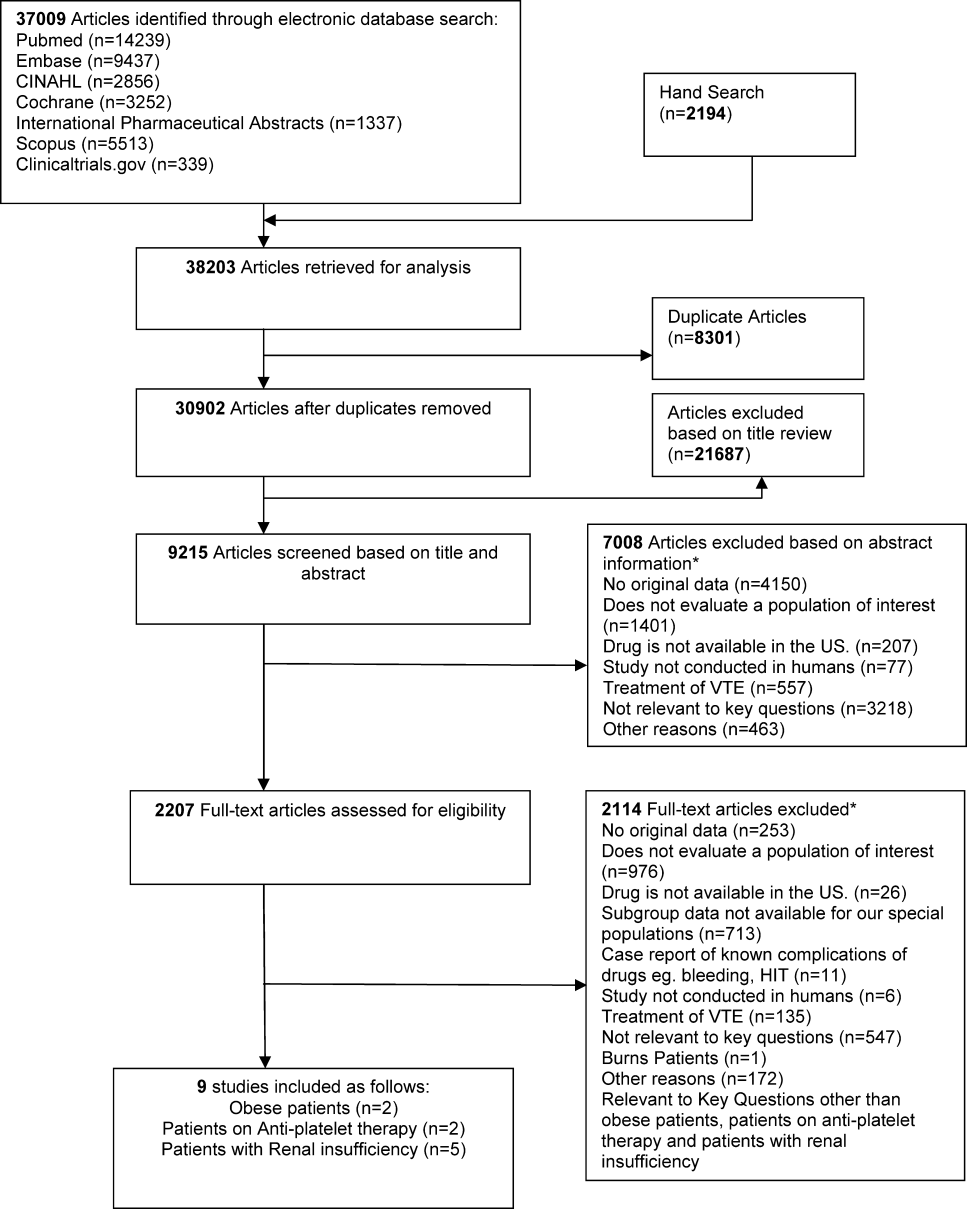
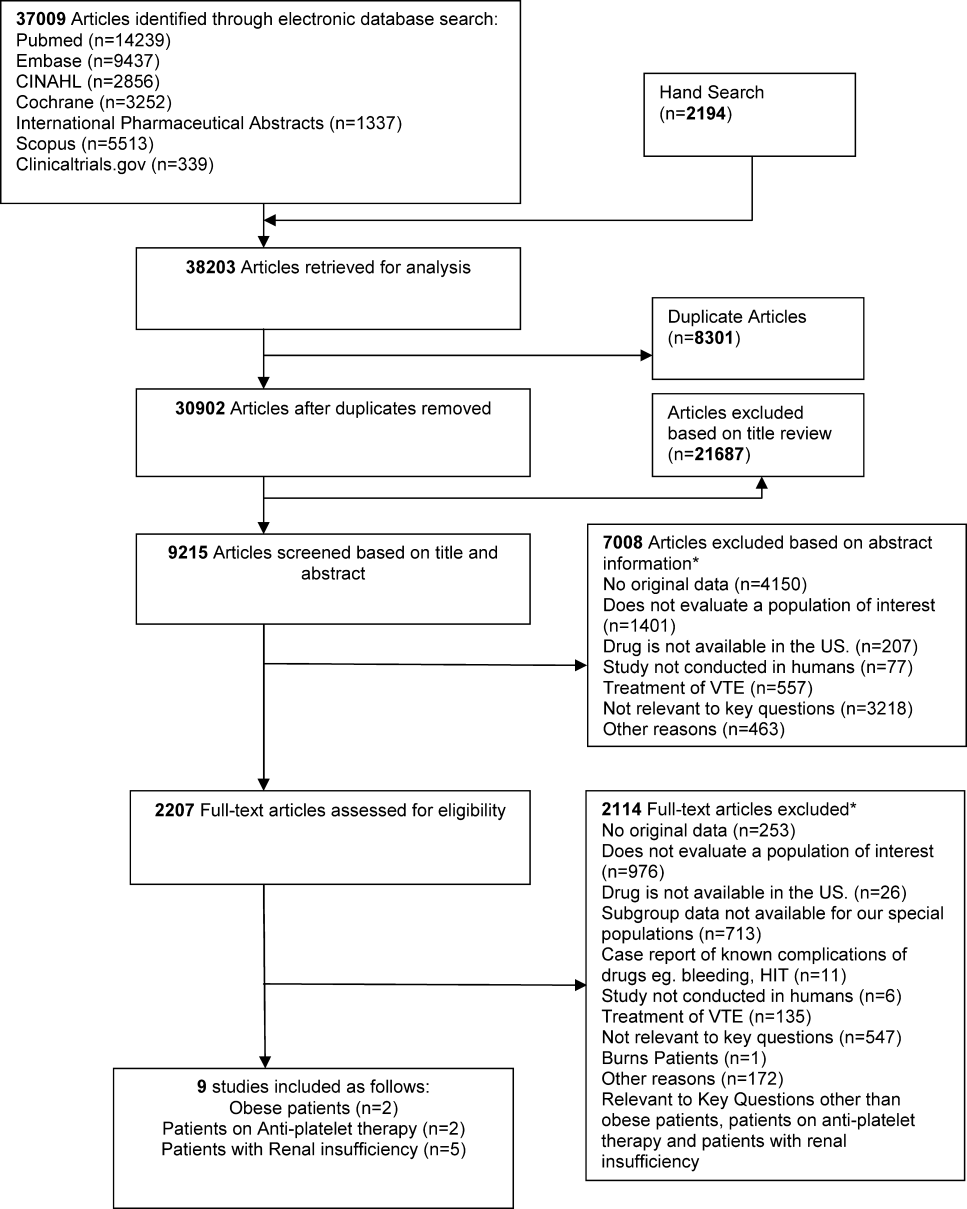
We identified 30,902 unique citations and included 9 studies (Figure 1). There were 5 RCTs with relevant subgroups and 4 observational studies (Table 1). Two studies reported on the risk of bleeding in patients given pharmacologic prophylaxis while they are concomitantly taking nonsteroidal anti‐inflammatory drugs (NSAIDS) or antiplatelet agents/aspirin, 1 RCT and 1 prospective observational study reported on obese patients, and 5 studies described outcomes of patients with renal insufficiency (see Supporting Information, Table 1, in the online version of this article). No study tested prophylaxis in underweight patients or those with liver disease.
| Study | Arm, n | Total VTE (DVT and PE) | Bleeding | Other Outcomes | |
|---|---|---|---|---|---|
| |||||
| Obese patients | |||||
| Kucher et al., 2005[11] | Arm 1 (dalteparin), 558 | 2.8% (95% CI: 1.34.3) | 0% | Mortality at 21 days: 4.6% | |
| Arm 2 (placebo), 560 | 4.3% (95% CI: 2.56.2) | 0.7% | Mortality at 21 days: 2.7% | ||
| Freeman et al., [12] | Arm 1 (fixed‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 19 % | |
| Arm 2 (lower‐dose enoxaparin), 9 | NR | NR | Peak anti‐factor Xa level 32 % | ||
| Arm 3 (higher‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 86 % | ||
| Patients on antiplatelet agents | |||||
| Eriksson et al., 2012[14] | Arm 1 (rivaroxaban), 563 | NR | 20 (3.6%), rate ratio for use vs nonuse: 1.32 (95% CI: 0.85‐2.05) | NR | |
| Arm 2 (enoxaparin/placebo), 526 | NR | 17 (3.2%), rate ratio for use vs nonuse: 1.40 (95% CI: 0.87‐2.25) | NR | ||
| Friedman et al., 2012[15] | Arm 2 (150 mg dabigatran, no ASA), 1149 | NR | 11 (1.0%)a | NR | |
| Arm 5 (150 mg dabigatran+ASA), 128 | NR | 2 (1.6%)a | NR | ||
| Arm 3 (enoxaparin, no ASA), 1167 | NR | 14 (1.2%)a | NR | ||
| Arm 6 (enoxaparin+ASA), 132 | NR | 4 (3.0%) | NR | ||
| 150 mg dabigatran compared with enoxaparinNo concomitant ASA therapy | NR | RR: 0.82 (95% CI: 0.37‐1.84) | NR | ||
| 150 mg dabigatran compared with enoxaparinWith concomitant ASA therapy | NR | RR: 0.55 (95% CI: 0.11‐2.78) | NR | ||
| Patients with renal insufficiency | |||||
| Bauersachs et al., 2011[16] | Arm 2 (GFR 30), 92 | Total DVT: 11.11%; Total PE: 0% | Major bleeding: 4/92 (4.35%), minor bleeding: 9/92 (9.78%) | Mortality: 5.81% | |
| Mah et al., 2007[17] | Arm 2 (tinzaparin), 27 | NR | Major bleeding: 2/27 (7.4%), minor bleeding: 3/27 (11.1%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.05 | |
| Arm 3 (enoxaparin), 28 | NR | Major bleeding: 1/28 (3.6%), minor bleeding: 3/28 (10.7%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.22 | ||
| Dahl et al., 2012[18] | Arm 1 (enoxaparin), 332 | Major VTE: 8 (9.0%) | Major bleeding: 6 (4.7%) | Infections and infestations: 25 (7.5%), Wound infection: 4 (1.2%) | |
| Arm 2 (dabigatran), 300 | Major VTE: 3 (4.3%) | Major bleeding: 0 (0%) | Infections and infestations: 21 (7.0%), Wound Infection: 3 (1.0%) | ||
| Shorr et al., 2012[19] | Arm 1 (enoxaparin, CrCL 60 mL/min), 353 | Total VTE: 17/275 (6.2%) | Major bleeding: 0/351 (0%) | NR | |
| Arm 2 (desirudin, CrCL 60 mL/min), 353 | Total VTE: 13/284 (4.3%) | Major bleeding: 2/349 (0.27%) | NR | ||
| Arm 3 (enoxaparin, CrCL 4559 mL/min), 369 | Total VTE: 18/282 (6.2%) | Major bleeding: 1/365 (0.27%) | NR | ||
| Arm 4 (desirudin, CrCL 4559 mL/min), 395 | Total VTE: 17/303 (5.6%) | Major bleeding: 1/393 (0.25%) | NR | ||
| Arm 5 (enoxaparin, CrCL 45 mL/min), 298 | Total VTE: 24/216 (11.1%) | Major bleeding: 1/294 (0.34%) | NR | ||
| Arm 6 (desirudin, CrCL 45 mL/min), 279 | Total VTE: 7/205 (3.4%) | Major bleeding: 5/275 (1.82%) | NR | ||
| Elsaid et al., 2012[20] | Arm 1 (enoxaparin, CrCL 60 mL/min), 17 | NR | Major bleeding: 2 (11.8%) | NR | |
| Arm 2 (enoxaparin, CrCL 3059 mL/min), 86 | NR | Major bleeding: 9 (10.5%) | NR | ||
| Arm 3 (enoxaparin, CrCL 30 mL/min), 53 | NR | Major bleeding: 10 (18.9%) | NR | ||
| Arm 4 (UFH, CrCL 60 mL/min), 19 | NR | Major bleeding: 2 (10.5%) | NR | ||
| Arm 5 (UFH, CrCL 3059 mL/min), 99 | NR | Major bleeding: 3 (3%) | NR | ||
| Arm 6 (UFH, CrCL 30 mL/min), 49 | NR | Major bleeding: 2 (4.1%) | NR | ||
Obese Patients
We found 1 subgroup analysis of an RCT (total 3706 patients, 2563 nonobese and 1118 obese patients) that reported on the comparative effectiveness and safety of fixed low‐dose dalteparin 5000 IU/day compared to placebo among 1118 hospitalized medically ill patients with body mass indices (BMI) greater than 30 kg/m2.11 Neither group received additional concurrent prophylactic therapies. The 3 most prevalent medical diagnoses prompting hospitalization were congestive heart failure, respiratory failure, and infectious diseases. Compression ultrasound was performed in all patients by day 21 of hospitalization. The primary end point was the composite of VTE, fatal PE, and sudden death, and secondary end points included DVT, bleeding, and thrombocytopenia by day 21 (Table 1). In obese patients, the primary end point occurred in 2.8% (95% confidence interval [CI]: 1.34.3) of the dalteparin group and in 4.3% (95% CI: 2.56.2) of the placebo group (relative risk [RR]: 0.64; 95% CI: 0.32‐1.28). In nonobese patients, the primary end point occurred in 2.8% (95% CI: 1.8‐3.8) and 5.2% (95% CI: 3.9‐6.6) of the dalteparin and placebo groups, respectively (RR: 0.53; 95% CI: 0.34‐0.82). When weight was modeled as a continuous variable, no statistically significant interaction between weight and dalteparin efficacy was observed (P=0.97). The authors calculated the RR in predefined BMI subgroups and found that dalteparin was effective in reducing VTE in patients with BMIs up to 40, with RRs of 1.0 for all (approximate range, 0.20.8). However, a fixed dose of dalteparin 5000 IU/day was not better than placebo for individuals with BMI >40 kg/m2. There was no significant difference in mortality or major hemorrhage by day 21 between treatment and placebo groups.
Freeman and colleagues prospectively assigned 31 medically ill patients with extreme obesity (BMI >40 kg/m2) to 1 of 3 dosing regimens of enoxaparin: a fixed dose of 40 mg daily enoxaparin (control group, n=11), enoxaparin at 0.4 mg/kg (n=9), or enoxaparin at 0.5 mg/kg (n=11).[12] The average BMI of the entire cohort was 62.1 kg/m2 (range, 40.582.4). All patients had anti‐factor Xa levels drawn on the day of enrollment and daily for 3 days (Table 2). The relationship between anti‐factor Xa levels and clinical efficacy of low‐molecular weight heparin (LMWH) in VTE prophylaxis is still unclear; however, an anti‐factor Xa level of 0.2 to 0.5 IU/mL, measured 4 hours after the fourth dose of LMWH, is the target level recommended for VTE prophylaxis.[13] Patients who received weight‐based enoxaparin at 0.5mg/kg achieved target anti‐factor Xa level 86% of the time compared to 32% of the time in those receiving 0.4 mg/kg and 19% of the time for those in the fixed‐dose group (P0.001). No clinical outcomes were reported in this study.
| Intervention | Outcome | Risk of Bias | Evidence Statement and Magnitude of Effect |
|---|---|---|---|
| |||
| Patients on antiplatelet agents | |||
| Rivaroxaban vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic rivaroxaban or enoxaparin in patients concomitantly treated with antiplatelet agents; 3.6% vs 3.25% |
| Dabigatran vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic dabigatran or enoxaparin in patients concomitantly treated with aspirin; 1.6% vs 3.0% |
| Obese patients | |||
| Dalteparin vs placebo | VTE | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing total VTE in obese patients; 2.8% vs 4.3%, RR: 0.64, 95% CI: 0.32‐1.28 |
| Dalteparin vs placebo | Mortality | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing mortality in obese patients; 9.9% vs 8.6%, P=0.36 |
| Dalteparin vs placebo | Major bleeding | Moderate | Insufficient evidence for safety of dalteparin vs placebo in reducing major bleeding in obese patients; 0% vs 0.7%, P>0.99 |
| Enoxaparin 40 mg daily vs 0.4 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.4 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 32%, P=NR |
| Enoxaparin 40 mg daily vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 86%, P0.001 |
| Enoxaparin 0.4 mg/kg vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 0.4 mg/kg versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 32% vs 86%, P=NR |
| Patients with renal insufficiency | |||
| Tinzaparin vs enoxaparin | VTE | High | Insufficient evidence about superiority of either drug for preventing VTE in patients with renal insufficiency, 0/27 vs 0/28* |
| Tinzaparin vs enoxaparin | Bleeding | High | Insufficient evidence about safety of either drug in patients with renal insufficiency; 5/27 vs 4/28, P=0.67 |
| Dabigatran vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of dabigatran in reducing VTE in severe renal compromise patients vs enoxaparin; 4.3% vs 9%, OR: 0.48, 95% CI: 0.13‐1.73, P=0.271 |
| Dabigatran vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of dabigatran vs enoxaparin in patients with renal impairment; 0 vs 4.7%, P=0.039 |
| Desirudin vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of desirudin vs enoxaparin in reducing VTE in patients with renal impairment; 4.9% vs 7.6%, P=0.019 |
| Desirudin vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of desirudin vs enoxaparin in patients with renal impairment; 0.8% vs 0.2%, P=0.109 |
| Enoxaparin vs UFH | Bleeding | High | Insufficient evidence for increased risk of bleeding with enoxaparin vs unfractionated heparin in patients with all levels of renal impairment, 13.5% vs 4.2%, RR: 3.2, 95% CI: 1.47.3; and for the subgroup of patients with creatinine clearance 30 mL/min; 18.9% vs 4.1%, RR: 4.68, 95% CI: 1.120.6 |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | VTE | Moderate | Insufficient evidence regarding differential benefit of unfractionated heparin by renal function; 2.6% of patients had a VTE event |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | Bleeding | Moderate | Insufficient evidence for differential harm from unfractionated heparin by renal function; 13 events in 92 patients |
Patients on Antiplatelet Drugs
We did not find studies that directly looked at the comparative effectiveness of VTE prophylaxis in patients who were on antiplatelet drugs including aspirin. However, there were 2 studies that looked at the risk of bleeding in patients who received VTE pharmacologic prophylaxis while concurrently taking antiplatelet agents including aspirin. Both studies used pooled data from large phase III trials.
The study by Eriksson et al. used data from the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) trial where over 12,000 patients undergoing elective total knee or hip replacement were randomized to receive VTE prophylaxis with oral rivaroxaban or subcutaneous enoxaparin.[14] Nine percent of participants in each arm (563 in rivaroxaban and 526 in enoxaparin/placebo) were concomitantly using antiplatelet agents or aspirin at least once during the at risk period, defined as starting at day 1 of surgery up to 2 days after the last intake of the study drug. The only end point evaluated was bleeding, and the authors found no statistically significant bleeding difference among the 2 arms (Table 1). Any bleeding event in the rivaroxaban with antiplatelets or aspirin arm was found in 20 (3.6%) patients, whereas in those on enoxaparin/placebo with antiplatelets or aspirin arm it was 17 (3.2%). The relative rate of bleeding among users versus nonusers of antiplatelet drugs or aspirin was 1.32 (95% CI: 0.85‐2.05) in the rivaroxaban group and 1.40 (95% CI: 0.87‐2.25) in the enoxaparin arm (Table 1).
Friedman et al. used pooled data from the RE‐MODEL, RENOVATE, and REMOBILIZE trials, where patients who were undergoing hip or knee arthroplasty were randomized to 220 mg of dabigatran once daily, 150 mg of dabigatran once daily (we focused on this lower dosage as this is the only available dose used in the US), 40 mg of enoxaparin once daily, or 30 mg of enoxaparin twice a day.[15] Of the 8135 patients, 4.7% were on concomitant aspirin. The baseline characteristics of those on aspirin were similar to the other enrollees. The primary outcome was major bleeding events requiring transfusion, symptomatic internal bleeding, or bleeding requiring surgery. Among patients receiving 150 mg of dabigatran, bleeding events with and without concomitant aspirin occurred in 1.6% and 1.0%, respectively (odds ratio [OR]: 1.64; 95% CI: 0.36‐7.49; P=0.523). The percentages of participants with bleeding who received enoxaparin, with and without aspirin, were 3.0% and 1.2%, respectively (OR: 2.57; 95% CI: 0.83‐7.94; P=0.101). The RR of bleeding on dabigatran compared to enoxaparin with and without aspirin therapy was 0.55 (95% CI: 0.11‐2.78) and 0.82 (95% CI: 0.37‐1.84), respectively (Table 1).
Patients With Renal Insufficiency
We found 5 studies that evaluated the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE in patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, or patients receiving dialysis. Four studies were RCTs,[16, 17, 18, 19] and 1 used a cohort design assessing separate cohorts before and after a quality improvement intervention.[20] Bauersachs and colleagues conducted an RCT comparing unfractionated heparin at 5000 IU, 3 times daily to certoparin, which is not approved in the United States and is not further discussed here.[16] The rate of DVT among patients treated with unfractionated heparin in patients with a glomerular filtration rate >30 mL/min was marginally lower than those with severe renal dysfunction (10.3 vs 11.1%) (Table 1).
Patients with severe renal dysfunction who received 5000 IU of unfractionated heparin 3 times a day were at increased risk of all bleeds (RR: 3.4; 95% CI: 2.05.9), major bleeds (RR: 7.3; 95% CI: 3.316), and minor bleeds (RR: 2.6; 95% CI: 1.4‐4.9) compared to patients treated with unfractionated heparin without severe renal dysfunction.[16]
A randomized trial by Mah and colleagues compared drug accumulation and anti‐Xa activity in elderly patients with renal dysfunction (defined as a glomerular filtration rate of 20 to 50 mL/min) who received either tinzaparin at 4500 IU once daily or enoxaparin at 4000 IU once daily.[17] Enoxaparin accumulated to a greater extent from day 1 to day 8 than did tinzaparin; the ratio of maximum concentration on day 8 compared to day 1 was 1.22 for enoxaparin and 1.05 for tinzaparin (P=0.016). No VTE events were reported in patients who received tinzaparin or enoxaparin. There was no statistical difference in the incidence of bleeding events between patients receiving tinzaparin (5, including 2 major events) and enoxaparin (4, including 3 major events, P=0.67) (Table 1).
The trial by Dahl and colleagues randomly assigned patients who were over 75 years of age and/or who had moderate renal dysfunction (defined as creatinine clearance between 30 and 49 mL/min) to receive enoxaparin 40 mg daily or dabigatran 150 mg daily.[18] There was no significant difference in the rate of major VTE events between patients receiving dabigatran (4.3%) and enoxaparin (9%) (OR: 0.48; 95% CI: 0.13‐1.73; P=0.271) (Table 1). The rate of major bleeding was significantly higher among patients randomly assigned to receive enoxaparin (4.7%) versus dabigatran (0%) (P=0.039).[18]
Shorr and colleagues published a post hoc subgroup analysis of a multicenter trial in which orthopedic patients were randomly assigned to receive desirudin 15 mg twice daily or enoxaparin 40 mg once daily.[19] Evaluable patients (1565 of the 2079 patients randomized in the trial) receiving desirudin experienced a significantly lower rate of major VTE compared with patients receiving enoxaparin (4.9% vs 7.6%, P=0.019). This relationship was particularly pronounced for evaluable patients whose creatinine clearance was between 30 and 44 mL/min. In evaluable patients with this degree of renal dysfunction, 11% of patients taking enoxaparin compared to 3.4% of those taking desirudin had a major VTE (OR: 3.52; 95% CI: 1.48‐8.4; P=0.004). There was no significant difference in the rates of major bleeding among a subset of patients assessed for safety outcomes (2078 of the 2079 patients randomized in the trial) who received desirudin (0.8%) or enoxaparin (0.2%) (Table 1).
Elsaid and Collins assessed VTE and bleeding events associated with the use of unfractionated heparin 5000U either 2 or 3 times daily and enoxaparin 30 mg once or twice daily across patients stratified by renal function (creatinine clearance 30, 3059, and 60 mL/min). The investigators made assessments before and after a quality improvement intervention that was designed to eliminate the use of enoxaparin in patients whose creatinine clearance was 30 mL/min. No VTE events were reported. Patients receiving enoxaparin were significantly more likely to experience a major bleeding episode compared with patients receiving unfractionated heparin (overall rates for all levels of renal function: 13.5% vs 4. 2%; RR: 3.2; 95% CI: 1.47.3) (Table 2). This association was largely driven by the subgroup of patients with a creatinine clearance 30 mL/min. For this subgroup with severe renal insufficiency, patients receiving enoxaparin were significantly more likely to have a bleed compared with patients receiving unfractionated heparin (18.9% vs 4.1%; RR: 4.68; 95% CI: 1.120.6) (Tables 1 and 2). There was no difference in the bleeding rates for patients whose creatinine clearances were >60 mL/min.[20]
Strength of Evidence
Obese Patients
Overall, we found that the strength of evidence was insufficient regarding the composite end point of DVT, PE, and sudden death, and the outcomes of mortality and bleeding (Table 2). This was based on a paucity of available data, and a moderate risk of bias in the reviewed studies. Additionally, 92% of the enrolled patients in the studies were white, limiting the generalizability of the results to other ethnic groups.
Patients on Antiplatelets
The strength of evidence was insufficient in the studies reviewed here to conclude that there is no difference in rates of bleeding in patients who are concomitantly taking antiplatelet drugs while getting VTE prophylaxis with rivaroxaban, dabigatran, or enoxaparin. We based this rating because of the imprecision of results and unknown consistencies across multiple studies.
Patients With Renal Insufficiency
One RCT had a high risk of bias for our key question because data from only 1 study arm were useful for our review.[16] The other RCTs were judged to have a moderate risk of bias. The analyses led by Dahl and Shorr[18, 19] were based on post hoc (ie, not prespecified) analysis of data from RCTs. Additionally, outcomes in the Shorr et al. trial were reported for evaluable subpopulations of the cohort that was initially randomized in the clinical trial.
We rated the strength of evidence as insufficient to know the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE during hospitalization of patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, and patients receiving dialysis. We based this rating on the risk of bias associated with published studies and a lack of consistent evidence regarding associations that were reported. Similarly, we rated the strength of evidence as insufficient that 5000 U of unfractionated heparin 3 times daily increases the risk of major and minor bleeding events in patients with severely compromised renal function compared to this dose in patients without severely compromised renal function. We based this rating on a high risk of bias of included studies and inconsistent evidence. Likewise, we rated the strength of evidence as insufficient that enoxaparin significantly increases the risk of major bleeding compared with unfractionated heparin in patients with severe renal insufficiency. We based this rating on a high risk of bias and inconsistent published evidence.
We similarly found insufficient evidence to guide treatment decisions for patients with renal insufficiency. Our findings are consistent with other recent reviews. The American College of Chest Physicians (ACCP) practice guidelines[21] make dosing recommendations for the therapeutic use of enoxaparin. However, their assessment is that the data are insufficient to make direct recommendations about prophylaxis. Their assessment of the indirect evidence regarding bioaccumulation and increased anti‐factor Xa levels are consistent with ours. The ACCP guidelines also suggest that decreased clearance of enoxaparin has been associated with increased risk of bleeding events for patients with severe renal insufficiency. However, the cited study[20] compares patients with and without severe renal dysfunction who received the same therapy. Therefore, it is not possible to determine the additional risk conveyed by enoxaparin therapy, that is, above the baseline increased risk of bleeding among patients with renal insufficiency, particularly those receiving an alternate pharmacologic VTE prevention strategy, such as unfractionated heparin.
DISCUSSION
We found that the evidence was very limited about prevention of VTE in these select and yet prevalent patient populations. Despite the fact that there is an increasing number of obese patients and patients who are on antiplatelet therapies, most clinical practice guidelines do not address the care of these populations, which may be entirely appropriate given the state of the evidence.
The ACCP practice guidelines[21] suggest using a higher dose of enoxaparin for the prevention of VTE in obese patients. The subgroup analysis by Kucher et al.[11] showed effect attenuation of dalteparin when given at a fixed dose of 5000 IU/mL to patients with a BMI of >40 kg/m2. The Freeman study[12] showed that extremely obese patients (average BMI >62.1 kg/m2) who are given a fixed dose of enoxaparin achieved target anti‐factor Xa levels significantly less often than those who received a higher dose of enoxaparin. The 2 separate findings, although not conclusive, lend some credence to the current ACCP guidelines.[21]
The studies we reviewed on VTE prophylaxis in patients who are concomitantly on antiplatelets including aspirin reported no major increased risk of bleeding; however, in the Friedman et al. study,[15] 3.0% of patients who were put on enoxaparin while still on aspirin had a bleeding event compared to 1.2% of those on enoxaparin alone. This difference is not statistically significant but is a trend possibly worth noting, especially when one looks at the lower RR of bleeding at 0.55 compared to 0.82 when dabigatran is compared with enoxaparin with and without concomitant aspirin therapy, respectively (Table 1). The highest dose of aspirin used in either of the studies was 160 mg/day, and neither study addressed other potent antiplatelets such as clopidogrel or ticlopidine separately, which limits the generalizability of the finding to all antiplatelets. Current ACCP guidelines do not recommend aspirin as a sole option for the prevention of VTE in orthopedic surgery patients.[22] Concerns remain among clinicians that antiplatelets, including aspirin, on their own are unlikely to be fully effective to thwart venous thrombotic processes for most patients, and yet the risk of bleeding is not fully known when these agents are combined with other anticoagulants for VTE prophylaxis.
Our review has several limitations, including the possibility that we may have missed some observational studies, as the identification of relevant observational studies in electronic searches is more challenging than that of RCTs. The few studies made it impossible to quantitatively pool results. These results, however, have important implications, namely that additional research on the comparative effectiveness and safety of pharmacologic and mechanical strategies to prevent VTE is needed for the optimal care of these patient subgroups. This might be achieved with trials dedicated to enrolling these patients or prespecified subgroup analyses within larger trials. Observational data may be appropriate as long as attention is paid to confounding.
APPENDIX
MEDLINE Search Strategy
((pulmonary embolism[mh] OR PE[tiab] OR Pulmonary embolism[tiab] OR thromboembolism[mh] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR Thrombosis[mh] OR thrombosis[tiab] OR DVT[tiab] OR VTE[tiab] OR clot[tiab]) AND (Anticoagulants[mh] OR Anticoagulants[tiab] OR Anticoagulant[tiab] OR thrombin inhibitors[tiab] OR Aspirin[mh] or aspirin[tiab] OR aspirins[tiab] or clopidogrel[nm] OR clopidogrel[tiab] OR Plavix[tiab] or ticlopidine[mh] or ticlopidine[tiab]OR ticlid[tiab] OR prasugrel[nm]Or prasugrel[tiab]OR effient[tiab]OR ticagrelor[NM] OR ticagrelor[tiab]OR Brilinta[tiab] OR cilostazol[NM] OR cilostazol[tiab]OR pletal[tiab] OR warfarin[mh]OR warfarin[tiab]OR coumadin[tiab] OR coumadine[tiab] OR Dipyridamole[mh]OR dipyridamole[tiab]OR persantine[tiab] OR dicoumarol[MH] OR dicoumarol[tiab] OR dicumarol[tiab] OR Dextran sulfate[mh] OR dextran sulfate[tiab] ORthrombin inhibitors[tiab] OR thrombin inhibitor[tiab] OR heparin[mh] OR Heparin[tiab] OR Heparins[tiab] OR LMWH[tiab] OR LDUH[tiab] OR Enoxaparin[mh] OR Enoxaparin[tiab] OR Lovenox[tiab] OR Dalteparin[tiab] OR Fragmin[tiab] OR Tinzaparin[tiab] OR innohep[tiab] OR Nadroparin[tiab] OR Fondaparinux[nm] OR Fondaparinux[tiab] OR Arixtra[tiab] OR Idraparinux[nm] OR Idraparinux[tiab] OR Rivaroxaban[nm] OR Rivaroxaban[tiab] OR novastan[tiab] OR Desirudin[nm] OR Desirudin[tiab] OR Iprivask[tiab]OR direct thrombin inhibitor[tiab] OR Argatroban[nm] OR Argatroban[tiab] OR Acova[tiab] OR Bivalirudin[nm] OR Bivalirudin[tiab] OR Angiomax[tiab] OR Lepirudin[nm] OR Lepirudin[tiab] OR Refludan[tiab] OR Dabigatran[nm] OR Dabigatran[tiab] OR Pradaxa[tiab] OR factor xa[mh] OR factor Xa[tiab] OR vena cava filters[mh] OR filters[tiab] OR filter[tiab] OR compression stockings[mh] OR intermittent pneumatic compression devices[mh] OR compression [tiab] OR Venous foot pump[tiab])) AND(prevent*[tiab] OR prophyla*[tiab] OR prevention and control[subheading]) NOT (animals[mh] NOT humans[mh]) NOT (editorial[pt] OR comment[pt]) NOT ((infant[mh] OR infant[tiab] OR child[mh] OR child[tiab] OR children[tiab] OR adolescent[mh] OR adolescent[tiab] OR teen‐age[tiab] OR pediatric[tiab] OR perinatal[tiab]) NOT (adult[tiab] OR adults[tiab] OR adult[mh])) NOT (mechanical valve[tiab] OR heart valve[tiab] OR atrial fibrillation[mh] OR atrial fibrillation[tiab] OR thrombophilia[mh] OR thrombophilia[tiab] OR pregnancy[mh])
- , , . Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the US. Blood. 2005;106:910.
- Institute of Medicine. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009.
- Lovenox (enoxaparin sodium injection for subcutaneous and intravenous use: prescribing information). Bridgewater, NJ: SanofiAventis; 2011. Available at: http://products.sanofi.us/lovenox/lovenox.html. Accessed October 17, 2012.
- Innohep (tinzaparin sodium injection). Ballerup, Denmark: LEO Pharmaceutical Products; 2008. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020484s011lbl.pdf. Accessed October 17, 2012.
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency‐ the IRIS trial. [50th ASH Annual Meeting and Exposition abstract 434]. Blood. 2008;11:112.
- Fragmin (dalteparin sodium injection). New York, NY: Pfizer Inc.; 2007. Available at: http://www.pfizer.com/files/products/uspi_fragmin.pdf. Accessed October 17, 2012.
- Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality; August 2011. AHRQ publication No. 10 (11)‐EHC063‐EF. Available at: http://www.effectivehealthcare.ahrq.gov. Accessed October 17, 2012.
- Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/928/VTE‐Special‐Populations_Protocol_20120112.pdf. Accessed April 17, 2012.
- , , , et al. Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Evidence Report/Technology Assessment (AHRQ). Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/1501/venous‐thromboembolism‐special‐populations‐report‐130529.pdf. 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Efficacy and safety of fixed low‐dose dalteparin in preventing venous thromboembolism among obese or elderly hospitalized patients: a subgroup analysis of the PREVENT trial. Arch Intern Med. 2005;165(3):341–345.
- , , , . Prospective comparison of three enoxaparin dosing regimens to achieve target anti‐factor Xa levels in hospitalized, medically ill patients with extreme obesity. Am J Hematol. 2012;87(7):740–743.
- , , . Effect of prophylactic dalteparin on anti‐factor xa levels in morbidly obese patients after bariatric surgery. Obes Surg. 2010;20(4):487–491.
- , , , , . Concomitant use of medication with antiplatelet effects in patients receiving either rivaroxaban or enoxaparin after total hip or knee arthroplasty. Thromb Res. 2012;130(2):147–151.
- , , , , , . Dabigatran etexilate and concomitant use of non‐steroidal anti‐inflammatory drugs or acetylsalicylic acid in patients undergoing total hip and total knee arthroplasty: No increased risk of bleeding. Thromb Haemost. 2012;108(1):183–190.
- , , , et al. CERTIFY: prophylaxis of venous thromboembolism in patients with severe renal insufficiency. Thromb Haemost. 2011;105(6):981–988.
- , , , et al. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost. 2007;97(4):581–586.
- , , , , , . Thromboprophylaxis in patients older than 75 years or with moderate renal impairment undergoing knee or hip replacement surgery [published correction appears in Int Orthop. 2012;36(5):1113]. Int Orthop. 2012;36(4):741–748.
- , , , . Impact of stage 3B chronic kidney disease on thrombosis and bleeding outcomes after orthopedic surgery in patients treated with desirudin or enoxaparin: insights from a randomized trial. J Thromb Haemost. 2012;10(8):1515–1520.
- , . Initiative to improve thromboprophylactic enoxaparin exposure in hospitalized patients with renal impairment. Am J Health Syst Pharm. 2012;69(5):390–396.
- , , , , ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , . Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74.
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is estimated to affect 900,000 Americans each year and is a cause of significant morbidity and mortality with associated high healthcare costs.[1] Accordingly, the comparative effectiveness and safety of interventions for the prevention and treatment of VTE are among the national priorities for comparative effectiveness research.[2] Whereas we have evidence‐based guidelines for the prophylaxis of VTE in the general population, there are no guidelines informing the care of select patient populations. Select populations are those patients in whom there is decisional uncertainty about the optimal choice, timing, and dose of VTE prophylaxis. Not only do these patients have an increased risk of DVT and PE, but most are also at high risk of bleeding, the most important complication of VTE prophylaxis.[3, 4, 5, 6]
The objectives of this systematic review were to define the comparative effectiveness and safety of pharmacologic and mechanical strategies for VTE prevention in some of these select medical populations including obese patients, patients on concomitant antiplatelet therapy, patients with renal insufficiency, patients who are underweight, and patients with coagulopathy due to liver disease.
METHODS
The methods for this comparative effectiveness review (CER) follow the guidelines suggested in the Agency for Healthcare Research and Quality (AHRQ) Methods Guide for Effectiveness and Comparative Effectiveness Reviews.[7] The protocol was publically posted.[8]
Search Strategy
We searched MEDLINE, EMBASE, and SCOPUS through August 2011, CINAHL, International Pharmaceutical Abstracts,
Study Selection
We reviewed titles followed by abstracts to identify randomized controlled trials (RCTs) or observational studies with comparison groups reporting on the effectiveness or safety of VTE prevention in our populations. Two investigators independently reviewed abstracts, and we excluded the abstracts if both investigators agreed that the article met 1 or more of the exclusion criteria. We included only English‐language articles that evaluated the effectiveness of pharmacological or mechanical interventions that have been approved for clinical use in the United States. To be eligible, the studies must have addressed relevant key questions in the population of our interest. We resolved disagreements by consensus. We used DistillerSR (Evidence Partners Inc., Ottawa, Ontario, Canada), a Web‐based database management program to manage the review process. Two investigators assessed the risk of bias in each study independently, using the Downs and Black instrument for observational studies and trials.[10]
Data Synthesis
For each select population, we created detailed evidence tables containing the information abstracted from the eligible studies. After synthesizing the evidence, we graded the quantity, quality, and consistency of the best available evidence for each select population by adapting an evidence‐grading scheme recommended in the Methods Guide for Conducting Comparative Effectiveness Reviews.[7]
RESULTS
We identified 30,902 unique citations and included 9 studies (Figure 1). There were 5 RCTs with relevant subgroups and 4 observational studies (Table 1). Two studies reported on the risk of bleeding in patients given pharmacologic prophylaxis while they are concomitantly taking nonsteroidal anti‐inflammatory drugs (NSAIDS) or antiplatelet agents/aspirin, 1 RCT and 1 prospective observational study reported on obese patients, and 5 studies described outcomes of patients with renal insufficiency (see Supporting Information, Table 1, in the online version of this article). No study tested prophylaxis in underweight patients or those with liver disease.

| Study | Arm, n | Total VTE (DVT and PE) | Bleeding | Other Outcomes | |
|---|---|---|---|---|---|
| |||||
| Obese patients | |||||
| Kucher et al., 2005[11] | Arm 1 (dalteparin), 558 | 2.8% (95% CI: 1.34.3) | 0% | Mortality at 21 days: 4.6% | |
| Arm 2 (placebo), 560 | 4.3% (95% CI: 2.56.2) | 0.7% | Mortality at 21 days: 2.7% | ||
| Freeman et al., [12] | Arm 1 (fixed‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 19 % | |
| Arm 2 (lower‐dose enoxaparin), 9 | NR | NR | Peak anti‐factor Xa level 32 % | ||
| Arm 3 (higher‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 86 % | ||
| Patients on antiplatelet agents | |||||
| Eriksson et al., 2012[14] | Arm 1 (rivaroxaban), 563 | NR | 20 (3.6%), rate ratio for use vs nonuse: 1.32 (95% CI: 0.85‐2.05) | NR | |
| Arm 2 (enoxaparin/placebo), 526 | NR | 17 (3.2%), rate ratio for use vs nonuse: 1.40 (95% CI: 0.87‐2.25) | NR | ||
| Friedman et al., 2012[15] | Arm 2 (150 mg dabigatran, no ASA), 1149 | NR | 11 (1.0%)a | NR | |
| Arm 5 (150 mg dabigatran+ASA), 128 | NR | 2 (1.6%)a | NR | ||
| Arm 3 (enoxaparin, no ASA), 1167 | NR | 14 (1.2%)a | NR | ||
| Arm 6 (enoxaparin+ASA), 132 | NR | 4 (3.0%) | NR | ||
| 150 mg dabigatran compared with enoxaparinNo concomitant ASA therapy | NR | RR: 0.82 (95% CI: 0.37‐1.84) | NR | ||
| 150 mg dabigatran compared with enoxaparinWith concomitant ASA therapy | NR | RR: 0.55 (95% CI: 0.11‐2.78) | NR | ||
| Patients with renal insufficiency | |||||
| Bauersachs et al., 2011[16] | Arm 2 (GFR 30), 92 | Total DVT: 11.11%; Total PE: 0% | Major bleeding: 4/92 (4.35%), minor bleeding: 9/92 (9.78%) | Mortality: 5.81% | |
| Mah et al., 2007[17] | Arm 2 (tinzaparin), 27 | NR | Major bleeding: 2/27 (7.4%), minor bleeding: 3/27 (11.1%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.05 | |
| Arm 3 (enoxaparin), 28 | NR | Major bleeding: 1/28 (3.6%), minor bleeding: 3/28 (10.7%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.22 | ||
| Dahl et al., 2012[18] | Arm 1 (enoxaparin), 332 | Major VTE: 8 (9.0%) | Major bleeding: 6 (4.7%) | Infections and infestations: 25 (7.5%), Wound infection: 4 (1.2%) | |
| Arm 2 (dabigatran), 300 | Major VTE: 3 (4.3%) | Major bleeding: 0 (0%) | Infections and infestations: 21 (7.0%), Wound Infection: 3 (1.0%) | ||
| Shorr et al., 2012[19] | Arm 1 (enoxaparin, CrCL 60 mL/min), 353 | Total VTE: 17/275 (6.2%) | Major bleeding: 0/351 (0%) | NR | |
| Arm 2 (desirudin, CrCL 60 mL/min), 353 | Total VTE: 13/284 (4.3%) | Major bleeding: 2/349 (0.27%) | NR | ||
| Arm 3 (enoxaparin, CrCL 4559 mL/min), 369 | Total VTE: 18/282 (6.2%) | Major bleeding: 1/365 (0.27%) | NR | ||
| Arm 4 (desirudin, CrCL 4559 mL/min), 395 | Total VTE: 17/303 (5.6%) | Major bleeding: 1/393 (0.25%) | NR | ||
| Arm 5 (enoxaparin, CrCL 45 mL/min), 298 | Total VTE: 24/216 (11.1%) | Major bleeding: 1/294 (0.34%) | NR | ||
| Arm 6 (desirudin, CrCL 45 mL/min), 279 | Total VTE: 7/205 (3.4%) | Major bleeding: 5/275 (1.82%) | NR | ||
| Elsaid et al., 2012[20] | Arm 1 (enoxaparin, CrCL 60 mL/min), 17 | NR | Major bleeding: 2 (11.8%) | NR | |
| Arm 2 (enoxaparin, CrCL 3059 mL/min), 86 | NR | Major bleeding: 9 (10.5%) | NR | ||
| Arm 3 (enoxaparin, CrCL 30 mL/min), 53 | NR | Major bleeding: 10 (18.9%) | NR | ||
| Arm 4 (UFH, CrCL 60 mL/min), 19 | NR | Major bleeding: 2 (10.5%) | NR | ||
| Arm 5 (UFH, CrCL 3059 mL/min), 99 | NR | Major bleeding: 3 (3%) | NR | ||
| Arm 6 (UFH, CrCL 30 mL/min), 49 | NR | Major bleeding: 2 (4.1%) | NR | ||
Obese Patients
We found 1 subgroup analysis of an RCT (total 3706 patients, 2563 nonobese and 1118 obese patients) that reported on the comparative effectiveness and safety of fixed low‐dose dalteparin 5000 IU/day compared to placebo among 1118 hospitalized medically ill patients with body mass indices (BMI) greater than 30 kg/m2.11 Neither group received additional concurrent prophylactic therapies. The 3 most prevalent medical diagnoses prompting hospitalization were congestive heart failure, respiratory failure, and infectious diseases. Compression ultrasound was performed in all patients by day 21 of hospitalization. The primary end point was the composite of VTE, fatal PE, and sudden death, and secondary end points included DVT, bleeding, and thrombocytopenia by day 21 (Table 1). In obese patients, the primary end point occurred in 2.8% (95% confidence interval [CI]: 1.34.3) of the dalteparin group and in 4.3% (95% CI: 2.56.2) of the placebo group (relative risk [RR]: 0.64; 95% CI: 0.32‐1.28). In nonobese patients, the primary end point occurred in 2.8% (95% CI: 1.8‐3.8) and 5.2% (95% CI: 3.9‐6.6) of the dalteparin and placebo groups, respectively (RR: 0.53; 95% CI: 0.34‐0.82). When weight was modeled as a continuous variable, no statistically significant interaction between weight and dalteparin efficacy was observed (P=0.97). The authors calculated the RR in predefined BMI subgroups and found that dalteparin was effective in reducing VTE in patients with BMIs up to 40, with RRs of 1.0 for all (approximate range, 0.20.8). However, a fixed dose of dalteparin 5000 IU/day was not better than placebo for individuals with BMI >40 kg/m2. There was no significant difference in mortality or major hemorrhage by day 21 between treatment and placebo groups.
Freeman and colleagues prospectively assigned 31 medically ill patients with extreme obesity (BMI >40 kg/m2) to 1 of 3 dosing regimens of enoxaparin: a fixed dose of 40 mg daily enoxaparin (control group, n=11), enoxaparin at 0.4 mg/kg (n=9), or enoxaparin at 0.5 mg/kg (n=11).[12] The average BMI of the entire cohort was 62.1 kg/m2 (range, 40.582.4). All patients had anti‐factor Xa levels drawn on the day of enrollment and daily for 3 days (Table 2). The relationship between anti‐factor Xa levels and clinical efficacy of low‐molecular weight heparin (LMWH) in VTE prophylaxis is still unclear; however, an anti‐factor Xa level of 0.2 to 0.5 IU/mL, measured 4 hours after the fourth dose of LMWH, is the target level recommended for VTE prophylaxis.[13] Patients who received weight‐based enoxaparin at 0.5mg/kg achieved target anti‐factor Xa level 86% of the time compared to 32% of the time in those receiving 0.4 mg/kg and 19% of the time for those in the fixed‐dose group (P0.001). No clinical outcomes were reported in this study.
| Intervention | Outcome | Risk of Bias | Evidence Statement and Magnitude of Effect |
|---|---|---|---|
| |||
| Patients on antiplatelet agents | |||
| Rivaroxaban vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic rivaroxaban or enoxaparin in patients concomitantly treated with antiplatelet agents; 3.6% vs 3.25% |
| Dabigatran vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic dabigatran or enoxaparin in patients concomitantly treated with aspirin; 1.6% vs 3.0% |
| Obese patients | |||
| Dalteparin vs placebo | VTE | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing total VTE in obese patients; 2.8% vs 4.3%, RR: 0.64, 95% CI: 0.32‐1.28 |
| Dalteparin vs placebo | Mortality | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing mortality in obese patients; 9.9% vs 8.6%, P=0.36 |
| Dalteparin vs placebo | Major bleeding | Moderate | Insufficient evidence for safety of dalteparin vs placebo in reducing major bleeding in obese patients; 0% vs 0.7%, P>0.99 |
| Enoxaparin 40 mg daily vs 0.4 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.4 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 32%, P=NR |
| Enoxaparin 40 mg daily vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 86%, P0.001 |
| Enoxaparin 0.4 mg/kg vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 0.4 mg/kg versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 32% vs 86%, P=NR |
| Patients with renal insufficiency | |||
| Tinzaparin vs enoxaparin | VTE | High | Insufficient evidence about superiority of either drug for preventing VTE in patients with renal insufficiency, 0/27 vs 0/28* |
| Tinzaparin vs enoxaparin | Bleeding | High | Insufficient evidence about safety of either drug in patients with renal insufficiency; 5/27 vs 4/28, P=0.67 |
| Dabigatran vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of dabigatran in reducing VTE in severe renal compromise patients vs enoxaparin; 4.3% vs 9%, OR: 0.48, 95% CI: 0.13‐1.73, P=0.271 |
| Dabigatran vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of dabigatran vs enoxaparin in patients with renal impairment; 0 vs 4.7%, P=0.039 |
| Desirudin vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of desirudin vs enoxaparin in reducing VTE in patients with renal impairment; 4.9% vs 7.6%, P=0.019 |
| Desirudin vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of desirudin vs enoxaparin in patients with renal impairment; 0.8% vs 0.2%, P=0.109 |
| Enoxaparin vs UFH | Bleeding | High | Insufficient evidence for increased risk of bleeding with enoxaparin vs unfractionated heparin in patients with all levels of renal impairment, 13.5% vs 4.2%, RR: 3.2, 95% CI: 1.47.3; and for the subgroup of patients with creatinine clearance 30 mL/min; 18.9% vs 4.1%, RR: 4.68, 95% CI: 1.120.6 |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | VTE | Moderate | Insufficient evidence regarding differential benefit of unfractionated heparin by renal function; 2.6% of patients had a VTE event |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | Bleeding | Moderate | Insufficient evidence for differential harm from unfractionated heparin by renal function; 13 events in 92 patients |
Patients on Antiplatelet Drugs
We did not find studies that directly looked at the comparative effectiveness of VTE prophylaxis in patients who were on antiplatelet drugs including aspirin. However, there were 2 studies that looked at the risk of bleeding in patients who received VTE pharmacologic prophylaxis while concurrently taking antiplatelet agents including aspirin. Both studies used pooled data from large phase III trials.
The study by Eriksson et al. used data from the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) trial where over 12,000 patients undergoing elective total knee or hip replacement were randomized to receive VTE prophylaxis with oral rivaroxaban or subcutaneous enoxaparin.[14] Nine percent of participants in each arm (563 in rivaroxaban and 526 in enoxaparin/placebo) were concomitantly using antiplatelet agents or aspirin at least once during the at risk period, defined as starting at day 1 of surgery up to 2 days after the last intake of the study drug. The only end point evaluated was bleeding, and the authors found no statistically significant bleeding difference among the 2 arms (Table 1). Any bleeding event in the rivaroxaban with antiplatelets or aspirin arm was found in 20 (3.6%) patients, whereas in those on enoxaparin/placebo with antiplatelets or aspirin arm it was 17 (3.2%). The relative rate of bleeding among users versus nonusers of antiplatelet drugs or aspirin was 1.32 (95% CI: 0.85‐2.05) in the rivaroxaban group and 1.40 (95% CI: 0.87‐2.25) in the enoxaparin arm (Table 1).
Friedman et al. used pooled data from the RE‐MODEL, RENOVATE, and REMOBILIZE trials, where patients who were undergoing hip or knee arthroplasty were randomized to 220 mg of dabigatran once daily, 150 mg of dabigatran once daily (we focused on this lower dosage as this is the only available dose used in the US), 40 mg of enoxaparin once daily, or 30 mg of enoxaparin twice a day.[15] Of the 8135 patients, 4.7% were on concomitant aspirin. The baseline characteristics of those on aspirin were similar to the other enrollees. The primary outcome was major bleeding events requiring transfusion, symptomatic internal bleeding, or bleeding requiring surgery. Among patients receiving 150 mg of dabigatran, bleeding events with and without concomitant aspirin occurred in 1.6% and 1.0%, respectively (odds ratio [OR]: 1.64; 95% CI: 0.36‐7.49; P=0.523). The percentages of participants with bleeding who received enoxaparin, with and without aspirin, were 3.0% and 1.2%, respectively (OR: 2.57; 95% CI: 0.83‐7.94; P=0.101). The RR of bleeding on dabigatran compared to enoxaparin with and without aspirin therapy was 0.55 (95% CI: 0.11‐2.78) and 0.82 (95% CI: 0.37‐1.84), respectively (Table 1).
Patients With Renal Insufficiency
We found 5 studies that evaluated the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE in patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, or patients receiving dialysis. Four studies were RCTs,[16, 17, 18, 19] and 1 used a cohort design assessing separate cohorts before and after a quality improvement intervention.[20] Bauersachs and colleagues conducted an RCT comparing unfractionated heparin at 5000 IU, 3 times daily to certoparin, which is not approved in the United States and is not further discussed here.[16] The rate of DVT among patients treated with unfractionated heparin in patients with a glomerular filtration rate >30 mL/min was marginally lower than those with severe renal dysfunction (10.3 vs 11.1%) (Table 1).
Patients with severe renal dysfunction who received 5000 IU of unfractionated heparin 3 times a day were at increased risk of all bleeds (RR: 3.4; 95% CI: 2.05.9), major bleeds (RR: 7.3; 95% CI: 3.316), and minor bleeds (RR: 2.6; 95% CI: 1.4‐4.9) compared to patients treated with unfractionated heparin without severe renal dysfunction.[16]
A randomized trial by Mah and colleagues compared drug accumulation and anti‐Xa activity in elderly patients with renal dysfunction (defined as a glomerular filtration rate of 20 to 50 mL/min) who received either tinzaparin at 4500 IU once daily or enoxaparin at 4000 IU once daily.[17] Enoxaparin accumulated to a greater extent from day 1 to day 8 than did tinzaparin; the ratio of maximum concentration on day 8 compared to day 1 was 1.22 for enoxaparin and 1.05 for tinzaparin (P=0.016). No VTE events were reported in patients who received tinzaparin or enoxaparin. There was no statistical difference in the incidence of bleeding events between patients receiving tinzaparin (5, including 2 major events) and enoxaparin (4, including 3 major events, P=0.67) (Table 1).
The trial by Dahl and colleagues randomly assigned patients who were over 75 years of age and/or who had moderate renal dysfunction (defined as creatinine clearance between 30 and 49 mL/min) to receive enoxaparin 40 mg daily or dabigatran 150 mg daily.[18] There was no significant difference in the rate of major VTE events between patients receiving dabigatran (4.3%) and enoxaparin (9%) (OR: 0.48; 95% CI: 0.13‐1.73; P=0.271) (Table 1). The rate of major bleeding was significantly higher among patients randomly assigned to receive enoxaparin (4.7%) versus dabigatran (0%) (P=0.039).[18]
Shorr and colleagues published a post hoc subgroup analysis of a multicenter trial in which orthopedic patients were randomly assigned to receive desirudin 15 mg twice daily or enoxaparin 40 mg once daily.[19] Evaluable patients (1565 of the 2079 patients randomized in the trial) receiving desirudin experienced a significantly lower rate of major VTE compared with patients receiving enoxaparin (4.9% vs 7.6%, P=0.019). This relationship was particularly pronounced for evaluable patients whose creatinine clearance was between 30 and 44 mL/min. In evaluable patients with this degree of renal dysfunction, 11% of patients taking enoxaparin compared to 3.4% of those taking desirudin had a major VTE (OR: 3.52; 95% CI: 1.48‐8.4; P=0.004). There was no significant difference in the rates of major bleeding among a subset of patients assessed for safety outcomes (2078 of the 2079 patients randomized in the trial) who received desirudin (0.8%) or enoxaparin (0.2%) (Table 1).
Elsaid and Collins assessed VTE and bleeding events associated with the use of unfractionated heparin 5000U either 2 or 3 times daily and enoxaparin 30 mg once or twice daily across patients stratified by renal function (creatinine clearance 30, 3059, and 60 mL/min). The investigators made assessments before and after a quality improvement intervention that was designed to eliminate the use of enoxaparin in patients whose creatinine clearance was 30 mL/min. No VTE events were reported. Patients receiving enoxaparin were significantly more likely to experience a major bleeding episode compared with patients receiving unfractionated heparin (overall rates for all levels of renal function: 13.5% vs 4. 2%; RR: 3.2; 95% CI: 1.47.3) (Table 2). This association was largely driven by the subgroup of patients with a creatinine clearance 30 mL/min. For this subgroup with severe renal insufficiency, patients receiving enoxaparin were significantly more likely to have a bleed compared with patients receiving unfractionated heparin (18.9% vs 4.1%; RR: 4.68; 95% CI: 1.120.6) (Tables 1 and 2). There was no difference in the bleeding rates for patients whose creatinine clearances were >60 mL/min.[20]
Strength of Evidence
Obese Patients
Overall, we found that the strength of evidence was insufficient regarding the composite end point of DVT, PE, and sudden death, and the outcomes of mortality and bleeding (Table 2). This was based on a paucity of available data, and a moderate risk of bias in the reviewed studies. Additionally, 92% of the enrolled patients in the studies were white, limiting the generalizability of the results to other ethnic groups.
Patients on Antiplatelets
The strength of evidence was insufficient in the studies reviewed here to conclude that there is no difference in rates of bleeding in patients who are concomitantly taking antiplatelet drugs while getting VTE prophylaxis with rivaroxaban, dabigatran, or enoxaparin. We based this rating because of the imprecision of results and unknown consistencies across multiple studies.
Patients With Renal Insufficiency
One RCT had a high risk of bias for our key question because data from only 1 study arm were useful for our review.[16] The other RCTs were judged to have a moderate risk of bias. The analyses led by Dahl and Shorr[18, 19] were based on post hoc (ie, not prespecified) analysis of data from RCTs. Additionally, outcomes in the Shorr et al. trial were reported for evaluable subpopulations of the cohort that was initially randomized in the clinical trial.
We rated the strength of evidence as insufficient to know the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE during hospitalization of patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, and patients receiving dialysis. We based this rating on the risk of bias associated with published studies and a lack of consistent evidence regarding associations that were reported. Similarly, we rated the strength of evidence as insufficient that 5000 U of unfractionated heparin 3 times daily increases the risk of major and minor bleeding events in patients with severely compromised renal function compared to this dose in patients without severely compromised renal function. We based this rating on a high risk of bias of included studies and inconsistent evidence. Likewise, we rated the strength of evidence as insufficient that enoxaparin significantly increases the risk of major bleeding compared with unfractionated heparin in patients with severe renal insufficiency. We based this rating on a high risk of bias and inconsistent published evidence.
We similarly found insufficient evidence to guide treatment decisions for patients with renal insufficiency. Our findings are consistent with other recent reviews. The American College of Chest Physicians (ACCP) practice guidelines[21] make dosing recommendations for the therapeutic use of enoxaparin. However, their assessment is that the data are insufficient to make direct recommendations about prophylaxis. Their assessment of the indirect evidence regarding bioaccumulation and increased anti‐factor Xa levels are consistent with ours. The ACCP guidelines also suggest that decreased clearance of enoxaparin has been associated with increased risk of bleeding events for patients with severe renal insufficiency. However, the cited study[20] compares patients with and without severe renal dysfunction who received the same therapy. Therefore, it is not possible to determine the additional risk conveyed by enoxaparin therapy, that is, above the baseline increased risk of bleeding among patients with renal insufficiency, particularly those receiving an alternate pharmacologic VTE prevention strategy, such as unfractionated heparin.
DISCUSSION
We found that the evidence was very limited about prevention of VTE in these select and yet prevalent patient populations. Despite the fact that there is an increasing number of obese patients and patients who are on antiplatelet therapies, most clinical practice guidelines do not address the care of these populations, which may be entirely appropriate given the state of the evidence.
The ACCP practice guidelines[21] suggest using a higher dose of enoxaparin for the prevention of VTE in obese patients. The subgroup analysis by Kucher et al.[11] showed effect attenuation of dalteparin when given at a fixed dose of 5000 IU/mL to patients with a BMI of >40 kg/m2. The Freeman study[12] showed that extremely obese patients (average BMI >62.1 kg/m2) who are given a fixed dose of enoxaparin achieved target anti‐factor Xa levels significantly less often than those who received a higher dose of enoxaparin. The 2 separate findings, although not conclusive, lend some credence to the current ACCP guidelines.[21]
The studies we reviewed on VTE prophylaxis in patients who are concomitantly on antiplatelets including aspirin reported no major increased risk of bleeding; however, in the Friedman et al. study,[15] 3.0% of patients who were put on enoxaparin while still on aspirin had a bleeding event compared to 1.2% of those on enoxaparin alone. This difference is not statistically significant but is a trend possibly worth noting, especially when one looks at the lower RR of bleeding at 0.55 compared to 0.82 when dabigatran is compared with enoxaparin with and without concomitant aspirin therapy, respectively (Table 1). The highest dose of aspirin used in either of the studies was 160 mg/day, and neither study addressed other potent antiplatelets such as clopidogrel or ticlopidine separately, which limits the generalizability of the finding to all antiplatelets. Current ACCP guidelines do not recommend aspirin as a sole option for the prevention of VTE in orthopedic surgery patients.[22] Concerns remain among clinicians that antiplatelets, including aspirin, on their own are unlikely to be fully effective to thwart venous thrombotic processes for most patients, and yet the risk of bleeding is not fully known when these agents are combined with other anticoagulants for VTE prophylaxis.
Our review has several limitations, including the possibility that we may have missed some observational studies, as the identification of relevant observational studies in electronic searches is more challenging than that of RCTs. The few studies made it impossible to quantitatively pool results. These results, however, have important implications, namely that additional research on the comparative effectiveness and safety of pharmacologic and mechanical strategies to prevent VTE is needed for the optimal care of these patient subgroups. This might be achieved with trials dedicated to enrolling these patients or prespecified subgroup analyses within larger trials. Observational data may be appropriate as long as attention is paid to confounding.
APPENDIX
MEDLINE Search Strategy
((pulmonary embolism[mh] OR PE[tiab] OR Pulmonary embolism[tiab] OR thromboembolism[mh] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR Thrombosis[mh] OR thrombosis[tiab] OR DVT[tiab] OR VTE[tiab] OR clot[tiab]) AND (Anticoagulants[mh] OR Anticoagulants[tiab] OR Anticoagulant[tiab] OR thrombin inhibitors[tiab] OR Aspirin[mh] or aspirin[tiab] OR aspirins[tiab] or clopidogrel[nm] OR clopidogrel[tiab] OR Plavix[tiab] or ticlopidine[mh] or ticlopidine[tiab]OR ticlid[tiab] OR prasugrel[nm]Or prasugrel[tiab]OR effient[tiab]OR ticagrelor[NM] OR ticagrelor[tiab]OR Brilinta[tiab] OR cilostazol[NM] OR cilostazol[tiab]OR pletal[tiab] OR warfarin[mh]OR warfarin[tiab]OR coumadin[tiab] OR coumadine[tiab] OR Dipyridamole[mh]OR dipyridamole[tiab]OR persantine[tiab] OR dicoumarol[MH] OR dicoumarol[tiab] OR dicumarol[tiab] OR Dextran sulfate[mh] OR dextran sulfate[tiab] ORthrombin inhibitors[tiab] OR thrombin inhibitor[tiab] OR heparin[mh] OR Heparin[tiab] OR Heparins[tiab] OR LMWH[tiab] OR LDUH[tiab] OR Enoxaparin[mh] OR Enoxaparin[tiab] OR Lovenox[tiab] OR Dalteparin[tiab] OR Fragmin[tiab] OR Tinzaparin[tiab] OR innohep[tiab] OR Nadroparin[tiab] OR Fondaparinux[nm] OR Fondaparinux[tiab] OR Arixtra[tiab] OR Idraparinux[nm] OR Idraparinux[tiab] OR Rivaroxaban[nm] OR Rivaroxaban[tiab] OR novastan[tiab] OR Desirudin[nm] OR Desirudin[tiab] OR Iprivask[tiab]OR direct thrombin inhibitor[tiab] OR Argatroban[nm] OR Argatroban[tiab] OR Acova[tiab] OR Bivalirudin[nm] OR Bivalirudin[tiab] OR Angiomax[tiab] OR Lepirudin[nm] OR Lepirudin[tiab] OR Refludan[tiab] OR Dabigatran[nm] OR Dabigatran[tiab] OR Pradaxa[tiab] OR factor xa[mh] OR factor Xa[tiab] OR vena cava filters[mh] OR filters[tiab] OR filter[tiab] OR compression stockings[mh] OR intermittent pneumatic compression devices[mh] OR compression [tiab] OR Venous foot pump[tiab])) AND(prevent*[tiab] OR prophyla*[tiab] OR prevention and control[subheading]) NOT (animals[mh] NOT humans[mh]) NOT (editorial[pt] OR comment[pt]) NOT ((infant[mh] OR infant[tiab] OR child[mh] OR child[tiab] OR children[tiab] OR adolescent[mh] OR adolescent[tiab] OR teen‐age[tiab] OR pediatric[tiab] OR perinatal[tiab]) NOT (adult[tiab] OR adults[tiab] OR adult[mh])) NOT (mechanical valve[tiab] OR heart valve[tiab] OR atrial fibrillation[mh] OR atrial fibrillation[tiab] OR thrombophilia[mh] OR thrombophilia[tiab] OR pregnancy[mh])
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is estimated to affect 900,000 Americans each year and is a cause of significant morbidity and mortality with associated high healthcare costs.[1] Accordingly, the comparative effectiveness and safety of interventions for the prevention and treatment of VTE are among the national priorities for comparative effectiveness research.[2] Whereas we have evidence‐based guidelines for the prophylaxis of VTE in the general population, there are no guidelines informing the care of select patient populations. Select populations are those patients in whom there is decisional uncertainty about the optimal choice, timing, and dose of VTE prophylaxis. Not only do these patients have an increased risk of DVT and PE, but most are also at high risk of bleeding, the most important complication of VTE prophylaxis.[3, 4, 5, 6]
The objectives of this systematic review were to define the comparative effectiveness and safety of pharmacologic and mechanical strategies for VTE prevention in some of these select medical populations including obese patients, patients on concomitant antiplatelet therapy, patients with renal insufficiency, patients who are underweight, and patients with coagulopathy due to liver disease.
METHODS
The methods for this comparative effectiveness review (CER) follow the guidelines suggested in the Agency for Healthcare Research and Quality (AHRQ) Methods Guide for Effectiveness and Comparative Effectiveness Reviews.[7] The protocol was publically posted.[8]
Search Strategy
We searched MEDLINE, EMBASE, and SCOPUS through August 2011, CINAHL, International Pharmaceutical Abstracts,
Study Selection
We reviewed titles followed by abstracts to identify randomized controlled trials (RCTs) or observational studies with comparison groups reporting on the effectiveness or safety of VTE prevention in our populations. Two investigators independently reviewed abstracts, and we excluded the abstracts if both investigators agreed that the article met 1 or more of the exclusion criteria. We included only English‐language articles that evaluated the effectiveness of pharmacological or mechanical interventions that have been approved for clinical use in the United States. To be eligible, the studies must have addressed relevant key questions in the population of our interest. We resolved disagreements by consensus. We used DistillerSR (Evidence Partners Inc., Ottawa, Ontario, Canada), a Web‐based database management program to manage the review process. Two investigators assessed the risk of bias in each study independently, using the Downs and Black instrument for observational studies and trials.[10]
Data Synthesis
For each select population, we created detailed evidence tables containing the information abstracted from the eligible studies. After synthesizing the evidence, we graded the quantity, quality, and consistency of the best available evidence for each select population by adapting an evidence‐grading scheme recommended in the Methods Guide for Conducting Comparative Effectiveness Reviews.[7]
RESULTS
We identified 30,902 unique citations and included 9 studies (Figure 1). There were 5 RCTs with relevant subgroups and 4 observational studies (Table 1). Two studies reported on the risk of bleeding in patients given pharmacologic prophylaxis while they are concomitantly taking nonsteroidal anti‐inflammatory drugs (NSAIDS) or antiplatelet agents/aspirin, 1 RCT and 1 prospective observational study reported on obese patients, and 5 studies described outcomes of patients with renal insufficiency (see Supporting Information, Table 1, in the online version of this article). No study tested prophylaxis in underweight patients or those with liver disease.

| Study | Arm, n | Total VTE (DVT and PE) | Bleeding | Other Outcomes | |
|---|---|---|---|---|---|
| |||||
| Obese patients | |||||
| Kucher et al., 2005[11] | Arm 1 (dalteparin), 558 | 2.8% (95% CI: 1.34.3) | 0% | Mortality at 21 days: 4.6% | |
| Arm 2 (placebo), 560 | 4.3% (95% CI: 2.56.2) | 0.7% | Mortality at 21 days: 2.7% | ||
| Freeman et al., [12] | Arm 1 (fixed‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 19 % | |
| Arm 2 (lower‐dose enoxaparin), 9 | NR | NR | Peak anti‐factor Xa level 32 % | ||
| Arm 3 (higher‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 86 % | ||
| Patients on antiplatelet agents | |||||
| Eriksson et al., 2012[14] | Arm 1 (rivaroxaban), 563 | NR | 20 (3.6%), rate ratio for use vs nonuse: 1.32 (95% CI: 0.85‐2.05) | NR | |
| Arm 2 (enoxaparin/placebo), 526 | NR | 17 (3.2%), rate ratio for use vs nonuse: 1.40 (95% CI: 0.87‐2.25) | NR | ||
| Friedman et al., 2012[15] | Arm 2 (150 mg dabigatran, no ASA), 1149 | NR | 11 (1.0%)a | NR | |
| Arm 5 (150 mg dabigatran+ASA), 128 | NR | 2 (1.6%)a | NR | ||
| Arm 3 (enoxaparin, no ASA), 1167 | NR | 14 (1.2%)a | NR | ||
| Arm 6 (enoxaparin+ASA), 132 | NR | 4 (3.0%) | NR | ||
| 150 mg dabigatran compared with enoxaparinNo concomitant ASA therapy | NR | RR: 0.82 (95% CI: 0.37‐1.84) | NR | ||
| 150 mg dabigatran compared with enoxaparinWith concomitant ASA therapy | NR | RR: 0.55 (95% CI: 0.11‐2.78) | NR | ||
| Patients with renal insufficiency | |||||
| Bauersachs et al., 2011[16] | Arm 2 (GFR 30), 92 | Total DVT: 11.11%; Total PE: 0% | Major bleeding: 4/92 (4.35%), minor bleeding: 9/92 (9.78%) | Mortality: 5.81% | |
| Mah et al., 2007[17] | Arm 2 (tinzaparin), 27 | NR | Major bleeding: 2/27 (7.4%), minor bleeding: 3/27 (11.1%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.05 | |
| Arm 3 (enoxaparin), 28 | NR | Major bleeding: 1/28 (3.6%), minor bleeding: 3/28 (10.7%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.22 | ||
| Dahl et al., 2012[18] | Arm 1 (enoxaparin), 332 | Major VTE: 8 (9.0%) | Major bleeding: 6 (4.7%) | Infections and infestations: 25 (7.5%), Wound infection: 4 (1.2%) | |
| Arm 2 (dabigatran), 300 | Major VTE: 3 (4.3%) | Major bleeding: 0 (0%) | Infections and infestations: 21 (7.0%), Wound Infection: 3 (1.0%) | ||
| Shorr et al., 2012[19] | Arm 1 (enoxaparin, CrCL 60 mL/min), 353 | Total VTE: 17/275 (6.2%) | Major bleeding: 0/351 (0%) | NR | |
| Arm 2 (desirudin, CrCL 60 mL/min), 353 | Total VTE: 13/284 (4.3%) | Major bleeding: 2/349 (0.27%) | NR | ||
| Arm 3 (enoxaparin, CrCL 4559 mL/min), 369 | Total VTE: 18/282 (6.2%) | Major bleeding: 1/365 (0.27%) | NR | ||
| Arm 4 (desirudin, CrCL 4559 mL/min), 395 | Total VTE: 17/303 (5.6%) | Major bleeding: 1/393 (0.25%) | NR | ||
| Arm 5 (enoxaparin, CrCL 45 mL/min), 298 | Total VTE: 24/216 (11.1%) | Major bleeding: 1/294 (0.34%) | NR | ||
| Arm 6 (desirudin, CrCL 45 mL/min), 279 | Total VTE: 7/205 (3.4%) | Major bleeding: 5/275 (1.82%) | NR | ||
| Elsaid et al., 2012[20] | Arm 1 (enoxaparin, CrCL 60 mL/min), 17 | NR | Major bleeding: 2 (11.8%) | NR | |
| Arm 2 (enoxaparin, CrCL 3059 mL/min), 86 | NR | Major bleeding: 9 (10.5%) | NR | ||
| Arm 3 (enoxaparin, CrCL 30 mL/min), 53 | NR | Major bleeding: 10 (18.9%) | NR | ||
| Arm 4 (UFH, CrCL 60 mL/min), 19 | NR | Major bleeding: 2 (10.5%) | NR | ||
| Arm 5 (UFH, CrCL 3059 mL/min), 99 | NR | Major bleeding: 3 (3%) | NR | ||
| Arm 6 (UFH, CrCL 30 mL/min), 49 | NR | Major bleeding: 2 (4.1%) | NR | ||
Obese Patients
We found 1 subgroup analysis of an RCT (total 3706 patients, 2563 nonobese and 1118 obese patients) that reported on the comparative effectiveness and safety of fixed low‐dose dalteparin 5000 IU/day compared to placebo among 1118 hospitalized medically ill patients with body mass indices (BMI) greater than 30 kg/m2.11 Neither group received additional concurrent prophylactic therapies. The 3 most prevalent medical diagnoses prompting hospitalization were congestive heart failure, respiratory failure, and infectious diseases. Compression ultrasound was performed in all patients by day 21 of hospitalization. The primary end point was the composite of VTE, fatal PE, and sudden death, and secondary end points included DVT, bleeding, and thrombocytopenia by day 21 (Table 1). In obese patients, the primary end point occurred in 2.8% (95% confidence interval [CI]: 1.34.3) of the dalteparin group and in 4.3% (95% CI: 2.56.2) of the placebo group (relative risk [RR]: 0.64; 95% CI: 0.32‐1.28). In nonobese patients, the primary end point occurred in 2.8% (95% CI: 1.8‐3.8) and 5.2% (95% CI: 3.9‐6.6) of the dalteparin and placebo groups, respectively (RR: 0.53; 95% CI: 0.34‐0.82). When weight was modeled as a continuous variable, no statistically significant interaction between weight and dalteparin efficacy was observed (P=0.97). The authors calculated the RR in predefined BMI subgroups and found that dalteparin was effective in reducing VTE in patients with BMIs up to 40, with RRs of 1.0 for all (approximate range, 0.20.8). However, a fixed dose of dalteparin 5000 IU/day was not better than placebo for individuals with BMI >40 kg/m2. There was no significant difference in mortality or major hemorrhage by day 21 between treatment and placebo groups.
Freeman and colleagues prospectively assigned 31 medically ill patients with extreme obesity (BMI >40 kg/m2) to 1 of 3 dosing regimens of enoxaparin: a fixed dose of 40 mg daily enoxaparin (control group, n=11), enoxaparin at 0.4 mg/kg (n=9), or enoxaparin at 0.5 mg/kg (n=11).[12] The average BMI of the entire cohort was 62.1 kg/m2 (range, 40.582.4). All patients had anti‐factor Xa levels drawn on the day of enrollment and daily for 3 days (Table 2). The relationship between anti‐factor Xa levels and clinical efficacy of low‐molecular weight heparin (LMWH) in VTE prophylaxis is still unclear; however, an anti‐factor Xa level of 0.2 to 0.5 IU/mL, measured 4 hours after the fourth dose of LMWH, is the target level recommended for VTE prophylaxis.[13] Patients who received weight‐based enoxaparin at 0.5mg/kg achieved target anti‐factor Xa level 86% of the time compared to 32% of the time in those receiving 0.4 mg/kg and 19% of the time for those in the fixed‐dose group (P0.001). No clinical outcomes were reported in this study.
| Intervention | Outcome | Risk of Bias | Evidence Statement and Magnitude of Effect |
|---|---|---|---|
| |||
| Patients on antiplatelet agents | |||
| Rivaroxaban vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic rivaroxaban or enoxaparin in patients concomitantly treated with antiplatelet agents; 3.6% vs 3.25% |
| Dabigatran vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic dabigatran or enoxaparin in patients concomitantly treated with aspirin; 1.6% vs 3.0% |
| Obese patients | |||
| Dalteparin vs placebo | VTE | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing total VTE in obese patients; 2.8% vs 4.3%, RR: 0.64, 95% CI: 0.32‐1.28 |
| Dalteparin vs placebo | Mortality | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing mortality in obese patients; 9.9% vs 8.6%, P=0.36 |
| Dalteparin vs placebo | Major bleeding | Moderate | Insufficient evidence for safety of dalteparin vs placebo in reducing major bleeding in obese patients; 0% vs 0.7%, P>0.99 |
| Enoxaparin 40 mg daily vs 0.4 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.4 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 32%, P=NR |
| Enoxaparin 40 mg daily vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 86%, P0.001 |
| Enoxaparin 0.4 mg/kg vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 0.4 mg/kg versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 32% vs 86%, P=NR |
| Patients with renal insufficiency | |||
| Tinzaparin vs enoxaparin | VTE | High | Insufficient evidence about superiority of either drug for preventing VTE in patients with renal insufficiency, 0/27 vs 0/28* |
| Tinzaparin vs enoxaparin | Bleeding | High | Insufficient evidence about safety of either drug in patients with renal insufficiency; 5/27 vs 4/28, P=0.67 |
| Dabigatran vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of dabigatran in reducing VTE in severe renal compromise patients vs enoxaparin; 4.3% vs 9%, OR: 0.48, 95% CI: 0.13‐1.73, P=0.271 |
| Dabigatran vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of dabigatran vs enoxaparin in patients with renal impairment; 0 vs 4.7%, P=0.039 |
| Desirudin vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of desirudin vs enoxaparin in reducing VTE in patients with renal impairment; 4.9% vs 7.6%, P=0.019 |
| Desirudin vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of desirudin vs enoxaparin in patients with renal impairment; 0.8% vs 0.2%, P=0.109 |
| Enoxaparin vs UFH | Bleeding | High | Insufficient evidence for increased risk of bleeding with enoxaparin vs unfractionated heparin in patients with all levels of renal impairment, 13.5% vs 4.2%, RR: 3.2, 95% CI: 1.47.3; and for the subgroup of patients with creatinine clearance 30 mL/min; 18.9% vs 4.1%, RR: 4.68, 95% CI: 1.120.6 |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | VTE | Moderate | Insufficient evidence regarding differential benefit of unfractionated heparin by renal function; 2.6% of patients had a VTE event |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | Bleeding | Moderate | Insufficient evidence for differential harm from unfractionated heparin by renal function; 13 events in 92 patients |
Patients on Antiplatelet Drugs
We did not find studies that directly looked at the comparative effectiveness of VTE prophylaxis in patients who were on antiplatelet drugs including aspirin. However, there were 2 studies that looked at the risk of bleeding in patients who received VTE pharmacologic prophylaxis while concurrently taking antiplatelet agents including aspirin. Both studies used pooled data from large phase III trials.
The study by Eriksson et al. used data from the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) trial where over 12,000 patients undergoing elective total knee or hip replacement were randomized to receive VTE prophylaxis with oral rivaroxaban or subcutaneous enoxaparin.[14] Nine percent of participants in each arm (563 in rivaroxaban and 526 in enoxaparin/placebo) were concomitantly using antiplatelet agents or aspirin at least once during the at risk period, defined as starting at day 1 of surgery up to 2 days after the last intake of the study drug. The only end point evaluated was bleeding, and the authors found no statistically significant bleeding difference among the 2 arms (Table 1). Any bleeding event in the rivaroxaban with antiplatelets or aspirin arm was found in 20 (3.6%) patients, whereas in those on enoxaparin/placebo with antiplatelets or aspirin arm it was 17 (3.2%). The relative rate of bleeding among users versus nonusers of antiplatelet drugs or aspirin was 1.32 (95% CI: 0.85‐2.05) in the rivaroxaban group and 1.40 (95% CI: 0.87‐2.25) in the enoxaparin arm (Table 1).
Friedman et al. used pooled data from the RE‐MODEL, RENOVATE, and REMOBILIZE trials, where patients who were undergoing hip or knee arthroplasty were randomized to 220 mg of dabigatran once daily, 150 mg of dabigatran once daily (we focused on this lower dosage as this is the only available dose used in the US), 40 mg of enoxaparin once daily, or 30 mg of enoxaparin twice a day.[15] Of the 8135 patients, 4.7% were on concomitant aspirin. The baseline characteristics of those on aspirin were similar to the other enrollees. The primary outcome was major bleeding events requiring transfusion, symptomatic internal bleeding, or bleeding requiring surgery. Among patients receiving 150 mg of dabigatran, bleeding events with and without concomitant aspirin occurred in 1.6% and 1.0%, respectively (odds ratio [OR]: 1.64; 95% CI: 0.36‐7.49; P=0.523). The percentages of participants with bleeding who received enoxaparin, with and without aspirin, were 3.0% and 1.2%, respectively (OR: 2.57; 95% CI: 0.83‐7.94; P=0.101). The RR of bleeding on dabigatran compared to enoxaparin with and without aspirin therapy was 0.55 (95% CI: 0.11‐2.78) and 0.82 (95% CI: 0.37‐1.84), respectively (Table 1).
Patients With Renal Insufficiency
We found 5 studies that evaluated the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE in patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, or patients receiving dialysis. Four studies were RCTs,[16, 17, 18, 19] and 1 used a cohort design assessing separate cohorts before and after a quality improvement intervention.[20] Bauersachs and colleagues conducted an RCT comparing unfractionated heparin at 5000 IU, 3 times daily to certoparin, which is not approved in the United States and is not further discussed here.[16] The rate of DVT among patients treated with unfractionated heparin in patients with a glomerular filtration rate >30 mL/min was marginally lower than those with severe renal dysfunction (10.3 vs 11.1%) (Table 1).
Patients with severe renal dysfunction who received 5000 IU of unfractionated heparin 3 times a day were at increased risk of all bleeds (RR: 3.4; 95% CI: 2.05.9), major bleeds (RR: 7.3; 95% CI: 3.316), and minor bleeds (RR: 2.6; 95% CI: 1.4‐4.9) compared to patients treated with unfractionated heparin without severe renal dysfunction.[16]
A randomized trial by Mah and colleagues compared drug accumulation and anti‐Xa activity in elderly patients with renal dysfunction (defined as a glomerular filtration rate of 20 to 50 mL/min) who received either tinzaparin at 4500 IU once daily or enoxaparin at 4000 IU once daily.[17] Enoxaparin accumulated to a greater extent from day 1 to day 8 than did tinzaparin; the ratio of maximum concentration on day 8 compared to day 1 was 1.22 for enoxaparin and 1.05 for tinzaparin (P=0.016). No VTE events were reported in patients who received tinzaparin or enoxaparin. There was no statistical difference in the incidence of bleeding events between patients receiving tinzaparin (5, including 2 major events) and enoxaparin (4, including 3 major events, P=0.67) (Table 1).
The trial by Dahl and colleagues randomly assigned patients who were over 75 years of age and/or who had moderate renal dysfunction (defined as creatinine clearance between 30 and 49 mL/min) to receive enoxaparin 40 mg daily or dabigatran 150 mg daily.[18] There was no significant difference in the rate of major VTE events between patients receiving dabigatran (4.3%) and enoxaparin (9%) (OR: 0.48; 95% CI: 0.13‐1.73; P=0.271) (Table 1). The rate of major bleeding was significantly higher among patients randomly assigned to receive enoxaparin (4.7%) versus dabigatran (0%) (P=0.039).[18]
Shorr and colleagues published a post hoc subgroup analysis of a multicenter trial in which orthopedic patients were randomly assigned to receive desirudin 15 mg twice daily or enoxaparin 40 mg once daily.[19] Evaluable patients (1565 of the 2079 patients randomized in the trial) receiving desirudin experienced a significantly lower rate of major VTE compared with patients receiving enoxaparin (4.9% vs 7.6%, P=0.019). This relationship was particularly pronounced for evaluable patients whose creatinine clearance was between 30 and 44 mL/min. In evaluable patients with this degree of renal dysfunction, 11% of patients taking enoxaparin compared to 3.4% of those taking desirudin had a major VTE (OR: 3.52; 95% CI: 1.48‐8.4; P=0.004). There was no significant difference in the rates of major bleeding among a subset of patients assessed for safety outcomes (2078 of the 2079 patients randomized in the trial) who received desirudin (0.8%) or enoxaparin (0.2%) (Table 1).
Elsaid and Collins assessed VTE and bleeding events associated with the use of unfractionated heparin 5000U either 2 or 3 times daily and enoxaparin 30 mg once or twice daily across patients stratified by renal function (creatinine clearance 30, 3059, and 60 mL/min). The investigators made assessments before and after a quality improvement intervention that was designed to eliminate the use of enoxaparin in patients whose creatinine clearance was 30 mL/min. No VTE events were reported. Patients receiving enoxaparin were significantly more likely to experience a major bleeding episode compared with patients receiving unfractionated heparin (overall rates for all levels of renal function: 13.5% vs 4. 2%; RR: 3.2; 95% CI: 1.47.3) (Table 2). This association was largely driven by the subgroup of patients with a creatinine clearance 30 mL/min. For this subgroup with severe renal insufficiency, patients receiving enoxaparin were significantly more likely to have a bleed compared with patients receiving unfractionated heparin (18.9% vs 4.1%; RR: 4.68; 95% CI: 1.120.6) (Tables 1 and 2). There was no difference in the bleeding rates for patients whose creatinine clearances were >60 mL/min.[20]
Strength of Evidence
Obese Patients
Overall, we found that the strength of evidence was insufficient regarding the composite end point of DVT, PE, and sudden death, and the outcomes of mortality and bleeding (Table 2). This was based on a paucity of available data, and a moderate risk of bias in the reviewed studies. Additionally, 92% of the enrolled patients in the studies were white, limiting the generalizability of the results to other ethnic groups.
Patients on Antiplatelets
The strength of evidence was insufficient in the studies reviewed here to conclude that there is no difference in rates of bleeding in patients who are concomitantly taking antiplatelet drugs while getting VTE prophylaxis with rivaroxaban, dabigatran, or enoxaparin. We based this rating because of the imprecision of results and unknown consistencies across multiple studies.
Patients With Renal Insufficiency
One RCT had a high risk of bias for our key question because data from only 1 study arm were useful for our review.[16] The other RCTs were judged to have a moderate risk of bias. The analyses led by Dahl and Shorr[18, 19] were based on post hoc (ie, not prespecified) analysis of data from RCTs. Additionally, outcomes in the Shorr et al. trial were reported for evaluable subpopulations of the cohort that was initially randomized in the clinical trial.
We rated the strength of evidence as insufficient to know the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE during hospitalization of patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, and patients receiving dialysis. We based this rating on the risk of bias associated with published studies and a lack of consistent evidence regarding associations that were reported. Similarly, we rated the strength of evidence as insufficient that 5000 U of unfractionated heparin 3 times daily increases the risk of major and minor bleeding events in patients with severely compromised renal function compared to this dose in patients without severely compromised renal function. We based this rating on a high risk of bias of included studies and inconsistent evidence. Likewise, we rated the strength of evidence as insufficient that enoxaparin significantly increases the risk of major bleeding compared with unfractionated heparin in patients with severe renal insufficiency. We based this rating on a high risk of bias and inconsistent published evidence.
We similarly found insufficient evidence to guide treatment decisions for patients with renal insufficiency. Our findings are consistent with other recent reviews. The American College of Chest Physicians (ACCP) practice guidelines[21] make dosing recommendations for the therapeutic use of enoxaparin. However, their assessment is that the data are insufficient to make direct recommendations about prophylaxis. Their assessment of the indirect evidence regarding bioaccumulation and increased anti‐factor Xa levels are consistent with ours. The ACCP guidelines also suggest that decreased clearance of enoxaparin has been associated with increased risk of bleeding events for patients with severe renal insufficiency. However, the cited study[20] compares patients with and without severe renal dysfunction who received the same therapy. Therefore, it is not possible to determine the additional risk conveyed by enoxaparin therapy, that is, above the baseline increased risk of bleeding among patients with renal insufficiency, particularly those receiving an alternate pharmacologic VTE prevention strategy, such as unfractionated heparin.
DISCUSSION
We found that the evidence was very limited about prevention of VTE in these select and yet prevalent patient populations. Despite the fact that there is an increasing number of obese patients and patients who are on antiplatelet therapies, most clinical practice guidelines do not address the care of these populations, which may be entirely appropriate given the state of the evidence.
The ACCP practice guidelines[21] suggest using a higher dose of enoxaparin for the prevention of VTE in obese patients. The subgroup analysis by Kucher et al.[11] showed effect attenuation of dalteparin when given at a fixed dose of 5000 IU/mL to patients with a BMI of >40 kg/m2. The Freeman study[12] showed that extremely obese patients (average BMI >62.1 kg/m2) who are given a fixed dose of enoxaparin achieved target anti‐factor Xa levels significantly less often than those who received a higher dose of enoxaparin. The 2 separate findings, although not conclusive, lend some credence to the current ACCP guidelines.[21]
The studies we reviewed on VTE prophylaxis in patients who are concomitantly on antiplatelets including aspirin reported no major increased risk of bleeding; however, in the Friedman et al. study,[15] 3.0% of patients who were put on enoxaparin while still on aspirin had a bleeding event compared to 1.2% of those on enoxaparin alone. This difference is not statistically significant but is a trend possibly worth noting, especially when one looks at the lower RR of bleeding at 0.55 compared to 0.82 when dabigatran is compared with enoxaparin with and without concomitant aspirin therapy, respectively (Table 1). The highest dose of aspirin used in either of the studies was 160 mg/day, and neither study addressed other potent antiplatelets such as clopidogrel or ticlopidine separately, which limits the generalizability of the finding to all antiplatelets. Current ACCP guidelines do not recommend aspirin as a sole option for the prevention of VTE in orthopedic surgery patients.[22] Concerns remain among clinicians that antiplatelets, including aspirin, on their own are unlikely to be fully effective to thwart venous thrombotic processes for most patients, and yet the risk of bleeding is not fully known when these agents are combined with other anticoagulants for VTE prophylaxis.
Our review has several limitations, including the possibility that we may have missed some observational studies, as the identification of relevant observational studies in electronic searches is more challenging than that of RCTs. The few studies made it impossible to quantitatively pool results. These results, however, have important implications, namely that additional research on the comparative effectiveness and safety of pharmacologic and mechanical strategies to prevent VTE is needed for the optimal care of these patient subgroups. This might be achieved with trials dedicated to enrolling these patients or prespecified subgroup analyses within larger trials. Observational data may be appropriate as long as attention is paid to confounding.
APPENDIX
MEDLINE Search Strategy
((pulmonary embolism[mh] OR PE[tiab] OR Pulmonary embolism[tiab] OR thromboembolism[mh] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR Thrombosis[mh] OR thrombosis[tiab] OR DVT[tiab] OR VTE[tiab] OR clot[tiab]) AND (Anticoagulants[mh] OR Anticoagulants[tiab] OR Anticoagulant[tiab] OR thrombin inhibitors[tiab] OR Aspirin[mh] or aspirin[tiab] OR aspirins[tiab] or clopidogrel[nm] OR clopidogrel[tiab] OR Plavix[tiab] or ticlopidine[mh] or ticlopidine[tiab]OR ticlid[tiab] OR prasugrel[nm]Or prasugrel[tiab]OR effient[tiab]OR ticagrelor[NM] OR ticagrelor[tiab]OR Brilinta[tiab] OR cilostazol[NM] OR cilostazol[tiab]OR pletal[tiab] OR warfarin[mh]OR warfarin[tiab]OR coumadin[tiab] OR coumadine[tiab] OR Dipyridamole[mh]OR dipyridamole[tiab]OR persantine[tiab] OR dicoumarol[MH] OR dicoumarol[tiab] OR dicumarol[tiab] OR Dextran sulfate[mh] OR dextran sulfate[tiab] ORthrombin inhibitors[tiab] OR thrombin inhibitor[tiab] OR heparin[mh] OR Heparin[tiab] OR Heparins[tiab] OR LMWH[tiab] OR LDUH[tiab] OR Enoxaparin[mh] OR Enoxaparin[tiab] OR Lovenox[tiab] OR Dalteparin[tiab] OR Fragmin[tiab] OR Tinzaparin[tiab] OR innohep[tiab] OR Nadroparin[tiab] OR Fondaparinux[nm] OR Fondaparinux[tiab] OR Arixtra[tiab] OR Idraparinux[nm] OR Idraparinux[tiab] OR Rivaroxaban[nm] OR Rivaroxaban[tiab] OR novastan[tiab] OR Desirudin[nm] OR Desirudin[tiab] OR Iprivask[tiab]OR direct thrombin inhibitor[tiab] OR Argatroban[nm] OR Argatroban[tiab] OR Acova[tiab] OR Bivalirudin[nm] OR Bivalirudin[tiab] OR Angiomax[tiab] OR Lepirudin[nm] OR Lepirudin[tiab] OR Refludan[tiab] OR Dabigatran[nm] OR Dabigatran[tiab] OR Pradaxa[tiab] OR factor xa[mh] OR factor Xa[tiab] OR vena cava filters[mh] OR filters[tiab] OR filter[tiab] OR compression stockings[mh] OR intermittent pneumatic compression devices[mh] OR compression [tiab] OR Venous foot pump[tiab])) AND(prevent*[tiab] OR prophyla*[tiab] OR prevention and control[subheading]) NOT (animals[mh] NOT humans[mh]) NOT (editorial[pt] OR comment[pt]) NOT ((infant[mh] OR infant[tiab] OR child[mh] OR child[tiab] OR children[tiab] OR adolescent[mh] OR adolescent[tiab] OR teen‐age[tiab] OR pediatric[tiab] OR perinatal[tiab]) NOT (adult[tiab] OR adults[tiab] OR adult[mh])) NOT (mechanical valve[tiab] OR heart valve[tiab] OR atrial fibrillation[mh] OR atrial fibrillation[tiab] OR thrombophilia[mh] OR thrombophilia[tiab] OR pregnancy[mh])
- , , . Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the US. Blood. 2005;106:910.
- Institute of Medicine. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009.
- Lovenox (enoxaparin sodium injection for subcutaneous and intravenous use: prescribing information). Bridgewater, NJ: SanofiAventis; 2011. Available at: http://products.sanofi.us/lovenox/lovenox.html. Accessed October 17, 2012.
- Innohep (tinzaparin sodium injection). Ballerup, Denmark: LEO Pharmaceutical Products; 2008. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020484s011lbl.pdf. Accessed October 17, 2012.
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency‐ the IRIS trial. [50th ASH Annual Meeting and Exposition abstract 434]. Blood. 2008;11:112.
- Fragmin (dalteparin sodium injection). New York, NY: Pfizer Inc.; 2007. Available at: http://www.pfizer.com/files/products/uspi_fragmin.pdf. Accessed October 17, 2012.
- Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality; August 2011. AHRQ publication No. 10 (11)‐EHC063‐EF. Available at: http://www.effectivehealthcare.ahrq.gov. Accessed October 17, 2012.
- Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/928/VTE‐Special‐Populations_Protocol_20120112.pdf. Accessed April 17, 2012.
- , , , et al. Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Evidence Report/Technology Assessment (AHRQ). Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/1501/venous‐thromboembolism‐special‐populations‐report‐130529.pdf. 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Efficacy and safety of fixed low‐dose dalteparin in preventing venous thromboembolism among obese or elderly hospitalized patients: a subgroup analysis of the PREVENT trial. Arch Intern Med. 2005;165(3):341–345.
- , , , . Prospective comparison of three enoxaparin dosing regimens to achieve target anti‐factor Xa levels in hospitalized, medically ill patients with extreme obesity. Am J Hematol. 2012;87(7):740–743.
- , , . Effect of prophylactic dalteparin on anti‐factor xa levels in morbidly obese patients after bariatric surgery. Obes Surg. 2010;20(4):487–491.
- , , , , . Concomitant use of medication with antiplatelet effects in patients receiving either rivaroxaban or enoxaparin after total hip or knee arthroplasty. Thromb Res. 2012;130(2):147–151.
- , , , , , . Dabigatran etexilate and concomitant use of non‐steroidal anti‐inflammatory drugs or acetylsalicylic acid in patients undergoing total hip and total knee arthroplasty: No increased risk of bleeding. Thromb Haemost. 2012;108(1):183–190.
- , , , et al. CERTIFY: prophylaxis of venous thromboembolism in patients with severe renal insufficiency. Thromb Haemost. 2011;105(6):981–988.
- , , , et al. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost. 2007;97(4):581–586.
- , , , , , . Thromboprophylaxis in patients older than 75 years or with moderate renal impairment undergoing knee or hip replacement surgery [published correction appears in Int Orthop. 2012;36(5):1113]. Int Orthop. 2012;36(4):741–748.
- , , , . Impact of stage 3B chronic kidney disease on thrombosis and bleeding outcomes after orthopedic surgery in patients treated with desirudin or enoxaparin: insights from a randomized trial. J Thromb Haemost. 2012;10(8):1515–1520.
- , . Initiative to improve thromboprophylactic enoxaparin exposure in hospitalized patients with renal impairment. Am J Health Syst Pharm. 2012;69(5):390–396.
- , , , , ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , . Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74.
- , , . Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the US. Blood. 2005;106:910.
- Institute of Medicine. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009.
- Lovenox (enoxaparin sodium injection for subcutaneous and intravenous use: prescribing information). Bridgewater, NJ: SanofiAventis; 2011. Available at: http://products.sanofi.us/lovenox/lovenox.html. Accessed October 17, 2012.
- Innohep (tinzaparin sodium injection). Ballerup, Denmark: LEO Pharmaceutical Products; 2008. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020484s011lbl.pdf. Accessed October 17, 2012.
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency‐ the IRIS trial. [50th ASH Annual Meeting and Exposition abstract 434]. Blood. 2008;11:112.
- Fragmin (dalteparin sodium injection). New York, NY: Pfizer Inc.; 2007. Available at: http://www.pfizer.com/files/products/uspi_fragmin.pdf. Accessed October 17, 2012.
- Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality; August 2011. AHRQ publication No. 10 (11)‐EHC063‐EF. Available at: http://www.effectivehealthcare.ahrq.gov. Accessed October 17, 2012.
- Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/928/VTE‐Special‐Populations_Protocol_20120112.pdf. Accessed April 17, 2012.
- , , , et al. Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Evidence Report/Technology Assessment (AHRQ). Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/1501/venous‐thromboembolism‐special‐populations‐report‐130529.pdf. 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Efficacy and safety of fixed low‐dose dalteparin in preventing venous thromboembolism among obese or elderly hospitalized patients: a subgroup analysis of the PREVENT trial. Arch Intern Med. 2005;165(3):341–345.
- , , , . Prospective comparison of three enoxaparin dosing regimens to achieve target anti‐factor Xa levels in hospitalized, medically ill patients with extreme obesity. Am J Hematol. 2012;87(7):740–743.
- , , . Effect of prophylactic dalteparin on anti‐factor xa levels in morbidly obese patients after bariatric surgery. Obes Surg. 2010;20(4):487–491.
- , , , , . Concomitant use of medication with antiplatelet effects in patients receiving either rivaroxaban or enoxaparin after total hip or knee arthroplasty. Thromb Res. 2012;130(2):147–151.
- , , , , , . Dabigatran etexilate and concomitant use of non‐steroidal anti‐inflammatory drugs or acetylsalicylic acid in patients undergoing total hip and total knee arthroplasty: No increased risk of bleeding. Thromb Haemost. 2012;108(1):183–190.
- , , , et al. CERTIFY: prophylaxis of venous thromboembolism in patients with severe renal insufficiency. Thromb Haemost. 2011;105(6):981–988.
- , , , et al. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost. 2007;97(4):581–586.
- , , , , , . Thromboprophylaxis in patients older than 75 years or with moderate renal impairment undergoing knee or hip replacement surgery [published correction appears in Int Orthop. 2012;36(5):1113]. Int Orthop. 2012;36(4):741–748.
- , , , . Impact of stage 3B chronic kidney disease on thrombosis and bleeding outcomes after orthopedic surgery in patients treated with desirudin or enoxaparin: insights from a randomized trial. J Thromb Haemost. 2012;10(8):1515–1520.
- , . Initiative to improve thromboprophylactic enoxaparin exposure in hospitalized patients with renal impairment. Am J Health Syst Pharm. 2012;69(5):390–396.
- , , , , ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , . Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74.
Study of Antimicrobial Scrubs
Healthcare workers' (HCWs) attire becomes contaminated with bacterial pathogens during the course of the workday,[1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12] and Munoz‐Price et al.[13] recently demonstrated that finding bacterial pathogens on HCWs' white coats correlated with finding the same pathogens on their hands. Because of concern for an association between attire colonization and nosocomial infection, governmental agencies in England and Scotland banned HCWs from wearing white coats or long‐sleeve garments,[14, 15] despite evidence that such an approach does not reduce contamination.[12]
Newly developed antimicrobial textiles have been incorporated into HCW scrubs,[16, 17, 18, 19, 20] and commercial Web sites and product inserts report that these products can reduce bacterial contamination by 80.9% at 8 hours to greater than 99% under laboratory conditions depending on the product and microbe studied.[16, 17, 19] Because there are limited clinical data pertaining to the effectiveness of antimicrobial scrubs, we performed a prospective study designed to determine whether wearing these products reduced bacterial contamination of HCWs' scrubs or skin at the end of an 8‐hour workday.
METHODS
Design
The study was a prospective, unblinded, randomized, controlled trial that was approved by the Colorado Multiple Institutional Review Board and conducted at Denver Health, a university‐affiliated public safety net hospital. No protocol changes occurred during the study.
Participants
Participants included hospitalist physicians, internal medicine residents, physician assistants, nurse practitioners, and nurses who directly cared for patients hospitalized on internal medicine units between March 12, 2012 and August 28, 2012. Participants known to be pregnant or those who refused to participate in the study were excluded.
Intervention
Standard scrubs issued by the hospital were tested along with 2 different antimicrobial scrubs (scrub A and scrub B). Scrub A was made with a polyester microfiber material embedded with a proprietary antimicrobial chemical. Scrub B was a polyestercotton blend scrub that included 2 proprietary antimicrobial chemicals and silver embedded into the fabric. The standard scrub was made of a polyestercotton blend with no antimicrobial properties. All scrubs consisted of pants and a short‐sleeved shirt, with either a pocket at the left breast or lower front surface, and all were tested new prior to any washing or wear. Preliminary cultures were done on 2 scrubs in each group to assess the extent of preuse contamination. All providers were instructed not to wear white coats at any time during the day that they were wearing the scrubs. Providers were not told the type of scrub they received, but the antimicrobial scrubs had a different appearance and texture than the standard scrubs, so blinding was not possible.
Outcomes
The primary end point was the total bacterial colony count of samples obtained from the breast or lower front pocket, the sleeve cuff of the dominant hand, and the pant leg at the midthigh of the dominant leg on all scrubs after an 8‐hour workday. Secondary outcomes were the bacterial colony counts of cultures obtained from the volar surface of the wrists of the HCWs' dominant arm, and the colony counts of methicillin‐resistant Staphylococcus aureus (MRSA), vancomycin‐resistant enterococci (VRE), and resistant Gram‐negative bacteria on the 3 scrub types, all obtained after the 8‐hour workday.
Cultures were collected using a standardized RODAC imprint method[21] with BBL RODAC plates containing blood agar (Becton Dickinson, Sparks, MD). Cultures were incubated in ambient air at 35 to 37C for 18 to 22 hours. After incubation, visible colonies were counted using a dissecting microscope to a maximum of 200 colonies as recommended by the manufacturer. Colonies morphologically consistent with Staphylococcus species were subsequently tested for coagulase using a BactiStaph rapid latex agglutination test (Remel, Lenexa, KS). If positive, these colonies were subcultured to sheep blood agar (Remel) and BBL MRSA CHROMagar (Becton Dickinson) and incubated for an additional 18 to 24 hours. Characteristic growth on blood agar that also produced mauve‐colored colonies on CHROMagar was taken to indicate MRSA. Colonies morphologically suspicious for being VRE were identified and confirmed as VRE using a positive identification and susceptibility panel (Microscan; Siemens, Deerfield, IL). A negative combination panel (Microscan, Siemens) was also used to identify and confirm resistant Gram‐negative rods.
Each participant completed a survey that included questions that identified their occupation, whether they had had contact with patients who were known to be colonized or infected with MRSA, VRE, or resistant Gram‐negative rods during the testing period, and whether they experienced any adverse events that might relate to wearing the uniform.
Sample Size
We assumed that cultures taken from the sleeve of the control scrubs would have a mean ( standard deviation) colony count of 69 (67) based on data from our previous study.[12] Although the companies making the antimicrobial scrubs indicated that their respective products provided between 80.9% at 8 hours and >99% reduction in bacterial colony counts in laboratory settings, we assumed that a 70% decrease in colony count compared with standard scrubs could be clinically important. After adjusting for multiple comparisons and accounting for using nonparametric analyses with an unknown distribution, we estimated a need to recruit 35 subjects in each of 3 groups.
Randomization
The principal investigator and coinvestigators enrolled and consented participants. After obtaining consent, block randomization, stratified by occupation, occurred 1 day prior to the study using a computer‐generated table of random numbers.
Statistics
Data were collected and managed using REDCap (Research Electronic Data Capture; Vanderbilt UniversityThe Institute for Medicine and Public Health, Nashville, TN) electronic data capture tools hosted at Denver Health. REDCap is a secure Web‐based application designed to support data collection for research studies, providing: (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources.[22]
Colony counts were compared using a Kruskal‐Wallis 1‐way analysis of variance by ranks. Bonferroni's correction for multiple comparisons resulted in a P<0.01 as indicating statistical significance. Proportions were compared using [2] analysis. All data are presented as medians with interquartile range (IQR) or proportions.
RESULTS
We screened 118 HCWs for participation and randomized 109, 37 in the control and antimicrobial scrub group A, and 35 in antimicrobial scrub group B (during the course of the study we neglected to culture the pockets of 2 participants in the standard scrub group and 2 in antimicrobial scrub group A). Because our primary end point was total colony count from cultures taken from 3 sites, data from these 4 subjects could not be used, and all the data from these 4 subjects were excluded from the primary analysis; 4 additional subjects were subsequently recruited allowing us to meet our block enrollment target (Figure 1). The first and last participants were studied on March 12, 2012 and August 28, 2012, respectively. The trial ended once the defined number of participants was enrolled. The occupations of the 105 participants are summarized in Table 1.
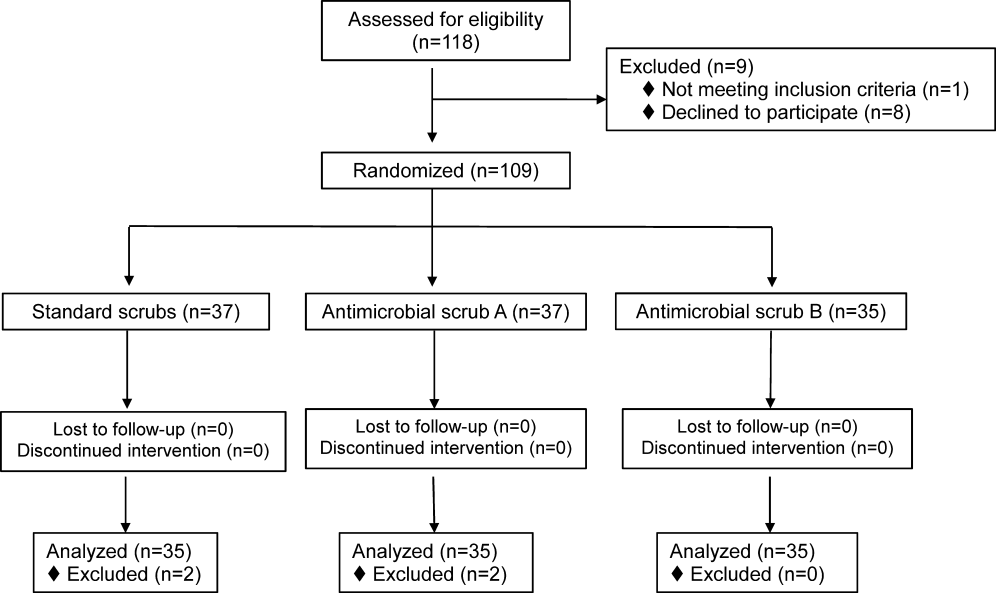
| All Subjects, N=105 | Standard Scrub, n=35 | Antimicrobial Scrub A, n=35 | Antimicrobial Scrub B, n=35 | |
|---|---|---|---|---|
| Healthcare worker type, n (%) | ||||
| Attending physician | 11 (10) | 5 (14) | 3 (9) | 3 (9) |
| Intern/resident | 51 (49) | 17 (49) | 16 (46) | 18 (51) |
| Midlevels | 6 (6) | 2 (6) | 2 (6) | 2 (6) |
| Nurse | 37 (35) | 11 (31) | 14 (40) | 12 (34) |
| Cared for colonized or infected patient with antibiotic resistant organism, n (%) | 55 (52) | 16 (46) | 20 (57) | 19 (54) |
| Number of colonized or infected patients cared for, n (%) | ||||
| 1 | 37 (67) | 10 (63) | 13 (65) | 14 (74) |
| 2 | 11 (20) | 4 (25) | 6 (30) | 1 (5) |
| 3 or more | 6 (11) | 2 (12) | 1 (5) | 3 (16) |
| Unknown | 1 (2) | 0 (0) | 0 (0) | 1 (5) |
Colony counts of all scrubs cultured prior to use never exceeded 10 colonies. The median (IQR) total colony counts from all sites on the scrubs was 99 (66182) for standard scrubs, 137 (84289) for antimicrobial scrub type A, and 138 (62274) for antimicrobial scrub type B (P=0.36). We found no significant differences between the colony counts cultured from any of the individual sites among the 3 groups, regardless of occupation (Table 2). No significant difference was observed with respect to colony counts cultured from the wrist among the 3 study groups (Table 2). Comparisons between groups were planned a priori if a difference across all groups was found. Given the nonsignificant P values across all scrub groups, no further comparisons were made.
| Total (From All Sites on Scrubs) | Sleeve Cuff | Thigh | Wrist | ||
|---|---|---|---|---|---|
| |||||
| All subjects, N=105 | |||||
| Standard scrub | 99 (66182) | 41 (2070) | 20 (944) | 32 (2161) | 16 (540) |
| Antimicrobial scrub A | 137 (84289) | 65 (35117) | 33 (16124) | 41 (1586) | 23 (442) |
| Antimicrobial scrub B | 138 (62274) | 41 (2299) | 21 (941) | 40 (18107) | 15 (654) |
| P value | 0.36 | 0.17 | 0.07 | 0.57 | 0.92 |
| Physicians and midlevels, n=68 | |||||
| Standard scrub | 115.5 (72.5173.5) | 44.5 (2270.5) | 27.5 (10.538.5) | 35 (2362.5) | 24.5 (755) |
| Antimicrobial scrub A | 210 (114289) | 86 (64120) | 39 (18129) | 49 (2486) | 24 (342) |
| Antimicrobial scrub B | 149 (68295) | 52 (26126) | 21 (1069) | 37 (18141) | 19 (872) |
| P value | 0.21 | 0.08 | 0.19 | 0.85 | 0.76 |
| Nurses, n=37 | |||||
| Standard scrub | 89 (31236) | 37 (1348) | 13 (552) | 28 (1342) | 9 (321) |
| Antimicrobial scrub A | 105 (43256) | 45.5 (2258) | 21.5 (1654) | 38.5 (1268) | 17 (643) |
| Antimicrobial scrub B | 91.5 (60174.5) | 27 (1340) | 16 (7.526) | 51 (2186.5) | 10 (3.543.5) |
| P value | 0.86 | 0.39 | 0.19 | 0.49 | 0.41 |
Fifty‐five participants (52%) reported caring for patients who were known to be colonized or infected with an antibiotic‐resistant organism, 16 (46%) randomized to wear standard scrubs, and 20 (57%) and 19 (54%) randomized to wear antimicrobial scrub A or B, respectively (P=0.61). Of these, however, antibiotic‐resistant organisms were only cultured from the scrubs of 2 providers (1 with 1 colony of MRSA from the breast pocket of antimicrobial scrub A, 1 with 1 colony of MRSA cultured from the pocket of antimicrobial scrub B [P=0.55]), and from the wrist of only 1 provider (a multiresistant Gram‐negative rod who wore antimicrobial scrub B).
Adverse Events
Six subjects (5.7%) reported adverse events, all of whom were wearing antimicrobial scrubs (P=0.18). For participants wearing antimicrobial scrub A, 1 (3%) reported itchiness and 2 (6%) reported heaviness or poor breathability. For participants wearing antimicrobial scrub B, 1 (3%) reported redness, 1 (3%) reported itchiness, and 1 (3%) reported heaviness or poor breathability.
DISCUSSION
The important findings of this study are that we found no evidence indicating that either of the 2 antimicrobial scrubs tested reduced bacterial contamination or antibiotic‐resistant contamination on HCWs' scrubs or wrists compared with standard scrubs at the end of an 8‐hour workday, and that despite many HCWs being exposed to patients who were colonized or infected with antibiotic‐resistant bacteria, these organisms were only rarely cultured from their uniforms.
We found that HCWs in all 3 arms of the study had bacterial contamination on their scrubs and skin, consistent with previous studies showing that HCWs' uniforms are frequently contaminated with bacteria, including MRSA, VRE, and other pathogens.[1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12] We previously found that bacterial contamination of HCWs' uniforms occurs within hours of putting on newly laundered uniforms.[12]
Literature on the effectiveness of antimicrobial HCW uniforms when tested in clinical settings is limited. Bearman and colleagues[23] recently published the results of a study of 31 subjects who wore either standard or antimicrobial scrubs, crossing over every 4 weeks for 4 months, with random culturing done weekly at the beginning and end of a work shift. Scrubs were laundered an average of 1.5 times/week, but the timing of the laundering relative to when cultures were obtained was not reported. Very few isolates of MRSA, Gram‐negative rods, or VRE were found (only 3.9%, 0.4%, and 0.05% of the 2000 samples obtained, respectively), and no differences were observed with respect to the number of HCWs who had antibiotic‐resistant organisms cultured when they were wearing standard versus antimicrobial scrubs. Those who had MRSA cultured, however, had lower mean log colony counts when they were wearing the antimicrobial scrubs. The small number of samples with positive isolates, together with differences in the extent of before‐shift contamination among groups complicates interpreting these data. The authors concluded that a prospective trial was needed. We attempted to include the scrub studied by Bearman and colleagues[23] in our study, but the company had insufficient stock available at the time we tried to purchase the product.
Gross and colleagues[24] found no difference in the mean colony counts of cultures taken from silver‐impregnated versus standard scrubs in a pilot crossover study done with 10 HCWs (although there were trends toward higher colony counts when the subjects wore antimicrobial scrubs).
Antibiotic‐resistant bacteria were only cultured from 3 participants (2.9%) in our current study, compared to 16% of those randomized to wearing white coats in our previous study and 20% of those randomized to wearing standard scrubs.[12] This difference may be explained by several recent studies reporting that rates of MRSA infections in hospitals are decreasing.[25, 26] The rate of hospital‐acquired MRSA infection or colonization at our own institution decreased 80% from 2007 to 2012. At the times of our previous and current studies, providers were expected to wear gowns and gloves when caring for patients as per standard contact precautions. Rates of infection and colonization of VRE and resistant Gram‐negative rods have remained low at our hospital, and our data are consistent with the rates reported on HCWs' uniforms in other studies.[2, 5, 10]
Only 6 of our subjects reported adverse reactions, but all were wearing antimicrobial scrubs (P=0.18). Several of the participants described that the fabrics of the 2 antimicrobial scrubs were heavier and less breathable than the standard scrubs. We believe this difference is more likely to explain the adverse reactions reported than is any type of reaction to the specific chemicals in the fabrics.
Our study has several limitations. Because it was conducted on the general internal medicine units of a single university‐affiliated public hospital, the results may not generalize to other types of institutions or other inpatient services.
As we previously described,[12] the RODAC imprint method only samples a small area of HCWs' uniforms and thus does not represent total bacterial contamination.[21] We specifically cultured areas that are known to be highly contaminated (ie, sleeve cuffs and pockets). Although imprint methods have limitations (as do other methods for culturing clothing), they have been commonly utilized in studies assessing bacterial contamination of HCW clothing.[2, 3, 5]
Although some of the bacterial load we cultured could have come from the providers themselves, previous studies have shown that 80% to 90% of the resistant bacteria cultured from HCWs' attire come from other sources.[1, 2]
Because our sample size was calculated on the basis of being able to detect a difference of 70% in total bacterial colony count, our study was not large enough to exclude a lower level of effectiveness. However, we saw no trends suggesting the antimicrobial products might have a lower level of effectiveness.
We did not observe the hand‐washing practices of the participants, and accordingly, cannot confirm that these practices were the same in each of our 3 study groups. Intermittent, surreptitious monitoring of hand‐washing practices on our internal medicine units over the last several years has found compliance with hand hygiene recommendations varying from 70% to 90%.
Although the participants in our study were not explicitly told to which scrub they were randomized, the colors, appearances, and textures of the antimicrobial fabrics were different from the standard scrubs such that blinding was impossible. Participants wearing antimicrobial scrubs could have changed their hand hygiene practices (ie, less careful hand hygiene). Lack of blinding could also have led to over‐reporting of adverse events by the subjects randomized to wear the antimicrobial scrubs.
In an effort to treat all the scrubs in the same fashion, all were tested new, prior to being washed or previously worn. Studying the scrubs prior to washing or wearing could have increased the reports of adverse effects, as the fabrics could have been stiffer and more uncomfortable than they might have been at a later stage in their use.
Our study also has some strengths. Our participants included physicians, residents, nurses, nurse practitioners, and physician assistants. Accordingly, our results should be generalizable to most HCWs. We also confirmed that the scrubs that were tested were nearly sterile prior to use.
In conclusion, we found no evidence suggesting that either of 2 antimicrobial scrubs tested decreased bacterial contamination of HCWs' scrubs or skin after an 8‐hour workday compared to standard scrubs. We also found that, although HCWs are frequently exposed to patients harboring antibiotic‐resistant bacteria, these bacteria were only rarely cultured from HCWs' scrubs or skin.
- , , , . Contamination of nurses' uniforms with Staphylococcus aureus. Lancet. 1969;2:233–235.
- , , . Contamination of protective clothing and nurses' uniforms in an isolation ward. J Hosp Infect. 1983;4:149–157.
- , , . Microbial flora on doctors' white coats. BMJ. 1991;303:1602–1604.
- . Bacterial contamination of nurses' uniforms: a study. Nursing Stand. 1998;13:37–42.
- , , . Bacterial flora on the white coats of medical students. J Hosp Infect. 2000;45:65–68.
- , , . Bacterial contamination of uniforms. J Hosp Infect. 2001;48:238–241.
- , , , et al. Significance of methicillin‐resistant Staphylococcus aureus (MRSA) survey in a university teaching hospital. J Infect Chemother. 2003;9:172–177.
- . Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007;65(suppl 2):50–54.
- , , , et al. Detection of methicillin‐resistant Staphylococcus aureus and vancomycin‐resistant enterococci on the gowns and gloves of healthcare workers. Infect Control Hosp Epidemiol. 2008;29:583–589.
- , , , , , . Bacterial contamination of health care workers' white coats. Am J Infect Control. 2009;37:101–105.
- , , , , , . Nursing and physician attire as possible source of nosocomial infections. Am J Infect Control. 2011;39:555–559.
- , , , , , . Newly cleaned physician uniforms and infrequently washed white coats have similar rates of bacterial contamination after an 8‐hour workday: a randomized controlled trial. J Hosp Med. 2011;6:177–182.
- , , , et al. Associations between bacterial contamination of health care workers' hands and contamination of white coats and scrubs. Am J Infect Control. 2012;40:e245–e248.
- Department of Health. Uniforms and workwear: an evidence base for developing local policy. National Health Service, 17 September 2007. Available at: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/Publicationspolicyandguidance/DH_078433. Accessed January 29, 2010.
- Scottish Government Health Directorates. NHS Scotland dress code. Available at: http://www.sehd.scot.nhs.uk/mels/CEL2008_53.pdf. Accessed February 10, 2010.
- Bio Shield Tech Web site. Bio Gardz–unisex scrub top–antimicrobial treatment. Available at: http://www.bioshieldtech.com/Bio_Gardz_Unisex_Scrub_Top_Antimicrobial_Tre_p/sbt01‐r‐p.htm. Accessed January 9, 2013.
- Doc Froc Web site and informational packet. Available at: http://www.docfroc.com. Accessed July 22, 2011.
- Vestagen Web site and informational packet. Available at: http://www.vestagen.com. Accessed July 22, 2011.
- Under Scrub apparel Web site. Testing. Available at: http://underscrub.com/testing. Accessed March 21, 2013.
- MediThreads Web site. Microban FAQ's. Available at: http://medithreads.com/faq/microban‐faqs. Accessed March 21, 2013.
- , , , , . Comparison of the Rodac imprint method to selective enrichment broth for recovery of vancomycin‐resistant enterococci and drug‐resistant Enterobacteriaceae from environmental surfaces. J Clin Microbiol. 2000;38:4646–4648.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
- , , , et al. A crossover trial of antimicrobial scrubs to reduce methicillin‐resistant Staphylococcus aureus burden on healthcare worker apparel. Infect Control Hosp Epidemiol. 2012;33:268–275.
- , , , , . Pilot study on the microbial contamination of conventional vs. silver‐impregnated uniforms worn by ambulance personnel during one week of emergency medical service. GMS Krankenhhyg Interdiszip. 2010;5.pii: Doc09.
- , , , et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA. 2012;308:50–59.
- , , , et al. Health care‐associated invasive MRSA infections, 2005–2008. JAMA. 2010;304:641–648.
Healthcare workers' (HCWs) attire becomes contaminated with bacterial pathogens during the course of the workday,[1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12] and Munoz‐Price et al.[13] recently demonstrated that finding bacterial pathogens on HCWs' white coats correlated with finding the same pathogens on their hands. Because of concern for an association between attire colonization and nosocomial infection, governmental agencies in England and Scotland banned HCWs from wearing white coats or long‐sleeve garments,[14, 15] despite evidence that such an approach does not reduce contamination.[12]
Newly developed antimicrobial textiles have been incorporated into HCW scrubs,[16, 17, 18, 19, 20] and commercial Web sites and product inserts report that these products can reduce bacterial contamination by 80.9% at 8 hours to greater than 99% under laboratory conditions depending on the product and microbe studied.[16, 17, 19] Because there are limited clinical data pertaining to the effectiveness of antimicrobial scrubs, we performed a prospective study designed to determine whether wearing these products reduced bacterial contamination of HCWs' scrubs or skin at the end of an 8‐hour workday.
METHODS
Design
The study was a prospective, unblinded, randomized, controlled trial that was approved by the Colorado Multiple Institutional Review Board and conducted at Denver Health, a university‐affiliated public safety net hospital. No protocol changes occurred during the study.
Participants
Participants included hospitalist physicians, internal medicine residents, physician assistants, nurse practitioners, and nurses who directly cared for patients hospitalized on internal medicine units between March 12, 2012 and August 28, 2012. Participants known to be pregnant or those who refused to participate in the study were excluded.
Intervention
Standard scrubs issued by the hospital were tested along with 2 different antimicrobial scrubs (scrub A and scrub B). Scrub A was made with a polyester microfiber material embedded with a proprietary antimicrobial chemical. Scrub B was a polyestercotton blend scrub that included 2 proprietary antimicrobial chemicals and silver embedded into the fabric. The standard scrub was made of a polyestercotton blend with no antimicrobial properties. All scrubs consisted of pants and a short‐sleeved shirt, with either a pocket at the left breast or lower front surface, and all were tested new prior to any washing or wear. Preliminary cultures were done on 2 scrubs in each group to assess the extent of preuse contamination. All providers were instructed not to wear white coats at any time during the day that they were wearing the scrubs. Providers were not told the type of scrub they received, but the antimicrobial scrubs had a different appearance and texture than the standard scrubs, so blinding was not possible.
Outcomes
The primary end point was the total bacterial colony count of samples obtained from the breast or lower front pocket, the sleeve cuff of the dominant hand, and the pant leg at the midthigh of the dominant leg on all scrubs after an 8‐hour workday. Secondary outcomes were the bacterial colony counts of cultures obtained from the volar surface of the wrists of the HCWs' dominant arm, and the colony counts of methicillin‐resistant Staphylococcus aureus (MRSA), vancomycin‐resistant enterococci (VRE), and resistant Gram‐negative bacteria on the 3 scrub types, all obtained after the 8‐hour workday.
Cultures were collected using a standardized RODAC imprint method[21] with BBL RODAC plates containing blood agar (Becton Dickinson, Sparks, MD). Cultures were incubated in ambient air at 35 to 37C for 18 to 22 hours. After incubation, visible colonies were counted using a dissecting microscope to a maximum of 200 colonies as recommended by the manufacturer. Colonies morphologically consistent with Staphylococcus species were subsequently tested for coagulase using a BactiStaph rapid latex agglutination test (Remel, Lenexa, KS). If positive, these colonies were subcultured to sheep blood agar (Remel) and BBL MRSA CHROMagar (Becton Dickinson) and incubated for an additional 18 to 24 hours. Characteristic growth on blood agar that also produced mauve‐colored colonies on CHROMagar was taken to indicate MRSA. Colonies morphologically suspicious for being VRE were identified and confirmed as VRE using a positive identification and susceptibility panel (Microscan; Siemens, Deerfield, IL). A negative combination panel (Microscan, Siemens) was also used to identify and confirm resistant Gram‐negative rods.
Each participant completed a survey that included questions that identified their occupation, whether they had had contact with patients who were known to be colonized or infected with MRSA, VRE, or resistant Gram‐negative rods during the testing period, and whether they experienced any adverse events that might relate to wearing the uniform.
Sample Size
We assumed that cultures taken from the sleeve of the control scrubs would have a mean ( standard deviation) colony count of 69 (67) based on data from our previous study.[12] Although the companies making the antimicrobial scrubs indicated that their respective products provided between 80.9% at 8 hours and >99% reduction in bacterial colony counts in laboratory settings, we assumed that a 70% decrease in colony count compared with standard scrubs could be clinically important. After adjusting for multiple comparisons and accounting for using nonparametric analyses with an unknown distribution, we estimated a need to recruit 35 subjects in each of 3 groups.
Randomization
The principal investigator and coinvestigators enrolled and consented participants. After obtaining consent, block randomization, stratified by occupation, occurred 1 day prior to the study using a computer‐generated table of random numbers.
Statistics
Data were collected and managed using REDCap (Research Electronic Data Capture; Vanderbilt UniversityThe Institute for Medicine and Public Health, Nashville, TN) electronic data capture tools hosted at Denver Health. REDCap is a secure Web‐based application designed to support data collection for research studies, providing: (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources.[22]
Colony counts were compared using a Kruskal‐Wallis 1‐way analysis of variance by ranks. Bonferroni's correction for multiple comparisons resulted in a P<0.01 as indicating statistical significance. Proportions were compared using [2] analysis. All data are presented as medians with interquartile range (IQR) or proportions.
RESULTS
We screened 118 HCWs for participation and randomized 109, 37 in the control and antimicrobial scrub group A, and 35 in antimicrobial scrub group B (during the course of the study we neglected to culture the pockets of 2 participants in the standard scrub group and 2 in antimicrobial scrub group A). Because our primary end point was total colony count from cultures taken from 3 sites, data from these 4 subjects could not be used, and all the data from these 4 subjects were excluded from the primary analysis; 4 additional subjects were subsequently recruited allowing us to meet our block enrollment target (Figure 1). The first and last participants were studied on March 12, 2012 and August 28, 2012, respectively. The trial ended once the defined number of participants was enrolled. The occupations of the 105 participants are summarized in Table 1.

| All Subjects, N=105 | Standard Scrub, n=35 | Antimicrobial Scrub A, n=35 | Antimicrobial Scrub B, n=35 | |
|---|---|---|---|---|
| Healthcare worker type, n (%) | ||||
| Attending physician | 11 (10) | 5 (14) | 3 (9) | 3 (9) |
| Intern/resident | 51 (49) | 17 (49) | 16 (46) | 18 (51) |
| Midlevels | 6 (6) | 2 (6) | 2 (6) | 2 (6) |
| Nurse | 37 (35) | 11 (31) | 14 (40) | 12 (34) |
| Cared for colonized or infected patient with antibiotic resistant organism, n (%) | 55 (52) | 16 (46) | 20 (57) | 19 (54) |
| Number of colonized or infected patients cared for, n (%) | ||||
| 1 | 37 (67) | 10 (63) | 13 (65) | 14 (74) |
| 2 | 11 (20) | 4 (25) | 6 (30) | 1 (5) |
| 3 or more | 6 (11) | 2 (12) | 1 (5) | 3 (16) |
| Unknown | 1 (2) | 0 (0) | 0 (0) | 1 (5) |
Colony counts of all scrubs cultured prior to use never exceeded 10 colonies. The median (IQR) total colony counts from all sites on the scrubs was 99 (66182) for standard scrubs, 137 (84289) for antimicrobial scrub type A, and 138 (62274) for antimicrobial scrub type B (P=0.36). We found no significant differences between the colony counts cultured from any of the individual sites among the 3 groups, regardless of occupation (Table 2). No significant difference was observed with respect to colony counts cultured from the wrist among the 3 study groups (Table 2). Comparisons between groups were planned a priori if a difference across all groups was found. Given the nonsignificant P values across all scrub groups, no further comparisons were made.
| Total (From All Sites on Scrubs) | Sleeve Cuff | Thigh | Wrist | ||
|---|---|---|---|---|---|
| |||||
| All subjects, N=105 | |||||
| Standard scrub | 99 (66182) | 41 (2070) | 20 (944) | 32 (2161) | 16 (540) |
| Antimicrobial scrub A | 137 (84289) | 65 (35117) | 33 (16124) | 41 (1586) | 23 (442) |
| Antimicrobial scrub B | 138 (62274) | 41 (2299) | 21 (941) | 40 (18107) | 15 (654) |
| P value | 0.36 | 0.17 | 0.07 | 0.57 | 0.92 |
| Physicians and midlevels, n=68 | |||||
| Standard scrub | 115.5 (72.5173.5) | 44.5 (2270.5) | 27.5 (10.538.5) | 35 (2362.5) | 24.5 (755) |
| Antimicrobial scrub A | 210 (114289) | 86 (64120) | 39 (18129) | 49 (2486) | 24 (342) |
| Antimicrobial scrub B | 149 (68295) | 52 (26126) | 21 (1069) | 37 (18141) | 19 (872) |
| P value | 0.21 | 0.08 | 0.19 | 0.85 | 0.76 |
| Nurses, n=37 | |||||
| Standard scrub | 89 (31236) | 37 (1348) | 13 (552) | 28 (1342) | 9 (321) |
| Antimicrobial scrub A | 105 (43256) | 45.5 (2258) | 21.5 (1654) | 38.5 (1268) | 17 (643) |
| Antimicrobial scrub B | 91.5 (60174.5) | 27 (1340) | 16 (7.526) | 51 (2186.5) | 10 (3.543.5) |
| P value | 0.86 | 0.39 | 0.19 | 0.49 | 0.41 |
Fifty‐five participants (52%) reported caring for patients who were known to be colonized or infected with an antibiotic‐resistant organism, 16 (46%) randomized to wear standard scrubs, and 20 (57%) and 19 (54%) randomized to wear antimicrobial scrub A or B, respectively (P=0.61). Of these, however, antibiotic‐resistant organisms were only cultured from the scrubs of 2 providers (1 with 1 colony of MRSA from the breast pocket of antimicrobial scrub A, 1 with 1 colony of MRSA cultured from the pocket of antimicrobial scrub B [P=0.55]), and from the wrist of only 1 provider (a multiresistant Gram‐negative rod who wore antimicrobial scrub B).
Adverse Events
Six subjects (5.7%) reported adverse events, all of whom were wearing antimicrobial scrubs (P=0.18). For participants wearing antimicrobial scrub A, 1 (3%) reported itchiness and 2 (6%) reported heaviness or poor breathability. For participants wearing antimicrobial scrub B, 1 (3%) reported redness, 1 (3%) reported itchiness, and 1 (3%) reported heaviness or poor breathability.
DISCUSSION
The important findings of this study are that we found no evidence indicating that either of the 2 antimicrobial scrubs tested reduced bacterial contamination or antibiotic‐resistant contamination on HCWs' scrubs or wrists compared with standard scrubs at the end of an 8‐hour workday, and that despite many HCWs being exposed to patients who were colonized or infected with antibiotic‐resistant bacteria, these organisms were only rarely cultured from their uniforms.
We found that HCWs in all 3 arms of the study had bacterial contamination on their scrubs and skin, consistent with previous studies showing that HCWs' uniforms are frequently contaminated with bacteria, including MRSA, VRE, and other pathogens.[1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12] We previously found that bacterial contamination of HCWs' uniforms occurs within hours of putting on newly laundered uniforms.[12]
Literature on the effectiveness of antimicrobial HCW uniforms when tested in clinical settings is limited. Bearman and colleagues[23] recently published the results of a study of 31 subjects who wore either standard or antimicrobial scrubs, crossing over every 4 weeks for 4 months, with random culturing done weekly at the beginning and end of a work shift. Scrubs were laundered an average of 1.5 times/week, but the timing of the laundering relative to when cultures were obtained was not reported. Very few isolates of MRSA, Gram‐negative rods, or VRE were found (only 3.9%, 0.4%, and 0.05% of the 2000 samples obtained, respectively), and no differences were observed with respect to the number of HCWs who had antibiotic‐resistant organisms cultured when they were wearing standard versus antimicrobial scrubs. Those who had MRSA cultured, however, had lower mean log colony counts when they were wearing the antimicrobial scrubs. The small number of samples with positive isolates, together with differences in the extent of before‐shift contamination among groups complicates interpreting these data. The authors concluded that a prospective trial was needed. We attempted to include the scrub studied by Bearman and colleagues[23] in our study, but the company had insufficient stock available at the time we tried to purchase the product.
Gross and colleagues[24] found no difference in the mean colony counts of cultures taken from silver‐impregnated versus standard scrubs in a pilot crossover study done with 10 HCWs (although there were trends toward higher colony counts when the subjects wore antimicrobial scrubs).
Antibiotic‐resistant bacteria were only cultured from 3 participants (2.9%) in our current study, compared to 16% of those randomized to wearing white coats in our previous study and 20% of those randomized to wearing standard scrubs.[12] This difference may be explained by several recent studies reporting that rates of MRSA infections in hospitals are decreasing.[25, 26] The rate of hospital‐acquired MRSA infection or colonization at our own institution decreased 80% from 2007 to 2012. At the times of our previous and current studies, providers were expected to wear gowns and gloves when caring for patients as per standard contact precautions. Rates of infection and colonization of VRE and resistant Gram‐negative rods have remained low at our hospital, and our data are consistent with the rates reported on HCWs' uniforms in other studies.[2, 5, 10]
Only 6 of our subjects reported adverse reactions, but all were wearing antimicrobial scrubs (P=0.18). Several of the participants described that the fabrics of the 2 antimicrobial scrubs were heavier and less breathable than the standard scrubs. We believe this difference is more likely to explain the adverse reactions reported than is any type of reaction to the specific chemicals in the fabrics.
Our study has several limitations. Because it was conducted on the general internal medicine units of a single university‐affiliated public hospital, the results may not generalize to other types of institutions or other inpatient services.
As we previously described,[12] the RODAC imprint method only samples a small area of HCWs' uniforms and thus does not represent total bacterial contamination.[21] We specifically cultured areas that are known to be highly contaminated (ie, sleeve cuffs and pockets). Although imprint methods have limitations (as do other methods for culturing clothing), they have been commonly utilized in studies assessing bacterial contamination of HCW clothing.[2, 3, 5]
Although some of the bacterial load we cultured could have come from the providers themselves, previous studies have shown that 80% to 90% of the resistant bacteria cultured from HCWs' attire come from other sources.[1, 2]
Because our sample size was calculated on the basis of being able to detect a difference of 70% in total bacterial colony count, our study was not large enough to exclude a lower level of effectiveness. However, we saw no trends suggesting the antimicrobial products might have a lower level of effectiveness.
We did not observe the hand‐washing practices of the participants, and accordingly, cannot confirm that these practices were the same in each of our 3 study groups. Intermittent, surreptitious monitoring of hand‐washing practices on our internal medicine units over the last several years has found compliance with hand hygiene recommendations varying from 70% to 90%.
Although the participants in our study were not explicitly told to which scrub they were randomized, the colors, appearances, and textures of the antimicrobial fabrics were different from the standard scrubs such that blinding was impossible. Participants wearing antimicrobial scrubs could have changed their hand hygiene practices (ie, less careful hand hygiene). Lack of blinding could also have led to over‐reporting of adverse events by the subjects randomized to wear the antimicrobial scrubs.
In an effort to treat all the scrubs in the same fashion, all were tested new, prior to being washed or previously worn. Studying the scrubs prior to washing or wearing could have increased the reports of adverse effects, as the fabrics could have been stiffer and more uncomfortable than they might have been at a later stage in their use.
Our study also has some strengths. Our participants included physicians, residents, nurses, nurse practitioners, and physician assistants. Accordingly, our results should be generalizable to most HCWs. We also confirmed that the scrubs that were tested were nearly sterile prior to use.
In conclusion, we found no evidence suggesting that either of 2 antimicrobial scrubs tested decreased bacterial contamination of HCWs' scrubs or skin after an 8‐hour workday compared to standard scrubs. We also found that, although HCWs are frequently exposed to patients harboring antibiotic‐resistant bacteria, these bacteria were only rarely cultured from HCWs' scrubs or skin.
Healthcare workers' (HCWs) attire becomes contaminated with bacterial pathogens during the course of the workday,[1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12] and Munoz‐Price et al.[13] recently demonstrated that finding bacterial pathogens on HCWs' white coats correlated with finding the same pathogens on their hands. Because of concern for an association between attire colonization and nosocomial infection, governmental agencies in England and Scotland banned HCWs from wearing white coats or long‐sleeve garments,[14, 15] despite evidence that such an approach does not reduce contamination.[12]
Newly developed antimicrobial textiles have been incorporated into HCW scrubs,[16, 17, 18, 19, 20] and commercial Web sites and product inserts report that these products can reduce bacterial contamination by 80.9% at 8 hours to greater than 99% under laboratory conditions depending on the product and microbe studied.[16, 17, 19] Because there are limited clinical data pertaining to the effectiveness of antimicrobial scrubs, we performed a prospective study designed to determine whether wearing these products reduced bacterial contamination of HCWs' scrubs or skin at the end of an 8‐hour workday.
METHODS
Design
The study was a prospective, unblinded, randomized, controlled trial that was approved by the Colorado Multiple Institutional Review Board and conducted at Denver Health, a university‐affiliated public safety net hospital. No protocol changes occurred during the study.
Participants
Participants included hospitalist physicians, internal medicine residents, physician assistants, nurse practitioners, and nurses who directly cared for patients hospitalized on internal medicine units between March 12, 2012 and August 28, 2012. Participants known to be pregnant or those who refused to participate in the study were excluded.
Intervention
Standard scrubs issued by the hospital were tested along with 2 different antimicrobial scrubs (scrub A and scrub B). Scrub A was made with a polyester microfiber material embedded with a proprietary antimicrobial chemical. Scrub B was a polyestercotton blend scrub that included 2 proprietary antimicrobial chemicals and silver embedded into the fabric. The standard scrub was made of a polyestercotton blend with no antimicrobial properties. All scrubs consisted of pants and a short‐sleeved shirt, with either a pocket at the left breast or lower front surface, and all were tested new prior to any washing or wear. Preliminary cultures were done on 2 scrubs in each group to assess the extent of preuse contamination. All providers were instructed not to wear white coats at any time during the day that they were wearing the scrubs. Providers were not told the type of scrub they received, but the antimicrobial scrubs had a different appearance and texture than the standard scrubs, so blinding was not possible.
Outcomes
The primary end point was the total bacterial colony count of samples obtained from the breast or lower front pocket, the sleeve cuff of the dominant hand, and the pant leg at the midthigh of the dominant leg on all scrubs after an 8‐hour workday. Secondary outcomes were the bacterial colony counts of cultures obtained from the volar surface of the wrists of the HCWs' dominant arm, and the colony counts of methicillin‐resistant Staphylococcus aureus (MRSA), vancomycin‐resistant enterococci (VRE), and resistant Gram‐negative bacteria on the 3 scrub types, all obtained after the 8‐hour workday.
Cultures were collected using a standardized RODAC imprint method[21] with BBL RODAC plates containing blood agar (Becton Dickinson, Sparks, MD). Cultures were incubated in ambient air at 35 to 37C for 18 to 22 hours. After incubation, visible colonies were counted using a dissecting microscope to a maximum of 200 colonies as recommended by the manufacturer. Colonies morphologically consistent with Staphylococcus species were subsequently tested for coagulase using a BactiStaph rapid latex agglutination test (Remel, Lenexa, KS). If positive, these colonies were subcultured to sheep blood agar (Remel) and BBL MRSA CHROMagar (Becton Dickinson) and incubated for an additional 18 to 24 hours. Characteristic growth on blood agar that also produced mauve‐colored colonies on CHROMagar was taken to indicate MRSA. Colonies morphologically suspicious for being VRE were identified and confirmed as VRE using a positive identification and susceptibility panel (Microscan; Siemens, Deerfield, IL). A negative combination panel (Microscan, Siemens) was also used to identify and confirm resistant Gram‐negative rods.
Each participant completed a survey that included questions that identified their occupation, whether they had had contact with patients who were known to be colonized or infected with MRSA, VRE, or resistant Gram‐negative rods during the testing period, and whether they experienced any adverse events that might relate to wearing the uniform.
Sample Size
We assumed that cultures taken from the sleeve of the control scrubs would have a mean ( standard deviation) colony count of 69 (67) based on data from our previous study.[12] Although the companies making the antimicrobial scrubs indicated that their respective products provided between 80.9% at 8 hours and >99% reduction in bacterial colony counts in laboratory settings, we assumed that a 70% decrease in colony count compared with standard scrubs could be clinically important. After adjusting for multiple comparisons and accounting for using nonparametric analyses with an unknown distribution, we estimated a need to recruit 35 subjects in each of 3 groups.
Randomization
The principal investigator and coinvestigators enrolled and consented participants. After obtaining consent, block randomization, stratified by occupation, occurred 1 day prior to the study using a computer‐generated table of random numbers.
Statistics
Data were collected and managed using REDCap (Research Electronic Data Capture; Vanderbilt UniversityThe Institute for Medicine and Public Health, Nashville, TN) electronic data capture tools hosted at Denver Health. REDCap is a secure Web‐based application designed to support data collection for research studies, providing: (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources.[22]
Colony counts were compared using a Kruskal‐Wallis 1‐way analysis of variance by ranks. Bonferroni's correction for multiple comparisons resulted in a P<0.01 as indicating statistical significance. Proportions were compared using [2] analysis. All data are presented as medians with interquartile range (IQR) or proportions.
RESULTS
We screened 118 HCWs for participation and randomized 109, 37 in the control and antimicrobial scrub group A, and 35 in antimicrobial scrub group B (during the course of the study we neglected to culture the pockets of 2 participants in the standard scrub group and 2 in antimicrobial scrub group A). Because our primary end point was total colony count from cultures taken from 3 sites, data from these 4 subjects could not be used, and all the data from these 4 subjects were excluded from the primary analysis; 4 additional subjects were subsequently recruited allowing us to meet our block enrollment target (Figure 1). The first and last participants were studied on March 12, 2012 and August 28, 2012, respectively. The trial ended once the defined number of participants was enrolled. The occupations of the 105 participants are summarized in Table 1.

| All Subjects, N=105 | Standard Scrub, n=35 | Antimicrobial Scrub A, n=35 | Antimicrobial Scrub B, n=35 | |
|---|---|---|---|---|
| Healthcare worker type, n (%) | ||||
| Attending physician | 11 (10) | 5 (14) | 3 (9) | 3 (9) |
| Intern/resident | 51 (49) | 17 (49) | 16 (46) | 18 (51) |
| Midlevels | 6 (6) | 2 (6) | 2 (6) | 2 (6) |
| Nurse | 37 (35) | 11 (31) | 14 (40) | 12 (34) |
| Cared for colonized or infected patient with antibiotic resistant organism, n (%) | 55 (52) | 16 (46) | 20 (57) | 19 (54) |
| Number of colonized or infected patients cared for, n (%) | ||||
| 1 | 37 (67) | 10 (63) | 13 (65) | 14 (74) |
| 2 | 11 (20) | 4 (25) | 6 (30) | 1 (5) |
| 3 or more | 6 (11) | 2 (12) | 1 (5) | 3 (16) |
| Unknown | 1 (2) | 0 (0) | 0 (0) | 1 (5) |
Colony counts of all scrubs cultured prior to use never exceeded 10 colonies. The median (IQR) total colony counts from all sites on the scrubs was 99 (66182) for standard scrubs, 137 (84289) for antimicrobial scrub type A, and 138 (62274) for antimicrobial scrub type B (P=0.36). We found no significant differences between the colony counts cultured from any of the individual sites among the 3 groups, regardless of occupation (Table 2). No significant difference was observed with respect to colony counts cultured from the wrist among the 3 study groups (Table 2). Comparisons between groups were planned a priori if a difference across all groups was found. Given the nonsignificant P values across all scrub groups, no further comparisons were made.
| Total (From All Sites on Scrubs) | Sleeve Cuff | Thigh | Wrist | ||
|---|---|---|---|---|---|
| |||||
| All subjects, N=105 | |||||
| Standard scrub | 99 (66182) | 41 (2070) | 20 (944) | 32 (2161) | 16 (540) |
| Antimicrobial scrub A | 137 (84289) | 65 (35117) | 33 (16124) | 41 (1586) | 23 (442) |
| Antimicrobial scrub B | 138 (62274) | 41 (2299) | 21 (941) | 40 (18107) | 15 (654) |
| P value | 0.36 | 0.17 | 0.07 | 0.57 | 0.92 |
| Physicians and midlevels, n=68 | |||||
| Standard scrub | 115.5 (72.5173.5) | 44.5 (2270.5) | 27.5 (10.538.5) | 35 (2362.5) | 24.5 (755) |
| Antimicrobial scrub A | 210 (114289) | 86 (64120) | 39 (18129) | 49 (2486) | 24 (342) |
| Antimicrobial scrub B | 149 (68295) | 52 (26126) | 21 (1069) | 37 (18141) | 19 (872) |
| P value | 0.21 | 0.08 | 0.19 | 0.85 | 0.76 |
| Nurses, n=37 | |||||
| Standard scrub | 89 (31236) | 37 (1348) | 13 (552) | 28 (1342) | 9 (321) |
| Antimicrobial scrub A | 105 (43256) | 45.5 (2258) | 21.5 (1654) | 38.5 (1268) | 17 (643) |
| Antimicrobial scrub B | 91.5 (60174.5) | 27 (1340) | 16 (7.526) | 51 (2186.5) | 10 (3.543.5) |
| P value | 0.86 | 0.39 | 0.19 | 0.49 | 0.41 |
Fifty‐five participants (52%) reported caring for patients who were known to be colonized or infected with an antibiotic‐resistant organism, 16 (46%) randomized to wear standard scrubs, and 20 (57%) and 19 (54%) randomized to wear antimicrobial scrub A or B, respectively (P=0.61). Of these, however, antibiotic‐resistant organisms were only cultured from the scrubs of 2 providers (1 with 1 colony of MRSA from the breast pocket of antimicrobial scrub A, 1 with 1 colony of MRSA cultured from the pocket of antimicrobial scrub B [P=0.55]), and from the wrist of only 1 provider (a multiresistant Gram‐negative rod who wore antimicrobial scrub B).
Adverse Events
Six subjects (5.7%) reported adverse events, all of whom were wearing antimicrobial scrubs (P=0.18). For participants wearing antimicrobial scrub A, 1 (3%) reported itchiness and 2 (6%) reported heaviness or poor breathability. For participants wearing antimicrobial scrub B, 1 (3%) reported redness, 1 (3%) reported itchiness, and 1 (3%) reported heaviness or poor breathability.
DISCUSSION
The important findings of this study are that we found no evidence indicating that either of the 2 antimicrobial scrubs tested reduced bacterial contamination or antibiotic‐resistant contamination on HCWs' scrubs or wrists compared with standard scrubs at the end of an 8‐hour workday, and that despite many HCWs being exposed to patients who were colonized or infected with antibiotic‐resistant bacteria, these organisms were only rarely cultured from their uniforms.
We found that HCWs in all 3 arms of the study had bacterial contamination on their scrubs and skin, consistent with previous studies showing that HCWs' uniforms are frequently contaminated with bacteria, including MRSA, VRE, and other pathogens.[1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12] We previously found that bacterial contamination of HCWs' uniforms occurs within hours of putting on newly laundered uniforms.[12]
Literature on the effectiveness of antimicrobial HCW uniforms when tested in clinical settings is limited. Bearman and colleagues[23] recently published the results of a study of 31 subjects who wore either standard or antimicrobial scrubs, crossing over every 4 weeks for 4 months, with random culturing done weekly at the beginning and end of a work shift. Scrubs were laundered an average of 1.5 times/week, but the timing of the laundering relative to when cultures were obtained was not reported. Very few isolates of MRSA, Gram‐negative rods, or VRE were found (only 3.9%, 0.4%, and 0.05% of the 2000 samples obtained, respectively), and no differences were observed with respect to the number of HCWs who had antibiotic‐resistant organisms cultured when they were wearing standard versus antimicrobial scrubs. Those who had MRSA cultured, however, had lower mean log colony counts when they were wearing the antimicrobial scrubs. The small number of samples with positive isolates, together with differences in the extent of before‐shift contamination among groups complicates interpreting these data. The authors concluded that a prospective trial was needed. We attempted to include the scrub studied by Bearman and colleagues[23] in our study, but the company had insufficient stock available at the time we tried to purchase the product.
Gross and colleagues[24] found no difference in the mean colony counts of cultures taken from silver‐impregnated versus standard scrubs in a pilot crossover study done with 10 HCWs (although there were trends toward higher colony counts when the subjects wore antimicrobial scrubs).
Antibiotic‐resistant bacteria were only cultured from 3 participants (2.9%) in our current study, compared to 16% of those randomized to wearing white coats in our previous study and 20% of those randomized to wearing standard scrubs.[12] This difference may be explained by several recent studies reporting that rates of MRSA infections in hospitals are decreasing.[25, 26] The rate of hospital‐acquired MRSA infection or colonization at our own institution decreased 80% from 2007 to 2012. At the times of our previous and current studies, providers were expected to wear gowns and gloves when caring for patients as per standard contact precautions. Rates of infection and colonization of VRE and resistant Gram‐negative rods have remained low at our hospital, and our data are consistent with the rates reported on HCWs' uniforms in other studies.[2, 5, 10]
Only 6 of our subjects reported adverse reactions, but all were wearing antimicrobial scrubs (P=0.18). Several of the participants described that the fabrics of the 2 antimicrobial scrubs were heavier and less breathable than the standard scrubs. We believe this difference is more likely to explain the adverse reactions reported than is any type of reaction to the specific chemicals in the fabrics.
Our study has several limitations. Because it was conducted on the general internal medicine units of a single university‐affiliated public hospital, the results may not generalize to other types of institutions or other inpatient services.
As we previously described,[12] the RODAC imprint method only samples a small area of HCWs' uniforms and thus does not represent total bacterial contamination.[21] We specifically cultured areas that are known to be highly contaminated (ie, sleeve cuffs and pockets). Although imprint methods have limitations (as do other methods for culturing clothing), they have been commonly utilized in studies assessing bacterial contamination of HCW clothing.[2, 3, 5]
Although some of the bacterial load we cultured could have come from the providers themselves, previous studies have shown that 80% to 90% of the resistant bacteria cultured from HCWs' attire come from other sources.[1, 2]
Because our sample size was calculated on the basis of being able to detect a difference of 70% in total bacterial colony count, our study was not large enough to exclude a lower level of effectiveness. However, we saw no trends suggesting the antimicrobial products might have a lower level of effectiveness.
We did not observe the hand‐washing practices of the participants, and accordingly, cannot confirm that these practices were the same in each of our 3 study groups. Intermittent, surreptitious monitoring of hand‐washing practices on our internal medicine units over the last several years has found compliance with hand hygiene recommendations varying from 70% to 90%.
Although the participants in our study were not explicitly told to which scrub they were randomized, the colors, appearances, and textures of the antimicrobial fabrics were different from the standard scrubs such that blinding was impossible. Participants wearing antimicrobial scrubs could have changed their hand hygiene practices (ie, less careful hand hygiene). Lack of blinding could also have led to over‐reporting of adverse events by the subjects randomized to wear the antimicrobial scrubs.
In an effort to treat all the scrubs in the same fashion, all were tested new, prior to being washed or previously worn. Studying the scrubs prior to washing or wearing could have increased the reports of adverse effects, as the fabrics could have been stiffer and more uncomfortable than they might have been at a later stage in their use.
Our study also has some strengths. Our participants included physicians, residents, nurses, nurse practitioners, and physician assistants. Accordingly, our results should be generalizable to most HCWs. We also confirmed that the scrubs that were tested were nearly sterile prior to use.
In conclusion, we found no evidence suggesting that either of 2 antimicrobial scrubs tested decreased bacterial contamination of HCWs' scrubs or skin after an 8‐hour workday compared to standard scrubs. We also found that, although HCWs are frequently exposed to patients harboring antibiotic‐resistant bacteria, these bacteria were only rarely cultured from HCWs' scrubs or skin.
- , , , . Contamination of nurses' uniforms with Staphylococcus aureus. Lancet. 1969;2:233–235.
- , , . Contamination of protective clothing and nurses' uniforms in an isolation ward. J Hosp Infect. 1983;4:149–157.
- , , . Microbial flora on doctors' white coats. BMJ. 1991;303:1602–1604.
- . Bacterial contamination of nurses' uniforms: a study. Nursing Stand. 1998;13:37–42.
- , , . Bacterial flora on the white coats of medical students. J Hosp Infect. 2000;45:65–68.
- , , . Bacterial contamination of uniforms. J Hosp Infect. 2001;48:238–241.
- , , , et al. Significance of methicillin‐resistant Staphylococcus aureus (MRSA) survey in a university teaching hospital. J Infect Chemother. 2003;9:172–177.
- . Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007;65(suppl 2):50–54.
- , , , et al. Detection of methicillin‐resistant Staphylococcus aureus and vancomycin‐resistant enterococci on the gowns and gloves of healthcare workers. Infect Control Hosp Epidemiol. 2008;29:583–589.
- , , , , , . Bacterial contamination of health care workers' white coats. Am J Infect Control. 2009;37:101–105.
- , , , , , . Nursing and physician attire as possible source of nosocomial infections. Am J Infect Control. 2011;39:555–559.
- , , , , , . Newly cleaned physician uniforms and infrequently washed white coats have similar rates of bacterial contamination after an 8‐hour workday: a randomized controlled trial. J Hosp Med. 2011;6:177–182.
- , , , et al. Associations between bacterial contamination of health care workers' hands and contamination of white coats and scrubs. Am J Infect Control. 2012;40:e245–e248.
- Department of Health. Uniforms and workwear: an evidence base for developing local policy. National Health Service, 17 September 2007. Available at: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/Publicationspolicyandguidance/DH_078433. Accessed January 29, 2010.
- Scottish Government Health Directorates. NHS Scotland dress code. Available at: http://www.sehd.scot.nhs.uk/mels/CEL2008_53.pdf. Accessed February 10, 2010.
- Bio Shield Tech Web site. Bio Gardz–unisex scrub top–antimicrobial treatment. Available at: http://www.bioshieldtech.com/Bio_Gardz_Unisex_Scrub_Top_Antimicrobial_Tre_p/sbt01‐r‐p.htm. Accessed January 9, 2013.
- Doc Froc Web site and informational packet. Available at: http://www.docfroc.com. Accessed July 22, 2011.
- Vestagen Web site and informational packet. Available at: http://www.vestagen.com. Accessed July 22, 2011.
- Under Scrub apparel Web site. Testing. Available at: http://underscrub.com/testing. Accessed March 21, 2013.
- MediThreads Web site. Microban FAQ's. Available at: http://medithreads.com/faq/microban‐faqs. Accessed March 21, 2013.
- , , , , . Comparison of the Rodac imprint method to selective enrichment broth for recovery of vancomycin‐resistant enterococci and drug‐resistant Enterobacteriaceae from environmental surfaces. J Clin Microbiol. 2000;38:4646–4648.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
- , , , et al. A crossover trial of antimicrobial scrubs to reduce methicillin‐resistant Staphylococcus aureus burden on healthcare worker apparel. Infect Control Hosp Epidemiol. 2012;33:268–275.
- , , , , . Pilot study on the microbial contamination of conventional vs. silver‐impregnated uniforms worn by ambulance personnel during one week of emergency medical service. GMS Krankenhhyg Interdiszip. 2010;5.pii: Doc09.
- , , , et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA. 2012;308:50–59.
- , , , et al. Health care‐associated invasive MRSA infections, 2005–2008. JAMA. 2010;304:641–648.
- , , , . Contamination of nurses' uniforms with Staphylococcus aureus. Lancet. 1969;2:233–235.
- , , . Contamination of protective clothing and nurses' uniforms in an isolation ward. J Hosp Infect. 1983;4:149–157.
- , , . Microbial flora on doctors' white coats. BMJ. 1991;303:1602–1604.
- . Bacterial contamination of nurses' uniforms: a study. Nursing Stand. 1998;13:37–42.
- , , . Bacterial flora on the white coats of medical students. J Hosp Infect. 2000;45:65–68.
- , , . Bacterial contamination of uniforms. J Hosp Infect. 2001;48:238–241.
- , , , et al. Significance of methicillin‐resistant Staphylococcus aureus (MRSA) survey in a university teaching hospital. J Infect Chemother. 2003;9:172–177.
- . Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007;65(suppl 2):50–54.
- , , , et al. Detection of methicillin‐resistant Staphylococcus aureus and vancomycin‐resistant enterococci on the gowns and gloves of healthcare workers. Infect Control Hosp Epidemiol. 2008;29:583–589.
- , , , , , . Bacterial contamination of health care workers' white coats. Am J Infect Control. 2009;37:101–105.
- , , , , , . Nursing and physician attire as possible source of nosocomial infections. Am J Infect Control. 2011;39:555–559.
- , , , , , . Newly cleaned physician uniforms and infrequently washed white coats have similar rates of bacterial contamination after an 8‐hour workday: a randomized controlled trial. J Hosp Med. 2011;6:177–182.
- , , , et al. Associations between bacterial contamination of health care workers' hands and contamination of white coats and scrubs. Am J Infect Control. 2012;40:e245–e248.
- Department of Health. Uniforms and workwear: an evidence base for developing local policy. National Health Service, 17 September 2007. Available at: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/Publicationspolicyandguidance/DH_078433. Accessed January 29, 2010.
- Scottish Government Health Directorates. NHS Scotland dress code. Available at: http://www.sehd.scot.nhs.uk/mels/CEL2008_53.pdf. Accessed February 10, 2010.
- Bio Shield Tech Web site. Bio Gardz–unisex scrub top–antimicrobial treatment. Available at: http://www.bioshieldtech.com/Bio_Gardz_Unisex_Scrub_Top_Antimicrobial_Tre_p/sbt01‐r‐p.htm. Accessed January 9, 2013.
- Doc Froc Web site and informational packet. Available at: http://www.docfroc.com. Accessed July 22, 2011.
- Vestagen Web site and informational packet. Available at: http://www.vestagen.com. Accessed July 22, 2011.
- Under Scrub apparel Web site. Testing. Available at: http://underscrub.com/testing. Accessed March 21, 2013.
- MediThreads Web site. Microban FAQ's. Available at: http://medithreads.com/faq/microban‐faqs. Accessed March 21, 2013.
- , , , , . Comparison of the Rodac imprint method to selective enrichment broth for recovery of vancomycin‐resistant enterococci and drug‐resistant Enterobacteriaceae from environmental surfaces. J Clin Microbiol. 2000;38:4646–4648.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
- , , , et al. A crossover trial of antimicrobial scrubs to reduce methicillin‐resistant Staphylococcus aureus burden on healthcare worker apparel. Infect Control Hosp Epidemiol. 2012;33:268–275.
- , , , , . Pilot study on the microbial contamination of conventional vs. silver‐impregnated uniforms worn by ambulance personnel during one week of emergency medical service. GMS Krankenhhyg Interdiszip. 2010;5.pii: Doc09.
- , , , et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA. 2012;308:50–59.
- , , , et al. Health care‐associated invasive MRSA infections, 2005–2008. JAMA. 2010;304:641–648.
© 2013 Society of Hospital Medicine
AUDIO EXCLUSIVE: Research, Innovation, and Clinical Vignette Competition Draws Rave Reviews
AUDIO EXCLUSIVE: Hospitalists Flock to HM13's Hands-On Medical Procedures Training
Click here to listen to Dr. Rosen.
Click here to listen to Dr. Rosen.
Click here to listen to Dr. Rosen.
New HIPAA requirements
I’m hearing a lot of concern about the impending changes in the Health Insurance Portability and Accountability Act (HIPAA) – which is understandable, since the Department of Health and Human Services has presented them as "the most sweeping ... since [the Act] was first implemented."
But after a careful perusal of the new rules – all 150 three-column pages of them – I can say with a modest degree of confidence that for most physicians, compliance will not be as challenging as some (such as those trying to sell you compliance-related materials) have warned.
However, you can’t simply ignore the new regulations; definitions will be more complex, security breaches more liberally defined, and potential penalties will be stiffer. Herewith the salient points:
• Business associates. The criteria for identifying "business associates" (BAs) remain the same: nonemployees, performing "functions or activities" on behalf of the "covered entity" (your practice), that involve "creating, receiving, maintaining, or transmitting" personal health information (PHI).
Typical BAs include answering and billing services, independent transcriptionists, hardware and software companies, and any other vendors involved in creating or maintaining your medical records. Practice management consultants, attorneys, companies that store or microfilm medical records, and record-shredding services are BAs if they must have direct access to PHI to do their jobs.
Mail carriers, package-delivery people, cleaning services, copier repairmen, bank employees, and the like are not considered BAs, even though they might conceivably come in contact with PHI on occasion. You are required to use "reasonable diligence" in limiting the PHI that these folks may encounter, but you do not need to enter into written BA agreements with them.
Independent contractors who work within your practice – aestheticians and physical therapists, for example – are not considered BAs either, and do not need to sign a BA agreement; just train them, as you do your employees. (I’ll have more on HIPAA and OSHA training in a future column.)
What is new is the additional onus placed on physicians for confidentiality breaches committed by their BAs. It’s not enough to simply have a BA contract. You are expected to use "reasonable diligence" in monitoring the work of your BAs. BAs and their subcontractors are directly responsible for their own actions, but the primary responsibility is ours. Let’s say that a contractor you hire to shred old medical records throws them into a trash bin instead; under the new rules, you must assume the worst-case scenario. Previously, you would only have to notify affected patients (and the government) if there was a "significant risk of financial or reputational harm," but now, any incident involving patient records is assumed to be a breach, and must be reported. Failure to do so could subject your practice, as well as the contractor, to significant fines – as high as $1 million in egregious cases.
• New patient rights. Patients will now be able to restrict the PHI shared with third-party insurers and health plans if they pay for the services themselves. They also have the right to request copies of their electronic health records, and you can bill the actual costs of responding to such a request. If you have EHR, now might be a good time to work out a system for doing this, because the response time has been decreased from 90 to 30 days – even less in some states.
• Marketing limitations. The new rule prohibits third-party-funded marketing to patients for products and services without their prior written authorization. You do not need prior authorization to market your own products and services, even when the communication is funded by a third party, but if there is any such funding, you will need to disclose it.
• Notice of privacy practices (NPP). You will need to revise your NPP to explain your relationships with BAs, and their status under the new rules. You will need to explain the breach notification process, too, as well as the new patient rights mentioned above. You must post your revised NPP in your office, and make copies available there, but you need not mail a copy to every patient.
• Get on it. The rules specify Sept. 23 as the effective date for the new regulations, although you have a year beyond that to revise your existing BA agreements. Extensions are possible, even likely.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J.
I’m hearing a lot of concern about the impending changes in the Health Insurance Portability and Accountability Act (HIPAA) – which is understandable, since the Department of Health and Human Services has presented them as "the most sweeping ... since [the Act] was first implemented."
But after a careful perusal of the new rules – all 150 three-column pages of them – I can say with a modest degree of confidence that for most physicians, compliance will not be as challenging as some (such as those trying to sell you compliance-related materials) have warned.
However, you can’t simply ignore the new regulations; definitions will be more complex, security breaches more liberally defined, and potential penalties will be stiffer. Herewith the salient points:
• Business associates. The criteria for identifying "business associates" (BAs) remain the same: nonemployees, performing "functions or activities" on behalf of the "covered entity" (your practice), that involve "creating, receiving, maintaining, or transmitting" personal health information (PHI).
Typical BAs include answering and billing services, independent transcriptionists, hardware and software companies, and any other vendors involved in creating or maintaining your medical records. Practice management consultants, attorneys, companies that store or microfilm medical records, and record-shredding services are BAs if they must have direct access to PHI to do their jobs.
Mail carriers, package-delivery people, cleaning services, copier repairmen, bank employees, and the like are not considered BAs, even though they might conceivably come in contact with PHI on occasion. You are required to use "reasonable diligence" in limiting the PHI that these folks may encounter, but you do not need to enter into written BA agreements with them.
Independent contractors who work within your practice – aestheticians and physical therapists, for example – are not considered BAs either, and do not need to sign a BA agreement; just train them, as you do your employees. (I’ll have more on HIPAA and OSHA training in a future column.)
What is new is the additional onus placed on physicians for confidentiality breaches committed by their BAs. It’s not enough to simply have a BA contract. You are expected to use "reasonable diligence" in monitoring the work of your BAs. BAs and their subcontractors are directly responsible for their own actions, but the primary responsibility is ours. Let’s say that a contractor you hire to shred old medical records throws them into a trash bin instead; under the new rules, you must assume the worst-case scenario. Previously, you would only have to notify affected patients (and the government) if there was a "significant risk of financial or reputational harm," but now, any incident involving patient records is assumed to be a breach, and must be reported. Failure to do so could subject your practice, as well as the contractor, to significant fines – as high as $1 million in egregious cases.
• New patient rights. Patients will now be able to restrict the PHI shared with third-party insurers and health plans if they pay for the services themselves. They also have the right to request copies of their electronic health records, and you can bill the actual costs of responding to such a request. If you have EHR, now might be a good time to work out a system for doing this, because the response time has been decreased from 90 to 30 days – even less in some states.
• Marketing limitations. The new rule prohibits third-party-funded marketing to patients for products and services without their prior written authorization. You do not need prior authorization to market your own products and services, even when the communication is funded by a third party, but if there is any such funding, you will need to disclose it.
• Notice of privacy practices (NPP). You will need to revise your NPP to explain your relationships with BAs, and their status under the new rules. You will need to explain the breach notification process, too, as well as the new patient rights mentioned above. You must post your revised NPP in your office, and make copies available there, but you need not mail a copy to every patient.
• Get on it. The rules specify Sept. 23 as the effective date for the new regulations, although you have a year beyond that to revise your existing BA agreements. Extensions are possible, even likely.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J.
I’m hearing a lot of concern about the impending changes in the Health Insurance Portability and Accountability Act (HIPAA) – which is understandable, since the Department of Health and Human Services has presented them as "the most sweeping ... since [the Act] was first implemented."
But after a careful perusal of the new rules – all 150 three-column pages of them – I can say with a modest degree of confidence that for most physicians, compliance will not be as challenging as some (such as those trying to sell you compliance-related materials) have warned.
However, you can’t simply ignore the new regulations; definitions will be more complex, security breaches more liberally defined, and potential penalties will be stiffer. Herewith the salient points:
• Business associates. The criteria for identifying "business associates" (BAs) remain the same: nonemployees, performing "functions or activities" on behalf of the "covered entity" (your practice), that involve "creating, receiving, maintaining, or transmitting" personal health information (PHI).
Typical BAs include answering and billing services, independent transcriptionists, hardware and software companies, and any other vendors involved in creating or maintaining your medical records. Practice management consultants, attorneys, companies that store or microfilm medical records, and record-shredding services are BAs if they must have direct access to PHI to do their jobs.
Mail carriers, package-delivery people, cleaning services, copier repairmen, bank employees, and the like are not considered BAs, even though they might conceivably come in contact with PHI on occasion. You are required to use "reasonable diligence" in limiting the PHI that these folks may encounter, but you do not need to enter into written BA agreements with them.
Independent contractors who work within your practice – aestheticians and physical therapists, for example – are not considered BAs either, and do not need to sign a BA agreement; just train them, as you do your employees. (I’ll have more on HIPAA and OSHA training in a future column.)
What is new is the additional onus placed on physicians for confidentiality breaches committed by their BAs. It’s not enough to simply have a BA contract. You are expected to use "reasonable diligence" in monitoring the work of your BAs. BAs and their subcontractors are directly responsible for their own actions, but the primary responsibility is ours. Let’s say that a contractor you hire to shred old medical records throws them into a trash bin instead; under the new rules, you must assume the worst-case scenario. Previously, you would only have to notify affected patients (and the government) if there was a "significant risk of financial or reputational harm," but now, any incident involving patient records is assumed to be a breach, and must be reported. Failure to do so could subject your practice, as well as the contractor, to significant fines – as high as $1 million in egregious cases.
• New patient rights. Patients will now be able to restrict the PHI shared with third-party insurers and health plans if they pay for the services themselves. They also have the right to request copies of their electronic health records, and you can bill the actual costs of responding to such a request. If you have EHR, now might be a good time to work out a system for doing this, because the response time has been decreased from 90 to 30 days – even less in some states.
• Marketing limitations. The new rule prohibits third-party-funded marketing to patients for products and services without their prior written authorization. You do not need prior authorization to market your own products and services, even when the communication is funded by a third party, but if there is any such funding, you will need to disclose it.
• Notice of privacy practices (NPP). You will need to revise your NPP to explain your relationships with BAs, and their status under the new rules. You will need to explain the breach notification process, too, as well as the new patient rights mentioned above. You must post your revised NPP in your office, and make copies available there, but you need not mail a copy to every patient.
• Get on it. The rules specify Sept. 23 as the effective date for the new regulations, although you have a year beyond that to revise your existing BA agreements. Extensions are possible, even likely.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J.
Concussion recovery takes longer if children have had one before
Children and teenagers take longer to recover from a concussion if they’ve had one before, especially within the past year, Boston Children’s Hospital emergency department physicians found in a study of 280 of their concussed patients published June 10 in Pediatrics.
The median duration of symptoms, assessed by the serial Rivermead Post-Concussion Symptoms Questionnaire (RPSQ) over a period of 3 months, climbed from 12 days in patients who hadn’t been concussed before to 24 days in those who had. The median symptom duration was 28 days in patients with multiple previous concussions, and 35 days in those who’d been concussed within the previous year, "nearly three times the median duration [for] those who had no previous concussions," according to Dr. Matthew A. Eisenberg and his associates at the hospital (Pediatrics 2013 [doi:10.1542/peds.2013-0432]).
"Similarly, patients with two or more previous concussions had more than double the median symptom duration [of] patients with zero or one previous concussion," they found.
On multivariate analysis, previous concussion, maintaining consciousness, being 13 years or older, and an initial RPSQ of 18 or higher all predicted prolonged recovery. Among all comers, 77% had symptoms at 1 week, 32% at 4 weeks, and 15% at 3 months. The mean age in the trial was 14.3 years (range, 11-22 years).
The findings were statistically significant and have "direct implications on the management of athletes and other at-risk individuals who sustain concussions, supporting the concept that sufficient time to recover from a concussion may improve long-term outcomes," the investigators said.
"However, we did not find an association between physician-advised cognitive or physical rest and duration of symptoms, which may reflect the limitations of our observational study," they added. "A randomized [controlled] trial will likely be necessary to address the utility of this intervention."
Sixty-six percent of the subjects were enrolled the day they were injured; 24.7% were enrolled 1 day later, 7.2% 2 days later, and 1.7% 3 days later. The majority (63.8%) had been injured playing hockey, soccer, football, basketball or some other sport.
The investigators defined concussion broadly to include either altered mental status following blunt head trauma or, within 4 hours of it, any of the following symptoms that were not present before the injury: headache, nausea, vomiting, dizziness/balance problems, fatigue, drowsiness, blurred vision, memory difficulty, or trouble concentrating.
The most common symptoms in the study were headache (85.1%), fatigue (64.7%), and dizziness (63.0%); 4.3% of subjects had altered gait or balance, and 2.4% had altered mental status. There were no abnormalities in the 20.8% of kids who got neuroimaging.
On discharge, 65.9% were prescribed a period of cognitive rest and 92.4% were told to take time off from sports; 63.8% were also told to follow up with their primary care doctor, 45.5% with a sports concussion clinic, and 6.2% with a specialist.
In contrast to prior studies, loss of consciousness seemed to protect against a prolonged recovery (HR, 0.648; P = .02). Maybe the 22% of kids who got knocked out were more likely to follow their doctors’ advice to rest, "thus speeding recovery from their injury. We cannot, however, eliminate the possibility that there is a biological basis to this finding," the team noted.
Subjects who were 13 years or older might have taken longer to recover (HR, 1.404; P = .04) because games "between older children involve more contact and higher-force impacts," although neurobiologic differences between older and younger kids might have played a role, as well, the investigators said.
"Female patients" – about 43% of the study total – "had more severe symptoms at presentation in our study (mean initial RPSQ of 21.3 vs. 17.0 in male patients, P = .02). ... Whether this finding is indicative of the fact that female patients have more severe symptoms from concussion in general, as suggested in several previous studies, or is due to referral bias in which female individuals preferentially present to the ED when symptoms are more severe ... cannot be ascertained from our data," they noted.
Female gender fell out on multivariate analysis as a predictor of prolonged recovery (HR, 1.294; P= 0.11).
The investigators said they had no relevant financial disclosures.
This study is "incredibly interesting. It’s amazing to think that as recently as 5-7 years ago, people were still operating under the advice that 90% of concussion patients get better within a week. You can still find that online every now and then. But clearly, whether they’ve had multiple concussions or not, recovery time is longer for teens and preteens than anyone has expected in the past. This backs up what I see in the clinic," said Dr. Kevin Walter.
So, if kids come to the office a week or 2 after a concussion and say they’re all better, they are "going to be the exception to the rule." More likely, they are not being honest with themselves or are a bit too eager to get back into the game or classroom, he said.
"You don’t want to let the athlete make the decision on their own that they’re better. [Sometimes] ERs [still] send them out saying that ‘if you still feel bad in a week, then go get seen. Otherwise, get back into sport[s],’ " he said.
Follow-up is critical to prevent that from happening. "The gold standard is moving towards multidisciplinary care with physicians and neuropsychologists, with the input of a school athletic trainer. [In my clinic,] the luxury of having a neuropsychologist is wonderful; they’ve got the cognitive function testing" to uncover subtle problems, "and they’ve got more time [to work with patients] and expertise on how to deliver the tests appropriately," Dr. Walter said.
No matter how hard it is for young patients to power down for a bit, "we know without a doubt that kids need some degree of cognitive rest and physical rest from activity and sports" after a concussion. It’s troubling in the study "that only 92% of people who had a concussion were told to refrain from athletics. That should be 100%; that’s the goal we need to shoot for," he said.
For now, it’s unclear if there’s a gap between when kids feel better and when they are truly physiologically recovered, and if they are especially vulnerable to another concussion in between. Also, although it’s been recognized before that kid concussions are different than ones in adults, what exactly that means for treatment is uncertain at this point.
Even so, "for most kids, we need to move a little bit more slowly" than in the past, he said.
Dr. Walter is an associate professor in the departments of orthopedic surgery and pediatrics at the Medical College of Wisconsin in Milwaukee, cofounder of the college’s Sports Concussion Program, and a member of the Institute of Medicine’s Committee on Sports-Related Concussions in Youth. He was lead author of the American Academy of Pediatrics’ clinical report "Sport-Related Concussion in Children and Adolescents."
This study is "incredibly interesting. It’s amazing to think that as recently as 5-7 years ago, people were still operating under the advice that 90% of concussion patients get better within a week. You can still find that online every now and then. But clearly, whether they’ve had multiple concussions or not, recovery time is longer for teens and preteens than anyone has expected in the past. This backs up what I see in the clinic," said Dr. Kevin Walter.
So, if kids come to the office a week or 2 after a concussion and say they’re all better, they are "going to be the exception to the rule." More likely, they are not being honest with themselves or are a bit too eager to get back into the game or classroom, he said.
"You don’t want to let the athlete make the decision on their own that they’re better. [Sometimes] ERs [still] send them out saying that ‘if you still feel bad in a week, then go get seen. Otherwise, get back into sport[s],’ " he said.
Follow-up is critical to prevent that from happening. "The gold standard is moving towards multidisciplinary care with physicians and neuropsychologists, with the input of a school athletic trainer. [In my clinic,] the luxury of having a neuropsychologist is wonderful; they’ve got the cognitive function testing" to uncover subtle problems, "and they’ve got more time [to work with patients] and expertise on how to deliver the tests appropriately," Dr. Walter said.
No matter how hard it is for young patients to power down for a bit, "we know without a doubt that kids need some degree of cognitive rest and physical rest from activity and sports" after a concussion. It’s troubling in the study "that only 92% of people who had a concussion were told to refrain from athletics. That should be 100%; that’s the goal we need to shoot for," he said.
For now, it’s unclear if there’s a gap between when kids feel better and when they are truly physiologically recovered, and if they are especially vulnerable to another concussion in between. Also, although it’s been recognized before that kid concussions are different than ones in adults, what exactly that means for treatment is uncertain at this point.
Even so, "for most kids, we need to move a little bit more slowly" than in the past, he said.
Dr. Walter is an associate professor in the departments of orthopedic surgery and pediatrics at the Medical College of Wisconsin in Milwaukee, cofounder of the college’s Sports Concussion Program, and a member of the Institute of Medicine’s Committee on Sports-Related Concussions in Youth. He was lead author of the American Academy of Pediatrics’ clinical report "Sport-Related Concussion in Children and Adolescents."
This study is "incredibly interesting. It’s amazing to think that as recently as 5-7 years ago, people were still operating under the advice that 90% of concussion patients get better within a week. You can still find that online every now and then. But clearly, whether they’ve had multiple concussions or not, recovery time is longer for teens and preteens than anyone has expected in the past. This backs up what I see in the clinic," said Dr. Kevin Walter.
So, if kids come to the office a week or 2 after a concussion and say they’re all better, they are "going to be the exception to the rule." More likely, they are not being honest with themselves or are a bit too eager to get back into the game or classroom, he said.
"You don’t want to let the athlete make the decision on their own that they’re better. [Sometimes] ERs [still] send them out saying that ‘if you still feel bad in a week, then go get seen. Otherwise, get back into sport[s],’ " he said.
Follow-up is critical to prevent that from happening. "The gold standard is moving towards multidisciplinary care with physicians and neuropsychologists, with the input of a school athletic trainer. [In my clinic,] the luxury of having a neuropsychologist is wonderful; they’ve got the cognitive function testing" to uncover subtle problems, "and they’ve got more time [to work with patients] and expertise on how to deliver the tests appropriately," Dr. Walter said.
No matter how hard it is for young patients to power down for a bit, "we know without a doubt that kids need some degree of cognitive rest and physical rest from activity and sports" after a concussion. It’s troubling in the study "that only 92% of people who had a concussion were told to refrain from athletics. That should be 100%; that’s the goal we need to shoot for," he said.
For now, it’s unclear if there’s a gap between when kids feel better and when they are truly physiologically recovered, and if they are especially vulnerable to another concussion in between. Also, although it’s been recognized before that kid concussions are different than ones in adults, what exactly that means for treatment is uncertain at this point.
Even so, "for most kids, we need to move a little bit more slowly" than in the past, he said.
Dr. Walter is an associate professor in the departments of orthopedic surgery and pediatrics at the Medical College of Wisconsin in Milwaukee, cofounder of the college’s Sports Concussion Program, and a member of the Institute of Medicine’s Committee on Sports-Related Concussions in Youth. He was lead author of the American Academy of Pediatrics’ clinical report "Sport-Related Concussion in Children and Adolescents."
Children and teenagers take longer to recover from a concussion if they’ve had one before, especially within the past year, Boston Children’s Hospital emergency department physicians found in a study of 280 of their concussed patients published June 10 in Pediatrics.
The median duration of symptoms, assessed by the serial Rivermead Post-Concussion Symptoms Questionnaire (RPSQ) over a period of 3 months, climbed from 12 days in patients who hadn’t been concussed before to 24 days in those who had. The median symptom duration was 28 days in patients with multiple previous concussions, and 35 days in those who’d been concussed within the previous year, "nearly three times the median duration [for] those who had no previous concussions," according to Dr. Matthew A. Eisenberg and his associates at the hospital (Pediatrics 2013 [doi:10.1542/peds.2013-0432]).
"Similarly, patients with two or more previous concussions had more than double the median symptom duration [of] patients with zero or one previous concussion," they found.
On multivariate analysis, previous concussion, maintaining consciousness, being 13 years or older, and an initial RPSQ of 18 or higher all predicted prolonged recovery. Among all comers, 77% had symptoms at 1 week, 32% at 4 weeks, and 15% at 3 months. The mean age in the trial was 14.3 years (range, 11-22 years).
The findings were statistically significant and have "direct implications on the management of athletes and other at-risk individuals who sustain concussions, supporting the concept that sufficient time to recover from a concussion may improve long-term outcomes," the investigators said.
"However, we did not find an association between physician-advised cognitive or physical rest and duration of symptoms, which may reflect the limitations of our observational study," they added. "A randomized [controlled] trial will likely be necessary to address the utility of this intervention."
Sixty-six percent of the subjects were enrolled the day they were injured; 24.7% were enrolled 1 day later, 7.2% 2 days later, and 1.7% 3 days later. The majority (63.8%) had been injured playing hockey, soccer, football, basketball or some other sport.
The investigators defined concussion broadly to include either altered mental status following blunt head trauma or, within 4 hours of it, any of the following symptoms that were not present before the injury: headache, nausea, vomiting, dizziness/balance problems, fatigue, drowsiness, blurred vision, memory difficulty, or trouble concentrating.
The most common symptoms in the study were headache (85.1%), fatigue (64.7%), and dizziness (63.0%); 4.3% of subjects had altered gait or balance, and 2.4% had altered mental status. There were no abnormalities in the 20.8% of kids who got neuroimaging.
On discharge, 65.9% were prescribed a period of cognitive rest and 92.4% were told to take time off from sports; 63.8% were also told to follow up with their primary care doctor, 45.5% with a sports concussion clinic, and 6.2% with a specialist.
In contrast to prior studies, loss of consciousness seemed to protect against a prolonged recovery (HR, 0.648; P = .02). Maybe the 22% of kids who got knocked out were more likely to follow their doctors’ advice to rest, "thus speeding recovery from their injury. We cannot, however, eliminate the possibility that there is a biological basis to this finding," the team noted.
Subjects who were 13 years or older might have taken longer to recover (HR, 1.404; P = .04) because games "between older children involve more contact and higher-force impacts," although neurobiologic differences between older and younger kids might have played a role, as well, the investigators said.
"Female patients" – about 43% of the study total – "had more severe symptoms at presentation in our study (mean initial RPSQ of 21.3 vs. 17.0 in male patients, P = .02). ... Whether this finding is indicative of the fact that female patients have more severe symptoms from concussion in general, as suggested in several previous studies, or is due to referral bias in which female individuals preferentially present to the ED when symptoms are more severe ... cannot be ascertained from our data," they noted.
Female gender fell out on multivariate analysis as a predictor of prolonged recovery (HR, 1.294; P= 0.11).
The investigators said they had no relevant financial disclosures.
Children and teenagers take longer to recover from a concussion if they’ve had one before, especially within the past year, Boston Children’s Hospital emergency department physicians found in a study of 280 of their concussed patients published June 10 in Pediatrics.
The median duration of symptoms, assessed by the serial Rivermead Post-Concussion Symptoms Questionnaire (RPSQ) over a period of 3 months, climbed from 12 days in patients who hadn’t been concussed before to 24 days in those who had. The median symptom duration was 28 days in patients with multiple previous concussions, and 35 days in those who’d been concussed within the previous year, "nearly three times the median duration [for] those who had no previous concussions," according to Dr. Matthew A. Eisenberg and his associates at the hospital (Pediatrics 2013 [doi:10.1542/peds.2013-0432]).
"Similarly, patients with two or more previous concussions had more than double the median symptom duration [of] patients with zero or one previous concussion," they found.
On multivariate analysis, previous concussion, maintaining consciousness, being 13 years or older, and an initial RPSQ of 18 or higher all predicted prolonged recovery. Among all comers, 77% had symptoms at 1 week, 32% at 4 weeks, and 15% at 3 months. The mean age in the trial was 14.3 years (range, 11-22 years).
The findings were statistically significant and have "direct implications on the management of athletes and other at-risk individuals who sustain concussions, supporting the concept that sufficient time to recover from a concussion may improve long-term outcomes," the investigators said.
"However, we did not find an association between physician-advised cognitive or physical rest and duration of symptoms, which may reflect the limitations of our observational study," they added. "A randomized [controlled] trial will likely be necessary to address the utility of this intervention."
Sixty-six percent of the subjects were enrolled the day they were injured; 24.7% were enrolled 1 day later, 7.2% 2 days later, and 1.7% 3 days later. The majority (63.8%) had been injured playing hockey, soccer, football, basketball or some other sport.
The investigators defined concussion broadly to include either altered mental status following blunt head trauma or, within 4 hours of it, any of the following symptoms that were not present before the injury: headache, nausea, vomiting, dizziness/balance problems, fatigue, drowsiness, blurred vision, memory difficulty, or trouble concentrating.
The most common symptoms in the study were headache (85.1%), fatigue (64.7%), and dizziness (63.0%); 4.3% of subjects had altered gait or balance, and 2.4% had altered mental status. There were no abnormalities in the 20.8% of kids who got neuroimaging.
On discharge, 65.9% were prescribed a period of cognitive rest and 92.4% were told to take time off from sports; 63.8% were also told to follow up with their primary care doctor, 45.5% with a sports concussion clinic, and 6.2% with a specialist.
In contrast to prior studies, loss of consciousness seemed to protect against a prolonged recovery (HR, 0.648; P = .02). Maybe the 22% of kids who got knocked out were more likely to follow their doctors’ advice to rest, "thus speeding recovery from their injury. We cannot, however, eliminate the possibility that there is a biological basis to this finding," the team noted.
Subjects who were 13 years or older might have taken longer to recover (HR, 1.404; P = .04) because games "between older children involve more contact and higher-force impacts," although neurobiologic differences between older and younger kids might have played a role, as well, the investigators said.
"Female patients" – about 43% of the study total – "had more severe symptoms at presentation in our study (mean initial RPSQ of 21.3 vs. 17.0 in male patients, P = .02). ... Whether this finding is indicative of the fact that female patients have more severe symptoms from concussion in general, as suggested in several previous studies, or is due to referral bias in which female individuals preferentially present to the ED when symptoms are more severe ... cannot be ascertained from our data," they noted.
Female gender fell out on multivariate analysis as a predictor of prolonged recovery (HR, 1.294; P= 0.11).
The investigators said they had no relevant financial disclosures.
FROM PEDIATRICS
Major finding: The median duration of concussion symptoms was 12 days in children and teens who hadn’t been concussed before, 24 days in those who had, and 35 days in those who had been concussed within the previous year.
Data source: A prospective cohort study of 280 concussed patients aged 11-22 years.
Disclosures: The study was funded by Boston Children’s Hospital, where it was conducted. The investigators said they had no relevant financial disclosures.
Simeprevir keeps HCV at bay in treatment-naive and experienced patients
ORLANDO – The investigational protease inhibitor simeprevir was associated with high levels of sustained virologic response in patients with both treatment-naive and relapsed hepatitis C viral infections, reported investigators at the annual Digestive Disease Week.
In the QUEST-2 phase III trial, 81.3% of previously untreated patients with hepatitis C (HCV) genotype 1 infections randomized to simeprevir (TMC435) and pegylated interferon-alfa (pegIFN/RBV) had a sustained virologic response following 12 weeks of therapy (SVR12, the primary endpoint), compared with 50% of those assigned to pegIFN/RBV and placebo (P less than .001), reported Dr. Fred Poordad from the University of Texas Health Science Center in San Antonio.
In the phase III PROMISE trial, 79.2% of patients with HCV genotype 1 infections who had a relapse following prior therapy with an interferon-based regimen had an SVR12 when treated with simeprevir, compared with 36.8% of patients treated with pegIFN/RBV and placebo (P less than .001), said Dr. Eric Lawitz, also from the University of Texas in San Antonio.
"Safety and tolerability appear to be comparable to placebo, and patient-reported outcomes support both the efficacy and the safety profiles of simeprevir," Dr. Poordad said.
QUEST-2
Simeprevir is a once-daily oral inhibitor of the HCV NS3/4A protease with demonstrated antiviral activity against HCV genotypes 1, 2, 4, 5, and 6.
In QUEST-2, 391 patients were randomized on a 2:1 basis to receive either simeprevir 150 mg daily plus pegIFN/RBV or placebo plus pegIFN/RBV for 12 weeks, followed by an additional 12 or 36 weeks of pegIFN/RBV depending on response-guided therapy criteria. If patients had HCV RNA less than 25 IU/mL at week 4 and undetectable at week 12, they received an additional 12 weeks of pegIFN/RBV. Patients outside of the response-guided criteria received a total of 36 additional weeks of pegIFN/RBV. In both treatment arms, patients were followed for an additional 24 months, for a total of 72 months.
A total of 235 of the 257 patients assigned to simeprevir (91.4%) met the response-guided criteria by week 24, completed therapy, and were then followed until study end. Of this group, 86% (202 patients) achieved SVR12.
Simeprevir was statistically significantly superior to placebo regardless of IL28B polymorphism genotype or METAVIR (fibrosis and inflammation) scores.
On-treatment failures, defined as a confirmed detectable HCV RNA level at the actual end of treatment, occurred in 7% of patients on simeprevir and 32.1% of controls. Relapses, defined as detectable HCV RNA on one or more follow-up visits following undetectable end-of-treatment levels, occurred in 12.7% and 23.9%, respectively (P values not shown).
Of the simeprevir-treated patients who did not achieve an SVR, 97.6% had emerging mutations in the NS3 protease domain at the time of treatment failure, Dr. Poordad said.
PROMISE
In the PROMISE trial, 393 patients who had experienced a relapse following interferon-based therapy were randomized to response guided therapy as described in the QUEST-2 study.
As noted before, 79.2% of patients assigned to simeprevir/pegIFN/RBV met the primary endpoint of SVR12, compared with 36.8% of patients assigned to placebo/pegIFN/RBV (P less than .001).
In this trial, simeprevir was significantly better than placebo in patients with both HCV genotypes 1a and 1b, and as in QUEST-2 was superior to placebo regardless of IL28B genotype or METAVIR score.
On-treatment failures occurred in 3.1% of simeprevir-treated patients and 27.1% of those on placebo and pegIFN/RBV. The respective relapse rates were 18.5% and 48.4%. As in QUEST-2, the large majority (92.3%) of simeprevir-treated patients who did not have an SVR had emerging mutations in the NS3 protease domain.
Safety
In QUEST-2, patients on simeprevir had more cases of rash, 27% vs. 20%, and photosensitivity, 4% vs. 1%. Anemia occurred in 13.6% and 15.7%, respectively. The incidences of other adverse events were similar between the groups.
In PROMISE, the most common adverse events were fatigue, influenzalike illness, pruritus, and headache. Anemia occurred in 17% of patients on the active drug plus pegIFN/RBV, compared with 20% for those on placebo/pegIFN/RBV. Neutropenia occurred in 18% and 22%, respectively. Rates of pruritus and rash were comparable between simeprevir and placebo.
The Food and Drug Administration has granted priority review status to simeprevir for the treatment of chronic HCV genotype 1.
The studies were funded by Janssen. Dr. Poordad and Dr. Lawitz have received grants and/or research support from the company, and several of their coauthors are employees of Janssen or its parent company Johnson & Johnson.
ORLANDO – The investigational protease inhibitor simeprevir was associated with high levels of sustained virologic response in patients with both treatment-naive and relapsed hepatitis C viral infections, reported investigators at the annual Digestive Disease Week.
In the QUEST-2 phase III trial, 81.3% of previously untreated patients with hepatitis C (HCV) genotype 1 infections randomized to simeprevir (TMC435) and pegylated interferon-alfa (pegIFN/RBV) had a sustained virologic response following 12 weeks of therapy (SVR12, the primary endpoint), compared with 50% of those assigned to pegIFN/RBV and placebo (P less than .001), reported Dr. Fred Poordad from the University of Texas Health Science Center in San Antonio.
In the phase III PROMISE trial, 79.2% of patients with HCV genotype 1 infections who had a relapse following prior therapy with an interferon-based regimen had an SVR12 when treated with simeprevir, compared with 36.8% of patients treated with pegIFN/RBV and placebo (P less than .001), said Dr. Eric Lawitz, also from the University of Texas in San Antonio.
"Safety and tolerability appear to be comparable to placebo, and patient-reported outcomes support both the efficacy and the safety profiles of simeprevir," Dr. Poordad said.
QUEST-2
Simeprevir is a once-daily oral inhibitor of the HCV NS3/4A protease with demonstrated antiviral activity against HCV genotypes 1, 2, 4, 5, and 6.
In QUEST-2, 391 patients were randomized on a 2:1 basis to receive either simeprevir 150 mg daily plus pegIFN/RBV or placebo plus pegIFN/RBV for 12 weeks, followed by an additional 12 or 36 weeks of pegIFN/RBV depending on response-guided therapy criteria. If patients had HCV RNA less than 25 IU/mL at week 4 and undetectable at week 12, they received an additional 12 weeks of pegIFN/RBV. Patients outside of the response-guided criteria received a total of 36 additional weeks of pegIFN/RBV. In both treatment arms, patients were followed for an additional 24 months, for a total of 72 months.
A total of 235 of the 257 patients assigned to simeprevir (91.4%) met the response-guided criteria by week 24, completed therapy, and were then followed until study end. Of this group, 86% (202 patients) achieved SVR12.
Simeprevir was statistically significantly superior to placebo regardless of IL28B polymorphism genotype or METAVIR (fibrosis and inflammation) scores.
On-treatment failures, defined as a confirmed detectable HCV RNA level at the actual end of treatment, occurred in 7% of patients on simeprevir and 32.1% of controls. Relapses, defined as detectable HCV RNA on one or more follow-up visits following undetectable end-of-treatment levels, occurred in 12.7% and 23.9%, respectively (P values not shown).
Of the simeprevir-treated patients who did not achieve an SVR, 97.6% had emerging mutations in the NS3 protease domain at the time of treatment failure, Dr. Poordad said.
PROMISE
In the PROMISE trial, 393 patients who had experienced a relapse following interferon-based therapy were randomized to response guided therapy as described in the QUEST-2 study.
As noted before, 79.2% of patients assigned to simeprevir/pegIFN/RBV met the primary endpoint of SVR12, compared with 36.8% of patients assigned to placebo/pegIFN/RBV (P less than .001).
In this trial, simeprevir was significantly better than placebo in patients with both HCV genotypes 1a and 1b, and as in QUEST-2 was superior to placebo regardless of IL28B genotype or METAVIR score.
On-treatment failures occurred in 3.1% of simeprevir-treated patients and 27.1% of those on placebo and pegIFN/RBV. The respective relapse rates were 18.5% and 48.4%. As in QUEST-2, the large majority (92.3%) of simeprevir-treated patients who did not have an SVR had emerging mutations in the NS3 protease domain.
Safety
In QUEST-2, patients on simeprevir had more cases of rash, 27% vs. 20%, and photosensitivity, 4% vs. 1%. Anemia occurred in 13.6% and 15.7%, respectively. The incidences of other adverse events were similar between the groups.
In PROMISE, the most common adverse events were fatigue, influenzalike illness, pruritus, and headache. Anemia occurred in 17% of patients on the active drug plus pegIFN/RBV, compared with 20% for those on placebo/pegIFN/RBV. Neutropenia occurred in 18% and 22%, respectively. Rates of pruritus and rash were comparable between simeprevir and placebo.
The Food and Drug Administration has granted priority review status to simeprevir for the treatment of chronic HCV genotype 1.
The studies were funded by Janssen. Dr. Poordad and Dr. Lawitz have received grants and/or research support from the company, and several of their coauthors are employees of Janssen or its parent company Johnson & Johnson.
ORLANDO – The investigational protease inhibitor simeprevir was associated with high levels of sustained virologic response in patients with both treatment-naive and relapsed hepatitis C viral infections, reported investigators at the annual Digestive Disease Week.
In the QUEST-2 phase III trial, 81.3% of previously untreated patients with hepatitis C (HCV) genotype 1 infections randomized to simeprevir (TMC435) and pegylated interferon-alfa (pegIFN/RBV) had a sustained virologic response following 12 weeks of therapy (SVR12, the primary endpoint), compared with 50% of those assigned to pegIFN/RBV and placebo (P less than .001), reported Dr. Fred Poordad from the University of Texas Health Science Center in San Antonio.
In the phase III PROMISE trial, 79.2% of patients with HCV genotype 1 infections who had a relapse following prior therapy with an interferon-based regimen had an SVR12 when treated with simeprevir, compared with 36.8% of patients treated with pegIFN/RBV and placebo (P less than .001), said Dr. Eric Lawitz, also from the University of Texas in San Antonio.
"Safety and tolerability appear to be comparable to placebo, and patient-reported outcomes support both the efficacy and the safety profiles of simeprevir," Dr. Poordad said.
QUEST-2
Simeprevir is a once-daily oral inhibitor of the HCV NS3/4A protease with demonstrated antiviral activity against HCV genotypes 1, 2, 4, 5, and 6.
In QUEST-2, 391 patients were randomized on a 2:1 basis to receive either simeprevir 150 mg daily plus pegIFN/RBV or placebo plus pegIFN/RBV for 12 weeks, followed by an additional 12 or 36 weeks of pegIFN/RBV depending on response-guided therapy criteria. If patients had HCV RNA less than 25 IU/mL at week 4 and undetectable at week 12, they received an additional 12 weeks of pegIFN/RBV. Patients outside of the response-guided criteria received a total of 36 additional weeks of pegIFN/RBV. In both treatment arms, patients were followed for an additional 24 months, for a total of 72 months.
A total of 235 of the 257 patients assigned to simeprevir (91.4%) met the response-guided criteria by week 24, completed therapy, and were then followed until study end. Of this group, 86% (202 patients) achieved SVR12.
Simeprevir was statistically significantly superior to placebo regardless of IL28B polymorphism genotype or METAVIR (fibrosis and inflammation) scores.
On-treatment failures, defined as a confirmed detectable HCV RNA level at the actual end of treatment, occurred in 7% of patients on simeprevir and 32.1% of controls. Relapses, defined as detectable HCV RNA on one or more follow-up visits following undetectable end-of-treatment levels, occurred in 12.7% and 23.9%, respectively (P values not shown).
Of the simeprevir-treated patients who did not achieve an SVR, 97.6% had emerging mutations in the NS3 protease domain at the time of treatment failure, Dr. Poordad said.
PROMISE
In the PROMISE trial, 393 patients who had experienced a relapse following interferon-based therapy were randomized to response guided therapy as described in the QUEST-2 study.
As noted before, 79.2% of patients assigned to simeprevir/pegIFN/RBV met the primary endpoint of SVR12, compared with 36.8% of patients assigned to placebo/pegIFN/RBV (P less than .001).
In this trial, simeprevir was significantly better than placebo in patients with both HCV genotypes 1a and 1b, and as in QUEST-2 was superior to placebo regardless of IL28B genotype or METAVIR score.
On-treatment failures occurred in 3.1% of simeprevir-treated patients and 27.1% of those on placebo and pegIFN/RBV. The respective relapse rates were 18.5% and 48.4%. As in QUEST-2, the large majority (92.3%) of simeprevir-treated patients who did not have an SVR had emerging mutations in the NS3 protease domain.
Safety
In QUEST-2, patients on simeprevir had more cases of rash, 27% vs. 20%, and photosensitivity, 4% vs. 1%. Anemia occurred in 13.6% and 15.7%, respectively. The incidences of other adverse events were similar between the groups.
In PROMISE, the most common adverse events were fatigue, influenzalike illness, pruritus, and headache. Anemia occurred in 17% of patients on the active drug plus pegIFN/RBV, compared with 20% for those on placebo/pegIFN/RBV. Neutropenia occurred in 18% and 22%, respectively. Rates of pruritus and rash were comparable between simeprevir and placebo.
The Food and Drug Administration has granted priority review status to simeprevir for the treatment of chronic HCV genotype 1.
The studies were funded by Janssen. Dr. Poordad and Dr. Lawitz have received grants and/or research support from the company, and several of their coauthors are employees of Janssen or its parent company Johnson & Johnson.
AT DDW 2013
Major finding: SVR 12 rates were 81.3% in treatment-naive patients with HCV genotype 1 treated with simeprevir/pegylated interferon/ribavirin, and 79.2% in relapsed patients, compared with 50% and 36.8% of patients treated with placebo and pegIFN/RBV.
Data source: Two randomized, controlled phase III studies involving 391 treatment-naive patients (QUEST-2), and 393 patients who had a relapse following prior interferon-based therapy (PROMISE).
Disclosures: The studies were funded by Janssen. Dr. Poordad and Dr. Lawitz have received grants and/or research support from the company, and several of their coauthors are employees of Janssen or its parent company Johnson & Johnson.
AMDEs in Children
Children with complex chronic conditions comprise an increasing proportion of hospital admissions, readmissions, and resource use.[1, 2, 3] Dependence on technology or medical devices is a frequent characteristic of children in this group.[4] Adverse medical device events (AMDEs) are estimated to occur in as many as 8% of all adult admissions, depending on the methods used to identify them.[5] These events may result in hospitalizations or complicate hospital stays. To date, however, the burden of AMDEs among hospitalized children is little described, even though children may be at increased risk for device events as compared to adults.[6] Although some medical devices are intended solely or primarily for use with children, most devices used with children have been initially developed for, tested with, and most frequently employed to treat adults.[6] Assessing the continued safety and effectiveness of medical devices marketed in the Unites States is the responsibility of the Center for Devices and Radiologic Health of the US Food and Drug Administration (FDA). Its existing mechanisms for postmarket device surveillance rely primarily on passive reporting systems and specific observational studies.[7]
The objective of this study was to utilize administrative data from children's hospitals to explore the prevalence and nature of AMDEs in tertiary care children's hospitals that treat significant numbers of children with complex needs requiring medical devices.
METHODS
Data were obtained from the Pediatric Health Information System (PHIS), an administrative database containing inpatient data from 44 not‐for‐profit, tertiary care, pediatric hospitals affiliated with the Children's Hospital Association. Data are deidentified at the time of submission, and are subjected to a number of reliability and validity checks.[8] Individual admission records have both a deidentified visit identification (ID) and patient ID, allowing for linkage of multiple admissions by the same patient.
AMDEs were defined by International Classification of Diseases, Ninth Revision (ICD‐9) codes, using a methodology developed by Samore et al., who identified a set of such codes that specified devices in their definitions and therefore were considered to have a high likelihood of indicating a device problem (see Supporting Information, Table S1, in the online version of this article).[5] The diagnosis codes were grouped into device categories (eg, nervous system, orthopedic, cardiac).
From the 44 hospitals, the primary study cohort consisted of any patient with an admission between January 1, 2004 and December 31, 2011 with 1 AMDE ICD‐9 code as a primary or secondary diagnosis.
Descriptive statistics for patient demographics and visit characteristics of AMDE admissions were generated and stratified by device category. We reported these as counts and percentages for categorical variables and as median and interquartile range for length of stay. We also reported on how frequently patients with AMDEs have a top 10 most common diagnosis and top 10 most common procedure during the AMDE admission. We also reported the presence or absence of a complex chronic condition.[9] We generated the list of most common principal diagnoses and procedures by a separate query of PHIS from 2004 to 2009. Our top 10 most common diagnoses included ICD‐9 codes 486 (pneumonia), 466.11 (acute bronchiolitis due to respiratory syncytial virus), V58.11 (chemotherapy encounter), 493.92 (asthma exacerbation), 493.91 (asthma with status asthmaticus), 466.19 (acute bronchiolitis due to other organism), 780.39 (other convulsions), 540.9 (acute appendicitis), 282.62 (sickle cell disease with crisis), and 276.51 (dehydration). Our top 10 most common procedures included ICD‐9 codes 38.93 (venous catheterization), 03.31 (spinal tap), 99.04 (packed blood cell transfusion), 99.15 (parenteral nutrition), 99.25 (cancer chemotherapy), 96.71 (invasive mechanical ventilation, 96 hours), 96.04 (endotracheal intubation), 96.72 (invasive mechanical ventilation,95 hours), 96.6 (enteral nutrition), and 99.05 (platelet transfusion). Analyses were performed using SAS Enterprise Guide version 4.2 for Windows (SAS Institute, Cary, NC).
This study was approved by Cincinnati Children's Hospital Medical Center Institutional Review Board.
RESULTS
Of the 4,115,755 admissions during the study period, 136,465 (3.3%) had at least 1 AMDE. Over our study period, AMDEs were associated with a mean 17,058 inpatient stays annually. The number of AMDE‐related admissions decreased the last 4 years of our study period despite generally increasing admissions at PHIS hospitals (Figure 1). For 55% of the admissions (75,206/136,465), this AMDE code represented the primary diagnosis. Of these visits with a primary AMDE diagnosis, 39,874 (53%) were related to nervous system devices. The visits associated with AMDEs were comprised of 88,908 unique patients, 55% of whom were male (Table 1). The median age on admission was 6 years, and the interquartile range was 1 to 14 years of age.
| Total, N=88,908 | |
|---|---|
| Age at first admission | |
| 2 years | 35,160 (40.0%) |
| 35 years | 9,352 (10.5%) |
| 611 years | 16,148 (18.2%) |
| 1218 years | 22,483 (25.3%) |
| 19 years | 5,765 (6.5%) |
| Gender | |
| Male | 49,172 (55.3%) |
| Female | 39,730 (44.7%) |
| Race | |
| White | 59,842 (67.3%) |
| Black | 14,747 (16.6%) |
| Asian | 1,910 (2.2%) |
| American Indian | 900 (1.0%) |
| Other | 8,732 (9.8%) |
| Missing | 2,777 (3.1%) |
| Number of admissions by patient | |
| 1 | 66,814 (75.1%) |
| 2 | 12,520 (14.1%) |
| 3 | 4,504 (5.1%) |
| 4 | 5,071 (5.7%) |
Among admissions with AMDEs, 2.9% ended in death. The mortality was 0.5% when an AMDE was the primary diagnosis and 5.7% when the AMDE was a secondary diagnosis. The median length of inpatient stays was 6 days, with an interquartile range of 2 to 17 days.
Vascular access AMDEs were the most common event associated with admissions (26.6%), followed by nervous system devices (17.8%) (Table 2). The majority (75.5%) of patients admitted with AMDEs had a complex chronic condition. Less than half (46.8%) of AMDE admissions had an associated code for 1 of the 10 most common principal procedures. A minority (14.3%) of admissions had an associated ICD‐9 code for 1 of the top 10 most common principal diagnoses.
| Device Category | Admissions, n=136,465 | Presence of Top 10 Most Common Principal Procedures, n=63,801 | Presence of Top 10 Most Common Principal Diagnoses, n=19,472 | Presence of 1 Complex Chronic Condition, n=103,003 |
|---|---|---|---|---|
| ||||
| Only 1 AMDE diagnosis | ||||
| Vascular access | 36,257 (26.6%) | 26,658 (41.8%) | 6,518 (33.5%) | 26,022 (25.3%) |
| Nervous system | 24,243 (17.8%) | 4,266 (6.7%) | 3,567 (18.3%) | 21,516 (20.9%) |
| Unspecified device | 21,222 (15.6%) | 11,368 (17.8%) | 2,512 (12.9%) | 13,826 (13.4%) |
| Cardiac | 4,384 (3.2%) | 1,959 (3.1%) | 309 (1.6%) | 3,962 (3.8%) |
| Orthopedic | 3,064 (2.2%) | 874 (1.4%) | 179 (0.9%) | 1,235 (1.2%) |
| Dialysis | 2,426 (1.8%) | 836 (1.3%) | 281 (1.4%) | 1,462 (1.4%) |
| Genitourinary | 1,165 (0.9%) | 388 (0.6%) | 166 (0.9%) | 668 (0.6%) |
| Prosthetic cardiac valve | 518 (0.4%) | 236 (0.4%) | 33 (0.2%) | 411 (0.4%) |
| Urologic catheters | 379 (0.3%) | 228 (0.4%) | 93 (0.5%) | 223 (0.2%) |
| Defibrillator | 197 (0.1%) | 11 (0.02%) | 4 (0.02%) | 18 (0.02%) |
| Ocular | 3 (0.002%) | 1 (0.002%) | 1 (0.005%) | 1 (0.001%) |
| Only 1 AMDE diagnosis subtotal | 93,861 (68.8%) | 46,825 (73.4%) | 13,663 (70.2%) | 69,344 (67.3%) |
| 2 AMDE diagnoses | 39,557 (29.0%) | 15,003 (23.5%) | 5,312 (27.3%) | 31,091 (30.2%) |
| >2 AMDE diagnoses | 3,047 (2.2%) | 1,973 (3.1%) | 497 (2.6%) | 2,568 (2.5%) |
DISCUSSION
To our knowledge, our study is the first to report the burden of AMDEs among children requiring hospitalization. AMDEs are common in this population of children cared for at tertiary care children's hospitals, accounting for or complicating 3.3% of inpatient stays in these 44 hospitals. AMDEs were associated with a mean of >17,000 total visits per year. Vascular access devices and nervous system devices were the most common device categories linked to AMDEs. Similar to published literature, we found that the youngest children accounted for the highest proportion of AMDEs.[10, 11]
The majority (>75%) of children with an AMDE admission had diagnoses indicating complex chronic conditions during the admission. Over a partially overlapping study period, Feudtner and colleagues found 25.2% of patients admitted to PHIS hospitals had complex chronic conditions.[12] This finding, combined with the uncommon association of the most prevalent diagnoses and procedures, suggests that the burden of AMDEs falls disproportionately on this population of children. Death occurred considerably less commonly when AMDE diagnosis was the primary versus a secondary diagnosis (0.5% vs 5.7%). This finding likely illustrates 2 distinct populations: children with an AMDE that causes admission who have a relatively low risk of mortality and a second group who have AMDE‐complicated hospitalizations that may have an already high risk of mortality.
Our findings complement those of Wang and colleagues who employed the National Electronic Injury Surveillance System All Injury Program database to provide national estimates of medical device‐associated adverse events.[11] Importantly, this group used a different population (patients presenting to the emergency department) and a different methodology. These authors reported on device‐associated events, as they did not collect information to discriminate the device's role in the event. A walker that malfunctioned leading to patient injury would be a device‐related event; however, a patient who has a walker suffering a fall would be device‐associated, even if the walker's role in the injury was uncertain. We believe our methodology, established by Samore et al., more accurately identifies device‐related events.[5] Wang et al. found that 6.3% of pediatric patients who presented to emergency departments with medical device‐associated events were admitted to the hospital.[11] This resulted in national estimates of 9,082 events with 95% confidence intervals of 2,990 to 25,373 hospitalizations. Our findings of >17,000 AMDE‐related inpatient stays per annum included not only AMDEs leading to admissions but also those that were complications during stays.
Our study has several limitations, most related to the possibility of misclassification present in administrative data. Our approach only captured events that led to or complicated admissions. We suspect that ICD‐9 codes likely missed some AMDEs and that our estimates may therefore under‐represent this problem in our population. Future studies should compare our methodology, which has produced the first across‐center estimates of AMDE admissions, to alternative event capture techniques. We were unable to determine which events were present on admission and which complicated hospital stays, and it is likely that differing interventions would be required to reduce these 2 types of AMDEs. Another important limitation is that the PHIS database, comprised of data on children receiving care at tertiary academic medical centers with large numbers of pediatric subspecialists, is not representative of the population of children overall. The individual ICD‐9 codes for AMDEs are sufficiently nonspecific to limit the ability to characterize device events from administrative data alone. The high prevalence of unspecified device‐related admissions is an additional limitation. Although the estimates of these types of AMDEs are important in describing the frequency of these events, the unspecified category limits the ability to fully stratify based on device type and then implement monitoring strategies and interventions based on each.
To our knowledge, this study is the first multicenter analysis of the spectrum of pediatric AMDEs in hospitalized children. The AMDE prevalence is substantial, and the burden of these events largely falls on children with complex chronic conditions. Despite its limitations, this study complements recent efforts to enhance postmarket surveillance of pediatric devices including that of the FDA's Office of Pediatric Therapeutics, the recent FDA report Strengthening Our National System for Medical Device Postmarket Surveillance (
Our description of AMDEs by device category and patient characteristics is a first and necessary step to understanding the public health burden associated with device use in the pediatric population. Further developments in refined coding and device designation (eg, UDI systems) are needed to refine these estimates.
Acknowledgments
The authors thank Amy Liu, with the Data Management Center, and Colleen Mangeot, with the Biostatistical Consulting Unit in the Division of Epidemiology and Biostatistics, for their assistance with the data pull and creation of the analytic dataset. The authors also thank Lilliam Ambroggio, PhD, and Joshua Schaffzin, MD, PhD, for their thoughtful review of draft manuscripts.
Disclosures: Dr. Brady was supported by funds from the Bureau of Health Professions (BHPr), Health Resources and Services Administration (HRSA), and Department of Health and Human Services (DHHS) under grant T32 HP10027. This project was supported by cooperative agreement number U18 HS016957‐03 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The study sponsors had no role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication. The opinions and assertions presented herein are the private views of the authors and are not to be construed as conveying either an official endorsement or criticism by the US Department of Health and Human Services, The Public Health Service, or the US Food and Drug Administration.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- , , , et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647–655.
- , , , , , . Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126(4):638–646.
- , , , et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538.
- , , , et al. Surveillance of medical device‐related hazards and adverse events in hospitalized patients. JAMA. 2004;291(3):325–334.
- Institute of Medicine (U.S.). Committee on Postmarket Surveillance of Pediatric Medical Devices. , . Safe medical devices for children. Washington, DC: National Academies Press; 2006.
- , . Pharmacovigilance. 2nd ed. Chichester, England ; Hoboken, NJ: John Wiley 2007.
- , , , . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055.
- , , . Pediatric deaths attributable to complex chronic conditions: a population‐based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 pt 2):205–209.
- , , , et al. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. J Neurosurg Pediatr. 2009;4(2):156–165.
- , , , et al. Emergency department visits for medical device‐associated adverse events among children. Pediatrics. 2010;126(2):247–259.
- , , , et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286–293.
- , . Unique device identification in the service of public health. N Engl J Med. 2012;367(17):1583–1585.
Children with complex chronic conditions comprise an increasing proportion of hospital admissions, readmissions, and resource use.[1, 2, 3] Dependence on technology or medical devices is a frequent characteristic of children in this group.[4] Adverse medical device events (AMDEs) are estimated to occur in as many as 8% of all adult admissions, depending on the methods used to identify them.[5] These events may result in hospitalizations or complicate hospital stays. To date, however, the burden of AMDEs among hospitalized children is little described, even though children may be at increased risk for device events as compared to adults.[6] Although some medical devices are intended solely or primarily for use with children, most devices used with children have been initially developed for, tested with, and most frequently employed to treat adults.[6] Assessing the continued safety and effectiveness of medical devices marketed in the Unites States is the responsibility of the Center for Devices and Radiologic Health of the US Food and Drug Administration (FDA). Its existing mechanisms for postmarket device surveillance rely primarily on passive reporting systems and specific observational studies.[7]
The objective of this study was to utilize administrative data from children's hospitals to explore the prevalence and nature of AMDEs in tertiary care children's hospitals that treat significant numbers of children with complex needs requiring medical devices.
METHODS
Data were obtained from the Pediatric Health Information System (PHIS), an administrative database containing inpatient data from 44 not‐for‐profit, tertiary care, pediatric hospitals affiliated with the Children's Hospital Association. Data are deidentified at the time of submission, and are subjected to a number of reliability and validity checks.[8] Individual admission records have both a deidentified visit identification (ID) and patient ID, allowing for linkage of multiple admissions by the same patient.
AMDEs were defined by International Classification of Diseases, Ninth Revision (ICD‐9) codes, using a methodology developed by Samore et al., who identified a set of such codes that specified devices in their definitions and therefore were considered to have a high likelihood of indicating a device problem (see Supporting Information, Table S1, in the online version of this article).[5] The diagnosis codes were grouped into device categories (eg, nervous system, orthopedic, cardiac).
From the 44 hospitals, the primary study cohort consisted of any patient with an admission between January 1, 2004 and December 31, 2011 with 1 AMDE ICD‐9 code as a primary or secondary diagnosis.
Descriptive statistics for patient demographics and visit characteristics of AMDE admissions were generated and stratified by device category. We reported these as counts and percentages for categorical variables and as median and interquartile range for length of stay. We also reported on how frequently patients with AMDEs have a top 10 most common diagnosis and top 10 most common procedure during the AMDE admission. We also reported the presence or absence of a complex chronic condition.[9] We generated the list of most common principal diagnoses and procedures by a separate query of PHIS from 2004 to 2009. Our top 10 most common diagnoses included ICD‐9 codes 486 (pneumonia), 466.11 (acute bronchiolitis due to respiratory syncytial virus), V58.11 (chemotherapy encounter), 493.92 (asthma exacerbation), 493.91 (asthma with status asthmaticus), 466.19 (acute bronchiolitis due to other organism), 780.39 (other convulsions), 540.9 (acute appendicitis), 282.62 (sickle cell disease with crisis), and 276.51 (dehydration). Our top 10 most common procedures included ICD‐9 codes 38.93 (venous catheterization), 03.31 (spinal tap), 99.04 (packed blood cell transfusion), 99.15 (parenteral nutrition), 99.25 (cancer chemotherapy), 96.71 (invasive mechanical ventilation, 96 hours), 96.04 (endotracheal intubation), 96.72 (invasive mechanical ventilation,95 hours), 96.6 (enteral nutrition), and 99.05 (platelet transfusion). Analyses were performed using SAS Enterprise Guide version 4.2 for Windows (SAS Institute, Cary, NC).
This study was approved by Cincinnati Children's Hospital Medical Center Institutional Review Board.
RESULTS
Of the 4,115,755 admissions during the study period, 136,465 (3.3%) had at least 1 AMDE. Over our study period, AMDEs were associated with a mean 17,058 inpatient stays annually. The number of AMDE‐related admissions decreased the last 4 years of our study period despite generally increasing admissions at PHIS hospitals (Figure 1). For 55% of the admissions (75,206/136,465), this AMDE code represented the primary diagnosis. Of these visits with a primary AMDE diagnosis, 39,874 (53%) were related to nervous system devices. The visits associated with AMDEs were comprised of 88,908 unique patients, 55% of whom were male (Table 1). The median age on admission was 6 years, and the interquartile range was 1 to 14 years of age.

| Total, N=88,908 | |
|---|---|
| Age at first admission | |
| 2 years | 35,160 (40.0%) |
| 35 years | 9,352 (10.5%) |
| 611 years | 16,148 (18.2%) |
| 1218 years | 22,483 (25.3%) |
| 19 years | 5,765 (6.5%) |
| Gender | |
| Male | 49,172 (55.3%) |
| Female | 39,730 (44.7%) |
| Race | |
| White | 59,842 (67.3%) |
| Black | 14,747 (16.6%) |
| Asian | 1,910 (2.2%) |
| American Indian | 900 (1.0%) |
| Other | 8,732 (9.8%) |
| Missing | 2,777 (3.1%) |
| Number of admissions by patient | |
| 1 | 66,814 (75.1%) |
| 2 | 12,520 (14.1%) |
| 3 | 4,504 (5.1%) |
| 4 | 5,071 (5.7%) |
Among admissions with AMDEs, 2.9% ended in death. The mortality was 0.5% when an AMDE was the primary diagnosis and 5.7% when the AMDE was a secondary diagnosis. The median length of inpatient stays was 6 days, with an interquartile range of 2 to 17 days.
Vascular access AMDEs were the most common event associated with admissions (26.6%), followed by nervous system devices (17.8%) (Table 2). The majority (75.5%) of patients admitted with AMDEs had a complex chronic condition. Less than half (46.8%) of AMDE admissions had an associated code for 1 of the 10 most common principal procedures. A minority (14.3%) of admissions had an associated ICD‐9 code for 1 of the top 10 most common principal diagnoses.
| Device Category | Admissions, n=136,465 | Presence of Top 10 Most Common Principal Procedures, n=63,801 | Presence of Top 10 Most Common Principal Diagnoses, n=19,472 | Presence of 1 Complex Chronic Condition, n=103,003 |
|---|---|---|---|---|
| ||||
| Only 1 AMDE diagnosis | ||||
| Vascular access | 36,257 (26.6%) | 26,658 (41.8%) | 6,518 (33.5%) | 26,022 (25.3%) |
| Nervous system | 24,243 (17.8%) | 4,266 (6.7%) | 3,567 (18.3%) | 21,516 (20.9%) |
| Unspecified device | 21,222 (15.6%) | 11,368 (17.8%) | 2,512 (12.9%) | 13,826 (13.4%) |
| Cardiac | 4,384 (3.2%) | 1,959 (3.1%) | 309 (1.6%) | 3,962 (3.8%) |
| Orthopedic | 3,064 (2.2%) | 874 (1.4%) | 179 (0.9%) | 1,235 (1.2%) |
| Dialysis | 2,426 (1.8%) | 836 (1.3%) | 281 (1.4%) | 1,462 (1.4%) |
| Genitourinary | 1,165 (0.9%) | 388 (0.6%) | 166 (0.9%) | 668 (0.6%) |
| Prosthetic cardiac valve | 518 (0.4%) | 236 (0.4%) | 33 (0.2%) | 411 (0.4%) |
| Urologic catheters | 379 (0.3%) | 228 (0.4%) | 93 (0.5%) | 223 (0.2%) |
| Defibrillator | 197 (0.1%) | 11 (0.02%) | 4 (0.02%) | 18 (0.02%) |
| Ocular | 3 (0.002%) | 1 (0.002%) | 1 (0.005%) | 1 (0.001%) |
| Only 1 AMDE diagnosis subtotal | 93,861 (68.8%) | 46,825 (73.4%) | 13,663 (70.2%) | 69,344 (67.3%) |
| 2 AMDE diagnoses | 39,557 (29.0%) | 15,003 (23.5%) | 5,312 (27.3%) | 31,091 (30.2%) |
| >2 AMDE diagnoses | 3,047 (2.2%) | 1,973 (3.1%) | 497 (2.6%) | 2,568 (2.5%) |
DISCUSSION
To our knowledge, our study is the first to report the burden of AMDEs among children requiring hospitalization. AMDEs are common in this population of children cared for at tertiary care children's hospitals, accounting for or complicating 3.3% of inpatient stays in these 44 hospitals. AMDEs were associated with a mean of >17,000 total visits per year. Vascular access devices and nervous system devices were the most common device categories linked to AMDEs. Similar to published literature, we found that the youngest children accounted for the highest proportion of AMDEs.[10, 11]
The majority (>75%) of children with an AMDE admission had diagnoses indicating complex chronic conditions during the admission. Over a partially overlapping study period, Feudtner and colleagues found 25.2% of patients admitted to PHIS hospitals had complex chronic conditions.[12] This finding, combined with the uncommon association of the most prevalent diagnoses and procedures, suggests that the burden of AMDEs falls disproportionately on this population of children. Death occurred considerably less commonly when AMDE diagnosis was the primary versus a secondary diagnosis (0.5% vs 5.7%). This finding likely illustrates 2 distinct populations: children with an AMDE that causes admission who have a relatively low risk of mortality and a second group who have AMDE‐complicated hospitalizations that may have an already high risk of mortality.
Our findings complement those of Wang and colleagues who employed the National Electronic Injury Surveillance System All Injury Program database to provide national estimates of medical device‐associated adverse events.[11] Importantly, this group used a different population (patients presenting to the emergency department) and a different methodology. These authors reported on device‐associated events, as they did not collect information to discriminate the device's role in the event. A walker that malfunctioned leading to patient injury would be a device‐related event; however, a patient who has a walker suffering a fall would be device‐associated, even if the walker's role in the injury was uncertain. We believe our methodology, established by Samore et al., more accurately identifies device‐related events.[5] Wang et al. found that 6.3% of pediatric patients who presented to emergency departments with medical device‐associated events were admitted to the hospital.[11] This resulted in national estimates of 9,082 events with 95% confidence intervals of 2,990 to 25,373 hospitalizations. Our findings of >17,000 AMDE‐related inpatient stays per annum included not only AMDEs leading to admissions but also those that were complications during stays.
Our study has several limitations, most related to the possibility of misclassification present in administrative data. Our approach only captured events that led to or complicated admissions. We suspect that ICD‐9 codes likely missed some AMDEs and that our estimates may therefore under‐represent this problem in our population. Future studies should compare our methodology, which has produced the first across‐center estimates of AMDE admissions, to alternative event capture techniques. We were unable to determine which events were present on admission and which complicated hospital stays, and it is likely that differing interventions would be required to reduce these 2 types of AMDEs. Another important limitation is that the PHIS database, comprised of data on children receiving care at tertiary academic medical centers with large numbers of pediatric subspecialists, is not representative of the population of children overall. The individual ICD‐9 codes for AMDEs are sufficiently nonspecific to limit the ability to characterize device events from administrative data alone. The high prevalence of unspecified device‐related admissions is an additional limitation. Although the estimates of these types of AMDEs are important in describing the frequency of these events, the unspecified category limits the ability to fully stratify based on device type and then implement monitoring strategies and interventions based on each.
To our knowledge, this study is the first multicenter analysis of the spectrum of pediatric AMDEs in hospitalized children. The AMDE prevalence is substantial, and the burden of these events largely falls on children with complex chronic conditions. Despite its limitations, this study complements recent efforts to enhance postmarket surveillance of pediatric devices including that of the FDA's Office of Pediatric Therapeutics, the recent FDA report Strengthening Our National System for Medical Device Postmarket Surveillance (
Our description of AMDEs by device category and patient characteristics is a first and necessary step to understanding the public health burden associated with device use in the pediatric population. Further developments in refined coding and device designation (eg, UDI systems) are needed to refine these estimates.
Acknowledgments
The authors thank Amy Liu, with the Data Management Center, and Colleen Mangeot, with the Biostatistical Consulting Unit in the Division of Epidemiology and Biostatistics, for their assistance with the data pull and creation of the analytic dataset. The authors also thank Lilliam Ambroggio, PhD, and Joshua Schaffzin, MD, PhD, for their thoughtful review of draft manuscripts.
Disclosures: Dr. Brady was supported by funds from the Bureau of Health Professions (BHPr), Health Resources and Services Administration (HRSA), and Department of Health and Human Services (DHHS) under grant T32 HP10027. This project was supported by cooperative agreement number U18 HS016957‐03 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The study sponsors had no role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication. The opinions and assertions presented herein are the private views of the authors and are not to be construed as conveying either an official endorsement or criticism by the US Department of Health and Human Services, The Public Health Service, or the US Food and Drug Administration.
Children with complex chronic conditions comprise an increasing proportion of hospital admissions, readmissions, and resource use.[1, 2, 3] Dependence on technology or medical devices is a frequent characteristic of children in this group.[4] Adverse medical device events (AMDEs) are estimated to occur in as many as 8% of all adult admissions, depending on the methods used to identify them.[5] These events may result in hospitalizations or complicate hospital stays. To date, however, the burden of AMDEs among hospitalized children is little described, even though children may be at increased risk for device events as compared to adults.[6] Although some medical devices are intended solely or primarily for use with children, most devices used with children have been initially developed for, tested with, and most frequently employed to treat adults.[6] Assessing the continued safety and effectiveness of medical devices marketed in the Unites States is the responsibility of the Center for Devices and Radiologic Health of the US Food and Drug Administration (FDA). Its existing mechanisms for postmarket device surveillance rely primarily on passive reporting systems and specific observational studies.[7]
The objective of this study was to utilize administrative data from children's hospitals to explore the prevalence and nature of AMDEs in tertiary care children's hospitals that treat significant numbers of children with complex needs requiring medical devices.
METHODS
Data were obtained from the Pediatric Health Information System (PHIS), an administrative database containing inpatient data from 44 not‐for‐profit, tertiary care, pediatric hospitals affiliated with the Children's Hospital Association. Data are deidentified at the time of submission, and are subjected to a number of reliability and validity checks.[8] Individual admission records have both a deidentified visit identification (ID) and patient ID, allowing for linkage of multiple admissions by the same patient.
AMDEs were defined by International Classification of Diseases, Ninth Revision (ICD‐9) codes, using a methodology developed by Samore et al., who identified a set of such codes that specified devices in their definitions and therefore were considered to have a high likelihood of indicating a device problem (see Supporting Information, Table S1, in the online version of this article).[5] The diagnosis codes were grouped into device categories (eg, nervous system, orthopedic, cardiac).
From the 44 hospitals, the primary study cohort consisted of any patient with an admission between January 1, 2004 and December 31, 2011 with 1 AMDE ICD‐9 code as a primary or secondary diagnosis.
Descriptive statistics for patient demographics and visit characteristics of AMDE admissions were generated and stratified by device category. We reported these as counts and percentages for categorical variables and as median and interquartile range for length of stay. We also reported on how frequently patients with AMDEs have a top 10 most common diagnosis and top 10 most common procedure during the AMDE admission. We also reported the presence or absence of a complex chronic condition.[9] We generated the list of most common principal diagnoses and procedures by a separate query of PHIS from 2004 to 2009. Our top 10 most common diagnoses included ICD‐9 codes 486 (pneumonia), 466.11 (acute bronchiolitis due to respiratory syncytial virus), V58.11 (chemotherapy encounter), 493.92 (asthma exacerbation), 493.91 (asthma with status asthmaticus), 466.19 (acute bronchiolitis due to other organism), 780.39 (other convulsions), 540.9 (acute appendicitis), 282.62 (sickle cell disease with crisis), and 276.51 (dehydration). Our top 10 most common procedures included ICD‐9 codes 38.93 (venous catheterization), 03.31 (spinal tap), 99.04 (packed blood cell transfusion), 99.15 (parenteral nutrition), 99.25 (cancer chemotherapy), 96.71 (invasive mechanical ventilation, 96 hours), 96.04 (endotracheal intubation), 96.72 (invasive mechanical ventilation,95 hours), 96.6 (enteral nutrition), and 99.05 (platelet transfusion). Analyses were performed using SAS Enterprise Guide version 4.2 for Windows (SAS Institute, Cary, NC).
This study was approved by Cincinnati Children's Hospital Medical Center Institutional Review Board.
RESULTS
Of the 4,115,755 admissions during the study period, 136,465 (3.3%) had at least 1 AMDE. Over our study period, AMDEs were associated with a mean 17,058 inpatient stays annually. The number of AMDE‐related admissions decreased the last 4 years of our study period despite generally increasing admissions at PHIS hospitals (Figure 1). For 55% of the admissions (75,206/136,465), this AMDE code represented the primary diagnosis. Of these visits with a primary AMDE diagnosis, 39,874 (53%) were related to nervous system devices. The visits associated with AMDEs were comprised of 88,908 unique patients, 55% of whom were male (Table 1). The median age on admission was 6 years, and the interquartile range was 1 to 14 years of age.

| Total, N=88,908 | |
|---|---|
| Age at first admission | |
| 2 years | 35,160 (40.0%) |
| 35 years | 9,352 (10.5%) |
| 611 years | 16,148 (18.2%) |
| 1218 years | 22,483 (25.3%) |
| 19 years | 5,765 (6.5%) |
| Gender | |
| Male | 49,172 (55.3%) |
| Female | 39,730 (44.7%) |
| Race | |
| White | 59,842 (67.3%) |
| Black | 14,747 (16.6%) |
| Asian | 1,910 (2.2%) |
| American Indian | 900 (1.0%) |
| Other | 8,732 (9.8%) |
| Missing | 2,777 (3.1%) |
| Number of admissions by patient | |
| 1 | 66,814 (75.1%) |
| 2 | 12,520 (14.1%) |
| 3 | 4,504 (5.1%) |
| 4 | 5,071 (5.7%) |
Among admissions with AMDEs, 2.9% ended in death. The mortality was 0.5% when an AMDE was the primary diagnosis and 5.7% when the AMDE was a secondary diagnosis. The median length of inpatient stays was 6 days, with an interquartile range of 2 to 17 days.
Vascular access AMDEs were the most common event associated with admissions (26.6%), followed by nervous system devices (17.8%) (Table 2). The majority (75.5%) of patients admitted with AMDEs had a complex chronic condition. Less than half (46.8%) of AMDE admissions had an associated code for 1 of the 10 most common principal procedures. A minority (14.3%) of admissions had an associated ICD‐9 code for 1 of the top 10 most common principal diagnoses.
| Device Category | Admissions, n=136,465 | Presence of Top 10 Most Common Principal Procedures, n=63,801 | Presence of Top 10 Most Common Principal Diagnoses, n=19,472 | Presence of 1 Complex Chronic Condition, n=103,003 |
|---|---|---|---|---|
| ||||
| Only 1 AMDE diagnosis | ||||
| Vascular access | 36,257 (26.6%) | 26,658 (41.8%) | 6,518 (33.5%) | 26,022 (25.3%) |
| Nervous system | 24,243 (17.8%) | 4,266 (6.7%) | 3,567 (18.3%) | 21,516 (20.9%) |
| Unspecified device | 21,222 (15.6%) | 11,368 (17.8%) | 2,512 (12.9%) | 13,826 (13.4%) |
| Cardiac | 4,384 (3.2%) | 1,959 (3.1%) | 309 (1.6%) | 3,962 (3.8%) |
| Orthopedic | 3,064 (2.2%) | 874 (1.4%) | 179 (0.9%) | 1,235 (1.2%) |
| Dialysis | 2,426 (1.8%) | 836 (1.3%) | 281 (1.4%) | 1,462 (1.4%) |
| Genitourinary | 1,165 (0.9%) | 388 (0.6%) | 166 (0.9%) | 668 (0.6%) |
| Prosthetic cardiac valve | 518 (0.4%) | 236 (0.4%) | 33 (0.2%) | 411 (0.4%) |
| Urologic catheters | 379 (0.3%) | 228 (0.4%) | 93 (0.5%) | 223 (0.2%) |
| Defibrillator | 197 (0.1%) | 11 (0.02%) | 4 (0.02%) | 18 (0.02%) |
| Ocular | 3 (0.002%) | 1 (0.002%) | 1 (0.005%) | 1 (0.001%) |
| Only 1 AMDE diagnosis subtotal | 93,861 (68.8%) | 46,825 (73.4%) | 13,663 (70.2%) | 69,344 (67.3%) |
| 2 AMDE diagnoses | 39,557 (29.0%) | 15,003 (23.5%) | 5,312 (27.3%) | 31,091 (30.2%) |
| >2 AMDE diagnoses | 3,047 (2.2%) | 1,973 (3.1%) | 497 (2.6%) | 2,568 (2.5%) |
DISCUSSION
To our knowledge, our study is the first to report the burden of AMDEs among children requiring hospitalization. AMDEs are common in this population of children cared for at tertiary care children's hospitals, accounting for or complicating 3.3% of inpatient stays in these 44 hospitals. AMDEs were associated with a mean of >17,000 total visits per year. Vascular access devices and nervous system devices were the most common device categories linked to AMDEs. Similar to published literature, we found that the youngest children accounted for the highest proportion of AMDEs.[10, 11]
The majority (>75%) of children with an AMDE admission had diagnoses indicating complex chronic conditions during the admission. Over a partially overlapping study period, Feudtner and colleagues found 25.2% of patients admitted to PHIS hospitals had complex chronic conditions.[12] This finding, combined with the uncommon association of the most prevalent diagnoses and procedures, suggests that the burden of AMDEs falls disproportionately on this population of children. Death occurred considerably less commonly when AMDE diagnosis was the primary versus a secondary diagnosis (0.5% vs 5.7%). This finding likely illustrates 2 distinct populations: children with an AMDE that causes admission who have a relatively low risk of mortality and a second group who have AMDE‐complicated hospitalizations that may have an already high risk of mortality.
Our findings complement those of Wang and colleagues who employed the National Electronic Injury Surveillance System All Injury Program database to provide national estimates of medical device‐associated adverse events.[11] Importantly, this group used a different population (patients presenting to the emergency department) and a different methodology. These authors reported on device‐associated events, as they did not collect information to discriminate the device's role in the event. A walker that malfunctioned leading to patient injury would be a device‐related event; however, a patient who has a walker suffering a fall would be device‐associated, even if the walker's role in the injury was uncertain. We believe our methodology, established by Samore et al., more accurately identifies device‐related events.[5] Wang et al. found that 6.3% of pediatric patients who presented to emergency departments with medical device‐associated events were admitted to the hospital.[11] This resulted in national estimates of 9,082 events with 95% confidence intervals of 2,990 to 25,373 hospitalizations. Our findings of >17,000 AMDE‐related inpatient stays per annum included not only AMDEs leading to admissions but also those that were complications during stays.
Our study has several limitations, most related to the possibility of misclassification present in administrative data. Our approach only captured events that led to or complicated admissions. We suspect that ICD‐9 codes likely missed some AMDEs and that our estimates may therefore under‐represent this problem in our population. Future studies should compare our methodology, which has produced the first across‐center estimates of AMDE admissions, to alternative event capture techniques. We were unable to determine which events were present on admission and which complicated hospital stays, and it is likely that differing interventions would be required to reduce these 2 types of AMDEs. Another important limitation is that the PHIS database, comprised of data on children receiving care at tertiary academic medical centers with large numbers of pediatric subspecialists, is not representative of the population of children overall. The individual ICD‐9 codes for AMDEs are sufficiently nonspecific to limit the ability to characterize device events from administrative data alone. The high prevalence of unspecified device‐related admissions is an additional limitation. Although the estimates of these types of AMDEs are important in describing the frequency of these events, the unspecified category limits the ability to fully stratify based on device type and then implement monitoring strategies and interventions based on each.
To our knowledge, this study is the first multicenter analysis of the spectrum of pediatric AMDEs in hospitalized children. The AMDE prevalence is substantial, and the burden of these events largely falls on children with complex chronic conditions. Despite its limitations, this study complements recent efforts to enhance postmarket surveillance of pediatric devices including that of the FDA's Office of Pediatric Therapeutics, the recent FDA report Strengthening Our National System for Medical Device Postmarket Surveillance (
Our description of AMDEs by device category and patient characteristics is a first and necessary step to understanding the public health burden associated with device use in the pediatric population. Further developments in refined coding and device designation (eg, UDI systems) are needed to refine these estimates.
Acknowledgments
The authors thank Amy Liu, with the Data Management Center, and Colleen Mangeot, with the Biostatistical Consulting Unit in the Division of Epidemiology and Biostatistics, for their assistance with the data pull and creation of the analytic dataset. The authors also thank Lilliam Ambroggio, PhD, and Joshua Schaffzin, MD, PhD, for their thoughtful review of draft manuscripts.
Disclosures: Dr. Brady was supported by funds from the Bureau of Health Professions (BHPr), Health Resources and Services Administration (HRSA), and Department of Health and Human Services (DHHS) under grant T32 HP10027. This project was supported by cooperative agreement number U18 HS016957‐03 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The study sponsors had no role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication. The opinions and assertions presented herein are the private views of the authors and are not to be construed as conveying either an official endorsement or criticism by the US Department of Health and Human Services, The Public Health Service, or the US Food and Drug Administration.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- , , , et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647–655.
- , , , , , . Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126(4):638–646.
- , , , et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538.
- , , , et al. Surveillance of medical device‐related hazards and adverse events in hospitalized patients. JAMA. 2004;291(3):325–334.
- Institute of Medicine (U.S.). Committee on Postmarket Surveillance of Pediatric Medical Devices. , . Safe medical devices for children. Washington, DC: National Academies Press; 2006.
- , . Pharmacovigilance. 2nd ed. Chichester, England ; Hoboken, NJ: John Wiley 2007.
- , , , . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055.
- , , . Pediatric deaths attributable to complex chronic conditions: a population‐based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 pt 2):205–209.
- , , , et al. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. J Neurosurg Pediatr. 2009;4(2):156–165.
- , , , et al. Emergency department visits for medical device‐associated adverse events among children. Pediatrics. 2010;126(2):247–259.
- , , , et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286–293.
- , . Unique device identification in the service of public health. N Engl J Med. 2012;367(17):1583–1585.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- , , , et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647–655.
- , , , , , . Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126(4):638–646.
- , , , et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538.
- , , , et al. Surveillance of medical device‐related hazards and adverse events in hospitalized patients. JAMA. 2004;291(3):325–334.
- Institute of Medicine (U.S.). Committee on Postmarket Surveillance of Pediatric Medical Devices. , . Safe medical devices for children. Washington, DC: National Academies Press; 2006.
- , . Pharmacovigilance. 2nd ed. Chichester, England ; Hoboken, NJ: John Wiley 2007.
- , , , . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055.
- , , . Pediatric deaths attributable to complex chronic conditions: a population‐based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 pt 2):205–209.
- , , , et al. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. J Neurosurg Pediatr. 2009;4(2):156–165.
- , , , et al. Emergency department visits for medical device‐associated adverse events among children. Pediatrics. 2010;126(2):247–259.
- , , , et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics. 2009;123(1):286–293.
- , . Unique device identification in the service of public health. N Engl J Med. 2012;367(17):1583–1585.
International travel - Focus on timely intervention
Many of your patients will head for international destinations this summer, where they may be exposed to infectious diseases and other health risks they normally do not encounter in the United States.
For the majority of patients, these exposures will be brief; however, several may be extended due to study abroad or parental job relocation. More and more adolescents also are traveling to resource-limited areas doing volunteer work or adventure travel, and many are residing with host families. Children with chronic diseases pose concerns directly related to their underlying conditions, susceptibility, and availability of medical care in the host country. While most international travel plans are made at least 3 months in advance, health precautions such as immunizations and preventive medication often are not considered as travel plans are being finalized. If you are lucky, your patients will have mentioned their plans to you prior to finalizing their trips. You may receive a call at the last minute for assistance in helping to prepare them for a safe and healthy journey.
The U.S. Office of Travel & Tourism reports that slightly more than 60 million Americans traveled outside of the United States in 2012, with 28.5 million of the final destinations being overseas. Children accounted for approximately 2.4 million travelers. While tourism was the most common reason for travel, children were more likely to be visiting friends and relatives (VFR). Studies have revealed significantly increased health risks among VFR travelers, who often stay in private homes and in less-developed areas, compared with vacationers or business travelers who are more likely to be staying in hotels and in urban areas (Pediatrics 2010;125:e1072-80).
Is it really necessary to seek pretravel advice? Some travelers are not convinced. To facilitate this discussion, I thought I would share a recent call.
You are informed via voicemail that a 3-year-old is traveling with his family to Madras, India, for 8 weeks. He is visiting relatives, and the family may visit rural areas. The accommodations are air conditioned and the family is departing in 5 days! They would like to schedule an appointment immediately. What can you do?
Vital information has already been provided. The destination, type of accommodations, activities, duration of stay, and that the patient is a VFR are all important details when making vaccine and other recommendations. First, determine if the child’s routine immunizations are up to date. Next, determine the potential exposures for this patient, and identify vaccine-preventable and nonpreventable diseases. If there is a travel medicine specialist in your area who also sees children, you can refer the patient. If one is not readily available or you prefer to manage the patient, a great resource is the Centers for Disease Control and Prevention Traveler's Health site.
Vaccine preventable diseases include hepatitis A, hepatitis B, Japanese encephalitis, polio, rabies, typhoid, and influenza. Nonvaccine preventable diseases include chikungunya and dengue fevers. Avian influenza, malaria, tuberculosis, and traveler’s diarrhea are also cause for concern.
If you determine the routine immunizations are up to date, remember that measles is still a concern in many countries, and current U.S. recommendations state that all children at least 12 months of age should have two doses prior to leaving the United States. Although routinely administered at 4 years of age, the second dose of MMR can be administered as early as 4 weeks after the first dose. Those aged 6-11 months should have one dose prior to leaving the country. The remaining two doses should be administered at the usual time. Therefore, a total of three doses will be required to complete the series. Since the immunizations are up to date, this patient will also be protected against hepatitis A and B in addition to polio. Hepatitis A is the most common vaccine preventable disease acquired by travelers.
Rabies is prevalent in India, and all animal bites should be taken seriously. Because the patient is in a major urban area, access to both rabies vaccine and immunoglobulin should not be a concern. Japanese encephalitis will be circulating (May-October), but is usually found in rural agricultural areas. Mosquito precautions utilizing DEET (30%) on exposed areas or Permethrine-containing sprays on clothes to repel mosquitoes and ticks should be emphasized if travel to rural areas occurs. Vaccines for rabies and Japanese encephalitis would not be recommended for this patient. If the itinerary were different, they may be considered. Ixiaro, an inactivated Japanese encephalitis (JE) vaccine was approved for use in children as young as 2 months of age in May 2013. Previously, it was approved for use only in those at least 17 years of age in the United States. Both rabies and JE require a minimum of 21 and 28 days, respectively, to complete, and JE should be completed at least 1 week prior to exposure.
Typhoid fever (enteric fever) occurs worldwide, with an estimated 22 million cases annually. In 2012, 343 cases were reported in the United States, most of which were in recent travelers. The risk for typhoid fever is highest for travelers to southern Asia (6-30 times higher) than for all other destinations (Centers for Disease Control and Prevention. CDC Health Information for International Travel 2012. New York: Oxford University Press; 2012). Two types of vaccine are available: an oral, live attenuated vaccine for those at least 6 years of age and an injectable polysaccharide vaccine for those at least 2 years of age. In this case there is only one option, the injectable vaccine. Ideally, it should be administered at least 2 weeks prior to travel. Although this patient will not have optimal benefit of vaccine for at least 2 weeks, he will be there an additional 6 weeks, staying with friends and relatives, and is traveling to a high-risk country. Vaccine administration is recommended, and the parent should be fully informed when maximum benefit will occur. Food and water precautions are essential, especially during the first 2 weeks.
Precautions such as consumption of only boiled or bottled water, avoidance of undercooked or raw meat and seafood, and avoidance of raw fruit and vegetables to minimize acquisition of traveler’s diarrhea should be discussed. Antimicrobials also can be provided.
Options for malaria prophylaxis are limited due to the ensuing departure date and the child’s age. Atovaquone-Proguanil can be prescribed because it can be initiated 1-2 days prior to departure. It is taken daily while in India and for 1 week after return. He is too young for doxycycline. Mefloquine, administered weekly, should begin at least 2 weeks prior to exposure, so it is not an option. There is no role for chloroquine because chloroquine-resistant malaria is present in this country. In contrast to malaria, where mosquitoes usually feed dusk to dawn, chikungunya and dengue fever are transmitted by mosquitoes during the daytime.
No specific prevention for tuberculosis is available. Avoidance of persons with chronic cough or known disease is recommended.
It can be challenging for a busy practitioner to stay abreast of the latest developments in non–routinely administered vaccines, disease outbreaks, or country-specific entry requirements. Many vaccines, such as those against typhoid or rabies, are not routinely available in the patient’s medical home.
Ideally, patients planning international travel should be referred to a travel medicine clinic 1 month prior to travel. Some vaccines take up to 2 weeks to become effective, while others – such as yellow fever – should be administered at least 10 days prior to travel. However, interventions are still available for the last-minute patient, as in this case. Counseling for a variety of issues is provided. It’s not just about the vaccines.
International travel among children and adolescents will continue to rise. It behooves every primary care practitioner to develop a system to determine the summertime plans/needs of their patients. Not all travel medicine clinics provide services to children. It’s a good idea to find out which ones do in your area. You can always locate a clinic through the International Society of Travel Medicine and the Centers for Disease Control and Prevention.
While this call is not the norm, it occurs frequently. In contrast, another call for a 2-month photography trip to Uganda was received the same day. Departure was 6 weeks later!
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
Many of your patients will head for international destinations this summer, where they may be exposed to infectious diseases and other health risks they normally do not encounter in the United States.
For the majority of patients, these exposures will be brief; however, several may be extended due to study abroad or parental job relocation. More and more adolescents also are traveling to resource-limited areas doing volunteer work or adventure travel, and many are residing with host families. Children with chronic diseases pose concerns directly related to their underlying conditions, susceptibility, and availability of medical care in the host country. While most international travel plans are made at least 3 months in advance, health precautions such as immunizations and preventive medication often are not considered as travel plans are being finalized. If you are lucky, your patients will have mentioned their plans to you prior to finalizing their trips. You may receive a call at the last minute for assistance in helping to prepare them for a safe and healthy journey.
The U.S. Office of Travel & Tourism reports that slightly more than 60 million Americans traveled outside of the United States in 2012, with 28.5 million of the final destinations being overseas. Children accounted for approximately 2.4 million travelers. While tourism was the most common reason for travel, children were more likely to be visiting friends and relatives (VFR). Studies have revealed significantly increased health risks among VFR travelers, who often stay in private homes and in less-developed areas, compared with vacationers or business travelers who are more likely to be staying in hotels and in urban areas (Pediatrics 2010;125:e1072-80).
Is it really necessary to seek pretravel advice? Some travelers are not convinced. To facilitate this discussion, I thought I would share a recent call.
You are informed via voicemail that a 3-year-old is traveling with his family to Madras, India, for 8 weeks. He is visiting relatives, and the family may visit rural areas. The accommodations are air conditioned and the family is departing in 5 days! They would like to schedule an appointment immediately. What can you do?
Vital information has already been provided. The destination, type of accommodations, activities, duration of stay, and that the patient is a VFR are all important details when making vaccine and other recommendations. First, determine if the child’s routine immunizations are up to date. Next, determine the potential exposures for this patient, and identify vaccine-preventable and nonpreventable diseases. If there is a travel medicine specialist in your area who also sees children, you can refer the patient. If one is not readily available or you prefer to manage the patient, a great resource is the Centers for Disease Control and Prevention Traveler's Health site.
Vaccine preventable diseases include hepatitis A, hepatitis B, Japanese encephalitis, polio, rabies, typhoid, and influenza. Nonvaccine preventable diseases include chikungunya and dengue fevers. Avian influenza, malaria, tuberculosis, and traveler’s diarrhea are also cause for concern.
If you determine the routine immunizations are up to date, remember that measles is still a concern in many countries, and current U.S. recommendations state that all children at least 12 months of age should have two doses prior to leaving the United States. Although routinely administered at 4 years of age, the second dose of MMR can be administered as early as 4 weeks after the first dose. Those aged 6-11 months should have one dose prior to leaving the country. The remaining two doses should be administered at the usual time. Therefore, a total of three doses will be required to complete the series. Since the immunizations are up to date, this patient will also be protected against hepatitis A and B in addition to polio. Hepatitis A is the most common vaccine preventable disease acquired by travelers.
Rabies is prevalent in India, and all animal bites should be taken seriously. Because the patient is in a major urban area, access to both rabies vaccine and immunoglobulin should not be a concern. Japanese encephalitis will be circulating (May-October), but is usually found in rural agricultural areas. Mosquito precautions utilizing DEET (30%) on exposed areas or Permethrine-containing sprays on clothes to repel mosquitoes and ticks should be emphasized if travel to rural areas occurs. Vaccines for rabies and Japanese encephalitis would not be recommended for this patient. If the itinerary were different, they may be considered. Ixiaro, an inactivated Japanese encephalitis (JE) vaccine was approved for use in children as young as 2 months of age in May 2013. Previously, it was approved for use only in those at least 17 years of age in the United States. Both rabies and JE require a minimum of 21 and 28 days, respectively, to complete, and JE should be completed at least 1 week prior to exposure.
Typhoid fever (enteric fever) occurs worldwide, with an estimated 22 million cases annually. In 2012, 343 cases were reported in the United States, most of which were in recent travelers. The risk for typhoid fever is highest for travelers to southern Asia (6-30 times higher) than for all other destinations (Centers for Disease Control and Prevention. CDC Health Information for International Travel 2012. New York: Oxford University Press; 2012). Two types of vaccine are available: an oral, live attenuated vaccine for those at least 6 years of age and an injectable polysaccharide vaccine for those at least 2 years of age. In this case there is only one option, the injectable vaccine. Ideally, it should be administered at least 2 weeks prior to travel. Although this patient will not have optimal benefit of vaccine for at least 2 weeks, he will be there an additional 6 weeks, staying with friends and relatives, and is traveling to a high-risk country. Vaccine administration is recommended, and the parent should be fully informed when maximum benefit will occur. Food and water precautions are essential, especially during the first 2 weeks.
Precautions such as consumption of only boiled or bottled water, avoidance of undercooked or raw meat and seafood, and avoidance of raw fruit and vegetables to minimize acquisition of traveler’s diarrhea should be discussed. Antimicrobials also can be provided.
Options for malaria prophylaxis are limited due to the ensuing departure date and the child’s age. Atovaquone-Proguanil can be prescribed because it can be initiated 1-2 days prior to departure. It is taken daily while in India and for 1 week after return. He is too young for doxycycline. Mefloquine, administered weekly, should begin at least 2 weeks prior to exposure, so it is not an option. There is no role for chloroquine because chloroquine-resistant malaria is present in this country. In contrast to malaria, where mosquitoes usually feed dusk to dawn, chikungunya and dengue fever are transmitted by mosquitoes during the daytime.
No specific prevention for tuberculosis is available. Avoidance of persons with chronic cough or known disease is recommended.
It can be challenging for a busy practitioner to stay abreast of the latest developments in non–routinely administered vaccines, disease outbreaks, or country-specific entry requirements. Many vaccines, such as those against typhoid or rabies, are not routinely available in the patient’s medical home.
Ideally, patients planning international travel should be referred to a travel medicine clinic 1 month prior to travel. Some vaccines take up to 2 weeks to become effective, while others – such as yellow fever – should be administered at least 10 days prior to travel. However, interventions are still available for the last-minute patient, as in this case. Counseling for a variety of issues is provided. It’s not just about the vaccines.
International travel among children and adolescents will continue to rise. It behooves every primary care practitioner to develop a system to determine the summertime plans/needs of their patients. Not all travel medicine clinics provide services to children. It’s a good idea to find out which ones do in your area. You can always locate a clinic through the International Society of Travel Medicine and the Centers for Disease Control and Prevention.
While this call is not the norm, it occurs frequently. In contrast, another call for a 2-month photography trip to Uganda was received the same day. Departure was 6 weeks later!
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
Many of your patients will head for international destinations this summer, where they may be exposed to infectious diseases and other health risks they normally do not encounter in the United States.
For the majority of patients, these exposures will be brief; however, several may be extended due to study abroad or parental job relocation. More and more adolescents also are traveling to resource-limited areas doing volunteer work or adventure travel, and many are residing with host families. Children with chronic diseases pose concerns directly related to their underlying conditions, susceptibility, and availability of medical care in the host country. While most international travel plans are made at least 3 months in advance, health precautions such as immunizations and preventive medication often are not considered as travel plans are being finalized. If you are lucky, your patients will have mentioned their plans to you prior to finalizing their trips. You may receive a call at the last minute for assistance in helping to prepare them for a safe and healthy journey.
The U.S. Office of Travel & Tourism reports that slightly more than 60 million Americans traveled outside of the United States in 2012, with 28.5 million of the final destinations being overseas. Children accounted for approximately 2.4 million travelers. While tourism was the most common reason for travel, children were more likely to be visiting friends and relatives (VFR). Studies have revealed significantly increased health risks among VFR travelers, who often stay in private homes and in less-developed areas, compared with vacationers or business travelers who are more likely to be staying in hotels and in urban areas (Pediatrics 2010;125:e1072-80).
Is it really necessary to seek pretravel advice? Some travelers are not convinced. To facilitate this discussion, I thought I would share a recent call.
You are informed via voicemail that a 3-year-old is traveling with his family to Madras, India, for 8 weeks. He is visiting relatives, and the family may visit rural areas. The accommodations are air conditioned and the family is departing in 5 days! They would like to schedule an appointment immediately. What can you do?
Vital information has already been provided. The destination, type of accommodations, activities, duration of stay, and that the patient is a VFR are all important details when making vaccine and other recommendations. First, determine if the child’s routine immunizations are up to date. Next, determine the potential exposures for this patient, and identify vaccine-preventable and nonpreventable diseases. If there is a travel medicine specialist in your area who also sees children, you can refer the patient. If one is not readily available or you prefer to manage the patient, a great resource is the Centers for Disease Control and Prevention Traveler's Health site.
Vaccine preventable diseases include hepatitis A, hepatitis B, Japanese encephalitis, polio, rabies, typhoid, and influenza. Nonvaccine preventable diseases include chikungunya and dengue fevers. Avian influenza, malaria, tuberculosis, and traveler’s diarrhea are also cause for concern.
If you determine the routine immunizations are up to date, remember that measles is still a concern in many countries, and current U.S. recommendations state that all children at least 12 months of age should have two doses prior to leaving the United States. Although routinely administered at 4 years of age, the second dose of MMR can be administered as early as 4 weeks after the first dose. Those aged 6-11 months should have one dose prior to leaving the country. The remaining two doses should be administered at the usual time. Therefore, a total of three doses will be required to complete the series. Since the immunizations are up to date, this patient will also be protected against hepatitis A and B in addition to polio. Hepatitis A is the most common vaccine preventable disease acquired by travelers.
Rabies is prevalent in India, and all animal bites should be taken seriously. Because the patient is in a major urban area, access to both rabies vaccine and immunoglobulin should not be a concern. Japanese encephalitis will be circulating (May-October), but is usually found in rural agricultural areas. Mosquito precautions utilizing DEET (30%) on exposed areas or Permethrine-containing sprays on clothes to repel mosquitoes and ticks should be emphasized if travel to rural areas occurs. Vaccines for rabies and Japanese encephalitis would not be recommended for this patient. If the itinerary were different, they may be considered. Ixiaro, an inactivated Japanese encephalitis (JE) vaccine was approved for use in children as young as 2 months of age in May 2013. Previously, it was approved for use only in those at least 17 years of age in the United States. Both rabies and JE require a minimum of 21 and 28 days, respectively, to complete, and JE should be completed at least 1 week prior to exposure.
Typhoid fever (enteric fever) occurs worldwide, with an estimated 22 million cases annually. In 2012, 343 cases were reported in the United States, most of which were in recent travelers. The risk for typhoid fever is highest for travelers to southern Asia (6-30 times higher) than for all other destinations (Centers for Disease Control and Prevention. CDC Health Information for International Travel 2012. New York: Oxford University Press; 2012). Two types of vaccine are available: an oral, live attenuated vaccine for those at least 6 years of age and an injectable polysaccharide vaccine for those at least 2 years of age. In this case there is only one option, the injectable vaccine. Ideally, it should be administered at least 2 weeks prior to travel. Although this patient will not have optimal benefit of vaccine for at least 2 weeks, he will be there an additional 6 weeks, staying with friends and relatives, and is traveling to a high-risk country. Vaccine administration is recommended, and the parent should be fully informed when maximum benefit will occur. Food and water precautions are essential, especially during the first 2 weeks.
Precautions such as consumption of only boiled or bottled water, avoidance of undercooked or raw meat and seafood, and avoidance of raw fruit and vegetables to minimize acquisition of traveler’s diarrhea should be discussed. Antimicrobials also can be provided.
Options for malaria prophylaxis are limited due to the ensuing departure date and the child’s age. Atovaquone-Proguanil can be prescribed because it can be initiated 1-2 days prior to departure. It is taken daily while in India and for 1 week after return. He is too young for doxycycline. Mefloquine, administered weekly, should begin at least 2 weeks prior to exposure, so it is not an option. There is no role for chloroquine because chloroquine-resistant malaria is present in this country. In contrast to malaria, where mosquitoes usually feed dusk to dawn, chikungunya and dengue fever are transmitted by mosquitoes during the daytime.
No specific prevention for tuberculosis is available. Avoidance of persons with chronic cough or known disease is recommended.
It can be challenging for a busy practitioner to stay abreast of the latest developments in non–routinely administered vaccines, disease outbreaks, or country-specific entry requirements. Many vaccines, such as those against typhoid or rabies, are not routinely available in the patient’s medical home.
Ideally, patients planning international travel should be referred to a travel medicine clinic 1 month prior to travel. Some vaccines take up to 2 weeks to become effective, while others – such as yellow fever – should be administered at least 10 days prior to travel. However, interventions are still available for the last-minute patient, as in this case. Counseling for a variety of issues is provided. It’s not just about the vaccines.
International travel among children and adolescents will continue to rise. It behooves every primary care practitioner to develop a system to determine the summertime plans/needs of their patients. Not all travel medicine clinics provide services to children. It’s a good idea to find out which ones do in your area. You can always locate a clinic through the International Society of Travel Medicine and the Centers for Disease Control and Prevention.
While this call is not the norm, it occurs frequently. In contrast, another call for a 2-month photography trip to Uganda was received the same day. Departure was 6 weeks later!
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].