User login
“Patchy” pneumonia
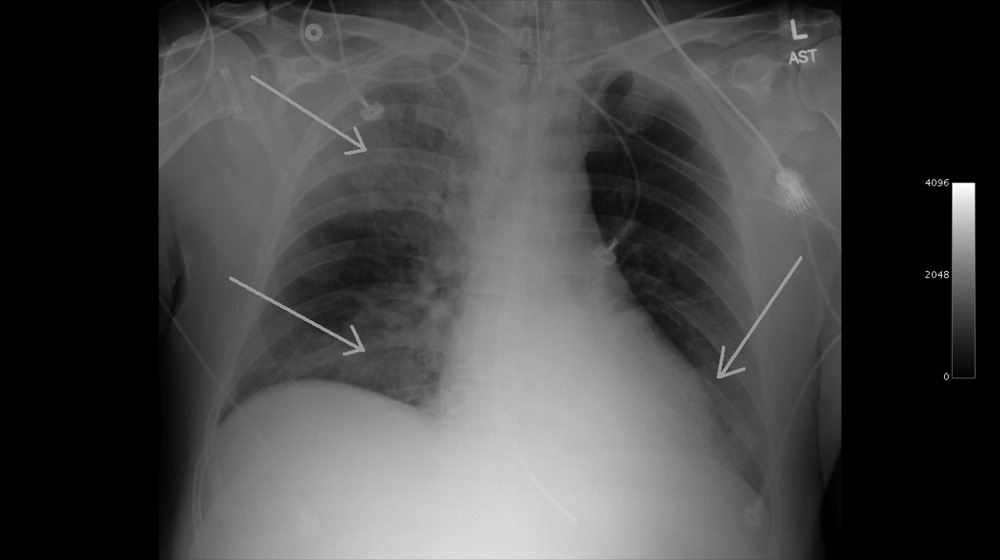
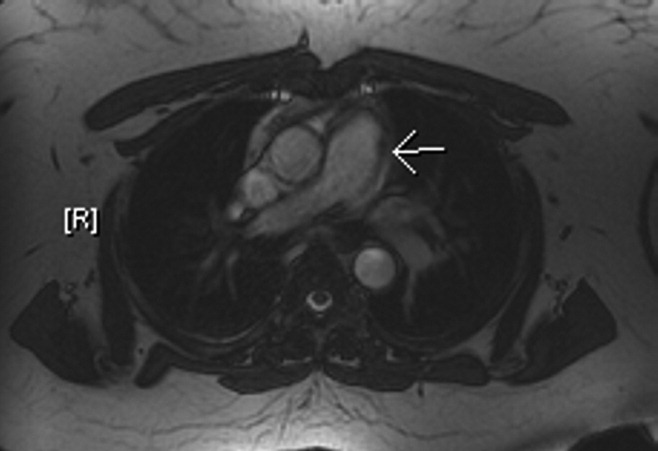
A 49‐year‐old retired air‐force officer presented with productive cough lasting 1 week. He was in good health except for back pain after a motor vehicle accident, 2 years ago, for which he took unspecified pain medications. His condition rapidly deteriorated necessitating mechanical ventilation. Chest x‐ray showed multilobar pneumonia (Figure 1). Blood and sputum cultures grew Serratia marcescens. Chest computed tomography (CT)‐scan showed multilobar involvement with debris in his airways that was thought to be respiratory secretions (Figure 2, arrows). He remained intubated for 2 weeks before his clinical status improved enough to permit extubation. Immediately following extubation, he coughed up a ball of crumpled, plastic material. His condition subsequently improved dramatically with complete radiological resolution of his pneumonia. On analyzing the foreign body, it turned out to be a fenestrated fentanyl patch (Figure 3). The patient then reported that he often cut‐up used fentanyl patches and sucked on them for an extra high. In retrospect, the endobronchial debris was actually the patch straddling the carina and had prevented rapid recovery. Aspiration of unusual foreign bodies have been reported in the literature. This foreign body masqueraded as respiratory secretions on imaging preventing early detection. This clinical encounter brings to light yet another innovative, but potentially deadly, form of drug abuse. It also emphasizes the importance of early bronchoscopy in nonresolving pneumonias.

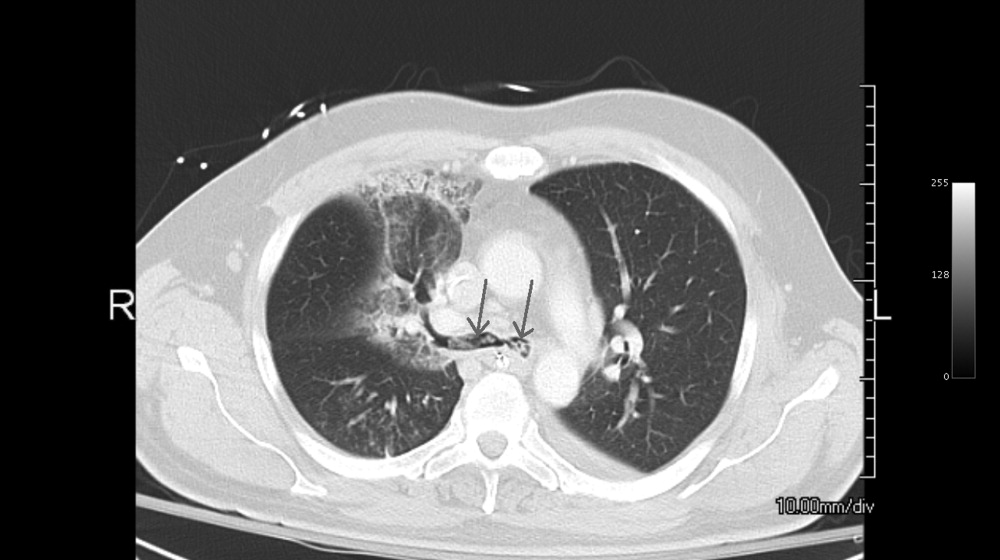
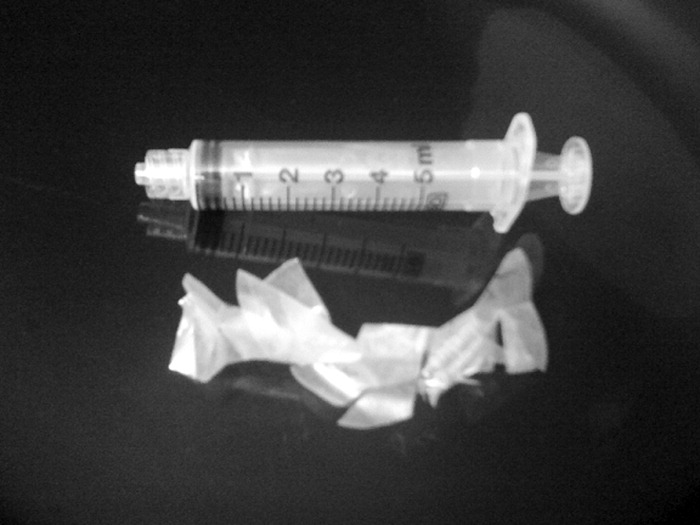
A 49‐year‐old retired air‐force officer presented with productive cough lasting 1 week. He was in good health except for back pain after a motor vehicle accident, 2 years ago, for which he took unspecified pain medications. His condition rapidly deteriorated necessitating mechanical ventilation. Chest x‐ray showed multilobar pneumonia (Figure 1). Blood and sputum cultures grew Serratia marcescens. Chest computed tomography (CT)‐scan showed multilobar involvement with debris in his airways that was thought to be respiratory secretions (Figure 2, arrows). He remained intubated for 2 weeks before his clinical status improved enough to permit extubation. Immediately following extubation, he coughed up a ball of crumpled, plastic material. His condition subsequently improved dramatically with complete radiological resolution of his pneumonia. On analyzing the foreign body, it turned out to be a fenestrated fentanyl patch (Figure 3). The patient then reported that he often cut‐up used fentanyl patches and sucked on them for an extra high. In retrospect, the endobronchial debris was actually the patch straddling the carina and had prevented rapid recovery. Aspiration of unusual foreign bodies have been reported in the literature. This foreign body masqueraded as respiratory secretions on imaging preventing early detection. This clinical encounter brings to light yet another innovative, but potentially deadly, form of drug abuse. It also emphasizes the importance of early bronchoscopy in nonresolving pneumonias.



A 49‐year‐old retired air‐force officer presented with productive cough lasting 1 week. He was in good health except for back pain after a motor vehicle accident, 2 years ago, for which he took unspecified pain medications. His condition rapidly deteriorated necessitating mechanical ventilation. Chest x‐ray showed multilobar pneumonia (Figure 1). Blood and sputum cultures grew Serratia marcescens. Chest computed tomography (CT)‐scan showed multilobar involvement with debris in his airways that was thought to be respiratory secretions (Figure 2, arrows). He remained intubated for 2 weeks before his clinical status improved enough to permit extubation. Immediately following extubation, he coughed up a ball of crumpled, plastic material. His condition subsequently improved dramatically with complete radiological resolution of his pneumonia. On analyzing the foreign body, it turned out to be a fenestrated fentanyl patch (Figure 3). The patient then reported that he often cut‐up used fentanyl patches and sucked on them for an extra high. In retrospect, the endobronchial debris was actually the patch straddling the carina and had prevented rapid recovery. Aspiration of unusual foreign bodies have been reported in the literature. This foreign body masqueraded as respiratory secretions on imaging preventing early detection. This clinical encounter brings to light yet another innovative, but potentially deadly, form of drug abuse. It also emphasizes the importance of early bronchoscopy in nonresolving pneumonias.



Language Barriers and Hospital Care
Forty‐five‐million Americans speak a language other than English and more than 19 million of these speak English less than very wellor are limited English proficient (LEP).1 The number of non‐English‐speaking and LEP people in the US has risen in recent decades, presenting a challenge to healthcare systems to provide high‐quality, patient‐centered care for these patients.2
For outpatients, language barriers are a fundamental contributor to gaps in health care. In the clinic setting, patients who do not speak English well have less access to a usual source of care and lower rates of physician visits and preventive services.36 Even when patients with language barriers do have access to care, they have poorer adherence, decreased comprehension of their diagnoses, decreased satisfaction with care, and increased medication complications.710
Few studies, however, have examined how language influences outcomes of hospital care. Compared to English‐speakers, patients who do not speak English well may experience longer lengths of stay,11 and have more adverse events while in the hospital.12 However, these previous studies have not investigated outcomes immediately post‐hospitalization, such as readmission rates and mortality, nor have they directly addressed the interaction between ethnicity and language.
To understand these questions, we analyzed data collected from a university‐based teaching hospital which cares for patients of diverse cultural and language backgrounds. Using these data, we examined how patients' primary language influenced hospital costs, length of stay (LOS), 30‐day readmission, and 30‐day mortality risk.
Patients and Methods
Patient Population and Setting
Our study examined patients admitted to the General Medicine Service at the University of California, San Francisco Medical Center (UCSF) between July 1, 2001 and June 30th, 2003, the time period during which UCSF participated in the Multicenter Hospitalist Trial (MHT) a prospective quasi‐randomized trial of hospitalist care for general medicine patients.13, 14
UCSF Moffitt‐Long Hospital is a 400‐bed urban academic medical center which provides services to the City and County of San Francisco, an ethnically and linguistically diverse area. UCSF employs staff language interpreters in Spanish, Chinese and Russian who travel to its many outpatient clinics, Comprehensive Cancer Center, Children's Hospital, as well as to Moffitt‐Long Hospital upon request; phone interpretation is also available when in‐person interpreters are not available, for off hours needs and for less common languages. During the period of this study there were no specific inpatient guidelines in place for use of interpretation services at UCSF, nor were there any specific interventions targeting LEP or non‐English speaking inpatients.
Patients were eligible for the MHT if they were 18 years of age or older and admitted at random to a hospitalist or non‐hospitalist physician (eg, outpatient general internist attending on average 1‐month/year); a minority of patients were cared for directly by their primary care physician while in the hospital, and were excluded. For purposes of our study, which merged MHT data with hospital administrative data on primary language, we further excluded all admissions for patients for whom primary language was missing (n = 5), whose listing was unknown or other language (n = 78), sign language (n = 3) or whose language was listed but was not one of the included languages (n = 258). Included languages were English, Chinese, Russian and Spanish. Because LOS and cost data were skewed, we excluded those admissions with the top 1% longest stays and the top 1% highest cost (n = 176); these exclusions did not alter the proportion of admissions across language and ethnicity. In addition, we excluded 102 admissions that were missing data on cost and 11 with costs <$500 and which were likely to be erroneous. Our research was approved by the UCSF Institutional Review Board.
Data Sources
We collected administrative data from Transition Systems Inc (TSI, Boston, MA) billing databases at UCSF as part of the MHT. These data include patient demographics, insurance, costs, ICD‐9CM diagnostic codes, admission and discharge dates in Uniform Bill 92 format. Patient mortality information was collected as part of the MHT using the National Death Index.14
Language data were collected from a separate patient‐registration database (STOR) at UCSF. Information on a patient's primary language is entered at the time each patient first registers at UCSF, whether for the index hospitalization or for prior clinic visits, and is based generally on patient self report. As part of our validation step, we cross‐checked 829 STOR language entries against patient reports and found 91% agreement with the majority of the errors classifying non‐English speakers as English‐speakers.
Measures
Predictor
Our primary language variable was derived using language designations collected from patient registration databases described above. Using these data we specified our key language groups as English, Chinese (Cantonese or Mandarin), Russian, or Spanish.
Outcomes
LOS and total cost of hospital stay for each hospitalization derived from administrative data sources. Readmissions were identified at the time patients were readmitted to UCSF (eg, flagged in administrative data). Mortality was determined by whether an individual patient with an admission in the database was recorded in the National Death Index as dead within 30‐days of admission.
Covariates
Additional covariates included age at admission, gender, ethnicity as recorded in registration databases (White, African American, Asian, Latino, Other), insurance, principal billing diagnosis, whether or not a patient received intensive care unit (ICU) care, type of admitting attending physician (Hospitalist/non‐Hospitalist), and an administrative Charlson comorbidity score.15 To collapse the principal diagnoses into categories, we used the Healthcare Cost and Utilization Project (HCUP)'s Clinical Classification System, which allowed us to classify each diagnosis in 1 of 14 generally accepted categories.16
Analysis
Statistical analyses were performed using STATA statistical software (STATACorp, Version 9, College Station, TX). We examined descriptive means and proportions for all variables, including sociodemographic, hospitalization, comorbidity and outcome variables. We compared English and non‐English speakers on all covariate and outcome variables using t‐tests for comparison of means and chi‐square for comparison of categorical variables.
It was not possible to fully test the language‐by‐ethnicity interactionwhether or not the impact of language varied by ethnic groupbecause many cells of the joint distribution were very sparse (eg, the sample contained very few non‐English‐speaking African Americans). Therefore, to better understand the influence of English vs. non‐English language usage across different ethnic groups, we created a combined language‐ethnicity predictor variable which categorized each subject first by language and then for the English‐speakers by ethnicity. For example, a Chinese, Spanish or Russian speaker would be categorized as such, and an English‐speaker could fall into the English‐White, English‐African American, English‐Asian or English‐Latino group. This allowed us to test whether there were any differences in language effects across the White, Asian, and Latino ethnicities, and any difference in ethnicity effects among English‐speakers.
Because cost and LOS were skewed, we used negative binomial models for LOS and log transformed costs. We performed a sensitivity analysis testing whether our results were robust to the exclusion of the admissions with the top 1% LOS and top 1% cost. We used logistic regression for the 30‐day readmission and mortality outcomes.
Our primary predictor was the language‐ethnicity variable described above. To determine the independent association between this predictor and our key outcomes, we then built models which included additional potential confounders selected either for face validity or because of observed confounding with other covariates. Our inclusion of potential confounders was limited by the variables available in the administrative database; thus, we were not able to pursue detailed analyses of communication and literacy factors and their interaction with our predictor or their independent impact on outcomes. Models also included a linear spline with a single knot at age 65 years as a further adjustment for age in Medicare recipients.1719 For the 30‐day readmission outcome model, we excluded those admissions for which the patient either died in the hospital or was discharged to hospice care. Within each model we tested the impact of a language barrier using custom contrasts. This allowed us to examine the language‐ethnicity effect aggregating all non‐English speakers compared to all English‐speakers, comparing each non‐English speaking group to all English‐speakers, comparing Chinese speakers to English‐speaking Asians and Spanish speakers to English‐speaking‐Latinos, as well as to test whether the effect of English language is the same across ethnicities.
Results
Admission Characteristics of the Sample
A total of 7023 patients were admitted to the General Medicine service, 5877 (84%) of whom were English‐speakers and 1146 (16%) non‐English‐speakers (Table 1). Overall, half of the admitted patients were women (50%), and the vast majority was insured (93%). The most common principal diagnoses were respiratory and gastrointestinal disorders. Only a small number of non‐English speakers 164 (14%) were recorded in the UCSF Interpreter Services database as having had any interaction with a professional staff interpreter during their hospitalization.
| English (n = 5877) n (%) | Non‐English (n = 1146) n (%) | |
|---|---|---|
| ||
| Socio‐economic variables | ||
| Language‐ethnicity | ||
| English | ||
| White | 3066 (52.2) | |
| African American | 1351 (23.0) | |
| Asian | 544 (9.3) | |
| Latino | 298 (5.1) | |
| Other | 618 (10.5) | |
| Chinese speakers | 584 (51.0) | |
| Spanish speakers | 272 (25.3) | |
| Russian speakers | 290 (23.7) | |
| Age mean (SD) (range 18‐105) | 58.8 (20.3) | 72.3 (15.5) |
| Gender | ||
| Male | 2967 (50.5) | 514 (44.8) |
| Female | 2910 (49.5) | 632 (55.2) |
| Insurance | ||
| Medicare | 2878 (49.0) | 800 (69.8) |
| Medicaid | 1201 (20.4) | 193 (16.8) |
| Commercial | 1358 (23.1) | 106 (9.3) |
| Charity/other | 440 (7.5) | 47 (4.1) |
| Hospitalization variables | ||
| Admitted to ICU | ||
| Yes | 721 (12.3) | 149 (13.0) |
| Attending physician | ||
| Hospitalist | 3950 (67.2) | 781 (68.2) |
| Comorbidity variables | ||
| Principal Diagnosis | ||
| Respiratory disorder | 1061 (18.1) | 225 (19.6) |
| Gastrointestinal disorder | 963 (16.4) | 205 (17.9) |
| Circulatory disorder | 613 (10.4) | 140 (12.2) |
| Endocrine/metabolism | 671 (11.4) | 80 (7.0) |
| Injury/poisoning | 475 (8.1) | 64 (5.6) |
| Malignancy | 395 (6.7) | 107 (9.3) |
| Renal/urinary disorder | 383 (6.5) | 108 (9.4) |
| Skin disorder | 278 (4.7) | 28 (2.9) |
| Infection/fatigue NOS | 206 (3.5) | 45 (3.4) |
| Blood disorder (non‐malignant) | 189 (3.2) | 38 (3.3) |
| Musculoskeletal/connective tissue disorder | 164 (2.8) | 33 (2.9) |
| Mental disorder/substance abuse | 171 (2.9) | 7 (0.6) |
| Nervous system/brain infection | 137 (2.3) | 26 (2.3) |
| Unclassified | 171 (2.9) | 40 (3.5) |
| Charlson Index score mean (SD) | 0.97 1.33 | 1.10 1.42 |
Among English speakers, Whites and African Americans were the most common ethnicities; however, more than 500 admissions were categorized as Asian ethnicity, and more than 600 as patients of other ethnicity. Close to 300 admissions were for Latinos. Among non‐English speakers, Chinese speakers had the largest number of admissions (n = 584), while Spanish and Russian speakers had similar numbers (n = 272 and 290 respectively).
Non‐English speakers were older, more likely to be female, more likely to be insured by Medicare, and more likely to have a higher comorbidity index score. While comorbidity scores were similar among non‐English speakers (Chinese 1.13 1.50; Russian 1.09 1.37; Spanish 1.06 1.30), they differed considerably among English speakers (White 0.94 1.29; African American 1.05 1.40; Asian 1.04 1.45; Latino 0.89 1.23; Other 0.91 1.29).
Hospital Outcome by Language‐Ethnicity Group (Table 2)
When aggregated together, non‐English speakers were somewhat more likely to be dead at 30‐days and have lower cost admissions; however, they did not differ from English speakers on LOS or readmission rates. While differences among disaggregated language‐ethnicity groups were not all statistically significant, English‐speaking Whites had the longest LOS (mean = 4.9 days) and highest costs (mean = $10,530). English‐speaking African Americans, Chinese and Spanish speakers had the highest 30‐day readmission rates; whereas, English‐speaking Latinos and Russian speakers had markedly lower 30‐day readmission rates (2.5% and 6.4%, respectively). Chinese speakers had the highest 30‐day mortality, followed by English speaking Whites and Asians.
| Language‐Ethnicity Groups | LOS* Mean #Days (SD) | Cost Mean Cost $ (SD) | 30‐Day Readmission, n (%) | 30‐Day Mortality, n (%) |
|---|---|---|---|---|
| ||||
| English speakers (all) | 4.7 (4.5) | 10,035 (15,041) | 648 (11.9) | 613 (10.4) |
| White | 4.9 (5.1) | 10,530 (15,894) | 322 (11.4) | 377 (12.3) |
| African American | 4.5 (4.8) | 9107 (13,314) | 227 (17.5) | 91 (6.7) |
| Asian | 4.3 (4.5) | 9933 (15,607) | 43 (8.8) | 67 (12.3) |
| Latino | 4.6 (4.8) | 9823 (14,113) | 7 (2.5) | 18 (6.0) |
| Other | 4.5 (4.8) | 9662 (14,016) | 49 (8.5) | 60 (9.7) |
| Non‐English speakers (all) | 4.5 (4.5) | 9515 (13,213) | 117 (11.0) | 147 (12.8) |
| Chinese speakers | 4.5 (4.6) | 9505 (12,841) | 69 (12.8) | 85 (14.6) |
| Spanish speakers | 4.5 (4.5) | 9115 (13,846) | 31 (12.0) | 28 (10.3) |
| Russian speakers | 4.7 (4.2) | 9846 (13,360) | 17 (6.4) | 34 (11.7) |
We further investigated differences among English speakers to better understand the very high rate of readmission for African Americans and the very low rate for English‐speaking Latinos. African Americans were on average younger than other English speakers (55 19 years vs. 60 21 years; P < 0.001); but, they had higher comorbidity scores than other English speakers (1.05 1.40 vs. 0.94 1.31; P = 0.008), and were more likely to be admitted for non‐malignant blood disorders (eg, sickle cell disease), endocrine disorders (eg, diabetes mellitus), and circulatory disorders (eg, stroke). In contrast, English‐speaking Latinos were also younger than other English speakers (53 21 years vs. 59 20 years; P < 0.001), but they trended toward lower comorbidity scores (0.87 1.23 vs. 0.97 1.33; P = 0.2), and were more likely to be admitted for gastrointestinal and musculoskeletal disorders, and less likely to be admitted for malignancy and endocrine disorders.
Multivariate Analyses: Association of Aggregated and Disaggregated Language‐Ethnicity Groups With Hospital Outcomes (Table 3)
In multivariate models examining aggregated language‐ethnicity groups, non‐English speakers had a trend toward higher odds of readmission at 30‐days post‐discharge than the English‐speaking group (odds ratio [OR], 1.3; 95% confidence interval [CI], 1.0‐1.7). There were no significant differences for LOS, cost, or 30‐day mortality. Compared to English speakers, Chinese and Spanish speakers had 70% and 50% higher adjusted odds of readmission at 30‐days post‐discharge respectively, while Russian speakers' odds of readmission was not increased. Additionally, Chinese speakers had 7% shorter LOS than English‐speakers. There were no significant differences among any of the language‐ethnicity groups for 30‐day mortality. The increased odds of readmission for Chinese and Spanish speakers compared to English speakers was robust to reinclusion of the admissions with the top 1% LOS and top 1% cost.
| Language Categorization | LOS, % Difference (95% CI) | Total Cost, % Difference (95% CI) | 30‐Day Readmission,* OR (95% CI) | Mortality, OR (95% CI) |
|---|---|---|---|---|
| ||||
| All English speakers | Reference | Reference | Reference | Reference |
| Non‐English speakers | 3.1 (8.7 to 3.1) | 2.5 (8.3 to 2.1) | 1.3 (1.0 to 1.7) | 0.9 (0.7 to 1.2) |
| All English speakers | Reference | Reference | Reference | Reference |
| Chinese speakers | 7.2 (13.9 to 0) | 5.3 (12.2 to 2.1) | 1.7 (1.2 to 2.3) | 1.0 (0.8 to 1.4) |
| Spanish speakers | 3.0 (12.6 to 7.6) | 3.0 (12.7 to 7.7) | 1.5 (1.0 to 2.3) | 0.9 (0.6 to 1.5) |
| Russian speakers | 1.5 (8.3 to 12.2) | 0.9 (8.9 to 11.8) | 0.8 (0.5 to 1.4) | 0.8 (0.5 to 1.2) |
Multivariate Analyses: Association of Language for Asians and Latinos, and of Ethnicity for English speakers, With Hospital Outcomes (Table 4)
Both Chinese and Spanish speakers had significantly higher odds of 30‐day readmission than their English speaking Asian and Latino counterparts. There were no significant differences in LOS, cost, or 30‐day mortality in this within‐ethnicity analysis. Among English speakers, admissions for patients with Asian ethnicity were 15% shorter and resulted in 9% lower costs than for Whites. While LOS and cost were similar for English‐speaking Latino and White admissions, English‐speaking Latinos had markedly lower odds of 30‐day readmission than their White counterparts. Whereas African‐Americans had 6% shorter LOS, 40% higher odds of readmission and 30% lower odds of mortality at 30‐days than English speaking Whites.
| Language‐Ethnicity Comparisons | LOS, % Difference (95% CI) | Total Cost, % Difference (95% CI) | 30‐Day Readmission,* OR (95% CI) | Mortality, OR (95% CI) |
|---|---|---|---|---|
| ||||
| English speaking Asians | Reference | Reference | Reference | Reference |
| Chinese speakers | 2.2 (7.4 to 12.7) | 0.3 (9.2 to 10.7) | 1.5 (1.0 to 2.3) | 0.8 (0.6 to 1.2) |
| English speaking Latinos | Reference | Reference | Reference | Reference |
| Spanish speakers | 4.5 (16.8 to 9.5) | 1.2 (14.0 to 13.5) | 5.7 (2.4 to 13.2) | 1.2 (0.6 to 2.4) |
| English‐White | Reference | Reference | Reference | Reference |
| English‐African American | 6.2 (11.3 to 0.9) | 4.4 (9.6 to 1.1) | 1.4 (1.1 to 1.7) | 0.6 (0.5 to 0.8) |
| English‐Asian | 14.6 (20.9 to 7.9) | 8.6 (15.4 to 1.4) | 0.8 (0.5 to 1.0) | 1.0 (0.7 to 1.4) |
| English‐Latino | 4.5 (13.5 to 5.4) | 5.0 (14.0 to 5.0) | 0.2 (0.1 to 0.4) | 0.6 (0.4 to 1.0) |
Conclusion/Discussion
Our results indicate that language barriers may contribute to higher readmission rates for non‐English speakers, but that they have less impact on care efficiency or mortality. This finding of an association between language and readmission, without a similar association with efficiency, suggests a potentially communication‐critical step in care.20, 21 Patients with language barriers are more likely to experience adverse events, and those events are often caused by errors in communication.12 It is conceivable that higher readmission risk for Chinese and Spanish speakers in our study was, at least in part, due to gaps in communication that are present in all patient groups, and exacerbated by the presence of a language barrier. This barrier is likely present during hospitalization but magnified at discharge, limiting caregivers' ability to understand patients' needs for home care, while simultaneously limiting patients' understanding of the discharge plan. After discharge, it is also possible that non‐English speakers are less able to communicate their needs as they arise, ormore subtlyfeel less supported by a primarily English speaking healthcare system. As in other clinical arenas,22 it is quite possible that increased access to professional interpreters in the hospital setting, and particularly at the time of discharge, would enhance communication and outcomes for LEP patients. Our interpreter services data showed that the patients in our study had quite limited access to staff professional interpreters.
Our findings differ somewhat from those of John‐Baptiste et al.,11 who found that language barriers contributed to increased LOS for patients with cardiac and major surgical diagnoses. Our study's findings are akin to recent research suggesting that being a monolingual Spanish speaker or receiving interpreter services may not significantly impact LOS or cost of hospitalization,23 and that LOS and in‐hospital mortality do not differ for non‐English speakers and English speakers after acute myocardial infarction.24 These studies, along with our results, suggest that that care efficiency in the hospital may be driven much more by clinical acuity (eg, the need to respond rapidly to urgent clinical signs such as hypotension, fever and respiratory distress) than by adequacy of communication. For example, elderly LEP patients may be even more likely than English speakers to have vigilant family members at the bedside throughout their hospitalization due to their need for communication assistance; these family members can quickly alert hospital staff to concerning changes in the patient's condition.
Our results also suggest the possibility that language and ethnicity are not monolithic concepts, and that even within language and ethnic groups there are potential differences in care pattern. For example, not speaking English may be a surrogate marker for unmeasured factors such as social supports and access to care. Language is intimately associated with culture; it remains plausible that cultural differences between highly acculturated and less acculturated members of a given ethno‐cultural group may have contributed to our observed differences in readmission rates. Differences in culture and associated factors, such as social support or use of multiple hospital systems, may account for lack of higher readmission risk in Russian speakers, while Chinese and Spanish speakers had higher readmission risk.
In addition, our finding that English‐speaking Latinos had lower readmission risk than any other group may be more consistent with their clinical characteristicseg, younger age, fewer comorbiditiesthan with cultural factors. Our finding that African American patients had the highest readmission risk in our hospital was both surprising and concerning. Some of this increased risk may be explained by clinical characteristics, such as higher comorbidities and higher rates of diagnoses leading to frequent admissions (eg, sickle cell disease); however, the reasons for this disparity deserves further investigation.
Our study has limitations. First, our data are administrative, and lack information about patients' educational attainment, social support, acculturation, utilization of other hospital systems, and usual source of care. Despite this, we were able to account for many significant covariates that might contribute to readmission rates, including age, insurance status, gender, comorbidities, and admission to the intensive care unit.2528 Second, our information about patients' English language proficiency is limited. While direct assessments of English proficiency are more accurate ways to determine a patient's ability to communicate with health care providers in English,29 our language validation work conducted in preparation for this study suggests that most of our patients recorded as having a non‐English primary language (87%) also have a low score on a language acculturation scale.
Third, only 14% of our non‐English speaking subjects utilized professional staff interpreters, and we had no information on the use of professional telephonic interpreters, or ad hoc interpretersfamily members, non‐interpreter staff membersand their impact on our results. It is well‐documented that ad hoc interpreters are used frequently in healthcare, particularly in the hospital setting, and thus we can assume this to be true in our study.30, 31 As noted above, it is likely that the advocacy of family members and friends at the bedside helped to minimize potential differences in care efficiency for patients with language barriers. Finally, our study was performed at a single university based hospital and may not produce results which are applicable to other care settings.
Our findings point to several avenues for future research on language barriers and hospitalized patients. First, the field would benefit from an examination of the impact of easy access to professional interpreters during hospitalization on outcomes of hospital care, in particular on readmission rates. Second, there is need for development and assessment of best practices for creating a culture of professional interpreter utilization in the hospital among physicians and nursing staff. Third, investigation of the role of caregiver presence in the hospital room and how this might differ by patient culture, age and language ability may further elucidate some of the differences across language groups observed in our study. Lastly, a more granular investigation of clinician‐patient communication and the importance of interpersonal processes of care on both patient satisfaction and understanding of and adherence to discharge instructions could lead to the development of detailed interventions to enhance this communication and these outcomes as it has done for communication‐sensitive outcomes in the outpatient arena.3234
In summary, our study suggests that higher risk for readmission can be added to the unfortunate list of outcomes which are worsened due to language barriers, pointing to transition from the hospital as a potentially communication‐critical step in care which may be amenable to intervention. Our findings also suggest that this risk can vary even between groups of patients who do not speak English primarily. Whether and to what degree language and communication barriers aloneincluding access to professional interpreters and patient‐centered communicationduring hospitalization, or differences in caregiver social support both during and after hospitalization as well as access to care post‐hospitalization contribute to these findings is a worthy subject of future research.
Acknowledgements
The authors acknowledge Dr. Eliseo J. Prez‐Stable for his mentorship on this project.
- ,. Language Use and English‐Speaking Ability:2000. Available at: http://www.census.gov/prod/2003pubs/c2kbr‐29.pdf. Accessed January 2010.
- U.S. Department of Health and Human Services.2006National Healthcare Disparities Report. AHRQ Publication No. 070012; 2006.
- ,,,.Disparities in health care by race, ethnicity, and language among the insured: findings from a national sample.Med Care.2002;40(1):52–59.
- ,.The effect of physician‐patient communication on mammography utilization by different ethnic groups.Med Care.1991;29(11):1065–1082.
- ,.Language of interview:relevance for research of Southwest Hispanics.Am J Pub Health.1991;81(11):1399–1404.
- ,,,.Is language a barrier to the use of preventive services?J Gen Intern Med.1997;12(8):472–477.
- ,,,.Impact of language barriers on patient satisfaction in an emergency department.JGIM.1999;14:82–87.
- .Patient comprehension of doctor‐patient communication on discharge from the emergency department.J Emerg Med.1997;15(1):1–7.
- ,,, et al.Drug complications in outpatients.J Gen Intern Med.2000;15:149–154.
- .Language concordance as a determinant of patient compliance and emergency room use in patients with asthma.Med Care.1988;26(12):1119–1128.
- ,,,et al.The effect of English language proficiency on length of stay and in‐hospital mortality.J Gen Intern Med.2004;19:221–228.
- ,,,.Language proficiency and adverse events in US hospitals: a pilot study.Int J Qual Health Care.2007;19(2):60–67.
- ,,, et al.Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study.J Hosp Med.2008;3(6):437–445.
- ,,, et al.Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non‐hospitalists.J Gen Intern Med.2008;23(9):1399–1406.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40(5):373–383.
- AHRQ. Healthcare Cost and Utilizlation Project: Tools 132(3):191–200.
- ,,.Free knot splines for logistic models and threshold selection.Comput Methods Programs Biomed.2005;77(1):1–9.
- ,,,,.Statistical methods in epidemiology: a comparison of statistical methods to analyze dose‐response and trend analysis in epidemiologic studies.J Clin Epidemiol.1998;51(12):1223–1233.
- The Care Transitions Project. Health Care Policy and Research, Practitioner Tools. Available at: http://www.caretransitions.org/practitioner_tools.asp.
- AHRQ. Improving safety at the point of care. Available at: http://www.ahrq.gov/qual/pips. Accessed January2010.
- ,,,.Do professional interpreters improve clinical care for patients with limited english proficiency? A systematic review of the literature.Health Serv Res.2007;42(2):727–754.
- ,,.The impact of an enhanced interpreter service intervention on hospital costs and patient satisfaction.J Gen Intern Med.2007;22 Suppl 2:306–311.
- ,,,,.Acute myocardial infarction length of stay and hospital mortality are not associated with language preference.J Gen Intern Med.2008;23(2):190–194.
- ,,.A systematic literature review of factors affecting outcome in older medical patients admitted to hospital.Age Ageing.2004;33(2):110–115.
- ,,,,,.Risk factors and prognostic predictors of unexpected intensive care unit admission within 3 days after ED discharge.Am J Emerg Med.2007;25(9):1009–1014.
- ,.Effect of gender, ethnicity, pulmonary disease, and symptom stability on rehospitalization in patients with heart failure.Am J Cardiol.2007;100(7):1139–1144.
- ,,,.Bouncing back: patterns and predictors of complicated transitions 30 days after hospitalization for acute ischemic stroke.J Am Geriatr Soc.2007;55(3):365–373.
- ,,,,.Identification of limited English proficient patients in clinical care.J Gen Intern Med.2008;23(10):1555–1560.
- ,,,,.Hospital langague services for patients with limited English proficiency: results from a national survey.Health Research October2006.
- ,.Hospitals, Language, and Culture: a Snapshot of the Nation. The Joint Commission and The California Endowment;2007.
- ,,, et al.A randomized controlled trial of interventions to enhance patient‐physician partnership, patient adherence and high blood pressure control among ethnic minorities and poor persons: study protocol NCT00123045.Implement Sci.2009;4:7.
- ,,,,.Interpersonal processes of care and patient satisfaction: do associations differ by race, ethnicity, and language?Health Serv Res.2009;44(4):1326–1344.
- ,,,.Understanding concordance in patient‐physician relationships: personal and ethnic dimensions of shared identity.Ann Fam Med.2008;6(3):198–205.
Forty‐five‐million Americans speak a language other than English and more than 19 million of these speak English less than very wellor are limited English proficient (LEP).1 The number of non‐English‐speaking and LEP people in the US has risen in recent decades, presenting a challenge to healthcare systems to provide high‐quality, patient‐centered care for these patients.2
For outpatients, language barriers are a fundamental contributor to gaps in health care. In the clinic setting, patients who do not speak English well have less access to a usual source of care and lower rates of physician visits and preventive services.36 Even when patients with language barriers do have access to care, they have poorer adherence, decreased comprehension of their diagnoses, decreased satisfaction with care, and increased medication complications.710
Few studies, however, have examined how language influences outcomes of hospital care. Compared to English‐speakers, patients who do not speak English well may experience longer lengths of stay,11 and have more adverse events while in the hospital.12 However, these previous studies have not investigated outcomes immediately post‐hospitalization, such as readmission rates and mortality, nor have they directly addressed the interaction between ethnicity and language.
To understand these questions, we analyzed data collected from a university‐based teaching hospital which cares for patients of diverse cultural and language backgrounds. Using these data, we examined how patients' primary language influenced hospital costs, length of stay (LOS), 30‐day readmission, and 30‐day mortality risk.
Patients and Methods
Patient Population and Setting
Our study examined patients admitted to the General Medicine Service at the University of California, San Francisco Medical Center (UCSF) between July 1, 2001 and June 30th, 2003, the time period during which UCSF participated in the Multicenter Hospitalist Trial (MHT) a prospective quasi‐randomized trial of hospitalist care for general medicine patients.13, 14
UCSF Moffitt‐Long Hospital is a 400‐bed urban academic medical center which provides services to the City and County of San Francisco, an ethnically and linguistically diverse area. UCSF employs staff language interpreters in Spanish, Chinese and Russian who travel to its many outpatient clinics, Comprehensive Cancer Center, Children's Hospital, as well as to Moffitt‐Long Hospital upon request; phone interpretation is also available when in‐person interpreters are not available, for off hours needs and for less common languages. During the period of this study there were no specific inpatient guidelines in place for use of interpretation services at UCSF, nor were there any specific interventions targeting LEP or non‐English speaking inpatients.
Patients were eligible for the MHT if they were 18 years of age or older and admitted at random to a hospitalist or non‐hospitalist physician (eg, outpatient general internist attending on average 1‐month/year); a minority of patients were cared for directly by their primary care physician while in the hospital, and were excluded. For purposes of our study, which merged MHT data with hospital administrative data on primary language, we further excluded all admissions for patients for whom primary language was missing (n = 5), whose listing was unknown or other language (n = 78), sign language (n = 3) or whose language was listed but was not one of the included languages (n = 258). Included languages were English, Chinese, Russian and Spanish. Because LOS and cost data were skewed, we excluded those admissions with the top 1% longest stays and the top 1% highest cost (n = 176); these exclusions did not alter the proportion of admissions across language and ethnicity. In addition, we excluded 102 admissions that were missing data on cost and 11 with costs <$500 and which were likely to be erroneous. Our research was approved by the UCSF Institutional Review Board.
Data Sources
We collected administrative data from Transition Systems Inc (TSI, Boston, MA) billing databases at UCSF as part of the MHT. These data include patient demographics, insurance, costs, ICD‐9CM diagnostic codes, admission and discharge dates in Uniform Bill 92 format. Patient mortality information was collected as part of the MHT using the National Death Index.14
Language data were collected from a separate patient‐registration database (STOR) at UCSF. Information on a patient's primary language is entered at the time each patient first registers at UCSF, whether for the index hospitalization or for prior clinic visits, and is based generally on patient self report. As part of our validation step, we cross‐checked 829 STOR language entries against patient reports and found 91% agreement with the majority of the errors classifying non‐English speakers as English‐speakers.
Measures
Predictor
Our primary language variable was derived using language designations collected from patient registration databases described above. Using these data we specified our key language groups as English, Chinese (Cantonese or Mandarin), Russian, or Spanish.
Outcomes
LOS and total cost of hospital stay for each hospitalization derived from administrative data sources. Readmissions were identified at the time patients were readmitted to UCSF (eg, flagged in administrative data). Mortality was determined by whether an individual patient with an admission in the database was recorded in the National Death Index as dead within 30‐days of admission.
Covariates
Additional covariates included age at admission, gender, ethnicity as recorded in registration databases (White, African American, Asian, Latino, Other), insurance, principal billing diagnosis, whether or not a patient received intensive care unit (ICU) care, type of admitting attending physician (Hospitalist/non‐Hospitalist), and an administrative Charlson comorbidity score.15 To collapse the principal diagnoses into categories, we used the Healthcare Cost and Utilization Project (HCUP)'s Clinical Classification System, which allowed us to classify each diagnosis in 1 of 14 generally accepted categories.16
Analysis
Statistical analyses were performed using STATA statistical software (STATACorp, Version 9, College Station, TX). We examined descriptive means and proportions for all variables, including sociodemographic, hospitalization, comorbidity and outcome variables. We compared English and non‐English speakers on all covariate and outcome variables using t‐tests for comparison of means and chi‐square for comparison of categorical variables.
It was not possible to fully test the language‐by‐ethnicity interactionwhether or not the impact of language varied by ethnic groupbecause many cells of the joint distribution were very sparse (eg, the sample contained very few non‐English‐speaking African Americans). Therefore, to better understand the influence of English vs. non‐English language usage across different ethnic groups, we created a combined language‐ethnicity predictor variable which categorized each subject first by language and then for the English‐speakers by ethnicity. For example, a Chinese, Spanish or Russian speaker would be categorized as such, and an English‐speaker could fall into the English‐White, English‐African American, English‐Asian or English‐Latino group. This allowed us to test whether there were any differences in language effects across the White, Asian, and Latino ethnicities, and any difference in ethnicity effects among English‐speakers.
Because cost and LOS were skewed, we used negative binomial models for LOS and log transformed costs. We performed a sensitivity analysis testing whether our results were robust to the exclusion of the admissions with the top 1% LOS and top 1% cost. We used logistic regression for the 30‐day readmission and mortality outcomes.
Our primary predictor was the language‐ethnicity variable described above. To determine the independent association between this predictor and our key outcomes, we then built models which included additional potential confounders selected either for face validity or because of observed confounding with other covariates. Our inclusion of potential confounders was limited by the variables available in the administrative database; thus, we were not able to pursue detailed analyses of communication and literacy factors and their interaction with our predictor or their independent impact on outcomes. Models also included a linear spline with a single knot at age 65 years as a further adjustment for age in Medicare recipients.1719 For the 30‐day readmission outcome model, we excluded those admissions for which the patient either died in the hospital or was discharged to hospice care. Within each model we tested the impact of a language barrier using custom contrasts. This allowed us to examine the language‐ethnicity effect aggregating all non‐English speakers compared to all English‐speakers, comparing each non‐English speaking group to all English‐speakers, comparing Chinese speakers to English‐speaking Asians and Spanish speakers to English‐speaking‐Latinos, as well as to test whether the effect of English language is the same across ethnicities.
Results
Admission Characteristics of the Sample
A total of 7023 patients were admitted to the General Medicine service, 5877 (84%) of whom were English‐speakers and 1146 (16%) non‐English‐speakers (Table 1). Overall, half of the admitted patients were women (50%), and the vast majority was insured (93%). The most common principal diagnoses were respiratory and gastrointestinal disorders. Only a small number of non‐English speakers 164 (14%) were recorded in the UCSF Interpreter Services database as having had any interaction with a professional staff interpreter during their hospitalization.
| English (n = 5877) n (%) | Non‐English (n = 1146) n (%) | |
|---|---|---|
| ||
| Socio‐economic variables | ||
| Language‐ethnicity | ||
| English | ||
| White | 3066 (52.2) | |
| African American | 1351 (23.0) | |
| Asian | 544 (9.3) | |
| Latino | 298 (5.1) | |
| Other | 618 (10.5) | |
| Chinese speakers | 584 (51.0) | |
| Spanish speakers | 272 (25.3) | |
| Russian speakers | 290 (23.7) | |
| Age mean (SD) (range 18‐105) | 58.8 (20.3) | 72.3 (15.5) |
| Gender | ||
| Male | 2967 (50.5) | 514 (44.8) |
| Female | 2910 (49.5) | 632 (55.2) |
| Insurance | ||
| Medicare | 2878 (49.0) | 800 (69.8) |
| Medicaid | 1201 (20.4) | 193 (16.8) |
| Commercial | 1358 (23.1) | 106 (9.3) |
| Charity/other | 440 (7.5) | 47 (4.1) |
| Hospitalization variables | ||
| Admitted to ICU | ||
| Yes | 721 (12.3) | 149 (13.0) |
| Attending physician | ||
| Hospitalist | 3950 (67.2) | 781 (68.2) |
| Comorbidity variables | ||
| Principal Diagnosis | ||
| Respiratory disorder | 1061 (18.1) | 225 (19.6) |
| Gastrointestinal disorder | 963 (16.4) | 205 (17.9) |
| Circulatory disorder | 613 (10.4) | 140 (12.2) |
| Endocrine/metabolism | 671 (11.4) | 80 (7.0) |
| Injury/poisoning | 475 (8.1) | 64 (5.6) |
| Malignancy | 395 (6.7) | 107 (9.3) |
| Renal/urinary disorder | 383 (6.5) | 108 (9.4) |
| Skin disorder | 278 (4.7) | 28 (2.9) |
| Infection/fatigue NOS | 206 (3.5) | 45 (3.4) |
| Blood disorder (non‐malignant) | 189 (3.2) | 38 (3.3) |
| Musculoskeletal/connective tissue disorder | 164 (2.8) | 33 (2.9) |
| Mental disorder/substance abuse | 171 (2.9) | 7 (0.6) |
| Nervous system/brain infection | 137 (2.3) | 26 (2.3) |
| Unclassified | 171 (2.9) | 40 (3.5) |
| Charlson Index score mean (SD) | 0.97 1.33 | 1.10 1.42 |
Among English speakers, Whites and African Americans were the most common ethnicities; however, more than 500 admissions were categorized as Asian ethnicity, and more than 600 as patients of other ethnicity. Close to 300 admissions were for Latinos. Among non‐English speakers, Chinese speakers had the largest number of admissions (n = 584), while Spanish and Russian speakers had similar numbers (n = 272 and 290 respectively).
Non‐English speakers were older, more likely to be female, more likely to be insured by Medicare, and more likely to have a higher comorbidity index score. While comorbidity scores were similar among non‐English speakers (Chinese 1.13 1.50; Russian 1.09 1.37; Spanish 1.06 1.30), they differed considerably among English speakers (White 0.94 1.29; African American 1.05 1.40; Asian 1.04 1.45; Latino 0.89 1.23; Other 0.91 1.29).
Hospital Outcome by Language‐Ethnicity Group (Table 2)
When aggregated together, non‐English speakers were somewhat more likely to be dead at 30‐days and have lower cost admissions; however, they did not differ from English speakers on LOS or readmission rates. While differences among disaggregated language‐ethnicity groups were not all statistically significant, English‐speaking Whites had the longest LOS (mean = 4.9 days) and highest costs (mean = $10,530). English‐speaking African Americans, Chinese and Spanish speakers had the highest 30‐day readmission rates; whereas, English‐speaking Latinos and Russian speakers had markedly lower 30‐day readmission rates (2.5% and 6.4%, respectively). Chinese speakers had the highest 30‐day mortality, followed by English speaking Whites and Asians.
| Language‐Ethnicity Groups | LOS* Mean #Days (SD) | Cost Mean Cost $ (SD) | 30‐Day Readmission, n (%) | 30‐Day Mortality, n (%) |
|---|---|---|---|---|
| ||||
| English speakers (all) | 4.7 (4.5) | 10,035 (15,041) | 648 (11.9) | 613 (10.4) |
| White | 4.9 (5.1) | 10,530 (15,894) | 322 (11.4) | 377 (12.3) |
| African American | 4.5 (4.8) | 9107 (13,314) | 227 (17.5) | 91 (6.7) |
| Asian | 4.3 (4.5) | 9933 (15,607) | 43 (8.8) | 67 (12.3) |
| Latino | 4.6 (4.8) | 9823 (14,113) | 7 (2.5) | 18 (6.0) |
| Other | 4.5 (4.8) | 9662 (14,016) | 49 (8.5) | 60 (9.7) |
| Non‐English speakers (all) | 4.5 (4.5) | 9515 (13,213) | 117 (11.0) | 147 (12.8) |
| Chinese speakers | 4.5 (4.6) | 9505 (12,841) | 69 (12.8) | 85 (14.6) |
| Spanish speakers | 4.5 (4.5) | 9115 (13,846) | 31 (12.0) | 28 (10.3) |
| Russian speakers | 4.7 (4.2) | 9846 (13,360) | 17 (6.4) | 34 (11.7) |
We further investigated differences among English speakers to better understand the very high rate of readmission for African Americans and the very low rate for English‐speaking Latinos. African Americans were on average younger than other English speakers (55 19 years vs. 60 21 years; P < 0.001); but, they had higher comorbidity scores than other English speakers (1.05 1.40 vs. 0.94 1.31; P = 0.008), and were more likely to be admitted for non‐malignant blood disorders (eg, sickle cell disease), endocrine disorders (eg, diabetes mellitus), and circulatory disorders (eg, stroke). In contrast, English‐speaking Latinos were also younger than other English speakers (53 21 years vs. 59 20 years; P < 0.001), but they trended toward lower comorbidity scores (0.87 1.23 vs. 0.97 1.33; P = 0.2), and were more likely to be admitted for gastrointestinal and musculoskeletal disorders, and less likely to be admitted for malignancy and endocrine disorders.
Multivariate Analyses: Association of Aggregated and Disaggregated Language‐Ethnicity Groups With Hospital Outcomes (Table 3)
In multivariate models examining aggregated language‐ethnicity groups, non‐English speakers had a trend toward higher odds of readmission at 30‐days post‐discharge than the English‐speaking group (odds ratio [OR], 1.3; 95% confidence interval [CI], 1.0‐1.7). There were no significant differences for LOS, cost, or 30‐day mortality. Compared to English speakers, Chinese and Spanish speakers had 70% and 50% higher adjusted odds of readmission at 30‐days post‐discharge respectively, while Russian speakers' odds of readmission was not increased. Additionally, Chinese speakers had 7% shorter LOS than English‐speakers. There were no significant differences among any of the language‐ethnicity groups for 30‐day mortality. The increased odds of readmission for Chinese and Spanish speakers compared to English speakers was robust to reinclusion of the admissions with the top 1% LOS and top 1% cost.
| Language Categorization | LOS, % Difference (95% CI) | Total Cost, % Difference (95% CI) | 30‐Day Readmission,* OR (95% CI) | Mortality, OR (95% CI) |
|---|---|---|---|---|
| ||||
| All English speakers | Reference | Reference | Reference | Reference |
| Non‐English speakers | 3.1 (8.7 to 3.1) | 2.5 (8.3 to 2.1) | 1.3 (1.0 to 1.7) | 0.9 (0.7 to 1.2) |
| All English speakers | Reference | Reference | Reference | Reference |
| Chinese speakers | 7.2 (13.9 to 0) | 5.3 (12.2 to 2.1) | 1.7 (1.2 to 2.3) | 1.0 (0.8 to 1.4) |
| Spanish speakers | 3.0 (12.6 to 7.6) | 3.0 (12.7 to 7.7) | 1.5 (1.0 to 2.3) | 0.9 (0.6 to 1.5) |
| Russian speakers | 1.5 (8.3 to 12.2) | 0.9 (8.9 to 11.8) | 0.8 (0.5 to 1.4) | 0.8 (0.5 to 1.2) |
Multivariate Analyses: Association of Language for Asians and Latinos, and of Ethnicity for English speakers, With Hospital Outcomes (Table 4)
Both Chinese and Spanish speakers had significantly higher odds of 30‐day readmission than their English speaking Asian and Latino counterparts. There were no significant differences in LOS, cost, or 30‐day mortality in this within‐ethnicity analysis. Among English speakers, admissions for patients with Asian ethnicity were 15% shorter and resulted in 9% lower costs than for Whites. While LOS and cost were similar for English‐speaking Latino and White admissions, English‐speaking Latinos had markedly lower odds of 30‐day readmission than their White counterparts. Whereas African‐Americans had 6% shorter LOS, 40% higher odds of readmission and 30% lower odds of mortality at 30‐days than English speaking Whites.
| Language‐Ethnicity Comparisons | LOS, % Difference (95% CI) | Total Cost, % Difference (95% CI) | 30‐Day Readmission,* OR (95% CI) | Mortality, OR (95% CI) |
|---|---|---|---|---|
| ||||
| English speaking Asians | Reference | Reference | Reference | Reference |
| Chinese speakers | 2.2 (7.4 to 12.7) | 0.3 (9.2 to 10.7) | 1.5 (1.0 to 2.3) | 0.8 (0.6 to 1.2) |
| English speaking Latinos | Reference | Reference | Reference | Reference |
| Spanish speakers | 4.5 (16.8 to 9.5) | 1.2 (14.0 to 13.5) | 5.7 (2.4 to 13.2) | 1.2 (0.6 to 2.4) |
| English‐White | Reference | Reference | Reference | Reference |
| English‐African American | 6.2 (11.3 to 0.9) | 4.4 (9.6 to 1.1) | 1.4 (1.1 to 1.7) | 0.6 (0.5 to 0.8) |
| English‐Asian | 14.6 (20.9 to 7.9) | 8.6 (15.4 to 1.4) | 0.8 (0.5 to 1.0) | 1.0 (0.7 to 1.4) |
| English‐Latino | 4.5 (13.5 to 5.4) | 5.0 (14.0 to 5.0) | 0.2 (0.1 to 0.4) | 0.6 (0.4 to 1.0) |
Conclusion/Discussion
Our results indicate that language barriers may contribute to higher readmission rates for non‐English speakers, but that they have less impact on care efficiency or mortality. This finding of an association between language and readmission, without a similar association with efficiency, suggests a potentially communication‐critical step in care.20, 21 Patients with language barriers are more likely to experience adverse events, and those events are often caused by errors in communication.12 It is conceivable that higher readmission risk for Chinese and Spanish speakers in our study was, at least in part, due to gaps in communication that are present in all patient groups, and exacerbated by the presence of a language barrier. This barrier is likely present during hospitalization but magnified at discharge, limiting caregivers' ability to understand patients' needs for home care, while simultaneously limiting patients' understanding of the discharge plan. After discharge, it is also possible that non‐English speakers are less able to communicate their needs as they arise, ormore subtlyfeel less supported by a primarily English speaking healthcare system. As in other clinical arenas,22 it is quite possible that increased access to professional interpreters in the hospital setting, and particularly at the time of discharge, would enhance communication and outcomes for LEP patients. Our interpreter services data showed that the patients in our study had quite limited access to staff professional interpreters.
Our findings differ somewhat from those of John‐Baptiste et al.,11 who found that language barriers contributed to increased LOS for patients with cardiac and major surgical diagnoses. Our study's findings are akin to recent research suggesting that being a monolingual Spanish speaker or receiving interpreter services may not significantly impact LOS or cost of hospitalization,23 and that LOS and in‐hospital mortality do not differ for non‐English speakers and English speakers after acute myocardial infarction.24 These studies, along with our results, suggest that that care efficiency in the hospital may be driven much more by clinical acuity (eg, the need to respond rapidly to urgent clinical signs such as hypotension, fever and respiratory distress) than by adequacy of communication. For example, elderly LEP patients may be even more likely than English speakers to have vigilant family members at the bedside throughout their hospitalization due to their need for communication assistance; these family members can quickly alert hospital staff to concerning changes in the patient's condition.
Our results also suggest the possibility that language and ethnicity are not monolithic concepts, and that even within language and ethnic groups there are potential differences in care pattern. For example, not speaking English may be a surrogate marker for unmeasured factors such as social supports and access to care. Language is intimately associated with culture; it remains plausible that cultural differences between highly acculturated and less acculturated members of a given ethno‐cultural group may have contributed to our observed differences in readmission rates. Differences in culture and associated factors, such as social support or use of multiple hospital systems, may account for lack of higher readmission risk in Russian speakers, while Chinese and Spanish speakers had higher readmission risk.
In addition, our finding that English‐speaking Latinos had lower readmission risk than any other group may be more consistent with their clinical characteristicseg, younger age, fewer comorbiditiesthan with cultural factors. Our finding that African American patients had the highest readmission risk in our hospital was both surprising and concerning. Some of this increased risk may be explained by clinical characteristics, such as higher comorbidities and higher rates of diagnoses leading to frequent admissions (eg, sickle cell disease); however, the reasons for this disparity deserves further investigation.
Our study has limitations. First, our data are administrative, and lack information about patients' educational attainment, social support, acculturation, utilization of other hospital systems, and usual source of care. Despite this, we were able to account for many significant covariates that might contribute to readmission rates, including age, insurance status, gender, comorbidities, and admission to the intensive care unit.2528 Second, our information about patients' English language proficiency is limited. While direct assessments of English proficiency are more accurate ways to determine a patient's ability to communicate with health care providers in English,29 our language validation work conducted in preparation for this study suggests that most of our patients recorded as having a non‐English primary language (87%) also have a low score on a language acculturation scale.
Third, only 14% of our non‐English speaking subjects utilized professional staff interpreters, and we had no information on the use of professional telephonic interpreters, or ad hoc interpretersfamily members, non‐interpreter staff membersand their impact on our results. It is well‐documented that ad hoc interpreters are used frequently in healthcare, particularly in the hospital setting, and thus we can assume this to be true in our study.30, 31 As noted above, it is likely that the advocacy of family members and friends at the bedside helped to minimize potential differences in care efficiency for patients with language barriers. Finally, our study was performed at a single university based hospital and may not produce results which are applicable to other care settings.
Our findings point to several avenues for future research on language barriers and hospitalized patients. First, the field would benefit from an examination of the impact of easy access to professional interpreters during hospitalization on outcomes of hospital care, in particular on readmission rates. Second, there is need for development and assessment of best practices for creating a culture of professional interpreter utilization in the hospital among physicians and nursing staff. Third, investigation of the role of caregiver presence in the hospital room and how this might differ by patient culture, age and language ability may further elucidate some of the differences across language groups observed in our study. Lastly, a more granular investigation of clinician‐patient communication and the importance of interpersonal processes of care on both patient satisfaction and understanding of and adherence to discharge instructions could lead to the development of detailed interventions to enhance this communication and these outcomes as it has done for communication‐sensitive outcomes in the outpatient arena.3234
In summary, our study suggests that higher risk for readmission can be added to the unfortunate list of outcomes which are worsened due to language barriers, pointing to transition from the hospital as a potentially communication‐critical step in care which may be amenable to intervention. Our findings also suggest that this risk can vary even between groups of patients who do not speak English primarily. Whether and to what degree language and communication barriers aloneincluding access to professional interpreters and patient‐centered communicationduring hospitalization, or differences in caregiver social support both during and after hospitalization as well as access to care post‐hospitalization contribute to these findings is a worthy subject of future research.
Acknowledgements
The authors acknowledge Dr. Eliseo J. Prez‐Stable for his mentorship on this project.
Forty‐five‐million Americans speak a language other than English and more than 19 million of these speak English less than very wellor are limited English proficient (LEP).1 The number of non‐English‐speaking and LEP people in the US has risen in recent decades, presenting a challenge to healthcare systems to provide high‐quality, patient‐centered care for these patients.2
For outpatients, language barriers are a fundamental contributor to gaps in health care. In the clinic setting, patients who do not speak English well have less access to a usual source of care and lower rates of physician visits and preventive services.36 Even when patients with language barriers do have access to care, they have poorer adherence, decreased comprehension of their diagnoses, decreased satisfaction with care, and increased medication complications.710
Few studies, however, have examined how language influences outcomes of hospital care. Compared to English‐speakers, patients who do not speak English well may experience longer lengths of stay,11 and have more adverse events while in the hospital.12 However, these previous studies have not investigated outcomes immediately post‐hospitalization, such as readmission rates and mortality, nor have they directly addressed the interaction between ethnicity and language.
To understand these questions, we analyzed data collected from a university‐based teaching hospital which cares for patients of diverse cultural and language backgrounds. Using these data, we examined how patients' primary language influenced hospital costs, length of stay (LOS), 30‐day readmission, and 30‐day mortality risk.
Patients and Methods
Patient Population and Setting
Our study examined patients admitted to the General Medicine Service at the University of California, San Francisco Medical Center (UCSF) between July 1, 2001 and June 30th, 2003, the time period during which UCSF participated in the Multicenter Hospitalist Trial (MHT) a prospective quasi‐randomized trial of hospitalist care for general medicine patients.13, 14
UCSF Moffitt‐Long Hospital is a 400‐bed urban academic medical center which provides services to the City and County of San Francisco, an ethnically and linguistically diverse area. UCSF employs staff language interpreters in Spanish, Chinese and Russian who travel to its many outpatient clinics, Comprehensive Cancer Center, Children's Hospital, as well as to Moffitt‐Long Hospital upon request; phone interpretation is also available when in‐person interpreters are not available, for off hours needs and for less common languages. During the period of this study there were no specific inpatient guidelines in place for use of interpretation services at UCSF, nor were there any specific interventions targeting LEP or non‐English speaking inpatients.
Patients were eligible for the MHT if they were 18 years of age or older and admitted at random to a hospitalist or non‐hospitalist physician (eg, outpatient general internist attending on average 1‐month/year); a minority of patients were cared for directly by their primary care physician while in the hospital, and were excluded. For purposes of our study, which merged MHT data with hospital administrative data on primary language, we further excluded all admissions for patients for whom primary language was missing (n = 5), whose listing was unknown or other language (n = 78), sign language (n = 3) or whose language was listed but was not one of the included languages (n = 258). Included languages were English, Chinese, Russian and Spanish. Because LOS and cost data were skewed, we excluded those admissions with the top 1% longest stays and the top 1% highest cost (n = 176); these exclusions did not alter the proportion of admissions across language and ethnicity. In addition, we excluded 102 admissions that were missing data on cost and 11 with costs <$500 and which were likely to be erroneous. Our research was approved by the UCSF Institutional Review Board.
Data Sources
We collected administrative data from Transition Systems Inc (TSI, Boston, MA) billing databases at UCSF as part of the MHT. These data include patient demographics, insurance, costs, ICD‐9CM diagnostic codes, admission and discharge dates in Uniform Bill 92 format. Patient mortality information was collected as part of the MHT using the National Death Index.14
Language data were collected from a separate patient‐registration database (STOR) at UCSF. Information on a patient's primary language is entered at the time each patient first registers at UCSF, whether for the index hospitalization or for prior clinic visits, and is based generally on patient self report. As part of our validation step, we cross‐checked 829 STOR language entries against patient reports and found 91% agreement with the majority of the errors classifying non‐English speakers as English‐speakers.
Measures
Predictor
Our primary language variable was derived using language designations collected from patient registration databases described above. Using these data we specified our key language groups as English, Chinese (Cantonese or Mandarin), Russian, or Spanish.
Outcomes
LOS and total cost of hospital stay for each hospitalization derived from administrative data sources. Readmissions were identified at the time patients were readmitted to UCSF (eg, flagged in administrative data). Mortality was determined by whether an individual patient with an admission in the database was recorded in the National Death Index as dead within 30‐days of admission.
Covariates
Additional covariates included age at admission, gender, ethnicity as recorded in registration databases (White, African American, Asian, Latino, Other), insurance, principal billing diagnosis, whether or not a patient received intensive care unit (ICU) care, type of admitting attending physician (Hospitalist/non‐Hospitalist), and an administrative Charlson comorbidity score.15 To collapse the principal diagnoses into categories, we used the Healthcare Cost and Utilization Project (HCUP)'s Clinical Classification System, which allowed us to classify each diagnosis in 1 of 14 generally accepted categories.16
Analysis
Statistical analyses were performed using STATA statistical software (STATACorp, Version 9, College Station, TX). We examined descriptive means and proportions for all variables, including sociodemographic, hospitalization, comorbidity and outcome variables. We compared English and non‐English speakers on all covariate and outcome variables using t‐tests for comparison of means and chi‐square for comparison of categorical variables.
It was not possible to fully test the language‐by‐ethnicity interactionwhether or not the impact of language varied by ethnic groupbecause many cells of the joint distribution were very sparse (eg, the sample contained very few non‐English‐speaking African Americans). Therefore, to better understand the influence of English vs. non‐English language usage across different ethnic groups, we created a combined language‐ethnicity predictor variable which categorized each subject first by language and then for the English‐speakers by ethnicity. For example, a Chinese, Spanish or Russian speaker would be categorized as such, and an English‐speaker could fall into the English‐White, English‐African American, English‐Asian or English‐Latino group. This allowed us to test whether there were any differences in language effects across the White, Asian, and Latino ethnicities, and any difference in ethnicity effects among English‐speakers.
Because cost and LOS were skewed, we used negative binomial models for LOS and log transformed costs. We performed a sensitivity analysis testing whether our results were robust to the exclusion of the admissions with the top 1% LOS and top 1% cost. We used logistic regression for the 30‐day readmission and mortality outcomes.
Our primary predictor was the language‐ethnicity variable described above. To determine the independent association between this predictor and our key outcomes, we then built models which included additional potential confounders selected either for face validity or because of observed confounding with other covariates. Our inclusion of potential confounders was limited by the variables available in the administrative database; thus, we were not able to pursue detailed analyses of communication and literacy factors and their interaction with our predictor or their independent impact on outcomes. Models also included a linear spline with a single knot at age 65 years as a further adjustment for age in Medicare recipients.1719 For the 30‐day readmission outcome model, we excluded those admissions for which the patient either died in the hospital or was discharged to hospice care. Within each model we tested the impact of a language barrier using custom contrasts. This allowed us to examine the language‐ethnicity effect aggregating all non‐English speakers compared to all English‐speakers, comparing each non‐English speaking group to all English‐speakers, comparing Chinese speakers to English‐speaking Asians and Spanish speakers to English‐speaking‐Latinos, as well as to test whether the effect of English language is the same across ethnicities.
Results
Admission Characteristics of the Sample
A total of 7023 patients were admitted to the General Medicine service, 5877 (84%) of whom were English‐speakers and 1146 (16%) non‐English‐speakers (Table 1). Overall, half of the admitted patients were women (50%), and the vast majority was insured (93%). The most common principal diagnoses were respiratory and gastrointestinal disorders. Only a small number of non‐English speakers 164 (14%) were recorded in the UCSF Interpreter Services database as having had any interaction with a professional staff interpreter during their hospitalization.
| English (n = 5877) n (%) | Non‐English (n = 1146) n (%) | |
|---|---|---|
| ||
| Socio‐economic variables | ||
| Language‐ethnicity | ||
| English | ||
| White | 3066 (52.2) | |
| African American | 1351 (23.0) | |
| Asian | 544 (9.3) | |
| Latino | 298 (5.1) | |
| Other | 618 (10.5) | |
| Chinese speakers | 584 (51.0) | |
| Spanish speakers | 272 (25.3) | |
| Russian speakers | 290 (23.7) | |
| Age mean (SD) (range 18‐105) | 58.8 (20.3) | 72.3 (15.5) |
| Gender | ||
| Male | 2967 (50.5) | 514 (44.8) |
| Female | 2910 (49.5) | 632 (55.2) |
| Insurance | ||
| Medicare | 2878 (49.0) | 800 (69.8) |
| Medicaid | 1201 (20.4) | 193 (16.8) |
| Commercial | 1358 (23.1) | 106 (9.3) |
| Charity/other | 440 (7.5) | 47 (4.1) |
| Hospitalization variables | ||
| Admitted to ICU | ||
| Yes | 721 (12.3) | 149 (13.0) |
| Attending physician | ||
| Hospitalist | 3950 (67.2) | 781 (68.2) |
| Comorbidity variables | ||
| Principal Diagnosis | ||
| Respiratory disorder | 1061 (18.1) | 225 (19.6) |
| Gastrointestinal disorder | 963 (16.4) | 205 (17.9) |
| Circulatory disorder | 613 (10.4) | 140 (12.2) |
| Endocrine/metabolism | 671 (11.4) | 80 (7.0) |
| Injury/poisoning | 475 (8.1) | 64 (5.6) |
| Malignancy | 395 (6.7) | 107 (9.3) |
| Renal/urinary disorder | 383 (6.5) | 108 (9.4) |
| Skin disorder | 278 (4.7) | 28 (2.9) |
| Infection/fatigue NOS | 206 (3.5) | 45 (3.4) |
| Blood disorder (non‐malignant) | 189 (3.2) | 38 (3.3) |
| Musculoskeletal/connective tissue disorder | 164 (2.8) | 33 (2.9) |
| Mental disorder/substance abuse | 171 (2.9) | 7 (0.6) |
| Nervous system/brain infection | 137 (2.3) | 26 (2.3) |
| Unclassified | 171 (2.9) | 40 (3.5) |
| Charlson Index score mean (SD) | 0.97 1.33 | 1.10 1.42 |
Among English speakers, Whites and African Americans were the most common ethnicities; however, more than 500 admissions were categorized as Asian ethnicity, and more than 600 as patients of other ethnicity. Close to 300 admissions were for Latinos. Among non‐English speakers, Chinese speakers had the largest number of admissions (n = 584), while Spanish and Russian speakers had similar numbers (n = 272 and 290 respectively).
Non‐English speakers were older, more likely to be female, more likely to be insured by Medicare, and more likely to have a higher comorbidity index score. While comorbidity scores were similar among non‐English speakers (Chinese 1.13 1.50; Russian 1.09 1.37; Spanish 1.06 1.30), they differed considerably among English speakers (White 0.94 1.29; African American 1.05 1.40; Asian 1.04 1.45; Latino 0.89 1.23; Other 0.91 1.29).
Hospital Outcome by Language‐Ethnicity Group (Table 2)
When aggregated together, non‐English speakers were somewhat more likely to be dead at 30‐days and have lower cost admissions; however, they did not differ from English speakers on LOS or readmission rates. While differences among disaggregated language‐ethnicity groups were not all statistically significant, English‐speaking Whites had the longest LOS (mean = 4.9 days) and highest costs (mean = $10,530). English‐speaking African Americans, Chinese and Spanish speakers had the highest 30‐day readmission rates; whereas, English‐speaking Latinos and Russian speakers had markedly lower 30‐day readmission rates (2.5% and 6.4%, respectively). Chinese speakers had the highest 30‐day mortality, followed by English speaking Whites and Asians.
| Language‐Ethnicity Groups | LOS* Mean #Days (SD) | Cost Mean Cost $ (SD) | 30‐Day Readmission, n (%) | 30‐Day Mortality, n (%) |
|---|---|---|---|---|
| ||||
| English speakers (all) | 4.7 (4.5) | 10,035 (15,041) | 648 (11.9) | 613 (10.4) |
| White | 4.9 (5.1) | 10,530 (15,894) | 322 (11.4) | 377 (12.3) |
| African American | 4.5 (4.8) | 9107 (13,314) | 227 (17.5) | 91 (6.7) |
| Asian | 4.3 (4.5) | 9933 (15,607) | 43 (8.8) | 67 (12.3) |
| Latino | 4.6 (4.8) | 9823 (14,113) | 7 (2.5) | 18 (6.0) |
| Other | 4.5 (4.8) | 9662 (14,016) | 49 (8.5) | 60 (9.7) |
| Non‐English speakers (all) | 4.5 (4.5) | 9515 (13,213) | 117 (11.0) | 147 (12.8) |
| Chinese speakers | 4.5 (4.6) | 9505 (12,841) | 69 (12.8) | 85 (14.6) |
| Spanish speakers | 4.5 (4.5) | 9115 (13,846) | 31 (12.0) | 28 (10.3) |
| Russian speakers | 4.7 (4.2) | 9846 (13,360) | 17 (6.4) | 34 (11.7) |
We further investigated differences among English speakers to better understand the very high rate of readmission for African Americans and the very low rate for English‐speaking Latinos. African Americans were on average younger than other English speakers (55 19 years vs. 60 21 years; P < 0.001); but, they had higher comorbidity scores than other English speakers (1.05 1.40 vs. 0.94 1.31; P = 0.008), and were more likely to be admitted for non‐malignant blood disorders (eg, sickle cell disease), endocrine disorders (eg, diabetes mellitus), and circulatory disorders (eg, stroke). In contrast, English‐speaking Latinos were also younger than other English speakers (53 21 years vs. 59 20 years; P < 0.001), but they trended toward lower comorbidity scores (0.87 1.23 vs. 0.97 1.33; P = 0.2), and were more likely to be admitted for gastrointestinal and musculoskeletal disorders, and less likely to be admitted for malignancy and endocrine disorders.
Multivariate Analyses: Association of Aggregated and Disaggregated Language‐Ethnicity Groups With Hospital Outcomes (Table 3)
In multivariate models examining aggregated language‐ethnicity groups, non‐English speakers had a trend toward higher odds of readmission at 30‐days post‐discharge than the English‐speaking group (odds ratio [OR], 1.3; 95% confidence interval [CI], 1.0‐1.7). There were no significant differences for LOS, cost, or 30‐day mortality. Compared to English speakers, Chinese and Spanish speakers had 70% and 50% higher adjusted odds of readmission at 30‐days post‐discharge respectively, while Russian speakers' odds of readmission was not increased. Additionally, Chinese speakers had 7% shorter LOS than English‐speakers. There were no significant differences among any of the language‐ethnicity groups for 30‐day mortality. The increased odds of readmission for Chinese and Spanish speakers compared to English speakers was robust to reinclusion of the admissions with the top 1% LOS and top 1% cost.
| Language Categorization | LOS, % Difference (95% CI) | Total Cost, % Difference (95% CI) | 30‐Day Readmission,* OR (95% CI) | Mortality, OR (95% CI) |
|---|---|---|---|---|
| ||||
| All English speakers | Reference | Reference | Reference | Reference |
| Non‐English speakers | 3.1 (8.7 to 3.1) | 2.5 (8.3 to 2.1) | 1.3 (1.0 to 1.7) | 0.9 (0.7 to 1.2) |
| All English speakers | Reference | Reference | Reference | Reference |
| Chinese speakers | 7.2 (13.9 to 0) | 5.3 (12.2 to 2.1) | 1.7 (1.2 to 2.3) | 1.0 (0.8 to 1.4) |
| Spanish speakers | 3.0 (12.6 to 7.6) | 3.0 (12.7 to 7.7) | 1.5 (1.0 to 2.3) | 0.9 (0.6 to 1.5) |
| Russian speakers | 1.5 (8.3 to 12.2) | 0.9 (8.9 to 11.8) | 0.8 (0.5 to 1.4) | 0.8 (0.5 to 1.2) |
Multivariate Analyses: Association of Language for Asians and Latinos, and of Ethnicity for English speakers, With Hospital Outcomes (Table 4)
Both Chinese and Spanish speakers had significantly higher odds of 30‐day readmission than their English speaking Asian and Latino counterparts. There were no significant differences in LOS, cost, or 30‐day mortality in this within‐ethnicity analysis. Among English speakers, admissions for patients with Asian ethnicity were 15% shorter and resulted in 9% lower costs than for Whites. While LOS and cost were similar for English‐speaking Latino and White admissions, English‐speaking Latinos had markedly lower odds of 30‐day readmission than their White counterparts. Whereas African‐Americans had 6% shorter LOS, 40% higher odds of readmission and 30% lower odds of mortality at 30‐days than English speaking Whites.
| Language‐Ethnicity Comparisons | LOS, % Difference (95% CI) | Total Cost, % Difference (95% CI) | 30‐Day Readmission,* OR (95% CI) | Mortality, OR (95% CI) |
|---|---|---|---|---|
| ||||
| English speaking Asians | Reference | Reference | Reference | Reference |
| Chinese speakers | 2.2 (7.4 to 12.7) | 0.3 (9.2 to 10.7) | 1.5 (1.0 to 2.3) | 0.8 (0.6 to 1.2) |
| English speaking Latinos | Reference | Reference | Reference | Reference |
| Spanish speakers | 4.5 (16.8 to 9.5) | 1.2 (14.0 to 13.5) | 5.7 (2.4 to 13.2) | 1.2 (0.6 to 2.4) |
| English‐White | Reference | Reference | Reference | Reference |
| English‐African American | 6.2 (11.3 to 0.9) | 4.4 (9.6 to 1.1) | 1.4 (1.1 to 1.7) | 0.6 (0.5 to 0.8) |
| English‐Asian | 14.6 (20.9 to 7.9) | 8.6 (15.4 to 1.4) | 0.8 (0.5 to 1.0) | 1.0 (0.7 to 1.4) |
| English‐Latino | 4.5 (13.5 to 5.4) | 5.0 (14.0 to 5.0) | 0.2 (0.1 to 0.4) | 0.6 (0.4 to 1.0) |
Conclusion/Discussion
Our results indicate that language barriers may contribute to higher readmission rates for non‐English speakers, but that they have less impact on care efficiency or mortality. This finding of an association between language and readmission, without a similar association with efficiency, suggests a potentially communication‐critical step in care.20, 21 Patients with language barriers are more likely to experience adverse events, and those events are often caused by errors in communication.12 It is conceivable that higher readmission risk for Chinese and Spanish speakers in our study was, at least in part, due to gaps in communication that are present in all patient groups, and exacerbated by the presence of a language barrier. This barrier is likely present during hospitalization but magnified at discharge, limiting caregivers' ability to understand patients' needs for home care, while simultaneously limiting patients' understanding of the discharge plan. After discharge, it is also possible that non‐English speakers are less able to communicate their needs as they arise, ormore subtlyfeel less supported by a primarily English speaking healthcare system. As in other clinical arenas,22 it is quite possible that increased access to professional interpreters in the hospital setting, and particularly at the time of discharge, would enhance communication and outcomes for LEP patients. Our interpreter services data showed that the patients in our study had quite limited access to staff professional interpreters.
Our findings differ somewhat from those of John‐Baptiste et al.,11 who found that language barriers contributed to increased LOS for patients with cardiac and major surgical diagnoses. Our study's findings are akin to recent research suggesting that being a monolingual Spanish speaker or receiving interpreter services may not significantly impact LOS or cost of hospitalization,23 and that LOS and in‐hospital mortality do not differ for non‐English speakers and English speakers after acute myocardial infarction.24 These studies, along with our results, suggest that that care efficiency in the hospital may be driven much more by clinical acuity (eg, the need to respond rapidly to urgent clinical signs such as hypotension, fever and respiratory distress) than by adequacy of communication. For example, elderly LEP patients may be even more likely than English speakers to have vigilant family members at the bedside throughout their hospitalization due to their need for communication assistance; these family members can quickly alert hospital staff to concerning changes in the patient's condition.
Our results also suggest the possibility that language and ethnicity are not monolithic concepts, and that even within language and ethnic groups there are potential differences in care pattern. For example, not speaking English may be a surrogate marker for unmeasured factors such as social supports and access to care. Language is intimately associated with culture; it remains plausible that cultural differences between highly acculturated and less acculturated members of a given ethno‐cultural group may have contributed to our observed differences in readmission rates. Differences in culture and associated factors, such as social support or use of multiple hospital systems, may account for lack of higher readmission risk in Russian speakers, while Chinese and Spanish speakers had higher readmission risk.
In addition, our finding that English‐speaking Latinos had lower readmission risk than any other group may be more consistent with their clinical characteristicseg, younger age, fewer comorbiditiesthan with cultural factors. Our finding that African American patients had the highest readmission risk in our hospital was both surprising and concerning. Some of this increased risk may be explained by clinical characteristics, such as higher comorbidities and higher rates of diagnoses leading to frequent admissions (eg, sickle cell disease); however, the reasons for this disparity deserves further investigation.
Our study has limitations. First, our data are administrative, and lack information about patients' educational attainment, social support, acculturation, utilization of other hospital systems, and usual source of care. Despite this, we were able to account for many significant covariates that might contribute to readmission rates, including age, insurance status, gender, comorbidities, and admission to the intensive care unit.2528 Second, our information about patients' English language proficiency is limited. While direct assessments of English proficiency are more accurate ways to determine a patient's ability to communicate with health care providers in English,29 our language validation work conducted in preparation for this study suggests that most of our patients recorded as having a non‐English primary language (87%) also have a low score on a language acculturation scale.
Third, only 14% of our non‐English speaking subjects utilized professional staff interpreters, and we had no information on the use of professional telephonic interpreters, or ad hoc interpretersfamily members, non‐interpreter staff membersand their impact on our results. It is well‐documented that ad hoc interpreters are used frequently in healthcare, particularly in the hospital setting, and thus we can assume this to be true in our study.30, 31 As noted above, it is likely that the advocacy of family members and friends at the bedside helped to minimize potential differences in care efficiency for patients with language barriers. Finally, our study was performed at a single university based hospital and may not produce results which are applicable to other care settings.
Our findings point to several avenues for future research on language barriers and hospitalized patients. First, the field would benefit from an examination of the impact of easy access to professional interpreters during hospitalization on outcomes of hospital care, in particular on readmission rates. Second, there is need for development and assessment of best practices for creating a culture of professional interpreter utilization in the hospital among physicians and nursing staff. Third, investigation of the role of caregiver presence in the hospital room and how this might differ by patient culture, age and language ability may further elucidate some of the differences across language groups observed in our study. Lastly, a more granular investigation of clinician‐patient communication and the importance of interpersonal processes of care on both patient satisfaction and understanding of and adherence to discharge instructions could lead to the development of detailed interventions to enhance this communication and these outcomes as it has done for communication‐sensitive outcomes in the outpatient arena.3234
In summary, our study suggests that higher risk for readmission can be added to the unfortunate list of outcomes which are worsened due to language barriers, pointing to transition from the hospital as a potentially communication‐critical step in care which may be amenable to intervention. Our findings also suggest that this risk can vary even between groups of patients who do not speak English primarily. Whether and to what degree language and communication barriers aloneincluding access to professional interpreters and patient‐centered communicationduring hospitalization, or differences in caregiver social support both during and after hospitalization as well as access to care post‐hospitalization contribute to these findings is a worthy subject of future research.
Acknowledgements
The authors acknowledge Dr. Eliseo J. Prez‐Stable for his mentorship on this project.
- ,. Language Use and English‐Speaking Ability:2000. Available at: http://www.census.gov/prod/2003pubs/c2kbr‐29.pdf. Accessed January 2010.
- U.S. Department of Health and Human Services.2006National Healthcare Disparities Report. AHRQ Publication No. 070012; 2006.
- ,,,.Disparities in health care by race, ethnicity, and language among the insured: findings from a national sample.Med Care.2002;40(1):52–59.
- ,.The effect of physician‐patient communication on mammography utilization by different ethnic groups.Med Care.1991;29(11):1065–1082.
- ,.Language of interview:relevance for research of Southwest Hispanics.Am J Pub Health.1991;81(11):1399–1404.
- ,,,.Is language a barrier to the use of preventive services?J Gen Intern Med.1997;12(8):472–477.
- ,,,.Impact of language barriers on patient satisfaction in an emergency department.JGIM.1999;14:82–87.
- .Patient comprehension of doctor‐patient communication on discharge from the emergency department.J Emerg Med.1997;15(1):1–7.
- ,,, et al.Drug complications in outpatients.J Gen Intern Med.2000;15:149–154.
- .Language concordance as a determinant of patient compliance and emergency room use in patients with asthma.Med Care.1988;26(12):1119–1128.
- ,,,et al.The effect of English language proficiency on length of stay and in‐hospital mortality.J Gen Intern Med.2004;19:221–228.
- ,,,.Language proficiency and adverse events in US hospitals: a pilot study.Int J Qual Health Care.2007;19(2):60–67.
- ,,, et al.Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study.J Hosp Med.2008;3(6):437–445.
- ,,, et al.Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non‐hospitalists.J Gen Intern Med.2008;23(9):1399–1406.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40(5):373–383.
- AHRQ. Healthcare Cost and Utilizlation Project: Tools 132(3):191–200.
- ,,.Free knot splines for logistic models and threshold selection.Comput Methods Programs Biomed.2005;77(1):1–9.
- ,,,,.Statistical methods in epidemiology: a comparison of statistical methods to analyze dose‐response and trend analysis in epidemiologic studies.J Clin Epidemiol.1998;51(12):1223–1233.
- The Care Transitions Project. Health Care Policy and Research, Practitioner Tools. Available at: http://www.caretransitions.org/practitioner_tools.asp.
- AHRQ. Improving safety at the point of care. Available at: http://www.ahrq.gov/qual/pips. Accessed January2010.
- ,,,.Do professional interpreters improve clinical care for patients with limited english proficiency? A systematic review of the literature.Health Serv Res.2007;42(2):727–754.
- ,,.The impact of an enhanced interpreter service intervention on hospital costs and patient satisfaction.J Gen Intern Med.2007;22 Suppl 2:306–311.
- ,,,,.Acute myocardial infarction length of stay and hospital mortality are not associated with language preference.J Gen Intern Med.2008;23(2):190–194.
- ,,.A systematic literature review of factors affecting outcome in older medical patients admitted to hospital.Age Ageing.2004;33(2):110–115.
- ,,,,,.Risk factors and prognostic predictors of unexpected intensive care unit admission within 3 days after ED discharge.Am J Emerg Med.2007;25(9):1009–1014.
- ,.Effect of gender, ethnicity, pulmonary disease, and symptom stability on rehospitalization in patients with heart failure.Am J Cardiol.2007;100(7):1139–1144.
- ,,,.Bouncing back: patterns and predictors of complicated transitions 30 days after hospitalization for acute ischemic stroke.J Am Geriatr Soc.2007;55(3):365–373.
- ,,,,.Identification of limited English proficient patients in clinical care.J Gen Intern Med.2008;23(10):1555–1560.
- ,,,,.Hospital langague services for patients with limited English proficiency: results from a national survey.Health Research October2006.
- ,.Hospitals, Language, and Culture: a Snapshot of the Nation. The Joint Commission and The California Endowment;2007.
- ,,, et al.A randomized controlled trial of interventions to enhance patient‐physician partnership, patient adherence and high blood pressure control among ethnic minorities and poor persons: study protocol NCT00123045.Implement Sci.2009;4:7.
- ,,,,.Interpersonal processes of care and patient satisfaction: do associations differ by race, ethnicity, and language?Health Serv Res.2009;44(4):1326–1344.
- ,,,.Understanding concordance in patient‐physician relationships: personal and ethnic dimensions of shared identity.Ann Fam Med.2008;6(3):198–205.
- ,. Language Use and English‐Speaking Ability:2000. Available at: http://www.census.gov/prod/2003pubs/c2kbr‐29.pdf. Accessed January 2010.
- U.S. Department of Health and Human Services.2006National Healthcare Disparities Report. AHRQ Publication No. 070012; 2006.
- ,,,.Disparities in health care by race, ethnicity, and language among the insured: findings from a national sample.Med Care.2002;40(1):52–59.
- ,.The effect of physician‐patient communication on mammography utilization by different ethnic groups.Med Care.1991;29(11):1065–1082.
- ,.Language of interview:relevance for research of Southwest Hispanics.Am J Pub Health.1991;81(11):1399–1404.
- ,,,.Is language a barrier to the use of preventive services?J Gen Intern Med.1997;12(8):472–477.
- ,,,.Impact of language barriers on patient satisfaction in an emergency department.JGIM.1999;14:82–87.
- .Patient comprehension of doctor‐patient communication on discharge from the emergency department.J Emerg Med.1997;15(1):1–7.
- ,,, et al.Drug complications in outpatients.J Gen Intern Med.2000;15:149–154.
- .Language concordance as a determinant of patient compliance and emergency room use in patients with asthma.Med Care.1988;26(12):1119–1128.
- ,,,et al.The effect of English language proficiency on length of stay and in‐hospital mortality.J Gen Intern Med.2004;19:221–228.
- ,,,.Language proficiency and adverse events in US hospitals: a pilot study.Int J Qual Health Care.2007;19(2):60–67.
- ,,, et al.Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study.J Hosp Med.2008;3(6):437–445.
- ,,, et al.Quality of care for decompensated heart failure: comparable performance between academic hospitalists and non‐hospitalists.J Gen Intern Med.2008;23(9):1399–1406.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40(5):373–383.
- AHRQ. Healthcare Cost and Utilizlation Project: Tools 132(3):191–200.
- ,,.Free knot splines for logistic models and threshold selection.Comput Methods Programs Biomed.2005;77(1):1–9.
- ,,,,.Statistical methods in epidemiology: a comparison of statistical methods to analyze dose‐response and trend analysis in epidemiologic studies.J Clin Epidemiol.1998;51(12):1223–1233.
- The Care Transitions Project. Health Care Policy and Research, Practitioner Tools. Available at: http://www.caretransitions.org/practitioner_tools.asp.
- AHRQ. Improving safety at the point of care. Available at: http://www.ahrq.gov/qual/pips. Accessed January2010.
- ,,,.Do professional interpreters improve clinical care for patients with limited english proficiency? A systematic review of the literature.Health Serv Res.2007;42(2):727–754.
- ,,.The impact of an enhanced interpreter service intervention on hospital costs and patient satisfaction.J Gen Intern Med.2007;22 Suppl 2:306–311.
- ,,,,.Acute myocardial infarction length of stay and hospital mortality are not associated with language preference.J Gen Intern Med.2008;23(2):190–194.
- ,,.A systematic literature review of factors affecting outcome in older medical patients admitted to hospital.Age Ageing.2004;33(2):110–115.
- ,,,,,.Risk factors and prognostic predictors of unexpected intensive care unit admission within 3 days after ED discharge.Am J Emerg Med.2007;25(9):1009–1014.
- ,.Effect of gender, ethnicity, pulmonary disease, and symptom stability on rehospitalization in patients with heart failure.Am J Cardiol.2007;100(7):1139–1144.
- ,,,.Bouncing back: patterns and predictors of complicated transitions 30 days after hospitalization for acute ischemic stroke.J Am Geriatr Soc.2007;55(3):365–373.
- ,,,,.Identification of limited English proficient patients in clinical care.J Gen Intern Med.2008;23(10):1555–1560.
- ,,,,.Hospital langague services for patients with limited English proficiency: results from a national survey.Health Research October2006.
- ,.Hospitals, Language, and Culture: a Snapshot of the Nation. The Joint Commission and The California Endowment;2007.
- ,,, et al.A randomized controlled trial of interventions to enhance patient‐physician partnership, patient adherence and high blood pressure control among ethnic minorities and poor persons: study protocol NCT00123045.Implement Sci.2009;4:7.
- ,,,,.Interpersonal processes of care and patient satisfaction: do associations differ by race, ethnicity, and language?Health Serv Res.2009;44(4):1326–1344.
- ,,,.Understanding concordance in patient‐physician relationships: personal and ethnic dimensions of shared identity.Ann Fam Med.2008;6(3):198–205.
Copyright © 2010 Society of Hospital Medicine
Hemoglobin Levels in Hospitalized Patients
Studies of the red blood cell mass performed in hospitalized patients were first done in the early 1970s.1 Thereafter, a decrease in the hemoglobin concentration ([Hb]) and its potential causes have been further reported, especially in critically ill patients.25 The decrease in [Hb] can occur as a result of blood draws for diagnostic testing; blood loss associated with invasive procedures and bleeding; occult gastrointestinal bleeding; hemolysis; shortening of red cell survival; iron, folic acid or cobalamine deficiencies; renal, liver or endocrine failure; hemodilution associated with fluid therapy; and the so‐called anemia of inflammation (AI).68 The latter would be a consequence of a blunted response of the bone marrow due to several factors such as inadequate secretion of erythropoietin, inhibition of the proliferation and differentiation of the erythroid precursors of the bone marrow and an hepcidin‐mediated functional iron deficiency.810
As mentioned, most studies were done in critically ill patients and scarce information is available about general ward admitted patients (GWAP) with less severe illnesses. In this scenario, some of the proposed mechanisms may have a less clear role. It is widely accepted that [Hb] may decrease without overt bleeding in GWAP. However, given the lack of information on this matter, laboratory controls and invasive procedures are undertaken to determine its potential causes.
The purpose of the present study is to describe [Hb] variation over time in nonbleeding GWAP, to estimate the proportion of patients with [Hb] decreases 1.5 g/dL, and to evaluate possible related variables.
Materials and Methods
A 16‐week (September 2004‐January 2005) prospective observational study was conducted in Internal Medicine GWAP at 2 Buenos Aires teaching hospitals.
All consecutive patients older than 16 years were evaluated. Patients admitted for the following reasons were excluded: bleeding, trauma, surgery, invasive procedures associated with blood loss (biopsies, biliary drainage, endovascular therapeutic procedures, and chest tubes), blood transfusions, anemia, chemotherapy, and acute renal failure.
Patients with a bleeding history, chemotherapy or radiation therapy within two months prior to admission, patients on dialysis and patients with current oncologic or hematologic disease were excluded, as well as those with length of stay less than 3 days, or with less than 2 [Hb] or hematocrit (HCT) measurements.
Patients were followed until discharge, death or transfer to a critical care unit. Patients were withdrawn from the study if they presented with bleeding or hemolysis, underwent red blood cell transfusion or therapy affecting hemoglobin levels (iron, chemotherapy or erythropoietin) or if either a surgical or invasive procedure associated with blood loss was performed.
Data were collected from patients' medical records and additional information was obtained from treating physicians and patients by the authors (AL, NC, SM, AN, MH). Standardized case report forms were completed during the hospitalization including: age, sex, admissions in the previous 3 months (readmissions) and whether or not the patient lived in a nursing home. Patients were categorized according to discharge diagnosis as reported by Nguyen et al.4 with modifications related to our general ward population. Upon admission Katz daily activity index (ADL),11, 12 APACHE II acute physiology score (APS),13, 14 and Charlson comorbidity score (Charlson)15, 16 were assessed. In all cases the [Hb] and HCT values were registered on admission and on days 3, 6, 10 and prior to discharge as well as any other determination required by the treating physician. Anemia was defined as [Hb] 13 g/dL for men and 12 g/dL for women.17 Based on previous reports a 1.5 g/dL decrease in [Hb] and of 4.5 points in HCT compared to admission values were considered a significant fall.1, 4 Acute renal failure was defined as an increase in creatinine level 0.5 mg/dL or a 50% increase from baseline.18, 19
Every procedure without significant blood loss (PWSBL) such as venous catheter placement, thoracentesis, lumbar punction, paracentesis, skin biopsy, arthrocentesis, and diagnostic angiogram was also recorded. The study was approved by the Hospital's ethical committee.
Data analysis and processing were performed with Excel 2000 and Stata version 8.0 (Stata Corp; USA). For continuous variables, results were expressed by the mean value its standard deviation (SD), and compared with Student's t‐test. The chi‐square test was used to compare categorical variables. A survival curve was developed with the Kaplan‐Meier method to analyze the time to a significant fall in [Hb]. Finally, Cox proportional hazard modeling was performed to assess the association between a significant fall in [Hb] and other variables. We accepted P < 0,05 as significant.
Results
A total of 338 patients were admitted to the Internal Medicine Inpatient Services in the study period. Thirty‐nine percent (131) of these patients were included. Exclusion criteria were: diagnosis at admission (n = 95, 45.9%), past medical history (n = 56, 27%) and length of stay less than 3 days, or less than 2 determinations of [Hb] or HCT (n = 56, 27%). Data collection was discontinued for the following reasons: discharge (81.7%), death (6.9%), surgery or procedures associated with blood loss (5.3%), transfer to a critical care unit (3%), transfusions (2.3%), and chemotherapy (0.8%). The baseline characteristics of the study patients are shown in Table 1.
| n | % | Mean (SD) | Median | Min/Max | |
|---|---|---|---|---|---|
| |||||
| Age, years | 71.9 (17.4) | 77 | 18/97 | ||
| 18‐40 | 11 | 8.4 | |||
| 41‐60 | 16 | 12.2 | |||
| 61‐80 | 52 | 39.7 | |||
| >80 | 52 | 39.7 | |||
| Gender | |||||
| Female | 75 | 57.2 | |||
| Lenght of stay (days) | 7 (4.8) | 6 | 3/28 | ||
| APS | 4.9 (4.2) | 4 | 0/22 | ||
| 0‐4 | 71 | 54.2 | |||
| 5‐8 | 36 | 27.5 | |||
| >8 | 24 | 18.3 | |||
| ADL | 4.5 (2.3) | 6 | 0/6 | ||
| 0‐2 | 33 | 25.2 | |||
| 3‐5 | 11 | 8.4 | |||
| 6 | 87 | 66.4 | |||
| CHARLSON | 2.2 (2.3) | 2 | 0/11 | ||
| 0 | 32 | 24.4 | |||
| 1 | 32 | 24.4 | |||
| 2 | 22 | 16.8 | |||
| 3 | 18 | 13.7 | |||
| >3 | 27 | 20.6 | |||
| Readmissions | 28 | 21.4 | |||
| PWSBL | 14 | 10.7 | |||
| Anemia at admission | 63 | 48.1 | |||
| [Hb] at admission | 12.5 (1.7) | 12.5 | 8.6/17 | ||
| [Hb] at admission males | 12.8 (1.9) | 12.6 | 8.7/17 | ||
| [Hb] at admission females | 12.3(1.5) | 12.3 | 8.6/15.5 | ||
Diagnoses at discharge were divided into the following categories: infections (25.2%), electrolyte disturbances (7.6%), cardiac diseases (9.9%), neurologic (19.1%), respiratory (16.8%), gastrointestinal (10.7%), and other diagnosis (10.7%).
No evidence of bleeding was found in the included patients. Bleeding was only observed in 4 of the initially evaluated patients who were excluded since they failed to meet the required number of [Hb] determinations before bleeding.
A mean decrease in [Hb] of 0.71 g/dL was found between admission and discharge day (P < 0.001; 95% CI, 0.47‐0.97). Mean nadir [Hb] was 1.45 g/dL lower than admission [Hb] (P < 0.001; 95% CI, 1.24‐1.67). Mean nadir day occurred between hospitalization days 3 and 4. Mean [Hb] at discharge was 11.8 1.8 g/dL. This value is higher than the mean concentration at nadir (0.74 g/dL P < 0.001; 95% CI, 0.60‐0.97) (Figure 1).
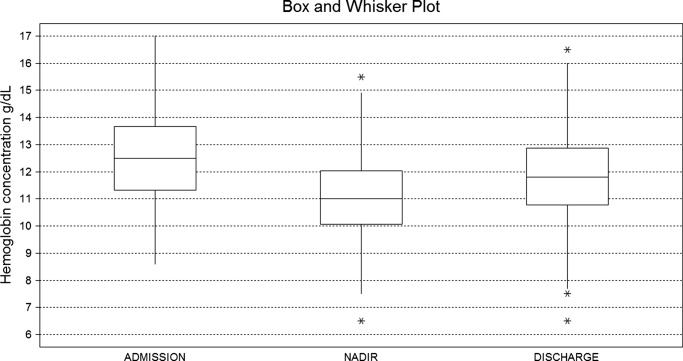
Table 2 shows the rate of patients with a decrease in the [Hb] for different cutoff levels. Forty‐five percent of the study population (59 patients) had a significant fall in [Hb] during hospitalization. This was observed on day 2 in 50% of the patients. Likewise, a significant fall in HCT value was found in 42.7% of patients. If [Hb] decrease during hospitalization is analyzed as a proportion of [Hb] at admission, 52.7% of patients had a 10% or greater [Hb] decrease during their hospital stay.
| [Hb] fall (g/dL) | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | 4.5 |
|---|---|---|---|---|---|---|---|---|---|
| % of patients | 80.9 | 60.3 | 45.0 | 28.2 | 17.6 | 9.9 | 5.3 | 3.8 | 2.3 |
Using Kaplan‐Meier analysis, the estimated proportion showing no significant fall of [Hb] was 0.63 (95% CI, 0.55‐0.71) on day 3 and 0.48 (95% CI, 0.29‐0.67) on day 10. By day 15, only 3.8% of the initially included patients were still hospitalized and showed no decrease in [Hb] 1.5 g/dl. These patients maintained their [Hb] stable for the rest of the follow up period (Figure 2).
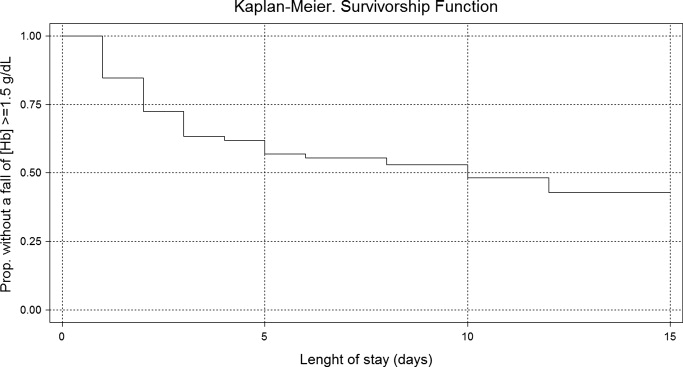
In the univariate analysis, comparing patients that experienced a decrease in [Hb] 1.5 g/dL with those who did not, significant differences were only found in the following variables: length of stay, APS, anemia at admission, [Hb] at admission, and infectious, gastrointestinal or cardiac diseases at discharge diagnosis (Table 3).
| Patients with a significant fall | Patients without a significant fall | P Value | |
|---|---|---|---|
| |||
| n | 59 (45%) | 72 (55%) | |
| Age, years | 73.15 (18.7) | 70.83 (16.2) | 0.448 |
| Gender, female | 32 (54.2%) | 43 (59.7%) | 0.527 |
| Length of stay (days) | 8.30 (5.6) | 5.91 (3.7) | <0.007 |
| APS | 6.13 (4.5) | 3.97 (3.7) | <0.004 |
| ADL | 4.33 (2.5) | 4.68 (2.1) | 0.410 |
| CHARLSON | 2.03 (1.8) | 2.37 (2.5) | 0.382 |
| Nurse home residents | 4 (6.8%) | 3 (4.2%) | 0.700 |
| Readmissions | 11 (18.6%) | 17 (23.6%) | 0.490 |
| PWSBL | 6 (10.2%) | 8 (11.1%) | 0.862 |
| Anemia at admission | 20 (33.9%) | 43 (59.7%) | <0.004 |
| [Hb] at admission | 13.09 (1.7) | 12.01 (1.5) | <0.001 |
| Diagnosis at discharge | |||
| Infectious | 20 (33.9%) | 13 (18.1%) | <0.05 |
| Respiratory | 8 (13.6%) | 14 (19.4%) | 0.370 |
| Neurologic | 9 (15.2%) | 16 (22.2%) | 0.312 |
| Gastrointestinal | 11 (18.6%) | 3 (4.2%) | <0.01 |
| Cardiac | 2 (3.4%) | 11(15.3%) | <0.05 |
| Electrolyte disturbances | 6 (10.2%) | 4 (5.6%) | 0.512 |
| Others | 3 (5.1%) | 11 (15.3%) | 0.087 |
In the Cox proportional hazard model adjusting for the variables shown in Table 4, a significant independent direct association was found between a significant fall in [Hb] during hospital stay and APS, [Hb] at admission, and either diagnosis of infectious or gastrointestinal disease at discharge. Similar results were found if a significant fall was redefined as a 12% decrease in [Hb] or as a 4.5 point decrease in HCT from baseline.
| Variable | HRR | P Value | 95% CI |
|---|---|---|---|
| |||
| APS | 1.07 | 0.007 | 1.02‐1.12 |
| ADL | 1.11 | 0.132 | 0.97‐1.27 |
| Charlson | 0.88 | 0.121 | 0.75‐1.03 |
| Nurse home resident | 1.52 | 0.361 | 0.62‐3.72 |
| PWSBL | 0.67 | 0.390 | 0.27‐1.66 |
| Readmission | 1.14 | 0.710 | 0.57‐2.29 |
| Female sex | 0.98 | 0.944 | 0.57‐1.69 |
| Age | 1.39 | 0.098 | 0.94‐2.07 |
| [Hb] at admission | 1.27 | 0.005 | 1.07‐1.51 |
| Diagnosis at discharge | |||
| Infectious | 2.70 | 0.015 | 1.21‐6.05 |
| Neurologic | 1.42 | 0.457 | 0.57‐3.55 |
| Gastrointestinal | 3.74 | 0.002 | 1.62‐8.64 |
| Cardiac | 0.41 | 0.289 | 0.08‐2.12 |
| Electrolyte dist. | 2.08 | 0.176 | 0.72‐6.05 |
| Others | 0.95 | 0.946 | 0.24‐3.81 |
Discussion
This study describes the variation of [Hb] and HCT values in GWAP without bleeding or other obvious medical conditions associated with a decrease in the red blood cell mass. As previously described,4, 20, 21 we found that [Hb] decreases during hospital stay are frequently observed. The mean [Hb] fall from admission was 1.45 g/dL, and was mainly recorded between hospitalization days 3 and 4. In approximately half of the study population, a 1.5 g/dL decrease in [Hb] was observed. In the survival analysis, 40% and 55% of patients are expected to present such a fall on day 4 and 12 respectively. In accordance with prior reports4, 21 a greater decrease in [Hb] occurred in the first days of hospitalization, and a high proportion of patients were already anemic at the time of admission.
The following variables were associated with a decrease in the [Hb] during hospitalization: higher APS score, higher [Hb] at admission, and diagnosis of infectious or gastrointestinal disease at discharge. Even though several mechanisms seem to contribute to the decrease in [Hb] during hospitalization our data suggest that 1 of these factors seems to be the severity of the disease, as previously proposed by Nguyen et al.4 This observation is supported by the association found in our study between [Hb] decrease and APS. No association was found with Charlson, ADL, and being a nursing home resident. These variables, which have not been previously analyzed, seem to indicate that patients with chronic illnesses are not more likely to have decreases in [Hb] during hospitalization.
Patients with higher [Hb] at admission had a greater [Hb] decrease during their hospital stay. This finding could suggest that the mechanism related to this decrease had less effect on patients with lower [Hb]. This is similar to that observed in the anemia of chronic diseases where [Hb] does not usually fall to extreme values. Our analysis reveals a greater decrease during the first days of hospitalization. Given the high rate of patients anemic at admission, it is possible that the decrease in [Hb] had begun prior to admission.
Previous papers describe a low prevalence of cyanocobalamine (2%), folic acid (2%), and iron (9%) deficiency in patients admitted to critical care units.3 In these patients an inadequate bone marrow response has been proposed as a mechanism for [Hb] decrease, a phenomenon that has been called AI or anemia of critical illness.8, 2228 Inflammatory response associated with acute disease causes this hypoproliferative anemia through 3 different pathways: relative erythropoietin deficit, a direct inhibition of the erythropoiesis in the bone marrow through different mediators (ie, interleukin [IL]‐1, IL‐6, tumor necrosis factor [TNF]) and a functional iron deficit. This relative iron deficiency is produced mainly by the IL‐6 induced overexpression of the hepcidin gene in hepatocytes. Hepcidin causes impaired intestinal iron absorption and an inadequate delivery of iron from the iron‐recycling macrophages to the erythroid precursors in the bone marrow.8, 9
AI could explain some of our findings, such as the greater decrease observed in the first hospital days when inflamatory mediators levels are expected to be higher. The association between APS and infectious disease diagnosis and a greater [Hb] decrease may also be explained by this mechanism. Nonetheless, the daily bone marrow production of red blood cells is small compared with the circulating red mass cell and therefore it is necessary to have a better explanation on how AI could be associated with this rapid decrease in the [Hb]. Rice et al.30, 31 have explored some mechanisms leading to a rapid decrease in the red mass cell related to acute variations in erytropoietin level. A role of this mechanism and other could be hypothesized.29
Overt bleeding was not found in our study population and procedures without significant blood loss (PWSBL) were infrequent, therefore it is unlikely that they could have had an impact on the decrease of [Hb]. The influence of other variables, such as blood volume drawn for diagnostic testing and occult blood losses (especially from the gastrointestinal tract) was not investigated. Parenteral hydration and hemodilution neither were evaluated in our study nor were extensively described in the literature. We think this last mentioned mechanism plays a role in the large [Hb] decrease during the first days of hospitalization. However a lack of association was mentioned in the only study that evaluated this issue in critically ill patients. Intravascular hemolysis has been cited as an infrequent event in these patients.2, 4
Only patients without a clear explanation for their [Hb] decrease were included because they represent a matter of concern for hospitalists and other treating physicians. Therefore 60% of the initially evaluated patients were excluded, since they were admitted for conditions likely to cause a decrease in [Hb]. Nevertheless, it seems likely that the mechanisms involved in the included patients may play a role in those with an obvious cause for [Hb] variation as well. Accordingly, the decrease of [Hb] expected in patients admitted with disorders known to cause a decrease in the red blood cell mass would be greater than the 1 observed in our study population.
This [Hb] drop during hospitalization may be clinically relevant in a number of ways: it could cause the attending physicians to order costly and invasive studies, it could have prognostic value as it occurs in patients admitted with myocardial infarction, and it could trigger an acute coronary syndrome.20 We observed an association between [Hb] drop and a higher APS in our patients. This score has been validated as a prognostic factor in previous studies. However, it is not possible to conclude that this [Hb] drop is associated with a worse prognosis.
Our study had several limitations. The sample's heterogeneity inherent to our general ward population could have altered our results and their generalization. To overcome this bias we used a categorization system based on discharge diagnosis. However, this particular system has several limitations. The small sample size and the absence of follow up data after discharge limited our capacity to detect any prognostic significance of [Hb] decrease. In the present study, the total decrease of [Hb] may have been underestimated because the onset of the acute illness precedes hospital admission by a variable time. Finally, a relatively small sample size limited our ability to detect other possible significant predictors of [Hb] decrease. However, this study was not designed to assess the mechanisms associated with the decrease of [Hb], but rather to establish its occurrence, measure it and explore related variables.
These results may be useful for further studies to evaluate [Hb] variation in admitted patients and its relation to other variables, such as bone marrow production, oxygen use, erythrocyte survival, nutritional deficiencies, and erythropoietin and inflammatory mediators levels.
In conclusion, our general medical inpatients had a mean 1.45 g/dL [Hb] decrease during hospitalization, which was greater in the first days of hospitalization, even though an evident cause was not present. These findings would help attending physicians in general wards make a rational and efficient approach when dealing with patients' decrease in [Hb].
Acknowledgements
The authors thank Jorge Lopez Camelo and Hugo Krupitzki for statistical advice and Valeria Melia for helping in the preparation of this manuscript.
- ,.Nosocomial anemia.JAMA.1973;223(1):73–74.
- ,,,,.Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients.Crit Care Med.1999;27(12):2630–2639.
- ,,,,,.Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness.J Crit Care.2001;16(1):36–41.
- ,,,.Time course of hemoglobin concentrations in nonbleeding intensive care unit patients.Crit Care Med.2003;31(2):406–410.
- ,,, et al.Erithropoietin response in critically ill patients.Crit Care Med.1997;25(Suppl1):a82.
- ,,.Acute event‐related anaemia.Br J Haematol.2001;115(4):739–743.
- ,,,,,.Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients.Crit Care Med.2000;28(8):2773–2778.
- .The anemia of inflammation/malignancy: mechanisms and management.Hematology Am Soc Hematol Educ Program.2008:159–165.
- .The regulation of hepcidin and its effects on systemic and cellular iron metabolism.Hematology Am Soc Hematol Educ Program.2008:151–158.
- ,,, et al.,Erythropoietin response is blunted in critically ill patients.Intensive Care Med.1997;23:159–162.
- ,,,,.Studies of illness in the aged. The index of ADL: standardized measure of biological and psychosocial function.JAMA.1963;185:914–919.
- ,,,Progress in the development of the index of ADL.Gerontologist.1970;10:20–30.
- ,,,,.Measuring prognosis and case mix in hospitalized elders. The importance of functional status.J Gen Intern Med.1997;12:203–208.
- ,,,.APACHE II: A severity of disease classification system.Crit Care Med.1985;12(10):818–829.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chron Dis.1997;40:373–383.
- ,,,.How to measure comorbidity: a critical review of available methods.J Clin Epidemiol.2003;56:221–229.
- ,,, et al.Nutritional anaemias. Report of a WHO Scientific Group.World Health Organ Tech Rep Ser.1968;405:1–40.
- ,,,.Relationship between hematocrit and renal function in men and women.Kidney Int.2001;59(2):725–731.
- ,,.Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey.J Am Soc Nephrol.2002;13(2):504–510.
- ,,, et al.,Changes in haemoglobin levels during hospital course and long‐term outcome after myocardial infarction.Eur Heart J.2007;28(11):1289–1296.
- ,,,,.Do blood test cause anemia in hospitalized patients?J Gen Intern Med.2005;20:520–524.
- ,.Red blood cell physiology in critical illness.Crit Care Med.2003;31(12 Suppl)( ):S651–S657.
- .Anemia in the critically ill.Crit Care Clin.2004;20:159–178.
- ,.Anemia of the critically ill: acute anemia of chronic disease.Crit Care Med.2000;28(8):3098–3099.
- .Anemia and blood transfusion in the critically ill patient: role of erythropoietin.Crit Care.2004;8(Suppl 2):S42–S44.
- .Anemia of critical illness – implications for understanding and treating rHuEPO resistance.Nephrol Dial Transplant.2002;17(Suppl 5):S48–S55.
- ,,.Transfusion practice in the critically ill.Crit Care Med.2003;31(Suppl):S668–S671.
- .Anemia in critically ill patients.Eur J Inter Med.2004;15(8):481–486.
- ,,,.Neocytolysis: a physiologic down‐regulator of red blood cell mass.Lancet.1997;349:1389–1390.
- ,,,,,.Neocytolysis contributes to the anemia of renal disease.Am J Kidney Dis.1999;33:59–62.
- ,,, et al.Neocytolysis on descent from altitude: a newly recognized mechanism for the control of red cell mass.Ann Intern Med.2001;134:652–656.
- ,.Phlebotomy for diagnostic laboratory test in adults. Pattern of use and effect on transfusion requirements.N Engl J Med.1986;314:1233–1235.
Studies of the red blood cell mass performed in hospitalized patients were first done in the early 1970s.1 Thereafter, a decrease in the hemoglobin concentration ([Hb]) and its potential causes have been further reported, especially in critically ill patients.25 The decrease in [Hb] can occur as a result of blood draws for diagnostic testing; blood loss associated with invasive procedures and bleeding; occult gastrointestinal bleeding; hemolysis; shortening of red cell survival; iron, folic acid or cobalamine deficiencies; renal, liver or endocrine failure; hemodilution associated with fluid therapy; and the so‐called anemia of inflammation (AI).68 The latter would be a consequence of a blunted response of the bone marrow due to several factors such as inadequate secretion of erythropoietin, inhibition of the proliferation and differentiation of the erythroid precursors of the bone marrow and an hepcidin‐mediated functional iron deficiency.810
As mentioned, most studies were done in critically ill patients and scarce information is available about general ward admitted patients (GWAP) with less severe illnesses. In this scenario, some of the proposed mechanisms may have a less clear role. It is widely accepted that [Hb] may decrease without overt bleeding in GWAP. However, given the lack of information on this matter, laboratory controls and invasive procedures are undertaken to determine its potential causes.
The purpose of the present study is to describe [Hb] variation over time in nonbleeding GWAP, to estimate the proportion of patients with [Hb] decreases 1.5 g/dL, and to evaluate possible related variables.
Materials and Methods
A 16‐week (September 2004‐January 2005) prospective observational study was conducted in Internal Medicine GWAP at 2 Buenos Aires teaching hospitals.
All consecutive patients older than 16 years were evaluated. Patients admitted for the following reasons were excluded: bleeding, trauma, surgery, invasive procedures associated with blood loss (biopsies, biliary drainage, endovascular therapeutic procedures, and chest tubes), blood transfusions, anemia, chemotherapy, and acute renal failure.
Patients with a bleeding history, chemotherapy or radiation therapy within two months prior to admission, patients on dialysis and patients with current oncologic or hematologic disease were excluded, as well as those with length of stay less than 3 days, or with less than 2 [Hb] or hematocrit (HCT) measurements.
Patients were followed until discharge, death or transfer to a critical care unit. Patients were withdrawn from the study if they presented with bleeding or hemolysis, underwent red blood cell transfusion or therapy affecting hemoglobin levels (iron, chemotherapy or erythropoietin) or if either a surgical or invasive procedure associated with blood loss was performed.
Data were collected from patients' medical records and additional information was obtained from treating physicians and patients by the authors (AL, NC, SM, AN, MH). Standardized case report forms were completed during the hospitalization including: age, sex, admissions in the previous 3 months (readmissions) and whether or not the patient lived in a nursing home. Patients were categorized according to discharge diagnosis as reported by Nguyen et al.4 with modifications related to our general ward population. Upon admission Katz daily activity index (ADL),11, 12 APACHE II acute physiology score (APS),13, 14 and Charlson comorbidity score (Charlson)15, 16 were assessed. In all cases the [Hb] and HCT values were registered on admission and on days 3, 6, 10 and prior to discharge as well as any other determination required by the treating physician. Anemia was defined as [Hb] 13 g/dL for men and 12 g/dL for women.17 Based on previous reports a 1.5 g/dL decrease in [Hb] and of 4.5 points in HCT compared to admission values were considered a significant fall.1, 4 Acute renal failure was defined as an increase in creatinine level 0.5 mg/dL or a 50% increase from baseline.18, 19
Every procedure without significant blood loss (PWSBL) such as venous catheter placement, thoracentesis, lumbar punction, paracentesis, skin biopsy, arthrocentesis, and diagnostic angiogram was also recorded. The study was approved by the Hospital's ethical committee.
Data analysis and processing were performed with Excel 2000 and Stata version 8.0 (Stata Corp; USA). For continuous variables, results were expressed by the mean value its standard deviation (SD), and compared with Student's t‐test. The chi‐square test was used to compare categorical variables. A survival curve was developed with the Kaplan‐Meier method to analyze the time to a significant fall in [Hb]. Finally, Cox proportional hazard modeling was performed to assess the association between a significant fall in [Hb] and other variables. We accepted P < 0,05 as significant.
Results
A total of 338 patients were admitted to the Internal Medicine Inpatient Services in the study period. Thirty‐nine percent (131) of these patients were included. Exclusion criteria were: diagnosis at admission (n = 95, 45.9%), past medical history (n = 56, 27%) and length of stay less than 3 days, or less than 2 determinations of [Hb] or HCT (n = 56, 27%). Data collection was discontinued for the following reasons: discharge (81.7%), death (6.9%), surgery or procedures associated with blood loss (5.3%), transfer to a critical care unit (3%), transfusions (2.3%), and chemotherapy (0.8%). The baseline characteristics of the study patients are shown in Table 1.
| n | % | Mean (SD) | Median | Min/Max | |
|---|---|---|---|---|---|
| |||||
| Age, years | 71.9 (17.4) | 77 | 18/97 | ||
| 18‐40 | 11 | 8.4 | |||
| 41‐60 | 16 | 12.2 | |||
| 61‐80 | 52 | 39.7 | |||
| >80 | 52 | 39.7 | |||
| Gender | |||||
| Female | 75 | 57.2 | |||
| Lenght of stay (days) | 7 (4.8) | 6 | 3/28 | ||
| APS | 4.9 (4.2) | 4 | 0/22 | ||
| 0‐4 | 71 | 54.2 | |||
| 5‐8 | 36 | 27.5 | |||
| >8 | 24 | 18.3 | |||
| ADL | 4.5 (2.3) | 6 | 0/6 | ||
| 0‐2 | 33 | 25.2 | |||
| 3‐5 | 11 | 8.4 | |||
| 6 | 87 | 66.4 | |||
| CHARLSON | 2.2 (2.3) | 2 | 0/11 | ||
| 0 | 32 | 24.4 | |||
| 1 | 32 | 24.4 | |||
| 2 | 22 | 16.8 | |||
| 3 | 18 | 13.7 | |||
| >3 | 27 | 20.6 | |||
| Readmissions | 28 | 21.4 | |||
| PWSBL | 14 | 10.7 | |||
| Anemia at admission | 63 | 48.1 | |||
| [Hb] at admission | 12.5 (1.7) | 12.5 | 8.6/17 | ||
| [Hb] at admission males | 12.8 (1.9) | 12.6 | 8.7/17 | ||
| [Hb] at admission females | 12.3(1.5) | 12.3 | 8.6/15.5 | ||
Diagnoses at discharge were divided into the following categories: infections (25.2%), electrolyte disturbances (7.6%), cardiac diseases (9.9%), neurologic (19.1%), respiratory (16.8%), gastrointestinal (10.7%), and other diagnosis (10.7%).
No evidence of bleeding was found in the included patients. Bleeding was only observed in 4 of the initially evaluated patients who were excluded since they failed to meet the required number of [Hb] determinations before bleeding.
A mean decrease in [Hb] of 0.71 g/dL was found between admission and discharge day (P < 0.001; 95% CI, 0.47‐0.97). Mean nadir [Hb] was 1.45 g/dL lower than admission [Hb] (P < 0.001; 95% CI, 1.24‐1.67). Mean nadir day occurred between hospitalization days 3 and 4. Mean [Hb] at discharge was 11.8 1.8 g/dL. This value is higher than the mean concentration at nadir (0.74 g/dL P < 0.001; 95% CI, 0.60‐0.97) (Figure 1).

Table 2 shows the rate of patients with a decrease in the [Hb] for different cutoff levels. Forty‐five percent of the study population (59 patients) had a significant fall in [Hb] during hospitalization. This was observed on day 2 in 50% of the patients. Likewise, a significant fall in HCT value was found in 42.7% of patients. If [Hb] decrease during hospitalization is analyzed as a proportion of [Hb] at admission, 52.7% of patients had a 10% or greater [Hb] decrease during their hospital stay.
| [Hb] fall (g/dL) | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | 4.5 |
|---|---|---|---|---|---|---|---|---|---|
| % of patients | 80.9 | 60.3 | 45.0 | 28.2 | 17.6 | 9.9 | 5.3 | 3.8 | 2.3 |
Using Kaplan‐Meier analysis, the estimated proportion showing no significant fall of [Hb] was 0.63 (95% CI, 0.55‐0.71) on day 3 and 0.48 (95% CI, 0.29‐0.67) on day 10. By day 15, only 3.8% of the initially included patients were still hospitalized and showed no decrease in [Hb] 1.5 g/dl. These patients maintained their [Hb] stable for the rest of the follow up period (Figure 2).

In the univariate analysis, comparing patients that experienced a decrease in [Hb] 1.5 g/dL with those who did not, significant differences were only found in the following variables: length of stay, APS, anemia at admission, [Hb] at admission, and infectious, gastrointestinal or cardiac diseases at discharge diagnosis (Table 3).
| Patients with a significant fall | Patients without a significant fall | P Value | |
|---|---|---|---|
| |||
| n | 59 (45%) | 72 (55%) | |
| Age, years | 73.15 (18.7) | 70.83 (16.2) | 0.448 |
| Gender, female | 32 (54.2%) | 43 (59.7%) | 0.527 |
| Length of stay (days) | 8.30 (5.6) | 5.91 (3.7) | <0.007 |
| APS | 6.13 (4.5) | 3.97 (3.7) | <0.004 |
| ADL | 4.33 (2.5) | 4.68 (2.1) | 0.410 |
| CHARLSON | 2.03 (1.8) | 2.37 (2.5) | 0.382 |
| Nurse home residents | 4 (6.8%) | 3 (4.2%) | 0.700 |
| Readmissions | 11 (18.6%) | 17 (23.6%) | 0.490 |
| PWSBL | 6 (10.2%) | 8 (11.1%) | 0.862 |
| Anemia at admission | 20 (33.9%) | 43 (59.7%) | <0.004 |
| [Hb] at admission | 13.09 (1.7) | 12.01 (1.5) | <0.001 |
| Diagnosis at discharge | |||
| Infectious | 20 (33.9%) | 13 (18.1%) | <0.05 |
| Respiratory | 8 (13.6%) | 14 (19.4%) | 0.370 |
| Neurologic | 9 (15.2%) | 16 (22.2%) | 0.312 |
| Gastrointestinal | 11 (18.6%) | 3 (4.2%) | <0.01 |
| Cardiac | 2 (3.4%) | 11(15.3%) | <0.05 |
| Electrolyte disturbances | 6 (10.2%) | 4 (5.6%) | 0.512 |
| Others | 3 (5.1%) | 11 (15.3%) | 0.087 |
In the Cox proportional hazard model adjusting for the variables shown in Table 4, a significant independent direct association was found between a significant fall in [Hb] during hospital stay and APS, [Hb] at admission, and either diagnosis of infectious or gastrointestinal disease at discharge. Similar results were found if a significant fall was redefined as a 12% decrease in [Hb] or as a 4.5 point decrease in HCT from baseline.
| Variable | HRR | P Value | 95% CI |
|---|---|---|---|
| |||
| APS | 1.07 | 0.007 | 1.02‐1.12 |
| ADL | 1.11 | 0.132 | 0.97‐1.27 |
| Charlson | 0.88 | 0.121 | 0.75‐1.03 |
| Nurse home resident | 1.52 | 0.361 | 0.62‐3.72 |
| PWSBL | 0.67 | 0.390 | 0.27‐1.66 |
| Readmission | 1.14 | 0.710 | 0.57‐2.29 |
| Female sex | 0.98 | 0.944 | 0.57‐1.69 |
| Age | 1.39 | 0.098 | 0.94‐2.07 |
| [Hb] at admission | 1.27 | 0.005 | 1.07‐1.51 |
| Diagnosis at discharge | |||
| Infectious | 2.70 | 0.015 | 1.21‐6.05 |
| Neurologic | 1.42 | 0.457 | 0.57‐3.55 |
| Gastrointestinal | 3.74 | 0.002 | 1.62‐8.64 |
| Cardiac | 0.41 | 0.289 | 0.08‐2.12 |
| Electrolyte dist. | 2.08 | 0.176 | 0.72‐6.05 |
| Others | 0.95 | 0.946 | 0.24‐3.81 |
Discussion
This study describes the variation of [Hb] and HCT values in GWAP without bleeding or other obvious medical conditions associated with a decrease in the red blood cell mass. As previously described,4, 20, 21 we found that [Hb] decreases during hospital stay are frequently observed. The mean [Hb] fall from admission was 1.45 g/dL, and was mainly recorded between hospitalization days 3 and 4. In approximately half of the study population, a 1.5 g/dL decrease in [Hb] was observed. In the survival analysis, 40% and 55% of patients are expected to present such a fall on day 4 and 12 respectively. In accordance with prior reports4, 21 a greater decrease in [Hb] occurred in the first days of hospitalization, and a high proportion of patients were already anemic at the time of admission.
The following variables were associated with a decrease in the [Hb] during hospitalization: higher APS score, higher [Hb] at admission, and diagnosis of infectious or gastrointestinal disease at discharge. Even though several mechanisms seem to contribute to the decrease in [Hb] during hospitalization our data suggest that 1 of these factors seems to be the severity of the disease, as previously proposed by Nguyen et al.4 This observation is supported by the association found in our study between [Hb] decrease and APS. No association was found with Charlson, ADL, and being a nursing home resident. These variables, which have not been previously analyzed, seem to indicate that patients with chronic illnesses are not more likely to have decreases in [Hb] during hospitalization.
Patients with higher [Hb] at admission had a greater [Hb] decrease during their hospital stay. This finding could suggest that the mechanism related to this decrease had less effect on patients with lower [Hb]. This is similar to that observed in the anemia of chronic diseases where [Hb] does not usually fall to extreme values. Our analysis reveals a greater decrease during the first days of hospitalization. Given the high rate of patients anemic at admission, it is possible that the decrease in [Hb] had begun prior to admission.
Previous papers describe a low prevalence of cyanocobalamine (2%), folic acid (2%), and iron (9%) deficiency in patients admitted to critical care units.3 In these patients an inadequate bone marrow response has been proposed as a mechanism for [Hb] decrease, a phenomenon that has been called AI or anemia of critical illness.8, 2228 Inflammatory response associated with acute disease causes this hypoproliferative anemia through 3 different pathways: relative erythropoietin deficit, a direct inhibition of the erythropoiesis in the bone marrow through different mediators (ie, interleukin [IL]‐1, IL‐6, tumor necrosis factor [TNF]) and a functional iron deficit. This relative iron deficiency is produced mainly by the IL‐6 induced overexpression of the hepcidin gene in hepatocytes. Hepcidin causes impaired intestinal iron absorption and an inadequate delivery of iron from the iron‐recycling macrophages to the erythroid precursors in the bone marrow.8, 9
AI could explain some of our findings, such as the greater decrease observed in the first hospital days when inflamatory mediators levels are expected to be higher. The association between APS and infectious disease diagnosis and a greater [Hb] decrease may also be explained by this mechanism. Nonetheless, the daily bone marrow production of red blood cells is small compared with the circulating red mass cell and therefore it is necessary to have a better explanation on how AI could be associated with this rapid decrease in the [Hb]. Rice et al.30, 31 have explored some mechanisms leading to a rapid decrease in the red mass cell related to acute variations in erytropoietin level. A role of this mechanism and other could be hypothesized.29
Overt bleeding was not found in our study population and procedures without significant blood loss (PWSBL) were infrequent, therefore it is unlikely that they could have had an impact on the decrease of [Hb]. The influence of other variables, such as blood volume drawn for diagnostic testing and occult blood losses (especially from the gastrointestinal tract) was not investigated. Parenteral hydration and hemodilution neither were evaluated in our study nor were extensively described in the literature. We think this last mentioned mechanism plays a role in the large [Hb] decrease during the first days of hospitalization. However a lack of association was mentioned in the only study that evaluated this issue in critically ill patients. Intravascular hemolysis has been cited as an infrequent event in these patients.2, 4
Only patients without a clear explanation for their [Hb] decrease were included because they represent a matter of concern for hospitalists and other treating physicians. Therefore 60% of the initially evaluated patients were excluded, since they were admitted for conditions likely to cause a decrease in [Hb]. Nevertheless, it seems likely that the mechanisms involved in the included patients may play a role in those with an obvious cause for [Hb] variation as well. Accordingly, the decrease of [Hb] expected in patients admitted with disorders known to cause a decrease in the red blood cell mass would be greater than the 1 observed in our study population.
This [Hb] drop during hospitalization may be clinically relevant in a number of ways: it could cause the attending physicians to order costly and invasive studies, it could have prognostic value as it occurs in patients admitted with myocardial infarction, and it could trigger an acute coronary syndrome.20 We observed an association between [Hb] drop and a higher APS in our patients. This score has been validated as a prognostic factor in previous studies. However, it is not possible to conclude that this [Hb] drop is associated with a worse prognosis.
Our study had several limitations. The sample's heterogeneity inherent to our general ward population could have altered our results and their generalization. To overcome this bias we used a categorization system based on discharge diagnosis. However, this particular system has several limitations. The small sample size and the absence of follow up data after discharge limited our capacity to detect any prognostic significance of [Hb] decrease. In the present study, the total decrease of [Hb] may have been underestimated because the onset of the acute illness precedes hospital admission by a variable time. Finally, a relatively small sample size limited our ability to detect other possible significant predictors of [Hb] decrease. However, this study was not designed to assess the mechanisms associated with the decrease of [Hb], but rather to establish its occurrence, measure it and explore related variables.
These results may be useful for further studies to evaluate [Hb] variation in admitted patients and its relation to other variables, such as bone marrow production, oxygen use, erythrocyte survival, nutritional deficiencies, and erythropoietin and inflammatory mediators levels.
In conclusion, our general medical inpatients had a mean 1.45 g/dL [Hb] decrease during hospitalization, which was greater in the first days of hospitalization, even though an evident cause was not present. These findings would help attending physicians in general wards make a rational and efficient approach when dealing with patients' decrease in [Hb].
Acknowledgements
The authors thank Jorge Lopez Camelo and Hugo Krupitzki for statistical advice and Valeria Melia for helping in the preparation of this manuscript.
Studies of the red blood cell mass performed in hospitalized patients were first done in the early 1970s.1 Thereafter, a decrease in the hemoglobin concentration ([Hb]) and its potential causes have been further reported, especially in critically ill patients.25 The decrease in [Hb] can occur as a result of blood draws for diagnostic testing; blood loss associated with invasive procedures and bleeding; occult gastrointestinal bleeding; hemolysis; shortening of red cell survival; iron, folic acid or cobalamine deficiencies; renal, liver or endocrine failure; hemodilution associated with fluid therapy; and the so‐called anemia of inflammation (AI).68 The latter would be a consequence of a blunted response of the bone marrow due to several factors such as inadequate secretion of erythropoietin, inhibition of the proliferation and differentiation of the erythroid precursors of the bone marrow and an hepcidin‐mediated functional iron deficiency.810
As mentioned, most studies were done in critically ill patients and scarce information is available about general ward admitted patients (GWAP) with less severe illnesses. In this scenario, some of the proposed mechanisms may have a less clear role. It is widely accepted that [Hb] may decrease without overt bleeding in GWAP. However, given the lack of information on this matter, laboratory controls and invasive procedures are undertaken to determine its potential causes.
The purpose of the present study is to describe [Hb] variation over time in nonbleeding GWAP, to estimate the proportion of patients with [Hb] decreases 1.5 g/dL, and to evaluate possible related variables.
Materials and Methods
A 16‐week (September 2004‐January 2005) prospective observational study was conducted in Internal Medicine GWAP at 2 Buenos Aires teaching hospitals.
All consecutive patients older than 16 years were evaluated. Patients admitted for the following reasons were excluded: bleeding, trauma, surgery, invasive procedures associated with blood loss (biopsies, biliary drainage, endovascular therapeutic procedures, and chest tubes), blood transfusions, anemia, chemotherapy, and acute renal failure.
Patients with a bleeding history, chemotherapy or radiation therapy within two months prior to admission, patients on dialysis and patients with current oncologic or hematologic disease were excluded, as well as those with length of stay less than 3 days, or with less than 2 [Hb] or hematocrit (HCT) measurements.
Patients were followed until discharge, death or transfer to a critical care unit. Patients were withdrawn from the study if they presented with bleeding or hemolysis, underwent red blood cell transfusion or therapy affecting hemoglobin levels (iron, chemotherapy or erythropoietin) or if either a surgical or invasive procedure associated with blood loss was performed.
Data were collected from patients' medical records and additional information was obtained from treating physicians and patients by the authors (AL, NC, SM, AN, MH). Standardized case report forms were completed during the hospitalization including: age, sex, admissions in the previous 3 months (readmissions) and whether or not the patient lived in a nursing home. Patients were categorized according to discharge diagnosis as reported by Nguyen et al.4 with modifications related to our general ward population. Upon admission Katz daily activity index (ADL),11, 12 APACHE II acute physiology score (APS),13, 14 and Charlson comorbidity score (Charlson)15, 16 were assessed. In all cases the [Hb] and HCT values were registered on admission and on days 3, 6, 10 and prior to discharge as well as any other determination required by the treating physician. Anemia was defined as [Hb] 13 g/dL for men and 12 g/dL for women.17 Based on previous reports a 1.5 g/dL decrease in [Hb] and of 4.5 points in HCT compared to admission values were considered a significant fall.1, 4 Acute renal failure was defined as an increase in creatinine level 0.5 mg/dL or a 50% increase from baseline.18, 19
Every procedure without significant blood loss (PWSBL) such as venous catheter placement, thoracentesis, lumbar punction, paracentesis, skin biopsy, arthrocentesis, and diagnostic angiogram was also recorded. The study was approved by the Hospital's ethical committee.
Data analysis and processing were performed with Excel 2000 and Stata version 8.0 (Stata Corp; USA). For continuous variables, results were expressed by the mean value its standard deviation (SD), and compared with Student's t‐test. The chi‐square test was used to compare categorical variables. A survival curve was developed with the Kaplan‐Meier method to analyze the time to a significant fall in [Hb]. Finally, Cox proportional hazard modeling was performed to assess the association between a significant fall in [Hb] and other variables. We accepted P < 0,05 as significant.
Results
A total of 338 patients were admitted to the Internal Medicine Inpatient Services in the study period. Thirty‐nine percent (131) of these patients were included. Exclusion criteria were: diagnosis at admission (n = 95, 45.9%), past medical history (n = 56, 27%) and length of stay less than 3 days, or less than 2 determinations of [Hb] or HCT (n = 56, 27%). Data collection was discontinued for the following reasons: discharge (81.7%), death (6.9%), surgery or procedures associated with blood loss (5.3%), transfer to a critical care unit (3%), transfusions (2.3%), and chemotherapy (0.8%). The baseline characteristics of the study patients are shown in Table 1.
| n | % | Mean (SD) | Median | Min/Max | |
|---|---|---|---|---|---|
| |||||
| Age, years | 71.9 (17.4) | 77 | 18/97 | ||
| 18‐40 | 11 | 8.4 | |||
| 41‐60 | 16 | 12.2 | |||
| 61‐80 | 52 | 39.7 | |||
| >80 | 52 | 39.7 | |||
| Gender | |||||
| Female | 75 | 57.2 | |||
| Lenght of stay (days) | 7 (4.8) | 6 | 3/28 | ||
| APS | 4.9 (4.2) | 4 | 0/22 | ||
| 0‐4 | 71 | 54.2 | |||
| 5‐8 | 36 | 27.5 | |||
| >8 | 24 | 18.3 | |||
| ADL | 4.5 (2.3) | 6 | 0/6 | ||
| 0‐2 | 33 | 25.2 | |||
| 3‐5 | 11 | 8.4 | |||
| 6 | 87 | 66.4 | |||
| CHARLSON | 2.2 (2.3) | 2 | 0/11 | ||
| 0 | 32 | 24.4 | |||
| 1 | 32 | 24.4 | |||
| 2 | 22 | 16.8 | |||
| 3 | 18 | 13.7 | |||
| >3 | 27 | 20.6 | |||
| Readmissions | 28 | 21.4 | |||
| PWSBL | 14 | 10.7 | |||
| Anemia at admission | 63 | 48.1 | |||
| [Hb] at admission | 12.5 (1.7) | 12.5 | 8.6/17 | ||
| [Hb] at admission males | 12.8 (1.9) | 12.6 | 8.7/17 | ||
| [Hb] at admission females | 12.3(1.5) | 12.3 | 8.6/15.5 | ||
Diagnoses at discharge were divided into the following categories: infections (25.2%), electrolyte disturbances (7.6%), cardiac diseases (9.9%), neurologic (19.1%), respiratory (16.8%), gastrointestinal (10.7%), and other diagnosis (10.7%).
No evidence of bleeding was found in the included patients. Bleeding was only observed in 4 of the initially evaluated patients who were excluded since they failed to meet the required number of [Hb] determinations before bleeding.
A mean decrease in [Hb] of 0.71 g/dL was found between admission and discharge day (P < 0.001; 95% CI, 0.47‐0.97). Mean nadir [Hb] was 1.45 g/dL lower than admission [Hb] (P < 0.001; 95% CI, 1.24‐1.67). Mean nadir day occurred between hospitalization days 3 and 4. Mean [Hb] at discharge was 11.8 1.8 g/dL. This value is higher than the mean concentration at nadir (0.74 g/dL P < 0.001; 95% CI, 0.60‐0.97) (Figure 1).

Table 2 shows the rate of patients with a decrease in the [Hb] for different cutoff levels. Forty‐five percent of the study population (59 patients) had a significant fall in [Hb] during hospitalization. This was observed on day 2 in 50% of the patients. Likewise, a significant fall in HCT value was found in 42.7% of patients. If [Hb] decrease during hospitalization is analyzed as a proportion of [Hb] at admission, 52.7% of patients had a 10% or greater [Hb] decrease during their hospital stay.
| [Hb] fall (g/dL) | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | 4.5 |
|---|---|---|---|---|---|---|---|---|---|
| % of patients | 80.9 | 60.3 | 45.0 | 28.2 | 17.6 | 9.9 | 5.3 | 3.8 | 2.3 |
Using Kaplan‐Meier analysis, the estimated proportion showing no significant fall of [Hb] was 0.63 (95% CI, 0.55‐0.71) on day 3 and 0.48 (95% CI, 0.29‐0.67) on day 10. By day 15, only 3.8% of the initially included patients were still hospitalized and showed no decrease in [Hb] 1.5 g/dl. These patients maintained their [Hb] stable for the rest of the follow up period (Figure 2).

In the univariate analysis, comparing patients that experienced a decrease in [Hb] 1.5 g/dL with those who did not, significant differences were only found in the following variables: length of stay, APS, anemia at admission, [Hb] at admission, and infectious, gastrointestinal or cardiac diseases at discharge diagnosis (Table 3).
| Patients with a significant fall | Patients without a significant fall | P Value | |
|---|---|---|---|
| |||
| n | 59 (45%) | 72 (55%) | |
| Age, years | 73.15 (18.7) | 70.83 (16.2) | 0.448 |
| Gender, female | 32 (54.2%) | 43 (59.7%) | 0.527 |
| Length of stay (days) | 8.30 (5.6) | 5.91 (3.7) | <0.007 |
| APS | 6.13 (4.5) | 3.97 (3.7) | <0.004 |
| ADL | 4.33 (2.5) | 4.68 (2.1) | 0.410 |
| CHARLSON | 2.03 (1.8) | 2.37 (2.5) | 0.382 |
| Nurse home residents | 4 (6.8%) | 3 (4.2%) | 0.700 |
| Readmissions | 11 (18.6%) | 17 (23.6%) | 0.490 |
| PWSBL | 6 (10.2%) | 8 (11.1%) | 0.862 |
| Anemia at admission | 20 (33.9%) | 43 (59.7%) | <0.004 |
| [Hb] at admission | 13.09 (1.7) | 12.01 (1.5) | <0.001 |
| Diagnosis at discharge | |||
| Infectious | 20 (33.9%) | 13 (18.1%) | <0.05 |
| Respiratory | 8 (13.6%) | 14 (19.4%) | 0.370 |
| Neurologic | 9 (15.2%) | 16 (22.2%) | 0.312 |
| Gastrointestinal | 11 (18.6%) | 3 (4.2%) | <0.01 |
| Cardiac | 2 (3.4%) | 11(15.3%) | <0.05 |
| Electrolyte disturbances | 6 (10.2%) | 4 (5.6%) | 0.512 |
| Others | 3 (5.1%) | 11 (15.3%) | 0.087 |
In the Cox proportional hazard model adjusting for the variables shown in Table 4, a significant independent direct association was found between a significant fall in [Hb] during hospital stay and APS, [Hb] at admission, and either diagnosis of infectious or gastrointestinal disease at discharge. Similar results were found if a significant fall was redefined as a 12% decrease in [Hb] or as a 4.5 point decrease in HCT from baseline.
| Variable | HRR | P Value | 95% CI |
|---|---|---|---|
| |||
| APS | 1.07 | 0.007 | 1.02‐1.12 |
| ADL | 1.11 | 0.132 | 0.97‐1.27 |
| Charlson | 0.88 | 0.121 | 0.75‐1.03 |
| Nurse home resident | 1.52 | 0.361 | 0.62‐3.72 |
| PWSBL | 0.67 | 0.390 | 0.27‐1.66 |
| Readmission | 1.14 | 0.710 | 0.57‐2.29 |
| Female sex | 0.98 | 0.944 | 0.57‐1.69 |
| Age | 1.39 | 0.098 | 0.94‐2.07 |
| [Hb] at admission | 1.27 | 0.005 | 1.07‐1.51 |
| Diagnosis at discharge | |||
| Infectious | 2.70 | 0.015 | 1.21‐6.05 |
| Neurologic | 1.42 | 0.457 | 0.57‐3.55 |
| Gastrointestinal | 3.74 | 0.002 | 1.62‐8.64 |
| Cardiac | 0.41 | 0.289 | 0.08‐2.12 |
| Electrolyte dist. | 2.08 | 0.176 | 0.72‐6.05 |
| Others | 0.95 | 0.946 | 0.24‐3.81 |
Discussion
This study describes the variation of [Hb] and HCT values in GWAP without bleeding or other obvious medical conditions associated with a decrease in the red blood cell mass. As previously described,4, 20, 21 we found that [Hb] decreases during hospital stay are frequently observed. The mean [Hb] fall from admission was 1.45 g/dL, and was mainly recorded between hospitalization days 3 and 4. In approximately half of the study population, a 1.5 g/dL decrease in [Hb] was observed. In the survival analysis, 40% and 55% of patients are expected to present such a fall on day 4 and 12 respectively. In accordance with prior reports4, 21 a greater decrease in [Hb] occurred in the first days of hospitalization, and a high proportion of patients were already anemic at the time of admission.
The following variables were associated with a decrease in the [Hb] during hospitalization: higher APS score, higher [Hb] at admission, and diagnosis of infectious or gastrointestinal disease at discharge. Even though several mechanisms seem to contribute to the decrease in [Hb] during hospitalization our data suggest that 1 of these factors seems to be the severity of the disease, as previously proposed by Nguyen et al.4 This observation is supported by the association found in our study between [Hb] decrease and APS. No association was found with Charlson, ADL, and being a nursing home resident. These variables, which have not been previously analyzed, seem to indicate that patients with chronic illnesses are not more likely to have decreases in [Hb] during hospitalization.
Patients with higher [Hb] at admission had a greater [Hb] decrease during their hospital stay. This finding could suggest that the mechanism related to this decrease had less effect on patients with lower [Hb]. This is similar to that observed in the anemia of chronic diseases where [Hb] does not usually fall to extreme values. Our analysis reveals a greater decrease during the first days of hospitalization. Given the high rate of patients anemic at admission, it is possible that the decrease in [Hb] had begun prior to admission.
Previous papers describe a low prevalence of cyanocobalamine (2%), folic acid (2%), and iron (9%) deficiency in patients admitted to critical care units.3 In these patients an inadequate bone marrow response has been proposed as a mechanism for [Hb] decrease, a phenomenon that has been called AI or anemia of critical illness.8, 2228 Inflammatory response associated with acute disease causes this hypoproliferative anemia through 3 different pathways: relative erythropoietin deficit, a direct inhibition of the erythropoiesis in the bone marrow through different mediators (ie, interleukin [IL]‐1, IL‐6, tumor necrosis factor [TNF]) and a functional iron deficit. This relative iron deficiency is produced mainly by the IL‐6 induced overexpression of the hepcidin gene in hepatocytes. Hepcidin causes impaired intestinal iron absorption and an inadequate delivery of iron from the iron‐recycling macrophages to the erythroid precursors in the bone marrow.8, 9
AI could explain some of our findings, such as the greater decrease observed in the first hospital days when inflamatory mediators levels are expected to be higher. The association between APS and infectious disease diagnosis and a greater [Hb] decrease may also be explained by this mechanism. Nonetheless, the daily bone marrow production of red blood cells is small compared with the circulating red mass cell and therefore it is necessary to have a better explanation on how AI could be associated with this rapid decrease in the [Hb]. Rice et al.30, 31 have explored some mechanisms leading to a rapid decrease in the red mass cell related to acute variations in erytropoietin level. A role of this mechanism and other could be hypothesized.29
Overt bleeding was not found in our study population and procedures without significant blood loss (PWSBL) were infrequent, therefore it is unlikely that they could have had an impact on the decrease of [Hb]. The influence of other variables, such as blood volume drawn for diagnostic testing and occult blood losses (especially from the gastrointestinal tract) was not investigated. Parenteral hydration and hemodilution neither were evaluated in our study nor were extensively described in the literature. We think this last mentioned mechanism plays a role in the large [Hb] decrease during the first days of hospitalization. However a lack of association was mentioned in the only study that evaluated this issue in critically ill patients. Intravascular hemolysis has been cited as an infrequent event in these patients.2, 4
Only patients without a clear explanation for their [Hb] decrease were included because they represent a matter of concern for hospitalists and other treating physicians. Therefore 60% of the initially evaluated patients were excluded, since they were admitted for conditions likely to cause a decrease in [Hb]. Nevertheless, it seems likely that the mechanisms involved in the included patients may play a role in those with an obvious cause for [Hb] variation as well. Accordingly, the decrease of [Hb] expected in patients admitted with disorders known to cause a decrease in the red blood cell mass would be greater than the 1 observed in our study population.
This [Hb] drop during hospitalization may be clinically relevant in a number of ways: it could cause the attending physicians to order costly and invasive studies, it could have prognostic value as it occurs in patients admitted with myocardial infarction, and it could trigger an acute coronary syndrome.20 We observed an association between [Hb] drop and a higher APS in our patients. This score has been validated as a prognostic factor in previous studies. However, it is not possible to conclude that this [Hb] drop is associated with a worse prognosis.
Our study had several limitations. The sample's heterogeneity inherent to our general ward population could have altered our results and their generalization. To overcome this bias we used a categorization system based on discharge diagnosis. However, this particular system has several limitations. The small sample size and the absence of follow up data after discharge limited our capacity to detect any prognostic significance of [Hb] decrease. In the present study, the total decrease of [Hb] may have been underestimated because the onset of the acute illness precedes hospital admission by a variable time. Finally, a relatively small sample size limited our ability to detect other possible significant predictors of [Hb] decrease. However, this study was not designed to assess the mechanisms associated with the decrease of [Hb], but rather to establish its occurrence, measure it and explore related variables.
These results may be useful for further studies to evaluate [Hb] variation in admitted patients and its relation to other variables, such as bone marrow production, oxygen use, erythrocyte survival, nutritional deficiencies, and erythropoietin and inflammatory mediators levels.
In conclusion, our general medical inpatients had a mean 1.45 g/dL [Hb] decrease during hospitalization, which was greater in the first days of hospitalization, even though an evident cause was not present. These findings would help attending physicians in general wards make a rational and efficient approach when dealing with patients' decrease in [Hb].
Acknowledgements
The authors thank Jorge Lopez Camelo and Hugo Krupitzki for statistical advice and Valeria Melia for helping in the preparation of this manuscript.
- ,.Nosocomial anemia.JAMA.1973;223(1):73–74.
- ,,,,.Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients.Crit Care Med.1999;27(12):2630–2639.
- ,,,,,.Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness.J Crit Care.2001;16(1):36–41.
- ,,,.Time course of hemoglobin concentrations in nonbleeding intensive care unit patients.Crit Care Med.2003;31(2):406–410.
- ,,, et al.Erithropoietin response in critically ill patients.Crit Care Med.1997;25(Suppl1):a82.
- ,,.Acute event‐related anaemia.Br J Haematol.2001;115(4):739–743.
- ,,,,,.Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients.Crit Care Med.2000;28(8):2773–2778.
- .The anemia of inflammation/malignancy: mechanisms and management.Hematology Am Soc Hematol Educ Program.2008:159–165.
- .The regulation of hepcidin and its effects on systemic and cellular iron metabolism.Hematology Am Soc Hematol Educ Program.2008:151–158.
- ,,, et al.,Erythropoietin response is blunted in critically ill patients.Intensive Care Med.1997;23:159–162.
- ,,,,.Studies of illness in the aged. The index of ADL: standardized measure of biological and psychosocial function.JAMA.1963;185:914–919.
- ,,,Progress in the development of the index of ADL.Gerontologist.1970;10:20–30.
- ,,,,.Measuring prognosis and case mix in hospitalized elders. The importance of functional status.J Gen Intern Med.1997;12:203–208.
- ,,,.APACHE II: A severity of disease classification system.Crit Care Med.1985;12(10):818–829.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chron Dis.1997;40:373–383.
- ,,,.How to measure comorbidity: a critical review of available methods.J Clin Epidemiol.2003;56:221–229.
- ,,, et al.Nutritional anaemias. Report of a WHO Scientific Group.World Health Organ Tech Rep Ser.1968;405:1–40.
- ,,,.Relationship between hematocrit and renal function in men and women.Kidney Int.2001;59(2):725–731.
- ,,.Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey.J Am Soc Nephrol.2002;13(2):504–510.
- ,,, et al.,Changes in haemoglobin levels during hospital course and long‐term outcome after myocardial infarction.Eur Heart J.2007;28(11):1289–1296.
- ,,,,.Do blood test cause anemia in hospitalized patients?J Gen Intern Med.2005;20:520–524.
- ,.Red blood cell physiology in critical illness.Crit Care Med.2003;31(12 Suppl)( ):S651–S657.
- .Anemia in the critically ill.Crit Care Clin.2004;20:159–178.
- ,.Anemia of the critically ill: acute anemia of chronic disease.Crit Care Med.2000;28(8):3098–3099.
- .Anemia and blood transfusion in the critically ill patient: role of erythropoietin.Crit Care.2004;8(Suppl 2):S42–S44.
- .Anemia of critical illness – implications for understanding and treating rHuEPO resistance.Nephrol Dial Transplant.2002;17(Suppl 5):S48–S55.
- ,,.Transfusion practice in the critically ill.Crit Care Med.2003;31(Suppl):S668–S671.
- .Anemia in critically ill patients.Eur J Inter Med.2004;15(8):481–486.
- ,,,.Neocytolysis: a physiologic down‐regulator of red blood cell mass.Lancet.1997;349:1389–1390.
- ,,,,,.Neocytolysis contributes to the anemia of renal disease.Am J Kidney Dis.1999;33:59–62.
- ,,, et al.Neocytolysis on descent from altitude: a newly recognized mechanism for the control of red cell mass.Ann Intern Med.2001;134:652–656.
- ,.Phlebotomy for diagnostic laboratory test in adults. Pattern of use and effect on transfusion requirements.N Engl J Med.1986;314:1233–1235.
- ,.Nosocomial anemia.JAMA.1973;223(1):73–74.
- ,,,,.Important role of nondiagnostic blood loss and blunted erythropoietic response in the anemia of medical intensive care patients.Crit Care Med.1999;27(12):2630–2639.
- ,,,,,.Nutritional deficiencies and blunted erythropoietin response as causes of the anemia of critical illness.J Crit Care.2001;16(1):36–41.
- ,,,.Time course of hemoglobin concentrations in nonbleeding intensive care unit patients.Crit Care Med.2003;31(2):406–410.
- ,,, et al.Erithropoietin response in critically ill patients.Crit Care Med.1997;25(Suppl1):a82.
- ,,.Acute event‐related anaemia.Br J Haematol.2001;115(4):739–743.
- ,,,,,.Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients.Crit Care Med.2000;28(8):2773–2778.
- .The anemia of inflammation/malignancy: mechanisms and management.Hematology Am Soc Hematol Educ Program.2008:159–165.
- .The regulation of hepcidin and its effects on systemic and cellular iron metabolism.Hematology Am Soc Hematol Educ Program.2008:151–158.
- ,,, et al.,Erythropoietin response is blunted in critically ill patients.Intensive Care Med.1997;23:159–162.
- ,,,,.Studies of illness in the aged. The index of ADL: standardized measure of biological and psychosocial function.JAMA.1963;185:914–919.
- ,,,Progress in the development of the index of ADL.Gerontologist.1970;10:20–30.
- ,,,,.Measuring prognosis and case mix in hospitalized elders. The importance of functional status.J Gen Intern Med.1997;12:203–208.
- ,,,.APACHE II: A severity of disease classification system.Crit Care Med.1985;12(10):818–829.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chron Dis.1997;40:373–383.
- ,,,.How to measure comorbidity: a critical review of available methods.J Clin Epidemiol.2003;56:221–229.
- ,,, et al.Nutritional anaemias. Report of a WHO Scientific Group.World Health Organ Tech Rep Ser.1968;405:1–40.
- ,,,.Relationship between hematocrit and renal function in men and women.Kidney Int.2001;59(2):725–731.
- ,,.Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey.J Am Soc Nephrol.2002;13(2):504–510.
- ,,, et al.,Changes in haemoglobin levels during hospital course and long‐term outcome after myocardial infarction.Eur Heart J.2007;28(11):1289–1296.
- ,,,,.Do blood test cause anemia in hospitalized patients?J Gen Intern Med.2005;20:520–524.
- ,.Red blood cell physiology in critical illness.Crit Care Med.2003;31(12 Suppl)( ):S651–S657.
- .Anemia in the critically ill.Crit Care Clin.2004;20:159–178.
- ,.Anemia of the critically ill: acute anemia of chronic disease.Crit Care Med.2000;28(8):3098–3099.
- .Anemia and blood transfusion in the critically ill patient: role of erythropoietin.Crit Care.2004;8(Suppl 2):S42–S44.
- .Anemia of critical illness – implications for understanding and treating rHuEPO resistance.Nephrol Dial Transplant.2002;17(Suppl 5):S48–S55.
- ,,.Transfusion practice in the critically ill.Crit Care Med.2003;31(Suppl):S668–S671.
- .Anemia in critically ill patients.Eur J Inter Med.2004;15(8):481–486.
- ,,,.Neocytolysis: a physiologic down‐regulator of red blood cell mass.Lancet.1997;349:1389–1390.
- ,,,,,.Neocytolysis contributes to the anemia of renal disease.Am J Kidney Dis.1999;33:59–62.
- ,,, et al.Neocytolysis on descent from altitude: a newly recognized mechanism for the control of red cell mass.Ann Intern Med.2001;134:652–656.
- ,.Phlebotomy for diagnostic laboratory test in adults. Pattern of use and effect on transfusion requirements.N Engl J Med.1986;314:1233–1235.
Copyright © 2010 Society of Hospital Medicine
Health Care‐Associated Candidemia
In the United States, candida now accounts for between 8% and 12% of all catheter‐associated blood stream infections (BSIs).1 Additionally, crude mortality rates in candidemia exceed 40%, and a recent systematic review demonstrated that the attributable mortality of candidemia ranges from 5% to 71%.2 Candidal BSIs also affect resource utilization. These infections independently increase length of stay and result in substantial excess costs.3 Most cases of candidemia arise in noncritically ill patients and thus may be managed by hospitalists.
Historically, the majority of candidal BSIs were caused by C. albicans. Presently, C. albicans accounts for only half of all yeast BSIs, and approximately 20% of these infections are caused by organisms such as C. glabrata and C. krusei.4 These 2 organisms have either variable or no susceptibility to agents, such as fluconazole, empirically employed against yeast. Parallel with the evolution in microbiology of candidemia has been recognition that inappropriate treatment of these infections independently increases mortality.5 These factors underscore the need for the clinician to treat suspected candidal BSIs aggressively in order to avoid the risks associated with inappropriate treatment.
Efforts to enhance rates of initial appropriate therapy for bacterial infections have encompassed the realization that health care‐associated infections (HAIs) represent a distinct syndrome.6, 7 Traditionally, infections were considered either community‐acquired or nosocomial in origin. However, with the spread of health care delivery beyond the hospital, multiple studies indicate that patients may now present to the emergency department with infections caused by pathogens such as Methicillin‐resistant Staphylococcus aureus (MRSA) and P. aeruginosaorganisms that were previously thought limited to hospital‐acquired processes.69 Furthermore, hospitalists often encounter subjects presenting to the hospital with suspected BSIs who have an active and ongoing interaction with the healthcare system.
The importance of candida as a health care‐associated pathogen in BSI remains unclear. We hypothesized that health care‐associated candidemia (HCAC) represented a distinct clinical entity. In order to confirm our theory, we conducted a retrospective analysis of all cases of candidal BSI at our institution over a 3‐year period.
Methods
We reviewed the records of all patients diagnosed with candidemia at our hospital between January 1, 2004 and December 31, 2006. Our institutional review board approved this study. We included adult patients diagnosed with candidemia. The diagnosis of candidemia was based on the isolation of yeast from the blood in at least one blood culture. We employ the BACTEC 9240 blood Culture System (Becton Dickinson Microbiology Systems, Sparks, MD). We excluded subjects who were admitted to the hospital within one month of a known diagnosis of candidemia.
We defined a nosocomial candidal BSI as the diagnosis of candidemia based on cultures drawn after the patient had been hospitalized for >48 hours. We considered HCAC to be present based on previously employed criteria for identifying HAI.69 Specifically, for patients with candidemia based on blood cultures obtained within 48 hours of hospitalization, a patient had to meet at least 1 of the following criteria: (1) receipt of intravenous therapy outside the hospital, (2) end stage renal disease necessitating hemodialysis (ESRD requiring HD), (3) hospitalization within previous 30 days, (4) residence in a nursing home or long term care facility, or (5) underwent an invasive procedure as an outpatient within 30 days of presentation. Community‐acquired candidemia was restricted to patients whose index culture was drawn within 48 hours of admission but who failed to meet the definition for HCAC.
The prevalence of the various forms of candidemia served as our primary endpoint. In addition, we compared patients with respect to demographic factors, comorbidities, and severity of illness. Severity of illness was calculated based on the Acute Physiology and Chronic Health Evaluation (APACHE) II score. We further noted rates of immune suppression in the cohort and defined this as treatment with corticosteroids (10 mg of prednisone or equivalent daily for more than 30 consecutive days), other immunosuppressants (eg, methotrexate), or chemotherapy. Those with acquired immune deficiency syndrome (AIDS) or another immunodeficiency syndrome were defined as immunosuppressed as well. We examined the distribution of yeast species across the 3 forms of candidemia. Finally, we assessed the prevalence of fluconazole resistance. Fluconazole susceptibilities were determined based on Etest (AB BIODISK, Solna, Sweden). An isolate was considered resistant to fluconazole if the minimum inhibitory concentration was >64 g/mL.
We compared categorical variables with the Fisher's exact test. Continuous variables were analyzed with either the Student's t‐test or a Mann‐Whitney test, as appropriate. All tests were 2 tailed and a P value of 0.05 was assumed to represent statistical significance. Analyses were performed with Stata 9.1 (Stata Corp., College Station, TX).
Results
The final cohort included 223 subjects. The mean age of the patients was 59.6 15.7 years and 49% were male. Nearly one quarter (n = 55) fulfilled our criteria for HCAC. The remainder met the definition for nosocomial candidemia. We observed no cases of community‐acquired candidemia. Most (n = 33) patients with HCAC had exposure to more than 1 health care‐related source and many were initially admitted to the medicine/hospitalist service as opposed to the intensive care unit (ICU). The most common criteria leading to categorization as HCAC was recent hospitalization (n = 30, 54.5% of all HCAC). The median time from recent hospitalization to admission was 17 days (Range: 5‐28 days). Other common reasons for classification as HCAC included ESRD requiring HD (30.9%), residence in a nursing home (25.5%), and undergoing an invasive outpatient procedure (16.4%). More than 75% of subjects with HCAC (n = 42) had central venous catheters in place at presentation. Between 2004 and 2006, the proportion of all candidemia due to HCAC increased from 20.9% to 26.9%, but this difference was not statistically significant.
Patients with HCAC were similar to those with nosocomial candidemia (Table 1). There was no difference in either severity of illness or the frequency of neutropenia. The prevalence of most comorbidities did not differ between those with nosocomial candidemia and persons with HCAC. However, immunosuppression was more prevalent among patients with HCAC (prevalence ratio, 1.67; 95% CI, 1.13‐3.08; P = 0.004). In part this finding is expected given that our definition of HCAC includes exposure to agents which may lead to immunosuppression, such as chemotherapy. Of patients with HCAC, the majority (n = 38, 69.1%) were initially admitted to the general medicine service and not to the ICU.
| Characteristic | Healthcare‐Associated Candidemia (n = 55) | Nosocomial Candidemia (n = 168) | P |
|---|---|---|---|
| |||
| Demographics | |||
| Age, mean SD | 61.0 12.9 | 59.1 16.6 | 0.45 |
| Male, % | 60.0 | 45.8 | 0.08 |
| Severity of illness | |||
| APACHE II score, mean SD | 15.9 6.8 | 14.6 6.3 | 0.21 |
| Co‐morbid illnesses | |||
| Diabetes mellitus, % | 36.4 | 32.7 | 0.87 |
| Malignancy, % | 36.4 | 22.6 | 0.04 |
| ESRD on HD, % | 30.9 | 23.2 | 0.25 |
| AIDS, % | 7.2 | 6.0 | 0.73 |
| Immunosupressed, % | 54.5 | 32.7 | 0.004 |
| White cell status | |||
| ANC, 1000/mm3, mean SD | 10.7 7.2 | 12.3 8.0 | 0.20 |
| Neutropenic, % | 2.0 | 2.2 | 0.91 |
A multitude of various yeast species were recovered (Figure 1). Overall, nonalbicans candida were responsible for nearly 60% of all infections. Nonalbicans yeast were as likely to be recovered in HCAC as in nosocomial yeast infection. Among both types of Candidemia, C. krusei was a rare culprit accounting for fewer than 2% of infections. C. glabrata, however, occurred more often in HCAC. Specifically, C. glabrata represented 1 in 5 cases of HCAC as opposed to approximately 10% of all nosocomial yeast BSIs (P = 0.05). In part reflecting this, fluconazole resistance was noted more often in HCAC (18.2% of patients vs. 7.7% among nosocomial candidemia, P = 0.036). There was no difference in the eventual diagnosis of deep‐seeded yeast infections (ie, endocarditis, endopthlamitis, or osteomyelitis) between those with HCAC and persons with nosocomial candidemia (3 cases in each group).
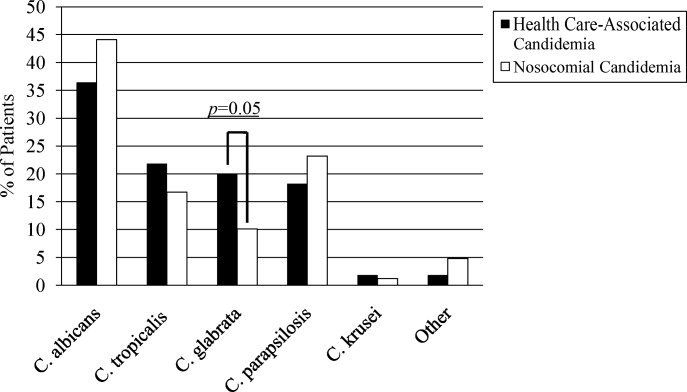
Discussion
This analysis demonstrates that HCAC accounts for approximately a quarter of all candidemia. Our findings underscore that candidemia can present to the emergency department as an HAI and may potentially be initially cared for by a hospitalist. In addition, patients with HCAC and nosocomial candidemia share many attributes. Furthermore, nonalbicans yeast are as prevalent in HCAC as in nosocomial candidal infection. Nonetheless, there appear to be important differences in these syndromes. Immunosuppression appears to be more common in HCAC as does infection due to C. glabrata.
Others have explored the concept of HCAC. Kung et al.10 described community‐onset candidemia at a single center over a 10‐year period. They described 56 patients and noted that the majority had been recently hospitalized or had ongoing interaction with the healthcare system. Sofair et al.11 followed subjects presenting to emergency departments with candidemia. Overall, more than one‐third met criteria for community‐onset infection. In this analysis, though, Sofair et al.11 did not distinguish between community‐acquired processes and HCAC. From a population perspective, Chen et al.12 explored candidemia in Australia. Among over 1000 patients, the noted that 11.6% represented HCAC and, as we note, that select nonalbicans yeast occurred more often in HCAC than in nosocomial candidemia. Our project builds on and adds to these earlier efforts. First, we confirm the general observation that candidemia is no longer solely a nosocomial pathogen. Second, unlike several of these earlier reports we examined a larger cohort of candidemia. Third, beyond the observations of Chen et al.,12 we note that currently, the proportion of Candidal BSI classified as HACA relative to nosocomial candidemia seems larger than reported in the past. Finally, a unique aspect of our report is that we employed express criteria to define HAI.
Our findings have several implications. First, hospitalists and emergency department physicians, along with others, must remain vigilant when approaching patients presenting to the hospital with signs and symptoms of BSI and multiple risk factors for candidal BSI. The fact that the patient has not been hospitalized should not preclude consideration of and treatment for candidemia. The current evidence does not support broad, empiric use of antifungal agents, as this would lead to excessive costs and potentially expose many patients to unnecessary antifungal coverage. On the other hand, given the association between delayed antifungal therapy and the risk for death in candidemia, failure to consider this infection in at‐risk subjects may have adverse consequences. Second, our observations emphasize the need for clinical risk stratification schemes and rapid diagnostic modalities. Such tools are urgently needed if physicians hope to target antifungal therapies more appropriately. Third, if the clinician opts to initiate therapy for possible HCAC, reliance on fluconazole alone may prove inadequate. As the generalizability of our conclusions is necessarily limited, we recommend that infection control practitioners review local epidemiologic data regarding HCAC so that physicians can have the best available guidance.
Our study has several important limitations. Its retrospective nature exposes it to several forms of bias. The single center design limits the generalizability of our findings. Prospective, multicenter studies are needed to validate our results. Additionally, no universally accepted criteria exist to define HAI syndromes. Nonetheless, the criteria we employed have been used by others. We also lacked data on exposure to recent broad spectrum antimicrobials. Selection pressure via exposure to such agents is a risk factor for candidemia and without this data we cannot gauge the impact of this on our findings. Finally, we cannot control for the possibility that some patients were miscategorized. This could have arisen because of: (1) either limitations inherent in the definition of HCAC or (2) because the clinician delayed the decision to obtain blood cultures. Some patients classified as nosocomial may actually have had HCAC or community‐acquired diseasebut for some reason blood cultures were not drawn at time of admission but were deferred until later. Although a difficult issue to address in any study of the epidemiology of infection, the significance of this misclassification bias must be considered a significant concern.
In summary, Candidemia can be the cause of BSI presenting to the hospital. Moreover, HCAC represents a significant proportion of all Candidemia. Although patients with HCAC and nosocomial candidemia share select characteristics, there appear to be some differences in the microbiology of these syndromes.
- CDC.National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990‐‐May 1999, issued June 1999.Am J Infect Control.1999;27:520–532.
- ,,.Attributable mortality of candidemia: a systematic review of matched cohort and case‐control studies.Eur J Clin Microbiol Infect Dis.2006;25:419–425.
- ,,, et al.Excess mortality, hospital stay, and cost due to candidemia: a case‐control study using data from population‐based candidemia surveillance.Infect Control Hosp Epidemiol.2005;26:540–547.
- .Shifting patterns in the epidemiology of nosocomial Candida infections.Chest.2003;123:500S–503S.
- ,,.Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality.Antimicrob Agents Chemother.2005;49:3640–3645.
- ,,, et al.Healthcare‐associated bloodstream infection: A distinct entity? Insights from a large U.S. database.Crit Care Med.2006;34:2588–2595.
- ,,, et al.Health care‐‐associated bloodstream infections in adults: a reason to change the accepted definition of community‐acquired infections.Ann Intern Med.2002;137:791–797.
- ,.Epidemiology of healthcare‐associated pneumonia (HCAP).Semin Respir Crit Care Med.2009;30:10–15.
- ,,, et al.Health care associated pneumonia and community‐acquired pneumonia: a single‐center experience.Antimicrob Agents Chemother.2007;51:3568–3573.
- ,,, et al.Communtiy‐onset candidemia at a university hospital, 1995‐2005.J Microbiol Immunol Infect.2007;40:355–363.
- ,,, et al.Epidemiology of community‐onset candidemia in Connecticut and Maryland.Clin Infect Dis.2006;43:32–39.
- ,,, et al.Active surveillance for candidemia, Australia.Emerg Infect Dis.2006;12:1508–1516.
In the United States, candida now accounts for between 8% and 12% of all catheter‐associated blood stream infections (BSIs).1 Additionally, crude mortality rates in candidemia exceed 40%, and a recent systematic review demonstrated that the attributable mortality of candidemia ranges from 5% to 71%.2 Candidal BSIs also affect resource utilization. These infections independently increase length of stay and result in substantial excess costs.3 Most cases of candidemia arise in noncritically ill patients and thus may be managed by hospitalists.
Historically, the majority of candidal BSIs were caused by C. albicans. Presently, C. albicans accounts for only half of all yeast BSIs, and approximately 20% of these infections are caused by organisms such as C. glabrata and C. krusei.4 These 2 organisms have either variable or no susceptibility to agents, such as fluconazole, empirically employed against yeast. Parallel with the evolution in microbiology of candidemia has been recognition that inappropriate treatment of these infections independently increases mortality.5 These factors underscore the need for the clinician to treat suspected candidal BSIs aggressively in order to avoid the risks associated with inappropriate treatment.
Efforts to enhance rates of initial appropriate therapy for bacterial infections have encompassed the realization that health care‐associated infections (HAIs) represent a distinct syndrome.6, 7 Traditionally, infections were considered either community‐acquired or nosocomial in origin. However, with the spread of health care delivery beyond the hospital, multiple studies indicate that patients may now present to the emergency department with infections caused by pathogens such as Methicillin‐resistant Staphylococcus aureus (MRSA) and P. aeruginosaorganisms that were previously thought limited to hospital‐acquired processes.69 Furthermore, hospitalists often encounter subjects presenting to the hospital with suspected BSIs who have an active and ongoing interaction with the healthcare system.
The importance of candida as a health care‐associated pathogen in BSI remains unclear. We hypothesized that health care‐associated candidemia (HCAC) represented a distinct clinical entity. In order to confirm our theory, we conducted a retrospective analysis of all cases of candidal BSI at our institution over a 3‐year period.
Methods
We reviewed the records of all patients diagnosed with candidemia at our hospital between January 1, 2004 and December 31, 2006. Our institutional review board approved this study. We included adult patients diagnosed with candidemia. The diagnosis of candidemia was based on the isolation of yeast from the blood in at least one blood culture. We employ the BACTEC 9240 blood Culture System (Becton Dickinson Microbiology Systems, Sparks, MD). We excluded subjects who were admitted to the hospital within one month of a known diagnosis of candidemia.
We defined a nosocomial candidal BSI as the diagnosis of candidemia based on cultures drawn after the patient had been hospitalized for >48 hours. We considered HCAC to be present based on previously employed criteria for identifying HAI.69 Specifically, for patients with candidemia based on blood cultures obtained within 48 hours of hospitalization, a patient had to meet at least 1 of the following criteria: (1) receipt of intravenous therapy outside the hospital, (2) end stage renal disease necessitating hemodialysis (ESRD requiring HD), (3) hospitalization within previous 30 days, (4) residence in a nursing home or long term care facility, or (5) underwent an invasive procedure as an outpatient within 30 days of presentation. Community‐acquired candidemia was restricted to patients whose index culture was drawn within 48 hours of admission but who failed to meet the definition for HCAC.
The prevalence of the various forms of candidemia served as our primary endpoint. In addition, we compared patients with respect to demographic factors, comorbidities, and severity of illness. Severity of illness was calculated based on the Acute Physiology and Chronic Health Evaluation (APACHE) II score. We further noted rates of immune suppression in the cohort and defined this as treatment with corticosteroids (10 mg of prednisone or equivalent daily for more than 30 consecutive days), other immunosuppressants (eg, methotrexate), or chemotherapy. Those with acquired immune deficiency syndrome (AIDS) or another immunodeficiency syndrome were defined as immunosuppressed as well. We examined the distribution of yeast species across the 3 forms of candidemia. Finally, we assessed the prevalence of fluconazole resistance. Fluconazole susceptibilities were determined based on Etest (AB BIODISK, Solna, Sweden). An isolate was considered resistant to fluconazole if the minimum inhibitory concentration was >64 g/mL.
We compared categorical variables with the Fisher's exact test. Continuous variables were analyzed with either the Student's t‐test or a Mann‐Whitney test, as appropriate. All tests were 2 tailed and a P value of 0.05 was assumed to represent statistical significance. Analyses were performed with Stata 9.1 (Stata Corp., College Station, TX).
Results
The final cohort included 223 subjects. The mean age of the patients was 59.6 15.7 years and 49% were male. Nearly one quarter (n = 55) fulfilled our criteria for HCAC. The remainder met the definition for nosocomial candidemia. We observed no cases of community‐acquired candidemia. Most (n = 33) patients with HCAC had exposure to more than 1 health care‐related source and many were initially admitted to the medicine/hospitalist service as opposed to the intensive care unit (ICU). The most common criteria leading to categorization as HCAC was recent hospitalization (n = 30, 54.5% of all HCAC). The median time from recent hospitalization to admission was 17 days (Range: 5‐28 days). Other common reasons for classification as HCAC included ESRD requiring HD (30.9%), residence in a nursing home (25.5%), and undergoing an invasive outpatient procedure (16.4%). More than 75% of subjects with HCAC (n = 42) had central venous catheters in place at presentation. Between 2004 and 2006, the proportion of all candidemia due to HCAC increased from 20.9% to 26.9%, but this difference was not statistically significant.
Patients with HCAC were similar to those with nosocomial candidemia (Table 1). There was no difference in either severity of illness or the frequency of neutropenia. The prevalence of most comorbidities did not differ between those with nosocomial candidemia and persons with HCAC. However, immunosuppression was more prevalent among patients with HCAC (prevalence ratio, 1.67; 95% CI, 1.13‐3.08; P = 0.004). In part this finding is expected given that our definition of HCAC includes exposure to agents which may lead to immunosuppression, such as chemotherapy. Of patients with HCAC, the majority (n = 38, 69.1%) were initially admitted to the general medicine service and not to the ICU.
| Characteristic | Healthcare‐Associated Candidemia (n = 55) | Nosocomial Candidemia (n = 168) | P |
|---|---|---|---|
| |||
| Demographics | |||
| Age, mean SD | 61.0 12.9 | 59.1 16.6 | 0.45 |
| Male, % | 60.0 | 45.8 | 0.08 |
| Severity of illness | |||
| APACHE II score, mean SD | 15.9 6.8 | 14.6 6.3 | 0.21 |
| Co‐morbid illnesses | |||
| Diabetes mellitus, % | 36.4 | 32.7 | 0.87 |
| Malignancy, % | 36.4 | 22.6 | 0.04 |
| ESRD on HD, % | 30.9 | 23.2 | 0.25 |
| AIDS, % | 7.2 | 6.0 | 0.73 |
| Immunosupressed, % | 54.5 | 32.7 | 0.004 |
| White cell status | |||
| ANC, 1000/mm3, mean SD | 10.7 7.2 | 12.3 8.0 | 0.20 |
| Neutropenic, % | 2.0 | 2.2 | 0.91 |
A multitude of various yeast species were recovered (Figure 1). Overall, nonalbicans candida were responsible for nearly 60% of all infections. Nonalbicans yeast were as likely to be recovered in HCAC as in nosocomial yeast infection. Among both types of Candidemia, C. krusei was a rare culprit accounting for fewer than 2% of infections. C. glabrata, however, occurred more often in HCAC. Specifically, C. glabrata represented 1 in 5 cases of HCAC as opposed to approximately 10% of all nosocomial yeast BSIs (P = 0.05). In part reflecting this, fluconazole resistance was noted more often in HCAC (18.2% of patients vs. 7.7% among nosocomial candidemia, P = 0.036). There was no difference in the eventual diagnosis of deep‐seeded yeast infections (ie, endocarditis, endopthlamitis, or osteomyelitis) between those with HCAC and persons with nosocomial candidemia (3 cases in each group).

Discussion
This analysis demonstrates that HCAC accounts for approximately a quarter of all candidemia. Our findings underscore that candidemia can present to the emergency department as an HAI and may potentially be initially cared for by a hospitalist. In addition, patients with HCAC and nosocomial candidemia share many attributes. Furthermore, nonalbicans yeast are as prevalent in HCAC as in nosocomial candidal infection. Nonetheless, there appear to be important differences in these syndromes. Immunosuppression appears to be more common in HCAC as does infection due to C. glabrata.
Others have explored the concept of HCAC. Kung et al.10 described community‐onset candidemia at a single center over a 10‐year period. They described 56 patients and noted that the majority had been recently hospitalized or had ongoing interaction with the healthcare system. Sofair et al.11 followed subjects presenting to emergency departments with candidemia. Overall, more than one‐third met criteria for community‐onset infection. In this analysis, though, Sofair et al.11 did not distinguish between community‐acquired processes and HCAC. From a population perspective, Chen et al.12 explored candidemia in Australia. Among over 1000 patients, the noted that 11.6% represented HCAC and, as we note, that select nonalbicans yeast occurred more often in HCAC than in nosocomial candidemia. Our project builds on and adds to these earlier efforts. First, we confirm the general observation that candidemia is no longer solely a nosocomial pathogen. Second, unlike several of these earlier reports we examined a larger cohort of candidemia. Third, beyond the observations of Chen et al.,12 we note that currently, the proportion of Candidal BSI classified as HACA relative to nosocomial candidemia seems larger than reported in the past. Finally, a unique aspect of our report is that we employed express criteria to define HAI.
Our findings have several implications. First, hospitalists and emergency department physicians, along with others, must remain vigilant when approaching patients presenting to the hospital with signs and symptoms of BSI and multiple risk factors for candidal BSI. The fact that the patient has not been hospitalized should not preclude consideration of and treatment for candidemia. The current evidence does not support broad, empiric use of antifungal agents, as this would lead to excessive costs and potentially expose many patients to unnecessary antifungal coverage. On the other hand, given the association between delayed antifungal therapy and the risk for death in candidemia, failure to consider this infection in at‐risk subjects may have adverse consequences. Second, our observations emphasize the need for clinical risk stratification schemes and rapid diagnostic modalities. Such tools are urgently needed if physicians hope to target antifungal therapies more appropriately. Third, if the clinician opts to initiate therapy for possible HCAC, reliance on fluconazole alone may prove inadequate. As the generalizability of our conclusions is necessarily limited, we recommend that infection control practitioners review local epidemiologic data regarding HCAC so that physicians can have the best available guidance.
Our study has several important limitations. Its retrospective nature exposes it to several forms of bias. The single center design limits the generalizability of our findings. Prospective, multicenter studies are needed to validate our results. Additionally, no universally accepted criteria exist to define HAI syndromes. Nonetheless, the criteria we employed have been used by others. We also lacked data on exposure to recent broad spectrum antimicrobials. Selection pressure via exposure to such agents is a risk factor for candidemia and without this data we cannot gauge the impact of this on our findings. Finally, we cannot control for the possibility that some patients were miscategorized. This could have arisen because of: (1) either limitations inherent in the definition of HCAC or (2) because the clinician delayed the decision to obtain blood cultures. Some patients classified as nosocomial may actually have had HCAC or community‐acquired diseasebut for some reason blood cultures were not drawn at time of admission but were deferred until later. Although a difficult issue to address in any study of the epidemiology of infection, the significance of this misclassification bias must be considered a significant concern.
In summary, Candidemia can be the cause of BSI presenting to the hospital. Moreover, HCAC represents a significant proportion of all Candidemia. Although patients with HCAC and nosocomial candidemia share select characteristics, there appear to be some differences in the microbiology of these syndromes.
In the United States, candida now accounts for between 8% and 12% of all catheter‐associated blood stream infections (BSIs).1 Additionally, crude mortality rates in candidemia exceed 40%, and a recent systematic review demonstrated that the attributable mortality of candidemia ranges from 5% to 71%.2 Candidal BSIs also affect resource utilization. These infections independently increase length of stay and result in substantial excess costs.3 Most cases of candidemia arise in noncritically ill patients and thus may be managed by hospitalists.
Historically, the majority of candidal BSIs were caused by C. albicans. Presently, C. albicans accounts for only half of all yeast BSIs, and approximately 20% of these infections are caused by organisms such as C. glabrata and C. krusei.4 These 2 organisms have either variable or no susceptibility to agents, such as fluconazole, empirically employed against yeast. Parallel with the evolution in microbiology of candidemia has been recognition that inappropriate treatment of these infections independently increases mortality.5 These factors underscore the need for the clinician to treat suspected candidal BSIs aggressively in order to avoid the risks associated with inappropriate treatment.
Efforts to enhance rates of initial appropriate therapy for bacterial infections have encompassed the realization that health care‐associated infections (HAIs) represent a distinct syndrome.6, 7 Traditionally, infections were considered either community‐acquired or nosocomial in origin. However, with the spread of health care delivery beyond the hospital, multiple studies indicate that patients may now present to the emergency department with infections caused by pathogens such as Methicillin‐resistant Staphylococcus aureus (MRSA) and P. aeruginosaorganisms that were previously thought limited to hospital‐acquired processes.69 Furthermore, hospitalists often encounter subjects presenting to the hospital with suspected BSIs who have an active and ongoing interaction with the healthcare system.
The importance of candida as a health care‐associated pathogen in BSI remains unclear. We hypothesized that health care‐associated candidemia (HCAC) represented a distinct clinical entity. In order to confirm our theory, we conducted a retrospective analysis of all cases of candidal BSI at our institution over a 3‐year period.
Methods
We reviewed the records of all patients diagnosed with candidemia at our hospital between January 1, 2004 and December 31, 2006. Our institutional review board approved this study. We included adult patients diagnosed with candidemia. The diagnosis of candidemia was based on the isolation of yeast from the blood in at least one blood culture. We employ the BACTEC 9240 blood Culture System (Becton Dickinson Microbiology Systems, Sparks, MD). We excluded subjects who were admitted to the hospital within one month of a known diagnosis of candidemia.
We defined a nosocomial candidal BSI as the diagnosis of candidemia based on cultures drawn after the patient had been hospitalized for >48 hours. We considered HCAC to be present based on previously employed criteria for identifying HAI.69 Specifically, for patients with candidemia based on blood cultures obtained within 48 hours of hospitalization, a patient had to meet at least 1 of the following criteria: (1) receipt of intravenous therapy outside the hospital, (2) end stage renal disease necessitating hemodialysis (ESRD requiring HD), (3) hospitalization within previous 30 days, (4) residence in a nursing home or long term care facility, or (5) underwent an invasive procedure as an outpatient within 30 days of presentation. Community‐acquired candidemia was restricted to patients whose index culture was drawn within 48 hours of admission but who failed to meet the definition for HCAC.
The prevalence of the various forms of candidemia served as our primary endpoint. In addition, we compared patients with respect to demographic factors, comorbidities, and severity of illness. Severity of illness was calculated based on the Acute Physiology and Chronic Health Evaluation (APACHE) II score. We further noted rates of immune suppression in the cohort and defined this as treatment with corticosteroids (10 mg of prednisone or equivalent daily for more than 30 consecutive days), other immunosuppressants (eg, methotrexate), or chemotherapy. Those with acquired immune deficiency syndrome (AIDS) or another immunodeficiency syndrome were defined as immunosuppressed as well. We examined the distribution of yeast species across the 3 forms of candidemia. Finally, we assessed the prevalence of fluconazole resistance. Fluconazole susceptibilities were determined based on Etest (AB BIODISK, Solna, Sweden). An isolate was considered resistant to fluconazole if the minimum inhibitory concentration was >64 g/mL.
We compared categorical variables with the Fisher's exact test. Continuous variables were analyzed with either the Student's t‐test or a Mann‐Whitney test, as appropriate. All tests were 2 tailed and a P value of 0.05 was assumed to represent statistical significance. Analyses were performed with Stata 9.1 (Stata Corp., College Station, TX).
Results
The final cohort included 223 subjects. The mean age of the patients was 59.6 15.7 years and 49% were male. Nearly one quarter (n = 55) fulfilled our criteria for HCAC. The remainder met the definition for nosocomial candidemia. We observed no cases of community‐acquired candidemia. Most (n = 33) patients with HCAC had exposure to more than 1 health care‐related source and many were initially admitted to the medicine/hospitalist service as opposed to the intensive care unit (ICU). The most common criteria leading to categorization as HCAC was recent hospitalization (n = 30, 54.5% of all HCAC). The median time from recent hospitalization to admission was 17 days (Range: 5‐28 days). Other common reasons for classification as HCAC included ESRD requiring HD (30.9%), residence in a nursing home (25.5%), and undergoing an invasive outpatient procedure (16.4%). More than 75% of subjects with HCAC (n = 42) had central venous catheters in place at presentation. Between 2004 and 2006, the proportion of all candidemia due to HCAC increased from 20.9% to 26.9%, but this difference was not statistically significant.
Patients with HCAC were similar to those with nosocomial candidemia (Table 1). There was no difference in either severity of illness or the frequency of neutropenia. The prevalence of most comorbidities did not differ between those with nosocomial candidemia and persons with HCAC. However, immunosuppression was more prevalent among patients with HCAC (prevalence ratio, 1.67; 95% CI, 1.13‐3.08; P = 0.004). In part this finding is expected given that our definition of HCAC includes exposure to agents which may lead to immunosuppression, such as chemotherapy. Of patients with HCAC, the majority (n = 38, 69.1%) were initially admitted to the general medicine service and not to the ICU.
| Characteristic | Healthcare‐Associated Candidemia (n = 55) | Nosocomial Candidemia (n = 168) | P |
|---|---|---|---|
| |||
| Demographics | |||
| Age, mean SD | 61.0 12.9 | 59.1 16.6 | 0.45 |
| Male, % | 60.0 | 45.8 | 0.08 |
| Severity of illness | |||
| APACHE II score, mean SD | 15.9 6.8 | 14.6 6.3 | 0.21 |
| Co‐morbid illnesses | |||
| Diabetes mellitus, % | 36.4 | 32.7 | 0.87 |
| Malignancy, % | 36.4 | 22.6 | 0.04 |
| ESRD on HD, % | 30.9 | 23.2 | 0.25 |
| AIDS, % | 7.2 | 6.0 | 0.73 |
| Immunosupressed, % | 54.5 | 32.7 | 0.004 |
| White cell status | |||
| ANC, 1000/mm3, mean SD | 10.7 7.2 | 12.3 8.0 | 0.20 |
| Neutropenic, % | 2.0 | 2.2 | 0.91 |
A multitude of various yeast species were recovered (Figure 1). Overall, nonalbicans candida were responsible for nearly 60% of all infections. Nonalbicans yeast were as likely to be recovered in HCAC as in nosocomial yeast infection. Among both types of Candidemia, C. krusei was a rare culprit accounting for fewer than 2% of infections. C. glabrata, however, occurred more often in HCAC. Specifically, C. glabrata represented 1 in 5 cases of HCAC as opposed to approximately 10% of all nosocomial yeast BSIs (P = 0.05). In part reflecting this, fluconazole resistance was noted more often in HCAC (18.2% of patients vs. 7.7% among nosocomial candidemia, P = 0.036). There was no difference in the eventual diagnosis of deep‐seeded yeast infections (ie, endocarditis, endopthlamitis, or osteomyelitis) between those with HCAC and persons with nosocomial candidemia (3 cases in each group).

Discussion
This analysis demonstrates that HCAC accounts for approximately a quarter of all candidemia. Our findings underscore that candidemia can present to the emergency department as an HAI and may potentially be initially cared for by a hospitalist. In addition, patients with HCAC and nosocomial candidemia share many attributes. Furthermore, nonalbicans yeast are as prevalent in HCAC as in nosocomial candidal infection. Nonetheless, there appear to be important differences in these syndromes. Immunosuppression appears to be more common in HCAC as does infection due to C. glabrata.
Others have explored the concept of HCAC. Kung et al.10 described community‐onset candidemia at a single center over a 10‐year period. They described 56 patients and noted that the majority had been recently hospitalized or had ongoing interaction with the healthcare system. Sofair et al.11 followed subjects presenting to emergency departments with candidemia. Overall, more than one‐third met criteria for community‐onset infection. In this analysis, though, Sofair et al.11 did not distinguish between community‐acquired processes and HCAC. From a population perspective, Chen et al.12 explored candidemia in Australia. Among over 1000 patients, the noted that 11.6% represented HCAC and, as we note, that select nonalbicans yeast occurred more often in HCAC than in nosocomial candidemia. Our project builds on and adds to these earlier efforts. First, we confirm the general observation that candidemia is no longer solely a nosocomial pathogen. Second, unlike several of these earlier reports we examined a larger cohort of candidemia. Third, beyond the observations of Chen et al.,12 we note that currently, the proportion of Candidal BSI classified as HACA relative to nosocomial candidemia seems larger than reported in the past. Finally, a unique aspect of our report is that we employed express criteria to define HAI.
Our findings have several implications. First, hospitalists and emergency department physicians, along with others, must remain vigilant when approaching patients presenting to the hospital with signs and symptoms of BSI and multiple risk factors for candidal BSI. The fact that the patient has not been hospitalized should not preclude consideration of and treatment for candidemia. The current evidence does not support broad, empiric use of antifungal agents, as this would lead to excessive costs and potentially expose many patients to unnecessary antifungal coverage. On the other hand, given the association between delayed antifungal therapy and the risk for death in candidemia, failure to consider this infection in at‐risk subjects may have adverse consequences. Second, our observations emphasize the need for clinical risk stratification schemes and rapid diagnostic modalities. Such tools are urgently needed if physicians hope to target antifungal therapies more appropriately. Third, if the clinician opts to initiate therapy for possible HCAC, reliance on fluconazole alone may prove inadequate. As the generalizability of our conclusions is necessarily limited, we recommend that infection control practitioners review local epidemiologic data regarding HCAC so that physicians can have the best available guidance.
Our study has several important limitations. Its retrospective nature exposes it to several forms of bias. The single center design limits the generalizability of our findings. Prospective, multicenter studies are needed to validate our results. Additionally, no universally accepted criteria exist to define HAI syndromes. Nonetheless, the criteria we employed have been used by others. We also lacked data on exposure to recent broad spectrum antimicrobials. Selection pressure via exposure to such agents is a risk factor for candidemia and without this data we cannot gauge the impact of this on our findings. Finally, we cannot control for the possibility that some patients were miscategorized. This could have arisen because of: (1) either limitations inherent in the definition of HCAC or (2) because the clinician delayed the decision to obtain blood cultures. Some patients classified as nosocomial may actually have had HCAC or community‐acquired diseasebut for some reason blood cultures were not drawn at time of admission but were deferred until later. Although a difficult issue to address in any study of the epidemiology of infection, the significance of this misclassification bias must be considered a significant concern.
In summary, Candidemia can be the cause of BSI presenting to the hospital. Moreover, HCAC represents a significant proportion of all Candidemia. Although patients with HCAC and nosocomial candidemia share select characteristics, there appear to be some differences in the microbiology of these syndromes.
- CDC.National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990‐‐May 1999, issued June 1999.Am J Infect Control.1999;27:520–532.
- ,,.Attributable mortality of candidemia: a systematic review of matched cohort and case‐control studies.Eur J Clin Microbiol Infect Dis.2006;25:419–425.
- ,,, et al.Excess mortality, hospital stay, and cost due to candidemia: a case‐control study using data from population‐based candidemia surveillance.Infect Control Hosp Epidemiol.2005;26:540–547.
- .Shifting patterns in the epidemiology of nosocomial Candida infections.Chest.2003;123:500S–503S.
- ,,.Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality.Antimicrob Agents Chemother.2005;49:3640–3645.
- ,,, et al.Healthcare‐associated bloodstream infection: A distinct entity? Insights from a large U.S. database.Crit Care Med.2006;34:2588–2595.
- ,,, et al.Health care‐‐associated bloodstream infections in adults: a reason to change the accepted definition of community‐acquired infections.Ann Intern Med.2002;137:791–797.
- ,.Epidemiology of healthcare‐associated pneumonia (HCAP).Semin Respir Crit Care Med.2009;30:10–15.
- ,,, et al.Health care associated pneumonia and community‐acquired pneumonia: a single‐center experience.Antimicrob Agents Chemother.2007;51:3568–3573.
- ,,, et al.Communtiy‐onset candidemia at a university hospital, 1995‐2005.J Microbiol Immunol Infect.2007;40:355–363.
- ,,, et al.Epidemiology of community‐onset candidemia in Connecticut and Maryland.Clin Infect Dis.2006;43:32–39.
- ,,, et al.Active surveillance for candidemia, Australia.Emerg Infect Dis.2006;12:1508–1516.
- CDC.National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990‐‐May 1999, issued June 1999.Am J Infect Control.1999;27:520–532.
- ,,.Attributable mortality of candidemia: a systematic review of matched cohort and case‐control studies.Eur J Clin Microbiol Infect Dis.2006;25:419–425.
- ,,, et al.Excess mortality, hospital stay, and cost due to candidemia: a case‐control study using data from population‐based candidemia surveillance.Infect Control Hosp Epidemiol.2005;26:540–547.
- .Shifting patterns in the epidemiology of nosocomial Candida infections.Chest.2003;123:500S–503S.
- ,,.Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality.Antimicrob Agents Chemother.2005;49:3640–3645.
- ,,, et al.Healthcare‐associated bloodstream infection: A distinct entity? Insights from a large U.S. database.Crit Care Med.2006;34:2588–2595.
- ,,, et al.Health care‐‐associated bloodstream infections in adults: a reason to change the accepted definition of community‐acquired infections.Ann Intern Med.2002;137:791–797.
- ,.Epidemiology of healthcare‐associated pneumonia (HCAP).Semin Respir Crit Care Med.2009;30:10–15.
- ,,, et al.Health care associated pneumonia and community‐acquired pneumonia: a single‐center experience.Antimicrob Agents Chemother.2007;51:3568–3573.
- ,,, et al.Communtiy‐onset candidemia at a university hospital, 1995‐2005.J Microbiol Immunol Infect.2007;40:355–363.
- ,,, et al.Epidemiology of community‐onset candidemia in Connecticut and Maryland.Clin Infect Dis.2006;43:32–39.
- ,,, et al.Active surveillance for candidemia, Australia.Emerg Infect Dis.2006;12:1508–1516.
Hospital Self‐Discharge and Patient Trust
Patients who leave a hospital against medical advice (AMA) have increased risks of negative clinical outcomes. Leaving a hospital AMA has been associated with increased risks of emergency department and hospital readmission, longer lengths of stay upon hospital readmission, increased risk of morbidities, and increased mortality.13 Ibrahim et al.4 estimated that 1.44% of all hospitalizations for adults in the United States end with the patient leaving AMA. Prior research, however, has shown that specific patient populations may experience greater AMA discharge rates, as up to 4.9% of asthma‐related hospitalizations,2 13% of human immunodeficiency virus (HIV)‐related hospitalizations,1 and 30% of psychiatric‐related hospitalizations have been shown to end with the patient leaving AMA.5
A small body of research examines the reasons that patients leave AMA. In prior studies, dissatisfaction with care and conflicts with medical staff were among the most commonly cited reasons given by patients.2, 6 In a study of healthcare provider reflections on recent patients who left AMA, providers identified patient mistrust, suboptimal physician‐patient communication, and physician‐patient conflict as important contributors to the patient leaving AMA.7
Sickle‐cell disease (SCD) is a painful genetic condition which in the United States affects mostly African Americans and leads to frequent hospital utilization. Patients with SCD frequently report having poor‐quality interpersonal relations with health care providers when the patient seeks treatment for his or her pain.8 Patients often report that their health care providers do not believe the patient's reports of pain, providers do not involve the patient to his or her satisfaction in setting the course of care, and providers typically stigmatize the patient as having a substance abuse problem.817 Indeed, a recent systematic review has shown that there is a high level of evidence that negative health care provider attitudes serve as a barrier to appropriate pain management in SCD.18
To our knowledge, the only study to date of hospital self‐discharge history among adults with SCD was conducted in the United Kingdom by Elander et al.19 These investigators found that 14% of their sample of patients reported ever having discharged themselves from a hospital. The most common reasons for this behavior given by these patients were that they grew tired of waiting for relief of their pain, there were other conflicts that occurred on the medical ward, and because they just simply wanted to go home.
Given the interpersonal conflicts and poor‐quality pain management often found in SCD care, additional examinations of hospital self‐discharge in this population are warranted. We hypothesized that SCD patient reports of interpersonal conflict during previous health care encounters would have an independent association with the likelihood of the patient having ever self‐discharged from a hospital. We also hypothesized that there would be an independent association between a patient's level of trust in the medical profession and their self‐discharge history. The aim of this study was to test these hypotheses.
Methods
Study Design, Setting, and Sample
We conducted a cross‐sectional study of patients with SCD seeking care at a mid‐Atlantic, urban academic medical center, from September 2006 to June 2007. Eligible patients were adults age 18 years or older with any sickle‐cell hemoglobinopathy (HbSS, HbSC, HbS/‐thalassemia, or HbS/‐thalassemia) who were seen at the medical center during the study period.
Data Collection Procedures
Eligible patients were recruited from the adult sickle‐cell and hematology outpatient clinics, the Emergency Department, the inpatient units, or within 5 days after discharge from an acute hospital visit. We collected data by patient interview and medical record abstraction. The interview assessed demographic characteristics (eg, age, sex, educational attainment), patient‐reported annual hospital utilization for pain, previous interpersonal healthcare experiences, and trust toward the medical profession. A trained interviewer conducted the patient interview, which lasted approximately 15 minutes. Patients received $10 for completion of the interview. We abstracted from the patient's medical record their hemoglobinopathy type, their previous complications from SCD, and the presence of other comorbidities. The academic medical center's institutional review board reviewed and approved the study procedures. All participating patients gave informed consent.
Measures
Patient History of Hospital Self‐Discharge
A patient's history of hospital self‐discharge, the dependent variable, was assessed using a single, dichotomous (yes/no) item developed by Elander et al.,19 which asks Have you ever discharged yourself from a hospital, or left suddenly or unexpectedly?
Previous Interpersonal Health Care Experiences
Previous interpersonal health care experiences were assessed using a single, dichotomous (yes/no) item developed by Elander et al.,19 which asks the patient to report whether or not they have ever had difficulty persuading medical staff about their sickle‐cell pain.
Trust in the Medical Profession
A patient's level of trust in the medical profession was assessed using the previously validated 5‐item Wake Forest Trust in the Medical Profession scale.20 There is evidence for the construct validity of this unidimensional scale through its positive associations with trust in a specific physician, general satisfaction with care, and following a doctor's recommendation, and its negative association with having had a prior dispute with a physician, having sought a second opinion, or having changed physicians. This measure uses 5‐point Likert scaling (strongly disagree to strongly agree) to assess patient agreement with the following statements: (1) sometimes doctors care more about what is convenient for them than about their patient's medical needs (reverse coded); (2) doctors are extremely thorough and careful; (3) you completely trust doctors' decisions about which treatments are best; (4) a doctor would never mislead you about anything; and (5) all in all, you trust doctors completely. Scores on each of the items were summed to form a composite score, and then transformed onto a 0 to 100 scale. Higher scores indicated greater levels of trust toward the medical profession. The factorial validity of this measure in the current study was assessed using confirmatory factor analyses (data not shown) which supported the unidimensionality of the measure in our sample. This measure demonstrated good internal consistency in the current sample with a Cronbach's alpha of 0.80.
Covariates
We assessed a number of additional characteristics that could confound the relationship between previous interpersonal health care experiences, trust, and patient history of hospital self‐discharge. We assessed demographic variables for patient age (continuous), sex, education (high‐school education or less vs. greater than a high‐school education), and annual household income (<$10,000, $10,000‐$35,000, and $35,000+). We used a categorical variable that examined the patient's self‐report of their annual hospital utilization for treatment of vasoocclusive crises (VOC) (3 per year vs. 3+ per year). We assessed the patient's clinical characteristics (hemoglobinopathy type, and histories of acute chest syndrome, pulmonary hypertension, avascular necrosis, renal complications, hypertension, and hepatitis C). We also used an indicator variable to identify patients who possessed a positive urine toxicology screen for cocaine or marijuana use upon a hospital admission at any time within the previous 5 years of the patient interview. We restricted the toxicology screen results to these 2 substances alone as the standard therapeutic regimen for pain relief for many patients in this population could lead to a positive toxicology screen for opioids. Finally, we included a categorical variable to represent whether or not the patient's interview occurred in the outpatient or inpatient setting to assess the potential that interview setting might be associated with hospital reported self‐discharge history.
Analytic Methods
We restricted all analyses to those patient records that had complete data on all of the variables of interest. Bivariate relationships between the primary independent variables, the covariates, and the dependent variable were examined using t tests and chi‐square tests as appropriate. Due to sample size considerations, only variables related to the dependent variable at a P value of 0.20 were retained for inclusion in subsequent regression models. We used exact logistic regression modeling to examine adjusted relationships between the primary independent variables of interest and the patient's history of hospital self‐discharge, while controlling for any covariates retained from the bivariate analyses. Exact logistic regression modeling is preferred over the maximum likelihood estimation found in traditional logistic regression models for data with sample sizes of less than 100.21, 22
Results
Patient Characteristics
Ninety‐five patients were enrolled into the study. Of these, 86 had complete data on all variables of interest and are thus the subjects of this analysis. Overall, 40 patients (46.5%) had a history of self‐discharge. Table 1 summarizes the patient characteristics and provides the bivariate comparisons between patients with and without a history of hospital self‐discharge. Patients with a history of hospital self‐discharge were more likely to report experiencing 3 or more hospitalizations each year for treatment of their sickle‐cell pain (62.5% vs. 34.8%; P = 0.01). Patients with a history of hospital self‐discharge were about twice as likely to have a positive toxicology screen in the past 5 years (27.5% vs. 13.0%; P = 0.09).
| History of Sudden Hospital Self‐Discharge | |||
|---|---|---|---|
| No (n = 46) | Yes (n = 40) | P Value | |
| |||
| Patient characteristics | |||
| Age (years) [mean (SD)] | 33.9 (12.1) | 31.8 (8.4) | 0.32 |
| Female (%) | 56.5 | 62.5 | 0.57 |
| With high school education or less (%) | 43.5 | 55.0 | 0.29 |
| Household income (%) | 0.51 | ||
| <$10,000 | 28.3 | 37.5 | |
| $10,000‐$35,000 | 30.4 | 32.5 | |
| $35,000+ | 41.3 | 30.0 | |
| Hospital visits per year for vasoocclusive crises (% with 3+ visits) | 34.8 | 62.5 | 0.01 |
| Positive toxicology screen in past 5 years (%) | 13.0 | 27.5 | 0.09 |
| Clinical characteristics (%) | |||
| Hemoglobin SS disease | 65.2 | 61.5 | 0.73 |
| History of acute chest syndrome | 56.5 | 60.0 | 0.74 |
| Avascular necrosis | 24.4 | 32.5 | 0.41 |
| Pulmonary hypertension | 36.9 | 30.0 | 0.49 |
| Renal complications | 13.3 | 20.5 | 0.38 |
| History of hypertension | 21.7 | 17.5 | 0.62 |
| History of hepatitis C | 10.9 | 15.0 | 0.58 |
| Interview location, inpatient (%) | 43.5 | 55.0 | 0.29 |
| Previous interpersonal experiences (% reporting previous difficulty persuading medical staff about pain) | 47.8 | 85.0 | <0.001 |
| Trust (interpersonal trust) [mean (SD)] | 62.3(19.8) | 44.8(19.2) | 0.0001 |
Associations Among Interpersonal Experiences, Trust, and Hospital Self‐Discharge
In unadjusted analyses, having a history of hospital self‐discharge was associated with a greater likelihood of reporting difficulty persuading medical staff about sickle‐cell pain (85% vs. 47.8%; P < 0.001) and with lower levels of trust in the medical profession (44.8 vs. 62.3; P = 0.0001).
Table 2 reports the results of a multivariate exact logistic regression analysis. Persons reporting difficulty persuading medical staff about sickle‐cell pain were more likely to report having ever self‐discharged from a hospital, even after controlling for patient trust, hospital utilization, and 5‐year toxicology screen history (adjusted odds ratio [AOR], 3.89; P = 0.04; 95% confidence interval [CI], 1.05‐16.26). Patients with greater levels of trust in the medical profession were less likely to have ever self‐discharged from a hospital, controlling for difficulty persuading medical staff about pain, hospital utilization, and 5‐year positive toxicology screen history (AOR, 0.96; P = 0.003; 95% CI, 0.93‐0.99). Independent associations between hospital utilization and self‐discharge history, or between 5‐year toxicology screen history and self‐discharge history, were not observed in this study.
| Adjusted Odds Ratio (95% confidence interval) (n = 86) | |
|---|---|
| |
| Difficulty persuading medical staff about pain | 3.89 (1.0516.26)* |
| Trust in the medical profession | 0.96 (0.930.99) |
| 3+ hospital visits/year due to VOC | 0.96 (0.263.36) |
| Positive toxicology screen in the past 5 years | 4.29 (0.8924.64) |
Discussion
In this study, we found that a high proportion of patients with SCD had a history of hospital self‐discharge. Patients with lower trust, and those who reported difficulty in persuading medical staff about sickle‐cell pain, were more likely to report having ever self‐discharged from a hospital, even after controlling for self‐reported hospital utilization for sickle‐cell pain, and the patient's 5‐year toxicology screen history. Because hospital self‐discharge is potentially dangerous,13 our study reveals an understudied aspect of how low trust and poor health care experiences may put patients with SCD at risk for poor outcomes.
In our study, 46.5% of our sample reported ever having self‐discharged from a hospital, which is much higher than the 14% found by Elander et al.19 in their United Kingdom (London‐based) sample. Other differences in our 2 patient populations may account for this discrepancy. Compared to the Elander sample, a much greater percentage of our patients reported ever having difficulty persuading medical staff about their pain (65% vs. 39%). As difficulty persuading medical staff about pain was independently associated with an increased hospital self‐discharge history in our study, one might expect that our sample, which had a higher percentage of patients reporting difficulty, would also be found to have a higher percentage of patients reporting a history of hospital self‐discharge. A second difference between the two patient samples is that our sample of patients experienced a greater number of hospital visits in the 12 months preceding the study compared to the Elander et al.19 sample. If this difference reflects an underlying difference in the overall hospital utilization experiences of the 2 groups, then our sample of adults would have greater opportunities, on average, than the Elander et al.19 sample to experience hospital self‐discharge. Other factors, such as patient behavioral or cultural differences between patients in the United Kingdom (with a national health system) and the United States (without a national health system), might be explored in future studies.
It is important to note that the wording of the hospital self‐discharge item as used both in our study and by Elander et al.19 would not only capture AMA discharges, but additionally may capture other sudden decisions about hospital discharge made by patients. A national‐level study of AMA discharges among adults with SCD in the United States that uses hospital records and/or chart review is needed in order to provide a more generalizable estimate of the prevalence of AMA discharge among this patient population in the United States.
Elander et al.19 suggest that while hospital self‐discharge among SCD patients may be interpreted by many as a sign that the patient engages in problematic use of opioids or other substances, it may be more appropriate to view this behavior as a sign that the patient has received inadequate management of their pain.19 In our study, hospital self‐discharge tended to be associated with having a history of substance abuse as operationalized by a positive toxicology screen for cocaine or marijuana use during any admission in the previous 5 years. Elander et al.19 found that hospital self‐discharge and other so‐called concern raising behaviors such as use of illicit substances were found to be significantly associated with patient attempts to obtain relief from their pain, but were not significantly associated with symptoms of actual substance dependence or addiction. For example, each instance of illicit substance use reported by the patients in the Elander et al.19 study described patient attempts to use marijuana in efforts to manage pain, to relax, or as alternatives to prescribed analgesics. Clinicians in the United States who observe positive toxicology screen results for SCD patients may see these results as casting doubt upon the legitimacy of the patient's pain reports, thus causing a reduction in the amount of pain medicine provided to the patient, when in fact, a substantial percentage of these results may reflect SCD patients attempts to manage their pain outside of a hospital setting. This potential discrepancy between clinician interpretations of the meaning of positive toxicology screen results for SCD patients, and the actual significance of these results for many patients as reflecting attempts to manage pain, could contribute to interpersonal conflicts between the clinicians and patients, and ultimately, patient self‐discharge and decreases in patient trust in clinicians. Further, to the extent that SCD patient positive toxicology screen results reflect use of illicit substances for reasons other than attempts to manage pain, this should signal for clinicians a need to refer the patient for substance abuse treatment and counseling in addition to (and not instead of) efforts to manage the patient's pain.
Our study is among the first to show empirically that persons with a history of hospital self‐discharge have lower levels of trust in the medical profession. Discharging himself or herself from a hospital could cause a patient to view future health care experiences in a more negative light, and cause the patient to have lower trust in the medical profession. Healthcare providers often label patients with a history of leaving AMA as challenging patients. Seeing in the medical record that a patient has left AMA before may bias the provider to view the patient in a more negative light, and consequently affect the quality of their communication with the patient, leading to lower patient trust. Alternatively, a patient could already possess lower trust in the medical profession due to poor‐quality interpersonal experiences, and thus be more likely to self‐discharge from a hospital during a future acute care visit due to a heightened wariness or greater level of anxiety.
The most consistent and robust predictors of trust found across studies in the literature are the quality of previous interactions with medical care.23 Poor physician communication, and experiences of conflict with staff have been associated with lower ratings of trust among a wide variety of patient populations.2427 Interestingly, we found a relationship between trust and hospital self‐discharge even after controlling for the quality of previous interpersonal experiences as measured by prior difficulty persuading staff about pain. Future studies should examine the relationship between trust and hospital self‐discharge history while controlling for other measures of previous interpersonal healthcare experiences among this patient population.
There are limitations to the current study that must be considered. First, as a single‐institution study, these results may not be generalizable to patients with SCD seeking care at other institutions. Also, we did not assess the actual reasons why patients chose to self‐discharge. Thus, while our data suggests that patient perceptions of poor‐quality care contributed to this behavior, we cannot state this definitively. The validity of a self‐reported annual hospital utilization measure as used in this study may be limited by inaccurate patient recall. However, we compared our patient's self‐report of annual hospital utilization with chart documented hospital utilization in the previous 12 months and found that the 2 measures were correlated in the appropriate direction, thereby giving us greater confidence in the validity of our self‐reported measure. Finally, the cross‐sectional nature of the data in this study makes it impossible to specify with certainty the causal directionality of the associations found here. Prospective research must be conducted to help tease apart the potentially complex relationships among trust, interpersonal experiences with care, and hospital self‐discharge.
Adults with SCD who have ever self‐discharged from a hospital have lower trust in the medical profession, and are more likely to report having had prior difficulty persuading medical staff about their sickle‐cell pain. The clinical consequences of hospital self‐discharge in this patient population must be examined, and the specific reasons behind this behavior must be further elucidated, so that clinicians and researchers may be able to design interventions to mitigate the occurrence of this potentially dangerous phenomenon.
- ,,,,,.Leaving hospital against medical advice among HIV‐positive patients.CMAJ.2002;167(6):633–637.
- ,,,,.Hospitalized patients with asthma who leave against medical advice: characteristics, reasons, and outcomes.J Allergy Clin Immunol.2007;119(4):924–929.
- ,,.Hospital discharge against advice after myocardial infarction: deaths and readmissions.Am J Med.2007;120(12):1047–1053.
- ,,.Factors associated with patients who leave acute‐care hospitals against medical advice.Am J Public Health.2007;97(12):2204–2208.
- .Discharge against medical advice: ethical considerations and professional obligations.J Hosp Med.2008;3(5):403–408.
- ,,,.Why patients sign out against medical advice (AMA): factors motivating patients to sign out AMA.Am J Drug Alcohol Abuse.2004;30(2):489–493.
- ,.Providers' perceptions of relationships and professional roles when caring for patients who leave the hospital against medical advice.J Gen Intern Med.2008;23(10):1698–1707.
- ,,.Experiences of hospital care and treatment seeking for pain from sickle cell disease: qualitative study.BMJ.1999;318(7198):1585–1590.
- ,.Painful crises in sickle cell disease—patients' perspectives.BMJ.1988;297(6646):452–454.
- ,,,,,.Health perceptions and medical care opinions of inner‐city adults with sickle cell disease or asthma compared with those of their siblings.South Med J.1989;82(1):9–12.
- ,,.Sickle cell mutual assistance groups and the health services delivery system.J Health Soc Policy.1994;5(3–4):243–259.
- ,.Functions of an adult sickle cell group: education, task orientation, and support.Health Soc Work.1993;18(1):49–56.
- ,.The management of sickle cell crisis pain as experienced by patients and their carers.J Adv Nurs.1994;19(4):725–732.
- ,,.Adults with sickle cell disease: psychological impact and experience of hospital services.Psychol Health Med.1998;3(2):171–179.
- ,,,,.Use of focus groups for pain and quality of life assessment in adults with sickle cell disease.J Natl Black Nurses Assoc.2001;12(2):36–43.
- ,.The psychosocial experience of people with sickle cell disease and its impact on quality of life: qualitative findings from focus groups.Br J Health Psychol.2002;7(part 3):345–363.
- ,,,.Pain management in sickle cell disease.Chronic Illn.2006;2(1):39–50.
- ,,, et al.A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease.J Natl Med Assoc.2009;101(10):1022–1033.
- ,,,,.Understanding the causes of problematic pain management in sickle cell disease: evidence that pseudoaddiction plays a more important role than genuine analgesic dependence.J Pain Symptom Manage.2004;27(2):156–169.
- ,,.Development of abbreviated measures to assess patient trust in a physician, a health insurer, and the medical profession.BMC Health Services Research.2005;5(1):64.
- ,.Applied Logistic Regression.2nd ed.New York:Wiley;2000.
- ,.Regression Models for Categorical Dependent Variables Using Stata.2nd ed.College Station, TX:StataCorp LP;2006.
- ,,,.Trust in physicians and medical institutions: what is it, can it be measured, and does it matter?Milbank Q.2001;79(4):613–639.
- ,,,,,.How are patients' specific ambulatory care experiences related to trust, satisfaction, and considering changing physicians?J Gen Intern Med.2002;17(1):29–39.
- ,,,,.Racial differences in trust and lung cancer patients' perceptions of physician communication.J Clin Oncol.2006;24(6):904–909.
- ,,.How patient‐physician encounters in critical medical situations affect trust: results of a national survey.BMC Health Serv Res.2004;4(1):24.
- ,,,.Trust in the medical profession: conceptual and measurement issues.Health Serv Res.2002;37(5):1419–1439.
Patients who leave a hospital against medical advice (AMA) have increased risks of negative clinical outcomes. Leaving a hospital AMA has been associated with increased risks of emergency department and hospital readmission, longer lengths of stay upon hospital readmission, increased risk of morbidities, and increased mortality.13 Ibrahim et al.4 estimated that 1.44% of all hospitalizations for adults in the United States end with the patient leaving AMA. Prior research, however, has shown that specific patient populations may experience greater AMA discharge rates, as up to 4.9% of asthma‐related hospitalizations,2 13% of human immunodeficiency virus (HIV)‐related hospitalizations,1 and 30% of psychiatric‐related hospitalizations have been shown to end with the patient leaving AMA.5
A small body of research examines the reasons that patients leave AMA. In prior studies, dissatisfaction with care and conflicts with medical staff were among the most commonly cited reasons given by patients.2, 6 In a study of healthcare provider reflections on recent patients who left AMA, providers identified patient mistrust, suboptimal physician‐patient communication, and physician‐patient conflict as important contributors to the patient leaving AMA.7
Sickle‐cell disease (SCD) is a painful genetic condition which in the United States affects mostly African Americans and leads to frequent hospital utilization. Patients with SCD frequently report having poor‐quality interpersonal relations with health care providers when the patient seeks treatment for his or her pain.8 Patients often report that their health care providers do not believe the patient's reports of pain, providers do not involve the patient to his or her satisfaction in setting the course of care, and providers typically stigmatize the patient as having a substance abuse problem.817 Indeed, a recent systematic review has shown that there is a high level of evidence that negative health care provider attitudes serve as a barrier to appropriate pain management in SCD.18
To our knowledge, the only study to date of hospital self‐discharge history among adults with SCD was conducted in the United Kingdom by Elander et al.19 These investigators found that 14% of their sample of patients reported ever having discharged themselves from a hospital. The most common reasons for this behavior given by these patients were that they grew tired of waiting for relief of their pain, there were other conflicts that occurred on the medical ward, and because they just simply wanted to go home.
Given the interpersonal conflicts and poor‐quality pain management often found in SCD care, additional examinations of hospital self‐discharge in this population are warranted. We hypothesized that SCD patient reports of interpersonal conflict during previous health care encounters would have an independent association with the likelihood of the patient having ever self‐discharged from a hospital. We also hypothesized that there would be an independent association between a patient's level of trust in the medical profession and their self‐discharge history. The aim of this study was to test these hypotheses.
Methods
Study Design, Setting, and Sample
We conducted a cross‐sectional study of patients with SCD seeking care at a mid‐Atlantic, urban academic medical center, from September 2006 to June 2007. Eligible patients were adults age 18 years or older with any sickle‐cell hemoglobinopathy (HbSS, HbSC, HbS/‐thalassemia, or HbS/‐thalassemia) who were seen at the medical center during the study period.
Data Collection Procedures
Eligible patients were recruited from the adult sickle‐cell and hematology outpatient clinics, the Emergency Department, the inpatient units, or within 5 days after discharge from an acute hospital visit. We collected data by patient interview and medical record abstraction. The interview assessed demographic characteristics (eg, age, sex, educational attainment), patient‐reported annual hospital utilization for pain, previous interpersonal healthcare experiences, and trust toward the medical profession. A trained interviewer conducted the patient interview, which lasted approximately 15 minutes. Patients received $10 for completion of the interview. We abstracted from the patient's medical record their hemoglobinopathy type, their previous complications from SCD, and the presence of other comorbidities. The academic medical center's institutional review board reviewed and approved the study procedures. All participating patients gave informed consent.
Measures
Patient History of Hospital Self‐Discharge
A patient's history of hospital self‐discharge, the dependent variable, was assessed using a single, dichotomous (yes/no) item developed by Elander et al.,19 which asks Have you ever discharged yourself from a hospital, or left suddenly or unexpectedly?
Previous Interpersonal Health Care Experiences
Previous interpersonal health care experiences were assessed using a single, dichotomous (yes/no) item developed by Elander et al.,19 which asks the patient to report whether or not they have ever had difficulty persuading medical staff about their sickle‐cell pain.
Trust in the Medical Profession
A patient's level of trust in the medical profession was assessed using the previously validated 5‐item Wake Forest Trust in the Medical Profession scale.20 There is evidence for the construct validity of this unidimensional scale through its positive associations with trust in a specific physician, general satisfaction with care, and following a doctor's recommendation, and its negative association with having had a prior dispute with a physician, having sought a second opinion, or having changed physicians. This measure uses 5‐point Likert scaling (strongly disagree to strongly agree) to assess patient agreement with the following statements: (1) sometimes doctors care more about what is convenient for them than about their patient's medical needs (reverse coded); (2) doctors are extremely thorough and careful; (3) you completely trust doctors' decisions about which treatments are best; (4) a doctor would never mislead you about anything; and (5) all in all, you trust doctors completely. Scores on each of the items were summed to form a composite score, and then transformed onto a 0 to 100 scale. Higher scores indicated greater levels of trust toward the medical profession. The factorial validity of this measure in the current study was assessed using confirmatory factor analyses (data not shown) which supported the unidimensionality of the measure in our sample. This measure demonstrated good internal consistency in the current sample with a Cronbach's alpha of 0.80.
Covariates
We assessed a number of additional characteristics that could confound the relationship between previous interpersonal health care experiences, trust, and patient history of hospital self‐discharge. We assessed demographic variables for patient age (continuous), sex, education (high‐school education or less vs. greater than a high‐school education), and annual household income (<$10,000, $10,000‐$35,000, and $35,000+). We used a categorical variable that examined the patient's self‐report of their annual hospital utilization for treatment of vasoocclusive crises (VOC) (3 per year vs. 3+ per year). We assessed the patient's clinical characteristics (hemoglobinopathy type, and histories of acute chest syndrome, pulmonary hypertension, avascular necrosis, renal complications, hypertension, and hepatitis C). We also used an indicator variable to identify patients who possessed a positive urine toxicology screen for cocaine or marijuana use upon a hospital admission at any time within the previous 5 years of the patient interview. We restricted the toxicology screen results to these 2 substances alone as the standard therapeutic regimen for pain relief for many patients in this population could lead to a positive toxicology screen for opioids. Finally, we included a categorical variable to represent whether or not the patient's interview occurred in the outpatient or inpatient setting to assess the potential that interview setting might be associated with hospital reported self‐discharge history.
Analytic Methods
We restricted all analyses to those patient records that had complete data on all of the variables of interest. Bivariate relationships between the primary independent variables, the covariates, and the dependent variable were examined using t tests and chi‐square tests as appropriate. Due to sample size considerations, only variables related to the dependent variable at a P value of 0.20 were retained for inclusion in subsequent regression models. We used exact logistic regression modeling to examine adjusted relationships between the primary independent variables of interest and the patient's history of hospital self‐discharge, while controlling for any covariates retained from the bivariate analyses. Exact logistic regression modeling is preferred over the maximum likelihood estimation found in traditional logistic regression models for data with sample sizes of less than 100.21, 22
Results
Patient Characteristics
Ninety‐five patients were enrolled into the study. Of these, 86 had complete data on all variables of interest and are thus the subjects of this analysis. Overall, 40 patients (46.5%) had a history of self‐discharge. Table 1 summarizes the patient characteristics and provides the bivariate comparisons between patients with and without a history of hospital self‐discharge. Patients with a history of hospital self‐discharge were more likely to report experiencing 3 or more hospitalizations each year for treatment of their sickle‐cell pain (62.5% vs. 34.8%; P = 0.01). Patients with a history of hospital self‐discharge were about twice as likely to have a positive toxicology screen in the past 5 years (27.5% vs. 13.0%; P = 0.09).
| History of Sudden Hospital Self‐Discharge | |||
|---|---|---|---|
| No (n = 46) | Yes (n = 40) | P Value | |
| |||
| Patient characteristics | |||
| Age (years) [mean (SD)] | 33.9 (12.1) | 31.8 (8.4) | 0.32 |
| Female (%) | 56.5 | 62.5 | 0.57 |
| With high school education or less (%) | 43.5 | 55.0 | 0.29 |
| Household income (%) | 0.51 | ||
| <$10,000 | 28.3 | 37.5 | |
| $10,000‐$35,000 | 30.4 | 32.5 | |
| $35,000+ | 41.3 | 30.0 | |
| Hospital visits per year for vasoocclusive crises (% with 3+ visits) | 34.8 | 62.5 | 0.01 |
| Positive toxicology screen in past 5 years (%) | 13.0 | 27.5 | 0.09 |
| Clinical characteristics (%) | |||
| Hemoglobin SS disease | 65.2 | 61.5 | 0.73 |
| History of acute chest syndrome | 56.5 | 60.0 | 0.74 |
| Avascular necrosis | 24.4 | 32.5 | 0.41 |
| Pulmonary hypertension | 36.9 | 30.0 | 0.49 |
| Renal complications | 13.3 | 20.5 | 0.38 |
| History of hypertension | 21.7 | 17.5 | 0.62 |
| History of hepatitis C | 10.9 | 15.0 | 0.58 |
| Interview location, inpatient (%) | 43.5 | 55.0 | 0.29 |
| Previous interpersonal experiences (% reporting previous difficulty persuading medical staff about pain) | 47.8 | 85.0 | <0.001 |
| Trust (interpersonal trust) [mean (SD)] | 62.3(19.8) | 44.8(19.2) | 0.0001 |
Associations Among Interpersonal Experiences, Trust, and Hospital Self‐Discharge
In unadjusted analyses, having a history of hospital self‐discharge was associated with a greater likelihood of reporting difficulty persuading medical staff about sickle‐cell pain (85% vs. 47.8%; P < 0.001) and with lower levels of trust in the medical profession (44.8 vs. 62.3; P = 0.0001).
Table 2 reports the results of a multivariate exact logistic regression analysis. Persons reporting difficulty persuading medical staff about sickle‐cell pain were more likely to report having ever self‐discharged from a hospital, even after controlling for patient trust, hospital utilization, and 5‐year toxicology screen history (adjusted odds ratio [AOR], 3.89; P = 0.04; 95% confidence interval [CI], 1.05‐16.26). Patients with greater levels of trust in the medical profession were less likely to have ever self‐discharged from a hospital, controlling for difficulty persuading medical staff about pain, hospital utilization, and 5‐year positive toxicology screen history (AOR, 0.96; P = 0.003; 95% CI, 0.93‐0.99). Independent associations between hospital utilization and self‐discharge history, or between 5‐year toxicology screen history and self‐discharge history, were not observed in this study.
| Adjusted Odds Ratio (95% confidence interval) (n = 86) | |
|---|---|
| |
| Difficulty persuading medical staff about pain | 3.89 (1.0516.26)* |
| Trust in the medical profession | 0.96 (0.930.99) |
| 3+ hospital visits/year due to VOC | 0.96 (0.263.36) |
| Positive toxicology screen in the past 5 years | 4.29 (0.8924.64) |
Discussion
In this study, we found that a high proportion of patients with SCD had a history of hospital self‐discharge. Patients with lower trust, and those who reported difficulty in persuading medical staff about sickle‐cell pain, were more likely to report having ever self‐discharged from a hospital, even after controlling for self‐reported hospital utilization for sickle‐cell pain, and the patient's 5‐year toxicology screen history. Because hospital self‐discharge is potentially dangerous,13 our study reveals an understudied aspect of how low trust and poor health care experiences may put patients with SCD at risk for poor outcomes.
In our study, 46.5% of our sample reported ever having self‐discharged from a hospital, which is much higher than the 14% found by Elander et al.19 in their United Kingdom (London‐based) sample. Other differences in our 2 patient populations may account for this discrepancy. Compared to the Elander sample, a much greater percentage of our patients reported ever having difficulty persuading medical staff about their pain (65% vs. 39%). As difficulty persuading medical staff about pain was independently associated with an increased hospital self‐discharge history in our study, one might expect that our sample, which had a higher percentage of patients reporting difficulty, would also be found to have a higher percentage of patients reporting a history of hospital self‐discharge. A second difference between the two patient samples is that our sample of patients experienced a greater number of hospital visits in the 12 months preceding the study compared to the Elander et al.19 sample. If this difference reflects an underlying difference in the overall hospital utilization experiences of the 2 groups, then our sample of adults would have greater opportunities, on average, than the Elander et al.19 sample to experience hospital self‐discharge. Other factors, such as patient behavioral or cultural differences between patients in the United Kingdom (with a national health system) and the United States (without a national health system), might be explored in future studies.
It is important to note that the wording of the hospital self‐discharge item as used both in our study and by Elander et al.19 would not only capture AMA discharges, but additionally may capture other sudden decisions about hospital discharge made by patients. A national‐level study of AMA discharges among adults with SCD in the United States that uses hospital records and/or chart review is needed in order to provide a more generalizable estimate of the prevalence of AMA discharge among this patient population in the United States.
Elander et al.19 suggest that while hospital self‐discharge among SCD patients may be interpreted by many as a sign that the patient engages in problematic use of opioids or other substances, it may be more appropriate to view this behavior as a sign that the patient has received inadequate management of their pain.19 In our study, hospital self‐discharge tended to be associated with having a history of substance abuse as operationalized by a positive toxicology screen for cocaine or marijuana use during any admission in the previous 5 years. Elander et al.19 found that hospital self‐discharge and other so‐called concern raising behaviors such as use of illicit substances were found to be significantly associated with patient attempts to obtain relief from their pain, but were not significantly associated with symptoms of actual substance dependence or addiction. For example, each instance of illicit substance use reported by the patients in the Elander et al.19 study described patient attempts to use marijuana in efforts to manage pain, to relax, or as alternatives to prescribed analgesics. Clinicians in the United States who observe positive toxicology screen results for SCD patients may see these results as casting doubt upon the legitimacy of the patient's pain reports, thus causing a reduction in the amount of pain medicine provided to the patient, when in fact, a substantial percentage of these results may reflect SCD patients attempts to manage their pain outside of a hospital setting. This potential discrepancy between clinician interpretations of the meaning of positive toxicology screen results for SCD patients, and the actual significance of these results for many patients as reflecting attempts to manage pain, could contribute to interpersonal conflicts between the clinicians and patients, and ultimately, patient self‐discharge and decreases in patient trust in clinicians. Further, to the extent that SCD patient positive toxicology screen results reflect use of illicit substances for reasons other than attempts to manage pain, this should signal for clinicians a need to refer the patient for substance abuse treatment and counseling in addition to (and not instead of) efforts to manage the patient's pain.
Our study is among the first to show empirically that persons with a history of hospital self‐discharge have lower levels of trust in the medical profession. Discharging himself or herself from a hospital could cause a patient to view future health care experiences in a more negative light, and cause the patient to have lower trust in the medical profession. Healthcare providers often label patients with a history of leaving AMA as challenging patients. Seeing in the medical record that a patient has left AMA before may bias the provider to view the patient in a more negative light, and consequently affect the quality of their communication with the patient, leading to lower patient trust. Alternatively, a patient could already possess lower trust in the medical profession due to poor‐quality interpersonal experiences, and thus be more likely to self‐discharge from a hospital during a future acute care visit due to a heightened wariness or greater level of anxiety.
The most consistent and robust predictors of trust found across studies in the literature are the quality of previous interactions with medical care.23 Poor physician communication, and experiences of conflict with staff have been associated with lower ratings of trust among a wide variety of patient populations.2427 Interestingly, we found a relationship between trust and hospital self‐discharge even after controlling for the quality of previous interpersonal experiences as measured by prior difficulty persuading staff about pain. Future studies should examine the relationship between trust and hospital self‐discharge history while controlling for other measures of previous interpersonal healthcare experiences among this patient population.
There are limitations to the current study that must be considered. First, as a single‐institution study, these results may not be generalizable to patients with SCD seeking care at other institutions. Also, we did not assess the actual reasons why patients chose to self‐discharge. Thus, while our data suggests that patient perceptions of poor‐quality care contributed to this behavior, we cannot state this definitively. The validity of a self‐reported annual hospital utilization measure as used in this study may be limited by inaccurate patient recall. However, we compared our patient's self‐report of annual hospital utilization with chart documented hospital utilization in the previous 12 months and found that the 2 measures were correlated in the appropriate direction, thereby giving us greater confidence in the validity of our self‐reported measure. Finally, the cross‐sectional nature of the data in this study makes it impossible to specify with certainty the causal directionality of the associations found here. Prospective research must be conducted to help tease apart the potentially complex relationships among trust, interpersonal experiences with care, and hospital self‐discharge.
Adults with SCD who have ever self‐discharged from a hospital have lower trust in the medical profession, and are more likely to report having had prior difficulty persuading medical staff about their sickle‐cell pain. The clinical consequences of hospital self‐discharge in this patient population must be examined, and the specific reasons behind this behavior must be further elucidated, so that clinicians and researchers may be able to design interventions to mitigate the occurrence of this potentially dangerous phenomenon.
Patients who leave a hospital against medical advice (AMA) have increased risks of negative clinical outcomes. Leaving a hospital AMA has been associated with increased risks of emergency department and hospital readmission, longer lengths of stay upon hospital readmission, increased risk of morbidities, and increased mortality.13 Ibrahim et al.4 estimated that 1.44% of all hospitalizations for adults in the United States end with the patient leaving AMA. Prior research, however, has shown that specific patient populations may experience greater AMA discharge rates, as up to 4.9% of asthma‐related hospitalizations,2 13% of human immunodeficiency virus (HIV)‐related hospitalizations,1 and 30% of psychiatric‐related hospitalizations have been shown to end with the patient leaving AMA.5
A small body of research examines the reasons that patients leave AMA. In prior studies, dissatisfaction with care and conflicts with medical staff were among the most commonly cited reasons given by patients.2, 6 In a study of healthcare provider reflections on recent patients who left AMA, providers identified patient mistrust, suboptimal physician‐patient communication, and physician‐patient conflict as important contributors to the patient leaving AMA.7
Sickle‐cell disease (SCD) is a painful genetic condition which in the United States affects mostly African Americans and leads to frequent hospital utilization. Patients with SCD frequently report having poor‐quality interpersonal relations with health care providers when the patient seeks treatment for his or her pain.8 Patients often report that their health care providers do not believe the patient's reports of pain, providers do not involve the patient to his or her satisfaction in setting the course of care, and providers typically stigmatize the patient as having a substance abuse problem.817 Indeed, a recent systematic review has shown that there is a high level of evidence that negative health care provider attitudes serve as a barrier to appropriate pain management in SCD.18
To our knowledge, the only study to date of hospital self‐discharge history among adults with SCD was conducted in the United Kingdom by Elander et al.19 These investigators found that 14% of their sample of patients reported ever having discharged themselves from a hospital. The most common reasons for this behavior given by these patients were that they grew tired of waiting for relief of their pain, there were other conflicts that occurred on the medical ward, and because they just simply wanted to go home.
Given the interpersonal conflicts and poor‐quality pain management often found in SCD care, additional examinations of hospital self‐discharge in this population are warranted. We hypothesized that SCD patient reports of interpersonal conflict during previous health care encounters would have an independent association with the likelihood of the patient having ever self‐discharged from a hospital. We also hypothesized that there would be an independent association between a patient's level of trust in the medical profession and their self‐discharge history. The aim of this study was to test these hypotheses.
Methods
Study Design, Setting, and Sample
We conducted a cross‐sectional study of patients with SCD seeking care at a mid‐Atlantic, urban academic medical center, from September 2006 to June 2007. Eligible patients were adults age 18 years or older with any sickle‐cell hemoglobinopathy (HbSS, HbSC, HbS/‐thalassemia, or HbS/‐thalassemia) who were seen at the medical center during the study period.
Data Collection Procedures
Eligible patients were recruited from the adult sickle‐cell and hematology outpatient clinics, the Emergency Department, the inpatient units, or within 5 days after discharge from an acute hospital visit. We collected data by patient interview and medical record abstraction. The interview assessed demographic characteristics (eg, age, sex, educational attainment), patient‐reported annual hospital utilization for pain, previous interpersonal healthcare experiences, and trust toward the medical profession. A trained interviewer conducted the patient interview, which lasted approximately 15 minutes. Patients received $10 for completion of the interview. We abstracted from the patient's medical record their hemoglobinopathy type, their previous complications from SCD, and the presence of other comorbidities. The academic medical center's institutional review board reviewed and approved the study procedures. All participating patients gave informed consent.
Measures
Patient History of Hospital Self‐Discharge
A patient's history of hospital self‐discharge, the dependent variable, was assessed using a single, dichotomous (yes/no) item developed by Elander et al.,19 which asks Have you ever discharged yourself from a hospital, or left suddenly or unexpectedly?
Previous Interpersonal Health Care Experiences
Previous interpersonal health care experiences were assessed using a single, dichotomous (yes/no) item developed by Elander et al.,19 which asks the patient to report whether or not they have ever had difficulty persuading medical staff about their sickle‐cell pain.
Trust in the Medical Profession
A patient's level of trust in the medical profession was assessed using the previously validated 5‐item Wake Forest Trust in the Medical Profession scale.20 There is evidence for the construct validity of this unidimensional scale through its positive associations with trust in a specific physician, general satisfaction with care, and following a doctor's recommendation, and its negative association with having had a prior dispute with a physician, having sought a second opinion, or having changed physicians. This measure uses 5‐point Likert scaling (strongly disagree to strongly agree) to assess patient agreement with the following statements: (1) sometimes doctors care more about what is convenient for them than about their patient's medical needs (reverse coded); (2) doctors are extremely thorough and careful; (3) you completely trust doctors' decisions about which treatments are best; (4) a doctor would never mislead you about anything; and (5) all in all, you trust doctors completely. Scores on each of the items were summed to form a composite score, and then transformed onto a 0 to 100 scale. Higher scores indicated greater levels of trust toward the medical profession. The factorial validity of this measure in the current study was assessed using confirmatory factor analyses (data not shown) which supported the unidimensionality of the measure in our sample. This measure demonstrated good internal consistency in the current sample with a Cronbach's alpha of 0.80.
Covariates
We assessed a number of additional characteristics that could confound the relationship between previous interpersonal health care experiences, trust, and patient history of hospital self‐discharge. We assessed demographic variables for patient age (continuous), sex, education (high‐school education or less vs. greater than a high‐school education), and annual household income (<$10,000, $10,000‐$35,000, and $35,000+). We used a categorical variable that examined the patient's self‐report of their annual hospital utilization for treatment of vasoocclusive crises (VOC) (3 per year vs. 3+ per year). We assessed the patient's clinical characteristics (hemoglobinopathy type, and histories of acute chest syndrome, pulmonary hypertension, avascular necrosis, renal complications, hypertension, and hepatitis C). We also used an indicator variable to identify patients who possessed a positive urine toxicology screen for cocaine or marijuana use upon a hospital admission at any time within the previous 5 years of the patient interview. We restricted the toxicology screen results to these 2 substances alone as the standard therapeutic regimen for pain relief for many patients in this population could lead to a positive toxicology screen for opioids. Finally, we included a categorical variable to represent whether or not the patient's interview occurred in the outpatient or inpatient setting to assess the potential that interview setting might be associated with hospital reported self‐discharge history.
Analytic Methods
We restricted all analyses to those patient records that had complete data on all of the variables of interest. Bivariate relationships between the primary independent variables, the covariates, and the dependent variable were examined using t tests and chi‐square tests as appropriate. Due to sample size considerations, only variables related to the dependent variable at a P value of 0.20 were retained for inclusion in subsequent regression models. We used exact logistic regression modeling to examine adjusted relationships between the primary independent variables of interest and the patient's history of hospital self‐discharge, while controlling for any covariates retained from the bivariate analyses. Exact logistic regression modeling is preferred over the maximum likelihood estimation found in traditional logistic regression models for data with sample sizes of less than 100.21, 22
Results
Patient Characteristics
Ninety‐five patients were enrolled into the study. Of these, 86 had complete data on all variables of interest and are thus the subjects of this analysis. Overall, 40 patients (46.5%) had a history of self‐discharge. Table 1 summarizes the patient characteristics and provides the bivariate comparisons between patients with and without a history of hospital self‐discharge. Patients with a history of hospital self‐discharge were more likely to report experiencing 3 or more hospitalizations each year for treatment of their sickle‐cell pain (62.5% vs. 34.8%; P = 0.01). Patients with a history of hospital self‐discharge were about twice as likely to have a positive toxicology screen in the past 5 years (27.5% vs. 13.0%; P = 0.09).
| History of Sudden Hospital Self‐Discharge | |||
|---|---|---|---|
| No (n = 46) | Yes (n = 40) | P Value | |
| |||
| Patient characteristics | |||
| Age (years) [mean (SD)] | 33.9 (12.1) | 31.8 (8.4) | 0.32 |
| Female (%) | 56.5 | 62.5 | 0.57 |
| With high school education or less (%) | 43.5 | 55.0 | 0.29 |
| Household income (%) | 0.51 | ||
| <$10,000 | 28.3 | 37.5 | |
| $10,000‐$35,000 | 30.4 | 32.5 | |
| $35,000+ | 41.3 | 30.0 | |
| Hospital visits per year for vasoocclusive crises (% with 3+ visits) | 34.8 | 62.5 | 0.01 |
| Positive toxicology screen in past 5 years (%) | 13.0 | 27.5 | 0.09 |
| Clinical characteristics (%) | |||
| Hemoglobin SS disease | 65.2 | 61.5 | 0.73 |
| History of acute chest syndrome | 56.5 | 60.0 | 0.74 |
| Avascular necrosis | 24.4 | 32.5 | 0.41 |
| Pulmonary hypertension | 36.9 | 30.0 | 0.49 |
| Renal complications | 13.3 | 20.5 | 0.38 |
| History of hypertension | 21.7 | 17.5 | 0.62 |
| History of hepatitis C | 10.9 | 15.0 | 0.58 |
| Interview location, inpatient (%) | 43.5 | 55.0 | 0.29 |
| Previous interpersonal experiences (% reporting previous difficulty persuading medical staff about pain) | 47.8 | 85.0 | <0.001 |
| Trust (interpersonal trust) [mean (SD)] | 62.3(19.8) | 44.8(19.2) | 0.0001 |
Associations Among Interpersonal Experiences, Trust, and Hospital Self‐Discharge
In unadjusted analyses, having a history of hospital self‐discharge was associated with a greater likelihood of reporting difficulty persuading medical staff about sickle‐cell pain (85% vs. 47.8%; P < 0.001) and with lower levels of trust in the medical profession (44.8 vs. 62.3; P = 0.0001).
Table 2 reports the results of a multivariate exact logistic regression analysis. Persons reporting difficulty persuading medical staff about sickle‐cell pain were more likely to report having ever self‐discharged from a hospital, even after controlling for patient trust, hospital utilization, and 5‐year toxicology screen history (adjusted odds ratio [AOR], 3.89; P = 0.04; 95% confidence interval [CI], 1.05‐16.26). Patients with greater levels of trust in the medical profession were less likely to have ever self‐discharged from a hospital, controlling for difficulty persuading medical staff about pain, hospital utilization, and 5‐year positive toxicology screen history (AOR, 0.96; P = 0.003; 95% CI, 0.93‐0.99). Independent associations between hospital utilization and self‐discharge history, or between 5‐year toxicology screen history and self‐discharge history, were not observed in this study.
| Adjusted Odds Ratio (95% confidence interval) (n = 86) | |
|---|---|
| |
| Difficulty persuading medical staff about pain | 3.89 (1.0516.26)* |
| Trust in the medical profession | 0.96 (0.930.99) |
| 3+ hospital visits/year due to VOC | 0.96 (0.263.36) |
| Positive toxicology screen in the past 5 years | 4.29 (0.8924.64) |
Discussion
In this study, we found that a high proportion of patients with SCD had a history of hospital self‐discharge. Patients with lower trust, and those who reported difficulty in persuading medical staff about sickle‐cell pain, were more likely to report having ever self‐discharged from a hospital, even after controlling for self‐reported hospital utilization for sickle‐cell pain, and the patient's 5‐year toxicology screen history. Because hospital self‐discharge is potentially dangerous,13 our study reveals an understudied aspect of how low trust and poor health care experiences may put patients with SCD at risk for poor outcomes.
In our study, 46.5% of our sample reported ever having self‐discharged from a hospital, which is much higher than the 14% found by Elander et al.19 in their United Kingdom (London‐based) sample. Other differences in our 2 patient populations may account for this discrepancy. Compared to the Elander sample, a much greater percentage of our patients reported ever having difficulty persuading medical staff about their pain (65% vs. 39%). As difficulty persuading medical staff about pain was independently associated with an increased hospital self‐discharge history in our study, one might expect that our sample, which had a higher percentage of patients reporting difficulty, would also be found to have a higher percentage of patients reporting a history of hospital self‐discharge. A second difference between the two patient samples is that our sample of patients experienced a greater number of hospital visits in the 12 months preceding the study compared to the Elander et al.19 sample. If this difference reflects an underlying difference in the overall hospital utilization experiences of the 2 groups, then our sample of adults would have greater opportunities, on average, than the Elander et al.19 sample to experience hospital self‐discharge. Other factors, such as patient behavioral or cultural differences between patients in the United Kingdom (with a national health system) and the United States (without a national health system), might be explored in future studies.
It is important to note that the wording of the hospital self‐discharge item as used both in our study and by Elander et al.19 would not only capture AMA discharges, but additionally may capture other sudden decisions about hospital discharge made by patients. A national‐level study of AMA discharges among adults with SCD in the United States that uses hospital records and/or chart review is needed in order to provide a more generalizable estimate of the prevalence of AMA discharge among this patient population in the United States.
Elander et al.19 suggest that while hospital self‐discharge among SCD patients may be interpreted by many as a sign that the patient engages in problematic use of opioids or other substances, it may be more appropriate to view this behavior as a sign that the patient has received inadequate management of their pain.19 In our study, hospital self‐discharge tended to be associated with having a history of substance abuse as operationalized by a positive toxicology screen for cocaine or marijuana use during any admission in the previous 5 years. Elander et al.19 found that hospital self‐discharge and other so‐called concern raising behaviors such as use of illicit substances were found to be significantly associated with patient attempts to obtain relief from their pain, but were not significantly associated with symptoms of actual substance dependence or addiction. For example, each instance of illicit substance use reported by the patients in the Elander et al.19 study described patient attempts to use marijuana in efforts to manage pain, to relax, or as alternatives to prescribed analgesics. Clinicians in the United States who observe positive toxicology screen results for SCD patients may see these results as casting doubt upon the legitimacy of the patient's pain reports, thus causing a reduction in the amount of pain medicine provided to the patient, when in fact, a substantial percentage of these results may reflect SCD patients attempts to manage their pain outside of a hospital setting. This potential discrepancy between clinician interpretations of the meaning of positive toxicology screen results for SCD patients, and the actual significance of these results for many patients as reflecting attempts to manage pain, could contribute to interpersonal conflicts between the clinicians and patients, and ultimately, patient self‐discharge and decreases in patient trust in clinicians. Further, to the extent that SCD patient positive toxicology screen results reflect use of illicit substances for reasons other than attempts to manage pain, this should signal for clinicians a need to refer the patient for substance abuse treatment and counseling in addition to (and not instead of) efforts to manage the patient's pain.
Our study is among the first to show empirically that persons with a history of hospital self‐discharge have lower levels of trust in the medical profession. Discharging himself or herself from a hospital could cause a patient to view future health care experiences in a more negative light, and cause the patient to have lower trust in the medical profession. Healthcare providers often label patients with a history of leaving AMA as challenging patients. Seeing in the medical record that a patient has left AMA before may bias the provider to view the patient in a more negative light, and consequently affect the quality of their communication with the patient, leading to lower patient trust. Alternatively, a patient could already possess lower trust in the medical profession due to poor‐quality interpersonal experiences, and thus be more likely to self‐discharge from a hospital during a future acute care visit due to a heightened wariness or greater level of anxiety.
The most consistent and robust predictors of trust found across studies in the literature are the quality of previous interactions with medical care.23 Poor physician communication, and experiences of conflict with staff have been associated with lower ratings of trust among a wide variety of patient populations.2427 Interestingly, we found a relationship between trust and hospital self‐discharge even after controlling for the quality of previous interpersonal experiences as measured by prior difficulty persuading staff about pain. Future studies should examine the relationship between trust and hospital self‐discharge history while controlling for other measures of previous interpersonal healthcare experiences among this patient population.
There are limitations to the current study that must be considered. First, as a single‐institution study, these results may not be generalizable to patients with SCD seeking care at other institutions. Also, we did not assess the actual reasons why patients chose to self‐discharge. Thus, while our data suggests that patient perceptions of poor‐quality care contributed to this behavior, we cannot state this definitively. The validity of a self‐reported annual hospital utilization measure as used in this study may be limited by inaccurate patient recall. However, we compared our patient's self‐report of annual hospital utilization with chart documented hospital utilization in the previous 12 months and found that the 2 measures were correlated in the appropriate direction, thereby giving us greater confidence in the validity of our self‐reported measure. Finally, the cross‐sectional nature of the data in this study makes it impossible to specify with certainty the causal directionality of the associations found here. Prospective research must be conducted to help tease apart the potentially complex relationships among trust, interpersonal experiences with care, and hospital self‐discharge.
Adults with SCD who have ever self‐discharged from a hospital have lower trust in the medical profession, and are more likely to report having had prior difficulty persuading medical staff about their sickle‐cell pain. The clinical consequences of hospital self‐discharge in this patient population must be examined, and the specific reasons behind this behavior must be further elucidated, so that clinicians and researchers may be able to design interventions to mitigate the occurrence of this potentially dangerous phenomenon.
- ,,,,,.Leaving hospital against medical advice among HIV‐positive patients.CMAJ.2002;167(6):633–637.
- ,,,,.Hospitalized patients with asthma who leave against medical advice: characteristics, reasons, and outcomes.J Allergy Clin Immunol.2007;119(4):924–929.
- ,,.Hospital discharge against advice after myocardial infarction: deaths and readmissions.Am J Med.2007;120(12):1047–1053.
- ,,.Factors associated with patients who leave acute‐care hospitals against medical advice.Am J Public Health.2007;97(12):2204–2208.
- .Discharge against medical advice: ethical considerations and professional obligations.J Hosp Med.2008;3(5):403–408.
- ,,,.Why patients sign out against medical advice (AMA): factors motivating patients to sign out AMA.Am J Drug Alcohol Abuse.2004;30(2):489–493.
- ,.Providers' perceptions of relationships and professional roles when caring for patients who leave the hospital against medical advice.J Gen Intern Med.2008;23(10):1698–1707.
- ,,.Experiences of hospital care and treatment seeking for pain from sickle cell disease: qualitative study.BMJ.1999;318(7198):1585–1590.
- ,.Painful crises in sickle cell disease—patients' perspectives.BMJ.1988;297(6646):452–454.
- ,,,,,.Health perceptions and medical care opinions of inner‐city adults with sickle cell disease or asthma compared with those of their siblings.South Med J.1989;82(1):9–12.
- ,,.Sickle cell mutual assistance groups and the health services delivery system.J Health Soc Policy.1994;5(3–4):243–259.
- ,.Functions of an adult sickle cell group: education, task orientation, and support.Health Soc Work.1993;18(1):49–56.
- ,.The management of sickle cell crisis pain as experienced by patients and their carers.J Adv Nurs.1994;19(4):725–732.
- ,,.Adults with sickle cell disease: psychological impact and experience of hospital services.Psychol Health Med.1998;3(2):171–179.
- ,,,,.Use of focus groups for pain and quality of life assessment in adults with sickle cell disease.J Natl Black Nurses Assoc.2001;12(2):36–43.
- ,.The psychosocial experience of people with sickle cell disease and its impact on quality of life: qualitative findings from focus groups.Br J Health Psychol.2002;7(part 3):345–363.
- ,,,.Pain management in sickle cell disease.Chronic Illn.2006;2(1):39–50.
- ,,, et al.A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease.J Natl Med Assoc.2009;101(10):1022–1033.
- ,,,,.Understanding the causes of problematic pain management in sickle cell disease: evidence that pseudoaddiction plays a more important role than genuine analgesic dependence.J Pain Symptom Manage.2004;27(2):156–169.
- ,,.Development of abbreviated measures to assess patient trust in a physician, a health insurer, and the medical profession.BMC Health Services Research.2005;5(1):64.
- ,.Applied Logistic Regression.2nd ed.New York:Wiley;2000.
- ,.Regression Models for Categorical Dependent Variables Using Stata.2nd ed.College Station, TX:StataCorp LP;2006.
- ,,,.Trust in physicians and medical institutions: what is it, can it be measured, and does it matter?Milbank Q.2001;79(4):613–639.
- ,,,,,.How are patients' specific ambulatory care experiences related to trust, satisfaction, and considering changing physicians?J Gen Intern Med.2002;17(1):29–39.
- ,,,,.Racial differences in trust and lung cancer patients' perceptions of physician communication.J Clin Oncol.2006;24(6):904–909.
- ,,.How patient‐physician encounters in critical medical situations affect trust: results of a national survey.BMC Health Serv Res.2004;4(1):24.
- ,,,.Trust in the medical profession: conceptual and measurement issues.Health Serv Res.2002;37(5):1419–1439.
- ,,,,,.Leaving hospital against medical advice among HIV‐positive patients.CMAJ.2002;167(6):633–637.
- ,,,,.Hospitalized patients with asthma who leave against medical advice: characteristics, reasons, and outcomes.J Allergy Clin Immunol.2007;119(4):924–929.
- ,,.Hospital discharge against advice after myocardial infarction: deaths and readmissions.Am J Med.2007;120(12):1047–1053.
- ,,.Factors associated with patients who leave acute‐care hospitals against medical advice.Am J Public Health.2007;97(12):2204–2208.
- .Discharge against medical advice: ethical considerations and professional obligations.J Hosp Med.2008;3(5):403–408.
- ,,,.Why patients sign out against medical advice (AMA): factors motivating patients to sign out AMA.Am J Drug Alcohol Abuse.2004;30(2):489–493.
- ,.Providers' perceptions of relationships and professional roles when caring for patients who leave the hospital against medical advice.J Gen Intern Med.2008;23(10):1698–1707.
- ,,.Experiences of hospital care and treatment seeking for pain from sickle cell disease: qualitative study.BMJ.1999;318(7198):1585–1590.
- ,.Painful crises in sickle cell disease—patients' perspectives.BMJ.1988;297(6646):452–454.
- ,,,,,.Health perceptions and medical care opinions of inner‐city adults with sickle cell disease or asthma compared with those of their siblings.South Med J.1989;82(1):9–12.
- ,,.Sickle cell mutual assistance groups and the health services delivery system.J Health Soc Policy.1994;5(3–4):243–259.
- ,.Functions of an adult sickle cell group: education, task orientation, and support.Health Soc Work.1993;18(1):49–56.
- ,.The management of sickle cell crisis pain as experienced by patients and their carers.J Adv Nurs.1994;19(4):725–732.
- ,,.Adults with sickle cell disease: psychological impact and experience of hospital services.Psychol Health Med.1998;3(2):171–179.
- ,,,,.Use of focus groups for pain and quality of life assessment in adults with sickle cell disease.J Natl Black Nurses Assoc.2001;12(2):36–43.
- ,.The psychosocial experience of people with sickle cell disease and its impact on quality of life: qualitative findings from focus groups.Br J Health Psychol.2002;7(part 3):345–363.
- ,,,.Pain management in sickle cell disease.Chronic Illn.2006;2(1):39–50.
- ,,, et al.A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease.J Natl Med Assoc.2009;101(10):1022–1033.
- ,,,,.Understanding the causes of problematic pain management in sickle cell disease: evidence that pseudoaddiction plays a more important role than genuine analgesic dependence.J Pain Symptom Manage.2004;27(2):156–169.
- ,,.Development of abbreviated measures to assess patient trust in a physician, a health insurer, and the medical profession.BMC Health Services Research.2005;5(1):64.
- ,.Applied Logistic Regression.2nd ed.New York:Wiley;2000.
- ,.Regression Models for Categorical Dependent Variables Using Stata.2nd ed.College Station, TX:StataCorp LP;2006.
- ,,,.Trust in physicians and medical institutions: what is it, can it be measured, and does it matter?Milbank Q.2001;79(4):613–639.
- ,,,,,.How are patients' specific ambulatory care experiences related to trust, satisfaction, and considering changing physicians?J Gen Intern Med.2002;17(1):29–39.
- ,,,,.Racial differences in trust and lung cancer patients' perceptions of physician communication.J Clin Oncol.2006;24(6):904–909.
- ,,.How patient‐physician encounters in critical medical situations affect trust: results of a national survey.BMC Health Serv Res.2004;4(1):24.
- ,,,.Trust in the medical profession: conceptual and measurement issues.Health Serv Res.2002;37(5):1419–1439.
Copyright © 2010 Society of Hospital Medicine
Wegener's Granulomatosis
A previously healthy 21‐year‐old man presented with 6 weeks of low‐grade fever, sore throat, red eyes, and hematuria. Physical examination revealed episcleral injection consistent with episcleritis (Figure 1), oral ulcers (Figure 2, black arrows), diffuse fine crackles on chest auscultation and testicular tenderness. Laboratory workup was significant for leukocytosis (14,000 cell/mL), hematuria with red blood cell (RBC) casts and serum creatinine level of 2.1 mg/dL, which subsequently rose rapidly to 4.1 mg/dL. Test for cytoplasmic‐stainingantineutrophil cytoplasmic antibody (C‐ANCA) was positive. Antiproteinase 3 (PR3) antibodies were also positive. Chest x‐ray showed bilateral pulmonary opacities and sinus computed tomography (CT) scan showed mucosal thickening of the sinuses consistent with sinusitis (Figure 3). Renal biopsy revealed segmental necrotizing glomerulonephritis that was pauci‐immune on immunofluorescence staining. The patient was diagnosed with Wegener's granulomatosis with rapidly progressive glomerulonephritis. He was treated with intravenous corticosteroids, cyclophosphamide, and trimethoprim‐sulfamethoxazole. The patient's symptoms and acute renal failure resolved with this medical regimen.
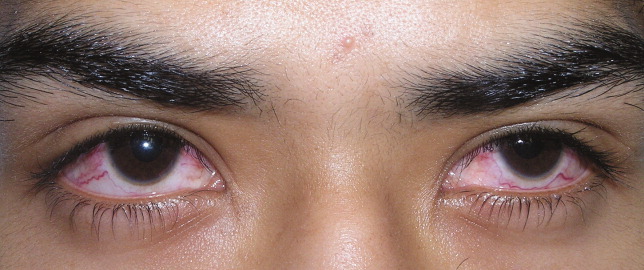
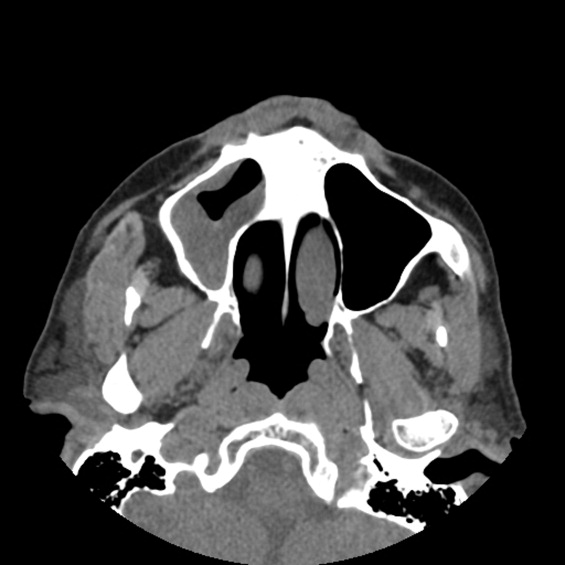
A previously healthy 21‐year‐old man presented with 6 weeks of low‐grade fever, sore throat, red eyes, and hematuria. Physical examination revealed episcleral injection consistent with episcleritis (Figure 1), oral ulcers (Figure 2, black arrows), diffuse fine crackles on chest auscultation and testicular tenderness. Laboratory workup was significant for leukocytosis (14,000 cell/mL), hematuria with red blood cell (RBC) casts and serum creatinine level of 2.1 mg/dL, which subsequently rose rapidly to 4.1 mg/dL. Test for cytoplasmic‐stainingantineutrophil cytoplasmic antibody (C‐ANCA) was positive. Antiproteinase 3 (PR3) antibodies were also positive. Chest x‐ray showed bilateral pulmonary opacities and sinus computed tomography (CT) scan showed mucosal thickening of the sinuses consistent with sinusitis (Figure 3). Renal biopsy revealed segmental necrotizing glomerulonephritis that was pauci‐immune on immunofluorescence staining. The patient was diagnosed with Wegener's granulomatosis with rapidly progressive glomerulonephritis. He was treated with intravenous corticosteroids, cyclophosphamide, and trimethoprim‐sulfamethoxazole. The patient's symptoms and acute renal failure resolved with this medical regimen.



A previously healthy 21‐year‐old man presented with 6 weeks of low‐grade fever, sore throat, red eyes, and hematuria. Physical examination revealed episcleral injection consistent with episcleritis (Figure 1), oral ulcers (Figure 2, black arrows), diffuse fine crackles on chest auscultation and testicular tenderness. Laboratory workup was significant for leukocytosis (14,000 cell/mL), hematuria with red blood cell (RBC) casts and serum creatinine level of 2.1 mg/dL, which subsequently rose rapidly to 4.1 mg/dL. Test for cytoplasmic‐stainingantineutrophil cytoplasmic antibody (C‐ANCA) was positive. Antiproteinase 3 (PR3) antibodies were also positive. Chest x‐ray showed bilateral pulmonary opacities and sinus computed tomography (CT) scan showed mucosal thickening of the sinuses consistent with sinusitis (Figure 3). Renal biopsy revealed segmental necrotizing glomerulonephritis that was pauci‐immune on immunofluorescence staining. The patient was diagnosed with Wegener's granulomatosis with rapidly progressive glomerulonephritis. He was treated with intravenous corticosteroids, cyclophosphamide, and trimethoprim‐sulfamethoxazole. The patient's symptoms and acute renal failure resolved with this medical regimen.



Pulmonary Artery Dissection
A 51‐year‐old African American woman with medical history of essential hypertension and chronic obstructive pulmonary disease (COPD) presented to the hospital with chest pain and shortness of breath. The chest pain was retrosternal and radiated to the back. It lasted for about an hour and resolved without any intervention. After some time, she again felt discomfort in the chest, which was a constant and dull ache.
She had similar episodes of chest pain 1 week prior, although less severe in intensity, for which she went to an outside hospital before coming to our hospital. Acute coronary syndrome was ruled out with serial cardiac enzymes measurements. An exercise stress test was also performed at that time, which failed to show any stress‐induced ischemia.
Her medications included lisinopril for hypertension and aspirin, which had been started 1 week prior to admission. She gave a 10‐pack‐year history of smoking tobacco. Family history was significant for hypertension in her father and coronary artery disease in her mother at the age of 58 years. A review of systems was negative for fever, cough, orthopnea, wheezing, palpitations, nausea, vomiting, recent surgery, or any significant trauma.
Assessment
The patient's physical examination was only remarkable for a blood pressure (BP) of 181/100 mm Hg. She did not have Marfanoid features, hyperflexible joints, or easy bruisability. Laboratory tests, including complete blood count, comprehensive metabolic panel, and cardiac enzymes, were within normal limits. A contrast‐enhanced computed tomography (CT) of the chest showed a linear hypodense area in the left lateral aspect of the main pulmonary trunk, which suggested an intimal dissection of the main pulmonary artery. Magnetic resonance angiography/emmaging (MRA/MRI) confirmed dissection of the main pulmonary artery extending into the proximal left pulmonary artery and associated with a 12 8 mm2 aneurysm (Figures 1 and 2). The entry site of dissection was located in the main pulmonary artery just after its origin and the exit site was located in the left pulmonary artery 5 mm distal to the bifurcation of main pulmonary artery. The pulmonary artery diameter at the dissection was 27 mm.
Diagnosis
To investigate possible etiologies, a transthoracic echocardiogram with Doppler was done to look for pulmonary hypertension. The echocardiogram showed normal pulmonary artery pressure with normal right ventricular systolic pressure. There was no evidence of pericardial effusion or structural cardiac abnormality on echocardiogram. Further investigations including work up for connective tissue diseases and infectious etiologies (Table 1) were normal.
| Variable | Reference Range | Patient's Result |
|---|---|---|
| ||
| ANA | Negative | Negative |
| C3 complement level | 88201 | 175 |
| C4 complement level | 1647 | 49 |
| RF | 20 | 20 |
| Anti‐centromere Ab | Negative | Negative |
| Anti‐Scl 70 Ab | Negative | Negative |
| Anti‐smooth muscle Ab | Negative | Negative |
| Anti‐mitochondrial Ab | Negative | Negative |
| Anti‐parietal cell Ab | Negative | Negative |
| TB skin test | 5 mm | |
| RPR | Negative | Negative |
According to Shilkin et al.,1 Helmbrecht first reported pulmonary artery dissection (PAD) in 1842. PAD is very rare and is usually diagnosed at autopsy. There are 71 other cases of PAD reported in the English literature, of which 16 cases are in living patients.217 Unlike aortic dissection, which is fairly common, the reentry circuit for PAD is formed by the rupture of the free wall of the pulmonary artery leading to hemopericardium, cardiac tamponade, and sudden death.2, 8, 9 There is wide variation in age of incidence, ranging from 26 to 85 years of age, with a slightly higher prevalence in females (male‐to‐female ratio 1:1.2).1, 2 The main pulmonary artery is usually involved, with or without involvement of its branches. Isolated left and right pulmonary artery involvement is seen in 6% and 4% of cases, respectively.2
Pulmonary hypertension, either primary or secondary (collagen vascular diseases, COPD, congenital heart diseases, etc.), is the most common underlying etiology. Other less common, but well‐described etiologies include: Marfan's syndrome, instrumentation of pulmonary artery, tuberculosis, syphilis, pregnancy, idiopathic cystic medial necrosis, and amyloidosis.2, 8
As noted earlier, underlying pulmonary hypertension is usually a major risk factor for PAD. More than 75% of the patients have underlying acute or chronic pulmonary hypertension.2 Our patient had COPD without pulmonary hypertension. Despite extensive investigation, no underlying cause of her pulmonary dissection was identified. The differential diagnosis includes cryptogenic cystic medionecrosis; however, because the patient refused surgery the diagnosis remains unknown. As in our case, idiopathic PAD is extremely rare, and only 4 other cases have been described in the literature.2 Underlying etiologies should always be ruled out to identify correctable causes such as congenital abnormalities of the heart leading to pulmonary hypertension.
Chest pain is a very common presenting complaint in the emergency department. Although rare, PAD should be suspected in a patient with retrosternal chest pain when other common causes of chest pain are excluded. Some of the more suggestive findings are the presence of a new diastolic murmur, a wide mediastinum on chest x‐ray, and CT scan of chest showing an intimal flap.2, 8 CT scan of the chest is an acceptable imaging modality to diagnose PAD.18 According to Neimatallah et al.,18 there are only 5 cases in the literature reported with detailed CT scans demonstrating PAD. If the diagnosis remains uncertain, it should be confirmed by MRI/MRA.16 Transthoracic echocardiography can be used for diagnosis and follow‐up of conservatively managed patients with PAD.3, 8, 19 The echocardiographic findings suggestive of PAD include direct or indirect evidence of pulmonary artery hypertension, with a large main pulmonary artery and an intimal flap across the pulmonary trunk.
Management
No consensus strategy is available for the management of PAD because of the rarity of this condition. In general, operative repair is the treatment of choice for PAD.2, 8, 9, 11 There are 16 cases diagnosed in living patients, out of which 6 were managed medically, 8 were managed surgically, and management was not discussed in 2 of the case reports (Table 2). In these case reports, medically managed patients were treated with oxygen, vasodilators (nitrates, angiotensin‐converting enzyme [ACE] inhibitors, dihydropyridine calcium channel blockers, sildenafil), diuretics and beta‐blockers. These patients did well on follow‐up, ranging from 3 weeks to 4 years, except for 1 who died on day 4 in the intensive care unit (ICU).
| Case Report | Etiology of PAD | Management | Outcome |
|---|---|---|---|
| |||
| Janus et al.3 | Balloon valvuloplasty for pulmonary stenosis | Medical (beta blocker) | Stable during 4 years of follow‐up |
| Khattar et al.8 | Secondary PH from COPD | Medical (diuretics, ACE inhibitor) | Stable during 1 year of follow‐up |
| Lobato et al.9 | Aortic valve replacement | Medical (vasodilators, diuretics) | Stable during 3 weeks of follow‐up |
| Smalcelj et al.10 | Primary PH | Medical (Sildenafil) | Stable during 8 months of follow‐up |
| Song and Kolecki11 | Secondary PH from VSD, Eisenmenger's syndrome | Medical (Nitroprusside) | Patient died on day 4 of admission |
| Steurer et al.15 | Primary PH | Medical (ACE inhibitor, CCB) | Stable during 1 year of follow‐up |
| Wuyts et al.4 | Secondary PH from VSD | Surgical (heart lung transplant) | Follow‐up not mentioned |
| Sakamaki et. al.5 | Primary PH | Surgical (reanastomosis) | Stable during 37 months of follow‐up |
| Westaby et al.7 | Secondary PH from VSD, Eisenmenger's syndrome | Surgical (vascular prosthesis) | Follow‐up not discussed, stable on discharge on tenth day |
| Senbaklavaci et al.12 | Primary PH | Surgical | Stable during 10 months of follow‐up |
| Inayama et al.2 | PH secondary to pulmonary thrombosis | Surgical | Follow‐up not discussed, stable at discharge |
| Wunderbaldinger et al.13 | Primary PH | Surgical | Follow‐up not discussed |
| Lopez‐Candales et al.14 | Secondary PH from partially corrected pulmonary stenosis | Surgical | Follow‐up not discussed, stable on discharge at 1 week |
| Khatchatourian and Vala17 | Associated with aortic dissection | Surgical | Stable during 3 months of follow‐up |
| Rosenson and Sutton6 | Secondary PH from mitral stenosis | Management not discussed | Follow‐up not discussed |
| Stern et al.16 | Secondary PH from hypersensitivity pneumonitis | Management not discussed | Follow‐up not discussed |
Conservative management may be tried in patients who are hemodynamically stable and do not have pericardial effusion.2, 9 The aim of conservative management is to decrease right ventricular preload and afterload. Preload reduction can be dangerous in patients with PAD and should be done in the intensive care setting as this can lead to profound hypotension. Nitrates for preload reduction should be used cautiously in patients taking sildenafil or similar agents for erectile dysfunction or pulmonary artery hypertension because of significant risk of cardiovascular collapse. The American Heart Association and American College of Cardiology both recommend that there should be a time gap of at least 24 hours between the last dose of sildenafil and the first dose of nitrates. Conservatively managed patients should be followed with interval CT scans2, 9, 18 or echocardiography.3, 19 In addition, the underlying etiology should always be investigated to predict prognosis and recommend future management strategies.
The patient was offered surgical repair but she declined. She was managed conservatively with nitrates and beta‐blockers and was pain free within 24 hours. Her BP was brought down to a systolic BP range of 130140 mm Hg. A repeat CT scan of the chest at 1‐month follow up was unchanged. The patient was doing well at 6‐month follow‐up.
Conclusions
PAD is an extremely rare cause of chest pain and a rare antemortem diagnosis. It is usually associated with underlying pulmonary hypertension. This case describes a patient with PAD in the absence of pulmonary hypertension. The patient was managed with conservative medical therapy and did well at 6‐month follow‐up. There are a total of 6 other case reports of patients with PAD managed conservatively, out of which 5 patients did well at follow‐up and 1 patient died. More case reports and longer follow‐up are needed to assess the effectiveness of conservative medical therapy in patients with PAD. To our knowledge, this is the first case report of idiopathic PAD diagnosed in a living patient and managed conservatively. This case also highlights better prognosis for patients with PAD without underlying pulmonary hypertension.
- ,,.Dissecting aneurysm of the pulmonary artery.J. Pathol.1969;98;25–29.
- ,,.Pulmonary artery dissection in patients without underlying pulmonary hypertension.Histopathology.2001;38:435–442.
- ,,,,,.Pulmonary artery dissection: a rare complication of pulmonary balloon valvuloplasty diagnosed 11 years after the procedure.J Am Soc Echocardiogr.2006;19:1191,e1195–e1198.
- ,,,,.Extensive dissection of the pulmonary artery treated with combined heart‐lung transplantation.J Thorac Cardiovasc Surg.2006;132:205–206.
- ,,, et al.Pulmonary artery dissection complicating lung transplantation for primary pulmonary hypertension.Ann Thorac Surg.2006;81:360–362.
- ,.Dissecting aneurysm of the pulmonary trunk in mitral stenosis.Am J Cardiol.1986;58:1140–1141.
- ,,.Pulmonary‐artery dissection in patients with Eisenmenger's syndrome.N Engl J Med.2007;356:2110–2112.
- ,,,.Pulmonary artery dissection: an emerging cardiovascular complication in surviving patients with chronic pulmonary hypertension.Heart.2005;91:142–145.
- ,,, et al.Pulmonary artery dissection and conservative medical management.Int J Cardiol.2007;119:e25–e26.
- ,,,,,.Giant, dissecting, high‐pressure pulmonary artery aneurysm: case report of a 1‐year natural course.Tex Heart Inst J.2005;32:589–594.
- ,.A case of pulmonary artery dissection diagnosed in the Emergency Department.J Emerg Med.2002;23:155–159.
- ,,, et al.Rupture and dissection in pulmonary artery aneurysms: incidence, cause, and treatment—review and case report.J Thorac Cardiovasc Surg.2001;121:1006–1008.
- ,,,,,.Acute pulmonary trunk dissection in a patient with primary pulmonary hypertension.J Comput Assist Tomogr.2000;24:92–95.
- ,,,,.Pulmonary artery aneurysm: review and case report.Clin Cardiol.1995;18:738–740.
- ,,,,,.Dissecting aneurysm of the pulmonary artery with pulmonary hypertension.Am Rev Respir Dis.1990;142:1219–1221.
- ,,,,.Pulmonary artery dissection: MR findings.J Comput Assist Tomogr.1992;16:481–483.
- ,.Images in cardiovascular medicine. Acute type I aortic dissection with concomitant pulmonary artery dissection.Circulation.2005;112:e313–314.
- ,,,.CT findings of pulmonary artery dissection.Br J Radiol.2007;80:e61–e63.
- ,.Pulmonary artery dissection: echocardiographic findings and diagnosis.Echocardiography.2003;20:375–377.
A 51‐year‐old African American woman with medical history of essential hypertension and chronic obstructive pulmonary disease (COPD) presented to the hospital with chest pain and shortness of breath. The chest pain was retrosternal and radiated to the back. It lasted for about an hour and resolved without any intervention. After some time, she again felt discomfort in the chest, which was a constant and dull ache.
She had similar episodes of chest pain 1 week prior, although less severe in intensity, for which she went to an outside hospital before coming to our hospital. Acute coronary syndrome was ruled out with serial cardiac enzymes measurements. An exercise stress test was also performed at that time, which failed to show any stress‐induced ischemia.
Her medications included lisinopril for hypertension and aspirin, which had been started 1 week prior to admission. She gave a 10‐pack‐year history of smoking tobacco. Family history was significant for hypertension in her father and coronary artery disease in her mother at the age of 58 years. A review of systems was negative for fever, cough, orthopnea, wheezing, palpitations, nausea, vomiting, recent surgery, or any significant trauma.
Assessment
The patient's physical examination was only remarkable for a blood pressure (BP) of 181/100 mm Hg. She did not have Marfanoid features, hyperflexible joints, or easy bruisability. Laboratory tests, including complete blood count, comprehensive metabolic panel, and cardiac enzymes, were within normal limits. A contrast‐enhanced computed tomography (CT) of the chest showed a linear hypodense area in the left lateral aspect of the main pulmonary trunk, which suggested an intimal dissection of the main pulmonary artery. Magnetic resonance angiography/emmaging (MRA/MRI) confirmed dissection of the main pulmonary artery extending into the proximal left pulmonary artery and associated with a 12 8 mm2 aneurysm (Figures 1 and 2). The entry site of dissection was located in the main pulmonary artery just after its origin and the exit site was located in the left pulmonary artery 5 mm distal to the bifurcation of main pulmonary artery. The pulmonary artery diameter at the dissection was 27 mm.


Diagnosis
To investigate possible etiologies, a transthoracic echocardiogram with Doppler was done to look for pulmonary hypertension. The echocardiogram showed normal pulmonary artery pressure with normal right ventricular systolic pressure. There was no evidence of pericardial effusion or structural cardiac abnormality on echocardiogram. Further investigations including work up for connective tissue diseases and infectious etiologies (Table 1) were normal.
| Variable | Reference Range | Patient's Result |
|---|---|---|
| ||
| ANA | Negative | Negative |
| C3 complement level | 88201 | 175 |
| C4 complement level | 1647 | 49 |
| RF | 20 | 20 |
| Anti‐centromere Ab | Negative | Negative |
| Anti‐Scl 70 Ab | Negative | Negative |
| Anti‐smooth muscle Ab | Negative | Negative |
| Anti‐mitochondrial Ab | Negative | Negative |
| Anti‐parietal cell Ab | Negative | Negative |
| TB skin test | 5 mm | |
| RPR | Negative | Negative |
According to Shilkin et al.,1 Helmbrecht first reported pulmonary artery dissection (PAD) in 1842. PAD is very rare and is usually diagnosed at autopsy. There are 71 other cases of PAD reported in the English literature, of which 16 cases are in living patients.217 Unlike aortic dissection, which is fairly common, the reentry circuit for PAD is formed by the rupture of the free wall of the pulmonary artery leading to hemopericardium, cardiac tamponade, and sudden death.2, 8, 9 There is wide variation in age of incidence, ranging from 26 to 85 years of age, with a slightly higher prevalence in females (male‐to‐female ratio 1:1.2).1, 2 The main pulmonary artery is usually involved, with or without involvement of its branches. Isolated left and right pulmonary artery involvement is seen in 6% and 4% of cases, respectively.2
Pulmonary hypertension, either primary or secondary (collagen vascular diseases, COPD, congenital heart diseases, etc.), is the most common underlying etiology. Other less common, but well‐described etiologies include: Marfan's syndrome, instrumentation of pulmonary artery, tuberculosis, syphilis, pregnancy, idiopathic cystic medial necrosis, and amyloidosis.2, 8
As noted earlier, underlying pulmonary hypertension is usually a major risk factor for PAD. More than 75% of the patients have underlying acute or chronic pulmonary hypertension.2 Our patient had COPD without pulmonary hypertension. Despite extensive investigation, no underlying cause of her pulmonary dissection was identified. The differential diagnosis includes cryptogenic cystic medionecrosis; however, because the patient refused surgery the diagnosis remains unknown. As in our case, idiopathic PAD is extremely rare, and only 4 other cases have been described in the literature.2 Underlying etiologies should always be ruled out to identify correctable causes such as congenital abnormalities of the heart leading to pulmonary hypertension.
Chest pain is a very common presenting complaint in the emergency department. Although rare, PAD should be suspected in a patient with retrosternal chest pain when other common causes of chest pain are excluded. Some of the more suggestive findings are the presence of a new diastolic murmur, a wide mediastinum on chest x‐ray, and CT scan of chest showing an intimal flap.2, 8 CT scan of the chest is an acceptable imaging modality to diagnose PAD.18 According to Neimatallah et al.,18 there are only 5 cases in the literature reported with detailed CT scans demonstrating PAD. If the diagnosis remains uncertain, it should be confirmed by MRI/MRA.16 Transthoracic echocardiography can be used for diagnosis and follow‐up of conservatively managed patients with PAD.3, 8, 19 The echocardiographic findings suggestive of PAD include direct or indirect evidence of pulmonary artery hypertension, with a large main pulmonary artery and an intimal flap across the pulmonary trunk.
Management
No consensus strategy is available for the management of PAD because of the rarity of this condition. In general, operative repair is the treatment of choice for PAD.2, 8, 9, 11 There are 16 cases diagnosed in living patients, out of which 6 were managed medically, 8 were managed surgically, and management was not discussed in 2 of the case reports (Table 2). In these case reports, medically managed patients were treated with oxygen, vasodilators (nitrates, angiotensin‐converting enzyme [ACE] inhibitors, dihydropyridine calcium channel blockers, sildenafil), diuretics and beta‐blockers. These patients did well on follow‐up, ranging from 3 weeks to 4 years, except for 1 who died on day 4 in the intensive care unit (ICU).
| Case Report | Etiology of PAD | Management | Outcome |
|---|---|---|---|
| |||
| Janus et al.3 | Balloon valvuloplasty for pulmonary stenosis | Medical (beta blocker) | Stable during 4 years of follow‐up |
| Khattar et al.8 | Secondary PH from COPD | Medical (diuretics, ACE inhibitor) | Stable during 1 year of follow‐up |
| Lobato et al.9 | Aortic valve replacement | Medical (vasodilators, diuretics) | Stable during 3 weeks of follow‐up |
| Smalcelj et al.10 | Primary PH | Medical (Sildenafil) | Stable during 8 months of follow‐up |
| Song and Kolecki11 | Secondary PH from VSD, Eisenmenger's syndrome | Medical (Nitroprusside) | Patient died on day 4 of admission |
| Steurer et al.15 | Primary PH | Medical (ACE inhibitor, CCB) | Stable during 1 year of follow‐up |
| Wuyts et al.4 | Secondary PH from VSD | Surgical (heart lung transplant) | Follow‐up not mentioned |
| Sakamaki et. al.5 | Primary PH | Surgical (reanastomosis) | Stable during 37 months of follow‐up |
| Westaby et al.7 | Secondary PH from VSD, Eisenmenger's syndrome | Surgical (vascular prosthesis) | Follow‐up not discussed, stable on discharge on tenth day |
| Senbaklavaci et al.12 | Primary PH | Surgical | Stable during 10 months of follow‐up |
| Inayama et al.2 | PH secondary to pulmonary thrombosis | Surgical | Follow‐up not discussed, stable at discharge |
| Wunderbaldinger et al.13 | Primary PH | Surgical | Follow‐up not discussed |
| Lopez‐Candales et al.14 | Secondary PH from partially corrected pulmonary stenosis | Surgical | Follow‐up not discussed, stable on discharge at 1 week |
| Khatchatourian and Vala17 | Associated with aortic dissection | Surgical | Stable during 3 months of follow‐up |
| Rosenson and Sutton6 | Secondary PH from mitral stenosis | Management not discussed | Follow‐up not discussed |
| Stern et al.16 | Secondary PH from hypersensitivity pneumonitis | Management not discussed | Follow‐up not discussed |
Conservative management may be tried in patients who are hemodynamically stable and do not have pericardial effusion.2, 9 The aim of conservative management is to decrease right ventricular preload and afterload. Preload reduction can be dangerous in patients with PAD and should be done in the intensive care setting as this can lead to profound hypotension. Nitrates for preload reduction should be used cautiously in patients taking sildenafil or similar agents for erectile dysfunction or pulmonary artery hypertension because of significant risk of cardiovascular collapse. The American Heart Association and American College of Cardiology both recommend that there should be a time gap of at least 24 hours between the last dose of sildenafil and the first dose of nitrates. Conservatively managed patients should be followed with interval CT scans2, 9, 18 or echocardiography.3, 19 In addition, the underlying etiology should always be investigated to predict prognosis and recommend future management strategies.
The patient was offered surgical repair but she declined. She was managed conservatively with nitrates and beta‐blockers and was pain free within 24 hours. Her BP was brought down to a systolic BP range of 130140 mm Hg. A repeat CT scan of the chest at 1‐month follow up was unchanged. The patient was doing well at 6‐month follow‐up.
Conclusions
PAD is an extremely rare cause of chest pain and a rare antemortem diagnosis. It is usually associated with underlying pulmonary hypertension. This case describes a patient with PAD in the absence of pulmonary hypertension. The patient was managed with conservative medical therapy and did well at 6‐month follow‐up. There are a total of 6 other case reports of patients with PAD managed conservatively, out of which 5 patients did well at follow‐up and 1 patient died. More case reports and longer follow‐up are needed to assess the effectiveness of conservative medical therapy in patients with PAD. To our knowledge, this is the first case report of idiopathic PAD diagnosed in a living patient and managed conservatively. This case also highlights better prognosis for patients with PAD without underlying pulmonary hypertension.
A 51‐year‐old African American woman with medical history of essential hypertension and chronic obstructive pulmonary disease (COPD) presented to the hospital with chest pain and shortness of breath. The chest pain was retrosternal and radiated to the back. It lasted for about an hour and resolved without any intervention. After some time, she again felt discomfort in the chest, which was a constant and dull ache.
She had similar episodes of chest pain 1 week prior, although less severe in intensity, for which she went to an outside hospital before coming to our hospital. Acute coronary syndrome was ruled out with serial cardiac enzymes measurements. An exercise stress test was also performed at that time, which failed to show any stress‐induced ischemia.
Her medications included lisinopril for hypertension and aspirin, which had been started 1 week prior to admission. She gave a 10‐pack‐year history of smoking tobacco. Family history was significant for hypertension in her father and coronary artery disease in her mother at the age of 58 years. A review of systems was negative for fever, cough, orthopnea, wheezing, palpitations, nausea, vomiting, recent surgery, or any significant trauma.
Assessment
The patient's physical examination was only remarkable for a blood pressure (BP) of 181/100 mm Hg. She did not have Marfanoid features, hyperflexible joints, or easy bruisability. Laboratory tests, including complete blood count, comprehensive metabolic panel, and cardiac enzymes, were within normal limits. A contrast‐enhanced computed tomography (CT) of the chest showed a linear hypodense area in the left lateral aspect of the main pulmonary trunk, which suggested an intimal dissection of the main pulmonary artery. Magnetic resonance angiography/emmaging (MRA/MRI) confirmed dissection of the main pulmonary artery extending into the proximal left pulmonary artery and associated with a 12 8 mm2 aneurysm (Figures 1 and 2). The entry site of dissection was located in the main pulmonary artery just after its origin and the exit site was located in the left pulmonary artery 5 mm distal to the bifurcation of main pulmonary artery. The pulmonary artery diameter at the dissection was 27 mm.


Diagnosis
To investigate possible etiologies, a transthoracic echocardiogram with Doppler was done to look for pulmonary hypertension. The echocardiogram showed normal pulmonary artery pressure with normal right ventricular systolic pressure. There was no evidence of pericardial effusion or structural cardiac abnormality on echocardiogram. Further investigations including work up for connective tissue diseases and infectious etiologies (Table 1) were normal.
| Variable | Reference Range | Patient's Result |
|---|---|---|
| ||
| ANA | Negative | Negative |
| C3 complement level | 88201 | 175 |
| C4 complement level | 1647 | 49 |
| RF | 20 | 20 |
| Anti‐centromere Ab | Negative | Negative |
| Anti‐Scl 70 Ab | Negative | Negative |
| Anti‐smooth muscle Ab | Negative | Negative |
| Anti‐mitochondrial Ab | Negative | Negative |
| Anti‐parietal cell Ab | Negative | Negative |
| TB skin test | 5 mm | |
| RPR | Negative | Negative |
According to Shilkin et al.,1 Helmbrecht first reported pulmonary artery dissection (PAD) in 1842. PAD is very rare and is usually diagnosed at autopsy. There are 71 other cases of PAD reported in the English literature, of which 16 cases are in living patients.217 Unlike aortic dissection, which is fairly common, the reentry circuit for PAD is formed by the rupture of the free wall of the pulmonary artery leading to hemopericardium, cardiac tamponade, and sudden death.2, 8, 9 There is wide variation in age of incidence, ranging from 26 to 85 years of age, with a slightly higher prevalence in females (male‐to‐female ratio 1:1.2).1, 2 The main pulmonary artery is usually involved, with or without involvement of its branches. Isolated left and right pulmonary artery involvement is seen in 6% and 4% of cases, respectively.2
Pulmonary hypertension, either primary or secondary (collagen vascular diseases, COPD, congenital heart diseases, etc.), is the most common underlying etiology. Other less common, but well‐described etiologies include: Marfan's syndrome, instrumentation of pulmonary artery, tuberculosis, syphilis, pregnancy, idiopathic cystic medial necrosis, and amyloidosis.2, 8
As noted earlier, underlying pulmonary hypertension is usually a major risk factor for PAD. More than 75% of the patients have underlying acute or chronic pulmonary hypertension.2 Our patient had COPD without pulmonary hypertension. Despite extensive investigation, no underlying cause of her pulmonary dissection was identified. The differential diagnosis includes cryptogenic cystic medionecrosis; however, because the patient refused surgery the diagnosis remains unknown. As in our case, idiopathic PAD is extremely rare, and only 4 other cases have been described in the literature.2 Underlying etiologies should always be ruled out to identify correctable causes such as congenital abnormalities of the heart leading to pulmonary hypertension.
Chest pain is a very common presenting complaint in the emergency department. Although rare, PAD should be suspected in a patient with retrosternal chest pain when other common causes of chest pain are excluded. Some of the more suggestive findings are the presence of a new diastolic murmur, a wide mediastinum on chest x‐ray, and CT scan of chest showing an intimal flap.2, 8 CT scan of the chest is an acceptable imaging modality to diagnose PAD.18 According to Neimatallah et al.,18 there are only 5 cases in the literature reported with detailed CT scans demonstrating PAD. If the diagnosis remains uncertain, it should be confirmed by MRI/MRA.16 Transthoracic echocardiography can be used for diagnosis and follow‐up of conservatively managed patients with PAD.3, 8, 19 The echocardiographic findings suggestive of PAD include direct or indirect evidence of pulmonary artery hypertension, with a large main pulmonary artery and an intimal flap across the pulmonary trunk.
Management
No consensus strategy is available for the management of PAD because of the rarity of this condition. In general, operative repair is the treatment of choice for PAD.2, 8, 9, 11 There are 16 cases diagnosed in living patients, out of which 6 were managed medically, 8 were managed surgically, and management was not discussed in 2 of the case reports (Table 2). In these case reports, medically managed patients were treated with oxygen, vasodilators (nitrates, angiotensin‐converting enzyme [ACE] inhibitors, dihydropyridine calcium channel blockers, sildenafil), diuretics and beta‐blockers. These patients did well on follow‐up, ranging from 3 weeks to 4 years, except for 1 who died on day 4 in the intensive care unit (ICU).
| Case Report | Etiology of PAD | Management | Outcome |
|---|---|---|---|
| |||
| Janus et al.3 | Balloon valvuloplasty for pulmonary stenosis | Medical (beta blocker) | Stable during 4 years of follow‐up |
| Khattar et al.8 | Secondary PH from COPD | Medical (diuretics, ACE inhibitor) | Stable during 1 year of follow‐up |
| Lobato et al.9 | Aortic valve replacement | Medical (vasodilators, diuretics) | Stable during 3 weeks of follow‐up |
| Smalcelj et al.10 | Primary PH | Medical (Sildenafil) | Stable during 8 months of follow‐up |
| Song and Kolecki11 | Secondary PH from VSD, Eisenmenger's syndrome | Medical (Nitroprusside) | Patient died on day 4 of admission |
| Steurer et al.15 | Primary PH | Medical (ACE inhibitor, CCB) | Stable during 1 year of follow‐up |
| Wuyts et al.4 | Secondary PH from VSD | Surgical (heart lung transplant) | Follow‐up not mentioned |
| Sakamaki et. al.5 | Primary PH | Surgical (reanastomosis) | Stable during 37 months of follow‐up |
| Westaby et al.7 | Secondary PH from VSD, Eisenmenger's syndrome | Surgical (vascular prosthesis) | Follow‐up not discussed, stable on discharge on tenth day |
| Senbaklavaci et al.12 | Primary PH | Surgical | Stable during 10 months of follow‐up |
| Inayama et al.2 | PH secondary to pulmonary thrombosis | Surgical | Follow‐up not discussed, stable at discharge |
| Wunderbaldinger et al.13 | Primary PH | Surgical | Follow‐up not discussed |
| Lopez‐Candales et al.14 | Secondary PH from partially corrected pulmonary stenosis | Surgical | Follow‐up not discussed, stable on discharge at 1 week |
| Khatchatourian and Vala17 | Associated with aortic dissection | Surgical | Stable during 3 months of follow‐up |
| Rosenson and Sutton6 | Secondary PH from mitral stenosis | Management not discussed | Follow‐up not discussed |
| Stern et al.16 | Secondary PH from hypersensitivity pneumonitis | Management not discussed | Follow‐up not discussed |
Conservative management may be tried in patients who are hemodynamically stable and do not have pericardial effusion.2, 9 The aim of conservative management is to decrease right ventricular preload and afterload. Preload reduction can be dangerous in patients with PAD and should be done in the intensive care setting as this can lead to profound hypotension. Nitrates for preload reduction should be used cautiously in patients taking sildenafil or similar agents for erectile dysfunction or pulmonary artery hypertension because of significant risk of cardiovascular collapse. The American Heart Association and American College of Cardiology both recommend that there should be a time gap of at least 24 hours between the last dose of sildenafil and the first dose of nitrates. Conservatively managed patients should be followed with interval CT scans2, 9, 18 or echocardiography.3, 19 In addition, the underlying etiology should always be investigated to predict prognosis and recommend future management strategies.
The patient was offered surgical repair but she declined. She was managed conservatively with nitrates and beta‐blockers and was pain free within 24 hours. Her BP was brought down to a systolic BP range of 130140 mm Hg. A repeat CT scan of the chest at 1‐month follow up was unchanged. The patient was doing well at 6‐month follow‐up.
Conclusions
PAD is an extremely rare cause of chest pain and a rare antemortem diagnosis. It is usually associated with underlying pulmonary hypertension. This case describes a patient with PAD in the absence of pulmonary hypertension. The patient was managed with conservative medical therapy and did well at 6‐month follow‐up. There are a total of 6 other case reports of patients with PAD managed conservatively, out of which 5 patients did well at follow‐up and 1 patient died. More case reports and longer follow‐up are needed to assess the effectiveness of conservative medical therapy in patients with PAD. To our knowledge, this is the first case report of idiopathic PAD diagnosed in a living patient and managed conservatively. This case also highlights better prognosis for patients with PAD without underlying pulmonary hypertension.
- ,,.Dissecting aneurysm of the pulmonary artery.J. Pathol.1969;98;25–29.
- ,,.Pulmonary artery dissection in patients without underlying pulmonary hypertension.Histopathology.2001;38:435–442.
- ,,,,,.Pulmonary artery dissection: a rare complication of pulmonary balloon valvuloplasty diagnosed 11 years after the procedure.J Am Soc Echocardiogr.2006;19:1191,e1195–e1198.
- ,,,,.Extensive dissection of the pulmonary artery treated with combined heart‐lung transplantation.J Thorac Cardiovasc Surg.2006;132:205–206.
- ,,, et al.Pulmonary artery dissection complicating lung transplantation for primary pulmonary hypertension.Ann Thorac Surg.2006;81:360–362.
- ,.Dissecting aneurysm of the pulmonary trunk in mitral stenosis.Am J Cardiol.1986;58:1140–1141.
- ,,.Pulmonary‐artery dissection in patients with Eisenmenger's syndrome.N Engl J Med.2007;356:2110–2112.
- ,,,.Pulmonary artery dissection: an emerging cardiovascular complication in surviving patients with chronic pulmonary hypertension.Heart.2005;91:142–145.
- ,,, et al.Pulmonary artery dissection and conservative medical management.Int J Cardiol.2007;119:e25–e26.
- ,,,,,.Giant, dissecting, high‐pressure pulmonary artery aneurysm: case report of a 1‐year natural course.Tex Heart Inst J.2005;32:589–594.
- ,.A case of pulmonary artery dissection diagnosed in the Emergency Department.J Emerg Med.2002;23:155–159.
- ,,, et al.Rupture and dissection in pulmonary artery aneurysms: incidence, cause, and treatment—review and case report.J Thorac Cardiovasc Surg.2001;121:1006–1008.
- ,,,,,.Acute pulmonary trunk dissection in a patient with primary pulmonary hypertension.J Comput Assist Tomogr.2000;24:92–95.
- ,,,,.Pulmonary artery aneurysm: review and case report.Clin Cardiol.1995;18:738–740.
- ,,,,,.Dissecting aneurysm of the pulmonary artery with pulmonary hypertension.Am Rev Respir Dis.1990;142:1219–1221.
- ,,,,.Pulmonary artery dissection: MR findings.J Comput Assist Tomogr.1992;16:481–483.
- ,.Images in cardiovascular medicine. Acute type I aortic dissection with concomitant pulmonary artery dissection.Circulation.2005;112:e313–314.
- ,,,.CT findings of pulmonary artery dissection.Br J Radiol.2007;80:e61–e63.
- ,.Pulmonary artery dissection: echocardiographic findings and diagnosis.Echocardiography.2003;20:375–377.
- ,,.Dissecting aneurysm of the pulmonary artery.J. Pathol.1969;98;25–29.
- ,,.Pulmonary artery dissection in patients without underlying pulmonary hypertension.Histopathology.2001;38:435–442.
- ,,,,,.Pulmonary artery dissection: a rare complication of pulmonary balloon valvuloplasty diagnosed 11 years after the procedure.J Am Soc Echocardiogr.2006;19:1191,e1195–e1198.
- ,,,,.Extensive dissection of the pulmonary artery treated with combined heart‐lung transplantation.J Thorac Cardiovasc Surg.2006;132:205–206.
- ,,, et al.Pulmonary artery dissection complicating lung transplantation for primary pulmonary hypertension.Ann Thorac Surg.2006;81:360–362.
- ,.Dissecting aneurysm of the pulmonary trunk in mitral stenosis.Am J Cardiol.1986;58:1140–1141.
- ,,.Pulmonary‐artery dissection in patients with Eisenmenger's syndrome.N Engl J Med.2007;356:2110–2112.
- ,,,.Pulmonary artery dissection: an emerging cardiovascular complication in surviving patients with chronic pulmonary hypertension.Heart.2005;91:142–145.
- ,,, et al.Pulmonary artery dissection and conservative medical management.Int J Cardiol.2007;119:e25–e26.
- ,,,,,.Giant, dissecting, high‐pressure pulmonary artery aneurysm: case report of a 1‐year natural course.Tex Heart Inst J.2005;32:589–594.
- ,.A case of pulmonary artery dissection diagnosed in the Emergency Department.J Emerg Med.2002;23:155–159.
- ,,, et al.Rupture and dissection in pulmonary artery aneurysms: incidence, cause, and treatment—review and case report.J Thorac Cardiovasc Surg.2001;121:1006–1008.
- ,,,,,.Acute pulmonary trunk dissection in a patient with primary pulmonary hypertension.J Comput Assist Tomogr.2000;24:92–95.
- ,,,,.Pulmonary artery aneurysm: review and case report.Clin Cardiol.1995;18:738–740.
- ,,,,,.Dissecting aneurysm of the pulmonary artery with pulmonary hypertension.Am Rev Respir Dis.1990;142:1219–1221.
- ,,,,.Pulmonary artery dissection: MR findings.J Comput Assist Tomogr.1992;16:481–483.
- ,.Images in cardiovascular medicine. Acute type I aortic dissection with concomitant pulmonary artery dissection.Circulation.2005;112:e313–314.
- ,,,.CT findings of pulmonary artery dissection.Br J Radiol.2007;80:e61–e63.
- ,.Pulmonary artery dissection: echocardiographic findings and diagnosis.Echocardiography.2003;20:375–377.
Hospitals and Recession
With the United States mired in its most severe recession in decades, stories of hospital struggles have emerged. Beaumont Hospital, located near the headquarters of major automakers and several assembly plants outside Detroit, recently cut hundreds of jobs and put major construction on indefinite hold.1 The CEO of Boston's Beth Israel Deaconess Medical Center made an agreement with employees to take large cuts in pay and vacation time to prevent laying off 10% of the staff.2 The University of Chicago Medical Center made plans to limit the number of emergency room beds, thereby decreasing low‐reimbursing emergency admissions while making beds available for higher‐paying elective hospitalizations.3
What is surprising about these stories is that hospitals have long been considered recession‐proof. Yet, with one‐half of US hospitals having reduced their staff to balance their budgets4 and with hospitals' financial margins falling dramatically,5 economic struggles are now a widespread problem.
Furthermore, it is difficult to determine if hospitals' clinical care has been damaged by the recession. The measurement of hospital quality is new and still under‐developed: there is virtually no reliable information on hospital quality from previous recessions, and even now it will be difficult to assess quality in real time.
Critics of waste and excess in the US health care system may see tough economic times as a Darwinian proving ground for hospitals, through which efficiency will improve and poor performers will close their doors. But more likely, hospital cutbacks will risk the quality and safety of health care delivery. For reasons of both public health and fiscal impact on communities, state and federal leaders may need to watch these trends closely to design and to be ready to implement potential government remedies for hospitals' fiscal woes.
In this commentary, we describe how hospitals have fared historically during recessions, how this recession could have different effectsfirst fiscally, then clinically, and we examine policy options to mitigate these untoward effects.
Decades of Recession‐Proof Hospitals
During the Great Depression, hospital insolvency was a national problem that prompted federal and state aid. Keeping hospitals alive was a critical policy goal and proved central to the early development of health insurance that focused on payment for hospital care.6
Since WWII, growth in America's hospitals has been only loosely related to national macroeconomic trends, with other changes like technological innovations and the advent of managed care far more influential to hospital finances. In fact, during recessions, hospital care spending growth often escalates in tandem with worsening unemployment (Figure 1). One explanation for this phenomenon is that economic pressures lead to declining primary care utilization, with adverse consequences for individuals' health.7
Hospitals' Current Fiscal Vulnerability
However, the current recession is the worst in 70 years. Every method of income generation available to hospitals appears at risk, including reimbursement per discharge (70% of hospitals report moderate or significant increases in uncompensated care), number of inpatient admissions (over one‐half report a moderate or significant decrease), difficulty obtaining bonds (60% report at least significant problems), and charitable donations.4 Over 50% of US hospitals had negative margins in the fourth quarter of 2008, though there has been some improvement since that time.8
Future hospital stability concerns remain. Growth in revenue per discharge is still below the norm.5 Because employment lags a recovering economy, further reimbursement decreases are possible from increasing proportions of patients with low‐reimbursing insurers or no coverage at all, decreasing payment rates from all payers, and decreasing elective care. The lower‐reimbursing payers, like state Medicaid programs, are experiencing increased enrollment as Americans lose their jobs and their better‐paying, employer‐sponsored private insurance.9 There's also evidence that reimbursement rates are declining from both Medicare and private insurers,10 which threatens the fragile cost‐shift through which hospitals have long used private insurance reimbursement to subsidize government reimbursements.11
Hospitals' specific financial challenges will likely vary across markets. The authors' state of Michigan has been hit particularly long and hard by the current recession. Unemployment rates exceeding 11% are expected to cause dramatic losses in private health insurance.9 Patients' increasing need with decreasing ability to pay will make markets in the deepest recession particularly vulnerable.
Hospital Quality and Safety at Risk?
The effect of the recession on the quality of hospital care is less clear. Until the 1990s, hospital quality was essentially assumed and virtually unmeasured. Even now, measuring hospital quality is difficult and rarely timely. Medicare data often take 1 to 2 years to become publicly available for analysis. Reports by trade organizations like the American Hospital Association are up‐to‐date but have conflicts of interest and are less rigorous. The most timely measures of hospitals' distressflawed as they may bewill come from the hospitals themselves, just like reports of economic woe from other businesses and government agencies during challenging economic times.
However, since the publication of the 1999 report To Err is Human,12 major improvements in hospital quality and safety have transformed the delivery of inpatient care. These improvements have taken the form of simple interventions like nationally consistent medical abbreviations, management initiatives like Six Sigma, and technological advances including computerized health records.
Nonetheless, during this recession and recovery, slashed hospital budgets may slow or even stop the momentum towards further improvements in quality and safety. Frontline care delivery could be at risk. Understaffed and under financed hospitals are rarely safe. Dissatisfaction and layoffs hurt the interactions between employees and patients. Robust nurse‐to‐patient ratios which have proven vital to patients' hospital outcomes could be at risk.13 Admittedly, recession‐induced threats to quality and safety are conjectures on our part: unfortunately, no recession measures of hospitals' specific spending on staffing, technology, or process improvements exist.
However, there are many small, evidence‐based changes that could improve hospital safety dramatically in the near future. Michigan's Keystone ICU Initiative showed that systematic interventions in routine care delivery could reduce the risk of catheter‐related bloodstream infections, which currently are implicated in the death of 28,000 Americans per year, to nearly zero.14 The Institute for Healthcare Improvement's 100,000 Lives Campaign also illustrated that dramatic improvements in hospital‐related mortality can occur with fairly focused interventions. In the month after discharge, more than one‐quarter of all hospitalized patients go to an emergency room or need to be rehospitalized. This rate can be cut by 30% by inserting a nurse discharge advocate into the discharge process.15 Instituting a simple safety checklist before surgery decreased surgery‐related mortality and complications by over one‐third.16
Such interventions are effective, reasonable, and widely accessible. Over the long‐term, many may even be cost‐saving. But, importantly, they all require an institutional investment in start‐up money and an organizational will to change how things have been done. In a period of recession with severe cost‐cutting, and a recovery period of cautious spending, this may not be possible.
A Possible Stimulus: Investing in Quality Initiatives at Fiscally Vulnerable Hospitals
It is not enough to keep hospitals' doors open in a recession. Hospitals must continue to improve the quality and safety of the care they delivervital for their future patients and also for their communities who depend on them as anchors of health systems. We believe there is a need for a new, federally supported alignment of hospital finance and hospital quality that can limit damage to hospitals, help community employment, and improve patient safety.
Timely, structural quality measures could speed the introduction of functional value‐based purchasing, promote hospital safety, and help local economies at the same time. There are many simple structural measures that could be examined, such as development of discharge coordinators, promoting effective nurse‐to‐patient ratios, and encouraging health information technology (IT). Importantly, this would not duplicate efforts already underway to promote quality with process measures. With effective financial monitoring in real time, these measures could focus on high‐risk, fiscally disadvantaged hospitals.
To its credit, the Obama administration has already reached out to support hospitals, although aid has not been targeted specifically to hospitals in the most dire financial circumstances. Along with support for Medicaid and community health centers to improve primary care during the recession, the administration has provided a $268 million increase in Disproportionate Share Hospital payments towards hospitals that care for vulnerable patients, an increase of about 3%.17 Concurrently, the Centers for Medicare and Medicaid Services are implementing a value‐based purchasing program that starts with a 5% withhold in reimbursement that institutions need to earn back through a combination of mortality, process, and patient satisfaction metrics.18 The administration also reserved $19 billion to promote improvement of health IT for American medicine.19
Using health IT investment to help hospitals is an appealing concept, but for many institutions the infrastructure required to make that transition directly competes with other patient needs, including bedside patient care. IT investments have large initial costs, at a time when bank loans are difficult to acquire and few organizations can make expensive capital improvements. In fact, one‐quarter of hospitals report scaling back health IT investments that they had already started, in spite of the stimulus funds available.4
Instead, the administration may have more influence on improving care delivery by focusing on connecting hospital safety with hospital financial stability, by appropriating stimulus funds to center on quality and safety programs like those described above. Here is how: a hospital that would receive stimulus money for employing nurse discharge advocates would preserve employment while advancing patient safety, as would a hospital that retains a nurse‐to‐patient ratio above a specified threshold. By focusing on measures of structural quality, the government could improve care in ways that are easy to measure and maximize local economic stimulus without difficult outcomes assessment, insurance reform, or duplicating process measure efforts. There could even be an innovation differential (ie, payment/reward) for hospitals that improve quality while holding flat or lowering overall costs.
Equally important is to use this national financial crisis as an opportunity to improve monitoring of hospital quality. While quality assessment of hospitals is difficult, increased federal awareness of local medical need, hospital financial stability, and government awareness of emergency services overcrowding, nurse‐to‐patient ratios, and IT utilization are all valuable and easy to measure.
None of these quality‐focused fiscal interventions would be guaranteed to prevent hospital closure. Especially in small population centers, hospital closures can affect an entire community's financial growth and clinical safety net,20 while leaving hundreds or even thousands unemployed. Hospital closure should be assessed by state and federal government officials in these larger terms, perhaps even encouraging closure when appropriate, and helping prevent it when necessary.
Conclusion
Hospitals, as complex pieces of America's health care system, are central to communities' safety and economic growth. While national health coverage reform, as currently being discussed in Washington, would make hospital infrastructure less sensitive to macroeconomic changes, major reform would not come fast enough if hospitals start closing. While the worst of the recession may be over, recovery and the continuing rise in unemployment is a tenuous lifeline for hospitals on the financial brink.
We are not arguing against all hospital layoffs, or even closures. Indeed, this recession is a lean time for most industries and is likely to lead to closures for hospitals that cannot compete on efficiency or quality. But a hospital closure is a major event for a community and should not be permitted to occur without thorough consideration of alternatives. Current data on hospitals' financial status and clinical safety are limited, potentially biased, and not timely enough for this rapidly changing economic crisis. Therefore, state and federal government officials should assess whether hospitals would be eligible not just for possible emergency loans, but for linking loans to quality of care and community need. In so doing, this difficult time could be an opportunity to help hospitals improve their care, rather than watching it diminish.
- Michigan's Health Care Safety Net: In Jeopardy.2009.
- .Final budget decisions.Running A Hospital. Vol 2009.Boston, MA;2009.
- .Doctors Plan to Limit Beds in ER.Wall Street Journal.2009.
- The Impact of the Economic Crisis on Health Services for Patients and Communities.Washington, DC2009.
- ,.Hospital Operational and Financial Performance Improving.Ann Arbor, MI:Thomson Reuters Center for Healthcare Improvement.2009.
- .The Social Transformation of American Medicine.New York, NY:Basic Books;1983.
- AAFP.Patient Care during the 2008‐2009 Recession – Online Survey.Leawood, KS:AAFP.2009.
- The Impact of the Economic Crisis on Health Services for Patients and Communities.Washington, D.C.:American Hospital Association.2009.
- The economic downturn and its impact on hospitals. American Hospital Association Trendwatch.2009.
- ,,.The Current Recession and U.S. Hospitals:Center for Healthcare Improvement.2009.
- ,,.The cost‐shift payment ‘hydraulic’: foundation, history, and implications.Health Aff (Millwood).2006;25(1):22–33.
- ,.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- ,,,,.Nurse‐staffing levels and the quality of care in hospitals.N Engl J Med.2002;346(22):1715–1722.
- ,,, et al.An intervention to decrease catheter‐related bloodstream infections in the ICU.N Engl J Med.2006;355(26):2725–2732.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150(3):178–187.
- ,,, et al.A surgical safety checklist to reduce morbidity and mortality in a global population.N Engl J Med.2009;360(5):491–499.
- Disproportionate Share Hospital (DSH). Available at: http://www.hhs. gov/recovery/cms/dsh.html. Accessed December 2009.
- ,,.Measuring outcomes and efficiency in medicare value‐based purchasing.Health Aff (Millwood).2009;28(2):w251–w261.
- .Stimulating the adoption of health information technology.N Engl J Med.2009;360(15):1477–1479.
- ,,,.The effect of rural hospital closures on community economic health.Health Serv Res.2006;41(2):467–485.
With the United States mired in its most severe recession in decades, stories of hospital struggles have emerged. Beaumont Hospital, located near the headquarters of major automakers and several assembly plants outside Detroit, recently cut hundreds of jobs and put major construction on indefinite hold.1 The CEO of Boston's Beth Israel Deaconess Medical Center made an agreement with employees to take large cuts in pay and vacation time to prevent laying off 10% of the staff.2 The University of Chicago Medical Center made plans to limit the number of emergency room beds, thereby decreasing low‐reimbursing emergency admissions while making beds available for higher‐paying elective hospitalizations.3
What is surprising about these stories is that hospitals have long been considered recession‐proof. Yet, with one‐half of US hospitals having reduced their staff to balance their budgets4 and with hospitals' financial margins falling dramatically,5 economic struggles are now a widespread problem.
Furthermore, it is difficult to determine if hospitals' clinical care has been damaged by the recession. The measurement of hospital quality is new and still under‐developed: there is virtually no reliable information on hospital quality from previous recessions, and even now it will be difficult to assess quality in real time.
Critics of waste and excess in the US health care system may see tough economic times as a Darwinian proving ground for hospitals, through which efficiency will improve and poor performers will close their doors. But more likely, hospital cutbacks will risk the quality and safety of health care delivery. For reasons of both public health and fiscal impact on communities, state and federal leaders may need to watch these trends closely to design and to be ready to implement potential government remedies for hospitals' fiscal woes.
In this commentary, we describe how hospitals have fared historically during recessions, how this recession could have different effectsfirst fiscally, then clinically, and we examine policy options to mitigate these untoward effects.
Decades of Recession‐Proof Hospitals
During the Great Depression, hospital insolvency was a national problem that prompted federal and state aid. Keeping hospitals alive was a critical policy goal and proved central to the early development of health insurance that focused on payment for hospital care.6
Since WWII, growth in America's hospitals has been only loosely related to national macroeconomic trends, with other changes like technological innovations and the advent of managed care far more influential to hospital finances. In fact, during recessions, hospital care spending growth often escalates in tandem with worsening unemployment (Figure 1). One explanation for this phenomenon is that economic pressures lead to declining primary care utilization, with adverse consequences for individuals' health.7

Hospitals' Current Fiscal Vulnerability
However, the current recession is the worst in 70 years. Every method of income generation available to hospitals appears at risk, including reimbursement per discharge (70% of hospitals report moderate or significant increases in uncompensated care), number of inpatient admissions (over one‐half report a moderate or significant decrease), difficulty obtaining bonds (60% report at least significant problems), and charitable donations.4 Over 50% of US hospitals had negative margins in the fourth quarter of 2008, though there has been some improvement since that time.8
Future hospital stability concerns remain. Growth in revenue per discharge is still below the norm.5 Because employment lags a recovering economy, further reimbursement decreases are possible from increasing proportions of patients with low‐reimbursing insurers or no coverage at all, decreasing payment rates from all payers, and decreasing elective care. The lower‐reimbursing payers, like state Medicaid programs, are experiencing increased enrollment as Americans lose their jobs and their better‐paying, employer‐sponsored private insurance.9 There's also evidence that reimbursement rates are declining from both Medicare and private insurers,10 which threatens the fragile cost‐shift through which hospitals have long used private insurance reimbursement to subsidize government reimbursements.11
Hospitals' specific financial challenges will likely vary across markets. The authors' state of Michigan has been hit particularly long and hard by the current recession. Unemployment rates exceeding 11% are expected to cause dramatic losses in private health insurance.9 Patients' increasing need with decreasing ability to pay will make markets in the deepest recession particularly vulnerable.
Hospital Quality and Safety at Risk?
The effect of the recession on the quality of hospital care is less clear. Until the 1990s, hospital quality was essentially assumed and virtually unmeasured. Even now, measuring hospital quality is difficult and rarely timely. Medicare data often take 1 to 2 years to become publicly available for analysis. Reports by trade organizations like the American Hospital Association are up‐to‐date but have conflicts of interest and are less rigorous. The most timely measures of hospitals' distressflawed as they may bewill come from the hospitals themselves, just like reports of economic woe from other businesses and government agencies during challenging economic times.
However, since the publication of the 1999 report To Err is Human,12 major improvements in hospital quality and safety have transformed the delivery of inpatient care. These improvements have taken the form of simple interventions like nationally consistent medical abbreviations, management initiatives like Six Sigma, and technological advances including computerized health records.
Nonetheless, during this recession and recovery, slashed hospital budgets may slow or even stop the momentum towards further improvements in quality and safety. Frontline care delivery could be at risk. Understaffed and under financed hospitals are rarely safe. Dissatisfaction and layoffs hurt the interactions between employees and patients. Robust nurse‐to‐patient ratios which have proven vital to patients' hospital outcomes could be at risk.13 Admittedly, recession‐induced threats to quality and safety are conjectures on our part: unfortunately, no recession measures of hospitals' specific spending on staffing, technology, or process improvements exist.
However, there are many small, evidence‐based changes that could improve hospital safety dramatically in the near future. Michigan's Keystone ICU Initiative showed that systematic interventions in routine care delivery could reduce the risk of catheter‐related bloodstream infections, which currently are implicated in the death of 28,000 Americans per year, to nearly zero.14 The Institute for Healthcare Improvement's 100,000 Lives Campaign also illustrated that dramatic improvements in hospital‐related mortality can occur with fairly focused interventions. In the month after discharge, more than one‐quarter of all hospitalized patients go to an emergency room or need to be rehospitalized. This rate can be cut by 30% by inserting a nurse discharge advocate into the discharge process.15 Instituting a simple safety checklist before surgery decreased surgery‐related mortality and complications by over one‐third.16
Such interventions are effective, reasonable, and widely accessible. Over the long‐term, many may even be cost‐saving. But, importantly, they all require an institutional investment in start‐up money and an organizational will to change how things have been done. In a period of recession with severe cost‐cutting, and a recovery period of cautious spending, this may not be possible.
A Possible Stimulus: Investing in Quality Initiatives at Fiscally Vulnerable Hospitals
It is not enough to keep hospitals' doors open in a recession. Hospitals must continue to improve the quality and safety of the care they delivervital for their future patients and also for their communities who depend on them as anchors of health systems. We believe there is a need for a new, federally supported alignment of hospital finance and hospital quality that can limit damage to hospitals, help community employment, and improve patient safety.
Timely, structural quality measures could speed the introduction of functional value‐based purchasing, promote hospital safety, and help local economies at the same time. There are many simple structural measures that could be examined, such as development of discharge coordinators, promoting effective nurse‐to‐patient ratios, and encouraging health information technology (IT). Importantly, this would not duplicate efforts already underway to promote quality with process measures. With effective financial monitoring in real time, these measures could focus on high‐risk, fiscally disadvantaged hospitals.
To its credit, the Obama administration has already reached out to support hospitals, although aid has not been targeted specifically to hospitals in the most dire financial circumstances. Along with support for Medicaid and community health centers to improve primary care during the recession, the administration has provided a $268 million increase in Disproportionate Share Hospital payments towards hospitals that care for vulnerable patients, an increase of about 3%.17 Concurrently, the Centers for Medicare and Medicaid Services are implementing a value‐based purchasing program that starts with a 5% withhold in reimbursement that institutions need to earn back through a combination of mortality, process, and patient satisfaction metrics.18 The administration also reserved $19 billion to promote improvement of health IT for American medicine.19
Using health IT investment to help hospitals is an appealing concept, but for many institutions the infrastructure required to make that transition directly competes with other patient needs, including bedside patient care. IT investments have large initial costs, at a time when bank loans are difficult to acquire and few organizations can make expensive capital improvements. In fact, one‐quarter of hospitals report scaling back health IT investments that they had already started, in spite of the stimulus funds available.4
Instead, the administration may have more influence on improving care delivery by focusing on connecting hospital safety with hospital financial stability, by appropriating stimulus funds to center on quality and safety programs like those described above. Here is how: a hospital that would receive stimulus money for employing nurse discharge advocates would preserve employment while advancing patient safety, as would a hospital that retains a nurse‐to‐patient ratio above a specified threshold. By focusing on measures of structural quality, the government could improve care in ways that are easy to measure and maximize local economic stimulus without difficult outcomes assessment, insurance reform, or duplicating process measure efforts. There could even be an innovation differential (ie, payment/reward) for hospitals that improve quality while holding flat or lowering overall costs.
Equally important is to use this national financial crisis as an opportunity to improve monitoring of hospital quality. While quality assessment of hospitals is difficult, increased federal awareness of local medical need, hospital financial stability, and government awareness of emergency services overcrowding, nurse‐to‐patient ratios, and IT utilization are all valuable and easy to measure.
None of these quality‐focused fiscal interventions would be guaranteed to prevent hospital closure. Especially in small population centers, hospital closures can affect an entire community's financial growth and clinical safety net,20 while leaving hundreds or even thousands unemployed. Hospital closure should be assessed by state and federal government officials in these larger terms, perhaps even encouraging closure when appropriate, and helping prevent it when necessary.
Conclusion
Hospitals, as complex pieces of America's health care system, are central to communities' safety and economic growth. While national health coverage reform, as currently being discussed in Washington, would make hospital infrastructure less sensitive to macroeconomic changes, major reform would not come fast enough if hospitals start closing. While the worst of the recession may be over, recovery and the continuing rise in unemployment is a tenuous lifeline for hospitals on the financial brink.
We are not arguing against all hospital layoffs, or even closures. Indeed, this recession is a lean time for most industries and is likely to lead to closures for hospitals that cannot compete on efficiency or quality. But a hospital closure is a major event for a community and should not be permitted to occur without thorough consideration of alternatives. Current data on hospitals' financial status and clinical safety are limited, potentially biased, and not timely enough for this rapidly changing economic crisis. Therefore, state and federal government officials should assess whether hospitals would be eligible not just for possible emergency loans, but for linking loans to quality of care and community need. In so doing, this difficult time could be an opportunity to help hospitals improve their care, rather than watching it diminish.
With the United States mired in its most severe recession in decades, stories of hospital struggles have emerged. Beaumont Hospital, located near the headquarters of major automakers and several assembly plants outside Detroit, recently cut hundreds of jobs and put major construction on indefinite hold.1 The CEO of Boston's Beth Israel Deaconess Medical Center made an agreement with employees to take large cuts in pay and vacation time to prevent laying off 10% of the staff.2 The University of Chicago Medical Center made plans to limit the number of emergency room beds, thereby decreasing low‐reimbursing emergency admissions while making beds available for higher‐paying elective hospitalizations.3
What is surprising about these stories is that hospitals have long been considered recession‐proof. Yet, with one‐half of US hospitals having reduced their staff to balance their budgets4 and with hospitals' financial margins falling dramatically,5 economic struggles are now a widespread problem.
Furthermore, it is difficult to determine if hospitals' clinical care has been damaged by the recession. The measurement of hospital quality is new and still under‐developed: there is virtually no reliable information on hospital quality from previous recessions, and even now it will be difficult to assess quality in real time.
Critics of waste and excess in the US health care system may see tough economic times as a Darwinian proving ground for hospitals, through which efficiency will improve and poor performers will close their doors. But more likely, hospital cutbacks will risk the quality and safety of health care delivery. For reasons of both public health and fiscal impact on communities, state and federal leaders may need to watch these trends closely to design and to be ready to implement potential government remedies for hospitals' fiscal woes.
In this commentary, we describe how hospitals have fared historically during recessions, how this recession could have different effectsfirst fiscally, then clinically, and we examine policy options to mitigate these untoward effects.
Decades of Recession‐Proof Hospitals
During the Great Depression, hospital insolvency was a national problem that prompted federal and state aid. Keeping hospitals alive was a critical policy goal and proved central to the early development of health insurance that focused on payment for hospital care.6
Since WWII, growth in America's hospitals has been only loosely related to national macroeconomic trends, with other changes like technological innovations and the advent of managed care far more influential to hospital finances. In fact, during recessions, hospital care spending growth often escalates in tandem with worsening unemployment (Figure 1). One explanation for this phenomenon is that economic pressures lead to declining primary care utilization, with adverse consequences for individuals' health.7

Hospitals' Current Fiscal Vulnerability
However, the current recession is the worst in 70 years. Every method of income generation available to hospitals appears at risk, including reimbursement per discharge (70% of hospitals report moderate or significant increases in uncompensated care), number of inpatient admissions (over one‐half report a moderate or significant decrease), difficulty obtaining bonds (60% report at least significant problems), and charitable donations.4 Over 50% of US hospitals had negative margins in the fourth quarter of 2008, though there has been some improvement since that time.8
Future hospital stability concerns remain. Growth in revenue per discharge is still below the norm.5 Because employment lags a recovering economy, further reimbursement decreases are possible from increasing proportions of patients with low‐reimbursing insurers or no coverage at all, decreasing payment rates from all payers, and decreasing elective care. The lower‐reimbursing payers, like state Medicaid programs, are experiencing increased enrollment as Americans lose their jobs and their better‐paying, employer‐sponsored private insurance.9 There's also evidence that reimbursement rates are declining from both Medicare and private insurers,10 which threatens the fragile cost‐shift through which hospitals have long used private insurance reimbursement to subsidize government reimbursements.11
Hospitals' specific financial challenges will likely vary across markets. The authors' state of Michigan has been hit particularly long and hard by the current recession. Unemployment rates exceeding 11% are expected to cause dramatic losses in private health insurance.9 Patients' increasing need with decreasing ability to pay will make markets in the deepest recession particularly vulnerable.
Hospital Quality and Safety at Risk?
The effect of the recession on the quality of hospital care is less clear. Until the 1990s, hospital quality was essentially assumed and virtually unmeasured. Even now, measuring hospital quality is difficult and rarely timely. Medicare data often take 1 to 2 years to become publicly available for analysis. Reports by trade organizations like the American Hospital Association are up‐to‐date but have conflicts of interest and are less rigorous. The most timely measures of hospitals' distressflawed as they may bewill come from the hospitals themselves, just like reports of economic woe from other businesses and government agencies during challenging economic times.
However, since the publication of the 1999 report To Err is Human,12 major improvements in hospital quality and safety have transformed the delivery of inpatient care. These improvements have taken the form of simple interventions like nationally consistent medical abbreviations, management initiatives like Six Sigma, and technological advances including computerized health records.
Nonetheless, during this recession and recovery, slashed hospital budgets may slow or even stop the momentum towards further improvements in quality and safety. Frontline care delivery could be at risk. Understaffed and under financed hospitals are rarely safe. Dissatisfaction and layoffs hurt the interactions between employees and patients. Robust nurse‐to‐patient ratios which have proven vital to patients' hospital outcomes could be at risk.13 Admittedly, recession‐induced threats to quality and safety are conjectures on our part: unfortunately, no recession measures of hospitals' specific spending on staffing, technology, or process improvements exist.
However, there are many small, evidence‐based changes that could improve hospital safety dramatically in the near future. Michigan's Keystone ICU Initiative showed that systematic interventions in routine care delivery could reduce the risk of catheter‐related bloodstream infections, which currently are implicated in the death of 28,000 Americans per year, to nearly zero.14 The Institute for Healthcare Improvement's 100,000 Lives Campaign also illustrated that dramatic improvements in hospital‐related mortality can occur with fairly focused interventions. In the month after discharge, more than one‐quarter of all hospitalized patients go to an emergency room or need to be rehospitalized. This rate can be cut by 30% by inserting a nurse discharge advocate into the discharge process.15 Instituting a simple safety checklist before surgery decreased surgery‐related mortality and complications by over one‐third.16
Such interventions are effective, reasonable, and widely accessible. Over the long‐term, many may even be cost‐saving. But, importantly, they all require an institutional investment in start‐up money and an organizational will to change how things have been done. In a period of recession with severe cost‐cutting, and a recovery period of cautious spending, this may not be possible.
A Possible Stimulus: Investing in Quality Initiatives at Fiscally Vulnerable Hospitals
It is not enough to keep hospitals' doors open in a recession. Hospitals must continue to improve the quality and safety of the care they delivervital for their future patients and also for their communities who depend on them as anchors of health systems. We believe there is a need for a new, federally supported alignment of hospital finance and hospital quality that can limit damage to hospitals, help community employment, and improve patient safety.
Timely, structural quality measures could speed the introduction of functional value‐based purchasing, promote hospital safety, and help local economies at the same time. There are many simple structural measures that could be examined, such as development of discharge coordinators, promoting effective nurse‐to‐patient ratios, and encouraging health information technology (IT). Importantly, this would not duplicate efforts already underway to promote quality with process measures. With effective financial monitoring in real time, these measures could focus on high‐risk, fiscally disadvantaged hospitals.
To its credit, the Obama administration has already reached out to support hospitals, although aid has not been targeted specifically to hospitals in the most dire financial circumstances. Along with support for Medicaid and community health centers to improve primary care during the recession, the administration has provided a $268 million increase in Disproportionate Share Hospital payments towards hospitals that care for vulnerable patients, an increase of about 3%.17 Concurrently, the Centers for Medicare and Medicaid Services are implementing a value‐based purchasing program that starts with a 5% withhold in reimbursement that institutions need to earn back through a combination of mortality, process, and patient satisfaction metrics.18 The administration also reserved $19 billion to promote improvement of health IT for American medicine.19
Using health IT investment to help hospitals is an appealing concept, but for many institutions the infrastructure required to make that transition directly competes with other patient needs, including bedside patient care. IT investments have large initial costs, at a time when bank loans are difficult to acquire and few organizations can make expensive capital improvements. In fact, one‐quarter of hospitals report scaling back health IT investments that they had already started, in spite of the stimulus funds available.4
Instead, the administration may have more influence on improving care delivery by focusing on connecting hospital safety with hospital financial stability, by appropriating stimulus funds to center on quality and safety programs like those described above. Here is how: a hospital that would receive stimulus money for employing nurse discharge advocates would preserve employment while advancing patient safety, as would a hospital that retains a nurse‐to‐patient ratio above a specified threshold. By focusing on measures of structural quality, the government could improve care in ways that are easy to measure and maximize local economic stimulus without difficult outcomes assessment, insurance reform, or duplicating process measure efforts. There could even be an innovation differential (ie, payment/reward) for hospitals that improve quality while holding flat or lowering overall costs.
Equally important is to use this national financial crisis as an opportunity to improve monitoring of hospital quality. While quality assessment of hospitals is difficult, increased federal awareness of local medical need, hospital financial stability, and government awareness of emergency services overcrowding, nurse‐to‐patient ratios, and IT utilization are all valuable and easy to measure.
None of these quality‐focused fiscal interventions would be guaranteed to prevent hospital closure. Especially in small population centers, hospital closures can affect an entire community's financial growth and clinical safety net,20 while leaving hundreds or even thousands unemployed. Hospital closure should be assessed by state and federal government officials in these larger terms, perhaps even encouraging closure when appropriate, and helping prevent it when necessary.
Conclusion
Hospitals, as complex pieces of America's health care system, are central to communities' safety and economic growth. While national health coverage reform, as currently being discussed in Washington, would make hospital infrastructure less sensitive to macroeconomic changes, major reform would not come fast enough if hospitals start closing. While the worst of the recession may be over, recovery and the continuing rise in unemployment is a tenuous lifeline for hospitals on the financial brink.
We are not arguing against all hospital layoffs, or even closures. Indeed, this recession is a lean time for most industries and is likely to lead to closures for hospitals that cannot compete on efficiency or quality. But a hospital closure is a major event for a community and should not be permitted to occur without thorough consideration of alternatives. Current data on hospitals' financial status and clinical safety are limited, potentially biased, and not timely enough for this rapidly changing economic crisis. Therefore, state and federal government officials should assess whether hospitals would be eligible not just for possible emergency loans, but for linking loans to quality of care and community need. In so doing, this difficult time could be an opportunity to help hospitals improve their care, rather than watching it diminish.
- Michigan's Health Care Safety Net: In Jeopardy.2009.
- .Final budget decisions.Running A Hospital. Vol 2009.Boston, MA;2009.
- .Doctors Plan to Limit Beds in ER.Wall Street Journal.2009.
- The Impact of the Economic Crisis on Health Services for Patients and Communities.Washington, DC2009.
- ,.Hospital Operational and Financial Performance Improving.Ann Arbor, MI:Thomson Reuters Center for Healthcare Improvement.2009.
- .The Social Transformation of American Medicine.New York, NY:Basic Books;1983.
- AAFP.Patient Care during the 2008‐2009 Recession – Online Survey.Leawood, KS:AAFP.2009.
- The Impact of the Economic Crisis on Health Services for Patients and Communities.Washington, D.C.:American Hospital Association.2009.
- The economic downturn and its impact on hospitals. American Hospital Association Trendwatch.2009.
- ,,.The Current Recession and U.S. Hospitals:Center for Healthcare Improvement.2009.
- ,,.The cost‐shift payment ‘hydraulic’: foundation, history, and implications.Health Aff (Millwood).2006;25(1):22–33.
- ,.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- ,,,,.Nurse‐staffing levels and the quality of care in hospitals.N Engl J Med.2002;346(22):1715–1722.
- ,,, et al.An intervention to decrease catheter‐related bloodstream infections in the ICU.N Engl J Med.2006;355(26):2725–2732.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150(3):178–187.
- ,,, et al.A surgical safety checklist to reduce morbidity and mortality in a global population.N Engl J Med.2009;360(5):491–499.
- Disproportionate Share Hospital (DSH). Available at: http://www.hhs. gov/recovery/cms/dsh.html. Accessed December 2009.
- ,,.Measuring outcomes and efficiency in medicare value‐based purchasing.Health Aff (Millwood).2009;28(2):w251–w261.
- .Stimulating the adoption of health information technology.N Engl J Med.2009;360(15):1477–1479.
- ,,,.The effect of rural hospital closures on community economic health.Health Serv Res.2006;41(2):467–485.
- Michigan's Health Care Safety Net: In Jeopardy.2009.
- .Final budget decisions.Running A Hospital. Vol 2009.Boston, MA;2009.
- .Doctors Plan to Limit Beds in ER.Wall Street Journal.2009.
- The Impact of the Economic Crisis on Health Services for Patients and Communities.Washington, DC2009.
- ,.Hospital Operational and Financial Performance Improving.Ann Arbor, MI:Thomson Reuters Center for Healthcare Improvement.2009.
- .The Social Transformation of American Medicine.New York, NY:Basic Books;1983.
- AAFP.Patient Care during the 2008‐2009 Recession – Online Survey.Leawood, KS:AAFP.2009.
- The Impact of the Economic Crisis on Health Services for Patients and Communities.Washington, D.C.:American Hospital Association.2009.
- The economic downturn and its impact on hospitals. American Hospital Association Trendwatch.2009.
- ,,.The Current Recession and U.S. Hospitals:Center for Healthcare Improvement.2009.
- ,,.The cost‐shift payment ‘hydraulic’: foundation, history, and implications.Health Aff (Millwood).2006;25(1):22–33.
- ,.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press;1999.
- ,,,,.Nurse‐staffing levels and the quality of care in hospitals.N Engl J Med.2002;346(22):1715–1722.
- ,,, et al.An intervention to decrease catheter‐related bloodstream infections in the ICU.N Engl J Med.2006;355(26):2725–2732.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150(3):178–187.
- ,,, et al.A surgical safety checklist to reduce morbidity and mortality in a global population.N Engl J Med.2009;360(5):491–499.
- Disproportionate Share Hospital (DSH). Available at: http://www.hhs. gov/recovery/cms/dsh.html. Accessed December 2009.
- ,,.Measuring outcomes and efficiency in medicare value‐based purchasing.Health Aff (Millwood).2009;28(2):w251–w261.
- .Stimulating the adoption of health information technology.N Engl J Med.2009;360(15):1477–1479.
- ,,,.The effect of rural hospital closures on community economic health.Health Serv Res.2006;41(2):467–485.
New Resident Regulations on the Horizon
The Accreditation Council for Graduate Medical Education (ACGME) task force is close to offering revised standards for medical resident work hours—a decision that could significantly change the landscape for academic hospitalist programs.
While no date has been set for the unveiling, a May 4 letter written by ACGME CEO Thomas Nasca, MD, MACP, says “the work of the task force is nearly complete.” Many expect the rules will be offered as a draft for public comment in the coming weeks. If approved, the new regulations would probably take effect in July 2011. ACGME formed the task force more than a year ago as the prescribed five-year update to the landmark 2003 duty-hour standards.
Medical experts say the new rules will in many ways mirror the recommendations of the Institute of Medicine’s 2008 report “Resident Duty Hours: Enhancing Sleep, Supervision and Safety.” The oft-quoted report recommended residents only treat patients for up to 16 hours during their shift, down from the current recommendation of 24 hours. It also suggests residents take an uninterrupted five hours for a continuous sleep period between 10 p.m. and 8 a.m.
Many HM physicians expect the new ACGME rules will include a 60-hour workweek cap, part of a growing trend to try to balance the educational requirements of medical school with the need to expose residents to practical experience. Dr. Nasca gave no hint as to what ACGME’s recommendations will be.
In an article in this month’s The Hospitalist, academic and community hospitalists say they have been keeping on eye on how the newest rules will change their playing fields: Will a wave of academics flee teaching hospitals, as additional clinical duties become an intrusion? Will teaching hospitals face financial pressure as they struggle to replace the lower-cost labor force that residents represent? And—perhaps most importantly from a medical perspective—will graduate trainees be as prepared as their predecessors when they enter practice?
“Hospitalists will always be involved in teaching—it will never go away,” says Julia Wright, MD, FHM, a member of Team Hospitalist and clinical associate professor of medicine and director of hospital medicine at the University of Wisconsin School of Medicine and Public Health in Madison. “But it will be a very different balance, a different kind of feel.”
The Accreditation Council for Graduate Medical Education (ACGME) task force is close to offering revised standards for medical resident work hours—a decision that could significantly change the landscape for academic hospitalist programs.
While no date has been set for the unveiling, a May 4 letter written by ACGME CEO Thomas Nasca, MD, MACP, says “the work of the task force is nearly complete.” Many expect the rules will be offered as a draft for public comment in the coming weeks. If approved, the new regulations would probably take effect in July 2011. ACGME formed the task force more than a year ago as the prescribed five-year update to the landmark 2003 duty-hour standards.
Medical experts say the new rules will in many ways mirror the recommendations of the Institute of Medicine’s 2008 report “Resident Duty Hours: Enhancing Sleep, Supervision and Safety.” The oft-quoted report recommended residents only treat patients for up to 16 hours during their shift, down from the current recommendation of 24 hours. It also suggests residents take an uninterrupted five hours for a continuous sleep period between 10 p.m. and 8 a.m.
Many HM physicians expect the new ACGME rules will include a 60-hour workweek cap, part of a growing trend to try to balance the educational requirements of medical school with the need to expose residents to practical experience. Dr. Nasca gave no hint as to what ACGME’s recommendations will be.
In an article in this month’s The Hospitalist, academic and community hospitalists say they have been keeping on eye on how the newest rules will change their playing fields: Will a wave of academics flee teaching hospitals, as additional clinical duties become an intrusion? Will teaching hospitals face financial pressure as they struggle to replace the lower-cost labor force that residents represent? And—perhaps most importantly from a medical perspective—will graduate trainees be as prepared as their predecessors when they enter practice?
“Hospitalists will always be involved in teaching—it will never go away,” says Julia Wright, MD, FHM, a member of Team Hospitalist and clinical associate professor of medicine and director of hospital medicine at the University of Wisconsin School of Medicine and Public Health in Madison. “But it will be a very different balance, a different kind of feel.”
The Accreditation Council for Graduate Medical Education (ACGME) task force is close to offering revised standards for medical resident work hours—a decision that could significantly change the landscape for academic hospitalist programs.
While no date has been set for the unveiling, a May 4 letter written by ACGME CEO Thomas Nasca, MD, MACP, says “the work of the task force is nearly complete.” Many expect the rules will be offered as a draft for public comment in the coming weeks. If approved, the new regulations would probably take effect in July 2011. ACGME formed the task force more than a year ago as the prescribed five-year update to the landmark 2003 duty-hour standards.
Medical experts say the new rules will in many ways mirror the recommendations of the Institute of Medicine’s 2008 report “Resident Duty Hours: Enhancing Sleep, Supervision and Safety.” The oft-quoted report recommended residents only treat patients for up to 16 hours during their shift, down from the current recommendation of 24 hours. It also suggests residents take an uninterrupted five hours for a continuous sleep period between 10 p.m. and 8 a.m.
Many HM physicians expect the new ACGME rules will include a 60-hour workweek cap, part of a growing trend to try to balance the educational requirements of medical school with the need to expose residents to practical experience. Dr. Nasca gave no hint as to what ACGME’s recommendations will be.
In an article in this month’s The Hospitalist, academic and community hospitalists say they have been keeping on eye on how the newest rules will change their playing fields: Will a wave of academics flee teaching hospitals, as additional clinical duties become an intrusion? Will teaching hospitals face financial pressure as they struggle to replace the lower-cost labor force that residents represent? And—perhaps most importantly from a medical perspective—will graduate trainees be as prepared as their predecessors when they enter practice?
“Hospitalists will always be involved in teaching—it will never go away,” says Julia Wright, MD, FHM, a member of Team Hospitalist and clinical associate professor of medicine and director of hospital medicine at the University of Wisconsin School of Medicine and Public Health in Madison. “But it will be a very different balance, a different kind of feel.”
In the Literature: Research You Need to Know
Clinical question: Is recombinant tissue-type plasminogen activator (rt-PA) at 50 mg/2 hr as effective and safe as 100 mg/2 hr for acute pulmonary thromboembolism (PTE)?
Background: The U.S. Food and Drug Administration approved a 100 mg/2 hr dose of rt-PA, which has been recommended as the standard regimen for PTE. Lower doses potentially have less bleeding but their clinical efficacy in PTE has not yet been evaluated. If efficacious, rt-PA at 50 mg/2 hr used for treating acute MI might prove to be a better regimen for acute PTE.
Study design: Prospective, randomized, open-label, multicenter trial.
Setting: Multiple centers in China.
Synopsis: 118 patients with PTE, with either hemodynamic instability or anatomically massive obstruction, were assigned to receive rt-PA at 50 mg/2 hr (n=65) or 100 mg/2 hr (n=53) and followed for 14 days. Clinical efficacy as serially measured by improvement in pulmonary artery pressure and right ventricular function on echocardiogram, lung perfusion on V/Q scan, and pulmonary artery obstruction by CTPA was not significantly different between the two groups.
Though mortality was not significantly different between both groups (three in the high-dose group and one in the low-dose group), there was one fatal ICH in the high-dose group. As can be expected, total bleeding prevalence (major and minor) was lower in the 50-mg group (17% vs. 32%, p=0.084), especially in patients with body weight <65 kgs or BMI <24 kg/m2.
The fact that two-thirds of the patients had only anatomically massive PTE without any hemodynamic instability limits the extrapolation of the efficacy of low-dose rt-PA because heparin alone is generally used in these cases. Also, patients with body weight >100 kg may need the higher dose but were not evaluated adequately in this study.
Bottom line: A lower-dose regimen of 50 mg/2 hr of rt-PA is as efficacious as 100 mg/2 hr in treatment of PTE but offers a better safety profile in patients with weight <65kg.
Citation: Wang C, Zhai Z, Yang Y, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. 2010;137(2):254-262.
Clinical question: Is recombinant tissue-type plasminogen activator (rt-PA) at 50 mg/2 hr as effective and safe as 100 mg/2 hr for acute pulmonary thromboembolism (PTE)?
Background: The U.S. Food and Drug Administration approved a 100 mg/2 hr dose of rt-PA, which has been recommended as the standard regimen for PTE. Lower doses potentially have less bleeding but their clinical efficacy in PTE has not yet been evaluated. If efficacious, rt-PA at 50 mg/2 hr used for treating acute MI might prove to be a better regimen for acute PTE.
Study design: Prospective, randomized, open-label, multicenter trial.
Setting: Multiple centers in China.
Synopsis: 118 patients with PTE, with either hemodynamic instability or anatomically massive obstruction, were assigned to receive rt-PA at 50 mg/2 hr (n=65) or 100 mg/2 hr (n=53) and followed for 14 days. Clinical efficacy as serially measured by improvement in pulmonary artery pressure and right ventricular function on echocardiogram, lung perfusion on V/Q scan, and pulmonary artery obstruction by CTPA was not significantly different between the two groups.
Though mortality was not significantly different between both groups (three in the high-dose group and one in the low-dose group), there was one fatal ICH in the high-dose group. As can be expected, total bleeding prevalence (major and minor) was lower in the 50-mg group (17% vs. 32%, p=0.084), especially in patients with body weight <65 kgs or BMI <24 kg/m2.
The fact that two-thirds of the patients had only anatomically massive PTE without any hemodynamic instability limits the extrapolation of the efficacy of low-dose rt-PA because heparin alone is generally used in these cases. Also, patients with body weight >100 kg may need the higher dose but were not evaluated adequately in this study.
Bottom line: A lower-dose regimen of 50 mg/2 hr of rt-PA is as efficacious as 100 mg/2 hr in treatment of PTE but offers a better safety profile in patients with weight <65kg.
Citation: Wang C, Zhai Z, Yang Y, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. 2010;137(2):254-262.
Clinical question: Is recombinant tissue-type plasminogen activator (rt-PA) at 50 mg/2 hr as effective and safe as 100 mg/2 hr for acute pulmonary thromboembolism (PTE)?
Background: The U.S. Food and Drug Administration approved a 100 mg/2 hr dose of rt-PA, which has been recommended as the standard regimen for PTE. Lower doses potentially have less bleeding but their clinical efficacy in PTE has not yet been evaluated. If efficacious, rt-PA at 50 mg/2 hr used for treating acute MI might prove to be a better regimen for acute PTE.
Study design: Prospective, randomized, open-label, multicenter trial.
Setting: Multiple centers in China.
Synopsis: 118 patients with PTE, with either hemodynamic instability or anatomically massive obstruction, were assigned to receive rt-PA at 50 mg/2 hr (n=65) or 100 mg/2 hr (n=53) and followed for 14 days. Clinical efficacy as serially measured by improvement in pulmonary artery pressure and right ventricular function on echocardiogram, lung perfusion on V/Q scan, and pulmonary artery obstruction by CTPA was not significantly different between the two groups.
Though mortality was not significantly different between both groups (three in the high-dose group and one in the low-dose group), there was one fatal ICH in the high-dose group. As can be expected, total bleeding prevalence (major and minor) was lower in the 50-mg group (17% vs. 32%, p=0.084), especially in patients with body weight <65 kgs or BMI <24 kg/m2.
The fact that two-thirds of the patients had only anatomically massive PTE without any hemodynamic instability limits the extrapolation of the efficacy of low-dose rt-PA because heparin alone is generally used in these cases. Also, patients with body weight >100 kg may need the higher dose but were not evaluated adequately in this study.
Bottom line: A lower-dose regimen of 50 mg/2 hr of rt-PA is as efficacious as 100 mg/2 hr in treatment of PTE but offers a better safety profile in patients with weight <65kg.
Citation: Wang C, Zhai Z, Yang Y, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. 2010;137(2):254-262.