User login
LVH and hypertension: Is treating the pressure not enough?
In patients with “borderline” hypertension or those in whom the duration of blood pressure elevation is hard to ascertain, the finding of end-organ damage has traditionally been used as an argument to institute aggressive antihypertensive therapy. In this setting, retinal hypertensive disease, an S4 gallop, and left ventricular hypertrophy (LVH) are often specifically sought.
LVH and otherwise unexplained chronic kidney disease in patients with hypertension have generally been believed to be products of the elevated arterial pressure, and primary treatment has targeted pressure control. Bauml and Underwood, in this issue of the Journal, emphasize some published clinical trial data indicating that LVH may be an independent risk factor for poorer cardiovascular outcome. Even more provocative is the suggestion that LVH can be reversed, as can the associated increased risk of cardiovascular morbidity, independently of the hypertension.
Given our current understanding that LVH, under some conditions, can be induced by products of the renin-angiotensin system, this would suggest that pharmacologic blockade of this enzyme system should have extra benefit, above that seen from other antihypertensive agents. Conceivably, this may be true only in patients with LVH, and the time course of benefit may not directly parallel that seen with the control of hypertension. That theoretically may explain the lack of uniform advantage of angiotensin blockade over other effective antihypertensive approaches.
Since electrocardiography is a specific but not very sensitive test for LVH, the authors suggest that patients with hypertension be routinely screened for LVH using echocardiography. I am not sure the weight of the evidence supports this approach at present, particularly in the current frenzy of cost containment. Nonetheless, this concept warrants consideration, and at the least, large patient databases might be screened retrospectively to further validate or refute the concept that hypertension-associated LVH is an independent, reversible risk factor for cardiovascular morbidity.
In patients with “borderline” hypertension or those in whom the duration of blood pressure elevation is hard to ascertain, the finding of end-organ damage has traditionally been used as an argument to institute aggressive antihypertensive therapy. In this setting, retinal hypertensive disease, an S4 gallop, and left ventricular hypertrophy (LVH) are often specifically sought.
LVH and otherwise unexplained chronic kidney disease in patients with hypertension have generally been believed to be products of the elevated arterial pressure, and primary treatment has targeted pressure control. Bauml and Underwood, in this issue of the Journal, emphasize some published clinical trial data indicating that LVH may be an independent risk factor for poorer cardiovascular outcome. Even more provocative is the suggestion that LVH can be reversed, as can the associated increased risk of cardiovascular morbidity, independently of the hypertension.
Given our current understanding that LVH, under some conditions, can be induced by products of the renin-angiotensin system, this would suggest that pharmacologic blockade of this enzyme system should have extra benefit, above that seen from other antihypertensive agents. Conceivably, this may be true only in patients with LVH, and the time course of benefit may not directly parallel that seen with the control of hypertension. That theoretically may explain the lack of uniform advantage of angiotensin blockade over other effective antihypertensive approaches.
Since electrocardiography is a specific but not very sensitive test for LVH, the authors suggest that patients with hypertension be routinely screened for LVH using echocardiography. I am not sure the weight of the evidence supports this approach at present, particularly in the current frenzy of cost containment. Nonetheless, this concept warrants consideration, and at the least, large patient databases might be screened retrospectively to further validate or refute the concept that hypertension-associated LVH is an independent, reversible risk factor for cardiovascular morbidity.
In patients with “borderline” hypertension or those in whom the duration of blood pressure elevation is hard to ascertain, the finding of end-organ damage has traditionally been used as an argument to institute aggressive antihypertensive therapy. In this setting, retinal hypertensive disease, an S4 gallop, and left ventricular hypertrophy (LVH) are often specifically sought.
LVH and otherwise unexplained chronic kidney disease in patients with hypertension have generally been believed to be products of the elevated arterial pressure, and primary treatment has targeted pressure control. Bauml and Underwood, in this issue of the Journal, emphasize some published clinical trial data indicating that LVH may be an independent risk factor for poorer cardiovascular outcome. Even more provocative is the suggestion that LVH can be reversed, as can the associated increased risk of cardiovascular morbidity, independently of the hypertension.
Given our current understanding that LVH, under some conditions, can be induced by products of the renin-angiotensin system, this would suggest that pharmacologic blockade of this enzyme system should have extra benefit, above that seen from other antihypertensive agents. Conceivably, this may be true only in patients with LVH, and the time course of benefit may not directly parallel that seen with the control of hypertension. That theoretically may explain the lack of uniform advantage of angiotensin blockade over other effective antihypertensive approaches.
Since electrocardiography is a specific but not very sensitive test for LVH, the authors suggest that patients with hypertension be routinely screened for LVH using echocardiography. I am not sure the weight of the evidence supports this approach at present, particularly in the current frenzy of cost containment. Nonetheless, this concept warrants consideration, and at the least, large patient databases might be screened retrospectively to further validate or refute the concept that hypertension-associated LVH is an independent, reversible risk factor for cardiovascular morbidity.
Left ventricular hypertrophy: An overlooked cardiovascular risk factor
Left ventricular hypertrophy (LVH) strongly predicts cardiovascular morbidity and overall mortality in hypertensive patients. 1–7 Antihypertensive treatment that causes LVH to regress decreases the rates of adverse cardiovascular events and improves survival, independent of how much the blood pressure is lowered.8–11 It is clinically important to recognize that LVH is a modifiable risk factor and that management is more complex than just blood pressure control.
This paper reviews the definition of LVH, compares the diagnostic tests for it, and discusses the current evidence-based approach to managing this dangerous risk factor.
A CHRONICALLY ELEVATED CARDIAC WORKLOAD CAUSES LVH
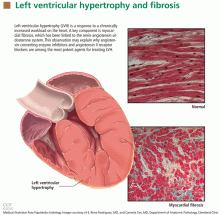
LVH is an abnormal increase in the mass of the left ventricular myocardium caused by a chronically increased workload on the heart.12 This most commonly results from the heart pumping against an elevated afterload, as in hypertension and aortic stenosis. Another notable cause is increased filling of the left ventricle (ie, diastolic overload), which is the underlying mechanism for LVH in patients with aortic or mitral regurgitation and dilated cardiomyopathy. Coronary artery disease can also play a role in the pathogenesis of LVH, as the normal myocardium attempts to compensate for the ischemic or infarcted tissue.13
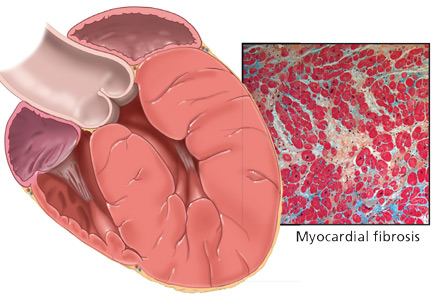
The development of myocardial fibrosis appears to be pathophysiologically linked to the renin-angiotensin-aldosterone system. Specifically, there is evidence that angiotensin II has a profibrotic effect on the myocardium of hypertensive patients.15 This may explain why angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are among the most potent agents for treating LVH, as we will discuss later in this review.
DIAGNOSIS BY ELECTROCARDIOGRAPHY, ECHOCARDIOGRAPHY, OR MRI
Many different criteria for electrocardiographic LVH have been proposed over the years. Most use the voltage in one or more leads, with or without additional factors such as QRS duration, secondary ST-T wave abnormalities, or left atrial abnormalities. The most well known electrocardiographic criteria are the Cornell voltage,21 the Cornell product,22 the Sokolow-Lyon index,23 and the Romhilt-Estes point score system (Table 1).24
- Cornell voltage—median sensitivity 15%, median specificity 96%
- Cornell product—median sensitivity 19.5%, median specificity 91%
- Sokolow-Lyon voltage—median sensitivity 21%, median specificity 89%
- Romhilt-Estes point score—median sensitivity 17%, median specificity 95%.
Of note, the ranges of the published values were extremely broad. For example, the ranges in sensitivity were:
- Cornell voltage—2% to 41%
- Cornell product—8% to 32%
- Sokolow-Lyon voltage—4% to 51%
- Romhilt-Estes point score—0% to 41%.
While the studies with the extreme values may have had issues of small sample size or poor study quality, the wide range in values may primarily be the result of diverse study populations as well as different validation methods and cutoff values to define LVH. Regardless, the overall message of high specificity and low sensitivity is indisputable.
Electrocardiography is insensitive for diagnosing LVH because it relies on measuring the electrical activity of the heart by electrodes on the surface of the skin to predict the left ventricular mass. The intracardiac electrical activity is problematic to measure externally because the measurements are affected by everything between the myocardium and the electrodes, most notably fat, fluid, and air. Because of this effect, electrocardiography underdiagnoses LVH in patients with obesity, pleural effusions, pericardial effusions, anasarca, or chronic obstructive pulmonary disease. In addition, the diagnosis of LVH by electrocardiography is strongly influenced by age and ethnicity.25–26
While electrocardiography is not sensitive and cannot be used to rule out LVH, it still has a role in its diagnosis and management. In the landmark Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study, regression of LVH (diagnosed electrocardiographically by the Sokolow-Lyon index or the Cornell product criteria) in response to losartan (Cozaar) improved cardiovascular outcomes independent of blood pressure.10 Based on this, it is reasonable that all hypertensive patients and other patients at risk of LVH who undergo electrocardiography be screened with these two criteria.
Echocardiography is the test of choice
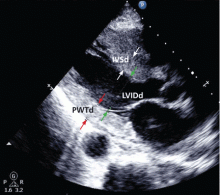
Echocardiography, if available, should be the test of choice to assess for LVH. It is much more sensitive than electrocardiography and can also detect other abnormalities such as left ventricular dysfunction and valvular disease.
This test uses transthoracic or transesophageal ultrasonography to measure the left ventricular end-diastolic diameter, posterior wall thickness, and interventricular septum thickness. From these measurements and the patient’s height and weight, the left ventricular mass index can be calculated.27
Several different cutoff values for the left ventricular mass index have been proposed; the LIFE study used values of > 104 g/m2 in women and > 116 g/m2 in men to define LVH.
When using echocardiography to assess for LVH, it is imperative that the left ventricular mass index be used and not just the left ventricular wall thickness, as often happens in clinical practice. This is necessary because diagnosis by wall thickness alone is not a good indicator of LVH, with a concordance between wall thickness and a left ventricular mass index of only 60%.28 In addition, wall thickness tends to underestimate LVH in women and overestimate it in men.
Is echocardiography cost-effective?
Despite its clear advantages, an important consideration about echocardiography as a screening test for all hypertensive patients is its cost.
A suggested way to reduce cost is to measure the left ventricular mass index only.29 A limited echocardiographic examination is much less expensive than a complete two-dimensional echocardiogram ($255 vs $431 per the 2009 Medicare Ambulatory Payment Classification30) and should be the examination performed if the patient has no other clinical indication for echocardiography.
Another way to control cost is to stratify patients by risk and to do echocardiography only in those who would benefit most from it. Based on the prevalence of LVH, one study concluded that echocardiography is most cost-effective in men 50 years or older.31
Further study is necessary to more precisely define the cost-effectiveness of echocardiographic screening for LVH in terms of potentially preventable cardiovascular morbidity and death.
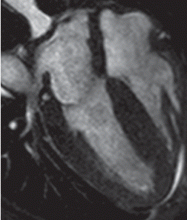
Cardiac MRI: The costly gold standard
Cardiac MRI is the gold standard test for LVH, as it is even more accurate and reproducible than echocardiography.32 It can precisely estimate a patient's left ventricular mass and assess for other structural cardiac abnormalities.
MRI’s use, however, is severely restricted in clinical practice due to its high cost and limited availability. While it may never be used for general screening for LVH, it certainly has a role in clinical research and for assessing cardiac anatomy in special clinical situations.
TREATMENT SHOULD INCLUDE AN ACE INHIBITOR OR ARB
Once LVH has been diagnosed, the next step is to decide on an appropriate treatment plan.
While the choice of therapy will always depend on other comorbidities, a 2003 metaanalysis of antihypertensive medications in the treatment of LVH (controlling for the degree of blood pressure lowering) showed that ARBs were the most efficacious class of agents for reducing the left ventricular mass.33 Specifically, ARBs decreased the mass by 13%, followed by calcium-channel blockers at 11%, ACE inhibitors at 10%, diuretics at 8%, and beta-blockers at 6%. In pairwise comparison, ARBs, calcium-channel blockers, and ACE inhibitors were all significantly more effective in reducing the left ventricular mass than beta-blockers.
As previously discussed, LVH appears to be pathophysiologically linked to myocardial fibrosis and the renin-angiotensin-aldosterone system. For this reason and based on the data presented above regarding the degree of LVH regression, ACE inhibitors or ARBs should be used as the first-line agents for LVH unless they are contraindicated in the individual patient.
The LIFE study
The LIFE study offers the strongest evidence that treating LVH is beneficial. It showed that in hypertensive patients with electrocardiographic LVH by the Cornell product or Sokolow-Lyon criteria, treatment with antihypertensive drugs that resulted in less-severe LVH on electrocardiography was associated with lower rates of cardiovascular morbidity and death, independent of the blood pressure achieved or the drug used.10
The end point in this study was a composite of stroke, myocardial infarction, and cardiovascular death. Regression of electrocardiographic LVH in hypertensive patients has also been shown to decrease the incidence of diabetes mellitus,34 atrial fibrillation,35 and hospitalizations for heart failure.36
The LIFE study also examined the prognostic implications of treating LVH detected by echocardiography. In this prospective cohort substudy, patients who had a lower left ventricular mass index during treatment with antihypertensive drugs had lower rates of cardiovascular morbidity and all-cause mortality, independent of the effects of blood pressure and treatment used.11
These results suggest that there may be a role not only for treating LVH, but also for monitoring for a reduction in the left ventricular mass index as a goal of therapy (similar to the way hemoglobin A1c is used in diabetic patients). If the index is used in this way, one could potentially adjust the dose of current drugs, switch classes, or add an additional drug based on a persistently elevated left ventricular mass index in order to optimize the patient's overall cardiovascular risk. A randomized controlled trial of therapy directed by the mass index vs conventional therapy of LVH would be necessary to assess the clinical utility of this approach.
RECOMMENDATIONS
LVH is a common and potentially modifiable cardiovascular risk factor often overlooked in clinical practice. Ideally, all hypertensive patients should be screened with echocardiography to look for LVH, using the calculated left ventricular mass index rather than wall thickness alone to make the diagnosis. While electrocardiography is specific and also has prognostic implications, it is not sensitive enough to be used alone to screen for LVH.
Once the diagnosis of LVH is made, the initial therapy should be an ARB or an ACE inhibitor. Response to therapy can be assessed by monitoring for a reduction in left ventricular mass index or regression of electrocardiographic LVH.
Treatment-induced regression of LVH decreases adverse cardiovascular events and improves overall survival. When modifying medications in hypertensive patients, it is important to remember that the treatment of LVH is not synonymous with blood pressure control.
- Casale PN, Devereux RB, Milner M, et al. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med 1986; 105:173–178.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322:1561–1566.
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991; 114:345–352.
- Verdecchia P, Carini G, Circo A, et al; MAVI (MAssa Ventricolare sinistra nell’Ipertensione) Study Group. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol 2001; 38:1829–1835.
- Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998; 32:1454–1459.
- Verdecchia P, Porcellati C, Reboldi G, et al. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 2001; 104:2039–2044.
- Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000; 35:580–586.
- Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation 1998; 97:48–54.
- Mathew J, Sleight P, Lonn E, et al; Heart Outcomes Prevention Evaluation (HOPE) Investigators. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001; 104:1615–1621.
- Okin PM, Devereux RB, Jern S, et al; LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004; 292:2343–2349.
- Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004; 292:2350–2356.
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 2000; 102:470–479.
- Zabalgoitia M, Berning J, Koren MJ, et al; LIFE Study Investigators. Impact of coronary artery disease on left ventricular systolic function and geometry in hypertensive patients with left ventricular hypertrophy (the LIFE study). Am J Cardiol 2001; 88:646–650.
- Weber KT, Janicki JS, Pick R, Capasso J, Anversa P. Myocardial fibrosis and pathologic hypertrophy in the rat with renovascular hypertension. Am J Cardiol 1990; 65:1G–7G.
- González A, López B, Querejeta R, Díez J. Regulation of myocardial fibrillar collagen by angiotensin II. A role in hypertensive heart disease? J Mol Cell Cardiol 2002; 34:1585–1593.
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002; 287:1308–1320.
- Liebson PR, Grandits G, Prineas R, et al. Echocardiographic correlates of left ventricular structure among 844 mildly hypertensive men and women in the Treatment of Mild Hypertension Study (TOMHS). Circulation 1993; 87:476–486.
- Martinez MA, Sancho T, Armada E, et al; Vascular Risk Working Group Grupo Monitorizacíon Ambulatoria de la Presión Arterial (MAPA)-Madrid. Prevalence of left ventricular hypertrophy in patients with mild hypertension in primary care: impact of echocardiography on cardiovascular risk stratification. Am J Hypertens 2003; 16:556–563.
- Pewsner D, Jüni P, Egger M, Battaglia M, Sundström J, Bachmann LM. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systematic review. BMJ 2007; 335:711.
- Devereux RB. Is the electrocardiogram still useful for detection of left ventricular hypertrophy? Circulation 1990; 81:1144–1146.
- Casale PN, Devereux RB, Kligfield P, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol 1985; 6:572–580.
- Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol 1992; 20:1180–1186.
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949; 37:161–186.
- Romhilt DW, Estes EH. A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J 1968; 75:752–758.
- Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation 1990; 81:815–820.
- Okin PM, Wright JT, Nieminen MS, et al. Ethnic differences in electrocardiographic criteria for left ventricular hypertrophy: the LIFE study. Losartan Intervention For Endpoint. Am J Hypertens 2002; 15:663–671.
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57:450–458.
- Leibowitz D, Planer D, Ben-Ibgi F, Rott D, Weiss AT, Bursztyn M. Measurement of wall thickness alone does not accurately assess the presence of left ventricular hypertrophy. Clin Exp Hypertens 2007; 29:119–125.
- Black HR, Weltin G, Jaffe CC. The limited echocardiogram: a modification of standard echocardiography for use in the routine evaluation of patients with systemic hypertension. Am J Cardiol 1991; 67:1027–1030.
- American Society of Echocardiography Coding and Reimbursement Newsletter, January 2009. http://www.asecho.org/files/public/CodingnewsJan09.pdf. Accessed May 13, 2010.
- Cuspidi C, Meani S, Valerio C, Fusi V, Sala C, Zanchetti A. Left ventricular hypertrophy and cardiovascular risk stratification: impact and cost-effectiveness of echocardiography in recently diagnosed essential hypertensives. J Hypertens 2006; 24:1671–1677.
- Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens 1995; 8:221–228.
- Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 2003; 115:41–46.
- Okin PM, Devereux RB, Harris KE, et al; LIFE Study Investigators. In-treatment resolution or absence of electrocardiographic left ventricular hypertrophy is associated with decreased incidence of new-onset diabetes mellitus in hypertensive patients: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) Study. Hypertension 2007; 50:984–990.
- Okin PM, Wachtell K, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA 2006; 296:1242–1248.
- Okin PM, Devereux RB, Harris KE, et al; LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med 2007; 147:311–319.
Left ventricular hypertrophy (LVH) strongly predicts cardiovascular morbidity and overall mortality in hypertensive patients. 1–7 Antihypertensive treatment that causes LVH to regress decreases the rates of adverse cardiovascular events and improves survival, independent of how much the blood pressure is lowered.8–11 It is clinically important to recognize that LVH is a modifiable risk factor and that management is more complex than just blood pressure control.
This paper reviews the definition of LVH, compares the diagnostic tests for it, and discusses the current evidence-based approach to managing this dangerous risk factor.
A CHRONICALLY ELEVATED CARDIAC WORKLOAD CAUSES LVH
LVH is an abnormal increase in the mass of the left ventricular myocardium caused by a chronically increased workload on the heart.12 This most commonly results from the heart pumping against an elevated afterload, as in hypertension and aortic stenosis. Another notable cause is increased filling of the left ventricle (ie, diastolic overload), which is the underlying mechanism for LVH in patients with aortic or mitral regurgitation and dilated cardiomyopathy. Coronary artery disease can also play a role in the pathogenesis of LVH, as the normal myocardium attempts to compensate for the ischemic or infarcted tissue.13
The development of myocardial fibrosis appears to be pathophysiologically linked to the renin-angiotensin-aldosterone system. Specifically, there is evidence that angiotensin II has a profibrotic effect on the myocardium of hypertensive patients.15 This may explain why angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are among the most potent agents for treating LVH, as we will discuss later in this review.
DIAGNOSIS BY ELECTROCARDIOGRAPHY, ECHOCARDIOGRAPHY, OR MRI
Many different criteria for electrocardiographic LVH have been proposed over the years. Most use the voltage in one or more leads, with or without additional factors such as QRS duration, secondary ST-T wave abnormalities, or left atrial abnormalities. The most well known electrocardiographic criteria are the Cornell voltage,21 the Cornell product,22 the Sokolow-Lyon index,23 and the Romhilt-Estes point score system (Table 1).24
- Cornell voltage—median sensitivity 15%, median specificity 96%
- Cornell product—median sensitivity 19.5%, median specificity 91%
- Sokolow-Lyon voltage—median sensitivity 21%, median specificity 89%
- Romhilt-Estes point score—median sensitivity 17%, median specificity 95%.
Of note, the ranges of the published values were extremely broad. For example, the ranges in sensitivity were:
- Cornell voltage—2% to 41%
- Cornell product—8% to 32%
- Sokolow-Lyon voltage—4% to 51%
- Romhilt-Estes point score—0% to 41%.
While the studies with the extreme values may have had issues of small sample size or poor study quality, the wide range in values may primarily be the result of diverse study populations as well as different validation methods and cutoff values to define LVH. Regardless, the overall message of high specificity and low sensitivity is indisputable.
Electrocardiography is insensitive for diagnosing LVH because it relies on measuring the electrical activity of the heart by electrodes on the surface of the skin to predict the left ventricular mass. The intracardiac electrical activity is problematic to measure externally because the measurements are affected by everything between the myocardium and the electrodes, most notably fat, fluid, and air. Because of this effect, electrocardiography underdiagnoses LVH in patients with obesity, pleural effusions, pericardial effusions, anasarca, or chronic obstructive pulmonary disease. In addition, the diagnosis of LVH by electrocardiography is strongly influenced by age and ethnicity.25–26
While electrocardiography is not sensitive and cannot be used to rule out LVH, it still has a role in its diagnosis and management. In the landmark Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study, regression of LVH (diagnosed electrocardiographically by the Sokolow-Lyon index or the Cornell product criteria) in response to losartan (Cozaar) improved cardiovascular outcomes independent of blood pressure.10 Based on this, it is reasonable that all hypertensive patients and other patients at risk of LVH who undergo electrocardiography be screened with these two criteria.
Echocardiography is the test of choice
Echocardiography, if available, should be the test of choice to assess for LVH. It is much more sensitive than electrocardiography and can also detect other abnormalities such as left ventricular dysfunction and valvular disease.
This test uses transthoracic or transesophageal ultrasonography to measure the left ventricular end-diastolic diameter, posterior wall thickness, and interventricular septum thickness. From these measurements and the patient’s height and weight, the left ventricular mass index can be calculated.27
Several different cutoff values for the left ventricular mass index have been proposed; the LIFE study used values of > 104 g/m2 in women and > 116 g/m2 in men to define LVH.
When using echocardiography to assess for LVH, it is imperative that the left ventricular mass index be used and not just the left ventricular wall thickness, as often happens in clinical practice. This is necessary because diagnosis by wall thickness alone is not a good indicator of LVH, with a concordance between wall thickness and a left ventricular mass index of only 60%.28 In addition, wall thickness tends to underestimate LVH in women and overestimate it in men.
Is echocardiography cost-effective?
Despite its clear advantages, an important consideration about echocardiography as a screening test for all hypertensive patients is its cost.
A suggested way to reduce cost is to measure the left ventricular mass index only.29 A limited echocardiographic examination is much less expensive than a complete two-dimensional echocardiogram ($255 vs $431 per the 2009 Medicare Ambulatory Payment Classification30) and should be the examination performed if the patient has no other clinical indication for echocardiography.
Another way to control cost is to stratify patients by risk and to do echocardiography only in those who would benefit most from it. Based on the prevalence of LVH, one study concluded that echocardiography is most cost-effective in men 50 years or older.31
Further study is necessary to more precisely define the cost-effectiveness of echocardiographic screening for LVH in terms of potentially preventable cardiovascular morbidity and death.
Cardiac MRI: The costly gold standard
Cardiac MRI is the gold standard test for LVH, as it is even more accurate and reproducible than echocardiography.32 It can precisely estimate a patient's left ventricular mass and assess for other structural cardiac abnormalities.
MRI’s use, however, is severely restricted in clinical practice due to its high cost and limited availability. While it may never be used for general screening for LVH, it certainly has a role in clinical research and for assessing cardiac anatomy in special clinical situations.
TREATMENT SHOULD INCLUDE AN ACE INHIBITOR OR ARB
Once LVH has been diagnosed, the next step is to decide on an appropriate treatment plan.
While the choice of therapy will always depend on other comorbidities, a 2003 metaanalysis of antihypertensive medications in the treatment of LVH (controlling for the degree of blood pressure lowering) showed that ARBs were the most efficacious class of agents for reducing the left ventricular mass.33 Specifically, ARBs decreased the mass by 13%, followed by calcium-channel blockers at 11%, ACE inhibitors at 10%, diuretics at 8%, and beta-blockers at 6%. In pairwise comparison, ARBs, calcium-channel blockers, and ACE inhibitors were all significantly more effective in reducing the left ventricular mass than beta-blockers.
As previously discussed, LVH appears to be pathophysiologically linked to myocardial fibrosis and the renin-angiotensin-aldosterone system. For this reason and based on the data presented above regarding the degree of LVH regression, ACE inhibitors or ARBs should be used as the first-line agents for LVH unless they are contraindicated in the individual patient.
The LIFE study
The LIFE study offers the strongest evidence that treating LVH is beneficial. It showed that in hypertensive patients with electrocardiographic LVH by the Cornell product or Sokolow-Lyon criteria, treatment with antihypertensive drugs that resulted in less-severe LVH on electrocardiography was associated with lower rates of cardiovascular morbidity and death, independent of the blood pressure achieved or the drug used.10
The end point in this study was a composite of stroke, myocardial infarction, and cardiovascular death. Regression of electrocardiographic LVH in hypertensive patients has also been shown to decrease the incidence of diabetes mellitus,34 atrial fibrillation,35 and hospitalizations for heart failure.36
The LIFE study also examined the prognostic implications of treating LVH detected by echocardiography. In this prospective cohort substudy, patients who had a lower left ventricular mass index during treatment with antihypertensive drugs had lower rates of cardiovascular morbidity and all-cause mortality, independent of the effects of blood pressure and treatment used.11
These results suggest that there may be a role not only for treating LVH, but also for monitoring for a reduction in the left ventricular mass index as a goal of therapy (similar to the way hemoglobin A1c is used in diabetic patients). If the index is used in this way, one could potentially adjust the dose of current drugs, switch classes, or add an additional drug based on a persistently elevated left ventricular mass index in order to optimize the patient's overall cardiovascular risk. A randomized controlled trial of therapy directed by the mass index vs conventional therapy of LVH would be necessary to assess the clinical utility of this approach.
RECOMMENDATIONS
LVH is a common and potentially modifiable cardiovascular risk factor often overlooked in clinical practice. Ideally, all hypertensive patients should be screened with echocardiography to look for LVH, using the calculated left ventricular mass index rather than wall thickness alone to make the diagnosis. While electrocardiography is specific and also has prognostic implications, it is not sensitive enough to be used alone to screen for LVH.
Once the diagnosis of LVH is made, the initial therapy should be an ARB or an ACE inhibitor. Response to therapy can be assessed by monitoring for a reduction in left ventricular mass index or regression of electrocardiographic LVH.
Treatment-induced regression of LVH decreases adverse cardiovascular events and improves overall survival. When modifying medications in hypertensive patients, it is important to remember that the treatment of LVH is not synonymous with blood pressure control.
Left ventricular hypertrophy (LVH) strongly predicts cardiovascular morbidity and overall mortality in hypertensive patients. 1–7 Antihypertensive treatment that causes LVH to regress decreases the rates of adverse cardiovascular events and improves survival, independent of how much the blood pressure is lowered.8–11 It is clinically important to recognize that LVH is a modifiable risk factor and that management is more complex than just blood pressure control.
This paper reviews the definition of LVH, compares the diagnostic tests for it, and discusses the current evidence-based approach to managing this dangerous risk factor.
A CHRONICALLY ELEVATED CARDIAC WORKLOAD CAUSES LVH
LVH is an abnormal increase in the mass of the left ventricular myocardium caused by a chronically increased workload on the heart.12 This most commonly results from the heart pumping against an elevated afterload, as in hypertension and aortic stenosis. Another notable cause is increased filling of the left ventricle (ie, diastolic overload), which is the underlying mechanism for LVH in patients with aortic or mitral regurgitation and dilated cardiomyopathy. Coronary artery disease can also play a role in the pathogenesis of LVH, as the normal myocardium attempts to compensate for the ischemic or infarcted tissue.13
The development of myocardial fibrosis appears to be pathophysiologically linked to the renin-angiotensin-aldosterone system. Specifically, there is evidence that angiotensin II has a profibrotic effect on the myocardium of hypertensive patients.15 This may explain why angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are among the most potent agents for treating LVH, as we will discuss later in this review.
DIAGNOSIS BY ELECTROCARDIOGRAPHY, ECHOCARDIOGRAPHY, OR MRI
Many different criteria for electrocardiographic LVH have been proposed over the years. Most use the voltage in one or more leads, with or without additional factors such as QRS duration, secondary ST-T wave abnormalities, or left atrial abnormalities. The most well known electrocardiographic criteria are the Cornell voltage,21 the Cornell product,22 the Sokolow-Lyon index,23 and the Romhilt-Estes point score system (Table 1).24
- Cornell voltage—median sensitivity 15%, median specificity 96%
- Cornell product—median sensitivity 19.5%, median specificity 91%
- Sokolow-Lyon voltage—median sensitivity 21%, median specificity 89%
- Romhilt-Estes point score—median sensitivity 17%, median specificity 95%.
Of note, the ranges of the published values were extremely broad. For example, the ranges in sensitivity were:
- Cornell voltage—2% to 41%
- Cornell product—8% to 32%
- Sokolow-Lyon voltage—4% to 51%
- Romhilt-Estes point score—0% to 41%.
While the studies with the extreme values may have had issues of small sample size or poor study quality, the wide range in values may primarily be the result of diverse study populations as well as different validation methods and cutoff values to define LVH. Regardless, the overall message of high specificity and low sensitivity is indisputable.
Electrocardiography is insensitive for diagnosing LVH because it relies on measuring the electrical activity of the heart by electrodes on the surface of the skin to predict the left ventricular mass. The intracardiac electrical activity is problematic to measure externally because the measurements are affected by everything between the myocardium and the electrodes, most notably fat, fluid, and air. Because of this effect, electrocardiography underdiagnoses LVH in patients with obesity, pleural effusions, pericardial effusions, anasarca, or chronic obstructive pulmonary disease. In addition, the diagnosis of LVH by electrocardiography is strongly influenced by age and ethnicity.25–26
While electrocardiography is not sensitive and cannot be used to rule out LVH, it still has a role in its diagnosis and management. In the landmark Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study, regression of LVH (diagnosed electrocardiographically by the Sokolow-Lyon index or the Cornell product criteria) in response to losartan (Cozaar) improved cardiovascular outcomes independent of blood pressure.10 Based on this, it is reasonable that all hypertensive patients and other patients at risk of LVH who undergo electrocardiography be screened with these two criteria.
Echocardiography is the test of choice
Echocardiography, if available, should be the test of choice to assess for LVH. It is much more sensitive than electrocardiography and can also detect other abnormalities such as left ventricular dysfunction and valvular disease.
This test uses transthoracic or transesophageal ultrasonography to measure the left ventricular end-diastolic diameter, posterior wall thickness, and interventricular septum thickness. From these measurements and the patient’s height and weight, the left ventricular mass index can be calculated.27
Several different cutoff values for the left ventricular mass index have been proposed; the LIFE study used values of > 104 g/m2 in women and > 116 g/m2 in men to define LVH.
When using echocardiography to assess for LVH, it is imperative that the left ventricular mass index be used and not just the left ventricular wall thickness, as often happens in clinical practice. This is necessary because diagnosis by wall thickness alone is not a good indicator of LVH, with a concordance between wall thickness and a left ventricular mass index of only 60%.28 In addition, wall thickness tends to underestimate LVH in women and overestimate it in men.
Is echocardiography cost-effective?
Despite its clear advantages, an important consideration about echocardiography as a screening test for all hypertensive patients is its cost.
A suggested way to reduce cost is to measure the left ventricular mass index only.29 A limited echocardiographic examination is much less expensive than a complete two-dimensional echocardiogram ($255 vs $431 per the 2009 Medicare Ambulatory Payment Classification30) and should be the examination performed if the patient has no other clinical indication for echocardiography.
Another way to control cost is to stratify patients by risk and to do echocardiography only in those who would benefit most from it. Based on the prevalence of LVH, one study concluded that echocardiography is most cost-effective in men 50 years or older.31
Further study is necessary to more precisely define the cost-effectiveness of echocardiographic screening for LVH in terms of potentially preventable cardiovascular morbidity and death.
Cardiac MRI: The costly gold standard
Cardiac MRI is the gold standard test for LVH, as it is even more accurate and reproducible than echocardiography.32 It can precisely estimate a patient's left ventricular mass and assess for other structural cardiac abnormalities.
MRI’s use, however, is severely restricted in clinical practice due to its high cost and limited availability. While it may never be used for general screening for LVH, it certainly has a role in clinical research and for assessing cardiac anatomy in special clinical situations.
TREATMENT SHOULD INCLUDE AN ACE INHIBITOR OR ARB
Once LVH has been diagnosed, the next step is to decide on an appropriate treatment plan.
While the choice of therapy will always depend on other comorbidities, a 2003 metaanalysis of antihypertensive medications in the treatment of LVH (controlling for the degree of blood pressure lowering) showed that ARBs were the most efficacious class of agents for reducing the left ventricular mass.33 Specifically, ARBs decreased the mass by 13%, followed by calcium-channel blockers at 11%, ACE inhibitors at 10%, diuretics at 8%, and beta-blockers at 6%. In pairwise comparison, ARBs, calcium-channel blockers, and ACE inhibitors were all significantly more effective in reducing the left ventricular mass than beta-blockers.
As previously discussed, LVH appears to be pathophysiologically linked to myocardial fibrosis and the renin-angiotensin-aldosterone system. For this reason and based on the data presented above regarding the degree of LVH regression, ACE inhibitors or ARBs should be used as the first-line agents for LVH unless they are contraindicated in the individual patient.
The LIFE study
The LIFE study offers the strongest evidence that treating LVH is beneficial. It showed that in hypertensive patients with electrocardiographic LVH by the Cornell product or Sokolow-Lyon criteria, treatment with antihypertensive drugs that resulted in less-severe LVH on electrocardiography was associated with lower rates of cardiovascular morbidity and death, independent of the blood pressure achieved or the drug used.10
The end point in this study was a composite of stroke, myocardial infarction, and cardiovascular death. Regression of electrocardiographic LVH in hypertensive patients has also been shown to decrease the incidence of diabetes mellitus,34 atrial fibrillation,35 and hospitalizations for heart failure.36
The LIFE study also examined the prognostic implications of treating LVH detected by echocardiography. In this prospective cohort substudy, patients who had a lower left ventricular mass index during treatment with antihypertensive drugs had lower rates of cardiovascular morbidity and all-cause mortality, independent of the effects of blood pressure and treatment used.11
These results suggest that there may be a role not only for treating LVH, but also for monitoring for a reduction in the left ventricular mass index as a goal of therapy (similar to the way hemoglobin A1c is used in diabetic patients). If the index is used in this way, one could potentially adjust the dose of current drugs, switch classes, or add an additional drug based on a persistently elevated left ventricular mass index in order to optimize the patient's overall cardiovascular risk. A randomized controlled trial of therapy directed by the mass index vs conventional therapy of LVH would be necessary to assess the clinical utility of this approach.
RECOMMENDATIONS
LVH is a common and potentially modifiable cardiovascular risk factor often overlooked in clinical practice. Ideally, all hypertensive patients should be screened with echocardiography to look for LVH, using the calculated left ventricular mass index rather than wall thickness alone to make the diagnosis. While electrocardiography is specific and also has prognostic implications, it is not sensitive enough to be used alone to screen for LVH.
Once the diagnosis of LVH is made, the initial therapy should be an ARB or an ACE inhibitor. Response to therapy can be assessed by monitoring for a reduction in left ventricular mass index or regression of electrocardiographic LVH.
Treatment-induced regression of LVH decreases adverse cardiovascular events and improves overall survival. When modifying medications in hypertensive patients, it is important to remember that the treatment of LVH is not synonymous with blood pressure control.
- Casale PN, Devereux RB, Milner M, et al. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med 1986; 105:173–178.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322:1561–1566.
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991; 114:345–352.
- Verdecchia P, Carini G, Circo A, et al; MAVI (MAssa Ventricolare sinistra nell’Ipertensione) Study Group. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol 2001; 38:1829–1835.
- Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998; 32:1454–1459.
- Verdecchia P, Porcellati C, Reboldi G, et al. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 2001; 104:2039–2044.
- Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000; 35:580–586.
- Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation 1998; 97:48–54.
- Mathew J, Sleight P, Lonn E, et al; Heart Outcomes Prevention Evaluation (HOPE) Investigators. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001; 104:1615–1621.
- Okin PM, Devereux RB, Jern S, et al; LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004; 292:2343–2349.
- Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004; 292:2350–2356.
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 2000; 102:470–479.
- Zabalgoitia M, Berning J, Koren MJ, et al; LIFE Study Investigators. Impact of coronary artery disease on left ventricular systolic function and geometry in hypertensive patients with left ventricular hypertrophy (the LIFE study). Am J Cardiol 2001; 88:646–650.
- Weber KT, Janicki JS, Pick R, Capasso J, Anversa P. Myocardial fibrosis and pathologic hypertrophy in the rat with renovascular hypertension. Am J Cardiol 1990; 65:1G–7G.
- González A, López B, Querejeta R, Díez J. Regulation of myocardial fibrillar collagen by angiotensin II. A role in hypertensive heart disease? J Mol Cell Cardiol 2002; 34:1585–1593.
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002; 287:1308–1320.
- Liebson PR, Grandits G, Prineas R, et al. Echocardiographic correlates of left ventricular structure among 844 mildly hypertensive men and women in the Treatment of Mild Hypertension Study (TOMHS). Circulation 1993; 87:476–486.
- Martinez MA, Sancho T, Armada E, et al; Vascular Risk Working Group Grupo Monitorizacíon Ambulatoria de la Presión Arterial (MAPA)-Madrid. Prevalence of left ventricular hypertrophy in patients with mild hypertension in primary care: impact of echocardiography on cardiovascular risk stratification. Am J Hypertens 2003; 16:556–563.
- Pewsner D, Jüni P, Egger M, Battaglia M, Sundström J, Bachmann LM. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systematic review. BMJ 2007; 335:711.
- Devereux RB. Is the electrocardiogram still useful for detection of left ventricular hypertrophy? Circulation 1990; 81:1144–1146.
- Casale PN, Devereux RB, Kligfield P, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol 1985; 6:572–580.
- Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol 1992; 20:1180–1186.
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949; 37:161–186.
- Romhilt DW, Estes EH. A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J 1968; 75:752–758.
- Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation 1990; 81:815–820.
- Okin PM, Wright JT, Nieminen MS, et al. Ethnic differences in electrocardiographic criteria for left ventricular hypertrophy: the LIFE study. Losartan Intervention For Endpoint. Am J Hypertens 2002; 15:663–671.
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57:450–458.
- Leibowitz D, Planer D, Ben-Ibgi F, Rott D, Weiss AT, Bursztyn M. Measurement of wall thickness alone does not accurately assess the presence of left ventricular hypertrophy. Clin Exp Hypertens 2007; 29:119–125.
- Black HR, Weltin G, Jaffe CC. The limited echocardiogram: a modification of standard echocardiography for use in the routine evaluation of patients with systemic hypertension. Am J Cardiol 1991; 67:1027–1030.
- American Society of Echocardiography Coding and Reimbursement Newsletter, January 2009. http://www.asecho.org/files/public/CodingnewsJan09.pdf. Accessed May 13, 2010.
- Cuspidi C, Meani S, Valerio C, Fusi V, Sala C, Zanchetti A. Left ventricular hypertrophy and cardiovascular risk stratification: impact and cost-effectiveness of echocardiography in recently diagnosed essential hypertensives. J Hypertens 2006; 24:1671–1677.
- Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens 1995; 8:221–228.
- Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 2003; 115:41–46.
- Okin PM, Devereux RB, Harris KE, et al; LIFE Study Investigators. In-treatment resolution or absence of electrocardiographic left ventricular hypertrophy is associated with decreased incidence of new-onset diabetes mellitus in hypertensive patients: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) Study. Hypertension 2007; 50:984–990.
- Okin PM, Wachtell K, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA 2006; 296:1242–1248.
- Okin PM, Devereux RB, Harris KE, et al; LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med 2007; 147:311–319.
- Casale PN, Devereux RB, Milner M, et al. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med 1986; 105:173–178.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322:1561–1566.
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991; 114:345–352.
- Verdecchia P, Carini G, Circo A, et al; MAVI (MAssa Ventricolare sinistra nell’Ipertensione) Study Group. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol 2001; 38:1829–1835.
- Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998; 32:1454–1459.
- Verdecchia P, Porcellati C, Reboldi G, et al. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 2001; 104:2039–2044.
- Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000; 35:580–586.
- Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation 1998; 97:48–54.
- Mathew J, Sleight P, Lonn E, et al; Heart Outcomes Prevention Evaluation (HOPE) Investigators. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001; 104:1615–1621.
- Okin PM, Devereux RB, Jern S, et al; LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004; 292:2343–2349.
- Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004; 292:2350–2356.
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 2000; 102:470–479.
- Zabalgoitia M, Berning J, Koren MJ, et al; LIFE Study Investigators. Impact of coronary artery disease on left ventricular systolic function and geometry in hypertensive patients with left ventricular hypertrophy (the LIFE study). Am J Cardiol 2001; 88:646–650.
- Weber KT, Janicki JS, Pick R, Capasso J, Anversa P. Myocardial fibrosis and pathologic hypertrophy in the rat with renovascular hypertension. Am J Cardiol 1990; 65:1G–7G.
- González A, López B, Querejeta R, Díez J. Regulation of myocardial fibrillar collagen by angiotensin II. A role in hypertensive heart disease? J Mol Cell Cardiol 2002; 34:1585–1593.
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002; 287:1308–1320.
- Liebson PR, Grandits G, Prineas R, et al. Echocardiographic correlates of left ventricular structure among 844 mildly hypertensive men and women in the Treatment of Mild Hypertension Study (TOMHS). Circulation 1993; 87:476–486.
- Martinez MA, Sancho T, Armada E, et al; Vascular Risk Working Group Grupo Monitorizacíon Ambulatoria de la Presión Arterial (MAPA)-Madrid. Prevalence of left ventricular hypertrophy in patients with mild hypertension in primary care: impact of echocardiography on cardiovascular risk stratification. Am J Hypertens 2003; 16:556–563.
- Pewsner D, Jüni P, Egger M, Battaglia M, Sundström J, Bachmann LM. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systematic review. BMJ 2007; 335:711.
- Devereux RB. Is the electrocardiogram still useful for detection of left ventricular hypertrophy? Circulation 1990; 81:1144–1146.
- Casale PN, Devereux RB, Kligfield P, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol 1985; 6:572–580.
- Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol 1992; 20:1180–1186.
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949; 37:161–186.
- Romhilt DW, Estes EH. A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J 1968; 75:752–758.
- Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation 1990; 81:815–820.
- Okin PM, Wright JT, Nieminen MS, et al. Ethnic differences in electrocardiographic criteria for left ventricular hypertrophy: the LIFE study. Losartan Intervention For Endpoint. Am J Hypertens 2002; 15:663–671.
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57:450–458.
- Leibowitz D, Planer D, Ben-Ibgi F, Rott D, Weiss AT, Bursztyn M. Measurement of wall thickness alone does not accurately assess the presence of left ventricular hypertrophy. Clin Exp Hypertens 2007; 29:119–125.
- Black HR, Weltin G, Jaffe CC. The limited echocardiogram: a modification of standard echocardiography for use in the routine evaluation of patients with systemic hypertension. Am J Cardiol 1991; 67:1027–1030.
- American Society of Echocardiography Coding and Reimbursement Newsletter, January 2009. http://www.asecho.org/files/public/CodingnewsJan09.pdf. Accessed May 13, 2010.
- Cuspidi C, Meani S, Valerio C, Fusi V, Sala C, Zanchetti A. Left ventricular hypertrophy and cardiovascular risk stratification: impact and cost-effectiveness of echocardiography in recently diagnosed essential hypertensives. J Hypertens 2006; 24:1671–1677.
- Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens 1995; 8:221–228.
- Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 2003; 115:41–46.
- Okin PM, Devereux RB, Harris KE, et al; LIFE Study Investigators. In-treatment resolution or absence of electrocardiographic left ventricular hypertrophy is associated with decreased incidence of new-onset diabetes mellitus in hypertensive patients: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) Study. Hypertension 2007; 50:984–990.
- Okin PM, Wachtell K, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA 2006; 296:1242–1248.
- Okin PM, Devereux RB, Harris KE, et al; LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med 2007; 147:311–319.
KEY POINTS
- LVH is caused by a chronically increased cardiac workload, most commonly from hypertension.
- Ideally, all hypertensive patients should undergo echocardiography to screen for LVH, using the calculated left ventricular mass index.
- Electrocardiography is too insensitive to be used alone to screen for LVH.
- In hypertensive patients, initial therapy of LVH should consist of an angiotensin II receptor blocker or an angiotensin-converting enzyme inhibitor.
- Treatment-induced regression of LVH improves cardiovascular outcomes independent of blood pressure.
- Further study is necessary to examine the utility of following the left ventricular mass index as a treatment goal.
Radiotherapy After Breast Cancer Surgery: Don't Wait
Grand Rounds: Woman, 80, With Hallucinations and Tremors
An 80-year-old Mandarin-speaking Chinese woman was referred to a mental health outpatient clinic for evaluation and treatment. The patient had a history of mild depression, for which she had been treated for many years with sertraline.
Five years earlier at age 75, the patient had been evaluated by a psychiatrist after she began to experience psychotic symptoms, including frequent repetitive auditory hallucinations of people counting, alternating with music from her childhood. At that time, she also had persecutory paranoid thoughts and delusional thinking that she was receiving messages in Mandarin while watching American TV programs. Initially, her only cognitive disturbance was an inability to differentiate among numbers on a calendar or a telephone keypad. No reports of memory problems were noted. Although the patient acknowledged auditory hallucinations, she denied experiencing command auditory hallucinations or hallucinations of other forms. The patient had no history of suicide attempts and denied suicidal or homicidal ideation. She had no history of psychiatric hospitalization.
The psychiatrist made a diagnosis of major depressive disorder with psychotic features, not otherwise specified1 and prescribed sertraline 50 mg/d. The patient was also started on risperidone 0.25 mg/d for management of her psychotic symptoms, with the dosage gradually increased to 2.0 mg/d over five years. While taking this combination, the patient experienced stable mood and fewer paranoid thoughts, although her auditory hallucinations continued.
Two months before the current visit, the patient moved into a retirement living facility, and she reported having adapted well to the new setting. She was sleeping well and had a good appetite. Her BMI was within normal range.
The patient described herself as a single parent for nearly 40 years, raising one daughter. Formerly high functioning, she had held a full-time clerical job until age 70. She appeared well-groomed, polite but anxious, and oriented to time, person, and place. Her speech was normal, her thought processes were coherent, and her mood was stable. However, her affect was constricted; she acknowledged auditory hallucinations, which impaired her thought content. The patient reported feeling increased anxiety prior to any nonroutine activity, such as a doctor’s appointment; this, she said, would cause insomnia, leaving her to pace in her room.
During the examination, fine tremors on upper and lower extremities were noted. The patient’s Abnormal Involuntary Movement Scale (AIMS) score2 was 13, which placed her in the highest risk category for antipsychotic-induced dopamine-blockade extrapyramidal symptoms (EPS). The patient was found to be negative for tardive dyskinesia, with no abnormal facial movements. She was aware of the tremors in her limbs and said she felt bothered by them.
The patient had an unsteady gait and used a four-point walker. Her Mini-Mental State Exam (MMSE) score3 was 28/30, which was normal for her age and education level (high school completed).
Apart from the described symptoms, the patient was healthy for her age and had no other medical diagnosis. Her vital signs were within normal range. The medical work-up to rule out other causes of dementia yielded negative results. Lab values were normal, including electrolyte levels and thyroid tests. The patient’s hearing test showed age-related hearing loss of full range, not limited to high pitch. She was able to engage in a meaningful conversation at a normal volume. Clinically, however, it was concerning to observe the possible signs of EPS and the relatively high risperidone dosage, considering the patient’s advanced age.
After the meeting with the patient, a treatment plan was created to 1) gradually reduce the dosage of antipsychotic medication, and 2) refer her to a neurologist for a complete work-up to rule out underlying neurologic disorders, such as dementia. Risperidone was tapered by increments of 0.25 mg/d every three to four weeks; throughout this process, the patient was closely monitored by the nursing staff at the retirement living facility. Monthly appointments were scheduled at the outpatient mental health clinic for evaluation and medication management.
Two months after the initial mental health clinic visit, the patient’s condition was pronounced stable on the current regimen of sertraline 50 mg/d and risperidone 1.0 mg/d. She was later seen by a neurologist, who made a diagnosis of Parkinson’s disease and placed her on carbidopa-levodopa (1 1/2 tablets, 25/100 mg, tid). The patient’s auditory hallucinations continued with the same intensity as at baseline, but fewer tremors were noted in her extremities. By six months into the tapering process (with risperidone reduced at that time to 0.25 mg/d and carbidopa-levodopa to 25/100 mg tid), the patient had begun to experience dissipation of the tremors, and her AIMS score2 was 0. She was able to replace her four-point walker with a cane.
One year after her initial visit to the mental health clinic, the patient’s neurologist suggested replacing risperidone with quetiapine (12.5 mg/d) for its improved tolerability and lower adverse effect profile.4 She continued to take sertraline and carbidopa-levodopa.
Improvement of symptoms was noted following the switch. After one month on the revised regimen, the patient reported that the number of auditory hallucinations persisted, but that their intensity had decreased dramatically. She had a brighter affect and appeared to feel uplifted and more energetic. She became involved in the social activities offered at the retirement living facility and the mental health clinic. She also maintained a steady gait without her cane. According to the patient’s daughter, her mother was at her best psychological state since the onset of psychotic symptoms six years earlier. The pharmacologic regimen had reached its maximum benefit.
At a mental health appointment at the outpatient clinic 18 months after her initial visit there, it was evident that the patient’s auditory hallucinations persisted as a major stressor. She began to complain about other residents in her facility. She said she disliked the resident with whom she shared meals, and she claimed that other residents often spit on the floor in front of her room. The nursing staff did not confirm these incidents, which they considered a delusion despite the patient’s “evidence” (the tissues she said she had used to clean up).
Additionally, a new theme had emerged in the patient’s auditory hallucinations. She reported hearing a male voice that announced changes in meal times. Although she knew there was no public address system in her room or in the hallway, the “announcement” was so convincing that she would go to the dining room and once there, realize that nothing had changed. She seemed to drift between reality and her hallucinations/delusions. According to her daughter, the patient’s independent and reserved personality forced her to internalize her stressors—in this case, her frustration about the other residents—which fed into her hallucinations and delusions.
In response to her worsening psychotic symptoms, the patient’s provider increased her quetiapine dosage from 12.5 mg/d to 25 mg/d. Her MMSE score3 at this visit was 25/30.
Two months later, the patient exhibited increasing symptoms of paranoia, delusions, and auditory hallucinations. She continued to respond to the “broadcast” messages about meal times, and she voiced her frustrations to others who spoke Mandarin. She became agitated in response to out-of-the-ordinary events. When her alarm clock battery ran out, for example, she insisted that “a man’s voice” kept reminding her to replace the battery; in response, she placed the alarm clock in the refrigerator, later explaining, “Now I don’t need to worry about it.”
Her cognitive status began to show obvious, progressive deterioration, with an MMSE score3 of 22/30 at this visit—a significant reduction from previous scores. Worsening of her short-term memory became apparent when she had difficulty playing bingo and was unable to remember her appointment or the current date. She became upset when others corrected her.
In a review of the trends in this patient’s clinical presentation, it became increasingly evident to the patient’s mental health care providers that she had Lewy body dementia.
DISCUSSION
Dementia with Lewy bodies (DLB), a progressive disease, is the second most common cause of neurodegenerative dementia after Alzheimer’s disease.5-7 It is estimated that DLB accounts for 20% of US cases of dementia (ie, about 800,000 patients).8,9 Although public awareness of DLB is on the rise, the disorder is still underrecognized and underdiagnosed because its clinical manifestations so closely resemble those of Alzheimer’s disease, Parkinson’s disease, and psychosis.10,11
Clinical symptoms of DLB include progressive cognitive decline, cognitive fluctuation, EPS, and parkinsonism; hallucinations involving all five senses, particularly sight; delusions; REM sleep disturbance, with or without vivid and frightening dreams; changes in mood and behavior; impaired judgment and insight; and autonomic dysfunction, such as orthostatic hypotension and carotid-sinus hypersensitivity.5,11-15
The symptoms of DLB are caused by the accumulations of Lewy bodies, that is, deposits of alpha-synuclein protein in the nuclei of neurons. Lewy bodies destroy neurons over time, resulting in the destruction of dopaminergic and acetylcholinergic pathways from the brain stem to areas of the cerebral cortex associated with cognition and motor functions.4,5,16
DLB is a spectrum disorder; it often coexists with Parkinson’s disease or Alzheimer’s disease, as Lewy bodies are also found in patients with these illnesses.7 This poses a challenge for formulating a differential diagnosis, particularly in patients with fluctuating cognition,10 and for attempting to establish disease prevalence.
Diagnosis
Currently, a conclusive diagnosis of DLB can be confirmed only through postmortem autopsy, although use of medial temporal lobe volume (via structural MRI) and regional blood flow (via single photon emission CT [SPECT] tracers) is being investigated.17
The diagnosis of DLB is currently based on the presenting clinical symptoms and the exclusion of other medical conditions whose symptoms mimic those of DLB.7 The screening assessment may include a neurologic/psychiatric assessment (MMSE, psychiatric evaluation, and interviews with family members or caretakers), neuroimaging such as MRI to rule out other organic causes, and laboratory evaluation to rule out potentially reversible causes of dementia, including electrolyte imbalance, vitamin deficiency (specifically vitamin B12), anemia, thyroid dysfunction, and kidney or liver impairment.18
The American Psychiatric Assocation1 categorizes DLB under “Dementia Due to Other General Medical Conditions” (294.1x). The World Health Organization19 includes it among “Other specified degenerative diseases of the nervous system” (G31.8).
Treatment
Lewy body dementia is an irreversible neurologic degenerative disorder. Treatment for DLB comprises symptom management, primarily through pharmacology; however, the response to medication is highly individualized. Treatment includes management of the following symptoms:
Cognitive impairment. Cholinesterase inhibitors, such as rivastigmine (3 to 12 mg/d), donepezil (10 mg/d), or galantamine (titrated up to 12 mg bid),20-23 improve attention and behavior and reduce apathy, anxiety, delusions, and hallucinations. As cognitive impairment worsens, memantine (10 mg bid) may be effective.24 The potential for anticholinergic adverse effects requires close monitoring in patients taking these agents.
Parkinsonian symptoms. Medications indicated for Parkinson’s disease and syndrome, such as carbidopa-levodopa (25/100 mg tid), can be effective; dosage may be slowly titrated upward as tolerated and if needed for symptom management.25,26 The dopaminergic effect of antiparkinson medications may intensify the psychotic symptoms and worsen the REM sleep pattern. In this case, a low-dose atypical antipsychotic is suggested27,28 (see below).
Psychotic symptoms. An atypical antipsychotic agent, such as quetiapine (12.5 mg), risperidone (0.25 mg), olanzapine (2.5 mg), ziprasidone (20 mg), aripiprazole (2 mg), or paliperidone (1.5 mg), may be used. Because of the DLB-associated risk of neuroleptic sensitivity, atypical antipsychotic agents should be initiated at a low dose with slow upward titration17,26,29; quetiapine appears less likely than risperidone or olanzapine to cause neuroleptic sensitivity or to trigger EPS.4 For Asian patients, who often respond to lower doses of these medications (and are more easily affected by associated adverse effects), Chen et al30 recommend a starting dose of about one-half the recommended dose.
Depression. An SSRI antidepressant with relatively simple pharmacologic properties and moderate half-life may be used to manage symptoms of depression.26,31,32 Long–half-life SSRIs (eg, fluoxetine) should be avoided in elderly patients; in response to SNRIs (serotonin-norepinephrine reuptake inhibitors), these patients may experience elevated blood pressures and pulses, with subsequent morbidity.33
REM sleep disturbances. Clonazepam (0.25 mg), melatonin (3.0 mg), or quetiapine (12.5 mg) may be administered at bedtime.34
Important Lessons
In general, providers should consider the benefits and risks of any pharmacologic treatment and avoid polypharmacy, if possible. Family and caretakers should be included in the treatment planning, with a focus on prioritizing and managing the most debilitating symptoms or dysfunctions that prompt concerns for safety.
For optimal homeostasis, some DLB patients may require joint pharmacologic modalities that appear counterintuitive—for example, an antiparkinsonism (dopaminergic) agent for parkinsonian symptoms or neuroleptic-induced EPS, versus an antipsychotic (eg, a dopamine antagonist) to treat profound hallucinations.26
As the response to treatment for DLB is highly individualized, it is essential to titrate and augment with care.
CONCLUSION
In DLB, as with other dementing illnesses, the onset of symptoms can be gradual and insidious, posing a great challenge to the clinician who seeks to confirm the diagnosis. In the illness’s early stages, the clinician may have to treat targeted symptoms and adjust the treatment plan once signs of the pathologic origins emerge.
It is critical to understand the mechanisms of psychotropic medications and targeted neurotransmitters when evaluating treatment for DLB. Titrating or augmenting these medications in elderly patients requires the clinician to follow a principle of start low and go slow, making only one change at a time.
It is always helpful to include family members in the patient’s care and to gather information on previous history, personality traits, family history, and cultural components. It is also important to communicate with other specialists to implement collaborative care.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed (text revision). Washington, DC: American Psychiatric Association; 2000:167.
2. National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS). www.atlantapsychia try.com/forms/AIMS.pdf. Accessed May 20, 2010.
3. Mini–Mental State Examination. www.nmaging .state.nm.us/pdf_files/Mini_Mental_Status_Exam.pdf. Accessed May 20, 2010.
4. Baskys A. Lewy body dementia: the litmus test for neuroleptic sensitivity and extrapyramidal symptoms. J Clin Psychiatry. 2004;65 suppl 11:16-22.
5. Weisman D, McKeith I. Dementia with Lewy bodies. Semin Neurol. 2007;27(1):42-47.
6. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3 suppl):417-423.
7. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology. 1996;47(5):1113-1124.
8. Hill C, Reiss N. Lewy body dementia (2008). www.mentalhelp.net/poc/view_doc.php?type=doc& id=13151&cn=231. Accessed May 20, 2010.
9. Lewy Body Dementia Association, Inc. Lewy body dementia: current issues in diagnosis and treatment. www.lewybodydementia.org. Accessed May 20, 2010.
10. Varanese S, Perfetti B, Monaco D, et al. Fluctuating cognition and different cognitive and behavioural profiles in Parkinson’s disease with dementia: comparison of dementia with Lewy bodies and Alzheimer’s disease. J Neurol. 2010 Jan 22. [Epub ahead of print]
11. Kurita A, Murakami M, Takagi S, et al. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disorder. 2010;25(2):167-171.
12. Gagnon JF, Postuma RB, Mazza S, et al. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5(5):424-432.
13. Dodel R, Csoti I, Ebersbach G, et al. Lewy body dementia and Parkinson’s disease with dementia. J Neurol. 2008;255 suppl 5:39-47.
14. Sonnesyn H, Nilsen DW, Rongve A, et al. High prevalence of orthostatic hypotension in mild dementia. Dement Geriatr Cogn Disord. 2009;28(4):307-313.
15. Kenny RA, Shaw FE, O’Brien JT, et al. Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiatry. 2004;75(7):966-971.
16. Hickey C, Chisholm T, Passmore MJ, et al. Differentiating the dementias: revisiting synucleinopathies and tauopathies. Curr Alzheimer Res. 2008;5(1):52-60.
17. McKeith IG, Burn DJ, Ballard CG, et al. Dementia with Lewy bodies. Semin Clin Neuropsychiatry. 2003; 8(1):46-57.
18. Bird TD, Miller BL. Dementia. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill Medical; 2008:2536-2549.
19. World Health Organization. International Classification of Diseases (ICD), Version 2007. Chapter VI: Diseases of the Central Nervous System. http://apps.who.int/classifications/apps/icd/icd10online/index.htm?kg00.htm+. Accessed May 20, 2010.
20. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
21. Emre M, Cummings JL, Lane RM. Rivastigmine in dementia associated with Parkinson’s disease and Alzheimer’s disease: similarities and differences. J Alzheimers Dis. 2007;11(4):509-519.
22. Lam B, Hollingdrake E, Kennedy JL, et al. Cholinesterase inhibitors in Alzheimer’s disease and Lewy body spectrum disorders: the emerging pharmacogenetic story. Hum Genomics. 2009;4(2):91-106.
23. Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev. 2003;(3):CD003672.
24. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
25. Merck & Co., Inc. Sinemet® CR (carbidopa-levodopa) sustained-release tablets. http://packageinserts.bms.com/pi/pi_sinemet_cr.pdf. Accessed May 20, 2010.
26. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
27. Kato K, Wada T, Kawakatsu S, Otani K. Improvement of both psychotic symptoms and Parkinsonism in a case of dementia with Lewy bodies by the combination therapy of risperidone and L-DOPA. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):201-203.
28. Yamauchi K, Takehisa M, Tsuno M, et al. Levodopa improved rapid eye movement sleep behavior disorder with diffuse Lewy body disease. Gen Hosp Psychiatry. 2003;25(2):140-142.
29. Stahl SM. The Prescriber’s Guide: Stahl’s Essential Psychopharmacology. Cambridge University Press; 2006:459.
30. Chen JP, Barron C, Lin KM, Chung H. Prescribing medication for Asians with mental disorders. West J Med. 2002;176(4):271-275.
31. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
32. Pollock BG, Mulsant BH, Rosen J, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159(3):460-465.
33. Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med. 2009;76(3):167-174.
34. Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67(5):742-747
An 80-year-old Mandarin-speaking Chinese woman was referred to a mental health outpatient clinic for evaluation and treatment. The patient had a history of mild depression, for which she had been treated for many years with sertraline.
Five years earlier at age 75, the patient had been evaluated by a psychiatrist after she began to experience psychotic symptoms, including frequent repetitive auditory hallucinations of people counting, alternating with music from her childhood. At that time, she also had persecutory paranoid thoughts and delusional thinking that she was receiving messages in Mandarin while watching American TV programs. Initially, her only cognitive disturbance was an inability to differentiate among numbers on a calendar or a telephone keypad. No reports of memory problems were noted. Although the patient acknowledged auditory hallucinations, she denied experiencing command auditory hallucinations or hallucinations of other forms. The patient had no history of suicide attempts and denied suicidal or homicidal ideation. She had no history of psychiatric hospitalization.
The psychiatrist made a diagnosis of major depressive disorder with psychotic features, not otherwise specified1 and prescribed sertraline 50 mg/d. The patient was also started on risperidone 0.25 mg/d for management of her psychotic symptoms, with the dosage gradually increased to 2.0 mg/d over five years. While taking this combination, the patient experienced stable mood and fewer paranoid thoughts, although her auditory hallucinations continued.
Two months before the current visit, the patient moved into a retirement living facility, and she reported having adapted well to the new setting. She was sleeping well and had a good appetite. Her BMI was within normal range.
The patient described herself as a single parent for nearly 40 years, raising one daughter. Formerly high functioning, she had held a full-time clerical job until age 70. She appeared well-groomed, polite but anxious, and oriented to time, person, and place. Her speech was normal, her thought processes were coherent, and her mood was stable. However, her affect was constricted; she acknowledged auditory hallucinations, which impaired her thought content. The patient reported feeling increased anxiety prior to any nonroutine activity, such as a doctor’s appointment; this, she said, would cause insomnia, leaving her to pace in her room.
During the examination, fine tremors on upper and lower extremities were noted. The patient’s Abnormal Involuntary Movement Scale (AIMS) score2 was 13, which placed her in the highest risk category for antipsychotic-induced dopamine-blockade extrapyramidal symptoms (EPS). The patient was found to be negative for tardive dyskinesia, with no abnormal facial movements. She was aware of the tremors in her limbs and said she felt bothered by them.
The patient had an unsteady gait and used a four-point walker. Her Mini-Mental State Exam (MMSE) score3 was 28/30, which was normal for her age and education level (high school completed).
Apart from the described symptoms, the patient was healthy for her age and had no other medical diagnosis. Her vital signs were within normal range. The medical work-up to rule out other causes of dementia yielded negative results. Lab values were normal, including electrolyte levels and thyroid tests. The patient’s hearing test showed age-related hearing loss of full range, not limited to high pitch. She was able to engage in a meaningful conversation at a normal volume. Clinically, however, it was concerning to observe the possible signs of EPS and the relatively high risperidone dosage, considering the patient’s advanced age.
After the meeting with the patient, a treatment plan was created to 1) gradually reduce the dosage of antipsychotic medication, and 2) refer her to a neurologist for a complete work-up to rule out underlying neurologic disorders, such as dementia. Risperidone was tapered by increments of 0.25 mg/d every three to four weeks; throughout this process, the patient was closely monitored by the nursing staff at the retirement living facility. Monthly appointments were scheduled at the outpatient mental health clinic for evaluation and medication management.
Two months after the initial mental health clinic visit, the patient’s condition was pronounced stable on the current regimen of sertraline 50 mg/d and risperidone 1.0 mg/d. She was later seen by a neurologist, who made a diagnosis of Parkinson’s disease and placed her on carbidopa-levodopa (1 1/2 tablets, 25/100 mg, tid). The patient’s auditory hallucinations continued with the same intensity as at baseline, but fewer tremors were noted in her extremities. By six months into the tapering process (with risperidone reduced at that time to 0.25 mg/d and carbidopa-levodopa to 25/100 mg tid), the patient had begun to experience dissipation of the tremors, and her AIMS score2 was 0. She was able to replace her four-point walker with a cane.
One year after her initial visit to the mental health clinic, the patient’s neurologist suggested replacing risperidone with quetiapine (12.5 mg/d) for its improved tolerability and lower adverse effect profile.4 She continued to take sertraline and carbidopa-levodopa.
Improvement of symptoms was noted following the switch. After one month on the revised regimen, the patient reported that the number of auditory hallucinations persisted, but that their intensity had decreased dramatically. She had a brighter affect and appeared to feel uplifted and more energetic. She became involved in the social activities offered at the retirement living facility and the mental health clinic. She also maintained a steady gait without her cane. According to the patient’s daughter, her mother was at her best psychological state since the onset of psychotic symptoms six years earlier. The pharmacologic regimen had reached its maximum benefit.
At a mental health appointment at the outpatient clinic 18 months after her initial visit there, it was evident that the patient’s auditory hallucinations persisted as a major stressor. She began to complain about other residents in her facility. She said she disliked the resident with whom she shared meals, and she claimed that other residents often spit on the floor in front of her room. The nursing staff did not confirm these incidents, which they considered a delusion despite the patient’s “evidence” (the tissues she said she had used to clean up).
Additionally, a new theme had emerged in the patient’s auditory hallucinations. She reported hearing a male voice that announced changes in meal times. Although she knew there was no public address system in her room or in the hallway, the “announcement” was so convincing that she would go to the dining room and once there, realize that nothing had changed. She seemed to drift between reality and her hallucinations/delusions. According to her daughter, the patient’s independent and reserved personality forced her to internalize her stressors—in this case, her frustration about the other residents—which fed into her hallucinations and delusions.
In response to her worsening psychotic symptoms, the patient’s provider increased her quetiapine dosage from 12.5 mg/d to 25 mg/d. Her MMSE score3 at this visit was 25/30.
Two months later, the patient exhibited increasing symptoms of paranoia, delusions, and auditory hallucinations. She continued to respond to the “broadcast” messages about meal times, and she voiced her frustrations to others who spoke Mandarin. She became agitated in response to out-of-the-ordinary events. When her alarm clock battery ran out, for example, she insisted that “a man’s voice” kept reminding her to replace the battery; in response, she placed the alarm clock in the refrigerator, later explaining, “Now I don’t need to worry about it.”
Her cognitive status began to show obvious, progressive deterioration, with an MMSE score3 of 22/30 at this visit—a significant reduction from previous scores. Worsening of her short-term memory became apparent when she had difficulty playing bingo and was unable to remember her appointment or the current date. She became upset when others corrected her.
In a review of the trends in this patient’s clinical presentation, it became increasingly evident to the patient’s mental health care providers that she had Lewy body dementia.
DISCUSSION
Dementia with Lewy bodies (DLB), a progressive disease, is the second most common cause of neurodegenerative dementia after Alzheimer’s disease.5-7 It is estimated that DLB accounts for 20% of US cases of dementia (ie, about 800,000 patients).8,9 Although public awareness of DLB is on the rise, the disorder is still underrecognized and underdiagnosed because its clinical manifestations so closely resemble those of Alzheimer’s disease, Parkinson’s disease, and psychosis.10,11
Clinical symptoms of DLB include progressive cognitive decline, cognitive fluctuation, EPS, and parkinsonism; hallucinations involving all five senses, particularly sight; delusions; REM sleep disturbance, with or without vivid and frightening dreams; changes in mood and behavior; impaired judgment and insight; and autonomic dysfunction, such as orthostatic hypotension and carotid-sinus hypersensitivity.5,11-15
The symptoms of DLB are caused by the accumulations of Lewy bodies, that is, deposits of alpha-synuclein protein in the nuclei of neurons. Lewy bodies destroy neurons over time, resulting in the destruction of dopaminergic and acetylcholinergic pathways from the brain stem to areas of the cerebral cortex associated with cognition and motor functions.4,5,16
DLB is a spectrum disorder; it often coexists with Parkinson’s disease or Alzheimer’s disease, as Lewy bodies are also found in patients with these illnesses.7 This poses a challenge for formulating a differential diagnosis, particularly in patients with fluctuating cognition,10 and for attempting to establish disease prevalence.
Diagnosis
Currently, a conclusive diagnosis of DLB can be confirmed only through postmortem autopsy, although use of medial temporal lobe volume (via structural MRI) and regional blood flow (via single photon emission CT [SPECT] tracers) is being investigated.17
The diagnosis of DLB is currently based on the presenting clinical symptoms and the exclusion of other medical conditions whose symptoms mimic those of DLB.7 The screening assessment may include a neurologic/psychiatric assessment (MMSE, psychiatric evaluation, and interviews with family members or caretakers), neuroimaging such as MRI to rule out other organic causes, and laboratory evaluation to rule out potentially reversible causes of dementia, including electrolyte imbalance, vitamin deficiency (specifically vitamin B12), anemia, thyroid dysfunction, and kidney or liver impairment.18
The American Psychiatric Assocation1 categorizes DLB under “Dementia Due to Other General Medical Conditions” (294.1x). The World Health Organization19 includes it among “Other specified degenerative diseases of the nervous system” (G31.8).
Treatment
Lewy body dementia is an irreversible neurologic degenerative disorder. Treatment for DLB comprises symptom management, primarily through pharmacology; however, the response to medication is highly individualized. Treatment includes management of the following symptoms:
Cognitive impairment. Cholinesterase inhibitors, such as rivastigmine (3 to 12 mg/d), donepezil (10 mg/d), or galantamine (titrated up to 12 mg bid),20-23 improve attention and behavior and reduce apathy, anxiety, delusions, and hallucinations. As cognitive impairment worsens, memantine (10 mg bid) may be effective.24 The potential for anticholinergic adverse effects requires close monitoring in patients taking these agents.
Parkinsonian symptoms. Medications indicated for Parkinson’s disease and syndrome, such as carbidopa-levodopa (25/100 mg tid), can be effective; dosage may be slowly titrated upward as tolerated and if needed for symptom management.25,26 The dopaminergic effect of antiparkinson medications may intensify the psychotic symptoms and worsen the REM sleep pattern. In this case, a low-dose atypical antipsychotic is suggested27,28 (see below).
Psychotic symptoms. An atypical antipsychotic agent, such as quetiapine (12.5 mg), risperidone (0.25 mg), olanzapine (2.5 mg), ziprasidone (20 mg), aripiprazole (2 mg), or paliperidone (1.5 mg), may be used. Because of the DLB-associated risk of neuroleptic sensitivity, atypical antipsychotic agents should be initiated at a low dose with slow upward titration17,26,29; quetiapine appears less likely than risperidone or olanzapine to cause neuroleptic sensitivity or to trigger EPS.4 For Asian patients, who often respond to lower doses of these medications (and are more easily affected by associated adverse effects), Chen et al30 recommend a starting dose of about one-half the recommended dose.
Depression. An SSRI antidepressant with relatively simple pharmacologic properties and moderate half-life may be used to manage symptoms of depression.26,31,32 Long–half-life SSRIs (eg, fluoxetine) should be avoided in elderly patients; in response to SNRIs (serotonin-norepinephrine reuptake inhibitors), these patients may experience elevated blood pressures and pulses, with subsequent morbidity.33
REM sleep disturbances. Clonazepam (0.25 mg), melatonin (3.0 mg), or quetiapine (12.5 mg) may be administered at bedtime.34
Important Lessons
In general, providers should consider the benefits and risks of any pharmacologic treatment and avoid polypharmacy, if possible. Family and caretakers should be included in the treatment planning, with a focus on prioritizing and managing the most debilitating symptoms or dysfunctions that prompt concerns for safety.
For optimal homeostasis, some DLB patients may require joint pharmacologic modalities that appear counterintuitive—for example, an antiparkinsonism (dopaminergic) agent for parkinsonian symptoms or neuroleptic-induced EPS, versus an antipsychotic (eg, a dopamine antagonist) to treat profound hallucinations.26
As the response to treatment for DLB is highly individualized, it is essential to titrate and augment with care.
CONCLUSION
In DLB, as with other dementing illnesses, the onset of symptoms can be gradual and insidious, posing a great challenge to the clinician who seeks to confirm the diagnosis. In the illness’s early stages, the clinician may have to treat targeted symptoms and adjust the treatment plan once signs of the pathologic origins emerge.
It is critical to understand the mechanisms of psychotropic medications and targeted neurotransmitters when evaluating treatment for DLB. Titrating or augmenting these medications in elderly patients requires the clinician to follow a principle of start low and go slow, making only one change at a time.
It is always helpful to include family members in the patient’s care and to gather information on previous history, personality traits, family history, and cultural components. It is also important to communicate with other specialists to implement collaborative care.
An 80-year-old Mandarin-speaking Chinese woman was referred to a mental health outpatient clinic for evaluation and treatment. The patient had a history of mild depression, for which she had been treated for many years with sertraline.
Five years earlier at age 75, the patient had been evaluated by a psychiatrist after she began to experience psychotic symptoms, including frequent repetitive auditory hallucinations of people counting, alternating with music from her childhood. At that time, she also had persecutory paranoid thoughts and delusional thinking that she was receiving messages in Mandarin while watching American TV programs. Initially, her only cognitive disturbance was an inability to differentiate among numbers on a calendar or a telephone keypad. No reports of memory problems were noted. Although the patient acknowledged auditory hallucinations, she denied experiencing command auditory hallucinations or hallucinations of other forms. The patient had no history of suicide attempts and denied suicidal or homicidal ideation. She had no history of psychiatric hospitalization.
The psychiatrist made a diagnosis of major depressive disorder with psychotic features, not otherwise specified1 and prescribed sertraline 50 mg/d. The patient was also started on risperidone 0.25 mg/d for management of her psychotic symptoms, with the dosage gradually increased to 2.0 mg/d over five years. While taking this combination, the patient experienced stable mood and fewer paranoid thoughts, although her auditory hallucinations continued.
Two months before the current visit, the patient moved into a retirement living facility, and she reported having adapted well to the new setting. She was sleeping well and had a good appetite. Her BMI was within normal range.
The patient described herself as a single parent for nearly 40 years, raising one daughter. Formerly high functioning, she had held a full-time clerical job until age 70. She appeared well-groomed, polite but anxious, and oriented to time, person, and place. Her speech was normal, her thought processes were coherent, and her mood was stable. However, her affect was constricted; she acknowledged auditory hallucinations, which impaired her thought content. The patient reported feeling increased anxiety prior to any nonroutine activity, such as a doctor’s appointment; this, she said, would cause insomnia, leaving her to pace in her room.
During the examination, fine tremors on upper and lower extremities were noted. The patient’s Abnormal Involuntary Movement Scale (AIMS) score2 was 13, which placed her in the highest risk category for antipsychotic-induced dopamine-blockade extrapyramidal symptoms (EPS). The patient was found to be negative for tardive dyskinesia, with no abnormal facial movements. She was aware of the tremors in her limbs and said she felt bothered by them.
The patient had an unsteady gait and used a four-point walker. Her Mini-Mental State Exam (MMSE) score3 was 28/30, which was normal for her age and education level (high school completed).
Apart from the described symptoms, the patient was healthy for her age and had no other medical diagnosis. Her vital signs were within normal range. The medical work-up to rule out other causes of dementia yielded negative results. Lab values were normal, including electrolyte levels and thyroid tests. The patient’s hearing test showed age-related hearing loss of full range, not limited to high pitch. She was able to engage in a meaningful conversation at a normal volume. Clinically, however, it was concerning to observe the possible signs of EPS and the relatively high risperidone dosage, considering the patient’s advanced age.
After the meeting with the patient, a treatment plan was created to 1) gradually reduce the dosage of antipsychotic medication, and 2) refer her to a neurologist for a complete work-up to rule out underlying neurologic disorders, such as dementia. Risperidone was tapered by increments of 0.25 mg/d every three to four weeks; throughout this process, the patient was closely monitored by the nursing staff at the retirement living facility. Monthly appointments were scheduled at the outpatient mental health clinic for evaluation and medication management.
Two months after the initial mental health clinic visit, the patient’s condition was pronounced stable on the current regimen of sertraline 50 mg/d and risperidone 1.0 mg/d. She was later seen by a neurologist, who made a diagnosis of Parkinson’s disease and placed her on carbidopa-levodopa (1 1/2 tablets, 25/100 mg, tid). The patient’s auditory hallucinations continued with the same intensity as at baseline, but fewer tremors were noted in her extremities. By six months into the tapering process (with risperidone reduced at that time to 0.25 mg/d and carbidopa-levodopa to 25/100 mg tid), the patient had begun to experience dissipation of the tremors, and her AIMS score2 was 0. She was able to replace her four-point walker with a cane.
One year after her initial visit to the mental health clinic, the patient’s neurologist suggested replacing risperidone with quetiapine (12.5 mg/d) for its improved tolerability and lower adverse effect profile.4 She continued to take sertraline and carbidopa-levodopa.
Improvement of symptoms was noted following the switch. After one month on the revised regimen, the patient reported that the number of auditory hallucinations persisted, but that their intensity had decreased dramatically. She had a brighter affect and appeared to feel uplifted and more energetic. She became involved in the social activities offered at the retirement living facility and the mental health clinic. She also maintained a steady gait without her cane. According to the patient’s daughter, her mother was at her best psychological state since the onset of psychotic symptoms six years earlier. The pharmacologic regimen had reached its maximum benefit.
At a mental health appointment at the outpatient clinic 18 months after her initial visit there, it was evident that the patient’s auditory hallucinations persisted as a major stressor. She began to complain about other residents in her facility. She said she disliked the resident with whom she shared meals, and she claimed that other residents often spit on the floor in front of her room. The nursing staff did not confirm these incidents, which they considered a delusion despite the patient’s “evidence” (the tissues she said she had used to clean up).
Additionally, a new theme had emerged in the patient’s auditory hallucinations. She reported hearing a male voice that announced changes in meal times. Although she knew there was no public address system in her room or in the hallway, the “announcement” was so convincing that she would go to the dining room and once there, realize that nothing had changed. She seemed to drift between reality and her hallucinations/delusions. According to her daughter, the patient’s independent and reserved personality forced her to internalize her stressors—in this case, her frustration about the other residents—which fed into her hallucinations and delusions.
In response to her worsening psychotic symptoms, the patient’s provider increased her quetiapine dosage from 12.5 mg/d to 25 mg/d. Her MMSE score3 at this visit was 25/30.
Two months later, the patient exhibited increasing symptoms of paranoia, delusions, and auditory hallucinations. She continued to respond to the “broadcast” messages about meal times, and she voiced her frustrations to others who spoke Mandarin. She became agitated in response to out-of-the-ordinary events. When her alarm clock battery ran out, for example, she insisted that “a man’s voice” kept reminding her to replace the battery; in response, she placed the alarm clock in the refrigerator, later explaining, “Now I don’t need to worry about it.”
Her cognitive status began to show obvious, progressive deterioration, with an MMSE score3 of 22/30 at this visit—a significant reduction from previous scores. Worsening of her short-term memory became apparent when she had difficulty playing bingo and was unable to remember her appointment or the current date. She became upset when others corrected her.
In a review of the trends in this patient’s clinical presentation, it became increasingly evident to the patient’s mental health care providers that she had Lewy body dementia.
DISCUSSION
Dementia with Lewy bodies (DLB), a progressive disease, is the second most common cause of neurodegenerative dementia after Alzheimer’s disease.5-7 It is estimated that DLB accounts for 20% of US cases of dementia (ie, about 800,000 patients).8,9 Although public awareness of DLB is on the rise, the disorder is still underrecognized and underdiagnosed because its clinical manifestations so closely resemble those of Alzheimer’s disease, Parkinson’s disease, and psychosis.10,11
Clinical symptoms of DLB include progressive cognitive decline, cognitive fluctuation, EPS, and parkinsonism; hallucinations involving all five senses, particularly sight; delusions; REM sleep disturbance, with or without vivid and frightening dreams; changes in mood and behavior; impaired judgment and insight; and autonomic dysfunction, such as orthostatic hypotension and carotid-sinus hypersensitivity.5,11-15
The symptoms of DLB are caused by the accumulations of Lewy bodies, that is, deposits of alpha-synuclein protein in the nuclei of neurons. Lewy bodies destroy neurons over time, resulting in the destruction of dopaminergic and acetylcholinergic pathways from the brain stem to areas of the cerebral cortex associated with cognition and motor functions.4,5,16
DLB is a spectrum disorder; it often coexists with Parkinson’s disease or Alzheimer’s disease, as Lewy bodies are also found in patients with these illnesses.7 This poses a challenge for formulating a differential diagnosis, particularly in patients with fluctuating cognition,10 and for attempting to establish disease prevalence.
Diagnosis
Currently, a conclusive diagnosis of DLB can be confirmed only through postmortem autopsy, although use of medial temporal lobe volume (via structural MRI) and regional blood flow (via single photon emission CT [SPECT] tracers) is being investigated.17
The diagnosis of DLB is currently based on the presenting clinical symptoms and the exclusion of other medical conditions whose symptoms mimic those of DLB.7 The screening assessment may include a neurologic/psychiatric assessment (MMSE, psychiatric evaluation, and interviews with family members or caretakers), neuroimaging such as MRI to rule out other organic causes, and laboratory evaluation to rule out potentially reversible causes of dementia, including electrolyte imbalance, vitamin deficiency (specifically vitamin B12), anemia, thyroid dysfunction, and kidney or liver impairment.18
The American Psychiatric Assocation1 categorizes DLB under “Dementia Due to Other General Medical Conditions” (294.1x). The World Health Organization19 includes it among “Other specified degenerative diseases of the nervous system” (G31.8).
Treatment
Lewy body dementia is an irreversible neurologic degenerative disorder. Treatment for DLB comprises symptom management, primarily through pharmacology; however, the response to medication is highly individualized. Treatment includes management of the following symptoms:
Cognitive impairment. Cholinesterase inhibitors, such as rivastigmine (3 to 12 mg/d), donepezil (10 mg/d), or galantamine (titrated up to 12 mg bid),20-23 improve attention and behavior and reduce apathy, anxiety, delusions, and hallucinations. As cognitive impairment worsens, memantine (10 mg bid) may be effective.24 The potential for anticholinergic adverse effects requires close monitoring in patients taking these agents.
Parkinsonian symptoms. Medications indicated for Parkinson’s disease and syndrome, such as carbidopa-levodopa (25/100 mg tid), can be effective; dosage may be slowly titrated upward as tolerated and if needed for symptom management.25,26 The dopaminergic effect of antiparkinson medications may intensify the psychotic symptoms and worsen the REM sleep pattern. In this case, a low-dose atypical antipsychotic is suggested27,28 (see below).
Psychotic symptoms. An atypical antipsychotic agent, such as quetiapine (12.5 mg), risperidone (0.25 mg), olanzapine (2.5 mg), ziprasidone (20 mg), aripiprazole (2 mg), or paliperidone (1.5 mg), may be used. Because of the DLB-associated risk of neuroleptic sensitivity, atypical antipsychotic agents should be initiated at a low dose with slow upward titration17,26,29; quetiapine appears less likely than risperidone or olanzapine to cause neuroleptic sensitivity or to trigger EPS.4 For Asian patients, who often respond to lower doses of these medications (and are more easily affected by associated adverse effects), Chen et al30 recommend a starting dose of about one-half the recommended dose.
Depression. An SSRI antidepressant with relatively simple pharmacologic properties and moderate half-life may be used to manage symptoms of depression.26,31,32 Long–half-life SSRIs (eg, fluoxetine) should be avoided in elderly patients; in response to SNRIs (serotonin-norepinephrine reuptake inhibitors), these patients may experience elevated blood pressures and pulses, with subsequent morbidity.33
REM sleep disturbances. Clonazepam (0.25 mg), melatonin (3.0 mg), or quetiapine (12.5 mg) may be administered at bedtime.34
Important Lessons
In general, providers should consider the benefits and risks of any pharmacologic treatment and avoid polypharmacy, if possible. Family and caretakers should be included in the treatment planning, with a focus on prioritizing and managing the most debilitating symptoms or dysfunctions that prompt concerns for safety.
For optimal homeostasis, some DLB patients may require joint pharmacologic modalities that appear counterintuitive—for example, an antiparkinsonism (dopaminergic) agent for parkinsonian symptoms or neuroleptic-induced EPS, versus an antipsychotic (eg, a dopamine antagonist) to treat profound hallucinations.26
As the response to treatment for DLB is highly individualized, it is essential to titrate and augment with care.
CONCLUSION
In DLB, as with other dementing illnesses, the onset of symptoms can be gradual and insidious, posing a great challenge to the clinician who seeks to confirm the diagnosis. In the illness’s early stages, the clinician may have to treat targeted symptoms and adjust the treatment plan once signs of the pathologic origins emerge.
It is critical to understand the mechanisms of psychotropic medications and targeted neurotransmitters when evaluating treatment for DLB. Titrating or augmenting these medications in elderly patients requires the clinician to follow a principle of start low and go slow, making only one change at a time.
It is always helpful to include family members in the patient’s care and to gather information on previous history, personality traits, family history, and cultural components. It is also important to communicate with other specialists to implement collaborative care.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed (text revision). Washington, DC: American Psychiatric Association; 2000:167.
2. National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS). www.atlantapsychia try.com/forms/AIMS.pdf. Accessed May 20, 2010.
3. Mini–Mental State Examination. www.nmaging .state.nm.us/pdf_files/Mini_Mental_Status_Exam.pdf. Accessed May 20, 2010.
4. Baskys A. Lewy body dementia: the litmus test for neuroleptic sensitivity and extrapyramidal symptoms. J Clin Psychiatry. 2004;65 suppl 11:16-22.
5. Weisman D, McKeith I. Dementia with Lewy bodies. Semin Neurol. 2007;27(1):42-47.
6. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3 suppl):417-423.
7. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology. 1996;47(5):1113-1124.
8. Hill C, Reiss N. Lewy body dementia (2008). www.mentalhelp.net/poc/view_doc.php?type=doc& id=13151&cn=231. Accessed May 20, 2010.
9. Lewy Body Dementia Association, Inc. Lewy body dementia: current issues in diagnosis and treatment. www.lewybodydementia.org. Accessed May 20, 2010.
10. Varanese S, Perfetti B, Monaco D, et al. Fluctuating cognition and different cognitive and behavioural profiles in Parkinson’s disease with dementia: comparison of dementia with Lewy bodies and Alzheimer’s disease. J Neurol. 2010 Jan 22. [Epub ahead of print]
11. Kurita A, Murakami M, Takagi S, et al. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disorder. 2010;25(2):167-171.
12. Gagnon JF, Postuma RB, Mazza S, et al. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5(5):424-432.
13. Dodel R, Csoti I, Ebersbach G, et al. Lewy body dementia and Parkinson’s disease with dementia. J Neurol. 2008;255 suppl 5:39-47.
14. Sonnesyn H, Nilsen DW, Rongve A, et al. High prevalence of orthostatic hypotension in mild dementia. Dement Geriatr Cogn Disord. 2009;28(4):307-313.
15. Kenny RA, Shaw FE, O’Brien JT, et al. Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiatry. 2004;75(7):966-971.
16. Hickey C, Chisholm T, Passmore MJ, et al. Differentiating the dementias: revisiting synucleinopathies and tauopathies. Curr Alzheimer Res. 2008;5(1):52-60.
17. McKeith IG, Burn DJ, Ballard CG, et al. Dementia with Lewy bodies. Semin Clin Neuropsychiatry. 2003; 8(1):46-57.
18. Bird TD, Miller BL. Dementia. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill Medical; 2008:2536-2549.
19. World Health Organization. International Classification of Diseases (ICD), Version 2007. Chapter VI: Diseases of the Central Nervous System. http://apps.who.int/classifications/apps/icd/icd10online/index.htm?kg00.htm+. Accessed May 20, 2010.
20. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
21. Emre M, Cummings JL, Lane RM. Rivastigmine in dementia associated with Parkinson’s disease and Alzheimer’s disease: similarities and differences. J Alzheimers Dis. 2007;11(4):509-519.
22. Lam B, Hollingdrake E, Kennedy JL, et al. Cholinesterase inhibitors in Alzheimer’s disease and Lewy body spectrum disorders: the emerging pharmacogenetic story. Hum Genomics. 2009;4(2):91-106.
23. Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev. 2003;(3):CD003672.
24. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
25. Merck & Co., Inc. Sinemet® CR (carbidopa-levodopa) sustained-release tablets. http://packageinserts.bms.com/pi/pi_sinemet_cr.pdf. Accessed May 20, 2010.
26. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
27. Kato K, Wada T, Kawakatsu S, Otani K. Improvement of both psychotic symptoms and Parkinsonism in a case of dementia with Lewy bodies by the combination therapy of risperidone and L-DOPA. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):201-203.
28. Yamauchi K, Takehisa M, Tsuno M, et al. Levodopa improved rapid eye movement sleep behavior disorder with diffuse Lewy body disease. Gen Hosp Psychiatry. 2003;25(2):140-142.
29. Stahl SM. The Prescriber’s Guide: Stahl’s Essential Psychopharmacology. Cambridge University Press; 2006:459.
30. Chen JP, Barron C, Lin KM, Chung H. Prescribing medication for Asians with mental disorders. West J Med. 2002;176(4):271-275.
31. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
32. Pollock BG, Mulsant BH, Rosen J, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159(3):460-465.
33. Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med. 2009;76(3):167-174.
34. Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67(5):742-747
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed (text revision). Washington, DC: American Psychiatric Association; 2000:167.
2. National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS). www.atlantapsychia try.com/forms/AIMS.pdf. Accessed May 20, 2010.
3. Mini–Mental State Examination. www.nmaging .state.nm.us/pdf_files/Mini_Mental_Status_Exam.pdf. Accessed May 20, 2010.
4. Baskys A. Lewy body dementia: the litmus test for neuroleptic sensitivity and extrapyramidal symptoms. J Clin Psychiatry. 2004;65 suppl 11:16-22.
5. Weisman D, McKeith I. Dementia with Lewy bodies. Semin Neurol. 2007;27(1):42-47.
6. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3 suppl):417-423.
7. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology. 1996;47(5):1113-1124.
8. Hill C, Reiss N. Lewy body dementia (2008). www.mentalhelp.net/poc/view_doc.php?type=doc& id=13151&cn=231. Accessed May 20, 2010.
9. Lewy Body Dementia Association, Inc. Lewy body dementia: current issues in diagnosis and treatment. www.lewybodydementia.org. Accessed May 20, 2010.
10. Varanese S, Perfetti B, Monaco D, et al. Fluctuating cognition and different cognitive and behavioural profiles in Parkinson’s disease with dementia: comparison of dementia with Lewy bodies and Alzheimer’s disease. J Neurol. 2010 Jan 22. [Epub ahead of print]
11. Kurita A, Murakami M, Takagi S, et al. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disorder. 2010;25(2):167-171.
12. Gagnon JF, Postuma RB, Mazza S, et al. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5(5):424-432.
13. Dodel R, Csoti I, Ebersbach G, et al. Lewy body dementia and Parkinson’s disease with dementia. J Neurol. 2008;255 suppl 5:39-47.
14. Sonnesyn H, Nilsen DW, Rongve A, et al. High prevalence of orthostatic hypotension in mild dementia. Dement Geriatr Cogn Disord. 2009;28(4):307-313.
15. Kenny RA, Shaw FE, O’Brien JT, et al. Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiatry. 2004;75(7):966-971.
16. Hickey C, Chisholm T, Passmore MJ, et al. Differentiating the dementias: revisiting synucleinopathies and tauopathies. Curr Alzheimer Res. 2008;5(1):52-60.
17. McKeith IG, Burn DJ, Ballard CG, et al. Dementia with Lewy bodies. Semin Clin Neuropsychiatry. 2003; 8(1):46-57.
18. Bird TD, Miller BL. Dementia. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill Medical; 2008:2536-2549.
19. World Health Organization. International Classification of Diseases (ICD), Version 2007. Chapter VI: Diseases of the Central Nervous System. http://apps.who.int/classifications/apps/icd/icd10online/index.htm?kg00.htm+. Accessed May 20, 2010.
20. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
21. Emre M, Cummings JL, Lane RM. Rivastigmine in dementia associated with Parkinson’s disease and Alzheimer’s disease: similarities and differences. J Alzheimers Dis. 2007;11(4):509-519.
22. Lam B, Hollingdrake E, Kennedy JL, et al. Cholinesterase inhibitors in Alzheimer’s disease and Lewy body spectrum disorders: the emerging pharmacogenetic story. Hum Genomics. 2009;4(2):91-106.
23. Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev. 2003;(3):CD003672.
24. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
25. Merck & Co., Inc. Sinemet® CR (carbidopa-levodopa) sustained-release tablets. http://packageinserts.bms.com/pi/pi_sinemet_cr.pdf. Accessed May 20, 2010.
26. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
27. Kato K, Wada T, Kawakatsu S, Otani K. Improvement of both psychotic symptoms and Parkinsonism in a case of dementia with Lewy bodies by the combination therapy of risperidone and L-DOPA. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):201-203.
28. Yamauchi K, Takehisa M, Tsuno M, et al. Levodopa improved rapid eye movement sleep behavior disorder with diffuse Lewy body disease. Gen Hosp Psychiatry. 2003;25(2):140-142.
29. Stahl SM. The Prescriber’s Guide: Stahl’s Essential Psychopharmacology. Cambridge University Press; 2006:459.
30. Chen JP, Barron C, Lin KM, Chung H. Prescribing medication for Asians with mental disorders. West J Med. 2002;176(4):271-275.
31. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
32. Pollock BG, Mulsant BH, Rosen J, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159(3):460-465.
33. Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med. 2009;76(3):167-174.
34. Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67(5):742-747
Two-Layer Repair of a Chronic Patellar Tendon Rupture: A Novel Technique and Literature Review
Vesiculopustular Eruption in a Neonate With Trisomy 21 Syndrome as a Clue of Transient Myeloproliferative Disorders
What's Eating You? Cat Flea (Ctenocephalides felis), Part 2: Prevention and Control
Successfully navigating the 15-minute ‘med check’
How to reduce malpractice risk with better documentation.
Tips to make documentation easier, faster, and more satisfying” (Current Psychiatry, February 2008), I discussed documentation techniques at length. Table 3 reprints principles that may be especially helpful in practices that consist primarily of med checks.
Table 3
Keys to better documentation
| Technique | Benefits |
|---|---|
| Time and date your notes | After an adverse event, establish when you saw the patient, recorded findings, wrote orders, reviewed lab results, or discussed problems with others can make a big difference in how your care is viewed |
| Sooner is better | Charting completed long after an adverse event occurred is vulnerable to accusations of fabrication |
| Brief quotes | Verbatim statements (‘I’ve never considered suicide’) quickly convey key factors in your therapeutic decision |
| Dictate or use speech recognition software | You speak faster than you write allowing you to document more |
| Provide handouts | Patients often do not remember or understand much of medication instructions doctors tell them |
| Use rating scales | Record more information in a scientifically validated format |
| Try macros and templates | These reduce documentation time and help you remember to cover everything you should |
| Source: Adapted from reference 18 | |
Acknowledgment
Thanks to James Knoll IV, MD for his helpful input on this article.
1. Mojtabai R, Olfson M. National trends in psychotherapy by office-based psychiatrists. Arch Gen Psychiatry. 2008;65:962-970.
2. Lewis MH, Gohagan JK, Merenstein DJ. The locality rule and the physician’s dilemma: local medical practices vs the national standard of care. JAMA. 2007;297(23):2633-2637.
3. Gabbard GO. Deconstructing the “med check.” Psychiatric Times. September 3, 2009. Available at: http://www.psychiatrictimes.com/display/article/10168/1444238. Accessed April 28, 2010.
4. Pies RW. Psychiatrists, physicians, and the prescriptive bond. Psychiatric Times. April 16, 2010. Available at: http://www.psychiatrictimes.com/blog/couchincrisis/content/article/10168/1555057. Accessed April 28, 2010.
5. Carlat DJ. Unhinged: the trouble with psychiatry—a doctor’s revelations about a profession in crisis. New York, NY: Free Press; 2010.
6. Nemeroff CB. The myth of the med check in psychopharmacology. Presented at: Presidential Symposium, Annual Meeting of the American Psychiatric Association; May 7, 2008; Washington, DC.
7. Rush W, Gochfeli L, Minkov K, et al. Medication visits: visit time and quality—the connection. Compliance Watch. 2009;2(2):13-15.
8. Fine P. Psychodynamic psychiatry in community settings. J Am Acad Psychoanal Dyn Psychiatry. 2007;35:431-441.
9. Sherman C. Don’t forget therapeutic skills even during a “med check.” Clinical Psychiatry News. 2002;30(7):390.-Available at: http://findarticles.com/p/articles/mi_hb4345/is_7_30/ai_n28933329. Accessed April 28, 2010.
10. Ackerman SJ, Hilsenroth MJ. A review of therapist characteristics and techniques positively impacting the therapeutic alliance. Clinical Psychology Rev. 2003;23:1-33.
11. Guggenheim FG. Prime time: maximizing the therapeutic experience—a primer for psychiatric clinicians. New York, NY: Routledge; 2009.
12. Saks ER. The center cannot hold: my journey through madness. New York, NY: Hyperion; 2007.
13. Pincus HA, Tanielian TL, Marcus SC, et al. Prescribing trends in psychotropic medications: primary care, psychiatry, and other medical specialties. JAMA. 1998;279(7):526-531.
14. Harman JS, Veazie PJ, Lyness JM. Primary care physician office visits for depression by older Americans. J Gen Intern Med. 2006;21:926-930.
15. Chen LM, Farwell WR, Jha AK. Primary care visit duration and quality: does good care take longer? Arch Intern Med. 2009;169:1866-1872.
16. Gilchrist VJ, Stange KC, Flocke SA, et al. A comparison of the National Ambulatory Medical Care Survey (NAMCS) measurement approach with direct observation of outpatient visits. Med Care. 2004;42(3):276-280.
17. Moffic HS. Make the most of the “15-minute med-check.” Current Psychiatry. 2006;5(9):116.-
18. Mossman D. Tips to make documentation easier, faster, and more satisfying. Current Psychiatry. 2008;7(2):84-86.
How to reduce malpractice risk with better documentation.
Tips to make documentation easier, faster, and more satisfying” (Current Psychiatry, February 2008), I discussed documentation techniques at length. Table 3 reprints principles that may be especially helpful in practices that consist primarily of med checks.
Table 3
Keys to better documentation
| Technique | Benefits |
|---|---|
| Time and date your notes | After an adverse event, establish when you saw the patient, recorded findings, wrote orders, reviewed lab results, or discussed problems with others can make a big difference in how your care is viewed |
| Sooner is better | Charting completed long after an adverse event occurred is vulnerable to accusations of fabrication |
| Brief quotes | Verbatim statements (‘I’ve never considered suicide’) quickly convey key factors in your therapeutic decision |
| Dictate or use speech recognition software | You speak faster than you write allowing you to document more |
| Provide handouts | Patients often do not remember or understand much of medication instructions doctors tell them |
| Use rating scales | Record more information in a scientifically validated format |
| Try macros and templates | These reduce documentation time and help you remember to cover everything you should |
| Source: Adapted from reference 18 | |
Acknowledgment
Thanks to James Knoll IV, MD for his helpful input on this article.
How to reduce malpractice risk with better documentation.
Tips to make documentation easier, faster, and more satisfying” (Current Psychiatry, February 2008), I discussed documentation techniques at length. Table 3 reprints principles that may be especially helpful in practices that consist primarily of med checks.
Table 3
Keys to better documentation
| Technique | Benefits |
|---|---|
| Time and date your notes | After an adverse event, establish when you saw the patient, recorded findings, wrote orders, reviewed lab results, or discussed problems with others can make a big difference in how your care is viewed |
| Sooner is better | Charting completed long after an adverse event occurred is vulnerable to accusations of fabrication |
| Brief quotes | Verbatim statements (‘I’ve never considered suicide’) quickly convey key factors in your therapeutic decision |
| Dictate or use speech recognition software | You speak faster than you write allowing you to document more |
| Provide handouts | Patients often do not remember or understand much of medication instructions doctors tell them |
| Use rating scales | Record more information in a scientifically validated format |
| Try macros and templates | These reduce documentation time and help you remember to cover everything you should |
| Source: Adapted from reference 18 | |
Acknowledgment
Thanks to James Knoll IV, MD for his helpful input on this article.
1. Mojtabai R, Olfson M. National trends in psychotherapy by office-based psychiatrists. Arch Gen Psychiatry. 2008;65:962-970.
2. Lewis MH, Gohagan JK, Merenstein DJ. The locality rule and the physician’s dilemma: local medical practices vs the national standard of care. JAMA. 2007;297(23):2633-2637.
3. Gabbard GO. Deconstructing the “med check.” Psychiatric Times. September 3, 2009. Available at: http://www.psychiatrictimes.com/display/article/10168/1444238. Accessed April 28, 2010.
4. Pies RW. Psychiatrists, physicians, and the prescriptive bond. Psychiatric Times. April 16, 2010. Available at: http://www.psychiatrictimes.com/blog/couchincrisis/content/article/10168/1555057. Accessed April 28, 2010.
5. Carlat DJ. Unhinged: the trouble with psychiatry—a doctor’s revelations about a profession in crisis. New York, NY: Free Press; 2010.
6. Nemeroff CB. The myth of the med check in psychopharmacology. Presented at: Presidential Symposium, Annual Meeting of the American Psychiatric Association; May 7, 2008; Washington, DC.
7. Rush W, Gochfeli L, Minkov K, et al. Medication visits: visit time and quality—the connection. Compliance Watch. 2009;2(2):13-15.
8. Fine P. Psychodynamic psychiatry in community settings. J Am Acad Psychoanal Dyn Psychiatry. 2007;35:431-441.
9. Sherman C. Don’t forget therapeutic skills even during a “med check.” Clinical Psychiatry News. 2002;30(7):390.-Available at: http://findarticles.com/p/articles/mi_hb4345/is_7_30/ai_n28933329. Accessed April 28, 2010.
10. Ackerman SJ, Hilsenroth MJ. A review of therapist characteristics and techniques positively impacting the therapeutic alliance. Clinical Psychology Rev. 2003;23:1-33.
11. Guggenheim FG. Prime time: maximizing the therapeutic experience—a primer for psychiatric clinicians. New York, NY: Routledge; 2009.
12. Saks ER. The center cannot hold: my journey through madness. New York, NY: Hyperion; 2007.
13. Pincus HA, Tanielian TL, Marcus SC, et al. Prescribing trends in psychotropic medications: primary care, psychiatry, and other medical specialties. JAMA. 1998;279(7):526-531.
14. Harman JS, Veazie PJ, Lyness JM. Primary care physician office visits for depression by older Americans. J Gen Intern Med. 2006;21:926-930.
15. Chen LM, Farwell WR, Jha AK. Primary care visit duration and quality: does good care take longer? Arch Intern Med. 2009;169:1866-1872.
16. Gilchrist VJ, Stange KC, Flocke SA, et al. A comparison of the National Ambulatory Medical Care Survey (NAMCS) measurement approach with direct observation of outpatient visits. Med Care. 2004;42(3):276-280.
17. Moffic HS. Make the most of the “15-minute med-check.” Current Psychiatry. 2006;5(9):116.-
18. Mossman D. Tips to make documentation easier, faster, and more satisfying. Current Psychiatry. 2008;7(2):84-86.
1. Mojtabai R, Olfson M. National trends in psychotherapy by office-based psychiatrists. Arch Gen Psychiatry. 2008;65:962-970.
2. Lewis MH, Gohagan JK, Merenstein DJ. The locality rule and the physician’s dilemma: local medical practices vs the national standard of care. JAMA. 2007;297(23):2633-2637.
3. Gabbard GO. Deconstructing the “med check.” Psychiatric Times. September 3, 2009. Available at: http://www.psychiatrictimes.com/display/article/10168/1444238. Accessed April 28, 2010.
4. Pies RW. Psychiatrists, physicians, and the prescriptive bond. Psychiatric Times. April 16, 2010. Available at: http://www.psychiatrictimes.com/blog/couchincrisis/content/article/10168/1555057. Accessed April 28, 2010.
5. Carlat DJ. Unhinged: the trouble with psychiatry—a doctor’s revelations about a profession in crisis. New York, NY: Free Press; 2010.
6. Nemeroff CB. The myth of the med check in psychopharmacology. Presented at: Presidential Symposium, Annual Meeting of the American Psychiatric Association; May 7, 2008; Washington, DC.
7. Rush W, Gochfeli L, Minkov K, et al. Medication visits: visit time and quality—the connection. Compliance Watch. 2009;2(2):13-15.
8. Fine P. Psychodynamic psychiatry in community settings. J Am Acad Psychoanal Dyn Psychiatry. 2007;35:431-441.
9. Sherman C. Don’t forget therapeutic skills even during a “med check.” Clinical Psychiatry News. 2002;30(7):390.-Available at: http://findarticles.com/p/articles/mi_hb4345/is_7_30/ai_n28933329. Accessed April 28, 2010.
10. Ackerman SJ, Hilsenroth MJ. A review of therapist characteristics and techniques positively impacting the therapeutic alliance. Clinical Psychology Rev. 2003;23:1-33.
11. Guggenheim FG. Prime time: maximizing the therapeutic experience—a primer for psychiatric clinicians. New York, NY: Routledge; 2009.
12. Saks ER. The center cannot hold: my journey through madness. New York, NY: Hyperion; 2007.
13. Pincus HA, Tanielian TL, Marcus SC, et al. Prescribing trends in psychotropic medications: primary care, psychiatry, and other medical specialties. JAMA. 1998;279(7):526-531.
14. Harman JS, Veazie PJ, Lyness JM. Primary care physician office visits for depression by older Americans. J Gen Intern Med. 2006;21:926-930.
15. Chen LM, Farwell WR, Jha AK. Primary care visit duration and quality: does good care take longer? Arch Intern Med. 2009;169:1866-1872.
16. Gilchrist VJ, Stange KC, Flocke SA, et al. A comparison of the National Ambulatory Medical Care Survey (NAMCS) measurement approach with direct observation of outpatient visits. Med Care. 2004;42(3):276-280.
17. Moffic HS. Make the most of the “15-minute med-check.” Current Psychiatry. 2006;5(9):116.-
18. Mossman D. Tips to make documentation easier, faster, and more satisfying. Current Psychiatry. 2008;7(2):84-86.
The manipulative self-harmer
CASE: Self-destructive behaviors
After being acquitted of 4 counts of second-degree forgery for writing checks from her mother’s bank account, Ms. L, age 52, is sent to the state hospital for a forensic examination to determine competency. Two years later she is granted conditional release from the hospital, transferred to our not-for-profit community mental health center, and enrolled in an intensive inpatient treatment program to monitor forensic patients. She is legally required to comply with treatment recommendations.
At admission, Ms. L is diagnosed with major depression, recurrent, and borderline personality disorder (BPD). She has no history of antisocial behavior or criminal acts other than forging checks and has never spent time in prison, which makes it unlikely she has co morbid antisocial personality disorder (Table 1).1
Over the next 5 years Ms. L tests limits with the treatment team and acts out by engaging in self-harming behaviors. In 1 instance, she cuts her forearm deeply, stuffs the wound with mayonnaise and paper towels, and wraps her arm with a bandage. She wears a long-sleeved shirt to hide her wound, which is not discovered until a severe infection develops.
Ms. L has difficulty with coping skills and interpersonal relationships. She approaches others with ambivalence and mistrust and consistently expects them to demean or take advantage of her. Ms. L is manipulative, at times injuring herself after perceived wrongdoings by staff. For example, after her therapist reschedules a meeting because of an emergency, Ms. L pours scalding water on her foot.
Table 1
Cluster B personality disorders: Differential diagnosis
| Diagnosis | Features |
|---|---|
| Borderline personality disorder | Self-destructiveness, angry disruptions in close relationships, and chronic feelings of deep emptiness and loneliness |
| Histrionic personality disorder | Attention seeking, manipulative behavior, and rapidly shifting emotions |
| Antisocial personality disorder | Manipulative to gain profit, power, or other material gratification |
| Source: Reference 1 | |
The authors’ observations
Ms. L consistently displays 3 common constructs of BPD:
- primitive defense mechanisms
- identity diffusion
- generally intact reality testing.2
Defense mechanisms are psychological attempts to deal with intrapsychic stress. Splitting—vacillating between extremes of idealization and devaluation—is a fundamental primitive defense mechanism that is the root of BPD.2 Identity diffusion causes confusion about life goals and values and feelings of boredom and emptiness. This internal world leads a patient to have the same perception of the external world, which explains many symptoms of BPD, such as rapidly shifting moods, intense anger, lack of clear sense of self, fear of abandonment, and unstable and intense interpersonal relationships.2
Early in treatment, Ms. L had difficulty breaking a cycle of self-defeating behavior, such as destroying personal items, trying to hang herself, and gluing an ear plug in her ear. During an argument with a staff member, Ms. L punched a wall and fractured her left hand. BPD patients sometimes will “up the ante” when acting out. For example, one of our patients claimed to have planted a bomb in an elementary school and another swallowed inedible objects, including spoons, forks, and butter knives. In Ms. L’s case, we addressed her self-harm behavior by helping her:
- develop less destructive coping skills such as drawing or painting
- identify irrational thoughts that contribute to self harm.
HISTORY: Troubled past
Raised by her biologic parents, Ms. L met all developmental milestones. She denies a history of childhood abuse but reports experiencing “depression and memory loss” and relationship problems with her parents during adolescence. As a child she often missed school because she “did not want anyone to know what a disgusting person I was” and “I should have my head cut open and cut into little pieces for thinking such mean thoughts.” Ms. L dropped out of school in the twelfth grade but obtained her general educational development certificate.
Notes and letters Ms. L wrote while in treatment consistently refer to her negative self-image. Ms. L writes that she feels she does not deserve to “be a part of this world,” is “never good enough for anyone,” and “should be thrown away with the garbage.”
Ms. L vacillates between desiring a closer relationship with her parents, especially her mother, and wanting to “cut them out of my life for good.” She has minimal contact with her older sister. Ms. L is divorced and has 2 adult sons. She was involved sporadically in her sons’ lives when they were children, but now has no contact with them.
BPD and crime
Ms. L is enrolled in the “911 program,” which monitors individuals who have been found not guilty by reason of mental defect. Individuals with BPD often are convicted of serious and violent crimes, which may be because of BPD features such as interpersonal hostility and self-harm. Impulsivity, substance abuse, and parental neglect—all of which are associated with BPD—can increase risk of criminality.3 There is no evidence to suggest a direct link between BPD and criminality; however, over-representation of BPD in prison populations suggest that in severe cases it may increase criminogenic risk.1,3
TREATMENT: Worsened depression
When Ms. L arrives at our facility, her medication regimen includes fluoxetine, 80 mg/d, risperidone, 2 mg/d, and buspirone, 20 mg/d. Risperidone and buspirone are discontinued because of perceived lack of efficacy. Venlafaxine XR is added and titrated to 300 mg/d, and Ms. L receives lorazepam, 1 and 2 mg as needed. However, lorazepam carries risks because impulsivity and impaired judgment—which are common in BPD—can lead to dependence and abuse. We feel that in a supervised setting the risks can be managed.
Recently, staff witnessed Ms. L experiencing an episode that appeared to be a grand mal seizure. After Ms. L is evaluated at the local emergency room, her EEG is normal, but a neurologic consult recommends discontinuing fluoxetine or venlafaxine XR because they may have contributed to the seizure. We taper and discontinue venlafaxine XR but Ms. L complains bitterly that she is getting increasingly depressed. On several occasions she attempts to pit team members against each other.
Ms. L falls, injures her back, and begins to abuse opiates. After her prescription runs out, she obtains more from an intellectually limited patient in her treatment program. Ms. L says she is getting more depressed, threatens suicide, and is placed in a more restrictive in-patient setting. We consider adding pregabalin to address her pain and help with anxiety and impulse control but the consulting neurologist prescribes carbamazepine, 400 mg/d, and her pain improves.5,6
The authors’ observations
BPD treatment primarily is psychotherapeutic and emphasizes skill building (Table 2) with focused, symptom-targeted pharmacotherapy as indicated.4 Pharmacotherapy typically targets 3 domains:
- affective dysregulation
- impulsive-behavioral dyscontrol symptoms
- cognitive-perceptual symptoms.
Patients with prominent anxiety may benefit from benzodiazepines, although research on these agents for BPD is limited. Recent studies show efficacy with fluoxetine, olanzapine, or a combination of both,7 and divalproex.8 Preliminary data supports the use of topiramate, quetiapine, risperidone, ziprasidone, lamotrigine, and clonidine (Table 3).9-14 A recent review and meta-analysis showed efficacy with topira-mate, lamotrigine, valproate, aripiprazole, and olanzapine.15
For Ms. L, we restart venlafaxine at a lower dose of 50 mg/d and titrate it to 150 mg/d, which is still lower than her previous dose of 300 mg/d. She has no recurrence of seizures and her depression improves.
Table 2
Features of psychotherapeutic modalities for BPD
| Description | Mode of treatment | Skills taught | |
|---|---|---|---|
| Dialectical behavior therapy | Manualized, time-limited, cognitive-behavioral approach based on the biosocial theory of BPD | Individual therapy, group skills training, telephone contact, and therapist consultation | Core mindfulness skills, interpersonal effectiveness skills, emotion modulation skills, and distress tolerance skills |
| Systems Training for Emotional Predictability and Problem Solving | Manual-based, group treatment that includes a systems component to train family members, friends, and significant others | 20-week basic skills group and a 1-year, twice-monthly advanced group program; utilizes a classroom ‘seminar’ format | Awareness of illness, emotion management skills, and behavior management skills |
| BPD: borderline personality disorder | |||
Table 3
Pharmacotherapy for BPD: What the evidence says
| Study | Design | Results |
|---|---|---|
| Hollander et al, 20039 | 96 patients with Cluster B personality disorders randomized to divalproex or placebo for 12 weeks | Divalproex was superior to placebo in treating impulsive aggression, irritability, and global severity |
| Hilger et al, 200310 | Case report of 2 women with BPD and severe self-mutilation receiving quetiapine monotherapy | Quetiapine resulted in a marked improvement of impulsive behavior and overall level of function |
| Rizvi, 200211 | Case report of a 14-year-old female with borderline personality traits admitted to an inpatient facility for suicide attempt, impulsive behavior, and mood lability. Lamotrigine was started at 25 mg/d and titrated to 200 mg/d. At admission, she was receiving clonazepam, valproic acid, quetiapine, and fluoxetine, which were tapered and discontinued | Over 6 months of inpatient treatment, suicidal behavior and ideation diminished and impulse control and mood lability improved; continued improvement at 1-year follow up |
| Rocca et al, 200212 | 15 BPD outpatients with aggressive behavior given risperidone (mean dose 3.27 mg/d) in an 8-week open-label study | Risperidone produced a significant reduction in aggression based on AQ scores, reduction in depressive symptoms, and an increase in energy and global functioning |
| Philipsen et al, 200413 | 14 women with BPD given oral clonidine, 75 and 150 µg, while experiencing strong aversive inner tension and urge to commit self-injury | Clonidine significantly decreased aversive inner tension, dissociative symptoms, and urge to commit self-injury as measured by self rated scales |
| Pascual et al, 200414 | A 2-week open-label study of 10 females and 2 males presenting to psychiatric emergency service for self-injurious behavior, aggression/hostility, loss of impulse control, and severe anxiety/depressive symptoms received IM ziprasidone, 20 mg, followed by flexible oral dosing between 40 mg/d and 160 mg/d | 9 patients who completed the study showed statistically significant improvements on CGI-S, HAM-D-17, HAM-A, BPRS, and BIS |
| AQ: Aggression Questionnaire; BIS: Barratt Impulsiveness Scale; BPD: borderline personality disorder; BPRS: Brief Psychiatric Rating Scale; CGI-S: Clinical Global Impressions-Severity of Illness; HAM-A: Hamilton Anxiety Rating scale; HAM-D-17: 17-item Hamilton Depression Rating scale | ||
OUTCOME: Some improvement
Ms. L has no dramatic suicidal gestures for 3 years. Although she continues to engage in self-injurious behaviors, the intensity and frequency are reduced and she does not inflict any serious injury for 18 months. Her mood and behavior continue to oscillate; she is relatively calm and satisfied 1 week, angry and assaultive the next. This stormy course is expected given her BPD diagnosis.
Initially, Ms. L resided in a locked residential unit and was minimally compliant with treatment recommendations and unit policies. As treatment progressed she moved to a different locked unit and eventually to an apartment. Recently, she was placed in a more restrictive setting because her hostile and self-destructive behavior escalated.
The authors’ observations
Ms. L is no different from most Axis II Cluster B disordered patients. During treatment she shows improvement by refraining from self-destructive behaviors for up to 18 months, but she then briefly reverts back to maladaptive behaviors. Ms. L resides in a very structured treatment setting. It is not clear if the gains she made in treatment would have been possible if she was living on her own in the community.
One year after finishing the court-mandated “911 program,” Ms. L lives in the community, draws and paints quite well, attends weekly individual and group therapy, and refrains from self-mutilation. She still experiences volatile moods, but can handle them without inflicting self injury.
Related resources
- Oldham JM. Guideline watch: practice guideline for the treatment of patients with borderline personality disorder. Arlington, VA: American Psychiatric Association; 2005. www.psychiatryonline.com/content.aspx?aID=148722.
- Koenigsberg HW, Kernberg OF, Stone MH, et al. Borderline patients: extending the limits of treatability. New York, NY: Basic Books; 2000.
Drug brand names
- Aripiprazole • Abilify
- Buspirone • Buspar
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Divalproex • Depakote
- Fluoxetine • Prozac
- Fluoxetine-olanzapine • Symbyax
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Pregabalin • Lyrica
- Risperidone • Risperdal
- Topiramate • Topamax
- Valproic acid • Depakene
- Venlafaxine XR • Effexor XR
- Ziprasidone • Geodon
Disclosures
Dr. Hashmi is on the speakers bureau for AstraZeneca, Eli Lilly and Company, and Janssen.
Dr. Vowell reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
2. Koenigsberg HW, Kernberg OF, Stone MH, et al. Borderline patients: extending the limits of treatability. New York, NY: Basic Books; 2000.
3. Nee C, Farman S. Female prisoners with borderline personality disorder: some promising treatment developments. Crim Behav Ment Health. 2005;15:2-16.
4. Oldham JM, Bender DS, Skodol AE, et al. Testing an APA practice guideline: symptom-targeted medication utilization for patients with borderline personality disorder. J Psychiatr Pract. 2004;10:156-161.
5. American Psychiatric Association Practice Guidelines. Practice guideline for the treatment of patients with borderline personality disorder. Am J Psychiatry. 2001;158(suppl 10):1-52.
6. Yatham LN. Newer anticonvulsants in the treatment of bipolar disorder. J Clin Psychiatry. 2004;65(suppl 10):28-35.
7. Rinne T, van den Brink W, Wouters L, et al. SSRI treatment of borderline personality disorder: a randomized, placebo-controlled clinical trial for female patients with borderline personality disorder. Am J Psychiatry. 2002;159(12):2048-2054.
8. Zanarini MC, Frankenburg FR, Parachini EA. A preliminary, randomized trial of fluoxetine, olanzapine, and the olanzapine-fluoxetine combination in women with borderline personality disorder. J Clin Psychiatry. 2004;65(7):903-907.
9. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186-1197.
10. Hilger E, Barnas C, Kasper S. Quetiapine in the treatment of borderline personality disorder. World J Biol Psychiatry. 2003;4(1):42-44.
11. Rizvi ST. Lamotrigine and borderline personality disorder. J Child Adolesc Psychopharmacol. 2002;12(4):365-366.
12. Rocca P, Marchiaro L, Cocuzza E, et al. Treatment of borderline personality disorder with risperidone. J Clin Psychiatry. 2002;63(3):241-244.
13. Philipsen A, Richter H, Schmahl C, et al. Clonidine in acute aversive inner tension and self-injurious behavior in female patients with borderline personality disorder. J Clin Psychiatry. 2004;65(10):1414-1419.
14. Pascual JC, Oller S, Soler J, et al. Ziprasidone in the acute treatment of borderline personality disorder in psychiatric emergency services. J Clin Psychiatry. 2004;65(9):1281-1282.
15. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
CASE: Self-destructive behaviors
After being acquitted of 4 counts of second-degree forgery for writing checks from her mother’s bank account, Ms. L, age 52, is sent to the state hospital for a forensic examination to determine competency. Two years later she is granted conditional release from the hospital, transferred to our not-for-profit community mental health center, and enrolled in an intensive inpatient treatment program to monitor forensic patients. She is legally required to comply with treatment recommendations.
At admission, Ms. L is diagnosed with major depression, recurrent, and borderline personality disorder (BPD). She has no history of antisocial behavior or criminal acts other than forging checks and has never spent time in prison, which makes it unlikely she has co morbid antisocial personality disorder (Table 1).1
Over the next 5 years Ms. L tests limits with the treatment team and acts out by engaging in self-harming behaviors. In 1 instance, she cuts her forearm deeply, stuffs the wound with mayonnaise and paper towels, and wraps her arm with a bandage. She wears a long-sleeved shirt to hide her wound, which is not discovered until a severe infection develops.
Ms. L has difficulty with coping skills and interpersonal relationships. She approaches others with ambivalence and mistrust and consistently expects them to demean or take advantage of her. Ms. L is manipulative, at times injuring herself after perceived wrongdoings by staff. For example, after her therapist reschedules a meeting because of an emergency, Ms. L pours scalding water on her foot.
Table 1
Cluster B personality disorders: Differential diagnosis
| Diagnosis | Features |
|---|---|
| Borderline personality disorder | Self-destructiveness, angry disruptions in close relationships, and chronic feelings of deep emptiness and loneliness |
| Histrionic personality disorder | Attention seeking, manipulative behavior, and rapidly shifting emotions |
| Antisocial personality disorder | Manipulative to gain profit, power, or other material gratification |
| Source: Reference 1 | |
The authors’ observations
Ms. L consistently displays 3 common constructs of BPD:
- primitive defense mechanisms
- identity diffusion
- generally intact reality testing.2
Defense mechanisms are psychological attempts to deal with intrapsychic stress. Splitting—vacillating between extremes of idealization and devaluation—is a fundamental primitive defense mechanism that is the root of BPD.2 Identity diffusion causes confusion about life goals and values and feelings of boredom and emptiness. This internal world leads a patient to have the same perception of the external world, which explains many symptoms of BPD, such as rapidly shifting moods, intense anger, lack of clear sense of self, fear of abandonment, and unstable and intense interpersonal relationships.2
Early in treatment, Ms. L had difficulty breaking a cycle of self-defeating behavior, such as destroying personal items, trying to hang herself, and gluing an ear plug in her ear. During an argument with a staff member, Ms. L punched a wall and fractured her left hand. BPD patients sometimes will “up the ante” when acting out. For example, one of our patients claimed to have planted a bomb in an elementary school and another swallowed inedible objects, including spoons, forks, and butter knives. In Ms. L’s case, we addressed her self-harm behavior by helping her:
- develop less destructive coping skills such as drawing or painting
- identify irrational thoughts that contribute to self harm.
HISTORY: Troubled past
Raised by her biologic parents, Ms. L met all developmental milestones. She denies a history of childhood abuse but reports experiencing “depression and memory loss” and relationship problems with her parents during adolescence. As a child she often missed school because she “did not want anyone to know what a disgusting person I was” and “I should have my head cut open and cut into little pieces for thinking such mean thoughts.” Ms. L dropped out of school in the twelfth grade but obtained her general educational development certificate.
Notes and letters Ms. L wrote while in treatment consistently refer to her negative self-image. Ms. L writes that she feels she does not deserve to “be a part of this world,” is “never good enough for anyone,” and “should be thrown away with the garbage.”
Ms. L vacillates between desiring a closer relationship with her parents, especially her mother, and wanting to “cut them out of my life for good.” She has minimal contact with her older sister. Ms. L is divorced and has 2 adult sons. She was involved sporadically in her sons’ lives when they were children, but now has no contact with them.
BPD and crime
Ms. L is enrolled in the “911 program,” which monitors individuals who have been found not guilty by reason of mental defect. Individuals with BPD often are convicted of serious and violent crimes, which may be because of BPD features such as interpersonal hostility and self-harm. Impulsivity, substance abuse, and parental neglect—all of which are associated with BPD—can increase risk of criminality.3 There is no evidence to suggest a direct link between BPD and criminality; however, over-representation of BPD in prison populations suggest that in severe cases it may increase criminogenic risk.1,3
TREATMENT: Worsened depression
When Ms. L arrives at our facility, her medication regimen includes fluoxetine, 80 mg/d, risperidone, 2 mg/d, and buspirone, 20 mg/d. Risperidone and buspirone are discontinued because of perceived lack of efficacy. Venlafaxine XR is added and titrated to 300 mg/d, and Ms. L receives lorazepam, 1 and 2 mg as needed. However, lorazepam carries risks because impulsivity and impaired judgment—which are common in BPD—can lead to dependence and abuse. We feel that in a supervised setting the risks can be managed.
Recently, staff witnessed Ms. L experiencing an episode that appeared to be a grand mal seizure. After Ms. L is evaluated at the local emergency room, her EEG is normal, but a neurologic consult recommends discontinuing fluoxetine or venlafaxine XR because they may have contributed to the seizure. We taper and discontinue venlafaxine XR but Ms. L complains bitterly that she is getting increasingly depressed. On several occasions she attempts to pit team members against each other.
Ms. L falls, injures her back, and begins to abuse opiates. After her prescription runs out, she obtains more from an intellectually limited patient in her treatment program. Ms. L says she is getting more depressed, threatens suicide, and is placed in a more restrictive in-patient setting. We consider adding pregabalin to address her pain and help with anxiety and impulse control but the consulting neurologist prescribes carbamazepine, 400 mg/d, and her pain improves.5,6
The authors’ observations
BPD treatment primarily is psychotherapeutic and emphasizes skill building (Table 2) with focused, symptom-targeted pharmacotherapy as indicated.4 Pharmacotherapy typically targets 3 domains:
- affective dysregulation
- impulsive-behavioral dyscontrol symptoms
- cognitive-perceptual symptoms.
Patients with prominent anxiety may benefit from benzodiazepines, although research on these agents for BPD is limited. Recent studies show efficacy with fluoxetine, olanzapine, or a combination of both,7 and divalproex.8 Preliminary data supports the use of topiramate, quetiapine, risperidone, ziprasidone, lamotrigine, and clonidine (Table 3).9-14 A recent review and meta-analysis showed efficacy with topira-mate, lamotrigine, valproate, aripiprazole, and olanzapine.15
For Ms. L, we restart venlafaxine at a lower dose of 50 mg/d and titrate it to 150 mg/d, which is still lower than her previous dose of 300 mg/d. She has no recurrence of seizures and her depression improves.
Table 2
Features of psychotherapeutic modalities for BPD
| Description | Mode of treatment | Skills taught | |
|---|---|---|---|
| Dialectical behavior therapy | Manualized, time-limited, cognitive-behavioral approach based on the biosocial theory of BPD | Individual therapy, group skills training, telephone contact, and therapist consultation | Core mindfulness skills, interpersonal effectiveness skills, emotion modulation skills, and distress tolerance skills |
| Systems Training for Emotional Predictability and Problem Solving | Manual-based, group treatment that includes a systems component to train family members, friends, and significant others | 20-week basic skills group and a 1-year, twice-monthly advanced group program; utilizes a classroom ‘seminar’ format | Awareness of illness, emotion management skills, and behavior management skills |
| BPD: borderline personality disorder | |||
Table 3
Pharmacotherapy for BPD: What the evidence says
| Study | Design | Results |
|---|---|---|
| Hollander et al, 20039 | 96 patients with Cluster B personality disorders randomized to divalproex or placebo for 12 weeks | Divalproex was superior to placebo in treating impulsive aggression, irritability, and global severity |
| Hilger et al, 200310 | Case report of 2 women with BPD and severe self-mutilation receiving quetiapine monotherapy | Quetiapine resulted in a marked improvement of impulsive behavior and overall level of function |
| Rizvi, 200211 | Case report of a 14-year-old female with borderline personality traits admitted to an inpatient facility for suicide attempt, impulsive behavior, and mood lability. Lamotrigine was started at 25 mg/d and titrated to 200 mg/d. At admission, she was receiving clonazepam, valproic acid, quetiapine, and fluoxetine, which were tapered and discontinued | Over 6 months of inpatient treatment, suicidal behavior and ideation diminished and impulse control and mood lability improved; continued improvement at 1-year follow up |
| Rocca et al, 200212 | 15 BPD outpatients with aggressive behavior given risperidone (mean dose 3.27 mg/d) in an 8-week open-label study | Risperidone produced a significant reduction in aggression based on AQ scores, reduction in depressive symptoms, and an increase in energy and global functioning |
| Philipsen et al, 200413 | 14 women with BPD given oral clonidine, 75 and 150 µg, while experiencing strong aversive inner tension and urge to commit self-injury | Clonidine significantly decreased aversive inner tension, dissociative symptoms, and urge to commit self-injury as measured by self rated scales |
| Pascual et al, 200414 | A 2-week open-label study of 10 females and 2 males presenting to psychiatric emergency service for self-injurious behavior, aggression/hostility, loss of impulse control, and severe anxiety/depressive symptoms received IM ziprasidone, 20 mg, followed by flexible oral dosing between 40 mg/d and 160 mg/d | 9 patients who completed the study showed statistically significant improvements on CGI-S, HAM-D-17, HAM-A, BPRS, and BIS |
| AQ: Aggression Questionnaire; BIS: Barratt Impulsiveness Scale; BPD: borderline personality disorder; BPRS: Brief Psychiatric Rating Scale; CGI-S: Clinical Global Impressions-Severity of Illness; HAM-A: Hamilton Anxiety Rating scale; HAM-D-17: 17-item Hamilton Depression Rating scale | ||
OUTCOME: Some improvement
Ms. L has no dramatic suicidal gestures for 3 years. Although she continues to engage in self-injurious behaviors, the intensity and frequency are reduced and she does not inflict any serious injury for 18 months. Her mood and behavior continue to oscillate; she is relatively calm and satisfied 1 week, angry and assaultive the next. This stormy course is expected given her BPD diagnosis.
Initially, Ms. L resided in a locked residential unit and was minimally compliant with treatment recommendations and unit policies. As treatment progressed she moved to a different locked unit and eventually to an apartment. Recently, she was placed in a more restrictive setting because her hostile and self-destructive behavior escalated.
The authors’ observations
Ms. L is no different from most Axis II Cluster B disordered patients. During treatment she shows improvement by refraining from self-destructive behaviors for up to 18 months, but she then briefly reverts back to maladaptive behaviors. Ms. L resides in a very structured treatment setting. It is not clear if the gains she made in treatment would have been possible if she was living on her own in the community.
One year after finishing the court-mandated “911 program,” Ms. L lives in the community, draws and paints quite well, attends weekly individual and group therapy, and refrains from self-mutilation. She still experiences volatile moods, but can handle them without inflicting self injury.
Related resources
- Oldham JM. Guideline watch: practice guideline for the treatment of patients with borderline personality disorder. Arlington, VA: American Psychiatric Association; 2005. www.psychiatryonline.com/content.aspx?aID=148722.
- Koenigsberg HW, Kernberg OF, Stone MH, et al. Borderline patients: extending the limits of treatability. New York, NY: Basic Books; 2000.
Drug brand names
- Aripiprazole • Abilify
- Buspirone • Buspar
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Divalproex • Depakote
- Fluoxetine • Prozac
- Fluoxetine-olanzapine • Symbyax
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Pregabalin • Lyrica
- Risperidone • Risperdal
- Topiramate • Topamax
- Valproic acid • Depakene
- Venlafaxine XR • Effexor XR
- Ziprasidone • Geodon
Disclosures
Dr. Hashmi is on the speakers bureau for AstraZeneca, Eli Lilly and Company, and Janssen.
Dr. Vowell reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Self-destructive behaviors
After being acquitted of 4 counts of second-degree forgery for writing checks from her mother’s bank account, Ms. L, age 52, is sent to the state hospital for a forensic examination to determine competency. Two years later she is granted conditional release from the hospital, transferred to our not-for-profit community mental health center, and enrolled in an intensive inpatient treatment program to monitor forensic patients. She is legally required to comply with treatment recommendations.
At admission, Ms. L is diagnosed with major depression, recurrent, and borderline personality disorder (BPD). She has no history of antisocial behavior or criminal acts other than forging checks and has never spent time in prison, which makes it unlikely she has co morbid antisocial personality disorder (Table 1).1
Over the next 5 years Ms. L tests limits with the treatment team and acts out by engaging in self-harming behaviors. In 1 instance, she cuts her forearm deeply, stuffs the wound with mayonnaise and paper towels, and wraps her arm with a bandage. She wears a long-sleeved shirt to hide her wound, which is not discovered until a severe infection develops.
Ms. L has difficulty with coping skills and interpersonal relationships. She approaches others with ambivalence and mistrust and consistently expects them to demean or take advantage of her. Ms. L is manipulative, at times injuring herself after perceived wrongdoings by staff. For example, after her therapist reschedules a meeting because of an emergency, Ms. L pours scalding water on her foot.
Table 1
Cluster B personality disorders: Differential diagnosis
| Diagnosis | Features |
|---|---|
| Borderline personality disorder | Self-destructiveness, angry disruptions in close relationships, and chronic feelings of deep emptiness and loneliness |
| Histrionic personality disorder | Attention seeking, manipulative behavior, and rapidly shifting emotions |
| Antisocial personality disorder | Manipulative to gain profit, power, or other material gratification |
| Source: Reference 1 | |
The authors’ observations
Ms. L consistently displays 3 common constructs of BPD:
- primitive defense mechanisms
- identity diffusion
- generally intact reality testing.2
Defense mechanisms are psychological attempts to deal with intrapsychic stress. Splitting—vacillating between extremes of idealization and devaluation—is a fundamental primitive defense mechanism that is the root of BPD.2 Identity diffusion causes confusion about life goals and values and feelings of boredom and emptiness. This internal world leads a patient to have the same perception of the external world, which explains many symptoms of BPD, such as rapidly shifting moods, intense anger, lack of clear sense of self, fear of abandonment, and unstable and intense interpersonal relationships.2
Early in treatment, Ms. L had difficulty breaking a cycle of self-defeating behavior, such as destroying personal items, trying to hang herself, and gluing an ear plug in her ear. During an argument with a staff member, Ms. L punched a wall and fractured her left hand. BPD patients sometimes will “up the ante” when acting out. For example, one of our patients claimed to have planted a bomb in an elementary school and another swallowed inedible objects, including spoons, forks, and butter knives. In Ms. L’s case, we addressed her self-harm behavior by helping her:
- develop less destructive coping skills such as drawing or painting
- identify irrational thoughts that contribute to self harm.
HISTORY: Troubled past
Raised by her biologic parents, Ms. L met all developmental milestones. She denies a history of childhood abuse but reports experiencing “depression and memory loss” and relationship problems with her parents during adolescence. As a child she often missed school because she “did not want anyone to know what a disgusting person I was” and “I should have my head cut open and cut into little pieces for thinking such mean thoughts.” Ms. L dropped out of school in the twelfth grade but obtained her general educational development certificate.
Notes and letters Ms. L wrote while in treatment consistently refer to her negative self-image. Ms. L writes that she feels she does not deserve to “be a part of this world,” is “never good enough for anyone,” and “should be thrown away with the garbage.”
Ms. L vacillates between desiring a closer relationship with her parents, especially her mother, and wanting to “cut them out of my life for good.” She has minimal contact with her older sister. Ms. L is divorced and has 2 adult sons. She was involved sporadically in her sons’ lives when they were children, but now has no contact with them.
BPD and crime
Ms. L is enrolled in the “911 program,” which monitors individuals who have been found not guilty by reason of mental defect. Individuals with BPD often are convicted of serious and violent crimes, which may be because of BPD features such as interpersonal hostility and self-harm. Impulsivity, substance abuse, and parental neglect—all of which are associated with BPD—can increase risk of criminality.3 There is no evidence to suggest a direct link between BPD and criminality; however, over-representation of BPD in prison populations suggest that in severe cases it may increase criminogenic risk.1,3
TREATMENT: Worsened depression
When Ms. L arrives at our facility, her medication regimen includes fluoxetine, 80 mg/d, risperidone, 2 mg/d, and buspirone, 20 mg/d. Risperidone and buspirone are discontinued because of perceived lack of efficacy. Venlafaxine XR is added and titrated to 300 mg/d, and Ms. L receives lorazepam, 1 and 2 mg as needed. However, lorazepam carries risks because impulsivity and impaired judgment—which are common in BPD—can lead to dependence and abuse. We feel that in a supervised setting the risks can be managed.
Recently, staff witnessed Ms. L experiencing an episode that appeared to be a grand mal seizure. After Ms. L is evaluated at the local emergency room, her EEG is normal, but a neurologic consult recommends discontinuing fluoxetine or venlafaxine XR because they may have contributed to the seizure. We taper and discontinue venlafaxine XR but Ms. L complains bitterly that she is getting increasingly depressed. On several occasions she attempts to pit team members against each other.
Ms. L falls, injures her back, and begins to abuse opiates. After her prescription runs out, she obtains more from an intellectually limited patient in her treatment program. Ms. L says she is getting more depressed, threatens suicide, and is placed in a more restrictive in-patient setting. We consider adding pregabalin to address her pain and help with anxiety and impulse control but the consulting neurologist prescribes carbamazepine, 400 mg/d, and her pain improves.5,6
The authors’ observations
BPD treatment primarily is psychotherapeutic and emphasizes skill building (Table 2) with focused, symptom-targeted pharmacotherapy as indicated.4 Pharmacotherapy typically targets 3 domains:
- affective dysregulation
- impulsive-behavioral dyscontrol symptoms
- cognitive-perceptual symptoms.
Patients with prominent anxiety may benefit from benzodiazepines, although research on these agents for BPD is limited. Recent studies show efficacy with fluoxetine, olanzapine, or a combination of both,7 and divalproex.8 Preliminary data supports the use of topiramate, quetiapine, risperidone, ziprasidone, lamotrigine, and clonidine (Table 3).9-14 A recent review and meta-analysis showed efficacy with topira-mate, lamotrigine, valproate, aripiprazole, and olanzapine.15
For Ms. L, we restart venlafaxine at a lower dose of 50 mg/d and titrate it to 150 mg/d, which is still lower than her previous dose of 300 mg/d. She has no recurrence of seizures and her depression improves.
Table 2
Features of psychotherapeutic modalities for BPD
| Description | Mode of treatment | Skills taught | |
|---|---|---|---|
| Dialectical behavior therapy | Manualized, time-limited, cognitive-behavioral approach based on the biosocial theory of BPD | Individual therapy, group skills training, telephone contact, and therapist consultation | Core mindfulness skills, interpersonal effectiveness skills, emotion modulation skills, and distress tolerance skills |
| Systems Training for Emotional Predictability and Problem Solving | Manual-based, group treatment that includes a systems component to train family members, friends, and significant others | 20-week basic skills group and a 1-year, twice-monthly advanced group program; utilizes a classroom ‘seminar’ format | Awareness of illness, emotion management skills, and behavior management skills |
| BPD: borderline personality disorder | |||
Table 3
Pharmacotherapy for BPD: What the evidence says
| Study | Design | Results |
|---|---|---|
| Hollander et al, 20039 | 96 patients with Cluster B personality disorders randomized to divalproex or placebo for 12 weeks | Divalproex was superior to placebo in treating impulsive aggression, irritability, and global severity |
| Hilger et al, 200310 | Case report of 2 women with BPD and severe self-mutilation receiving quetiapine monotherapy | Quetiapine resulted in a marked improvement of impulsive behavior and overall level of function |
| Rizvi, 200211 | Case report of a 14-year-old female with borderline personality traits admitted to an inpatient facility for suicide attempt, impulsive behavior, and mood lability. Lamotrigine was started at 25 mg/d and titrated to 200 mg/d. At admission, she was receiving clonazepam, valproic acid, quetiapine, and fluoxetine, which were tapered and discontinued | Over 6 months of inpatient treatment, suicidal behavior and ideation diminished and impulse control and mood lability improved; continued improvement at 1-year follow up |
| Rocca et al, 200212 | 15 BPD outpatients with aggressive behavior given risperidone (mean dose 3.27 mg/d) in an 8-week open-label study | Risperidone produced a significant reduction in aggression based on AQ scores, reduction in depressive symptoms, and an increase in energy and global functioning |
| Philipsen et al, 200413 | 14 women with BPD given oral clonidine, 75 and 150 µg, while experiencing strong aversive inner tension and urge to commit self-injury | Clonidine significantly decreased aversive inner tension, dissociative symptoms, and urge to commit self-injury as measured by self rated scales |
| Pascual et al, 200414 | A 2-week open-label study of 10 females and 2 males presenting to psychiatric emergency service for self-injurious behavior, aggression/hostility, loss of impulse control, and severe anxiety/depressive symptoms received IM ziprasidone, 20 mg, followed by flexible oral dosing between 40 mg/d and 160 mg/d | 9 patients who completed the study showed statistically significant improvements on CGI-S, HAM-D-17, HAM-A, BPRS, and BIS |
| AQ: Aggression Questionnaire; BIS: Barratt Impulsiveness Scale; BPD: borderline personality disorder; BPRS: Brief Psychiatric Rating Scale; CGI-S: Clinical Global Impressions-Severity of Illness; HAM-A: Hamilton Anxiety Rating scale; HAM-D-17: 17-item Hamilton Depression Rating scale | ||
OUTCOME: Some improvement
Ms. L has no dramatic suicidal gestures for 3 years. Although she continues to engage in self-injurious behaviors, the intensity and frequency are reduced and she does not inflict any serious injury for 18 months. Her mood and behavior continue to oscillate; she is relatively calm and satisfied 1 week, angry and assaultive the next. This stormy course is expected given her BPD diagnosis.
Initially, Ms. L resided in a locked residential unit and was minimally compliant with treatment recommendations and unit policies. As treatment progressed she moved to a different locked unit and eventually to an apartment. Recently, she was placed in a more restrictive setting because her hostile and self-destructive behavior escalated.
The authors’ observations
Ms. L is no different from most Axis II Cluster B disordered patients. During treatment she shows improvement by refraining from self-destructive behaviors for up to 18 months, but she then briefly reverts back to maladaptive behaviors. Ms. L resides in a very structured treatment setting. It is not clear if the gains she made in treatment would have been possible if she was living on her own in the community.
One year after finishing the court-mandated “911 program,” Ms. L lives in the community, draws and paints quite well, attends weekly individual and group therapy, and refrains from self-mutilation. She still experiences volatile moods, but can handle them without inflicting self injury.
Related resources
- Oldham JM. Guideline watch: practice guideline for the treatment of patients with borderline personality disorder. Arlington, VA: American Psychiatric Association; 2005. www.psychiatryonline.com/content.aspx?aID=148722.
- Koenigsberg HW, Kernberg OF, Stone MH, et al. Borderline patients: extending the limits of treatability. New York, NY: Basic Books; 2000.
Drug brand names
- Aripiprazole • Abilify
- Buspirone • Buspar
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Divalproex • Depakote
- Fluoxetine • Prozac
- Fluoxetine-olanzapine • Symbyax
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Pregabalin • Lyrica
- Risperidone • Risperdal
- Topiramate • Topamax
- Valproic acid • Depakene
- Venlafaxine XR • Effexor XR
- Ziprasidone • Geodon
Disclosures
Dr. Hashmi is on the speakers bureau for AstraZeneca, Eli Lilly and Company, and Janssen.
Dr. Vowell reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
2. Koenigsberg HW, Kernberg OF, Stone MH, et al. Borderline patients: extending the limits of treatability. New York, NY: Basic Books; 2000.
3. Nee C, Farman S. Female prisoners with borderline personality disorder: some promising treatment developments. Crim Behav Ment Health. 2005;15:2-16.
4. Oldham JM, Bender DS, Skodol AE, et al. Testing an APA practice guideline: symptom-targeted medication utilization for patients with borderline personality disorder. J Psychiatr Pract. 2004;10:156-161.
5. American Psychiatric Association Practice Guidelines. Practice guideline for the treatment of patients with borderline personality disorder. Am J Psychiatry. 2001;158(suppl 10):1-52.
6. Yatham LN. Newer anticonvulsants in the treatment of bipolar disorder. J Clin Psychiatry. 2004;65(suppl 10):28-35.
7. Rinne T, van den Brink W, Wouters L, et al. SSRI treatment of borderline personality disorder: a randomized, placebo-controlled clinical trial for female patients with borderline personality disorder. Am J Psychiatry. 2002;159(12):2048-2054.
8. Zanarini MC, Frankenburg FR, Parachini EA. A preliminary, randomized trial of fluoxetine, olanzapine, and the olanzapine-fluoxetine combination in women with borderline personality disorder. J Clin Psychiatry. 2004;65(7):903-907.
9. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186-1197.
10. Hilger E, Barnas C, Kasper S. Quetiapine in the treatment of borderline personality disorder. World J Biol Psychiatry. 2003;4(1):42-44.
11. Rizvi ST. Lamotrigine and borderline personality disorder. J Child Adolesc Psychopharmacol. 2002;12(4):365-366.
12. Rocca P, Marchiaro L, Cocuzza E, et al. Treatment of borderline personality disorder with risperidone. J Clin Psychiatry. 2002;63(3):241-244.
13. Philipsen A, Richter H, Schmahl C, et al. Clonidine in acute aversive inner tension and self-injurious behavior in female patients with borderline personality disorder. J Clin Psychiatry. 2004;65(10):1414-1419.
14. Pascual JC, Oller S, Soler J, et al. Ziprasidone in the acute treatment of borderline personality disorder in psychiatric emergency services. J Clin Psychiatry. 2004;65(9):1281-1282.
15. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
2. Koenigsberg HW, Kernberg OF, Stone MH, et al. Borderline patients: extending the limits of treatability. New York, NY: Basic Books; 2000.
3. Nee C, Farman S. Female prisoners with borderline personality disorder: some promising treatment developments. Crim Behav Ment Health. 2005;15:2-16.
4. Oldham JM, Bender DS, Skodol AE, et al. Testing an APA practice guideline: symptom-targeted medication utilization for patients with borderline personality disorder. J Psychiatr Pract. 2004;10:156-161.
5. American Psychiatric Association Practice Guidelines. Practice guideline for the treatment of patients with borderline personality disorder. Am J Psychiatry. 2001;158(suppl 10):1-52.
6. Yatham LN. Newer anticonvulsants in the treatment of bipolar disorder. J Clin Psychiatry. 2004;65(suppl 10):28-35.
7. Rinne T, van den Brink W, Wouters L, et al. SSRI treatment of borderline personality disorder: a randomized, placebo-controlled clinical trial for female patients with borderline personality disorder. Am J Psychiatry. 2002;159(12):2048-2054.
8. Zanarini MC, Frankenburg FR, Parachini EA. A preliminary, randomized trial of fluoxetine, olanzapine, and the olanzapine-fluoxetine combination in women with borderline personality disorder. J Clin Psychiatry. 2004;65(7):903-907.
9. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186-1197.
10. Hilger E, Barnas C, Kasper S. Quetiapine in the treatment of borderline personality disorder. World J Biol Psychiatry. 2003;4(1):42-44.
11. Rizvi ST. Lamotrigine and borderline personality disorder. J Child Adolesc Psychopharmacol. 2002;12(4):365-366.
12. Rocca P, Marchiaro L, Cocuzza E, et al. Treatment of borderline personality disorder with risperidone. J Clin Psychiatry. 2002;63(3):241-244.
13. Philipsen A, Richter H, Schmahl C, et al. Clonidine in acute aversive inner tension and self-injurious behavior in female patients with borderline personality disorder. J Clin Psychiatry. 2004;65(10):1414-1419.
14. Pascual JC, Oller S, Soler J, et al. Ziprasidone in the acute treatment of borderline personality disorder in psychiatric emergency services. J Clin Psychiatry. 2004;65(9):1281-1282.
15. Lieb K, Völlm B, Rücker G, et al. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry. 2010;196(1):4-12.
Lowering risk of Alzheimer’s disease
Pharmacologic treatments for Alzheimer’s disease (AD) may improve symptoms but have not been shown to prevent AD onset. Primary prevention therefore remains the goal. Although preventing AD by managing risk factors such as age or genetics is beyond our control (Box 1), we can do something about other factors.
This article summarizes the findings of many studies that address AD prevention and includes an online-only bibliography for readers seeking an in-depth review. The evidence does not support a firm recommendation for any specific form of primary prevention and has revealed hazards associated with estrogen therapy and nonsteroidal anti-inflammatory drugs (Box 2). Most important, it suggests that you could reduce your patients’ risk of developing AD by routinely supporting their mental, physical, and social health.
The potential benefits of modifying an individual’s AD risk factors likely will depend on his or her genetic makeup, environment, and lifestyle. Even so, counseling patients to exercise more and improve their diets—such as by eating more fish, fruits, and vegetables and less saturated fat—might help protect the brain. Your ongoing efforts to manage hypertension, hypercholesterolemia, and diabetes also may reduce their AD risk.
Age remains the strongest risk factor for dementia, particularly for Alzheimer’s disease (AD).a The risk of developing AD doubles every 5 years after age 65 and approaches 50% after age 85.b
Family history is a risk factor for AD, although true familial AD accounts for <5% of cases.c When diseases show a familial pattern, either genetics, environmental factors, or both may play a role. Patients with a first-degree relative with dementia have a 10% to 30% increased risk of developing the disorder.d
Genetic factors play a role in both early-onset and late-onset AD. Early-onset AD (before age 65) accounts for 6% to 7% of cases.e From this small pool of patients, only 13% exhibit clear autosomal dominant transmission over >1 generation.f Three gene mutations have been associated with early-onset AD:
- 30% to 70% are in the presenilin-1 gene
- 10% to 15% are in the amyloid precursor protein gene
- <5% are in the presenilin-2 gene.g,h
For late-onset AD (after age 65), the strongest evidence for a genetic risk factor exists for the epsilon 4 allele of the apolipoprotein E gene (APOE e4).i This genotype has been linked to the development of AD and possibly to vascular dementia.j,k In contrast, the epsilon 2 allele of APOE may exert a protective effect in AD.l APOE e3, the most common allele, appears to play a neutral role in the development of AD.
References
a. Evans DA. The epidemiology of dementia and Alzheimer’s disease: an evolving field. J Am Geriatr Soc. 1996;44:1482-1483.
b. Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728-733.
c. van Duijn CM, Clayton D, Chandra V, et al. Familial aggregation of Alzheimer’s disease and related disorders: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(suppl 2):S13-S20.
d. Chang JB, Wang PN, Chen WT, et al. ApoE epsilon4 allele is associated with incidental hallucinations and delusions in patients with AD. Neurology. 2004;63:1105-1107.
e. Sleegers K, Roks G, Theuns J, et al. Familial clustering and genetic risk for dementia in a genetically isolated Dutch population. Brain. 2004;127:1641-1649.
f. Schoenberg BS, Anderson DW, Haerer AF. Severe dementia. Prevalence and clinical features in a biracial US population. Arch Neurol. 1985;42:740-743.
g. Hsiung GY, Sadovnick AD. Genetics and dementia: risk factors, diagnosis and management. Alzheimers Dement. 2007;3:418-427.
h. GeneTests database. Available at: http://www.genetests.org. Accessed March 19, 2010.
i. Li H, Wetten S, Li L, et al. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch Neurol. 2008;65:45-53.
j. Graff-Radford NR, Green RC, Go RC, et al. Association between apolipoprotein E genotype and Alzheimer disease in African American subjects. Arch Neurol. 2002;59:594-600.
k. Slooter AJ, Cruts M, Hofman A, et al. The impact of APOE on myocardial infarction, stroke, and dementia: the Rotterdam Study. Neurology. 2004;62:1196-1198.
l. Tiraboschi P, Hansen LA, Masliah E, et al. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62:1977-1983.
Estrogen. Before the Women’s Health Initiative (WHI) study, various trials of the effects of estrogen therapy on the development of Alzheimer’s disease (AD) in women age ≥65 showed inconsistent results. In the randomized, placebo-controlled WHI Memory Study, conjugated equine estrogen, 0.625 mg/d, plus medroxyprogesterone acetate, 2.5 mg/d, did not prevent mild cognitive impairment or improve global cognitive function and was associated with an increased risk for probable dementia.a Based on this evidence, conjugated equine estrogen with or without medroxyprogesterone is not recommended as therapy to protect cognitive function in older women.
NSAID therapy. Cytokine-mediated inflammation may play a role in neurodegenerative disorders and cognitive impairment in the elderly. Nonsteroidal anti-inflammatory drugs (NSAIDs), including cyclooxygenase-2 (COX-2) inhibitors, have been studied for a possible protective effect against AD and cognitive decline,b possibly by lowering amyloidogenic proteins.c A 1-year randomized controlled trial by the Alzheimer’s Disease Cooperative Consortium found no significant differences in cognition scores of patients treated with once-daily rofecoxib, 25 mg, or twice-daily naproxen sodium, 220 mg, when compared with placebo.d Similarly, naproxen and celecoxib did not prevent AD in the randomized, controlled Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT).e Rofecoxib has been withdrawn from the market, and celecoxib labeling carries a warning of potential for increased risk of cardiovascular events and life-threatening gastrointestinal bleeding associated with its use.
NSAIDs and COX-2 inhibitors are not recommended for the treatment or prevention of dementia or cognitive impairment. Their use for AD prevention is not supported by randomized clinical trialsd,e and they may have serious adverse effects.
References
a. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
b. Szekely CA, Breitner JC, Fitzpatrick AL, et al. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology. 2008;70:17-24.
c. Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212-216.
d. Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289(21):2819-2826.
e. ADAPT Research Group, Martin BK, Szekely C, Brandt J, et al. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65(7):896-905.
Cardiovascular risk factors
The risk of developing AD or vascular dementia appears to be increased by conditions that damage the heart or blood vessels. Recent evidence suggests that successfully managing cardiovascular risk factors may decrease the likelihood of dementia in later life.
Hypertension is associated with a higher risk of AD and all-cause dementia. Curiously, some studies have shown that low blood pressure also increases dementia risk, suggesting a U-shaped relationship between blood pressure and cognitive decline. Systolic hypertension in midlife may be associated with dementia 20 years later.
One might assume that antihypertensive therapy would help prevent dementia, but the data are conflicting. The Systolic Hypertension in Europe (SYST-EUR) study1 showed a 53% reduction in vascular dementia or mixed dementia among patients receiving antihypertensive medication and a 60% reduction in AD. Similarly, the PROGRESS2 clinical trial of prevention of recurrent stroke by antihypertensive treatment reported a 34% reduction in a composite measure of cognitive impairment and dementia. On the other hand, cognitive function neither improved nor worsened in the Hypertension in the Very Elderly Trial (HYVET-COG),3 whether patients received blood pressure treatment or placebo.
Hyperlipidemia. Lipid metabolism likely is an important pathway in amyloid beta-protein deposition, tau phosphorylation, and disruption of synaptic plasticity and neurodegenerative endpoints. Cognitive decline and incident dementia have been associated with higher dietary intake of saturated fats, partially hydrogenated unsaturated fatty acids (trans fats), and cholesterol. Not all studies have found this association, however. This could be because serum cholesterol levels may decrease in early dementia, limiting the ability to detect an effect of hypercholesterolemia on dementia risk when measurements are made later in life.
Using statins (3-hydroxy-3-methylglutaryl–coenzyme A reductase inhibitors) to treat hypercholesterolemia has been hypothesized to impede large vessel atherosclerosis and its consequences and to trigger metabolic effects in the brain related to AD pathogenesis. Mechanisms by which statins might help prevent dementia include:
- a direct association between amyloid processing and cholesterol in the brain
- an indirect effect by decreasing the risk of stroke, as even small cerebral infarcts worsen AD severity.
Nonrandomized epidemiologic studies such as the Cardiovascular Health Study4 and MRC/BHF Heart Protection Study5 suggested that statin treatment might reduce the incidence of dementia, the degree of age-related cognitive decline, and AD’s neuropathologic burden. Large, randomized, controlled trials have not supported these observations, however. Statins failed to reduce the incidence of dementia in:
- the Heart Protection Study, testing simvastatin for 5 years in 20,536 subjects age 40 to 805
- the 3-year Preventive Study of Pravastatin in the Elderly at Risk (PROSPER) of 5,800 subjects.6
Similarly, patients receiving adjunctive atorvastatin or placebo showed no significant differences in cognition assessments after 72 weeks in the Lipitor’s Effect in Alzheimer’s Dementia (LEADe) study. This trial enrolled 640 subjects age 50 to 90 with mild-to-moderate dementia who were treated with donepezil.7 A recent Cochrane review concluded that high serum cholesterol may contribute to the development of AD and vascular dementia, but lowering cholesterol levels with statins does not prevent these problems.8
Diabetes mellitus. Diabetes and cognitive decline are closely associated. Diabetes is associated with a 50% to 100% increase in risk of AD and dementia overall and a 100% to 150% increased risk of vascular dementia. The mechanism by which diabetes increases dementia risk is uncertain but does not appear to be mediated entirely through vascular disease. High and low insulin levels may increase the risk of dementia, independent of diabetes and blood glucose. Increased peripheral insulin levels are associated with reduced brain atrophy and cognitive impairment in patients with early AD, suggesting a role for insulin signaling in AD pathophysiology. A possible relationship between insulin and beta amyloid metabolism is being studied.
Elevated postprandial plasma glucose has been associated with accelerated declines in cognitive performance.9 An inverse correlation has been noted between some cognitive measures and hemoglobin A1C levels.10 It is not clear that treating diabetes reduces the risk of dementia. In addition, in the prospective, population-based Rotterdam study, elderly patients with type 2 diabetes treated with insulin had the highest incidence of dementia.11
Tobacco smoke directly affects neuronal function, integrity, and survival. Chronic smoking has been linked to decreased global cerebral blood flow, accelerated cerebral atrophy, and ventricular enlargement.
Some studies suggest an increased risk of dementia in middle-aged and elderly smokers, possibly through a cerebrovascular mechanism such as stroke. Other studies found no association between smoking and dementia risk, and 1 suggested that nicotine may protect against AD by reducing senile plaque formation. Any protective effect of smoking would be offset by increased risks of lung cancer, chronic obstructive pulmonary disease, and vascular dementia.
The apolipoprotein E epsilon 4 (APOE e4) gene may explain, at least in part, the conflicting results of these studies. In 2 population-based cohorts,12,13 smoking was associated with memory decline in patients without, but not with, the APOE e4 genotype.
Dietary factors
Antioxidants. The brains of patients with AD contain elevated levels of endogenous antioxidants. In vitro studies show exogenous antioxidants can reduce the toxicity of beta-amyloid in brain tissue of persons with AD. These findings have led to interest in assessing the role of dietary antioxidants such as vitamins E and C for AD prevention.
High-dose alpha-tocopherol (vitamin E, 2,000 IU/d) may slow disease progression in patients with AD, but this association is not consistently found. Furthermore, a meta-analysis of 19 randomized controlled trials (RCTs) totaling >135,000 patients found an association between vitamin E doses >400 IU/d and increased all-cause mortality.14 High-dose vitamin E supplementation for primary or secondary prevention of AD may be dangerous and is not recommended.
The lack of consistent efficacy data for vitamin C in preventing or treating AD may discourage its routine use for this purpose.15
Homocysteine is a risk factor for stroke and heart disease. It also could play a role in vascular dementia through its association with large- and small-vessel disease.
Low folate and hyperhomocysteinemia have been associated with dementia or cognitive impairment, although a cause-effect relationship is not clear. In non-demented elderly populations, plasma homocysteine is inversely associated with poor performance in tests of global cognitive function, particularly in measures of psychomotor speed.
In a recent double-blind RCT, folic acid supplementation for 3 years significantly improved domains of cognitive function that tend to decline with age, especially information processing and sensorimotor speed.16 No other good evidence, however, has shown that homocysteine-lowering therapy using folic acid or other vitamin B supplements improves cognitive function or prevents cognitive decline.
Fish and omega-3 fatty acids. High total fat, saturated fat, and total cholesterol intake increases the risk for incident dementia. In epidemiologic studies, low omega-3 fatty acid serum levels have been linked to increased dementia risk.
Fish consumption may be beneficial in reducing the risk of dementia or cognitive decline. A prospective study of 815 elderly persons found 60% less risk of developing AD in those who ate ≥1 fish meal per week, compared with those who rarely or never ate fish.17 In the Framingham study, individuals who at baseline were in the top quartile of docosahexaenoic acid consumption had lower dementia rates over 9 years of follow-up.18 Results from cross-sectional and longitudinal studies have been inconsistent; some have shown that high intake of n-3 polyunsaturated fatty acids is associated with less cognitive decline,19 whereas others have not.20
Although we cannot offer unequivocal advice regarding seafood or omega-3 fatty acid intake for primary prevention of dementia without evidence from RCTs, these uncontrolled studies show promise.
Mediterranean diet (MeDi) components include abundant fruits and vegetables, fish or shellfish at least twice weekly, very limited red meat, olive oil or canola oil instead of butter or margarine, tree nuts such as walnuts or pecans, red wine in moderation, and using herbs and spices instead of salt to season food. High adherence to the MeDi has been associated with a significantly lower risk for incident AD. The MeDi may affect the risk of developing AD21 as well as subsequent disease course, with a possible dose-response relationship in lower mortality.22
Eating fruits and vegetables has been associated with improved cognitive performance22 and decreased incident dementia in elderly subjects.18
Alcohol. A U-shaped relationship exists between alcohol consumption and dementia risk. High alcohol intake is associated with clinical problem drinking and alcoholism and can lead to cognitive decline. Conversely, moderate wine consumption (250 to 500 mL/d) may be protective—compared with more or less than this amount—and is associated with approximately 50% less risk of dementia.
Alcohol use may increase the risk of dementia in persons carrying the APOE e4 allele, according to the population-based Cardiovascular Risk Factors, Aging and Dementia (CAIDE) study from Sweden.23 After an average 21 years of follow-up of 1,449 individuals, researchers found that environmental factors—such as physical inactivity, dietary fat intake, alcohol consumption, and smoking at midlife—were associated with an increased risk of dementia at age 65 to 79 in APOE e4 carriers compared with noncarriers. The study also found that physical inactivity, dietary fat intake, and smoking at midlife increase AD risk, especially among APOE e4 carriers.
In the absence of evidence from RCTs, we cannot recommend alcohol to reduce the risk of AD.
Lifestyle and activity
Three components of lifestyle—social, mental, and physical activity—are inversely associated with the risk for dementia, AD, and cognitive impairment.
Physical exercise has been thought to enhance brain neurotrophic factor and modify apoptosis. Exercise can deter stroke and microvascular disease and improve regional cerebral blood flow. In the Cardiovascular Health Study, participants who expended the highest quartile of energy had a lower risk of all-cause dementia and AD compared with participants who expended the lowest quartile of energy.24
Mental and social activity. Epidemiologic studies have shown associations between higher educational achievement and other socioeconomic factors and reduced AD risk. Advanced education is believed to represent a cognitive reserve that delays presentation of AD’s effects on memory and cognitive function, rather than providing a protective effect against accumulation of AD pathology. Higher-educated individuals appear to experience a somewhat more rapid rate of cognitive decline when AD does become apparent, perhaps because they have accumulated a greater degree of AD pathology at that point compared with less-educated persons.
Among 117 persons with dementia in the Bronx Aging Study, each additional year of formal education delayed the time of accelerated decline by 0.21 years. After accelerated decline began, each year of additional formal education was associated with a slightly faster rate of memory decline.25
The longitudinal, population-based Kungsholmen Project in Stockholm, Sweden, found an association between daily mentally stimulating activities and decreased risk of all-cause dementia.26 Similarly, higher levels of leisure activity were linked to reduced risk of all-cause dementia in a longitudinal study of 1,772 persons age ≥65 living in Manhattan, NY.27 In a randomized, single-controlled study of the long-term effects of cognitive training, elderly individuals from 6 U.S. cities showed sustained improvement in specific cognitive performance up to 5 years after training sessions began, including memory, reasoning, and speed of processing.28
It seems reasonable to encourage older patients to maintain or increase physical, cognitive, and leisure activities as well as social interaction. These interventions can improve the quality of life and lower the risk of depression, which may be a response to cognitive decline or an independent risk factor for dementia (Box 3). The Table lists “brain exercises” you can suggest to patients to increase their mental and social activity.
Head trauma. The Multi-Institutional Research in Alzheimer’s Genetic Epidemiology (MIRAGE) project found an association between AD risk and a history of head trauma, especially in persons with APOE e4 alleles.29 Conversely, the Rotterdam Study showed no change in dementia risk for persons with a history of head trauma.30
Even in the absence of conclusive evidence supporting AD prevention, protecting the head by buckling seat belts while driving, wearing helmets when participating in sports, and “fall-proofing” the home is recommended.
Depression often occurs before or as a coexisting condition with Alzheimer’s disease (AD).a Although depression has been considered a response to cognitive decline or an early manifestation of dementia,b it also could be an independent risk factor.c,d
The pathologic mechanism linking depression and subsequent dementia is not well understood. Hypotheses include an indirect neurotoxic effect of depression mediated by cortisol-induced hippocampal atrophy or lowered brain-derived neurotrophic factor levels.e Depression and dementia might share genetic links, although a cohort study of 404 individuals with AD detected no association between apolipoprotein E genotypes or alleles and depressive symptoms.f
References
a. Lupien SJ, Nair NP, Brière S, et al. Increased cortisol levels and impaired cognition in human aging: implication for depression and dementia in later life. Rev Neurosci. 1999;10(2):117-139.
b. Preuss UW, Siafarikas N, Petrucci M, et al. Depressive disorders in dementia and mild cognitive impairments: is comorbidity a cause or a risk factor? Fortschr Neurol Psychiatr. 2009;77:399-406.
c. Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60(5):753-759.
d. Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer’s disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530-538.
e. Meeks TW, Ropacki SA, Jeste DV. The neurobiology of neuropsychiatric syndromes in dementia. Curr Opin Psychiatry. 2006;19(6):581-586.
f. Craig D, Hart DJ, McIlroy SP, et al. Association analysis of apolipoprotein E genotype and risk of depressive symptoms in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19(2-3):154-157.
Table
Brain exercises to suggest to patients
| Learn something new (how to play a musical instrument, a foreign language, or a new hobby) |
| Play memory games |
| Practice using the opposite hand to perform tasks you usually do with your dominant hand |
| Read, especially challenging material |
| Join a book discussion group |
| Write; if not a book or article, write a diary, letters, or emails or start your memoirs |
| Do crossword, Sudoku, or jigsaw puzzles |
| Play board games, card games, and other strategy games |
| Debate or discuss topics |
Related resource
- For an extensive bibliography of literature on Alzheimer’s disease risk factors and prevention, see this article at CurrentPsychiatry.com.
Drug brand names
- Atorvastatin • Lipitor
- Celecoxib • Celebrex
- Donepezil • Aricept
- Medroxyprogesterone • Provera
- Pravastatin • Pravachol
- Rofecoxib • Vioxx
- Simvastatin • Zocor
Disclosures
Dr. Bassil reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Dr. Grossberg receives research/grant support from and is a consultant to Bristol-Myers Squibb, Forest Pharmaceuticals, Novartis, Pfizer Inc., and Wyeth Pharmaceuticals. He also receives research/grant support from Baxter.
1. Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the systolic hypertension in Europe (Syst-Eur) study. Arch Intern Med. 2002;162:2046-2052.
2. Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069-1075.
3. Peters R, Beckett N, Forette F. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG). Lancet Neurol. 2008;7(8):683-689.
4. Rea TD, Breitner JC, Psaty BM, et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol. 2005;62:1047-1051.
5. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7-22.
6. Kulbertus H, Scheen AJ. [The PROSPER Study (PROspective study of pravastatin in the elderly at risk)]. Rev Med Liege. 2002;57(12):809-813.
7. Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74(12):956-964.
8. McGuinness B, Bullock R, Craig D, et al. Statins for the treatment of Alzheimer’s disease and dementia (protocol). Cochrane Database Syst Rev. 2009;1:CD007514.-
9. Abbatecola AM, Rizzo MR, Barbieri M, et al. Postprandial plasmaglucose excursions and cognitive functioning in aged type 2 diabetics. Neurology. 2006;67:235-240.
10. Munshi M, Grande L, Hayes M, et al. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care. 2006;29:1794-1799.
11. Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia. The Rotterdam study. Neurology. 1999;53:1937-1942.
12. Reitz C, Luchsinger J, Tang MX, et al. Effect of smoking and time on cognitive function in the elderly without dementia. Neurology. 2005;65:870-875.
13. Reitz C, den Heijer T, van Duijn C, et al. Relation between smoking and risk of dementia and Alzheimer disease: the Rotterdam Study. Neurology. 2007;69:998-1005.
14. Miller ER, III, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37-46.
15. Boothby LA, Doering PL. Vitamin C and vitamin E for Alzheimer’s disease. Ann Pharmacother. 2005;39(12):2073-2080.
16. Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369:208-216.
17. Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940-946.
18. Schaefer EJ, Bongard V, Beiser AS, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545-1550.
19. Kalmijn S, Launer LJ, Ott A, et al. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776-782.
20. van Gelder BM, Tijhuis M, Kalmijn S, et al. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007;85:1142-1147.
21. Solfrizzi V, Capurso C, Panza F. Adherence to a Mediterranean dietary pattern and risk of Alzheimer’s disease. Ann Neurol. 2006;60:620.-
22. Scarmeas N, Luchsinger JA, Mayeux R, et al. Mediterranean diet and Alzheimer disease mortality. Neurology. 2007;69(11):1084-1093.
23. Kivipelto M, Rovio S, Ngandu T, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12(6B):2762-2771.
24. Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639-651.
25. Hall CB, Derby C, LeValley A, et al. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69:1657-1664.
26. Wang HX, Karp A, Winblad B, et al. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen Project. Am J Epidemiol. 2002;155:1081-1087.
27. Scarmeas N, Levy G, Tang MX, et al. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236-2242.
28. Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805-2814.
29. Guo Z, Cupples LA, Kurz A, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316-1323.
30. Ruitenberg A, van Swieten JC, Witteman JC, et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281-286.
Pharmacologic treatments for Alzheimer’s disease (AD) may improve symptoms but have not been shown to prevent AD onset. Primary prevention therefore remains the goal. Although preventing AD by managing risk factors such as age or genetics is beyond our control (Box 1), we can do something about other factors.
This article summarizes the findings of many studies that address AD prevention and includes an online-only bibliography for readers seeking an in-depth review. The evidence does not support a firm recommendation for any specific form of primary prevention and has revealed hazards associated with estrogen therapy and nonsteroidal anti-inflammatory drugs (Box 2). Most important, it suggests that you could reduce your patients’ risk of developing AD by routinely supporting their mental, physical, and social health.
The potential benefits of modifying an individual’s AD risk factors likely will depend on his or her genetic makeup, environment, and lifestyle. Even so, counseling patients to exercise more and improve their diets—such as by eating more fish, fruits, and vegetables and less saturated fat—might help protect the brain. Your ongoing efforts to manage hypertension, hypercholesterolemia, and diabetes also may reduce their AD risk.
Age remains the strongest risk factor for dementia, particularly for Alzheimer’s disease (AD).a The risk of developing AD doubles every 5 years after age 65 and approaches 50% after age 85.b
Family history is a risk factor for AD, although true familial AD accounts for <5% of cases.c When diseases show a familial pattern, either genetics, environmental factors, or both may play a role. Patients with a first-degree relative with dementia have a 10% to 30% increased risk of developing the disorder.d
Genetic factors play a role in both early-onset and late-onset AD. Early-onset AD (before age 65) accounts for 6% to 7% of cases.e From this small pool of patients, only 13% exhibit clear autosomal dominant transmission over >1 generation.f Three gene mutations have been associated with early-onset AD:
- 30% to 70% are in the presenilin-1 gene
- 10% to 15% are in the amyloid precursor protein gene
- <5% are in the presenilin-2 gene.g,h
For late-onset AD (after age 65), the strongest evidence for a genetic risk factor exists for the epsilon 4 allele of the apolipoprotein E gene (APOE e4).i This genotype has been linked to the development of AD and possibly to vascular dementia.j,k In contrast, the epsilon 2 allele of APOE may exert a protective effect in AD.l APOE e3, the most common allele, appears to play a neutral role in the development of AD.
References
a. Evans DA. The epidemiology of dementia and Alzheimer’s disease: an evolving field. J Am Geriatr Soc. 1996;44:1482-1483.
b. Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728-733.
c. van Duijn CM, Clayton D, Chandra V, et al. Familial aggregation of Alzheimer’s disease and related disorders: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(suppl 2):S13-S20.
d. Chang JB, Wang PN, Chen WT, et al. ApoE epsilon4 allele is associated with incidental hallucinations and delusions in patients with AD. Neurology. 2004;63:1105-1107.
e. Sleegers K, Roks G, Theuns J, et al. Familial clustering and genetic risk for dementia in a genetically isolated Dutch population. Brain. 2004;127:1641-1649.
f. Schoenberg BS, Anderson DW, Haerer AF. Severe dementia. Prevalence and clinical features in a biracial US population. Arch Neurol. 1985;42:740-743.
g. Hsiung GY, Sadovnick AD. Genetics and dementia: risk factors, diagnosis and management. Alzheimers Dement. 2007;3:418-427.
h. GeneTests database. Available at: http://www.genetests.org. Accessed March 19, 2010.
i. Li H, Wetten S, Li L, et al. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch Neurol. 2008;65:45-53.
j. Graff-Radford NR, Green RC, Go RC, et al. Association between apolipoprotein E genotype and Alzheimer disease in African American subjects. Arch Neurol. 2002;59:594-600.
k. Slooter AJ, Cruts M, Hofman A, et al. The impact of APOE on myocardial infarction, stroke, and dementia: the Rotterdam Study. Neurology. 2004;62:1196-1198.
l. Tiraboschi P, Hansen LA, Masliah E, et al. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62:1977-1983.
Estrogen. Before the Women’s Health Initiative (WHI) study, various trials of the effects of estrogen therapy on the development of Alzheimer’s disease (AD) in women age ≥65 showed inconsistent results. In the randomized, placebo-controlled WHI Memory Study, conjugated equine estrogen, 0.625 mg/d, plus medroxyprogesterone acetate, 2.5 mg/d, did not prevent mild cognitive impairment or improve global cognitive function and was associated with an increased risk for probable dementia.a Based on this evidence, conjugated equine estrogen with or without medroxyprogesterone is not recommended as therapy to protect cognitive function in older women.
NSAID therapy. Cytokine-mediated inflammation may play a role in neurodegenerative disorders and cognitive impairment in the elderly. Nonsteroidal anti-inflammatory drugs (NSAIDs), including cyclooxygenase-2 (COX-2) inhibitors, have been studied for a possible protective effect against AD and cognitive decline,b possibly by lowering amyloidogenic proteins.c A 1-year randomized controlled trial by the Alzheimer’s Disease Cooperative Consortium found no significant differences in cognition scores of patients treated with once-daily rofecoxib, 25 mg, or twice-daily naproxen sodium, 220 mg, when compared with placebo.d Similarly, naproxen and celecoxib did not prevent AD in the randomized, controlled Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT).e Rofecoxib has been withdrawn from the market, and celecoxib labeling carries a warning of potential for increased risk of cardiovascular events and life-threatening gastrointestinal bleeding associated with its use.
NSAIDs and COX-2 inhibitors are not recommended for the treatment or prevention of dementia or cognitive impairment. Their use for AD prevention is not supported by randomized clinical trialsd,e and they may have serious adverse effects.
References
a. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
b. Szekely CA, Breitner JC, Fitzpatrick AL, et al. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology. 2008;70:17-24.
c. Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212-216.
d. Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289(21):2819-2826.
e. ADAPT Research Group, Martin BK, Szekely C, Brandt J, et al. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65(7):896-905.
Cardiovascular risk factors
The risk of developing AD or vascular dementia appears to be increased by conditions that damage the heart or blood vessels. Recent evidence suggests that successfully managing cardiovascular risk factors may decrease the likelihood of dementia in later life.
Hypertension is associated with a higher risk of AD and all-cause dementia. Curiously, some studies have shown that low blood pressure also increases dementia risk, suggesting a U-shaped relationship between blood pressure and cognitive decline. Systolic hypertension in midlife may be associated with dementia 20 years later.
One might assume that antihypertensive therapy would help prevent dementia, but the data are conflicting. The Systolic Hypertension in Europe (SYST-EUR) study1 showed a 53% reduction in vascular dementia or mixed dementia among patients receiving antihypertensive medication and a 60% reduction in AD. Similarly, the PROGRESS2 clinical trial of prevention of recurrent stroke by antihypertensive treatment reported a 34% reduction in a composite measure of cognitive impairment and dementia. On the other hand, cognitive function neither improved nor worsened in the Hypertension in the Very Elderly Trial (HYVET-COG),3 whether patients received blood pressure treatment or placebo.
Hyperlipidemia. Lipid metabolism likely is an important pathway in amyloid beta-protein deposition, tau phosphorylation, and disruption of synaptic plasticity and neurodegenerative endpoints. Cognitive decline and incident dementia have been associated with higher dietary intake of saturated fats, partially hydrogenated unsaturated fatty acids (trans fats), and cholesterol. Not all studies have found this association, however. This could be because serum cholesterol levels may decrease in early dementia, limiting the ability to detect an effect of hypercholesterolemia on dementia risk when measurements are made later in life.
Using statins (3-hydroxy-3-methylglutaryl–coenzyme A reductase inhibitors) to treat hypercholesterolemia has been hypothesized to impede large vessel atherosclerosis and its consequences and to trigger metabolic effects in the brain related to AD pathogenesis. Mechanisms by which statins might help prevent dementia include:
- a direct association between amyloid processing and cholesterol in the brain
- an indirect effect by decreasing the risk of stroke, as even small cerebral infarcts worsen AD severity.
Nonrandomized epidemiologic studies such as the Cardiovascular Health Study4 and MRC/BHF Heart Protection Study5 suggested that statin treatment might reduce the incidence of dementia, the degree of age-related cognitive decline, and AD’s neuropathologic burden. Large, randomized, controlled trials have not supported these observations, however. Statins failed to reduce the incidence of dementia in:
- the Heart Protection Study, testing simvastatin for 5 years in 20,536 subjects age 40 to 805
- the 3-year Preventive Study of Pravastatin in the Elderly at Risk (PROSPER) of 5,800 subjects.6
Similarly, patients receiving adjunctive atorvastatin or placebo showed no significant differences in cognition assessments after 72 weeks in the Lipitor’s Effect in Alzheimer’s Dementia (LEADe) study. This trial enrolled 640 subjects age 50 to 90 with mild-to-moderate dementia who were treated with donepezil.7 A recent Cochrane review concluded that high serum cholesterol may contribute to the development of AD and vascular dementia, but lowering cholesterol levels with statins does not prevent these problems.8
Diabetes mellitus. Diabetes and cognitive decline are closely associated. Diabetes is associated with a 50% to 100% increase in risk of AD and dementia overall and a 100% to 150% increased risk of vascular dementia. The mechanism by which diabetes increases dementia risk is uncertain but does not appear to be mediated entirely through vascular disease. High and low insulin levels may increase the risk of dementia, independent of diabetes and blood glucose. Increased peripheral insulin levels are associated with reduced brain atrophy and cognitive impairment in patients with early AD, suggesting a role for insulin signaling in AD pathophysiology. A possible relationship between insulin and beta amyloid metabolism is being studied.
Elevated postprandial plasma glucose has been associated with accelerated declines in cognitive performance.9 An inverse correlation has been noted between some cognitive measures and hemoglobin A1C levels.10 It is not clear that treating diabetes reduces the risk of dementia. In addition, in the prospective, population-based Rotterdam study, elderly patients with type 2 diabetes treated with insulin had the highest incidence of dementia.11
Tobacco smoke directly affects neuronal function, integrity, and survival. Chronic smoking has been linked to decreased global cerebral blood flow, accelerated cerebral atrophy, and ventricular enlargement.
Some studies suggest an increased risk of dementia in middle-aged and elderly smokers, possibly through a cerebrovascular mechanism such as stroke. Other studies found no association between smoking and dementia risk, and 1 suggested that nicotine may protect against AD by reducing senile plaque formation. Any protective effect of smoking would be offset by increased risks of lung cancer, chronic obstructive pulmonary disease, and vascular dementia.
The apolipoprotein E epsilon 4 (APOE e4) gene may explain, at least in part, the conflicting results of these studies. In 2 population-based cohorts,12,13 smoking was associated with memory decline in patients without, but not with, the APOE e4 genotype.
Dietary factors
Antioxidants. The brains of patients with AD contain elevated levels of endogenous antioxidants. In vitro studies show exogenous antioxidants can reduce the toxicity of beta-amyloid in brain tissue of persons with AD. These findings have led to interest in assessing the role of dietary antioxidants such as vitamins E and C for AD prevention.
High-dose alpha-tocopherol (vitamin E, 2,000 IU/d) may slow disease progression in patients with AD, but this association is not consistently found. Furthermore, a meta-analysis of 19 randomized controlled trials (RCTs) totaling >135,000 patients found an association between vitamin E doses >400 IU/d and increased all-cause mortality.14 High-dose vitamin E supplementation for primary or secondary prevention of AD may be dangerous and is not recommended.
The lack of consistent efficacy data for vitamin C in preventing or treating AD may discourage its routine use for this purpose.15
Homocysteine is a risk factor for stroke and heart disease. It also could play a role in vascular dementia through its association with large- and small-vessel disease.
Low folate and hyperhomocysteinemia have been associated with dementia or cognitive impairment, although a cause-effect relationship is not clear. In non-demented elderly populations, plasma homocysteine is inversely associated with poor performance in tests of global cognitive function, particularly in measures of psychomotor speed.
In a recent double-blind RCT, folic acid supplementation for 3 years significantly improved domains of cognitive function that tend to decline with age, especially information processing and sensorimotor speed.16 No other good evidence, however, has shown that homocysteine-lowering therapy using folic acid or other vitamin B supplements improves cognitive function or prevents cognitive decline.
Fish and omega-3 fatty acids. High total fat, saturated fat, and total cholesterol intake increases the risk for incident dementia. In epidemiologic studies, low omega-3 fatty acid serum levels have been linked to increased dementia risk.
Fish consumption may be beneficial in reducing the risk of dementia or cognitive decline. A prospective study of 815 elderly persons found 60% less risk of developing AD in those who ate ≥1 fish meal per week, compared with those who rarely or never ate fish.17 In the Framingham study, individuals who at baseline were in the top quartile of docosahexaenoic acid consumption had lower dementia rates over 9 years of follow-up.18 Results from cross-sectional and longitudinal studies have been inconsistent; some have shown that high intake of n-3 polyunsaturated fatty acids is associated with less cognitive decline,19 whereas others have not.20
Although we cannot offer unequivocal advice regarding seafood or omega-3 fatty acid intake for primary prevention of dementia without evidence from RCTs, these uncontrolled studies show promise.
Mediterranean diet (MeDi) components include abundant fruits and vegetables, fish or shellfish at least twice weekly, very limited red meat, olive oil or canola oil instead of butter or margarine, tree nuts such as walnuts or pecans, red wine in moderation, and using herbs and spices instead of salt to season food. High adherence to the MeDi has been associated with a significantly lower risk for incident AD. The MeDi may affect the risk of developing AD21 as well as subsequent disease course, with a possible dose-response relationship in lower mortality.22
Eating fruits and vegetables has been associated with improved cognitive performance22 and decreased incident dementia in elderly subjects.18
Alcohol. A U-shaped relationship exists between alcohol consumption and dementia risk. High alcohol intake is associated with clinical problem drinking and alcoholism and can lead to cognitive decline. Conversely, moderate wine consumption (250 to 500 mL/d) may be protective—compared with more or less than this amount—and is associated with approximately 50% less risk of dementia.
Alcohol use may increase the risk of dementia in persons carrying the APOE e4 allele, according to the population-based Cardiovascular Risk Factors, Aging and Dementia (CAIDE) study from Sweden.23 After an average 21 years of follow-up of 1,449 individuals, researchers found that environmental factors—such as physical inactivity, dietary fat intake, alcohol consumption, and smoking at midlife—were associated with an increased risk of dementia at age 65 to 79 in APOE e4 carriers compared with noncarriers. The study also found that physical inactivity, dietary fat intake, and smoking at midlife increase AD risk, especially among APOE e4 carriers.
In the absence of evidence from RCTs, we cannot recommend alcohol to reduce the risk of AD.
Lifestyle and activity
Three components of lifestyle—social, mental, and physical activity—are inversely associated with the risk for dementia, AD, and cognitive impairment.
Physical exercise has been thought to enhance brain neurotrophic factor and modify apoptosis. Exercise can deter stroke and microvascular disease and improve regional cerebral blood flow. In the Cardiovascular Health Study, participants who expended the highest quartile of energy had a lower risk of all-cause dementia and AD compared with participants who expended the lowest quartile of energy.24
Mental and social activity. Epidemiologic studies have shown associations between higher educational achievement and other socioeconomic factors and reduced AD risk. Advanced education is believed to represent a cognitive reserve that delays presentation of AD’s effects on memory and cognitive function, rather than providing a protective effect against accumulation of AD pathology. Higher-educated individuals appear to experience a somewhat more rapid rate of cognitive decline when AD does become apparent, perhaps because they have accumulated a greater degree of AD pathology at that point compared with less-educated persons.
Among 117 persons with dementia in the Bronx Aging Study, each additional year of formal education delayed the time of accelerated decline by 0.21 years. After accelerated decline began, each year of additional formal education was associated with a slightly faster rate of memory decline.25
The longitudinal, population-based Kungsholmen Project in Stockholm, Sweden, found an association between daily mentally stimulating activities and decreased risk of all-cause dementia.26 Similarly, higher levels of leisure activity were linked to reduced risk of all-cause dementia in a longitudinal study of 1,772 persons age ≥65 living in Manhattan, NY.27 In a randomized, single-controlled study of the long-term effects of cognitive training, elderly individuals from 6 U.S. cities showed sustained improvement in specific cognitive performance up to 5 years after training sessions began, including memory, reasoning, and speed of processing.28
It seems reasonable to encourage older patients to maintain or increase physical, cognitive, and leisure activities as well as social interaction. These interventions can improve the quality of life and lower the risk of depression, which may be a response to cognitive decline or an independent risk factor for dementia (Box 3). The Table lists “brain exercises” you can suggest to patients to increase their mental and social activity.
Head trauma. The Multi-Institutional Research in Alzheimer’s Genetic Epidemiology (MIRAGE) project found an association between AD risk and a history of head trauma, especially in persons with APOE e4 alleles.29 Conversely, the Rotterdam Study showed no change in dementia risk for persons with a history of head trauma.30
Even in the absence of conclusive evidence supporting AD prevention, protecting the head by buckling seat belts while driving, wearing helmets when participating in sports, and “fall-proofing” the home is recommended.
Depression often occurs before or as a coexisting condition with Alzheimer’s disease (AD).a Although depression has been considered a response to cognitive decline or an early manifestation of dementia,b it also could be an independent risk factor.c,d
The pathologic mechanism linking depression and subsequent dementia is not well understood. Hypotheses include an indirect neurotoxic effect of depression mediated by cortisol-induced hippocampal atrophy or lowered brain-derived neurotrophic factor levels.e Depression and dementia might share genetic links, although a cohort study of 404 individuals with AD detected no association between apolipoprotein E genotypes or alleles and depressive symptoms.f
References
a. Lupien SJ, Nair NP, Brière S, et al. Increased cortisol levels and impaired cognition in human aging: implication for depression and dementia in later life. Rev Neurosci. 1999;10(2):117-139.
b. Preuss UW, Siafarikas N, Petrucci M, et al. Depressive disorders in dementia and mild cognitive impairments: is comorbidity a cause or a risk factor? Fortschr Neurol Psychiatr. 2009;77:399-406.
c. Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60(5):753-759.
d. Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer’s disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530-538.
e. Meeks TW, Ropacki SA, Jeste DV. The neurobiology of neuropsychiatric syndromes in dementia. Curr Opin Psychiatry. 2006;19(6):581-586.
f. Craig D, Hart DJ, McIlroy SP, et al. Association analysis of apolipoprotein E genotype and risk of depressive symptoms in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19(2-3):154-157.
Table
Brain exercises to suggest to patients
| Learn something new (how to play a musical instrument, a foreign language, or a new hobby) |
| Play memory games |
| Practice using the opposite hand to perform tasks you usually do with your dominant hand |
| Read, especially challenging material |
| Join a book discussion group |
| Write; if not a book or article, write a diary, letters, or emails or start your memoirs |
| Do crossword, Sudoku, or jigsaw puzzles |
| Play board games, card games, and other strategy games |
| Debate or discuss topics |
Related resource
- For an extensive bibliography of literature on Alzheimer’s disease risk factors and prevention, see this article at CurrentPsychiatry.com.
Drug brand names
- Atorvastatin • Lipitor
- Celecoxib • Celebrex
- Donepezil • Aricept
- Medroxyprogesterone • Provera
- Pravastatin • Pravachol
- Rofecoxib • Vioxx
- Simvastatin • Zocor
Disclosures
Dr. Bassil reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Dr. Grossberg receives research/grant support from and is a consultant to Bristol-Myers Squibb, Forest Pharmaceuticals, Novartis, Pfizer Inc., and Wyeth Pharmaceuticals. He also receives research/grant support from Baxter.
Pharmacologic treatments for Alzheimer’s disease (AD) may improve symptoms but have not been shown to prevent AD onset. Primary prevention therefore remains the goal. Although preventing AD by managing risk factors such as age or genetics is beyond our control (Box 1), we can do something about other factors.
This article summarizes the findings of many studies that address AD prevention and includes an online-only bibliography for readers seeking an in-depth review. The evidence does not support a firm recommendation for any specific form of primary prevention and has revealed hazards associated with estrogen therapy and nonsteroidal anti-inflammatory drugs (Box 2). Most important, it suggests that you could reduce your patients’ risk of developing AD by routinely supporting their mental, physical, and social health.
The potential benefits of modifying an individual’s AD risk factors likely will depend on his or her genetic makeup, environment, and lifestyle. Even so, counseling patients to exercise more and improve their diets—such as by eating more fish, fruits, and vegetables and less saturated fat—might help protect the brain. Your ongoing efforts to manage hypertension, hypercholesterolemia, and diabetes also may reduce their AD risk.
Age remains the strongest risk factor for dementia, particularly for Alzheimer’s disease (AD).a The risk of developing AD doubles every 5 years after age 65 and approaches 50% after age 85.b
Family history is a risk factor for AD, although true familial AD accounts for <5% of cases.c When diseases show a familial pattern, either genetics, environmental factors, or both may play a role. Patients with a first-degree relative with dementia have a 10% to 30% increased risk of developing the disorder.d
Genetic factors play a role in both early-onset and late-onset AD. Early-onset AD (before age 65) accounts for 6% to 7% of cases.e From this small pool of patients, only 13% exhibit clear autosomal dominant transmission over >1 generation.f Three gene mutations have been associated with early-onset AD:
- 30% to 70% are in the presenilin-1 gene
- 10% to 15% are in the amyloid precursor protein gene
- <5% are in the presenilin-2 gene.g,h
For late-onset AD (after age 65), the strongest evidence for a genetic risk factor exists for the epsilon 4 allele of the apolipoprotein E gene (APOE e4).i This genotype has been linked to the development of AD and possibly to vascular dementia.j,k In contrast, the epsilon 2 allele of APOE may exert a protective effect in AD.l APOE e3, the most common allele, appears to play a neutral role in the development of AD.
References
a. Evans DA. The epidemiology of dementia and Alzheimer’s disease: an evolving field. J Am Geriatr Soc. 1996;44:1482-1483.
b. Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728-733.
c. van Duijn CM, Clayton D, Chandra V, et al. Familial aggregation of Alzheimer’s disease and related disorders: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(suppl 2):S13-S20.
d. Chang JB, Wang PN, Chen WT, et al. ApoE epsilon4 allele is associated with incidental hallucinations and delusions in patients with AD. Neurology. 2004;63:1105-1107.
e. Sleegers K, Roks G, Theuns J, et al. Familial clustering and genetic risk for dementia in a genetically isolated Dutch population. Brain. 2004;127:1641-1649.
f. Schoenberg BS, Anderson DW, Haerer AF. Severe dementia. Prevalence and clinical features in a biracial US population. Arch Neurol. 1985;42:740-743.
g. Hsiung GY, Sadovnick AD. Genetics and dementia: risk factors, diagnosis and management. Alzheimers Dement. 2007;3:418-427.
h. GeneTests database. Available at: http://www.genetests.org. Accessed March 19, 2010.
i. Li H, Wetten S, Li L, et al. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch Neurol. 2008;65:45-53.
j. Graff-Radford NR, Green RC, Go RC, et al. Association between apolipoprotein E genotype and Alzheimer disease in African American subjects. Arch Neurol. 2002;59:594-600.
k. Slooter AJ, Cruts M, Hofman A, et al. The impact of APOE on myocardial infarction, stroke, and dementia: the Rotterdam Study. Neurology. 2004;62:1196-1198.
l. Tiraboschi P, Hansen LA, Masliah E, et al. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62:1977-1983.
Estrogen. Before the Women’s Health Initiative (WHI) study, various trials of the effects of estrogen therapy on the development of Alzheimer’s disease (AD) in women age ≥65 showed inconsistent results. In the randomized, placebo-controlled WHI Memory Study, conjugated equine estrogen, 0.625 mg/d, plus medroxyprogesterone acetate, 2.5 mg/d, did not prevent mild cognitive impairment or improve global cognitive function and was associated with an increased risk for probable dementia.a Based on this evidence, conjugated equine estrogen with or without medroxyprogesterone is not recommended as therapy to protect cognitive function in older women.
NSAID therapy. Cytokine-mediated inflammation may play a role in neurodegenerative disorders and cognitive impairment in the elderly. Nonsteroidal anti-inflammatory drugs (NSAIDs), including cyclooxygenase-2 (COX-2) inhibitors, have been studied for a possible protective effect against AD and cognitive decline,b possibly by lowering amyloidogenic proteins.c A 1-year randomized controlled trial by the Alzheimer’s Disease Cooperative Consortium found no significant differences in cognition scores of patients treated with once-daily rofecoxib, 25 mg, or twice-daily naproxen sodium, 220 mg, when compared with placebo.d Similarly, naproxen and celecoxib did not prevent AD in the randomized, controlled Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT).e Rofecoxib has been withdrawn from the market, and celecoxib labeling carries a warning of potential for increased risk of cardiovascular events and life-threatening gastrointestinal bleeding associated with its use.
NSAIDs and COX-2 inhibitors are not recommended for the treatment or prevention of dementia or cognitive impairment. Their use for AD prevention is not supported by randomized clinical trialsd,e and they may have serious adverse effects.
References
a. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947-2958.
b. Szekely CA, Breitner JC, Fitzpatrick AL, et al. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology. 2008;70:17-24.
c. Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212-216.
d. Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289(21):2819-2826.
e. ADAPT Research Group, Martin BK, Szekely C, Brandt J, et al. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65(7):896-905.
Cardiovascular risk factors
The risk of developing AD or vascular dementia appears to be increased by conditions that damage the heart or blood vessels. Recent evidence suggests that successfully managing cardiovascular risk factors may decrease the likelihood of dementia in later life.
Hypertension is associated with a higher risk of AD and all-cause dementia. Curiously, some studies have shown that low blood pressure also increases dementia risk, suggesting a U-shaped relationship between blood pressure and cognitive decline. Systolic hypertension in midlife may be associated with dementia 20 years later.
One might assume that antihypertensive therapy would help prevent dementia, but the data are conflicting. The Systolic Hypertension in Europe (SYST-EUR) study1 showed a 53% reduction in vascular dementia or mixed dementia among patients receiving antihypertensive medication and a 60% reduction in AD. Similarly, the PROGRESS2 clinical trial of prevention of recurrent stroke by antihypertensive treatment reported a 34% reduction in a composite measure of cognitive impairment and dementia. On the other hand, cognitive function neither improved nor worsened in the Hypertension in the Very Elderly Trial (HYVET-COG),3 whether patients received blood pressure treatment or placebo.
Hyperlipidemia. Lipid metabolism likely is an important pathway in amyloid beta-protein deposition, tau phosphorylation, and disruption of synaptic plasticity and neurodegenerative endpoints. Cognitive decline and incident dementia have been associated with higher dietary intake of saturated fats, partially hydrogenated unsaturated fatty acids (trans fats), and cholesterol. Not all studies have found this association, however. This could be because serum cholesterol levels may decrease in early dementia, limiting the ability to detect an effect of hypercholesterolemia on dementia risk when measurements are made later in life.
Using statins (3-hydroxy-3-methylglutaryl–coenzyme A reductase inhibitors) to treat hypercholesterolemia has been hypothesized to impede large vessel atherosclerosis and its consequences and to trigger metabolic effects in the brain related to AD pathogenesis. Mechanisms by which statins might help prevent dementia include:
- a direct association between amyloid processing and cholesterol in the brain
- an indirect effect by decreasing the risk of stroke, as even small cerebral infarcts worsen AD severity.
Nonrandomized epidemiologic studies such as the Cardiovascular Health Study4 and MRC/BHF Heart Protection Study5 suggested that statin treatment might reduce the incidence of dementia, the degree of age-related cognitive decline, and AD’s neuropathologic burden. Large, randomized, controlled trials have not supported these observations, however. Statins failed to reduce the incidence of dementia in:
- the Heart Protection Study, testing simvastatin for 5 years in 20,536 subjects age 40 to 805
- the 3-year Preventive Study of Pravastatin in the Elderly at Risk (PROSPER) of 5,800 subjects.6
Similarly, patients receiving adjunctive atorvastatin or placebo showed no significant differences in cognition assessments after 72 weeks in the Lipitor’s Effect in Alzheimer’s Dementia (LEADe) study. This trial enrolled 640 subjects age 50 to 90 with mild-to-moderate dementia who were treated with donepezil.7 A recent Cochrane review concluded that high serum cholesterol may contribute to the development of AD and vascular dementia, but lowering cholesterol levels with statins does not prevent these problems.8
Diabetes mellitus. Diabetes and cognitive decline are closely associated. Diabetes is associated with a 50% to 100% increase in risk of AD and dementia overall and a 100% to 150% increased risk of vascular dementia. The mechanism by which diabetes increases dementia risk is uncertain but does not appear to be mediated entirely through vascular disease. High and low insulin levels may increase the risk of dementia, independent of diabetes and blood glucose. Increased peripheral insulin levels are associated with reduced brain atrophy and cognitive impairment in patients with early AD, suggesting a role for insulin signaling in AD pathophysiology. A possible relationship between insulin and beta amyloid metabolism is being studied.
Elevated postprandial plasma glucose has been associated with accelerated declines in cognitive performance.9 An inverse correlation has been noted between some cognitive measures and hemoglobin A1C levels.10 It is not clear that treating diabetes reduces the risk of dementia. In addition, in the prospective, population-based Rotterdam study, elderly patients with type 2 diabetes treated with insulin had the highest incidence of dementia.11
Tobacco smoke directly affects neuronal function, integrity, and survival. Chronic smoking has been linked to decreased global cerebral blood flow, accelerated cerebral atrophy, and ventricular enlargement.
Some studies suggest an increased risk of dementia in middle-aged and elderly smokers, possibly through a cerebrovascular mechanism such as stroke. Other studies found no association between smoking and dementia risk, and 1 suggested that nicotine may protect against AD by reducing senile plaque formation. Any protective effect of smoking would be offset by increased risks of lung cancer, chronic obstructive pulmonary disease, and vascular dementia.
The apolipoprotein E epsilon 4 (APOE e4) gene may explain, at least in part, the conflicting results of these studies. In 2 population-based cohorts,12,13 smoking was associated with memory decline in patients without, but not with, the APOE e4 genotype.
Dietary factors
Antioxidants. The brains of patients with AD contain elevated levels of endogenous antioxidants. In vitro studies show exogenous antioxidants can reduce the toxicity of beta-amyloid in brain tissue of persons with AD. These findings have led to interest in assessing the role of dietary antioxidants such as vitamins E and C for AD prevention.
High-dose alpha-tocopherol (vitamin E, 2,000 IU/d) may slow disease progression in patients with AD, but this association is not consistently found. Furthermore, a meta-analysis of 19 randomized controlled trials (RCTs) totaling >135,000 patients found an association between vitamin E doses >400 IU/d and increased all-cause mortality.14 High-dose vitamin E supplementation for primary or secondary prevention of AD may be dangerous and is not recommended.
The lack of consistent efficacy data for vitamin C in preventing or treating AD may discourage its routine use for this purpose.15
Homocysteine is a risk factor for stroke and heart disease. It also could play a role in vascular dementia through its association with large- and small-vessel disease.
Low folate and hyperhomocysteinemia have been associated with dementia or cognitive impairment, although a cause-effect relationship is not clear. In non-demented elderly populations, plasma homocysteine is inversely associated with poor performance in tests of global cognitive function, particularly in measures of psychomotor speed.
In a recent double-blind RCT, folic acid supplementation for 3 years significantly improved domains of cognitive function that tend to decline with age, especially information processing and sensorimotor speed.16 No other good evidence, however, has shown that homocysteine-lowering therapy using folic acid or other vitamin B supplements improves cognitive function or prevents cognitive decline.
Fish and omega-3 fatty acids. High total fat, saturated fat, and total cholesterol intake increases the risk for incident dementia. In epidemiologic studies, low omega-3 fatty acid serum levels have been linked to increased dementia risk.
Fish consumption may be beneficial in reducing the risk of dementia or cognitive decline. A prospective study of 815 elderly persons found 60% less risk of developing AD in those who ate ≥1 fish meal per week, compared with those who rarely or never ate fish.17 In the Framingham study, individuals who at baseline were in the top quartile of docosahexaenoic acid consumption had lower dementia rates over 9 years of follow-up.18 Results from cross-sectional and longitudinal studies have been inconsistent; some have shown that high intake of n-3 polyunsaturated fatty acids is associated with less cognitive decline,19 whereas others have not.20
Although we cannot offer unequivocal advice regarding seafood or omega-3 fatty acid intake for primary prevention of dementia without evidence from RCTs, these uncontrolled studies show promise.
Mediterranean diet (MeDi) components include abundant fruits and vegetables, fish or shellfish at least twice weekly, very limited red meat, olive oil or canola oil instead of butter or margarine, tree nuts such as walnuts or pecans, red wine in moderation, and using herbs and spices instead of salt to season food. High adherence to the MeDi has been associated with a significantly lower risk for incident AD. The MeDi may affect the risk of developing AD21 as well as subsequent disease course, with a possible dose-response relationship in lower mortality.22
Eating fruits and vegetables has been associated with improved cognitive performance22 and decreased incident dementia in elderly subjects.18
Alcohol. A U-shaped relationship exists between alcohol consumption and dementia risk. High alcohol intake is associated with clinical problem drinking and alcoholism and can lead to cognitive decline. Conversely, moderate wine consumption (250 to 500 mL/d) may be protective—compared with more or less than this amount—and is associated with approximately 50% less risk of dementia.
Alcohol use may increase the risk of dementia in persons carrying the APOE e4 allele, according to the population-based Cardiovascular Risk Factors, Aging and Dementia (CAIDE) study from Sweden.23 After an average 21 years of follow-up of 1,449 individuals, researchers found that environmental factors—such as physical inactivity, dietary fat intake, alcohol consumption, and smoking at midlife—were associated with an increased risk of dementia at age 65 to 79 in APOE e4 carriers compared with noncarriers. The study also found that physical inactivity, dietary fat intake, and smoking at midlife increase AD risk, especially among APOE e4 carriers.
In the absence of evidence from RCTs, we cannot recommend alcohol to reduce the risk of AD.
Lifestyle and activity
Three components of lifestyle—social, mental, and physical activity—are inversely associated with the risk for dementia, AD, and cognitive impairment.
Physical exercise has been thought to enhance brain neurotrophic factor and modify apoptosis. Exercise can deter stroke and microvascular disease and improve regional cerebral blood flow. In the Cardiovascular Health Study, participants who expended the highest quartile of energy had a lower risk of all-cause dementia and AD compared with participants who expended the lowest quartile of energy.24
Mental and social activity. Epidemiologic studies have shown associations between higher educational achievement and other socioeconomic factors and reduced AD risk. Advanced education is believed to represent a cognitive reserve that delays presentation of AD’s effects on memory and cognitive function, rather than providing a protective effect against accumulation of AD pathology. Higher-educated individuals appear to experience a somewhat more rapid rate of cognitive decline when AD does become apparent, perhaps because they have accumulated a greater degree of AD pathology at that point compared with less-educated persons.
Among 117 persons with dementia in the Bronx Aging Study, each additional year of formal education delayed the time of accelerated decline by 0.21 years. After accelerated decline began, each year of additional formal education was associated with a slightly faster rate of memory decline.25
The longitudinal, population-based Kungsholmen Project in Stockholm, Sweden, found an association between daily mentally stimulating activities and decreased risk of all-cause dementia.26 Similarly, higher levels of leisure activity were linked to reduced risk of all-cause dementia in a longitudinal study of 1,772 persons age ≥65 living in Manhattan, NY.27 In a randomized, single-controlled study of the long-term effects of cognitive training, elderly individuals from 6 U.S. cities showed sustained improvement in specific cognitive performance up to 5 years after training sessions began, including memory, reasoning, and speed of processing.28
It seems reasonable to encourage older patients to maintain or increase physical, cognitive, and leisure activities as well as social interaction. These interventions can improve the quality of life and lower the risk of depression, which may be a response to cognitive decline or an independent risk factor for dementia (Box 3). The Table lists “brain exercises” you can suggest to patients to increase their mental and social activity.
Head trauma. The Multi-Institutional Research in Alzheimer’s Genetic Epidemiology (MIRAGE) project found an association between AD risk and a history of head trauma, especially in persons with APOE e4 alleles.29 Conversely, the Rotterdam Study showed no change in dementia risk for persons with a history of head trauma.30
Even in the absence of conclusive evidence supporting AD prevention, protecting the head by buckling seat belts while driving, wearing helmets when participating in sports, and “fall-proofing” the home is recommended.
Depression often occurs before or as a coexisting condition with Alzheimer’s disease (AD).a Although depression has been considered a response to cognitive decline or an early manifestation of dementia,b it also could be an independent risk factor.c,d
The pathologic mechanism linking depression and subsequent dementia is not well understood. Hypotheses include an indirect neurotoxic effect of depression mediated by cortisol-induced hippocampal atrophy or lowered brain-derived neurotrophic factor levels.e Depression and dementia might share genetic links, although a cohort study of 404 individuals with AD detected no association between apolipoprotein E genotypes or alleles and depressive symptoms.f
References
a. Lupien SJ, Nair NP, Brière S, et al. Increased cortisol levels and impaired cognition in human aging: implication for depression and dementia in later life. Rev Neurosci. 1999;10(2):117-139.
b. Preuss UW, Siafarikas N, Petrucci M, et al. Depressive disorders in dementia and mild cognitive impairments: is comorbidity a cause or a risk factor? Fortschr Neurol Psychiatr. 2009;77:399-406.
c. Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60(5):753-759.
d. Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer’s disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530-538.
e. Meeks TW, Ropacki SA, Jeste DV. The neurobiology of neuropsychiatric syndromes in dementia. Curr Opin Psychiatry. 2006;19(6):581-586.
f. Craig D, Hart DJ, McIlroy SP, et al. Association analysis of apolipoprotein E genotype and risk of depressive symptoms in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19(2-3):154-157.
Table
Brain exercises to suggest to patients
| Learn something new (how to play a musical instrument, a foreign language, or a new hobby) |
| Play memory games |
| Practice using the opposite hand to perform tasks you usually do with your dominant hand |
| Read, especially challenging material |
| Join a book discussion group |
| Write; if not a book or article, write a diary, letters, or emails or start your memoirs |
| Do crossword, Sudoku, or jigsaw puzzles |
| Play board games, card games, and other strategy games |
| Debate or discuss topics |
Related resource
- For an extensive bibliography of literature on Alzheimer’s disease risk factors and prevention, see this article at CurrentPsychiatry.com.
Drug brand names
- Atorvastatin • Lipitor
- Celecoxib • Celebrex
- Donepezil • Aricept
- Medroxyprogesterone • Provera
- Pravastatin • Pravachol
- Rofecoxib • Vioxx
- Simvastatin • Zocor
Disclosures
Dr. Bassil reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Dr. Grossberg receives research/grant support from and is a consultant to Bristol-Myers Squibb, Forest Pharmaceuticals, Novartis, Pfizer Inc., and Wyeth Pharmaceuticals. He also receives research/grant support from Baxter.
1. Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the systolic hypertension in Europe (Syst-Eur) study. Arch Intern Med. 2002;162:2046-2052.
2. Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069-1075.
3. Peters R, Beckett N, Forette F. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG). Lancet Neurol. 2008;7(8):683-689.
4. Rea TD, Breitner JC, Psaty BM, et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol. 2005;62:1047-1051.
5. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7-22.
6. Kulbertus H, Scheen AJ. [The PROSPER Study (PROspective study of pravastatin in the elderly at risk)]. Rev Med Liege. 2002;57(12):809-813.
7. Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74(12):956-964.
8. McGuinness B, Bullock R, Craig D, et al. Statins for the treatment of Alzheimer’s disease and dementia (protocol). Cochrane Database Syst Rev. 2009;1:CD007514.-
9. Abbatecola AM, Rizzo MR, Barbieri M, et al. Postprandial plasmaglucose excursions and cognitive functioning in aged type 2 diabetics. Neurology. 2006;67:235-240.
10. Munshi M, Grande L, Hayes M, et al. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care. 2006;29:1794-1799.
11. Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia. The Rotterdam study. Neurology. 1999;53:1937-1942.
12. Reitz C, Luchsinger J, Tang MX, et al. Effect of smoking and time on cognitive function in the elderly without dementia. Neurology. 2005;65:870-875.
13. Reitz C, den Heijer T, van Duijn C, et al. Relation between smoking and risk of dementia and Alzheimer disease: the Rotterdam Study. Neurology. 2007;69:998-1005.
14. Miller ER, III, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37-46.
15. Boothby LA, Doering PL. Vitamin C and vitamin E for Alzheimer’s disease. Ann Pharmacother. 2005;39(12):2073-2080.
16. Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369:208-216.
17. Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940-946.
18. Schaefer EJ, Bongard V, Beiser AS, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545-1550.
19. Kalmijn S, Launer LJ, Ott A, et al. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776-782.
20. van Gelder BM, Tijhuis M, Kalmijn S, et al. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007;85:1142-1147.
21. Solfrizzi V, Capurso C, Panza F. Adherence to a Mediterranean dietary pattern and risk of Alzheimer’s disease. Ann Neurol. 2006;60:620.-
22. Scarmeas N, Luchsinger JA, Mayeux R, et al. Mediterranean diet and Alzheimer disease mortality. Neurology. 2007;69(11):1084-1093.
23. Kivipelto M, Rovio S, Ngandu T, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12(6B):2762-2771.
24. Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639-651.
25. Hall CB, Derby C, LeValley A, et al. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69:1657-1664.
26. Wang HX, Karp A, Winblad B, et al. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen Project. Am J Epidemiol. 2002;155:1081-1087.
27. Scarmeas N, Levy G, Tang MX, et al. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236-2242.
28. Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805-2814.
29. Guo Z, Cupples LA, Kurz A, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316-1323.
30. Ruitenberg A, van Swieten JC, Witteman JC, et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281-286.
1. Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the systolic hypertension in Europe (Syst-Eur) study. Arch Intern Med. 2002;162:2046-2052.
2. Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069-1075.
3. Peters R, Beckett N, Forette F. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG). Lancet Neurol. 2008;7(8):683-689.
4. Rea TD, Breitner JC, Psaty BM, et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol. 2005;62:1047-1051.
5. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7-22.
6. Kulbertus H, Scheen AJ. [The PROSPER Study (PROspective study of pravastatin in the elderly at risk)]. Rev Med Liege. 2002;57(12):809-813.
7. Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74(12):956-964.
8. McGuinness B, Bullock R, Craig D, et al. Statins for the treatment of Alzheimer’s disease and dementia (protocol). Cochrane Database Syst Rev. 2009;1:CD007514.-
9. Abbatecola AM, Rizzo MR, Barbieri M, et al. Postprandial plasmaglucose excursions and cognitive functioning in aged type 2 diabetics. Neurology. 2006;67:235-240.
10. Munshi M, Grande L, Hayes M, et al. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes Care. 2006;29:1794-1799.
11. Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia. The Rotterdam study. Neurology. 1999;53:1937-1942.
12. Reitz C, Luchsinger J, Tang MX, et al. Effect of smoking and time on cognitive function in the elderly without dementia. Neurology. 2005;65:870-875.
13. Reitz C, den Heijer T, van Duijn C, et al. Relation between smoking and risk of dementia and Alzheimer disease: the Rotterdam Study. Neurology. 2007;69:998-1005.
14. Miller ER, III, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37-46.
15. Boothby LA, Doering PL. Vitamin C and vitamin E for Alzheimer’s disease. Ann Pharmacother. 2005;39(12):2073-2080.
16. Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369:208-216.
17. Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940-946.
18. Schaefer EJ, Bongard V, Beiser AS, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545-1550.
19. Kalmijn S, Launer LJ, Ott A, et al. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776-782.
20. van Gelder BM, Tijhuis M, Kalmijn S, et al. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007;85:1142-1147.
21. Solfrizzi V, Capurso C, Panza F. Adherence to a Mediterranean dietary pattern and risk of Alzheimer’s disease. Ann Neurol. 2006;60:620.-
22. Scarmeas N, Luchsinger JA, Mayeux R, et al. Mediterranean diet and Alzheimer disease mortality. Neurology. 2007;69(11):1084-1093.
23. Kivipelto M, Rovio S, Ngandu T, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12(6B):2762-2771.
24. Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639-651.
25. Hall CB, Derby C, LeValley A, et al. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69:1657-1664.
26. Wang HX, Karp A, Winblad B, et al. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen Project. Am J Epidemiol. 2002;155:1081-1087.
27. Scarmeas N, Levy G, Tang MX, et al. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236-2242.
28. Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805-2814.
29. Guo Z, Cupples LA, Kurz A, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316-1323.
30. Ruitenberg A, van Swieten JC, Witteman JC, et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281-286.