User login
Iliac vein compression syndrome: An underdiagnosed cause of lower extremity deep venous thrombosis
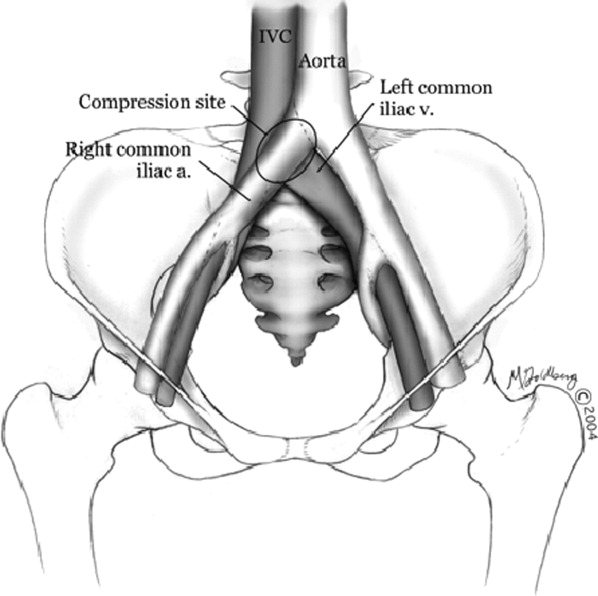
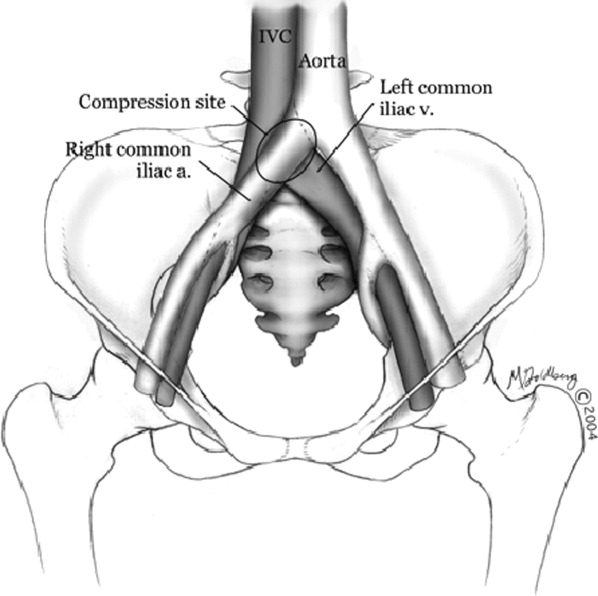
Hospitalists frequently diagnose and treat lower extremity deep venous thrombosis (DVT). Patients presenting with acute DVT or chronic venous stasis of the left leg can have an underlying anatomic anomaly known as iliac vein compression syndrome (ICS), May‐Thurner syndrome, or Cockett syndrome in Europe. In this condition, the right iliac artery overlies the left iliac vein, causing extrinsic compression of the vein (Figure 1). 1 This compression and accompanying intraluminal changes predisposes patients to left‐sided lower extremity DVT.2 Failure to recognize and treat this anomaly in patients with acute thrombosis can result in serious vascular sequelae and chronic left leg symptoms.3 A high clinical suspicion should be maintained in young individuals presenting with proximal left leg DVT with or without hypercoagulable risk factors. The following report is a case of ICS in a young male recognized and treated early by aggressive diagnostic and therapeutic interventions.
11‐ Grunwald et al.
Case Report
A 19‐year‐old man presented to the ER with sudden onset of left lower extremity swelling and pain 5 days after a fall. He had no known risk factors for DVT. On physical examination his left leg was dusky, swollen, and tender from his groin to his ankle, with good arterial pulses. Duplex ultra‐sonogram of the leg showed a clot in the femoral vein extending up the popliteal vein. Following a venogram, he underwent mechanical thrombectomy and regional thrombolysis. A repeat venogram showed an irregular narrowing of the left iliac vein and a tubular filling defect at the junction of the inferior vena cava and common iliac veins, suggestive of external compression from the right common iliac artery. The patient underwent successful angioplasty and stenting of the common iliac vein. He was treated with intravenous heparin, warfarin and clopidogrel. His hypercoagulable work‐up was inconclusive.
Discussion
In 1956, May and Thurner 1 brought clinical attention to ICS. They hypothesized that an abnormal compression of the left iliac vein by an overriding right iliac arterypresent in 22% of a series of 430 cadaversled to an intraluminal filling defect in the vein. The chronic extrinsic compression and pulsing force from the overlying artery results in endothelial irritation and formation of venous spurs (fibrous vascular lesions) in the intimal layer of the vein.1 Following the principles of Virchow's triad, this endothelial injury propagates the formation of a thrombus. Subsequent studies by Kim et al.4 suggest that there are 3 stages involved in the pathogenesis of thrombosis in ICS: asymptomatic vein compression, venous spur formation, and finally DVT formation.4, 5 It is estimated that 1 to 3 out of 1000 individuals with this malformation develop DVT each year.5, 6
Patients with ICS may present to the emergency or ambulatory setting in either an acute or chronic phase. The acute phase is the actual episode of thrombosis. Symptoms include left leg pain and swelling up to the groin. In rare cases, pulmonary emboli may be the initial presentation. A lifelong chronic phase can follow if undiagnosed, resulting in pain and swelling of the entire left leg, venous claudication, recurrent thrombosis, pigmentation changes, and ulceration. 3
The typical ICS patient is a woman between 18 and 30 years old, 3 possibly due to the developmental changes in the pelvic structures in preparation for child‐bearing.2 Many patients also present after pregnancy; increased lordosis during pregnancy may put additional strain on the anatomic lesion.3 Nevertheless, Steinberg and Jacocks7 reported that out of 127 patients, 38 (30%) were male. Thus, it is critical not to overlook ICS as a possible cause of thrombosis in male patients.
The urgency in diagnosing this anatomic variation lies in the distinct need for more aggressive treatment than that required for a typical DVT. While Doppler ultrasound is typically the first diagnostic test performed in this patient population, it is not specific. For patients with physical exam findings highly suspicious of ICS, venography and magnetic resonance venography are superior modalities to make a definitive diagnosis of the syndrome. 8 In ICS, these studies will reveal left common iliac vein narrowing with intraluminal changes suggestive of spur formation.2
Due to the mechanical nature of ICS pathology, anticoagulation therapy alone is ineffective. ICS prevents recanalization in 70% to 80% of patients and up to 40% will have continued clot propagation. 5, 7 More aggressive treatment using endovascular techniques such as the combination of thrombectomy, angioplasty, and intraluminal stenting have proven to be the most efficacious treatment modality for ICS.9 A study by AbuRahma et al.10 demonstrated that one year following this aggressive combination, patency rate was 83% (vs. 24% following thrombectomy alone).
Conclusion
The anatomic anomaly present in ICS was identified by CT in as many as two‐thirds of an asymptomatic patient population studied by Kibbe et al. 12 Although a common structural anomaly, it is important to note that only 1 to 3 out of 1000 individuals with this malformation develop DVT annually. ICS should be included in the differential diagnosis of all young individuals presenting with proximal left leg DVT with or without hypercoagulable risk factors. If the mechanical compression is not diagnosed and treated, the syndrome can develop into a life‐long chronic phase with multiple complications.2 It is therefore critical that aggressive diagnostic and therapeutic interventions be implemented immediately upon suspicion of ICS.
- , . A vascular spur in the vena iliaca communis sinistra as a cause of predominantly left‐sided thrombosis of the pelvic veins. Z Kreislaufforsch. 1956;45:912–922.
- , , , , . Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2008;19:366–370; quiz 71.
- . The iliac compression syndrome alias ‘Iliofemoral thrombosis’ or ‘white leg’. Proc R Soc Med. 1966;59:360–361.
- , , . Venographic anatomy, technique and interpretation. Pheripheral Vascular Imaging and Intervention. St. Louis (MO): Mosby‐Year Book; 1992. p. 269–349.
- , , , , . Symptomatic ileofemoral DVT after onset of oral contraceptive use in women with previously undiagnosed May‐Thurner Syndrome. J Vasc Surg. 2009;49:697–703.
- , , . A prospective study of the incidence of deep vein thrombosis within a defined urban population. J Intern Med. 1992;232:152–160.
- , . May‐Thurner syndrome: a previously unreported variant. Ann Vasc Surg. 1993;7:577–581.
- , , , , , . Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34:106–113.
- , , , et al. Endovascular management of iliac vein compression (May‐Thurner) syndrome. J Vasc Interv Radiol. 2000;11:823–836.
- , , , . Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg. 2001;233:752–760.
- , , . Endovascular management of May‐Thurner Syndrome. Am J Roentgenol. 2004;183:1523–1524.
- , , , , , . Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004:39:937–943.
Hospitalists frequently diagnose and treat lower extremity deep venous thrombosis (DVT). Patients presenting with acute DVT or chronic venous stasis of the left leg can have an underlying anatomic anomaly known as iliac vein compression syndrome (ICS), May‐Thurner syndrome, or Cockett syndrome in Europe. In this condition, the right iliac artery overlies the left iliac vein, causing extrinsic compression of the vein (Figure 1). 1 This compression and accompanying intraluminal changes predisposes patients to left‐sided lower extremity DVT.2 Failure to recognize and treat this anomaly in patients with acute thrombosis can result in serious vascular sequelae and chronic left leg symptoms.3 A high clinical suspicion should be maintained in young individuals presenting with proximal left leg DVT with or without hypercoagulable risk factors. The following report is a case of ICS in a young male recognized and treated early by aggressive diagnostic and therapeutic interventions.

11‐ Grunwald et al.
Case Report
A 19‐year‐old man presented to the ER with sudden onset of left lower extremity swelling and pain 5 days after a fall. He had no known risk factors for DVT. On physical examination his left leg was dusky, swollen, and tender from his groin to his ankle, with good arterial pulses. Duplex ultra‐sonogram of the leg showed a clot in the femoral vein extending up the popliteal vein. Following a venogram, he underwent mechanical thrombectomy and regional thrombolysis. A repeat venogram showed an irregular narrowing of the left iliac vein and a tubular filling defect at the junction of the inferior vena cava and common iliac veins, suggestive of external compression from the right common iliac artery. The patient underwent successful angioplasty and stenting of the common iliac vein. He was treated with intravenous heparin, warfarin and clopidogrel. His hypercoagulable work‐up was inconclusive.
Discussion
In 1956, May and Thurner 1 brought clinical attention to ICS. They hypothesized that an abnormal compression of the left iliac vein by an overriding right iliac arterypresent in 22% of a series of 430 cadaversled to an intraluminal filling defect in the vein. The chronic extrinsic compression and pulsing force from the overlying artery results in endothelial irritation and formation of venous spurs (fibrous vascular lesions) in the intimal layer of the vein.1 Following the principles of Virchow's triad, this endothelial injury propagates the formation of a thrombus. Subsequent studies by Kim et al.4 suggest that there are 3 stages involved in the pathogenesis of thrombosis in ICS: asymptomatic vein compression, venous spur formation, and finally DVT formation.4, 5 It is estimated that 1 to 3 out of 1000 individuals with this malformation develop DVT each year.5, 6
Patients with ICS may present to the emergency or ambulatory setting in either an acute or chronic phase. The acute phase is the actual episode of thrombosis. Symptoms include left leg pain and swelling up to the groin. In rare cases, pulmonary emboli may be the initial presentation. A lifelong chronic phase can follow if undiagnosed, resulting in pain and swelling of the entire left leg, venous claudication, recurrent thrombosis, pigmentation changes, and ulceration. 3
The typical ICS patient is a woman between 18 and 30 years old, 3 possibly due to the developmental changes in the pelvic structures in preparation for child‐bearing.2 Many patients also present after pregnancy; increased lordosis during pregnancy may put additional strain on the anatomic lesion.3 Nevertheless, Steinberg and Jacocks7 reported that out of 127 patients, 38 (30%) were male. Thus, it is critical not to overlook ICS as a possible cause of thrombosis in male patients.
The urgency in diagnosing this anatomic variation lies in the distinct need for more aggressive treatment than that required for a typical DVT. While Doppler ultrasound is typically the first diagnostic test performed in this patient population, it is not specific. For patients with physical exam findings highly suspicious of ICS, venography and magnetic resonance venography are superior modalities to make a definitive diagnosis of the syndrome. 8 In ICS, these studies will reveal left common iliac vein narrowing with intraluminal changes suggestive of spur formation.2
Due to the mechanical nature of ICS pathology, anticoagulation therapy alone is ineffective. ICS prevents recanalization in 70% to 80% of patients and up to 40% will have continued clot propagation. 5, 7 More aggressive treatment using endovascular techniques such as the combination of thrombectomy, angioplasty, and intraluminal stenting have proven to be the most efficacious treatment modality for ICS.9 A study by AbuRahma et al.10 demonstrated that one year following this aggressive combination, patency rate was 83% (vs. 24% following thrombectomy alone).
Conclusion
The anatomic anomaly present in ICS was identified by CT in as many as two‐thirds of an asymptomatic patient population studied by Kibbe et al. 12 Although a common structural anomaly, it is important to note that only 1 to 3 out of 1000 individuals with this malformation develop DVT annually. ICS should be included in the differential diagnosis of all young individuals presenting with proximal left leg DVT with or without hypercoagulable risk factors. If the mechanical compression is not diagnosed and treated, the syndrome can develop into a life‐long chronic phase with multiple complications.2 It is therefore critical that aggressive diagnostic and therapeutic interventions be implemented immediately upon suspicion of ICS.
Hospitalists frequently diagnose and treat lower extremity deep venous thrombosis (DVT). Patients presenting with acute DVT or chronic venous stasis of the left leg can have an underlying anatomic anomaly known as iliac vein compression syndrome (ICS), May‐Thurner syndrome, or Cockett syndrome in Europe. In this condition, the right iliac artery overlies the left iliac vein, causing extrinsic compression of the vein (Figure 1). 1 This compression and accompanying intraluminal changes predisposes patients to left‐sided lower extremity DVT.2 Failure to recognize and treat this anomaly in patients with acute thrombosis can result in serious vascular sequelae and chronic left leg symptoms.3 A high clinical suspicion should be maintained in young individuals presenting with proximal left leg DVT with or without hypercoagulable risk factors. The following report is a case of ICS in a young male recognized and treated early by aggressive diagnostic and therapeutic interventions.

11‐ Grunwald et al.
Case Report
A 19‐year‐old man presented to the ER with sudden onset of left lower extremity swelling and pain 5 days after a fall. He had no known risk factors for DVT. On physical examination his left leg was dusky, swollen, and tender from his groin to his ankle, with good arterial pulses. Duplex ultra‐sonogram of the leg showed a clot in the femoral vein extending up the popliteal vein. Following a venogram, he underwent mechanical thrombectomy and regional thrombolysis. A repeat venogram showed an irregular narrowing of the left iliac vein and a tubular filling defect at the junction of the inferior vena cava and common iliac veins, suggestive of external compression from the right common iliac artery. The patient underwent successful angioplasty and stenting of the common iliac vein. He was treated with intravenous heparin, warfarin and clopidogrel. His hypercoagulable work‐up was inconclusive.
Discussion
In 1956, May and Thurner 1 brought clinical attention to ICS. They hypothesized that an abnormal compression of the left iliac vein by an overriding right iliac arterypresent in 22% of a series of 430 cadaversled to an intraluminal filling defect in the vein. The chronic extrinsic compression and pulsing force from the overlying artery results in endothelial irritation and formation of venous spurs (fibrous vascular lesions) in the intimal layer of the vein.1 Following the principles of Virchow's triad, this endothelial injury propagates the formation of a thrombus. Subsequent studies by Kim et al.4 suggest that there are 3 stages involved in the pathogenesis of thrombosis in ICS: asymptomatic vein compression, venous spur formation, and finally DVT formation.4, 5 It is estimated that 1 to 3 out of 1000 individuals with this malformation develop DVT each year.5, 6
Patients with ICS may present to the emergency or ambulatory setting in either an acute or chronic phase. The acute phase is the actual episode of thrombosis. Symptoms include left leg pain and swelling up to the groin. In rare cases, pulmonary emboli may be the initial presentation. A lifelong chronic phase can follow if undiagnosed, resulting in pain and swelling of the entire left leg, venous claudication, recurrent thrombosis, pigmentation changes, and ulceration. 3
The typical ICS patient is a woman between 18 and 30 years old, 3 possibly due to the developmental changes in the pelvic structures in preparation for child‐bearing.2 Many patients also present after pregnancy; increased lordosis during pregnancy may put additional strain on the anatomic lesion.3 Nevertheless, Steinberg and Jacocks7 reported that out of 127 patients, 38 (30%) were male. Thus, it is critical not to overlook ICS as a possible cause of thrombosis in male patients.
The urgency in diagnosing this anatomic variation lies in the distinct need for more aggressive treatment than that required for a typical DVT. While Doppler ultrasound is typically the first diagnostic test performed in this patient population, it is not specific. For patients with physical exam findings highly suspicious of ICS, venography and magnetic resonance venography are superior modalities to make a definitive diagnosis of the syndrome. 8 In ICS, these studies will reveal left common iliac vein narrowing with intraluminal changes suggestive of spur formation.2
Due to the mechanical nature of ICS pathology, anticoagulation therapy alone is ineffective. ICS prevents recanalization in 70% to 80% of patients and up to 40% will have continued clot propagation. 5, 7 More aggressive treatment using endovascular techniques such as the combination of thrombectomy, angioplasty, and intraluminal stenting have proven to be the most efficacious treatment modality for ICS.9 A study by AbuRahma et al.10 demonstrated that one year following this aggressive combination, patency rate was 83% (vs. 24% following thrombectomy alone).
Conclusion
The anatomic anomaly present in ICS was identified by CT in as many as two‐thirds of an asymptomatic patient population studied by Kibbe et al. 12 Although a common structural anomaly, it is important to note that only 1 to 3 out of 1000 individuals with this malformation develop DVT annually. ICS should be included in the differential diagnosis of all young individuals presenting with proximal left leg DVT with or without hypercoagulable risk factors. If the mechanical compression is not diagnosed and treated, the syndrome can develop into a life‐long chronic phase with multiple complications.2 It is therefore critical that aggressive diagnostic and therapeutic interventions be implemented immediately upon suspicion of ICS.
- , . A vascular spur in the vena iliaca communis sinistra as a cause of predominantly left‐sided thrombosis of the pelvic veins. Z Kreislaufforsch. 1956;45:912–922.
- , , , , . Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2008;19:366–370; quiz 71.
- . The iliac compression syndrome alias ‘Iliofemoral thrombosis’ or ‘white leg’. Proc R Soc Med. 1966;59:360–361.
- , , . Venographic anatomy, technique and interpretation. Pheripheral Vascular Imaging and Intervention. St. Louis (MO): Mosby‐Year Book; 1992. p. 269–349.
- , , , , . Symptomatic ileofemoral DVT after onset of oral contraceptive use in women with previously undiagnosed May‐Thurner Syndrome. J Vasc Surg. 2009;49:697–703.
- , , . A prospective study of the incidence of deep vein thrombosis within a defined urban population. J Intern Med. 1992;232:152–160.
- , . May‐Thurner syndrome: a previously unreported variant. Ann Vasc Surg. 1993;7:577–581.
- , , , , , . Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34:106–113.
- , , , et al. Endovascular management of iliac vein compression (May‐Thurner) syndrome. J Vasc Interv Radiol. 2000;11:823–836.
- , , , . Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg. 2001;233:752–760.
- , , . Endovascular management of May‐Thurner Syndrome. Am J Roentgenol. 2004;183:1523–1524.
- , , , , , . Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004:39:937–943.
- , . A vascular spur in the vena iliaca communis sinistra as a cause of predominantly left‐sided thrombosis of the pelvic veins. Z Kreislaufforsch. 1956;45:912–922.
- , , , , . Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2008;19:366–370; quiz 71.
- . The iliac compression syndrome alias ‘Iliofemoral thrombosis’ or ‘white leg’. Proc R Soc Med. 1966;59:360–361.
- , , . Venographic anatomy, technique and interpretation. Pheripheral Vascular Imaging and Intervention. St. Louis (MO): Mosby‐Year Book; 1992. p. 269–349.
- , , , , . Symptomatic ileofemoral DVT after onset of oral contraceptive use in women with previously undiagnosed May‐Thurner Syndrome. J Vasc Surg. 2009;49:697–703.
- , , . A prospective study of the incidence of deep vein thrombosis within a defined urban population. J Intern Med. 1992;232:152–160.
- , . May‐Thurner syndrome: a previously unreported variant. Ann Vasc Surg. 1993;7:577–581.
- , , , , , . Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34:106–113.
- , , , et al. Endovascular management of iliac vein compression (May‐Thurner) syndrome. J Vasc Interv Radiol. 2000;11:823–836.
- , , , . Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg. 2001;233:752–760.
- , , . Endovascular management of May‐Thurner Syndrome. Am J Roentgenol. 2004;183:1523–1524.
- , , , , , . Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004:39:937–943.
Stevens‐Johnson and mycoplasma pneumoniae: A scary duo
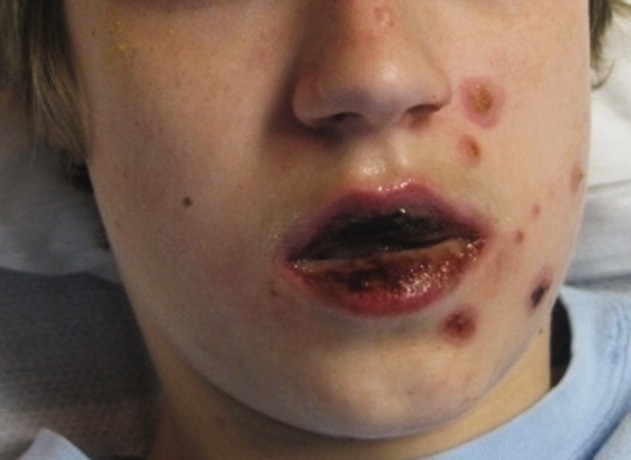
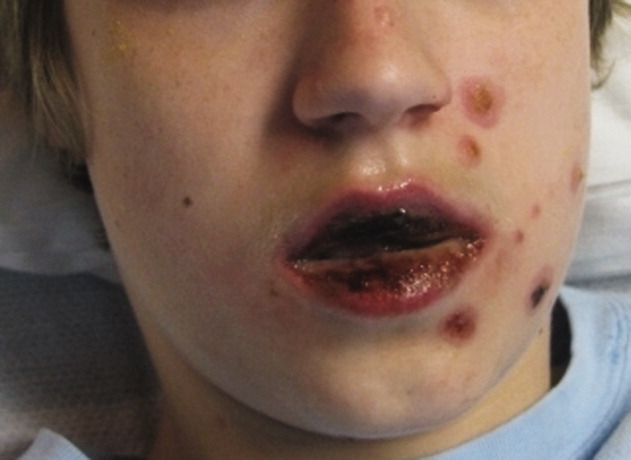
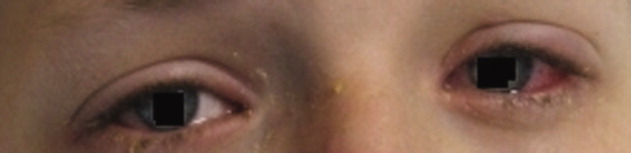
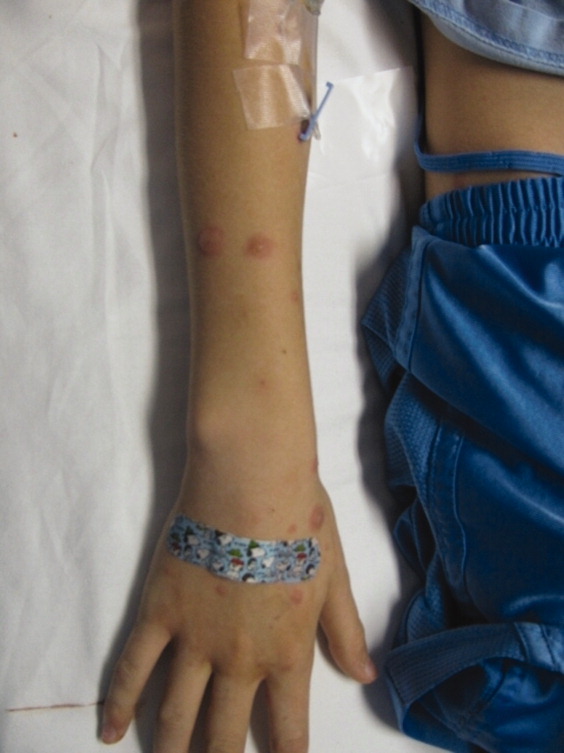
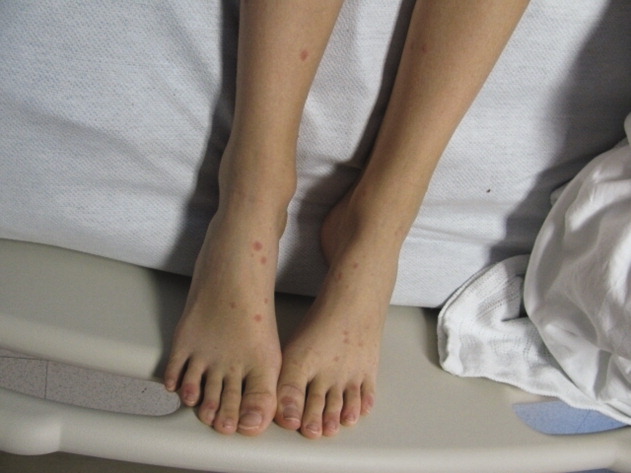
A 15‐year‐old male was hospitalized with painful blisters on the lips and ulcers in the oral mucosa that were preceded by upper respiratory infection symptoms for 1 week. He had not been treated with antimicrobials. He subsequently developed conjunctival injection and painful blisters at the urethral meatus and symmetric scattered target lesions in the extremities. Examination demonstrated low‐grade fever, mild conjunctival injection (Figure 2), and oral vesicular lesions affecting the lips (Figure 1) and both the hard and soft palate; he had vesicular lesions affecting the glans penis, a ruptured vesicle at the urethral meatus and target lesions in the arms (Figure 3) and legs (Figure 4). His cardiopulmonary exam was normal. He was started on acyclovir and azithromycin, and symptomatic treatment with oral lidocaine and morphine. Serologies for Epstein‐Barr virus (EBV), cytomegalovirus (CMV) and Coxsackievirus and cultures for herpes simplex virus (HSV) were negative. Mycoplasma pneumoniae immunoglobulin G (IgG) and IgM titers were significantly elevated (>4‐fold) and the diagnosis made of Stevens‐Johnson syndrome (SJS) secondary to Mycoplasma pneumoniae infection. He was able to tolerate oral intake after a 1‐week hospital course.
M. pneumoniae infection can cause mucocutaneous involvement varying from mild mucositis to SJS with significant morbidity and mortality, 1, 2 mostly in the pediatric population. The differential diagnosis includes HSV, Kawasaki, and Streptococcal toxic shock syndrome, as well as other viral infections (eg, Coxsackievirus).3 Pharmacologic causesespecially antibiotics, non steroidal anti‐inflammatory drug (NSAIDS) and anticonvulsantsshould also be considered in the etiology of SJS4 especially in the adult population.
- . Erythema multiforme due to Mycoplasma pneumoniae infection in two children. Pediatr Dermatol. 2006;23(6):546–555.
- . Mycoplasma pneumoniae infection complicated by severe mucocutaneous lesions. Lancet Infect Dis. 2008;8:268.
- , , , , , . Mycoplasma pneumoniae and atypical Stevens‐Johnson syndrome: a case series. Pediatrics. 2007;119:e1002–e1005.
- , , , . Mycoplasma pneumoniae associated with Stevens Johnson syndrome. Anaesth Intensive Care. 2007;35:414–417.
A 15‐year‐old male was hospitalized with painful blisters on the lips and ulcers in the oral mucosa that were preceded by upper respiratory infection symptoms for 1 week. He had not been treated with antimicrobials. He subsequently developed conjunctival injection and painful blisters at the urethral meatus and symmetric scattered target lesions in the extremities. Examination demonstrated low‐grade fever, mild conjunctival injection (Figure 2), and oral vesicular lesions affecting the lips (Figure 1) and both the hard and soft palate; he had vesicular lesions affecting the glans penis, a ruptured vesicle at the urethral meatus and target lesions in the arms (Figure 3) and legs (Figure 4). His cardiopulmonary exam was normal. He was started on acyclovir and azithromycin, and symptomatic treatment with oral lidocaine and morphine. Serologies for Epstein‐Barr virus (EBV), cytomegalovirus (CMV) and Coxsackievirus and cultures for herpes simplex virus (HSV) were negative. Mycoplasma pneumoniae immunoglobulin G (IgG) and IgM titers were significantly elevated (>4‐fold) and the diagnosis made of Stevens‐Johnson syndrome (SJS) secondary to Mycoplasma pneumoniae infection. He was able to tolerate oral intake after a 1‐week hospital course.
M. pneumoniae infection can cause mucocutaneous involvement varying from mild mucositis to SJS with significant morbidity and mortality, 1, 2 mostly in the pediatric population. The differential diagnosis includes HSV, Kawasaki, and Streptococcal toxic shock syndrome, as well as other viral infections (eg, Coxsackievirus).3 Pharmacologic causesespecially antibiotics, non steroidal anti‐inflammatory drug (NSAIDS) and anticonvulsantsshould also be considered in the etiology of SJS4 especially in the adult population.




A 15‐year‐old male was hospitalized with painful blisters on the lips and ulcers in the oral mucosa that were preceded by upper respiratory infection symptoms for 1 week. He had not been treated with antimicrobials. He subsequently developed conjunctival injection and painful blisters at the urethral meatus and symmetric scattered target lesions in the extremities. Examination demonstrated low‐grade fever, mild conjunctival injection (Figure 2), and oral vesicular lesions affecting the lips (Figure 1) and both the hard and soft palate; he had vesicular lesions affecting the glans penis, a ruptured vesicle at the urethral meatus and target lesions in the arms (Figure 3) and legs (Figure 4). His cardiopulmonary exam was normal. He was started on acyclovir and azithromycin, and symptomatic treatment with oral lidocaine and morphine. Serologies for Epstein‐Barr virus (EBV), cytomegalovirus (CMV) and Coxsackievirus and cultures for herpes simplex virus (HSV) were negative. Mycoplasma pneumoniae immunoglobulin G (IgG) and IgM titers were significantly elevated (>4‐fold) and the diagnosis made of Stevens‐Johnson syndrome (SJS) secondary to Mycoplasma pneumoniae infection. He was able to tolerate oral intake after a 1‐week hospital course.
M. pneumoniae infection can cause mucocutaneous involvement varying from mild mucositis to SJS with significant morbidity and mortality, 1, 2 mostly in the pediatric population. The differential diagnosis includes HSV, Kawasaki, and Streptococcal toxic shock syndrome, as well as other viral infections (eg, Coxsackievirus).3 Pharmacologic causesespecially antibiotics, non steroidal anti‐inflammatory drug (NSAIDS) and anticonvulsantsshould also be considered in the etiology of SJS4 especially in the adult population.




- . Erythema multiforme due to Mycoplasma pneumoniae infection in two children. Pediatr Dermatol. 2006;23(6):546–555.
- . Mycoplasma pneumoniae infection complicated by severe mucocutaneous lesions. Lancet Infect Dis. 2008;8:268.
- , , , , , . Mycoplasma pneumoniae and atypical Stevens‐Johnson syndrome: a case series. Pediatrics. 2007;119:e1002–e1005.
- , , , . Mycoplasma pneumoniae associated with Stevens Johnson syndrome. Anaesth Intensive Care. 2007;35:414–417.
- . Erythema multiforme due to Mycoplasma pneumoniae infection in two children. Pediatr Dermatol. 2006;23(6):546–555.
- . Mycoplasma pneumoniae infection complicated by severe mucocutaneous lesions. Lancet Infect Dis. 2008;8:268.
- , , , , , . Mycoplasma pneumoniae and atypical Stevens‐Johnson syndrome: a case series. Pediatrics. 2007;119:e1002–e1005.
- , , , . Mycoplasma pneumoniae associated with Stevens Johnson syndrome. Anaesth Intensive Care. 2007;35:414–417.
New Research Target
A report in this month’s Journal of Hospital Medicine shows macrolide and quinolone antibiotics are associated with similar rates of treatment failure in acute exacerbation of chronic pulmonary disease (AECOPD). The lead author says the study could be a precursor to, say, an intrepid HM researcher working on a randomized trial of the antibiotics’ effectiveness.
“It’s a perfect thing for hospitalists to study because they’re the ones treating it,” says Michael Rothberg, MD, MPH, associate professor of medicine at Tufts University School of Medicine in Boston, and lead author of "Comparative Effectiveness of Macrolides and Quinolones for Patients Hospitalized with Acute Exacerbations of Chronic Obstructive Pulmonary Disease (AECOPD)."
The retrospective cohort review reported that out of nearly 20,000 patients, 6,139 (31%) were treated initially with a macrolide and 13,469 (69%) with a quinolone. “Those who received macrolides had a lower risk of treatment failure (6.8% vs. 8.1%, p<0.01), a finding that was attenuated after multivariable adjustment (OR=0.89, 95% CI 0.78-1.01), and disappeared in a grouped-treatment analysis (OR=1.01, 95% CI 0.75-1.35),” the authors wrote. The study found no differences in adjusted length of stay or cost. However, antibiotic-associated diarrhea was more common with quinolones (1.2% vs. 0.6%, p<0.001).
Dr. Rothberg, who is affiliated with the Center for Quality of Care Research at Baystate Medical Center in Springfield, Mass., says the data, while a point in the right direction, should be viewed as a first step in doing more search to determine the best treatment for AECOPD.
“If you look at the guidelines, the recommendations are all over the map,” Dr. Rothberg says. “This is really because there are no randomized trials in COPD patients. … There are so many unanswered questions. There’s been so much focus on pneumonia, heart failure, and acute myocardial infarction. COPD kind of has a dearth of research.”
Dr. Rothberg hopes to further that research via the COPD Outcomes-Based Network for Clinical Effectiveness & Research Translation (CONCERT), a team of physicians and researchers from centers around the country who are advocating for improvements to COPD treatment. Baystate is one of CONCERT’s outposts.
A report in this month’s Journal of Hospital Medicine shows macrolide and quinolone antibiotics are associated with similar rates of treatment failure in acute exacerbation of chronic pulmonary disease (AECOPD). The lead author says the study could be a precursor to, say, an intrepid HM researcher working on a randomized trial of the antibiotics’ effectiveness.
“It’s a perfect thing for hospitalists to study because they’re the ones treating it,” says Michael Rothberg, MD, MPH, associate professor of medicine at Tufts University School of Medicine in Boston, and lead author of "Comparative Effectiveness of Macrolides and Quinolones for Patients Hospitalized with Acute Exacerbations of Chronic Obstructive Pulmonary Disease (AECOPD)."
The retrospective cohort review reported that out of nearly 20,000 patients, 6,139 (31%) were treated initially with a macrolide and 13,469 (69%) with a quinolone. “Those who received macrolides had a lower risk of treatment failure (6.8% vs. 8.1%, p<0.01), a finding that was attenuated after multivariable adjustment (OR=0.89, 95% CI 0.78-1.01), and disappeared in a grouped-treatment analysis (OR=1.01, 95% CI 0.75-1.35),” the authors wrote. The study found no differences in adjusted length of stay or cost. However, antibiotic-associated diarrhea was more common with quinolones (1.2% vs. 0.6%, p<0.001).
Dr. Rothberg, who is affiliated with the Center for Quality of Care Research at Baystate Medical Center in Springfield, Mass., says the data, while a point in the right direction, should be viewed as a first step in doing more search to determine the best treatment for AECOPD.
“If you look at the guidelines, the recommendations are all over the map,” Dr. Rothberg says. “This is really because there are no randomized trials in COPD patients. … There are so many unanswered questions. There’s been so much focus on pneumonia, heart failure, and acute myocardial infarction. COPD kind of has a dearth of research.”
Dr. Rothberg hopes to further that research via the COPD Outcomes-Based Network for Clinical Effectiveness & Research Translation (CONCERT), a team of physicians and researchers from centers around the country who are advocating for improvements to COPD treatment. Baystate is one of CONCERT’s outposts.
A report in this month’s Journal of Hospital Medicine shows macrolide and quinolone antibiotics are associated with similar rates of treatment failure in acute exacerbation of chronic pulmonary disease (AECOPD). The lead author says the study could be a precursor to, say, an intrepid HM researcher working on a randomized trial of the antibiotics’ effectiveness.
“It’s a perfect thing for hospitalists to study because they’re the ones treating it,” says Michael Rothberg, MD, MPH, associate professor of medicine at Tufts University School of Medicine in Boston, and lead author of "Comparative Effectiveness of Macrolides and Quinolones for Patients Hospitalized with Acute Exacerbations of Chronic Obstructive Pulmonary Disease (AECOPD)."
The retrospective cohort review reported that out of nearly 20,000 patients, 6,139 (31%) were treated initially with a macrolide and 13,469 (69%) with a quinolone. “Those who received macrolides had a lower risk of treatment failure (6.8% vs. 8.1%, p<0.01), a finding that was attenuated after multivariable adjustment (OR=0.89, 95% CI 0.78-1.01), and disappeared in a grouped-treatment analysis (OR=1.01, 95% CI 0.75-1.35),” the authors wrote. The study found no differences in adjusted length of stay or cost. However, antibiotic-associated diarrhea was more common with quinolones (1.2% vs. 0.6%, p<0.001).
Dr. Rothberg, who is affiliated with the Center for Quality of Care Research at Baystate Medical Center in Springfield, Mass., says the data, while a point in the right direction, should be viewed as a first step in doing more search to determine the best treatment for AECOPD.
“If you look at the guidelines, the recommendations are all over the map,” Dr. Rothberg says. “This is really because there are no randomized trials in COPD patients. … There are so many unanswered questions. There’s been so much focus on pneumonia, heart failure, and acute myocardial infarction. COPD kind of has a dearth of research.”
Dr. Rothberg hopes to further that research via the COPD Outcomes-Based Network for Clinical Effectiveness & Research Translation (CONCERT), a team of physicians and researchers from centers around the country who are advocating for improvements to COPD treatment. Baystate is one of CONCERT’s outposts.
In the Literature: Research You Need to Know
Clinical question: Is glucose variability associated with increased mortality independent of mean glucose values in intensive-care-unit (ICU) patients on a strict glucose-control algorithm?
Background: Initial studies demonstrating that strict glycemic control in the ICU improves mortality have not been reproduced in more recent trials and meta-analyses. This inconsistency may be due to unstudied aspects of glycemic control, such as glucose variability.
Study design: Retrospective cohort.
Setting: Eighteen-bed medical/surgical ICU in a teaching hospital in Amsterdam, Netherlands.
Synopsis: Data were collected on 5,728 patients admitted to the ICU from January 2004 to December 2007, all of whom were treated with a computerized intensive insulin protocol. Mean glucose, standard deviation in glucose, and mean absolute glucose change per hour (glucose variability) were calculated for each patient stay in the ICU. The results from these three calculated values were divided into quartiles and evaluated for their predictive value of ICU death and in-hospital death.
Within each mean glucose quartile, the uppermost glucose variability quartile was associated with increased risk of death. Compared with the lowest glucose variability quartile, the highest quartile had a 3.3-fold increased risk of ICU death and a 2.8-fold increased risk of in-hospital death. Patients in the highest glucose quartile with the highest glucose variability had a 12.4-fold increased risk of ICU death.
Bottom line: High glucose variability is associated with increased ICU and in-hospital mortality independent of mean glucose values in patients on a strict glucose control algorithm.
Reviewed for TH eWire by Dimitriy Levin, MD, Jeffrey Carter, MD, Erin Egan, MD, JD, Jonathan Pell, MD, Laura Rosenthal, MSN, ACNP, Nichole Zehnder, MD, Hospital Medicine Group, University of Colorado Denver
For more reviews of HM-related literature, visit our website.
Clinical question: Is glucose variability associated with increased mortality independent of mean glucose values in intensive-care-unit (ICU) patients on a strict glucose-control algorithm?
Background: Initial studies demonstrating that strict glycemic control in the ICU improves mortality have not been reproduced in more recent trials and meta-analyses. This inconsistency may be due to unstudied aspects of glycemic control, such as glucose variability.
Study design: Retrospective cohort.
Setting: Eighteen-bed medical/surgical ICU in a teaching hospital in Amsterdam, Netherlands.
Synopsis: Data were collected on 5,728 patients admitted to the ICU from January 2004 to December 2007, all of whom were treated with a computerized intensive insulin protocol. Mean glucose, standard deviation in glucose, and mean absolute glucose change per hour (glucose variability) were calculated for each patient stay in the ICU. The results from these three calculated values were divided into quartiles and evaluated for their predictive value of ICU death and in-hospital death.
Within each mean glucose quartile, the uppermost glucose variability quartile was associated with increased risk of death. Compared with the lowest glucose variability quartile, the highest quartile had a 3.3-fold increased risk of ICU death and a 2.8-fold increased risk of in-hospital death. Patients in the highest glucose quartile with the highest glucose variability had a 12.4-fold increased risk of ICU death.
Bottom line: High glucose variability is associated with increased ICU and in-hospital mortality independent of mean glucose values in patients on a strict glucose control algorithm.
Reviewed for TH eWire by Dimitriy Levin, MD, Jeffrey Carter, MD, Erin Egan, MD, JD, Jonathan Pell, MD, Laura Rosenthal, MSN, ACNP, Nichole Zehnder, MD, Hospital Medicine Group, University of Colorado Denver
For more reviews of HM-related literature, visit our website.
Clinical question: Is glucose variability associated with increased mortality independent of mean glucose values in intensive-care-unit (ICU) patients on a strict glucose-control algorithm?
Background: Initial studies demonstrating that strict glycemic control in the ICU improves mortality have not been reproduced in more recent trials and meta-analyses. This inconsistency may be due to unstudied aspects of glycemic control, such as glucose variability.
Study design: Retrospective cohort.
Setting: Eighteen-bed medical/surgical ICU in a teaching hospital in Amsterdam, Netherlands.
Synopsis: Data were collected on 5,728 patients admitted to the ICU from January 2004 to December 2007, all of whom were treated with a computerized intensive insulin protocol. Mean glucose, standard deviation in glucose, and mean absolute glucose change per hour (glucose variability) were calculated for each patient stay in the ICU. The results from these three calculated values were divided into quartiles and evaluated for their predictive value of ICU death and in-hospital death.
Within each mean glucose quartile, the uppermost glucose variability quartile was associated with increased risk of death. Compared with the lowest glucose variability quartile, the highest quartile had a 3.3-fold increased risk of ICU death and a 2.8-fold increased risk of in-hospital death. Patients in the highest glucose quartile with the highest glucose variability had a 12.4-fold increased risk of ICU death.
Bottom line: High glucose variability is associated with increased ICU and in-hospital mortality independent of mean glucose values in patients on a strict glucose control algorithm.
Reviewed for TH eWire by Dimitriy Levin, MD, Jeffrey Carter, MD, Erin Egan, MD, JD, Jonathan Pell, MD, Laura Rosenthal, MSN, ACNP, Nichole Zehnder, MD, Hospital Medicine Group, University of Colorado Denver
For more reviews of HM-related literature, visit our website.
Insurance Status and Hospital Care
With about 1 in 5 working‐age Americans (age 18‐64 years) currently uninsured and a large number relying on Medicaid, adequate access to quality health care services is becoming increasingly difficult.1 Substantial literature has accumulated over the years suggesting that access and quality in health care are closely linked to an individual's health insurance status.211 Some studies indicate that being uninsured or publicly insured is associated with negative health consequences.12, 13 Although the Medicaid program has improved access for qualifying low‐income individuals, significant gaps in access and quality remain.2, 5, 11, 1419 These issues are likely to become more pervasive should there be further modifications to state Medicaid funding in response to the unfolding economic crisis.
Although numerous studies have focused on insurance‐related disparities in the outpatient setting, few nationally representative studies have examined such disparities among hospitalized patients. A cross‐sectional study of a large hospital database from 1987 reported higher risk‐adjusted in‐hospital mortality, shorter length of stay (LOS), and lower procedure use among uninsured patients.9 A more recent analysis, limited to patients admitted with stroke, reported significant variation in hospital care associated with insurance status.15 Other studies reporting myocardial infarction registry and quality improvement program data are biased by the self‐selection of large urban teaching hospitals.1618 To our knowledge, no nationally representative study has focused on the impact of insurance coverage on hospital care for common medical conditions among working‐age Americans, the fastest growing segment of the uninsured population.
To address this gap in knowledge, we analyzed a nationally representative hospital database to determine whether there are significant insurance‐related disparities in in‐hospital mortality, LOS, and cost per hospitalization for 3 common medical conditions among working‐age adults, and, if present, to determine whether these disparities are due to differences in disease severity and comorbidities, and whether these disparities are affected by the proportion of uninsured and Medicaid patients receiving care in each hospital.
Methods
Design and Subjects
We examined data from the 2005 Nationwide Inpatient Sample (NIS), a nationally representative database of hospital inpatient stays maintained by the Agency for Healthcare Research and Quality (AHRQ) as part of the Healthcare Cost and Utilization Project (HCUP).20, 21 The NIS is a stratified probability sample of 20% of all US community hospitals, including public hospitals, academic medical centers, and specialty hospitals. Long‐term care hospitals, psychiatric hospitals, and alcoholism/chemical‐dependency treatment facilities are excluded. The 2005 NIS contains data on 7,995,048 discharges from 1054 hospitals located in 37 States and is designed to be representative of all acute care discharges from all US community hospitals.21
We identified discharges with a principal diagnosis of acute myocardial infarction (AMI), stroke, and pneumonia using International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) codes specified in the AHRQ definitions of Inpatient Quality Indicators (Supporting Information Appendix).22 These 3 conditions are among the leading causes of noncancer inpatient deaths in patients under 65 years old,23 and evidence suggests that high mortality may be associated with deficiencies in the quality of inpatient care.24
We confined our analysis to patients 18 to 64 years of age, since this population is most at risk of being uninsured or underinsured.25 We excluded pregnant women because they account for an unusually high proportion of uninsured discharges and were relatively few in our cohort.26 In addition, we excluded patients transferred to another acute care hospital and patients missing payer source and discharge disposition. Our study protocol was approved by the Partners Human Research Committee.
Study Variables
We categorized insurance status as privately insured, uninsured, Medicaid, or Medicare. We defined privately insured patients as those having either Blue Cross or another commercial carrier listed as the primary payer and uninsured patients as those having either no charge or self‐pay listed as the primary payer.27 Other governmental payer categories were noted to share several characteristics with Medicare patients and comprised only a small proportion of the sample, and were thus included with Medicare. In order to account for NIS's sampling scheme and accurately apply sample weights in our analysis, we used Medicare as a separate category. However, since Medicare patients age 18 to 64 years represent a fundamentally different population that is primarily disabled or very ill, only results of privately insured, uninsured, and Medicaid patients are reported.
We selected in‐hospital mortality as the outcome measure and LOS and cost per hospitalization as measures of resource utilization. The NIS includes a binary indicator variable for in‐hospital mortality and specifies inpatient LOS in integers, with same‐day stays coded as 0. NIS's cost estimates are based on hospital cost reports submitted to the Centers for Medicare and Medicaid Services. To test the validity of our cost analyses, we performed parallel analyses using hospital charges as a measure of utilization (charges include hospital overhead, charity care, and bad debt). The resulting adjusted ratios differed little from cost ratios and we opted to report only the details of our cost analyses.
In order to assess the independent association between insurance status and the outcome measures listed above, we selected covariates for inclusion in multivariable models based on the existing literature. Patient covariates included: age group (18‐34 years, 35‐49 years, 50‐64 years), sex (male/female), race/ethnicity (non‐Hispanic white, non‐Hispanic black, Hispanic, other, missing), median income by zip code of residence (less than $37,000, $37,000‐$45,999, $46,000‐$60,999, $61,000 or more), admission through the emergency department (yes/no), admission on a weekend (yes/no), measures of disease severity, and comorbidity indicators. Measures of disease severity specific to each outcome are assigned in the NIS using criteria developed by Medstat (Medstat Disease Staging Software Version 5.2, Thomson Medstat Inc., Ann Arbor, MI). Severity is categorized into 5 levels, with a higher level indicating greater risk. We recorded comorbidities for each patient in our sample using HCUP Comorbidity Software, Version 3.2 (
Hospital covariates included: bed size (small, medium, large), ownership/control (private, government, private or government), geographic region (northeast, midwest, south, west), teaching status (teaching, non‐teaching), and the proportion of uninsured and Medicaid patients (combined) admitted to each hospital for AMI, stroke, or pneumonia. The actual number of hospital beds in each bed size category varies according to a hospital's geographic region and teaching status.27 Ownership/control, geographic region, and teaching status are assigned according to information from the American Hospital Association Annual Survey of Hospitals. The proportion of uninsured and Medicaid patients admitted to each hospital was found to have a nonmonotonic relationship with the outcomes being assessed and was thus treated as a 6‐level categorical variable with the following levels: 0% to 10%, 11% to 20%, 21% to 30%, 31% to 40%, 41% to 50%, and 51% to 100%.
Statistical Analysis
Summary statistics were constructed at the patient level and differences in proportions were evaluated with the chi‐square test. We employed direct standardization, using the age and sex distribution of the entire cohort, to compute age‐standardized and sex‐standardized estimates for each insurance group and compared them using the chi‐square test for in‐hospital mortality and t test for log transformed LOS and cost per hospitalization. For each condition, we developed multivariable logistic regression models for in‐hospital mortality and multivariable linear regression models for log transformed LOS and cost. The patient was the unit of analysis in all models.
In order to elucidate the contribution of disease severity and comorbidities and the proportion of uninsured and Medicaid patients admitted to each hospital, we fitted 3 sequential models for each outcome measure: Model 1 adjusted for patient sociodemographic characteristics and hospital characteristics with the exception of the covariate for the proportion of uninsured and Medicaid patients, Model 2 adjusted for all covariates in the preceding model as well as patients' comorbidities and severity of principal diagnosis, and Model 3 adjusted for all previously mentioned covariates as well as the proportion of uninsured and Medicaid patients admitted to each hospital. We excluded patients who died during hospitalization from the models for LOS and cost. We exponentiated the effect estimates from the log transformed linear regression models so that the adjusted ratio represents the percentage change in the mean LOS or mean cost.
To determine whether the association between patients' insurance status and in‐hospital mortality was modified by seeking care in hospitals treating a smaller or larger proportion of uninsured and Medicaid patients, we entered an interaction term for insurance status and proportion of uninsured and Medicaid patients in the final models (Model 3) for our primary outcome of in‐hospital mortality. However, since no significant interaction was found for any of the 3 conditions, this term was removed from the models and results from the interaction models are not described. In order to assess model specification for the linear regression models, we evaluated the normality of model residuals and found that these were approximately normally distributed. Lastly, we attempted to test the robustness of our analyses by creating fixed effects models that controlled for hospital site but were unable to do so due to the computational limitations of available software packages that could not render fixed effects models with more than 1000 hospital sites.
For all variables except race/ethnicity, data were missing for less than 3% of patients, so we excluded these individuals from adjusted analyses. However, race/ethnicity data were missing for 29% of the sample and were analyzed in 3 different ways, namely, with the missing data treated as a separate covariate level, with the missing data removed from the analysis, and with the missing data assigned to the majority covariate level (white race). The results of our analysis were unchanged no matter how the missing values were assigned. As a result, missing values for race/ethnicity were treated as a separate covariate level in the final analysis.15 Sociodemographic characteristics of patients with missing race/ethnicity information were similar to those with complete data.
We used SUDAAN (Release 9.0.1, Research Triangle Institute, NC) to account for NIS's sampling scheme and generalized estimating equations to adjust for the clustering of patients within hospitals and hospitals within sampling strata.29 In order to account for NIS's stratified probability sampling scheme, SUDAAN uses Taylor series linearization for robust variance estimation of descriptive statistics and regression parameters.30, 31 We present 2‐tailed P values or 95% confidence intervals (CIs) for all statistical comparisons.
Results
Patient and Hospital Characteristics
The final cohort comprised of 154,381 patients discharged from 1018 hospitals in 37 states during calendar year 2005 (Table 1). This cohort was representative of 755,346 working‐age Americans, representing approximately 225,947 cases of AMI (29.9%), 151,812 cases of stroke (20.1%), and 377,588 cases of pneumonia (50.0%). Of these patients, 47.5% were privately insured, 12.0% were uninsured, 17.0% received Medicaid, and 23.5% were assigned to Medicare. Compared with the privately insured, uninsured and Medicaid patients were generally younger, less likely to be white, more likely to have lower income, and more likely to be admitted through the emergency department. Of the 1018 hospitals included in our study, close to half (44.3%) were small, with bedsize ranging from 24 to 249. A large number of hospitals were located in the South (39.9%), and 14.9% were designated teaching hospitals.
| Characteristic* | Privately insured (n = 73,256) | Uninsured (n = 18,531) | Medicaid (n = 26,222) |
|---|---|---|---|
| |||
| Principal diagnosis (%) | |||
| Acute myocardial infarction | 36.7 | 31.2 | 19.7 |
| Stroke | 20.6 | 23.7 | 19.9 |
| Pneumonia | 42.7 | 45.2 | 60.4 |
| Age group (%) | |||
| 18‐34 years | 6.8 | 13.0 | 13.7 |
| 35‐49 years | 27.6 | 36.9 | 33.2 |
| 50‐64 years | 65.7 | 50.1 | 53.2 |
| Male sex (%) | 59.3 | 62.3 | 46.6 |
| Race or ethnicity (%) | |||
| White | 55.7 | 41.5 | 38.0 |
| Black | 7.6 | 14.8 | 16.6 |
| Hispanic | 4.8 | 10.5 | 10.4 |
| Other race | 3.6 | 4.7 | 5.2 |
| Missing | 28.4 | 29.0 | 29.7 |
| Median income by ZIP code (%) | |||
| <$37,000 | 21.5 | 36.7 | 43.0 |
| $37,000‐$45,999 | 25.2 | 27.8 | 27.1 |
| $46,000‐$60,999 | 26.3 | 20.3 | 17.6 |
| $61,000 | 24.8 | 11.5 | 8.4 |
| Emergency admission (%) | 63.3 | 75.6 | 72.9 |
| Weekend admission (%) | 24.5 | 26.2 | 25.1 |
| Hospital bed size (%) | |||
| Small | 8.9 | 10.3 | 11.4 |
| Medium | 24.0 | 22.3 | 25.9 |
| Large | 67.1 | 67.5 | 62.8 |
| Hospital control (%) | |||
| Private | 33.8 | 34.8 | 34.4 |
| Government (nonfederal) | 6.7 | 9.7 | 8.3 |
| Private or government | 59.5 | 55.5 | 57.3 |
| Hospital region (%) | |||
| Northeast | 17.4 | 12.5 | 17.6 |
| Midwest | 25.7 | 19.4 | 20.9 |
| South | 39.5 | 56.8 | 42.4 |
| West | 17.4 | 11.3 | 19.2 |
| Teaching hospital (%) | 41.7 | 43.8 | 43.3 |
Compared with privately insured patients, a larger proportion of uninsured and Medicaid patients had higher predicted mortality levels (Table 2). Medicaid patients had a disproportionately higher predicted LOS, whereas predicted resource demand was higher among privately insured patients. Hypertension (48%), chronic pulmonary disease (29.5%), and uncomplicated diabetes (21.5%) were the 3 most common comorbidities in the study cohort, with a generally higher prevalence of comorbidities among Medicaid patients.
| Characteristic* | Privately insured (n = 73,256) | Uninsured (n = 18,531) | Medicaid (n = 26,222) |
|---|---|---|---|
| |||
| Medstat disease staging (%) | |||
| Mortality level 1 | 50.8 | 45.4 | 36.7 |
| Mortality level 2 | 44.0 | 49.1 | 56.7 |
| Mortality level 3 | 5.3 | 5.5 | 6.7 |
| Length of stay level 1 | 66.8 | 71.6 | 53.8 |
| Length of stay level 2 | 28.5 | 24.5 | 39.3 |
| Length of stay level 3 | 4.8 | 3.8 | 6.9 |
| Resource demand level 1 | 45.2 | 54.2 | 48.5 |
| Resource demand level 2 | 40.5 | 34.2 | 39.2 |
| Resource demand level 3 | 14.2 | 11.7 | 12.3 |
| Coexisting medical conditions (%) | |||
| Congestive heart failure | 4.7 | 4.8 | 10.1 |
| Valvular disease | 2.8 | 2.0 | 2.7 |
| Pulmonary circulation disease | 0.8 | 0.6 | 1.5 |
| Peripheral vascular disease | 3.2 | 2.2 | 3.2 |
| Paralysis | 1.2 | 0.8 | 3.5 |
| Other neurological disorders | 2.4 | 1.9 | 7.3 |
| Chronic pulmonary disease | 23.6 | 22.4 | 37.7 |
| Uncomplicated diabetes | 19.6 | 18.6 | 23.4 |
| Complicated diabetes | 3.3 | 2.1 | 4.9 |
| Hypothyroidism | 5.6 | 2.7 | 4.7 |
| Renal failure | 3.0 | 1.9 | 5.6 |
| Liver disease | 1.6 | 2.5 | 4.4 |
| Peptic ulcer disease | <0.5 | <0.5 | <0.5 |
| AIDS | 0.1 | 0.1 | 0.4 |
| Lymphoma | 1.1 | 0.4 | 0.6 |
| Metastatic cancer | 2.1 | 0.7 | 2.2 |
| Non‐metastatic solid tumor | 1.5 | 0.8 | 2.1 |
| Collagen vascular diseases | 2.3 | 0.9 | 2.3 |
| Coagulopathy | 2.7 | 2.4 | 3.4 |
| Obesity | 10.3 | 8.2 | 9.3 |
| Weight loss | 1.6 | 1.8 | 3.3 |
| Fluid and electrolyte disorders | 18.3 | 19.4 | 23.8 |
| Chronic blood loss anemia | 0.6 | 0.6 | 0.8 |
| Deficiency anemias | 8.6 | 8.5 | 13.4 |
| Alcohol abuse | 3.3 | 9.8 | 8.3 |
| Drug abuse | 1.9 | 10.2 | 9.8 |
| Psychoses | 1.5 | 1.9 | 6.8 |
| Depression | 7.2 | 4.8 | 9.9 |
| Hypertension | 48.0 | 44.1 | 45.7 |
In‐Hospital Mortality
Compared with the privately insured, age‐standardized and sex‐standardized in‐hospital mortality for AMI and stroke was significantly higher for uninsured and Medicaid patients (Table 3). Among pneumonia patients, Medicaid recipients had significantly higher in‐hospital mortality compared with privately insured and uninsured patients.
| Privately Insured | Uninsured | Medicaid | |
|---|---|---|---|
| |||
| In‐hospital mortality, rate per 100 discharges (SE) | |||
| Acute myocardial infarction | 2.22 (0.10) | 4.03 (0.31)* | 4.57 (0.34)* |
| Stroke | 7.49 (0.27) | 10.46 (0.64)* | 9.89 (0.45)* |
| Pneumonia | 1.75 (0.09) | 1.74 (0.18) | 2.48 (0.14)* |
| Length of stay, mean (SE), days | |||
| Acute myocardial infarction | 4.17 (0.06) | 4.46 (0.09) | 5.85 (0.16) |
| Stroke | 6.37 (0.13) | 7.15 (0.25) | 9.28 (0.30) |
| Pneumonia | 4.89 (0.05) | 4.64 (0.10) | 5.80 (0.08) |
| Cost per episode, mean (SE), dollars | |||
| Acute myocardial infarction | 21,077 (512) | 19,977 (833) | 22,452 (841) |
| Stroke | 16,022 (679) | 14,571 (1,036) | 18,462 (824) |
| Pneumonia | 8,223 (192) | 7,086 (293) | 9,479 (271) |
After multivariable adjustment for additional patient and hospital characteristics, uninsured AMI and stroke patients continued to have significantly higher in‐hospital mortality compared with the privately insured (Table 4). Among pneumonia patients, Medicaid recipients persisted in having significantly higher in‐hospital mortality than the privately insured.
| Model 1* | Model 2 | Model 3 | |
|---|---|---|---|
| |||
| In‐hospital mortality, adjusted odds ratio (95% CI) | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 1.59 (1.35‐1.88) | 1.58 (1.30‐1.93) | 1.52 (1.24‐1.85) |
| Medicaid vs. privately insured | 1.83 (1.54‐2.18) | 1.22 (0.99‐1.50) | 1.15 (0.94‐1.42) |
| Stroke | |||
| Uninsured vs. privately insured | 1.56 (1.35‐1.80) | 1.50 (1.30‐1.73) | 1.49 (1.29‐1.72) |
| Medicaid vs. privately insured | 1.32 (1.15‐1.52) | 1.09 (0.93‐1.27) | 1.08 (0.93‐1.26) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.99 (0.81‐1.21) | 1.12 (0.91‐1.39) | 1.10 (0.89‐1.36) |
| Medicaid vs. privately insured | 1.41 (1.20‐1.65) | 1.24 (1.04‐1.48) | 1.21 (1.01‐1.45) |
| Length of stay, adjusted ratio (95% CI)| | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 1.00 (0.98‐1.02) | 1.00 (0.98‐1.02) | 1.00 (0.98‐1.02) |
| Medicaid vs. privately insured | 1.17 (1.14‐1.21) | 1.07 (1.05‐1.09) | 1.07 (1.05‐1.09) |
| Stroke | |||
| Uninsured vs. privately insured | 1.06 (1.02‐1.10) | 1.08 (1.04‐1.11) | 1.07 (1.04‐1.11) |
| Medicaid vs. privately insured | 1.30 (1.26‐1.34) | 1.17 (1.14‐1.20) | 1.17 (1.14‐1.20) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.95 (0.93‐0.97) | 0.96 (0.94‐0.99) | 0.96 (0.94‐0.98) |
| Medicaid vs. privately insured | 1.15 (1.13‐1.17) | 1.04 (1.03‐1.06) | 1.04 (1.03‐1.06) |
| Cost per episode, adjusted ratio (95% CI)| | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 0.97 (0.95‐0.99) | 0.99 (0.97‐1.00) | 0.99 (0.97‐1.00) |
| Medicaid vs. privately insured | 1.01 (0.98‐1.04) | 0.99 (0.97‐1.01) | 0.99 (0.97‐1.01) |
| Stroke | |||
| Uninsured vs. privately insured | 0.97 (0.93‐1.02) | 1.00 (0.96‐1.03) | 1.00 (0.97‐1.03) |
| Medicaid vs. privately insured | 1.17 (1.13‐1.21) | 1.06 (1.04‐1.09) | 1.06 (1.04‐1.09) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.95 (0.92‐0.97) | 0.98 (0.96‐1.00) | 0.98 (0.96‐1.00) |
| Medicaid vs. privately insured | 1.17 (1.15‐1.19) | 1.05 (1.04‐1.07) | 1.05 (1.04‐1.07) |
LOS
Among AMI and stroke patients, age‐standardized and sex‐standardized mean LOS was significantly longer for the uninsured and Medicaid recipients compared with the privately insured (Table 3). Among pneumonia patients, the uninsured had a slightly shorter mean LOS compared with the privately insured whereas Medicaid recipients averaged the longest LOS.
These insurance‐related disparities in LOS among pneumonia patients persisted after multivariable adjustment (Table 4). Among AMI patients, only Medicaid recipients persisted in having a significantly longer LOS than the privately insured. Among stroke patients, both the uninsured and Medicaid recipients averaged a longer LOS compared with the privately insured.
Cost per Episode
For all 3 conditions, the uninsured had significantly lower age‐standardized and sex‐standardized costs compared with the privately insured (Table 3). However, Medicaid patients had higher costs than the privately insured for all three conditions, significantly so among patients with stroke and pneumonia.
These insurance‐related disparities in costs persisted in multivariable analyses (Table 4). The uninsured continued to have lower costs compared with the privately insured, significantly so for patients with AMI and pneumonia. Among stroke and pneumonia patients, Medicaid recipients continued to accrue higher costs than the uninsured or privately insured.
Discussion
In this nationally representative study of working‐age Americans hospitalized for 3 common medical conditions, we found that insurance status was associated with significant variations in in‐hospital mortality and resource use. Whereas privately insured patients experienced comparatively lower in‐hospital mortality in most cases, mortality risk was highest among the uninsured for 2 of the 3 common causes of noncancer inpatient deaths. Although previous studies have examined insurance‐related disparities in inpatient care for individual diagnoses and specific populations, no broad overview of this important issue has been published in the past decade. In light of the current economic recession and national healthcare debate, these findings may be a prescient indication of a widening insurance gap in the quality of hospital care.
There are several potential mechanisms for these disparities. For instance, Hadley et al.9 reported significant underuse of high‐cost or high‐discretion procedures among the uninsured in their analysis of a nationally representative sample of 592,598 hospitalized patients. Similarly, Burstin et al.10 found that among a population of 30,195 hospitalized patients with diverse diagnoses, the uninsured were at greater risk for receiving substandard care regardless of hospital characteristics. These, and other similar findings,7, 8, 19 are suggestive of differences in the way uninsured patients are generally managed in the hospital that may partly explain the disparities reported herein.
More specifically, analyses of national registries of AMI have documented lower rates of utilization of invasive, potentially life‐saving, cardiac interventions among the uninsured.16, 17 Similarly, a lower rate of carotid endarterectomy was reported among uninsured stroke patients from an analysis of the 2002 NIS.15 Other differences in inpatient management unmeasured by administrative data, such as the use of subspecialists and allied health professionals, may also contribute.32 Unfortunately, limitations in the available data prevented us from being able to appropriately address the important issue of insurance related differences in the utilization of specific inpatient procedures.
These disparities may also be indicative of differences in severity of illness that are not captured fully by the MedStat disease staging criteria. The uninsured might have more severe illness at admission, either due to the presence of more advanced chronic disease or delay in seeking care for the acute episode. AMI and stroke are usually the culmination of longstanding atherosclerosis that is amenable to improvement through timely and consistent risk‐factor modification. Not having a usual source of medical care,6, 33 inadequate screening and management of known risk‐factors,3, 34 and difficulties in obtaining specialty care5 among the uninsured likely increases their risk of being hospitalized with more advanced disease. The higher likelihood of being admitted through the emergency department19 and on weekends9 among the uninsured lends credence to the possibility of delays in seeking care. All of these are potential mediators of higher AMI and stroke mortality in uninsured patients.
Finally, these mortality differences could also be due to the additional risks imposed by poorly managed comorbidities among uninsured patients. Although we controlled for the presence of comorbidities in our analysis, we lacked data about the severity of individual comorbidities. A recent study reported significant lapses in follow‐up care after the onset of a chronic condition in uninsured individuals under 65 years of age.34 Other studies have also documented insurance related disparities in the care of chronic diseases3, 35 that were among the most common comorbidities in our cohort.
Most of the reasons for insurance‐related disparities noted above for the uninsured are also applicable to Medicaid patients. Differences in the intensity of inpatient care,7, 8, 1519 limited access to health care services,2, 14 unmet health needs,5 and suboptimal management of chronic medical conditions35 were also reported for Medicaid patients in prior research. These factors likely contributed to the higher in‐hospital mortality in this patient population, evidenced by the sequential decrease in odds after adjusting for comorbidities and disease severity. Medicaid patients hospitalized for stroke were noted to have significantly longer LOS, which could plausibly be due to difficulties with arranging appropriate discharge disposition; the higher likelihood of paralysis among these patients15 would likely necessitate a higher frequency of rehabilitation facility placement. The higher costs for Medicaid patients with stroke and pneumonia may potentially be the result of these patients longer LOS. Although cost differences between the uninsured and privately insured were statistically significant, these were not large enough to be of material significance.
Limitations
Our study has several limitations. Since the NIS does not assign unique patient identifiers that would permit tracking of readmissions, we excluded patients transferred to another acute‐care hospital from our study to avoid counting the same patient twice. However, only 10% of hospitalized patients underwent transfer for cardiac procedures in the National Registry of Myocardial Infarction, with privately insured patients more likely to be transferred than other insurance groups.17 Since these patients are also more likely to have better survival, their exclusion likely biased our study toward the null. The same is probable for stroke patients as well.
Some uninsured patients begin Medicaid coverage during hospitalization and should ideally be counted as uninsured but were included under Medicaid in our analysis. They are also likely to be state‐ and plan‐specific variations in Medicaid and private payer coverage that we could not incorporate into our analysis. In addition, we were unable to include deaths that may have occurred shortly after discharge, even though these may have been related to the quality of hospital care. Furthermore, although the 3 conditions we studied are common and responsible for a large number of hospital deaths, they make up about 8% of total annual hospital discharges,23 and caution should be exercised in generalizing our findings to the full spectrum of hospitalizations. Lastly, it is possible that unmeasured confounding could be responsible for the observed associations. Uninsured and Medicaid patients are likely to have more severe disease, which may not be adequately captured by the administrative data available in the NIS. If so, this would explain the mortality association rather than insurance status.36, 37
Conclusions
Significant insurance‐related differences in mortality exist for 2 of the leading causes of noncancer inpatient deaths among working‐age Americans. Further studies are needed to determine whether provider sensitivity to insurance status or unmeasured sociodemographic and clinical prognostic factors are responsible for these disparities. Policy makers, hospital administrators, and physicians should be cognizant of these disparities and consider policies to address potential insurance related gaps in the quality of inpatient care.
- , .The U.S. economy and changes in health insurance coverage, 2000‐2006.Health Aff (Millwood).2008;27(2):w135‐w144.
- , , .Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland.JAMA.1992;268(17):2388‐2394.
- , , , et al.Unmet health needs of uninsured adults in the United States.JAMA.2000;284(16):2061‐2069.
- , , .Health insurance and access to care for symptomatic conditions.Arch Intern Med.2000;160(9):1269‐1274.
- , , , et al.Access to specialty care and medical services in community health centers.Health Aff (Millwood).2007;26(5):1459‐1468.
- , , , et al.A national study of chronic disease prevalence and access to care in uninsured U.S. adults.Ann Intern Med.2008;149:170–176.
- , , , .Relationship between patient source of payment and the intensity of hospital services.Med Care.1988;26(11):1111‐1114.
- , , .The association of payer with utilization of cardiac procedures in Massachusetts.JAMA.1990;264(10):1255‐1260.
- , , .Comparison of uninsured and privately insured hospital patients: condition on admission, resource use, and outcome.JAMA.1991;265:374–379.
- , , .Socioeconomic status and risk for substandard medical care.JAMA.1992;268(17):2383‐2387.
- , , , .The relation between health insurance coverage and clinical outcomes among women with breast cancer.N Engl J Med.1993;329(5):326‐331.
- , , .Health insurance and mortality. Evidence from a national cohort.JAMA.1993;270(6):737‐741.
- , , , .Mortality in the uninsured compared with that in persons with public and private health insurance.Arch Intern Med.1994;154(21):2409‐2416.
- .Medicaid policy and the substitution of hospital outpatient care for physician care.Health Serv Res.1989;24:33–66.
- , .Disparities in outcomes among patients with stroke associated with insurance status.Stroke.2007;38(3):1010‐1016.
- , , , et al.Influence of payor on use of invasive cardiac procedures and patient outcome after myocardial infarction in the United States. Participants in the National Registry of Myocardial Infarction.J Am Coll Cardiol.1998;31(7):1474‐1480.
- , , , et al.Payer status and the utilization of hospital resources in acute myocardial infarction: a report from the National Registry of Myocardial Infarction 2.Arch Intern Med.2000;160(6):817–823.
- , , , et al.Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes.Ann Intern Med.2006;145(10):739–748.
- , , .Comparing uninsured and privately insured hospital patients: Admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2005. Rockville, MD: Agency for Healthcare Research and Quality; 2007:6. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/NIS_Introduction_2005.pdf. Accessed February2010.
- Healthcare Cost and Utilization Project. Design of the Nationwide Inpatient Sample (NIS) 2005. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/reports/NIS_2005_Design_Report.pdf. Accessed February2010.
- AHRQ Quality Indicators. Inpatient Quality Indicators: Technical Specifications. Version 3.1 (March 12, 2007). Available at: www.qualityindicators.ahrq.gov/downloads/iqi/iqi_technical_specs_v31.pdf. Accessed February2010.
- , , . National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Washington, DC: National Center for Health Statistics; 2007. Vital and Health Statistics 13(165). Available at: www.cdc.gov/nchs/data/series/sr_13/sr13_165.pdf. Accessed February2010.
- AHRQ Quality Indicators. Guide to Inpatient Quality Indicators: Quality of Care in Hospitals—Volume, Mortality, and Utilization. Version 3.1 (March 12, 2007). Available at: www.qualityindicators.ahrq.gov/downloads/iqi/iqi_guide_v31.pdf. Accessed February2010.
- , , .Income, poverty, and health insurance coverage in the United States: 2006.Washington, DC:US Census Bureau. Current Population Reports;2007:60–233.
- , . Conditions Related to Uninsured Hospitalizations, 2003. HCUP Statistical Brief #8. Rockville, MD: Agency for Healthcare Research and Quality; 2006:6. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb8.pdf. Accessed February2010.
- Healthcare Cost and Utilization Project. NIS Description of Data Elements. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/nisdde.jsp. Accessed February2010.
- , , , .Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- SUDAAN User's Manual, Release 9.0. Research TrianglePark, NC:Research Triangle Institute;2006.
- , . Final Report on Calculating Nationwide Inpatient Sample (NIS) Variances, 2001. HCUP Methods Series Report #2003‐2. Online June 2005 (revised June 6, 2005). U.S. Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/CalculatingNISVariances200106092005.pdf. Accessed February2010.
- .On the variances of asymptotically normal estimators from complex surveys.Int Stat Rev.1983;51:279–292.
- , , , et al.Patient characteristics associated with care by a cardiologist among adults hospitalized with severe congestive heart failure.J Am Coll Cardiol.2000;36:2119–2125.
- , . Summary health statistics for U.S. adults: National Health Interview Survey, 2006. Washington, DC: National Center for Health Statistics; 2007:12. Vital and Health Statistics 10(235). Available at: www.cdc.gov/nchs/data/series/sr_10/sr10_235.pdf. Accessed February2010.
- .Insurance coverage, medical care use, and short‐term health changes following an unintentional injury or the onset of a chronic condition.JAMA.2007;297(10):1073–1084.
- , , , et al.The quality of chronic disease care in U.S. community health centers.Health Aff (Millwood).2006;25(6):1712–1723.
- , , , , , .Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases.Med Care.2008;46(3):232–239.
- , , , .The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population.J Clin Epidemiol. [E‐pub ahead of print].
With about 1 in 5 working‐age Americans (age 18‐64 years) currently uninsured and a large number relying on Medicaid, adequate access to quality health care services is becoming increasingly difficult.1 Substantial literature has accumulated over the years suggesting that access and quality in health care are closely linked to an individual's health insurance status.211 Some studies indicate that being uninsured or publicly insured is associated with negative health consequences.12, 13 Although the Medicaid program has improved access for qualifying low‐income individuals, significant gaps in access and quality remain.2, 5, 11, 1419 These issues are likely to become more pervasive should there be further modifications to state Medicaid funding in response to the unfolding economic crisis.
Although numerous studies have focused on insurance‐related disparities in the outpatient setting, few nationally representative studies have examined such disparities among hospitalized patients. A cross‐sectional study of a large hospital database from 1987 reported higher risk‐adjusted in‐hospital mortality, shorter length of stay (LOS), and lower procedure use among uninsured patients.9 A more recent analysis, limited to patients admitted with stroke, reported significant variation in hospital care associated with insurance status.15 Other studies reporting myocardial infarction registry and quality improvement program data are biased by the self‐selection of large urban teaching hospitals.1618 To our knowledge, no nationally representative study has focused on the impact of insurance coverage on hospital care for common medical conditions among working‐age Americans, the fastest growing segment of the uninsured population.
To address this gap in knowledge, we analyzed a nationally representative hospital database to determine whether there are significant insurance‐related disparities in in‐hospital mortality, LOS, and cost per hospitalization for 3 common medical conditions among working‐age adults, and, if present, to determine whether these disparities are due to differences in disease severity and comorbidities, and whether these disparities are affected by the proportion of uninsured and Medicaid patients receiving care in each hospital.
Methods
Design and Subjects
We examined data from the 2005 Nationwide Inpatient Sample (NIS), a nationally representative database of hospital inpatient stays maintained by the Agency for Healthcare Research and Quality (AHRQ) as part of the Healthcare Cost and Utilization Project (HCUP).20, 21 The NIS is a stratified probability sample of 20% of all US community hospitals, including public hospitals, academic medical centers, and specialty hospitals. Long‐term care hospitals, psychiatric hospitals, and alcoholism/chemical‐dependency treatment facilities are excluded. The 2005 NIS contains data on 7,995,048 discharges from 1054 hospitals located in 37 States and is designed to be representative of all acute care discharges from all US community hospitals.21
We identified discharges with a principal diagnosis of acute myocardial infarction (AMI), stroke, and pneumonia using International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) codes specified in the AHRQ definitions of Inpatient Quality Indicators (Supporting Information Appendix).22 These 3 conditions are among the leading causes of noncancer inpatient deaths in patients under 65 years old,23 and evidence suggests that high mortality may be associated with deficiencies in the quality of inpatient care.24
We confined our analysis to patients 18 to 64 years of age, since this population is most at risk of being uninsured or underinsured.25 We excluded pregnant women because they account for an unusually high proportion of uninsured discharges and were relatively few in our cohort.26 In addition, we excluded patients transferred to another acute care hospital and patients missing payer source and discharge disposition. Our study protocol was approved by the Partners Human Research Committee.
Study Variables
We categorized insurance status as privately insured, uninsured, Medicaid, or Medicare. We defined privately insured patients as those having either Blue Cross or another commercial carrier listed as the primary payer and uninsured patients as those having either no charge or self‐pay listed as the primary payer.27 Other governmental payer categories were noted to share several characteristics with Medicare patients and comprised only a small proportion of the sample, and were thus included with Medicare. In order to account for NIS's sampling scheme and accurately apply sample weights in our analysis, we used Medicare as a separate category. However, since Medicare patients age 18 to 64 years represent a fundamentally different population that is primarily disabled or very ill, only results of privately insured, uninsured, and Medicaid patients are reported.
We selected in‐hospital mortality as the outcome measure and LOS and cost per hospitalization as measures of resource utilization. The NIS includes a binary indicator variable for in‐hospital mortality and specifies inpatient LOS in integers, with same‐day stays coded as 0. NIS's cost estimates are based on hospital cost reports submitted to the Centers for Medicare and Medicaid Services. To test the validity of our cost analyses, we performed parallel analyses using hospital charges as a measure of utilization (charges include hospital overhead, charity care, and bad debt). The resulting adjusted ratios differed little from cost ratios and we opted to report only the details of our cost analyses.
In order to assess the independent association between insurance status and the outcome measures listed above, we selected covariates for inclusion in multivariable models based on the existing literature. Patient covariates included: age group (18‐34 years, 35‐49 years, 50‐64 years), sex (male/female), race/ethnicity (non‐Hispanic white, non‐Hispanic black, Hispanic, other, missing), median income by zip code of residence (less than $37,000, $37,000‐$45,999, $46,000‐$60,999, $61,000 or more), admission through the emergency department (yes/no), admission on a weekend (yes/no), measures of disease severity, and comorbidity indicators. Measures of disease severity specific to each outcome are assigned in the NIS using criteria developed by Medstat (Medstat Disease Staging Software Version 5.2, Thomson Medstat Inc., Ann Arbor, MI). Severity is categorized into 5 levels, with a higher level indicating greater risk. We recorded comorbidities for each patient in our sample using HCUP Comorbidity Software, Version 3.2 (
Hospital covariates included: bed size (small, medium, large), ownership/control (private, government, private or government), geographic region (northeast, midwest, south, west), teaching status (teaching, non‐teaching), and the proportion of uninsured and Medicaid patients (combined) admitted to each hospital for AMI, stroke, or pneumonia. The actual number of hospital beds in each bed size category varies according to a hospital's geographic region and teaching status.27 Ownership/control, geographic region, and teaching status are assigned according to information from the American Hospital Association Annual Survey of Hospitals. The proportion of uninsured and Medicaid patients admitted to each hospital was found to have a nonmonotonic relationship with the outcomes being assessed and was thus treated as a 6‐level categorical variable with the following levels: 0% to 10%, 11% to 20%, 21% to 30%, 31% to 40%, 41% to 50%, and 51% to 100%.
Statistical Analysis
Summary statistics were constructed at the patient level and differences in proportions were evaluated with the chi‐square test. We employed direct standardization, using the age and sex distribution of the entire cohort, to compute age‐standardized and sex‐standardized estimates for each insurance group and compared them using the chi‐square test for in‐hospital mortality and t test for log transformed LOS and cost per hospitalization. For each condition, we developed multivariable logistic regression models for in‐hospital mortality and multivariable linear regression models for log transformed LOS and cost. The patient was the unit of analysis in all models.
In order to elucidate the contribution of disease severity and comorbidities and the proportion of uninsured and Medicaid patients admitted to each hospital, we fitted 3 sequential models for each outcome measure: Model 1 adjusted for patient sociodemographic characteristics and hospital characteristics with the exception of the covariate for the proportion of uninsured and Medicaid patients, Model 2 adjusted for all covariates in the preceding model as well as patients' comorbidities and severity of principal diagnosis, and Model 3 adjusted for all previously mentioned covariates as well as the proportion of uninsured and Medicaid patients admitted to each hospital. We excluded patients who died during hospitalization from the models for LOS and cost. We exponentiated the effect estimates from the log transformed linear regression models so that the adjusted ratio represents the percentage change in the mean LOS or mean cost.
To determine whether the association between patients' insurance status and in‐hospital mortality was modified by seeking care in hospitals treating a smaller or larger proportion of uninsured and Medicaid patients, we entered an interaction term for insurance status and proportion of uninsured and Medicaid patients in the final models (Model 3) for our primary outcome of in‐hospital mortality. However, since no significant interaction was found for any of the 3 conditions, this term was removed from the models and results from the interaction models are not described. In order to assess model specification for the linear regression models, we evaluated the normality of model residuals and found that these were approximately normally distributed. Lastly, we attempted to test the robustness of our analyses by creating fixed effects models that controlled for hospital site but were unable to do so due to the computational limitations of available software packages that could not render fixed effects models with more than 1000 hospital sites.
For all variables except race/ethnicity, data were missing for less than 3% of patients, so we excluded these individuals from adjusted analyses. However, race/ethnicity data were missing for 29% of the sample and were analyzed in 3 different ways, namely, with the missing data treated as a separate covariate level, with the missing data removed from the analysis, and with the missing data assigned to the majority covariate level (white race). The results of our analysis were unchanged no matter how the missing values were assigned. As a result, missing values for race/ethnicity were treated as a separate covariate level in the final analysis.15 Sociodemographic characteristics of patients with missing race/ethnicity information were similar to those with complete data.
We used SUDAAN (Release 9.0.1, Research Triangle Institute, NC) to account for NIS's sampling scheme and generalized estimating equations to adjust for the clustering of patients within hospitals and hospitals within sampling strata.29 In order to account for NIS's stratified probability sampling scheme, SUDAAN uses Taylor series linearization for robust variance estimation of descriptive statistics and regression parameters.30, 31 We present 2‐tailed P values or 95% confidence intervals (CIs) for all statistical comparisons.
Results
Patient and Hospital Characteristics
The final cohort comprised of 154,381 patients discharged from 1018 hospitals in 37 states during calendar year 2005 (Table 1). This cohort was representative of 755,346 working‐age Americans, representing approximately 225,947 cases of AMI (29.9%), 151,812 cases of stroke (20.1%), and 377,588 cases of pneumonia (50.0%). Of these patients, 47.5% were privately insured, 12.0% were uninsured, 17.0% received Medicaid, and 23.5% were assigned to Medicare. Compared with the privately insured, uninsured and Medicaid patients were generally younger, less likely to be white, more likely to have lower income, and more likely to be admitted through the emergency department. Of the 1018 hospitals included in our study, close to half (44.3%) were small, with bedsize ranging from 24 to 249. A large number of hospitals were located in the South (39.9%), and 14.9% were designated teaching hospitals.
| Characteristic* | Privately insured (n = 73,256) | Uninsured (n = 18,531) | Medicaid (n = 26,222) |
|---|---|---|---|
| |||
| Principal diagnosis (%) | |||
| Acute myocardial infarction | 36.7 | 31.2 | 19.7 |
| Stroke | 20.6 | 23.7 | 19.9 |
| Pneumonia | 42.7 | 45.2 | 60.4 |
| Age group (%) | |||
| 18‐34 years | 6.8 | 13.0 | 13.7 |
| 35‐49 years | 27.6 | 36.9 | 33.2 |
| 50‐64 years | 65.7 | 50.1 | 53.2 |
| Male sex (%) | 59.3 | 62.3 | 46.6 |
| Race or ethnicity (%) | |||
| White | 55.7 | 41.5 | 38.0 |
| Black | 7.6 | 14.8 | 16.6 |
| Hispanic | 4.8 | 10.5 | 10.4 |
| Other race | 3.6 | 4.7 | 5.2 |
| Missing | 28.4 | 29.0 | 29.7 |
| Median income by ZIP code (%) | |||
| <$37,000 | 21.5 | 36.7 | 43.0 |
| $37,000‐$45,999 | 25.2 | 27.8 | 27.1 |
| $46,000‐$60,999 | 26.3 | 20.3 | 17.6 |
| $61,000 | 24.8 | 11.5 | 8.4 |
| Emergency admission (%) | 63.3 | 75.6 | 72.9 |
| Weekend admission (%) | 24.5 | 26.2 | 25.1 |
| Hospital bed size (%) | |||
| Small | 8.9 | 10.3 | 11.4 |
| Medium | 24.0 | 22.3 | 25.9 |
| Large | 67.1 | 67.5 | 62.8 |
| Hospital control (%) | |||
| Private | 33.8 | 34.8 | 34.4 |
| Government (nonfederal) | 6.7 | 9.7 | 8.3 |
| Private or government | 59.5 | 55.5 | 57.3 |
| Hospital region (%) | |||
| Northeast | 17.4 | 12.5 | 17.6 |
| Midwest | 25.7 | 19.4 | 20.9 |
| South | 39.5 | 56.8 | 42.4 |
| West | 17.4 | 11.3 | 19.2 |
| Teaching hospital (%) | 41.7 | 43.8 | 43.3 |
Compared with privately insured patients, a larger proportion of uninsured and Medicaid patients had higher predicted mortality levels (Table 2). Medicaid patients had a disproportionately higher predicted LOS, whereas predicted resource demand was higher among privately insured patients. Hypertension (48%), chronic pulmonary disease (29.5%), and uncomplicated diabetes (21.5%) were the 3 most common comorbidities in the study cohort, with a generally higher prevalence of comorbidities among Medicaid patients.
| Characteristic* | Privately insured (n = 73,256) | Uninsured (n = 18,531) | Medicaid (n = 26,222) |
|---|---|---|---|
| |||
| Medstat disease staging (%) | |||
| Mortality level 1 | 50.8 | 45.4 | 36.7 |
| Mortality level 2 | 44.0 | 49.1 | 56.7 |
| Mortality level 3 | 5.3 | 5.5 | 6.7 |
| Length of stay level 1 | 66.8 | 71.6 | 53.8 |
| Length of stay level 2 | 28.5 | 24.5 | 39.3 |
| Length of stay level 3 | 4.8 | 3.8 | 6.9 |
| Resource demand level 1 | 45.2 | 54.2 | 48.5 |
| Resource demand level 2 | 40.5 | 34.2 | 39.2 |
| Resource demand level 3 | 14.2 | 11.7 | 12.3 |
| Coexisting medical conditions (%) | |||
| Congestive heart failure | 4.7 | 4.8 | 10.1 |
| Valvular disease | 2.8 | 2.0 | 2.7 |
| Pulmonary circulation disease | 0.8 | 0.6 | 1.5 |
| Peripheral vascular disease | 3.2 | 2.2 | 3.2 |
| Paralysis | 1.2 | 0.8 | 3.5 |
| Other neurological disorders | 2.4 | 1.9 | 7.3 |
| Chronic pulmonary disease | 23.6 | 22.4 | 37.7 |
| Uncomplicated diabetes | 19.6 | 18.6 | 23.4 |
| Complicated diabetes | 3.3 | 2.1 | 4.9 |
| Hypothyroidism | 5.6 | 2.7 | 4.7 |
| Renal failure | 3.0 | 1.9 | 5.6 |
| Liver disease | 1.6 | 2.5 | 4.4 |
| Peptic ulcer disease | <0.5 | <0.5 | <0.5 |
| AIDS | 0.1 | 0.1 | 0.4 |
| Lymphoma | 1.1 | 0.4 | 0.6 |
| Metastatic cancer | 2.1 | 0.7 | 2.2 |
| Non‐metastatic solid tumor | 1.5 | 0.8 | 2.1 |
| Collagen vascular diseases | 2.3 | 0.9 | 2.3 |
| Coagulopathy | 2.7 | 2.4 | 3.4 |
| Obesity | 10.3 | 8.2 | 9.3 |
| Weight loss | 1.6 | 1.8 | 3.3 |
| Fluid and electrolyte disorders | 18.3 | 19.4 | 23.8 |
| Chronic blood loss anemia | 0.6 | 0.6 | 0.8 |
| Deficiency anemias | 8.6 | 8.5 | 13.4 |
| Alcohol abuse | 3.3 | 9.8 | 8.3 |
| Drug abuse | 1.9 | 10.2 | 9.8 |
| Psychoses | 1.5 | 1.9 | 6.8 |
| Depression | 7.2 | 4.8 | 9.9 |
| Hypertension | 48.0 | 44.1 | 45.7 |
In‐Hospital Mortality
Compared with the privately insured, age‐standardized and sex‐standardized in‐hospital mortality for AMI and stroke was significantly higher for uninsured and Medicaid patients (Table 3). Among pneumonia patients, Medicaid recipients had significantly higher in‐hospital mortality compared with privately insured and uninsured patients.
| Privately Insured | Uninsured | Medicaid | |
|---|---|---|---|
| |||
| In‐hospital mortality, rate per 100 discharges (SE) | |||
| Acute myocardial infarction | 2.22 (0.10) | 4.03 (0.31)* | 4.57 (0.34)* |
| Stroke | 7.49 (0.27) | 10.46 (0.64)* | 9.89 (0.45)* |
| Pneumonia | 1.75 (0.09) | 1.74 (0.18) | 2.48 (0.14)* |
| Length of stay, mean (SE), days | |||
| Acute myocardial infarction | 4.17 (0.06) | 4.46 (0.09) | 5.85 (0.16) |
| Stroke | 6.37 (0.13) | 7.15 (0.25) | 9.28 (0.30) |
| Pneumonia | 4.89 (0.05) | 4.64 (0.10) | 5.80 (0.08) |
| Cost per episode, mean (SE), dollars | |||
| Acute myocardial infarction | 21,077 (512) | 19,977 (833) | 22,452 (841) |
| Stroke | 16,022 (679) | 14,571 (1,036) | 18,462 (824) |
| Pneumonia | 8,223 (192) | 7,086 (293) | 9,479 (271) |
After multivariable adjustment for additional patient and hospital characteristics, uninsured AMI and stroke patients continued to have significantly higher in‐hospital mortality compared with the privately insured (Table 4). Among pneumonia patients, Medicaid recipients persisted in having significantly higher in‐hospital mortality than the privately insured.
| Model 1* | Model 2 | Model 3 | |
|---|---|---|---|
| |||
| In‐hospital mortality, adjusted odds ratio (95% CI) | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 1.59 (1.35‐1.88) | 1.58 (1.30‐1.93) | 1.52 (1.24‐1.85) |
| Medicaid vs. privately insured | 1.83 (1.54‐2.18) | 1.22 (0.99‐1.50) | 1.15 (0.94‐1.42) |
| Stroke | |||
| Uninsured vs. privately insured | 1.56 (1.35‐1.80) | 1.50 (1.30‐1.73) | 1.49 (1.29‐1.72) |
| Medicaid vs. privately insured | 1.32 (1.15‐1.52) | 1.09 (0.93‐1.27) | 1.08 (0.93‐1.26) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.99 (0.81‐1.21) | 1.12 (0.91‐1.39) | 1.10 (0.89‐1.36) |
| Medicaid vs. privately insured | 1.41 (1.20‐1.65) | 1.24 (1.04‐1.48) | 1.21 (1.01‐1.45) |
| Length of stay, adjusted ratio (95% CI)| | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 1.00 (0.98‐1.02) | 1.00 (0.98‐1.02) | 1.00 (0.98‐1.02) |
| Medicaid vs. privately insured | 1.17 (1.14‐1.21) | 1.07 (1.05‐1.09) | 1.07 (1.05‐1.09) |
| Stroke | |||
| Uninsured vs. privately insured | 1.06 (1.02‐1.10) | 1.08 (1.04‐1.11) | 1.07 (1.04‐1.11) |
| Medicaid vs. privately insured | 1.30 (1.26‐1.34) | 1.17 (1.14‐1.20) | 1.17 (1.14‐1.20) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.95 (0.93‐0.97) | 0.96 (0.94‐0.99) | 0.96 (0.94‐0.98) |
| Medicaid vs. privately insured | 1.15 (1.13‐1.17) | 1.04 (1.03‐1.06) | 1.04 (1.03‐1.06) |
| Cost per episode, adjusted ratio (95% CI)| | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 0.97 (0.95‐0.99) | 0.99 (0.97‐1.00) | 0.99 (0.97‐1.00) |
| Medicaid vs. privately insured | 1.01 (0.98‐1.04) | 0.99 (0.97‐1.01) | 0.99 (0.97‐1.01) |
| Stroke | |||
| Uninsured vs. privately insured | 0.97 (0.93‐1.02) | 1.00 (0.96‐1.03) | 1.00 (0.97‐1.03) |
| Medicaid vs. privately insured | 1.17 (1.13‐1.21) | 1.06 (1.04‐1.09) | 1.06 (1.04‐1.09) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.95 (0.92‐0.97) | 0.98 (0.96‐1.00) | 0.98 (0.96‐1.00) |
| Medicaid vs. privately insured | 1.17 (1.15‐1.19) | 1.05 (1.04‐1.07) | 1.05 (1.04‐1.07) |
LOS
Among AMI and stroke patients, age‐standardized and sex‐standardized mean LOS was significantly longer for the uninsured and Medicaid recipients compared with the privately insured (Table 3). Among pneumonia patients, the uninsured had a slightly shorter mean LOS compared with the privately insured whereas Medicaid recipients averaged the longest LOS.
These insurance‐related disparities in LOS among pneumonia patients persisted after multivariable adjustment (Table 4). Among AMI patients, only Medicaid recipients persisted in having a significantly longer LOS than the privately insured. Among stroke patients, both the uninsured and Medicaid recipients averaged a longer LOS compared with the privately insured.
Cost per Episode
For all 3 conditions, the uninsured had significantly lower age‐standardized and sex‐standardized costs compared with the privately insured (Table 3). However, Medicaid patients had higher costs than the privately insured for all three conditions, significantly so among patients with stroke and pneumonia.
These insurance‐related disparities in costs persisted in multivariable analyses (Table 4). The uninsured continued to have lower costs compared with the privately insured, significantly so for patients with AMI and pneumonia. Among stroke and pneumonia patients, Medicaid recipients continued to accrue higher costs than the uninsured or privately insured.
Discussion
In this nationally representative study of working‐age Americans hospitalized for 3 common medical conditions, we found that insurance status was associated with significant variations in in‐hospital mortality and resource use. Whereas privately insured patients experienced comparatively lower in‐hospital mortality in most cases, mortality risk was highest among the uninsured for 2 of the 3 common causes of noncancer inpatient deaths. Although previous studies have examined insurance‐related disparities in inpatient care for individual diagnoses and specific populations, no broad overview of this important issue has been published in the past decade. In light of the current economic recession and national healthcare debate, these findings may be a prescient indication of a widening insurance gap in the quality of hospital care.
There are several potential mechanisms for these disparities. For instance, Hadley et al.9 reported significant underuse of high‐cost or high‐discretion procedures among the uninsured in their analysis of a nationally representative sample of 592,598 hospitalized patients. Similarly, Burstin et al.10 found that among a population of 30,195 hospitalized patients with diverse diagnoses, the uninsured were at greater risk for receiving substandard care regardless of hospital characteristics. These, and other similar findings,7, 8, 19 are suggestive of differences in the way uninsured patients are generally managed in the hospital that may partly explain the disparities reported herein.
More specifically, analyses of national registries of AMI have documented lower rates of utilization of invasive, potentially life‐saving, cardiac interventions among the uninsured.16, 17 Similarly, a lower rate of carotid endarterectomy was reported among uninsured stroke patients from an analysis of the 2002 NIS.15 Other differences in inpatient management unmeasured by administrative data, such as the use of subspecialists and allied health professionals, may also contribute.32 Unfortunately, limitations in the available data prevented us from being able to appropriately address the important issue of insurance related differences in the utilization of specific inpatient procedures.
These disparities may also be indicative of differences in severity of illness that are not captured fully by the MedStat disease staging criteria. The uninsured might have more severe illness at admission, either due to the presence of more advanced chronic disease or delay in seeking care for the acute episode. AMI and stroke are usually the culmination of longstanding atherosclerosis that is amenable to improvement through timely and consistent risk‐factor modification. Not having a usual source of medical care,6, 33 inadequate screening and management of known risk‐factors,3, 34 and difficulties in obtaining specialty care5 among the uninsured likely increases their risk of being hospitalized with more advanced disease. The higher likelihood of being admitted through the emergency department19 and on weekends9 among the uninsured lends credence to the possibility of delays in seeking care. All of these are potential mediators of higher AMI and stroke mortality in uninsured patients.
Finally, these mortality differences could also be due to the additional risks imposed by poorly managed comorbidities among uninsured patients. Although we controlled for the presence of comorbidities in our analysis, we lacked data about the severity of individual comorbidities. A recent study reported significant lapses in follow‐up care after the onset of a chronic condition in uninsured individuals under 65 years of age.34 Other studies have also documented insurance related disparities in the care of chronic diseases3, 35 that were among the most common comorbidities in our cohort.
Most of the reasons for insurance‐related disparities noted above for the uninsured are also applicable to Medicaid patients. Differences in the intensity of inpatient care,7, 8, 1519 limited access to health care services,2, 14 unmet health needs,5 and suboptimal management of chronic medical conditions35 were also reported for Medicaid patients in prior research. These factors likely contributed to the higher in‐hospital mortality in this patient population, evidenced by the sequential decrease in odds after adjusting for comorbidities and disease severity. Medicaid patients hospitalized for stroke were noted to have significantly longer LOS, which could plausibly be due to difficulties with arranging appropriate discharge disposition; the higher likelihood of paralysis among these patients15 would likely necessitate a higher frequency of rehabilitation facility placement. The higher costs for Medicaid patients with stroke and pneumonia may potentially be the result of these patients longer LOS. Although cost differences between the uninsured and privately insured were statistically significant, these were not large enough to be of material significance.
Limitations
Our study has several limitations. Since the NIS does not assign unique patient identifiers that would permit tracking of readmissions, we excluded patients transferred to another acute‐care hospital from our study to avoid counting the same patient twice. However, only 10% of hospitalized patients underwent transfer for cardiac procedures in the National Registry of Myocardial Infarction, with privately insured patients more likely to be transferred than other insurance groups.17 Since these patients are also more likely to have better survival, their exclusion likely biased our study toward the null. The same is probable for stroke patients as well.
Some uninsured patients begin Medicaid coverage during hospitalization and should ideally be counted as uninsured but were included under Medicaid in our analysis. They are also likely to be state‐ and plan‐specific variations in Medicaid and private payer coverage that we could not incorporate into our analysis. In addition, we were unable to include deaths that may have occurred shortly after discharge, even though these may have been related to the quality of hospital care. Furthermore, although the 3 conditions we studied are common and responsible for a large number of hospital deaths, they make up about 8% of total annual hospital discharges,23 and caution should be exercised in generalizing our findings to the full spectrum of hospitalizations. Lastly, it is possible that unmeasured confounding could be responsible for the observed associations. Uninsured and Medicaid patients are likely to have more severe disease, which may not be adequately captured by the administrative data available in the NIS. If so, this would explain the mortality association rather than insurance status.36, 37
Conclusions
Significant insurance‐related differences in mortality exist for 2 of the leading causes of noncancer inpatient deaths among working‐age Americans. Further studies are needed to determine whether provider sensitivity to insurance status or unmeasured sociodemographic and clinical prognostic factors are responsible for these disparities. Policy makers, hospital administrators, and physicians should be cognizant of these disparities and consider policies to address potential insurance related gaps in the quality of inpatient care.
With about 1 in 5 working‐age Americans (age 18‐64 years) currently uninsured and a large number relying on Medicaid, adequate access to quality health care services is becoming increasingly difficult.1 Substantial literature has accumulated over the years suggesting that access and quality in health care are closely linked to an individual's health insurance status.211 Some studies indicate that being uninsured or publicly insured is associated with negative health consequences.12, 13 Although the Medicaid program has improved access for qualifying low‐income individuals, significant gaps in access and quality remain.2, 5, 11, 1419 These issues are likely to become more pervasive should there be further modifications to state Medicaid funding in response to the unfolding economic crisis.
Although numerous studies have focused on insurance‐related disparities in the outpatient setting, few nationally representative studies have examined such disparities among hospitalized patients. A cross‐sectional study of a large hospital database from 1987 reported higher risk‐adjusted in‐hospital mortality, shorter length of stay (LOS), and lower procedure use among uninsured patients.9 A more recent analysis, limited to patients admitted with stroke, reported significant variation in hospital care associated with insurance status.15 Other studies reporting myocardial infarction registry and quality improvement program data are biased by the self‐selection of large urban teaching hospitals.1618 To our knowledge, no nationally representative study has focused on the impact of insurance coverage on hospital care for common medical conditions among working‐age Americans, the fastest growing segment of the uninsured population.
To address this gap in knowledge, we analyzed a nationally representative hospital database to determine whether there are significant insurance‐related disparities in in‐hospital mortality, LOS, and cost per hospitalization for 3 common medical conditions among working‐age adults, and, if present, to determine whether these disparities are due to differences in disease severity and comorbidities, and whether these disparities are affected by the proportion of uninsured and Medicaid patients receiving care in each hospital.
Methods
Design and Subjects
We examined data from the 2005 Nationwide Inpatient Sample (NIS), a nationally representative database of hospital inpatient stays maintained by the Agency for Healthcare Research and Quality (AHRQ) as part of the Healthcare Cost and Utilization Project (HCUP).20, 21 The NIS is a stratified probability sample of 20% of all US community hospitals, including public hospitals, academic medical centers, and specialty hospitals. Long‐term care hospitals, psychiatric hospitals, and alcoholism/chemical‐dependency treatment facilities are excluded. The 2005 NIS contains data on 7,995,048 discharges from 1054 hospitals located in 37 States and is designed to be representative of all acute care discharges from all US community hospitals.21
We identified discharges with a principal diagnosis of acute myocardial infarction (AMI), stroke, and pneumonia using International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) codes specified in the AHRQ definitions of Inpatient Quality Indicators (Supporting Information Appendix).22 These 3 conditions are among the leading causes of noncancer inpatient deaths in patients under 65 years old,23 and evidence suggests that high mortality may be associated with deficiencies in the quality of inpatient care.24
We confined our analysis to patients 18 to 64 years of age, since this population is most at risk of being uninsured or underinsured.25 We excluded pregnant women because they account for an unusually high proportion of uninsured discharges and were relatively few in our cohort.26 In addition, we excluded patients transferred to another acute care hospital and patients missing payer source and discharge disposition. Our study protocol was approved by the Partners Human Research Committee.
Study Variables
We categorized insurance status as privately insured, uninsured, Medicaid, or Medicare. We defined privately insured patients as those having either Blue Cross or another commercial carrier listed as the primary payer and uninsured patients as those having either no charge or self‐pay listed as the primary payer.27 Other governmental payer categories were noted to share several characteristics with Medicare patients and comprised only a small proportion of the sample, and were thus included with Medicare. In order to account for NIS's sampling scheme and accurately apply sample weights in our analysis, we used Medicare as a separate category. However, since Medicare patients age 18 to 64 years represent a fundamentally different population that is primarily disabled or very ill, only results of privately insured, uninsured, and Medicaid patients are reported.
We selected in‐hospital mortality as the outcome measure and LOS and cost per hospitalization as measures of resource utilization. The NIS includes a binary indicator variable for in‐hospital mortality and specifies inpatient LOS in integers, with same‐day stays coded as 0. NIS's cost estimates are based on hospital cost reports submitted to the Centers for Medicare and Medicaid Services. To test the validity of our cost analyses, we performed parallel analyses using hospital charges as a measure of utilization (charges include hospital overhead, charity care, and bad debt). The resulting adjusted ratios differed little from cost ratios and we opted to report only the details of our cost analyses.
In order to assess the independent association between insurance status and the outcome measures listed above, we selected covariates for inclusion in multivariable models based on the existing literature. Patient covariates included: age group (18‐34 years, 35‐49 years, 50‐64 years), sex (male/female), race/ethnicity (non‐Hispanic white, non‐Hispanic black, Hispanic, other, missing), median income by zip code of residence (less than $37,000, $37,000‐$45,999, $46,000‐$60,999, $61,000 or more), admission through the emergency department (yes/no), admission on a weekend (yes/no), measures of disease severity, and comorbidity indicators. Measures of disease severity specific to each outcome are assigned in the NIS using criteria developed by Medstat (Medstat Disease Staging Software Version 5.2, Thomson Medstat Inc., Ann Arbor, MI). Severity is categorized into 5 levels, with a higher level indicating greater risk. We recorded comorbidities for each patient in our sample using HCUP Comorbidity Software, Version 3.2 (
Hospital covariates included: bed size (small, medium, large), ownership/control (private, government, private or government), geographic region (northeast, midwest, south, west), teaching status (teaching, non‐teaching), and the proportion of uninsured and Medicaid patients (combined) admitted to each hospital for AMI, stroke, or pneumonia. The actual number of hospital beds in each bed size category varies according to a hospital's geographic region and teaching status.27 Ownership/control, geographic region, and teaching status are assigned according to information from the American Hospital Association Annual Survey of Hospitals. The proportion of uninsured and Medicaid patients admitted to each hospital was found to have a nonmonotonic relationship with the outcomes being assessed and was thus treated as a 6‐level categorical variable with the following levels: 0% to 10%, 11% to 20%, 21% to 30%, 31% to 40%, 41% to 50%, and 51% to 100%.
Statistical Analysis
Summary statistics were constructed at the patient level and differences in proportions were evaluated with the chi‐square test. We employed direct standardization, using the age and sex distribution of the entire cohort, to compute age‐standardized and sex‐standardized estimates for each insurance group and compared them using the chi‐square test for in‐hospital mortality and t test for log transformed LOS and cost per hospitalization. For each condition, we developed multivariable logistic regression models for in‐hospital mortality and multivariable linear regression models for log transformed LOS and cost. The patient was the unit of analysis in all models.
In order to elucidate the contribution of disease severity and comorbidities and the proportion of uninsured and Medicaid patients admitted to each hospital, we fitted 3 sequential models for each outcome measure: Model 1 adjusted for patient sociodemographic characteristics and hospital characteristics with the exception of the covariate for the proportion of uninsured and Medicaid patients, Model 2 adjusted for all covariates in the preceding model as well as patients' comorbidities and severity of principal diagnosis, and Model 3 adjusted for all previously mentioned covariates as well as the proportion of uninsured and Medicaid patients admitted to each hospital. We excluded patients who died during hospitalization from the models for LOS and cost. We exponentiated the effect estimates from the log transformed linear regression models so that the adjusted ratio represents the percentage change in the mean LOS or mean cost.
To determine whether the association between patients' insurance status and in‐hospital mortality was modified by seeking care in hospitals treating a smaller or larger proportion of uninsured and Medicaid patients, we entered an interaction term for insurance status and proportion of uninsured and Medicaid patients in the final models (Model 3) for our primary outcome of in‐hospital mortality. However, since no significant interaction was found for any of the 3 conditions, this term was removed from the models and results from the interaction models are not described. In order to assess model specification for the linear regression models, we evaluated the normality of model residuals and found that these were approximately normally distributed. Lastly, we attempted to test the robustness of our analyses by creating fixed effects models that controlled for hospital site but were unable to do so due to the computational limitations of available software packages that could not render fixed effects models with more than 1000 hospital sites.
For all variables except race/ethnicity, data were missing for less than 3% of patients, so we excluded these individuals from adjusted analyses. However, race/ethnicity data were missing for 29% of the sample and were analyzed in 3 different ways, namely, with the missing data treated as a separate covariate level, with the missing data removed from the analysis, and with the missing data assigned to the majority covariate level (white race). The results of our analysis were unchanged no matter how the missing values were assigned. As a result, missing values for race/ethnicity were treated as a separate covariate level in the final analysis.15 Sociodemographic characteristics of patients with missing race/ethnicity information were similar to those with complete data.
We used SUDAAN (Release 9.0.1, Research Triangle Institute, NC) to account for NIS's sampling scheme and generalized estimating equations to adjust for the clustering of patients within hospitals and hospitals within sampling strata.29 In order to account for NIS's stratified probability sampling scheme, SUDAAN uses Taylor series linearization for robust variance estimation of descriptive statistics and regression parameters.30, 31 We present 2‐tailed P values or 95% confidence intervals (CIs) for all statistical comparisons.
Results
Patient and Hospital Characteristics
The final cohort comprised of 154,381 patients discharged from 1018 hospitals in 37 states during calendar year 2005 (Table 1). This cohort was representative of 755,346 working‐age Americans, representing approximately 225,947 cases of AMI (29.9%), 151,812 cases of stroke (20.1%), and 377,588 cases of pneumonia (50.0%). Of these patients, 47.5% were privately insured, 12.0% were uninsured, 17.0% received Medicaid, and 23.5% were assigned to Medicare. Compared with the privately insured, uninsured and Medicaid patients were generally younger, less likely to be white, more likely to have lower income, and more likely to be admitted through the emergency department. Of the 1018 hospitals included in our study, close to half (44.3%) were small, with bedsize ranging from 24 to 249. A large number of hospitals were located in the South (39.9%), and 14.9% were designated teaching hospitals.
| Characteristic* | Privately insured (n = 73,256) | Uninsured (n = 18,531) | Medicaid (n = 26,222) |
|---|---|---|---|
| |||
| Principal diagnosis (%) | |||
| Acute myocardial infarction | 36.7 | 31.2 | 19.7 |
| Stroke | 20.6 | 23.7 | 19.9 |
| Pneumonia | 42.7 | 45.2 | 60.4 |
| Age group (%) | |||
| 18‐34 years | 6.8 | 13.0 | 13.7 |
| 35‐49 years | 27.6 | 36.9 | 33.2 |
| 50‐64 years | 65.7 | 50.1 | 53.2 |
| Male sex (%) | 59.3 | 62.3 | 46.6 |
| Race or ethnicity (%) | |||
| White | 55.7 | 41.5 | 38.0 |
| Black | 7.6 | 14.8 | 16.6 |
| Hispanic | 4.8 | 10.5 | 10.4 |
| Other race | 3.6 | 4.7 | 5.2 |
| Missing | 28.4 | 29.0 | 29.7 |
| Median income by ZIP code (%) | |||
| <$37,000 | 21.5 | 36.7 | 43.0 |
| $37,000‐$45,999 | 25.2 | 27.8 | 27.1 |
| $46,000‐$60,999 | 26.3 | 20.3 | 17.6 |
| $61,000 | 24.8 | 11.5 | 8.4 |
| Emergency admission (%) | 63.3 | 75.6 | 72.9 |
| Weekend admission (%) | 24.5 | 26.2 | 25.1 |
| Hospital bed size (%) | |||
| Small | 8.9 | 10.3 | 11.4 |
| Medium | 24.0 | 22.3 | 25.9 |
| Large | 67.1 | 67.5 | 62.8 |
| Hospital control (%) | |||
| Private | 33.8 | 34.8 | 34.4 |
| Government (nonfederal) | 6.7 | 9.7 | 8.3 |
| Private or government | 59.5 | 55.5 | 57.3 |
| Hospital region (%) | |||
| Northeast | 17.4 | 12.5 | 17.6 |
| Midwest | 25.7 | 19.4 | 20.9 |
| South | 39.5 | 56.8 | 42.4 |
| West | 17.4 | 11.3 | 19.2 |
| Teaching hospital (%) | 41.7 | 43.8 | 43.3 |
Compared with privately insured patients, a larger proportion of uninsured and Medicaid patients had higher predicted mortality levels (Table 2). Medicaid patients had a disproportionately higher predicted LOS, whereas predicted resource demand was higher among privately insured patients. Hypertension (48%), chronic pulmonary disease (29.5%), and uncomplicated diabetes (21.5%) were the 3 most common comorbidities in the study cohort, with a generally higher prevalence of comorbidities among Medicaid patients.
| Characteristic* | Privately insured (n = 73,256) | Uninsured (n = 18,531) | Medicaid (n = 26,222) |
|---|---|---|---|
| |||
| Medstat disease staging (%) | |||
| Mortality level 1 | 50.8 | 45.4 | 36.7 |
| Mortality level 2 | 44.0 | 49.1 | 56.7 |
| Mortality level 3 | 5.3 | 5.5 | 6.7 |
| Length of stay level 1 | 66.8 | 71.6 | 53.8 |
| Length of stay level 2 | 28.5 | 24.5 | 39.3 |
| Length of stay level 3 | 4.8 | 3.8 | 6.9 |
| Resource demand level 1 | 45.2 | 54.2 | 48.5 |
| Resource demand level 2 | 40.5 | 34.2 | 39.2 |
| Resource demand level 3 | 14.2 | 11.7 | 12.3 |
| Coexisting medical conditions (%) | |||
| Congestive heart failure | 4.7 | 4.8 | 10.1 |
| Valvular disease | 2.8 | 2.0 | 2.7 |
| Pulmonary circulation disease | 0.8 | 0.6 | 1.5 |
| Peripheral vascular disease | 3.2 | 2.2 | 3.2 |
| Paralysis | 1.2 | 0.8 | 3.5 |
| Other neurological disorders | 2.4 | 1.9 | 7.3 |
| Chronic pulmonary disease | 23.6 | 22.4 | 37.7 |
| Uncomplicated diabetes | 19.6 | 18.6 | 23.4 |
| Complicated diabetes | 3.3 | 2.1 | 4.9 |
| Hypothyroidism | 5.6 | 2.7 | 4.7 |
| Renal failure | 3.0 | 1.9 | 5.6 |
| Liver disease | 1.6 | 2.5 | 4.4 |
| Peptic ulcer disease | <0.5 | <0.5 | <0.5 |
| AIDS | 0.1 | 0.1 | 0.4 |
| Lymphoma | 1.1 | 0.4 | 0.6 |
| Metastatic cancer | 2.1 | 0.7 | 2.2 |
| Non‐metastatic solid tumor | 1.5 | 0.8 | 2.1 |
| Collagen vascular diseases | 2.3 | 0.9 | 2.3 |
| Coagulopathy | 2.7 | 2.4 | 3.4 |
| Obesity | 10.3 | 8.2 | 9.3 |
| Weight loss | 1.6 | 1.8 | 3.3 |
| Fluid and electrolyte disorders | 18.3 | 19.4 | 23.8 |
| Chronic blood loss anemia | 0.6 | 0.6 | 0.8 |
| Deficiency anemias | 8.6 | 8.5 | 13.4 |
| Alcohol abuse | 3.3 | 9.8 | 8.3 |
| Drug abuse | 1.9 | 10.2 | 9.8 |
| Psychoses | 1.5 | 1.9 | 6.8 |
| Depression | 7.2 | 4.8 | 9.9 |
| Hypertension | 48.0 | 44.1 | 45.7 |
In‐Hospital Mortality
Compared with the privately insured, age‐standardized and sex‐standardized in‐hospital mortality for AMI and stroke was significantly higher for uninsured and Medicaid patients (Table 3). Among pneumonia patients, Medicaid recipients had significantly higher in‐hospital mortality compared with privately insured and uninsured patients.
| Privately Insured | Uninsured | Medicaid | |
|---|---|---|---|
| |||
| In‐hospital mortality, rate per 100 discharges (SE) | |||
| Acute myocardial infarction | 2.22 (0.10) | 4.03 (0.31)* | 4.57 (0.34)* |
| Stroke | 7.49 (0.27) | 10.46 (0.64)* | 9.89 (0.45)* |
| Pneumonia | 1.75 (0.09) | 1.74 (0.18) | 2.48 (0.14)* |
| Length of stay, mean (SE), days | |||
| Acute myocardial infarction | 4.17 (0.06) | 4.46 (0.09) | 5.85 (0.16) |
| Stroke | 6.37 (0.13) | 7.15 (0.25) | 9.28 (0.30) |
| Pneumonia | 4.89 (0.05) | 4.64 (0.10) | 5.80 (0.08) |
| Cost per episode, mean (SE), dollars | |||
| Acute myocardial infarction | 21,077 (512) | 19,977 (833) | 22,452 (841) |
| Stroke | 16,022 (679) | 14,571 (1,036) | 18,462 (824) |
| Pneumonia | 8,223 (192) | 7,086 (293) | 9,479 (271) |
After multivariable adjustment for additional patient and hospital characteristics, uninsured AMI and stroke patients continued to have significantly higher in‐hospital mortality compared with the privately insured (Table 4). Among pneumonia patients, Medicaid recipients persisted in having significantly higher in‐hospital mortality than the privately insured.
| Model 1* | Model 2 | Model 3 | |
|---|---|---|---|
| |||
| In‐hospital mortality, adjusted odds ratio (95% CI) | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 1.59 (1.35‐1.88) | 1.58 (1.30‐1.93) | 1.52 (1.24‐1.85) |
| Medicaid vs. privately insured | 1.83 (1.54‐2.18) | 1.22 (0.99‐1.50) | 1.15 (0.94‐1.42) |
| Stroke | |||
| Uninsured vs. privately insured | 1.56 (1.35‐1.80) | 1.50 (1.30‐1.73) | 1.49 (1.29‐1.72) |
| Medicaid vs. privately insured | 1.32 (1.15‐1.52) | 1.09 (0.93‐1.27) | 1.08 (0.93‐1.26) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.99 (0.81‐1.21) | 1.12 (0.91‐1.39) | 1.10 (0.89‐1.36) |
| Medicaid vs. privately insured | 1.41 (1.20‐1.65) | 1.24 (1.04‐1.48) | 1.21 (1.01‐1.45) |
| Length of stay, adjusted ratio (95% CI)| | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 1.00 (0.98‐1.02) | 1.00 (0.98‐1.02) | 1.00 (0.98‐1.02) |
| Medicaid vs. privately insured | 1.17 (1.14‐1.21) | 1.07 (1.05‐1.09) | 1.07 (1.05‐1.09) |
| Stroke | |||
| Uninsured vs. privately insured | 1.06 (1.02‐1.10) | 1.08 (1.04‐1.11) | 1.07 (1.04‐1.11) |
| Medicaid vs. privately insured | 1.30 (1.26‐1.34) | 1.17 (1.14‐1.20) | 1.17 (1.14‐1.20) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.95 (0.93‐0.97) | 0.96 (0.94‐0.99) | 0.96 (0.94‐0.98) |
| Medicaid vs. privately insured | 1.15 (1.13‐1.17) | 1.04 (1.03‐1.06) | 1.04 (1.03‐1.06) |
| Cost per episode, adjusted ratio (95% CI)| | |||
| Acute Myocardial Infarction | |||
| Uninsured vs. privately insured | 0.97 (0.95‐0.99) | 0.99 (0.97‐1.00) | 0.99 (0.97‐1.00) |
| Medicaid vs. privately insured | 1.01 (0.98‐1.04) | 0.99 (0.97‐1.01) | 0.99 (0.97‐1.01) |
| Stroke | |||
| Uninsured vs. privately insured | 0.97 (0.93‐1.02) | 1.00 (0.96‐1.03) | 1.00 (0.97‐1.03) |
| Medicaid vs. privately insured | 1.17 (1.13‐1.21) | 1.06 (1.04‐1.09) | 1.06 (1.04‐1.09) |
| Pneumonia | |||
| Uninsured vs. privately insured | 0.95 (0.92‐0.97) | 0.98 (0.96‐1.00) | 0.98 (0.96‐1.00) |
| Medicaid vs. privately insured | 1.17 (1.15‐1.19) | 1.05 (1.04‐1.07) | 1.05 (1.04‐1.07) |
LOS
Among AMI and stroke patients, age‐standardized and sex‐standardized mean LOS was significantly longer for the uninsured and Medicaid recipients compared with the privately insured (Table 3). Among pneumonia patients, the uninsured had a slightly shorter mean LOS compared with the privately insured whereas Medicaid recipients averaged the longest LOS.
These insurance‐related disparities in LOS among pneumonia patients persisted after multivariable adjustment (Table 4). Among AMI patients, only Medicaid recipients persisted in having a significantly longer LOS than the privately insured. Among stroke patients, both the uninsured and Medicaid recipients averaged a longer LOS compared with the privately insured.
Cost per Episode
For all 3 conditions, the uninsured had significantly lower age‐standardized and sex‐standardized costs compared with the privately insured (Table 3). However, Medicaid patients had higher costs than the privately insured for all three conditions, significantly so among patients with stroke and pneumonia.
These insurance‐related disparities in costs persisted in multivariable analyses (Table 4). The uninsured continued to have lower costs compared with the privately insured, significantly so for patients with AMI and pneumonia. Among stroke and pneumonia patients, Medicaid recipients continued to accrue higher costs than the uninsured or privately insured.
Discussion
In this nationally representative study of working‐age Americans hospitalized for 3 common medical conditions, we found that insurance status was associated with significant variations in in‐hospital mortality and resource use. Whereas privately insured patients experienced comparatively lower in‐hospital mortality in most cases, mortality risk was highest among the uninsured for 2 of the 3 common causes of noncancer inpatient deaths. Although previous studies have examined insurance‐related disparities in inpatient care for individual diagnoses and specific populations, no broad overview of this important issue has been published in the past decade. In light of the current economic recession and national healthcare debate, these findings may be a prescient indication of a widening insurance gap in the quality of hospital care.
There are several potential mechanisms for these disparities. For instance, Hadley et al.9 reported significant underuse of high‐cost or high‐discretion procedures among the uninsured in their analysis of a nationally representative sample of 592,598 hospitalized patients. Similarly, Burstin et al.10 found that among a population of 30,195 hospitalized patients with diverse diagnoses, the uninsured were at greater risk for receiving substandard care regardless of hospital characteristics. These, and other similar findings,7, 8, 19 are suggestive of differences in the way uninsured patients are generally managed in the hospital that may partly explain the disparities reported herein.
More specifically, analyses of national registries of AMI have documented lower rates of utilization of invasive, potentially life‐saving, cardiac interventions among the uninsured.16, 17 Similarly, a lower rate of carotid endarterectomy was reported among uninsured stroke patients from an analysis of the 2002 NIS.15 Other differences in inpatient management unmeasured by administrative data, such as the use of subspecialists and allied health professionals, may also contribute.32 Unfortunately, limitations in the available data prevented us from being able to appropriately address the important issue of insurance related differences in the utilization of specific inpatient procedures.
These disparities may also be indicative of differences in severity of illness that are not captured fully by the MedStat disease staging criteria. The uninsured might have more severe illness at admission, either due to the presence of more advanced chronic disease or delay in seeking care for the acute episode. AMI and stroke are usually the culmination of longstanding atherosclerosis that is amenable to improvement through timely and consistent risk‐factor modification. Not having a usual source of medical care,6, 33 inadequate screening and management of known risk‐factors,3, 34 and difficulties in obtaining specialty care5 among the uninsured likely increases their risk of being hospitalized with more advanced disease. The higher likelihood of being admitted through the emergency department19 and on weekends9 among the uninsured lends credence to the possibility of delays in seeking care. All of these are potential mediators of higher AMI and stroke mortality in uninsured patients.
Finally, these mortality differences could also be due to the additional risks imposed by poorly managed comorbidities among uninsured patients. Although we controlled for the presence of comorbidities in our analysis, we lacked data about the severity of individual comorbidities. A recent study reported significant lapses in follow‐up care after the onset of a chronic condition in uninsured individuals under 65 years of age.34 Other studies have also documented insurance related disparities in the care of chronic diseases3, 35 that were among the most common comorbidities in our cohort.
Most of the reasons for insurance‐related disparities noted above for the uninsured are also applicable to Medicaid patients. Differences in the intensity of inpatient care,7, 8, 1519 limited access to health care services,2, 14 unmet health needs,5 and suboptimal management of chronic medical conditions35 were also reported for Medicaid patients in prior research. These factors likely contributed to the higher in‐hospital mortality in this patient population, evidenced by the sequential decrease in odds after adjusting for comorbidities and disease severity. Medicaid patients hospitalized for stroke were noted to have significantly longer LOS, which could plausibly be due to difficulties with arranging appropriate discharge disposition; the higher likelihood of paralysis among these patients15 would likely necessitate a higher frequency of rehabilitation facility placement. The higher costs for Medicaid patients with stroke and pneumonia may potentially be the result of these patients longer LOS. Although cost differences between the uninsured and privately insured were statistically significant, these were not large enough to be of material significance.
Limitations
Our study has several limitations. Since the NIS does not assign unique patient identifiers that would permit tracking of readmissions, we excluded patients transferred to another acute‐care hospital from our study to avoid counting the same patient twice. However, only 10% of hospitalized patients underwent transfer for cardiac procedures in the National Registry of Myocardial Infarction, with privately insured patients more likely to be transferred than other insurance groups.17 Since these patients are also more likely to have better survival, their exclusion likely biased our study toward the null. The same is probable for stroke patients as well.
Some uninsured patients begin Medicaid coverage during hospitalization and should ideally be counted as uninsured but were included under Medicaid in our analysis. They are also likely to be state‐ and plan‐specific variations in Medicaid and private payer coverage that we could not incorporate into our analysis. In addition, we were unable to include deaths that may have occurred shortly after discharge, even though these may have been related to the quality of hospital care. Furthermore, although the 3 conditions we studied are common and responsible for a large number of hospital deaths, they make up about 8% of total annual hospital discharges,23 and caution should be exercised in generalizing our findings to the full spectrum of hospitalizations. Lastly, it is possible that unmeasured confounding could be responsible for the observed associations. Uninsured and Medicaid patients are likely to have more severe disease, which may not be adequately captured by the administrative data available in the NIS. If so, this would explain the mortality association rather than insurance status.36, 37
Conclusions
Significant insurance‐related differences in mortality exist for 2 of the leading causes of noncancer inpatient deaths among working‐age Americans. Further studies are needed to determine whether provider sensitivity to insurance status or unmeasured sociodemographic and clinical prognostic factors are responsible for these disparities. Policy makers, hospital administrators, and physicians should be cognizant of these disparities and consider policies to address potential insurance related gaps in the quality of inpatient care.
- , .The U.S. economy and changes in health insurance coverage, 2000‐2006.Health Aff (Millwood).2008;27(2):w135‐w144.
- , , .Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland.JAMA.1992;268(17):2388‐2394.
- , , , et al.Unmet health needs of uninsured adults in the United States.JAMA.2000;284(16):2061‐2069.
- , , .Health insurance and access to care for symptomatic conditions.Arch Intern Med.2000;160(9):1269‐1274.
- , , , et al.Access to specialty care and medical services in community health centers.Health Aff (Millwood).2007;26(5):1459‐1468.
- , , , et al.A national study of chronic disease prevalence and access to care in uninsured U.S. adults.Ann Intern Med.2008;149:170–176.
- , , , .Relationship between patient source of payment and the intensity of hospital services.Med Care.1988;26(11):1111‐1114.
- , , .The association of payer with utilization of cardiac procedures in Massachusetts.JAMA.1990;264(10):1255‐1260.
- , , .Comparison of uninsured and privately insured hospital patients: condition on admission, resource use, and outcome.JAMA.1991;265:374–379.
- , , .Socioeconomic status and risk for substandard medical care.JAMA.1992;268(17):2383‐2387.
- , , , .The relation between health insurance coverage and clinical outcomes among women with breast cancer.N Engl J Med.1993;329(5):326‐331.
- , , .Health insurance and mortality. Evidence from a national cohort.JAMA.1993;270(6):737‐741.
- , , , .Mortality in the uninsured compared with that in persons with public and private health insurance.Arch Intern Med.1994;154(21):2409‐2416.
- .Medicaid policy and the substitution of hospital outpatient care for physician care.Health Serv Res.1989;24:33–66.
- , .Disparities in outcomes among patients with stroke associated with insurance status.Stroke.2007;38(3):1010‐1016.
- , , , et al.Influence of payor on use of invasive cardiac procedures and patient outcome after myocardial infarction in the United States. Participants in the National Registry of Myocardial Infarction.J Am Coll Cardiol.1998;31(7):1474‐1480.
- , , , et al.Payer status and the utilization of hospital resources in acute myocardial infarction: a report from the National Registry of Myocardial Infarction 2.Arch Intern Med.2000;160(6):817–823.
- , , , et al.Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes.Ann Intern Med.2006;145(10):739–748.
- , , .Comparing uninsured and privately insured hospital patients: Admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2005. Rockville, MD: Agency for Healthcare Research and Quality; 2007:6. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/NIS_Introduction_2005.pdf. Accessed February2010.
- Healthcare Cost and Utilization Project. Design of the Nationwide Inpatient Sample (NIS) 2005. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/reports/NIS_2005_Design_Report.pdf. Accessed February2010.
- AHRQ Quality Indicators. Inpatient Quality Indicators: Technical Specifications. Version 3.1 (March 12, 2007). Available at: www.qualityindicators.ahrq.gov/downloads/iqi/iqi_technical_specs_v31.pdf. Accessed February2010.
- , , . National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Washington, DC: National Center for Health Statistics; 2007. Vital and Health Statistics 13(165). Available at: www.cdc.gov/nchs/data/series/sr_13/sr13_165.pdf. Accessed February2010.
- AHRQ Quality Indicators. Guide to Inpatient Quality Indicators: Quality of Care in Hospitals—Volume, Mortality, and Utilization. Version 3.1 (March 12, 2007). Available at: www.qualityindicators.ahrq.gov/downloads/iqi/iqi_guide_v31.pdf. Accessed February2010.
- , , .Income, poverty, and health insurance coverage in the United States: 2006.Washington, DC:US Census Bureau. Current Population Reports;2007:60–233.
- , . Conditions Related to Uninsured Hospitalizations, 2003. HCUP Statistical Brief #8. Rockville, MD: Agency for Healthcare Research and Quality; 2006:6. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb8.pdf. Accessed February2010.
- Healthcare Cost and Utilization Project. NIS Description of Data Elements. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/nisdde.jsp. Accessed February2010.
- , , , .Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- SUDAAN User's Manual, Release 9.0. Research TrianglePark, NC:Research Triangle Institute;2006.
- , . Final Report on Calculating Nationwide Inpatient Sample (NIS) Variances, 2001. HCUP Methods Series Report #2003‐2. Online June 2005 (revised June 6, 2005). U.S. Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/CalculatingNISVariances200106092005.pdf. Accessed February2010.
- .On the variances of asymptotically normal estimators from complex surveys.Int Stat Rev.1983;51:279–292.
- , , , et al.Patient characteristics associated with care by a cardiologist among adults hospitalized with severe congestive heart failure.J Am Coll Cardiol.2000;36:2119–2125.
- , . Summary health statistics for U.S. adults: National Health Interview Survey, 2006. Washington, DC: National Center for Health Statistics; 2007:12. Vital and Health Statistics 10(235). Available at: www.cdc.gov/nchs/data/series/sr_10/sr10_235.pdf. Accessed February2010.
- .Insurance coverage, medical care use, and short‐term health changes following an unintentional injury or the onset of a chronic condition.JAMA.2007;297(10):1073–1084.
- , , , et al.The quality of chronic disease care in U.S. community health centers.Health Aff (Millwood).2006;25(6):1712–1723.
- , , , , , .Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases.Med Care.2008;46(3):232–239.
- , , , .The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population.J Clin Epidemiol. [E‐pub ahead of print].
- , .The U.S. economy and changes in health insurance coverage, 2000‐2006.Health Aff (Millwood).2008;27(2):w135‐w144.
- , , .Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland.JAMA.1992;268(17):2388‐2394.
- , , , et al.Unmet health needs of uninsured adults in the United States.JAMA.2000;284(16):2061‐2069.
- , , .Health insurance and access to care for symptomatic conditions.Arch Intern Med.2000;160(9):1269‐1274.
- , , , et al.Access to specialty care and medical services in community health centers.Health Aff (Millwood).2007;26(5):1459‐1468.
- , , , et al.A national study of chronic disease prevalence and access to care in uninsured U.S. adults.Ann Intern Med.2008;149:170–176.
- , , , .Relationship between patient source of payment and the intensity of hospital services.Med Care.1988;26(11):1111‐1114.
- , , .The association of payer with utilization of cardiac procedures in Massachusetts.JAMA.1990;264(10):1255‐1260.
- , , .Comparison of uninsured and privately insured hospital patients: condition on admission, resource use, and outcome.JAMA.1991;265:374–379.
- , , .Socioeconomic status and risk for substandard medical care.JAMA.1992;268(17):2383‐2387.
- , , , .The relation between health insurance coverage and clinical outcomes among women with breast cancer.N Engl J Med.1993;329(5):326‐331.
- , , .Health insurance and mortality. Evidence from a national cohort.JAMA.1993;270(6):737‐741.
- , , , .Mortality in the uninsured compared with that in persons with public and private health insurance.Arch Intern Med.1994;154(21):2409‐2416.
- .Medicaid policy and the substitution of hospital outpatient care for physician care.Health Serv Res.1989;24:33–66.
- , .Disparities in outcomes among patients with stroke associated with insurance status.Stroke.2007;38(3):1010‐1016.
- , , , et al.Influence of payor on use of invasive cardiac procedures and patient outcome after myocardial infarction in the United States. Participants in the National Registry of Myocardial Infarction.J Am Coll Cardiol.1998;31(7):1474‐1480.
- , , , et al.Payer status and the utilization of hospital resources in acute myocardial infarction: a report from the National Registry of Myocardial Infarction 2.Arch Intern Med.2000;160(6):817–823.
- , , , et al.Insurance coverage and care of patients with non‐ST‐segment elevation acute coronary syndromes.Ann Intern Med.2006;145(10):739–748.
- , , .Comparing uninsured and privately insured hospital patients: Admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- Healthcare Cost and Utilization Project. Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2005. Rockville, MD: Agency for Healthcare Research and Quality; 2007:6. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/NIS_Introduction_2005.pdf. Accessed February2010.
- Healthcare Cost and Utilization Project. Design of the Nationwide Inpatient Sample (NIS) 2005. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/reports/NIS_2005_Design_Report.pdf. Accessed February2010.
- AHRQ Quality Indicators. Inpatient Quality Indicators: Technical Specifications. Version 3.1 (March 12, 2007). Available at: www.qualityindicators.ahrq.gov/downloads/iqi/iqi_technical_specs_v31.pdf. Accessed February2010.
- , , . National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Washington, DC: National Center for Health Statistics; 2007. Vital and Health Statistics 13(165). Available at: www.cdc.gov/nchs/data/series/sr_13/sr13_165.pdf. Accessed February2010.
- AHRQ Quality Indicators. Guide to Inpatient Quality Indicators: Quality of Care in Hospitals—Volume, Mortality, and Utilization. Version 3.1 (March 12, 2007). Available at: www.qualityindicators.ahrq.gov/downloads/iqi/iqi_guide_v31.pdf. Accessed February2010.
- , , .Income, poverty, and health insurance coverage in the United States: 2006.Washington, DC:US Census Bureau. Current Population Reports;2007:60–233.
- , . Conditions Related to Uninsured Hospitalizations, 2003. HCUP Statistical Brief #8. Rockville, MD: Agency for Healthcare Research and Quality; 2006:6. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb8.pdf. Accessed February2010.
- Healthcare Cost and Utilization Project. NIS Description of Data Elements. Available at: www.hcup‐us.ahrq.gov/db/nation/nis/nisdde.jsp. Accessed February2010.
- , , , .Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- SUDAAN User's Manual, Release 9.0. Research TrianglePark, NC:Research Triangle Institute;2006.
- , . Final Report on Calculating Nationwide Inpatient Sample (NIS) Variances, 2001. HCUP Methods Series Report #2003‐2. Online June 2005 (revised June 6, 2005). U.S. Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/CalculatingNISVariances200106092005.pdf. Accessed February2010.
- .On the variances of asymptotically normal estimators from complex surveys.Int Stat Rev.1983;51:279–292.
- , , , et al.Patient characteristics associated with care by a cardiologist among adults hospitalized with severe congestive heart failure.J Am Coll Cardiol.2000;36:2119–2125.
- , . Summary health statistics for U.S. adults: National Health Interview Survey, 2006. Washington, DC: National Center for Health Statistics; 2007:12. Vital and Health Statistics 10(235). Available at: www.cdc.gov/nchs/data/series/sr_10/sr10_235.pdf. Accessed February2010.
- .Insurance coverage, medical care use, and short‐term health changes following an unintentional injury or the onset of a chronic condition.JAMA.2007;297(10):1073–1084.
- , , , et al.The quality of chronic disease care in U.S. community health centers.Health Aff (Millwood).2006;25(6):1712–1723.
- , , , , , .Risk‐adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases.Med Care.2008;46(3):232–239.
- , , , .The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population.J Clin Epidemiol. [E‐pub ahead of print].
Copyright © 2010 Society of Hospital Medicine
Talking the Talk
On May 26, members of SHM’s Public Policy Committee visited Capitol Hill to meet with members of Congress and their legislative staffs. Now in their fifth year, the annual “Hill Day” meetings have evolved from explaining “What is a hospitalist?” to substantive discussions about HM’s impact on improving the quality and efficiency of healthcare and reducing preventable rehospitalizations.
“We don’t go in with an agenda to ask for things” or to guard professional turf, says Eric Siegal, MD, SFHM, committee chair and a critical care fellow at the University of Wisconsin School of Medicine in Madison. “People on the Hill who talk to us recognize that we are the experts [on improving the quality of hospital care]. They say to us: ‘You are different than the other medical associations; you really represent the solution.’ ”
The committee highlighted Project BOOST (Better Outcomes for Older Adults through Safe Transitions), SHM’s national quality initiative aimed at helping to improve care transitions and reduce rehospitalizations—–a major focus in the healthcare reform legislation.
Committee members also discussed SHM's support for a permanent repeal of the Sustainable Growth Rate (SGR). Physician reimbursement to Medicare patients technically was cut by 21% on June 1. The House passed another stopgap measure, but the Senate adjourned before a vote and is expected to raise the topic again early next week.
Committee member Patrick Torcson, MD, SFHM, a hospitalist at St. Tammany Parish Hospital in Covington, La., spent 30 minutes with Sen. David Vitter (R-La.), among his other Congressional encounters. “I had to be respectful and mindful of our senators’ and representatives’ different positions on healthcare reform,” Dr. Torcson explains, adding Vitter voted against the Patient Protection and Affordable Care Act, which was passed in March. “But he couldn’t have been more engaging with me. We talked about all aspects of hospital medicine.”
Find out the latest information on SGR reform and contact your legislators in support of permanent repeal through SHM's Legislative Action Center.
On May 26, members of SHM’s Public Policy Committee visited Capitol Hill to meet with members of Congress and their legislative staffs. Now in their fifth year, the annual “Hill Day” meetings have evolved from explaining “What is a hospitalist?” to substantive discussions about HM’s impact on improving the quality and efficiency of healthcare and reducing preventable rehospitalizations.
“We don’t go in with an agenda to ask for things” or to guard professional turf, says Eric Siegal, MD, SFHM, committee chair and a critical care fellow at the University of Wisconsin School of Medicine in Madison. “People on the Hill who talk to us recognize that we are the experts [on improving the quality of hospital care]. They say to us: ‘You are different than the other medical associations; you really represent the solution.’ ”
The committee highlighted Project BOOST (Better Outcomes for Older Adults through Safe Transitions), SHM’s national quality initiative aimed at helping to improve care transitions and reduce rehospitalizations—–a major focus in the healthcare reform legislation.
Committee members also discussed SHM's support for a permanent repeal of the Sustainable Growth Rate (SGR). Physician reimbursement to Medicare patients technically was cut by 21% on June 1. The House passed another stopgap measure, but the Senate adjourned before a vote and is expected to raise the topic again early next week.
Committee member Patrick Torcson, MD, SFHM, a hospitalist at St. Tammany Parish Hospital in Covington, La., spent 30 minutes with Sen. David Vitter (R-La.), among his other Congressional encounters. “I had to be respectful and mindful of our senators’ and representatives’ different positions on healthcare reform,” Dr. Torcson explains, adding Vitter voted against the Patient Protection and Affordable Care Act, which was passed in March. “But he couldn’t have been more engaging with me. We talked about all aspects of hospital medicine.”
Find out the latest information on SGR reform and contact your legislators in support of permanent repeal through SHM's Legislative Action Center.
On May 26, members of SHM’s Public Policy Committee visited Capitol Hill to meet with members of Congress and their legislative staffs. Now in their fifth year, the annual “Hill Day” meetings have evolved from explaining “What is a hospitalist?” to substantive discussions about HM’s impact on improving the quality and efficiency of healthcare and reducing preventable rehospitalizations.
“We don’t go in with an agenda to ask for things” or to guard professional turf, says Eric Siegal, MD, SFHM, committee chair and a critical care fellow at the University of Wisconsin School of Medicine in Madison. “People on the Hill who talk to us recognize that we are the experts [on improving the quality of hospital care]. They say to us: ‘You are different than the other medical associations; you really represent the solution.’ ”
The committee highlighted Project BOOST (Better Outcomes for Older Adults through Safe Transitions), SHM’s national quality initiative aimed at helping to improve care transitions and reduce rehospitalizations—–a major focus in the healthcare reform legislation.
Committee members also discussed SHM's support for a permanent repeal of the Sustainable Growth Rate (SGR). Physician reimbursement to Medicare patients technically was cut by 21% on June 1. The House passed another stopgap measure, but the Senate adjourned before a vote and is expected to raise the topic again early next week.
Committee member Patrick Torcson, MD, SFHM, a hospitalist at St. Tammany Parish Hospital in Covington, La., spent 30 minutes with Sen. David Vitter (R-La.), among his other Congressional encounters. “I had to be respectful and mindful of our senators’ and representatives’ different positions on healthcare reform,” Dr. Torcson explains, adding Vitter voted against the Patient Protection and Affordable Care Act, which was passed in March. “But he couldn’t have been more engaging with me. We talked about all aspects of hospital medicine.”
Find out the latest information on SGR reform and contact your legislators in support of permanent repeal through SHM's Legislative Action Center.
HM-pedia
Ever think there has to be a better way to update your group’s 100-plus-page program manual? The HM group at Beth Israel Deaconess Medical Center in Boston offers a solution: Post all of the information on a secure wiki page.
That’s what hospitalist Roger Yu, MD, did a few months ago. He transferred his group’s manual to a wiki that allows members to access, add, and edit information about referring physicians, schedules, and more.
Dr. Yu says the page, hosted by Microsoft’s SharePoint platform, allows the 33-hospitalist group to stay up to date on new policies without forcing them to sift through a barrage of e-mails. “We probably have at least eight to 10 clinical rotations,” Dr. Yu says. “The hospital policy is always changing, the way we do our work constantly changes, and [using a wiki platform] is a way of getting this information across.”
The program was especially helpful, he adds, when his group recently developed a new referral relationship with a large, multispecialty group that had some unique needs. “There are specific ways they want us to consult their specialists, and they have different pager numbers and contact people, so we were able to post all of that in a page on the wiki,” Dr. Yu explains, adding any member of his group can add or edit information on the wiki.
Dr. Yu says his group hopes to eventually use the wiki to post calendar events and share teaching materials.
Joe Li, MD, director of the Beth Israel HM program, who tasked Dr. Yu with developing the wiki, says the program has, for the most part, run smoothly: “I wouldn’t say there have been glitches, per se, but I think people in the group have different levels of willingness to adopt new technology,” says Dr. Li, SHM’s president-elect. “Some [of our] people have accessed it less than others.”
Dr. Yu says that while creating a wiki doesn’t require extensive HTML knowledge or even computer savviness, it does involve some initial planning. “I would say if you’re going to start it up, it’s more about thinking about how to organize the table of contents,” he says. Once the table of contents is created, he says, expect to spend a few hours a day plugging in the information. It took him about three days to input the 120-plus-page manual.
For more tips on how to create your own wiki, visit Microsoft's help page.
Ever think there has to be a better way to update your group’s 100-plus-page program manual? The HM group at Beth Israel Deaconess Medical Center in Boston offers a solution: Post all of the information on a secure wiki page.
That’s what hospitalist Roger Yu, MD, did a few months ago. He transferred his group’s manual to a wiki that allows members to access, add, and edit information about referring physicians, schedules, and more.
Dr. Yu says the page, hosted by Microsoft’s SharePoint platform, allows the 33-hospitalist group to stay up to date on new policies without forcing them to sift through a barrage of e-mails. “We probably have at least eight to 10 clinical rotations,” Dr. Yu says. “The hospital policy is always changing, the way we do our work constantly changes, and [using a wiki platform] is a way of getting this information across.”
The program was especially helpful, he adds, when his group recently developed a new referral relationship with a large, multispecialty group that had some unique needs. “There are specific ways they want us to consult their specialists, and they have different pager numbers and contact people, so we were able to post all of that in a page on the wiki,” Dr. Yu explains, adding any member of his group can add or edit information on the wiki.
Dr. Yu says his group hopes to eventually use the wiki to post calendar events and share teaching materials.
Joe Li, MD, director of the Beth Israel HM program, who tasked Dr. Yu with developing the wiki, says the program has, for the most part, run smoothly: “I wouldn’t say there have been glitches, per se, but I think people in the group have different levels of willingness to adopt new technology,” says Dr. Li, SHM’s president-elect. “Some [of our] people have accessed it less than others.”
Dr. Yu says that while creating a wiki doesn’t require extensive HTML knowledge or even computer savviness, it does involve some initial planning. “I would say if you’re going to start it up, it’s more about thinking about how to organize the table of contents,” he says. Once the table of contents is created, he says, expect to spend a few hours a day plugging in the information. It took him about three days to input the 120-plus-page manual.
For more tips on how to create your own wiki, visit Microsoft's help page.
Ever think there has to be a better way to update your group’s 100-plus-page program manual? The HM group at Beth Israel Deaconess Medical Center in Boston offers a solution: Post all of the information on a secure wiki page.
That’s what hospitalist Roger Yu, MD, did a few months ago. He transferred his group’s manual to a wiki that allows members to access, add, and edit information about referring physicians, schedules, and more.
Dr. Yu says the page, hosted by Microsoft’s SharePoint platform, allows the 33-hospitalist group to stay up to date on new policies without forcing them to sift through a barrage of e-mails. “We probably have at least eight to 10 clinical rotations,” Dr. Yu says. “The hospital policy is always changing, the way we do our work constantly changes, and [using a wiki platform] is a way of getting this information across.”
The program was especially helpful, he adds, when his group recently developed a new referral relationship with a large, multispecialty group that had some unique needs. “There are specific ways they want us to consult their specialists, and they have different pager numbers and contact people, so we were able to post all of that in a page on the wiki,” Dr. Yu explains, adding any member of his group can add or edit information on the wiki.
Dr. Yu says his group hopes to eventually use the wiki to post calendar events and share teaching materials.
Joe Li, MD, director of the Beth Israel HM program, who tasked Dr. Yu with developing the wiki, says the program has, for the most part, run smoothly: “I wouldn’t say there have been glitches, per se, but I think people in the group have different levels of willingness to adopt new technology,” says Dr. Li, SHM’s president-elect. “Some [of our] people have accessed it less than others.”
Dr. Yu says that while creating a wiki doesn’t require extensive HTML knowledge or even computer savviness, it does involve some initial planning. “I would say if you’re going to start it up, it’s more about thinking about how to organize the table of contents,” he says. Once the table of contents is created, he says, expect to spend a few hours a day plugging in the information. It took him about three days to input the 120-plus-page manual.
For more tips on how to create your own wiki, visit Microsoft's help page.
Second-line CML drugs prove better than first

Credit: UC San Diego
Nilotinib and dasatinib, currently approved for the treatment of drug-resistant chronic myeloid leukemia (CML), provide quicker and better responses as front-line therapy than the current standard, imatinib, according to 2 international, phase 3 trials.
Complete cytogenetic responses and major molecular responses were higher among newly diagnosed CML patients treated with nilotinib or dasatinib first, compared to newly diagnosed patients treated with imatinib first.
Also, more patients treated with imatinib experienced disease progression than patients treated with second-line drugs.
In the DASISION trial, 519 treatment-naïve CML patients received 100 mg of dasatinib or 400 mg of imatinib, once daily, as front-line therapy.
In the dasatinib arm, 77% of patients had confirmed cytogenetic responses (CCyR), compared to 66% in the imatinib arm. Patients on dasatinib also reached CCyR faster than those on imatinib, 54% in 3 months vs 31% in 3 months, respectively.
Forty-six percent of patients in the dasatinib arm reached a major molecular response (MMR), compared to 28% in the imatinib group.
In the imatinib arm, 3.5% of patients experienced disease progression, compared to 1.9% in the dasatinib group.
Side effects were mostly low-grade with both drugs. However hematologic side-effects were more common in dasatinib-treated patients and low-grade side effects such as vomiting, muscle pain, and inflammation were more common in patients using imatinib.
“We’ve learned in cancer therapy that it’s important to use your big guns up front,” said Hagop Kantarjian, MD, of The University of Texas MD Anderson Cancer Center in Houston. Dr Kantarjian is the corresponding author of the DASISION study and coauthor of the ENEST study.
“We know that achieving complete cytogenetic response or major molecular response within a year of starting treatment is associated with more favorable long-term survival. Using these second-generation drugs will likely improve outcomes for patients with chronic myeloid leukemia.”
The ENEST trial of nilotinib and imatinib yielded similar results in favor of second-generation treatments.
Eight hundred thirty-six newly diagnosed CML patients were treated with 300 mg or 400 mg of nilotinib twice daily or 100 mg of imatinib once daily.
The 300 mg nilotinib arm experienced CCyR in 80% of patients, MMR in 44% of patients, and progression in less than 1% of patients. In the 400 mg nilotinib arm, 78% of patients experienced CCyR, 43% experienced MMR ,and again, less than 1% experienced disease progression.
In the same trial, only 65% of patients experienced CCyR, 22% experienced MMR, and 4% saw progression in their disease when treated with imatinib.
The median time to reach MMR was 5.7 months in the 300 mg nilotinib group, 5.8 months in the 400 mg nilotinib group, and 8.3 months in the imatinib group.
Serious side effects were uncommon for both drugs. This time, hematologic side effects were more common in patients taking imatinib, and patients taking nilotinib were more likely to experience low-grade side effects like vomiting and inflammation.
“Findings from both of these studies confirm the single-arm trials done at MD Anderson, which had shown superiority of second-generation drugs in a front-line setting,” said Dr Kantarjian.
Their findings are published online in The New England Journal of Medicine.
Currently, Jorge Cortez, MD, at MD Anderson Cancer Center, is conducting 2 single-arm clinical trials to compare the performance of the second-generation CML drugs against historical imatinib trial results. ![]()

Credit: UC San Diego
Nilotinib and dasatinib, currently approved for the treatment of drug-resistant chronic myeloid leukemia (CML), provide quicker and better responses as front-line therapy than the current standard, imatinib, according to 2 international, phase 3 trials.
Complete cytogenetic responses and major molecular responses were higher among newly diagnosed CML patients treated with nilotinib or dasatinib first, compared to newly diagnosed patients treated with imatinib first.
Also, more patients treated with imatinib experienced disease progression than patients treated with second-line drugs.
In the DASISION trial, 519 treatment-naïve CML patients received 100 mg of dasatinib or 400 mg of imatinib, once daily, as front-line therapy.
In the dasatinib arm, 77% of patients had confirmed cytogenetic responses (CCyR), compared to 66% in the imatinib arm. Patients on dasatinib also reached CCyR faster than those on imatinib, 54% in 3 months vs 31% in 3 months, respectively.
Forty-six percent of patients in the dasatinib arm reached a major molecular response (MMR), compared to 28% in the imatinib group.
In the imatinib arm, 3.5% of patients experienced disease progression, compared to 1.9% in the dasatinib group.
Side effects were mostly low-grade with both drugs. However hematologic side-effects were more common in dasatinib-treated patients and low-grade side effects such as vomiting, muscle pain, and inflammation were more common in patients using imatinib.
“We’ve learned in cancer therapy that it’s important to use your big guns up front,” said Hagop Kantarjian, MD, of The University of Texas MD Anderson Cancer Center in Houston. Dr Kantarjian is the corresponding author of the DASISION study and coauthor of the ENEST study.
“We know that achieving complete cytogenetic response or major molecular response within a year of starting treatment is associated with more favorable long-term survival. Using these second-generation drugs will likely improve outcomes for patients with chronic myeloid leukemia.”
The ENEST trial of nilotinib and imatinib yielded similar results in favor of second-generation treatments.
Eight hundred thirty-six newly diagnosed CML patients were treated with 300 mg or 400 mg of nilotinib twice daily or 100 mg of imatinib once daily.
The 300 mg nilotinib arm experienced CCyR in 80% of patients, MMR in 44% of patients, and progression in less than 1% of patients. In the 400 mg nilotinib arm, 78% of patients experienced CCyR, 43% experienced MMR ,and again, less than 1% experienced disease progression.
In the same trial, only 65% of patients experienced CCyR, 22% experienced MMR, and 4% saw progression in their disease when treated with imatinib.
The median time to reach MMR was 5.7 months in the 300 mg nilotinib group, 5.8 months in the 400 mg nilotinib group, and 8.3 months in the imatinib group.
Serious side effects were uncommon for both drugs. This time, hematologic side effects were more common in patients taking imatinib, and patients taking nilotinib were more likely to experience low-grade side effects like vomiting and inflammation.
“Findings from both of these studies confirm the single-arm trials done at MD Anderson, which had shown superiority of second-generation drugs in a front-line setting,” said Dr Kantarjian.
Their findings are published online in The New England Journal of Medicine.
Currently, Jorge Cortez, MD, at MD Anderson Cancer Center, is conducting 2 single-arm clinical trials to compare the performance of the second-generation CML drugs against historical imatinib trial results. ![]()

Credit: UC San Diego
Nilotinib and dasatinib, currently approved for the treatment of drug-resistant chronic myeloid leukemia (CML), provide quicker and better responses as front-line therapy than the current standard, imatinib, according to 2 international, phase 3 trials.
Complete cytogenetic responses and major molecular responses were higher among newly diagnosed CML patients treated with nilotinib or dasatinib first, compared to newly diagnosed patients treated with imatinib first.
Also, more patients treated with imatinib experienced disease progression than patients treated with second-line drugs.
In the DASISION trial, 519 treatment-naïve CML patients received 100 mg of dasatinib or 400 mg of imatinib, once daily, as front-line therapy.
In the dasatinib arm, 77% of patients had confirmed cytogenetic responses (CCyR), compared to 66% in the imatinib arm. Patients on dasatinib also reached CCyR faster than those on imatinib, 54% in 3 months vs 31% in 3 months, respectively.
Forty-six percent of patients in the dasatinib arm reached a major molecular response (MMR), compared to 28% in the imatinib group.
In the imatinib arm, 3.5% of patients experienced disease progression, compared to 1.9% in the dasatinib group.
Side effects were mostly low-grade with both drugs. However hematologic side-effects were more common in dasatinib-treated patients and low-grade side effects such as vomiting, muscle pain, and inflammation were more common in patients using imatinib.
“We’ve learned in cancer therapy that it’s important to use your big guns up front,” said Hagop Kantarjian, MD, of The University of Texas MD Anderson Cancer Center in Houston. Dr Kantarjian is the corresponding author of the DASISION study and coauthor of the ENEST study.
“We know that achieving complete cytogenetic response or major molecular response within a year of starting treatment is associated with more favorable long-term survival. Using these second-generation drugs will likely improve outcomes for patients with chronic myeloid leukemia.”
The ENEST trial of nilotinib and imatinib yielded similar results in favor of second-generation treatments.
Eight hundred thirty-six newly diagnosed CML patients were treated with 300 mg or 400 mg of nilotinib twice daily or 100 mg of imatinib once daily.
The 300 mg nilotinib arm experienced CCyR in 80% of patients, MMR in 44% of patients, and progression in less than 1% of patients. In the 400 mg nilotinib arm, 78% of patients experienced CCyR, 43% experienced MMR ,and again, less than 1% experienced disease progression.
In the same trial, only 65% of patients experienced CCyR, 22% experienced MMR, and 4% saw progression in their disease when treated with imatinib.
The median time to reach MMR was 5.7 months in the 300 mg nilotinib group, 5.8 months in the 400 mg nilotinib group, and 8.3 months in the imatinib group.
Serious side effects were uncommon for both drugs. This time, hematologic side effects were more common in patients taking imatinib, and patients taking nilotinib were more likely to experience low-grade side effects like vomiting and inflammation.
“Findings from both of these studies confirm the single-arm trials done at MD Anderson, which had shown superiority of second-generation drugs in a front-line setting,” said Dr Kantarjian.
Their findings are published online in The New England Journal of Medicine.
Currently, Jorge Cortez, MD, at MD Anderson Cancer Center, is conducting 2 single-arm clinical trials to compare the performance of the second-generation CML drugs against historical imatinib trial results. ![]()
CER and Hospital Medicine
The topic of comparative effectiveness research (CER) has recently gained prominence within the context of the national focus on health reform. This article provides a brief overview and history of CER, and discusses the implications of CER for hospitalists in each of four major career roles: research, clinical practice, education and training, and hospital leadership. Both medical journals and lay media have produced a flurry of articles recently on a variety of health reform subjects. One topic that has achieved prominence within this growing body of literature is comparative effectiveness research (CER). For many hospitalists, this particular brand of research may be unfamiliar. As discussions about CER priorities, the controversy surrounding CER, and even the definition of CER gain visibility, hospitalists may be left wondering, What exactly is CER and what does it mean for me?
Until recently no common definition for CER existed, and the very concept was identified only in relatively narrow policy and research circles. However, CER is not a new idea. Its ancestor is the notion of medical technology assessment (MTA), which garnered enthusiasm and support in the 1970s. In 1978, Congress established the National Center for Health Technology Assessment (which, over time, evolved into the Agency for Healthcare Research and Quality [AHRQ]), whose charge was to coordinate efforts within the government to assess the safety, efficacy, effectiveness, and cost‐effectiveness of medical technologies. The recognition of a need for technology assessment at that time is mirrored by the widespread interest in CER seen today. Part of the reason that MTA did not take hold is that then, as now, this type of evaluation is challenging and time consuming, requiring large, well‐designed effectiveness studies. These studies require rigorous methods, typically long‐term follow‐up, and acceptance via editors and the medical literature that effectiveness is as important as efficacy demonstrated in a randomized trial. With the spread of antiregulatory sentiment and the lack of an economic imperative to reduce costs, the national focus on technology assessment waned. The current economic crisis has refocused the government and private sector on the soaring cost of health care and the need to improve quality, and the stimulus package passed in February of 2009 placed CER once again in the forefront. The American Recovery and Reinvestment Act (ARRA) of 2009 allocated $1.1 billion for CER.1 On June 30, 2009, 2 reports delineating the strategy and priorities for CER were released. The report from the ARRA‐mandated Federal Coordinating Council (FCC) for CER includes a broad definition of CER and outlines a high‐level strategic framework for priorities and investments in CER.2 Simultaneously, the report from the Institute of Medicine (IOM) lists 100 priority research topics, and gives 10 general recommendations for the CER enterprise going forward.3
So what is CER and why is it important? How is it different from standard research that hospitalists use every day to inform their clinical decision‐making? Unfortunately, patients and providers confront medical decisions daily that are not evidence based. All too frequently it is unclear what therapeutic option works best for which patient under which circumstances. For example, what is the best inpatient diabetes management strategy for an African American woman with multiple medical problems? What is the best discharge process for an elderly man with heart disease in order to prevent readmission? CER seeks to fill the gaps in evidence needed by patients and clinicians in order to make appropriate medical decisions. It differs from standard efficacy research in that it compares interventions or management strategies in real world settings, allows identification of effectiveness in patient subgroups, and is more patient‐centered, focusing on the decisions confronting patients and their physicians. The following definition of CER was developed by the FCC for CER:
CER is the conduct and synthesis of research comparing the benefits and harms of different interventions and strategies to prevent, diagnose, treat and monitor health conditions in real world settings. The purpose of this research is to improve health outcomes by developing and disseminating evidence‐based information to patients, clinicians, and other decision‐makers, responding to their expressed needs about which interventions are most effective for which patients under specific circumstances.
-
To provide this information, CER must assess a comprehensive array of health‐related outcomes for diverse patient populations and sub‐groups.
-
Defined interventions compared may include medications, procedures, medical and assistive devices and technologies, diagnostic testing, behavioral change and delivery system strategies.
-
This research necessitates the development, expansion and use of a variety of data sources and methods to assess comparative effectiveness and actively disseminate the results.
While CER is an evolving field requiring continued methodological development (such as enhancement of methods for practical, or pragmatic trials and complex analyses of large, linked databases), examples of rigorous comparative studies do exist. The Veterans Administration (VA) COURAGE trial compared optimal medical therapy (OMT) with or without percutaneous coronary intervention (PCI) for patients with stable coronary disease, finding that PCI did not reduce the risk of death or cardiovascular events compared to OMT alone.4 Another example is the Diabetes Prevention Program which compared placebo, metformin, and a lifestyle modification program to prevent or delay the onset of type 2 diabetes. This study famously showed that lifestyle modification was more effective than metformin or placebo in reducing the incidence of diabetes.5
CER holds the promise of significantly improving the health of Americans through the ability to target treatments and other interventions to individual patients. As noted by the FCC, CER can allow for the delivery of the right treatment to the right patient at the right time2 even as the field continues to evolve. To quote Fineberg and Hiatt6 in describing technology assessment in 1979, we cannot expect CER to lead to perfect decisions, but we can expect even imperfect methods to facilitate better informed decisions than would otherwise be possible.
CER has important implications for hospitalists in all roles and settings. As the field of hospital medicine has grown, hospitalists have increasingly assumed more responsibilities than just patient care. In academic and community hospitals, hospitalists take on leadership roles, particularly in quality improvement (QI) and patient safety, and educational roles in the training of housestaff, medical students, and physician extenders. The last several years have also seen a significant increase in hospitalists participating in research. The relevance of CER to each of these 4 major activities is described below and in the accompanying Table 1.
| Primary Role | Potential Implications of CER |
|---|---|
| |
| Research | New availability of funds for hospital‐based CER |
| Enhanced data infrastructure to conduct CER | |
| Opportunity to apply CER to issues unique to hospital medicine | |
| Opportunity to develop methodologic skills | |
| Clinical practice | End users of CER evidence |
| Responsibility for translation of CER into practice | |
| Targets of Federal and non‐Federal dissemination efforts | |
| Education and training | Development of a workforce to conduct hospital‐based CER |
| Responsibility for teaching physician and nonphysician trainees about CER concepts and review of CER literature | |
| Hospital leadership | Direct hospital‐wide efforts to implement emerging CER evidence into practice through a multidisciplinary approach |
| Education and empowerment of clinician and nonclinician staff to translate CER information into practice | |
Hospitalists and Research
Many comparative effectiveness questions about clinical care, processes of care, and quality of care within the inpatient setting are in need of answers. Hospitalist researchers have the opportunity to make a significant impact on care by pursuing answers to questions that are unique to the field of hospital medicine. With the new availability of funds for CER, now is the time to address many of these questions head‐on. For example, there is a lack of evidence about best practices for a large number of inpatient acute conditions. What is the best strategy to manage acute hospital delirium in an elderly patient? What is the best approach to treating acute pain in an elderly woman on multiple medications? Overwhelmingly the patients that hospitalists care for are elderly and/or have multiple chronic conditions, including children with special health care needs. Many are from racial or ethnic minority backgrounds. These subgroups of patients have been historically under‐represented in clinical trials, yet represent exactly the priority populations that the Federal CER effort targets. The field of hospital medicine can be transformed with a substantial investment in research to address common inpatient clinical conditions in real world settings focused on the kinds of patients hospitalists actually care for.
One of the most vexing and frustrating care delivery issues for hospitalists, clinicians and researchers alike, is the discharge process. This problem received increased attention after a recent article highlighted the high rate of readmissions in the Medicare population.7 Research on the discharge process has grown substantially in recent years, and has become an area of intense focus and attention for hospitalists, nurses, researchers, hospital administrators and policymakers. Without question, hospitalists are uniquely poised to conduct research on this critically important topic, and CER is an ideal vehicle for moving this field forward. In collaboration with nurses, primary care physicians, pharmacists, case managers and others, hospitalists should take advantage of the Federal investment in studying care delivery systems interventions, and develop innovative methods and strategies for studying and improving this crucial transition in care. CER is also applicable to other care transitions, including the admission process, transitions within the hospital, and discharge to nursing facilities. Other examples of comparative effectiveness topics that hospitalist researchers are particularly suited for include comparing methods for implementing inpatient treatment protocols or clinical pathways, comparison of health information technology (IT) systems to reduce medical error, and QI approaches.
What are the methodologies that hospitalists should use to conduct CER? While randomized pragmatic real world trials are appealing, this method may not always be practical. Other methodologies are available for rigorous use, including cohort studies, comparative QI interventions, clustered and factorial design, systematic reviews, and analysis of registries, administrative claims, or other databases. Databases currently available for analysis on priority populations and subgroups are limited, and include the VA and Medicare databases. To address this need, one of the primary Federal investments in CER is for the enhancement and expansion of data infrastructure. Data infrastructure tools that are likely to be available to hospitalist researchers for CER include expanded longitudinal administrative claims databases with linkages to electronic health records (EHRs), expanded patient registries with linkages to other forms of data, and distributed data networks that are populated by EHRs in provider and practice settings. Hospitalist researchers should take advantage of these resources as they become available, as they have tremendous potential to inform decision‐making for providers and patients alike.
Hospitalists and Clinical Practice
As with all providers, hospitalists will be end‐users of CER evidence, and will have the responsibility of translating new knowledge into practice. This process will not be easy. How are hospitalists to reliably access and incorporate new comparative effectiveness information into their daily practice? How should they deal with some of the potential unintended consequences of CER, such as information overload or conflicting evidence? While hospitalists have a professional responsibility to search for and apply CER findings, the future development of CER‐based practice guidelines will encourage evidence translation. The development of a common platform for the dissemination of CER relevant to hospitalists would significantly enhance the uptake of new evidence by practicing hospitalists and other hospital‐based providers such as physician assistants or nurse practitioners. Medical societies such as the Society of Hospital Medicine and the American Academy of Pediatrics should consider developing committees for CER and leading coordinated educational efforts specifically focused on CER results through publications and presentations at local, regional, and national meetings. In addition, other dissemination tools for CER will soon emerge and existing tools will be enhanced, such as the Effective Health Care Program and Eisenberg Center housed at the AHRQ. The coming years will see an expansion of these and other dissemination efforts to both providers and patients, and hospitalists must be vigilant about accessing these resources and integrating comparative effectiveness evidence into practice. As Federal dissemination efforts to consumers spread, patients will increasingly expect physicians to discuss comparative effectiveness evidence in describing options for their individual health needs. Finally, a key lever for translating CER into practice will be payment models that place accountability for performance on physicians and hospitals, with a significant proportion of payment based on the delivery of high quality, efficient care.
Education and Training
Investment in the training and development of a skilled workforce to conduct CER is an important priority. Hospitalist researchers should take advantage of education and training programs to support the development of methodologies and skills for conducting CER that will become available. These programs will enable hospitalists to learn such skills as the use of the newly enhanced data infrastructure discussed above. The national investment in human and scientific capital for CER can promote the training of a corps of hospitalist researchers focused on this research which, in turn, could support the growth of the academic hospitalist field. Hospitalists who have responsibilities in medical education and residency training programs should take the lead in teaching CER concepts that are relevant to inpatient care. They will need to train the next generation of medical students and residents to read and understand comparative effectiveness literature and its application in clinical practice. Hospitalist educators are also best positioned to teach medical trainees comparative effectiveness evidence about inpatient QI methods and care processes.
Hospital Leadership
As front‐line providers and team leaders, hospitalists are well placed to direct the efforts within their hospitals to implement new CER evidence. For example, suppose new comparative effectiveness evidence about best practices for the discharge process for community‐dwelling older adults with multiple chronic conditions were to emerge. Hospitalists could lead efforts within their hospital to establish a multidisciplinary team to address this development, create standard protocols for implementing the new discharge process that align with their hospital's unique systems and organizational structure, advocate for necessary resources for the team to accomplish the goal of safely discharging these patients, ensure a method to track outcomes such as readmissions once the new discharge process is implemented, and provide data feedback to the team, hospital staff, and administrative leadership of the hospital. All of these activities should include a variety of disciplines working together, but as physician leaders, hospitalists can take the initiative to spearhead these endeavors. The inpatient setting is one that requires teamwork and coordination, and as team leaders, hospitalists can strongly influence the spread and adoption of CER results. Similarly, hospitalists are in a position to affect this dissemination and translation process by actively educating and empowering other clinicians and hospital staff within their local environment. Finally, as hospitalists increasingly take on leadership roles in QI departments and as chief medical officers within both community and university‐affiliated hospitals8, they are in a unique position to lead efforts to implement CER‐based QI activities. These may range from the implementation of IT functions to reduce medical error to strategies to reduce hospital‐acquired infections or falls.
Conclusion
As a result of the stimulus funds directed towards CER, the coming years will see a vast increase in the generation of comparative effectiveness evidence and the application of that evidence into practice.9 The national CER endeavor is particularly germane to the field of hospital medicine, as uncertainty about best practices is common, and the patients hospitalists serve represent priority populations for CER investments. Hospitalists can play a central role in both generating CER and implementing its findings in settings in which patients are highly vulnerable, and existing information is insufficient. In addition to clinical questions, hospitalist researchers are particularly suited to answering important questions about quality of care and inpatient processes such as transitions of care and care coordination. Having evidence on the best practices for care transitions or strategies to reduce medical error, for example, could have a significant impact on patient outcomes, quality of life, and cost of care. However, none of this new evidence will be of any value if it is not used by front‐line providers.10 Practicing hospitalists should lead efforts within their hospital to disseminate new CER findings to their hospitalist and non‐hospitalist colleagues, and to leverage their position as hospital and team leaders to implement inpatient‐based CER findings. All of these combined efforts have the potential to significantly move the field of hospital medicine forward, with the end result being improved health and better outcomes for patients.
- American Recovery and Reinvestment Act. Available at: http://frwebgate.access.gpo.gov/cgi‐bin/getdoc.cgi?dbname=111_cong_bills356:1503–1516.
- ,,, et al.Reduction in the Incidence of Type 2 diabetes with lifestyle intervention or metformin.N Engl J Med.2002;346:393–403.
- ,,Evaluation of medical practices: the case for technology assessment.N Engl J Med.1979;301:1086–1091.
- ,,,Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360:1418–1428.
- 2005–2006 Society of Hospital Medicine Survey. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2361:328–330.
- ,,Transformation of health care at the front line.JAMA.2009;301:763–765.
The topic of comparative effectiveness research (CER) has recently gained prominence within the context of the national focus on health reform. This article provides a brief overview and history of CER, and discusses the implications of CER for hospitalists in each of four major career roles: research, clinical practice, education and training, and hospital leadership. Both medical journals and lay media have produced a flurry of articles recently on a variety of health reform subjects. One topic that has achieved prominence within this growing body of literature is comparative effectiveness research (CER). For many hospitalists, this particular brand of research may be unfamiliar. As discussions about CER priorities, the controversy surrounding CER, and even the definition of CER gain visibility, hospitalists may be left wondering, What exactly is CER and what does it mean for me?
Until recently no common definition for CER existed, and the very concept was identified only in relatively narrow policy and research circles. However, CER is not a new idea. Its ancestor is the notion of medical technology assessment (MTA), which garnered enthusiasm and support in the 1970s. In 1978, Congress established the National Center for Health Technology Assessment (which, over time, evolved into the Agency for Healthcare Research and Quality [AHRQ]), whose charge was to coordinate efforts within the government to assess the safety, efficacy, effectiveness, and cost‐effectiveness of medical technologies. The recognition of a need for technology assessment at that time is mirrored by the widespread interest in CER seen today. Part of the reason that MTA did not take hold is that then, as now, this type of evaluation is challenging and time consuming, requiring large, well‐designed effectiveness studies. These studies require rigorous methods, typically long‐term follow‐up, and acceptance via editors and the medical literature that effectiveness is as important as efficacy demonstrated in a randomized trial. With the spread of antiregulatory sentiment and the lack of an economic imperative to reduce costs, the national focus on technology assessment waned. The current economic crisis has refocused the government and private sector on the soaring cost of health care and the need to improve quality, and the stimulus package passed in February of 2009 placed CER once again in the forefront. The American Recovery and Reinvestment Act (ARRA) of 2009 allocated $1.1 billion for CER.1 On June 30, 2009, 2 reports delineating the strategy and priorities for CER were released. The report from the ARRA‐mandated Federal Coordinating Council (FCC) for CER includes a broad definition of CER and outlines a high‐level strategic framework for priorities and investments in CER.2 Simultaneously, the report from the Institute of Medicine (IOM) lists 100 priority research topics, and gives 10 general recommendations for the CER enterprise going forward.3
So what is CER and why is it important? How is it different from standard research that hospitalists use every day to inform their clinical decision‐making? Unfortunately, patients and providers confront medical decisions daily that are not evidence based. All too frequently it is unclear what therapeutic option works best for which patient under which circumstances. For example, what is the best inpatient diabetes management strategy for an African American woman with multiple medical problems? What is the best discharge process for an elderly man with heart disease in order to prevent readmission? CER seeks to fill the gaps in evidence needed by patients and clinicians in order to make appropriate medical decisions. It differs from standard efficacy research in that it compares interventions or management strategies in real world settings, allows identification of effectiveness in patient subgroups, and is more patient‐centered, focusing on the decisions confronting patients and their physicians. The following definition of CER was developed by the FCC for CER:
CER is the conduct and synthesis of research comparing the benefits and harms of different interventions and strategies to prevent, diagnose, treat and monitor health conditions in real world settings. The purpose of this research is to improve health outcomes by developing and disseminating evidence‐based information to patients, clinicians, and other decision‐makers, responding to their expressed needs about which interventions are most effective for which patients under specific circumstances.
-
To provide this information, CER must assess a comprehensive array of health‐related outcomes for diverse patient populations and sub‐groups.
-
Defined interventions compared may include medications, procedures, medical and assistive devices and technologies, diagnostic testing, behavioral change and delivery system strategies.
-
This research necessitates the development, expansion and use of a variety of data sources and methods to assess comparative effectiveness and actively disseminate the results.
While CER is an evolving field requiring continued methodological development (such as enhancement of methods for practical, or pragmatic trials and complex analyses of large, linked databases), examples of rigorous comparative studies do exist. The Veterans Administration (VA) COURAGE trial compared optimal medical therapy (OMT) with or without percutaneous coronary intervention (PCI) for patients with stable coronary disease, finding that PCI did not reduce the risk of death or cardiovascular events compared to OMT alone.4 Another example is the Diabetes Prevention Program which compared placebo, metformin, and a lifestyle modification program to prevent or delay the onset of type 2 diabetes. This study famously showed that lifestyle modification was more effective than metformin or placebo in reducing the incidence of diabetes.5
CER holds the promise of significantly improving the health of Americans through the ability to target treatments and other interventions to individual patients. As noted by the FCC, CER can allow for the delivery of the right treatment to the right patient at the right time2 even as the field continues to evolve. To quote Fineberg and Hiatt6 in describing technology assessment in 1979, we cannot expect CER to lead to perfect decisions, but we can expect even imperfect methods to facilitate better informed decisions than would otherwise be possible.
CER has important implications for hospitalists in all roles and settings. As the field of hospital medicine has grown, hospitalists have increasingly assumed more responsibilities than just patient care. In academic and community hospitals, hospitalists take on leadership roles, particularly in quality improvement (QI) and patient safety, and educational roles in the training of housestaff, medical students, and physician extenders. The last several years have also seen a significant increase in hospitalists participating in research. The relevance of CER to each of these 4 major activities is described below and in the accompanying Table 1.
| Primary Role | Potential Implications of CER |
|---|---|
| |
| Research | New availability of funds for hospital‐based CER |
| Enhanced data infrastructure to conduct CER | |
| Opportunity to apply CER to issues unique to hospital medicine | |
| Opportunity to develop methodologic skills | |
| Clinical practice | End users of CER evidence |
| Responsibility for translation of CER into practice | |
| Targets of Federal and non‐Federal dissemination efforts | |
| Education and training | Development of a workforce to conduct hospital‐based CER |
| Responsibility for teaching physician and nonphysician trainees about CER concepts and review of CER literature | |
| Hospital leadership | Direct hospital‐wide efforts to implement emerging CER evidence into practice through a multidisciplinary approach |
| Education and empowerment of clinician and nonclinician staff to translate CER information into practice | |
Hospitalists and Research
Many comparative effectiveness questions about clinical care, processes of care, and quality of care within the inpatient setting are in need of answers. Hospitalist researchers have the opportunity to make a significant impact on care by pursuing answers to questions that are unique to the field of hospital medicine. With the new availability of funds for CER, now is the time to address many of these questions head‐on. For example, there is a lack of evidence about best practices for a large number of inpatient acute conditions. What is the best strategy to manage acute hospital delirium in an elderly patient? What is the best approach to treating acute pain in an elderly woman on multiple medications? Overwhelmingly the patients that hospitalists care for are elderly and/or have multiple chronic conditions, including children with special health care needs. Many are from racial or ethnic minority backgrounds. These subgroups of patients have been historically under‐represented in clinical trials, yet represent exactly the priority populations that the Federal CER effort targets. The field of hospital medicine can be transformed with a substantial investment in research to address common inpatient clinical conditions in real world settings focused on the kinds of patients hospitalists actually care for.
One of the most vexing and frustrating care delivery issues for hospitalists, clinicians and researchers alike, is the discharge process. This problem received increased attention after a recent article highlighted the high rate of readmissions in the Medicare population.7 Research on the discharge process has grown substantially in recent years, and has become an area of intense focus and attention for hospitalists, nurses, researchers, hospital administrators and policymakers. Without question, hospitalists are uniquely poised to conduct research on this critically important topic, and CER is an ideal vehicle for moving this field forward. In collaboration with nurses, primary care physicians, pharmacists, case managers and others, hospitalists should take advantage of the Federal investment in studying care delivery systems interventions, and develop innovative methods and strategies for studying and improving this crucial transition in care. CER is also applicable to other care transitions, including the admission process, transitions within the hospital, and discharge to nursing facilities. Other examples of comparative effectiveness topics that hospitalist researchers are particularly suited for include comparing methods for implementing inpatient treatment protocols or clinical pathways, comparison of health information technology (IT) systems to reduce medical error, and QI approaches.
What are the methodologies that hospitalists should use to conduct CER? While randomized pragmatic real world trials are appealing, this method may not always be practical. Other methodologies are available for rigorous use, including cohort studies, comparative QI interventions, clustered and factorial design, systematic reviews, and analysis of registries, administrative claims, or other databases. Databases currently available for analysis on priority populations and subgroups are limited, and include the VA and Medicare databases. To address this need, one of the primary Federal investments in CER is for the enhancement and expansion of data infrastructure. Data infrastructure tools that are likely to be available to hospitalist researchers for CER include expanded longitudinal administrative claims databases with linkages to electronic health records (EHRs), expanded patient registries with linkages to other forms of data, and distributed data networks that are populated by EHRs in provider and practice settings. Hospitalist researchers should take advantage of these resources as they become available, as they have tremendous potential to inform decision‐making for providers and patients alike.
Hospitalists and Clinical Practice
As with all providers, hospitalists will be end‐users of CER evidence, and will have the responsibility of translating new knowledge into practice. This process will not be easy. How are hospitalists to reliably access and incorporate new comparative effectiveness information into their daily practice? How should they deal with some of the potential unintended consequences of CER, such as information overload or conflicting evidence? While hospitalists have a professional responsibility to search for and apply CER findings, the future development of CER‐based practice guidelines will encourage evidence translation. The development of a common platform for the dissemination of CER relevant to hospitalists would significantly enhance the uptake of new evidence by practicing hospitalists and other hospital‐based providers such as physician assistants or nurse practitioners. Medical societies such as the Society of Hospital Medicine and the American Academy of Pediatrics should consider developing committees for CER and leading coordinated educational efforts specifically focused on CER results through publications and presentations at local, regional, and national meetings. In addition, other dissemination tools for CER will soon emerge and existing tools will be enhanced, such as the Effective Health Care Program and Eisenberg Center housed at the AHRQ. The coming years will see an expansion of these and other dissemination efforts to both providers and patients, and hospitalists must be vigilant about accessing these resources and integrating comparative effectiveness evidence into practice. As Federal dissemination efforts to consumers spread, patients will increasingly expect physicians to discuss comparative effectiveness evidence in describing options for their individual health needs. Finally, a key lever for translating CER into practice will be payment models that place accountability for performance on physicians and hospitals, with a significant proportion of payment based on the delivery of high quality, efficient care.
Education and Training
Investment in the training and development of a skilled workforce to conduct CER is an important priority. Hospitalist researchers should take advantage of education and training programs to support the development of methodologies and skills for conducting CER that will become available. These programs will enable hospitalists to learn such skills as the use of the newly enhanced data infrastructure discussed above. The national investment in human and scientific capital for CER can promote the training of a corps of hospitalist researchers focused on this research which, in turn, could support the growth of the academic hospitalist field. Hospitalists who have responsibilities in medical education and residency training programs should take the lead in teaching CER concepts that are relevant to inpatient care. They will need to train the next generation of medical students and residents to read and understand comparative effectiveness literature and its application in clinical practice. Hospitalist educators are also best positioned to teach medical trainees comparative effectiveness evidence about inpatient QI methods and care processes.
Hospital Leadership
As front‐line providers and team leaders, hospitalists are well placed to direct the efforts within their hospitals to implement new CER evidence. For example, suppose new comparative effectiveness evidence about best practices for the discharge process for community‐dwelling older adults with multiple chronic conditions were to emerge. Hospitalists could lead efforts within their hospital to establish a multidisciplinary team to address this development, create standard protocols for implementing the new discharge process that align with their hospital's unique systems and organizational structure, advocate for necessary resources for the team to accomplish the goal of safely discharging these patients, ensure a method to track outcomes such as readmissions once the new discharge process is implemented, and provide data feedback to the team, hospital staff, and administrative leadership of the hospital. All of these activities should include a variety of disciplines working together, but as physician leaders, hospitalists can take the initiative to spearhead these endeavors. The inpatient setting is one that requires teamwork and coordination, and as team leaders, hospitalists can strongly influence the spread and adoption of CER results. Similarly, hospitalists are in a position to affect this dissemination and translation process by actively educating and empowering other clinicians and hospital staff within their local environment. Finally, as hospitalists increasingly take on leadership roles in QI departments and as chief medical officers within both community and university‐affiliated hospitals8, they are in a unique position to lead efforts to implement CER‐based QI activities. These may range from the implementation of IT functions to reduce medical error to strategies to reduce hospital‐acquired infections or falls.
Conclusion
As a result of the stimulus funds directed towards CER, the coming years will see a vast increase in the generation of comparative effectiveness evidence and the application of that evidence into practice.9 The national CER endeavor is particularly germane to the field of hospital medicine, as uncertainty about best practices is common, and the patients hospitalists serve represent priority populations for CER investments. Hospitalists can play a central role in both generating CER and implementing its findings in settings in which patients are highly vulnerable, and existing information is insufficient. In addition to clinical questions, hospitalist researchers are particularly suited to answering important questions about quality of care and inpatient processes such as transitions of care and care coordination. Having evidence on the best practices for care transitions or strategies to reduce medical error, for example, could have a significant impact on patient outcomes, quality of life, and cost of care. However, none of this new evidence will be of any value if it is not used by front‐line providers.10 Practicing hospitalists should lead efforts within their hospital to disseminate new CER findings to their hospitalist and non‐hospitalist colleagues, and to leverage their position as hospital and team leaders to implement inpatient‐based CER findings. All of these combined efforts have the potential to significantly move the field of hospital medicine forward, with the end result being improved health and better outcomes for patients.
The topic of comparative effectiveness research (CER) has recently gained prominence within the context of the national focus on health reform. This article provides a brief overview and history of CER, and discusses the implications of CER for hospitalists in each of four major career roles: research, clinical practice, education and training, and hospital leadership. Both medical journals and lay media have produced a flurry of articles recently on a variety of health reform subjects. One topic that has achieved prominence within this growing body of literature is comparative effectiveness research (CER). For many hospitalists, this particular brand of research may be unfamiliar. As discussions about CER priorities, the controversy surrounding CER, and even the definition of CER gain visibility, hospitalists may be left wondering, What exactly is CER and what does it mean for me?
Until recently no common definition for CER existed, and the very concept was identified only in relatively narrow policy and research circles. However, CER is not a new idea. Its ancestor is the notion of medical technology assessment (MTA), which garnered enthusiasm and support in the 1970s. In 1978, Congress established the National Center for Health Technology Assessment (which, over time, evolved into the Agency for Healthcare Research and Quality [AHRQ]), whose charge was to coordinate efforts within the government to assess the safety, efficacy, effectiveness, and cost‐effectiveness of medical technologies. The recognition of a need for technology assessment at that time is mirrored by the widespread interest in CER seen today. Part of the reason that MTA did not take hold is that then, as now, this type of evaluation is challenging and time consuming, requiring large, well‐designed effectiveness studies. These studies require rigorous methods, typically long‐term follow‐up, and acceptance via editors and the medical literature that effectiveness is as important as efficacy demonstrated in a randomized trial. With the spread of antiregulatory sentiment and the lack of an economic imperative to reduce costs, the national focus on technology assessment waned. The current economic crisis has refocused the government and private sector on the soaring cost of health care and the need to improve quality, and the stimulus package passed in February of 2009 placed CER once again in the forefront. The American Recovery and Reinvestment Act (ARRA) of 2009 allocated $1.1 billion for CER.1 On June 30, 2009, 2 reports delineating the strategy and priorities for CER were released. The report from the ARRA‐mandated Federal Coordinating Council (FCC) for CER includes a broad definition of CER and outlines a high‐level strategic framework for priorities and investments in CER.2 Simultaneously, the report from the Institute of Medicine (IOM) lists 100 priority research topics, and gives 10 general recommendations for the CER enterprise going forward.3
So what is CER and why is it important? How is it different from standard research that hospitalists use every day to inform their clinical decision‐making? Unfortunately, patients and providers confront medical decisions daily that are not evidence based. All too frequently it is unclear what therapeutic option works best for which patient under which circumstances. For example, what is the best inpatient diabetes management strategy for an African American woman with multiple medical problems? What is the best discharge process for an elderly man with heart disease in order to prevent readmission? CER seeks to fill the gaps in evidence needed by patients and clinicians in order to make appropriate medical decisions. It differs from standard efficacy research in that it compares interventions or management strategies in real world settings, allows identification of effectiveness in patient subgroups, and is more patient‐centered, focusing on the decisions confronting patients and their physicians. The following definition of CER was developed by the FCC for CER:
CER is the conduct and synthesis of research comparing the benefits and harms of different interventions and strategies to prevent, diagnose, treat and monitor health conditions in real world settings. The purpose of this research is to improve health outcomes by developing and disseminating evidence‐based information to patients, clinicians, and other decision‐makers, responding to their expressed needs about which interventions are most effective for which patients under specific circumstances.
-
To provide this information, CER must assess a comprehensive array of health‐related outcomes for diverse patient populations and sub‐groups.
-
Defined interventions compared may include medications, procedures, medical and assistive devices and technologies, diagnostic testing, behavioral change and delivery system strategies.
-
This research necessitates the development, expansion and use of a variety of data sources and methods to assess comparative effectiveness and actively disseminate the results.
While CER is an evolving field requiring continued methodological development (such as enhancement of methods for practical, or pragmatic trials and complex analyses of large, linked databases), examples of rigorous comparative studies do exist. The Veterans Administration (VA) COURAGE trial compared optimal medical therapy (OMT) with or without percutaneous coronary intervention (PCI) for patients with stable coronary disease, finding that PCI did not reduce the risk of death or cardiovascular events compared to OMT alone.4 Another example is the Diabetes Prevention Program which compared placebo, metformin, and a lifestyle modification program to prevent or delay the onset of type 2 diabetes. This study famously showed that lifestyle modification was more effective than metformin or placebo in reducing the incidence of diabetes.5
CER holds the promise of significantly improving the health of Americans through the ability to target treatments and other interventions to individual patients. As noted by the FCC, CER can allow for the delivery of the right treatment to the right patient at the right time2 even as the field continues to evolve. To quote Fineberg and Hiatt6 in describing technology assessment in 1979, we cannot expect CER to lead to perfect decisions, but we can expect even imperfect methods to facilitate better informed decisions than would otherwise be possible.
CER has important implications for hospitalists in all roles and settings. As the field of hospital medicine has grown, hospitalists have increasingly assumed more responsibilities than just patient care. In academic and community hospitals, hospitalists take on leadership roles, particularly in quality improvement (QI) and patient safety, and educational roles in the training of housestaff, medical students, and physician extenders. The last several years have also seen a significant increase in hospitalists participating in research. The relevance of CER to each of these 4 major activities is described below and in the accompanying Table 1.
| Primary Role | Potential Implications of CER |
|---|---|
| |
| Research | New availability of funds for hospital‐based CER |
| Enhanced data infrastructure to conduct CER | |
| Opportunity to apply CER to issues unique to hospital medicine | |
| Opportunity to develop methodologic skills | |
| Clinical practice | End users of CER evidence |
| Responsibility for translation of CER into practice | |
| Targets of Federal and non‐Federal dissemination efforts | |
| Education and training | Development of a workforce to conduct hospital‐based CER |
| Responsibility for teaching physician and nonphysician trainees about CER concepts and review of CER literature | |
| Hospital leadership | Direct hospital‐wide efforts to implement emerging CER evidence into practice through a multidisciplinary approach |
| Education and empowerment of clinician and nonclinician staff to translate CER information into practice | |
Hospitalists and Research
Many comparative effectiveness questions about clinical care, processes of care, and quality of care within the inpatient setting are in need of answers. Hospitalist researchers have the opportunity to make a significant impact on care by pursuing answers to questions that are unique to the field of hospital medicine. With the new availability of funds for CER, now is the time to address many of these questions head‐on. For example, there is a lack of evidence about best practices for a large number of inpatient acute conditions. What is the best strategy to manage acute hospital delirium in an elderly patient? What is the best approach to treating acute pain in an elderly woman on multiple medications? Overwhelmingly the patients that hospitalists care for are elderly and/or have multiple chronic conditions, including children with special health care needs. Many are from racial or ethnic minority backgrounds. These subgroups of patients have been historically under‐represented in clinical trials, yet represent exactly the priority populations that the Federal CER effort targets. The field of hospital medicine can be transformed with a substantial investment in research to address common inpatient clinical conditions in real world settings focused on the kinds of patients hospitalists actually care for.
One of the most vexing and frustrating care delivery issues for hospitalists, clinicians and researchers alike, is the discharge process. This problem received increased attention after a recent article highlighted the high rate of readmissions in the Medicare population.7 Research on the discharge process has grown substantially in recent years, and has become an area of intense focus and attention for hospitalists, nurses, researchers, hospital administrators and policymakers. Without question, hospitalists are uniquely poised to conduct research on this critically important topic, and CER is an ideal vehicle for moving this field forward. In collaboration with nurses, primary care physicians, pharmacists, case managers and others, hospitalists should take advantage of the Federal investment in studying care delivery systems interventions, and develop innovative methods and strategies for studying and improving this crucial transition in care. CER is also applicable to other care transitions, including the admission process, transitions within the hospital, and discharge to nursing facilities. Other examples of comparative effectiveness topics that hospitalist researchers are particularly suited for include comparing methods for implementing inpatient treatment protocols or clinical pathways, comparison of health information technology (IT) systems to reduce medical error, and QI approaches.
What are the methodologies that hospitalists should use to conduct CER? While randomized pragmatic real world trials are appealing, this method may not always be practical. Other methodologies are available for rigorous use, including cohort studies, comparative QI interventions, clustered and factorial design, systematic reviews, and analysis of registries, administrative claims, or other databases. Databases currently available for analysis on priority populations and subgroups are limited, and include the VA and Medicare databases. To address this need, one of the primary Federal investments in CER is for the enhancement and expansion of data infrastructure. Data infrastructure tools that are likely to be available to hospitalist researchers for CER include expanded longitudinal administrative claims databases with linkages to electronic health records (EHRs), expanded patient registries with linkages to other forms of data, and distributed data networks that are populated by EHRs in provider and practice settings. Hospitalist researchers should take advantage of these resources as they become available, as they have tremendous potential to inform decision‐making for providers and patients alike.
Hospitalists and Clinical Practice
As with all providers, hospitalists will be end‐users of CER evidence, and will have the responsibility of translating new knowledge into practice. This process will not be easy. How are hospitalists to reliably access and incorporate new comparative effectiveness information into their daily practice? How should they deal with some of the potential unintended consequences of CER, such as information overload or conflicting evidence? While hospitalists have a professional responsibility to search for and apply CER findings, the future development of CER‐based practice guidelines will encourage evidence translation. The development of a common platform for the dissemination of CER relevant to hospitalists would significantly enhance the uptake of new evidence by practicing hospitalists and other hospital‐based providers such as physician assistants or nurse practitioners. Medical societies such as the Society of Hospital Medicine and the American Academy of Pediatrics should consider developing committees for CER and leading coordinated educational efforts specifically focused on CER results through publications and presentations at local, regional, and national meetings. In addition, other dissemination tools for CER will soon emerge and existing tools will be enhanced, such as the Effective Health Care Program and Eisenberg Center housed at the AHRQ. The coming years will see an expansion of these and other dissemination efforts to both providers and patients, and hospitalists must be vigilant about accessing these resources and integrating comparative effectiveness evidence into practice. As Federal dissemination efforts to consumers spread, patients will increasingly expect physicians to discuss comparative effectiveness evidence in describing options for their individual health needs. Finally, a key lever for translating CER into practice will be payment models that place accountability for performance on physicians and hospitals, with a significant proportion of payment based on the delivery of high quality, efficient care.
Education and Training
Investment in the training and development of a skilled workforce to conduct CER is an important priority. Hospitalist researchers should take advantage of education and training programs to support the development of methodologies and skills for conducting CER that will become available. These programs will enable hospitalists to learn such skills as the use of the newly enhanced data infrastructure discussed above. The national investment in human and scientific capital for CER can promote the training of a corps of hospitalist researchers focused on this research which, in turn, could support the growth of the academic hospitalist field. Hospitalists who have responsibilities in medical education and residency training programs should take the lead in teaching CER concepts that are relevant to inpatient care. They will need to train the next generation of medical students and residents to read and understand comparative effectiveness literature and its application in clinical practice. Hospitalist educators are also best positioned to teach medical trainees comparative effectiveness evidence about inpatient QI methods and care processes.
Hospital Leadership
As front‐line providers and team leaders, hospitalists are well placed to direct the efforts within their hospitals to implement new CER evidence. For example, suppose new comparative effectiveness evidence about best practices for the discharge process for community‐dwelling older adults with multiple chronic conditions were to emerge. Hospitalists could lead efforts within their hospital to establish a multidisciplinary team to address this development, create standard protocols for implementing the new discharge process that align with their hospital's unique systems and organizational structure, advocate for necessary resources for the team to accomplish the goal of safely discharging these patients, ensure a method to track outcomes such as readmissions once the new discharge process is implemented, and provide data feedback to the team, hospital staff, and administrative leadership of the hospital. All of these activities should include a variety of disciplines working together, but as physician leaders, hospitalists can take the initiative to spearhead these endeavors. The inpatient setting is one that requires teamwork and coordination, and as team leaders, hospitalists can strongly influence the spread and adoption of CER results. Similarly, hospitalists are in a position to affect this dissemination and translation process by actively educating and empowering other clinicians and hospital staff within their local environment. Finally, as hospitalists increasingly take on leadership roles in QI departments and as chief medical officers within both community and university‐affiliated hospitals8, they are in a unique position to lead efforts to implement CER‐based QI activities. These may range from the implementation of IT functions to reduce medical error to strategies to reduce hospital‐acquired infections or falls.
Conclusion
As a result of the stimulus funds directed towards CER, the coming years will see a vast increase in the generation of comparative effectiveness evidence and the application of that evidence into practice.9 The national CER endeavor is particularly germane to the field of hospital medicine, as uncertainty about best practices is common, and the patients hospitalists serve represent priority populations for CER investments. Hospitalists can play a central role in both generating CER and implementing its findings in settings in which patients are highly vulnerable, and existing information is insufficient. In addition to clinical questions, hospitalist researchers are particularly suited to answering important questions about quality of care and inpatient processes such as transitions of care and care coordination. Having evidence on the best practices for care transitions or strategies to reduce medical error, for example, could have a significant impact on patient outcomes, quality of life, and cost of care. However, none of this new evidence will be of any value if it is not used by front‐line providers.10 Practicing hospitalists should lead efforts within their hospital to disseminate new CER findings to their hospitalist and non‐hospitalist colleagues, and to leverage their position as hospital and team leaders to implement inpatient‐based CER findings. All of these combined efforts have the potential to significantly move the field of hospital medicine forward, with the end result being improved health and better outcomes for patients.
- American Recovery and Reinvestment Act. Available at: http://frwebgate.access.gpo.gov/cgi‐bin/getdoc.cgi?dbname=111_cong_bills356:1503–1516.
- ,,, et al.Reduction in the Incidence of Type 2 diabetes with lifestyle intervention or metformin.N Engl J Med.2002;346:393–403.
- ,,Evaluation of medical practices: the case for technology assessment.N Engl J Med.1979;301:1086–1091.
- ,,,Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360:1418–1428.
- 2005–2006 Society of Hospital Medicine Survey. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2361:328–330.
- ,,Transformation of health care at the front line.JAMA.2009;301:763–765.
- American Recovery and Reinvestment Act. Available at: http://frwebgate.access.gpo.gov/cgi‐bin/getdoc.cgi?dbname=111_cong_bills356:1503–1516.
- ,,, et al.Reduction in the Incidence of Type 2 diabetes with lifestyle intervention or metformin.N Engl J Med.2002;346:393–403.
- ,,Evaluation of medical practices: the case for technology assessment.N Engl J Med.1979;301:1086–1091.
- ,,,Rehospitalizations among patients in the Medicare Fee‐for‐Service Program.N Engl J Med.2009;360:1418–1428.
- 2005–2006 Society of Hospital Medicine Survey. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2361:328–330.
- ,,Transformation of health care at the front line.JAMA.2009;301:763–765.
Plummer‐Vinson (Patterson‐Kelly) Syndrome
A 41‐year‐old woman with menorrhagia presented with dysphagia and fatigue. An examination revealed koilonychia (Figure 1), cheilosis, atrophic glossitis, and conjunctival pallor. The hemoglobin level was 4.3 g/dL, the mean corpuscular volume was 48.3, the iron level was 6 g/dL, and the ferritin level was undetectable (1 ng/mL). A barium esophagram demonstrated probable esophageal webs (Figure 2). Esophageal webs were confirmed by upper endoscopy (Figure 3) and successfully dilated with a balloon dilator sequentially at 8, 9, and 10 mm. She was transfused, treated for menorrhagia, and given ferrous sulfate and ascorbic acid (to improve iron absorption).
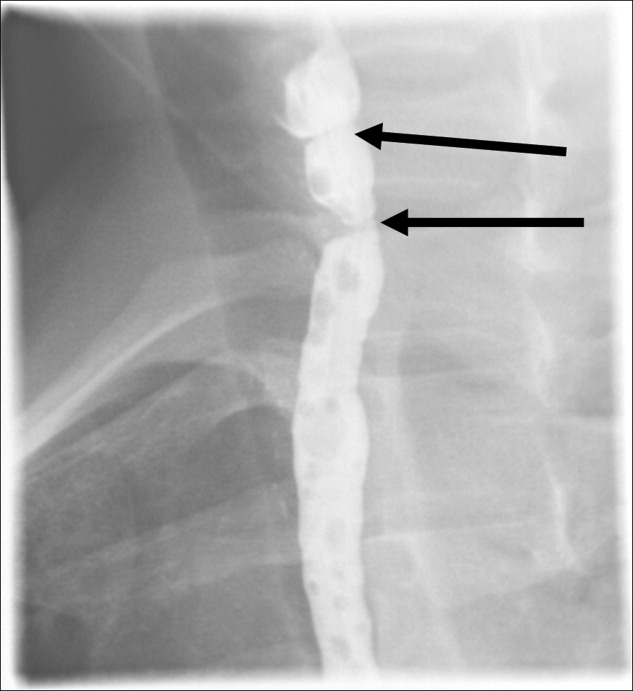
Koilonychia (or spoon nails) is derived from the Greek word for hollow nails. Spoon nails are associated with iron deficiency, thyroid dysfunction, trauma, chronic solvent exposure, and nail‐patella syndrome, but they can be normal in infants.1 Patterson and Kelly independently described the triad of iron‐deficiency anemia, dysphagia, and upper esophageal webs in 1919 following Plummer's (1912) and Vinson's (1919) similar but less comprehensive descriptions of hysterical dysphagia.2 Plummer‐Vinson syndrome is rare, but precise prevalence data are unavailable.2 Whether iron deficiency truly causes esophageal webs is debated, but iron deficiency is thought to weaken esophageal musculature and cause epithelial cell atrophy.2 Autoimmunity and genetic predisposition are other putative causes. Dilation of esophageal webs is usually curative, although their association with an increased risk of upper alimentary cancers may justify surveillance endoscopy.3
- ,.Nails: Diagnosis, Therapy, Surgery.3rd ed.Philadelphia, PA:Elsevier Saunders;2005.
- .Plummer‐Vinson syndrome.Orphanet J Rare Dis.2006;1:36–39.
- .Squamous cell cancer of the oesophagus.Best Pract Res Clin Gastroenterol.2001;15(2):249–265.
A 41‐year‐old woman with menorrhagia presented with dysphagia and fatigue. An examination revealed koilonychia (Figure 1), cheilosis, atrophic glossitis, and conjunctival pallor. The hemoglobin level was 4.3 g/dL, the mean corpuscular volume was 48.3, the iron level was 6 g/dL, and the ferritin level was undetectable (1 ng/mL). A barium esophagram demonstrated probable esophageal webs (Figure 2). Esophageal webs were confirmed by upper endoscopy (Figure 3) and successfully dilated with a balloon dilator sequentially at 8, 9, and 10 mm. She was transfused, treated for menorrhagia, and given ferrous sulfate and ascorbic acid (to improve iron absorption).



Koilonychia (or spoon nails) is derived from the Greek word for hollow nails. Spoon nails are associated with iron deficiency, thyroid dysfunction, trauma, chronic solvent exposure, and nail‐patella syndrome, but they can be normal in infants.1 Patterson and Kelly independently described the triad of iron‐deficiency anemia, dysphagia, and upper esophageal webs in 1919 following Plummer's (1912) and Vinson's (1919) similar but less comprehensive descriptions of hysterical dysphagia.2 Plummer‐Vinson syndrome is rare, but precise prevalence data are unavailable.2 Whether iron deficiency truly causes esophageal webs is debated, but iron deficiency is thought to weaken esophageal musculature and cause epithelial cell atrophy.2 Autoimmunity and genetic predisposition are other putative causes. Dilation of esophageal webs is usually curative, although their association with an increased risk of upper alimentary cancers may justify surveillance endoscopy.3
A 41‐year‐old woman with menorrhagia presented with dysphagia and fatigue. An examination revealed koilonychia (Figure 1), cheilosis, atrophic glossitis, and conjunctival pallor. The hemoglobin level was 4.3 g/dL, the mean corpuscular volume was 48.3, the iron level was 6 g/dL, and the ferritin level was undetectable (1 ng/mL). A barium esophagram demonstrated probable esophageal webs (Figure 2). Esophageal webs were confirmed by upper endoscopy (Figure 3) and successfully dilated with a balloon dilator sequentially at 8, 9, and 10 mm. She was transfused, treated for menorrhagia, and given ferrous sulfate and ascorbic acid (to improve iron absorption).



Koilonychia (or spoon nails) is derived from the Greek word for hollow nails. Spoon nails are associated with iron deficiency, thyroid dysfunction, trauma, chronic solvent exposure, and nail‐patella syndrome, but they can be normal in infants.1 Patterson and Kelly independently described the triad of iron‐deficiency anemia, dysphagia, and upper esophageal webs in 1919 following Plummer's (1912) and Vinson's (1919) similar but less comprehensive descriptions of hysterical dysphagia.2 Plummer‐Vinson syndrome is rare, but precise prevalence data are unavailable.2 Whether iron deficiency truly causes esophageal webs is debated, but iron deficiency is thought to weaken esophageal musculature and cause epithelial cell atrophy.2 Autoimmunity and genetic predisposition are other putative causes. Dilation of esophageal webs is usually curative, although their association with an increased risk of upper alimentary cancers may justify surveillance endoscopy.3
- ,.Nails: Diagnosis, Therapy, Surgery.3rd ed.Philadelphia, PA:Elsevier Saunders;2005.
- .Plummer‐Vinson syndrome.Orphanet J Rare Dis.2006;1:36–39.
- .Squamous cell cancer of the oesophagus.Best Pract Res Clin Gastroenterol.2001;15(2):249–265.
- ,.Nails: Diagnosis, Therapy, Surgery.3rd ed.Philadelphia, PA:Elsevier Saunders;2005.
- .Plummer‐Vinson syndrome.Orphanet J Rare Dis.2006;1:36–39.
- .Squamous cell cancer of the oesophagus.Best Pract Res Clin Gastroenterol.2001;15(2):249–265.