User login
Should patients with mild asthma use inhaled steroids?
Yes. A number of large randomized controlled trials have shown inhaled corticosteroids to be beneficial in low doses for patients who have mild persistent asthma, and therefore these drugs are strongly recommended in this situation.1
Asthma care providers should, however, consider this “yes” in the context of asthma severity, the goals of therapy, and the benefits and risks associated with inhaled corticosteroids.
CLASSIFICATION OF ASTHMA SEVERITY
Although the studies of asthma prevalence had methodologic limitations and therefore the true prevalence of mild persistent asthma cannot be determined, it is common. Fuhlbrigge et al2 reported that most asthma patients have some form of persistent asthma. In contrast, Dusser et al3 reviewed available studies and concluded that most patients with asthma have either intermittent or mild persistent asthma.
GOALS: REDUCE IMPAIRMENT AND RISK
The goals of asthma management are to:
Reduce impairment by controlling symptoms so that normal activity levels can be maintained, by minimizing the need for short-acting bronchodilator use, and by maintaining normal pulmonary function; and to
Reduce risk by preventing progressive loss of lung function and recurrent exacerbations, and by optimizing pharmacotherapy while minimizing potential adverse effects.1
EVIDENCE OF BENEFIT
The OPTIMA trial4 (Low Dose Inhaled Budesonide and Formoterol in Mild Persistent Asthma) was a double-blind, randomized trial carried out in 198 centers in 17 countries. Compared with those randomized to receive placebo, patients who were randomized to receive an inhaled corticosteroid, ie, budesonide (Pulmicort) 100 μg twice daily, had 60% fewer severe exacerbations (relative risk [RR] 0.4, 95% confidence interval [CI] 0.27–0.59) and 48% fewer days when their asthma was poorly controlled (RR 0.52, 95% CI 0.4–0.67). Adding a long-acting beta-agonist did not change this outcome.
The START study5 (Inhaled Steroid Treatment as Regular Therapy in Early Asthma) showed that, compared with placebo, starting inhaled budesonide within the first 2 years of asthma symptoms in patients with mild persistent asthma was associated with better asthma control and less need for additional asthma medication.
The IMPACT study6 (Improving Asthma Control Trial) showed that inhaled steroids need to be taken daily, on a regular schedule, rather than intermittently as needed. Patients received either inhaled budesonide as needed, budesonide 200 μg twice daily every day, or zafirlukast (Accolate) 20 mg twice daily. Daily budesonide therapy resulted in better asthma control, less bronchial hyperresponsiveness, and less airway inflammation compared with intermittent use, zafirlukast therapy, or placebo. Daily zafirlukast and intermittent steroid treatment produced similar results for all outcomes measured.
Despite this strong evidence supporting regular use of inhaled corticosteroids in patients with mild persistent asthma, many patients choose to take them intermittently.
Suissa et al7 found, in a large observational cohort study, that fewer patients died of asthma if they were receiving low-dose inhaled corticosteroids than if they were not. The rate of death due to asthma was lower in patients who had used more inhaled corticosteroids over the previous year, and the death rate was higher in those who had discontinued inhaled corticosteroids in the previous 3 months than in those who continued using them.
STEROIDS DO NOT SLOW THE LOSS OF LUNG FUNCTION
Compared with people without asthma, asthma patients have substantially lower values of forced expiratory volume in the first second of expiration (FEV1). They also have a faster rate of functional decline: the average decrease in FEV1 in asthma patients is 38 mL per year, compared with 22 mL per year in nonasthmatic people.9
Although inhaled corticosteroids have been shown to increase lung function in asthma patients in the short term, there is little convincing evidence to suggest that they affect the rate of decline in the long term.10 In fact, airway inflammation and bronchial hyperresponsiveness return to baseline within 2 weeks after inhaled corticosteroids are discontinued.10
DO INHALED CORTICOSTEROIDS STUNT CHILDREN’S GROWTH?
The safety of long-term low-dose inhaled corticosteroids is well established in adults. However, two large randomized controlled trials found that children treated with low-dose inhaled steroids (budesonide 200–400 μg per day) grew 1 to 1.5 cm less over 3 to 5 years of treatment than children receiving placebo.11 However, this effect was primarily evident within the first year of therapy, and growth velocity was similar to that with placebo at the end of the treatment period (4 to 6 years).12
Agertoft and Pedersen13 found that taking inhaled corticosteroids long-term is unlikely to have an effect on final height. Children who took inhaled budesonide (up to an average daily dose of 500 μg) into adulthood ended up no shorter than those who did not.
Based on these and other data, inhaled corticosteroids are generally considered safe at recommended doses. However, the decision to prescribe them for long-term therapy should be based on the risks and benefits to the individual patient.1
ALTERNATIVE DRUGS FOR MILD PERSISTENT ASTHMA
Leukotriene-modifying drugs include the leukotriene receptor antagonists montelukast (Singulair) and zafirlukast and the 5-lipoxygenase inhibitor zileuton (Zyflo CR). These drugs have been associated with statistically significant improvement in FEV1 compared with placebo in patients with mild to moderate asthma, reductions in both blood and sputum eosinophils,14 and attenuation of bronchoconstriction with exercise.11
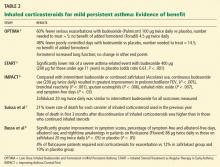
Large randomized trials comparing leukotriene modifier therapy with low-dose inhaled steroids in adults and children with mild persistent asthma have found that although outcomes improve with either therapy, the improvement is statistically superior with inhaled steroids for most asthma-control measures. 6,8 Low-dose inhaled steroid therapy in patients with mild persistent and moderate persistent asthma has been associated with superior clinical outcomes as well as greater improvement in pulmonary function than treatment with antileukotriene drugs (Table 2).8
Asthma is heterogeneous, and properly selected patients with mild persistent asthma may achieve good control with leukotrienemodifier monotherapy.15 Alternatives for patients with mild persistent asthma include the methylxanthine theophylline, but this drug is less desirable due to its narrow therapeutic index. 1 The inhaled cromones nedocromil (Tilade) and cromolyn (Intal) were other options in this patient population, but their short half-lives made them less practical, and US production has been discontinued.
THE BOTTOM LINE
Though leukotriene receptor antagonists can be effective, the daily use of inhaled corticosteroids results in higher asthma control test scores, more symptom-free days, greater pre-bronchodilator FEV1, and decreased percentage of sputum eosinophils6 in patients with mild persistent asthma, and the addition of a long-acting beta agonist does not provide additional benefit.4 Furthermore, daily use of inhaled corticosteroids in these patients has also been associated with a lower rate of asthma-related deaths and with less need for systemic corticosteroid therapy,7,8 even though inhaled corticosteroids have not yet been shown to alter the progressive loss of lung function.10
- National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma (EPR-3). www.nhlbi.nih.gov/guidelines/asthma/. Accessed March 26, 2010.
- Fuhlbrigge AL, Adams RJ, Guilbert TW, et al. The burden of asthma in the United States: level and distribution are dependent on interpretation of the National Asthma Education and Prevention Program. Am J Respir Crit Care Med 2002; 166:1044–1049.
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007; 62:591–604.
- O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164:1392–1397.
- Busse WW, Pedersen S, Pauwels RA, et al; START Investigators Group. The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol 2008; 121:1167–1174.
- Boushey HA, Sorkness CA, King TS, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med 2005; 352:1519–1528.
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343:332–356.
- Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001; 50:595–602.
- Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339:1194–1200.
- Fanta CH. Asthma. N Engl J Med 2009; 360:1002–1014.
- O’Byrne PM, Parameswaran K. Pharmacological management of mild or moderate persistent asthma. Lancet 2006; 368:794–803.
- The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000; 343:1054–1063.
- Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000; 343:1064–1069.
- Pizzichini E, Leff JA, Reiss TF, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J 1999; 14:12–18.
- Kraft M, Israel E, O’Connor GT. Clinical decisions. Treatment of mild persistent asthma. N Engl J Med 2007; 356:2096–2100.
Yes. A number of large randomized controlled trials have shown inhaled corticosteroids to be beneficial in low doses for patients who have mild persistent asthma, and therefore these drugs are strongly recommended in this situation.1
Asthma care providers should, however, consider this “yes” in the context of asthma severity, the goals of therapy, and the benefits and risks associated with inhaled corticosteroids.
CLASSIFICATION OF ASTHMA SEVERITY
Although the studies of asthma prevalence had methodologic limitations and therefore the true prevalence of mild persistent asthma cannot be determined, it is common. Fuhlbrigge et al2 reported that most asthma patients have some form of persistent asthma. In contrast, Dusser et al3 reviewed available studies and concluded that most patients with asthma have either intermittent or mild persistent asthma.
GOALS: REDUCE IMPAIRMENT AND RISK
The goals of asthma management are to:
Reduce impairment by controlling symptoms so that normal activity levels can be maintained, by minimizing the need for short-acting bronchodilator use, and by maintaining normal pulmonary function; and to
Reduce risk by preventing progressive loss of lung function and recurrent exacerbations, and by optimizing pharmacotherapy while minimizing potential adverse effects.1
EVIDENCE OF BENEFIT
The OPTIMA trial4 (Low Dose Inhaled Budesonide and Formoterol in Mild Persistent Asthma) was a double-blind, randomized trial carried out in 198 centers in 17 countries. Compared with those randomized to receive placebo, patients who were randomized to receive an inhaled corticosteroid, ie, budesonide (Pulmicort) 100 μg twice daily, had 60% fewer severe exacerbations (relative risk [RR] 0.4, 95% confidence interval [CI] 0.27–0.59) and 48% fewer days when their asthma was poorly controlled (RR 0.52, 95% CI 0.4–0.67). Adding a long-acting beta-agonist did not change this outcome.
The START study5 (Inhaled Steroid Treatment as Regular Therapy in Early Asthma) showed that, compared with placebo, starting inhaled budesonide within the first 2 years of asthma symptoms in patients with mild persistent asthma was associated with better asthma control and less need for additional asthma medication.
The IMPACT study6 (Improving Asthma Control Trial) showed that inhaled steroids need to be taken daily, on a regular schedule, rather than intermittently as needed. Patients received either inhaled budesonide as needed, budesonide 200 μg twice daily every day, or zafirlukast (Accolate) 20 mg twice daily. Daily budesonide therapy resulted in better asthma control, less bronchial hyperresponsiveness, and less airway inflammation compared with intermittent use, zafirlukast therapy, or placebo. Daily zafirlukast and intermittent steroid treatment produced similar results for all outcomes measured.
Despite this strong evidence supporting regular use of inhaled corticosteroids in patients with mild persistent asthma, many patients choose to take them intermittently.
Suissa et al7 found, in a large observational cohort study, that fewer patients died of asthma if they were receiving low-dose inhaled corticosteroids than if they were not. The rate of death due to asthma was lower in patients who had used more inhaled corticosteroids over the previous year, and the death rate was higher in those who had discontinued inhaled corticosteroids in the previous 3 months than in those who continued using them.
STEROIDS DO NOT SLOW THE LOSS OF LUNG FUNCTION
Compared with people without asthma, asthma patients have substantially lower values of forced expiratory volume in the first second of expiration (FEV1). They also have a faster rate of functional decline: the average decrease in FEV1 in asthma patients is 38 mL per year, compared with 22 mL per year in nonasthmatic people.9
Although inhaled corticosteroids have been shown to increase lung function in asthma patients in the short term, there is little convincing evidence to suggest that they affect the rate of decline in the long term.10 In fact, airway inflammation and bronchial hyperresponsiveness return to baseline within 2 weeks after inhaled corticosteroids are discontinued.10
DO INHALED CORTICOSTEROIDS STUNT CHILDREN’S GROWTH?
The safety of long-term low-dose inhaled corticosteroids is well established in adults. However, two large randomized controlled trials found that children treated with low-dose inhaled steroids (budesonide 200–400 μg per day) grew 1 to 1.5 cm less over 3 to 5 years of treatment than children receiving placebo.11 However, this effect was primarily evident within the first year of therapy, and growth velocity was similar to that with placebo at the end of the treatment period (4 to 6 years).12
Agertoft and Pedersen13 found that taking inhaled corticosteroids long-term is unlikely to have an effect on final height. Children who took inhaled budesonide (up to an average daily dose of 500 μg) into adulthood ended up no shorter than those who did not.
Based on these and other data, inhaled corticosteroids are generally considered safe at recommended doses. However, the decision to prescribe them for long-term therapy should be based on the risks and benefits to the individual patient.1
ALTERNATIVE DRUGS FOR MILD PERSISTENT ASTHMA
Leukotriene-modifying drugs include the leukotriene receptor antagonists montelukast (Singulair) and zafirlukast and the 5-lipoxygenase inhibitor zileuton (Zyflo CR). These drugs have been associated with statistically significant improvement in FEV1 compared with placebo in patients with mild to moderate asthma, reductions in both blood and sputum eosinophils,14 and attenuation of bronchoconstriction with exercise.11
Large randomized trials comparing leukotriene modifier therapy with low-dose inhaled steroids in adults and children with mild persistent asthma have found that although outcomes improve with either therapy, the improvement is statistically superior with inhaled steroids for most asthma-control measures. 6,8 Low-dose inhaled steroid therapy in patients with mild persistent and moderate persistent asthma has been associated with superior clinical outcomes as well as greater improvement in pulmonary function than treatment with antileukotriene drugs (Table 2).8
Asthma is heterogeneous, and properly selected patients with mild persistent asthma may achieve good control with leukotrienemodifier monotherapy.15 Alternatives for patients with mild persistent asthma include the methylxanthine theophylline, but this drug is less desirable due to its narrow therapeutic index. 1 The inhaled cromones nedocromil (Tilade) and cromolyn (Intal) were other options in this patient population, but their short half-lives made them less practical, and US production has been discontinued.
THE BOTTOM LINE
Though leukotriene receptor antagonists can be effective, the daily use of inhaled corticosteroids results in higher asthma control test scores, more symptom-free days, greater pre-bronchodilator FEV1, and decreased percentage of sputum eosinophils6 in patients with mild persistent asthma, and the addition of a long-acting beta agonist does not provide additional benefit.4 Furthermore, daily use of inhaled corticosteroids in these patients has also been associated with a lower rate of asthma-related deaths and with less need for systemic corticosteroid therapy,7,8 even though inhaled corticosteroids have not yet been shown to alter the progressive loss of lung function.10
Yes. A number of large randomized controlled trials have shown inhaled corticosteroids to be beneficial in low doses for patients who have mild persistent asthma, and therefore these drugs are strongly recommended in this situation.1
Asthma care providers should, however, consider this “yes” in the context of asthma severity, the goals of therapy, and the benefits and risks associated with inhaled corticosteroids.
CLASSIFICATION OF ASTHMA SEVERITY
Although the studies of asthma prevalence had methodologic limitations and therefore the true prevalence of mild persistent asthma cannot be determined, it is common. Fuhlbrigge et al2 reported that most asthma patients have some form of persistent asthma. In contrast, Dusser et al3 reviewed available studies and concluded that most patients with asthma have either intermittent or mild persistent asthma.
GOALS: REDUCE IMPAIRMENT AND RISK
The goals of asthma management are to:
Reduce impairment by controlling symptoms so that normal activity levels can be maintained, by minimizing the need for short-acting bronchodilator use, and by maintaining normal pulmonary function; and to
Reduce risk by preventing progressive loss of lung function and recurrent exacerbations, and by optimizing pharmacotherapy while minimizing potential adverse effects.1
EVIDENCE OF BENEFIT
The OPTIMA trial4 (Low Dose Inhaled Budesonide and Formoterol in Mild Persistent Asthma) was a double-blind, randomized trial carried out in 198 centers in 17 countries. Compared with those randomized to receive placebo, patients who were randomized to receive an inhaled corticosteroid, ie, budesonide (Pulmicort) 100 μg twice daily, had 60% fewer severe exacerbations (relative risk [RR] 0.4, 95% confidence interval [CI] 0.27–0.59) and 48% fewer days when their asthma was poorly controlled (RR 0.52, 95% CI 0.4–0.67). Adding a long-acting beta-agonist did not change this outcome.
The START study5 (Inhaled Steroid Treatment as Regular Therapy in Early Asthma) showed that, compared with placebo, starting inhaled budesonide within the first 2 years of asthma symptoms in patients with mild persistent asthma was associated with better asthma control and less need for additional asthma medication.
The IMPACT study6 (Improving Asthma Control Trial) showed that inhaled steroids need to be taken daily, on a regular schedule, rather than intermittently as needed. Patients received either inhaled budesonide as needed, budesonide 200 μg twice daily every day, or zafirlukast (Accolate) 20 mg twice daily. Daily budesonide therapy resulted in better asthma control, less bronchial hyperresponsiveness, and less airway inflammation compared with intermittent use, zafirlukast therapy, or placebo. Daily zafirlukast and intermittent steroid treatment produced similar results for all outcomes measured.
Despite this strong evidence supporting regular use of inhaled corticosteroids in patients with mild persistent asthma, many patients choose to take them intermittently.
Suissa et al7 found, in a large observational cohort study, that fewer patients died of asthma if they were receiving low-dose inhaled corticosteroids than if they were not. The rate of death due to asthma was lower in patients who had used more inhaled corticosteroids over the previous year, and the death rate was higher in those who had discontinued inhaled corticosteroids in the previous 3 months than in those who continued using them.
STEROIDS DO NOT SLOW THE LOSS OF LUNG FUNCTION
Compared with people without asthma, asthma patients have substantially lower values of forced expiratory volume in the first second of expiration (FEV1). They also have a faster rate of functional decline: the average decrease in FEV1 in asthma patients is 38 mL per year, compared with 22 mL per year in nonasthmatic people.9
Although inhaled corticosteroids have been shown to increase lung function in asthma patients in the short term, there is little convincing evidence to suggest that they affect the rate of decline in the long term.10 In fact, airway inflammation and bronchial hyperresponsiveness return to baseline within 2 weeks after inhaled corticosteroids are discontinued.10
DO INHALED CORTICOSTEROIDS STUNT CHILDREN’S GROWTH?
The safety of long-term low-dose inhaled corticosteroids is well established in adults. However, two large randomized controlled trials found that children treated with low-dose inhaled steroids (budesonide 200–400 μg per day) grew 1 to 1.5 cm less over 3 to 5 years of treatment than children receiving placebo.11 However, this effect was primarily evident within the first year of therapy, and growth velocity was similar to that with placebo at the end of the treatment period (4 to 6 years).12
Agertoft and Pedersen13 found that taking inhaled corticosteroids long-term is unlikely to have an effect on final height. Children who took inhaled budesonide (up to an average daily dose of 500 μg) into adulthood ended up no shorter than those who did not.
Based on these and other data, inhaled corticosteroids are generally considered safe at recommended doses. However, the decision to prescribe them for long-term therapy should be based on the risks and benefits to the individual patient.1
ALTERNATIVE DRUGS FOR MILD PERSISTENT ASTHMA
Leukotriene-modifying drugs include the leukotriene receptor antagonists montelukast (Singulair) and zafirlukast and the 5-lipoxygenase inhibitor zileuton (Zyflo CR). These drugs have been associated with statistically significant improvement in FEV1 compared with placebo in patients with mild to moderate asthma, reductions in both blood and sputum eosinophils,14 and attenuation of bronchoconstriction with exercise.11
Large randomized trials comparing leukotriene modifier therapy with low-dose inhaled steroids in adults and children with mild persistent asthma have found that although outcomes improve with either therapy, the improvement is statistically superior with inhaled steroids for most asthma-control measures. 6,8 Low-dose inhaled steroid therapy in patients with mild persistent and moderate persistent asthma has been associated with superior clinical outcomes as well as greater improvement in pulmonary function than treatment with antileukotriene drugs (Table 2).8
Asthma is heterogeneous, and properly selected patients with mild persistent asthma may achieve good control with leukotrienemodifier monotherapy.15 Alternatives for patients with mild persistent asthma include the methylxanthine theophylline, but this drug is less desirable due to its narrow therapeutic index. 1 The inhaled cromones nedocromil (Tilade) and cromolyn (Intal) were other options in this patient population, but their short half-lives made them less practical, and US production has been discontinued.
THE BOTTOM LINE
Though leukotriene receptor antagonists can be effective, the daily use of inhaled corticosteroids results in higher asthma control test scores, more symptom-free days, greater pre-bronchodilator FEV1, and decreased percentage of sputum eosinophils6 in patients with mild persistent asthma, and the addition of a long-acting beta agonist does not provide additional benefit.4 Furthermore, daily use of inhaled corticosteroids in these patients has also been associated with a lower rate of asthma-related deaths and with less need for systemic corticosteroid therapy,7,8 even though inhaled corticosteroids have not yet been shown to alter the progressive loss of lung function.10
- National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma (EPR-3). www.nhlbi.nih.gov/guidelines/asthma/. Accessed March 26, 2010.
- Fuhlbrigge AL, Adams RJ, Guilbert TW, et al. The burden of asthma in the United States: level and distribution are dependent on interpretation of the National Asthma Education and Prevention Program. Am J Respir Crit Care Med 2002; 166:1044–1049.
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007; 62:591–604.
- O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164:1392–1397.
- Busse WW, Pedersen S, Pauwels RA, et al; START Investigators Group. The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol 2008; 121:1167–1174.
- Boushey HA, Sorkness CA, King TS, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med 2005; 352:1519–1528.
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343:332–356.
- Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001; 50:595–602.
- Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339:1194–1200.
- Fanta CH. Asthma. N Engl J Med 2009; 360:1002–1014.
- O’Byrne PM, Parameswaran K. Pharmacological management of mild or moderate persistent asthma. Lancet 2006; 368:794–803.
- The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000; 343:1054–1063.
- Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000; 343:1064–1069.
- Pizzichini E, Leff JA, Reiss TF, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J 1999; 14:12–18.
- Kraft M, Israel E, O’Connor GT. Clinical decisions. Treatment of mild persistent asthma. N Engl J Med 2007; 356:2096–2100.
- National Heart, Lung, and Blood Institute. Guidelines for the Diagnosis and Management of Asthma (EPR-3). www.nhlbi.nih.gov/guidelines/asthma/. Accessed March 26, 2010.
- Fuhlbrigge AL, Adams RJ, Guilbert TW, et al. The burden of asthma in the United States: level and distribution are dependent on interpretation of the National Asthma Education and Prevention Program. Am J Respir Crit Care Med 2002; 166:1044–1049.
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007; 62:591–604.
- O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001; 164:1392–1397.
- Busse WW, Pedersen S, Pauwels RA, et al; START Investigators Group. The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol 2008; 121:1167–1174.
- Boushey HA, Sorkness CA, King TS, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med 2005; 352:1519–1528.
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med 2000; 343:332–356.
- Busse W, Wolfe J, Storms W, et al. Fluticasone propionate compared with zafirlukast in controlling persistent asthma: a randomized double-blind, placebo-controlled trial. J Fam Pract 2001; 50:595–602.
- Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339:1194–1200.
- Fanta CH. Asthma. N Engl J Med 2009; 360:1002–1014.
- O’Byrne PM, Parameswaran K. Pharmacological management of mild or moderate persistent asthma. Lancet 2006; 368:794–803.
- The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000; 343:1054–1063.
- Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med 2000; 343:1064–1069.
- Pizzichini E, Leff JA, Reiss TF, et al. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. Eur Respir J 1999; 14:12–18.
- Kraft M, Israel E, O’Connor GT. Clinical decisions. Treatment of mild persistent asthma. N Engl J Med 2007; 356:2096–2100.
Difficulty swallowing solid foods; food ‘getting stuck in the chest’
A 61-year-old woman presents to her primary care physician because for the last 4 weeks she has had difficulty swallowing solid food and a feeling of food “getting stuck in the chest.” She also reports having nausea, mild epigastric pain, and heartburn. She denies having fevers, chills, night sweats, weight loss, vomiting, diarrhea, hematochezia, or melena.
Medical history
For the past 20 years, she has had gastroesophageal reflux disease (GERD), intermittently treated with a proton pump inhibitor. She also has arthritis, hyperlipidemia, hypertension, and asthma, and she has undergone right hip replacement for a hip fracture. She has no known allergies.
She lives in the Midwest region of the United States and is on disability due to her arthritis. She is divorced and has three children.
She quit smoking 3 years ago after smoking half a pack per day for 30 years. She drinks socially; she has never used recreational drugs.
She recalls that an uncle had cancer, but she does not know the specific type.
Physical examination
The patient’s temperature is 96.7°F (35.9°C), heart rate 86 per minute, blood pressure 150/92 mm Hg, respiratory rate 16 per minute, and oxygen saturation 100% on room air.
Differential diagnosis
Although the differential diagnosis at this stage is broad, a few conditions that commonly present like this are:
- Esophageal cancer
- Esophageal stricture
- Esophageal webs
- Esophagitis (infectious, inflammatory)
- Peptic ulcer disease.
WHICH TEST SHOULD BE ORDERED?
1. Which test will you order now for this patient?
- Endoscopy (esophagogastroduodenoscopy)
- Serum Helicobacter pylori antibody testing
- Wireless pH monitoring
- Barium swallow
Endoscopy would be the best test to order. Esophageal cancer and esophageal stricture must be ruled out, in view of her long history of GERD, gastritis, and smoking and her alarming symptoms of difficulty swallowing and food sticking. In this situation, endoscopy is the first test recommended. In addition to its diagnostic value, it offers an opportunity to obtain tissue samples and to perform a therapeutic intervention, if necessary.1,2
H pyloriantibody testing is used in the “test-and-treat approach” for H pylori infection, an established management strategy for patients who have uninvestigated dyspepsia and who are younger than 55 years and have no “alarm features,” ie, red flags for cancer. The alarm features commonly described are anemia, early satiety, unexplained weight loss, bleeding, odynophagia, progressive dysphagia, unexplained vomiting, and a family history or prior history of gastrointestinal malignancy.3
Our patient’s symptoms raise the possibility of cancer, so that H pylori testing would not be the best test to order at this point.
Ambulatory wireless pH monitoring with a wireless pH capsule is useful for confirming GERD in those with persistent symptoms (whether typical or atypical) who do not have evidence of mucosal damage on initial endoscopy, particularly if a trial of acid suppression has failed.4–6
Barium swallow is an x-ray examination of the esophagus with contrast. It can show both the anatomy and the function of the esophagus, and it would be the initial diagnostic procedure of choice for patients with dysphagia who have no alarm symptoms.7 However, our patient does have alarm symptoms.
First highlight point
- Endoscopy is the first test in patients with dysphagia with alarm symptoms.
CASE CONTINUES: ENDOSCOPY
Multiple biopsies of the nodules show atypical lymphoid infiltrates with small cleaved lymphocytes that are mostly positive for CD5, CD20, and CD43 and negative for CD10 and CD23 by flow cytometry. In addition, a staining test for H pylori is positive.
Comment. Our patient has had GERD for the past 20 years, intermittently treated with a proton pump inhibitor. Acid suppressive therapy with a proton pump inhibitor is the standard of care of patients with erosive esophagitis. In standard doses, these drugs control symptoms in most cases and heal esophagitis in almost 90% of cases within 4 to 8 weeks.9 Proton pump inhibitors are also effective for maintaining healing of esophagitis and controlling symptoms in patients who respond to an acute course of therapy for a period of 6 to 12 months.10
WHAT IS THE DIAGNOSIS?
2. Which is the most likely diagnosis for our patient?
- Fundic gland polyps
- Gastric hyperplastic polyps
- Gastric adenomas
- Mucosa-associated lymphoid tissue (MALT) lymphoma
Fundic gland polyps are small (0.1–0.8 cm), hyperemic, sessile, flat, nodular lesions that have a smooth surface. They occur exclusively in the gastric corpus and are composed of normal gastric corpus-type epithelium arranged in a disorderly or microcystic configuration. 11 This pattern does not match our patient’s findings.
Gastric hyperplastic polyps are elongated, cystic, and distorted foveolar epithelium with marked regeneration. Other histologic findings are stromal inflammation, edema, and smooth muscle hyperplasia.12 This does not match our patient’s findings.
Adenomas can be flat or polypoid and range in size from a few millimeters to several centimeters. Endoscopically, adenomatous polyps have a velvety, lobulated appearance. Most are solitary (82% of cases), located in the antrum, and less than 2 cm in diameter.13 This does not match our patient’s findings.
MALT lymphoma, the correct answer, is characterized by small cleaved lymphocytes positive for CD4, CD20, and CD43. Although CD5 positivity is not characteristic, rare cases of MALT lymphoma may be CD5-positive and may be more aggressive.14
Other common features of MALT lymphoma are erosions, small nodules, thickening of gastric folds—generally suggesting a benign condition—or hyperemic or even normal gastric mucosa.15 Our patient’s complaint of food sticking in her chest and difficulty swallowing was most likely related to the erosive esophagitis found on endoscopy.
A TYPE OF NON-HODGKIN LYMPHOMA
Normal gastric mucosa contains no lymphoid tissue.16,17 Primary gastric lymphoma, of which MALT lymphoma is a subtype, accounts for around 5% of gastric malignancies, with an annual incidence rate of 0.5 per 100,000 people. 18–20 Although rare, it accounts for 60% to 70% of cases of non-Hodgkin lymphoma of the gastrointestinal tract and can involve the perigastric or abdominal lymph nodes or both.21–23 Although earlier studies suggested that its incidence was increasing, recent data indicate the incidence may be decreasing, thanks to active H pylori treatment.24–26
Two subtypes of primary gastric non-Hodgkin lymphoma commonly described are MALT lymphoma and diffuse large B-cell (DLBC) lymphoma. In the Revised European-American Lymphoma Classification, high-grade MALT lymphoma is comparable to DLBC lymphoma and may have transformed from low-grade MALT lymphoma.27,28 Another reported subtype, mantle cell lymphoma with MALT lymphoma features, should be considered in the differential diagnosis, although it is rare.29
MALT lymphoma is linked to H pylori
H pylori infection is a factor in the development of MALT lymphoma,30 as multiple lines of evidence show:
- H pylori infection has been reported in more than 90% of patients with MALT lymphoma.31–35
- H pylori antibodies have been found in stored serum drawn from patients who subsequently developed MALT lymphoma.35
- In response to H pylori antigens, T cells from MALT lymphoma proliferate and cause an increase in tumor immunoglobulin production.36
- In animals experimentally infected with H pylori, around one-third develop lymphoid follicles and lymphoepithelial lesions including B cells, which are similar to human MALT lymphoma.37
However, only a minority of patients with H pylori develop lymphoma, owing to a host immune response that is not well defined.
Second highlight point
- Gastric MALT lymphoma is associated with H pylori.
Associated genetic translocations
Three translocations, t(11;18), t(1;14), and t(14;18), are specifically associated with MALT lymphoma, and the genes involved have been characterized.
The t(11;18) translocation, seen in gastric and nongastric MALT lymphoma, is not seen in H pylori gastritis.38 This translocation is usually associated with extension of the disease outside the stomach (ie, to regional lymph nodes or distal sites).27 Most cases that do not respond to H pylori eradication involve the t(11;18) and t(1;14) translocations.28
Clinical presentation of gastric MALT lymphoma
The average age at presentation with gastric MALT lymphoma is 54 to 58 years.
The most common complaint is nonspecific abdominal pain in the epigastric region, sometimes accompanied by weight loss, nausea, vomiting, and, in a quarter of cases, acute or chronic bleeding.39–41 Weight loss is common, and its extent is associated with the location and the grade of the disease.
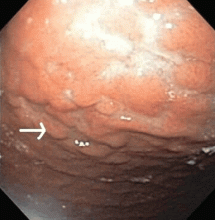
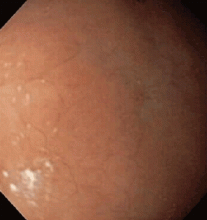
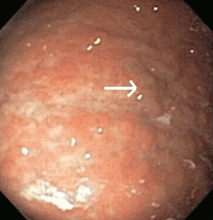
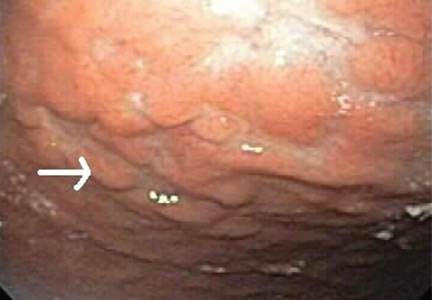
Most cases of MALT lymphoma are found serendipitously during endoscopy, on which the appearance of the lesions ranges from small ulcerations to polypoid masses with infiltrated, thickened folds involving predominantly the antrum or prepyloric region.15,41
MANAGING MALT LYMPHOMA
Our patient undergoes endoscopic ultrasonography, which reveals she has stage I disease, ie, it is limited to the stomach without involving the lymph nodes (stage II), adjacent organs (stage III), or distant organs (stage IV).
3. How will you treat this patient, given the present information?
- Chemotherapy
- Radiation therapy
- Surgery
- Antibiotics with a proton pump inhibitor
Antibiotics with a proton pump inhibitor would be best. According to the Maastricht III Consensus Report,42H pylori eradication is the treatment of first choice for H pylori infection in patients with stage I low-grade gastric MALT lymphoma. This therapy can induce complete histologic remission in 80% to 100% of patients with MALT lymphoma. 43 Several studies have shown regression44 or complete remission of low-grade gastric MALT lymphoma after eradication of H pylori with antibiotics, making it a reasonable initial treatment.45–49
Several regimens are used. The first choice in populations in which the prevalence of resistance to clarithromycin (Biaxin) is less than 15% to 20% is a proton pump inhibitor, clarithromycin, and either amoxicillin or metronidazole (Flagyl). (Metronidazole is preferable to amoxicillin if the prevalence of resistance to metronidazole is less than 40%.)
Sequential treatment—ie, 5 days of a proton pump inhibitor plus amoxicillin followed by 5 additional days of a proton pump inhibitor plus clarithromycin plus tinidazole (Tindamax)— may be better than a 7-day course of the combination of a proton pump inhibitor, amoxicillin, and clarithromycin.50,51
Treatment with a proton pump inhibitor, clarithromycin (500 mg twice a day), and either amoxicillin (1,000 mg twice a day) or metronidazole (400 or 500 mg twice a day) for 14 days is more effective than treatment for 7 days.52
H pylori reinfection in the general population is quite rare, with an estimated yearly rate as low as 2%.53 Recurrence of the infection is a risk factor for lymphoma relapse.17,54
Several predictors of the response of MALT lymphoma to eradication therapy have been recognized: H pylori positivity, stage I, lymphoma confined to the stomach; gastric wall invasion confined to mucosa and submucosa, and the absence of the t(11;18) translocation.
The time between H pylori eradication and complete remission of primary gastric lymphoma varies and can be longer than 12 months.55
Chemotherapy. In a single study,56 complete remission was achieved with oral cyclophosphamide (Cytoxan) in cases of antibiotic-refractory gastric MALT lymphoma. Comparable results were achieved after radiation therapy (see below); hence, oral monotherapy with cyclophosphamide might also be a suitable second-line therapy.57
The regimen of cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone (CHOP) has been recommended for patients with stage III and IV disease.41,58
Rituximab (Rituxan) has been proven effective in treating t(11;18)-positive MALT lymphoma.59
Radiation therapy. Two studies have shown a 100% complete response rate after radiation therapy with a median dose of 30 Gy.57,60 Tsang et al61 reported complete remission in up to 90% of patients receiving radiation therapy alone, with excellent 5-year progression-free and overall survival rates of 98% and 77%, respectively.
Although surgery, radiotherapy, and chemotherapy have been used in cases in which eradication therapy failed and in more advanced stages of MALT lymphoma, there is no consensus about their use, so therapy must be individualized.
Fourth highlight point
- Antibiotic treatment for eradication of H pylori infection is the recommended treatment only for stage I low-grade MALT lymphoma.
FOLOW-UP
4. How should you follow patients with MALT lymphoma?
- Endoscopy
- H pylori testing
- Computed tomography and magnetic resonance imaging
- No surveillance required after treatment
Endoscopy is the correct answer. As initial diagnostic biopsies do not exclude aggressive lymphoma, careful endoscopic follow-up is recommended. A recommended schedule is a breath test for H pylori every 2 months in conjunction with repeat endoscopy with biopsies every 3 to 6 months for the first 2 years, and then annually.62
Although H pylori may be eradicated within 1 month of drug therapy, lymphoma may take several months to disappear histologically. In patients with stage I disease with residual lymphoma after H pylori eradication therapy, a simple wait-and-watch strategy is a suitable alternative to oncologic therapy.63,64
Local relapse may occur after many years of complete remission; thus, patients should be followed closely long-term with endoscopy and possibly endoscopic ultrasonography. 47–49,63
Patients who fail to attain a complete remission within 12 months should undergo radiation therapy, with or without chemotherapy. The same therapy should be started as soon as possible in patients with progressive disease after antibiotic therapy. Patients negative for H pylori, patients with stage II disease, and patients with t(11;18) translocation should receive antibiotic treatment with endoscopic surveillance every 3 months.
Fifth highlight point
- Surveillance endoscopy is recommended for follow-up of MALT lymphoma.
CASE CONTINUES: HER CONDITION IMPROVES, THEN WORSENS
Computed tomography of the chest, abdomen, and pelvis reveals no evidence of additional sites of tumor. Positron emission tomography reveals increased uptake in the left tonsillar region, for which she has undergoes an ear, nose, and throat evaluation, and no pathology is found.
Due to recurrence of her marginal zone Bcell lymphoma of MALT type of the stomach, the patient is referred to an oncology service. She is treated with radiation, receiving 15 sessions of 30 Gy localized to the stomach. Three months after radiation therapy, she undergoes endoscopy again, which shows no evidence of the previously described nodules. Repeat biopsies are negative for H pylori and MALT lymphoma.
Annual surveillance endoscopy and computed tomography for the past 3 years have been negative for any tumor recurrence.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102:1808–1825.
- Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98:740–749.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Furlow B. Barium swallow. Radiol Technol 2004; 76:49–58.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45:172–180.
- Havelund T, Laursen LS, Skoubo-Kristensen E, et al. Omeprazole and ranitidine in treatment of reflux oesophagitis: double blind comparative trial. Br Med J (Clin Res Ed) 1988; 296:89–92.
- Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1392–1413.
- Odze RD, Marcial MA, Antonioli D. Gastric fundic gland polyps: a morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum Pathol 1996; 27:896–903.
- Snover DC. Benign epithelial polyps of the stomach. Pathol Annu 1985; 20:303–329.
- Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 2009; 6:331–341.
- Wenzel C, Dieckmann K, Fiebiger W, Mannhalter C, Chott A, Raderer M. CD5 expression in a lymphoma of the mucosa-associated lymphoid tissue (MALT)-type as a marker for early dissemination and aggressive clinical behaviour. Leuk Lymphoma 2001; 42:823–829.
- Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol 2003; 98:975–986.
- Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med 1999; 131:88–95.
- Stolte M, Bayerdörffer E, Morgner A, et al. Helicobacter and gastric MALT lymphoma. Gut 2002; 50(suppl 3):III19–III24.
- Ducreux M, Boutron MC, Piard F, Carli PM, Faivre J. A 15-year series of gastrointestinal non-Hodgkin’s lymphomas: a population-based study. Br J Cancer 1998; 77:511–514.
- Gurney KA, Cartwright RA, Gilman EA. Descriptive epidemiology of gastrointestinal non-Hodgkin’s lymphoma in a population-based registry. Br J Cancer 1999; 79:1929–1934.
- d’Amore F, Brincker H, Grønbaek K, et al. Non-Hodgkin’s lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol 1994; 12:1673–1684.
- Koch P, del Valle F, Berdel WE, et al; German Multicenter Study Group. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001; 19:3861–3873.
- Papaxoinis G, Papageorgiou S, Rontogianni D, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma 2006; 47:2140–2146.
- Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology 1992; 20:29–34.
- Wotherspoon AC. Choosing the right MALT. Gut 1996; 39:617–618.
- Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003; 97:2462–2473.
- Luminari S, Cesaretti M, Marcheselli L, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol 2009; epub ahead of print.
- Liu H, Ye H, Dogan A, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001; 98:1182–1187.
- Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001; 357:39–40.
- Shibata K, Shimamoto Y, Nakano S, Miyahara M, Nakano H, Yamaguchi M. Mantle cell lymphoma with the features of mucosa-associated lymphoid tissue (MALT) lymphoma in an HTLV-I-seropositive patient. Ann Hematol 1995; 70:47–51.
- Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol 2005; 23:6370–6378.
- de Jong D, Boot H, van Heerde P, Hart GA, Taal BG. Histological grading in gastric lymphoma: pretreatment criteria and clinical relevance. Gastroenterology 1997; 112:1466–1474.
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338:1175–1176.
- Eidt S, Stolte M, Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin’s lymphomas. J Clin Pathol 1994; 47:436–439.
- Doglioni C, Wotherspoon AC, Moschini A, de Boni M, Isaacson PG. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992; 339:834–835.
- Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330:1267–1271.
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 1993; 342:571–574.
- Lee A, O’Rourke J, Enno A. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations—mouse models of gastric lymphoma. Recent Results Cancer Res 2000; 156:42–51.
- Auer IA, Gascoyne RD, Connors JM, et al. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997; 8:979–985.
- Morgner A, Bayerdörffer E, Neubauer A, Stolte M. Malignant tumors of the stomach. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori. Gastroenterol Clin North Am 2000; 29:593–607.
- Ruskoné-Fourmestraux A, Aegerter P, Delmer A, Brousse N, Galian A, Rambaud JC. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d’Etude des Lymphomes Digestifs. Gastroenterology 1993; 105:1662–1671.
- Cogliatti SB, Schmid U, Schumacher U, et al. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology 1991; 101:1159–1170.
- Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56:772–781.
- Boot H, de Jong D. Gastric lymphoma: the revolution of the past decade. Scand J Gastroenterol Suppl 2002; 236:27–36.
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993; 342:575–577.
- Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995; 345:1591–1594.
- Roggero E, Zucca E, Pinotti G, et al. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med 1995; 122:767–769.
- Ruskoné-Fourmestraux A. Gastrointestinal lymphomas: the French experience of the Groupe d’Étude des Lymphomes Digestifs (GELD). Recent Results Cancer Res 2000; 156:99–103.
- Wündisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol 2005; 23:8018–8024.
- Wündisch T, Mösch C, Neubauer A, Stolte M. Helicobacter pylori eradication in gastric mucosa-associated lymphoid tissue lymphoma: results of a 196-patient series. Leuk Lymphoma 2006; 47:2110–2114.
- De Francesco V, Zullo A, Margiotta M, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther 2004; 19:407–414.
- Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003; 17:719–726.
- Paoluzi P, Iacopini F, Crispino P, et al. 2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter 2006; 11:562–568.
- Gisbert JP, Olivares D, Jimenez I, Pajares JM. Long-term follow-up of 13C-urea breath test results after Helicobacter pylori eradication: frequency and significance of borderline delta13CO2 values. Aliment Pharmacol Ther 2006; 23:275–280.
- Bayerdörffer E, Morgner A. Gastric marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type: management of the disease. Dig Liver Dis 2000; 32:192–194.
- Savio A, Zamboni G, Capelli P, et al. Relapse of low-grade gastric MALT lymphoma after Helicobacter pylori eradication: true relapse or persistence? Long-term post-treatment follow-up of a multicenter trial in the north-east of Italy and evaluation of the diagnostic protocol’s adequacy. Recent Results Cancer Res 2000; 156:116–124.
- Nakamura S, Matsumoto T, Suekane H, et al. Long-term clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer 2005; 104:532–540.
- Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol 1998; 16:1916–1921.
- Solidoro A, Payet C, Sanchez-Lihon J, Montalbetti JA. Gastric lymphomas: chemotherapy as a primary treatment. Semin Surg Oncol 1990; 6:218–225.
- Lévy M, Copie-Bergman C, Molinier-Frenkel V, et al. Treatment of t(11;18)-positive gastric mucosa-associated lymphoid tissue lymphoma with rituximab and chlorambucil: clinical, histological, and molecular follow-up. Leuk Lymphoma 2010; 51:284–290.
- Yahalom J. MALT lymphomas: a radiation oncology viewpoint. Ann Hematol 2001; 80(suppl 3):B100–B105.
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosaassociated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003; 21:4157–4164.
- Hung PD, Schubert ML, Mihas AA. Marginal zone B-cell lymphoma (MALT lymphoma). Curr Treat Options Gastroenterol 2004; 7:133–138.
- Zucca E, Cavalli F. Are antibiotics the treatment of choice for gastric lymphoma? Curr Hematol Rep 2004; 3:11–16.
- Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, et al; EGI LS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut 2007; 56:1685–1687.
A 61-year-old woman presents to her primary care physician because for the last 4 weeks she has had difficulty swallowing solid food and a feeling of food “getting stuck in the chest.” She also reports having nausea, mild epigastric pain, and heartburn. She denies having fevers, chills, night sweats, weight loss, vomiting, diarrhea, hematochezia, or melena.
Medical history
For the past 20 years, she has had gastroesophageal reflux disease (GERD), intermittently treated with a proton pump inhibitor. She also has arthritis, hyperlipidemia, hypertension, and asthma, and she has undergone right hip replacement for a hip fracture. She has no known allergies.
She lives in the Midwest region of the United States and is on disability due to her arthritis. She is divorced and has three children.
She quit smoking 3 years ago after smoking half a pack per day for 30 years. She drinks socially; she has never used recreational drugs.
She recalls that an uncle had cancer, but she does not know the specific type.
Physical examination
The patient’s temperature is 96.7°F (35.9°C), heart rate 86 per minute, blood pressure 150/92 mm Hg, respiratory rate 16 per minute, and oxygen saturation 100% on room air.
Differential diagnosis
Although the differential diagnosis at this stage is broad, a few conditions that commonly present like this are:
- Esophageal cancer
- Esophageal stricture
- Esophageal webs
- Esophagitis (infectious, inflammatory)
- Peptic ulcer disease.
WHICH TEST SHOULD BE ORDERED?
1. Which test will you order now for this patient?
- Endoscopy (esophagogastroduodenoscopy)
- Serum Helicobacter pylori antibody testing
- Wireless pH monitoring
- Barium swallow
Endoscopy would be the best test to order. Esophageal cancer and esophageal stricture must be ruled out, in view of her long history of GERD, gastritis, and smoking and her alarming symptoms of difficulty swallowing and food sticking. In this situation, endoscopy is the first test recommended. In addition to its diagnostic value, it offers an opportunity to obtain tissue samples and to perform a therapeutic intervention, if necessary.1,2
H pyloriantibody testing is used in the “test-and-treat approach” for H pylori infection, an established management strategy for patients who have uninvestigated dyspepsia and who are younger than 55 years and have no “alarm features,” ie, red flags for cancer. The alarm features commonly described are anemia, early satiety, unexplained weight loss, bleeding, odynophagia, progressive dysphagia, unexplained vomiting, and a family history or prior history of gastrointestinal malignancy.3
Our patient’s symptoms raise the possibility of cancer, so that H pylori testing would not be the best test to order at this point.
Ambulatory wireless pH monitoring with a wireless pH capsule is useful for confirming GERD in those with persistent symptoms (whether typical or atypical) who do not have evidence of mucosal damage on initial endoscopy, particularly if a trial of acid suppression has failed.4–6
Barium swallow is an x-ray examination of the esophagus with contrast. It can show both the anatomy and the function of the esophagus, and it would be the initial diagnostic procedure of choice for patients with dysphagia who have no alarm symptoms.7 However, our patient does have alarm symptoms.
First highlight point
- Endoscopy is the first test in patients with dysphagia with alarm symptoms.
CASE CONTINUES: ENDOSCOPY
Multiple biopsies of the nodules show atypical lymphoid infiltrates with small cleaved lymphocytes that are mostly positive for CD5, CD20, and CD43 and negative for CD10 and CD23 by flow cytometry. In addition, a staining test for H pylori is positive.
Comment. Our patient has had GERD for the past 20 years, intermittently treated with a proton pump inhibitor. Acid suppressive therapy with a proton pump inhibitor is the standard of care of patients with erosive esophagitis. In standard doses, these drugs control symptoms in most cases and heal esophagitis in almost 90% of cases within 4 to 8 weeks.9 Proton pump inhibitors are also effective for maintaining healing of esophagitis and controlling symptoms in patients who respond to an acute course of therapy for a period of 6 to 12 months.10
WHAT IS THE DIAGNOSIS?
2. Which is the most likely diagnosis for our patient?
- Fundic gland polyps
- Gastric hyperplastic polyps
- Gastric adenomas
- Mucosa-associated lymphoid tissue (MALT) lymphoma
Fundic gland polyps are small (0.1–0.8 cm), hyperemic, sessile, flat, nodular lesions that have a smooth surface. They occur exclusively in the gastric corpus and are composed of normal gastric corpus-type epithelium arranged in a disorderly or microcystic configuration. 11 This pattern does not match our patient’s findings.
Gastric hyperplastic polyps are elongated, cystic, and distorted foveolar epithelium with marked regeneration. Other histologic findings are stromal inflammation, edema, and smooth muscle hyperplasia.12 This does not match our patient’s findings.
Adenomas can be flat or polypoid and range in size from a few millimeters to several centimeters. Endoscopically, adenomatous polyps have a velvety, lobulated appearance. Most are solitary (82% of cases), located in the antrum, and less than 2 cm in diameter.13 This does not match our patient’s findings.
MALT lymphoma, the correct answer, is characterized by small cleaved lymphocytes positive for CD4, CD20, and CD43. Although CD5 positivity is not characteristic, rare cases of MALT lymphoma may be CD5-positive and may be more aggressive.14
Other common features of MALT lymphoma are erosions, small nodules, thickening of gastric folds—generally suggesting a benign condition—or hyperemic or even normal gastric mucosa.15 Our patient’s complaint of food sticking in her chest and difficulty swallowing was most likely related to the erosive esophagitis found on endoscopy.
A TYPE OF NON-HODGKIN LYMPHOMA
Normal gastric mucosa contains no lymphoid tissue.16,17 Primary gastric lymphoma, of which MALT lymphoma is a subtype, accounts for around 5% of gastric malignancies, with an annual incidence rate of 0.5 per 100,000 people. 18–20 Although rare, it accounts for 60% to 70% of cases of non-Hodgkin lymphoma of the gastrointestinal tract and can involve the perigastric or abdominal lymph nodes or both.21–23 Although earlier studies suggested that its incidence was increasing, recent data indicate the incidence may be decreasing, thanks to active H pylori treatment.24–26
Two subtypes of primary gastric non-Hodgkin lymphoma commonly described are MALT lymphoma and diffuse large B-cell (DLBC) lymphoma. In the Revised European-American Lymphoma Classification, high-grade MALT lymphoma is comparable to DLBC lymphoma and may have transformed from low-grade MALT lymphoma.27,28 Another reported subtype, mantle cell lymphoma with MALT lymphoma features, should be considered in the differential diagnosis, although it is rare.29
MALT lymphoma is linked to H pylori
H pylori infection is a factor in the development of MALT lymphoma,30 as multiple lines of evidence show:
- H pylori infection has been reported in more than 90% of patients with MALT lymphoma.31–35
- H pylori antibodies have been found in stored serum drawn from patients who subsequently developed MALT lymphoma.35
- In response to H pylori antigens, T cells from MALT lymphoma proliferate and cause an increase in tumor immunoglobulin production.36
- In animals experimentally infected with H pylori, around one-third develop lymphoid follicles and lymphoepithelial lesions including B cells, which are similar to human MALT lymphoma.37
However, only a minority of patients with H pylori develop lymphoma, owing to a host immune response that is not well defined.
Second highlight point
- Gastric MALT lymphoma is associated with H pylori.
Associated genetic translocations
Three translocations, t(11;18), t(1;14), and t(14;18), are specifically associated with MALT lymphoma, and the genes involved have been characterized.
The t(11;18) translocation, seen in gastric and nongastric MALT lymphoma, is not seen in H pylori gastritis.38 This translocation is usually associated with extension of the disease outside the stomach (ie, to regional lymph nodes or distal sites).27 Most cases that do not respond to H pylori eradication involve the t(11;18) and t(1;14) translocations.28
Clinical presentation of gastric MALT lymphoma
The average age at presentation with gastric MALT lymphoma is 54 to 58 years.
The most common complaint is nonspecific abdominal pain in the epigastric region, sometimes accompanied by weight loss, nausea, vomiting, and, in a quarter of cases, acute or chronic bleeding.39–41 Weight loss is common, and its extent is associated with the location and the grade of the disease.
Most cases of MALT lymphoma are found serendipitously during endoscopy, on which the appearance of the lesions ranges from small ulcerations to polypoid masses with infiltrated, thickened folds involving predominantly the antrum or prepyloric region.15,41
MANAGING MALT LYMPHOMA
Our patient undergoes endoscopic ultrasonography, which reveals she has stage I disease, ie, it is limited to the stomach without involving the lymph nodes (stage II), adjacent organs (stage III), or distant organs (stage IV).
3. How will you treat this patient, given the present information?
- Chemotherapy
- Radiation therapy
- Surgery
- Antibiotics with a proton pump inhibitor
Antibiotics with a proton pump inhibitor would be best. According to the Maastricht III Consensus Report,42H pylori eradication is the treatment of first choice for H pylori infection in patients with stage I low-grade gastric MALT lymphoma. This therapy can induce complete histologic remission in 80% to 100% of patients with MALT lymphoma. 43 Several studies have shown regression44 or complete remission of low-grade gastric MALT lymphoma after eradication of H pylori with antibiotics, making it a reasonable initial treatment.45–49
Several regimens are used. The first choice in populations in which the prevalence of resistance to clarithromycin (Biaxin) is less than 15% to 20% is a proton pump inhibitor, clarithromycin, and either amoxicillin or metronidazole (Flagyl). (Metronidazole is preferable to amoxicillin if the prevalence of resistance to metronidazole is less than 40%.)
Sequential treatment—ie, 5 days of a proton pump inhibitor plus amoxicillin followed by 5 additional days of a proton pump inhibitor plus clarithromycin plus tinidazole (Tindamax)— may be better than a 7-day course of the combination of a proton pump inhibitor, amoxicillin, and clarithromycin.50,51
Treatment with a proton pump inhibitor, clarithromycin (500 mg twice a day), and either amoxicillin (1,000 mg twice a day) or metronidazole (400 or 500 mg twice a day) for 14 days is more effective than treatment for 7 days.52
H pylori reinfection in the general population is quite rare, with an estimated yearly rate as low as 2%.53 Recurrence of the infection is a risk factor for lymphoma relapse.17,54
Several predictors of the response of MALT lymphoma to eradication therapy have been recognized: H pylori positivity, stage I, lymphoma confined to the stomach; gastric wall invasion confined to mucosa and submucosa, and the absence of the t(11;18) translocation.
The time between H pylori eradication and complete remission of primary gastric lymphoma varies and can be longer than 12 months.55
Chemotherapy. In a single study,56 complete remission was achieved with oral cyclophosphamide (Cytoxan) in cases of antibiotic-refractory gastric MALT lymphoma. Comparable results were achieved after radiation therapy (see below); hence, oral monotherapy with cyclophosphamide might also be a suitable second-line therapy.57
The regimen of cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone (CHOP) has been recommended for patients with stage III and IV disease.41,58
Rituximab (Rituxan) has been proven effective in treating t(11;18)-positive MALT lymphoma.59
Radiation therapy. Two studies have shown a 100% complete response rate after radiation therapy with a median dose of 30 Gy.57,60 Tsang et al61 reported complete remission in up to 90% of patients receiving radiation therapy alone, with excellent 5-year progression-free and overall survival rates of 98% and 77%, respectively.
Although surgery, radiotherapy, and chemotherapy have been used in cases in which eradication therapy failed and in more advanced stages of MALT lymphoma, there is no consensus about their use, so therapy must be individualized.
Fourth highlight point
- Antibiotic treatment for eradication of H pylori infection is the recommended treatment only for stage I low-grade MALT lymphoma.
FOLOW-UP
4. How should you follow patients with MALT lymphoma?
- Endoscopy
- H pylori testing
- Computed tomography and magnetic resonance imaging
- No surveillance required after treatment
Endoscopy is the correct answer. As initial diagnostic biopsies do not exclude aggressive lymphoma, careful endoscopic follow-up is recommended. A recommended schedule is a breath test for H pylori every 2 months in conjunction with repeat endoscopy with biopsies every 3 to 6 months for the first 2 years, and then annually.62
Although H pylori may be eradicated within 1 month of drug therapy, lymphoma may take several months to disappear histologically. In patients with stage I disease with residual lymphoma after H pylori eradication therapy, a simple wait-and-watch strategy is a suitable alternative to oncologic therapy.63,64
Local relapse may occur after many years of complete remission; thus, patients should be followed closely long-term with endoscopy and possibly endoscopic ultrasonography. 47–49,63
Patients who fail to attain a complete remission within 12 months should undergo radiation therapy, with or without chemotherapy. The same therapy should be started as soon as possible in patients with progressive disease after antibiotic therapy. Patients negative for H pylori, patients with stage II disease, and patients with t(11;18) translocation should receive antibiotic treatment with endoscopic surveillance every 3 months.
Fifth highlight point
- Surveillance endoscopy is recommended for follow-up of MALT lymphoma.
CASE CONTINUES: HER CONDITION IMPROVES, THEN WORSENS
Computed tomography of the chest, abdomen, and pelvis reveals no evidence of additional sites of tumor. Positron emission tomography reveals increased uptake in the left tonsillar region, for which she has undergoes an ear, nose, and throat evaluation, and no pathology is found.
Due to recurrence of her marginal zone Bcell lymphoma of MALT type of the stomach, the patient is referred to an oncology service. She is treated with radiation, receiving 15 sessions of 30 Gy localized to the stomach. Three months after radiation therapy, she undergoes endoscopy again, which shows no evidence of the previously described nodules. Repeat biopsies are negative for H pylori and MALT lymphoma.
Annual surveillance endoscopy and computed tomography for the past 3 years have been negative for any tumor recurrence.
A 61-year-old woman presents to her primary care physician because for the last 4 weeks she has had difficulty swallowing solid food and a feeling of food “getting stuck in the chest.” She also reports having nausea, mild epigastric pain, and heartburn. She denies having fevers, chills, night sweats, weight loss, vomiting, diarrhea, hematochezia, or melena.
Medical history
For the past 20 years, she has had gastroesophageal reflux disease (GERD), intermittently treated with a proton pump inhibitor. She also has arthritis, hyperlipidemia, hypertension, and asthma, and she has undergone right hip replacement for a hip fracture. She has no known allergies.
She lives in the Midwest region of the United States and is on disability due to her arthritis. She is divorced and has three children.
She quit smoking 3 years ago after smoking half a pack per day for 30 years. She drinks socially; she has never used recreational drugs.
She recalls that an uncle had cancer, but she does not know the specific type.
Physical examination
The patient’s temperature is 96.7°F (35.9°C), heart rate 86 per minute, blood pressure 150/92 mm Hg, respiratory rate 16 per minute, and oxygen saturation 100% on room air.
Differential diagnosis
Although the differential diagnosis at this stage is broad, a few conditions that commonly present like this are:
- Esophageal cancer
- Esophageal stricture
- Esophageal webs
- Esophagitis (infectious, inflammatory)
- Peptic ulcer disease.
WHICH TEST SHOULD BE ORDERED?
1. Which test will you order now for this patient?
- Endoscopy (esophagogastroduodenoscopy)
- Serum Helicobacter pylori antibody testing
- Wireless pH monitoring
- Barium swallow
Endoscopy would be the best test to order. Esophageal cancer and esophageal stricture must be ruled out, in view of her long history of GERD, gastritis, and smoking and her alarming symptoms of difficulty swallowing and food sticking. In this situation, endoscopy is the first test recommended. In addition to its diagnostic value, it offers an opportunity to obtain tissue samples and to perform a therapeutic intervention, if necessary.1,2
H pyloriantibody testing is used in the “test-and-treat approach” for H pylori infection, an established management strategy for patients who have uninvestigated dyspepsia and who are younger than 55 years and have no “alarm features,” ie, red flags for cancer. The alarm features commonly described are anemia, early satiety, unexplained weight loss, bleeding, odynophagia, progressive dysphagia, unexplained vomiting, and a family history or prior history of gastrointestinal malignancy.3
Our patient’s symptoms raise the possibility of cancer, so that H pylori testing would not be the best test to order at this point.
Ambulatory wireless pH monitoring with a wireless pH capsule is useful for confirming GERD in those with persistent symptoms (whether typical or atypical) who do not have evidence of mucosal damage on initial endoscopy, particularly if a trial of acid suppression has failed.4–6
Barium swallow is an x-ray examination of the esophagus with contrast. It can show both the anatomy and the function of the esophagus, and it would be the initial diagnostic procedure of choice for patients with dysphagia who have no alarm symptoms.7 However, our patient does have alarm symptoms.
First highlight point
- Endoscopy is the first test in patients with dysphagia with alarm symptoms.
CASE CONTINUES: ENDOSCOPY
Multiple biopsies of the nodules show atypical lymphoid infiltrates with small cleaved lymphocytes that are mostly positive for CD5, CD20, and CD43 and negative for CD10 and CD23 by flow cytometry. In addition, a staining test for H pylori is positive.
Comment. Our patient has had GERD for the past 20 years, intermittently treated with a proton pump inhibitor. Acid suppressive therapy with a proton pump inhibitor is the standard of care of patients with erosive esophagitis. In standard doses, these drugs control symptoms in most cases and heal esophagitis in almost 90% of cases within 4 to 8 weeks.9 Proton pump inhibitors are also effective for maintaining healing of esophagitis and controlling symptoms in patients who respond to an acute course of therapy for a period of 6 to 12 months.10
WHAT IS THE DIAGNOSIS?
2. Which is the most likely diagnosis for our patient?
- Fundic gland polyps
- Gastric hyperplastic polyps
- Gastric adenomas
- Mucosa-associated lymphoid tissue (MALT) lymphoma
Fundic gland polyps are small (0.1–0.8 cm), hyperemic, sessile, flat, nodular lesions that have a smooth surface. They occur exclusively in the gastric corpus and are composed of normal gastric corpus-type epithelium arranged in a disorderly or microcystic configuration. 11 This pattern does not match our patient’s findings.
Gastric hyperplastic polyps are elongated, cystic, and distorted foveolar epithelium with marked regeneration. Other histologic findings are stromal inflammation, edema, and smooth muscle hyperplasia.12 This does not match our patient’s findings.
Adenomas can be flat or polypoid and range in size from a few millimeters to several centimeters. Endoscopically, adenomatous polyps have a velvety, lobulated appearance. Most are solitary (82% of cases), located in the antrum, and less than 2 cm in diameter.13 This does not match our patient’s findings.
MALT lymphoma, the correct answer, is characterized by small cleaved lymphocytes positive for CD4, CD20, and CD43. Although CD5 positivity is not characteristic, rare cases of MALT lymphoma may be CD5-positive and may be more aggressive.14
Other common features of MALT lymphoma are erosions, small nodules, thickening of gastric folds—generally suggesting a benign condition—or hyperemic or even normal gastric mucosa.15 Our patient’s complaint of food sticking in her chest and difficulty swallowing was most likely related to the erosive esophagitis found on endoscopy.
A TYPE OF NON-HODGKIN LYMPHOMA
Normal gastric mucosa contains no lymphoid tissue.16,17 Primary gastric lymphoma, of which MALT lymphoma is a subtype, accounts for around 5% of gastric malignancies, with an annual incidence rate of 0.5 per 100,000 people. 18–20 Although rare, it accounts for 60% to 70% of cases of non-Hodgkin lymphoma of the gastrointestinal tract and can involve the perigastric or abdominal lymph nodes or both.21–23 Although earlier studies suggested that its incidence was increasing, recent data indicate the incidence may be decreasing, thanks to active H pylori treatment.24–26
Two subtypes of primary gastric non-Hodgkin lymphoma commonly described are MALT lymphoma and diffuse large B-cell (DLBC) lymphoma. In the Revised European-American Lymphoma Classification, high-grade MALT lymphoma is comparable to DLBC lymphoma and may have transformed from low-grade MALT lymphoma.27,28 Another reported subtype, mantle cell lymphoma with MALT lymphoma features, should be considered in the differential diagnosis, although it is rare.29
MALT lymphoma is linked to H pylori
H pylori infection is a factor in the development of MALT lymphoma,30 as multiple lines of evidence show:
- H pylori infection has been reported in more than 90% of patients with MALT lymphoma.31–35
- H pylori antibodies have been found in stored serum drawn from patients who subsequently developed MALT lymphoma.35
- In response to H pylori antigens, T cells from MALT lymphoma proliferate and cause an increase in tumor immunoglobulin production.36
- In animals experimentally infected with H pylori, around one-third develop lymphoid follicles and lymphoepithelial lesions including B cells, which are similar to human MALT lymphoma.37
However, only a minority of patients with H pylori develop lymphoma, owing to a host immune response that is not well defined.
Second highlight point
- Gastric MALT lymphoma is associated with H pylori.
Associated genetic translocations
Three translocations, t(11;18), t(1;14), and t(14;18), are specifically associated with MALT lymphoma, and the genes involved have been characterized.
The t(11;18) translocation, seen in gastric and nongastric MALT lymphoma, is not seen in H pylori gastritis.38 This translocation is usually associated with extension of the disease outside the stomach (ie, to regional lymph nodes or distal sites).27 Most cases that do not respond to H pylori eradication involve the t(11;18) and t(1;14) translocations.28
Clinical presentation of gastric MALT lymphoma
The average age at presentation with gastric MALT lymphoma is 54 to 58 years.
The most common complaint is nonspecific abdominal pain in the epigastric region, sometimes accompanied by weight loss, nausea, vomiting, and, in a quarter of cases, acute or chronic bleeding.39–41 Weight loss is common, and its extent is associated with the location and the grade of the disease.
Most cases of MALT lymphoma are found serendipitously during endoscopy, on which the appearance of the lesions ranges from small ulcerations to polypoid masses with infiltrated, thickened folds involving predominantly the antrum or prepyloric region.15,41
MANAGING MALT LYMPHOMA
Our patient undergoes endoscopic ultrasonography, which reveals she has stage I disease, ie, it is limited to the stomach without involving the lymph nodes (stage II), adjacent organs (stage III), or distant organs (stage IV).
3. How will you treat this patient, given the present information?
- Chemotherapy
- Radiation therapy
- Surgery
- Antibiotics with a proton pump inhibitor
Antibiotics with a proton pump inhibitor would be best. According to the Maastricht III Consensus Report,42H pylori eradication is the treatment of first choice for H pylori infection in patients with stage I low-grade gastric MALT lymphoma. This therapy can induce complete histologic remission in 80% to 100% of patients with MALT lymphoma. 43 Several studies have shown regression44 or complete remission of low-grade gastric MALT lymphoma after eradication of H pylori with antibiotics, making it a reasonable initial treatment.45–49
Several regimens are used. The first choice in populations in which the prevalence of resistance to clarithromycin (Biaxin) is less than 15% to 20% is a proton pump inhibitor, clarithromycin, and either amoxicillin or metronidazole (Flagyl). (Metronidazole is preferable to amoxicillin if the prevalence of resistance to metronidazole is less than 40%.)
Sequential treatment—ie, 5 days of a proton pump inhibitor plus amoxicillin followed by 5 additional days of a proton pump inhibitor plus clarithromycin plus tinidazole (Tindamax)— may be better than a 7-day course of the combination of a proton pump inhibitor, amoxicillin, and clarithromycin.50,51
Treatment with a proton pump inhibitor, clarithromycin (500 mg twice a day), and either amoxicillin (1,000 mg twice a day) or metronidazole (400 or 500 mg twice a day) for 14 days is more effective than treatment for 7 days.52
H pylori reinfection in the general population is quite rare, with an estimated yearly rate as low as 2%.53 Recurrence of the infection is a risk factor for lymphoma relapse.17,54
Several predictors of the response of MALT lymphoma to eradication therapy have been recognized: H pylori positivity, stage I, lymphoma confined to the stomach; gastric wall invasion confined to mucosa and submucosa, and the absence of the t(11;18) translocation.
The time between H pylori eradication and complete remission of primary gastric lymphoma varies and can be longer than 12 months.55
Chemotherapy. In a single study,56 complete remission was achieved with oral cyclophosphamide (Cytoxan) in cases of antibiotic-refractory gastric MALT lymphoma. Comparable results were achieved after radiation therapy (see below); hence, oral monotherapy with cyclophosphamide might also be a suitable second-line therapy.57
The regimen of cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone (CHOP) has been recommended for patients with stage III and IV disease.41,58
Rituximab (Rituxan) has been proven effective in treating t(11;18)-positive MALT lymphoma.59
Radiation therapy. Two studies have shown a 100% complete response rate after radiation therapy with a median dose of 30 Gy.57,60 Tsang et al61 reported complete remission in up to 90% of patients receiving radiation therapy alone, with excellent 5-year progression-free and overall survival rates of 98% and 77%, respectively.
Although surgery, radiotherapy, and chemotherapy have been used in cases in which eradication therapy failed and in more advanced stages of MALT lymphoma, there is no consensus about their use, so therapy must be individualized.
Fourth highlight point
- Antibiotic treatment for eradication of H pylori infection is the recommended treatment only for stage I low-grade MALT lymphoma.
FOLOW-UP
4. How should you follow patients with MALT lymphoma?
- Endoscopy
- H pylori testing
- Computed tomography and magnetic resonance imaging
- No surveillance required after treatment
Endoscopy is the correct answer. As initial diagnostic biopsies do not exclude aggressive lymphoma, careful endoscopic follow-up is recommended. A recommended schedule is a breath test for H pylori every 2 months in conjunction with repeat endoscopy with biopsies every 3 to 6 months for the first 2 years, and then annually.62
Although H pylori may be eradicated within 1 month of drug therapy, lymphoma may take several months to disappear histologically. In patients with stage I disease with residual lymphoma after H pylori eradication therapy, a simple wait-and-watch strategy is a suitable alternative to oncologic therapy.63,64
Local relapse may occur after many years of complete remission; thus, patients should be followed closely long-term with endoscopy and possibly endoscopic ultrasonography. 47–49,63
Patients who fail to attain a complete remission within 12 months should undergo radiation therapy, with or without chemotherapy. The same therapy should be started as soon as possible in patients with progressive disease after antibiotic therapy. Patients negative for H pylori, patients with stage II disease, and patients with t(11;18) translocation should receive antibiotic treatment with endoscopic surveillance every 3 months.
Fifth highlight point
- Surveillance endoscopy is recommended for follow-up of MALT lymphoma.
CASE CONTINUES: HER CONDITION IMPROVES, THEN WORSENS
Computed tomography of the chest, abdomen, and pelvis reveals no evidence of additional sites of tumor. Positron emission tomography reveals increased uptake in the left tonsillar region, for which she has undergoes an ear, nose, and throat evaluation, and no pathology is found.
Due to recurrence of her marginal zone Bcell lymphoma of MALT type of the stomach, the patient is referred to an oncology service. She is treated with radiation, receiving 15 sessions of 30 Gy localized to the stomach. Three months after radiation therapy, she undergoes endoscopy again, which shows no evidence of the previously described nodules. Repeat biopsies are negative for H pylori and MALT lymphoma.
Annual surveillance endoscopy and computed tomography for the past 3 years have been negative for any tumor recurrence.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102:1808–1825.
- Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98:740–749.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Furlow B. Barium swallow. Radiol Technol 2004; 76:49–58.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45:172–180.
- Havelund T, Laursen LS, Skoubo-Kristensen E, et al. Omeprazole and ranitidine in treatment of reflux oesophagitis: double blind comparative trial. Br Med J (Clin Res Ed) 1988; 296:89–92.
- Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1392–1413.
- Odze RD, Marcial MA, Antonioli D. Gastric fundic gland polyps: a morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum Pathol 1996; 27:896–903.
- Snover DC. Benign epithelial polyps of the stomach. Pathol Annu 1985; 20:303–329.
- Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 2009; 6:331–341.
- Wenzel C, Dieckmann K, Fiebiger W, Mannhalter C, Chott A, Raderer M. CD5 expression in a lymphoma of the mucosa-associated lymphoid tissue (MALT)-type as a marker for early dissemination and aggressive clinical behaviour. Leuk Lymphoma 2001; 42:823–829.
- Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol 2003; 98:975–986.
- Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med 1999; 131:88–95.
- Stolte M, Bayerdörffer E, Morgner A, et al. Helicobacter and gastric MALT lymphoma. Gut 2002; 50(suppl 3):III19–III24.
- Ducreux M, Boutron MC, Piard F, Carli PM, Faivre J. A 15-year series of gastrointestinal non-Hodgkin’s lymphomas: a population-based study. Br J Cancer 1998; 77:511–514.
- Gurney KA, Cartwright RA, Gilman EA. Descriptive epidemiology of gastrointestinal non-Hodgkin’s lymphoma in a population-based registry. Br J Cancer 1999; 79:1929–1934.
- d’Amore F, Brincker H, Grønbaek K, et al. Non-Hodgkin’s lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol 1994; 12:1673–1684.
- Koch P, del Valle F, Berdel WE, et al; German Multicenter Study Group. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001; 19:3861–3873.
- Papaxoinis G, Papageorgiou S, Rontogianni D, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma 2006; 47:2140–2146.
- Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology 1992; 20:29–34.
- Wotherspoon AC. Choosing the right MALT. Gut 1996; 39:617–618.
- Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003; 97:2462–2473.
- Luminari S, Cesaretti M, Marcheselli L, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol 2009; epub ahead of print.
- Liu H, Ye H, Dogan A, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001; 98:1182–1187.
- Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001; 357:39–40.
- Shibata K, Shimamoto Y, Nakano S, Miyahara M, Nakano H, Yamaguchi M. Mantle cell lymphoma with the features of mucosa-associated lymphoid tissue (MALT) lymphoma in an HTLV-I-seropositive patient. Ann Hematol 1995; 70:47–51.
- Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol 2005; 23:6370–6378.
- de Jong D, Boot H, van Heerde P, Hart GA, Taal BG. Histological grading in gastric lymphoma: pretreatment criteria and clinical relevance. Gastroenterology 1997; 112:1466–1474.
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338:1175–1176.
- Eidt S, Stolte M, Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin’s lymphomas. J Clin Pathol 1994; 47:436–439.
- Doglioni C, Wotherspoon AC, Moschini A, de Boni M, Isaacson PG. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992; 339:834–835.
- Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330:1267–1271.
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 1993; 342:571–574.
- Lee A, O’Rourke J, Enno A. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations—mouse models of gastric lymphoma. Recent Results Cancer Res 2000; 156:42–51.
- Auer IA, Gascoyne RD, Connors JM, et al. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997; 8:979–985.
- Morgner A, Bayerdörffer E, Neubauer A, Stolte M. Malignant tumors of the stomach. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori. Gastroenterol Clin North Am 2000; 29:593–607.
- Ruskoné-Fourmestraux A, Aegerter P, Delmer A, Brousse N, Galian A, Rambaud JC. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d’Etude des Lymphomes Digestifs. Gastroenterology 1993; 105:1662–1671.
- Cogliatti SB, Schmid U, Schumacher U, et al. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology 1991; 101:1159–1170.
- Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56:772–781.
- Boot H, de Jong D. Gastric lymphoma: the revolution of the past decade. Scand J Gastroenterol Suppl 2002; 236:27–36.
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993; 342:575–577.
- Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995; 345:1591–1594.
- Roggero E, Zucca E, Pinotti G, et al. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med 1995; 122:767–769.
- Ruskoné-Fourmestraux A. Gastrointestinal lymphomas: the French experience of the Groupe d’Étude des Lymphomes Digestifs (GELD). Recent Results Cancer Res 2000; 156:99–103.
- Wündisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol 2005; 23:8018–8024.
- Wündisch T, Mösch C, Neubauer A, Stolte M. Helicobacter pylori eradication in gastric mucosa-associated lymphoid tissue lymphoma: results of a 196-patient series. Leuk Lymphoma 2006; 47:2110–2114.
- De Francesco V, Zullo A, Margiotta M, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther 2004; 19:407–414.
- Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003; 17:719–726.
- Paoluzi P, Iacopini F, Crispino P, et al. 2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter 2006; 11:562–568.
- Gisbert JP, Olivares D, Jimenez I, Pajares JM. Long-term follow-up of 13C-urea breath test results after Helicobacter pylori eradication: frequency and significance of borderline delta13CO2 values. Aliment Pharmacol Ther 2006; 23:275–280.
- Bayerdörffer E, Morgner A. Gastric marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type: management of the disease. Dig Liver Dis 2000; 32:192–194.
- Savio A, Zamboni G, Capelli P, et al. Relapse of low-grade gastric MALT lymphoma after Helicobacter pylori eradication: true relapse or persistence? Long-term post-treatment follow-up of a multicenter trial in the north-east of Italy and evaluation of the diagnostic protocol’s adequacy. Recent Results Cancer Res 2000; 156:116–124.
- Nakamura S, Matsumoto T, Suekane H, et al. Long-term clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer 2005; 104:532–540.
- Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol 1998; 16:1916–1921.
- Solidoro A, Payet C, Sanchez-Lihon J, Montalbetti JA. Gastric lymphomas: chemotherapy as a primary treatment. Semin Surg Oncol 1990; 6:218–225.
- Lévy M, Copie-Bergman C, Molinier-Frenkel V, et al. Treatment of t(11;18)-positive gastric mucosa-associated lymphoid tissue lymphoma with rituximab and chlorambucil: clinical, histological, and molecular follow-up. Leuk Lymphoma 2010; 51:284–290.
- Yahalom J. MALT lymphomas: a radiation oncology viewpoint. Ann Hematol 2001; 80(suppl 3):B100–B105.
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosaassociated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003; 21:4157–4164.
- Hung PD, Schubert ML, Mihas AA. Marginal zone B-cell lymphoma (MALT lymphoma). Curr Treat Options Gastroenterol 2004; 7:133–138.
- Zucca E, Cavalli F. Are antibiotics the treatment of choice for gastric lymphoma? Curr Hematol Rep 2004; 3:11–16.
- Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, et al; EGI LS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut 2007; 56:1685–1687.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102:1808–1825.
- Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol 2003; 98:740–749.
- DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005; 100:190–200.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920.
- Furlow B. Barium swallow. Radiol Technol 2004; 76:49–58.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45:172–180.
- Havelund T, Laursen LS, Skoubo-Kristensen E, et al. Omeprazole and ranitidine in treatment of reflux oesophagitis: double blind comparative trial. Br Med J (Clin Res Ed) 1988; 296:89–92.
- Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1392–1413.
- Odze RD, Marcial MA, Antonioli D. Gastric fundic gland polyps: a morphological study including mucin histochemistry, stereometry, and MIB-1 immunohistochemistry. Hum Pathol 1996; 27:896–903.
- Snover DC. Benign epithelial polyps of the stomach. Pathol Annu 1985; 20:303–329.
- Carmack SW, Genta RM, Graham DY, Lauwers GY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 2009; 6:331–341.
- Wenzel C, Dieckmann K, Fiebiger W, Mannhalter C, Chott A, Raderer M. CD5 expression in a lymphoma of the mucosa-associated lymphoid tissue (MALT)-type as a marker for early dissemination and aggressive clinical behaviour. Leuk Lymphoma 2001; 42:823–829.
- Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol 2003; 98:975–986.
- Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med 1999; 131:88–95.
- Stolte M, Bayerdörffer E, Morgner A, et al. Helicobacter and gastric MALT lymphoma. Gut 2002; 50(suppl 3):III19–III24.
- Ducreux M, Boutron MC, Piard F, Carli PM, Faivre J. A 15-year series of gastrointestinal non-Hodgkin’s lymphomas: a population-based study. Br J Cancer 1998; 77:511–514.
- Gurney KA, Cartwright RA, Gilman EA. Descriptive epidemiology of gastrointestinal non-Hodgkin’s lymphoma in a population-based registry. Br J Cancer 1999; 79:1929–1934.
- d’Amore F, Brincker H, Grønbaek K, et al. Non-Hodgkin’s lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol 1994; 12:1673–1684.
- Koch P, del Valle F, Berdel WE, et al; German Multicenter Study Group. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001; 19:3861–3873.
- Papaxoinis G, Papageorgiou S, Rontogianni D, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma 2006; 47:2140–2146.
- Wotherspoon AC, Doglioni C, Isaacson PG. Low-grade gastric B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): a multifocal disease. Histopathology 1992; 20:29–34.
- Wotherspoon AC. Choosing the right MALT. Gut 1996; 39:617–618.
- Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer 2003; 97:2462–2473.
- Luminari S, Cesaretti M, Marcheselli L, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol 2009; epub ahead of print.
- Liu H, Ye H, Dogan A, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001; 98:1182–1187.
- Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001; 357:39–40.
- Shibata K, Shimamoto Y, Nakano S, Miyahara M, Nakano H, Yamaguchi M. Mantle cell lymphoma with the features of mucosa-associated lymphoid tissue (MALT) lymphoma in an HTLV-I-seropositive patient. Ann Hematol 1995; 70:47–51.
- Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol 2005; 23:6370–6378.
- de Jong D, Boot H, van Heerde P, Hart GA, Taal BG. Histological grading in gastric lymphoma: pretreatment criteria and clinical relevance. Gastroenterology 1997; 112:1466–1474.
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991; 338:1175–1176.
- Eidt S, Stolte M, Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin’s lymphomas. J Clin Pathol 1994; 47:436–439.
- Doglioni C, Wotherspoon AC, Moschini A, de Boni M, Isaacson PG. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992; 339:834–835.
- Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330:1267–1271.
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet 1993; 342:571–574.
- Lee A, O’Rourke J, Enno A. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations—mouse models of gastric lymphoma. Recent Results Cancer Res 2000; 156:42–51.
- Auer IA, Gascoyne RD, Connors JM, et al. t(11;18)(q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol 1997; 8:979–985.
- Morgner A, Bayerdörffer E, Neubauer A, Stolte M. Malignant tumors of the stomach. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori. Gastroenterol Clin North Am 2000; 29:593–607.
- Ruskoné-Fourmestraux A, Aegerter P, Delmer A, Brousse N, Galian A, Rambaud JC. Primary digestive tract lymphoma: a prospective multicentric study of 91 patients. Groupe d’Etude des Lymphomes Digestifs. Gastroenterology 1993; 105:1662–1671.
- Cogliatti SB, Schmid U, Schumacher U, et al. Primary B-cell gastric lymphoma: a clinicopathological study of 145 patients. Gastroenterology 1991; 101:1159–1170.
- Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56:772–781.
- Boot H, de Jong D. Gastric lymphoma: the revolution of the past decade. Scand J Gastroenterol Suppl 2002; 236:27–36.
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993; 342:575–577.
- Bayerdörffer E, Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995; 345:1591–1594.
- Roggero E, Zucca E, Pinotti G, et al. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med 1995; 122:767–769.
- Ruskoné-Fourmestraux A. Gastrointestinal lymphomas: the French experience of the Groupe d’Étude des Lymphomes Digestifs (GELD). Recent Results Cancer Res 2000; 156:99–103.
- Wündisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol 2005; 23:8018–8024.
- Wündisch T, Mösch C, Neubauer A, Stolte M. Helicobacter pylori eradication in gastric mucosa-associated lymphoid tissue lymphoma: results of a 196-patient series. Leuk Lymphoma 2006; 47:2110–2114.
- De Francesco V, Zullo A, Margiotta M, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther 2004; 19:407–414.
- Zullo A, Vaira D, Vakil N, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003; 17:719–726.
- Paoluzi P, Iacopini F, Crispino P, et al. 2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter 2006; 11:562–568.
- Gisbert JP, Olivares D, Jimenez I, Pajares JM. Long-term follow-up of 13C-urea breath test results after Helicobacter pylori eradication: frequency and significance of borderline delta13CO2 values. Aliment Pharmacol Ther 2006; 23:275–280.
- Bayerdörffer E, Morgner A. Gastric marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type: management of the disease. Dig Liver Dis 2000; 32:192–194.
- Savio A, Zamboni G, Capelli P, et al. Relapse of low-grade gastric MALT lymphoma after Helicobacter pylori eradication: true relapse or persistence? Long-term post-treatment follow-up of a multicenter trial in the north-east of Italy and evaluation of the diagnostic protocol’s adequacy. Recent Results Cancer Res 2000; 156:116–124.
- Nakamura S, Matsumoto T, Suekane H, et al. Long-term clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer 2005; 104:532–540.
- Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol 1998; 16:1916–1921.
- Solidoro A, Payet C, Sanchez-Lihon J, Montalbetti JA. Gastric lymphomas: chemotherapy as a primary treatment. Semin Surg Oncol 1990; 6:218–225.
- Lévy M, Copie-Bergman C, Molinier-Frenkel V, et al. Treatment of t(11;18)-positive gastric mucosa-associated lymphoid tissue lymphoma with rituximab and chlorambucil: clinical, histological, and molecular follow-up. Leuk Lymphoma 2010; 51:284–290.
- Yahalom J. MALT lymphomas: a radiation oncology viewpoint. Ann Hematol 2001; 80(suppl 3):B100–B105.
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosaassociated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003; 21:4157–4164.
- Hung PD, Schubert ML, Mihas AA. Marginal zone B-cell lymphoma (MALT lymphoma). Curr Treat Options Gastroenterol 2004; 7:133–138.
- Zucca E, Cavalli F. Are antibiotics the treatment of choice for gastric lymphoma? Curr Hematol Rep 2004; 3:11–16.
- Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, et al; EGI LS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut 2007; 56:1685–1687.
Recurrent spontaneous pneumothorax
Q: Which is the most likely diagnosis?
- Pulmonary Langerhans cell histiocytosis
- Cystic fibrosis
- Pneumocystis jirovecii pneumonia
- Alpha-1 antitrypsin deficiency
- Tuberous sclerosis complex with lymphangioleiomyomatosis
A: The correct diagnosis is tuberous sclerosis complex with lymphangioleiomyomatosis. The lymphangioleiomyomatosis was suggested by the CT findings, by the recurrence of pneumothorax, and, later, by biopsy results. Lymphangioleiomyomatosis occurs in about 30% of women with the tuberous sclerosis complex.1 However, 10% to 15% of women with lymphangioleiomyomatosis do not have tuberous sclerosis complex, 2 in which case the condition is called sporadic lymphangioleiomyomatosis.
Tuberous sclerosis complex can involve the nerves (seizures, brain tumors), the lungs (lymphangioleiomyomatosis, causing pneumothorax or chylothorax), and the skin; skin lesions include facial angiofibromas (Figure 1), ash-leaf spot (Figure 2), and shagreen patch (Figure 3). It is also associated with abdominal involvement (lymphangiomyomas, renal angiomyolipomas).3
Lymphangioleiomyomatosis usually presents as spontaneous pneumothorax in women of childbearing age. After initial stabilization of pneumothorax with simple aspiration or thoracostomy, the patient should undergo ipsilateral chemical or surgical pleurodesis, as the risk of recurrent pneumothorax is greater than 70%.4 Single or bilateral lung transplantation has been accepted as therapy for end-stage pulmonary lymphangioleiomyomatosis, characterized by recurrent pneumothoraces and chylous pleural fluid collections causing respiratory failure (marked dyspnea, hypoxemia, and reductions in forced expiratory volume in the first second of expiration and in diffusing capacity for carbon monoxide. Recurrence of lymphangioleiomyomatosis in the allograft lung is rare.5 Hormone therapies such as intramuscular progesterone, oral progestins, or gonadotropin-releasing hormone agonists have been used for lymphangioleiomyomatosis (not pneumothorax), but they are no longer recommended.
Pulmonary Langerhans cell histiocytosis, cystic fibrosis, Pneumocystis jirovecii pneumonia, and alpha-1 antitrypsin deficiency have all been associated with spontaneous pneumothorax.
Pulmonary Langerhans cell histiocytosis can affect multiple systems, including lung, skin, bone, and the pituitary gland. More than 90% of patients have a history of smoking.6 Chest radiography reveals a reticulonodular pattern with involvement of the middle and upper lobes. Later, the nodules tend to cavitate and form contiguous cysts that may mimic lymphangioleiomyomatosis on a high-resolution chest CT.
Cystic fibrosis is most often diagnosed before the age of 3.7 In adults, it can present as sinus and pulmonary disease (chronic cough with sputum production, chronic sinusitis with nasal polyposis, radiographic evidence of bronchiectasis and, less commonly, pneumothorax); as a gastrointestinal tract and nutritional abnormality (pancreatic insufficiency, distal intestinal obstruction, focal biliary cirrhosis); and as male infertility.
Pneumocystis jiroveciipneumonia occurs mainly in patients on chronic immunosuppressive drugs or with immune deficiency due to HIV infection. Typical radiographic features are bilateral perihilar interstitial infiltrates that become increasingly homogeneous and diffuse as the disease progresses. Less common findings include solitary or multiple nodules, upper-lobe infiltrates in patients receiving aerosolized pentamidine (NebuPent), pneumatoceles, and pneumothorax.8
Alpha-1 antitrypsin is an inhibitor of neutrophil elastase. Deficiency is associated with severe, early-onset panacinar emphysema with a basilar predominance, with chronic liver disease including cirrhosis, and less commonly with panniculitis and vasculitis associated with antineutrophil cytoplasmic antibody.9 Coalescence of panacinar emphysema leads to the formation of bullae and is important in the development of spontaneous pneumothorax.10
The patient underwent bilateral talc pleurodesis. Lung biopsy at the same time confirmed lymphangioleiomyomatosis. One month later, the right pneumothorax recurred, and she underwent pleurodesis in the right hemithorax with tetracycline. Six months after the second pleurodesis, she was asymptomatic.
- Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc 2000; 75:591–594.
- Strizheva GD, Carsillo T, Kruger WD, Sullivan EJ, Ryu JH, Henske EP. The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Respir Crit Care Med 2001; 163:253–258.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Almoosa KF, Ryu JH, Mendez J, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest 2006; 129:1274–1281.
- Benden C, Rea F, Behr J, et al. Lung transplantation for lymphangioleiomyomatosis: the European Experience. J Heart Lung Transplant 2009; 28:1–7.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Boyle MP. Adult cystic fibrosis. JAMA 2007; 298:1787–1793.
- Thomas CF, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004; 350:2487–2498.
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med 2009; 360:2749–2757.
- Anderson AE, Furlaneto JA, Foraker AG. Bronchopulmonary derangements in nonsmokers. Am Rev Respir Dis 1970; 101:518–527.
Q: Which is the most likely diagnosis?
- Pulmonary Langerhans cell histiocytosis
- Cystic fibrosis
- Pneumocystis jirovecii pneumonia
- Alpha-1 antitrypsin deficiency
- Tuberous sclerosis complex with lymphangioleiomyomatosis
A: The correct diagnosis is tuberous sclerosis complex with lymphangioleiomyomatosis. The lymphangioleiomyomatosis was suggested by the CT findings, by the recurrence of pneumothorax, and, later, by biopsy results. Lymphangioleiomyomatosis occurs in about 30% of women with the tuberous sclerosis complex.1 However, 10% to 15% of women with lymphangioleiomyomatosis do not have tuberous sclerosis complex, 2 in which case the condition is called sporadic lymphangioleiomyomatosis.
Tuberous sclerosis complex can involve the nerves (seizures, brain tumors), the lungs (lymphangioleiomyomatosis, causing pneumothorax or chylothorax), and the skin; skin lesions include facial angiofibromas (Figure 1), ash-leaf spot (Figure 2), and shagreen patch (Figure 3). It is also associated with abdominal involvement (lymphangiomyomas, renal angiomyolipomas).3
Lymphangioleiomyomatosis usually presents as spontaneous pneumothorax in women of childbearing age. After initial stabilization of pneumothorax with simple aspiration or thoracostomy, the patient should undergo ipsilateral chemical or surgical pleurodesis, as the risk of recurrent pneumothorax is greater than 70%.4 Single or bilateral lung transplantation has been accepted as therapy for end-stage pulmonary lymphangioleiomyomatosis, characterized by recurrent pneumothoraces and chylous pleural fluid collections causing respiratory failure (marked dyspnea, hypoxemia, and reductions in forced expiratory volume in the first second of expiration and in diffusing capacity for carbon monoxide. Recurrence of lymphangioleiomyomatosis in the allograft lung is rare.5 Hormone therapies such as intramuscular progesterone, oral progestins, or gonadotropin-releasing hormone agonists have been used for lymphangioleiomyomatosis (not pneumothorax), but they are no longer recommended.
Pulmonary Langerhans cell histiocytosis, cystic fibrosis, Pneumocystis jirovecii pneumonia, and alpha-1 antitrypsin deficiency have all been associated with spontaneous pneumothorax.
Pulmonary Langerhans cell histiocytosis can affect multiple systems, including lung, skin, bone, and the pituitary gland. More than 90% of patients have a history of smoking.6 Chest radiography reveals a reticulonodular pattern with involvement of the middle and upper lobes. Later, the nodules tend to cavitate and form contiguous cysts that may mimic lymphangioleiomyomatosis on a high-resolution chest CT.
Cystic fibrosis is most often diagnosed before the age of 3.7 In adults, it can present as sinus and pulmonary disease (chronic cough with sputum production, chronic sinusitis with nasal polyposis, radiographic evidence of bronchiectasis and, less commonly, pneumothorax); as a gastrointestinal tract and nutritional abnormality (pancreatic insufficiency, distal intestinal obstruction, focal biliary cirrhosis); and as male infertility.
Pneumocystis jiroveciipneumonia occurs mainly in patients on chronic immunosuppressive drugs or with immune deficiency due to HIV infection. Typical radiographic features are bilateral perihilar interstitial infiltrates that become increasingly homogeneous and diffuse as the disease progresses. Less common findings include solitary or multiple nodules, upper-lobe infiltrates in patients receiving aerosolized pentamidine (NebuPent), pneumatoceles, and pneumothorax.8
Alpha-1 antitrypsin is an inhibitor of neutrophil elastase. Deficiency is associated with severe, early-onset panacinar emphysema with a basilar predominance, with chronic liver disease including cirrhosis, and less commonly with panniculitis and vasculitis associated with antineutrophil cytoplasmic antibody.9 Coalescence of panacinar emphysema leads to the formation of bullae and is important in the development of spontaneous pneumothorax.10
The patient underwent bilateral talc pleurodesis. Lung biopsy at the same time confirmed lymphangioleiomyomatosis. One month later, the right pneumothorax recurred, and she underwent pleurodesis in the right hemithorax with tetracycline. Six months after the second pleurodesis, she was asymptomatic.
Q: Which is the most likely diagnosis?
- Pulmonary Langerhans cell histiocytosis
- Cystic fibrosis
- Pneumocystis jirovecii pneumonia
- Alpha-1 antitrypsin deficiency
- Tuberous sclerosis complex with lymphangioleiomyomatosis
A: The correct diagnosis is tuberous sclerosis complex with lymphangioleiomyomatosis. The lymphangioleiomyomatosis was suggested by the CT findings, by the recurrence of pneumothorax, and, later, by biopsy results. Lymphangioleiomyomatosis occurs in about 30% of women with the tuberous sclerosis complex.1 However, 10% to 15% of women with lymphangioleiomyomatosis do not have tuberous sclerosis complex, 2 in which case the condition is called sporadic lymphangioleiomyomatosis.
Tuberous sclerosis complex can involve the nerves (seizures, brain tumors), the lungs (lymphangioleiomyomatosis, causing pneumothorax or chylothorax), and the skin; skin lesions include facial angiofibromas (Figure 1), ash-leaf spot (Figure 2), and shagreen patch (Figure 3). It is also associated with abdominal involvement (lymphangiomyomas, renal angiomyolipomas).3
Lymphangioleiomyomatosis usually presents as spontaneous pneumothorax in women of childbearing age. After initial stabilization of pneumothorax with simple aspiration or thoracostomy, the patient should undergo ipsilateral chemical or surgical pleurodesis, as the risk of recurrent pneumothorax is greater than 70%.4 Single or bilateral lung transplantation has been accepted as therapy for end-stage pulmonary lymphangioleiomyomatosis, characterized by recurrent pneumothoraces and chylous pleural fluid collections causing respiratory failure (marked dyspnea, hypoxemia, and reductions in forced expiratory volume in the first second of expiration and in diffusing capacity for carbon monoxide. Recurrence of lymphangioleiomyomatosis in the allograft lung is rare.5 Hormone therapies such as intramuscular progesterone, oral progestins, or gonadotropin-releasing hormone agonists have been used for lymphangioleiomyomatosis (not pneumothorax), but they are no longer recommended.
Pulmonary Langerhans cell histiocytosis, cystic fibrosis, Pneumocystis jirovecii pneumonia, and alpha-1 antitrypsin deficiency have all been associated with spontaneous pneumothorax.
Pulmonary Langerhans cell histiocytosis can affect multiple systems, including lung, skin, bone, and the pituitary gland. More than 90% of patients have a history of smoking.6 Chest radiography reveals a reticulonodular pattern with involvement of the middle and upper lobes. Later, the nodules tend to cavitate and form contiguous cysts that may mimic lymphangioleiomyomatosis on a high-resolution chest CT.
Cystic fibrosis is most often diagnosed before the age of 3.7 In adults, it can present as sinus and pulmonary disease (chronic cough with sputum production, chronic sinusitis with nasal polyposis, radiographic evidence of bronchiectasis and, less commonly, pneumothorax); as a gastrointestinal tract and nutritional abnormality (pancreatic insufficiency, distal intestinal obstruction, focal biliary cirrhosis); and as male infertility.
Pneumocystis jiroveciipneumonia occurs mainly in patients on chronic immunosuppressive drugs or with immune deficiency due to HIV infection. Typical radiographic features are bilateral perihilar interstitial infiltrates that become increasingly homogeneous and diffuse as the disease progresses. Less common findings include solitary or multiple nodules, upper-lobe infiltrates in patients receiving aerosolized pentamidine (NebuPent), pneumatoceles, and pneumothorax.8
Alpha-1 antitrypsin is an inhibitor of neutrophil elastase. Deficiency is associated with severe, early-onset panacinar emphysema with a basilar predominance, with chronic liver disease including cirrhosis, and less commonly with panniculitis and vasculitis associated with antineutrophil cytoplasmic antibody.9 Coalescence of panacinar emphysema leads to the formation of bullae and is important in the development of spontaneous pneumothorax.10
The patient underwent bilateral talc pleurodesis. Lung biopsy at the same time confirmed lymphangioleiomyomatosis. One month later, the right pneumothorax recurred, and she underwent pleurodesis in the right hemithorax with tetracycline. Six months after the second pleurodesis, she was asymptomatic.
- Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc 2000; 75:591–594.
- Strizheva GD, Carsillo T, Kruger WD, Sullivan EJ, Ryu JH, Henske EP. The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Respir Crit Care Med 2001; 163:253–258.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Almoosa KF, Ryu JH, Mendez J, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest 2006; 129:1274–1281.
- Benden C, Rea F, Behr J, et al. Lung transplantation for lymphangioleiomyomatosis: the European Experience. J Heart Lung Transplant 2009; 28:1–7.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Boyle MP. Adult cystic fibrosis. JAMA 2007; 298:1787–1793.
- Thomas CF, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004; 350:2487–2498.
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med 2009; 360:2749–2757.
- Anderson AE, Furlaneto JA, Foraker AG. Bronchopulmonary derangements in nonsmokers. Am Rev Respir Dis 1970; 101:518–527.
- Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc 2000; 75:591–594.
- Strizheva GD, Carsillo T, Kruger WD, Sullivan EJ, Ryu JH, Henske EP. The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Respir Crit Care Med 2001; 163:253–258.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Almoosa KF, Ryu JH, Mendez J, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest 2006; 129:1274–1281.
- Benden C, Rea F, Behr J, et al. Lung transplantation for lymphangioleiomyomatosis: the European Experience. J Heart Lung Transplant 2009; 28:1–7.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Boyle MP. Adult cystic fibrosis. JAMA 2007; 298:1787–1793.
- Thomas CF, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004; 350:2487–2498.
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med 2009; 360:2749–2757.
- Anderson AE, Furlaneto JA, Foraker AG. Bronchopulmonary derangements in nonsmokers. Am Rev Respir Dis 1970; 101:518–527.
LVH and hypertension: Is treating the pressure not enough?
In patients with “borderline” hypertension or those in whom the duration of blood pressure elevation is hard to ascertain, the finding of end-organ damage has traditionally been used as an argument to institute aggressive antihypertensive therapy. In this setting, retinal hypertensive disease, an S4 gallop, and left ventricular hypertrophy (LVH) are often specifically sought.
LVH and otherwise unexplained chronic kidney disease in patients with hypertension have generally been believed to be products of the elevated arterial pressure, and primary treatment has targeted pressure control. Bauml and Underwood, in this issue of the Journal, emphasize some published clinical trial data indicating that LVH may be an independent risk factor for poorer cardiovascular outcome. Even more provocative is the suggestion that LVH can be reversed, as can the associated increased risk of cardiovascular morbidity, independently of the hypertension.
Given our current understanding that LVH, under some conditions, can be induced by products of the renin-angiotensin system, this would suggest that pharmacologic blockade of this enzyme system should have extra benefit, above that seen from other antihypertensive agents. Conceivably, this may be true only in patients with LVH, and the time course of benefit may not directly parallel that seen with the control of hypertension. That theoretically may explain the lack of uniform advantage of angiotensin blockade over other effective antihypertensive approaches.
Since electrocardiography is a specific but not very sensitive test for LVH, the authors suggest that patients with hypertension be routinely screened for LVH using echocardiography. I am not sure the weight of the evidence supports this approach at present, particularly in the current frenzy of cost containment. Nonetheless, this concept warrants consideration, and at the least, large patient databases might be screened retrospectively to further validate or refute the concept that hypertension-associated LVH is an independent, reversible risk factor for cardiovascular morbidity.
In patients with “borderline” hypertension or those in whom the duration of blood pressure elevation is hard to ascertain, the finding of end-organ damage has traditionally been used as an argument to institute aggressive antihypertensive therapy. In this setting, retinal hypertensive disease, an S4 gallop, and left ventricular hypertrophy (LVH) are often specifically sought.
LVH and otherwise unexplained chronic kidney disease in patients with hypertension have generally been believed to be products of the elevated arterial pressure, and primary treatment has targeted pressure control. Bauml and Underwood, in this issue of the Journal, emphasize some published clinical trial data indicating that LVH may be an independent risk factor for poorer cardiovascular outcome. Even more provocative is the suggestion that LVH can be reversed, as can the associated increased risk of cardiovascular morbidity, independently of the hypertension.
Given our current understanding that LVH, under some conditions, can be induced by products of the renin-angiotensin system, this would suggest that pharmacologic blockade of this enzyme system should have extra benefit, above that seen from other antihypertensive agents. Conceivably, this may be true only in patients with LVH, and the time course of benefit may not directly parallel that seen with the control of hypertension. That theoretically may explain the lack of uniform advantage of angiotensin blockade over other effective antihypertensive approaches.
Since electrocardiography is a specific but not very sensitive test for LVH, the authors suggest that patients with hypertension be routinely screened for LVH using echocardiography. I am not sure the weight of the evidence supports this approach at present, particularly in the current frenzy of cost containment. Nonetheless, this concept warrants consideration, and at the least, large patient databases might be screened retrospectively to further validate or refute the concept that hypertension-associated LVH is an independent, reversible risk factor for cardiovascular morbidity.
In patients with “borderline” hypertension or those in whom the duration of blood pressure elevation is hard to ascertain, the finding of end-organ damage has traditionally been used as an argument to institute aggressive antihypertensive therapy. In this setting, retinal hypertensive disease, an S4 gallop, and left ventricular hypertrophy (LVH) are often specifically sought.
LVH and otherwise unexplained chronic kidney disease in patients with hypertension have generally been believed to be products of the elevated arterial pressure, and primary treatment has targeted pressure control. Bauml and Underwood, in this issue of the Journal, emphasize some published clinical trial data indicating that LVH may be an independent risk factor for poorer cardiovascular outcome. Even more provocative is the suggestion that LVH can be reversed, as can the associated increased risk of cardiovascular morbidity, independently of the hypertension.
Given our current understanding that LVH, under some conditions, can be induced by products of the renin-angiotensin system, this would suggest that pharmacologic blockade of this enzyme system should have extra benefit, above that seen from other antihypertensive agents. Conceivably, this may be true only in patients with LVH, and the time course of benefit may not directly parallel that seen with the control of hypertension. That theoretically may explain the lack of uniform advantage of angiotensin blockade over other effective antihypertensive approaches.
Since electrocardiography is a specific but not very sensitive test for LVH, the authors suggest that patients with hypertension be routinely screened for LVH using echocardiography. I am not sure the weight of the evidence supports this approach at present, particularly in the current frenzy of cost containment. Nonetheless, this concept warrants consideration, and at the least, large patient databases might be screened retrospectively to further validate or refute the concept that hypertension-associated LVH is an independent, reversible risk factor for cardiovascular morbidity.
Left ventricular hypertrophy: An overlooked cardiovascular risk factor
Left ventricular hypertrophy (LVH) strongly predicts cardiovascular morbidity and overall mortality in hypertensive patients. 1–7 Antihypertensive treatment that causes LVH to regress decreases the rates of adverse cardiovascular events and improves survival, independent of how much the blood pressure is lowered.8–11 It is clinically important to recognize that LVH is a modifiable risk factor and that management is more complex than just blood pressure control.
This paper reviews the definition of LVH, compares the diagnostic tests for it, and discusses the current evidence-based approach to managing this dangerous risk factor.
A CHRONICALLY ELEVATED CARDIAC WORKLOAD CAUSES LVH
LVH is an abnormal increase in the mass of the left ventricular myocardium caused by a chronically increased workload on the heart.12 This most commonly results from the heart pumping against an elevated afterload, as in hypertension and aortic stenosis. Another notable cause is increased filling of the left ventricle (ie, diastolic overload), which is the underlying mechanism for LVH in patients with aortic or mitral regurgitation and dilated cardiomyopathy. Coronary artery disease can also play a role in the pathogenesis of LVH, as the normal myocardium attempts to compensate for the ischemic or infarcted tissue.13
The development of myocardial fibrosis appears to be pathophysiologically linked to the renin-angiotensin-aldosterone system. Specifically, there is evidence that angiotensin II has a profibrotic effect on the myocardium of hypertensive patients.15 This may explain why angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are among the most potent agents for treating LVH, as we will discuss later in this review.
DIAGNOSIS BY ELECTROCARDIOGRAPHY, ECHOCARDIOGRAPHY, OR MRI
Many different criteria for electrocardiographic LVH have been proposed over the years. Most use the voltage in one or more leads, with or without additional factors such as QRS duration, secondary ST-T wave abnormalities, or left atrial abnormalities. The most well known electrocardiographic criteria are the Cornell voltage,21 the Cornell product,22 the Sokolow-Lyon index,23 and the Romhilt-Estes point score system (Table 1).24
- Cornell voltage—median sensitivity 15%, median specificity 96%
- Cornell product—median sensitivity 19.5%, median specificity 91%
- Sokolow-Lyon voltage—median sensitivity 21%, median specificity 89%
- Romhilt-Estes point score—median sensitivity 17%, median specificity 95%.
Of note, the ranges of the published values were extremely broad. For example, the ranges in sensitivity were:
- Cornell voltage—2% to 41%
- Cornell product—8% to 32%
- Sokolow-Lyon voltage—4% to 51%
- Romhilt-Estes point score—0% to 41%.
While the studies with the extreme values may have had issues of small sample size or poor study quality, the wide range in values may primarily be the result of diverse study populations as well as different validation methods and cutoff values to define LVH. Regardless, the overall message of high specificity and low sensitivity is indisputable.
Electrocardiography is insensitive for diagnosing LVH because it relies on measuring the electrical activity of the heart by electrodes on the surface of the skin to predict the left ventricular mass. The intracardiac electrical activity is problematic to measure externally because the measurements are affected by everything between the myocardium and the electrodes, most notably fat, fluid, and air. Because of this effect, electrocardiography underdiagnoses LVH in patients with obesity, pleural effusions, pericardial effusions, anasarca, or chronic obstructive pulmonary disease. In addition, the diagnosis of LVH by electrocardiography is strongly influenced by age and ethnicity.25–26
While electrocardiography is not sensitive and cannot be used to rule out LVH, it still has a role in its diagnosis and management. In the landmark Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study, regression of LVH (diagnosed electrocardiographically by the Sokolow-Lyon index or the Cornell product criteria) in response to losartan (Cozaar) improved cardiovascular outcomes independent of blood pressure.10 Based on this, it is reasonable that all hypertensive patients and other patients at risk of LVH who undergo electrocardiography be screened with these two criteria.
Echocardiography is the test of choice
Echocardiography, if available, should be the test of choice to assess for LVH. It is much more sensitive than electrocardiography and can also detect other abnormalities such as left ventricular dysfunction and valvular disease.
This test uses transthoracic or transesophageal ultrasonography to measure the left ventricular end-diastolic diameter, posterior wall thickness, and interventricular septum thickness. From these measurements and the patient’s height and weight, the left ventricular mass index can be calculated.27
Several different cutoff values for the left ventricular mass index have been proposed; the LIFE study used values of > 104 g/m2 in women and > 116 g/m2 in men to define LVH.
When using echocardiography to assess for LVH, it is imperative that the left ventricular mass index be used and not just the left ventricular wall thickness, as often happens in clinical practice. This is necessary because diagnosis by wall thickness alone is not a good indicator of LVH, with a concordance between wall thickness and a left ventricular mass index of only 60%.28 In addition, wall thickness tends to underestimate LVH in women and overestimate it in men.
Is echocardiography cost-effective?
Despite its clear advantages, an important consideration about echocardiography as a screening test for all hypertensive patients is its cost.
A suggested way to reduce cost is to measure the left ventricular mass index only.29 A limited echocardiographic examination is much less expensive than a complete two-dimensional echocardiogram ($255 vs $431 per the 2009 Medicare Ambulatory Payment Classification30) and should be the examination performed if the patient has no other clinical indication for echocardiography.
Another way to control cost is to stratify patients by risk and to do echocardiography only in those who would benefit most from it. Based on the prevalence of LVH, one study concluded that echocardiography is most cost-effective in men 50 years or older.31
Further study is necessary to more precisely define the cost-effectiveness of echocardiographic screening for LVH in terms of potentially preventable cardiovascular morbidity and death.
Cardiac MRI: The costly gold standard
Cardiac MRI is the gold standard test for LVH, as it is even more accurate and reproducible than echocardiography.32 It can precisely estimate a patient's left ventricular mass and assess for other structural cardiac abnormalities.
MRI’s use, however, is severely restricted in clinical practice due to its high cost and limited availability. While it may never be used for general screening for LVH, it certainly has a role in clinical research and for assessing cardiac anatomy in special clinical situations.
TREATMENT SHOULD INCLUDE AN ACE INHIBITOR OR ARB
Once LVH has been diagnosed, the next step is to decide on an appropriate treatment plan.
While the choice of therapy will always depend on other comorbidities, a 2003 metaanalysis of antihypertensive medications in the treatment of LVH (controlling for the degree of blood pressure lowering) showed that ARBs were the most efficacious class of agents for reducing the left ventricular mass.33 Specifically, ARBs decreased the mass by 13%, followed by calcium-channel blockers at 11%, ACE inhibitors at 10%, diuretics at 8%, and beta-blockers at 6%. In pairwise comparison, ARBs, calcium-channel blockers, and ACE inhibitors were all significantly more effective in reducing the left ventricular mass than beta-blockers.
As previously discussed, LVH appears to be pathophysiologically linked to myocardial fibrosis and the renin-angiotensin-aldosterone system. For this reason and based on the data presented above regarding the degree of LVH regression, ACE inhibitors or ARBs should be used as the first-line agents for LVH unless they are contraindicated in the individual patient.
The LIFE study
The LIFE study offers the strongest evidence that treating LVH is beneficial. It showed that in hypertensive patients with electrocardiographic LVH by the Cornell product or Sokolow-Lyon criteria, treatment with antihypertensive drugs that resulted in less-severe LVH on electrocardiography was associated with lower rates of cardiovascular morbidity and death, independent of the blood pressure achieved or the drug used.10
The end point in this study was a composite of stroke, myocardial infarction, and cardiovascular death. Regression of electrocardiographic LVH in hypertensive patients has also been shown to decrease the incidence of diabetes mellitus,34 atrial fibrillation,35 and hospitalizations for heart failure.36
The LIFE study also examined the prognostic implications of treating LVH detected by echocardiography. In this prospective cohort substudy, patients who had a lower left ventricular mass index during treatment with antihypertensive drugs had lower rates of cardiovascular morbidity and all-cause mortality, independent of the effects of blood pressure and treatment used.11
These results suggest that there may be a role not only for treating LVH, but also for monitoring for a reduction in the left ventricular mass index as a goal of therapy (similar to the way hemoglobin A1c is used in diabetic patients). If the index is used in this way, one could potentially adjust the dose of current drugs, switch classes, or add an additional drug based on a persistently elevated left ventricular mass index in order to optimize the patient's overall cardiovascular risk. A randomized controlled trial of therapy directed by the mass index vs conventional therapy of LVH would be necessary to assess the clinical utility of this approach.
RECOMMENDATIONS
LVH is a common and potentially modifiable cardiovascular risk factor often overlooked in clinical practice. Ideally, all hypertensive patients should be screened with echocardiography to look for LVH, using the calculated left ventricular mass index rather than wall thickness alone to make the diagnosis. While electrocardiography is specific and also has prognostic implications, it is not sensitive enough to be used alone to screen for LVH.
Once the diagnosis of LVH is made, the initial therapy should be an ARB or an ACE inhibitor. Response to therapy can be assessed by monitoring for a reduction in left ventricular mass index or regression of electrocardiographic LVH.
Treatment-induced regression of LVH decreases adverse cardiovascular events and improves overall survival. When modifying medications in hypertensive patients, it is important to remember that the treatment of LVH is not synonymous with blood pressure control.
- Casale PN, Devereux RB, Milner M, et al. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med 1986; 105:173–178.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322:1561–1566.
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991; 114:345–352.
- Verdecchia P, Carini G, Circo A, et al; MAVI (MAssa Ventricolare sinistra nell’Ipertensione) Study Group. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol 2001; 38:1829–1835.
- Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998; 32:1454–1459.
- Verdecchia P, Porcellati C, Reboldi G, et al. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 2001; 104:2039–2044.
- Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000; 35:580–586.
- Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation 1998; 97:48–54.
- Mathew J, Sleight P, Lonn E, et al; Heart Outcomes Prevention Evaluation (HOPE) Investigators. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001; 104:1615–1621.
- Okin PM, Devereux RB, Jern S, et al; LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004; 292:2343–2349.
- Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004; 292:2350–2356.
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 2000; 102:470–479.
- Zabalgoitia M, Berning J, Koren MJ, et al; LIFE Study Investigators. Impact of coronary artery disease on left ventricular systolic function and geometry in hypertensive patients with left ventricular hypertrophy (the LIFE study). Am J Cardiol 2001; 88:646–650.
- Weber KT, Janicki JS, Pick R, Capasso J, Anversa P. Myocardial fibrosis and pathologic hypertrophy in the rat with renovascular hypertension. Am J Cardiol 1990; 65:1G–7G.
- González A, López B, Querejeta R, Díez J. Regulation of myocardial fibrillar collagen by angiotensin II. A role in hypertensive heart disease? J Mol Cell Cardiol 2002; 34:1585–1593.
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002; 287:1308–1320.
- Liebson PR, Grandits G, Prineas R, et al. Echocardiographic correlates of left ventricular structure among 844 mildly hypertensive men and women in the Treatment of Mild Hypertension Study (TOMHS). Circulation 1993; 87:476–486.
- Martinez MA, Sancho T, Armada E, et al; Vascular Risk Working Group Grupo Monitorizacíon Ambulatoria de la Presión Arterial (MAPA)-Madrid. Prevalence of left ventricular hypertrophy in patients with mild hypertension in primary care: impact of echocardiography on cardiovascular risk stratification. Am J Hypertens 2003; 16:556–563.
- Pewsner D, Jüni P, Egger M, Battaglia M, Sundström J, Bachmann LM. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systematic review. BMJ 2007; 335:711.
- Devereux RB. Is the electrocardiogram still useful for detection of left ventricular hypertrophy? Circulation 1990; 81:1144–1146.
- Casale PN, Devereux RB, Kligfield P, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol 1985; 6:572–580.
- Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol 1992; 20:1180–1186.
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949; 37:161–186.
- Romhilt DW, Estes EH. A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J 1968; 75:752–758.
- Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation 1990; 81:815–820.
- Okin PM, Wright JT, Nieminen MS, et al. Ethnic differences in electrocardiographic criteria for left ventricular hypertrophy: the LIFE study. Losartan Intervention For Endpoint. Am J Hypertens 2002; 15:663–671.
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57:450–458.
- Leibowitz D, Planer D, Ben-Ibgi F, Rott D, Weiss AT, Bursztyn M. Measurement of wall thickness alone does not accurately assess the presence of left ventricular hypertrophy. Clin Exp Hypertens 2007; 29:119–125.
- Black HR, Weltin G, Jaffe CC. The limited echocardiogram: a modification of standard echocardiography for use in the routine evaluation of patients with systemic hypertension. Am J Cardiol 1991; 67:1027–1030.
- American Society of Echocardiography Coding and Reimbursement Newsletter, January 2009. http://www.asecho.org/files/public/CodingnewsJan09.pdf. Accessed May 13, 2010.
- Cuspidi C, Meani S, Valerio C, Fusi V, Sala C, Zanchetti A. Left ventricular hypertrophy and cardiovascular risk stratification: impact and cost-effectiveness of echocardiography in recently diagnosed essential hypertensives. J Hypertens 2006; 24:1671–1677.
- Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens 1995; 8:221–228.
- Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 2003; 115:41–46.
- Okin PM, Devereux RB, Harris KE, et al; LIFE Study Investigators. In-treatment resolution or absence of electrocardiographic left ventricular hypertrophy is associated with decreased incidence of new-onset diabetes mellitus in hypertensive patients: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) Study. Hypertension 2007; 50:984–990.
- Okin PM, Wachtell K, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA 2006; 296:1242–1248.
- Okin PM, Devereux RB, Harris KE, et al; LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med 2007; 147:311–319.
Left ventricular hypertrophy (LVH) strongly predicts cardiovascular morbidity and overall mortality in hypertensive patients. 1–7 Antihypertensive treatment that causes LVH to regress decreases the rates of adverse cardiovascular events and improves survival, independent of how much the blood pressure is lowered.8–11 It is clinically important to recognize that LVH is a modifiable risk factor and that management is more complex than just blood pressure control.
This paper reviews the definition of LVH, compares the diagnostic tests for it, and discusses the current evidence-based approach to managing this dangerous risk factor.
A CHRONICALLY ELEVATED CARDIAC WORKLOAD CAUSES LVH
LVH is an abnormal increase in the mass of the left ventricular myocardium caused by a chronically increased workload on the heart.12 This most commonly results from the heart pumping against an elevated afterload, as in hypertension and aortic stenosis. Another notable cause is increased filling of the left ventricle (ie, diastolic overload), which is the underlying mechanism for LVH in patients with aortic or mitral regurgitation and dilated cardiomyopathy. Coronary artery disease can also play a role in the pathogenesis of LVH, as the normal myocardium attempts to compensate for the ischemic or infarcted tissue.13
The development of myocardial fibrosis appears to be pathophysiologically linked to the renin-angiotensin-aldosterone system. Specifically, there is evidence that angiotensin II has a profibrotic effect on the myocardium of hypertensive patients.15 This may explain why angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are among the most potent agents for treating LVH, as we will discuss later in this review.
DIAGNOSIS BY ELECTROCARDIOGRAPHY, ECHOCARDIOGRAPHY, OR MRI
Many different criteria for electrocardiographic LVH have been proposed over the years. Most use the voltage in one or more leads, with or without additional factors such as QRS duration, secondary ST-T wave abnormalities, or left atrial abnormalities. The most well known electrocardiographic criteria are the Cornell voltage,21 the Cornell product,22 the Sokolow-Lyon index,23 and the Romhilt-Estes point score system (Table 1).24
- Cornell voltage—median sensitivity 15%, median specificity 96%
- Cornell product—median sensitivity 19.5%, median specificity 91%
- Sokolow-Lyon voltage—median sensitivity 21%, median specificity 89%
- Romhilt-Estes point score—median sensitivity 17%, median specificity 95%.
Of note, the ranges of the published values were extremely broad. For example, the ranges in sensitivity were:
- Cornell voltage—2% to 41%
- Cornell product—8% to 32%
- Sokolow-Lyon voltage—4% to 51%
- Romhilt-Estes point score—0% to 41%.
While the studies with the extreme values may have had issues of small sample size or poor study quality, the wide range in values may primarily be the result of diverse study populations as well as different validation methods and cutoff values to define LVH. Regardless, the overall message of high specificity and low sensitivity is indisputable.
Electrocardiography is insensitive for diagnosing LVH because it relies on measuring the electrical activity of the heart by electrodes on the surface of the skin to predict the left ventricular mass. The intracardiac electrical activity is problematic to measure externally because the measurements are affected by everything between the myocardium and the electrodes, most notably fat, fluid, and air. Because of this effect, electrocardiography underdiagnoses LVH in patients with obesity, pleural effusions, pericardial effusions, anasarca, or chronic obstructive pulmonary disease. In addition, the diagnosis of LVH by electrocardiography is strongly influenced by age and ethnicity.25–26
While electrocardiography is not sensitive and cannot be used to rule out LVH, it still has a role in its diagnosis and management. In the landmark Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study, regression of LVH (diagnosed electrocardiographically by the Sokolow-Lyon index or the Cornell product criteria) in response to losartan (Cozaar) improved cardiovascular outcomes independent of blood pressure.10 Based on this, it is reasonable that all hypertensive patients and other patients at risk of LVH who undergo electrocardiography be screened with these two criteria.
Echocardiography is the test of choice
Echocardiography, if available, should be the test of choice to assess for LVH. It is much more sensitive than electrocardiography and can also detect other abnormalities such as left ventricular dysfunction and valvular disease.
This test uses transthoracic or transesophageal ultrasonography to measure the left ventricular end-diastolic diameter, posterior wall thickness, and interventricular septum thickness. From these measurements and the patient’s height and weight, the left ventricular mass index can be calculated.27
Several different cutoff values for the left ventricular mass index have been proposed; the LIFE study used values of > 104 g/m2 in women and > 116 g/m2 in men to define LVH.
When using echocardiography to assess for LVH, it is imperative that the left ventricular mass index be used and not just the left ventricular wall thickness, as often happens in clinical practice. This is necessary because diagnosis by wall thickness alone is not a good indicator of LVH, with a concordance between wall thickness and a left ventricular mass index of only 60%.28 In addition, wall thickness tends to underestimate LVH in women and overestimate it in men.
Is echocardiography cost-effective?
Despite its clear advantages, an important consideration about echocardiography as a screening test for all hypertensive patients is its cost.
A suggested way to reduce cost is to measure the left ventricular mass index only.29 A limited echocardiographic examination is much less expensive than a complete two-dimensional echocardiogram ($255 vs $431 per the 2009 Medicare Ambulatory Payment Classification30) and should be the examination performed if the patient has no other clinical indication for echocardiography.
Another way to control cost is to stratify patients by risk and to do echocardiography only in those who would benefit most from it. Based on the prevalence of LVH, one study concluded that echocardiography is most cost-effective in men 50 years or older.31
Further study is necessary to more precisely define the cost-effectiveness of echocardiographic screening for LVH in terms of potentially preventable cardiovascular morbidity and death.
Cardiac MRI: The costly gold standard
Cardiac MRI is the gold standard test for LVH, as it is even more accurate and reproducible than echocardiography.32 It can precisely estimate a patient's left ventricular mass and assess for other structural cardiac abnormalities.
MRI’s use, however, is severely restricted in clinical practice due to its high cost and limited availability. While it may never be used for general screening for LVH, it certainly has a role in clinical research and for assessing cardiac anatomy in special clinical situations.
TREATMENT SHOULD INCLUDE AN ACE INHIBITOR OR ARB
Once LVH has been diagnosed, the next step is to decide on an appropriate treatment plan.
While the choice of therapy will always depend on other comorbidities, a 2003 metaanalysis of antihypertensive medications in the treatment of LVH (controlling for the degree of blood pressure lowering) showed that ARBs were the most efficacious class of agents for reducing the left ventricular mass.33 Specifically, ARBs decreased the mass by 13%, followed by calcium-channel blockers at 11%, ACE inhibitors at 10%, diuretics at 8%, and beta-blockers at 6%. In pairwise comparison, ARBs, calcium-channel blockers, and ACE inhibitors were all significantly more effective in reducing the left ventricular mass than beta-blockers.
As previously discussed, LVH appears to be pathophysiologically linked to myocardial fibrosis and the renin-angiotensin-aldosterone system. For this reason and based on the data presented above regarding the degree of LVH regression, ACE inhibitors or ARBs should be used as the first-line agents for LVH unless they are contraindicated in the individual patient.
The LIFE study
The LIFE study offers the strongest evidence that treating LVH is beneficial. It showed that in hypertensive patients with electrocardiographic LVH by the Cornell product or Sokolow-Lyon criteria, treatment with antihypertensive drugs that resulted in less-severe LVH on electrocardiography was associated with lower rates of cardiovascular morbidity and death, independent of the blood pressure achieved or the drug used.10
The end point in this study was a composite of stroke, myocardial infarction, and cardiovascular death. Regression of electrocardiographic LVH in hypertensive patients has also been shown to decrease the incidence of diabetes mellitus,34 atrial fibrillation,35 and hospitalizations for heart failure.36
The LIFE study also examined the prognostic implications of treating LVH detected by echocardiography. In this prospective cohort substudy, patients who had a lower left ventricular mass index during treatment with antihypertensive drugs had lower rates of cardiovascular morbidity and all-cause mortality, independent of the effects of blood pressure and treatment used.11
These results suggest that there may be a role not only for treating LVH, but also for monitoring for a reduction in the left ventricular mass index as a goal of therapy (similar to the way hemoglobin A1c is used in diabetic patients). If the index is used in this way, one could potentially adjust the dose of current drugs, switch classes, or add an additional drug based on a persistently elevated left ventricular mass index in order to optimize the patient's overall cardiovascular risk. A randomized controlled trial of therapy directed by the mass index vs conventional therapy of LVH would be necessary to assess the clinical utility of this approach.
RECOMMENDATIONS
LVH is a common and potentially modifiable cardiovascular risk factor often overlooked in clinical practice. Ideally, all hypertensive patients should be screened with echocardiography to look for LVH, using the calculated left ventricular mass index rather than wall thickness alone to make the diagnosis. While electrocardiography is specific and also has prognostic implications, it is not sensitive enough to be used alone to screen for LVH.
Once the diagnosis of LVH is made, the initial therapy should be an ARB or an ACE inhibitor. Response to therapy can be assessed by monitoring for a reduction in left ventricular mass index or regression of electrocardiographic LVH.
Treatment-induced regression of LVH decreases adverse cardiovascular events and improves overall survival. When modifying medications in hypertensive patients, it is important to remember that the treatment of LVH is not synonymous with blood pressure control.
Left ventricular hypertrophy (LVH) strongly predicts cardiovascular morbidity and overall mortality in hypertensive patients. 1–7 Antihypertensive treatment that causes LVH to regress decreases the rates of adverse cardiovascular events and improves survival, independent of how much the blood pressure is lowered.8–11 It is clinically important to recognize that LVH is a modifiable risk factor and that management is more complex than just blood pressure control.
This paper reviews the definition of LVH, compares the diagnostic tests for it, and discusses the current evidence-based approach to managing this dangerous risk factor.
A CHRONICALLY ELEVATED CARDIAC WORKLOAD CAUSES LVH
LVH is an abnormal increase in the mass of the left ventricular myocardium caused by a chronically increased workload on the heart.12 This most commonly results from the heart pumping against an elevated afterload, as in hypertension and aortic stenosis. Another notable cause is increased filling of the left ventricle (ie, diastolic overload), which is the underlying mechanism for LVH in patients with aortic or mitral regurgitation and dilated cardiomyopathy. Coronary artery disease can also play a role in the pathogenesis of LVH, as the normal myocardium attempts to compensate for the ischemic or infarcted tissue.13
The development of myocardial fibrosis appears to be pathophysiologically linked to the renin-angiotensin-aldosterone system. Specifically, there is evidence that angiotensin II has a profibrotic effect on the myocardium of hypertensive patients.15 This may explain why angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are among the most potent agents for treating LVH, as we will discuss later in this review.
DIAGNOSIS BY ELECTROCARDIOGRAPHY, ECHOCARDIOGRAPHY, OR MRI
Many different criteria for electrocardiographic LVH have been proposed over the years. Most use the voltage in one or more leads, with or without additional factors such as QRS duration, secondary ST-T wave abnormalities, or left atrial abnormalities. The most well known electrocardiographic criteria are the Cornell voltage,21 the Cornell product,22 the Sokolow-Lyon index,23 and the Romhilt-Estes point score system (Table 1).24
- Cornell voltage—median sensitivity 15%, median specificity 96%
- Cornell product—median sensitivity 19.5%, median specificity 91%
- Sokolow-Lyon voltage—median sensitivity 21%, median specificity 89%
- Romhilt-Estes point score—median sensitivity 17%, median specificity 95%.
Of note, the ranges of the published values were extremely broad. For example, the ranges in sensitivity were:
- Cornell voltage—2% to 41%
- Cornell product—8% to 32%
- Sokolow-Lyon voltage—4% to 51%
- Romhilt-Estes point score—0% to 41%.
While the studies with the extreme values may have had issues of small sample size or poor study quality, the wide range in values may primarily be the result of diverse study populations as well as different validation methods and cutoff values to define LVH. Regardless, the overall message of high specificity and low sensitivity is indisputable.
Electrocardiography is insensitive for diagnosing LVH because it relies on measuring the electrical activity of the heart by electrodes on the surface of the skin to predict the left ventricular mass. The intracardiac electrical activity is problematic to measure externally because the measurements are affected by everything between the myocardium and the electrodes, most notably fat, fluid, and air. Because of this effect, electrocardiography underdiagnoses LVH in patients with obesity, pleural effusions, pericardial effusions, anasarca, or chronic obstructive pulmonary disease. In addition, the diagnosis of LVH by electrocardiography is strongly influenced by age and ethnicity.25–26
While electrocardiography is not sensitive and cannot be used to rule out LVH, it still has a role in its diagnosis and management. In the landmark Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study, regression of LVH (diagnosed electrocardiographically by the Sokolow-Lyon index or the Cornell product criteria) in response to losartan (Cozaar) improved cardiovascular outcomes independent of blood pressure.10 Based on this, it is reasonable that all hypertensive patients and other patients at risk of LVH who undergo electrocardiography be screened with these two criteria.
Echocardiography is the test of choice
Echocardiography, if available, should be the test of choice to assess for LVH. It is much more sensitive than electrocardiography and can also detect other abnormalities such as left ventricular dysfunction and valvular disease.
This test uses transthoracic or transesophageal ultrasonography to measure the left ventricular end-diastolic diameter, posterior wall thickness, and interventricular septum thickness. From these measurements and the patient’s height and weight, the left ventricular mass index can be calculated.27
Several different cutoff values for the left ventricular mass index have been proposed; the LIFE study used values of > 104 g/m2 in women and > 116 g/m2 in men to define LVH.
When using echocardiography to assess for LVH, it is imperative that the left ventricular mass index be used and not just the left ventricular wall thickness, as often happens in clinical practice. This is necessary because diagnosis by wall thickness alone is not a good indicator of LVH, with a concordance between wall thickness and a left ventricular mass index of only 60%.28 In addition, wall thickness tends to underestimate LVH in women and overestimate it in men.
Is echocardiography cost-effective?
Despite its clear advantages, an important consideration about echocardiography as a screening test for all hypertensive patients is its cost.
A suggested way to reduce cost is to measure the left ventricular mass index only.29 A limited echocardiographic examination is much less expensive than a complete two-dimensional echocardiogram ($255 vs $431 per the 2009 Medicare Ambulatory Payment Classification30) and should be the examination performed if the patient has no other clinical indication for echocardiography.
Another way to control cost is to stratify patients by risk and to do echocardiography only in those who would benefit most from it. Based on the prevalence of LVH, one study concluded that echocardiography is most cost-effective in men 50 years or older.31
Further study is necessary to more precisely define the cost-effectiveness of echocardiographic screening for LVH in terms of potentially preventable cardiovascular morbidity and death.
Cardiac MRI: The costly gold standard
Cardiac MRI is the gold standard test for LVH, as it is even more accurate and reproducible than echocardiography.32 It can precisely estimate a patient's left ventricular mass and assess for other structural cardiac abnormalities.
MRI’s use, however, is severely restricted in clinical practice due to its high cost and limited availability. While it may never be used for general screening for LVH, it certainly has a role in clinical research and for assessing cardiac anatomy in special clinical situations.
TREATMENT SHOULD INCLUDE AN ACE INHIBITOR OR ARB
Once LVH has been diagnosed, the next step is to decide on an appropriate treatment plan.
While the choice of therapy will always depend on other comorbidities, a 2003 metaanalysis of antihypertensive medications in the treatment of LVH (controlling for the degree of blood pressure lowering) showed that ARBs were the most efficacious class of agents for reducing the left ventricular mass.33 Specifically, ARBs decreased the mass by 13%, followed by calcium-channel blockers at 11%, ACE inhibitors at 10%, diuretics at 8%, and beta-blockers at 6%. In pairwise comparison, ARBs, calcium-channel blockers, and ACE inhibitors were all significantly more effective in reducing the left ventricular mass than beta-blockers.
As previously discussed, LVH appears to be pathophysiologically linked to myocardial fibrosis and the renin-angiotensin-aldosterone system. For this reason and based on the data presented above regarding the degree of LVH regression, ACE inhibitors or ARBs should be used as the first-line agents for LVH unless they are contraindicated in the individual patient.
The LIFE study
The LIFE study offers the strongest evidence that treating LVH is beneficial. It showed that in hypertensive patients with electrocardiographic LVH by the Cornell product or Sokolow-Lyon criteria, treatment with antihypertensive drugs that resulted in less-severe LVH on electrocardiography was associated with lower rates of cardiovascular morbidity and death, independent of the blood pressure achieved or the drug used.10
The end point in this study was a composite of stroke, myocardial infarction, and cardiovascular death. Regression of electrocardiographic LVH in hypertensive patients has also been shown to decrease the incidence of diabetes mellitus,34 atrial fibrillation,35 and hospitalizations for heart failure.36
The LIFE study also examined the prognostic implications of treating LVH detected by echocardiography. In this prospective cohort substudy, patients who had a lower left ventricular mass index during treatment with antihypertensive drugs had lower rates of cardiovascular morbidity and all-cause mortality, independent of the effects of blood pressure and treatment used.11
These results suggest that there may be a role not only for treating LVH, but also for monitoring for a reduction in the left ventricular mass index as a goal of therapy (similar to the way hemoglobin A1c is used in diabetic patients). If the index is used in this way, one could potentially adjust the dose of current drugs, switch classes, or add an additional drug based on a persistently elevated left ventricular mass index in order to optimize the patient's overall cardiovascular risk. A randomized controlled trial of therapy directed by the mass index vs conventional therapy of LVH would be necessary to assess the clinical utility of this approach.
RECOMMENDATIONS
LVH is a common and potentially modifiable cardiovascular risk factor often overlooked in clinical practice. Ideally, all hypertensive patients should be screened with echocardiography to look for LVH, using the calculated left ventricular mass index rather than wall thickness alone to make the diagnosis. While electrocardiography is specific and also has prognostic implications, it is not sensitive enough to be used alone to screen for LVH.
Once the diagnosis of LVH is made, the initial therapy should be an ARB or an ACE inhibitor. Response to therapy can be assessed by monitoring for a reduction in left ventricular mass index or regression of electrocardiographic LVH.
Treatment-induced regression of LVH decreases adverse cardiovascular events and improves overall survival. When modifying medications in hypertensive patients, it is important to remember that the treatment of LVH is not synonymous with blood pressure control.
- Casale PN, Devereux RB, Milner M, et al. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med 1986; 105:173–178.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322:1561–1566.
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991; 114:345–352.
- Verdecchia P, Carini G, Circo A, et al; MAVI (MAssa Ventricolare sinistra nell’Ipertensione) Study Group. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol 2001; 38:1829–1835.
- Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998; 32:1454–1459.
- Verdecchia P, Porcellati C, Reboldi G, et al. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 2001; 104:2039–2044.
- Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000; 35:580–586.
- Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation 1998; 97:48–54.
- Mathew J, Sleight P, Lonn E, et al; Heart Outcomes Prevention Evaluation (HOPE) Investigators. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001; 104:1615–1621.
- Okin PM, Devereux RB, Jern S, et al; LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004; 292:2343–2349.
- Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004; 292:2350–2356.
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 2000; 102:470–479.
- Zabalgoitia M, Berning J, Koren MJ, et al; LIFE Study Investigators. Impact of coronary artery disease on left ventricular systolic function and geometry in hypertensive patients with left ventricular hypertrophy (the LIFE study). Am J Cardiol 2001; 88:646–650.
- Weber KT, Janicki JS, Pick R, Capasso J, Anversa P. Myocardial fibrosis and pathologic hypertrophy in the rat with renovascular hypertension. Am J Cardiol 1990; 65:1G–7G.
- González A, López B, Querejeta R, Díez J. Regulation of myocardial fibrillar collagen by angiotensin II. A role in hypertensive heart disease? J Mol Cell Cardiol 2002; 34:1585–1593.
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002; 287:1308–1320.
- Liebson PR, Grandits G, Prineas R, et al. Echocardiographic correlates of left ventricular structure among 844 mildly hypertensive men and women in the Treatment of Mild Hypertension Study (TOMHS). Circulation 1993; 87:476–486.
- Martinez MA, Sancho T, Armada E, et al; Vascular Risk Working Group Grupo Monitorizacíon Ambulatoria de la Presión Arterial (MAPA)-Madrid. Prevalence of left ventricular hypertrophy in patients with mild hypertension in primary care: impact of echocardiography on cardiovascular risk stratification. Am J Hypertens 2003; 16:556–563.
- Pewsner D, Jüni P, Egger M, Battaglia M, Sundström J, Bachmann LM. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systematic review. BMJ 2007; 335:711.
- Devereux RB. Is the electrocardiogram still useful for detection of left ventricular hypertrophy? Circulation 1990; 81:1144–1146.
- Casale PN, Devereux RB, Kligfield P, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol 1985; 6:572–580.
- Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol 1992; 20:1180–1186.
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949; 37:161–186.
- Romhilt DW, Estes EH. A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J 1968; 75:752–758.
- Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation 1990; 81:815–820.
- Okin PM, Wright JT, Nieminen MS, et al. Ethnic differences in electrocardiographic criteria for left ventricular hypertrophy: the LIFE study. Losartan Intervention For Endpoint. Am J Hypertens 2002; 15:663–671.
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57:450–458.
- Leibowitz D, Planer D, Ben-Ibgi F, Rott D, Weiss AT, Bursztyn M. Measurement of wall thickness alone does not accurately assess the presence of left ventricular hypertrophy. Clin Exp Hypertens 2007; 29:119–125.
- Black HR, Weltin G, Jaffe CC. The limited echocardiogram: a modification of standard echocardiography for use in the routine evaluation of patients with systemic hypertension. Am J Cardiol 1991; 67:1027–1030.
- American Society of Echocardiography Coding and Reimbursement Newsletter, January 2009. http://www.asecho.org/files/public/CodingnewsJan09.pdf. Accessed May 13, 2010.
- Cuspidi C, Meani S, Valerio C, Fusi V, Sala C, Zanchetti A. Left ventricular hypertrophy and cardiovascular risk stratification: impact and cost-effectiveness of echocardiography in recently diagnosed essential hypertensives. J Hypertens 2006; 24:1671–1677.
- Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens 1995; 8:221–228.
- Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 2003; 115:41–46.
- Okin PM, Devereux RB, Harris KE, et al; LIFE Study Investigators. In-treatment resolution or absence of electrocardiographic left ventricular hypertrophy is associated with decreased incidence of new-onset diabetes mellitus in hypertensive patients: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) Study. Hypertension 2007; 50:984–990.
- Okin PM, Wachtell K, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA 2006; 296:1242–1248.
- Okin PM, Devereux RB, Harris KE, et al; LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med 2007; 147:311–319.
- Casale PN, Devereux RB, Milner M, et al. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med 1986; 105:173–178.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322:1561–1566.
- Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 1991; 114:345–352.
- Verdecchia P, Carini G, Circo A, et al; MAVI (MAssa Ventricolare sinistra nell’Ipertensione) Study Group. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol 2001; 38:1829–1835.
- Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol 1998; 32:1454–1459.
- Verdecchia P, Porcellati C, Reboldi G, et al. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 2001; 104:2039–2044.
- Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 2000; 35:580–586.
- Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation 1998; 97:48–54.
- Mathew J, Sleight P, Lonn E, et al; Heart Outcomes Prevention Evaluation (HOPE) Investigators. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 2001; 104:1615–1621.
- Okin PM, Devereux RB, Jern S, et al; LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA 2004; 292:2343–2349.
- Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 2004; 292:2350–2356.
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 2000; 102:470–479.
- Zabalgoitia M, Berning J, Koren MJ, et al; LIFE Study Investigators. Impact of coronary artery disease on left ventricular systolic function and geometry in hypertensive patients with left ventricular hypertrophy (the LIFE study). Am J Cardiol 2001; 88:646–650.
- Weber KT, Janicki JS, Pick R, Capasso J, Anversa P. Myocardial fibrosis and pathologic hypertrophy in the rat with renovascular hypertension. Am J Cardiol 1990; 65:1G–7G.
- González A, López B, Querejeta R, Díez J. Regulation of myocardial fibrillar collagen by angiotensin II. A role in hypertensive heart disease? J Mol Cell Cardiol 2002; 34:1585–1593.
- Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002; 287:1308–1320.
- Liebson PR, Grandits G, Prineas R, et al. Echocardiographic correlates of left ventricular structure among 844 mildly hypertensive men and women in the Treatment of Mild Hypertension Study (TOMHS). Circulation 1993; 87:476–486.
- Martinez MA, Sancho T, Armada E, et al; Vascular Risk Working Group Grupo Monitorizacíon Ambulatoria de la Presión Arterial (MAPA)-Madrid. Prevalence of left ventricular hypertrophy in patients with mild hypertension in primary care: impact of echocardiography on cardiovascular risk stratification. Am J Hypertens 2003; 16:556–563.
- Pewsner D, Jüni P, Egger M, Battaglia M, Sundström J, Bachmann LM. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systematic review. BMJ 2007; 335:711.
- Devereux RB. Is the electrocardiogram still useful for detection of left ventricular hypertrophy? Circulation 1990; 81:1144–1146.
- Casale PN, Devereux RB, Kligfield P, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol 1985; 6:572–580.
- Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol 1992; 20:1180–1186.
- Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949; 37:161–186.
- Romhilt DW, Estes EH. A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J 1968; 75:752–758.
- Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation 1990; 81:815–820.
- Okin PM, Wright JT, Nieminen MS, et al. Ethnic differences in electrocardiographic criteria for left ventricular hypertrophy: the LIFE study. Losartan Intervention For Endpoint. Am J Hypertens 2002; 15:663–671.
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986; 57:450–458.
- Leibowitz D, Planer D, Ben-Ibgi F, Rott D, Weiss AT, Bursztyn M. Measurement of wall thickness alone does not accurately assess the presence of left ventricular hypertrophy. Clin Exp Hypertens 2007; 29:119–125.
- Black HR, Weltin G, Jaffe CC. The limited echocardiogram: a modification of standard echocardiography for use in the routine evaluation of patients with systemic hypertension. Am J Cardiol 1991; 67:1027–1030.
- American Society of Echocardiography Coding and Reimbursement Newsletter, January 2009. http://www.asecho.org/files/public/CodingnewsJan09.pdf. Accessed May 13, 2010.
- Cuspidi C, Meani S, Valerio C, Fusi V, Sala C, Zanchetti A. Left ventricular hypertrophy and cardiovascular risk stratification: impact and cost-effectiveness of echocardiography in recently diagnosed essential hypertensives. J Hypertens 2006; 24:1671–1677.
- Bottini PB, Carr AA, Prisant LM, Flickinger FW, Allison JD, Gottdiener JS. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am J Hypertens 1995; 8:221–228.
- Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med 2003; 115:41–46.
- Okin PM, Devereux RB, Harris KE, et al; LIFE Study Investigators. In-treatment resolution or absence of electrocardiographic left ventricular hypertrophy is associated with decreased incidence of new-onset diabetes mellitus in hypertensive patients: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) Study. Hypertension 2007; 50:984–990.
- Okin PM, Wachtell K, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA 2006; 296:1242–1248.
- Okin PM, Devereux RB, Harris KE, et al; LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med 2007; 147:311–319.
KEY POINTS
- LVH is caused by a chronically increased cardiac workload, most commonly from hypertension.
- Ideally, all hypertensive patients should undergo echocardiography to screen for LVH, using the calculated left ventricular mass index.
- Electrocardiography is too insensitive to be used alone to screen for LVH.
- In hypertensive patients, initial therapy of LVH should consist of an angiotensin II receptor blocker or an angiotensin-converting enzyme inhibitor.
- Treatment-induced regression of LVH improves cardiovascular outcomes independent of blood pressure.
- Further study is necessary to examine the utility of following the left ventricular mass index as a treatment goal.
Radiotherapy After Breast Cancer Surgery: Don't Wait
Grand Rounds: Woman, 80, With Hallucinations and Tremors
An 80-year-old Mandarin-speaking Chinese woman was referred to a mental health outpatient clinic for evaluation and treatment. The patient had a history of mild depression, for which she had been treated for many years with sertraline.
Five years earlier at age 75, the patient had been evaluated by a psychiatrist after she began to experience psychotic symptoms, including frequent repetitive auditory hallucinations of people counting, alternating with music from her childhood. At that time, she also had persecutory paranoid thoughts and delusional thinking that she was receiving messages in Mandarin while watching American TV programs. Initially, her only cognitive disturbance was an inability to differentiate among numbers on a calendar or a telephone keypad. No reports of memory problems were noted. Although the patient acknowledged auditory hallucinations, she denied experiencing command auditory hallucinations or hallucinations of other forms. The patient had no history of suicide attempts and denied suicidal or homicidal ideation. She had no history of psychiatric hospitalization.
The psychiatrist made a diagnosis of major depressive disorder with psychotic features, not otherwise specified1 and prescribed sertraline 50 mg/d. The patient was also started on risperidone 0.25 mg/d for management of her psychotic symptoms, with the dosage gradually increased to 2.0 mg/d over five years. While taking this combination, the patient experienced stable mood and fewer paranoid thoughts, although her auditory hallucinations continued.
Two months before the current visit, the patient moved into a retirement living facility, and she reported having adapted well to the new setting. She was sleeping well and had a good appetite. Her BMI was within normal range.
The patient described herself as a single parent for nearly 40 years, raising one daughter. Formerly high functioning, she had held a full-time clerical job until age 70. She appeared well-groomed, polite but anxious, and oriented to time, person, and place. Her speech was normal, her thought processes were coherent, and her mood was stable. However, her affect was constricted; she acknowledged auditory hallucinations, which impaired her thought content. The patient reported feeling increased anxiety prior to any nonroutine activity, such as a doctor’s appointment; this, she said, would cause insomnia, leaving her to pace in her room.
During the examination, fine tremors on upper and lower extremities were noted. The patient’s Abnormal Involuntary Movement Scale (AIMS) score2 was 13, which placed her in the highest risk category for antipsychotic-induced dopamine-blockade extrapyramidal symptoms (EPS). The patient was found to be negative for tardive dyskinesia, with no abnormal facial movements. She was aware of the tremors in her limbs and said she felt bothered by them.
The patient had an unsteady gait and used a four-point walker. Her Mini-Mental State Exam (MMSE) score3 was 28/30, which was normal for her age and education level (high school completed).
Apart from the described symptoms, the patient was healthy for her age and had no other medical diagnosis. Her vital signs were within normal range. The medical work-up to rule out other causes of dementia yielded negative results. Lab values were normal, including electrolyte levels and thyroid tests. The patient’s hearing test showed age-related hearing loss of full range, not limited to high pitch. She was able to engage in a meaningful conversation at a normal volume. Clinically, however, it was concerning to observe the possible signs of EPS and the relatively high risperidone dosage, considering the patient’s advanced age.
After the meeting with the patient, a treatment plan was created to 1) gradually reduce the dosage of antipsychotic medication, and 2) refer her to a neurologist for a complete work-up to rule out underlying neurologic disorders, such as dementia. Risperidone was tapered by increments of 0.25 mg/d every three to four weeks; throughout this process, the patient was closely monitored by the nursing staff at the retirement living facility. Monthly appointments were scheduled at the outpatient mental health clinic for evaluation and medication management.
Two months after the initial mental health clinic visit, the patient’s condition was pronounced stable on the current regimen of sertraline 50 mg/d and risperidone 1.0 mg/d. She was later seen by a neurologist, who made a diagnosis of Parkinson’s disease and placed her on carbidopa-levodopa (1 1/2 tablets, 25/100 mg, tid). The patient’s auditory hallucinations continued with the same intensity as at baseline, but fewer tremors were noted in her extremities. By six months into the tapering process (with risperidone reduced at that time to 0.25 mg/d and carbidopa-levodopa to 25/100 mg tid), the patient had begun to experience dissipation of the tremors, and her AIMS score2 was 0. She was able to replace her four-point walker with a cane.
One year after her initial visit to the mental health clinic, the patient’s neurologist suggested replacing risperidone with quetiapine (12.5 mg/d) for its improved tolerability and lower adverse effect profile.4 She continued to take sertraline and carbidopa-levodopa.
Improvement of symptoms was noted following the switch. After one month on the revised regimen, the patient reported that the number of auditory hallucinations persisted, but that their intensity had decreased dramatically. She had a brighter affect and appeared to feel uplifted and more energetic. She became involved in the social activities offered at the retirement living facility and the mental health clinic. She also maintained a steady gait without her cane. According to the patient’s daughter, her mother was at her best psychological state since the onset of psychotic symptoms six years earlier. The pharmacologic regimen had reached its maximum benefit.
At a mental health appointment at the outpatient clinic 18 months after her initial visit there, it was evident that the patient’s auditory hallucinations persisted as a major stressor. She began to complain about other residents in her facility. She said she disliked the resident with whom she shared meals, and she claimed that other residents often spit on the floor in front of her room. The nursing staff did not confirm these incidents, which they considered a delusion despite the patient’s “evidence” (the tissues she said she had used to clean up).
Additionally, a new theme had emerged in the patient’s auditory hallucinations. She reported hearing a male voice that announced changes in meal times. Although she knew there was no public address system in her room or in the hallway, the “announcement” was so convincing that she would go to the dining room and once there, realize that nothing had changed. She seemed to drift between reality and her hallucinations/delusions. According to her daughter, the patient’s independent and reserved personality forced her to internalize her stressors—in this case, her frustration about the other residents—which fed into her hallucinations and delusions.
In response to her worsening psychotic symptoms, the patient’s provider increased her quetiapine dosage from 12.5 mg/d to 25 mg/d. Her MMSE score3 at this visit was 25/30.
Two months later, the patient exhibited increasing symptoms of paranoia, delusions, and auditory hallucinations. She continued to respond to the “broadcast” messages about meal times, and she voiced her frustrations to others who spoke Mandarin. She became agitated in response to out-of-the-ordinary events. When her alarm clock battery ran out, for example, she insisted that “a man’s voice” kept reminding her to replace the battery; in response, she placed the alarm clock in the refrigerator, later explaining, “Now I don’t need to worry about it.”
Her cognitive status began to show obvious, progressive deterioration, with an MMSE score3 of 22/30 at this visit—a significant reduction from previous scores. Worsening of her short-term memory became apparent when she had difficulty playing bingo and was unable to remember her appointment or the current date. She became upset when others corrected her.
In a review of the trends in this patient’s clinical presentation, it became increasingly evident to the patient’s mental health care providers that she had Lewy body dementia.
DISCUSSION
Dementia with Lewy bodies (DLB), a progressive disease, is the second most common cause of neurodegenerative dementia after Alzheimer’s disease.5-7 It is estimated that DLB accounts for 20% of US cases of dementia (ie, about 800,000 patients).8,9 Although public awareness of DLB is on the rise, the disorder is still underrecognized and underdiagnosed because its clinical manifestations so closely resemble those of Alzheimer’s disease, Parkinson’s disease, and psychosis.10,11
Clinical symptoms of DLB include progressive cognitive decline, cognitive fluctuation, EPS, and parkinsonism; hallucinations involving all five senses, particularly sight; delusions; REM sleep disturbance, with or without vivid and frightening dreams; changes in mood and behavior; impaired judgment and insight; and autonomic dysfunction, such as orthostatic hypotension and carotid-sinus hypersensitivity.5,11-15
The symptoms of DLB are caused by the accumulations of Lewy bodies, that is, deposits of alpha-synuclein protein in the nuclei of neurons. Lewy bodies destroy neurons over time, resulting in the destruction of dopaminergic and acetylcholinergic pathways from the brain stem to areas of the cerebral cortex associated with cognition and motor functions.4,5,16
DLB is a spectrum disorder; it often coexists with Parkinson’s disease or Alzheimer’s disease, as Lewy bodies are also found in patients with these illnesses.7 This poses a challenge for formulating a differential diagnosis, particularly in patients with fluctuating cognition,10 and for attempting to establish disease prevalence.
Diagnosis
Currently, a conclusive diagnosis of DLB can be confirmed only through postmortem autopsy, although use of medial temporal lobe volume (via structural MRI) and regional blood flow (via single photon emission CT [SPECT] tracers) is being investigated.17
The diagnosis of DLB is currently based on the presenting clinical symptoms and the exclusion of other medical conditions whose symptoms mimic those of DLB.7 The screening assessment may include a neurologic/psychiatric assessment (MMSE, psychiatric evaluation, and interviews with family members or caretakers), neuroimaging such as MRI to rule out other organic causes, and laboratory evaluation to rule out potentially reversible causes of dementia, including electrolyte imbalance, vitamin deficiency (specifically vitamin B12), anemia, thyroid dysfunction, and kidney or liver impairment.18
The American Psychiatric Assocation1 categorizes DLB under “Dementia Due to Other General Medical Conditions” (294.1x). The World Health Organization19 includes it among “Other specified degenerative diseases of the nervous system” (G31.8).
Treatment
Lewy body dementia is an irreversible neurologic degenerative disorder. Treatment for DLB comprises symptom management, primarily through pharmacology; however, the response to medication is highly individualized. Treatment includes management of the following symptoms:
Cognitive impairment. Cholinesterase inhibitors, such as rivastigmine (3 to 12 mg/d), donepezil (10 mg/d), or galantamine (titrated up to 12 mg bid),20-23 improve attention and behavior and reduce apathy, anxiety, delusions, and hallucinations. As cognitive impairment worsens, memantine (10 mg bid) may be effective.24 The potential for anticholinergic adverse effects requires close monitoring in patients taking these agents.
Parkinsonian symptoms. Medications indicated for Parkinson’s disease and syndrome, such as carbidopa-levodopa (25/100 mg tid), can be effective; dosage may be slowly titrated upward as tolerated and if needed for symptom management.25,26 The dopaminergic effect of antiparkinson medications may intensify the psychotic symptoms and worsen the REM sleep pattern. In this case, a low-dose atypical antipsychotic is suggested27,28 (see below).
Psychotic symptoms. An atypical antipsychotic agent, such as quetiapine (12.5 mg), risperidone (0.25 mg), olanzapine (2.5 mg), ziprasidone (20 mg), aripiprazole (2 mg), or paliperidone (1.5 mg), may be used. Because of the DLB-associated risk of neuroleptic sensitivity, atypical antipsychotic agents should be initiated at a low dose with slow upward titration17,26,29; quetiapine appears less likely than risperidone or olanzapine to cause neuroleptic sensitivity or to trigger EPS.4 For Asian patients, who often respond to lower doses of these medications (and are more easily affected by associated adverse effects), Chen et al30 recommend a starting dose of about one-half the recommended dose.
Depression. An SSRI antidepressant with relatively simple pharmacologic properties and moderate half-life may be used to manage symptoms of depression.26,31,32 Long–half-life SSRIs (eg, fluoxetine) should be avoided in elderly patients; in response to SNRIs (serotonin-norepinephrine reuptake inhibitors), these patients may experience elevated blood pressures and pulses, with subsequent morbidity.33
REM sleep disturbances. Clonazepam (0.25 mg), melatonin (3.0 mg), or quetiapine (12.5 mg) may be administered at bedtime.34
Important Lessons
In general, providers should consider the benefits and risks of any pharmacologic treatment and avoid polypharmacy, if possible. Family and caretakers should be included in the treatment planning, with a focus on prioritizing and managing the most debilitating symptoms or dysfunctions that prompt concerns for safety.
For optimal homeostasis, some DLB patients may require joint pharmacologic modalities that appear counterintuitive—for example, an antiparkinsonism (dopaminergic) agent for parkinsonian symptoms or neuroleptic-induced EPS, versus an antipsychotic (eg, a dopamine antagonist) to treat profound hallucinations.26
As the response to treatment for DLB is highly individualized, it is essential to titrate and augment with care.
CONCLUSION
In DLB, as with other dementing illnesses, the onset of symptoms can be gradual and insidious, posing a great challenge to the clinician who seeks to confirm the diagnosis. In the illness’s early stages, the clinician may have to treat targeted symptoms and adjust the treatment plan once signs of the pathologic origins emerge.
It is critical to understand the mechanisms of psychotropic medications and targeted neurotransmitters when evaluating treatment for DLB. Titrating or augmenting these medications in elderly patients requires the clinician to follow a principle of start low and go slow, making only one change at a time.
It is always helpful to include family members in the patient’s care and to gather information on previous history, personality traits, family history, and cultural components. It is also important to communicate with other specialists to implement collaborative care.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed (text revision). Washington, DC: American Psychiatric Association; 2000:167.
2. National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS). www.atlantapsychia try.com/forms/AIMS.pdf. Accessed May 20, 2010.
3. Mini–Mental State Examination. www.nmaging .state.nm.us/pdf_files/Mini_Mental_Status_Exam.pdf. Accessed May 20, 2010.
4. Baskys A. Lewy body dementia: the litmus test for neuroleptic sensitivity and extrapyramidal symptoms. J Clin Psychiatry. 2004;65 suppl 11:16-22.
5. Weisman D, McKeith I. Dementia with Lewy bodies. Semin Neurol. 2007;27(1):42-47.
6. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3 suppl):417-423.
7. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology. 1996;47(5):1113-1124.
8. Hill C, Reiss N. Lewy body dementia (2008). www.mentalhelp.net/poc/view_doc.php?type=doc& id=13151&cn=231. Accessed May 20, 2010.
9. Lewy Body Dementia Association, Inc. Lewy body dementia: current issues in diagnosis and treatment. www.lewybodydementia.org. Accessed May 20, 2010.
10. Varanese S, Perfetti B, Monaco D, et al. Fluctuating cognition and different cognitive and behavioural profiles in Parkinson’s disease with dementia: comparison of dementia with Lewy bodies and Alzheimer’s disease. J Neurol. 2010 Jan 22. [Epub ahead of print]
11. Kurita A, Murakami M, Takagi S, et al. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disorder. 2010;25(2):167-171.
12. Gagnon JF, Postuma RB, Mazza S, et al. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5(5):424-432.
13. Dodel R, Csoti I, Ebersbach G, et al. Lewy body dementia and Parkinson’s disease with dementia. J Neurol. 2008;255 suppl 5:39-47.
14. Sonnesyn H, Nilsen DW, Rongve A, et al. High prevalence of orthostatic hypotension in mild dementia. Dement Geriatr Cogn Disord. 2009;28(4):307-313.
15. Kenny RA, Shaw FE, O’Brien JT, et al. Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiatry. 2004;75(7):966-971.
16. Hickey C, Chisholm T, Passmore MJ, et al. Differentiating the dementias: revisiting synucleinopathies and tauopathies. Curr Alzheimer Res. 2008;5(1):52-60.
17. McKeith IG, Burn DJ, Ballard CG, et al. Dementia with Lewy bodies. Semin Clin Neuropsychiatry. 2003; 8(1):46-57.
18. Bird TD, Miller BL. Dementia. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill Medical; 2008:2536-2549.
19. World Health Organization. International Classification of Diseases (ICD), Version 2007. Chapter VI: Diseases of the Central Nervous System. http://apps.who.int/classifications/apps/icd/icd10online/index.htm?kg00.htm+. Accessed May 20, 2010.
20. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
21. Emre M, Cummings JL, Lane RM. Rivastigmine in dementia associated with Parkinson’s disease and Alzheimer’s disease: similarities and differences. J Alzheimers Dis. 2007;11(4):509-519.
22. Lam B, Hollingdrake E, Kennedy JL, et al. Cholinesterase inhibitors in Alzheimer’s disease and Lewy body spectrum disorders: the emerging pharmacogenetic story. Hum Genomics. 2009;4(2):91-106.
23. Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev. 2003;(3):CD003672.
24. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
25. Merck & Co., Inc. Sinemet® CR (carbidopa-levodopa) sustained-release tablets. http://packageinserts.bms.com/pi/pi_sinemet_cr.pdf. Accessed May 20, 2010.
26. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
27. Kato K, Wada T, Kawakatsu S, Otani K. Improvement of both psychotic symptoms and Parkinsonism in a case of dementia with Lewy bodies by the combination therapy of risperidone and L-DOPA. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):201-203.
28. Yamauchi K, Takehisa M, Tsuno M, et al. Levodopa improved rapid eye movement sleep behavior disorder with diffuse Lewy body disease. Gen Hosp Psychiatry. 2003;25(2):140-142.
29. Stahl SM. The Prescriber’s Guide: Stahl’s Essential Psychopharmacology. Cambridge University Press; 2006:459.
30. Chen JP, Barron C, Lin KM, Chung H. Prescribing medication for Asians with mental disorders. West J Med. 2002;176(4):271-275.
31. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
32. Pollock BG, Mulsant BH, Rosen J, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159(3):460-465.
33. Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med. 2009;76(3):167-174.
34. Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67(5):742-747
An 80-year-old Mandarin-speaking Chinese woman was referred to a mental health outpatient clinic for evaluation and treatment. The patient had a history of mild depression, for which she had been treated for many years with sertraline.
Five years earlier at age 75, the patient had been evaluated by a psychiatrist after she began to experience psychotic symptoms, including frequent repetitive auditory hallucinations of people counting, alternating with music from her childhood. At that time, she also had persecutory paranoid thoughts and delusional thinking that she was receiving messages in Mandarin while watching American TV programs. Initially, her only cognitive disturbance was an inability to differentiate among numbers on a calendar or a telephone keypad. No reports of memory problems were noted. Although the patient acknowledged auditory hallucinations, she denied experiencing command auditory hallucinations or hallucinations of other forms. The patient had no history of suicide attempts and denied suicidal or homicidal ideation. She had no history of psychiatric hospitalization.
The psychiatrist made a diagnosis of major depressive disorder with psychotic features, not otherwise specified1 and prescribed sertraline 50 mg/d. The patient was also started on risperidone 0.25 mg/d for management of her psychotic symptoms, with the dosage gradually increased to 2.0 mg/d over five years. While taking this combination, the patient experienced stable mood and fewer paranoid thoughts, although her auditory hallucinations continued.
Two months before the current visit, the patient moved into a retirement living facility, and she reported having adapted well to the new setting. She was sleeping well and had a good appetite. Her BMI was within normal range.
The patient described herself as a single parent for nearly 40 years, raising one daughter. Formerly high functioning, she had held a full-time clerical job until age 70. She appeared well-groomed, polite but anxious, and oriented to time, person, and place. Her speech was normal, her thought processes were coherent, and her mood was stable. However, her affect was constricted; she acknowledged auditory hallucinations, which impaired her thought content. The patient reported feeling increased anxiety prior to any nonroutine activity, such as a doctor’s appointment; this, she said, would cause insomnia, leaving her to pace in her room.
During the examination, fine tremors on upper and lower extremities were noted. The patient’s Abnormal Involuntary Movement Scale (AIMS) score2 was 13, which placed her in the highest risk category for antipsychotic-induced dopamine-blockade extrapyramidal symptoms (EPS). The patient was found to be negative for tardive dyskinesia, with no abnormal facial movements. She was aware of the tremors in her limbs and said she felt bothered by them.
The patient had an unsteady gait and used a four-point walker. Her Mini-Mental State Exam (MMSE) score3 was 28/30, which was normal for her age and education level (high school completed).
Apart from the described symptoms, the patient was healthy for her age and had no other medical diagnosis. Her vital signs were within normal range. The medical work-up to rule out other causes of dementia yielded negative results. Lab values were normal, including electrolyte levels and thyroid tests. The patient’s hearing test showed age-related hearing loss of full range, not limited to high pitch. She was able to engage in a meaningful conversation at a normal volume. Clinically, however, it was concerning to observe the possible signs of EPS and the relatively high risperidone dosage, considering the patient’s advanced age.
After the meeting with the patient, a treatment plan was created to 1) gradually reduce the dosage of antipsychotic medication, and 2) refer her to a neurologist for a complete work-up to rule out underlying neurologic disorders, such as dementia. Risperidone was tapered by increments of 0.25 mg/d every three to four weeks; throughout this process, the patient was closely monitored by the nursing staff at the retirement living facility. Monthly appointments were scheduled at the outpatient mental health clinic for evaluation and medication management.
Two months after the initial mental health clinic visit, the patient’s condition was pronounced stable on the current regimen of sertraline 50 mg/d and risperidone 1.0 mg/d. She was later seen by a neurologist, who made a diagnosis of Parkinson’s disease and placed her on carbidopa-levodopa (1 1/2 tablets, 25/100 mg, tid). The patient’s auditory hallucinations continued with the same intensity as at baseline, but fewer tremors were noted in her extremities. By six months into the tapering process (with risperidone reduced at that time to 0.25 mg/d and carbidopa-levodopa to 25/100 mg tid), the patient had begun to experience dissipation of the tremors, and her AIMS score2 was 0. She was able to replace her four-point walker with a cane.
One year after her initial visit to the mental health clinic, the patient’s neurologist suggested replacing risperidone with quetiapine (12.5 mg/d) for its improved tolerability and lower adverse effect profile.4 She continued to take sertraline and carbidopa-levodopa.
Improvement of symptoms was noted following the switch. After one month on the revised regimen, the patient reported that the number of auditory hallucinations persisted, but that their intensity had decreased dramatically. She had a brighter affect and appeared to feel uplifted and more energetic. She became involved in the social activities offered at the retirement living facility and the mental health clinic. She also maintained a steady gait without her cane. According to the patient’s daughter, her mother was at her best psychological state since the onset of psychotic symptoms six years earlier. The pharmacologic regimen had reached its maximum benefit.
At a mental health appointment at the outpatient clinic 18 months after her initial visit there, it was evident that the patient’s auditory hallucinations persisted as a major stressor. She began to complain about other residents in her facility. She said she disliked the resident with whom she shared meals, and she claimed that other residents often spit on the floor in front of her room. The nursing staff did not confirm these incidents, which they considered a delusion despite the patient’s “evidence” (the tissues she said she had used to clean up).
Additionally, a new theme had emerged in the patient’s auditory hallucinations. She reported hearing a male voice that announced changes in meal times. Although she knew there was no public address system in her room or in the hallway, the “announcement” was so convincing that she would go to the dining room and once there, realize that nothing had changed. She seemed to drift between reality and her hallucinations/delusions. According to her daughter, the patient’s independent and reserved personality forced her to internalize her stressors—in this case, her frustration about the other residents—which fed into her hallucinations and delusions.
In response to her worsening psychotic symptoms, the patient’s provider increased her quetiapine dosage from 12.5 mg/d to 25 mg/d. Her MMSE score3 at this visit was 25/30.
Two months later, the patient exhibited increasing symptoms of paranoia, delusions, and auditory hallucinations. She continued to respond to the “broadcast” messages about meal times, and she voiced her frustrations to others who spoke Mandarin. She became agitated in response to out-of-the-ordinary events. When her alarm clock battery ran out, for example, she insisted that “a man’s voice” kept reminding her to replace the battery; in response, she placed the alarm clock in the refrigerator, later explaining, “Now I don’t need to worry about it.”
Her cognitive status began to show obvious, progressive deterioration, with an MMSE score3 of 22/30 at this visit—a significant reduction from previous scores. Worsening of her short-term memory became apparent when she had difficulty playing bingo and was unable to remember her appointment or the current date. She became upset when others corrected her.
In a review of the trends in this patient’s clinical presentation, it became increasingly evident to the patient’s mental health care providers that she had Lewy body dementia.
DISCUSSION
Dementia with Lewy bodies (DLB), a progressive disease, is the second most common cause of neurodegenerative dementia after Alzheimer’s disease.5-7 It is estimated that DLB accounts for 20% of US cases of dementia (ie, about 800,000 patients).8,9 Although public awareness of DLB is on the rise, the disorder is still underrecognized and underdiagnosed because its clinical manifestations so closely resemble those of Alzheimer’s disease, Parkinson’s disease, and psychosis.10,11
Clinical symptoms of DLB include progressive cognitive decline, cognitive fluctuation, EPS, and parkinsonism; hallucinations involving all five senses, particularly sight; delusions; REM sleep disturbance, with or without vivid and frightening dreams; changes in mood and behavior; impaired judgment and insight; and autonomic dysfunction, such as orthostatic hypotension and carotid-sinus hypersensitivity.5,11-15
The symptoms of DLB are caused by the accumulations of Lewy bodies, that is, deposits of alpha-synuclein protein in the nuclei of neurons. Lewy bodies destroy neurons over time, resulting in the destruction of dopaminergic and acetylcholinergic pathways from the brain stem to areas of the cerebral cortex associated with cognition and motor functions.4,5,16
DLB is a spectrum disorder; it often coexists with Parkinson’s disease or Alzheimer’s disease, as Lewy bodies are also found in patients with these illnesses.7 This poses a challenge for formulating a differential diagnosis, particularly in patients with fluctuating cognition,10 and for attempting to establish disease prevalence.
Diagnosis
Currently, a conclusive diagnosis of DLB can be confirmed only through postmortem autopsy, although use of medial temporal lobe volume (via structural MRI) and regional blood flow (via single photon emission CT [SPECT] tracers) is being investigated.17
The diagnosis of DLB is currently based on the presenting clinical symptoms and the exclusion of other medical conditions whose symptoms mimic those of DLB.7 The screening assessment may include a neurologic/psychiatric assessment (MMSE, psychiatric evaluation, and interviews with family members or caretakers), neuroimaging such as MRI to rule out other organic causes, and laboratory evaluation to rule out potentially reversible causes of dementia, including electrolyte imbalance, vitamin deficiency (specifically vitamin B12), anemia, thyroid dysfunction, and kidney or liver impairment.18
The American Psychiatric Assocation1 categorizes DLB under “Dementia Due to Other General Medical Conditions” (294.1x). The World Health Organization19 includes it among “Other specified degenerative diseases of the nervous system” (G31.8).
Treatment
Lewy body dementia is an irreversible neurologic degenerative disorder. Treatment for DLB comprises symptom management, primarily through pharmacology; however, the response to medication is highly individualized. Treatment includes management of the following symptoms:
Cognitive impairment. Cholinesterase inhibitors, such as rivastigmine (3 to 12 mg/d), donepezil (10 mg/d), or galantamine (titrated up to 12 mg bid),20-23 improve attention and behavior and reduce apathy, anxiety, delusions, and hallucinations. As cognitive impairment worsens, memantine (10 mg bid) may be effective.24 The potential for anticholinergic adverse effects requires close monitoring in patients taking these agents.
Parkinsonian symptoms. Medications indicated for Parkinson’s disease and syndrome, such as carbidopa-levodopa (25/100 mg tid), can be effective; dosage may be slowly titrated upward as tolerated and if needed for symptom management.25,26 The dopaminergic effect of antiparkinson medications may intensify the psychotic symptoms and worsen the REM sleep pattern. In this case, a low-dose atypical antipsychotic is suggested27,28 (see below).
Psychotic symptoms. An atypical antipsychotic agent, such as quetiapine (12.5 mg), risperidone (0.25 mg), olanzapine (2.5 mg), ziprasidone (20 mg), aripiprazole (2 mg), or paliperidone (1.5 mg), may be used. Because of the DLB-associated risk of neuroleptic sensitivity, atypical antipsychotic agents should be initiated at a low dose with slow upward titration17,26,29; quetiapine appears less likely than risperidone or olanzapine to cause neuroleptic sensitivity or to trigger EPS.4 For Asian patients, who often respond to lower doses of these medications (and are more easily affected by associated adverse effects), Chen et al30 recommend a starting dose of about one-half the recommended dose.
Depression. An SSRI antidepressant with relatively simple pharmacologic properties and moderate half-life may be used to manage symptoms of depression.26,31,32 Long–half-life SSRIs (eg, fluoxetine) should be avoided in elderly patients; in response to SNRIs (serotonin-norepinephrine reuptake inhibitors), these patients may experience elevated blood pressures and pulses, with subsequent morbidity.33
REM sleep disturbances. Clonazepam (0.25 mg), melatonin (3.0 mg), or quetiapine (12.5 mg) may be administered at bedtime.34
Important Lessons
In general, providers should consider the benefits and risks of any pharmacologic treatment and avoid polypharmacy, if possible. Family and caretakers should be included in the treatment planning, with a focus on prioritizing and managing the most debilitating symptoms or dysfunctions that prompt concerns for safety.
For optimal homeostasis, some DLB patients may require joint pharmacologic modalities that appear counterintuitive—for example, an antiparkinsonism (dopaminergic) agent for parkinsonian symptoms or neuroleptic-induced EPS, versus an antipsychotic (eg, a dopamine antagonist) to treat profound hallucinations.26
As the response to treatment for DLB is highly individualized, it is essential to titrate and augment with care.
CONCLUSION
In DLB, as with other dementing illnesses, the onset of symptoms can be gradual and insidious, posing a great challenge to the clinician who seeks to confirm the diagnosis. In the illness’s early stages, the clinician may have to treat targeted symptoms and adjust the treatment plan once signs of the pathologic origins emerge.
It is critical to understand the mechanisms of psychotropic medications and targeted neurotransmitters when evaluating treatment for DLB. Titrating or augmenting these medications in elderly patients requires the clinician to follow a principle of start low and go slow, making only one change at a time.
It is always helpful to include family members in the patient’s care and to gather information on previous history, personality traits, family history, and cultural components. It is also important to communicate with other specialists to implement collaborative care.
An 80-year-old Mandarin-speaking Chinese woman was referred to a mental health outpatient clinic for evaluation and treatment. The patient had a history of mild depression, for which she had been treated for many years with sertraline.
Five years earlier at age 75, the patient had been evaluated by a psychiatrist after she began to experience psychotic symptoms, including frequent repetitive auditory hallucinations of people counting, alternating with music from her childhood. At that time, she also had persecutory paranoid thoughts and delusional thinking that she was receiving messages in Mandarin while watching American TV programs. Initially, her only cognitive disturbance was an inability to differentiate among numbers on a calendar or a telephone keypad. No reports of memory problems were noted. Although the patient acknowledged auditory hallucinations, she denied experiencing command auditory hallucinations or hallucinations of other forms. The patient had no history of suicide attempts and denied suicidal or homicidal ideation. She had no history of psychiatric hospitalization.
The psychiatrist made a diagnosis of major depressive disorder with psychotic features, not otherwise specified1 and prescribed sertraline 50 mg/d. The patient was also started on risperidone 0.25 mg/d for management of her psychotic symptoms, with the dosage gradually increased to 2.0 mg/d over five years. While taking this combination, the patient experienced stable mood and fewer paranoid thoughts, although her auditory hallucinations continued.
Two months before the current visit, the patient moved into a retirement living facility, and she reported having adapted well to the new setting. She was sleeping well and had a good appetite. Her BMI was within normal range.
The patient described herself as a single parent for nearly 40 years, raising one daughter. Formerly high functioning, she had held a full-time clerical job until age 70. She appeared well-groomed, polite but anxious, and oriented to time, person, and place. Her speech was normal, her thought processes were coherent, and her mood was stable. However, her affect was constricted; she acknowledged auditory hallucinations, which impaired her thought content. The patient reported feeling increased anxiety prior to any nonroutine activity, such as a doctor’s appointment; this, she said, would cause insomnia, leaving her to pace in her room.
During the examination, fine tremors on upper and lower extremities were noted. The patient’s Abnormal Involuntary Movement Scale (AIMS) score2 was 13, which placed her in the highest risk category for antipsychotic-induced dopamine-blockade extrapyramidal symptoms (EPS). The patient was found to be negative for tardive dyskinesia, with no abnormal facial movements. She was aware of the tremors in her limbs and said she felt bothered by them.
The patient had an unsteady gait and used a four-point walker. Her Mini-Mental State Exam (MMSE) score3 was 28/30, which was normal for her age and education level (high school completed).
Apart from the described symptoms, the patient was healthy for her age and had no other medical diagnosis. Her vital signs were within normal range. The medical work-up to rule out other causes of dementia yielded negative results. Lab values were normal, including electrolyte levels and thyroid tests. The patient’s hearing test showed age-related hearing loss of full range, not limited to high pitch. She was able to engage in a meaningful conversation at a normal volume. Clinically, however, it was concerning to observe the possible signs of EPS and the relatively high risperidone dosage, considering the patient’s advanced age.
After the meeting with the patient, a treatment plan was created to 1) gradually reduce the dosage of antipsychotic medication, and 2) refer her to a neurologist for a complete work-up to rule out underlying neurologic disorders, such as dementia. Risperidone was tapered by increments of 0.25 mg/d every three to four weeks; throughout this process, the patient was closely monitored by the nursing staff at the retirement living facility. Monthly appointments were scheduled at the outpatient mental health clinic for evaluation and medication management.
Two months after the initial mental health clinic visit, the patient’s condition was pronounced stable on the current regimen of sertraline 50 mg/d and risperidone 1.0 mg/d. She was later seen by a neurologist, who made a diagnosis of Parkinson’s disease and placed her on carbidopa-levodopa (1 1/2 tablets, 25/100 mg, tid). The patient’s auditory hallucinations continued with the same intensity as at baseline, but fewer tremors were noted in her extremities. By six months into the tapering process (with risperidone reduced at that time to 0.25 mg/d and carbidopa-levodopa to 25/100 mg tid), the patient had begun to experience dissipation of the tremors, and her AIMS score2 was 0. She was able to replace her four-point walker with a cane.
One year after her initial visit to the mental health clinic, the patient’s neurologist suggested replacing risperidone with quetiapine (12.5 mg/d) for its improved tolerability and lower adverse effect profile.4 She continued to take sertraline and carbidopa-levodopa.
Improvement of symptoms was noted following the switch. After one month on the revised regimen, the patient reported that the number of auditory hallucinations persisted, but that their intensity had decreased dramatically. She had a brighter affect and appeared to feel uplifted and more energetic. She became involved in the social activities offered at the retirement living facility and the mental health clinic. She also maintained a steady gait without her cane. According to the patient’s daughter, her mother was at her best psychological state since the onset of psychotic symptoms six years earlier. The pharmacologic regimen had reached its maximum benefit.
At a mental health appointment at the outpatient clinic 18 months after her initial visit there, it was evident that the patient’s auditory hallucinations persisted as a major stressor. She began to complain about other residents in her facility. She said she disliked the resident with whom she shared meals, and she claimed that other residents often spit on the floor in front of her room. The nursing staff did not confirm these incidents, which they considered a delusion despite the patient’s “evidence” (the tissues she said she had used to clean up).
Additionally, a new theme had emerged in the patient’s auditory hallucinations. She reported hearing a male voice that announced changes in meal times. Although she knew there was no public address system in her room or in the hallway, the “announcement” was so convincing that she would go to the dining room and once there, realize that nothing had changed. She seemed to drift between reality and her hallucinations/delusions. According to her daughter, the patient’s independent and reserved personality forced her to internalize her stressors—in this case, her frustration about the other residents—which fed into her hallucinations and delusions.
In response to her worsening psychotic symptoms, the patient’s provider increased her quetiapine dosage from 12.5 mg/d to 25 mg/d. Her MMSE score3 at this visit was 25/30.
Two months later, the patient exhibited increasing symptoms of paranoia, delusions, and auditory hallucinations. She continued to respond to the “broadcast” messages about meal times, and she voiced her frustrations to others who spoke Mandarin. She became agitated in response to out-of-the-ordinary events. When her alarm clock battery ran out, for example, she insisted that “a man’s voice” kept reminding her to replace the battery; in response, she placed the alarm clock in the refrigerator, later explaining, “Now I don’t need to worry about it.”
Her cognitive status began to show obvious, progressive deterioration, with an MMSE score3 of 22/30 at this visit—a significant reduction from previous scores. Worsening of her short-term memory became apparent when she had difficulty playing bingo and was unable to remember her appointment or the current date. She became upset when others corrected her.
In a review of the trends in this patient’s clinical presentation, it became increasingly evident to the patient’s mental health care providers that she had Lewy body dementia.
DISCUSSION
Dementia with Lewy bodies (DLB), a progressive disease, is the second most common cause of neurodegenerative dementia after Alzheimer’s disease.5-7 It is estimated that DLB accounts for 20% of US cases of dementia (ie, about 800,000 patients).8,9 Although public awareness of DLB is on the rise, the disorder is still underrecognized and underdiagnosed because its clinical manifestations so closely resemble those of Alzheimer’s disease, Parkinson’s disease, and psychosis.10,11
Clinical symptoms of DLB include progressive cognitive decline, cognitive fluctuation, EPS, and parkinsonism; hallucinations involving all five senses, particularly sight; delusions; REM sleep disturbance, with or without vivid and frightening dreams; changes in mood and behavior; impaired judgment and insight; and autonomic dysfunction, such as orthostatic hypotension and carotid-sinus hypersensitivity.5,11-15
The symptoms of DLB are caused by the accumulations of Lewy bodies, that is, deposits of alpha-synuclein protein in the nuclei of neurons. Lewy bodies destroy neurons over time, resulting in the destruction of dopaminergic and acetylcholinergic pathways from the brain stem to areas of the cerebral cortex associated with cognition and motor functions.4,5,16
DLB is a spectrum disorder; it often coexists with Parkinson’s disease or Alzheimer’s disease, as Lewy bodies are also found in patients with these illnesses.7 This poses a challenge for formulating a differential diagnosis, particularly in patients with fluctuating cognition,10 and for attempting to establish disease prevalence.
Diagnosis
Currently, a conclusive diagnosis of DLB can be confirmed only through postmortem autopsy, although use of medial temporal lobe volume (via structural MRI) and regional blood flow (via single photon emission CT [SPECT] tracers) is being investigated.17
The diagnosis of DLB is currently based on the presenting clinical symptoms and the exclusion of other medical conditions whose symptoms mimic those of DLB.7 The screening assessment may include a neurologic/psychiatric assessment (MMSE, psychiatric evaluation, and interviews with family members or caretakers), neuroimaging such as MRI to rule out other organic causes, and laboratory evaluation to rule out potentially reversible causes of dementia, including electrolyte imbalance, vitamin deficiency (specifically vitamin B12), anemia, thyroid dysfunction, and kidney or liver impairment.18
The American Psychiatric Assocation1 categorizes DLB under “Dementia Due to Other General Medical Conditions” (294.1x). The World Health Organization19 includes it among “Other specified degenerative diseases of the nervous system” (G31.8).
Treatment
Lewy body dementia is an irreversible neurologic degenerative disorder. Treatment for DLB comprises symptom management, primarily through pharmacology; however, the response to medication is highly individualized. Treatment includes management of the following symptoms:
Cognitive impairment. Cholinesterase inhibitors, such as rivastigmine (3 to 12 mg/d), donepezil (10 mg/d), or galantamine (titrated up to 12 mg bid),20-23 improve attention and behavior and reduce apathy, anxiety, delusions, and hallucinations. As cognitive impairment worsens, memantine (10 mg bid) may be effective.24 The potential for anticholinergic adverse effects requires close monitoring in patients taking these agents.
Parkinsonian symptoms. Medications indicated for Parkinson’s disease and syndrome, such as carbidopa-levodopa (25/100 mg tid), can be effective; dosage may be slowly titrated upward as tolerated and if needed for symptom management.25,26 The dopaminergic effect of antiparkinson medications may intensify the psychotic symptoms and worsen the REM sleep pattern. In this case, a low-dose atypical antipsychotic is suggested27,28 (see below).
Psychotic symptoms. An atypical antipsychotic agent, such as quetiapine (12.5 mg), risperidone (0.25 mg), olanzapine (2.5 mg), ziprasidone (20 mg), aripiprazole (2 mg), or paliperidone (1.5 mg), may be used. Because of the DLB-associated risk of neuroleptic sensitivity, atypical antipsychotic agents should be initiated at a low dose with slow upward titration17,26,29; quetiapine appears less likely than risperidone or olanzapine to cause neuroleptic sensitivity or to trigger EPS.4 For Asian patients, who often respond to lower doses of these medications (and are more easily affected by associated adverse effects), Chen et al30 recommend a starting dose of about one-half the recommended dose.
Depression. An SSRI antidepressant with relatively simple pharmacologic properties and moderate half-life may be used to manage symptoms of depression.26,31,32 Long–half-life SSRIs (eg, fluoxetine) should be avoided in elderly patients; in response to SNRIs (serotonin-norepinephrine reuptake inhibitors), these patients may experience elevated blood pressures and pulses, with subsequent morbidity.33
REM sleep disturbances. Clonazepam (0.25 mg), melatonin (3.0 mg), or quetiapine (12.5 mg) may be administered at bedtime.34
Important Lessons
In general, providers should consider the benefits and risks of any pharmacologic treatment and avoid polypharmacy, if possible. Family and caretakers should be included in the treatment planning, with a focus on prioritizing and managing the most debilitating symptoms or dysfunctions that prompt concerns for safety.
For optimal homeostasis, some DLB patients may require joint pharmacologic modalities that appear counterintuitive—for example, an antiparkinsonism (dopaminergic) agent for parkinsonian symptoms or neuroleptic-induced EPS, versus an antipsychotic (eg, a dopamine antagonist) to treat profound hallucinations.26
As the response to treatment for DLB is highly individualized, it is essential to titrate and augment with care.
CONCLUSION
In DLB, as with other dementing illnesses, the onset of symptoms can be gradual and insidious, posing a great challenge to the clinician who seeks to confirm the diagnosis. In the illness’s early stages, the clinician may have to treat targeted symptoms and adjust the treatment plan once signs of the pathologic origins emerge.
It is critical to understand the mechanisms of psychotropic medications and targeted neurotransmitters when evaluating treatment for DLB. Titrating or augmenting these medications in elderly patients requires the clinician to follow a principle of start low and go slow, making only one change at a time.
It is always helpful to include family members in the patient’s care and to gather information on previous history, personality traits, family history, and cultural components. It is also important to communicate with other specialists to implement collaborative care.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed (text revision). Washington, DC: American Psychiatric Association; 2000:167.
2. National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS). www.atlantapsychia try.com/forms/AIMS.pdf. Accessed May 20, 2010.
3. Mini–Mental State Examination. www.nmaging .state.nm.us/pdf_files/Mini_Mental_Status_Exam.pdf. Accessed May 20, 2010.
4. Baskys A. Lewy body dementia: the litmus test for neuroleptic sensitivity and extrapyramidal symptoms. J Clin Psychiatry. 2004;65 suppl 11:16-22.
5. Weisman D, McKeith I. Dementia with Lewy bodies. Semin Neurol. 2007;27(1):42-47.
6. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3 suppl):417-423.
7. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology. 1996;47(5):1113-1124.
8. Hill C, Reiss N. Lewy body dementia (2008). www.mentalhelp.net/poc/view_doc.php?type=doc& id=13151&cn=231. Accessed May 20, 2010.
9. Lewy Body Dementia Association, Inc. Lewy body dementia: current issues in diagnosis and treatment. www.lewybodydementia.org. Accessed May 20, 2010.
10. Varanese S, Perfetti B, Monaco D, et al. Fluctuating cognition and different cognitive and behavioural profiles in Parkinson’s disease with dementia: comparison of dementia with Lewy bodies and Alzheimer’s disease. J Neurol. 2010 Jan 22. [Epub ahead of print]
11. Kurita A, Murakami M, Takagi S, et al. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disorder. 2010;25(2):167-171.
12. Gagnon JF, Postuma RB, Mazza S, et al. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5(5):424-432.
13. Dodel R, Csoti I, Ebersbach G, et al. Lewy body dementia and Parkinson’s disease with dementia. J Neurol. 2008;255 suppl 5:39-47.
14. Sonnesyn H, Nilsen DW, Rongve A, et al. High prevalence of orthostatic hypotension in mild dementia. Dement Geriatr Cogn Disord. 2009;28(4):307-313.
15. Kenny RA, Shaw FE, O’Brien JT, et al. Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiatry. 2004;75(7):966-971.
16. Hickey C, Chisholm T, Passmore MJ, et al. Differentiating the dementias: revisiting synucleinopathies and tauopathies. Curr Alzheimer Res. 2008;5(1):52-60.
17. McKeith IG, Burn DJ, Ballard CG, et al. Dementia with Lewy bodies. Semin Clin Neuropsychiatry. 2003; 8(1):46-57.
18. Bird TD, Miller BL. Dementia. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill Medical; 2008:2536-2549.
19. World Health Organization. International Classification of Diseases (ICD), Version 2007. Chapter VI: Diseases of the Central Nervous System. http://apps.who.int/classifications/apps/icd/icd10online/index.htm?kg00.htm+. Accessed May 20, 2010.
20. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
21. Emre M, Cummings JL, Lane RM. Rivastigmine in dementia associated with Parkinson’s disease and Alzheimer’s disease: similarities and differences. J Alzheimers Dis. 2007;11(4):509-519.
22. Lam B, Hollingdrake E, Kennedy JL, et al. Cholinesterase inhibitors in Alzheimer’s disease and Lewy body spectrum disorders: the emerging pharmacogenetic story. Hum Genomics. 2009;4(2):91-106.
23. Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev. 2003;(3):CD003672.
24. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
25. Merck & Co., Inc. Sinemet® CR (carbidopa-levodopa) sustained-release tablets. http://packageinserts.bms.com/pi/pi_sinemet_cr.pdf. Accessed May 20, 2010.
26. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
27. Kato K, Wada T, Kawakatsu S, Otani K. Improvement of both psychotic symptoms and Parkinsonism in a case of dementia with Lewy bodies by the combination therapy of risperidone and L-DOPA. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):201-203.
28. Yamauchi K, Takehisa M, Tsuno M, et al. Levodopa improved rapid eye movement sleep behavior disorder with diffuse Lewy body disease. Gen Hosp Psychiatry. 2003;25(2):140-142.
29. Stahl SM. The Prescriber’s Guide: Stahl’s Essential Psychopharmacology. Cambridge University Press; 2006:459.
30. Chen JP, Barron C, Lin KM, Chung H. Prescribing medication for Asians with mental disorders. West J Med. 2002;176(4):271-275.
31. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
32. Pollock BG, Mulsant BH, Rosen J, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159(3):460-465.
33. Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med. 2009;76(3):167-174.
34. Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67(5):742-747
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed (text revision). Washington, DC: American Psychiatric Association; 2000:167.
2. National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS). www.atlantapsychia try.com/forms/AIMS.pdf. Accessed May 20, 2010.
3. Mini–Mental State Examination. www.nmaging .state.nm.us/pdf_files/Mini_Mental_Status_Exam.pdf. Accessed May 20, 2010.
4. Baskys A. Lewy body dementia: the litmus test for neuroleptic sensitivity and extrapyramidal symptoms. J Clin Psychiatry. 2004;65 suppl 11:16-22.
5. Weisman D, McKeith I. Dementia with Lewy bodies. Semin Neurol. 2007;27(1):42-47.
6. McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3 suppl):417-423.
7. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology. 1996;47(5):1113-1124.
8. Hill C, Reiss N. Lewy body dementia (2008). www.mentalhelp.net/poc/view_doc.php?type=doc& id=13151&cn=231. Accessed May 20, 2010.
9. Lewy Body Dementia Association, Inc. Lewy body dementia: current issues in diagnosis and treatment. www.lewybodydementia.org. Accessed May 20, 2010.
10. Varanese S, Perfetti B, Monaco D, et al. Fluctuating cognition and different cognitive and behavioural profiles in Parkinson’s disease with dementia: comparison of dementia with Lewy bodies and Alzheimer’s disease. J Neurol. 2010 Jan 22. [Epub ahead of print]
11. Kurita A, Murakami M, Takagi S, et al. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disorder. 2010;25(2):167-171.
12. Gagnon JF, Postuma RB, Mazza S, et al. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol. 2006;5(5):424-432.
13. Dodel R, Csoti I, Ebersbach G, et al. Lewy body dementia and Parkinson’s disease with dementia. J Neurol. 2008;255 suppl 5:39-47.
14. Sonnesyn H, Nilsen DW, Rongve A, et al. High prevalence of orthostatic hypotension in mild dementia. Dement Geriatr Cogn Disord. 2009;28(4):307-313.
15. Kenny RA, Shaw FE, O’Brien JT, et al. Carotid sinus syndrome is common in dementia with Lewy bodies and correlates with deep white matter lesions. J Neurol Neurosurg Psychiatry. 2004;75(7):966-971.
16. Hickey C, Chisholm T, Passmore MJ, et al. Differentiating the dementias: revisiting synucleinopathies and tauopathies. Curr Alzheimer Res. 2008;5(1):52-60.
17. McKeith IG, Burn DJ, Ballard CG, et al. Dementia with Lewy bodies. Semin Clin Neuropsychiatry. 2003; 8(1):46-57.
18. Bird TD, Miller BL. Dementia. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw Hill Medical; 2008:2536-2549.
19. World Health Organization. International Classification of Diseases (ICD), Version 2007. Chapter VI: Diseases of the Central Nervous System. http://apps.who.int/classifications/apps/icd/icd10online/index.htm?kg00.htm+. Accessed May 20, 2010.
20. McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
21. Emre M, Cummings JL, Lane RM. Rivastigmine in dementia associated with Parkinson’s disease and Alzheimer’s disease: similarities and differences. J Alzheimers Dis. 2007;11(4):509-519.
22. Lam B, Hollingdrake E, Kennedy JL, et al. Cholinesterase inhibitors in Alzheimer’s disease and Lewy body spectrum disorders: the emerging pharmacogenetic story. Hum Genomics. 2009;4(2):91-106.
23. Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database Syst Rev. 2003;(3):CD003672.
24. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613-618.
25. Merck & Co., Inc. Sinemet® CR (carbidopa-levodopa) sustained-release tablets. http://packageinserts.bms.com/pi/pi_sinemet_cr.pdf. Accessed May 20, 2010.
26. Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027-2037.
27. Kato K, Wada T, Kawakatsu S, Otani K. Improvement of both psychotic symptoms and Parkinsonism in a case of dementia with Lewy bodies by the combination therapy of risperidone and L-DOPA. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):201-203.
28. Yamauchi K, Takehisa M, Tsuno M, et al. Levodopa improved rapid eye movement sleep behavior disorder with diffuse Lewy body disease. Gen Hosp Psychiatry. 2003;25(2):140-142.
29. Stahl SM. The Prescriber’s Guide: Stahl’s Essential Psychopharmacology. Cambridge University Press; 2006:459.
30. Chen JP, Barron C, Lin KM, Chung H. Prescribing medication for Asians with mental disorders. West J Med. 2002;176(4):271-275.
31. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
32. Pollock BG, Mulsant BH, Rosen J, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159(3):460-465.
33. Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med. 2009;76(3):167-174.
34. Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67(5):742-747