User login
Admit Documentation
In light of the recent elimination of consultation codes from the Medicare Physician Fee Schedule, physicians of all specialties are being asked to report initial hospital care services (99221-99223) for their first encounter with a patient.1 This leaves hospitalists with questions about the billing and financial implications of reporting admissions services.
Here’s a typical scenario: Dr. A admits a Medicare patient to the hospital from the ED for hyperglycemia and dehydration in the setting of uncontrolled diabetes. He performs and documents an initial hospital-care service on day one of the admission. On day two, another hospitalist, Dr. B, who works in the same HM group, sees the patient for the first time. What should each of the physicians report for their first encounter with the patient?
Each hospitalist should select the CPT code that best fits the service and their role in the case. Remember, only one physician is named “attending of record” or “admitting physician.”
When billing during the course of the hospitalization, consider all physicians of the same specialty in the same provider group as the “admitting physician/group.”
Admissions Service
On day one, Dr. A admits the patient. He performs and documents a comprehensive history, a comprehensive exam, and medical decision-making of high complexity. The documentation corresponds to the highest initial admission service, 99223. Given the recent Medicare billing changes, the attending of record is required to append modifier “AI” (principal physician of record) to the admission service (e.g., 99223-AI).
The purpose of this modifier is “to identify the physician who oversees the patient’s care from all other physicians who may be furnishing specialty care.”2 This modifier has no financial implications. It does not increase or decrease the payment associated with the reported visit level (i.e., 99223 is reimbursed at a national rate of approximately $190, with or without modifier AI).
Initial Encounter by Team Members
As previously stated, the elimination of consultation services requires physicians to report their initial hospital encounter with an initial hospital-care code (i.e., 99221-99223). However, Medicare states that “physicians in the same group practice who are in the same specialty must bill and be paid as though they were a single physician.”3 This means followup services performed on days subsequent to a group member’s initial admission service must be reported with subsequent hospital-care codes (99231-99233). Therefore, in the scenario above, Dr. B is obligated to report the appropriate subsequent hospital-care code for his patient encounter on day two.
Incomplete Documentation
Initial hospital-care services (99221-99223) require the physician to obtain, perform, and document the necessary elements of history, physical exam, and medical decision-making in support of the code reported on the claim. There are occasions when the physician’s documentation does not support the lowest code (i.e., 99221). A reasonable approach is to report the service with an unlisted E&M code (99499). “Unlisted” codes do not have a payor-recognized code description or fee. When reporting an unlisted code, the biller must manually enter a charge description (e.g., expanded problem-focused admissions service) and a fee. A payor-prompted request for documentation is likely before payment is made.
Some payors have more specific references to the situation and allow for options. Two options exist for coding services that do not meet the work and/or medical necessity requirements of 99221-99223: report an unlisted E&M service (99499); or report a subsequent hospital care code (99231-99233) that appropriately reflects physician work and medical necessity for the service, and avoids mandatory medical record submission and manual medical review.4
In fact, Medicare Administrator Contractor TrailBlazer Health’s Web site (www.trailblazerhealth.com) offers guidance to physicians who are unsure if subsequent hospital care is an appropriate choice for this dilemma: “TrailBlazer recognizes provider reluctance to miscode initial hospital care as subsequent hospital care. However, doing so is preferable in that it allows Medicare to process and pay the claims much more efficiently. For those concerned about miscoding these services, please understand that TrailBlazer will not find fault with providers who choose this option when records appropriately demonstrate the work and medical necessity of the subsequent code chosen.”4 TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is faculty for SHM’s inpatient coding course.
References
- CMS announces payment, policy changes for physicians services to Medicare beneficiaries in 2010. Centers for Medicare and Medicaid Services Web site. Available at: www.cms.hhs.gov/apps/media/ press/release.asp?Counter=3539&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=1%2C+2%2C+3%2C+4%2C+5&intPage=&showAll=&pYear=&year=&desc=&cboOrder=date. Accessed Nov. 12, 2009.
- Revisions to Consultation Services Payment Policy. Medicare Learning Network Web site. Available at: www.cms.hhs.gov/MLNMattersArticles/downloads/ MM6740.pdf. Accessed Jan. 16, 2010.
- Medicare Claims Processing Manual: Chapter 12, Section 30.6.5. CMS Web site. Available at: www.cms.hhs.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 16, 2010.
- Update-evaluation and management services formerly coded as consultations. Trailblazer Health Enterprises Web site. Available at: www.trailblazerhealth.com/Tools/Notices.aspx?DomainID=1. Accessed Jan. 17, 2010.
- Beebe M, Dalton J, Espronceda M, Evans D, Glenn R. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2009;14-15.
In light of the recent elimination of consultation codes from the Medicare Physician Fee Schedule, physicians of all specialties are being asked to report initial hospital care services (99221-99223) for their first encounter with a patient.1 This leaves hospitalists with questions about the billing and financial implications of reporting admissions services.
Here’s a typical scenario: Dr. A admits a Medicare patient to the hospital from the ED for hyperglycemia and dehydration in the setting of uncontrolled diabetes. He performs and documents an initial hospital-care service on day one of the admission. On day two, another hospitalist, Dr. B, who works in the same HM group, sees the patient for the first time. What should each of the physicians report for their first encounter with the patient?
Each hospitalist should select the CPT code that best fits the service and their role in the case. Remember, only one physician is named “attending of record” or “admitting physician.”
When billing during the course of the hospitalization, consider all physicians of the same specialty in the same provider group as the “admitting physician/group.”
Admissions Service
On day one, Dr. A admits the patient. He performs and documents a comprehensive history, a comprehensive exam, and medical decision-making of high complexity. The documentation corresponds to the highest initial admission service, 99223. Given the recent Medicare billing changes, the attending of record is required to append modifier “AI” (principal physician of record) to the admission service (e.g., 99223-AI).
The purpose of this modifier is “to identify the physician who oversees the patient’s care from all other physicians who may be furnishing specialty care.”2 This modifier has no financial implications. It does not increase or decrease the payment associated with the reported visit level (i.e., 99223 is reimbursed at a national rate of approximately $190, with or without modifier AI).
Initial Encounter by Team Members
As previously stated, the elimination of consultation services requires physicians to report their initial hospital encounter with an initial hospital-care code (i.e., 99221-99223). However, Medicare states that “physicians in the same group practice who are in the same specialty must bill and be paid as though they were a single physician.”3 This means followup services performed on days subsequent to a group member’s initial admission service must be reported with subsequent hospital-care codes (99231-99233). Therefore, in the scenario above, Dr. B is obligated to report the appropriate subsequent hospital-care code for his patient encounter on day two.
Incomplete Documentation
Initial hospital-care services (99221-99223) require the physician to obtain, perform, and document the necessary elements of history, physical exam, and medical decision-making in support of the code reported on the claim. There are occasions when the physician’s documentation does not support the lowest code (i.e., 99221). A reasonable approach is to report the service with an unlisted E&M code (99499). “Unlisted” codes do not have a payor-recognized code description or fee. When reporting an unlisted code, the biller must manually enter a charge description (e.g., expanded problem-focused admissions service) and a fee. A payor-prompted request for documentation is likely before payment is made.
Some payors have more specific references to the situation and allow for options. Two options exist for coding services that do not meet the work and/or medical necessity requirements of 99221-99223: report an unlisted E&M service (99499); or report a subsequent hospital care code (99231-99233) that appropriately reflects physician work and medical necessity for the service, and avoids mandatory medical record submission and manual medical review.4
In fact, Medicare Administrator Contractor TrailBlazer Health’s Web site (www.trailblazerhealth.com) offers guidance to physicians who are unsure if subsequent hospital care is an appropriate choice for this dilemma: “TrailBlazer recognizes provider reluctance to miscode initial hospital care as subsequent hospital care. However, doing so is preferable in that it allows Medicare to process and pay the claims much more efficiently. For those concerned about miscoding these services, please understand that TrailBlazer will not find fault with providers who choose this option when records appropriately demonstrate the work and medical necessity of the subsequent code chosen.”4 TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is faculty for SHM’s inpatient coding course.
References
- CMS announces payment, policy changes for physicians services to Medicare beneficiaries in 2010. Centers for Medicare and Medicaid Services Web site. Available at: www.cms.hhs.gov/apps/media/ press/release.asp?Counter=3539&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=1%2C+2%2C+3%2C+4%2C+5&intPage=&showAll=&pYear=&year=&desc=&cboOrder=date. Accessed Nov. 12, 2009.
- Revisions to Consultation Services Payment Policy. Medicare Learning Network Web site. Available at: www.cms.hhs.gov/MLNMattersArticles/downloads/ MM6740.pdf. Accessed Jan. 16, 2010.
- Medicare Claims Processing Manual: Chapter 12, Section 30.6.5. CMS Web site. Available at: www.cms.hhs.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 16, 2010.
- Update-evaluation and management services formerly coded as consultations. Trailblazer Health Enterprises Web site. Available at: www.trailblazerhealth.com/Tools/Notices.aspx?DomainID=1. Accessed Jan. 17, 2010.
- Beebe M, Dalton J, Espronceda M, Evans D, Glenn R. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2009;14-15.
In light of the recent elimination of consultation codes from the Medicare Physician Fee Schedule, physicians of all specialties are being asked to report initial hospital care services (99221-99223) for their first encounter with a patient.1 This leaves hospitalists with questions about the billing and financial implications of reporting admissions services.
Here’s a typical scenario: Dr. A admits a Medicare patient to the hospital from the ED for hyperglycemia and dehydration in the setting of uncontrolled diabetes. He performs and documents an initial hospital-care service on day one of the admission. On day two, another hospitalist, Dr. B, who works in the same HM group, sees the patient for the first time. What should each of the physicians report for their first encounter with the patient?
Each hospitalist should select the CPT code that best fits the service and their role in the case. Remember, only one physician is named “attending of record” or “admitting physician.”
When billing during the course of the hospitalization, consider all physicians of the same specialty in the same provider group as the “admitting physician/group.”
Admissions Service
On day one, Dr. A admits the patient. He performs and documents a comprehensive history, a comprehensive exam, and medical decision-making of high complexity. The documentation corresponds to the highest initial admission service, 99223. Given the recent Medicare billing changes, the attending of record is required to append modifier “AI” (principal physician of record) to the admission service (e.g., 99223-AI).
The purpose of this modifier is “to identify the physician who oversees the patient’s care from all other physicians who may be furnishing specialty care.”2 This modifier has no financial implications. It does not increase or decrease the payment associated with the reported visit level (i.e., 99223 is reimbursed at a national rate of approximately $190, with or without modifier AI).
Initial Encounter by Team Members
As previously stated, the elimination of consultation services requires physicians to report their initial hospital encounter with an initial hospital-care code (i.e., 99221-99223). However, Medicare states that “physicians in the same group practice who are in the same specialty must bill and be paid as though they were a single physician.”3 This means followup services performed on days subsequent to a group member’s initial admission service must be reported with subsequent hospital-care codes (99231-99233). Therefore, in the scenario above, Dr. B is obligated to report the appropriate subsequent hospital-care code for his patient encounter on day two.
Incomplete Documentation
Initial hospital-care services (99221-99223) require the physician to obtain, perform, and document the necessary elements of history, physical exam, and medical decision-making in support of the code reported on the claim. There are occasions when the physician’s documentation does not support the lowest code (i.e., 99221). A reasonable approach is to report the service with an unlisted E&M code (99499). “Unlisted” codes do not have a payor-recognized code description or fee. When reporting an unlisted code, the biller must manually enter a charge description (e.g., expanded problem-focused admissions service) and a fee. A payor-prompted request for documentation is likely before payment is made.
Some payors have more specific references to the situation and allow for options. Two options exist for coding services that do not meet the work and/or medical necessity requirements of 99221-99223: report an unlisted E&M service (99499); or report a subsequent hospital care code (99231-99233) that appropriately reflects physician work and medical necessity for the service, and avoids mandatory medical record submission and manual medical review.4
In fact, Medicare Administrator Contractor TrailBlazer Health’s Web site (www.trailblazerhealth.com) offers guidance to physicians who are unsure if subsequent hospital care is an appropriate choice for this dilemma: “TrailBlazer recognizes provider reluctance to miscode initial hospital care as subsequent hospital care. However, doing so is preferable in that it allows Medicare to process and pay the claims much more efficiently. For those concerned about miscoding these services, please understand that TrailBlazer will not find fault with providers who choose this option when records appropriately demonstrate the work and medical necessity of the subsequent code chosen.”4 TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is faculty for SHM’s inpatient coding course.
References
- CMS announces payment, policy changes for physicians services to Medicare beneficiaries in 2010. Centers for Medicare and Medicaid Services Web site. Available at: www.cms.hhs.gov/apps/media/ press/release.asp?Counter=3539&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=1%2C+2%2C+3%2C+4%2C+5&intPage=&showAll=&pYear=&year=&desc=&cboOrder=date. Accessed Nov. 12, 2009.
- Revisions to Consultation Services Payment Policy. Medicare Learning Network Web site. Available at: www.cms.hhs.gov/MLNMattersArticles/downloads/ MM6740.pdf. Accessed Jan. 16, 2010.
- Medicare Claims Processing Manual: Chapter 12, Section 30.6.5. CMS Web site. Available at: www.cms.hhs.gov/manuals/downloads/clm104c12.pdf. Accessed Jan. 16, 2010.
- Update-evaluation and management services formerly coded as consultations. Trailblazer Health Enterprises Web site. Available at: www.trailblazerhealth.com/Tools/Notices.aspx?DomainID=1. Accessed Jan. 17, 2010.
- Beebe M, Dalton J, Espronceda M, Evans D, Glenn R. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2009;14-15.
When Should an IVC Filter Be Used to Treat a DVT?
Case
A 67-year-old man with a history of hypertension presents with a swollen right lower extremity. An ultrasound reveals a DVT, and he is commenced on low-molecular-weight heparin and warfarin. Two days later, he develops slurred speech and right-sided weakness. A head CT reveals an intracranial hemorrhage. When should an inferior vena cava (IVC) filter be utilized for treatment of DVT?
Overview
It is estimated that 350,000 to 600,000 Americans develop a VTE each year.1 Patients with a DVT are at high risk of developing a pulmonary embolism (PE). In a multicenter study, nearly 40% of patients admitted with a DVT had evidence of a PE on ventilation perfusion scan.2 Treatment of a DVT is aimed at preventing the extension of the DVT and embolization.3 The American College of Chest Physicians (ACCP) recommends anticoagulation as the primary DVT treatment (Grade 1A).4 However, IVC filters might be considered when anticoagulation is contraindicated.
In 1868, Trousseau created the conceptual model of surgical interruption of the IVC to prevent PE. However, it wasn’t until 1959 by Bottini that the surgical interruption was successfully performed.5 The Mobin-Uddin filter was introduced in 1967 as the first mechanical IVC filter.6 IVC filters mechanically trap the DVT, preventing emboli from traveling into the pulmonary vasculature.7
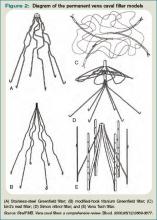
There are two classes of IVC filters: permanent filters and removable filters. Removable filters include both temporary filters and retrievable filters. Temporary filters are attached to a catheter that exits the skin and therefore must be removed due to the risk of infection and embolization.7 Retrievable filters are similar in design to permanent filters but are designed to be removed. However, this must be done with caution, as neointimal hyperplasia can prevent removal or cause vessel wall damage upon removal.8
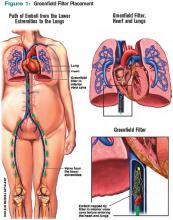
IVC filters are inserted into the vena cava percutaneously via the femoral or jugular approach under fluoroscopy or ultrasound guidance (see Figure 1, p. 16). The filters typically are placed infrarenally, unless there is an indication for a suprarenal filter (e.g., renal vein thrombosis or IVC thrombus extending above the renal veins).7 Complete IVC thrombosis is an absolute contraindication to IVC filter placement, and the relative contraindications include significant coagulopathy and bacteremia.9
The incidence of complications related to IVC filter placement is 4% to 11%. Complications include:
- Insertion-site thrombosis;
- IVC thrombosis;
- Recurrent DVT postphlebitic syndrome;
- Filter migration;
- Erosion of the filter through the vessel wall; and
- Vena caval obstruction.10
A review of the National Hospital Discharge Survey database for trends in IVC filter use in the U.S. found a dramatic increase in the use of IVC filters from 1979 to 1999—to 49,000 patients from 2,000 patients with IVC filters in place. The indications for IVC filter use vary such that it is imperative there are well-designed trials and guidelines to guide appropriate use.11
The Evidence
The 2008 ACCP guidelines on VTE management follow a grading system that classifies recommendations as Grade 1 (strong) or Grade 2 (weak), and classifies the quality of evidence as A (high), B (moderate), or C (low).12 The ACCP guidelines’ recommended first-line treatment for a confirmed DVT is anticoagulation with subcutaneous low-molecular-weight heparin, intravenous unfractionated heparin, monitored subcutaneous heparin, fixed-dose subcutaneous unfractionated heparin, or subcutaneous fondaparinux (all Grade 1A recommendations). The ACCP recommends against the routine use of an IVC filter in addition to anticoagulants (Grade 1A). However, for patients with acute proximal DVT, if anticoagulant therapy is not possible because of the risk of bleeding, IVC filter placement is recommended (Grade 1C). If a patient requires an IVC filter for treatment of an acute DVT as an alternative to anticoagulation, it is recommended to start anticoagulant therapy once the risk of bleeding resolves (Grade 1C).4
The 2008 ACCP guidelines for IVC filter use have a few important changes from the 2004 version. First, the IVC filter placement recommendation for patients with contraindications to anticoagulation was strengthened from Grade 2C to Grade 1C. Second, the 2008 guidelines omitted the early recommendation of IVC filter use for recurrent VTE, despite adequate anticoagulation (Grade 2C).13
Only one randomized study has evaluated the efficacy of IVC filters. All other studies of IVC filters are retrospective or prospective case series.
The PREPIC study randomized 400 patients with proximal DVT considered to be at high risk for PE to receive either an IVC filter or no IVC filter. Additionally, patients were randomized to receive enoxaparin or unfractionated heparin as a bridge to warfarin therapy, which was continued for at least three months. The primary endpoints were recurrent DVT, PE, major bleeding, or death. The patients were followed up at day 12, two years, and then annually up to eight years following randomization.14 At day 12, there were fewer PEs in the group that received filters (OR 0.22, 95% CI, 0.05-0.90). However, at year two, there was no significant difference in PE development in the filter group compared with the no-filter group (OR 0.50, 95% CI, 0.19-1.33).
Additionally, at year two, the filter group was more likely to develop recurrent DVT (OR 1.87, 95% CI, 1.10-3.20). At year eight, there was a significant reduction in the number of PEs in the filter group versus the no-filter group (6.2% vs.15.1%, P=0.008). However, at eight-year followup, IVC filter use was associated with increased DVT (35.7% vs. 27.5%, P=0.042). There was no difference in mortality between the two groups.
In summary, the use of IVC filters was associated with decreased incidence of PE at eight years, offset by higher rates of recurrent DVT and no overall mortality benefit.14,15 Importantly, the indications for IVC filter use in this study differ from the current ACCP guidelines; all patients were given concomitant anticoagulation for at least three months, which might not be possible in patients for whom the ACCP recommends IVC filters.
There are no randomized studies to compare the efficacy of permanent IVC filters and retrievable filters for PE prevention. A retrospective study comparing the clinical effectiveness of the two filter types reported no difference in the rates of symptomatic PE (permanent filter 4% vs. retrievable filter 4.7%, P=0.67) or DVT (11.3% vs. 12.6%, P=0.59). In addition, the frequency of symptomatic IVC thrombosis was similar (1.1% vs. 0.5%, p=0.39).16 A paper reviewing the efficacy of IVC filters reported that permanent filters were associated with a 0%-6.2% rate of PE versus a 0%-1.9% rate with retrievable filters.7 Notably, these studies were not randomized controlled trials—rather, case series—and the indications for IVC filters were not necessarily those currently recommended by the ACCP.
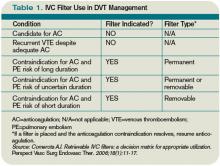
Due to the long-term complications of permanent IVC filters, it is suggested that a retrievable IVC filter be used for patients with temporary contraindications to anticoagulation.17 Comerata et al created a clinical decision-making tool for picking the type of filter to employ. If the duration of contraindication to anticoagulation is short or uncertain, a retrievable filter is recommended.18 Table 1 (p. 15) outlines the recommendations for IVC filter placement.
There are no randomized controlled trials to guide the use of concomitant anticoagulation after filter insertion, although this intervention may be beneficial to prevent DVT propagation, recurrence, or IVC filter thrombosis.5 A meta-analysis of 14 studies evaluating the rates of VTE after IVC filter placement demonstrated a non-statistically significant trend toward fewer VTE events in the patients with an IVC filter and concomitant anticoagulation in comparison with those who solely had an IVC filter (OR 0.64, 95% CI, 0.35-1.2). The duration and degree of anticoagulation was not presented in all of the studies in the meta-analysis, therefore limiting the analysis.19
In addition to the ACCP guidelines, there have been other proposed indications for IVC filter use, including recurrent VTE despite anticoagulation, chronic recurrent PE with pulmonary hypertension, extensive free-floating iliofemoral thrombus, and thrombolysis of ilio-caval thrombus.20 The ACCP guidelines do not specifically address these individual indications, and at this time there are no randomized controlled trials to guide IVC filter use in these cases.
Back to the Case
Our patient developed a significant complication from anticoagulation. Current ACCP guidelines recommend an IVC filter if anticoagulant therapy is contraindicated (Grade 1C). The anticoagulation was discontinued and a retrievable IVC filter was placed. Once a patient no longer has a contraindication for anticoagulation, the ACCP recommends restarting a conventional course of anticoagulation. Thus, once the patient can tolerate anticoagulation, consideration will be given to removal of the retrievable filter.
Bottom Line
An IVC filter should be considered in patients with a DVT who have a contraindication to anticoagulation. Other indications for IVC filter use are not supported by the current literature. TH
Drs. Bhogal and Eid are hospitalist fellows and instructors at Johns Hopkins Bayview Medical Center in Baltimore. Dr. Kantsiper is a hospitalist and assistant professor at Bayview Medical Center.
References
- The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. U.S. Department of Health & Human Services Web site. Available at: www.surgeongeneral.gov/topics/deepvein/. Accessed Jan. 25, 2010.
- Moser KM, Fedullo PR, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271(3):223-225.
- Bates SM, Ginsberg JS. Treatment of deep vein thrombosis. N Engl J Med. 2004;351:268-277.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians. Antithrombotic therapy for venous theomboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S.
- Becker DM, Philbrick JT, Selby JB. Inferior vena cava filters. Indications, safety, effectiveness. Arch Intern Med. 1992;152(10):1985-1994.
- Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000;95(12):3669-3677.
- Chung J, Owen RJ. Using inferior vena cava filters to prevent pulmonary embolism. Can Fam Physician. 2008;54(1):49-55.
- Ku GH. Billett HH. Long lives, short indications. The case for removable inferior cava filters. Thromb Haemost. 2005;93(1):17-22.
- Stavropoulos WS. Inferior vena cava filters. Tech Vasc Interv Radiol. 2004;7(2):91-95.
- Crowther MA. Inferior vena cava filters in the management of venous thromboembolism. Am J Med. 2007;120(10 Suppl 2):S13–S17.
- Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541-1545.
- Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129(1):174-181.
- Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):401S-428S.
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7):409-415.
- Decousus H, Barral F, Buchmuller-Cordier A, et al. Participating centers eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC randomization croup. Circulation. 2005;112:416-422.
- Kim HS, Young MJ, Narayan AK, Liddell RP, Streiff MB. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol. 2008:19(3):393-399.
- Houman Fekrazad M, Lopes RD, Stashenko GJ, Alexander JH, Garcia D. Treatment of venous thromboembolism: guidelines translated for the clinician. J Thromb Thrombolysis. 2009; 28(3):270–275.
- Comerota AJ. Retrievable IVC filters: a decision matrix for appropriate utilization. Perspect Vasc Surg Endovasc Ther. 2006;18(1):11-17.
- Ray CE Jr, Prochazka A. The need for anticoagulation following inferior vena cava filter placement: systematic review. Cardiovasc Intervent Radiol. 2008; 31(2):316-324.
- Hajduk B, Tomkowski WZ, Malek G, Davidson BL. Vena cava filter occlusion and venous thromboembolism risk in persistently anticoagulated patients: A prospective, observational cohort study. Chest. 2009.
Case
A 67-year-old man with a history of hypertension presents with a swollen right lower extremity. An ultrasound reveals a DVT, and he is commenced on low-molecular-weight heparin and warfarin. Two days later, he develops slurred speech and right-sided weakness. A head CT reveals an intracranial hemorrhage. When should an inferior vena cava (IVC) filter be utilized for treatment of DVT?
Overview
It is estimated that 350,000 to 600,000 Americans develop a VTE each year.1 Patients with a DVT are at high risk of developing a pulmonary embolism (PE). In a multicenter study, nearly 40% of patients admitted with a DVT had evidence of a PE on ventilation perfusion scan.2 Treatment of a DVT is aimed at preventing the extension of the DVT and embolization.3 The American College of Chest Physicians (ACCP) recommends anticoagulation as the primary DVT treatment (Grade 1A).4 However, IVC filters might be considered when anticoagulation is contraindicated.
In 1868, Trousseau created the conceptual model of surgical interruption of the IVC to prevent PE. However, it wasn’t until 1959 by Bottini that the surgical interruption was successfully performed.5 The Mobin-Uddin filter was introduced in 1967 as the first mechanical IVC filter.6 IVC filters mechanically trap the DVT, preventing emboli from traveling into the pulmonary vasculature.7
There are two classes of IVC filters: permanent filters and removable filters. Removable filters include both temporary filters and retrievable filters. Temporary filters are attached to a catheter that exits the skin and therefore must be removed due to the risk of infection and embolization.7 Retrievable filters are similar in design to permanent filters but are designed to be removed. However, this must be done with caution, as neointimal hyperplasia can prevent removal or cause vessel wall damage upon removal.8
IVC filters are inserted into the vena cava percutaneously via the femoral or jugular approach under fluoroscopy or ultrasound guidance (see Figure 1, p. 16). The filters typically are placed infrarenally, unless there is an indication for a suprarenal filter (e.g., renal vein thrombosis or IVC thrombus extending above the renal veins).7 Complete IVC thrombosis is an absolute contraindication to IVC filter placement, and the relative contraindications include significant coagulopathy and bacteremia.9
The incidence of complications related to IVC filter placement is 4% to 11%. Complications include:
- Insertion-site thrombosis;
- IVC thrombosis;
- Recurrent DVT postphlebitic syndrome;
- Filter migration;
- Erosion of the filter through the vessel wall; and
- Vena caval obstruction.10
A review of the National Hospital Discharge Survey database for trends in IVC filter use in the U.S. found a dramatic increase in the use of IVC filters from 1979 to 1999—to 49,000 patients from 2,000 patients with IVC filters in place. The indications for IVC filter use vary such that it is imperative there are well-designed trials and guidelines to guide appropriate use.11
The Evidence
The 2008 ACCP guidelines on VTE management follow a grading system that classifies recommendations as Grade 1 (strong) or Grade 2 (weak), and classifies the quality of evidence as A (high), B (moderate), or C (low).12 The ACCP guidelines’ recommended first-line treatment for a confirmed DVT is anticoagulation with subcutaneous low-molecular-weight heparin, intravenous unfractionated heparin, monitored subcutaneous heparin, fixed-dose subcutaneous unfractionated heparin, or subcutaneous fondaparinux (all Grade 1A recommendations). The ACCP recommends against the routine use of an IVC filter in addition to anticoagulants (Grade 1A). However, for patients with acute proximal DVT, if anticoagulant therapy is not possible because of the risk of bleeding, IVC filter placement is recommended (Grade 1C). If a patient requires an IVC filter for treatment of an acute DVT as an alternative to anticoagulation, it is recommended to start anticoagulant therapy once the risk of bleeding resolves (Grade 1C).4
The 2008 ACCP guidelines for IVC filter use have a few important changes from the 2004 version. First, the IVC filter placement recommendation for patients with contraindications to anticoagulation was strengthened from Grade 2C to Grade 1C. Second, the 2008 guidelines omitted the early recommendation of IVC filter use for recurrent VTE, despite adequate anticoagulation (Grade 2C).13
Only one randomized study has evaluated the efficacy of IVC filters. All other studies of IVC filters are retrospective or prospective case series.
The PREPIC study randomized 400 patients with proximal DVT considered to be at high risk for PE to receive either an IVC filter or no IVC filter. Additionally, patients were randomized to receive enoxaparin or unfractionated heparin as a bridge to warfarin therapy, which was continued for at least three months. The primary endpoints were recurrent DVT, PE, major bleeding, or death. The patients were followed up at day 12, two years, and then annually up to eight years following randomization.14 At day 12, there were fewer PEs in the group that received filters (OR 0.22, 95% CI, 0.05-0.90). However, at year two, there was no significant difference in PE development in the filter group compared with the no-filter group (OR 0.50, 95% CI, 0.19-1.33).
Additionally, at year two, the filter group was more likely to develop recurrent DVT (OR 1.87, 95% CI, 1.10-3.20). At year eight, there was a significant reduction in the number of PEs in the filter group versus the no-filter group (6.2% vs.15.1%, P=0.008). However, at eight-year followup, IVC filter use was associated with increased DVT (35.7% vs. 27.5%, P=0.042). There was no difference in mortality between the two groups.
In summary, the use of IVC filters was associated with decreased incidence of PE at eight years, offset by higher rates of recurrent DVT and no overall mortality benefit.14,15 Importantly, the indications for IVC filter use in this study differ from the current ACCP guidelines; all patients were given concomitant anticoagulation for at least three months, which might not be possible in patients for whom the ACCP recommends IVC filters.
There are no randomized studies to compare the efficacy of permanent IVC filters and retrievable filters for PE prevention. A retrospective study comparing the clinical effectiveness of the two filter types reported no difference in the rates of symptomatic PE (permanent filter 4% vs. retrievable filter 4.7%, P=0.67) or DVT (11.3% vs. 12.6%, P=0.59). In addition, the frequency of symptomatic IVC thrombosis was similar (1.1% vs. 0.5%, p=0.39).16 A paper reviewing the efficacy of IVC filters reported that permanent filters were associated with a 0%-6.2% rate of PE versus a 0%-1.9% rate with retrievable filters.7 Notably, these studies were not randomized controlled trials—rather, case series—and the indications for IVC filters were not necessarily those currently recommended by the ACCP.
Due to the long-term complications of permanent IVC filters, it is suggested that a retrievable IVC filter be used for patients with temporary contraindications to anticoagulation.17 Comerata et al created a clinical decision-making tool for picking the type of filter to employ. If the duration of contraindication to anticoagulation is short or uncertain, a retrievable filter is recommended.18 Table 1 (p. 15) outlines the recommendations for IVC filter placement.
There are no randomized controlled trials to guide the use of concomitant anticoagulation after filter insertion, although this intervention may be beneficial to prevent DVT propagation, recurrence, or IVC filter thrombosis.5 A meta-analysis of 14 studies evaluating the rates of VTE after IVC filter placement demonstrated a non-statistically significant trend toward fewer VTE events in the patients with an IVC filter and concomitant anticoagulation in comparison with those who solely had an IVC filter (OR 0.64, 95% CI, 0.35-1.2). The duration and degree of anticoagulation was not presented in all of the studies in the meta-analysis, therefore limiting the analysis.19
In addition to the ACCP guidelines, there have been other proposed indications for IVC filter use, including recurrent VTE despite anticoagulation, chronic recurrent PE with pulmonary hypertension, extensive free-floating iliofemoral thrombus, and thrombolysis of ilio-caval thrombus.20 The ACCP guidelines do not specifically address these individual indications, and at this time there are no randomized controlled trials to guide IVC filter use in these cases.
Back to the Case
Our patient developed a significant complication from anticoagulation. Current ACCP guidelines recommend an IVC filter if anticoagulant therapy is contraindicated (Grade 1C). The anticoagulation was discontinued and a retrievable IVC filter was placed. Once a patient no longer has a contraindication for anticoagulation, the ACCP recommends restarting a conventional course of anticoagulation. Thus, once the patient can tolerate anticoagulation, consideration will be given to removal of the retrievable filter.
Bottom Line
An IVC filter should be considered in patients with a DVT who have a contraindication to anticoagulation. Other indications for IVC filter use are not supported by the current literature. TH
Drs. Bhogal and Eid are hospitalist fellows and instructors at Johns Hopkins Bayview Medical Center in Baltimore. Dr. Kantsiper is a hospitalist and assistant professor at Bayview Medical Center.
References
- The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. U.S. Department of Health & Human Services Web site. Available at: www.surgeongeneral.gov/topics/deepvein/. Accessed Jan. 25, 2010.
- Moser KM, Fedullo PR, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271(3):223-225.
- Bates SM, Ginsberg JS. Treatment of deep vein thrombosis. N Engl J Med. 2004;351:268-277.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians. Antithrombotic therapy for venous theomboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S.
- Becker DM, Philbrick JT, Selby JB. Inferior vena cava filters. Indications, safety, effectiveness. Arch Intern Med. 1992;152(10):1985-1994.
- Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000;95(12):3669-3677.
- Chung J, Owen RJ. Using inferior vena cava filters to prevent pulmonary embolism. Can Fam Physician. 2008;54(1):49-55.
- Ku GH. Billett HH. Long lives, short indications. The case for removable inferior cava filters. Thromb Haemost. 2005;93(1):17-22.
- Stavropoulos WS. Inferior vena cava filters. Tech Vasc Interv Radiol. 2004;7(2):91-95.
- Crowther MA. Inferior vena cava filters in the management of venous thromboembolism. Am J Med. 2007;120(10 Suppl 2):S13–S17.
- Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541-1545.
- Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129(1):174-181.
- Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):401S-428S.
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7):409-415.
- Decousus H, Barral F, Buchmuller-Cordier A, et al. Participating centers eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC randomization croup. Circulation. 2005;112:416-422.
- Kim HS, Young MJ, Narayan AK, Liddell RP, Streiff MB. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol. 2008:19(3):393-399.
- Houman Fekrazad M, Lopes RD, Stashenko GJ, Alexander JH, Garcia D. Treatment of venous thromboembolism: guidelines translated for the clinician. J Thromb Thrombolysis. 2009; 28(3):270–275.
- Comerota AJ. Retrievable IVC filters: a decision matrix for appropriate utilization. Perspect Vasc Surg Endovasc Ther. 2006;18(1):11-17.
- Ray CE Jr, Prochazka A. The need for anticoagulation following inferior vena cava filter placement: systematic review. Cardiovasc Intervent Radiol. 2008; 31(2):316-324.
- Hajduk B, Tomkowski WZ, Malek G, Davidson BL. Vena cava filter occlusion and venous thromboembolism risk in persistently anticoagulated patients: A prospective, observational cohort study. Chest. 2009.
Case
A 67-year-old man with a history of hypertension presents with a swollen right lower extremity. An ultrasound reveals a DVT, and he is commenced on low-molecular-weight heparin and warfarin. Two days later, he develops slurred speech and right-sided weakness. A head CT reveals an intracranial hemorrhage. When should an inferior vena cava (IVC) filter be utilized for treatment of DVT?
Overview
It is estimated that 350,000 to 600,000 Americans develop a VTE each year.1 Patients with a DVT are at high risk of developing a pulmonary embolism (PE). In a multicenter study, nearly 40% of patients admitted with a DVT had evidence of a PE on ventilation perfusion scan.2 Treatment of a DVT is aimed at preventing the extension of the DVT and embolization.3 The American College of Chest Physicians (ACCP) recommends anticoagulation as the primary DVT treatment (Grade 1A).4 However, IVC filters might be considered when anticoagulation is contraindicated.
In 1868, Trousseau created the conceptual model of surgical interruption of the IVC to prevent PE. However, it wasn’t until 1959 by Bottini that the surgical interruption was successfully performed.5 The Mobin-Uddin filter was introduced in 1967 as the first mechanical IVC filter.6 IVC filters mechanically trap the DVT, preventing emboli from traveling into the pulmonary vasculature.7
There are two classes of IVC filters: permanent filters and removable filters. Removable filters include both temporary filters and retrievable filters. Temporary filters are attached to a catheter that exits the skin and therefore must be removed due to the risk of infection and embolization.7 Retrievable filters are similar in design to permanent filters but are designed to be removed. However, this must be done with caution, as neointimal hyperplasia can prevent removal or cause vessel wall damage upon removal.8
IVC filters are inserted into the vena cava percutaneously via the femoral or jugular approach under fluoroscopy or ultrasound guidance (see Figure 1, p. 16). The filters typically are placed infrarenally, unless there is an indication for a suprarenal filter (e.g., renal vein thrombosis or IVC thrombus extending above the renal veins).7 Complete IVC thrombosis is an absolute contraindication to IVC filter placement, and the relative contraindications include significant coagulopathy and bacteremia.9
The incidence of complications related to IVC filter placement is 4% to 11%. Complications include:
- Insertion-site thrombosis;
- IVC thrombosis;
- Recurrent DVT postphlebitic syndrome;
- Filter migration;
- Erosion of the filter through the vessel wall; and
- Vena caval obstruction.10
A review of the National Hospital Discharge Survey database for trends in IVC filter use in the U.S. found a dramatic increase in the use of IVC filters from 1979 to 1999—to 49,000 patients from 2,000 patients with IVC filters in place. The indications for IVC filter use vary such that it is imperative there are well-designed trials and guidelines to guide appropriate use.11
The Evidence
The 2008 ACCP guidelines on VTE management follow a grading system that classifies recommendations as Grade 1 (strong) or Grade 2 (weak), and classifies the quality of evidence as A (high), B (moderate), or C (low).12 The ACCP guidelines’ recommended first-line treatment for a confirmed DVT is anticoagulation with subcutaneous low-molecular-weight heparin, intravenous unfractionated heparin, monitored subcutaneous heparin, fixed-dose subcutaneous unfractionated heparin, or subcutaneous fondaparinux (all Grade 1A recommendations). The ACCP recommends against the routine use of an IVC filter in addition to anticoagulants (Grade 1A). However, for patients with acute proximal DVT, if anticoagulant therapy is not possible because of the risk of bleeding, IVC filter placement is recommended (Grade 1C). If a patient requires an IVC filter for treatment of an acute DVT as an alternative to anticoagulation, it is recommended to start anticoagulant therapy once the risk of bleeding resolves (Grade 1C).4
The 2008 ACCP guidelines for IVC filter use have a few important changes from the 2004 version. First, the IVC filter placement recommendation for patients with contraindications to anticoagulation was strengthened from Grade 2C to Grade 1C. Second, the 2008 guidelines omitted the early recommendation of IVC filter use for recurrent VTE, despite adequate anticoagulation (Grade 2C).13
Only one randomized study has evaluated the efficacy of IVC filters. All other studies of IVC filters are retrospective or prospective case series.
The PREPIC study randomized 400 patients with proximal DVT considered to be at high risk for PE to receive either an IVC filter or no IVC filter. Additionally, patients were randomized to receive enoxaparin or unfractionated heparin as a bridge to warfarin therapy, which was continued for at least three months. The primary endpoints were recurrent DVT, PE, major bleeding, or death. The patients were followed up at day 12, two years, and then annually up to eight years following randomization.14 At day 12, there were fewer PEs in the group that received filters (OR 0.22, 95% CI, 0.05-0.90). However, at year two, there was no significant difference in PE development in the filter group compared with the no-filter group (OR 0.50, 95% CI, 0.19-1.33).
Additionally, at year two, the filter group was more likely to develop recurrent DVT (OR 1.87, 95% CI, 1.10-3.20). At year eight, there was a significant reduction in the number of PEs in the filter group versus the no-filter group (6.2% vs.15.1%, P=0.008). However, at eight-year followup, IVC filter use was associated with increased DVT (35.7% vs. 27.5%, P=0.042). There was no difference in mortality between the two groups.
In summary, the use of IVC filters was associated with decreased incidence of PE at eight years, offset by higher rates of recurrent DVT and no overall mortality benefit.14,15 Importantly, the indications for IVC filter use in this study differ from the current ACCP guidelines; all patients were given concomitant anticoagulation for at least three months, which might not be possible in patients for whom the ACCP recommends IVC filters.
There are no randomized studies to compare the efficacy of permanent IVC filters and retrievable filters for PE prevention. A retrospective study comparing the clinical effectiveness of the two filter types reported no difference in the rates of symptomatic PE (permanent filter 4% vs. retrievable filter 4.7%, P=0.67) or DVT (11.3% vs. 12.6%, P=0.59). In addition, the frequency of symptomatic IVC thrombosis was similar (1.1% vs. 0.5%, p=0.39).16 A paper reviewing the efficacy of IVC filters reported that permanent filters were associated with a 0%-6.2% rate of PE versus a 0%-1.9% rate with retrievable filters.7 Notably, these studies were not randomized controlled trials—rather, case series—and the indications for IVC filters were not necessarily those currently recommended by the ACCP.
Due to the long-term complications of permanent IVC filters, it is suggested that a retrievable IVC filter be used for patients with temporary contraindications to anticoagulation.17 Comerata et al created a clinical decision-making tool for picking the type of filter to employ. If the duration of contraindication to anticoagulation is short or uncertain, a retrievable filter is recommended.18 Table 1 (p. 15) outlines the recommendations for IVC filter placement.
There are no randomized controlled trials to guide the use of concomitant anticoagulation after filter insertion, although this intervention may be beneficial to prevent DVT propagation, recurrence, or IVC filter thrombosis.5 A meta-analysis of 14 studies evaluating the rates of VTE after IVC filter placement demonstrated a non-statistically significant trend toward fewer VTE events in the patients with an IVC filter and concomitant anticoagulation in comparison with those who solely had an IVC filter (OR 0.64, 95% CI, 0.35-1.2). The duration and degree of anticoagulation was not presented in all of the studies in the meta-analysis, therefore limiting the analysis.19
In addition to the ACCP guidelines, there have been other proposed indications for IVC filter use, including recurrent VTE despite anticoagulation, chronic recurrent PE with pulmonary hypertension, extensive free-floating iliofemoral thrombus, and thrombolysis of ilio-caval thrombus.20 The ACCP guidelines do not specifically address these individual indications, and at this time there are no randomized controlled trials to guide IVC filter use in these cases.
Back to the Case
Our patient developed a significant complication from anticoagulation. Current ACCP guidelines recommend an IVC filter if anticoagulant therapy is contraindicated (Grade 1C). The anticoagulation was discontinued and a retrievable IVC filter was placed. Once a patient no longer has a contraindication for anticoagulation, the ACCP recommends restarting a conventional course of anticoagulation. Thus, once the patient can tolerate anticoagulation, consideration will be given to removal of the retrievable filter.
Bottom Line
An IVC filter should be considered in patients with a DVT who have a contraindication to anticoagulation. Other indications for IVC filter use are not supported by the current literature. TH
Drs. Bhogal and Eid are hospitalist fellows and instructors at Johns Hopkins Bayview Medical Center in Baltimore. Dr. Kantsiper is a hospitalist and assistant professor at Bayview Medical Center.
References
- The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. U.S. Department of Health & Human Services Web site. Available at: www.surgeongeneral.gov/topics/deepvein/. Accessed Jan. 25, 2010.
- Moser KM, Fedullo PR, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271(3):223-225.
- Bates SM, Ginsberg JS. Treatment of deep vein thrombosis. N Engl J Med. 2004;351:268-277.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians. Antithrombotic therapy for venous theomboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):454S-545S.
- Becker DM, Philbrick JT, Selby JB. Inferior vena cava filters. Indications, safety, effectiveness. Arch Intern Med. 1992;152(10):1985-1994.
- Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000;95(12):3669-3677.
- Chung J, Owen RJ. Using inferior vena cava filters to prevent pulmonary embolism. Can Fam Physician. 2008;54(1):49-55.
- Ku GH. Billett HH. Long lives, short indications. The case for removable inferior cava filters. Thromb Haemost. 2005;93(1):17-22.
- Stavropoulos WS. Inferior vena cava filters. Tech Vasc Interv Radiol. 2004;7(2):91-95.
- Crowther MA. Inferior vena cava filters in the management of venous thromboembolism. Am J Med. 2007;120(10 Suppl 2):S13–S17.
- Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541-1545.
- Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129(1):174-181.
- Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):401S-428S.
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7):409-415.
- Decousus H, Barral F, Buchmuller-Cordier A, et al. Participating centers eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC randomization croup. Circulation. 2005;112:416-422.
- Kim HS, Young MJ, Narayan AK, Liddell RP, Streiff MB. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol. 2008:19(3):393-399.
- Houman Fekrazad M, Lopes RD, Stashenko GJ, Alexander JH, Garcia D. Treatment of venous thromboembolism: guidelines translated for the clinician. J Thromb Thrombolysis. 2009; 28(3):270–275.
- Comerota AJ. Retrievable IVC filters: a decision matrix for appropriate utilization. Perspect Vasc Surg Endovasc Ther. 2006;18(1):11-17.
- Ray CE Jr, Prochazka A. The need for anticoagulation following inferior vena cava filter placement: systematic review. Cardiovasc Intervent Radiol. 2008; 31(2):316-324.
- Hajduk B, Tomkowski WZ, Malek G, Davidson BL. Vena cava filter occlusion and venous thromboembolism risk in persistently anticoagulated patients: A prospective, observational cohort study. Chest. 2009.
Market Watch
New Generics
- Donepezil orally disintegrating tablets (generic Aricept ODT)1
- Nizatadine oral solution (generic Axid oral solution), 15mg/mL.2 It is available in peppermint flavor.
New Drugs, Indications, and Dosage Forms
- Clonidine ER tablets and suspension (Clonidine ER suspension and Clonidine ER tablets) have been approved by the FDA.3
- Estradiol 10 mcg vaginal (Vagifem) low-dose tablets have been approved by the FDA for treating atrophic vaginitis due to menopause.4
- Olanzapine (Zyprexa) has been approved by the FDA to treat schizophrenia and manic or mixed episodes associated with bipolar I disorder in patients aged 13 to 17 years old.5 Prescribers must consider the potential for weight gain and hyperlipidemia, as well as other long-term risks that might occur in adolescents compared with adult patients.
- Olanzapine injection (Zyprexa Relprevv) has been approved by the FDA for treating schizophrenia in adults.6 A risk-evaluation and mitigation strategy (REMS) will be implemented with this agent. It is a long-acting, intramuscular depot injection given every two to four weeks, depending on the dose.7
- Quetiapine extended-release (Zyprexa) has been approved by the FDA as add-on therapy to antidepressants in managing adults with major depressive disorder.8 AstraZeneca, the drug manufacturer, also is seeking approval for a monotherapy indication to manage depression in the acute and maintenance phases.
- Sildenafil intravenous (Revatio IV) has been approved by the FDA for treating pulmonary arterial hypertension for patients who are temporarily unable to take the oral medication.9 The injection is administered as a single-dose of 10 mg up to three times daily. According to the manufacturer, this is bioequivalent to 20 mg three times a day for the oral formulation.
- Tiotropium bromide inhalation powder (Spiriva HandiHaler) has been approved by the FDA for reducing exacerbations in patients with chronic obstructive pulmonary disease (COPD).10
Pipeline
- Approval is pending for Aztreonam lysine inhaled (Cayston) for the treatment of Pseudomonas aeruginosa infections in patients with cystic fibrosis.11 The FDA’s Anti-Infectives Drugs Advisory Committee voted 15-2 in favor of the drug’s safety and effectiveness, and 17-0 in favor of a regimen of 75 mg three times a day. The FDA usually approves drugs recommended by its panels.
- Darapladib, a selective and orally active LpPLA2 inhibitor, has begun Phase 3 clinical trials in the management of acute coronary syndrome (ACS).12 The study will include 11,500 male and female patients from 40 countries. It is a double-blind, randomized, placebo-controlled clinical efficacy trial of the long-term use of darapladib when added to standard of care. The study will test whether darapladib affects the chances of having a cardiovascular event, such as a myocardial infarction or a stroke, when treatment is started within 30 days after an ACS.
- Denosumab has received a positive opinion from the European Union for treating osteoporosis in postmenopausal women at increased risk of fractures, and also for treating bone loss in men with prostate cancer who are at increased risk of fractures.13 In the U.S., approval by the FDA is pending for management of osteoporosis in postmenopausal women.14 The FDA’s reproductive health advisory committee, which evaluated the agent, voted 12-1 to require the drug to carry a REMS.
- Ocrelizumab, a Phase 3 humanized anti-CD20 monoclonal antibody for treating rheumatoid arthritis (RA), recently reported positive results when given in combination with methotrexate (MTX) in an international, randomized, multicenter, double-blind trial.15 Ocrelizumab or placebo administered by intravenous infusion on days one and 15 met the primary endpoint of improving the signs and symptoms of RA in patients with an inadequate response to MTX.
- A response to an FDA complete response letter dated May 2009 was expected for rivaroxaban, an oral, direct Factor Xa inhibitor for preventing DVT and pulmonary embolism in patients undergoing hip or knee surgery. Complete review of rivaroxaban data was deferred by its manufacturers until February.16,17
- The FDA is considering a new indication for rosuvastatin (Crestor), following recommendations of the Endocrinologic and Metabolic Drugs Advisory Committee on Dec. 15, 2009.18
Safety Information
Pay attention to two agents manufactured by AstraZeneca: Dexlansoprazole (Kapidex), a new formulation of the proton-pump inhibitor lansoprazole, and bicalutamide (Casodex), which is used in combination with a hormone treatment for prostate cancer, have had medication mixups. The agent names look alike and sound alike when written and verbalized. Both written and verbal prescriptions have been dispensed in error. Bicalutamide is available as 50-mg tablets; dexlansoprazole is available as 30-mg and 60-mg capsules. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City and a clinical pharmacist at New York Downtown Hospital.
References
- Walsh S. FDA Approves Generic Aricept to Treat Dementia Related to Alzheimer’s Disease. U.S. Food and Drug Administration Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm194173.htm. Accessed Dec. 28, 2009.
- Amenal Receives FDA Approval For Nizatidine Oral Solution, the First Oral Solution for Axid in the Market. Medical News Today Web site. Available at: www.medicalnewstoday.com/articles/173591.php. Accessed Dec. 28, 2009.
- Clonidine ER Suspension and Clonidine ER Tablets approved. Monthly Prescribing Reference Web site. Available at: www.empr.com/clonidine-er-suspension-and-clonidine-er-tablets-approved/article/159148/. Accessed Dec. 28, 2009.
- FDA Approves Mcg Dose of Vagifem For the Treatment of Atrophic Vaginitis Due to Menopause. Medical News Today Web site. Available at: www.medicalnewstoday.com/articles/172804.php. Accessed Dec. 28, 2009.
- Todoruk M. Eli Lilly’s Zyprexa approved in US for adolescents. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=B5699B38AADB46AF85E7B34F508DB943&logRowId=340534. Accessed Dec. 28, 2009.
- Dennis M. FDA approves Eli Lilly’s long-acting Zyprexa injection. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=30C504823E42425FA0C3B9BB8490D5AA&logRowId=341775. Accessed Dec. 28, 2009.
- Gever J. FDA Approves Long-Acting Olanzapine. MedPage Today Web site. Available at: www.medpagetoday.com/ProductAlert/Prescriptions/17539. Accessed Dec. 28, 2009.
- Dennis M. FDA approves AstraZeneca’s Seroquel XR as add-on therapy; requires more data as monotherapy. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=0530A906ABCB44B5A32EC05E148E0220&logRowId=340531. Accessed Dec. 28, 2009.
- Petrochko C. FDA Approves IV Sildenafil for Hypertension. MedPage Today Web site. Available at: www.medpagetoday.com/ProductAlert/Prescriptions/17296. Accessed Dec. 28, 2009.
- FDA Approves Spiriva HandiHaler for the Reduction of COPD Exacerbations. Pfizer Web site. Available at: mediaroom.pfizer.com/portal/site/pfizer/?ndmViewId=news_view&newsId=20091217006384&newsLang=en. Accessed Dec. 28, 2009.
- Leuty R. Gilead wins panel OK for cystic fibrosis drug. San Francisco Business Times Web site. Available at: sanfrancisco.bizjournals.com/sanfrancisco/stories/2009/12/07/daily59.html?surround=etf&ana=e_article. Accessed Dec. 28, 2009.
- GSK initiates second pivotal Phase III trial for investigational cardiovascular medication Darapladib. GlaxoSmithKline Web site. Available at: www.gsk.com/media/pressreleases/2009/2009_pressrelease_10141.htm. Accessed Dec. 29, 2009.
- Todoruk M. EU panel issues positive opinion for Amgen’s Prolia. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=A9F30DD044A14893899DF7D880FB8AB0&logRowId=342650. Accessed Dec. 29, 2009.
- Walker EP. FDA Panel Backs Denosumab for Osteoporosis, But Not Osteopenia. MedPage Web site. Available at: www.medpagetoday.com/Endocrinology/Osteoporosis/15530. Accessed Dec. 29, 2009.
- Phase 3 study of ocrelizumab for rheumatoid arthritis (RA). Monthly Prescribing Reference Web site. Available at: www.empr.com/phase-3-study-of-ocrelizumab-for-rheumatoid-arthritis-ra/article/159474/. Accessed Dec. 29, 2009.
- Phase III EINSTEIN-Extension Study of Bayer’s Rivaroxaban Shows Significant Benefit in the Prevention of Secondary Symptomatic VTE. Bayer Web site. Available at: www.bayer.com/en/News-Detail.aspx?id=12554. Accessed Dec. 20, 2009.
- Todoruk M. Bayer, Johnson & Johnson provide update on Xarelto complete response to FDA. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=8F8AEC62E7DB46C3809C1E77A8384F30&logRowId=340535. Accessed Dec. 29, 2009.
- O’Riordan M. FDA advisory panel votes in favor of broadened rosuvastatin indication. TheHeart.org Web site. Available at: www.theheart.org/article/1035155/print.do. Accessed Dec. 29, 2009.
- Kapidex-Casodex confusion. Institute for Safe Medication Practices Web site. Available at: www.ismp.org/newsletters/ambulatory/archives/200907_1.asp. Accessed Dec. 28, 2009.
New Generics
- Donepezil orally disintegrating tablets (generic Aricept ODT)1
- Nizatadine oral solution (generic Axid oral solution), 15mg/mL.2 It is available in peppermint flavor.
New Drugs, Indications, and Dosage Forms
- Clonidine ER tablets and suspension (Clonidine ER suspension and Clonidine ER tablets) have been approved by the FDA.3
- Estradiol 10 mcg vaginal (Vagifem) low-dose tablets have been approved by the FDA for treating atrophic vaginitis due to menopause.4
- Olanzapine (Zyprexa) has been approved by the FDA to treat schizophrenia and manic or mixed episodes associated with bipolar I disorder in patients aged 13 to 17 years old.5 Prescribers must consider the potential for weight gain and hyperlipidemia, as well as other long-term risks that might occur in adolescents compared with adult patients.
- Olanzapine injection (Zyprexa Relprevv) has been approved by the FDA for treating schizophrenia in adults.6 A risk-evaluation and mitigation strategy (REMS) will be implemented with this agent. It is a long-acting, intramuscular depot injection given every two to four weeks, depending on the dose.7
- Quetiapine extended-release (Zyprexa) has been approved by the FDA as add-on therapy to antidepressants in managing adults with major depressive disorder.8 AstraZeneca, the drug manufacturer, also is seeking approval for a monotherapy indication to manage depression in the acute and maintenance phases.
- Sildenafil intravenous (Revatio IV) has been approved by the FDA for treating pulmonary arterial hypertension for patients who are temporarily unable to take the oral medication.9 The injection is administered as a single-dose of 10 mg up to three times daily. According to the manufacturer, this is bioequivalent to 20 mg three times a day for the oral formulation.
- Tiotropium bromide inhalation powder (Spiriva HandiHaler) has been approved by the FDA for reducing exacerbations in patients with chronic obstructive pulmonary disease (COPD).10
Pipeline
- Approval is pending for Aztreonam lysine inhaled (Cayston) for the treatment of Pseudomonas aeruginosa infections in patients with cystic fibrosis.11 The FDA’s Anti-Infectives Drugs Advisory Committee voted 15-2 in favor of the drug’s safety and effectiveness, and 17-0 in favor of a regimen of 75 mg three times a day. The FDA usually approves drugs recommended by its panels.
- Darapladib, a selective and orally active LpPLA2 inhibitor, has begun Phase 3 clinical trials in the management of acute coronary syndrome (ACS).12 The study will include 11,500 male and female patients from 40 countries. It is a double-blind, randomized, placebo-controlled clinical efficacy trial of the long-term use of darapladib when added to standard of care. The study will test whether darapladib affects the chances of having a cardiovascular event, such as a myocardial infarction or a stroke, when treatment is started within 30 days after an ACS.
- Denosumab has received a positive opinion from the European Union for treating osteoporosis in postmenopausal women at increased risk of fractures, and also for treating bone loss in men with prostate cancer who are at increased risk of fractures.13 In the U.S., approval by the FDA is pending for management of osteoporosis in postmenopausal women.14 The FDA’s reproductive health advisory committee, which evaluated the agent, voted 12-1 to require the drug to carry a REMS.
- Ocrelizumab, a Phase 3 humanized anti-CD20 monoclonal antibody for treating rheumatoid arthritis (RA), recently reported positive results when given in combination with methotrexate (MTX) in an international, randomized, multicenter, double-blind trial.15 Ocrelizumab or placebo administered by intravenous infusion on days one and 15 met the primary endpoint of improving the signs and symptoms of RA in patients with an inadequate response to MTX.
- A response to an FDA complete response letter dated May 2009 was expected for rivaroxaban, an oral, direct Factor Xa inhibitor for preventing DVT and pulmonary embolism in patients undergoing hip or knee surgery. Complete review of rivaroxaban data was deferred by its manufacturers until February.16,17
- The FDA is considering a new indication for rosuvastatin (Crestor), following recommendations of the Endocrinologic and Metabolic Drugs Advisory Committee on Dec. 15, 2009.18
Safety Information
Pay attention to two agents manufactured by AstraZeneca: Dexlansoprazole (Kapidex), a new formulation of the proton-pump inhibitor lansoprazole, and bicalutamide (Casodex), which is used in combination with a hormone treatment for prostate cancer, have had medication mixups. The agent names look alike and sound alike when written and verbalized. Both written and verbal prescriptions have been dispensed in error. Bicalutamide is available as 50-mg tablets; dexlansoprazole is available as 30-mg and 60-mg capsules. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City and a clinical pharmacist at New York Downtown Hospital.
References
- Walsh S. FDA Approves Generic Aricept to Treat Dementia Related to Alzheimer’s Disease. U.S. Food and Drug Administration Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm194173.htm. Accessed Dec. 28, 2009.
- Amenal Receives FDA Approval For Nizatidine Oral Solution, the First Oral Solution for Axid in the Market. Medical News Today Web site. Available at: www.medicalnewstoday.com/articles/173591.php. Accessed Dec. 28, 2009.
- Clonidine ER Suspension and Clonidine ER Tablets approved. Monthly Prescribing Reference Web site. Available at: www.empr.com/clonidine-er-suspension-and-clonidine-er-tablets-approved/article/159148/. Accessed Dec. 28, 2009.
- FDA Approves Mcg Dose of Vagifem For the Treatment of Atrophic Vaginitis Due to Menopause. Medical News Today Web site. Available at: www.medicalnewstoday.com/articles/172804.php. Accessed Dec. 28, 2009.
- Todoruk M. Eli Lilly’s Zyprexa approved in US for adolescents. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=B5699B38AADB46AF85E7B34F508DB943&logRowId=340534. Accessed Dec. 28, 2009.
- Dennis M. FDA approves Eli Lilly’s long-acting Zyprexa injection. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=30C504823E42425FA0C3B9BB8490D5AA&logRowId=341775. Accessed Dec. 28, 2009.
- Gever J. FDA Approves Long-Acting Olanzapine. MedPage Today Web site. Available at: www.medpagetoday.com/ProductAlert/Prescriptions/17539. Accessed Dec. 28, 2009.
- Dennis M. FDA approves AstraZeneca’s Seroquel XR as add-on therapy; requires more data as monotherapy. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=0530A906ABCB44B5A32EC05E148E0220&logRowId=340531. Accessed Dec. 28, 2009.
- Petrochko C. FDA Approves IV Sildenafil for Hypertension. MedPage Today Web site. Available at: www.medpagetoday.com/ProductAlert/Prescriptions/17296. Accessed Dec. 28, 2009.
- FDA Approves Spiriva HandiHaler for the Reduction of COPD Exacerbations. Pfizer Web site. Available at: mediaroom.pfizer.com/portal/site/pfizer/?ndmViewId=news_view&newsId=20091217006384&newsLang=en. Accessed Dec. 28, 2009.
- Leuty R. Gilead wins panel OK for cystic fibrosis drug. San Francisco Business Times Web site. Available at: sanfrancisco.bizjournals.com/sanfrancisco/stories/2009/12/07/daily59.html?surround=etf&ana=e_article. Accessed Dec. 28, 2009.
- GSK initiates second pivotal Phase III trial for investigational cardiovascular medication Darapladib. GlaxoSmithKline Web site. Available at: www.gsk.com/media/pressreleases/2009/2009_pressrelease_10141.htm. Accessed Dec. 29, 2009.
- Todoruk M. EU panel issues positive opinion for Amgen’s Prolia. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=A9F30DD044A14893899DF7D880FB8AB0&logRowId=342650. Accessed Dec. 29, 2009.
- Walker EP. FDA Panel Backs Denosumab for Osteoporosis, But Not Osteopenia. MedPage Web site. Available at: www.medpagetoday.com/Endocrinology/Osteoporosis/15530. Accessed Dec. 29, 2009.
- Phase 3 study of ocrelizumab for rheumatoid arthritis (RA). Monthly Prescribing Reference Web site. Available at: www.empr.com/phase-3-study-of-ocrelizumab-for-rheumatoid-arthritis-ra/article/159474/. Accessed Dec. 29, 2009.
- Phase III EINSTEIN-Extension Study of Bayer’s Rivaroxaban Shows Significant Benefit in the Prevention of Secondary Symptomatic VTE. Bayer Web site. Available at: www.bayer.com/en/News-Detail.aspx?id=12554. Accessed Dec. 20, 2009.
- Todoruk M. Bayer, Johnson & Johnson provide update on Xarelto complete response to FDA. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=8F8AEC62E7DB46C3809C1E77A8384F30&logRowId=340535. Accessed Dec. 29, 2009.
- O’Riordan M. FDA advisory panel votes in favor of broadened rosuvastatin indication. TheHeart.org Web site. Available at: www.theheart.org/article/1035155/print.do. Accessed Dec. 29, 2009.
- Kapidex-Casodex confusion. Institute for Safe Medication Practices Web site. Available at: www.ismp.org/newsletters/ambulatory/archives/200907_1.asp. Accessed Dec. 28, 2009.
New Generics
- Donepezil orally disintegrating tablets (generic Aricept ODT)1
- Nizatadine oral solution (generic Axid oral solution), 15mg/mL.2 It is available in peppermint flavor.
New Drugs, Indications, and Dosage Forms
- Clonidine ER tablets and suspension (Clonidine ER suspension and Clonidine ER tablets) have been approved by the FDA.3
- Estradiol 10 mcg vaginal (Vagifem) low-dose tablets have been approved by the FDA for treating atrophic vaginitis due to menopause.4
- Olanzapine (Zyprexa) has been approved by the FDA to treat schizophrenia and manic or mixed episodes associated with bipolar I disorder in patients aged 13 to 17 years old.5 Prescribers must consider the potential for weight gain and hyperlipidemia, as well as other long-term risks that might occur in adolescents compared with adult patients.
- Olanzapine injection (Zyprexa Relprevv) has been approved by the FDA for treating schizophrenia in adults.6 A risk-evaluation and mitigation strategy (REMS) will be implemented with this agent. It is a long-acting, intramuscular depot injection given every two to four weeks, depending on the dose.7
- Quetiapine extended-release (Zyprexa) has been approved by the FDA as add-on therapy to antidepressants in managing adults with major depressive disorder.8 AstraZeneca, the drug manufacturer, also is seeking approval for a monotherapy indication to manage depression in the acute and maintenance phases.
- Sildenafil intravenous (Revatio IV) has been approved by the FDA for treating pulmonary arterial hypertension for patients who are temporarily unable to take the oral medication.9 The injection is administered as a single-dose of 10 mg up to three times daily. According to the manufacturer, this is bioequivalent to 20 mg three times a day for the oral formulation.
- Tiotropium bromide inhalation powder (Spiriva HandiHaler) has been approved by the FDA for reducing exacerbations in patients with chronic obstructive pulmonary disease (COPD).10
Pipeline
- Approval is pending for Aztreonam lysine inhaled (Cayston) for the treatment of Pseudomonas aeruginosa infections in patients with cystic fibrosis.11 The FDA’s Anti-Infectives Drugs Advisory Committee voted 15-2 in favor of the drug’s safety and effectiveness, and 17-0 in favor of a regimen of 75 mg three times a day. The FDA usually approves drugs recommended by its panels.
- Darapladib, a selective and orally active LpPLA2 inhibitor, has begun Phase 3 clinical trials in the management of acute coronary syndrome (ACS).12 The study will include 11,500 male and female patients from 40 countries. It is a double-blind, randomized, placebo-controlled clinical efficacy trial of the long-term use of darapladib when added to standard of care. The study will test whether darapladib affects the chances of having a cardiovascular event, such as a myocardial infarction or a stroke, when treatment is started within 30 days after an ACS.
- Denosumab has received a positive opinion from the European Union for treating osteoporosis in postmenopausal women at increased risk of fractures, and also for treating bone loss in men with prostate cancer who are at increased risk of fractures.13 In the U.S., approval by the FDA is pending for management of osteoporosis in postmenopausal women.14 The FDA’s reproductive health advisory committee, which evaluated the agent, voted 12-1 to require the drug to carry a REMS.
- Ocrelizumab, a Phase 3 humanized anti-CD20 monoclonal antibody for treating rheumatoid arthritis (RA), recently reported positive results when given in combination with methotrexate (MTX) in an international, randomized, multicenter, double-blind trial.15 Ocrelizumab or placebo administered by intravenous infusion on days one and 15 met the primary endpoint of improving the signs and symptoms of RA in patients with an inadequate response to MTX.
- A response to an FDA complete response letter dated May 2009 was expected for rivaroxaban, an oral, direct Factor Xa inhibitor for preventing DVT and pulmonary embolism in patients undergoing hip or knee surgery. Complete review of rivaroxaban data was deferred by its manufacturers until February.16,17
- The FDA is considering a new indication for rosuvastatin (Crestor), following recommendations of the Endocrinologic and Metabolic Drugs Advisory Committee on Dec. 15, 2009.18
Safety Information
Pay attention to two agents manufactured by AstraZeneca: Dexlansoprazole (Kapidex), a new formulation of the proton-pump inhibitor lansoprazole, and bicalutamide (Casodex), which is used in combination with a hormone treatment for prostate cancer, have had medication mixups. The agent names look alike and sound alike when written and verbalized. Both written and verbal prescriptions have been dispensed in error. Bicalutamide is available as 50-mg tablets; dexlansoprazole is available as 30-mg and 60-mg capsules. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City and a clinical pharmacist at New York Downtown Hospital.
References
- Walsh S. FDA Approves Generic Aricept to Treat Dementia Related to Alzheimer’s Disease. U.S. Food and Drug Administration Web site. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm194173.htm. Accessed Dec. 28, 2009.
- Amenal Receives FDA Approval For Nizatidine Oral Solution, the First Oral Solution for Axid in the Market. Medical News Today Web site. Available at: www.medicalnewstoday.com/articles/173591.php. Accessed Dec. 28, 2009.
- Clonidine ER Suspension and Clonidine ER Tablets approved. Monthly Prescribing Reference Web site. Available at: www.empr.com/clonidine-er-suspension-and-clonidine-er-tablets-approved/article/159148/. Accessed Dec. 28, 2009.
- FDA Approves Mcg Dose of Vagifem For the Treatment of Atrophic Vaginitis Due to Menopause. Medical News Today Web site. Available at: www.medicalnewstoday.com/articles/172804.php. Accessed Dec. 28, 2009.
- Todoruk M. Eli Lilly’s Zyprexa approved in US for adolescents. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=B5699B38AADB46AF85E7B34F508DB943&logRowId=340534. Accessed Dec. 28, 2009.
- Dennis M. FDA approves Eli Lilly’s long-acting Zyprexa injection. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=30C504823E42425FA0C3B9BB8490D5AA&logRowId=341775. Accessed Dec. 28, 2009.
- Gever J. FDA Approves Long-Acting Olanzapine. MedPage Today Web site. Available at: www.medpagetoday.com/ProductAlert/Prescriptions/17539. Accessed Dec. 28, 2009.
- Dennis M. FDA approves AstraZeneca’s Seroquel XR as add-on therapy; requires more data as monotherapy. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=0530A906ABCB44B5A32EC05E148E0220&logRowId=340531. Accessed Dec. 28, 2009.
- Petrochko C. FDA Approves IV Sildenafil for Hypertension. MedPage Today Web site. Available at: www.medpagetoday.com/ProductAlert/Prescriptions/17296. Accessed Dec. 28, 2009.
- FDA Approves Spiriva HandiHaler for the Reduction of COPD Exacerbations. Pfizer Web site. Available at: mediaroom.pfizer.com/portal/site/pfizer/?ndmViewId=news_view&newsId=20091217006384&newsLang=en. Accessed Dec. 28, 2009.
- Leuty R. Gilead wins panel OK for cystic fibrosis drug. San Francisco Business Times Web site. Available at: sanfrancisco.bizjournals.com/sanfrancisco/stories/2009/12/07/daily59.html?surround=etf&ana=e_article. Accessed Dec. 28, 2009.
- GSK initiates second pivotal Phase III trial for investigational cardiovascular medication Darapladib. GlaxoSmithKline Web site. Available at: www.gsk.com/media/pressreleases/2009/2009_pressrelease_10141.htm. Accessed Dec. 29, 2009.
- Todoruk M. EU panel issues positive opinion for Amgen’s Prolia. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=A9F30DD044A14893899DF7D880FB8AB0&logRowId=342650. Accessed Dec. 29, 2009.
- Walker EP. FDA Panel Backs Denosumab for Osteoporosis, But Not Osteopenia. MedPage Web site. Available at: www.medpagetoday.com/Endocrinology/Osteoporosis/15530. Accessed Dec. 29, 2009.
- Phase 3 study of ocrelizumab for rheumatoid arthritis (RA). Monthly Prescribing Reference Web site. Available at: www.empr.com/phase-3-study-of-ocrelizumab-for-rheumatoid-arthritis-ra/article/159474/. Accessed Dec. 29, 2009.
- Phase III EINSTEIN-Extension Study of Bayer’s Rivaroxaban Shows Significant Benefit in the Prevention of Secondary Symptomatic VTE. Bayer Web site. Available at: www.bayer.com/en/News-Detail.aspx?id=12554. Accessed Dec. 20, 2009.
- Todoruk M. Bayer, Johnson & Johnson provide update on Xarelto complete response to FDA. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=8F8AEC62E7DB46C3809C1E77A8384F30&logRowId=340535. Accessed Dec. 29, 2009.
- O’Riordan M. FDA advisory panel votes in favor of broadened rosuvastatin indication. TheHeart.org Web site. Available at: www.theheart.org/article/1035155/print.do. Accessed Dec. 29, 2009.
- Kapidex-Casodex confusion. Institute for Safe Medication Practices Web site. Available at: www.ismp.org/newsletters/ambulatory/archives/200907_1.asp. Accessed Dec. 28, 2009.
In the Literature: March 2010
In This Edition
Literature at a Glance
A guide to this month’s studies
- Statins and postoperative cardiac outcomes
- Cardiac resynchronization therapy in patients with mild CHF symptoms
- Oral direct thrombin inhibitor versus warfarin for stroke prevention in atrial fibrillation
- Association of fatigue and medical error
- Effects of chronic inhaled steroid and beta-agonist use in COPD
- Dialysis and functional status in nursing home patients
- Outcomes with different insulin-dosing regimens
- Understanding of disease severity and outcomes in advanced dementia
Fluvastatin Improves Postoperative Cardiac Outcomes in Patients Undergoing Vascular Surgery
Clinical question: Does perioperative fluvastatin decrease adverse cardiac events after vascular surgery?
Background: Patients with atherosclerotic vascular disease who undergo vascular surgery are at high risk for postoperative cardiac events. Studies in nonsurgical populations have shown the beneficial effects of statin therapy on cardiac outcomes. However, no placebo-controlled trials have addressed the effect of statins on postoperative cardiac outcomes.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: Single large academic medical center in the Netherlands.
Synopsis: The study looked at 497 statin-naïve patients 40 years or older undergoing non-cardiac vascular surgery. The patients were randomized to 80 mg of extended-release fluvastatin versus placebo; all patients received a beta-blocker. Therapy began preoperatively (median of 37 days) and continued for at least 30 days after surgery. Outcomes were assessed at 30 days post-surgery.
Postoperative myocardial infarction (MI) was significantly less common in the fluvastatin group than with placebo (10.8% vs. 19%, hazard ratio (HR) 0.55, P=0.01). In addition, the treatment group had a lower frequency of death from cardiovascular causes (4.8% vs. 10.1%, HR 0.47, P=0.03). Statin therapy was not associated with an increased rate of adverse events.
Notably, all of the patients enrolled in this study were high-risk patients undergoing high-risk (vascular) surgery. Patients already on statins were excluded.
Further studies are needed to determine whether the findings can be extrapolated to other populations, including nonvascular surgery patients.
Bottom line: Perioperative statin therapy resulted in a significant decrease in postoperative MI and death within 30 days of vascular surgery.
Citation: Schouten O, Boersma E, Hoeks SE, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361(10):980-989.
Cardiac Resynchronization Therapy with Implantable Cardioverter Defibrillator Placement Decreases Heart Failure
Clinical question: Does cardiac resynchronization therapy (CRT) with biventricular pacing decrease cardiac events in patients with reduced ejection fraction (EF) and wide QRS complex but only mild cardiac symptoms?
Background: In patients with severely reduced EF, implantable cardioverter defibrillators (ICDs) have been shown to improve survival. Meanwhile, CRT decreases heart-failure-related hospitalizations for patients with advanced heart-failure symptoms, EF less than 35%, and intraventricular conduction delay. It is not as clear whether patients with less-severe symptoms benefit from CRT.
Study design: Randomized, controlled trial.
Setting: 110 medical centers in the U.S., Canada, and Europe.
Synopsis: This Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) study randomly assigned 1,820 adults with EF less than 30%, New York Health Association Class I or II congestive heart failure, and in sinus rhythm with QRS greater than 130 msec to receive ICD with CRT or ICD alone. The primary endpoint was all-cause mortality or nonfatal heart-failure events. Average followup was 2.4 years.
A 34% reduction in the primary endpoint was found in the ICD-CRT group when compared with the ICD-only group, primarily due to a 41% reduction in heart-failure events. In a subgroup analysis, women and patients with QRS greater than 150 msec experienced particular benefit. Echocardiography one year after device implantation demonstrated significant reductions in left ventricular end-systolic and end-diastolic volume, and a significant increase in EF with ICD-CRT versus ICD-only (P<0.001).
Bottom line: Compared with ICD alone, CRT in combination with ICD prevented heart-failure events in relatively asymptomatic heart-failure patients with low EF and prolonged QRS.
Citation: Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329-1338.
Dabigatran Is Not Inferior to Warfarin in Atrial Fibrillation
Clinical question: Is dabigatran, an oral thrombin inhibitor, an effective and safe alternative to warfarin in patients with atrial fibrillation?
Background: Warfarin reduces the risk of stroke among patients with atrial fibrillation (AF) but requires frequent laboratory monitoring. Dabigatran is an oral direct thrombin inhibitor given in fixed dosages without laboratory monitoring.
Study design: Randomized, multicenter, open-label, noninferiority trial.
Setting: 951 clinical centers in 44 countries.
Synopsis: More than 18,000 patients 65 and older with AF and at least one stroke risk factor were enrolled. The average CHADS2 score was 2.1. Patients were randomized to receive fixed doses of dabigatran (110 mg or 150 mg, twice daily) or warfarin adjusted to an INR of 2.0-3.0. The primary outcomes were a) stroke or systemic embolism and b) major hemorrhage. Median followup was two years.
The annual rates of stroke or systemic embolism for both doses of dabigatran were noninferior to warfarin (P<0.001); higher-dose dabigatran was statistically superior to warfarin (relative risk (RR)=0.66, P<0.001). The annual rate of major hemorrhage was lowest in the lower-dose dabigatran group (RR=0.80, P=0.003 compared with warfarin); the higher-dose dabigatran and warfarin groups had equivalent rates of major bleeding. No increased risk of liver function abnormalities was noted.
Bottom line: Dabigatran appears to be an effective and safe alternative to warfarin in AF patients. If the drug were to be FDA-approved, appropriate patient selection and cost will need to be established.
Citation: Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
Resident Fatigue and Distress Contribute to Perceived Medical Errors
Clinical question: Do resident fatigue and distress contribute to medical errors?
Background: In recent years, such measures as work-hour limitations have been implemented to decrease resident fatigue and, it is presumed, medical errors. However, few studies address the relationship between residents’ well-being and self-reported medical errors.
Study design: Prospective six-year longitudinal cohort study.
Setting: Single academic medical center.
Synopsis: The authors had 380 internal-medicine residents complete quarterly surveys to assess fatigue, quality of life, burnout, symptoms of depression, and frequency of perceived medical errors. In a univariate analysis, fatigue/sleepiness, burnout, depression, and overall quality of life measures correlated significantly with self-reported major medical errors. Fatigue/sleepiness and measures of distress additively increased the risk of self-reported errors. Increases in one or both domains were estimated to increase the risk of self-reported errors by as much as 15% to 28%.
The authors studied only self-reported medical errors. It is difficult to know whether these errors directly affected patient outcomes. Additionally, results of this single-site study might not be able to be generalized.
Bottom line: Fatigue and distress contribute to self-perceived medical errors among residents.
Citation: West CP, Tan AD, Habermann TM, Sloan JA, Shanafelt TD. Association of resident fatigue and distress with perceived medical errors. JAMA. 2009;302(12):1294-1300.
Inhaled Corticosteroids Decrease Inflammation in Moderate to Severe COPD
Clinical question: Does long-term inhaled corticosteroid therapy, with and without long-acting beta-agonists, decrease airway inflammation and improve lung function in patients with moderate to severe chronic obstructive pulmonary disease (COPD)?
Background: Guideline-recommended treatment of COPD with inhaled corticosteroids and long-acting beta-agonists improves symptoms and exacerbation rates; little is known about the impact of these therapies on inflammation and long-term lung function.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: Two university medical centers in the Netherlands.
Synopsis: One hundred one steroid-naïve patients, ages 45 to 75 who were current or former smokers with moderate to severe COPD, were randomized to one of four regimens: 1) fluticasone for six months, then placebo for 24 months; 2) fluticasone for 30 months; 3) fluticasone and salmeterol for 30 months; or 4) placebo for 30 months. The primary outcome was inflammatory cell counts in bronchial biopsies/induced sputum. Secondary outcomes included postbronchodilator spirometry, methacholine hyperresponsiveness, and self-reported symptoms and health status. Patients with asthma were excluded.
Short-term fluticasone therapy decreased inflammation and improved forced expiratory volume in one second (FEV1). Long-term therapy also decreased the rate of FEV1 decline, reduced dyspnea, and improved health status. Discontinuation of therapy at six months led to inflammation relapse with worsened symptoms and increased rate of FEV1 decline. The addition of long-acting beta-agonists did not provide additional anti-inflammatory benefits, but it did improve FEV1 and dyspnea at six months.
Additional studies are needed to further define clinical outcomes and assess the cost benefit of these therapies.
Bottom line: Inhaled corticosteroids decrease inflammation in steroid-naïve patients with moderate to severe COPD and might decrease the rate of lung function decline. Long-acting beta-agonists do not offer additional anti-inflammatory benefit.
Citation: Lapperre TS, Snoeck-Stroband JB, Gosman MM, et al. Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2009;151(8):517-527.
Initiation of Dialysis Does Not Help Maintain Functional Status in Elderly
Clinical question: Is functional status in the elderly maintained over time after initiating long-term dialysis?
Background: Quality-of-life maintenance often is used as a goal when initiating long-term dialysis in elderly patients with end-stage renal disease. More elderly patients are being offered long-term dialysis treatment. Little is known about the functional status of elderly patients on long-term dialysis.
Study design: Retrospective cohort study.
Setting: U.S. nursing homes.
Synopsis: By cross-linking data from two population-based administrative datasets, this study identified 3,702 nursing home patients (mean 73.4 years) who had started long-term dialysis and whose functional status had been assessed. Activities of daily living assessments before and at three-month intervals after dialysis initiation were compared to see if functional status was maintained.
Within three months of starting dialysis, 61% of patients had a decline in functional status or had died. By one year, only 1 in 8 patients had maintained their pre-dialysis functional status.
Decline in functional status cannot be attributed solely to dialysis because study patients were not compared to patients with chronic kidney disease who were not dialyzed. In addition, these results might not apply to all elderly patients on dialysis, as the functional status of elderly nursing home patients might differ significantly from those living at home.
Bottom line: Functional status is not maintained in most elderly nursing home patients in the first 12 months after long-term dialysis is initiated. Elderly patients considering dialysis treatment should be aware that dialysis might not help maintain functional status and quality of life.
Citation: Kurella Tamura MK, Covinsky KE, Chertow GM, Yaffe C, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547.
Adding Basal Insulin to Oral Agents in Type 2 Diabetes Might Offer Best Glycemic Control
Clinical question: When added to oral diabetic agents, which insulin regimen (biphasic, prandial or basal) best achieves glycemic control in patients with Type 2 diabetes?
Background: Most patients with Type 2 diabetes mellitus (DM2) require insulin when oral agents provide suboptimal glycemic control. Little is known about which insulin regimen is most effective.
Study design: Three-year, open-label, multicenter trial.
Setting: Fifty-eight clinical centers in the United Kingdom and Ireland.
Synopsis: The authors randomized 708 insulin-naïve DM2 patients (median age 62 years) with HgbA1c 7% to 10% on maximum-dose metformin or sulfonylurea to one of three regimens: biphasic insulin twice daily; prandial insulin three times daily; or basal insulin once daily. Outcomes were HgbA1c, hypoglycemia rates, and weight gain. Sulfonylureas were replaced by another insulin if glycemic control was unacceptable.
The patients were mostly Caucasian and overweight. At three years of followup, median HgbA1c was similar in all groups (7.1% biphasic, 6.8% prandial, 6.9% basal); however, more patients who received prandial or basal insulin achieved HgbA1c less than 6.5% (45% and 43%, respectively) than in the biphasic group (32%).
Hypoglycemia was significantly less frequent in the basal insulin group (1.7 per patient per year versus 3.0 and 5.5 with biphasic and prandial, respectively). Patients gained weight in all groups; the greatest gain was with prandial insulin. At three years, there were no significant between-group differences in blood pressure, cholesterol, albuminuria, or quality of life.
Bottom line: Adding insulin to oral diabetic regimens improves glycemic control. Basal or prandial insulin regimens achieve glycemic targets more frequently than biphasic dosing.
Citation: Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361(18):1736-1747.
Advanced Dementia Is a Terminal Illness with High Morbidity and Mortality
Clinical question: Does understanding the expected clinical course of advanced dementia influence end-of-life decisions by proxy decision-makers?
Background: Advanced dementia is a leading cause of death in the United States, but the clinical course of advanced dementia has not been described in a rigorous, prospective manner. The lack of information might cause risk to be underestimated, and patients might receive suboptimal palliative care.
Study design: Multicenter prospective cohort study.
Setting: Twenty-two nursing homes in a single U.S. city.
Synopsis: The survey examined 323 nursing home residents with advanced dementia. The patients were clinically assessed at baseline and quarterly for 18 months through chart reviews, nursing interviews, and physical examinations. Additionally, their proxies were surveyed regarding their understanding of the subjects’ prognoses.
During the survey period, 41.1% of patients developed pneumonia, 52.6% of patients experienced a febrile episode, and 85.8% of patients developed an eating problem; cumulative all-cause mortality was 54.8%. Adjusted for age, sex, and disease duration, the six-month mortality rate for subjects who had pneumonia was 46.7%; a febrile episode, 44.5%; and an eating problem, 38.6%.
Distressing symptoms, including dyspnea (46.0%) and pain (39.1%), were common. In the last three months of life, 40.7% of subjects underwent at least one burdensome intervention (defined as hospitalization, ED visit, parenteral therapy, or tube feeding).
Subjects whose proxies reported an understanding of the poor prognosis and expected clinical complications of advanced dementia underwent significantly fewer burdensome interventions (adjusted odds ratio 0.12).
Bottom line: Advanced dementia is associated with frequent complications, including infections and eating problems, with high six-month mortality and significant associated morbidity. Patients whose healthcare proxies have a good understanding of the expected clinical course and prognosis receive less-aggressive end-of-life care.
Citation: Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529-1538. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Statins and postoperative cardiac outcomes
- Cardiac resynchronization therapy in patients with mild CHF symptoms
- Oral direct thrombin inhibitor versus warfarin for stroke prevention in atrial fibrillation
- Association of fatigue and medical error
- Effects of chronic inhaled steroid and beta-agonist use in COPD
- Dialysis and functional status in nursing home patients
- Outcomes with different insulin-dosing regimens
- Understanding of disease severity and outcomes in advanced dementia
Fluvastatin Improves Postoperative Cardiac Outcomes in Patients Undergoing Vascular Surgery
Clinical question: Does perioperative fluvastatin decrease adverse cardiac events after vascular surgery?
Background: Patients with atherosclerotic vascular disease who undergo vascular surgery are at high risk for postoperative cardiac events. Studies in nonsurgical populations have shown the beneficial effects of statin therapy on cardiac outcomes. However, no placebo-controlled trials have addressed the effect of statins on postoperative cardiac outcomes.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: Single large academic medical center in the Netherlands.
Synopsis: The study looked at 497 statin-naïve patients 40 years or older undergoing non-cardiac vascular surgery. The patients were randomized to 80 mg of extended-release fluvastatin versus placebo; all patients received a beta-blocker. Therapy began preoperatively (median of 37 days) and continued for at least 30 days after surgery. Outcomes were assessed at 30 days post-surgery.
Postoperative myocardial infarction (MI) was significantly less common in the fluvastatin group than with placebo (10.8% vs. 19%, hazard ratio (HR) 0.55, P=0.01). In addition, the treatment group had a lower frequency of death from cardiovascular causes (4.8% vs. 10.1%, HR 0.47, P=0.03). Statin therapy was not associated with an increased rate of adverse events.
Notably, all of the patients enrolled in this study were high-risk patients undergoing high-risk (vascular) surgery. Patients already on statins were excluded.
Further studies are needed to determine whether the findings can be extrapolated to other populations, including nonvascular surgery patients.
Bottom line: Perioperative statin therapy resulted in a significant decrease in postoperative MI and death within 30 days of vascular surgery.
Citation: Schouten O, Boersma E, Hoeks SE, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361(10):980-989.
Cardiac Resynchronization Therapy with Implantable Cardioverter Defibrillator Placement Decreases Heart Failure
Clinical question: Does cardiac resynchronization therapy (CRT) with biventricular pacing decrease cardiac events in patients with reduced ejection fraction (EF) and wide QRS complex but only mild cardiac symptoms?
Background: In patients with severely reduced EF, implantable cardioverter defibrillators (ICDs) have been shown to improve survival. Meanwhile, CRT decreases heart-failure-related hospitalizations for patients with advanced heart-failure symptoms, EF less than 35%, and intraventricular conduction delay. It is not as clear whether patients with less-severe symptoms benefit from CRT.
Study design: Randomized, controlled trial.
Setting: 110 medical centers in the U.S., Canada, and Europe.
Synopsis: This Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) study randomly assigned 1,820 adults with EF less than 30%, New York Health Association Class I or II congestive heart failure, and in sinus rhythm with QRS greater than 130 msec to receive ICD with CRT or ICD alone. The primary endpoint was all-cause mortality or nonfatal heart-failure events. Average followup was 2.4 years.
A 34% reduction in the primary endpoint was found in the ICD-CRT group when compared with the ICD-only group, primarily due to a 41% reduction in heart-failure events. In a subgroup analysis, women and patients with QRS greater than 150 msec experienced particular benefit. Echocardiography one year after device implantation demonstrated significant reductions in left ventricular end-systolic and end-diastolic volume, and a significant increase in EF with ICD-CRT versus ICD-only (P<0.001).
Bottom line: Compared with ICD alone, CRT in combination with ICD prevented heart-failure events in relatively asymptomatic heart-failure patients with low EF and prolonged QRS.
Citation: Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329-1338.
Dabigatran Is Not Inferior to Warfarin in Atrial Fibrillation
Clinical question: Is dabigatran, an oral thrombin inhibitor, an effective and safe alternative to warfarin in patients with atrial fibrillation?
Background: Warfarin reduces the risk of stroke among patients with atrial fibrillation (AF) but requires frequent laboratory monitoring. Dabigatran is an oral direct thrombin inhibitor given in fixed dosages without laboratory monitoring.
Study design: Randomized, multicenter, open-label, noninferiority trial.
Setting: 951 clinical centers in 44 countries.
Synopsis: More than 18,000 patients 65 and older with AF and at least one stroke risk factor were enrolled. The average CHADS2 score was 2.1. Patients were randomized to receive fixed doses of dabigatran (110 mg or 150 mg, twice daily) or warfarin adjusted to an INR of 2.0-3.0. The primary outcomes were a) stroke or systemic embolism and b) major hemorrhage. Median followup was two years.
The annual rates of stroke or systemic embolism for both doses of dabigatran were noninferior to warfarin (P<0.001); higher-dose dabigatran was statistically superior to warfarin (relative risk (RR)=0.66, P<0.001). The annual rate of major hemorrhage was lowest in the lower-dose dabigatran group (RR=0.80, P=0.003 compared with warfarin); the higher-dose dabigatran and warfarin groups had equivalent rates of major bleeding. No increased risk of liver function abnormalities was noted.
Bottom line: Dabigatran appears to be an effective and safe alternative to warfarin in AF patients. If the drug were to be FDA-approved, appropriate patient selection and cost will need to be established.
Citation: Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
Resident Fatigue and Distress Contribute to Perceived Medical Errors
Clinical question: Do resident fatigue and distress contribute to medical errors?
Background: In recent years, such measures as work-hour limitations have been implemented to decrease resident fatigue and, it is presumed, medical errors. However, few studies address the relationship between residents’ well-being and self-reported medical errors.
Study design: Prospective six-year longitudinal cohort study.
Setting: Single academic medical center.
Synopsis: The authors had 380 internal-medicine residents complete quarterly surveys to assess fatigue, quality of life, burnout, symptoms of depression, and frequency of perceived medical errors. In a univariate analysis, fatigue/sleepiness, burnout, depression, and overall quality of life measures correlated significantly with self-reported major medical errors. Fatigue/sleepiness and measures of distress additively increased the risk of self-reported errors. Increases in one or both domains were estimated to increase the risk of self-reported errors by as much as 15% to 28%.
The authors studied only self-reported medical errors. It is difficult to know whether these errors directly affected patient outcomes. Additionally, results of this single-site study might not be able to be generalized.
Bottom line: Fatigue and distress contribute to self-perceived medical errors among residents.
Citation: West CP, Tan AD, Habermann TM, Sloan JA, Shanafelt TD. Association of resident fatigue and distress with perceived medical errors. JAMA. 2009;302(12):1294-1300.
Inhaled Corticosteroids Decrease Inflammation in Moderate to Severe COPD
Clinical question: Does long-term inhaled corticosteroid therapy, with and without long-acting beta-agonists, decrease airway inflammation and improve lung function in patients with moderate to severe chronic obstructive pulmonary disease (COPD)?
Background: Guideline-recommended treatment of COPD with inhaled corticosteroids and long-acting beta-agonists improves symptoms and exacerbation rates; little is known about the impact of these therapies on inflammation and long-term lung function.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: Two university medical centers in the Netherlands.
Synopsis: One hundred one steroid-naïve patients, ages 45 to 75 who were current or former smokers with moderate to severe COPD, were randomized to one of four regimens: 1) fluticasone for six months, then placebo for 24 months; 2) fluticasone for 30 months; 3) fluticasone and salmeterol for 30 months; or 4) placebo for 30 months. The primary outcome was inflammatory cell counts in bronchial biopsies/induced sputum. Secondary outcomes included postbronchodilator spirometry, methacholine hyperresponsiveness, and self-reported symptoms and health status. Patients with asthma were excluded.
Short-term fluticasone therapy decreased inflammation and improved forced expiratory volume in one second (FEV1). Long-term therapy also decreased the rate of FEV1 decline, reduced dyspnea, and improved health status. Discontinuation of therapy at six months led to inflammation relapse with worsened symptoms and increased rate of FEV1 decline. The addition of long-acting beta-agonists did not provide additional anti-inflammatory benefits, but it did improve FEV1 and dyspnea at six months.
Additional studies are needed to further define clinical outcomes and assess the cost benefit of these therapies.
Bottom line: Inhaled corticosteroids decrease inflammation in steroid-naïve patients with moderate to severe COPD and might decrease the rate of lung function decline. Long-acting beta-agonists do not offer additional anti-inflammatory benefit.
Citation: Lapperre TS, Snoeck-Stroband JB, Gosman MM, et al. Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2009;151(8):517-527.
Initiation of Dialysis Does Not Help Maintain Functional Status in Elderly
Clinical question: Is functional status in the elderly maintained over time after initiating long-term dialysis?
Background: Quality-of-life maintenance often is used as a goal when initiating long-term dialysis in elderly patients with end-stage renal disease. More elderly patients are being offered long-term dialysis treatment. Little is known about the functional status of elderly patients on long-term dialysis.
Study design: Retrospective cohort study.
Setting: U.S. nursing homes.
Synopsis: By cross-linking data from two population-based administrative datasets, this study identified 3,702 nursing home patients (mean 73.4 years) who had started long-term dialysis and whose functional status had been assessed. Activities of daily living assessments before and at three-month intervals after dialysis initiation were compared to see if functional status was maintained.
Within three months of starting dialysis, 61% of patients had a decline in functional status or had died. By one year, only 1 in 8 patients had maintained their pre-dialysis functional status.
Decline in functional status cannot be attributed solely to dialysis because study patients were not compared to patients with chronic kidney disease who were not dialyzed. In addition, these results might not apply to all elderly patients on dialysis, as the functional status of elderly nursing home patients might differ significantly from those living at home.
Bottom line: Functional status is not maintained in most elderly nursing home patients in the first 12 months after long-term dialysis is initiated. Elderly patients considering dialysis treatment should be aware that dialysis might not help maintain functional status and quality of life.
Citation: Kurella Tamura MK, Covinsky KE, Chertow GM, Yaffe C, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547.
Adding Basal Insulin to Oral Agents in Type 2 Diabetes Might Offer Best Glycemic Control
Clinical question: When added to oral diabetic agents, which insulin regimen (biphasic, prandial or basal) best achieves glycemic control in patients with Type 2 diabetes?
Background: Most patients with Type 2 diabetes mellitus (DM2) require insulin when oral agents provide suboptimal glycemic control. Little is known about which insulin regimen is most effective.
Study design: Three-year, open-label, multicenter trial.
Setting: Fifty-eight clinical centers in the United Kingdom and Ireland.
Synopsis: The authors randomized 708 insulin-naïve DM2 patients (median age 62 years) with HgbA1c 7% to 10% on maximum-dose metformin or sulfonylurea to one of three regimens: biphasic insulin twice daily; prandial insulin three times daily; or basal insulin once daily. Outcomes were HgbA1c, hypoglycemia rates, and weight gain. Sulfonylureas were replaced by another insulin if glycemic control was unacceptable.
The patients were mostly Caucasian and overweight. At three years of followup, median HgbA1c was similar in all groups (7.1% biphasic, 6.8% prandial, 6.9% basal); however, more patients who received prandial or basal insulin achieved HgbA1c less than 6.5% (45% and 43%, respectively) than in the biphasic group (32%).
Hypoglycemia was significantly less frequent in the basal insulin group (1.7 per patient per year versus 3.0 and 5.5 with biphasic and prandial, respectively). Patients gained weight in all groups; the greatest gain was with prandial insulin. At three years, there were no significant between-group differences in blood pressure, cholesterol, albuminuria, or quality of life.
Bottom line: Adding insulin to oral diabetic regimens improves glycemic control. Basal or prandial insulin regimens achieve glycemic targets more frequently than biphasic dosing.
Citation: Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361(18):1736-1747.
Advanced Dementia Is a Terminal Illness with High Morbidity and Mortality
Clinical question: Does understanding the expected clinical course of advanced dementia influence end-of-life decisions by proxy decision-makers?
Background: Advanced dementia is a leading cause of death in the United States, but the clinical course of advanced dementia has not been described in a rigorous, prospective manner. The lack of information might cause risk to be underestimated, and patients might receive suboptimal palliative care.
Study design: Multicenter prospective cohort study.
Setting: Twenty-two nursing homes in a single U.S. city.
Synopsis: The survey examined 323 nursing home residents with advanced dementia. The patients were clinically assessed at baseline and quarterly for 18 months through chart reviews, nursing interviews, and physical examinations. Additionally, their proxies were surveyed regarding their understanding of the subjects’ prognoses.
During the survey period, 41.1% of patients developed pneumonia, 52.6% of patients experienced a febrile episode, and 85.8% of patients developed an eating problem; cumulative all-cause mortality was 54.8%. Adjusted for age, sex, and disease duration, the six-month mortality rate for subjects who had pneumonia was 46.7%; a febrile episode, 44.5%; and an eating problem, 38.6%.
Distressing symptoms, including dyspnea (46.0%) and pain (39.1%), were common. In the last three months of life, 40.7% of subjects underwent at least one burdensome intervention (defined as hospitalization, ED visit, parenteral therapy, or tube feeding).
Subjects whose proxies reported an understanding of the poor prognosis and expected clinical complications of advanced dementia underwent significantly fewer burdensome interventions (adjusted odds ratio 0.12).
Bottom line: Advanced dementia is associated with frequent complications, including infections and eating problems, with high six-month mortality and significant associated morbidity. Patients whose healthcare proxies have a good understanding of the expected clinical course and prognosis receive less-aggressive end-of-life care.
Citation: Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529-1538. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Statins and postoperative cardiac outcomes
- Cardiac resynchronization therapy in patients with mild CHF symptoms
- Oral direct thrombin inhibitor versus warfarin for stroke prevention in atrial fibrillation
- Association of fatigue and medical error
- Effects of chronic inhaled steroid and beta-agonist use in COPD
- Dialysis and functional status in nursing home patients
- Outcomes with different insulin-dosing regimens
- Understanding of disease severity and outcomes in advanced dementia
Fluvastatin Improves Postoperative Cardiac Outcomes in Patients Undergoing Vascular Surgery
Clinical question: Does perioperative fluvastatin decrease adverse cardiac events after vascular surgery?
Background: Patients with atherosclerotic vascular disease who undergo vascular surgery are at high risk for postoperative cardiac events. Studies in nonsurgical populations have shown the beneficial effects of statin therapy on cardiac outcomes. However, no placebo-controlled trials have addressed the effect of statins on postoperative cardiac outcomes.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: Single large academic medical center in the Netherlands.
Synopsis: The study looked at 497 statin-naïve patients 40 years or older undergoing non-cardiac vascular surgery. The patients were randomized to 80 mg of extended-release fluvastatin versus placebo; all patients received a beta-blocker. Therapy began preoperatively (median of 37 days) and continued for at least 30 days after surgery. Outcomes were assessed at 30 days post-surgery.
Postoperative myocardial infarction (MI) was significantly less common in the fluvastatin group than with placebo (10.8% vs. 19%, hazard ratio (HR) 0.55, P=0.01). In addition, the treatment group had a lower frequency of death from cardiovascular causes (4.8% vs. 10.1%, HR 0.47, P=0.03). Statin therapy was not associated with an increased rate of adverse events.
Notably, all of the patients enrolled in this study were high-risk patients undergoing high-risk (vascular) surgery. Patients already on statins were excluded.
Further studies are needed to determine whether the findings can be extrapolated to other populations, including nonvascular surgery patients.
Bottom line: Perioperative statin therapy resulted in a significant decrease in postoperative MI and death within 30 days of vascular surgery.
Citation: Schouten O, Boersma E, Hoeks SE, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361(10):980-989.
Cardiac Resynchronization Therapy with Implantable Cardioverter Defibrillator Placement Decreases Heart Failure
Clinical question: Does cardiac resynchronization therapy (CRT) with biventricular pacing decrease cardiac events in patients with reduced ejection fraction (EF) and wide QRS complex but only mild cardiac symptoms?
Background: In patients with severely reduced EF, implantable cardioverter defibrillators (ICDs) have been shown to improve survival. Meanwhile, CRT decreases heart-failure-related hospitalizations for patients with advanced heart-failure symptoms, EF less than 35%, and intraventricular conduction delay. It is not as clear whether patients with less-severe symptoms benefit from CRT.
Study design: Randomized, controlled trial.
Setting: 110 medical centers in the U.S., Canada, and Europe.
Synopsis: This Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) study randomly assigned 1,820 adults with EF less than 30%, New York Health Association Class I or II congestive heart failure, and in sinus rhythm with QRS greater than 130 msec to receive ICD with CRT or ICD alone. The primary endpoint was all-cause mortality or nonfatal heart-failure events. Average followup was 2.4 years.
A 34% reduction in the primary endpoint was found in the ICD-CRT group when compared with the ICD-only group, primarily due to a 41% reduction in heart-failure events. In a subgroup analysis, women and patients with QRS greater than 150 msec experienced particular benefit. Echocardiography one year after device implantation demonstrated significant reductions in left ventricular end-systolic and end-diastolic volume, and a significant increase in EF with ICD-CRT versus ICD-only (P<0.001).
Bottom line: Compared with ICD alone, CRT in combination with ICD prevented heart-failure events in relatively asymptomatic heart-failure patients with low EF and prolonged QRS.
Citation: Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329-1338.
Dabigatran Is Not Inferior to Warfarin in Atrial Fibrillation
Clinical question: Is dabigatran, an oral thrombin inhibitor, an effective and safe alternative to warfarin in patients with atrial fibrillation?
Background: Warfarin reduces the risk of stroke among patients with atrial fibrillation (AF) but requires frequent laboratory monitoring. Dabigatran is an oral direct thrombin inhibitor given in fixed dosages without laboratory monitoring.
Study design: Randomized, multicenter, open-label, noninferiority trial.
Setting: 951 clinical centers in 44 countries.
Synopsis: More than 18,000 patients 65 and older with AF and at least one stroke risk factor were enrolled. The average CHADS2 score was 2.1. Patients were randomized to receive fixed doses of dabigatran (110 mg or 150 mg, twice daily) or warfarin adjusted to an INR of 2.0-3.0. The primary outcomes were a) stroke or systemic embolism and b) major hemorrhage. Median followup was two years.
The annual rates of stroke or systemic embolism for both doses of dabigatran were noninferior to warfarin (P<0.001); higher-dose dabigatran was statistically superior to warfarin (relative risk (RR)=0.66, P<0.001). The annual rate of major hemorrhage was lowest in the lower-dose dabigatran group (RR=0.80, P=0.003 compared with warfarin); the higher-dose dabigatran and warfarin groups had equivalent rates of major bleeding. No increased risk of liver function abnormalities was noted.
Bottom line: Dabigatran appears to be an effective and safe alternative to warfarin in AF patients. If the drug were to be FDA-approved, appropriate patient selection and cost will need to be established.
Citation: Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
Resident Fatigue and Distress Contribute to Perceived Medical Errors
Clinical question: Do resident fatigue and distress contribute to medical errors?
Background: In recent years, such measures as work-hour limitations have been implemented to decrease resident fatigue and, it is presumed, medical errors. However, few studies address the relationship between residents’ well-being and self-reported medical errors.
Study design: Prospective six-year longitudinal cohort study.
Setting: Single academic medical center.
Synopsis: The authors had 380 internal-medicine residents complete quarterly surveys to assess fatigue, quality of life, burnout, symptoms of depression, and frequency of perceived medical errors. In a univariate analysis, fatigue/sleepiness, burnout, depression, and overall quality of life measures correlated significantly with self-reported major medical errors. Fatigue/sleepiness and measures of distress additively increased the risk of self-reported errors. Increases in one or both domains were estimated to increase the risk of self-reported errors by as much as 15% to 28%.
The authors studied only self-reported medical errors. It is difficult to know whether these errors directly affected patient outcomes. Additionally, results of this single-site study might not be able to be generalized.
Bottom line: Fatigue and distress contribute to self-perceived medical errors among residents.
Citation: West CP, Tan AD, Habermann TM, Sloan JA, Shanafelt TD. Association of resident fatigue and distress with perceived medical errors. JAMA. 2009;302(12):1294-1300.
Inhaled Corticosteroids Decrease Inflammation in Moderate to Severe COPD
Clinical question: Does long-term inhaled corticosteroid therapy, with and without long-acting beta-agonists, decrease airway inflammation and improve lung function in patients with moderate to severe chronic obstructive pulmonary disease (COPD)?
Background: Guideline-recommended treatment of COPD with inhaled corticosteroids and long-acting beta-agonists improves symptoms and exacerbation rates; little is known about the impact of these therapies on inflammation and long-term lung function.
Study design: Randomized, double-blind, placebo-controlled trial.
Setting: Two university medical centers in the Netherlands.
Synopsis: One hundred one steroid-naïve patients, ages 45 to 75 who were current or former smokers with moderate to severe COPD, were randomized to one of four regimens: 1) fluticasone for six months, then placebo for 24 months; 2) fluticasone for 30 months; 3) fluticasone and salmeterol for 30 months; or 4) placebo for 30 months. The primary outcome was inflammatory cell counts in bronchial biopsies/induced sputum. Secondary outcomes included postbronchodilator spirometry, methacholine hyperresponsiveness, and self-reported symptoms and health status. Patients with asthma were excluded.
Short-term fluticasone therapy decreased inflammation and improved forced expiratory volume in one second (FEV1). Long-term therapy also decreased the rate of FEV1 decline, reduced dyspnea, and improved health status. Discontinuation of therapy at six months led to inflammation relapse with worsened symptoms and increased rate of FEV1 decline. The addition of long-acting beta-agonists did not provide additional anti-inflammatory benefits, but it did improve FEV1 and dyspnea at six months.
Additional studies are needed to further define clinical outcomes and assess the cost benefit of these therapies.
Bottom line: Inhaled corticosteroids decrease inflammation in steroid-naïve patients with moderate to severe COPD and might decrease the rate of lung function decline. Long-acting beta-agonists do not offer additional anti-inflammatory benefit.
Citation: Lapperre TS, Snoeck-Stroband JB, Gosman MM, et al. Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2009;151(8):517-527.
Initiation of Dialysis Does Not Help Maintain Functional Status in Elderly
Clinical question: Is functional status in the elderly maintained over time after initiating long-term dialysis?
Background: Quality-of-life maintenance often is used as a goal when initiating long-term dialysis in elderly patients with end-stage renal disease. More elderly patients are being offered long-term dialysis treatment. Little is known about the functional status of elderly patients on long-term dialysis.
Study design: Retrospective cohort study.
Setting: U.S. nursing homes.
Synopsis: By cross-linking data from two population-based administrative datasets, this study identified 3,702 nursing home patients (mean 73.4 years) who had started long-term dialysis and whose functional status had been assessed. Activities of daily living assessments before and at three-month intervals after dialysis initiation were compared to see if functional status was maintained.
Within three months of starting dialysis, 61% of patients had a decline in functional status or had died. By one year, only 1 in 8 patients had maintained their pre-dialysis functional status.
Decline in functional status cannot be attributed solely to dialysis because study patients were not compared to patients with chronic kidney disease who were not dialyzed. In addition, these results might not apply to all elderly patients on dialysis, as the functional status of elderly nursing home patients might differ significantly from those living at home.
Bottom line: Functional status is not maintained in most elderly nursing home patients in the first 12 months after long-term dialysis is initiated. Elderly patients considering dialysis treatment should be aware that dialysis might not help maintain functional status and quality of life.
Citation: Kurella Tamura MK, Covinsky KE, Chertow GM, Yaffe C, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547.
Adding Basal Insulin to Oral Agents in Type 2 Diabetes Might Offer Best Glycemic Control
Clinical question: When added to oral diabetic agents, which insulin regimen (biphasic, prandial or basal) best achieves glycemic control in patients with Type 2 diabetes?
Background: Most patients with Type 2 diabetes mellitus (DM2) require insulin when oral agents provide suboptimal glycemic control. Little is known about which insulin regimen is most effective.
Study design: Three-year, open-label, multicenter trial.
Setting: Fifty-eight clinical centers in the United Kingdom and Ireland.
Synopsis: The authors randomized 708 insulin-naïve DM2 patients (median age 62 years) with HgbA1c 7% to 10% on maximum-dose metformin or sulfonylurea to one of three regimens: biphasic insulin twice daily; prandial insulin three times daily; or basal insulin once daily. Outcomes were HgbA1c, hypoglycemia rates, and weight gain. Sulfonylureas were replaced by another insulin if glycemic control was unacceptable.
The patients were mostly Caucasian and overweight. At three years of followup, median HgbA1c was similar in all groups (7.1% biphasic, 6.8% prandial, 6.9% basal); however, more patients who received prandial or basal insulin achieved HgbA1c less than 6.5% (45% and 43%, respectively) than in the biphasic group (32%).
Hypoglycemia was significantly less frequent in the basal insulin group (1.7 per patient per year versus 3.0 and 5.5 with biphasic and prandial, respectively). Patients gained weight in all groups; the greatest gain was with prandial insulin. At three years, there were no significant between-group differences in blood pressure, cholesterol, albuminuria, or quality of life.
Bottom line: Adding insulin to oral diabetic regimens improves glycemic control. Basal or prandial insulin regimens achieve glycemic targets more frequently than biphasic dosing.
Citation: Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361(18):1736-1747.
Advanced Dementia Is a Terminal Illness with High Morbidity and Mortality
Clinical question: Does understanding the expected clinical course of advanced dementia influence end-of-life decisions by proxy decision-makers?
Background: Advanced dementia is a leading cause of death in the United States, but the clinical course of advanced dementia has not been described in a rigorous, prospective manner. The lack of information might cause risk to be underestimated, and patients might receive suboptimal palliative care.
Study design: Multicenter prospective cohort study.
Setting: Twenty-two nursing homes in a single U.S. city.
Synopsis: The survey examined 323 nursing home residents with advanced dementia. The patients were clinically assessed at baseline and quarterly for 18 months through chart reviews, nursing interviews, and physical examinations. Additionally, their proxies were surveyed regarding their understanding of the subjects’ prognoses.
During the survey period, 41.1% of patients developed pneumonia, 52.6% of patients experienced a febrile episode, and 85.8% of patients developed an eating problem; cumulative all-cause mortality was 54.8%. Adjusted for age, sex, and disease duration, the six-month mortality rate for subjects who had pneumonia was 46.7%; a febrile episode, 44.5%; and an eating problem, 38.6%.
Distressing symptoms, including dyspnea (46.0%) and pain (39.1%), were common. In the last three months of life, 40.7% of subjects underwent at least one burdensome intervention (defined as hospitalization, ED visit, parenteral therapy, or tube feeding).
Subjects whose proxies reported an understanding of the poor prognosis and expected clinical complications of advanced dementia underwent significantly fewer burdensome interventions (adjusted odds ratio 0.12).
Bottom line: Advanced dementia is associated with frequent complications, including infections and eating problems, with high six-month mortality and significant associated morbidity. Patients whose healthcare proxies have a good understanding of the expected clinical course and prognosis receive less-aggressive end-of-life care.
Citation: Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529-1538. TH
A Time to Be Recognized
Like so many things in HM, the story of how hospitalists first learned about the focused practice program is a modern one.
It started with a text message, which led to a blog post, which reached thousands of readers, many of them hospitalists interested in how to bolster their bona fides in a specialty known for its explosive growth in recent years.
Now, hospitalists certified in internal medicine have the opportunity to reinforce their commitment to the specialty by maintaining their certification through the Focused Practice in Hospital Medicine pathway offered by the American Board of Internal Medicine (ABIM). The Focused Practice in Hospital Medicine (FPHM) Maintenance of Certification (MOC) program enables hospitalists to distinguish their practice within the larger specialty of internal medicine.
The Evolution of FPHM
The new pathway has been years in the making, and it reflects the growing influence of HM in healthcare, according to ABIM Chief Medical Officer Eric Holmboe, MD. He sees the FPHM as the result of a combination of factors, including the fact that the specialty now has more than 30,000 hospitalists practicing nationwide. “If you look at the past years, this has been a viable and vibrant practice,” he says. “If you look at the number of people doing hospital medicine, it’s a factor.”
For Holmboe, it also is a shift in how individuals are recognized based on their practice areas. “This is an acknowledgement by ABIM and the American Board of Medical Specialties to look at Maintenance of Certification in terms of what the individual actually does,” he explains. “Hospitalists play a very important role in the hospital.”
He also credits the leadership of the HM movement—especially pioneers like Robert Wachter, MD, FHM. One of HM’s most ardent champions, Dr. Wachter, chief of the hospital medicine division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, worked with ABIM to find a way to recognize hospitalists’ specialized skill sets and their commitment to inpatient medicine. After more than a decade of advocating for a board-certified process to recognize the field, Dr. Wachter, an ABIM board member, began receiving multiple text messages from colleagues announcing that ABIM had approved the focused-practice program. He wrote a post on his blog, Wachter’s World (www.wachtersworld.com), that outlined the need for the FPHM and the significance for aspiring hospitalists.
“In any case, this is an important milestone for the field,” Dr. Wachter wrote in his Sept. 23, 2009, blog entry, “Board Certification for Hospitalists: It’s Heeeere!” “In fact, when I first began speaking to groups of hospitalists nearly 15 years ago, I often showed a slide listing the elements of a true specialty, and one by one we’ve ticked them off,” wrote Dr. Wachter, a former SHM president. “The only unchecked box was recognition of the field as a legitimate ‘specialty,’ as codified by the ABMS board certification process.”
Unchecked, that is, until now.
In early 2011, the medical world will be introduced to the first internists recognized for their focus in HM. For Holmboe, the FPHM is the beginning of an even larger movement.
“The goal is continued interest: getting people involved in quality in their hospital and encouraging people to change behaviors and be recognized by patients and credentialists as valuable,” he says. “That’s the primary mission of ABIM: using certification to improve care.”
Requirements and Process
Shortly after the program’s approval, ABIM, which administers the FPHM program, went to work in defining the process for the FPHM application and building infrastructure to support the tests. Holmboe expects ABIM will be ready to process pre-applications by April or May. While some details may change, the FPHM application will dovetail with ABIM’s MOC process.
Although hospitalists’ MOC must be current in order to apply for FPHM, hospitalists can begin the FPHM application process at any time. Hospitalists do not need to wait until their next MOC renewal.
Before beginning the application process, hospitalists should ensure that they are eligible. ABIM requires FPHM candidates to have:
- A current or previous ABIM certification in internal medicine;
- A valid, unrestricted medical license and confirmation of good standing in the local practice community;
- ACLS certification; and
- At least three years of hospital medicine practice experience.
Candidates who meet the requirements can then begin the enrollment process by:
- Submitting attestations. Both the hospitalist and a senior officer at the hospital must provide attestations that demonstrate the hospitalist’s experience in HM and his or her commitment to the principles of the specialty.
- Performing a self-assessment. Hospitalists must quantify their experience in HM through an MOC self-assessment. Candidates must achieve at least 100 MOC points. Successful applicants must submit a new self-assessment every three years. The self-assessment can be conducted before or after the exam.
- Taking the MOC examination in Hospital Medicine. Registration for the first HM examination will begin in May. The exam will be conducted in October, and diplomates can take the exam at any time in the process.
Passing the exam and completing the other requirements will earn ABIM diplomats recognition as “Board Certified in Internal Medicine with a Focused Practice in Hospital Medicine.” ABIM will notify successful applicants in late 2010 and ship personalized certificates in early 2011. TH
Brendon Shank is a freelance writer based in Philadelphia.
Like so many things in HM, the story of how hospitalists first learned about the focused practice program is a modern one.
It started with a text message, which led to a blog post, which reached thousands of readers, many of them hospitalists interested in how to bolster their bona fides in a specialty known for its explosive growth in recent years.
Now, hospitalists certified in internal medicine have the opportunity to reinforce their commitment to the specialty by maintaining their certification through the Focused Practice in Hospital Medicine pathway offered by the American Board of Internal Medicine (ABIM). The Focused Practice in Hospital Medicine (FPHM) Maintenance of Certification (MOC) program enables hospitalists to distinguish their practice within the larger specialty of internal medicine.
The Evolution of FPHM
The new pathway has been years in the making, and it reflects the growing influence of HM in healthcare, according to ABIM Chief Medical Officer Eric Holmboe, MD. He sees the FPHM as the result of a combination of factors, including the fact that the specialty now has more than 30,000 hospitalists practicing nationwide. “If you look at the past years, this has been a viable and vibrant practice,” he says. “If you look at the number of people doing hospital medicine, it’s a factor.”
For Holmboe, it also is a shift in how individuals are recognized based on their practice areas. “This is an acknowledgement by ABIM and the American Board of Medical Specialties to look at Maintenance of Certification in terms of what the individual actually does,” he explains. “Hospitalists play a very important role in the hospital.”
He also credits the leadership of the HM movement—especially pioneers like Robert Wachter, MD, FHM. One of HM’s most ardent champions, Dr. Wachter, chief of the hospital medicine division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, worked with ABIM to find a way to recognize hospitalists’ specialized skill sets and their commitment to inpatient medicine. After more than a decade of advocating for a board-certified process to recognize the field, Dr. Wachter, an ABIM board member, began receiving multiple text messages from colleagues announcing that ABIM had approved the focused-practice program. He wrote a post on his blog, Wachter’s World (www.wachtersworld.com), that outlined the need for the FPHM and the significance for aspiring hospitalists.
“In any case, this is an important milestone for the field,” Dr. Wachter wrote in his Sept. 23, 2009, blog entry, “Board Certification for Hospitalists: It’s Heeeere!” “In fact, when I first began speaking to groups of hospitalists nearly 15 years ago, I often showed a slide listing the elements of a true specialty, and one by one we’ve ticked them off,” wrote Dr. Wachter, a former SHM president. “The only unchecked box was recognition of the field as a legitimate ‘specialty,’ as codified by the ABMS board certification process.”
Unchecked, that is, until now.
In early 2011, the medical world will be introduced to the first internists recognized for their focus in HM. For Holmboe, the FPHM is the beginning of an even larger movement.
“The goal is continued interest: getting people involved in quality in their hospital and encouraging people to change behaviors and be recognized by patients and credentialists as valuable,” he says. “That’s the primary mission of ABIM: using certification to improve care.”
Requirements and Process
Shortly after the program’s approval, ABIM, which administers the FPHM program, went to work in defining the process for the FPHM application and building infrastructure to support the tests. Holmboe expects ABIM will be ready to process pre-applications by April or May. While some details may change, the FPHM application will dovetail with ABIM’s MOC process.
Although hospitalists’ MOC must be current in order to apply for FPHM, hospitalists can begin the FPHM application process at any time. Hospitalists do not need to wait until their next MOC renewal.
Before beginning the application process, hospitalists should ensure that they are eligible. ABIM requires FPHM candidates to have:
- A current or previous ABIM certification in internal medicine;
- A valid, unrestricted medical license and confirmation of good standing in the local practice community;
- ACLS certification; and
- At least three years of hospital medicine practice experience.
Candidates who meet the requirements can then begin the enrollment process by:
- Submitting attestations. Both the hospitalist and a senior officer at the hospital must provide attestations that demonstrate the hospitalist’s experience in HM and his or her commitment to the principles of the specialty.
- Performing a self-assessment. Hospitalists must quantify their experience in HM through an MOC self-assessment. Candidates must achieve at least 100 MOC points. Successful applicants must submit a new self-assessment every three years. The self-assessment can be conducted before or after the exam.
- Taking the MOC examination in Hospital Medicine. Registration for the first HM examination will begin in May. The exam will be conducted in October, and diplomates can take the exam at any time in the process.
Passing the exam and completing the other requirements will earn ABIM diplomats recognition as “Board Certified in Internal Medicine with a Focused Practice in Hospital Medicine.” ABIM will notify successful applicants in late 2010 and ship personalized certificates in early 2011. TH
Brendon Shank is a freelance writer based in Philadelphia.
Like so many things in HM, the story of how hospitalists first learned about the focused practice program is a modern one.
It started with a text message, which led to a blog post, which reached thousands of readers, many of them hospitalists interested in how to bolster their bona fides in a specialty known for its explosive growth in recent years.
Now, hospitalists certified in internal medicine have the opportunity to reinforce their commitment to the specialty by maintaining their certification through the Focused Practice in Hospital Medicine pathway offered by the American Board of Internal Medicine (ABIM). The Focused Practice in Hospital Medicine (FPHM) Maintenance of Certification (MOC) program enables hospitalists to distinguish their practice within the larger specialty of internal medicine.
The Evolution of FPHM
The new pathway has been years in the making, and it reflects the growing influence of HM in healthcare, according to ABIM Chief Medical Officer Eric Holmboe, MD. He sees the FPHM as the result of a combination of factors, including the fact that the specialty now has more than 30,000 hospitalists practicing nationwide. “If you look at the past years, this has been a viable and vibrant practice,” he says. “If you look at the number of people doing hospital medicine, it’s a factor.”
For Holmboe, it also is a shift in how individuals are recognized based on their practice areas. “This is an acknowledgement by ABIM and the American Board of Medical Specialties to look at Maintenance of Certification in terms of what the individual actually does,” he explains. “Hospitalists play a very important role in the hospital.”
He also credits the leadership of the HM movement—especially pioneers like Robert Wachter, MD, FHM. One of HM’s most ardent champions, Dr. Wachter, chief of the hospital medicine division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco, worked with ABIM to find a way to recognize hospitalists’ specialized skill sets and their commitment to inpatient medicine. After more than a decade of advocating for a board-certified process to recognize the field, Dr. Wachter, an ABIM board member, began receiving multiple text messages from colleagues announcing that ABIM had approved the focused-practice program. He wrote a post on his blog, Wachter’s World (www.wachtersworld.com), that outlined the need for the FPHM and the significance for aspiring hospitalists.
“In any case, this is an important milestone for the field,” Dr. Wachter wrote in his Sept. 23, 2009, blog entry, “Board Certification for Hospitalists: It’s Heeeere!” “In fact, when I first began speaking to groups of hospitalists nearly 15 years ago, I often showed a slide listing the elements of a true specialty, and one by one we’ve ticked them off,” wrote Dr. Wachter, a former SHM president. “The only unchecked box was recognition of the field as a legitimate ‘specialty,’ as codified by the ABMS board certification process.”
Unchecked, that is, until now.
In early 2011, the medical world will be introduced to the first internists recognized for their focus in HM. For Holmboe, the FPHM is the beginning of an even larger movement.
“The goal is continued interest: getting people involved in quality in their hospital and encouraging people to change behaviors and be recognized by patients and credentialists as valuable,” he says. “That’s the primary mission of ABIM: using certification to improve care.”
Requirements and Process
Shortly after the program’s approval, ABIM, which administers the FPHM program, went to work in defining the process for the FPHM application and building infrastructure to support the tests. Holmboe expects ABIM will be ready to process pre-applications by April or May. While some details may change, the FPHM application will dovetail with ABIM’s MOC process.
Although hospitalists’ MOC must be current in order to apply for FPHM, hospitalists can begin the FPHM application process at any time. Hospitalists do not need to wait until their next MOC renewal.
Before beginning the application process, hospitalists should ensure that they are eligible. ABIM requires FPHM candidates to have:
- A current or previous ABIM certification in internal medicine;
- A valid, unrestricted medical license and confirmation of good standing in the local practice community;
- ACLS certification; and
- At least three years of hospital medicine practice experience.
Candidates who meet the requirements can then begin the enrollment process by:
- Submitting attestations. Both the hospitalist and a senior officer at the hospital must provide attestations that demonstrate the hospitalist’s experience in HM and his or her commitment to the principles of the specialty.
- Performing a self-assessment. Hospitalists must quantify their experience in HM through an MOC self-assessment. Candidates must achieve at least 100 MOC points. Successful applicants must submit a new self-assessment every three years. The self-assessment can be conducted before or after the exam.
- Taking the MOC examination in Hospital Medicine. Registration for the first HM examination will begin in May. The exam will be conducted in October, and diplomates can take the exam at any time in the process.
Passing the exam and completing the other requirements will earn ABIM diplomats recognition as “Board Certified in Internal Medicine with a Focused Practice in Hospital Medicine.” ABIM will notify successful applicants in late 2010 and ship personalized certificates in early 2011. TH
Brendon Shank is a freelance writer based in Philadelphia.
Group Leaders Can Shift the HM Negotiation Paradigm
Whether hospitalists like it or not, the art of negotiation has a significant impact on their daily activities. Negotiations take place with consultants over what the perceived optimal plan of care should be. Discussions are held with patients on how best to overcome the social, financial, and psychological barriers that may impede their health. Hospitalists negotiate with administrators over schedules, benefits, and responsibilities.
Quite frequently, negotiation is viewed as a process where one party wins and the other loses, a zero-sum game, like chess. The spoils may be financial (e.g., better reimbursements) or they may be cognitive (e.g., success in convincing someone of your particular viewpoint). Significant value that could potentially benefit both parties may be lost if the negotiation is approached with a win-loss mentality. However, with proper preparation and insight, a hospitalist can create value in a negotiation that otherwise may be lost by shifting their negotiation paradigm to a collaborative strategy.
A collaborative strategy is when the relationship—and not just the outcome—is important. This would apply to most negotiations that hospitalists take part in.
A significant part of this strategy involves listening and allowing the other side to divulge their interests and positions. Information must flow freely. Once the problem is identified, it must then be detailed further, ensuring both parties understand each other.
Only once both party’s issues are presented can an alternative solution be contemplated that will be win-win in nature. The parties then must both agree to choose that solution and move forward.
The optimal result is that the chosen solution appeases both parties and has a greater total value than if both sides were solely vying for their own interests.
Riyad Fares, MD,
hospitalist,
Adventist Hospital, Portland, Ore.
Whether hospitalists like it or not, the art of negotiation has a significant impact on their daily activities. Negotiations take place with consultants over what the perceived optimal plan of care should be. Discussions are held with patients on how best to overcome the social, financial, and psychological barriers that may impede their health. Hospitalists negotiate with administrators over schedules, benefits, and responsibilities.
Quite frequently, negotiation is viewed as a process where one party wins and the other loses, a zero-sum game, like chess. The spoils may be financial (e.g., better reimbursements) or they may be cognitive (e.g., success in convincing someone of your particular viewpoint). Significant value that could potentially benefit both parties may be lost if the negotiation is approached with a win-loss mentality. However, with proper preparation and insight, a hospitalist can create value in a negotiation that otherwise may be lost by shifting their negotiation paradigm to a collaborative strategy.
A collaborative strategy is when the relationship—and not just the outcome—is important. This would apply to most negotiations that hospitalists take part in.
A significant part of this strategy involves listening and allowing the other side to divulge their interests and positions. Information must flow freely. Once the problem is identified, it must then be detailed further, ensuring both parties understand each other.
Only once both party’s issues are presented can an alternative solution be contemplated that will be win-win in nature. The parties then must both agree to choose that solution and move forward.
The optimal result is that the chosen solution appeases both parties and has a greater total value than if both sides were solely vying for their own interests.
Riyad Fares, MD,
hospitalist,
Adventist Hospital, Portland, Ore.
Whether hospitalists like it or not, the art of negotiation has a significant impact on their daily activities. Negotiations take place with consultants over what the perceived optimal plan of care should be. Discussions are held with patients on how best to overcome the social, financial, and psychological barriers that may impede their health. Hospitalists negotiate with administrators over schedules, benefits, and responsibilities.
Quite frequently, negotiation is viewed as a process where one party wins and the other loses, a zero-sum game, like chess. The spoils may be financial (e.g., better reimbursements) or they may be cognitive (e.g., success in convincing someone of your particular viewpoint). Significant value that could potentially benefit both parties may be lost if the negotiation is approached with a win-loss mentality. However, with proper preparation and insight, a hospitalist can create value in a negotiation that otherwise may be lost by shifting their negotiation paradigm to a collaborative strategy.
A collaborative strategy is when the relationship—and not just the outcome—is important. This would apply to most negotiations that hospitalists take part in.
A significant part of this strategy involves listening and allowing the other side to divulge their interests and positions. Information must flow freely. Once the problem is identified, it must then be detailed further, ensuring both parties understand each other.
Only once both party’s issues are presented can an alternative solution be contemplated that will be win-win in nature. The parties then must both agree to choose that solution and move forward.
The optimal result is that the chosen solution appeases both parties and has a greater total value than if both sides were solely vying for their own interests.
Riyad Fares, MD,
hospitalist,
Adventist Hospital, Portland, Ore.
Prevent Defense
Three U.S. medical centers have been recognized for innovative approaches to preventing DVT and its potentially fatal complications, which include pulmonary embolism (PE). Central to each of the DVT prevention strategies is a risk assessment tool that is easy to use, built directly into routine care, and linked directly to guideline-recommended choices for prophylaxis.
The University of California at San Diego (UCSD) Medical Center, Johns Hopkins Hospital in Baltimore, and the Veterans Affairs (VA) Medical Center in Washington, D.C., each received the first DVTeamCare Hospital Award. The North American Thrombosis Forum (NATF), in conjunction with pharmaceutical company Eisai Inc., recognized each center’s accomplishment based upon an evaluation by an independent panel of expert judges.

—Gregory A. Maynard, MD, FHM, hospital medicine division chief, University of California at San Diego
The award reflects NATF’s goal of enhancing thrombosis education, prevention, diagnosis, and treatment to improve patient outcomes, says NATF Executive Director Ilene Sussman, PhD. Dr. Sussman notes that DVT affects more than 600,000 Americans annually, kills more than 100,000, and is one of the leading causes of preventable deaths in hospitals. Preventable DVT-related complication is on Medicare’s list of “never events,” for which hospitals will no longer be reimbursed.
UCSD, representing medical centers with more than 200 beds, imbedded its VTE prevention protocol into admission, transfer, and perioperative order sets across all medical and surgical services, says Gregory A. Maynard, MD, FHM, hospital medicine division chief. The protocol flags three levels of DVT risk, notes possible contraindications for a particular kind of patient, and presents a set of options for guideline-recommended prophylaxis. The protocol can be paper- or computer-based. Prompting concurrent intervention is a central component of UCSD’s implementation strategy, “identifying in real-time patients who are not receiving the right DVT prophylaxis and having a front-line nurse or pharmacist intervene appropriately,” Dr. Maynard explains.
The percent of UCSD’s patients on adequate prophylaxis rose to more than 98% in the past two years, up from about 50% before the intervention, while preventable VTE dropped by 85%—about 50 fewer cases per year in a hospital with fewer than 300 beds. “Having DVT prevention protocols such as these in place allows hospitalists to provide better care with less effort by leaving hospitalists free to focus on more complicated patient-care issues,” Dr. Maynard says.
UCSD has partnered with SHM to develop DVT prevention toolkits and mentored collaboratives, with which hospitalists can take the lead on QI projects at their local institutions. SHM’s online VTE Implementation Guide is available at www.hospitalmedicine.org/ResourceRoomRedesign/RR_VTE/VTE_Home.cfm.
Johns Hopkins Hospital, representing medical centers with more than 200 beds, developed a mandatory computer-based decision-support system to facilitate specialty-specific risk-factor assessment and the application of risk-appropriate VTE prophylaxis, says Michael Streiff, MD, FACP, director of Johns Hopkins’ Anticoagulation Management Service and Outpatient Clinic, and a member of its Evidence-Based Practice Center. Before a physician can issue any orders—medications, lab tests, nursing instructions, etc.—using a physician transfer order set, the computerized order-entry system automatically guides them through a concise set of questions about a patient’s DVT risk factors, contraindications for blood thinners, and guideline-recommended prophylaxis choices, Dr. Streiff says.
Since implementing the system, the percent of patients being DVT-risk-stratified within 24 hours of hospital admission rose to more than 90%, and nearly 9 in 10 of the appropriate patients are now receiving risk-appropriate, American College of Chest Physicians-approved DVT prophylaxis, up from about 26% before the intervention, Dr. Streiff notes.
The VA Medical Center in Washington, D.C., representing medical centers with fewer than 200 beds, participated in a mentorship collaborative with UCSD’s Dr. Maynard and designed a seven-step process that walks providers through an evidence-based risk-factor assessment to determine appropriate thromboprophylactic therapy, says Divya Shroff, MD, associate chief of staff, Informatics. The guideline-driven steps are integrated into the VA’s computerized patient medical record system and take no more than 60 seconds to follow, says pharmacy practice resident Jovonne H. Jones, PharmD. The steps include:
- Assess patient DVT risk level;
- Educate patient about the order;
- Identify contraindications, if any;
- Choose prophylaxis drug or device;
- Accept order for drug or device;
- Check if additional prophylactic method is needed; and
- Accept the final order.
After the intervention, the rate at which patients receive appropriate prophylaxis upon admission more than doubled. Twenty VA medical centers around the country are in the process of implementing the system, Jones says.
The award-winning protocols will be presented at an NATF-hosted program April 9 at Harvard Medical School. The protocols and implementation plans will be made available at www.DVTeamCareAward.com to help other hospitals enhance their efforts to prevent DVT. TH
Chris Guadagnino is a freelance medical writer based in Philadelphia.
Three U.S. medical centers have been recognized for innovative approaches to preventing DVT and its potentially fatal complications, which include pulmonary embolism (PE). Central to each of the DVT prevention strategies is a risk assessment tool that is easy to use, built directly into routine care, and linked directly to guideline-recommended choices for prophylaxis.
The University of California at San Diego (UCSD) Medical Center, Johns Hopkins Hospital in Baltimore, and the Veterans Affairs (VA) Medical Center in Washington, D.C., each received the first DVTeamCare Hospital Award. The North American Thrombosis Forum (NATF), in conjunction with pharmaceutical company Eisai Inc., recognized each center’s accomplishment based upon an evaluation by an independent panel of expert judges.

—Gregory A. Maynard, MD, FHM, hospital medicine division chief, University of California at San Diego
The award reflects NATF’s goal of enhancing thrombosis education, prevention, diagnosis, and treatment to improve patient outcomes, says NATF Executive Director Ilene Sussman, PhD. Dr. Sussman notes that DVT affects more than 600,000 Americans annually, kills more than 100,000, and is one of the leading causes of preventable deaths in hospitals. Preventable DVT-related complication is on Medicare’s list of “never events,” for which hospitals will no longer be reimbursed.
UCSD, representing medical centers with more than 200 beds, imbedded its VTE prevention protocol into admission, transfer, and perioperative order sets across all medical and surgical services, says Gregory A. Maynard, MD, FHM, hospital medicine division chief. The protocol flags three levels of DVT risk, notes possible contraindications for a particular kind of patient, and presents a set of options for guideline-recommended prophylaxis. The protocol can be paper- or computer-based. Prompting concurrent intervention is a central component of UCSD’s implementation strategy, “identifying in real-time patients who are not receiving the right DVT prophylaxis and having a front-line nurse or pharmacist intervene appropriately,” Dr. Maynard explains.
The percent of UCSD’s patients on adequate prophylaxis rose to more than 98% in the past two years, up from about 50% before the intervention, while preventable VTE dropped by 85%—about 50 fewer cases per year in a hospital with fewer than 300 beds. “Having DVT prevention protocols such as these in place allows hospitalists to provide better care with less effort by leaving hospitalists free to focus on more complicated patient-care issues,” Dr. Maynard says.
UCSD has partnered with SHM to develop DVT prevention toolkits and mentored collaboratives, with which hospitalists can take the lead on QI projects at their local institutions. SHM’s online VTE Implementation Guide is available at www.hospitalmedicine.org/ResourceRoomRedesign/RR_VTE/VTE_Home.cfm.
Johns Hopkins Hospital, representing medical centers with more than 200 beds, developed a mandatory computer-based decision-support system to facilitate specialty-specific risk-factor assessment and the application of risk-appropriate VTE prophylaxis, says Michael Streiff, MD, FACP, director of Johns Hopkins’ Anticoagulation Management Service and Outpatient Clinic, and a member of its Evidence-Based Practice Center. Before a physician can issue any orders—medications, lab tests, nursing instructions, etc.—using a physician transfer order set, the computerized order-entry system automatically guides them through a concise set of questions about a patient’s DVT risk factors, contraindications for blood thinners, and guideline-recommended prophylaxis choices, Dr. Streiff says.
Since implementing the system, the percent of patients being DVT-risk-stratified within 24 hours of hospital admission rose to more than 90%, and nearly 9 in 10 of the appropriate patients are now receiving risk-appropriate, American College of Chest Physicians-approved DVT prophylaxis, up from about 26% before the intervention, Dr. Streiff notes.
The VA Medical Center in Washington, D.C., representing medical centers with fewer than 200 beds, participated in a mentorship collaborative with UCSD’s Dr. Maynard and designed a seven-step process that walks providers through an evidence-based risk-factor assessment to determine appropriate thromboprophylactic therapy, says Divya Shroff, MD, associate chief of staff, Informatics. The guideline-driven steps are integrated into the VA’s computerized patient medical record system and take no more than 60 seconds to follow, says pharmacy practice resident Jovonne H. Jones, PharmD. The steps include:
- Assess patient DVT risk level;
- Educate patient about the order;
- Identify contraindications, if any;
- Choose prophylaxis drug or device;
- Accept order for drug or device;
- Check if additional prophylactic method is needed; and
- Accept the final order.
After the intervention, the rate at which patients receive appropriate prophylaxis upon admission more than doubled. Twenty VA medical centers around the country are in the process of implementing the system, Jones says.
The award-winning protocols will be presented at an NATF-hosted program April 9 at Harvard Medical School. The protocols and implementation plans will be made available at www.DVTeamCareAward.com to help other hospitals enhance their efforts to prevent DVT. TH
Chris Guadagnino is a freelance medical writer based in Philadelphia.
Three U.S. medical centers have been recognized for innovative approaches to preventing DVT and its potentially fatal complications, which include pulmonary embolism (PE). Central to each of the DVT prevention strategies is a risk assessment tool that is easy to use, built directly into routine care, and linked directly to guideline-recommended choices for prophylaxis.
The University of California at San Diego (UCSD) Medical Center, Johns Hopkins Hospital in Baltimore, and the Veterans Affairs (VA) Medical Center in Washington, D.C., each received the first DVTeamCare Hospital Award. The North American Thrombosis Forum (NATF), in conjunction with pharmaceutical company Eisai Inc., recognized each center’s accomplishment based upon an evaluation by an independent panel of expert judges.

—Gregory A. Maynard, MD, FHM, hospital medicine division chief, University of California at San Diego
The award reflects NATF’s goal of enhancing thrombosis education, prevention, diagnosis, and treatment to improve patient outcomes, says NATF Executive Director Ilene Sussman, PhD. Dr. Sussman notes that DVT affects more than 600,000 Americans annually, kills more than 100,000, and is one of the leading causes of preventable deaths in hospitals. Preventable DVT-related complication is on Medicare’s list of “never events,” for which hospitals will no longer be reimbursed.
UCSD, representing medical centers with more than 200 beds, imbedded its VTE prevention protocol into admission, transfer, and perioperative order sets across all medical and surgical services, says Gregory A. Maynard, MD, FHM, hospital medicine division chief. The protocol flags three levels of DVT risk, notes possible contraindications for a particular kind of patient, and presents a set of options for guideline-recommended prophylaxis. The protocol can be paper- or computer-based. Prompting concurrent intervention is a central component of UCSD’s implementation strategy, “identifying in real-time patients who are not receiving the right DVT prophylaxis and having a front-line nurse or pharmacist intervene appropriately,” Dr. Maynard explains.
The percent of UCSD’s patients on adequate prophylaxis rose to more than 98% in the past two years, up from about 50% before the intervention, while preventable VTE dropped by 85%—about 50 fewer cases per year in a hospital with fewer than 300 beds. “Having DVT prevention protocols such as these in place allows hospitalists to provide better care with less effort by leaving hospitalists free to focus on more complicated patient-care issues,” Dr. Maynard says.
UCSD has partnered with SHM to develop DVT prevention toolkits and mentored collaboratives, with which hospitalists can take the lead on QI projects at their local institutions. SHM’s online VTE Implementation Guide is available at www.hospitalmedicine.org/ResourceRoomRedesign/RR_VTE/VTE_Home.cfm.
Johns Hopkins Hospital, representing medical centers with more than 200 beds, developed a mandatory computer-based decision-support system to facilitate specialty-specific risk-factor assessment and the application of risk-appropriate VTE prophylaxis, says Michael Streiff, MD, FACP, director of Johns Hopkins’ Anticoagulation Management Service and Outpatient Clinic, and a member of its Evidence-Based Practice Center. Before a physician can issue any orders—medications, lab tests, nursing instructions, etc.—using a physician transfer order set, the computerized order-entry system automatically guides them through a concise set of questions about a patient’s DVT risk factors, contraindications for blood thinners, and guideline-recommended prophylaxis choices, Dr. Streiff says.
Since implementing the system, the percent of patients being DVT-risk-stratified within 24 hours of hospital admission rose to more than 90%, and nearly 9 in 10 of the appropriate patients are now receiving risk-appropriate, American College of Chest Physicians-approved DVT prophylaxis, up from about 26% before the intervention, Dr. Streiff notes.
The VA Medical Center in Washington, D.C., representing medical centers with fewer than 200 beds, participated in a mentorship collaborative with UCSD’s Dr. Maynard and designed a seven-step process that walks providers through an evidence-based risk-factor assessment to determine appropriate thromboprophylactic therapy, says Divya Shroff, MD, associate chief of staff, Informatics. The guideline-driven steps are integrated into the VA’s computerized patient medical record system and take no more than 60 seconds to follow, says pharmacy practice resident Jovonne H. Jones, PharmD. The steps include:
- Assess patient DVT risk level;
- Educate patient about the order;
- Identify contraindications, if any;
- Choose prophylaxis drug or device;
- Accept order for drug or device;
- Check if additional prophylactic method is needed; and
- Accept the final order.
After the intervention, the rate at which patients receive appropriate prophylaxis upon admission more than doubled. Twenty VA medical centers around the country are in the process of implementing the system, Jones says.
The award-winning protocols will be presented at an NATF-hosted program April 9 at Harvard Medical School. The protocols and implementation plans will be made available at www.DVTeamCareAward.com to help other hospitals enhance their efforts to prevent DVT. TH
Chris Guadagnino is a freelance medical writer based in Philadelphia.
HM10 PREVIEW: Still Taking Reservations
HM10 is planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Blackwell Futura Media Services (BFMS) and SHM. BFMS is accredited by ACCME to provide CME for physicians.
Regular registration is available on SHM’s Web site (www.hospitalmedicine.org) and remains available until March 28. SHM also permits walk-up registration during HM10 at the Gaylord National Resort & Convention Center in National Harbor, Md. However, remember that last year’s meeting sold out a couple weeks ahead of time.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
BFMS designates the educational activity for SHM’s annual meeting at a maximum of 20 Category 1 credits toward the AMA Physician’s Recognition Award. Each physician should claim only those hours of credit they actually spend in each educational activity. BFMS has designated a credit schedule for HM10’s pre-courses on April 8 as follows:
- ABIM MOC learning session, 6.5 credits;
- Critical Care, 7.25 credits;
- Documentation and Coding, 7.5 credits;
- Early Career Hospitalist, 7.5 credits;
- Essential Neurology, 7.25 credits;
- Essential Procedures, 3.75 credits;
- Practice Management, 7.5 credits; and
- Quality Improvement Skills, 7.75 credits.
Source: www.hospitalmedicine.org
PHOTO MATT FENSTERMACHER
HM10 is planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Blackwell Futura Media Services (BFMS) and SHM. BFMS is accredited by ACCME to provide CME for physicians.
Regular registration is available on SHM’s Web site (www.hospitalmedicine.org) and remains available until March 28. SHM also permits walk-up registration during HM10 at the Gaylord National Resort & Convention Center in National Harbor, Md. However, remember that last year’s meeting sold out a couple weeks ahead of time.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
BFMS designates the educational activity for SHM’s annual meeting at a maximum of 20 Category 1 credits toward the AMA Physician’s Recognition Award. Each physician should claim only those hours of credit they actually spend in each educational activity. BFMS has designated a credit schedule for HM10’s pre-courses on April 8 as follows:
- ABIM MOC learning session, 6.5 credits;
- Critical Care, 7.25 credits;
- Documentation and Coding, 7.5 credits;
- Early Career Hospitalist, 7.5 credits;
- Essential Neurology, 7.25 credits;
- Essential Procedures, 3.75 credits;
- Practice Management, 7.5 credits; and
- Quality Improvement Skills, 7.75 credits.
Source: www.hospitalmedicine.org
PHOTO MATT FENSTERMACHER
HM10 is planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Blackwell Futura Media Services (BFMS) and SHM. BFMS is accredited by ACCME to provide CME for physicians.
Regular registration is available on SHM’s Web site (www.hospitalmedicine.org) and remains available until March 28. SHM also permits walk-up registration during HM10 at the Gaylord National Resort & Convention Center in National Harbor, Md. However, remember that last year’s meeting sold out a couple weeks ahead of time.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
BFMS designates the educational activity for SHM’s annual meeting at a maximum of 20 Category 1 credits toward the AMA Physician’s Recognition Award. Each physician should claim only those hours of credit they actually spend in each educational activity. BFMS has designated a credit schedule for HM10’s pre-courses on April 8 as follows:
- ABIM MOC learning session, 6.5 credits;
- Critical Care, 7.25 credits;
- Documentation and Coding, 7.5 credits;
- Early Career Hospitalist, 7.5 credits;
- Essential Neurology, 7.25 credits;
- Essential Procedures, 3.75 credits;
- Practice Management, 7.5 credits; and
- Quality Improvement Skills, 7.75 credits.
Source: www.hospitalmedicine.org
PHOTO MATT FENSTERMACHER
HM10 PREVIEW: National Treasures
It’s often said that only death and taxes are certainties. Add to that short list the beauty of Washington’s National Cherry Blossom Festival in early April.
This year’s festival parade is April 10, which happens to be the third day of HM10 at the Gaylord National Resort & Convention Center in National Harbor, Md., along the banks of the Potomac River outside the nation’s capital. This year’s two-week festival celebrates the 98th anniversary of Tokyo donating 3,000 cherry trees to the people of Washington.
“It gets a little bit crowded, but it’s definitely impressive,” says pediatric hospitalist Patrick Conway, MD, a chief medical officer at U.S. Department of Health and Human Services in Washington. “It’s pretty cool.”
HM10 is the perfect opportunity to get to know D.C., which offers a plethora of museums and attractions for those with time to explore. One of the most famous is the National Mall, which extends from the Lincoln Memorial to the U.S. Capitol. In between is a catalog of American history—the Washington Monument, the National Gallery of Art, and the Smithsonian Institution.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
“The good thing about the National Mall is it’s pretty walkable,” says Dr. Conway, who also uses the city’s subway system, known as the Metro, to get around the city.
Closer to the host hotel, HM10 attendees can take a water taxi from the Potomac Riverboat Co. to visit Old Town Alexandria, George Washington’s Home at Mount Vernon Estate & Gardens, and historic Georgetown. Arlington National Cemetery and its landmark Tomb of the Unknowns is also nearby. “It’s sort of a somber thought, but we took our family there,” Dr. Conway says. “We thought it was moving.”
The Gaylord offers an assortment of casual and upscale restaurants, along with the two-story Pose Ultra Lounge, an infusion bar with nightly entertainment. For those looking for a night on the town, Dr. Conway suggests Zaytinya, a Mediterranean tapas restaurant at the corner of 9th and G streets; BLT Steak off Farragut Square; and Vidalia, an eatery that specializes in Southern cuisine, at 1990 M Street NW (try the shrimp and grits). For a nightcap, get a drink at the Rooftop Sky Terrace at the Hotel Washington, which overlooks the White House.
“We love D.C.,” Dr. Conway says. “There’s a ton to do. It’s very metropolitan. It’s very easy to get around and doesn’t feel like a massive city.”
The average high temperature in April is 66 degrees, so plan to bring a jacket. For more information, visit www.washington.org or www.frommers.com (see “72 Hours in D.C.,” p. 10). For information about HM10’s Family Programs, visit www.hospitalmedicine.org/HM10family.
Richard Quinn is a freelance writer based in New Jersey.
IMAGES SOURCE: TDOES1/DREAMSTIME.COM
It’s often said that only death and taxes are certainties. Add to that short list the beauty of Washington’s National Cherry Blossom Festival in early April.
This year’s festival parade is April 10, which happens to be the third day of HM10 at the Gaylord National Resort & Convention Center in National Harbor, Md., along the banks of the Potomac River outside the nation’s capital. This year’s two-week festival celebrates the 98th anniversary of Tokyo donating 3,000 cherry trees to the people of Washington.
“It gets a little bit crowded, but it’s definitely impressive,” says pediatric hospitalist Patrick Conway, MD, a chief medical officer at U.S. Department of Health and Human Services in Washington. “It’s pretty cool.”
HM10 is the perfect opportunity to get to know D.C., which offers a plethora of museums and attractions for those with time to explore. One of the most famous is the National Mall, which extends from the Lincoln Memorial to the U.S. Capitol. In between is a catalog of American history—the Washington Monument, the National Gallery of Art, and the Smithsonian Institution.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
“The good thing about the National Mall is it’s pretty walkable,” says Dr. Conway, who also uses the city’s subway system, known as the Metro, to get around the city.
Closer to the host hotel, HM10 attendees can take a water taxi from the Potomac Riverboat Co. to visit Old Town Alexandria, George Washington’s Home at Mount Vernon Estate & Gardens, and historic Georgetown. Arlington National Cemetery and its landmark Tomb of the Unknowns is also nearby. “It’s sort of a somber thought, but we took our family there,” Dr. Conway says. “We thought it was moving.”
The Gaylord offers an assortment of casual and upscale restaurants, along with the two-story Pose Ultra Lounge, an infusion bar with nightly entertainment. For those looking for a night on the town, Dr. Conway suggests Zaytinya, a Mediterranean tapas restaurant at the corner of 9th and G streets; BLT Steak off Farragut Square; and Vidalia, an eatery that specializes in Southern cuisine, at 1990 M Street NW (try the shrimp and grits). For a nightcap, get a drink at the Rooftop Sky Terrace at the Hotel Washington, which overlooks the White House.
“We love D.C.,” Dr. Conway says. “There’s a ton to do. It’s very metropolitan. It’s very easy to get around and doesn’t feel like a massive city.”
The average high temperature in April is 66 degrees, so plan to bring a jacket. For more information, visit www.washington.org or www.frommers.com (see “72 Hours in D.C.,” p. 10). For information about HM10’s Family Programs, visit www.hospitalmedicine.org/HM10family.
Richard Quinn is a freelance writer based in New Jersey.
IMAGES SOURCE: TDOES1/DREAMSTIME.COM
It’s often said that only death and taxes are certainties. Add to that short list the beauty of Washington’s National Cherry Blossom Festival in early April.
This year’s festival parade is April 10, which happens to be the third day of HM10 at the Gaylord National Resort & Convention Center in National Harbor, Md., along the banks of the Potomac River outside the nation’s capital. This year’s two-week festival celebrates the 98th anniversary of Tokyo donating 3,000 cherry trees to the people of Washington.
“It gets a little bit crowded, but it’s definitely impressive,” says pediatric hospitalist Patrick Conway, MD, a chief medical officer at U.S. Department of Health and Human Services in Washington. “It’s pretty cool.”
HM10 is the perfect opportunity to get to know D.C., which offers a plethora of museums and attractions for those with time to explore. One of the most famous is the National Mall, which extends from the Lincoln Memorial to the U.S. Capitol. In between is a catalog of American history—the Washington Monument, the National Gallery of Art, and the Smithsonian Institution.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
“The good thing about the National Mall is it’s pretty walkable,” says Dr. Conway, who also uses the city’s subway system, known as the Metro, to get around the city.
Closer to the host hotel, HM10 attendees can take a water taxi from the Potomac Riverboat Co. to visit Old Town Alexandria, George Washington’s Home at Mount Vernon Estate & Gardens, and historic Georgetown. Arlington National Cemetery and its landmark Tomb of the Unknowns is also nearby. “It’s sort of a somber thought, but we took our family there,” Dr. Conway says. “We thought it was moving.”
The Gaylord offers an assortment of casual and upscale restaurants, along with the two-story Pose Ultra Lounge, an infusion bar with nightly entertainment. For those looking for a night on the town, Dr. Conway suggests Zaytinya, a Mediterranean tapas restaurant at the corner of 9th and G streets; BLT Steak off Farragut Square; and Vidalia, an eatery that specializes in Southern cuisine, at 1990 M Street NW (try the shrimp and grits). For a nightcap, get a drink at the Rooftop Sky Terrace at the Hotel Washington, which overlooks the White House.
“We love D.C.,” Dr. Conway says. “There’s a ton to do. It’s very metropolitan. It’s very easy to get around and doesn’t feel like a massive city.”
The average high temperature in April is 66 degrees, so plan to bring a jacket. For more information, visit www.washington.org or www.frommers.com (see “72 Hours in D.C.,” p. 10). For information about HM10’s Family Programs, visit www.hospitalmedicine.org/HM10family.
Richard Quinn is a freelance writer based in New Jersey.
IMAGES SOURCE: TDOES1/DREAMSTIME.COM
HM10 PREVIEW: The Last Word
When you’re a fixture on the HM speaking circuit, it can be challenging to hold the attention of the crowd year after year—especially when your address is at lunchtime on the final day of SHM’s annual powwow, when a fair number of physicians will be checked out or looking to catch a cab to the airport.
The circumstances rarely faze Robert Wachter, MD, FHM, chief of the hospitalist division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco. In fact, since helping pioneer the use of the term “hospitalist,” Dr. Wachter has become the signature closing presenter at SHM’s annual meetings. Sometimes the talk is a window into patient-safety issues; sometimes it’s an oral white paper on policy issues; and, just once, it was a message in a bottle from the future.
This year, though, Dr. Wachter, former SHM president and author of the blog Wachter’s World (www.wachters world.com), is keeping it local.
“It just strikes me that this year, more than any other in recent memory, what is happening in Washington will have a major impact on what hospitalists do for a living and what hospitalists will do for a living,” he says.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Few would disagree.
“Bob has his fingers on the pulse of how hospital medicine is evolving, where hospital medicine started,” says Amir Jaffer, MD, FHM, chair of the annual meeting committee, associate professor, and division chief of hospital medicine at the University of Miami Miller School of Medicine. “Bob’s really been great in terms of predicting what happens next.”
In light of the fluid realities of healthcare reform, Dr. Wachter’s exact words are likely to change several times before he speaks. Still, his themes have remained relatively constant over the years: He boasts of HM’s growth as a specialty and challenges hospitalists to act as leaders.
“I’ve always seen our field as not just reactive, but at the cutting edge of moving things forward,” he says. “This is an extremely important time because if (hospitalists) can be motivational, they can move the whole system forward.”
Hospitalists and group leaders will have to navigate the intricacies of Washington’s reform package. With that in mind, Dr. Wachter will speak about a number of hot policy topics:
- The future of bundled payments. Hospitalists are wary of how the government will reimburse the industry for delivery of care. Whether a new payment system rewards hospitals directly or physicians continue to bill for their services, HM leaders will need to keep abreast of changes.
- Continued migration toward “accountable-care organizations,” also known as ACOs, where providers partner and share responsibility for both the quality and cost of healthcare for a specific population of beneficiaries.
- Healthcare IT improvements. As the industry continues to embrace electronic health records, and industrywide metrics solidify, hospitalists can take leading roles as quality-improvement (QI) leaders.
- Dr. Watcher says hospitalists should use the changing nature of healthcare reform as an opportunity to take a leading role in the discussion.
“It’s not entirely clear how this will play out, but it is clear some piece of the legislation will push for more efficiency,” he says, “and hospitalists will be a major leader in that area.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTOS MATT FENSTERMACHER
When you’re a fixture on the HM speaking circuit, it can be challenging to hold the attention of the crowd year after year—especially when your address is at lunchtime on the final day of SHM’s annual powwow, when a fair number of physicians will be checked out or looking to catch a cab to the airport.
The circumstances rarely faze Robert Wachter, MD, FHM, chief of the hospitalist division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco. In fact, since helping pioneer the use of the term “hospitalist,” Dr. Wachter has become the signature closing presenter at SHM’s annual meetings. Sometimes the talk is a window into patient-safety issues; sometimes it’s an oral white paper on policy issues; and, just once, it was a message in a bottle from the future.
This year, though, Dr. Wachter, former SHM president and author of the blog Wachter’s World (www.wachters world.com), is keeping it local.
“It just strikes me that this year, more than any other in recent memory, what is happening in Washington will have a major impact on what hospitalists do for a living and what hospitalists will do for a living,” he says.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Few would disagree.
“Bob has his fingers on the pulse of how hospital medicine is evolving, where hospital medicine started,” says Amir Jaffer, MD, FHM, chair of the annual meeting committee, associate professor, and division chief of hospital medicine at the University of Miami Miller School of Medicine. “Bob’s really been great in terms of predicting what happens next.”
In light of the fluid realities of healthcare reform, Dr. Wachter’s exact words are likely to change several times before he speaks. Still, his themes have remained relatively constant over the years: He boasts of HM’s growth as a specialty and challenges hospitalists to act as leaders.
“I’ve always seen our field as not just reactive, but at the cutting edge of moving things forward,” he says. “This is an extremely important time because if (hospitalists) can be motivational, they can move the whole system forward.”
Hospitalists and group leaders will have to navigate the intricacies of Washington’s reform package. With that in mind, Dr. Wachter will speak about a number of hot policy topics:
- The future of bundled payments. Hospitalists are wary of how the government will reimburse the industry for delivery of care. Whether a new payment system rewards hospitals directly or physicians continue to bill for their services, HM leaders will need to keep abreast of changes.
- Continued migration toward “accountable-care organizations,” also known as ACOs, where providers partner and share responsibility for both the quality and cost of healthcare for a specific population of beneficiaries.
- Healthcare IT improvements. As the industry continues to embrace electronic health records, and industrywide metrics solidify, hospitalists can take leading roles as quality-improvement (QI) leaders.
- Dr. Watcher says hospitalists should use the changing nature of healthcare reform as an opportunity to take a leading role in the discussion.
“It’s not entirely clear how this will play out, but it is clear some piece of the legislation will push for more efficiency,” he says, “and hospitalists will be a major leader in that area.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTOS MATT FENSTERMACHER
When you’re a fixture on the HM speaking circuit, it can be challenging to hold the attention of the crowd year after year—especially when your address is at lunchtime on the final day of SHM’s annual powwow, when a fair number of physicians will be checked out or looking to catch a cab to the airport.
The circumstances rarely faze Robert Wachter, MD, FHM, chief of the hospitalist division, professor, and associate chair of the Department of Medicine at the University of California at San Francisco. In fact, since helping pioneer the use of the term “hospitalist,” Dr. Wachter has become the signature closing presenter at SHM’s annual meetings. Sometimes the talk is a window into patient-safety issues; sometimes it’s an oral white paper on policy issues; and, just once, it was a message in a bottle from the future.
This year, though, Dr. Wachter, former SHM president and author of the blog Wachter’s World (www.wachters world.com), is keeping it local.
“It just strikes me that this year, more than any other in recent memory, what is happening in Washington will have a major impact on what hospitalists do for a living and what hospitalists will do for a living,” he says.
More HM10 Preview
Hospitalists will gather in the shadows of the national healthcare reform debate
Plan ahead to maximize your HM10 experience
Hospital CEO, HM10 keynote speaker sees bright future for hospitalists
Annual meetings sell out, so get your ticket now
Washington, D.C., is ripe with sights to see and cherry blossoms to admire
HM10 expands hospitalist opportunities for education and interaction
HM pioneer Bob Wachter to address Washington’s impact on hospitalists
You may also
HM10 PREVIEW SUPPLEMENT
in pdf format.
Few would disagree.
“Bob has his fingers on the pulse of how hospital medicine is evolving, where hospital medicine started,” says Amir Jaffer, MD, FHM, chair of the annual meeting committee, associate professor, and division chief of hospital medicine at the University of Miami Miller School of Medicine. “Bob’s really been great in terms of predicting what happens next.”
In light of the fluid realities of healthcare reform, Dr. Wachter’s exact words are likely to change several times before he speaks. Still, his themes have remained relatively constant over the years: He boasts of HM’s growth as a specialty and challenges hospitalists to act as leaders.
“I’ve always seen our field as not just reactive, but at the cutting edge of moving things forward,” he says. “This is an extremely important time because if (hospitalists) can be motivational, they can move the whole system forward.”
Hospitalists and group leaders will have to navigate the intricacies of Washington’s reform package. With that in mind, Dr. Wachter will speak about a number of hot policy topics:
- The future of bundled payments. Hospitalists are wary of how the government will reimburse the industry for delivery of care. Whether a new payment system rewards hospitals directly or physicians continue to bill for their services, HM leaders will need to keep abreast of changes.
- Continued migration toward “accountable-care organizations,” also known as ACOs, where providers partner and share responsibility for both the quality and cost of healthcare for a specific population of beneficiaries.
- Healthcare IT improvements. As the industry continues to embrace electronic health records, and industrywide metrics solidify, hospitalists can take leading roles as quality-improvement (QI) leaders.
- Dr. Watcher says hospitalists should use the changing nature of healthcare reform as an opportunity to take a leading role in the discussion.
“It’s not entirely clear how this will play out, but it is clear some piece of the legislation will push for more efficiency,” he says, “and hospitalists will be a major leader in that area.”
Richard Quinn is a freelance writer based in New Jersey.
PHOTOS MATT FENSTERMACHER