User login
The More, The Merrier
Organizers of HM09 hope bigger means better.
SHM’s annual meeting—May 14-17 in Chicago—has been expanded to four days. A task force led by SHM board member and course director Joseph Ming-Wah Li, MD, FHM, recommended the change.
“The sense was, with a one-day pre-course and the annual meeting at two days, we didn’t really have enough time to put in all the content we wanted to put in,” says Dr. Li, an SHM board member and director of the hospital medicine program at Harvard Medical School, and associate chief of the division of general medicine and primary care at Beth Israel Deaconess Medical Center in Boston. “Unlike many other professional society meetings, we have several specialties. We have some physicians taking care of adults and others who take care of children. We also have nurse practitioners and physician assistants. The extra day really allows us to take a ‘big tent’ approach and make sure we have content of interest to a variety of different physicians.”
The longer meeting will allow for additional educational programming as well as two new tracks. The “second chance” track will give meeting participants an opportunity to attend sessions they missed earlier in the week. The research track is designed to give both novice and experienced researchers the chance to hone their skills.
“We’re a very young movement, and research lends credibility to any field,” Dr. Li says. “We wanted the SHM annual meeting to be a venue for HM research. In order to do that, we have to do that in a very public way.”
Also new this year is the American Board of Internal Medicine Maintenance of Certification learning session.
Continuing medical education sessions, typically offered in a block schedule, will be staggered this year. Course lengths will be tailored to specific topics, and the variations should create a better meeting experience by reducing lines in the dining area, exhibit halls, and other areas during breaks, Dr. Li says.
Based on early registration, HM09 will set attendance records this year. More than 2,000 physicians are expected to attend.
Mark Chassin, MD, MPP, MPH, president of the Joint Commission, will deliver the keynote address at 9 a.m. Friday. Dr. Chassin, whose organization accredits and certifies more than 15,000 healthcare organizations and programs, says the time has come for the commission to connect with physicians—specifically hospitalists—in a “better, more effective way.”
“The emergence of hospital medicine as a specialty is probably the most important structural change in medical practice in 20 or 25 years,” Dr. Chassin says. “Since many hospitalists are becoming more engaged in not just practicing medicine one patient at a time, but taking responsibility for oversight of quality programs in their hospitals, we certainly want to hear from them about how we can help them achieve their quality and safety goals.”
Robert Wachter, MD, FHM, professor and chief of the division of hospital medicine at the University of California at San Francisco, a former SHM president, and author of the blog Wachter’s World (www.wachtersworld.com), will speak at noon Sunday. His program is titled “Creating ‘Accountability’ in a ‘No-Blame’ Culture: The Yin and Yang of the Quality and Safety Revolutions.” TH
Mark Leiser is a freelance writer based in New Jersey.
Organizers of HM09 hope bigger means better.
SHM’s annual meeting—May 14-17 in Chicago—has been expanded to four days. A task force led by SHM board member and course director Joseph Ming-Wah Li, MD, FHM, recommended the change.
“The sense was, with a one-day pre-course and the annual meeting at two days, we didn’t really have enough time to put in all the content we wanted to put in,” says Dr. Li, an SHM board member and director of the hospital medicine program at Harvard Medical School, and associate chief of the division of general medicine and primary care at Beth Israel Deaconess Medical Center in Boston. “Unlike many other professional society meetings, we have several specialties. We have some physicians taking care of adults and others who take care of children. We also have nurse practitioners and physician assistants. The extra day really allows us to take a ‘big tent’ approach and make sure we have content of interest to a variety of different physicians.”
The longer meeting will allow for additional educational programming as well as two new tracks. The “second chance” track will give meeting participants an opportunity to attend sessions they missed earlier in the week. The research track is designed to give both novice and experienced researchers the chance to hone their skills.
“We’re a very young movement, and research lends credibility to any field,” Dr. Li says. “We wanted the SHM annual meeting to be a venue for HM research. In order to do that, we have to do that in a very public way.”
Also new this year is the American Board of Internal Medicine Maintenance of Certification learning session.
Continuing medical education sessions, typically offered in a block schedule, will be staggered this year. Course lengths will be tailored to specific topics, and the variations should create a better meeting experience by reducing lines in the dining area, exhibit halls, and other areas during breaks, Dr. Li says.
Based on early registration, HM09 will set attendance records this year. More than 2,000 physicians are expected to attend.
Mark Chassin, MD, MPP, MPH, president of the Joint Commission, will deliver the keynote address at 9 a.m. Friday. Dr. Chassin, whose organization accredits and certifies more than 15,000 healthcare organizations and programs, says the time has come for the commission to connect with physicians—specifically hospitalists—in a “better, more effective way.”
“The emergence of hospital medicine as a specialty is probably the most important structural change in medical practice in 20 or 25 years,” Dr. Chassin says. “Since many hospitalists are becoming more engaged in not just practicing medicine one patient at a time, but taking responsibility for oversight of quality programs in their hospitals, we certainly want to hear from them about how we can help them achieve their quality and safety goals.”
Robert Wachter, MD, FHM, professor and chief of the division of hospital medicine at the University of California at San Francisco, a former SHM president, and author of the blog Wachter’s World (www.wachtersworld.com), will speak at noon Sunday. His program is titled “Creating ‘Accountability’ in a ‘No-Blame’ Culture: The Yin and Yang of the Quality and Safety Revolutions.” TH
Mark Leiser is a freelance writer based in New Jersey.
Organizers of HM09 hope bigger means better.
SHM’s annual meeting—May 14-17 in Chicago—has been expanded to four days. A task force led by SHM board member and course director Joseph Ming-Wah Li, MD, FHM, recommended the change.
“The sense was, with a one-day pre-course and the annual meeting at two days, we didn’t really have enough time to put in all the content we wanted to put in,” says Dr. Li, an SHM board member and director of the hospital medicine program at Harvard Medical School, and associate chief of the division of general medicine and primary care at Beth Israel Deaconess Medical Center in Boston. “Unlike many other professional society meetings, we have several specialties. We have some physicians taking care of adults and others who take care of children. We also have nurse practitioners and physician assistants. The extra day really allows us to take a ‘big tent’ approach and make sure we have content of interest to a variety of different physicians.”
The longer meeting will allow for additional educational programming as well as two new tracks. The “second chance” track will give meeting participants an opportunity to attend sessions they missed earlier in the week. The research track is designed to give both novice and experienced researchers the chance to hone their skills.
“We’re a very young movement, and research lends credibility to any field,” Dr. Li says. “We wanted the SHM annual meeting to be a venue for HM research. In order to do that, we have to do that in a very public way.”
Also new this year is the American Board of Internal Medicine Maintenance of Certification learning session.
Continuing medical education sessions, typically offered in a block schedule, will be staggered this year. Course lengths will be tailored to specific topics, and the variations should create a better meeting experience by reducing lines in the dining area, exhibit halls, and other areas during breaks, Dr. Li says.
Based on early registration, HM09 will set attendance records this year. More than 2,000 physicians are expected to attend.
Mark Chassin, MD, MPP, MPH, president of the Joint Commission, will deliver the keynote address at 9 a.m. Friday. Dr. Chassin, whose organization accredits and certifies more than 15,000 healthcare organizations and programs, says the time has come for the commission to connect with physicians—specifically hospitalists—in a “better, more effective way.”
“The emergence of hospital medicine as a specialty is probably the most important structural change in medical practice in 20 or 25 years,” Dr. Chassin says. “Since many hospitalists are becoming more engaged in not just practicing medicine one patient at a time, but taking responsibility for oversight of quality programs in their hospitals, we certainly want to hear from them about how we can help them achieve their quality and safety goals.”
Robert Wachter, MD, FHM, professor and chief of the division of hospital medicine at the University of California at San Francisco, a former SHM president, and author of the blog Wachter’s World (www.wachtersworld.com), will speak at noon Sunday. His program is titled “Creating ‘Accountability’ in a ‘No-Blame’ Culture: The Yin and Yang of the Quality and Safety Revolutions.” TH
Mark Leiser is a freelance writer based in New Jersey.
What is the best approach to treat an upper extremity DVT?
Case
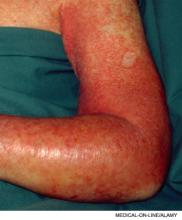
A 45-year-old female with a history of cellulitis requiring peripheral inserted central catheter (PICC) line placement for intravenous antibiotics presents two weeks after line removal with persistent, dull, aching pain in her right shoulder and difficulty removing the rings on her right hand. The pain worsens with exercise and is relieved with rest. The physical exam reveals nonpitting edema of her hand. The ultrasound shows subclavian vein thrombosis. What is the best approach to treating her upper extremity deep venous thrombosis (UEDVT)?
Background
DVT and pulmonary embolism (PE) have been subject to increased publicity recently, and both conditions are recognized as serious entities with life-threatening consequences. In fact, more people die annually from blood clots than breast cancer and AIDS combined.1,2 Still, the increased DVT and PE awareness is primarily focused on lower extremity DVT (LEDVT)), while UEDVT is thought of as a more benign entity. However, current data suggest that UEDVT is associated with equally significant morbidity and mortality.
UEDVT prevalence has increased in step with the increased use of central venous catheters (CVCs) and pacemakers. Although most patients present with pain, swelling, parathesias, and prominent veins throughout the arm or shoulder, many patients will not display any local DVT symptoms. For example, Kabani et al recently presented data for 1,275 patients admitted to the surgical ICU over a 12-month period. They found the incidence of UEDVT was higher than that of LEDVT (17% vs. 11%; P=0.11). They also determined that scanning all four extremities diagnosed more DVT than two-extremity scans (33% vs. 7%; P<0.001).3
While current medical literature has pushed for increased UEDVT attention, there is no consensus on its treatment. Recent American College of Chest Physicians (ACCP) guidelines addressed UEDVT treatment specifically and recommended analogous treatment to LEDVT with heparin and warfarin.4 This follows prospective studies that have shown patients with UEDVT and LEDVT have similar three-month clinical outcomes. The ACCP guidelines do not specifically recommend different treatment courses based on whether the UEDVT is catheter-related or not. Furthermore, while one might assume that removal of an associated catheter might reduce the treatment duration, there is limited data to support shorter courses in this scenario.
Review of the Data
Incidence: UEDVT is becoming more common secondary to increased interventions in the upper extremity (CVC, pacemaker), and is more easily recognized due to improvement in noninvasive ultrasound technology. UEDVT accounts for up to 10% of all DVT, with an incidence of approximately three per 100,000 persons in the general population.5-8 Because UEDVT can also be asymptomatic, it is believed that the incidence likely is higher than previously reported, but prospective data are lacking.
Risk factors: UEDVT is further categorized as either primary or secondary, depending upon the cause. First described in the late 1800s, spontaneous primary thrombosis of the upper extremity, or Paget-Schroetter syndrome, accounts for approximately 20% of UEDVT.9 Primary UEDVT includes both idiopathic and “effort-related” thrombosis. Effort-related thrombosis usually develops among young people after strenuous or repetitive exercise, such as pitching a baseball. Some hypothesize that effort-related thrombosis is related to a hypercoaguable state or anatomic abnormalities, although a specific cause, such as thoracic outlet syndrome, is found in only 5% of these cases.10,11
Secondary UEDVT characterizes thrombosis in which an endogenous or exogenous risk factor is present. Endogenous risk factors include coagulation abnormalities, such as antithrombin, protein C and protein S deficiencies; factor V Leiden gene mutation; hyperhomocysteinemia; and antiphospholipid antibody syndrome. Exogenous risk factors include CVC pacemakers, intracardiac defibrillators, malignancy, previous or concurrent LEDVT, oral contraceptives, some artificial reproductive technologies (women can develop ovarian hyperstimulation syndrome, which is associated with increased hypercoaguability), trauma, and IV drug use (especially cocaine).5,12-14
Clinical presentation and diagnosis: Swelling (80% of patients) and pain (40% of patients) are the most common UEDVT symptoms at presentation.2 Other clinical features include new, prominent veins throughout the shoulder girdle, erythema, increased warmth, functional impairment, parathesias, and non-specific feelings of arm heaviness or discomfort. Symptoms typically worsen with arm use and improve with rest and elevation.15 Patients with UEDVT related to CVC are more likely to be asymptomatic and may present only with PE.16 The differential diagnosis includes superficial phlebitis, lymphatic edema, hematoma, contusions, venous compression, and muscle tears.17
Contrast venography is the gold standard for the UEDVT diagnosis. However, it is more expensive and invasive than ultrasound, and thus serial compression ultrasound is now the standard test in UEDVT evaluation. Then again, contrast venography remains the test of choice in patients with high pre-test probability and negative ultrasound results.18,19
Prevention: Nearly 70% of secondary UEDVT is associated with a CVC.5 Further, CVC use is the most powerful predictor of UEDVT (adjusted odds ratio (OR), 9.7; 95% CI, 7.8 to 12.2).2 Despite the association between CVCs and UEDVT, anticoagulant prophylaxis is not recommended. Studies evaluating the results of 1-mg warfarin conflict and include small populations. Warfarin’s potential interaction with antibiotics and dosing variance based on nutritional intake logically prompted studies on the potential benefit of low-weight molecular heparain (LWMH); however, these studies have failed to show benefit.20,21
Treatment: Recent ACCP guidelines recommend treating UEDVT patients with unfractionated heparin (UFH) or LMWH and warfarin, with an INR goal of 2 to 3 for at least three months depending upon the overall clinical scenario. Two small studies evaluating catheter-related thrombosis (15 patients in each trial) reported no subsequent embolic phenomenon.22,23 Some authors interpreted this data to mean UEDVT was not as morbid as LEDVT and, subsequently, that catheter-related UEDVTs require only one month of therapy. Since the small studies were published, the increasing incidence and relevance of UEDVT have become more widely recognized, and most authors are recommending three months of treatment.
Still, it’s important to note that there aren’t any published data directly comparing the one-month and the three-month anticoagulation therapies. The RIETE registry, which is the largest ongoing published registry of patients with confirmed DVT or PE, reports similar three-month clinical outcomes between those with UEDVT and LEDVT.
Small, single-center trials have shown that active intervention, such as thrombolysis, surgery, or multi-staged approaches are associated with increased vein patency and decreased rates of post-thrombotic syndrome.24,25 However, ACCP has withheld general recommendations for these interventions based on a lack of sufficient data to comment on their overall safety and efficacy, as well as comparable rates of post-thrombotic syndrome (15% to 50%) in studies that directly compared surgical and medical intervention. In fact, the ACCP recommends against interventional treatments unless the patient has failed anticoagulation therapy, has severe symptoms, and expertise is available.4
Superior vena cava filters are available at some centers for patients in whom anticoagulation is contraindicated, but efficacy data is limited. While the data for filter use in UEDVT is limited, its use should be considered in patients who have a contraindication to anticoagulation and remain high risk for UEDVT (e.g., prolonged central line placement).
Complications: Post-thrombotic syndrome (PTS) is the most significant local complication of UEDVT. PTS characteristics are edema, pain, venous ulcers, and skin pigmentation changes, and it is the result of chronic venous insufficiency due to the clot. A meta-analysis of clinical studies on UEDVT noted that PTS occurs in 7% to 46% (mean 15%) of patients.26 One hypothesis for the wide range in frequency is the lack of clear diagnostic criteria for PTS.27 No clear beneficial treatment or prevention for PTS exists, but many recommend graduated compression stockings for the arm.
Residual and recurrent thrombosis are associated with increased PTS risk, which emphasizes the need for further study of interventional treatment because preliminary work has shown increased rates of vein patency in comparison to anticoagulants alone. Recurrent venous thromboembolism (VTE), another local complication, appears to occur less often than it does in patients with LEDVTs, but reaches 8% after five years of followup.28
PE is less common on presentation among patients with UEDVT when compared to patients with LEDVT, but when PE occurs, the three-month outcome is similar.3 PE appears to be more frequent in patients who have a CVC, with an incidence as high as 36% of DVT patients.4,13,21,29
Increased mortality: The mortality among UEDVT patients has been described as 10% to 50% in the 12 months after diagnosis, which is much higher than the ratio in LEDVT patients.21,30 This in part is due to sicker cohorts getting UEDVT. For example, patients with distant metastasis are more likely to develop UEDVT than those with confined malignancy (adjusted OR 11.5; 95% CI, 1.6 to 80.2).31
Occult malignancy, most commonly lung cancer or lymphoma, has been found in as many as 24% of UEDVT patients.32 The high rate of mortality associated with UEDVT appears to be related more with the patient's overall poor clinical condition rather than directly related to complications from the DVT. However, its presence should alert hospitalists to the patient's potentially poorer prognosis and prompt evaluation for occult malignancy if no risk factor is present.
Back to the Case
This patient should be started on either UFH or LMWH while simultaneously beginning warfarin. She should continue warfarin treatment for at least three months, with a goal INR of 2.0 to 3.0, similar to treatment for LEDVT. The ultimate treatment duration with warfarin follows the same guidelines as treatment with a LEDVT. Although prophylaxis is not routinely recommended, dosing 1 mg of warfarin beginning three days before subsequent CVC placement should be considered if this patient requires a future CVC. Additionally, an evaluation for occult malignancy should be considered in this patient.
Bottom Line
Upper extremity DVT is not a benign condition, and is associated with a general increase in mortality. It should be treated similarly to LEDVT in order to decrease PTS, recurrent DVT, and PE. TH
Dr. Hollberg is a clinical instructor in the section of hospital medicine at Emory University Hospital in Atlanta.
References
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis, American Heart Association. Circulation. 1996;93(12):2212-2245.
- Gerotziafas GT, Samama MM. Prophylaxis of venous thromboembolism in medical patients. Curr Opin Pulm Med. 2004;10(5):356-365.
- Kabani L, et al. Upper extremity DVT as prevalent as lower extremity DVT in ICU patients. Society of Critical Care Medicine (SCCM) 38th annual Critical Care Congress: Abstract 305. Presented Feb. 2, 2009.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6Suppl):454S-545S.
- Joffe HV, Kucher N, Tapson VF, Goldhaber SZ. Upper extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110:1605.
- Munoz FJ, Mismetti P, Poggio R, et al. Clinical outcome of patients with an upper-extremity deep vein thrombosis: results from the RIETE registry. Chest. 2008,133:143-148.
- Coon WW, Willis PW. Thrombosis of axillary and subclavian veins. Arch Surg. 1967;94(5):657-663.
- Horattas MC, Wright DJ, Fenton AH, et al. Changing concepts of deep venous thrombosis of the upper extremity—a report of a series and review of the literature. Surgery. 1988;104(3):561-567.
- Bernardi E, Piccioli A, Marchiori A, Girolami B, Prandoni P. Upper extremity deep vein thrombosis: risk factors, diagnosis, and management. Semin Vasc Med. 2001;1(1):105;110.
- Heron E, Lozinguez O, Alhenc-Gelas M, Emmerich J, Flessinger JN. Hypercoagulable states in primary upper-extremity deep vein thrombosis. Arch Intern Med. 2000;160:382-386.
- Ninet J, Demolombe-Rague S, Bureau Du Colombier P, Coppere B. Les thromboses veineuses profondes des members superieurs. Sang Thromb Vaisseaux. 1994;6:103-114.
- Painter TD, Kerpf M. Deep venous thrombosis of the upper extremity five years experience at a university hospital. Angiology. 1984;35(35):743-749.
- Chan WS, Ginsberg JS. A review of upper extremity deep vein thrombosis in pregnancy: unmasking the “ART” behind the clot. J Thromb Haemost. 2006;4(8):1673-1677.
- Hughes MJ, D’Agostino JC. Upper extremity deep vein thrombosis: a case report and review of current diagnostic/therapeutic modalities. Am J Emerg Med. 1994;12(6):631-635.
- Prandoni P, Polistena P, Bernardi E, et al. Upper extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157:57-62.
- Van Rooden CJ, Tesslar ME, Osanto S, Rosendal FR, Huisman MV. Deep vein thrombosis associated with central venous catheters—a review. J Thromb Haemost. 2005;3:2049-2419.
- Horattas MC, Wright DJ, Fenton AH, et al. Changing concepts of deep venous thrombosis of the upper extremity—report of a series and review of the literature. Surgery. 1988;104(3):561-567.
- Bernardi E, Pesavento R, Prandoni P. Upper extremity deep venous thrombosis. Semin Thromb Hemost. 2006;32(7):729-736.
- Baxter GM, McKechnie S, Duffy P. Colour Doppler ultrasound in deep venous thrombosis: a comparison with venography. Clin Radiol. 1990;42(1):32-36.
- Bern MM, Lokich JJ, Wallach SR, et al. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990;112(6):423-428.
- Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23(18):4063-4069.
- Lokich JJ, Both A, Benotti P. Complications and management of implanted central venous catheters. J Clin Oncol. 1985;3:710-717.
- Moss JF, Wagman LD, Rijhmaki DU, Terz JJ. Central venous thrombosis related to the silastic Hickman-Broviac catheter in an oncologic population. J Parenter Enteral Nutr. 1989;13:397.
- Machleder HI. Evaluation of a new treatment strategy for Paget-Schroetter syndrome: spontaneous thrombosis of the axillary-subclavian vein. J Vasc Surg. 1993;17:305-315.
- Malcynski J, O’Donnell TF, Mackey WC. Long-term results of treatment for axillary subclavian vein thrombosis. Can J Surg. 1993;36:365-371.
- Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep vein thrombosis in adults: a systematic review. Thromb Res. 2006;117(6):609-614.
- Baarslag HJ, Koopman MM, Hutten BA, et al. Long-term follow up of patients with suspected deep vein thrombosis of the upper extremity: survival, risk factors and post-thrombotic syndrome. Eur J Intern Med. 2004;15:503-507.
- Prandoni P, Bernardi E, Marchiori A, et al. The long term clinical consequence of acute deep venous thrombosis of the arm: prospective cohort study. BMJ. 2004;329:484-485.
- Monreal M, Raventos A, Lerma R, et al. Pulmonary embolism in patients with upper extremity DVT associated to venous central lines—a prospective study. Thromb Haemost. 1994;72(4):548-550.
- Hingorani A, Ascher E, Lorenson E, et al. Upper extremity deep venous thrombosis and its impact on morbidity and mortality rates in a hospital-based population. J Vasc Surg. 1997;26:853-860.
- Blom JW, Doggen CM, Osanto S, Rosendaal FR. Old and new risk factors for upper extremity deep vein thrombosis. J Thromb Haemost. 2005;3:2471-2478.
- Girolami A, Prandoni P, Zanon E, Bagatella P, Girolami B. Venous thromboses of upper limbs are more frequently associated with occult cancer as compared with those of lower limbs. Blood Coagul Fibrinolysis. 1999;10(8):455-457.
Case
A 45-year-old female with a history of cellulitis requiring peripheral inserted central catheter (PICC) line placement for intravenous antibiotics presents two weeks after line removal with persistent, dull, aching pain in her right shoulder and difficulty removing the rings on her right hand. The pain worsens with exercise and is relieved with rest. The physical exam reveals nonpitting edema of her hand. The ultrasound shows subclavian vein thrombosis. What is the best approach to treating her upper extremity deep venous thrombosis (UEDVT)?
Background
DVT and pulmonary embolism (PE) have been subject to increased publicity recently, and both conditions are recognized as serious entities with life-threatening consequences. In fact, more people die annually from blood clots than breast cancer and AIDS combined.1,2 Still, the increased DVT and PE awareness is primarily focused on lower extremity DVT (LEDVT)), while UEDVT is thought of as a more benign entity. However, current data suggest that UEDVT is associated with equally significant morbidity and mortality.
UEDVT prevalence has increased in step with the increased use of central venous catheters (CVCs) and pacemakers. Although most patients present with pain, swelling, parathesias, and prominent veins throughout the arm or shoulder, many patients will not display any local DVT symptoms. For example, Kabani et al recently presented data for 1,275 patients admitted to the surgical ICU over a 12-month period. They found the incidence of UEDVT was higher than that of LEDVT (17% vs. 11%; P=0.11). They also determined that scanning all four extremities diagnosed more DVT than two-extremity scans (33% vs. 7%; P<0.001).3
While current medical literature has pushed for increased UEDVT attention, there is no consensus on its treatment. Recent American College of Chest Physicians (ACCP) guidelines addressed UEDVT treatment specifically and recommended analogous treatment to LEDVT with heparin and warfarin.4 This follows prospective studies that have shown patients with UEDVT and LEDVT have similar three-month clinical outcomes. The ACCP guidelines do not specifically recommend different treatment courses based on whether the UEDVT is catheter-related or not. Furthermore, while one might assume that removal of an associated catheter might reduce the treatment duration, there is limited data to support shorter courses in this scenario.
Review of the Data
Incidence: UEDVT is becoming more common secondary to increased interventions in the upper extremity (CVC, pacemaker), and is more easily recognized due to improvement in noninvasive ultrasound technology. UEDVT accounts for up to 10% of all DVT, with an incidence of approximately three per 100,000 persons in the general population.5-8 Because UEDVT can also be asymptomatic, it is believed that the incidence likely is higher than previously reported, but prospective data are lacking.
Risk factors: UEDVT is further categorized as either primary or secondary, depending upon the cause. First described in the late 1800s, spontaneous primary thrombosis of the upper extremity, or Paget-Schroetter syndrome, accounts for approximately 20% of UEDVT.9 Primary UEDVT includes both idiopathic and “effort-related” thrombosis. Effort-related thrombosis usually develops among young people after strenuous or repetitive exercise, such as pitching a baseball. Some hypothesize that effort-related thrombosis is related to a hypercoaguable state or anatomic abnormalities, although a specific cause, such as thoracic outlet syndrome, is found in only 5% of these cases.10,11
Secondary UEDVT characterizes thrombosis in which an endogenous or exogenous risk factor is present. Endogenous risk factors include coagulation abnormalities, such as antithrombin, protein C and protein S deficiencies; factor V Leiden gene mutation; hyperhomocysteinemia; and antiphospholipid antibody syndrome. Exogenous risk factors include CVC pacemakers, intracardiac defibrillators, malignancy, previous or concurrent LEDVT, oral contraceptives, some artificial reproductive technologies (women can develop ovarian hyperstimulation syndrome, which is associated with increased hypercoaguability), trauma, and IV drug use (especially cocaine).5,12-14
Clinical presentation and diagnosis: Swelling (80% of patients) and pain (40% of patients) are the most common UEDVT symptoms at presentation.2 Other clinical features include new, prominent veins throughout the shoulder girdle, erythema, increased warmth, functional impairment, parathesias, and non-specific feelings of arm heaviness or discomfort. Symptoms typically worsen with arm use and improve with rest and elevation.15 Patients with UEDVT related to CVC are more likely to be asymptomatic and may present only with PE.16 The differential diagnosis includes superficial phlebitis, lymphatic edema, hematoma, contusions, venous compression, and muscle tears.17
Contrast venography is the gold standard for the UEDVT diagnosis. However, it is more expensive and invasive than ultrasound, and thus serial compression ultrasound is now the standard test in UEDVT evaluation. Then again, contrast venography remains the test of choice in patients with high pre-test probability and negative ultrasound results.18,19
Prevention: Nearly 70% of secondary UEDVT is associated with a CVC.5 Further, CVC use is the most powerful predictor of UEDVT (adjusted odds ratio (OR), 9.7; 95% CI, 7.8 to 12.2).2 Despite the association between CVCs and UEDVT, anticoagulant prophylaxis is not recommended. Studies evaluating the results of 1-mg warfarin conflict and include small populations. Warfarin’s potential interaction with antibiotics and dosing variance based on nutritional intake logically prompted studies on the potential benefit of low-weight molecular heparain (LWMH); however, these studies have failed to show benefit.20,21
Treatment: Recent ACCP guidelines recommend treating UEDVT patients with unfractionated heparin (UFH) or LMWH and warfarin, with an INR goal of 2 to 3 for at least three months depending upon the overall clinical scenario. Two small studies evaluating catheter-related thrombosis (15 patients in each trial) reported no subsequent embolic phenomenon.22,23 Some authors interpreted this data to mean UEDVT was not as morbid as LEDVT and, subsequently, that catheter-related UEDVTs require only one month of therapy. Since the small studies were published, the increasing incidence and relevance of UEDVT have become more widely recognized, and most authors are recommending three months of treatment.
Still, it’s important to note that there aren’t any published data directly comparing the one-month and the three-month anticoagulation therapies. The RIETE registry, which is the largest ongoing published registry of patients with confirmed DVT or PE, reports similar three-month clinical outcomes between those with UEDVT and LEDVT.
Small, single-center trials have shown that active intervention, such as thrombolysis, surgery, or multi-staged approaches are associated with increased vein patency and decreased rates of post-thrombotic syndrome.24,25 However, ACCP has withheld general recommendations for these interventions based on a lack of sufficient data to comment on their overall safety and efficacy, as well as comparable rates of post-thrombotic syndrome (15% to 50%) in studies that directly compared surgical and medical intervention. In fact, the ACCP recommends against interventional treatments unless the patient has failed anticoagulation therapy, has severe symptoms, and expertise is available.4
Superior vena cava filters are available at some centers for patients in whom anticoagulation is contraindicated, but efficacy data is limited. While the data for filter use in UEDVT is limited, its use should be considered in patients who have a contraindication to anticoagulation and remain high risk for UEDVT (e.g., prolonged central line placement).
Complications: Post-thrombotic syndrome (PTS) is the most significant local complication of UEDVT. PTS characteristics are edema, pain, venous ulcers, and skin pigmentation changes, and it is the result of chronic venous insufficiency due to the clot. A meta-analysis of clinical studies on UEDVT noted that PTS occurs in 7% to 46% (mean 15%) of patients.26 One hypothesis for the wide range in frequency is the lack of clear diagnostic criteria for PTS.27 No clear beneficial treatment or prevention for PTS exists, but many recommend graduated compression stockings for the arm.
Residual and recurrent thrombosis are associated with increased PTS risk, which emphasizes the need for further study of interventional treatment because preliminary work has shown increased rates of vein patency in comparison to anticoagulants alone. Recurrent venous thromboembolism (VTE), another local complication, appears to occur less often than it does in patients with LEDVTs, but reaches 8% after five years of followup.28
PE is less common on presentation among patients with UEDVT when compared to patients with LEDVT, but when PE occurs, the three-month outcome is similar.3 PE appears to be more frequent in patients who have a CVC, with an incidence as high as 36% of DVT patients.4,13,21,29
Increased mortality: The mortality among UEDVT patients has been described as 10% to 50% in the 12 months after diagnosis, which is much higher than the ratio in LEDVT patients.21,30 This in part is due to sicker cohorts getting UEDVT. For example, patients with distant metastasis are more likely to develop UEDVT than those with confined malignancy (adjusted OR 11.5; 95% CI, 1.6 to 80.2).31
Occult malignancy, most commonly lung cancer or lymphoma, has been found in as many as 24% of UEDVT patients.32 The high rate of mortality associated with UEDVT appears to be related more with the patient's overall poor clinical condition rather than directly related to complications from the DVT. However, its presence should alert hospitalists to the patient's potentially poorer prognosis and prompt evaluation for occult malignancy if no risk factor is present.
Back to the Case
This patient should be started on either UFH or LMWH while simultaneously beginning warfarin. She should continue warfarin treatment for at least three months, with a goal INR of 2.0 to 3.0, similar to treatment for LEDVT. The ultimate treatment duration with warfarin follows the same guidelines as treatment with a LEDVT. Although prophylaxis is not routinely recommended, dosing 1 mg of warfarin beginning three days before subsequent CVC placement should be considered if this patient requires a future CVC. Additionally, an evaluation for occult malignancy should be considered in this patient.
Bottom Line
Upper extremity DVT is not a benign condition, and is associated with a general increase in mortality. It should be treated similarly to LEDVT in order to decrease PTS, recurrent DVT, and PE. TH
Dr. Hollberg is a clinical instructor in the section of hospital medicine at Emory University Hospital in Atlanta.
References
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis, American Heart Association. Circulation. 1996;93(12):2212-2245.
- Gerotziafas GT, Samama MM. Prophylaxis of venous thromboembolism in medical patients. Curr Opin Pulm Med. 2004;10(5):356-365.
- Kabani L, et al. Upper extremity DVT as prevalent as lower extremity DVT in ICU patients. Society of Critical Care Medicine (SCCM) 38th annual Critical Care Congress: Abstract 305. Presented Feb. 2, 2009.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6Suppl):454S-545S.
- Joffe HV, Kucher N, Tapson VF, Goldhaber SZ. Upper extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110:1605.
- Munoz FJ, Mismetti P, Poggio R, et al. Clinical outcome of patients with an upper-extremity deep vein thrombosis: results from the RIETE registry. Chest. 2008,133:143-148.
- Coon WW, Willis PW. Thrombosis of axillary and subclavian veins. Arch Surg. 1967;94(5):657-663.
- Horattas MC, Wright DJ, Fenton AH, et al. Changing concepts of deep venous thrombosis of the upper extremity—a report of a series and review of the literature. Surgery. 1988;104(3):561-567.
- Bernardi E, Piccioli A, Marchiori A, Girolami B, Prandoni P. Upper extremity deep vein thrombosis: risk factors, diagnosis, and management. Semin Vasc Med. 2001;1(1):105;110.
- Heron E, Lozinguez O, Alhenc-Gelas M, Emmerich J, Flessinger JN. Hypercoagulable states in primary upper-extremity deep vein thrombosis. Arch Intern Med. 2000;160:382-386.
- Ninet J, Demolombe-Rague S, Bureau Du Colombier P, Coppere B. Les thromboses veineuses profondes des members superieurs. Sang Thromb Vaisseaux. 1994;6:103-114.
- Painter TD, Kerpf M. Deep venous thrombosis of the upper extremity five years experience at a university hospital. Angiology. 1984;35(35):743-749.
- Chan WS, Ginsberg JS. A review of upper extremity deep vein thrombosis in pregnancy: unmasking the “ART” behind the clot. J Thromb Haemost. 2006;4(8):1673-1677.
- Hughes MJ, D’Agostino JC. Upper extremity deep vein thrombosis: a case report and review of current diagnostic/therapeutic modalities. Am J Emerg Med. 1994;12(6):631-635.
- Prandoni P, Polistena P, Bernardi E, et al. Upper extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157:57-62.
- Van Rooden CJ, Tesslar ME, Osanto S, Rosendal FR, Huisman MV. Deep vein thrombosis associated with central venous catheters—a review. J Thromb Haemost. 2005;3:2049-2419.
- Horattas MC, Wright DJ, Fenton AH, et al. Changing concepts of deep venous thrombosis of the upper extremity—report of a series and review of the literature. Surgery. 1988;104(3):561-567.
- Bernardi E, Pesavento R, Prandoni P. Upper extremity deep venous thrombosis. Semin Thromb Hemost. 2006;32(7):729-736.
- Baxter GM, McKechnie S, Duffy P. Colour Doppler ultrasound in deep venous thrombosis: a comparison with venography. Clin Radiol. 1990;42(1):32-36.
- Bern MM, Lokich JJ, Wallach SR, et al. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990;112(6):423-428.
- Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23(18):4063-4069.
- Lokich JJ, Both A, Benotti P. Complications and management of implanted central venous catheters. J Clin Oncol. 1985;3:710-717.
- Moss JF, Wagman LD, Rijhmaki DU, Terz JJ. Central venous thrombosis related to the silastic Hickman-Broviac catheter in an oncologic population. J Parenter Enteral Nutr. 1989;13:397.
- Machleder HI. Evaluation of a new treatment strategy for Paget-Schroetter syndrome: spontaneous thrombosis of the axillary-subclavian vein. J Vasc Surg. 1993;17:305-315.
- Malcynski J, O’Donnell TF, Mackey WC. Long-term results of treatment for axillary subclavian vein thrombosis. Can J Surg. 1993;36:365-371.
- Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep vein thrombosis in adults: a systematic review. Thromb Res. 2006;117(6):609-614.
- Baarslag HJ, Koopman MM, Hutten BA, et al. Long-term follow up of patients with suspected deep vein thrombosis of the upper extremity: survival, risk factors and post-thrombotic syndrome. Eur J Intern Med. 2004;15:503-507.
- Prandoni P, Bernardi E, Marchiori A, et al. The long term clinical consequence of acute deep venous thrombosis of the arm: prospective cohort study. BMJ. 2004;329:484-485.
- Monreal M, Raventos A, Lerma R, et al. Pulmonary embolism in patients with upper extremity DVT associated to venous central lines—a prospective study. Thromb Haemost. 1994;72(4):548-550.
- Hingorani A, Ascher E, Lorenson E, et al. Upper extremity deep venous thrombosis and its impact on morbidity and mortality rates in a hospital-based population. J Vasc Surg. 1997;26:853-860.
- Blom JW, Doggen CM, Osanto S, Rosendaal FR. Old and new risk factors for upper extremity deep vein thrombosis. J Thromb Haemost. 2005;3:2471-2478.
- Girolami A, Prandoni P, Zanon E, Bagatella P, Girolami B. Venous thromboses of upper limbs are more frequently associated with occult cancer as compared with those of lower limbs. Blood Coagul Fibrinolysis. 1999;10(8):455-457.
Case
A 45-year-old female with a history of cellulitis requiring peripheral inserted central catheter (PICC) line placement for intravenous antibiotics presents two weeks after line removal with persistent, dull, aching pain in her right shoulder and difficulty removing the rings on her right hand. The pain worsens with exercise and is relieved with rest. The physical exam reveals nonpitting edema of her hand. The ultrasound shows subclavian vein thrombosis. What is the best approach to treating her upper extremity deep venous thrombosis (UEDVT)?
Background
DVT and pulmonary embolism (PE) have been subject to increased publicity recently, and both conditions are recognized as serious entities with life-threatening consequences. In fact, more people die annually from blood clots than breast cancer and AIDS combined.1,2 Still, the increased DVT and PE awareness is primarily focused on lower extremity DVT (LEDVT)), while UEDVT is thought of as a more benign entity. However, current data suggest that UEDVT is associated with equally significant morbidity and mortality.
UEDVT prevalence has increased in step with the increased use of central venous catheters (CVCs) and pacemakers. Although most patients present with pain, swelling, parathesias, and prominent veins throughout the arm or shoulder, many patients will not display any local DVT symptoms. For example, Kabani et al recently presented data for 1,275 patients admitted to the surgical ICU over a 12-month period. They found the incidence of UEDVT was higher than that of LEDVT (17% vs. 11%; P=0.11). They also determined that scanning all four extremities diagnosed more DVT than two-extremity scans (33% vs. 7%; P<0.001).3
While current medical literature has pushed for increased UEDVT attention, there is no consensus on its treatment. Recent American College of Chest Physicians (ACCP) guidelines addressed UEDVT treatment specifically and recommended analogous treatment to LEDVT with heparin and warfarin.4 This follows prospective studies that have shown patients with UEDVT and LEDVT have similar three-month clinical outcomes. The ACCP guidelines do not specifically recommend different treatment courses based on whether the UEDVT is catheter-related or not. Furthermore, while one might assume that removal of an associated catheter might reduce the treatment duration, there is limited data to support shorter courses in this scenario.
Review of the Data
Incidence: UEDVT is becoming more common secondary to increased interventions in the upper extremity (CVC, pacemaker), and is more easily recognized due to improvement in noninvasive ultrasound technology. UEDVT accounts for up to 10% of all DVT, with an incidence of approximately three per 100,000 persons in the general population.5-8 Because UEDVT can also be asymptomatic, it is believed that the incidence likely is higher than previously reported, but prospective data are lacking.
Risk factors: UEDVT is further categorized as either primary or secondary, depending upon the cause. First described in the late 1800s, spontaneous primary thrombosis of the upper extremity, or Paget-Schroetter syndrome, accounts for approximately 20% of UEDVT.9 Primary UEDVT includes both idiopathic and “effort-related” thrombosis. Effort-related thrombosis usually develops among young people after strenuous or repetitive exercise, such as pitching a baseball. Some hypothesize that effort-related thrombosis is related to a hypercoaguable state or anatomic abnormalities, although a specific cause, such as thoracic outlet syndrome, is found in only 5% of these cases.10,11
Secondary UEDVT characterizes thrombosis in which an endogenous or exogenous risk factor is present. Endogenous risk factors include coagulation abnormalities, such as antithrombin, protein C and protein S deficiencies; factor V Leiden gene mutation; hyperhomocysteinemia; and antiphospholipid antibody syndrome. Exogenous risk factors include CVC pacemakers, intracardiac defibrillators, malignancy, previous or concurrent LEDVT, oral contraceptives, some artificial reproductive technologies (women can develop ovarian hyperstimulation syndrome, which is associated with increased hypercoaguability), trauma, and IV drug use (especially cocaine).5,12-14
Clinical presentation and diagnosis: Swelling (80% of patients) and pain (40% of patients) are the most common UEDVT symptoms at presentation.2 Other clinical features include new, prominent veins throughout the shoulder girdle, erythema, increased warmth, functional impairment, parathesias, and non-specific feelings of arm heaviness or discomfort. Symptoms typically worsen with arm use and improve with rest and elevation.15 Patients with UEDVT related to CVC are more likely to be asymptomatic and may present only with PE.16 The differential diagnosis includes superficial phlebitis, lymphatic edema, hematoma, contusions, venous compression, and muscle tears.17
Contrast venography is the gold standard for the UEDVT diagnosis. However, it is more expensive and invasive than ultrasound, and thus serial compression ultrasound is now the standard test in UEDVT evaluation. Then again, contrast venography remains the test of choice in patients with high pre-test probability and negative ultrasound results.18,19
Prevention: Nearly 70% of secondary UEDVT is associated with a CVC.5 Further, CVC use is the most powerful predictor of UEDVT (adjusted odds ratio (OR), 9.7; 95% CI, 7.8 to 12.2).2 Despite the association between CVCs and UEDVT, anticoagulant prophylaxis is not recommended. Studies evaluating the results of 1-mg warfarin conflict and include small populations. Warfarin’s potential interaction with antibiotics and dosing variance based on nutritional intake logically prompted studies on the potential benefit of low-weight molecular heparain (LWMH); however, these studies have failed to show benefit.20,21
Treatment: Recent ACCP guidelines recommend treating UEDVT patients with unfractionated heparin (UFH) or LMWH and warfarin, with an INR goal of 2 to 3 for at least three months depending upon the overall clinical scenario. Two small studies evaluating catheter-related thrombosis (15 patients in each trial) reported no subsequent embolic phenomenon.22,23 Some authors interpreted this data to mean UEDVT was not as morbid as LEDVT and, subsequently, that catheter-related UEDVTs require only one month of therapy. Since the small studies were published, the increasing incidence and relevance of UEDVT have become more widely recognized, and most authors are recommending three months of treatment.
Still, it’s important to note that there aren’t any published data directly comparing the one-month and the three-month anticoagulation therapies. The RIETE registry, which is the largest ongoing published registry of patients with confirmed DVT or PE, reports similar three-month clinical outcomes between those with UEDVT and LEDVT.
Small, single-center trials have shown that active intervention, such as thrombolysis, surgery, or multi-staged approaches are associated with increased vein patency and decreased rates of post-thrombotic syndrome.24,25 However, ACCP has withheld general recommendations for these interventions based on a lack of sufficient data to comment on their overall safety and efficacy, as well as comparable rates of post-thrombotic syndrome (15% to 50%) in studies that directly compared surgical and medical intervention. In fact, the ACCP recommends against interventional treatments unless the patient has failed anticoagulation therapy, has severe symptoms, and expertise is available.4
Superior vena cava filters are available at some centers for patients in whom anticoagulation is contraindicated, but efficacy data is limited. While the data for filter use in UEDVT is limited, its use should be considered in patients who have a contraindication to anticoagulation and remain high risk for UEDVT (e.g., prolonged central line placement).
Complications: Post-thrombotic syndrome (PTS) is the most significant local complication of UEDVT. PTS characteristics are edema, pain, venous ulcers, and skin pigmentation changes, and it is the result of chronic venous insufficiency due to the clot. A meta-analysis of clinical studies on UEDVT noted that PTS occurs in 7% to 46% (mean 15%) of patients.26 One hypothesis for the wide range in frequency is the lack of clear diagnostic criteria for PTS.27 No clear beneficial treatment or prevention for PTS exists, but many recommend graduated compression stockings for the arm.
Residual and recurrent thrombosis are associated with increased PTS risk, which emphasizes the need for further study of interventional treatment because preliminary work has shown increased rates of vein patency in comparison to anticoagulants alone. Recurrent venous thromboembolism (VTE), another local complication, appears to occur less often than it does in patients with LEDVTs, but reaches 8% after five years of followup.28
PE is less common on presentation among patients with UEDVT when compared to patients with LEDVT, but when PE occurs, the three-month outcome is similar.3 PE appears to be more frequent in patients who have a CVC, with an incidence as high as 36% of DVT patients.4,13,21,29
Increased mortality: The mortality among UEDVT patients has been described as 10% to 50% in the 12 months after diagnosis, which is much higher than the ratio in LEDVT patients.21,30 This in part is due to sicker cohorts getting UEDVT. For example, patients with distant metastasis are more likely to develop UEDVT than those with confined malignancy (adjusted OR 11.5; 95% CI, 1.6 to 80.2).31
Occult malignancy, most commonly lung cancer or lymphoma, has been found in as many as 24% of UEDVT patients.32 The high rate of mortality associated with UEDVT appears to be related more with the patient's overall poor clinical condition rather than directly related to complications from the DVT. However, its presence should alert hospitalists to the patient's potentially poorer prognosis and prompt evaluation for occult malignancy if no risk factor is present.
Back to the Case
This patient should be started on either UFH or LMWH while simultaneously beginning warfarin. She should continue warfarin treatment for at least three months, with a goal INR of 2.0 to 3.0, similar to treatment for LEDVT. The ultimate treatment duration with warfarin follows the same guidelines as treatment with a LEDVT. Although prophylaxis is not routinely recommended, dosing 1 mg of warfarin beginning three days before subsequent CVC placement should be considered if this patient requires a future CVC. Additionally, an evaluation for occult malignancy should be considered in this patient.
Bottom Line
Upper extremity DVT is not a benign condition, and is associated with a general increase in mortality. It should be treated similarly to LEDVT in order to decrease PTS, recurrent DVT, and PE. TH
Dr. Hollberg is a clinical instructor in the section of hospital medicine at Emory University Hospital in Atlanta.
References
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis, American Heart Association. Circulation. 1996;93(12):2212-2245.
- Gerotziafas GT, Samama MM. Prophylaxis of venous thromboembolism in medical patients. Curr Opin Pulm Med. 2004;10(5):356-365.
- Kabani L, et al. Upper extremity DVT as prevalent as lower extremity DVT in ICU patients. Society of Critical Care Medicine (SCCM) 38th annual Critical Care Congress: Abstract 305. Presented Feb. 2, 2009.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133(6Suppl):454S-545S.
- Joffe HV, Kucher N, Tapson VF, Goldhaber SZ. Upper extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110:1605.
- Munoz FJ, Mismetti P, Poggio R, et al. Clinical outcome of patients with an upper-extremity deep vein thrombosis: results from the RIETE registry. Chest. 2008,133:143-148.
- Coon WW, Willis PW. Thrombosis of axillary and subclavian veins. Arch Surg. 1967;94(5):657-663.
- Horattas MC, Wright DJ, Fenton AH, et al. Changing concepts of deep venous thrombosis of the upper extremity—a report of a series and review of the literature. Surgery. 1988;104(3):561-567.
- Bernardi E, Piccioli A, Marchiori A, Girolami B, Prandoni P. Upper extremity deep vein thrombosis: risk factors, diagnosis, and management. Semin Vasc Med. 2001;1(1):105;110.
- Heron E, Lozinguez O, Alhenc-Gelas M, Emmerich J, Flessinger JN. Hypercoagulable states in primary upper-extremity deep vein thrombosis. Arch Intern Med. 2000;160:382-386.
- Ninet J, Demolombe-Rague S, Bureau Du Colombier P, Coppere B. Les thromboses veineuses profondes des members superieurs. Sang Thromb Vaisseaux. 1994;6:103-114.
- Painter TD, Kerpf M. Deep venous thrombosis of the upper extremity five years experience at a university hospital. Angiology. 1984;35(35):743-749.
- Chan WS, Ginsberg JS. A review of upper extremity deep vein thrombosis in pregnancy: unmasking the “ART” behind the clot. J Thromb Haemost. 2006;4(8):1673-1677.
- Hughes MJ, D’Agostino JC. Upper extremity deep vein thrombosis: a case report and review of current diagnostic/therapeutic modalities. Am J Emerg Med. 1994;12(6):631-635.
- Prandoni P, Polistena P, Bernardi E, et al. Upper extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157:57-62.
- Van Rooden CJ, Tesslar ME, Osanto S, Rosendal FR, Huisman MV. Deep vein thrombosis associated with central venous catheters—a review. J Thromb Haemost. 2005;3:2049-2419.
- Horattas MC, Wright DJ, Fenton AH, et al. Changing concepts of deep venous thrombosis of the upper extremity—report of a series and review of the literature. Surgery. 1988;104(3):561-567.
- Bernardi E, Pesavento R, Prandoni P. Upper extremity deep venous thrombosis. Semin Thromb Hemost. 2006;32(7):729-736.
- Baxter GM, McKechnie S, Duffy P. Colour Doppler ultrasound in deep venous thrombosis: a comparison with venography. Clin Radiol. 1990;42(1):32-36.
- Bern MM, Lokich JJ, Wallach SR, et al. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990;112(6):423-428.
- Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23(18):4063-4069.
- Lokich JJ, Both A, Benotti P. Complications and management of implanted central venous catheters. J Clin Oncol. 1985;3:710-717.
- Moss JF, Wagman LD, Rijhmaki DU, Terz JJ. Central venous thrombosis related to the silastic Hickman-Broviac catheter in an oncologic population. J Parenter Enteral Nutr. 1989;13:397.
- Machleder HI. Evaluation of a new treatment strategy for Paget-Schroetter syndrome: spontaneous thrombosis of the axillary-subclavian vein. J Vasc Surg. 1993;17:305-315.
- Malcynski J, O’Donnell TF, Mackey WC. Long-term results of treatment for axillary subclavian vein thrombosis. Can J Surg. 1993;36:365-371.
- Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep vein thrombosis in adults: a systematic review. Thromb Res. 2006;117(6):609-614.
- Baarslag HJ, Koopman MM, Hutten BA, et al. Long-term follow up of patients with suspected deep vein thrombosis of the upper extremity: survival, risk factors and post-thrombotic syndrome. Eur J Intern Med. 2004;15:503-507.
- Prandoni P, Bernardi E, Marchiori A, et al. The long term clinical consequence of acute deep venous thrombosis of the arm: prospective cohort study. BMJ. 2004;329:484-485.
- Monreal M, Raventos A, Lerma R, et al. Pulmonary embolism in patients with upper extremity DVT associated to venous central lines—a prospective study. Thromb Haemost. 1994;72(4):548-550.
- Hingorani A, Ascher E, Lorenson E, et al. Upper extremity deep venous thrombosis and its impact on morbidity and mortality rates in a hospital-based population. J Vasc Surg. 1997;26:853-860.
- Blom JW, Doggen CM, Osanto S, Rosendaal FR. Old and new risk factors for upper extremity deep vein thrombosis. J Thromb Haemost. 2005;3:2471-2478.
- Girolami A, Prandoni P, Zanon E, Bagatella P, Girolami B. Venous thromboses of upper limbs are more frequently associated with occult cancer as compared with those of lower limbs. Blood Coagul Fibrinolysis. 1999;10(8):455-457.
He’s on HM’s Fast Track
William Ford, MD, FHM, was just three years removed from residency when he assumed his first HM leadership role. His qualifications were impressive, but because he was just in his early 30s, his soon-to-be bosses needed some convincing that he was right for the job.
But Dr. Ford—now medical director at Cogent Healthcare and director of the HM program at Temple University in Philadelphia—quickly proved that ability, attitude, and work ethic mean as much as, if not more than, a lengthy résumé. “I don’t think you need a title to lead or a position to lead,” Dr. Ford says. “You need the will to lead.”
Q: You spent a little more than a year in private practice before you became a hospitalist. What motivated you to make the switch?
A: I really enjoy the mix of responsibilities. There’s a lot more to HM than just treating the patient. You’re actually treating the hospital. And, ultimately, it’s more fast-paced. I like the ability to be on the cutting edge of medicine, and just being in a hospital keeps you on your toes from a medical perspective.
Q: Your first two clinical sites—Lehigh Valley Hospital in Allentown, Pa., and Union Hospital in Elkton, Md.—are more suburban settings. What made Temple the right fit?
A: I trained at Drexel University (also in Philadelphia), and I wanted to get back to the urban setting. I find that environment to be very challenging.
Q: How so?
A: The biggest challenge is the socioeconomic problems. Eighty percent of our patients are on Medicare or Medicaid. … In a nutshell, the challenge comes down to basic access to care.
Q: How frustrating is that for you?
A: It’s very frustrating, and it angers me. If I write a prescription for a patient, there’s a good chance the person won’t take it. If I tell them they need follow-up treatment, there’s a good chance they won’t get it. It’s not that they don’t want to. Maybe they can’t afford the co-pay, or maybe, if they haven’t been monitored by a primary-care physician (PCP), they can’t get an appointment for three months. I know we, as a group, can care for patients much better if they would follow up with our instructions. But because of the hoops they have to go through, whether for economic reasons or access reasons, many of them are coming back to the ED.
Q: What keeps you going in spite of those challenges?
A: The patients. They are a very grateful population. They know they are underserved, and they are appreciative of the care.
Q: Temple partnered with Cogent Healthcare in 2006 to manage its hospitalist program. Were you excited about being able to put your stamp on a program and really help it develop?
A: That was enormously appealing. If you’re a true leader, you’re never satisfied with the way your boss is running the program. If you really have the qualities to lead a group, you always think you can do better. … I was intrigued by the opportunity to start a group in a major teaching center that, for the first time, was outsourcing its hospitalist program and trying to solidify its teaching mission.
Q: How quickly has the program grown?
A: We’ve grown from four physicians to 27, and we treat about 15,000 inpatients annually.
Q: What advice would you give to the director of a program experiencing similar growth?
A: Be very stringent on the doctors you choose. For a lot of groups, retention/recruitment is the No. 1, No. 2, and No. 3 problem. We’ve been fortunate we haven’t made many bad hires. But the time and effort it takes to get rid of a bad hire can really end up bogging you down. I’d rather have everyone pull up their bootstraps and work a bit harder and take an extra few months to find the right person than go ahead with a bad hire simply to have another body.
Q: Were there other keys behind the program’s success?
A: There are several. I owe a great deal of the success of the program to the great doctors I work with. I received tremendous support from the department of internal medicine when I arrived, and that ensured a smooth transition. Another big component is good communication.
Q: What role has communication played?
A: Hospitals are very siloed. One group doesn’t speak to another. We’re taught to stick our head in the sand, fix the problem, and move on to the next problem. That gets you crucified in the world of HM. As hospitalists, we have to be the glue that brings all these silos together. In our profession, to be a good leader, you don’t have to be the smartest or best clinician. But you do have to have the attributes of communication and teambuilding. The key is to meet people and talk to them. Try to get to know every key hospital administrator. Don’t just write an order and go away; talk to the nurse. If you forge relationships and try to get the group more fully implemented, it will be more likely to reach its full potential.
Q: At 35, you are slightly younger than the average U.S. hospitalist, yet you’re nearly three years into your first true leadership role. Has your age ever been an issue?
A: Initially, it was a hindrance. It took four months for Temple to interview me. The biggest negative they gave to Cogent was, “He’s so young.” In any other field, 35 would not be considered a child. We’d be in the workforce for 13 years, and we’d be considered middle or senior management. Medicine in general is steeped in, “If you don’t have gray hair, you’re not able to sit at the table.” In our specialty, you can. … It doesn’t have to hinder you, but you have to be willing and able to do the right things. If you are, you will be noticed.
Q: You consider HM program marketing and branding one of your specialties. Why are those efforts necessary?
A: If you don’t market yourself, you’ll die, particularly in a competitive market. Whether you are at an academic center or a small community hospital or even a larger hospital, you could have two or three hospitalist groups all vying for the same patient volume. You need to give yourself a differential advantage.
Q: How do you do that?
A: You have to get out and meet people and shake some hands. You have to meet all of your customers, and you have to find out if they are happy or displeased. You have to communicate with them. You have to think about your customers, and they’re not just the patients in the bed. Your customers also are your administration, your PCPs, your subspecialists. … It’s no different than a vendor selling fax machines. We are a business, and if doctors don’t think that, they’re very naive.
Q: You’re also a big proponent of team-building within groups.
A: Definitely. That’s the foundation. Groups are going to coalesce differently. In my group at Lehigh Valley, we all had a Fourth of July party. We were never so close as after that one experience when we shared dinner together. It may be as simple as that. At Temple, I had all 25 of us meet and go over a teambuilding exercise to understand what values people have and why they come to work. I asked them to tell me something I didn’t know about them. I heard everything from “I changed my name when I was 5” to “I played basketball in college.”
You’re more willing to cover for a colleague if he or she is sick if you get to know them on a personal level. And if that happens, you’re less likely to leave, and that decreases turnover. On top of everything else, you become a group. You see group buy-in and goal recognition, and you start to see those goals attained.
Q: On top of your administrative duties and teaching responsibilities, you’re still doing 10 clinical shifts per month. Why?
A: It’s hugely important for two reasons. No. 1 is respect among members of your team. No. 2 is knowledge of your service. It’s not until you get your hands dirty that you can really understand what physicians in your group are going through and figure out ways to make life better. And at the end of the day, we’re all still physicians. TH
Mark Leiser is a freelance writer in New Jersey.
William Ford, MD, FHM, was just three years removed from residency when he assumed his first HM leadership role. His qualifications were impressive, but because he was just in his early 30s, his soon-to-be bosses needed some convincing that he was right for the job.
But Dr. Ford—now medical director at Cogent Healthcare and director of the HM program at Temple University in Philadelphia—quickly proved that ability, attitude, and work ethic mean as much as, if not more than, a lengthy résumé. “I don’t think you need a title to lead or a position to lead,” Dr. Ford says. “You need the will to lead.”
Q: You spent a little more than a year in private practice before you became a hospitalist. What motivated you to make the switch?
A: I really enjoy the mix of responsibilities. There’s a lot more to HM than just treating the patient. You’re actually treating the hospital. And, ultimately, it’s more fast-paced. I like the ability to be on the cutting edge of medicine, and just being in a hospital keeps you on your toes from a medical perspective.
Q: Your first two clinical sites—Lehigh Valley Hospital in Allentown, Pa., and Union Hospital in Elkton, Md.—are more suburban settings. What made Temple the right fit?
A: I trained at Drexel University (also in Philadelphia), and I wanted to get back to the urban setting. I find that environment to be very challenging.
Q: How so?
A: The biggest challenge is the socioeconomic problems. Eighty percent of our patients are on Medicare or Medicaid. … In a nutshell, the challenge comes down to basic access to care.
Q: How frustrating is that for you?
A: It’s very frustrating, and it angers me. If I write a prescription for a patient, there’s a good chance the person won’t take it. If I tell them they need follow-up treatment, there’s a good chance they won’t get it. It’s not that they don’t want to. Maybe they can’t afford the co-pay, or maybe, if they haven’t been monitored by a primary-care physician (PCP), they can’t get an appointment for three months. I know we, as a group, can care for patients much better if they would follow up with our instructions. But because of the hoops they have to go through, whether for economic reasons or access reasons, many of them are coming back to the ED.
Q: What keeps you going in spite of those challenges?
A: The patients. They are a very grateful population. They know they are underserved, and they are appreciative of the care.
Q: Temple partnered with Cogent Healthcare in 2006 to manage its hospitalist program. Were you excited about being able to put your stamp on a program and really help it develop?
A: That was enormously appealing. If you’re a true leader, you’re never satisfied with the way your boss is running the program. If you really have the qualities to lead a group, you always think you can do better. … I was intrigued by the opportunity to start a group in a major teaching center that, for the first time, was outsourcing its hospitalist program and trying to solidify its teaching mission.
Q: How quickly has the program grown?
A: We’ve grown from four physicians to 27, and we treat about 15,000 inpatients annually.
Q: What advice would you give to the director of a program experiencing similar growth?
A: Be very stringent on the doctors you choose. For a lot of groups, retention/recruitment is the No. 1, No. 2, and No. 3 problem. We’ve been fortunate we haven’t made many bad hires. But the time and effort it takes to get rid of a bad hire can really end up bogging you down. I’d rather have everyone pull up their bootstraps and work a bit harder and take an extra few months to find the right person than go ahead with a bad hire simply to have another body.
Q: Were there other keys behind the program’s success?
A: There are several. I owe a great deal of the success of the program to the great doctors I work with. I received tremendous support from the department of internal medicine when I arrived, and that ensured a smooth transition. Another big component is good communication.
Q: What role has communication played?
A: Hospitals are very siloed. One group doesn’t speak to another. We’re taught to stick our head in the sand, fix the problem, and move on to the next problem. That gets you crucified in the world of HM. As hospitalists, we have to be the glue that brings all these silos together. In our profession, to be a good leader, you don’t have to be the smartest or best clinician. But you do have to have the attributes of communication and teambuilding. The key is to meet people and talk to them. Try to get to know every key hospital administrator. Don’t just write an order and go away; talk to the nurse. If you forge relationships and try to get the group more fully implemented, it will be more likely to reach its full potential.
Q: At 35, you are slightly younger than the average U.S. hospitalist, yet you’re nearly three years into your first true leadership role. Has your age ever been an issue?
A: Initially, it was a hindrance. It took four months for Temple to interview me. The biggest negative they gave to Cogent was, “He’s so young.” In any other field, 35 would not be considered a child. We’d be in the workforce for 13 years, and we’d be considered middle or senior management. Medicine in general is steeped in, “If you don’t have gray hair, you’re not able to sit at the table.” In our specialty, you can. … It doesn’t have to hinder you, but you have to be willing and able to do the right things. If you are, you will be noticed.
Q: You consider HM program marketing and branding one of your specialties. Why are those efforts necessary?
A: If you don’t market yourself, you’ll die, particularly in a competitive market. Whether you are at an academic center or a small community hospital or even a larger hospital, you could have two or three hospitalist groups all vying for the same patient volume. You need to give yourself a differential advantage.
Q: How do you do that?
A: You have to get out and meet people and shake some hands. You have to meet all of your customers, and you have to find out if they are happy or displeased. You have to communicate with them. You have to think about your customers, and they’re not just the patients in the bed. Your customers also are your administration, your PCPs, your subspecialists. … It’s no different than a vendor selling fax machines. We are a business, and if doctors don’t think that, they’re very naive.
Q: You’re also a big proponent of team-building within groups.
A: Definitely. That’s the foundation. Groups are going to coalesce differently. In my group at Lehigh Valley, we all had a Fourth of July party. We were never so close as after that one experience when we shared dinner together. It may be as simple as that. At Temple, I had all 25 of us meet and go over a teambuilding exercise to understand what values people have and why they come to work. I asked them to tell me something I didn’t know about them. I heard everything from “I changed my name when I was 5” to “I played basketball in college.”
You’re more willing to cover for a colleague if he or she is sick if you get to know them on a personal level. And if that happens, you’re less likely to leave, and that decreases turnover. On top of everything else, you become a group. You see group buy-in and goal recognition, and you start to see those goals attained.
Q: On top of your administrative duties and teaching responsibilities, you’re still doing 10 clinical shifts per month. Why?
A: It’s hugely important for two reasons. No. 1 is respect among members of your team. No. 2 is knowledge of your service. It’s not until you get your hands dirty that you can really understand what physicians in your group are going through and figure out ways to make life better. And at the end of the day, we’re all still physicians. TH
Mark Leiser is a freelance writer in New Jersey.
William Ford, MD, FHM, was just three years removed from residency when he assumed his first HM leadership role. His qualifications were impressive, but because he was just in his early 30s, his soon-to-be bosses needed some convincing that he was right for the job.
But Dr. Ford—now medical director at Cogent Healthcare and director of the HM program at Temple University in Philadelphia—quickly proved that ability, attitude, and work ethic mean as much as, if not more than, a lengthy résumé. “I don’t think you need a title to lead or a position to lead,” Dr. Ford says. “You need the will to lead.”
Q: You spent a little more than a year in private practice before you became a hospitalist. What motivated you to make the switch?
A: I really enjoy the mix of responsibilities. There’s a lot more to HM than just treating the patient. You’re actually treating the hospital. And, ultimately, it’s more fast-paced. I like the ability to be on the cutting edge of medicine, and just being in a hospital keeps you on your toes from a medical perspective.
Q: Your first two clinical sites—Lehigh Valley Hospital in Allentown, Pa., and Union Hospital in Elkton, Md.—are more suburban settings. What made Temple the right fit?
A: I trained at Drexel University (also in Philadelphia), and I wanted to get back to the urban setting. I find that environment to be very challenging.
Q: How so?
A: The biggest challenge is the socioeconomic problems. Eighty percent of our patients are on Medicare or Medicaid. … In a nutshell, the challenge comes down to basic access to care.
Q: How frustrating is that for you?
A: It’s very frustrating, and it angers me. If I write a prescription for a patient, there’s a good chance the person won’t take it. If I tell them they need follow-up treatment, there’s a good chance they won’t get it. It’s not that they don’t want to. Maybe they can’t afford the co-pay, or maybe, if they haven’t been monitored by a primary-care physician (PCP), they can’t get an appointment for three months. I know we, as a group, can care for patients much better if they would follow up with our instructions. But because of the hoops they have to go through, whether for economic reasons or access reasons, many of them are coming back to the ED.
Q: What keeps you going in spite of those challenges?
A: The patients. They are a very grateful population. They know they are underserved, and they are appreciative of the care.
Q: Temple partnered with Cogent Healthcare in 2006 to manage its hospitalist program. Were you excited about being able to put your stamp on a program and really help it develop?
A: That was enormously appealing. If you’re a true leader, you’re never satisfied with the way your boss is running the program. If you really have the qualities to lead a group, you always think you can do better. … I was intrigued by the opportunity to start a group in a major teaching center that, for the first time, was outsourcing its hospitalist program and trying to solidify its teaching mission.
Q: How quickly has the program grown?
A: We’ve grown from four physicians to 27, and we treat about 15,000 inpatients annually.
Q: What advice would you give to the director of a program experiencing similar growth?
A: Be very stringent on the doctors you choose. For a lot of groups, retention/recruitment is the No. 1, No. 2, and No. 3 problem. We’ve been fortunate we haven’t made many bad hires. But the time and effort it takes to get rid of a bad hire can really end up bogging you down. I’d rather have everyone pull up their bootstraps and work a bit harder and take an extra few months to find the right person than go ahead with a bad hire simply to have another body.
Q: Were there other keys behind the program’s success?
A: There are several. I owe a great deal of the success of the program to the great doctors I work with. I received tremendous support from the department of internal medicine when I arrived, and that ensured a smooth transition. Another big component is good communication.
Q: What role has communication played?
A: Hospitals are very siloed. One group doesn’t speak to another. We’re taught to stick our head in the sand, fix the problem, and move on to the next problem. That gets you crucified in the world of HM. As hospitalists, we have to be the glue that brings all these silos together. In our profession, to be a good leader, you don’t have to be the smartest or best clinician. But you do have to have the attributes of communication and teambuilding. The key is to meet people and talk to them. Try to get to know every key hospital administrator. Don’t just write an order and go away; talk to the nurse. If you forge relationships and try to get the group more fully implemented, it will be more likely to reach its full potential.
Q: At 35, you are slightly younger than the average U.S. hospitalist, yet you’re nearly three years into your first true leadership role. Has your age ever been an issue?
A: Initially, it was a hindrance. It took four months for Temple to interview me. The biggest negative they gave to Cogent was, “He’s so young.” In any other field, 35 would not be considered a child. We’d be in the workforce for 13 years, and we’d be considered middle or senior management. Medicine in general is steeped in, “If you don’t have gray hair, you’re not able to sit at the table.” In our specialty, you can. … It doesn’t have to hinder you, but you have to be willing and able to do the right things. If you are, you will be noticed.
Q: You consider HM program marketing and branding one of your specialties. Why are those efforts necessary?
A: If you don’t market yourself, you’ll die, particularly in a competitive market. Whether you are at an academic center or a small community hospital or even a larger hospital, you could have two or three hospitalist groups all vying for the same patient volume. You need to give yourself a differential advantage.
Q: How do you do that?
A: You have to get out and meet people and shake some hands. You have to meet all of your customers, and you have to find out if they are happy or displeased. You have to communicate with them. You have to think about your customers, and they’re not just the patients in the bed. Your customers also are your administration, your PCPs, your subspecialists. … It’s no different than a vendor selling fax machines. We are a business, and if doctors don’t think that, they’re very naive.
Q: You’re also a big proponent of team-building within groups.
A: Definitely. That’s the foundation. Groups are going to coalesce differently. In my group at Lehigh Valley, we all had a Fourth of July party. We were never so close as after that one experience when we shared dinner together. It may be as simple as that. At Temple, I had all 25 of us meet and go over a teambuilding exercise to understand what values people have and why they come to work. I asked them to tell me something I didn’t know about them. I heard everything from “I changed my name when I was 5” to “I played basketball in college.”
You’re more willing to cover for a colleague if he or she is sick if you get to know them on a personal level. And if that happens, you’re less likely to leave, and that decreases turnover. On top of everything else, you become a group. You see group buy-in and goal recognition, and you start to see those goals attained.
Q: On top of your administrative duties and teaching responsibilities, you’re still doing 10 clinical shifts per month. Why?
A: It’s hugely important for two reasons. No. 1 is respect among members of your team. No. 2 is knowledge of your service. It’s not until you get your hands dirty that you can really understand what physicians in your group are going through and figure out ways to make life better. And at the end of the day, we’re all still physicians. TH
Mark Leiser is a freelance writer in New Jersey.
Should Patients Be Informed of Better Care Elsewhere?
Editors’ note: Hospitalists face difficult decisions every day, including situations that don’t always have clear-cut answers. Beginning with this month’s “HM Debate,” The Hospitalist stares down the tough questions and presents all sides of the issues. This month’s question: You are discharging a patient after treatment for a non-ST segment myocardial infarction (NSTEMI). The cardiology team recommends nonemergent coronary artery bypass grafting (CABG) for the patient’s three-vessel disease. You set up a referral for surgery, but you know the CABG morbidity and mortality rates are higher at your hospital than at a hospital 30 miles away. Should you disclose this information to your patient?
PRO
If you would tell your relative, you should tell your patient
If a patient at your hospital needs surgery or another invasive intervention, are you obligated to inform them of your hospital’s record with that procedure—particularly if the record is not as good as the one of the hospital down the street? Should loyalty to your hospital trump the risk to the patient?
In our scenario, the patient is being referred for elective surgery, and it is known that the cardiovascular team at a neighboring hospital has a better record for this procedure. It is the hospitalist’s job to present this information to the patient so that an intelligent and informed decision can be made. If the hospitalist believes the outcomes data, then an obligation exists to share that information with the patient.
If the data are subtle, one might argue that confusing the patient with more levels of decision-making is unnecessary. On the other hand, if data on performance outcomes between two institutions are clear, it presents an ethical position.
Let us assume the hospitalist is aware of poor outcomes in coronary bypass surgery at their hospital. Perhaps the mortality rates were unusually high and the hospitalist knew external consultants were brought in to identify the problems. Would you refer your patient for bypass surgery in that situation? A better question might be: Would you let a family member undergo coronary artery bypass grafting (CABG) in your hospital? Probably not. So if you would inform a family member, shouldn’t you tell your patient?
A situation like this occurred in September 2005 at the University of Massachusetts Medical Center in Worcester. Media coverage was intense, and statistics showed that thoracic surgery mortality at UMass was the highest in the state two years running. The service at the hospital was closed temporarily.1 Extensive reorganization, adoption of QI protocols, and development of oversight committees resulted in much-improved patient outcomes when the program reopened a few weeks later.
The higher-than-expected complication rate had persisted at UMass for four years before the closure and reorganization. One wonders if hospitalists and cardiologists suspected a problem with surgical outcomes before the hospital was thrust into the national spotlight. Is it our job to know our hospital’s track record in surgery and invasive procedures? Yes, it probably is. This is why a hospital’s quality-assurance committee is so important. As keeper of the outcomes data, the committee is charged with sounding an alarm when a problem is brewing.
Tech-savvy patients have access to detailed reporting on performance measures for hospitals and physicians. Interpretation of the data can be complex. High surgical complication rates might be the result of a higher-than-expected patient acuity mix—patients who were older and sicker than usual—and may not represent a system or surgeon problem. Hospitalists need to guide patients through the interpretation of this data.
Patients trust their physicians. They trust that hospitalists will provide the best advice and make recommendations with their interests at heart. To not do so violates the public trust in physicians as patient advocates. Required by law, transparency of hospital quality data is the basis of a truthful relationship between the healthcare system and the public.
HM’s reputation will be tarnished if patients perceive that the physicians are more interested in the well-being of the hospital than the well-being of the patients.
Reference
- Kowalczyk L, Smith S. Hospital halts heart surgeries due to deaths. Boston Globe Web site. Available at: www.boston.com/news/local/articles/2005/09/22/hospital_halts_heart_surgeries_due_to_deaths. Accessed March 31, 2009.
CON
Outcome disclosure is impractical, unnecessary
At first glance, disclosing information about better outcomes at another hospital seems reasonable—even ethically obligatory. However, there are several competing interests, and in the end, the existing precedent for requiring reasonable disclosure in informed consent makes more sense.
The first issue is practicality. How much is a hospitalist obligated to know, and what degree of difference must be disclosed? In the case example, the hospitalist knows better outcomes are available at a nearby facility. However, if a duty to disclose this information exists, it can’t be limited to the information that an individual hospitalist has available to them. If such a duty exists, there is a corresponding burden on providers to have consistent and accurate information to disclose. If disclosure of differing outcomes is the ethical standard, then the reasonable disclosure needs to meet some uniform criteria for when a differing outcome rises to the level that the disclosure is compelled.
Data exist for pneumonia outcomes, readmission rates, and medication errors, as well as data for physicians relative to their colleagues. The fact that a hospitalist might have incidental knowledge of differing outcomes is not sufficient to create an ethical obligation, but there must be some uniformity to a disclosure requirement.
It is easy to envision a hospitalist spending as much time disclosing outcomes data as disclosing medical information and prognosis in the process of obtaining informed consent. Hospitalists can’t be expected to manage all of that information, much less make a meaningful disclosure. Physicians’ information-management skills should focus on medical knowledge—not outcomes data.
The test case has more implications for the professionalism of the hospitalist. Ultimately, they should act in the best interest of the patient. The recommended course of treatment should maximize benefit and minimize harm. Enough information should be provided that the patient can participate in weighing risks and benefits. The hospitalist needs to decide if it is unsafe to perform the procedure at their institution, and if so, the patient should be referred out. If there is a small but real benefit to having the procedure done elsewhere, the hospitalist cannot be responsible for determining what threshold of incremental benefit warrants disclosure. Existing ethical responsibilities to protect the patient and act in their best interests already addresses the issues of disparate outcomes more effectively than a blanket disclosure policy. Patients need to trust their hospitalists, and we need to be worthy of that trust.
Mandating disclosure of better outcomes would create a conflict of interest for physicians and hospitals. This conflict would be difficult to manage. Large referral centers exist because physicians recognize their own limits and act in patients’ best interests. Requiring a new level of disclosure would mean that many hospitals (save for an elite few) would recommend patients go elsewhere a substantial part of the time.
Conflicts of interest usually are managed more than eliminated, and the current management of the tension between caring for a patient personally and referring them out based on a perception of the patient’s need for a higher level of care achieves a reasonable and balanced result. Disrupting this result with a mandated level of disclosure will result in disruption of a functional process.
Measured disclosure of relevant information is a good thing. A high level of communication and shared decision-making between physicians and patients is a good thing. Discussion of disparate outcomes may be an ethically important part of a treatment plan, but saying it is advisable in all cases is unnecessary, unmanageable, and inadvisable.
The opinions expressed herein are those of the authors and do not represent those of the Society of Hospital Medicine or The Hospitalist.
Editors’ note: Hospitalists face difficult decisions every day, including situations that don’t always have clear-cut answers. Beginning with this month’s “HM Debate,” The Hospitalist stares down the tough questions and presents all sides of the issues. This month’s question: You are discharging a patient after treatment for a non-ST segment myocardial infarction (NSTEMI). The cardiology team recommends nonemergent coronary artery bypass grafting (CABG) for the patient’s three-vessel disease. You set up a referral for surgery, but you know the CABG morbidity and mortality rates are higher at your hospital than at a hospital 30 miles away. Should you disclose this information to your patient?
PRO
If you would tell your relative, you should tell your patient
If a patient at your hospital needs surgery or another invasive intervention, are you obligated to inform them of your hospital’s record with that procedure—particularly if the record is not as good as the one of the hospital down the street? Should loyalty to your hospital trump the risk to the patient?
In our scenario, the patient is being referred for elective surgery, and it is known that the cardiovascular team at a neighboring hospital has a better record for this procedure. It is the hospitalist’s job to present this information to the patient so that an intelligent and informed decision can be made. If the hospitalist believes the outcomes data, then an obligation exists to share that information with the patient.
If the data are subtle, one might argue that confusing the patient with more levels of decision-making is unnecessary. On the other hand, if data on performance outcomes between two institutions are clear, it presents an ethical position.
Let us assume the hospitalist is aware of poor outcomes in coronary bypass surgery at their hospital. Perhaps the mortality rates were unusually high and the hospitalist knew external consultants were brought in to identify the problems. Would you refer your patient for bypass surgery in that situation? A better question might be: Would you let a family member undergo coronary artery bypass grafting (CABG) in your hospital? Probably not. So if you would inform a family member, shouldn’t you tell your patient?
A situation like this occurred in September 2005 at the University of Massachusetts Medical Center in Worcester. Media coverage was intense, and statistics showed that thoracic surgery mortality at UMass was the highest in the state two years running. The service at the hospital was closed temporarily.1 Extensive reorganization, adoption of QI protocols, and development of oversight committees resulted in much-improved patient outcomes when the program reopened a few weeks later.
The higher-than-expected complication rate had persisted at UMass for four years before the closure and reorganization. One wonders if hospitalists and cardiologists suspected a problem with surgical outcomes before the hospital was thrust into the national spotlight. Is it our job to know our hospital’s track record in surgery and invasive procedures? Yes, it probably is. This is why a hospital’s quality-assurance committee is so important. As keeper of the outcomes data, the committee is charged with sounding an alarm when a problem is brewing.
Tech-savvy patients have access to detailed reporting on performance measures for hospitals and physicians. Interpretation of the data can be complex. High surgical complication rates might be the result of a higher-than-expected patient acuity mix—patients who were older and sicker than usual—and may not represent a system or surgeon problem. Hospitalists need to guide patients through the interpretation of this data.
Patients trust their physicians. They trust that hospitalists will provide the best advice and make recommendations with their interests at heart. To not do so violates the public trust in physicians as patient advocates. Required by law, transparency of hospital quality data is the basis of a truthful relationship between the healthcare system and the public.
HM’s reputation will be tarnished if patients perceive that the physicians are more interested in the well-being of the hospital than the well-being of the patients.
Reference
- Kowalczyk L, Smith S. Hospital halts heart surgeries due to deaths. Boston Globe Web site. Available at: www.boston.com/news/local/articles/2005/09/22/hospital_halts_heart_surgeries_due_to_deaths. Accessed March 31, 2009.
CON
Outcome disclosure is impractical, unnecessary
At first glance, disclosing information about better outcomes at another hospital seems reasonable—even ethically obligatory. However, there are several competing interests, and in the end, the existing precedent for requiring reasonable disclosure in informed consent makes more sense.
The first issue is practicality. How much is a hospitalist obligated to know, and what degree of difference must be disclosed? In the case example, the hospitalist knows better outcomes are available at a nearby facility. However, if a duty to disclose this information exists, it can’t be limited to the information that an individual hospitalist has available to them. If such a duty exists, there is a corresponding burden on providers to have consistent and accurate information to disclose. If disclosure of differing outcomes is the ethical standard, then the reasonable disclosure needs to meet some uniform criteria for when a differing outcome rises to the level that the disclosure is compelled.
Data exist for pneumonia outcomes, readmission rates, and medication errors, as well as data for physicians relative to their colleagues. The fact that a hospitalist might have incidental knowledge of differing outcomes is not sufficient to create an ethical obligation, but there must be some uniformity to a disclosure requirement.
It is easy to envision a hospitalist spending as much time disclosing outcomes data as disclosing medical information and prognosis in the process of obtaining informed consent. Hospitalists can’t be expected to manage all of that information, much less make a meaningful disclosure. Physicians’ information-management skills should focus on medical knowledge—not outcomes data.
The test case has more implications for the professionalism of the hospitalist. Ultimately, they should act in the best interest of the patient. The recommended course of treatment should maximize benefit and minimize harm. Enough information should be provided that the patient can participate in weighing risks and benefits. The hospitalist needs to decide if it is unsafe to perform the procedure at their institution, and if so, the patient should be referred out. If there is a small but real benefit to having the procedure done elsewhere, the hospitalist cannot be responsible for determining what threshold of incremental benefit warrants disclosure. Existing ethical responsibilities to protect the patient and act in their best interests already addresses the issues of disparate outcomes more effectively than a blanket disclosure policy. Patients need to trust their hospitalists, and we need to be worthy of that trust.
Mandating disclosure of better outcomes would create a conflict of interest for physicians and hospitals. This conflict would be difficult to manage. Large referral centers exist because physicians recognize their own limits and act in patients’ best interests. Requiring a new level of disclosure would mean that many hospitals (save for an elite few) would recommend patients go elsewhere a substantial part of the time.
Conflicts of interest usually are managed more than eliminated, and the current management of the tension between caring for a patient personally and referring them out based on a perception of the patient’s need for a higher level of care achieves a reasonable and balanced result. Disrupting this result with a mandated level of disclosure will result in disruption of a functional process.
Measured disclosure of relevant information is a good thing. A high level of communication and shared decision-making between physicians and patients is a good thing. Discussion of disparate outcomes may be an ethically important part of a treatment plan, but saying it is advisable in all cases is unnecessary, unmanageable, and inadvisable.
The opinions expressed herein are those of the authors and do not represent those of the Society of Hospital Medicine or The Hospitalist.
Editors’ note: Hospitalists face difficult decisions every day, including situations that don’t always have clear-cut answers. Beginning with this month’s “HM Debate,” The Hospitalist stares down the tough questions and presents all sides of the issues. This month’s question: You are discharging a patient after treatment for a non-ST segment myocardial infarction (NSTEMI). The cardiology team recommends nonemergent coronary artery bypass grafting (CABG) for the patient’s three-vessel disease. You set up a referral for surgery, but you know the CABG morbidity and mortality rates are higher at your hospital than at a hospital 30 miles away. Should you disclose this information to your patient?
PRO
If you would tell your relative, you should tell your patient
If a patient at your hospital needs surgery or another invasive intervention, are you obligated to inform them of your hospital’s record with that procedure—particularly if the record is not as good as the one of the hospital down the street? Should loyalty to your hospital trump the risk to the patient?
In our scenario, the patient is being referred for elective surgery, and it is known that the cardiovascular team at a neighboring hospital has a better record for this procedure. It is the hospitalist’s job to present this information to the patient so that an intelligent and informed decision can be made. If the hospitalist believes the outcomes data, then an obligation exists to share that information with the patient.
If the data are subtle, one might argue that confusing the patient with more levels of decision-making is unnecessary. On the other hand, if data on performance outcomes between two institutions are clear, it presents an ethical position.
Let us assume the hospitalist is aware of poor outcomes in coronary bypass surgery at their hospital. Perhaps the mortality rates were unusually high and the hospitalist knew external consultants were brought in to identify the problems. Would you refer your patient for bypass surgery in that situation? A better question might be: Would you let a family member undergo coronary artery bypass grafting (CABG) in your hospital? Probably not. So if you would inform a family member, shouldn’t you tell your patient?
A situation like this occurred in September 2005 at the University of Massachusetts Medical Center in Worcester. Media coverage was intense, and statistics showed that thoracic surgery mortality at UMass was the highest in the state two years running. The service at the hospital was closed temporarily.1 Extensive reorganization, adoption of QI protocols, and development of oversight committees resulted in much-improved patient outcomes when the program reopened a few weeks later.
The higher-than-expected complication rate had persisted at UMass for four years before the closure and reorganization. One wonders if hospitalists and cardiologists suspected a problem with surgical outcomes before the hospital was thrust into the national spotlight. Is it our job to know our hospital’s track record in surgery and invasive procedures? Yes, it probably is. This is why a hospital’s quality-assurance committee is so important. As keeper of the outcomes data, the committee is charged with sounding an alarm when a problem is brewing.
Tech-savvy patients have access to detailed reporting on performance measures for hospitals and physicians. Interpretation of the data can be complex. High surgical complication rates might be the result of a higher-than-expected patient acuity mix—patients who were older and sicker than usual—and may not represent a system or surgeon problem. Hospitalists need to guide patients through the interpretation of this data.
Patients trust their physicians. They trust that hospitalists will provide the best advice and make recommendations with their interests at heart. To not do so violates the public trust in physicians as patient advocates. Required by law, transparency of hospital quality data is the basis of a truthful relationship between the healthcare system and the public.
HM’s reputation will be tarnished if patients perceive that the physicians are more interested in the well-being of the hospital than the well-being of the patients.
Reference
- Kowalczyk L, Smith S. Hospital halts heart surgeries due to deaths. Boston Globe Web site. Available at: www.boston.com/news/local/articles/2005/09/22/hospital_halts_heart_surgeries_due_to_deaths. Accessed March 31, 2009.
CON
Outcome disclosure is impractical, unnecessary
At first glance, disclosing information about better outcomes at another hospital seems reasonable—even ethically obligatory. However, there are several competing interests, and in the end, the existing precedent for requiring reasonable disclosure in informed consent makes more sense.
The first issue is practicality. How much is a hospitalist obligated to know, and what degree of difference must be disclosed? In the case example, the hospitalist knows better outcomes are available at a nearby facility. However, if a duty to disclose this information exists, it can’t be limited to the information that an individual hospitalist has available to them. If such a duty exists, there is a corresponding burden on providers to have consistent and accurate information to disclose. If disclosure of differing outcomes is the ethical standard, then the reasonable disclosure needs to meet some uniform criteria for when a differing outcome rises to the level that the disclosure is compelled.
Data exist for pneumonia outcomes, readmission rates, and medication errors, as well as data for physicians relative to their colleagues. The fact that a hospitalist might have incidental knowledge of differing outcomes is not sufficient to create an ethical obligation, but there must be some uniformity to a disclosure requirement.
It is easy to envision a hospitalist spending as much time disclosing outcomes data as disclosing medical information and prognosis in the process of obtaining informed consent. Hospitalists can’t be expected to manage all of that information, much less make a meaningful disclosure. Physicians’ information-management skills should focus on medical knowledge—not outcomes data.
The test case has more implications for the professionalism of the hospitalist. Ultimately, they should act in the best interest of the patient. The recommended course of treatment should maximize benefit and minimize harm. Enough information should be provided that the patient can participate in weighing risks and benefits. The hospitalist needs to decide if it is unsafe to perform the procedure at their institution, and if so, the patient should be referred out. If there is a small but real benefit to having the procedure done elsewhere, the hospitalist cannot be responsible for determining what threshold of incremental benefit warrants disclosure. Existing ethical responsibilities to protect the patient and act in their best interests already addresses the issues of disparate outcomes more effectively than a blanket disclosure policy. Patients need to trust their hospitalists, and we need to be worthy of that trust.
Mandating disclosure of better outcomes would create a conflict of interest for physicians and hospitals. This conflict would be difficult to manage. Large referral centers exist because physicians recognize their own limits and act in patients’ best interests. Requiring a new level of disclosure would mean that many hospitals (save for an elite few) would recommend patients go elsewhere a substantial part of the time.
Conflicts of interest usually are managed more than eliminated, and the current management of the tension between caring for a patient personally and referring them out based on a perception of the patient’s need for a higher level of care achieves a reasonable and balanced result. Disrupting this result with a mandated level of disclosure will result in disruption of a functional process.
Measured disclosure of relevant information is a good thing. A high level of communication and shared decision-making between physicians and patients is a good thing. Discussion of disparate outcomes may be an ethically important part of a treatment plan, but saying it is advisable in all cases is unnecessary, unmanageable, and inadvisable.
The opinions expressed herein are those of the authors and do not represent those of the Society of Hospital Medicine or The Hospitalist.
Medicine’s Change Agent
Sen. Max Baucus (D-Mont.) might be the most devoted champion of healthcare reform on Capitol Hill today. He chairs the Senate Finance Committee, which has jurisdiction over Medicare, Medicaid, the State Children’s Health Insurance Program (SCHIP), and other healthcare entitlement programs. He has worked for healthcare reform in large and small measures, and recently put forth a comprehensive—and controversial—plan for changing the U.S. healthcare system.
Sen. Baucus penned a white paper, “Call to Action: Health Care Reform 2009,” which was published after the November election and outlines his proposals for universal healthcare coverage. Although he hopes to introduce some form of his white paper as a Senate bill, as of press time, he had not done so.
A Stanford Law graduate, Sen. Baucus was the executive director of the 1972 Montana Constitutional Convention, which rewrote that state’s constitution. He has served in public office since 1973, including six consecutive terms in the U.S. Senate.
The Hospitalist caught up with Sen. Baucus to discuss national healthcare reform and how hospitalists can—and will—factor into the changes.
Question: You are pushing for action on healthcare reform in 2009. What’s the status of your first piece of healthcare legislation this year?
Answer: My goal is to craft consensus legislation and move it through the Congress and into law. Now that Congress has passed the American Recovery and Reinvestment Act, I intend to return the attention of the Finance Committee to health reform.
Q: Where will funding come from for some of the immediate healthcare initiatives you’d like to see?
A: Healthcare reform will require an upfront investment in order to achieve the savings we all know are possible. It is my intention that after 10 years, the U.S. will spend no more on healthcare than is currently projected, but we will spend those resources more efficiently and will provide better-quality coverage to all Americans. One of the reasons healthcare reform is so important is that if we ignore the problems in the system and fail to act, healthcare costs will only grow. Acting now is a cost-saving proposition.
Q: As coverage is provided for more Americans, what steps should be taken to ensure an adequate number of physicians, hospital beds, clinics, etc.?
A: With more people in the healthcare system, we will need more physicians and resources. My plan increases the number of primary-care doctors by strengthening the role of primary care. Today, America’s system undervalues primary care relative to specialty care. This has caused fewer medical students to choose careers in primary care. My plan will increase the supply of primary-care practitioners by using federal reimbursement systems and other means to improve the value placed on their work.
My plan also builds on existing resources that have been successful in delivering primary-care services, like community health centers. The proposal I’ve put forward increases funding for low-income and rural clinics designated by Medicare as Federally Qualified Health Centers. [It would provide] more funding for Rural Health Clinics. By strengthening community and rural providers, we can improve access to primary care and better manage conditions before they become serious. That will keep people healthier and save money in the long run.
Q: You call for refocusing payment incentives from quantity of care to quality of care. Do you have CMS’ Physician Quality Reporting Initiative (PQRI) in mind to help move the focus of compensation?
A: My plan builds on a number of programs that are already in place to improve the quality of care, including PQRI, which provides incentives for physicians who track and report data on the quality of care they provide. My plan would expand this initiative using cutting-edge technology to collect and analyze data on quality, and improve it by increasing outreach, information, and assistance to doctors who participate in the program. ... My plan would expand gain-sharing programs, which allow providers to share savings from improved efficiency and quality.
Q: SHM has identified improvements in care coordination, particularly as patients transition from the hospital to the home, as an important element of health reform. You, too, have identified this as priority. Can you elaborate on the types of proposals to improve care transitions that we might see in your healthcare reform bill?
—Sen. Max Baucus
A: Today’s healthcare system doesn’t do enough to encourage healthcare providers to work together, which can be particularly detrimental for patients who are transitioning from hospital to home. According to some estimates, 18% of Medicare hospital admissions result in a rehospitalization within 30 days. This is simply unacceptable, and it is avoidable. Providers can work better together to ensure that patients receive proper follow-up care post-discharge.
In my blueprint for reform, I laid out a series of proposals to encourage greater collaboration among providers. These proposals include a plan to reduce hospital readmissions through increasing public disclosure around readmission rates and, in later years, reducing payment rates for hospitals with readmissions above a certain benchmark. My plan also identifies “bundling” hospital and physician payments under Medicare as a way to encourage greater provider collaboration across a patient’s episode of care, and other concepts like the development of accountable care organizations. My hope is that these proposals will encourage and reward health providers who work together to provide patients the best possible care.
Q: Regarding value-based purchasing, your paper states, “Every effort must be made to align hospital and physician quality goals.” Would this alignment apply to bonus payments, and if so, will it necessitate loosening current restrictions on gain-sharing?
A: Successful implementation of new payment and delivery system models may require changes to the regulatory structure governing provider collaboration. ... It is critical that we strike an appropriate balance between offering providers incentives to work together while also protecting against financial conflicts of interest that could negatively impact quality of care. Regarding value-based purchasing, we are continuing to explore ways to encourage hospitals and doctors to work together to improve quality and are evaluating the best way to align payment incentives to meet this goal.
Q: How can hospitalists help with healthcare reform efforts?
A: As is true with all members of the healthcare community, I encourage hospitalists to work with me and my colleagues throughout the reform process. It is certain to take significant cooperation to create a more accessible, lower-cost, higher-quality system, but I’m confident that working together, we will succeed. I’m asking everyone in the healthcare community to help me create a “can-do” environment for healthcare reform. All stakeholders have a particular focus, and I am willing to listen to every issue. But our collective focus should be on [making] the health system better for everyone.
As always, I appreciate all questions, comments, and concerns, and I look forward to working with all stakeholders throughout this process. TH
Jane Jerrard is a medical writer based in Chicago.
Sen. Max Baucus (D-Mont.) might be the most devoted champion of healthcare reform on Capitol Hill today. He chairs the Senate Finance Committee, which has jurisdiction over Medicare, Medicaid, the State Children’s Health Insurance Program (SCHIP), and other healthcare entitlement programs. He has worked for healthcare reform in large and small measures, and recently put forth a comprehensive—and controversial—plan for changing the U.S. healthcare system.
Sen. Baucus penned a white paper, “Call to Action: Health Care Reform 2009,” which was published after the November election and outlines his proposals for universal healthcare coverage. Although he hopes to introduce some form of his white paper as a Senate bill, as of press time, he had not done so.
A Stanford Law graduate, Sen. Baucus was the executive director of the 1972 Montana Constitutional Convention, which rewrote that state’s constitution. He has served in public office since 1973, including six consecutive terms in the U.S. Senate.
The Hospitalist caught up with Sen. Baucus to discuss national healthcare reform and how hospitalists can—and will—factor into the changes.
Question: You are pushing for action on healthcare reform in 2009. What’s the status of your first piece of healthcare legislation this year?
Answer: My goal is to craft consensus legislation and move it through the Congress and into law. Now that Congress has passed the American Recovery and Reinvestment Act, I intend to return the attention of the Finance Committee to health reform.
Q: Where will funding come from for some of the immediate healthcare initiatives you’d like to see?
A: Healthcare reform will require an upfront investment in order to achieve the savings we all know are possible. It is my intention that after 10 years, the U.S. will spend no more on healthcare than is currently projected, but we will spend those resources more efficiently and will provide better-quality coverage to all Americans. One of the reasons healthcare reform is so important is that if we ignore the problems in the system and fail to act, healthcare costs will only grow. Acting now is a cost-saving proposition.
Q: As coverage is provided for more Americans, what steps should be taken to ensure an adequate number of physicians, hospital beds, clinics, etc.?
A: With more people in the healthcare system, we will need more physicians and resources. My plan increases the number of primary-care doctors by strengthening the role of primary care. Today, America’s system undervalues primary care relative to specialty care. This has caused fewer medical students to choose careers in primary care. My plan will increase the supply of primary-care practitioners by using federal reimbursement systems and other means to improve the value placed on their work.
My plan also builds on existing resources that have been successful in delivering primary-care services, like community health centers. The proposal I’ve put forward increases funding for low-income and rural clinics designated by Medicare as Federally Qualified Health Centers. [It would provide] more funding for Rural Health Clinics. By strengthening community and rural providers, we can improve access to primary care and better manage conditions before they become serious. That will keep people healthier and save money in the long run.
Q: You call for refocusing payment incentives from quantity of care to quality of care. Do you have CMS’ Physician Quality Reporting Initiative (PQRI) in mind to help move the focus of compensation?
A: My plan builds on a number of programs that are already in place to improve the quality of care, including PQRI, which provides incentives for physicians who track and report data on the quality of care they provide. My plan would expand this initiative using cutting-edge technology to collect and analyze data on quality, and improve it by increasing outreach, information, and assistance to doctors who participate in the program. ... My plan would expand gain-sharing programs, which allow providers to share savings from improved efficiency and quality.
Q: SHM has identified improvements in care coordination, particularly as patients transition from the hospital to the home, as an important element of health reform. You, too, have identified this as priority. Can you elaborate on the types of proposals to improve care transitions that we might see in your healthcare reform bill?
—Sen. Max Baucus
A: Today’s healthcare system doesn’t do enough to encourage healthcare providers to work together, which can be particularly detrimental for patients who are transitioning from hospital to home. According to some estimates, 18% of Medicare hospital admissions result in a rehospitalization within 30 days. This is simply unacceptable, and it is avoidable. Providers can work better together to ensure that patients receive proper follow-up care post-discharge.
In my blueprint for reform, I laid out a series of proposals to encourage greater collaboration among providers. These proposals include a plan to reduce hospital readmissions through increasing public disclosure around readmission rates and, in later years, reducing payment rates for hospitals with readmissions above a certain benchmark. My plan also identifies “bundling” hospital and physician payments under Medicare as a way to encourage greater provider collaboration across a patient’s episode of care, and other concepts like the development of accountable care organizations. My hope is that these proposals will encourage and reward health providers who work together to provide patients the best possible care.
Q: Regarding value-based purchasing, your paper states, “Every effort must be made to align hospital and physician quality goals.” Would this alignment apply to bonus payments, and if so, will it necessitate loosening current restrictions on gain-sharing?
A: Successful implementation of new payment and delivery system models may require changes to the regulatory structure governing provider collaboration. ... It is critical that we strike an appropriate balance between offering providers incentives to work together while also protecting against financial conflicts of interest that could negatively impact quality of care. Regarding value-based purchasing, we are continuing to explore ways to encourage hospitals and doctors to work together to improve quality and are evaluating the best way to align payment incentives to meet this goal.
Q: How can hospitalists help with healthcare reform efforts?
A: As is true with all members of the healthcare community, I encourage hospitalists to work with me and my colleagues throughout the reform process. It is certain to take significant cooperation to create a more accessible, lower-cost, higher-quality system, but I’m confident that working together, we will succeed. I’m asking everyone in the healthcare community to help me create a “can-do” environment for healthcare reform. All stakeholders have a particular focus, and I am willing to listen to every issue. But our collective focus should be on [making] the health system better for everyone.
As always, I appreciate all questions, comments, and concerns, and I look forward to working with all stakeholders throughout this process. TH
Jane Jerrard is a medical writer based in Chicago.
Sen. Max Baucus (D-Mont.) might be the most devoted champion of healthcare reform on Capitol Hill today. He chairs the Senate Finance Committee, which has jurisdiction over Medicare, Medicaid, the State Children’s Health Insurance Program (SCHIP), and other healthcare entitlement programs. He has worked for healthcare reform in large and small measures, and recently put forth a comprehensive—and controversial—plan for changing the U.S. healthcare system.
Sen. Baucus penned a white paper, “Call to Action: Health Care Reform 2009,” which was published after the November election and outlines his proposals for universal healthcare coverage. Although he hopes to introduce some form of his white paper as a Senate bill, as of press time, he had not done so.
A Stanford Law graduate, Sen. Baucus was the executive director of the 1972 Montana Constitutional Convention, which rewrote that state’s constitution. He has served in public office since 1973, including six consecutive terms in the U.S. Senate.
The Hospitalist caught up with Sen. Baucus to discuss national healthcare reform and how hospitalists can—and will—factor into the changes.
Question: You are pushing for action on healthcare reform in 2009. What’s the status of your first piece of healthcare legislation this year?
Answer: My goal is to craft consensus legislation and move it through the Congress and into law. Now that Congress has passed the American Recovery and Reinvestment Act, I intend to return the attention of the Finance Committee to health reform.
Q: Where will funding come from for some of the immediate healthcare initiatives you’d like to see?
A: Healthcare reform will require an upfront investment in order to achieve the savings we all know are possible. It is my intention that after 10 years, the U.S. will spend no more on healthcare than is currently projected, but we will spend those resources more efficiently and will provide better-quality coverage to all Americans. One of the reasons healthcare reform is so important is that if we ignore the problems in the system and fail to act, healthcare costs will only grow. Acting now is a cost-saving proposition.
Q: As coverage is provided for more Americans, what steps should be taken to ensure an adequate number of physicians, hospital beds, clinics, etc.?
A: With more people in the healthcare system, we will need more physicians and resources. My plan increases the number of primary-care doctors by strengthening the role of primary care. Today, America’s system undervalues primary care relative to specialty care. This has caused fewer medical students to choose careers in primary care. My plan will increase the supply of primary-care practitioners by using federal reimbursement systems and other means to improve the value placed on their work.
My plan also builds on existing resources that have been successful in delivering primary-care services, like community health centers. The proposal I’ve put forward increases funding for low-income and rural clinics designated by Medicare as Federally Qualified Health Centers. [It would provide] more funding for Rural Health Clinics. By strengthening community and rural providers, we can improve access to primary care and better manage conditions before they become serious. That will keep people healthier and save money in the long run.
Q: You call for refocusing payment incentives from quantity of care to quality of care. Do you have CMS’ Physician Quality Reporting Initiative (PQRI) in mind to help move the focus of compensation?
A: My plan builds on a number of programs that are already in place to improve the quality of care, including PQRI, which provides incentives for physicians who track and report data on the quality of care they provide. My plan would expand this initiative using cutting-edge technology to collect and analyze data on quality, and improve it by increasing outreach, information, and assistance to doctors who participate in the program. ... My plan would expand gain-sharing programs, which allow providers to share savings from improved efficiency and quality.
Q: SHM has identified improvements in care coordination, particularly as patients transition from the hospital to the home, as an important element of health reform. You, too, have identified this as priority. Can you elaborate on the types of proposals to improve care transitions that we might see in your healthcare reform bill?
—Sen. Max Baucus
A: Today’s healthcare system doesn’t do enough to encourage healthcare providers to work together, which can be particularly detrimental for patients who are transitioning from hospital to home. According to some estimates, 18% of Medicare hospital admissions result in a rehospitalization within 30 days. This is simply unacceptable, and it is avoidable. Providers can work better together to ensure that patients receive proper follow-up care post-discharge.
In my blueprint for reform, I laid out a series of proposals to encourage greater collaboration among providers. These proposals include a plan to reduce hospital readmissions through increasing public disclosure around readmission rates and, in later years, reducing payment rates for hospitals with readmissions above a certain benchmark. My plan also identifies “bundling” hospital and physician payments under Medicare as a way to encourage greater provider collaboration across a patient’s episode of care, and other concepts like the development of accountable care organizations. My hope is that these proposals will encourage and reward health providers who work together to provide patients the best possible care.
Q: Regarding value-based purchasing, your paper states, “Every effort must be made to align hospital and physician quality goals.” Would this alignment apply to bonus payments, and if so, will it necessitate loosening current restrictions on gain-sharing?
A: Successful implementation of new payment and delivery system models may require changes to the regulatory structure governing provider collaboration. ... It is critical that we strike an appropriate balance between offering providers incentives to work together while also protecting against financial conflicts of interest that could negatively impact quality of care. Regarding value-based purchasing, we are continuing to explore ways to encourage hospitals and doctors to work together to improve quality and are evaluating the best way to align payment incentives to meet this goal.
Q: How can hospitalists help with healthcare reform efforts?
A: As is true with all members of the healthcare community, I encourage hospitalists to work with me and my colleagues throughout the reform process. It is certain to take significant cooperation to create a more accessible, lower-cost, higher-quality system, but I’m confident that working together, we will succeed. I’m asking everyone in the healthcare community to help me create a “can-do” environment for healthcare reform. All stakeholders have a particular focus, and I am willing to listen to every issue. But our collective focus should be on [making] the health system better for everyone.
As always, I appreciate all questions, comments, and concerns, and I look forward to working with all stakeholders throughout this process. TH
Jane Jerrard is a medical writer based in Chicago.
Promotional Pursuits
Hospitalists who are planning on advancing their careers—particularly those working toward leadership roles—need to acquire or sharpen their skills through additional training: conferences and seminars, online courses, self-study, or university classes.
The Hospitalist spoke with several HM leaders and a physician executive coach about what makes an employee promotion material. Here are their “continuing education” suggestions for ambitious hospitalists.
Invest in Yourself
The first step in selecting a training venue is to identify your goal. What do you need to learn? How much time, effort, and money do you want to devote to the training? “There are avenues for physicians to pursue if they want to develop some leadership skills,” says Francine R. Gaillour, MD, MBA, FACPE, executive director of the Physician Coaching Institute in Bellevue, Wash.
One route, which requires a considerable investment, is to pursue a master’s of business administration (MBA) degree. “I don’t recommend this for most physicians. However, a lot of physicians choose this,” Dr. Gaillour says. “It will help mainly on the business side of becoming a leader, and there are several MBA programs that cater specifically to physicians, or to healthcare.”
A number of the nation’s top universities offer advanced degrees for physicians, including:
- The University of Tennessee offers a physician executive MBA;
- The University of California at Irvine offers a healthcare executive MBA;
- The University of South Florida offers an executive MBA for physicians; and
- The University of Massachusetts offers an MBA program through the American College of Physician Executives (ACPE).
For many hospitalist leaders, an MBA is not necessary. Instead, you might prefer to sign up for a certificate program or short-term course in physician leadership. “For example, here in my area, the University of Washington offers a nine-month course in medical management,” Dr. Gaillour explains. “You attend one evening a week, and it covers the essential concepts in being a leader in the medical field. The course kind of skims the surface of a number of important topics.”
A practical—and popular—way to acquire targeted training is by taking focused leadership courses and workshops offered by such organizations as SHM or ACPE.
Start with SHM
As the chair of SHM’s Leadership Committee, Eric Howell, MD, FHM, SHM board member and director of Collaborative Inpatient Medicine Service in the Department of Medicine at Johns Hopkins Bayview Medical Center in Baltimore, is closely involved in the society’s Leadership Academy. “Anybody can sign up for this—hospitalists, nonphysicians, even administrators,” he explains. “Level 1 has no prerequisites, and Level 2 requires only that you’ve completed Level 1 or something equivalent.”
The Level 1 Academy is “probably best for those looking to improve their leadership skills in whatever venue they’re in—an HM group, nursing unit, you name it,” Dr. Howell says. “You can use it to figure out what you need more help with and then branch out to an ACPE [course] or something like that—even an MBA program.”
The next Leadership Academy is Sept. 14-17 in Miami.
Physician-Specific Leadership Courses
ACPE offers a wealth of physician leadership education options, including live and online courses. The core curriculum includes courses that cover the basics of negotiation, managing physicians, finance, and more. ACPE also offers courses that count toward four different medical management degrees, including an MBA.
“The ACPE is probably the No. 1 resource for physicians who want to develop skills in leadership,” says Dr. Gaillour, who is an ACPE fellow. “Their core courses are valuable, as well as fun and interesting. Beyond the basics, you can go as deep as you want in a specific area. About one-third of their curriculum is newer topics for more experienced physicians.”
Dr. Howell says ACPE courses “get a lot of traction among the leadership [committee]. They have courses relevant to hospitalists and hospital leaders.”
Patience Agborbesong, MD, has completed several ACPE courses. Currently an assistant professor as well as the medical director of the hospitalist program at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., Dr. Agborbesong first discovered ACPE courses as a newly promoted HM group director. “I took ‘Managing Physician Performance,’ a Web-based class with an actual instructor,” she says. “That course was particularly helpful to me. It covered interviewing job candidates, giving feedback and performance reviews, and dealing with disruptive individuals.”
One difference between SHM’s Leadership Academy and ACPE courses is class makeup. SHM’s Academy attracts a hospitalist crowd; physicians from all specialties attend ACPE courses. “I like the SHM Leadership Academy because it focuses on the hospital environment,” Dr. Agborbesong says, “but the ACPE is good, too, because I like to know how other worlds work—like private practice.”
Teach the Teacher
For academic hospitalists, a whole subset of specialized training exists, including the new Academic Hospitalist Academy. Co-sponsored by SHM, the Society of General Internal Medicine (SGIM), and the Association of Chiefs of General Internal Medicine (ACGIM), the academy will teach the practical knowledge, skills, and attitudes necessary to succeed as an academic hospitalist.
“The first one will take place in November,” says Jeffrey Wiese, MD, FACP, FHM, SHM board member and associate professor of medicine at Tulane University Health Sciences Center in New Orleans, as well as associate chairman of medicine and director of the Tulane Internal Medicine Residency Program. The four-day course “covers teaching, working on research, and generally putting together a portfolio of academic work. It will also include some education on quality, knowing that academic hospitalists do a lot of research on this.”
Dr. Weise also recommends the Teaching Hospital Educators (THE) Course: “What Clinical Teachers in Hospital Medicine Need to Know.” It is offered as a pre-course at HM09 this month in Chicago. It debuted at the 2008 annual meeting and drew rave reviews, Dr. Wiese says. “This is a one-day course that focuses on the teaching component of being an academic hospitalist,” he says.
In-House Opportunities
Don’t overlook training opportunities offered by your group or institution, as they can help you save on travel and registration costs. “Investing yourself in whatever you have available is essential,” Dr. Howell stresses. “Most organizations have some leadership training, or some mentorship program. Even if it’s something like a course on dealing with difficult people offered by your human resources department, this is a great place to start, especially for those hospitalists just beginning to think about leadership.”
A side benefit of taking training offered by your employer is that you’ll position yourself for further training at your organization’s expense: “Many groups are willing to invest in their leaders, and I think they would give you CME money for leadership training … if you’ve demonstrated your interest by going to those free [in-house] courses, or taken it upon yourself to take a community college class or an online course,” Dr. Howell says. “This shows you’re ready to invest in yourself.”
Dr. Agborbesong helped compile a list of leadership training resources, which is available on SHM’s Web site at www.hospitalmedicine.org/LeadershipSpecialInterest. TH
Jane Jerrard is a freelance writer based in Chicago. She also writes Public Policy for The Hospitalist.
Hospitalists who are planning on advancing their careers—particularly those working toward leadership roles—need to acquire or sharpen their skills through additional training: conferences and seminars, online courses, self-study, or university classes.
The Hospitalist spoke with several HM leaders and a physician executive coach about what makes an employee promotion material. Here are their “continuing education” suggestions for ambitious hospitalists.
Invest in Yourself
The first step in selecting a training venue is to identify your goal. What do you need to learn? How much time, effort, and money do you want to devote to the training? “There are avenues for physicians to pursue if they want to develop some leadership skills,” says Francine R. Gaillour, MD, MBA, FACPE, executive director of the Physician Coaching Institute in Bellevue, Wash.
One route, which requires a considerable investment, is to pursue a master’s of business administration (MBA) degree. “I don’t recommend this for most physicians. However, a lot of physicians choose this,” Dr. Gaillour says. “It will help mainly on the business side of becoming a leader, and there are several MBA programs that cater specifically to physicians, or to healthcare.”
A number of the nation’s top universities offer advanced degrees for physicians, including:
- The University of Tennessee offers a physician executive MBA;
- The University of California at Irvine offers a healthcare executive MBA;
- The University of South Florida offers an executive MBA for physicians; and
- The University of Massachusetts offers an MBA program through the American College of Physician Executives (ACPE).
For many hospitalist leaders, an MBA is not necessary. Instead, you might prefer to sign up for a certificate program or short-term course in physician leadership. “For example, here in my area, the University of Washington offers a nine-month course in medical management,” Dr. Gaillour explains. “You attend one evening a week, and it covers the essential concepts in being a leader in the medical field. The course kind of skims the surface of a number of important topics.”
A practical—and popular—way to acquire targeted training is by taking focused leadership courses and workshops offered by such organizations as SHM or ACPE.
Start with SHM
As the chair of SHM’s Leadership Committee, Eric Howell, MD, FHM, SHM board member and director of Collaborative Inpatient Medicine Service in the Department of Medicine at Johns Hopkins Bayview Medical Center in Baltimore, is closely involved in the society’s Leadership Academy. “Anybody can sign up for this—hospitalists, nonphysicians, even administrators,” he explains. “Level 1 has no prerequisites, and Level 2 requires only that you’ve completed Level 1 or something equivalent.”
The Level 1 Academy is “probably best for those looking to improve their leadership skills in whatever venue they’re in—an HM group, nursing unit, you name it,” Dr. Howell says. “You can use it to figure out what you need more help with and then branch out to an ACPE [course] or something like that—even an MBA program.”
The next Leadership Academy is Sept. 14-17 in Miami.
Physician-Specific Leadership Courses
ACPE offers a wealth of physician leadership education options, including live and online courses. The core curriculum includes courses that cover the basics of negotiation, managing physicians, finance, and more. ACPE also offers courses that count toward four different medical management degrees, including an MBA.
“The ACPE is probably the No. 1 resource for physicians who want to develop skills in leadership,” says Dr. Gaillour, who is an ACPE fellow. “Their core courses are valuable, as well as fun and interesting. Beyond the basics, you can go as deep as you want in a specific area. About one-third of their curriculum is newer topics for more experienced physicians.”
Dr. Howell says ACPE courses “get a lot of traction among the leadership [committee]. They have courses relevant to hospitalists and hospital leaders.”
Patience Agborbesong, MD, has completed several ACPE courses. Currently an assistant professor as well as the medical director of the hospitalist program at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., Dr. Agborbesong first discovered ACPE courses as a newly promoted HM group director. “I took ‘Managing Physician Performance,’ a Web-based class with an actual instructor,” she says. “That course was particularly helpful to me. It covered interviewing job candidates, giving feedback and performance reviews, and dealing with disruptive individuals.”
One difference between SHM’s Leadership Academy and ACPE courses is class makeup. SHM’s Academy attracts a hospitalist crowd; physicians from all specialties attend ACPE courses. “I like the SHM Leadership Academy because it focuses on the hospital environment,” Dr. Agborbesong says, “but the ACPE is good, too, because I like to know how other worlds work—like private practice.”
Teach the Teacher
For academic hospitalists, a whole subset of specialized training exists, including the new Academic Hospitalist Academy. Co-sponsored by SHM, the Society of General Internal Medicine (SGIM), and the Association of Chiefs of General Internal Medicine (ACGIM), the academy will teach the practical knowledge, skills, and attitudes necessary to succeed as an academic hospitalist.
“The first one will take place in November,” says Jeffrey Wiese, MD, FACP, FHM, SHM board member and associate professor of medicine at Tulane University Health Sciences Center in New Orleans, as well as associate chairman of medicine and director of the Tulane Internal Medicine Residency Program. The four-day course “covers teaching, working on research, and generally putting together a portfolio of academic work. It will also include some education on quality, knowing that academic hospitalists do a lot of research on this.”
Dr. Weise also recommends the Teaching Hospital Educators (THE) Course: “What Clinical Teachers in Hospital Medicine Need to Know.” It is offered as a pre-course at HM09 this month in Chicago. It debuted at the 2008 annual meeting and drew rave reviews, Dr. Wiese says. “This is a one-day course that focuses on the teaching component of being an academic hospitalist,” he says.
In-House Opportunities
Don’t overlook training opportunities offered by your group or institution, as they can help you save on travel and registration costs. “Investing yourself in whatever you have available is essential,” Dr. Howell stresses. “Most organizations have some leadership training, or some mentorship program. Even if it’s something like a course on dealing with difficult people offered by your human resources department, this is a great place to start, especially for those hospitalists just beginning to think about leadership.”
A side benefit of taking training offered by your employer is that you’ll position yourself for further training at your organization’s expense: “Many groups are willing to invest in their leaders, and I think they would give you CME money for leadership training … if you’ve demonstrated your interest by going to those free [in-house] courses, or taken it upon yourself to take a community college class or an online course,” Dr. Howell says. “This shows you’re ready to invest in yourself.”
Dr. Agborbesong helped compile a list of leadership training resources, which is available on SHM’s Web site at www.hospitalmedicine.org/LeadershipSpecialInterest. TH
Jane Jerrard is a freelance writer based in Chicago. She also writes Public Policy for The Hospitalist.
Hospitalists who are planning on advancing their careers—particularly those working toward leadership roles—need to acquire or sharpen their skills through additional training: conferences and seminars, online courses, self-study, or university classes.
The Hospitalist spoke with several HM leaders and a physician executive coach about what makes an employee promotion material. Here are their “continuing education” suggestions for ambitious hospitalists.
Invest in Yourself
The first step in selecting a training venue is to identify your goal. What do you need to learn? How much time, effort, and money do you want to devote to the training? “There are avenues for physicians to pursue if they want to develop some leadership skills,” says Francine R. Gaillour, MD, MBA, FACPE, executive director of the Physician Coaching Institute in Bellevue, Wash.
One route, which requires a considerable investment, is to pursue a master’s of business administration (MBA) degree. “I don’t recommend this for most physicians. However, a lot of physicians choose this,” Dr. Gaillour says. “It will help mainly on the business side of becoming a leader, and there are several MBA programs that cater specifically to physicians, or to healthcare.”
A number of the nation’s top universities offer advanced degrees for physicians, including:
- The University of Tennessee offers a physician executive MBA;
- The University of California at Irvine offers a healthcare executive MBA;
- The University of South Florida offers an executive MBA for physicians; and
- The University of Massachusetts offers an MBA program through the American College of Physician Executives (ACPE).
For many hospitalist leaders, an MBA is not necessary. Instead, you might prefer to sign up for a certificate program or short-term course in physician leadership. “For example, here in my area, the University of Washington offers a nine-month course in medical management,” Dr. Gaillour explains. “You attend one evening a week, and it covers the essential concepts in being a leader in the medical field. The course kind of skims the surface of a number of important topics.”
A practical—and popular—way to acquire targeted training is by taking focused leadership courses and workshops offered by such organizations as SHM or ACPE.
Start with SHM
As the chair of SHM’s Leadership Committee, Eric Howell, MD, FHM, SHM board member and director of Collaborative Inpatient Medicine Service in the Department of Medicine at Johns Hopkins Bayview Medical Center in Baltimore, is closely involved in the society’s Leadership Academy. “Anybody can sign up for this—hospitalists, nonphysicians, even administrators,” he explains. “Level 1 has no prerequisites, and Level 2 requires only that you’ve completed Level 1 or something equivalent.”
The Level 1 Academy is “probably best for those looking to improve their leadership skills in whatever venue they’re in—an HM group, nursing unit, you name it,” Dr. Howell says. “You can use it to figure out what you need more help with and then branch out to an ACPE [course] or something like that—even an MBA program.”
The next Leadership Academy is Sept. 14-17 in Miami.
Physician-Specific Leadership Courses
ACPE offers a wealth of physician leadership education options, including live and online courses. The core curriculum includes courses that cover the basics of negotiation, managing physicians, finance, and more. ACPE also offers courses that count toward four different medical management degrees, including an MBA.
“The ACPE is probably the No. 1 resource for physicians who want to develop skills in leadership,” says Dr. Gaillour, who is an ACPE fellow. “Their core courses are valuable, as well as fun and interesting. Beyond the basics, you can go as deep as you want in a specific area. About one-third of their curriculum is newer topics for more experienced physicians.”
Dr. Howell says ACPE courses “get a lot of traction among the leadership [committee]. They have courses relevant to hospitalists and hospital leaders.”
Patience Agborbesong, MD, has completed several ACPE courses. Currently an assistant professor as well as the medical director of the hospitalist program at Wake Forest University Baptist Medical Center in Winston-Salem, N.C., Dr. Agborbesong first discovered ACPE courses as a newly promoted HM group director. “I took ‘Managing Physician Performance,’ a Web-based class with an actual instructor,” she says. “That course was particularly helpful to me. It covered interviewing job candidates, giving feedback and performance reviews, and dealing with disruptive individuals.”
One difference between SHM’s Leadership Academy and ACPE courses is class makeup. SHM’s Academy attracts a hospitalist crowd; physicians from all specialties attend ACPE courses. “I like the SHM Leadership Academy because it focuses on the hospital environment,” Dr. Agborbesong says, “but the ACPE is good, too, because I like to know how other worlds work—like private practice.”
Teach the Teacher
For academic hospitalists, a whole subset of specialized training exists, including the new Academic Hospitalist Academy. Co-sponsored by SHM, the Society of General Internal Medicine (SGIM), and the Association of Chiefs of General Internal Medicine (ACGIM), the academy will teach the practical knowledge, skills, and attitudes necessary to succeed as an academic hospitalist.
“The first one will take place in November,” says Jeffrey Wiese, MD, FACP, FHM, SHM board member and associate professor of medicine at Tulane University Health Sciences Center in New Orleans, as well as associate chairman of medicine and director of the Tulane Internal Medicine Residency Program. The four-day course “covers teaching, working on research, and generally putting together a portfolio of academic work. It will also include some education on quality, knowing that academic hospitalists do a lot of research on this.”
Dr. Weise also recommends the Teaching Hospital Educators (THE) Course: “What Clinical Teachers in Hospital Medicine Need to Know.” It is offered as a pre-course at HM09 this month in Chicago. It debuted at the 2008 annual meeting and drew rave reviews, Dr. Wiese says. “This is a one-day course that focuses on the teaching component of being an academic hospitalist,” he says.
In-House Opportunities
Don’t overlook training opportunities offered by your group or institution, as they can help you save on travel and registration costs. “Investing yourself in whatever you have available is essential,” Dr. Howell stresses. “Most organizations have some leadership training, or some mentorship program. Even if it’s something like a course on dealing with difficult people offered by your human resources department, this is a great place to start, especially for those hospitalists just beginning to think about leadership.”
A side benefit of taking training offered by your employer is that you’ll position yourself for further training at your organization’s expense: “Many groups are willing to invest in their leaders, and I think they would give you CME money for leadership training … if you’ve demonstrated your interest by going to those free [in-house] courses, or taken it upon yourself to take a community college class or an online course,” Dr. Howell says. “This shows you’re ready to invest in yourself.”
Dr. Agborbesong helped compile a list of leadership training resources, which is available on SHM’s Web site at www.hospitalmedicine.org/LeadershipSpecialInterest. TH
Jane Jerrard is a freelance writer based in Chicago. She also writes Public Policy for The Hospitalist.
Self-Serve SSU Study
When the hospitalist-run short-stay unit (SSU) debuted at Cook County Hospital in Chicago seven years ago, a dearth of clinical research made it difficult to show the efficacy of such programs. Only a handful of such studies existed, and none had been conducted in the U.S. So while the hospitalists behind the nascent Cook County SSU thought their approach worked, Brian Lucas, MD, FHM, MS, wanted more evidentiary proof.
“We accept patients the emergency department sends to us without argument,” says Dr. Lucas, a hospitalist in the Department of Medicine at Cook County. “We wanted to be able to convey to the ED docs with data what kind of patients actually are best suited for the short-stay. We didn’t want it to be anecdotal or based on hunches a couple of us had. … We thought it would be nice to contribute something to the literature.”
Now they have.
Their prospective, observational, cohort study, “A Hospitalist-Run Short Stay Unit: Features that Predict Length-of-Stay and Eventual Admission to Traditional Inpatient Services,” can be found in the May-June Journal of Hospital Medicine. The study found that 79% of 738 eligible patients had successful SSU stays. Success was defined as discharge from the unit within 72 hours without admission to a general hospital unit.
The authors also found that in a multivariable model, the provisional diagnosis of heart failure predicted stays of longer than 72 hours (P=0.007), but risk assessments were unimportant. Patients who received specialty consultations were most likely to need eventual admission, and the likelihood of long stays was inversely proportional to the accessibility of diagnostic tests.
“In our hospital-run SSU, the inaccessibility of diagnostic tests and the need for specialty consultations were the most important predictors of unsuccessful stays,” the authors concluded. “Designs for other SSUs that care for mostly low-risk patients should focus on matching patients’ diagnostic and consultative needs with readily accessible services.”
—Brian Lucas, MD, FHM, MS, hospitalist, Cook County Hospital, Chicago
Dr. Lucas thinks the study could help HM groups establish or refine hospitalist-led SSUs and understand the best way to administer programs. He also points out that minimal funding was needed to complete the review, as the study mostly required the time of participating hospitalists to record their own data.
“Hospitalists are increasingly involved with quality-improvement projects at their hospitals,” Dr. Lucas says. “In order to actually decide whether it’s working right, you need data, and usually data costs a lot of money. In this case, it was free.”
Cook County’s 14-bed SSU was formed in 2002, when the hospital moved into new facilities and reduced its bed count from about 650 to 500. The decreased number of beds led to the short-term unit approach to handle potential overflows and diagnoses that required shorter lengths of stay. Dr. Lucas ran the unit at inception and later handed it off to Rudolf Kumapley, MBChB, its current medical director.
But questions on the operational parameters of the unit arose quickly. What types of admissions should the SSU allow? What risk levels would it focus on? And because one of the main benefits of an SSU is to alleviate pressure and backlogs in the ED, how should the wants of ED physicians be balanced against the success rate of the SSU?
“This was an extremely useful unit,” Dr. Kumapley says, and he thought, “Why don’t we get ourselves some data?”
Study Structure
While ED physicians can be admitted to the SSU without approval of a unit-assigned physician, Cook County’s departments of medicine and emergency medicine have promoted five guidelines for admission, although none are statutory:
- Patients should have anticipated stays of less than 72 hours;
- Patients should not be expected to require traditional inpatient services;
- Patients with provisional cardiovascular diagnoses should be preferentially admitted to the SSU over general medical units;
- No patients should be admitted with a risk level higher than intermediate; and
- Patients shouldn’t require advanced ancillary services, including bedside procedures, time-intensive nursing, and complex social services.
Once the study began, attending physician investigators would interview, examine, and review the health records of enrolled patients within 12 hours of admission to the unit. When diagnoses included possible acute coronary syndrome (ACS) or decompensated heart failure, additional data was gathered. ACS and decompensated heart failure are two of the most common provisional diagnoses admitted to the SSU, in large part because the unit is equipped with continuous telemetry monitors, a treadmill testing laboratory, and other reserved cardiac tests.
“We built an online database that allowed the physicians to enter the data on all of their patients in real time,” Dr. Lucas explains. “We didn’t have any research assistants. We gathered all the data ourselves.”
Length of Stay
Of the 21% of unsuccessful stays, the most common reason was a hospital length of stay (LOS) longer than 72 hours (71% of 156 patients), although the median LOS was 42 hours. Sixty-six patients eventually required traditional inpatient services, nearly half of those after a specialty consult. The study concluded that the types of services patients received during their SSU stays were stronger predictors of success than the patients’ characteristics upon admission.
“I was surprised by some of the findings, in the sense that I’ve worked and I’ve seen the kind of patients that are admitted into ED-run short-stay units … and for the most part, that is observation medicine,” Dr. Lucas says. “I got the immediate sense in our unit you’re actively managing sick patients. They’re just discharged within 72 hours.
“One of the whole reasons to have hospitalists run this unit, as opposed to ED docs, is because the hospital should be able to handle any diagnoses that come their way because they’re handling any diagnoses that come their way upstairs. But the ED doctors are more limited in what they’re able to do.”
Dr. Kumpaley adds that the hospitalist-run SSU works best when there is open communication between ED physicians who are doing the admitting and SSU physicians who must deal with the repercussions of those decisions.
In the case of a hospitalist-run unit, the earlier the two departments start a dialogue, the more successful the unit will be in determining whether patients should be admitted to the SSU in the first place, Dr. Lucas says.
“Every time you have to hand off a patient to a new doctor, there’s risk involved,” he says. “One of the ideas of HM right now is how transitions should be improved upon. The best way to improve on care transitions is to make them unnecessary altogether.” TH
Richard Quinn is a freelance writer based in New Jersey.
When the hospitalist-run short-stay unit (SSU) debuted at Cook County Hospital in Chicago seven years ago, a dearth of clinical research made it difficult to show the efficacy of such programs. Only a handful of such studies existed, and none had been conducted in the U.S. So while the hospitalists behind the nascent Cook County SSU thought their approach worked, Brian Lucas, MD, FHM, MS, wanted more evidentiary proof.
“We accept patients the emergency department sends to us without argument,” says Dr. Lucas, a hospitalist in the Department of Medicine at Cook County. “We wanted to be able to convey to the ED docs with data what kind of patients actually are best suited for the short-stay. We didn’t want it to be anecdotal or based on hunches a couple of us had. … We thought it would be nice to contribute something to the literature.”
Now they have.
Their prospective, observational, cohort study, “A Hospitalist-Run Short Stay Unit: Features that Predict Length-of-Stay and Eventual Admission to Traditional Inpatient Services,” can be found in the May-June Journal of Hospital Medicine. The study found that 79% of 738 eligible patients had successful SSU stays. Success was defined as discharge from the unit within 72 hours without admission to a general hospital unit.
The authors also found that in a multivariable model, the provisional diagnosis of heart failure predicted stays of longer than 72 hours (P=0.007), but risk assessments were unimportant. Patients who received specialty consultations were most likely to need eventual admission, and the likelihood of long stays was inversely proportional to the accessibility of diagnostic tests.
“In our hospital-run SSU, the inaccessibility of diagnostic tests and the need for specialty consultations were the most important predictors of unsuccessful stays,” the authors concluded. “Designs for other SSUs that care for mostly low-risk patients should focus on matching patients’ diagnostic and consultative needs with readily accessible services.”
—Brian Lucas, MD, FHM, MS, hospitalist, Cook County Hospital, Chicago
Dr. Lucas thinks the study could help HM groups establish or refine hospitalist-led SSUs and understand the best way to administer programs. He also points out that minimal funding was needed to complete the review, as the study mostly required the time of participating hospitalists to record their own data.
“Hospitalists are increasingly involved with quality-improvement projects at their hospitals,” Dr. Lucas says. “In order to actually decide whether it’s working right, you need data, and usually data costs a lot of money. In this case, it was free.”
Cook County’s 14-bed SSU was formed in 2002, when the hospital moved into new facilities and reduced its bed count from about 650 to 500. The decreased number of beds led to the short-term unit approach to handle potential overflows and diagnoses that required shorter lengths of stay. Dr. Lucas ran the unit at inception and later handed it off to Rudolf Kumapley, MBChB, its current medical director.
But questions on the operational parameters of the unit arose quickly. What types of admissions should the SSU allow? What risk levels would it focus on? And because one of the main benefits of an SSU is to alleviate pressure and backlogs in the ED, how should the wants of ED physicians be balanced against the success rate of the SSU?
“This was an extremely useful unit,” Dr. Kumapley says, and he thought, “Why don’t we get ourselves some data?”
Study Structure
While ED physicians can be admitted to the SSU without approval of a unit-assigned physician, Cook County’s departments of medicine and emergency medicine have promoted five guidelines for admission, although none are statutory:
- Patients should have anticipated stays of less than 72 hours;
- Patients should not be expected to require traditional inpatient services;
- Patients with provisional cardiovascular diagnoses should be preferentially admitted to the SSU over general medical units;
- No patients should be admitted with a risk level higher than intermediate; and
- Patients shouldn’t require advanced ancillary services, including bedside procedures, time-intensive nursing, and complex social services.
Once the study began, attending physician investigators would interview, examine, and review the health records of enrolled patients within 12 hours of admission to the unit. When diagnoses included possible acute coronary syndrome (ACS) or decompensated heart failure, additional data was gathered. ACS and decompensated heart failure are two of the most common provisional diagnoses admitted to the SSU, in large part because the unit is equipped with continuous telemetry monitors, a treadmill testing laboratory, and other reserved cardiac tests.
“We built an online database that allowed the physicians to enter the data on all of their patients in real time,” Dr. Lucas explains. “We didn’t have any research assistants. We gathered all the data ourselves.”
Length of Stay
Of the 21% of unsuccessful stays, the most common reason was a hospital length of stay (LOS) longer than 72 hours (71% of 156 patients), although the median LOS was 42 hours. Sixty-six patients eventually required traditional inpatient services, nearly half of those after a specialty consult. The study concluded that the types of services patients received during their SSU stays were stronger predictors of success than the patients’ characteristics upon admission.
“I was surprised by some of the findings, in the sense that I’ve worked and I’ve seen the kind of patients that are admitted into ED-run short-stay units … and for the most part, that is observation medicine,” Dr. Lucas says. “I got the immediate sense in our unit you’re actively managing sick patients. They’re just discharged within 72 hours.
“One of the whole reasons to have hospitalists run this unit, as opposed to ED docs, is because the hospital should be able to handle any diagnoses that come their way because they’re handling any diagnoses that come their way upstairs. But the ED doctors are more limited in what they’re able to do.”
Dr. Kumpaley adds that the hospitalist-run SSU works best when there is open communication between ED physicians who are doing the admitting and SSU physicians who must deal with the repercussions of those decisions.
In the case of a hospitalist-run unit, the earlier the two departments start a dialogue, the more successful the unit will be in determining whether patients should be admitted to the SSU in the first place, Dr. Lucas says.
“Every time you have to hand off a patient to a new doctor, there’s risk involved,” he says. “One of the ideas of HM right now is how transitions should be improved upon. The best way to improve on care transitions is to make them unnecessary altogether.” TH
Richard Quinn is a freelance writer based in New Jersey.
When the hospitalist-run short-stay unit (SSU) debuted at Cook County Hospital in Chicago seven years ago, a dearth of clinical research made it difficult to show the efficacy of such programs. Only a handful of such studies existed, and none had been conducted in the U.S. So while the hospitalists behind the nascent Cook County SSU thought their approach worked, Brian Lucas, MD, FHM, MS, wanted more evidentiary proof.
“We accept patients the emergency department sends to us without argument,” says Dr. Lucas, a hospitalist in the Department of Medicine at Cook County. “We wanted to be able to convey to the ED docs with data what kind of patients actually are best suited for the short-stay. We didn’t want it to be anecdotal or based on hunches a couple of us had. … We thought it would be nice to contribute something to the literature.”
Now they have.
Their prospective, observational, cohort study, “A Hospitalist-Run Short Stay Unit: Features that Predict Length-of-Stay and Eventual Admission to Traditional Inpatient Services,” can be found in the May-June Journal of Hospital Medicine. The study found that 79% of 738 eligible patients had successful SSU stays. Success was defined as discharge from the unit within 72 hours without admission to a general hospital unit.
The authors also found that in a multivariable model, the provisional diagnosis of heart failure predicted stays of longer than 72 hours (P=0.007), but risk assessments were unimportant. Patients who received specialty consultations were most likely to need eventual admission, and the likelihood of long stays was inversely proportional to the accessibility of diagnostic tests.
“In our hospital-run SSU, the inaccessibility of diagnostic tests and the need for specialty consultations were the most important predictors of unsuccessful stays,” the authors concluded. “Designs for other SSUs that care for mostly low-risk patients should focus on matching patients’ diagnostic and consultative needs with readily accessible services.”
—Brian Lucas, MD, FHM, MS, hospitalist, Cook County Hospital, Chicago
Dr. Lucas thinks the study could help HM groups establish or refine hospitalist-led SSUs and understand the best way to administer programs. He also points out that minimal funding was needed to complete the review, as the study mostly required the time of participating hospitalists to record their own data.
“Hospitalists are increasingly involved with quality-improvement projects at their hospitals,” Dr. Lucas says. “In order to actually decide whether it’s working right, you need data, and usually data costs a lot of money. In this case, it was free.”
Cook County’s 14-bed SSU was formed in 2002, when the hospital moved into new facilities and reduced its bed count from about 650 to 500. The decreased number of beds led to the short-term unit approach to handle potential overflows and diagnoses that required shorter lengths of stay. Dr. Lucas ran the unit at inception and later handed it off to Rudolf Kumapley, MBChB, its current medical director.
But questions on the operational parameters of the unit arose quickly. What types of admissions should the SSU allow? What risk levels would it focus on? And because one of the main benefits of an SSU is to alleviate pressure and backlogs in the ED, how should the wants of ED physicians be balanced against the success rate of the SSU?
“This was an extremely useful unit,” Dr. Kumapley says, and he thought, “Why don’t we get ourselves some data?”
Study Structure
While ED physicians can be admitted to the SSU without approval of a unit-assigned physician, Cook County’s departments of medicine and emergency medicine have promoted five guidelines for admission, although none are statutory:
- Patients should have anticipated stays of less than 72 hours;
- Patients should not be expected to require traditional inpatient services;
- Patients with provisional cardiovascular diagnoses should be preferentially admitted to the SSU over general medical units;
- No patients should be admitted with a risk level higher than intermediate; and
- Patients shouldn’t require advanced ancillary services, including bedside procedures, time-intensive nursing, and complex social services.
Once the study began, attending physician investigators would interview, examine, and review the health records of enrolled patients within 12 hours of admission to the unit. When diagnoses included possible acute coronary syndrome (ACS) or decompensated heart failure, additional data was gathered. ACS and decompensated heart failure are two of the most common provisional diagnoses admitted to the SSU, in large part because the unit is equipped with continuous telemetry monitors, a treadmill testing laboratory, and other reserved cardiac tests.
“We built an online database that allowed the physicians to enter the data on all of their patients in real time,” Dr. Lucas explains. “We didn’t have any research assistants. We gathered all the data ourselves.”
Length of Stay
Of the 21% of unsuccessful stays, the most common reason was a hospital length of stay (LOS) longer than 72 hours (71% of 156 patients), although the median LOS was 42 hours. Sixty-six patients eventually required traditional inpatient services, nearly half of those after a specialty consult. The study concluded that the types of services patients received during their SSU stays were stronger predictors of success than the patients’ characteristics upon admission.
“I was surprised by some of the findings, in the sense that I’ve worked and I’ve seen the kind of patients that are admitted into ED-run short-stay units … and for the most part, that is observation medicine,” Dr. Lucas says. “I got the immediate sense in our unit you’re actively managing sick patients. They’re just discharged within 72 hours.
“One of the whole reasons to have hospitalists run this unit, as opposed to ED docs, is because the hospital should be able to handle any diagnoses that come their way because they’re handling any diagnoses that come their way upstairs. But the ED doctors are more limited in what they’re able to do.”
Dr. Kumpaley adds that the hospitalist-run SSU works best when there is open communication between ED physicians who are doing the admitting and SSU physicians who must deal with the repercussions of those decisions.
In the case of a hospitalist-run unit, the earlier the two departments start a dialogue, the more successful the unit will be in determining whether patients should be admitted to the SSU in the first place, Dr. Lucas says.
“Every time you have to hand off a patient to a new doctor, there’s risk involved,” he says. “One of the ideas of HM right now is how transitions should be improved upon. The best way to improve on care transitions is to make them unnecessary altogether.” TH
Richard Quinn is a freelance writer based in New Jersey.
Up Close and Personal
There has been a lot of talk recently about pharmacogenomics and personalized medicine. Pharmaco-genomics—the way an individual responds to a medication—includes both positive and negative reactions, and how individual genetic differences affect drug response. It also examines the inherited variations in genes that dictate drug response and explores how these variations can be used to predict what type of response a patient will have to a particular drug, whether that is a good response, a bad response, or no response at all.1
In the race to catalog all the different gene variations, variations known as single nucleotide polymorphisms (SNPs, or “snips”) are used diagnostically to predict a patient’s response to a drug. In the future, pharmaceutical companies could use pharmacogenomics to predict which patients will have a negative response to a particular drug in clinical trials and, therefore, not study the medication in those patients.2 In essence, it would be a way to “streamline” therapy to those in most need of it or for those who likely will have a positive response with minimal adverse events.
By pre-screening patients, clinical trials could be smaller, faster, and less costly. The capability to pre-assess whether a patient will benefit from a particular medication before it is prescribed is a major advantage when it comes to medication use. It also might increase a prescriber’s confidence before starting a patient on a medication, and it might improve a patient’s confidence in taking the medication, which could increase medication adherence and lead to better patient outcomes.
Researchers have found that when patients with certain SNP variants of cytochrome P450 (CYP) take warfarin, metabolism and patient sensitivity are affected. Once this was discovered, the Food and Drug Administration (FDA) subsequently approved prescribing label changes for warfarin that incorporated pharmacogenomics information.3 This change included information on increased bleeding risk for patients carrying either the CYP2C9*2 or CYP2C9*3 alleles.4
More recently, Eckman et al published a cost-effectiveness analysis evaluating pharmacogenetic information related to warfarin dosing in patients with nonvalvular atrial fibrillation.5 In the study, the authors concluded that currently limited data exist utilizing pharmacogenomics for dosing of warfarin and its effects on major bleeding. They did note, however, that genotyping may be beneficial and cost-effective in patients who are at high risk of hemorrhage.
Additionally, there has been recent media coverage of the genetic variations of CYP2C19 affecting clopidogrel metabolism and efficacy.6,7 Both Mega et al and Collet et al noted that patients carrying at least one genetic variation of CYP2C19 had diminished platelet inhibition. Published earlier this year, both studies noted that patients carrying the genetic variation of CYP2C19 exhibited a higher rate of major cardiovascular events—including death, stent thrombosis, myocardial infarction, or stroke—than did noncarriers.
The FDA warned healthcare providers in November 2008 about using phenytoin or fosphenytoin in patients with the HLA-B*1502 allele due to a potentially increased risk of Stevens-Johnson syndrome and toxic epidermal necrolysis. A similar warning exists for carbamazepine in the same patient population. This is another example of pharmacogenomics information at work.8
Patient-specific variables have been identified that can help determine how an individual will respond to certain medications. Ultimately, this could decrease healthcare costs. It is a slow process and might be the wave of the future, but we are not there yet. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City.
References
- Just the facts: a basic introduction to the science underlying NCBI resources. National Center for Biotechnology Information Web site. Available at: www.ncbi.nlm.nih.gov/About/primer/pharm.html. Accessed Jan. 31, 2009.
- Gulseth MP, Grice GR, Dager WE. Pharmacogenomics of warfarin: uncovering a piece of the warfarin mystery. Am J Health Syst Pharm. 2009;66:123-133.
- FDA letter on approval of pharmacogenomics information for Coumadin label. Food and Drug Administration Web site. Available at: www.fda.gov/cder/foi/appletter/2007/009218s105ltr.pdf. Accessed Feb. 4, 2009.
- Coumadin label updated. Food and Drug Administration Web site. Available at: www.fda.gov/cder/foi/label/2007/009218s105lblv2.pdf. Accessed Feb. 4, 2009.
- Eckman MH, Rosand J, Greenberg SM, Gage BF. Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med. 2009;150:73-83.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354-362.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450) 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309-317.
- Phenytoin and fosphenytoin information. Food and Drug Administration Web site. Available at: www.fda.gov/cder/drug/infopage/phenytoin_fosphenytoin/default.htm. Accessed Feb. 4, 2009.
- Mylan receives final approval for first-to-file generic version of antiepileptic Keppra and launches immediately. Mylan Web site. Available at: investor.mylan.com/phoenix.zhtml?c=66563&p=irol-newsArticle&ID=1221778. Accessed Feb. 4, 2009.
- Prism pharmaceuticals receives FDA approval of Nexterone for life-threatening ventricular fibrillation and ventricular tachycardia. Prism Pharmaceuticals Web site. Available at: www.prismpharma.com/news.html. Accessed Feb. 4, 2009.
- Now in double concentration. E-Pharm/alert Web site. Available at: whatcounts.jobson.com/dm?id=0CCEED6291EC025CFED437DC0261D862. Accessed Feb. 4, 2009.
- Meeting of the Cardiovascular and Renal Drugs Advisory Committee. Food and Drug Administration Web site. Available at: www.fda.gov/cder/audiences/acspage/meetings/crdac_meeting_20090203.htm. Accessed Feb. 4, 2009.
- Bratulic, A. FDA panel recommends Eli Lilly’s, Daiichi Sankyo’s Effient. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=F4B502BDC684486986AE56DED7B532F3&logRowId=281890. Accessed Feb. 4, 2009.
- Now available 12-mg tablets. E-Pharm/alert Web site. Available at: whatcounts.jobson.com/dm?id=0CCEED6291EC025C1D5D3BD762CE3ECB. Accessed Feb. 4, 2009.
- Tom WC. New venlafaxine extended-release formulation. Pharmacist’s Letter/Prescriber’s Letter. 2009;25(1)250108.
There has been a lot of talk recently about pharmacogenomics and personalized medicine. Pharmaco-genomics—the way an individual responds to a medication—includes both positive and negative reactions, and how individual genetic differences affect drug response. It also examines the inherited variations in genes that dictate drug response and explores how these variations can be used to predict what type of response a patient will have to a particular drug, whether that is a good response, a bad response, or no response at all.1
In the race to catalog all the different gene variations, variations known as single nucleotide polymorphisms (SNPs, or “snips”) are used diagnostically to predict a patient’s response to a drug. In the future, pharmaceutical companies could use pharmacogenomics to predict which patients will have a negative response to a particular drug in clinical trials and, therefore, not study the medication in those patients.2 In essence, it would be a way to “streamline” therapy to those in most need of it or for those who likely will have a positive response with minimal adverse events.
By pre-screening patients, clinical trials could be smaller, faster, and less costly. The capability to pre-assess whether a patient will benefit from a particular medication before it is prescribed is a major advantage when it comes to medication use. It also might increase a prescriber’s confidence before starting a patient on a medication, and it might improve a patient’s confidence in taking the medication, which could increase medication adherence and lead to better patient outcomes.
Researchers have found that when patients with certain SNP variants of cytochrome P450 (CYP) take warfarin, metabolism and patient sensitivity are affected. Once this was discovered, the Food and Drug Administration (FDA) subsequently approved prescribing label changes for warfarin that incorporated pharmacogenomics information.3 This change included information on increased bleeding risk for patients carrying either the CYP2C9*2 or CYP2C9*3 alleles.4
More recently, Eckman et al published a cost-effectiveness analysis evaluating pharmacogenetic information related to warfarin dosing in patients with nonvalvular atrial fibrillation.5 In the study, the authors concluded that currently limited data exist utilizing pharmacogenomics for dosing of warfarin and its effects on major bleeding. They did note, however, that genotyping may be beneficial and cost-effective in patients who are at high risk of hemorrhage.
Additionally, there has been recent media coverage of the genetic variations of CYP2C19 affecting clopidogrel metabolism and efficacy.6,7 Both Mega et al and Collet et al noted that patients carrying at least one genetic variation of CYP2C19 had diminished platelet inhibition. Published earlier this year, both studies noted that patients carrying the genetic variation of CYP2C19 exhibited a higher rate of major cardiovascular events—including death, stent thrombosis, myocardial infarction, or stroke—than did noncarriers.
The FDA warned healthcare providers in November 2008 about using phenytoin or fosphenytoin in patients with the HLA-B*1502 allele due to a potentially increased risk of Stevens-Johnson syndrome and toxic epidermal necrolysis. A similar warning exists for carbamazepine in the same patient population. This is another example of pharmacogenomics information at work.8
Patient-specific variables have been identified that can help determine how an individual will respond to certain medications. Ultimately, this could decrease healthcare costs. It is a slow process and might be the wave of the future, but we are not there yet. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City.
References
- Just the facts: a basic introduction to the science underlying NCBI resources. National Center for Biotechnology Information Web site. Available at: www.ncbi.nlm.nih.gov/About/primer/pharm.html. Accessed Jan. 31, 2009.
- Gulseth MP, Grice GR, Dager WE. Pharmacogenomics of warfarin: uncovering a piece of the warfarin mystery. Am J Health Syst Pharm. 2009;66:123-133.
- FDA letter on approval of pharmacogenomics information for Coumadin label. Food and Drug Administration Web site. Available at: www.fda.gov/cder/foi/appletter/2007/009218s105ltr.pdf. Accessed Feb. 4, 2009.
- Coumadin label updated. Food and Drug Administration Web site. Available at: www.fda.gov/cder/foi/label/2007/009218s105lblv2.pdf. Accessed Feb. 4, 2009.
- Eckman MH, Rosand J, Greenberg SM, Gage BF. Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med. 2009;150:73-83.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354-362.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450) 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309-317.
- Phenytoin and fosphenytoin information. Food and Drug Administration Web site. Available at: www.fda.gov/cder/drug/infopage/phenytoin_fosphenytoin/default.htm. Accessed Feb. 4, 2009.
- Mylan receives final approval for first-to-file generic version of antiepileptic Keppra and launches immediately. Mylan Web site. Available at: investor.mylan.com/phoenix.zhtml?c=66563&p=irol-newsArticle&ID=1221778. Accessed Feb. 4, 2009.
- Prism pharmaceuticals receives FDA approval of Nexterone for life-threatening ventricular fibrillation and ventricular tachycardia. Prism Pharmaceuticals Web site. Available at: www.prismpharma.com/news.html. Accessed Feb. 4, 2009.
- Now in double concentration. E-Pharm/alert Web site. Available at: whatcounts.jobson.com/dm?id=0CCEED6291EC025CFED437DC0261D862. Accessed Feb. 4, 2009.
- Meeting of the Cardiovascular and Renal Drugs Advisory Committee. Food and Drug Administration Web site. Available at: www.fda.gov/cder/audiences/acspage/meetings/crdac_meeting_20090203.htm. Accessed Feb. 4, 2009.
- Bratulic, A. FDA panel recommends Eli Lilly’s, Daiichi Sankyo’s Effient. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=F4B502BDC684486986AE56DED7B532F3&logRowId=281890. Accessed Feb. 4, 2009.
- Now available 12-mg tablets. E-Pharm/alert Web site. Available at: whatcounts.jobson.com/dm?id=0CCEED6291EC025C1D5D3BD762CE3ECB. Accessed Feb. 4, 2009.
- Tom WC. New venlafaxine extended-release formulation. Pharmacist’s Letter/Prescriber’s Letter. 2009;25(1)250108.
There has been a lot of talk recently about pharmacogenomics and personalized medicine. Pharmaco-genomics—the way an individual responds to a medication—includes both positive and negative reactions, and how individual genetic differences affect drug response. It also examines the inherited variations in genes that dictate drug response and explores how these variations can be used to predict what type of response a patient will have to a particular drug, whether that is a good response, a bad response, or no response at all.1
In the race to catalog all the different gene variations, variations known as single nucleotide polymorphisms (SNPs, or “snips”) are used diagnostically to predict a patient’s response to a drug. In the future, pharmaceutical companies could use pharmacogenomics to predict which patients will have a negative response to a particular drug in clinical trials and, therefore, not study the medication in those patients.2 In essence, it would be a way to “streamline” therapy to those in most need of it or for those who likely will have a positive response with minimal adverse events.
By pre-screening patients, clinical trials could be smaller, faster, and less costly. The capability to pre-assess whether a patient will benefit from a particular medication before it is prescribed is a major advantage when it comes to medication use. It also might increase a prescriber’s confidence before starting a patient on a medication, and it might improve a patient’s confidence in taking the medication, which could increase medication adherence and lead to better patient outcomes.
Researchers have found that when patients with certain SNP variants of cytochrome P450 (CYP) take warfarin, metabolism and patient sensitivity are affected. Once this was discovered, the Food and Drug Administration (FDA) subsequently approved prescribing label changes for warfarin that incorporated pharmacogenomics information.3 This change included information on increased bleeding risk for patients carrying either the CYP2C9*2 or CYP2C9*3 alleles.4
More recently, Eckman et al published a cost-effectiveness analysis evaluating pharmacogenetic information related to warfarin dosing in patients with nonvalvular atrial fibrillation.5 In the study, the authors concluded that currently limited data exist utilizing pharmacogenomics for dosing of warfarin and its effects on major bleeding. They did note, however, that genotyping may be beneficial and cost-effective in patients who are at high risk of hemorrhage.
Additionally, there has been recent media coverage of the genetic variations of CYP2C19 affecting clopidogrel metabolism and efficacy.6,7 Both Mega et al and Collet et al noted that patients carrying at least one genetic variation of CYP2C19 had diminished platelet inhibition. Published earlier this year, both studies noted that patients carrying the genetic variation of CYP2C19 exhibited a higher rate of major cardiovascular events—including death, stent thrombosis, myocardial infarction, or stroke—than did noncarriers.
The FDA warned healthcare providers in November 2008 about using phenytoin or fosphenytoin in patients with the HLA-B*1502 allele due to a potentially increased risk of Stevens-Johnson syndrome and toxic epidermal necrolysis. A similar warning exists for carbamazepine in the same patient population. This is another example of pharmacogenomics information at work.8
Patient-specific variables have been identified that can help determine how an individual will respond to certain medications. Ultimately, this could decrease healthcare costs. It is a slow process and might be the wave of the future, but we are not there yet. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a freelance medical writer based in New York City.
References
- Just the facts: a basic introduction to the science underlying NCBI resources. National Center for Biotechnology Information Web site. Available at: www.ncbi.nlm.nih.gov/About/primer/pharm.html. Accessed Jan. 31, 2009.
- Gulseth MP, Grice GR, Dager WE. Pharmacogenomics of warfarin: uncovering a piece of the warfarin mystery. Am J Health Syst Pharm. 2009;66:123-133.
- FDA letter on approval of pharmacogenomics information for Coumadin label. Food and Drug Administration Web site. Available at: www.fda.gov/cder/foi/appletter/2007/009218s105ltr.pdf. Accessed Feb. 4, 2009.
- Coumadin label updated. Food and Drug Administration Web site. Available at: www.fda.gov/cder/foi/label/2007/009218s105lblv2.pdf. Accessed Feb. 4, 2009.
- Eckman MH, Rosand J, Greenberg SM, Gage BF. Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med. 2009;150:73-83.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354-362.
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450) 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309-317.
- Phenytoin and fosphenytoin information. Food and Drug Administration Web site. Available at: www.fda.gov/cder/drug/infopage/phenytoin_fosphenytoin/default.htm. Accessed Feb. 4, 2009.
- Mylan receives final approval for first-to-file generic version of antiepileptic Keppra and launches immediately. Mylan Web site. Available at: investor.mylan.com/phoenix.zhtml?c=66563&p=irol-newsArticle&ID=1221778. Accessed Feb. 4, 2009.
- Prism pharmaceuticals receives FDA approval of Nexterone for life-threatening ventricular fibrillation and ventricular tachycardia. Prism Pharmaceuticals Web site. Available at: www.prismpharma.com/news.html. Accessed Feb. 4, 2009.
- Now in double concentration. E-Pharm/alert Web site. Available at: whatcounts.jobson.com/dm?id=0CCEED6291EC025CFED437DC0261D862. Accessed Feb. 4, 2009.
- Meeting of the Cardiovascular and Renal Drugs Advisory Committee. Food and Drug Administration Web site. Available at: www.fda.gov/cder/audiences/acspage/meetings/crdac_meeting_20090203.htm. Accessed Feb. 4, 2009.
- Bratulic, A. FDA panel recommends Eli Lilly’s, Daiichi Sankyo’s Effient. FirstWord Web site. Available at: www.firstwordplus.com/Fws.do?articleid=F4B502BDC684486986AE56DED7B532F3&logRowId=281890. Accessed Feb. 4, 2009.
- Now available 12-mg tablets. E-Pharm/alert Web site. Available at: whatcounts.jobson.com/dm?id=0CCEED6291EC025C1D5D3BD762CE3ECB. Accessed Feb. 4, 2009.
- Tom WC. New venlafaxine extended-release formulation. Pharmacist’s Letter/Prescriber’s Letter. 2009;25(1)250108.
The latest research you need to know
Literature at a Glance
- Discharge innovation and readmission rates.
- Electronic medical records and outcomes.
- CXR findings predict outcomes.
- NSAIDs and congestive heart failure morbidity.
- Outcomes of interpreter use.
- Predictors and outcomes of postoperative delirium.
- Perioperative beta-blockers.
- Perioperative stroke risk.
Standardized Discharge Intervention Decreases Readmission Rates
Clinical question: Does a standardized discharge intervention lead to a decrease in ED visits and readmission rates following hospital discharge?
Background: Hospital discharge is a complex process that is not standardized at many institutions. Deficiencies in the process can lead to poor outcomes, unnecessary rehospitalizations, and increased costs. Previous studies of peridischarge interventions have yielded mixed results and typically focus on specific patient populations.
Study design: Randomized trial.
Setting: Boston Medical Center, a large, urban, academic medical center.
Synopsis: In this single-institution study, 749 English-speaking hospitalized adults were randomly assigned one of two discharge plans: a multidisciplinary package of discharge services or the usual discharge process. Patients in the intervention group were assigned a nurse discharge advocate who performed patient education, medication reconciliation, discharge coordination, and scheduled follow-up appointments. A pharmacist also telephoned participants two to four days after discharge to reinforce the discharge plan and review medications.
Participants in the intervention group had a 30% relative reduction in hospital utilization (defined as ED visit or hospital readmission) at 30 days. Overall, 21.6% of intervention patients and 26.9% of usual-discharge patients had at least one hospital utilization within 30 days of discharge.
This study was limited to a single center, and 27% of the patients did not meet eligibility criteria. The applicability also is limited by the resource utilization required for the intervention. The authors estimated that 0.5 full-time-equivalent (FTE) nursing time and 0.15 FTE pharmacist time was required to maintain 14 patients per week.
Bottom line: A systematic, intensive approach to discharges can reduce ED return visits and readmission rates.
Citation: Jack B, Chetty V, Anthony D, et al. A re-engineered hospital discharge program to decrease re-hospitalization. Ann Intern Med. 2009:150(3):178-187.
EMR Equals Lower Mortality, Fewer Complications, Lower Costs
Clinical question: Is improved automation of hospital information associated with reduced rates of inpatient mortality, complications, cost, and length of stay (LOS)?
Background: Clinical information technologies, including electronic medical records (EMR), are touted as an antidote for the fragmented, unsafe, and expensive American healthcare system. Most studies on the effect of such technologies are limited to a single site, and few involve commercially available information systems.
Study design: Cross-sectional study.
Setting: Urban hospitals in Texas.
Synopsis: Researchers used the previously validated Clinical Information Technology Assessment Tool to survey physicians providing inpatient care in 72 Texas hospitals. This tool measures the degree to which clinical information processes are computerized. Automation is divided into four subdomains: test results, notes and records, order entry, and decision support. To achieve a high score, a process must be fully computerized, the physician must know how to activate it, and the physician must choose the computerized process over alternatives. The authors examined the association between a hospital’s degree of automation and mortality, costs, and LOS among patients with myocardial infarction, congestive heart failure, coronary artery bypass grafting, and pneumonia.
Overall, greater automation was associated with lower mortality, fewer complications, and lower costs. No clear impact on LOS was found. Higher scores in the notes and records subdomains were most associated with lower mortality. Higher decision-support scores were most associated with lower complication rates and costs.
This study is one of the first to demonstrate the benefits of clinical information technologies across a variety of institutions using different information systems.
Bottom line: Hospitals with EMR, order entry, and clinical decision support have lower mortality rates, fewer complications, and lower costs.
Citation: Amarasingham R, Plantinga L, Diener-West M, et al. Clinical information technologies and inpatient outcomes. Arch Intern Med. 2009;169(2):108-114.
Radiologic Progression of Pulmonary Infiltrates Portends Worse Prognosis in Severe CAP Patients
Clinical question: In patients admitted to the ICU with severe community-acquired pneumonia (CAP), do bacteremia and rapid radiologic progression of pulmonary infiltrates increase the risk of shock and mortality?
Background: Severe CAP is associated with considerable morbidity and mortality; however, data focusing on short-term outcomes is limited. The role of the chest radiograph is established in diagnosis but is unclear as a prognostic tool. Bacteremia is associated with higher mortality risk but also is more common in patients with comorbid illnesses.
Study design: Retrospective cohort.
Setting: 33 hospitals in Spain.
Synopsis: This study retrospectively analyzed 457 patients with severe CAP admitted to the ICU between Dec. 1, 2000, and Feb. 28, 2002. Patients were classified into four groups according to the presence or absence of rapid radiographic spread of pulmonary infiltrates and CAP-associated bacteremia. Patients demonstrating significant worsening by chest radiography within the first 48 hours after admission had a threefold increase in the risk of death. Bacteremia was not associated with increased mortality.
The retrospective nature of this study is its major limitation. Other limitations are the probable inclusion of unrecognized bacteremia in the nonbacteremic groups, the fact that repeat chest radiographs were obtained only once (at 48 hours), and that the cause of radiographic deterioration was not examined.
This study contributes to the literature by identifying a subset of patients (those with worsening chest radiographs at 48 hours) who may benefit from further study and targeted interventions.
Bottom line: In severe CAP patients, radiographic worsening at 48 hours is a negative prognostic factor, while bacteremia is not associated with worse outcomes.
Citation: Lisboa T, Blot S, Waterer G, et al. Radiologic progression of pulmonary infiltrates predicts a worse prognosis in severe community-acquired pneumonia than bacteremia. Chest. 2009;135(1):165-172.
NSAIDs Increase Risk of Death and Cardiovascular Morbidity in CHF Patients
Clinical question: Is NSAID use by patients with congestive heart failure (CHF) associated with a higher risk of death or hospitalization due to acute myocardial infarction (MI) or heart failure?
Background: NSAID use is widespread and generally perceived to be low-risk given their over-the-counter availability. However, clinical guidelines discourage the use of NSAIDs in patients with chronic heart failure due to the risk of fluid retention and worsening heart failure.
Study design: Retrospective cohort.
Setting: All hospitals in Denmark.
Synopsis: This study identified 107,092 patients who survived their first hospitalizations due to heart failure between 1995 and 2004. Subsequent use of NSAIDs was determined from a national prescription registry. Patient records were retrospectively analyzed to assess mortality and hospitalization due to MI or heart failure.
At least one NSAID prescription was claimed by 33.9% of the patients after discharge. All NSAIDs were associated with higher death rates, and there was a dose-dependent increase in the risk of death. Ibuprofen and naproxen demonstrated increased mortality only at high doses. All NSAIDs that were studied increased the risk of hospitalization for MI or heart failure.
The observational design is the study’s major limitation. Other important limitations include lack of detailed information about heart failure diagnoses and indication for starting NSAID therapy.
This study intensifies the debate regarding the increased risk of cardiovascular events in NSAID patients, which has been ongoing since the publication of the VIGOR Study in 2000.
Bottom line: In patients with a history of heart failure, NSAIDs are associated with an increased risk of death and cardiovascular morbidity.
Citation: Gislason G, Rasmussen J, Abildstrom S, et al. Increased mortality and cardiovascular morbidity associated with use of non-steroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169(2):141-149.
Language Barriers Present Obstacle during ICU Family Meetings
Clinical question: Do family meetings that require the use of an interpreter have different characteristics than those in which an interpreter is not needed?
Background: Communication about end-of-life care is essential in the ICU, yet limited English proficiency (LEP) can be a barrier to effective discussions. Overall, outcomes and satisfaction are improved when interpreters are used, but specific effects on ICU family conferences are unknown.
Study design: Cross-sectional evaluation of family meetings.
Setting: Four hospitals in Seattle.
Synopsis: Fifty-one noninterpreted (English-speaking members only) and 10 interpreted (non-English-speaking members present) ICU family meetings were recorded and analyzed for the amount of speaking time and content. The total duration was similar for interpreted versus noninterpreted conferences (26.3 minutes vs. 32.0 minutes, P=0.25), but clinician speech was significantly less in the interpreted group (10.9 minutes vs. 19.6 minutes, P=0.001). Family speaking time was similar in both interpreted and noninterpreted conversations (7.1 minutes vs. 8.2 minutes, P=0.66). Clinicians used more emotional support for families in noninterpreted meetings, including active listening and pausing for questions.
This study is limited by the use of an audio recorder; a video recorder would have provided researchers with participants’ physical interaction and expressions. Additionally, the study does not differentiate between cultural and linguistic difficulties, or provide a reference for the degree of complexity of each conference.
Bottom line: Family meetings in the ICU that require the use of an interpreter provide less information and emotional support to family members than those in which an interpreter is not required.
Citation: Thornton J, Pham K, Engelberg R, et al. Families with limited English proficiency receive less information and support in interpreted intensive care unit family conferences. Crit Care Med. 2009;37(1):89-95.
Postoperative Delirium and Poor Outcomes
Clinical question: In patients 50 and older, what are the risk factors for the development of postoperative delirium, and how are outcomes affected by delirium?
Background: Delirium in the elderly postoperative patient is common. It results in increased costs, morbidity, and mortality. As the population ages, more elderly patients will undergo surgical procedures, so identification of delirium risk factors is essential.
Study design: Prospective, observational, cohort study.
Setting: Veterans Affairs Medical Center, Denver.
Synopsis: Researchers assessed 144 patients 50 and older who were scheduled to undergo surgical procedures with a planned, postoperative ICU stay for cognitive function, overall functional status, and comorbidities. Postoperatively, patients were assessed daily for the development of dementia using the cognitive assessment method-ICU instrument (CAM-ICU). Additionally, a validated chart review method for diagnosing delirium was used. The overall delirium incidence was 44%, and only 12% of cases had an identifiable etiology. The mean onset of delirium was 2.4 days; duration was 4.5 days. The incidence of delirium increased with age, reaching 92% in the 80- to 89-year-old group. In multivariate analysis, preoperative cognitive dysfunction was the strongest predictor of delirium.
Delirium development in patients 50 and older was associated with marked increases in costs ($50,000 vs. $32,000), length of stay (16 days vs. eight days), discharge to a facility (33% vs. 1%), and mortality (9% vs. 1%).
Limitations of this study included the patient population studied (97% men) and lack of data regarding medication use during hospitalization.
Bottom line: In older patients undergoing surgery requiring postoperative ICU care, delirium is common, is associated with prior cognitive dysfunction, and results in significant increases in LOS and mortality.
Citation: Robinson T, Raeburn C, Tran Z, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249(1):173-178.
Meta-Analysis of Perioperative Beta-Blockers Doesn’t Support Routine Use
Clinical question: Are perioperative beta-blockers effective in preventing cardiac events in noncardiac surgery?
Background: The American College of Cardiology (ACC) and American Heart Association (AHA) guidelines for noncardiac surgery recommend beta-blockers for high-risk patients undergoing intermediate or high-risk surgery. The results of previous randomized controlled trials have been inconsistent.
Study design: Meta-analysis.
Setting: Literature search of PubMed, Embase, and the Cochrane Library.
Synopsis: Thirty-three randomized, controlled trials—which included 12,306 patients—were selected for statistical analysis. In these trials, beta-blockers were initiated in the perioperative period with 30-day followup for outcomes of interest. Overall, perioperative beta-blocker use was associated with a 35% risk reduction in nonfatal MI, a 116% increased risk of nonfatal stroke, and no significant difference in all-cause mortality. Trials with higher levels of bias showed greater statistical benefit of beta-blockers. Of note, the POISE trial carried the largest amount of weight, accounting for nearly two-thirds of the total number of patients included in treatment arms.
The application of this data is challenging, as studies differ in several important variables, such as timing of beta-blocker initiation, dosing regimen, and duration of treatment. The POISE trial, in particular, employed a very large dose of metoprolol, compared with doses of beta-blockers used in other studies. There is less data regarding the perioperative efficacy of beta-blockers when therapy is started well before surgery.
Bottom line: Current evidence does not support the routine use of beta-blockers started immediately prior to noncardiac surgery to prevent perioperative cardiac events.
Citation: Bangalore S, Wetterslev J, Pranesh S, et al. Perioperative beta-blockers in patients having non-cardiac surgery: a meta-analysis. Lancet. 2008;372:1962-1976.
Perioperative Ischemic Stroke an Important Cause of Morbidity and Mortality in Noncardiac Surgery
Clinical question: What are the incidence, risk factors, and outcomes of acute ischemic stroke in nonvascular surgery?
Background: Ischemic stroke is a well-understood complication of cardiovascular surgery. However, little data exist in medical literature regarding the frequency, associations, and outcomes of perioperative ischemic stroke in noncardiac surgery.
Study design: Observational chart review using administrative data.
Setting: The Nationwide Inpatient Sample, a public-use database, in which approximately 1,000 hospitals submit data from nonfederal acute-care hospitals.
Synopsis: Three common surgeries were sampled from an administrative database to characterize the epidemiology of perioperative ischemic stroke in noncardiac surgery. This outcome occurred in 0.7% of hemicolectomy patients; 0.2% of total hip replacement patients; and 0.6% of lobectomy/segmental lung resection patients. Studying the rate of perioperative ischemic stroke in coronary artery bypass graft (CABG) patients validated the authors’ method of data extraction. This rate was consistent with prior studies of this outcome in cardiovascular surgery patients.
Multivariate analysis showed that renal disease (odds ratio 3.0), atrial fibrillation (OR 2.0), prior stroke (OR 1.6), and valvular disease (OR 1.5) are statistically associated with an increased risk of perioperative ischemic stroke. The primary outcome was associated with a marked increase in the odds of in-hospital mortality or need for chronic care upon hospital discharge.
This study is limited by its use of administrative coding data, as well as potential bias introduced by the use of only three major types of surgery.
Bottom line: Ischemic stroke is a serious complication of intermediate and major noncardiovascular surgery. It is associated with poor patient outcomes; more evidence is needed to confirm associations with this outcome and to discover strategies to reduce risk.
Citation: Bateman B, Schum-acher H, Wang S, et al. Perioperative acute ischemic stroke in non-cardiac and nonvascular surgery. Anesthesiology. 2009;110:231-238.
Literature at a Glance
- Discharge innovation and readmission rates.
- Electronic medical records and outcomes.
- CXR findings predict outcomes.
- NSAIDs and congestive heart failure morbidity.
- Outcomes of interpreter use.
- Predictors and outcomes of postoperative delirium.
- Perioperative beta-blockers.
- Perioperative stroke risk.
Standardized Discharge Intervention Decreases Readmission Rates
Clinical question: Does a standardized discharge intervention lead to a decrease in ED visits and readmission rates following hospital discharge?
Background: Hospital discharge is a complex process that is not standardized at many institutions. Deficiencies in the process can lead to poor outcomes, unnecessary rehospitalizations, and increased costs. Previous studies of peridischarge interventions have yielded mixed results and typically focus on specific patient populations.
Study design: Randomized trial.
Setting: Boston Medical Center, a large, urban, academic medical center.
Synopsis: In this single-institution study, 749 English-speaking hospitalized adults were randomly assigned one of two discharge plans: a multidisciplinary package of discharge services or the usual discharge process. Patients in the intervention group were assigned a nurse discharge advocate who performed patient education, medication reconciliation, discharge coordination, and scheduled follow-up appointments. A pharmacist also telephoned participants two to four days after discharge to reinforce the discharge plan and review medications.
Participants in the intervention group had a 30% relative reduction in hospital utilization (defined as ED visit or hospital readmission) at 30 days. Overall, 21.6% of intervention patients and 26.9% of usual-discharge patients had at least one hospital utilization within 30 days of discharge.
This study was limited to a single center, and 27% of the patients did not meet eligibility criteria. The applicability also is limited by the resource utilization required for the intervention. The authors estimated that 0.5 full-time-equivalent (FTE) nursing time and 0.15 FTE pharmacist time was required to maintain 14 patients per week.
Bottom line: A systematic, intensive approach to discharges can reduce ED return visits and readmission rates.
Citation: Jack B, Chetty V, Anthony D, et al. A re-engineered hospital discharge program to decrease re-hospitalization. Ann Intern Med. 2009:150(3):178-187.
EMR Equals Lower Mortality, Fewer Complications, Lower Costs
Clinical question: Is improved automation of hospital information associated with reduced rates of inpatient mortality, complications, cost, and length of stay (LOS)?
Background: Clinical information technologies, including electronic medical records (EMR), are touted as an antidote for the fragmented, unsafe, and expensive American healthcare system. Most studies on the effect of such technologies are limited to a single site, and few involve commercially available information systems.
Study design: Cross-sectional study.
Setting: Urban hospitals in Texas.
Synopsis: Researchers used the previously validated Clinical Information Technology Assessment Tool to survey physicians providing inpatient care in 72 Texas hospitals. This tool measures the degree to which clinical information processes are computerized. Automation is divided into four subdomains: test results, notes and records, order entry, and decision support. To achieve a high score, a process must be fully computerized, the physician must know how to activate it, and the physician must choose the computerized process over alternatives. The authors examined the association between a hospital’s degree of automation and mortality, costs, and LOS among patients with myocardial infarction, congestive heart failure, coronary artery bypass grafting, and pneumonia.
Overall, greater automation was associated with lower mortality, fewer complications, and lower costs. No clear impact on LOS was found. Higher scores in the notes and records subdomains were most associated with lower mortality. Higher decision-support scores were most associated with lower complication rates and costs.
This study is one of the first to demonstrate the benefits of clinical information technologies across a variety of institutions using different information systems.
Bottom line: Hospitals with EMR, order entry, and clinical decision support have lower mortality rates, fewer complications, and lower costs.
Citation: Amarasingham R, Plantinga L, Diener-West M, et al. Clinical information technologies and inpatient outcomes. Arch Intern Med. 2009;169(2):108-114.
Radiologic Progression of Pulmonary Infiltrates Portends Worse Prognosis in Severe CAP Patients
Clinical question: In patients admitted to the ICU with severe community-acquired pneumonia (CAP), do bacteremia and rapid radiologic progression of pulmonary infiltrates increase the risk of shock and mortality?
Background: Severe CAP is associated with considerable morbidity and mortality; however, data focusing on short-term outcomes is limited. The role of the chest radiograph is established in diagnosis but is unclear as a prognostic tool. Bacteremia is associated with higher mortality risk but also is more common in patients with comorbid illnesses.
Study design: Retrospective cohort.
Setting: 33 hospitals in Spain.
Synopsis: This study retrospectively analyzed 457 patients with severe CAP admitted to the ICU between Dec. 1, 2000, and Feb. 28, 2002. Patients were classified into four groups according to the presence or absence of rapid radiographic spread of pulmonary infiltrates and CAP-associated bacteremia. Patients demonstrating significant worsening by chest radiography within the first 48 hours after admission had a threefold increase in the risk of death. Bacteremia was not associated with increased mortality.
The retrospective nature of this study is its major limitation. Other limitations are the probable inclusion of unrecognized bacteremia in the nonbacteremic groups, the fact that repeat chest radiographs were obtained only once (at 48 hours), and that the cause of radiographic deterioration was not examined.
This study contributes to the literature by identifying a subset of patients (those with worsening chest radiographs at 48 hours) who may benefit from further study and targeted interventions.
Bottom line: In severe CAP patients, radiographic worsening at 48 hours is a negative prognostic factor, while bacteremia is not associated with worse outcomes.
Citation: Lisboa T, Blot S, Waterer G, et al. Radiologic progression of pulmonary infiltrates predicts a worse prognosis in severe community-acquired pneumonia than bacteremia. Chest. 2009;135(1):165-172.
NSAIDs Increase Risk of Death and Cardiovascular Morbidity in CHF Patients
Clinical question: Is NSAID use by patients with congestive heart failure (CHF) associated with a higher risk of death or hospitalization due to acute myocardial infarction (MI) or heart failure?
Background: NSAID use is widespread and generally perceived to be low-risk given their over-the-counter availability. However, clinical guidelines discourage the use of NSAIDs in patients with chronic heart failure due to the risk of fluid retention and worsening heart failure.
Study design: Retrospective cohort.
Setting: All hospitals in Denmark.
Synopsis: This study identified 107,092 patients who survived their first hospitalizations due to heart failure between 1995 and 2004. Subsequent use of NSAIDs was determined from a national prescription registry. Patient records were retrospectively analyzed to assess mortality and hospitalization due to MI or heart failure.
At least one NSAID prescription was claimed by 33.9% of the patients after discharge. All NSAIDs were associated with higher death rates, and there was a dose-dependent increase in the risk of death. Ibuprofen and naproxen demonstrated increased mortality only at high doses. All NSAIDs that were studied increased the risk of hospitalization for MI or heart failure.
The observational design is the study’s major limitation. Other important limitations include lack of detailed information about heart failure diagnoses and indication for starting NSAID therapy.
This study intensifies the debate regarding the increased risk of cardiovascular events in NSAID patients, which has been ongoing since the publication of the VIGOR Study in 2000.
Bottom line: In patients with a history of heart failure, NSAIDs are associated with an increased risk of death and cardiovascular morbidity.
Citation: Gislason G, Rasmussen J, Abildstrom S, et al. Increased mortality and cardiovascular morbidity associated with use of non-steroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169(2):141-149.
Language Barriers Present Obstacle during ICU Family Meetings
Clinical question: Do family meetings that require the use of an interpreter have different characteristics than those in which an interpreter is not needed?
Background: Communication about end-of-life care is essential in the ICU, yet limited English proficiency (LEP) can be a barrier to effective discussions. Overall, outcomes and satisfaction are improved when interpreters are used, but specific effects on ICU family conferences are unknown.
Study design: Cross-sectional evaluation of family meetings.
Setting: Four hospitals in Seattle.
Synopsis: Fifty-one noninterpreted (English-speaking members only) and 10 interpreted (non-English-speaking members present) ICU family meetings were recorded and analyzed for the amount of speaking time and content. The total duration was similar for interpreted versus noninterpreted conferences (26.3 minutes vs. 32.0 minutes, P=0.25), but clinician speech was significantly less in the interpreted group (10.9 minutes vs. 19.6 minutes, P=0.001). Family speaking time was similar in both interpreted and noninterpreted conversations (7.1 minutes vs. 8.2 minutes, P=0.66). Clinicians used more emotional support for families in noninterpreted meetings, including active listening and pausing for questions.
This study is limited by the use of an audio recorder; a video recorder would have provided researchers with participants’ physical interaction and expressions. Additionally, the study does not differentiate between cultural and linguistic difficulties, or provide a reference for the degree of complexity of each conference.
Bottom line: Family meetings in the ICU that require the use of an interpreter provide less information and emotional support to family members than those in which an interpreter is not required.
Citation: Thornton J, Pham K, Engelberg R, et al. Families with limited English proficiency receive less information and support in interpreted intensive care unit family conferences. Crit Care Med. 2009;37(1):89-95.
Postoperative Delirium and Poor Outcomes
Clinical question: In patients 50 and older, what are the risk factors for the development of postoperative delirium, and how are outcomes affected by delirium?
Background: Delirium in the elderly postoperative patient is common. It results in increased costs, morbidity, and mortality. As the population ages, more elderly patients will undergo surgical procedures, so identification of delirium risk factors is essential.
Study design: Prospective, observational, cohort study.
Setting: Veterans Affairs Medical Center, Denver.
Synopsis: Researchers assessed 144 patients 50 and older who were scheduled to undergo surgical procedures with a planned, postoperative ICU stay for cognitive function, overall functional status, and comorbidities. Postoperatively, patients were assessed daily for the development of dementia using the cognitive assessment method-ICU instrument (CAM-ICU). Additionally, a validated chart review method for diagnosing delirium was used. The overall delirium incidence was 44%, and only 12% of cases had an identifiable etiology. The mean onset of delirium was 2.4 days; duration was 4.5 days. The incidence of delirium increased with age, reaching 92% in the 80- to 89-year-old group. In multivariate analysis, preoperative cognitive dysfunction was the strongest predictor of delirium.
Delirium development in patients 50 and older was associated with marked increases in costs ($50,000 vs. $32,000), length of stay (16 days vs. eight days), discharge to a facility (33% vs. 1%), and mortality (9% vs. 1%).
Limitations of this study included the patient population studied (97% men) and lack of data regarding medication use during hospitalization.
Bottom line: In older patients undergoing surgery requiring postoperative ICU care, delirium is common, is associated with prior cognitive dysfunction, and results in significant increases in LOS and mortality.
Citation: Robinson T, Raeburn C, Tran Z, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249(1):173-178.
Meta-Analysis of Perioperative Beta-Blockers Doesn’t Support Routine Use
Clinical question: Are perioperative beta-blockers effective in preventing cardiac events in noncardiac surgery?
Background: The American College of Cardiology (ACC) and American Heart Association (AHA) guidelines for noncardiac surgery recommend beta-blockers for high-risk patients undergoing intermediate or high-risk surgery. The results of previous randomized controlled trials have been inconsistent.
Study design: Meta-analysis.
Setting: Literature search of PubMed, Embase, and the Cochrane Library.
Synopsis: Thirty-three randomized, controlled trials—which included 12,306 patients—were selected for statistical analysis. In these trials, beta-blockers were initiated in the perioperative period with 30-day followup for outcomes of interest. Overall, perioperative beta-blocker use was associated with a 35% risk reduction in nonfatal MI, a 116% increased risk of nonfatal stroke, and no significant difference in all-cause mortality. Trials with higher levels of bias showed greater statistical benefit of beta-blockers. Of note, the POISE trial carried the largest amount of weight, accounting for nearly two-thirds of the total number of patients included in treatment arms.
The application of this data is challenging, as studies differ in several important variables, such as timing of beta-blocker initiation, dosing regimen, and duration of treatment. The POISE trial, in particular, employed a very large dose of metoprolol, compared with doses of beta-blockers used in other studies. There is less data regarding the perioperative efficacy of beta-blockers when therapy is started well before surgery.
Bottom line: Current evidence does not support the routine use of beta-blockers started immediately prior to noncardiac surgery to prevent perioperative cardiac events.
Citation: Bangalore S, Wetterslev J, Pranesh S, et al. Perioperative beta-blockers in patients having non-cardiac surgery: a meta-analysis. Lancet. 2008;372:1962-1976.
Perioperative Ischemic Stroke an Important Cause of Morbidity and Mortality in Noncardiac Surgery
Clinical question: What are the incidence, risk factors, and outcomes of acute ischemic stroke in nonvascular surgery?
Background: Ischemic stroke is a well-understood complication of cardiovascular surgery. However, little data exist in medical literature regarding the frequency, associations, and outcomes of perioperative ischemic stroke in noncardiac surgery.
Study design: Observational chart review using administrative data.
Setting: The Nationwide Inpatient Sample, a public-use database, in which approximately 1,000 hospitals submit data from nonfederal acute-care hospitals.
Synopsis: Three common surgeries were sampled from an administrative database to characterize the epidemiology of perioperative ischemic stroke in noncardiac surgery. This outcome occurred in 0.7% of hemicolectomy patients; 0.2% of total hip replacement patients; and 0.6% of lobectomy/segmental lung resection patients. Studying the rate of perioperative ischemic stroke in coronary artery bypass graft (CABG) patients validated the authors’ method of data extraction. This rate was consistent with prior studies of this outcome in cardiovascular surgery patients.
Multivariate analysis showed that renal disease (odds ratio 3.0), atrial fibrillation (OR 2.0), prior stroke (OR 1.6), and valvular disease (OR 1.5) are statistically associated with an increased risk of perioperative ischemic stroke. The primary outcome was associated with a marked increase in the odds of in-hospital mortality or need for chronic care upon hospital discharge.
This study is limited by its use of administrative coding data, as well as potential bias introduced by the use of only three major types of surgery.
Bottom line: Ischemic stroke is a serious complication of intermediate and major noncardiovascular surgery. It is associated with poor patient outcomes; more evidence is needed to confirm associations with this outcome and to discover strategies to reduce risk.
Citation: Bateman B, Schum-acher H, Wang S, et al. Perioperative acute ischemic stroke in non-cardiac and nonvascular surgery. Anesthesiology. 2009;110:231-238.
Literature at a Glance
- Discharge innovation and readmission rates.
- Electronic medical records and outcomes.
- CXR findings predict outcomes.
- NSAIDs and congestive heart failure morbidity.
- Outcomes of interpreter use.
- Predictors and outcomes of postoperative delirium.
- Perioperative beta-blockers.
- Perioperative stroke risk.
Standardized Discharge Intervention Decreases Readmission Rates
Clinical question: Does a standardized discharge intervention lead to a decrease in ED visits and readmission rates following hospital discharge?
Background: Hospital discharge is a complex process that is not standardized at many institutions. Deficiencies in the process can lead to poor outcomes, unnecessary rehospitalizations, and increased costs. Previous studies of peridischarge interventions have yielded mixed results and typically focus on specific patient populations.
Study design: Randomized trial.
Setting: Boston Medical Center, a large, urban, academic medical center.
Synopsis: In this single-institution study, 749 English-speaking hospitalized adults were randomly assigned one of two discharge plans: a multidisciplinary package of discharge services or the usual discharge process. Patients in the intervention group were assigned a nurse discharge advocate who performed patient education, medication reconciliation, discharge coordination, and scheduled follow-up appointments. A pharmacist also telephoned participants two to four days after discharge to reinforce the discharge plan and review medications.
Participants in the intervention group had a 30% relative reduction in hospital utilization (defined as ED visit or hospital readmission) at 30 days. Overall, 21.6% of intervention patients and 26.9% of usual-discharge patients had at least one hospital utilization within 30 days of discharge.
This study was limited to a single center, and 27% of the patients did not meet eligibility criteria. The applicability also is limited by the resource utilization required for the intervention. The authors estimated that 0.5 full-time-equivalent (FTE) nursing time and 0.15 FTE pharmacist time was required to maintain 14 patients per week.
Bottom line: A systematic, intensive approach to discharges can reduce ED return visits and readmission rates.
Citation: Jack B, Chetty V, Anthony D, et al. A re-engineered hospital discharge program to decrease re-hospitalization. Ann Intern Med. 2009:150(3):178-187.
EMR Equals Lower Mortality, Fewer Complications, Lower Costs
Clinical question: Is improved automation of hospital information associated with reduced rates of inpatient mortality, complications, cost, and length of stay (LOS)?
Background: Clinical information technologies, including electronic medical records (EMR), are touted as an antidote for the fragmented, unsafe, and expensive American healthcare system. Most studies on the effect of such technologies are limited to a single site, and few involve commercially available information systems.
Study design: Cross-sectional study.
Setting: Urban hospitals in Texas.
Synopsis: Researchers used the previously validated Clinical Information Technology Assessment Tool to survey physicians providing inpatient care in 72 Texas hospitals. This tool measures the degree to which clinical information processes are computerized. Automation is divided into four subdomains: test results, notes and records, order entry, and decision support. To achieve a high score, a process must be fully computerized, the physician must know how to activate it, and the physician must choose the computerized process over alternatives. The authors examined the association between a hospital’s degree of automation and mortality, costs, and LOS among patients with myocardial infarction, congestive heart failure, coronary artery bypass grafting, and pneumonia.
Overall, greater automation was associated with lower mortality, fewer complications, and lower costs. No clear impact on LOS was found. Higher scores in the notes and records subdomains were most associated with lower mortality. Higher decision-support scores were most associated with lower complication rates and costs.
This study is one of the first to demonstrate the benefits of clinical information technologies across a variety of institutions using different information systems.
Bottom line: Hospitals with EMR, order entry, and clinical decision support have lower mortality rates, fewer complications, and lower costs.
Citation: Amarasingham R, Plantinga L, Diener-West M, et al. Clinical information technologies and inpatient outcomes. Arch Intern Med. 2009;169(2):108-114.
Radiologic Progression of Pulmonary Infiltrates Portends Worse Prognosis in Severe CAP Patients
Clinical question: In patients admitted to the ICU with severe community-acquired pneumonia (CAP), do bacteremia and rapid radiologic progression of pulmonary infiltrates increase the risk of shock and mortality?
Background: Severe CAP is associated with considerable morbidity and mortality; however, data focusing on short-term outcomes is limited. The role of the chest radiograph is established in diagnosis but is unclear as a prognostic tool. Bacteremia is associated with higher mortality risk but also is more common in patients with comorbid illnesses.
Study design: Retrospective cohort.
Setting: 33 hospitals in Spain.
Synopsis: This study retrospectively analyzed 457 patients with severe CAP admitted to the ICU between Dec. 1, 2000, and Feb. 28, 2002. Patients were classified into four groups according to the presence or absence of rapid radiographic spread of pulmonary infiltrates and CAP-associated bacteremia. Patients demonstrating significant worsening by chest radiography within the first 48 hours after admission had a threefold increase in the risk of death. Bacteremia was not associated with increased mortality.
The retrospective nature of this study is its major limitation. Other limitations are the probable inclusion of unrecognized bacteremia in the nonbacteremic groups, the fact that repeat chest radiographs were obtained only once (at 48 hours), and that the cause of radiographic deterioration was not examined.
This study contributes to the literature by identifying a subset of patients (those with worsening chest radiographs at 48 hours) who may benefit from further study and targeted interventions.
Bottom line: In severe CAP patients, radiographic worsening at 48 hours is a negative prognostic factor, while bacteremia is not associated with worse outcomes.
Citation: Lisboa T, Blot S, Waterer G, et al. Radiologic progression of pulmonary infiltrates predicts a worse prognosis in severe community-acquired pneumonia than bacteremia. Chest. 2009;135(1):165-172.
NSAIDs Increase Risk of Death and Cardiovascular Morbidity in CHF Patients
Clinical question: Is NSAID use by patients with congestive heart failure (CHF) associated with a higher risk of death or hospitalization due to acute myocardial infarction (MI) or heart failure?
Background: NSAID use is widespread and generally perceived to be low-risk given their over-the-counter availability. However, clinical guidelines discourage the use of NSAIDs in patients with chronic heart failure due to the risk of fluid retention and worsening heart failure.
Study design: Retrospective cohort.
Setting: All hospitals in Denmark.
Synopsis: This study identified 107,092 patients who survived their first hospitalizations due to heart failure between 1995 and 2004. Subsequent use of NSAIDs was determined from a national prescription registry. Patient records were retrospectively analyzed to assess mortality and hospitalization due to MI or heart failure.
At least one NSAID prescription was claimed by 33.9% of the patients after discharge. All NSAIDs were associated with higher death rates, and there was a dose-dependent increase in the risk of death. Ibuprofen and naproxen demonstrated increased mortality only at high doses. All NSAIDs that were studied increased the risk of hospitalization for MI or heart failure.
The observational design is the study’s major limitation. Other important limitations include lack of detailed information about heart failure diagnoses and indication for starting NSAID therapy.
This study intensifies the debate regarding the increased risk of cardiovascular events in NSAID patients, which has been ongoing since the publication of the VIGOR Study in 2000.
Bottom line: In patients with a history of heart failure, NSAIDs are associated with an increased risk of death and cardiovascular morbidity.
Citation: Gislason G, Rasmussen J, Abildstrom S, et al. Increased mortality and cardiovascular morbidity associated with use of non-steroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169(2):141-149.
Language Barriers Present Obstacle during ICU Family Meetings
Clinical question: Do family meetings that require the use of an interpreter have different characteristics than those in which an interpreter is not needed?
Background: Communication about end-of-life care is essential in the ICU, yet limited English proficiency (LEP) can be a barrier to effective discussions. Overall, outcomes and satisfaction are improved when interpreters are used, but specific effects on ICU family conferences are unknown.
Study design: Cross-sectional evaluation of family meetings.
Setting: Four hospitals in Seattle.
Synopsis: Fifty-one noninterpreted (English-speaking members only) and 10 interpreted (non-English-speaking members present) ICU family meetings were recorded and analyzed for the amount of speaking time and content. The total duration was similar for interpreted versus noninterpreted conferences (26.3 minutes vs. 32.0 minutes, P=0.25), but clinician speech was significantly less in the interpreted group (10.9 minutes vs. 19.6 minutes, P=0.001). Family speaking time was similar in both interpreted and noninterpreted conversations (7.1 minutes vs. 8.2 minutes, P=0.66). Clinicians used more emotional support for families in noninterpreted meetings, including active listening and pausing for questions.
This study is limited by the use of an audio recorder; a video recorder would have provided researchers with participants’ physical interaction and expressions. Additionally, the study does not differentiate between cultural and linguistic difficulties, or provide a reference for the degree of complexity of each conference.
Bottom line: Family meetings in the ICU that require the use of an interpreter provide less information and emotional support to family members than those in which an interpreter is not required.
Citation: Thornton J, Pham K, Engelberg R, et al. Families with limited English proficiency receive less information and support in interpreted intensive care unit family conferences. Crit Care Med. 2009;37(1):89-95.
Postoperative Delirium and Poor Outcomes
Clinical question: In patients 50 and older, what are the risk factors for the development of postoperative delirium, and how are outcomes affected by delirium?
Background: Delirium in the elderly postoperative patient is common. It results in increased costs, morbidity, and mortality. As the population ages, more elderly patients will undergo surgical procedures, so identification of delirium risk factors is essential.
Study design: Prospective, observational, cohort study.
Setting: Veterans Affairs Medical Center, Denver.
Synopsis: Researchers assessed 144 patients 50 and older who were scheduled to undergo surgical procedures with a planned, postoperative ICU stay for cognitive function, overall functional status, and comorbidities. Postoperatively, patients were assessed daily for the development of dementia using the cognitive assessment method-ICU instrument (CAM-ICU). Additionally, a validated chart review method for diagnosing delirium was used. The overall delirium incidence was 44%, and only 12% of cases had an identifiable etiology. The mean onset of delirium was 2.4 days; duration was 4.5 days. The incidence of delirium increased with age, reaching 92% in the 80- to 89-year-old group. In multivariate analysis, preoperative cognitive dysfunction was the strongest predictor of delirium.
Delirium development in patients 50 and older was associated with marked increases in costs ($50,000 vs. $32,000), length of stay (16 days vs. eight days), discharge to a facility (33% vs. 1%), and mortality (9% vs. 1%).
Limitations of this study included the patient population studied (97% men) and lack of data regarding medication use during hospitalization.
Bottom line: In older patients undergoing surgery requiring postoperative ICU care, delirium is common, is associated with prior cognitive dysfunction, and results in significant increases in LOS and mortality.
Citation: Robinson T, Raeburn C, Tran Z, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249(1):173-178.
Meta-Analysis of Perioperative Beta-Blockers Doesn’t Support Routine Use
Clinical question: Are perioperative beta-blockers effective in preventing cardiac events in noncardiac surgery?
Background: The American College of Cardiology (ACC) and American Heart Association (AHA) guidelines for noncardiac surgery recommend beta-blockers for high-risk patients undergoing intermediate or high-risk surgery. The results of previous randomized controlled trials have been inconsistent.
Study design: Meta-analysis.
Setting: Literature search of PubMed, Embase, and the Cochrane Library.
Synopsis: Thirty-three randomized, controlled trials—which included 12,306 patients—were selected for statistical analysis. In these trials, beta-blockers were initiated in the perioperative period with 30-day followup for outcomes of interest. Overall, perioperative beta-blocker use was associated with a 35% risk reduction in nonfatal MI, a 116% increased risk of nonfatal stroke, and no significant difference in all-cause mortality. Trials with higher levels of bias showed greater statistical benefit of beta-blockers. Of note, the POISE trial carried the largest amount of weight, accounting for nearly two-thirds of the total number of patients included in treatment arms.
The application of this data is challenging, as studies differ in several important variables, such as timing of beta-blocker initiation, dosing regimen, and duration of treatment. The POISE trial, in particular, employed a very large dose of metoprolol, compared with doses of beta-blockers used in other studies. There is less data regarding the perioperative efficacy of beta-blockers when therapy is started well before surgery.
Bottom line: Current evidence does not support the routine use of beta-blockers started immediately prior to noncardiac surgery to prevent perioperative cardiac events.
Citation: Bangalore S, Wetterslev J, Pranesh S, et al. Perioperative beta-blockers in patients having non-cardiac surgery: a meta-analysis. Lancet. 2008;372:1962-1976.
Perioperative Ischemic Stroke an Important Cause of Morbidity and Mortality in Noncardiac Surgery
Clinical question: What are the incidence, risk factors, and outcomes of acute ischemic stroke in nonvascular surgery?
Background: Ischemic stroke is a well-understood complication of cardiovascular surgery. However, little data exist in medical literature regarding the frequency, associations, and outcomes of perioperative ischemic stroke in noncardiac surgery.
Study design: Observational chart review using administrative data.
Setting: The Nationwide Inpatient Sample, a public-use database, in which approximately 1,000 hospitals submit data from nonfederal acute-care hospitals.
Synopsis: Three common surgeries were sampled from an administrative database to characterize the epidemiology of perioperative ischemic stroke in noncardiac surgery. This outcome occurred in 0.7% of hemicolectomy patients; 0.2% of total hip replacement patients; and 0.6% of lobectomy/segmental lung resection patients. Studying the rate of perioperative ischemic stroke in coronary artery bypass graft (CABG) patients validated the authors’ method of data extraction. This rate was consistent with prior studies of this outcome in cardiovascular surgery patients.
Multivariate analysis showed that renal disease (odds ratio 3.0), atrial fibrillation (OR 2.0), prior stroke (OR 1.6), and valvular disease (OR 1.5) are statistically associated with an increased risk of perioperative ischemic stroke. The primary outcome was associated with a marked increase in the odds of in-hospital mortality or need for chronic care upon hospital discharge.
This study is limited by its use of administrative coding data, as well as potential bias introduced by the use of only three major types of surgery.
Bottom line: Ischemic stroke is a serious complication of intermediate and major noncardiovascular surgery. It is associated with poor patient outcomes; more evidence is needed to confirm associations with this outcome and to discover strategies to reduce risk.
Citation: Bateman B, Schum-acher H, Wang S, et al. Perioperative acute ischemic stroke in non-cardiac and nonvascular surgery. Anesthesiology. 2009;110:231-238.
2010: HM Goes to Washington
Enter text here
Enter text here
Enter text here