User login
SHM’s Down with Digital
When hospitalist Robert Wachter, MD, FHM, started his HM blog almost two years ago, he didn’t anticipate that one of his blog entries would be about pop-music icon Britney Spears. Or that it would become his most popular, attracting nearly double the number of readers as his next-most-popular post.
Dr. Wachter—professor and chief of the division of hospital medicine at the University of California at San Francisco, a former SHM president, and author of the blog “Wachter’s World” (www.wachtersworld.com)—attributes the popularity of that post partly to Spears, but also to the fact it touched on a topic that always sparks interest among hospitalists, other healthcare providers, and hospital executives: the relationship between doctors and nurses in a hospital setting. Dr. Wachter’s most-popular post used Spears’ hospitalization in early 2008 and the controversy surrounding care providers who sneaked a peek at her medical records to make a point about how physicians and nurses often are treated differently in a hospital setting.
—Robert Chang, MD, hospitalist, University of Michigan Medical Center, Ann Arbor
But that was just one story. In the first year alone, Dr. Wachter wrote 76 blog posts, each of which easily exceeded 1,000 words. During the first year of blogging, the average post was read more than 1,800 times and the Web site attracted nearly 140,000 views.
“This has been one of the most gratifying things I’ve done in my career,” Dr. Wachter says. “I’ve published hundreds of articles in journals, but something about this form has an immediacy and connection to the audience that feels very important.”
And he isn’t alone. Blogs and HM have experienced similar growth trajectories in recent years. Now they are coming together to help hospitalists understand the most pressing issues in the specialty and provide the best care to hospitalized patients.
A Blog Primer
Blogs are Web sites that feature regular articles, or “posts.” The topic, length, and regularity of the posts are entirely at the discretion of the author, also known as the blogger. Some blogs are updated dozens of times a day; others, such as Wachter’s World, only feature new posts every week or so, but often with more depth and insight.
Although initially dismissed by many as outlets for trivial information, blogs are now recognized by experts in nearly every field as an important and cost-effective way to spark conversation and take positions on issues of the day.
Compared with more traditional media outlets, the ability to create dialogue is perhaps the most distinctive blog characteristic. Bloggers often invite readers to post or e-mail comments, creating interactivity between author and reader. In addition, many blogs automatically e-mail and distribute new blog posts to subscribers.
The “viral” aspect of blogs is a major contributor to their success. For instance, say Dr. Wachter writes a new blog post at 8:30 in the morning. Shortly thereafter, his readers will receive an automatically generated e-mail from the blog informing readers that Dr. Wachter has posted a new blog entry. When the reader visits the blog and reads the new post, they might think it could be of interest to a colleague, so they forward it to a colleague via e-mail. The colleague not only reads the article, but they also are impressed and post a comment for the rest of the blog’s readers to view.
SHM Blogs Advance the Specialty
The feedback loop of blogs isn’t limited to the on-screen world. That’s the lesson learned by clinical hospitalist Danielle Scheurer, MD, MSCR, SHM’s Web editor and director of General Medical Services at Brigham and Women’s Hospital in Boston.
As the author of SHM’s new clinical practice blog, “Hospital Medicine Quick Hits,” Dr. Scheurer knew the fledgling blog provided a valuable resource to busy clinicians, but she didn’t expect it to get back to her. She recalls that one day, “my blog was quoted to me by one of my house staff, who said, ‘I found this great hospital medicine blog today,’ and he didn’t realize I was the author.”
For the blog, Scheurer scours through 50 of the top medical journals for articles that are relevant to practicing clinical hospitalists. She posts concise overviews of the articles, along with links to the original research.
For hospitalists who have an interest in practice management, SHM offers another new blog, “The Hospitalist Leader.” It shares perspectives and ideas on the day-to-day interactions that hospitalists encounter and how best to administer a hospital practice. Four hospitalist co-authors—Robert Chang, MD, FHM, a hospitalist at the University of Michigan Medical Center in Ann Arbor; Rusty Holman, MD, FHM, chief operating officer of Brentwood, Tenn.-based Cogent Healthcare; John Nelson, MD, FHM, principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm; and Robert Bessler, MD, FHM, a hospitalist with Sound Inpatient Physicians in Tacoma, Wash.—use many of their own experiences in the hospital as raw material for the blog.
Dr. Chang views the blog as another way to move HM forward: “I trust and hope that we can use the blog to help the professional status of our profession, as this ultimately will determine the choices we make, large and small,” he says.
What’s Next
If you’re attending HM09 this year, don’t be surprised if you see someone else in the crowd excitedly typing into an iPhone or BlackBerry. You just might find a new blog post on the session you just attended.
Gone are the old images of a blogger in slippers and pajamas stealthily typing on the computer in the basement. These days, posting at or during an event, on site and in real time, is standard practice for many bloggers. In fact, SHM made a concerted effort to invite the most influential bloggers in the industry to HM09.
And if the person typing away isn’t “live blogging,” he may be “tweeting,” or adding super-short updates to the popular Web site Twitter. For many bloggers, it’s a way of communicating instantaneously with their audiences; once they post a blog article, they “tweet”—or send out—the link to thousands of readers.
SHM has its own Twitter account—@SHMLive—and uses the account to keep interested hospitalists updated on new blog posts, society news, and other HM developments.
“Hospital medicine is constantly evolving,” says Heather Abdel-Salam, SHM’s public relations and marketing coordinator, “and so do our efforts t.o communicate the best practices in the specialty. Blogs, Twitter feeds, and other online outreach are a big part of how we promote hospital medicine and help it grow within the healthcare arena.” TH
Brendon Shank is a freelance writer based in Philadelphia.
When hospitalist Robert Wachter, MD, FHM, started his HM blog almost two years ago, he didn’t anticipate that one of his blog entries would be about pop-music icon Britney Spears. Or that it would become his most popular, attracting nearly double the number of readers as his next-most-popular post.
Dr. Wachter—professor and chief of the division of hospital medicine at the University of California at San Francisco, a former SHM president, and author of the blog “Wachter’s World” (www.wachtersworld.com)—attributes the popularity of that post partly to Spears, but also to the fact it touched on a topic that always sparks interest among hospitalists, other healthcare providers, and hospital executives: the relationship between doctors and nurses in a hospital setting. Dr. Wachter’s most-popular post used Spears’ hospitalization in early 2008 and the controversy surrounding care providers who sneaked a peek at her medical records to make a point about how physicians and nurses often are treated differently in a hospital setting.
—Robert Chang, MD, hospitalist, University of Michigan Medical Center, Ann Arbor
But that was just one story. In the first year alone, Dr. Wachter wrote 76 blog posts, each of which easily exceeded 1,000 words. During the first year of blogging, the average post was read more than 1,800 times and the Web site attracted nearly 140,000 views.
“This has been one of the most gratifying things I’ve done in my career,” Dr. Wachter says. “I’ve published hundreds of articles in journals, but something about this form has an immediacy and connection to the audience that feels very important.”
And he isn’t alone. Blogs and HM have experienced similar growth trajectories in recent years. Now they are coming together to help hospitalists understand the most pressing issues in the specialty and provide the best care to hospitalized patients.
A Blog Primer
Blogs are Web sites that feature regular articles, or “posts.” The topic, length, and regularity of the posts are entirely at the discretion of the author, also known as the blogger. Some blogs are updated dozens of times a day; others, such as Wachter’s World, only feature new posts every week or so, but often with more depth and insight.
Although initially dismissed by many as outlets for trivial information, blogs are now recognized by experts in nearly every field as an important and cost-effective way to spark conversation and take positions on issues of the day.
Compared with more traditional media outlets, the ability to create dialogue is perhaps the most distinctive blog characteristic. Bloggers often invite readers to post or e-mail comments, creating interactivity between author and reader. In addition, many blogs automatically e-mail and distribute new blog posts to subscribers.
The “viral” aspect of blogs is a major contributor to their success. For instance, say Dr. Wachter writes a new blog post at 8:30 in the morning. Shortly thereafter, his readers will receive an automatically generated e-mail from the blog informing readers that Dr. Wachter has posted a new blog entry. When the reader visits the blog and reads the new post, they might think it could be of interest to a colleague, so they forward it to a colleague via e-mail. The colleague not only reads the article, but they also are impressed and post a comment for the rest of the blog’s readers to view.
SHM Blogs Advance the Specialty
The feedback loop of blogs isn’t limited to the on-screen world. That’s the lesson learned by clinical hospitalist Danielle Scheurer, MD, MSCR, SHM’s Web editor and director of General Medical Services at Brigham and Women’s Hospital in Boston.
As the author of SHM’s new clinical practice blog, “Hospital Medicine Quick Hits,” Dr. Scheurer knew the fledgling blog provided a valuable resource to busy clinicians, but she didn’t expect it to get back to her. She recalls that one day, “my blog was quoted to me by one of my house staff, who said, ‘I found this great hospital medicine blog today,’ and he didn’t realize I was the author.”
For the blog, Scheurer scours through 50 of the top medical journals for articles that are relevant to practicing clinical hospitalists. She posts concise overviews of the articles, along with links to the original research.
For hospitalists who have an interest in practice management, SHM offers another new blog, “The Hospitalist Leader.” It shares perspectives and ideas on the day-to-day interactions that hospitalists encounter and how best to administer a hospital practice. Four hospitalist co-authors—Robert Chang, MD, FHM, a hospitalist at the University of Michigan Medical Center in Ann Arbor; Rusty Holman, MD, FHM, chief operating officer of Brentwood, Tenn.-based Cogent Healthcare; John Nelson, MD, FHM, principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm; and Robert Bessler, MD, FHM, a hospitalist with Sound Inpatient Physicians in Tacoma, Wash.—use many of their own experiences in the hospital as raw material for the blog.
Dr. Chang views the blog as another way to move HM forward: “I trust and hope that we can use the blog to help the professional status of our profession, as this ultimately will determine the choices we make, large and small,” he says.
What’s Next
If you’re attending HM09 this year, don’t be surprised if you see someone else in the crowd excitedly typing into an iPhone or BlackBerry. You just might find a new blog post on the session you just attended.
Gone are the old images of a blogger in slippers and pajamas stealthily typing on the computer in the basement. These days, posting at or during an event, on site and in real time, is standard practice for many bloggers. In fact, SHM made a concerted effort to invite the most influential bloggers in the industry to HM09.
And if the person typing away isn’t “live blogging,” he may be “tweeting,” or adding super-short updates to the popular Web site Twitter. For many bloggers, it’s a way of communicating instantaneously with their audiences; once they post a blog article, they “tweet”—or send out—the link to thousands of readers.
SHM has its own Twitter account—@SHMLive—and uses the account to keep interested hospitalists updated on new blog posts, society news, and other HM developments.
“Hospital medicine is constantly evolving,” says Heather Abdel-Salam, SHM’s public relations and marketing coordinator, “and so do our efforts t.o communicate the best practices in the specialty. Blogs, Twitter feeds, and other online outreach are a big part of how we promote hospital medicine and help it grow within the healthcare arena.” TH
Brendon Shank is a freelance writer based in Philadelphia.
When hospitalist Robert Wachter, MD, FHM, started his HM blog almost two years ago, he didn’t anticipate that one of his blog entries would be about pop-music icon Britney Spears. Or that it would become his most popular, attracting nearly double the number of readers as his next-most-popular post.
Dr. Wachter—professor and chief of the division of hospital medicine at the University of California at San Francisco, a former SHM president, and author of the blog “Wachter’s World” (www.wachtersworld.com)—attributes the popularity of that post partly to Spears, but also to the fact it touched on a topic that always sparks interest among hospitalists, other healthcare providers, and hospital executives: the relationship between doctors and nurses in a hospital setting. Dr. Wachter’s most-popular post used Spears’ hospitalization in early 2008 and the controversy surrounding care providers who sneaked a peek at her medical records to make a point about how physicians and nurses often are treated differently in a hospital setting.
—Robert Chang, MD, hospitalist, University of Michigan Medical Center, Ann Arbor
But that was just one story. In the first year alone, Dr. Wachter wrote 76 blog posts, each of which easily exceeded 1,000 words. During the first year of blogging, the average post was read more than 1,800 times and the Web site attracted nearly 140,000 views.
“This has been one of the most gratifying things I’ve done in my career,” Dr. Wachter says. “I’ve published hundreds of articles in journals, but something about this form has an immediacy and connection to the audience that feels very important.”
And he isn’t alone. Blogs and HM have experienced similar growth trajectories in recent years. Now they are coming together to help hospitalists understand the most pressing issues in the specialty and provide the best care to hospitalized patients.
A Blog Primer
Blogs are Web sites that feature regular articles, or “posts.” The topic, length, and regularity of the posts are entirely at the discretion of the author, also known as the blogger. Some blogs are updated dozens of times a day; others, such as Wachter’s World, only feature new posts every week or so, but often with more depth and insight.
Although initially dismissed by many as outlets for trivial information, blogs are now recognized by experts in nearly every field as an important and cost-effective way to spark conversation and take positions on issues of the day.
Compared with more traditional media outlets, the ability to create dialogue is perhaps the most distinctive blog characteristic. Bloggers often invite readers to post or e-mail comments, creating interactivity between author and reader. In addition, many blogs automatically e-mail and distribute new blog posts to subscribers.
The “viral” aspect of blogs is a major contributor to their success. For instance, say Dr. Wachter writes a new blog post at 8:30 in the morning. Shortly thereafter, his readers will receive an automatically generated e-mail from the blog informing readers that Dr. Wachter has posted a new blog entry. When the reader visits the blog and reads the new post, they might think it could be of interest to a colleague, so they forward it to a colleague via e-mail. The colleague not only reads the article, but they also are impressed and post a comment for the rest of the blog’s readers to view.
SHM Blogs Advance the Specialty
The feedback loop of blogs isn’t limited to the on-screen world. That’s the lesson learned by clinical hospitalist Danielle Scheurer, MD, MSCR, SHM’s Web editor and director of General Medical Services at Brigham and Women’s Hospital in Boston.
As the author of SHM’s new clinical practice blog, “Hospital Medicine Quick Hits,” Dr. Scheurer knew the fledgling blog provided a valuable resource to busy clinicians, but she didn’t expect it to get back to her. She recalls that one day, “my blog was quoted to me by one of my house staff, who said, ‘I found this great hospital medicine blog today,’ and he didn’t realize I was the author.”
For the blog, Scheurer scours through 50 of the top medical journals for articles that are relevant to practicing clinical hospitalists. She posts concise overviews of the articles, along with links to the original research.
For hospitalists who have an interest in practice management, SHM offers another new blog, “The Hospitalist Leader.” It shares perspectives and ideas on the day-to-day interactions that hospitalists encounter and how best to administer a hospital practice. Four hospitalist co-authors—Robert Chang, MD, FHM, a hospitalist at the University of Michigan Medical Center in Ann Arbor; Rusty Holman, MD, FHM, chief operating officer of Brentwood, Tenn.-based Cogent Healthcare; John Nelson, MD, FHM, principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm; and Robert Bessler, MD, FHM, a hospitalist with Sound Inpatient Physicians in Tacoma, Wash.—use many of their own experiences in the hospital as raw material for the blog.
Dr. Chang views the blog as another way to move HM forward: “I trust and hope that we can use the blog to help the professional status of our profession, as this ultimately will determine the choices we make, large and small,” he says.
What’s Next
If you’re attending HM09 this year, don’t be surprised if you see someone else in the crowd excitedly typing into an iPhone or BlackBerry. You just might find a new blog post on the session you just attended.
Gone are the old images of a blogger in slippers and pajamas stealthily typing on the computer in the basement. These days, posting at or during an event, on site and in real time, is standard practice for many bloggers. In fact, SHM made a concerted effort to invite the most influential bloggers in the industry to HM09.
And if the person typing away isn’t “live blogging,” he may be “tweeting,” or adding super-short updates to the popular Web site Twitter. For many bloggers, it’s a way of communicating instantaneously with their audiences; once they post a blog article, they “tweet”—or send out—the link to thousands of readers.
SHM has its own Twitter account—@SHMLive—and uses the account to keep interested hospitalists updated on new blog posts, society news, and other HM developments.
“Hospital medicine is constantly evolving,” says Heather Abdel-Salam, SHM’s public relations and marketing coordinator, “and so do our efforts t.o communicate the best practices in the specialty. Blogs, Twitter feeds, and other online outreach are a big part of how we promote hospital medicine and help it grow within the healthcare arena.” TH
Brendon Shank is a freelance writer based in Philadelphia.
Clinical tips for managing safety in patients with borderline personality disorder
Issues in Hepatitis B Virus Infection
Supplement Editor:
William D. Carey, MD
Contents
The prevalence and natural history of hepatitis B in the 21st century
William D. Carey, MD
Risk of hepatocellular carcinoma in hepatitis B and prevention through treatment
Morris Sherman, MD, PhD
Understanding cultural barriers in hepatitis B virus infection
Tram T. Tran, MD
Hepatitis B treatment: Current best practices, avoiding resistance
Robert G. Gish, MD
Monotherapy vs multiple-drug therapy: The experts debate
Robert G. Gish, MD, and Pierre M. Gholam,MD
Management of hepatitis B in pregnancy: Weighing the options
Tram T. Tran, MD
Strategies for managing coinfection with hepatitis B virus and HIV
Morris Sherman, MD, PhD
Supplement Editor:
William D. Carey, MD
Contents
The prevalence and natural history of hepatitis B in the 21st century
William D. Carey, MD
Risk of hepatocellular carcinoma in hepatitis B and prevention through treatment
Morris Sherman, MD, PhD
Understanding cultural barriers in hepatitis B virus infection
Tram T. Tran, MD
Hepatitis B treatment: Current best practices, avoiding resistance
Robert G. Gish, MD
Monotherapy vs multiple-drug therapy: The experts debate
Robert G. Gish, MD, and Pierre M. Gholam,MD
Management of hepatitis B in pregnancy: Weighing the options
Tram T. Tran, MD
Strategies for managing coinfection with hepatitis B virus and HIV
Morris Sherman, MD, PhD
Supplement Editor:
William D. Carey, MD
Contents
The prevalence and natural history of hepatitis B in the 21st century
William D. Carey, MD
Risk of hepatocellular carcinoma in hepatitis B and prevention through treatment
Morris Sherman, MD, PhD
Understanding cultural barriers in hepatitis B virus infection
Tram T. Tran, MD
Hepatitis B treatment: Current best practices, avoiding resistance
Robert G. Gish, MD
Monotherapy vs multiple-drug therapy: The experts debate
Robert G. Gish, MD, and Pierre M. Gholam,MD
Management of hepatitis B in pregnancy: Weighing the options
Tram T. Tran, MD
Strategies for managing coinfection with hepatitis B virus and HIV
Morris Sherman, MD, PhD
The prevalence and natural history of hepatitis B in the 21st century
Hepatitis B virus (HBV) infection is highly prevalent worldwide and is a major cause of morbidity and death. Two billion people globally have been infected with HBV, 350 to 400 million are chronic carriers, and tens of millions of new cases occur annually. Of those infected, 15% to 40% develop HBV complications, namely cirrhosis or hepatocellular carcinoma (HCC).1–3
The high prevalence of HBV infection represents an enormous failure of public health, considering that HBV immunization has been available for an entire generation, and where it has been employed it has been highly effective at reducing the incidence of HBV infection. Immunization, however, has been underused.
This supplement to the Cleveland Clinic Journal of Medicine, derived from a live symposium, aims to enhance awareness of the natural history of HBV infection and clarify its management recommendations with illustrative case histories. The supplement starts with a brief review of HBV terminology, natural history, and epidemiology.
CHRONIC HBV INFECTION TERMINOLOGY
Familiarity with the terms commonly used to describe chronic HBV infection will help clinicians in the management of the disease4:
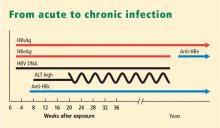
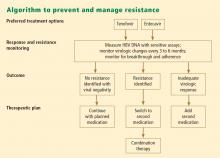
- Chronic HBV infection is defined as presence of hepatitis B surface antigen (HBsAg) for more than 6 months. Those with infection may also express another antigen, HB e antigen (HBeAg), a marker of heightened infectivity. At the same time, those who are HBeAg positive are better responders to antiviral therapy compared with those who are HBeAg negative.
- An inactive HBsAg carrier is an individual who is HBsAg positive with a very low level of circulating virus, liver enzyme levels within normal limits, and a low likelihood of having chronic progressive disease.
- Resolved HBV infection is defined as previous HBV infection with no remaining evidence of active disease. Such individuals test negative for HBsAg and positive for antibody to HBsAg (anti-HBs) and to HB core antigen (anti-HBc). They also have no detectable viral load, or HBV DNA, in their blood. In most instances, they are protected from reinfection.
- Reactivation is the reappearance of HBV infection in someone who is known to be an inactive HBsAg carrier or whose previous HBV infection had resolved (see “Case: Recurrence despite anti-HBs and HBsAg negativity”).
- HBeAg seroconversion is the transition from HBeAg-positive to HBeAg-negative status and development of antibody to HBeAg (anti-HBe), usually accompanied by less active liver disease and lower viral loads.
- HBeAg clearance is disappearance of HBeAg without the development of anti-HBe; reactivation or reversion to HBeAg-positive status can occur.
GEOGRAPHIC DISTRIBUTION OF CHRONIC HBV INFECTION
The global prevalence of HBV varies widely. Regions are divided into areas of low, intermediate, and high prevalence, defined as follows4:
- High prevalence implies that at least 8% of the population is currently infected, with a lifetime likelihood of active or resolved infection greater than 60%. About 45% of the world’s population lives in regions of high prevalence. Among this group, early childhood infections are common, with the virus usually transmitted from mother to infant during the perinatal period.
- Intermediate prevalence is defined as 2% to 7%, with a lifetime risk of infection of 20% to 60%. These regions represent about 43% of the global population. In intermediate-prevalence areas, infections occur in all age groups.
- Low prevalence is defined as less than 2% and represents only 12% of the global population. In these regions, the lifetime risk of infection is less than 20%.
North America is a low-prevalence area except for the northern rim, where Inuit and Yupik Eskimos have a high prevalence, and communities that have a substantial immigrant population from high-prevalence areas, such as sub-Saharan Africa and many parts of Asia.
Chronic HBV infection in the United States
Approximately 1.25 million individuals in the United States are HBsAg carriers.2,4 In Asian Americans and Alaskan natives, the prevalence of HBsAg positivity, or chronic disease, is 5% to 15%.5,6 Similarly, US health statistics sources estimate that among those who are chronically infected, approximately half are Asian American.7 As the Asian American population continues to increase (1.5 million to 7 million from 1970 to 19905,8; 11.9 million in the 2000 US Census8), the total prevalence of chronic HBV infection will increase as well.
NATURAL HISTORY OF CHRONIC HBV INFECTION
Chronic HBV usually causes microinflammatory changes that evoke a fibrotic response in the liver, and many infected individuals will eventually develop cirrhosis and are at risk for the development of HCC. Inactive HBsAg carriers often bypass the development of cirrhosis but remain at risk for HCC if their viral load is very high. This is particularly true when infection is acquired in infancy.
The age at acquisition of HBV has a large impact on the likelihood of the disease becoming chronic. The chance of chronic infection is 90% or greater among neonates who become infected with HBV through perinatal transmission. Exposure during adolescence or young adulthood is associated with a 95% or greater likelihood that the disease will be self-limiting.
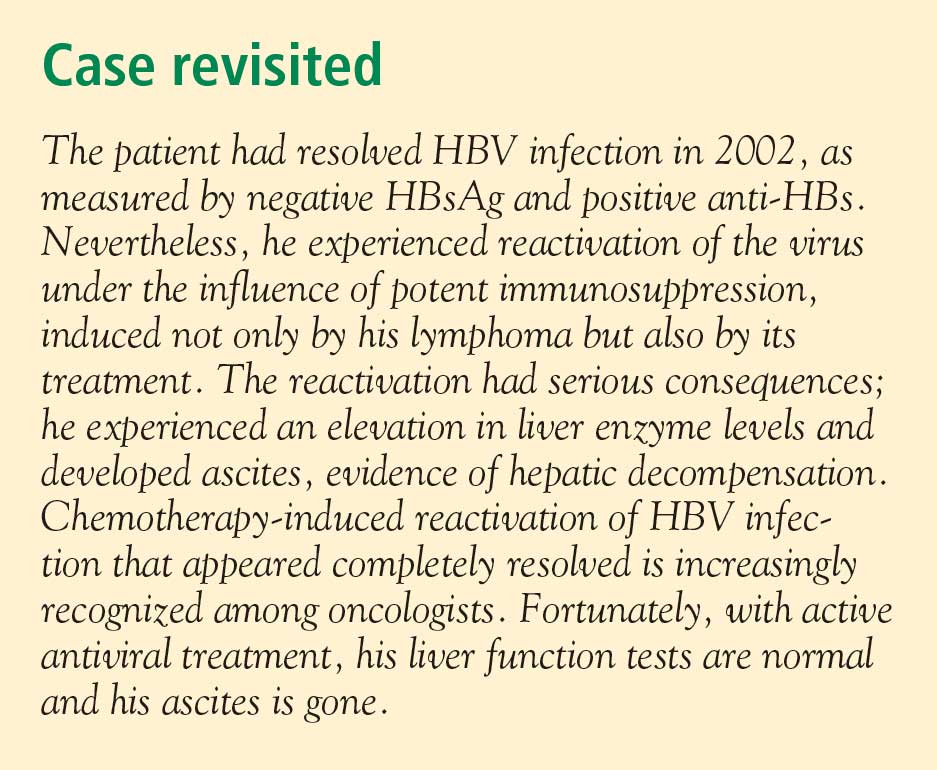
The typical North American patient with HBV acquires the infection as an adolescent or young adult and is not at risk of HCC unless cirrhosis develops. In most patients who acquire the disease in adolescence or adulthood, the infection resolves after weeks or a few months and they are not at risk of either cirrhosis or HCC. However, an individual such as the one described in the accompanying case, who becomes immunocompromised, is at risk of reactivation of HBV infection (see “Case revisited”).
HBV MODES OF TRANSMISSION
In low-prevalence areas, such as most of North America, most cases of HBV infection are acquired during adolescence to midadulthood, a period during which behaviors that increase the risk of HBV infection (ie, intravenous drug abuse or unprotected sexual activity) are most likely.9,10 Sex workers and homosexuals are at particular risk of sexual transmission of HBV. Intravenous drug abusers and health workers are at risk of parenteral transmission.
In high-prevalence areas, HBV is mostly transmitted during the perinatal period from mother to infant, conferring a high likelihood of chronicity.9,10 Mothers who are HBsAg positive, particularly those who are also HBeAg positive, are much more likely than others to transmit HBV to their offspring.
FACTORS THAT INFLUENCE THE COURSE OF HBV INFECTION
Viral load has emerged as the most significant factor implicated in the development of cirrhosis or HCC. Iloeje et al11 found that viral load predicted progression to cirrhosis among a cohort of nearly 4,000 Taiwanese. Other factors that can influence the course of HBV infection include age at onset, male sex, and comorbidities (ie, alcohol use, human immunodeficiency virus infection, hepatitis C virus infection). Core promoter and precore mutants may affect the likelihood of developing HCC. A genetic signature that predisposes liver cells to proliferate, termed field effects, may also lead to the development of HCC. The influence of smoking and diabetes on the development of HCC in HBV-infected individuals is not well documented.
Reduction or elimination of measurable virus is the current holy grail of treatment; available antiviral therapies are potent tools that lower viral load with the hope of reducing the likelihood of either cirrhosis or HCC.
HBV genotypes may be implicated in the progression of liver disease or the risk of development of HCC. HBV genotypes differ by region and may correlate with ethnicity and disease progression. In a study of 694 US patients with chronic HBV, Chu et al12 found that genotypes A and C were associated with a higher prevalence of HBsAg positivity than other genotypes. Genotypes B and C were the most common among Asian American patients, while genotype A was the most common among Caucasian and African American patients. The authors suggested that HBV genotypes may explain the heterogeneity in the manifestation of the disease.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004; 11:97–107.
- Hepatitis B Foundation. Statistics. Hepatitis B Foundation Web site. http://www.hepb.org/hepb/statistics.htm. Published 2003–2008. Accessed January 9, 2009.
- Hepatitis Foundation International. The ABC’s of hepatitis. Hepatitis Foundation International Web site. http://www.hepfi.org/living/liv_abc.html. Published 2003. Accessed January 9, 2009.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Tong MJ, Hwang S-J. Hepatitis B virus infection in Asian Americans. Gastroenterol Clin North Am 1994; 23:523–536.
- McMahon BJ, Schoenberg S, Bulkow L, et al. Seroprevalence of hepatitis B viral markers in 52,000 Alaska natives. Am J Epidemiol 1993; 138:544–549.
- US Department of Health and Human Services. Hepatitis and Asian Americans. The Office of Minority Health Web site. http://www.omhrc.gov/templates/content.aspx?lvl=3&lvlid=541&ID=6495. Updated May 5, 2008. Accessed January 12, 2009.
- Barnes JS, Bennett CE. The Asian population: 2000. Census 2000 brief. United States Census 2000 Web site. http://www.census.gov/prod/2002pubs/c2kbr01-16.pdf. Published February 2002. Accessed January 12, 2009.
- Lok AS, McMahon BJ; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Chronic hepatitis B. Hepatology 2001; 34:1225–1241.
- Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000—summary of a workshop. Gastroenterology 2001; 120:1828–1853.
- Iloeje UH, Yang H-I, Su J, Jen C-L, You S-L, Chen C-J, and The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-in HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006; 130:678–686.
- Chu CJ, Keeffe EB, Han SH, et al. Hepatitis B virus genotypes in the United States: results of a nationwide study. Gastroenterology 2003; 125:444–451.
Hepatitis B virus (HBV) infection is highly prevalent worldwide and is a major cause of morbidity and death. Two billion people globally have been infected with HBV, 350 to 400 million are chronic carriers, and tens of millions of new cases occur annually. Of those infected, 15% to 40% develop HBV complications, namely cirrhosis or hepatocellular carcinoma (HCC).1–3
The high prevalence of HBV infection represents an enormous failure of public health, considering that HBV immunization has been available for an entire generation, and where it has been employed it has been highly effective at reducing the incidence of HBV infection. Immunization, however, has been underused.
This supplement to the Cleveland Clinic Journal of Medicine, derived from a live symposium, aims to enhance awareness of the natural history of HBV infection and clarify its management recommendations with illustrative case histories. The supplement starts with a brief review of HBV terminology, natural history, and epidemiology.
CHRONIC HBV INFECTION TERMINOLOGY
Familiarity with the terms commonly used to describe chronic HBV infection will help clinicians in the management of the disease4:
- Chronic HBV infection is defined as presence of hepatitis B surface antigen (HBsAg) for more than 6 months. Those with infection may also express another antigen, HB e antigen (HBeAg), a marker of heightened infectivity. At the same time, those who are HBeAg positive are better responders to antiviral therapy compared with those who are HBeAg negative.
- An inactive HBsAg carrier is an individual who is HBsAg positive with a very low level of circulating virus, liver enzyme levels within normal limits, and a low likelihood of having chronic progressive disease.
- Resolved HBV infection is defined as previous HBV infection with no remaining evidence of active disease. Such individuals test negative for HBsAg and positive for antibody to HBsAg (anti-HBs) and to HB core antigen (anti-HBc). They also have no detectable viral load, or HBV DNA, in their blood. In most instances, they are protected from reinfection.
- Reactivation is the reappearance of HBV infection in someone who is known to be an inactive HBsAg carrier or whose previous HBV infection had resolved (see “Case: Recurrence despite anti-HBs and HBsAg negativity”).
- HBeAg seroconversion is the transition from HBeAg-positive to HBeAg-negative status and development of antibody to HBeAg (anti-HBe), usually accompanied by less active liver disease and lower viral loads.
- HBeAg clearance is disappearance of HBeAg without the development of anti-HBe; reactivation or reversion to HBeAg-positive status can occur.

GEOGRAPHIC DISTRIBUTION OF CHRONIC HBV INFECTION
The global prevalence of HBV varies widely. Regions are divided into areas of low, intermediate, and high prevalence, defined as follows4:
- High prevalence implies that at least 8% of the population is currently infected, with a lifetime likelihood of active or resolved infection greater than 60%. About 45% of the world’s population lives in regions of high prevalence. Among this group, early childhood infections are common, with the virus usually transmitted from mother to infant during the perinatal period.
- Intermediate prevalence is defined as 2% to 7%, with a lifetime risk of infection of 20% to 60%. These regions represent about 43% of the global population. In intermediate-prevalence areas, infections occur in all age groups.
- Low prevalence is defined as less than 2% and represents only 12% of the global population. In these regions, the lifetime risk of infection is less than 20%.
North America is a low-prevalence area except for the northern rim, where Inuit and Yupik Eskimos have a high prevalence, and communities that have a substantial immigrant population from high-prevalence areas, such as sub-Saharan Africa and many parts of Asia.
Chronic HBV infection in the United States
Approximately 1.25 million individuals in the United States are HBsAg carriers.2,4 In Asian Americans and Alaskan natives, the prevalence of HBsAg positivity, or chronic disease, is 5% to 15%.5,6 Similarly, US health statistics sources estimate that among those who are chronically infected, approximately half are Asian American.7 As the Asian American population continues to increase (1.5 million to 7 million from 1970 to 19905,8; 11.9 million in the 2000 US Census8), the total prevalence of chronic HBV infection will increase as well.
NATURAL HISTORY OF CHRONIC HBV INFECTION
Chronic HBV usually causes microinflammatory changes that evoke a fibrotic response in the liver, and many infected individuals will eventually develop cirrhosis and are at risk for the development of HCC. Inactive HBsAg carriers often bypass the development of cirrhosis but remain at risk for HCC if their viral load is very high. This is particularly true when infection is acquired in infancy.
The age at acquisition of HBV has a large impact on the likelihood of the disease becoming chronic. The chance of chronic infection is 90% or greater among neonates who become infected with HBV through perinatal transmission. Exposure during adolescence or young adulthood is associated with a 95% or greater likelihood that the disease will be self-limiting.
The typical North American patient with HBV acquires the infection as an adolescent or young adult and is not at risk of HCC unless cirrhosis develops. In most patients who acquire the disease in adolescence or adulthood, the infection resolves after weeks or a few months and they are not at risk of either cirrhosis or HCC. However, an individual such as the one described in the accompanying case, who becomes immunocompromised, is at risk of reactivation of HBV infection (see “Case revisited”).

HBV MODES OF TRANSMISSION
In low-prevalence areas, such as most of North America, most cases of HBV infection are acquired during adolescence to midadulthood, a period during which behaviors that increase the risk of HBV infection (ie, intravenous drug abuse or unprotected sexual activity) are most likely.9,10 Sex workers and homosexuals are at particular risk of sexual transmission of HBV. Intravenous drug abusers and health workers are at risk of parenteral transmission.
In high-prevalence areas, HBV is mostly transmitted during the perinatal period from mother to infant, conferring a high likelihood of chronicity.9,10 Mothers who are HBsAg positive, particularly those who are also HBeAg positive, are much more likely than others to transmit HBV to their offspring.
FACTORS THAT INFLUENCE THE COURSE OF HBV INFECTION
Viral load has emerged as the most significant factor implicated in the development of cirrhosis or HCC. Iloeje et al11 found that viral load predicted progression to cirrhosis among a cohort of nearly 4,000 Taiwanese. Other factors that can influence the course of HBV infection include age at onset, male sex, and comorbidities (ie, alcohol use, human immunodeficiency virus infection, hepatitis C virus infection). Core promoter and precore mutants may affect the likelihood of developing HCC. A genetic signature that predisposes liver cells to proliferate, termed field effects, may also lead to the development of HCC. The influence of smoking and diabetes on the development of HCC in HBV-infected individuals is not well documented.
Reduction or elimination of measurable virus is the current holy grail of treatment; available antiviral therapies are potent tools that lower viral load with the hope of reducing the likelihood of either cirrhosis or HCC.
HBV genotypes may be implicated in the progression of liver disease or the risk of development of HCC. HBV genotypes differ by region and may correlate with ethnicity and disease progression. In a study of 694 US patients with chronic HBV, Chu et al12 found that genotypes A and C were associated with a higher prevalence of HBsAg positivity than other genotypes. Genotypes B and C were the most common among Asian American patients, while genotype A was the most common among Caucasian and African American patients. The authors suggested that HBV genotypes may explain the heterogeneity in the manifestation of the disease.
Hepatitis B virus (HBV) infection is highly prevalent worldwide and is a major cause of morbidity and death. Two billion people globally have been infected with HBV, 350 to 400 million are chronic carriers, and tens of millions of new cases occur annually. Of those infected, 15% to 40% develop HBV complications, namely cirrhosis or hepatocellular carcinoma (HCC).1–3
The high prevalence of HBV infection represents an enormous failure of public health, considering that HBV immunization has been available for an entire generation, and where it has been employed it has been highly effective at reducing the incidence of HBV infection. Immunization, however, has been underused.
This supplement to the Cleveland Clinic Journal of Medicine, derived from a live symposium, aims to enhance awareness of the natural history of HBV infection and clarify its management recommendations with illustrative case histories. The supplement starts with a brief review of HBV terminology, natural history, and epidemiology.
CHRONIC HBV INFECTION TERMINOLOGY
Familiarity with the terms commonly used to describe chronic HBV infection will help clinicians in the management of the disease4:
- Chronic HBV infection is defined as presence of hepatitis B surface antigen (HBsAg) for more than 6 months. Those with infection may also express another antigen, HB e antigen (HBeAg), a marker of heightened infectivity. At the same time, those who are HBeAg positive are better responders to antiviral therapy compared with those who are HBeAg negative.
- An inactive HBsAg carrier is an individual who is HBsAg positive with a very low level of circulating virus, liver enzyme levels within normal limits, and a low likelihood of having chronic progressive disease.
- Resolved HBV infection is defined as previous HBV infection with no remaining evidence of active disease. Such individuals test negative for HBsAg and positive for antibody to HBsAg (anti-HBs) and to HB core antigen (anti-HBc). They also have no detectable viral load, or HBV DNA, in their blood. In most instances, they are protected from reinfection.
- Reactivation is the reappearance of HBV infection in someone who is known to be an inactive HBsAg carrier or whose previous HBV infection had resolved (see “Case: Recurrence despite anti-HBs and HBsAg negativity”).
- HBeAg seroconversion is the transition from HBeAg-positive to HBeAg-negative status and development of antibody to HBeAg (anti-HBe), usually accompanied by less active liver disease and lower viral loads.
- HBeAg clearance is disappearance of HBeAg without the development of anti-HBe; reactivation or reversion to HBeAg-positive status can occur.

GEOGRAPHIC DISTRIBUTION OF CHRONIC HBV INFECTION
The global prevalence of HBV varies widely. Regions are divided into areas of low, intermediate, and high prevalence, defined as follows4:
- High prevalence implies that at least 8% of the population is currently infected, with a lifetime likelihood of active or resolved infection greater than 60%. About 45% of the world’s population lives in regions of high prevalence. Among this group, early childhood infections are common, with the virus usually transmitted from mother to infant during the perinatal period.
- Intermediate prevalence is defined as 2% to 7%, with a lifetime risk of infection of 20% to 60%. These regions represent about 43% of the global population. In intermediate-prevalence areas, infections occur in all age groups.
- Low prevalence is defined as less than 2% and represents only 12% of the global population. In these regions, the lifetime risk of infection is less than 20%.
North America is a low-prevalence area except for the northern rim, where Inuit and Yupik Eskimos have a high prevalence, and communities that have a substantial immigrant population from high-prevalence areas, such as sub-Saharan Africa and many parts of Asia.
Chronic HBV infection in the United States
Approximately 1.25 million individuals in the United States are HBsAg carriers.2,4 In Asian Americans and Alaskan natives, the prevalence of HBsAg positivity, or chronic disease, is 5% to 15%.5,6 Similarly, US health statistics sources estimate that among those who are chronically infected, approximately half are Asian American.7 As the Asian American population continues to increase (1.5 million to 7 million from 1970 to 19905,8; 11.9 million in the 2000 US Census8), the total prevalence of chronic HBV infection will increase as well.
NATURAL HISTORY OF CHRONIC HBV INFECTION
Chronic HBV usually causes microinflammatory changes that evoke a fibrotic response in the liver, and many infected individuals will eventually develop cirrhosis and are at risk for the development of HCC. Inactive HBsAg carriers often bypass the development of cirrhosis but remain at risk for HCC if their viral load is very high. This is particularly true when infection is acquired in infancy.
The age at acquisition of HBV has a large impact on the likelihood of the disease becoming chronic. The chance of chronic infection is 90% or greater among neonates who become infected with HBV through perinatal transmission. Exposure during adolescence or young adulthood is associated with a 95% or greater likelihood that the disease will be self-limiting.
The typical North American patient with HBV acquires the infection as an adolescent or young adult and is not at risk of HCC unless cirrhosis develops. In most patients who acquire the disease in adolescence or adulthood, the infection resolves after weeks or a few months and they are not at risk of either cirrhosis or HCC. However, an individual such as the one described in the accompanying case, who becomes immunocompromised, is at risk of reactivation of HBV infection (see “Case revisited”).

HBV MODES OF TRANSMISSION
In low-prevalence areas, such as most of North America, most cases of HBV infection are acquired during adolescence to midadulthood, a period during which behaviors that increase the risk of HBV infection (ie, intravenous drug abuse or unprotected sexual activity) are most likely.9,10 Sex workers and homosexuals are at particular risk of sexual transmission of HBV. Intravenous drug abusers and health workers are at risk of parenteral transmission.
In high-prevalence areas, HBV is mostly transmitted during the perinatal period from mother to infant, conferring a high likelihood of chronicity.9,10 Mothers who are HBsAg positive, particularly those who are also HBeAg positive, are much more likely than others to transmit HBV to their offspring.
FACTORS THAT INFLUENCE THE COURSE OF HBV INFECTION
Viral load has emerged as the most significant factor implicated in the development of cirrhosis or HCC. Iloeje et al11 found that viral load predicted progression to cirrhosis among a cohort of nearly 4,000 Taiwanese. Other factors that can influence the course of HBV infection include age at onset, male sex, and comorbidities (ie, alcohol use, human immunodeficiency virus infection, hepatitis C virus infection). Core promoter and precore mutants may affect the likelihood of developing HCC. A genetic signature that predisposes liver cells to proliferate, termed field effects, may also lead to the development of HCC. The influence of smoking and diabetes on the development of HCC in HBV-infected individuals is not well documented.
Reduction or elimination of measurable virus is the current holy grail of treatment; available antiviral therapies are potent tools that lower viral load with the hope of reducing the likelihood of either cirrhosis or HCC.
HBV genotypes may be implicated in the progression of liver disease or the risk of development of HCC. HBV genotypes differ by region and may correlate with ethnicity and disease progression. In a study of 694 US patients with chronic HBV, Chu et al12 found that genotypes A and C were associated with a higher prevalence of HBsAg positivity than other genotypes. Genotypes B and C were the most common among Asian American patients, while genotype A was the most common among Caucasian and African American patients. The authors suggested that HBV genotypes may explain the heterogeneity in the manifestation of the disease.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004; 11:97–107.
- Hepatitis B Foundation. Statistics. Hepatitis B Foundation Web site. http://www.hepb.org/hepb/statistics.htm. Published 2003–2008. Accessed January 9, 2009.
- Hepatitis Foundation International. The ABC’s of hepatitis. Hepatitis Foundation International Web site. http://www.hepfi.org/living/liv_abc.html. Published 2003. Accessed January 9, 2009.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Tong MJ, Hwang S-J. Hepatitis B virus infection in Asian Americans. Gastroenterol Clin North Am 1994; 23:523–536.
- McMahon BJ, Schoenberg S, Bulkow L, et al. Seroprevalence of hepatitis B viral markers in 52,000 Alaska natives. Am J Epidemiol 1993; 138:544–549.
- US Department of Health and Human Services. Hepatitis and Asian Americans. The Office of Minority Health Web site. http://www.omhrc.gov/templates/content.aspx?lvl=3&lvlid=541&ID=6495. Updated May 5, 2008. Accessed January 12, 2009.
- Barnes JS, Bennett CE. The Asian population: 2000. Census 2000 brief. United States Census 2000 Web site. http://www.census.gov/prod/2002pubs/c2kbr01-16.pdf. Published February 2002. Accessed January 12, 2009.
- Lok AS, McMahon BJ; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Chronic hepatitis B. Hepatology 2001; 34:1225–1241.
- Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000—summary of a workshop. Gastroenterology 2001; 120:1828–1853.
- Iloeje UH, Yang H-I, Su J, Jen C-L, You S-L, Chen C-J, and The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-in HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006; 130:678–686.
- Chu CJ, Keeffe EB, Han SH, et al. Hepatitis B virus genotypes in the United States: results of a nationwide study. Gastroenterology 2003; 125:444–451.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004; 11:97–107.
- Hepatitis B Foundation. Statistics. Hepatitis B Foundation Web site. http://www.hepb.org/hepb/statistics.htm. Published 2003–2008. Accessed January 9, 2009.
- Hepatitis Foundation International. The ABC’s of hepatitis. Hepatitis Foundation International Web site. http://www.hepfi.org/living/liv_abc.html. Published 2003. Accessed January 9, 2009.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Tong MJ, Hwang S-J. Hepatitis B virus infection in Asian Americans. Gastroenterol Clin North Am 1994; 23:523–536.
- McMahon BJ, Schoenberg S, Bulkow L, et al. Seroprevalence of hepatitis B viral markers in 52,000 Alaska natives. Am J Epidemiol 1993; 138:544–549.
- US Department of Health and Human Services. Hepatitis and Asian Americans. The Office of Minority Health Web site. http://www.omhrc.gov/templates/content.aspx?lvl=3&lvlid=541&ID=6495. Updated May 5, 2008. Accessed January 12, 2009.
- Barnes JS, Bennett CE. The Asian population: 2000. Census 2000 brief. United States Census 2000 Web site. http://www.census.gov/prod/2002pubs/c2kbr01-16.pdf. Published February 2002. Accessed January 12, 2009.
- Lok AS, McMahon BJ; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Chronic hepatitis B. Hepatology 2001; 34:1225–1241.
- Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000—summary of a workshop. Gastroenterology 2001; 120:1828–1853.
- Iloeje UH, Yang H-I, Su J, Jen C-L, You S-L, Chen C-J, and The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-in HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006; 130:678–686.
- Chu CJ, Keeffe EB, Han SH, et al. Hepatitis B virus genotypes in the United States: results of a nationwide study. Gastroenterology 2003; 125:444–451.
KEY POINTS
- The prevalence of chronic HBV infection in the United States is expected to increase as Asian immigrants constitute a larger proportion of the US population.
- The chance of chronic infection is 90% or greater with perinatal transmission; conversely, the risk of chronic disease is less than 10% with adult-acquired infection.
- In addition to viral load, predictors of disease progression include age at onset, male sex, and comorbidities.
Risk of hepatocellular carcinoma in hepatitis B and prevention through treatment
The role of hepatitis B virus (HBV) as a risk factor for the development of hepatocellular carcinoma (HCC) is well established. Not every patient with HBV infection develops HCC; yet, the current guidelines issued by the American Association for the Study of Liver Diseases1 recommend screening all patients who have HBV infection when they reach certain ages associated with increased risk. Improved identification of risk factors specifically associated with the likelihood of developing HCC may spare some patients the burden of unnecessary testing. This article reviews up-to-date information that will help identify patients who are at risk of HCC based on factors with more specificity than age, and considers whether treatment can alter their risk.
ASSESSING RISK
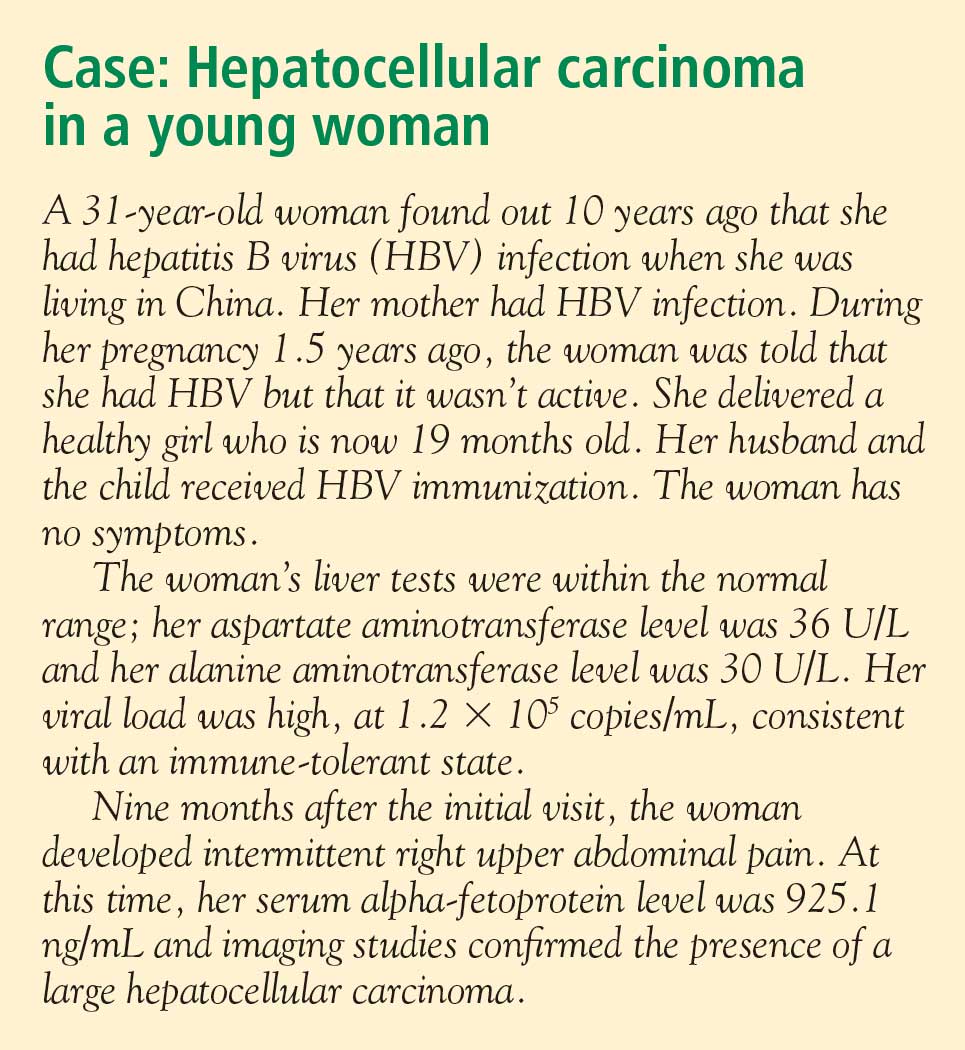
Several factors are associated with increased risk of developing HCC (see “Case: Hepatocellular carcinoma in a young woman”):
- An elevated serum alanine aminotransferase (ALT) level signifies the presence of active disease and increases risk, particularly if the ALT is persistently or intermittently elevated over years.
- Persistently elevated alpha-fetoprotein level is a reflection of enhanced regenerative state in the liver; the increased rate of cell division increases the risk of mutation, leading to increased risk of HCC.
- A low platelet count suggests the presence of cirrhosis, which itself increases the risk of HCC.
- Histologic risk factors revealed at biopsy include dysplasia, geographic morphologic changes that suggest clonal populations of cells, and a positive stain for proliferating cell nuclear antigen.
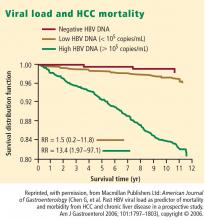
- Viral load (HBV DNA) is a significant predictor of HCC; two recent large, prospective studies—the Haimen City study2,3 and the REVEAL-HBV (Risk Evaluation of Viral Load Evaluation and Associated Liver Disease/Cancer-Hepatitis B Virus) study4—support the importance of this risk factor.
Haimen City study
The REVEAL-HBV study
The REVEAL-HBV study was a multicenter observational cohort study of 23,820 Taiwanese individuals aged 30 to 65 years old who were free of HCC at baseline.4 Of these, 3,653 were seropositive for HBsAg and seronegative for antibodies to hepatitis C virus.
Some 1,619 men and women had serum HBV DNA levels greater than or equal to 104 copies/mL at study entry.4 A direct correlation was observed between baseline HBV DNA levels and the incidence of HCC.During a mean follow-up period of 11.4 years, there were 164 new cases of HCC. In a model that integrated baseline and follow-up HBV DNA levels, the cumulative incidence of HCC ranged from 1.3% of those with undetectable levels of HBV DNA to 14.9% of those with HBV DNA levels of 106 copies/mL or greater. The same association between viral load and incidence of HCC was evident in patients who upon study entry had normal ALT levels and were hepatitis B e antigen (HBeAg) negative, a group previously considered to be inactive carriers of HBV.
The incidence of HCC was higher in the subjects with persistent viremia than in those whose viral load decreased over time, representing a biologic gradient of risk. Compared with the reference group (baseline HBV DNA < 104 copies/mL), the adjusted relative risk was nine times greater in those who maintained HBV DNA levels of 105 copies/mL or greater.
Genotype further defines risk
In addition to viral load, genotype may further define the risk of HCC in HBV carriers aged 30 years or older. In a nested case-control study, genotype C was associated with fivefold increased risk of HCC compared with other genotypes.5 Consistent with other studies, the risk of HCC increased with increasing viral load.
Caveats to the viral load–HCC link
The association between viral load and the development of HCC applies to patients aged 30 years or older, the subjects of the aforementioned studies. Younger patients who present with a high viral load and are HBeAg positive are likely to be in an immune-tolerant phase of HBV infection. Among patients aged 30 years or older, the association between viral load and HCC applies to HBeAg-positive as well as HBeAg-negative status. The longer the HBeAg-positive state is maintained, the greater the risk of developing cirrhosis and HCC, which is a reflection of active disease over a prolonged period. The association applies equally to patients with normal or elevated ALT levels. A risk nomogram is being developed that will help identify patients at highest risk of HCC.6
ALT AS PROGNOSTIC DETERMINANT
The risk of developing liver complications from chronic HBV infection increases with increasing concentrations of ALT. Yuen et al7 followed 3,233 Chinese patients with chronic HBV infection for approximately 4 years. The risk of developing complications from liver disease increased as ALT concentration increased from less than 0.5 times the upper limit of normal (ULN) to two to six times the ULN; ALT levels one to two times the ULN were associated with the highest risk of development of complications.
Interestingly, an ALT level greater than six times the ULN was associated with a significantly lower risk of liver complications. The speculation is that this phenomenon represents inactivation of disease following HBeAg seroconversion.
VIRAL LOAD SUPPRESSION LIMITS DISEASE PROGRESSION
Disease activity may flare during the natural course of chronic HBV infection, and repeated episodes may lead to progressive fibrosis, cirrhosis, and end-stage liver disease, as well as HCC. Patients whose cirrhosis has progressed to end-stage liver disease are candidates for transplant.
Continuous antiviral therapy with lamivudine has been shown to dramatically reduce the risk of complications and disease progression in patients with chronic HBV infection. In a placebo-controlled trial of 651 patients with chronic HBV infection and advanced fibrosis or cirrhosis, those randomized to lamivudine who remained sensitive to the drug had a 7.8% risk of complications over approximately 3 years, compared with a 17.7% risk in the patients randomized to placebo.8 The difference was significant and sizeable enough that the study was terminated after a mean duration of 32.4 months. Patients who developed resistance to lamivudine, caused by a mutation in HBV (YMDD mutation, a sign of lamivudine resistance), lost the protection provided by viral suppression.
The risk of disease progression to cirrhosis or HCC was also significantly lower among HBeAg-positive patients without cirrhosis who were treated with lamivudine for a median of 89.9 months compared with placebo, Yuen et al found.9 As in other studies, patients in whom the YMDD mutation developed lost the protection of viral suppression.
In a retrospective study, Di Marco et al10 also found that a loss of response to lamivudine was associated with higher risk of development of HCC, whereas patients who maintained a response to lamivudine were much less likely to develop progressive disease. The authors found that cirrhosis and loss of antiviral response were independently related to mortality and development of HCC.
SUMMARY
Patients with HBV are at risk for cancer, and the risk factors can be identified. Although not yet fully evaluated, awareness of these factors will make the screening process more efficient and less burdensome than current guidelines recommend. The publication and eventual validation of a risk nomogram will facilitate the determination of risk. An especially strong predictor of adverse outcomes, including HCC, is HBV DNA concentration higher than 104 copies/mL, as shown by two recent large studies; further, investigators observed a correlation between HBV DNA level and incidence of HCC.
Antiviral therapy has dramatically reduced the risk of complications and progression of HBV infection. Those who develop resistance to therapy lose the protection provided by viral suppression.
DISCUSSION
William D. Carey, MD: Does biopsy of nontumorous portions of the liver have value, either by showing dysplasia or perhaps through a staining technique, in predicting the development of liver cancer?
Morris Sherman, MD, PhD: I believe that you’re referring to a recent study in which microarray technology was used to identify patients at risk for the development of a de novo tumor after a resection of the first tumor.11 Liver tissue surrounding the tumor was analyzed by microarray technology, and gene expression profiling accurately predicted the development of a new tumor in another part of the liver more than 2 years later. This discovery suggests the presence of a field defect, or a propensity for the development of new tumors in a damaged organ. Patients who have a field defect identified by the microarray technique are at much higher risk of developing a subsequent cancer. These patients might be candidates for liver transplant despite apparent surgical cure of their HCC. However, because the subsequent liver malignancy occurs some time later and is a new primary tumor, the need for transplant is less urgent than it is for a patient with a progressive hepatoma, for example.
Pierre M. Gholam, MD: Do you consider ethnicity in addition to age, viral load, and other factors in your decision to screen patients for HCC?
Dr. Sherman: We traditionally think of ethnicity as a major factor because HBV is concentrated in Asian and African populations. I’m not entirely sure whether ethnicity or the viral genotypes are more important, because the viral genotypes are distributed along ethnic lines. We know that genotypes B and C, which are common in the Far East, are associated with a high rate of progressive liver disease. Genotype D, observed mainly in Middle Eastern and Greek populations, is associated with a much higher rate of progressive liver disease than genotypes common in Western Europe and most of North America. I believe that genotype should be a factor in decisions to screen.
Robert G. Gish, MD: In your case presentation you described the aspartate aminotransferase (AST) and ALT as being normal. New criteria define an AST/ALT of 20 as being “healthy” for a woman. I like the word “healthy” better than “normal.” How would you have described those test results to the patient?
Dr. Sherman: I would have told her that although her AST and ALT levels were within the laboratory reference range, ideally for a young woman the ALT should be closer to 20 U/L. Her actual levels were at least twice the upper range of ideal, and therefore, I believe a biopsy to determine the extent of injury in the liver would be important.
Tram T. Tran, MD: Are there any new serum markers for liver cancer that have promise?
Dr. Sherman: The problem with serum markers, or biomarkers in general, is the confusion over their intended use, such as for screening, risk stratification, or diagnosis.
I assume that your question refers to their potential use in screening, and so far none of the existing biomarkers is adequate to find small tumors. For screening purposes, you ideally want to find tumors that are 2 cm or smaller, and none of the biomarkers is efficient with those small tumors. A biomarker is not needed to identify tumors that are 5 or 6 cm.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. AASLD Practice Guideline. Hepatology 2005; 42:1208–1235.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 2006; 101:1797–1803.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Chronic hepatitis B virus infection and mortality from nonliver causes: results from the Haimen City cohort study [published online ahead of print January 19, 2005]. Int J Epidemiol 2005; 34:132–137.
- Chen C-J, Yang H-I, Su J, et al; for the REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73.
- Yu M-W, Yeh S-H, Chen P-J, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005; 97:265–272.
- Chen C-J, Yang H-I, Iloeje UH, et al. A risk function nomogram for predicting HCC in patients with chronic hepatitis B: the REVEAL-HBV study [AASLD abstract S1766]. Gastroenterology 2007; 132(suppl 2):A761.
- Yuen M-F, Yuan H-J, Wong DK-H, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut 2005; 54:1610–1614.
- Liaw Y-F, Sung JJY, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351:1521–1531.
- Yuen MF, Seto WK, Chow DHF, et al. Long-term beneficial outcome of Chinese asymptomatic patients with HBeAg-positive chronic hepatitis B on continuous lamivudine therapy: 7-year experience [AASLD abstract 985]. Hepatology 2005; 42(suppl 1):583A.
- Di Marco V, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004; 40:883–891.
- Hoshida Y, Villaneuva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008; 359:1995–2004.
The role of hepatitis B virus (HBV) as a risk factor for the development of hepatocellular carcinoma (HCC) is well established. Not every patient with HBV infection develops HCC; yet, the current guidelines issued by the American Association for the Study of Liver Diseases1 recommend screening all patients who have HBV infection when they reach certain ages associated with increased risk. Improved identification of risk factors specifically associated with the likelihood of developing HCC may spare some patients the burden of unnecessary testing. This article reviews up-to-date information that will help identify patients who are at risk of HCC based on factors with more specificity than age, and considers whether treatment can alter their risk.
ASSESSING RISK
Several factors are associated with increased risk of developing HCC (see “Case: Hepatocellular carcinoma in a young woman”):
- An elevated serum alanine aminotransferase (ALT) level signifies the presence of active disease and increases risk, particularly if the ALT is persistently or intermittently elevated over years.
- Persistently elevated alpha-fetoprotein level is a reflection of enhanced regenerative state in the liver; the increased rate of cell division increases the risk of mutation, leading to increased risk of HCC.
- A low platelet count suggests the presence of cirrhosis, which itself increases the risk of HCC.
- Histologic risk factors revealed at biopsy include dysplasia, geographic morphologic changes that suggest clonal populations of cells, and a positive stain for proliferating cell nuclear antigen.
- Viral load (HBV DNA) is a significant predictor of HCC; two recent large, prospective studies—the Haimen City study2,3 and the REVEAL-HBV (Risk Evaluation of Viral Load Evaluation and Associated Liver Disease/Cancer-Hepatitis B Virus) study4—support the importance of this risk factor.

Haimen City study
The REVEAL-HBV study
The REVEAL-HBV study was a multicenter observational cohort study of 23,820 Taiwanese individuals aged 30 to 65 years old who were free of HCC at baseline.4 Of these, 3,653 were seropositive for HBsAg and seronegative for antibodies to hepatitis C virus.
Some 1,619 men and women had serum HBV DNA levels greater than or equal to 104 copies/mL at study entry.4 A direct correlation was observed between baseline HBV DNA levels and the incidence of HCC.During a mean follow-up period of 11.4 years, there were 164 new cases of HCC. In a model that integrated baseline and follow-up HBV DNA levels, the cumulative incidence of HCC ranged from 1.3% of those with undetectable levels of HBV DNA to 14.9% of those with HBV DNA levels of 106 copies/mL or greater. The same association between viral load and incidence of HCC was evident in patients who upon study entry had normal ALT levels and were hepatitis B e antigen (HBeAg) negative, a group previously considered to be inactive carriers of HBV.
The incidence of HCC was higher in the subjects with persistent viremia than in those whose viral load decreased over time, representing a biologic gradient of risk. Compared with the reference group (baseline HBV DNA < 104 copies/mL), the adjusted relative risk was nine times greater in those who maintained HBV DNA levels of 105 copies/mL or greater.
Genotype further defines risk
In addition to viral load, genotype may further define the risk of HCC in HBV carriers aged 30 years or older. In a nested case-control study, genotype C was associated with fivefold increased risk of HCC compared with other genotypes.5 Consistent with other studies, the risk of HCC increased with increasing viral load.
Caveats to the viral load–HCC link
The association between viral load and the development of HCC applies to patients aged 30 years or older, the subjects of the aforementioned studies. Younger patients who present with a high viral load and are HBeAg positive are likely to be in an immune-tolerant phase of HBV infection. Among patients aged 30 years or older, the association between viral load and HCC applies to HBeAg-positive as well as HBeAg-negative status. The longer the HBeAg-positive state is maintained, the greater the risk of developing cirrhosis and HCC, which is a reflection of active disease over a prolonged period. The association applies equally to patients with normal or elevated ALT levels. A risk nomogram is being developed that will help identify patients at highest risk of HCC.6
ALT AS PROGNOSTIC DETERMINANT
The risk of developing liver complications from chronic HBV infection increases with increasing concentrations of ALT. Yuen et al7 followed 3,233 Chinese patients with chronic HBV infection for approximately 4 years. The risk of developing complications from liver disease increased as ALT concentration increased from less than 0.5 times the upper limit of normal (ULN) to two to six times the ULN; ALT levels one to two times the ULN were associated with the highest risk of development of complications.
Interestingly, an ALT level greater than six times the ULN was associated with a significantly lower risk of liver complications. The speculation is that this phenomenon represents inactivation of disease following HBeAg seroconversion.
VIRAL LOAD SUPPRESSION LIMITS DISEASE PROGRESSION
Disease activity may flare during the natural course of chronic HBV infection, and repeated episodes may lead to progressive fibrosis, cirrhosis, and end-stage liver disease, as well as HCC. Patients whose cirrhosis has progressed to end-stage liver disease are candidates for transplant.
Continuous antiviral therapy with lamivudine has been shown to dramatically reduce the risk of complications and disease progression in patients with chronic HBV infection. In a placebo-controlled trial of 651 patients with chronic HBV infection and advanced fibrosis or cirrhosis, those randomized to lamivudine who remained sensitive to the drug had a 7.8% risk of complications over approximately 3 years, compared with a 17.7% risk in the patients randomized to placebo.8 The difference was significant and sizeable enough that the study was terminated after a mean duration of 32.4 months. Patients who developed resistance to lamivudine, caused by a mutation in HBV (YMDD mutation, a sign of lamivudine resistance), lost the protection provided by viral suppression.
The risk of disease progression to cirrhosis or HCC was also significantly lower among HBeAg-positive patients without cirrhosis who were treated with lamivudine for a median of 89.9 months compared with placebo, Yuen et al found.9 As in other studies, patients in whom the YMDD mutation developed lost the protection of viral suppression.
In a retrospective study, Di Marco et al10 also found that a loss of response to lamivudine was associated with higher risk of development of HCC, whereas patients who maintained a response to lamivudine were much less likely to develop progressive disease. The authors found that cirrhosis and loss of antiviral response were independently related to mortality and development of HCC.
SUMMARY
Patients with HBV are at risk for cancer, and the risk factors can be identified. Although not yet fully evaluated, awareness of these factors will make the screening process more efficient and less burdensome than current guidelines recommend. The publication and eventual validation of a risk nomogram will facilitate the determination of risk. An especially strong predictor of adverse outcomes, including HCC, is HBV DNA concentration higher than 104 copies/mL, as shown by two recent large studies; further, investigators observed a correlation between HBV DNA level and incidence of HCC.
Antiviral therapy has dramatically reduced the risk of complications and progression of HBV infection. Those who develop resistance to therapy lose the protection provided by viral suppression.
DISCUSSION
William D. Carey, MD: Does biopsy of nontumorous portions of the liver have value, either by showing dysplasia or perhaps through a staining technique, in predicting the development of liver cancer?
Morris Sherman, MD, PhD: I believe that you’re referring to a recent study in which microarray technology was used to identify patients at risk for the development of a de novo tumor after a resection of the first tumor.11 Liver tissue surrounding the tumor was analyzed by microarray technology, and gene expression profiling accurately predicted the development of a new tumor in another part of the liver more than 2 years later. This discovery suggests the presence of a field defect, or a propensity for the development of new tumors in a damaged organ. Patients who have a field defect identified by the microarray technique are at much higher risk of developing a subsequent cancer. These patients might be candidates for liver transplant despite apparent surgical cure of their HCC. However, because the subsequent liver malignancy occurs some time later and is a new primary tumor, the need for transplant is less urgent than it is for a patient with a progressive hepatoma, for example.
Pierre M. Gholam, MD: Do you consider ethnicity in addition to age, viral load, and other factors in your decision to screen patients for HCC?
Dr. Sherman: We traditionally think of ethnicity as a major factor because HBV is concentrated in Asian and African populations. I’m not entirely sure whether ethnicity or the viral genotypes are more important, because the viral genotypes are distributed along ethnic lines. We know that genotypes B and C, which are common in the Far East, are associated with a high rate of progressive liver disease. Genotype D, observed mainly in Middle Eastern and Greek populations, is associated with a much higher rate of progressive liver disease than genotypes common in Western Europe and most of North America. I believe that genotype should be a factor in decisions to screen.
Robert G. Gish, MD: In your case presentation you described the aspartate aminotransferase (AST) and ALT as being normal. New criteria define an AST/ALT of 20 as being “healthy” for a woman. I like the word “healthy” better than “normal.” How would you have described those test results to the patient?
Dr. Sherman: I would have told her that although her AST and ALT levels were within the laboratory reference range, ideally for a young woman the ALT should be closer to 20 U/L. Her actual levels were at least twice the upper range of ideal, and therefore, I believe a biopsy to determine the extent of injury in the liver would be important.
Tram T. Tran, MD: Are there any new serum markers for liver cancer that have promise?
Dr. Sherman: The problem with serum markers, or biomarkers in general, is the confusion over their intended use, such as for screening, risk stratification, or diagnosis.
I assume that your question refers to their potential use in screening, and so far none of the existing biomarkers is adequate to find small tumors. For screening purposes, you ideally want to find tumors that are 2 cm or smaller, and none of the biomarkers is efficient with those small tumors. A biomarker is not needed to identify tumors that are 5 or 6 cm.
The role of hepatitis B virus (HBV) as a risk factor for the development of hepatocellular carcinoma (HCC) is well established. Not every patient with HBV infection develops HCC; yet, the current guidelines issued by the American Association for the Study of Liver Diseases1 recommend screening all patients who have HBV infection when they reach certain ages associated with increased risk. Improved identification of risk factors specifically associated with the likelihood of developing HCC may spare some patients the burden of unnecessary testing. This article reviews up-to-date information that will help identify patients who are at risk of HCC based on factors with more specificity than age, and considers whether treatment can alter their risk.
ASSESSING RISK
Several factors are associated with increased risk of developing HCC (see “Case: Hepatocellular carcinoma in a young woman”):
- An elevated serum alanine aminotransferase (ALT) level signifies the presence of active disease and increases risk, particularly if the ALT is persistently or intermittently elevated over years.
- Persistently elevated alpha-fetoprotein level is a reflection of enhanced regenerative state in the liver; the increased rate of cell division increases the risk of mutation, leading to increased risk of HCC.
- A low platelet count suggests the presence of cirrhosis, which itself increases the risk of HCC.
- Histologic risk factors revealed at biopsy include dysplasia, geographic morphologic changes that suggest clonal populations of cells, and a positive stain for proliferating cell nuclear antigen.
- Viral load (HBV DNA) is a significant predictor of HCC; two recent large, prospective studies—the Haimen City study2,3 and the REVEAL-HBV (Risk Evaluation of Viral Load Evaluation and Associated Liver Disease/Cancer-Hepatitis B Virus) study4—support the importance of this risk factor.

Haimen City study
The REVEAL-HBV study
The REVEAL-HBV study was a multicenter observational cohort study of 23,820 Taiwanese individuals aged 30 to 65 years old who were free of HCC at baseline.4 Of these, 3,653 were seropositive for HBsAg and seronegative for antibodies to hepatitis C virus.
Some 1,619 men and women had serum HBV DNA levels greater than or equal to 104 copies/mL at study entry.4 A direct correlation was observed between baseline HBV DNA levels and the incidence of HCC.During a mean follow-up period of 11.4 years, there were 164 new cases of HCC. In a model that integrated baseline and follow-up HBV DNA levels, the cumulative incidence of HCC ranged from 1.3% of those with undetectable levels of HBV DNA to 14.9% of those with HBV DNA levels of 106 copies/mL or greater. The same association between viral load and incidence of HCC was evident in patients who upon study entry had normal ALT levels and were hepatitis B e antigen (HBeAg) negative, a group previously considered to be inactive carriers of HBV.
The incidence of HCC was higher in the subjects with persistent viremia than in those whose viral load decreased over time, representing a biologic gradient of risk. Compared with the reference group (baseline HBV DNA < 104 copies/mL), the adjusted relative risk was nine times greater in those who maintained HBV DNA levels of 105 copies/mL or greater.
Genotype further defines risk
In addition to viral load, genotype may further define the risk of HCC in HBV carriers aged 30 years or older. In a nested case-control study, genotype C was associated with fivefold increased risk of HCC compared with other genotypes.5 Consistent with other studies, the risk of HCC increased with increasing viral load.
Caveats to the viral load–HCC link
The association between viral load and the development of HCC applies to patients aged 30 years or older, the subjects of the aforementioned studies. Younger patients who present with a high viral load and are HBeAg positive are likely to be in an immune-tolerant phase of HBV infection. Among patients aged 30 years or older, the association between viral load and HCC applies to HBeAg-positive as well as HBeAg-negative status. The longer the HBeAg-positive state is maintained, the greater the risk of developing cirrhosis and HCC, which is a reflection of active disease over a prolonged period. The association applies equally to patients with normal or elevated ALT levels. A risk nomogram is being developed that will help identify patients at highest risk of HCC.6
ALT AS PROGNOSTIC DETERMINANT
The risk of developing liver complications from chronic HBV infection increases with increasing concentrations of ALT. Yuen et al7 followed 3,233 Chinese patients with chronic HBV infection for approximately 4 years. The risk of developing complications from liver disease increased as ALT concentration increased from less than 0.5 times the upper limit of normal (ULN) to two to six times the ULN; ALT levels one to two times the ULN were associated with the highest risk of development of complications.
Interestingly, an ALT level greater than six times the ULN was associated with a significantly lower risk of liver complications. The speculation is that this phenomenon represents inactivation of disease following HBeAg seroconversion.
VIRAL LOAD SUPPRESSION LIMITS DISEASE PROGRESSION
Disease activity may flare during the natural course of chronic HBV infection, and repeated episodes may lead to progressive fibrosis, cirrhosis, and end-stage liver disease, as well as HCC. Patients whose cirrhosis has progressed to end-stage liver disease are candidates for transplant.
Continuous antiviral therapy with lamivudine has been shown to dramatically reduce the risk of complications and disease progression in patients with chronic HBV infection. In a placebo-controlled trial of 651 patients with chronic HBV infection and advanced fibrosis or cirrhosis, those randomized to lamivudine who remained sensitive to the drug had a 7.8% risk of complications over approximately 3 years, compared with a 17.7% risk in the patients randomized to placebo.8 The difference was significant and sizeable enough that the study was terminated after a mean duration of 32.4 months. Patients who developed resistance to lamivudine, caused by a mutation in HBV (YMDD mutation, a sign of lamivudine resistance), lost the protection provided by viral suppression.
The risk of disease progression to cirrhosis or HCC was also significantly lower among HBeAg-positive patients without cirrhosis who were treated with lamivudine for a median of 89.9 months compared with placebo, Yuen et al found.9 As in other studies, patients in whom the YMDD mutation developed lost the protection of viral suppression.
In a retrospective study, Di Marco et al10 also found that a loss of response to lamivudine was associated with higher risk of development of HCC, whereas patients who maintained a response to lamivudine were much less likely to develop progressive disease. The authors found that cirrhosis and loss of antiviral response were independently related to mortality and development of HCC.
SUMMARY
Patients with HBV are at risk for cancer, and the risk factors can be identified. Although not yet fully evaluated, awareness of these factors will make the screening process more efficient and less burdensome than current guidelines recommend. The publication and eventual validation of a risk nomogram will facilitate the determination of risk. An especially strong predictor of adverse outcomes, including HCC, is HBV DNA concentration higher than 104 copies/mL, as shown by two recent large studies; further, investigators observed a correlation between HBV DNA level and incidence of HCC.
Antiviral therapy has dramatically reduced the risk of complications and progression of HBV infection. Those who develop resistance to therapy lose the protection provided by viral suppression.
DISCUSSION
William D. Carey, MD: Does biopsy of nontumorous portions of the liver have value, either by showing dysplasia or perhaps through a staining technique, in predicting the development of liver cancer?
Morris Sherman, MD, PhD: I believe that you’re referring to a recent study in which microarray technology was used to identify patients at risk for the development of a de novo tumor after a resection of the first tumor.11 Liver tissue surrounding the tumor was analyzed by microarray technology, and gene expression profiling accurately predicted the development of a new tumor in another part of the liver more than 2 years later. This discovery suggests the presence of a field defect, or a propensity for the development of new tumors in a damaged organ. Patients who have a field defect identified by the microarray technique are at much higher risk of developing a subsequent cancer. These patients might be candidates for liver transplant despite apparent surgical cure of their HCC. However, because the subsequent liver malignancy occurs some time later and is a new primary tumor, the need for transplant is less urgent than it is for a patient with a progressive hepatoma, for example.
Pierre M. Gholam, MD: Do you consider ethnicity in addition to age, viral load, and other factors in your decision to screen patients for HCC?
Dr. Sherman: We traditionally think of ethnicity as a major factor because HBV is concentrated in Asian and African populations. I’m not entirely sure whether ethnicity or the viral genotypes are more important, because the viral genotypes are distributed along ethnic lines. We know that genotypes B and C, which are common in the Far East, are associated with a high rate of progressive liver disease. Genotype D, observed mainly in Middle Eastern and Greek populations, is associated with a much higher rate of progressive liver disease than genotypes common in Western Europe and most of North America. I believe that genotype should be a factor in decisions to screen.
Robert G. Gish, MD: In your case presentation you described the aspartate aminotransferase (AST) and ALT as being normal. New criteria define an AST/ALT of 20 as being “healthy” for a woman. I like the word “healthy” better than “normal.” How would you have described those test results to the patient?
Dr. Sherman: I would have told her that although her AST and ALT levels were within the laboratory reference range, ideally for a young woman the ALT should be closer to 20 U/L. Her actual levels were at least twice the upper range of ideal, and therefore, I believe a biopsy to determine the extent of injury in the liver would be important.
Tram T. Tran, MD: Are there any new serum markers for liver cancer that have promise?
Dr. Sherman: The problem with serum markers, or biomarkers in general, is the confusion over their intended use, such as for screening, risk stratification, or diagnosis.
I assume that your question refers to their potential use in screening, and so far none of the existing biomarkers is adequate to find small tumors. For screening purposes, you ideally want to find tumors that are 2 cm or smaller, and none of the biomarkers is efficient with those small tumors. A biomarker is not needed to identify tumors that are 5 or 6 cm.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. AASLD Practice Guideline. Hepatology 2005; 42:1208–1235.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 2006; 101:1797–1803.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Chronic hepatitis B virus infection and mortality from nonliver causes: results from the Haimen City cohort study [published online ahead of print January 19, 2005]. Int J Epidemiol 2005; 34:132–137.
- Chen C-J, Yang H-I, Su J, et al; for the REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73.
- Yu M-W, Yeh S-H, Chen P-J, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005; 97:265–272.
- Chen C-J, Yang H-I, Iloeje UH, et al. A risk function nomogram for predicting HCC in patients with chronic hepatitis B: the REVEAL-HBV study [AASLD abstract S1766]. Gastroenterology 2007; 132(suppl 2):A761.
- Yuen M-F, Yuan H-J, Wong DK-H, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut 2005; 54:1610–1614.
- Liaw Y-F, Sung JJY, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351:1521–1531.
- Yuen MF, Seto WK, Chow DHF, et al. Long-term beneficial outcome of Chinese asymptomatic patients with HBeAg-positive chronic hepatitis B on continuous lamivudine therapy: 7-year experience [AASLD abstract 985]. Hepatology 2005; 42(suppl 1):583A.
- Di Marco V, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004; 40:883–891.
- Hoshida Y, Villaneuva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008; 359:1995–2004.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. AASLD Practice Guideline. Hepatology 2005; 42:1208–1235.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 2006; 101:1797–1803.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, Evans AA. Chronic hepatitis B virus infection and mortality from nonliver causes: results from the Haimen City cohort study [published online ahead of print January 19, 2005]. Int J Epidemiol 2005; 34:132–137.
- Chen C-J, Yang H-I, Su J, et al; for the REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73.
- Yu M-W, Yeh S-H, Chen P-J, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005; 97:265–272.
- Chen C-J, Yang H-I, Iloeje UH, et al. A risk function nomogram for predicting HCC in patients with chronic hepatitis B: the REVEAL-HBV study [AASLD abstract S1766]. Gastroenterology 2007; 132(suppl 2):A761.
- Yuen M-F, Yuan H-J, Wong DK-H, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut 2005; 54:1610–1614.
- Liaw Y-F, Sung JJY, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351:1521–1531.
- Yuen MF, Seto WK, Chow DHF, et al. Long-term beneficial outcome of Chinese asymptomatic patients with HBeAg-positive chronic hepatitis B on continuous lamivudine therapy: 7-year experience [AASLD abstract 985]. Hepatology 2005; 42(suppl 1):583A.
- Di Marco V, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004; 40:883–891.
- Hoshida Y, Villaneuva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008; 359:1995–2004.
KEY POINTS
- A high viral load is a significant predictor of the development of hepatocellular carcinoma in patients aged 30 years or older with chronic HBV infection.
- The risk of developing liver complications from chronic HBV infection increases with increasing concentrations of alanine aminotransferase.
- Continuous antiviral therapy to suppress viral load dramatically reduces the risk of complications from HBV infection and reduces the rate of disease progression, as long as patients maintain a therapeutic response.
Understanding cultural barriers in hepatitis B virus infection
Asian Americans represent 4% of the population in the United States, and their share of the US population is projected to grow to 9% by 2050.1 These numbers are significant because of the high prevalence of hepatitis B virus (HBV) infection in this community and the cultural barriers to its effective management.
Appreciating the impact of cultural barriers on health care among Asian Americans requires an understanding of the diversity of the Asian continent, which is composed of 52 countries where 100 languages and dialects are spoken. Within each region are religious, cultural, and societal differences. Asians have immigrated to the United States over the course of several generations, and the era in which they immigrated may affect their ability to understand English, integrate into American culture, and navigate the US health care system. Successful integration into American life favors those whose families immigrated several generations earlier.
The overall prevalence of HBV infection in the United States is 0.4%2; however, estimates of prevalence range from 5% to 15% in Asian American populations, and are as high as 20% in some Pacific Rim populations.3,4 The prevalence of HBV infection in Asian Americans differs by subpopulation, with the highest prevalence among immigrants from Vietnam, Laos, and China, and the lowest among those from Japan.
Of the approximately 1 million Americans estimated to be infected with HBV as of 2005, more than 750,000 had access to health care; of these, 205,000 were diagnosed with HBV infection,5 suggesting substantial underdiagnosis. Referrals to specialists were even fewer (175,000), and only about 31,000 patients chronically infected with HBV received antiviral treatment, a figure that has likely increased with greater awareness of HBV and the availability of new antiviral medications.
BARRIERS TO DIAGNOSIS AND TREATMENT
The barriers to effective management of HBV infection in Asian Americans include cultural, socioeconomic, and accessibility issues (see “Case: Stigma and cultural barriers lead to inadequate care”).

Language and linguistic isolation
Limited proficiency in English is a large, if not the largest, barrier to effective management of chronic HBV infection. According to the US Census Bureau, a person with limited English proficiency is one who does not speak English “very well.”6 This terminology has implications for allocation of federal government resources; ie, the percentage of a community’s residents with limited English proficiency is a criterion for receipt of governmental grants and other forms of assistance, including translation services.6
Linguistic isolation, another barrier to medical care, is lack of an English-speaking household member who is older than 14 years.7 By this definition, more than one-third of Korean, Taiwanese, Chinese, Hmong, and Bangladeshi households, and almost half of Vietnamese households, are linguistically isolated, with limited ability to communicate with health care providers.8
Lack of health insurance and its correlates
The high percentage of Asian immigrants without health insurance is a challenge to providing adequate health care. Health insurance coverage is lacking for about one-third of Korean immigrants, about one in five immigrants from Southeast Asia and South Asia, and about 15% of Filipino and Chinese immigrants.9
One reason for the large proportion of uninsured among these groups is the high rate of small business ownership among Asian Americans and the difficulty that small business owners have in obtaining affordable health insurance coverage. In addition, although Asian Americans are as likely as other US residents to be employed full time, their employment options may be less likely to include health insurance benefits.
Poverty affects the ability to acquire health insurance. Although the popular image of the Asian immigrant is an educated person with high earning potential, the reality is that poverty strikes immigrants from Southeast Asia at a high rate. Almost 40% of the Hmong population, for example, lives below the poverty level, and poverty rates among the Cambodian, Bangladeshi, Malaysian, and several other Asian subpopulations are nearly as high.8
Citizenship correlates with the ability to obtain health insurance; it is estimated that 42% to 57% of noncitizens lack health insurance, compared with 15% of citizens.8 Only half of Asian immigrants become naturalized citizens, with wide variability among subgroups. Two-thirds of Filipinos who immigrate to the United States eventually become naturalized compared with less than one-third of Malaysian, Japanese, Indonesian, and Hmong immigrants.8
Educational achievement is associated with attainment of financial security and health insurance. The vast majority of Taiwanese, Japanese, Filipino, and Korean Americans obtain a high school education or higher, with correspondingly higher rates of health insurance coverage. Among those from Southeast Asia (Hmong, Cambodians, Laotians, and Vietnamese), whose immigration to this country is relatively recent, fewer than half complete a high school education.8
Health care workforce representation
Certain Asian subgroups are underrepresented in the racial composition of the US health care workforce; this imbalance may affect accessibility to the health care system and adherence to medical prescriptions and instructions among underrepresented groups. Racial concordance between patient and health care provider is associated with greater patient participation in care, according to the Institute of Medicine.10 In addition to racial similarity, linguistic similarity enhances communication and adherence to instructions.
Belief systems and attitudes toward health care
An immigrant patient’s religious beliefs and cultural attitudes toward Western medicine may pose difficulties in successfully managing disease. Many Asian Americans are Buddhists, who may believe that suffering is an integral part of life; proactively seeking medical care may not be imperative for them. Confucianism, the worship of ancestors and the subjugation of the self to the well-being of the family, is a common belief system among Asians that may inhibit the desire to seek needed medical care. For example, a family elder may instruct a young man not to seek medical care for his HBV infection because this would jeopardize his siblings’ marriage prospects. Taoism involves the belief that perfection is achieved when events are allowed to take the more natural course. Intervention is therefore frowned upon.
Some belief systems may impede care because they incorporate indifference toward suffering. Many Hmong believe that the length of life is predetermined, so lifesaving care is pointless. Cultural value may be placed on stoicism, discouraging visits to health care providers. A belief that disease is caused by supernatural events rather than organic etiologies is another perception that serves as a barrier to seeking medical care.
Distrust of, or unfamiliarity with, Western medicine may delay care, and the resulting poor outcomes may be falsely attributed to Western medicine itself. In some cultures, there is a pervasive belief that a physician can touch the pulse and identify the problem. Some Laotians believe that immunizations are dangerous for a baby’s spirit, and therefore forgo immunization against HBV when it is indicated.
The patient’s relationship with his or her health care provider is an important determinant of quality of care and willingness to continue to receive care. The best possible scenario is concordance in language and culture. Asian cultures emphasize politeness, respect for authority, filial piety, and avoidance of shame. Because Asian patients often view physicians as authority figures, they may not ask questions or voice reservations or fears about their treatment regimens; instead, they may express their agreement with physicians’ advice, but with no intent to return or follow instructions.
Infection with HBV carries a stigma about the mode of transmission that can interfere with patients’ daily lives. A study of attitudes about HBV found that HBV-infected patients feel less welcome to stay overnight or share the same bathroom at friends’ or relatives’ houses, that noninfected persons fear that the disease may be passed to them by HBV-positive friends, and that HBV-infected patients are concerned about whether their choices may have led to the infection.11
OVERCOMING BARRIERS
Sensitivity to cultural attitudes may enhance communication and the likelihood that patients will accept physicians’ recommendations. Several office visits may be necessary to confirm that a patient is receptive to the health care provider’s instructions and is adhering to them. Referral to access programs can aid communication. For example, most cities have community centers where patients can seek medical advice from physicians who speak the patients’ language; these centers also may provide native-language materials and interpreters.
Offering reassurance to patients in their own language and in a culturally sensitive setting will help break down barriers and improve care. Patients who are educated about HBV transmission and the availability of an effective vaccine may be instrumental in preventing transmission of the disease to household members.
Cultural sensitivity training will benefit health care providers and staffs whose patients include Asian Americans. Educational programs should be specific to the needs of the community, as different subpopulations have different needs. Resource materials are available for such training; for example, the federal government’s Office of Minority Health Web site (http://www.omhrc.gov/) offers links to resources for cultural training. In addition to educating themselves and their staffs, health care providers have a responsibility to advocate for funding and equal access to care, and for the creation of more cultural and community health centers that can serve as resources to overcome cultural barriers.
DISCUSSION
Robert G. Gish, MD: How often are herbal remedies tried for chronic HBV infection in the patients you see, especially in the Vietnamese population?
Tram T. Tran, MD: Once patients are diagnosed with chronic HBV infection, the use of herbal remedies is very high; it approaches 80% in my practice. Patients may not admit to it unless you ask them specifically, because they know herbal remedies may be somewhat frowned upon by Western physicians. If you are careful and ask very gently about their use of herbals, they will tell you that they do believe in herbal medicines pretty strongly.
Morris Sherman, MD, PhD: I’d like to emphasize the need to be able to communicate with patients in their own language. In Toronto, 50% of the population was born outside of Canada. We have a huge immigrant population; given the nature of hepatology, we have many patients from Southeast and South Asia, and from all over the world, who don’t speak English. My hospital has a multilingual interpreter service, which we use freely. Scarcely a day goes by without two or three interpreters coming to the clinic to talk to patients, and as a result it’s rare that I can’t make myself understood. Maybe what I’ve said hasn’t been accepted, but patients can at least understand what I’m saying.
William D. Carey, MD: I interview many applicants for our medical school, and many of them are Asians, including Hmong and Vietnamese. With the high value that most of these groups put on education and their success with educational attainment, is their access to care improving? Are we doing a better job of training nurses, allied health personnel, and physicians to deal with this problem?
Dr. Tran: I think so, yes. For instance, the Southeast Asian immigrant population arrived in two different eras. The Vietnamese who immigrated in 1975 have been in the United States longer and in general have been able to attain a higher level of education than those who came later. The group that arrived earlier is therefore more likely to have health insurance, and it has been easier to get them into the health care system. More recent immigrants have had more difficulty navigating the system. In general, their socioeconomic status and therefore access to care is directly related to how long they’ve been in the country.
- President’s Advisory Commission on Asian Americans and Pacific Islanders. Asian Americans and Pacific Islanders: a people looking forward. Action for access and partnerships in the 21st century. Interim report to the president and the nation. http://permanent.access.gpo.gov/lps17931/www.aapi.gov/intreport.htm. Published January 2001. Accessed December 21, 2008.
- National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Hepatitis B index. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/hepatitis/HBV/HBVfaq.htm. Updated July 8, 2008. Accessed January 21, 2009.
- Do S. The natural history of hepatitis B in Asian Americans. Asian Am Pac Isl J Health 2001; 9:141–153.
- Stanford University School of Medicine. FAQ about hepatitis B. Asian Liver Center Web site. http://liver.stanford.edu/Education/faq.html. Updated July 10, 2008. Accessed January 21, 2009.
- Di Bisceglie AM, Keeffe E, Atillasoy E, Varshneya R, Bergstein G. Management of chronic hepatitis B—an analysis of physician practices [DDW abstract M918]. Gastroenterology 2005; 128(suppl 2):A739.
- US Census Bureau. American community survey. US Census Bureau Web site. http://www.census.gov/acs/www/SBasics/SQuest/fact_pdf/P%2013%20factsheetlanguageathome2.pdf. Published January 29, 2004. Accessed January 21, 2009.
- Lestina FA. Analysis of the linguistically isolated population in Census 2000. http://www.census.gov/pred/www/rpts/A.5a.pdf. Published September 30, 2003. Accessed January 21, 2009.
- Asian & Pacific Islander American Health Forum. Diverse communities, diverse experiences: the status of Asian Americans and Pacific Islanders in the U.S. http://www.apiahf.org/resources/pdf/Diverse%20Communities%20Diverse%20Experiences.pdf. Accessed January 21, 2009.
- Asian & Pacific Islander American Health Forum. Race, ethnicity and health care fact sheet. Henry J. Kaiser Family Foundation Web site. http://www.kff.org/minorityhealth/upload/7745.pdf. Published April 2008. Accessed January 21, 2009.
- Smedley BD, Stith AY, Nelson AR, eds; Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Board on Health Sciences Policy, Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. http://www.nap.edu/openbook.php?isbn=030908265X. Published 2003. Accessed January 21, 2009.
- Speigel BMR, Bollus R, Han S, et al. Development and validation of a disease-targeted quality of life instrument in chronic hepatitis B: the hepatitis B quality of life instrument, version 1.0. Hepatology 2007; 46:113–121.
Asian Americans represent 4% of the population in the United States, and their share of the US population is projected to grow to 9% by 2050.1 These numbers are significant because of the high prevalence of hepatitis B virus (HBV) infection in this community and the cultural barriers to its effective management.
Appreciating the impact of cultural barriers on health care among Asian Americans requires an understanding of the diversity of the Asian continent, which is composed of 52 countries where 100 languages and dialects are spoken. Within each region are religious, cultural, and societal differences. Asians have immigrated to the United States over the course of several generations, and the era in which they immigrated may affect their ability to understand English, integrate into American culture, and navigate the US health care system. Successful integration into American life favors those whose families immigrated several generations earlier.
The overall prevalence of HBV infection in the United States is 0.4%2; however, estimates of prevalence range from 5% to 15% in Asian American populations, and are as high as 20% in some Pacific Rim populations.3,4 The prevalence of HBV infection in Asian Americans differs by subpopulation, with the highest prevalence among immigrants from Vietnam, Laos, and China, and the lowest among those from Japan.
Of the approximately 1 million Americans estimated to be infected with HBV as of 2005, more than 750,000 had access to health care; of these, 205,000 were diagnosed with HBV infection,5 suggesting substantial underdiagnosis. Referrals to specialists were even fewer (175,000), and only about 31,000 patients chronically infected with HBV received antiviral treatment, a figure that has likely increased with greater awareness of HBV and the availability of new antiviral medications.
BARRIERS TO DIAGNOSIS AND TREATMENT
The barriers to effective management of HBV infection in Asian Americans include cultural, socioeconomic, and accessibility issues (see “Case: Stigma and cultural barriers lead to inadequate care”).

Language and linguistic isolation
Limited proficiency in English is a large, if not the largest, barrier to effective management of chronic HBV infection. According to the US Census Bureau, a person with limited English proficiency is one who does not speak English “very well.”6 This terminology has implications for allocation of federal government resources; ie, the percentage of a community’s residents with limited English proficiency is a criterion for receipt of governmental grants and other forms of assistance, including translation services.6
Linguistic isolation, another barrier to medical care, is lack of an English-speaking household member who is older than 14 years.7 By this definition, more than one-third of Korean, Taiwanese, Chinese, Hmong, and Bangladeshi households, and almost half of Vietnamese households, are linguistically isolated, with limited ability to communicate with health care providers.8
Lack of health insurance and its correlates
The high percentage of Asian immigrants without health insurance is a challenge to providing adequate health care. Health insurance coverage is lacking for about one-third of Korean immigrants, about one in five immigrants from Southeast Asia and South Asia, and about 15% of Filipino and Chinese immigrants.9
One reason for the large proportion of uninsured among these groups is the high rate of small business ownership among Asian Americans and the difficulty that small business owners have in obtaining affordable health insurance coverage. In addition, although Asian Americans are as likely as other US residents to be employed full time, their employment options may be less likely to include health insurance benefits.
Poverty affects the ability to acquire health insurance. Although the popular image of the Asian immigrant is an educated person with high earning potential, the reality is that poverty strikes immigrants from Southeast Asia at a high rate. Almost 40% of the Hmong population, for example, lives below the poverty level, and poverty rates among the Cambodian, Bangladeshi, Malaysian, and several other Asian subpopulations are nearly as high.8
Citizenship correlates with the ability to obtain health insurance; it is estimated that 42% to 57% of noncitizens lack health insurance, compared with 15% of citizens.8 Only half of Asian immigrants become naturalized citizens, with wide variability among subgroups. Two-thirds of Filipinos who immigrate to the United States eventually become naturalized compared with less than one-third of Malaysian, Japanese, Indonesian, and Hmong immigrants.8
Educational achievement is associated with attainment of financial security and health insurance. The vast majority of Taiwanese, Japanese, Filipino, and Korean Americans obtain a high school education or higher, with correspondingly higher rates of health insurance coverage. Among those from Southeast Asia (Hmong, Cambodians, Laotians, and Vietnamese), whose immigration to this country is relatively recent, fewer than half complete a high school education.8
Health care workforce representation
Certain Asian subgroups are underrepresented in the racial composition of the US health care workforce; this imbalance may affect accessibility to the health care system and adherence to medical prescriptions and instructions among underrepresented groups. Racial concordance between patient and health care provider is associated with greater patient participation in care, according to the Institute of Medicine.10 In addition to racial similarity, linguistic similarity enhances communication and adherence to instructions.
Belief systems and attitudes toward health care
An immigrant patient’s religious beliefs and cultural attitudes toward Western medicine may pose difficulties in successfully managing disease. Many Asian Americans are Buddhists, who may believe that suffering is an integral part of life; proactively seeking medical care may not be imperative for them. Confucianism, the worship of ancestors and the subjugation of the self to the well-being of the family, is a common belief system among Asians that may inhibit the desire to seek needed medical care. For example, a family elder may instruct a young man not to seek medical care for his HBV infection because this would jeopardize his siblings’ marriage prospects. Taoism involves the belief that perfection is achieved when events are allowed to take the more natural course. Intervention is therefore frowned upon.
Some belief systems may impede care because they incorporate indifference toward suffering. Many Hmong believe that the length of life is predetermined, so lifesaving care is pointless. Cultural value may be placed on stoicism, discouraging visits to health care providers. A belief that disease is caused by supernatural events rather than organic etiologies is another perception that serves as a barrier to seeking medical care.
Distrust of, or unfamiliarity with, Western medicine may delay care, and the resulting poor outcomes may be falsely attributed to Western medicine itself. In some cultures, there is a pervasive belief that a physician can touch the pulse and identify the problem. Some Laotians believe that immunizations are dangerous for a baby’s spirit, and therefore forgo immunization against HBV when it is indicated.
The patient’s relationship with his or her health care provider is an important determinant of quality of care and willingness to continue to receive care. The best possible scenario is concordance in language and culture. Asian cultures emphasize politeness, respect for authority, filial piety, and avoidance of shame. Because Asian patients often view physicians as authority figures, they may not ask questions or voice reservations or fears about their treatment regimens; instead, they may express their agreement with physicians’ advice, but with no intent to return or follow instructions.
Infection with HBV carries a stigma about the mode of transmission that can interfere with patients’ daily lives. A study of attitudes about HBV found that HBV-infected patients feel less welcome to stay overnight or share the same bathroom at friends’ or relatives’ houses, that noninfected persons fear that the disease may be passed to them by HBV-positive friends, and that HBV-infected patients are concerned about whether their choices may have led to the infection.11
OVERCOMING BARRIERS
Sensitivity to cultural attitudes may enhance communication and the likelihood that patients will accept physicians’ recommendations. Several office visits may be necessary to confirm that a patient is receptive to the health care provider’s instructions and is adhering to them. Referral to access programs can aid communication. For example, most cities have community centers where patients can seek medical advice from physicians who speak the patients’ language; these centers also may provide native-language materials and interpreters.
Offering reassurance to patients in their own language and in a culturally sensitive setting will help break down barriers and improve care. Patients who are educated about HBV transmission and the availability of an effective vaccine may be instrumental in preventing transmission of the disease to household members.
Cultural sensitivity training will benefit health care providers and staffs whose patients include Asian Americans. Educational programs should be specific to the needs of the community, as different subpopulations have different needs. Resource materials are available for such training; for example, the federal government’s Office of Minority Health Web site (http://www.omhrc.gov/) offers links to resources for cultural training. In addition to educating themselves and their staffs, health care providers have a responsibility to advocate for funding and equal access to care, and for the creation of more cultural and community health centers that can serve as resources to overcome cultural barriers.
DISCUSSION
Robert G. Gish, MD: How often are herbal remedies tried for chronic HBV infection in the patients you see, especially in the Vietnamese population?
Tram T. Tran, MD: Once patients are diagnosed with chronic HBV infection, the use of herbal remedies is very high; it approaches 80% in my practice. Patients may not admit to it unless you ask them specifically, because they know herbal remedies may be somewhat frowned upon by Western physicians. If you are careful and ask very gently about their use of herbals, they will tell you that they do believe in herbal medicines pretty strongly.
Morris Sherman, MD, PhD: I’d like to emphasize the need to be able to communicate with patients in their own language. In Toronto, 50% of the population was born outside of Canada. We have a huge immigrant population; given the nature of hepatology, we have many patients from Southeast and South Asia, and from all over the world, who don’t speak English. My hospital has a multilingual interpreter service, which we use freely. Scarcely a day goes by without two or three interpreters coming to the clinic to talk to patients, and as a result it’s rare that I can’t make myself understood. Maybe what I’ve said hasn’t been accepted, but patients can at least understand what I’m saying.
William D. Carey, MD: I interview many applicants for our medical school, and many of them are Asians, including Hmong and Vietnamese. With the high value that most of these groups put on education and their success with educational attainment, is their access to care improving? Are we doing a better job of training nurses, allied health personnel, and physicians to deal with this problem?
Dr. Tran: I think so, yes. For instance, the Southeast Asian immigrant population arrived in two different eras. The Vietnamese who immigrated in 1975 have been in the United States longer and in general have been able to attain a higher level of education than those who came later. The group that arrived earlier is therefore more likely to have health insurance, and it has been easier to get them into the health care system. More recent immigrants have had more difficulty navigating the system. In general, their socioeconomic status and therefore access to care is directly related to how long they’ve been in the country.
Asian Americans represent 4% of the population in the United States, and their share of the US population is projected to grow to 9% by 2050.1 These numbers are significant because of the high prevalence of hepatitis B virus (HBV) infection in this community and the cultural barriers to its effective management.
Appreciating the impact of cultural barriers on health care among Asian Americans requires an understanding of the diversity of the Asian continent, which is composed of 52 countries where 100 languages and dialects are spoken. Within each region are religious, cultural, and societal differences. Asians have immigrated to the United States over the course of several generations, and the era in which they immigrated may affect their ability to understand English, integrate into American culture, and navigate the US health care system. Successful integration into American life favors those whose families immigrated several generations earlier.
The overall prevalence of HBV infection in the United States is 0.4%2; however, estimates of prevalence range from 5% to 15% in Asian American populations, and are as high as 20% in some Pacific Rim populations.3,4 The prevalence of HBV infection in Asian Americans differs by subpopulation, with the highest prevalence among immigrants from Vietnam, Laos, and China, and the lowest among those from Japan.
Of the approximately 1 million Americans estimated to be infected with HBV as of 2005, more than 750,000 had access to health care; of these, 205,000 were diagnosed with HBV infection,5 suggesting substantial underdiagnosis. Referrals to specialists were even fewer (175,000), and only about 31,000 patients chronically infected with HBV received antiviral treatment, a figure that has likely increased with greater awareness of HBV and the availability of new antiviral medications.
BARRIERS TO DIAGNOSIS AND TREATMENT
The barriers to effective management of HBV infection in Asian Americans include cultural, socioeconomic, and accessibility issues (see “Case: Stigma and cultural barriers lead to inadequate care”).

Language and linguistic isolation
Limited proficiency in English is a large, if not the largest, barrier to effective management of chronic HBV infection. According to the US Census Bureau, a person with limited English proficiency is one who does not speak English “very well.”6 This terminology has implications for allocation of federal government resources; ie, the percentage of a community’s residents with limited English proficiency is a criterion for receipt of governmental grants and other forms of assistance, including translation services.6
Linguistic isolation, another barrier to medical care, is lack of an English-speaking household member who is older than 14 years.7 By this definition, more than one-third of Korean, Taiwanese, Chinese, Hmong, and Bangladeshi households, and almost half of Vietnamese households, are linguistically isolated, with limited ability to communicate with health care providers.8
Lack of health insurance and its correlates
The high percentage of Asian immigrants without health insurance is a challenge to providing adequate health care. Health insurance coverage is lacking for about one-third of Korean immigrants, about one in five immigrants from Southeast Asia and South Asia, and about 15% of Filipino and Chinese immigrants.9
One reason for the large proportion of uninsured among these groups is the high rate of small business ownership among Asian Americans and the difficulty that small business owners have in obtaining affordable health insurance coverage. In addition, although Asian Americans are as likely as other US residents to be employed full time, their employment options may be less likely to include health insurance benefits.
Poverty affects the ability to acquire health insurance. Although the popular image of the Asian immigrant is an educated person with high earning potential, the reality is that poverty strikes immigrants from Southeast Asia at a high rate. Almost 40% of the Hmong population, for example, lives below the poverty level, and poverty rates among the Cambodian, Bangladeshi, Malaysian, and several other Asian subpopulations are nearly as high.8
Citizenship correlates with the ability to obtain health insurance; it is estimated that 42% to 57% of noncitizens lack health insurance, compared with 15% of citizens.8 Only half of Asian immigrants become naturalized citizens, with wide variability among subgroups. Two-thirds of Filipinos who immigrate to the United States eventually become naturalized compared with less than one-third of Malaysian, Japanese, Indonesian, and Hmong immigrants.8
Educational achievement is associated with attainment of financial security and health insurance. The vast majority of Taiwanese, Japanese, Filipino, and Korean Americans obtain a high school education or higher, with correspondingly higher rates of health insurance coverage. Among those from Southeast Asia (Hmong, Cambodians, Laotians, and Vietnamese), whose immigration to this country is relatively recent, fewer than half complete a high school education.8
Health care workforce representation
Certain Asian subgroups are underrepresented in the racial composition of the US health care workforce; this imbalance may affect accessibility to the health care system and adherence to medical prescriptions and instructions among underrepresented groups. Racial concordance between patient and health care provider is associated with greater patient participation in care, according to the Institute of Medicine.10 In addition to racial similarity, linguistic similarity enhances communication and adherence to instructions.
Belief systems and attitudes toward health care
An immigrant patient’s religious beliefs and cultural attitudes toward Western medicine may pose difficulties in successfully managing disease. Many Asian Americans are Buddhists, who may believe that suffering is an integral part of life; proactively seeking medical care may not be imperative for them. Confucianism, the worship of ancestors and the subjugation of the self to the well-being of the family, is a common belief system among Asians that may inhibit the desire to seek needed medical care. For example, a family elder may instruct a young man not to seek medical care for his HBV infection because this would jeopardize his siblings’ marriage prospects. Taoism involves the belief that perfection is achieved when events are allowed to take the more natural course. Intervention is therefore frowned upon.
Some belief systems may impede care because they incorporate indifference toward suffering. Many Hmong believe that the length of life is predetermined, so lifesaving care is pointless. Cultural value may be placed on stoicism, discouraging visits to health care providers. A belief that disease is caused by supernatural events rather than organic etiologies is another perception that serves as a barrier to seeking medical care.
Distrust of, or unfamiliarity with, Western medicine may delay care, and the resulting poor outcomes may be falsely attributed to Western medicine itself. In some cultures, there is a pervasive belief that a physician can touch the pulse and identify the problem. Some Laotians believe that immunizations are dangerous for a baby’s spirit, and therefore forgo immunization against HBV when it is indicated.
The patient’s relationship with his or her health care provider is an important determinant of quality of care and willingness to continue to receive care. The best possible scenario is concordance in language and culture. Asian cultures emphasize politeness, respect for authority, filial piety, and avoidance of shame. Because Asian patients often view physicians as authority figures, they may not ask questions or voice reservations or fears about their treatment regimens; instead, they may express their agreement with physicians’ advice, but with no intent to return or follow instructions.
Infection with HBV carries a stigma about the mode of transmission that can interfere with patients’ daily lives. A study of attitudes about HBV found that HBV-infected patients feel less welcome to stay overnight or share the same bathroom at friends’ or relatives’ houses, that noninfected persons fear that the disease may be passed to them by HBV-positive friends, and that HBV-infected patients are concerned about whether their choices may have led to the infection.11
OVERCOMING BARRIERS
Sensitivity to cultural attitudes may enhance communication and the likelihood that patients will accept physicians’ recommendations. Several office visits may be necessary to confirm that a patient is receptive to the health care provider’s instructions and is adhering to them. Referral to access programs can aid communication. For example, most cities have community centers where patients can seek medical advice from physicians who speak the patients’ language; these centers also may provide native-language materials and interpreters.
Offering reassurance to patients in their own language and in a culturally sensitive setting will help break down barriers and improve care. Patients who are educated about HBV transmission and the availability of an effective vaccine may be instrumental in preventing transmission of the disease to household members.
Cultural sensitivity training will benefit health care providers and staffs whose patients include Asian Americans. Educational programs should be specific to the needs of the community, as different subpopulations have different needs. Resource materials are available for such training; for example, the federal government’s Office of Minority Health Web site (http://www.omhrc.gov/) offers links to resources for cultural training. In addition to educating themselves and their staffs, health care providers have a responsibility to advocate for funding and equal access to care, and for the creation of more cultural and community health centers that can serve as resources to overcome cultural barriers.
DISCUSSION
Robert G. Gish, MD: How often are herbal remedies tried for chronic HBV infection in the patients you see, especially in the Vietnamese population?
Tram T. Tran, MD: Once patients are diagnosed with chronic HBV infection, the use of herbal remedies is very high; it approaches 80% in my practice. Patients may not admit to it unless you ask them specifically, because they know herbal remedies may be somewhat frowned upon by Western physicians. If you are careful and ask very gently about their use of herbals, they will tell you that they do believe in herbal medicines pretty strongly.
Morris Sherman, MD, PhD: I’d like to emphasize the need to be able to communicate with patients in their own language. In Toronto, 50% of the population was born outside of Canada. We have a huge immigrant population; given the nature of hepatology, we have many patients from Southeast and South Asia, and from all over the world, who don’t speak English. My hospital has a multilingual interpreter service, which we use freely. Scarcely a day goes by without two or three interpreters coming to the clinic to talk to patients, and as a result it’s rare that I can’t make myself understood. Maybe what I’ve said hasn’t been accepted, but patients can at least understand what I’m saying.
William D. Carey, MD: I interview many applicants for our medical school, and many of them are Asians, including Hmong and Vietnamese. With the high value that most of these groups put on education and their success with educational attainment, is their access to care improving? Are we doing a better job of training nurses, allied health personnel, and physicians to deal with this problem?
Dr. Tran: I think so, yes. For instance, the Southeast Asian immigrant population arrived in two different eras. The Vietnamese who immigrated in 1975 have been in the United States longer and in general have been able to attain a higher level of education than those who came later. The group that arrived earlier is therefore more likely to have health insurance, and it has been easier to get them into the health care system. More recent immigrants have had more difficulty navigating the system. In general, their socioeconomic status and therefore access to care is directly related to how long they’ve been in the country.
- President’s Advisory Commission on Asian Americans and Pacific Islanders. Asian Americans and Pacific Islanders: a people looking forward. Action for access and partnerships in the 21st century. Interim report to the president and the nation. http://permanent.access.gpo.gov/lps17931/www.aapi.gov/intreport.htm. Published January 2001. Accessed December 21, 2008.
- National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Hepatitis B index. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/hepatitis/HBV/HBVfaq.htm. Updated July 8, 2008. Accessed January 21, 2009.
- Do S. The natural history of hepatitis B in Asian Americans. Asian Am Pac Isl J Health 2001; 9:141–153.
- Stanford University School of Medicine. FAQ about hepatitis B. Asian Liver Center Web site. http://liver.stanford.edu/Education/faq.html. Updated July 10, 2008. Accessed January 21, 2009.
- Di Bisceglie AM, Keeffe E, Atillasoy E, Varshneya R, Bergstein G. Management of chronic hepatitis B—an analysis of physician practices [DDW abstract M918]. Gastroenterology 2005; 128(suppl 2):A739.
- US Census Bureau. American community survey. US Census Bureau Web site. http://www.census.gov/acs/www/SBasics/SQuest/fact_pdf/P%2013%20factsheetlanguageathome2.pdf. Published January 29, 2004. Accessed January 21, 2009.
- Lestina FA. Analysis of the linguistically isolated population in Census 2000. http://www.census.gov/pred/www/rpts/A.5a.pdf. Published September 30, 2003. Accessed January 21, 2009.
- Asian & Pacific Islander American Health Forum. Diverse communities, diverse experiences: the status of Asian Americans and Pacific Islanders in the U.S. http://www.apiahf.org/resources/pdf/Diverse%20Communities%20Diverse%20Experiences.pdf. Accessed January 21, 2009.
- Asian & Pacific Islander American Health Forum. Race, ethnicity and health care fact sheet. Henry J. Kaiser Family Foundation Web site. http://www.kff.org/minorityhealth/upload/7745.pdf. Published April 2008. Accessed January 21, 2009.
- Smedley BD, Stith AY, Nelson AR, eds; Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Board on Health Sciences Policy, Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. http://www.nap.edu/openbook.php?isbn=030908265X. Published 2003. Accessed January 21, 2009.
- Speigel BMR, Bollus R, Han S, et al. Development and validation of a disease-targeted quality of life instrument in chronic hepatitis B: the hepatitis B quality of life instrument, version 1.0. Hepatology 2007; 46:113–121.
- President’s Advisory Commission on Asian Americans and Pacific Islanders. Asian Americans and Pacific Islanders: a people looking forward. Action for access and partnerships in the 21st century. Interim report to the president and the nation. http://permanent.access.gpo.gov/lps17931/www.aapi.gov/intreport.htm. Published January 2001. Accessed December 21, 2008.
- National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Hepatitis B index. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/hepatitis/HBV/HBVfaq.htm. Updated July 8, 2008. Accessed January 21, 2009.
- Do S. The natural history of hepatitis B in Asian Americans. Asian Am Pac Isl J Health 2001; 9:141–153.
- Stanford University School of Medicine. FAQ about hepatitis B. Asian Liver Center Web site. http://liver.stanford.edu/Education/faq.html. Updated July 10, 2008. Accessed January 21, 2009.
- Di Bisceglie AM, Keeffe E, Atillasoy E, Varshneya R, Bergstein G. Management of chronic hepatitis B—an analysis of physician practices [DDW abstract M918]. Gastroenterology 2005; 128(suppl 2):A739.
- US Census Bureau. American community survey. US Census Bureau Web site. http://www.census.gov/acs/www/SBasics/SQuest/fact_pdf/P%2013%20factsheetlanguageathome2.pdf. Published January 29, 2004. Accessed January 21, 2009.
- Lestina FA. Analysis of the linguistically isolated population in Census 2000. http://www.census.gov/pred/www/rpts/A.5a.pdf. Published September 30, 2003. Accessed January 21, 2009.
- Asian & Pacific Islander American Health Forum. Diverse communities, diverse experiences: the status of Asian Americans and Pacific Islanders in the U.S. http://www.apiahf.org/resources/pdf/Diverse%20Communities%20Diverse%20Experiences.pdf. Accessed January 21, 2009.
- Asian & Pacific Islander American Health Forum. Race, ethnicity and health care fact sheet. Henry J. Kaiser Family Foundation Web site. http://www.kff.org/minorityhealth/upload/7745.pdf. Published April 2008. Accessed January 21, 2009.
- Smedley BD, Stith AY, Nelson AR, eds; Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Board on Health Sciences Policy, Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in health care. http://www.nap.edu/openbook.php?isbn=030908265X. Published 2003. Accessed January 21, 2009.
- Speigel BMR, Bollus R, Han S, et al. Development and validation of a disease-targeted quality of life instrument in chronic hepatitis B: the hepatitis B quality of life instrument, version 1.0. Hepatology 2007; 46:113–121.
KEY POINTS
- Some Asian Americans have limited proficiency in English and are isolated linguistically, limiting their ability to communicate with health care providers.
- Asian Americans may view Western medicine with suspicion, causing delays in seeking care and making it difficult to successfully manage chronic HBV infection.
- Sensitivity to cultural attitudes may enhance communication and the likelihood that immigrant patients will accept health care providers’ recommendations; cultural sensitivity training may be helpful.
Hepatitis B treatment: Current best practices, avoiding resistance
Guidelines for the management of hepatitis B virus (HBV) infection can be daunting to clinicians. Further, although established practice guidelines can provide direction, treatment of chronic HBV infection is characterized by uncertainties that can hinder optimal patient care. Reservations about when to initiate and terminate therapy, cost issues, and the development of resistance to therapy are among the factors that impede adequate treatment. This article offers a straightforward roadmap for the management of chronic HBV infection, based on interpretation of recently released guidelines,1–3 and strategies for preventing and managing resistance to antiviral therapy.
DECIDING TO TREAT
Key factors: Viral load and ALT
Two important factors influencing the decision to treat are viral load (HBV DNA) and alanine aminotransferase (ALT) level; although these are relatively straightforward measures, other factors can cause clinicians to avoid or delay treatment.
A simple guideline is to discuss treatment with any patient who is positive for HBV DNA. The most recent guidelines for the treatment of HBV infection, published by the European Association for the Study of the Liver (EASL), recommend an HBV DNA level of 2,000 copies/mL as a threshold for initiating therapy; this recommendation applies to patients who are either positive or negative for hepatitis B e antigen (HBeAg).3
The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus (REVEAL-HBV) study investigators used ultrasensitive polymerase chain reaction (PCR) to quantify HBV DNA levels and conducted a time-dependent multiple Cox regression analysis of HBV DNA level and the risk of hepatocellular carcinoma (HCC).4,5 The length of time at a given DNA level was weighted in determining the adjusted hazard ratio. With an HBV DNA level less than 300 copies/mL defined as the reference group, risk of HCC increased commensurate with increasing HBV DNA level; even at levels ranging from 300 to 10,000 copies/mL, longer duration of HBV DNA positivity increased risk. This group also found HBV DNA level to be an independent risk factor for cirrhosis.
Patients who are HBV DNA negative are at much lower risk of cirrhosis and HCC than HBV DNA–positive patients; HBV DNA–negative patients being treated with antiviral drugs are much less likely to develop resistance to treatment, provided that first-line medications such as tenofovir or entecavir are used.
The definition of a “healthy” ALT level is controversial. In my opinion, an abnormal ALT is greater than 19 IU/mL for women and greater than 25 IU/mL for men; in either setting, treatment should be instituted if the patient is HBV DNA positive. This position is supported by a recently published algorithm,6 a recent National Institutes of Health conference on management of HBV,7 and other sources.8–12
Barriers to optimal treatment
Patient reluctance to undergo invasive tests, concerns about resistance, confusion about when to initiate therapy, cost, and other issues can impede timely and effective treatment of HBV infection.
Invasive studies. Liver histology is a key driver for initiating treatment, but many patients resist undergoing a liver biopsy. Ultrasonography has enabled noninvasive determination of spleen size, portal vein size, and liver tissue and surface heterogeneity; noninvasive assessments such as measurement of aspartate aminotransferase, varices, serum markers of fibrosis, and platelet count may provide clues to advanced liver fibrosis. Eventually, ultrasonographic elastography to measure liver stiffness and magnetic resonance scans may be common in clinical practice for noninvasive evaluation of liver damage. Ultimately, however, liver biopsy remains a valuable tool to motivate patients with chronic HBV infection to initiate and continue antiviral therapy.
Rationales for avoiding or delaying treatment. Concern about the development of resistance to treatment, as with antiviral therapy directed against human immunodeficiency virus (HIV), is one reason not to treat. The absence of clear guidelines regarding the appropriate time to terminate therapy has also led to avoidance or delay of treatment. The lack of risk calculators similar to the Framingham risk score, which estimates the risk of coronary heart disease, has limited the treatment of chronic HBV infection.
Cost. Cost must be examined in relation to the cost of resistance developing and the cost of treating complications. Lamivudine, considered a third-line treatment for chronic HBV infection, is an inexpensive drug. However, up to 70% of patients will develop resistance to lamivudine over 5 years3,6; most will require combination therapy, with its attendant costs, and may eventually require transplants or experience poor clinical outcomes. Although the initial costs of potent first-line therapies (tenofovir, entecavir, and pegylated interferon) are high, cost modeling shows that they are less expensive over the long term when the overall cost of care is considered.13,14
GOALS OF THERAPY: VIRAL LOAD SUPPRESSION, SEROCONVERSION
Profound suppression of viral load reduces the risk of resistance and is the ultimate goal of therapy for HBV infection. We can infer from recent data15 that achieving HBV DNA negativity has led to improved outcomes in patients with chronic HBV infection; ie, with the increased use of antiviral drugs in the United States over the past 2 decades, the number of liver transplants for end-stage liver disease has fallen dramatically,15 suggesting that profound suppression of viral loads has translated into fewer cases of liver failure and less need for transplants.
Over the same period, the number of patients diagnosed annually with HCC has increased by 146%.15 One interpretation of these data is that patients with chronic HBV infection are living longer, allowing time for HCC to develop. In addition, aggressive surveillance guidelines may account for the increased number of HCC cases since 1990. If detected early, HCC is curable by liver transplant at a rate exceeding 80%.16–18
In discussing treatment duration with patients, I present the ultimate goal of therapy as loss of HB surface antigen (HBsAg), or seroconversion to anti-HBs. At our clinic, we monitor HBsAg at least annually when patients are on long-term therapy.
The cost-effectiveness of treating all patients until they are HBsAg negative needs to be assessed. Incremental cost-effectiveness ratios per quality-adjusted life-year are key to identifying the best course of action.
TREATMENT OPTIONS
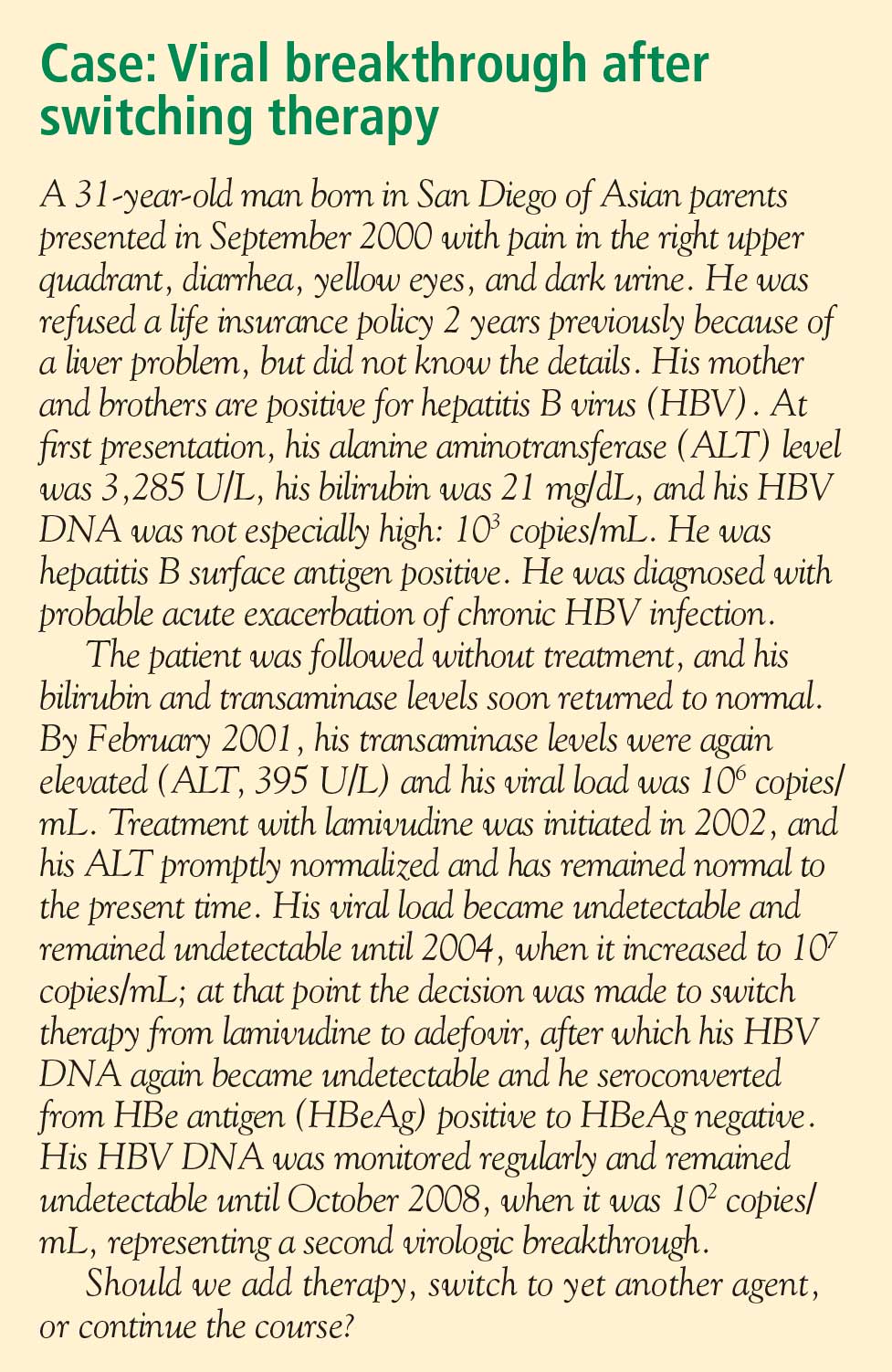
Nucleoside analogues
Lamivudine. The incidence of lamivudine resistance increases with increased treatment duration, reaching a peak of 80% after 5 years of treatment19–22; use of this agent eventually requires combination therapy. For this reason, lamivudine is considered a third-line drug and is not recommended as a first-line therapy.
Entecavir. Entecavir induces profound suppression of HBV DNA (to undetectable levels by weeks 24 to 36) in patients who are HBeAg positive or negative, regardless of baseline HBV DNA levels; resistance rates are very low in treatment-naïve patients,23 and entecavir is therefore considered first-line therapy. More than 90% of HBeAg-positive or -negative patients who are adherent to entecavir are HBV DNA negative at 5 years.24 Loss of HBsAg is 5% in entecavir-treated patients at follow-up of approximately 80 weeks, which is roughly double the rate of HBsAg loss with lamivudine.32
Telbivudine. Telbivudine has a secondary role in treatment of HBV infection. In a study by Lai et al,25,26 the cumulative incidence of telbivudine resistance and virologic breakthrough in HBeAg-positive patients rose from nearly 5% after 1 year to 22% after 2 years of treatment. Although the incidence was lower in HBeAg-negative patients, rates of genotypic resistance with virologic breakthrough rose to 9% in this population.
Since these results report genotypic resistance and virologic breakthrough, the rates of genotypic resistance in these patients may actually be higher than reported. Indeed, genotypic resistance was detected in 6.8% of the entire study population after 1 year of treatment. In this study, it must be remembered that patients with HBV DNA levels that were detectable by PCR (≥ 300 copies/mL) but were less than 1,000 copies/mL were not assessed for resistance.
Because of high rates of resistance associated with telbivudine, its role in the treatment of chronic HBV is secondary. I may use it in pregnant patients because most other nucleoside analogues are category C drugs and telbivudine is a category B agent (see “Management of hepatitis B in pregnancy: Weighing the options”). There are risks of myositis and neuropathy with telbivudine; although these risks are low, I mention them to patients when discussing a treatment plan.
Nucleotide analogues
Adefovir. Adefovir is considered second-line or add-on therapy when resistance to lamivudine develops because of its low potency in suppressing viral load. At 48 weeks, only 12% of HBeAg-positive patients are HBV DNA negative when treated with adefovir monotherapy.33,34
In a phase 3 clinical trial, genotypic resistance to adefovir was detected in 29% of HBeAg-negative patients treated for up to 5 years.27 The probability of resistance with virologic breakthrough was 3%, 8%, 14%, and 20% after 2, 3, 4, and 5 years of treatment, respectively.
In patients infected with lamivudine-resistant HBV, the probability of adefovir resistance is reduced by adding adefovir to ongoing lamivudine therapy, according to data from a large retrospective comparative study.35 In patients treated with adefovir monotherapy, the probability of virologic breakthrough (defined as > 1 log10 rebound in HBV DNA compared with on-treatment nadir) reached 30% over 36 months. In patients treated with add-on adefovir, the probability of virologic breakthrough was reduced to 6%. Similarly, the probability of adefovir resistance over 36 months of treatment was greater in the adefovir monotherapy group (16%) than in the add-on adefovir group (0%).
Although adefovir resistance is observed infrequently when adefovir is added to lamivudine, the effectiveness of adding adefovir is still limited by its low potency.
Tenofovir. More than 90% of HBeAg-negative patients and nearly 80% of HBeAg-positive patients treated with tenofovir have persistent virologic responses and HBV DNA levels less than 400 copies/mL by 72 weeks, with minimal side effects.33,34 Marcellin et al reported no development of resistance to tenofovir after 48 weeks of treatment.31 Although the nucleotide analogues have been associated with renal toxicity,36 the risk of renal toxicity associated with tenofovir is 1% or less per year; it can be reduced even further by calculating renal function through the use of the Cockroft-Gault equation or the Modification of Diet in Renal Disease equation prior to therapy and adjusting the dosage accordingly.37
With profound HBV DNA suppression, HBsAg loss occurs in about 5% of tenofovir-treated patients at 64 weeks.33
Treatment with tenofovir in treatment-experienced patients leads to potent suppression of HBV DNA independent of HBV genotype, HBV mutations (YMDD mutations) that signal lamivudine resistance, or HBeAg status at baseline.38 Patients with genotypic resistance to adefovir at baseline had a lower probability of achieving HBV DNA suppression during treatment with tenofovir.
Pegylated interferon
Pegylated interferon has proven useful in subsets of HBV DNA–positive patients. These include patients with genotype A or B who are young, those with high ALT levels (≥ 2 or 3 times the upper limit of normal) and low viral load (< 107 copies/mL), and patients without significant comorbidities.6 Pegylated interferon is also an option for patients who require a defined treatment period (eg, a woman wishing to become pregnant in 1 to 2 years). The patients who would benefit from pegylated interferon as first-line therapy must be better defined, and early markers of virologic response need to be identified.
PREVENTING AND MANAGING RESISTANCE
Antiviral drug resistance has a negative impact on the treatment of patients with chronic HBV infection. The development of resistance can result in virologic breakthrough (a confirmed 1 log10 increase in plasma HBV DNA levels)1; increased ALT levels1,39; and the progression of liver disease,40 including hepatic decompensation, development of HCC, and need for liver transplant. In addition, resistance mutations may re-emerge, with covalently closed circular DNA representing a genetic archive for development of resistance; this can significantly limit future treatment options.41 Early detection and regular monitoring are critical to prevention and management of resistance.
Detection
Detecting virologic breakthrough as early as possible increases the likelihood of achieving virologic response. In a study by Rapti and colleagues,42 patients with lamivudine-resistant chronic HBV were treated with a combination of lamivudine and adefovir. The 3-year cumulative probability of virologic response (< 103 copies/mL) was 99% with the addition of adefovir when baseline viral load levels were less than 5 log10 copies/mL, but only 71% when baseline viral loads were greater than 6 log10 copies/mL.
Monitoring
Patient response must be defined correctly. In adherent patients who show an early favorable response to therapy, I advise HBV DNA testing every 3 to 6 months. For those whose response flattens and whose viral load remains high, switching therapy or adding on should be considered. We continue therapy and monitor regularly after HBV DNA reaches an undetectable level. If the response is suboptimal, the treatment regimen is adapted by adding a new agent or switching to an alternative therapy (see “Case revisited”).
For patients who are being treated with tenofovir or entecavir, I typically extend the interval of measuring DNA levels to every 6 months because rates of resistance with these agents are low. If response is suboptimal but resistance is absent, I consider switching to the opposite drug. In those patients with a resistance mutation, I add the other agent.
Managing resistance
Combination therapy has a role in individuals in whom medication has failed to suppress viral load, in the setting of drug resistance, after liver transplant, and in individuals coinfected with HIV (see “Strategies for managing coinfection with hepatitis B virus and HIV”). If patients demonstrate resistance to their current therapy, we examine viral factors, adherence to therapy, and medication availability (eg, cost and insurance coverage). Switching to entecavir in adefovir-resistant patients produces profound suppression of HBV DNA. Patients in whom entecavir or lamivudine have failed may respond to tenofovir, depending on the resistance mutations.
A POTENTIAL FUTURE OPTION
Clevudine is a nucleoside analogue in phase 3 clinical studies in the United States. Its potential role in therapy is not yet clear. To be determined is whether it will induce a long-term, off-treatment viral response, in which case treatment may be able to be terminated earlier, and whether it will show clinically important cross-resistance with other nucleoside analogues. The availability of more sensitive assays to demonstrate the emergence of early viral resistance would enable earlier changes in treatment for more successful outcomes.
SUMMARY
Preventing resistance is crucial to the success of antiviral drug therapy for treatment of chronic HBV; a persistently high viral load increases the risk of cirrhosis and HCC, and resistance is associated with increased HBV DNA levels. The best chance for long-term success depends on initiating therapy before cirrhosis develops, when viral load is still low; profound suppression of viral load using the most potent agents as first-line therapy; and long-term monitoring of HBV DNA. The development of resistance can result in virologic breakthrough and liver complications. Entecavir and tenofovir represent the most effective first-line options to suppress HBV DNA. Because cross-resistance can occur, adding another agent is preferred to switching agents if resistance to initial therapy develops.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Liaw Y-F, Leung N, Kao J-H, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008; 2:263–283.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242.
- Chen CJ, Yang HI, Su J, et al. Serial monitoring of viral load and serum alanine aminotransferase level and the risk of hepatocellular carcinoma (HCC): R.E.V.E.A.L.-HBV study update [abstract 141]. J Hepatol 2008; 48(suppl 2):S61.
- Chen JD, Yang HI, Iloeje UH, et al. Liver disease progression in chronic hepatitis B infected persons with normal serum alanine amino transferase level: update from the R.E.V.E.A.L.-HBV study [abstract 644]. J Hepatol 2008; 48(suppl 2):S240.
- Keeffe EG, Dieterich DT, Han S-H B, et al. Special report. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008; 6:1315–1341.
- Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health Consensus Development Conference statement: management of hepatitis B. Ann Intern Med 2009; 150:104–112.
- Piton A, Poynard T, Imbert-Bismut F, et al. Factors associated with serum alanine aminotransaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC group. Hepatology 1998; 27:1213–1219.
- Kim CH, Nam CM, Jee SH, Khan KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004; 328:983–986.
- Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology 2006; 43:1145–1151.
- Puoti C, Magrini A, Stati TN, et al. Clinical, histological, and virological features of hepatitis C virus carriers with persistently normal or abnormal alanine transaminase levels. Hepatology 1997; 26:1393–1398.
- Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002; 137:1–9.
- Deniz B, Buti M, Brosa M, et al. Cost-effectiveness simulation analysis of tenofovir disoproxil fumarate, lamivudine, adefovir dipivoxil, and entecavir of HbeAg negative patients with chronic hepatitis B in Spain [EASL abstract 558]. J Hepatol 2008; 48(suppl 2):S209.
- Deniz B, Everhard R. Cost-effectiveness simulation analysis of tenofovir disoproxil fumarate in HBeAg negative patients with chronic hepatitis B in Italy and France [EASL abstract 559]. J Hepatol 2008; 48(suppl 2):S210.
- Kim W, Benson JT, Hindman A, Brosgart C, Fortner-Burton C. Decline in the need for liver transplantation for end stage liver disease secondary to hepatitis B in the US. Paper presented at: 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. Abstract 12.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–700.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl 2003; 9:684–692.
- Lai CL, Ratziu V, Yuen M-F, Poynard T. Viral hepatitis B. Lancet 2003; 362:2089–2094.
- Leung NW, Lai C-L, Chang T-T, et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 2001; 33:1527–1532.
- Benhamou Y, Bochet M, Thibault V, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology 1999; 30:1302–1306.
- Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125:1714–1722.
- Colonno RJ, Rose R, Baldick CJ, et al. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology 2006; 44:1656–1665.
- Perrillo RP. Current treatment of chronic hepatitis B: benefits and limitations. Semin Liver Dis 2005; 25(suppl 1):20–28.
- Lai C-L, Gane E, Liaw Y-F, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 2007; 357:2576–2588.
- Lai C-L, Gane E, Hsu C-W, et al. Two-year results from the GLOBE trial in patients with hepatitis B: greater clinical and antiviral efficacy for telbivudine (LDT) vs. lamivudine [AASLD abstract 91]. Hepatology 2006; 44(suppl 1):222A.
- Locarnini S, Qi X, Arterburn S, et al. Incidence and predictors of emergence of adefovir resistant HBV during four years of adefovir dipivoxil therapy for patients with chronic hepatitis B [EASL abstract 36]. J Hepatol 2005; 42(suppl 2):17.
- Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al; Adefovir Dipivoxil 438 Study Group. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med 2005; 352:2673–2681.
- Hepsera [package insert]. Foster City, CA: Gilead Sciences, Inc; 2008.
- Lee YS, Suh DJ, Lim YS, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology 2006; 43:1385–1391.
- Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–2455.
- Gish R, Chang T-T, Lai C-L, et al. Hepatitis B surface antigen loss in antiviral-treated patients with HBeAg(+) chronic hepatitis B infection: observations from antiviral-naïve patients treated with entecavir or lamivudine. Paper presented at: 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. Abstract 992.
- Heathcote J, George J, Gordon S, et al. Tenofovir disoproxil fumarate (TDF) for the treatment of HBeAg-positive chronic hepatitis B: week 72 TDF data and week 24 adefovir dipivoxil switch data (study 103) [EASL abstract 71]. J Hepatol 2008; 48(suppl 2):S32.
- Marcellin P, Jacobson I, Habersetzer F, et al. Tenofovir disoproxil fumarate (TDF) for the treatment of HBeAg-negative chronic hepatitis B: week 72 TDF data and week 24 adefovir dipivoxil switch data (study 102) [EASL abstract 57]. J Hepatol 2008; 48(suppl 2):S26.
- Lampertico P, Marzano A, Levrero M, et al. Adefovir and lamivudine combination therapy is superior to adefovir monotherapy for lamivudine-resistant patients with HBeAg-negative chronic hepatitis B [EASL abstract 502]. J Hepatol 2007; 46(suppl 1):S191.
- Ha NB, Ha NB, Trinh HN. Changes in creatinine clearance (CRCL) in chronic hepatitis B (CHB) patients treated with adefovir dipivoxil (ADV) [AASLD abstract 901]. Hepatology 2008; 48:709A–710A.
- Gallant J, Staszewski S, Pozniak AL, et al; for the 903 Study Team. Similar renal safety profile between tenofovir DF (TDF) and stavudine (d4T) using modification of diet in renal disease (MDRD) and Cockcroft-Gault (CG) estimations of glomerular filtration rate (GFR) in antiretroviral-naïve patients through 144 weeks. In: Program and Abstracts of the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19, 2005; Washington, DC. Abstract H-350.
- van Bömmel F, de Man RA, Stein K, et al. A multicenter analysis of antiviral response after one year of tenofovir monotherapy in HBV-monoinfected patients with prior nucleos(t)ide analog experience [EASL abstract 73]. J Hepatol 2008; 48(suppl 2):S32.
- Fung SK, Lok AS. Management of hepatitis B patients with antiviral resistance. Antivir Ther 2004; 9:1013–1026.
- Gish RG. Chronic hepatitis B virus: treating patients to prevent and manage resistance. US Gastroenterology Review 2007; March:51–54.
- Zoulim F. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antiviral Res 2004; 64:1–15.
- Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 2007; 45:307–313.
Guidelines for the management of hepatitis B virus (HBV) infection can be daunting to clinicians. Further, although established practice guidelines can provide direction, treatment of chronic HBV infection is characterized by uncertainties that can hinder optimal patient care. Reservations about when to initiate and terminate therapy, cost issues, and the development of resistance to therapy are among the factors that impede adequate treatment. This article offers a straightforward roadmap for the management of chronic HBV infection, based on interpretation of recently released guidelines,1–3 and strategies for preventing and managing resistance to antiviral therapy.
DECIDING TO TREAT
Key factors: Viral load and ALT
Two important factors influencing the decision to treat are viral load (HBV DNA) and alanine aminotransferase (ALT) level; although these are relatively straightforward measures, other factors can cause clinicians to avoid or delay treatment.
A simple guideline is to discuss treatment with any patient who is positive for HBV DNA. The most recent guidelines for the treatment of HBV infection, published by the European Association for the Study of the Liver (EASL), recommend an HBV DNA level of 2,000 copies/mL as a threshold for initiating therapy; this recommendation applies to patients who are either positive or negative for hepatitis B e antigen (HBeAg).3
The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus (REVEAL-HBV) study investigators used ultrasensitive polymerase chain reaction (PCR) to quantify HBV DNA levels and conducted a time-dependent multiple Cox regression analysis of HBV DNA level and the risk of hepatocellular carcinoma (HCC).4,5 The length of time at a given DNA level was weighted in determining the adjusted hazard ratio. With an HBV DNA level less than 300 copies/mL defined as the reference group, risk of HCC increased commensurate with increasing HBV DNA level; even at levels ranging from 300 to 10,000 copies/mL, longer duration of HBV DNA positivity increased risk. This group also found HBV DNA level to be an independent risk factor for cirrhosis.
Patients who are HBV DNA negative are at much lower risk of cirrhosis and HCC than HBV DNA–positive patients; HBV DNA–negative patients being treated with antiviral drugs are much less likely to develop resistance to treatment, provided that first-line medications such as tenofovir or entecavir are used.
The definition of a “healthy” ALT level is controversial. In my opinion, an abnormal ALT is greater than 19 IU/mL for women and greater than 25 IU/mL for men; in either setting, treatment should be instituted if the patient is HBV DNA positive. This position is supported by a recently published algorithm,6 a recent National Institutes of Health conference on management of HBV,7 and other sources.8–12
Barriers to optimal treatment
Patient reluctance to undergo invasive tests, concerns about resistance, confusion about when to initiate therapy, cost, and other issues can impede timely and effective treatment of HBV infection.
Invasive studies. Liver histology is a key driver for initiating treatment, but many patients resist undergoing a liver biopsy. Ultrasonography has enabled noninvasive determination of spleen size, portal vein size, and liver tissue and surface heterogeneity; noninvasive assessments such as measurement of aspartate aminotransferase, varices, serum markers of fibrosis, and platelet count may provide clues to advanced liver fibrosis. Eventually, ultrasonographic elastography to measure liver stiffness and magnetic resonance scans may be common in clinical practice for noninvasive evaluation of liver damage. Ultimately, however, liver biopsy remains a valuable tool to motivate patients with chronic HBV infection to initiate and continue antiviral therapy.
Rationales for avoiding or delaying treatment. Concern about the development of resistance to treatment, as with antiviral therapy directed against human immunodeficiency virus (HIV), is one reason not to treat. The absence of clear guidelines regarding the appropriate time to terminate therapy has also led to avoidance or delay of treatment. The lack of risk calculators similar to the Framingham risk score, which estimates the risk of coronary heart disease, has limited the treatment of chronic HBV infection.
Cost. Cost must be examined in relation to the cost of resistance developing and the cost of treating complications. Lamivudine, considered a third-line treatment for chronic HBV infection, is an inexpensive drug. However, up to 70% of patients will develop resistance to lamivudine over 5 years3,6; most will require combination therapy, with its attendant costs, and may eventually require transplants or experience poor clinical outcomes. Although the initial costs of potent first-line therapies (tenofovir, entecavir, and pegylated interferon) are high, cost modeling shows that they are less expensive over the long term when the overall cost of care is considered.13,14
GOALS OF THERAPY: VIRAL LOAD SUPPRESSION, SEROCONVERSION
Profound suppression of viral load reduces the risk of resistance and is the ultimate goal of therapy for HBV infection. We can infer from recent data15 that achieving HBV DNA negativity has led to improved outcomes in patients with chronic HBV infection; ie, with the increased use of antiviral drugs in the United States over the past 2 decades, the number of liver transplants for end-stage liver disease has fallen dramatically,15 suggesting that profound suppression of viral loads has translated into fewer cases of liver failure and less need for transplants.
Over the same period, the number of patients diagnosed annually with HCC has increased by 146%.15 One interpretation of these data is that patients with chronic HBV infection are living longer, allowing time for HCC to develop. In addition, aggressive surveillance guidelines may account for the increased number of HCC cases since 1990. If detected early, HCC is curable by liver transplant at a rate exceeding 80%.16–18
In discussing treatment duration with patients, I present the ultimate goal of therapy as loss of HB surface antigen (HBsAg), or seroconversion to anti-HBs. At our clinic, we monitor HBsAg at least annually when patients are on long-term therapy.
The cost-effectiveness of treating all patients until they are HBsAg negative needs to be assessed. Incremental cost-effectiveness ratios per quality-adjusted life-year are key to identifying the best course of action.
TREATMENT OPTIONS

Nucleoside analogues
Lamivudine. The incidence of lamivudine resistance increases with increased treatment duration, reaching a peak of 80% after 5 years of treatment19–22; use of this agent eventually requires combination therapy. For this reason, lamivudine is considered a third-line drug and is not recommended as a first-line therapy.
Entecavir. Entecavir induces profound suppression of HBV DNA (to undetectable levels by weeks 24 to 36) in patients who are HBeAg positive or negative, regardless of baseline HBV DNA levels; resistance rates are very low in treatment-naïve patients,23 and entecavir is therefore considered first-line therapy. More than 90% of HBeAg-positive or -negative patients who are adherent to entecavir are HBV DNA negative at 5 years.24 Loss of HBsAg is 5% in entecavir-treated patients at follow-up of approximately 80 weeks, which is roughly double the rate of HBsAg loss with lamivudine.32
Telbivudine. Telbivudine has a secondary role in treatment of HBV infection. In a study by Lai et al,25,26 the cumulative incidence of telbivudine resistance and virologic breakthrough in HBeAg-positive patients rose from nearly 5% after 1 year to 22% after 2 years of treatment. Although the incidence was lower in HBeAg-negative patients, rates of genotypic resistance with virologic breakthrough rose to 9% in this population.
Since these results report genotypic resistance and virologic breakthrough, the rates of genotypic resistance in these patients may actually be higher than reported. Indeed, genotypic resistance was detected in 6.8% of the entire study population after 1 year of treatment. In this study, it must be remembered that patients with HBV DNA levels that were detectable by PCR (≥ 300 copies/mL) but were less than 1,000 copies/mL were not assessed for resistance.
Because of high rates of resistance associated with telbivudine, its role in the treatment of chronic HBV is secondary. I may use it in pregnant patients because most other nucleoside analogues are category C drugs and telbivudine is a category B agent (see “Management of hepatitis B in pregnancy: Weighing the options”). There are risks of myositis and neuropathy with telbivudine; although these risks are low, I mention them to patients when discussing a treatment plan.
Nucleotide analogues
Adefovir. Adefovir is considered second-line or add-on therapy when resistance to lamivudine develops because of its low potency in suppressing viral load. At 48 weeks, only 12% of HBeAg-positive patients are HBV DNA negative when treated with adefovir monotherapy.33,34
In a phase 3 clinical trial, genotypic resistance to adefovir was detected in 29% of HBeAg-negative patients treated for up to 5 years.27 The probability of resistance with virologic breakthrough was 3%, 8%, 14%, and 20% after 2, 3, 4, and 5 years of treatment, respectively.
In patients infected with lamivudine-resistant HBV, the probability of adefovir resistance is reduced by adding adefovir to ongoing lamivudine therapy, according to data from a large retrospective comparative study.35 In patients treated with adefovir monotherapy, the probability of virologic breakthrough (defined as > 1 log10 rebound in HBV DNA compared with on-treatment nadir) reached 30% over 36 months. In patients treated with add-on adefovir, the probability of virologic breakthrough was reduced to 6%. Similarly, the probability of adefovir resistance over 36 months of treatment was greater in the adefovir monotherapy group (16%) than in the add-on adefovir group (0%).
Although adefovir resistance is observed infrequently when adefovir is added to lamivudine, the effectiveness of adding adefovir is still limited by its low potency.
Tenofovir. More than 90% of HBeAg-negative patients and nearly 80% of HBeAg-positive patients treated with tenofovir have persistent virologic responses and HBV DNA levels less than 400 copies/mL by 72 weeks, with minimal side effects.33,34 Marcellin et al reported no development of resistance to tenofovir after 48 weeks of treatment.31 Although the nucleotide analogues have been associated with renal toxicity,36 the risk of renal toxicity associated with tenofovir is 1% or less per year; it can be reduced even further by calculating renal function through the use of the Cockroft-Gault equation or the Modification of Diet in Renal Disease equation prior to therapy and adjusting the dosage accordingly.37
With profound HBV DNA suppression, HBsAg loss occurs in about 5% of tenofovir-treated patients at 64 weeks.33
Treatment with tenofovir in treatment-experienced patients leads to potent suppression of HBV DNA independent of HBV genotype, HBV mutations (YMDD mutations) that signal lamivudine resistance, or HBeAg status at baseline.38 Patients with genotypic resistance to adefovir at baseline had a lower probability of achieving HBV DNA suppression during treatment with tenofovir.
Pegylated interferon
Pegylated interferon has proven useful in subsets of HBV DNA–positive patients. These include patients with genotype A or B who are young, those with high ALT levels (≥ 2 or 3 times the upper limit of normal) and low viral load (< 107 copies/mL), and patients without significant comorbidities.6 Pegylated interferon is also an option for patients who require a defined treatment period (eg, a woman wishing to become pregnant in 1 to 2 years). The patients who would benefit from pegylated interferon as first-line therapy must be better defined, and early markers of virologic response need to be identified.
PREVENTING AND MANAGING RESISTANCE
Antiviral drug resistance has a negative impact on the treatment of patients with chronic HBV infection. The development of resistance can result in virologic breakthrough (a confirmed 1 log10 increase in plasma HBV DNA levels)1; increased ALT levels1,39; and the progression of liver disease,40 including hepatic decompensation, development of HCC, and need for liver transplant. In addition, resistance mutations may re-emerge, with covalently closed circular DNA representing a genetic archive for development of resistance; this can significantly limit future treatment options.41 Early detection and regular monitoring are critical to prevention and management of resistance.
Detection
Detecting virologic breakthrough as early as possible increases the likelihood of achieving virologic response. In a study by Rapti and colleagues,42 patients with lamivudine-resistant chronic HBV were treated with a combination of lamivudine and adefovir. The 3-year cumulative probability of virologic response (< 103 copies/mL) was 99% with the addition of adefovir when baseline viral load levels were less than 5 log10 copies/mL, but only 71% when baseline viral loads were greater than 6 log10 copies/mL.
Monitoring
Patient response must be defined correctly. In adherent patients who show an early favorable response to therapy, I advise HBV DNA testing every 3 to 6 months. For those whose response flattens and whose viral load remains high, switching therapy or adding on should be considered. We continue therapy and monitor regularly after HBV DNA reaches an undetectable level. If the response is suboptimal, the treatment regimen is adapted by adding a new agent or switching to an alternative therapy (see “Case revisited”).
For patients who are being treated with tenofovir or entecavir, I typically extend the interval of measuring DNA levels to every 6 months because rates of resistance with these agents are low. If response is suboptimal but resistance is absent, I consider switching to the opposite drug. In those patients with a resistance mutation, I add the other agent.

Managing resistance
Combination therapy has a role in individuals in whom medication has failed to suppress viral load, in the setting of drug resistance, after liver transplant, and in individuals coinfected with HIV (see “Strategies for managing coinfection with hepatitis B virus and HIV”). If patients demonstrate resistance to their current therapy, we examine viral factors, adherence to therapy, and medication availability (eg, cost and insurance coverage). Switching to entecavir in adefovir-resistant patients produces profound suppression of HBV DNA. Patients in whom entecavir or lamivudine have failed may respond to tenofovir, depending on the resistance mutations.
A POTENTIAL FUTURE OPTION
Clevudine is a nucleoside analogue in phase 3 clinical studies in the United States. Its potential role in therapy is not yet clear. To be determined is whether it will induce a long-term, off-treatment viral response, in which case treatment may be able to be terminated earlier, and whether it will show clinically important cross-resistance with other nucleoside analogues. The availability of more sensitive assays to demonstrate the emergence of early viral resistance would enable earlier changes in treatment for more successful outcomes.
SUMMARY
Preventing resistance is crucial to the success of antiviral drug therapy for treatment of chronic HBV; a persistently high viral load increases the risk of cirrhosis and HCC, and resistance is associated with increased HBV DNA levels. The best chance for long-term success depends on initiating therapy before cirrhosis develops, when viral load is still low; profound suppression of viral load using the most potent agents as first-line therapy; and long-term monitoring of HBV DNA. The development of resistance can result in virologic breakthrough and liver complications. Entecavir and tenofovir represent the most effective first-line options to suppress HBV DNA. Because cross-resistance can occur, adding another agent is preferred to switching agents if resistance to initial therapy develops.
Guidelines for the management of hepatitis B virus (HBV) infection can be daunting to clinicians. Further, although established practice guidelines can provide direction, treatment of chronic HBV infection is characterized by uncertainties that can hinder optimal patient care. Reservations about when to initiate and terminate therapy, cost issues, and the development of resistance to therapy are among the factors that impede adequate treatment. This article offers a straightforward roadmap for the management of chronic HBV infection, based on interpretation of recently released guidelines,1–3 and strategies for preventing and managing resistance to antiviral therapy.
DECIDING TO TREAT
Key factors: Viral load and ALT
Two important factors influencing the decision to treat are viral load (HBV DNA) and alanine aminotransferase (ALT) level; although these are relatively straightforward measures, other factors can cause clinicians to avoid or delay treatment.
A simple guideline is to discuss treatment with any patient who is positive for HBV DNA. The most recent guidelines for the treatment of HBV infection, published by the European Association for the Study of the Liver (EASL), recommend an HBV DNA level of 2,000 copies/mL as a threshold for initiating therapy; this recommendation applies to patients who are either positive or negative for hepatitis B e antigen (HBeAg).3
The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus (REVEAL-HBV) study investigators used ultrasensitive polymerase chain reaction (PCR) to quantify HBV DNA levels and conducted a time-dependent multiple Cox regression analysis of HBV DNA level and the risk of hepatocellular carcinoma (HCC).4,5 The length of time at a given DNA level was weighted in determining the adjusted hazard ratio. With an HBV DNA level less than 300 copies/mL defined as the reference group, risk of HCC increased commensurate with increasing HBV DNA level; even at levels ranging from 300 to 10,000 copies/mL, longer duration of HBV DNA positivity increased risk. This group also found HBV DNA level to be an independent risk factor for cirrhosis.
Patients who are HBV DNA negative are at much lower risk of cirrhosis and HCC than HBV DNA–positive patients; HBV DNA–negative patients being treated with antiviral drugs are much less likely to develop resistance to treatment, provided that first-line medications such as tenofovir or entecavir are used.
The definition of a “healthy” ALT level is controversial. In my opinion, an abnormal ALT is greater than 19 IU/mL for women and greater than 25 IU/mL for men; in either setting, treatment should be instituted if the patient is HBV DNA positive. This position is supported by a recently published algorithm,6 a recent National Institutes of Health conference on management of HBV,7 and other sources.8–12
Barriers to optimal treatment
Patient reluctance to undergo invasive tests, concerns about resistance, confusion about when to initiate therapy, cost, and other issues can impede timely and effective treatment of HBV infection.
Invasive studies. Liver histology is a key driver for initiating treatment, but many patients resist undergoing a liver biopsy. Ultrasonography has enabled noninvasive determination of spleen size, portal vein size, and liver tissue and surface heterogeneity; noninvasive assessments such as measurement of aspartate aminotransferase, varices, serum markers of fibrosis, and platelet count may provide clues to advanced liver fibrosis. Eventually, ultrasonographic elastography to measure liver stiffness and magnetic resonance scans may be common in clinical practice for noninvasive evaluation of liver damage. Ultimately, however, liver biopsy remains a valuable tool to motivate patients with chronic HBV infection to initiate and continue antiviral therapy.
Rationales for avoiding or delaying treatment. Concern about the development of resistance to treatment, as with antiviral therapy directed against human immunodeficiency virus (HIV), is one reason not to treat. The absence of clear guidelines regarding the appropriate time to terminate therapy has also led to avoidance or delay of treatment. The lack of risk calculators similar to the Framingham risk score, which estimates the risk of coronary heart disease, has limited the treatment of chronic HBV infection.
Cost. Cost must be examined in relation to the cost of resistance developing and the cost of treating complications. Lamivudine, considered a third-line treatment for chronic HBV infection, is an inexpensive drug. However, up to 70% of patients will develop resistance to lamivudine over 5 years3,6; most will require combination therapy, with its attendant costs, and may eventually require transplants or experience poor clinical outcomes. Although the initial costs of potent first-line therapies (tenofovir, entecavir, and pegylated interferon) are high, cost modeling shows that they are less expensive over the long term when the overall cost of care is considered.13,14
GOALS OF THERAPY: VIRAL LOAD SUPPRESSION, SEROCONVERSION
Profound suppression of viral load reduces the risk of resistance and is the ultimate goal of therapy for HBV infection. We can infer from recent data15 that achieving HBV DNA negativity has led to improved outcomes in patients with chronic HBV infection; ie, with the increased use of antiviral drugs in the United States over the past 2 decades, the number of liver transplants for end-stage liver disease has fallen dramatically,15 suggesting that profound suppression of viral loads has translated into fewer cases of liver failure and less need for transplants.
Over the same period, the number of patients diagnosed annually with HCC has increased by 146%.15 One interpretation of these data is that patients with chronic HBV infection are living longer, allowing time for HCC to develop. In addition, aggressive surveillance guidelines may account for the increased number of HCC cases since 1990. If detected early, HCC is curable by liver transplant at a rate exceeding 80%.16–18
In discussing treatment duration with patients, I present the ultimate goal of therapy as loss of HB surface antigen (HBsAg), or seroconversion to anti-HBs. At our clinic, we monitor HBsAg at least annually when patients are on long-term therapy.
The cost-effectiveness of treating all patients until they are HBsAg negative needs to be assessed. Incremental cost-effectiveness ratios per quality-adjusted life-year are key to identifying the best course of action.
TREATMENT OPTIONS

Nucleoside analogues
Lamivudine. The incidence of lamivudine resistance increases with increased treatment duration, reaching a peak of 80% after 5 years of treatment19–22; use of this agent eventually requires combination therapy. For this reason, lamivudine is considered a third-line drug and is not recommended as a first-line therapy.
Entecavir. Entecavir induces profound suppression of HBV DNA (to undetectable levels by weeks 24 to 36) in patients who are HBeAg positive or negative, regardless of baseline HBV DNA levels; resistance rates are very low in treatment-naïve patients,23 and entecavir is therefore considered first-line therapy. More than 90% of HBeAg-positive or -negative patients who are adherent to entecavir are HBV DNA negative at 5 years.24 Loss of HBsAg is 5% in entecavir-treated patients at follow-up of approximately 80 weeks, which is roughly double the rate of HBsAg loss with lamivudine.32
Telbivudine. Telbivudine has a secondary role in treatment of HBV infection. In a study by Lai et al,25,26 the cumulative incidence of telbivudine resistance and virologic breakthrough in HBeAg-positive patients rose from nearly 5% after 1 year to 22% after 2 years of treatment. Although the incidence was lower in HBeAg-negative patients, rates of genotypic resistance with virologic breakthrough rose to 9% in this population.
Since these results report genotypic resistance and virologic breakthrough, the rates of genotypic resistance in these patients may actually be higher than reported. Indeed, genotypic resistance was detected in 6.8% of the entire study population after 1 year of treatment. In this study, it must be remembered that patients with HBV DNA levels that were detectable by PCR (≥ 300 copies/mL) but were less than 1,000 copies/mL were not assessed for resistance.
Because of high rates of resistance associated with telbivudine, its role in the treatment of chronic HBV is secondary. I may use it in pregnant patients because most other nucleoside analogues are category C drugs and telbivudine is a category B agent (see “Management of hepatitis B in pregnancy: Weighing the options”). There are risks of myositis and neuropathy with telbivudine; although these risks are low, I mention them to patients when discussing a treatment plan.
Nucleotide analogues
Adefovir. Adefovir is considered second-line or add-on therapy when resistance to lamivudine develops because of its low potency in suppressing viral load. At 48 weeks, only 12% of HBeAg-positive patients are HBV DNA negative when treated with adefovir monotherapy.33,34
In a phase 3 clinical trial, genotypic resistance to adefovir was detected in 29% of HBeAg-negative patients treated for up to 5 years.27 The probability of resistance with virologic breakthrough was 3%, 8%, 14%, and 20% after 2, 3, 4, and 5 years of treatment, respectively.
In patients infected with lamivudine-resistant HBV, the probability of adefovir resistance is reduced by adding adefovir to ongoing lamivudine therapy, according to data from a large retrospective comparative study.35 In patients treated with adefovir monotherapy, the probability of virologic breakthrough (defined as > 1 log10 rebound in HBV DNA compared with on-treatment nadir) reached 30% over 36 months. In patients treated with add-on adefovir, the probability of virologic breakthrough was reduced to 6%. Similarly, the probability of adefovir resistance over 36 months of treatment was greater in the adefovir monotherapy group (16%) than in the add-on adefovir group (0%).
Although adefovir resistance is observed infrequently when adefovir is added to lamivudine, the effectiveness of adding adefovir is still limited by its low potency.
Tenofovir. More than 90% of HBeAg-negative patients and nearly 80% of HBeAg-positive patients treated with tenofovir have persistent virologic responses and HBV DNA levels less than 400 copies/mL by 72 weeks, with minimal side effects.33,34 Marcellin et al reported no development of resistance to tenofovir after 48 weeks of treatment.31 Although the nucleotide analogues have been associated with renal toxicity,36 the risk of renal toxicity associated with tenofovir is 1% or less per year; it can be reduced even further by calculating renal function through the use of the Cockroft-Gault equation or the Modification of Diet in Renal Disease equation prior to therapy and adjusting the dosage accordingly.37
With profound HBV DNA suppression, HBsAg loss occurs in about 5% of tenofovir-treated patients at 64 weeks.33
Treatment with tenofovir in treatment-experienced patients leads to potent suppression of HBV DNA independent of HBV genotype, HBV mutations (YMDD mutations) that signal lamivudine resistance, or HBeAg status at baseline.38 Patients with genotypic resistance to adefovir at baseline had a lower probability of achieving HBV DNA suppression during treatment with tenofovir.
Pegylated interferon
Pegylated interferon has proven useful in subsets of HBV DNA–positive patients. These include patients with genotype A or B who are young, those with high ALT levels (≥ 2 or 3 times the upper limit of normal) and low viral load (< 107 copies/mL), and patients without significant comorbidities.6 Pegylated interferon is also an option for patients who require a defined treatment period (eg, a woman wishing to become pregnant in 1 to 2 years). The patients who would benefit from pegylated interferon as first-line therapy must be better defined, and early markers of virologic response need to be identified.
PREVENTING AND MANAGING RESISTANCE
Antiviral drug resistance has a negative impact on the treatment of patients with chronic HBV infection. The development of resistance can result in virologic breakthrough (a confirmed 1 log10 increase in plasma HBV DNA levels)1; increased ALT levels1,39; and the progression of liver disease,40 including hepatic decompensation, development of HCC, and need for liver transplant. In addition, resistance mutations may re-emerge, with covalently closed circular DNA representing a genetic archive for development of resistance; this can significantly limit future treatment options.41 Early detection and regular monitoring are critical to prevention and management of resistance.
Detection
Detecting virologic breakthrough as early as possible increases the likelihood of achieving virologic response. In a study by Rapti and colleagues,42 patients with lamivudine-resistant chronic HBV were treated with a combination of lamivudine and adefovir. The 3-year cumulative probability of virologic response (< 103 copies/mL) was 99% with the addition of adefovir when baseline viral load levels were less than 5 log10 copies/mL, but only 71% when baseline viral loads were greater than 6 log10 copies/mL.
Monitoring
Patient response must be defined correctly. In adherent patients who show an early favorable response to therapy, I advise HBV DNA testing every 3 to 6 months. For those whose response flattens and whose viral load remains high, switching therapy or adding on should be considered. We continue therapy and monitor regularly after HBV DNA reaches an undetectable level. If the response is suboptimal, the treatment regimen is adapted by adding a new agent or switching to an alternative therapy (see “Case revisited”).
For patients who are being treated with tenofovir or entecavir, I typically extend the interval of measuring DNA levels to every 6 months because rates of resistance with these agents are low. If response is suboptimal but resistance is absent, I consider switching to the opposite drug. In those patients with a resistance mutation, I add the other agent.

Managing resistance
Combination therapy has a role in individuals in whom medication has failed to suppress viral load, in the setting of drug resistance, after liver transplant, and in individuals coinfected with HIV (see “Strategies for managing coinfection with hepatitis B virus and HIV”). If patients demonstrate resistance to their current therapy, we examine viral factors, adherence to therapy, and medication availability (eg, cost and insurance coverage). Switching to entecavir in adefovir-resistant patients produces profound suppression of HBV DNA. Patients in whom entecavir or lamivudine have failed may respond to tenofovir, depending on the resistance mutations.
A POTENTIAL FUTURE OPTION
Clevudine is a nucleoside analogue in phase 3 clinical studies in the United States. Its potential role in therapy is not yet clear. To be determined is whether it will induce a long-term, off-treatment viral response, in which case treatment may be able to be terminated earlier, and whether it will show clinically important cross-resistance with other nucleoside analogues. The availability of more sensitive assays to demonstrate the emergence of early viral resistance would enable earlier changes in treatment for more successful outcomes.
SUMMARY
Preventing resistance is crucial to the success of antiviral drug therapy for treatment of chronic HBV; a persistently high viral load increases the risk of cirrhosis and HCC, and resistance is associated with increased HBV DNA levels. The best chance for long-term success depends on initiating therapy before cirrhosis develops, when viral load is still low; profound suppression of viral load using the most potent agents as first-line therapy; and long-term monitoring of HBV DNA. The development of resistance can result in virologic breakthrough and liver complications. Entecavir and tenofovir represent the most effective first-line options to suppress HBV DNA. Because cross-resistance can occur, adding another agent is preferred to switching agents if resistance to initial therapy develops.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Liaw Y-F, Leung N, Kao J-H, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008; 2:263–283.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242.
- Chen CJ, Yang HI, Su J, et al. Serial monitoring of viral load and serum alanine aminotransferase level and the risk of hepatocellular carcinoma (HCC): R.E.V.E.A.L.-HBV study update [abstract 141]. J Hepatol 2008; 48(suppl 2):S61.
- Chen JD, Yang HI, Iloeje UH, et al. Liver disease progression in chronic hepatitis B infected persons with normal serum alanine amino transferase level: update from the R.E.V.E.A.L.-HBV study [abstract 644]. J Hepatol 2008; 48(suppl 2):S240.
- Keeffe EG, Dieterich DT, Han S-H B, et al. Special report. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008; 6:1315–1341.
- Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health Consensus Development Conference statement: management of hepatitis B. Ann Intern Med 2009; 150:104–112.
- Piton A, Poynard T, Imbert-Bismut F, et al. Factors associated with serum alanine aminotransaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC group. Hepatology 1998; 27:1213–1219.
- Kim CH, Nam CM, Jee SH, Khan KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004; 328:983–986.
- Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology 2006; 43:1145–1151.
- Puoti C, Magrini A, Stati TN, et al. Clinical, histological, and virological features of hepatitis C virus carriers with persistently normal or abnormal alanine transaminase levels. Hepatology 1997; 26:1393–1398.
- Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002; 137:1–9.
- Deniz B, Buti M, Brosa M, et al. Cost-effectiveness simulation analysis of tenofovir disoproxil fumarate, lamivudine, adefovir dipivoxil, and entecavir of HbeAg negative patients with chronic hepatitis B in Spain [EASL abstract 558]. J Hepatol 2008; 48(suppl 2):S209.
- Deniz B, Everhard R. Cost-effectiveness simulation analysis of tenofovir disoproxil fumarate in HBeAg negative patients with chronic hepatitis B in Italy and France [EASL abstract 559]. J Hepatol 2008; 48(suppl 2):S210.
- Kim W, Benson JT, Hindman A, Brosgart C, Fortner-Burton C. Decline in the need for liver transplantation for end stage liver disease secondary to hepatitis B in the US. Paper presented at: 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. Abstract 12.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–700.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl 2003; 9:684–692.
- Lai CL, Ratziu V, Yuen M-F, Poynard T. Viral hepatitis B. Lancet 2003; 362:2089–2094.
- Leung NW, Lai C-L, Chang T-T, et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 2001; 33:1527–1532.
- Benhamou Y, Bochet M, Thibault V, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology 1999; 30:1302–1306.
- Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125:1714–1722.
- Colonno RJ, Rose R, Baldick CJ, et al. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology 2006; 44:1656–1665.
- Perrillo RP. Current treatment of chronic hepatitis B: benefits and limitations. Semin Liver Dis 2005; 25(suppl 1):20–28.
- Lai C-L, Gane E, Liaw Y-F, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 2007; 357:2576–2588.
- Lai C-L, Gane E, Hsu C-W, et al. Two-year results from the GLOBE trial in patients with hepatitis B: greater clinical and antiviral efficacy for telbivudine (LDT) vs. lamivudine [AASLD abstract 91]. Hepatology 2006; 44(suppl 1):222A.
- Locarnini S, Qi X, Arterburn S, et al. Incidence and predictors of emergence of adefovir resistant HBV during four years of adefovir dipivoxil therapy for patients with chronic hepatitis B [EASL abstract 36]. J Hepatol 2005; 42(suppl 2):17.
- Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al; Adefovir Dipivoxil 438 Study Group. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med 2005; 352:2673–2681.
- Hepsera [package insert]. Foster City, CA: Gilead Sciences, Inc; 2008.
- Lee YS, Suh DJ, Lim YS, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology 2006; 43:1385–1391.
- Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–2455.
- Gish R, Chang T-T, Lai C-L, et al. Hepatitis B surface antigen loss in antiviral-treated patients with HBeAg(+) chronic hepatitis B infection: observations from antiviral-naïve patients treated with entecavir or lamivudine. Paper presented at: 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. Abstract 992.
- Heathcote J, George J, Gordon S, et al. Tenofovir disoproxil fumarate (TDF) for the treatment of HBeAg-positive chronic hepatitis B: week 72 TDF data and week 24 adefovir dipivoxil switch data (study 103) [EASL abstract 71]. J Hepatol 2008; 48(suppl 2):S32.
- Marcellin P, Jacobson I, Habersetzer F, et al. Tenofovir disoproxil fumarate (TDF) for the treatment of HBeAg-negative chronic hepatitis B: week 72 TDF data and week 24 adefovir dipivoxil switch data (study 102) [EASL abstract 57]. J Hepatol 2008; 48(suppl 2):S26.
- Lampertico P, Marzano A, Levrero M, et al. Adefovir and lamivudine combination therapy is superior to adefovir monotherapy for lamivudine-resistant patients with HBeAg-negative chronic hepatitis B [EASL abstract 502]. J Hepatol 2007; 46(suppl 1):S191.
- Ha NB, Ha NB, Trinh HN. Changes in creatinine clearance (CRCL) in chronic hepatitis B (CHB) patients treated with adefovir dipivoxil (ADV) [AASLD abstract 901]. Hepatology 2008; 48:709A–710A.
- Gallant J, Staszewski S, Pozniak AL, et al; for the 903 Study Team. Similar renal safety profile between tenofovir DF (TDF) and stavudine (d4T) using modification of diet in renal disease (MDRD) and Cockcroft-Gault (CG) estimations of glomerular filtration rate (GFR) in antiretroviral-naïve patients through 144 weeks. In: Program and Abstracts of the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19, 2005; Washington, DC. Abstract H-350.
- van Bömmel F, de Man RA, Stein K, et al. A multicenter analysis of antiviral response after one year of tenofovir monotherapy in HBV-monoinfected patients with prior nucleos(t)ide analog experience [EASL abstract 73]. J Hepatol 2008; 48(suppl 2):S32.
- Fung SK, Lok AS. Management of hepatitis B patients with antiviral resistance. Antivir Ther 2004; 9:1013–1026.
- Gish RG. Chronic hepatitis B virus: treating patients to prevent and manage resistance. US Gastroenterology Review 2007; March:51–54.
- Zoulim F. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antiviral Res 2004; 64:1–15.
- Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 2007; 45:307–313.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Liaw Y-F, Leung N, Kao J-H, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008; 2:263–283.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242.
- Chen CJ, Yang HI, Su J, et al. Serial monitoring of viral load and serum alanine aminotransferase level and the risk of hepatocellular carcinoma (HCC): R.E.V.E.A.L.-HBV study update [abstract 141]. J Hepatol 2008; 48(suppl 2):S61.
- Chen JD, Yang HI, Iloeje UH, et al. Liver disease progression in chronic hepatitis B infected persons with normal serum alanine amino transferase level: update from the R.E.V.E.A.L.-HBV study [abstract 644]. J Hepatol 2008; 48(suppl 2):S240.
- Keeffe EG, Dieterich DT, Han S-H B, et al. Special report. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008; 6:1315–1341.
- Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health Consensus Development Conference statement: management of hepatitis B. Ann Intern Med 2009; 150:104–112.
- Piton A, Poynard T, Imbert-Bismut F, et al. Factors associated with serum alanine aminotransaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC group. Hepatology 1998; 27:1213–1219.
- Kim CH, Nam CM, Jee SH, Khan KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 2004; 328:983–986.
- Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology 2006; 43:1145–1151.
- Puoti C, Magrini A, Stati TN, et al. Clinical, histological, and virological features of hepatitis C virus carriers with persistently normal or abnormal alanine transaminase levels. Hepatology 1997; 26:1393–1398.
- Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002; 137:1–9.
- Deniz B, Buti M, Brosa M, et al. Cost-effectiveness simulation analysis of tenofovir disoproxil fumarate, lamivudine, adefovir dipivoxil, and entecavir of HbeAg negative patients with chronic hepatitis B in Spain [EASL abstract 558]. J Hepatol 2008; 48(suppl 2):S209.
- Deniz B, Everhard R. Cost-effectiveness simulation analysis of tenofovir disoproxil fumarate in HBeAg negative patients with chronic hepatitis B in Italy and France [EASL abstract 559]. J Hepatol 2008; 48(suppl 2):S210.
- Kim W, Benson JT, Hindman A, Brosgart C, Fortner-Burton C. Decline in the need for liver transplantation for end stage liver disease secondary to hepatitis B in the US. Paper presented at: 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. Abstract 12.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–700.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl 2003; 9:684–692.
- Lai CL, Ratziu V, Yuen M-F, Poynard T. Viral hepatitis B. Lancet 2003; 362:2089–2094.
- Leung NW, Lai C-L, Chang T-T, et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 2001; 33:1527–1532.
- Benhamou Y, Bochet M, Thibault V, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology 1999; 30:1302–1306.
- Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125:1714–1722.
- Colonno RJ, Rose R, Baldick CJ, et al. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology 2006; 44:1656–1665.
- Perrillo RP. Current treatment of chronic hepatitis B: benefits and limitations. Semin Liver Dis 2005; 25(suppl 1):20–28.
- Lai C-L, Gane E, Liaw Y-F, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 2007; 357:2576–2588.
- Lai C-L, Gane E, Hsu C-W, et al. Two-year results from the GLOBE trial in patients with hepatitis B: greater clinical and antiviral efficacy for telbivudine (LDT) vs. lamivudine [AASLD abstract 91]. Hepatology 2006; 44(suppl 1):222A.
- Locarnini S, Qi X, Arterburn S, et al. Incidence and predictors of emergence of adefovir resistant HBV during four years of adefovir dipivoxil therapy for patients with chronic hepatitis B [EASL abstract 36]. J Hepatol 2005; 42(suppl 2):17.
- Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al; Adefovir Dipivoxil 438 Study Group. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med 2005; 352:2673–2681.
- Hepsera [package insert]. Foster City, CA: Gilead Sciences, Inc; 2008.
- Lee YS, Suh DJ, Lim YS, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology 2006; 43:1385–1391.
- Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–2455.
- Gish R, Chang T-T, Lai C-L, et al. Hepatitis B surface antigen loss in antiviral-treated patients with HBeAg(+) chronic hepatitis B infection: observations from antiviral-naïve patients treated with entecavir or lamivudine. Paper presented at: 58th Annual Meeting of the American Association for the Study of Liver Diseases; November 2–6, 2007; Boston, MA. Abstract 992.
- Heathcote J, George J, Gordon S, et al. Tenofovir disoproxil fumarate (TDF) for the treatment of HBeAg-positive chronic hepatitis B: week 72 TDF data and week 24 adefovir dipivoxil switch data (study 103) [EASL abstract 71]. J Hepatol 2008; 48(suppl 2):S32.
- Marcellin P, Jacobson I, Habersetzer F, et al. Tenofovir disoproxil fumarate (TDF) for the treatment of HBeAg-negative chronic hepatitis B: week 72 TDF data and week 24 adefovir dipivoxil switch data (study 102) [EASL abstract 57]. J Hepatol 2008; 48(suppl 2):S26.
- Lampertico P, Marzano A, Levrero M, et al. Adefovir and lamivudine combination therapy is superior to adefovir monotherapy for lamivudine-resistant patients with HBeAg-negative chronic hepatitis B [EASL abstract 502]. J Hepatol 2007; 46(suppl 1):S191.
- Ha NB, Ha NB, Trinh HN. Changes in creatinine clearance (CRCL) in chronic hepatitis B (CHB) patients treated with adefovir dipivoxil (ADV) [AASLD abstract 901]. Hepatology 2008; 48:709A–710A.
- Gallant J, Staszewski S, Pozniak AL, et al; for the 903 Study Team. Similar renal safety profile between tenofovir DF (TDF) and stavudine (d4T) using modification of diet in renal disease (MDRD) and Cockcroft-Gault (CG) estimations of glomerular filtration rate (GFR) in antiretroviral-naïve patients through 144 weeks. In: Program and Abstracts of the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19, 2005; Washington, DC. Abstract H-350.
- van Bömmel F, de Man RA, Stein K, et al. A multicenter analysis of antiviral response after one year of tenofovir monotherapy in HBV-monoinfected patients with prior nucleos(t)ide analog experience [EASL abstract 73]. J Hepatol 2008; 48(suppl 2):S32.
- Fung SK, Lok AS. Management of hepatitis B patients with antiviral resistance. Antivir Ther 2004; 9:1013–1026.
- Gish RG. Chronic hepatitis B virus: treating patients to prevent and manage resistance. US Gastroenterology Review 2007; March:51–54.
- Zoulim F. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antiviral Res 2004; 64:1–15.
- Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 2007; 45:307–313.
KEY POINTS
- Consider treatment for chronic HBV infection for all patients who are positive for HBV DNA, as viral load levels as low as 300 copies/mL confer a risk for hepatocellular carcinoma.
- The goal of therapy is an undetectable level of HBV DNA; initiate therapy with the most potent agent to limit the possibility of resistance.
- Preventing resistance to therapy is crucial for successful treatment of chronic HBV infection.
Monotherapy vs multiple-drug therapy: The experts debate
Monotherapy for treatment-naïve patients
By Robert G. Gish, MD
Powerful antiviral medicines with activity against hepatitis B virus (HBV) have long-term records of potency and safety, supporting the case for monotherapy in treatment-naïve patients. Combination therapy has a limited role in the management of HBV infection; if the approach to treatment is rational from the start, then combination therapy can be reserved for cases of treatment failure or resistance.
THE CASE FOR MONOTHERAPY
Three arguments that favor monotherapy with potent medications are cost, low risk of resistance, and unproven benefit of combination therapy.
Cost
The cost of dual-medication therapy is nearly double that of single-drug therapy, while the benefit is unknown in treatment-naïve patients. My choices for first-line therapy are tenofovir or entecavir, highly potent nucleoside/nucleotide analogues that can cost up to $5,500 and $8,000, respectively, per year of treatment.1 The two in combination would cost nearly $14,000 per year, and benefits have not been proven in the treatment-naïve population.
Low risk of resistance
Potent medications have low rates of resistance, in the range of 1% over 2 to 5 years.2–4 If one starts therapy with the highly potent entecavir, discussions about switching or adding on therapy would be superfluous because of the low rates of resistance and failure associated with entecavir monotherapy. At 5 years, the cumulative rate of entecavir resistance in patients with positive HBV DNA at baseline is 1.2%.5 Tenofovir also produces potent inhibition of HBV DNA and is associated with low rates of resistance,6 although follow-up data with tenofovir extend only to 2 years. Starting therapy with the less potent adefovir, followed by the development of resistance, decreases the probability that tenofovir will achieve HBV DNA suppression during treatment.7 The main driver of resistance is nonadherence with therapy, not treatment failure.
Resistance to pegylated interferon has not been encountered. The therapy is limited in duration (24 to 48 weeks), with durable suppression of HBV DNA and high rates of seroconversion from hepatitis B e antigen (HBeAg)-positive to HBeAg-negative status. Parameters for the use of pegylated interferon as first-line therapy have been established, and include patients with genotype A or B who are young, have HBV DNA levels less than 107 copies/mL, have serum alanine aminotransferase (ALT) levels two to three times the upper limit of normal, and lack significant comorbidities.3,4
Unproven benefit of combination therapy
Perhaps the most convincing argument against combination therapy is that numerous studies of combinations have failed to demonstrate a benefit compared with monotherapy in treatment-naïve patients:
- Interferon in combination with lamivudine has not been shown to be significantly more effective than lamivudine monotherapy.8,9 Further, because of limited information on the safety of interferon in combination with nucleoside or nucleotide analogues, use of the combination is not recommended.4 Neuropathy has been reported with the combination of interferon and telbivudine,4 leading to the release of a warning about its use.10
- A 1-year trial by Lai et al failed to show an improvement in virologic and biochemical responses with the combination of telbivudine and lamivudine compared with telbivudine alone.11
- In patients with lamivudine-resistant chronic HBV infection, adefovir reduced serum HBV DNA levels by 4 weeks whether or not lamivudine therapy was ongoing.12
- Although more patients taking a combination of adefovir and the nucleoside reverse transcriptase inhibitor emtricitabine had normalization of ALT and suppression of HBV DNA to less than 300 copies compared with adefovir monotherapy, rates of HBeAg seroconversion were comparable in the two arms.13
- A recent study that compared tenofovir monotherapy with tenofovir and emtricitabine in combination showed comparable effectiveness for both regimens; the authors concluded that further study is necessary before either choice can be recommended as superior to the other.14
RESISTANCE: IDENTIFY EARLY, ADD ON
To minimize the likelihood of resistance and its impact, HBV DNA levels should be monitored every 3 months; at the first sign of a virologic breakthrough, therapy should be added or switched. Resistance to lamivudine is apparent early; models of treatment response indicate that resistance to lamivudine is likely if HBV DNA does not become undetectable by week 4.
In cases of lamivudine failure, adding adefovir early, when the viral load is less than 107 copies/mL, increases the probability of a virologic response.15 In the situation of lamivudine failure, I prefer adding on to switching to reduce the risk of resistance—a practice supported by the study just cited.15 In lamivudine-resistant patients, adefovir monotherapy was associated with virologic breakthrough and resistance to adefovir in 21% of patients, whereas no patient experienced virologic breakthrough or resistance when adefovir was added to lamivudine.
Successful management involves choosing the best medication up front and educating patients about the importance of taking their medication as instructed. For example, entecavir should be taken without food to maximize its bioavailability. With tenofovir, the risk of renal toxicity is low (1%),16 and can be reduced even further with a pretreatment assessment of the patient.
Multiple-drug therapy is the wave of the future
By Pierre M. Gholam, MD
A concise rationale for multiple-drug therapy is that resistance to monotherapy will occur eventually, with serious consequences in some patients and grave public health implications over the long term. Data from France and Australia indicate that multidrug-resistant HBV is a reality in individual cases. Resistance may be less likely when combinations are used, although little evidence exists at present to support this contention.
COMBINATION THERAPY IS COMMON SENSE
Much of the evidence supporting combination therapy for HBV is common sense:
- Most patients with HBV infection require treatment indefinitely, and duration of therapy that is not finite will inevitably lead to resistance.
- Your first shot is your best shot. Once resistance develops, treatment response will eventually decline.
- Sometimes the stakes are too high to risk breakthroughs. In particular, in patients who have cirrhosis and in those awaiting or following liver transplant, flares and recurrences can have disastrous consequences.
Treatment duration and resistance
As Dr. Gish demonstrated, tenofovir and entecavir are highly potent drugs that suppress viral loads effectively and have high genetic barriers to resistance. On an intent-to-treat basis, HBV DNA levels below the threshold level of detection are achieved at impressive rates with tenofovir and entecavir at 2 years in patients who are either HBeAg negative or positive.5,6,17 When the analyses are limited to patients who actually received the drugs, suppression of HBV DNA to undetectable levels exceeds 90%. Resistance to tenofovir is 0% at 2 years,3 and resistance to entecavir is 1.2% at 5 years.5
Although such data appear to favor monotherapy, most HBV-infected patients who commit to treatment will be treated indefinitely; this applies to patients who are HBeAg negative, who constitute most HBV-infected individuals in the United States and worldwide, or HBeAg positive. There are no established end points for treatment termination in HBeAg-negative patients. The only treatment termination end point that is deemed acceptable in HBeAg-positive patients is a period 6 to 12 months after the loss of HBeAg and the development of antibody to HBeAg, or e antigen seroconversion. Even after many years of treatment that includes the first-line agents tenofovir and entecavir, the likelihood of achieving this end point is fairly low.2,5,18
Adherence is also a consideration. Studies of patients with hypertension, heart disease, and other chronic diseases have shown that strict adherence to therapy over decades is unlikely. The same adherence pattern probably applies to the treatment of chronic HBV infection.
Antiviral drugs used in the treatment of chronic HBV infection are associated with certain resistance mutations that confer additional risk of developing resistance to a subsequent drug. Furthermore, with indefinite duration of therapy, it is realistic to expect that resistance will develop.
Other factors play roles in the development of resistance:
- Mutant viruses. We do not fully understand the potential problem of transmission of mutant viruses. This phenomenon is becoming apparent in endemic areas where treatment-naïve patients harbor mutant viruses acquired through sexual contact with HBV-infected patients who have been treated and in whom the virus has subsequently mutated.
- Barriers to resistance. The genetic barrier to resistance for a single drug will eventually be overcome. It may take longer than it took for adefovir, which is associated with a 30% rate of resistance at 5 years.3 It may take a much longer time for entecavir or tenofovir, but resistance is a biological certainty and we need to contend with it. With human immunodeficiency virus (HIV) infection, we are able to genotype for mutations and tailor treatment accordingly. This strategy is not currently recommended for HBV infection, partly because it is expensive and not routinely available.
- Misuse of therapy. Finally, wider use of antiviral agents for the treatment of HBV may lead to wider misuse, and therefore more resistance. Realistically, not every practitioner will start therapy with entecavir or tenofovir; many of the less potent agents have associated rates of resistance, and these in turn may confer an additional risk of resistance if tenofovir or entecavir is eventually used.
Declining response
Colonno et al19 studied the likelihood of entecavir resistance developing in patients with existing lamivudine resistance. The likelihood of resistance to entecavir at 3 years was 1.2% among patients who had never been exposed to lamivudine. Among patients in whom lamivudine resistance had developed and who were subsequently started on entecavir, resistance to entecavir was 32% at 3 years.19 Resistance has consequences; 25% of lamivudine-resistant patients develop viral breakthrough.
Dr. Gish and I agree that the addition of adefovir to lamivudine is better than switching to adefovir monotherapy in the case of lamivudine failure. Compared with switching, the adefovir-lamivudine combination leads to a lower incidence of virologic breakthrough, a lower likelihood of adefovir resistance over time, a greater probability of achieving undetectable levels of HBV DNA (< 35 copies/mL), and a lower cumulative rate of resistance.20 The superiority of combination therapy in achieving undetectable levels of HBV DNA confers a lower risk of developing resistance over time; by year 4, the likelihood of adefovir resistance is only 4% among lamivudine-resistant patients treated with the combination of adefovir and lamivudine.20
In a study of nucleoside analogue–experienced patients who did not achieve viral suppression, response to tenofovir, defined as HBV DNA less than 400 copies/mL at month 12, was 85% overall and only 30% in adefovir-resistant patients.7 These data demonstrate that, if not starting with combination therapy, it is preferable to initiate treatment with a potent drug that is highly successful at HBV DNA suppression. A second monotherapy will be less successful than the initial attempt.
Consequences of resistance
The consequences of resistance in patients with cirrhosis are significant, prompting strong consideration of combination therapy as a potential means to avoid resistance.
One consequence is a well-documented potential for decompensation in the setting of new-onset resistance as a result of flares. Another is post-transplantation recurrence of HBV, leading to poor outcomes. These risks converge in the patient who is awaiting liver transplantation, in whom combination therapy seems to make the most sense to prevent the development of a flare and a recurrence of HBV infection after transplantation.
WHO SHOULD RECEIVE MULTIPLE-DRUG THERAPY?
The American Association for the Study of Liver Diseases recommends combination therapy as the preferred rescue therapy for primary failure of a first-line agent, citing the possibility of resistance with switching in some circumstances and the superiority of adding on as opposed to switching.2 No data clearly support de novo multiple-drug therapy. Although a number of studies have failed to show an advantage of combination therapy over monotherapy, they were of relatively short duration and focused primarily on viral suppression rather than the occurrence of resistance over time. Long-term studies are needed to determine whether combination therapy is an option de novo.
De novo multiple-drug therapy might be reasonable if a patient is at high risk for resistance—for example, for patients with extraordinarily high levels of HBV DNA or in whom resistance can lead to dire consequences, such as patients with cirrhosis or pretransplant patients.
The HIV pandemic serves as a paradigm for combination therapy. Many agents used to treat HBV infection also have anti-HIV effects; their use as monotherapy should be avoided in order to prevent the development of HIV drug resistance. HIV regimens that include only one HBV antiviral agent with a low genetic barrier to resistance (eg, lamivudine) should also be avoided in order to minimize the risk of HBV drug resistance.
I agree with Dr. Gish that cost and potential toxicity, especially renal toxicity, may limit the widespread use of combination therapies.
Discussion
William D. Carey, MD: I hear more agreement than not between the debaters. Are there any comments from the panel?
Morris Sherman, MD, PhD: I’ll comment on the guidelines for the treatment of HBV infection. Tong et al21 recently examined whether a group of HBV-infected patients who developed cirrhosis and hepatoma would have qualified for treatment under four current sets of guidelines. A startlingly large proportion of patients who developed adverse consequences from their liver disease would not have met the criteria for treatment under any of these major guidelines. As many as one-fourth of patients with chronic HBV infection die as a consequence of their liver disease, and in order to prevent these deaths up to one-half of the patients have to be treated. In the long run, overtreatment may be preferable to undertreatment to reduce the incidence of hepatitis-related deaths. My point is that the treatment guidelines probably exclude many patients who should be treated.
The factors I consider important in my decision to treat are a high viral load, which is indicative of active viral replication, and evidence of liver injury. Patients who have a high viral load and no liver injury won’t experience complications. What do I consider evidence of liver injury? Prolonged elevation of ALT is suggestive, although not necessarily as high as 200 or 300 U/L; it could be in the range of 50 to 80 U/L if fibrosis is significant, which I define as stage 2 or greater on the biopsy. If a high viral load and evidence of significant liver injury are present, I treat the patient regardless of the precise level of the viral load or the ALT.
Dr. Carey: Can you clarify your position? Some of our earlier discussion emphasized the importance of treating when the viral load is high, regardless of other factors. A high viral load by itself may be associated with increased risk of cirrhosis or hepatocellular carcinoma without cirrhosis, so why would a biopsy make a difference?
Dr. Sherman: We can’t predict which younger HBeAg-positive patients with a very high viral load are going to run into trouble down the road. Many will seroconvert spontaneously and never have problems thereafter. In contrast, a patient in his 40s with a high viral load, even if HBeAg positive, and without major fibrosis should be considered for therapy. I tell my patients and the physicians who refer them that once I’m finished with the evaluation, it’s not good-bye. They have to be followed for life because things change.
Tram T. Tran, MD: In the paper by Tong et al,21 all of the patients who subsequently had poor outcomes had low platelet counts. I therefore recommend considering the entire picture in the decision to treat. If physicians followed the treatment guidelines strictly, they would not have treated those patients, but had they noticed thrombocytopenia they would have considered the possibility of advanced fibrosis and considered screening or a biopsy.
- Wong JB. Costs of antiviral therapy of chronic hepatitis B. Paper presented at: Management of Hepatitis B: 2006. National Institutes of Health Workshop. April 6–8, 2006; Bethesda, MD.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Keeffe EG, Dieterich DT, Han S-H B, et al. Special report. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008; 6:1315–1341.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242.
- Tenney DJ, Pokomowski KA, Rose RE, et al. Entecavir at five years shows long-term maintenance of high genetic barrier to hepatitis B virus resistance [abstract OL-107]. Hepatol Int 2008; 2:S76–S77.
- Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–2455.
- van Bömmel F, de Man RA, Stein K, et al. A multicenter analysis of antiviral response after one year of tenofovir monotherapy in HBV-monoinfected patients with prior nucleos(t)ide analog experience [EASL abstract 73]. J Hepatol 2008; 48(suppl 2):S32.
- Schalm SW, Heathcote J, Cianciara J, et al. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomized trial. Gut 2000; 46:562–568.
- Chan HL-Y, Leung NW-Y, Hui AY, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-a2b and lamivudine with lamivudine alone. Ann Intern Med 2005; 142:240–250.
- Novartis Pharmaceuticals Canada Inc. Risk of peripheral neuropathy in patients treated with telbivudine (SEBIVO®) in combination with interferon. Health Canada Web site. http://www.hc-sc.gc.ca/dhp-mps/alt_formats/hpfb-dgpsa/pdf/medeff/sebivo_pc-cp-eng.pdf. March 12, 2008. Accessed March 12, 2009.
- Lai C-L, Leung N, Teo E-K, et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology 2005; 129:528–536.
- Peters MG, Hann HW, Martin P, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 2004; 126:91–101.
- Hui C-K, Zhang H-Y, Bowden S, et al. 96 weeks combination of adefovir dipivoxil plus emtricitabine vs. adefovir dipivoxil monotherapy in the treatment of chronic hepatitis B. J Hepatol 2008; 48:714–720.
- Berg T, Moller B, Trinh H, et al. Tenofovir disoproxil fumarate (TDF) versus emtricitabine plus TDF for treatment of chronic hepatitis B (CHB) in subjects with persistent viral replication receiving adefovir dipivoxil (ADV). Paper presented at: 43rd Annual Meeting of the European Association for the Study of the Liver; April 23–27, 2008; Milan, Italy.
- Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 2007; 45:307–313.
- Szczech LA. Tenofovir nephrotoxicity: focusing research questions and putting them into clinical context. J Infect Dis 2008; 197:7–9.
- Shouval D, Lai C-L, Chang T-T, et al. Three years of entecavir (ETV) retreatment of HBeAg(–) ETV patients who previously discontinued treatment: results from study ETV-901. Poster presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA. Poster 927.
- Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125:1714–1722.
- Colonno RJ, Rose RE, Pokornowski K, Baldick CJ, Klesczewski K, Tenney D. Assessment at three years shows high barrier to resistance is maintained in entecavir-treated nucleoside naïve patients while resistance emergence increases over time in lamivudine refractory patients [AASLD abstract 110]. Hepatology 2006; 44(suppl 1):229A–230A.
- Lampertico P, Viganò M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology 2007; 133:1445–1451.
- Tong MJ, Hsien C, Hsu L, Sun HE, Blatt LM. Treatment recommendations for chronic hepatitis B: an evaluation of current guidelines based on a natural history study in the United States. Hepatology 2008; 48:1070–1078.
Monotherapy for treatment-naïve patients
By Robert G. Gish, MD
Powerful antiviral medicines with activity against hepatitis B virus (HBV) have long-term records of potency and safety, supporting the case for monotherapy in treatment-naïve patients. Combination therapy has a limited role in the management of HBV infection; if the approach to treatment is rational from the start, then combination therapy can be reserved for cases of treatment failure or resistance.
THE CASE FOR MONOTHERAPY
Three arguments that favor monotherapy with potent medications are cost, low risk of resistance, and unproven benefit of combination therapy.
Cost
The cost of dual-medication therapy is nearly double that of single-drug therapy, while the benefit is unknown in treatment-naïve patients. My choices for first-line therapy are tenofovir or entecavir, highly potent nucleoside/nucleotide analogues that can cost up to $5,500 and $8,000, respectively, per year of treatment.1 The two in combination would cost nearly $14,000 per year, and benefits have not been proven in the treatment-naïve population.
Low risk of resistance
Potent medications have low rates of resistance, in the range of 1% over 2 to 5 years.2–4 If one starts therapy with the highly potent entecavir, discussions about switching or adding on therapy would be superfluous because of the low rates of resistance and failure associated with entecavir monotherapy. At 5 years, the cumulative rate of entecavir resistance in patients with positive HBV DNA at baseline is 1.2%.5 Tenofovir also produces potent inhibition of HBV DNA and is associated with low rates of resistance,6 although follow-up data with tenofovir extend only to 2 years. Starting therapy with the less potent adefovir, followed by the development of resistance, decreases the probability that tenofovir will achieve HBV DNA suppression during treatment.7 The main driver of resistance is nonadherence with therapy, not treatment failure.
Resistance to pegylated interferon has not been encountered. The therapy is limited in duration (24 to 48 weeks), with durable suppression of HBV DNA and high rates of seroconversion from hepatitis B e antigen (HBeAg)-positive to HBeAg-negative status. Parameters for the use of pegylated interferon as first-line therapy have been established, and include patients with genotype A or B who are young, have HBV DNA levels less than 107 copies/mL, have serum alanine aminotransferase (ALT) levels two to three times the upper limit of normal, and lack significant comorbidities.3,4
Unproven benefit of combination therapy
Perhaps the most convincing argument against combination therapy is that numerous studies of combinations have failed to demonstrate a benefit compared with monotherapy in treatment-naïve patients:
- Interferon in combination with lamivudine has not been shown to be significantly more effective than lamivudine monotherapy.8,9 Further, because of limited information on the safety of interferon in combination with nucleoside or nucleotide analogues, use of the combination is not recommended.4 Neuropathy has been reported with the combination of interferon and telbivudine,4 leading to the release of a warning about its use.10
- A 1-year trial by Lai et al failed to show an improvement in virologic and biochemical responses with the combination of telbivudine and lamivudine compared with telbivudine alone.11
- In patients with lamivudine-resistant chronic HBV infection, adefovir reduced serum HBV DNA levels by 4 weeks whether or not lamivudine therapy was ongoing.12
- Although more patients taking a combination of adefovir and the nucleoside reverse transcriptase inhibitor emtricitabine had normalization of ALT and suppression of HBV DNA to less than 300 copies compared with adefovir monotherapy, rates of HBeAg seroconversion were comparable in the two arms.13
- A recent study that compared tenofovir monotherapy with tenofovir and emtricitabine in combination showed comparable effectiveness for both regimens; the authors concluded that further study is necessary before either choice can be recommended as superior to the other.14
RESISTANCE: IDENTIFY EARLY, ADD ON
To minimize the likelihood of resistance and its impact, HBV DNA levels should be monitored every 3 months; at the first sign of a virologic breakthrough, therapy should be added or switched. Resistance to lamivudine is apparent early; models of treatment response indicate that resistance to lamivudine is likely if HBV DNA does not become undetectable by week 4.
In cases of lamivudine failure, adding adefovir early, when the viral load is less than 107 copies/mL, increases the probability of a virologic response.15 In the situation of lamivudine failure, I prefer adding on to switching to reduce the risk of resistance—a practice supported by the study just cited.15 In lamivudine-resistant patients, adefovir monotherapy was associated with virologic breakthrough and resistance to adefovir in 21% of patients, whereas no patient experienced virologic breakthrough or resistance when adefovir was added to lamivudine.
Successful management involves choosing the best medication up front and educating patients about the importance of taking their medication as instructed. For example, entecavir should be taken without food to maximize its bioavailability. With tenofovir, the risk of renal toxicity is low (1%),16 and can be reduced even further with a pretreatment assessment of the patient.
Multiple-drug therapy is the wave of the future
By Pierre M. Gholam, MD
A concise rationale for multiple-drug therapy is that resistance to monotherapy will occur eventually, with serious consequences in some patients and grave public health implications over the long term. Data from France and Australia indicate that multidrug-resistant HBV is a reality in individual cases. Resistance may be less likely when combinations are used, although little evidence exists at present to support this contention.
COMBINATION THERAPY IS COMMON SENSE
Much of the evidence supporting combination therapy for HBV is common sense:
- Most patients with HBV infection require treatment indefinitely, and duration of therapy that is not finite will inevitably lead to resistance.
- Your first shot is your best shot. Once resistance develops, treatment response will eventually decline.
- Sometimes the stakes are too high to risk breakthroughs. In particular, in patients who have cirrhosis and in those awaiting or following liver transplant, flares and recurrences can have disastrous consequences.
Treatment duration and resistance
As Dr. Gish demonstrated, tenofovir and entecavir are highly potent drugs that suppress viral loads effectively and have high genetic barriers to resistance. On an intent-to-treat basis, HBV DNA levels below the threshold level of detection are achieved at impressive rates with tenofovir and entecavir at 2 years in patients who are either HBeAg negative or positive.5,6,17 When the analyses are limited to patients who actually received the drugs, suppression of HBV DNA to undetectable levels exceeds 90%. Resistance to tenofovir is 0% at 2 years,3 and resistance to entecavir is 1.2% at 5 years.5
Although such data appear to favor monotherapy, most HBV-infected patients who commit to treatment will be treated indefinitely; this applies to patients who are HBeAg negative, who constitute most HBV-infected individuals in the United States and worldwide, or HBeAg positive. There are no established end points for treatment termination in HBeAg-negative patients. The only treatment termination end point that is deemed acceptable in HBeAg-positive patients is a period 6 to 12 months after the loss of HBeAg and the development of antibody to HBeAg, or e antigen seroconversion. Even after many years of treatment that includes the first-line agents tenofovir and entecavir, the likelihood of achieving this end point is fairly low.2,5,18
Adherence is also a consideration. Studies of patients with hypertension, heart disease, and other chronic diseases have shown that strict adherence to therapy over decades is unlikely. The same adherence pattern probably applies to the treatment of chronic HBV infection.
Antiviral drugs used in the treatment of chronic HBV infection are associated with certain resistance mutations that confer additional risk of developing resistance to a subsequent drug. Furthermore, with indefinite duration of therapy, it is realistic to expect that resistance will develop.
Other factors play roles in the development of resistance:
- Mutant viruses. We do not fully understand the potential problem of transmission of mutant viruses. This phenomenon is becoming apparent in endemic areas where treatment-naïve patients harbor mutant viruses acquired through sexual contact with HBV-infected patients who have been treated and in whom the virus has subsequently mutated.
- Barriers to resistance. The genetic barrier to resistance for a single drug will eventually be overcome. It may take longer than it took for adefovir, which is associated with a 30% rate of resistance at 5 years.3 It may take a much longer time for entecavir or tenofovir, but resistance is a biological certainty and we need to contend with it. With human immunodeficiency virus (HIV) infection, we are able to genotype for mutations and tailor treatment accordingly. This strategy is not currently recommended for HBV infection, partly because it is expensive and not routinely available.
- Misuse of therapy. Finally, wider use of antiviral agents for the treatment of HBV may lead to wider misuse, and therefore more resistance. Realistically, not every practitioner will start therapy with entecavir or tenofovir; many of the less potent agents have associated rates of resistance, and these in turn may confer an additional risk of resistance if tenofovir or entecavir is eventually used.
Declining response
Colonno et al19 studied the likelihood of entecavir resistance developing in patients with existing lamivudine resistance. The likelihood of resistance to entecavir at 3 years was 1.2% among patients who had never been exposed to lamivudine. Among patients in whom lamivudine resistance had developed and who were subsequently started on entecavir, resistance to entecavir was 32% at 3 years.19 Resistance has consequences; 25% of lamivudine-resistant patients develop viral breakthrough.
Dr. Gish and I agree that the addition of adefovir to lamivudine is better than switching to adefovir monotherapy in the case of lamivudine failure. Compared with switching, the adefovir-lamivudine combination leads to a lower incidence of virologic breakthrough, a lower likelihood of adefovir resistance over time, a greater probability of achieving undetectable levels of HBV DNA (< 35 copies/mL), and a lower cumulative rate of resistance.20 The superiority of combination therapy in achieving undetectable levels of HBV DNA confers a lower risk of developing resistance over time; by year 4, the likelihood of adefovir resistance is only 4% among lamivudine-resistant patients treated with the combination of adefovir and lamivudine.20
In a study of nucleoside analogue–experienced patients who did not achieve viral suppression, response to tenofovir, defined as HBV DNA less than 400 copies/mL at month 12, was 85% overall and only 30% in adefovir-resistant patients.7 These data demonstrate that, if not starting with combination therapy, it is preferable to initiate treatment with a potent drug that is highly successful at HBV DNA suppression. A second monotherapy will be less successful than the initial attempt.
Consequences of resistance
The consequences of resistance in patients with cirrhosis are significant, prompting strong consideration of combination therapy as a potential means to avoid resistance.
One consequence is a well-documented potential for decompensation in the setting of new-onset resistance as a result of flares. Another is post-transplantation recurrence of HBV, leading to poor outcomes. These risks converge in the patient who is awaiting liver transplantation, in whom combination therapy seems to make the most sense to prevent the development of a flare and a recurrence of HBV infection after transplantation.
WHO SHOULD RECEIVE MULTIPLE-DRUG THERAPY?
The American Association for the Study of Liver Diseases recommends combination therapy as the preferred rescue therapy for primary failure of a first-line agent, citing the possibility of resistance with switching in some circumstances and the superiority of adding on as opposed to switching.2 No data clearly support de novo multiple-drug therapy. Although a number of studies have failed to show an advantage of combination therapy over monotherapy, they were of relatively short duration and focused primarily on viral suppression rather than the occurrence of resistance over time. Long-term studies are needed to determine whether combination therapy is an option de novo.
De novo multiple-drug therapy might be reasonable if a patient is at high risk for resistance—for example, for patients with extraordinarily high levels of HBV DNA or in whom resistance can lead to dire consequences, such as patients with cirrhosis or pretransplant patients.
The HIV pandemic serves as a paradigm for combination therapy. Many agents used to treat HBV infection also have anti-HIV effects; their use as monotherapy should be avoided in order to prevent the development of HIV drug resistance. HIV regimens that include only one HBV antiviral agent with a low genetic barrier to resistance (eg, lamivudine) should also be avoided in order to minimize the risk of HBV drug resistance.
I agree with Dr. Gish that cost and potential toxicity, especially renal toxicity, may limit the widespread use of combination therapies.
Discussion
William D. Carey, MD: I hear more agreement than not between the debaters. Are there any comments from the panel?
Morris Sherman, MD, PhD: I’ll comment on the guidelines for the treatment of HBV infection. Tong et al21 recently examined whether a group of HBV-infected patients who developed cirrhosis and hepatoma would have qualified for treatment under four current sets of guidelines. A startlingly large proportion of patients who developed adverse consequences from their liver disease would not have met the criteria for treatment under any of these major guidelines. As many as one-fourth of patients with chronic HBV infection die as a consequence of their liver disease, and in order to prevent these deaths up to one-half of the patients have to be treated. In the long run, overtreatment may be preferable to undertreatment to reduce the incidence of hepatitis-related deaths. My point is that the treatment guidelines probably exclude many patients who should be treated.
The factors I consider important in my decision to treat are a high viral load, which is indicative of active viral replication, and evidence of liver injury. Patients who have a high viral load and no liver injury won’t experience complications. What do I consider evidence of liver injury? Prolonged elevation of ALT is suggestive, although not necessarily as high as 200 or 300 U/L; it could be in the range of 50 to 80 U/L if fibrosis is significant, which I define as stage 2 or greater on the biopsy. If a high viral load and evidence of significant liver injury are present, I treat the patient regardless of the precise level of the viral load or the ALT.
Dr. Carey: Can you clarify your position? Some of our earlier discussion emphasized the importance of treating when the viral load is high, regardless of other factors. A high viral load by itself may be associated with increased risk of cirrhosis or hepatocellular carcinoma without cirrhosis, so why would a biopsy make a difference?
Dr. Sherman: We can’t predict which younger HBeAg-positive patients with a very high viral load are going to run into trouble down the road. Many will seroconvert spontaneously and never have problems thereafter. In contrast, a patient in his 40s with a high viral load, even if HBeAg positive, and without major fibrosis should be considered for therapy. I tell my patients and the physicians who refer them that once I’m finished with the evaluation, it’s not good-bye. They have to be followed for life because things change.
Tram T. Tran, MD: In the paper by Tong et al,21 all of the patients who subsequently had poor outcomes had low platelet counts. I therefore recommend considering the entire picture in the decision to treat. If physicians followed the treatment guidelines strictly, they would not have treated those patients, but had they noticed thrombocytopenia they would have considered the possibility of advanced fibrosis and considered screening or a biopsy.
Monotherapy for treatment-naïve patients
By Robert G. Gish, MD
Powerful antiviral medicines with activity against hepatitis B virus (HBV) have long-term records of potency and safety, supporting the case for monotherapy in treatment-naïve patients. Combination therapy has a limited role in the management of HBV infection; if the approach to treatment is rational from the start, then combination therapy can be reserved for cases of treatment failure or resistance.
THE CASE FOR MONOTHERAPY
Three arguments that favor monotherapy with potent medications are cost, low risk of resistance, and unproven benefit of combination therapy.
Cost
The cost of dual-medication therapy is nearly double that of single-drug therapy, while the benefit is unknown in treatment-naïve patients. My choices for first-line therapy are tenofovir or entecavir, highly potent nucleoside/nucleotide analogues that can cost up to $5,500 and $8,000, respectively, per year of treatment.1 The two in combination would cost nearly $14,000 per year, and benefits have not been proven in the treatment-naïve population.
Low risk of resistance
Potent medications have low rates of resistance, in the range of 1% over 2 to 5 years.2–4 If one starts therapy with the highly potent entecavir, discussions about switching or adding on therapy would be superfluous because of the low rates of resistance and failure associated with entecavir monotherapy. At 5 years, the cumulative rate of entecavir resistance in patients with positive HBV DNA at baseline is 1.2%.5 Tenofovir also produces potent inhibition of HBV DNA and is associated with low rates of resistance,6 although follow-up data with tenofovir extend only to 2 years. Starting therapy with the less potent adefovir, followed by the development of resistance, decreases the probability that tenofovir will achieve HBV DNA suppression during treatment.7 The main driver of resistance is nonadherence with therapy, not treatment failure.
Resistance to pegylated interferon has not been encountered. The therapy is limited in duration (24 to 48 weeks), with durable suppression of HBV DNA and high rates of seroconversion from hepatitis B e antigen (HBeAg)-positive to HBeAg-negative status. Parameters for the use of pegylated interferon as first-line therapy have been established, and include patients with genotype A or B who are young, have HBV DNA levels less than 107 copies/mL, have serum alanine aminotransferase (ALT) levels two to three times the upper limit of normal, and lack significant comorbidities.3,4
Unproven benefit of combination therapy
Perhaps the most convincing argument against combination therapy is that numerous studies of combinations have failed to demonstrate a benefit compared with monotherapy in treatment-naïve patients:
- Interferon in combination with lamivudine has not been shown to be significantly more effective than lamivudine monotherapy.8,9 Further, because of limited information on the safety of interferon in combination with nucleoside or nucleotide analogues, use of the combination is not recommended.4 Neuropathy has been reported with the combination of interferon and telbivudine,4 leading to the release of a warning about its use.10
- A 1-year trial by Lai et al failed to show an improvement in virologic and biochemical responses with the combination of telbivudine and lamivudine compared with telbivudine alone.11
- In patients with lamivudine-resistant chronic HBV infection, adefovir reduced serum HBV DNA levels by 4 weeks whether or not lamivudine therapy was ongoing.12
- Although more patients taking a combination of adefovir and the nucleoside reverse transcriptase inhibitor emtricitabine had normalization of ALT and suppression of HBV DNA to less than 300 copies compared with adefovir monotherapy, rates of HBeAg seroconversion were comparable in the two arms.13
- A recent study that compared tenofovir monotherapy with tenofovir and emtricitabine in combination showed comparable effectiveness for both regimens; the authors concluded that further study is necessary before either choice can be recommended as superior to the other.14
RESISTANCE: IDENTIFY EARLY, ADD ON
To minimize the likelihood of resistance and its impact, HBV DNA levels should be monitored every 3 months; at the first sign of a virologic breakthrough, therapy should be added or switched. Resistance to lamivudine is apparent early; models of treatment response indicate that resistance to lamivudine is likely if HBV DNA does not become undetectable by week 4.
In cases of lamivudine failure, adding adefovir early, when the viral load is less than 107 copies/mL, increases the probability of a virologic response.15 In the situation of lamivudine failure, I prefer adding on to switching to reduce the risk of resistance—a practice supported by the study just cited.15 In lamivudine-resistant patients, adefovir monotherapy was associated with virologic breakthrough and resistance to adefovir in 21% of patients, whereas no patient experienced virologic breakthrough or resistance when adefovir was added to lamivudine.
Successful management involves choosing the best medication up front and educating patients about the importance of taking their medication as instructed. For example, entecavir should be taken without food to maximize its bioavailability. With tenofovir, the risk of renal toxicity is low (1%),16 and can be reduced even further with a pretreatment assessment of the patient.
Multiple-drug therapy is the wave of the future
By Pierre M. Gholam, MD
A concise rationale for multiple-drug therapy is that resistance to monotherapy will occur eventually, with serious consequences in some patients and grave public health implications over the long term. Data from France and Australia indicate that multidrug-resistant HBV is a reality in individual cases. Resistance may be less likely when combinations are used, although little evidence exists at present to support this contention.
COMBINATION THERAPY IS COMMON SENSE
Much of the evidence supporting combination therapy for HBV is common sense:
- Most patients with HBV infection require treatment indefinitely, and duration of therapy that is not finite will inevitably lead to resistance.
- Your first shot is your best shot. Once resistance develops, treatment response will eventually decline.
- Sometimes the stakes are too high to risk breakthroughs. In particular, in patients who have cirrhosis and in those awaiting or following liver transplant, flares and recurrences can have disastrous consequences.
Treatment duration and resistance
As Dr. Gish demonstrated, tenofovir and entecavir are highly potent drugs that suppress viral loads effectively and have high genetic barriers to resistance. On an intent-to-treat basis, HBV DNA levels below the threshold level of detection are achieved at impressive rates with tenofovir and entecavir at 2 years in patients who are either HBeAg negative or positive.5,6,17 When the analyses are limited to patients who actually received the drugs, suppression of HBV DNA to undetectable levels exceeds 90%. Resistance to tenofovir is 0% at 2 years,3 and resistance to entecavir is 1.2% at 5 years.5
Although such data appear to favor monotherapy, most HBV-infected patients who commit to treatment will be treated indefinitely; this applies to patients who are HBeAg negative, who constitute most HBV-infected individuals in the United States and worldwide, or HBeAg positive. There are no established end points for treatment termination in HBeAg-negative patients. The only treatment termination end point that is deemed acceptable in HBeAg-positive patients is a period 6 to 12 months after the loss of HBeAg and the development of antibody to HBeAg, or e antigen seroconversion. Even after many years of treatment that includes the first-line agents tenofovir and entecavir, the likelihood of achieving this end point is fairly low.2,5,18
Adherence is also a consideration. Studies of patients with hypertension, heart disease, and other chronic diseases have shown that strict adherence to therapy over decades is unlikely. The same adherence pattern probably applies to the treatment of chronic HBV infection.
Antiviral drugs used in the treatment of chronic HBV infection are associated with certain resistance mutations that confer additional risk of developing resistance to a subsequent drug. Furthermore, with indefinite duration of therapy, it is realistic to expect that resistance will develop.
Other factors play roles in the development of resistance:
- Mutant viruses. We do not fully understand the potential problem of transmission of mutant viruses. This phenomenon is becoming apparent in endemic areas where treatment-naïve patients harbor mutant viruses acquired through sexual contact with HBV-infected patients who have been treated and in whom the virus has subsequently mutated.
- Barriers to resistance. The genetic barrier to resistance for a single drug will eventually be overcome. It may take longer than it took for adefovir, which is associated with a 30% rate of resistance at 5 years.3 It may take a much longer time for entecavir or tenofovir, but resistance is a biological certainty and we need to contend with it. With human immunodeficiency virus (HIV) infection, we are able to genotype for mutations and tailor treatment accordingly. This strategy is not currently recommended for HBV infection, partly because it is expensive and not routinely available.
- Misuse of therapy. Finally, wider use of antiviral agents for the treatment of HBV may lead to wider misuse, and therefore more resistance. Realistically, not every practitioner will start therapy with entecavir or tenofovir; many of the less potent agents have associated rates of resistance, and these in turn may confer an additional risk of resistance if tenofovir or entecavir is eventually used.
Declining response
Colonno et al19 studied the likelihood of entecavir resistance developing in patients with existing lamivudine resistance. The likelihood of resistance to entecavir at 3 years was 1.2% among patients who had never been exposed to lamivudine. Among patients in whom lamivudine resistance had developed and who were subsequently started on entecavir, resistance to entecavir was 32% at 3 years.19 Resistance has consequences; 25% of lamivudine-resistant patients develop viral breakthrough.
Dr. Gish and I agree that the addition of adefovir to lamivudine is better than switching to adefovir monotherapy in the case of lamivudine failure. Compared with switching, the adefovir-lamivudine combination leads to a lower incidence of virologic breakthrough, a lower likelihood of adefovir resistance over time, a greater probability of achieving undetectable levels of HBV DNA (< 35 copies/mL), and a lower cumulative rate of resistance.20 The superiority of combination therapy in achieving undetectable levels of HBV DNA confers a lower risk of developing resistance over time; by year 4, the likelihood of adefovir resistance is only 4% among lamivudine-resistant patients treated with the combination of adefovir and lamivudine.20
In a study of nucleoside analogue–experienced patients who did not achieve viral suppression, response to tenofovir, defined as HBV DNA less than 400 copies/mL at month 12, was 85% overall and only 30% in adefovir-resistant patients.7 These data demonstrate that, if not starting with combination therapy, it is preferable to initiate treatment with a potent drug that is highly successful at HBV DNA suppression. A second monotherapy will be less successful than the initial attempt.
Consequences of resistance
The consequences of resistance in patients with cirrhosis are significant, prompting strong consideration of combination therapy as a potential means to avoid resistance.
One consequence is a well-documented potential for decompensation in the setting of new-onset resistance as a result of flares. Another is post-transplantation recurrence of HBV, leading to poor outcomes. These risks converge in the patient who is awaiting liver transplantation, in whom combination therapy seems to make the most sense to prevent the development of a flare and a recurrence of HBV infection after transplantation.
WHO SHOULD RECEIVE MULTIPLE-DRUG THERAPY?
The American Association for the Study of Liver Diseases recommends combination therapy as the preferred rescue therapy for primary failure of a first-line agent, citing the possibility of resistance with switching in some circumstances and the superiority of adding on as opposed to switching.2 No data clearly support de novo multiple-drug therapy. Although a number of studies have failed to show an advantage of combination therapy over monotherapy, they were of relatively short duration and focused primarily on viral suppression rather than the occurrence of resistance over time. Long-term studies are needed to determine whether combination therapy is an option de novo.
De novo multiple-drug therapy might be reasonable if a patient is at high risk for resistance—for example, for patients with extraordinarily high levels of HBV DNA or in whom resistance can lead to dire consequences, such as patients with cirrhosis or pretransplant patients.
The HIV pandemic serves as a paradigm for combination therapy. Many agents used to treat HBV infection also have anti-HIV effects; their use as monotherapy should be avoided in order to prevent the development of HIV drug resistance. HIV regimens that include only one HBV antiviral agent with a low genetic barrier to resistance (eg, lamivudine) should also be avoided in order to minimize the risk of HBV drug resistance.
I agree with Dr. Gish that cost and potential toxicity, especially renal toxicity, may limit the widespread use of combination therapies.
Discussion
William D. Carey, MD: I hear more agreement than not between the debaters. Are there any comments from the panel?
Morris Sherman, MD, PhD: I’ll comment on the guidelines for the treatment of HBV infection. Tong et al21 recently examined whether a group of HBV-infected patients who developed cirrhosis and hepatoma would have qualified for treatment under four current sets of guidelines. A startlingly large proportion of patients who developed adverse consequences from their liver disease would not have met the criteria for treatment under any of these major guidelines. As many as one-fourth of patients with chronic HBV infection die as a consequence of their liver disease, and in order to prevent these deaths up to one-half of the patients have to be treated. In the long run, overtreatment may be preferable to undertreatment to reduce the incidence of hepatitis-related deaths. My point is that the treatment guidelines probably exclude many patients who should be treated.
The factors I consider important in my decision to treat are a high viral load, which is indicative of active viral replication, and evidence of liver injury. Patients who have a high viral load and no liver injury won’t experience complications. What do I consider evidence of liver injury? Prolonged elevation of ALT is suggestive, although not necessarily as high as 200 or 300 U/L; it could be in the range of 50 to 80 U/L if fibrosis is significant, which I define as stage 2 or greater on the biopsy. If a high viral load and evidence of significant liver injury are present, I treat the patient regardless of the precise level of the viral load or the ALT.
Dr. Carey: Can you clarify your position? Some of our earlier discussion emphasized the importance of treating when the viral load is high, regardless of other factors. A high viral load by itself may be associated with increased risk of cirrhosis or hepatocellular carcinoma without cirrhosis, so why would a biopsy make a difference?
Dr. Sherman: We can’t predict which younger HBeAg-positive patients with a very high viral load are going to run into trouble down the road. Many will seroconvert spontaneously and never have problems thereafter. In contrast, a patient in his 40s with a high viral load, even if HBeAg positive, and without major fibrosis should be considered for therapy. I tell my patients and the physicians who refer them that once I’m finished with the evaluation, it’s not good-bye. They have to be followed for life because things change.
Tram T. Tran, MD: In the paper by Tong et al,21 all of the patients who subsequently had poor outcomes had low platelet counts. I therefore recommend considering the entire picture in the decision to treat. If physicians followed the treatment guidelines strictly, they would not have treated those patients, but had they noticed thrombocytopenia they would have considered the possibility of advanced fibrosis and considered screening or a biopsy.
- Wong JB. Costs of antiviral therapy of chronic hepatitis B. Paper presented at: Management of Hepatitis B: 2006. National Institutes of Health Workshop. April 6–8, 2006; Bethesda, MD.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Keeffe EG, Dieterich DT, Han S-H B, et al. Special report. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008; 6:1315–1341.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242.
- Tenney DJ, Pokomowski KA, Rose RE, et al. Entecavir at five years shows long-term maintenance of high genetic barrier to hepatitis B virus resistance [abstract OL-107]. Hepatol Int 2008; 2:S76–S77.
- Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–2455.
- van Bömmel F, de Man RA, Stein K, et al. A multicenter analysis of antiviral response after one year of tenofovir monotherapy in HBV-monoinfected patients with prior nucleos(t)ide analog experience [EASL abstract 73]. J Hepatol 2008; 48(suppl 2):S32.
- Schalm SW, Heathcote J, Cianciara J, et al. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomized trial. Gut 2000; 46:562–568.
- Chan HL-Y, Leung NW-Y, Hui AY, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-a2b and lamivudine with lamivudine alone. Ann Intern Med 2005; 142:240–250.
- Novartis Pharmaceuticals Canada Inc. Risk of peripheral neuropathy in patients treated with telbivudine (SEBIVO®) in combination with interferon. Health Canada Web site. http://www.hc-sc.gc.ca/dhp-mps/alt_formats/hpfb-dgpsa/pdf/medeff/sebivo_pc-cp-eng.pdf. March 12, 2008. Accessed March 12, 2009.
- Lai C-L, Leung N, Teo E-K, et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology 2005; 129:528–536.
- Peters MG, Hann HW, Martin P, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 2004; 126:91–101.
- Hui C-K, Zhang H-Y, Bowden S, et al. 96 weeks combination of adefovir dipivoxil plus emtricitabine vs. adefovir dipivoxil monotherapy in the treatment of chronic hepatitis B. J Hepatol 2008; 48:714–720.
- Berg T, Moller B, Trinh H, et al. Tenofovir disoproxil fumarate (TDF) versus emtricitabine plus TDF for treatment of chronic hepatitis B (CHB) in subjects with persistent viral replication receiving adefovir dipivoxil (ADV). Paper presented at: 43rd Annual Meeting of the European Association for the Study of the Liver; April 23–27, 2008; Milan, Italy.
- Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 2007; 45:307–313.
- Szczech LA. Tenofovir nephrotoxicity: focusing research questions and putting them into clinical context. J Infect Dis 2008; 197:7–9.
- Shouval D, Lai C-L, Chang T-T, et al. Three years of entecavir (ETV) retreatment of HBeAg(–) ETV patients who previously discontinued treatment: results from study ETV-901. Poster presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA. Poster 927.
- Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125:1714–1722.
- Colonno RJ, Rose RE, Pokornowski K, Baldick CJ, Klesczewski K, Tenney D. Assessment at three years shows high barrier to resistance is maintained in entecavir-treated nucleoside naïve patients while resistance emergence increases over time in lamivudine refractory patients [AASLD abstract 110]. Hepatology 2006; 44(suppl 1):229A–230A.
- Lampertico P, Viganò M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology 2007; 133:1445–1451.
- Tong MJ, Hsien C, Hsu L, Sun HE, Blatt LM. Treatment recommendations for chronic hepatitis B: an evaluation of current guidelines based on a natural history study in the United States. Hepatology 2008; 48:1070–1078.
- Wong JB. Costs of antiviral therapy of chronic hepatitis B. Paper presented at: Management of Hepatitis B: 2006. National Institutes of Health Workshop. April 6–8, 2006; Bethesda, MD.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539.
- Keeffe EG, Dieterich DT, Han S-H B, et al. Special report. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol 2008; 6:1315–1341.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol 2009; 50:227–242.
- Tenney DJ, Pokomowski KA, Rose RE, et al. Entecavir at five years shows long-term maintenance of high genetic barrier to hepatitis B virus resistance [abstract OL-107]. Hepatol Int 2008; 2:S76–S77.
- Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–2455.
- van Bömmel F, de Man RA, Stein K, et al. A multicenter analysis of antiviral response after one year of tenofovir monotherapy in HBV-monoinfected patients with prior nucleos(t)ide analog experience [EASL abstract 73]. J Hepatol 2008; 48(suppl 2):S32.
- Schalm SW, Heathcote J, Cianciara J, et al. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomized trial. Gut 2000; 46:562–568.
- Chan HL-Y, Leung NW-Y, Hui AY, et al. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-a2b and lamivudine with lamivudine alone. Ann Intern Med 2005; 142:240–250.
- Novartis Pharmaceuticals Canada Inc. Risk of peripheral neuropathy in patients treated with telbivudine (SEBIVO®) in combination with interferon. Health Canada Web site. http://www.hc-sc.gc.ca/dhp-mps/alt_formats/hpfb-dgpsa/pdf/medeff/sebivo_pc-cp-eng.pdf. March 12, 2008. Accessed March 12, 2009.
- Lai C-L, Leung N, Teo E-K, et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology 2005; 129:528–536.
- Peters MG, Hann HW, Martin P, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 2004; 126:91–101.
- Hui C-K, Zhang H-Y, Bowden S, et al. 96 weeks combination of adefovir dipivoxil plus emtricitabine vs. adefovir dipivoxil monotherapy in the treatment of chronic hepatitis B. J Hepatol 2008; 48:714–720.
- Berg T, Moller B, Trinh H, et al. Tenofovir disoproxil fumarate (TDF) versus emtricitabine plus TDF for treatment of chronic hepatitis B (CHB) in subjects with persistent viral replication receiving adefovir dipivoxil (ADV). Paper presented at: 43rd Annual Meeting of the European Association for the Study of the Liver; April 23–27, 2008; Milan, Italy.
- Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 2007; 45:307–313.
- Szczech LA. Tenofovir nephrotoxicity: focusing research questions and putting them into clinical context. J Infect Dis 2008; 197:7–9.
- Shouval D, Lai C-L, Chang T-T, et al. Three years of entecavir (ETV) retreatment of HBeAg(–) ETV patients who previously discontinued treatment: results from study ETV-901. Poster presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA. Poster 927.
- Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125:1714–1722.
- Colonno RJ, Rose RE, Pokornowski K, Baldick CJ, Klesczewski K, Tenney D. Assessment at three years shows high barrier to resistance is maintained in entecavir-treated nucleoside naïve patients while resistance emergence increases over time in lamivudine refractory patients [AASLD abstract 110]. Hepatology 2006; 44(suppl 1):229A–230A.
- Lampertico P, Viganò M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology 2007; 133:1445–1451.
- Tong MJ, Hsien C, Hsu L, Sun HE, Blatt LM. Treatment recommendations for chronic hepatitis B: an evaluation of current guidelines based on a natural history study in the United States. Hepatology 2008; 48:1070–1078.
Management of hepatitis B in pregnancy: Weighing the options
The management of hepatitis B virus (HBV) infection in pregnancy is complex. Because infection with HBV in infancy often leads to chronic disease, prevention of perinatal, or vertical, transmission is a worthy goal; yet, prophylactic therapy during pregnancy is not well studied. This article explores the consequences of HBV infection during pregnancy, the specific risks imposed by high viral load, the evidence to support preemptive antiviral therapy, and the timing of therapy during pregnancy.
PERINATAL TRANSMISSION
Perinatal transmission is the most common mode of HBV transmission worldwide; however, the maternal screening programs and universal vaccination in newborns with active and passive immunoprophylaxis have dramatically reduced HBV transmission rates. According to recent data from the US Centers for Disease Control and Prevention, prenatal screening for hepatitis B surface antigen (HBsAg) in the United States is nearly universal; 97% of pregnant women undergo screening before delivery.1 Further, among infants at risk of acquiring HBV infection, 92% complete the three-dose vaccination series by the time they are 3 years old. There is some nationwide variation, however, in the appropriate administration of immunoprophylaxis to infants exposed perinatally, ranging from 78% in Louisiana to 99.8% in one California health maintenance organization.2
Perinatal transmission of HBV infection has declined steadily in the United States over the past 2 decades, consistent with the successful implementation of universal screening of pregnant women and vaccination policies.3 Outside the United States, however, many high-prevalence countries lack vaccination coverage and perinatal transmission is common. In 87 countries with a prevalence of HBV infection that exceeds 8%, the infant vaccine coverage was only 36%.4
Risk of chronic infection
The risk of progression to chronic HBV infection is inversely proportional to the age at which the infection was acquired. Without immunoprophylaxis, up to 90% of infants born to hepatitis B e antigen (HBeAg)-positive mothers become HBV carriers. In comparison, 20% to 30% of children infected between age 1 year and 5 years, and fewer than 5% of immunocompetent adults, become HBV carriers.5–7
If the mother is positive for both HBsAg and HBeAg and her baby does not receive immunoprophylaxis, the risk of the baby developing chronic HBV infection by age 6 months is 70% to 90%.8–10 Of those exposed in early childhood, 28.8% are HBsAg positive by age 4 years.5 These data underscore the need for early vaccination.
In a study of 402 HBsAg-positive pregnant women in China, Xu et al11 found that 3.7% of their newborn infants were HBsAg positive within 24 hours of birth. Of the women who were HBeAg positive, the intrauterine infection rate was 9.8%. Analysis of placental tissue for HBsAg, hepatitis B core antigen (HBcAg), and viral load (HBV DNA) uncovered an overall placental infection rate of 44.6%.
Transplacental transmission of HBV has been observed in multiple studies, especially when mothers are positive for HBsAg and HBeAg and have high viral loads. Among mothers positive for HBeAg, Burk et al12 found an odds ratio of 147 for a persistently infected infant when the maternal HBV DNA level was at least 1.4 ng/mL compared with less than .005 ng/mL. Among the HBeAg-negative mothers, the odds ratio for a persistently infected infant was 19.2 with high versus low maternal HBV DNA levels.
Importance of maternal viremia
Despite successful screening and vaccination programs, high maternal HBV DNA correlates in some studies with perinatal transmission. Wiseman et al13 studied 298 chronically HBV-infected women and their infants, who were tested for HBV at age 9 months. Interim analysis showed a transmission rate of 8.5% for infants born to mothers with virus levels greater than 8 log10 copies/mL. These data suggest that perinatal transmission may still be occurring despite the use of effective active and passive immunoprophylaxis. Additional studies are needed to assess the potential risk reduction associated with treatment of high maternal viremia during pregnancy.
Maternal HBV DNA positivity was associated with a high rate of intrauterine transmission of HBV in a program in India in which 11,524 woman were screened for HBV infection.14 Babies of the 133 women found to be positive at the time of birth were screened for HBsAg, HBeAg, and HBV DNA in serum and cord blood. Of 127 deliveries in which the mothers were positive for HBV DNA, 66% of infants had HBV DNA in their cord blood and 41% had serum markers that were positive at birth. Maternal HBV DNA greater than 1.5 X 105 copies/mL was significantly associated with intrauterine transmission (P = .025), whereas mode of delivery and maternal HBeAg status were not. This study adds to the concern that in some cases, the vaccine and hepatitis B immune globulin (HBIg) given at the time of birth may not prevent infection in those born already infected and further supports the need to assess the treatment of pregnant women with high viral titers.
TREATMENT DURING PREGNANCY
The use of active and passive immunoprophylaxis to reduce the risk of perinatal transmission of HBV is well accepted in clinical practice. HBIg given at the time of birth in combination with three doses of the recombinant hepatitis B vaccine given over the first 6 months of life has been up to 95% effective in preventing perinatal transmission. As noted above, however, the risk of perinatal transmission of HBV increases as the mother’s viral load increases. In one series of mothers with high viral loads, this risk was as high as 28%.15
It stands to reason that if the mother’s viral load can be reduced at the time of birth, the risk of perinatal transmission could also be reduced (see “Case: Minimizing risk in a 29-year-old woman”). In fact, lamivudine treatment of highly viremic HBsAg-positive women during the final months of pregnancy appears safe and may effectively reduce the risk of perinatal transmission of HBV, even in the setting of HBV vaccination plus HBIg.

Evidence for third-trimester treatment
van Zonneveld et al15 studied eight HBeAg-positive women with HBV DNA levels of 1.2 X 109 copies/mL or greater who were treated with 150 mg/day of lamivudine after the 34th week of pregnancy, and compared the rates of perinatal transmission between them and 24 matched historical controls who did not receive treatment. All children received standard immunoprophylaxis at birth and were followed for 12 months. In five of the eight treated mothers, viral load declined to less than 1.2 X 108 copies/mL. Of the eight infants born to treated mothers, four were HBsAg positive at birth, but only one remained positive at 1 year. This 12.5% rate of perinatal transmission was substantially lower than the 28% rate observed among the controls. No adverse events occurred with lamivudine in this study.
In a multicenter, randomized, double-blind, placebo-controlled study in China and the Philippines, Xu et al16 assessed outcomes among 114 HBsAg-positive pregnant women who had high viral loads (HBV DNA > 1,000 mEq/mL). The women were randomized to placebo or treatment with lamivudine starting at 32 weeks of gestation and continuing until 4 weeks postpartum. All of the infants received standard vaccine plus HBIg.
The mothers treated with lamivudine were more likely (98%) to have a reduction in their viral loads to less than 1,000 mEq/mL than the controls (31%). This reduction in viral load translated to improved outcomes for the infants of mothers receiving lamivudine. At 1 year, 18% of infants born to mothers treated with lamivudine were HBsAg positive compared with 39% of infants born to mothers randomized to placebo (P = .014). The rate of HBV DNA positivity at 1 year was reduced by more than half among the infants born to actively treated mothers compared with those who received placebo (20% vs 46%, respectively; P = .003). There was no difference in the rate of adverse events between the treatment and control groups in either the mothers or the infants.
The Xu study suggests that the use of lamivudine in the third trimester in mothers with high viral loads may effectively reduce the risk of perinatal transmission beyond what can be achieved with active and passive immunoprophylaxis. As this study has been presented in abstract form only, we await the final analysis of these data. This therapy appears to be relatively safe for both mother and infant, although the optimal timing and duration of therapy is still unclear.
Treatment options during pregnancy
Most human experience with antiviral drug therapy in pregnancy has been with lamivudine. More than 4,600 women have been exposed to the drug during their second or third trimesters.17 Even though lamivudine is classified as FDA pregnancy risk category C, it is associated with a risk of birth defects (2.2% to 2.4%) that is no higher than the baseline birth defect rate.17
Of the two agents classified as FDA pregnancy risk category B, only tenofovir received this classification based on data collected in human exposure. The experience with tenofovir in pregnant women consists of 606 women in their first trimester and 336 in their second trimester.17 The rate of birth defects associated with tenofovir ranges from 1.5% (second-trimester use) to 2.3% (first-trimester use), which is similar to the background rate.17 Telbivudine received its pregnancy risk category B rating based on animal studies; there are few human pregnancy registry data.
Nonpegylated interferon alfa-2b has been shown to have abortifacient effects in rhesus monkeys at 15 and 30 million IU/kg (estimated human equivalent of 5 and 10 million IU/kg, based on body surface area adjustment for a 60-kg adult). Peginterferon alfa-2b should therefore be assumed to also have abortifacient potential, as there are no adequate and well-controlled studies in pregnant women. Peginterferon alfa-2b is to be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is recommended for use in fertile women only when they are using effective contraception during the treatment period. Pegylated interferon alfa-2a is approved for treatment of chronic HBV infection, but is not recommended for use during pregnancy.
MANAGEMENT STRATEGY
If the mother is HBsAg positive in the first trimester, history of perinatal transmission and an assessment of viral load at week 28 guide further management decisions. All children of HBsAg-positive mothers receive HBIg in addition to vaccination at birth.
Women with high viral loads can be considered for treatment with antiviral therapy, but a comprehensive discussion of risks and benefits needs to take place before opting for treatment as the data are too limited at this time to advocate therapy. One strategy for therapy is the use of lamivudine, tenofovir, or telbivudine starting at 32 weeks of pregnancy; the HBV DNA level that warrants treatment depends on the presence or absence of a history of perinatal transmission. If a previous child was HBV positive, concerns about the risk of perinatal transmission may be higher, so the threshold for treatment may be lower (HBV DNA > 106 copies/mL) than if the previous child were not positive for HBV. If the previous child was not HBV positive, treatment might be considered with HBV DNA levels greater than 108 copies/mL.
SUMMARY
Although a case can be made for treatment of HBV infection during pregnancy, the risks and benefits must be weighed carefully. The benefits of treatment appear to be most pronounced in cases with high maternal viremia; in such instances, treatment should be considered and discussed with the patient at the start of the third trimester. Viable treatment choices are limited to lamivudine, tenofovir, and telbivudine. Of these, lamivudine and tenofovir appear to be the therapeutic options with reasonable human exposure and safety data in pregnancy.
DISCUSSION
William D. Carey, MD: Referring to your case patient, assume that you treat her with tenofovir and her viral load declines. She delivers her baby and then undergoes a thorough workup, including a liver biopsy, that shows no particular liver damage. What would you do?
Tram T. Tran, MD: There are two separate issues: treating the baby and treating the mother. When you’re treating a mother in her third trimester, your goal is to prevent perinatal transmission of HBV. Once the baby is delivered, treated with HBIg, and vaccinated, then your attention turns to the mother. You can then decide based on treatment guidelines and your clinical judgment whether you want to treat the mom.
The period immediately after birth is a time of treatment uncertainty in mothers who choose to breastfeed, because the nucleoside analogues are likely passed in breast milk to some unknown degree, and it’s probably unwise to expose the child this way. In a mother who chooses to breastfeed, I would stop the medication after the delivery, by which time the baby will have received HBIg and the vaccine. When treatment is stopped, you have to think about the potential for a flare; although clinically significant flares are uncommon, the mother should be monitored after stopping treatment. After she stops breastfeeding, you can decide whether to treat her.
Robert G. Gish, MD: What are the effects of tenofovir on bone? Do you talk to your patients about it, and is it an issue during pregnancy or after the baby is delivered?
Dr. Tran: Some data show a decrease in bone mineral density with tenofovir in the human immunodeficiency virus patient population. I definitely talk to my patients about all the potential risks associated with these medicines as, naturally, pregnant women will be very sensitive to any possible risk to their unborn child. Lamivudine probably has the safest profile in pregnancy, given its large body of human experience; however, it is now classified as an FDA pregnancy risk category C drug, whereas tenofovir is classified as category B. This may make a difference to some clinicians.
- Schrag SJ, Arnold KE, Mohle-Boetani JC, et al. Prenatal screening for infectious diseases and opportunities for prevention. Obstet Gynecol 2003; 102:753–760.
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006; 28:112–125.
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55(RR-16):1–33.
- Centers for Disease Control and Prevention (CDC). Implementation of newborn hepatitis B vaccination—worldwide, 2006. MMWR Morb Mortal Wkly Rep 2008; 57:1249–1252.
- McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985; 151:599–603.
- Tassopoulos NC, Papaevangelou GJ, Sjogren MH, Roumeliotou-Karayannis A, Gerin JL, Purcell RH. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology 1987; 92:1844–1850.
- Chang MH. Natural history of hepatitis B virus infection in children. J Gastroenterol Hepatol 2000; 15(suppl):E16–E19.
- Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol 1977; 105:94–98.
- Wong VC, Ip HM, Reesink HW, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin: double-blind randomised placebo-controlled study. Lancet 1984; 1(8383):921–926.
- Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med 1976; 294:746–749.
- Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol 2002; 67:20–26.
- Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis 1994; 170:1418–1423.
- Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: viral load and HBeAg status are significant risk factors. Presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA. Abstract 827.
- Pande C, Kumar A, Patra S, Trivedi SS, Dutta AK, Sarin SK. High maternal hepatitis B virus DNA levels but not HBeAg positivity predicts perinatal transmission of hepatitis B to the newborn. Presented at: Digestive Disease Week; May 17–22, 2008; San Diego, CA. Abstract 252.
- van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat 2003; 10:294–297.
- Xu W-M, Cui Y-T, Wang L, et al. Efficacy and safety of lamivudine in late pregnancy for the prevention of mother-child transmission of hepatitis B: a multicentre, randomised, double-blind, placebo-controlled study [AASLD abstract 246]. Hepatology 2004; 40:272A.
- Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral pregnancy registry international interim report for 1 January 1989 through 31 July 2008. Wilmington, NC: Registry Coordinating Center; 2008. Antiretroviral Pregnancy Registry Web site. http://www.apregistry.com/forms/interim_report.pdf. Accessed December 17, 2008.
- Tran TT, Keeffe EB. Management of the pregnant hepatitis B patient. Current Hepatitis Reports 2008; 7:12–17.
The management of hepatitis B virus (HBV) infection in pregnancy is complex. Because infection with HBV in infancy often leads to chronic disease, prevention of perinatal, or vertical, transmission is a worthy goal; yet, prophylactic therapy during pregnancy is not well studied. This article explores the consequences of HBV infection during pregnancy, the specific risks imposed by high viral load, the evidence to support preemptive antiviral therapy, and the timing of therapy during pregnancy.
PERINATAL TRANSMISSION
Perinatal transmission is the most common mode of HBV transmission worldwide; however, the maternal screening programs and universal vaccination in newborns with active and passive immunoprophylaxis have dramatically reduced HBV transmission rates. According to recent data from the US Centers for Disease Control and Prevention, prenatal screening for hepatitis B surface antigen (HBsAg) in the United States is nearly universal; 97% of pregnant women undergo screening before delivery.1 Further, among infants at risk of acquiring HBV infection, 92% complete the three-dose vaccination series by the time they are 3 years old. There is some nationwide variation, however, in the appropriate administration of immunoprophylaxis to infants exposed perinatally, ranging from 78% in Louisiana to 99.8% in one California health maintenance organization.2
Perinatal transmission of HBV infection has declined steadily in the United States over the past 2 decades, consistent with the successful implementation of universal screening of pregnant women and vaccination policies.3 Outside the United States, however, many high-prevalence countries lack vaccination coverage and perinatal transmission is common. In 87 countries with a prevalence of HBV infection that exceeds 8%, the infant vaccine coverage was only 36%.4
Risk of chronic infection
The risk of progression to chronic HBV infection is inversely proportional to the age at which the infection was acquired. Without immunoprophylaxis, up to 90% of infants born to hepatitis B e antigen (HBeAg)-positive mothers become HBV carriers. In comparison, 20% to 30% of children infected between age 1 year and 5 years, and fewer than 5% of immunocompetent adults, become HBV carriers.5–7
If the mother is positive for both HBsAg and HBeAg and her baby does not receive immunoprophylaxis, the risk of the baby developing chronic HBV infection by age 6 months is 70% to 90%.8–10 Of those exposed in early childhood, 28.8% are HBsAg positive by age 4 years.5 These data underscore the need for early vaccination.
In a study of 402 HBsAg-positive pregnant women in China, Xu et al11 found that 3.7% of their newborn infants were HBsAg positive within 24 hours of birth. Of the women who were HBeAg positive, the intrauterine infection rate was 9.8%. Analysis of placental tissue for HBsAg, hepatitis B core antigen (HBcAg), and viral load (HBV DNA) uncovered an overall placental infection rate of 44.6%.
Transplacental transmission of HBV has been observed in multiple studies, especially when mothers are positive for HBsAg and HBeAg and have high viral loads. Among mothers positive for HBeAg, Burk et al12 found an odds ratio of 147 for a persistently infected infant when the maternal HBV DNA level was at least 1.4 ng/mL compared with less than .005 ng/mL. Among the HBeAg-negative mothers, the odds ratio for a persistently infected infant was 19.2 with high versus low maternal HBV DNA levels.
Importance of maternal viremia
Despite successful screening and vaccination programs, high maternal HBV DNA correlates in some studies with perinatal transmission. Wiseman et al13 studied 298 chronically HBV-infected women and their infants, who were tested for HBV at age 9 months. Interim analysis showed a transmission rate of 8.5% for infants born to mothers with virus levels greater than 8 log10 copies/mL. These data suggest that perinatal transmission may still be occurring despite the use of effective active and passive immunoprophylaxis. Additional studies are needed to assess the potential risk reduction associated with treatment of high maternal viremia during pregnancy.
Maternal HBV DNA positivity was associated with a high rate of intrauterine transmission of HBV in a program in India in which 11,524 woman were screened for HBV infection.14 Babies of the 133 women found to be positive at the time of birth were screened for HBsAg, HBeAg, and HBV DNA in serum and cord blood. Of 127 deliveries in which the mothers were positive for HBV DNA, 66% of infants had HBV DNA in their cord blood and 41% had serum markers that were positive at birth. Maternal HBV DNA greater than 1.5 X 105 copies/mL was significantly associated with intrauterine transmission (P = .025), whereas mode of delivery and maternal HBeAg status were not. This study adds to the concern that in some cases, the vaccine and hepatitis B immune globulin (HBIg) given at the time of birth may not prevent infection in those born already infected and further supports the need to assess the treatment of pregnant women with high viral titers.
TREATMENT DURING PREGNANCY
The use of active and passive immunoprophylaxis to reduce the risk of perinatal transmission of HBV is well accepted in clinical practice. HBIg given at the time of birth in combination with three doses of the recombinant hepatitis B vaccine given over the first 6 months of life has been up to 95% effective in preventing perinatal transmission. As noted above, however, the risk of perinatal transmission of HBV increases as the mother’s viral load increases. In one series of mothers with high viral loads, this risk was as high as 28%.15
It stands to reason that if the mother’s viral load can be reduced at the time of birth, the risk of perinatal transmission could also be reduced (see “Case: Minimizing risk in a 29-year-old woman”). In fact, lamivudine treatment of highly viremic HBsAg-positive women during the final months of pregnancy appears safe and may effectively reduce the risk of perinatal transmission of HBV, even in the setting of HBV vaccination plus HBIg.

Evidence for third-trimester treatment
van Zonneveld et al15 studied eight HBeAg-positive women with HBV DNA levels of 1.2 X 109 copies/mL or greater who were treated with 150 mg/day of lamivudine after the 34th week of pregnancy, and compared the rates of perinatal transmission between them and 24 matched historical controls who did not receive treatment. All children received standard immunoprophylaxis at birth and were followed for 12 months. In five of the eight treated mothers, viral load declined to less than 1.2 X 108 copies/mL. Of the eight infants born to treated mothers, four were HBsAg positive at birth, but only one remained positive at 1 year. This 12.5% rate of perinatal transmission was substantially lower than the 28% rate observed among the controls. No adverse events occurred with lamivudine in this study.
In a multicenter, randomized, double-blind, placebo-controlled study in China and the Philippines, Xu et al16 assessed outcomes among 114 HBsAg-positive pregnant women who had high viral loads (HBV DNA > 1,000 mEq/mL). The women were randomized to placebo or treatment with lamivudine starting at 32 weeks of gestation and continuing until 4 weeks postpartum. All of the infants received standard vaccine plus HBIg.
The mothers treated with lamivudine were more likely (98%) to have a reduction in their viral loads to less than 1,000 mEq/mL than the controls (31%). This reduction in viral load translated to improved outcomes for the infants of mothers receiving lamivudine. At 1 year, 18% of infants born to mothers treated with lamivudine were HBsAg positive compared with 39% of infants born to mothers randomized to placebo (P = .014). The rate of HBV DNA positivity at 1 year was reduced by more than half among the infants born to actively treated mothers compared with those who received placebo (20% vs 46%, respectively; P = .003). There was no difference in the rate of adverse events between the treatment and control groups in either the mothers or the infants.
The Xu study suggests that the use of lamivudine in the third trimester in mothers with high viral loads may effectively reduce the risk of perinatal transmission beyond what can be achieved with active and passive immunoprophylaxis. As this study has been presented in abstract form only, we await the final analysis of these data. This therapy appears to be relatively safe for both mother and infant, although the optimal timing and duration of therapy is still unclear.
Treatment options during pregnancy
Most human experience with antiviral drug therapy in pregnancy has been with lamivudine. More than 4,600 women have been exposed to the drug during their second or third trimesters.17 Even though lamivudine is classified as FDA pregnancy risk category C, it is associated with a risk of birth defects (2.2% to 2.4%) that is no higher than the baseline birth defect rate.17
Of the two agents classified as FDA pregnancy risk category B, only tenofovir received this classification based on data collected in human exposure. The experience with tenofovir in pregnant women consists of 606 women in their first trimester and 336 in their second trimester.17 The rate of birth defects associated with tenofovir ranges from 1.5% (second-trimester use) to 2.3% (first-trimester use), which is similar to the background rate.17 Telbivudine received its pregnancy risk category B rating based on animal studies; there are few human pregnancy registry data.
Nonpegylated interferon alfa-2b has been shown to have abortifacient effects in rhesus monkeys at 15 and 30 million IU/kg (estimated human equivalent of 5 and 10 million IU/kg, based on body surface area adjustment for a 60-kg adult). Peginterferon alfa-2b should therefore be assumed to also have abortifacient potential, as there are no adequate and well-controlled studies in pregnant women. Peginterferon alfa-2b is to be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is recommended for use in fertile women only when they are using effective contraception during the treatment period. Pegylated interferon alfa-2a is approved for treatment of chronic HBV infection, but is not recommended for use during pregnancy.
MANAGEMENT STRATEGY
If the mother is HBsAg positive in the first trimester, history of perinatal transmission and an assessment of viral load at week 28 guide further management decisions. All children of HBsAg-positive mothers receive HBIg in addition to vaccination at birth.
Women with high viral loads can be considered for treatment with antiviral therapy, but a comprehensive discussion of risks and benefits needs to take place before opting for treatment as the data are too limited at this time to advocate therapy. One strategy for therapy is the use of lamivudine, tenofovir, or telbivudine starting at 32 weeks of pregnancy; the HBV DNA level that warrants treatment depends on the presence or absence of a history of perinatal transmission. If a previous child was HBV positive, concerns about the risk of perinatal transmission may be higher, so the threshold for treatment may be lower (HBV DNA > 106 copies/mL) than if the previous child were not positive for HBV. If the previous child was not HBV positive, treatment might be considered with HBV DNA levels greater than 108 copies/mL.
SUMMARY
Although a case can be made for treatment of HBV infection during pregnancy, the risks and benefits must be weighed carefully. The benefits of treatment appear to be most pronounced in cases with high maternal viremia; in such instances, treatment should be considered and discussed with the patient at the start of the third trimester. Viable treatment choices are limited to lamivudine, tenofovir, and telbivudine. Of these, lamivudine and tenofovir appear to be the therapeutic options with reasonable human exposure and safety data in pregnancy.
DISCUSSION
William D. Carey, MD: Referring to your case patient, assume that you treat her with tenofovir and her viral load declines. She delivers her baby and then undergoes a thorough workup, including a liver biopsy, that shows no particular liver damage. What would you do?
Tram T. Tran, MD: There are two separate issues: treating the baby and treating the mother. When you’re treating a mother in her third trimester, your goal is to prevent perinatal transmission of HBV. Once the baby is delivered, treated with HBIg, and vaccinated, then your attention turns to the mother. You can then decide based on treatment guidelines and your clinical judgment whether you want to treat the mom.
The period immediately after birth is a time of treatment uncertainty in mothers who choose to breastfeed, because the nucleoside analogues are likely passed in breast milk to some unknown degree, and it’s probably unwise to expose the child this way. In a mother who chooses to breastfeed, I would stop the medication after the delivery, by which time the baby will have received HBIg and the vaccine. When treatment is stopped, you have to think about the potential for a flare; although clinically significant flares are uncommon, the mother should be monitored after stopping treatment. After she stops breastfeeding, you can decide whether to treat her.
Robert G. Gish, MD: What are the effects of tenofovir on bone? Do you talk to your patients about it, and is it an issue during pregnancy or after the baby is delivered?
Dr. Tran: Some data show a decrease in bone mineral density with tenofovir in the human immunodeficiency virus patient population. I definitely talk to my patients about all the potential risks associated with these medicines as, naturally, pregnant women will be very sensitive to any possible risk to their unborn child. Lamivudine probably has the safest profile in pregnancy, given its large body of human experience; however, it is now classified as an FDA pregnancy risk category C drug, whereas tenofovir is classified as category B. This may make a difference to some clinicians.
The management of hepatitis B virus (HBV) infection in pregnancy is complex. Because infection with HBV in infancy often leads to chronic disease, prevention of perinatal, or vertical, transmission is a worthy goal; yet, prophylactic therapy during pregnancy is not well studied. This article explores the consequences of HBV infection during pregnancy, the specific risks imposed by high viral load, the evidence to support preemptive antiviral therapy, and the timing of therapy during pregnancy.
PERINATAL TRANSMISSION
Perinatal transmission is the most common mode of HBV transmission worldwide; however, the maternal screening programs and universal vaccination in newborns with active and passive immunoprophylaxis have dramatically reduced HBV transmission rates. According to recent data from the US Centers for Disease Control and Prevention, prenatal screening for hepatitis B surface antigen (HBsAg) in the United States is nearly universal; 97% of pregnant women undergo screening before delivery.1 Further, among infants at risk of acquiring HBV infection, 92% complete the three-dose vaccination series by the time they are 3 years old. There is some nationwide variation, however, in the appropriate administration of immunoprophylaxis to infants exposed perinatally, ranging from 78% in Louisiana to 99.8% in one California health maintenance organization.2
Perinatal transmission of HBV infection has declined steadily in the United States over the past 2 decades, consistent with the successful implementation of universal screening of pregnant women and vaccination policies.3 Outside the United States, however, many high-prevalence countries lack vaccination coverage and perinatal transmission is common. In 87 countries with a prevalence of HBV infection that exceeds 8%, the infant vaccine coverage was only 36%.4
Risk of chronic infection
The risk of progression to chronic HBV infection is inversely proportional to the age at which the infection was acquired. Without immunoprophylaxis, up to 90% of infants born to hepatitis B e antigen (HBeAg)-positive mothers become HBV carriers. In comparison, 20% to 30% of children infected between age 1 year and 5 years, and fewer than 5% of immunocompetent adults, become HBV carriers.5–7
If the mother is positive for both HBsAg and HBeAg and her baby does not receive immunoprophylaxis, the risk of the baby developing chronic HBV infection by age 6 months is 70% to 90%.8–10 Of those exposed in early childhood, 28.8% are HBsAg positive by age 4 years.5 These data underscore the need for early vaccination.
In a study of 402 HBsAg-positive pregnant women in China, Xu et al11 found that 3.7% of their newborn infants were HBsAg positive within 24 hours of birth. Of the women who were HBeAg positive, the intrauterine infection rate was 9.8%. Analysis of placental tissue for HBsAg, hepatitis B core antigen (HBcAg), and viral load (HBV DNA) uncovered an overall placental infection rate of 44.6%.
Transplacental transmission of HBV has been observed in multiple studies, especially when mothers are positive for HBsAg and HBeAg and have high viral loads. Among mothers positive for HBeAg, Burk et al12 found an odds ratio of 147 for a persistently infected infant when the maternal HBV DNA level was at least 1.4 ng/mL compared with less than .005 ng/mL. Among the HBeAg-negative mothers, the odds ratio for a persistently infected infant was 19.2 with high versus low maternal HBV DNA levels.
Importance of maternal viremia
Despite successful screening and vaccination programs, high maternal HBV DNA correlates in some studies with perinatal transmission. Wiseman et al13 studied 298 chronically HBV-infected women and their infants, who were tested for HBV at age 9 months. Interim analysis showed a transmission rate of 8.5% for infants born to mothers with virus levels greater than 8 log10 copies/mL. These data suggest that perinatal transmission may still be occurring despite the use of effective active and passive immunoprophylaxis. Additional studies are needed to assess the potential risk reduction associated with treatment of high maternal viremia during pregnancy.
Maternal HBV DNA positivity was associated with a high rate of intrauterine transmission of HBV in a program in India in which 11,524 woman were screened for HBV infection.14 Babies of the 133 women found to be positive at the time of birth were screened for HBsAg, HBeAg, and HBV DNA in serum and cord blood. Of 127 deliveries in which the mothers were positive for HBV DNA, 66% of infants had HBV DNA in their cord blood and 41% had serum markers that were positive at birth. Maternal HBV DNA greater than 1.5 X 105 copies/mL was significantly associated with intrauterine transmission (P = .025), whereas mode of delivery and maternal HBeAg status were not. This study adds to the concern that in some cases, the vaccine and hepatitis B immune globulin (HBIg) given at the time of birth may not prevent infection in those born already infected and further supports the need to assess the treatment of pregnant women with high viral titers.
TREATMENT DURING PREGNANCY
The use of active and passive immunoprophylaxis to reduce the risk of perinatal transmission of HBV is well accepted in clinical practice. HBIg given at the time of birth in combination with three doses of the recombinant hepatitis B vaccine given over the first 6 months of life has been up to 95% effective in preventing perinatal transmission. As noted above, however, the risk of perinatal transmission of HBV increases as the mother’s viral load increases. In one series of mothers with high viral loads, this risk was as high as 28%.15
It stands to reason that if the mother’s viral load can be reduced at the time of birth, the risk of perinatal transmission could also be reduced (see “Case: Minimizing risk in a 29-year-old woman”). In fact, lamivudine treatment of highly viremic HBsAg-positive women during the final months of pregnancy appears safe and may effectively reduce the risk of perinatal transmission of HBV, even in the setting of HBV vaccination plus HBIg.

Evidence for third-trimester treatment
van Zonneveld et al15 studied eight HBeAg-positive women with HBV DNA levels of 1.2 X 109 copies/mL or greater who were treated with 150 mg/day of lamivudine after the 34th week of pregnancy, and compared the rates of perinatal transmission between them and 24 matched historical controls who did not receive treatment. All children received standard immunoprophylaxis at birth and were followed for 12 months. In five of the eight treated mothers, viral load declined to less than 1.2 X 108 copies/mL. Of the eight infants born to treated mothers, four were HBsAg positive at birth, but only one remained positive at 1 year. This 12.5% rate of perinatal transmission was substantially lower than the 28% rate observed among the controls. No adverse events occurred with lamivudine in this study.
In a multicenter, randomized, double-blind, placebo-controlled study in China and the Philippines, Xu et al16 assessed outcomes among 114 HBsAg-positive pregnant women who had high viral loads (HBV DNA > 1,000 mEq/mL). The women were randomized to placebo or treatment with lamivudine starting at 32 weeks of gestation and continuing until 4 weeks postpartum. All of the infants received standard vaccine plus HBIg.
The mothers treated with lamivudine were more likely (98%) to have a reduction in their viral loads to less than 1,000 mEq/mL than the controls (31%). This reduction in viral load translated to improved outcomes for the infants of mothers receiving lamivudine. At 1 year, 18% of infants born to mothers treated with lamivudine were HBsAg positive compared with 39% of infants born to mothers randomized to placebo (P = .014). The rate of HBV DNA positivity at 1 year was reduced by more than half among the infants born to actively treated mothers compared with those who received placebo (20% vs 46%, respectively; P = .003). There was no difference in the rate of adverse events between the treatment and control groups in either the mothers or the infants.
The Xu study suggests that the use of lamivudine in the third trimester in mothers with high viral loads may effectively reduce the risk of perinatal transmission beyond what can be achieved with active and passive immunoprophylaxis. As this study has been presented in abstract form only, we await the final analysis of these data. This therapy appears to be relatively safe for both mother and infant, although the optimal timing and duration of therapy is still unclear.
Treatment options during pregnancy
Most human experience with antiviral drug therapy in pregnancy has been with lamivudine. More than 4,600 women have been exposed to the drug during their second or third trimesters.17 Even though lamivudine is classified as FDA pregnancy risk category C, it is associated with a risk of birth defects (2.2% to 2.4%) that is no higher than the baseline birth defect rate.17
Of the two agents classified as FDA pregnancy risk category B, only tenofovir received this classification based on data collected in human exposure. The experience with tenofovir in pregnant women consists of 606 women in their first trimester and 336 in their second trimester.17 The rate of birth defects associated with tenofovir ranges from 1.5% (second-trimester use) to 2.3% (first-trimester use), which is similar to the background rate.17 Telbivudine received its pregnancy risk category B rating based on animal studies; there are few human pregnancy registry data.
Nonpegylated interferon alfa-2b has been shown to have abortifacient effects in rhesus monkeys at 15 and 30 million IU/kg (estimated human equivalent of 5 and 10 million IU/kg, based on body surface area adjustment for a 60-kg adult). Peginterferon alfa-2b should therefore be assumed to also have abortifacient potential, as there are no adequate and well-controlled studies in pregnant women. Peginterferon alfa-2b is to be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It is recommended for use in fertile women only when they are using effective contraception during the treatment period. Pegylated interferon alfa-2a is approved for treatment of chronic HBV infection, but is not recommended for use during pregnancy.
MANAGEMENT STRATEGY
If the mother is HBsAg positive in the first trimester, history of perinatal transmission and an assessment of viral load at week 28 guide further management decisions. All children of HBsAg-positive mothers receive HBIg in addition to vaccination at birth.
Women with high viral loads can be considered for treatment with antiviral therapy, but a comprehensive discussion of risks and benefits needs to take place before opting for treatment as the data are too limited at this time to advocate therapy. One strategy for therapy is the use of lamivudine, tenofovir, or telbivudine starting at 32 weeks of pregnancy; the HBV DNA level that warrants treatment depends on the presence or absence of a history of perinatal transmission. If a previous child was HBV positive, concerns about the risk of perinatal transmission may be higher, so the threshold for treatment may be lower (HBV DNA > 106 copies/mL) than if the previous child were not positive for HBV. If the previous child was not HBV positive, treatment might be considered with HBV DNA levels greater than 108 copies/mL.
SUMMARY
Although a case can be made for treatment of HBV infection during pregnancy, the risks and benefits must be weighed carefully. The benefits of treatment appear to be most pronounced in cases with high maternal viremia; in such instances, treatment should be considered and discussed with the patient at the start of the third trimester. Viable treatment choices are limited to lamivudine, tenofovir, and telbivudine. Of these, lamivudine and tenofovir appear to be the therapeutic options with reasonable human exposure and safety data in pregnancy.
DISCUSSION
William D. Carey, MD: Referring to your case patient, assume that you treat her with tenofovir and her viral load declines. She delivers her baby and then undergoes a thorough workup, including a liver biopsy, that shows no particular liver damage. What would you do?
Tram T. Tran, MD: There are two separate issues: treating the baby and treating the mother. When you’re treating a mother in her third trimester, your goal is to prevent perinatal transmission of HBV. Once the baby is delivered, treated with HBIg, and vaccinated, then your attention turns to the mother. You can then decide based on treatment guidelines and your clinical judgment whether you want to treat the mom.
The period immediately after birth is a time of treatment uncertainty in mothers who choose to breastfeed, because the nucleoside analogues are likely passed in breast milk to some unknown degree, and it’s probably unwise to expose the child this way. In a mother who chooses to breastfeed, I would stop the medication after the delivery, by which time the baby will have received HBIg and the vaccine. When treatment is stopped, you have to think about the potential for a flare; although clinically significant flares are uncommon, the mother should be monitored after stopping treatment. After she stops breastfeeding, you can decide whether to treat her.
Robert G. Gish, MD: What are the effects of tenofovir on bone? Do you talk to your patients about it, and is it an issue during pregnancy or after the baby is delivered?
Dr. Tran: Some data show a decrease in bone mineral density with tenofovir in the human immunodeficiency virus patient population. I definitely talk to my patients about all the potential risks associated with these medicines as, naturally, pregnant women will be very sensitive to any possible risk to their unborn child. Lamivudine probably has the safest profile in pregnancy, given its large body of human experience; however, it is now classified as an FDA pregnancy risk category C drug, whereas tenofovir is classified as category B. This may make a difference to some clinicians.
- Schrag SJ, Arnold KE, Mohle-Boetani JC, et al. Prenatal screening for infectious diseases and opportunities for prevention. Obstet Gynecol 2003; 102:753–760.
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006; 28:112–125.
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55(RR-16):1–33.
- Centers for Disease Control and Prevention (CDC). Implementation of newborn hepatitis B vaccination—worldwide, 2006. MMWR Morb Mortal Wkly Rep 2008; 57:1249–1252.
- McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985; 151:599–603.
- Tassopoulos NC, Papaevangelou GJ, Sjogren MH, Roumeliotou-Karayannis A, Gerin JL, Purcell RH. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology 1987; 92:1844–1850.
- Chang MH. Natural history of hepatitis B virus infection in children. J Gastroenterol Hepatol 2000; 15(suppl):E16–E19.
- Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol 1977; 105:94–98.
- Wong VC, Ip HM, Reesink HW, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin: double-blind randomised placebo-controlled study. Lancet 1984; 1(8383):921–926.
- Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med 1976; 294:746–749.
- Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol 2002; 67:20–26.
- Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis 1994; 170:1418–1423.
- Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: viral load and HBeAg status are significant risk factors. Presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA. Abstract 827.
- Pande C, Kumar A, Patra S, Trivedi SS, Dutta AK, Sarin SK. High maternal hepatitis B virus DNA levels but not HBeAg positivity predicts perinatal transmission of hepatitis B to the newborn. Presented at: Digestive Disease Week; May 17–22, 2008; San Diego, CA. Abstract 252.
- van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat 2003; 10:294–297.
- Xu W-M, Cui Y-T, Wang L, et al. Efficacy and safety of lamivudine in late pregnancy for the prevention of mother-child transmission of hepatitis B: a multicentre, randomised, double-blind, placebo-controlled study [AASLD abstract 246]. Hepatology 2004; 40:272A.
- Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral pregnancy registry international interim report for 1 January 1989 through 31 July 2008. Wilmington, NC: Registry Coordinating Center; 2008. Antiretroviral Pregnancy Registry Web site. http://www.apregistry.com/forms/interim_report.pdf. Accessed December 17, 2008.
- Tran TT, Keeffe EB. Management of the pregnant hepatitis B patient. Current Hepatitis Reports 2008; 7:12–17.
- Schrag SJ, Arnold KE, Mohle-Boetani JC, et al. Prenatal screening for infectious diseases and opportunities for prevention. Obstet Gynecol 2003; 102:753–760.
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 2006; 28:112–125.
- Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55(RR-16):1–33.
- Centers for Disease Control and Prevention (CDC). Implementation of newborn hepatitis B vaccination—worldwide, 2006. MMWR Morb Mortal Wkly Rep 2008; 57:1249–1252.
- McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985; 151:599–603.
- Tassopoulos NC, Papaevangelou GJ, Sjogren MH, Roumeliotou-Karayannis A, Gerin JL, Purcell RH. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology 1987; 92:1844–1850.
- Chang MH. Natural history of hepatitis B virus infection in children. J Gastroenterol Hepatol 2000; 15(suppl):E16–E19.
- Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol 1977; 105:94–98.
- Wong VC, Ip HM, Reesink HW, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin: double-blind randomised placebo-controlled study. Lancet 1984; 1(8383):921–926.
- Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med 1976; 294:746–749.
- Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol 2002; 67:20–26.
- Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis 1994; 170:1418–1423.
- Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: viral load and HBeAg status are significant risk factors. Presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA. Abstract 827.
- Pande C, Kumar A, Patra S, Trivedi SS, Dutta AK, Sarin SK. High maternal hepatitis B virus DNA levels but not HBeAg positivity predicts perinatal transmission of hepatitis B to the newborn. Presented at: Digestive Disease Week; May 17–22, 2008; San Diego, CA. Abstract 252.
- van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat 2003; 10:294–297.
- Xu W-M, Cui Y-T, Wang L, et al. Efficacy and safety of lamivudine in late pregnancy for the prevention of mother-child transmission of hepatitis B: a multicentre, randomised, double-blind, placebo-controlled study [AASLD abstract 246]. Hepatology 2004; 40:272A.
- Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral pregnancy registry international interim report for 1 January 1989 through 31 July 2008. Wilmington, NC: Registry Coordinating Center; 2008. Antiretroviral Pregnancy Registry Web site. http://www.apregistry.com/forms/interim_report.pdf. Accessed December 17, 2008.
- Tran TT, Keeffe EB. Management of the pregnant hepatitis B patient. Current Hepatitis Reports 2008; 7:12–17.
KEY POINTS
- Hepatitis B immune globulin at the time of birth plus three doses of the recombinant hepatitis B vaccine over the first 6 months of life is up to 95% effective in
preventing perinatal transmission. - Despite successful screening and vaccination, perinatal transmission of HBV is still possible if maternal viral load is high.
- Antiviral treatment during the third trimester of pregnancy may reduce perinatal transmission of HBV; the benefit appears most pronounced with high maternal viremia.
Strategies for managing coinfection with hepatitis B virus and HIV
Worldwide, 40 million people are infected with the human immunodeficiency virus (HIV). As many as 4 million of them are coinfected with hepatitis B virus (HBV).1 In North America and Europe, the highest prevalence of HBV/HIV coinfection is in men who have sex with men. Approximately half of HIV-positive men who have sex with men have evidence of prior or active HBV infection, and 5% to 10% have chronic HBV infection. Among those who acquire HIV through injected drug use or through heterosexual transmission, the coinfection rate is much lower.2,3
Coinfection with HBV and HIV follows a different course elsewhere in the world. For example, in Africa and Asia, HBV is usually acquired first through neonatal or childhood infection, with either vertical or horizontal transmission after birth.4,5 In parts of Africa, ritual scarification is likely a major player in the adolescent transmission of HBV. (Ritual scarification is the practice of creating small incisions in the skin of adolescents and rubbing black ash in the wounds to form scars; the cutting instruments are not sterilized between rituals.)
NATURAL HISTORY
In general, HBV tends to be more aggressive in HIV-positive individuals than in monoinfected individuals,2,6 with higher HBV carrier rates, higher levels of HBV viremia, more frequent episodes of activation, and faster progression to cirrhosis.
Hepatocellular carcinoma occurs more often, its onset is earlier, and its course is more aggressive in coinfected individuals than in monoinfected individuals.7,8 Using data from a prospective cohort study, Thio et al9 found that among men coinfected with HIV and HBV, liver-related mortality was almost 19 times greater compared with men infected with HBV only and more than seven times greater compared with those infected with HIV only.
In an observational longitudinal cohort study,10 the risk of death from liver disease in HIV-positive persons was nearly three times greater among those also infected with HBV (P < .0001).
ASSESSING WHEN TO TREAT
The objectives of HBV therapy in individuals coinfected with HIV are similar to those in the population infected with HBV alone. Suppression of viral replication is the major goal. Ideally, the viral load should be reduced to an undetectable level, which will result in normalization of alanine aminotransferase (ALT) level, improved liver histology, reduced risk of progression to cirrhosis and liver failure (although supportive evidence from controlled clinical trials is lacking), and likely reduced incidence of hepatocellular carcinoma.
For those who are hepatitis B e antigen (HBeAg) positive, seroconversion may be a convenient end point for treatment, although for many patients seroconversion is not associated with remission of disease activity or viral replication.
Treatment decisions depend on whether or not the patient requires highly active antiretroviral therapy (HAART) for HIV infection. If HAART is indicated, then HIV agents that have HBV activity are incorporated into the regimen. If the patient does not yet require HAART for HIV but requires treatment for hepatitis B, this is itself an indication for HAART, since monotherapy for HBV is associated with the development of HIV resistance.
Viral load and ALT
Liver biopsy
If the ALT is normal in the presence of a high HBV DNA level, a liver biopsy is recommended, partly because the ALT level is an inadequate indicator of the severity of liver disease. If significant fibrosis is present, treatment is recommended. No treatment is required if fibrosis is mild, but liver biopsies should be repeated every 3 to 5 years in this group because a hallmark of HBV infection is its variability in time to progression. The extent of fibrosis may influence the choice of therapy.
Often in the coinfected patient, HBV-related liver injury must be distinguished from other forms of liver injury. For instance, some of the drugs used to treat HIV infection can induce nonalcoholic fatty liver disease and lipodystrophy. Because the risk of advanced fibrosis is higher in the coinfected patient than in the patient infected only with HBV, the threshold for biopsy in the coinfected patient should be lower.
At present, noninvasive tools to assess the extent of liver injury have not been validated in chronic HBV infection, unlike in hepatitis C virus infection.
TREATMENT OPTIONS
The potential therapies for HBV in the coinfected patient are the same as those for the patient infected with HBV alone (see “Hepatitis B treatment: Current best practices, avoiding resistance”), with the addition of tenofovir and emtricitabine in combination.
Interferon
Early studies of interferon for the treatment of chronic HBV infection included many patients who were also HIV positive. These early studies revealed a lower rate of HBeAg seroconversion in HIV-positive patients compared with HIV-negative patients. Di Martino et al11 found that approximately half (26 of 50) of HIV-negative patients treated with interferon seroconverted at 6 years, compared with only 4 of 26 interferon-treated patients with chronic HBV who were coinfected with HIV. Based on results such as these, interferon therapy in the HBV/HIV-coinfected patient should be limited to patients who are likely to seroconvert: ie, those who are female and younger than 40 years with high ALT levels, low serum HBV DNA levels, and active liver histology (a subgroup that is more likely to undergo spontaneous seroconversion than other HBV-infected groups).
Standard or pegylated interferon is a treatment option for coinfected patients who do not yet require HAART, especially patients who have high ALT levels, low viral loads, and positive HBeAg status without liver decompensation.12
Nucleoside and nucleotide analogues
The nucleoside and nucleotide analogues used for HBV therapy have different degrees of effectiveness against HIV polymerase, but none can be used as monotherapy because of the risk of inducing HIV resistance. Lamivudine and tenofovir are used as part of the standard cocktail for the treatment of HIV (see “Case: HIV/HBV with resistance to tenofovir”). Entecavir is now recognized as a partial inhibitor of HIV replication. Both lamivudine and entecavir induce the YMDD mutation, an indication of resistance to therapy, in HIV polymerase. Tenofovir also may select for resistance mutations in HIV polymerase.
At dosages used for the treatment of HBV infection, adefovir has weak activity against HIV, and therefore HIV would not be under significant selective pressure to develop resistance mutations. In HIV polymerase, the mutations that confer resistance to adefovir also confer resistance to tenofovir, and therefore use of adefovir may induce tenofovir resistance.
Telbivudine has not been studied in HIV-infected patients, but its resistance profile is similar to that of lamivudine.
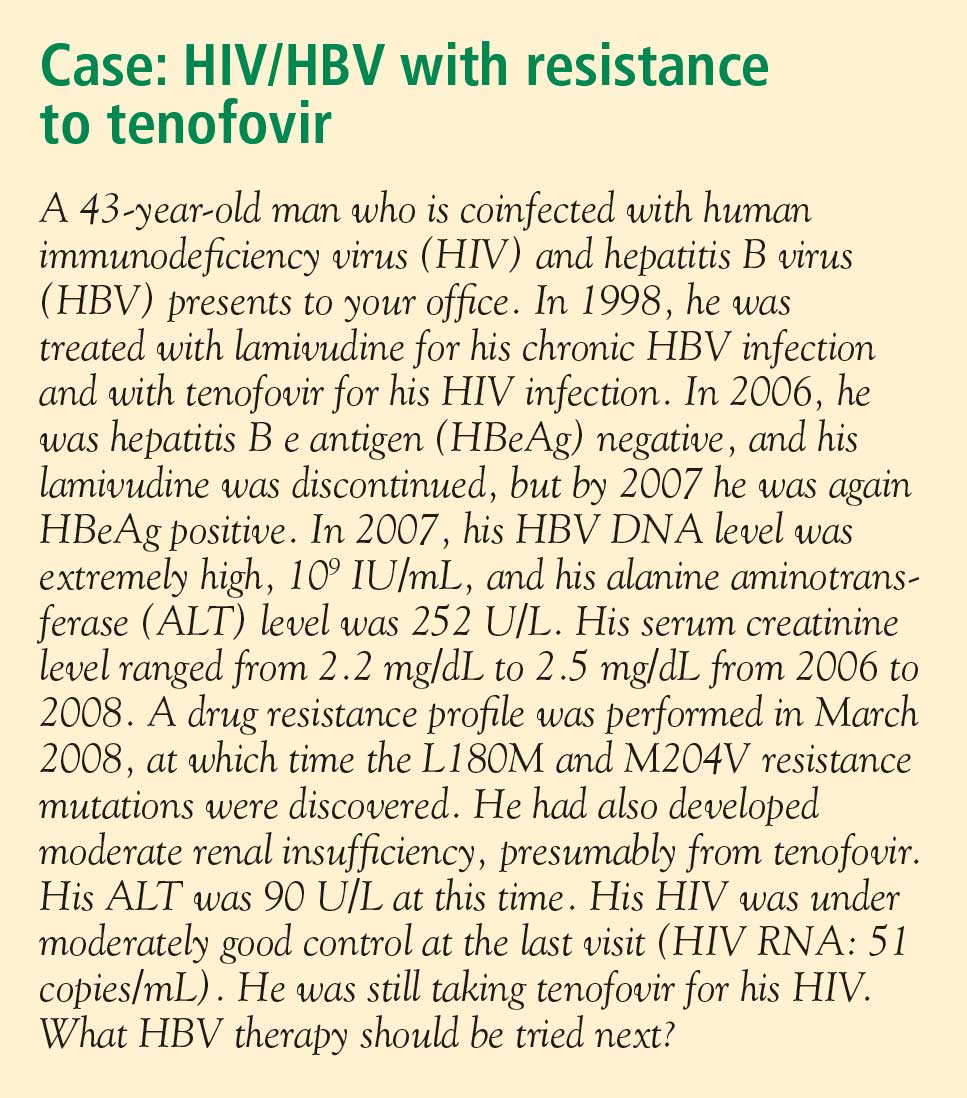
Treating both infections
When both HBV and HIV infections require treatment, HAART is necessary for HIV.12 The treatment strategy for coinfection is to use standard therapy for HIV, selecting two agents that are effective against HBV infection.
The need to avoid antiviral resistance complicates the selection of active agents. Resistance to HIV therapy limits the choices for treatment of HBV infection. The immediate aim of therapy, an undetectable level of HBV DNA, eliminates the use of less potent agents. The best choice for therapy is the most potent agent that can be used, such as tenofovir plus lamivudine or tenofovir plus emtricitabine.
Antiviral resistance
For the coinfected patient who develops resistance to lamivudine, the recommendation is to treat with tenofovir plus entecavir (the preferable choice because of absence of cross-reactivity between the two agents) or tenofovir plus lamivudine or emtricitabine. There is some evidence that lamivudine resistance predisposes to entecavir resistance, but the studies that generated these results were conducted in patients who had very high baseline viral loads13; the effectiveness of entecavir in patients with low baseline viral loads is unknown. Presumably, when entecavir is used in combination with another potent nucleoside analogue in coinfected patients, the sensitivity of HBV will be more durable than when entecavir is used as monotherapy.
Long-term monitoring
Long-term monitoring for the coinfected patient is similar to that for the patient infected with HBV only. HBV DNA levels should be monitored every 3 months for signs of resistance until levels have plateaued or become undetectable. Once the HBV DNA level is stable or undetectable, the monitoring interval can be extended. Ultrasonographic screening for hepatocellular carcinoma should be conducted every 6 months. Patients with cirrhosis should be screened for esophageal varices.

SUMMARY
HBV in the setting of HIV is more aggressive than in a patient infected with HBV only, and treatment must be comparably aggressive and carefully selected. The primary goal of HBV treatment in a coinfected patient is the same as in a patient with HBV infection only: reduction of viral load to undetectable levels. Treatment decisions are based on viral load, ALT level, findings on liver biopsy, the need for HAART, and the drug’s resistance profile. None of the nucleoside or nucleotide analogues can be used as monotherapy in the coinfected patient because of the risk of inducing resistance to HIV therapy. When the patient requires HAART, then the general recommendation is to select a combination of two drugs that have activity against HIV. If resistance develops, the preferred strategy is treatment with tenofovir plus entecavir. Monitoring includes measurement of HBV DNA levels every 3 months and ultrasonographic screening for hepatocellular carcinoma every 6 months.
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006; 44(suppl 1):S6–S9.
- Soriano V, Puoti M, Peters M, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS 2008; 22:1399–1410.
- Núñez M, Soriano V. Management of patients co-infected with hepatitis B virus and HIV. Lancet Infect Dis 2005; 5:374–382.
- Modi AA, Feld JJ. Viral hepatitis and HIV in Africa. AIDS Rev 2007; 9:25–39.
- Hoffman CJ, Thio CL. Clinical implications of HIV and hepatitis B coinfection in Asia and Africa. Lancet Infect Dis 2007; 7:402–409.
- Puoti M, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in co-infected patients. J Hepatol 2006; 44(suppl 1):S65–S70.
- Puoti M, Bruno R, Soriano V, et al. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS 2004; 18:2285–2293.
- Bräu N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a US-Canadian multicenter study. J Hepatol 2007; 47:527–537.
- Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360:1921–1926.
- Mocroft A, Soriano V, Rockstroh J, et al. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS 2005; 19:2117–2125.
- Di Martino V, Thevenot T, Colin J-F, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002; 123:1812–1822.
- Iser DM, Sasadeusz JJ. Current treatment of HIV/hepatitis B virus coinfection. J Gastroenterol Hepatol 2008; 23:699–706.
- Sherman M, Yurdaydin C, Sollano J, et al; AI463026 BEHoLD Study Group. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology 2006; 130:2039–2049.
Worldwide, 40 million people are infected with the human immunodeficiency virus (HIV). As many as 4 million of them are coinfected with hepatitis B virus (HBV).1 In North America and Europe, the highest prevalence of HBV/HIV coinfection is in men who have sex with men. Approximately half of HIV-positive men who have sex with men have evidence of prior or active HBV infection, and 5% to 10% have chronic HBV infection. Among those who acquire HIV through injected drug use or through heterosexual transmission, the coinfection rate is much lower.2,3
Coinfection with HBV and HIV follows a different course elsewhere in the world. For example, in Africa and Asia, HBV is usually acquired first through neonatal or childhood infection, with either vertical or horizontal transmission after birth.4,5 In parts of Africa, ritual scarification is likely a major player in the adolescent transmission of HBV. (Ritual scarification is the practice of creating small incisions in the skin of adolescents and rubbing black ash in the wounds to form scars; the cutting instruments are not sterilized between rituals.)
NATURAL HISTORY
In general, HBV tends to be more aggressive in HIV-positive individuals than in monoinfected individuals,2,6 with higher HBV carrier rates, higher levels of HBV viremia, more frequent episodes of activation, and faster progression to cirrhosis.
Hepatocellular carcinoma occurs more often, its onset is earlier, and its course is more aggressive in coinfected individuals than in monoinfected individuals.7,8 Using data from a prospective cohort study, Thio et al9 found that among men coinfected with HIV and HBV, liver-related mortality was almost 19 times greater compared with men infected with HBV only and more than seven times greater compared with those infected with HIV only.
In an observational longitudinal cohort study,10 the risk of death from liver disease in HIV-positive persons was nearly three times greater among those also infected with HBV (P < .0001).
ASSESSING WHEN TO TREAT
The objectives of HBV therapy in individuals coinfected with HIV are similar to those in the population infected with HBV alone. Suppression of viral replication is the major goal. Ideally, the viral load should be reduced to an undetectable level, which will result in normalization of alanine aminotransferase (ALT) level, improved liver histology, reduced risk of progression to cirrhosis and liver failure (although supportive evidence from controlled clinical trials is lacking), and likely reduced incidence of hepatocellular carcinoma.
For those who are hepatitis B e antigen (HBeAg) positive, seroconversion may be a convenient end point for treatment, although for many patients seroconversion is not associated with remission of disease activity or viral replication.
Treatment decisions depend on whether or not the patient requires highly active antiretroviral therapy (HAART) for HIV infection. If HAART is indicated, then HIV agents that have HBV activity are incorporated into the regimen. If the patient does not yet require HAART for HIV but requires treatment for hepatitis B, this is itself an indication for HAART, since monotherapy for HBV is associated with the development of HIV resistance.
Viral load and ALT
Liver biopsy
If the ALT is normal in the presence of a high HBV DNA level, a liver biopsy is recommended, partly because the ALT level is an inadequate indicator of the severity of liver disease. If significant fibrosis is present, treatment is recommended. No treatment is required if fibrosis is mild, but liver biopsies should be repeated every 3 to 5 years in this group because a hallmark of HBV infection is its variability in time to progression. The extent of fibrosis may influence the choice of therapy.
Often in the coinfected patient, HBV-related liver injury must be distinguished from other forms of liver injury. For instance, some of the drugs used to treat HIV infection can induce nonalcoholic fatty liver disease and lipodystrophy. Because the risk of advanced fibrosis is higher in the coinfected patient than in the patient infected only with HBV, the threshold for biopsy in the coinfected patient should be lower.
At present, noninvasive tools to assess the extent of liver injury have not been validated in chronic HBV infection, unlike in hepatitis C virus infection.
TREATMENT OPTIONS
The potential therapies for HBV in the coinfected patient are the same as those for the patient infected with HBV alone (see “Hepatitis B treatment: Current best practices, avoiding resistance”), with the addition of tenofovir and emtricitabine in combination.
Interferon
Early studies of interferon for the treatment of chronic HBV infection included many patients who were also HIV positive. These early studies revealed a lower rate of HBeAg seroconversion in HIV-positive patients compared with HIV-negative patients. Di Martino et al11 found that approximately half (26 of 50) of HIV-negative patients treated with interferon seroconverted at 6 years, compared with only 4 of 26 interferon-treated patients with chronic HBV who were coinfected with HIV. Based on results such as these, interferon therapy in the HBV/HIV-coinfected patient should be limited to patients who are likely to seroconvert: ie, those who are female and younger than 40 years with high ALT levels, low serum HBV DNA levels, and active liver histology (a subgroup that is more likely to undergo spontaneous seroconversion than other HBV-infected groups).
Standard or pegylated interferon is a treatment option for coinfected patients who do not yet require HAART, especially patients who have high ALT levels, low viral loads, and positive HBeAg status without liver decompensation.12
Nucleoside and nucleotide analogues
The nucleoside and nucleotide analogues used for HBV therapy have different degrees of effectiveness against HIV polymerase, but none can be used as monotherapy because of the risk of inducing HIV resistance. Lamivudine and tenofovir are used as part of the standard cocktail for the treatment of HIV (see “Case: HIV/HBV with resistance to tenofovir”). Entecavir is now recognized as a partial inhibitor of HIV replication. Both lamivudine and entecavir induce the YMDD mutation, an indication of resistance to therapy, in HIV polymerase. Tenofovir also may select for resistance mutations in HIV polymerase.
At dosages used for the treatment of HBV infection, adefovir has weak activity against HIV, and therefore HIV would not be under significant selective pressure to develop resistance mutations. In HIV polymerase, the mutations that confer resistance to adefovir also confer resistance to tenofovir, and therefore use of adefovir may induce tenofovir resistance.
Telbivudine has not been studied in HIV-infected patients, but its resistance profile is similar to that of lamivudine.

Treating both infections
When both HBV and HIV infections require treatment, HAART is necessary for HIV.12 The treatment strategy for coinfection is to use standard therapy for HIV, selecting two agents that are effective against HBV infection.
The need to avoid antiviral resistance complicates the selection of active agents. Resistance to HIV therapy limits the choices for treatment of HBV infection. The immediate aim of therapy, an undetectable level of HBV DNA, eliminates the use of less potent agents. The best choice for therapy is the most potent agent that can be used, such as tenofovir plus lamivudine or tenofovir plus emtricitabine.
Antiviral resistance
For the coinfected patient who develops resistance to lamivudine, the recommendation is to treat with tenofovir plus entecavir (the preferable choice because of absence of cross-reactivity between the two agents) or tenofovir plus lamivudine or emtricitabine. There is some evidence that lamivudine resistance predisposes to entecavir resistance, but the studies that generated these results were conducted in patients who had very high baseline viral loads13; the effectiveness of entecavir in patients with low baseline viral loads is unknown. Presumably, when entecavir is used in combination with another potent nucleoside analogue in coinfected patients, the sensitivity of HBV will be more durable than when entecavir is used as monotherapy.
Long-term monitoring
Long-term monitoring for the coinfected patient is similar to that for the patient infected with HBV only. HBV DNA levels should be monitored every 3 months for signs of resistance until levels have plateaued or become undetectable. Once the HBV DNA level is stable or undetectable, the monitoring interval can be extended. Ultrasonographic screening for hepatocellular carcinoma should be conducted every 6 months. Patients with cirrhosis should be screened for esophageal varices.

SUMMARY
HBV in the setting of HIV is more aggressive than in a patient infected with HBV only, and treatment must be comparably aggressive and carefully selected. The primary goal of HBV treatment in a coinfected patient is the same as in a patient with HBV infection only: reduction of viral load to undetectable levels. Treatment decisions are based on viral load, ALT level, findings on liver biopsy, the need for HAART, and the drug’s resistance profile. None of the nucleoside or nucleotide analogues can be used as monotherapy in the coinfected patient because of the risk of inducing resistance to HIV therapy. When the patient requires HAART, then the general recommendation is to select a combination of two drugs that have activity against HIV. If resistance develops, the preferred strategy is treatment with tenofovir plus entecavir. Monitoring includes measurement of HBV DNA levels every 3 months and ultrasonographic screening for hepatocellular carcinoma every 6 months.
Worldwide, 40 million people are infected with the human immunodeficiency virus (HIV). As many as 4 million of them are coinfected with hepatitis B virus (HBV).1 In North America and Europe, the highest prevalence of HBV/HIV coinfection is in men who have sex with men. Approximately half of HIV-positive men who have sex with men have evidence of prior or active HBV infection, and 5% to 10% have chronic HBV infection. Among those who acquire HIV through injected drug use or through heterosexual transmission, the coinfection rate is much lower.2,3
Coinfection with HBV and HIV follows a different course elsewhere in the world. For example, in Africa and Asia, HBV is usually acquired first through neonatal or childhood infection, with either vertical or horizontal transmission after birth.4,5 In parts of Africa, ritual scarification is likely a major player in the adolescent transmission of HBV. (Ritual scarification is the practice of creating small incisions in the skin of adolescents and rubbing black ash in the wounds to form scars; the cutting instruments are not sterilized between rituals.)
NATURAL HISTORY
In general, HBV tends to be more aggressive in HIV-positive individuals than in monoinfected individuals,2,6 with higher HBV carrier rates, higher levels of HBV viremia, more frequent episodes of activation, and faster progression to cirrhosis.
Hepatocellular carcinoma occurs more often, its onset is earlier, and its course is more aggressive in coinfected individuals than in monoinfected individuals.7,8 Using data from a prospective cohort study, Thio et al9 found that among men coinfected with HIV and HBV, liver-related mortality was almost 19 times greater compared with men infected with HBV only and more than seven times greater compared with those infected with HIV only.
In an observational longitudinal cohort study,10 the risk of death from liver disease in HIV-positive persons was nearly three times greater among those also infected with HBV (P < .0001).
ASSESSING WHEN TO TREAT
The objectives of HBV therapy in individuals coinfected with HIV are similar to those in the population infected with HBV alone. Suppression of viral replication is the major goal. Ideally, the viral load should be reduced to an undetectable level, which will result in normalization of alanine aminotransferase (ALT) level, improved liver histology, reduced risk of progression to cirrhosis and liver failure (although supportive evidence from controlled clinical trials is lacking), and likely reduced incidence of hepatocellular carcinoma.
For those who are hepatitis B e antigen (HBeAg) positive, seroconversion may be a convenient end point for treatment, although for many patients seroconversion is not associated with remission of disease activity or viral replication.
Treatment decisions depend on whether or not the patient requires highly active antiretroviral therapy (HAART) for HIV infection. If HAART is indicated, then HIV agents that have HBV activity are incorporated into the regimen. If the patient does not yet require HAART for HIV but requires treatment for hepatitis B, this is itself an indication for HAART, since monotherapy for HBV is associated with the development of HIV resistance.
Viral load and ALT
Liver biopsy
If the ALT is normal in the presence of a high HBV DNA level, a liver biopsy is recommended, partly because the ALT level is an inadequate indicator of the severity of liver disease. If significant fibrosis is present, treatment is recommended. No treatment is required if fibrosis is mild, but liver biopsies should be repeated every 3 to 5 years in this group because a hallmark of HBV infection is its variability in time to progression. The extent of fibrosis may influence the choice of therapy.
Often in the coinfected patient, HBV-related liver injury must be distinguished from other forms of liver injury. For instance, some of the drugs used to treat HIV infection can induce nonalcoholic fatty liver disease and lipodystrophy. Because the risk of advanced fibrosis is higher in the coinfected patient than in the patient infected only with HBV, the threshold for biopsy in the coinfected patient should be lower.
At present, noninvasive tools to assess the extent of liver injury have not been validated in chronic HBV infection, unlike in hepatitis C virus infection.
TREATMENT OPTIONS
The potential therapies for HBV in the coinfected patient are the same as those for the patient infected with HBV alone (see “Hepatitis B treatment: Current best practices, avoiding resistance”), with the addition of tenofovir and emtricitabine in combination.
Interferon
Early studies of interferon for the treatment of chronic HBV infection included many patients who were also HIV positive. These early studies revealed a lower rate of HBeAg seroconversion in HIV-positive patients compared with HIV-negative patients. Di Martino et al11 found that approximately half (26 of 50) of HIV-negative patients treated with interferon seroconverted at 6 years, compared with only 4 of 26 interferon-treated patients with chronic HBV who were coinfected with HIV. Based on results such as these, interferon therapy in the HBV/HIV-coinfected patient should be limited to patients who are likely to seroconvert: ie, those who are female and younger than 40 years with high ALT levels, low serum HBV DNA levels, and active liver histology (a subgroup that is more likely to undergo spontaneous seroconversion than other HBV-infected groups).
Standard or pegylated interferon is a treatment option for coinfected patients who do not yet require HAART, especially patients who have high ALT levels, low viral loads, and positive HBeAg status without liver decompensation.12
Nucleoside and nucleotide analogues
The nucleoside and nucleotide analogues used for HBV therapy have different degrees of effectiveness against HIV polymerase, but none can be used as monotherapy because of the risk of inducing HIV resistance. Lamivudine and tenofovir are used as part of the standard cocktail for the treatment of HIV (see “Case: HIV/HBV with resistance to tenofovir”). Entecavir is now recognized as a partial inhibitor of HIV replication. Both lamivudine and entecavir induce the YMDD mutation, an indication of resistance to therapy, in HIV polymerase. Tenofovir also may select for resistance mutations in HIV polymerase.
At dosages used for the treatment of HBV infection, adefovir has weak activity against HIV, and therefore HIV would not be under significant selective pressure to develop resistance mutations. In HIV polymerase, the mutations that confer resistance to adefovir also confer resistance to tenofovir, and therefore use of adefovir may induce tenofovir resistance.
Telbivudine has not been studied in HIV-infected patients, but its resistance profile is similar to that of lamivudine.

Treating both infections
When both HBV and HIV infections require treatment, HAART is necessary for HIV.12 The treatment strategy for coinfection is to use standard therapy for HIV, selecting two agents that are effective against HBV infection.
The need to avoid antiviral resistance complicates the selection of active agents. Resistance to HIV therapy limits the choices for treatment of HBV infection. The immediate aim of therapy, an undetectable level of HBV DNA, eliminates the use of less potent agents. The best choice for therapy is the most potent agent that can be used, such as tenofovir plus lamivudine or tenofovir plus emtricitabine.
Antiviral resistance
For the coinfected patient who develops resistance to lamivudine, the recommendation is to treat with tenofovir plus entecavir (the preferable choice because of absence of cross-reactivity between the two agents) or tenofovir plus lamivudine or emtricitabine. There is some evidence that lamivudine resistance predisposes to entecavir resistance, but the studies that generated these results were conducted in patients who had very high baseline viral loads13; the effectiveness of entecavir in patients with low baseline viral loads is unknown. Presumably, when entecavir is used in combination with another potent nucleoside analogue in coinfected patients, the sensitivity of HBV will be more durable than when entecavir is used as monotherapy.
Long-term monitoring
Long-term monitoring for the coinfected patient is similar to that for the patient infected with HBV only. HBV DNA levels should be monitored every 3 months for signs of resistance until levels have plateaued or become undetectable. Once the HBV DNA level is stable or undetectable, the monitoring interval can be extended. Ultrasonographic screening for hepatocellular carcinoma should be conducted every 6 months. Patients with cirrhosis should be screened for esophageal varices.

SUMMARY
HBV in the setting of HIV is more aggressive than in a patient infected with HBV only, and treatment must be comparably aggressive and carefully selected. The primary goal of HBV treatment in a coinfected patient is the same as in a patient with HBV infection only: reduction of viral load to undetectable levels. Treatment decisions are based on viral load, ALT level, findings on liver biopsy, the need for HAART, and the drug’s resistance profile. None of the nucleoside or nucleotide analogues can be used as monotherapy in the coinfected patient because of the risk of inducing resistance to HIV therapy. When the patient requires HAART, then the general recommendation is to select a combination of two drugs that have activity against HIV. If resistance develops, the preferred strategy is treatment with tenofovir plus entecavir. Monitoring includes measurement of HBV DNA levels every 3 months and ultrasonographic screening for hepatocellular carcinoma every 6 months.
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006; 44(suppl 1):S6–S9.
- Soriano V, Puoti M, Peters M, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS 2008; 22:1399–1410.
- Núñez M, Soriano V. Management of patients co-infected with hepatitis B virus and HIV. Lancet Infect Dis 2005; 5:374–382.
- Modi AA, Feld JJ. Viral hepatitis and HIV in Africa. AIDS Rev 2007; 9:25–39.
- Hoffman CJ, Thio CL. Clinical implications of HIV and hepatitis B coinfection in Asia and Africa. Lancet Infect Dis 2007; 7:402–409.
- Puoti M, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in co-infected patients. J Hepatol 2006; 44(suppl 1):S65–S70.
- Puoti M, Bruno R, Soriano V, et al. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS 2004; 18:2285–2293.
- Bräu N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a US-Canadian multicenter study. J Hepatol 2007; 47:527–537.
- Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360:1921–1926.
- Mocroft A, Soriano V, Rockstroh J, et al. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS 2005; 19:2117–2125.
- Di Martino V, Thevenot T, Colin J-F, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002; 123:1812–1822.
- Iser DM, Sasadeusz JJ. Current treatment of HIV/hepatitis B virus coinfection. J Gastroenterol Hepatol 2008; 23:699–706.
- Sherman M, Yurdaydin C, Sollano J, et al; AI463026 BEHoLD Study Group. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology 2006; 130:2039–2049.
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006; 44(suppl 1):S6–S9.
- Soriano V, Puoti M, Peters M, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS 2008; 22:1399–1410.
- Núñez M, Soriano V. Management of patients co-infected with hepatitis B virus and HIV. Lancet Infect Dis 2005; 5:374–382.
- Modi AA, Feld JJ. Viral hepatitis and HIV in Africa. AIDS Rev 2007; 9:25–39.
- Hoffman CJ, Thio CL. Clinical implications of HIV and hepatitis B coinfection in Asia and Africa. Lancet Infect Dis 2007; 7:402–409.
- Puoti M, Torti C, Bruno R, Filice G, Carosi G. Natural history of chronic hepatitis B in co-infected patients. J Hepatol 2006; 44(suppl 1):S65–S70.
- Puoti M, Bruno R, Soriano V, et al. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS 2004; 18:2285–2293.
- Bräu N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a US-Canadian multicenter study. J Hepatol 2007; 47:527–537.
- Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360:1921–1926.
- Mocroft A, Soriano V, Rockstroh J, et al. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS 2005; 19:2117–2125.
- Di Martino V, Thevenot T, Colin J-F, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002; 123:1812–1822.
- Iser DM, Sasadeusz JJ. Current treatment of HIV/hepatitis B virus coinfection. J Gastroenterol Hepatol 2008; 23:699–706.
- Sherman M, Yurdaydin C, Sollano J, et al; AI463026 BEHoLD Study Group. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology 2006; 130:2039–2049.
KEY POINTS
- Patients with HBV/HIV coinfection are at relatively high risk of frequent HBV activation, progression to cirrhosis, and death from liver-related causes.
- If the patient does not yet require HAART but requires treatment for HBV, this is itself an indication for HAART, since monotherapy for HBV is associated with development of resistance to HIV therapy.
- Nucleoside and nucleotide analogues should not be used as monotherapy in the HBV/HIV-coinfected patient because of the risk of inducing HIV resistance.