User login
Patient Satisfaction: the Hospitalist's Role
Introduction
Patient satisfaction is a highly desirable outcome of clinical care in the hospital and may even be an element of health status itself (1). A patient’s expression of satisfaction or dissatisfaction is a judgment on the quality of hospital care in all of its aspects. Whatever its strengths and limitations, patient satisfaction is an indicator that should be indispensable to the assessment of the quality of care in hospitals.
The word “hospital” comes from the Latin for both “guest” and “host,” and the true spirit of hospitality is at the core of the hospital experience (2). The original mission of hospitals was to serve as houses of mercy, refuge, and dying for pilgrims returning from the Holy Land at the time of the late Christian antiquity (3). The striving to please patients is in harmony with the service calling of medicine and is certainly the right thing to do.
Current Reality
From the patient’s perspective, hospitals can be scary and unfriendly places. The American Hospital Association’s Reality Check (4) evaluated the public’s perceptions of hospitals and hospital care using a time-honored technique of asking focus group participants to imagine that the hospital was an animal and a car. Two out of 3 respondents chose animals that would be seen as aggressive, scary, or lumbering to suggest traits such as arrogance, uncontrolled power, and sluggishness. For cars, no respondent chose the Toyota Camry or any other model that would likely make the Consumer Reports list of best values. Instead, the cars chosen were representatives of unreasonable overpricing, waste, and outdated engineering.
“Imagine that the hospital is …”
A Car
“Volkswagen bus...Old, very noisy, just not a real great car.”
“A Rolls Royce, because of the expense.”
“A Pinto, because it was run down.”
“Ford Escort, just barely passing the test.”
“A Cadillac…big and expensive.”
An Animal
“A bear…a grizzly…horrible.”
“Elephant…large…cumbersome.”
“A leech.…I’m sure all hospitals aren’t that lowly.”
“A snake, kind of slithery and sneaky, because of what hospitals charge.”
The same AHA survey showed that patients felt that insurance companies and not physicians were in charge of their care in the hospital. A follow-up question revealed that patients clearly want to be in charge of their own hospital care. Additionally, patients do not see hospitals as part of a planned or consumer-focused health care system. In fact, they see quite the opposite: a confusing, expensive, unreliable, and often impersonal disassembly of medical professionals and institutions. If they see any system at all, it is one devoted to maximizing profits by blocking access, reducing quality, and limiting spending, all at the expense of the patient.
The American Customer Satisfaction Index (5) gave hospitals an overall 67% satisfaction rating, ranking 27th out of 31 industries. This ranking placed hospitals 10 percentage points below the tobacco industry and just above the Internal Revenue Service. A National Coalition on Health Care survey (6) found that 80% of respondents believe hospitals cut corners to save money, and 77% believe that these cuts have endangered patients. Quality of care and patient safety have become significant public concerns recently. “To Err is Human,” the 1999 report from the Institute of Medicine, highlighted the potential for serious injury and death in U.S. hospitals (7). Estimates are that 44,000 to 98,000 Americans die each year in hospitals as a result of medical errors and unsafe practices.
Patient Satisfaction Is Important
Patient satisfaction is the health care recipient’s reaction to aspects of his or her service experience (8). Patient satisfaction belongs to the service dimension as opposed to the technical dimension of quality of care. Most patients report few problems related to technical quality of care in hospitals and moreover do not feel qualified to judge technical quality and therefore assume technical competence (9).
From 1986 to 1992, the Health Care Financing Administration publicly reported hospital mortality rates as an effort to aid consumers in selecting hospitals (10). Hospitals with over twice the expected mortality rate saw very little change in volume during that time. Patients do not seem to be affected by morbidity and mortality statistics but more by personal stories of care. Patient perception of quality is assessed through dimensions of what is personally valued, and often they do not distinguish between the provider of the service and the service received.
Being treated with respect and dignity and involvement in treatment decisions are intangible issues of patient satisfaction that are paramount issues for patients (11).
As many as one quarter of 13,000 patients discharged from 51 Massachusetts hospitals reported problems with patient satisfaction issues such as the involvement of families in their care, communication and coordination of care, and the transition from hospital to home (12). Patients who had been admitted to academic health centers and teaching hospitals generally reported more problems than those cared for in community hospitals. As it turns out, service outcomes such as patient satisfaction drive market share and profitability for hospitals (13). With the average medical/surgery hospital charge amounting to $12,083, hospitals cannot afford to lose patients and, therefore, revenue due to issues of patient dissatisfaction (14). Recruiting new patients as customers is 5 times more costly than retaining an existing customer base (15).
There is compelling evidence from well-developed lines of research demonstrating that increasing patient satisfaction improves clinical outcomes, such as functional status and physiologic measures of health (16,17). Finally, it is important for clinicians to know that it has been clearly demonstrated that satisfied patients improve physician satisfaction (18,19).
Psychology of Satisfaction
To create a culture of customer service excellence in hospitals and achieve outstanding patient satisfaction, it is necessary to understand the intangible aspects of perception and expectation that contribute to patient satisfaction. The “First Law of Service” provides a useful, simple mathematical model of satisfaction (20). The formula for this model is Satisfaction = Perception – Expectation. If a patient’s perception of their hospital experience meets or exceeds the expectation, there will be a corresponding degree of satisfaction. However, if the perception does not meet the expectation, there will be resulting dissatisfaction. Thus, patient satisfaction results from meeting or exceeding patients’ expectations. Patient perceptions of care can be measured directly from patient satisfaction surveys, focus groups, and telephone surveys. A hospital’s reputation and market share are indirect measurements of patient perceptions. There are 2 main directions in which patient satisfaction can be influenced: by working on what the patient perceives and on what the patient expects.
Expectations are integral to the experience of being a customer. There has been confusion and controversy in health care as to whether patients are in fact customers. This confusion may be at the root of the overall service failing of hospitals (21). “The more horizontal they are, the more they are a patient. The more vertical they are, the more they are a customer” (22). Using a technical definition, a customer is anyone who has expectations about process operations or outputs (23). Therefore, all patients are customers, but not all customers are patients (21). Hospitals have a whole list of primary and secondary customers, each of whom has his or her own set of expectations. Patients and their families can be seen as primary customers, and referring physicians, third-party payers, external overseers, communities, shareholders, and employees are all secondary customers of hospitals.
Expectations are psychological phenomena that can be defined as beliefs created and sustained by cognitive processes (24). Expectations of patients as hospital customers rise from past experiences of their own or of others, as well as from current needs and unique internal preferences that form the basis of a value system. Expectations cannot be mandated, because they are based in self-gratification. However, expectations may change over time, and, very importantly, they can be measured and perhaps modified through education. For most people, illness and hospitalization is a rare event. Patients will know something is wrong and that treatment is needed, but most won’t know the nature of their disease, the diagnostic and therapeutic options, and the likely outcomes. Therefore, patients seek out health care professionals who have the opportunity to inform them on what to expect as a way to begin the process of managing expectations.
The Kano Model
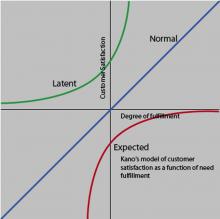
The Kano Model provides a useful tool for studying different levels of patient expectations (23).
This model is useful to examine the voice of the customer in the relationship between satisfaction and quality, and it is relevant for hospital encounters. According to the model, patients will have a basic set of subconscious expectations about their care that will be taken for granted. These expectations are so routine and expected that patients don’t recognize them as comparative quality factors, but they will be shocked if they are absent. For example, patients assume that physicians are basically competent, and that hospitals are capable of providing safe, courteous, lifesaving medical care (25). Although these expectations are in the patient’s subconscious, if they are not met, the patient will be dissatisfied. Providing this level of basic quality isn’t necessarily enough to create satisfied patients.
There is a normal level of service that patients consciously consider, they have to do with the anticipated issues of hospital care related to access, wait times, scheduling, and billing. The model shows that satisfaction increases as more of these expectations are met and that patients will be dissatisfied if these quality expectations are not met. Patients use comparisons of these expectations to recognize differences among competitors and to make choices.
The latent quality curve lies entirely in the satisfaction region and represents supraconscious, unexpected quality items that patients didn’t know they wanted and result in a delightful surprise when present. In this experience, patients receive more than they had expected, often as the result of innovation that can raise patient expectations and provide a significant competitive advantage. The data from hospitals show that this is achieved primarily through the patient’s perception of personalized, customized service, provided by caring and concerned clinicians (26).
There is a tremendous opportunity in hospital care to modify patient expectations through education and to create high levels of patient satisfaction. Hospitals that are successful in this endeavor will have a significant competitive advantage. Hospital patients have a whole list of issues about which they have expectations: the smoothness of the admission and discharge process, accuracy and clarity of billing statements, courtesy of hospital employees, response time for calls and requests, the level of technology available in the hospital, nurse competency, taste and temperature of the food, and price. Most of these issues are not directly related to clinical care and certainly not under the control or influence of the hospitalist. What are the expectations that patients have for their clinical care by hospitalists, and how can we give it to them?
The Hospitalist as a Caring and Concerned Clinician
A useful model to define the hospitalist’s role in patient satisfaction is that of a caring and concerned clinician. This caring and concern for patients is exemplified by attentiveness, dignity and respect, effective information transfer, and shared decision making (23).
The outcome service chain for hospitals begins with the patient’s perception of caring and concerned clinicians who demonstrate these attributes of attentiveness, dignity and respect, effective information transfer, and shared decision making. This leads to the degree of patient satisfaction and loyalty that results in patients who will return to the hospital, seek related business, and refer additional business. This drives market share and financial success for hospitals (9).
The characteristics of the caring and concerned clinician begin with attentiveness. This is the practice of establishing a person-to-person connection with patients and involves attending to them as unique individuals and not just in their role as patients. The constant interruptions that occur in physician/patient encounters, control issues, discontinuity of care, and the often overwhelming complexity of a patient’s illness can be obstacles to the perception of attentiveness that result in patients feeling connected with the clinician. Some effective tools for attentiveness are demonstrating curiosity about the patient as a person, using open-ended questions to gather clinical data, orienting patients to the process of care, and actively eliciting a patient’s agenda for their care and then summarizing their concerns.
The demonstration of dignity and respect results in a patient’s feeling understood and accepted as a person. This is the practice of empathy, which is often confused with sympathy (27). Sympathy is an expression of one’s own feelings (“I’m sorry”). Empathy is the demonstration of an understanding of the patient’s feelings (“You must be very sad”). The confusion of medical terminology as well as the time constraints of modern hospital-care encounters can be obstacles to achieving the type of dignity and respect that results in a high degree of patient satisfaction. A number of effective tools are available to facilitate this important result. Sitting down during patient encounters greatly enhances the perception of time and caring of hospitalists on the part of patients. Eye contact and appropriate touch are demonstrations of dignity and respect, as is seeing patients fully clothed. It is important to pay attention to nonverbal communication issues with patients. An important element of how hospitalists are perceived by patients has to do with nonverbal issues such as demeanor, body posture, and verbal tone. Using a patient’s own words and addressing underlying feelings facilitates the practice of dignity and respect (28). Patients perceive statements of assured understanding as confirmation that they have been listened to. Remember the words of Sir William Osler: “Listening is unspoken caring.”
The effective transfer of information is at the core of physician/patient communication (29). Patients have the need to provide complete information to physicians to facilitate an accurate diagnosis. The physician’s role is to provide information that addresses the cognitive, behavioral, and affective needs of patients and their families concerning their illness. The discrepancy of language, time constraints, and the ability of patients to remember are all barriers to the effective transfer of information. Some useful techniques for effective information transfer include assessing a patient’s current level of understanding and asking about their self-diagnosis. Timeliness in providing results of diagnostic tests is an important issue to patients who are often waiting expectantly. Studies have shown that the majority of patients have questions about the so-called “mysteries of medicine,” related to the diagnosis, etiology, and prognosis of their illness (30).
Patients may not specifically ask these questions; however, they are present, and patient satisfaction will increase if these questions are answered. Patients have decisively indicated their desire for shared decision making regarding their health care and for patient and family control of all-important choices (26). The process of shared decision making can be facilitated by collaboration between patients and the hospitalist around goals ands plans for treatment in the hospital (31). A barrier to shared decision making is a patient/physician relationship based on a model of paternalism (“I’m the doctor, and I know best”). A more productive model for the therapeutic relationship is that of a partnership between the hospitalist and the patient, particularly in the present era of web-educated, sophisticated consumer patients.
An important tool to achieving this type of collaboration involves the approach of presenting patients and families with treatment options and then actively soliciting patient preferences. A question is whether patients will actively participate in treatment decisions and then adhere to treatment plans. This is in large part determined by the interpersonal relationship skills of the clinician and can be further facilitated with simplified regimens that have been agreed upon by the patient and the hospitalist (32).
A complete model of the hospital-care encounter provided by hospitalists has an opening and a closing. In between there is a series of moments of truth that can potentially be imbued with attentiveness, dignity and respect, effective information transfer, and shared decision making. The opening is a brief moment that will set the stage for the remainder of the encounter. Greeting patients by name and maintaining eye contact will help in establishing the early perception of being a caring and concerned clinician. It is important to close hospital encounters with a sense of hope and optimism, making sure that all of the patient’s issues have been addressed, as well as planning for the next steps.
The development and growth of hospital medicine is the latest site-specific evolution of practice specialization and focuses on the complex care of hospitalized patients. Hospitalists spend most of their professional time in the hospital providing care for general medical patients and are well positioned and uniquely committed to improving the care of hospitalized patients. Exceptional patient satisfaction is a key outcome that should result from the care provided by hospitalists.
References
- Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743-8.
- Zaleski P. Knights Hospitaller: the rise and fall of a chivalric order of Christian caregiving. Parabola. 1990;15:55-62.
- Risse GB. Mending Bodies, Saving Souls. New York, NY: Oxford University Press; 1999.
- Reality Check: Public Perceptions of Health Care and Hospitals. The American Hospital Association. 1996.
- Now are you satisfied? Fortune. February 1998:166.
- National Coalition on Health Care. How Americans perceive the health care system. www.nchc.org/perceive.html. Accessed August 2004.
- To Err Is Human: Building a Safer Health Care System. Committee on Quality of Health Care in America, Institute of Medicine. Washington, DC: National Academy Press; 1998.
- Pascoe GC. Patient satisfaction in primary health care: a literature review and analysis. Eval Prog Plann. 1983;6:185-210.
- Kenagy JW, Berwick DM, Shore MF. Service quality in heath care. JAMA. 1999;281:661-5.
- Mennemeyer ST, Morrisey MA, Howard LZ. Death and reputation: how consumers acted upon HCFA mortality information. Inquiry. 1997;34:117-28.
- Cleary PD, Edgman-Levitan S. Health care quality. Incorporating consumer perspectives. JAMA. 1997;278:1608-12.
- Rogers G, Smith DP. Reporting comparative results from hospital surveys. Int J Qual Health Care. 1999;11:251-9.
- Schlesinger LA, Heskett JL. The service-driven service company. Harvard Business Review. 1991;Sept-Oct:1-19.
- Modern Health care. 2000;May:70.
- Mittal B, Lassar W. Why do customers switch? The dynamics of satisfaction versus loyalty. Journal of Services Marketing. 1998;12:177-191.
- Greenfield S, Kaplan S, Ware WE Jr. Expanding patient involvement in care: Effects on patient outcomes. Ann Intern Med. 1985;102:520-8.
- Sobel DS. Rethinking medicine: improving health outcomes with cost-effective psychosocial interventions. Psychosom Med. 1995;57:234-44.
- Novack DH, Suchman AL, Clark W, Epstein RM, Najberg E, Kaplan C. Calibrating the physician. Personal awareness and effective patient care. Working Group on Promoting Physician Personal Awareness, American Academy on Physician and Patient. JAMA. 1997;278:502-9.
- Suchman AL, Roter D, Green M, Lipkin M Jr. Physician satisfaction with primary care office visits. Collaborative Study Group of the American Academy on Physician and Patient. Med Care. 1993;31:1083-92.
- Maister DH. The Psychology of Waiting Lines. Case No. 9-684-064. Boston, Mass: Harvard Business School Publishing; 1984.
- Fottler MD, Ford RC, Heaton CP. Achieving Service Excellence. Chicago, Ill.: Health Administration Press; 2002.
- Mayer T, Cates RJ. Service excellence in health care. JAMA. 1999;282:1281-83.
- James BC. Curing vs. Caring: The Art of Service Quality. Institute for Health Care Delivery Research. Intermountain Health Care. Salt Lake City, Utah; 2003.
- Thompson AG, Sunol R. Expectations as determinants of patient satisfaction: concepts, theory and evidence. Int J Qual Health Care. 1995;7:127-41.
- Larson CO, Nelson EC, Gustafson D, Batalden PB. The relationship between meeting patient’s information needs and their satisfaction with hospital care and general health status outcomes. Int J Qual Health Care. 1996;8:447-56.
- Gerteis M, Edgman-Levitan S, Daley J, Deblanco T, eds. Through the Patient’s Eyes. San Francisco, Calif.: Jossey-Bass; 1993.
- Spiro H, Peschel E, Curnen MG, St. James D, eds. Empathy and the Practice of Medicine: Beyond Pills and the Scalpel. New Haven, Conn.: Yale University Press; 1993.
- Branch WT, Malik TK. Using ‘windows of opportunities’ in brief interviews to understand patients’ concerns. JAMA. 1993;269:1667-68.
- Worthlin Group. Communication and the physician/patient relationship: A physician and communication survey. Bayer Institute for Health Care Communication. New Haven, CT; 1995.
- Clinician Patient Communication to Enhance Health Outcomes. Bayer Institute for Health Care Communication Workshop. Bayer Institute, New Haven, Conn.; 1999.
- Donovan J, Blake R. Patient non-compliance: deviance or reasoned decision-making? Soc Sci Med. 1992;34:507-13.
- DiMatteo MR, Reiter RC, Gambone JC. Enhancing medication adherence through communication and informed collaborative choice. Health Commun. 1994;6:253-65.
Introduction
Patient satisfaction is a highly desirable outcome of clinical care in the hospital and may even be an element of health status itself (1). A patient’s expression of satisfaction or dissatisfaction is a judgment on the quality of hospital care in all of its aspects. Whatever its strengths and limitations, patient satisfaction is an indicator that should be indispensable to the assessment of the quality of care in hospitals.
The word “hospital” comes from the Latin for both “guest” and “host,” and the true spirit of hospitality is at the core of the hospital experience (2). The original mission of hospitals was to serve as houses of mercy, refuge, and dying for pilgrims returning from the Holy Land at the time of the late Christian antiquity (3). The striving to please patients is in harmony with the service calling of medicine and is certainly the right thing to do.
Current Reality
From the patient’s perspective, hospitals can be scary and unfriendly places. The American Hospital Association’s Reality Check (4) evaluated the public’s perceptions of hospitals and hospital care using a time-honored technique of asking focus group participants to imagine that the hospital was an animal and a car. Two out of 3 respondents chose animals that would be seen as aggressive, scary, or lumbering to suggest traits such as arrogance, uncontrolled power, and sluggishness. For cars, no respondent chose the Toyota Camry or any other model that would likely make the Consumer Reports list of best values. Instead, the cars chosen were representatives of unreasonable overpricing, waste, and outdated engineering.
“Imagine that the hospital is …”
A Car
“Volkswagen bus...Old, very noisy, just not a real great car.”
“A Rolls Royce, because of the expense.”
“A Pinto, because it was run down.”
“Ford Escort, just barely passing the test.”
“A Cadillac…big and expensive.”
An Animal
“A bear…a grizzly…horrible.”
“Elephant…large…cumbersome.”
“A leech.…I’m sure all hospitals aren’t that lowly.”
“A snake, kind of slithery and sneaky, because of what hospitals charge.”
The same AHA survey showed that patients felt that insurance companies and not physicians were in charge of their care in the hospital. A follow-up question revealed that patients clearly want to be in charge of their own hospital care. Additionally, patients do not see hospitals as part of a planned or consumer-focused health care system. In fact, they see quite the opposite: a confusing, expensive, unreliable, and often impersonal disassembly of medical professionals and institutions. If they see any system at all, it is one devoted to maximizing profits by blocking access, reducing quality, and limiting spending, all at the expense of the patient.
The American Customer Satisfaction Index (5) gave hospitals an overall 67% satisfaction rating, ranking 27th out of 31 industries. This ranking placed hospitals 10 percentage points below the tobacco industry and just above the Internal Revenue Service. A National Coalition on Health Care survey (6) found that 80% of respondents believe hospitals cut corners to save money, and 77% believe that these cuts have endangered patients. Quality of care and patient safety have become significant public concerns recently. “To Err is Human,” the 1999 report from the Institute of Medicine, highlighted the potential for serious injury and death in U.S. hospitals (7). Estimates are that 44,000 to 98,000 Americans die each year in hospitals as a result of medical errors and unsafe practices.
Patient Satisfaction Is Important
Patient satisfaction is the health care recipient’s reaction to aspects of his or her service experience (8). Patient satisfaction belongs to the service dimension as opposed to the technical dimension of quality of care. Most patients report few problems related to technical quality of care in hospitals and moreover do not feel qualified to judge technical quality and therefore assume technical competence (9).
From 1986 to 1992, the Health Care Financing Administration publicly reported hospital mortality rates as an effort to aid consumers in selecting hospitals (10). Hospitals with over twice the expected mortality rate saw very little change in volume during that time. Patients do not seem to be affected by morbidity and mortality statistics but more by personal stories of care. Patient perception of quality is assessed through dimensions of what is personally valued, and often they do not distinguish between the provider of the service and the service received.
Being treated with respect and dignity and involvement in treatment decisions are intangible issues of patient satisfaction that are paramount issues for patients (11).
As many as one quarter of 13,000 patients discharged from 51 Massachusetts hospitals reported problems with patient satisfaction issues such as the involvement of families in their care, communication and coordination of care, and the transition from hospital to home (12). Patients who had been admitted to academic health centers and teaching hospitals generally reported more problems than those cared for in community hospitals. As it turns out, service outcomes such as patient satisfaction drive market share and profitability for hospitals (13). With the average medical/surgery hospital charge amounting to $12,083, hospitals cannot afford to lose patients and, therefore, revenue due to issues of patient dissatisfaction (14). Recruiting new patients as customers is 5 times more costly than retaining an existing customer base (15).
There is compelling evidence from well-developed lines of research demonstrating that increasing patient satisfaction improves clinical outcomes, such as functional status and physiologic measures of health (16,17). Finally, it is important for clinicians to know that it has been clearly demonstrated that satisfied patients improve physician satisfaction (18,19).
Psychology of Satisfaction
To create a culture of customer service excellence in hospitals and achieve outstanding patient satisfaction, it is necessary to understand the intangible aspects of perception and expectation that contribute to patient satisfaction. The “First Law of Service” provides a useful, simple mathematical model of satisfaction (20). The formula for this model is Satisfaction = Perception – Expectation. If a patient’s perception of their hospital experience meets or exceeds the expectation, there will be a corresponding degree of satisfaction. However, if the perception does not meet the expectation, there will be resulting dissatisfaction. Thus, patient satisfaction results from meeting or exceeding patients’ expectations. Patient perceptions of care can be measured directly from patient satisfaction surveys, focus groups, and telephone surveys. A hospital’s reputation and market share are indirect measurements of patient perceptions. There are 2 main directions in which patient satisfaction can be influenced: by working on what the patient perceives and on what the patient expects.
Expectations are integral to the experience of being a customer. There has been confusion and controversy in health care as to whether patients are in fact customers. This confusion may be at the root of the overall service failing of hospitals (21). “The more horizontal they are, the more they are a patient. The more vertical they are, the more they are a customer” (22). Using a technical definition, a customer is anyone who has expectations about process operations or outputs (23). Therefore, all patients are customers, but not all customers are patients (21). Hospitals have a whole list of primary and secondary customers, each of whom has his or her own set of expectations. Patients and their families can be seen as primary customers, and referring physicians, third-party payers, external overseers, communities, shareholders, and employees are all secondary customers of hospitals.
Expectations are psychological phenomena that can be defined as beliefs created and sustained by cognitive processes (24). Expectations of patients as hospital customers rise from past experiences of their own or of others, as well as from current needs and unique internal preferences that form the basis of a value system. Expectations cannot be mandated, because they are based in self-gratification. However, expectations may change over time, and, very importantly, they can be measured and perhaps modified through education. For most people, illness and hospitalization is a rare event. Patients will know something is wrong and that treatment is needed, but most won’t know the nature of their disease, the diagnostic and therapeutic options, and the likely outcomes. Therefore, patients seek out health care professionals who have the opportunity to inform them on what to expect as a way to begin the process of managing expectations.
The Kano Model
The Kano Model provides a useful tool for studying different levels of patient expectations (23).
This model is useful to examine the voice of the customer in the relationship between satisfaction and quality, and it is relevant for hospital encounters. According to the model, patients will have a basic set of subconscious expectations about their care that will be taken for granted. These expectations are so routine and expected that patients don’t recognize them as comparative quality factors, but they will be shocked if they are absent. For example, patients assume that physicians are basically competent, and that hospitals are capable of providing safe, courteous, lifesaving medical care (25). Although these expectations are in the patient’s subconscious, if they are not met, the patient will be dissatisfied. Providing this level of basic quality isn’t necessarily enough to create satisfied patients.
There is a normal level of service that patients consciously consider, they have to do with the anticipated issues of hospital care related to access, wait times, scheduling, and billing. The model shows that satisfaction increases as more of these expectations are met and that patients will be dissatisfied if these quality expectations are not met. Patients use comparisons of these expectations to recognize differences among competitors and to make choices.
The latent quality curve lies entirely in the satisfaction region and represents supraconscious, unexpected quality items that patients didn’t know they wanted and result in a delightful surprise when present. In this experience, patients receive more than they had expected, often as the result of innovation that can raise patient expectations and provide a significant competitive advantage. The data from hospitals show that this is achieved primarily through the patient’s perception of personalized, customized service, provided by caring and concerned clinicians (26).
There is a tremendous opportunity in hospital care to modify patient expectations through education and to create high levels of patient satisfaction. Hospitals that are successful in this endeavor will have a significant competitive advantage. Hospital patients have a whole list of issues about which they have expectations: the smoothness of the admission and discharge process, accuracy and clarity of billing statements, courtesy of hospital employees, response time for calls and requests, the level of technology available in the hospital, nurse competency, taste and temperature of the food, and price. Most of these issues are not directly related to clinical care and certainly not under the control or influence of the hospitalist. What are the expectations that patients have for their clinical care by hospitalists, and how can we give it to them?
The Hospitalist as a Caring and Concerned Clinician
A useful model to define the hospitalist’s role in patient satisfaction is that of a caring and concerned clinician. This caring and concern for patients is exemplified by attentiveness, dignity and respect, effective information transfer, and shared decision making (23).
The outcome service chain for hospitals begins with the patient’s perception of caring and concerned clinicians who demonstrate these attributes of attentiveness, dignity and respect, effective information transfer, and shared decision making. This leads to the degree of patient satisfaction and loyalty that results in patients who will return to the hospital, seek related business, and refer additional business. This drives market share and financial success for hospitals (9).
The characteristics of the caring and concerned clinician begin with attentiveness. This is the practice of establishing a person-to-person connection with patients and involves attending to them as unique individuals and not just in their role as patients. The constant interruptions that occur in physician/patient encounters, control issues, discontinuity of care, and the often overwhelming complexity of a patient’s illness can be obstacles to the perception of attentiveness that result in patients feeling connected with the clinician. Some effective tools for attentiveness are demonstrating curiosity about the patient as a person, using open-ended questions to gather clinical data, orienting patients to the process of care, and actively eliciting a patient’s agenda for their care and then summarizing their concerns.
The demonstration of dignity and respect results in a patient’s feeling understood and accepted as a person. This is the practice of empathy, which is often confused with sympathy (27). Sympathy is an expression of one’s own feelings (“I’m sorry”). Empathy is the demonstration of an understanding of the patient’s feelings (“You must be very sad”). The confusion of medical terminology as well as the time constraints of modern hospital-care encounters can be obstacles to achieving the type of dignity and respect that results in a high degree of patient satisfaction. A number of effective tools are available to facilitate this important result. Sitting down during patient encounters greatly enhances the perception of time and caring of hospitalists on the part of patients. Eye contact and appropriate touch are demonstrations of dignity and respect, as is seeing patients fully clothed. It is important to pay attention to nonverbal communication issues with patients. An important element of how hospitalists are perceived by patients has to do with nonverbal issues such as demeanor, body posture, and verbal tone. Using a patient’s own words and addressing underlying feelings facilitates the practice of dignity and respect (28). Patients perceive statements of assured understanding as confirmation that they have been listened to. Remember the words of Sir William Osler: “Listening is unspoken caring.”
The effective transfer of information is at the core of physician/patient communication (29). Patients have the need to provide complete information to physicians to facilitate an accurate diagnosis. The physician’s role is to provide information that addresses the cognitive, behavioral, and affective needs of patients and their families concerning their illness. The discrepancy of language, time constraints, and the ability of patients to remember are all barriers to the effective transfer of information. Some useful techniques for effective information transfer include assessing a patient’s current level of understanding and asking about their self-diagnosis. Timeliness in providing results of diagnostic tests is an important issue to patients who are often waiting expectantly. Studies have shown that the majority of patients have questions about the so-called “mysteries of medicine,” related to the diagnosis, etiology, and prognosis of their illness (30).
Patients may not specifically ask these questions; however, they are present, and patient satisfaction will increase if these questions are answered. Patients have decisively indicated their desire for shared decision making regarding their health care and for patient and family control of all-important choices (26). The process of shared decision making can be facilitated by collaboration between patients and the hospitalist around goals ands plans for treatment in the hospital (31). A barrier to shared decision making is a patient/physician relationship based on a model of paternalism (“I’m the doctor, and I know best”). A more productive model for the therapeutic relationship is that of a partnership between the hospitalist and the patient, particularly in the present era of web-educated, sophisticated consumer patients.
An important tool to achieving this type of collaboration involves the approach of presenting patients and families with treatment options and then actively soliciting patient preferences. A question is whether patients will actively participate in treatment decisions and then adhere to treatment plans. This is in large part determined by the interpersonal relationship skills of the clinician and can be further facilitated with simplified regimens that have been agreed upon by the patient and the hospitalist (32).
A complete model of the hospital-care encounter provided by hospitalists has an opening and a closing. In between there is a series of moments of truth that can potentially be imbued with attentiveness, dignity and respect, effective information transfer, and shared decision making. The opening is a brief moment that will set the stage for the remainder of the encounter. Greeting patients by name and maintaining eye contact will help in establishing the early perception of being a caring and concerned clinician. It is important to close hospital encounters with a sense of hope and optimism, making sure that all of the patient’s issues have been addressed, as well as planning for the next steps.
The development and growth of hospital medicine is the latest site-specific evolution of practice specialization and focuses on the complex care of hospitalized patients. Hospitalists spend most of their professional time in the hospital providing care for general medical patients and are well positioned and uniquely committed to improving the care of hospitalized patients. Exceptional patient satisfaction is a key outcome that should result from the care provided by hospitalists.
References
- Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743-8.
- Zaleski P. Knights Hospitaller: the rise and fall of a chivalric order of Christian caregiving. Parabola. 1990;15:55-62.
- Risse GB. Mending Bodies, Saving Souls. New York, NY: Oxford University Press; 1999.
- Reality Check: Public Perceptions of Health Care and Hospitals. The American Hospital Association. 1996.
- Now are you satisfied? Fortune. February 1998:166.
- National Coalition on Health Care. How Americans perceive the health care system. www.nchc.org/perceive.html. Accessed August 2004.
- To Err Is Human: Building a Safer Health Care System. Committee on Quality of Health Care in America, Institute of Medicine. Washington, DC: National Academy Press; 1998.
- Pascoe GC. Patient satisfaction in primary health care: a literature review and analysis. Eval Prog Plann. 1983;6:185-210.
- Kenagy JW, Berwick DM, Shore MF. Service quality in heath care. JAMA. 1999;281:661-5.
- Mennemeyer ST, Morrisey MA, Howard LZ. Death and reputation: how consumers acted upon HCFA mortality information. Inquiry. 1997;34:117-28.
- Cleary PD, Edgman-Levitan S. Health care quality. Incorporating consumer perspectives. JAMA. 1997;278:1608-12.
- Rogers G, Smith DP. Reporting comparative results from hospital surveys. Int J Qual Health Care. 1999;11:251-9.
- Schlesinger LA, Heskett JL. The service-driven service company. Harvard Business Review. 1991;Sept-Oct:1-19.
- Modern Health care. 2000;May:70.
- Mittal B, Lassar W. Why do customers switch? The dynamics of satisfaction versus loyalty. Journal of Services Marketing. 1998;12:177-191.
- Greenfield S, Kaplan S, Ware WE Jr. Expanding patient involvement in care: Effects on patient outcomes. Ann Intern Med. 1985;102:520-8.
- Sobel DS. Rethinking medicine: improving health outcomes with cost-effective psychosocial interventions. Psychosom Med. 1995;57:234-44.
- Novack DH, Suchman AL, Clark W, Epstein RM, Najberg E, Kaplan C. Calibrating the physician. Personal awareness and effective patient care. Working Group on Promoting Physician Personal Awareness, American Academy on Physician and Patient. JAMA. 1997;278:502-9.
- Suchman AL, Roter D, Green M, Lipkin M Jr. Physician satisfaction with primary care office visits. Collaborative Study Group of the American Academy on Physician and Patient. Med Care. 1993;31:1083-92.
- Maister DH. The Psychology of Waiting Lines. Case No. 9-684-064. Boston, Mass: Harvard Business School Publishing; 1984.
- Fottler MD, Ford RC, Heaton CP. Achieving Service Excellence. Chicago, Ill.: Health Administration Press; 2002.
- Mayer T, Cates RJ. Service excellence in health care. JAMA. 1999;282:1281-83.
- James BC. Curing vs. Caring: The Art of Service Quality. Institute for Health Care Delivery Research. Intermountain Health Care. Salt Lake City, Utah; 2003.
- Thompson AG, Sunol R. Expectations as determinants of patient satisfaction: concepts, theory and evidence. Int J Qual Health Care. 1995;7:127-41.
- Larson CO, Nelson EC, Gustafson D, Batalden PB. The relationship between meeting patient’s information needs and their satisfaction with hospital care and general health status outcomes. Int J Qual Health Care. 1996;8:447-56.
- Gerteis M, Edgman-Levitan S, Daley J, Deblanco T, eds. Through the Patient’s Eyes. San Francisco, Calif.: Jossey-Bass; 1993.
- Spiro H, Peschel E, Curnen MG, St. James D, eds. Empathy and the Practice of Medicine: Beyond Pills and the Scalpel. New Haven, Conn.: Yale University Press; 1993.
- Branch WT, Malik TK. Using ‘windows of opportunities’ in brief interviews to understand patients’ concerns. JAMA. 1993;269:1667-68.
- Worthlin Group. Communication and the physician/patient relationship: A physician and communication survey. Bayer Institute for Health Care Communication. New Haven, CT; 1995.
- Clinician Patient Communication to Enhance Health Outcomes. Bayer Institute for Health Care Communication Workshop. Bayer Institute, New Haven, Conn.; 1999.
- Donovan J, Blake R. Patient non-compliance: deviance or reasoned decision-making? Soc Sci Med. 1992;34:507-13.
- DiMatteo MR, Reiter RC, Gambone JC. Enhancing medication adherence through communication and informed collaborative choice. Health Commun. 1994;6:253-65.
Introduction
Patient satisfaction is a highly desirable outcome of clinical care in the hospital and may even be an element of health status itself (1). A patient’s expression of satisfaction or dissatisfaction is a judgment on the quality of hospital care in all of its aspects. Whatever its strengths and limitations, patient satisfaction is an indicator that should be indispensable to the assessment of the quality of care in hospitals.
The word “hospital” comes from the Latin for both “guest” and “host,” and the true spirit of hospitality is at the core of the hospital experience (2). The original mission of hospitals was to serve as houses of mercy, refuge, and dying for pilgrims returning from the Holy Land at the time of the late Christian antiquity (3). The striving to please patients is in harmony with the service calling of medicine and is certainly the right thing to do.
Current Reality
From the patient’s perspective, hospitals can be scary and unfriendly places. The American Hospital Association’s Reality Check (4) evaluated the public’s perceptions of hospitals and hospital care using a time-honored technique of asking focus group participants to imagine that the hospital was an animal and a car. Two out of 3 respondents chose animals that would be seen as aggressive, scary, or lumbering to suggest traits such as arrogance, uncontrolled power, and sluggishness. For cars, no respondent chose the Toyota Camry or any other model that would likely make the Consumer Reports list of best values. Instead, the cars chosen were representatives of unreasonable overpricing, waste, and outdated engineering.
“Imagine that the hospital is …”
A Car
“Volkswagen bus...Old, very noisy, just not a real great car.”
“A Rolls Royce, because of the expense.”
“A Pinto, because it was run down.”
“Ford Escort, just barely passing the test.”
“A Cadillac…big and expensive.”
An Animal
“A bear…a grizzly…horrible.”
“Elephant…large…cumbersome.”
“A leech.…I’m sure all hospitals aren’t that lowly.”
“A snake, kind of slithery and sneaky, because of what hospitals charge.”
The same AHA survey showed that patients felt that insurance companies and not physicians were in charge of their care in the hospital. A follow-up question revealed that patients clearly want to be in charge of their own hospital care. Additionally, patients do not see hospitals as part of a planned or consumer-focused health care system. In fact, they see quite the opposite: a confusing, expensive, unreliable, and often impersonal disassembly of medical professionals and institutions. If they see any system at all, it is one devoted to maximizing profits by blocking access, reducing quality, and limiting spending, all at the expense of the patient.
The American Customer Satisfaction Index (5) gave hospitals an overall 67% satisfaction rating, ranking 27th out of 31 industries. This ranking placed hospitals 10 percentage points below the tobacco industry and just above the Internal Revenue Service. A National Coalition on Health Care survey (6) found that 80% of respondents believe hospitals cut corners to save money, and 77% believe that these cuts have endangered patients. Quality of care and patient safety have become significant public concerns recently. “To Err is Human,” the 1999 report from the Institute of Medicine, highlighted the potential for serious injury and death in U.S. hospitals (7). Estimates are that 44,000 to 98,000 Americans die each year in hospitals as a result of medical errors and unsafe practices.
Patient Satisfaction Is Important
Patient satisfaction is the health care recipient’s reaction to aspects of his or her service experience (8). Patient satisfaction belongs to the service dimension as opposed to the technical dimension of quality of care. Most patients report few problems related to technical quality of care in hospitals and moreover do not feel qualified to judge technical quality and therefore assume technical competence (9).
From 1986 to 1992, the Health Care Financing Administration publicly reported hospital mortality rates as an effort to aid consumers in selecting hospitals (10). Hospitals with over twice the expected mortality rate saw very little change in volume during that time. Patients do not seem to be affected by morbidity and mortality statistics but more by personal stories of care. Patient perception of quality is assessed through dimensions of what is personally valued, and often they do not distinguish between the provider of the service and the service received.
Being treated with respect and dignity and involvement in treatment decisions are intangible issues of patient satisfaction that are paramount issues for patients (11).
As many as one quarter of 13,000 patients discharged from 51 Massachusetts hospitals reported problems with patient satisfaction issues such as the involvement of families in their care, communication and coordination of care, and the transition from hospital to home (12). Patients who had been admitted to academic health centers and teaching hospitals generally reported more problems than those cared for in community hospitals. As it turns out, service outcomes such as patient satisfaction drive market share and profitability for hospitals (13). With the average medical/surgery hospital charge amounting to $12,083, hospitals cannot afford to lose patients and, therefore, revenue due to issues of patient dissatisfaction (14). Recruiting new patients as customers is 5 times more costly than retaining an existing customer base (15).
There is compelling evidence from well-developed lines of research demonstrating that increasing patient satisfaction improves clinical outcomes, such as functional status and physiologic measures of health (16,17). Finally, it is important for clinicians to know that it has been clearly demonstrated that satisfied patients improve physician satisfaction (18,19).
Psychology of Satisfaction
To create a culture of customer service excellence in hospitals and achieve outstanding patient satisfaction, it is necessary to understand the intangible aspects of perception and expectation that contribute to patient satisfaction. The “First Law of Service” provides a useful, simple mathematical model of satisfaction (20). The formula for this model is Satisfaction = Perception – Expectation. If a patient’s perception of their hospital experience meets or exceeds the expectation, there will be a corresponding degree of satisfaction. However, if the perception does not meet the expectation, there will be resulting dissatisfaction. Thus, patient satisfaction results from meeting or exceeding patients’ expectations. Patient perceptions of care can be measured directly from patient satisfaction surveys, focus groups, and telephone surveys. A hospital’s reputation and market share are indirect measurements of patient perceptions. There are 2 main directions in which patient satisfaction can be influenced: by working on what the patient perceives and on what the patient expects.
Expectations are integral to the experience of being a customer. There has been confusion and controversy in health care as to whether patients are in fact customers. This confusion may be at the root of the overall service failing of hospitals (21). “The more horizontal they are, the more they are a patient. The more vertical they are, the more they are a customer” (22). Using a technical definition, a customer is anyone who has expectations about process operations or outputs (23). Therefore, all patients are customers, but not all customers are patients (21). Hospitals have a whole list of primary and secondary customers, each of whom has his or her own set of expectations. Patients and their families can be seen as primary customers, and referring physicians, third-party payers, external overseers, communities, shareholders, and employees are all secondary customers of hospitals.
Expectations are psychological phenomena that can be defined as beliefs created and sustained by cognitive processes (24). Expectations of patients as hospital customers rise from past experiences of their own or of others, as well as from current needs and unique internal preferences that form the basis of a value system. Expectations cannot be mandated, because they are based in self-gratification. However, expectations may change over time, and, very importantly, they can be measured and perhaps modified through education. For most people, illness and hospitalization is a rare event. Patients will know something is wrong and that treatment is needed, but most won’t know the nature of their disease, the diagnostic and therapeutic options, and the likely outcomes. Therefore, patients seek out health care professionals who have the opportunity to inform them on what to expect as a way to begin the process of managing expectations.
The Kano Model
The Kano Model provides a useful tool for studying different levels of patient expectations (23).
This model is useful to examine the voice of the customer in the relationship between satisfaction and quality, and it is relevant for hospital encounters. According to the model, patients will have a basic set of subconscious expectations about their care that will be taken for granted. These expectations are so routine and expected that patients don’t recognize them as comparative quality factors, but they will be shocked if they are absent. For example, patients assume that physicians are basically competent, and that hospitals are capable of providing safe, courteous, lifesaving medical care (25). Although these expectations are in the patient’s subconscious, if they are not met, the patient will be dissatisfied. Providing this level of basic quality isn’t necessarily enough to create satisfied patients.
There is a normal level of service that patients consciously consider, they have to do with the anticipated issues of hospital care related to access, wait times, scheduling, and billing. The model shows that satisfaction increases as more of these expectations are met and that patients will be dissatisfied if these quality expectations are not met. Patients use comparisons of these expectations to recognize differences among competitors and to make choices.
The latent quality curve lies entirely in the satisfaction region and represents supraconscious, unexpected quality items that patients didn’t know they wanted and result in a delightful surprise when present. In this experience, patients receive more than they had expected, often as the result of innovation that can raise patient expectations and provide a significant competitive advantage. The data from hospitals show that this is achieved primarily through the patient’s perception of personalized, customized service, provided by caring and concerned clinicians (26).
There is a tremendous opportunity in hospital care to modify patient expectations through education and to create high levels of patient satisfaction. Hospitals that are successful in this endeavor will have a significant competitive advantage. Hospital patients have a whole list of issues about which they have expectations: the smoothness of the admission and discharge process, accuracy and clarity of billing statements, courtesy of hospital employees, response time for calls and requests, the level of technology available in the hospital, nurse competency, taste and temperature of the food, and price. Most of these issues are not directly related to clinical care and certainly not under the control or influence of the hospitalist. What are the expectations that patients have for their clinical care by hospitalists, and how can we give it to them?
The Hospitalist as a Caring and Concerned Clinician
A useful model to define the hospitalist’s role in patient satisfaction is that of a caring and concerned clinician. This caring and concern for patients is exemplified by attentiveness, dignity and respect, effective information transfer, and shared decision making (23).
The outcome service chain for hospitals begins with the patient’s perception of caring and concerned clinicians who demonstrate these attributes of attentiveness, dignity and respect, effective information transfer, and shared decision making. This leads to the degree of patient satisfaction and loyalty that results in patients who will return to the hospital, seek related business, and refer additional business. This drives market share and financial success for hospitals (9).
The characteristics of the caring and concerned clinician begin with attentiveness. This is the practice of establishing a person-to-person connection with patients and involves attending to them as unique individuals and not just in their role as patients. The constant interruptions that occur in physician/patient encounters, control issues, discontinuity of care, and the often overwhelming complexity of a patient’s illness can be obstacles to the perception of attentiveness that result in patients feeling connected with the clinician. Some effective tools for attentiveness are demonstrating curiosity about the patient as a person, using open-ended questions to gather clinical data, orienting patients to the process of care, and actively eliciting a patient’s agenda for their care and then summarizing their concerns.
The demonstration of dignity and respect results in a patient’s feeling understood and accepted as a person. This is the practice of empathy, which is often confused with sympathy (27). Sympathy is an expression of one’s own feelings (“I’m sorry”). Empathy is the demonstration of an understanding of the patient’s feelings (“You must be very sad”). The confusion of medical terminology as well as the time constraints of modern hospital-care encounters can be obstacles to achieving the type of dignity and respect that results in a high degree of patient satisfaction. A number of effective tools are available to facilitate this important result. Sitting down during patient encounters greatly enhances the perception of time and caring of hospitalists on the part of patients. Eye contact and appropriate touch are demonstrations of dignity and respect, as is seeing patients fully clothed. It is important to pay attention to nonverbal communication issues with patients. An important element of how hospitalists are perceived by patients has to do with nonverbal issues such as demeanor, body posture, and verbal tone. Using a patient’s own words and addressing underlying feelings facilitates the practice of dignity and respect (28). Patients perceive statements of assured understanding as confirmation that they have been listened to. Remember the words of Sir William Osler: “Listening is unspoken caring.”
The effective transfer of information is at the core of physician/patient communication (29). Patients have the need to provide complete information to physicians to facilitate an accurate diagnosis. The physician’s role is to provide information that addresses the cognitive, behavioral, and affective needs of patients and their families concerning their illness. The discrepancy of language, time constraints, and the ability of patients to remember are all barriers to the effective transfer of information. Some useful techniques for effective information transfer include assessing a patient’s current level of understanding and asking about their self-diagnosis. Timeliness in providing results of diagnostic tests is an important issue to patients who are often waiting expectantly. Studies have shown that the majority of patients have questions about the so-called “mysteries of medicine,” related to the diagnosis, etiology, and prognosis of their illness (30).
Patients may not specifically ask these questions; however, they are present, and patient satisfaction will increase if these questions are answered. Patients have decisively indicated their desire for shared decision making regarding their health care and for patient and family control of all-important choices (26). The process of shared decision making can be facilitated by collaboration between patients and the hospitalist around goals ands plans for treatment in the hospital (31). A barrier to shared decision making is a patient/physician relationship based on a model of paternalism (“I’m the doctor, and I know best”). A more productive model for the therapeutic relationship is that of a partnership between the hospitalist and the patient, particularly in the present era of web-educated, sophisticated consumer patients.
An important tool to achieving this type of collaboration involves the approach of presenting patients and families with treatment options and then actively soliciting patient preferences. A question is whether patients will actively participate in treatment decisions and then adhere to treatment plans. This is in large part determined by the interpersonal relationship skills of the clinician and can be further facilitated with simplified regimens that have been agreed upon by the patient and the hospitalist (32).
A complete model of the hospital-care encounter provided by hospitalists has an opening and a closing. In between there is a series of moments of truth that can potentially be imbued with attentiveness, dignity and respect, effective information transfer, and shared decision making. The opening is a brief moment that will set the stage for the remainder of the encounter. Greeting patients by name and maintaining eye contact will help in establishing the early perception of being a caring and concerned clinician. It is important to close hospital encounters with a sense of hope and optimism, making sure that all of the patient’s issues have been addressed, as well as planning for the next steps.
The development and growth of hospital medicine is the latest site-specific evolution of practice specialization and focuses on the complex care of hospitalized patients. Hospitalists spend most of their professional time in the hospital providing care for general medical patients and are well positioned and uniquely committed to improving the care of hospitalized patients. Exceptional patient satisfaction is a key outcome that should result from the care provided by hospitalists.
References
- Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743-8.
- Zaleski P. Knights Hospitaller: the rise and fall of a chivalric order of Christian caregiving. Parabola. 1990;15:55-62.
- Risse GB. Mending Bodies, Saving Souls. New York, NY: Oxford University Press; 1999.
- Reality Check: Public Perceptions of Health Care and Hospitals. The American Hospital Association. 1996.
- Now are you satisfied? Fortune. February 1998:166.
- National Coalition on Health Care. How Americans perceive the health care system. www.nchc.org/perceive.html. Accessed August 2004.
- To Err Is Human: Building a Safer Health Care System. Committee on Quality of Health Care in America, Institute of Medicine. Washington, DC: National Academy Press; 1998.
- Pascoe GC. Patient satisfaction in primary health care: a literature review and analysis. Eval Prog Plann. 1983;6:185-210.
- Kenagy JW, Berwick DM, Shore MF. Service quality in heath care. JAMA. 1999;281:661-5.
- Mennemeyer ST, Morrisey MA, Howard LZ. Death and reputation: how consumers acted upon HCFA mortality information. Inquiry. 1997;34:117-28.
- Cleary PD, Edgman-Levitan S. Health care quality. Incorporating consumer perspectives. JAMA. 1997;278:1608-12.
- Rogers G, Smith DP. Reporting comparative results from hospital surveys. Int J Qual Health Care. 1999;11:251-9.
- Schlesinger LA, Heskett JL. The service-driven service company. Harvard Business Review. 1991;Sept-Oct:1-19.
- Modern Health care. 2000;May:70.
- Mittal B, Lassar W. Why do customers switch? The dynamics of satisfaction versus loyalty. Journal of Services Marketing. 1998;12:177-191.
- Greenfield S, Kaplan S, Ware WE Jr. Expanding patient involvement in care: Effects on patient outcomes. Ann Intern Med. 1985;102:520-8.
- Sobel DS. Rethinking medicine: improving health outcomes with cost-effective psychosocial interventions. Psychosom Med. 1995;57:234-44.
- Novack DH, Suchman AL, Clark W, Epstein RM, Najberg E, Kaplan C. Calibrating the physician. Personal awareness and effective patient care. Working Group on Promoting Physician Personal Awareness, American Academy on Physician and Patient. JAMA. 1997;278:502-9.
- Suchman AL, Roter D, Green M, Lipkin M Jr. Physician satisfaction with primary care office visits. Collaborative Study Group of the American Academy on Physician and Patient. Med Care. 1993;31:1083-92.
- Maister DH. The Psychology of Waiting Lines. Case No. 9-684-064. Boston, Mass: Harvard Business School Publishing; 1984.
- Fottler MD, Ford RC, Heaton CP. Achieving Service Excellence. Chicago, Ill.: Health Administration Press; 2002.
- Mayer T, Cates RJ. Service excellence in health care. JAMA. 1999;282:1281-83.
- James BC. Curing vs. Caring: The Art of Service Quality. Institute for Health Care Delivery Research. Intermountain Health Care. Salt Lake City, Utah; 2003.
- Thompson AG, Sunol R. Expectations as determinants of patient satisfaction: concepts, theory and evidence. Int J Qual Health Care. 1995;7:127-41.
- Larson CO, Nelson EC, Gustafson D, Batalden PB. The relationship between meeting patient’s information needs and their satisfaction with hospital care and general health status outcomes. Int J Qual Health Care. 1996;8:447-56.
- Gerteis M, Edgman-Levitan S, Daley J, Deblanco T, eds. Through the Patient’s Eyes. San Francisco, Calif.: Jossey-Bass; 1993.
- Spiro H, Peschel E, Curnen MG, St. James D, eds. Empathy and the Practice of Medicine: Beyond Pills and the Scalpel. New Haven, Conn.: Yale University Press; 1993.
- Branch WT, Malik TK. Using ‘windows of opportunities’ in brief interviews to understand patients’ concerns. JAMA. 1993;269:1667-68.
- Worthlin Group. Communication and the physician/patient relationship: A physician and communication survey. Bayer Institute for Health Care Communication. New Haven, CT; 1995.
- Clinician Patient Communication to Enhance Health Outcomes. Bayer Institute for Health Care Communication Workshop. Bayer Institute, New Haven, Conn.; 1999.
- Donovan J, Blake R. Patient non-compliance: deviance or reasoned decision-making? Soc Sci Med. 1992;34:507-13.
- DiMatteo MR, Reiter RC, Gambone JC. Enhancing medication adherence through communication and informed collaborative choice. Health Commun. 1994;6:253-65.
Feeling a Little Blue
Case Presentation
A 68-year-old man presented to a university hospital with a 4-day history of sudden, progressive finger ischemia. His past medical history was significant for type II diabetes mellitus and hyperlipidemia. He also suffered from severe vascular disease. Four years prior to admission, he underwent several surgeries, including right carotid endarterectomy, coronary artery bypass, and right-lower-extremity revascularization. One year prior, he also required a left below-the-knee amputation due to vascular insufficiency. Additional history revealed long-standing asthma, hearing loss due to chronic bilateral otitis media, and multiple sinus surgeries in attempts to relieve recurrent infections. He also had lower-extremity peripheral neuropathy, attributed to diabetes and frequent steroid use for asthma control.
On admission, vital signs were stable. Physical exam demonstrated mild cyanosis of digits 2 through 5 on both hands. There were also scattered splinter hemorrhages and petechiae on the involved fingers. Rales were noted in the left lung base with diffuse wheezes. Cardiac and vascular exams were unremarkable. Chronic ulceration of the right toes was also noted. Laboratory studies were significant for a white blood cell count of 26,700 cells/mL with 52% eosinophils and a positive perinuclear antineutrophil cytoplasmic antibody (p-ANCA).
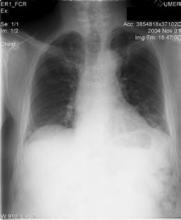
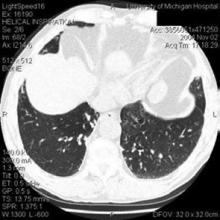
A chest radiograph revealed hazy, bilateral perihilar and left lower lobe infiltrates (Figure 1). Computed tomography of the chest was then performed and showed bronchiectasis of the left lung (Figure 2). This prompted a transbronchial biopsy that yielded tissue consistent with chronic inflammation.
What Is the Diagnosis?
Churg-Strauss Syndrome
Discussion
Churg-Strauss syndrome (CSS) is an allergic and granulomatous vasculitic illness affecting multiple organ systems. It typically follows 3 phases of progression. The first occurs in the second or third decade of life and includes the development of asthma in addition to chronic ear, nose, and sinus inflammation or infection. This is followed by eosinophilic infiltration of the lungs, skin, and other organs. The third phase commonly occurs 10 to 20 years after initial presentation, and it is heralded by small- and medium-vessel vasculitis.
In 1990, the American College of Rheumatology developed 6 diagnostic criteria for CSS and showed that having at least 4 of the 6 predicted the presence of CSS with a sensitivity of 85% and a specificity of 99.7%. They include:
- Asthma;
- Eosinophilia of >10% on a peripheral white blood cell count;
- Paranasal sinus disease;
- Mononeuropathy or polyneuropathy;
- Migratory or transient pulmonary opacities seen radiographically; and
- A blood vessel showing the accumulation of eosinophils in extravascular areas, as revealed by a biopsy.
Other helpful, but nonspecific diagnostic tests include a significantly elevated sedimentation rate, a positive p-ANCA with low titers of rheumatoid factor, high circulating IgE levels, and normocytic, normochromic anemia. CSS typically responds quite well to immunosuppressive therapy. The usual regimen consists of corticosteroids, and cyclophosphamide is frequently added. Before the advent of such therapies, CSS was consistently fatal, often within 3 months of the onset of vasculitis. Currently, 5-year survival rates exceed 70%.
In this patient, a diagnosis of CSS was based on history, clinical presentation, and laboratory results. Highdose methylprednisolone was initiated, and complete resolution of finger cyanosis and pain occurred in 48 hours. Oral cyclophosphamide was added the following day, and the patient was discharged home to complete 6 months of aggressive immunosuppressive therapy.
This presentation of CSS was rather unusual. Digital ischemia is uncommon in CSS, although it is consistent with the small-vessel vasculitis seen in this syndrome. Similarly, the late onset of the patient’s vasculitis is also unusual. The intermittent use of prednisone for asthma perhaps delayed the declaration of systemic symptoms.
Suggested Reading
- Noth I, Strek ME, Leff AR. Churg-Strauss syndrome. Lancet. 2003;361:587-94.
- Abril A, Calamia KT, Cohen MD. The Churg-Strauss syndrome (allergic granulomatous angiitis): review and update. Semin Arthritis Rheum. 2003; 33:106-14.
- Gross WL. Churg-Strauss syndrome: update on recent developments. Curr Opin Rheumatol. 2002;14:11-4.
- Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 Criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990;33:1094-100.
Case Presentation
A 68-year-old man presented to a university hospital with a 4-day history of sudden, progressive finger ischemia. His past medical history was significant for type II diabetes mellitus and hyperlipidemia. He also suffered from severe vascular disease. Four years prior to admission, he underwent several surgeries, including right carotid endarterectomy, coronary artery bypass, and right-lower-extremity revascularization. One year prior, he also required a left below-the-knee amputation due to vascular insufficiency. Additional history revealed long-standing asthma, hearing loss due to chronic bilateral otitis media, and multiple sinus surgeries in attempts to relieve recurrent infections. He also had lower-extremity peripheral neuropathy, attributed to diabetes and frequent steroid use for asthma control.
On admission, vital signs were stable. Physical exam demonstrated mild cyanosis of digits 2 through 5 on both hands. There were also scattered splinter hemorrhages and petechiae on the involved fingers. Rales were noted in the left lung base with diffuse wheezes. Cardiac and vascular exams were unremarkable. Chronic ulceration of the right toes was also noted. Laboratory studies were significant for a white blood cell count of 26,700 cells/mL with 52% eosinophils and a positive perinuclear antineutrophil cytoplasmic antibody (p-ANCA).
A chest radiograph revealed hazy, bilateral perihilar and left lower lobe infiltrates (Figure 1). Computed tomography of the chest was then performed and showed bronchiectasis of the left lung (Figure 2). This prompted a transbronchial biopsy that yielded tissue consistent with chronic inflammation.
What Is the Diagnosis?
Churg-Strauss Syndrome
Discussion
Churg-Strauss syndrome (CSS) is an allergic and granulomatous vasculitic illness affecting multiple organ systems. It typically follows 3 phases of progression. The first occurs in the second or third decade of life and includes the development of asthma in addition to chronic ear, nose, and sinus inflammation or infection. This is followed by eosinophilic infiltration of the lungs, skin, and other organs. The third phase commonly occurs 10 to 20 years after initial presentation, and it is heralded by small- and medium-vessel vasculitis.
In 1990, the American College of Rheumatology developed 6 diagnostic criteria for CSS and showed that having at least 4 of the 6 predicted the presence of CSS with a sensitivity of 85% and a specificity of 99.7%. They include:
- Asthma;
- Eosinophilia of >10% on a peripheral white blood cell count;
- Paranasal sinus disease;
- Mononeuropathy or polyneuropathy;
- Migratory or transient pulmonary opacities seen radiographically; and
- A blood vessel showing the accumulation of eosinophils in extravascular areas, as revealed by a biopsy.
Other helpful, but nonspecific diagnostic tests include a significantly elevated sedimentation rate, a positive p-ANCA with low titers of rheumatoid factor, high circulating IgE levels, and normocytic, normochromic anemia. CSS typically responds quite well to immunosuppressive therapy. The usual regimen consists of corticosteroids, and cyclophosphamide is frequently added. Before the advent of such therapies, CSS was consistently fatal, often within 3 months of the onset of vasculitis. Currently, 5-year survival rates exceed 70%.
In this patient, a diagnosis of CSS was based on history, clinical presentation, and laboratory results. Highdose methylprednisolone was initiated, and complete resolution of finger cyanosis and pain occurred in 48 hours. Oral cyclophosphamide was added the following day, and the patient was discharged home to complete 6 months of aggressive immunosuppressive therapy.
This presentation of CSS was rather unusual. Digital ischemia is uncommon in CSS, although it is consistent with the small-vessel vasculitis seen in this syndrome. Similarly, the late onset of the patient’s vasculitis is also unusual. The intermittent use of prednisone for asthma perhaps delayed the declaration of systemic symptoms.
Suggested Reading
- Noth I, Strek ME, Leff AR. Churg-Strauss syndrome. Lancet. 2003;361:587-94.
- Abril A, Calamia KT, Cohen MD. The Churg-Strauss syndrome (allergic granulomatous angiitis): review and update. Semin Arthritis Rheum. 2003; 33:106-14.
- Gross WL. Churg-Strauss syndrome: update on recent developments. Curr Opin Rheumatol. 2002;14:11-4.
- Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 Criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990;33:1094-100.
Case Presentation
A 68-year-old man presented to a university hospital with a 4-day history of sudden, progressive finger ischemia. His past medical history was significant for type II diabetes mellitus and hyperlipidemia. He also suffered from severe vascular disease. Four years prior to admission, he underwent several surgeries, including right carotid endarterectomy, coronary artery bypass, and right-lower-extremity revascularization. One year prior, he also required a left below-the-knee amputation due to vascular insufficiency. Additional history revealed long-standing asthma, hearing loss due to chronic bilateral otitis media, and multiple sinus surgeries in attempts to relieve recurrent infections. He also had lower-extremity peripheral neuropathy, attributed to diabetes and frequent steroid use for asthma control.
On admission, vital signs were stable. Physical exam demonstrated mild cyanosis of digits 2 through 5 on both hands. There were also scattered splinter hemorrhages and petechiae on the involved fingers. Rales were noted in the left lung base with diffuse wheezes. Cardiac and vascular exams were unremarkable. Chronic ulceration of the right toes was also noted. Laboratory studies were significant for a white blood cell count of 26,700 cells/mL with 52% eosinophils and a positive perinuclear antineutrophil cytoplasmic antibody (p-ANCA).
A chest radiograph revealed hazy, bilateral perihilar and left lower lobe infiltrates (Figure 1). Computed tomography of the chest was then performed and showed bronchiectasis of the left lung (Figure 2). This prompted a transbronchial biopsy that yielded tissue consistent with chronic inflammation.
What Is the Diagnosis?
Churg-Strauss Syndrome
Discussion
Churg-Strauss syndrome (CSS) is an allergic and granulomatous vasculitic illness affecting multiple organ systems. It typically follows 3 phases of progression. The first occurs in the second or third decade of life and includes the development of asthma in addition to chronic ear, nose, and sinus inflammation or infection. This is followed by eosinophilic infiltration of the lungs, skin, and other organs. The third phase commonly occurs 10 to 20 years after initial presentation, and it is heralded by small- and medium-vessel vasculitis.
In 1990, the American College of Rheumatology developed 6 diagnostic criteria for CSS and showed that having at least 4 of the 6 predicted the presence of CSS with a sensitivity of 85% and a specificity of 99.7%. They include:
- Asthma;
- Eosinophilia of >10% on a peripheral white blood cell count;
- Paranasal sinus disease;
- Mononeuropathy or polyneuropathy;
- Migratory or transient pulmonary opacities seen radiographically; and
- A blood vessel showing the accumulation of eosinophils in extravascular areas, as revealed by a biopsy.
Other helpful, but nonspecific diagnostic tests include a significantly elevated sedimentation rate, a positive p-ANCA with low titers of rheumatoid factor, high circulating IgE levels, and normocytic, normochromic anemia. CSS typically responds quite well to immunosuppressive therapy. The usual regimen consists of corticosteroids, and cyclophosphamide is frequently added. Before the advent of such therapies, CSS was consistently fatal, often within 3 months of the onset of vasculitis. Currently, 5-year survival rates exceed 70%.
In this patient, a diagnosis of CSS was based on history, clinical presentation, and laboratory results. Highdose methylprednisolone was initiated, and complete resolution of finger cyanosis and pain occurred in 48 hours. Oral cyclophosphamide was added the following day, and the patient was discharged home to complete 6 months of aggressive immunosuppressive therapy.
This presentation of CSS was rather unusual. Digital ischemia is uncommon in CSS, although it is consistent with the small-vessel vasculitis seen in this syndrome. Similarly, the late onset of the patient’s vasculitis is also unusual. The intermittent use of prednisone for asthma perhaps delayed the declaration of systemic symptoms.
Suggested Reading
- Noth I, Strek ME, Leff AR. Churg-Strauss syndrome. Lancet. 2003;361:587-94.
- Abril A, Calamia KT, Cohen MD. The Churg-Strauss syndrome (allergic granulomatous angiitis): review and update. Semin Arthritis Rheum. 2003; 33:106-14.
- Gross WL. Churg-Strauss syndrome: update on recent developments. Curr Opin Rheumatol. 2002;14:11-4.
- Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 Criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990;33:1094-100.
Effects of Hospitalists on Outcomes and Costs in a Multicenter Trial of Academic Hospitalists
Background: Several studies suggest that hospitalists can improve costs or outcomes in academic medical centers, but almost all of these studies have nonrandom assignment of patients to hospitalists, and no multi-center studies exist. We studied patients assigned to hospitalist or non-hospitalist physicians based only on day of admission to determine the effects of hospitalists on outcomes and costs in 6 academic medical centers.
Methods: From July 2001 to June 2003, 31,891 general medicine inpatients were assigned to hospitalist or non-hospitalist physicians according to a predetermined daily call schedule. Patient interviews at admission and 1 month after discharge and administrative data were used to study effects on outcomes and costs.
Results: Twelve thousand and one patients were cared for by hospitalists and 19,890 by non-hospitalists. There were no statistically significant differences in age, race, gender, Charlson Index, or distribution of primary diagnosis between the 2 groups. There were no statistically significant differences in in-hospital mortality, 30-day readmission and emergency room use, 30-day self-reported health status, or patient satisfaction. Mortality data up to 1 year after admission are pending. Average length of stay was 0.05 days shorter for hospitalist patients but this difference was not statistically significant. Costs were also similar between the groups. Individual center analyses had large confidence intervals on outcomes and costs and failed to show statistically significant effects on any measure of outcomes or costs except for 1 of the larger centers, which had lower length of stay and costs for hospitalists.
Conclusions: Hospitalists had small effects on selected outcome measures available to date, but did not produce the large resource savings that had been suggested by some earlier studies. The effectiveness of hospitalists appeared to vary by site, but was difficult to assess due to limited statistical power for site-specific analyses. Understanding the factors, such as physician experience, that may influence the effectiveness of hospitalists is important for maximizing the efficacy of hospitalist programs, because effects on outcomes may be small, vary by site, and be difficult to distinguish from chance in a specific clinical setting.
Background: Several studies suggest that hospitalists can improve costs or outcomes in academic medical centers, but almost all of these studies have nonrandom assignment of patients to hospitalists, and no multi-center studies exist. We studied patients assigned to hospitalist or non-hospitalist physicians based only on day of admission to determine the effects of hospitalists on outcomes and costs in 6 academic medical centers.
Methods: From July 2001 to June 2003, 31,891 general medicine inpatients were assigned to hospitalist or non-hospitalist physicians according to a predetermined daily call schedule. Patient interviews at admission and 1 month after discharge and administrative data were used to study effects on outcomes and costs.
Results: Twelve thousand and one patients were cared for by hospitalists and 19,890 by non-hospitalists. There were no statistically significant differences in age, race, gender, Charlson Index, or distribution of primary diagnosis between the 2 groups. There were no statistically significant differences in in-hospital mortality, 30-day readmission and emergency room use, 30-day self-reported health status, or patient satisfaction. Mortality data up to 1 year after admission are pending. Average length of stay was 0.05 days shorter for hospitalist patients but this difference was not statistically significant. Costs were also similar between the groups. Individual center analyses had large confidence intervals on outcomes and costs and failed to show statistically significant effects on any measure of outcomes or costs except for 1 of the larger centers, which had lower length of stay and costs for hospitalists.
Conclusions: Hospitalists had small effects on selected outcome measures available to date, but did not produce the large resource savings that had been suggested by some earlier studies. The effectiveness of hospitalists appeared to vary by site, but was difficult to assess due to limited statistical power for site-specific analyses. Understanding the factors, such as physician experience, that may influence the effectiveness of hospitalists is important for maximizing the efficacy of hospitalist programs, because effects on outcomes may be small, vary by site, and be difficult to distinguish from chance in a specific clinical setting.
Background: Several studies suggest that hospitalists can improve costs or outcomes in academic medical centers, but almost all of these studies have nonrandom assignment of patients to hospitalists, and no multi-center studies exist. We studied patients assigned to hospitalist or non-hospitalist physicians based only on day of admission to determine the effects of hospitalists on outcomes and costs in 6 academic medical centers.
Methods: From July 2001 to June 2003, 31,891 general medicine inpatients were assigned to hospitalist or non-hospitalist physicians according to a predetermined daily call schedule. Patient interviews at admission and 1 month after discharge and administrative data were used to study effects on outcomes and costs.
Results: Twelve thousand and one patients were cared for by hospitalists and 19,890 by non-hospitalists. There were no statistically significant differences in age, race, gender, Charlson Index, or distribution of primary diagnosis between the 2 groups. There were no statistically significant differences in in-hospital mortality, 30-day readmission and emergency room use, 30-day self-reported health status, or patient satisfaction. Mortality data up to 1 year after admission are pending. Average length of stay was 0.05 days shorter for hospitalist patients but this difference was not statistically significant. Costs were also similar between the groups. Individual center analyses had large confidence intervals on outcomes and costs and failed to show statistically significant effects on any measure of outcomes or costs except for 1 of the larger centers, which had lower length of stay and costs for hospitalists.
Conclusions: Hospitalists had small effects on selected outcome measures available to date, but did not produce the large resource savings that had been suggested by some earlier studies. The effectiveness of hospitalists appeared to vary by site, but was difficult to assess due to limited statistical power for site-specific analyses. Understanding the factors, such as physician experience, that may influence the effectiveness of hospitalists is important for maximizing the efficacy of hospitalist programs, because effects on outcomes may be small, vary by site, and be difficult to distinguish from chance in a specific clinical setting.
Observation and Discharge Codes
1. When should the observation code be used? Do the provider and facility need to use the same codes in order to be reimbursed for observation? What are the restrictions, if any, on what diagnoses may be used to bill for observation?
Observation status is an “outpatient status” even if the patient is located in an inpatient bed. The purpose of observation is to allow the physician time to make a decision about whether the patient should be admitted, and then rapidly move the patient to the most appropriate setting—i.e., the patient should either be admitted as an inpatient or sent home.
Therefore, only the physician who writes the order that places the patient in “observation status” and is responsible for the patient during his or her stay should use the observation codes. Always date and time the “admitting order,” because this information is needed to meet the minimum 8-hours rule if the patient is admitted and discharged on the same calendar date.
If a patient is both admitted and discharged on the same calendar date, the code range of 99234-99236 are used; however, the following criteria must be met:
- The patient must be in observation for a minimum of 8 hours.
- The billing physician must be present and show active involvement by charting condition updates, orders, etc.
- Both the admission and discharge notes are written by the billing physician (or may be billed by 2 physicians within the same group practice).
The specific CPT observation codes (99218-99220 and 99234-99236) do not have to match those used by the facility, because the physician codes are based on the physician E&M criteria (i.e., extent of history, exam, and decision making). The facility’s use of these codes is based on facility-specific criteria that measure the resources used by the facility’s employees and does not relate to the physician’s evaluation.
There are diagnosis/condition restrictions for separate payment to facilities for observation under the Outpatient Prospective Payment System (OPPS) reimbursement program (i.e., payment is based on Ambulatory Patient Classification [APC]). Even though separate payments for observation charges are made only for chest pain, asthma, and congestive heart failure, the facilities still code and report charges for all patients admitted to observation status. Note, however, that there are no such restrictions for the physician professional services billed. Only hospital facilities are subject to the diagnosis restrictions because of APC payment rules.
2. How should a change in status from observation to full admission affect coding (i.e., when this occurs, what should the appropriate coding be for the initial hospital day or for the second hospital day)?
The best way to answer this question is with some scenarios.
Scenario #1:
The patient is admitted to observation status after being evaluated in the ED. The attending physician writes an order “admit to observation status;” writes an admit note, which includes the intent of observation; and writes orders to help determine if the patient is to be admitted or sent home. After test results return, the physician decides to admit the patient on the same calendar date:
Code: Initial Hospital Care code (99221-99223) that incorporates all services (observation and admission note) provided and documented that day.
Scenario #2:
The patient is admitted in the evening (Day 1) to observation status, tests are performed, and results are pending. The following morning (Day 2), based on the results of tests, the physician evaluates the patient and decides to admit (writes admit order). On Day 3 the patient is evaluated and discharged home.
Code:
Day 1: Initial Observation Care (99218-99220)
Day 2: Initial Hospital Care (99221-99223)
Day 3: Discharge Management (99238 or 99239)
3. Is it acceptable to bill for a d/c day if the patient is not examined that day, but activities such as d/c planning and dictation occur?
Discharge management codes do require the face-to-face evaluation/examination of the patient. Also included is the time spent on instructions to the patient/family, coordination of care with other providers, preparation of discharge records, prescriptions, referrals and/or certification forms, etc. The dictation of discharge summary is not typically included in this definition, because it is usually considered a hospital requirement as opposed to something needed for the patient’s care.
4. How frequently should discharge code 99239 be used? What elements of the d/c process can/should actually be used toward the “greater than 30 minutes” definition? (e.g., do filling out the d/c paperwork, dictating d/c summary, phone time arranging f/u, etc., count?)
There is not a specific “frequency” for any code, although most payers will compare utilization of codes to “peers” of the same specialty. While this helps them identify outliers, it does not necessarily mean someone is coding incorrectly. It does mean that high utilization by a physician will probably result in some sort of “audit” or request for supporting documentation. For instance, if a physician has a high volume of patients who go to nursing homes requiring a lot of coordination of care, referral forms, etc., it may be expected that the physician may have a higher frequency of 99239 discharge management codes. For patients who are going home with great family support and are relatively healthy, it may not seem as “reasonable and necessary” to have greater than 30 minutes of discharge management, especially if every chart is documented with the same “35 minutes.” Therefore, try to keep track of the time devoted to these services as accurately as you can, and document the actual time and sufficient information to support the use of 99239.
Dr. Pfeiffer can be contacted at [email protected].
1. When should the observation code be used? Do the provider and facility need to use the same codes in order to be reimbursed for observation? What are the restrictions, if any, on what diagnoses may be used to bill for observation?
Observation status is an “outpatient status” even if the patient is located in an inpatient bed. The purpose of observation is to allow the physician time to make a decision about whether the patient should be admitted, and then rapidly move the patient to the most appropriate setting—i.e., the patient should either be admitted as an inpatient or sent home.
Therefore, only the physician who writes the order that places the patient in “observation status” and is responsible for the patient during his or her stay should use the observation codes. Always date and time the “admitting order,” because this information is needed to meet the minimum 8-hours rule if the patient is admitted and discharged on the same calendar date.
If a patient is both admitted and discharged on the same calendar date, the code range of 99234-99236 are used; however, the following criteria must be met:
- The patient must be in observation for a minimum of 8 hours.
- The billing physician must be present and show active involvement by charting condition updates, orders, etc.
- Both the admission and discharge notes are written by the billing physician (or may be billed by 2 physicians within the same group practice).
The specific CPT observation codes (99218-99220 and 99234-99236) do not have to match those used by the facility, because the physician codes are based on the physician E&M criteria (i.e., extent of history, exam, and decision making). The facility’s use of these codes is based on facility-specific criteria that measure the resources used by the facility’s employees and does not relate to the physician’s evaluation.
There are diagnosis/condition restrictions for separate payment to facilities for observation under the Outpatient Prospective Payment System (OPPS) reimbursement program (i.e., payment is based on Ambulatory Patient Classification [APC]). Even though separate payments for observation charges are made only for chest pain, asthma, and congestive heart failure, the facilities still code and report charges for all patients admitted to observation status. Note, however, that there are no such restrictions for the physician professional services billed. Only hospital facilities are subject to the diagnosis restrictions because of APC payment rules.
2. How should a change in status from observation to full admission affect coding (i.e., when this occurs, what should the appropriate coding be for the initial hospital day or for the second hospital day)?
The best way to answer this question is with some scenarios.
Scenario #1:
The patient is admitted to observation status after being evaluated in the ED. The attending physician writes an order “admit to observation status;” writes an admit note, which includes the intent of observation; and writes orders to help determine if the patient is to be admitted or sent home. After test results return, the physician decides to admit the patient on the same calendar date:
Code: Initial Hospital Care code (99221-99223) that incorporates all services (observation and admission note) provided and documented that day.
Scenario #2:
The patient is admitted in the evening (Day 1) to observation status, tests are performed, and results are pending. The following morning (Day 2), based on the results of tests, the physician evaluates the patient and decides to admit (writes admit order). On Day 3 the patient is evaluated and discharged home.
Code:
Day 1: Initial Observation Care (99218-99220)
Day 2: Initial Hospital Care (99221-99223)
Day 3: Discharge Management (99238 or 99239)
3. Is it acceptable to bill for a d/c day if the patient is not examined that day, but activities such as d/c planning and dictation occur?
Discharge management codes do require the face-to-face evaluation/examination of the patient. Also included is the time spent on instructions to the patient/family, coordination of care with other providers, preparation of discharge records, prescriptions, referrals and/or certification forms, etc. The dictation of discharge summary is not typically included in this definition, because it is usually considered a hospital requirement as opposed to something needed for the patient’s care.
4. How frequently should discharge code 99239 be used? What elements of the d/c process can/should actually be used toward the “greater than 30 minutes” definition? (e.g., do filling out the d/c paperwork, dictating d/c summary, phone time arranging f/u, etc., count?)
There is not a specific “frequency” for any code, although most payers will compare utilization of codes to “peers” of the same specialty. While this helps them identify outliers, it does not necessarily mean someone is coding incorrectly. It does mean that high utilization by a physician will probably result in some sort of “audit” or request for supporting documentation. For instance, if a physician has a high volume of patients who go to nursing homes requiring a lot of coordination of care, referral forms, etc., it may be expected that the physician may have a higher frequency of 99239 discharge management codes. For patients who are going home with great family support and are relatively healthy, it may not seem as “reasonable and necessary” to have greater than 30 minutes of discharge management, especially if every chart is documented with the same “35 minutes.” Therefore, try to keep track of the time devoted to these services as accurately as you can, and document the actual time and sufficient information to support the use of 99239.
Dr. Pfeiffer can be contacted at [email protected].
1. When should the observation code be used? Do the provider and facility need to use the same codes in order to be reimbursed for observation? What are the restrictions, if any, on what diagnoses may be used to bill for observation?
Observation status is an “outpatient status” even if the patient is located in an inpatient bed. The purpose of observation is to allow the physician time to make a decision about whether the patient should be admitted, and then rapidly move the patient to the most appropriate setting—i.e., the patient should either be admitted as an inpatient or sent home.
Therefore, only the physician who writes the order that places the patient in “observation status” and is responsible for the patient during his or her stay should use the observation codes. Always date and time the “admitting order,” because this information is needed to meet the minimum 8-hours rule if the patient is admitted and discharged on the same calendar date.
If a patient is both admitted and discharged on the same calendar date, the code range of 99234-99236 are used; however, the following criteria must be met:
- The patient must be in observation for a minimum of 8 hours.
- The billing physician must be present and show active involvement by charting condition updates, orders, etc.
- Both the admission and discharge notes are written by the billing physician (or may be billed by 2 physicians within the same group practice).
The specific CPT observation codes (99218-99220 and 99234-99236) do not have to match those used by the facility, because the physician codes are based on the physician E&M criteria (i.e., extent of history, exam, and decision making). The facility’s use of these codes is based on facility-specific criteria that measure the resources used by the facility’s employees and does not relate to the physician’s evaluation.
There are diagnosis/condition restrictions for separate payment to facilities for observation under the Outpatient Prospective Payment System (OPPS) reimbursement program (i.e., payment is based on Ambulatory Patient Classification [APC]). Even though separate payments for observation charges are made only for chest pain, asthma, and congestive heart failure, the facilities still code and report charges for all patients admitted to observation status. Note, however, that there are no such restrictions for the physician professional services billed. Only hospital facilities are subject to the diagnosis restrictions because of APC payment rules.
2. How should a change in status from observation to full admission affect coding (i.e., when this occurs, what should the appropriate coding be for the initial hospital day or for the second hospital day)?
The best way to answer this question is with some scenarios.
Scenario #1:
The patient is admitted to observation status after being evaluated in the ED. The attending physician writes an order “admit to observation status;” writes an admit note, which includes the intent of observation; and writes orders to help determine if the patient is to be admitted or sent home. After test results return, the physician decides to admit the patient on the same calendar date:
Code: Initial Hospital Care code (99221-99223) that incorporates all services (observation and admission note) provided and documented that day.
Scenario #2:
The patient is admitted in the evening (Day 1) to observation status, tests are performed, and results are pending. The following morning (Day 2), based on the results of tests, the physician evaluates the patient and decides to admit (writes admit order). On Day 3 the patient is evaluated and discharged home.
Code:
Day 1: Initial Observation Care (99218-99220)
Day 2: Initial Hospital Care (99221-99223)
Day 3: Discharge Management (99238 or 99239)
3. Is it acceptable to bill for a d/c day if the patient is not examined that day, but activities such as d/c planning and dictation occur?
Discharge management codes do require the face-to-face evaluation/examination of the patient. Also included is the time spent on instructions to the patient/family, coordination of care with other providers, preparation of discharge records, prescriptions, referrals and/or certification forms, etc. The dictation of discharge summary is not typically included in this definition, because it is usually considered a hospital requirement as opposed to something needed for the patient’s care.
4. How frequently should discharge code 99239 be used? What elements of the d/c process can/should actually be used toward the “greater than 30 minutes” definition? (e.g., do filling out the d/c paperwork, dictating d/c summary, phone time arranging f/u, etc., count?)
There is not a specific “frequency” for any code, although most payers will compare utilization of codes to “peers” of the same specialty. While this helps them identify outliers, it does not necessarily mean someone is coding incorrectly. It does mean that high utilization by a physician will probably result in some sort of “audit” or request for supporting documentation. For instance, if a physician has a high volume of patients who go to nursing homes requiring a lot of coordination of care, referral forms, etc., it may be expected that the physician may have a higher frequency of 99239 discharge management codes. For patients who are going home with great family support and are relatively healthy, it may not seem as “reasonable and necessary” to have greater than 30 minutes of discharge management, especially if every chart is documented with the same “35 minutes.” Therefore, try to keep track of the time devoted to these services as accurately as you can, and document the actual time and sufficient information to support the use of 99239.
Dr. Pfeiffer can be contacted at [email protected].
“Medical Terrorism” or Best Interests?:
Teresa Marie Schiavo died on March 31, 2005, 15 years after sustaining a cardiac arrest and entering a persistent vegetative state (PVS). Her saga ignited national debates over the rights of the incapacitated, the outcomes of patients in persistent vegetative states, the basic requirements for human life, the distinction between nutrition and other medical treatments, and the involvement of the courts and politicians in our most private affairs. Sadly, Terri’s plight is not unique, and such a tragic predicament poses special challenges for hospitalists. The goals of this article are to review the definition and prognosis of PVS, and to outline the ethical argument for deciding to withdraw or withhold treatment from patients in PVS.
Clinical Features of the Persistent Vegetative State
Comatose patients may experience one of three general outcomes: recovery (partial or complete), death, or a prolonged or irreversible period of unconsciousness. As life support measures improved throughout the 1960s, this latter group represented an increasingly large yet ill-defined subgroup. In an attempt to characterize this population of severely brain-damaged patients who progress from coma to a state of wakefulness without awareness, Jennett and Plum coined the term “vegetative state” in 1972 (1). An estimated 10,000 to 25,000 adults in the United States exist in this manner for at least 1 month and are said to be in a persistent vegetative state.
In 1991, the Multi-Society Task Force on PVS was established and identified several key components of the condition (Table 1) (2). Hospitalists encounter patients who transition into PVS after an acute injury due to head trauma, or following nontraumatic diffuse bilateral cortical insult from prolonged hypoxic-ischemic periods. Less commonly, PVS is diagnosed in the hospital as an end-stage manifestation of a neurodegenerative illness. The cornerstone of PVS is diffuse cortical damage with relative preservation of brainstem and hypothalamic functions. Lacking cortical function, patients in PVS remain unaware of themselves or their environment, and are not thought to suffer. Many autonomic functions remain intact, however. PVS patients exhibit sleep-wake patterns, thermoregulate, maintain stable hemodynamics, respond with reflexive movements, and often live independent of a ventilator.
Several conditions have been confused with PVS (2). Coma is often applied inappropriately to patients in PVS. A lack of self-awareness characterizes both conditions; however, comatose patients (eyes closed, unresponsive) do not have recognizable sleep-wake cycles, whereas PVS patients exhibit wakefulness with open eyes. Key points of distinction between PVS, coma, brain death, and the locked-in syndrome are listed in Table 2.
Physical movements have traditionally been a source of confusion in PVS, and Terri Schiavo’s case was no exception. Politicians with medical backgrounds pointed to publicized video footage as evidence of her potential for recovery, or as an indication that a diagnosis of PVS had been made erroneously. In fact, PVS patients frequently exhibit truncal and limb movement, and they may smile, grimace, grunt, moan, or even cry on occasion. Some demonstrate a startle myoclonus and have preserved gag and cough reflexes. However, PVS patients do not exhibit sustained visual pursuit, visual fixation, or reproducible responses to threatening gestures. Because their ability to coordinate swallowing is impaired, most rely on alternative means to oral feeding and hydration for sustenance.
Prognosis in PVS
After 1 month has elapsed and a diagnosis of PVS is firmly established, attention focuses on prognosis. The Multi-Society Task Force reviewed the outcomes of 603 adults in PVS from traumatic and nontraumatic causes. Although PVS following trauma has a better prognosis than PVS following nontraumatic injury, the outcomes of both are poor (Table 3). Irreversibility is implied the longer a person remains in a vegetative state (3).
When does PVS become permanent?
In their comprehensive review, the Task Force defined durations of PVS after which meaningful recovery is near impossible: 12 months following trauma and 3 months after nontraumatic injury (Figure) (3).
Twenty-four percent of patients who entered PVS following trauma improved to a point of moderate disability. However, once the duration of PVS exceeded 12 months, only 7 of 434 patients recovered. In all 7 cases, recovery was noted between 23 and 36 months after traumatic injury, but the patients’ functional status remained quite poor: 5 remained severely disabled, 1 was moderately disabled, and the status of the 7th could not be determined.
PVS following acute nontraumatic injury portends an even worse prognosis. Among 169 PVS patients in this category, 15% regained consciousness, and only 1 patient experienced a good recovery. After 3 months, the probability of recovery was less than 1%.
Based on the dismal outcomes after several months, how do we account for the occasional media reports of recovery after many years in vegetative states? The Task Force reviewed all such accounts and identified 5 patients with verified recovery after prolonged PVS, ranging in age from 18 to 61; all but 1 remained severely disabled. Following nontraumatic anoxic injury, the longest duration of PVS prior to regaining consciousness was 22 months (3). Given the prevalence of PVS, late recovery after PVS appears to be exceptionally rare. Following her anoxic injury, Terri Schiavo remained in PVS for 15 years—well beyond the 3-month cut-off for potential reversibility.
The Ethical Basis for Withdrawing Support in Patients with PVS
Life-sustaining treatment (LST) is most commonly withdrawn or withheld when this is known to be the patient’s preference either because of advance directives or through a surrogate representing the best interests of the patient. Conflicts arise when physicians recommend withdrawal of LST over the objections of surrogates. Faced with this dilemma, physicians caring for patients in PVS may cede to the wishes of the family. Alternatively, they may pursue withdrawal of LST based on 1 of the following 3 arguments:
- The perceived futility of ongoing LST;
- The presumption that patients in PVS have very poor quality of life and would opt for withdrawal if they could communicate their wishes; and
- The belief that patients in PVS no longer possess the minimal requirements for human existence and therefore have no interests to advance.
Is life-sustaining treatment futile for patients in PVS?
In its narrowest sense, futility implies the inability to achieve a particular physiologic goal because the treatment has no pathophysiologic basis, because the treatment has already been tried and failed in a patient, or because maximal therapy is failing (4). Invoking futility as a reason to withhold or withdraw care unilaterally in PVS is problematic. Because patients in PVS can remain alive for months to years with supportive measures, interventions (such as antibiotics or nutrition) are not futile, because the goal of prolonging life can be achieved, though at a markedly diminished level of quality.
Does PVS imply a presumption to withdraw LST?
Because futility cannot be invoked, some experts argue that PVS represents such a dismal quality of life that LST cannot be consistent with a patient’s best interests. While society generally errs on the side of prolonging the lives of incapacitated patients whose preferences are unknown, some ethicists argue that it should be presumed patients in PVS would not desire LST unless they have expressly stated preferences to the contrary. Public opinion polls support this notion, because the majority of people surveyed would not want LST if they were in PVS (5). This flexible position respects the divergent beliefs of the minority, permitting previously competent patients to continue LST when they choose.
Physicians who invoke this line of reasoning to override a surrogate’s decision explore relatively uncharted legal terrain. In the 1991 case of Helga Wanglie, an 87-year-old woman in a vegetative state, her husband wished to continue LST. Objecting to ongoing LST, her treating physicians attempted to remove the husband as legal guardian, but were rebuked (6). Without addressing the presumed desires of adults in PVS, the courts uphold the legal standing of surrogate decision-makers as long as they are acting in the patient’s best interests. On the other hand, a Massachusetts jury found that physicians were not guilty of malpractice when they entered a do-not-resuscitate order for a 71-year-old comatose woman without surrogate permission (5).
Do patients in PVS meet the minimal criteria for human existence?
A third ethical argument for withdrawing and withholding treatment in vegetative patients is unique to PVS. This line of reasoning challenges our assumptions about “patient interests” and resurrects the philosophical debate over the essence of human life. By virtue of being in PVS without hope for recovery, these patients have lost the minimal requirements of being human and have no hope of regaining them. In this view, prolonging mere biologic life is pointless because essential human qualities cannot be restored. This applies to patients in PVS for such duration that the probability of regaining consciousness is exceptionally rare (i.e., 12 months after trauma, or 3 months after nontraumatic injury). In such cases because no patient interests can be served and no medical goals are obtainable, no duty exists to provide life-sustaining treatment (7).
Physicians who choose to invoke this last argument should be aware of its uncertain legal and moral acceptance. Legally, the ethical argument that patients in PVS have no interests to advance has not been challenged directly in the courts. Groups rejecting the notion that patients in PVS lack minimal requirements for human life were galvanized by Terri Schiavo’s plight. Although public opinion polls determined that a clear majority would want their guardian to remove the feeding tube if they were in Terri’s predicament, a vocal minority was opposed (5). Politicians such as Congressman Tom DeLay entered the fray, declaring the removal of Terri’s feeding tube an “act of medical terrorism.” This right-to-life movement found an ally in the Roman Catholic Church when Pope John Paul II avowed that patients in PVS do, in fact, meet minimal criteria for human life and as such deserve nutrition and hydration (8). His opinion has yet to be adopted as doctrine, and political and moral consensus have not been achieved.
Conclusions
PVS is diagnosed 1 month after a patient enters a state of wakefulness without awareness. Movements are common in these patients, but they are not purposeful or reproducible in response to stimuli. Once PVS has exceeded durations of 3 months following nontraumatic injury or 12 months following trauma, the probability of meaningful recovery is exceptionally rare.
Armed with the above knowledge, what practical recourse exists to hospitalists caring for patients in PVS whose hope for regaining consciousness is exceedingly remote? First, inquire about advance directives that may direct care in such circumstances. If advance directives are not present, identify the surrogate decision maker, keeping in mind the state-to-state legal variations in the surrogate hierarchy. If the appropriate surrogate wishes to continue care and the treating physician objects, attempt to achieve a consensus. Time to adjust to the devastating plight and repeated nonjudgmental discussions focusing on the patient’s wishes often lead surrogates to accept withdrawal, or at least to establish limits on care (e.g., do-not-resuscitate order, withholding antibiotics and nutrition). Involvement of hospital ethics committees, primary care physicians, social workers, and religious or cultural ombudsmen may facilitate this process. Barring a mutually acceptable solution or progress in this direction, physicians may decide to cede to the wishes of the surrogates or, as a last recourse, involve the judicial system to achieve resolution.
References
- Jennett B. Plum F. Persistent vegetative state after brain damage: a syndrome in search of a name. Lancet. 1972; 1:734-7.
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state—first of two parts. NEJM. 1994; 330:1499-1508.
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state—second of two parts. NEJM. 1994; 330: 1572-9.
- Lo B. Resolving Ethical Dilemmas. A Guide for Clinicians. 2nd ed. Philadelphia, Pa: Lippincott Williams and Wilkins; p. 73.
- Eisenberg D. Lessons of the Schiavo battle. Time. April 4, 2005. pp. 26-27.
- Cantor NL. Can health care providers obtain judicial intervention against surrogates who demand “medically inappropriate” life support for incompetent patients? Crit Care Med. 1996; 24:883-87.
- Jonsen AR, Siegler M, and Winslade WJ. Clinical Ethics. A practical approach to ethical decisions in clinical practice. 5th ed. McGraw-Hill; p. 129.
- Address of Pope John Paul II to the participants in the international congress on “life-sustaining treatments and vegetative state: scientific advances and ethical dilemmas.” 20 March, 2004 available at: www.vatican.va/holy_father/john_paul_ii/speeches/2004/march/documents/hf_jp-ii_spe_20040320_congress-fiamc_en.html, Last accessed on May 11, 2005.
- Doble JE, Haig AJ, Anderson C, Katz R. Impairment, activity, participation, life satisfaction and survival in persons with locked-in syndrome for over a decade. J Head Trauma Rehabil. 2003;18:435-44.
- Booth CM, Boone RH, Tomlinson J, and Detsky AS. Is this patient dead, vegetative or severely impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004; 291:870-9.
- Levy DE, Caronna JJ, et al. Predicting coma from hypoxic-schemic coma. JAMA. 1985; 253:1420-6.
- Zandbergen EG, de Haan RJ, et al. Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet. 1998; 352:1808-12.
Teresa Marie Schiavo died on March 31, 2005, 15 years after sustaining a cardiac arrest and entering a persistent vegetative state (PVS). Her saga ignited national debates over the rights of the incapacitated, the outcomes of patients in persistent vegetative states, the basic requirements for human life, the distinction between nutrition and other medical treatments, and the involvement of the courts and politicians in our most private affairs. Sadly, Terri’s plight is not unique, and such a tragic predicament poses special challenges for hospitalists. The goals of this article are to review the definition and prognosis of PVS, and to outline the ethical argument for deciding to withdraw or withhold treatment from patients in PVS.
Clinical Features of the Persistent Vegetative State
Comatose patients may experience one of three general outcomes: recovery (partial or complete), death, or a prolonged or irreversible period of unconsciousness. As life support measures improved throughout the 1960s, this latter group represented an increasingly large yet ill-defined subgroup. In an attempt to characterize this population of severely brain-damaged patients who progress from coma to a state of wakefulness without awareness, Jennett and Plum coined the term “vegetative state” in 1972 (1). An estimated 10,000 to 25,000 adults in the United States exist in this manner for at least 1 month and are said to be in a persistent vegetative state.
In 1991, the Multi-Society Task Force on PVS was established and identified several key components of the condition (Table 1) (2). Hospitalists encounter patients who transition into PVS after an acute injury due to head trauma, or following nontraumatic diffuse bilateral cortical insult from prolonged hypoxic-ischemic periods. Less commonly, PVS is diagnosed in the hospital as an end-stage manifestation of a neurodegenerative illness. The cornerstone of PVS is diffuse cortical damage with relative preservation of brainstem and hypothalamic functions. Lacking cortical function, patients in PVS remain unaware of themselves or their environment, and are not thought to suffer. Many autonomic functions remain intact, however. PVS patients exhibit sleep-wake patterns, thermoregulate, maintain stable hemodynamics, respond with reflexive movements, and often live independent of a ventilator.
Several conditions have been confused with PVS (2). Coma is often applied inappropriately to patients in PVS. A lack of self-awareness characterizes both conditions; however, comatose patients (eyes closed, unresponsive) do not have recognizable sleep-wake cycles, whereas PVS patients exhibit wakefulness with open eyes. Key points of distinction between PVS, coma, brain death, and the locked-in syndrome are listed in Table 2.
Physical movements have traditionally been a source of confusion in PVS, and Terri Schiavo’s case was no exception. Politicians with medical backgrounds pointed to publicized video footage as evidence of her potential for recovery, or as an indication that a diagnosis of PVS had been made erroneously. In fact, PVS patients frequently exhibit truncal and limb movement, and they may smile, grimace, grunt, moan, or even cry on occasion. Some demonstrate a startle myoclonus and have preserved gag and cough reflexes. However, PVS patients do not exhibit sustained visual pursuit, visual fixation, or reproducible responses to threatening gestures. Because their ability to coordinate swallowing is impaired, most rely on alternative means to oral feeding and hydration for sustenance.
Prognosis in PVS
After 1 month has elapsed and a diagnosis of PVS is firmly established, attention focuses on prognosis. The Multi-Society Task Force reviewed the outcomes of 603 adults in PVS from traumatic and nontraumatic causes. Although PVS following trauma has a better prognosis than PVS following nontraumatic injury, the outcomes of both are poor (Table 3). Irreversibility is implied the longer a person remains in a vegetative state (3).
When does PVS become permanent?
In their comprehensive review, the Task Force defined durations of PVS after which meaningful recovery is near impossible: 12 months following trauma and 3 months after nontraumatic injury (Figure) (3).
Twenty-four percent of patients who entered PVS following trauma improved to a point of moderate disability. However, once the duration of PVS exceeded 12 months, only 7 of 434 patients recovered. In all 7 cases, recovery was noted between 23 and 36 months after traumatic injury, but the patients’ functional status remained quite poor: 5 remained severely disabled, 1 was moderately disabled, and the status of the 7th could not be determined.
PVS following acute nontraumatic injury portends an even worse prognosis. Among 169 PVS patients in this category, 15% regained consciousness, and only 1 patient experienced a good recovery. After 3 months, the probability of recovery was less than 1%.
Based on the dismal outcomes after several months, how do we account for the occasional media reports of recovery after many years in vegetative states? The Task Force reviewed all such accounts and identified 5 patients with verified recovery after prolonged PVS, ranging in age from 18 to 61; all but 1 remained severely disabled. Following nontraumatic anoxic injury, the longest duration of PVS prior to regaining consciousness was 22 months (3). Given the prevalence of PVS, late recovery after PVS appears to be exceptionally rare. Following her anoxic injury, Terri Schiavo remained in PVS for 15 years—well beyond the 3-month cut-off for potential reversibility.
The Ethical Basis for Withdrawing Support in Patients with PVS
Life-sustaining treatment (LST) is most commonly withdrawn or withheld when this is known to be the patient’s preference either because of advance directives or through a surrogate representing the best interests of the patient. Conflicts arise when physicians recommend withdrawal of LST over the objections of surrogates. Faced with this dilemma, physicians caring for patients in PVS may cede to the wishes of the family. Alternatively, they may pursue withdrawal of LST based on 1 of the following 3 arguments:
- The perceived futility of ongoing LST;
- The presumption that patients in PVS have very poor quality of life and would opt for withdrawal if they could communicate their wishes; and
- The belief that patients in PVS no longer possess the minimal requirements for human existence and therefore have no interests to advance.
Is life-sustaining treatment futile for patients in PVS?
In its narrowest sense, futility implies the inability to achieve a particular physiologic goal because the treatment has no pathophysiologic basis, because the treatment has already been tried and failed in a patient, or because maximal therapy is failing (4). Invoking futility as a reason to withhold or withdraw care unilaterally in PVS is problematic. Because patients in PVS can remain alive for months to years with supportive measures, interventions (such as antibiotics or nutrition) are not futile, because the goal of prolonging life can be achieved, though at a markedly diminished level of quality.
Does PVS imply a presumption to withdraw LST?
Because futility cannot be invoked, some experts argue that PVS represents such a dismal quality of life that LST cannot be consistent with a patient’s best interests. While society generally errs on the side of prolonging the lives of incapacitated patients whose preferences are unknown, some ethicists argue that it should be presumed patients in PVS would not desire LST unless they have expressly stated preferences to the contrary. Public opinion polls support this notion, because the majority of people surveyed would not want LST if they were in PVS (5). This flexible position respects the divergent beliefs of the minority, permitting previously competent patients to continue LST when they choose.
Physicians who invoke this line of reasoning to override a surrogate’s decision explore relatively uncharted legal terrain. In the 1991 case of Helga Wanglie, an 87-year-old woman in a vegetative state, her husband wished to continue LST. Objecting to ongoing LST, her treating physicians attempted to remove the husband as legal guardian, but were rebuked (6). Without addressing the presumed desires of adults in PVS, the courts uphold the legal standing of surrogate decision-makers as long as they are acting in the patient’s best interests. On the other hand, a Massachusetts jury found that physicians were not guilty of malpractice when they entered a do-not-resuscitate order for a 71-year-old comatose woman without surrogate permission (5).
Do patients in PVS meet the minimal criteria for human existence?
A third ethical argument for withdrawing and withholding treatment in vegetative patients is unique to PVS. This line of reasoning challenges our assumptions about “patient interests” and resurrects the philosophical debate over the essence of human life. By virtue of being in PVS without hope for recovery, these patients have lost the minimal requirements of being human and have no hope of regaining them. In this view, prolonging mere biologic life is pointless because essential human qualities cannot be restored. This applies to patients in PVS for such duration that the probability of regaining consciousness is exceptionally rare (i.e., 12 months after trauma, or 3 months after nontraumatic injury). In such cases because no patient interests can be served and no medical goals are obtainable, no duty exists to provide life-sustaining treatment (7).
Physicians who choose to invoke this last argument should be aware of its uncertain legal and moral acceptance. Legally, the ethical argument that patients in PVS have no interests to advance has not been challenged directly in the courts. Groups rejecting the notion that patients in PVS lack minimal requirements for human life were galvanized by Terri Schiavo’s plight. Although public opinion polls determined that a clear majority would want their guardian to remove the feeding tube if they were in Terri’s predicament, a vocal minority was opposed (5). Politicians such as Congressman Tom DeLay entered the fray, declaring the removal of Terri’s feeding tube an “act of medical terrorism.” This right-to-life movement found an ally in the Roman Catholic Church when Pope John Paul II avowed that patients in PVS do, in fact, meet minimal criteria for human life and as such deserve nutrition and hydration (8). His opinion has yet to be adopted as doctrine, and political and moral consensus have not been achieved.
Conclusions
PVS is diagnosed 1 month after a patient enters a state of wakefulness without awareness. Movements are common in these patients, but they are not purposeful or reproducible in response to stimuli. Once PVS has exceeded durations of 3 months following nontraumatic injury or 12 months following trauma, the probability of meaningful recovery is exceptionally rare.
Armed with the above knowledge, what practical recourse exists to hospitalists caring for patients in PVS whose hope for regaining consciousness is exceedingly remote? First, inquire about advance directives that may direct care in such circumstances. If advance directives are not present, identify the surrogate decision maker, keeping in mind the state-to-state legal variations in the surrogate hierarchy. If the appropriate surrogate wishes to continue care and the treating physician objects, attempt to achieve a consensus. Time to adjust to the devastating plight and repeated nonjudgmental discussions focusing on the patient’s wishes often lead surrogates to accept withdrawal, or at least to establish limits on care (e.g., do-not-resuscitate order, withholding antibiotics and nutrition). Involvement of hospital ethics committees, primary care physicians, social workers, and religious or cultural ombudsmen may facilitate this process. Barring a mutually acceptable solution or progress in this direction, physicians may decide to cede to the wishes of the surrogates or, as a last recourse, involve the judicial system to achieve resolution.
References
- Jennett B. Plum F. Persistent vegetative state after brain damage: a syndrome in search of a name. Lancet. 1972; 1:734-7.
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state—first of two parts. NEJM. 1994; 330:1499-1508.
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state—second of two parts. NEJM. 1994; 330: 1572-9.
- Lo B. Resolving Ethical Dilemmas. A Guide for Clinicians. 2nd ed. Philadelphia, Pa: Lippincott Williams and Wilkins; p. 73.
- Eisenberg D. Lessons of the Schiavo battle. Time. April 4, 2005. pp. 26-27.
- Cantor NL. Can health care providers obtain judicial intervention against surrogates who demand “medically inappropriate” life support for incompetent patients? Crit Care Med. 1996; 24:883-87.
- Jonsen AR, Siegler M, and Winslade WJ. Clinical Ethics. A practical approach to ethical decisions in clinical practice. 5th ed. McGraw-Hill; p. 129.
- Address of Pope John Paul II to the participants in the international congress on “life-sustaining treatments and vegetative state: scientific advances and ethical dilemmas.” 20 March, 2004 available at: www.vatican.va/holy_father/john_paul_ii/speeches/2004/march/documents/hf_jp-ii_spe_20040320_congress-fiamc_en.html, Last accessed on May 11, 2005.
- Doble JE, Haig AJ, Anderson C, Katz R. Impairment, activity, participation, life satisfaction and survival in persons with locked-in syndrome for over a decade. J Head Trauma Rehabil. 2003;18:435-44.
- Booth CM, Boone RH, Tomlinson J, and Detsky AS. Is this patient dead, vegetative or severely impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004; 291:870-9.
- Levy DE, Caronna JJ, et al. Predicting coma from hypoxic-schemic coma. JAMA. 1985; 253:1420-6.
- Zandbergen EG, de Haan RJ, et al. Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet. 1998; 352:1808-12.
Teresa Marie Schiavo died on March 31, 2005, 15 years after sustaining a cardiac arrest and entering a persistent vegetative state (PVS). Her saga ignited national debates over the rights of the incapacitated, the outcomes of patients in persistent vegetative states, the basic requirements for human life, the distinction between nutrition and other medical treatments, and the involvement of the courts and politicians in our most private affairs. Sadly, Terri’s plight is not unique, and such a tragic predicament poses special challenges for hospitalists. The goals of this article are to review the definition and prognosis of PVS, and to outline the ethical argument for deciding to withdraw or withhold treatment from patients in PVS.
Clinical Features of the Persistent Vegetative State
Comatose patients may experience one of three general outcomes: recovery (partial or complete), death, or a prolonged or irreversible period of unconsciousness. As life support measures improved throughout the 1960s, this latter group represented an increasingly large yet ill-defined subgroup. In an attempt to characterize this population of severely brain-damaged patients who progress from coma to a state of wakefulness without awareness, Jennett and Plum coined the term “vegetative state” in 1972 (1). An estimated 10,000 to 25,000 adults in the United States exist in this manner for at least 1 month and are said to be in a persistent vegetative state.
In 1991, the Multi-Society Task Force on PVS was established and identified several key components of the condition (Table 1) (2). Hospitalists encounter patients who transition into PVS after an acute injury due to head trauma, or following nontraumatic diffuse bilateral cortical insult from prolonged hypoxic-ischemic periods. Less commonly, PVS is diagnosed in the hospital as an end-stage manifestation of a neurodegenerative illness. The cornerstone of PVS is diffuse cortical damage with relative preservation of brainstem and hypothalamic functions. Lacking cortical function, patients in PVS remain unaware of themselves or their environment, and are not thought to suffer. Many autonomic functions remain intact, however. PVS patients exhibit sleep-wake patterns, thermoregulate, maintain stable hemodynamics, respond with reflexive movements, and often live independent of a ventilator.
Several conditions have been confused with PVS (2). Coma is often applied inappropriately to patients in PVS. A lack of self-awareness characterizes both conditions; however, comatose patients (eyes closed, unresponsive) do not have recognizable sleep-wake cycles, whereas PVS patients exhibit wakefulness with open eyes. Key points of distinction between PVS, coma, brain death, and the locked-in syndrome are listed in Table 2.
Physical movements have traditionally been a source of confusion in PVS, and Terri Schiavo’s case was no exception. Politicians with medical backgrounds pointed to publicized video footage as evidence of her potential for recovery, or as an indication that a diagnosis of PVS had been made erroneously. In fact, PVS patients frequently exhibit truncal and limb movement, and they may smile, grimace, grunt, moan, or even cry on occasion. Some demonstrate a startle myoclonus and have preserved gag and cough reflexes. However, PVS patients do not exhibit sustained visual pursuit, visual fixation, or reproducible responses to threatening gestures. Because their ability to coordinate swallowing is impaired, most rely on alternative means to oral feeding and hydration for sustenance.
Prognosis in PVS
After 1 month has elapsed and a diagnosis of PVS is firmly established, attention focuses on prognosis. The Multi-Society Task Force reviewed the outcomes of 603 adults in PVS from traumatic and nontraumatic causes. Although PVS following trauma has a better prognosis than PVS following nontraumatic injury, the outcomes of both are poor (Table 3). Irreversibility is implied the longer a person remains in a vegetative state (3).
When does PVS become permanent?
In their comprehensive review, the Task Force defined durations of PVS after which meaningful recovery is near impossible: 12 months following trauma and 3 months after nontraumatic injury (Figure) (3).
Twenty-four percent of patients who entered PVS following trauma improved to a point of moderate disability. However, once the duration of PVS exceeded 12 months, only 7 of 434 patients recovered. In all 7 cases, recovery was noted between 23 and 36 months after traumatic injury, but the patients’ functional status remained quite poor: 5 remained severely disabled, 1 was moderately disabled, and the status of the 7th could not be determined.
PVS following acute nontraumatic injury portends an even worse prognosis. Among 169 PVS patients in this category, 15% regained consciousness, and only 1 patient experienced a good recovery. After 3 months, the probability of recovery was less than 1%.
Based on the dismal outcomes after several months, how do we account for the occasional media reports of recovery after many years in vegetative states? The Task Force reviewed all such accounts and identified 5 patients with verified recovery after prolonged PVS, ranging in age from 18 to 61; all but 1 remained severely disabled. Following nontraumatic anoxic injury, the longest duration of PVS prior to regaining consciousness was 22 months (3). Given the prevalence of PVS, late recovery after PVS appears to be exceptionally rare. Following her anoxic injury, Terri Schiavo remained in PVS for 15 years—well beyond the 3-month cut-off for potential reversibility.
The Ethical Basis for Withdrawing Support in Patients with PVS
Life-sustaining treatment (LST) is most commonly withdrawn or withheld when this is known to be the patient’s preference either because of advance directives or through a surrogate representing the best interests of the patient. Conflicts arise when physicians recommend withdrawal of LST over the objections of surrogates. Faced with this dilemma, physicians caring for patients in PVS may cede to the wishes of the family. Alternatively, they may pursue withdrawal of LST based on 1 of the following 3 arguments:
- The perceived futility of ongoing LST;
- The presumption that patients in PVS have very poor quality of life and would opt for withdrawal if they could communicate their wishes; and
- The belief that patients in PVS no longer possess the minimal requirements for human existence and therefore have no interests to advance.
Is life-sustaining treatment futile for patients in PVS?
In its narrowest sense, futility implies the inability to achieve a particular physiologic goal because the treatment has no pathophysiologic basis, because the treatment has already been tried and failed in a patient, or because maximal therapy is failing (4). Invoking futility as a reason to withhold or withdraw care unilaterally in PVS is problematic. Because patients in PVS can remain alive for months to years with supportive measures, interventions (such as antibiotics or nutrition) are not futile, because the goal of prolonging life can be achieved, though at a markedly diminished level of quality.
Does PVS imply a presumption to withdraw LST?
Because futility cannot be invoked, some experts argue that PVS represents such a dismal quality of life that LST cannot be consistent with a patient’s best interests. While society generally errs on the side of prolonging the lives of incapacitated patients whose preferences are unknown, some ethicists argue that it should be presumed patients in PVS would not desire LST unless they have expressly stated preferences to the contrary. Public opinion polls support this notion, because the majority of people surveyed would not want LST if they were in PVS (5). This flexible position respects the divergent beliefs of the minority, permitting previously competent patients to continue LST when they choose.
Physicians who invoke this line of reasoning to override a surrogate’s decision explore relatively uncharted legal terrain. In the 1991 case of Helga Wanglie, an 87-year-old woman in a vegetative state, her husband wished to continue LST. Objecting to ongoing LST, her treating physicians attempted to remove the husband as legal guardian, but were rebuked (6). Without addressing the presumed desires of adults in PVS, the courts uphold the legal standing of surrogate decision-makers as long as they are acting in the patient’s best interests. On the other hand, a Massachusetts jury found that physicians were not guilty of malpractice when they entered a do-not-resuscitate order for a 71-year-old comatose woman without surrogate permission (5).
Do patients in PVS meet the minimal criteria for human existence?
A third ethical argument for withdrawing and withholding treatment in vegetative patients is unique to PVS. This line of reasoning challenges our assumptions about “patient interests” and resurrects the philosophical debate over the essence of human life. By virtue of being in PVS without hope for recovery, these patients have lost the minimal requirements of being human and have no hope of regaining them. In this view, prolonging mere biologic life is pointless because essential human qualities cannot be restored. This applies to patients in PVS for such duration that the probability of regaining consciousness is exceptionally rare (i.e., 12 months after trauma, or 3 months after nontraumatic injury). In such cases because no patient interests can be served and no medical goals are obtainable, no duty exists to provide life-sustaining treatment (7).
Physicians who choose to invoke this last argument should be aware of its uncertain legal and moral acceptance. Legally, the ethical argument that patients in PVS have no interests to advance has not been challenged directly in the courts. Groups rejecting the notion that patients in PVS lack minimal requirements for human life were galvanized by Terri Schiavo’s plight. Although public opinion polls determined that a clear majority would want their guardian to remove the feeding tube if they were in Terri’s predicament, a vocal minority was opposed (5). Politicians such as Congressman Tom DeLay entered the fray, declaring the removal of Terri’s feeding tube an “act of medical terrorism.” This right-to-life movement found an ally in the Roman Catholic Church when Pope John Paul II avowed that patients in PVS do, in fact, meet minimal criteria for human life and as such deserve nutrition and hydration (8). His opinion has yet to be adopted as doctrine, and political and moral consensus have not been achieved.
Conclusions
PVS is diagnosed 1 month after a patient enters a state of wakefulness without awareness. Movements are common in these patients, but they are not purposeful or reproducible in response to stimuli. Once PVS has exceeded durations of 3 months following nontraumatic injury or 12 months following trauma, the probability of meaningful recovery is exceptionally rare.
Armed with the above knowledge, what practical recourse exists to hospitalists caring for patients in PVS whose hope for regaining consciousness is exceedingly remote? First, inquire about advance directives that may direct care in such circumstances. If advance directives are not present, identify the surrogate decision maker, keeping in mind the state-to-state legal variations in the surrogate hierarchy. If the appropriate surrogate wishes to continue care and the treating physician objects, attempt to achieve a consensus. Time to adjust to the devastating plight and repeated nonjudgmental discussions focusing on the patient’s wishes often lead surrogates to accept withdrawal, or at least to establish limits on care (e.g., do-not-resuscitate order, withholding antibiotics and nutrition). Involvement of hospital ethics committees, primary care physicians, social workers, and religious or cultural ombudsmen may facilitate this process. Barring a mutually acceptable solution or progress in this direction, physicians may decide to cede to the wishes of the surrogates or, as a last recourse, involve the judicial system to achieve resolution.
References
- Jennett B. Plum F. Persistent vegetative state after brain damage: a syndrome in search of a name. Lancet. 1972; 1:734-7.
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state—first of two parts. NEJM. 1994; 330:1499-1508.
- The Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state—second of two parts. NEJM. 1994; 330: 1572-9.
- Lo B. Resolving Ethical Dilemmas. A Guide for Clinicians. 2nd ed. Philadelphia, Pa: Lippincott Williams and Wilkins; p. 73.
- Eisenberg D. Lessons of the Schiavo battle. Time. April 4, 2005. pp. 26-27.
- Cantor NL. Can health care providers obtain judicial intervention against surrogates who demand “medically inappropriate” life support for incompetent patients? Crit Care Med. 1996; 24:883-87.
- Jonsen AR, Siegler M, and Winslade WJ. Clinical Ethics. A practical approach to ethical decisions in clinical practice. 5th ed. McGraw-Hill; p. 129.
- Address of Pope John Paul II to the participants in the international congress on “life-sustaining treatments and vegetative state: scientific advances and ethical dilemmas.” 20 March, 2004 available at: www.vatican.va/holy_father/john_paul_ii/speeches/2004/march/documents/hf_jp-ii_spe_20040320_congress-fiamc_en.html, Last accessed on May 11, 2005.
- Doble JE, Haig AJ, Anderson C, Katz R. Impairment, activity, participation, life satisfaction and survival in persons with locked-in syndrome for over a decade. J Head Trauma Rehabil. 2003;18:435-44.
- Booth CM, Boone RH, Tomlinson J, and Detsky AS. Is this patient dead, vegetative or severely impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004; 291:870-9.
- Levy DE, Caronna JJ, et al. Predicting coma from hypoxic-schemic coma. JAMA. 1985; 253:1420-6.
- Zandbergen EG, de Haan RJ, et al. Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet. 1998; 352:1808-12.
Physician Pay-for-Performance Comes to the Hospital
In January 2004, the Mercy Inpatient Medicine Service embarked on a quality-based incentive program, or “pay-for-performance.” This was spurred on by Blue Cross of Massachusetts, which contracted with all hospitals in the state to receive a substantial financial bonus for agreed-upon quality indicators.
Prior to the start date, representatives from Mercy Medical Center negotiated with Blue Cross, arriving at the following quality indicators and targets for all hospital patients for the period of January to December 2004:
- For pneumonia patients, 45% rate of pneumococcal vaccine screening and administration;
- For heart failure patients, 85% rate of documentation of ejection fraction; and
- For heart failure patients with ejection fraction <40%, prescription of an ACE-inhibitor (or documentation of a contraindication) upon discharge.
The Mercy Inpatient Medicine Service, which consisted of 10 full-time hospitalists in 2004, cared for approximately 75% of all medical inpatients at the hospital. We created a quality-based incentive program for this hospital medicine group that mirrored the incentives mentioned above for the entire hospital. We postulated that superior performance by the hospitalists would “raise the bar” for the remainder of the patients, some of whom were cared for by a separate hospital medicine group and some by traditional PCPs.
Mechanics of the Pay for Performance Program
The quality-based bonus was structured so that it would be paid out at 6-month intervals in equal parts to all full-time hospitalists. The magnitude of the bonus was set in excess of 7.5% of the hospitalists’ base salary, an amount that we felt would be large enough to influence behavior change.
From the outset of the bonus period (2004), as medical director of the group, I convened a weekly meeting with hospitalists and representatives from the Quality Improvement department, nursing, and case management. The meeting was being held amid the backdrop of a renewed institutional emphasis on all the JCAHO Core Measures and other quality/patient safety initiatives. The purpose of the meeting was to review hospital performance on all JCAHO Core Measures.
A nurse from the Quality Improvement department reviewed all charts of pneumonia and heart failure patients. For cases where pneumovax was not screened/administered, ejection fraction not assessed, and ACE-I not given, a hospitalist would review the chart and provide feedback to the physician-of-record regarding the nature of the problem. Further, I provided regular feedback to the hospital medicine group regarding their performance on the 3 indicators.
Results
The results in Figures 1–3 are for the hospitalist group, with results for the hospital as a whole slightly lower, but still in excess of the targets agreed upon with Blue Cross.
Summary
Mercy’s experience in 2004 is among the first hospitalist pay-for-performance programs reported. This quality-based incentive resulted in marked improvement in 3 quality indicators, resulting in the hospital’s attainment of the bonus paid by Blue Cross. The hospitalists were satisfied with the incentive program, because they felt appropriately rewarded for high-quality care. It is interesting to note that less-aggressive measures had been undertaken in the past to improve these indicators, but with little success. In 2004, with financial rewards at stake, more robust processes—such as a weekly meeting, ongoing chart review, and direct feedback to physicians—were put in place to ensure quality improvement.
Win Whitcomb, MD, cofounded the Society of Hospital Medicine with John Nelson, MD, in 1996. He is director of Performance Improvement at Mercy Medical Center, Springfield, MA, and can be reached at [email protected].
In January 2004, the Mercy Inpatient Medicine Service embarked on a quality-based incentive program, or “pay-for-performance.” This was spurred on by Blue Cross of Massachusetts, which contracted with all hospitals in the state to receive a substantial financial bonus for agreed-upon quality indicators.
Prior to the start date, representatives from Mercy Medical Center negotiated with Blue Cross, arriving at the following quality indicators and targets for all hospital patients for the period of January to December 2004:
- For pneumonia patients, 45% rate of pneumococcal vaccine screening and administration;
- For heart failure patients, 85% rate of documentation of ejection fraction; and
- For heart failure patients with ejection fraction <40%, prescription of an ACE-inhibitor (or documentation of a contraindication) upon discharge.
The Mercy Inpatient Medicine Service, which consisted of 10 full-time hospitalists in 2004, cared for approximately 75% of all medical inpatients at the hospital. We created a quality-based incentive program for this hospital medicine group that mirrored the incentives mentioned above for the entire hospital. We postulated that superior performance by the hospitalists would “raise the bar” for the remainder of the patients, some of whom were cared for by a separate hospital medicine group and some by traditional PCPs.
Mechanics of the Pay for Performance Program
The quality-based bonus was structured so that it would be paid out at 6-month intervals in equal parts to all full-time hospitalists. The magnitude of the bonus was set in excess of 7.5% of the hospitalists’ base salary, an amount that we felt would be large enough to influence behavior change.
From the outset of the bonus period (2004), as medical director of the group, I convened a weekly meeting with hospitalists and representatives from the Quality Improvement department, nursing, and case management. The meeting was being held amid the backdrop of a renewed institutional emphasis on all the JCAHO Core Measures and other quality/patient safety initiatives. The purpose of the meeting was to review hospital performance on all JCAHO Core Measures.
A nurse from the Quality Improvement department reviewed all charts of pneumonia and heart failure patients. For cases where pneumovax was not screened/administered, ejection fraction not assessed, and ACE-I not given, a hospitalist would review the chart and provide feedback to the physician-of-record regarding the nature of the problem. Further, I provided regular feedback to the hospital medicine group regarding their performance on the 3 indicators.
Results
The results in Figures 1–3 are for the hospitalist group, with results for the hospital as a whole slightly lower, but still in excess of the targets agreed upon with Blue Cross.
Summary
Mercy’s experience in 2004 is among the first hospitalist pay-for-performance programs reported. This quality-based incentive resulted in marked improvement in 3 quality indicators, resulting in the hospital’s attainment of the bonus paid by Blue Cross. The hospitalists were satisfied with the incentive program, because they felt appropriately rewarded for high-quality care. It is interesting to note that less-aggressive measures had been undertaken in the past to improve these indicators, but with little success. In 2004, with financial rewards at stake, more robust processes—such as a weekly meeting, ongoing chart review, and direct feedback to physicians—were put in place to ensure quality improvement.
Win Whitcomb, MD, cofounded the Society of Hospital Medicine with John Nelson, MD, in 1996. He is director of Performance Improvement at Mercy Medical Center, Springfield, MA, and can be reached at [email protected].
In January 2004, the Mercy Inpatient Medicine Service embarked on a quality-based incentive program, or “pay-for-performance.” This was spurred on by Blue Cross of Massachusetts, which contracted with all hospitals in the state to receive a substantial financial bonus for agreed-upon quality indicators.
Prior to the start date, representatives from Mercy Medical Center negotiated with Blue Cross, arriving at the following quality indicators and targets for all hospital patients for the period of January to December 2004:
- For pneumonia patients, 45% rate of pneumococcal vaccine screening and administration;
- For heart failure patients, 85% rate of documentation of ejection fraction; and
- For heart failure patients with ejection fraction <40%, prescription of an ACE-inhibitor (or documentation of a contraindication) upon discharge.
The Mercy Inpatient Medicine Service, which consisted of 10 full-time hospitalists in 2004, cared for approximately 75% of all medical inpatients at the hospital. We created a quality-based incentive program for this hospital medicine group that mirrored the incentives mentioned above for the entire hospital. We postulated that superior performance by the hospitalists would “raise the bar” for the remainder of the patients, some of whom were cared for by a separate hospital medicine group and some by traditional PCPs.
Mechanics of the Pay for Performance Program
The quality-based bonus was structured so that it would be paid out at 6-month intervals in equal parts to all full-time hospitalists. The magnitude of the bonus was set in excess of 7.5% of the hospitalists’ base salary, an amount that we felt would be large enough to influence behavior change.
From the outset of the bonus period (2004), as medical director of the group, I convened a weekly meeting with hospitalists and representatives from the Quality Improvement department, nursing, and case management. The meeting was being held amid the backdrop of a renewed institutional emphasis on all the JCAHO Core Measures and other quality/patient safety initiatives. The purpose of the meeting was to review hospital performance on all JCAHO Core Measures.
A nurse from the Quality Improvement department reviewed all charts of pneumonia and heart failure patients. For cases where pneumovax was not screened/administered, ejection fraction not assessed, and ACE-I not given, a hospitalist would review the chart and provide feedback to the physician-of-record regarding the nature of the problem. Further, I provided regular feedback to the hospital medicine group regarding their performance on the 3 indicators.
Results
The results in Figures 1–3 are for the hospitalist group, with results for the hospital as a whole slightly lower, but still in excess of the targets agreed upon with Blue Cross.
Summary
Mercy’s experience in 2004 is among the first hospitalist pay-for-performance programs reported. This quality-based incentive resulted in marked improvement in 3 quality indicators, resulting in the hospital’s attainment of the bonus paid by Blue Cross. The hospitalists were satisfied with the incentive program, because they felt appropriately rewarded for high-quality care. It is interesting to note that less-aggressive measures had been undertaken in the past to improve these indicators, but with little success. In 2004, with financial rewards at stake, more robust processes—such as a weekly meeting, ongoing chart review, and direct feedback to physicians—were put in place to ensure quality improvement.
Win Whitcomb, MD, cofounded the Society of Hospital Medicine with John Nelson, MD, in 1996. He is director of Performance Improvement at Mercy Medical Center, Springfield, MA, and can be reached at [email protected].
SHM Member in the Spotlight
SHM member David Feinbloom, MD, testified before the Massachusetts Joint Committee on Healthcare Financing and Economic Development and Emerging Technologies on May 5, 2005. Dr. Feinbloom was part of a panel of Massachusetts’ healthcare and information systems leaders advocating for additional funding of a statewide initiative to install Computerized Physician Order Entry (CPOE) systems and other advanced information technologies in each hospital across Massachusetts. Dr. Feinbloom is the director of clinical resource management, Department of Medicine, and physician liaison for Clinical Information Systems Development at Beth Israel Deaconess Medical Center in Boston. Under the leadership of John Halamka, MD, MS, and chief information office of Harvard Medical School and BIDMC, the medical center is a nationally recognized leader in medical information technology.
“The goal of the hearing was to share views about the implementation of advanced technologies like CPOE, one of a series of initiatives to create a statewide medical information technology infrastructure,” says Dr. Feinbloom. “Ultimately, this will include applications such as e-prescribing, online physician-patient communications, and regional data sharing networks, which will improve quality, patient satisfaction, and reduce costs.” He says that currently a parallel initiative for related technologies has a $50 million commitment from Blue Cross and Blue Shield. An additional $210 million is needed to bring inpatient CPOE to all of the hospitals in the state. “We wanted to make sure that the committee understood that despite the seemingly high initial outlay of capital, there is a projected savings of $275 million annually.” The dramatic savings, Dr. Feinbloom says, come from efficiencies in patient throughput, reductions in medication errors and adverse drug events, and improved utilization of inpatient resources. The Massachusetts Technology Collaborative and New England Healthcare Institute are coordinating statewide efforts to remove barriers to inpatient CPOE.
Currently, only 5% to 10% percent of hospitals nationwide have CPOE systems, but that is destined to change, says Dr. Feinbloom, especially if hospitalists lead the charge. “Hospitalists are the natural choice to champion these initiatives,” Dr. Feinbloom says. “We are the experts on inpatient care and hospital systems, and we understand how important information technology is for managing complicated patients during an acute hospitalization. In addition, these technologies have proven indispensable for communicating among care providers and managing the transition from the inpatient to the outpatient setting—a process that is notorious for errors.”
For more information on CPOE implementation or funding, contact Dr. Feinbloom at [email protected].
SHM member David Feinbloom, MD, testified before the Massachusetts Joint Committee on Healthcare Financing and Economic Development and Emerging Technologies on May 5, 2005. Dr. Feinbloom was part of a panel of Massachusetts’ healthcare and information systems leaders advocating for additional funding of a statewide initiative to install Computerized Physician Order Entry (CPOE) systems and other advanced information technologies in each hospital across Massachusetts. Dr. Feinbloom is the director of clinical resource management, Department of Medicine, and physician liaison for Clinical Information Systems Development at Beth Israel Deaconess Medical Center in Boston. Under the leadership of John Halamka, MD, MS, and chief information office of Harvard Medical School and BIDMC, the medical center is a nationally recognized leader in medical information technology.
“The goal of the hearing was to share views about the implementation of advanced technologies like CPOE, one of a series of initiatives to create a statewide medical information technology infrastructure,” says Dr. Feinbloom. “Ultimately, this will include applications such as e-prescribing, online physician-patient communications, and regional data sharing networks, which will improve quality, patient satisfaction, and reduce costs.” He says that currently a parallel initiative for related technologies has a $50 million commitment from Blue Cross and Blue Shield. An additional $210 million is needed to bring inpatient CPOE to all of the hospitals in the state. “We wanted to make sure that the committee understood that despite the seemingly high initial outlay of capital, there is a projected savings of $275 million annually.” The dramatic savings, Dr. Feinbloom says, come from efficiencies in patient throughput, reductions in medication errors and adverse drug events, and improved utilization of inpatient resources. The Massachusetts Technology Collaborative and New England Healthcare Institute are coordinating statewide efforts to remove barriers to inpatient CPOE.
Currently, only 5% to 10% percent of hospitals nationwide have CPOE systems, but that is destined to change, says Dr. Feinbloom, especially if hospitalists lead the charge. “Hospitalists are the natural choice to champion these initiatives,” Dr. Feinbloom says. “We are the experts on inpatient care and hospital systems, and we understand how important information technology is for managing complicated patients during an acute hospitalization. In addition, these technologies have proven indispensable for communicating among care providers and managing the transition from the inpatient to the outpatient setting—a process that is notorious for errors.”
For more information on CPOE implementation or funding, contact Dr. Feinbloom at [email protected].
SHM member David Feinbloom, MD, testified before the Massachusetts Joint Committee on Healthcare Financing and Economic Development and Emerging Technologies on May 5, 2005. Dr. Feinbloom was part of a panel of Massachusetts’ healthcare and information systems leaders advocating for additional funding of a statewide initiative to install Computerized Physician Order Entry (CPOE) systems and other advanced information technologies in each hospital across Massachusetts. Dr. Feinbloom is the director of clinical resource management, Department of Medicine, and physician liaison for Clinical Information Systems Development at Beth Israel Deaconess Medical Center in Boston. Under the leadership of John Halamka, MD, MS, and chief information office of Harvard Medical School and BIDMC, the medical center is a nationally recognized leader in medical information technology.
“The goal of the hearing was to share views about the implementation of advanced technologies like CPOE, one of a series of initiatives to create a statewide medical information technology infrastructure,” says Dr. Feinbloom. “Ultimately, this will include applications such as e-prescribing, online physician-patient communications, and regional data sharing networks, which will improve quality, patient satisfaction, and reduce costs.” He says that currently a parallel initiative for related technologies has a $50 million commitment from Blue Cross and Blue Shield. An additional $210 million is needed to bring inpatient CPOE to all of the hospitals in the state. “We wanted to make sure that the committee understood that despite the seemingly high initial outlay of capital, there is a projected savings of $275 million annually.” The dramatic savings, Dr. Feinbloom says, come from efficiencies in patient throughput, reductions in medication errors and adverse drug events, and improved utilization of inpatient resources. The Massachusetts Technology Collaborative and New England Healthcare Institute are coordinating statewide efforts to remove barriers to inpatient CPOE.
Currently, only 5% to 10% percent of hospitals nationwide have CPOE systems, but that is destined to change, says Dr. Feinbloom, especially if hospitalists lead the charge. “Hospitalists are the natural choice to champion these initiatives,” Dr. Feinbloom says. “We are the experts on inpatient care and hospital systems, and we understand how important information technology is for managing complicated patients during an acute hospitalization. In addition, these technologies have proven indispensable for communicating among care providers and managing the transition from the inpatient to the outpatient setting—a process that is notorious for errors.”
For more information on CPOE implementation or funding, contact Dr. Feinbloom at [email protected].
SHM’s Jamie Newman, MD, Appointed Editor of The Hospitalist
After several months of planning, interviewing, and lively debate, the SHM editorial board has appointed Jamie Newman, MD, as editor of its publication, The Hospitalist, effective September 1, 2005. The Hospitalist is the nation’s only society-sanctioned news publication for hospitalists, and it contains the latest in hospital medicine news and industry issues, along with updates on society-sponsored programs, activities, and educational opportunities.
Dr. Newman is assistant professor of Internal Medicine and Medical History at the Mayo Clinic College of Medicine in Rochester, MN, where he has been a member of the Hospital Internal Medicine Program since 2001. Prior to joining the Mayo Clinic College, Dr. Newman practiced medicine in several single and multispecialty group practices. From 1989 to 2001 he taught at the University of Texas Medical Branch in Galveston, where he received several teaching awards. He also was the host of a local medical radio show, “Onward and Upward.” He has been an editor and a writer of numerous papers and book chapters.
“We’re very pleased to have Dr. Newman accept the position of editor for The Hospitalist,” said Jim Pile, MD, outgoing editor of the publication. “He is highly respected, well-published, and has a broad view of the industry and where hospitalists fit in. SHM’s Editorial Board was particularly impressed by his creativity, drive, and energy, and felt that he would bring a new vision that would steer The Hospitalist in a positive direction.”
Dr. Newman was one of a number of very qualified and interesting candidates vying for the editor position. “We were very fortunate to have so many excellent candidates to interview for this high-profile and important job,” said Editorial Board member Peter Lindenauer, MD. “I think that says a lot about just how much The Hospitalist has grown and is valued by our members.”
“I’m happy to have this opportunity to serve SHM and the field of hospital medicine through my contributions as editor of The Hospitalist,” said Dr. Newman. “With the continued rapid expansion of hospital medicine, The Hospitalist must provide vital and relevant information to a wide range of practitioners, from solo to group practice, from small local hospital to tertiary referral center, all with one common goal: improving the care of the hospitalized patient.”
As SHM readies its launch of the new Journal of Hospital Medicine in January 2006, Dr. Newman says it is more important than ever to distinguish the role of The Hospitalist from that of the journal. “While the Journal of Hospital Medicine will provide authors a place for peer reviewed publication, The Hospitalist will continue to present timely information in other areas that impact our industry, from government regulation to patient safety to education and medical innovation,” explained Dr. Newman. “Members can stay informed about these things as well as activities of the society and other members, and hopefully be entertained as well. I plan to create new sections on palliative care, geriatrics, communication, and quality, as well as a surprise or two.”
Dr. Newman earned his undergraduate degree from Johns Hopkins University and obtained his MD from Mayo Medical School. A member of SHM since 2001, Dr. Newman has served in a variety of leadership roles, including the Communication Committee and the Medical History Forum.
Launched in 1997, The Hospitalist newsletter is published 6 times a year and provided free to approximately 10,000 hospitalists in the United States. In September 2005, The Hospitalist will be issued 10 times per year.
To contact Dr. Newman about story or column ideas for the Hospitalist, please email him at [email protected].
After several months of planning, interviewing, and lively debate, the SHM editorial board has appointed Jamie Newman, MD, as editor of its publication, The Hospitalist, effective September 1, 2005. The Hospitalist is the nation’s only society-sanctioned news publication for hospitalists, and it contains the latest in hospital medicine news and industry issues, along with updates on society-sponsored programs, activities, and educational opportunities.
Dr. Newman is assistant professor of Internal Medicine and Medical History at the Mayo Clinic College of Medicine in Rochester, MN, where he has been a member of the Hospital Internal Medicine Program since 2001. Prior to joining the Mayo Clinic College, Dr. Newman practiced medicine in several single and multispecialty group practices. From 1989 to 2001 he taught at the University of Texas Medical Branch in Galveston, where he received several teaching awards. He also was the host of a local medical radio show, “Onward and Upward.” He has been an editor and a writer of numerous papers and book chapters.
“We’re very pleased to have Dr. Newman accept the position of editor for The Hospitalist,” said Jim Pile, MD, outgoing editor of the publication. “He is highly respected, well-published, and has a broad view of the industry and where hospitalists fit in. SHM’s Editorial Board was particularly impressed by his creativity, drive, and energy, and felt that he would bring a new vision that would steer The Hospitalist in a positive direction.”
Dr. Newman was one of a number of very qualified and interesting candidates vying for the editor position. “We were very fortunate to have so many excellent candidates to interview for this high-profile and important job,” said Editorial Board member Peter Lindenauer, MD. “I think that says a lot about just how much The Hospitalist has grown and is valued by our members.”
“I’m happy to have this opportunity to serve SHM and the field of hospital medicine through my contributions as editor of The Hospitalist,” said Dr. Newman. “With the continued rapid expansion of hospital medicine, The Hospitalist must provide vital and relevant information to a wide range of practitioners, from solo to group practice, from small local hospital to tertiary referral center, all with one common goal: improving the care of the hospitalized patient.”
As SHM readies its launch of the new Journal of Hospital Medicine in January 2006, Dr. Newman says it is more important than ever to distinguish the role of The Hospitalist from that of the journal. “While the Journal of Hospital Medicine will provide authors a place for peer reviewed publication, The Hospitalist will continue to present timely information in other areas that impact our industry, from government regulation to patient safety to education and medical innovation,” explained Dr. Newman. “Members can stay informed about these things as well as activities of the society and other members, and hopefully be entertained as well. I plan to create new sections on palliative care, geriatrics, communication, and quality, as well as a surprise or two.”
Dr. Newman earned his undergraduate degree from Johns Hopkins University and obtained his MD from Mayo Medical School. A member of SHM since 2001, Dr. Newman has served in a variety of leadership roles, including the Communication Committee and the Medical History Forum.
Launched in 1997, The Hospitalist newsletter is published 6 times a year and provided free to approximately 10,000 hospitalists in the United States. In September 2005, The Hospitalist will be issued 10 times per year.
To contact Dr. Newman about story or column ideas for the Hospitalist, please email him at [email protected].
After several months of planning, interviewing, and lively debate, the SHM editorial board has appointed Jamie Newman, MD, as editor of its publication, The Hospitalist, effective September 1, 2005. The Hospitalist is the nation’s only society-sanctioned news publication for hospitalists, and it contains the latest in hospital medicine news and industry issues, along with updates on society-sponsored programs, activities, and educational opportunities.
Dr. Newman is assistant professor of Internal Medicine and Medical History at the Mayo Clinic College of Medicine in Rochester, MN, where he has been a member of the Hospital Internal Medicine Program since 2001. Prior to joining the Mayo Clinic College, Dr. Newman practiced medicine in several single and multispecialty group practices. From 1989 to 2001 he taught at the University of Texas Medical Branch in Galveston, where he received several teaching awards. He also was the host of a local medical radio show, “Onward and Upward.” He has been an editor and a writer of numerous papers and book chapters.
“We’re very pleased to have Dr. Newman accept the position of editor for The Hospitalist,” said Jim Pile, MD, outgoing editor of the publication. “He is highly respected, well-published, and has a broad view of the industry and where hospitalists fit in. SHM’s Editorial Board was particularly impressed by his creativity, drive, and energy, and felt that he would bring a new vision that would steer The Hospitalist in a positive direction.”
Dr. Newman was one of a number of very qualified and interesting candidates vying for the editor position. “We were very fortunate to have so many excellent candidates to interview for this high-profile and important job,” said Editorial Board member Peter Lindenauer, MD. “I think that says a lot about just how much The Hospitalist has grown and is valued by our members.”
“I’m happy to have this opportunity to serve SHM and the field of hospital medicine through my contributions as editor of The Hospitalist,” said Dr. Newman. “With the continued rapid expansion of hospital medicine, The Hospitalist must provide vital and relevant information to a wide range of practitioners, from solo to group practice, from small local hospital to tertiary referral center, all with one common goal: improving the care of the hospitalized patient.”
As SHM readies its launch of the new Journal of Hospital Medicine in January 2006, Dr. Newman says it is more important than ever to distinguish the role of The Hospitalist from that of the journal. “While the Journal of Hospital Medicine will provide authors a place for peer reviewed publication, The Hospitalist will continue to present timely information in other areas that impact our industry, from government regulation to patient safety to education and medical innovation,” explained Dr. Newman. “Members can stay informed about these things as well as activities of the society and other members, and hopefully be entertained as well. I plan to create new sections on palliative care, geriatrics, communication, and quality, as well as a surprise or two.”
Dr. Newman earned his undergraduate degree from Johns Hopkins University and obtained his MD from Mayo Medical School. A member of SHM since 2001, Dr. Newman has served in a variety of leadership roles, including the Communication Committee and the Medical History Forum.
Launched in 1997, The Hospitalist newsletter is published 6 times a year and provided free to approximately 10,000 hospitalists in the United States. In September 2005, The Hospitalist will be issued 10 times per year.
To contact Dr. Newman about story or column ideas for the Hospitalist, please email him at [email protected].
Palliative Care Services Offer New Horizons for Hospitalists
Howard Epstein, a hospitalist at Regions Hospital in St. Paul, MN, spent nearly 2 years planning an inpatient palliative care consultation service for Regions before its launch in January of this year. A multidisciplinary advisory committee met monthly to help with the planning, and Dr. Epstein, the embryonic program’s medical director, went before the hospital’s administration to make the clinical and financial case for supporting it.
“What we’re trying to do is to take the basic interdisciplinary approach pioneered in hospice and move it upstream,” to help relieve suffering in seriously ill patients before they need or qualify for hospice care, he explains. “I just knew I wanted to incorporate it into a hospitalist model,” and into the Hospitalist Services Division’s weekly block schedule.
After the service was launched, it became clear that the schedule did not allow for the significant time commitment required to do palliative care, so a new approach is planned for July. Dr. Epstein and 6 other hospitalists participating in the palliative care service will divide up weekly blocks of time. Half of their duties while on service will be devoted to palliative care and the other half to covering 1 hospital unit as a hospitalist, rather than the usual 2 units for hospitalists at Regions.
The palliative care service at Regions, which includes a half-time chaplain and social worker and a full-time nurse practitioner, responds to consultation requests by doctors and nurses from all of the hospital’s adult services. The service also admits patients from HealthPartners’ affiliated hospice program when they are hospitalized at Regions for short-term symptom management or respite care.
Key to long-term success lies in documenting improved clinical outcomes, patient, family, and provider satisfaction, financial savings, and enhanced patient throughput. “I’m optimistic we’ll be able to demonstrate significant value, but if we can’t, we’ll be hard-pressed to get continued support,” he says. This challenge, he adds, is similar to what the hospitalist service at Regions faced when it was launched in 1998.
Palliative care is not a new concept in medicine, but it has enjoyed dramatic growth in recent years. The American Hospital Association estimates that 17% of community hospitals and 26% of academic teaching hospitals in the United States now have either a palliative care consultation service or a dedicated unit, although the former is more common because it can be established with a smaller fiscal outlay.
Palliative care aims to relieve suffering, broadly defined, for patients living with chronic, advanced illnesses. State-of-the-art pain management is a major emphasis for the interdisciplinary palliative care team, but so are addressing the patient and family’s emotional, psychological and spiritual concerns related to the illness and offering guidance for making informed treatment decisions that reflect their values and goals for care. Palliative care services generally target all patients with advanced illness from the point of diagnosis, simultaneous with any other medical treatment regimens.
Two Medical Fields Growing Together
“I believe hospitalist practices and palliative care services are of necessity growing closer together,” says Susan Block, codirector of the Harvard Medical School Center for Palliative Care in Boston, MA. The Harvard Center provides intensive palliative care training for clinicians who also have an interest in teaching.
“If you run a palliative care consult service or a palliative care unit, you are operating much like a hospitalist, with a focus on hospital systems and workload issues, communication, and getting people out of the hospital,” Dr. Block says. At the same time, most hospitalists deal with end-of-life issues and the challenges of relieving symptoms such as pain, delirium, or anxiety every day, whether they view their role in those terms or not.
Stephanie Grossman, a hospitalist at Emory Healthcare in Atlanta, GA, says she discovered personal satisfaction as a young physician in having meaningful conversations about care goals with seriously ill patients and leading family conferences, despite the time pressures of the job. The head of the hospital medicine service at Emory, Mark Williams, MD, told Dr. Grossman there was a name for what she enjoyed doing: palliative care. He encouraged her, along with colleague Melissa Mahoney, to obtain additional training in developing such a program, starting with a 2003 conference in San Diego sponsored by the Center to Advance Palliative Care.
“I came back from San Diego feeling swept away by how it really was possible to develop a financially feasible program,” says Dr. Grossman. Back at Emory, she and Dr. Mahoney joined a palliative care task force formed by Dr. Williams, with representatives from geriatrics, nursing, social work, chaplaincy, finance, and administration. This group provided input for a business plan for the palliative care service that will start in September at Emory Crawford Long and Emory University hospitals.
The 2 hospitalists, who have become certified in palliative medicine, will divide a full-time position as codirectors of the inpatient palliative care service in alternating monthly blocks, along with additional teaching responsibilities. Their 4-year plan is to add additional staffing as the program grows and to work with a geriatrician to develop a palliative care fellowship program. The palliative care team, including a nurse, social worker, and chaplain, will conduct daily palliative care rounds and biweekly interdisciplinary case conferences at the 2 hospitals.
“We have a nurse practitioner involved to help us coordinate between the 2 sites. We’ll go to various departments and do some grand rounds to introduce and market the program,” Dr. Grossman notes. In addition to practicing a style of medicine that offers deeper personal interactions with patients, she is excited to be part of creating a new program. However, she emphasizes the importance of having an executive champion within the hospital who understands financing, institutional politics, and how to recruit other champions. “We’ve been lucky to enjoy the support of Dr. Williams and [Emory Chief Operating Officer] Pete Basler. Dr. Mahoney and I have been working with hospitalists for several years, but our work has all been clinical,” she says.
Another challenge for hospitalists interested in pursuing palliative care include the need to make sure their new responsibilities are not just an add-on to a full-time job. The hospital needs to commit resources for planning and implementing a palliative care program, including a percentage of the hospitalist’s time, Dr. Block says. “Zero FTEs is not viable in the long run,” she adds.
Physician billing for palliative care consults can help offset the costs of running a service, but it is unlikely to break even on billing alone, says Eva Chittenden, a hospitalist and palliative care physician at the University of California-San Francisco, which has operated a palliative care service since 1999. Dr. Chittenden is also part of the Palliative Care Leadership Center at UCSF, which offers 2-day intensive training programs 4 times a year for hospital teams that want to start or strengthen inpatient palliative care services.
In most cases, palliative care requires financial support from the hospital, although it’s not difficult to justify that support by showing cost avoidance, reduced lengths of stay, and improved clinical outcomes, with the help of tools developed by the Center to Advance Palliative Care, Dr. Chittenden says. Program development also challenges the hospitalist’s leadership and marketing skills.
A Process of Growing Involvement
“What often happens with hospitalists is that they start out exploring palliative care, and it becomes very compelling,” Dr. Block adds. “The more competent you get at it, the more compelling it becomes. They find deeper meaning in their work. And then they’re hooked.”
A hospitalist can seek additional training and then incorporate palliative care tools, concepts, and perspectives into his or her daily work. An interest in palliative care may lead to involvement with the hospital ethics committee, a seat on a palliative care advisory committee, or a role in standards or protocol development, as well as pursuit of specialty certification in hospice and palliative medicine.
Although hospitalists may be obvious candidates to participate in more formal palliative care program development, “incorporating palliative care into a routine hospitalist practice is not a trivial thing,” Dr. Block adds. For starters, it requires additional training. “Most hospitalists don’t have the competencies to practice expert palliative care if they don’t seek them out,” she says. But the opportunities are increasing, with growing palliative care fellowship opportunities nationwide.
Two hospitalists at Chandler Regional Medical Center in Chandler, AZ, are among the 4 physicians who serve on that facility’s 12-member interdisciplinary palliative care team, attending weekly team meetings to review active cases and brainstorm program development. Both have attended national palliative care conferences, reports the palliative care service’s nurse practitioner, Donna Nolde. The service consulted on 89 patients in March, and about 70% of those referrals came from hospitalists.
“As hospitalists, we often deal with issues of death and dying,” especially when working in the ICU or with referring oncologists, notes Chandler’s Mahmood Shahlapour. “We can sometimes step back and see the big picture when other doctors have trouble letting go.” Dr. Shahlapour believes palliative care is a logical extension of good internal medicine and will eventually become a bigger part of the training of internists.
An atypical path to palliative care is that of Glenda Hickman, MD, who was a hospitalist for the Denver, CO–based HealthONE system until one of the system’s hospitals asked her to take on the role of freelance palliative care consultant. Hickman, who also works part-time for Hospice of Metro Denver as a team medical director and picks up lecturing and teaching assignments, accepts consultations from 3 HealthONE hospitals and bills third-party payers for her consultations. Her husband is office manager and biller for her home-based business, and she carries a cell phone and pager to promptly answer referrals.
“I had a reputation for the touchy-feely aspects of medicine at the hospitals where I worked,” Dr. Hickman relates. “Dying patients would often get referred to me.” Based on her interests, Dr. Hickman sought training in palliative care, but she found it difficult to juggle with her full-time job as a hospitalist. “The heart of palliative care in a hospital is talking with patients and families. These conversations take a long time,” she says. When the hospital asked for her help in meeting its JCAHO requirements in pain management and palliative care, Dr. Hickman was willing to explore a model for how she could hang out a shingle as a solo practitioner.
Business is growing, although the workload fluctuates widely. However, while Dr. Hickman works alongside social workers and chaplains at the hospitals, the biggest drawback has been the lack of a formal, interdisciplinary team. “This is high-maintenance, high-emotion work. It can be a big drain, and I don’t have a designated team with which to share the burden. My goal is to run a full palliative care team for the hospital,” she says, and there are signs that HealthONE eventually may move in that direction.
“It’s not that hospitalists can’t do palliative care. I did. I was so drawn to it and to trying to do it right, which meant I was trying to do 2 jobs at once,” she adds. Hospitalists can also participate by recognizing when their patients need the extra attention of a palliative care specialist. “Identifying who those patients are is a huge skill by itself.”
Resources for Getting Started in Palliative Care
The Center to Advance Palliative Care (CAPC) at Mount Sinai School of Medicine in New York City offers a comprehensive national resource for palliative care development in hospitals, including how to make the financial case. Its next national seminar is October 17 to 19 in San Diego, CA. CAPC also supports 6 regional Palliative Care Leadership Centers, including one with a hospitalist emphasis at the University of California-San Francisco, scheduled to run through June 2006. For more information on CAPC’s resources and leadership centers, call 202-201-2670 or visit www.capc.org.
Larry Beresford can be contacted at [email protected].
Other Helpful Resources
- For information on the Education in Palliative and End-of-Life Care (EPEC) curriculum, visit www.epec.net.
- The American Board of Hospice and Palliative Medicine will offer its next specialty certifying examination in November of 2005. For eligibility or other questions, call 301-439-8001 or visit www.abhpm.org.
- The American Association of Hospice and Palliative Medicine offers education and training resources, including an annual assembly scheduled for February 8 to 11, 2006, in Nashville, TN; visit www.aahpm.org.
- Harvard’s Center for Palliative Care offers a 2-week intensive training course, with an emphasis on teaching, in April and November every year. For information, call 617-724-9509, send email to [email protected], or visit www.hms.harvard.edu/cdi/pallcare/.
- The Veterans Administration also offers palliative care resources, fellowship opportunities and other information; visit www.hospice.va.gov.
Howard Epstein, a hospitalist at Regions Hospital in St. Paul, MN, spent nearly 2 years planning an inpatient palliative care consultation service for Regions before its launch in January of this year. A multidisciplinary advisory committee met monthly to help with the planning, and Dr. Epstein, the embryonic program’s medical director, went before the hospital’s administration to make the clinical and financial case for supporting it.
“What we’re trying to do is to take the basic interdisciplinary approach pioneered in hospice and move it upstream,” to help relieve suffering in seriously ill patients before they need or qualify for hospice care, he explains. “I just knew I wanted to incorporate it into a hospitalist model,” and into the Hospitalist Services Division’s weekly block schedule.
After the service was launched, it became clear that the schedule did not allow for the significant time commitment required to do palliative care, so a new approach is planned for July. Dr. Epstein and 6 other hospitalists participating in the palliative care service will divide up weekly blocks of time. Half of their duties while on service will be devoted to palliative care and the other half to covering 1 hospital unit as a hospitalist, rather than the usual 2 units for hospitalists at Regions.
The palliative care service at Regions, which includes a half-time chaplain and social worker and a full-time nurse practitioner, responds to consultation requests by doctors and nurses from all of the hospital’s adult services. The service also admits patients from HealthPartners’ affiliated hospice program when they are hospitalized at Regions for short-term symptom management or respite care.
Key to long-term success lies in documenting improved clinical outcomes, patient, family, and provider satisfaction, financial savings, and enhanced patient throughput. “I’m optimistic we’ll be able to demonstrate significant value, but if we can’t, we’ll be hard-pressed to get continued support,” he says. This challenge, he adds, is similar to what the hospitalist service at Regions faced when it was launched in 1998.
Palliative care is not a new concept in medicine, but it has enjoyed dramatic growth in recent years. The American Hospital Association estimates that 17% of community hospitals and 26% of academic teaching hospitals in the United States now have either a palliative care consultation service or a dedicated unit, although the former is more common because it can be established with a smaller fiscal outlay.
Palliative care aims to relieve suffering, broadly defined, for patients living with chronic, advanced illnesses. State-of-the-art pain management is a major emphasis for the interdisciplinary palliative care team, but so are addressing the patient and family’s emotional, psychological and spiritual concerns related to the illness and offering guidance for making informed treatment decisions that reflect their values and goals for care. Palliative care services generally target all patients with advanced illness from the point of diagnosis, simultaneous with any other medical treatment regimens.
Two Medical Fields Growing Together
“I believe hospitalist practices and palliative care services are of necessity growing closer together,” says Susan Block, codirector of the Harvard Medical School Center for Palliative Care in Boston, MA. The Harvard Center provides intensive palliative care training for clinicians who also have an interest in teaching.
“If you run a palliative care consult service or a palliative care unit, you are operating much like a hospitalist, with a focus on hospital systems and workload issues, communication, and getting people out of the hospital,” Dr. Block says. At the same time, most hospitalists deal with end-of-life issues and the challenges of relieving symptoms such as pain, delirium, or anxiety every day, whether they view their role in those terms or not.
Stephanie Grossman, a hospitalist at Emory Healthcare in Atlanta, GA, says she discovered personal satisfaction as a young physician in having meaningful conversations about care goals with seriously ill patients and leading family conferences, despite the time pressures of the job. The head of the hospital medicine service at Emory, Mark Williams, MD, told Dr. Grossman there was a name for what she enjoyed doing: palliative care. He encouraged her, along with colleague Melissa Mahoney, to obtain additional training in developing such a program, starting with a 2003 conference in San Diego sponsored by the Center to Advance Palliative Care.
“I came back from San Diego feeling swept away by how it really was possible to develop a financially feasible program,” says Dr. Grossman. Back at Emory, she and Dr. Mahoney joined a palliative care task force formed by Dr. Williams, with representatives from geriatrics, nursing, social work, chaplaincy, finance, and administration. This group provided input for a business plan for the palliative care service that will start in September at Emory Crawford Long and Emory University hospitals.
The 2 hospitalists, who have become certified in palliative medicine, will divide a full-time position as codirectors of the inpatient palliative care service in alternating monthly blocks, along with additional teaching responsibilities. Their 4-year plan is to add additional staffing as the program grows and to work with a geriatrician to develop a palliative care fellowship program. The palliative care team, including a nurse, social worker, and chaplain, will conduct daily palliative care rounds and biweekly interdisciplinary case conferences at the 2 hospitals.
“We have a nurse practitioner involved to help us coordinate between the 2 sites. We’ll go to various departments and do some grand rounds to introduce and market the program,” Dr. Grossman notes. In addition to practicing a style of medicine that offers deeper personal interactions with patients, she is excited to be part of creating a new program. However, she emphasizes the importance of having an executive champion within the hospital who understands financing, institutional politics, and how to recruit other champions. “We’ve been lucky to enjoy the support of Dr. Williams and [Emory Chief Operating Officer] Pete Basler. Dr. Mahoney and I have been working with hospitalists for several years, but our work has all been clinical,” she says.
Another challenge for hospitalists interested in pursuing palliative care include the need to make sure their new responsibilities are not just an add-on to a full-time job. The hospital needs to commit resources for planning and implementing a palliative care program, including a percentage of the hospitalist’s time, Dr. Block says. “Zero FTEs is not viable in the long run,” she adds.
Physician billing for palliative care consults can help offset the costs of running a service, but it is unlikely to break even on billing alone, says Eva Chittenden, a hospitalist and palliative care physician at the University of California-San Francisco, which has operated a palliative care service since 1999. Dr. Chittenden is also part of the Palliative Care Leadership Center at UCSF, which offers 2-day intensive training programs 4 times a year for hospital teams that want to start or strengthen inpatient palliative care services.
In most cases, palliative care requires financial support from the hospital, although it’s not difficult to justify that support by showing cost avoidance, reduced lengths of stay, and improved clinical outcomes, with the help of tools developed by the Center to Advance Palliative Care, Dr. Chittenden says. Program development also challenges the hospitalist’s leadership and marketing skills.
A Process of Growing Involvement
“What often happens with hospitalists is that they start out exploring palliative care, and it becomes very compelling,” Dr. Block adds. “The more competent you get at it, the more compelling it becomes. They find deeper meaning in their work. And then they’re hooked.”
A hospitalist can seek additional training and then incorporate palliative care tools, concepts, and perspectives into his or her daily work. An interest in palliative care may lead to involvement with the hospital ethics committee, a seat on a palliative care advisory committee, or a role in standards or protocol development, as well as pursuit of specialty certification in hospice and palliative medicine.
Although hospitalists may be obvious candidates to participate in more formal palliative care program development, “incorporating palliative care into a routine hospitalist practice is not a trivial thing,” Dr. Block adds. For starters, it requires additional training. “Most hospitalists don’t have the competencies to practice expert palliative care if they don’t seek them out,” she says. But the opportunities are increasing, with growing palliative care fellowship opportunities nationwide.
Two hospitalists at Chandler Regional Medical Center in Chandler, AZ, are among the 4 physicians who serve on that facility’s 12-member interdisciplinary palliative care team, attending weekly team meetings to review active cases and brainstorm program development. Both have attended national palliative care conferences, reports the palliative care service’s nurse practitioner, Donna Nolde. The service consulted on 89 patients in March, and about 70% of those referrals came from hospitalists.
“As hospitalists, we often deal with issues of death and dying,” especially when working in the ICU or with referring oncologists, notes Chandler’s Mahmood Shahlapour. “We can sometimes step back and see the big picture when other doctors have trouble letting go.” Dr. Shahlapour believes palliative care is a logical extension of good internal medicine and will eventually become a bigger part of the training of internists.
An atypical path to palliative care is that of Glenda Hickman, MD, who was a hospitalist for the Denver, CO–based HealthONE system until one of the system’s hospitals asked her to take on the role of freelance palliative care consultant. Hickman, who also works part-time for Hospice of Metro Denver as a team medical director and picks up lecturing and teaching assignments, accepts consultations from 3 HealthONE hospitals and bills third-party payers for her consultations. Her husband is office manager and biller for her home-based business, and she carries a cell phone and pager to promptly answer referrals.
“I had a reputation for the touchy-feely aspects of medicine at the hospitals where I worked,” Dr. Hickman relates. “Dying patients would often get referred to me.” Based on her interests, Dr. Hickman sought training in palliative care, but she found it difficult to juggle with her full-time job as a hospitalist. “The heart of palliative care in a hospital is talking with patients and families. These conversations take a long time,” she says. When the hospital asked for her help in meeting its JCAHO requirements in pain management and palliative care, Dr. Hickman was willing to explore a model for how she could hang out a shingle as a solo practitioner.
Business is growing, although the workload fluctuates widely. However, while Dr. Hickman works alongside social workers and chaplains at the hospitals, the biggest drawback has been the lack of a formal, interdisciplinary team. “This is high-maintenance, high-emotion work. It can be a big drain, and I don’t have a designated team with which to share the burden. My goal is to run a full palliative care team for the hospital,” she says, and there are signs that HealthONE eventually may move in that direction.
“It’s not that hospitalists can’t do palliative care. I did. I was so drawn to it and to trying to do it right, which meant I was trying to do 2 jobs at once,” she adds. Hospitalists can also participate by recognizing when their patients need the extra attention of a palliative care specialist. “Identifying who those patients are is a huge skill by itself.”
Resources for Getting Started in Palliative Care
The Center to Advance Palliative Care (CAPC) at Mount Sinai School of Medicine in New York City offers a comprehensive national resource for palliative care development in hospitals, including how to make the financial case. Its next national seminar is October 17 to 19 in San Diego, CA. CAPC also supports 6 regional Palliative Care Leadership Centers, including one with a hospitalist emphasis at the University of California-San Francisco, scheduled to run through June 2006. For more information on CAPC’s resources and leadership centers, call 202-201-2670 or visit www.capc.org.
Larry Beresford can be contacted at [email protected].
Other Helpful Resources
- For information on the Education in Palliative and End-of-Life Care (EPEC) curriculum, visit www.epec.net.
- The American Board of Hospice and Palliative Medicine will offer its next specialty certifying examination in November of 2005. For eligibility or other questions, call 301-439-8001 or visit www.abhpm.org.
- The American Association of Hospice and Palliative Medicine offers education and training resources, including an annual assembly scheduled for February 8 to 11, 2006, in Nashville, TN; visit www.aahpm.org.
- Harvard’s Center for Palliative Care offers a 2-week intensive training course, with an emphasis on teaching, in April and November every year. For information, call 617-724-9509, send email to [email protected], or visit www.hms.harvard.edu/cdi/pallcare/.
- The Veterans Administration also offers palliative care resources, fellowship opportunities and other information; visit www.hospice.va.gov.
Howard Epstein, a hospitalist at Regions Hospital in St. Paul, MN, spent nearly 2 years planning an inpatient palliative care consultation service for Regions before its launch in January of this year. A multidisciplinary advisory committee met monthly to help with the planning, and Dr. Epstein, the embryonic program’s medical director, went before the hospital’s administration to make the clinical and financial case for supporting it.
“What we’re trying to do is to take the basic interdisciplinary approach pioneered in hospice and move it upstream,” to help relieve suffering in seriously ill patients before they need or qualify for hospice care, he explains. “I just knew I wanted to incorporate it into a hospitalist model,” and into the Hospitalist Services Division’s weekly block schedule.
After the service was launched, it became clear that the schedule did not allow for the significant time commitment required to do palliative care, so a new approach is planned for July. Dr. Epstein and 6 other hospitalists participating in the palliative care service will divide up weekly blocks of time. Half of their duties while on service will be devoted to palliative care and the other half to covering 1 hospital unit as a hospitalist, rather than the usual 2 units for hospitalists at Regions.
The palliative care service at Regions, which includes a half-time chaplain and social worker and a full-time nurse practitioner, responds to consultation requests by doctors and nurses from all of the hospital’s adult services. The service also admits patients from HealthPartners’ affiliated hospice program when they are hospitalized at Regions for short-term symptom management or respite care.
Key to long-term success lies in documenting improved clinical outcomes, patient, family, and provider satisfaction, financial savings, and enhanced patient throughput. “I’m optimistic we’ll be able to demonstrate significant value, but if we can’t, we’ll be hard-pressed to get continued support,” he says. This challenge, he adds, is similar to what the hospitalist service at Regions faced when it was launched in 1998.
Palliative care is not a new concept in medicine, but it has enjoyed dramatic growth in recent years. The American Hospital Association estimates that 17% of community hospitals and 26% of academic teaching hospitals in the United States now have either a palliative care consultation service or a dedicated unit, although the former is more common because it can be established with a smaller fiscal outlay.
Palliative care aims to relieve suffering, broadly defined, for patients living with chronic, advanced illnesses. State-of-the-art pain management is a major emphasis for the interdisciplinary palliative care team, but so are addressing the patient and family’s emotional, psychological and spiritual concerns related to the illness and offering guidance for making informed treatment decisions that reflect their values and goals for care. Palliative care services generally target all patients with advanced illness from the point of diagnosis, simultaneous with any other medical treatment regimens.
Two Medical Fields Growing Together
“I believe hospitalist practices and palliative care services are of necessity growing closer together,” says Susan Block, codirector of the Harvard Medical School Center for Palliative Care in Boston, MA. The Harvard Center provides intensive palliative care training for clinicians who also have an interest in teaching.
“If you run a palliative care consult service or a palliative care unit, you are operating much like a hospitalist, with a focus on hospital systems and workload issues, communication, and getting people out of the hospital,” Dr. Block says. At the same time, most hospitalists deal with end-of-life issues and the challenges of relieving symptoms such as pain, delirium, or anxiety every day, whether they view their role in those terms or not.
Stephanie Grossman, a hospitalist at Emory Healthcare in Atlanta, GA, says she discovered personal satisfaction as a young physician in having meaningful conversations about care goals with seriously ill patients and leading family conferences, despite the time pressures of the job. The head of the hospital medicine service at Emory, Mark Williams, MD, told Dr. Grossman there was a name for what she enjoyed doing: palliative care. He encouraged her, along with colleague Melissa Mahoney, to obtain additional training in developing such a program, starting with a 2003 conference in San Diego sponsored by the Center to Advance Palliative Care.
“I came back from San Diego feeling swept away by how it really was possible to develop a financially feasible program,” says Dr. Grossman. Back at Emory, she and Dr. Mahoney joined a palliative care task force formed by Dr. Williams, with representatives from geriatrics, nursing, social work, chaplaincy, finance, and administration. This group provided input for a business plan for the palliative care service that will start in September at Emory Crawford Long and Emory University hospitals.
The 2 hospitalists, who have become certified in palliative medicine, will divide a full-time position as codirectors of the inpatient palliative care service in alternating monthly blocks, along with additional teaching responsibilities. Their 4-year plan is to add additional staffing as the program grows and to work with a geriatrician to develop a palliative care fellowship program. The palliative care team, including a nurse, social worker, and chaplain, will conduct daily palliative care rounds and biweekly interdisciplinary case conferences at the 2 hospitals.
“We have a nurse practitioner involved to help us coordinate between the 2 sites. We’ll go to various departments and do some grand rounds to introduce and market the program,” Dr. Grossman notes. In addition to practicing a style of medicine that offers deeper personal interactions with patients, she is excited to be part of creating a new program. However, she emphasizes the importance of having an executive champion within the hospital who understands financing, institutional politics, and how to recruit other champions. “We’ve been lucky to enjoy the support of Dr. Williams and [Emory Chief Operating Officer] Pete Basler. Dr. Mahoney and I have been working with hospitalists for several years, but our work has all been clinical,” she says.
Another challenge for hospitalists interested in pursuing palliative care include the need to make sure their new responsibilities are not just an add-on to a full-time job. The hospital needs to commit resources for planning and implementing a palliative care program, including a percentage of the hospitalist’s time, Dr. Block says. “Zero FTEs is not viable in the long run,” she adds.
Physician billing for palliative care consults can help offset the costs of running a service, but it is unlikely to break even on billing alone, says Eva Chittenden, a hospitalist and palliative care physician at the University of California-San Francisco, which has operated a palliative care service since 1999. Dr. Chittenden is also part of the Palliative Care Leadership Center at UCSF, which offers 2-day intensive training programs 4 times a year for hospital teams that want to start or strengthen inpatient palliative care services.
In most cases, palliative care requires financial support from the hospital, although it’s not difficult to justify that support by showing cost avoidance, reduced lengths of stay, and improved clinical outcomes, with the help of tools developed by the Center to Advance Palliative Care, Dr. Chittenden says. Program development also challenges the hospitalist’s leadership and marketing skills.
A Process of Growing Involvement
“What often happens with hospitalists is that they start out exploring palliative care, and it becomes very compelling,” Dr. Block adds. “The more competent you get at it, the more compelling it becomes. They find deeper meaning in their work. And then they’re hooked.”
A hospitalist can seek additional training and then incorporate palliative care tools, concepts, and perspectives into his or her daily work. An interest in palliative care may lead to involvement with the hospital ethics committee, a seat on a palliative care advisory committee, or a role in standards or protocol development, as well as pursuit of specialty certification in hospice and palliative medicine.
Although hospitalists may be obvious candidates to participate in more formal palliative care program development, “incorporating palliative care into a routine hospitalist practice is not a trivial thing,” Dr. Block adds. For starters, it requires additional training. “Most hospitalists don’t have the competencies to practice expert palliative care if they don’t seek them out,” she says. But the opportunities are increasing, with growing palliative care fellowship opportunities nationwide.
Two hospitalists at Chandler Regional Medical Center in Chandler, AZ, are among the 4 physicians who serve on that facility’s 12-member interdisciplinary palliative care team, attending weekly team meetings to review active cases and brainstorm program development. Both have attended national palliative care conferences, reports the palliative care service’s nurse practitioner, Donna Nolde. The service consulted on 89 patients in March, and about 70% of those referrals came from hospitalists.
“As hospitalists, we often deal with issues of death and dying,” especially when working in the ICU or with referring oncologists, notes Chandler’s Mahmood Shahlapour. “We can sometimes step back and see the big picture when other doctors have trouble letting go.” Dr. Shahlapour believes palliative care is a logical extension of good internal medicine and will eventually become a bigger part of the training of internists.
An atypical path to palliative care is that of Glenda Hickman, MD, who was a hospitalist for the Denver, CO–based HealthONE system until one of the system’s hospitals asked her to take on the role of freelance palliative care consultant. Hickman, who also works part-time for Hospice of Metro Denver as a team medical director and picks up lecturing and teaching assignments, accepts consultations from 3 HealthONE hospitals and bills third-party payers for her consultations. Her husband is office manager and biller for her home-based business, and she carries a cell phone and pager to promptly answer referrals.
“I had a reputation for the touchy-feely aspects of medicine at the hospitals where I worked,” Dr. Hickman relates. “Dying patients would often get referred to me.” Based on her interests, Dr. Hickman sought training in palliative care, but she found it difficult to juggle with her full-time job as a hospitalist. “The heart of palliative care in a hospital is talking with patients and families. These conversations take a long time,” she says. When the hospital asked for her help in meeting its JCAHO requirements in pain management and palliative care, Dr. Hickman was willing to explore a model for how she could hang out a shingle as a solo practitioner.
Business is growing, although the workload fluctuates widely. However, while Dr. Hickman works alongside social workers and chaplains at the hospitals, the biggest drawback has been the lack of a formal, interdisciplinary team. “This is high-maintenance, high-emotion work. It can be a big drain, and I don’t have a designated team with which to share the burden. My goal is to run a full palliative care team for the hospital,” she says, and there are signs that HealthONE eventually may move in that direction.
“It’s not that hospitalists can’t do palliative care. I did. I was so drawn to it and to trying to do it right, which meant I was trying to do 2 jobs at once,” she adds. Hospitalists can also participate by recognizing when their patients need the extra attention of a palliative care specialist. “Identifying who those patients are is a huge skill by itself.”
Resources for Getting Started in Palliative Care
The Center to Advance Palliative Care (CAPC) at Mount Sinai School of Medicine in New York City offers a comprehensive national resource for palliative care development in hospitals, including how to make the financial case. Its next national seminar is October 17 to 19 in San Diego, CA. CAPC also supports 6 regional Palliative Care Leadership Centers, including one with a hospitalist emphasis at the University of California-San Francisco, scheduled to run through June 2006. For more information on CAPC’s resources and leadership centers, call 202-201-2670 or visit www.capc.org.
Larry Beresford can be contacted at [email protected].
Other Helpful Resources
- For information on the Education in Palliative and End-of-Life Care (EPEC) curriculum, visit www.epec.net.
- The American Board of Hospice and Palliative Medicine will offer its next specialty certifying examination in November of 2005. For eligibility or other questions, call 301-439-8001 or visit www.abhpm.org.
- The American Association of Hospice and Palliative Medicine offers education and training resources, including an annual assembly scheduled for February 8 to 11, 2006, in Nashville, TN; visit www.aahpm.org.
- Harvard’s Center for Palliative Care offers a 2-week intensive training course, with an emphasis on teaching, in April and November every year. For information, call 617-724-9509, send email to [email protected], or visit www.hms.harvard.edu/cdi/pallcare/.
- The Veterans Administration also offers palliative care resources, fellowship opportunities and other information; visit www.hospice.va.gov.
A New Debut
Here’s a question for you: When was the first issue of The Hospitalist published?
The answer: Winter (early) 1997.
In the late ’90s, The Hospitalist was little more than a bifold eight-page newsletter mailed to members of the then-National Association of Inpatient Physicians. The newsletter contained one page of classified advertising.
Contrast that first issue of The Hospitalist to the one you’re reading now: a whopping 45 pages of editorial content, with about the same number of advertising pages to match!
Nine years and dozens of changes later, The Hospitalist is about to transform itself again—a transformation that will put it at the forefront of professionally published, business-to- business titles. Sounds exciting, to be sure. But I bet you’re wondering, “Why another change?”
Simply put, The Hospitalist must reflect the progressive growth of SHM and of hospital medicine overall.
Partnering for the Future
In January SHM endeavored to elevate SHM’s publishing program on the national publishing stage and so partnered with John Wiley & Sons. SHM brought hospital medicine knowledge and expertise to the table; Wiley brought editorial and publishing knowledge and expertise.
Together, the two organizations determined that The Hospitalist needed to further grow—in format, frequency, and editorial excellence—to meet the needs of members and to respond to industry interest in The Hospitalist as the most trusted, credible source of news, issues, and trends in hospital medicine. SHM and Wiley also partnered to launch the first peer-reviewed hospital medicine journal (the Journal of Hospital Medicine—coming in January).
These initiatives, along with the commitment to maintaining strict ethical publishing guidelines (meaning—among other things—separation of editorial content and advertising) in both titles, pack the one-two punch that SHM members deserve.
What You’ll See
Beginning with the September 2005 issue, you’ll notice quite a few improvements in The Hospitalist. Here’s my short list of what you can expect:
- Size change: The publication will grow from its current iteration (a standard or 8.5” x 11” size) to a “tabloid” (or 11” by 17” size);
- Frequency change: We’ll begin publishing The Hospitalist on a monthly basis. Instead of six issue per year, you’ll receive 12; • The “look:” The design of the publication will evolve from a text-driven format to a more diverse, visually appealing format;
- Who’s who: The editorial staff will grow from a sole physician editor to a two-editor team: A professional editor from SHM publishing partner John Wiley & Sons—that’s me—will pair with Physician Editor Jamie Newman, MD, from the Mayo Clinic Rochester, to create each issue. In addition, we’ll utilize hospital medicine experts from around the country, as well as tap professional health-care writers to report on and investigate the most pressing issues in hospital medicine; and
- Content changes: Each month we’ll continue to present the quality articles you’ve come to expect from us. Our singular editorial goal is to be the voice of hospital medicine. And, thanks to the format changes, we’ll be able to offer you even more each issue. In the coming months, look for such enhancements as
- Balance: a blend of clinical, management/leadership, administrative, and policy articles in each issue—all written and edited with one question in mind, “Why is this article meaningful to you, the hospitalist reader?”;
- More strategies on how to get information on subjects: extended references, resources, and other bonus guidance in each article;
- Fast reads: “bottom line” sections that condense articles into at-a-glance summaries and shorter articles;
- SHM focus: organized, easy-to read news specific to SHM members and activities;
- Voices, voices: hospitalists from all parts of the country used as sources and in quoted material throughout the publication; and
- Much more!
Growth and Change = Great
Every once in a while you get lucky enough to be involved in a project that’s good as it is—but that teeters on the cusp of catapulting into something truly great. That’s how I feel about the impending growth and change of The Hospitalist. Incoming Physician Editor Jamie Newman and I welcome your ideas on how we can best shape the future of The Hospitalist. E-mail us at [email protected] or [email protected]. Onward!
Editor Lisa Dionne has been involved in magazine publishing for more than 14 years. She’s helped create, edit, and write award-winning editorial content for audiences ranging from long-term care physicians to EMS personnel to children.
Here’s a question for you: When was the first issue of The Hospitalist published?
The answer: Winter (early) 1997.
In the late ’90s, The Hospitalist was little more than a bifold eight-page newsletter mailed to members of the then-National Association of Inpatient Physicians. The newsletter contained one page of classified advertising.
Contrast that first issue of The Hospitalist to the one you’re reading now: a whopping 45 pages of editorial content, with about the same number of advertising pages to match!
Nine years and dozens of changes later, The Hospitalist is about to transform itself again—a transformation that will put it at the forefront of professionally published, business-to- business titles. Sounds exciting, to be sure. But I bet you’re wondering, “Why another change?”
Simply put, The Hospitalist must reflect the progressive growth of SHM and of hospital medicine overall.
Partnering for the Future
In January SHM endeavored to elevate SHM’s publishing program on the national publishing stage and so partnered with John Wiley & Sons. SHM brought hospital medicine knowledge and expertise to the table; Wiley brought editorial and publishing knowledge and expertise.
Together, the two organizations determined that The Hospitalist needed to further grow—in format, frequency, and editorial excellence—to meet the needs of members and to respond to industry interest in The Hospitalist as the most trusted, credible source of news, issues, and trends in hospital medicine. SHM and Wiley also partnered to launch the first peer-reviewed hospital medicine journal (the Journal of Hospital Medicine—coming in January).
These initiatives, along with the commitment to maintaining strict ethical publishing guidelines (meaning—among other things—separation of editorial content and advertising) in both titles, pack the one-two punch that SHM members deserve.
What You’ll See
Beginning with the September 2005 issue, you’ll notice quite a few improvements in The Hospitalist. Here’s my short list of what you can expect:
- Size change: The publication will grow from its current iteration (a standard or 8.5” x 11” size) to a “tabloid” (or 11” by 17” size);
- Frequency change: We’ll begin publishing The Hospitalist on a monthly basis. Instead of six issue per year, you’ll receive 12; • The “look:” The design of the publication will evolve from a text-driven format to a more diverse, visually appealing format;
- Who’s who: The editorial staff will grow from a sole physician editor to a two-editor team: A professional editor from SHM publishing partner John Wiley & Sons—that’s me—will pair with Physician Editor Jamie Newman, MD, from the Mayo Clinic Rochester, to create each issue. In addition, we’ll utilize hospital medicine experts from around the country, as well as tap professional health-care writers to report on and investigate the most pressing issues in hospital medicine; and
- Content changes: Each month we’ll continue to present the quality articles you’ve come to expect from us. Our singular editorial goal is to be the voice of hospital medicine. And, thanks to the format changes, we’ll be able to offer you even more each issue. In the coming months, look for such enhancements as
- Balance: a blend of clinical, management/leadership, administrative, and policy articles in each issue—all written and edited with one question in mind, “Why is this article meaningful to you, the hospitalist reader?”;
- More strategies on how to get information on subjects: extended references, resources, and other bonus guidance in each article;
- Fast reads: “bottom line” sections that condense articles into at-a-glance summaries and shorter articles;
- SHM focus: organized, easy-to read news specific to SHM members and activities;
- Voices, voices: hospitalists from all parts of the country used as sources and in quoted material throughout the publication; and
- Much more!
Growth and Change = Great
Every once in a while you get lucky enough to be involved in a project that’s good as it is—but that teeters on the cusp of catapulting into something truly great. That’s how I feel about the impending growth and change of The Hospitalist. Incoming Physician Editor Jamie Newman and I welcome your ideas on how we can best shape the future of The Hospitalist. E-mail us at [email protected] or [email protected]. Onward!
Editor Lisa Dionne has been involved in magazine publishing for more than 14 years. She’s helped create, edit, and write award-winning editorial content for audiences ranging from long-term care physicians to EMS personnel to children.
Here’s a question for you: When was the first issue of The Hospitalist published?
The answer: Winter (early) 1997.
In the late ’90s, The Hospitalist was little more than a bifold eight-page newsletter mailed to members of the then-National Association of Inpatient Physicians. The newsletter contained one page of classified advertising.
Contrast that first issue of The Hospitalist to the one you’re reading now: a whopping 45 pages of editorial content, with about the same number of advertising pages to match!
Nine years and dozens of changes later, The Hospitalist is about to transform itself again—a transformation that will put it at the forefront of professionally published, business-to- business titles. Sounds exciting, to be sure. But I bet you’re wondering, “Why another change?”
Simply put, The Hospitalist must reflect the progressive growth of SHM and of hospital medicine overall.
Partnering for the Future
In January SHM endeavored to elevate SHM’s publishing program on the national publishing stage and so partnered with John Wiley & Sons. SHM brought hospital medicine knowledge and expertise to the table; Wiley brought editorial and publishing knowledge and expertise.
Together, the two organizations determined that The Hospitalist needed to further grow—in format, frequency, and editorial excellence—to meet the needs of members and to respond to industry interest in The Hospitalist as the most trusted, credible source of news, issues, and trends in hospital medicine. SHM and Wiley also partnered to launch the first peer-reviewed hospital medicine journal (the Journal of Hospital Medicine—coming in January).
These initiatives, along with the commitment to maintaining strict ethical publishing guidelines (meaning—among other things—separation of editorial content and advertising) in both titles, pack the one-two punch that SHM members deserve.
What You’ll See
Beginning with the September 2005 issue, you’ll notice quite a few improvements in The Hospitalist. Here’s my short list of what you can expect:
- Size change: The publication will grow from its current iteration (a standard or 8.5” x 11” size) to a “tabloid” (or 11” by 17” size);
- Frequency change: We’ll begin publishing The Hospitalist on a monthly basis. Instead of six issue per year, you’ll receive 12; • The “look:” The design of the publication will evolve from a text-driven format to a more diverse, visually appealing format;
- Who’s who: The editorial staff will grow from a sole physician editor to a two-editor team: A professional editor from SHM publishing partner John Wiley & Sons—that’s me—will pair with Physician Editor Jamie Newman, MD, from the Mayo Clinic Rochester, to create each issue. In addition, we’ll utilize hospital medicine experts from around the country, as well as tap professional health-care writers to report on and investigate the most pressing issues in hospital medicine; and
- Content changes: Each month we’ll continue to present the quality articles you’ve come to expect from us. Our singular editorial goal is to be the voice of hospital medicine. And, thanks to the format changes, we’ll be able to offer you even more each issue. In the coming months, look for such enhancements as
- Balance: a blend of clinical, management/leadership, administrative, and policy articles in each issue—all written and edited with one question in mind, “Why is this article meaningful to you, the hospitalist reader?”;
- More strategies on how to get information on subjects: extended references, resources, and other bonus guidance in each article;
- Fast reads: “bottom line” sections that condense articles into at-a-glance summaries and shorter articles;
- SHM focus: organized, easy-to read news specific to SHM members and activities;
- Voices, voices: hospitalists from all parts of the country used as sources and in quoted material throughout the publication; and
- Much more!
Growth and Change = Great
Every once in a while you get lucky enough to be involved in a project that’s good as it is—but that teeters on the cusp of catapulting into something truly great. That’s how I feel about the impending growth and change of The Hospitalist. Incoming Physician Editor Jamie Newman and I welcome your ideas on how we can best shape the future of The Hospitalist. E-mail us at [email protected] or [email protected]. Onward!
Editor Lisa Dionne has been involved in magazine publishing for more than 14 years. She’s helped create, edit, and write award-winning editorial content for audiences ranging from long-term care physicians to EMS personnel to children.