User login
Clostridium difficile–Associated Diarrhea and Colitis: A Significant Cause of Nosocomial Infection
Introduction
Clostridium difficile–associated diarrhea (CDAD) has been recognized with increased frequency as a cause of nosocomial illness. The frequency and incidence of CDAD varies widely, and is influenced by multiple factors including nosocomial outbreaks, patterns of antimicrobial use, and individual susceptibility. There are no reports of prospective studies by hospitals tracking positive toxin A or A/B and the outcomes of CDAD and its complications.
The Centers for Disease Control and Prevention (CDC) has analyzed secular trends in the incidence of CDAD, and it reported a steady increase from 1987 to 2001 (1). In this report, 30% of 440 infectious disease physicians who participated in a Web-based poll reported that they are seeing higher rates of CDAD, more severe CDAD, and more relapsing CDAD than in the past. There is an overall impression that there has been an increase in the proportion of cases with severe and fatal complications, and an increase in the relapse rate among affected patients.
In addition to morbidity and mortality, the economic burden of C. difficile infection in terms of delayed discharge and other hospital costs is considerable.
Epidemiology
The frequency and incidence of CDAD varies between hospitals and within a given institution over time. The risk for disease increases in patients with antibiotic exposure, gastrointestinal surgery, increasing length of stay in healthcare settings, serious underlying illness, immuno-compromising conditions, and advanced age.
C. difficile is shed in feces. Any surface, device, or material (e.g., commode, bathing tub, and electronic rectal thermometer) that becomes contaminated with feces may serve as a reservoir for C. difficile spores. Spores are transferred to patients mainly via the hands of healthcare personnel who have touched a contaminated surface or item (2-6).
The Organism and Pathophysiology of C. difficile Diarrhea
C. difficile is a gram-positive, anaerobic, spore-forming bacillus that is responsible for the development of antibiotic-associated diarrhea and colitis. C. difficile was first described in 1935 as a component of the fecal flora of healthy newborns and was initially not thought to be pathogenic (7). The bacillus was named difficile because it grows slowly and is difficult to culture. C. difficile is presently responsible for nearly all causes of pseudomembranous colitis and as many as 20% of cases of antibiotic-associated diarrhea without colitis. Although found in the stool of only 5% of the general population, as many as 21% of adults become colonized with this organism while hospitalized (2,6).
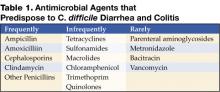
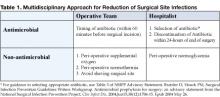
An alteration of the normal colonic microflora, usually caused by antibiotic therapy, is the main factor that predisposes to infection with C. difficile. Almost all antibiotics have been associated with C. difficile diarrhea and colitis. The antibiotics most frequently associated include clindamycin, cephalosporins, ampicillin, and amoxicillin (Table 1) (8).
In addition to antibiotic therapy, older age and severity of underlying disease are important risk factors for C. difficile infection. Other risk factors include the presence of a nasogastric tube, gastrointestinal procedures, acid antisecretory medications, intensive care unit stay, and duration of hospitalization (9).
C. difficile diarrhea is caused primarily by the elaboration of toxins A and B produced by bacterial multiplication within the intestinal lumen. These toxins bind to the colonic mucosa and exert their deleterious effects upon it. The organism rarely damages the colon by direct invasion, and diarrhea is caused by the effects of toxins produced within the intestinal lumen that adhere to the mucosal surface. Most toxigenic isolates produce both toxins, and about 5–25% of isolates produce neither toxin A nor B, and do not cause colitis or diarrhea (3-5).
Clinical Manifestations
Infection with C. difficile may produce a wide range of clinical manifestations, including asymptomatic carriage, mild-to-moderate diarrhea, and fulminant disease with pseudomembranous colitis (10). In patients who develop CDAD, symptoms usually begin soon after colonization. Colonization may occur during antibiotic treatment or up to several weeks after a course of antibiotics. CDAD typically is associated with the passage of frequent, loose bowel movements consistent with proctocolitis. Mucus or occult blood may be present, but visible blood is rare.
Diagnosis
The diagnosis of CDAD is based on a history of recent or current antibiotic therapy, development of diarrhea or other evidence of acute colitis, and demonstration of infection by toxigenic C. difficile, usually by detection of toxin A or toxin B in stool sample.
Practical Guidelines for Diagnosis of C. difficile Diarrheal Syndromes
- The diagnosis should be suspected in anyone with diarrhea who has received antibiotics within the previous 2 months and/or whose diarrhea begins 72 hours or more after hospitalization.
- When the diagnosis is suspected, a single stool specimen should be sent to the laboratory for testing for the presence of C. difficile and/or its toxins.
- When diarrhea persists despite a negative stool toxin result, one or two additional samples may be sent for testing with the same or different tests (4). Endoscopy is reserved for special situations, such as when a rapid diagnosis is needed and test results are delayed or the test is not highly sensitive, when the patient has ileus and stool is not available, or when other colonic diseases are also a consideration.
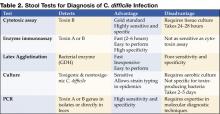
There is as yet no simple, inexpensive, rapid, sensitive and specific test for diagnosing C. difficile diarrhea and colitis, nor are all the available tests suitable for adoption by every laboratory (Table 2) (11).
Endoscopic Diagnosis of C. difficile Diarrhea and Colitis
Sigmoidoscopy and colonoscopy are not indicated for most patients with CDAD (10,12). Endoscopy is helpful, however, in special situations, such as when the diagnosis is in doubt or the clinical situation demands rapid diagnosis. The results of endoscopic examination may be normal in patients with mild diarrhea or may show nonspecific colitis in moderate cases. The finding of colonic pseudomembranes in a patient with antibiotic-associated diarrhea is virtually pathognomonic for C. difficile colitis. A few patients without any diagnostic features in the rectosigmoid have pseudomembranes in the more proximal areas of the colon (13). Other endoscopic findings include erythema, edema, friability, and nonspecific colitis with small ulcerations or erosions.
Treatment
The first step in the management of C. difficile diarrhea and colitis is to discontinue the precipitating antibiotics if possible (10,12). Diarrhea resolves in approximately 15–25% of patients without specific anti–C. difficile therapy (14,15). Conservative management alone may not be indicated, however, in patients who are systemically ill or who have multiple medical problems, since it is difficult to predict which patients will improve spontaneously. If it is not possible to discontinue the precipitating antibiotic because of other active infections, the patient’s antibiotic regimen should be altered if possible to make use of agents less likely to cause CDAD (e.g., aminoglycosides, trimethoprim, rifampin, or a quinolone).
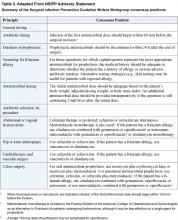
Antiperistaltic agents, such as diphenoxylate plus atropine (Lomotil), or loperamide (Imodium), and narcotic analgesics should be avoided because they may delay clearance of toxins from the colon and thereby exacerbate toxin-induced colonic injury or precipitate ileus and toxic dilatation (12,16). Specific therapy to eradicate C. difficile should be used in patients with initially severe symptoms and in patients whose symptoms persist despite discontinuation of antibiotic treatment. Although the diagnosis of C. difficile colitis should ideally be established before antimicrobial therapy is implemented, current ACG guidelines recommend that empiric therapy should be initiated in highly suggestive cases of severely ill patients (Table 3 on page 54) (12).
Currently, oral vancomycin or metronidazole, used for 7 to 10 days, are considered first-line therapy by most authors and current guidelines. Metronidazole at a dose of 250 mg 4 times daily is recommended by most authors and ACG guidelines as the drug of choice for the initial treatment of C. difficile colitis (12). These recommendations are largely based on efficacy, lower costs, and concerns about the development of vancomycin-resistant strains. Major disadvantages of metronidazole include a less desirable drug profile and contraindications in children and pregnant women.
Vancomycin, on the other hand, at a dose of 125 mg 4 times daily, is safe and well tolerated and achieves stool levels 20 times the required minimal inhibitory concentration for the treatment of C. difficile. Drawbacks to the use of vancomycin are cost and potential development of vancomycin-resistant strains. The current ACG guidelines consider vancomycin the drug of choice in severely ill patients and in cases in which the use of metronidazole is precluded.
Controlled clinical trials are lacking for patients with fulminant colitis who may not tolerate oral therapy. Administration of metronidazole intravenously or administration of vancomycin by nasogastric tube or rectal enema has been described in small case series (17-20). Intravenous administration of vancomycin is not recommended, because the drug is not excreted in the colon (17).
Management of Recurrent C. difficile Diarrhea
Despite successful initial treatment of CDAD, 15–20% of patients have recurrence of diarrhea in association with a positive stool test for C. difficile toxin. Symptomatic recurrence is rarely due to treatment failure or antimicrobial resistance to metronidazole or vancomycin. Approaches to management include conservative therapy (however, many patients are elderly and infirm and unable to tolerate diarrhea), therapy with specific anti–C. difficile antibiotics, the use of anion-binding resins, therapy with microorganisms (probiotics), and immunoglobulin therapy.
The most common therapy for recurrent C. difficile diarrhea is a second course of the same antibiotic used to treat the initial episode (12). In a large observational study in the United States, 92% of patients with recurrent CDAD responded successfully to a single repeated course of therapy, usually with metronidazole or vancomycin (14). There is evidence to suggest that patients with a history of recurrence have a high risk of further episodes of CDAD after antibiotic therapy is discontinued. There are no data to suggest that sequential episodes become progressively more severe or complicated (21). A variety of treatment schedules have been suggested for patients with multiple recurrences of C. difficile diarrhea. One approach is to give a prolonged course of vancomycin (or metronidazole) using a decreasing dosage schedule followed by pulse therapy (Table 4).
Cholestyramine, an anionexchange resin administered at a dose of 4 grams 3 or 4 times daily for 1 to 2 weeks, binds C. difficile toxins and may be used in conjunction with antibiotics to treat repeated relapses. Because cholestyramine may bind vancomycin as well as toxins, it should be taken at least 2 to 3 hours apart from the vancomycin.
Severe C. difficile Colitis
The incidence of fulminant C. difficile colitis has been reported to be 1.6–3.2% (22). Although recent precise figures from other centers are lacking, it is being recognized as an increasing cause of complications and death. The clinical syndrome of fulminant C. difficile colitis can be recognized with a proper knowledge of the spectrum of disease presentation.
A. Diarrhea: Although diarrhea is the hallmark of C. difficile colitis, it is not invariably present, and its absence may lead to diagnostic confusion. When diarrhea is absent, this appears to be secondary to severe colonic dysmotility. Even when present, diarrhea may be perceived to be a minor component of a nonspecific septic picture.
B. Severe Disease: Fulminant colitis is an unusual form of C. difficile infection, occurring in only 3% of patients but accounting for virtually all serious complications. Patients with more severe forms of the disease may present with or without diarrhea. When patients develop colitis localized to the cecum and right side of the colon, diarrhea may be minimal or absent. In the absence of diarrhea, the only clues to diagnosis may be systemic signs of toxicity (fever, tachycardia, leukocytosis, and/or volume depletion).
An elevated white blood cell count may be an important clue to impending fulminant C. difficile colitis. The rapid elevation of the peripheral white cell count (commonly as high as 30,000 to 50,000) with a significant excess of bands and sometimes more immature forms often precedes hemodynamic instability and the development of organ dysfunction. Even in patients who are mildly symptomatic for an extended period, sudden and unexpected progression to shock may occur. It is difficult to predict those patients who may not respond to medical treatment. Hence, early warning signs such as a leukemoid reaction may be invaluable.
Hypotension is a late finding and can be resistant to vasopressor support. Abdominal signs range from distention to generalized tenderness with guarding. Colonic perforation is usually accompanied by abdominal rigidity, involuntary guarding, rebound tenderness, and absent bowel sounds. Free air may be revealed on abdominal radiographs. Any suspicion of perforation in this setting should prompt immediate surgical consultation. Death generally occurs before free air and perforation can occur. In one study, contrary to most other literature, perforation was found to be rare (22).
Abdominal radiography may reveal a dilated colon (>7 cm in its greatest diameter), consistent with toxic megacolon. Patients with megacolon may have an associated small bowel ileus with dilated small intestine on plain abdominal radiographs, with air-fluid levels mimicking small intestinal obstruction or ischemia. CT without contrast and endoscopy can quickly diagnose or at least strongly suggest fulminant C.difficile colitis. CT scan findings include evidence of ascites, colonic wall thickening and/or dilatation. These findings may prove helpful in categorizing the severity of the colitis.
More aggressive intervention in medically unresponsive patients, including rapid identification of patients failing to respond to medical therapy, is crucial to a positive outcome, and early surgical intervention should be done in this group (Figures 1-3).
It is important that everyone involved with patient care in hospitals, nursing homes, and skilled nursing facilities be educated about the organism and its epidemiology, rational approaches to the treatment and care of patients with C. difficile diarrhea, the importance of hand washing between contact with patients, the use of gloves when caring for a patient with C. difficile diarrhea, and the avoidance of the unnecessary use of antimicrobials.
Conclusion
Recent years have raised concerns over rising incidence and serious complication rates of CDAD in North American hospitals (22,23). The Canadian Medical Association journal published a report in 2004 detailing an outbreak of CDAD involving several hospitals in Montreal. The introduction of new hypervirulent and highly transmissible strains of C. difficile has been postulated as the possible cause for the outbreak (24). A deteriorating infrastructure, inadequate infection control practices, the increasing number of debilitated patients, an aging population, and hypervirulent strains were all felt to be likely contributors to recent outbreaks in Canada (25).
Two epidemiological investigations in the United States and Canada (24,26) independently examined samples of C. difficile and found that a mutated version of the “wild” strain was responsible for outbreaks in Quebec and increased rates of CDAD in hospitals in the United States recently (22,23). Clinical epidemiologists at the CDC investigated C. difficile isolates from hospitals in the United States with recent (i.e., 2001–2004) CDAD outbreaks (22,23). The report indicates the emergence of a new epidemic strain, “BI” (distinct from the “J” strain of 1989–1992), which may be responsible for the recent increase in rates and apparent severity of CDAD (26).
CDAD and colitis in most cases can be treated by the administration of metronidazole or vancomycin. In some patients severe life-threatening toxicity develops despite appropriate and timely medical treatment, and surgical intervention is necessary. Systemic symptoms of infection with C. difficile are reported not to derive from bacteremia, colonic perforation or ischemia, but from toxin-induced inflammatory mediators released from the colon (27-29). Early surgical intervention should be employed in refractory cases of severe disease. Surgical intervention is far from ideal, however, and carries a very high rate of complications and significant risk of mortality (22). The future clinical approach to the treatment of nosocomial C. difficile colitis may eventually involve specific antitoxin hyperimmunoglobulins and inhibitors of the inflammatory cascade (28,30,31).
References
- Archibald LK, Banerjee SN, Jarvis WR. Secular trend in hospital-acquired Clostridium difficile disease in the United States; 1987-2001. J Infect Dis. 2004;189:1585-9.
- Fekety R. Antibiotic-associated colitis. In: Mandell G, Bennet JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 4th ed. New York: Churchill Livingston; 1996:978-806.
- Mitty RD, LaMont T. Clostridium difficile diarrhea: Pathogenesis, epidemiology, and treatment. Gastroenterologist. 1994;2:61-9.
- Bartlett JG. Clostridium difficile: History of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis. 1994;18(Suppl 4):265-72.
- Johnson S, Gerding D. Clostridium difficile. In: Mayhall CG, ed. Hospital Epidemiology and Infection Control. Baltimore, Md: Williams & Wilkins; 1996:99-408.
- Mcfarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile. N Engl J Med. 1989;320:204-10.
- Hall IC, O Toole E. Intestinal Flora in new-born infants: With a description of a new pathogenic anaerobe, Bacillus difficile. Am J Dis Child. 1935;49:390-402.
- Kelly CP, LaMont JT. Treatment of Clostridium difficile diarrhea and colitis. In: Wolfe MM, ed. Gastrointestinal Pharmacotherapy. Philadelphia, Pa.: WB Saunders; 1993:199-212.
- Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40:1-15.
- Kelly CP, Pothoulakas C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257-62.
- Linevsky JK, Kelly CP. Clostridium difficile colitis. In: Lamont JT, ed. Gastrointestinal Infections: Diagnosis and Management. New York: Marcel Dekker; 1997:293-325.
- Fekety R. Guidelines for the diagnosis and management of Clostridium difficile associated diarrhea and colitis. American College of Gastroenetrology, Practice Parameters Committee. Am J Gastroenterol. 1997;92:739-50.
- Tedesco FJ, Corless JK, Brownstein RE. Rectal sparing in antibiotic-associated pseudomembranous colitis: A prospective study. Gastroenterology. 1982;83:1259-60.
- Olson MM, Shanholtzer CJ, Lee JT Jr, Gerding DN. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982-1991. Infect Control Hosp Epidemiol. 1994;15: 371-81.
- Teasley DG, Gerding DN, Olson MM, et al. Prospective randomized trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-6.
- Walley T, Milson D. Loperamide-related toxic megacolon in Clostridium difficile colitis. Postgrad Med J. 1990;66:582.
- Malnick SD, Zimhony O. Treatment of Clostridium difficile associated diarrhea. Ann Pharmacother. 2002;36:1767-75.
- Sehgal M, Kyne L. Clostridium difficile disease. Curr Treatment Options Infect Dis. 2002;4:201-10.
- Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature. Clin Infect Dis. 2002;35:690-6.
- Friendenberg F, Fernandez A, Kaul V, Niami P, Levine GM. Intravenous metronidazole for the treatment of Clostridium difficile colitis. Dis Colon Rectum. 2001;44:1176-80.
- Fekety R, McFarland LV, Surawicz CMGreenberg, RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blind trial. Clin Infect Dis. 1997;24:324-33.
- Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363-72.
- Morris AM, Jobe BA, Sontey, M, Sheppard BC, Deveney CW, Deveney KE. Clostridium difficile colitis: an increasingly aggressive iatrogenic disease? Arch Surg. 2002;137:1096-100.
- Eggerston L, Sibbald B. Hospitals battling outbreaks of C. difficile. CMAJ. 2004;171:19-21.
- Valiquette L, Low DE, Pepin J, McGeer A. Clostridium difficile infection in hospitals: a brewing storm. CMAJ. 2004;171:27-9.
- McDonald LC, Killgore GE, Thompson A, et al. Emergence of an epidemic strain of Clostridium difficile in the United States, 2001-4: Potential role for virulence factors and antimicrobial resistance traits. Infectious Diseases Society of America 42th Annual Meeting. Boston, MA, September 30 – October 3, 2004. Abstract # LB-2.
- Flegel W, Muller F, Daubener W, Fischer HG, Hadding U, Northoff H. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect Immun. 1991;59:3659-66.
- Castagliuolo I, Keates A, Qiu B, et al. Increased substance P responses in dorsal root ganglia, intestinal macrophages during Clostridium difficile toxin A enteritis in rats. Proc Natl Acad Sci U S A. 1997;94:4788-93.
- Castagliuolo I, Keates A, Wang C, et al. Clostridium difficile toxin A stimulates macrophage-inflammatory protein-2 production in rat intestinal epithelial cells. J Immunol. 1998;160:6039-45.
- Kelly C, Chetham S, Keates S, et al. Survival of anti-Clostridium difficile bovine immunoglobulin concentrate in the human gastrointestinal tract. Antimicrob Agents Chemother. 1997;41:236-41.
- Salcedo J, Keates S, Pothoulakis C, et. al. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut. 1997;41:366-70.
General References
- Shea Position Paper Gerding DN,Johnson S, Peterson LR, Mulligan ME Silva J. SHEA Position Paper:Clostridium difficile-associated diarrhea and colitis; Infect Control Hosp Epidemiol 1995, 16:459-77
- Kyne L, Farrell RJ, Kelly CP. Clostridium difficile. Gastroenterol Clin North Am 2001; 30:753-77.
Introduction
Clostridium difficile–associated diarrhea (CDAD) has been recognized with increased frequency as a cause of nosocomial illness. The frequency and incidence of CDAD varies widely, and is influenced by multiple factors including nosocomial outbreaks, patterns of antimicrobial use, and individual susceptibility. There are no reports of prospective studies by hospitals tracking positive toxin A or A/B and the outcomes of CDAD and its complications.
The Centers for Disease Control and Prevention (CDC) has analyzed secular trends in the incidence of CDAD, and it reported a steady increase from 1987 to 2001 (1). In this report, 30% of 440 infectious disease physicians who participated in a Web-based poll reported that they are seeing higher rates of CDAD, more severe CDAD, and more relapsing CDAD than in the past. There is an overall impression that there has been an increase in the proportion of cases with severe and fatal complications, and an increase in the relapse rate among affected patients.
In addition to morbidity and mortality, the economic burden of C. difficile infection in terms of delayed discharge and other hospital costs is considerable.
Epidemiology
The frequency and incidence of CDAD varies between hospitals and within a given institution over time. The risk for disease increases in patients with antibiotic exposure, gastrointestinal surgery, increasing length of stay in healthcare settings, serious underlying illness, immuno-compromising conditions, and advanced age.
C. difficile is shed in feces. Any surface, device, or material (e.g., commode, bathing tub, and electronic rectal thermometer) that becomes contaminated with feces may serve as a reservoir for C. difficile spores. Spores are transferred to patients mainly via the hands of healthcare personnel who have touched a contaminated surface or item (2-6).
The Organism and Pathophysiology of C. difficile Diarrhea
C. difficile is a gram-positive, anaerobic, spore-forming bacillus that is responsible for the development of antibiotic-associated diarrhea and colitis. C. difficile was first described in 1935 as a component of the fecal flora of healthy newborns and was initially not thought to be pathogenic (7). The bacillus was named difficile because it grows slowly and is difficult to culture. C. difficile is presently responsible for nearly all causes of pseudomembranous colitis and as many as 20% of cases of antibiotic-associated diarrhea without colitis. Although found in the stool of only 5% of the general population, as many as 21% of adults become colonized with this organism while hospitalized (2,6).
An alteration of the normal colonic microflora, usually caused by antibiotic therapy, is the main factor that predisposes to infection with C. difficile. Almost all antibiotics have been associated with C. difficile diarrhea and colitis. The antibiotics most frequently associated include clindamycin, cephalosporins, ampicillin, and amoxicillin (Table 1) (8).
In addition to antibiotic therapy, older age and severity of underlying disease are important risk factors for C. difficile infection. Other risk factors include the presence of a nasogastric tube, gastrointestinal procedures, acid antisecretory medications, intensive care unit stay, and duration of hospitalization (9).
C. difficile diarrhea is caused primarily by the elaboration of toxins A and B produced by bacterial multiplication within the intestinal lumen. These toxins bind to the colonic mucosa and exert their deleterious effects upon it. The organism rarely damages the colon by direct invasion, and diarrhea is caused by the effects of toxins produced within the intestinal lumen that adhere to the mucosal surface. Most toxigenic isolates produce both toxins, and about 5–25% of isolates produce neither toxin A nor B, and do not cause colitis or diarrhea (3-5).
Clinical Manifestations
Infection with C. difficile may produce a wide range of clinical manifestations, including asymptomatic carriage, mild-to-moderate diarrhea, and fulminant disease with pseudomembranous colitis (10). In patients who develop CDAD, symptoms usually begin soon after colonization. Colonization may occur during antibiotic treatment or up to several weeks after a course of antibiotics. CDAD typically is associated with the passage of frequent, loose bowel movements consistent with proctocolitis. Mucus or occult blood may be present, but visible blood is rare.
Diagnosis
The diagnosis of CDAD is based on a history of recent or current antibiotic therapy, development of diarrhea or other evidence of acute colitis, and demonstration of infection by toxigenic C. difficile, usually by detection of toxin A or toxin B in stool sample.
Practical Guidelines for Diagnosis of C. difficile Diarrheal Syndromes
- The diagnosis should be suspected in anyone with diarrhea who has received antibiotics within the previous 2 months and/or whose diarrhea begins 72 hours or more after hospitalization.
- When the diagnosis is suspected, a single stool specimen should be sent to the laboratory for testing for the presence of C. difficile and/or its toxins.
- When diarrhea persists despite a negative stool toxin result, one or two additional samples may be sent for testing with the same or different tests (4). Endoscopy is reserved for special situations, such as when a rapid diagnosis is needed and test results are delayed or the test is not highly sensitive, when the patient has ileus and stool is not available, or when other colonic diseases are also a consideration.
There is as yet no simple, inexpensive, rapid, sensitive and specific test for diagnosing C. difficile diarrhea and colitis, nor are all the available tests suitable for adoption by every laboratory (Table 2) (11).
Endoscopic Diagnosis of C. difficile Diarrhea and Colitis
Sigmoidoscopy and colonoscopy are not indicated for most patients with CDAD (10,12). Endoscopy is helpful, however, in special situations, such as when the diagnosis is in doubt or the clinical situation demands rapid diagnosis. The results of endoscopic examination may be normal in patients with mild diarrhea or may show nonspecific colitis in moderate cases. The finding of colonic pseudomembranes in a patient with antibiotic-associated diarrhea is virtually pathognomonic for C. difficile colitis. A few patients without any diagnostic features in the rectosigmoid have pseudomembranes in the more proximal areas of the colon (13). Other endoscopic findings include erythema, edema, friability, and nonspecific colitis with small ulcerations or erosions.
Treatment
The first step in the management of C. difficile diarrhea and colitis is to discontinue the precipitating antibiotics if possible (10,12). Diarrhea resolves in approximately 15–25% of patients without specific anti–C. difficile therapy (14,15). Conservative management alone may not be indicated, however, in patients who are systemically ill or who have multiple medical problems, since it is difficult to predict which patients will improve spontaneously. If it is not possible to discontinue the precipitating antibiotic because of other active infections, the patient’s antibiotic regimen should be altered if possible to make use of agents less likely to cause CDAD (e.g., aminoglycosides, trimethoprim, rifampin, or a quinolone).
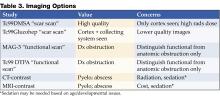
Antiperistaltic agents, such as diphenoxylate plus atropine (Lomotil), or loperamide (Imodium), and narcotic analgesics should be avoided because they may delay clearance of toxins from the colon and thereby exacerbate toxin-induced colonic injury or precipitate ileus and toxic dilatation (12,16). Specific therapy to eradicate C. difficile should be used in patients with initially severe symptoms and in patients whose symptoms persist despite discontinuation of antibiotic treatment. Although the diagnosis of C. difficile colitis should ideally be established before antimicrobial therapy is implemented, current ACG guidelines recommend that empiric therapy should be initiated in highly suggestive cases of severely ill patients (Table 3 on page 54) (12).
Currently, oral vancomycin or metronidazole, used for 7 to 10 days, are considered first-line therapy by most authors and current guidelines. Metronidazole at a dose of 250 mg 4 times daily is recommended by most authors and ACG guidelines as the drug of choice for the initial treatment of C. difficile colitis (12). These recommendations are largely based on efficacy, lower costs, and concerns about the development of vancomycin-resistant strains. Major disadvantages of metronidazole include a less desirable drug profile and contraindications in children and pregnant women.
Vancomycin, on the other hand, at a dose of 125 mg 4 times daily, is safe and well tolerated and achieves stool levels 20 times the required minimal inhibitory concentration for the treatment of C. difficile. Drawbacks to the use of vancomycin are cost and potential development of vancomycin-resistant strains. The current ACG guidelines consider vancomycin the drug of choice in severely ill patients and in cases in which the use of metronidazole is precluded.
Controlled clinical trials are lacking for patients with fulminant colitis who may not tolerate oral therapy. Administration of metronidazole intravenously or administration of vancomycin by nasogastric tube or rectal enema has been described in small case series (17-20). Intravenous administration of vancomycin is not recommended, because the drug is not excreted in the colon (17).
Management of Recurrent C. difficile Diarrhea
Despite successful initial treatment of CDAD, 15–20% of patients have recurrence of diarrhea in association with a positive stool test for C. difficile toxin. Symptomatic recurrence is rarely due to treatment failure or antimicrobial resistance to metronidazole or vancomycin. Approaches to management include conservative therapy (however, many patients are elderly and infirm and unable to tolerate diarrhea), therapy with specific anti–C. difficile antibiotics, the use of anion-binding resins, therapy with microorganisms (probiotics), and immunoglobulin therapy.
The most common therapy for recurrent C. difficile diarrhea is a second course of the same antibiotic used to treat the initial episode (12). In a large observational study in the United States, 92% of patients with recurrent CDAD responded successfully to a single repeated course of therapy, usually with metronidazole or vancomycin (14). There is evidence to suggest that patients with a history of recurrence have a high risk of further episodes of CDAD after antibiotic therapy is discontinued. There are no data to suggest that sequential episodes become progressively more severe or complicated (21). A variety of treatment schedules have been suggested for patients with multiple recurrences of C. difficile diarrhea. One approach is to give a prolonged course of vancomycin (or metronidazole) using a decreasing dosage schedule followed by pulse therapy (Table 4).
Cholestyramine, an anionexchange resin administered at a dose of 4 grams 3 or 4 times daily for 1 to 2 weeks, binds C. difficile toxins and may be used in conjunction with antibiotics to treat repeated relapses. Because cholestyramine may bind vancomycin as well as toxins, it should be taken at least 2 to 3 hours apart from the vancomycin.
Severe C. difficile Colitis
The incidence of fulminant C. difficile colitis has been reported to be 1.6–3.2% (22). Although recent precise figures from other centers are lacking, it is being recognized as an increasing cause of complications and death. The clinical syndrome of fulminant C. difficile colitis can be recognized with a proper knowledge of the spectrum of disease presentation.
A. Diarrhea: Although diarrhea is the hallmark of C. difficile colitis, it is not invariably present, and its absence may lead to diagnostic confusion. When diarrhea is absent, this appears to be secondary to severe colonic dysmotility. Even when present, diarrhea may be perceived to be a minor component of a nonspecific septic picture.
B. Severe Disease: Fulminant colitis is an unusual form of C. difficile infection, occurring in only 3% of patients but accounting for virtually all serious complications. Patients with more severe forms of the disease may present with or without diarrhea. When patients develop colitis localized to the cecum and right side of the colon, diarrhea may be minimal or absent. In the absence of diarrhea, the only clues to diagnosis may be systemic signs of toxicity (fever, tachycardia, leukocytosis, and/or volume depletion).
An elevated white blood cell count may be an important clue to impending fulminant C. difficile colitis. The rapid elevation of the peripheral white cell count (commonly as high as 30,000 to 50,000) with a significant excess of bands and sometimes more immature forms often precedes hemodynamic instability and the development of organ dysfunction. Even in patients who are mildly symptomatic for an extended period, sudden and unexpected progression to shock may occur. It is difficult to predict those patients who may not respond to medical treatment. Hence, early warning signs such as a leukemoid reaction may be invaluable.
Hypotension is a late finding and can be resistant to vasopressor support. Abdominal signs range from distention to generalized tenderness with guarding. Colonic perforation is usually accompanied by abdominal rigidity, involuntary guarding, rebound tenderness, and absent bowel sounds. Free air may be revealed on abdominal radiographs. Any suspicion of perforation in this setting should prompt immediate surgical consultation. Death generally occurs before free air and perforation can occur. In one study, contrary to most other literature, perforation was found to be rare (22).
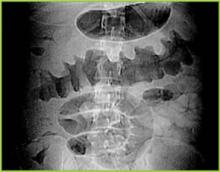
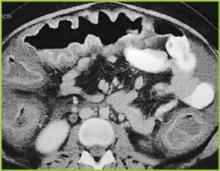
Abdominal radiography may reveal a dilated colon (>7 cm in its greatest diameter), consistent with toxic megacolon. Patients with megacolon may have an associated small bowel ileus with dilated small intestine on plain abdominal radiographs, with air-fluid levels mimicking small intestinal obstruction or ischemia. CT without contrast and endoscopy can quickly diagnose or at least strongly suggest fulminant C.difficile colitis. CT scan findings include evidence of ascites, colonic wall thickening and/or dilatation. These findings may prove helpful in categorizing the severity of the colitis.
More aggressive intervention in medically unresponsive patients, including rapid identification of patients failing to respond to medical therapy, is crucial to a positive outcome, and early surgical intervention should be done in this group (Figures 1-3).
It is important that everyone involved with patient care in hospitals, nursing homes, and skilled nursing facilities be educated about the organism and its epidemiology, rational approaches to the treatment and care of patients with C. difficile diarrhea, the importance of hand washing between contact with patients, the use of gloves when caring for a patient with C. difficile diarrhea, and the avoidance of the unnecessary use of antimicrobials.
Conclusion
Recent years have raised concerns over rising incidence and serious complication rates of CDAD in North American hospitals (22,23). The Canadian Medical Association journal published a report in 2004 detailing an outbreak of CDAD involving several hospitals in Montreal. The introduction of new hypervirulent and highly transmissible strains of C. difficile has been postulated as the possible cause for the outbreak (24). A deteriorating infrastructure, inadequate infection control practices, the increasing number of debilitated patients, an aging population, and hypervirulent strains were all felt to be likely contributors to recent outbreaks in Canada (25).
Two epidemiological investigations in the United States and Canada (24,26) independently examined samples of C. difficile and found that a mutated version of the “wild” strain was responsible for outbreaks in Quebec and increased rates of CDAD in hospitals in the United States recently (22,23). Clinical epidemiologists at the CDC investigated C. difficile isolates from hospitals in the United States with recent (i.e., 2001–2004) CDAD outbreaks (22,23). The report indicates the emergence of a new epidemic strain, “BI” (distinct from the “J” strain of 1989–1992), which may be responsible for the recent increase in rates and apparent severity of CDAD (26).
CDAD and colitis in most cases can be treated by the administration of metronidazole or vancomycin. In some patients severe life-threatening toxicity develops despite appropriate and timely medical treatment, and surgical intervention is necessary. Systemic symptoms of infection with C. difficile are reported not to derive from bacteremia, colonic perforation or ischemia, but from toxin-induced inflammatory mediators released from the colon (27-29). Early surgical intervention should be employed in refractory cases of severe disease. Surgical intervention is far from ideal, however, and carries a very high rate of complications and significant risk of mortality (22). The future clinical approach to the treatment of nosocomial C. difficile colitis may eventually involve specific antitoxin hyperimmunoglobulins and inhibitors of the inflammatory cascade (28,30,31).
References
- Archibald LK, Banerjee SN, Jarvis WR. Secular trend in hospital-acquired Clostridium difficile disease in the United States; 1987-2001. J Infect Dis. 2004;189:1585-9.
- Fekety R. Antibiotic-associated colitis. In: Mandell G, Bennet JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 4th ed. New York: Churchill Livingston; 1996:978-806.
- Mitty RD, LaMont T. Clostridium difficile diarrhea: Pathogenesis, epidemiology, and treatment. Gastroenterologist. 1994;2:61-9.
- Bartlett JG. Clostridium difficile: History of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis. 1994;18(Suppl 4):265-72.
- Johnson S, Gerding D. Clostridium difficile. In: Mayhall CG, ed. Hospital Epidemiology and Infection Control. Baltimore, Md: Williams & Wilkins; 1996:99-408.
- Mcfarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile. N Engl J Med. 1989;320:204-10.
- Hall IC, O Toole E. Intestinal Flora in new-born infants: With a description of a new pathogenic anaerobe, Bacillus difficile. Am J Dis Child. 1935;49:390-402.
- Kelly CP, LaMont JT. Treatment of Clostridium difficile diarrhea and colitis. In: Wolfe MM, ed. Gastrointestinal Pharmacotherapy. Philadelphia, Pa.: WB Saunders; 1993:199-212.
- Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40:1-15.
- Kelly CP, Pothoulakas C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257-62.
- Linevsky JK, Kelly CP. Clostridium difficile colitis. In: Lamont JT, ed. Gastrointestinal Infections: Diagnosis and Management. New York: Marcel Dekker; 1997:293-325.
- Fekety R. Guidelines for the diagnosis and management of Clostridium difficile associated diarrhea and colitis. American College of Gastroenetrology, Practice Parameters Committee. Am J Gastroenterol. 1997;92:739-50.
- Tedesco FJ, Corless JK, Brownstein RE. Rectal sparing in antibiotic-associated pseudomembranous colitis: A prospective study. Gastroenterology. 1982;83:1259-60.
- Olson MM, Shanholtzer CJ, Lee JT Jr, Gerding DN. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982-1991. Infect Control Hosp Epidemiol. 1994;15: 371-81.
- Teasley DG, Gerding DN, Olson MM, et al. Prospective randomized trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-6.
- Walley T, Milson D. Loperamide-related toxic megacolon in Clostridium difficile colitis. Postgrad Med J. 1990;66:582.
- Malnick SD, Zimhony O. Treatment of Clostridium difficile associated diarrhea. Ann Pharmacother. 2002;36:1767-75.
- Sehgal M, Kyne L. Clostridium difficile disease. Curr Treatment Options Infect Dis. 2002;4:201-10.
- Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature. Clin Infect Dis. 2002;35:690-6.
- Friendenberg F, Fernandez A, Kaul V, Niami P, Levine GM. Intravenous metronidazole for the treatment of Clostridium difficile colitis. Dis Colon Rectum. 2001;44:1176-80.
- Fekety R, McFarland LV, Surawicz CMGreenberg, RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blind trial. Clin Infect Dis. 1997;24:324-33.
- Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363-72.
- Morris AM, Jobe BA, Sontey, M, Sheppard BC, Deveney CW, Deveney KE. Clostridium difficile colitis: an increasingly aggressive iatrogenic disease? Arch Surg. 2002;137:1096-100.
- Eggerston L, Sibbald B. Hospitals battling outbreaks of C. difficile. CMAJ. 2004;171:19-21.
- Valiquette L, Low DE, Pepin J, McGeer A. Clostridium difficile infection in hospitals: a brewing storm. CMAJ. 2004;171:27-9.
- McDonald LC, Killgore GE, Thompson A, et al. Emergence of an epidemic strain of Clostridium difficile in the United States, 2001-4: Potential role for virulence factors and antimicrobial resistance traits. Infectious Diseases Society of America 42th Annual Meeting. Boston, MA, September 30 – October 3, 2004. Abstract # LB-2.
- Flegel W, Muller F, Daubener W, Fischer HG, Hadding U, Northoff H. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect Immun. 1991;59:3659-66.
- Castagliuolo I, Keates A, Qiu B, et al. Increased substance P responses in dorsal root ganglia, intestinal macrophages during Clostridium difficile toxin A enteritis in rats. Proc Natl Acad Sci U S A. 1997;94:4788-93.
- Castagliuolo I, Keates A, Wang C, et al. Clostridium difficile toxin A stimulates macrophage-inflammatory protein-2 production in rat intestinal epithelial cells. J Immunol. 1998;160:6039-45.
- Kelly C, Chetham S, Keates S, et al. Survival of anti-Clostridium difficile bovine immunoglobulin concentrate in the human gastrointestinal tract. Antimicrob Agents Chemother. 1997;41:236-41.
- Salcedo J, Keates S, Pothoulakis C, et. al. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut. 1997;41:366-70.
General References
- Shea Position Paper Gerding DN,Johnson S, Peterson LR, Mulligan ME Silva J. SHEA Position Paper:Clostridium difficile-associated diarrhea and colitis; Infect Control Hosp Epidemiol 1995, 16:459-77
- Kyne L, Farrell RJ, Kelly CP. Clostridium difficile. Gastroenterol Clin North Am 2001; 30:753-77.
Introduction
Clostridium difficile–associated diarrhea (CDAD) has been recognized with increased frequency as a cause of nosocomial illness. The frequency and incidence of CDAD varies widely, and is influenced by multiple factors including nosocomial outbreaks, patterns of antimicrobial use, and individual susceptibility. There are no reports of prospective studies by hospitals tracking positive toxin A or A/B and the outcomes of CDAD and its complications.
The Centers for Disease Control and Prevention (CDC) has analyzed secular trends in the incidence of CDAD, and it reported a steady increase from 1987 to 2001 (1). In this report, 30% of 440 infectious disease physicians who participated in a Web-based poll reported that they are seeing higher rates of CDAD, more severe CDAD, and more relapsing CDAD than in the past. There is an overall impression that there has been an increase in the proportion of cases with severe and fatal complications, and an increase in the relapse rate among affected patients.
In addition to morbidity and mortality, the economic burden of C. difficile infection in terms of delayed discharge and other hospital costs is considerable.
Epidemiology
The frequency and incidence of CDAD varies between hospitals and within a given institution over time. The risk for disease increases in patients with antibiotic exposure, gastrointestinal surgery, increasing length of stay in healthcare settings, serious underlying illness, immuno-compromising conditions, and advanced age.
C. difficile is shed in feces. Any surface, device, or material (e.g., commode, bathing tub, and electronic rectal thermometer) that becomes contaminated with feces may serve as a reservoir for C. difficile spores. Spores are transferred to patients mainly via the hands of healthcare personnel who have touched a contaminated surface or item (2-6).
The Organism and Pathophysiology of C. difficile Diarrhea
C. difficile is a gram-positive, anaerobic, spore-forming bacillus that is responsible for the development of antibiotic-associated diarrhea and colitis. C. difficile was first described in 1935 as a component of the fecal flora of healthy newborns and was initially not thought to be pathogenic (7). The bacillus was named difficile because it grows slowly and is difficult to culture. C. difficile is presently responsible for nearly all causes of pseudomembranous colitis and as many as 20% of cases of antibiotic-associated diarrhea without colitis. Although found in the stool of only 5% of the general population, as many as 21% of adults become colonized with this organism while hospitalized (2,6).
An alteration of the normal colonic microflora, usually caused by antibiotic therapy, is the main factor that predisposes to infection with C. difficile. Almost all antibiotics have been associated with C. difficile diarrhea and colitis. The antibiotics most frequently associated include clindamycin, cephalosporins, ampicillin, and amoxicillin (Table 1) (8).
In addition to antibiotic therapy, older age and severity of underlying disease are important risk factors for C. difficile infection. Other risk factors include the presence of a nasogastric tube, gastrointestinal procedures, acid antisecretory medications, intensive care unit stay, and duration of hospitalization (9).
C. difficile diarrhea is caused primarily by the elaboration of toxins A and B produced by bacterial multiplication within the intestinal lumen. These toxins bind to the colonic mucosa and exert their deleterious effects upon it. The organism rarely damages the colon by direct invasion, and diarrhea is caused by the effects of toxins produced within the intestinal lumen that adhere to the mucosal surface. Most toxigenic isolates produce both toxins, and about 5–25% of isolates produce neither toxin A nor B, and do not cause colitis or diarrhea (3-5).
Clinical Manifestations
Infection with C. difficile may produce a wide range of clinical manifestations, including asymptomatic carriage, mild-to-moderate diarrhea, and fulminant disease with pseudomembranous colitis (10). In patients who develop CDAD, symptoms usually begin soon after colonization. Colonization may occur during antibiotic treatment or up to several weeks after a course of antibiotics. CDAD typically is associated with the passage of frequent, loose bowel movements consistent with proctocolitis. Mucus or occult blood may be present, but visible blood is rare.
Diagnosis
The diagnosis of CDAD is based on a history of recent or current antibiotic therapy, development of diarrhea or other evidence of acute colitis, and demonstration of infection by toxigenic C. difficile, usually by detection of toxin A or toxin B in stool sample.
Practical Guidelines for Diagnosis of C. difficile Diarrheal Syndromes
- The diagnosis should be suspected in anyone with diarrhea who has received antibiotics within the previous 2 months and/or whose diarrhea begins 72 hours or more after hospitalization.
- When the diagnosis is suspected, a single stool specimen should be sent to the laboratory for testing for the presence of C. difficile and/or its toxins.
- When diarrhea persists despite a negative stool toxin result, one or two additional samples may be sent for testing with the same or different tests (4). Endoscopy is reserved for special situations, such as when a rapid diagnosis is needed and test results are delayed or the test is not highly sensitive, when the patient has ileus and stool is not available, or when other colonic diseases are also a consideration.
There is as yet no simple, inexpensive, rapid, sensitive and specific test for diagnosing C. difficile diarrhea and colitis, nor are all the available tests suitable for adoption by every laboratory (Table 2) (11).
Endoscopic Diagnosis of C. difficile Diarrhea and Colitis
Sigmoidoscopy and colonoscopy are not indicated for most patients with CDAD (10,12). Endoscopy is helpful, however, in special situations, such as when the diagnosis is in doubt or the clinical situation demands rapid diagnosis. The results of endoscopic examination may be normal in patients with mild diarrhea or may show nonspecific colitis in moderate cases. The finding of colonic pseudomembranes in a patient with antibiotic-associated diarrhea is virtually pathognomonic for C. difficile colitis. A few patients without any diagnostic features in the rectosigmoid have pseudomembranes in the more proximal areas of the colon (13). Other endoscopic findings include erythema, edema, friability, and nonspecific colitis with small ulcerations or erosions.
Treatment
The first step in the management of C. difficile diarrhea and colitis is to discontinue the precipitating antibiotics if possible (10,12). Diarrhea resolves in approximately 15–25% of patients without specific anti–C. difficile therapy (14,15). Conservative management alone may not be indicated, however, in patients who are systemically ill or who have multiple medical problems, since it is difficult to predict which patients will improve spontaneously. If it is not possible to discontinue the precipitating antibiotic because of other active infections, the patient’s antibiotic regimen should be altered if possible to make use of agents less likely to cause CDAD (e.g., aminoglycosides, trimethoprim, rifampin, or a quinolone).
Antiperistaltic agents, such as diphenoxylate plus atropine (Lomotil), or loperamide (Imodium), and narcotic analgesics should be avoided because they may delay clearance of toxins from the colon and thereby exacerbate toxin-induced colonic injury or precipitate ileus and toxic dilatation (12,16). Specific therapy to eradicate C. difficile should be used in patients with initially severe symptoms and in patients whose symptoms persist despite discontinuation of antibiotic treatment. Although the diagnosis of C. difficile colitis should ideally be established before antimicrobial therapy is implemented, current ACG guidelines recommend that empiric therapy should be initiated in highly suggestive cases of severely ill patients (Table 3 on page 54) (12).
Currently, oral vancomycin or metronidazole, used for 7 to 10 days, are considered first-line therapy by most authors and current guidelines. Metronidazole at a dose of 250 mg 4 times daily is recommended by most authors and ACG guidelines as the drug of choice for the initial treatment of C. difficile colitis (12). These recommendations are largely based on efficacy, lower costs, and concerns about the development of vancomycin-resistant strains. Major disadvantages of metronidazole include a less desirable drug profile and contraindications in children and pregnant women.
Vancomycin, on the other hand, at a dose of 125 mg 4 times daily, is safe and well tolerated and achieves stool levels 20 times the required minimal inhibitory concentration for the treatment of C. difficile. Drawbacks to the use of vancomycin are cost and potential development of vancomycin-resistant strains. The current ACG guidelines consider vancomycin the drug of choice in severely ill patients and in cases in which the use of metronidazole is precluded.
Controlled clinical trials are lacking for patients with fulminant colitis who may not tolerate oral therapy. Administration of metronidazole intravenously or administration of vancomycin by nasogastric tube or rectal enema has been described in small case series (17-20). Intravenous administration of vancomycin is not recommended, because the drug is not excreted in the colon (17).
Management of Recurrent C. difficile Diarrhea
Despite successful initial treatment of CDAD, 15–20% of patients have recurrence of diarrhea in association with a positive stool test for C. difficile toxin. Symptomatic recurrence is rarely due to treatment failure or antimicrobial resistance to metronidazole or vancomycin. Approaches to management include conservative therapy (however, many patients are elderly and infirm and unable to tolerate diarrhea), therapy with specific anti–C. difficile antibiotics, the use of anion-binding resins, therapy with microorganisms (probiotics), and immunoglobulin therapy.
The most common therapy for recurrent C. difficile diarrhea is a second course of the same antibiotic used to treat the initial episode (12). In a large observational study in the United States, 92% of patients with recurrent CDAD responded successfully to a single repeated course of therapy, usually with metronidazole or vancomycin (14). There is evidence to suggest that patients with a history of recurrence have a high risk of further episodes of CDAD after antibiotic therapy is discontinued. There are no data to suggest that sequential episodes become progressively more severe or complicated (21). A variety of treatment schedules have been suggested for patients with multiple recurrences of C. difficile diarrhea. One approach is to give a prolonged course of vancomycin (or metronidazole) using a decreasing dosage schedule followed by pulse therapy (Table 4).
Cholestyramine, an anionexchange resin administered at a dose of 4 grams 3 or 4 times daily for 1 to 2 weeks, binds C. difficile toxins and may be used in conjunction with antibiotics to treat repeated relapses. Because cholestyramine may bind vancomycin as well as toxins, it should be taken at least 2 to 3 hours apart from the vancomycin.
Severe C. difficile Colitis
The incidence of fulminant C. difficile colitis has been reported to be 1.6–3.2% (22). Although recent precise figures from other centers are lacking, it is being recognized as an increasing cause of complications and death. The clinical syndrome of fulminant C. difficile colitis can be recognized with a proper knowledge of the spectrum of disease presentation.
A. Diarrhea: Although diarrhea is the hallmark of C. difficile colitis, it is not invariably present, and its absence may lead to diagnostic confusion. When diarrhea is absent, this appears to be secondary to severe colonic dysmotility. Even when present, diarrhea may be perceived to be a minor component of a nonspecific septic picture.
B. Severe Disease: Fulminant colitis is an unusual form of C. difficile infection, occurring in only 3% of patients but accounting for virtually all serious complications. Patients with more severe forms of the disease may present with or without diarrhea. When patients develop colitis localized to the cecum and right side of the colon, diarrhea may be minimal or absent. In the absence of diarrhea, the only clues to diagnosis may be systemic signs of toxicity (fever, tachycardia, leukocytosis, and/or volume depletion).
An elevated white blood cell count may be an important clue to impending fulminant C. difficile colitis. The rapid elevation of the peripheral white cell count (commonly as high as 30,000 to 50,000) with a significant excess of bands and sometimes more immature forms often precedes hemodynamic instability and the development of organ dysfunction. Even in patients who are mildly symptomatic for an extended period, sudden and unexpected progression to shock may occur. It is difficult to predict those patients who may not respond to medical treatment. Hence, early warning signs such as a leukemoid reaction may be invaluable.
Hypotension is a late finding and can be resistant to vasopressor support. Abdominal signs range from distention to generalized tenderness with guarding. Colonic perforation is usually accompanied by abdominal rigidity, involuntary guarding, rebound tenderness, and absent bowel sounds. Free air may be revealed on abdominal radiographs. Any suspicion of perforation in this setting should prompt immediate surgical consultation. Death generally occurs before free air and perforation can occur. In one study, contrary to most other literature, perforation was found to be rare (22).
Abdominal radiography may reveal a dilated colon (>7 cm in its greatest diameter), consistent with toxic megacolon. Patients with megacolon may have an associated small bowel ileus with dilated small intestine on plain abdominal radiographs, with air-fluid levels mimicking small intestinal obstruction or ischemia. CT without contrast and endoscopy can quickly diagnose or at least strongly suggest fulminant C.difficile colitis. CT scan findings include evidence of ascites, colonic wall thickening and/or dilatation. These findings may prove helpful in categorizing the severity of the colitis.
More aggressive intervention in medically unresponsive patients, including rapid identification of patients failing to respond to medical therapy, is crucial to a positive outcome, and early surgical intervention should be done in this group (Figures 1-3).
It is important that everyone involved with patient care in hospitals, nursing homes, and skilled nursing facilities be educated about the organism and its epidemiology, rational approaches to the treatment and care of patients with C. difficile diarrhea, the importance of hand washing between contact with patients, the use of gloves when caring for a patient with C. difficile diarrhea, and the avoidance of the unnecessary use of antimicrobials.
Conclusion
Recent years have raised concerns over rising incidence and serious complication rates of CDAD in North American hospitals (22,23). The Canadian Medical Association journal published a report in 2004 detailing an outbreak of CDAD involving several hospitals in Montreal. The introduction of new hypervirulent and highly transmissible strains of C. difficile has been postulated as the possible cause for the outbreak (24). A deteriorating infrastructure, inadequate infection control practices, the increasing number of debilitated patients, an aging population, and hypervirulent strains were all felt to be likely contributors to recent outbreaks in Canada (25).
Two epidemiological investigations in the United States and Canada (24,26) independently examined samples of C. difficile and found that a mutated version of the “wild” strain was responsible for outbreaks in Quebec and increased rates of CDAD in hospitals in the United States recently (22,23). Clinical epidemiologists at the CDC investigated C. difficile isolates from hospitals in the United States with recent (i.e., 2001–2004) CDAD outbreaks (22,23). The report indicates the emergence of a new epidemic strain, “BI” (distinct from the “J” strain of 1989–1992), which may be responsible for the recent increase in rates and apparent severity of CDAD (26).
CDAD and colitis in most cases can be treated by the administration of metronidazole or vancomycin. In some patients severe life-threatening toxicity develops despite appropriate and timely medical treatment, and surgical intervention is necessary. Systemic symptoms of infection with C. difficile are reported not to derive from bacteremia, colonic perforation or ischemia, but from toxin-induced inflammatory mediators released from the colon (27-29). Early surgical intervention should be employed in refractory cases of severe disease. Surgical intervention is far from ideal, however, and carries a very high rate of complications and significant risk of mortality (22). The future clinical approach to the treatment of nosocomial C. difficile colitis may eventually involve specific antitoxin hyperimmunoglobulins and inhibitors of the inflammatory cascade (28,30,31).
References
- Archibald LK, Banerjee SN, Jarvis WR. Secular trend in hospital-acquired Clostridium difficile disease in the United States; 1987-2001. J Infect Dis. 2004;189:1585-9.
- Fekety R. Antibiotic-associated colitis. In: Mandell G, Bennet JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 4th ed. New York: Churchill Livingston; 1996:978-806.
- Mitty RD, LaMont T. Clostridium difficile diarrhea: Pathogenesis, epidemiology, and treatment. Gastroenterologist. 1994;2:61-9.
- Bartlett JG. Clostridium difficile: History of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis. 1994;18(Suppl 4):265-72.
- Johnson S, Gerding D. Clostridium difficile. In: Mayhall CG, ed. Hospital Epidemiology and Infection Control. Baltimore, Md: Williams & Wilkins; 1996:99-408.
- Mcfarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile. N Engl J Med. 1989;320:204-10.
- Hall IC, O Toole E. Intestinal Flora in new-born infants: With a description of a new pathogenic anaerobe, Bacillus difficile. Am J Dis Child. 1935;49:390-402.
- Kelly CP, LaMont JT. Treatment of Clostridium difficile diarrhea and colitis. In: Wolfe MM, ed. Gastrointestinal Pharmacotherapy. Philadelphia, Pa.: WB Saunders; 1993:199-212.
- Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40:1-15.
- Kelly CP, Pothoulakas C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257-62.
- Linevsky JK, Kelly CP. Clostridium difficile colitis. In: Lamont JT, ed. Gastrointestinal Infections: Diagnosis and Management. New York: Marcel Dekker; 1997:293-325.
- Fekety R. Guidelines for the diagnosis and management of Clostridium difficile associated diarrhea and colitis. American College of Gastroenetrology, Practice Parameters Committee. Am J Gastroenterol. 1997;92:739-50.
- Tedesco FJ, Corless JK, Brownstein RE. Rectal sparing in antibiotic-associated pseudomembranous colitis: A prospective study. Gastroenterology. 1982;83:1259-60.
- Olson MM, Shanholtzer CJ, Lee JT Jr, Gerding DN. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982-1991. Infect Control Hosp Epidemiol. 1994;15: 371-81.
- Teasley DG, Gerding DN, Olson MM, et al. Prospective randomized trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043-6.
- Walley T, Milson D. Loperamide-related toxic megacolon in Clostridium difficile colitis. Postgrad Med J. 1990;66:582.
- Malnick SD, Zimhony O. Treatment of Clostridium difficile associated diarrhea. Ann Pharmacother. 2002;36:1767-75.
- Sehgal M, Kyne L. Clostridium difficile disease. Curr Treatment Options Infect Dis. 2002;4:201-10.
- Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature. Clin Infect Dis. 2002;35:690-6.
- Friendenberg F, Fernandez A, Kaul V, Niami P, Levine GM. Intravenous metronidazole for the treatment of Clostridium difficile colitis. Dis Colon Rectum. 2001;44:1176-80.
- Fekety R, McFarland LV, Surawicz CMGreenberg, RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blind trial. Clin Infect Dis. 1997;24:324-33.
- Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363-72.
- Morris AM, Jobe BA, Sontey, M, Sheppard BC, Deveney CW, Deveney KE. Clostridium difficile colitis: an increasingly aggressive iatrogenic disease? Arch Surg. 2002;137:1096-100.
- Eggerston L, Sibbald B. Hospitals battling outbreaks of C. difficile. CMAJ. 2004;171:19-21.
- Valiquette L, Low DE, Pepin J, McGeer A. Clostridium difficile infection in hospitals: a brewing storm. CMAJ. 2004;171:27-9.
- McDonald LC, Killgore GE, Thompson A, et al. Emergence of an epidemic strain of Clostridium difficile in the United States, 2001-4: Potential role for virulence factors and antimicrobial resistance traits. Infectious Diseases Society of America 42th Annual Meeting. Boston, MA, September 30 – October 3, 2004. Abstract # LB-2.
- Flegel W, Muller F, Daubener W, Fischer HG, Hadding U, Northoff H. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect Immun. 1991;59:3659-66.
- Castagliuolo I, Keates A, Qiu B, et al. Increased substance P responses in dorsal root ganglia, intestinal macrophages during Clostridium difficile toxin A enteritis in rats. Proc Natl Acad Sci U S A. 1997;94:4788-93.
- Castagliuolo I, Keates A, Wang C, et al. Clostridium difficile toxin A stimulates macrophage-inflammatory protein-2 production in rat intestinal epithelial cells. J Immunol. 1998;160:6039-45.
- Kelly C, Chetham S, Keates S, et al. Survival of anti-Clostridium difficile bovine immunoglobulin concentrate in the human gastrointestinal tract. Antimicrob Agents Chemother. 1997;41:236-41.
- Salcedo J, Keates S, Pothoulakis C, et. al. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut. 1997;41:366-70.
General References
- Shea Position Paper Gerding DN,Johnson S, Peterson LR, Mulligan ME Silva J. SHEA Position Paper:Clostridium difficile-associated diarrhea and colitis; Infect Control Hosp Epidemiol 1995, 16:459-77
- Kyne L, Farrell RJ, Kelly CP. Clostridium difficile. Gastroenterol Clin North Am 2001; 30:753-77.
Inpatient Management of Urinary Tract Infections in Infants and Young Children
Introduction
Urinary tract infections (UTIs) are serious bacterial infections and a common cause for hospital admission of infants and young children. The prevalence of UTI in infants younger than 1 year of age ranges from 3.3% to 6.5%, and between 1 and 2 years of age from 1.9% to 8.1%. Females outpace males across all age groups, with the exception of the first 3 months of life (1). Without appropriate treatment and management, UTI can result in dehydration, urosepsis, and long-term medical problems including hypertension, renal scarring, and decreased renal function. This review will focus on the inpatient management of first-episode UTI in infants and young children.
Diagnosis
Presenting symptoms in older children include urgency, frequency, dysuria, and complaints of back pain. In contrast, symptoms in infants and young children are often nonspecific and include irritability, diarrhea, vomiting, poor feeding, poor weight gain, crying on urination, and foul-smelling urine. The presence of a fever in infants and young children with UTI has been accepted as a marker of pyelonephritis, which occurs when infection has ascended to the upper collecting system of the kidney. Urinalysis (UA) and culture should be collected by suprapubic aspiration or transurethral catheterization, or by appropriately performed clean catch method for children of appropriate age and developmental ability. The use of a bag-collected urine specimen is insufficient and unreliable and should not be used in making the diagnosis of UTI. While suprapubic aspiration is considered the gold standard with a specificity and sensitivity of 100%, there is often resistance from parents and from physicians who are not properly trained to do this procedure.
The most accepted method of obtaining urine is sterile transurethral catheterization, results of which have 95% sensitivity and 99% specificity (2). When interpreting the UA, the most useful components for the diagnosis of a UTI include a positive leukocyte esterase, nitrite test, or gram stain on unspun urine, and microscopy revealing >10 white blood cells per high-powered field of spun urine. However, neonates under 30 days old may have no abnormality noted on initial UA (3,4). The presence of any bacteria on gram-stained urine offers the best sensitivity and specificity (5). Final diagnosis depends upon isolation of >105 of a single organism from a clean-catch specimen, or >104 of a single organism from a catheterized specimen.
Admission Criteria
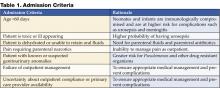
Guidelines for evaluation of serious bacterial infection and parenteral antibiotic use for febrile infants under 60 days of age should be followed. All febrile neonates less than 30 days of age should be admitted for parenteral antibiotics (6–11). Controversy exists on the need to use corrected or postconceptual age when evaluating and determining need for admission for febrile preterm infants, particularly for those under 35 weeks of gestation. Factors that can be considered by the practitioner include severity of Neonatal ICU course, severity of prematurity, and combined disease burden of UTI with common preterm comorbidities (anemia, apnea of prematurity, chronic lung disease).
Consider admitting and initiating parenteral antibiotic treatment using Table 1 as a guideline. Exercise a lower threshold for admitting infants and toxic-appearing young children due to concern for urosepsis, complications, and the need for appropriate and aggressive initial therapy.
Initial Inpatient Management
The 3 goals of inhospital treatment of UTIs are to effectively treat and eliminate the acute infection, prevent urosepsis in infants and immunocompromised children, and prevent and reduce long-term complications such as renal scarring, hypertension, and decreased renal function. Initial antibiotic treatment should be administered parenterally to ensure optimal antimicrobial levels and aimed at the most common organisms, including Escherichia coli, Klebsiella, Proteus, and Enterobacter spp. Less common organisms to consider include Pseudomonas, Enterococcus, Staphylococcus aureus, and group B Streptococcus. Organisms will differ on many factors, such as age, underlying disease, prior colonization, and antibiotic exposure.
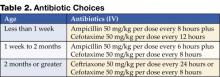
Table 2 outlines the initial choices of antibiotics until culture and sensitivities are known.
The choices and dosage of antibiotics are dependent on the age of the patient and are selected based on the other most likely organisms and their expected sensitivities (12). Ampicillin is added to the less than 2-month age group not only to cover Enterococcus, but also as part of broader neonatal sepsis coverage for Listeria. The third-generation cephalosporins are felt to be adequate initial coverage for most of the common organisms causing UTI. Children with congenital anomalies known to be associated with genitourinary abnormalities may be infected with less common organisms. In these situations, consider tailoring initial antibiotic coverage.
Complications
The major complication of UTIs in infants is bacteremia. The rate of bacteremia in infants 0–3 months has variably been reported as 10% (13), 21–31% (14), and 36% (15). Infants with and without bacteremia are often clinically indistinguishable, making early determination of bacteremia difficult. A recent comprehensive review of 17 studies by Malik noted both C-reactive protein (CRP) and procalcitonin (PCT) results were highly variable in infants under 90 days old with known positive bacterial cultures (16). These inflammatory markers are therefore currently not useful to predict bacteremia. In addition to blood stream infection, other acute complications include meningitis, renal and perinephric abscess, and infected calculi. Longer-term complications include reflux nephropathy, renal scarring, hypertension, decreased renal function, and renal failure.
Duration of Antibiotic Therapy
While most uncomplicated UTIs are successfully treated with a 10-day treatment course, many experts prefer a 14-day course for neonates, infants, and ill-appearing young children. Despite effectiveness in adults, very-short-course therapy (≤3 days) for pediatric patients is associated with more treatment failures and reinfections (17). Although it may be considered in older children with cystitis, at this time it is not appropriate for treating infants and younger children in whom pyelonephritis cannot be distinguished from isolated lower tract infection (17,18).
Total treatment time and total days of parenteral therapy needed continue to be debated. Hoberman randomized children as young as 1 month of age to either entirely oral treatment or parenteral therapy for 3 days followed by oral treatment (19). In both arms of this study, children received 14 days of total therapy as was the standard at the time. He suggested, however, that a 10-day course of antibiotics should be adequate therapy for noncomplicated acute pyelonephritis. Of the 306 children, only 13 were under 2 months of age. Although only 13 positive blood cultures were reported, 10 of these occurred in children under 6 months of age. Given the limited number of children less than 2 months of age and the prevalence of positive blood cultures noted, conclusions cannot be drawn on the safety of entirely oral treatment for young infants. Parenteral antibiotic therapy should be continued for all hospitalized children until the patient is afebrile and free from signs of toxicity. Most hospitalized pediatric patients defervesce quickly on parenteral therapy—89% within 48 hours and 97% within 72 hours (20). Longer parenteral therapy of at least 10 days should be considered for neonates and infants with urosepsis, because they are immunologically immature, at greater risk of complications, have higher incidence of urinary tract anomalies, and have less reliable absorption of oral antibiotics.
Delayed or lack of response to antibiotic therapy may indicate the presence of urinary tract obstruction, resistant organisms, or renal or perinephric abscess. A repeat urine culture and immediate renal ultrasound or CT should be performed if the patient is not improving within 48 hours.
Radiological Studies
Renal Ultrasound (RUS)
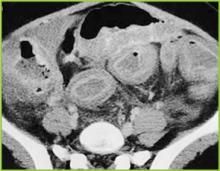
Recent studies have questioned the value of performing routine RUS after a first-time UTI because of the low sensitivity in detecting vesicoureteral reflux (VUR) and a lack of significant influence in altering management (21,22). Patients who have had a normal late (30-32 weeks’ gestation) prenatal ultrasound with a good view of the kidneys may not require a repeat postnatal renal ultrasound (21,22). Further studies are needed to evaluate the costs and value of routine RUS. Until these studies are completed, renal and bladder ultrasound early during hospitalization continues to be recommended for all patients admitted with a first-time UTI to identify hydronephrosis, duplicating collecting systems, ureteral dilatation, calculi, and other structural anomalies.
Voiding Cystourethrogram (VCUG) or Radionuclide Cystography (RNC)
Either a VCUG or RNC should be performed to detect vesico-ureteral reflux in infants and young children. The AAP practice parameter and more recent literature clearly state the need for this evaluation in children under the age of 2 years (2,21). Additional data on incidence of anomalies by age suggest studying children under the age of 6 years (23,24). Recommendations for evaluation of children over age 6 may vary depending on age, patient, and family history, and comorbidities. Alternate methods such as voiding sonogram may also be options for this age group, and is not part of this discussion (25).
RNC exposes the patient to less radiation but does not show urethral or bladder anomalies. RNC is more often used for females with normal RUS and no voiding dysfunction, or to follow the progress of known VUR. The VCUG is often preferred because it provides more anatomic detail and is better for grading VUR and demonstrating posterior urethral vales in males (26). It is suggested that infants with antenatal renal pelvis dilation who have 2 normal renal sonograms in the first month of life are at low risk for abnormalities and may not require a VCUG (27). The rate of detection of VUR with a first episode of UTI does not increase when the VCUG is done early, within the first 7 days of diagnosis (28,29). Performing the VCUG as an inpatient should be considered if outpatient follow-up is of significant concern, or if the RUS suggests bilateral ureteral obstruction. If done as an inpatient procedure, it should be performed preferably during day 3–5 of antibiotic therapy and when the patient is clinically responding to the appropriate antibiotic. The overall value of the VCUG is being reviewed, as its usefulness is most significant only if VUR antimicrobial prophylaxis is effective in reducing reinfections and renal scarring (21,30). Until further studies are performed, the VCUG should continue as part of the initial UTI evaluation for infants and young children.
Renal Cortical Scintigraphy (RCS)
This is the imaging study of choice for the detection of acute pyelonephritis and renal scarring. As children are treated for presumptive upper-tract infection empirically, DMSA scan for diagnosis of pyelonephritis has limited utility (21). Scans have more often been performed at 6 months’ postinfection to document scar formation. Hoberman demonstrated that only 15% of children with abnormal scintigraphy at diagnosis have renal scarring on repeat RCS at 6 months. The importance of these scars is unclear. Association of scars with ultimate development of hypertension, renal insufficiency, and end-stage renal disease is based on studies performed in the 1980s using intravenous pyelogram. RCS is much more sensitive, finding more minor scars of uncertain significance.
Table 3 may be of value when considering imaging options.
Other Considerations
CRP and PCT use in UTI have been evaluated by Pratt. Values at diagnosis are potentially helpful in ruling out scar formation at 6 months’ postinfection. Values under 1.0 ng/mL for PCT and 20 mg/L for CRP had a negative predictive value of 97.5% and 95%, respectively (31). Further studies are warranted to confirm the usefulness of these inflammatory markers to rule out future scar formation.
Consultations
Consider urology consultation if the RUS, VCUG, voiding history, or examination demonstrated concern for significant genitourinary abnormalities, abnormal voiding function or neurogenic bladder (23,32). Consider infectious disease consultation if the patient is not responding to conventional therapy without obstruction, unusual organisms are identified, or the patient is having recurrent urinary tract infections in the presence of normal urological structure and function.
Discharge Criteria and Processes
Consider discharge under the following conditions:
- The patient is comfortable and tolerating oral fluids well.
- The patient has been afebrile or has significantly decreasing fever for 24 hours.
- Appropriate radiological studies and consultations have been completed or arranged for as an outpatient.
- For patients requiring parenterally administered medications at home, long-term IV access must be obtained to assessment of home care service availability, benefits, family home resources, and caregiver education completed.
- Appropriate prophylactic antibiotic prescription has been given to the caregiver with education on use after completion of acute antibiotic therapy. Prophylactic antibiotics should be administered until imaging studies have been completed and assessed.
Conclusion
UTI is a common bacterial infection requiring hospital admission for infants and young children. Admission decisions should take into consideration goals for inpatient care and special age or clinical circumstances. Treatment mode and duration must address avoidance of both acute and chronic complications. Radiologic studies offer both anatomic view and functional information. Clinical relevance of scars, utility of radiologic studies, and value of inflammatory markers are some of the many areas requiring further study.
References
- Long SS, Klein J. Bacterial infections of the urinary tract. In: Remington JSand Klein JO(eds.). Infectious Diseases of the Fetus and Newborn Infant. 5th ed. Philadelphia, Pa: WB Saunders; 2001:1035-46.
- Committee on Quality Improvement, Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103:843-52.
- Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine gram stain in infants ≤60 days of age with fever. Pediatr Emerg Care. 2002;18:12-4.
- Huicho L, Campos-Sanchez M, Alamo C. Metaanalysis of urine screening tests for determining the risk of urinary tract infection in children. Pediatr Infect Dis J. 2002;21:1-11.
- Gorelick M, Shaw KN. Screening tests for urinary tract infections in Children: a meta-analysis. Pediatrics. 1999;104:e54.
- Byington CL, Enriquez F, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004:113:1662-6.
- Baraff L. Management of fever without source in infants and children. Ann Emerg Med. 2000;36:602-14.
- Baraff LJ, Oslund SA, Schriger D, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11:257-64.
- Klein JO. Management of the febrile child without a focus of infection in the era of universal pneumococcal immunization. Pediatr Infect Dis J. 2002;21:584-8.
- Syrogiannopoulos G, Grieva I, Anastassiou E, Triga M, Dimitracopoulos G, Beratis N. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infections. Pediatr Infect Dis J. 2001;20:927-30.
- Jaskiewicz JA, Mc Carthy CA, Richardson AC, ET AL. Febrile infants at low risk for serious bacterial infection--an appraisal of the Rochester criteria and implications for management. Febrile Collaborative Study Group. Pediatrics. 1994;94:390-6.
- AAP Redbook. Report of the committee on infectious diseases, 2003:700.
- Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in the office setting: the Pediatric Research in Office Settings’ Febrile Infant Study Arch Pediatr Adolesc Med. 2002;156:44-54.
- Ginsberg CM, McCracken GH Jr. Urinary tract infection in young infants. Pediatrics. 1982;69:409-12.
- Wiswell T, Geschke D. Risks from circumcision during the first month of life compared to uncircumcised boys. Pediatrics. 1989;83:1011-15.
- Malik A, Hui C, Pennie RA, Kirpalani H. Beyond the complete blood cell count and C-reactive protein: a systematic review of modern diagnostic tests for neonatal sepsis. Arch Pediatr Adolesc Med. 2003;157:511-6.
- Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109:e70.
- Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database of Syst Rev.2003.
- Hoberman A, Wald ER, Hickey RW, et al. Oral versus intravenous therapy for urinary tract infections in young children. Pediatrics. 1999;104:79-86.
- Bachur R. Nonresponders: prolonged fever among infants with urinary tract infections. Pediatrics. 2000;105:E59.
- Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348:195-202.
- Zamir G, Sakran W, Horowitz Y, Koren A, Miron D. Urinary tract infection: is there a need for routine renal ultrasonography? Arch Dis Child. 2004;89:466-8.
- Johnson CE. New advances in childhood urinary tract infections. Pediatr Rev. 1999:20:335-43.
- Thompson M, Simon S, Sharma V, Alon US. Timing of follow-up voiding cystourethrogram in children with primary vesicoureteral reflux: development and application of a clinical algorithm. Pediatrics. 2005:115:426-34.
- Darge K, Moeller RT, Trusen A, BuĴer F, Gordjani N, Riedmiller H. Diagnosis of vesicoureteral reflux with low-dose contrast-enhanced harmonic ultrasound imaging. Pediatr Radiol. 2005:35:73-8.
- Kraus S. Genitourinary imaging in children. Pediatr Clin North Am. 2001;48:1381-1424.
- Ismaili K, Avni F, Hall M; Brussels Free University Perinatal Nephrology (BFUPN) Study Group. Results of systematic voiding cystourethrography in infants with antenatally diagnosed renal pelvis dilation. J Pediatr. 2002;141: 21-4.
- Mahant S, To T, Friedman J. Timing of voiding cystourethrogram in the investigation of urinary tract infections in children. J Pediatr. 2001;39:568-71.
- McDonald A, Scranton M, Gillespie R, Mahajan V, Edwards GA. Voiding cystourethrograms and urinary tract infections: how long to wait? Pediatrics. 2000:105:E50.
- Williams G, Lee A, Craig J. Antibiotics for the prevention of urinary tract infection in children: a systematic review of randomized controlled trials. J Pediatr. 2001;138:868-74.
- Prat C, Dominguez J, Rodrigo C, et al. Elevated serum procalcitonin values correlate with renal scarring in children with urinary tract infection. Pediatr Infect Dis J. 2003;22:438-42.
- Roberts KB. Urinary tract infection treatment and evaluation update. Pediatr Infect Dis J. 2004: 23:1163-4.
Introduction
Urinary tract infections (UTIs) are serious bacterial infections and a common cause for hospital admission of infants and young children. The prevalence of UTI in infants younger than 1 year of age ranges from 3.3% to 6.5%, and between 1 and 2 years of age from 1.9% to 8.1%. Females outpace males across all age groups, with the exception of the first 3 months of life (1). Without appropriate treatment and management, UTI can result in dehydration, urosepsis, and long-term medical problems including hypertension, renal scarring, and decreased renal function. This review will focus on the inpatient management of first-episode UTI in infants and young children.
Diagnosis
Presenting symptoms in older children include urgency, frequency, dysuria, and complaints of back pain. In contrast, symptoms in infants and young children are often nonspecific and include irritability, diarrhea, vomiting, poor feeding, poor weight gain, crying on urination, and foul-smelling urine. The presence of a fever in infants and young children with UTI has been accepted as a marker of pyelonephritis, which occurs when infection has ascended to the upper collecting system of the kidney. Urinalysis (UA) and culture should be collected by suprapubic aspiration or transurethral catheterization, or by appropriately performed clean catch method for children of appropriate age and developmental ability. The use of a bag-collected urine specimen is insufficient and unreliable and should not be used in making the diagnosis of UTI. While suprapubic aspiration is considered the gold standard with a specificity and sensitivity of 100%, there is often resistance from parents and from physicians who are not properly trained to do this procedure.
The most accepted method of obtaining urine is sterile transurethral catheterization, results of which have 95% sensitivity and 99% specificity (2). When interpreting the UA, the most useful components for the diagnosis of a UTI include a positive leukocyte esterase, nitrite test, or gram stain on unspun urine, and microscopy revealing >10 white blood cells per high-powered field of spun urine. However, neonates under 30 days old may have no abnormality noted on initial UA (3,4). The presence of any bacteria on gram-stained urine offers the best sensitivity and specificity (5). Final diagnosis depends upon isolation of >105 of a single organism from a clean-catch specimen, or >104 of a single organism from a catheterized specimen.
Admission Criteria
Guidelines for evaluation of serious bacterial infection and parenteral antibiotic use for febrile infants under 60 days of age should be followed. All febrile neonates less than 30 days of age should be admitted for parenteral antibiotics (6–11). Controversy exists on the need to use corrected or postconceptual age when evaluating and determining need for admission for febrile preterm infants, particularly for those under 35 weeks of gestation. Factors that can be considered by the practitioner include severity of Neonatal ICU course, severity of prematurity, and combined disease burden of UTI with common preterm comorbidities (anemia, apnea of prematurity, chronic lung disease).
Consider admitting and initiating parenteral antibiotic treatment using Table 1 as a guideline. Exercise a lower threshold for admitting infants and toxic-appearing young children due to concern for urosepsis, complications, and the need for appropriate and aggressive initial therapy.
Initial Inpatient Management
The 3 goals of inhospital treatment of UTIs are to effectively treat and eliminate the acute infection, prevent urosepsis in infants and immunocompromised children, and prevent and reduce long-term complications such as renal scarring, hypertension, and decreased renal function. Initial antibiotic treatment should be administered parenterally to ensure optimal antimicrobial levels and aimed at the most common organisms, including Escherichia coli, Klebsiella, Proteus, and Enterobacter spp. Less common organisms to consider include Pseudomonas, Enterococcus, Staphylococcus aureus, and group B Streptococcus. Organisms will differ on many factors, such as age, underlying disease, prior colonization, and antibiotic exposure.
Table 2 outlines the initial choices of antibiotics until culture and sensitivities are known.
The choices and dosage of antibiotics are dependent on the age of the patient and are selected based on the other most likely organisms and their expected sensitivities (12). Ampicillin is added to the less than 2-month age group not only to cover Enterococcus, but also as part of broader neonatal sepsis coverage for Listeria. The third-generation cephalosporins are felt to be adequate initial coverage for most of the common organisms causing UTI. Children with congenital anomalies known to be associated with genitourinary abnormalities may be infected with less common organisms. In these situations, consider tailoring initial antibiotic coverage.
Complications
The major complication of UTIs in infants is bacteremia. The rate of bacteremia in infants 0–3 months has variably been reported as 10% (13), 21–31% (14), and 36% (15). Infants with and without bacteremia are often clinically indistinguishable, making early determination of bacteremia difficult. A recent comprehensive review of 17 studies by Malik noted both C-reactive protein (CRP) and procalcitonin (PCT) results were highly variable in infants under 90 days old with known positive bacterial cultures (16). These inflammatory markers are therefore currently not useful to predict bacteremia. In addition to blood stream infection, other acute complications include meningitis, renal and perinephric abscess, and infected calculi. Longer-term complications include reflux nephropathy, renal scarring, hypertension, decreased renal function, and renal failure.
Duration of Antibiotic Therapy
While most uncomplicated UTIs are successfully treated with a 10-day treatment course, many experts prefer a 14-day course for neonates, infants, and ill-appearing young children. Despite effectiveness in adults, very-short-course therapy (≤3 days) for pediatric patients is associated with more treatment failures and reinfections (17). Although it may be considered in older children with cystitis, at this time it is not appropriate for treating infants and younger children in whom pyelonephritis cannot be distinguished from isolated lower tract infection (17,18).
Total treatment time and total days of parenteral therapy needed continue to be debated. Hoberman randomized children as young as 1 month of age to either entirely oral treatment or parenteral therapy for 3 days followed by oral treatment (19). In both arms of this study, children received 14 days of total therapy as was the standard at the time. He suggested, however, that a 10-day course of antibiotics should be adequate therapy for noncomplicated acute pyelonephritis. Of the 306 children, only 13 were under 2 months of age. Although only 13 positive blood cultures were reported, 10 of these occurred in children under 6 months of age. Given the limited number of children less than 2 months of age and the prevalence of positive blood cultures noted, conclusions cannot be drawn on the safety of entirely oral treatment for young infants. Parenteral antibiotic therapy should be continued for all hospitalized children until the patient is afebrile and free from signs of toxicity. Most hospitalized pediatric patients defervesce quickly on parenteral therapy—89% within 48 hours and 97% within 72 hours (20). Longer parenteral therapy of at least 10 days should be considered for neonates and infants with urosepsis, because they are immunologically immature, at greater risk of complications, have higher incidence of urinary tract anomalies, and have less reliable absorption of oral antibiotics.
Delayed or lack of response to antibiotic therapy may indicate the presence of urinary tract obstruction, resistant organisms, or renal or perinephric abscess. A repeat urine culture and immediate renal ultrasound or CT should be performed if the patient is not improving within 48 hours.
Radiological Studies
Renal Ultrasound (RUS)
Recent studies have questioned the value of performing routine RUS after a first-time UTI because of the low sensitivity in detecting vesicoureteral reflux (VUR) and a lack of significant influence in altering management (21,22). Patients who have had a normal late (30-32 weeks’ gestation) prenatal ultrasound with a good view of the kidneys may not require a repeat postnatal renal ultrasound (21,22). Further studies are needed to evaluate the costs and value of routine RUS. Until these studies are completed, renal and bladder ultrasound early during hospitalization continues to be recommended for all patients admitted with a first-time UTI to identify hydronephrosis, duplicating collecting systems, ureteral dilatation, calculi, and other structural anomalies.
Voiding Cystourethrogram (VCUG) or Radionuclide Cystography (RNC)
Either a VCUG or RNC should be performed to detect vesico-ureteral reflux in infants and young children. The AAP practice parameter and more recent literature clearly state the need for this evaluation in children under the age of 2 years (2,21). Additional data on incidence of anomalies by age suggest studying children under the age of 6 years (23,24). Recommendations for evaluation of children over age 6 may vary depending on age, patient, and family history, and comorbidities. Alternate methods such as voiding sonogram may also be options for this age group, and is not part of this discussion (25).
RNC exposes the patient to less radiation but does not show urethral or bladder anomalies. RNC is more often used for females with normal RUS and no voiding dysfunction, or to follow the progress of known VUR. The VCUG is often preferred because it provides more anatomic detail and is better for grading VUR and demonstrating posterior urethral vales in males (26). It is suggested that infants with antenatal renal pelvis dilation who have 2 normal renal sonograms in the first month of life are at low risk for abnormalities and may not require a VCUG (27). The rate of detection of VUR with a first episode of UTI does not increase when the VCUG is done early, within the first 7 days of diagnosis (28,29). Performing the VCUG as an inpatient should be considered if outpatient follow-up is of significant concern, or if the RUS suggests bilateral ureteral obstruction. If done as an inpatient procedure, it should be performed preferably during day 3–5 of antibiotic therapy and when the patient is clinically responding to the appropriate antibiotic. The overall value of the VCUG is being reviewed, as its usefulness is most significant only if VUR antimicrobial prophylaxis is effective in reducing reinfections and renal scarring (21,30). Until further studies are performed, the VCUG should continue as part of the initial UTI evaluation for infants and young children.
Renal Cortical Scintigraphy (RCS)
This is the imaging study of choice for the detection of acute pyelonephritis and renal scarring. As children are treated for presumptive upper-tract infection empirically, DMSA scan for diagnosis of pyelonephritis has limited utility (21). Scans have more often been performed at 6 months’ postinfection to document scar formation. Hoberman demonstrated that only 15% of children with abnormal scintigraphy at diagnosis have renal scarring on repeat RCS at 6 months. The importance of these scars is unclear. Association of scars with ultimate development of hypertension, renal insufficiency, and end-stage renal disease is based on studies performed in the 1980s using intravenous pyelogram. RCS is much more sensitive, finding more minor scars of uncertain significance.
Table 3 may be of value when considering imaging options.
Other Considerations
CRP and PCT use in UTI have been evaluated by Pratt. Values at diagnosis are potentially helpful in ruling out scar formation at 6 months’ postinfection. Values under 1.0 ng/mL for PCT and 20 mg/L for CRP had a negative predictive value of 97.5% and 95%, respectively (31). Further studies are warranted to confirm the usefulness of these inflammatory markers to rule out future scar formation.
Consultations
Consider urology consultation if the RUS, VCUG, voiding history, or examination demonstrated concern for significant genitourinary abnormalities, abnormal voiding function or neurogenic bladder (23,32). Consider infectious disease consultation if the patient is not responding to conventional therapy without obstruction, unusual organisms are identified, or the patient is having recurrent urinary tract infections in the presence of normal urological structure and function.
Discharge Criteria and Processes
Consider discharge under the following conditions:
- The patient is comfortable and tolerating oral fluids well.
- The patient has been afebrile or has significantly decreasing fever for 24 hours.
- Appropriate radiological studies and consultations have been completed or arranged for as an outpatient.
- For patients requiring parenterally administered medications at home, long-term IV access must be obtained to assessment of home care service availability, benefits, family home resources, and caregiver education completed.
- Appropriate prophylactic antibiotic prescription has been given to the caregiver with education on use after completion of acute antibiotic therapy. Prophylactic antibiotics should be administered until imaging studies have been completed and assessed.
Conclusion
UTI is a common bacterial infection requiring hospital admission for infants and young children. Admission decisions should take into consideration goals for inpatient care and special age or clinical circumstances. Treatment mode and duration must address avoidance of both acute and chronic complications. Radiologic studies offer both anatomic view and functional information. Clinical relevance of scars, utility of radiologic studies, and value of inflammatory markers are some of the many areas requiring further study.
References
- Long SS, Klein J. Bacterial infections of the urinary tract. In: Remington JSand Klein JO(eds.). Infectious Diseases of the Fetus and Newborn Infant. 5th ed. Philadelphia, Pa: WB Saunders; 2001:1035-46.
- Committee on Quality Improvement, Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103:843-52.
- Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine gram stain in infants ≤60 days of age with fever. Pediatr Emerg Care. 2002;18:12-4.
- Huicho L, Campos-Sanchez M, Alamo C. Metaanalysis of urine screening tests for determining the risk of urinary tract infection in children. Pediatr Infect Dis J. 2002;21:1-11.
- Gorelick M, Shaw KN. Screening tests for urinary tract infections in Children: a meta-analysis. Pediatrics. 1999;104:e54.
- Byington CL, Enriquez F, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004:113:1662-6.
- Baraff L. Management of fever without source in infants and children. Ann Emerg Med. 2000;36:602-14.
- Baraff LJ, Oslund SA, Schriger D, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11:257-64.
- Klein JO. Management of the febrile child without a focus of infection in the era of universal pneumococcal immunization. Pediatr Infect Dis J. 2002;21:584-8.
- Syrogiannopoulos G, Grieva I, Anastassiou E, Triga M, Dimitracopoulos G, Beratis N. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infections. Pediatr Infect Dis J. 2001;20:927-30.
- Jaskiewicz JA, Mc Carthy CA, Richardson AC, ET AL. Febrile infants at low risk for serious bacterial infection--an appraisal of the Rochester criteria and implications for management. Febrile Collaborative Study Group. Pediatrics. 1994;94:390-6.
- AAP Redbook. Report of the committee on infectious diseases, 2003:700.
- Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in the office setting: the Pediatric Research in Office Settings’ Febrile Infant Study Arch Pediatr Adolesc Med. 2002;156:44-54.
- Ginsberg CM, McCracken GH Jr. Urinary tract infection in young infants. Pediatrics. 1982;69:409-12.
- Wiswell T, Geschke D. Risks from circumcision during the first month of life compared to uncircumcised boys. Pediatrics. 1989;83:1011-15.
- Malik A, Hui C, Pennie RA, Kirpalani H. Beyond the complete blood cell count and C-reactive protein: a systematic review of modern diagnostic tests for neonatal sepsis. Arch Pediatr Adolesc Med. 2003;157:511-6.
- Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109:e70.
- Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database of Syst Rev.2003.
- Hoberman A, Wald ER, Hickey RW, et al. Oral versus intravenous therapy for urinary tract infections in young children. Pediatrics. 1999;104:79-86.
- Bachur R. Nonresponders: prolonged fever among infants with urinary tract infections. Pediatrics. 2000;105:E59.
- Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348:195-202.
- Zamir G, Sakran W, Horowitz Y, Koren A, Miron D. Urinary tract infection: is there a need for routine renal ultrasonography? Arch Dis Child. 2004;89:466-8.
- Johnson CE. New advances in childhood urinary tract infections. Pediatr Rev. 1999:20:335-43.
- Thompson M, Simon S, Sharma V, Alon US. Timing of follow-up voiding cystourethrogram in children with primary vesicoureteral reflux: development and application of a clinical algorithm. Pediatrics. 2005:115:426-34.
- Darge K, Moeller RT, Trusen A, BuĴer F, Gordjani N, Riedmiller H. Diagnosis of vesicoureteral reflux with low-dose contrast-enhanced harmonic ultrasound imaging. Pediatr Radiol. 2005:35:73-8.
- Kraus S. Genitourinary imaging in children. Pediatr Clin North Am. 2001;48:1381-1424.
- Ismaili K, Avni F, Hall M; Brussels Free University Perinatal Nephrology (BFUPN) Study Group. Results of systematic voiding cystourethrography in infants with antenatally diagnosed renal pelvis dilation. J Pediatr. 2002;141: 21-4.
- Mahant S, To T, Friedman J. Timing of voiding cystourethrogram in the investigation of urinary tract infections in children. J Pediatr. 2001;39:568-71.
- McDonald A, Scranton M, Gillespie R, Mahajan V, Edwards GA. Voiding cystourethrograms and urinary tract infections: how long to wait? Pediatrics. 2000:105:E50.
- Williams G, Lee A, Craig J. Antibiotics for the prevention of urinary tract infection in children: a systematic review of randomized controlled trials. J Pediatr. 2001;138:868-74.
- Prat C, Dominguez J, Rodrigo C, et al. Elevated serum procalcitonin values correlate with renal scarring in children with urinary tract infection. Pediatr Infect Dis J. 2003;22:438-42.
- Roberts KB. Urinary tract infection treatment and evaluation update. Pediatr Infect Dis J. 2004: 23:1163-4.
Introduction
Urinary tract infections (UTIs) are serious bacterial infections and a common cause for hospital admission of infants and young children. The prevalence of UTI in infants younger than 1 year of age ranges from 3.3% to 6.5%, and between 1 and 2 years of age from 1.9% to 8.1%. Females outpace males across all age groups, with the exception of the first 3 months of life (1). Without appropriate treatment and management, UTI can result in dehydration, urosepsis, and long-term medical problems including hypertension, renal scarring, and decreased renal function. This review will focus on the inpatient management of first-episode UTI in infants and young children.
Diagnosis
Presenting symptoms in older children include urgency, frequency, dysuria, and complaints of back pain. In contrast, symptoms in infants and young children are often nonspecific and include irritability, diarrhea, vomiting, poor feeding, poor weight gain, crying on urination, and foul-smelling urine. The presence of a fever in infants and young children with UTI has been accepted as a marker of pyelonephritis, which occurs when infection has ascended to the upper collecting system of the kidney. Urinalysis (UA) and culture should be collected by suprapubic aspiration or transurethral catheterization, or by appropriately performed clean catch method for children of appropriate age and developmental ability. The use of a bag-collected urine specimen is insufficient and unreliable and should not be used in making the diagnosis of UTI. While suprapubic aspiration is considered the gold standard with a specificity and sensitivity of 100%, there is often resistance from parents and from physicians who are not properly trained to do this procedure.
The most accepted method of obtaining urine is sterile transurethral catheterization, results of which have 95% sensitivity and 99% specificity (2). When interpreting the UA, the most useful components for the diagnosis of a UTI include a positive leukocyte esterase, nitrite test, or gram stain on unspun urine, and microscopy revealing >10 white blood cells per high-powered field of spun urine. However, neonates under 30 days old may have no abnormality noted on initial UA (3,4). The presence of any bacteria on gram-stained urine offers the best sensitivity and specificity (5). Final diagnosis depends upon isolation of >105 of a single organism from a clean-catch specimen, or >104 of a single organism from a catheterized specimen.
Admission Criteria
Guidelines for evaluation of serious bacterial infection and parenteral antibiotic use for febrile infants under 60 days of age should be followed. All febrile neonates less than 30 days of age should be admitted for parenteral antibiotics (6–11). Controversy exists on the need to use corrected or postconceptual age when evaluating and determining need for admission for febrile preterm infants, particularly for those under 35 weeks of gestation. Factors that can be considered by the practitioner include severity of Neonatal ICU course, severity of prematurity, and combined disease burden of UTI with common preterm comorbidities (anemia, apnea of prematurity, chronic lung disease).
Consider admitting and initiating parenteral antibiotic treatment using Table 1 as a guideline. Exercise a lower threshold for admitting infants and toxic-appearing young children due to concern for urosepsis, complications, and the need for appropriate and aggressive initial therapy.
Initial Inpatient Management
The 3 goals of inhospital treatment of UTIs are to effectively treat and eliminate the acute infection, prevent urosepsis in infants and immunocompromised children, and prevent and reduce long-term complications such as renal scarring, hypertension, and decreased renal function. Initial antibiotic treatment should be administered parenterally to ensure optimal antimicrobial levels and aimed at the most common organisms, including Escherichia coli, Klebsiella, Proteus, and Enterobacter spp. Less common organisms to consider include Pseudomonas, Enterococcus, Staphylococcus aureus, and group B Streptococcus. Organisms will differ on many factors, such as age, underlying disease, prior colonization, and antibiotic exposure.
Table 2 outlines the initial choices of antibiotics until culture and sensitivities are known.
The choices and dosage of antibiotics are dependent on the age of the patient and are selected based on the other most likely organisms and their expected sensitivities (12). Ampicillin is added to the less than 2-month age group not only to cover Enterococcus, but also as part of broader neonatal sepsis coverage for Listeria. The third-generation cephalosporins are felt to be adequate initial coverage for most of the common organisms causing UTI. Children with congenital anomalies known to be associated with genitourinary abnormalities may be infected with less common organisms. In these situations, consider tailoring initial antibiotic coverage.
Complications
The major complication of UTIs in infants is bacteremia. The rate of bacteremia in infants 0–3 months has variably been reported as 10% (13), 21–31% (14), and 36% (15). Infants with and without bacteremia are often clinically indistinguishable, making early determination of bacteremia difficult. A recent comprehensive review of 17 studies by Malik noted both C-reactive protein (CRP) and procalcitonin (PCT) results were highly variable in infants under 90 days old with known positive bacterial cultures (16). These inflammatory markers are therefore currently not useful to predict bacteremia. In addition to blood stream infection, other acute complications include meningitis, renal and perinephric abscess, and infected calculi. Longer-term complications include reflux nephropathy, renal scarring, hypertension, decreased renal function, and renal failure.
Duration of Antibiotic Therapy
While most uncomplicated UTIs are successfully treated with a 10-day treatment course, many experts prefer a 14-day course for neonates, infants, and ill-appearing young children. Despite effectiveness in adults, very-short-course therapy (≤3 days) for pediatric patients is associated with more treatment failures and reinfections (17). Although it may be considered in older children with cystitis, at this time it is not appropriate for treating infants and younger children in whom pyelonephritis cannot be distinguished from isolated lower tract infection (17,18).
Total treatment time and total days of parenteral therapy needed continue to be debated. Hoberman randomized children as young as 1 month of age to either entirely oral treatment or parenteral therapy for 3 days followed by oral treatment (19). In both arms of this study, children received 14 days of total therapy as was the standard at the time. He suggested, however, that a 10-day course of antibiotics should be adequate therapy for noncomplicated acute pyelonephritis. Of the 306 children, only 13 were under 2 months of age. Although only 13 positive blood cultures were reported, 10 of these occurred in children under 6 months of age. Given the limited number of children less than 2 months of age and the prevalence of positive blood cultures noted, conclusions cannot be drawn on the safety of entirely oral treatment for young infants. Parenteral antibiotic therapy should be continued for all hospitalized children until the patient is afebrile and free from signs of toxicity. Most hospitalized pediatric patients defervesce quickly on parenteral therapy—89% within 48 hours and 97% within 72 hours (20). Longer parenteral therapy of at least 10 days should be considered for neonates and infants with urosepsis, because they are immunologically immature, at greater risk of complications, have higher incidence of urinary tract anomalies, and have less reliable absorption of oral antibiotics.
Delayed or lack of response to antibiotic therapy may indicate the presence of urinary tract obstruction, resistant organisms, or renal or perinephric abscess. A repeat urine culture and immediate renal ultrasound or CT should be performed if the patient is not improving within 48 hours.
Radiological Studies
Renal Ultrasound (RUS)
Recent studies have questioned the value of performing routine RUS after a first-time UTI because of the low sensitivity in detecting vesicoureteral reflux (VUR) and a lack of significant influence in altering management (21,22). Patients who have had a normal late (30-32 weeks’ gestation) prenatal ultrasound with a good view of the kidneys may not require a repeat postnatal renal ultrasound (21,22). Further studies are needed to evaluate the costs and value of routine RUS. Until these studies are completed, renal and bladder ultrasound early during hospitalization continues to be recommended for all patients admitted with a first-time UTI to identify hydronephrosis, duplicating collecting systems, ureteral dilatation, calculi, and other structural anomalies.
Voiding Cystourethrogram (VCUG) or Radionuclide Cystography (RNC)
Either a VCUG or RNC should be performed to detect vesico-ureteral reflux in infants and young children. The AAP practice parameter and more recent literature clearly state the need for this evaluation in children under the age of 2 years (2,21). Additional data on incidence of anomalies by age suggest studying children under the age of 6 years (23,24). Recommendations for evaluation of children over age 6 may vary depending on age, patient, and family history, and comorbidities. Alternate methods such as voiding sonogram may also be options for this age group, and is not part of this discussion (25).
RNC exposes the patient to less radiation but does not show urethral or bladder anomalies. RNC is more often used for females with normal RUS and no voiding dysfunction, or to follow the progress of known VUR. The VCUG is often preferred because it provides more anatomic detail and is better for grading VUR and demonstrating posterior urethral vales in males (26). It is suggested that infants with antenatal renal pelvis dilation who have 2 normal renal sonograms in the first month of life are at low risk for abnormalities and may not require a VCUG (27). The rate of detection of VUR with a first episode of UTI does not increase when the VCUG is done early, within the first 7 days of diagnosis (28,29). Performing the VCUG as an inpatient should be considered if outpatient follow-up is of significant concern, or if the RUS suggests bilateral ureteral obstruction. If done as an inpatient procedure, it should be performed preferably during day 3–5 of antibiotic therapy and when the patient is clinically responding to the appropriate antibiotic. The overall value of the VCUG is being reviewed, as its usefulness is most significant only if VUR antimicrobial prophylaxis is effective in reducing reinfections and renal scarring (21,30). Until further studies are performed, the VCUG should continue as part of the initial UTI evaluation for infants and young children.
Renal Cortical Scintigraphy (RCS)
This is the imaging study of choice for the detection of acute pyelonephritis and renal scarring. As children are treated for presumptive upper-tract infection empirically, DMSA scan for diagnosis of pyelonephritis has limited utility (21). Scans have more often been performed at 6 months’ postinfection to document scar formation. Hoberman demonstrated that only 15% of children with abnormal scintigraphy at diagnosis have renal scarring on repeat RCS at 6 months. The importance of these scars is unclear. Association of scars with ultimate development of hypertension, renal insufficiency, and end-stage renal disease is based on studies performed in the 1980s using intravenous pyelogram. RCS is much more sensitive, finding more minor scars of uncertain significance.
Table 3 may be of value when considering imaging options.
Other Considerations
CRP and PCT use in UTI have been evaluated by Pratt. Values at diagnosis are potentially helpful in ruling out scar formation at 6 months’ postinfection. Values under 1.0 ng/mL for PCT and 20 mg/L for CRP had a negative predictive value of 97.5% and 95%, respectively (31). Further studies are warranted to confirm the usefulness of these inflammatory markers to rule out future scar formation.
Consultations
Consider urology consultation if the RUS, VCUG, voiding history, or examination demonstrated concern for significant genitourinary abnormalities, abnormal voiding function or neurogenic bladder (23,32). Consider infectious disease consultation if the patient is not responding to conventional therapy without obstruction, unusual organisms are identified, or the patient is having recurrent urinary tract infections in the presence of normal urological structure and function.
Discharge Criteria and Processes
Consider discharge under the following conditions:
- The patient is comfortable and tolerating oral fluids well.
- The patient has been afebrile or has significantly decreasing fever for 24 hours.
- Appropriate radiological studies and consultations have been completed or arranged for as an outpatient.
- For patients requiring parenterally administered medications at home, long-term IV access must be obtained to assessment of home care service availability, benefits, family home resources, and caregiver education completed.
- Appropriate prophylactic antibiotic prescription has been given to the caregiver with education on use after completion of acute antibiotic therapy. Prophylactic antibiotics should be administered until imaging studies have been completed and assessed.
Conclusion
UTI is a common bacterial infection requiring hospital admission for infants and young children. Admission decisions should take into consideration goals for inpatient care and special age or clinical circumstances. Treatment mode and duration must address avoidance of both acute and chronic complications. Radiologic studies offer both anatomic view and functional information. Clinical relevance of scars, utility of radiologic studies, and value of inflammatory markers are some of the many areas requiring further study.
References
- Long SS, Klein J. Bacterial infections of the urinary tract. In: Remington JSand Klein JO(eds.). Infectious Diseases of the Fetus and Newborn Infant. 5th ed. Philadelphia, Pa: WB Saunders; 2001:1035-46.
- Committee on Quality Improvement, Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103:843-52.
- Dayan PS, Bennett J, Best R, et al. Test characteristics of the urine gram stain in infants ≤60 days of age with fever. Pediatr Emerg Care. 2002;18:12-4.
- Huicho L, Campos-Sanchez M, Alamo C. Metaanalysis of urine screening tests for determining the risk of urinary tract infection in children. Pediatr Infect Dis J. 2002;21:1-11.
- Gorelick M, Shaw KN. Screening tests for urinary tract infections in Children: a meta-analysis. Pediatrics. 1999;104:e54.
- Byington CL, Enriquez F, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004:113:1662-6.
- Baraff L. Management of fever without source in infants and children. Ann Emerg Med. 2000;36:602-14.
- Baraff LJ, Oslund SA, Schriger D, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11:257-64.
- Klein JO. Management of the febrile child without a focus of infection in the era of universal pneumococcal immunization. Pediatr Infect Dis J. 2002;21:584-8.
- Syrogiannopoulos G, Grieva I, Anastassiou E, Triga M, Dimitracopoulos G, Beratis N. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infections. Pediatr Infect Dis J. 2001;20:927-30.
- Jaskiewicz JA, Mc Carthy CA, Richardson AC, ET AL. Febrile infants at low risk for serious bacterial infection--an appraisal of the Rochester criteria and implications for management. Febrile Collaborative Study Group. Pediatrics. 1994;94:390-6.
- AAP Redbook. Report of the committee on infectious diseases, 2003:700.
- Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in the office setting: the Pediatric Research in Office Settings’ Febrile Infant Study Arch Pediatr Adolesc Med. 2002;156:44-54.
- Ginsberg CM, McCracken GH Jr. Urinary tract infection in young infants. Pediatrics. 1982;69:409-12.
- Wiswell T, Geschke D. Risks from circumcision during the first month of life compared to uncircumcised boys. Pediatrics. 1989;83:1011-15.
- Malik A, Hui C, Pennie RA, Kirpalani H. Beyond the complete blood cell count and C-reactive protein: a systematic review of modern diagnostic tests for neonatal sepsis. Arch Pediatr Adolesc Med. 2003;157:511-6.
- Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109:e70.
- Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database of Syst Rev.2003.
- Hoberman A, Wald ER, Hickey RW, et al. Oral versus intravenous therapy for urinary tract infections in young children. Pediatrics. 1999;104:79-86.
- Bachur R. Nonresponders: prolonged fever among infants with urinary tract infections. Pediatrics. 2000;105:E59.
- Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348:195-202.
- Zamir G, Sakran W, Horowitz Y, Koren A, Miron D. Urinary tract infection: is there a need for routine renal ultrasonography? Arch Dis Child. 2004;89:466-8.
- Johnson CE. New advances in childhood urinary tract infections. Pediatr Rev. 1999:20:335-43.
- Thompson M, Simon S, Sharma V, Alon US. Timing of follow-up voiding cystourethrogram in children with primary vesicoureteral reflux: development and application of a clinical algorithm. Pediatrics. 2005:115:426-34.
- Darge K, Moeller RT, Trusen A, BuĴer F, Gordjani N, Riedmiller H. Diagnosis of vesicoureteral reflux with low-dose contrast-enhanced harmonic ultrasound imaging. Pediatr Radiol. 2005:35:73-8.
- Kraus S. Genitourinary imaging in children. Pediatr Clin North Am. 2001;48:1381-1424.
- Ismaili K, Avni F, Hall M; Brussels Free University Perinatal Nephrology (BFUPN) Study Group. Results of systematic voiding cystourethrography in infants with antenatally diagnosed renal pelvis dilation. J Pediatr. 2002;141: 21-4.
- Mahant S, To T, Friedman J. Timing of voiding cystourethrogram in the investigation of urinary tract infections in children. J Pediatr. 2001;39:568-71.
- McDonald A, Scranton M, Gillespie R, Mahajan V, Edwards GA. Voiding cystourethrograms and urinary tract infections: how long to wait? Pediatrics. 2000:105:E50.
- Williams G, Lee A, Craig J. Antibiotics for the prevention of urinary tract infection in children: a systematic review of randomized controlled trials. J Pediatr. 2001;138:868-74.
- Prat C, Dominguez J, Rodrigo C, et al. Elevated serum procalcitonin values correlate with renal scarring in children with urinary tract infection. Pediatr Infect Dis J. 2003;22:438-42.
- Roberts KB. Urinary tract infection treatment and evaluation update. Pediatr Infect Dis J. 2004: 23:1163-4.
Infective Endocarditis
Introduction
A 55-year-old heroin addict presented to the emergency department, complaining of shaking chills and fevers for 2 weeks. On examination, there was a loud holosystolic murmur, maximally audible in the epigastric space, and a pulsatile liver. Subcutaneous nodular lesions were noted on his palms. Blood cultures grew Pseudomonas aeruginosa. After nearly completing a prolonged course of intravenous antibiotic therapy, the patient died in his washroom from an overdose of heroin. This sad tale, often tragically repeated, represents a continuing challenge to the medical community. The patients’ palm lesions noted were
Osler’s nodes, originally described in 1908 by Sir William Osler, considered by many the father of internal medicine. Osler was born in 1849 and died in 1919. He was an astute clinician and educator, with professorships at McGill University, University of Pennsylvania, Johns Hopkins University, and Oxford University. Osler defined “chronic” infectious endocarditis as an illness lasting longer than 3 months and characterized by low grade fevers. This syndrome was distinct from a “malignant” form, which resulted in early death. Blood cultures usually grew streptococci or, occasionally, staphylococci. Osler made a practice of following his patients to the autopsy table. Vegetations on valves from patients who died of the chronic form looked more like “warts,” and were neither ”ulcerating or very large.” Osler thought anti-streptococcal vaccines might be of some benefit. There was little else to offer. Regardless of the form, nearly all patients died.
In this review, I discuss current methods for the diagnosis and management of infective endocarditis. Cases seen in recent years will illustrate key points.
Case 1 A 39-year-old computer programmer complained of occipital headaches, migratory muscle pains, afternoon fevers, and a 15-pound weight loss for 2 months. He had previously enjoyed excellent health. On examination his temperature was 38.0°C. An apical systolic heart murmur was noted. A transthoracic echocardiogram (TTE) showed mitral regurgitation, with a probable vegetation on the mitral valve. Blood cultures were drawn and the patient was admitted to the hospital. The next day, a transesophageal echocardiogram (TEE) showed perforation of the posterior mitral leaflet. That evening, the patient developed severe right flank pain. CT scan showed infarcts in the right kidney and spleen. The next day the patient underwent urgent mitral valve repair and was dismissed 5 days later to complete a course of intravenous ceftriaxone. All blood cultures grew viridans streptococci, exquisitely susceptible to penicillin.
Comment: This patient represents classic “subacute” bacterial endocarditis with fever, weight loss, and a heart murmur. In most cases, he would be cured with medical therapy alone. However, a TEE showed a lesion that was not appreciated on the initial TTE, and he required urgent surgery to restore a failing mitral valve.
Although the patient had no identified skin or mucosal lesions, when present these suggest the diagnosis. The subconjunctivial sacs and soft palate should be examined for petechiae, the nail beds for splinter hemorrhages, and digits for Janeway lesions.
Osler’s definition of endocarditis included remittent fever, history of valvular heart disease, embolic features, skin lesions, and heart failure. These remain useful bedside observations, and positive blood cultures usually clinch the diagnosis. Perhaps the most important technical advance in recent years for diagnosis is the echocardiogram. The major Duke criteria, published in 1994, include a predictable bacterial organism in blood culture, multiple positive blood cultures with the same organism, or an echocardiogram with definite vegetation, abscess, or valve dehiscence. Any two of the above suffice for diagnosis of probable endocarditis. Accepted minor criteria consist of predisposing lesions, history of intravenous drug abuse, temperature higher than 38°C, vasculitis, skin lesions, or “suggestive” echocardiographic or microbiologic findings. Five of these, or three with one major criterion, support the diagnosis. Transesophageal is superior to transthoracic echocardiography and should be performed if the TTE is equivocal or non-diagnostic.
Case 2 A 31-year-old warehouse manager with progressive dyspnea was transferred from an outside hospital. His illness began 8 months earlier with a dry cough and progressive fatigue. His past history was negative except for an asymptomatic heart murmur. On examination, he was pale and diaphoretic with a temperature of 36°C, pulse 110, and blood pressure of 108/56mm Hg. Neck veins were distended beats/min; loud heart murmurs and diffuse airway crackles were heard. The spleen was palpable. Blood cultures were drawn and antibiotics started.
As the patient was being wheeled for urgent heart surgery, he suffered a huge left-sided stroke. Contrast studies showed a leaking basilar artery aneurysm with subarachnoid hemorrhage. Once his neurologic problem stabilized, urgent mitral and aortic valve replacement was performed. Both valves were severely damaged and rife with vegetations. Admission blood cultures grew viridans streptococci, susceptible to penicillin. After prolonged hospitalization, the patient was transferred for continued care to a rehabilitation unit closer to home.
Comment: Neurologic complications of endocarditis are more common than generally appreciated, and occur in at least one third of patients at the time of diagnosis. Stroke is the most frequent finding, but encephalopathy, retinal embolic lesions, mycotic aneurysm, brain abscess, and meningitis can also occur. Fortunately, most neurologic problems resolve with medical management, but as seen in this patient, some are devastating and have permanent sequelae.
Organisms responsible for the majority of cases of native valve endocarditis are streptococci, as was true in Osler’s time. Staphylococcus aureus is next in frequency, followed by gram-negative bacilli, fungi, coagulase-negative staphylococci, and a poorly-defined category of “culture negative” cases. Therapy for infection caused by penicillin-susceptible streptococci is straightforward. The preferred agent is intravenous penicillin or ampicillin, with ceftraxione or vancomycin as alternatives. Streptococci less susceptible to penicillin, including nutritionally variant organisms, are treated more vigorously with a penicillin and low-dose aminoglycoside.
The HACEK group of gram-negative bacteria (Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella species) often produce large vegetations. Embolic lesions to major organs or extremities are a fairly common presenting feature. Treatment with ceftriaxone or ampicillin plus gentamicin is usually successful.
“Culture negative” endocarditis includes infections due to microorganisms difficult to culture on standard media. These uncommon pathogens include Bartonella, Brucella, Chlamydia, Coxiella, Francisella, Legionella, and Tropheryma whippeli.
Bartonella endocarditis has been reported in the homeless population. Blood cultures are usually negative. Serology is helpful. More recently, polymerase drain reactions (PCR) from resected valve tissue have proven useful. Treatment of choice is ampicillin plus gentamicin, but mortality remains approximately 25%.
Patients with endocarditis due to Coxiella burnetii (Q fever) are likewise difficult to diagnose. They may not have fever. However, there is generally underlying valvular heart disease, and frequently patients are immunosuppressed. Vegetations are rarely detected on echocardiogram. Routine blood cultures are negative. Fortunately, serology is quite specific for the diagnosis. A combination of doxycycline and chloroquine is the current treatment of choice.
PCR and special immunohistochemical techniques may be useful in the diagnosis of these unusual etiologies. Unfortunately, the methodology is not currently available at most hospitals. Broad-range PCR on surgical tissue help to identify more typical organisms (staphylococci and streptococci), whose growth may be suppressed by conventional antibiotic therapy. Although promising, PCR technology may lack specificity in these cases.
Case 3 A 61-year-old executive was admitted with a 4 week history of fevers and fatigue. Three months earlier he had undergone a bovine aortic valve replacement with mitral valve repair. Blood cultures drawn by a local physician grew methicillin-resistant Staphylococcus epidermidis (MRSE). Despite antibiotics, the patient’s fatigue persisted and he returned for further evaluation. On examination, he was afebrile, with a resting pulse of 71 beats/min and a blood pressure of 135/63mm Hg. However, he was very pale. Loud systolic and diastolic murmurs were heard throughout the precordium. His spleen was enlarged and very soft.
The patient underwent urgent reoperation. At surgery, partial aortic valve dehiscence as a result of a large subprosthetic abscess was discovered. Both aortic and mitral valve were replaced. Admission and operative cultures were negative on antibiotic therapy.
Comment: This is a classic presentation of early-onset prosthetic valve endocarditis. Usual organisms are S. epidermidis and S. aureus Streptococci, vancomycin-resistant enterococci (VRE), diphtheroids, gram-negative bacilli, and fungi (yeast and molds) are all seen in this setting, albeit less frequently.
S. epidermidis is of special interest because it produces hemolysins, grows very slowly on cell surfaces, and binds to host and foreign proteins. This biofilm creates a barrier to host defenses and appears to neutralize certain antibiotics. In addition there is clonal variability, with some isolates fully susceptible to oxacillin, while other clones are resistant.
Standard therapy for staphylococcal prosthetic valve endocarditis is oxacillin with gentamicin and rifampin. For oxacillin-resistant species, vancomycin is substituted. Prosthetic valve enterococcal endocarditis resistant to both penicillin and vancomycin is a growing concern. Some medical centers report VRE colonization rates as high as 30%. Therapy is daunting. For strains with a minimum inhibitory concentrations (MIC) less than 128 gr/mL to ampicillin, ampicillin/sulbactam plus an aminoglycoside has been recommended. For strains totally resistant to ampicillin, quinupristin/dalfopristin, linezolid, or daptomycin may be tried, but the overall success rate is probably no better than 50%.
Case 4 A 31-year-old automobile mechanic underwent aortic valve and graft replacement for severe aortic regurgitation with a large aneurysm of the ascending aorta. His post-operative course was complicated by massive bleeding at the distal graft anastomosis, and respiratory failure. After prolonged hospitalization, the patient was discharged improved, but 2 days later he complained of blurred vision and fevers. His wife noted a green hue from his right pupil. The patient was readmitted and started on intravenous acyclovir for presumed acute retinal necrosis. However, several days later, vitrectomy fluid grew Pseudoallescheria boydii.
Therapy was switched to intravenous miconazole but, shortly afterward, the patient suffered a cardiac arrest. Although his pulse and blood pressure were restored, he remained comatose and support was withdrawn. At autopsy, invasive prosthetic aortic valve and graft endocarditis was noted. Blood and tissue cultures also grew P. boydii.
Comment: Fungal prosthetic valve endocarditis is a devastating disease. Predisposing factors are prolonged use of central vascular catheters, often for antibiotic therapy or parenteral nutrition, and immunosuppression. Most success has been reported combining surgery with intravenous antifungal therapy. Patients should be continued on oral suppressive therapy afterward to prevent relapse later in life.
“Pacemaker endocarditis,” seen with increasing frequency, applies to pacemakers, defibrillators, or combinations thereof. Usual causes are skin flora microbes (staphylococci and Propionibacterium species) that gain access through a generator pocket wound. An echocardiogram may not show vegetations unless they extend to the tricuspid valve. Removal of all hardware, combined with intravenous antibiotic therapy, is necessary for cure. Some impacted leads require open heart surgery for removal.
Hospital-associated bacteremia from another source may spread to a heart valve or pacemaker lead, causing endocarditis. S. aureus bacteremia from intravenous catheters, hemodialysis fistula, and surgical wounds is most likely to do this. Patients on hemodialysis may be colonized with methicillin-resistant S. aureus (MRSA), a risk factor for infection. While intra-nasal mupirocin ointment may reduce MRSA colonization transiently, it is probably not effective for long-term prophylaxis.
Case 5 A 54-year-old accountant was admitted with chills and palpitations for several days. A bovine aortic valve prosthesis had been implanted 2 years earlier. The patient had complained of intermittent fevers for 6 months. A single blood culture had grown Propionibacterium acnes. Although a TEE was interpreted as normal, he was treated with intravenous vancomycin. Follow-up blood cultures were negative and a TTE was read as normal.
On examination, the patient was acutely ill with distended neck veins. His pulse was 50 beats/min and blood pressure 110/50mm Hg. Systolic and diastolic murmurs were present. Blood cultures were drawn, and antibiotics started.
An electrocardiogram showed heart block. A temporary pacemaker was placed. A TEE revealed a huge atrial septal abscess with a fistula from the right atrium to the aorta. The patient was taken emergently to surgery, where the prosthesis was found to have nearly completely dehisced. The fistula was resected and the aortic valve replaced with a homograft. Postoperatively the patient remained in cardiogenic shock and died. Admission blood and valve cultures subsequently grew P. acnes.
Comment and Conclusions
Continued fevers despite appropriate antibiotic and medical management are cause for alarm. Ring abscesses may develop. This is a clear indication for surgical intervention. Fevers may also be caused by embolic events (arterial or venous), drug reactions, and intravascular catheter-related infections. Close monitoring is necessary to avoid major events. Vigilance should be maintained for widening pulse pressures and rhythm disturbances as these are ominous signs of progressive infection.
Indications for urgent surgery include progressive valvular dysfunction; aortic root, ring or septal abscesses; large vegetations (greater than l cm in diameter); and organisms such as VRE, MRSA, Pseudomonas species, and fungi refractive to antimicrobial therapy. It is important to note that, even with appropriate therapy and a bacteriologic “cure,” about one half of patients will have enough valve damage to require surgery later in life.
Despite our best efforts, the death rate from infective endocarditis remains in the range of 10–20%. Death is more likely with prosthetic valve endocarditis and when the organism is S. aureus. Patients still succumb from congestive heart failure, embolic phenomenon, and ruptured mycotic aneurysms, just as they did during Osler’s time.
It is clear there is room for improvement in the diagnosis and management of endocarditis. First, we must continue to refine microbiologic techniques, to allow diagnosis more quickly and accurately. Second, we must develop more effective antimicrobial therapy, especially for pathogens resistant to conventional antimicrobials. Third, we must learn how to combat biofilms. Perhaps in the future we can avoid removal of foreign materials. Finally, we must follow our patients closely and pursue timely surgical intervention when indicated. In recent years this has become more difficult, because patients, once stabilized, are often discharged home or to a skilled nursing facility to complete antibiotic therapy.
While we have learned more about infective endocarditis over the past quarter century, the challenges we face today are greater than ever before.
References
- Osler W. Chronic infectious endocarditis. Q J Med. 1909;2: 219-30.
- Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-30.
- Durack DT, Lukes AS, Bright KD, et al: New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200-9.
- Salgado AV, Furlan AJ, Keys TF. Neurologic complications of endocarditis: a 12 year experience. Neurology. 1989;39:173-8.
- Wilson WR, Karchmer AW, Dajani AS, et al: Antibiotic treatment of adults with infective endocarditis due to streptococci, enterococci, staphylococci and HACEK micro organisms. JAMA. 1995;274:1706-13.
- Lepidi H, Houpikian P, Liang Z, Raoult D. Cardiac valves in patients with Q-fever endocarditis. J Infect Dis. 2003;187: 1097-106.
- Bosshard PP, Kronenberg A, Zbinden R, et al. Etiologic diagnosis of infective endocarditis by broad-range PCR. Clin Infect Dis. 2003;37:167-72.
- Keys TF. Early-onset prosthetic valve endocarditis. Cleve Clin J Med. 1993;60:455-9.
- Proctor RA. Coagulase-negative staphylococcal infection: a diagnostic and therapeutic challenge. Clin Infect Dis. 2000;31:31-3.
- Melgar GR, Nasser RM, Gordon SM, et al. Fungal prosthetic-valve endocarditis in 16 patients: an 11-year experience in a tertiary care hospital. Medicine. 1997;76:94-103.
- Fowler VG, Sanders LL, Kong LK, et al. Infective endocarditis due to Staphylococcus aureus. Clin Infect Dis. 1999;28:106-14.
- Douglas A, Moore-Gillon J, Eykyn S. Fever during treatment of infective endocarditis. Lancet. 1986;1:1341-3.
- Tornos MP, Permanyer-Miralda G, Olona J, et al. Long term complications of native valve endocarditis in non-addicts: a 15 year follow up study. Ann Intern Med. 1992;117:567-72.
- Andrews MM, Von Reyn CF. Patients election criteria and management guidelines for outpatient parenteral antibiotic therapy for native valve infective endocarditis. Clin Infect Dis. 2001;32:203-9.
Introduction
A 55-year-old heroin addict presented to the emergency department, complaining of shaking chills and fevers for 2 weeks. On examination, there was a loud holosystolic murmur, maximally audible in the epigastric space, and a pulsatile liver. Subcutaneous nodular lesions were noted on his palms. Blood cultures grew Pseudomonas aeruginosa. After nearly completing a prolonged course of intravenous antibiotic therapy, the patient died in his washroom from an overdose of heroin. This sad tale, often tragically repeated, represents a continuing challenge to the medical community. The patients’ palm lesions noted were
Osler’s nodes, originally described in 1908 by Sir William Osler, considered by many the father of internal medicine. Osler was born in 1849 and died in 1919. He was an astute clinician and educator, with professorships at McGill University, University of Pennsylvania, Johns Hopkins University, and Oxford University. Osler defined “chronic” infectious endocarditis as an illness lasting longer than 3 months and characterized by low grade fevers. This syndrome was distinct from a “malignant” form, which resulted in early death. Blood cultures usually grew streptococci or, occasionally, staphylococci. Osler made a practice of following his patients to the autopsy table. Vegetations on valves from patients who died of the chronic form looked more like “warts,” and were neither ”ulcerating or very large.” Osler thought anti-streptococcal vaccines might be of some benefit. There was little else to offer. Regardless of the form, nearly all patients died.
In this review, I discuss current methods for the diagnosis and management of infective endocarditis. Cases seen in recent years will illustrate key points.
Case 1 A 39-year-old computer programmer complained of occipital headaches, migratory muscle pains, afternoon fevers, and a 15-pound weight loss for 2 months. He had previously enjoyed excellent health. On examination his temperature was 38.0°C. An apical systolic heart murmur was noted. A transthoracic echocardiogram (TTE) showed mitral regurgitation, with a probable vegetation on the mitral valve. Blood cultures were drawn and the patient was admitted to the hospital. The next day, a transesophageal echocardiogram (TEE) showed perforation of the posterior mitral leaflet. That evening, the patient developed severe right flank pain. CT scan showed infarcts in the right kidney and spleen. The next day the patient underwent urgent mitral valve repair and was dismissed 5 days later to complete a course of intravenous ceftriaxone. All blood cultures grew viridans streptococci, exquisitely susceptible to penicillin.
Comment: This patient represents classic “subacute” bacterial endocarditis with fever, weight loss, and a heart murmur. In most cases, he would be cured with medical therapy alone. However, a TEE showed a lesion that was not appreciated on the initial TTE, and he required urgent surgery to restore a failing mitral valve.
Although the patient had no identified skin or mucosal lesions, when present these suggest the diagnosis. The subconjunctivial sacs and soft palate should be examined for petechiae, the nail beds for splinter hemorrhages, and digits for Janeway lesions.
Osler’s definition of endocarditis included remittent fever, history of valvular heart disease, embolic features, skin lesions, and heart failure. These remain useful bedside observations, and positive blood cultures usually clinch the diagnosis. Perhaps the most important technical advance in recent years for diagnosis is the echocardiogram. The major Duke criteria, published in 1994, include a predictable bacterial organism in blood culture, multiple positive blood cultures with the same organism, or an echocardiogram with definite vegetation, abscess, or valve dehiscence. Any two of the above suffice for diagnosis of probable endocarditis. Accepted minor criteria consist of predisposing lesions, history of intravenous drug abuse, temperature higher than 38°C, vasculitis, skin lesions, or “suggestive” echocardiographic or microbiologic findings. Five of these, or three with one major criterion, support the diagnosis. Transesophageal is superior to transthoracic echocardiography and should be performed if the TTE is equivocal or non-diagnostic.
Case 2 A 31-year-old warehouse manager with progressive dyspnea was transferred from an outside hospital. His illness began 8 months earlier with a dry cough and progressive fatigue. His past history was negative except for an asymptomatic heart murmur. On examination, he was pale and diaphoretic with a temperature of 36°C, pulse 110, and blood pressure of 108/56mm Hg. Neck veins were distended beats/min; loud heart murmurs and diffuse airway crackles were heard. The spleen was palpable. Blood cultures were drawn and antibiotics started.
As the patient was being wheeled for urgent heart surgery, he suffered a huge left-sided stroke. Contrast studies showed a leaking basilar artery aneurysm with subarachnoid hemorrhage. Once his neurologic problem stabilized, urgent mitral and aortic valve replacement was performed. Both valves were severely damaged and rife with vegetations. Admission blood cultures grew viridans streptococci, susceptible to penicillin. After prolonged hospitalization, the patient was transferred for continued care to a rehabilitation unit closer to home.
Comment: Neurologic complications of endocarditis are more common than generally appreciated, and occur in at least one third of patients at the time of diagnosis. Stroke is the most frequent finding, but encephalopathy, retinal embolic lesions, mycotic aneurysm, brain abscess, and meningitis can also occur. Fortunately, most neurologic problems resolve with medical management, but as seen in this patient, some are devastating and have permanent sequelae.
Organisms responsible for the majority of cases of native valve endocarditis are streptococci, as was true in Osler’s time. Staphylococcus aureus is next in frequency, followed by gram-negative bacilli, fungi, coagulase-negative staphylococci, and a poorly-defined category of “culture negative” cases. Therapy for infection caused by penicillin-susceptible streptococci is straightforward. The preferred agent is intravenous penicillin or ampicillin, with ceftraxione or vancomycin as alternatives. Streptococci less susceptible to penicillin, including nutritionally variant organisms, are treated more vigorously with a penicillin and low-dose aminoglycoside.
The HACEK group of gram-negative bacteria (Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella species) often produce large vegetations. Embolic lesions to major organs or extremities are a fairly common presenting feature. Treatment with ceftriaxone or ampicillin plus gentamicin is usually successful.
“Culture negative” endocarditis includes infections due to microorganisms difficult to culture on standard media. These uncommon pathogens include Bartonella, Brucella, Chlamydia, Coxiella, Francisella, Legionella, and Tropheryma whippeli.
Bartonella endocarditis has been reported in the homeless population. Blood cultures are usually negative. Serology is helpful. More recently, polymerase drain reactions (PCR) from resected valve tissue have proven useful. Treatment of choice is ampicillin plus gentamicin, but mortality remains approximately 25%.
Patients with endocarditis due to Coxiella burnetii (Q fever) are likewise difficult to diagnose. They may not have fever. However, there is generally underlying valvular heart disease, and frequently patients are immunosuppressed. Vegetations are rarely detected on echocardiogram. Routine blood cultures are negative. Fortunately, serology is quite specific for the diagnosis. A combination of doxycycline and chloroquine is the current treatment of choice.
PCR and special immunohistochemical techniques may be useful in the diagnosis of these unusual etiologies. Unfortunately, the methodology is not currently available at most hospitals. Broad-range PCR on surgical tissue help to identify more typical organisms (staphylococci and streptococci), whose growth may be suppressed by conventional antibiotic therapy. Although promising, PCR technology may lack specificity in these cases.
Case 3 A 61-year-old executive was admitted with a 4 week history of fevers and fatigue. Three months earlier he had undergone a bovine aortic valve replacement with mitral valve repair. Blood cultures drawn by a local physician grew methicillin-resistant Staphylococcus epidermidis (MRSE). Despite antibiotics, the patient’s fatigue persisted and he returned for further evaluation. On examination, he was afebrile, with a resting pulse of 71 beats/min and a blood pressure of 135/63mm Hg. However, he was very pale. Loud systolic and diastolic murmurs were heard throughout the precordium. His spleen was enlarged and very soft.
The patient underwent urgent reoperation. At surgery, partial aortic valve dehiscence as a result of a large subprosthetic abscess was discovered. Both aortic and mitral valve were replaced. Admission and operative cultures were negative on antibiotic therapy.
Comment: This is a classic presentation of early-onset prosthetic valve endocarditis. Usual organisms are S. epidermidis and S. aureus Streptococci, vancomycin-resistant enterococci (VRE), diphtheroids, gram-negative bacilli, and fungi (yeast and molds) are all seen in this setting, albeit less frequently.
S. epidermidis is of special interest because it produces hemolysins, grows very slowly on cell surfaces, and binds to host and foreign proteins. This biofilm creates a barrier to host defenses and appears to neutralize certain antibiotics. In addition there is clonal variability, with some isolates fully susceptible to oxacillin, while other clones are resistant.
Standard therapy for staphylococcal prosthetic valve endocarditis is oxacillin with gentamicin and rifampin. For oxacillin-resistant species, vancomycin is substituted. Prosthetic valve enterococcal endocarditis resistant to both penicillin and vancomycin is a growing concern. Some medical centers report VRE colonization rates as high as 30%. Therapy is daunting. For strains with a minimum inhibitory concentrations (MIC) less than 128 gr/mL to ampicillin, ampicillin/sulbactam plus an aminoglycoside has been recommended. For strains totally resistant to ampicillin, quinupristin/dalfopristin, linezolid, or daptomycin may be tried, but the overall success rate is probably no better than 50%.
Case 4 A 31-year-old automobile mechanic underwent aortic valve and graft replacement for severe aortic regurgitation with a large aneurysm of the ascending aorta. His post-operative course was complicated by massive bleeding at the distal graft anastomosis, and respiratory failure. After prolonged hospitalization, the patient was discharged improved, but 2 days later he complained of blurred vision and fevers. His wife noted a green hue from his right pupil. The patient was readmitted and started on intravenous acyclovir for presumed acute retinal necrosis. However, several days later, vitrectomy fluid grew Pseudoallescheria boydii.
Therapy was switched to intravenous miconazole but, shortly afterward, the patient suffered a cardiac arrest. Although his pulse and blood pressure were restored, he remained comatose and support was withdrawn. At autopsy, invasive prosthetic aortic valve and graft endocarditis was noted. Blood and tissue cultures also grew P. boydii.
Comment: Fungal prosthetic valve endocarditis is a devastating disease. Predisposing factors are prolonged use of central vascular catheters, often for antibiotic therapy or parenteral nutrition, and immunosuppression. Most success has been reported combining surgery with intravenous antifungal therapy. Patients should be continued on oral suppressive therapy afterward to prevent relapse later in life.
“Pacemaker endocarditis,” seen with increasing frequency, applies to pacemakers, defibrillators, or combinations thereof. Usual causes are skin flora microbes (staphylococci and Propionibacterium species) that gain access through a generator pocket wound. An echocardiogram may not show vegetations unless they extend to the tricuspid valve. Removal of all hardware, combined with intravenous antibiotic therapy, is necessary for cure. Some impacted leads require open heart surgery for removal.
Hospital-associated bacteremia from another source may spread to a heart valve or pacemaker lead, causing endocarditis. S. aureus bacteremia from intravenous catheters, hemodialysis fistula, and surgical wounds is most likely to do this. Patients on hemodialysis may be colonized with methicillin-resistant S. aureus (MRSA), a risk factor for infection. While intra-nasal mupirocin ointment may reduce MRSA colonization transiently, it is probably not effective for long-term prophylaxis.
Case 5 A 54-year-old accountant was admitted with chills and palpitations for several days. A bovine aortic valve prosthesis had been implanted 2 years earlier. The patient had complained of intermittent fevers for 6 months. A single blood culture had grown Propionibacterium acnes. Although a TEE was interpreted as normal, he was treated with intravenous vancomycin. Follow-up blood cultures were negative and a TTE was read as normal.
On examination, the patient was acutely ill with distended neck veins. His pulse was 50 beats/min and blood pressure 110/50mm Hg. Systolic and diastolic murmurs were present. Blood cultures were drawn, and antibiotics started.
An electrocardiogram showed heart block. A temporary pacemaker was placed. A TEE revealed a huge atrial septal abscess with a fistula from the right atrium to the aorta. The patient was taken emergently to surgery, where the prosthesis was found to have nearly completely dehisced. The fistula was resected and the aortic valve replaced with a homograft. Postoperatively the patient remained in cardiogenic shock and died. Admission blood and valve cultures subsequently grew P. acnes.
Comment and Conclusions
Continued fevers despite appropriate antibiotic and medical management are cause for alarm. Ring abscesses may develop. This is a clear indication for surgical intervention. Fevers may also be caused by embolic events (arterial or venous), drug reactions, and intravascular catheter-related infections. Close monitoring is necessary to avoid major events. Vigilance should be maintained for widening pulse pressures and rhythm disturbances as these are ominous signs of progressive infection.
Indications for urgent surgery include progressive valvular dysfunction; aortic root, ring or septal abscesses; large vegetations (greater than l cm in diameter); and organisms such as VRE, MRSA, Pseudomonas species, and fungi refractive to antimicrobial therapy. It is important to note that, even with appropriate therapy and a bacteriologic “cure,” about one half of patients will have enough valve damage to require surgery later in life.
Despite our best efforts, the death rate from infective endocarditis remains in the range of 10–20%. Death is more likely with prosthetic valve endocarditis and when the organism is S. aureus. Patients still succumb from congestive heart failure, embolic phenomenon, and ruptured mycotic aneurysms, just as they did during Osler’s time.
It is clear there is room for improvement in the diagnosis and management of endocarditis. First, we must continue to refine microbiologic techniques, to allow diagnosis more quickly and accurately. Second, we must develop more effective antimicrobial therapy, especially for pathogens resistant to conventional antimicrobials. Third, we must learn how to combat biofilms. Perhaps in the future we can avoid removal of foreign materials. Finally, we must follow our patients closely and pursue timely surgical intervention when indicated. In recent years this has become more difficult, because patients, once stabilized, are often discharged home or to a skilled nursing facility to complete antibiotic therapy.
While we have learned more about infective endocarditis over the past quarter century, the challenges we face today are greater than ever before.
References
- Osler W. Chronic infectious endocarditis. Q J Med. 1909;2: 219-30.
- Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-30.
- Durack DT, Lukes AS, Bright KD, et al: New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200-9.
- Salgado AV, Furlan AJ, Keys TF. Neurologic complications of endocarditis: a 12 year experience. Neurology. 1989;39:173-8.
- Wilson WR, Karchmer AW, Dajani AS, et al: Antibiotic treatment of adults with infective endocarditis due to streptococci, enterococci, staphylococci and HACEK micro organisms. JAMA. 1995;274:1706-13.
- Lepidi H, Houpikian P, Liang Z, Raoult D. Cardiac valves in patients with Q-fever endocarditis. J Infect Dis. 2003;187: 1097-106.
- Bosshard PP, Kronenberg A, Zbinden R, et al. Etiologic diagnosis of infective endocarditis by broad-range PCR. Clin Infect Dis. 2003;37:167-72.
- Keys TF. Early-onset prosthetic valve endocarditis. Cleve Clin J Med. 1993;60:455-9.
- Proctor RA. Coagulase-negative staphylococcal infection: a diagnostic and therapeutic challenge. Clin Infect Dis. 2000;31:31-3.
- Melgar GR, Nasser RM, Gordon SM, et al. Fungal prosthetic-valve endocarditis in 16 patients: an 11-year experience in a tertiary care hospital. Medicine. 1997;76:94-103.
- Fowler VG, Sanders LL, Kong LK, et al. Infective endocarditis due to Staphylococcus aureus. Clin Infect Dis. 1999;28:106-14.
- Douglas A, Moore-Gillon J, Eykyn S. Fever during treatment of infective endocarditis. Lancet. 1986;1:1341-3.
- Tornos MP, Permanyer-Miralda G, Olona J, et al. Long term complications of native valve endocarditis in non-addicts: a 15 year follow up study. Ann Intern Med. 1992;117:567-72.
- Andrews MM, Von Reyn CF. Patients election criteria and management guidelines for outpatient parenteral antibiotic therapy for native valve infective endocarditis. Clin Infect Dis. 2001;32:203-9.
Introduction
A 55-year-old heroin addict presented to the emergency department, complaining of shaking chills and fevers for 2 weeks. On examination, there was a loud holosystolic murmur, maximally audible in the epigastric space, and a pulsatile liver. Subcutaneous nodular lesions were noted on his palms. Blood cultures grew Pseudomonas aeruginosa. After nearly completing a prolonged course of intravenous antibiotic therapy, the patient died in his washroom from an overdose of heroin. This sad tale, often tragically repeated, represents a continuing challenge to the medical community. The patients’ palm lesions noted were
Osler’s nodes, originally described in 1908 by Sir William Osler, considered by many the father of internal medicine. Osler was born in 1849 and died in 1919. He was an astute clinician and educator, with professorships at McGill University, University of Pennsylvania, Johns Hopkins University, and Oxford University. Osler defined “chronic” infectious endocarditis as an illness lasting longer than 3 months and characterized by low grade fevers. This syndrome was distinct from a “malignant” form, which resulted in early death. Blood cultures usually grew streptococci or, occasionally, staphylococci. Osler made a practice of following his patients to the autopsy table. Vegetations on valves from patients who died of the chronic form looked more like “warts,” and were neither ”ulcerating or very large.” Osler thought anti-streptococcal vaccines might be of some benefit. There was little else to offer. Regardless of the form, nearly all patients died.
In this review, I discuss current methods for the diagnosis and management of infective endocarditis. Cases seen in recent years will illustrate key points.
Case 1 A 39-year-old computer programmer complained of occipital headaches, migratory muscle pains, afternoon fevers, and a 15-pound weight loss for 2 months. He had previously enjoyed excellent health. On examination his temperature was 38.0°C. An apical systolic heart murmur was noted. A transthoracic echocardiogram (TTE) showed mitral regurgitation, with a probable vegetation on the mitral valve. Blood cultures were drawn and the patient was admitted to the hospital. The next day, a transesophageal echocardiogram (TEE) showed perforation of the posterior mitral leaflet. That evening, the patient developed severe right flank pain. CT scan showed infarcts in the right kidney and spleen. The next day the patient underwent urgent mitral valve repair and was dismissed 5 days later to complete a course of intravenous ceftriaxone. All blood cultures grew viridans streptococci, exquisitely susceptible to penicillin.
Comment: This patient represents classic “subacute” bacterial endocarditis with fever, weight loss, and a heart murmur. In most cases, he would be cured with medical therapy alone. However, a TEE showed a lesion that was not appreciated on the initial TTE, and he required urgent surgery to restore a failing mitral valve.
Although the patient had no identified skin or mucosal lesions, when present these suggest the diagnosis. The subconjunctivial sacs and soft palate should be examined for petechiae, the nail beds for splinter hemorrhages, and digits for Janeway lesions.
Osler’s definition of endocarditis included remittent fever, history of valvular heart disease, embolic features, skin lesions, and heart failure. These remain useful bedside observations, and positive blood cultures usually clinch the diagnosis. Perhaps the most important technical advance in recent years for diagnosis is the echocardiogram. The major Duke criteria, published in 1994, include a predictable bacterial organism in blood culture, multiple positive blood cultures with the same organism, or an echocardiogram with definite vegetation, abscess, or valve dehiscence. Any two of the above suffice for diagnosis of probable endocarditis. Accepted minor criteria consist of predisposing lesions, history of intravenous drug abuse, temperature higher than 38°C, vasculitis, skin lesions, or “suggestive” echocardiographic or microbiologic findings. Five of these, or three with one major criterion, support the diagnosis. Transesophageal is superior to transthoracic echocardiography and should be performed if the TTE is equivocal or non-diagnostic.
Case 2 A 31-year-old warehouse manager with progressive dyspnea was transferred from an outside hospital. His illness began 8 months earlier with a dry cough and progressive fatigue. His past history was negative except for an asymptomatic heart murmur. On examination, he was pale and diaphoretic with a temperature of 36°C, pulse 110, and blood pressure of 108/56mm Hg. Neck veins were distended beats/min; loud heart murmurs and diffuse airway crackles were heard. The spleen was palpable. Blood cultures were drawn and antibiotics started.
As the patient was being wheeled for urgent heart surgery, he suffered a huge left-sided stroke. Contrast studies showed a leaking basilar artery aneurysm with subarachnoid hemorrhage. Once his neurologic problem stabilized, urgent mitral and aortic valve replacement was performed. Both valves were severely damaged and rife with vegetations. Admission blood cultures grew viridans streptococci, susceptible to penicillin. After prolonged hospitalization, the patient was transferred for continued care to a rehabilitation unit closer to home.
Comment: Neurologic complications of endocarditis are more common than generally appreciated, and occur in at least one third of patients at the time of diagnosis. Stroke is the most frequent finding, but encephalopathy, retinal embolic lesions, mycotic aneurysm, brain abscess, and meningitis can also occur. Fortunately, most neurologic problems resolve with medical management, but as seen in this patient, some are devastating and have permanent sequelae.
Organisms responsible for the majority of cases of native valve endocarditis are streptococci, as was true in Osler’s time. Staphylococcus aureus is next in frequency, followed by gram-negative bacilli, fungi, coagulase-negative staphylococci, and a poorly-defined category of “culture negative” cases. Therapy for infection caused by penicillin-susceptible streptococci is straightforward. The preferred agent is intravenous penicillin or ampicillin, with ceftraxione or vancomycin as alternatives. Streptococci less susceptible to penicillin, including nutritionally variant organisms, are treated more vigorously with a penicillin and low-dose aminoglycoside.
The HACEK group of gram-negative bacteria (Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella species) often produce large vegetations. Embolic lesions to major organs or extremities are a fairly common presenting feature. Treatment with ceftriaxone or ampicillin plus gentamicin is usually successful.
“Culture negative” endocarditis includes infections due to microorganisms difficult to culture on standard media. These uncommon pathogens include Bartonella, Brucella, Chlamydia, Coxiella, Francisella, Legionella, and Tropheryma whippeli.
Bartonella endocarditis has been reported in the homeless population. Blood cultures are usually negative. Serology is helpful. More recently, polymerase drain reactions (PCR) from resected valve tissue have proven useful. Treatment of choice is ampicillin plus gentamicin, but mortality remains approximately 25%.
Patients with endocarditis due to Coxiella burnetii (Q fever) are likewise difficult to diagnose. They may not have fever. However, there is generally underlying valvular heart disease, and frequently patients are immunosuppressed. Vegetations are rarely detected on echocardiogram. Routine blood cultures are negative. Fortunately, serology is quite specific for the diagnosis. A combination of doxycycline and chloroquine is the current treatment of choice.
PCR and special immunohistochemical techniques may be useful in the diagnosis of these unusual etiologies. Unfortunately, the methodology is not currently available at most hospitals. Broad-range PCR on surgical tissue help to identify more typical organisms (staphylococci and streptococci), whose growth may be suppressed by conventional antibiotic therapy. Although promising, PCR technology may lack specificity in these cases.
Case 3 A 61-year-old executive was admitted with a 4 week history of fevers and fatigue. Three months earlier he had undergone a bovine aortic valve replacement with mitral valve repair. Blood cultures drawn by a local physician grew methicillin-resistant Staphylococcus epidermidis (MRSE). Despite antibiotics, the patient’s fatigue persisted and he returned for further evaluation. On examination, he was afebrile, with a resting pulse of 71 beats/min and a blood pressure of 135/63mm Hg. However, he was very pale. Loud systolic and diastolic murmurs were heard throughout the precordium. His spleen was enlarged and very soft.
The patient underwent urgent reoperation. At surgery, partial aortic valve dehiscence as a result of a large subprosthetic abscess was discovered. Both aortic and mitral valve were replaced. Admission and operative cultures were negative on antibiotic therapy.
Comment: This is a classic presentation of early-onset prosthetic valve endocarditis. Usual organisms are S. epidermidis and S. aureus Streptococci, vancomycin-resistant enterococci (VRE), diphtheroids, gram-negative bacilli, and fungi (yeast and molds) are all seen in this setting, albeit less frequently.
S. epidermidis is of special interest because it produces hemolysins, grows very slowly on cell surfaces, and binds to host and foreign proteins. This biofilm creates a barrier to host defenses and appears to neutralize certain antibiotics. In addition there is clonal variability, with some isolates fully susceptible to oxacillin, while other clones are resistant.
Standard therapy for staphylococcal prosthetic valve endocarditis is oxacillin with gentamicin and rifampin. For oxacillin-resistant species, vancomycin is substituted. Prosthetic valve enterococcal endocarditis resistant to both penicillin and vancomycin is a growing concern. Some medical centers report VRE colonization rates as high as 30%. Therapy is daunting. For strains with a minimum inhibitory concentrations (MIC) less than 128 gr/mL to ampicillin, ampicillin/sulbactam plus an aminoglycoside has been recommended. For strains totally resistant to ampicillin, quinupristin/dalfopristin, linezolid, or daptomycin may be tried, but the overall success rate is probably no better than 50%.
Case 4 A 31-year-old automobile mechanic underwent aortic valve and graft replacement for severe aortic regurgitation with a large aneurysm of the ascending aorta. His post-operative course was complicated by massive bleeding at the distal graft anastomosis, and respiratory failure. After prolonged hospitalization, the patient was discharged improved, but 2 days later he complained of blurred vision and fevers. His wife noted a green hue from his right pupil. The patient was readmitted and started on intravenous acyclovir for presumed acute retinal necrosis. However, several days later, vitrectomy fluid grew Pseudoallescheria boydii.
Therapy was switched to intravenous miconazole but, shortly afterward, the patient suffered a cardiac arrest. Although his pulse and blood pressure were restored, he remained comatose and support was withdrawn. At autopsy, invasive prosthetic aortic valve and graft endocarditis was noted. Blood and tissue cultures also grew P. boydii.
Comment: Fungal prosthetic valve endocarditis is a devastating disease. Predisposing factors are prolonged use of central vascular catheters, often for antibiotic therapy or parenteral nutrition, and immunosuppression. Most success has been reported combining surgery with intravenous antifungal therapy. Patients should be continued on oral suppressive therapy afterward to prevent relapse later in life.
“Pacemaker endocarditis,” seen with increasing frequency, applies to pacemakers, defibrillators, or combinations thereof. Usual causes are skin flora microbes (staphylococci and Propionibacterium species) that gain access through a generator pocket wound. An echocardiogram may not show vegetations unless they extend to the tricuspid valve. Removal of all hardware, combined with intravenous antibiotic therapy, is necessary for cure. Some impacted leads require open heart surgery for removal.
Hospital-associated bacteremia from another source may spread to a heart valve or pacemaker lead, causing endocarditis. S. aureus bacteremia from intravenous catheters, hemodialysis fistula, and surgical wounds is most likely to do this. Patients on hemodialysis may be colonized with methicillin-resistant S. aureus (MRSA), a risk factor for infection. While intra-nasal mupirocin ointment may reduce MRSA colonization transiently, it is probably not effective for long-term prophylaxis.
Case 5 A 54-year-old accountant was admitted with chills and palpitations for several days. A bovine aortic valve prosthesis had been implanted 2 years earlier. The patient had complained of intermittent fevers for 6 months. A single blood culture had grown Propionibacterium acnes. Although a TEE was interpreted as normal, he was treated with intravenous vancomycin. Follow-up blood cultures were negative and a TTE was read as normal.
On examination, the patient was acutely ill with distended neck veins. His pulse was 50 beats/min and blood pressure 110/50mm Hg. Systolic and diastolic murmurs were present. Blood cultures were drawn, and antibiotics started.
An electrocardiogram showed heart block. A temporary pacemaker was placed. A TEE revealed a huge atrial septal abscess with a fistula from the right atrium to the aorta. The patient was taken emergently to surgery, where the prosthesis was found to have nearly completely dehisced. The fistula was resected and the aortic valve replaced with a homograft. Postoperatively the patient remained in cardiogenic shock and died. Admission blood and valve cultures subsequently grew P. acnes.
Comment and Conclusions
Continued fevers despite appropriate antibiotic and medical management are cause for alarm. Ring abscesses may develop. This is a clear indication for surgical intervention. Fevers may also be caused by embolic events (arterial or venous), drug reactions, and intravascular catheter-related infections. Close monitoring is necessary to avoid major events. Vigilance should be maintained for widening pulse pressures and rhythm disturbances as these are ominous signs of progressive infection.
Indications for urgent surgery include progressive valvular dysfunction; aortic root, ring or septal abscesses; large vegetations (greater than l cm in diameter); and organisms such as VRE, MRSA, Pseudomonas species, and fungi refractive to antimicrobial therapy. It is important to note that, even with appropriate therapy and a bacteriologic “cure,” about one half of patients will have enough valve damage to require surgery later in life.
Despite our best efforts, the death rate from infective endocarditis remains in the range of 10–20%. Death is more likely with prosthetic valve endocarditis and when the organism is S. aureus. Patients still succumb from congestive heart failure, embolic phenomenon, and ruptured mycotic aneurysms, just as they did during Osler’s time.
It is clear there is room for improvement in the diagnosis and management of endocarditis. First, we must continue to refine microbiologic techniques, to allow diagnosis more quickly and accurately. Second, we must develop more effective antimicrobial therapy, especially for pathogens resistant to conventional antimicrobials. Third, we must learn how to combat biofilms. Perhaps in the future we can avoid removal of foreign materials. Finally, we must follow our patients closely and pursue timely surgical intervention when indicated. In recent years this has become more difficult, because patients, once stabilized, are often discharged home or to a skilled nursing facility to complete antibiotic therapy.
While we have learned more about infective endocarditis over the past quarter century, the challenges we face today are greater than ever before.
References
- Osler W. Chronic infectious endocarditis. Q J Med. 1909;2: 219-30.
- Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-30.
- Durack DT, Lukes AS, Bright KD, et al: New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200-9.
- Salgado AV, Furlan AJ, Keys TF. Neurologic complications of endocarditis: a 12 year experience. Neurology. 1989;39:173-8.
- Wilson WR, Karchmer AW, Dajani AS, et al: Antibiotic treatment of adults with infective endocarditis due to streptococci, enterococci, staphylococci and HACEK micro organisms. JAMA. 1995;274:1706-13.
- Lepidi H, Houpikian P, Liang Z, Raoult D. Cardiac valves in patients with Q-fever endocarditis. J Infect Dis. 2003;187: 1097-106.
- Bosshard PP, Kronenberg A, Zbinden R, et al. Etiologic diagnosis of infective endocarditis by broad-range PCR. Clin Infect Dis. 2003;37:167-72.
- Keys TF. Early-onset prosthetic valve endocarditis. Cleve Clin J Med. 1993;60:455-9.
- Proctor RA. Coagulase-negative staphylococcal infection: a diagnostic and therapeutic challenge. Clin Infect Dis. 2000;31:31-3.
- Melgar GR, Nasser RM, Gordon SM, et al. Fungal prosthetic-valve endocarditis in 16 patients: an 11-year experience in a tertiary care hospital. Medicine. 1997;76:94-103.
- Fowler VG, Sanders LL, Kong LK, et al. Infective endocarditis due to Staphylococcus aureus. Clin Infect Dis. 1999;28:106-14.
- Douglas A, Moore-Gillon J, Eykyn S. Fever during treatment of infective endocarditis. Lancet. 1986;1:1341-3.
- Tornos MP, Permanyer-Miralda G, Olona J, et al. Long term complications of native valve endocarditis in non-addicts: a 15 year follow up study. Ann Intern Med. 1992;117:567-72.
- Andrews MM, Von Reyn CF. Patients election criteria and management guidelines for outpatient parenteral antibiotic therapy for native valve infective endocarditis. Clin Infect Dis. 2001;32:203-9.
Preventing Surgical Site Infections
Background
An appropriately feared complication of operations, surgical site infections (SSIs) are infections associated with high economic costs and significantly worse clinical outcomes (1). Defined as infections of the superficial incision site, deep incision space, or organ space, SSIs add additional cost ranging from $2,700 to $26,000 per episode according to CDC’s National Nosocomial Infections Surveillance System. Patients who develop an SSI have hospital lengths of stay (LOS) in excess of 7 days longer and are 60% more likely to spend time in the intensive care unit than are patients without an SSI. A patient with an SSI is five times more likely to be readmitted to the hospital and is twice as likely to die (2).
Unfortunately, surgical site infections are common. Among healthcare-acquired infections, SSIs rank second only to urinary tract infections in frequency, making them more common than bloodstream infections and nosocomial pneumonia (3). There are approximately 30 million operations annually in the United States and an SSI complicates 2–5% of clean extra-abdominal sites. The rate is much higher for intra-abdominal operations, approaching 20% (1). Because most SSIs begin within 2 hours of contamination, the perioperative period is the most crucial for development of an SSI (4). By offering clinical expertise in the practice guidelines that reduce the risk of SSIs, hospital medicine programs can help patients and hospital systems lower morbidity, mortality, and costs associated with this complication. Adherence to best practices will likely require coordinated, multidisciplinary process improvement.
Several important interventions fall directly under the control of the anesthesia and surgical teams, such as administering perioperative oxygen, ensuring perioperative normothermia, and avoiding shaving of the surgical site. In coordinated quality improvement efforts, members of the operative team should assume direct responsibility for the performance of these measures. But the performance of two important interventions in this decisive period is likely to be significantly enhanced by the presence of focused hospitalist surgical co-management: antimicrobial prophylaxis and perioperative glycemic control (Table 1).
Antimicrobial Prophylaxis
Studies overwhelmingly show a marked reduction in the relative risk of SSIs with the use of antibiotic prophylaxis (1). In June 2004, the National Surgical Infection Prevention Project (NSIPP) published an advisory statement on antimicrobial prophylaxis in which it outlined three performance measures for quality improvement in prevention of SSIs:
- The proportion of patients who have parenteral antimicrobial prophylaxis initiated within 1 hour before surgical incision
- The proportion of patients provided with a prophylactic antimicrobial agent that is consistent with currently published guidelines, and
- The proportion of patients whose prophylactic antimicrobial therapy is discontinued within 24 hours after the end of surgery (5)
Pooled data suggest that attention to timing makes a favorable difference in SSI rates (1). Fully administering the appropriate antibiotic within 60 minutes of incision ensures that serum and tissue drug levels exceed the MICs of the most likely contaminating organisms. Dosing the antibiotic immediately prior to the start of surgery also provides the best opportunity to extend therapeutic levels for the duration of the surgery. The fact that anesthesia and surgical teams are in the most practical time-space positions to apply this measure underscores the multi-disciplinary and process-level efforts necessary to reduce SSI rates.
When it comes to the choice of antimicrobial and the duration of its use, hospitalists may find themselves in superior positions of impact. Familiarity with recommendations of the NSIPP advisory statement (summarized in Table 2) promotes evidence-based selection of antibiotic prophylaxis based on patient-specific factors: type of operation and presence of true drug allergies (5). Compared with other members of the surgical co-management team, hospitalists are more likely to be aware of relevant patient-specific risk factors such as the likelihood of colonization with methicillin-resistant Staphylococcus aureus (MRSA). For example, in patients colonized with MRSA, hospitalists might consider vancomycin as the alternative agent for prophylaxis. Free access to the NSIPP advisory statement is available at www.journals.uchicago.edu/CID/journal/issues/v38n12/33257/33257.html.
Antimicrobial prophylaxis after wound closure is unnecessary; published evidence demonstrates the non-inferiority of single dose prophylaxis when compared with multiple dose prophylaxis (5). Further, prolonged use of antimicrobial prophylaxis is associated with the emergence of resistant organisms (6-8). By ensuring that the duration of prophylaxis does not exceed 24 hours past the end of the operation, hospitalists can make valuable contributions to public health and cost containment.
Non-Antimicrobial Measures
Several non-antimicrobial measures also significantly reduce SSI rates. Those that fall outside the domain of the hospitalist and into the direct purview of the operative team include high levels of inspired oxygen, maintenance of perioperative normothermia, and use of clippers rather than a razor when hair removal is necessary. The risk of SSIs is directly related to tissue oxygenation. Bacterial infectivity is enhanced and cellular immunity is compromised in hypoperfused, poorly oxygenated tissue (9). The practice of administering perioperative supplemental oxygen (at least 80% FIO2 in intubated patients) reduces the risk of SSI by nearly one-half (1). For non-intubated patients, oxygen at 12 L/min by non-rebreathing face mask applied intra-operatively and for at least 2 hours following surgery leads to similar reductions of SSI rates. Besides being effective, this intervention is inexpensive, has no recognized adverse effects, and carries the added benefit of significantly reducing post-operative nausea and vomiting (4).
Hypothermia also predisposes the surgical wound to infection. Even mild perioperative hypothermia (i.e., core temperature 35-36.5°C) typically occurs in the absence of specific measures to prevent net heat loss. Perioperative hypothermia is the combined result of exposure and anesthetic-induced thermo-dysregulation, with redistribution of core body heat to the periphery (4). Even mild hypothermia causes vasoconstriction which diminishes perfusion, dropping tissue oxygen tension which impairs phagocytosis and oxidative killing by neutrophils (10). Hypothermia also blunts scar formation which further diminishes wound integrity. Active warming of the patient to maintain a core temperature near 36.5°C constitutes the intra-operative standard of care and is effective at reducing the risk of SSIs by as much as two-thirds (1).
Hyperglycemia, an established independent risk factor for an array of adverse outcomes in hospitalized patients, is also an independent risk factor for SSIs across a range of surgical patients (1). Short-term hyperglycemia depresses immune function through nonenzymatic glycosylation of immunoglobulin and by impairing normal leukocyte performance (11). Among diabetic cardiac surgery patients, reduction of hyperglycemia with an intravenous insulin infusion lowered the incidence of deep sternal wound infection by as much as two-thirds (12). While the value of achieving glycemic targets has already been established for a variety of important endpoints and across a range of inpatient populations, hospitalists should stay tuned. As high quality studies emerge proving that glycemic control lowers SSIs among non-cardiac surgical subpopulations, hospitalists may increasingly be relied upon to achieve strict glycemic targets.
By recognizing and coordinating practices known to reduce SSIs, hospitalists can elevate the level of care provided for surgical patients. At the same time, hospitalists can help lower costs and keep the hospital system mindful of public health goals, such as prevention of antimicrobial resistance. While individual hospitalists have key roles to play, the overall approach to SSI reduction calls for a coordinated, multidisciplinary team approach with process and system-level efforts.
Dr. Stein can be contacted at [email protected].
References
- Auerbach AD. Prevention of surgical site infections. In: Shojania KG, Duncan BW, McDonald KM, et al., eds. Making health care safer: a critical analysis of patient safety practices. Evidence report/technology assessment no. 43. AHRQ publication no. 01-E058. Rockville, MD: Agency for Healthcare Research and Quality, 20 July 2001:221-44.
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-30.
- National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996: a report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996;24:380-8.
- Sessler DI, Akca O. Nonpharmacologic prevention of surgical wound infections. Clin Infect Dis. 2002;35:1397 404.
- Bratzler D, Houck PM. Surgical Infection Prevention Guidelines Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;Jun15;38(12):1706-15. E-pub 2004 May 26.
- Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916-21.
- Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059-93.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972-8.
- Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997-1005.
- Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-15.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;Mar-Apr;10Suppl2:4-9.
- Furnary AP, Zerr K, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures [with discussion]. Ann Thorac Surg. 1999;67:352-62.
Background
An appropriately feared complication of operations, surgical site infections (SSIs) are infections associated with high economic costs and significantly worse clinical outcomes (1). Defined as infections of the superficial incision site, deep incision space, or organ space, SSIs add additional cost ranging from $2,700 to $26,000 per episode according to CDC’s National Nosocomial Infections Surveillance System. Patients who develop an SSI have hospital lengths of stay (LOS) in excess of 7 days longer and are 60% more likely to spend time in the intensive care unit than are patients without an SSI. A patient with an SSI is five times more likely to be readmitted to the hospital and is twice as likely to die (2).
Unfortunately, surgical site infections are common. Among healthcare-acquired infections, SSIs rank second only to urinary tract infections in frequency, making them more common than bloodstream infections and nosocomial pneumonia (3). There are approximately 30 million operations annually in the United States and an SSI complicates 2–5% of clean extra-abdominal sites. The rate is much higher for intra-abdominal operations, approaching 20% (1). Because most SSIs begin within 2 hours of contamination, the perioperative period is the most crucial for development of an SSI (4). By offering clinical expertise in the practice guidelines that reduce the risk of SSIs, hospital medicine programs can help patients and hospital systems lower morbidity, mortality, and costs associated with this complication. Adherence to best practices will likely require coordinated, multidisciplinary process improvement.
Several important interventions fall directly under the control of the anesthesia and surgical teams, such as administering perioperative oxygen, ensuring perioperative normothermia, and avoiding shaving of the surgical site. In coordinated quality improvement efforts, members of the operative team should assume direct responsibility for the performance of these measures. But the performance of two important interventions in this decisive period is likely to be significantly enhanced by the presence of focused hospitalist surgical co-management: antimicrobial prophylaxis and perioperative glycemic control (Table 1).
Antimicrobial Prophylaxis
Studies overwhelmingly show a marked reduction in the relative risk of SSIs with the use of antibiotic prophylaxis (1). In June 2004, the National Surgical Infection Prevention Project (NSIPP) published an advisory statement on antimicrobial prophylaxis in which it outlined three performance measures for quality improvement in prevention of SSIs:
- The proportion of patients who have parenteral antimicrobial prophylaxis initiated within 1 hour before surgical incision
- The proportion of patients provided with a prophylactic antimicrobial agent that is consistent with currently published guidelines, and
- The proportion of patients whose prophylactic antimicrobial therapy is discontinued within 24 hours after the end of surgery (5)
Pooled data suggest that attention to timing makes a favorable difference in SSI rates (1). Fully administering the appropriate antibiotic within 60 minutes of incision ensures that serum and tissue drug levels exceed the MICs of the most likely contaminating organisms. Dosing the antibiotic immediately prior to the start of surgery also provides the best opportunity to extend therapeutic levels for the duration of the surgery. The fact that anesthesia and surgical teams are in the most practical time-space positions to apply this measure underscores the multi-disciplinary and process-level efforts necessary to reduce SSI rates.
When it comes to the choice of antimicrobial and the duration of its use, hospitalists may find themselves in superior positions of impact. Familiarity with recommendations of the NSIPP advisory statement (summarized in Table 2) promotes evidence-based selection of antibiotic prophylaxis based on patient-specific factors: type of operation and presence of true drug allergies (5). Compared with other members of the surgical co-management team, hospitalists are more likely to be aware of relevant patient-specific risk factors such as the likelihood of colonization with methicillin-resistant Staphylococcus aureus (MRSA). For example, in patients colonized with MRSA, hospitalists might consider vancomycin as the alternative agent for prophylaxis. Free access to the NSIPP advisory statement is available at www.journals.uchicago.edu/CID/journal/issues/v38n12/33257/33257.html.
Antimicrobial prophylaxis after wound closure is unnecessary; published evidence demonstrates the non-inferiority of single dose prophylaxis when compared with multiple dose prophylaxis (5). Further, prolonged use of antimicrobial prophylaxis is associated with the emergence of resistant organisms (6-8). By ensuring that the duration of prophylaxis does not exceed 24 hours past the end of the operation, hospitalists can make valuable contributions to public health and cost containment.
Non-Antimicrobial Measures
Several non-antimicrobial measures also significantly reduce SSI rates. Those that fall outside the domain of the hospitalist and into the direct purview of the operative team include high levels of inspired oxygen, maintenance of perioperative normothermia, and use of clippers rather than a razor when hair removal is necessary. The risk of SSIs is directly related to tissue oxygenation. Bacterial infectivity is enhanced and cellular immunity is compromised in hypoperfused, poorly oxygenated tissue (9). The practice of administering perioperative supplemental oxygen (at least 80% FIO2 in intubated patients) reduces the risk of SSI by nearly one-half (1). For non-intubated patients, oxygen at 12 L/min by non-rebreathing face mask applied intra-operatively and for at least 2 hours following surgery leads to similar reductions of SSI rates. Besides being effective, this intervention is inexpensive, has no recognized adverse effects, and carries the added benefit of significantly reducing post-operative nausea and vomiting (4).
Hypothermia also predisposes the surgical wound to infection. Even mild perioperative hypothermia (i.e., core temperature 35-36.5°C) typically occurs in the absence of specific measures to prevent net heat loss. Perioperative hypothermia is the combined result of exposure and anesthetic-induced thermo-dysregulation, with redistribution of core body heat to the periphery (4). Even mild hypothermia causes vasoconstriction which diminishes perfusion, dropping tissue oxygen tension which impairs phagocytosis and oxidative killing by neutrophils (10). Hypothermia also blunts scar formation which further diminishes wound integrity. Active warming of the patient to maintain a core temperature near 36.5°C constitutes the intra-operative standard of care and is effective at reducing the risk of SSIs by as much as two-thirds (1).
Hyperglycemia, an established independent risk factor for an array of adverse outcomes in hospitalized patients, is also an independent risk factor for SSIs across a range of surgical patients (1). Short-term hyperglycemia depresses immune function through nonenzymatic glycosylation of immunoglobulin and by impairing normal leukocyte performance (11). Among diabetic cardiac surgery patients, reduction of hyperglycemia with an intravenous insulin infusion lowered the incidence of deep sternal wound infection by as much as two-thirds (12). While the value of achieving glycemic targets has already been established for a variety of important endpoints and across a range of inpatient populations, hospitalists should stay tuned. As high quality studies emerge proving that glycemic control lowers SSIs among non-cardiac surgical subpopulations, hospitalists may increasingly be relied upon to achieve strict glycemic targets.
By recognizing and coordinating practices known to reduce SSIs, hospitalists can elevate the level of care provided for surgical patients. At the same time, hospitalists can help lower costs and keep the hospital system mindful of public health goals, such as prevention of antimicrobial resistance. While individual hospitalists have key roles to play, the overall approach to SSI reduction calls for a coordinated, multidisciplinary team approach with process and system-level efforts.
Dr. Stein can be contacted at [email protected].
References
- Auerbach AD. Prevention of surgical site infections. In: Shojania KG, Duncan BW, McDonald KM, et al., eds. Making health care safer: a critical analysis of patient safety practices. Evidence report/technology assessment no. 43. AHRQ publication no. 01-E058. Rockville, MD: Agency for Healthcare Research and Quality, 20 July 2001:221-44.
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-30.
- National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996: a report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996;24:380-8.
- Sessler DI, Akca O. Nonpharmacologic prevention of surgical wound infections. Clin Infect Dis. 2002;35:1397 404.
- Bratzler D, Houck PM. Surgical Infection Prevention Guidelines Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;Jun15;38(12):1706-15. E-pub 2004 May 26.
- Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916-21.
- Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059-93.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972-8.
- Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997-1005.
- Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-15.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;Mar-Apr;10Suppl2:4-9.
- Furnary AP, Zerr K, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures [with discussion]. Ann Thorac Surg. 1999;67:352-62.
Background
An appropriately feared complication of operations, surgical site infections (SSIs) are infections associated with high economic costs and significantly worse clinical outcomes (1). Defined as infections of the superficial incision site, deep incision space, or organ space, SSIs add additional cost ranging from $2,700 to $26,000 per episode according to CDC’s National Nosocomial Infections Surveillance System. Patients who develop an SSI have hospital lengths of stay (LOS) in excess of 7 days longer and are 60% more likely to spend time in the intensive care unit than are patients without an SSI. A patient with an SSI is five times more likely to be readmitted to the hospital and is twice as likely to die (2).
Unfortunately, surgical site infections are common. Among healthcare-acquired infections, SSIs rank second only to urinary tract infections in frequency, making them more common than bloodstream infections and nosocomial pneumonia (3). There are approximately 30 million operations annually in the United States and an SSI complicates 2–5% of clean extra-abdominal sites. The rate is much higher for intra-abdominal operations, approaching 20% (1). Because most SSIs begin within 2 hours of contamination, the perioperative period is the most crucial for development of an SSI (4). By offering clinical expertise in the practice guidelines that reduce the risk of SSIs, hospital medicine programs can help patients and hospital systems lower morbidity, mortality, and costs associated with this complication. Adherence to best practices will likely require coordinated, multidisciplinary process improvement.
Several important interventions fall directly under the control of the anesthesia and surgical teams, such as administering perioperative oxygen, ensuring perioperative normothermia, and avoiding shaving of the surgical site. In coordinated quality improvement efforts, members of the operative team should assume direct responsibility for the performance of these measures. But the performance of two important interventions in this decisive period is likely to be significantly enhanced by the presence of focused hospitalist surgical co-management: antimicrobial prophylaxis and perioperative glycemic control (Table 1).
Antimicrobial Prophylaxis
Studies overwhelmingly show a marked reduction in the relative risk of SSIs with the use of antibiotic prophylaxis (1). In June 2004, the National Surgical Infection Prevention Project (NSIPP) published an advisory statement on antimicrobial prophylaxis in which it outlined three performance measures for quality improvement in prevention of SSIs:
- The proportion of patients who have parenteral antimicrobial prophylaxis initiated within 1 hour before surgical incision
- The proportion of patients provided with a prophylactic antimicrobial agent that is consistent with currently published guidelines, and
- The proportion of patients whose prophylactic antimicrobial therapy is discontinued within 24 hours after the end of surgery (5)
Pooled data suggest that attention to timing makes a favorable difference in SSI rates (1). Fully administering the appropriate antibiotic within 60 minutes of incision ensures that serum and tissue drug levels exceed the MICs of the most likely contaminating organisms. Dosing the antibiotic immediately prior to the start of surgery also provides the best opportunity to extend therapeutic levels for the duration of the surgery. The fact that anesthesia and surgical teams are in the most practical time-space positions to apply this measure underscores the multi-disciplinary and process-level efforts necessary to reduce SSI rates.
When it comes to the choice of antimicrobial and the duration of its use, hospitalists may find themselves in superior positions of impact. Familiarity with recommendations of the NSIPP advisory statement (summarized in Table 2) promotes evidence-based selection of antibiotic prophylaxis based on patient-specific factors: type of operation and presence of true drug allergies (5). Compared with other members of the surgical co-management team, hospitalists are more likely to be aware of relevant patient-specific risk factors such as the likelihood of colonization with methicillin-resistant Staphylococcus aureus (MRSA). For example, in patients colonized with MRSA, hospitalists might consider vancomycin as the alternative agent for prophylaxis. Free access to the NSIPP advisory statement is available at www.journals.uchicago.edu/CID/journal/issues/v38n12/33257/33257.html.
Antimicrobial prophylaxis after wound closure is unnecessary; published evidence demonstrates the non-inferiority of single dose prophylaxis when compared with multiple dose prophylaxis (5). Further, prolonged use of antimicrobial prophylaxis is associated with the emergence of resistant organisms (6-8). By ensuring that the duration of prophylaxis does not exceed 24 hours past the end of the operation, hospitalists can make valuable contributions to public health and cost containment.
Non-Antimicrobial Measures
Several non-antimicrobial measures also significantly reduce SSI rates. Those that fall outside the domain of the hospitalist and into the direct purview of the operative team include high levels of inspired oxygen, maintenance of perioperative normothermia, and use of clippers rather than a razor when hair removal is necessary. The risk of SSIs is directly related to tissue oxygenation. Bacterial infectivity is enhanced and cellular immunity is compromised in hypoperfused, poorly oxygenated tissue (9). The practice of administering perioperative supplemental oxygen (at least 80% FIO2 in intubated patients) reduces the risk of SSI by nearly one-half (1). For non-intubated patients, oxygen at 12 L/min by non-rebreathing face mask applied intra-operatively and for at least 2 hours following surgery leads to similar reductions of SSI rates. Besides being effective, this intervention is inexpensive, has no recognized adverse effects, and carries the added benefit of significantly reducing post-operative nausea and vomiting (4).
Hypothermia also predisposes the surgical wound to infection. Even mild perioperative hypothermia (i.e., core temperature 35-36.5°C) typically occurs in the absence of specific measures to prevent net heat loss. Perioperative hypothermia is the combined result of exposure and anesthetic-induced thermo-dysregulation, with redistribution of core body heat to the periphery (4). Even mild hypothermia causes vasoconstriction which diminishes perfusion, dropping tissue oxygen tension which impairs phagocytosis and oxidative killing by neutrophils (10). Hypothermia also blunts scar formation which further diminishes wound integrity. Active warming of the patient to maintain a core temperature near 36.5°C constitutes the intra-operative standard of care and is effective at reducing the risk of SSIs by as much as two-thirds (1).
Hyperglycemia, an established independent risk factor for an array of adverse outcomes in hospitalized patients, is also an independent risk factor for SSIs across a range of surgical patients (1). Short-term hyperglycemia depresses immune function through nonenzymatic glycosylation of immunoglobulin and by impairing normal leukocyte performance (11). Among diabetic cardiac surgery patients, reduction of hyperglycemia with an intravenous insulin infusion lowered the incidence of deep sternal wound infection by as much as two-thirds (12). While the value of achieving glycemic targets has already been established for a variety of important endpoints and across a range of inpatient populations, hospitalists should stay tuned. As high quality studies emerge proving that glycemic control lowers SSIs among non-cardiac surgical subpopulations, hospitalists may increasingly be relied upon to achieve strict glycemic targets.
By recognizing and coordinating practices known to reduce SSIs, hospitalists can elevate the level of care provided for surgical patients. At the same time, hospitalists can help lower costs and keep the hospital system mindful of public health goals, such as prevention of antimicrobial resistance. While individual hospitalists have key roles to play, the overall approach to SSI reduction calls for a coordinated, multidisciplinary team approach with process and system-level efforts.
Dr. Stein can be contacted at [email protected].
References
- Auerbach AD. Prevention of surgical site infections. In: Shojania KG, Duncan BW, McDonald KM, et al., eds. Making health care safer: a critical analysis of patient safety practices. Evidence report/technology assessment no. 43. AHRQ publication no. 01-E058. Rockville, MD: Agency for Healthcare Research and Quality, 20 July 2001:221-44.
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-30.
- National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996: a report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996;24:380-8.
- Sessler DI, Akca O. Nonpharmacologic prevention of surgical wound infections. Clin Infect Dis. 2002;35:1397 404.
- Bratzler D, Houck PM. Surgical Infection Prevention Guidelines Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;Jun15;38(12):1706-15. E-pub 2004 May 26.
- Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916-21.
- Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059-93.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972-8.
- Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997-1005.
- Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-15.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;Mar-Apr;10Suppl2:4-9.
- Furnary AP, Zerr K, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures [with discussion]. Ann Thorac Surg. 1999;67:352-62.
Acute Bacterial Meningitis in Adults
Background Acute bacterial meningitis is an inflammation of the meninges, which results from bacterially mediated recruitment and activation of inflammatory cells in the cerebrospinal fluid (CSF). Bacterial meningitis was an almost invariably fatal disease at the start of the 20th century. With the development of and advancements in antimicrobial therapy, however, there has been a significant reduction in the mortality rate, although this has remained stable during the past 20 years (1). One large study of adults with community-acquired bacterial meningitis reported an overall mortality rate of 21%, including a 30% mortality rate associated with Streptococcus pneumoniae meningitis and a 7% mortality rate for Neisseria meningitidis (2). In adults, the most commonly identified organisms are S. pneumoniae (40–50%), Neisseria meningitidis (14–37%), and Listeria monocytogenes (4–10%) (2-4).
Clinical Presentation
Bacterial meningitis is a serious illness that often progresses rapidly. The classic clinical presentation consists of fever, nuchal rigidity, and mental status change (3). One large review of 10 critically appraised studies showed that almost all (99–100%) of the patients with bacterial meningitis presented with at least one of these clinical findings; and 95% of the patients had at least 2 of the clinical findings (5). In contrast, less than half of the patients presented with all 3 findings. Thus, in the absence of all 3 of these classic findings, the diagnosis of meningitis can virtually be dismissed, and further evaluation for meningitis need not be pursued. Individually, fever was the most common presenting finding, with a sensitivity of 85%. Nuchal rigidity had a sensitivity of 70%, and mental status change was 67%. While these physical examination findings may be of value in determining the diagnosis of bacterial meningitis, the accuracy of the clinical history including features such as headache, nausea and vomiting, and neck pain was too low to be of use clinically.
Signs of meningeal irritation may be of benefit in the clinical diagnosis of bacterial meningitis. Kernig’s and Brudzinski’s signs were first described nearly a century ago and have been used by most clinicians in the clinical realm; however, their diagnostic utility has been evaluated only in a limited number of studies. Kernig’s sign is positive when a patient in the supine position with his/her hips flexed at 90 degrees develops pain in the lower back or posterior thigh during an attempt to extend the knee. Brudzinski’s sign is positive when a patient in the supine position whose neck is passively flexed responds with flexion of his/her knees and hips. Recently, a bedside maneuver called jolt accentuation of headache was found to be potentially useful. In this maneuver, the patient is asked to turn his/her head horizontally 2–3 times per second, and a worsening headache is considered a positive sign. A small study showed that this maneuver had 97% sensitivity and 60% specificity for patients with CSF pleocytosis (6).
Other clinical manifestations in patients with bacterial meningitis include photophobia, seizure, rash, focal neurologic deficits, and signs of increased intracranial pressure. While these various findings may be present in many patients with bacterial meningitis, their sensitivities have been found to be low. Thus, their clinical utility in ruling out the diagnosis of bacterial meningitis is limited (5).
Laboratory Findings
Any patient who presents with a reasonable likelihood of having bacterial meningitis should undergo a lumbar puncture (LP) to evaluate the CSF as soon as possible. The initial CSF study should measure the opening pressure. One study demonstrated that 39% of patients with bacterial meningitis had opening pressures greater than 300 mg H20 (3). Other CSF laboratory studies should be sent for analysis in 4 sterile tubes filled with approximately 1 mL of CSF each. The first tube is typically reserved for gram stain and culture. The gram stain is positive in about 70% of patients with bacterial meningitis, and the culture will be positive in about 80% of cases. The second tube is sent for protein and glucose levels. Patients who have markedly elevated CSF protein counts (>500 mg/dL) and low glucose levels (<45 mg/dL, or ratio of serum: CSF glucose levels <0.4) are likely to have bacterial meningitis. The third tube is sent for cell count and differential. Patients with bacterial meningitis are likely to have >10 WBC/μL that are predominantly polymorphonucleocytes and have few or no red blood cells in the absence of a traumatic LP. We recommend the fourth tube be used for any viral, fungal, or other miscellaneous studies. In addition to the CSF studies, other diagnostic evaluations should include blood cultures, complete blood count with platelets and differential (CBCPD), and basic chemistry labs.
The CSF studies described above are the primary tools in diagnosing bacterial meningitis; however, there are other studies that may be helpful in certain clinical settings. Latex agglutination tests for bacterial antigens may be used in cases in which bacterial meningitis remains a possible diagnosis despite negative CSF studies. This test is available for S. pneumoniae, N. meningitidis, H. influenzae type B, group B Streptococcus, and E. coli. The polymerase chain reaction (PCR) test of the CSF has been developed for some bacterial pathogens including S. pneumoniae, N. meningitidis, H. influenzae type B, and Mycobacterium tuberculosis. The limulus amebocyte lysate assay is a very sensitive test for gram-negative endotoxins, which may aid in identifying gram-negative organisms as potential pathogens in the CSF. While these alternative CSF diagnostic tests are available, many laboratories do not perform the tests on site and require send-out to a specialty laboratory. The time required for this may negate the clinical utility of these tests.
Role of Brain Imaging
The decision to obtain a brain imaging study prior to performing an LP has been a controversial issue for both patient safety and medical-legal reasons. Two large studies have been published in an attempt to derive a clinically useful decision analysis tool (7,8). In summary, the studies found that 5 clinical features were associated with an abnormal head cranial tomography (CT) scan. These were:
- Age >60 years
- Immunocompromised state
- Any history of central nervous system (CNS) disease
- A history of seizure within 1 week prior to presentation
- Presence of a focal neurologic abnormality, including altered level of consciousness, inability to answer or follow 2 consecutive requests, gaze palsy, abnormal visual fields, facial palsy, arm or leg drift, and abnormal language.
In patients with none of these findings, there was a 97% negative predictive value of having an abnormal CT scan, with the few patients with positive scans nonetheless tolerating LP without adverse effects. Thus, in patients with none of these findings, it appears that an LP can safely be performed without obtaining a CT scan. One study also demonstrated that patients who underwent a CT scan prior to their LP waited, on average, 2 hours longer to get an LP; with antibiotic administration delayed by an average of 1 hour (8). Antibiotic administration should not be delayed in any patient suspected of having bacterial meningitis, whether brain imaging is performed or not.
Differential Diagnosis
Given the severe nature of this disease, the diagnosis of bacterial meningitis must be differentiated from other conditions that may present in similar ways. Infectious causes that may present similarly to bacterial meningitis include other types of meningitis (viral, tuberculous, Lyme disease, syphilitic), viral encephalitis, Rocky Mountain spotted fever, fungal meningitis, parasitic causes, brain abscess, and epidural and subdural empyema. Other infectious etiologies not originating from the CNS may be mistaken for bacterial meningitis when these patients present with concomitant mental-status changes. This is especially common in elderly patients with pneumonia and urinary tract infections. Other noninfectious considerations include a CNS bleed such as a subarachnoid hemorrhage, drug-induced aseptic meningitis, and CNS vasculitis.
Treatment
When the patient’s presentation is suggestive of bacterial meningitis, empiric antibiotics should be administered without delay, while awaiting diagnostic evaluation. The initial dose of antibiotics should not alter the results of the diagnostic studies significantly. The choice of antibiotics is based upon the most likely offending organism from epidemiologic data and underlying predisposing conditions. S. pneumoniae and N. meningitidis are the 2 most common causes of bacterial meningitis in adults.
The development of antibiotic resistance by S. pneumoniae to penicillin and cephalosporins has been one of the major developments in the past 20 years. Due to this resistance, the recommended empiric therapy is a combination of a third-generation cephalosporin (ceftriaxone or cefotaxime) and vancomycin. For special cases, additional or alternative therapy should be given. Ampicillin should be added for patients at risk for Listeria monocytogenes; and postsurgical or post-trauma patients should have expanded coverage to include staphylococcal and gram-negative infections. Table 1 lists the recommended antibiotic therapy for patients with possible bacterial meningitis, along with the most commonly associated organisms.
Once the offending organism has been identified, antibiotic therapy should be narrowed to target the bacteria based on laboratory minimal inhibitory concentrations (MIC). The antibiotic should also have excellent CSF penetration and bactericidal activity. For S. pneumoniae that are susceptible to penicillin, penicillin G and ampicillin remain the therapy of choice (9). The increasing trend toward antibiotic resistance by S. pneumoniae has increased the use of vancomycin as therapy. In patients with resistant strains of S. pneumoniae, however, vancomycin should not be used alone. Vancomycin should be used in combination with a third-generation cephalosporin while keeping the serum vancomycin levels in the range of 15–20 μg/mL (10). It is imperative that the treatment course outlined be completed through its full duration. Table 2 lists specific antibiotic therapy with dosages and recommended duration of therapy based on isolated organisms.
Adjunctive Therapy
The release and production of inflammatory cytokines in bacterial meningitis is thought to be a major cause of adverse outcomes. To counteract this inflammatory process, use of adjunctive steroids in patients with bacterial meningitis has been evaluated. Initial data from children with bacterial meningitis, mostly due to H. influenzae and S. pneumoniae, demonstrated improved neurologic outcomes, with significant reductions in deafness, in patients treated with dexamethasone as an adjunctive therapy to antibiotics (11). In adults with bacterial meningitis, a recent major trial demonstrated that treatment with adjunctive steroids, along with antibiotics, led to significant improvement in mortality and morbidity in patients with meningitis due to S. pneumoniae (12). Among patients with meningococcal meningitis, there was a trend toward improved outcomes. Patients with suspected pneumococcal meningitis should receive their first dose of dexamethasone 20–30 minutes prior to or at the same time as the initial antibiotic administration. The recommended dose and duration is 0.15 mg/kg every 6 hours for 2 to 4 days. The use of dexamethasone appears to have no benefit if administered after antibiotics have already been given, and data are lacking for patients with meningitis due to organisms other than S. pneumoniae. Most experts recommend against the use of adjunctive corticosteroids in these cases (10-13). Several questions, however, remain unanswered with regard to adjunctive corticosteroid use. These include the optimal duration of treatment, whether the penetration of vancomycin into the CSF is significantly decreased by dexamethasone, and whether they should be administered to immunocompromised patients (14).
Prevention
Currently, prevention of some types of bacterial meningitis can be accomplished by appropriate use of vaccines, or through antibiotic chemoprophylaxis in certain situations. For adults, vaccines are available against the 2 most common causes of bacterial meningitis. The 23 polyvalent pneumococcal vaccine is recommended for all adults >65 years of age and for anyone age >2 with a compromised immune status. The meningococcal vaccine is available as a quadravalent vaccine (serotypes A, C, Y, and W-135) and should be administered to anyone with functional asplenia, terminal complement deficiencies, those traveling to endemic areas of meningococcal meningitis, and any college freshman requesting the vaccine who will be living in college dormitories (15).
Antibiotic chemoprophylaxis can be administered to individuals who have had close contact with an index patient with meningococcal meningitis. Antibiotics should be administered as soon as exposure has been determined. There are several options available for meningococcal meningitis exposure. Ciprofloxacin is probably the simplest regimen due to its 1-time 500-mg oral dose. Other options include rifampin 600 mg every 12 hours ×4 doses and ceftriaxone 250 mg IM as a 1-time dose. Pregnant women should avoid ciprofloxacin and rifampin due to their potential teratogenic effects.
Prognosis and Follow-up
Prognosis of bacterial meningitis is closely linked to the causative organism, the severity of disease at the time of presentation, and the speed at which the disease progresses. One large retrospective study demonstrated in-hospital mortality rates of 25% for S. pneumoniae, 10% for N. meningitidis, and 21% for L. monocytogenes. Conditions associated with an increased risk of mortality included age >60, state of obtundation on admission, and development of seizure within 24 hours of admission. This study also showed that 21% of patients developed some type of neurologic deficits, and, overall, 9% had persistence of these deficits at time of discharge (3). Another study showed that baseline features of hypotension, mental status changes, and seizures were associated with increased mortality and neurologic morbidity (16). A more recent large study evaluating the efficacy of adjunctive corticosteroids reported a mortality rate of 15% in the control arm, with mortality of 34% in patients infected with S. pneumoniae (12). Another study suggested that if patients had a rapid progression of their disease, this seemed to correlate with worse outcomes. These investigators found an uncertain correlation between antibiotic timing and unfavorable outcomes (16).
Patients discharged from the hospital should have close follow-up with their primary care physician or infectious disease specialist. Evaluation in the short-term should focus on any complications that may have developed as a result of the bacterial meningitis; such as mental status change, seizure, focal neurologic deficits, and hearing loss. Long-term evaluations should also address cognitive functioning and the neuropsychiatric well-being of the patient, in addition to those issues addressed during short-term follow-up (11, 12).
Dr. Kim may be reached at [email protected].
References
- Swartz, Morton N. Bacterial Meningitis—A View of the Past 90 Years. N Engl J Med. 2004;351:1826-8.
- van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849-59.
- Durand ML, Calderwood SB, Weber DJ, et al. Acute Bacterial Meningitis in Adults. A review of 493 episodes. N Engl J Med. 1993;328:21-8.
- Schuchat A, Robinson K, Wenger J, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970-6.
- Attia J, Hatala R, Cook DJ, Wong JG. The rational clinical examination. Does this adult patient have acute meningitis? JAMA. 1999;282:175-181.
- Uchihara T, Tsukagoshi H. Jolt accentuation of headache: the most sensitive sign of CSF pleocytosis. Headache. 1991;31:167-71.
- Gopal AK, Whitehouse JD, Simel DL, Corey RG. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med. 1999;159:2681-5.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before LP in adults with suspected meningitis. N Engl J Med. 2001;345:1727-33.
- Quagliarello VJ, Scheld WM. Treatment of bacterial meningitis. N Engl J Med. 1997;336:708-16.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice Guidelines for the Management of Bacterial Meningitis. Clin Infect Dis. 2004;39:1267-84.
- McIntyre PB, Berkey CS, King SM, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278:928-31.
- de Gans J, van de Beek D Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549-56.
- van de Beek D, de Gans J, McIntyre P, Prasad K. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect Dis 2004;4: 139-43.
- Pile JC, Longworth DL. Should adults with suspected acute bacterial meningitis get adjunctive corticosteroids? Cleve Clin J Med. 2005;72:67-70.
- Control and prevention of meningococcal disease and control and prevention of serogroup C meningococcal disease: evaluation and management of suspected outbreaks: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;4613-21.
- Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129:862-9.
Background Acute bacterial meningitis is an inflammation of the meninges, which results from bacterially mediated recruitment and activation of inflammatory cells in the cerebrospinal fluid (CSF). Bacterial meningitis was an almost invariably fatal disease at the start of the 20th century. With the development of and advancements in antimicrobial therapy, however, there has been a significant reduction in the mortality rate, although this has remained stable during the past 20 years (1). One large study of adults with community-acquired bacterial meningitis reported an overall mortality rate of 21%, including a 30% mortality rate associated with Streptococcus pneumoniae meningitis and a 7% mortality rate for Neisseria meningitidis (2). In adults, the most commonly identified organisms are S. pneumoniae (40–50%), Neisseria meningitidis (14–37%), and Listeria monocytogenes (4–10%) (2-4).
Clinical Presentation
Bacterial meningitis is a serious illness that often progresses rapidly. The classic clinical presentation consists of fever, nuchal rigidity, and mental status change (3). One large review of 10 critically appraised studies showed that almost all (99–100%) of the patients with bacterial meningitis presented with at least one of these clinical findings; and 95% of the patients had at least 2 of the clinical findings (5). In contrast, less than half of the patients presented with all 3 findings. Thus, in the absence of all 3 of these classic findings, the diagnosis of meningitis can virtually be dismissed, and further evaluation for meningitis need not be pursued. Individually, fever was the most common presenting finding, with a sensitivity of 85%. Nuchal rigidity had a sensitivity of 70%, and mental status change was 67%. While these physical examination findings may be of value in determining the diagnosis of bacterial meningitis, the accuracy of the clinical history including features such as headache, nausea and vomiting, and neck pain was too low to be of use clinically.
Signs of meningeal irritation may be of benefit in the clinical diagnosis of bacterial meningitis. Kernig’s and Brudzinski’s signs were first described nearly a century ago and have been used by most clinicians in the clinical realm; however, their diagnostic utility has been evaluated only in a limited number of studies. Kernig’s sign is positive when a patient in the supine position with his/her hips flexed at 90 degrees develops pain in the lower back or posterior thigh during an attempt to extend the knee. Brudzinski’s sign is positive when a patient in the supine position whose neck is passively flexed responds with flexion of his/her knees and hips. Recently, a bedside maneuver called jolt accentuation of headache was found to be potentially useful. In this maneuver, the patient is asked to turn his/her head horizontally 2–3 times per second, and a worsening headache is considered a positive sign. A small study showed that this maneuver had 97% sensitivity and 60% specificity for patients with CSF pleocytosis (6).
Other clinical manifestations in patients with bacterial meningitis include photophobia, seizure, rash, focal neurologic deficits, and signs of increased intracranial pressure. While these various findings may be present in many patients with bacterial meningitis, their sensitivities have been found to be low. Thus, their clinical utility in ruling out the diagnosis of bacterial meningitis is limited (5).
Laboratory Findings
Any patient who presents with a reasonable likelihood of having bacterial meningitis should undergo a lumbar puncture (LP) to evaluate the CSF as soon as possible. The initial CSF study should measure the opening pressure. One study demonstrated that 39% of patients with bacterial meningitis had opening pressures greater than 300 mg H20 (3). Other CSF laboratory studies should be sent for analysis in 4 sterile tubes filled with approximately 1 mL of CSF each. The first tube is typically reserved for gram stain and culture. The gram stain is positive in about 70% of patients with bacterial meningitis, and the culture will be positive in about 80% of cases. The second tube is sent for protein and glucose levels. Patients who have markedly elevated CSF protein counts (>500 mg/dL) and low glucose levels (<45 mg/dL, or ratio of serum: CSF glucose levels <0.4) are likely to have bacterial meningitis. The third tube is sent for cell count and differential. Patients with bacterial meningitis are likely to have >10 WBC/μL that are predominantly polymorphonucleocytes and have few or no red blood cells in the absence of a traumatic LP. We recommend the fourth tube be used for any viral, fungal, or other miscellaneous studies. In addition to the CSF studies, other diagnostic evaluations should include blood cultures, complete blood count with platelets and differential (CBCPD), and basic chemistry labs.
The CSF studies described above are the primary tools in diagnosing bacterial meningitis; however, there are other studies that may be helpful in certain clinical settings. Latex agglutination tests for bacterial antigens may be used in cases in which bacterial meningitis remains a possible diagnosis despite negative CSF studies. This test is available for S. pneumoniae, N. meningitidis, H. influenzae type B, group B Streptococcus, and E. coli. The polymerase chain reaction (PCR) test of the CSF has been developed for some bacterial pathogens including S. pneumoniae, N. meningitidis, H. influenzae type B, and Mycobacterium tuberculosis. The limulus amebocyte lysate assay is a very sensitive test for gram-negative endotoxins, which may aid in identifying gram-negative organisms as potential pathogens in the CSF. While these alternative CSF diagnostic tests are available, many laboratories do not perform the tests on site and require send-out to a specialty laboratory. The time required for this may negate the clinical utility of these tests.
Role of Brain Imaging
The decision to obtain a brain imaging study prior to performing an LP has been a controversial issue for both patient safety and medical-legal reasons. Two large studies have been published in an attempt to derive a clinically useful decision analysis tool (7,8). In summary, the studies found that 5 clinical features were associated with an abnormal head cranial tomography (CT) scan. These were:
- Age >60 years
- Immunocompromised state
- Any history of central nervous system (CNS) disease
- A history of seizure within 1 week prior to presentation
- Presence of a focal neurologic abnormality, including altered level of consciousness, inability to answer or follow 2 consecutive requests, gaze palsy, abnormal visual fields, facial palsy, arm or leg drift, and abnormal language.
In patients with none of these findings, there was a 97% negative predictive value of having an abnormal CT scan, with the few patients with positive scans nonetheless tolerating LP without adverse effects. Thus, in patients with none of these findings, it appears that an LP can safely be performed without obtaining a CT scan. One study also demonstrated that patients who underwent a CT scan prior to their LP waited, on average, 2 hours longer to get an LP; with antibiotic administration delayed by an average of 1 hour (8). Antibiotic administration should not be delayed in any patient suspected of having bacterial meningitis, whether brain imaging is performed or not.
Differential Diagnosis
Given the severe nature of this disease, the diagnosis of bacterial meningitis must be differentiated from other conditions that may present in similar ways. Infectious causes that may present similarly to bacterial meningitis include other types of meningitis (viral, tuberculous, Lyme disease, syphilitic), viral encephalitis, Rocky Mountain spotted fever, fungal meningitis, parasitic causes, brain abscess, and epidural and subdural empyema. Other infectious etiologies not originating from the CNS may be mistaken for bacterial meningitis when these patients present with concomitant mental-status changes. This is especially common in elderly patients with pneumonia and urinary tract infections. Other noninfectious considerations include a CNS bleed such as a subarachnoid hemorrhage, drug-induced aseptic meningitis, and CNS vasculitis.
Treatment
When the patient’s presentation is suggestive of bacterial meningitis, empiric antibiotics should be administered without delay, while awaiting diagnostic evaluation. The initial dose of antibiotics should not alter the results of the diagnostic studies significantly. The choice of antibiotics is based upon the most likely offending organism from epidemiologic data and underlying predisposing conditions. S. pneumoniae and N. meningitidis are the 2 most common causes of bacterial meningitis in adults.
The development of antibiotic resistance by S. pneumoniae to penicillin and cephalosporins has been one of the major developments in the past 20 years. Due to this resistance, the recommended empiric therapy is a combination of a third-generation cephalosporin (ceftriaxone or cefotaxime) and vancomycin. For special cases, additional or alternative therapy should be given. Ampicillin should be added for patients at risk for Listeria monocytogenes; and postsurgical or post-trauma patients should have expanded coverage to include staphylococcal and gram-negative infections. Table 1 lists the recommended antibiotic therapy for patients with possible bacterial meningitis, along with the most commonly associated organisms.
Once the offending organism has been identified, antibiotic therapy should be narrowed to target the bacteria based on laboratory minimal inhibitory concentrations (MIC). The antibiotic should also have excellent CSF penetration and bactericidal activity. For S. pneumoniae that are susceptible to penicillin, penicillin G and ampicillin remain the therapy of choice (9). The increasing trend toward antibiotic resistance by S. pneumoniae has increased the use of vancomycin as therapy. In patients with resistant strains of S. pneumoniae, however, vancomycin should not be used alone. Vancomycin should be used in combination with a third-generation cephalosporin while keeping the serum vancomycin levels in the range of 15–20 μg/mL (10). It is imperative that the treatment course outlined be completed through its full duration. Table 2 lists specific antibiotic therapy with dosages and recommended duration of therapy based on isolated organisms.
Adjunctive Therapy
The release and production of inflammatory cytokines in bacterial meningitis is thought to be a major cause of adverse outcomes. To counteract this inflammatory process, use of adjunctive steroids in patients with bacterial meningitis has been evaluated. Initial data from children with bacterial meningitis, mostly due to H. influenzae and S. pneumoniae, demonstrated improved neurologic outcomes, with significant reductions in deafness, in patients treated with dexamethasone as an adjunctive therapy to antibiotics (11). In adults with bacterial meningitis, a recent major trial demonstrated that treatment with adjunctive steroids, along with antibiotics, led to significant improvement in mortality and morbidity in patients with meningitis due to S. pneumoniae (12). Among patients with meningococcal meningitis, there was a trend toward improved outcomes. Patients with suspected pneumococcal meningitis should receive their first dose of dexamethasone 20–30 minutes prior to or at the same time as the initial antibiotic administration. The recommended dose and duration is 0.15 mg/kg every 6 hours for 2 to 4 days. The use of dexamethasone appears to have no benefit if administered after antibiotics have already been given, and data are lacking for patients with meningitis due to organisms other than S. pneumoniae. Most experts recommend against the use of adjunctive corticosteroids in these cases (10-13). Several questions, however, remain unanswered with regard to adjunctive corticosteroid use. These include the optimal duration of treatment, whether the penetration of vancomycin into the CSF is significantly decreased by dexamethasone, and whether they should be administered to immunocompromised patients (14).
Prevention
Currently, prevention of some types of bacterial meningitis can be accomplished by appropriate use of vaccines, or through antibiotic chemoprophylaxis in certain situations. For adults, vaccines are available against the 2 most common causes of bacterial meningitis. The 23 polyvalent pneumococcal vaccine is recommended for all adults >65 years of age and for anyone age >2 with a compromised immune status. The meningococcal vaccine is available as a quadravalent vaccine (serotypes A, C, Y, and W-135) and should be administered to anyone with functional asplenia, terminal complement deficiencies, those traveling to endemic areas of meningococcal meningitis, and any college freshman requesting the vaccine who will be living in college dormitories (15).
Antibiotic chemoprophylaxis can be administered to individuals who have had close contact with an index patient with meningococcal meningitis. Antibiotics should be administered as soon as exposure has been determined. There are several options available for meningococcal meningitis exposure. Ciprofloxacin is probably the simplest regimen due to its 1-time 500-mg oral dose. Other options include rifampin 600 mg every 12 hours ×4 doses and ceftriaxone 250 mg IM as a 1-time dose. Pregnant women should avoid ciprofloxacin and rifampin due to their potential teratogenic effects.
Prognosis and Follow-up
Prognosis of bacterial meningitis is closely linked to the causative organism, the severity of disease at the time of presentation, and the speed at which the disease progresses. One large retrospective study demonstrated in-hospital mortality rates of 25% for S. pneumoniae, 10% for N. meningitidis, and 21% for L. monocytogenes. Conditions associated with an increased risk of mortality included age >60, state of obtundation on admission, and development of seizure within 24 hours of admission. This study also showed that 21% of patients developed some type of neurologic deficits, and, overall, 9% had persistence of these deficits at time of discharge (3). Another study showed that baseline features of hypotension, mental status changes, and seizures were associated with increased mortality and neurologic morbidity (16). A more recent large study evaluating the efficacy of adjunctive corticosteroids reported a mortality rate of 15% in the control arm, with mortality of 34% in patients infected with S. pneumoniae (12). Another study suggested that if patients had a rapid progression of their disease, this seemed to correlate with worse outcomes. These investigators found an uncertain correlation between antibiotic timing and unfavorable outcomes (16).
Patients discharged from the hospital should have close follow-up with their primary care physician or infectious disease specialist. Evaluation in the short-term should focus on any complications that may have developed as a result of the bacterial meningitis; such as mental status change, seizure, focal neurologic deficits, and hearing loss. Long-term evaluations should also address cognitive functioning and the neuropsychiatric well-being of the patient, in addition to those issues addressed during short-term follow-up (11, 12).
Dr. Kim may be reached at [email protected].
References
- Swartz, Morton N. Bacterial Meningitis—A View of the Past 90 Years. N Engl J Med. 2004;351:1826-8.
- van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849-59.
- Durand ML, Calderwood SB, Weber DJ, et al. Acute Bacterial Meningitis in Adults. A review of 493 episodes. N Engl J Med. 1993;328:21-8.
- Schuchat A, Robinson K, Wenger J, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970-6.
- Attia J, Hatala R, Cook DJ, Wong JG. The rational clinical examination. Does this adult patient have acute meningitis? JAMA. 1999;282:175-181.
- Uchihara T, Tsukagoshi H. Jolt accentuation of headache: the most sensitive sign of CSF pleocytosis. Headache. 1991;31:167-71.
- Gopal AK, Whitehouse JD, Simel DL, Corey RG. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med. 1999;159:2681-5.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before LP in adults with suspected meningitis. N Engl J Med. 2001;345:1727-33.
- Quagliarello VJ, Scheld WM. Treatment of bacterial meningitis. N Engl J Med. 1997;336:708-16.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice Guidelines for the Management of Bacterial Meningitis. Clin Infect Dis. 2004;39:1267-84.
- McIntyre PB, Berkey CS, King SM, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278:928-31.
- de Gans J, van de Beek D Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549-56.
- van de Beek D, de Gans J, McIntyre P, Prasad K. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect Dis 2004;4: 139-43.
- Pile JC, Longworth DL. Should adults with suspected acute bacterial meningitis get adjunctive corticosteroids? Cleve Clin J Med. 2005;72:67-70.
- Control and prevention of meningococcal disease and control and prevention of serogroup C meningococcal disease: evaluation and management of suspected outbreaks: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;4613-21.
- Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129:862-9.
Background Acute bacterial meningitis is an inflammation of the meninges, which results from bacterially mediated recruitment and activation of inflammatory cells in the cerebrospinal fluid (CSF). Bacterial meningitis was an almost invariably fatal disease at the start of the 20th century. With the development of and advancements in antimicrobial therapy, however, there has been a significant reduction in the mortality rate, although this has remained stable during the past 20 years (1). One large study of adults with community-acquired bacterial meningitis reported an overall mortality rate of 21%, including a 30% mortality rate associated with Streptococcus pneumoniae meningitis and a 7% mortality rate for Neisseria meningitidis (2). In adults, the most commonly identified organisms are S. pneumoniae (40–50%), Neisseria meningitidis (14–37%), and Listeria monocytogenes (4–10%) (2-4).
Clinical Presentation
Bacterial meningitis is a serious illness that often progresses rapidly. The classic clinical presentation consists of fever, nuchal rigidity, and mental status change (3). One large review of 10 critically appraised studies showed that almost all (99–100%) of the patients with bacterial meningitis presented with at least one of these clinical findings; and 95% of the patients had at least 2 of the clinical findings (5). In contrast, less than half of the patients presented with all 3 findings. Thus, in the absence of all 3 of these classic findings, the diagnosis of meningitis can virtually be dismissed, and further evaluation for meningitis need not be pursued. Individually, fever was the most common presenting finding, with a sensitivity of 85%. Nuchal rigidity had a sensitivity of 70%, and mental status change was 67%. While these physical examination findings may be of value in determining the diagnosis of bacterial meningitis, the accuracy of the clinical history including features such as headache, nausea and vomiting, and neck pain was too low to be of use clinically.
Signs of meningeal irritation may be of benefit in the clinical diagnosis of bacterial meningitis. Kernig’s and Brudzinski’s signs were first described nearly a century ago and have been used by most clinicians in the clinical realm; however, their diagnostic utility has been evaluated only in a limited number of studies. Kernig’s sign is positive when a patient in the supine position with his/her hips flexed at 90 degrees develops pain in the lower back or posterior thigh during an attempt to extend the knee. Brudzinski’s sign is positive when a patient in the supine position whose neck is passively flexed responds with flexion of his/her knees and hips. Recently, a bedside maneuver called jolt accentuation of headache was found to be potentially useful. In this maneuver, the patient is asked to turn his/her head horizontally 2–3 times per second, and a worsening headache is considered a positive sign. A small study showed that this maneuver had 97% sensitivity and 60% specificity for patients with CSF pleocytosis (6).
Other clinical manifestations in patients with bacterial meningitis include photophobia, seizure, rash, focal neurologic deficits, and signs of increased intracranial pressure. While these various findings may be present in many patients with bacterial meningitis, their sensitivities have been found to be low. Thus, their clinical utility in ruling out the diagnosis of bacterial meningitis is limited (5).
Laboratory Findings
Any patient who presents with a reasonable likelihood of having bacterial meningitis should undergo a lumbar puncture (LP) to evaluate the CSF as soon as possible. The initial CSF study should measure the opening pressure. One study demonstrated that 39% of patients with bacterial meningitis had opening pressures greater than 300 mg H20 (3). Other CSF laboratory studies should be sent for analysis in 4 sterile tubes filled with approximately 1 mL of CSF each. The first tube is typically reserved for gram stain and culture. The gram stain is positive in about 70% of patients with bacterial meningitis, and the culture will be positive in about 80% of cases. The second tube is sent for protein and glucose levels. Patients who have markedly elevated CSF protein counts (>500 mg/dL) and low glucose levels (<45 mg/dL, or ratio of serum: CSF glucose levels <0.4) are likely to have bacterial meningitis. The third tube is sent for cell count and differential. Patients with bacterial meningitis are likely to have >10 WBC/μL that are predominantly polymorphonucleocytes and have few or no red blood cells in the absence of a traumatic LP. We recommend the fourth tube be used for any viral, fungal, or other miscellaneous studies. In addition to the CSF studies, other diagnostic evaluations should include blood cultures, complete blood count with platelets and differential (CBCPD), and basic chemistry labs.
The CSF studies described above are the primary tools in diagnosing bacterial meningitis; however, there are other studies that may be helpful in certain clinical settings. Latex agglutination tests for bacterial antigens may be used in cases in which bacterial meningitis remains a possible diagnosis despite negative CSF studies. This test is available for S. pneumoniae, N. meningitidis, H. influenzae type B, group B Streptococcus, and E. coli. The polymerase chain reaction (PCR) test of the CSF has been developed for some bacterial pathogens including S. pneumoniae, N. meningitidis, H. influenzae type B, and Mycobacterium tuberculosis. The limulus amebocyte lysate assay is a very sensitive test for gram-negative endotoxins, which may aid in identifying gram-negative organisms as potential pathogens in the CSF. While these alternative CSF diagnostic tests are available, many laboratories do not perform the tests on site and require send-out to a specialty laboratory. The time required for this may negate the clinical utility of these tests.
Role of Brain Imaging
The decision to obtain a brain imaging study prior to performing an LP has been a controversial issue for both patient safety and medical-legal reasons. Two large studies have been published in an attempt to derive a clinically useful decision analysis tool (7,8). In summary, the studies found that 5 clinical features were associated with an abnormal head cranial tomography (CT) scan. These were:
- Age >60 years
- Immunocompromised state
- Any history of central nervous system (CNS) disease
- A history of seizure within 1 week prior to presentation
- Presence of a focal neurologic abnormality, including altered level of consciousness, inability to answer or follow 2 consecutive requests, gaze palsy, abnormal visual fields, facial palsy, arm or leg drift, and abnormal language.
In patients with none of these findings, there was a 97% negative predictive value of having an abnormal CT scan, with the few patients with positive scans nonetheless tolerating LP without adverse effects. Thus, in patients with none of these findings, it appears that an LP can safely be performed without obtaining a CT scan. One study also demonstrated that patients who underwent a CT scan prior to their LP waited, on average, 2 hours longer to get an LP; with antibiotic administration delayed by an average of 1 hour (8). Antibiotic administration should not be delayed in any patient suspected of having bacterial meningitis, whether brain imaging is performed or not.
Differential Diagnosis
Given the severe nature of this disease, the diagnosis of bacterial meningitis must be differentiated from other conditions that may present in similar ways. Infectious causes that may present similarly to bacterial meningitis include other types of meningitis (viral, tuberculous, Lyme disease, syphilitic), viral encephalitis, Rocky Mountain spotted fever, fungal meningitis, parasitic causes, brain abscess, and epidural and subdural empyema. Other infectious etiologies not originating from the CNS may be mistaken for bacterial meningitis when these patients present with concomitant mental-status changes. This is especially common in elderly patients with pneumonia and urinary tract infections. Other noninfectious considerations include a CNS bleed such as a subarachnoid hemorrhage, drug-induced aseptic meningitis, and CNS vasculitis.
Treatment
When the patient’s presentation is suggestive of bacterial meningitis, empiric antibiotics should be administered without delay, while awaiting diagnostic evaluation. The initial dose of antibiotics should not alter the results of the diagnostic studies significantly. The choice of antibiotics is based upon the most likely offending organism from epidemiologic data and underlying predisposing conditions. S. pneumoniae and N. meningitidis are the 2 most common causes of bacterial meningitis in adults.
The development of antibiotic resistance by S. pneumoniae to penicillin and cephalosporins has been one of the major developments in the past 20 years. Due to this resistance, the recommended empiric therapy is a combination of a third-generation cephalosporin (ceftriaxone or cefotaxime) and vancomycin. For special cases, additional or alternative therapy should be given. Ampicillin should be added for patients at risk for Listeria monocytogenes; and postsurgical or post-trauma patients should have expanded coverage to include staphylococcal and gram-negative infections. Table 1 lists the recommended antibiotic therapy for patients with possible bacterial meningitis, along with the most commonly associated organisms.
Once the offending organism has been identified, antibiotic therapy should be narrowed to target the bacteria based on laboratory minimal inhibitory concentrations (MIC). The antibiotic should also have excellent CSF penetration and bactericidal activity. For S. pneumoniae that are susceptible to penicillin, penicillin G and ampicillin remain the therapy of choice (9). The increasing trend toward antibiotic resistance by S. pneumoniae has increased the use of vancomycin as therapy. In patients with resistant strains of S. pneumoniae, however, vancomycin should not be used alone. Vancomycin should be used in combination with a third-generation cephalosporin while keeping the serum vancomycin levels in the range of 15–20 μg/mL (10). It is imperative that the treatment course outlined be completed through its full duration. Table 2 lists specific antibiotic therapy with dosages and recommended duration of therapy based on isolated organisms.
Adjunctive Therapy
The release and production of inflammatory cytokines in bacterial meningitis is thought to be a major cause of adverse outcomes. To counteract this inflammatory process, use of adjunctive steroids in patients with bacterial meningitis has been evaluated. Initial data from children with bacterial meningitis, mostly due to H. influenzae and S. pneumoniae, demonstrated improved neurologic outcomes, with significant reductions in deafness, in patients treated with dexamethasone as an adjunctive therapy to antibiotics (11). In adults with bacterial meningitis, a recent major trial demonstrated that treatment with adjunctive steroids, along with antibiotics, led to significant improvement in mortality and morbidity in patients with meningitis due to S. pneumoniae (12). Among patients with meningococcal meningitis, there was a trend toward improved outcomes. Patients with suspected pneumococcal meningitis should receive their first dose of dexamethasone 20–30 minutes prior to or at the same time as the initial antibiotic administration. The recommended dose and duration is 0.15 mg/kg every 6 hours for 2 to 4 days. The use of dexamethasone appears to have no benefit if administered after antibiotics have already been given, and data are lacking for patients with meningitis due to organisms other than S. pneumoniae. Most experts recommend against the use of adjunctive corticosteroids in these cases (10-13). Several questions, however, remain unanswered with regard to adjunctive corticosteroid use. These include the optimal duration of treatment, whether the penetration of vancomycin into the CSF is significantly decreased by dexamethasone, and whether they should be administered to immunocompromised patients (14).
Prevention
Currently, prevention of some types of bacterial meningitis can be accomplished by appropriate use of vaccines, or through antibiotic chemoprophylaxis in certain situations. For adults, vaccines are available against the 2 most common causes of bacterial meningitis. The 23 polyvalent pneumococcal vaccine is recommended for all adults >65 years of age and for anyone age >2 with a compromised immune status. The meningococcal vaccine is available as a quadravalent vaccine (serotypes A, C, Y, and W-135) and should be administered to anyone with functional asplenia, terminal complement deficiencies, those traveling to endemic areas of meningococcal meningitis, and any college freshman requesting the vaccine who will be living in college dormitories (15).
Antibiotic chemoprophylaxis can be administered to individuals who have had close contact with an index patient with meningococcal meningitis. Antibiotics should be administered as soon as exposure has been determined. There are several options available for meningococcal meningitis exposure. Ciprofloxacin is probably the simplest regimen due to its 1-time 500-mg oral dose. Other options include rifampin 600 mg every 12 hours ×4 doses and ceftriaxone 250 mg IM as a 1-time dose. Pregnant women should avoid ciprofloxacin and rifampin due to their potential teratogenic effects.
Prognosis and Follow-up
Prognosis of bacterial meningitis is closely linked to the causative organism, the severity of disease at the time of presentation, and the speed at which the disease progresses. One large retrospective study demonstrated in-hospital mortality rates of 25% for S. pneumoniae, 10% for N. meningitidis, and 21% for L. monocytogenes. Conditions associated with an increased risk of mortality included age >60, state of obtundation on admission, and development of seizure within 24 hours of admission. This study also showed that 21% of patients developed some type of neurologic deficits, and, overall, 9% had persistence of these deficits at time of discharge (3). Another study showed that baseline features of hypotension, mental status changes, and seizures were associated with increased mortality and neurologic morbidity (16). A more recent large study evaluating the efficacy of adjunctive corticosteroids reported a mortality rate of 15% in the control arm, with mortality of 34% in patients infected with S. pneumoniae (12). Another study suggested that if patients had a rapid progression of their disease, this seemed to correlate with worse outcomes. These investigators found an uncertain correlation between antibiotic timing and unfavorable outcomes (16).
Patients discharged from the hospital should have close follow-up with their primary care physician or infectious disease specialist. Evaluation in the short-term should focus on any complications that may have developed as a result of the bacterial meningitis; such as mental status change, seizure, focal neurologic deficits, and hearing loss. Long-term evaluations should also address cognitive functioning and the neuropsychiatric well-being of the patient, in addition to those issues addressed during short-term follow-up (11, 12).
Dr. Kim may be reached at [email protected].
References
- Swartz, Morton N. Bacterial Meningitis—A View of the Past 90 Years. N Engl J Med. 2004;351:1826-8.
- van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849-59.
- Durand ML, Calderwood SB, Weber DJ, et al. Acute Bacterial Meningitis in Adults. A review of 493 episodes. N Engl J Med. 1993;328:21-8.
- Schuchat A, Robinson K, Wenger J, et al. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med. 1997;337:970-6.
- Attia J, Hatala R, Cook DJ, Wong JG. The rational clinical examination. Does this adult patient have acute meningitis? JAMA. 1999;282:175-181.
- Uchihara T, Tsukagoshi H. Jolt accentuation of headache: the most sensitive sign of CSF pleocytosis. Headache. 1991;31:167-71.
- Gopal AK, Whitehouse JD, Simel DL, Corey RG. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med. 1999;159:2681-5.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before LP in adults with suspected meningitis. N Engl J Med. 2001;345:1727-33.
- Quagliarello VJ, Scheld WM. Treatment of bacterial meningitis. N Engl J Med. 1997;336:708-16.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice Guidelines for the Management of Bacterial Meningitis. Clin Infect Dis. 2004;39:1267-84.
- McIntyre PB, Berkey CS, King SM, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278:928-31.
- de Gans J, van de Beek D Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549-56.
- van de Beek D, de Gans J, McIntyre P, Prasad K. Steroids in adults with acute bacterial meningitis: a systematic review. Lancet Infect Dis 2004;4: 139-43.
- Pile JC, Longworth DL. Should adults with suspected acute bacterial meningitis get adjunctive corticosteroids? Cleve Clin J Med. 2005;72:67-70.
- Control and prevention of meningococcal disease and control and prevention of serogroup C meningococcal disease: evaluation and management of suspected outbreaks: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;4613-21.
- Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129:862-9.