User login
Improving Physicians’ Practices: Hospitalists Add Value
When Robert Lee, MD, an internist affiliated with Iowa Health Physicians, a multi-specialty group in Des Moines, was called to the hospital to see one of his patients, he faced a 50-minute round trip plus additional time to find a parking place and catch an elevator before reaching the inpatient unit. In the time it took for him to see a couple of his patients in the hospital, he could have treated five patients in the office (1).
David McAtee, MD, an osteopath at Murdock Family Medicine, a group practice of eight family physicians in Port Charlotte, Florida, estimates its doctors were spending 30% of their time at the hospital caring for only 5% of their patients (2).
With an eye toward enhancing their office practices and offering patients efficient and effective inpatient treatment, both the Des Moines and Port Charlotte medical groups pursued a growing trend in the health care industry: they turned to hospitalists. Lee notes that the change allows him to enjoy a more normal lifestyle with his family and enhances his income (1). The Murdock group’s decision to contract with hospitalists in 2003 resulted in an expansion of office hours. With more available time, the group is in the process of developing a series of programs targeting various diseases as a means of educating patients in better self-care. Additionally, McAtee expresses the hope that medical malpractice insurance premiums will decrease as a result of less time spent on inpatient care (2).
Hospitalist Impact on Primary Care Physicians
Primary care physicians (PCPs) do have reservations regarding the involvement of hospitalists in the care of their patients. Some PCPs voice concerns about the potential reduction in income if they opt to use hospitalists. According to one estimate, primary care doctors may incur an average annual decrease in income of $25,000 by forgoing hospital rounds. However, studies indicate that PCPs have the potential to earn as much as $50,000 more by spending time in the office instead of seeing inpatients (3). Hospitalist programs that offer on-site, 24-hour availability provide other benefits. When a crisis strikes, PCPs may be difficult to reach as they are seeing office patients. The hurricanes that hit Florida in September and October 2004 clearly demonstrated the value of having continuous inpatient care by qualified physicians already at the hospital. Treacherous weather conditions prevented PCPs from driving to the hospital to see their patients. Although the hospital was unable to perform lab tests, surgeries, or diagnostic imaging procedures owing to power outages, hospitalists were already on-site and stabilized patients with their basic clinical skills (3). Patients who may not have heard of the term “hospitalist” were pleased that a physician was available to answer questions, address unexpected medical issues, and offer immediate support and comfort.
Admittedly, not all PCPs have embraced the hospitalist model. The perception that they might lose skill and prestige by giving up inpatient visits might prevent them from utilizing hospitalist services. In some cases, PCPs might perceive a reduction in continuity of care. These concerns are valid and warrant consideration. However, a well-run hospitalist program will keep communication lines open between hospitalists and PCPs, so that patients receive optimal care as both inpatients and outpatients.
Hospitalists and Surgeons/Specialists
Robert T. Trousdale, MD, orthopedic surgeon at the Mayo Clinic in Rochester, Minnesota, spends most of his day in the operating room or evaluating patients for surgery. An expert in hip and knee surgery, he admits that many orthopedic surgeons have insufficient knowledge when it comes to treating some of the common medical problems that may occur postoperatively. “Hospitalists help us co-manage patients in this area. They bring an increased level of experience to the management of the patient,” he says. Trousdale notes the added benefits of time and hospitalist availability.
“I am in the operating room for 5 hours at a time. If a nurse calls to report that one of my patients has developed post-op dizziness or chest pain, I might not be able to see him for 2 hours,” he says. Hospitalists have both the expertise and the availability to address medical issues in a timelier manner and expedite recovery time.
Additionally, Trousdale admits that, although he is quite familiar with the intricacies of the musculoskeletal system, he is less certain of the necessary tests a patient might need post-operatively. “We might take a ‘shotgun’ approach and order 15 expensive tests, which is an unnecessary use of the hospital’s resources,” he says (4).
Jeanne Huddleston, MD, director of the Inpatient Internal Medicine Program at Mayo Clinic and assistant professor of medicine at the Mayo College of Medicine, led a study to determine the impact hospitalists have on the co-management of patients having hip and knee surgery. The findings, published in 2004, reveal that of 526 patients in the study, more of those managed by hospitalist-orthopedic teams were discharged with no complications (61.6% for hospitalist-orthopedic teams vs. 48.8% for traditional orthopedic surgical teams). Only 30.2% of patients co-managed by hospitalists experienced minor complications, while 44.3% of patients managed by traditional orthopedic surgical teams had similar difficulties. Huddleston notes also that most orthopedic surgeons and nurses responding to a satisfaction survey preferred the hospitalist orthopedic model (5).
Hospitalists and Emergency Department Physicians
Brent R. Asplin, MD, MPH, research director in the department of emergency medicine at Regions Hospital in St. Paul, Minnesota, cites three ways in which hospitalists positively affect the emergency department (ED): through extraordinary availability, consistent and reliable care, and their focus on the hospital. “Hospitalists are available 24 hours a day,” he says. “It’s nice to know when you send a patient to the floor, there is an experienced physician in-house to take care of them. You do not have to try to reach a PCP on the phone.” He reports that capacity is a major problem for EDs. Bottlenecks result when there are patients who are ready to be admitted from the ED but must wait for other patients to be discharged. Hospitalists are always available to maintain a smooth patient flow and facilitate throughput, according to Asplin.
As a group, hospitalists adhere to a consistent approach to patient care. Once a patient is admitted, efficient, reliable in-house care will ensure a quick recovery and discharge. Asplin says, “Hospitalists are more likely to embrace clinical pathways for the most common clinical diagnoses. This reduces variability across the board and increases patient outcome and flow.” Also, hospitalists focus exclusively on inpatient care, enabling them to devote all their attention to servicing the patient while they are hospitalized without the distractions that might divert a PCP’s concentration. Asplin says, “Regarding clinical care, operations, and quality improvement, it helps to have a group dedicated and focused on the hospital” (6).
In teaching hospitals, residents also benefit from the presence of hospitalists. According to Barbara LeTourneau, MD, an ED physician and professional physician executive consultant also based at Regions, residents have the continuous supervision of experienced practitioners who can answer questions and teach on an ongoing basis. “With hospitalists there is much quicker and better patient care,” she says.
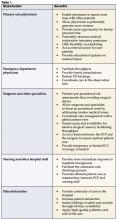
In her role as administrator, LeTourneau has an historical perspective on the delivery of inpatient care at her hospital. Prior to the implementation of hospital medicine programs, positive changes took a longer period of time to reach agreement and execution, she reports. “Having hospitalists here provides one group of experienced physicians who see a large percentage of patients,” says LeTourneau. Managing a significant caseload enables the hospitalist to understand the system in depth. “Hospitalists can provide good feedback and make it easier to implement necessary changes,” LeTourneau says (7) (Table 1, page 26).
Stakeholder Analysis
Studies reveal that hospitalists improve the practices of physicians and several subspecialties in a number of ways. Not only do PCPs benefit from the presence of hospitalists, but other medical specialists, patients, families, and medical facilities gain advantages as well.
Research Studies
Since 1996 when the term “hospitalist” was first used, a number of studies have been conducted to evaluate the benefits they bring to PCPs and other physicians. In the past decade, the number of hospitalists has increased dramatically, lending credence to their value in an inpatient medical setting. In 2005, the Society of Hospital Medicine (SHM) estimates that there are 12,000 hospitalists in the U.S.
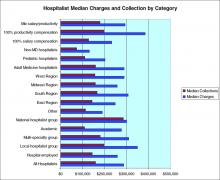
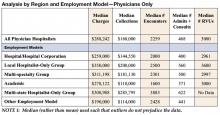
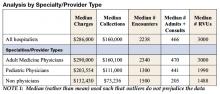
In a survey by Mitretek Healthcare , researchers asked hospital leaders to rate a number of strategies that affect hospital–medical staff relations. Sixty-two percent of the leaders surveyed gave hospitalist programs a high rating pertaining to hospital-physician alignment (8). Other studies also support the growing belief that hospitalists can effectively and efficiently enhance physician practices (Table 2).
Conclusion
Joseph Li, MD, director of the hospitalist program at Beth Israel Deaconess Medical Center in Boston, hopes to build a career based on the belief that hospitalists are leading the way in “preventing medical errors and hospital-acquired infections, managing the complex hospital environment, finding the right transition to home care or rehabilitation, and providing palliative and end-of-life care” (9). As hospital medicine programs become more prevalent and accepted, more and more PCPs are seeing the value in their presence. A major national hospitalist management company surveyed PCPs in five markets on their experiences with hospitalists. The responses revealed a 100% satisfaction rating on the quality of inpatient care (10). In the future, hospitalists like Li will strive to maintain that rating while they help improve physician practices and enhance patient care.
Dr. Kealey can be contact at [email protected].
References
- Jackson C. Doctors find hospitalists save time, money: primary care physicians are seeing that turning over their hospital business allows them to make more income. Amednews.com, February 19, 2001.
- Trendy hospital medicine comes to Charlotte. Sunherald.com, February 13, 2004.
- Landro L. Medicine’s fastest-growing specialty: hospitalbound doctors take the place of your physician; effort to reduce costs, errors. The Wall Street Journal Online, October 6, 2004.
- Trousdale RT. Department of Orthopedics, Mayo Clinic, Rochester, MN. Telephone interview, January 3, 2005.
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized controlled trial. Ann Intern Med. 2004;141:28-38.
- Asplin BR. research director, Department of Emergency Medicine, Regions Hospital, St. Paul, MN. Telephone interview, January 5, 2005.
- LeTourneau B. Emergency department physician, professional physician executive consultant, Regions Hospital, St. Paul, MN. Telephone interview, January 7, 2005.
- McGowan RA. Strengthening hospital-physician relationships. Healthcare Financial Management Association. December 2004. www.hfma.org/publications/HFMMagazine/business.htm
- Barnard A. Medical profession, patients have warmed to the ‘hospitalist’. The Boston Globe. January 30, 2002.
- PCPs and hospitalists: a new attitude? Cogent Quarterly. 1;4:Fall 2001.
- Auerbach AD, Aronson MD, Davis RB, Phillips RS. How physicians perceive hospitalist services after implementation: anticipation vs. reality. Arch Intern Med. 2003;163:2330-6.
- Auerbach AD, Nelson EA, Lindenauer PK, Pantilat SZ, Katz PP, Wachter RM. Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey. Am J Med. 2000;109(8):648-53.
- Halpert AP, Pearson SD, LeWine HE, et al. The impact of an inpatient physician program on quality, utilization, and satisfaction. Am J Manag Care. 2000;6:549-55.
- Fernandez A, Grumbach K, Goetein L, et al. Friend or foe? How primary care physicians perceive hospitalists. Arch Intern Med. 2000;160:2902-8.
When Robert Lee, MD, an internist affiliated with Iowa Health Physicians, a multi-specialty group in Des Moines, was called to the hospital to see one of his patients, he faced a 50-minute round trip plus additional time to find a parking place and catch an elevator before reaching the inpatient unit. In the time it took for him to see a couple of his patients in the hospital, he could have treated five patients in the office (1).
David McAtee, MD, an osteopath at Murdock Family Medicine, a group practice of eight family physicians in Port Charlotte, Florida, estimates its doctors were spending 30% of their time at the hospital caring for only 5% of their patients (2).
With an eye toward enhancing their office practices and offering patients efficient and effective inpatient treatment, both the Des Moines and Port Charlotte medical groups pursued a growing trend in the health care industry: they turned to hospitalists. Lee notes that the change allows him to enjoy a more normal lifestyle with his family and enhances his income (1). The Murdock group’s decision to contract with hospitalists in 2003 resulted in an expansion of office hours. With more available time, the group is in the process of developing a series of programs targeting various diseases as a means of educating patients in better self-care. Additionally, McAtee expresses the hope that medical malpractice insurance premiums will decrease as a result of less time spent on inpatient care (2).
Hospitalist Impact on Primary Care Physicians
Primary care physicians (PCPs) do have reservations regarding the involvement of hospitalists in the care of their patients. Some PCPs voice concerns about the potential reduction in income if they opt to use hospitalists. According to one estimate, primary care doctors may incur an average annual decrease in income of $25,000 by forgoing hospital rounds. However, studies indicate that PCPs have the potential to earn as much as $50,000 more by spending time in the office instead of seeing inpatients (3). Hospitalist programs that offer on-site, 24-hour availability provide other benefits. When a crisis strikes, PCPs may be difficult to reach as they are seeing office patients. The hurricanes that hit Florida in September and October 2004 clearly demonstrated the value of having continuous inpatient care by qualified physicians already at the hospital. Treacherous weather conditions prevented PCPs from driving to the hospital to see their patients. Although the hospital was unable to perform lab tests, surgeries, or diagnostic imaging procedures owing to power outages, hospitalists were already on-site and stabilized patients with their basic clinical skills (3). Patients who may not have heard of the term “hospitalist” were pleased that a physician was available to answer questions, address unexpected medical issues, and offer immediate support and comfort.
Admittedly, not all PCPs have embraced the hospitalist model. The perception that they might lose skill and prestige by giving up inpatient visits might prevent them from utilizing hospitalist services. In some cases, PCPs might perceive a reduction in continuity of care. These concerns are valid and warrant consideration. However, a well-run hospitalist program will keep communication lines open between hospitalists and PCPs, so that patients receive optimal care as both inpatients and outpatients.
Hospitalists and Surgeons/Specialists
Robert T. Trousdale, MD, orthopedic surgeon at the Mayo Clinic in Rochester, Minnesota, spends most of his day in the operating room or evaluating patients for surgery. An expert in hip and knee surgery, he admits that many orthopedic surgeons have insufficient knowledge when it comes to treating some of the common medical problems that may occur postoperatively. “Hospitalists help us co-manage patients in this area. They bring an increased level of experience to the management of the patient,” he says. Trousdale notes the added benefits of time and hospitalist availability.
“I am in the operating room for 5 hours at a time. If a nurse calls to report that one of my patients has developed post-op dizziness or chest pain, I might not be able to see him for 2 hours,” he says. Hospitalists have both the expertise and the availability to address medical issues in a timelier manner and expedite recovery time.
Additionally, Trousdale admits that, although he is quite familiar with the intricacies of the musculoskeletal system, he is less certain of the necessary tests a patient might need post-operatively. “We might take a ‘shotgun’ approach and order 15 expensive tests, which is an unnecessary use of the hospital’s resources,” he says (4).
Jeanne Huddleston, MD, director of the Inpatient Internal Medicine Program at Mayo Clinic and assistant professor of medicine at the Mayo College of Medicine, led a study to determine the impact hospitalists have on the co-management of patients having hip and knee surgery. The findings, published in 2004, reveal that of 526 patients in the study, more of those managed by hospitalist-orthopedic teams were discharged with no complications (61.6% for hospitalist-orthopedic teams vs. 48.8% for traditional orthopedic surgical teams). Only 30.2% of patients co-managed by hospitalists experienced minor complications, while 44.3% of patients managed by traditional orthopedic surgical teams had similar difficulties. Huddleston notes also that most orthopedic surgeons and nurses responding to a satisfaction survey preferred the hospitalist orthopedic model (5).
Hospitalists and Emergency Department Physicians
Brent R. Asplin, MD, MPH, research director in the department of emergency medicine at Regions Hospital in St. Paul, Minnesota, cites three ways in which hospitalists positively affect the emergency department (ED): through extraordinary availability, consistent and reliable care, and their focus on the hospital. “Hospitalists are available 24 hours a day,” he says. “It’s nice to know when you send a patient to the floor, there is an experienced physician in-house to take care of them. You do not have to try to reach a PCP on the phone.” He reports that capacity is a major problem for EDs. Bottlenecks result when there are patients who are ready to be admitted from the ED but must wait for other patients to be discharged. Hospitalists are always available to maintain a smooth patient flow and facilitate throughput, according to Asplin.
As a group, hospitalists adhere to a consistent approach to patient care. Once a patient is admitted, efficient, reliable in-house care will ensure a quick recovery and discharge. Asplin says, “Hospitalists are more likely to embrace clinical pathways for the most common clinical diagnoses. This reduces variability across the board and increases patient outcome and flow.” Also, hospitalists focus exclusively on inpatient care, enabling them to devote all their attention to servicing the patient while they are hospitalized without the distractions that might divert a PCP’s concentration. Asplin says, “Regarding clinical care, operations, and quality improvement, it helps to have a group dedicated and focused on the hospital” (6).
In teaching hospitals, residents also benefit from the presence of hospitalists. According to Barbara LeTourneau, MD, an ED physician and professional physician executive consultant also based at Regions, residents have the continuous supervision of experienced practitioners who can answer questions and teach on an ongoing basis. “With hospitalists there is much quicker and better patient care,” she says.
In her role as administrator, LeTourneau has an historical perspective on the delivery of inpatient care at her hospital. Prior to the implementation of hospital medicine programs, positive changes took a longer period of time to reach agreement and execution, she reports. “Having hospitalists here provides one group of experienced physicians who see a large percentage of patients,” says LeTourneau. Managing a significant caseload enables the hospitalist to understand the system in depth. “Hospitalists can provide good feedback and make it easier to implement necessary changes,” LeTourneau says (7) (Table 1, page 26).
Stakeholder Analysis
Studies reveal that hospitalists improve the practices of physicians and several subspecialties in a number of ways. Not only do PCPs benefit from the presence of hospitalists, but other medical specialists, patients, families, and medical facilities gain advantages as well.
Research Studies
Since 1996 when the term “hospitalist” was first used, a number of studies have been conducted to evaluate the benefits they bring to PCPs and other physicians. In the past decade, the number of hospitalists has increased dramatically, lending credence to their value in an inpatient medical setting. In 2005, the Society of Hospital Medicine (SHM) estimates that there are 12,000 hospitalists in the U.S.
In a survey by Mitretek Healthcare , researchers asked hospital leaders to rate a number of strategies that affect hospital–medical staff relations. Sixty-two percent of the leaders surveyed gave hospitalist programs a high rating pertaining to hospital-physician alignment (8). Other studies also support the growing belief that hospitalists can effectively and efficiently enhance physician practices (Table 2).
Conclusion
Joseph Li, MD, director of the hospitalist program at Beth Israel Deaconess Medical Center in Boston, hopes to build a career based on the belief that hospitalists are leading the way in “preventing medical errors and hospital-acquired infections, managing the complex hospital environment, finding the right transition to home care or rehabilitation, and providing palliative and end-of-life care” (9). As hospital medicine programs become more prevalent and accepted, more and more PCPs are seeing the value in their presence. A major national hospitalist management company surveyed PCPs in five markets on their experiences with hospitalists. The responses revealed a 100% satisfaction rating on the quality of inpatient care (10). In the future, hospitalists like Li will strive to maintain that rating while they help improve physician practices and enhance patient care.
Dr. Kealey can be contact at [email protected].
References
- Jackson C. Doctors find hospitalists save time, money: primary care physicians are seeing that turning over their hospital business allows them to make more income. Amednews.com, February 19, 2001.
- Trendy hospital medicine comes to Charlotte. Sunherald.com, February 13, 2004.
- Landro L. Medicine’s fastest-growing specialty: hospitalbound doctors take the place of your physician; effort to reduce costs, errors. The Wall Street Journal Online, October 6, 2004.
- Trousdale RT. Department of Orthopedics, Mayo Clinic, Rochester, MN. Telephone interview, January 3, 2005.
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized controlled trial. Ann Intern Med. 2004;141:28-38.
- Asplin BR. research director, Department of Emergency Medicine, Regions Hospital, St. Paul, MN. Telephone interview, January 5, 2005.
- LeTourneau B. Emergency department physician, professional physician executive consultant, Regions Hospital, St. Paul, MN. Telephone interview, January 7, 2005.
- McGowan RA. Strengthening hospital-physician relationships. Healthcare Financial Management Association. December 2004. www.hfma.org/publications/HFMMagazine/business.htm
- Barnard A. Medical profession, patients have warmed to the ‘hospitalist’. The Boston Globe. January 30, 2002.
- PCPs and hospitalists: a new attitude? Cogent Quarterly. 1;4:Fall 2001.
- Auerbach AD, Aronson MD, Davis RB, Phillips RS. How physicians perceive hospitalist services after implementation: anticipation vs. reality. Arch Intern Med. 2003;163:2330-6.
- Auerbach AD, Nelson EA, Lindenauer PK, Pantilat SZ, Katz PP, Wachter RM. Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey. Am J Med. 2000;109(8):648-53.
- Halpert AP, Pearson SD, LeWine HE, et al. The impact of an inpatient physician program on quality, utilization, and satisfaction. Am J Manag Care. 2000;6:549-55.
- Fernandez A, Grumbach K, Goetein L, et al. Friend or foe? How primary care physicians perceive hospitalists. Arch Intern Med. 2000;160:2902-8.
When Robert Lee, MD, an internist affiliated with Iowa Health Physicians, a multi-specialty group in Des Moines, was called to the hospital to see one of his patients, he faced a 50-minute round trip plus additional time to find a parking place and catch an elevator before reaching the inpatient unit. In the time it took for him to see a couple of his patients in the hospital, he could have treated five patients in the office (1).
David McAtee, MD, an osteopath at Murdock Family Medicine, a group practice of eight family physicians in Port Charlotte, Florida, estimates its doctors were spending 30% of their time at the hospital caring for only 5% of their patients (2).
With an eye toward enhancing their office practices and offering patients efficient and effective inpatient treatment, both the Des Moines and Port Charlotte medical groups pursued a growing trend in the health care industry: they turned to hospitalists. Lee notes that the change allows him to enjoy a more normal lifestyle with his family and enhances his income (1). The Murdock group’s decision to contract with hospitalists in 2003 resulted in an expansion of office hours. With more available time, the group is in the process of developing a series of programs targeting various diseases as a means of educating patients in better self-care. Additionally, McAtee expresses the hope that medical malpractice insurance premiums will decrease as a result of less time spent on inpatient care (2).
Hospitalist Impact on Primary Care Physicians
Primary care physicians (PCPs) do have reservations regarding the involvement of hospitalists in the care of their patients. Some PCPs voice concerns about the potential reduction in income if they opt to use hospitalists. According to one estimate, primary care doctors may incur an average annual decrease in income of $25,000 by forgoing hospital rounds. However, studies indicate that PCPs have the potential to earn as much as $50,000 more by spending time in the office instead of seeing inpatients (3). Hospitalist programs that offer on-site, 24-hour availability provide other benefits. When a crisis strikes, PCPs may be difficult to reach as they are seeing office patients. The hurricanes that hit Florida in September and October 2004 clearly demonstrated the value of having continuous inpatient care by qualified physicians already at the hospital. Treacherous weather conditions prevented PCPs from driving to the hospital to see their patients. Although the hospital was unable to perform lab tests, surgeries, or diagnostic imaging procedures owing to power outages, hospitalists were already on-site and stabilized patients with their basic clinical skills (3). Patients who may not have heard of the term “hospitalist” were pleased that a physician was available to answer questions, address unexpected medical issues, and offer immediate support and comfort.
Admittedly, not all PCPs have embraced the hospitalist model. The perception that they might lose skill and prestige by giving up inpatient visits might prevent them from utilizing hospitalist services. In some cases, PCPs might perceive a reduction in continuity of care. These concerns are valid and warrant consideration. However, a well-run hospitalist program will keep communication lines open between hospitalists and PCPs, so that patients receive optimal care as both inpatients and outpatients.
Hospitalists and Surgeons/Specialists
Robert T. Trousdale, MD, orthopedic surgeon at the Mayo Clinic in Rochester, Minnesota, spends most of his day in the operating room or evaluating patients for surgery. An expert in hip and knee surgery, he admits that many orthopedic surgeons have insufficient knowledge when it comes to treating some of the common medical problems that may occur postoperatively. “Hospitalists help us co-manage patients in this area. They bring an increased level of experience to the management of the patient,” he says. Trousdale notes the added benefits of time and hospitalist availability.
“I am in the operating room for 5 hours at a time. If a nurse calls to report that one of my patients has developed post-op dizziness or chest pain, I might not be able to see him for 2 hours,” he says. Hospitalists have both the expertise and the availability to address medical issues in a timelier manner and expedite recovery time.
Additionally, Trousdale admits that, although he is quite familiar with the intricacies of the musculoskeletal system, he is less certain of the necessary tests a patient might need post-operatively. “We might take a ‘shotgun’ approach and order 15 expensive tests, which is an unnecessary use of the hospital’s resources,” he says (4).
Jeanne Huddleston, MD, director of the Inpatient Internal Medicine Program at Mayo Clinic and assistant professor of medicine at the Mayo College of Medicine, led a study to determine the impact hospitalists have on the co-management of patients having hip and knee surgery. The findings, published in 2004, reveal that of 526 patients in the study, more of those managed by hospitalist-orthopedic teams were discharged with no complications (61.6% for hospitalist-orthopedic teams vs. 48.8% for traditional orthopedic surgical teams). Only 30.2% of patients co-managed by hospitalists experienced minor complications, while 44.3% of patients managed by traditional orthopedic surgical teams had similar difficulties. Huddleston notes also that most orthopedic surgeons and nurses responding to a satisfaction survey preferred the hospitalist orthopedic model (5).
Hospitalists and Emergency Department Physicians
Brent R. Asplin, MD, MPH, research director in the department of emergency medicine at Regions Hospital in St. Paul, Minnesota, cites three ways in which hospitalists positively affect the emergency department (ED): through extraordinary availability, consistent and reliable care, and their focus on the hospital. “Hospitalists are available 24 hours a day,” he says. “It’s nice to know when you send a patient to the floor, there is an experienced physician in-house to take care of them. You do not have to try to reach a PCP on the phone.” He reports that capacity is a major problem for EDs. Bottlenecks result when there are patients who are ready to be admitted from the ED but must wait for other patients to be discharged. Hospitalists are always available to maintain a smooth patient flow and facilitate throughput, according to Asplin.
As a group, hospitalists adhere to a consistent approach to patient care. Once a patient is admitted, efficient, reliable in-house care will ensure a quick recovery and discharge. Asplin says, “Hospitalists are more likely to embrace clinical pathways for the most common clinical diagnoses. This reduces variability across the board and increases patient outcome and flow.” Also, hospitalists focus exclusively on inpatient care, enabling them to devote all their attention to servicing the patient while they are hospitalized without the distractions that might divert a PCP’s concentration. Asplin says, “Regarding clinical care, operations, and quality improvement, it helps to have a group dedicated and focused on the hospital” (6).
In teaching hospitals, residents also benefit from the presence of hospitalists. According to Barbara LeTourneau, MD, an ED physician and professional physician executive consultant also based at Regions, residents have the continuous supervision of experienced practitioners who can answer questions and teach on an ongoing basis. “With hospitalists there is much quicker and better patient care,” she says.
In her role as administrator, LeTourneau has an historical perspective on the delivery of inpatient care at her hospital. Prior to the implementation of hospital medicine programs, positive changes took a longer period of time to reach agreement and execution, she reports. “Having hospitalists here provides one group of experienced physicians who see a large percentage of patients,” says LeTourneau. Managing a significant caseload enables the hospitalist to understand the system in depth. “Hospitalists can provide good feedback and make it easier to implement necessary changes,” LeTourneau says (7) (Table 1, page 26).
Stakeholder Analysis
Studies reveal that hospitalists improve the practices of physicians and several subspecialties in a number of ways. Not only do PCPs benefit from the presence of hospitalists, but other medical specialists, patients, families, and medical facilities gain advantages as well.
Research Studies
Since 1996 when the term “hospitalist” was first used, a number of studies have been conducted to evaluate the benefits they bring to PCPs and other physicians. In the past decade, the number of hospitalists has increased dramatically, lending credence to their value in an inpatient medical setting. In 2005, the Society of Hospital Medicine (SHM) estimates that there are 12,000 hospitalists in the U.S.
In a survey by Mitretek Healthcare , researchers asked hospital leaders to rate a number of strategies that affect hospital–medical staff relations. Sixty-two percent of the leaders surveyed gave hospitalist programs a high rating pertaining to hospital-physician alignment (8). Other studies also support the growing belief that hospitalists can effectively and efficiently enhance physician practices (Table 2).
Conclusion
Joseph Li, MD, director of the hospitalist program at Beth Israel Deaconess Medical Center in Boston, hopes to build a career based on the belief that hospitalists are leading the way in “preventing medical errors and hospital-acquired infections, managing the complex hospital environment, finding the right transition to home care or rehabilitation, and providing palliative and end-of-life care” (9). As hospital medicine programs become more prevalent and accepted, more and more PCPs are seeing the value in their presence. A major national hospitalist management company surveyed PCPs in five markets on their experiences with hospitalists. The responses revealed a 100% satisfaction rating on the quality of inpatient care (10). In the future, hospitalists like Li will strive to maintain that rating while they help improve physician practices and enhance patient care.
Dr. Kealey can be contact at [email protected].
References
- Jackson C. Doctors find hospitalists save time, money: primary care physicians are seeing that turning over their hospital business allows them to make more income. Amednews.com, February 19, 2001.
- Trendy hospital medicine comes to Charlotte. Sunherald.com, February 13, 2004.
- Landro L. Medicine’s fastest-growing specialty: hospitalbound doctors take the place of your physician; effort to reduce costs, errors. The Wall Street Journal Online, October 6, 2004.
- Trousdale RT. Department of Orthopedics, Mayo Clinic, Rochester, MN. Telephone interview, January 3, 2005.
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized controlled trial. Ann Intern Med. 2004;141:28-38.
- Asplin BR. research director, Department of Emergency Medicine, Regions Hospital, St. Paul, MN. Telephone interview, January 5, 2005.
- LeTourneau B. Emergency department physician, professional physician executive consultant, Regions Hospital, St. Paul, MN. Telephone interview, January 7, 2005.
- McGowan RA. Strengthening hospital-physician relationships. Healthcare Financial Management Association. December 2004. www.hfma.org/publications/HFMMagazine/business.htm
- Barnard A. Medical profession, patients have warmed to the ‘hospitalist’. The Boston Globe. January 30, 2002.
- PCPs and hospitalists: a new attitude? Cogent Quarterly. 1;4:Fall 2001.
- Auerbach AD, Aronson MD, Davis RB, Phillips RS. How physicians perceive hospitalist services after implementation: anticipation vs. reality. Arch Intern Med. 2003;163:2330-6.
- Auerbach AD, Nelson EA, Lindenauer PK, Pantilat SZ, Katz PP, Wachter RM. Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey. Am J Med. 2000;109(8):648-53.
- Halpert AP, Pearson SD, LeWine HE, et al. The impact of an inpatient physician program on quality, utilization, and satisfaction. Am J Manag Care. 2000;6:549-55.
- Fernandez A, Grumbach K, Goetein L, et al. Friend or foe? How primary care physicians perceive hospitalists. Arch Intern Med. 2000;160:2902-8.
First Ever SHM Leadership Academy— A Rousing Success
What would prompt someone to exclaim “fantastic, inspirational, and motivational,” and trigger adults to hug each other when it is time to say goodbye? The first-ever SHM Leadership Academy welcomed 110 hospitalist leaders to the Westin La Paloma Resort in Tucson, AZ on January 10–13, 2005. A resounding success, the Leadership Academy offered instruction in leading change, communicating effectively, handling conflict and negotiation, strategic planning, and interpreting hospital business drivers. Two years in the making, this course combined an outstanding national faculty with small group learning exercises to begin the process of training hospitalists who will lead important initiatives as we shape the hospital of the future.
At the 2003 SHM Annual Meeting in San Diego, a standing room crowd of about 200 hospitalists at the Leadership Forum expressed their need for advanced leadership training. Responding to obvious demand, SHM developed a successful, soldout 1-day Leadership Pre-Course held in New Orleans at the 2004 SHM Annual Meeting. Building on this pre-course and again reacting to the requests of SHM members, Co-Directors Russell Holman and Mark Williams designed the Leadership Academy to provide more in-depth training over 4 days. Assisted by Tina Budnitz, SHM Senior Advisor for Planning and Development, this course was developed to address the leadership training needs of hospitalists. As an example of its resounding success, one participant made the following comment. “Even with 18 years of clinical/administrative experience as well as an MBA, this course was a learning experience and I gained and reinforced critical areas of thinking and actions.”
Credit for this success deservedly should be attributed to the outstanding faculty. SHM’s CEO Larry Wellikson led the first day, eloquently delineating the leadership challenges in hospital medicine. The audience appreciated how hospital medicine is evolving rapidly, still defining itself, and how hospitalists will be developing metrics for success. The remainder of the first day allowed participants to evaluate their own strengths and assess how their unique styles impact interactions with others. Using the Strength Deployment Inventory®, David Javitch, PhD explored how “reds,” “greens,” and “blues” approach situations and communicate with their colleagues. Javitch, an organizational psychologist from Harvard, demonstrated how individual styles of communication and interaction influence success at management and leadership. SHM Member Eric Howell, MD moderated a discussion featuring a movie capturing common hospital-based examples of conflict and led participants through techniques for conflict resolution and negotiation.
On the second day, Michael Guthrie, MD, MBA identified business drivers for hospital survival and success. Guthrie currently serves as a senior executive for a large national health alliance and has experience as a health system CEO, medical director, and consultant on performance improvement. Guthrie finished the morning by helping attendees interpret hospital performance reports and associated metrics and determine how such measures should guide leadership planning and decision making.
The next day was highlighted by sessions led by Jack Silversin, DMD, DrPH from Harvard, using table exercises and real world examples to demonstrate how to lead change. A nationally recognized expert in change management and co-author of “Leading Physicians through Change: How to Achieve and Sustain Results,” Dr. Silversin actively stimulated attendees to appraise their situations at home. He showed participants how to develop shared organizational vision, strengthen leadership, and accelerate implementation of change. Afterwards, Holman and Williams coordinated a series of sessions on strategic planning. They used multiple examples and exercises to aid attendees in developing vision and mission statements, as well as “SMART” goals.
The final day focused on communication. An experienced educator, Kathleen Miner, PhD, MPH, MEd, reviewed communication theory and how it applies to our everyday conversations and interactions. Miner, an Associate Dean for Public Health at Emory University, brought decades of experience to her presentation. The course ended with Holman recapping how to use what we learned to achieve success as a leader.
Overall, the course was structured to facilitate interaction and small group exercises. The interactive sessions provided opportunities for participants to apply concepts.
Use of facilitators greatly augmented the impact of this training. Participants in the course sat ten to a table, and each table was led by an experienced hospitalist leader trained to be a facilitator. We were extraordinarily fortunate to have leaders in hospital medicine as facilitators including: Mary Jo Gorman, Bill Atchley, Pat Cawley, Lisa Kettering, Alpesh Amin, Ron Greeno, Burke Kealey, Eric Siegal, Stacy Goldsholl, and Eric Howell.
The impact of the meeting was powerfully described by a facilitator, “I’ve never before experienced such sustained energy and enthusiasm at a meeting. People literally spent hours after the didactic sessions talking, sharing ideas, and commiserating. Speaks to the pent-up need for this, and the effectiveness of the curriculum in galvanizing the group.”
No meeting can be such a success without tremendous support from SHM staff. Angela Musial and Erica Pearson deserve our sincere thanks for handling all the logistical issues and guaranteeing a terrific time for everyone who attended. They ensured that everything worked without a hitch including two wonderful receptions, which fostered networking and opportunities to share challenges and success stories.
The Society of Hospital Medicine will hold another Leadership Academy this Fall: September 12–15 in Vail, Colorado. The learning objectives for the Leadership Academy highlight the skills hospitalists can gain by attending.
- Evaluate personal leadership strengths and weaknesses and apply them to everyday leadership and management challenges
- Effectively advocate the value of their Hospital Medicine program
- Predict and plan for the near-term challenges affecting the viability of their Hospital Medicine program
- Improve patient outcomes through successful planning, allocation of resources, collaboration, teamwork, and execution
- Create and execute a communication strategy for all key constituencies
- Interpret key hospital drivers
- Examine how hospital performance metrics are derived and how hospital medicine practices can influence and impact these metrics
- Implement methods of effective change through leadership, shared vision, and managing the organizational culture
- Utilize strategic planning to define a vision for their program, prioritize efforts, and achieve designated goals
Registration will again be limited to 100 hospitalist leaders and we expect this to fill quickly. The first Leadership Academy was sold out months before it was held, and interest in the September 2005 Leadership Academy in Vail is equally as strong after the rousing success of the January meeting in Tucson. If you are interested in attending, registration information can be found on page 19, at the SHM Web site at www.hospitalmedicine.org, or by calling SHM at 800-843-3360. We look forward to seeing you there.
What would prompt someone to exclaim “fantastic, inspirational, and motivational,” and trigger adults to hug each other when it is time to say goodbye? The first-ever SHM Leadership Academy welcomed 110 hospitalist leaders to the Westin La Paloma Resort in Tucson, AZ on January 10–13, 2005. A resounding success, the Leadership Academy offered instruction in leading change, communicating effectively, handling conflict and negotiation, strategic planning, and interpreting hospital business drivers. Two years in the making, this course combined an outstanding national faculty with small group learning exercises to begin the process of training hospitalists who will lead important initiatives as we shape the hospital of the future.
At the 2003 SHM Annual Meeting in San Diego, a standing room crowd of about 200 hospitalists at the Leadership Forum expressed their need for advanced leadership training. Responding to obvious demand, SHM developed a successful, soldout 1-day Leadership Pre-Course held in New Orleans at the 2004 SHM Annual Meeting. Building on this pre-course and again reacting to the requests of SHM members, Co-Directors Russell Holman and Mark Williams designed the Leadership Academy to provide more in-depth training over 4 days. Assisted by Tina Budnitz, SHM Senior Advisor for Planning and Development, this course was developed to address the leadership training needs of hospitalists. As an example of its resounding success, one participant made the following comment. “Even with 18 years of clinical/administrative experience as well as an MBA, this course was a learning experience and I gained and reinforced critical areas of thinking and actions.”
Credit for this success deservedly should be attributed to the outstanding faculty. SHM’s CEO Larry Wellikson led the first day, eloquently delineating the leadership challenges in hospital medicine. The audience appreciated how hospital medicine is evolving rapidly, still defining itself, and how hospitalists will be developing metrics for success. The remainder of the first day allowed participants to evaluate their own strengths and assess how their unique styles impact interactions with others. Using the Strength Deployment Inventory®, David Javitch, PhD explored how “reds,” “greens,” and “blues” approach situations and communicate with their colleagues. Javitch, an organizational psychologist from Harvard, demonstrated how individual styles of communication and interaction influence success at management and leadership. SHM Member Eric Howell, MD moderated a discussion featuring a movie capturing common hospital-based examples of conflict and led participants through techniques for conflict resolution and negotiation.
On the second day, Michael Guthrie, MD, MBA identified business drivers for hospital survival and success. Guthrie currently serves as a senior executive for a large national health alliance and has experience as a health system CEO, medical director, and consultant on performance improvement. Guthrie finished the morning by helping attendees interpret hospital performance reports and associated metrics and determine how such measures should guide leadership planning and decision making.
The next day was highlighted by sessions led by Jack Silversin, DMD, DrPH from Harvard, using table exercises and real world examples to demonstrate how to lead change. A nationally recognized expert in change management and co-author of “Leading Physicians through Change: How to Achieve and Sustain Results,” Dr. Silversin actively stimulated attendees to appraise their situations at home. He showed participants how to develop shared organizational vision, strengthen leadership, and accelerate implementation of change. Afterwards, Holman and Williams coordinated a series of sessions on strategic planning. They used multiple examples and exercises to aid attendees in developing vision and mission statements, as well as “SMART” goals.
The final day focused on communication. An experienced educator, Kathleen Miner, PhD, MPH, MEd, reviewed communication theory and how it applies to our everyday conversations and interactions. Miner, an Associate Dean for Public Health at Emory University, brought decades of experience to her presentation. The course ended with Holman recapping how to use what we learned to achieve success as a leader.
Overall, the course was structured to facilitate interaction and small group exercises. The interactive sessions provided opportunities for participants to apply concepts.
Use of facilitators greatly augmented the impact of this training. Participants in the course sat ten to a table, and each table was led by an experienced hospitalist leader trained to be a facilitator. We were extraordinarily fortunate to have leaders in hospital medicine as facilitators including: Mary Jo Gorman, Bill Atchley, Pat Cawley, Lisa Kettering, Alpesh Amin, Ron Greeno, Burke Kealey, Eric Siegal, Stacy Goldsholl, and Eric Howell.
The impact of the meeting was powerfully described by a facilitator, “I’ve never before experienced such sustained energy and enthusiasm at a meeting. People literally spent hours after the didactic sessions talking, sharing ideas, and commiserating. Speaks to the pent-up need for this, and the effectiveness of the curriculum in galvanizing the group.”
No meeting can be such a success without tremendous support from SHM staff. Angela Musial and Erica Pearson deserve our sincere thanks for handling all the logistical issues and guaranteeing a terrific time for everyone who attended. They ensured that everything worked without a hitch including two wonderful receptions, which fostered networking and opportunities to share challenges and success stories.
The Society of Hospital Medicine will hold another Leadership Academy this Fall: September 12–15 in Vail, Colorado. The learning objectives for the Leadership Academy highlight the skills hospitalists can gain by attending.
- Evaluate personal leadership strengths and weaknesses and apply them to everyday leadership and management challenges
- Effectively advocate the value of their Hospital Medicine program
- Predict and plan for the near-term challenges affecting the viability of their Hospital Medicine program
- Improve patient outcomes through successful planning, allocation of resources, collaboration, teamwork, and execution
- Create and execute a communication strategy for all key constituencies
- Interpret key hospital drivers
- Examine how hospital performance metrics are derived and how hospital medicine practices can influence and impact these metrics
- Implement methods of effective change through leadership, shared vision, and managing the organizational culture
- Utilize strategic planning to define a vision for their program, prioritize efforts, and achieve designated goals
Registration will again be limited to 100 hospitalist leaders and we expect this to fill quickly. The first Leadership Academy was sold out months before it was held, and interest in the September 2005 Leadership Academy in Vail is equally as strong after the rousing success of the January meeting in Tucson. If you are interested in attending, registration information can be found on page 19, at the SHM Web site at www.hospitalmedicine.org, or by calling SHM at 800-843-3360. We look forward to seeing you there.
What would prompt someone to exclaim “fantastic, inspirational, and motivational,” and trigger adults to hug each other when it is time to say goodbye? The first-ever SHM Leadership Academy welcomed 110 hospitalist leaders to the Westin La Paloma Resort in Tucson, AZ on January 10–13, 2005. A resounding success, the Leadership Academy offered instruction in leading change, communicating effectively, handling conflict and negotiation, strategic planning, and interpreting hospital business drivers. Two years in the making, this course combined an outstanding national faculty with small group learning exercises to begin the process of training hospitalists who will lead important initiatives as we shape the hospital of the future.
At the 2003 SHM Annual Meeting in San Diego, a standing room crowd of about 200 hospitalists at the Leadership Forum expressed their need for advanced leadership training. Responding to obvious demand, SHM developed a successful, soldout 1-day Leadership Pre-Course held in New Orleans at the 2004 SHM Annual Meeting. Building on this pre-course and again reacting to the requests of SHM members, Co-Directors Russell Holman and Mark Williams designed the Leadership Academy to provide more in-depth training over 4 days. Assisted by Tina Budnitz, SHM Senior Advisor for Planning and Development, this course was developed to address the leadership training needs of hospitalists. As an example of its resounding success, one participant made the following comment. “Even with 18 years of clinical/administrative experience as well as an MBA, this course was a learning experience and I gained and reinforced critical areas of thinking and actions.”
Credit for this success deservedly should be attributed to the outstanding faculty. SHM’s CEO Larry Wellikson led the first day, eloquently delineating the leadership challenges in hospital medicine. The audience appreciated how hospital medicine is evolving rapidly, still defining itself, and how hospitalists will be developing metrics for success. The remainder of the first day allowed participants to evaluate their own strengths and assess how their unique styles impact interactions with others. Using the Strength Deployment Inventory®, David Javitch, PhD explored how “reds,” “greens,” and “blues” approach situations and communicate with their colleagues. Javitch, an organizational psychologist from Harvard, demonstrated how individual styles of communication and interaction influence success at management and leadership. SHM Member Eric Howell, MD moderated a discussion featuring a movie capturing common hospital-based examples of conflict and led participants through techniques for conflict resolution and negotiation.
On the second day, Michael Guthrie, MD, MBA identified business drivers for hospital survival and success. Guthrie currently serves as a senior executive for a large national health alliance and has experience as a health system CEO, medical director, and consultant on performance improvement. Guthrie finished the morning by helping attendees interpret hospital performance reports and associated metrics and determine how such measures should guide leadership planning and decision making.
The next day was highlighted by sessions led by Jack Silversin, DMD, DrPH from Harvard, using table exercises and real world examples to demonstrate how to lead change. A nationally recognized expert in change management and co-author of “Leading Physicians through Change: How to Achieve and Sustain Results,” Dr. Silversin actively stimulated attendees to appraise their situations at home. He showed participants how to develop shared organizational vision, strengthen leadership, and accelerate implementation of change. Afterwards, Holman and Williams coordinated a series of sessions on strategic planning. They used multiple examples and exercises to aid attendees in developing vision and mission statements, as well as “SMART” goals.
The final day focused on communication. An experienced educator, Kathleen Miner, PhD, MPH, MEd, reviewed communication theory and how it applies to our everyday conversations and interactions. Miner, an Associate Dean for Public Health at Emory University, brought decades of experience to her presentation. The course ended with Holman recapping how to use what we learned to achieve success as a leader.
Overall, the course was structured to facilitate interaction and small group exercises. The interactive sessions provided opportunities for participants to apply concepts.
Use of facilitators greatly augmented the impact of this training. Participants in the course sat ten to a table, and each table was led by an experienced hospitalist leader trained to be a facilitator. We were extraordinarily fortunate to have leaders in hospital medicine as facilitators including: Mary Jo Gorman, Bill Atchley, Pat Cawley, Lisa Kettering, Alpesh Amin, Ron Greeno, Burke Kealey, Eric Siegal, Stacy Goldsholl, and Eric Howell.
The impact of the meeting was powerfully described by a facilitator, “I’ve never before experienced such sustained energy and enthusiasm at a meeting. People literally spent hours after the didactic sessions talking, sharing ideas, and commiserating. Speaks to the pent-up need for this, and the effectiveness of the curriculum in galvanizing the group.”
No meeting can be such a success without tremendous support from SHM staff. Angela Musial and Erica Pearson deserve our sincere thanks for handling all the logistical issues and guaranteeing a terrific time for everyone who attended. They ensured that everything worked without a hitch including two wonderful receptions, which fostered networking and opportunities to share challenges and success stories.
The Society of Hospital Medicine will hold another Leadership Academy this Fall: September 12–15 in Vail, Colorado. The learning objectives for the Leadership Academy highlight the skills hospitalists can gain by attending.
- Evaluate personal leadership strengths and weaknesses and apply them to everyday leadership and management challenges
- Effectively advocate the value of their Hospital Medicine program
- Predict and plan for the near-term challenges affecting the viability of their Hospital Medicine program
- Improve patient outcomes through successful planning, allocation of resources, collaboration, teamwork, and execution
- Create and execute a communication strategy for all key constituencies
- Interpret key hospital drivers
- Examine how hospital performance metrics are derived and how hospital medicine practices can influence and impact these metrics
- Implement methods of effective change through leadership, shared vision, and managing the organizational culture
- Utilize strategic planning to define a vision for their program, prioritize efforts, and achieve designated goals
Registration will again be limited to 100 hospitalist leaders and we expect this to fill quickly. The first Leadership Academy was sold out months before it was held, and interest in the September 2005 Leadership Academy in Vail is equally as strong after the rousing success of the January meeting in Tucson. If you are interested in attending, registration information can be found on page 19, at the SHM Web site at www.hospitalmedicine.org, or by calling SHM at 800-843-3360. We look forward to seeing you there.
Drs. Goldsholl, Amin, and Flanders Elected to SHM Board
SHM has elected Scott A. Flanders, MD, Alpesh Amin, MD, MBA, and Stacy Goldsholl, MD, to serve a 3-year term on the board of directors, beginning April 29, 2005. The new board members replace outgoing board members Jeff Dichter, MD, David Zipes, MD, and Peter Lindenauer, MD.
“Our new board members are all accomplished physicians who have consistently demonstrated their commitment to the field of hospital medicine,” said SHM president Jeanne Huddleston, MD. “Each of these new board members brings a vast range of experience, leadership, and passion to the Board that is sure to stimulate new thinking and new goals that will strengthen the role of hospitalists. We look forward to their insights and vision as we continue to expand the role of hospitalists as leaders and change agents in transforming patient care and quality.”
Scott A. Flanders, MD, FACP was a founding member of SHM’s Board of Directors in 1997 and served on the board for 6 years. He has since served on many of SHM’s committees and was editor of the organization’s newsletter, The Hospitalist, from 1997 through 2003.
Dr. Flanders currently is a clinical associate professor in the Division of General Internal Medicine at the University of Michigan in Ann Arbor, where he also serves as associate division chief of General Medicine for Inpatient Programs and associate director of Inpatient Programs for the Department of Internal Medicine. He is the director of the University of Michigan’s Hospitalist Program. He was formerly an associate professor of medicine at the University of California, San Francisco and director of the Hospitalist Residency Track there . Dr. Flanders, in collaboration with other University of California faculty, developed the content for the nation’s first Hospitalist Residency Track. This track has become a model that has been widely disseminated to other academic centers starting similar programs and formed the basis of a recent chapter for the Association of Program Directors in Internal Medicine (APDIM) Manual. Dr. Flanders regularly consults with both academic and community hospitals on issues related to curriculum development in the inpatient setting.
In addition to these activities, Dr. Flanders has been active in guideline development, quality improvement, and patient safety both at the University of Michigan and the University of California, San Francisco. His research interests are related to hospitalists, dissemination of patient safety practices, and the diagnosis and treatment of lower respiratory infections. He speaks regularly at national conferences on the topics of hospitalists and community-acquired and nosocomial pneumonia. He served as associate editor of AHRQ’s Web M&M online journal of patient safety from its inception until 2004.
Dr. Flanders earned his medical degree from the University of Chicago in 1993 and completed his residency training in Internal Medicine at the University of California, San Francisco.
Alpesh Amin, MD, MBA, FACP became a charter member of SHM in 1998 and also serves as chair of SHM’s Education Committee. Under his leadership, the committee developed three task forces: the Core Curricuum, Leadership, and Geriatrics. Dr. Amin serves as a member of each of these (as well as the SHM Journal Task Force), where he provides guidance in developing education curriculum to improve SHM member skills in these areas. Dr. Amin also is one of the co-authors of the first Core Curriculum for Hospital Medicine, soon to be published. He also served on the 2004 Annual Program Committee and will be the program director for the 2006 Annual Meeting.
Dr. Amin is the executive director for the Hospitalist Program at the University of California Irvine Medical Center in Irvine, a program he started in June 1998. Over the last 7 years, he has grown the program to 15 academic hospitalists. He is also vice-chair for Clinical Affairs, associate program director for the Internal Medicine Residency Program, and clerkship director for the Medicine Clerkship at the University of California, Irvine. Through these different roles, he has been involved in clinical care, administrative and hospital based committee work, and curriculum development. He has also been involved in developing the Hospitalist/Consultative Curriculum, Palliative & Hospice Care Curriculum, and Business of Medicine Curriculum at UCI.
Dr. Amin’s research interests are related to the field of hospital medicine, patient safety and quality, and medical education. He is an invited speaker at national conferences on community acquired and hospital acquired pneumonia, deep vein thrombosis and venous thromboembolism, and heart failure.
Dr. Amin earned his medical degree from Northwestern University Medical School, Chicago, IL in 1994. He did his residency training in Internal Medicine at the University of California, Irvine, and went on to earn an MBA in Health Care from that school in 2000.
Stacy Goldsholl, MD, BC, IM has been a practicing hospitalist for 10 years and is a charter member of SHM. Since joining SHM, she has participated as SHM’s Michigan State Regional Councilor, a member of the Practice Management Committee, and faculty for the Midwest Regional Meeting. Currently she is the 2004/5 Chair of the Benchmarks and Productivity Task Force, a faculty member for the 2004/5 Annual Meeting Committee, and facilitator for the 2005 SHM Leadership Academy.
Dr. Goldsholl is currently national medical director for Cogent Healthcare and is the owner of Catalyst Inpatient Solutions, LLC, a consulting firm she founded in 2002 for hospital medicine program development and education.
Dr. Goldsholl began her career in hospital medicine at a community hospital in Atlanta, where she served as a physician advisor for medical management and utilization review. In 2000, she initiated a hospitalist division for a large multi-specialty group in Wilmington, North Carolina. She has spent the last 4 years implementing hospital medicine programs for two large (700 bed) non-profit hospitals and establishing Catalyst Inpatient Solutions to serve the needs of various hospitals, including for-profit and critical access hospital designations, ranging in size from 75 to 700 beds.
Dr. Goldsholl’s clinical interests include partnering Palliative Medicine and Pastoral Services with care of the hospitalized patient, and she participated as a faculty scholar with the 2004 Harvard Medical School’s Program in Palliative Care Education and Practice.
In addition to Dr. Goldsholl’s clinical practice, she served as co-author for “The Hospitalist Program Management Guide” (HCPro), as well as consultant to the Clinical Advisory Board of the Advisory Board Company for the publication “Second Generation Hospitalist Programs.”
Dr. Goldsholl earned her MD at the University of North Carolina at Chapel Hill in 1992 and went on to complete her residency training there. She earned a Bachelor of Science degree in Biology from York College of Pennsylvania in 1985 and attended a Medical Scholars Program at the University of Illinois in Urbana from 1986-1990. She is Board Certified in Internal Medicine and a member of the American College of Physician Executives.
Please join us in congratulating all of the new board members, and also in thanking our outgoing board members for their dedication and service.
SHM has elected Scott A. Flanders, MD, Alpesh Amin, MD, MBA, and Stacy Goldsholl, MD, to serve a 3-year term on the board of directors, beginning April 29, 2005. The new board members replace outgoing board members Jeff Dichter, MD, David Zipes, MD, and Peter Lindenauer, MD.
“Our new board members are all accomplished physicians who have consistently demonstrated their commitment to the field of hospital medicine,” said SHM president Jeanne Huddleston, MD. “Each of these new board members brings a vast range of experience, leadership, and passion to the Board that is sure to stimulate new thinking and new goals that will strengthen the role of hospitalists. We look forward to their insights and vision as we continue to expand the role of hospitalists as leaders and change agents in transforming patient care and quality.”
Scott A. Flanders, MD, FACP was a founding member of SHM’s Board of Directors in 1997 and served on the board for 6 years. He has since served on many of SHM’s committees and was editor of the organization’s newsletter, The Hospitalist, from 1997 through 2003.
Dr. Flanders currently is a clinical associate professor in the Division of General Internal Medicine at the University of Michigan in Ann Arbor, where he also serves as associate division chief of General Medicine for Inpatient Programs and associate director of Inpatient Programs for the Department of Internal Medicine. He is the director of the University of Michigan’s Hospitalist Program. He was formerly an associate professor of medicine at the University of California, San Francisco and director of the Hospitalist Residency Track there . Dr. Flanders, in collaboration with other University of California faculty, developed the content for the nation’s first Hospitalist Residency Track. This track has become a model that has been widely disseminated to other academic centers starting similar programs and formed the basis of a recent chapter for the Association of Program Directors in Internal Medicine (APDIM) Manual. Dr. Flanders regularly consults with both academic and community hospitals on issues related to curriculum development in the inpatient setting.
In addition to these activities, Dr. Flanders has been active in guideline development, quality improvement, and patient safety both at the University of Michigan and the University of California, San Francisco. His research interests are related to hospitalists, dissemination of patient safety practices, and the diagnosis and treatment of lower respiratory infections. He speaks regularly at national conferences on the topics of hospitalists and community-acquired and nosocomial pneumonia. He served as associate editor of AHRQ’s Web M&M online journal of patient safety from its inception until 2004.
Dr. Flanders earned his medical degree from the University of Chicago in 1993 and completed his residency training in Internal Medicine at the University of California, San Francisco.
Alpesh Amin, MD, MBA, FACP became a charter member of SHM in 1998 and also serves as chair of SHM’s Education Committee. Under his leadership, the committee developed three task forces: the Core Curricuum, Leadership, and Geriatrics. Dr. Amin serves as a member of each of these (as well as the SHM Journal Task Force), where he provides guidance in developing education curriculum to improve SHM member skills in these areas. Dr. Amin also is one of the co-authors of the first Core Curriculum for Hospital Medicine, soon to be published. He also served on the 2004 Annual Program Committee and will be the program director for the 2006 Annual Meeting.
Dr. Amin is the executive director for the Hospitalist Program at the University of California Irvine Medical Center in Irvine, a program he started in June 1998. Over the last 7 years, he has grown the program to 15 academic hospitalists. He is also vice-chair for Clinical Affairs, associate program director for the Internal Medicine Residency Program, and clerkship director for the Medicine Clerkship at the University of California, Irvine. Through these different roles, he has been involved in clinical care, administrative and hospital based committee work, and curriculum development. He has also been involved in developing the Hospitalist/Consultative Curriculum, Palliative & Hospice Care Curriculum, and Business of Medicine Curriculum at UCI.
Dr. Amin’s research interests are related to the field of hospital medicine, patient safety and quality, and medical education. He is an invited speaker at national conferences on community acquired and hospital acquired pneumonia, deep vein thrombosis and venous thromboembolism, and heart failure.
Dr. Amin earned his medical degree from Northwestern University Medical School, Chicago, IL in 1994. He did his residency training in Internal Medicine at the University of California, Irvine, and went on to earn an MBA in Health Care from that school in 2000.
Stacy Goldsholl, MD, BC, IM has been a practicing hospitalist for 10 years and is a charter member of SHM. Since joining SHM, she has participated as SHM’s Michigan State Regional Councilor, a member of the Practice Management Committee, and faculty for the Midwest Regional Meeting. Currently she is the 2004/5 Chair of the Benchmarks and Productivity Task Force, a faculty member for the 2004/5 Annual Meeting Committee, and facilitator for the 2005 SHM Leadership Academy.
Dr. Goldsholl is currently national medical director for Cogent Healthcare and is the owner of Catalyst Inpatient Solutions, LLC, a consulting firm she founded in 2002 for hospital medicine program development and education.
Dr. Goldsholl began her career in hospital medicine at a community hospital in Atlanta, where she served as a physician advisor for medical management and utilization review. In 2000, she initiated a hospitalist division for a large multi-specialty group in Wilmington, North Carolina. She has spent the last 4 years implementing hospital medicine programs for two large (700 bed) non-profit hospitals and establishing Catalyst Inpatient Solutions to serve the needs of various hospitals, including for-profit and critical access hospital designations, ranging in size from 75 to 700 beds.
Dr. Goldsholl’s clinical interests include partnering Palliative Medicine and Pastoral Services with care of the hospitalized patient, and she participated as a faculty scholar with the 2004 Harvard Medical School’s Program in Palliative Care Education and Practice.
In addition to Dr. Goldsholl’s clinical practice, she served as co-author for “The Hospitalist Program Management Guide” (HCPro), as well as consultant to the Clinical Advisory Board of the Advisory Board Company for the publication “Second Generation Hospitalist Programs.”
Dr. Goldsholl earned her MD at the University of North Carolina at Chapel Hill in 1992 and went on to complete her residency training there. She earned a Bachelor of Science degree in Biology from York College of Pennsylvania in 1985 and attended a Medical Scholars Program at the University of Illinois in Urbana from 1986-1990. She is Board Certified in Internal Medicine and a member of the American College of Physician Executives.
Please join us in congratulating all of the new board members, and also in thanking our outgoing board members for their dedication and service.
SHM has elected Scott A. Flanders, MD, Alpesh Amin, MD, MBA, and Stacy Goldsholl, MD, to serve a 3-year term on the board of directors, beginning April 29, 2005. The new board members replace outgoing board members Jeff Dichter, MD, David Zipes, MD, and Peter Lindenauer, MD.
“Our new board members are all accomplished physicians who have consistently demonstrated their commitment to the field of hospital medicine,” said SHM president Jeanne Huddleston, MD. “Each of these new board members brings a vast range of experience, leadership, and passion to the Board that is sure to stimulate new thinking and new goals that will strengthen the role of hospitalists. We look forward to their insights and vision as we continue to expand the role of hospitalists as leaders and change agents in transforming patient care and quality.”
Scott A. Flanders, MD, FACP was a founding member of SHM’s Board of Directors in 1997 and served on the board for 6 years. He has since served on many of SHM’s committees and was editor of the organization’s newsletter, The Hospitalist, from 1997 through 2003.
Dr. Flanders currently is a clinical associate professor in the Division of General Internal Medicine at the University of Michigan in Ann Arbor, where he also serves as associate division chief of General Medicine for Inpatient Programs and associate director of Inpatient Programs for the Department of Internal Medicine. He is the director of the University of Michigan’s Hospitalist Program. He was formerly an associate professor of medicine at the University of California, San Francisco and director of the Hospitalist Residency Track there . Dr. Flanders, in collaboration with other University of California faculty, developed the content for the nation’s first Hospitalist Residency Track. This track has become a model that has been widely disseminated to other academic centers starting similar programs and formed the basis of a recent chapter for the Association of Program Directors in Internal Medicine (APDIM) Manual. Dr. Flanders regularly consults with both academic and community hospitals on issues related to curriculum development in the inpatient setting.
In addition to these activities, Dr. Flanders has been active in guideline development, quality improvement, and patient safety both at the University of Michigan and the University of California, San Francisco. His research interests are related to hospitalists, dissemination of patient safety practices, and the diagnosis and treatment of lower respiratory infections. He speaks regularly at national conferences on the topics of hospitalists and community-acquired and nosocomial pneumonia. He served as associate editor of AHRQ’s Web M&M online journal of patient safety from its inception until 2004.
Dr. Flanders earned his medical degree from the University of Chicago in 1993 and completed his residency training in Internal Medicine at the University of California, San Francisco.
Alpesh Amin, MD, MBA, FACP became a charter member of SHM in 1998 and also serves as chair of SHM’s Education Committee. Under his leadership, the committee developed three task forces: the Core Curricuum, Leadership, and Geriatrics. Dr. Amin serves as a member of each of these (as well as the SHM Journal Task Force), where he provides guidance in developing education curriculum to improve SHM member skills in these areas. Dr. Amin also is one of the co-authors of the first Core Curriculum for Hospital Medicine, soon to be published. He also served on the 2004 Annual Program Committee and will be the program director for the 2006 Annual Meeting.
Dr. Amin is the executive director for the Hospitalist Program at the University of California Irvine Medical Center in Irvine, a program he started in June 1998. Over the last 7 years, he has grown the program to 15 academic hospitalists. He is also vice-chair for Clinical Affairs, associate program director for the Internal Medicine Residency Program, and clerkship director for the Medicine Clerkship at the University of California, Irvine. Through these different roles, he has been involved in clinical care, administrative and hospital based committee work, and curriculum development. He has also been involved in developing the Hospitalist/Consultative Curriculum, Palliative & Hospice Care Curriculum, and Business of Medicine Curriculum at UCI.
Dr. Amin’s research interests are related to the field of hospital medicine, patient safety and quality, and medical education. He is an invited speaker at national conferences on community acquired and hospital acquired pneumonia, deep vein thrombosis and venous thromboembolism, and heart failure.
Dr. Amin earned his medical degree from Northwestern University Medical School, Chicago, IL in 1994. He did his residency training in Internal Medicine at the University of California, Irvine, and went on to earn an MBA in Health Care from that school in 2000.
Stacy Goldsholl, MD, BC, IM has been a practicing hospitalist for 10 years and is a charter member of SHM. Since joining SHM, she has participated as SHM’s Michigan State Regional Councilor, a member of the Practice Management Committee, and faculty for the Midwest Regional Meeting. Currently she is the 2004/5 Chair of the Benchmarks and Productivity Task Force, a faculty member for the 2004/5 Annual Meeting Committee, and facilitator for the 2005 SHM Leadership Academy.
Dr. Goldsholl is currently national medical director for Cogent Healthcare and is the owner of Catalyst Inpatient Solutions, LLC, a consulting firm she founded in 2002 for hospital medicine program development and education.
Dr. Goldsholl began her career in hospital medicine at a community hospital in Atlanta, where she served as a physician advisor for medical management and utilization review. In 2000, she initiated a hospitalist division for a large multi-specialty group in Wilmington, North Carolina. She has spent the last 4 years implementing hospital medicine programs for two large (700 bed) non-profit hospitals and establishing Catalyst Inpatient Solutions to serve the needs of various hospitals, including for-profit and critical access hospital designations, ranging in size from 75 to 700 beds.
Dr. Goldsholl’s clinical interests include partnering Palliative Medicine and Pastoral Services with care of the hospitalized patient, and she participated as a faculty scholar with the 2004 Harvard Medical School’s Program in Palliative Care Education and Practice.
In addition to Dr. Goldsholl’s clinical practice, she served as co-author for “The Hospitalist Program Management Guide” (HCPro), as well as consultant to the Clinical Advisory Board of the Advisory Board Company for the publication “Second Generation Hospitalist Programs.”
Dr. Goldsholl earned her MD at the University of North Carolina at Chapel Hill in 1992 and went on to complete her residency training there. She earned a Bachelor of Science degree in Biology from York College of Pennsylvania in 1985 and attended a Medical Scholars Program at the University of Illinois in Urbana from 1986-1990. She is Board Certified in Internal Medicine and a member of the American College of Physician Executives.
Please join us in congratulating all of the new board members, and also in thanking our outgoing board members for their dedication and service.
An Ongoing Analysis of the 2003–04 SHM Productivity and Compensation Survey
This chapter looks at work generated by the surveyed hospitalists. Overall, the median charges per year for physician hospitalists are $288,242 and the median collections are $160,000 (a 56% collection rate). The typical surveyed physician had a median of 2,259 encounters, 468 admissions and consultations, and 3,000 RVUs. The analyses below examine these measures from the following perspectives: region, employment model, specialty/provider type, and compensation model.
- Consistent with the compensation and productivity input measures, academic hospitalists have low median collections ($110,000 vs. $160,000 overall), low encounters (1,600 vs. 2,259 overall), low admissions and consults (371 vs. 468). However, academic hospitalists generate an equivalent number of RVUs (3,000) indicating that they are delivering more complex services. NOTE: At 40% ($110,000/$278,122), the median collection rate for academic groups is the lowest of all employment categories. This may be explained by the payer mix at academic medical centers.
- Hospitalist-only groups, both local and multi-state, generate the most work output. With regard to median charges generated, local groups are 21% higher ($350,000 vs. $288,242) and multi-state groups are 4% higher ($300,988 vs. $288,242). Hospitalists in multi-state groups have remarkable performance with regard to collections, achieving a 95% collection rate compared to a overall average of 56%. These hospitalist-only groups also have much higher encounters, admissions/consults, and RVUs.
- As in the Productivity Inputs, Eastern hospitalists have the lowest measures of Productivity Outputs. Hospitalists in the Southern region generate the most work in all five categories of outputs.
- Compared with adult medicine hospitalists, pediatricians generate fewer charges ($203,554 vs. $290,000), collections ($111,000 vs. $160,100), encounters (1,300 vs. 2,340), admissions/consults (441 vs. 470), and RVUs (1,990 vs. 3,000).
- Non-physicians generate about half the charges, collections, admissions/consults, and RVUs of physician hospitalists.
- Productivity–based compensation appears to have a positive impact on Productivity Output (the incentives appear to work).
- Compared with hospitalists with a 100% salary model, hospitalists that have a 100% productivity model have median charges 65% higher ($385,200 vs. $233,251), median collections that are 56% higher ($200,000 vs. $128,063), encounters that are 64% higher (3,000 vs. 1,831), admissions/consults that are 44% higher (575 vs. 400), and more than twice the median number of RVUs (4,967 vs. 2,395). In all cases hospitalists with a mixed compensation model fall in the middle of the two medians.
This chapter looks at work generated by the surveyed hospitalists. Overall, the median charges per year for physician hospitalists are $288,242 and the median collections are $160,000 (a 56% collection rate). The typical surveyed physician had a median of 2,259 encounters, 468 admissions and consultations, and 3,000 RVUs. The analyses below examine these measures from the following perspectives: region, employment model, specialty/provider type, and compensation model.
- Consistent with the compensation and productivity input measures, academic hospitalists have low median collections ($110,000 vs. $160,000 overall), low encounters (1,600 vs. 2,259 overall), low admissions and consults (371 vs. 468). However, academic hospitalists generate an equivalent number of RVUs (3,000) indicating that they are delivering more complex services. NOTE: At 40% ($110,000/$278,122), the median collection rate for academic groups is the lowest of all employment categories. This may be explained by the payer mix at academic medical centers.
- Hospitalist-only groups, both local and multi-state, generate the most work output. With regard to median charges generated, local groups are 21% higher ($350,000 vs. $288,242) and multi-state groups are 4% higher ($300,988 vs. $288,242). Hospitalists in multi-state groups have remarkable performance with regard to collections, achieving a 95% collection rate compared to a overall average of 56%. These hospitalist-only groups also have much higher encounters, admissions/consults, and RVUs.
- As in the Productivity Inputs, Eastern hospitalists have the lowest measures of Productivity Outputs. Hospitalists in the Southern region generate the most work in all five categories of outputs.
- Compared with adult medicine hospitalists, pediatricians generate fewer charges ($203,554 vs. $290,000), collections ($111,000 vs. $160,100), encounters (1,300 vs. 2,340), admissions/consults (441 vs. 470), and RVUs (1,990 vs. 3,000).
- Non-physicians generate about half the charges, collections, admissions/consults, and RVUs of physician hospitalists.
- Productivity–based compensation appears to have a positive impact on Productivity Output (the incentives appear to work).
- Compared with hospitalists with a 100% salary model, hospitalists that have a 100% productivity model have median charges 65% higher ($385,200 vs. $233,251), median collections that are 56% higher ($200,000 vs. $128,063), encounters that are 64% higher (3,000 vs. 1,831), admissions/consults that are 44% higher (575 vs. 400), and more than twice the median number of RVUs (4,967 vs. 2,395). In all cases hospitalists with a mixed compensation model fall in the middle of the two medians.
This chapter looks at work generated by the surveyed hospitalists. Overall, the median charges per year for physician hospitalists are $288,242 and the median collections are $160,000 (a 56% collection rate). The typical surveyed physician had a median of 2,259 encounters, 468 admissions and consultations, and 3,000 RVUs. The analyses below examine these measures from the following perspectives: region, employment model, specialty/provider type, and compensation model.
- Consistent with the compensation and productivity input measures, academic hospitalists have low median collections ($110,000 vs. $160,000 overall), low encounters (1,600 vs. 2,259 overall), low admissions and consults (371 vs. 468). However, academic hospitalists generate an equivalent number of RVUs (3,000) indicating that they are delivering more complex services. NOTE: At 40% ($110,000/$278,122), the median collection rate for academic groups is the lowest of all employment categories. This may be explained by the payer mix at academic medical centers.
- Hospitalist-only groups, both local and multi-state, generate the most work output. With regard to median charges generated, local groups are 21% higher ($350,000 vs. $288,242) and multi-state groups are 4% higher ($300,988 vs. $288,242). Hospitalists in multi-state groups have remarkable performance with regard to collections, achieving a 95% collection rate compared to a overall average of 56%. These hospitalist-only groups also have much higher encounters, admissions/consults, and RVUs.
- As in the Productivity Inputs, Eastern hospitalists have the lowest measures of Productivity Outputs. Hospitalists in the Southern region generate the most work in all five categories of outputs.
- Compared with adult medicine hospitalists, pediatricians generate fewer charges ($203,554 vs. $290,000), collections ($111,000 vs. $160,100), encounters (1,300 vs. 2,340), admissions/consults (441 vs. 470), and RVUs (1,990 vs. 3,000).
- Non-physicians generate about half the charges, collections, admissions/consults, and RVUs of physician hospitalists.
- Productivity–based compensation appears to have a positive impact on Productivity Output (the incentives appear to work).
- Compared with hospitalists with a 100% salary model, hospitalists that have a 100% productivity model have median charges 65% higher ($385,200 vs. $233,251), median collections that are 56% higher ($200,000 vs. $128,063), encounters that are 64% higher (3,000 vs. 1,831), admissions/consults that are 44% higher (575 vs. 400), and more than twice the median number of RVUs (4,967 vs. 2,395). In all cases hospitalists with a mixed compensation model fall in the middle of the two medians.
What Is a Hospitalist?
The next Annual Meeting is near. This means that the year, serving as your President, has nearly come to an end. As a result, this will be my last column. It is a good time for reflection.
First, I must thank the thousands of hospitalists who have joined SHM, filled out surveys, and attended meetings (either nationally or locally). You see, SHM’s data on hospitalists—where they are, what they do, and numbers in practice—is the source of information people outside the profession are using to understand and make decisions about our growing specialty. There is strength in numbers and your participation at every level infuses the energy necessary for us to continue the journey of becoming a legitimate specialty. Thank you.
As an organization, we have made significant progress toward our mission of making quality and safety core to what it means to practice hospital medicine. We have joined a number of other organizations, associations, and foundations to create various initiatives aimed at improving the quality and safety of care delivered in our hospitals. Specific areas of focus to date have been in the care of geriatric, diabetic, cardiac, and critically ill patients. We have also begun to address key preventative strategies such as antibiotic resistance and thromboembolic disease prophylaxis. These national partnerships SHM has formed with such organizations as the American College of Chest Physicians, American Association of Critical Care Nurses, American Hospital Association, Hartford Foundation, American College of Cardiology, Institute of Health Care Improvement, Joint Commission, and many others, demonstrate the breadth of teamwork that is necessary to care for patients across the continuum. Hospitalists are a crucial part of that team as more and more of our nation’s patients are cared for by hospitalists during their acute illnesses.
It has been an amazing year of watching this organization grow and mature. But what do I worry about as I leave my post? What is the kernel of concern that I must ensure is passed on with this next “transition of care” of our organization? It is that which defines us. I mentioned in my speech at lunch last year that we (the Society of Hospital Medicine) would fail if what hospitalists became known as were simply those pediatricians or internists who spent more time than their peers in the hospital. I don’t know about you, but at least once each month someone will approach me and say “I spend 25% of my time seeing patients in the hospital—So I must be a hospitalist!” (Quoting the original work of Drs. Robert Wachter and Lee Goldman in their 1996 New England Journal of Medicine article.) Well, as time has passed and the field has clearly evolved with more than 10,000 hospitalists practicing, the definition has clearly evolved.
But is our defi nition of what we do still based on time in the hospital? Or is it a more substantial definition? Is it about one’s professional focus? Is it about where one’s passion for medicine lies? What one wakes up in the middle of the night worrying about? What is a hospitalist?
The definition set forth by the Society of Hospital Medicine (adopted in the spring of 2000) is the following: Hospitalists are doctors whose primary professional focus is the general medical care of hospitalized patients. Their activities may include patient care, teaching, research, and leadership related to hospital care. Hospital medicine is a specialty organized around a site of care (the hospital) rather than an organ (like cardiology), a disease (like oncology), or a patient’s age (like pediatrics).
It seems silly to be asking this question of what defines us after writing about the burgeoning specialty. But as was pointed out in February’s JGIM issue, “varied employment relationships create diverse practice structures, priorities and roles.” Our practices have each grown so quickly out of local market pressures and individual hospital needs that we have evolved in slightly different directions. This is a natural consequence of such rapid growth. Just like any other specialty, practice structures and employment models will differ across the country. But I would challenge us to ensure that our higher priorities do not differ.
The American health care system needs an entire army of physicians and other providers to give whole heartedly and completely of their professional time, creativity, and energy to the hospital… to fix current system problems. It is no different than the cardiologists of today spending their energy and time discovering at the molecular level what causes ventricular dysfunction and then translating that knowledge to bedside care. Hospitalists, are specialists of in hospital medicine, and must do for the hospital what cardiologists do to the heart. We must study and learn what causes the health care delivered by the hospital to fail. Then we must translate that knowledge to the care of all patients in the hospital by improving the systems of care delivery.
According to Dr. Charles Mayo, “The definition of a specialist as one who ‘knows more and more about less and less. Its truth makes essential that the specialist, to do efficient work, must have some association with others who, taken altogether, represent the whole of which the specialty is only a part.” Our generalist colleagues care about the hospital as much as we do but do not necessarily have the time to concentrate specifically on hospital systems. There are so many other things that require their attention at the same time, but we need them to be there.
So we are only a part of what is needed to deliver the best care to all patients. But we have become a critical part of that team in the hospital. It is the hospitalists who, through their chosen focus on one aspect of medicine, will need to give the energy, creativity, and time to improving our systems of health care delivery in the hospital and across key transitions of care. Regardless of where we practice or how our practice is structured, our higher calling as a specialty is to determine the root causes of what ails the hospitals of this country. Then, as a specialty we must discover new mechanisms that would provide care at the level of quality called for by our patients. If we as hospitalists are truly specialists… If we are experts at delivering hospital based care… Then we have a vested interest in addressing these higher priorities. The improvements in health care that will be achieved, because a group of providers have dedicated their careers to making the hospital a better place for our patients, will ultimately make the definition of a hospitalist quite clear.
The next Annual Meeting is near. This means that the year, serving as your President, has nearly come to an end. As a result, this will be my last column. It is a good time for reflection.
First, I must thank the thousands of hospitalists who have joined SHM, filled out surveys, and attended meetings (either nationally or locally). You see, SHM’s data on hospitalists—where they are, what they do, and numbers in practice—is the source of information people outside the profession are using to understand and make decisions about our growing specialty. There is strength in numbers and your participation at every level infuses the energy necessary for us to continue the journey of becoming a legitimate specialty. Thank you.
As an organization, we have made significant progress toward our mission of making quality and safety core to what it means to practice hospital medicine. We have joined a number of other organizations, associations, and foundations to create various initiatives aimed at improving the quality and safety of care delivered in our hospitals. Specific areas of focus to date have been in the care of geriatric, diabetic, cardiac, and critically ill patients. We have also begun to address key preventative strategies such as antibiotic resistance and thromboembolic disease prophylaxis. These national partnerships SHM has formed with such organizations as the American College of Chest Physicians, American Association of Critical Care Nurses, American Hospital Association, Hartford Foundation, American College of Cardiology, Institute of Health Care Improvement, Joint Commission, and many others, demonstrate the breadth of teamwork that is necessary to care for patients across the continuum. Hospitalists are a crucial part of that team as more and more of our nation’s patients are cared for by hospitalists during their acute illnesses.
It has been an amazing year of watching this organization grow and mature. But what do I worry about as I leave my post? What is the kernel of concern that I must ensure is passed on with this next “transition of care” of our organization? It is that which defines us. I mentioned in my speech at lunch last year that we (the Society of Hospital Medicine) would fail if what hospitalists became known as were simply those pediatricians or internists who spent more time than their peers in the hospital. I don’t know about you, but at least once each month someone will approach me and say “I spend 25% of my time seeing patients in the hospital—So I must be a hospitalist!” (Quoting the original work of Drs. Robert Wachter and Lee Goldman in their 1996 New England Journal of Medicine article.) Well, as time has passed and the field has clearly evolved with more than 10,000 hospitalists practicing, the definition has clearly evolved.
But is our defi nition of what we do still based on time in the hospital? Or is it a more substantial definition? Is it about one’s professional focus? Is it about where one’s passion for medicine lies? What one wakes up in the middle of the night worrying about? What is a hospitalist?
The definition set forth by the Society of Hospital Medicine (adopted in the spring of 2000) is the following: Hospitalists are doctors whose primary professional focus is the general medical care of hospitalized patients. Their activities may include patient care, teaching, research, and leadership related to hospital care. Hospital medicine is a specialty organized around a site of care (the hospital) rather than an organ (like cardiology), a disease (like oncology), or a patient’s age (like pediatrics).
It seems silly to be asking this question of what defines us after writing about the burgeoning specialty. But as was pointed out in February’s JGIM issue, “varied employment relationships create diverse practice structures, priorities and roles.” Our practices have each grown so quickly out of local market pressures and individual hospital needs that we have evolved in slightly different directions. This is a natural consequence of such rapid growth. Just like any other specialty, practice structures and employment models will differ across the country. But I would challenge us to ensure that our higher priorities do not differ.
The American health care system needs an entire army of physicians and other providers to give whole heartedly and completely of their professional time, creativity, and energy to the hospital… to fix current system problems. It is no different than the cardiologists of today spending their energy and time discovering at the molecular level what causes ventricular dysfunction and then translating that knowledge to bedside care. Hospitalists, are specialists of in hospital medicine, and must do for the hospital what cardiologists do to the heart. We must study and learn what causes the health care delivered by the hospital to fail. Then we must translate that knowledge to the care of all patients in the hospital by improving the systems of care delivery.
According to Dr. Charles Mayo, “The definition of a specialist as one who ‘knows more and more about less and less. Its truth makes essential that the specialist, to do efficient work, must have some association with others who, taken altogether, represent the whole of which the specialty is only a part.” Our generalist colleagues care about the hospital as much as we do but do not necessarily have the time to concentrate specifically on hospital systems. There are so many other things that require their attention at the same time, but we need them to be there.
So we are only a part of what is needed to deliver the best care to all patients. But we have become a critical part of that team in the hospital. It is the hospitalists who, through their chosen focus on one aspect of medicine, will need to give the energy, creativity, and time to improving our systems of health care delivery in the hospital and across key transitions of care. Regardless of where we practice or how our practice is structured, our higher calling as a specialty is to determine the root causes of what ails the hospitals of this country. Then, as a specialty we must discover new mechanisms that would provide care at the level of quality called for by our patients. If we as hospitalists are truly specialists… If we are experts at delivering hospital based care… Then we have a vested interest in addressing these higher priorities. The improvements in health care that will be achieved, because a group of providers have dedicated their careers to making the hospital a better place for our patients, will ultimately make the definition of a hospitalist quite clear.
The next Annual Meeting is near. This means that the year, serving as your President, has nearly come to an end. As a result, this will be my last column. It is a good time for reflection.
First, I must thank the thousands of hospitalists who have joined SHM, filled out surveys, and attended meetings (either nationally or locally). You see, SHM’s data on hospitalists—where they are, what they do, and numbers in practice—is the source of information people outside the profession are using to understand and make decisions about our growing specialty. There is strength in numbers and your participation at every level infuses the energy necessary for us to continue the journey of becoming a legitimate specialty. Thank you.
As an organization, we have made significant progress toward our mission of making quality and safety core to what it means to practice hospital medicine. We have joined a number of other organizations, associations, and foundations to create various initiatives aimed at improving the quality and safety of care delivered in our hospitals. Specific areas of focus to date have been in the care of geriatric, diabetic, cardiac, and critically ill patients. We have also begun to address key preventative strategies such as antibiotic resistance and thromboembolic disease prophylaxis. These national partnerships SHM has formed with such organizations as the American College of Chest Physicians, American Association of Critical Care Nurses, American Hospital Association, Hartford Foundation, American College of Cardiology, Institute of Health Care Improvement, Joint Commission, and many others, demonstrate the breadth of teamwork that is necessary to care for patients across the continuum. Hospitalists are a crucial part of that team as more and more of our nation’s patients are cared for by hospitalists during their acute illnesses.
It has been an amazing year of watching this organization grow and mature. But what do I worry about as I leave my post? What is the kernel of concern that I must ensure is passed on with this next “transition of care” of our organization? It is that which defines us. I mentioned in my speech at lunch last year that we (the Society of Hospital Medicine) would fail if what hospitalists became known as were simply those pediatricians or internists who spent more time than their peers in the hospital. I don’t know about you, but at least once each month someone will approach me and say “I spend 25% of my time seeing patients in the hospital—So I must be a hospitalist!” (Quoting the original work of Drs. Robert Wachter and Lee Goldman in their 1996 New England Journal of Medicine article.) Well, as time has passed and the field has clearly evolved with more than 10,000 hospitalists practicing, the definition has clearly evolved.
But is our defi nition of what we do still based on time in the hospital? Or is it a more substantial definition? Is it about one’s professional focus? Is it about where one’s passion for medicine lies? What one wakes up in the middle of the night worrying about? What is a hospitalist?
The definition set forth by the Society of Hospital Medicine (adopted in the spring of 2000) is the following: Hospitalists are doctors whose primary professional focus is the general medical care of hospitalized patients. Their activities may include patient care, teaching, research, and leadership related to hospital care. Hospital medicine is a specialty organized around a site of care (the hospital) rather than an organ (like cardiology), a disease (like oncology), or a patient’s age (like pediatrics).
It seems silly to be asking this question of what defines us after writing about the burgeoning specialty. But as was pointed out in February’s JGIM issue, “varied employment relationships create diverse practice structures, priorities and roles.” Our practices have each grown so quickly out of local market pressures and individual hospital needs that we have evolved in slightly different directions. This is a natural consequence of such rapid growth. Just like any other specialty, practice structures and employment models will differ across the country. But I would challenge us to ensure that our higher priorities do not differ.
The American health care system needs an entire army of physicians and other providers to give whole heartedly and completely of their professional time, creativity, and energy to the hospital… to fix current system problems. It is no different than the cardiologists of today spending their energy and time discovering at the molecular level what causes ventricular dysfunction and then translating that knowledge to bedside care. Hospitalists, are specialists of in hospital medicine, and must do for the hospital what cardiologists do to the heart. We must study and learn what causes the health care delivered by the hospital to fail. Then we must translate that knowledge to the care of all patients in the hospital by improving the systems of care delivery.
According to Dr. Charles Mayo, “The definition of a specialist as one who ‘knows more and more about less and less. Its truth makes essential that the specialist, to do efficient work, must have some association with others who, taken altogether, represent the whole of which the specialty is only a part.” Our generalist colleagues care about the hospital as much as we do but do not necessarily have the time to concentrate specifically on hospital systems. There are so many other things that require their attention at the same time, but we need them to be there.
So we are only a part of what is needed to deliver the best care to all patients. But we have become a critical part of that team in the hospital. It is the hospitalists who, through their chosen focus on one aspect of medicine, will need to give the energy, creativity, and time to improving our systems of health care delivery in the hospital and across key transitions of care. Regardless of where we practice or how our practice is structured, our higher calling as a specialty is to determine the root causes of what ails the hospitals of this country. Then, as a specialty we must discover new mechanisms that would provide care at the level of quality called for by our patients. If we as hospitalists are truly specialists… If we are experts at delivering hospital based care… Then we have a vested interest in addressing these higher priorities. The improvements in health care that will be achieved, because a group of providers have dedicated their careers to making the hospital a better place for our patients, will ultimately make the definition of a hospitalist quite clear.
Cholesterol guidelines update: More aggressive therapy for higher-risk patients
Osteonecrosis and HAART
How to avoid ‘foreseeable’ harm
When a psychiatrist is sued for negligence, the legal system asks, “Did the doctor depart from the standard of care, and—if so—did that departure proximately cause the harm?” To determine proximate cause, the legal system then asks whether the harm was a “reasonably foreseeable” result of the negligent act.
Inadequate evaluation leads to patient’s suicide, plaintiff alleges
Dallas County (TX) District Court
In 1997, 1 year after suffering a stroke, a 56-year-old man was admitted to a rehabilitation center and seen by a psychiatrist for depression. In February 2000, the patient died after jumping from the center’s fifth-floor window.
The patient’s estate charged that the psychiatrist was negligent and did not adequately evaluate or treat the patient. The defense disputed the charge.
- The jury found for the defense.
Dr. Grant’s observations
In this case, the psychiatrist presumably assessed the patient, determined whether he was depressed, and made appropriate treatment interventions. Three years later, the patient killed himself.
Although 3 years passed between the consultation and the suicide, under the law an intervening act that is the reasonably foreseeable result of negligence does not break the causal chain. For example, if the psychiatrist told the patient he needed no treatment and did not need to follow-up with another clinician, the causal chain arguably would still exist. Thus, proper care, not time, will prevent such a suit.
To win a negligence case, the plaintiff must show that the psychiatrist’s actions proximately caused the harm. In order to defend your treatment decisions, document and discuss with the patient:
- the diagnosis and illness severity
- possible illness course based on patient history and the illness in question
- the need to monitor mood symptoms
- basis for treatment recommendations
- the possible need for continued treatment and to arrange follow-up care.
Plaintiff: Improper treatment caused fatal altercation
Cuyahoga County (OH) Court of Common Pleas
A man in his early 30s was admitted to the hospital’s psychiatric unit for depression, mood disorder, and adjustment disorder secondary to myasthenia gravis. He was estranged from his wife, who was dating another man. The treating psychiatrist discharged the patient after 5 days.
Approximately 40 hours after discharge, the patient went to the other man’s home and confronted him about his relationship with the patient’s wife. The two men then fought, and the other man fatally shot the patient. During the fight, the patient stabbed the other man in the neck with a knife; this man required care and subsequently developed a scar.
The other man and the patient’s estate charged that the psychiatrist did not appropriately evaluate and treat the patient. They claimed that a more thorough evaluation would have resulted in continued hospitalization and prevented the fight.
The defendant argued that the evaluation was thorough, that the discharge met the guidelines of appropriate care, and that the patient posed no risk to himself or others when he was discharged.
- The jury decided for the defense.
Dr. Grant’s observations
This case raises the clinically difficult issue of assessing a patient’s danger to self or others and whether this danger is reasonably foreseeable. Although Hughes reports that 17% of psychiatric emergency room patients are homicidal,1 the American Psychiatric Association notes that 2 of 3 predictions of patient violence are wrong.2
In Tarasoff v Regents of the University of California, the California Supreme Court ruled that clinicians must warn potential victims of, and protect them from, a patient’s intent to harm.3 You should become familiar with the Tarasoff-type legislations in your state. But even if the patient shows no violent intent, the possibility of future violence cannot be ruled out.
During admission, assess and document an inpatient’s risk of violence.4 Factors that may increase a depressed patient’s risk of becoming homicidal include:
- past violence
- current substance abuse
- psychopathy
- having suffered physical abuse as a child
- violent thoughts.4-6
Check the patient’s records for a history of recurrent violence. Culling this information from the chart is necessary because:
- past violent behavior may predict future violence
- patients rarely reveal homicidal thoughts or behavior spontaneously.6
A psychiatrist may be found negligent after a patient’s violent act if the violence was foreseeable. However, after having seen the patient for 5 days in an inpatient setting, the psychiatrist in this case apparently could clearly document that the patient was not dangerous to himself or others before discharge.
1. Hughes DH. Suicide and violence assessment in psychiatry. Gen Hosp Psychiatry 1996;18:416-21.
2. American Psychiatric Association fact sheet. Violence and mental illness. January 1998:1-5. Available at: http://www.psych.org/public_info/violence.pdf. Accessed Feb. 3, 2005.
3. Tarasoff v Regents of the University of California, 551 P2d 334 (Cal 1976)
4. Green B, Pedley R, Whittingham D. A structured clinical model for violence risk intervention. Int J Law Psychiatry 2004;27:349-59.
5. Appelbaum PS, Robbins PC, Monahan J. Violence and delusions: data from the MacArthur Violence Risk Assessment Study. Am J Psychiatry 2000;157:566-72.
6. Rosenbaum M, Bennett B. Homicide and depression. Am J Psychiatry 1986;143:367-70.
7. Appelbaum PS. Can a psychiatrist be held responsible when a patient commits murder? Psychiatr Serv 2002;53:27-9.
When a psychiatrist is sued for negligence, the legal system asks, “Did the doctor depart from the standard of care, and—if so—did that departure proximately cause the harm?” To determine proximate cause, the legal system then asks whether the harm was a “reasonably foreseeable” result of the negligent act.
Inadequate evaluation leads to patient’s suicide, plaintiff alleges
Dallas County (TX) District Court
In 1997, 1 year after suffering a stroke, a 56-year-old man was admitted to a rehabilitation center and seen by a psychiatrist for depression. In February 2000, the patient died after jumping from the center’s fifth-floor window.
The patient’s estate charged that the psychiatrist was negligent and did not adequately evaluate or treat the patient. The defense disputed the charge.
- The jury found for the defense.
Dr. Grant’s observations
In this case, the psychiatrist presumably assessed the patient, determined whether he was depressed, and made appropriate treatment interventions. Three years later, the patient killed himself.
Although 3 years passed between the consultation and the suicide, under the law an intervening act that is the reasonably foreseeable result of negligence does not break the causal chain. For example, if the psychiatrist told the patient he needed no treatment and did not need to follow-up with another clinician, the causal chain arguably would still exist. Thus, proper care, not time, will prevent such a suit.
To win a negligence case, the plaintiff must show that the psychiatrist’s actions proximately caused the harm. In order to defend your treatment decisions, document and discuss with the patient:
- the diagnosis and illness severity
- possible illness course based on patient history and the illness in question
- the need to monitor mood symptoms
- basis for treatment recommendations
- the possible need for continued treatment and to arrange follow-up care.
Plaintiff: Improper treatment caused fatal altercation
Cuyahoga County (OH) Court of Common Pleas
A man in his early 30s was admitted to the hospital’s psychiatric unit for depression, mood disorder, and adjustment disorder secondary to myasthenia gravis. He was estranged from his wife, who was dating another man. The treating psychiatrist discharged the patient after 5 days.
Approximately 40 hours after discharge, the patient went to the other man’s home and confronted him about his relationship with the patient’s wife. The two men then fought, and the other man fatally shot the patient. During the fight, the patient stabbed the other man in the neck with a knife; this man required care and subsequently developed a scar.
The other man and the patient’s estate charged that the psychiatrist did not appropriately evaluate and treat the patient. They claimed that a more thorough evaluation would have resulted in continued hospitalization and prevented the fight.
The defendant argued that the evaluation was thorough, that the discharge met the guidelines of appropriate care, and that the patient posed no risk to himself or others when he was discharged.
- The jury decided for the defense.
Dr. Grant’s observations
This case raises the clinically difficult issue of assessing a patient’s danger to self or others and whether this danger is reasonably foreseeable. Although Hughes reports that 17% of psychiatric emergency room patients are homicidal,1 the American Psychiatric Association notes that 2 of 3 predictions of patient violence are wrong.2
In Tarasoff v Regents of the University of California, the California Supreme Court ruled that clinicians must warn potential victims of, and protect them from, a patient’s intent to harm.3 You should become familiar with the Tarasoff-type legislations in your state. But even if the patient shows no violent intent, the possibility of future violence cannot be ruled out.
During admission, assess and document an inpatient’s risk of violence.4 Factors that may increase a depressed patient’s risk of becoming homicidal include:
- past violence
- current substance abuse
- psychopathy
- having suffered physical abuse as a child
- violent thoughts.4-6
Check the patient’s records for a history of recurrent violence. Culling this information from the chart is necessary because:
- past violent behavior may predict future violence
- patients rarely reveal homicidal thoughts or behavior spontaneously.6
A psychiatrist may be found negligent after a patient’s violent act if the violence was foreseeable. However, after having seen the patient for 5 days in an inpatient setting, the psychiatrist in this case apparently could clearly document that the patient was not dangerous to himself or others before discharge.
When a psychiatrist is sued for negligence, the legal system asks, “Did the doctor depart from the standard of care, and—if so—did that departure proximately cause the harm?” To determine proximate cause, the legal system then asks whether the harm was a “reasonably foreseeable” result of the negligent act.
Inadequate evaluation leads to patient’s suicide, plaintiff alleges
Dallas County (TX) District Court
In 1997, 1 year after suffering a stroke, a 56-year-old man was admitted to a rehabilitation center and seen by a psychiatrist for depression. In February 2000, the patient died after jumping from the center’s fifth-floor window.
The patient’s estate charged that the psychiatrist was negligent and did not adequately evaluate or treat the patient. The defense disputed the charge.
- The jury found for the defense.
Dr. Grant’s observations
In this case, the psychiatrist presumably assessed the patient, determined whether he was depressed, and made appropriate treatment interventions. Three years later, the patient killed himself.
Although 3 years passed between the consultation and the suicide, under the law an intervening act that is the reasonably foreseeable result of negligence does not break the causal chain. For example, if the psychiatrist told the patient he needed no treatment and did not need to follow-up with another clinician, the causal chain arguably would still exist. Thus, proper care, not time, will prevent such a suit.
To win a negligence case, the plaintiff must show that the psychiatrist’s actions proximately caused the harm. In order to defend your treatment decisions, document and discuss with the patient:
- the diagnosis and illness severity
- possible illness course based on patient history and the illness in question
- the need to monitor mood symptoms
- basis for treatment recommendations
- the possible need for continued treatment and to arrange follow-up care.
Plaintiff: Improper treatment caused fatal altercation
Cuyahoga County (OH) Court of Common Pleas
A man in his early 30s was admitted to the hospital’s psychiatric unit for depression, mood disorder, and adjustment disorder secondary to myasthenia gravis. He was estranged from his wife, who was dating another man. The treating psychiatrist discharged the patient after 5 days.
Approximately 40 hours after discharge, the patient went to the other man’s home and confronted him about his relationship with the patient’s wife. The two men then fought, and the other man fatally shot the patient. During the fight, the patient stabbed the other man in the neck with a knife; this man required care and subsequently developed a scar.
The other man and the patient’s estate charged that the psychiatrist did not appropriately evaluate and treat the patient. They claimed that a more thorough evaluation would have resulted in continued hospitalization and prevented the fight.
The defendant argued that the evaluation was thorough, that the discharge met the guidelines of appropriate care, and that the patient posed no risk to himself or others when he was discharged.
- The jury decided for the defense.
Dr. Grant’s observations
This case raises the clinically difficult issue of assessing a patient’s danger to self or others and whether this danger is reasonably foreseeable. Although Hughes reports that 17% of psychiatric emergency room patients are homicidal,1 the American Psychiatric Association notes that 2 of 3 predictions of patient violence are wrong.2
In Tarasoff v Regents of the University of California, the California Supreme Court ruled that clinicians must warn potential victims of, and protect them from, a patient’s intent to harm.3 You should become familiar with the Tarasoff-type legislations in your state. But even if the patient shows no violent intent, the possibility of future violence cannot be ruled out.
During admission, assess and document an inpatient’s risk of violence.4 Factors that may increase a depressed patient’s risk of becoming homicidal include:
- past violence
- current substance abuse
- psychopathy
- having suffered physical abuse as a child
- violent thoughts.4-6
Check the patient’s records for a history of recurrent violence. Culling this information from the chart is necessary because:
- past violent behavior may predict future violence
- patients rarely reveal homicidal thoughts or behavior spontaneously.6
A psychiatrist may be found negligent after a patient’s violent act if the violence was foreseeable. However, after having seen the patient for 5 days in an inpatient setting, the psychiatrist in this case apparently could clearly document that the patient was not dangerous to himself or others before discharge.
1. Hughes DH. Suicide and violence assessment in psychiatry. Gen Hosp Psychiatry 1996;18:416-21.
2. American Psychiatric Association fact sheet. Violence and mental illness. January 1998:1-5. Available at: http://www.psych.org/public_info/violence.pdf. Accessed Feb. 3, 2005.
3. Tarasoff v Regents of the University of California, 551 P2d 334 (Cal 1976)
4. Green B, Pedley R, Whittingham D. A structured clinical model for violence risk intervention. Int J Law Psychiatry 2004;27:349-59.
5. Appelbaum PS, Robbins PC, Monahan J. Violence and delusions: data from the MacArthur Violence Risk Assessment Study. Am J Psychiatry 2000;157:566-72.
6. Rosenbaum M, Bennett B. Homicide and depression. Am J Psychiatry 1986;143:367-70.
7. Appelbaum PS. Can a psychiatrist be held responsible when a patient commits murder? Psychiatr Serv 2002;53:27-9.
1. Hughes DH. Suicide and violence assessment in psychiatry. Gen Hosp Psychiatry 1996;18:416-21.
2. American Psychiatric Association fact sheet. Violence and mental illness. January 1998:1-5. Available at: http://www.psych.org/public_info/violence.pdf. Accessed Feb. 3, 2005.
3. Tarasoff v Regents of the University of California, 551 P2d 334 (Cal 1976)
4. Green B, Pedley R, Whittingham D. A structured clinical model for violence risk intervention. Int J Law Psychiatry 2004;27:349-59.
5. Appelbaum PS, Robbins PC, Monahan J. Violence and delusions: data from the MacArthur Violence Risk Assessment Study. Am J Psychiatry 2000;157:566-72.
6. Rosenbaum M, Bennett B. Homicide and depression. Am J Psychiatry 1986;143:367-70.
7. Appelbaum PS. Can a psychiatrist be held responsible when a patient commits murder? Psychiatr Serv 2002;53:27-9.
A Rare Scleroderma Look-Alike: Nephrogenic Fibrosing Dermopathy
A recently described cutaneous fibrosing disorder could be mistaken for scleroderma, but there are some key differences, said Dr. Collier.
Worldwide, there have been only 170 cases of nephrogenic fibrosing dermopathy (NFD) reported since it was first described in 1997, he said. Yet “I think it's far more common than we're led to believe,” he added.
The typical presentation of NFD consists of acute, lumpy, plaquelike indurations involving the lower limbs and occasionally the upper limbs and torso, he said.
Usually, scleroderma starts on the hands and face. But in NFD, these are almost always spared, he said.
The most common distribution of NFD skin presentation is between the ankles and the mid-thighs and between the wrists and mid-upper arms bilaterally, he said. Skin-colored to erythematous papules coalesce into brawny plaques with a peau d'orange appearance. There is a distinctive, irregular edge with amoeboid projections and islands of sparing within the indurated plaque. Eventually, the skin becomes markedly thickened and woody. Pruritis and causalgia are prominent features.
Unlike scleroderma, NFD often causes severe sharp pains in the affected areas, and renal insufficiency is necessary for the diagnosis.
The biopsy will show deposits of collagen and elastin—spindle cells, dendritic cells, and mucin deposits—“which is different from what we see in scleroderma.”
Although NFD was initially thought to be only a cutaneous disease, there now appears to be a severe myopathic component. Joint contractures may develop within days or weeks of onset, likely resulting from facial and muscle fibrosis, Dr. Collier noted.
The abrupt emergence of this disease suggests that toxic exposures, infectious agents, or medical techniques may be involved.
NFD plaques typically take on a peau d'orange appearence. Courtesy Dr. David H. Collier
A recently described cutaneous fibrosing disorder could be mistaken for scleroderma, but there are some key differences, said Dr. Collier.
Worldwide, there have been only 170 cases of nephrogenic fibrosing dermopathy (NFD) reported since it was first described in 1997, he said. Yet “I think it's far more common than we're led to believe,” he added.
The typical presentation of NFD consists of acute, lumpy, plaquelike indurations involving the lower limbs and occasionally the upper limbs and torso, he said.
Usually, scleroderma starts on the hands and face. But in NFD, these are almost always spared, he said.
The most common distribution of NFD skin presentation is between the ankles and the mid-thighs and between the wrists and mid-upper arms bilaterally, he said. Skin-colored to erythematous papules coalesce into brawny plaques with a peau d'orange appearance. There is a distinctive, irregular edge with amoeboid projections and islands of sparing within the indurated plaque. Eventually, the skin becomes markedly thickened and woody. Pruritis and causalgia are prominent features.
Unlike scleroderma, NFD often causes severe sharp pains in the affected areas, and renal insufficiency is necessary for the diagnosis.
The biopsy will show deposits of collagen and elastin—spindle cells, dendritic cells, and mucin deposits—“which is different from what we see in scleroderma.”
Although NFD was initially thought to be only a cutaneous disease, there now appears to be a severe myopathic component. Joint contractures may develop within days or weeks of onset, likely resulting from facial and muscle fibrosis, Dr. Collier noted.
The abrupt emergence of this disease suggests that toxic exposures, infectious agents, or medical techniques may be involved.
NFD plaques typically take on a peau d'orange appearence. Courtesy Dr. David H. Collier
A recently described cutaneous fibrosing disorder could be mistaken for scleroderma, but there are some key differences, said Dr. Collier.
Worldwide, there have been only 170 cases of nephrogenic fibrosing dermopathy (NFD) reported since it was first described in 1997, he said. Yet “I think it's far more common than we're led to believe,” he added.
The typical presentation of NFD consists of acute, lumpy, plaquelike indurations involving the lower limbs and occasionally the upper limbs and torso, he said.
Usually, scleroderma starts on the hands and face. But in NFD, these are almost always spared, he said.
The most common distribution of NFD skin presentation is between the ankles and the mid-thighs and between the wrists and mid-upper arms bilaterally, he said. Skin-colored to erythematous papules coalesce into brawny plaques with a peau d'orange appearance. There is a distinctive, irregular edge with amoeboid projections and islands of sparing within the indurated plaque. Eventually, the skin becomes markedly thickened and woody. Pruritis and causalgia are prominent features.
Unlike scleroderma, NFD often causes severe sharp pains in the affected areas, and renal insufficiency is necessary for the diagnosis.
The biopsy will show deposits of collagen and elastin—spindle cells, dendritic cells, and mucin deposits—“which is different from what we see in scleroderma.”
Although NFD was initially thought to be only a cutaneous disease, there now appears to be a severe myopathic component. Joint contractures may develop within days or weeks of onset, likely resulting from facial and muscle fibrosis, Dr. Collier noted.
The abrupt emergence of this disease suggests that toxic exposures, infectious agents, or medical techniques may be involved.
NFD plaques typically take on a peau d'orange appearence. Courtesy Dr. David H. Collier
New Raynaud's: Nail Folds Predict Scleroderma
SNOWMASS, COLO — The most significant predictor of progression to scleroderma in a patient with new onset Raynaud's phenomenon is the presence of capillary abnormalities at the proximal nail fold, according to David H. Collier, M.D.
Although scleroderma is primarily managed by rheumatologists, it is dermatologists who most commonly identify the early skin manifestations of the disorder, said Dr. Collier of the University of Colorado, Denver, and chief of rheumatology at Denver Health Medical Center.
In addition to Raynaud's, these manifestations include skin thickening, ulceration, telangiectases, calcinosis, and pigmentation changes.
Speaking at a clinical dermatology seminar sponsored by Medicis, Dr. Collier explained that Raynaud's phenomenon is an almost universal component of systemic sclerosis, and yet the vast majority of patients with Raynaud's never progress to scleroderma.
“Up to 10% of adult women can have Raynaud's, and less then 0.1% can go on to develop scleroderma,” he said in an interview, adding that about 77% of Raynaud's patients are female.
By examining the periungual area of the finger, under gel with an opthalmoscope, the physician can easily assess capillary abnormalities at the proximal nail fold, he said.
“Instead of thin little loops of capillaries that you would see in a normal patient, you see capillary dilation and areas that are denuded or dropped out altogether,” he said, explaining that capillary dilation occurs early in the disease, and after about 10 years, only denudation is typically visible.
“A patient with abnormal capilloscopy should be followed every 3-6 months for signs of progression to systemic sclerosis,” he advised, adding that early identification of scleroderma and referral can allow for a prompt pulmonary evaluation and establishment of gastroesophageal reflux prevention/management.
Pitting or ulceration of the fingertips is another indication that a Raynaud's patient has scleroderma, said Dr. Collier.
“Primary Raynaud's disease does not give you pitting. So if you see pits—especially fingertip pits and ulceration—that's a red light [indicating] that you're dealing with an autoimmune disease. It's almost always Raynaud's secondary to scleroderma or mixed connective tissue disease, or occasionally lupus,” he said.
In addition to the evaluation for capillary abnormalities, the scleroderma work-up for patients presenting with Raynaud's should also include autoantibody testing, he said.
“If they also have the antibodies, that's the subgroup that I worry about the most for progressing to scleroderma, but it's not universal. I've certainly followed people with autoantibodies, and they didn't progress.”
Anticentromere antibodies are seen in 20%-30% of scleroderma patients and are the most predictive of risk to progression to limited systemic sclerosis, although they are also commonly seen in primary biliary cirrhosis and, rarely, in other connective tissue diseases, such as rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, and polymyositis, he said.
Anti-topoisomerase-1 antibodies (e.g., anti-scleroderma [Scl]-70) are present in 9%-20% of scleroderma patients. Anti-RNA polymerase 1-3 antibodies are seen in 20%. Antifibrillarin/anti-U3 ribonucleoprotein (anti-U3 RNP) antibodies are seen in 10%, and anti-neutrophilic cytoplasmic antibodies (ANCA) are seen in about 4%, though mostly in patients with diffuse systemic sclerosis. Finally, antipolymyositis /Scl (anti-PM Scl) and anti-Th/To (which recognizes certain RNA processing enzymes) antibodies are seen in about 2% of scleroderma patients.
SNOWMASS, COLO — The most significant predictor of progression to scleroderma in a patient with new onset Raynaud's phenomenon is the presence of capillary abnormalities at the proximal nail fold, according to David H. Collier, M.D.
Although scleroderma is primarily managed by rheumatologists, it is dermatologists who most commonly identify the early skin manifestations of the disorder, said Dr. Collier of the University of Colorado, Denver, and chief of rheumatology at Denver Health Medical Center.
In addition to Raynaud's, these manifestations include skin thickening, ulceration, telangiectases, calcinosis, and pigmentation changes.
Speaking at a clinical dermatology seminar sponsored by Medicis, Dr. Collier explained that Raynaud's phenomenon is an almost universal component of systemic sclerosis, and yet the vast majority of patients with Raynaud's never progress to scleroderma.
“Up to 10% of adult women can have Raynaud's, and less then 0.1% can go on to develop scleroderma,” he said in an interview, adding that about 77% of Raynaud's patients are female.
By examining the periungual area of the finger, under gel with an opthalmoscope, the physician can easily assess capillary abnormalities at the proximal nail fold, he said.
“Instead of thin little loops of capillaries that you would see in a normal patient, you see capillary dilation and areas that are denuded or dropped out altogether,” he said, explaining that capillary dilation occurs early in the disease, and after about 10 years, only denudation is typically visible.
“A patient with abnormal capilloscopy should be followed every 3-6 months for signs of progression to systemic sclerosis,” he advised, adding that early identification of scleroderma and referral can allow for a prompt pulmonary evaluation and establishment of gastroesophageal reflux prevention/management.
Pitting or ulceration of the fingertips is another indication that a Raynaud's patient has scleroderma, said Dr. Collier.
“Primary Raynaud's disease does not give you pitting. So if you see pits—especially fingertip pits and ulceration—that's a red light [indicating] that you're dealing with an autoimmune disease. It's almost always Raynaud's secondary to scleroderma or mixed connective tissue disease, or occasionally lupus,” he said.
In addition to the evaluation for capillary abnormalities, the scleroderma work-up for patients presenting with Raynaud's should also include autoantibody testing, he said.
“If they also have the antibodies, that's the subgroup that I worry about the most for progressing to scleroderma, but it's not universal. I've certainly followed people with autoantibodies, and they didn't progress.”
Anticentromere antibodies are seen in 20%-30% of scleroderma patients and are the most predictive of risk to progression to limited systemic sclerosis, although they are also commonly seen in primary biliary cirrhosis and, rarely, in other connective tissue diseases, such as rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, and polymyositis, he said.
Anti-topoisomerase-1 antibodies (e.g., anti-scleroderma [Scl]-70) are present in 9%-20% of scleroderma patients. Anti-RNA polymerase 1-3 antibodies are seen in 20%. Antifibrillarin/anti-U3 ribonucleoprotein (anti-U3 RNP) antibodies are seen in 10%, and anti-neutrophilic cytoplasmic antibodies (ANCA) are seen in about 4%, though mostly in patients with diffuse systemic sclerosis. Finally, antipolymyositis /Scl (anti-PM Scl) and anti-Th/To (which recognizes certain RNA processing enzymes) antibodies are seen in about 2% of scleroderma patients.
SNOWMASS, COLO — The most significant predictor of progression to scleroderma in a patient with new onset Raynaud's phenomenon is the presence of capillary abnormalities at the proximal nail fold, according to David H. Collier, M.D.
Although scleroderma is primarily managed by rheumatologists, it is dermatologists who most commonly identify the early skin manifestations of the disorder, said Dr. Collier of the University of Colorado, Denver, and chief of rheumatology at Denver Health Medical Center.
In addition to Raynaud's, these manifestations include skin thickening, ulceration, telangiectases, calcinosis, and pigmentation changes.
Speaking at a clinical dermatology seminar sponsored by Medicis, Dr. Collier explained that Raynaud's phenomenon is an almost universal component of systemic sclerosis, and yet the vast majority of patients with Raynaud's never progress to scleroderma.
“Up to 10% of adult women can have Raynaud's, and less then 0.1% can go on to develop scleroderma,” he said in an interview, adding that about 77% of Raynaud's patients are female.
By examining the periungual area of the finger, under gel with an opthalmoscope, the physician can easily assess capillary abnormalities at the proximal nail fold, he said.
“Instead of thin little loops of capillaries that you would see in a normal patient, you see capillary dilation and areas that are denuded or dropped out altogether,” he said, explaining that capillary dilation occurs early in the disease, and after about 10 years, only denudation is typically visible.
“A patient with abnormal capilloscopy should be followed every 3-6 months for signs of progression to systemic sclerosis,” he advised, adding that early identification of scleroderma and referral can allow for a prompt pulmonary evaluation and establishment of gastroesophageal reflux prevention/management.
Pitting or ulceration of the fingertips is another indication that a Raynaud's patient has scleroderma, said Dr. Collier.
“Primary Raynaud's disease does not give you pitting. So if you see pits—especially fingertip pits and ulceration—that's a red light [indicating] that you're dealing with an autoimmune disease. It's almost always Raynaud's secondary to scleroderma or mixed connective tissue disease, or occasionally lupus,” he said.
In addition to the evaluation for capillary abnormalities, the scleroderma work-up for patients presenting with Raynaud's should also include autoantibody testing, he said.
“If they also have the antibodies, that's the subgroup that I worry about the most for progressing to scleroderma, but it's not universal. I've certainly followed people with autoantibodies, and they didn't progress.”
Anticentromere antibodies are seen in 20%-30% of scleroderma patients and are the most predictive of risk to progression to limited systemic sclerosis, although they are also commonly seen in primary biliary cirrhosis and, rarely, in other connective tissue diseases, such as rheumatoid arthritis, systemic lupus erythematosus, mixed connective tissue disease, and polymyositis, he said.
Anti-topoisomerase-1 antibodies (e.g., anti-scleroderma [Scl]-70) are present in 9%-20% of scleroderma patients. Anti-RNA polymerase 1-3 antibodies are seen in 20%. Antifibrillarin/anti-U3 ribonucleoprotein (anti-U3 RNP) antibodies are seen in 10%, and anti-neutrophilic cytoplasmic antibodies (ANCA) are seen in about 4%, though mostly in patients with diffuse systemic sclerosis. Finally, antipolymyositis /Scl (anti-PM Scl) and anti-Th/To (which recognizes certain RNA processing enzymes) antibodies are seen in about 2% of scleroderma patients.