User login
FDA warns of potential problems with Abbott Trifecta valves
There is a potential risk of early structural valve deterioration with the Abbott Trifecta valve and Trifecta valve with glide technology (Trifecta GT), the U.S. Food and Drug Administration says in a letter to health care professionals posted on its website.
Evidence in the literature suggests a higher cumulative incidence of early structural valve deterioration (SVD) and a lower freedom from reintervention due to SVD with the Trifecta valves, compared with other commercially available bovine pericardial valves, the FDA says.
The Trifecta and Trifecta GT valves are heart valve replacement devices intended to treat diseased, damaged, or malfunctioning native or prosthetic aortic heart valves, the letter notes. The first-generation Trifecta valve was approved in 2011 but is no longer marketed in the United States. The Trifecta GT valve was approved in 2016.
Medical device reports (MDRs) received by the FDA describe early SVD with Trifecta valves, with a peak time to SVD of 3 to 4 years post-implant. “Reported outcomes include surgical valve explant/replacement, transcatheter valve-in-valve intervention, and in some cases death,” the FDA notes.
In a letter to customers, Abbott says a “complaint analysis has shown that most cases of early SVD which occur within 5 years post-implant are characterized as a non-calcific leaflet tear, while most cases of late SVD which occur beyond 5 years post-implant are characterized as a fibrous-calcific SVD.”
The FDA recommends that health care providers take the following actions:
- Be aware of the potential risk of early SVD with Trifecta valves, and current patient management considerations, as communicated by Abbott.
- Discuss the risks and benefits of all available aortic valve treatment options with patients and caregivers as part of shared clinical decision-making prior to surgery.
- Read and carefully follow the Instructions for Use when implanting a Trifecta GT valve.
- Monitor patients who have undergone implantation with Trifecta valves for signs and symptoms of potential SVD.
- Instruct patients to seek medical attention with new onset of symptoms such as shortness of breath or fatigue.
- Ensure lifelong follow-up visits, conducted at least yearly, including transthoracic echocardiogram assessment of the valve beginning 1-year post-implant.
The FDA is working with Abbott to further evaluate the issue and develop additional patient management strategies, if needed. The FDA says it will continue to monitor the literature and reports of adverse events related to the issue.
Clinicians are encouraged to report any adverse events or quality problems with the Trifecta valves to their local Abbott representative or the customer service department at 1-800-544-1664.
Health care professionals can also report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
There is a potential risk of early structural valve deterioration with the Abbott Trifecta valve and Trifecta valve with glide technology (Trifecta GT), the U.S. Food and Drug Administration says in a letter to health care professionals posted on its website.
Evidence in the literature suggests a higher cumulative incidence of early structural valve deterioration (SVD) and a lower freedom from reintervention due to SVD with the Trifecta valves, compared with other commercially available bovine pericardial valves, the FDA says.
The Trifecta and Trifecta GT valves are heart valve replacement devices intended to treat diseased, damaged, or malfunctioning native or prosthetic aortic heart valves, the letter notes. The first-generation Trifecta valve was approved in 2011 but is no longer marketed in the United States. The Trifecta GT valve was approved in 2016.
Medical device reports (MDRs) received by the FDA describe early SVD with Trifecta valves, with a peak time to SVD of 3 to 4 years post-implant. “Reported outcomes include surgical valve explant/replacement, transcatheter valve-in-valve intervention, and in some cases death,” the FDA notes.
In a letter to customers, Abbott says a “complaint analysis has shown that most cases of early SVD which occur within 5 years post-implant are characterized as a non-calcific leaflet tear, while most cases of late SVD which occur beyond 5 years post-implant are characterized as a fibrous-calcific SVD.”
The FDA recommends that health care providers take the following actions:
- Be aware of the potential risk of early SVD with Trifecta valves, and current patient management considerations, as communicated by Abbott.
- Discuss the risks and benefits of all available aortic valve treatment options with patients and caregivers as part of shared clinical decision-making prior to surgery.
- Read and carefully follow the Instructions for Use when implanting a Trifecta GT valve.
- Monitor patients who have undergone implantation with Trifecta valves for signs and symptoms of potential SVD.
- Instruct patients to seek medical attention with new onset of symptoms such as shortness of breath or fatigue.
- Ensure lifelong follow-up visits, conducted at least yearly, including transthoracic echocardiogram assessment of the valve beginning 1-year post-implant.
The FDA is working with Abbott to further evaluate the issue and develop additional patient management strategies, if needed. The FDA says it will continue to monitor the literature and reports of adverse events related to the issue.
Clinicians are encouraged to report any adverse events or quality problems with the Trifecta valves to their local Abbott representative or the customer service department at 1-800-544-1664.
Health care professionals can also report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
There is a potential risk of early structural valve deterioration with the Abbott Trifecta valve and Trifecta valve with glide technology (Trifecta GT), the U.S. Food and Drug Administration says in a letter to health care professionals posted on its website.
Evidence in the literature suggests a higher cumulative incidence of early structural valve deterioration (SVD) and a lower freedom from reintervention due to SVD with the Trifecta valves, compared with other commercially available bovine pericardial valves, the FDA says.
The Trifecta and Trifecta GT valves are heart valve replacement devices intended to treat diseased, damaged, or malfunctioning native or prosthetic aortic heart valves, the letter notes. The first-generation Trifecta valve was approved in 2011 but is no longer marketed in the United States. The Trifecta GT valve was approved in 2016.
Medical device reports (MDRs) received by the FDA describe early SVD with Trifecta valves, with a peak time to SVD of 3 to 4 years post-implant. “Reported outcomes include surgical valve explant/replacement, transcatheter valve-in-valve intervention, and in some cases death,” the FDA notes.
In a letter to customers, Abbott says a “complaint analysis has shown that most cases of early SVD which occur within 5 years post-implant are characterized as a non-calcific leaflet tear, while most cases of late SVD which occur beyond 5 years post-implant are characterized as a fibrous-calcific SVD.”
The FDA recommends that health care providers take the following actions:
- Be aware of the potential risk of early SVD with Trifecta valves, and current patient management considerations, as communicated by Abbott.
- Discuss the risks and benefits of all available aortic valve treatment options with patients and caregivers as part of shared clinical decision-making prior to surgery.
- Read and carefully follow the Instructions for Use when implanting a Trifecta GT valve.
- Monitor patients who have undergone implantation with Trifecta valves for signs and symptoms of potential SVD.
- Instruct patients to seek medical attention with new onset of symptoms such as shortness of breath or fatigue.
- Ensure lifelong follow-up visits, conducted at least yearly, including transthoracic echocardiogram assessment of the valve beginning 1-year post-implant.
The FDA is working with Abbott to further evaluate the issue and develop additional patient management strategies, if needed. The FDA says it will continue to monitor the literature and reports of adverse events related to the issue.
Clinicians are encouraged to report any adverse events or quality problems with the Trifecta valves to their local Abbott representative or the customer service department at 1-800-544-1664.
Health care professionals can also report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
To prevent MS, should we target EBV?
SAN DIEGO – Although most adults have been exposed, it is very rare to find MS in an individual with no prior EBV exposure.
That apparent relationship has driven interest in a vaccine against EBV in an effort to reduce MS incidence on a population level.
At a session at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS), two researchers debated the potential benefits and pitfalls of such a program. The issues included the possible benefit in MS and other EBV-related conditions such as mononucleosis and various cancers, and whether EBV infection is a sufficient cause for MS, as well as concerns about vaccinating a healthy at-risk population.
Reducing the risk of MS by targeting EBV
Jeffrey I. Cohen, MD, spoke first, and cited several lines of evidence supporting the importance of EBV in MS. One study showed a 32-fold increased risk of MS following primary infection with EBV, and another showed that higher EBV nuclear antigen (EBNA) antibody titers were associated with a 36-fold higher risk of MS. “So we have two completely independent studies suggesting that EBV is really very important as a cofactor for development of MS,” said Dr. Cohen, chief of the laboratory of infectious diseases and chief of the medical virology section at the National Institutes of Health, Bethesda, Md.
EBV is also latent in B cells, and anti-B cell therapy is an effective therapeutic strategy for MS. However, the mechanism remains unknown.
Targeting EBV could involve attacking infected cells, or a therapeutic vaccine could be employed to treat EVB-infected individuals, similar to the shingles vaccine. “In all of these methods, one would end up with fewer EBV infected B cells and as a result, presumably you’d have reduced antigenic stimulation of EBV-infected B cells to stimulate either antibodies or T cells that could damage the nervous system. By reducing this, one might be able to [treat] multiple sclerosis,” said Dr. Cohen.
He did acknowledge concerns. It isn’t yet understood whether destroying EBV-infected cells would actually improve outcomes. It also may be more difficult to reduce a latent infection than to prevent infection, since almost all B cells become latently infected. “Thus we think perhaps a role for preventing infection or modifying the initial infection could be important,” said Dr. Cohen.
The most advanced vaccine candidate is a soluble form of EBV glycoprotein gp 350, which is the dominant glycoprotein on the surface of the virus and infected cells. It reduced the risk of mononucleosis by 78%, but it did not prevent EBV infection. There were no safety concerns. Two more vaccines are currently in clinical trials – an mRNA vaccine against a gp 350 sponsored by Moderna, and a gp 350 nanoparticle vaccine by the NIH.
Dr. Cohen acknowledged that safety is the most important factor, since it would be given to healthy individuals, and probably children. There are worries that a vaccine using EBV proteins could worsen MS. In particular, higher titers of antibodies against EBNA have been linked to developing MS and the anti-EBNA antibody has been implicated in molecular mimicry related to MS. However, the current vaccines avoid EBNA. Another worry is that a vaccine could delay onset of disease to an older age, when infection might be more dangerous. However, no delay in onset has been noted with the varicella vaccine or polio vaccines, which prompted similar concerns.
Vaccinating against EBV could also reduce other conditions such as mononucleosis and several cancers.
Does EBV infection even matter?
In his talk, Peter Calabresi, MD, made the case that EBV is not the sole cause of MS, and thus targeting it may prove ineffective. Dr. Calabresi is director of the division of neuroimmunology at Johns Hopkins Medicine, Baltimore.
Why was he asked to provide a rebuttal? “About this time last year, I commented at a meeting that we should be thoughtful as we think about what to do about EBV and MS. I do believe that constructive dialogue is the foundation of science,” he said. He also stated that he is not opposed to vaccines. “I congratulate Dr. Cohen on all of his vaccine successes,” he said.
Still, he is unconvinced that EBV is solely responsible for MS. “I think it’s hard to draw a straight line between EBV and MS as one might with HPV [human papillomavirus] and cervical cancer. For example, we know that EBV accounts for more than 1% of all cancers, and EBV can also cause other autoimmune diseases such as lupus and Sjogren’s, so it’s complicated. And MS of course has genetic susceptibility that’s not limited to the major histocompatibility complex (MHC) genes that are associated with presenting viral peptides,” said Dr. Calabresi.
Evidence relating MS vulnerability to other genetic and environmental factors, including diet, sunlight, smoking, and even pollution, calls into question a direct causal relationship between EBV and MS, he said.
The age prevalence of EBV would complicate efforts to eradicate it. Seroprevalence is 55% by age 5-11 and 75% among university students. “This is important because the duration of the vaccine response–induced protection in young seronegative children is not lengthy. Vaccinated individuals may become susceptible to natural infection at an age where the consequences of infection are more severe, especially leading to infectious mononucleosis, and hopefully not MS. This then raises the issue of the need for boosters, which we’re all well aware of during the COVID pandemic. This may be a problem, especially in young adults due to noncompliance,” said Dr. Calabresi.
He pointed out that not all vaccine attempts went well. In the 1960s, early respiratory syncytial virus (RSV) vaccines caused enhanced respiratory disease and 2 deaths. “We need to be careful when we think about targeting healthy at-risk young people,” said Dr. Calabresi.
Rather than pursue vaccination, Dr. Calabresi favors research into EBV latency in B cells as well as how EBV-infected B cells may cause or exacerbate MS, with the hopes of developing interventions. “It’s tempting to speculate that the success of the anti-CD 20 monoclonal antibody therapies is related to depletion of EBV infected B cells. In fact, I think that may be the case,” he said.
Dr. Cohen has no relevant financial disclosures. Dr. Calabresi has served on a scientific advisory board or data monitoring board for Biogen and Disarm Therapeutics.
SAN DIEGO – Although most adults have been exposed, it is very rare to find MS in an individual with no prior EBV exposure.
That apparent relationship has driven interest in a vaccine against EBV in an effort to reduce MS incidence on a population level.
At a session at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS), two researchers debated the potential benefits and pitfalls of such a program. The issues included the possible benefit in MS and other EBV-related conditions such as mononucleosis and various cancers, and whether EBV infection is a sufficient cause for MS, as well as concerns about vaccinating a healthy at-risk population.
Reducing the risk of MS by targeting EBV
Jeffrey I. Cohen, MD, spoke first, and cited several lines of evidence supporting the importance of EBV in MS. One study showed a 32-fold increased risk of MS following primary infection with EBV, and another showed that higher EBV nuclear antigen (EBNA) antibody titers were associated with a 36-fold higher risk of MS. “So we have two completely independent studies suggesting that EBV is really very important as a cofactor for development of MS,” said Dr. Cohen, chief of the laboratory of infectious diseases and chief of the medical virology section at the National Institutes of Health, Bethesda, Md.
EBV is also latent in B cells, and anti-B cell therapy is an effective therapeutic strategy for MS. However, the mechanism remains unknown.
Targeting EBV could involve attacking infected cells, or a therapeutic vaccine could be employed to treat EVB-infected individuals, similar to the shingles vaccine. “In all of these methods, one would end up with fewer EBV infected B cells and as a result, presumably you’d have reduced antigenic stimulation of EBV-infected B cells to stimulate either antibodies or T cells that could damage the nervous system. By reducing this, one might be able to [treat] multiple sclerosis,” said Dr. Cohen.
He did acknowledge concerns. It isn’t yet understood whether destroying EBV-infected cells would actually improve outcomes. It also may be more difficult to reduce a latent infection than to prevent infection, since almost all B cells become latently infected. “Thus we think perhaps a role for preventing infection or modifying the initial infection could be important,” said Dr. Cohen.
The most advanced vaccine candidate is a soluble form of EBV glycoprotein gp 350, which is the dominant glycoprotein on the surface of the virus and infected cells. It reduced the risk of mononucleosis by 78%, but it did not prevent EBV infection. There were no safety concerns. Two more vaccines are currently in clinical trials – an mRNA vaccine against a gp 350 sponsored by Moderna, and a gp 350 nanoparticle vaccine by the NIH.
Dr. Cohen acknowledged that safety is the most important factor, since it would be given to healthy individuals, and probably children. There are worries that a vaccine using EBV proteins could worsen MS. In particular, higher titers of antibodies against EBNA have been linked to developing MS and the anti-EBNA antibody has been implicated in molecular mimicry related to MS. However, the current vaccines avoid EBNA. Another worry is that a vaccine could delay onset of disease to an older age, when infection might be more dangerous. However, no delay in onset has been noted with the varicella vaccine or polio vaccines, which prompted similar concerns.
Vaccinating against EBV could also reduce other conditions such as mononucleosis and several cancers.
Does EBV infection even matter?
In his talk, Peter Calabresi, MD, made the case that EBV is not the sole cause of MS, and thus targeting it may prove ineffective. Dr. Calabresi is director of the division of neuroimmunology at Johns Hopkins Medicine, Baltimore.
Why was he asked to provide a rebuttal? “About this time last year, I commented at a meeting that we should be thoughtful as we think about what to do about EBV and MS. I do believe that constructive dialogue is the foundation of science,” he said. He also stated that he is not opposed to vaccines. “I congratulate Dr. Cohen on all of his vaccine successes,” he said.
Still, he is unconvinced that EBV is solely responsible for MS. “I think it’s hard to draw a straight line between EBV and MS as one might with HPV [human papillomavirus] and cervical cancer. For example, we know that EBV accounts for more than 1% of all cancers, and EBV can also cause other autoimmune diseases such as lupus and Sjogren’s, so it’s complicated. And MS of course has genetic susceptibility that’s not limited to the major histocompatibility complex (MHC) genes that are associated with presenting viral peptides,” said Dr. Calabresi.
Evidence relating MS vulnerability to other genetic and environmental factors, including diet, sunlight, smoking, and even pollution, calls into question a direct causal relationship between EBV and MS, he said.
The age prevalence of EBV would complicate efforts to eradicate it. Seroprevalence is 55% by age 5-11 and 75% among university students. “This is important because the duration of the vaccine response–induced protection in young seronegative children is not lengthy. Vaccinated individuals may become susceptible to natural infection at an age where the consequences of infection are more severe, especially leading to infectious mononucleosis, and hopefully not MS. This then raises the issue of the need for boosters, which we’re all well aware of during the COVID pandemic. This may be a problem, especially in young adults due to noncompliance,” said Dr. Calabresi.
He pointed out that not all vaccine attempts went well. In the 1960s, early respiratory syncytial virus (RSV) vaccines caused enhanced respiratory disease and 2 deaths. “We need to be careful when we think about targeting healthy at-risk young people,” said Dr. Calabresi.
Rather than pursue vaccination, Dr. Calabresi favors research into EBV latency in B cells as well as how EBV-infected B cells may cause or exacerbate MS, with the hopes of developing interventions. “It’s tempting to speculate that the success of the anti-CD 20 monoclonal antibody therapies is related to depletion of EBV infected B cells. In fact, I think that may be the case,” he said.
Dr. Cohen has no relevant financial disclosures. Dr. Calabresi has served on a scientific advisory board or data monitoring board for Biogen and Disarm Therapeutics.
SAN DIEGO – Although most adults have been exposed, it is very rare to find MS in an individual with no prior EBV exposure.
That apparent relationship has driven interest in a vaccine against EBV in an effort to reduce MS incidence on a population level.
At a session at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS), two researchers debated the potential benefits and pitfalls of such a program. The issues included the possible benefit in MS and other EBV-related conditions such as mononucleosis and various cancers, and whether EBV infection is a sufficient cause for MS, as well as concerns about vaccinating a healthy at-risk population.
Reducing the risk of MS by targeting EBV
Jeffrey I. Cohen, MD, spoke first, and cited several lines of evidence supporting the importance of EBV in MS. One study showed a 32-fold increased risk of MS following primary infection with EBV, and another showed that higher EBV nuclear antigen (EBNA) antibody titers were associated with a 36-fold higher risk of MS. “So we have two completely independent studies suggesting that EBV is really very important as a cofactor for development of MS,” said Dr. Cohen, chief of the laboratory of infectious diseases and chief of the medical virology section at the National Institutes of Health, Bethesda, Md.
EBV is also latent in B cells, and anti-B cell therapy is an effective therapeutic strategy for MS. However, the mechanism remains unknown.
Targeting EBV could involve attacking infected cells, or a therapeutic vaccine could be employed to treat EVB-infected individuals, similar to the shingles vaccine. “In all of these methods, one would end up with fewer EBV infected B cells and as a result, presumably you’d have reduced antigenic stimulation of EBV-infected B cells to stimulate either antibodies or T cells that could damage the nervous system. By reducing this, one might be able to [treat] multiple sclerosis,” said Dr. Cohen.
He did acknowledge concerns. It isn’t yet understood whether destroying EBV-infected cells would actually improve outcomes. It also may be more difficult to reduce a latent infection than to prevent infection, since almost all B cells become latently infected. “Thus we think perhaps a role for preventing infection or modifying the initial infection could be important,” said Dr. Cohen.
The most advanced vaccine candidate is a soluble form of EBV glycoprotein gp 350, which is the dominant glycoprotein on the surface of the virus and infected cells. It reduced the risk of mononucleosis by 78%, but it did not prevent EBV infection. There were no safety concerns. Two more vaccines are currently in clinical trials – an mRNA vaccine against a gp 350 sponsored by Moderna, and a gp 350 nanoparticle vaccine by the NIH.
Dr. Cohen acknowledged that safety is the most important factor, since it would be given to healthy individuals, and probably children. There are worries that a vaccine using EBV proteins could worsen MS. In particular, higher titers of antibodies against EBNA have been linked to developing MS and the anti-EBNA antibody has been implicated in molecular mimicry related to MS. However, the current vaccines avoid EBNA. Another worry is that a vaccine could delay onset of disease to an older age, when infection might be more dangerous. However, no delay in onset has been noted with the varicella vaccine or polio vaccines, which prompted similar concerns.
Vaccinating against EBV could also reduce other conditions such as mononucleosis and several cancers.
Does EBV infection even matter?
In his talk, Peter Calabresi, MD, made the case that EBV is not the sole cause of MS, and thus targeting it may prove ineffective. Dr. Calabresi is director of the division of neuroimmunology at Johns Hopkins Medicine, Baltimore.
Why was he asked to provide a rebuttal? “About this time last year, I commented at a meeting that we should be thoughtful as we think about what to do about EBV and MS. I do believe that constructive dialogue is the foundation of science,” he said. He also stated that he is not opposed to vaccines. “I congratulate Dr. Cohen on all of his vaccine successes,” he said.
Still, he is unconvinced that EBV is solely responsible for MS. “I think it’s hard to draw a straight line between EBV and MS as one might with HPV [human papillomavirus] and cervical cancer. For example, we know that EBV accounts for more than 1% of all cancers, and EBV can also cause other autoimmune diseases such as lupus and Sjogren’s, so it’s complicated. And MS of course has genetic susceptibility that’s not limited to the major histocompatibility complex (MHC) genes that are associated with presenting viral peptides,” said Dr. Calabresi.
Evidence relating MS vulnerability to other genetic and environmental factors, including diet, sunlight, smoking, and even pollution, calls into question a direct causal relationship between EBV and MS, he said.
The age prevalence of EBV would complicate efforts to eradicate it. Seroprevalence is 55% by age 5-11 and 75% among university students. “This is important because the duration of the vaccine response–induced protection in young seronegative children is not lengthy. Vaccinated individuals may become susceptible to natural infection at an age where the consequences of infection are more severe, especially leading to infectious mononucleosis, and hopefully not MS. This then raises the issue of the need for boosters, which we’re all well aware of during the COVID pandemic. This may be a problem, especially in young adults due to noncompliance,” said Dr. Calabresi.
He pointed out that not all vaccine attempts went well. In the 1960s, early respiratory syncytial virus (RSV) vaccines caused enhanced respiratory disease and 2 deaths. “We need to be careful when we think about targeting healthy at-risk young people,” said Dr. Calabresi.
Rather than pursue vaccination, Dr. Calabresi favors research into EBV latency in B cells as well as how EBV-infected B cells may cause or exacerbate MS, with the hopes of developing interventions. “It’s tempting to speculate that the success of the anti-CD 20 monoclonal antibody therapies is related to depletion of EBV infected B cells. In fact, I think that may be the case,” he said.
Dr. Cohen has no relevant financial disclosures. Dr. Calabresi has served on a scientific advisory board or data monitoring board for Biogen and Disarm Therapeutics.
FROM ACTRIMS FORUM 2023
Fewer than 10% of eligible type 2 diabetes patients get new, pricey drugs
Fewer than 10% of American adults with type 2 diabetes who qualified for treatment with newer agents – such as an SGLT2 inhibitor or GLP-1 agonist – actually received treatment with at least one drug from drug class in 2017-2020, based on a new analysis of just over a thousand adults who participated in a representative, biannual survey and self-reported a diabetes diagnosis.
The cost of these agents, and their uncertain cost-effectiveness at current prices, is likely a key driver of the low usage rate, say the authors of a brief report published in Annals of Internal Medicine.
“Clinical studies have shown that both GLP-1 [glucagonlike peptide–1] receptor agonists and SGLT2 [sodium-glucose cotransporter 2] inhibitors yield additional clinical benefits, compared with older treatments in reducing body weight and progression of cardiovascular disease and chronic kidney disease,” write Shichao Tang, PhD, from the U.S. Centers for Disease Control and Prevention, Atlanta, and colleagues.
“However, these medications come at a substantially higher cost,” they stress.
Dr. Tang explained in an interview that the new study “points to prior studies about the high cost of these medications as a potential barrier to use, but more research is needed to understand cost-effectiveness and any potential barriers to use, including cost.”
The work “did not include research into cost-effectiveness or why the percentage of people already using these medications was low,” he emphasized.
Dr. Tang and colleagues used data collected by the U.S. National Health and Nutrition Examination Survey during two 2-year cycles between 2017 and 2020 that included 1,417 people who self-identified a diagnosis of diabetes.
Excluding those who likely had type 1 diabetes and those with incomplete data left 1,330 survey participants, including 1,133 (85%) who fit criteria for the treatment of type 2 diabetes with an agent from one of the two studied classes, as recommended in 2022 by a panel representing the American Diabetes Association and the European Association for the Study of Diabetes.
Among these 1,133 people – who represent more than 22 million American adults with type 2 diabetes who fit the 2022 criteria – a scant 3.7% were actually taking a GLP-1 agonist and 5.3% were taking an SGLT2 inhibitor.
“While it’s important to note that our data predate the 2022 recommendations, these drugs were offered as second-line therapy for patients with certain diabetes-related complications in 2017-2020” and hence provide potentially useful insights, noted Dr. Tang, a health economist with the CDC National Center for Chronic Disease Prevention and Health Promotion.
Based on retail prices listed on a United States–based website, a 30-day supply of an oral SGLT2 inhibitor can cost about $550-$600 per month, while common subcutaneously injected GLP-1 receptor agonists can run from a few hundred dollars for a daily injection or close to $1,000 for a formulation administered weekly.
“Cost-effectiveness was not formally considered in the current guideline, but an assessment of cost-effectiveness may assist better targeting of interventions to achieve the greatest effect at a sustainable cost,” the researchers conclude.
The study received no commercial funding. None of the authors had relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fewer than 10% of American adults with type 2 diabetes who qualified for treatment with newer agents – such as an SGLT2 inhibitor or GLP-1 agonist – actually received treatment with at least one drug from drug class in 2017-2020, based on a new analysis of just over a thousand adults who participated in a representative, biannual survey and self-reported a diabetes diagnosis.
The cost of these agents, and their uncertain cost-effectiveness at current prices, is likely a key driver of the low usage rate, say the authors of a brief report published in Annals of Internal Medicine.
“Clinical studies have shown that both GLP-1 [glucagonlike peptide–1] receptor agonists and SGLT2 [sodium-glucose cotransporter 2] inhibitors yield additional clinical benefits, compared with older treatments in reducing body weight and progression of cardiovascular disease and chronic kidney disease,” write Shichao Tang, PhD, from the U.S. Centers for Disease Control and Prevention, Atlanta, and colleagues.
“However, these medications come at a substantially higher cost,” they stress.
Dr. Tang explained in an interview that the new study “points to prior studies about the high cost of these medications as a potential barrier to use, but more research is needed to understand cost-effectiveness and any potential barriers to use, including cost.”
The work “did not include research into cost-effectiveness or why the percentage of people already using these medications was low,” he emphasized.
Dr. Tang and colleagues used data collected by the U.S. National Health and Nutrition Examination Survey during two 2-year cycles between 2017 and 2020 that included 1,417 people who self-identified a diagnosis of diabetes.
Excluding those who likely had type 1 diabetes and those with incomplete data left 1,330 survey participants, including 1,133 (85%) who fit criteria for the treatment of type 2 diabetes with an agent from one of the two studied classes, as recommended in 2022 by a panel representing the American Diabetes Association and the European Association for the Study of Diabetes.
Among these 1,133 people – who represent more than 22 million American adults with type 2 diabetes who fit the 2022 criteria – a scant 3.7% were actually taking a GLP-1 agonist and 5.3% were taking an SGLT2 inhibitor.
“While it’s important to note that our data predate the 2022 recommendations, these drugs were offered as second-line therapy for patients with certain diabetes-related complications in 2017-2020” and hence provide potentially useful insights, noted Dr. Tang, a health economist with the CDC National Center for Chronic Disease Prevention and Health Promotion.
Based on retail prices listed on a United States–based website, a 30-day supply of an oral SGLT2 inhibitor can cost about $550-$600 per month, while common subcutaneously injected GLP-1 receptor agonists can run from a few hundred dollars for a daily injection or close to $1,000 for a formulation administered weekly.
“Cost-effectiveness was not formally considered in the current guideline, but an assessment of cost-effectiveness may assist better targeting of interventions to achieve the greatest effect at a sustainable cost,” the researchers conclude.
The study received no commercial funding. None of the authors had relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fewer than 10% of American adults with type 2 diabetes who qualified for treatment with newer agents – such as an SGLT2 inhibitor or GLP-1 agonist – actually received treatment with at least one drug from drug class in 2017-2020, based on a new analysis of just over a thousand adults who participated in a representative, biannual survey and self-reported a diabetes diagnosis.
The cost of these agents, and their uncertain cost-effectiveness at current prices, is likely a key driver of the low usage rate, say the authors of a brief report published in Annals of Internal Medicine.
“Clinical studies have shown that both GLP-1 [glucagonlike peptide–1] receptor agonists and SGLT2 [sodium-glucose cotransporter 2] inhibitors yield additional clinical benefits, compared with older treatments in reducing body weight and progression of cardiovascular disease and chronic kidney disease,” write Shichao Tang, PhD, from the U.S. Centers for Disease Control and Prevention, Atlanta, and colleagues.
“However, these medications come at a substantially higher cost,” they stress.
Dr. Tang explained in an interview that the new study “points to prior studies about the high cost of these medications as a potential barrier to use, but more research is needed to understand cost-effectiveness and any potential barriers to use, including cost.”
The work “did not include research into cost-effectiveness or why the percentage of people already using these medications was low,” he emphasized.
Dr. Tang and colleagues used data collected by the U.S. National Health and Nutrition Examination Survey during two 2-year cycles between 2017 and 2020 that included 1,417 people who self-identified a diagnosis of diabetes.
Excluding those who likely had type 1 diabetes and those with incomplete data left 1,330 survey participants, including 1,133 (85%) who fit criteria for the treatment of type 2 diabetes with an agent from one of the two studied classes, as recommended in 2022 by a panel representing the American Diabetes Association and the European Association for the Study of Diabetes.
Among these 1,133 people – who represent more than 22 million American adults with type 2 diabetes who fit the 2022 criteria – a scant 3.7% were actually taking a GLP-1 agonist and 5.3% were taking an SGLT2 inhibitor.
“While it’s important to note that our data predate the 2022 recommendations, these drugs were offered as second-line therapy for patients with certain diabetes-related complications in 2017-2020” and hence provide potentially useful insights, noted Dr. Tang, a health economist with the CDC National Center for Chronic Disease Prevention and Health Promotion.
Based on retail prices listed on a United States–based website, a 30-day supply of an oral SGLT2 inhibitor can cost about $550-$600 per month, while common subcutaneously injected GLP-1 receptor agonists can run from a few hundred dollars for a daily injection or close to $1,000 for a formulation administered weekly.
“Cost-effectiveness was not formally considered in the current guideline, but an assessment of cost-effectiveness may assist better targeting of interventions to achieve the greatest effect at a sustainable cost,” the researchers conclude.
The study received no commercial funding. None of the authors had relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Optimal Use of Disease-Modifying Therapies in Spinal Muscular Atrophy
Spinal muscular atrophy (SMA) is a hereditary neuromuscular disease that typically begins in infancy or childhood but can manifest at any age.
It is characterized by the irreversible and progressive degeneration of motor neurons in the spinal cord and brainstem. This results in a wide range of symptoms, in addition to which there is substantial variation in the rate of progression and disease prognosis.
Although early diagnosis and timely therapy can slow or prevent disease progression, disease-modifying therapies since 2016 have significantly advanced the management of SMA.
In a clinically focused program, Dr Perry Shieh, a neuromuscular neurologist from the University of California, Los Angeles, discusses the three medications currently approved by the US Food and Drug Administration: nusinersen, risdiplam, and onasemnogene abeparvovec.
He weighs the clinical benefits and key considerations for the use of each drug and emphasizes the need for shared decision-making with the patient.
--
Professor, Departments of Neurology and Pediatrics, University of California, Los Angeles; Neuromuscular Neurologist, Ronald Reagan UCLA Medical Center, Los Angeles, California
Perry Shieh, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Grifolis; Biogen; Genentech; CSL Behring; Alexion; Argenx; Catalyst
Received income in an amount equal to or greater than $250 from: Sarepta; Novartis; Biogen; Genentech; Alexion; Argenx; Catalyst; UCB
Spinal muscular atrophy (SMA) is a hereditary neuromuscular disease that typically begins in infancy or childhood but can manifest at any age.
It is characterized by the irreversible and progressive degeneration of motor neurons in the spinal cord and brainstem. This results in a wide range of symptoms, in addition to which there is substantial variation in the rate of progression and disease prognosis.
Although early diagnosis and timely therapy can slow or prevent disease progression, disease-modifying therapies since 2016 have significantly advanced the management of SMA.
In a clinically focused program, Dr Perry Shieh, a neuromuscular neurologist from the University of California, Los Angeles, discusses the three medications currently approved by the US Food and Drug Administration: nusinersen, risdiplam, and onasemnogene abeparvovec.
He weighs the clinical benefits and key considerations for the use of each drug and emphasizes the need for shared decision-making with the patient.
--
Professor, Departments of Neurology and Pediatrics, University of California, Los Angeles; Neuromuscular Neurologist, Ronald Reagan UCLA Medical Center, Los Angeles, California
Perry Shieh, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Grifolis; Biogen; Genentech; CSL Behring; Alexion; Argenx; Catalyst
Received income in an amount equal to or greater than $250 from: Sarepta; Novartis; Biogen; Genentech; Alexion; Argenx; Catalyst; UCB
Spinal muscular atrophy (SMA) is a hereditary neuromuscular disease that typically begins in infancy or childhood but can manifest at any age.
It is characterized by the irreversible and progressive degeneration of motor neurons in the spinal cord and brainstem. This results in a wide range of symptoms, in addition to which there is substantial variation in the rate of progression and disease prognosis.
Although early diagnosis and timely therapy can slow or prevent disease progression, disease-modifying therapies since 2016 have significantly advanced the management of SMA.
In a clinically focused program, Dr Perry Shieh, a neuromuscular neurologist from the University of California, Los Angeles, discusses the three medications currently approved by the US Food and Drug Administration: nusinersen, risdiplam, and onasemnogene abeparvovec.
He weighs the clinical benefits and key considerations for the use of each drug and emphasizes the need for shared decision-making with the patient.
--
Professor, Departments of Neurology and Pediatrics, University of California, Los Angeles; Neuromuscular Neurologist, Ronald Reagan UCLA Medical Center, Los Angeles, California
Perry Shieh, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Grifolis; Biogen; Genentech; CSL Behring; Alexion; Argenx; Catalyst
Received income in an amount equal to or greater than $250 from: Sarepta; Novartis; Biogen; Genentech; Alexion; Argenx; Catalyst; UCB

Isolated nail psoriasis may bring arthritis into play
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
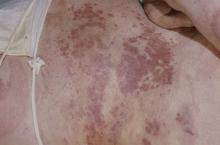
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.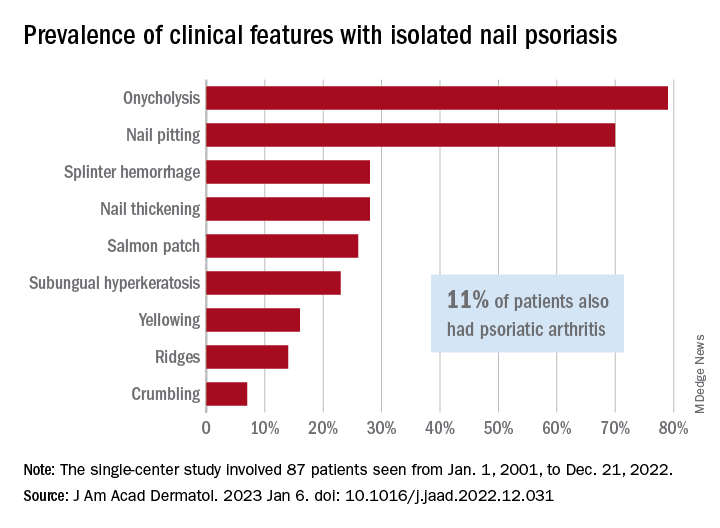
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Intermittent abdominal pain
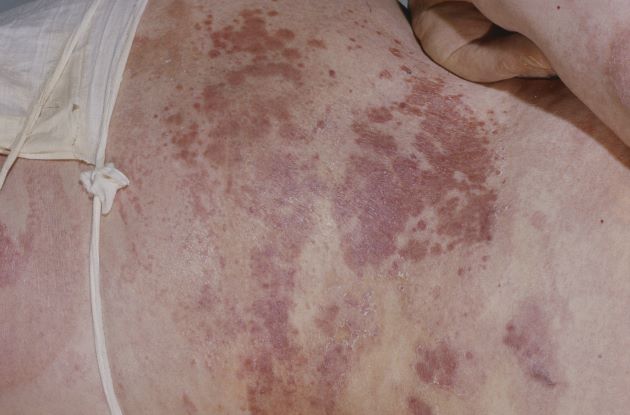
Mantle cell lymphoma (MCL) is an aggressive form of non-Hodgkin lymphoma characterized by the proliferation of CD5-positive B cells within the mantle zone that surrounds normal germinal center follicles. MCL is a rare disease that most commonly presents in adult men (male to female ratio > 2:1) in the fifth and sixth decades of life. Individuals diagnosed with MCL typically present with constitutional symptoms, such as weight loss, night sweats, persistent fever, and fatigue. Approximately 25% of cases present with extranodal involvement with the bone marrow; peripheral blood and gastrointestinal tract are most often involved. In patients with extensive node involvement in the gastrointestinal tract, additional symptoms at presentation often include abdominal pain, abdominal fullness, and bloating. Skin involvement in MCL is rare and usually indicates widespread disease.
According to the guidelines of the World Health Organization–European Organization for Research and Treatment of Cancer, a diagnosis of MCL is established on the basis of the morphologic examination findings and immunophenotyping.
Immunohistochemically, expression of cyclin D1 in normal lymphoid cells is very low and often undetectable; only hairy cell leukemia shows moderate expression of cyclin D1. Therefore, positive immunohistochemistry for cyclin D1 is pathognomonic for MCL. Increased expression of cyclin D1 protein leads to dysregulation of the cell cycle and stimulates uncontrolled cell proliferation. It is also indirect evidence of the chromosomal translocation (11;14)(q13;q32) on the CCND1 gene, which is detected in 95% of cases of MCL. In addition, negative expression of antigens may also help to differentiate MCL from other lymphomas. MCL does not usually express the antigens that are associated with germinal centers, such as CD10, CD23, and BCL6. Thus, these antigens can be used to distinguish MCL from B-cell lymphomas of germinal center origin, including follicular lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma.
The National Comprehensive Cancer Network recommends chemotherapy followed by radiation for stage I or II disease. In general, patients with advanced-stage disease benefit from systemic chemotherapy. Because MCL is clinically heterogeneous, treatment may require adjustment on the basis of the patient's age, underlying comorbidities, and underlying MCL biology such as TP53 mutations. During induction therapy, prophylaxis and monitoring for tumor lysis syndrome is strongly recommended to be considered. Before treatment, hepatitis B virus testing is recommended because of an increased risk for viral reactivation with use of immunotherapy regimens for treatment.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Mantle cell lymphoma (MCL) is an aggressive form of non-Hodgkin lymphoma characterized by the proliferation of CD5-positive B cells within the mantle zone that surrounds normal germinal center follicles. MCL is a rare disease that most commonly presents in adult men (male to female ratio > 2:1) in the fifth and sixth decades of life. Individuals diagnosed with MCL typically present with constitutional symptoms, such as weight loss, night sweats, persistent fever, and fatigue. Approximately 25% of cases present with extranodal involvement with the bone marrow; peripheral blood and gastrointestinal tract are most often involved. In patients with extensive node involvement in the gastrointestinal tract, additional symptoms at presentation often include abdominal pain, abdominal fullness, and bloating. Skin involvement in MCL is rare and usually indicates widespread disease.
According to the guidelines of the World Health Organization–European Organization for Research and Treatment of Cancer, a diagnosis of MCL is established on the basis of the morphologic examination findings and immunophenotyping.
Immunohistochemically, expression of cyclin D1 in normal lymphoid cells is very low and often undetectable; only hairy cell leukemia shows moderate expression of cyclin D1. Therefore, positive immunohistochemistry for cyclin D1 is pathognomonic for MCL. Increased expression of cyclin D1 protein leads to dysregulation of the cell cycle and stimulates uncontrolled cell proliferation. It is also indirect evidence of the chromosomal translocation (11;14)(q13;q32) on the CCND1 gene, which is detected in 95% of cases of MCL. In addition, negative expression of antigens may also help to differentiate MCL from other lymphomas. MCL does not usually express the antigens that are associated with germinal centers, such as CD10, CD23, and BCL6. Thus, these antigens can be used to distinguish MCL from B-cell lymphomas of germinal center origin, including follicular lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma.
The National Comprehensive Cancer Network recommends chemotherapy followed by radiation for stage I or II disease. In general, patients with advanced-stage disease benefit from systemic chemotherapy. Because MCL is clinically heterogeneous, treatment may require adjustment on the basis of the patient's age, underlying comorbidities, and underlying MCL biology such as TP53 mutations. During induction therapy, prophylaxis and monitoring for tumor lysis syndrome is strongly recommended to be considered. Before treatment, hepatitis B virus testing is recommended because of an increased risk for viral reactivation with use of immunotherapy regimens for treatment.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Mantle cell lymphoma (MCL) is an aggressive form of non-Hodgkin lymphoma characterized by the proliferation of CD5-positive B cells within the mantle zone that surrounds normal germinal center follicles. MCL is a rare disease that most commonly presents in adult men (male to female ratio > 2:1) in the fifth and sixth decades of life. Individuals diagnosed with MCL typically present with constitutional symptoms, such as weight loss, night sweats, persistent fever, and fatigue. Approximately 25% of cases present with extranodal involvement with the bone marrow; peripheral blood and gastrointestinal tract are most often involved. In patients with extensive node involvement in the gastrointestinal tract, additional symptoms at presentation often include abdominal pain, abdominal fullness, and bloating. Skin involvement in MCL is rare and usually indicates widespread disease.
According to the guidelines of the World Health Organization–European Organization for Research and Treatment of Cancer, a diagnosis of MCL is established on the basis of the morphologic examination findings and immunophenotyping.
Immunohistochemically, expression of cyclin D1 in normal lymphoid cells is very low and often undetectable; only hairy cell leukemia shows moderate expression of cyclin D1. Therefore, positive immunohistochemistry for cyclin D1 is pathognomonic for MCL. Increased expression of cyclin D1 protein leads to dysregulation of the cell cycle and stimulates uncontrolled cell proliferation. It is also indirect evidence of the chromosomal translocation (11;14)(q13;q32) on the CCND1 gene, which is detected in 95% of cases of MCL. In addition, negative expression of antigens may also help to differentiate MCL from other lymphomas. MCL does not usually express the antigens that are associated with germinal centers, such as CD10, CD23, and BCL6. Thus, these antigens can be used to distinguish MCL from B-cell lymphomas of germinal center origin, including follicular lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma.
The National Comprehensive Cancer Network recommends chemotherapy followed by radiation for stage I or II disease. In general, patients with advanced-stage disease benefit from systemic chemotherapy. Because MCL is clinically heterogeneous, treatment may require adjustment on the basis of the patient's age, underlying comorbidities, and underlying MCL biology such as TP53 mutations. During induction therapy, prophylaxis and monitoring for tumor lysis syndrome is strongly recommended to be considered. Before treatment, hepatitis B virus testing is recommended because of an increased risk for viral reactivation with use of immunotherapy regimens for treatment.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 69-year-old man presents for an evaluation for a 3-month history of generalized intermittent abdominal pain with occasional dark blood with bowel movements. He does not report experiencing any fever, chills, diarrhea, or obstructive symptoms. However, he does note a 10-lb weight loss over the past few months. He underwent routine screening colonoscopy 5 years ago, which was unremarkable. Complete blood count reveals normocytic anemia. CT of the chest, abdomen, and pelvis demonstrated extensive mesenteric lymphadenopathy and bilateral axillary lymphadenopathy. Physical examination reveals an enlarged right inguinal lymph node, diffuse cutaneous erythematous plaques, and nodules with irregular borders on the upper back. Lesion diameters range from 0.5 to 1.5 cm, with the largest having a central ulceration.
A biopsy of one of the skin lesions was performed. Histopathologic examination demonstrated diffuse lymphoid infiltrate composed predominately of small, mature lymphocytes. Immunohistochemistry showed expression of cyclin D1, CD5, CD20, SOX11, and BCL2. Lesions were negative for CD10, CD23, and BCL6.
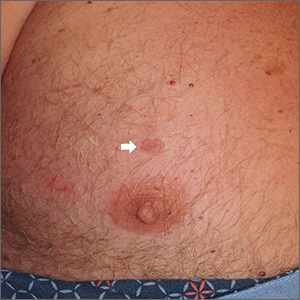
Chest lesion
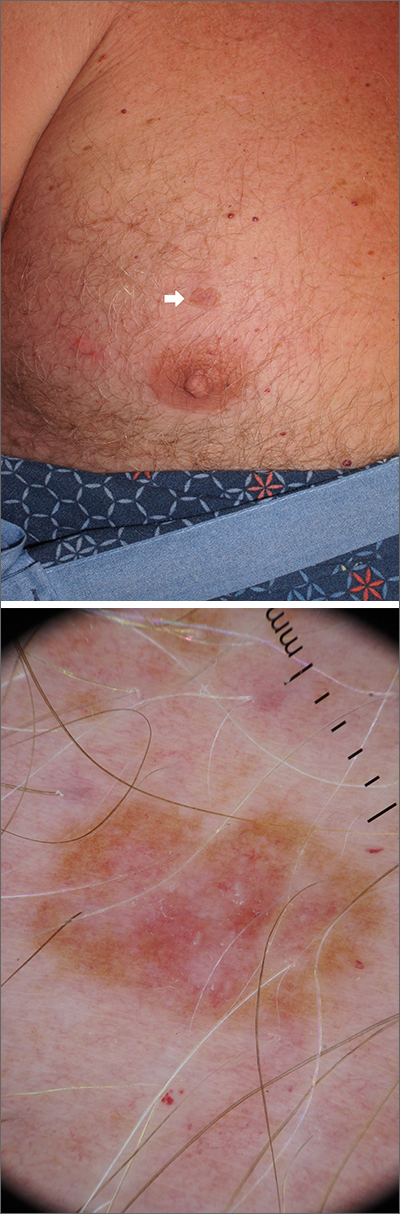
A scoop shave biopsy was performed, including at least a 1-mm margin of normal-looking skin. Pathology was consistent with melanoma in situ.
Melanoma in situ, also called Stage 0 melanoma, is defined by atypical melanocytes that have not begun to invade the dermis and, therefore, have a Breslow thickness of 0 mm. While invasive melanoma is responsible for the largest number of skin cancer deaths in the United States (estimated to be 7990 in 2023), melanoma in situ maintains a very high cure rate when treated appropriately.1
Dermoscopy can help differentiate melanoma from benign nevi or other benign skin lesions. In this case, dermoscopy revealed a fine pigment network at the periphery that indicated this lesion was made up of melanocytes. There were also atypical vascular markings (the milky red color) in the center. Taken together, these findings were strongly indicative of melanoma.
Standard of care for melanoma in situ is a wide local excision with a margin of 5 to 10 mm. Melanoma in situ does not require sentinel lymph node biopsy. However, a lymph node biopsy would have been necessary if the melanoma had been ≥ 1 mm in thickness or if it had been ≥ 0.8 mm in thickness with higher-risk features, such as an increased number of mitoses per high-power field on pathology. Mohs micrographic surgery (MMS) is emerging as an alternative method to wide local excision to treat melanoma in situ. However, it can only be done in specialized centers that can do rapid immunohistochemical staining on frozen sections. MMS is especially useful in cosmetically sensitive areas of the body and in areas where the true size of the melanoma in situ is unclear.
This patient subsequently underwent a wide local excision in the office with a margin of 6 mm. A sentinel lymph node biopsy was not performed. The patient will continue with skin surveillance consisting of full skin exams 3 to 4 times in the first year of diagnosis, then twice annually for Years 2 to 5. He will then come in for annual skin exams after that.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. American Cancer Society. Cancer Facts & Figures 2023. Atlanta: American Cancer Society; 2023. Accessed February 20, 2023. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf

A scoop shave biopsy was performed, including at least a 1-mm margin of normal-looking skin. Pathology was consistent with melanoma in situ.
Melanoma in situ, also called Stage 0 melanoma, is defined by atypical melanocytes that have not begun to invade the dermis and, therefore, have a Breslow thickness of 0 mm. While invasive melanoma is responsible for the largest number of skin cancer deaths in the United States (estimated to be 7990 in 2023), melanoma in situ maintains a very high cure rate when treated appropriately.1
Dermoscopy can help differentiate melanoma from benign nevi or other benign skin lesions. In this case, dermoscopy revealed a fine pigment network at the periphery that indicated this lesion was made up of melanocytes. There were also atypical vascular markings (the milky red color) in the center. Taken together, these findings were strongly indicative of melanoma.
Standard of care for melanoma in situ is a wide local excision with a margin of 5 to 10 mm. Melanoma in situ does not require sentinel lymph node biopsy. However, a lymph node biopsy would have been necessary if the melanoma had been ≥ 1 mm in thickness or if it had been ≥ 0.8 mm in thickness with higher-risk features, such as an increased number of mitoses per high-power field on pathology. Mohs micrographic surgery (MMS) is emerging as an alternative method to wide local excision to treat melanoma in situ. However, it can only be done in specialized centers that can do rapid immunohistochemical staining on frozen sections. MMS is especially useful in cosmetically sensitive areas of the body and in areas where the true size of the melanoma in situ is unclear.
This patient subsequently underwent a wide local excision in the office with a margin of 6 mm. A sentinel lymph node biopsy was not performed. The patient will continue with skin surveillance consisting of full skin exams 3 to 4 times in the first year of diagnosis, then twice annually for Years 2 to 5. He will then come in for annual skin exams after that.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A scoop shave biopsy was performed, including at least a 1-mm margin of normal-looking skin. Pathology was consistent with melanoma in situ.
Melanoma in situ, also called Stage 0 melanoma, is defined by atypical melanocytes that have not begun to invade the dermis and, therefore, have a Breslow thickness of 0 mm. While invasive melanoma is responsible for the largest number of skin cancer deaths in the United States (estimated to be 7990 in 2023), melanoma in situ maintains a very high cure rate when treated appropriately.1
Dermoscopy can help differentiate melanoma from benign nevi or other benign skin lesions. In this case, dermoscopy revealed a fine pigment network at the periphery that indicated this lesion was made up of melanocytes. There were also atypical vascular markings (the milky red color) in the center. Taken together, these findings were strongly indicative of melanoma.
Standard of care for melanoma in situ is a wide local excision with a margin of 5 to 10 mm. Melanoma in situ does not require sentinel lymph node biopsy. However, a lymph node biopsy would have been necessary if the melanoma had been ≥ 1 mm in thickness or if it had been ≥ 0.8 mm in thickness with higher-risk features, such as an increased number of mitoses per high-power field on pathology. Mohs micrographic surgery (MMS) is emerging as an alternative method to wide local excision to treat melanoma in situ. However, it can only be done in specialized centers that can do rapid immunohistochemical staining on frozen sections. MMS is especially useful in cosmetically sensitive areas of the body and in areas where the true size of the melanoma in situ is unclear.
This patient subsequently underwent a wide local excision in the office with a margin of 6 mm. A sentinel lymph node biopsy was not performed. The patient will continue with skin surveillance consisting of full skin exams 3 to 4 times in the first year of diagnosis, then twice annually for Years 2 to 5. He will then come in for annual skin exams after that.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. American Cancer Society. Cancer Facts & Figures 2023. Atlanta: American Cancer Society; 2023. Accessed February 20, 2023. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
1. American Cancer Society. Cancer Facts & Figures 2023. Atlanta: American Cancer Society; 2023. Accessed February 20, 2023. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
Can California solve its ob.gyn. shortage?
Three patients were waiting in a queue for their telemedicine visit. Four others were in exam rooms, waiting for their appointments. Another patient was on the phone, requesting a prescription renewal.
On a sunny Wednesday afternoon in February, David Ahdoot, MD, FACOG, an ob.gyn. in Burbank, Calif., about 10 miles north of downtown Los Angeles, knows he’ll be working late.
“Normally, we would be closed on Wednesday afternoon,” he said. That time would ordinarily be used to schedule surgeries, make dictation, and perform other tasks. But those were the old days, before the COVID-19 pandemic, before the ob.gyn. shortage got even worse, and before many of the other obstacles that make his practice more burdensome worsened.
Those Wednesday afternoon tasks must be done another time. “There are too many patients to see in the office,” said Dr. Ahdoot, who’s also an assistant clinical professor at UCLA. Because of the shortage of primary care physicians, he has taken on new patients, although he said he would like to focus on his existing ones.
Many of those existing patients have been coming to Dr. Ahdoot for years. “I love my job,” he said, and it shows.
His patient reviews online include the usual grumblings about waiting time and being rushed, but many, especially those from new parents, praise him as caring, compassionate, exceptional – the kind of doctor women trust to deliver their first baby and their next ones, then guide them through menopause and other issues.
The shortage of ob.gyns. in California, as elsewhere, is real, as Dr. Ahdoot’s day-to-day attests. The implications are in evidence well beyond his higher patient loads. Lately, Dr. Ahdoot said, the calls from headhunters seeking to fill positions for locum tenens have increased from twice a month to three times a day. Despite his love for his practice, he admits he thinks about stepping away. He is 56, 8 years short of the average retirement age for ob.gyns. nationally, according to a 2018 report.
Projected shortages
The shortage of primary care doctors, including ob.gyns., is nationwide. Dr. Ahdoot is one of many faces behind the statistics. According to a 2021 update from the U.S. Department of Health & Human Services, the number of ob.gyns. nationwide is expected to decrease 7% between 2018 and 2030, from 50,850 to 47,490. Meanwhile, demand is headed in the other direction – it is projected to rise 4%, from 50,850 to 52,660 ob.gyns. needed. The need for nurse-midwives, nurse-practitioners, and physician assistants who provide women’s health care is also expected to exceed the supply in coming years.
Some areas are harder hit. The Northeast is expected to have enough maternal health care providers to meet the current average level of care nationally but the West, Midwest, and South will not, according to HHS.
California will likely need an additional 4,700 primary care clinicians by 2025, according to projections by the HealthForce Center at the University of California, San Francisco.
Solutions in sight?
Efforts are increasing to make it easier or more appealing for ob.gyns. to practice, or remain in practice, in California. Some existing programs have received funding, while new initiatives to improve the situation are launching.
Some of these efforts and programs will be viewed as a model by some other states, said Janet Coffman, PhD, associate professor at UC San Francisco and a health policy expert who is familiar with new programs and established ones.
“I would say that California offers an example of a multifaceted approach to addressing the shortage of reproductive health providers in general and abortion providers in particular.”
The state has not sat idly in the face of dire predictions of shortfalls in the number of ob.gyns. Over the past decade, Dr. Coffman said, the legislature has “substantially” boosted funding for grants to support ob.gyn. residency programs through CalMedForce and the Song-Brown Healthcare Workforce Training Program. The result: an 18% increase in the number of residents entering the field over the past decade.
“These programs have also substantially increased funding for family medicine residency programs, which are important because family physicians are trained to provide preventive reproductive health services and manage low-risk deliveries,” she added. “Funding for midwifery, nurse midwifery, and nurse practitioner education has been more modest, which I find disappointing because they are qualified to provide many reproductive health services and are more likely to care for underserved populations.”
Other new programs and legislation are focused on expanding the scope of practice for nonphysician health care providers who care for women. Many of these measures are meant to ensure continued access to abortion services not just for California residents, who are guaranteed that right in the state constitution, but for the influx of women expected from states that limited or prohibited abortion after the overturn of Roe v. Wade.
Gavin Newsom, the state’s Democratic governor, has promoted California as a safe haven for women seeking abortions. In September, Gov. Newsom’s reelection campaign rented billboards in six states that have restrictive abortion laws with messages directing women to a website informing them “abortion is legal and protected in California.” The website includes a search function for women looking for providers – representing a further potential strain on the already stressed pool of clinicians. Each year, an estimated 8,000 to 16,100 more people are expected to travel to California for abortions, according to projections made in 2022 by the UCLA Center on Reproductive Health, Law, and Policy.
The questions are, will the efforts be enough to stall or reverse the shortage, and will the efforts to expand other health care providers’ scope of practice be met with cooperation or resistance by MDs?
Just launched: California reproductive health service corps
Brand new, as of January 2023, is the California Reproductive Health Service Corps, created by a bill Gov. Newsom signed into law last September. The program operates within the Department of Health Care Access and Information. Rajeena Victoria Bisla, a spokesperson for assemblywoman Cottie Petrie-Norris (D-Irvine), who authored the bill, said: “The Corps will be responsible for recruiting, training, and retaining a diverse workforce of health care professionals who will be part of reproductive health care teams assigned to work in underserved areas.”
The teams will include MDs as well as licensed midwives, nurses, physician’s assistants, doulas, and medical assistants. They will provide abortion care, contraception, perinatal care, gynecology services, and gender-affirming care, among other needs, Ms. Bisla said.
The California Medical Association’s philanthropic arm, Physicians for a Healthy California (PHC), has two programs that aim to grow and diversify the physician workforce and invest in the state’s underserved areas, according to Lupe Alonzo-Diaz, CEO and president of PHC.
CalMedForce gives annual grants to fund new residency positions at graduate medical education (GME) programs throughout the state. The goal, Ms. Alonzo-Diaz said, is to expand the physician training pool. Funds were generated by Proposition 56, which was passed in 2016. The legislation generates tax on tobacco products. To date, GME programs have received more than $112 million to retain and expand primary care GME programs.
A second program, CalHealthCares, also funded by Proposition 56, offers a loan repayment program of up to $300,000 for physicians who meet certain criteria. “We are incentivizing young physicians and dentists to practice in Medi-Cal communities,” Ms. Alonzo-Diaz said, referring to the state’s Medicaid program. Clinicians must have graduated within the past 5 years (since Jan. 1, 2018) or will be graduating from a residency or fellowship program no later than June 30, 2023. Dentists applying for the practice support grant must have graduated from dental school or residency program within the past 15 years (since Jan. 1, 2008).
In exchange for the loan repayment, the health care providers are asked to commit to 5 years of service in the underserved community. So far, about 800 providers are part of the program, she said. According to Ms. Alonzo-Diaz, the average educational debt for health care providers in California is $315,000 to $350,000. That is as much as $100,000 above the national average.
What else is needed? Shannan Velayas, a spokesperson for the California Medical Association, said the state should invest in the Medi-Cal system to improve “meaningful access” to health care services and to expand loan repayment and residency programs like CalHealthCares and CalMedForce.
“Workforce shortages are not a reason to sacrifice quality of care or compromise patient safety but do warrant additional investment to increase access to medical providers working within their scope of practice,” Ms. Velayas said.
Widening scopes
Efforts are also underway to expand the scope of practice for nurse-practitioners, certified nurse-midwives, and physician assistants. Triggering these efforts has been the fallout and expected consequences of the overturning of Roe v. Wade, removing the federal right to abortion care.
Effective January 2023, trained and qualified nurse-practitioners and certified nurse-midwives in California can perform first-trimester abortions without a doctor’s supervision. Toni Atkins (D-San Diego), now president pro tempore of the California State Senate, authored the bill, SB1375. The measure builds on a 2013 law she spearheaded that allowed certain advanced-practice providers to perform first-trimester abortions with physician supervision.
On Feb. 13, Ms. Atkins introduced SB385, which gives physician assistants the same ability to become qualified in abortion care.
Ms. Atkins expressed confidence that teamwork would prevail in the efforts to have enough providers in the state. “One of the biggest lessons I learned working at a women’s health clinic [prior to her assuming her legislative positions] is that providers put their patients above all else, whether they are doctors, registered nurses, nurse practitioners, certified nurse-midwives, or physician assistants,” she said. “Everyone is on the same team when it comes to breaking down barriers and ensuring all Californians get the care they need without delay.”
Will other states follow suit? “This is pure speculation, but I believe states in which the political leadership supports abortion rights may see the California Reproductive Service Corps and the changes to scope-of-practice laws that allow specially trained CNMs, NPs, and PAs to provide abortions as a model for preserving access to abortion in their states,” Dr. Coffman said.
However, she said, “other states are less likely to view CalMedForce and CalHealthCares as models, because other states have had similar programs for many years, and some have historically invested larger shares of state budget resources into these programs, especially some rural states.”
Reports from the trenches
Laurie Love, DNP, RN, is a family nurse practitioner in Valencia and a clinical instructor and lecturer at the UCLA School of Nursing. When a patient becomes pregnant, she refers her to one of four local ob.gyns.
The working relationships she has with them, she said, “are extremely collaborative. There is no animosity or lack of respect because I don’t have an MD behind my name.”
One of those doctors is Dr. Ahdoot, who said he welcomes the expansion of scope of practice for non-MD health care providers. Some of his colleagues, he said, have tried to fight it, but many have come to the point of welcoming the help. “The consensus is you can’t practice without a nurse practitioner anymore,” Dr. Ahdoot told this news organization.
Expanding the scope of practice for other clinicians helps everyone, including patients, he said. He thinks about how the shortage affects them. “For patients, there is frustration,” he said. He said he often hears women saying they can’t schedule a pap smear for 3 months, or they can’t get a return call from their doctor.
Nalo Hamilton, PhD, an ob.gyn. nurse practitioner and associate professor at UCLA, said the physicians she interacts with support the expanded scope of practice. “Many are confused about details, about what it means and how it will impact them,” she said. “Those who understand it, yes, they agree with it. Doctors will simply have more health care providers who are able to do independent practice.” And she makes another point clear: “We won’t replace ob.gyns.”
None of the persons quoted in this story have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
Three patients were waiting in a queue for their telemedicine visit. Four others were in exam rooms, waiting for their appointments. Another patient was on the phone, requesting a prescription renewal.
On a sunny Wednesday afternoon in February, David Ahdoot, MD, FACOG, an ob.gyn. in Burbank, Calif., about 10 miles north of downtown Los Angeles, knows he’ll be working late.
“Normally, we would be closed on Wednesday afternoon,” he said. That time would ordinarily be used to schedule surgeries, make dictation, and perform other tasks. But those were the old days, before the COVID-19 pandemic, before the ob.gyn. shortage got even worse, and before many of the other obstacles that make his practice more burdensome worsened.
Those Wednesday afternoon tasks must be done another time. “There are too many patients to see in the office,” said Dr. Ahdoot, who’s also an assistant clinical professor at UCLA. Because of the shortage of primary care physicians, he has taken on new patients, although he said he would like to focus on his existing ones.
Many of those existing patients have been coming to Dr. Ahdoot for years. “I love my job,” he said, and it shows.
His patient reviews online include the usual grumblings about waiting time and being rushed, but many, especially those from new parents, praise him as caring, compassionate, exceptional – the kind of doctor women trust to deliver their first baby and their next ones, then guide them through menopause and other issues.
The shortage of ob.gyns. in California, as elsewhere, is real, as Dr. Ahdoot’s day-to-day attests. The implications are in evidence well beyond his higher patient loads. Lately, Dr. Ahdoot said, the calls from headhunters seeking to fill positions for locum tenens have increased from twice a month to three times a day. Despite his love for his practice, he admits he thinks about stepping away. He is 56, 8 years short of the average retirement age for ob.gyns. nationally, according to a 2018 report.
Projected shortages
The shortage of primary care doctors, including ob.gyns., is nationwide. Dr. Ahdoot is one of many faces behind the statistics. According to a 2021 update from the U.S. Department of Health & Human Services, the number of ob.gyns. nationwide is expected to decrease 7% between 2018 and 2030, from 50,850 to 47,490. Meanwhile, demand is headed in the other direction – it is projected to rise 4%, from 50,850 to 52,660 ob.gyns. needed. The need for nurse-midwives, nurse-practitioners, and physician assistants who provide women’s health care is also expected to exceed the supply in coming years.
Some areas are harder hit. The Northeast is expected to have enough maternal health care providers to meet the current average level of care nationally but the West, Midwest, and South will not, according to HHS.
California will likely need an additional 4,700 primary care clinicians by 2025, according to projections by the HealthForce Center at the University of California, San Francisco.
Solutions in sight?
Efforts are increasing to make it easier or more appealing for ob.gyns. to practice, or remain in practice, in California. Some existing programs have received funding, while new initiatives to improve the situation are launching.
Some of these efforts and programs will be viewed as a model by some other states, said Janet Coffman, PhD, associate professor at UC San Francisco and a health policy expert who is familiar with new programs and established ones.
“I would say that California offers an example of a multifaceted approach to addressing the shortage of reproductive health providers in general and abortion providers in particular.”
The state has not sat idly in the face of dire predictions of shortfalls in the number of ob.gyns. Over the past decade, Dr. Coffman said, the legislature has “substantially” boosted funding for grants to support ob.gyn. residency programs through CalMedForce and the Song-Brown Healthcare Workforce Training Program. The result: an 18% increase in the number of residents entering the field over the past decade.
“These programs have also substantially increased funding for family medicine residency programs, which are important because family physicians are trained to provide preventive reproductive health services and manage low-risk deliveries,” she added. “Funding for midwifery, nurse midwifery, and nurse practitioner education has been more modest, which I find disappointing because they are qualified to provide many reproductive health services and are more likely to care for underserved populations.”
Other new programs and legislation are focused on expanding the scope of practice for nonphysician health care providers who care for women. Many of these measures are meant to ensure continued access to abortion services not just for California residents, who are guaranteed that right in the state constitution, but for the influx of women expected from states that limited or prohibited abortion after the overturn of Roe v. Wade.
Gavin Newsom, the state’s Democratic governor, has promoted California as a safe haven for women seeking abortions. In September, Gov. Newsom’s reelection campaign rented billboards in six states that have restrictive abortion laws with messages directing women to a website informing them “abortion is legal and protected in California.” The website includes a search function for women looking for providers – representing a further potential strain on the already stressed pool of clinicians. Each year, an estimated 8,000 to 16,100 more people are expected to travel to California for abortions, according to projections made in 2022 by the UCLA Center on Reproductive Health, Law, and Policy.
The questions are, will the efforts be enough to stall or reverse the shortage, and will the efforts to expand other health care providers’ scope of practice be met with cooperation or resistance by MDs?
Just launched: California reproductive health service corps
Brand new, as of January 2023, is the California Reproductive Health Service Corps, created by a bill Gov. Newsom signed into law last September. The program operates within the Department of Health Care Access and Information. Rajeena Victoria Bisla, a spokesperson for assemblywoman Cottie Petrie-Norris (D-Irvine), who authored the bill, said: “The Corps will be responsible for recruiting, training, and retaining a diverse workforce of health care professionals who will be part of reproductive health care teams assigned to work in underserved areas.”
The teams will include MDs as well as licensed midwives, nurses, physician’s assistants, doulas, and medical assistants. They will provide abortion care, contraception, perinatal care, gynecology services, and gender-affirming care, among other needs, Ms. Bisla said.
The California Medical Association’s philanthropic arm, Physicians for a Healthy California (PHC), has two programs that aim to grow and diversify the physician workforce and invest in the state’s underserved areas, according to Lupe Alonzo-Diaz, CEO and president of PHC.
CalMedForce gives annual grants to fund new residency positions at graduate medical education (GME) programs throughout the state. The goal, Ms. Alonzo-Diaz said, is to expand the physician training pool. Funds were generated by Proposition 56, which was passed in 2016. The legislation generates tax on tobacco products. To date, GME programs have received more than $112 million to retain and expand primary care GME programs.
A second program, CalHealthCares, also funded by Proposition 56, offers a loan repayment program of up to $300,000 for physicians who meet certain criteria. “We are incentivizing young physicians and dentists to practice in Medi-Cal communities,” Ms. Alonzo-Diaz said, referring to the state’s Medicaid program. Clinicians must have graduated within the past 5 years (since Jan. 1, 2018) or will be graduating from a residency or fellowship program no later than June 30, 2023. Dentists applying for the practice support grant must have graduated from dental school or residency program within the past 15 years (since Jan. 1, 2008).
In exchange for the loan repayment, the health care providers are asked to commit to 5 years of service in the underserved community. So far, about 800 providers are part of the program, she said. According to Ms. Alonzo-Diaz, the average educational debt for health care providers in California is $315,000 to $350,000. That is as much as $100,000 above the national average.
What else is needed? Shannan Velayas, a spokesperson for the California Medical Association, said the state should invest in the Medi-Cal system to improve “meaningful access” to health care services and to expand loan repayment and residency programs like CalHealthCares and CalMedForce.
“Workforce shortages are not a reason to sacrifice quality of care or compromise patient safety but do warrant additional investment to increase access to medical providers working within their scope of practice,” Ms. Velayas said.
Widening scopes
Efforts are also underway to expand the scope of practice for nurse-practitioners, certified nurse-midwives, and physician assistants. Triggering these efforts has been the fallout and expected consequences of the overturning of Roe v. Wade, removing the federal right to abortion care.
Effective January 2023, trained and qualified nurse-practitioners and certified nurse-midwives in California can perform first-trimester abortions without a doctor’s supervision. Toni Atkins (D-San Diego), now president pro tempore of the California State Senate, authored the bill, SB1375. The measure builds on a 2013 law she spearheaded that allowed certain advanced-practice providers to perform first-trimester abortions with physician supervision.
On Feb. 13, Ms. Atkins introduced SB385, which gives physician assistants the same ability to become qualified in abortion care.
Ms. Atkins expressed confidence that teamwork would prevail in the efforts to have enough providers in the state. “One of the biggest lessons I learned working at a women’s health clinic [prior to her assuming her legislative positions] is that providers put their patients above all else, whether they are doctors, registered nurses, nurse practitioners, certified nurse-midwives, or physician assistants,” she said. “Everyone is on the same team when it comes to breaking down barriers and ensuring all Californians get the care they need without delay.”
Will other states follow suit? “This is pure speculation, but I believe states in which the political leadership supports abortion rights may see the California Reproductive Service Corps and the changes to scope-of-practice laws that allow specially trained CNMs, NPs, and PAs to provide abortions as a model for preserving access to abortion in their states,” Dr. Coffman said.
However, she said, “other states are less likely to view CalMedForce and CalHealthCares as models, because other states have had similar programs for many years, and some have historically invested larger shares of state budget resources into these programs, especially some rural states.”
Reports from the trenches
Laurie Love, DNP, RN, is a family nurse practitioner in Valencia and a clinical instructor and lecturer at the UCLA School of Nursing. When a patient becomes pregnant, she refers her to one of four local ob.gyns.
The working relationships she has with them, she said, “are extremely collaborative. There is no animosity or lack of respect because I don’t have an MD behind my name.”
One of those doctors is Dr. Ahdoot, who said he welcomes the expansion of scope of practice for non-MD health care providers. Some of his colleagues, he said, have tried to fight it, but many have come to the point of welcoming the help. “The consensus is you can’t practice without a nurse practitioner anymore,” Dr. Ahdoot told this news organization.
Expanding the scope of practice for other clinicians helps everyone, including patients, he said. He thinks about how the shortage affects them. “For patients, there is frustration,” he said. He said he often hears women saying they can’t schedule a pap smear for 3 months, or they can’t get a return call from their doctor.
Nalo Hamilton, PhD, an ob.gyn. nurse practitioner and associate professor at UCLA, said the physicians she interacts with support the expanded scope of practice. “Many are confused about details, about what it means and how it will impact them,” she said. “Those who understand it, yes, they agree with it. Doctors will simply have more health care providers who are able to do independent practice.” And she makes another point clear: “We won’t replace ob.gyns.”
None of the persons quoted in this story have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
Three patients were waiting in a queue for their telemedicine visit. Four others were in exam rooms, waiting for their appointments. Another patient was on the phone, requesting a prescription renewal.
On a sunny Wednesday afternoon in February, David Ahdoot, MD, FACOG, an ob.gyn. in Burbank, Calif., about 10 miles north of downtown Los Angeles, knows he’ll be working late.
“Normally, we would be closed on Wednesday afternoon,” he said. That time would ordinarily be used to schedule surgeries, make dictation, and perform other tasks. But those were the old days, before the COVID-19 pandemic, before the ob.gyn. shortage got even worse, and before many of the other obstacles that make his practice more burdensome worsened.
Those Wednesday afternoon tasks must be done another time. “There are too many patients to see in the office,” said Dr. Ahdoot, who’s also an assistant clinical professor at UCLA. Because of the shortage of primary care physicians, he has taken on new patients, although he said he would like to focus on his existing ones.
Many of those existing patients have been coming to Dr. Ahdoot for years. “I love my job,” he said, and it shows.
His patient reviews online include the usual grumblings about waiting time and being rushed, but many, especially those from new parents, praise him as caring, compassionate, exceptional – the kind of doctor women trust to deliver their first baby and their next ones, then guide them through menopause and other issues.
The shortage of ob.gyns. in California, as elsewhere, is real, as Dr. Ahdoot’s day-to-day attests. The implications are in evidence well beyond his higher patient loads. Lately, Dr. Ahdoot said, the calls from headhunters seeking to fill positions for locum tenens have increased from twice a month to three times a day. Despite his love for his practice, he admits he thinks about stepping away. He is 56, 8 years short of the average retirement age for ob.gyns. nationally, according to a 2018 report.
Projected shortages
The shortage of primary care doctors, including ob.gyns., is nationwide. Dr. Ahdoot is one of many faces behind the statistics. According to a 2021 update from the U.S. Department of Health & Human Services, the number of ob.gyns. nationwide is expected to decrease 7% between 2018 and 2030, from 50,850 to 47,490. Meanwhile, demand is headed in the other direction – it is projected to rise 4%, from 50,850 to 52,660 ob.gyns. needed. The need for nurse-midwives, nurse-practitioners, and physician assistants who provide women’s health care is also expected to exceed the supply in coming years.
Some areas are harder hit. The Northeast is expected to have enough maternal health care providers to meet the current average level of care nationally but the West, Midwest, and South will not, according to HHS.
California will likely need an additional 4,700 primary care clinicians by 2025, according to projections by the HealthForce Center at the University of California, San Francisco.
Solutions in sight?
Efforts are increasing to make it easier or more appealing for ob.gyns. to practice, or remain in practice, in California. Some existing programs have received funding, while new initiatives to improve the situation are launching.
Some of these efforts and programs will be viewed as a model by some other states, said Janet Coffman, PhD, associate professor at UC San Francisco and a health policy expert who is familiar with new programs and established ones.
“I would say that California offers an example of a multifaceted approach to addressing the shortage of reproductive health providers in general and abortion providers in particular.”
The state has not sat idly in the face of dire predictions of shortfalls in the number of ob.gyns. Over the past decade, Dr. Coffman said, the legislature has “substantially” boosted funding for grants to support ob.gyn. residency programs through CalMedForce and the Song-Brown Healthcare Workforce Training Program. The result: an 18% increase in the number of residents entering the field over the past decade.
“These programs have also substantially increased funding for family medicine residency programs, which are important because family physicians are trained to provide preventive reproductive health services and manage low-risk deliveries,” she added. “Funding for midwifery, nurse midwifery, and nurse practitioner education has been more modest, which I find disappointing because they are qualified to provide many reproductive health services and are more likely to care for underserved populations.”
Other new programs and legislation are focused on expanding the scope of practice for nonphysician health care providers who care for women. Many of these measures are meant to ensure continued access to abortion services not just for California residents, who are guaranteed that right in the state constitution, but for the influx of women expected from states that limited or prohibited abortion after the overturn of Roe v. Wade.
Gavin Newsom, the state’s Democratic governor, has promoted California as a safe haven for women seeking abortions. In September, Gov. Newsom’s reelection campaign rented billboards in six states that have restrictive abortion laws with messages directing women to a website informing them “abortion is legal and protected in California.” The website includes a search function for women looking for providers – representing a further potential strain on the already stressed pool of clinicians. Each year, an estimated 8,000 to 16,100 more people are expected to travel to California for abortions, according to projections made in 2022 by the UCLA Center on Reproductive Health, Law, and Policy.
The questions are, will the efforts be enough to stall or reverse the shortage, and will the efforts to expand other health care providers’ scope of practice be met with cooperation or resistance by MDs?
Just launched: California reproductive health service corps
Brand new, as of January 2023, is the California Reproductive Health Service Corps, created by a bill Gov. Newsom signed into law last September. The program operates within the Department of Health Care Access and Information. Rajeena Victoria Bisla, a spokesperson for assemblywoman Cottie Petrie-Norris (D-Irvine), who authored the bill, said: “The Corps will be responsible for recruiting, training, and retaining a diverse workforce of health care professionals who will be part of reproductive health care teams assigned to work in underserved areas.”
The teams will include MDs as well as licensed midwives, nurses, physician’s assistants, doulas, and medical assistants. They will provide abortion care, contraception, perinatal care, gynecology services, and gender-affirming care, among other needs, Ms. Bisla said.
The California Medical Association’s philanthropic arm, Physicians for a Healthy California (PHC), has two programs that aim to grow and diversify the physician workforce and invest in the state’s underserved areas, according to Lupe Alonzo-Diaz, CEO and president of PHC.
CalMedForce gives annual grants to fund new residency positions at graduate medical education (GME) programs throughout the state. The goal, Ms. Alonzo-Diaz said, is to expand the physician training pool. Funds were generated by Proposition 56, which was passed in 2016. The legislation generates tax on tobacco products. To date, GME programs have received more than $112 million to retain and expand primary care GME programs.
A second program, CalHealthCares, also funded by Proposition 56, offers a loan repayment program of up to $300,000 for physicians who meet certain criteria. “We are incentivizing young physicians and dentists to practice in Medi-Cal communities,” Ms. Alonzo-Diaz said, referring to the state’s Medicaid program. Clinicians must have graduated within the past 5 years (since Jan. 1, 2018) or will be graduating from a residency or fellowship program no later than June 30, 2023. Dentists applying for the practice support grant must have graduated from dental school or residency program within the past 15 years (since Jan. 1, 2008).
In exchange for the loan repayment, the health care providers are asked to commit to 5 years of service in the underserved community. So far, about 800 providers are part of the program, she said. According to Ms. Alonzo-Diaz, the average educational debt for health care providers in California is $315,000 to $350,000. That is as much as $100,000 above the national average.
What else is needed? Shannan Velayas, a spokesperson for the California Medical Association, said the state should invest in the Medi-Cal system to improve “meaningful access” to health care services and to expand loan repayment and residency programs like CalHealthCares and CalMedForce.
“Workforce shortages are not a reason to sacrifice quality of care or compromise patient safety but do warrant additional investment to increase access to medical providers working within their scope of practice,” Ms. Velayas said.
Widening scopes
Efforts are also underway to expand the scope of practice for nurse-practitioners, certified nurse-midwives, and physician assistants. Triggering these efforts has been the fallout and expected consequences of the overturning of Roe v. Wade, removing the federal right to abortion care.
Effective January 2023, trained and qualified nurse-practitioners and certified nurse-midwives in California can perform first-trimester abortions without a doctor’s supervision. Toni Atkins (D-San Diego), now president pro tempore of the California State Senate, authored the bill, SB1375. The measure builds on a 2013 law she spearheaded that allowed certain advanced-practice providers to perform first-trimester abortions with physician supervision.
On Feb. 13, Ms. Atkins introduced SB385, which gives physician assistants the same ability to become qualified in abortion care.
Ms. Atkins expressed confidence that teamwork would prevail in the efforts to have enough providers in the state. “One of the biggest lessons I learned working at a women’s health clinic [prior to her assuming her legislative positions] is that providers put their patients above all else, whether they are doctors, registered nurses, nurse practitioners, certified nurse-midwives, or physician assistants,” she said. “Everyone is on the same team when it comes to breaking down barriers and ensuring all Californians get the care they need without delay.”
Will other states follow suit? “This is pure speculation, but I believe states in which the political leadership supports abortion rights may see the California Reproductive Service Corps and the changes to scope-of-practice laws that allow specially trained CNMs, NPs, and PAs to provide abortions as a model for preserving access to abortion in their states,” Dr. Coffman said.
However, she said, “other states are less likely to view CalMedForce and CalHealthCares as models, because other states have had similar programs for many years, and some have historically invested larger shares of state budget resources into these programs, especially some rural states.”
Reports from the trenches
Laurie Love, DNP, RN, is a family nurse practitioner in Valencia and a clinical instructor and lecturer at the UCLA School of Nursing. When a patient becomes pregnant, she refers her to one of four local ob.gyns.
The working relationships she has with them, she said, “are extremely collaborative. There is no animosity or lack of respect because I don’t have an MD behind my name.”
One of those doctors is Dr. Ahdoot, who said he welcomes the expansion of scope of practice for non-MD health care providers. Some of his colleagues, he said, have tried to fight it, but many have come to the point of welcoming the help. “The consensus is you can’t practice without a nurse practitioner anymore,” Dr. Ahdoot told this news organization.
Expanding the scope of practice for other clinicians helps everyone, including patients, he said. He thinks about how the shortage affects them. “For patients, there is frustration,” he said. He said he often hears women saying they can’t schedule a pap smear for 3 months, or they can’t get a return call from their doctor.
Nalo Hamilton, PhD, an ob.gyn. nurse practitioner and associate professor at UCLA, said the physicians she interacts with support the expanded scope of practice. “Many are confused about details, about what it means and how it will impact them,” she said. “Those who understand it, yes, they agree with it. Doctors will simply have more health care providers who are able to do independent practice.” And she makes another point clear: “We won’t replace ob.gyns.”
None of the persons quoted in this story have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prepare for endometriosis excision surgery with a multidisciplinary approach
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Introduction: The preoperative evaluation for endometriosis – more than meets the eye
It is well known that it often takes 6-10 years for endometriosis to be diagnosed in patients who have the disease, depending on where the patient lives. I certainly am not surprised. During my residency at Parkland Memorial Hospital, if a patient had chronic pelvic pain and no fibroids, her diagnosis was usually pelvic inflammatory disease. Later, during my fellowship in reproductive endocrinology at the University of Pennsylvania, the diagnosis became endometriosis.
As I gained more interest and expertise in the treatment of endometriosis, I became aware of several articles concluding that if a woman sought treatment for chronic pelvic pain with an internist, the diagnosis would be irritable bowel syndrome (IBS); with a urologist, it would be interstitial cystitis; and with a gynecologist, endometriosis. Moreover, there is an increased propensity for IBS and IC in patients with endometriosis. There also is an increased risk of small intestine bacterial overgrowth (SIBO), as noted by our guest author for this latest installment of the Master Class in Gynecologic Surgery, Iris Orbuch, MD.
Like our guest author, I have also noted increased risk of pelvic floor myalgia. Dr. Orbuch clearly outlines why this occurs. In fact, we can now understand why many patients have multiple pelvic pain–inducing issues compounding their pain secondary to endometriosis and leading to remodeling of the central nervous system. Therefore, it certainly makes sense to follow Dr. Orbuch’s recommendation for a multidisciplinary pre- and postsurgical approach “to downregulate the pain generators.”
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in the treatment of patients diagnosed with endometriosis. Dr. Orbuch serves on the Board of Directors of the Foundation of the American Association of Gynecologic Laparoscopists and has served as the chair of the AAGL’s Special Interest Group on Endometriosis and Reproductive Surgery. She is the coauthor of the book “Beating Endo – How to Reclaim Your Life From Endometriosis” (New York: HarperCollins; 2019). The book is written for patients but addresses many issues discussed in this installment of the Master Class in Gynecologic Surgery.
Dr. Miller, MD, FACOG, is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. He has no conflicts of interest to report.
Patients with endometriosis and the all-too-often decade-long diagnostic delay have a variety of coexisting conditions that are pain generators – from painful bladder syndrome and pelvic floor dysfunction to a small intestine bacterial system that is significantly upregulated and sensitized.
For optimal surgical outcomes, and to help our patients recover from years of this inflammatory, systemic disease, we must treat our patients holistically and work to downregulate their pain as much as possible before excision surgery. I work with patients a few months prior to surgery, often for 4-5 months, during which time they not only see me for informative follow-ups, but also pelvic floor physical therapists, gastroenterologists, mental health professionals, integrative nutritionists, and physiatrists or pain specialists, depending on their needs.1
By identifying coexisting conditions in an initial consult and employing a presurgical multidisciplinary approach to downregulate the pain generators, my patients recover well from excision surgery, with greater and faster relief from pain, compared with those using standard approaches, and with little to no use of opioids.
At a minimum, given the unfortunate time constraints and productivity demands of working within health systems – and considering that surgeries are often scheduled a couple of months out – the surgeon could ensure that patients are engaged in at least 6-8 weeks of pelvic floor physical therapy before surgery to sufficiently lengthen the pelvic muscles and loosen surrounding fascia.
Short, tight pelvic floor muscles are almost universal in patients with delayed diagnosis of endometriosis and are significant generators of pain.
Appreciating sequelae of diagnostic delay
After my fellowship in advanced laparoscopic and pelvic surgery with Harry Reich, MD, and C. Y. Liu, MD, pioneers of endometriosis excision surgery, and as I did my residency in the early 2000s, I noticed puzzlement in the literature about why some patients still had lasting pain after thorough excision.
I didn’t doubt the efficacy of excision. It is the cornerstone of treatment, and at least one randomized double-blind trial2 and a systematic review and meta-analysis3 have demonstrated its superior efficacy over ablation in symptom reduction. What I did doubt was any presumption that surgery alone was enough. I knew there was more to healing when a disease process wreaks havoc on the body for more than a decade and that there were other generators of pain in addition to the endometriosis implants themselves.
As I began to focus on endometriosis in my own surgical practice, I strove to detect and treat endometriosis in teens. But in those patients with longstanding disease, I recognized patterns and began to more fully appreciate the systemic sequelae of endometriosis.
To cope with dysmenorrhea, patients curl up and assume a fetal position, tensing the abdominal muscles, inner thigh muscles, and pelvic floor muscles. Over time, these muscles come to maintain a short, tight, and painful state. (Hence the need for physical therapy to undo this decade-long pattern.)
Endometriosis implants on or near the gastrointestinal tract tug on fascia and muscles and commonly cause constipation, leading women to further overwork the pelvic floor muscles. In the case of diarrhea-predominant dysfunction, our patients squeeze pelvic floor muscles to prevent leakage. And in the case of urinary urgency, they squeeze muscles to release urine that isn’t really there.
As the chronic inflammation of the disease grows, and as pain worsens, the patient is increasingly in sympathetic overdrive (also known as ”fight or flight”), as opposed to a parasympathetic state (also known as “rest and digest”). The bowel’s motility slows, allowing the bacteria of the small intestine to grow beyond what is normal, leading to SIBO, a condition increasingly recognized by gastroenterologists and others that can impede nutrient absorption and cause bloat and pain and exacerbate constipation and diarrhea.
Key to my conceptualization of pain was a review published in 2011 by Pam Stratton, MD, of the National Institutes of Health, and Karen J. Berkley, PhD, then of Florida State University, on chronic pain and endometriosis.4 They detailed how endometriotic lesions can develop their own nerve supply that interacts directly and in a two-way fashion with the CNS – and how the lesions can engage the nervous system in ways that create comorbid conditions and pain that becomes “independent of the disease itself.”
Sensitized peripheral nerve fibers innervating a deeply infiltrating lesion on the left uterosacral ligament, for instance, can sensitize neurons in the spinal sacral segment. Branches of these nerve fibers can extend to other segments of the spinal cord, and, once sensitized themselves, turn on neurons in these other segments. There is a resultant remodeling of the central nervous system, in essence, and what is called “remote central sensitization.” The CNS becomes independent from peripheral neural processes.
I now explain to both patients and physicians that those who have had endometriosis for years have had an enduring “hand on the stove,” with a persistent signal to the CNS. Tight muscles are a hand on the stove, painful bladder syndrome is another hand on the stove, and SIBO is yet another. So are anxiety and depression.
The CNS becomes so upregulated and overloaded that messages branch out through the spinal cord to other available pathways and to other organs, muscles, and nerves. The CNS also starts firing on its own – and once it becomes its own pain generator, taking one hand off the stove (for instance, excising implants) while leaving multiple other hands on the hot stove won’t remove all pain. We must downregulate the CNS more broadly.
As I began addressing pain generators and instigators of CNS sensitization – and waiting for excision surgery until the CNS had sufficiently cooled – I saw that my patients had a better chance of more significant and lasting pain relief.
Pearls for a multimodal approach
My initial physical exam includes an assessment of the pelvic floor for overly tight musculature. An abdominal exam will usually reveal whether there is asymmetry of the abdominal wall muscles, which typically informs me of the likelihood of tightness and pulling on either side of the pelvic anatomy. On the internal exam, then, the pelvic floor muscles can be palpated and assessed. These findings will guide my referrals and my discussions with patients about the value of pelvic floor physical therapy. The cervix should be in the midline of the vagina – equidistant from the left and right vaginal fornices. If the cervix is pulled away from this midline, and a palpation of a thickened uterosacral ligament reproduces pain, endometriosis is 90% likely.
Patients who report significant “burning” pain that’s suggestive of neuropathic pain should be referred to a physical medicine rehabilitation physician or a pain specialist who can help downregulate their CNS. And patients who have symptoms of depression, anxiety disorders (including obsessive-compulsive disorder), or posttraumatic stress disorder should be referred to pain therapists, psychologists, or other mental health professionals, preferably well before surgery. I will also often discuss mindfulness practices and give my patients “meditation challenges” to achieve during the presurgical phase.
Additional points of emphasis about a multidisciplinary, multimodal approach include:
Advanced pelvic floor therapy: Therapists with specialized training in pelvic health and manual therapy utilize a range of techniques and modalities to release tension in affected muscles, fascia, nerves, and bone, and in doing so, they help to downregulate the CNS. Myofascial release, myofascial trigger point release, neural mobilization, and visceral mobilization are among these techniques. In addition to using manual therapy, many of these therapists may also employ neuromuscular reeducation and other techniques that will be helpful for the longer term.
It is important to identify physical therapists who have training in this approach; women with endometriosis often have a history of treatment by physical therapists whose focus is on incontinence and muscle strengthening (that is, Kegel exercises), which is the opposite of what endometriosis patients need.
Treating SIBO: Symptoms commonly associated with SIBO often overlap with symptoms of irritable bowel syndrome (IBS) – namely constipation, diarrhea (or both), and bloating. Indeed, many patients with undiagnosed endometriosis have been diagnosed with IBS. I send every patient who has one of these symptoms for SIBO breath testing, which utilizes carbohydrate substrates (glucose or lactulose) and measures hydrogen and/or methane in the breath.
SIBO is typically treated with rifampin, which stays in the small bowel and will not negatively affect beneficial bacteria, with or without neomycin. Gastroenterologists with more integrative practices also consider the use of herbals in addition to – or instead of – antibiotics. It can sometimes take months or a couple of years to correct SIBO, depending on how long the patient has been affected, but with presurgical diagnosis and a start on treatment, we can remove or at least tone down another instigator of CNS sensitization.
I estimate that 80% of my patients have tested positive for SIBO. Notably, in a testament to the systemic nature of endometriosis, a study published in 2009 of 355 women undergoing operative laparoscopy for suspected endometriosis found that 90% had gastrointestinal symptoms, but only 7.6% of the vast majority whose endometriosis was confirmed were found to have endometrial implants on the bowel itself.5
Addressing bladder issues: I routinely administer the PUF (Pain, Urgency, Frequency) questionnaire as part of my intake package and follow it up with conversation. For just about every patient with painful bladder syndrome, pelvic floor physical therapy in combination with a low-acid, low-potassium diet will work effectively together to reduce symptoms and pain. The IC Network offers a helpful food list, and patients can be counseled to choose foods that are also anti-inflammatory. When referrals to a urologist for bladder instillations are possible, these can be helpful as well.
Our communication with patients
Our patients need to have their symptoms and pain validated and to understand why we’re recommending these measures before surgery. Some education is necessary. Few patients will go to an integrative nutritionist, for example, if we just write a referral without explaining how years of inflammation and disruption in the gut can affect the whole body – including mental health – and that it can be corrected over time.
Also necessary is an appreciation of the fact that patients with delayed diagnoses have lived with gastrointestinal and other symptoms and patterns for so long – and often have mothers whose endometriosis caused similar symptoms – that some of their own experiences can seem almost “normal.” A patient whose mother had bowel movements every 7 days may think that 4-5 day intervals are acceptable, for instance. This means we have to carefully consider how we ask our questions.
I always ask my patients as we’re going into surgery, what percentage better are you? I’ve long aimed for at least 30% improvement, but most of the time, with pelvic floor therapy and as many other pain-generator–focused measures as possible, we’re getting them 70% better.
Excision surgery will remove the inflammation that has helped fuel the SIBO and other coconditions. Then, everything done to prepare the body must continue for some time. Certain practices, such as eating an anti-inflammatory diet, should be lifelong.
One day, it is hoped, a pediatrician or other physician will suspect endometriosis early on. The patient will see the surgeon within several months of the onset of pain, and we won’t need to unravel layers of pain generation and CNS upregulation before operating. But until this happens and we shorten the diagnostic delay, we must consider the benefits of presurgical preparation.
References
1. Orbuch I, Stein A. Beating Endo: How to Reclaim Your Life From Endometriosis. (New York: HarperCollins, 2019).
2. Healey M et al. J Minim Invasive Gynecol. 2014;21(6):999-1004.
3. Pundir J et al. J Minim Invasive Gynecol. 2017;24(5):747-56.
4. Stratton P, Berkley KJ. Hum Repro Update. 2011;17(3):327-46.
5. Maroun P et al. Aust N Z J Obstet Gynaecol. 2009;49(4):411-4.
Dr. Orbuch is a minimally invasive gynecologic surgeon in Los Angeles who specializes in endometriosis. She has no conflicts of interest to report.
Artificial sweetener in ‘keto foods’ tied to cardiovascular risk
Erythritol is one of the most widely used artificial sweeteners with rapidly increasing prevalence in processed and “keto-related” foods. Artificial sweeteners are “generally recognized as safe” (GRAS) by the U.S. Food and Drug Administration, so there is no requirement for long-term safety studies, and little is known about the long-term health effects.
The current research, published online in Nature Medicine by Marco Witkowski, MD, of the Lerner Research Institute at Cleveland Clinic and colleagues, had multiple parts.
First, in a group of patients undergoing cardiac risk assessment, the researchers found that high levels of polyols, especially erythritol, were associated with increased 3-year risk of MACE, defined as cardiovascular death or nonfatal myocardial infarction or stroke.
Next, the association of erythritol with this outcome was reproduced in two large U.S. and European groups of stable patients undergoing elective cardiac evaluation.
Next, adding erythritol to whole blood or platelets led to clot activation. And lastly, in eight healthy volunteers, ingesting 30 g of an erythritol-sweetened drink – comparable to a single can of commercially available beverage or a pint of keto ice cream – induced marked and sustained (> 2 day) increases in levels of plasma erythritol.
“Our study shows that when participants consumed an artificially sweetened beverage with an amount of erythritol found in many processed foods, markedly elevated levels in the blood are observed for days – levels well above those observed to enhance clotting risks,” said senior author Stanley L. Hazen, MD, PhD.
“It is important that further safety studies are conducted to examine the long-term effects of artificial sweeteners in general, and erythritol specifically, on risks for heart attack and stroke, particularly in people at higher risk for cardiovascular disease,” Dr. Hazen, co–section head of preventive cardiology at Cleveland Clinic, said in a press release from his institution.
“Sweeteners like erythritol have rapidly increased in popularity in recent years, but there needs to be more in-depth research into their long-term effects. Cardiovascular disease builds over time, and heart disease is the leading cause of death globally. We need to make sure the foods we eat aren’t hidden contributors,” Dr. Hazen urged.
The topic remains controversial.
Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University, Birmingham, England, told the U.K. Science Media Centre: “This paper effectively shows multiple pieces of a jigsaw exploring the effects of erythritol – although it claims to show an associated risk with the use of erythritol as an artificial sweetener and cardiovascular disease, I believe it fails to do so, as ultimately, erythritol can be made inside our bodies and the intake in most people’s diet is much lower than the amount given in this study.”
Dr. Hazen countered that data from the 2013-2014 National Health and Nutrition Examination Survey (NHANES) in the United States show that, in some individuals, daily intake of erythritol is estimated to reach 30 g/day.
“Many try and reduce sugar intake by taking many teaspoons of erythritol in their tea, coffee, etc., instead of sugar,” Dr. Hazen added. “Or they eat keto processed foods that have significant quantities of erythritol within it.”
“These studies are a warning for how our processed food (keto and zero sugar, especially) may inadvertently be causing risk/harm. … in the very subset of subjects who are most vulnerable,” according to Dr. Hazen.
Erythritol marketed as ‘zero calorie’, ‘non-nutritive’, or ‘natural’
Patients with type 2 diabetes and obesity are often advised to replace sugar with artificial sweeteners for better glucose control and weight loss, but growing epidemiologic evidence links artificial sweetener consumption with weight gain, insulin resistance, type 2 diabetes, and cardiovascular disease, the researchers write.
Erythritol is naturally present in low amounts in fruits and vegetables; the artificial sweetener erythritol that is produced from corn is only 70% as sweet as sugar.
Upon ingestion it is poorly metabolized, and most is excreted in the urine, so it is characterized as a “zero-calorie,” “non-nutritive,” or “natural sweetener.” It is predicted to double in marketshare in the sweetener sector in the next 5 years.
Multipart study
In the first part of their study, in a discovery cohort in 1,157 patients undergoing cardiovascular assessment with 3-year outcomes, the researchers identified polyols that were associated with MACE, and erythritol was among the top MACE-associated molecules.
Next, in a U.S. validation cohort of 2,149 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 1.8-fold higher risk of MACE than patients in the lowest quartile (P = .007), after adjusting for cardiovascular risk factors.
In a European validation cohort of 833 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 2.21-fold higher risk of MACE than patients in the lowest quartile (P = .010, after adjustment).
At physiologic levels, erythritol enhanced platelet reactivity in vitro and thrombosis formation in vivo.
Finally, in a prospective pilot intervention study, erythritol ingestion in healthy volunteers induced marked and sustained increases in plasma erythritol levels well above thresholds associated with heightened platelet reactivity and thrombosis potential in in vitro and in vivo studies.
Others weigh in
“While I think the finding certainly warrants further investigation, don’t throw out your sweeteners just yet,” commented Oliver Jones, PhD, professor of chemistry at the Royal Melbourne Institute of Technology.
“This study only looks at erythritol, and artificial sweeteners are generally considered safe. Any possible (and, as yet unproven) risks of excess erythritol would also need to be balanced against the very real health risks of excess glucose consumption,” he said.
Dr. Hazen responded: “True enough. Erythritol is but one of many artificial sweeteners. That is why it is important to read labels. This study can make patients be informed about how to potentially avoid something that might cause them inadvertent harm.”
“The key findings of this study are that high blood levels of erythritol are strongly associated with cardiovascular outcomes in high-risk patients, which has been replicated in separate validation studies,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London.
“Diabetes UK currently advises diabetes patients not to use polyols,” he added.
Dr. Hazen noted that “About three-quarters of the participants had coronary disease, high blood pressure, and about a fifth had diabetes.”
The researchers acknowledge, however, that the observational studies cannot show cause and effect.
The study was supported by the Office of Dietary Supplements at the National Institutes of Health, the Leducq Foundation, and the German Research Foundation (Deutsche Forschungsgemeinschaft). Dr. Mellor, Dr. Jones, and Dr. Sanders have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Erythritol is one of the most widely used artificial sweeteners with rapidly increasing prevalence in processed and “keto-related” foods. Artificial sweeteners are “generally recognized as safe” (GRAS) by the U.S. Food and Drug Administration, so there is no requirement for long-term safety studies, and little is known about the long-term health effects.
The current research, published online in Nature Medicine by Marco Witkowski, MD, of the Lerner Research Institute at Cleveland Clinic and colleagues, had multiple parts.
First, in a group of patients undergoing cardiac risk assessment, the researchers found that high levels of polyols, especially erythritol, were associated with increased 3-year risk of MACE, defined as cardiovascular death or nonfatal myocardial infarction or stroke.
Next, the association of erythritol with this outcome was reproduced in two large U.S. and European groups of stable patients undergoing elective cardiac evaluation.
Next, adding erythritol to whole blood or platelets led to clot activation. And lastly, in eight healthy volunteers, ingesting 30 g of an erythritol-sweetened drink – comparable to a single can of commercially available beverage or a pint of keto ice cream – induced marked and sustained (> 2 day) increases in levels of plasma erythritol.
“Our study shows that when participants consumed an artificially sweetened beverage with an amount of erythritol found in many processed foods, markedly elevated levels in the blood are observed for days – levels well above those observed to enhance clotting risks,” said senior author Stanley L. Hazen, MD, PhD.
“It is important that further safety studies are conducted to examine the long-term effects of artificial sweeteners in general, and erythritol specifically, on risks for heart attack and stroke, particularly in people at higher risk for cardiovascular disease,” Dr. Hazen, co–section head of preventive cardiology at Cleveland Clinic, said in a press release from his institution.
“Sweeteners like erythritol have rapidly increased in popularity in recent years, but there needs to be more in-depth research into their long-term effects. Cardiovascular disease builds over time, and heart disease is the leading cause of death globally. We need to make sure the foods we eat aren’t hidden contributors,” Dr. Hazen urged.
The topic remains controversial.
Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University, Birmingham, England, told the U.K. Science Media Centre: “This paper effectively shows multiple pieces of a jigsaw exploring the effects of erythritol – although it claims to show an associated risk with the use of erythritol as an artificial sweetener and cardiovascular disease, I believe it fails to do so, as ultimately, erythritol can be made inside our bodies and the intake in most people’s diet is much lower than the amount given in this study.”
Dr. Hazen countered that data from the 2013-2014 National Health and Nutrition Examination Survey (NHANES) in the United States show that, in some individuals, daily intake of erythritol is estimated to reach 30 g/day.
“Many try and reduce sugar intake by taking many teaspoons of erythritol in their tea, coffee, etc., instead of sugar,” Dr. Hazen added. “Or they eat keto processed foods that have significant quantities of erythritol within it.”
“These studies are a warning for how our processed food (keto and zero sugar, especially) may inadvertently be causing risk/harm. … in the very subset of subjects who are most vulnerable,” according to Dr. Hazen.
Erythritol marketed as ‘zero calorie’, ‘non-nutritive’, or ‘natural’
Patients with type 2 diabetes and obesity are often advised to replace sugar with artificial sweeteners for better glucose control and weight loss, but growing epidemiologic evidence links artificial sweetener consumption with weight gain, insulin resistance, type 2 diabetes, and cardiovascular disease, the researchers write.
Erythritol is naturally present in low amounts in fruits and vegetables; the artificial sweetener erythritol that is produced from corn is only 70% as sweet as sugar.
Upon ingestion it is poorly metabolized, and most is excreted in the urine, so it is characterized as a “zero-calorie,” “non-nutritive,” or “natural sweetener.” It is predicted to double in marketshare in the sweetener sector in the next 5 years.
Multipart study
In the first part of their study, in a discovery cohort in 1,157 patients undergoing cardiovascular assessment with 3-year outcomes, the researchers identified polyols that were associated with MACE, and erythritol was among the top MACE-associated molecules.
Next, in a U.S. validation cohort of 2,149 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 1.8-fold higher risk of MACE than patients in the lowest quartile (P = .007), after adjusting for cardiovascular risk factors.
In a European validation cohort of 833 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 2.21-fold higher risk of MACE than patients in the lowest quartile (P = .010, after adjustment).
At physiologic levels, erythritol enhanced platelet reactivity in vitro and thrombosis formation in vivo.
Finally, in a prospective pilot intervention study, erythritol ingestion in healthy volunteers induced marked and sustained increases in plasma erythritol levels well above thresholds associated with heightened platelet reactivity and thrombosis potential in in vitro and in vivo studies.
Others weigh in
“While I think the finding certainly warrants further investigation, don’t throw out your sweeteners just yet,” commented Oliver Jones, PhD, professor of chemistry at the Royal Melbourne Institute of Technology.
“This study only looks at erythritol, and artificial sweeteners are generally considered safe. Any possible (and, as yet unproven) risks of excess erythritol would also need to be balanced against the very real health risks of excess glucose consumption,” he said.
Dr. Hazen responded: “True enough. Erythritol is but one of many artificial sweeteners. That is why it is important to read labels. This study can make patients be informed about how to potentially avoid something that might cause them inadvertent harm.”
“The key findings of this study are that high blood levels of erythritol are strongly associated with cardiovascular outcomes in high-risk patients, which has been replicated in separate validation studies,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London.
“Diabetes UK currently advises diabetes patients not to use polyols,” he added.
Dr. Hazen noted that “About three-quarters of the participants had coronary disease, high blood pressure, and about a fifth had diabetes.”
The researchers acknowledge, however, that the observational studies cannot show cause and effect.
The study was supported by the Office of Dietary Supplements at the National Institutes of Health, the Leducq Foundation, and the German Research Foundation (Deutsche Forschungsgemeinschaft). Dr. Mellor, Dr. Jones, and Dr. Sanders have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Erythritol is one of the most widely used artificial sweeteners with rapidly increasing prevalence in processed and “keto-related” foods. Artificial sweeteners are “generally recognized as safe” (GRAS) by the U.S. Food and Drug Administration, so there is no requirement for long-term safety studies, and little is known about the long-term health effects.
The current research, published online in Nature Medicine by Marco Witkowski, MD, of the Lerner Research Institute at Cleveland Clinic and colleagues, had multiple parts.
First, in a group of patients undergoing cardiac risk assessment, the researchers found that high levels of polyols, especially erythritol, were associated with increased 3-year risk of MACE, defined as cardiovascular death or nonfatal myocardial infarction or stroke.
Next, the association of erythritol with this outcome was reproduced in two large U.S. and European groups of stable patients undergoing elective cardiac evaluation.
Next, adding erythritol to whole blood or platelets led to clot activation. And lastly, in eight healthy volunteers, ingesting 30 g of an erythritol-sweetened drink – comparable to a single can of commercially available beverage or a pint of keto ice cream – induced marked and sustained (> 2 day) increases in levels of plasma erythritol.
“Our study shows that when participants consumed an artificially sweetened beverage with an amount of erythritol found in many processed foods, markedly elevated levels in the blood are observed for days – levels well above those observed to enhance clotting risks,” said senior author Stanley L. Hazen, MD, PhD.
“It is important that further safety studies are conducted to examine the long-term effects of artificial sweeteners in general, and erythritol specifically, on risks for heart attack and stroke, particularly in people at higher risk for cardiovascular disease,” Dr. Hazen, co–section head of preventive cardiology at Cleveland Clinic, said in a press release from his institution.
“Sweeteners like erythritol have rapidly increased in popularity in recent years, but there needs to be more in-depth research into their long-term effects. Cardiovascular disease builds over time, and heart disease is the leading cause of death globally. We need to make sure the foods we eat aren’t hidden contributors,” Dr. Hazen urged.
The topic remains controversial.
Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University, Birmingham, England, told the U.K. Science Media Centre: “This paper effectively shows multiple pieces of a jigsaw exploring the effects of erythritol – although it claims to show an associated risk with the use of erythritol as an artificial sweetener and cardiovascular disease, I believe it fails to do so, as ultimately, erythritol can be made inside our bodies and the intake in most people’s diet is much lower than the amount given in this study.”
Dr. Hazen countered that data from the 2013-2014 National Health and Nutrition Examination Survey (NHANES) in the United States show that, in some individuals, daily intake of erythritol is estimated to reach 30 g/day.
“Many try and reduce sugar intake by taking many teaspoons of erythritol in their tea, coffee, etc., instead of sugar,” Dr. Hazen added. “Or they eat keto processed foods that have significant quantities of erythritol within it.”
“These studies are a warning for how our processed food (keto and zero sugar, especially) may inadvertently be causing risk/harm. … in the very subset of subjects who are most vulnerable,” according to Dr. Hazen.
Erythritol marketed as ‘zero calorie’, ‘non-nutritive’, or ‘natural’
Patients with type 2 diabetes and obesity are often advised to replace sugar with artificial sweeteners for better glucose control and weight loss, but growing epidemiologic evidence links artificial sweetener consumption with weight gain, insulin resistance, type 2 diabetes, and cardiovascular disease, the researchers write.
Erythritol is naturally present in low amounts in fruits and vegetables; the artificial sweetener erythritol that is produced from corn is only 70% as sweet as sugar.
Upon ingestion it is poorly metabolized, and most is excreted in the urine, so it is characterized as a “zero-calorie,” “non-nutritive,” or “natural sweetener.” It is predicted to double in marketshare in the sweetener sector in the next 5 years.
Multipart study
In the first part of their study, in a discovery cohort in 1,157 patients undergoing cardiovascular assessment with 3-year outcomes, the researchers identified polyols that were associated with MACE, and erythritol was among the top MACE-associated molecules.
Next, in a U.S. validation cohort of 2,149 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 1.8-fold higher risk of MACE than patients in the lowest quartile (P = .007), after adjusting for cardiovascular risk factors.
In a European validation cohort of 833 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 2.21-fold higher risk of MACE than patients in the lowest quartile (P = .010, after adjustment).
At physiologic levels, erythritol enhanced platelet reactivity in vitro and thrombosis formation in vivo.
Finally, in a prospective pilot intervention study, erythritol ingestion in healthy volunteers induced marked and sustained increases in plasma erythritol levels well above thresholds associated with heightened platelet reactivity and thrombosis potential in in vitro and in vivo studies.
Others weigh in
“While I think the finding certainly warrants further investigation, don’t throw out your sweeteners just yet,” commented Oliver Jones, PhD, professor of chemistry at the Royal Melbourne Institute of Technology.
“This study only looks at erythritol, and artificial sweeteners are generally considered safe. Any possible (and, as yet unproven) risks of excess erythritol would also need to be balanced against the very real health risks of excess glucose consumption,” he said.
Dr. Hazen responded: “True enough. Erythritol is but one of many artificial sweeteners. That is why it is important to read labels. This study can make patients be informed about how to potentially avoid something that might cause them inadvertent harm.”
“The key findings of this study are that high blood levels of erythritol are strongly associated with cardiovascular outcomes in high-risk patients, which has been replicated in separate validation studies,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London.
“Diabetes UK currently advises diabetes patients not to use polyols,” he added.
Dr. Hazen noted that “About three-quarters of the participants had coronary disease, high blood pressure, and about a fifth had diabetes.”
The researchers acknowledge, however, that the observational studies cannot show cause and effect.
The study was supported by the Office of Dietary Supplements at the National Institutes of Health, the Leducq Foundation, and the German Research Foundation (Deutsche Forschungsgemeinschaft). Dr. Mellor, Dr. Jones, and Dr. Sanders have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE