User login
Therapeutic drug monitoring pays off for arthritis patients
Therapeutic drug monitoring allowed patients with rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis to reduce their dosage of tumor necrosis factor–alpha (TNF) inhibitors, based on data from 239 individuals.
Use of TNF-alpha inhibitors improves treatment response for many arthritis patients but dosage is rarely adjusted on an individual level, which may lead to unnecessary overdosing in some patients, Mogens Pfeiffer-Jensen, MD, of Aarhus (Denmark) University Hospital, and colleagues wrote.
Data from previous studies suggest that therapeutic drug monitoring (TDM) based on serum trough levels may allow for dose optimization and dose reduction in inflammatory bowel disease patients, but data in patients with arthritis are lacking, they wrote.
In a study published in the Scandinavian Journal of Rheumatology, the researchers recruited 99 patients with RA, 48 with psoriatic arthritis (PsA), and 92 with spondyloarthritis (SpA). The participants were randomized to standard care or standard care plus TDM. Serum trough levels were assessed at baseline and at every 4 months, and prescription changes or drug switches were implemented based on these levels. At baseline, 81 patients were being treated with infliximab (Remicade and biosimilars), 79 with etanercept (Enbrel), and 79 with adalimumab (Humira).
The primary endpoint was reduced drug prescription after 48 weeks.
Overall, TDM significantly reduced prescription of infliximab by 12% (P = .001) and prescription of etanercept by 15% (P = .01), compared with standard care. TDM also prolonged the interdosing intervals of etanercept by 235% (P = .02) and of adalimumab by 28% (P = .04), compared with standard care.
TDM patients taking infliximab had more frequent dose reduction and less frequent dose increases during and after the study when compared with patients who stayed with standard care; similar trends were seen with adalimumab. TDM also accelerated the switch to other biologics for patients on all three medications.
No significant differences occurred in adverse events or hospitalizations between the TDM and standard care patients.
Clinical composite scores (Disease Activity Score based on 28 joints with C-reactive protein) were reduced in patients with RA and PsA who were taking adalimumab and randomized to TDM, but no other clinical outcome differences were noted. Scores on the Health Assessment Questionnaire and global Visual Analog Scale for pain were significantly lower in patients in the TDM group who were taking infliximab and adalimumab, “indicating equally or superior sustained remission across diagnoses,” the researchers emphasized.
The findings were limited by several factors, including the variations in pathophysiology and open-label design. “However, since the TDM was based on an objective serum value and decision procedures were clear, we do not consider the potential of unconscious bias to outweigh the benefits of dose-changing abilities,” they wrote.
The researchers expressed surprise that the reduced use of TNF-alpha inhibitors did not significantly reduce adverse events or serious adverse events, compared with standard care, but they proposed that standard of care may have taken adverse events into account, because all patients had received prescriptions at least 3 months before the study.
As for clinical implications, the current costs of the biochemical assays necessary for TDM may be a barrier to implementing TDM as a standard part of daily clinical practice, the researchers added. However, the study was strengthened by the inclusion of patients with RA, PsA, and SpA, and is the first known to include patients receiving etanercept or adalimumab in an examination of TDM.
“Our data support TDM based solely on serum trough levels in [TNF-alpha inhibitors] with different pharmacokinetics as a future key player in personalized medicine for chronic rheumatoid diseases treated with biologics,” they concluded.
The study was supported by Spydspidspuljen, Region Midt, Denmark, and Department of Rheumatology, Aarhus University Hospital. The researchers had no financial conflicts to disclose.
Therapeutic drug monitoring allowed patients with rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis to reduce their dosage of tumor necrosis factor–alpha (TNF) inhibitors, based on data from 239 individuals.
Use of TNF-alpha inhibitors improves treatment response for many arthritis patients but dosage is rarely adjusted on an individual level, which may lead to unnecessary overdosing in some patients, Mogens Pfeiffer-Jensen, MD, of Aarhus (Denmark) University Hospital, and colleagues wrote.
Data from previous studies suggest that therapeutic drug monitoring (TDM) based on serum trough levels may allow for dose optimization and dose reduction in inflammatory bowel disease patients, but data in patients with arthritis are lacking, they wrote.
In a study published in the Scandinavian Journal of Rheumatology, the researchers recruited 99 patients with RA, 48 with psoriatic arthritis (PsA), and 92 with spondyloarthritis (SpA). The participants were randomized to standard care or standard care plus TDM. Serum trough levels were assessed at baseline and at every 4 months, and prescription changes or drug switches were implemented based on these levels. At baseline, 81 patients were being treated with infliximab (Remicade and biosimilars), 79 with etanercept (Enbrel), and 79 with adalimumab (Humira).
The primary endpoint was reduced drug prescription after 48 weeks.
Overall, TDM significantly reduced prescription of infliximab by 12% (P = .001) and prescription of etanercept by 15% (P = .01), compared with standard care. TDM also prolonged the interdosing intervals of etanercept by 235% (P = .02) and of adalimumab by 28% (P = .04), compared with standard care.
TDM patients taking infliximab had more frequent dose reduction and less frequent dose increases during and after the study when compared with patients who stayed with standard care; similar trends were seen with adalimumab. TDM also accelerated the switch to other biologics for patients on all three medications.
No significant differences occurred in adverse events or hospitalizations between the TDM and standard care patients.
Clinical composite scores (Disease Activity Score based on 28 joints with C-reactive protein) were reduced in patients with RA and PsA who were taking adalimumab and randomized to TDM, but no other clinical outcome differences were noted. Scores on the Health Assessment Questionnaire and global Visual Analog Scale for pain were significantly lower in patients in the TDM group who were taking infliximab and adalimumab, “indicating equally or superior sustained remission across diagnoses,” the researchers emphasized.
The findings were limited by several factors, including the variations in pathophysiology and open-label design. “However, since the TDM was based on an objective serum value and decision procedures were clear, we do not consider the potential of unconscious bias to outweigh the benefits of dose-changing abilities,” they wrote.
The researchers expressed surprise that the reduced use of TNF-alpha inhibitors did not significantly reduce adverse events or serious adverse events, compared with standard care, but they proposed that standard of care may have taken adverse events into account, because all patients had received prescriptions at least 3 months before the study.
As for clinical implications, the current costs of the biochemical assays necessary for TDM may be a barrier to implementing TDM as a standard part of daily clinical practice, the researchers added. However, the study was strengthened by the inclusion of patients with RA, PsA, and SpA, and is the first known to include patients receiving etanercept or adalimumab in an examination of TDM.
“Our data support TDM based solely on serum trough levels in [TNF-alpha inhibitors] with different pharmacokinetics as a future key player in personalized medicine for chronic rheumatoid diseases treated with biologics,” they concluded.
The study was supported by Spydspidspuljen, Region Midt, Denmark, and Department of Rheumatology, Aarhus University Hospital. The researchers had no financial conflicts to disclose.
Therapeutic drug monitoring allowed patients with rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis to reduce their dosage of tumor necrosis factor–alpha (TNF) inhibitors, based on data from 239 individuals.
Use of TNF-alpha inhibitors improves treatment response for many arthritis patients but dosage is rarely adjusted on an individual level, which may lead to unnecessary overdosing in some patients, Mogens Pfeiffer-Jensen, MD, of Aarhus (Denmark) University Hospital, and colleagues wrote.
Data from previous studies suggest that therapeutic drug monitoring (TDM) based on serum trough levels may allow for dose optimization and dose reduction in inflammatory bowel disease patients, but data in patients with arthritis are lacking, they wrote.
In a study published in the Scandinavian Journal of Rheumatology, the researchers recruited 99 patients with RA, 48 with psoriatic arthritis (PsA), and 92 with spondyloarthritis (SpA). The participants were randomized to standard care or standard care plus TDM. Serum trough levels were assessed at baseline and at every 4 months, and prescription changes or drug switches were implemented based on these levels. At baseline, 81 patients were being treated with infliximab (Remicade and biosimilars), 79 with etanercept (Enbrel), and 79 with adalimumab (Humira).
The primary endpoint was reduced drug prescription after 48 weeks.
Overall, TDM significantly reduced prescription of infliximab by 12% (P = .001) and prescription of etanercept by 15% (P = .01), compared with standard care. TDM also prolonged the interdosing intervals of etanercept by 235% (P = .02) and of adalimumab by 28% (P = .04), compared with standard care.
TDM patients taking infliximab had more frequent dose reduction and less frequent dose increases during and after the study when compared with patients who stayed with standard care; similar trends were seen with adalimumab. TDM also accelerated the switch to other biologics for patients on all three medications.
No significant differences occurred in adverse events or hospitalizations between the TDM and standard care patients.
Clinical composite scores (Disease Activity Score based on 28 joints with C-reactive protein) were reduced in patients with RA and PsA who were taking adalimumab and randomized to TDM, but no other clinical outcome differences were noted. Scores on the Health Assessment Questionnaire and global Visual Analog Scale for pain were significantly lower in patients in the TDM group who were taking infliximab and adalimumab, “indicating equally or superior sustained remission across diagnoses,” the researchers emphasized.
The findings were limited by several factors, including the variations in pathophysiology and open-label design. “However, since the TDM was based on an objective serum value and decision procedures were clear, we do not consider the potential of unconscious bias to outweigh the benefits of dose-changing abilities,” they wrote.
The researchers expressed surprise that the reduced use of TNF-alpha inhibitors did not significantly reduce adverse events or serious adverse events, compared with standard care, but they proposed that standard of care may have taken adverse events into account, because all patients had received prescriptions at least 3 months before the study.
As for clinical implications, the current costs of the biochemical assays necessary for TDM may be a barrier to implementing TDM as a standard part of daily clinical practice, the researchers added. However, the study was strengthened by the inclusion of patients with RA, PsA, and SpA, and is the first known to include patients receiving etanercept or adalimumab in an examination of TDM.
“Our data support TDM based solely on serum trough levels in [TNF-alpha inhibitors] with different pharmacokinetics as a future key player in personalized medicine for chronic rheumatoid diseases treated with biologics,” they concluded.
The study was supported by Spydspidspuljen, Region Midt, Denmark, and Department of Rheumatology, Aarhus University Hospital. The researchers had no financial conflicts to disclose.
FROM THE SCANDINAVIAN JOURNAL OF RHEUMATOLOGY
Commentary: Drug efficacy and comorbid factors in PsA, November 2022
The effectiveness and safety of advanced therapies for psoriatic arthritis (PsA) was a focus of many published studies last month. Janus kinase inhibitors (JAKi) are a recent class of drugs made available to treat PsA and related diseases, and several clinical trials have been published. Sarabia and colleagues reported the results of a meta-analysis of 15 randomized controlled trials including 6757 patients with psoriasis or PsA who received treatment with a JAKi or placebo. Their analyses revealed that treatment with JAKi vs placebo was associated with higher odds of achieving American College of Rheumatology 20 (ACR20) response (odds ratio [OR] 4.45; 95% CI 3.64-5.44), with similar outcomes observed with tofacitinib vs placebo (OR 2.96; 95% CI 2.01-4.35) and non-tofacitinib JAKi vs placebo (OR 5.41; 95% CI 3.95-7.40). Serious adverse event rates were low (1%-7% in the maximum-dose intervention group).
Interleukin-23i (guselkumab, tildrakizumab, or risankizumab) are another class of biologics recently approved for the treatment of PsA. Preliminary results from a real-world study demonstrate the efficacy of these drugs for PsA. In a retrospective observational study including 80 patients with psoriasis (22 with PsA) who received guselkumab, tildrakizumab, or risankizumab, Elgaard and colleagues demonstrated that 40.9% or 36.4% of the PsA patients achieved complete or partial remission, respectively, compared with only 18.2% of patients with no improvement.
Regarding drug safety, a recent study demonstrated low rates of opportunistic infections with biologic disease-modifying antirheumatic drugs (bDMARD) and targeted synthetic DMARD (tsDMARD). Vassilopoulos and colleagues conducted a meta-analysis of 47 randomized controlled trials and 26 follow-up extension studies that included patients with PsA who received at least one dose of a bDMARD or a tsDMARD (n = 11,790) or placebo (n = 6425) during the placebo-controlled period, and 17,197 patients who received at least one dose of a bDMARD or a tsDMARD in the long-term extension period.
The cumulative incidence of opportunistic infections was < 3% when stratified by the mechanism of action: JAKi (2.72%; 95% CI 1.05%-5.04%), anti-interleukin (IL)-17i (1.18%; 95% CI 0.60%-1.90%), anti-IL-23i (0.24%; 95% CI 0.04%-0.54%), and TNFi (0.01%; 95% CI 0.00%-0.21%). These results are consistent with my own observations in my clinic. Thus, currently available advanced therapies, including JAKi and IL-23i, are effective and safe for the management of patients with PsA when used as monotherapy with or without conventional synthetic DMARD (csDMARD). Ongoing studies on combination therapy will provide us with guidance on the efficacy and safety of combining these drugs for the treatment of resistant disease.
Many patients do not respond to treatment, however. Actionable risk factors for lack of response are of clinical interest. One such factor is obesity. In an observational study of 774 adult PsA patients who started their first b/tsDMARD, Vallejo-Yague and colleagues reported that the odds of achieving minimal disease activity (adjusted OR [aOR] 0.45; 95% CI 0.24-0.82) and Disease Activity Index for Psoriatic Arthritis (DAPSA)-remission (aOR 0.42; 95% CI 0.21-0.85) were lower in the obese vs normal-weight group within the first year. Thus, obese patients had ~50% lower likelihood of achieving a state of low disease activity. Comprehensive management of PsA must include management of obesity and other comorbid conditions to achieve optimal outcomes.
Finally, an interesting study by Freuer and colleagues used bidirectional two-sample Mendelian randomization in 12,882 patients with inflammatory bowel disease (IBD), 21,770 matched controls, 5621 patients with psoriasis, 2063 patients with PsA, and 252,323 controls. The study found that genetically predicted IBD was associated with a higher risk for PsA (pooled OR 1.11; P = .003) with the risk being majorly mediated by Crohn's disease (OR 1.12; P = .002) and not ulcerative colitis (P = .70). Thus, patients with Crohn's disease need to be carefully evaluated for the development of PsA.
The effectiveness and safety of advanced therapies for psoriatic arthritis (PsA) was a focus of many published studies last month. Janus kinase inhibitors (JAKi) are a recent class of drugs made available to treat PsA and related diseases, and several clinical trials have been published. Sarabia and colleagues reported the results of a meta-analysis of 15 randomized controlled trials including 6757 patients with psoriasis or PsA who received treatment with a JAKi or placebo. Their analyses revealed that treatment with JAKi vs placebo was associated with higher odds of achieving American College of Rheumatology 20 (ACR20) response (odds ratio [OR] 4.45; 95% CI 3.64-5.44), with similar outcomes observed with tofacitinib vs placebo (OR 2.96; 95% CI 2.01-4.35) and non-tofacitinib JAKi vs placebo (OR 5.41; 95% CI 3.95-7.40). Serious adverse event rates were low (1%-7% in the maximum-dose intervention group).
Interleukin-23i (guselkumab, tildrakizumab, or risankizumab) are another class of biologics recently approved for the treatment of PsA. Preliminary results from a real-world study demonstrate the efficacy of these drugs for PsA. In a retrospective observational study including 80 patients with psoriasis (22 with PsA) who received guselkumab, tildrakizumab, or risankizumab, Elgaard and colleagues demonstrated that 40.9% or 36.4% of the PsA patients achieved complete or partial remission, respectively, compared with only 18.2% of patients with no improvement.
Regarding drug safety, a recent study demonstrated low rates of opportunistic infections with biologic disease-modifying antirheumatic drugs (bDMARD) and targeted synthetic DMARD (tsDMARD). Vassilopoulos and colleagues conducted a meta-analysis of 47 randomized controlled trials and 26 follow-up extension studies that included patients with PsA who received at least one dose of a bDMARD or a tsDMARD (n = 11,790) or placebo (n = 6425) during the placebo-controlled period, and 17,197 patients who received at least one dose of a bDMARD or a tsDMARD in the long-term extension period.
The cumulative incidence of opportunistic infections was < 3% when stratified by the mechanism of action: JAKi (2.72%; 95% CI 1.05%-5.04%), anti-interleukin (IL)-17i (1.18%; 95% CI 0.60%-1.90%), anti-IL-23i (0.24%; 95% CI 0.04%-0.54%), and TNFi (0.01%; 95% CI 0.00%-0.21%). These results are consistent with my own observations in my clinic. Thus, currently available advanced therapies, including JAKi and IL-23i, are effective and safe for the management of patients with PsA when used as monotherapy with or without conventional synthetic DMARD (csDMARD). Ongoing studies on combination therapy will provide us with guidance on the efficacy and safety of combining these drugs for the treatment of resistant disease.
Many patients do not respond to treatment, however. Actionable risk factors for lack of response are of clinical interest. One such factor is obesity. In an observational study of 774 adult PsA patients who started their first b/tsDMARD, Vallejo-Yague and colleagues reported that the odds of achieving minimal disease activity (adjusted OR [aOR] 0.45; 95% CI 0.24-0.82) and Disease Activity Index for Psoriatic Arthritis (DAPSA)-remission (aOR 0.42; 95% CI 0.21-0.85) were lower in the obese vs normal-weight group within the first year. Thus, obese patients had ~50% lower likelihood of achieving a state of low disease activity. Comprehensive management of PsA must include management of obesity and other comorbid conditions to achieve optimal outcomes.
Finally, an interesting study by Freuer and colleagues used bidirectional two-sample Mendelian randomization in 12,882 patients with inflammatory bowel disease (IBD), 21,770 matched controls, 5621 patients with psoriasis, 2063 patients with PsA, and 252,323 controls. The study found that genetically predicted IBD was associated with a higher risk for PsA (pooled OR 1.11; P = .003) with the risk being majorly mediated by Crohn's disease (OR 1.12; P = .002) and not ulcerative colitis (P = .70). Thus, patients with Crohn's disease need to be carefully evaluated for the development of PsA.
The effectiveness and safety of advanced therapies for psoriatic arthritis (PsA) was a focus of many published studies last month. Janus kinase inhibitors (JAKi) are a recent class of drugs made available to treat PsA and related diseases, and several clinical trials have been published. Sarabia and colleagues reported the results of a meta-analysis of 15 randomized controlled trials including 6757 patients with psoriasis or PsA who received treatment with a JAKi or placebo. Their analyses revealed that treatment with JAKi vs placebo was associated with higher odds of achieving American College of Rheumatology 20 (ACR20) response (odds ratio [OR] 4.45; 95% CI 3.64-5.44), with similar outcomes observed with tofacitinib vs placebo (OR 2.96; 95% CI 2.01-4.35) and non-tofacitinib JAKi vs placebo (OR 5.41; 95% CI 3.95-7.40). Serious adverse event rates were low (1%-7% in the maximum-dose intervention group).
Interleukin-23i (guselkumab, tildrakizumab, or risankizumab) are another class of biologics recently approved for the treatment of PsA. Preliminary results from a real-world study demonstrate the efficacy of these drugs for PsA. In a retrospective observational study including 80 patients with psoriasis (22 with PsA) who received guselkumab, tildrakizumab, or risankizumab, Elgaard and colleagues demonstrated that 40.9% or 36.4% of the PsA patients achieved complete or partial remission, respectively, compared with only 18.2% of patients with no improvement.
Regarding drug safety, a recent study demonstrated low rates of opportunistic infections with biologic disease-modifying antirheumatic drugs (bDMARD) and targeted synthetic DMARD (tsDMARD). Vassilopoulos and colleagues conducted a meta-analysis of 47 randomized controlled trials and 26 follow-up extension studies that included patients with PsA who received at least one dose of a bDMARD or a tsDMARD (n = 11,790) or placebo (n = 6425) during the placebo-controlled period, and 17,197 patients who received at least one dose of a bDMARD or a tsDMARD in the long-term extension period.
The cumulative incidence of opportunistic infections was < 3% when stratified by the mechanism of action: JAKi (2.72%; 95% CI 1.05%-5.04%), anti-interleukin (IL)-17i (1.18%; 95% CI 0.60%-1.90%), anti-IL-23i (0.24%; 95% CI 0.04%-0.54%), and TNFi (0.01%; 95% CI 0.00%-0.21%). These results are consistent with my own observations in my clinic. Thus, currently available advanced therapies, including JAKi and IL-23i, are effective and safe for the management of patients with PsA when used as monotherapy with or without conventional synthetic DMARD (csDMARD). Ongoing studies on combination therapy will provide us with guidance on the efficacy and safety of combining these drugs for the treatment of resistant disease.
Many patients do not respond to treatment, however. Actionable risk factors for lack of response are of clinical interest. One such factor is obesity. In an observational study of 774 adult PsA patients who started their first b/tsDMARD, Vallejo-Yague and colleagues reported that the odds of achieving minimal disease activity (adjusted OR [aOR] 0.45; 95% CI 0.24-0.82) and Disease Activity Index for Psoriatic Arthritis (DAPSA)-remission (aOR 0.42; 95% CI 0.21-0.85) were lower in the obese vs normal-weight group within the first year. Thus, obese patients had ~50% lower likelihood of achieving a state of low disease activity. Comprehensive management of PsA must include management of obesity and other comorbid conditions to achieve optimal outcomes.
Finally, an interesting study by Freuer and colleagues used bidirectional two-sample Mendelian randomization in 12,882 patients with inflammatory bowel disease (IBD), 21,770 matched controls, 5621 patients with psoriasis, 2063 patients with PsA, and 252,323 controls. The study found that genetically predicted IBD was associated with a higher risk for PsA (pooled OR 1.11; P = .003) with the risk being majorly mediated by Crohn's disease (OR 1.12; P = .002) and not ulcerative colitis (P = .70). Thus, patients with Crohn's disease need to be carefully evaluated for the development of PsA.
Two biologics equally effective for extraintestinal manifestations of IBD
Vedolizumab (Entyvio) and ustekinumab (Stelara) appear to be equally effective for extraintestinal manifestation (EIM) of inflammatory bowel disease (IBD), according to results of a retrospective study published online in Digestive and Liver Disease.
Between 25% and 40% of patients with IBD experience EIM, which reduces quality of life, according to the Crohn’s & Colitis Foundation. EIM commonly involves the joints, skin, bones, eyes, kidney, and liver. Anemia is another extraintestinal complication.
Until now, it’s been unclear whether vedolizumab and ustekinumab are equally effective for treating EIM.
Vedolizumab specifically targets the gastrointestinal tract, a potential disadvantage in reducing EIM, while ustekinumab is thought to have a systemic effect, a potential treatment advantage, Moran Livne-Margolin, MD, and colleagues, Chaim Sheba Medical Center, Ramat Gan, Israel, point out.
To investigate, they included 111 adults with IBD who were treated at the medical center between 2015 and 2021 – 53 with vedolizumab and 58 with ustekinumab. Before starting treatment, all of them had active EIM, most commonly arthralgia (84%).
After 6 weeks of treatment, 66% of patients in both groups had a clinical response to their intestinal disease.
After 14 and 26 weeks of treatment, clinical response rates were 59% and 50%, respectively, with vedolizumab, and 48% and 41%, respectively, with ustekinumab.
Over 52 weeks, both biologics were equally effective against the intestinal disease, with clinical response rates of 42% with vedolizumab and 44% with ustekinumab.
A similar pattern emerged when looking at improvement in EIM.
At week 6, 44% of patients taking vedolizumab and 35% taking ustekinumab had improvement in EIM, with no significant difference between the two biologics (P = .4).
At week 14, rates of improvement in EIM were 43% for vedolizumab and 33% for ustekinumab (P = .39); at 26 weeks, rates were 39% and 33%, respectively (P = .6); and at 52 weeks, rates were 34% and 36% (P = .9).
Researchers also found a significant positive correlation between improvement of the intestinal disease and clinical improvement of EIM at each time point.
Ustekinumab is usually preferred in patients with EIM, Dr. Livne-Margolin and colleagues note. But their findings “may raise some questions whether ustekinumab is, in fact, a better choice in those specific patients.”
Limitations of the study include its retrospective design and small cohort size.
Additionally, vedolizumab is given intravenously in the clinic and mandates patients to have a routine checkup every 1-2 months, whereas ustekinumab can be given at home. As a result, data were missing on some of the patients treated with ustekinumab during the follow-up.
Another limitation is that most of the patients had articular complaints with a small presentation of other EIM.
Also, most of the patients had Crohn’s disease, with only one patient with ulcerative colitis in the ustekinumab group, compared with 12 in the vedolizumab group.
Finally, patients treated with ustekinumab had more experience with anti-TNF treatment, compared with the vedolizumab group, which might have influenced the results with a negative bias toward ustekinumab.
The study had no specific funding. Three authors have disclosed relationships with Janssen, which makes ustekinumab.
A version of this article first appeared on Medscape.com.
Vedolizumab (Entyvio) and ustekinumab (Stelara) appear to be equally effective for extraintestinal manifestation (EIM) of inflammatory bowel disease (IBD), according to results of a retrospective study published online in Digestive and Liver Disease.
Between 25% and 40% of patients with IBD experience EIM, which reduces quality of life, according to the Crohn’s & Colitis Foundation. EIM commonly involves the joints, skin, bones, eyes, kidney, and liver. Anemia is another extraintestinal complication.
Until now, it’s been unclear whether vedolizumab and ustekinumab are equally effective for treating EIM.
Vedolizumab specifically targets the gastrointestinal tract, a potential disadvantage in reducing EIM, while ustekinumab is thought to have a systemic effect, a potential treatment advantage, Moran Livne-Margolin, MD, and colleagues, Chaim Sheba Medical Center, Ramat Gan, Israel, point out.
To investigate, they included 111 adults with IBD who were treated at the medical center between 2015 and 2021 – 53 with vedolizumab and 58 with ustekinumab. Before starting treatment, all of them had active EIM, most commonly arthralgia (84%).
After 6 weeks of treatment, 66% of patients in both groups had a clinical response to their intestinal disease.
After 14 and 26 weeks of treatment, clinical response rates were 59% and 50%, respectively, with vedolizumab, and 48% and 41%, respectively, with ustekinumab.
Over 52 weeks, both biologics were equally effective against the intestinal disease, with clinical response rates of 42% with vedolizumab and 44% with ustekinumab.
A similar pattern emerged when looking at improvement in EIM.
At week 6, 44% of patients taking vedolizumab and 35% taking ustekinumab had improvement in EIM, with no significant difference between the two biologics (P = .4).
At week 14, rates of improvement in EIM were 43% for vedolizumab and 33% for ustekinumab (P = .39); at 26 weeks, rates were 39% and 33%, respectively (P = .6); and at 52 weeks, rates were 34% and 36% (P = .9).
Researchers also found a significant positive correlation between improvement of the intestinal disease and clinical improvement of EIM at each time point.
Ustekinumab is usually preferred in patients with EIM, Dr. Livne-Margolin and colleagues note. But their findings “may raise some questions whether ustekinumab is, in fact, a better choice in those specific patients.”
Limitations of the study include its retrospective design and small cohort size.
Additionally, vedolizumab is given intravenously in the clinic and mandates patients to have a routine checkup every 1-2 months, whereas ustekinumab can be given at home. As a result, data were missing on some of the patients treated with ustekinumab during the follow-up.
Another limitation is that most of the patients had articular complaints with a small presentation of other EIM.
Also, most of the patients had Crohn’s disease, with only one patient with ulcerative colitis in the ustekinumab group, compared with 12 in the vedolizumab group.
Finally, patients treated with ustekinumab had more experience with anti-TNF treatment, compared with the vedolizumab group, which might have influenced the results with a negative bias toward ustekinumab.
The study had no specific funding. Three authors have disclosed relationships with Janssen, which makes ustekinumab.
A version of this article first appeared on Medscape.com.
Vedolizumab (Entyvio) and ustekinumab (Stelara) appear to be equally effective for extraintestinal manifestation (EIM) of inflammatory bowel disease (IBD), according to results of a retrospective study published online in Digestive and Liver Disease.
Between 25% and 40% of patients with IBD experience EIM, which reduces quality of life, according to the Crohn’s & Colitis Foundation. EIM commonly involves the joints, skin, bones, eyes, kidney, and liver. Anemia is another extraintestinal complication.
Until now, it’s been unclear whether vedolizumab and ustekinumab are equally effective for treating EIM.
Vedolizumab specifically targets the gastrointestinal tract, a potential disadvantage in reducing EIM, while ustekinumab is thought to have a systemic effect, a potential treatment advantage, Moran Livne-Margolin, MD, and colleagues, Chaim Sheba Medical Center, Ramat Gan, Israel, point out.
To investigate, they included 111 adults with IBD who were treated at the medical center between 2015 and 2021 – 53 with vedolizumab and 58 with ustekinumab. Before starting treatment, all of them had active EIM, most commonly arthralgia (84%).
After 6 weeks of treatment, 66% of patients in both groups had a clinical response to their intestinal disease.
After 14 and 26 weeks of treatment, clinical response rates were 59% and 50%, respectively, with vedolizumab, and 48% and 41%, respectively, with ustekinumab.
Over 52 weeks, both biologics were equally effective against the intestinal disease, with clinical response rates of 42% with vedolizumab and 44% with ustekinumab.
A similar pattern emerged when looking at improvement in EIM.
At week 6, 44% of patients taking vedolizumab and 35% taking ustekinumab had improvement in EIM, with no significant difference between the two biologics (P = .4).
At week 14, rates of improvement in EIM were 43% for vedolizumab and 33% for ustekinumab (P = .39); at 26 weeks, rates were 39% and 33%, respectively (P = .6); and at 52 weeks, rates were 34% and 36% (P = .9).
Researchers also found a significant positive correlation between improvement of the intestinal disease and clinical improvement of EIM at each time point.
Ustekinumab is usually preferred in patients with EIM, Dr. Livne-Margolin and colleagues note. But their findings “may raise some questions whether ustekinumab is, in fact, a better choice in those specific patients.”
Limitations of the study include its retrospective design and small cohort size.
Additionally, vedolizumab is given intravenously in the clinic and mandates patients to have a routine checkup every 1-2 months, whereas ustekinumab can be given at home. As a result, data were missing on some of the patients treated with ustekinumab during the follow-up.
Another limitation is that most of the patients had articular complaints with a small presentation of other EIM.
Also, most of the patients had Crohn’s disease, with only one patient with ulcerative colitis in the ustekinumab group, compared with 12 in the vedolizumab group.
Finally, patients treated with ustekinumab had more experience with anti-TNF treatment, compared with the vedolizumab group, which might have influenced the results with a negative bias toward ustekinumab.
The study had no specific funding. Three authors have disclosed relationships with Janssen, which makes ustekinumab.
A version of this article first appeared on Medscape.com.
FROM DIGESTIVE AND LIVER DISEASE
IgA Vasculitis in the Setting of Biologic Therapy for Psoriasis and Recurrent Cutaneous Methicillin-Resistant Staphylococcus aureus Colonization
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
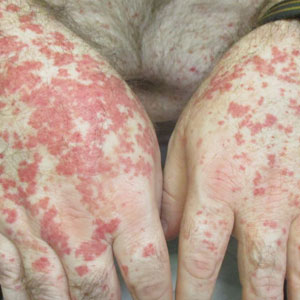
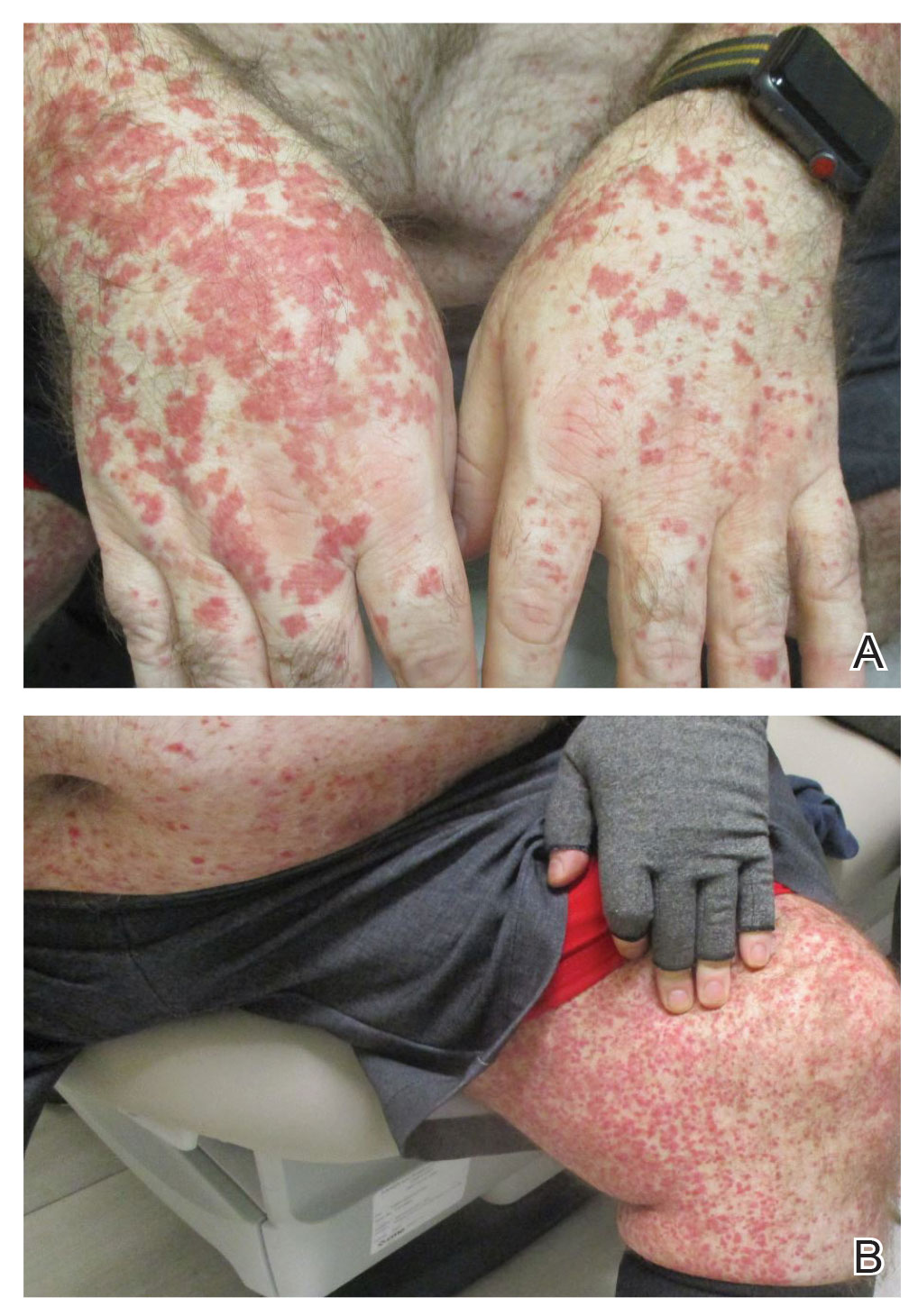
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.
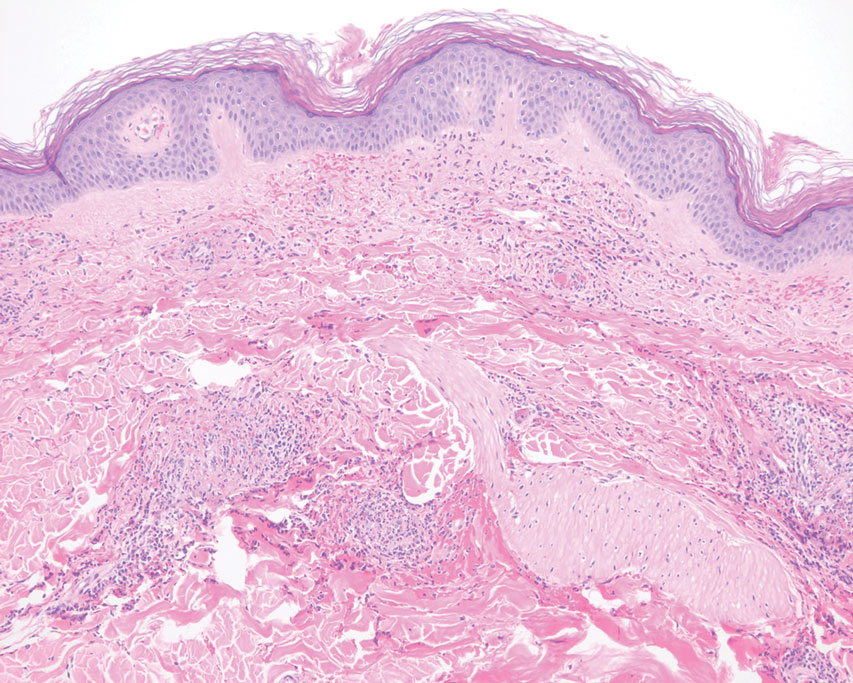
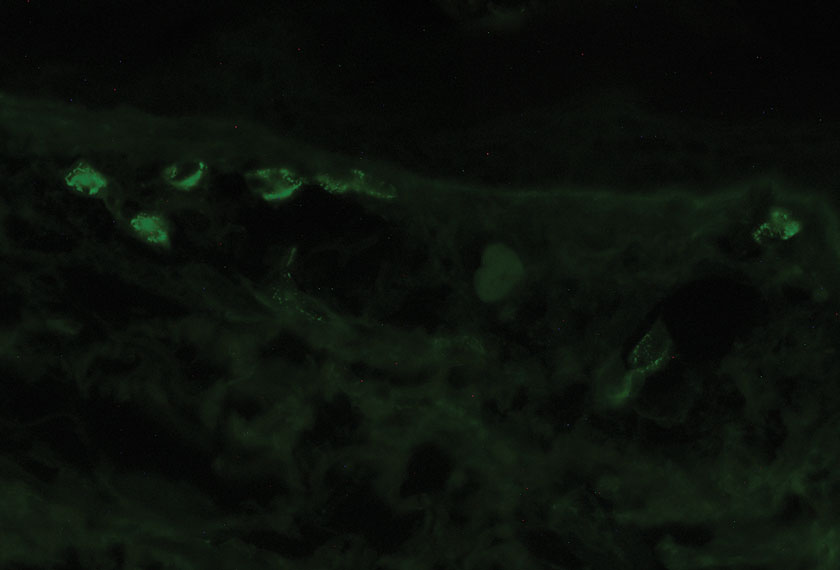
Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.
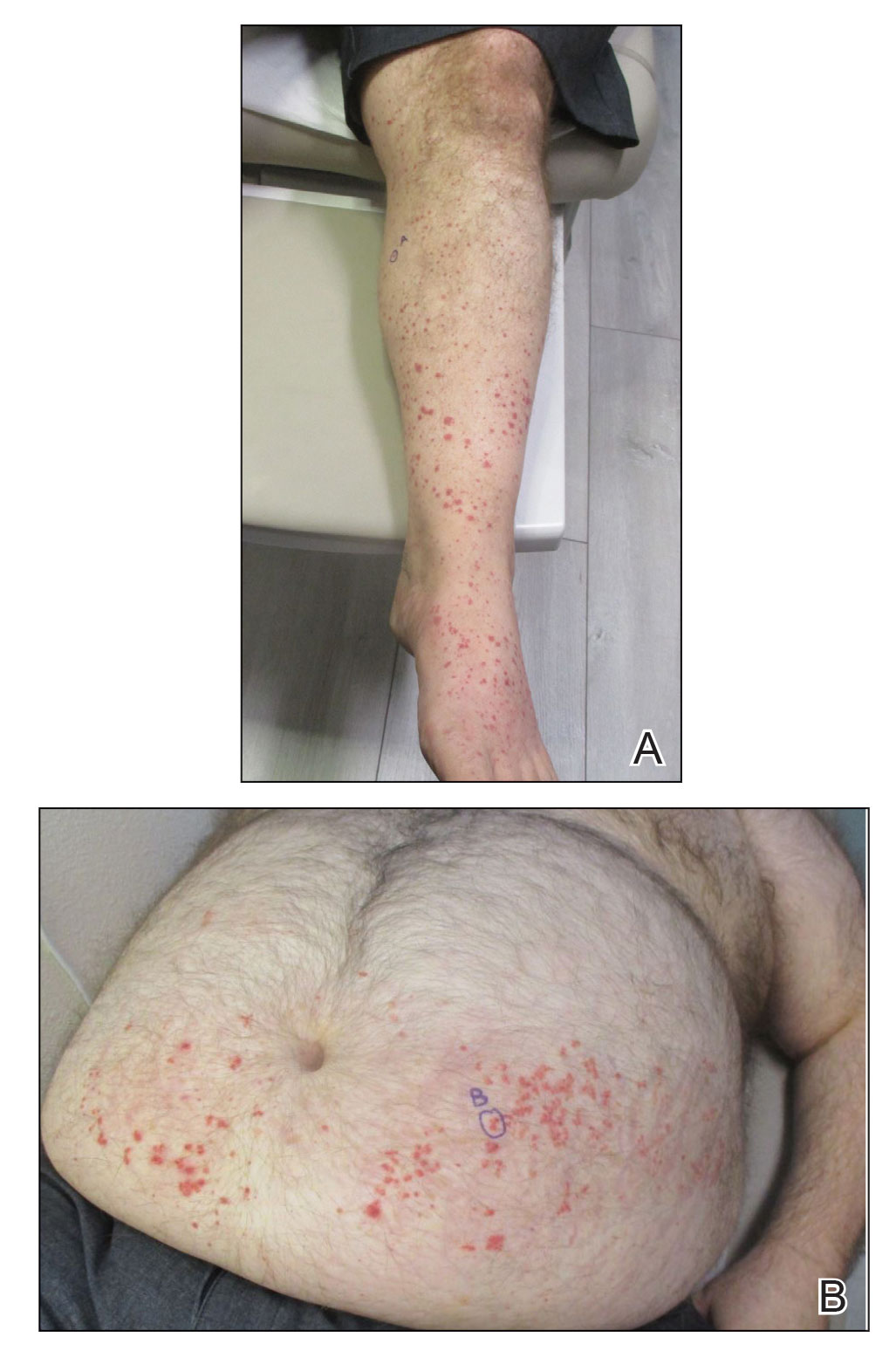
Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.
Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.
Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
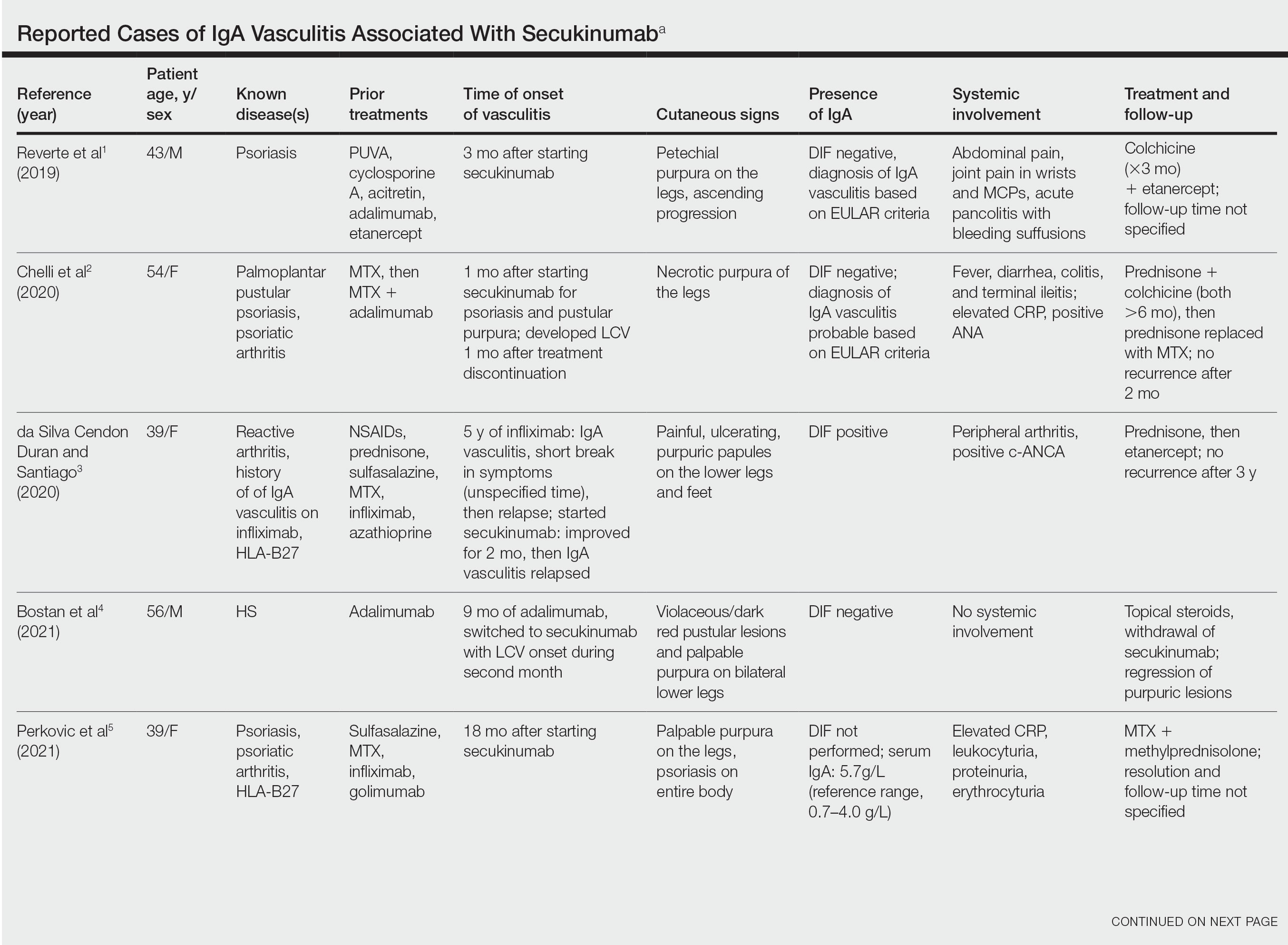
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7
The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10
There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.

Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.

Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.

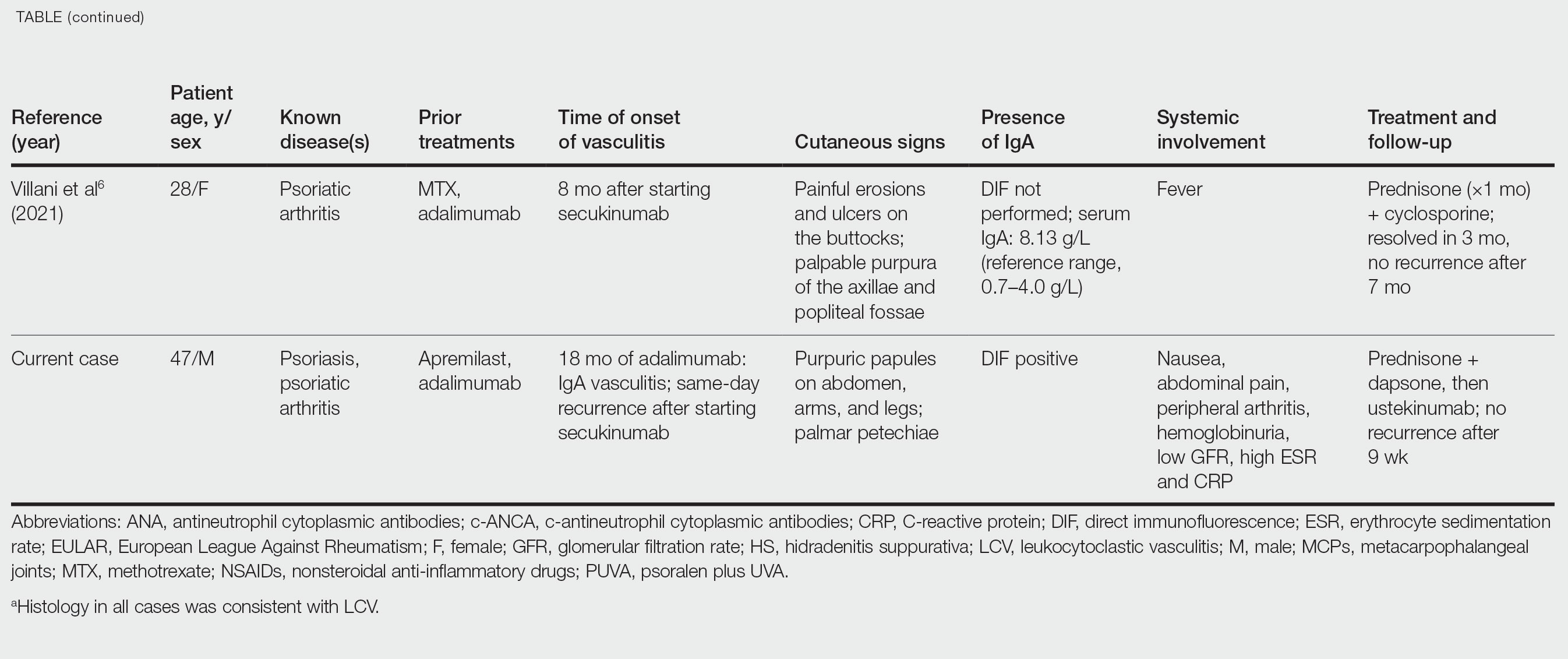
Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.

Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7

The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10

There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
Case Report
A 47-year-old man presented with a sudden-onset rash consisting of red bumps on the abdomen and legs that had been ongoing for several days. He had known psoriasis and psoriatic arthritis that had been well controlled with adalimumab for the last 18 months. He reported concurrent onset of nausea but denied fevers, chills, night sweats, unintentional weight loss, abdominal pain, and pruritus. He endorsed prior cutaneous infections of methicillin-resistant Staphylococcus aureus (MRSA). His medical history also included diabetes mellitus, hypertension, and obesity. His other medications included oral losartan-hydrochlorothiazide, amlodipine, naproxen, and atorvastatin.
Physical examination revealed numerous thin purpuric papules—some with adherent scale—distributed on the lower legs, extensor forearms, and abdomen. Abdominal lesions were confined to weight-related striae (Figure 1). The palms, soles, oral mucosa, and face were spared. Three punch biopsies were performed, including 1 for direct immunofluorescence (DIF), and the patient was instructed to apply clobetasol to the affected areas twice daily until further notice.

Pathology showed perivascular extravasation of erythrocytes, neutrophils, eosinophils, and leukocytoclasis surrounding blood vessels associated with fibrin (Figure 2). Direct immunofluorescence showed granular deposition of IgA, complement component 3, and fibrinogen in a superficial dermal vascular pattern (Figure 3). These results were consistent with IgA small-vessel vasculitis. One specimen was consistent with the patient’s known psoriasis.

Urinalysis revealed moderate hemoglobinuria, and urine microscopy showed 174 red blood cells per high-power field. Creatinine was high at 1.87 mg/dL (reference range, <1.34 mg/dL; patient’s baseline, 0.81 mg/dL) and glomerular filtration rate was low (42 mL/min, patient’s baseline, >60 mL/min [reference range, 90–120 mL/min]). Erythrocyte sedimentation rate (21 mm/h [reference range, 0–22 mm/h]) and C-reactive protein were elevated (2.2 mg/dL [reference range, 0.3–1.0 mg/dL]). Given his history of cutaneous MRSA infections, a bacterial culture swab was collected from the skin surface to check for colonization, which showed moderate growth of MRSA. Naproxen was discontinued over concern of worsening the patient’s renal status. The patient was instructed to rest at home with his legs elevated, wear compression socks when ambulatory, use chlorhexidine antiseptic daily as a body wash when showering, and apply mupirocin three times daily to the biopsy sites. He was referred to urology for his microhematuria, where cystoscopy revealed no abnormalities.A month passed with no improvement of the patient’s cutaneous vasculitis, and his psoriatic arthritis worsened without his usual use of naproxen. He developed abdominal pain and loss of appetite. A prednisone taper was ordered starting at 40 mg/d (28.8 mg/kg), which provided relief of the skin and joint symptoms only until the course was completed 12 days later.

Five weeks after the initial presentation, the patient returned with a more severe eruption consisting of innumerable purpuric papules that coalesced in plaques on the abdomen, arms, and legs. He also had erythematous facial pustules and mild palmar petechiae (Figure 4). Three biopsies were performed, including 1 for DIF and 1 from a pustule on the forehead. Histology and DIF were again consistent with IgA small-vessel vasculitis. The forehead biopsy was compatible with steroid acne (attributed to recent prednisone use) and psoriasis.

Rheumatology was consulted, and adalimumab was discontinued 6 weeks after the initial presentation out of concern for drug-induced cutaneous vasculitis. Vasculitis work-up was unremarkable, including antineutrophil cytoplasmic antibodies, rheumatoid factor, cyclic citrullinated peptide, and serum protein electrophoresis. Oral dapsone was started at 100 mg/d, with the tentative plan of starting secukinumab if cutaneous symptoms improved. For 3 weeks, the patient’s cutaneous symptoms steadily improved.
Nine weeks after initial presentation to dermatology (3 weeks after discontinuing adalimumab) the patient self-administered his first dose of secukinumab at home. Several hours later, he reported sudden reappearance of vasculitis. He denied diarrhea, abdominal pain, bowel movement urgency, fevers, fatigue, and unintentional weight loss. Antistreptolysin O and hepatitis A antibodies were negative. He was instructed to hold secukinumab indefinitely.
Four weeks after his only secukinumab injection, the patient reported another episode of acute worsening cutaneous symptoms. A 4-week prednisone taper starting at 40 mg/d was ordered. Computed tomography of the chest, abdomen, and pelvis to rule out internal malignancy was unremarkable. Around this time, the patient reported major emotional distress related to an unexpected death in his family, which added to a gradual increase in his stress level related to the COVID-19 pandemic.
Three weeks later, dapsone was increased to 100 mg twice daily on account of the patient’s adiposity and lack of cutaneous improvement on the lower dose. Subsequently, the vasculitis rapidly improved for 2 weeks. The patient then reported symptoms of headache, dizziness, and chills. He was tested for COVID-19 and was negative. Six weeks after increasing the dapsone dose (5 months after initial presentation), the skin was normalizing, showing only faintly hyperpigmented macules confined to areas of resolved vasculitis (forearms, abdomen, legs).
The patient had been on dapsone 100 mg twice daily for 3 months when he was started on ustekinumab (90 mg at weeks 0 and 4, with planned doses every 12 weeks) for psoriatic arthritis in hopes of withdrawing dapsone. His cutaneous symptoms have remained well controlled on this regimen for 18 months. Lowering of dapsone below 100 mg daily has resulted in recurrent mild vasculitis symptoms; he now maintains the once-daily dosing without negative side effects.
Comment
IgA vasculitis is a form of cutaneous small-vessel leukocytoclastic vasculitis (LCV) characterized by episodes of palpable purpura on the extensor surfaces of the arms and legs that may be associated with arthritis, abdominal pain, and/or hematuria. Although vasculitis is a known potential adverse effect of anti–tumor necrosis factor (TNF) α therapy, cases of adalimumab-induced IgA vasculitis are uncommon. As use of more targeted therapies for psoriasis and psoriatic arthritis, such as the IL-17 inhibitor secukinumab, increases so do reports of associated adverse events. Of 6 previously reported cases of secukinumab-associated vasculitis, at least 4 were IgA vasculitis (Table).1-6 Another case described one patient with rheumatoid arthritis undergoing secukinumab treatment who experienced necrotizing glomerulonephritis; however, the authors concluded secukinumab likely was not causative in that case, as serologies and urinalyses suggested gradual onset of the process prior to initiating the medication.7

The exact pathogenesis of IgA vasculitis is unclear, but a prevailing theory involves the dysregulation of IgA synthesis and metabolism. Other than increased serum levels of transforming growth factor β, which is a major stimulating factor for IgA production, it also has been hypothesized that the presence of aberrantly hypoglycosylated IgA exposes an autoepitope for recognition by other pathogenic IgG and IgA, leading to the formation of large immune complexes that can readily deposit in postcapillary venules. The deposition of IgA immune complexes in postcapillary venules and the subsequent activation of the complement system causes direct damage to the endothelial cells of vessel walls. This complement activation is evidenced by vascular complement component 3 deposition on DIF (a nonspecific feature of LCV). Chemotaxis of neutrophils ensues, followed by their firm adherence and transendothelial migration (mediated by monocyte chemoattractant protein 1 [MCP-1]). Neutrophil degranulation releases reactive oxygen species and cytokines, which in turn recruit additional leukocytes to the area of inflammation, subsequently undergoing degeneration (leukocytoclasis). Microvascular permeability also is enhanced by MCP-1, allowing exudation of serum, erythrocytes, and fibrin. In the setting of elevated circulating TNF and IL-1, endothelium is stimulated to activate the intrinsic and extrinsic coagulation pathways. This decreases endothelial fibrinolytic activity, leading to thrombosis. The high venous pressure and low fibrinolytic activity in the lower legs explains why vasculitic lesions often are confined to or begin in this distribution.1,8-10

There also are noteworthy roles for cytokines in LCV. Circulating transforming growth factor β and IL-6—which are necessary for development of T helper 17 (TH17) cells and production of IL-17—are higher in patients with LCV compared to controls. Peripheral blood monocytes in patients with LCV demonstrate higher production of IL-17. Once TH17 cells develop, their survival and phenotype are maintained by IL-23 (considered the master regulator of TH17 differentiation). IL-17 is a potent chemoattractant of IL-8 (CXCL8) and MCP-1, both of which promote neutrophil-mediated perivascular inflammation. The IL-23 and IL-17 pathways implicated in the pathogenesis of psoriasis also cause neutrophil activation and upregulate transcription of proinflammatory cytokines (IL-1, IL-6, IL-8, and TNF-α), which overlap with those implicated in LCV. Autoimmune disease generally entails some positive feedback loop of progressively severe self-recognition and tissue destruction by the immune system. These shared cytokinetic processes may explain how the internal environment of psoriasis could perpetuate IgA vasculitis.1,2,8,10-12
The mechanisms underlying vasculitis associated with adalimumab are unclear, but hypotheses involve direct toxicity on vessels, capillary deposition of anti-TNF/TNF immune complexes, or an inflammatory process resulting in autoantibodies. Similar hypotheses are posited for secukinumab-associated vasculitis, including deposition of secukinumab–IL-17 complexes. Anti–TNF-α medications may increase TH17 cell numbers, leading to increased production of IL-22 and a resultant immunologic microenvironment conducive to vasculitis. All 6 published cases of secukinumab-associated vasculitis that we found had received prior treatment with a TNF-α blocker, but only 1 had occurrence of vasculitis during that treatment.1-6,10
In the 6 cases we reviewed, the time from starting secukinumab to onset of vasculitis ranged from 1 to 18 months. Our patient’s same-day re-emergence of vasculitis after his first secukinumab dose was so acute that we were skeptical of secukinumab as a potential trigger; this may simply have been coincident to the natural waxing and waning of the vasculitis (although onset of IgA vasculitis within 1 day of starting anti–TNF-α therapy has been reported).1-6,13
Specific associations of IgA vasculitis are many and can include bacterial organisms such as Helicobacter pylori, streptococci, and staphylococci. Although internal mucous membrane infections are considered more linked because of the surveillance role of IgA predominantly in mucosal tissues, it is possible that our patient with cutaneous MRSA harbored the same within the nasal mucosa. Our patient also received multiple vaccinations outside our department throughout his clinical course (2 hepatitis B and 1 pneumococcal conjugate), which are known potential triggers for vasculitis. Psychological stress is a known trigger for psoriasis, and given the cytokinetic relationship of psoriasis to vasculitis described previously, it may have indirectly contributed to vasculitis in our case. The anxiety associated with being immunosuppressed during the COVID-19 pandemic and bereavement of losing a family member may have contributed to the refractory nature of our patient’s condition. Renal involvement is relatively common in adults with IgA vasculitis and so should be ruled out, as should occult internal malignancy.8,10,14
It is unclear which of the above factors was causative in our case, but a multifactorial process is likely. Treatment of monoclonal antibody–associated vasculitis entails investigating for triggers and systemic involvement, removing the most likely culprit, quelling the vasculitis acutely, avoiding known potential exacerbators, and introducing an alternative long-term immunomodulant. In all 6 reported similar cases, discontinuation of secukinumab and initiation of prednisone or colchicine led to resolution.1-6 Dapsone also is acceptable for acute control of IgA vasculitis, although this medication is highly lipid soluble and penetrates well into various tissues.15 Thus, lower doses may prove ineffective for obese patients, as was demonstrated in our case. Given the known potential of vaccinations, infections, and other factors (eg, alcohol, penicillin) to trigger IgA vasculitis, these should be avoided.10
Blockade of IL-23 with ustekinumab has been suggested by other authors encountering secukinumab-associated vasculitis, as IL-23 is the main driver and sustainer of TH17 cell differentiation.8 Although 6 previously reported cases of secukinumab-associated vasculitis achieved resolution without long-term recurrence, none did so using an IL-23 inhibitor (nor had any of the described patients received IL-23 inhibitors previously).1-6 Given the established safety of IL-23 inhibitors and that they theoretically are well suited for this unique circumstance (by ceasing the main causative cytokine cascades “upstream”) and were efficacious in quickly resolving our patient’s vasculitis, we suggest that ustekinumab may represent
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
- Reverte M, Etienne M, Fouchard M, et al. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. J Eur Acad Dermatol Venereol. 2019;33:E455-E457.
- Chelli C, Loget J, Vanhaecke C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. Acta Derm Venereol. 2020;100:adv00077.
- da Silva Cendon Duran C, Santiago MB. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815.
- Bostan E, Gulseren D, Yalici-Armagan B, et al. Vasculitis during certolizumab pegol and secukinumab treatment: report of two cases. Dermatol Ther. 2021;34:E15007.
- Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071-2073.
- Villani A, DE Fata Salvatores G, Nappa P, et al. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. Ital J Dermatol Venerol. 2021;156(suppl 1 to no. 6):9-10.
- Góis M, Messias A, Carvalho D, et al. MPO-ANCA-associated necrotizing glomerulonephritis in rheumatoid arthritis; a case report and review of literature. J Nephropathol. 2017;6:58-62.
- Jen HY, Chuang YH, Lin SC, et al. Increased serum interleukin-17 and peripheral Th17 cells in children with acute Henoch-Schönlein purpura. Pediatr Allergy Immunol. 2011;22:862-868.
- Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017;97:1160-1166.
- Weedon D. The vasculopathic reaction pattern. In: Houston M, Davie B, eds. Weedon’s Skin Pathology. 3rd ed. Elsevier Limited; 2010:207-211.
- Puig L. Paradoxical reactions: anti-TNFα ants, ustekinumab, secukinumab, ixekizumab, and others. Curr Probl Dermatol. 2018;53:49-63.
- Nestle F, Kaplan D, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNFα therapy: a fortuitous event or an indication to stop therapy? Eur J Dermatol. 2017;27:304-305.
- Hello CL, Cohen P, Bousser MG, et al. Suspected hepatitis B vaccination related vasculitis. J Rheumatol. 1999;26:191-194.
- Wolverton SE. Dapsone. In: Wolverton SE, Wu JJ, eds. Comprehensive Dermatologic Drug Therapy. 4th ed. Elsevier, Inc; 2021:222-231.
Practice Points
- Biologic medications including adalimumab and more rarely secukinumab may be associated with leukocytoclastic vasculitis; a smaller subset of patients may experience IgA vasculitis.
- The IL-23 blocker ustekinumab may represent an ideal therapeutic agent when secukinumabassociated vasculitis is suspected. Because IL-23 is the main driver and sustainer of TH17 cell differentiation, it may cease the main causative cytokine cascades “upstream.”
bDMARDs, especially anti-TNF agents, reduce radiographic progression in PsA
Key clinical point: Some biologic disease-modifying antirheumatic drugs (bDMARDs), especially anti-tumor necrosis factor (TNF) agents, may prevent radiographic progression in patients with psoriatic arthritis (PsA).
Major finding: Anti-TNF agents like infliximab (standardized mean difference [SMD], −0.59; 95% CI, −0.87 to −0.3), etanercept (SMD, −0.51; 95% CI, −0.78 to −0.23), and adalimumab (SMD, −0.45; 95% CI, −0.64 to −0.26) followed by interleukin inhibitors like ixekizumab (SMD, −0.37; 95% CI, −0.62 to −0.12) and secukinumab 300 mg (SMD, −0.33; 95% CI, −0.50 to −0.15) were more effective than placebo in reducing the total radiographic score for structural damage.
Study details: Finding are from a meta-analysis of 11 randomized controlled trials including 4,010 patients with PsA who received bDMARDs or placebo.
Disclosures: This study did not receive any external funding. CH Yang declared receiving speaking fees from several sources.
Source: Wang SH et al. Biologic disease-modifying antirheumatic drugs for preventing radiographic progression in psoriatic arthritis: A systematic review and network meta-analysis. Pharmaceutics. 2022;14(10):2140 (Oct 8). Doi: 10.3390/pharmaceutics14102140.
Key clinical point: Some biologic disease-modifying antirheumatic drugs (bDMARDs), especially anti-tumor necrosis factor (TNF) agents, may prevent radiographic progression in patients with psoriatic arthritis (PsA).
Major finding: Anti-TNF agents like infliximab (standardized mean difference [SMD], −0.59; 95% CI, −0.87 to −0.3), etanercept (SMD, −0.51; 95% CI, −0.78 to −0.23), and adalimumab (SMD, −0.45; 95% CI, −0.64 to −0.26) followed by interleukin inhibitors like ixekizumab (SMD, −0.37; 95% CI, −0.62 to −0.12) and secukinumab 300 mg (SMD, −0.33; 95% CI, −0.50 to −0.15) were more effective than placebo in reducing the total radiographic score for structural damage.
Study details: Finding are from a meta-analysis of 11 randomized controlled trials including 4,010 patients with PsA who received bDMARDs or placebo.
Disclosures: This study did not receive any external funding. CH Yang declared receiving speaking fees from several sources.
Source: Wang SH et al. Biologic disease-modifying antirheumatic drugs for preventing radiographic progression in psoriatic arthritis: A systematic review and network meta-analysis. Pharmaceutics. 2022;14(10):2140 (Oct 8). Doi: 10.3390/pharmaceutics14102140.
Key clinical point: Some biologic disease-modifying antirheumatic drugs (bDMARDs), especially anti-tumor necrosis factor (TNF) agents, may prevent radiographic progression in patients with psoriatic arthritis (PsA).
Major finding: Anti-TNF agents like infliximab (standardized mean difference [SMD], −0.59; 95% CI, −0.87 to −0.3), etanercept (SMD, −0.51; 95% CI, −0.78 to −0.23), and adalimumab (SMD, −0.45; 95% CI, −0.64 to −0.26) followed by interleukin inhibitors like ixekizumab (SMD, −0.37; 95% CI, −0.62 to −0.12) and secukinumab 300 mg (SMD, −0.33; 95% CI, −0.50 to −0.15) were more effective than placebo in reducing the total radiographic score for structural damage.
Study details: Finding are from a meta-analysis of 11 randomized controlled trials including 4,010 patients with PsA who received bDMARDs or placebo.
Disclosures: This study did not receive any external funding. CH Yang declared receiving speaking fees from several sources.
Source: Wang SH et al. Biologic disease-modifying antirheumatic drugs for preventing radiographic progression in psoriatic arthritis: A systematic review and network meta-analysis. Pharmaceutics. 2022;14(10):2140 (Oct 8). Doi: 10.3390/pharmaceutics14102140.
PsA: Meta-analysis demonstrates low rate of opportunistic infections with bDMARDs and tsDMARDs
Key clinical point: Patients with psoriatic arthritis (PsA) reported a low cumulative incidence of opportunistic infections (OIs) after treatment with either biologic (bDMARDs) or targeted synthetic (tsDMARDs) disease-modifying antirheumatic drugs.
Major finding: The cumulative incidence of OIs was <3% when stratified by the mechanism of action: Janus Kinase inhibitors (2.72%; 95% CI, 1.05%-5.04%), anti-interleukin (IL)-17s (1.18%; 95% CI, 0.60%-1.90%), anti-IL-23s (0.24%; 95% CI, 0.04%-0.54%), and anti-tumor necrosis factors (0.01%; 95% CI, 0.00%-0.21%).
Study details: Findings are from a meta-analysis of 47 randomized controlled trials and 26 follow-up extension studies including patients with PsA who were assigned to receive ≥1 dose of bDMARD or tsDMARD (n=11,790) or placebo (n=6,425) during the placebo-controlled period; 17,197 patients received ≥1 dose of bDMARD or tsDMARD considering the extension period.
Disclosures: This study did not report any funding source. The authors declared no conflicts of interest.
Source: Vassilopoulos A et al. The incidence of opportunistic infections in patients with psoriatic arthritis treated with biologic and targeted synthetic agents: A systematic review and meta-analysis. Front. Pharmacol. 2022;13:992713 (Oct 5). Doi: 10.3389/fphar.2022.992713.
Key clinical point: Patients with psoriatic arthritis (PsA) reported a low cumulative incidence of opportunistic infections (OIs) after treatment with either biologic (bDMARDs) or targeted synthetic (tsDMARDs) disease-modifying antirheumatic drugs.
Major finding: The cumulative incidence of OIs was <3% when stratified by the mechanism of action: Janus Kinase inhibitors (2.72%; 95% CI, 1.05%-5.04%), anti-interleukin (IL)-17s (1.18%; 95% CI, 0.60%-1.90%), anti-IL-23s (0.24%; 95% CI, 0.04%-0.54%), and anti-tumor necrosis factors (0.01%; 95% CI, 0.00%-0.21%).
Study details: Findings are from a meta-analysis of 47 randomized controlled trials and 26 follow-up extension studies including patients with PsA who were assigned to receive ≥1 dose of bDMARD or tsDMARD (n=11,790) or placebo (n=6,425) during the placebo-controlled period; 17,197 patients received ≥1 dose of bDMARD or tsDMARD considering the extension period.
Disclosures: This study did not report any funding source. The authors declared no conflicts of interest.
Source: Vassilopoulos A et al. The incidence of opportunistic infections in patients with psoriatic arthritis treated with biologic and targeted synthetic agents: A systematic review and meta-analysis. Front. Pharmacol. 2022;13:992713 (Oct 5). Doi: 10.3389/fphar.2022.992713.
Key clinical point: Patients with psoriatic arthritis (PsA) reported a low cumulative incidence of opportunistic infections (OIs) after treatment with either biologic (bDMARDs) or targeted synthetic (tsDMARDs) disease-modifying antirheumatic drugs.
Major finding: The cumulative incidence of OIs was <3% when stratified by the mechanism of action: Janus Kinase inhibitors (2.72%; 95% CI, 1.05%-5.04%), anti-interleukin (IL)-17s (1.18%; 95% CI, 0.60%-1.90%), anti-IL-23s (0.24%; 95% CI, 0.04%-0.54%), and anti-tumor necrosis factors (0.01%; 95% CI, 0.00%-0.21%).
Study details: Findings are from a meta-analysis of 47 randomized controlled trials and 26 follow-up extension studies including patients with PsA who were assigned to receive ≥1 dose of bDMARD or tsDMARD (n=11,790) or placebo (n=6,425) during the placebo-controlled period; 17,197 patients received ≥1 dose of bDMARD or tsDMARD considering the extension period.
Disclosures: This study did not report any funding source. The authors declared no conflicts of interest.
Source: Vassilopoulos A et al. The incidence of opportunistic infections in patients with psoriatic arthritis treated with biologic and targeted synthetic agents: A systematic review and meta-analysis. Front. Pharmacol. 2022;13:992713 (Oct 5). Doi: 10.3389/fphar.2022.992713.
Meta-analysis demonstrates efficacy of JAK inhibitors in treating PsA
Key clinical point: Janus Kinase (JAK) inhibitors demonstrated promising efficacy in reducing disease severity in patients with psoriatic arthritis (PsA).
Major finding: Treatment with JAK inhibitors vs. placebo was associated with higher odds of achieving ≥20% improvement in American College of Rheumatology (ACR) score (odds ratio [OR], 4.45; 95% CI, 3.64-5.44), with similar outcomes observed with tofacitinib vs. placebo (OR, 2.96; 95% CI, 2.01-4.35) and non-tofacitinib JAK inhibitors vs. placebo (OR, 5.41; 95% CI, 3.95-7.40).
Study details: Findings are from a meta-analysis of 15 randomized controlled trials including 6,757 patients with moderate-to-severe plaque psoriasis or PsA who received treatment with a JAK inhibitor or placebo.
Disclosures: S Sarabia declared receiving summer student grant from the Queen’s University Faculty of Medicine. The authors declared no conflicts of interest.
Source: Sarabia S et al. Efficacy and safety of JAK inhibitors in the treatment of psoriasis and psoriatic arthritis: A systematic review and meta-analysis. BMC Rheumatol. 2022;6(1):71 (Sep 27). Doi: 10.1186/s41927-022-00287-7.
Key clinical point: Janus Kinase (JAK) inhibitors demonstrated promising efficacy in reducing disease severity in patients with psoriatic arthritis (PsA).
Major finding: Treatment with JAK inhibitors vs. placebo was associated with higher odds of achieving ≥20% improvement in American College of Rheumatology (ACR) score (odds ratio [OR], 4.45; 95% CI, 3.64-5.44), with similar outcomes observed with tofacitinib vs. placebo (OR, 2.96; 95% CI, 2.01-4.35) and non-tofacitinib JAK inhibitors vs. placebo (OR, 5.41; 95% CI, 3.95-7.40).
Study details: Findings are from a meta-analysis of 15 randomized controlled trials including 6,757 patients with moderate-to-severe plaque psoriasis or PsA who received treatment with a JAK inhibitor or placebo.
Disclosures: S Sarabia declared receiving summer student grant from the Queen’s University Faculty of Medicine. The authors declared no conflicts of interest.
Source: Sarabia S et al. Efficacy and safety of JAK inhibitors in the treatment of psoriasis and psoriatic arthritis: A systematic review and meta-analysis. BMC Rheumatol. 2022;6(1):71 (Sep 27). Doi: 10.1186/s41927-022-00287-7.
Key clinical point: Janus Kinase (JAK) inhibitors demonstrated promising efficacy in reducing disease severity in patients with psoriatic arthritis (PsA).
Major finding: Treatment with JAK inhibitors vs. placebo was associated with higher odds of achieving ≥20% improvement in American College of Rheumatology (ACR) score (odds ratio [OR], 4.45; 95% CI, 3.64-5.44), with similar outcomes observed with tofacitinib vs. placebo (OR, 2.96; 95% CI, 2.01-4.35) and non-tofacitinib JAK inhibitors vs. placebo (OR, 5.41; 95% CI, 3.95-7.40).
Study details: Findings are from a meta-analysis of 15 randomized controlled trials including 6,757 patients with moderate-to-severe plaque psoriasis or PsA who received treatment with a JAK inhibitor or placebo.
Disclosures: S Sarabia declared receiving summer student grant from the Queen’s University Faculty of Medicine. The authors declared no conflicts of interest.
Source: Sarabia S et al. Efficacy and safety of JAK inhibitors in the treatment of psoriasis and psoriatic arthritis: A systematic review and meta-analysis. BMC Rheumatol. 2022;6(1):71 (Sep 27). Doi: 10.1186/s41927-022-00287-7.
PsA: Tofacitinib reduces pain by improving itch and other symptoms
Key clinical point: Pain alleviation by tofacitinib in patients with psoriatic arthritis (PsA) was mostly due to improvement in itch, enthesitis, and C-reactive protein (CRP) levels.
Major finding: Tofacitinib predominantly reduced pain indirectly (70.5%; P < .0001), as indicated by improvements in the Itch Severity Item score (37.4%; P = .0002), Leeds Enthesitis Index score (17.8%; P = .0157), and CRP levels (15.3%; P = .0107).
Study details: Findings are from a mediation analysis of pooled data from 2 phase 3 studies (OPAL Broaden and OPAL Beyond) including 474 patients with active PsA who received tofacitinib 5 mg twice daily or placebo.
Disclosures: This study was funded by Pfizer Inc. Four authors declared being employees and shareholders of Pfizer, and the other authors reported ties with several sources.
Source: de Vlam K et al. Identifying and quantifying the role of inflammation in pain reduction for patients with psoriatic arthritis treated with tofacitinib: A mediation analysis. Rheumatol Ther. 2022;9(5):1451-1464 (Sep 8). Doi: 10.1007/s40744-022-00482-5.
Key clinical point: Pain alleviation by tofacitinib in patients with psoriatic arthritis (PsA) was mostly due to improvement in itch, enthesitis, and C-reactive protein (CRP) levels.
Major finding: Tofacitinib predominantly reduced pain indirectly (70.5%; P < .0001), as indicated by improvements in the Itch Severity Item score (37.4%; P = .0002), Leeds Enthesitis Index score (17.8%; P = .0157), and CRP levels (15.3%; P = .0107).
Study details: Findings are from a mediation analysis of pooled data from 2 phase 3 studies (OPAL Broaden and OPAL Beyond) including 474 patients with active PsA who received tofacitinib 5 mg twice daily or placebo.
Disclosures: This study was funded by Pfizer Inc. Four authors declared being employees and shareholders of Pfizer, and the other authors reported ties with several sources.
Source: de Vlam K et al. Identifying and quantifying the role of inflammation in pain reduction for patients with psoriatic arthritis treated with tofacitinib: A mediation analysis. Rheumatol Ther. 2022;9(5):1451-1464 (Sep 8). Doi: 10.1007/s40744-022-00482-5.
Key clinical point: Pain alleviation by tofacitinib in patients with psoriatic arthritis (PsA) was mostly due to improvement in itch, enthesitis, and C-reactive protein (CRP) levels.
Major finding: Tofacitinib predominantly reduced pain indirectly (70.5%; P < .0001), as indicated by improvements in the Itch Severity Item score (37.4%; P = .0002), Leeds Enthesitis Index score (17.8%; P = .0157), and CRP levels (15.3%; P = .0107).
Study details: Findings are from a mediation analysis of pooled data from 2 phase 3 studies (OPAL Broaden and OPAL Beyond) including 474 patients with active PsA who received tofacitinib 5 mg twice daily or placebo.
Disclosures: This study was funded by Pfizer Inc. Four authors declared being employees and shareholders of Pfizer, and the other authors reported ties with several sources.
Source: de Vlam K et al. Identifying and quantifying the role of inflammation in pain reduction for patients with psoriatic arthritis treated with tofacitinib: A mediation analysis. Rheumatol Ther. 2022;9(5):1451-1464 (Sep 8). Doi: 10.1007/s40744-022-00482-5.
Biologic treatments effective against PsA in the real world
Key clinical point: Biologics improved disease activity (DA) in a real-world population of patients with psoriatic arthritis (PsA) in Italy.
Major finding: At 6 months, a substantially high proportion of patients receiving biologics achieved the European league against rheumatism (EULAR) DA Score 28 (71.8%; 95% CI, 66.7%-76.8%), with outcomes being similar with secukinumab (73.4%; 95% CI, 65.8%-81.1%) and tumor necrosis factor-inhibitors (71.9%; 95% CI, 64.9%-78.8%). The responder rate was 68.0% at year 1.
Study details: Findings are from a multicenter, noninterventional, cohort study including 399 patients with PsA, of which 308 were evaluable at 6 months and 297 patients were evaluable at 1 year. Most patients received biologics for ≥6 months.
Disclosures: This study was supported by a grant from Novartis Farma S.p.A. Origgio. Three authors declared being part-time/regular employees of Novartis Farma or MediNeos Observational Research, hired by Novartis Farma. The authors declared receiving honoraria, speaker fees, and/or scientific support from other sources.
Source: Colombo D et al. Real-world evidence of biologic treatments in psoriatic arthritis in Italy: Results of the CHRONOS (EffeCtiveness of biologic treatments for psoriatic artHRitis in Italy: An ObservatioNal lOngitudinal Study of real-life clinical practice) observational longitudinal study. BMC Rheumatol. 2022;6(1):57 (Sep 12). Doi: 10.1186/s41927-022-00284-w.
Key clinical point: Biologics improved disease activity (DA) in a real-world population of patients with psoriatic arthritis (PsA) in Italy.
Major finding: At 6 months, a substantially high proportion of patients receiving biologics achieved the European league against rheumatism (EULAR) DA Score 28 (71.8%; 95% CI, 66.7%-76.8%), with outcomes being similar with secukinumab (73.4%; 95% CI, 65.8%-81.1%) and tumor necrosis factor-inhibitors (71.9%; 95% CI, 64.9%-78.8%). The responder rate was 68.0% at year 1.
Study details: Findings are from a multicenter, noninterventional, cohort study including 399 patients with PsA, of which 308 were evaluable at 6 months and 297 patients were evaluable at 1 year. Most patients received biologics for ≥6 months.
Disclosures: This study was supported by a grant from Novartis Farma S.p.A. Origgio. Three authors declared being part-time/regular employees of Novartis Farma or MediNeos Observational Research, hired by Novartis Farma. The authors declared receiving honoraria, speaker fees, and/or scientific support from other sources.
Source: Colombo D et al. Real-world evidence of biologic treatments in psoriatic arthritis in Italy: Results of the CHRONOS (EffeCtiveness of biologic treatments for psoriatic artHRitis in Italy: An ObservatioNal lOngitudinal Study of real-life clinical practice) observational longitudinal study. BMC Rheumatol. 2022;6(1):57 (Sep 12). Doi: 10.1186/s41927-022-00284-w.
Key clinical point: Biologics improved disease activity (DA) in a real-world population of patients with psoriatic arthritis (PsA) in Italy.
Major finding: At 6 months, a substantially high proportion of patients receiving biologics achieved the European league against rheumatism (EULAR) DA Score 28 (71.8%; 95% CI, 66.7%-76.8%), with outcomes being similar with secukinumab (73.4%; 95% CI, 65.8%-81.1%) and tumor necrosis factor-inhibitors (71.9%; 95% CI, 64.9%-78.8%). The responder rate was 68.0% at year 1.
Study details: Findings are from a multicenter, noninterventional, cohort study including 399 patients with PsA, of which 308 were evaluable at 6 months and 297 patients were evaluable at 1 year. Most patients received biologics for ≥6 months.
Disclosures: This study was supported by a grant from Novartis Farma S.p.A. Origgio. Three authors declared being part-time/regular employees of Novartis Farma or MediNeos Observational Research, hired by Novartis Farma. The authors declared receiving honoraria, speaker fees, and/or scientific support from other sources.
Source: Colombo D et al. Real-world evidence of biologic treatments in psoriatic arthritis in Italy: Results of the CHRONOS (EffeCtiveness of biologic treatments for psoriatic artHRitis in Italy: An ObservatioNal lOngitudinal Study of real-life clinical practice) observational longitudinal study. BMC Rheumatol. 2022;6(1):57 (Sep 12). Doi: 10.1186/s41927-022-00284-w.
Nail psoriasis increases disease burden in PsA
Key clinical point: Patients with psoriatic arthritis (PsA) who had nail psoriasis were older and had higher disease burden and lower quality of life (QoL) than those without nail psoriasis.
Major finding: Patients with vs. without nail psoriasis had a higher median age (48 vs. 46 years; P = .001), body mass index (29 vs. 28 kg/m2; P = .02), tender (P < .001) and swollen (P = .011) joint counts, and PsAQoL score (6 vs. 4; P = .001).
Study details: Findings are from a cross-sectional, multicenter study including 1,22 patients with PsA, of which 57.5% had nail psoriasis.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Cengiz G et al. The impact of nail psoriasis on disease activity, quality of life, and clinical variables in patients with psoriatic arthritis: A cross-sectional multicenter study. Int J Rheum Dis. 2022 (Sep 27). Doi:10.1111/1756-185X.14442
Key clinical point: Patients with psoriatic arthritis (PsA) who had nail psoriasis were older and had higher disease burden and lower quality of life (QoL) than those without nail psoriasis.
Major finding: Patients with vs. without nail psoriasis had a higher median age (48 vs. 46 years; P = .001), body mass index (29 vs. 28 kg/m2; P = .02), tender (P < .001) and swollen (P = .011) joint counts, and PsAQoL score (6 vs. 4; P = .001).
Study details: Findings are from a cross-sectional, multicenter study including 1,22 patients with PsA, of which 57.5% had nail psoriasis.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Cengiz G et al. The impact of nail psoriasis on disease activity, quality of life, and clinical variables in patients with psoriatic arthritis: A cross-sectional multicenter study. Int J Rheum Dis. 2022 (Sep 27). Doi:10.1111/1756-185X.14442
Key clinical point: Patients with psoriatic arthritis (PsA) who had nail psoriasis were older and had higher disease burden and lower quality of life (QoL) than those without nail psoriasis.
Major finding: Patients with vs. without nail psoriasis had a higher median age (48 vs. 46 years; P = .001), body mass index (29 vs. 28 kg/m2; P = .02), tender (P < .001) and swollen (P = .011) joint counts, and PsAQoL score (6 vs. 4; P = .001).
Study details: Findings are from a cross-sectional, multicenter study including 1,22 patients with PsA, of which 57.5% had nail psoriasis.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Cengiz G et al. The impact of nail psoriasis on disease activity, quality of life, and clinical variables in patients with psoriatic arthritis: A cross-sectional multicenter study. Int J Rheum Dis. 2022 (Sep 27). Doi:10.1111/1756-185X.14442