User login
Greater odds of metabolic syndrome and cardiovascular disease in PsA vs RA
Key clinical point: Patients with psoriatic arthritis (PsA) were more likely to have cardiovascular diseases (CVD) and metabolic syndrome (MS) than patients with rheumatoid arthritis (RA).
Major finding: MS (odds ratio [OR] 1.54; P = .002) and CVD (OR 6.13; P = .001) were more frequently observed in patients with PsA vs RA.
Study details: Findings are from a cross-sectional study including 197 patients with PsA and 279 patients with RA.
Disclosures: This study was supported by Peking University First Hospital, China, and other sources. The authors declared no conflict of interests.
Source: Li B et al. Discrepancy in metabolic syndrome between psoriatic arthritis and rheumatoid arthritis: A direct comparison of two cohorts in one center. Rheumatol Ther. 2022 (Oct 20). Doi: 10.1007/s40744-022-00502-4
Key clinical point: Patients with psoriatic arthritis (PsA) were more likely to have cardiovascular diseases (CVD) and metabolic syndrome (MS) than patients with rheumatoid arthritis (RA).
Major finding: MS (odds ratio [OR] 1.54; P = .002) and CVD (OR 6.13; P = .001) were more frequently observed in patients with PsA vs RA.
Study details: Findings are from a cross-sectional study including 197 patients with PsA and 279 patients with RA.
Disclosures: This study was supported by Peking University First Hospital, China, and other sources. The authors declared no conflict of interests.
Source: Li B et al. Discrepancy in metabolic syndrome between psoriatic arthritis and rheumatoid arthritis: A direct comparison of two cohorts in one center. Rheumatol Ther. 2022 (Oct 20). Doi: 10.1007/s40744-022-00502-4
Key clinical point: Patients with psoriatic arthritis (PsA) were more likely to have cardiovascular diseases (CVD) and metabolic syndrome (MS) than patients with rheumatoid arthritis (RA).
Major finding: MS (odds ratio [OR] 1.54; P = .002) and CVD (OR 6.13; P = .001) were more frequently observed in patients with PsA vs RA.
Study details: Findings are from a cross-sectional study including 197 patients with PsA and 279 patients with RA.
Disclosures: This study was supported by Peking University First Hospital, China, and other sources. The authors declared no conflict of interests.
Source: Li B et al. Discrepancy in metabolic syndrome between psoriatic arthritis and rheumatoid arthritis: A direct comparison of two cohorts in one center. Rheumatol Ther. 2022 (Oct 20). Doi: 10.1007/s40744-022-00502-4
Higher prevalence of sonographic enthesitis in men than in women with PsA
Key clinical point: Among patients with psoriatic arthritis (PsA), sonographic enthesitis was more prevalent in men than women.
Major finding: Compared with women, men had higher total ultrasound and gray scale enthesitis scores (both P = .01) and sonographic active inflammatory score (P = .005), as reflected by higher hypoechogenicity, thickening, and enthesophytes (P < .05), with the male sex being associated with a significantly higher ultrasound inflammatory enthesitis score (P = .02).
Study details: Findings are from a prospective study including 158 patients with PsA, of which 70 patients were men and 88 were women.
Disclosures: This study did not receive any funding. The authors declared no conflict of interests.
Source: Furer V et al. Sex-based differences in sonographic and clinical findings among patients with psoriatic arthritis. J Rheumatol. 2022 (Oct 15). Doi: 10.3899/jrheum.220547
Key clinical point: Among patients with psoriatic arthritis (PsA), sonographic enthesitis was more prevalent in men than women.
Major finding: Compared with women, men had higher total ultrasound and gray scale enthesitis scores (both P = .01) and sonographic active inflammatory score (P = .005), as reflected by higher hypoechogenicity, thickening, and enthesophytes (P < .05), with the male sex being associated with a significantly higher ultrasound inflammatory enthesitis score (P = .02).
Study details: Findings are from a prospective study including 158 patients with PsA, of which 70 patients were men and 88 were women.
Disclosures: This study did not receive any funding. The authors declared no conflict of interests.
Source: Furer V et al. Sex-based differences in sonographic and clinical findings among patients with psoriatic arthritis. J Rheumatol. 2022 (Oct 15). Doi: 10.3899/jrheum.220547
Key clinical point: Among patients with psoriatic arthritis (PsA), sonographic enthesitis was more prevalent in men than women.
Major finding: Compared with women, men had higher total ultrasound and gray scale enthesitis scores (both P = .01) and sonographic active inflammatory score (P = .005), as reflected by higher hypoechogenicity, thickening, and enthesophytes (P < .05), with the male sex being associated with a significantly higher ultrasound inflammatory enthesitis score (P = .02).
Study details: Findings are from a prospective study including 158 patients with PsA, of which 70 patients were men and 88 were women.
Disclosures: This study did not receive any funding. The authors declared no conflict of interests.
Source: Furer V et al. Sex-based differences in sonographic and clinical findings among patients with psoriatic arthritis. J Rheumatol. 2022 (Oct 15). Doi: 10.3899/jrheum.220547
Etanercept slows radiographic progression of PsA in the real world
Key clinical point: Patients with psoriatic arthritis (PsA) experienced a slowing of radiographic progression during treatment with etanercept.
Major finding: In patients with available pre-etanercept radiographic data, the annualized radiographic progression was significantly lower with etanercept during the first 18 months than during the pre-etanercept treatment period (P < .005), and a higher proportion of patients showed no progression during etanercept treatment (61.7%) than during the pre-etanercept period (55.3%).
Study details: Findings are from a real-world, noninterventional study including 1821 participants who started treatment with etanercept, of which 1378 patients had rheumatoid arthritis and 440 patients had PsA.
Disclosures: This study was funded by Pfizer. Five authors declared being current or former employees of Pfizer, and the other authors reported ties with several sources, including Pfizer.
Source: Wassenberg S et al. Etanercept is effective and halts radiographic progression in rheumatoid arthritis and psoriatic arthritis: Final results from a German non-interventional study (PRERA). Rheumatol Ther. 2022 (Oct 17). Doi: 10.1007/s40744-022-00491-4
Key clinical point: Patients with psoriatic arthritis (PsA) experienced a slowing of radiographic progression during treatment with etanercept.
Major finding: In patients with available pre-etanercept radiographic data, the annualized radiographic progression was significantly lower with etanercept during the first 18 months than during the pre-etanercept treatment period (P < .005), and a higher proportion of patients showed no progression during etanercept treatment (61.7%) than during the pre-etanercept period (55.3%).
Study details: Findings are from a real-world, noninterventional study including 1821 participants who started treatment with etanercept, of which 1378 patients had rheumatoid arthritis and 440 patients had PsA.
Disclosures: This study was funded by Pfizer. Five authors declared being current or former employees of Pfizer, and the other authors reported ties with several sources, including Pfizer.
Source: Wassenberg S et al. Etanercept is effective and halts radiographic progression in rheumatoid arthritis and psoriatic arthritis: Final results from a German non-interventional study (PRERA). Rheumatol Ther. 2022 (Oct 17). Doi: 10.1007/s40744-022-00491-4
Key clinical point: Patients with psoriatic arthritis (PsA) experienced a slowing of radiographic progression during treatment with etanercept.
Major finding: In patients with available pre-etanercept radiographic data, the annualized radiographic progression was significantly lower with etanercept during the first 18 months than during the pre-etanercept treatment period (P < .005), and a higher proportion of patients showed no progression during etanercept treatment (61.7%) than during the pre-etanercept period (55.3%).
Study details: Findings are from a real-world, noninterventional study including 1821 participants who started treatment with etanercept, of which 1378 patients had rheumatoid arthritis and 440 patients had PsA.
Disclosures: This study was funded by Pfizer. Five authors declared being current or former employees of Pfizer, and the other authors reported ties with several sources, including Pfizer.
Source: Wassenberg S et al. Etanercept is effective and halts radiographic progression in rheumatoid arthritis and psoriatic arthritis: Final results from a German non-interventional study (PRERA). Rheumatol Ther. 2022 (Oct 17). Doi: 10.1007/s40744-022-00491-4
Upadacitinib as effective and safe as adalimumab in the long term treatment of PsA
Key clinical point: Compared with adalimumab, both 15 mg and 30 mg upadacitinib showed similar or better efficacy through 2 years and a similar safety profile in patients with psoriatic arthritis (PsA).
Major finding: At week 104, a similar proportion of patients receiving 15/30 mg upadacitinib vs adalimumab achieved ≥20% improvement in the American College of Rheumatology criteria (69.0%/69.5% vs 63.4%), whereas significantly more patients receiving 30 mg upadacitinib vs adalimumab achieved minimal disease activity (45.9% vs 37.8%; P < .05). The safety profiles of upadacitinib and adalimumab were comparable.
Study details: Findings are from an exploratory analysis of the SELECT-PsA 1 study including 1704 patients with active PsA and inadequate response/intolerance to ≥1 non-biologic disease-modifying antirheumatic drug who were randomly assigned to receive upadacitinib (15 or 30 mg) or adalimumab.
Disclosures: This study was funded by AbbVie. Six authors declared being current or former employees or stockholders of AbbVie. The other authors reported ties with several sources, including AbbVie.
Source: McInnes IB et al. Efficacy and safety of upadacitinib in patients with psoriatic arthritis: 2-year results from the phase 3 SELECT-PsA 1 study. Rheumatol Ther. 2022 (Oct 15). Doi: 10.1007/s40744-022-00499-w
Key clinical point: Compared with adalimumab, both 15 mg and 30 mg upadacitinib showed similar or better efficacy through 2 years and a similar safety profile in patients with psoriatic arthritis (PsA).
Major finding: At week 104, a similar proportion of patients receiving 15/30 mg upadacitinib vs adalimumab achieved ≥20% improvement in the American College of Rheumatology criteria (69.0%/69.5% vs 63.4%), whereas significantly more patients receiving 30 mg upadacitinib vs adalimumab achieved minimal disease activity (45.9% vs 37.8%; P < .05). The safety profiles of upadacitinib and adalimumab were comparable.
Study details: Findings are from an exploratory analysis of the SELECT-PsA 1 study including 1704 patients with active PsA and inadequate response/intolerance to ≥1 non-biologic disease-modifying antirheumatic drug who were randomly assigned to receive upadacitinib (15 or 30 mg) or adalimumab.
Disclosures: This study was funded by AbbVie. Six authors declared being current or former employees or stockholders of AbbVie. The other authors reported ties with several sources, including AbbVie.
Source: McInnes IB et al. Efficacy and safety of upadacitinib in patients with psoriatic arthritis: 2-year results from the phase 3 SELECT-PsA 1 study. Rheumatol Ther. 2022 (Oct 15). Doi: 10.1007/s40744-022-00499-w
Key clinical point: Compared with adalimumab, both 15 mg and 30 mg upadacitinib showed similar or better efficacy through 2 years and a similar safety profile in patients with psoriatic arthritis (PsA).
Major finding: At week 104, a similar proportion of patients receiving 15/30 mg upadacitinib vs adalimumab achieved ≥20% improvement in the American College of Rheumatology criteria (69.0%/69.5% vs 63.4%), whereas significantly more patients receiving 30 mg upadacitinib vs adalimumab achieved minimal disease activity (45.9% vs 37.8%; P < .05). The safety profiles of upadacitinib and adalimumab were comparable.
Study details: Findings are from an exploratory analysis of the SELECT-PsA 1 study including 1704 patients with active PsA and inadequate response/intolerance to ≥1 non-biologic disease-modifying antirheumatic drug who were randomly assigned to receive upadacitinib (15 or 30 mg) or adalimumab.
Disclosures: This study was funded by AbbVie. Six authors declared being current or former employees or stockholders of AbbVie. The other authors reported ties with several sources, including AbbVie.
Source: McInnes IB et al. Efficacy and safety of upadacitinib in patients with psoriatic arthritis: 2-year results from the phase 3 SELECT-PsA 1 study. Rheumatol Ther. 2022 (Oct 15). Doi: 10.1007/s40744-022-00499-w
Increased risk for preeclampsia in women with PsA
Key clinical point: Singleton pregnant women with psoriatic arthritis (PsA) had a significantly higher risk for preeclampsia than matched control pregnant women without PsA.
Major finding: Compared with control women, the risk for preeclampsia was much higher in women with PsA (adjusted odds ratio [aOR] 1.85; 95% CI 1.10-3.12), with the risk being primarily driven by the receipt of monotherapy for PsA before pregnancy (aOR 2.72; 95% CI 1.44-5.13).
Study details: Findings are from an analysis of a register-based cohort study including singleton pregnant women with rheumatoid arthritis (n = 1739), axial spondyloarthritis (n = 819), and PsA (n = 489) who were matched with 17,390, 8190, and 4890 control pregnant women, respectively.
Disclosures: This study was supported by NordForsk and other sources. Some authors declared serving on speakers’ bureaus, receiving research grants, or receiving consulting fees from several sources.
Source: Secher AEP et al. Risk of pre-eclampsia and impact of disease activity and antirheumatic treatment in women with rheumatoid arthritis, axial spondylarthritis and psoriatic arthritis: A collaborative matched cohort study from Sweden and Denmark. RMD Open. 2022;8:e002445 (Nov 3). Doi: 10.1136/rmdopen-2022-002445
Key clinical point: Singleton pregnant women with psoriatic arthritis (PsA) had a significantly higher risk for preeclampsia than matched control pregnant women without PsA.
Major finding: Compared with control women, the risk for preeclampsia was much higher in women with PsA (adjusted odds ratio [aOR] 1.85; 95% CI 1.10-3.12), with the risk being primarily driven by the receipt of monotherapy for PsA before pregnancy (aOR 2.72; 95% CI 1.44-5.13).
Study details: Findings are from an analysis of a register-based cohort study including singleton pregnant women with rheumatoid arthritis (n = 1739), axial spondyloarthritis (n = 819), and PsA (n = 489) who were matched with 17,390, 8190, and 4890 control pregnant women, respectively.
Disclosures: This study was supported by NordForsk and other sources. Some authors declared serving on speakers’ bureaus, receiving research grants, or receiving consulting fees from several sources.
Source: Secher AEP et al. Risk of pre-eclampsia and impact of disease activity and antirheumatic treatment in women with rheumatoid arthritis, axial spondylarthritis and psoriatic arthritis: A collaborative matched cohort study from Sweden and Denmark. RMD Open. 2022;8:e002445 (Nov 3). Doi: 10.1136/rmdopen-2022-002445
Key clinical point: Singleton pregnant women with psoriatic arthritis (PsA) had a significantly higher risk for preeclampsia than matched control pregnant women without PsA.
Major finding: Compared with control women, the risk for preeclampsia was much higher in women with PsA (adjusted odds ratio [aOR] 1.85; 95% CI 1.10-3.12), with the risk being primarily driven by the receipt of monotherapy for PsA before pregnancy (aOR 2.72; 95% CI 1.44-5.13).
Study details: Findings are from an analysis of a register-based cohort study including singleton pregnant women with rheumatoid arthritis (n = 1739), axial spondyloarthritis (n = 819), and PsA (n = 489) who were matched with 17,390, 8190, and 4890 control pregnant women, respectively.
Disclosures: This study was supported by NordForsk and other sources. Some authors declared serving on speakers’ bureaus, receiving research grants, or receiving consulting fees from several sources.
Source: Secher AEP et al. Risk of pre-eclampsia and impact of disease activity and antirheumatic treatment in women with rheumatoid arthritis, axial spondylarthritis and psoriatic arthritis: A collaborative matched cohort study from Sweden and Denmark. RMD Open. 2022;8:e002445 (Nov 3). Doi: 10.1136/rmdopen-2022-002445
Long-term efficacy and safety of risankizumab in PsA patients with inadequate response to csDMARD
Key clinical point: Risankizumab demonstrated long-term (52 weeks) efficacy in reducing disease severity and showed a consistent safety profile in patients with psoriatic arthritis (PsA) and previous inadequate response or intolerance to ≥1 conventional synthetic disease-modifying antirheumatic drug (csDMARD-IR).
Major finding: Among patients who received risankizumab continuously, those achieving ≥20% improvement in American College of Rheumatology (ACR20) response increased from 57.3% at week 24 to 70.0% at week 52; meanwhile, 63.0% of patients who switched from placebo to risankizumab achieved ACR20 at week 52. No new safety signals were identified.
Study details: Findings are from the ongoing phase 3 KEEPsAKE 1 study including 964 patients with active PsA who were csDMARD-IR and were randomly assigned to receive risankizumab or placebo for 24 weeks, of which 940 patients were eligible to receive open-label risankizumab through 208 weeks.
Disclosures: This study was funded by AbbVie. Four authors declared being employees or holding stock options in AbbVie, and the other authors reported ties with several sources, including AbbVie.
Source: Kristensen LE et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 52-week results from the KEEPsAKE 1 study. Rheumatology (Oxford). 2022 (Oct 25). Doi: 10.1093/rheumatology/keac607
Key clinical point: Risankizumab demonstrated long-term (52 weeks) efficacy in reducing disease severity and showed a consistent safety profile in patients with psoriatic arthritis (PsA) and previous inadequate response or intolerance to ≥1 conventional synthetic disease-modifying antirheumatic drug (csDMARD-IR).
Major finding: Among patients who received risankizumab continuously, those achieving ≥20% improvement in American College of Rheumatology (ACR20) response increased from 57.3% at week 24 to 70.0% at week 52; meanwhile, 63.0% of patients who switched from placebo to risankizumab achieved ACR20 at week 52. No new safety signals were identified.
Study details: Findings are from the ongoing phase 3 KEEPsAKE 1 study including 964 patients with active PsA who were csDMARD-IR and were randomly assigned to receive risankizumab or placebo for 24 weeks, of which 940 patients were eligible to receive open-label risankizumab through 208 weeks.
Disclosures: This study was funded by AbbVie. Four authors declared being employees or holding stock options in AbbVie, and the other authors reported ties with several sources, including AbbVie.
Source: Kristensen LE et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 52-week results from the KEEPsAKE 1 study. Rheumatology (Oxford). 2022 (Oct 25). Doi: 10.1093/rheumatology/keac607
Key clinical point: Risankizumab demonstrated long-term (52 weeks) efficacy in reducing disease severity and showed a consistent safety profile in patients with psoriatic arthritis (PsA) and previous inadequate response or intolerance to ≥1 conventional synthetic disease-modifying antirheumatic drug (csDMARD-IR).
Major finding: Among patients who received risankizumab continuously, those achieving ≥20% improvement in American College of Rheumatology (ACR20) response increased from 57.3% at week 24 to 70.0% at week 52; meanwhile, 63.0% of patients who switched from placebo to risankizumab achieved ACR20 at week 52. No new safety signals were identified.
Study details: Findings are from the ongoing phase 3 KEEPsAKE 1 study including 964 patients with active PsA who were csDMARD-IR and were randomly assigned to receive risankizumab or placebo for 24 weeks, of which 940 patients were eligible to receive open-label risankizumab through 208 weeks.
Disclosures: This study was funded by AbbVie. Four authors declared being employees or holding stock options in AbbVie, and the other authors reported ties with several sources, including AbbVie.
Source: Kristensen LE et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 52-week results from the KEEPsAKE 1 study. Rheumatology (Oxford). 2022 (Oct 25). Doi: 10.1093/rheumatology/keac607
New ACR vaccination guideline: Take your best shot
PHILADELPHIA – The new American College of Rheumatology Guideline for Vaccinations in Patients with Rheumatic and Musculoskeletal Diseases (RMDs) emphasizes that both adult and pediatric patients should receive recommended vaccinations whenever possible.
But the guideline, currently in press, also offers recommendations about whether and when to withhold vaccines from patients with RMDs, such as avoiding the use of live attenuated virus vaccines in patients who are on immunosuppressive drug regimens, such as conventional synthetic disease-modifying antirheumatic drugs (DMARDs), biologic DMARDs, or targeted synthetic DMARDs.
The new consensus guideline was formulated with the understanding that patients with RMDs are at increased risk for vaccine-preventable infections and more serious complications from infections, compared with the general population.
However, the guideline also acknowledges that the immunogenicity and safety of vaccines may differ among patients with RMDs, and that, depending on the patient age and disease state, individuals may benefit from modified vaccine indications, schedules, or modified medication schedules, said guideline panel member Anne Bass, MD, a rheumatologist at Hospital for Special Surgery and a professor of clinical medicine at Weill Cornell Medicine in New York, who presented the guideline with other panel members in a session outlining the recommendations at the annual meeting of the ACR.
“In addition, vaccination recommendations – since much of it relates to medications – really applies across diseases, and so the ACR felt that, rather than having vaccine recommendations tacked onto the end of treatment guidelines for each individual disease, that the topic should be discussed or tackled as a whole,” she said.
The guideline does not cover vaccinations in patients taking nonsteroidal anti-inflammatory drugs because this class of agents has minimal or no impact on antibody responses to vaccines. The guideline also does not address vaccinations against COVID-19 infections since the rapidly changing formulations would make the recommendations obsolete before they were even published, and because the U.S. Centers for Disease Control and Prevention provides up-to-date guidance on COVID-19 vaccinations in patients with compromised immunity, she said.
Guiding principles
The overarching principles of the guideline are to give indicated vaccines to patients with RMD whenever possible and that any decision to hold medications before or after vaccination consider the dosage used, RMD disease activity, and the patient’s risk for vaccine-preventable infection.
The guideline also states that “shared decision-making with patients is a key component of any vaccination strategy.”
Panel member Clifton O. Bingham III, MD, professor of medicine at Johns Hopkins University in Baltimore, outlined expanded indications for vaccinations against influenza, pneumococcal infections, varicella zoster virus (VZV) and human papillomavirus (HPV).
Influenza
The guideline conditionally recommends that patients with RMD aged 65 years and older and adults older than age 18 years who are on immunosuppressive medications should receive either high-dose or adjuvanted influenza vaccination rather than regular-dose vaccines.
“It’s recognized that the high-dose or adjuvanted vaccinations may be unavailable for patients when they’re seen in your practice,” Dr. Bingham said,” and we came out with two additional statements within the guidelines that said that any flu vaccine is recommended over no flu vaccinations, because we do know that responses are elicited, and a flu vaccination today is preferred over a flu vaccination delay.”
Pneumococcal vaccination
The panelists strongly recommended that patients with RMD younger than age 65 years who are on immunosuppressive medication receive pneumococcal vaccinations.
The ACR guideline is in sync with those issued by the CDC’s Advisory Committee on Immunization Practices, Dr. Bingham said. He urged audience members to visit a CDC-ACIP web page for more information on who should receive pneumococcal vaccination and when.
Recombinant varicella zoster
The recommendations strongly support that patients aged 18 years and over who are on immunosuppressive therapies should receive the recombinant VZV vaccine (Shingrix).
HPV
A less robust, conditional recommendation is for patients with RMDs who are between the ages of 26 and 45 years and on immunosuppressive medications to receive the HPV vaccine (if they have not already received the vaccine).
Non-live attenuated vaccines
Kevin Winthrop, MD, MPH, professor of infectious diseases and public health at Oregon Health & Science University, Portland, summarized the recommendations for managing immunosuppressive therapies in patients scheduled to receive vaccinations using killed or nonactive antigens.
“In influenza season, don’t pass up the opportunity to vaccinate,” he said, adding, “if you can wait on rituximab dosing, do it, and if you can’t, go ahead and vaccinate.”
The guidelines also recommend a 2-week methotrexate hold at the time of influenza vaccination; other DMARD dosing changes are likely not necessary at the time of vaccination, “but this is an area of fervent study, and I think in a year or two we’ll have more experimental hold data with regard to other DMARDs,” Dr. Winthrop said.
For other nonlive attenuated vaccinations, recommendations are similar to those for influenza, except with more flexible timing because these vaccinations are not seasonal. When and how to hold methotrexate is still up in the air, he said.
Additionally, it’s recommended that vaccinations be delayed in patients on high-dose prednisone until the drug is tapered to below 20 mg per day, and ideally to less than 10 mg per day, he said.
Live-attenuated vaccines
The guideline conditionally recommends deferring live-attenuated vaccines in patients on immunosuppressive drugs. It also recommends holding these medications “for an appropriate period before” vaccination and for 4 weeks afterward.
“Although the evidence around conventional synthetic DMARDs and TNF inhibitors is reassuring in terms of their safety at the time of live attenuated vaccines, as you can see the number of studies is quite small, and so the voting panel conditionally recommend against administering live-attenuated virus vaccines to patients who are on conventional synthetics, biologic, or targeted DMARDs,” Dr. Bass said.
In utero exposures
Most women with RMD who have recently given birth will consult their general pediatricians rather than rheumatologists for infant vaccinations, but pediatricians may not be aware of the affect that in utero exposures to biologic DMARDs can have on vaccine safety and immunogenicity in infants, Dr, Bass said.
“It’s important that you, as a provider, give your recommendations regarding infant rotavirus vaccination after in utero exposure to the pregnant rheumatic disease patient prior to delivery, and let that patient know that this is something that they should share with their pediatrician to be,” she advised audience members.
Getting the message out
In an interview, session moderator and guidelines panelist Lisa F. Imundo, MD, director of the center for adolescent rheumatology at Columbia University in New York, noted that rheumatologists don’t usually have the full schedule of pediatric vaccinations in stock and often leave the decisions about what to give – and when – to general practitioners.
“Pediatric rheumatologists sometimes will give patients flu vaccinations because they’re a high-risk population of patients, and we want to make sure that they’re getting it in a timely manner,” she said.
In addition, because pneumococcal polysaccharide vaccines are not indicated in the general pediatric population, children on biologic DMARDs who have completed their standard series of pneumococcal conjugate vaccines (PCV13 or PVC15) are recommended to get a 23-valent pneumococcal polysaccharide vaccine, Dr. Imundo said.
She also noted that communication between pediatric rheumatologists and general practitioners about vaccine recommendations can be challenging.
“It’s a huge issue, figuring out how we’re going to communicate all of this information to our pediatric colleagues,” she said. “With individual patients, we may sometimes remind doctors, especially with our younger patients who haven’t gotten their live vaccines, that they really shouldn’t get live vaccines until they’re off medication or until we arrange holding medication for some period of time.”
She said that ACR vaccine committee members are working with infectious disease specialists and guideline developers for the American Academy of Pediatrics to ensure guidelines include the most important vaccination recommendations for pediatric patients with RMDs.
The development process for the guidelines was supported by the ACR. Dr. Bass reported no relevant disclosures, Dr. Bingham disclosed consulting activities, grant/research support, and royalties from various corporate entities. Dr. Winthrop disclosed consulting activities for and research funding from various companies. Dr. Imundo reported no relevant financial relationships.
PHILADELPHIA – The new American College of Rheumatology Guideline for Vaccinations in Patients with Rheumatic and Musculoskeletal Diseases (RMDs) emphasizes that both adult and pediatric patients should receive recommended vaccinations whenever possible.
But the guideline, currently in press, also offers recommendations about whether and when to withhold vaccines from patients with RMDs, such as avoiding the use of live attenuated virus vaccines in patients who are on immunosuppressive drug regimens, such as conventional synthetic disease-modifying antirheumatic drugs (DMARDs), biologic DMARDs, or targeted synthetic DMARDs.
The new consensus guideline was formulated with the understanding that patients with RMDs are at increased risk for vaccine-preventable infections and more serious complications from infections, compared with the general population.
However, the guideline also acknowledges that the immunogenicity and safety of vaccines may differ among patients with RMDs, and that, depending on the patient age and disease state, individuals may benefit from modified vaccine indications, schedules, or modified medication schedules, said guideline panel member Anne Bass, MD, a rheumatologist at Hospital for Special Surgery and a professor of clinical medicine at Weill Cornell Medicine in New York, who presented the guideline with other panel members in a session outlining the recommendations at the annual meeting of the ACR.
“In addition, vaccination recommendations – since much of it relates to medications – really applies across diseases, and so the ACR felt that, rather than having vaccine recommendations tacked onto the end of treatment guidelines for each individual disease, that the topic should be discussed or tackled as a whole,” she said.
The guideline does not cover vaccinations in patients taking nonsteroidal anti-inflammatory drugs because this class of agents has minimal or no impact on antibody responses to vaccines. The guideline also does not address vaccinations against COVID-19 infections since the rapidly changing formulations would make the recommendations obsolete before they were even published, and because the U.S. Centers for Disease Control and Prevention provides up-to-date guidance on COVID-19 vaccinations in patients with compromised immunity, she said.
Guiding principles
The overarching principles of the guideline are to give indicated vaccines to patients with RMD whenever possible and that any decision to hold medications before or after vaccination consider the dosage used, RMD disease activity, and the patient’s risk for vaccine-preventable infection.
The guideline also states that “shared decision-making with patients is a key component of any vaccination strategy.”
Panel member Clifton O. Bingham III, MD, professor of medicine at Johns Hopkins University in Baltimore, outlined expanded indications for vaccinations against influenza, pneumococcal infections, varicella zoster virus (VZV) and human papillomavirus (HPV).
Influenza
The guideline conditionally recommends that patients with RMD aged 65 years and older and adults older than age 18 years who are on immunosuppressive medications should receive either high-dose or adjuvanted influenza vaccination rather than regular-dose vaccines.
“It’s recognized that the high-dose or adjuvanted vaccinations may be unavailable for patients when they’re seen in your practice,” Dr. Bingham said,” and we came out with two additional statements within the guidelines that said that any flu vaccine is recommended over no flu vaccinations, because we do know that responses are elicited, and a flu vaccination today is preferred over a flu vaccination delay.”
Pneumococcal vaccination
The panelists strongly recommended that patients with RMD younger than age 65 years who are on immunosuppressive medication receive pneumococcal vaccinations.
The ACR guideline is in sync with those issued by the CDC’s Advisory Committee on Immunization Practices, Dr. Bingham said. He urged audience members to visit a CDC-ACIP web page for more information on who should receive pneumococcal vaccination and when.
Recombinant varicella zoster
The recommendations strongly support that patients aged 18 years and over who are on immunosuppressive therapies should receive the recombinant VZV vaccine (Shingrix).
HPV
A less robust, conditional recommendation is for patients with RMDs who are between the ages of 26 and 45 years and on immunosuppressive medications to receive the HPV vaccine (if they have not already received the vaccine).
Non-live attenuated vaccines
Kevin Winthrop, MD, MPH, professor of infectious diseases and public health at Oregon Health & Science University, Portland, summarized the recommendations for managing immunosuppressive therapies in patients scheduled to receive vaccinations using killed or nonactive antigens.
“In influenza season, don’t pass up the opportunity to vaccinate,” he said, adding, “if you can wait on rituximab dosing, do it, and if you can’t, go ahead and vaccinate.”
The guidelines also recommend a 2-week methotrexate hold at the time of influenza vaccination; other DMARD dosing changes are likely not necessary at the time of vaccination, “but this is an area of fervent study, and I think in a year or two we’ll have more experimental hold data with regard to other DMARDs,” Dr. Winthrop said.
For other nonlive attenuated vaccinations, recommendations are similar to those for influenza, except with more flexible timing because these vaccinations are not seasonal. When and how to hold methotrexate is still up in the air, he said.
Additionally, it’s recommended that vaccinations be delayed in patients on high-dose prednisone until the drug is tapered to below 20 mg per day, and ideally to less than 10 mg per day, he said.
Live-attenuated vaccines
The guideline conditionally recommends deferring live-attenuated vaccines in patients on immunosuppressive drugs. It also recommends holding these medications “for an appropriate period before” vaccination and for 4 weeks afterward.
“Although the evidence around conventional synthetic DMARDs and TNF inhibitors is reassuring in terms of their safety at the time of live attenuated vaccines, as you can see the number of studies is quite small, and so the voting panel conditionally recommend against administering live-attenuated virus vaccines to patients who are on conventional synthetics, biologic, or targeted DMARDs,” Dr. Bass said.
In utero exposures
Most women with RMD who have recently given birth will consult their general pediatricians rather than rheumatologists for infant vaccinations, but pediatricians may not be aware of the affect that in utero exposures to biologic DMARDs can have on vaccine safety and immunogenicity in infants, Dr, Bass said.
“It’s important that you, as a provider, give your recommendations regarding infant rotavirus vaccination after in utero exposure to the pregnant rheumatic disease patient prior to delivery, and let that patient know that this is something that they should share with their pediatrician to be,” she advised audience members.
Getting the message out
In an interview, session moderator and guidelines panelist Lisa F. Imundo, MD, director of the center for adolescent rheumatology at Columbia University in New York, noted that rheumatologists don’t usually have the full schedule of pediatric vaccinations in stock and often leave the decisions about what to give – and when – to general practitioners.
“Pediatric rheumatologists sometimes will give patients flu vaccinations because they’re a high-risk population of patients, and we want to make sure that they’re getting it in a timely manner,” she said.
In addition, because pneumococcal polysaccharide vaccines are not indicated in the general pediatric population, children on biologic DMARDs who have completed their standard series of pneumococcal conjugate vaccines (PCV13 or PVC15) are recommended to get a 23-valent pneumococcal polysaccharide vaccine, Dr. Imundo said.
She also noted that communication between pediatric rheumatologists and general practitioners about vaccine recommendations can be challenging.
“It’s a huge issue, figuring out how we’re going to communicate all of this information to our pediatric colleagues,” she said. “With individual patients, we may sometimes remind doctors, especially with our younger patients who haven’t gotten their live vaccines, that they really shouldn’t get live vaccines until they’re off medication or until we arrange holding medication for some period of time.”
She said that ACR vaccine committee members are working with infectious disease specialists and guideline developers for the American Academy of Pediatrics to ensure guidelines include the most important vaccination recommendations for pediatric patients with RMDs.
The development process for the guidelines was supported by the ACR. Dr. Bass reported no relevant disclosures, Dr. Bingham disclosed consulting activities, grant/research support, and royalties from various corporate entities. Dr. Winthrop disclosed consulting activities for and research funding from various companies. Dr. Imundo reported no relevant financial relationships.
PHILADELPHIA – The new American College of Rheumatology Guideline for Vaccinations in Patients with Rheumatic and Musculoskeletal Diseases (RMDs) emphasizes that both adult and pediatric patients should receive recommended vaccinations whenever possible.
But the guideline, currently in press, also offers recommendations about whether and when to withhold vaccines from patients with RMDs, such as avoiding the use of live attenuated virus vaccines in patients who are on immunosuppressive drug regimens, such as conventional synthetic disease-modifying antirheumatic drugs (DMARDs), biologic DMARDs, or targeted synthetic DMARDs.
The new consensus guideline was formulated with the understanding that patients with RMDs are at increased risk for vaccine-preventable infections and more serious complications from infections, compared with the general population.
However, the guideline also acknowledges that the immunogenicity and safety of vaccines may differ among patients with RMDs, and that, depending on the patient age and disease state, individuals may benefit from modified vaccine indications, schedules, or modified medication schedules, said guideline panel member Anne Bass, MD, a rheumatologist at Hospital for Special Surgery and a professor of clinical medicine at Weill Cornell Medicine in New York, who presented the guideline with other panel members in a session outlining the recommendations at the annual meeting of the ACR.
“In addition, vaccination recommendations – since much of it relates to medications – really applies across diseases, and so the ACR felt that, rather than having vaccine recommendations tacked onto the end of treatment guidelines for each individual disease, that the topic should be discussed or tackled as a whole,” she said.
The guideline does not cover vaccinations in patients taking nonsteroidal anti-inflammatory drugs because this class of agents has minimal or no impact on antibody responses to vaccines. The guideline also does not address vaccinations against COVID-19 infections since the rapidly changing formulations would make the recommendations obsolete before they were even published, and because the U.S. Centers for Disease Control and Prevention provides up-to-date guidance on COVID-19 vaccinations in patients with compromised immunity, she said.
Guiding principles
The overarching principles of the guideline are to give indicated vaccines to patients with RMD whenever possible and that any decision to hold medications before or after vaccination consider the dosage used, RMD disease activity, and the patient’s risk for vaccine-preventable infection.
The guideline also states that “shared decision-making with patients is a key component of any vaccination strategy.”
Panel member Clifton O. Bingham III, MD, professor of medicine at Johns Hopkins University in Baltimore, outlined expanded indications for vaccinations against influenza, pneumococcal infections, varicella zoster virus (VZV) and human papillomavirus (HPV).
Influenza
The guideline conditionally recommends that patients with RMD aged 65 years and older and adults older than age 18 years who are on immunosuppressive medications should receive either high-dose or adjuvanted influenza vaccination rather than regular-dose vaccines.
“It’s recognized that the high-dose or adjuvanted vaccinations may be unavailable for patients when they’re seen in your practice,” Dr. Bingham said,” and we came out with two additional statements within the guidelines that said that any flu vaccine is recommended over no flu vaccinations, because we do know that responses are elicited, and a flu vaccination today is preferred over a flu vaccination delay.”
Pneumococcal vaccination
The panelists strongly recommended that patients with RMD younger than age 65 years who are on immunosuppressive medication receive pneumococcal vaccinations.
The ACR guideline is in sync with those issued by the CDC’s Advisory Committee on Immunization Practices, Dr. Bingham said. He urged audience members to visit a CDC-ACIP web page for more information on who should receive pneumococcal vaccination and when.
Recombinant varicella zoster
The recommendations strongly support that patients aged 18 years and over who are on immunosuppressive therapies should receive the recombinant VZV vaccine (Shingrix).
HPV
A less robust, conditional recommendation is for patients with RMDs who are between the ages of 26 and 45 years and on immunosuppressive medications to receive the HPV vaccine (if they have not already received the vaccine).
Non-live attenuated vaccines
Kevin Winthrop, MD, MPH, professor of infectious diseases and public health at Oregon Health & Science University, Portland, summarized the recommendations for managing immunosuppressive therapies in patients scheduled to receive vaccinations using killed or nonactive antigens.
“In influenza season, don’t pass up the opportunity to vaccinate,” he said, adding, “if you can wait on rituximab dosing, do it, and if you can’t, go ahead and vaccinate.”
The guidelines also recommend a 2-week methotrexate hold at the time of influenza vaccination; other DMARD dosing changes are likely not necessary at the time of vaccination, “but this is an area of fervent study, and I think in a year or two we’ll have more experimental hold data with regard to other DMARDs,” Dr. Winthrop said.
For other nonlive attenuated vaccinations, recommendations are similar to those for influenza, except with more flexible timing because these vaccinations are not seasonal. When and how to hold methotrexate is still up in the air, he said.
Additionally, it’s recommended that vaccinations be delayed in patients on high-dose prednisone until the drug is tapered to below 20 mg per day, and ideally to less than 10 mg per day, he said.
Live-attenuated vaccines
The guideline conditionally recommends deferring live-attenuated vaccines in patients on immunosuppressive drugs. It also recommends holding these medications “for an appropriate period before” vaccination and for 4 weeks afterward.
“Although the evidence around conventional synthetic DMARDs and TNF inhibitors is reassuring in terms of their safety at the time of live attenuated vaccines, as you can see the number of studies is quite small, and so the voting panel conditionally recommend against administering live-attenuated virus vaccines to patients who are on conventional synthetics, biologic, or targeted DMARDs,” Dr. Bass said.
In utero exposures
Most women with RMD who have recently given birth will consult their general pediatricians rather than rheumatologists for infant vaccinations, but pediatricians may not be aware of the affect that in utero exposures to biologic DMARDs can have on vaccine safety and immunogenicity in infants, Dr, Bass said.
“It’s important that you, as a provider, give your recommendations regarding infant rotavirus vaccination after in utero exposure to the pregnant rheumatic disease patient prior to delivery, and let that patient know that this is something that they should share with their pediatrician to be,” she advised audience members.
Getting the message out
In an interview, session moderator and guidelines panelist Lisa F. Imundo, MD, director of the center for adolescent rheumatology at Columbia University in New York, noted that rheumatologists don’t usually have the full schedule of pediatric vaccinations in stock and often leave the decisions about what to give – and when – to general practitioners.
“Pediatric rheumatologists sometimes will give patients flu vaccinations because they’re a high-risk population of patients, and we want to make sure that they’re getting it in a timely manner,” she said.
In addition, because pneumococcal polysaccharide vaccines are not indicated in the general pediatric population, children on biologic DMARDs who have completed their standard series of pneumococcal conjugate vaccines (PCV13 or PVC15) are recommended to get a 23-valent pneumococcal polysaccharide vaccine, Dr. Imundo said.
She also noted that communication between pediatric rheumatologists and general practitioners about vaccine recommendations can be challenging.
“It’s a huge issue, figuring out how we’re going to communicate all of this information to our pediatric colleagues,” she said. “With individual patients, we may sometimes remind doctors, especially with our younger patients who haven’t gotten their live vaccines, that they really shouldn’t get live vaccines until they’re off medication or until we arrange holding medication for some period of time.”
She said that ACR vaccine committee members are working with infectious disease specialists and guideline developers for the American Academy of Pediatrics to ensure guidelines include the most important vaccination recommendations for pediatric patients with RMDs.
The development process for the guidelines was supported by the ACR. Dr. Bass reported no relevant disclosures, Dr. Bingham disclosed consulting activities, grant/research support, and royalties from various corporate entities. Dr. Winthrop disclosed consulting activities for and research funding from various companies. Dr. Imundo reported no relevant financial relationships.
AT ACR 2022
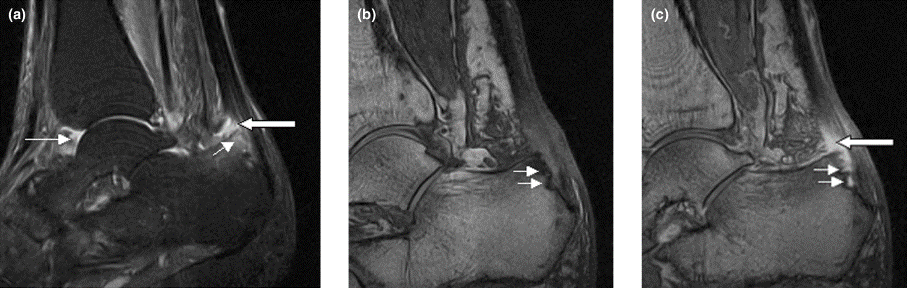
Right ankle pain and swelling
This patient's findings are consistent with a diagnosis of psoriatic enthesitis.
Enthesitis is a hallmark manifestation of psoriatic arthritis (PsA). Approximately 30% of patients with psoriasis are estimated to be affected by PsA, which belongs to the spondyloarthritis (SpA) family of inflammatory rheumatic diseases.
An enthesis is an attachment site of ligaments, tendons, and joint capsules to bone and is a key inflammatory target in SpA. It is a complex structure that dissipates biomechanical stress to preserve homeostasis. Entheses are anatomically and functionally integrated with bursa, fibrocartilage, and synovium in a synovial entheseal complex; biomechanical stress in this area may trigger inflammation. Enthesitis is an early manifestation of PsA that has been associated with radiographic peripheral/axial joint damage and severe disease, as well as reduced quality of life.
Enthesitis can be difficult to diagnose in clinical practice. Symptoms include tenderness, soreness, and pain at entheses on palpation, often without overt clinical evidence of inflammation. In contrast, dactylitis, another hallmark manifestation of PsA, can be recognized by swelling of an entire digit that is different from adjacent digits. Fibromyalgia frequently coexists with enthesitis, and it can be difficult to distinguish the two given the anatomic overlap between the tender points of fibromyalgia and many entheseal sites. Long-lasting morning stiffness and a sustained response to a course of steroids is more suggestive of enthesitis, whereas a higher number of somatoform symptoms is more suggestive of fibromyalgia.
Enthesitis is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as a hallmark of PsA. While it can be diagnosed clinically, imaging studies may be required, particularly in patients in whom symptoms may be difficult to discern. Evidence of enthesitis by conventional radiography includes bone cortex irregularities, erosions, entheseal soft tissue calcifications, and new bone formation; however, entheseal bone changes detected with conventional radiography appear relatively late in the disease process. Ultrasound is highly sensitive for assessing inflammation and can detect various features of enthesitis, such as increased thickness of tendon insertion, hypoechogenicity, erosions, enthesophytes, and subclinical enthesitis in people with PsA. MRI has the advantage of identifying perientheseal inflammation with adjacent bone marrow edema. Fat-suppressed MRI with or without gadolinium enhancement is a highly sensitive method for visualizing active enthesitis and can identify perientheseal inflammation with adjacent bone marrow edema.
Delayed treatment of PsA can result in irreversible joint damage and reduced quality of life; thus, patients with psoriasis should be closely monitored for early signs of its development, such as enthesitis. A thorough evaluation of the key clinical features of PsA (psoriasis, arthritis, enthesitis, dactylitis, and spondylitis), including evaluation of severity of each feature and impact on physical function and quality of life, is encouraged at each clinical encounter. Because patients may not understand the link between psoriasis and joint pain, specific probing questions can be helpful. Screening questionnaires to detect early signs and symptoms of PsA are available, such as the Psoriasis Epidemiology Screening Tool (PEST), Psoriatic Arthritis Screening and Evaluation (PASE) questionnaire, and Toronto Psoriatic Arthritis Screening (ToPAS) questionnaire. These and many others may be used to help dermatologists detect early signs and symptoms of PsA. Although these questionnaires all have limitations in sensitivity and specificity for the diagnosis of PsA, their use can still improve early diagnosis.
The treatment of PsA focuses on achieving the least amount of disease activity and inflammation possible; optimizing functional status, quality of life, and well-being; and preventing structural damage. Treatment decisions are based on the specific domains affected. Nonsteroidal anti-inflammatory drugs and corticosteroid injections are first-line treatments for enthesitis. Early use of tumor necrosis factor inhibitors (TNF) (adalimumab, certolizumab pegol, etanercept, infliximab, and golimumab) is recommended. Alternative biologic disease-modifying agents are indicated when these TNF inhibitors provide an inadequate response. They include ustekinumab (dual interleukin [IL]-12 and IL-23 inhibitor), secukinumab (IL-17A inhibitor), and apremilast (phosphodiesterase-4 inhibitor) and may be considered for patients with predominantly entheseal manifestations of PsA or dactylitis. Biological disease-modifying agents approved for PsA that have shown efficacy for enthesitis include ixekizumab (which targets IL-17A), abatacept (a T-cell inhibitor), guselkumab (monoclonal antibody), and ustekinumab (monoclonal antibody). Tofacitinib and upadacitinib, both oral Janus kinase inhibitors, may also be considered.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's findings are consistent with a diagnosis of psoriatic enthesitis.
Enthesitis is a hallmark manifestation of psoriatic arthritis (PsA). Approximately 30% of patients with psoriasis are estimated to be affected by PsA, which belongs to the spondyloarthritis (SpA) family of inflammatory rheumatic diseases.
An enthesis is an attachment site of ligaments, tendons, and joint capsules to bone and is a key inflammatory target in SpA. It is a complex structure that dissipates biomechanical stress to preserve homeostasis. Entheses are anatomically and functionally integrated with bursa, fibrocartilage, and synovium in a synovial entheseal complex; biomechanical stress in this area may trigger inflammation. Enthesitis is an early manifestation of PsA that has been associated with radiographic peripheral/axial joint damage and severe disease, as well as reduced quality of life.
Enthesitis can be difficult to diagnose in clinical practice. Symptoms include tenderness, soreness, and pain at entheses on palpation, often without overt clinical evidence of inflammation. In contrast, dactylitis, another hallmark manifestation of PsA, can be recognized by swelling of an entire digit that is different from adjacent digits. Fibromyalgia frequently coexists with enthesitis, and it can be difficult to distinguish the two given the anatomic overlap between the tender points of fibromyalgia and many entheseal sites. Long-lasting morning stiffness and a sustained response to a course of steroids is more suggestive of enthesitis, whereas a higher number of somatoform symptoms is more suggestive of fibromyalgia.
Enthesitis is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as a hallmark of PsA. While it can be diagnosed clinically, imaging studies may be required, particularly in patients in whom symptoms may be difficult to discern. Evidence of enthesitis by conventional radiography includes bone cortex irregularities, erosions, entheseal soft tissue calcifications, and new bone formation; however, entheseal bone changes detected with conventional radiography appear relatively late in the disease process. Ultrasound is highly sensitive for assessing inflammation and can detect various features of enthesitis, such as increased thickness of tendon insertion, hypoechogenicity, erosions, enthesophytes, and subclinical enthesitis in people with PsA. MRI has the advantage of identifying perientheseal inflammation with adjacent bone marrow edema. Fat-suppressed MRI with or without gadolinium enhancement is a highly sensitive method for visualizing active enthesitis and can identify perientheseal inflammation with adjacent bone marrow edema.
Delayed treatment of PsA can result in irreversible joint damage and reduced quality of life; thus, patients with psoriasis should be closely monitored for early signs of its development, such as enthesitis. A thorough evaluation of the key clinical features of PsA (psoriasis, arthritis, enthesitis, dactylitis, and spondylitis), including evaluation of severity of each feature and impact on physical function and quality of life, is encouraged at each clinical encounter. Because patients may not understand the link between psoriasis and joint pain, specific probing questions can be helpful. Screening questionnaires to detect early signs and symptoms of PsA are available, such as the Psoriasis Epidemiology Screening Tool (PEST), Psoriatic Arthritis Screening and Evaluation (PASE) questionnaire, and Toronto Psoriatic Arthritis Screening (ToPAS) questionnaire. These and many others may be used to help dermatologists detect early signs and symptoms of PsA. Although these questionnaires all have limitations in sensitivity and specificity for the diagnosis of PsA, their use can still improve early diagnosis.
The treatment of PsA focuses on achieving the least amount of disease activity and inflammation possible; optimizing functional status, quality of life, and well-being; and preventing structural damage. Treatment decisions are based on the specific domains affected. Nonsteroidal anti-inflammatory drugs and corticosteroid injections are first-line treatments for enthesitis. Early use of tumor necrosis factor inhibitors (TNF) (adalimumab, certolizumab pegol, etanercept, infliximab, and golimumab) is recommended. Alternative biologic disease-modifying agents are indicated when these TNF inhibitors provide an inadequate response. They include ustekinumab (dual interleukin [IL]-12 and IL-23 inhibitor), secukinumab (IL-17A inhibitor), and apremilast (phosphodiesterase-4 inhibitor) and may be considered for patients with predominantly entheseal manifestations of PsA or dactylitis. Biological disease-modifying agents approved for PsA that have shown efficacy for enthesitis include ixekizumab (which targets IL-17A), abatacept (a T-cell inhibitor), guselkumab (monoclonal antibody), and ustekinumab (monoclonal antibody). Tofacitinib and upadacitinib, both oral Janus kinase inhibitors, may also be considered.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's findings are consistent with a diagnosis of psoriatic enthesitis.
Enthesitis is a hallmark manifestation of psoriatic arthritis (PsA). Approximately 30% of patients with psoriasis are estimated to be affected by PsA, which belongs to the spondyloarthritis (SpA) family of inflammatory rheumatic diseases.
An enthesis is an attachment site of ligaments, tendons, and joint capsules to bone and is a key inflammatory target in SpA. It is a complex structure that dissipates biomechanical stress to preserve homeostasis. Entheses are anatomically and functionally integrated with bursa, fibrocartilage, and synovium in a synovial entheseal complex; biomechanical stress in this area may trigger inflammation. Enthesitis is an early manifestation of PsA that has been associated with radiographic peripheral/axial joint damage and severe disease, as well as reduced quality of life.
Enthesitis can be difficult to diagnose in clinical practice. Symptoms include tenderness, soreness, and pain at entheses on palpation, often without overt clinical evidence of inflammation. In contrast, dactylitis, another hallmark manifestation of PsA, can be recognized by swelling of an entire digit that is different from adjacent digits. Fibromyalgia frequently coexists with enthesitis, and it can be difficult to distinguish the two given the anatomic overlap between the tender points of fibromyalgia and many entheseal sites. Long-lasting morning stiffness and a sustained response to a course of steroids is more suggestive of enthesitis, whereas a higher number of somatoform symptoms is more suggestive of fibromyalgia.
Enthesitis is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as a hallmark of PsA. While it can be diagnosed clinically, imaging studies may be required, particularly in patients in whom symptoms may be difficult to discern. Evidence of enthesitis by conventional radiography includes bone cortex irregularities, erosions, entheseal soft tissue calcifications, and new bone formation; however, entheseal bone changes detected with conventional radiography appear relatively late in the disease process. Ultrasound is highly sensitive for assessing inflammation and can detect various features of enthesitis, such as increased thickness of tendon insertion, hypoechogenicity, erosions, enthesophytes, and subclinical enthesitis in people with PsA. MRI has the advantage of identifying perientheseal inflammation with adjacent bone marrow edema. Fat-suppressed MRI with or without gadolinium enhancement is a highly sensitive method for visualizing active enthesitis and can identify perientheseal inflammation with adjacent bone marrow edema.
Delayed treatment of PsA can result in irreversible joint damage and reduced quality of life; thus, patients with psoriasis should be closely monitored for early signs of its development, such as enthesitis. A thorough evaluation of the key clinical features of PsA (psoriasis, arthritis, enthesitis, dactylitis, and spondylitis), including evaluation of severity of each feature and impact on physical function and quality of life, is encouraged at each clinical encounter. Because patients may not understand the link between psoriasis and joint pain, specific probing questions can be helpful. Screening questionnaires to detect early signs and symptoms of PsA are available, such as the Psoriasis Epidemiology Screening Tool (PEST), Psoriatic Arthritis Screening and Evaluation (PASE) questionnaire, and Toronto Psoriatic Arthritis Screening (ToPAS) questionnaire. These and many others may be used to help dermatologists detect early signs and symptoms of PsA. Although these questionnaires all have limitations in sensitivity and specificity for the diagnosis of PsA, their use can still improve early diagnosis.
The treatment of PsA focuses on achieving the least amount of disease activity and inflammation possible; optimizing functional status, quality of life, and well-being; and preventing structural damage. Treatment decisions are based on the specific domains affected. Nonsteroidal anti-inflammatory drugs and corticosteroid injections are first-line treatments for enthesitis. Early use of tumor necrosis factor inhibitors (TNF) (adalimumab, certolizumab pegol, etanercept, infliximab, and golimumab) is recommended. Alternative biologic disease-modifying agents are indicated when these TNF inhibitors provide an inadequate response. They include ustekinumab (dual interleukin [IL]-12 and IL-23 inhibitor), secukinumab (IL-17A inhibitor), and apremilast (phosphodiesterase-4 inhibitor) and may be considered for patients with predominantly entheseal manifestations of PsA or dactylitis. Biological disease-modifying agents approved for PsA that have shown efficacy for enthesitis include ixekizumab (which targets IL-17A), abatacept (a T-cell inhibitor), guselkumab (monoclonal antibody), and ustekinumab (monoclonal antibody). Tofacitinib and upadacitinib, both oral Janus kinase inhibitors, may also be considered.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 42-year-old woman with a 20-year history of plaque psoriasis presents with complaints of a 3-month history of pain, tenderness, and swelling in her right ankle and foot, of unknown origin. Physical examination reveals active psoriasis, with a Psoriasis Area and Severity Index (PASI) score of 6.7 and psoriatic nail dystrophy, including onycholysis, pitting, and hyperkeratosis. Tenderness and swelling are noted at the back of the heel. The patient denies any other complaints. Laboratory tests are normal, including negative rheumatoid factor and antinuclear factor. MRI reveals soft tissue and bone marrow edema below the Achilles insertion.
Retention rates high after biosimilar-to-biosimilar switch for inflammatory arthritis
PHILADELPHIA – When patients with inflammatory rheumatic diseases were switched from one biosimilar agent to another, treatment retention rates were high, investigators in Denmark reported.
The findings suggest patient-related factors rather than drug-related factors appear to determine whether patients will stay on the new drug, the researchers said.
One year after a Danish government-mandated switch from one infliximab (Remicade) biosimilar to another equally efficacious but less costly biosimilar, 83% of patients who had started therapy on a biosimilar (so-called “originator-naive” patients) stayed on the newly assigned therapy. And so did 92% of patients who had started on the original infliximab (“originator experienced”) before they were switched to one biosimilar and then another.
“In regards to potential baseline predictors, we found that treatment withdrawal was more frequent among originator-naive switchers and patients with higher baseline disease activity, especially [in] patient-reported outcomes, which may indicate that treatment-related outcomes may be more affected by patient-related rather than drug-related factors,” said lead author Hafsah Nabi, MD from the Danish biosimilar registry DANBIO and a PhD candidate at the Copenhagen Center for Arthritis Research.
Dr. Nabi reported the results in an oral abstract session at the annual meeting of the American College of Rheumatology.
Annual review of biologic agents
In Denmark, health authorities issue annual recommendations for the use of biologic agents. “And since patients receive this treatment free from the hospital, based on the tax system, the switches are made due to these cost considerations,” Dr. Nabi said in an interview.
To get the nod from Danish pharmaceutical regulators, pharmaceutical manufacturers submit drugs that have already been approved by the European Medicines Agency for consideration for treatment of specific indications, explained coauthor Merete Lund Hetland, MD, PhD, DMSc, from Rigshospitalet in Copenhagen.
“Those drugs that are then considered equally safe and effective are invited to this process where they will give their bid, and then the cheapest one will win,” she said.
The winning formulation will be able to capture about 80% of prescriptions for that indication for the coming year.
Awake at the switch
Dr. Nabi, Dr. Hetland, and colleagues studied how one such recent government-mandated switch from one biosimilar to another affected efficacy and patterns of care among patients with rheumatoid arthritis, psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).
To identify prior comorbidities, they drew data from the DANBIO registry, which is linked to patient specific but anonymous data from other comprehensive birth-to-death patient registries in Denmark.
They looked at all patients with RA, PsA, or axSpA who were switched from CT-P13 (Remsira, Inflectra) to GP1111 (Zessly) from April 1, 2019, to Feb. 1, 2020.
They identified a total of 1,605 patients, including 685 with RA, 314 with PsA, and 606 with axSpa. The median disease duration was 9 years, and 37% of all patients were in remission according to Clinical Disease Activity Index or Ankylosing Spondylitis Disease Activity Scale.
Of this group, 1,171 had started therapy on a biosimilar.
As noted above, 83% of patients who had never received original infliximab, and 92% of those who were originator experienced were still on the new biosimilar 1 year after the switch.
In a multivariate analysis controlling for demographic and clinical factors at baseline, the variables significantly associated with treatment withdrawal from the new biosimilar (GP11110) included previous Remicade exposure (hazard ratio, 0.36), methotrexate use (HR, 0.60), and patient-reported global visual analog scale (HR, 1.02).
Among all patients, disease activity was stable 6 months before and after the switch, Dr. Nabi said, although she did not show data to support it.
Patient education benefit
During the session, Jonathan Kay, MD, professor of rheumatology and chair of the division of rheumatology at the University of Massachusetts, Worcester, who was not involved the study, asked Dr. Nabi whether patients were educated about equivalent efficacy and safety of biosimilars prior to the switch. He noted that education prior to switching led to a much lower patient withdrawal rate in a similar switching study conducted in The Netherlands.
“In this study, we haven’t looked more specifically into the education and which strategies have been used prior to switching, and we also conclude in the study that there may be the presence of a nocebo effect, which can be handled by better educating the patients,” she replied.
The nocebo effect refers to the phenomenon in which a patient’s belief that a specific intervention may cause harm actually can lead to negative outcomes – in other words, the opposite of the placebo effect.
In an interview, Dr. Kay said that he is confident about the efficacy, safety, and equivalency of approved biosimilar agents.
“A biosimilar that has been reviewed and approved by a regulatory agency such as the [Food and Drug Administration or the [European Medicines Agency] should be equivalent in efficacy and comparable in safety and immunogenicity. I would be fully confident in switching from the reference product to the biosimilar,” he said.
Dr. Nabi reported that the study was partly funded by a research grant from Sandoz, the maker of GP1111. Dr. Hetland has disclosed grants from various companies, not including Sandoz. Dr. Kay disclosed consulting fees from various companies, not including Sandoz.
PHILADELPHIA – When patients with inflammatory rheumatic diseases were switched from one biosimilar agent to another, treatment retention rates were high, investigators in Denmark reported.
The findings suggest patient-related factors rather than drug-related factors appear to determine whether patients will stay on the new drug, the researchers said.
One year after a Danish government-mandated switch from one infliximab (Remicade) biosimilar to another equally efficacious but less costly biosimilar, 83% of patients who had started therapy on a biosimilar (so-called “originator-naive” patients) stayed on the newly assigned therapy. And so did 92% of patients who had started on the original infliximab (“originator experienced”) before they were switched to one biosimilar and then another.
“In regards to potential baseline predictors, we found that treatment withdrawal was more frequent among originator-naive switchers and patients with higher baseline disease activity, especially [in] patient-reported outcomes, which may indicate that treatment-related outcomes may be more affected by patient-related rather than drug-related factors,” said lead author Hafsah Nabi, MD from the Danish biosimilar registry DANBIO and a PhD candidate at the Copenhagen Center for Arthritis Research.
Dr. Nabi reported the results in an oral abstract session at the annual meeting of the American College of Rheumatology.
Annual review of biologic agents
In Denmark, health authorities issue annual recommendations for the use of biologic agents. “And since patients receive this treatment free from the hospital, based on the tax system, the switches are made due to these cost considerations,” Dr. Nabi said in an interview.
To get the nod from Danish pharmaceutical regulators, pharmaceutical manufacturers submit drugs that have already been approved by the European Medicines Agency for consideration for treatment of specific indications, explained coauthor Merete Lund Hetland, MD, PhD, DMSc, from Rigshospitalet in Copenhagen.
“Those drugs that are then considered equally safe and effective are invited to this process where they will give their bid, and then the cheapest one will win,” she said.
The winning formulation will be able to capture about 80% of prescriptions for that indication for the coming year.
Awake at the switch
Dr. Nabi, Dr. Hetland, and colleagues studied how one such recent government-mandated switch from one biosimilar to another affected efficacy and patterns of care among patients with rheumatoid arthritis, psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).
To identify prior comorbidities, they drew data from the DANBIO registry, which is linked to patient specific but anonymous data from other comprehensive birth-to-death patient registries in Denmark.
They looked at all patients with RA, PsA, or axSpA who were switched from CT-P13 (Remsira, Inflectra) to GP1111 (Zessly) from April 1, 2019, to Feb. 1, 2020.
They identified a total of 1,605 patients, including 685 with RA, 314 with PsA, and 606 with axSpa. The median disease duration was 9 years, and 37% of all patients were in remission according to Clinical Disease Activity Index or Ankylosing Spondylitis Disease Activity Scale.
Of this group, 1,171 had started therapy on a biosimilar.
As noted above, 83% of patients who had never received original infliximab, and 92% of those who were originator experienced were still on the new biosimilar 1 year after the switch.
In a multivariate analysis controlling for demographic and clinical factors at baseline, the variables significantly associated with treatment withdrawal from the new biosimilar (GP11110) included previous Remicade exposure (hazard ratio, 0.36), methotrexate use (HR, 0.60), and patient-reported global visual analog scale (HR, 1.02).
Among all patients, disease activity was stable 6 months before and after the switch, Dr. Nabi said, although she did not show data to support it.
Patient education benefit
During the session, Jonathan Kay, MD, professor of rheumatology and chair of the division of rheumatology at the University of Massachusetts, Worcester, who was not involved the study, asked Dr. Nabi whether patients were educated about equivalent efficacy and safety of biosimilars prior to the switch. He noted that education prior to switching led to a much lower patient withdrawal rate in a similar switching study conducted in The Netherlands.
“In this study, we haven’t looked more specifically into the education and which strategies have been used prior to switching, and we also conclude in the study that there may be the presence of a nocebo effect, which can be handled by better educating the patients,” she replied.
The nocebo effect refers to the phenomenon in which a patient’s belief that a specific intervention may cause harm actually can lead to negative outcomes – in other words, the opposite of the placebo effect.
In an interview, Dr. Kay said that he is confident about the efficacy, safety, and equivalency of approved biosimilar agents.
“A biosimilar that has been reviewed and approved by a regulatory agency such as the [Food and Drug Administration or the [European Medicines Agency] should be equivalent in efficacy and comparable in safety and immunogenicity. I would be fully confident in switching from the reference product to the biosimilar,” he said.
Dr. Nabi reported that the study was partly funded by a research grant from Sandoz, the maker of GP1111. Dr. Hetland has disclosed grants from various companies, not including Sandoz. Dr. Kay disclosed consulting fees from various companies, not including Sandoz.
PHILADELPHIA – When patients with inflammatory rheumatic diseases were switched from one biosimilar agent to another, treatment retention rates were high, investigators in Denmark reported.
The findings suggest patient-related factors rather than drug-related factors appear to determine whether patients will stay on the new drug, the researchers said.
One year after a Danish government-mandated switch from one infliximab (Remicade) biosimilar to another equally efficacious but less costly biosimilar, 83% of patients who had started therapy on a biosimilar (so-called “originator-naive” patients) stayed on the newly assigned therapy. And so did 92% of patients who had started on the original infliximab (“originator experienced”) before they were switched to one biosimilar and then another.
“In regards to potential baseline predictors, we found that treatment withdrawal was more frequent among originator-naive switchers and patients with higher baseline disease activity, especially [in] patient-reported outcomes, which may indicate that treatment-related outcomes may be more affected by patient-related rather than drug-related factors,” said lead author Hafsah Nabi, MD from the Danish biosimilar registry DANBIO and a PhD candidate at the Copenhagen Center for Arthritis Research.
Dr. Nabi reported the results in an oral abstract session at the annual meeting of the American College of Rheumatology.
Annual review of biologic agents
In Denmark, health authorities issue annual recommendations for the use of biologic agents. “And since patients receive this treatment free from the hospital, based on the tax system, the switches are made due to these cost considerations,” Dr. Nabi said in an interview.
To get the nod from Danish pharmaceutical regulators, pharmaceutical manufacturers submit drugs that have already been approved by the European Medicines Agency for consideration for treatment of specific indications, explained coauthor Merete Lund Hetland, MD, PhD, DMSc, from Rigshospitalet in Copenhagen.
“Those drugs that are then considered equally safe and effective are invited to this process where they will give their bid, and then the cheapest one will win,” she said.
The winning formulation will be able to capture about 80% of prescriptions for that indication for the coming year.
Awake at the switch
Dr. Nabi, Dr. Hetland, and colleagues studied how one such recent government-mandated switch from one biosimilar to another affected efficacy and patterns of care among patients with rheumatoid arthritis, psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).
To identify prior comorbidities, they drew data from the DANBIO registry, which is linked to patient specific but anonymous data from other comprehensive birth-to-death patient registries in Denmark.
They looked at all patients with RA, PsA, or axSpA who were switched from CT-P13 (Remsira, Inflectra) to GP1111 (Zessly) from April 1, 2019, to Feb. 1, 2020.
They identified a total of 1,605 patients, including 685 with RA, 314 with PsA, and 606 with axSpa. The median disease duration was 9 years, and 37% of all patients were in remission according to Clinical Disease Activity Index or Ankylosing Spondylitis Disease Activity Scale.
Of this group, 1,171 had started therapy on a biosimilar.
As noted above, 83% of patients who had never received original infliximab, and 92% of those who were originator experienced were still on the new biosimilar 1 year after the switch.
In a multivariate analysis controlling for demographic and clinical factors at baseline, the variables significantly associated with treatment withdrawal from the new biosimilar (GP11110) included previous Remicade exposure (hazard ratio, 0.36), methotrexate use (HR, 0.60), and patient-reported global visual analog scale (HR, 1.02).
Among all patients, disease activity was stable 6 months before and after the switch, Dr. Nabi said, although she did not show data to support it.
Patient education benefit
During the session, Jonathan Kay, MD, professor of rheumatology and chair of the division of rheumatology at the University of Massachusetts, Worcester, who was not involved the study, asked Dr. Nabi whether patients were educated about equivalent efficacy and safety of biosimilars prior to the switch. He noted that education prior to switching led to a much lower patient withdrawal rate in a similar switching study conducted in The Netherlands.
“In this study, we haven’t looked more specifically into the education and which strategies have been used prior to switching, and we also conclude in the study that there may be the presence of a nocebo effect, which can be handled by better educating the patients,” she replied.
The nocebo effect refers to the phenomenon in which a patient’s belief that a specific intervention may cause harm actually can lead to negative outcomes – in other words, the opposite of the placebo effect.
In an interview, Dr. Kay said that he is confident about the efficacy, safety, and equivalency of approved biosimilar agents.
“A biosimilar that has been reviewed and approved by a regulatory agency such as the [Food and Drug Administration or the [European Medicines Agency] should be equivalent in efficacy and comparable in safety and immunogenicity. I would be fully confident in switching from the reference product to the biosimilar,” he said.
Dr. Nabi reported that the study was partly funded by a research grant from Sandoz, the maker of GP1111. Dr. Hetland has disclosed grants from various companies, not including Sandoz. Dr. Kay disclosed consulting fees from various companies, not including Sandoz.
AT ACR 2022
Practical pearls guide treatment of psoriasis in tricky areas
LAS VEGAS – With the right regimen, a majority of patients with psoriasis can achieve at least a Psoriasis Area and Severity Index (PASI) 75 score, Jennifer Soung, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
The array of treatment options includes mainstay topicals, new nonsteroidals, traditional oral systemics, new oral systemics, biologics, and light therapy, said Dr. Soung, director of clinical research at Harbor-UCLA Medical Center, Santa Ana, Calif. However, she said.
For these areas, make sure the diagnosis of psoriasis is correct, to avoid wasting time on the wrong course of treatment, Dr. Soung emphasized.
Scalp strategies
The scalp is often the first area of the body affected by psoriasis, and patients with severe scalp psoriasis may have minimal plaques on the body, Dr. Soung said. However, a differential diagnosis should include seborrheic dermatitis, she noted.
For most cases of scalp psoriasis, “start with localized topical treatment,” such as vitamin D and corticosteroid combination therapy, or excimer laser, Dr. Soung advised.
Systemic treatments with demonstrated effectiveness on scalp psoriasis in post hoc analyses of patients with moderate to severe plaque psoriasis include adalimumab, etanercept, ixekizumab, and secukinumab. Studies specifically focused on treatment of scalp psoriasis have shown success with secukinumab and apremilast, she noted.
Roflumilast foam, 0.3%, is in development and is an emerging option for scalp psoriasis. (A cream formulation of roflumilast, a topical phosphodiesterase 4 inhibitor, was approved for treatment of plaque psoriasis in July 2022.) A phase 2b study of roflumilast foam showed that approximately one-third of patients with scalp psoriasis achieved a status of clear based on scalp-investigator global assessment, compared with approximately 3% of those on vehicle, and similar results were seen in a recently completed phase 3 trial for scalp and body psoriasis, she added.
Facial psoriasis
Patients with facial psoriasis tend to be younger, and they may have more severe disease overall, Dr. Soung said. Given the sensitivity of facial skin, “it is nice to have a nonsteroidal option,” she noted. Current novel nonsteroidal therapies include a cream formulation of tapinarof, an aryl hydrocarbon receptor agonist, which was approved earlier this year for plaque psoriasis in adults, and roflumilast cream. Vitamin D and topical calcineurin inhibitors are options as well, she said.
Intertriginous tricks
Intertriginous (inverse) psoriasis is distinct from other areas in that the plaques are usually smooth and well-demarcated, with little or no scaling, Dr. Soung said. Erosions or signs of maceration may be present. The prevalence of inverse psoriasis is approximately 30%, but the prevalence on external genitalia is 80%, she noted. For inverse psoriasis, topical corticosteroids can result in adverse events such as poor wound healing and skin fragility, and some patients resist the idea of a steroid and don’t adhere to the medication, she added. Dr. Soung recommended topical corticosteroids for the short term, and topical calcineurin inhibitors or calcipotriol for the long term.
New topical options for inverse psoriasis include tapinarof and roflumilast, Dr. Soung said. For tapinarof, the phase 3 PSOARING program included assessment of tolerability in sensitive skin areas and found little to no irritation. Similarly, treatment with roflumilast cream was effective and well tolerated by patients with intertriginous plaque psoriasis in the DERMIS-1 and DERMIS-2 studies, she said.
Genital psoriasis
Ask patients with psoriasis about genital psoriasis, because they often are too embarrassed to provide that information on their own, said Dr. Soung. In fact, 63% of patients with psoriasis report ever experiencing genital psoriasis, but it often goes undiagnosed and undertreated, which has a significant impact on patient quality of life and sexual health.
A differential diagnosis of genital psoriasis should include dermatitis, tinea or candidiasis, and even squamous cell carcinoma, she noted. Other considerations include fixed drug eruption, lichen nitidus, lichen sclerosus, and scabies.
Dr. Soung’s first line of treatment for genital psoriasis is low-potency topical corticosteroids for 2-4 weeks. If long-term topical therapy is needed, alternatives include calcineurin inhibitors and vitamin D analogs, she said. The new topicals roflumilast and tapinarof are options as well, she said.
For those patients with severe and extensive genital psoriasis, consider systemic therapy, possibly with ixekizumab or secukinumab, she added. Patients with moderate to severe genital psoriasis treated with apremilast have shown improvement at week 16, in an ongoing clinical trial, she noted.
Palmoplantar involvement
For patients with palmar plantar psoriasis, “don’t underestimate the impact on quality of life,” said Dr. Soung. Approximately 12%-16% of patients with psoriasis report palmoplantar involvement, she noted.
Palmoplantar psoriasis can be stubborn, and many patients will need combination therapy with topicals and systemics, she said. “I am very curious about how well our new topical nonsteroidals will work in these areas,” she added.
Dr. Soung starts patients with palmoplantar psoriasis with a “potent to super-potent” twice daily topical corticosteroid, with or without occlusion. Her first-line systemic therapy is acitretin, 10-50 mg daily. However, keep in mind that acitretin is contraindicated in pregnancy, and also may cause side effects including cheilitis, alopecia, and peeling skin, she cautioned.
During the question and answer session, Dr. Soung was asked whether she routinely biopsies patients with palmoplantar psoriasis. “Not always,” was her answer. Instead, she looks for clues elsewhere on the body to confirm the diagnosis.
Nail know-how
Approximately 23%-27% of patients with psoriasis experience nail involvement, said Dr. Soung. Nail psoriasis can appear on the nail plate as pitting, onycholysis, or subungual hyperkeratosis, or in the nail bed as splinter hemorrhages or oil spots, she said.
For patients with psoriasis of the nails only, Dr. Soung described the use of high-potency topical corticosteroids, with or without calcipotriol. In her experience, she said that intralesional steroids for nail psoriasis are torturous to patients. For patients who have failed topical therapy or have psoriasis in other areas, with or without psoriatic arthritis, she advised the use of either IL-17 antagonists (secukinumab, ixekizumab, brodalumab) or IL-23 antagonists (risankizumab, guselkumab).
Dr. Soung disclosed serving as a consultant or advisor for Arcutis, Bristol Myers Squibb Company, Dermavant, and Novartis. She also disclosed serving as a speaker or member of the speakers’ bureau for AbbVie, Amgen, Arcutis, Bristol Myers Squibb Company, Celgene, Leo Pharma, Eli Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi, as well as research funding from AbbVie, Amgen, Arcutis, Castle Biosciences, Dermavant, KoBio, Kyowa Kirin, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – With the right regimen, a majority of patients with psoriasis can achieve at least a Psoriasis Area and Severity Index (PASI) 75 score, Jennifer Soung, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
The array of treatment options includes mainstay topicals, new nonsteroidals, traditional oral systemics, new oral systemics, biologics, and light therapy, said Dr. Soung, director of clinical research at Harbor-UCLA Medical Center, Santa Ana, Calif. However, she said.
For these areas, make sure the diagnosis of psoriasis is correct, to avoid wasting time on the wrong course of treatment, Dr. Soung emphasized.
Scalp strategies
The scalp is often the first area of the body affected by psoriasis, and patients with severe scalp psoriasis may have minimal plaques on the body, Dr. Soung said. However, a differential diagnosis should include seborrheic dermatitis, she noted.
For most cases of scalp psoriasis, “start with localized topical treatment,” such as vitamin D and corticosteroid combination therapy, or excimer laser, Dr. Soung advised.
Systemic treatments with demonstrated effectiveness on scalp psoriasis in post hoc analyses of patients with moderate to severe plaque psoriasis include adalimumab, etanercept, ixekizumab, and secukinumab. Studies specifically focused on treatment of scalp psoriasis have shown success with secukinumab and apremilast, she noted.
Roflumilast foam, 0.3%, is in development and is an emerging option for scalp psoriasis. (A cream formulation of roflumilast, a topical phosphodiesterase 4 inhibitor, was approved for treatment of plaque psoriasis in July 2022.) A phase 2b study of roflumilast foam showed that approximately one-third of patients with scalp psoriasis achieved a status of clear based on scalp-investigator global assessment, compared with approximately 3% of those on vehicle, and similar results were seen in a recently completed phase 3 trial for scalp and body psoriasis, she added.
Facial psoriasis
Patients with facial psoriasis tend to be younger, and they may have more severe disease overall, Dr. Soung said. Given the sensitivity of facial skin, “it is nice to have a nonsteroidal option,” she noted. Current novel nonsteroidal therapies include a cream formulation of tapinarof, an aryl hydrocarbon receptor agonist, which was approved earlier this year for plaque psoriasis in adults, and roflumilast cream. Vitamin D and topical calcineurin inhibitors are options as well, she said.
Intertriginous tricks
Intertriginous (inverse) psoriasis is distinct from other areas in that the plaques are usually smooth and well-demarcated, with little or no scaling, Dr. Soung said. Erosions or signs of maceration may be present. The prevalence of inverse psoriasis is approximately 30%, but the prevalence on external genitalia is 80%, she noted. For inverse psoriasis, topical corticosteroids can result in adverse events such as poor wound healing and skin fragility, and some patients resist the idea of a steroid and don’t adhere to the medication, she added. Dr. Soung recommended topical corticosteroids for the short term, and topical calcineurin inhibitors or calcipotriol for the long term.
New topical options for inverse psoriasis include tapinarof and roflumilast, Dr. Soung said. For tapinarof, the phase 3 PSOARING program included assessment of tolerability in sensitive skin areas and found little to no irritation. Similarly, treatment with roflumilast cream was effective and well tolerated by patients with intertriginous plaque psoriasis in the DERMIS-1 and DERMIS-2 studies, she said.
Genital psoriasis
Ask patients with psoriasis about genital psoriasis, because they often are too embarrassed to provide that information on their own, said Dr. Soung. In fact, 63% of patients with psoriasis report ever experiencing genital psoriasis, but it often goes undiagnosed and undertreated, which has a significant impact on patient quality of life and sexual health.
A differential diagnosis of genital psoriasis should include dermatitis, tinea or candidiasis, and even squamous cell carcinoma, she noted. Other considerations include fixed drug eruption, lichen nitidus, lichen sclerosus, and scabies.
Dr. Soung’s first line of treatment for genital psoriasis is low-potency topical corticosteroids for 2-4 weeks. If long-term topical therapy is needed, alternatives include calcineurin inhibitors and vitamin D analogs, she said. The new topicals roflumilast and tapinarof are options as well, she said.
For those patients with severe and extensive genital psoriasis, consider systemic therapy, possibly with ixekizumab or secukinumab, she added. Patients with moderate to severe genital psoriasis treated with apremilast have shown improvement at week 16, in an ongoing clinical trial, she noted.
Palmoplantar involvement
For patients with palmar plantar psoriasis, “don’t underestimate the impact on quality of life,” said Dr. Soung. Approximately 12%-16% of patients with psoriasis report palmoplantar involvement, she noted.
Palmoplantar psoriasis can be stubborn, and many patients will need combination therapy with topicals and systemics, she said. “I am very curious about how well our new topical nonsteroidals will work in these areas,” she added.
Dr. Soung starts patients with palmoplantar psoriasis with a “potent to super-potent” twice daily topical corticosteroid, with or without occlusion. Her first-line systemic therapy is acitretin, 10-50 mg daily. However, keep in mind that acitretin is contraindicated in pregnancy, and also may cause side effects including cheilitis, alopecia, and peeling skin, she cautioned.
During the question and answer session, Dr. Soung was asked whether she routinely biopsies patients with palmoplantar psoriasis. “Not always,” was her answer. Instead, she looks for clues elsewhere on the body to confirm the diagnosis.
Nail know-how
Approximately 23%-27% of patients with psoriasis experience nail involvement, said Dr. Soung. Nail psoriasis can appear on the nail plate as pitting, onycholysis, or subungual hyperkeratosis, or in the nail bed as splinter hemorrhages or oil spots, she said.
For patients with psoriasis of the nails only, Dr. Soung described the use of high-potency topical corticosteroids, with or without calcipotriol. In her experience, she said that intralesional steroids for nail psoriasis are torturous to patients. For patients who have failed topical therapy or have psoriasis in other areas, with or without psoriatic arthritis, she advised the use of either IL-17 antagonists (secukinumab, ixekizumab, brodalumab) or IL-23 antagonists (risankizumab, guselkumab).
Dr. Soung disclosed serving as a consultant or advisor for Arcutis, Bristol Myers Squibb Company, Dermavant, and Novartis. She also disclosed serving as a speaker or member of the speakers’ bureau for AbbVie, Amgen, Arcutis, Bristol Myers Squibb Company, Celgene, Leo Pharma, Eli Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi, as well as research funding from AbbVie, Amgen, Arcutis, Castle Biosciences, Dermavant, KoBio, Kyowa Kirin, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – With the right regimen, a majority of patients with psoriasis can achieve at least a Psoriasis Area and Severity Index (PASI) 75 score, Jennifer Soung, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
The array of treatment options includes mainstay topicals, new nonsteroidals, traditional oral systemics, new oral systemics, biologics, and light therapy, said Dr. Soung, director of clinical research at Harbor-UCLA Medical Center, Santa Ana, Calif. However, she said.
For these areas, make sure the diagnosis of psoriasis is correct, to avoid wasting time on the wrong course of treatment, Dr. Soung emphasized.
Scalp strategies
The scalp is often the first area of the body affected by psoriasis, and patients with severe scalp psoriasis may have minimal plaques on the body, Dr. Soung said. However, a differential diagnosis should include seborrheic dermatitis, she noted.
For most cases of scalp psoriasis, “start with localized topical treatment,” such as vitamin D and corticosteroid combination therapy, or excimer laser, Dr. Soung advised.
Systemic treatments with demonstrated effectiveness on scalp psoriasis in post hoc analyses of patients with moderate to severe plaque psoriasis include adalimumab, etanercept, ixekizumab, and secukinumab. Studies specifically focused on treatment of scalp psoriasis have shown success with secukinumab and apremilast, she noted.
Roflumilast foam, 0.3%, is in development and is an emerging option for scalp psoriasis. (A cream formulation of roflumilast, a topical phosphodiesterase 4 inhibitor, was approved for treatment of plaque psoriasis in July 2022.) A phase 2b study of roflumilast foam showed that approximately one-third of patients with scalp psoriasis achieved a status of clear based on scalp-investigator global assessment, compared with approximately 3% of those on vehicle, and similar results were seen in a recently completed phase 3 trial for scalp and body psoriasis, she added.
Facial psoriasis
Patients with facial psoriasis tend to be younger, and they may have more severe disease overall, Dr. Soung said. Given the sensitivity of facial skin, “it is nice to have a nonsteroidal option,” she noted. Current novel nonsteroidal therapies include a cream formulation of tapinarof, an aryl hydrocarbon receptor agonist, which was approved earlier this year for plaque psoriasis in adults, and roflumilast cream. Vitamin D and topical calcineurin inhibitors are options as well, she said.
Intertriginous tricks
Intertriginous (inverse) psoriasis is distinct from other areas in that the plaques are usually smooth and well-demarcated, with little or no scaling, Dr. Soung said. Erosions or signs of maceration may be present. The prevalence of inverse psoriasis is approximately 30%, but the prevalence on external genitalia is 80%, she noted. For inverse psoriasis, topical corticosteroids can result in adverse events such as poor wound healing and skin fragility, and some patients resist the idea of a steroid and don’t adhere to the medication, she added. Dr. Soung recommended topical corticosteroids for the short term, and topical calcineurin inhibitors or calcipotriol for the long term.
New topical options for inverse psoriasis include tapinarof and roflumilast, Dr. Soung said. For tapinarof, the phase 3 PSOARING program included assessment of tolerability in sensitive skin areas and found little to no irritation. Similarly, treatment with roflumilast cream was effective and well tolerated by patients with intertriginous plaque psoriasis in the DERMIS-1 and DERMIS-2 studies, she said.
Genital psoriasis
Ask patients with psoriasis about genital psoriasis, because they often are too embarrassed to provide that information on their own, said Dr. Soung. In fact, 63% of patients with psoriasis report ever experiencing genital psoriasis, but it often goes undiagnosed and undertreated, which has a significant impact on patient quality of life and sexual health.
A differential diagnosis of genital psoriasis should include dermatitis, tinea or candidiasis, and even squamous cell carcinoma, she noted. Other considerations include fixed drug eruption, lichen nitidus, lichen sclerosus, and scabies.
Dr. Soung’s first line of treatment for genital psoriasis is low-potency topical corticosteroids for 2-4 weeks. If long-term topical therapy is needed, alternatives include calcineurin inhibitors and vitamin D analogs, she said. The new topicals roflumilast and tapinarof are options as well, she said.
For those patients with severe and extensive genital psoriasis, consider systemic therapy, possibly with ixekizumab or secukinumab, she added. Patients with moderate to severe genital psoriasis treated with apremilast have shown improvement at week 16, in an ongoing clinical trial, she noted.
Palmoplantar involvement
For patients with palmar plantar psoriasis, “don’t underestimate the impact on quality of life,” said Dr. Soung. Approximately 12%-16% of patients with psoriasis report palmoplantar involvement, she noted.
Palmoplantar psoriasis can be stubborn, and many patients will need combination therapy with topicals and systemics, she said. “I am very curious about how well our new topical nonsteroidals will work in these areas,” she added.
Dr. Soung starts patients with palmoplantar psoriasis with a “potent to super-potent” twice daily topical corticosteroid, with or without occlusion. Her first-line systemic therapy is acitretin, 10-50 mg daily. However, keep in mind that acitretin is contraindicated in pregnancy, and also may cause side effects including cheilitis, alopecia, and peeling skin, she cautioned.
During the question and answer session, Dr. Soung was asked whether she routinely biopsies patients with palmoplantar psoriasis. “Not always,” was her answer. Instead, she looks for clues elsewhere on the body to confirm the diagnosis.
Nail know-how
Approximately 23%-27% of patients with psoriasis experience nail involvement, said Dr. Soung. Nail psoriasis can appear on the nail plate as pitting, onycholysis, or subungual hyperkeratosis, or in the nail bed as splinter hemorrhages or oil spots, she said.
For patients with psoriasis of the nails only, Dr. Soung described the use of high-potency topical corticosteroids, with or without calcipotriol. In her experience, she said that intralesional steroids for nail psoriasis are torturous to patients. For patients who have failed topical therapy or have psoriasis in other areas, with or without psoriatic arthritis, she advised the use of either IL-17 antagonists (secukinumab, ixekizumab, brodalumab) or IL-23 antagonists (risankizumab, guselkumab).
Dr. Soung disclosed serving as a consultant or advisor for Arcutis, Bristol Myers Squibb Company, Dermavant, and Novartis. She also disclosed serving as a speaker or member of the speakers’ bureau for AbbVie, Amgen, Arcutis, Bristol Myers Squibb Company, Celgene, Leo Pharma, Eli Lilly, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi, as well as research funding from AbbVie, Amgen, Arcutis, Castle Biosciences, Dermavant, KoBio, Kyowa Kirin, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY