User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Asthma-COPD overlap: Patients have high disease burden
Patients with asthma–chronic obstructive pulmonary disease overlap (ACO) experienced a higher burden of disease than patients with either asthma or COPD alone, a recent study has found.
Approximately 20% of chronic obstructive airway disease cases are ACO, but data on these patients are limited, as they are often excluded from clinical trials, wrote Sarah A. Hiles, MD, of the University of Newcastle (Australia) and colleagues.
“Comparing the burden of eosinophilic ACO, eosinophilic severe asthma, and eosinophilic COPD may also help contextualize findings from phenotype-targeted treatments in different diagnostic groups, such as the limited success of anti-IL [interleukin]–5 monoclonal antibodies as therapy in eosinophilic COPD,” they said.
In a cross-sectional, observational study published in Respirology the researchers recruited patients aged 18 years and older with a confirmed diagnosis of COPD only (153) severe asthma only (64), or ACO (106). Patients were assessed for demographic and clinical factors including health-related quality of life, past-year exacerbation, and other indicators of disease burden. In addition, patients were identified as having eosinophilic airway disease based on a blood eosinophil count of at least 0.3x109/L.
Overall, eosinophilic airway disease was present in 41% of the patients; 55%, 44%, and 29% for those with ACO, severe asthma, and COPD, respectively. Reports of poor health-related quality of life and past-year exacerbations were similar for eosinophilic patients across all three conditions.
However, patients with eosinophilic ACO experienced significantly more past-year exacerbations, notably those requiring oral corticosteroids, compared with patients with asthma alone. In addition, the cumulative number of past-year exacerbations in patient with eosinophilic disease was 164 in those with ACO, compared with severe asthma alone (44) and COPD alone (59).
Patients with ACO also had significantly higher disease burden based on the St. George’s Respiratory Questionnaire (SGRQ), which assessed functional limitation. “For 100 patients, the cumulative SGRQ score attributable to eosinophilic airways disease in ACO was 2,872.8, which was higher than in severe asthma (1,942.5) or COPD (1,638.1),” the researchers said.
The study was limited by several factors including the cross-sectional design and use of a single measurement to classify eosinophilia, the researchers noted. “The non-eosinophilic group likely included a mix of patients with treated eosinophilia and patients without eosinophilia, regardless of treatment, which is a limitation to consider when interpreting the disease burden estimates in this group,” they added.
However, the results add to the understanding of blood eosinophils in airway disease and the study “supports eosinophilia as a phenotype that spans across disease labels of severe asthma and COPD, and their overlap,” they concluded.
The study was supported by AstraZeneca; lead author Dr. Hiles received a salary through a grant from AstraZeneca to the University of Newcastle while conducting the study. Other coauthors disclosed relationships with companies including AstraZeneca, GlaxoSmithKline, Menarini, and Novartis.
Patients with asthma–chronic obstructive pulmonary disease overlap (ACO) experienced a higher burden of disease than patients with either asthma or COPD alone, a recent study has found.
Approximately 20% of chronic obstructive airway disease cases are ACO, but data on these patients are limited, as they are often excluded from clinical trials, wrote Sarah A. Hiles, MD, of the University of Newcastle (Australia) and colleagues.
“Comparing the burden of eosinophilic ACO, eosinophilic severe asthma, and eosinophilic COPD may also help contextualize findings from phenotype-targeted treatments in different diagnostic groups, such as the limited success of anti-IL [interleukin]–5 monoclonal antibodies as therapy in eosinophilic COPD,” they said.
In a cross-sectional, observational study published in Respirology the researchers recruited patients aged 18 years and older with a confirmed diagnosis of COPD only (153) severe asthma only (64), or ACO (106). Patients were assessed for demographic and clinical factors including health-related quality of life, past-year exacerbation, and other indicators of disease burden. In addition, patients were identified as having eosinophilic airway disease based on a blood eosinophil count of at least 0.3x109/L.
Overall, eosinophilic airway disease was present in 41% of the patients; 55%, 44%, and 29% for those with ACO, severe asthma, and COPD, respectively. Reports of poor health-related quality of life and past-year exacerbations were similar for eosinophilic patients across all three conditions.
However, patients with eosinophilic ACO experienced significantly more past-year exacerbations, notably those requiring oral corticosteroids, compared with patients with asthma alone. In addition, the cumulative number of past-year exacerbations in patient with eosinophilic disease was 164 in those with ACO, compared with severe asthma alone (44) and COPD alone (59).
Patients with ACO also had significantly higher disease burden based on the St. George’s Respiratory Questionnaire (SGRQ), which assessed functional limitation. “For 100 patients, the cumulative SGRQ score attributable to eosinophilic airways disease in ACO was 2,872.8, which was higher than in severe asthma (1,942.5) or COPD (1,638.1),” the researchers said.
The study was limited by several factors including the cross-sectional design and use of a single measurement to classify eosinophilia, the researchers noted. “The non-eosinophilic group likely included a mix of patients with treated eosinophilia and patients without eosinophilia, regardless of treatment, which is a limitation to consider when interpreting the disease burden estimates in this group,” they added.
However, the results add to the understanding of blood eosinophils in airway disease and the study “supports eosinophilia as a phenotype that spans across disease labels of severe asthma and COPD, and their overlap,” they concluded.
The study was supported by AstraZeneca; lead author Dr. Hiles received a salary through a grant from AstraZeneca to the University of Newcastle while conducting the study. Other coauthors disclosed relationships with companies including AstraZeneca, GlaxoSmithKline, Menarini, and Novartis.
Patients with asthma–chronic obstructive pulmonary disease overlap (ACO) experienced a higher burden of disease than patients with either asthma or COPD alone, a recent study has found.
Approximately 20% of chronic obstructive airway disease cases are ACO, but data on these patients are limited, as they are often excluded from clinical trials, wrote Sarah A. Hiles, MD, of the University of Newcastle (Australia) and colleagues.
“Comparing the burden of eosinophilic ACO, eosinophilic severe asthma, and eosinophilic COPD may also help contextualize findings from phenotype-targeted treatments in different diagnostic groups, such as the limited success of anti-IL [interleukin]–5 monoclonal antibodies as therapy in eosinophilic COPD,” they said.
In a cross-sectional, observational study published in Respirology the researchers recruited patients aged 18 years and older with a confirmed diagnosis of COPD only (153) severe asthma only (64), or ACO (106). Patients were assessed for demographic and clinical factors including health-related quality of life, past-year exacerbation, and other indicators of disease burden. In addition, patients were identified as having eosinophilic airway disease based on a blood eosinophil count of at least 0.3x109/L.
Overall, eosinophilic airway disease was present in 41% of the patients; 55%, 44%, and 29% for those with ACO, severe asthma, and COPD, respectively. Reports of poor health-related quality of life and past-year exacerbations were similar for eosinophilic patients across all three conditions.
However, patients with eosinophilic ACO experienced significantly more past-year exacerbations, notably those requiring oral corticosteroids, compared with patients with asthma alone. In addition, the cumulative number of past-year exacerbations in patient with eosinophilic disease was 164 in those with ACO, compared with severe asthma alone (44) and COPD alone (59).
Patients with ACO also had significantly higher disease burden based on the St. George’s Respiratory Questionnaire (SGRQ), which assessed functional limitation. “For 100 patients, the cumulative SGRQ score attributable to eosinophilic airways disease in ACO was 2,872.8, which was higher than in severe asthma (1,942.5) or COPD (1,638.1),” the researchers said.
The study was limited by several factors including the cross-sectional design and use of a single measurement to classify eosinophilia, the researchers noted. “The non-eosinophilic group likely included a mix of patients with treated eosinophilia and patients without eosinophilia, regardless of treatment, which is a limitation to consider when interpreting the disease burden estimates in this group,” they added.
However, the results add to the understanding of blood eosinophils in airway disease and the study “supports eosinophilia as a phenotype that spans across disease labels of severe asthma and COPD, and their overlap,” they concluded.
The study was supported by AstraZeneca; lead author Dr. Hiles received a salary through a grant from AstraZeneca to the University of Newcastle while conducting the study. Other coauthors disclosed relationships with companies including AstraZeneca, GlaxoSmithKline, Menarini, and Novartis.
FROM RESPIROLOGY
CVD deaths rose, imaging declined during pandemic
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Natural immunity from COVID-19 ‘may last months’
Infection with the SARS-CoV-2 virus may provide some immunity for at least 5 months, interim results from a study has found.
The first report from the Sarscov2 Immunity & Reinfection Evaluation (SIREN) study suggested that antibodies from people who had recovered from COVID-19 gave at least 83% protection against reinfection compared with people who had not had the disease before.
However, Public Health England (PHE) researchers said some people with antibodies may still be able to carry and transmit the SARS-CoV-2 virus.
‘Strongly encouraged’
Susan Hopkins, PhD, senior medical advisor at PHE, who is leading the study, said the overall findings were good news. She told a briefing hosted by the Science Media Centre: “I am strongly encouraged that people have immunity that is lasting much more than the few months that was speculated before the summer.”
She added: “It allows people to feel that their prior infection will protect them from future infections but at the same time it is not complete protection, and therefore they still need to be careful when they are out and about.”
PHE scientists said they would continue to assess whether protection might last longer than 5 months.
Eleanor Riley, PhD, professor of immunology and infectious disease at the University of Edinburgh, said the report suggested that “natural infection provides short-term protection against COVID-19 that is very similar to that conferred by vaccination.”
Simon Clarke, PhD, associate professor in cellular microbiology at the University of Reading, said: “The concerning finding is that some people who have COVID antibodies appear to still be able to carry the coronavirus and could spread it to others. This means that the vast majority of the population will either need to have natural immunity or have been immunised for us to fully lift restrictions on our lives.”
The analysis took place before the new variant of SARS-CoV-2 became widespread in the UK. The PHE scientists said that further work was underway to establish whether and to what extent antibodies also provide protection from the VOC202012/01 variant.
Healthcare Workers
The SIREN preprint analysed data from 20,787 health care workers from 102 NHS trusts who had undergone antibody and PCR testing from June 18 to November 9, 2020.
Of those, 6614 tested positive for COVID-19 antibodies.
Of the 44 potential reinfections identified, two were designated ‘probable’ and 42 ‘possible’, based on available evidence.
Both of the two individuals classified as probable reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic but were not tested at the time. Both reported that their symptoms were less severe the second time.
None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the time they were recruited to the study.
Tom Wingfield, PhD, senior clinical lecturer at the Liverpool School of Tropical Medicine, said that given the high risk of SARS-CoV-2 infection for frontline NHS staff, it was “vital that we do all that we can to understand, predict, and prevent risk of SARS-CoV-2 amongst healthcare workers”.
The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines, and to what extent people with immunity are able to carry and transmit the virus.
A version of this article first appeared on Medscape.com.
Infection with the SARS-CoV-2 virus may provide some immunity for at least 5 months, interim results from a study has found.
The first report from the Sarscov2 Immunity & Reinfection Evaluation (SIREN) study suggested that antibodies from people who had recovered from COVID-19 gave at least 83% protection against reinfection compared with people who had not had the disease before.
However, Public Health England (PHE) researchers said some people with antibodies may still be able to carry and transmit the SARS-CoV-2 virus.
‘Strongly encouraged’
Susan Hopkins, PhD, senior medical advisor at PHE, who is leading the study, said the overall findings were good news. She told a briefing hosted by the Science Media Centre: “I am strongly encouraged that people have immunity that is lasting much more than the few months that was speculated before the summer.”
She added: “It allows people to feel that their prior infection will protect them from future infections but at the same time it is not complete protection, and therefore they still need to be careful when they are out and about.”
PHE scientists said they would continue to assess whether protection might last longer than 5 months.
Eleanor Riley, PhD, professor of immunology and infectious disease at the University of Edinburgh, said the report suggested that “natural infection provides short-term protection against COVID-19 that is very similar to that conferred by vaccination.”
Simon Clarke, PhD, associate professor in cellular microbiology at the University of Reading, said: “The concerning finding is that some people who have COVID antibodies appear to still be able to carry the coronavirus and could spread it to others. This means that the vast majority of the population will either need to have natural immunity or have been immunised for us to fully lift restrictions on our lives.”
The analysis took place before the new variant of SARS-CoV-2 became widespread in the UK. The PHE scientists said that further work was underway to establish whether and to what extent antibodies also provide protection from the VOC202012/01 variant.
Healthcare Workers
The SIREN preprint analysed data from 20,787 health care workers from 102 NHS trusts who had undergone antibody and PCR testing from June 18 to November 9, 2020.
Of those, 6614 tested positive for COVID-19 antibodies.
Of the 44 potential reinfections identified, two were designated ‘probable’ and 42 ‘possible’, based on available evidence.
Both of the two individuals classified as probable reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic but were not tested at the time. Both reported that their symptoms were less severe the second time.
None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the time they were recruited to the study.
Tom Wingfield, PhD, senior clinical lecturer at the Liverpool School of Tropical Medicine, said that given the high risk of SARS-CoV-2 infection for frontline NHS staff, it was “vital that we do all that we can to understand, predict, and prevent risk of SARS-CoV-2 amongst healthcare workers”.
The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines, and to what extent people with immunity are able to carry and transmit the virus.
A version of this article first appeared on Medscape.com.
Infection with the SARS-CoV-2 virus may provide some immunity for at least 5 months, interim results from a study has found.
The first report from the Sarscov2 Immunity & Reinfection Evaluation (SIREN) study suggested that antibodies from people who had recovered from COVID-19 gave at least 83% protection against reinfection compared with people who had not had the disease before.
However, Public Health England (PHE) researchers said some people with antibodies may still be able to carry and transmit the SARS-CoV-2 virus.
‘Strongly encouraged’
Susan Hopkins, PhD, senior medical advisor at PHE, who is leading the study, said the overall findings were good news. She told a briefing hosted by the Science Media Centre: “I am strongly encouraged that people have immunity that is lasting much more than the few months that was speculated before the summer.”
She added: “It allows people to feel that their prior infection will protect them from future infections but at the same time it is not complete protection, and therefore they still need to be careful when they are out and about.”
PHE scientists said they would continue to assess whether protection might last longer than 5 months.
Eleanor Riley, PhD, professor of immunology and infectious disease at the University of Edinburgh, said the report suggested that “natural infection provides short-term protection against COVID-19 that is very similar to that conferred by vaccination.”
Simon Clarke, PhD, associate professor in cellular microbiology at the University of Reading, said: “The concerning finding is that some people who have COVID antibodies appear to still be able to carry the coronavirus and could spread it to others. This means that the vast majority of the population will either need to have natural immunity or have been immunised for us to fully lift restrictions on our lives.”
The analysis took place before the new variant of SARS-CoV-2 became widespread in the UK. The PHE scientists said that further work was underway to establish whether and to what extent antibodies also provide protection from the VOC202012/01 variant.
Healthcare Workers
The SIREN preprint analysed data from 20,787 health care workers from 102 NHS trusts who had undergone antibody and PCR testing from June 18 to November 9, 2020.
Of those, 6614 tested positive for COVID-19 antibodies.
Of the 44 potential reinfections identified, two were designated ‘probable’ and 42 ‘possible’, based on available evidence.
Both of the two individuals classified as probable reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic but were not tested at the time. Both reported that their symptoms were less severe the second time.
None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the time they were recruited to the study.
Tom Wingfield, PhD, senior clinical lecturer at the Liverpool School of Tropical Medicine, said that given the high risk of SARS-CoV-2 infection for frontline NHS staff, it was “vital that we do all that we can to understand, predict, and prevent risk of SARS-CoV-2 amongst healthcare workers”.
The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines, and to what extent people with immunity are able to carry and transmit the virus.
A version of this article first appeared on Medscape.com.
COVID protections suppressed flu season in U.S.
Last fall, health experts said it was possible the United States could experience an easy 2020-21 flu season because health measures to fight COVID-19 would also thwart the spread of influenza.
It looks like that happened – and then some. Numbers are strikingly low for cases of the flu and other common respiratory and gastrointestinal viruses, health experts told the Washington Post.
“It’s crazy,” Lynnette Brammer, MPH, who leads the domestic influenza surveillance team at the Centers for Disease Control and Prevention, told the Washington Post. “This is my 30th flu season. I never would have expected to see flu activity this low.”
Influenza A, influenza B, parainfluenza, norovirus, respiratory syncytial virus, human metapneumovirus, and the bacteria that cause whooping cough and pneumonia are circulating at near-record-low levels.
As an example, the Washington Post said in the third week of December 2019, the CDC’s network of clinical labs reported 16.2% of almost 30,000 samples tested positive for influenza A. During the same period in 2020, only 0.3% tested positive.
But there’s a possible downside to this suppression of viruses, because flu and other viruses may rebound once the coronavirus is brought under control.
“The best analogy is to a forest fire,” Bryan Grenfell, PhD, an epidemiologist and population biologist at Princeton (N.J.) University, told the Washington Post. “For the fire to spread, it needs to have unburned wood. For epidemics to spread, they require people who haven’t previously been infected. So if people don’t get infected this year by these viruses, they likely will at some point later on.”
American health experts like Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Disease, said last fall that they noticed Australia and other nations in the southern hemisphere had easy flu seasons, apparently because of COVID protection measures. The flu season there runs March through August.
COVID-19 now has a very low presence in Australia, but in recent months the flu has been making a comeback. Flu cases among children aged 5 and younger rose sixfold by December, when such cases are usually at their lowest, the Washington Post said.
“That’s an important cautionary tale for us,” said Kevin Messacar, MD, an infectious disease doctor at Children’s Hospital Colorado, Aurora. “Just because we get through the winter and don’t see much RSV or influenza doesn’t mean we’ll be out of the woods.”
A version of this article first appeared on WebMD.com.
Last fall, health experts said it was possible the United States could experience an easy 2020-21 flu season because health measures to fight COVID-19 would also thwart the spread of influenza.
It looks like that happened – and then some. Numbers are strikingly low for cases of the flu and other common respiratory and gastrointestinal viruses, health experts told the Washington Post.
“It’s crazy,” Lynnette Brammer, MPH, who leads the domestic influenza surveillance team at the Centers for Disease Control and Prevention, told the Washington Post. “This is my 30th flu season. I never would have expected to see flu activity this low.”
Influenza A, influenza B, parainfluenza, norovirus, respiratory syncytial virus, human metapneumovirus, and the bacteria that cause whooping cough and pneumonia are circulating at near-record-low levels.
As an example, the Washington Post said in the third week of December 2019, the CDC’s network of clinical labs reported 16.2% of almost 30,000 samples tested positive for influenza A. During the same period in 2020, only 0.3% tested positive.
But there’s a possible downside to this suppression of viruses, because flu and other viruses may rebound once the coronavirus is brought under control.
“The best analogy is to a forest fire,” Bryan Grenfell, PhD, an epidemiologist and population biologist at Princeton (N.J.) University, told the Washington Post. “For the fire to spread, it needs to have unburned wood. For epidemics to spread, they require people who haven’t previously been infected. So if people don’t get infected this year by these viruses, they likely will at some point later on.”
American health experts like Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Disease, said last fall that they noticed Australia and other nations in the southern hemisphere had easy flu seasons, apparently because of COVID protection measures. The flu season there runs March through August.
COVID-19 now has a very low presence in Australia, but in recent months the flu has been making a comeback. Flu cases among children aged 5 and younger rose sixfold by December, when such cases are usually at their lowest, the Washington Post said.
“That’s an important cautionary tale for us,” said Kevin Messacar, MD, an infectious disease doctor at Children’s Hospital Colorado, Aurora. “Just because we get through the winter and don’t see much RSV or influenza doesn’t mean we’ll be out of the woods.”
A version of this article first appeared on WebMD.com.
Last fall, health experts said it was possible the United States could experience an easy 2020-21 flu season because health measures to fight COVID-19 would also thwart the spread of influenza.
It looks like that happened – and then some. Numbers are strikingly low for cases of the flu and other common respiratory and gastrointestinal viruses, health experts told the Washington Post.
“It’s crazy,” Lynnette Brammer, MPH, who leads the domestic influenza surveillance team at the Centers for Disease Control and Prevention, told the Washington Post. “This is my 30th flu season. I never would have expected to see flu activity this low.”
Influenza A, influenza B, parainfluenza, norovirus, respiratory syncytial virus, human metapneumovirus, and the bacteria that cause whooping cough and pneumonia are circulating at near-record-low levels.
As an example, the Washington Post said in the third week of December 2019, the CDC’s network of clinical labs reported 16.2% of almost 30,000 samples tested positive for influenza A. During the same period in 2020, only 0.3% tested positive.
But there’s a possible downside to this suppression of viruses, because flu and other viruses may rebound once the coronavirus is brought under control.
“The best analogy is to a forest fire,” Bryan Grenfell, PhD, an epidemiologist and population biologist at Princeton (N.J.) University, told the Washington Post. “For the fire to spread, it needs to have unburned wood. For epidemics to spread, they require people who haven’t previously been infected. So if people don’t get infected this year by these viruses, they likely will at some point later on.”
American health experts like Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Disease, said last fall that they noticed Australia and other nations in the southern hemisphere had easy flu seasons, apparently because of COVID protection measures. The flu season there runs March through August.
COVID-19 now has a very low presence in Australia, but in recent months the flu has been making a comeback. Flu cases among children aged 5 and younger rose sixfold by December, when such cases are usually at their lowest, the Washington Post said.
“That’s an important cautionary tale for us,” said Kevin Messacar, MD, an infectious disease doctor at Children’s Hospital Colorado, Aurora. “Just because we get through the winter and don’t see much RSV or influenza doesn’t mean we’ll be out of the woods.”
A version of this article first appeared on WebMD.com.
Treprostinil offers some benefits for patients with ILD-associated pulmonary hypertension
and was associated with some additional clinical benefits, according to a new study published in the New England Journal of Medicine.
To investigate treprostinil therapy for pulmonary hypertension in this subset of patients with lung disease, Aaron Waxman, MD, PhD, of Brigham and Women’s Hospital in Boston, and his fellow researchers launched the multicenter, randomized, double-blind, placebo-controlled INCREASE trial. They assigned 163 patients to the inhaled treprostinil group – administered via an ultrasonic, pulsed-delivery nebulizer over 16 weeks – and 163 patients to the placebo group. Their average age was 66.5 years, 73% were white, and 47% were female
At baseline, the mean 6-minute walk distance (6MWD) for all patients was 259.6 m. After 16 weeks, the treprostinil group gained a mean of 21.08 m in 6MWD, and the placebo group lost 10.04 m. The least-squares mean difference between the groups from baseline in the 6MWD was 31.12 m (95% confidence interval, 16.85-45.39; P < .001). After sensitivity analysis with multiple imputation, the difference remained significant at 30.97 m (95% CI, 16.53-45.41; P < .001).
In a comparison of N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels from baseline to 16 weeks, the treprostinil group saw a decrease of 15% while the placebo group’s levels increased by 46% (treatment ratio 0.58; 95% CI, 0.47-0.72; P < .001). Clinical worsening occurred in 37 patients (23%) in the treprostinil group and 54 patients (33%) in the placebo group (hazard ratio, 0.61; 95% CI, 0.40-0.92; P = .04), while serious adverse events occurred in 23.3% of the patients on treprostinil and 25.8% of the patients on placebo. There was no significant difference between groups in patient-reported quality of life, as assessed via the St. George’s Respiratory Questionnaire.
“There was no guarantee that this was going to work in this condition,” said Adriano Tonelli, MD, of the department of pulmonary medicine at the Cleveland Clinic, in an interview. “Several small studies have tried different medications, for pulmonary hypertension or otherwise, in patients with interstitial lung disease with minimal effect, if any. Given that all the prior studies were not categorically positive, the expectation, at least on my end, was that we needed to wait and see.” Dr. Tonelli and coauthors published a post hoc analysis of inhaled treprostinil studied in the TRIUMPH and BEAT trials.
Next steps: Assess clinical outcomes after inhaled treprostinil
Although the results of this study by Waxman et al, are encouraging, and the need for a treatment in this type of pulmonary hypertension is very real, more narrowing down will be needed to confirm the benefits of inhaled treprostinil, wrote Darren B. Taichman, MD, PhD, of the University of Pennsylvania in an accompanying editorial. He wrote, “After all, patients and physicians may reason, ‘It can’t hurt.’ Unfortunately, however, it could. Therapies approved for pulmonary arterial hypertension have been studied in patients with [ILD]-associated pulmonary hypertension and have shown inconsistent results, with some studies showing no benefit or suggesting harm.”
While the 6MWD has been used as an end point in previous drug trials for pulmonary arterial hypertension, Dr. Taichman wrote that improvements in such a variable were “probably too modest to be unequivocally consequential for many patients.” To confirm the benefits – and detriments – of treatments like inhaled treprostinil, it’s time for studies to focus on clinical end points, he stated, including hospitalizations, disease progression, and death.
He also highlighted the disparity between a treatment that led to increased walk distance and decreased clinical worsening yet did not register an improvement in health-related quality of life. He noted that the oft-cited minimal clinically important difference for 6MWD is approximately 30 m – similar to the difference recorded here. That said, he wrote, “prevention of deterioration is not to be ignored, even if it does not make a patient feel better.”
Regarding quality of life, Dr. Tonelli observed that this questionnaire, standard fare in respiratory research, may not have been perfectly suited for this particular study.
“You have to put it in the context of, ‘How good is the questionnaire to capture a difference in this particular disease over a 16-week period?’ ” he said. “It might not be sensitive enough to capture a significant change. The questionnaire was not developed for pulmonary hypertension in interstitial lung disease, of course. It was developed more generically. It may not capture all that you need to show significance.”
The investigators acknowledged the study’s other potential limitations, including a short duration, a notable percentage of patients who discontinued the trial early, and the fact that clinical worsening and exacerbation of disease were investigator reported and not confirmed by an independent committee.
As for next steps in assessing pulmonary hypertension treatments, Dr. Tonelli pointed to the direction of future research. “The other big study that needs to come out in our field, and I believe it’s being worked on, is inhaled treprostinil in pulmonary hypertension due to chronic obstructive pulmonary disease [COPD],” he said. “That’s a major unmet need; the COPD population is larger than the population for interstitial lung disease, and one would wonder whether inhaled treprostinil would benefit those patients as well. At the moment, we have no treatments for that condition. In the future, a COPD study will be needed.”
The study was supported by United Therapeutics. Author disclosures are listed on the New England Journal of Medicine website.
and was associated with some additional clinical benefits, according to a new study published in the New England Journal of Medicine.
To investigate treprostinil therapy for pulmonary hypertension in this subset of patients with lung disease, Aaron Waxman, MD, PhD, of Brigham and Women’s Hospital in Boston, and his fellow researchers launched the multicenter, randomized, double-blind, placebo-controlled INCREASE trial. They assigned 163 patients to the inhaled treprostinil group – administered via an ultrasonic, pulsed-delivery nebulizer over 16 weeks – and 163 patients to the placebo group. Their average age was 66.5 years, 73% were white, and 47% were female
At baseline, the mean 6-minute walk distance (6MWD) for all patients was 259.6 m. After 16 weeks, the treprostinil group gained a mean of 21.08 m in 6MWD, and the placebo group lost 10.04 m. The least-squares mean difference between the groups from baseline in the 6MWD was 31.12 m (95% confidence interval, 16.85-45.39; P < .001). After sensitivity analysis with multiple imputation, the difference remained significant at 30.97 m (95% CI, 16.53-45.41; P < .001).
In a comparison of N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels from baseline to 16 weeks, the treprostinil group saw a decrease of 15% while the placebo group’s levels increased by 46% (treatment ratio 0.58; 95% CI, 0.47-0.72; P < .001). Clinical worsening occurred in 37 patients (23%) in the treprostinil group and 54 patients (33%) in the placebo group (hazard ratio, 0.61; 95% CI, 0.40-0.92; P = .04), while serious adverse events occurred in 23.3% of the patients on treprostinil and 25.8% of the patients on placebo. There was no significant difference between groups in patient-reported quality of life, as assessed via the St. George’s Respiratory Questionnaire.
“There was no guarantee that this was going to work in this condition,” said Adriano Tonelli, MD, of the department of pulmonary medicine at the Cleveland Clinic, in an interview. “Several small studies have tried different medications, for pulmonary hypertension or otherwise, in patients with interstitial lung disease with minimal effect, if any. Given that all the prior studies were not categorically positive, the expectation, at least on my end, was that we needed to wait and see.” Dr. Tonelli and coauthors published a post hoc analysis of inhaled treprostinil studied in the TRIUMPH and BEAT trials.
Next steps: Assess clinical outcomes after inhaled treprostinil
Although the results of this study by Waxman et al, are encouraging, and the need for a treatment in this type of pulmonary hypertension is very real, more narrowing down will be needed to confirm the benefits of inhaled treprostinil, wrote Darren B. Taichman, MD, PhD, of the University of Pennsylvania in an accompanying editorial. He wrote, “After all, patients and physicians may reason, ‘It can’t hurt.’ Unfortunately, however, it could. Therapies approved for pulmonary arterial hypertension have been studied in patients with [ILD]-associated pulmonary hypertension and have shown inconsistent results, with some studies showing no benefit or suggesting harm.”
While the 6MWD has been used as an end point in previous drug trials for pulmonary arterial hypertension, Dr. Taichman wrote that improvements in such a variable were “probably too modest to be unequivocally consequential for many patients.” To confirm the benefits – and detriments – of treatments like inhaled treprostinil, it’s time for studies to focus on clinical end points, he stated, including hospitalizations, disease progression, and death.
He also highlighted the disparity between a treatment that led to increased walk distance and decreased clinical worsening yet did not register an improvement in health-related quality of life. He noted that the oft-cited minimal clinically important difference for 6MWD is approximately 30 m – similar to the difference recorded here. That said, he wrote, “prevention of deterioration is not to be ignored, even if it does not make a patient feel better.”
Regarding quality of life, Dr. Tonelli observed that this questionnaire, standard fare in respiratory research, may not have been perfectly suited for this particular study.
“You have to put it in the context of, ‘How good is the questionnaire to capture a difference in this particular disease over a 16-week period?’ ” he said. “It might not be sensitive enough to capture a significant change. The questionnaire was not developed for pulmonary hypertension in interstitial lung disease, of course. It was developed more generically. It may not capture all that you need to show significance.”
The investigators acknowledged the study’s other potential limitations, including a short duration, a notable percentage of patients who discontinued the trial early, and the fact that clinical worsening and exacerbation of disease were investigator reported and not confirmed by an independent committee.
As for next steps in assessing pulmonary hypertension treatments, Dr. Tonelli pointed to the direction of future research. “The other big study that needs to come out in our field, and I believe it’s being worked on, is inhaled treprostinil in pulmonary hypertension due to chronic obstructive pulmonary disease [COPD],” he said. “That’s a major unmet need; the COPD population is larger than the population for interstitial lung disease, and one would wonder whether inhaled treprostinil would benefit those patients as well. At the moment, we have no treatments for that condition. In the future, a COPD study will be needed.”
The study was supported by United Therapeutics. Author disclosures are listed on the New England Journal of Medicine website.
and was associated with some additional clinical benefits, according to a new study published in the New England Journal of Medicine.
To investigate treprostinil therapy for pulmonary hypertension in this subset of patients with lung disease, Aaron Waxman, MD, PhD, of Brigham and Women’s Hospital in Boston, and his fellow researchers launched the multicenter, randomized, double-blind, placebo-controlled INCREASE trial. They assigned 163 patients to the inhaled treprostinil group – administered via an ultrasonic, pulsed-delivery nebulizer over 16 weeks – and 163 patients to the placebo group. Their average age was 66.5 years, 73% were white, and 47% were female
At baseline, the mean 6-minute walk distance (6MWD) for all patients was 259.6 m. After 16 weeks, the treprostinil group gained a mean of 21.08 m in 6MWD, and the placebo group lost 10.04 m. The least-squares mean difference between the groups from baseline in the 6MWD was 31.12 m (95% confidence interval, 16.85-45.39; P < .001). After sensitivity analysis with multiple imputation, the difference remained significant at 30.97 m (95% CI, 16.53-45.41; P < .001).
In a comparison of N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels from baseline to 16 weeks, the treprostinil group saw a decrease of 15% while the placebo group’s levels increased by 46% (treatment ratio 0.58; 95% CI, 0.47-0.72; P < .001). Clinical worsening occurred in 37 patients (23%) in the treprostinil group and 54 patients (33%) in the placebo group (hazard ratio, 0.61; 95% CI, 0.40-0.92; P = .04), while serious adverse events occurred in 23.3% of the patients on treprostinil and 25.8% of the patients on placebo. There was no significant difference between groups in patient-reported quality of life, as assessed via the St. George’s Respiratory Questionnaire.
“There was no guarantee that this was going to work in this condition,” said Adriano Tonelli, MD, of the department of pulmonary medicine at the Cleveland Clinic, in an interview. “Several small studies have tried different medications, for pulmonary hypertension or otherwise, in patients with interstitial lung disease with minimal effect, if any. Given that all the prior studies were not categorically positive, the expectation, at least on my end, was that we needed to wait and see.” Dr. Tonelli and coauthors published a post hoc analysis of inhaled treprostinil studied in the TRIUMPH and BEAT trials.
Next steps: Assess clinical outcomes after inhaled treprostinil
Although the results of this study by Waxman et al, are encouraging, and the need for a treatment in this type of pulmonary hypertension is very real, more narrowing down will be needed to confirm the benefits of inhaled treprostinil, wrote Darren B. Taichman, MD, PhD, of the University of Pennsylvania in an accompanying editorial. He wrote, “After all, patients and physicians may reason, ‘It can’t hurt.’ Unfortunately, however, it could. Therapies approved for pulmonary arterial hypertension have been studied in patients with [ILD]-associated pulmonary hypertension and have shown inconsistent results, with some studies showing no benefit or suggesting harm.”
While the 6MWD has been used as an end point in previous drug trials for pulmonary arterial hypertension, Dr. Taichman wrote that improvements in such a variable were “probably too modest to be unequivocally consequential for many patients.” To confirm the benefits – and detriments – of treatments like inhaled treprostinil, it’s time for studies to focus on clinical end points, he stated, including hospitalizations, disease progression, and death.
He also highlighted the disparity between a treatment that led to increased walk distance and decreased clinical worsening yet did not register an improvement in health-related quality of life. He noted that the oft-cited minimal clinically important difference for 6MWD is approximately 30 m – similar to the difference recorded here. That said, he wrote, “prevention of deterioration is not to be ignored, even if it does not make a patient feel better.”
Regarding quality of life, Dr. Tonelli observed that this questionnaire, standard fare in respiratory research, may not have been perfectly suited for this particular study.
“You have to put it in the context of, ‘How good is the questionnaire to capture a difference in this particular disease over a 16-week period?’ ” he said. “It might not be sensitive enough to capture a significant change. The questionnaire was not developed for pulmonary hypertension in interstitial lung disease, of course. It was developed more generically. It may not capture all that you need to show significance.”
The investigators acknowledged the study’s other potential limitations, including a short duration, a notable percentage of patients who discontinued the trial early, and the fact that clinical worsening and exacerbation of disease were investigator reported and not confirmed by an independent committee.
As for next steps in assessing pulmonary hypertension treatments, Dr. Tonelli pointed to the direction of future research. “The other big study that needs to come out in our field, and I believe it’s being worked on, is inhaled treprostinil in pulmonary hypertension due to chronic obstructive pulmonary disease [COPD],” he said. “That’s a major unmet need; the COPD population is larger than the population for interstitial lung disease, and one would wonder whether inhaled treprostinil would benefit those patients as well. At the moment, we have no treatments for that condition. In the future, a COPD study will be needed.”
The study was supported by United Therapeutics. Author disclosures are listed on the New England Journal of Medicine website.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Independent physicians finally get vaccine for selves, but not patients
Physicians unaffiliated with health care systems continue to have difficulties obtaining COVID-19 vaccinations for themselves and their staffs, but that challenge appears to be fading in some states. Yet, in many places, primary care physicians (PCPs) still aren’t being enlisted in the national vaccination effort, despite their numbers and their relationships with patients.
In the first few weeks after the Pfizer and Moderna vaccines received emergency-use authorizations from the Food and Drug Administration, they were distributed mostly to hospitals, pharmacies, and long-term care facilities. Naturally, the hospitals and health care systems vaccinated their own staffs and employed physicians first.
So, even though the guidelines from the Centers for Disease Control and Prevention specify that all frontline health care workers should be included in the first vaccination group, many non–hospital-affiliated private practices have been left out in the cold. Non–patient-facing hospital staff members in some facilities, as well as first responders such as police officers and firefighters, have taken precedence over independent primary care physicians.
In Florida, residents older than 65 years were invited to get vaccinated before some physicians had received shots, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, said in an interview.
While the Department of Health & Human Services is now telling states to give vaccinations to everyone over 65, that wasn’t the case back then.
Community doctors in some areas are still finding it hard to get vaccinated or even find out how to get shots. Yul Ejnes, MD, an internist and partner in Coastal Medical, an independent medical group based in Cranston, R.I., said in an interview that he and his practice staff haven’t been vaccinated, while the staffs of local hospitals have received their shots.
In response to repeated inquiries from his group, he said, the state health department recently said independent practice staffs will start getting vaccinated the week of Jan. 25.
Dr. Ejnes said he understood why hospital personnel went first: Hospitals have the necessary infrastructure, “and the staff in the emergency department and the ICU are caring for the sickest of the sick.”
For primary care doctors like himself who don’t work for the hospital, he said, “I don’t think an infrastructure to get us the vaccine in a timely manner was developed – or if it was developed, it hasn’t been communicated to us.”
Nevertheless, Dr. Ejnes stressed that primary care physicians in the community are just as vulnerable to the coronavirus as hospital clinicians. “We’re seeing patients who have COVID but don’t know they have it. I’m seeing 15 patients a day, and we screen them – as everyone else does – for symptoms and contact and travel, and check their temp,” he said. “But not a day goes by that one of the clinicians in this office doesn’t get a phone call from a patient who was seen a day or 2 earlier to tell them it turns out they were COVID positive. I’m spending 15 minutes in a 100–sq ft room with a patient for a routine visit. And as much as we’re masking and gloving and wearing eye protection, I wouldn’t consider us to be at low risk, especially with the high prevalence of disease.”
In some other states, the situation seems to be improving. Ada Stewart, MD, president of the American Academy of Family Physicians, said that she and her colleagues in a community health center in Columbia, S.C., are in the process of being vaccinated. She got her own shot Jan. 6 at a local hospital.
Her clinic’s staff hadn’t been vaccinated earlier, she said, because nobody in the practice knew the contact person at the hospital who could help access the vaccine doses. Other independent practices in her state are now getting vaccinated, she said, after Gov. Henry McMaster of South Carolina ordered that all health care providers in the top priority category be inoculated by Jan. 15. “At this point, the issues have been diminished.”
However, Dr. Stewart added, independent doctors in some states are still unable to get their shots. AAFP state chapters, as well as the national organization, are trying to persuade governors to ensure all of these physicians are vaccinated. “We’re trying to make sure that the voices of physicians not affiliated with health systems are being heard,” she said.
Lucky shot for doctor
David Boles, DO, a family doctor in Clarksville, Tenn., was able to get his first dose of vaccine just before Christmas, he said in an interview, because he was medical director of a hospice that had received vaccine doses for first responders. When some firefighters and police officers failed to show up for their appointments, the hospice called him and said he had 45 minutes to get to the site if he wanted to be vaccinated.
In early January, his colleagues and staff were also vaccinated, he said, after they were notified of their eligibility as frontline health care workers.
Dr. Boles agreed with Dr. Ejnes that community physicians and nurses are as much at risk as hospital clinicians, except for those intubating patients in the ICU. They may be even more vulnerable, he added, because they have less personal protective equipment than hospital doctors and nurses.
Jennifer Brull, MD, a family physician in Plainville, Kan., said there have been plenty of COVID-19 cases in her small rural community, and the local critical access hospital nearly ran out of beds at one point. Through a collaborative relationship among her clinic (the lone one in the area), the hospital, and the county health department, nearly every frontline health care worker has been vaccinated, and most clinicians in her group have gotten their second doses.
Both the hospital and the health department received vaccine supplies, she said, and everyone in the high-priority category was offered shots. So far, about 170 health care workers have been vaccinated, and only a few declined. More than 300 other people – most of them essential non–health care workers and people older than 65 – have signed up for the next round of shots.
Expanding vaccination effort
Dr. Brull’s practice is the exception among private medical groups around the country. Mr. Gilberg said the MGMA is “concerned that independent practices are playing second fiddle because they’ve been left behind.” Physicians and patient-facing staff in private groups should be getting vaccinated before hospital information technology workers and other non–patient-facing staffers.
Medical practices also can and should play a much bigger role in the overall vaccination effort. Mr. Gilberg has spoken to leaders of several large primary care groups “that have the freezers [for vaccines] and the capacity but haven’t been folded into the distribution plan, especially if they’re not part of the hospital system.”
While hospitals have the storage, he said, they’re not set up to distribute vaccines throughout their communities. “Most health care in this country is delivered outside of the hospital setting. That’s how you’re going to get people vaccinated.”
Ironically, he added, “the same PCPs that are having trouble getting themselves and their staffs vaccinated would be the physicians who could help with vaccine distribution.”
Dr. Brull’s clinic stands ready to help the hospital and health department vaccinate the local population. When sufficient vaccine supplies arrive, she said, she envisioned the doctors and staff administering 200-400 shots per day on Saturdays or weekends.
Dr. Brull was the exception – the other physicians interviewed hadn’t been invited to participate in vaccination efforts.
Dr. Ejnes said his group is capable of vaccinating its patients if it uses the Moderna vaccine, which doesn’t require a super-cold freezer. There are logistical challenges, including social distancing and finding space to observe vaccinated patients for 15 minutes after their shots, he noted. “We’re ready and willing, but realistic about how much we’ll be able to do in this effort.”
The fact that doctors haven’t been enlisted yet in this campaign speaks volumes about “the neglect of the public health infrastructure,” Dr. Ejnes said. “We’re not mobilizing as quickly as we should.”
Alternative routes
Dr. Boles’ group has a refrigerator for pediatric vaccines, which could be used to store the Moderna vaccine, he noted. Shots could be administered to patients in their cars in the parking lot, and they could wait for a while afterward until a nurse came out to verify they were okay.
Mass vaccination sites might also be deployed, as Los Angeles is doing with Dodger Stadium, and physicians could take shifts there in their spare time, Dr. Boles said. But for right now, he views pharmacies as the primary venues for community vaccination.
Of course, the number of pharmacists and pharmacy-employed advanced practice nurses is tiny, compared with the number of primary care doctors, mid-level practitioners, and nurses in ambulatory care practices. Moreover, Mr. Gilberg said, practices know from their electronic health records which patients are most at risk and should be vaccinated first. “Walgreens and CVS don’t know that.”
Physicians should also take the lead in vaccinations because of their patient relationships, he noted. “They can help educate [vaccine-hesitant] patients on why it’s important and dispel some of the rumors and the misinformation that has been politicized. That’s why we should engage physicians in an outpatient setting. And we have to vaccinate them and their staffs. Otherwise, we’re never going to get this rollout underway.”
Dr. Stewart agreed. “We are really the foundation of how we’re going to accomplish this. Most folks are seen by a primary care physician. We touch millions of lives,” she said. “We’re part of the community. Our patients trust us. We’re out there doing it already. We’re doing prevention, giving flu shots, and we’re trying to encourage people to get the COVID vaccine.”
A version of this article first appeared on Medscape.com.
Physicians unaffiliated with health care systems continue to have difficulties obtaining COVID-19 vaccinations for themselves and their staffs, but that challenge appears to be fading in some states. Yet, in many places, primary care physicians (PCPs) still aren’t being enlisted in the national vaccination effort, despite their numbers and their relationships with patients.
In the first few weeks after the Pfizer and Moderna vaccines received emergency-use authorizations from the Food and Drug Administration, they were distributed mostly to hospitals, pharmacies, and long-term care facilities. Naturally, the hospitals and health care systems vaccinated their own staffs and employed physicians first.
So, even though the guidelines from the Centers for Disease Control and Prevention specify that all frontline health care workers should be included in the first vaccination group, many non–hospital-affiliated private practices have been left out in the cold. Non–patient-facing hospital staff members in some facilities, as well as first responders such as police officers and firefighters, have taken precedence over independent primary care physicians.
In Florida, residents older than 65 years were invited to get vaccinated before some physicians had received shots, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, said in an interview.
While the Department of Health & Human Services is now telling states to give vaccinations to everyone over 65, that wasn’t the case back then.
Community doctors in some areas are still finding it hard to get vaccinated or even find out how to get shots. Yul Ejnes, MD, an internist and partner in Coastal Medical, an independent medical group based in Cranston, R.I., said in an interview that he and his practice staff haven’t been vaccinated, while the staffs of local hospitals have received their shots.
In response to repeated inquiries from his group, he said, the state health department recently said independent practice staffs will start getting vaccinated the week of Jan. 25.
Dr. Ejnes said he understood why hospital personnel went first: Hospitals have the necessary infrastructure, “and the staff in the emergency department and the ICU are caring for the sickest of the sick.”
For primary care doctors like himself who don’t work for the hospital, he said, “I don’t think an infrastructure to get us the vaccine in a timely manner was developed – or if it was developed, it hasn’t been communicated to us.”
Nevertheless, Dr. Ejnes stressed that primary care physicians in the community are just as vulnerable to the coronavirus as hospital clinicians. “We’re seeing patients who have COVID but don’t know they have it. I’m seeing 15 patients a day, and we screen them – as everyone else does – for symptoms and contact and travel, and check their temp,” he said. “But not a day goes by that one of the clinicians in this office doesn’t get a phone call from a patient who was seen a day or 2 earlier to tell them it turns out they were COVID positive. I’m spending 15 minutes in a 100–sq ft room with a patient for a routine visit. And as much as we’re masking and gloving and wearing eye protection, I wouldn’t consider us to be at low risk, especially with the high prevalence of disease.”
In some other states, the situation seems to be improving. Ada Stewart, MD, president of the American Academy of Family Physicians, said that she and her colleagues in a community health center in Columbia, S.C., are in the process of being vaccinated. She got her own shot Jan. 6 at a local hospital.
Her clinic’s staff hadn’t been vaccinated earlier, she said, because nobody in the practice knew the contact person at the hospital who could help access the vaccine doses. Other independent practices in her state are now getting vaccinated, she said, after Gov. Henry McMaster of South Carolina ordered that all health care providers in the top priority category be inoculated by Jan. 15. “At this point, the issues have been diminished.”
However, Dr. Stewart added, independent doctors in some states are still unable to get their shots. AAFP state chapters, as well as the national organization, are trying to persuade governors to ensure all of these physicians are vaccinated. “We’re trying to make sure that the voices of physicians not affiliated with health systems are being heard,” she said.
Lucky shot for doctor
David Boles, DO, a family doctor in Clarksville, Tenn., was able to get his first dose of vaccine just before Christmas, he said in an interview, because he was medical director of a hospice that had received vaccine doses for first responders. When some firefighters and police officers failed to show up for their appointments, the hospice called him and said he had 45 minutes to get to the site if he wanted to be vaccinated.
In early January, his colleagues and staff were also vaccinated, he said, after they were notified of their eligibility as frontline health care workers.
Dr. Boles agreed with Dr. Ejnes that community physicians and nurses are as much at risk as hospital clinicians, except for those intubating patients in the ICU. They may be even more vulnerable, he added, because they have less personal protective equipment than hospital doctors and nurses.
Jennifer Brull, MD, a family physician in Plainville, Kan., said there have been plenty of COVID-19 cases in her small rural community, and the local critical access hospital nearly ran out of beds at one point. Through a collaborative relationship among her clinic (the lone one in the area), the hospital, and the county health department, nearly every frontline health care worker has been vaccinated, and most clinicians in her group have gotten their second doses.
Both the hospital and the health department received vaccine supplies, she said, and everyone in the high-priority category was offered shots. So far, about 170 health care workers have been vaccinated, and only a few declined. More than 300 other people – most of them essential non–health care workers and people older than 65 – have signed up for the next round of shots.
Expanding vaccination effort
Dr. Brull’s practice is the exception among private medical groups around the country. Mr. Gilberg said the MGMA is “concerned that independent practices are playing second fiddle because they’ve been left behind.” Physicians and patient-facing staff in private groups should be getting vaccinated before hospital information technology workers and other non–patient-facing staffers.
Medical practices also can and should play a much bigger role in the overall vaccination effort. Mr. Gilberg has spoken to leaders of several large primary care groups “that have the freezers [for vaccines] and the capacity but haven’t been folded into the distribution plan, especially if they’re not part of the hospital system.”
While hospitals have the storage, he said, they’re not set up to distribute vaccines throughout their communities. “Most health care in this country is delivered outside of the hospital setting. That’s how you’re going to get people vaccinated.”
Ironically, he added, “the same PCPs that are having trouble getting themselves and their staffs vaccinated would be the physicians who could help with vaccine distribution.”
Dr. Brull’s clinic stands ready to help the hospital and health department vaccinate the local population. When sufficient vaccine supplies arrive, she said, she envisioned the doctors and staff administering 200-400 shots per day on Saturdays or weekends.
Dr. Brull was the exception – the other physicians interviewed hadn’t been invited to participate in vaccination efforts.
Dr. Ejnes said his group is capable of vaccinating its patients if it uses the Moderna vaccine, which doesn’t require a super-cold freezer. There are logistical challenges, including social distancing and finding space to observe vaccinated patients for 15 minutes after their shots, he noted. “We’re ready and willing, but realistic about how much we’ll be able to do in this effort.”
The fact that doctors haven’t been enlisted yet in this campaign speaks volumes about “the neglect of the public health infrastructure,” Dr. Ejnes said. “We’re not mobilizing as quickly as we should.”
Alternative routes
Dr. Boles’ group has a refrigerator for pediatric vaccines, which could be used to store the Moderna vaccine, he noted. Shots could be administered to patients in their cars in the parking lot, and they could wait for a while afterward until a nurse came out to verify they were okay.
Mass vaccination sites might also be deployed, as Los Angeles is doing with Dodger Stadium, and physicians could take shifts there in their spare time, Dr. Boles said. But for right now, he views pharmacies as the primary venues for community vaccination.
Of course, the number of pharmacists and pharmacy-employed advanced practice nurses is tiny, compared with the number of primary care doctors, mid-level practitioners, and nurses in ambulatory care practices. Moreover, Mr. Gilberg said, practices know from their electronic health records which patients are most at risk and should be vaccinated first. “Walgreens and CVS don’t know that.”
Physicians should also take the lead in vaccinations because of their patient relationships, he noted. “They can help educate [vaccine-hesitant] patients on why it’s important and dispel some of the rumors and the misinformation that has been politicized. That’s why we should engage physicians in an outpatient setting. And we have to vaccinate them and their staffs. Otherwise, we’re never going to get this rollout underway.”
Dr. Stewart agreed. “We are really the foundation of how we’re going to accomplish this. Most folks are seen by a primary care physician. We touch millions of lives,” she said. “We’re part of the community. Our patients trust us. We’re out there doing it already. We’re doing prevention, giving flu shots, and we’re trying to encourage people to get the COVID vaccine.”
A version of this article first appeared on Medscape.com.
Physicians unaffiliated with health care systems continue to have difficulties obtaining COVID-19 vaccinations for themselves and their staffs, but that challenge appears to be fading in some states. Yet, in many places, primary care physicians (PCPs) still aren’t being enlisted in the national vaccination effort, despite their numbers and their relationships with patients.
In the first few weeks after the Pfizer and Moderna vaccines received emergency-use authorizations from the Food and Drug Administration, they were distributed mostly to hospitals, pharmacies, and long-term care facilities. Naturally, the hospitals and health care systems vaccinated their own staffs and employed physicians first.
So, even though the guidelines from the Centers for Disease Control and Prevention specify that all frontline health care workers should be included in the first vaccination group, many non–hospital-affiliated private practices have been left out in the cold. Non–patient-facing hospital staff members in some facilities, as well as first responders such as police officers and firefighters, have taken precedence over independent primary care physicians.
In Florida, residents older than 65 years were invited to get vaccinated before some physicians had received shots, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, said in an interview.
While the Department of Health & Human Services is now telling states to give vaccinations to everyone over 65, that wasn’t the case back then.
Community doctors in some areas are still finding it hard to get vaccinated or even find out how to get shots. Yul Ejnes, MD, an internist and partner in Coastal Medical, an independent medical group based in Cranston, R.I., said in an interview that he and his practice staff haven’t been vaccinated, while the staffs of local hospitals have received their shots.
In response to repeated inquiries from his group, he said, the state health department recently said independent practice staffs will start getting vaccinated the week of Jan. 25.
Dr. Ejnes said he understood why hospital personnel went first: Hospitals have the necessary infrastructure, “and the staff in the emergency department and the ICU are caring for the sickest of the sick.”
For primary care doctors like himself who don’t work for the hospital, he said, “I don’t think an infrastructure to get us the vaccine in a timely manner was developed – or if it was developed, it hasn’t been communicated to us.”
Nevertheless, Dr. Ejnes stressed that primary care physicians in the community are just as vulnerable to the coronavirus as hospital clinicians. “We’re seeing patients who have COVID but don’t know they have it. I’m seeing 15 patients a day, and we screen them – as everyone else does – for symptoms and contact and travel, and check their temp,” he said. “But not a day goes by that one of the clinicians in this office doesn’t get a phone call from a patient who was seen a day or 2 earlier to tell them it turns out they were COVID positive. I’m spending 15 minutes in a 100–sq ft room with a patient for a routine visit. And as much as we’re masking and gloving and wearing eye protection, I wouldn’t consider us to be at low risk, especially with the high prevalence of disease.”
In some other states, the situation seems to be improving. Ada Stewart, MD, president of the American Academy of Family Physicians, said that she and her colleagues in a community health center in Columbia, S.C., are in the process of being vaccinated. She got her own shot Jan. 6 at a local hospital.
Her clinic’s staff hadn’t been vaccinated earlier, she said, because nobody in the practice knew the contact person at the hospital who could help access the vaccine doses. Other independent practices in her state are now getting vaccinated, she said, after Gov. Henry McMaster of South Carolina ordered that all health care providers in the top priority category be inoculated by Jan. 15. “At this point, the issues have been diminished.”
However, Dr. Stewart added, independent doctors in some states are still unable to get their shots. AAFP state chapters, as well as the national organization, are trying to persuade governors to ensure all of these physicians are vaccinated. “We’re trying to make sure that the voices of physicians not affiliated with health systems are being heard,” she said.
Lucky shot for doctor
David Boles, DO, a family doctor in Clarksville, Tenn., was able to get his first dose of vaccine just before Christmas, he said in an interview, because he was medical director of a hospice that had received vaccine doses for first responders. When some firefighters and police officers failed to show up for their appointments, the hospice called him and said he had 45 minutes to get to the site if he wanted to be vaccinated.
In early January, his colleagues and staff were also vaccinated, he said, after they were notified of their eligibility as frontline health care workers.
Dr. Boles agreed with Dr. Ejnes that community physicians and nurses are as much at risk as hospital clinicians, except for those intubating patients in the ICU. They may be even more vulnerable, he added, because they have less personal protective equipment than hospital doctors and nurses.
Jennifer Brull, MD, a family physician in Plainville, Kan., said there have been plenty of COVID-19 cases in her small rural community, and the local critical access hospital nearly ran out of beds at one point. Through a collaborative relationship among her clinic (the lone one in the area), the hospital, and the county health department, nearly every frontline health care worker has been vaccinated, and most clinicians in her group have gotten their second doses.
Both the hospital and the health department received vaccine supplies, she said, and everyone in the high-priority category was offered shots. So far, about 170 health care workers have been vaccinated, and only a few declined. More than 300 other people – most of them essential non–health care workers and people older than 65 – have signed up for the next round of shots.
Expanding vaccination effort
Dr. Brull’s practice is the exception among private medical groups around the country. Mr. Gilberg said the MGMA is “concerned that independent practices are playing second fiddle because they’ve been left behind.” Physicians and patient-facing staff in private groups should be getting vaccinated before hospital information technology workers and other non–patient-facing staffers.
Medical practices also can and should play a much bigger role in the overall vaccination effort. Mr. Gilberg has spoken to leaders of several large primary care groups “that have the freezers [for vaccines] and the capacity but haven’t been folded into the distribution plan, especially if they’re not part of the hospital system.”
While hospitals have the storage, he said, they’re not set up to distribute vaccines throughout their communities. “Most health care in this country is delivered outside of the hospital setting. That’s how you’re going to get people vaccinated.”
Ironically, he added, “the same PCPs that are having trouble getting themselves and their staffs vaccinated would be the physicians who could help with vaccine distribution.”
Dr. Brull’s clinic stands ready to help the hospital and health department vaccinate the local population. When sufficient vaccine supplies arrive, she said, she envisioned the doctors and staff administering 200-400 shots per day on Saturdays or weekends.
Dr. Brull was the exception – the other physicians interviewed hadn’t been invited to participate in vaccination efforts.
Dr. Ejnes said his group is capable of vaccinating its patients if it uses the Moderna vaccine, which doesn’t require a super-cold freezer. There are logistical challenges, including social distancing and finding space to observe vaccinated patients for 15 minutes after their shots, he noted. “We’re ready and willing, but realistic about how much we’ll be able to do in this effort.”
The fact that doctors haven’t been enlisted yet in this campaign speaks volumes about “the neglect of the public health infrastructure,” Dr. Ejnes said. “We’re not mobilizing as quickly as we should.”
Alternative routes
Dr. Boles’ group has a refrigerator for pediatric vaccines, which could be used to store the Moderna vaccine, he noted. Shots could be administered to patients in their cars in the parking lot, and they could wait for a while afterward until a nurse came out to verify they were okay.
Mass vaccination sites might also be deployed, as Los Angeles is doing with Dodger Stadium, and physicians could take shifts there in their spare time, Dr. Boles said. But for right now, he views pharmacies as the primary venues for community vaccination.
Of course, the number of pharmacists and pharmacy-employed advanced practice nurses is tiny, compared with the number of primary care doctors, mid-level practitioners, and nurses in ambulatory care practices. Moreover, Mr. Gilberg said, practices know from their electronic health records which patients are most at risk and should be vaccinated first. “Walgreens and CVS don’t know that.”
Physicians should also take the lead in vaccinations because of their patient relationships, he noted. “They can help educate [vaccine-hesitant] patients on why it’s important and dispel some of the rumors and the misinformation that has been politicized. That’s why we should engage physicians in an outpatient setting. And we have to vaccinate them and their staffs. Otherwise, we’re never going to get this rollout underway.”
Dr. Stewart agreed. “We are really the foundation of how we’re going to accomplish this. Most folks are seen by a primary care physician. We touch millions of lives,” she said. “We’re part of the community. Our patients trust us. We’re out there doing it already. We’re doing prevention, giving flu shots, and we’re trying to encourage people to get the COVID vaccine.”
A version of this article first appeared on Medscape.com.
AMA president: Biden team must create national pandemic strategy
The incoming Biden administration must formulate an effective national strategy for the COVID-19 pandemic, Susan R. Bailey, MD, president of the American Medical Association (AMA), said in a speech delivered Jan. 12 at the National Press Club in Washington.
Dr. Bailey noted that America’s fight against the pandemic is in a critical phase, as evidenced by the escalation in cases, hospitalizations, and deaths in recent weeks. Emergency departments and ICUs are overwhelmed; many frontline clinicians are burned out; and the state- and local-level mechanisms for vaccine distribution have been slow and inconsistent, she said.
“The most important lesson for this moment, and for the year ahead, is that leaving state and local officials to shoulder this burden alone without adequate support from the federal government is not going to work,” Dr. Bailey emphasized.
She called on the Biden administration, which takes over on Jan. 20, to “provide states and local jurisdictions with additional resources, guidance, and support to enable rapid distribution and administration of vaccines.”
In addition, she said, the incoming administration needs to develop a more robust, national strategy for continued COVID-19 testing and PPE production “by tapping into the full powers of the Defense Production Act.”
Biden vaccine distribution policy
In a question-and-answer period following her speech, however, Dr. Bailey said she opposed the president-elect’s decision to release nearly all available vaccine supplies immediately, rather than hold back some doses for the second shots that the Pfizer and Moderna vaccines require. On Jan. 12, the Trump administration announced that it plans to do the same thing.
“We’re a little bit concerned about the announcement that [the Department of Health and Human Services] will not hold back vaccine doses to make sure that everyone who’s gotten their first dose will have a second dose in reserve,” Dr. Bailey said. “We don’t have adequate data to tell us that one dose is sufficient – we don’t think it is – and how long you can wait for the second dose without losing the benefits of the first dose.”
She added that it’s not recommended that people mix the two vaccines in the first and second doses. “Since the Pfizer vaccine has such rigid storage requirements, I want to make sure there’s plenty of vaccine for frontline health care workers who got the Pfizer vaccine because it was the first one to come out in December. I want to make sure they get their second dose on time and [do] not have to wait.”
Dr. Bailey said she hoped there will be plenty of vaccine supply. But she suggested that state and local health authorities be in communication with the federal government about whether there will be enough vaccine to guarantee people can get both doses.
Bolstering public health
In her speech, Dr. Bailey outlined five areas in which steps should be taken to improve the health system so that it isn’t overwhelmed the next time the United States has a public health crisis:
- Restore trust in science and science-based decision making. Make sure that scientific institutions such as the Centers for Disease Control and Prevention and the Food and Drug Administration are “free from political pressure, and that their actions are guided by the best available scientific evidence.”
- Ensure that the health system provides all Americans with affordable access to comprehensive health care. Dr. Bailey wasn’t talking about Medicare for All; she suggested that perhaps there be a second enrollment period for the Affordable Care Act’s individual insurance exchanges.
- Work to remove health care inequities that have hurt communities of color, who have been disproportionately impacted by the pandemic. She referred to a recent AMA that recognized racism as a public health threat.
- Improve public health domestically and globally. Among other things, she noted, the public health infrastructure needs to be revitalized after “decades of disinvestment and neglect,” which has contributed to the slow vaccine rollout.
- Recognize the global health community and restore America’s leadership in global efforts to combat disease, which are critical to preventing future threats. She praised President-Elect Biden for his promise that the United States will rejoin the World Health Organization.
At several points in her presentation, Dr. Bailey rejected political interference with science and health care. Among other things, she said public health could be improved by protecting the doctor-patient relationship from political interference.
Answering a question about how to separate politics from the pandemic, she replied, “The key is in sticking to the science and listening to our public health authorities. They all have to deliver the same message. Also, leaders at all levels, including in our communities, our schools, churches and college campuses, should wear masks and socially distance. This isn’t about anything other than the desire to get out of the pandemic and get our country on the right track again. Masks shouldn’t be political. Going back to school shouldn’t be political. Taking a certain medication or not shouldn’t be political. We need to stick to the science and listen to our public health authorities. That’s the quickest way out.”
Asked when she thought that life might get back to normal again in the United States, Dr. Bailey said a lot depends on the extent of vaccine uptake and how much self-discipline people exhibit in following public health advice. “I think we’re looking at the end of this year. I’m hopeful that by fall, things will have opened up quite a bit as the Venn diagrams of those who’ve gotten vaccines grow larger.”
A version of this article first appeared on Medscape.com.
The incoming Biden administration must formulate an effective national strategy for the COVID-19 pandemic, Susan R. Bailey, MD, president of the American Medical Association (AMA), said in a speech delivered Jan. 12 at the National Press Club in Washington.
Dr. Bailey noted that America’s fight against the pandemic is in a critical phase, as evidenced by the escalation in cases, hospitalizations, and deaths in recent weeks. Emergency departments and ICUs are overwhelmed; many frontline clinicians are burned out; and the state- and local-level mechanisms for vaccine distribution have been slow and inconsistent, she said.
“The most important lesson for this moment, and for the year ahead, is that leaving state and local officials to shoulder this burden alone without adequate support from the federal government is not going to work,” Dr. Bailey emphasized.
She called on the Biden administration, which takes over on Jan. 20, to “provide states and local jurisdictions with additional resources, guidance, and support to enable rapid distribution and administration of vaccines.”
In addition, she said, the incoming administration needs to develop a more robust, national strategy for continued COVID-19 testing and PPE production “by tapping into the full powers of the Defense Production Act.”
Biden vaccine distribution policy
In a question-and-answer period following her speech, however, Dr. Bailey said she opposed the president-elect’s decision to release nearly all available vaccine supplies immediately, rather than hold back some doses for the second shots that the Pfizer and Moderna vaccines require. On Jan. 12, the Trump administration announced that it plans to do the same thing.
“We’re a little bit concerned about the announcement that [the Department of Health and Human Services] will not hold back vaccine doses to make sure that everyone who’s gotten their first dose will have a second dose in reserve,” Dr. Bailey said. “We don’t have adequate data to tell us that one dose is sufficient – we don’t think it is – and how long you can wait for the second dose without losing the benefits of the first dose.”
She added that it’s not recommended that people mix the two vaccines in the first and second doses. “Since the Pfizer vaccine has such rigid storage requirements, I want to make sure there’s plenty of vaccine for frontline health care workers who got the Pfizer vaccine because it was the first one to come out in December. I want to make sure they get their second dose on time and [do] not have to wait.”
Dr. Bailey said she hoped there will be plenty of vaccine supply. But she suggested that state and local health authorities be in communication with the federal government about whether there will be enough vaccine to guarantee people can get both doses.
Bolstering public health
In her speech, Dr. Bailey outlined five areas in which steps should be taken to improve the health system so that it isn’t overwhelmed the next time the United States has a public health crisis:
- Restore trust in science and science-based decision making. Make sure that scientific institutions such as the Centers for Disease Control and Prevention and the Food and Drug Administration are “free from political pressure, and that their actions are guided by the best available scientific evidence.”
- Ensure that the health system provides all Americans with affordable access to comprehensive health care. Dr. Bailey wasn’t talking about Medicare for All; she suggested that perhaps there be a second enrollment period for the Affordable Care Act’s individual insurance exchanges.
- Work to remove health care inequities that have hurt communities of color, who have been disproportionately impacted by the pandemic. She referred to a recent AMA that recognized racism as a public health threat.
- Improve public health domestically and globally. Among other things, she noted, the public health infrastructure needs to be revitalized after “decades of disinvestment and neglect,” which has contributed to the slow vaccine rollout.
- Recognize the global health community and restore America’s leadership in global efforts to combat disease, which are critical to preventing future threats. She praised President-Elect Biden for his promise that the United States will rejoin the World Health Organization.
At several points in her presentation, Dr. Bailey rejected political interference with science and health care. Among other things, she said public health could be improved by protecting the doctor-patient relationship from political interference.
Answering a question about how to separate politics from the pandemic, she replied, “The key is in sticking to the science and listening to our public health authorities. They all have to deliver the same message. Also, leaders at all levels, including in our communities, our schools, churches and college campuses, should wear masks and socially distance. This isn’t about anything other than the desire to get out of the pandemic and get our country on the right track again. Masks shouldn’t be political. Going back to school shouldn’t be political. Taking a certain medication or not shouldn’t be political. We need to stick to the science and listen to our public health authorities. That’s the quickest way out.”
Asked when she thought that life might get back to normal again in the United States, Dr. Bailey said a lot depends on the extent of vaccine uptake and how much self-discipline people exhibit in following public health advice. “I think we’re looking at the end of this year. I’m hopeful that by fall, things will have opened up quite a bit as the Venn diagrams of those who’ve gotten vaccines grow larger.”
A version of this article first appeared on Medscape.com.
The incoming Biden administration must formulate an effective national strategy for the COVID-19 pandemic, Susan R. Bailey, MD, president of the American Medical Association (AMA), said in a speech delivered Jan. 12 at the National Press Club in Washington.
Dr. Bailey noted that America’s fight against the pandemic is in a critical phase, as evidenced by the escalation in cases, hospitalizations, and deaths in recent weeks. Emergency departments and ICUs are overwhelmed; many frontline clinicians are burned out; and the state- and local-level mechanisms for vaccine distribution have been slow and inconsistent, she said.
“The most important lesson for this moment, and for the year ahead, is that leaving state and local officials to shoulder this burden alone without adequate support from the federal government is not going to work,” Dr. Bailey emphasized.
She called on the Biden administration, which takes over on Jan. 20, to “provide states and local jurisdictions with additional resources, guidance, and support to enable rapid distribution and administration of vaccines.”
In addition, she said, the incoming administration needs to develop a more robust, national strategy for continued COVID-19 testing and PPE production “by tapping into the full powers of the Defense Production Act.”
Biden vaccine distribution policy
In a question-and-answer period following her speech, however, Dr. Bailey said she opposed the president-elect’s decision to release nearly all available vaccine supplies immediately, rather than hold back some doses for the second shots that the Pfizer and Moderna vaccines require. On Jan. 12, the Trump administration announced that it plans to do the same thing.
“We’re a little bit concerned about the announcement that [the Department of Health and Human Services] will not hold back vaccine doses to make sure that everyone who’s gotten their first dose will have a second dose in reserve,” Dr. Bailey said. “We don’t have adequate data to tell us that one dose is sufficient – we don’t think it is – and how long you can wait for the second dose without losing the benefits of the first dose.”
She added that it’s not recommended that people mix the two vaccines in the first and second doses. “Since the Pfizer vaccine has such rigid storage requirements, I want to make sure there’s plenty of vaccine for frontline health care workers who got the Pfizer vaccine because it was the first one to come out in December. I want to make sure they get their second dose on time and [do] not have to wait.”
Dr. Bailey said she hoped there will be plenty of vaccine supply. But she suggested that state and local health authorities be in communication with the federal government about whether there will be enough vaccine to guarantee people can get both doses.
Bolstering public health
In her speech, Dr. Bailey outlined five areas in which steps should be taken to improve the health system so that it isn’t overwhelmed the next time the United States has a public health crisis:
- Restore trust in science and science-based decision making. Make sure that scientific institutions such as the Centers for Disease Control and Prevention and the Food and Drug Administration are “free from political pressure, and that their actions are guided by the best available scientific evidence.”
- Ensure that the health system provides all Americans with affordable access to comprehensive health care. Dr. Bailey wasn’t talking about Medicare for All; she suggested that perhaps there be a second enrollment period for the Affordable Care Act’s individual insurance exchanges.
- Work to remove health care inequities that have hurt communities of color, who have been disproportionately impacted by the pandemic. She referred to a recent AMA that recognized racism as a public health threat.
- Improve public health domestically and globally. Among other things, she noted, the public health infrastructure needs to be revitalized after “decades of disinvestment and neglect,” which has contributed to the slow vaccine rollout.
- Recognize the global health community and restore America’s leadership in global efforts to combat disease, which are critical to preventing future threats. She praised President-Elect Biden for his promise that the United States will rejoin the World Health Organization.
At several points in her presentation, Dr. Bailey rejected political interference with science and health care. Among other things, she said public health could be improved by protecting the doctor-patient relationship from political interference.
Answering a question about how to separate politics from the pandemic, she replied, “The key is in sticking to the science and listening to our public health authorities. They all have to deliver the same message. Also, leaders at all levels, including in our communities, our schools, churches and college campuses, should wear masks and socially distance. This isn’t about anything other than the desire to get out of the pandemic and get our country on the right track again. Masks shouldn’t be political. Going back to school shouldn’t be political. Taking a certain medication or not shouldn’t be political. We need to stick to the science and listen to our public health authorities. That’s the quickest way out.”
Asked when she thought that life might get back to normal again in the United States, Dr. Bailey said a lot depends on the extent of vaccine uptake and how much self-discipline people exhibit in following public health advice. “I think we’re looking at the end of this year. I’m hopeful that by fall, things will have opened up quite a bit as the Venn diagrams of those who’ve gotten vaccines grow larger.”
A version of this article first appeared on Medscape.com.
Feds to states: Give COVID-19 vaccine to 65+ and those with comorbidities
Federal health officials are urging states to vaccinate all Americans over age 65 and those aged 16-64 who have a documented underlying health condition that makes them more vulnerable to COVID-19.
U.S. Department of Health and Human Services (HHS) Secretary Alex Azar and Centers for Disease Control and Prevention Director Robert Redfield, MD, made the recommendation in a briefing with reporters on Jan. 12, saying that the current vaccine supply was sufficient to meet demand for the next phase of immunization as recommended by the CDC’s Advisory Committee on Immunization Practices.
“We are ready for a transition that we outlined last September in the playbook we sent to states,” Mr. Azar said. Both he and U.S. Army General Gustave F. Perna, chief operations officer for Operation Warp Speed, said that confidence in the distribution system had led to the decision to urge wider access.
The federal government will also increase the number of sites eligible to receive vaccine – including some 13,000 federally qualified community health centers – and will not keep doses in reserve as insurance against issues that might prevent people from receiving a second dose on a timely basis.
“We don’t need to hold back reserve doses,” Mr. Azar said, noting that if there were any “glitches in production” the federal government would move to fulfill obligations for second doses first and delay initial doses.
Azar: Use it or lose it
In a move that is sure to generate pushback, Mr. Azar said that states that don’t quickly administer vaccines will receive fewer doses in the future. That policy will not go into effect until later in February, which leaves open the possibility that it could be reversed by the incoming Biden administration.
“We have too much vaccine sitting in freezers at hospitals with hospitals not using it,” said Mr. Azar, who also blamed the slow administration process on a reporting lag and states being what he called “overly prescriptive” in who has been eligible to receive a shot.
“I would rather have people working to get appointments to get vaccinated than having vaccine going to waste sitting in freezers,” he told reporters.
Mr. Azar had already been pushing for broader vaccination, telling states to do so in an Operation Warp Speed briefing on Jan. 6. At that briefing, he also said that the federal government would be stepping up vaccination through an “early launch” of a federal partnership with 19 pharmacy chains, which will let states allocate vaccines directly to some 40,000 pharmacy sites.
Gen. Perna said during the Jan. 12 briefing that the aim is to further expand that to some 70,000 locations total.
The CDC reported that as of Jan. 11 some 25.4 million doses have been distributed, with 8.9 million administered. An additional 4.2 million doses were distributed to long-term care facilities, and 937,000 residents and staff have received a dose.
“Pace of administration”
Alaska, Connecticut, North Dakota, South Dakota, the District of Columbia, West Virginia, and the Northern Mariana Islands have administered the most vaccines per capita, according to the CDC. But even these locations have immunized only 4%-5% of their populations, the New York Times reports. At the bottom: Alabama, Arizona, Arkansas, Georgia, Mississippi, and South Carolina.
The federal government can encourage but not require states to move on to new phases of vaccination.
“States ultimately determine how they will proceed with vaccination,” said Marcus Plescia, MD, MPH, chief medical officer for the Association of State and Territorial Health Officials. “Most will be cautious about assuring there are doses for those needing a second dose,” he said in an interview.
Dr. Plescia said that ensuring a second dose is available is especially important for health care workers “who need to be confident that they are protected and not inadvertently transmitting the disease themselves.”
He added that “once we reach a steady state of supply and administration, the rate-limiting factor will be supply of vaccine.”
That supply could now be threatened if states don’t comply with a just-announced federal action that will change how doses are allocated.
Beginning in late February, vaccine allocations to states will be based on “the pace of administration reported by states,” and the size of the 65-and-older population, said Mr. Azar, who has previously criticized New York Governor Andrew Cuomo for fining hospitals that didn’t use up vaccine supply within a week.
“This new system gives states a strong incentive to ensure that all vaccinations are being promptly reported, which they currently are not,” he said.
Currently, allocations are based on a state’s or territory’s population.
Prepandemic, states were required to report vaccinations within 30 days. Since COVID-19 vaccines became available, the CDC has required reporting of shots within 72 hours.
Dr. Redfield said the requirement has caused some difficulty, and that the CDC is investigating why some states have reported using only 15% of doses while others have used 80%.
States have been scrambling to ramp up vaccinations.
Just ahead of the federal briefing, Gov. Cuomo tweeted that New York would be opening up vaccinations to anyone older than 65.
The Associated Press is reporting that some states have started mass vaccination sites.
Arizona has begun operating a 24/7 appointment-only vaccination program at State Farm Stadium outside of Phoenix, with the aim of immunizing 6,000 people each day, according to local radio station KJZZ.
California and Florida have also taken steps to use stadiums, while Michigan, New Jersey, New York, and Texas will use convention centers and fairgrounds, Axios has reported.
In Florida, Palm Beach County Health Director Alina Alonso, MD, told county commissioners on Jan. 12 that there isn’t enough vaccine to meet demand, WPTV reported. “We need to realize that there’s a shortage of vaccine. So it’s not the plan, it’s not our ability to do it. It’s simply supply and demand at this point,” Dr. Alonso said, according to the TV station report.
A version of this article first appeared on Medscape.com.
Federal health officials are urging states to vaccinate all Americans over age 65 and those aged 16-64 who have a documented underlying health condition that makes them more vulnerable to COVID-19.
U.S. Department of Health and Human Services (HHS) Secretary Alex Azar and Centers for Disease Control and Prevention Director Robert Redfield, MD, made the recommendation in a briefing with reporters on Jan. 12, saying that the current vaccine supply was sufficient to meet demand for the next phase of immunization as recommended by the CDC’s Advisory Committee on Immunization Practices.
“We are ready for a transition that we outlined last September in the playbook we sent to states,” Mr. Azar said. Both he and U.S. Army General Gustave F. Perna, chief operations officer for Operation Warp Speed, said that confidence in the distribution system had led to the decision to urge wider access.
The federal government will also increase the number of sites eligible to receive vaccine – including some 13,000 federally qualified community health centers – and will not keep doses in reserve as insurance against issues that might prevent people from receiving a second dose on a timely basis.
“We don’t need to hold back reserve doses,” Mr. Azar said, noting that if there were any “glitches in production” the federal government would move to fulfill obligations for second doses first and delay initial doses.
Azar: Use it or lose it
In a move that is sure to generate pushback, Mr. Azar said that states that don’t quickly administer vaccines will receive fewer doses in the future. That policy will not go into effect until later in February, which leaves open the possibility that it could be reversed by the incoming Biden administration.
“We have too much vaccine sitting in freezers at hospitals with hospitals not using it,” said Mr. Azar, who also blamed the slow administration process on a reporting lag and states being what he called “overly prescriptive” in who has been eligible to receive a shot.
“I would rather have people working to get appointments to get vaccinated than having vaccine going to waste sitting in freezers,” he told reporters.
Mr. Azar had already been pushing for broader vaccination, telling states to do so in an Operation Warp Speed briefing on Jan. 6. At that briefing, he also said that the federal government would be stepping up vaccination through an “early launch” of a federal partnership with 19 pharmacy chains, which will let states allocate vaccines directly to some 40,000 pharmacy sites.
Gen. Perna said during the Jan. 12 briefing that the aim is to further expand that to some 70,000 locations total.
The CDC reported that as of Jan. 11 some 25.4 million doses have been distributed, with 8.9 million administered. An additional 4.2 million doses were distributed to long-term care facilities, and 937,000 residents and staff have received a dose.
“Pace of administration”
Alaska, Connecticut, North Dakota, South Dakota, the District of Columbia, West Virginia, and the Northern Mariana Islands have administered the most vaccines per capita, according to the CDC. But even these locations have immunized only 4%-5% of their populations, the New York Times reports. At the bottom: Alabama, Arizona, Arkansas, Georgia, Mississippi, and South Carolina.
The federal government can encourage but not require states to move on to new phases of vaccination.
“States ultimately determine how they will proceed with vaccination,” said Marcus Plescia, MD, MPH, chief medical officer for the Association of State and Territorial Health Officials. “Most will be cautious about assuring there are doses for those needing a second dose,” he said in an interview.
Dr. Plescia said that ensuring a second dose is available is especially important for health care workers “who need to be confident that they are protected and not inadvertently transmitting the disease themselves.”
He added that “once we reach a steady state of supply and administration, the rate-limiting factor will be supply of vaccine.”
That supply could now be threatened if states don’t comply with a just-announced federal action that will change how doses are allocated.
Beginning in late February, vaccine allocations to states will be based on “the pace of administration reported by states,” and the size of the 65-and-older population, said Mr. Azar, who has previously criticized New York Governor Andrew Cuomo for fining hospitals that didn’t use up vaccine supply within a week.
“This new system gives states a strong incentive to ensure that all vaccinations are being promptly reported, which they currently are not,” he said.
Currently, allocations are based on a state’s or territory’s population.
Prepandemic, states were required to report vaccinations within 30 days. Since COVID-19 vaccines became available, the CDC has required reporting of shots within 72 hours.
Dr. Redfield said the requirement has caused some difficulty, and that the CDC is investigating why some states have reported using only 15% of doses while others have used 80%.
States have been scrambling to ramp up vaccinations.
Just ahead of the federal briefing, Gov. Cuomo tweeted that New York would be opening up vaccinations to anyone older than 65.
The Associated Press is reporting that some states have started mass vaccination sites.
Arizona has begun operating a 24/7 appointment-only vaccination program at State Farm Stadium outside of Phoenix, with the aim of immunizing 6,000 people each day, according to local radio station KJZZ.
California and Florida have also taken steps to use stadiums, while Michigan, New Jersey, New York, and Texas will use convention centers and fairgrounds, Axios has reported.
In Florida, Palm Beach County Health Director Alina Alonso, MD, told county commissioners on Jan. 12 that there isn’t enough vaccine to meet demand, WPTV reported. “We need to realize that there’s a shortage of vaccine. So it’s not the plan, it’s not our ability to do it. It’s simply supply and demand at this point,” Dr. Alonso said, according to the TV station report.
A version of this article first appeared on Medscape.com.
Federal health officials are urging states to vaccinate all Americans over age 65 and those aged 16-64 who have a documented underlying health condition that makes them more vulnerable to COVID-19.
U.S. Department of Health and Human Services (HHS) Secretary Alex Azar and Centers for Disease Control and Prevention Director Robert Redfield, MD, made the recommendation in a briefing with reporters on Jan. 12, saying that the current vaccine supply was sufficient to meet demand for the next phase of immunization as recommended by the CDC’s Advisory Committee on Immunization Practices.
“We are ready for a transition that we outlined last September in the playbook we sent to states,” Mr. Azar said. Both he and U.S. Army General Gustave F. Perna, chief operations officer for Operation Warp Speed, said that confidence in the distribution system had led to the decision to urge wider access.
The federal government will also increase the number of sites eligible to receive vaccine – including some 13,000 federally qualified community health centers – and will not keep doses in reserve as insurance against issues that might prevent people from receiving a second dose on a timely basis.
“We don’t need to hold back reserve doses,” Mr. Azar said, noting that if there were any “glitches in production” the federal government would move to fulfill obligations for second doses first and delay initial doses.
Azar: Use it or lose it
In a move that is sure to generate pushback, Mr. Azar said that states that don’t quickly administer vaccines will receive fewer doses in the future. That policy will not go into effect until later in February, which leaves open the possibility that it could be reversed by the incoming Biden administration.
“We have too much vaccine sitting in freezers at hospitals with hospitals not using it,” said Mr. Azar, who also blamed the slow administration process on a reporting lag and states being what he called “overly prescriptive” in who has been eligible to receive a shot.
“I would rather have people working to get appointments to get vaccinated than having vaccine going to waste sitting in freezers,” he told reporters.
Mr. Azar had already been pushing for broader vaccination, telling states to do so in an Operation Warp Speed briefing on Jan. 6. At that briefing, he also said that the federal government would be stepping up vaccination through an “early launch” of a federal partnership with 19 pharmacy chains, which will let states allocate vaccines directly to some 40,000 pharmacy sites.
Gen. Perna said during the Jan. 12 briefing that the aim is to further expand that to some 70,000 locations total.
The CDC reported that as of Jan. 11 some 25.4 million doses have been distributed, with 8.9 million administered. An additional 4.2 million doses were distributed to long-term care facilities, and 937,000 residents and staff have received a dose.
“Pace of administration”
Alaska, Connecticut, North Dakota, South Dakota, the District of Columbia, West Virginia, and the Northern Mariana Islands have administered the most vaccines per capita, according to the CDC. But even these locations have immunized only 4%-5% of their populations, the New York Times reports. At the bottom: Alabama, Arizona, Arkansas, Georgia, Mississippi, and South Carolina.
The federal government can encourage but not require states to move on to new phases of vaccination.
“States ultimately determine how they will proceed with vaccination,” said Marcus Plescia, MD, MPH, chief medical officer for the Association of State and Territorial Health Officials. “Most will be cautious about assuring there are doses for those needing a second dose,” he said in an interview.
Dr. Plescia said that ensuring a second dose is available is especially important for health care workers “who need to be confident that they are protected and not inadvertently transmitting the disease themselves.”
He added that “once we reach a steady state of supply and administration, the rate-limiting factor will be supply of vaccine.”
That supply could now be threatened if states don’t comply with a just-announced federal action that will change how doses are allocated.
Beginning in late February, vaccine allocations to states will be based on “the pace of administration reported by states,” and the size of the 65-and-older population, said Mr. Azar, who has previously criticized New York Governor Andrew Cuomo for fining hospitals that didn’t use up vaccine supply within a week.
“This new system gives states a strong incentive to ensure that all vaccinations are being promptly reported, which they currently are not,” he said.
Currently, allocations are based on a state’s or territory’s population.
Prepandemic, states were required to report vaccinations within 30 days. Since COVID-19 vaccines became available, the CDC has required reporting of shots within 72 hours.
Dr. Redfield said the requirement has caused some difficulty, and that the CDC is investigating why some states have reported using only 15% of doses while others have used 80%.
States have been scrambling to ramp up vaccinations.
Just ahead of the federal briefing, Gov. Cuomo tweeted that New York would be opening up vaccinations to anyone older than 65.
The Associated Press is reporting that some states have started mass vaccination sites.
Arizona has begun operating a 24/7 appointment-only vaccination program at State Farm Stadium outside of Phoenix, with the aim of immunizing 6,000 people each day, according to local radio station KJZZ.
California and Florida have also taken steps to use stadiums, while Michigan, New Jersey, New York, and Texas will use convention centers and fairgrounds, Axios has reported.
In Florida, Palm Beach County Health Director Alina Alonso, MD, told county commissioners on Jan. 12 that there isn’t enough vaccine to meet demand, WPTV reported. “We need to realize that there’s a shortage of vaccine. So it’s not the plan, it’s not our ability to do it. It’s simply supply and demand at this point,” Dr. Alonso said, according to the TV station report.
A version of this article first appeared on Medscape.com.
Averting COVID hospitalizations with monoclonal antibodies
The United States has allocated more than 641,000 monoclonal antibody treatments for outpatients to ease pressure on strained hospitals, but officials from Operation Warp Speed report that more than half of that reserve sits unused as clinicians grapple with best practices.
There are space and personnel limitations in hospitals right now, Janet Woodcock, MD, therapeutics lead on Operation Warp Speed, acknowledges in an interview with this news organization. “Special areas and procedures must be set up.” And the operation is in the process of broadening availability beyond hospitals, she points out.
But for frontline clinicians, questions about treatment efficacy and the logistics of administering intravenous drugs to infectious outpatients loom large.
More than 50 monoclonal antibody products that target SARS-CoV-2 are now in development. The U.S. Food and Drug Administration has already issued Emergency Use Authorization (EUA) for two such drugs on the basis of phase 2 trial data – bamlanivimab, made by Eli Lilly, and a cocktail of casirivimab plus imdevimab, made by Regeneron – and another two-antibody cocktail from AstraZeneca, AZD7442, has started phase 3 clinical trials. The Regeneron combination was used to treat President Donald Trump when he contracted COVID-19 in October.
Monoclonal antibody drugs are based on the natural antibodies that the body uses to fight infections. They work by binding to a specific target and then blocking its action or flagging it for destruction by other parts of the immune system. Both bamlanivimab and the casirivimab plus imdevimab combination target the spike protein of the virus and stop it from attaching to and entering human cells.
Targeting the spike protein out of the hospital
The antibody drugs covered by EUAs do not cure COVID-19, but they have been shown to reduce hospitalizations and visits to the emergency department for patients at high risk for disease progression. They are approved to treat patients older than 12 years with mild to moderate COVID-19 who are at high risk of progressing to severe disease or hospitalization. They are not authorized for use in patients who have been hospitalized or who are on ventilators. The hope is that antibody drugs will reduce the number of severe cases of COVID-19 and ease pressure on overstretched hospitals.
Most COVID-19 patients are outpatients, so we need something to keep them from getting worse.
This is important because it targets the greatest need in COVID-19 therapeutics, says Rajesh Gandhi, MD, an infectious disease physician at Harvard Medical School in Boston, who is a member of two panels evaluating COVID-19 treatments: one for the Infectious Disease Society of America and the other for the National Institutes of Health. “Up to now, most of the focus has been on hospitalized patients,” he says, but “most COVID-19 patients are outpatients, so we need something to keep them from getting worse.”
Both panels have said that, despite the EUAs, more evidence is needed to be sure of the efficacy of the drugs and to determine which patients will benefit the most from them.
These aren’t the mature data from drug development that guideline groups are accustomed to working with, Dr. Woodcock points out. “But this is an emergency and the data taken as a whole are pretty convincing,” she says. “As I look at the totality of the evidence, monoclonal antibodies will have a big effect in keeping people out of the hospital and helping them recover faster.”
High-risk patients are eligible for treatment, especially those older than 65 years and those with comorbidities who are younger. Access to the drugs is increasing for clinicians who are able to infuse safely or work with a site that will.
In the Boston area, several hospitals, including Massachusetts General where Dr. Gandhi works, have set up infusion centers where newly diagnosed patients can get the antibody treatment if their doctor thinks it will benefit them. And Coram, a provider of at-home infusion therapy owned by the CVS pharmacy chain, is running a pilot program offering the Eli Lilly drug to people in seven cities – including Boston, Chicago, Los Angeles, and Tampa – and their surrounding communities with a physician referral.
Getting that referral could be tricky, however, for patients without a primary care physician or for those whose doctor isn’t already connected to one of the institutions providing the infusions. The hospitals are sending out communications on how patients and physicians can get the therapy, but Dr. Gandhi says that making information about access available should be a priority. The window for the effective treatment is small – the drugs appear to work best before patients begin to make their own antibodies, says Dr. Gandhi – so it’s vital that doctors act quickly if they have a patient who is eligible.
And rolling out the new therapies to patients around the world will be a major logistical undertaking.
The first hurdle will be making enough of them to go around. Case numbers are skyrocketing around the globe, and producing the drugs is a complex time- and labor-intensive process that requires specialized facilities. Antibodies are produced by cell lines in bioreactors, so a plant that churns out generic aspirin tablets can’t simply be converted into an antibody factory.
“These types of drugs are manufactured in a sterile injectables plant, which is different from a plant where oral solids are made,” says Kim Crabtree, senior director of pharma portfolio management for Henry Schein Medical, a medical supplies distributor. “Those are not as plentiful as a standard pill factory.”
The doses required are also relatively high – 1.2 g of each antibody in Regeneron’s cocktail – which will further strain production capacity. Leah Lipsich, PhD, vice president of strategic program direction at Regeneron, says the company is prepared for high demand and has been able to respond, thanks to its rapid development and manufacturing technology, known as VelociSuite, which allows it to rapidly scale-up from discovery to productions in weeks instead of months.
“We knew supply would be a huge problem for COVID-19, but because we had such confidence in our technology, we went immediately from research-scale to our largest-scale manufacturing,” she says. “We’ve been manufacturing our cocktail for months now.”
The company has also partnered with Roche, the biggest manufacturer and vendor of monoclonal antibodies in the world, to manufacture and supply the drugs. Once full manufacturing capacity is reached in 2021, the companies expect to produce at least 2 million doses a year.
Then there is the issue of getting the drugs from the factories to the places they will be used.
Antibodies are temperature sensitive and need to be refrigerated during transport and storage, so a cold-chain-compliant supply chain is required. Fortunately, they can be kept at standard refrigerator temperatures, ranging from 2° C to 8° C, rather than the ultra-low temperatures required by some COVID-19 vaccines.
Two million doses a year
Medical logistics companies have a lot of experience dealing with products like these and are well prepared to handle the new antibody drugs. “There are quite a few products like these on the market, and the supply chain is used to shipping them,” Ms. Crabtree says.
They will be shipped to distribution centers in refrigerated trucks, repacked into smaller lots that can sustain the correct temperature for 24 hours, and then sent to their final destination, often in something as simple as a Styrofoam cooler filled with dry ice.
The expected rise in demand shouldn’t be too much of an issue for distributors either, says Ms. Crabtree; they have built systems that can deal with short-term surges in volume. The annual flu vaccine, for example, involves shipping a lot of product in a very short time, usually from August to November. “The distribution system is used to seasonal variations and peaks in demand,” she says.
The next question is how the treatments will be administered. Although most patients who will receive monoclonal antibodies will be ambulatory and not hospitalized, the administration requires intravenous infusion. Hospitals, of course, have a lot of experience with intravenous drugs, but typically give them only to inpatients. Most other monoclonal antibody drugs – such as those for cancer and autoimmune disorders – are given in specialized suites in doctor’s offices or in stand-alone infusion clinics.
That means that the places best suited to treat COVID-19 patients with antibodies are those that regularly deal with people who are immunocompromised, and such patients should not be interacting with people who have an infectious disease. “How do we protect the staff and other patients?” Dr. Gandhi asks.
Protecting staff and other patients
This is not an insurmountable obstacle, he points out, but it is one that requires careful thought and planning to accommodate COVID-19 patients without unduly disrupting life-saving treatments for other patients. It might involve, for example, treating COVID-19 patients in sequestered parts of the clinic or at different times of day, with even greater attention paid to cleaning, he explains. “We now have many months of experience with infection control, so we know how to do this; it’s just a question of logistics.”
But even once all the details around manufacturing, transporting, and administering the drugs are sorted out, there is still the issue of how they will be distributed fairly and equitably.
Despite multiple companies working to produce an array of different antibody drugs, demand is still expected to exceed supply for many months. “With more than 200,000 new cases a day in the United States, there won’t be enough antibodies to treat all of the high-risk patients,” says Dr. Gandhi. “Most of us are worried that demand will far outstrip supply. People are talking about lotteries to determine who gets them.”
The Department of Health and Human Services will continue to distribute the drugs to states on the basis of their COVID-19 burdens, and the states will then decide how much to provide to each health care facility.
Although the HHS goal is to ensure that the drugs reach as many patients as possible, no matter where they live and regardless of their income, there are still concerns that larger facilities serving more affluent areas will end up being favored, if only because they are the ones best equipped to deal with the drugs right now.
“We are all aware that this has affected certain communities more, so we need to make sure that the drugs are used equitably and made available to the communities that were hardest hit,” says Dr. Gandhi. The ability to monitor drug distribution should be built into the rollout, so that institutions and governments will have some sense of whether they are being doled out evenly, he adds.
Equity in distribution will be an issue for the rest of the world as well. Currently, 80% of monoclonal antibodies are sold in Canada, Europe, and the United States; few, if any, are available in low- and middle-income countries. The treatments are expensive: the cost of producing one g of marketed monoclonal antibodies is between $95 and $200, which does not include the cost of R&D, packaging, shipping, or administration. The median price for antibody treatment not related to COVID-19 runs from $15,000 to $200,000 per year in the United States.
Regeneron’s Dr. Lipsich says that the company has not yet set a price for its antibody cocktail. The government paid $450 million for its 300,000 doses, but that price includes the costs of research, manufacturing, and distribution, so is not a useful indicator of the eventual per-dose price. “We’re not in a position to talk about how it will be priced yet, but we will do our best to make it affordable and accessible to all,” she says.
There are some projects underway to ensure that the drugs are made available in poorer countries. In April, the COVID-19 Therapeutics Accelerator – an initiative launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard to speed-up the response to the global pandemic – reserved manufacturing capacity with Fujifilm Diosynth Biotechnologies in Denmark for future monoclonal antibody therapies that will supply low- and middle-income countries. In October, the initiative announced that Eli Lilly would use that reserved capacity to produce its antibody drug starting in April 2021.
In the meantime, Lilly will make some of its product manufactured in other facilities available to lower-income countries. To help keep costs down, the company’s collaborators have agreed to waive their royalties on antibodies distributed in low- and middle-income countries.
“Everyone is looking carefully at how the drugs are distributed to ensure all will get access,” said Dr. Lipsich.
A version of this article first appeared on Medscape.com.
The United States has allocated more than 641,000 monoclonal antibody treatments for outpatients to ease pressure on strained hospitals, but officials from Operation Warp Speed report that more than half of that reserve sits unused as clinicians grapple with best practices.
There are space and personnel limitations in hospitals right now, Janet Woodcock, MD, therapeutics lead on Operation Warp Speed, acknowledges in an interview with this news organization. “Special areas and procedures must be set up.” And the operation is in the process of broadening availability beyond hospitals, she points out.
But for frontline clinicians, questions about treatment efficacy and the logistics of administering intravenous drugs to infectious outpatients loom large.
More than 50 monoclonal antibody products that target SARS-CoV-2 are now in development. The U.S. Food and Drug Administration has already issued Emergency Use Authorization (EUA) for two such drugs on the basis of phase 2 trial data – bamlanivimab, made by Eli Lilly, and a cocktail of casirivimab plus imdevimab, made by Regeneron – and another two-antibody cocktail from AstraZeneca, AZD7442, has started phase 3 clinical trials. The Regeneron combination was used to treat President Donald Trump when he contracted COVID-19 in October.
Monoclonal antibody drugs are based on the natural antibodies that the body uses to fight infections. They work by binding to a specific target and then blocking its action or flagging it for destruction by other parts of the immune system. Both bamlanivimab and the casirivimab plus imdevimab combination target the spike protein of the virus and stop it from attaching to and entering human cells.
Targeting the spike protein out of the hospital
The antibody drugs covered by EUAs do not cure COVID-19, but they have been shown to reduce hospitalizations and visits to the emergency department for patients at high risk for disease progression. They are approved to treat patients older than 12 years with mild to moderate COVID-19 who are at high risk of progressing to severe disease or hospitalization. They are not authorized for use in patients who have been hospitalized or who are on ventilators. The hope is that antibody drugs will reduce the number of severe cases of COVID-19 and ease pressure on overstretched hospitals.
Most COVID-19 patients are outpatients, so we need something to keep them from getting worse.
This is important because it targets the greatest need in COVID-19 therapeutics, says Rajesh Gandhi, MD, an infectious disease physician at Harvard Medical School in Boston, who is a member of two panels evaluating COVID-19 treatments: one for the Infectious Disease Society of America and the other for the National Institutes of Health. “Up to now, most of the focus has been on hospitalized patients,” he says, but “most COVID-19 patients are outpatients, so we need something to keep them from getting worse.”
Both panels have said that, despite the EUAs, more evidence is needed to be sure of the efficacy of the drugs and to determine which patients will benefit the most from them.
These aren’t the mature data from drug development that guideline groups are accustomed to working with, Dr. Woodcock points out. “But this is an emergency and the data taken as a whole are pretty convincing,” she says. “As I look at the totality of the evidence, monoclonal antibodies will have a big effect in keeping people out of the hospital and helping them recover faster.”
High-risk patients are eligible for treatment, especially those older than 65 years and those with comorbidities who are younger. Access to the drugs is increasing for clinicians who are able to infuse safely or work with a site that will.
In the Boston area, several hospitals, including Massachusetts General where Dr. Gandhi works, have set up infusion centers where newly diagnosed patients can get the antibody treatment if their doctor thinks it will benefit them. And Coram, a provider of at-home infusion therapy owned by the CVS pharmacy chain, is running a pilot program offering the Eli Lilly drug to people in seven cities – including Boston, Chicago, Los Angeles, and Tampa – and their surrounding communities with a physician referral.
Getting that referral could be tricky, however, for patients without a primary care physician or for those whose doctor isn’t already connected to one of the institutions providing the infusions. The hospitals are sending out communications on how patients and physicians can get the therapy, but Dr. Gandhi says that making information about access available should be a priority. The window for the effective treatment is small – the drugs appear to work best before patients begin to make their own antibodies, says Dr. Gandhi – so it’s vital that doctors act quickly if they have a patient who is eligible.
And rolling out the new therapies to patients around the world will be a major logistical undertaking.
The first hurdle will be making enough of them to go around. Case numbers are skyrocketing around the globe, and producing the drugs is a complex time- and labor-intensive process that requires specialized facilities. Antibodies are produced by cell lines in bioreactors, so a plant that churns out generic aspirin tablets can’t simply be converted into an antibody factory.
“These types of drugs are manufactured in a sterile injectables plant, which is different from a plant where oral solids are made,” says Kim Crabtree, senior director of pharma portfolio management for Henry Schein Medical, a medical supplies distributor. “Those are not as plentiful as a standard pill factory.”
The doses required are also relatively high – 1.2 g of each antibody in Regeneron’s cocktail – which will further strain production capacity. Leah Lipsich, PhD, vice president of strategic program direction at Regeneron, says the company is prepared for high demand and has been able to respond, thanks to its rapid development and manufacturing technology, known as VelociSuite, which allows it to rapidly scale-up from discovery to productions in weeks instead of months.
“We knew supply would be a huge problem for COVID-19, but because we had such confidence in our technology, we went immediately from research-scale to our largest-scale manufacturing,” she says. “We’ve been manufacturing our cocktail for months now.”
The company has also partnered with Roche, the biggest manufacturer and vendor of monoclonal antibodies in the world, to manufacture and supply the drugs. Once full manufacturing capacity is reached in 2021, the companies expect to produce at least 2 million doses a year.
Then there is the issue of getting the drugs from the factories to the places they will be used.
Antibodies are temperature sensitive and need to be refrigerated during transport and storage, so a cold-chain-compliant supply chain is required. Fortunately, they can be kept at standard refrigerator temperatures, ranging from 2° C to 8° C, rather than the ultra-low temperatures required by some COVID-19 vaccines.
Two million doses a year
Medical logistics companies have a lot of experience dealing with products like these and are well prepared to handle the new antibody drugs. “There are quite a few products like these on the market, and the supply chain is used to shipping them,” Ms. Crabtree says.
They will be shipped to distribution centers in refrigerated trucks, repacked into smaller lots that can sustain the correct temperature for 24 hours, and then sent to their final destination, often in something as simple as a Styrofoam cooler filled with dry ice.
The expected rise in demand shouldn’t be too much of an issue for distributors either, says Ms. Crabtree; they have built systems that can deal with short-term surges in volume. The annual flu vaccine, for example, involves shipping a lot of product in a very short time, usually from August to November. “The distribution system is used to seasonal variations and peaks in demand,” she says.
The next question is how the treatments will be administered. Although most patients who will receive monoclonal antibodies will be ambulatory and not hospitalized, the administration requires intravenous infusion. Hospitals, of course, have a lot of experience with intravenous drugs, but typically give them only to inpatients. Most other monoclonal antibody drugs – such as those for cancer and autoimmune disorders – are given in specialized suites in doctor’s offices or in stand-alone infusion clinics.
That means that the places best suited to treat COVID-19 patients with antibodies are those that regularly deal with people who are immunocompromised, and such patients should not be interacting with people who have an infectious disease. “How do we protect the staff and other patients?” Dr. Gandhi asks.
Protecting staff and other patients
This is not an insurmountable obstacle, he points out, but it is one that requires careful thought and planning to accommodate COVID-19 patients without unduly disrupting life-saving treatments for other patients. It might involve, for example, treating COVID-19 patients in sequestered parts of the clinic or at different times of day, with even greater attention paid to cleaning, he explains. “We now have many months of experience with infection control, so we know how to do this; it’s just a question of logistics.”
But even once all the details around manufacturing, transporting, and administering the drugs are sorted out, there is still the issue of how they will be distributed fairly and equitably.
Despite multiple companies working to produce an array of different antibody drugs, demand is still expected to exceed supply for many months. “With more than 200,000 new cases a day in the United States, there won’t be enough antibodies to treat all of the high-risk patients,” says Dr. Gandhi. “Most of us are worried that demand will far outstrip supply. People are talking about lotteries to determine who gets them.”
The Department of Health and Human Services will continue to distribute the drugs to states on the basis of their COVID-19 burdens, and the states will then decide how much to provide to each health care facility.
Although the HHS goal is to ensure that the drugs reach as many patients as possible, no matter where they live and regardless of their income, there are still concerns that larger facilities serving more affluent areas will end up being favored, if only because they are the ones best equipped to deal with the drugs right now.
“We are all aware that this has affected certain communities more, so we need to make sure that the drugs are used equitably and made available to the communities that were hardest hit,” says Dr. Gandhi. The ability to monitor drug distribution should be built into the rollout, so that institutions and governments will have some sense of whether they are being doled out evenly, he adds.
Equity in distribution will be an issue for the rest of the world as well. Currently, 80% of monoclonal antibodies are sold in Canada, Europe, and the United States; few, if any, are available in low- and middle-income countries. The treatments are expensive: the cost of producing one g of marketed monoclonal antibodies is between $95 and $200, which does not include the cost of R&D, packaging, shipping, or administration. The median price for antibody treatment not related to COVID-19 runs from $15,000 to $200,000 per year in the United States.
Regeneron’s Dr. Lipsich says that the company has not yet set a price for its antibody cocktail. The government paid $450 million for its 300,000 doses, but that price includes the costs of research, manufacturing, and distribution, so is not a useful indicator of the eventual per-dose price. “We’re not in a position to talk about how it will be priced yet, but we will do our best to make it affordable and accessible to all,” she says.
There are some projects underway to ensure that the drugs are made available in poorer countries. In April, the COVID-19 Therapeutics Accelerator – an initiative launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard to speed-up the response to the global pandemic – reserved manufacturing capacity with Fujifilm Diosynth Biotechnologies in Denmark for future monoclonal antibody therapies that will supply low- and middle-income countries. In October, the initiative announced that Eli Lilly would use that reserved capacity to produce its antibody drug starting in April 2021.
In the meantime, Lilly will make some of its product manufactured in other facilities available to lower-income countries. To help keep costs down, the company’s collaborators have agreed to waive their royalties on antibodies distributed in low- and middle-income countries.
“Everyone is looking carefully at how the drugs are distributed to ensure all will get access,” said Dr. Lipsich.
A version of this article first appeared on Medscape.com.
The United States has allocated more than 641,000 monoclonal antibody treatments for outpatients to ease pressure on strained hospitals, but officials from Operation Warp Speed report that more than half of that reserve sits unused as clinicians grapple with best practices.
There are space and personnel limitations in hospitals right now, Janet Woodcock, MD, therapeutics lead on Operation Warp Speed, acknowledges in an interview with this news organization. “Special areas and procedures must be set up.” And the operation is in the process of broadening availability beyond hospitals, she points out.
But for frontline clinicians, questions about treatment efficacy and the logistics of administering intravenous drugs to infectious outpatients loom large.
More than 50 monoclonal antibody products that target SARS-CoV-2 are now in development. The U.S. Food and Drug Administration has already issued Emergency Use Authorization (EUA) for two such drugs on the basis of phase 2 trial data – bamlanivimab, made by Eli Lilly, and a cocktail of casirivimab plus imdevimab, made by Regeneron – and another two-antibody cocktail from AstraZeneca, AZD7442, has started phase 3 clinical trials. The Regeneron combination was used to treat President Donald Trump when he contracted COVID-19 in October.
Monoclonal antibody drugs are based on the natural antibodies that the body uses to fight infections. They work by binding to a specific target and then blocking its action or flagging it for destruction by other parts of the immune system. Both bamlanivimab and the casirivimab plus imdevimab combination target the spike protein of the virus and stop it from attaching to and entering human cells.
Targeting the spike protein out of the hospital
The antibody drugs covered by EUAs do not cure COVID-19, but they have been shown to reduce hospitalizations and visits to the emergency department for patients at high risk for disease progression. They are approved to treat patients older than 12 years with mild to moderate COVID-19 who are at high risk of progressing to severe disease or hospitalization. They are not authorized for use in patients who have been hospitalized or who are on ventilators. The hope is that antibody drugs will reduce the number of severe cases of COVID-19 and ease pressure on overstretched hospitals.
Most COVID-19 patients are outpatients, so we need something to keep them from getting worse.
This is important because it targets the greatest need in COVID-19 therapeutics, says Rajesh Gandhi, MD, an infectious disease physician at Harvard Medical School in Boston, who is a member of two panels evaluating COVID-19 treatments: one for the Infectious Disease Society of America and the other for the National Institutes of Health. “Up to now, most of the focus has been on hospitalized patients,” he says, but “most COVID-19 patients are outpatients, so we need something to keep them from getting worse.”
Both panels have said that, despite the EUAs, more evidence is needed to be sure of the efficacy of the drugs and to determine which patients will benefit the most from them.
These aren’t the mature data from drug development that guideline groups are accustomed to working with, Dr. Woodcock points out. “But this is an emergency and the data taken as a whole are pretty convincing,” she says. “As I look at the totality of the evidence, monoclonal antibodies will have a big effect in keeping people out of the hospital and helping them recover faster.”
High-risk patients are eligible for treatment, especially those older than 65 years and those with comorbidities who are younger. Access to the drugs is increasing for clinicians who are able to infuse safely or work with a site that will.
In the Boston area, several hospitals, including Massachusetts General where Dr. Gandhi works, have set up infusion centers where newly diagnosed patients can get the antibody treatment if their doctor thinks it will benefit them. And Coram, a provider of at-home infusion therapy owned by the CVS pharmacy chain, is running a pilot program offering the Eli Lilly drug to people in seven cities – including Boston, Chicago, Los Angeles, and Tampa – and their surrounding communities with a physician referral.
Getting that referral could be tricky, however, for patients without a primary care physician or for those whose doctor isn’t already connected to one of the institutions providing the infusions. The hospitals are sending out communications on how patients and physicians can get the therapy, but Dr. Gandhi says that making information about access available should be a priority. The window for the effective treatment is small – the drugs appear to work best before patients begin to make their own antibodies, says Dr. Gandhi – so it’s vital that doctors act quickly if they have a patient who is eligible.
And rolling out the new therapies to patients around the world will be a major logistical undertaking.
The first hurdle will be making enough of them to go around. Case numbers are skyrocketing around the globe, and producing the drugs is a complex time- and labor-intensive process that requires specialized facilities. Antibodies are produced by cell lines in bioreactors, so a plant that churns out generic aspirin tablets can’t simply be converted into an antibody factory.
“These types of drugs are manufactured in a sterile injectables plant, which is different from a plant where oral solids are made,” says Kim Crabtree, senior director of pharma portfolio management for Henry Schein Medical, a medical supplies distributor. “Those are not as plentiful as a standard pill factory.”
The doses required are also relatively high – 1.2 g of each antibody in Regeneron’s cocktail – which will further strain production capacity. Leah Lipsich, PhD, vice president of strategic program direction at Regeneron, says the company is prepared for high demand and has been able to respond, thanks to its rapid development and manufacturing technology, known as VelociSuite, which allows it to rapidly scale-up from discovery to productions in weeks instead of months.
“We knew supply would be a huge problem for COVID-19, but because we had such confidence in our technology, we went immediately from research-scale to our largest-scale manufacturing,” she says. “We’ve been manufacturing our cocktail for months now.”
The company has also partnered with Roche, the biggest manufacturer and vendor of monoclonal antibodies in the world, to manufacture and supply the drugs. Once full manufacturing capacity is reached in 2021, the companies expect to produce at least 2 million doses a year.
Then there is the issue of getting the drugs from the factories to the places they will be used.
Antibodies are temperature sensitive and need to be refrigerated during transport and storage, so a cold-chain-compliant supply chain is required. Fortunately, they can be kept at standard refrigerator temperatures, ranging from 2° C to 8° C, rather than the ultra-low temperatures required by some COVID-19 vaccines.
Two million doses a year
Medical logistics companies have a lot of experience dealing with products like these and are well prepared to handle the new antibody drugs. “There are quite a few products like these on the market, and the supply chain is used to shipping them,” Ms. Crabtree says.
They will be shipped to distribution centers in refrigerated trucks, repacked into smaller lots that can sustain the correct temperature for 24 hours, and then sent to their final destination, often in something as simple as a Styrofoam cooler filled with dry ice.
The expected rise in demand shouldn’t be too much of an issue for distributors either, says Ms. Crabtree; they have built systems that can deal with short-term surges in volume. The annual flu vaccine, for example, involves shipping a lot of product in a very short time, usually from August to November. “The distribution system is used to seasonal variations and peaks in demand,” she says.
The next question is how the treatments will be administered. Although most patients who will receive monoclonal antibodies will be ambulatory and not hospitalized, the administration requires intravenous infusion. Hospitals, of course, have a lot of experience with intravenous drugs, but typically give them only to inpatients. Most other monoclonal antibody drugs – such as those for cancer and autoimmune disorders – are given in specialized suites in doctor’s offices or in stand-alone infusion clinics.
That means that the places best suited to treat COVID-19 patients with antibodies are those that regularly deal with people who are immunocompromised, and such patients should not be interacting with people who have an infectious disease. “How do we protect the staff and other patients?” Dr. Gandhi asks.
Protecting staff and other patients
This is not an insurmountable obstacle, he points out, but it is one that requires careful thought and planning to accommodate COVID-19 patients without unduly disrupting life-saving treatments for other patients. It might involve, for example, treating COVID-19 patients in sequestered parts of the clinic or at different times of day, with even greater attention paid to cleaning, he explains. “We now have many months of experience with infection control, so we know how to do this; it’s just a question of logistics.”
But even once all the details around manufacturing, transporting, and administering the drugs are sorted out, there is still the issue of how they will be distributed fairly and equitably.
Despite multiple companies working to produce an array of different antibody drugs, demand is still expected to exceed supply for many months. “With more than 200,000 new cases a day in the United States, there won’t be enough antibodies to treat all of the high-risk patients,” says Dr. Gandhi. “Most of us are worried that demand will far outstrip supply. People are talking about lotteries to determine who gets them.”
The Department of Health and Human Services will continue to distribute the drugs to states on the basis of their COVID-19 burdens, and the states will then decide how much to provide to each health care facility.
Although the HHS goal is to ensure that the drugs reach as many patients as possible, no matter where they live and regardless of their income, there are still concerns that larger facilities serving more affluent areas will end up being favored, if only because they are the ones best equipped to deal with the drugs right now.
“We are all aware that this has affected certain communities more, so we need to make sure that the drugs are used equitably and made available to the communities that were hardest hit,” says Dr. Gandhi. The ability to monitor drug distribution should be built into the rollout, so that institutions and governments will have some sense of whether they are being doled out evenly, he adds.
Equity in distribution will be an issue for the rest of the world as well. Currently, 80% of monoclonal antibodies are sold in Canada, Europe, and the United States; few, if any, are available in low- and middle-income countries. The treatments are expensive: the cost of producing one g of marketed monoclonal antibodies is between $95 and $200, which does not include the cost of R&D, packaging, shipping, or administration. The median price for antibody treatment not related to COVID-19 runs from $15,000 to $200,000 per year in the United States.
Regeneron’s Dr. Lipsich says that the company has not yet set a price for its antibody cocktail. The government paid $450 million for its 300,000 doses, but that price includes the costs of research, manufacturing, and distribution, so is not a useful indicator of the eventual per-dose price. “We’re not in a position to talk about how it will be priced yet, but we will do our best to make it affordable and accessible to all,” she says.
There are some projects underway to ensure that the drugs are made available in poorer countries. In April, the COVID-19 Therapeutics Accelerator – an initiative launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard to speed-up the response to the global pandemic – reserved manufacturing capacity with Fujifilm Diosynth Biotechnologies in Denmark for future monoclonal antibody therapies that will supply low- and middle-income countries. In October, the initiative announced that Eli Lilly would use that reserved capacity to produce its antibody drug starting in April 2021.
In the meantime, Lilly will make some of its product manufactured in other facilities available to lower-income countries. To help keep costs down, the company’s collaborators have agreed to waive their royalties on antibodies distributed in low- and middle-income countries.
“Everyone is looking carefully at how the drugs are distributed to ensure all will get access,” said Dr. Lipsich.
A version of this article first appeared on Medscape.com.
COVID-19 in children: Weekly cases trending downward
The United States added over 171,000 new COVID-19 cases in children during the week ending Jan. 7, but that figure is lower than 3 of the previous 4 weeks, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
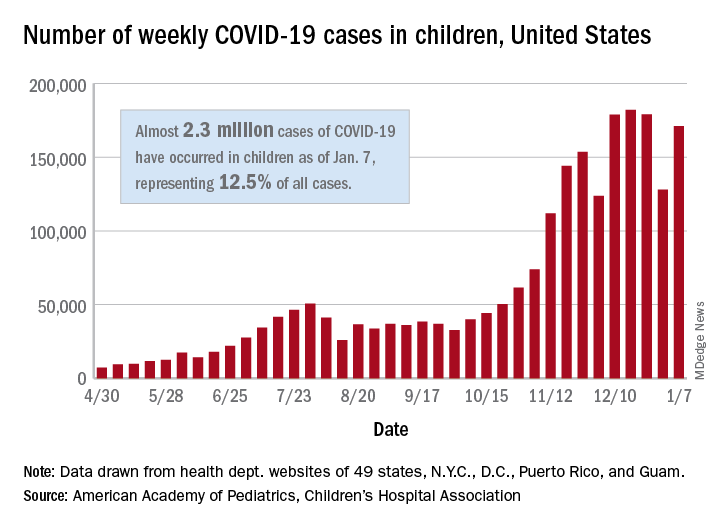
Despite an increase compared with the week ending Dec. 31, the most recent weekly total is down from the high of 182,000 cases reported for the week ending Dec. 17, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Those jurisdictions have recorded a total of almost 2.3 million COVID-19 cases in children since the beginning of the pandemic, which amounts to 12.5% of reported cases among all ages. The 171,000 child cases for the most recent week represented 12.9% of the more than 1.3 million cases nationwide, the AAP and CHA said in their latest weekly update.
The United States now has a rate of 3,055 COVID-19 cases per 100,000 children in the population, the report shows, with 31 states above that figure and 14 states reporting rates above 4,500 per 100,000 children.
Severe illness, however, continues to be rare among children. So far, children represent 1.8% of all hospitalizations in the jurisdictions reporting such data (24 states and New York City), and just 0.9% of infected children have been hospitalized. There have been 188 deaths among children in 42 states and New York City, which makes up just 0.06% of the total for all ages in those jurisdictions, the AAP and CHA reported.
There are 13 states that have reported no coronavirus-related deaths in children, while Texas (34), New York City (21), Arizona (17), and Illinois (11) are the only jurisdictions with 10 or more. Nevada has the highest proportion of child deaths to all deaths at 0.2%, with Arizona and Nebraska next at 0.18%, according to the AAP/CHA report.
The United States added over 171,000 new COVID-19 cases in children during the week ending Jan. 7, but that figure is lower than 3 of the previous 4 weeks, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Despite an increase compared with the week ending Dec. 31, the most recent weekly total is down from the high of 182,000 cases reported for the week ending Dec. 17, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Those jurisdictions have recorded a total of almost 2.3 million COVID-19 cases in children since the beginning of the pandemic, which amounts to 12.5% of reported cases among all ages. The 171,000 child cases for the most recent week represented 12.9% of the more than 1.3 million cases nationwide, the AAP and CHA said in their latest weekly update.
The United States now has a rate of 3,055 COVID-19 cases per 100,000 children in the population, the report shows, with 31 states above that figure and 14 states reporting rates above 4,500 per 100,000 children.
Severe illness, however, continues to be rare among children. So far, children represent 1.8% of all hospitalizations in the jurisdictions reporting such data (24 states and New York City), and just 0.9% of infected children have been hospitalized. There have been 188 deaths among children in 42 states and New York City, which makes up just 0.06% of the total for all ages in those jurisdictions, the AAP and CHA reported.
There are 13 states that have reported no coronavirus-related deaths in children, while Texas (34), New York City (21), Arizona (17), and Illinois (11) are the only jurisdictions with 10 or more. Nevada has the highest proportion of child deaths to all deaths at 0.2%, with Arizona and Nebraska next at 0.18%, according to the AAP/CHA report.
The United States added over 171,000 new COVID-19 cases in children during the week ending Jan. 7, but that figure is lower than 3 of the previous 4 weeks, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Despite an increase compared with the week ending Dec. 31, the most recent weekly total is down from the high of 182,000 cases reported for the week ending Dec. 17, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Those jurisdictions have recorded a total of almost 2.3 million COVID-19 cases in children since the beginning of the pandemic, which amounts to 12.5% of reported cases among all ages. The 171,000 child cases for the most recent week represented 12.9% of the more than 1.3 million cases nationwide, the AAP and CHA said in their latest weekly update.
The United States now has a rate of 3,055 COVID-19 cases per 100,000 children in the population, the report shows, with 31 states above that figure and 14 states reporting rates above 4,500 per 100,000 children.
Severe illness, however, continues to be rare among children. So far, children represent 1.8% of all hospitalizations in the jurisdictions reporting such data (24 states and New York City), and just 0.9% of infected children have been hospitalized. There have been 188 deaths among children in 42 states and New York City, which makes up just 0.06% of the total for all ages in those jurisdictions, the AAP and CHA reported.
There are 13 states that have reported no coronavirus-related deaths in children, while Texas (34), New York City (21), Arizona (17), and Illinois (11) are the only jurisdictions with 10 or more. Nevada has the highest proportion of child deaths to all deaths at 0.2%, with Arizona and Nebraska next at 0.18%, according to the AAP/CHA report.