User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
ACEIs, ARBs safe to continue in COVID-19: Trial published
The BRACE-CORONA trial, the first randomized trial to address the question of whether patients with COVID-19 should continue to take ACE inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) – has now been published.
The study, which was conducted in patients hospitalized with COVID-19 who were taking ACEIs or ARBs before hospitalization, showed no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue versus those assigned to continue these medications.
There were, however, hints that continuing to take ACEIs or ARBs may be beneficial for patients with more severe COVID-19.
The study was first presented at last year’s European Society of Cardiology Congress and was reported by this news organization at that time. The study was published online in JAMA on Jan. 19, 2021.
“These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment,” the authors concluded.
Led by Renato D. Lopes, MD, Duke Clinical Research Institute, Durham, N.C., the researchers explained that there has been conflicting speculation about the effect of renin-angiotensin-aldosterone system (RAAS) inhibitors on the course of COVID-19.
On the one hand, observations from animal models suggest that ACEIs and ARBs up-regulate the expression of ACE2, a receptor involved in SARS-CoV-2 infection of host target cells. This led to suggestions that these medications may enhance viral binding and cell entry. Conversely, RAAS inhibitors could benefit patients with COVID-19 through effects on angiotensin II expression and subsequent increases in angiotensin 1-7 and 1-9, which have vasodilatory and anti-inflammatory effects that might attenuate lung injury.
The BRACE-CORONA trial included 659 patients hospitalized in Brazil with mild to moderate COVID-19 who were taking ACEIs or ARBs prior to hospitalization. The median age of the patients was 55 years. Of these patients, 57.1% were considered to have mild cases at hospital admission, and 42.9% were considered to have moderate cases.
Results showed no significant difference in the number of days alive and out of the hospital for patients in the discontinuation group (mean, 21.9 days) in comparison with patients in the continuation group (mean, 22.9 days). The mean ratio was 0.95 (95% confidence interval, 0.90-1.01).
There also was no statistically significant difference in deaths (2.7% of the discontinuation group vs. 2.8% for the continuation group); cardiovascular death (0.6% vs. 0.3%), or COVID-19 progression (38.3% vs. 32.3%).
The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinuation group vs. 7.7% in the continuation group), shock requiring vasopressors (8.4% vs. 7.1%), acute MI (7.5% vs. 4.6%), new or worsening heart failure (4.2% vs. 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs. 2.8%).
The authors note that hypertension is an important comorbidity in patients with COVID-19. Recent data suggest that immune dysfunction may contribute to poor outcomes among patients who have COVID-19 and hypertension.
It has been shown that, when use of long-term medications is discontinued during hospitalization, the use of those medications is often not resumed, owing to clinical inertia. Long-term outcomes worsen as a result, the authors reported. In the current study, all patients had hypertension, and more than 50% were obese; both of these comorbidities increase the risk for poor outcomes with COVID-19.
The investigators pointed out that a sensitivity analysis in which site was regarded as a random effect showed a statistically significant finding in favor of the group that continued ACEIs or ARBs. This finding was similar to that of the on-treatment analysis. There were also statistically significant interactions between treatment effect and some subgroups, such as patients with lower oxygen saturation and greater disease severity at hospital admission. For these patients, continuing ACEIs or ARBs may be beneficial.
“The primary analyses with the null results but wide 95% confidence intervals suggest that the study might have been underpowered to detect a statistically significant benefit of continuing ACEIs or ARBs,” they said.
Dr. Lopes has received grant support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola, and Sanofi.
A version of this article first appeared on Medscape.com.
The BRACE-CORONA trial, the first randomized trial to address the question of whether patients with COVID-19 should continue to take ACE inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) – has now been published.
The study, which was conducted in patients hospitalized with COVID-19 who were taking ACEIs or ARBs before hospitalization, showed no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue versus those assigned to continue these medications.
There were, however, hints that continuing to take ACEIs or ARBs may be beneficial for patients with more severe COVID-19.
The study was first presented at last year’s European Society of Cardiology Congress and was reported by this news organization at that time. The study was published online in JAMA on Jan. 19, 2021.
“These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment,” the authors concluded.
Led by Renato D. Lopes, MD, Duke Clinical Research Institute, Durham, N.C., the researchers explained that there has been conflicting speculation about the effect of renin-angiotensin-aldosterone system (RAAS) inhibitors on the course of COVID-19.
On the one hand, observations from animal models suggest that ACEIs and ARBs up-regulate the expression of ACE2, a receptor involved in SARS-CoV-2 infection of host target cells. This led to suggestions that these medications may enhance viral binding and cell entry. Conversely, RAAS inhibitors could benefit patients with COVID-19 through effects on angiotensin II expression and subsequent increases in angiotensin 1-7 and 1-9, which have vasodilatory and anti-inflammatory effects that might attenuate lung injury.
The BRACE-CORONA trial included 659 patients hospitalized in Brazil with mild to moderate COVID-19 who were taking ACEIs or ARBs prior to hospitalization. The median age of the patients was 55 years. Of these patients, 57.1% were considered to have mild cases at hospital admission, and 42.9% were considered to have moderate cases.
Results showed no significant difference in the number of days alive and out of the hospital for patients in the discontinuation group (mean, 21.9 days) in comparison with patients in the continuation group (mean, 22.9 days). The mean ratio was 0.95 (95% confidence interval, 0.90-1.01).
There also was no statistically significant difference in deaths (2.7% of the discontinuation group vs. 2.8% for the continuation group); cardiovascular death (0.6% vs. 0.3%), or COVID-19 progression (38.3% vs. 32.3%).
The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinuation group vs. 7.7% in the continuation group), shock requiring vasopressors (8.4% vs. 7.1%), acute MI (7.5% vs. 4.6%), new or worsening heart failure (4.2% vs. 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs. 2.8%).
The authors note that hypertension is an important comorbidity in patients with COVID-19. Recent data suggest that immune dysfunction may contribute to poor outcomes among patients who have COVID-19 and hypertension.
It has been shown that, when use of long-term medications is discontinued during hospitalization, the use of those medications is often not resumed, owing to clinical inertia. Long-term outcomes worsen as a result, the authors reported. In the current study, all patients had hypertension, and more than 50% were obese; both of these comorbidities increase the risk for poor outcomes with COVID-19.
The investigators pointed out that a sensitivity analysis in which site was regarded as a random effect showed a statistically significant finding in favor of the group that continued ACEIs or ARBs. This finding was similar to that of the on-treatment analysis. There were also statistically significant interactions between treatment effect and some subgroups, such as patients with lower oxygen saturation and greater disease severity at hospital admission. For these patients, continuing ACEIs or ARBs may be beneficial.
“The primary analyses with the null results but wide 95% confidence intervals suggest that the study might have been underpowered to detect a statistically significant benefit of continuing ACEIs or ARBs,” they said.
Dr. Lopes has received grant support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola, and Sanofi.
A version of this article first appeared on Medscape.com.
The BRACE-CORONA trial, the first randomized trial to address the question of whether patients with COVID-19 should continue to take ACE inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) – has now been published.
The study, which was conducted in patients hospitalized with COVID-19 who were taking ACEIs or ARBs before hospitalization, showed no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue versus those assigned to continue these medications.
There were, however, hints that continuing to take ACEIs or ARBs may be beneficial for patients with more severe COVID-19.
The study was first presented at last year’s European Society of Cardiology Congress and was reported by this news organization at that time. The study was published online in JAMA on Jan. 19, 2021.
“These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment,” the authors concluded.
Led by Renato D. Lopes, MD, Duke Clinical Research Institute, Durham, N.C., the researchers explained that there has been conflicting speculation about the effect of renin-angiotensin-aldosterone system (RAAS) inhibitors on the course of COVID-19.
On the one hand, observations from animal models suggest that ACEIs and ARBs up-regulate the expression of ACE2, a receptor involved in SARS-CoV-2 infection of host target cells. This led to suggestions that these medications may enhance viral binding and cell entry. Conversely, RAAS inhibitors could benefit patients with COVID-19 through effects on angiotensin II expression and subsequent increases in angiotensin 1-7 and 1-9, which have vasodilatory and anti-inflammatory effects that might attenuate lung injury.
The BRACE-CORONA trial included 659 patients hospitalized in Brazil with mild to moderate COVID-19 who were taking ACEIs or ARBs prior to hospitalization. The median age of the patients was 55 years. Of these patients, 57.1% were considered to have mild cases at hospital admission, and 42.9% were considered to have moderate cases.
Results showed no significant difference in the number of days alive and out of the hospital for patients in the discontinuation group (mean, 21.9 days) in comparison with patients in the continuation group (mean, 22.9 days). The mean ratio was 0.95 (95% confidence interval, 0.90-1.01).
There also was no statistically significant difference in deaths (2.7% of the discontinuation group vs. 2.8% for the continuation group); cardiovascular death (0.6% vs. 0.3%), or COVID-19 progression (38.3% vs. 32.3%).
The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinuation group vs. 7.7% in the continuation group), shock requiring vasopressors (8.4% vs. 7.1%), acute MI (7.5% vs. 4.6%), new or worsening heart failure (4.2% vs. 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs. 2.8%).
The authors note that hypertension is an important comorbidity in patients with COVID-19. Recent data suggest that immune dysfunction may contribute to poor outcomes among patients who have COVID-19 and hypertension.
It has been shown that, when use of long-term medications is discontinued during hospitalization, the use of those medications is often not resumed, owing to clinical inertia. Long-term outcomes worsen as a result, the authors reported. In the current study, all patients had hypertension, and more than 50% were obese; both of these comorbidities increase the risk for poor outcomes with COVID-19.
The investigators pointed out that a sensitivity analysis in which site was regarded as a random effect showed a statistically significant finding in favor of the group that continued ACEIs or ARBs. This finding was similar to that of the on-treatment analysis. There were also statistically significant interactions between treatment effect and some subgroups, such as patients with lower oxygen saturation and greater disease severity at hospital admission. For these patients, continuing ACEIs or ARBs may be beneficial.
“The primary analyses with the null results but wide 95% confidence intervals suggest that the study might have been underpowered to detect a statistically significant benefit of continuing ACEIs or ARBs,” they said.
Dr. Lopes has received grant support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola, and Sanofi.
A version of this article first appeared on Medscape.com.
President Biden signs 10 new orders to help fight COVID-19

“For the past year, we couldn’t rely on the federal government to act with the urgency and focus and coordination we needed, and we have seen the tragic cost of that failure,” Mr. Biden said in remarks from the White House, unveiling his 198-page National Strategy for the COVID-19 Response and Pandemic Preparedness.
He said as many as 500,000 Americans will have died by February. “It’s going to take months for us to turn things around,” he said.
“Our national strategy is comprehensive – it’s based on science, not politics; it’s based on truth, not denial,” Mr. Biden said. He also promised to restore public trust, in part by having scientists and public health experts speak to the public. “That’s why you’ll be hearing a lot more from Dr. Fauci again, not from the president,” he said, adding that the experts will be “free from political interference.”
While the president’s executive orders can help accomplish some of the plan’s proposals, the majority will require new funding from Congress and will be included in the $1.9 trillion American Rescue package that Mr. Biden hopes legislators will approve.
Ten new orders
The 10 new pandemic-related orders Biden signed on Jan. 21 follow two he signed on his first day in office.
One establishes a COVID-19 Response Office responsible for coordinating the pandemic response across all federal departments and agencies and also reestablishes the White House Directorate on Global Health Security and Biodefense, which was disabled by the Trump administration.
The other order requires masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
Among the new orders will be directives that:
- Require individuals to also wear masks in airports and planes, and when using other modes of public transportation including trains, boats, and intercity buses, and also require international travelers to produce proof of a recent negative COVID-19 test prior to entry and to quarantine after entry.
- Federal agencies use all powers, including the Defense Production Act, to accelerate manufacturing and delivery of supplies such as N95 masks, gowns, gloves, swabs, reagents, pipette tips, rapid test kits, and nitrocellulose material for rapid antigen tests, and all equipment and material needed to accelerate manufacture, delivery, and administration of COVID-19 vaccine.
- Create a Pandemic Testing Board to expand supply and access, to promote more surge capacity, and to ensure equitable access to tests.
- Facilitate discovery, development, and trials of potential COVID-19 treatments, as well as expand access to programs that can meet the long-term health needs of those recovering from the disease.
- Facilitate more and better data sharing that will allow businesses, schools, hospitals, and individuals to make real-time decisions based on spread in their community.
- Direct the Education and Health & Human Services departments to provide schools and child-care operations guidance on how to reopen and operate safely.
- Direct the Occupational Safety and Health Administration (OSHA) to immediately release clear guidance for employers to help keep workers safe and to enforce health and safety requirements.
The plan also sets goals for vaccination – including 100 million shots in the administration’s first 100 days. President Biden had already previewed his goals for vaccination, including setting up mass vaccination sites and mobile vaccination sites. During his remarks, Mr. Biden said that he had already directed the Federal Emergency Management Agency (FEMA) to begin setting up the vaccination centers.
The administration is also going to look into improving reimbursement for giving vaccines. As a start, the HHS will ask the Centers for Medicare & Medicaid Services to consider if a higher rate “may more accurately compensate providers,” according to the Biden plan.
“But the brutal truth is it will take months before we can get the majority of Americans vaccinated,” said Mr. Biden.
As part of the goal of ensuring an equitable pandemic response, the president will sign an order that establishes a COVID-19 Health Equity Task Force. The task force is charged with providing recommendations for allocating resources and funding in communities with inequities in COVID-19 outcomes by race, ethnicity, geography, disability, and other considerations.
Finally, the administration has committed to being more transparent and sharing more information. The national plan calls for the federal government to conduct regular, expert-led, science-based public briefings and to release regular reports on the pandemic. The administration said it will launch massive science-based public information campaigns – in multiple languages – to educate Americans on masks, testing, and vaccines, and also work to counter misinformation and disinformation.
The American Academy of Family Physicians (AAFP) applauded Mr. Biden’s initiative. “If enacted, this bold legislative agenda will provide much-needed support to American families struggling during the pandemic – especially communities of color and those hardest hit by the virus,” Ada D. Stewart, MD, AAFP president, said in a statement.
Dr. Stewart also noted that family physicians “are uniquely positioned in their communities to educate patients, prioritize access, and coordinate administration of the COVID-19 vaccines,” and urged the administration to ensure that family physicians and staff be vaccinated as soon as possible, to help them “more safely provide care to their communities.”
A version of this article first appeared on Medscape.com.

“For the past year, we couldn’t rely on the federal government to act with the urgency and focus and coordination we needed, and we have seen the tragic cost of that failure,” Mr. Biden said in remarks from the White House, unveiling his 198-page National Strategy for the COVID-19 Response and Pandemic Preparedness.
He said as many as 500,000 Americans will have died by February. “It’s going to take months for us to turn things around,” he said.
“Our national strategy is comprehensive – it’s based on science, not politics; it’s based on truth, not denial,” Mr. Biden said. He also promised to restore public trust, in part by having scientists and public health experts speak to the public. “That’s why you’ll be hearing a lot more from Dr. Fauci again, not from the president,” he said, adding that the experts will be “free from political interference.”
While the president’s executive orders can help accomplish some of the plan’s proposals, the majority will require new funding from Congress and will be included in the $1.9 trillion American Rescue package that Mr. Biden hopes legislators will approve.
Ten new orders
The 10 new pandemic-related orders Biden signed on Jan. 21 follow two he signed on his first day in office.
One establishes a COVID-19 Response Office responsible for coordinating the pandemic response across all federal departments and agencies and also reestablishes the White House Directorate on Global Health Security and Biodefense, which was disabled by the Trump administration.
The other order requires masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
Among the new orders will be directives that:
- Require individuals to also wear masks in airports and planes, and when using other modes of public transportation including trains, boats, and intercity buses, and also require international travelers to produce proof of a recent negative COVID-19 test prior to entry and to quarantine after entry.
- Federal agencies use all powers, including the Defense Production Act, to accelerate manufacturing and delivery of supplies such as N95 masks, gowns, gloves, swabs, reagents, pipette tips, rapid test kits, and nitrocellulose material for rapid antigen tests, and all equipment and material needed to accelerate manufacture, delivery, and administration of COVID-19 vaccine.
- Create a Pandemic Testing Board to expand supply and access, to promote more surge capacity, and to ensure equitable access to tests.
- Facilitate discovery, development, and trials of potential COVID-19 treatments, as well as expand access to programs that can meet the long-term health needs of those recovering from the disease.
- Facilitate more and better data sharing that will allow businesses, schools, hospitals, and individuals to make real-time decisions based on spread in their community.
- Direct the Education and Health & Human Services departments to provide schools and child-care operations guidance on how to reopen and operate safely.
- Direct the Occupational Safety and Health Administration (OSHA) to immediately release clear guidance for employers to help keep workers safe and to enforce health and safety requirements.
The plan also sets goals for vaccination – including 100 million shots in the administration’s first 100 days. President Biden had already previewed his goals for vaccination, including setting up mass vaccination sites and mobile vaccination sites. During his remarks, Mr. Biden said that he had already directed the Federal Emergency Management Agency (FEMA) to begin setting up the vaccination centers.
The administration is also going to look into improving reimbursement for giving vaccines. As a start, the HHS will ask the Centers for Medicare & Medicaid Services to consider if a higher rate “may more accurately compensate providers,” according to the Biden plan.
“But the brutal truth is it will take months before we can get the majority of Americans vaccinated,” said Mr. Biden.
As part of the goal of ensuring an equitable pandemic response, the president will sign an order that establishes a COVID-19 Health Equity Task Force. The task force is charged with providing recommendations for allocating resources and funding in communities with inequities in COVID-19 outcomes by race, ethnicity, geography, disability, and other considerations.
Finally, the administration has committed to being more transparent and sharing more information. The national plan calls for the federal government to conduct regular, expert-led, science-based public briefings and to release regular reports on the pandemic. The administration said it will launch massive science-based public information campaigns – in multiple languages – to educate Americans on masks, testing, and vaccines, and also work to counter misinformation and disinformation.
The American Academy of Family Physicians (AAFP) applauded Mr. Biden’s initiative. “If enacted, this bold legislative agenda will provide much-needed support to American families struggling during the pandemic – especially communities of color and those hardest hit by the virus,” Ada D. Stewart, MD, AAFP president, said in a statement.
Dr. Stewart also noted that family physicians “are uniquely positioned in their communities to educate patients, prioritize access, and coordinate administration of the COVID-19 vaccines,” and urged the administration to ensure that family physicians and staff be vaccinated as soon as possible, to help them “more safely provide care to their communities.”
A version of this article first appeared on Medscape.com.

“For the past year, we couldn’t rely on the federal government to act with the urgency and focus and coordination we needed, and we have seen the tragic cost of that failure,” Mr. Biden said in remarks from the White House, unveiling his 198-page National Strategy for the COVID-19 Response and Pandemic Preparedness.
He said as many as 500,000 Americans will have died by February. “It’s going to take months for us to turn things around,” he said.
“Our national strategy is comprehensive – it’s based on science, not politics; it’s based on truth, not denial,” Mr. Biden said. He also promised to restore public trust, in part by having scientists and public health experts speak to the public. “That’s why you’ll be hearing a lot more from Dr. Fauci again, not from the president,” he said, adding that the experts will be “free from political interference.”
While the president’s executive orders can help accomplish some of the plan’s proposals, the majority will require new funding from Congress and will be included in the $1.9 trillion American Rescue package that Mr. Biden hopes legislators will approve.
Ten new orders
The 10 new pandemic-related orders Biden signed on Jan. 21 follow two he signed on his first day in office.
One establishes a COVID-19 Response Office responsible for coordinating the pandemic response across all federal departments and agencies and also reestablishes the White House Directorate on Global Health Security and Biodefense, which was disabled by the Trump administration.
The other order requires masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
Among the new orders will be directives that:
- Require individuals to also wear masks in airports and planes, and when using other modes of public transportation including trains, boats, and intercity buses, and also require international travelers to produce proof of a recent negative COVID-19 test prior to entry and to quarantine after entry.
- Federal agencies use all powers, including the Defense Production Act, to accelerate manufacturing and delivery of supplies such as N95 masks, gowns, gloves, swabs, reagents, pipette tips, rapid test kits, and nitrocellulose material for rapid antigen tests, and all equipment and material needed to accelerate manufacture, delivery, and administration of COVID-19 vaccine.
- Create a Pandemic Testing Board to expand supply and access, to promote more surge capacity, and to ensure equitable access to tests.
- Facilitate discovery, development, and trials of potential COVID-19 treatments, as well as expand access to programs that can meet the long-term health needs of those recovering from the disease.
- Facilitate more and better data sharing that will allow businesses, schools, hospitals, and individuals to make real-time decisions based on spread in their community.
- Direct the Education and Health & Human Services departments to provide schools and child-care operations guidance on how to reopen and operate safely.
- Direct the Occupational Safety and Health Administration (OSHA) to immediately release clear guidance for employers to help keep workers safe and to enforce health and safety requirements.
The plan also sets goals for vaccination – including 100 million shots in the administration’s first 100 days. President Biden had already previewed his goals for vaccination, including setting up mass vaccination sites and mobile vaccination sites. During his remarks, Mr. Biden said that he had already directed the Federal Emergency Management Agency (FEMA) to begin setting up the vaccination centers.
The administration is also going to look into improving reimbursement for giving vaccines. As a start, the HHS will ask the Centers for Medicare & Medicaid Services to consider if a higher rate “may more accurately compensate providers,” according to the Biden plan.
“But the brutal truth is it will take months before we can get the majority of Americans vaccinated,” said Mr. Biden.
As part of the goal of ensuring an equitable pandemic response, the president will sign an order that establishes a COVID-19 Health Equity Task Force. The task force is charged with providing recommendations for allocating resources and funding in communities with inequities in COVID-19 outcomes by race, ethnicity, geography, disability, and other considerations.
Finally, the administration has committed to being more transparent and sharing more information. The national plan calls for the federal government to conduct regular, expert-led, science-based public briefings and to release regular reports on the pandemic. The administration said it will launch massive science-based public information campaigns – in multiple languages – to educate Americans on masks, testing, and vaccines, and also work to counter misinformation and disinformation.
The American Academy of Family Physicians (AAFP) applauded Mr. Biden’s initiative. “If enacted, this bold legislative agenda will provide much-needed support to American families struggling during the pandemic – especially communities of color and those hardest hit by the virus,” Ada D. Stewart, MD, AAFP president, said in a statement.
Dr. Stewart also noted that family physicians “are uniquely positioned in their communities to educate patients, prioritize access, and coordinate administration of the COVID-19 vaccines,” and urged the administration to ensure that family physicians and staff be vaccinated as soon as possible, to help them “more safely provide care to their communities.”
A version of this article first appeared on Medscape.com.
Patients fend for themselves to access highly touted COVID antibody treatments
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Biden’s COVID-19 challenge: 100 million vaccinations in the first 100 days. It won’t be easy.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
Many EM docs have treated COVID-19 patients without proper PPE: Survey
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.
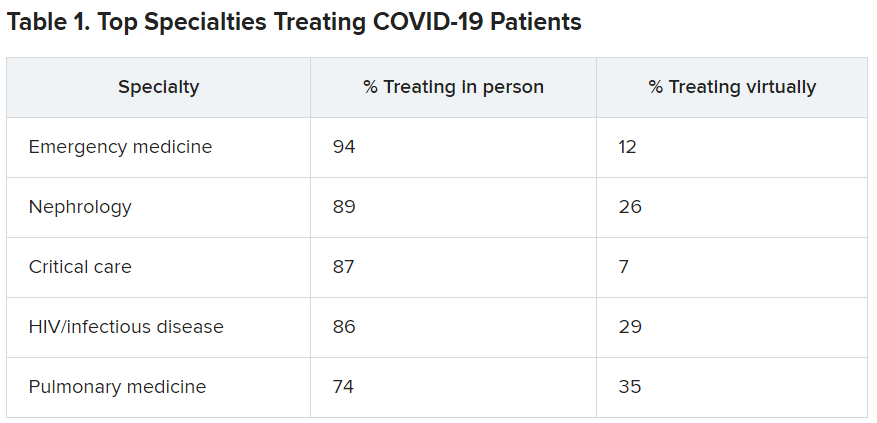
For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.
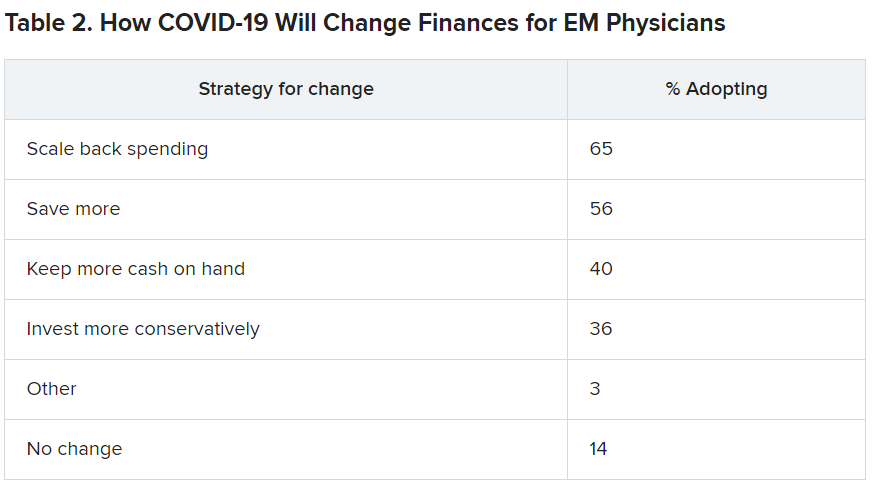
Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.

For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.

Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.

For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.

Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
COVID-19 in children: Latest weekly increase is largest yet
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
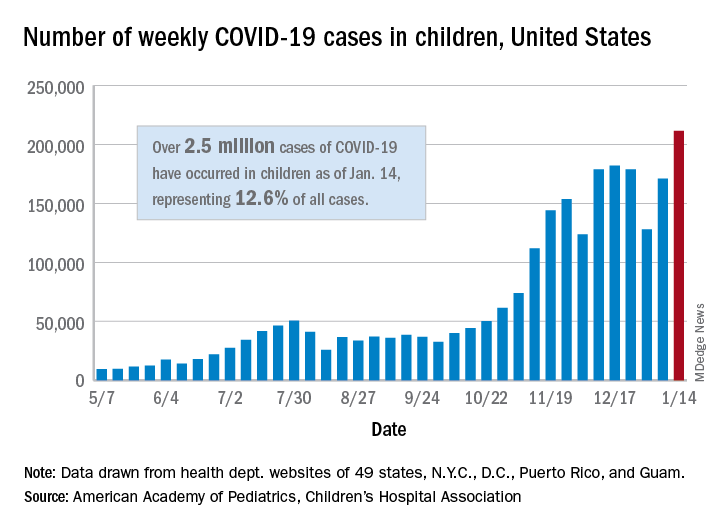
There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
Arthritis drugs ‘impressive’ for severe COVID but not ‘magic cure’
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
Long-haul COVID-19 cases rise as stigma of chronic fatigue taunts
When Margot Gage-Witvliet began feeling run down after her family returned from a trip to the Netherlands in late February 2020, she initially chalked up her symptoms to jet lag. Three days later, however, her situation went from concerning to alarming as she struggled to breathe. “It felt like there was an elephant sitting on my chest,” she said.
Her husband and daughters also became ill with COVID-19, but Ms. Gage-Witvliet was the only one in her family who didn’t get better. After an early improvement, a rare coronavirus-induced tonic-clonic seizure in early April sent her spiraling back down. Ms. Gage-Witvliet spent the next several weeks in bed with the curtains drawn, unable to tolerate light or sound.
Today, Ms. Gage-Witvliet’s life looks nothing like it did 6 months ago when she first got sick. As one of COVID-19’s so called long-haulers, she continues to struggle with crushing fatigue, brain fog, and headaches – symptoms that worsen when she pushes herself to do more. Across the country, as many as 1 in 10 COVID-19 patients are reporting illnesses that continue for weeks and months after their initial diagnosis. Nearly all report neurologic issues like Ms. Gage-Witvliet, as well as shortness of breath and psychiatric concerns.
For Avindra Nath, MD, a neurologist at the National Institutes of Health, the experience of these long-haul COVID-19 patients feels familiar and reminds him of myalgic encephalomyelitis, also known as chronic fatigue syndrome.
Dr. Nath has long been interested in the lingering neurologic issues connected to chronic fatigue. An estimated three-quarters of all patients with chronic fatigue syndrome report that their symptoms started after a viral infection, and they suffer unrelenting exhaustion, difficulties regulating pulse and blood pressure, aches and pains, and brain fog. When Dr. Nath first read about the novel coronavirus, he began to worry that the virus would trigger symptoms in a subset of those infected. Hearing about the experiences of long-haulers like Ms. Gage-Witvliet raised his suspicions even more.
Unlike COVID-19 long-haulers, however, many patients with chronic fatigue syndrome go at least a year with these symptoms before receiving a diagnosis, according to a British survey. That means researchers have had few opportunities to study the early stages of the syndrome. “When we see patients with myalgic encephalomyelitis, whatever infection they might have had occurred in the remote past, so there’s no way for us to know how they got infected with it, what the infection was, or what the effects of it were in that early phase. We’re seeing them 2 years afterward,” Dr. Nath said.
Dr. Nath quickly realized that studying patients like Ms. Gage-Witvliet would give physicians and scientists a unique opportunity to understand not only long-term outcomes of COVID-19 infections, but also other postviral syndromes, including chronic fatigue syndrome at their earliest stages. It’s why Dr. Nath has spent the past several months scrambling to launch two NIH studies to examine the phenomenon.
Although Dr. Nath said that the parallels between COVID-19 long-haulers and those with chronic fatigue syndrome are obvious, he cautions against assuming that they are the same phenomenon. Some long-haulers might simply be taking a much slower path to recovery, or they might have a condition that looks similar on the surface but differs from chronic fatigue syndrome on a molecular level. But even if Dr. Nath fails to see links to chronic fatigue syndrome, with more than 92.5 million documented cases of COVID-19 around the world, the work will be relevant to the substantial number of infected individuals who don’t recover quickly.
“With so many people having exposure to the same virus over a similar time period, we really have the opportunity to look at these manifestations and at the very least to understand postviral syndromes,” said Mady Hornig, MD, a psychiatrist at Columbia University, New York.
The origins of chronic fatigue syndrome date back to 1985, when the Centers for Disease Control and Prevention received a request from two physicians – Paul Cheney, MD, and Daniel Peterson, MD – to investigate a mysterious disease outbreak in Nevada. In November 1984, residents in and around the idyllic vacation spot of Incline Village, a small town tucked into the north shore of Lake Tahoe, had begun reporting flu-like symptoms that persisted for weeks, even months. The doctors had searched high and low for a cause, but they couldn’t figure out what was making their patients sick.
They reported a range of symptoms – including muscle aches and pains, low-grade fevers, sore throats, and headaches – but everyone said that crippling fatigue was the most debilitating issue. This wasn’t the kind of fatigue that could be cured by a nap or even a long holiday. No matter how much their patients slept – and some were almost completely bedbound – their fatigue didn’t abate. What’s more, the fatigue got worse whenever they tried to push themselves to do more. Puzzled, the CDC sent two epidemic intelligence service (EIS) officers to try to get to the bottom of what might be happening.
Muscle aches and pains with crippling fatigue
After their visit to Incline Village, however, the CDC was just as perplexed as Dr. Cheney and Dr. Peterson. Many of the people with the condition reported flu-like symptoms right around the time they first got sick, and the physicians’ leading hypothesis was that the outbreak and its lasting symptoms were caused by chronic Epstein-Barr virus infection. But neither the CDC nor anyone else could identify the infection or any other microbial cause. The two EIS officers duly wrote up a report for the CDC’s flagship publication, Morbidity and Mortality Weekly ReportI, titled “Chronic Fatigue Possibly Related to Epstein-Barr Virus – Nevada”.
That investigators focused on the fatigue aspect made sense, says Leonard A. Jason, PhD, professor of psychology at DePaul University and director of the Center for Community Research, both in Chicago, because it was one of the few symptoms shared by all the individuals studied and it was also the most debilitating. But that focus – and the name “chronic fatigue syndrome” – led to broad public dismissal of the condition’s severity, as did an editorial note in MMWR urging physicians to look for “more definable, and possibly treatable, conditions.” Subsequent research failed to confirm a specific link to the Epstein-Barr virus, which only added to the condition’s phony reputation. Rather than being considered a potentially disabling illness, it was disregarded as a “yuppie flu” or a fancy name for malingering.
“It’s not a surprise that patients are being dismissed because there’s already this sort of grandfathered-in sense that fatigue is not real,” said Jennifer Frankovich, MD, a pediatric rheumatologist at Stanford (Calif.) University’s Lucile Packard Children’s Hospital in Palo Alto. “I’m sure that’s frustrating for them to be tired and then to have the clinician not believe them or dismiss them or think they’re making it up. It would be more helpful to the families to say: ‘You know what, we don’t know, we do not have the answer, and we believe you.’ ”
A syndrome’s shame
As time passed, patient advocacy groups began pushing back against the negative way the condition was being perceived. This criticism came as organizations like the CDC worked to develop a set of diagnostic criteria that researchers and clinicians dealing with chronic fatigue syndrome could use. With such a heterogeneous group of patients and symptoms, the task was no small challenge. The discussions, which took place over nearly 2 decades, played a key role in helping scientists home in on the single factor that was central to chronic fatigue: postexertional malaise.
“This is quite unique for chronic fatigue syndrome. With other diseases, yes, you may have fatigue as one of the components of the disease, but postexertional fatigue is very specific,” said Alain Moreau, PhD, a molecular biologist at the University of Montreal.
Of course, plenty of people have pushed themselves too hard physically and paid the price the next day. But those with chronic fatigue syndrome weren’t running marathons. To them, exertion could be anything from getting the mail to reading a book. Nor could the resulting exhaustion be resolved by an afternoon on the couch or a long vacation.
“If they do these activities, they can crash for weeks, even months,” Dr. Moreau said. It was deep, persistent, and – for 40% of those with chronic fatigue syndrome – disabling. In 2015, a study group from the Institute of Medicine proposed renaming chronic fatigue to “systemic exercise intolerance disease” because of the centrality of this symptom. Although that effort mostly stalled, their report did bring the condition out of its historic place as a scientific backwater. What resulted was an uptick in research on chronic fatigue syndrome, which helped define some of the physiological issues that either contribute to or result from the condition.
Researchers had long known about the link between infection and fatigue, said Dr. Frankovich. Work included mysterious outbreaks like the one in Lake Tahoe and well-documented issues like the wave of encephalitis lethargica (a condition that leaves patients in an almost vegetative state) that followed the 1918 H1N1 influenza pandemic.
“As a clinician, when you see someone who comes in with a chronic infection, they’re tired. I think that’s why, in the chronic-fatigue world, people are desperately looking for the infection so we can treat it, and maybe these poor suffering people will feel better,” Dr. Frankovich added. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Immunologic symptoms
Given the close link between a nonspecific viral illness and the onset of symptoms in chronic fatigue syndrome, scientists like Dr. Hornig opted to focus on immunologic symptoms. In a 2015 analysis published in Science, Dr. Hornig and colleagues showed that immune problems can be found in the earliest stages of chronic fatigue syndrome, and that they change as the illness progresses. Patients who had been sick for less than 3 years showed significant increases in levels of both pro- and anti-inflammatory cytokines, and the factor most strongly correlated to this inability to regulate cytokine levels was the duration of symptoms, not their severity. A series of other studies also revealed problems with regulation of the immune system, although no one could show what might have set these problems in motion.
Other researchers found signs of mitochondrial dysfunction in those with chronic fatigue syndrome. Because mitochondria make energy for cells, it wasn’t an intellectual stretch to believe that glitches in this process could contribute to fatigue. As early as 1991, scientists had discovered signs of mitochondrial degeneration in muscle biopsies from people with chronic fatigue syndrome. Subsequent studies showed that those affected by chronic fatigue were missing segments of mitochondrial DNA and had significantly reduced levels of mitochondrial activity. Although exercise normally improves mitochondrial functioning, the opposite appears to happen in chronic fatigue.
To Dr. Nath, these dual hypotheses aren’t necessarily mutually exclusive. Some studies have hinted that infection with the common human herpesvirus–6 (HHV-6) can lead to an autoimmune condition in which the body makes antibodies against the mitochondria. Mitochondria also play a key role in the ability of the innate immune system to produce interferon and other proinflammatory cytokines. It might also be that the link between immune and mitochondrial problems is more convoluted than originally thought, or that the two systems are affected independent of one another, Dr. Nath said.
Finding answers, especially those that could lead to potential treatments, wouldn’t be easy, however. In 2016, the NIH launched an in-depth study of a small number of individuals with chronic fatigue, hoping to find clues about what the condition was and how it might be treated.
For scientists like Dr. Nath, the NIH study provided a way to get at the underlying biology of chronic fatigue syndrome. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Chronic post-SARS syndrome
In March 2020, retired physician Harvey Moldofsky, MD, began receiving inquiries about a 2011 study he and his colleague, John Patcai, MD, had published in BMC Neurology about something they dubbed “chronic post-SARS syndrome.” The small case-control study, which involved mainly health care workers in Toronto, received little attention when it was first published, but with COVID-19, it was suddenly relevant.
Early clusters of similar cases in Miami made local physicians desperate for Dr. Moldofsky’s expertise. Luckily, he was nearby; he had fled the frigid Canadian winter for the warmth of Sarasota, Fla.
“I had people from various countries around the world writing to me and asking what they should do. And of course I don’t have any answers,” he said. But the study contained one of the world’s only references to the syndrome.
In 2003, a woman arrived in Toronto from Hong Kong. She didn’t know it at the time, but her preairport stay at the Hotel Metropole had infected her with the first SARS (severe acute respiratory syndrome) coronavirus. Her subsequent hospitalization in Toronto sparked a city-wide outbreak of SARS in which 273 people became ill and 44 died. Many of those affected were health care workers, including nurses and respiratory therapists. Although most eventually returned to work, a subset couldn’t. They complained of energy-sapping fatigue, poor sleep, brain fog, and assorted body aches and pains that persisted for more than 18 months. The aches and pains brought them to the attention of Dr. Moldofsky, then director of the Centre for the Study of Pain at the University of Toronto.
His primary interest at the time was fibromyalgia, which caused symptoms similar to those reported by the original SARS long-haulers. Intrigued, Dr. Moldofsky agreed to take a look. Their chest x-rays were clear and the nurses showed no signs of lingering viral infection. Dr. Moldofsky could see that the nurses were ill and suffering, but no lab tests or anything else could identify what was causing their symptoms.
In 2011, Dr. Moldofsky and Dr. Patcai found a strong overlap between chronic SARS, fibromyalgia, and chronic fatigue syndrome when they compared 22 patients with long-term SARS issues with 21 who had fibromyalgia. “Their problems are exactly the same. They have strange symptoms and nobody can figure out what they’re about. And these symptoms are aches and pains, and they have trouble thinking and concentrating,” Dr. Moldofsky said. Reports of COVID-19 long-haulers didn’t surprise Dr. Moldofsky, and he immediately recognized that Nath’s intention to follow these patients could provide insights into both fibromyalgia and chronic fatigue syndrome.
That’s exactly what Dr. Nath is proposing with the two NIH studies. One will focus solely on the neurologic impacts of COVID-19, including stroke, loss of taste and smell, and brain fog. The other will bring patients who have had COVID-19 symptoms for at least 6 months to the NIH Clinical Center for an inpatient stay during which they will undergo detailed physiologic tests.
Scientists around the world are launching their own post–COVID-19 studies. Dr. Moreau’s group in Montreal has laid the groundwork for such an endeavor, and the CoroNerve group in the United Kingdom is monitoring neurologic complications from the coronavirus. Many of them have the same goals as the NIH studies: Leverage the large number of COVID-19 long-haulers to better understand the earliest stages of postviral syndrome.
“At this juncture, after all the reports that we’ve seen so far, I think it’s very unlikely that there will be no relationship whatsoever between COVID-19 and chronic fatigue syndrome,” Dr. Hornig said. “I think there certainly will be some, but again, what’s the scope, what’s the size? And then, of course, even more importantly, if it is happening, what is the mechanism and how is it happening?”
For people like Ms. Gage-Witvliet, the answers can’t come soon enough. For the first time in more than a decade, the full-time professor of epidemiology didn’t prepare to teach this year because she simply can’t. It’s too taxing for her brain to deal with impromptu student questions. Ms. Gage-Witvliet hopes that, by sharing her own experiences with post COVID-19, she can help others.
“In my work, I use data to give a voice to people who don’t have a voice,” she said. “Now, I am one of those people.”
A version of this article first appeared on Medscape.com.
When Margot Gage-Witvliet began feeling run down after her family returned from a trip to the Netherlands in late February 2020, she initially chalked up her symptoms to jet lag. Three days later, however, her situation went from concerning to alarming as she struggled to breathe. “It felt like there was an elephant sitting on my chest,” she said.
Her husband and daughters also became ill with COVID-19, but Ms. Gage-Witvliet was the only one in her family who didn’t get better. After an early improvement, a rare coronavirus-induced tonic-clonic seizure in early April sent her spiraling back down. Ms. Gage-Witvliet spent the next several weeks in bed with the curtains drawn, unable to tolerate light or sound.
Today, Ms. Gage-Witvliet’s life looks nothing like it did 6 months ago when she first got sick. As one of COVID-19’s so called long-haulers, she continues to struggle with crushing fatigue, brain fog, and headaches – symptoms that worsen when she pushes herself to do more. Across the country, as many as 1 in 10 COVID-19 patients are reporting illnesses that continue for weeks and months after their initial diagnosis. Nearly all report neurologic issues like Ms. Gage-Witvliet, as well as shortness of breath and psychiatric concerns.
For Avindra Nath, MD, a neurologist at the National Institutes of Health, the experience of these long-haul COVID-19 patients feels familiar and reminds him of myalgic encephalomyelitis, also known as chronic fatigue syndrome.
Dr. Nath has long been interested in the lingering neurologic issues connected to chronic fatigue. An estimated three-quarters of all patients with chronic fatigue syndrome report that their symptoms started after a viral infection, and they suffer unrelenting exhaustion, difficulties regulating pulse and blood pressure, aches and pains, and brain fog. When Dr. Nath first read about the novel coronavirus, he began to worry that the virus would trigger symptoms in a subset of those infected. Hearing about the experiences of long-haulers like Ms. Gage-Witvliet raised his suspicions even more.
Unlike COVID-19 long-haulers, however, many patients with chronic fatigue syndrome go at least a year with these symptoms before receiving a diagnosis, according to a British survey. That means researchers have had few opportunities to study the early stages of the syndrome. “When we see patients with myalgic encephalomyelitis, whatever infection they might have had occurred in the remote past, so there’s no way for us to know how they got infected with it, what the infection was, or what the effects of it were in that early phase. We’re seeing them 2 years afterward,” Dr. Nath said.
Dr. Nath quickly realized that studying patients like Ms. Gage-Witvliet would give physicians and scientists a unique opportunity to understand not only long-term outcomes of COVID-19 infections, but also other postviral syndromes, including chronic fatigue syndrome at their earliest stages. It’s why Dr. Nath has spent the past several months scrambling to launch two NIH studies to examine the phenomenon.
Although Dr. Nath said that the parallels between COVID-19 long-haulers and those with chronic fatigue syndrome are obvious, he cautions against assuming that they are the same phenomenon. Some long-haulers might simply be taking a much slower path to recovery, or they might have a condition that looks similar on the surface but differs from chronic fatigue syndrome on a molecular level. But even if Dr. Nath fails to see links to chronic fatigue syndrome, with more than 92.5 million documented cases of COVID-19 around the world, the work will be relevant to the substantial number of infected individuals who don’t recover quickly.
“With so many people having exposure to the same virus over a similar time period, we really have the opportunity to look at these manifestations and at the very least to understand postviral syndromes,” said Mady Hornig, MD, a psychiatrist at Columbia University, New York.
The origins of chronic fatigue syndrome date back to 1985, when the Centers for Disease Control and Prevention received a request from two physicians – Paul Cheney, MD, and Daniel Peterson, MD – to investigate a mysterious disease outbreak in Nevada. In November 1984, residents in and around the idyllic vacation spot of Incline Village, a small town tucked into the north shore of Lake Tahoe, had begun reporting flu-like symptoms that persisted for weeks, even months. The doctors had searched high and low for a cause, but they couldn’t figure out what was making their patients sick.
They reported a range of symptoms – including muscle aches and pains, low-grade fevers, sore throats, and headaches – but everyone said that crippling fatigue was the most debilitating issue. This wasn’t the kind of fatigue that could be cured by a nap or even a long holiday. No matter how much their patients slept – and some were almost completely bedbound – their fatigue didn’t abate. What’s more, the fatigue got worse whenever they tried to push themselves to do more. Puzzled, the CDC sent two epidemic intelligence service (EIS) officers to try to get to the bottom of what might be happening.
Muscle aches and pains with crippling fatigue
After their visit to Incline Village, however, the CDC was just as perplexed as Dr. Cheney and Dr. Peterson. Many of the people with the condition reported flu-like symptoms right around the time they first got sick, and the physicians’ leading hypothesis was that the outbreak and its lasting symptoms were caused by chronic Epstein-Barr virus infection. But neither the CDC nor anyone else could identify the infection or any other microbial cause. The two EIS officers duly wrote up a report for the CDC’s flagship publication, Morbidity and Mortality Weekly ReportI, titled “Chronic Fatigue Possibly Related to Epstein-Barr Virus – Nevada”.
That investigators focused on the fatigue aspect made sense, says Leonard A. Jason, PhD, professor of psychology at DePaul University and director of the Center for Community Research, both in Chicago, because it was one of the few symptoms shared by all the individuals studied and it was also the most debilitating. But that focus – and the name “chronic fatigue syndrome” – led to broad public dismissal of the condition’s severity, as did an editorial note in MMWR urging physicians to look for “more definable, and possibly treatable, conditions.” Subsequent research failed to confirm a specific link to the Epstein-Barr virus, which only added to the condition’s phony reputation. Rather than being considered a potentially disabling illness, it was disregarded as a “yuppie flu” or a fancy name for malingering.
“It’s not a surprise that patients are being dismissed because there’s already this sort of grandfathered-in sense that fatigue is not real,” said Jennifer Frankovich, MD, a pediatric rheumatologist at Stanford (Calif.) University’s Lucile Packard Children’s Hospital in Palo Alto. “I’m sure that’s frustrating for them to be tired and then to have the clinician not believe them or dismiss them or think they’re making it up. It would be more helpful to the families to say: ‘You know what, we don’t know, we do not have the answer, and we believe you.’ ”
A syndrome’s shame
As time passed, patient advocacy groups began pushing back against the negative way the condition was being perceived. This criticism came as organizations like the CDC worked to develop a set of diagnostic criteria that researchers and clinicians dealing with chronic fatigue syndrome could use. With such a heterogeneous group of patients and symptoms, the task was no small challenge. The discussions, which took place over nearly 2 decades, played a key role in helping scientists home in on the single factor that was central to chronic fatigue: postexertional malaise.
“This is quite unique for chronic fatigue syndrome. With other diseases, yes, you may have fatigue as one of the components of the disease, but postexertional fatigue is very specific,” said Alain Moreau, PhD, a molecular biologist at the University of Montreal.
Of course, plenty of people have pushed themselves too hard physically and paid the price the next day. But those with chronic fatigue syndrome weren’t running marathons. To them, exertion could be anything from getting the mail to reading a book. Nor could the resulting exhaustion be resolved by an afternoon on the couch or a long vacation.
“If they do these activities, they can crash for weeks, even months,” Dr. Moreau said. It was deep, persistent, and – for 40% of those with chronic fatigue syndrome – disabling. In 2015, a study group from the Institute of Medicine proposed renaming chronic fatigue to “systemic exercise intolerance disease” because of the centrality of this symptom. Although that effort mostly stalled, their report did bring the condition out of its historic place as a scientific backwater. What resulted was an uptick in research on chronic fatigue syndrome, which helped define some of the physiological issues that either contribute to or result from the condition.
Researchers had long known about the link between infection and fatigue, said Dr. Frankovich. Work included mysterious outbreaks like the one in Lake Tahoe and well-documented issues like the wave of encephalitis lethargica (a condition that leaves patients in an almost vegetative state) that followed the 1918 H1N1 influenza pandemic.
“As a clinician, when you see someone who comes in with a chronic infection, they’re tired. I think that’s why, in the chronic-fatigue world, people are desperately looking for the infection so we can treat it, and maybe these poor suffering people will feel better,” Dr. Frankovich added. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Immunologic symptoms
Given the close link between a nonspecific viral illness and the onset of symptoms in chronic fatigue syndrome, scientists like Dr. Hornig opted to focus on immunologic symptoms. In a 2015 analysis published in Science, Dr. Hornig and colleagues showed that immune problems can be found in the earliest stages of chronic fatigue syndrome, and that they change as the illness progresses. Patients who had been sick for less than 3 years showed significant increases in levels of both pro- and anti-inflammatory cytokines, and the factor most strongly correlated to this inability to regulate cytokine levels was the duration of symptoms, not their severity. A series of other studies also revealed problems with regulation of the immune system, although no one could show what might have set these problems in motion.
Other researchers found signs of mitochondrial dysfunction in those with chronic fatigue syndrome. Because mitochondria make energy for cells, it wasn’t an intellectual stretch to believe that glitches in this process could contribute to fatigue. As early as 1991, scientists had discovered signs of mitochondrial degeneration in muscle biopsies from people with chronic fatigue syndrome. Subsequent studies showed that those affected by chronic fatigue were missing segments of mitochondrial DNA and had significantly reduced levels of mitochondrial activity. Although exercise normally improves mitochondrial functioning, the opposite appears to happen in chronic fatigue.
To Dr. Nath, these dual hypotheses aren’t necessarily mutually exclusive. Some studies have hinted that infection with the common human herpesvirus–6 (HHV-6) can lead to an autoimmune condition in which the body makes antibodies against the mitochondria. Mitochondria also play a key role in the ability of the innate immune system to produce interferon and other proinflammatory cytokines. It might also be that the link between immune and mitochondrial problems is more convoluted than originally thought, or that the two systems are affected independent of one another, Dr. Nath said.
Finding answers, especially those that could lead to potential treatments, wouldn’t be easy, however. In 2016, the NIH launched an in-depth study of a small number of individuals with chronic fatigue, hoping to find clues about what the condition was and how it might be treated.
For scientists like Dr. Nath, the NIH study provided a way to get at the underlying biology of chronic fatigue syndrome. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Chronic post-SARS syndrome
In March 2020, retired physician Harvey Moldofsky, MD, began receiving inquiries about a 2011 study he and his colleague, John Patcai, MD, had published in BMC Neurology about something they dubbed “chronic post-SARS syndrome.” The small case-control study, which involved mainly health care workers in Toronto, received little attention when it was first published, but with COVID-19, it was suddenly relevant.
Early clusters of similar cases in Miami made local physicians desperate for Dr. Moldofsky’s expertise. Luckily, he was nearby; he had fled the frigid Canadian winter for the warmth of Sarasota, Fla.
“I had people from various countries around the world writing to me and asking what they should do. And of course I don’t have any answers,” he said. But the study contained one of the world’s only references to the syndrome.
In 2003, a woman arrived in Toronto from Hong Kong. She didn’t know it at the time, but her preairport stay at the Hotel Metropole had infected her with the first SARS (severe acute respiratory syndrome) coronavirus. Her subsequent hospitalization in Toronto sparked a city-wide outbreak of SARS in which 273 people became ill and 44 died. Many of those affected were health care workers, including nurses and respiratory therapists. Although most eventually returned to work, a subset couldn’t. They complained of energy-sapping fatigue, poor sleep, brain fog, and assorted body aches and pains that persisted for more than 18 months. The aches and pains brought them to the attention of Dr. Moldofsky, then director of the Centre for the Study of Pain at the University of Toronto.
His primary interest at the time was fibromyalgia, which caused symptoms similar to those reported by the original SARS long-haulers. Intrigued, Dr. Moldofsky agreed to take a look. Their chest x-rays were clear and the nurses showed no signs of lingering viral infection. Dr. Moldofsky could see that the nurses were ill and suffering, but no lab tests or anything else could identify what was causing their symptoms.
In 2011, Dr. Moldofsky and Dr. Patcai found a strong overlap between chronic SARS, fibromyalgia, and chronic fatigue syndrome when they compared 22 patients with long-term SARS issues with 21 who had fibromyalgia. “Their problems are exactly the same. They have strange symptoms and nobody can figure out what they’re about. And these symptoms are aches and pains, and they have trouble thinking and concentrating,” Dr. Moldofsky said. Reports of COVID-19 long-haulers didn’t surprise Dr. Moldofsky, and he immediately recognized that Nath’s intention to follow these patients could provide insights into both fibromyalgia and chronic fatigue syndrome.
That’s exactly what Dr. Nath is proposing with the two NIH studies. One will focus solely on the neurologic impacts of COVID-19, including stroke, loss of taste and smell, and brain fog. The other will bring patients who have had COVID-19 symptoms for at least 6 months to the NIH Clinical Center for an inpatient stay during which they will undergo detailed physiologic tests.
Scientists around the world are launching their own post–COVID-19 studies. Dr. Moreau’s group in Montreal has laid the groundwork for such an endeavor, and the CoroNerve group in the United Kingdom is monitoring neurologic complications from the coronavirus. Many of them have the same goals as the NIH studies: Leverage the large number of COVID-19 long-haulers to better understand the earliest stages of postviral syndrome.
“At this juncture, after all the reports that we’ve seen so far, I think it’s very unlikely that there will be no relationship whatsoever between COVID-19 and chronic fatigue syndrome,” Dr. Hornig said. “I think there certainly will be some, but again, what’s the scope, what’s the size? And then, of course, even more importantly, if it is happening, what is the mechanism and how is it happening?”
For people like Ms. Gage-Witvliet, the answers can’t come soon enough. For the first time in more than a decade, the full-time professor of epidemiology didn’t prepare to teach this year because she simply can’t. It’s too taxing for her brain to deal with impromptu student questions. Ms. Gage-Witvliet hopes that, by sharing her own experiences with post COVID-19, she can help others.
“In my work, I use data to give a voice to people who don’t have a voice,” she said. “Now, I am one of those people.”
A version of this article first appeared on Medscape.com.
When Margot Gage-Witvliet began feeling run down after her family returned from a trip to the Netherlands in late February 2020, she initially chalked up her symptoms to jet lag. Three days later, however, her situation went from concerning to alarming as she struggled to breathe. “It felt like there was an elephant sitting on my chest,” she said.
Her husband and daughters also became ill with COVID-19, but Ms. Gage-Witvliet was the only one in her family who didn’t get better. After an early improvement, a rare coronavirus-induced tonic-clonic seizure in early April sent her spiraling back down. Ms. Gage-Witvliet spent the next several weeks in bed with the curtains drawn, unable to tolerate light or sound.
Today, Ms. Gage-Witvliet’s life looks nothing like it did 6 months ago when she first got sick. As one of COVID-19’s so called long-haulers, she continues to struggle with crushing fatigue, brain fog, and headaches – symptoms that worsen when she pushes herself to do more. Across the country, as many as 1 in 10 COVID-19 patients are reporting illnesses that continue for weeks and months after their initial diagnosis. Nearly all report neurologic issues like Ms. Gage-Witvliet, as well as shortness of breath and psychiatric concerns.
For Avindra Nath, MD, a neurologist at the National Institutes of Health, the experience of these long-haul COVID-19 patients feels familiar and reminds him of myalgic encephalomyelitis, also known as chronic fatigue syndrome.
Dr. Nath has long been interested in the lingering neurologic issues connected to chronic fatigue. An estimated three-quarters of all patients with chronic fatigue syndrome report that their symptoms started after a viral infection, and they suffer unrelenting exhaustion, difficulties regulating pulse and blood pressure, aches and pains, and brain fog. When Dr. Nath first read about the novel coronavirus, he began to worry that the virus would trigger symptoms in a subset of those infected. Hearing about the experiences of long-haulers like Ms. Gage-Witvliet raised his suspicions even more.
Unlike COVID-19 long-haulers, however, many patients with chronic fatigue syndrome go at least a year with these symptoms before receiving a diagnosis, according to a British survey. That means researchers have had few opportunities to study the early stages of the syndrome. “When we see patients with myalgic encephalomyelitis, whatever infection they might have had occurred in the remote past, so there’s no way for us to know how they got infected with it, what the infection was, or what the effects of it were in that early phase. We’re seeing them 2 years afterward,” Dr. Nath said.
Dr. Nath quickly realized that studying patients like Ms. Gage-Witvliet would give physicians and scientists a unique opportunity to understand not only long-term outcomes of COVID-19 infections, but also other postviral syndromes, including chronic fatigue syndrome at their earliest stages. It’s why Dr. Nath has spent the past several months scrambling to launch two NIH studies to examine the phenomenon.
Although Dr. Nath said that the parallels between COVID-19 long-haulers and those with chronic fatigue syndrome are obvious, he cautions against assuming that they are the same phenomenon. Some long-haulers might simply be taking a much slower path to recovery, or they might have a condition that looks similar on the surface but differs from chronic fatigue syndrome on a molecular level. But even if Dr. Nath fails to see links to chronic fatigue syndrome, with more than 92.5 million documented cases of COVID-19 around the world, the work will be relevant to the substantial number of infected individuals who don’t recover quickly.
“With so many people having exposure to the same virus over a similar time period, we really have the opportunity to look at these manifestations and at the very least to understand postviral syndromes,” said Mady Hornig, MD, a psychiatrist at Columbia University, New York.
The origins of chronic fatigue syndrome date back to 1985, when the Centers for Disease Control and Prevention received a request from two physicians – Paul Cheney, MD, and Daniel Peterson, MD – to investigate a mysterious disease outbreak in Nevada. In November 1984, residents in and around the idyllic vacation spot of Incline Village, a small town tucked into the north shore of Lake Tahoe, had begun reporting flu-like symptoms that persisted for weeks, even months. The doctors had searched high and low for a cause, but they couldn’t figure out what was making their patients sick.
They reported a range of symptoms – including muscle aches and pains, low-grade fevers, sore throats, and headaches – but everyone said that crippling fatigue was the most debilitating issue. This wasn’t the kind of fatigue that could be cured by a nap or even a long holiday. No matter how much their patients slept – and some were almost completely bedbound – their fatigue didn’t abate. What’s more, the fatigue got worse whenever they tried to push themselves to do more. Puzzled, the CDC sent two epidemic intelligence service (EIS) officers to try to get to the bottom of what might be happening.
Muscle aches and pains with crippling fatigue
After their visit to Incline Village, however, the CDC was just as perplexed as Dr. Cheney and Dr. Peterson. Many of the people with the condition reported flu-like symptoms right around the time they first got sick, and the physicians’ leading hypothesis was that the outbreak and its lasting symptoms were caused by chronic Epstein-Barr virus infection. But neither the CDC nor anyone else could identify the infection or any other microbial cause. The two EIS officers duly wrote up a report for the CDC’s flagship publication, Morbidity and Mortality Weekly ReportI, titled “Chronic Fatigue Possibly Related to Epstein-Barr Virus – Nevada”.
That investigators focused on the fatigue aspect made sense, says Leonard A. Jason, PhD, professor of psychology at DePaul University and director of the Center for Community Research, both in Chicago, because it was one of the few symptoms shared by all the individuals studied and it was also the most debilitating. But that focus – and the name “chronic fatigue syndrome” – led to broad public dismissal of the condition’s severity, as did an editorial note in MMWR urging physicians to look for “more definable, and possibly treatable, conditions.” Subsequent research failed to confirm a specific link to the Epstein-Barr virus, which only added to the condition’s phony reputation. Rather than being considered a potentially disabling illness, it was disregarded as a “yuppie flu” or a fancy name for malingering.
“It’s not a surprise that patients are being dismissed because there’s already this sort of grandfathered-in sense that fatigue is not real,” said Jennifer Frankovich, MD, a pediatric rheumatologist at Stanford (Calif.) University’s Lucile Packard Children’s Hospital in Palo Alto. “I’m sure that’s frustrating for them to be tired and then to have the clinician not believe them or dismiss them or think they’re making it up. It would be more helpful to the families to say: ‘You know what, we don’t know, we do not have the answer, and we believe you.’ ”
A syndrome’s shame
As time passed, patient advocacy groups began pushing back against the negative way the condition was being perceived. This criticism came as organizations like the CDC worked to develop a set of diagnostic criteria that researchers and clinicians dealing with chronic fatigue syndrome could use. With such a heterogeneous group of patients and symptoms, the task was no small challenge. The discussions, which took place over nearly 2 decades, played a key role in helping scientists home in on the single factor that was central to chronic fatigue: postexertional malaise.
“This is quite unique for chronic fatigue syndrome. With other diseases, yes, you may have fatigue as one of the components of the disease, but postexertional fatigue is very specific,” said Alain Moreau, PhD, a molecular biologist at the University of Montreal.
Of course, plenty of people have pushed themselves too hard physically and paid the price the next day. But those with chronic fatigue syndrome weren’t running marathons. To them, exertion could be anything from getting the mail to reading a book. Nor could the resulting exhaustion be resolved by an afternoon on the couch or a long vacation.
“If they do these activities, they can crash for weeks, even months,” Dr. Moreau said. It was deep, persistent, and – for 40% of those with chronic fatigue syndrome – disabling. In 2015, a study group from the Institute of Medicine proposed renaming chronic fatigue to “systemic exercise intolerance disease” because of the centrality of this symptom. Although that effort mostly stalled, their report did bring the condition out of its historic place as a scientific backwater. What resulted was an uptick in research on chronic fatigue syndrome, which helped define some of the physiological issues that either contribute to or result from the condition.
Researchers had long known about the link between infection and fatigue, said Dr. Frankovich. Work included mysterious outbreaks like the one in Lake Tahoe and well-documented issues like the wave of encephalitis lethargica (a condition that leaves patients in an almost vegetative state) that followed the 1918 H1N1 influenza pandemic.
“As a clinician, when you see someone who comes in with a chronic infection, they’re tired. I think that’s why, in the chronic-fatigue world, people are desperately looking for the infection so we can treat it, and maybe these poor suffering people will feel better,” Dr. Frankovich added. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Immunologic symptoms
Given the close link between a nonspecific viral illness and the onset of symptoms in chronic fatigue syndrome, scientists like Dr. Hornig opted to focus on immunologic symptoms. In a 2015 analysis published in Science, Dr. Hornig and colleagues showed that immune problems can be found in the earliest stages of chronic fatigue syndrome, and that they change as the illness progresses. Patients who had been sick for less than 3 years showed significant increases in levels of both pro- and anti-inflammatory cytokines, and the factor most strongly correlated to this inability to regulate cytokine levels was the duration of symptoms, not their severity. A series of other studies also revealed problems with regulation of the immune system, although no one could show what might have set these problems in motion.
Other researchers found signs of mitochondrial dysfunction in those with chronic fatigue syndrome. Because mitochondria make energy for cells, it wasn’t an intellectual stretch to believe that glitches in this process could contribute to fatigue. As early as 1991, scientists had discovered signs of mitochondrial degeneration in muscle biopsies from people with chronic fatigue syndrome. Subsequent studies showed that those affected by chronic fatigue were missing segments of mitochondrial DNA and had significantly reduced levels of mitochondrial activity. Although exercise normally improves mitochondrial functioning, the opposite appears to happen in chronic fatigue.
To Dr. Nath, these dual hypotheses aren’t necessarily mutually exclusive. Some studies have hinted that infection with the common human herpesvirus–6 (HHV-6) can lead to an autoimmune condition in which the body makes antibodies against the mitochondria. Mitochondria also play a key role in the ability of the innate immune system to produce interferon and other proinflammatory cytokines. It might also be that the link between immune and mitochondrial problems is more convoluted than originally thought, or that the two systems are affected independent of one another, Dr. Nath said.
Finding answers, especially those that could lead to potential treatments, wouldn’t be easy, however. In 2016, the NIH launched an in-depth study of a small number of individuals with chronic fatigue, hoping to find clues about what the condition was and how it might be treated.
For scientists like Dr. Nath, the NIH study provided a way to get at the underlying biology of chronic fatigue syndrome. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Chronic post-SARS syndrome
In March 2020, retired physician Harvey Moldofsky, MD, began receiving inquiries about a 2011 study he and his colleague, John Patcai, MD, had published in BMC Neurology about something they dubbed “chronic post-SARS syndrome.” The small case-control study, which involved mainly health care workers in Toronto, received little attention when it was first published, but with COVID-19, it was suddenly relevant.
Early clusters of similar cases in Miami made local physicians desperate for Dr. Moldofsky’s expertise. Luckily, he was nearby; he had fled the frigid Canadian winter for the warmth of Sarasota, Fla.
“I had people from various countries around the world writing to me and asking what they should do. And of course I don’t have any answers,” he said. But the study contained one of the world’s only references to the syndrome.
In 2003, a woman arrived in Toronto from Hong Kong. She didn’t know it at the time, but her preairport stay at the Hotel Metropole had infected her with the first SARS (severe acute respiratory syndrome) coronavirus. Her subsequent hospitalization in Toronto sparked a city-wide outbreak of SARS in which 273 people became ill and 44 died. Many of those affected were health care workers, including nurses and respiratory therapists. Although most eventually returned to work, a subset couldn’t. They complained of energy-sapping fatigue, poor sleep, brain fog, and assorted body aches and pains that persisted for more than 18 months. The aches and pains brought them to the attention of Dr. Moldofsky, then director of the Centre for the Study of Pain at the University of Toronto.
His primary interest at the time was fibromyalgia, which caused symptoms similar to those reported by the original SARS long-haulers. Intrigued, Dr. Moldofsky agreed to take a look. Their chest x-rays were clear and the nurses showed no signs of lingering viral infection. Dr. Moldofsky could see that the nurses were ill and suffering, but no lab tests or anything else could identify what was causing their symptoms.
In 2011, Dr. Moldofsky and Dr. Patcai found a strong overlap between chronic SARS, fibromyalgia, and chronic fatigue syndrome when they compared 22 patients with long-term SARS issues with 21 who had fibromyalgia. “Their problems are exactly the same. They have strange symptoms and nobody can figure out what they’re about. And these symptoms are aches and pains, and they have trouble thinking and concentrating,” Dr. Moldofsky said. Reports of COVID-19 long-haulers didn’t surprise Dr. Moldofsky, and he immediately recognized that Nath’s intention to follow these patients could provide insights into both fibromyalgia and chronic fatigue syndrome.
That’s exactly what Dr. Nath is proposing with the two NIH studies. One will focus solely on the neurologic impacts of COVID-19, including stroke, loss of taste and smell, and brain fog. The other will bring patients who have had COVID-19 symptoms for at least 6 months to the NIH Clinical Center for an inpatient stay during which they will undergo detailed physiologic tests.
Scientists around the world are launching their own post–COVID-19 studies. Dr. Moreau’s group in Montreal has laid the groundwork for such an endeavor, and the CoroNerve group in the United Kingdom is monitoring neurologic complications from the coronavirus. Many of them have the same goals as the NIH studies: Leverage the large number of COVID-19 long-haulers to better understand the earliest stages of postviral syndrome.
“At this juncture, after all the reports that we’ve seen so far, I think it’s very unlikely that there will be no relationship whatsoever between COVID-19 and chronic fatigue syndrome,” Dr. Hornig said. “I think there certainly will be some, but again, what’s the scope, what’s the size? And then, of course, even more importantly, if it is happening, what is the mechanism and how is it happening?”
For people like Ms. Gage-Witvliet, the answers can’t come soon enough. For the first time in more than a decade, the full-time professor of epidemiology didn’t prepare to teach this year because she simply can’t. It’s too taxing for her brain to deal with impromptu student questions. Ms. Gage-Witvliet hopes that, by sharing her own experiences with post COVID-19, she can help others.
“In my work, I use data to give a voice to people who don’t have a voice,” she said. “Now, I am one of those people.”
A version of this article first appeared on Medscape.com.
Pressure builds on CDC to prioritize both diabetes types for vaccine
The American Diabetes Association, along with 18 other organizations, has sent a letter to the U.S. Centers for Disease Control and Prevention urging them to rank people with type 1 diabetes as equally high risk for COVID-19 severity, and therefore vaccination, as those with type 2 diabetes.
On Jan. 12, the CDC recommended states vaccinate all Americans over age 65 and those with underlying health conditions that make them more vulnerable to COVID-19.
Currently, type 2 diabetes is listed among 12 conditions that place adults “at increased risk of severe illness from the virus that causes COVID-19,” with the latter defined as “hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
On the other hand, the autoimmune condition type 1 diabetes is among 11 conditions the CDC says “might be at increased risk” for COVID-19, but limited data were available at the time of the last update on Dec. 23, 2020.
“States are utilizing the CDC risk classification when designing their vaccine distribution plans. This raises an obvious concern as it could result in the approximately 1.6 million with type 1 diabetes receiving the vaccination later than others with the same risk,” states the ADA letter, sent to the CDC on Jan. 13.
Representatives from the Endocrine Society, American Association of Clinical Endocrinology, Pediatric Endocrine Society, Association of Diabetes Care & Education Specialists, and JDRF, among others, cosigned the letter.
Newer data show those with type 1 diabetes at equally high risk
While acknowledging that “early data did not provide as much clarity about the extent to which those with type 1 diabetes are at high risk,” the ADA says newer evidence has emerged, as previously reported by this news organization, that “convincingly demonstrates that COVID-19 severity is more than tripled in individuals with type 1 diabetes.”
The letter also cites another study showing that people with type 1 diabetes “have a 3.3-fold greater risk of severe illness, are 3.9 times more likely to be hospitalized with COVID-19, and have a 3-fold increase in mortality compared to those without type 1 diabetes.”
Those risks, they note, are comparable to the increased risk established for those with type 2 diabetes, as shown in a third study from Scotland, published last month.
Asked for comment, CDC representative Kirsten Nordlund said in an interview, “This list is a living document that will be periodically updated by CDC, and it could rapidly change as the science evolves.”
In addition, Ms. Nordlund said, “Decisions about transitioning to subsequent phases should depend on supply; demand; equitable vaccine distribution; and local, state, or territorial context.”
“Phased vaccine recommendations are meant to be fluid and not restrictive for jurisdictions. It is not necessary to vaccinate all individuals in one phase before initiating the next phase; phases may overlap,” she noted. More information is available here.
Tennessee gives type 1 and type 2 diabetes equal priority for vaccination
Meanwhile, at least one state, Tennessee, has updated its guidance to include both types of diabetes as being priority for COVID-19 vaccination.
Vanderbilt University pediatric endocrinologist Justin M. Gregory, MD, said in an interview: “I was thrilled when our state modified its guidance on December 30th to include both type 1 and type 2 diabetes in the ‘high-risk category.’ Other states have not modified that guidance though.”
It’s unclear how this might play out on the ground, noted Dr. Gregory, who led one of the three studies demonstrating increased COVID-19 risk for people with type 1 diabetes.
“To tell you the truth, I don’t really know how individual organizations dispensing the vaccination [will handle] people who come to their facility saying they have ‘diabetes.’ Individual states set the vaccine-dispensing guidance and individual county health departments and health care systems mirror that guidance,” he said.
Thus, he added, “Although it’s possible an individual nurse may take the ‘I’ll ask you no questions, and you’ll tell me no lies’ approach if someone with type 1 diabetes says they have ‘diabetes’, websites and health department–recorded telephone messages are going to tell people with type 1 diabetes they have to wait further back in line if that is what their state’s guidance directs.”
A version of this article first appeared on Medscape.com.
The American Diabetes Association, along with 18 other organizations, has sent a letter to the U.S. Centers for Disease Control and Prevention urging them to rank people with type 1 diabetes as equally high risk for COVID-19 severity, and therefore vaccination, as those with type 2 diabetes.
On Jan. 12, the CDC recommended states vaccinate all Americans over age 65 and those with underlying health conditions that make them more vulnerable to COVID-19.
Currently, type 2 diabetes is listed among 12 conditions that place adults “at increased risk of severe illness from the virus that causes COVID-19,” with the latter defined as “hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
On the other hand, the autoimmune condition type 1 diabetes is among 11 conditions the CDC says “might be at increased risk” for COVID-19, but limited data were available at the time of the last update on Dec. 23, 2020.
“States are utilizing the CDC risk classification when designing their vaccine distribution plans. This raises an obvious concern as it could result in the approximately 1.6 million with type 1 diabetes receiving the vaccination later than others with the same risk,” states the ADA letter, sent to the CDC on Jan. 13.
Representatives from the Endocrine Society, American Association of Clinical Endocrinology, Pediatric Endocrine Society, Association of Diabetes Care & Education Specialists, and JDRF, among others, cosigned the letter.
Newer data show those with type 1 diabetes at equally high risk
While acknowledging that “early data did not provide as much clarity about the extent to which those with type 1 diabetes are at high risk,” the ADA says newer evidence has emerged, as previously reported by this news organization, that “convincingly demonstrates that COVID-19 severity is more than tripled in individuals with type 1 diabetes.”
The letter also cites another study showing that people with type 1 diabetes “have a 3.3-fold greater risk of severe illness, are 3.9 times more likely to be hospitalized with COVID-19, and have a 3-fold increase in mortality compared to those without type 1 diabetes.”
Those risks, they note, are comparable to the increased risk established for those with type 2 diabetes, as shown in a third study from Scotland, published last month.
Asked for comment, CDC representative Kirsten Nordlund said in an interview, “This list is a living document that will be periodically updated by CDC, and it could rapidly change as the science evolves.”
In addition, Ms. Nordlund said, “Decisions about transitioning to subsequent phases should depend on supply; demand; equitable vaccine distribution; and local, state, or territorial context.”
“Phased vaccine recommendations are meant to be fluid and not restrictive for jurisdictions. It is not necessary to vaccinate all individuals in one phase before initiating the next phase; phases may overlap,” she noted. More information is available here.
Tennessee gives type 1 and type 2 diabetes equal priority for vaccination
Meanwhile, at least one state, Tennessee, has updated its guidance to include both types of diabetes as being priority for COVID-19 vaccination.
Vanderbilt University pediatric endocrinologist Justin M. Gregory, MD, said in an interview: “I was thrilled when our state modified its guidance on December 30th to include both type 1 and type 2 diabetes in the ‘high-risk category.’ Other states have not modified that guidance though.”
It’s unclear how this might play out on the ground, noted Dr. Gregory, who led one of the three studies demonstrating increased COVID-19 risk for people with type 1 diabetes.
“To tell you the truth, I don’t really know how individual organizations dispensing the vaccination [will handle] people who come to their facility saying they have ‘diabetes.’ Individual states set the vaccine-dispensing guidance and individual county health departments and health care systems mirror that guidance,” he said.
Thus, he added, “Although it’s possible an individual nurse may take the ‘I’ll ask you no questions, and you’ll tell me no lies’ approach if someone with type 1 diabetes says they have ‘diabetes’, websites and health department–recorded telephone messages are going to tell people with type 1 diabetes they have to wait further back in line if that is what their state’s guidance directs.”
A version of this article first appeared on Medscape.com.
The American Diabetes Association, along with 18 other organizations, has sent a letter to the U.S. Centers for Disease Control and Prevention urging them to rank people with type 1 diabetes as equally high risk for COVID-19 severity, and therefore vaccination, as those with type 2 diabetes.
On Jan. 12, the CDC recommended states vaccinate all Americans over age 65 and those with underlying health conditions that make them more vulnerable to COVID-19.
Currently, type 2 diabetes is listed among 12 conditions that place adults “at increased risk of severe illness from the virus that causes COVID-19,” with the latter defined as “hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
On the other hand, the autoimmune condition type 1 diabetes is among 11 conditions the CDC says “might be at increased risk” for COVID-19, but limited data were available at the time of the last update on Dec. 23, 2020.
“States are utilizing the CDC risk classification when designing their vaccine distribution plans. This raises an obvious concern as it could result in the approximately 1.6 million with type 1 diabetes receiving the vaccination later than others with the same risk,” states the ADA letter, sent to the CDC on Jan. 13.
Representatives from the Endocrine Society, American Association of Clinical Endocrinology, Pediatric Endocrine Society, Association of Diabetes Care & Education Specialists, and JDRF, among others, cosigned the letter.
Newer data show those with type 1 diabetes at equally high risk
While acknowledging that “early data did not provide as much clarity about the extent to which those with type 1 diabetes are at high risk,” the ADA says newer evidence has emerged, as previously reported by this news organization, that “convincingly demonstrates that COVID-19 severity is more than tripled in individuals with type 1 diabetes.”
The letter also cites another study showing that people with type 1 diabetes “have a 3.3-fold greater risk of severe illness, are 3.9 times more likely to be hospitalized with COVID-19, and have a 3-fold increase in mortality compared to those without type 1 diabetes.”
Those risks, they note, are comparable to the increased risk established for those with type 2 diabetes, as shown in a third study from Scotland, published last month.
Asked for comment, CDC representative Kirsten Nordlund said in an interview, “This list is a living document that will be periodically updated by CDC, and it could rapidly change as the science evolves.”
In addition, Ms. Nordlund said, “Decisions about transitioning to subsequent phases should depend on supply; demand; equitable vaccine distribution; and local, state, or territorial context.”
“Phased vaccine recommendations are meant to be fluid and not restrictive for jurisdictions. It is not necessary to vaccinate all individuals in one phase before initiating the next phase; phases may overlap,” she noted. More information is available here.
Tennessee gives type 1 and type 2 diabetes equal priority for vaccination
Meanwhile, at least one state, Tennessee, has updated its guidance to include both types of diabetes as being priority for COVID-19 vaccination.
Vanderbilt University pediatric endocrinologist Justin M. Gregory, MD, said in an interview: “I was thrilled when our state modified its guidance on December 30th to include both type 1 and type 2 diabetes in the ‘high-risk category.’ Other states have not modified that guidance though.”
It’s unclear how this might play out on the ground, noted Dr. Gregory, who led one of the three studies demonstrating increased COVID-19 risk for people with type 1 diabetes.
“To tell you the truth, I don’t really know how individual organizations dispensing the vaccination [will handle] people who come to their facility saying they have ‘diabetes.’ Individual states set the vaccine-dispensing guidance and individual county health departments and health care systems mirror that guidance,” he said.
Thus, he added, “Although it’s possible an individual nurse may take the ‘I’ll ask you no questions, and you’ll tell me no lies’ approach if someone with type 1 diabetes says they have ‘diabetes’, websites and health department–recorded telephone messages are going to tell people with type 1 diabetes they have to wait further back in line if that is what their state’s guidance directs.”
A version of this article first appeared on Medscape.com.
COVID-19 symptoms persist months after acute infection
, according to a follow-up study involving 1,733 patients.
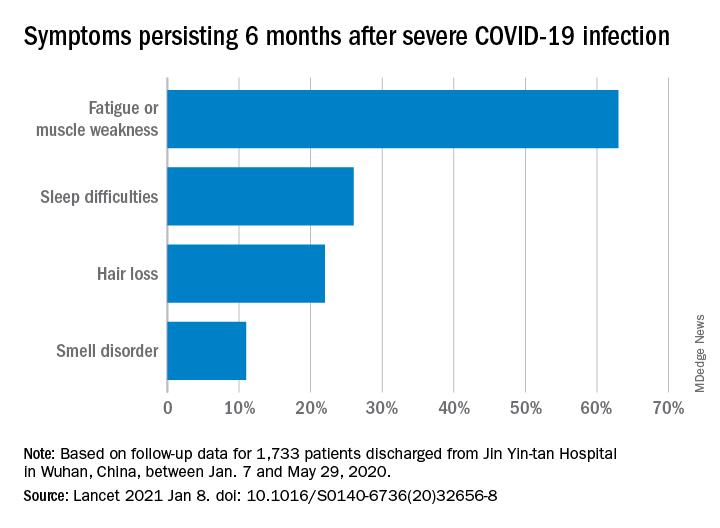
“Patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression,” and those with “more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations,” Chaolin Huang, MD, of Jin Yin-tan Hospital in Wuhan, China, and associates wrote in the Lancet.
Fatigue or muscle weakness, reported by 63% of patients, was the most common symptom, followed by sleep difficulties, hair loss, and smell disorder. Altogether, 76% of those examined 6 months after discharge from Jin Yin-tan hospital – the first designated for patients with COVID-19 in Wuhan – reported at least one symptom, they said.
Symptoms were more common in women than men: 81% vs. 73% had at least one symptom, and 66% vs. 59% had fatigue or muscle weakness. Women were also more likely than men to report anxiety or depression at follow-up: 28% vs. 18% (23% overall), the investigators said.
Patients with the most severe COVID-19 were 2.4 times as likely to report any symptom later, compared with those who had the least severe levels of infection. Among the 349 participants who completed a lung function test at follow-up, lung diffusion impairment was seen in 56% of those with the most severe illness and 22% of those with the lowest level, Dr. Huang and associates reported.
In a different subset of 94 patients from whom plasma samples were collected, the “seropositivity and median titres of the neutralising antibodies were significantly lower than at the acute phase,” raising concern for reinfection, they said.
The results of the study, the investigators noted, “support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.”
, according to a follow-up study involving 1,733 patients.

“Patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression,” and those with “more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations,” Chaolin Huang, MD, of Jin Yin-tan Hospital in Wuhan, China, and associates wrote in the Lancet.
Fatigue or muscle weakness, reported by 63% of patients, was the most common symptom, followed by sleep difficulties, hair loss, and smell disorder. Altogether, 76% of those examined 6 months after discharge from Jin Yin-tan hospital – the first designated for patients with COVID-19 in Wuhan – reported at least one symptom, they said.
Symptoms were more common in women than men: 81% vs. 73% had at least one symptom, and 66% vs. 59% had fatigue or muscle weakness. Women were also more likely than men to report anxiety or depression at follow-up: 28% vs. 18% (23% overall), the investigators said.
Patients with the most severe COVID-19 were 2.4 times as likely to report any symptom later, compared with those who had the least severe levels of infection. Among the 349 participants who completed a lung function test at follow-up, lung diffusion impairment was seen in 56% of those with the most severe illness and 22% of those with the lowest level, Dr. Huang and associates reported.
In a different subset of 94 patients from whom plasma samples were collected, the “seropositivity and median titres of the neutralising antibodies were significantly lower than at the acute phase,” raising concern for reinfection, they said.
The results of the study, the investigators noted, “support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.”
, according to a follow-up study involving 1,733 patients.

“Patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression,” and those with “more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations,” Chaolin Huang, MD, of Jin Yin-tan Hospital in Wuhan, China, and associates wrote in the Lancet.
Fatigue or muscle weakness, reported by 63% of patients, was the most common symptom, followed by sleep difficulties, hair loss, and smell disorder. Altogether, 76% of those examined 6 months after discharge from Jin Yin-tan hospital – the first designated for patients with COVID-19 in Wuhan – reported at least one symptom, they said.
Symptoms were more common in women than men: 81% vs. 73% had at least one symptom, and 66% vs. 59% had fatigue or muscle weakness. Women were also more likely than men to report anxiety or depression at follow-up: 28% vs. 18% (23% overall), the investigators said.
Patients with the most severe COVID-19 were 2.4 times as likely to report any symptom later, compared with those who had the least severe levels of infection. Among the 349 participants who completed a lung function test at follow-up, lung diffusion impairment was seen in 56% of those with the most severe illness and 22% of those with the lowest level, Dr. Huang and associates reported.
In a different subset of 94 patients from whom plasma samples were collected, the “seropositivity and median titres of the neutralising antibodies were significantly lower than at the acute phase,” raising concern for reinfection, they said.
The results of the study, the investigators noted, “support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.”
FROM THE LANCET



