User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
50.6 million tobacco users are not a homogeneous group
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.
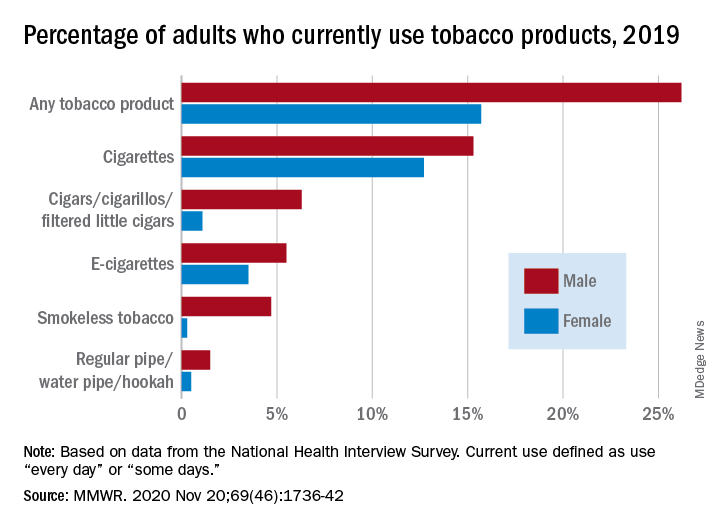
with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.

with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.

with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
FROM MMWR
Liquid oxygen recommended for mobile patients with lung disease
People with chronic lung disease who need significant amounts of oxygen should be able to take it in liquid form when they are able to leave home, according to a new guideline from the American Thoracic Society.
“For those patients, often the other types of devices either can’t supply enough oxygen or are not portable enough,” said Anne Holland, PT, PhD, a professor of physiotherapy at Monash University and Alfred Hospital in Melbourne. “They’re heavy and cumbersome to use.”
Dr. Holland and colleagues also gave a more general recommendation to prescribe ambulatory oxygen – though not necessarily in liquid form – for adults with chronic obstructive pulmonary disease (COPD) or interstitial lung disease (ILD) who have severe exertional room air hypoxemia.
They published the recommendations as part of the ATS’ first-ever guideline on home oxygen therapy for adults with chronic lung disease in the American Journal of Respiratory and Critical Care Medicine.
The ATS identified the need for an updated guideline because of new research, and because an online survey of almost 2,000 U.S. oxygen users showed they were having problems accessing and using oxygen.
For long-term oxygen therapy, the guideline reinforces what most practitioners are already doing, Dr. Holland said. It recommends that adults with COPD or ILD who have severe chronic resting room air hypoxemia receive oxygen therapy at least 15 hours per day.
On the other hand, in adults with COPD who have moderate chronic resting room-air hypoxemia, the guideline recommends against long-term oxygen therapy.
The recommendation to prescribe ambulatory oxygen for people with severe exertional room-air hypoxemia may have more effect on practice, Dr. Holland said. Laboratory-based tests have suggested oxygen can improve exercise capacity, but clinical trials used during daily life have had inconsistent results.
The evidence is particularly lacking for patients with ILD, Dr. Holland said in an interview. “It’s such an important part of practice to maintain oxygen therapy that it’s ethically very difficult to conduct such a trial. So, we did have to make use of indirect evidence from patients with COPD” for the guidelines.
The portable equipment comes with burdens, including managing its weight and bulk, social stigma, fear of cylinders running out, and equipment noise.
“We tried to clearly set out both the benefits and burdens of that therapy and made a conditional recommendation, and also a really strong call for shared decision-making with patients and health professionals,” Dr. Holland said.
In addition to looking at the evidence, the panel took into consideration the concerns identified by patients. This included the challenge of figuring out how to use the equipment. “All the oxygen equipment was ‘dumped’ on me,” wrote one oxygen user quoted in the guideline. “I knew nothing and was in a daze. I am sure that the delivery guy gave me some instructions when it was delivered but I retained nothing.”
For this reason, the guideline describes instruction and training on the use and maintenance of the equipment, including smoking cessation, fire prevention, and tripping hazards, as a “best practice.”
Nothing about the guideline is surprising, said MeiLan K. Han, MD, a spokesperson for the American Lung Association and professor of pulmonary and critical care medicine at the University of Michigan Health System in Ann Arbor. “I don’t think they’ve actually come to any new conclusion,” she said in an interview. “This is pretty much how I practice already.”
But the guideline could have an effect on policy, she said. The panel noted research showing that lower Medicare reimbursement to durable medical equipment companies since 2011 has forced many patients to switch from small, easily portable liquid oxygen to home-fill oxygen systems that include heavy cylinders.
“The impact of this decline in the availability and adequacy of portable oxygen devices in the United States has been profound,” Dr. Holland and colleagues wrote. “Supplemental oxygen users reported numerous problems, with the overarching theme being restricted mobility and isolation due to inadequate portable options.”
For this reason, the guideline recommends liquid oxygen for patients with chronic lung disease who are mobile outside of the home and require continuous oxygen flow rates of >3 L/min during exertion.
Many of Dr. Han’s patients have struggled with this problem, she said. “The clunkiest, most painful form of ‘ambulatory oxygen’ are these really large metal cylinders. They’re huge. And you have to carry them on a cart. It’s portable in theory only.”
Some of her patients have resorted to buying their own equipment on eBay, she said.
The authors report multiple disclosures including serving as advisory board members to foundations and pharmaceutical companies, and some are company employees or stockholders.
A version of this article originally appeared on Medscape.com.
People with chronic lung disease who need significant amounts of oxygen should be able to take it in liquid form when they are able to leave home, according to a new guideline from the American Thoracic Society.
“For those patients, often the other types of devices either can’t supply enough oxygen or are not portable enough,” said Anne Holland, PT, PhD, a professor of physiotherapy at Monash University and Alfred Hospital in Melbourne. “They’re heavy and cumbersome to use.”
Dr. Holland and colleagues also gave a more general recommendation to prescribe ambulatory oxygen – though not necessarily in liquid form – for adults with chronic obstructive pulmonary disease (COPD) or interstitial lung disease (ILD) who have severe exertional room air hypoxemia.
They published the recommendations as part of the ATS’ first-ever guideline on home oxygen therapy for adults with chronic lung disease in the American Journal of Respiratory and Critical Care Medicine.
The ATS identified the need for an updated guideline because of new research, and because an online survey of almost 2,000 U.S. oxygen users showed they were having problems accessing and using oxygen.
For long-term oxygen therapy, the guideline reinforces what most practitioners are already doing, Dr. Holland said. It recommends that adults with COPD or ILD who have severe chronic resting room air hypoxemia receive oxygen therapy at least 15 hours per day.
On the other hand, in adults with COPD who have moderate chronic resting room-air hypoxemia, the guideline recommends against long-term oxygen therapy.
The recommendation to prescribe ambulatory oxygen for people with severe exertional room-air hypoxemia may have more effect on practice, Dr. Holland said. Laboratory-based tests have suggested oxygen can improve exercise capacity, but clinical trials used during daily life have had inconsistent results.
The evidence is particularly lacking for patients with ILD, Dr. Holland said in an interview. “It’s such an important part of practice to maintain oxygen therapy that it’s ethically very difficult to conduct such a trial. So, we did have to make use of indirect evidence from patients with COPD” for the guidelines.
The portable equipment comes with burdens, including managing its weight and bulk, social stigma, fear of cylinders running out, and equipment noise.
“We tried to clearly set out both the benefits and burdens of that therapy and made a conditional recommendation, and also a really strong call for shared decision-making with patients and health professionals,” Dr. Holland said.
In addition to looking at the evidence, the panel took into consideration the concerns identified by patients. This included the challenge of figuring out how to use the equipment. “All the oxygen equipment was ‘dumped’ on me,” wrote one oxygen user quoted in the guideline. “I knew nothing and was in a daze. I am sure that the delivery guy gave me some instructions when it was delivered but I retained nothing.”
For this reason, the guideline describes instruction and training on the use and maintenance of the equipment, including smoking cessation, fire prevention, and tripping hazards, as a “best practice.”
Nothing about the guideline is surprising, said MeiLan K. Han, MD, a spokesperson for the American Lung Association and professor of pulmonary and critical care medicine at the University of Michigan Health System in Ann Arbor. “I don’t think they’ve actually come to any new conclusion,” she said in an interview. “This is pretty much how I practice already.”
But the guideline could have an effect on policy, she said. The panel noted research showing that lower Medicare reimbursement to durable medical equipment companies since 2011 has forced many patients to switch from small, easily portable liquid oxygen to home-fill oxygen systems that include heavy cylinders.
“The impact of this decline in the availability and adequacy of portable oxygen devices in the United States has been profound,” Dr. Holland and colleagues wrote. “Supplemental oxygen users reported numerous problems, with the overarching theme being restricted mobility and isolation due to inadequate portable options.”
For this reason, the guideline recommends liquid oxygen for patients with chronic lung disease who are mobile outside of the home and require continuous oxygen flow rates of >3 L/min during exertion.
Many of Dr. Han’s patients have struggled with this problem, she said. “The clunkiest, most painful form of ‘ambulatory oxygen’ are these really large metal cylinders. They’re huge. And you have to carry them on a cart. It’s portable in theory only.”
Some of her patients have resorted to buying their own equipment on eBay, she said.
The authors report multiple disclosures including serving as advisory board members to foundations and pharmaceutical companies, and some are company employees or stockholders.
A version of this article originally appeared on Medscape.com.
People with chronic lung disease who need significant amounts of oxygen should be able to take it in liquid form when they are able to leave home, according to a new guideline from the American Thoracic Society.
“For those patients, often the other types of devices either can’t supply enough oxygen or are not portable enough,” said Anne Holland, PT, PhD, a professor of physiotherapy at Monash University and Alfred Hospital in Melbourne. “They’re heavy and cumbersome to use.”
Dr. Holland and colleagues also gave a more general recommendation to prescribe ambulatory oxygen – though not necessarily in liquid form – for adults with chronic obstructive pulmonary disease (COPD) or interstitial lung disease (ILD) who have severe exertional room air hypoxemia.
They published the recommendations as part of the ATS’ first-ever guideline on home oxygen therapy for adults with chronic lung disease in the American Journal of Respiratory and Critical Care Medicine.
The ATS identified the need for an updated guideline because of new research, and because an online survey of almost 2,000 U.S. oxygen users showed they were having problems accessing and using oxygen.
For long-term oxygen therapy, the guideline reinforces what most practitioners are already doing, Dr. Holland said. It recommends that adults with COPD or ILD who have severe chronic resting room air hypoxemia receive oxygen therapy at least 15 hours per day.
On the other hand, in adults with COPD who have moderate chronic resting room-air hypoxemia, the guideline recommends against long-term oxygen therapy.
The recommendation to prescribe ambulatory oxygen for people with severe exertional room-air hypoxemia may have more effect on practice, Dr. Holland said. Laboratory-based tests have suggested oxygen can improve exercise capacity, but clinical trials used during daily life have had inconsistent results.
The evidence is particularly lacking for patients with ILD, Dr. Holland said in an interview. “It’s such an important part of practice to maintain oxygen therapy that it’s ethically very difficult to conduct such a trial. So, we did have to make use of indirect evidence from patients with COPD” for the guidelines.
The portable equipment comes with burdens, including managing its weight and bulk, social stigma, fear of cylinders running out, and equipment noise.
“We tried to clearly set out both the benefits and burdens of that therapy and made a conditional recommendation, and also a really strong call for shared decision-making with patients and health professionals,” Dr. Holland said.
In addition to looking at the evidence, the panel took into consideration the concerns identified by patients. This included the challenge of figuring out how to use the equipment. “All the oxygen equipment was ‘dumped’ on me,” wrote one oxygen user quoted in the guideline. “I knew nothing and was in a daze. I am sure that the delivery guy gave me some instructions when it was delivered but I retained nothing.”
For this reason, the guideline describes instruction and training on the use and maintenance of the equipment, including smoking cessation, fire prevention, and tripping hazards, as a “best practice.”
Nothing about the guideline is surprising, said MeiLan K. Han, MD, a spokesperson for the American Lung Association and professor of pulmonary and critical care medicine at the University of Michigan Health System in Ann Arbor. “I don’t think they’ve actually come to any new conclusion,” she said in an interview. “This is pretty much how I practice already.”
But the guideline could have an effect on policy, she said. The panel noted research showing that lower Medicare reimbursement to durable medical equipment companies since 2011 has forced many patients to switch from small, easily portable liquid oxygen to home-fill oxygen systems that include heavy cylinders.
“The impact of this decline in the availability and adequacy of portable oxygen devices in the United States has been profound,” Dr. Holland and colleagues wrote. “Supplemental oxygen users reported numerous problems, with the overarching theme being restricted mobility and isolation due to inadequate portable options.”
For this reason, the guideline recommends liquid oxygen for patients with chronic lung disease who are mobile outside of the home and require continuous oxygen flow rates of >3 L/min during exertion.
Many of Dr. Han’s patients have struggled with this problem, she said. “The clunkiest, most painful form of ‘ambulatory oxygen’ are these really large metal cylinders. They’re huge. And you have to carry them on a cart. It’s portable in theory only.”
Some of her patients have resorted to buying their own equipment on eBay, she said.
The authors report multiple disclosures including serving as advisory board members to foundations and pharmaceutical companies, and some are company employees or stockholders.
A version of this article originally appeared on Medscape.com.
Add delirium to checklist of COVID-19 symptoms in seniors
Delirium should be included on checklists of the presenting signs and symptoms of COVID-19, particularly in elderly adults, according to a multicenter study of seniors visiting emergency departments.
Overall, 28% of the 817 older adults who presented to the emergency department and were diagnosed with COVID-19 had delirium, according to a study published online November 19 in JAMA Network Open. Moreover, 16% of these patients had delirium that was not accompanied by typical symptoms or signs of SARS-CoV-2 infection.
Among patients with delirium, there was a greater probability of admission to the intensive care unit compared with patients who presented without delirium (adjusted relative risk [aRR], 1.67; 95% CI, 1.30 – 2.15), as well as a greater probability of death (aRR, 1.24; 95% CI, 1.00 – 1.55).
“These findings suggest the clinical importance of including delirium on checklists of presenting signs and symptoms of COVID-19 that guide screening, testing, and evaluation,” write Maura Kennedy, MD, MPH, and colleagues.
“I was absolutely seeing cases of delirium where there were no other symptoms of COVID-19, but we didn’t have lot of data on the frequency of this,” explained Kennedy, an emergency department physician at Massachusetts General Hospital and an assistant professor of emergency medicine at Harvard Medical School, Boston.
“And the rate was somewhat surprising compared with that seen in non-COVID studies of delirium, but then our study population was more at risk, coming from long-term care facilities and having prior stroke or dementia,” she said. The most common form of delirium was hypoactive sleepiness and nonresponsiveness, although hyperactivity and agitation were also seen.
Kennedy thinks the addition of delirium as a common presenting symptom to diagnostic checklists would prevent some cases from being missed and allow earlier identification and management of COVID-19 patients at high risk for poor outcomes. “We certainly don’t want to send them back undiagnosed to a long-term care facility or promote transmission within the hospital,” she told Medscape Medical News.
That step has already been implemented in some US centers. “Delirium is something we’ve been looking at since the early summer,” said geriatrician Angela Catic, MD, an assistant professor at Baylor College of Medicine’s Huffington Center on Aging and the Michael E. DeBakey VA Medical Center, Houston, Texas.
“If we see delirium, we’re looking for COVID-19,” said Catic, who was not involved in the study.
In Catic’s experience, it is “not at all atypical” to see patients whose only symptom of COVID-19 is delirium. As with other infections and diseases, “the aging brain is incredibly vulnerable,” she said.
According to William W. Hung, MD, MPH, an assistant professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, New York City, delirium is “generally a common sign of something seriously wrong” in older adults. “In the case of COVID-19, low oxygenation caused by the infection may play a role,” he told Medscape Medical News. Although he agreed that delirium should be included in the differential diagnosis of COVID-19, how frequently it is the only symptom at presentation would need to be determined in a considerably larger population, he said.
Joining the company of those observing this COVID-19 manifestation is Christopher R. Carpenter, MD, a professor of emergency medicine at Washington University in St. Louis, St. Louis, Missouri. He was not a participant in the current study.
“I have absolutely seen and documented delirium as the presenting complaint in older adult patients who were ultimately diagnosed with SARS-CoV-2, and since March, I contemplate SARS-CoV-2 each time I identify delirium,” Carpenter told Medscape Medical News. “Honestly, I ― and most of my colleagues ― are considering SARS-CoV-2 for a range of symptoms and complaints these days, because of the odd presentations we’ve all encountered.”
Study details
For the study, Kennedy and colleagues enrolled consecutive adults aged 65 years and older who were diagnosed with active COVID-19 and who presented to emergency departments at seven centers in Massachusetts, Maine, Connecticut, Michigan, and North Carolina on or after March 13, 2020. Active infection with SARS-CoV-2 was determined on the basis of results of nasal swab polymerase chain reaction tests (99% of cases) or the appearance and distribution of ground-glass opacities on chest radiography or CT (1%).
Of the 817 patients enrolled, 386 (47%) were men, 493 (62%) were White, 215 (27%) were Black, and 54 (7%) were Hispanic or Latinx. The mean age of patients was 77.7 years (standard deviation, 8.2). Their age placed them at risk for chronic comorbidities and cognitive problems; indeed, 15% had at least four chronic conditions, and 30% had existing cognitive impairment.
The authors note that among the 226 patients (28%) who had delirium at presentation, 60 (27%) had experienced delirium for a duration of 2 to 7 days.
Additionally, of the 226 patients who exhibited delirium as a primary symptom, 84 (37%) showed no typical COVID-19 symptoms or signs, such as cough, fever, or shortness of breath.
The presence of delirium did not correlate with any of the typical COVID-19 symptoms in particular; Kennedy noted that only 56% of patients in the cohort had a fever at presentation.
Delirium at presentation was significantly associated with a median hospital stay of more than 8 days (aRR, 1.14; 95% CI, .97 – 1.35) and a greater risk for discharge to a rehabilitation facility (aRR, 1.55; 95% CI, 1.07 – 2.26). Factors associated with delirium included age older than 75 years, residence in a nursing home or assisted-living facility, previous use of psychoactive medications, vision impairment, hearing impairment, stroke, and Parkinson’s disease.
Kennedy noted that the rate of delirium observed in this study is much higher than that generally reported in emergency department studies conducted before the COVID-19 pandemic. In those studies, the delirium rate ranged from 7% to 20%. The associated risk factors, however, are comparable.
“Mounting evidence supports the high occurrence of delirium and other neuropsychiatric manifestations with COVID-19, with previously reported rates of 22% to 33% among hospitalized patients,” Kennedy and associates write.
In Carpenter’s opinion, the development of incident delirium while receiving care in the emergency department, as opposed to delirium at the time of presentation, has been exacerbated by the no-visitor policies mandated by the pandemic, which have prevented visits even from personal caregivers of patients with moderate to severe dementia. “Although healthcare systems need to be cognizant of the risk of spread to uninfected caregivers, there’s a risk-benefit balance that must be found, because having one caregiver at the bedside can prevent delirium in cognitively impaired patients,” said Carpenter, who was not involved in the current study.
Among the barriers to improving the situation, Carpenter cited the lack of routine delirium screening and the absence of high-quality evidence to support emergency department interventions to mitigate delirium.
“Layer those challenges on top of COVID-19’s rapidly evolving diagnostic landscape, frequent atypical presentations, and asymptomatic carriers across all age groups and the negative impact of delirium is magnified,” Carpenter said.
Once elderly patients are hospitalized, Kennedy recommends the nonpharmacologic guidelines of the Hospital Elder Life Program for reducing delirium risk. Recommendations include the providing of adequate sleep, hydration, and nutrition, as well as function restoration, precipitant avoidance, and reorientation.
The study was supported in part by the National Institute on Aging and the Massachusetts Medical School. The authors, Carpenter, Hung, and Catic have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Delirium should be included on checklists of the presenting signs and symptoms of COVID-19, particularly in elderly adults, according to a multicenter study of seniors visiting emergency departments.
Overall, 28% of the 817 older adults who presented to the emergency department and were diagnosed with COVID-19 had delirium, according to a study published online November 19 in JAMA Network Open. Moreover, 16% of these patients had delirium that was not accompanied by typical symptoms or signs of SARS-CoV-2 infection.
Among patients with delirium, there was a greater probability of admission to the intensive care unit compared with patients who presented without delirium (adjusted relative risk [aRR], 1.67; 95% CI, 1.30 – 2.15), as well as a greater probability of death (aRR, 1.24; 95% CI, 1.00 – 1.55).
“These findings suggest the clinical importance of including delirium on checklists of presenting signs and symptoms of COVID-19 that guide screening, testing, and evaluation,” write Maura Kennedy, MD, MPH, and colleagues.
“I was absolutely seeing cases of delirium where there were no other symptoms of COVID-19, but we didn’t have lot of data on the frequency of this,” explained Kennedy, an emergency department physician at Massachusetts General Hospital and an assistant professor of emergency medicine at Harvard Medical School, Boston.
“And the rate was somewhat surprising compared with that seen in non-COVID studies of delirium, but then our study population was more at risk, coming from long-term care facilities and having prior stroke or dementia,” she said. The most common form of delirium was hypoactive sleepiness and nonresponsiveness, although hyperactivity and agitation were also seen.
Kennedy thinks the addition of delirium as a common presenting symptom to diagnostic checklists would prevent some cases from being missed and allow earlier identification and management of COVID-19 patients at high risk for poor outcomes. “We certainly don’t want to send them back undiagnosed to a long-term care facility or promote transmission within the hospital,” she told Medscape Medical News.
That step has already been implemented in some US centers. “Delirium is something we’ve been looking at since the early summer,” said geriatrician Angela Catic, MD, an assistant professor at Baylor College of Medicine’s Huffington Center on Aging and the Michael E. DeBakey VA Medical Center, Houston, Texas.
“If we see delirium, we’re looking for COVID-19,” said Catic, who was not involved in the study.
In Catic’s experience, it is “not at all atypical” to see patients whose only symptom of COVID-19 is delirium. As with other infections and diseases, “the aging brain is incredibly vulnerable,” she said.
According to William W. Hung, MD, MPH, an assistant professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, New York City, delirium is “generally a common sign of something seriously wrong” in older adults. “In the case of COVID-19, low oxygenation caused by the infection may play a role,” he told Medscape Medical News. Although he agreed that delirium should be included in the differential diagnosis of COVID-19, how frequently it is the only symptom at presentation would need to be determined in a considerably larger population, he said.
Joining the company of those observing this COVID-19 manifestation is Christopher R. Carpenter, MD, a professor of emergency medicine at Washington University in St. Louis, St. Louis, Missouri. He was not a participant in the current study.
“I have absolutely seen and documented delirium as the presenting complaint in older adult patients who were ultimately diagnosed with SARS-CoV-2, and since March, I contemplate SARS-CoV-2 each time I identify delirium,” Carpenter told Medscape Medical News. “Honestly, I ― and most of my colleagues ― are considering SARS-CoV-2 for a range of symptoms and complaints these days, because of the odd presentations we’ve all encountered.”
Study details
For the study, Kennedy and colleagues enrolled consecutive adults aged 65 years and older who were diagnosed with active COVID-19 and who presented to emergency departments at seven centers in Massachusetts, Maine, Connecticut, Michigan, and North Carolina on or after March 13, 2020. Active infection with SARS-CoV-2 was determined on the basis of results of nasal swab polymerase chain reaction tests (99% of cases) or the appearance and distribution of ground-glass opacities on chest radiography or CT (1%).
Of the 817 patients enrolled, 386 (47%) were men, 493 (62%) were White, 215 (27%) were Black, and 54 (7%) were Hispanic or Latinx. The mean age of patients was 77.7 years (standard deviation, 8.2). Their age placed them at risk for chronic comorbidities and cognitive problems; indeed, 15% had at least four chronic conditions, and 30% had existing cognitive impairment.
The authors note that among the 226 patients (28%) who had delirium at presentation, 60 (27%) had experienced delirium for a duration of 2 to 7 days.
Additionally, of the 226 patients who exhibited delirium as a primary symptom, 84 (37%) showed no typical COVID-19 symptoms or signs, such as cough, fever, or shortness of breath.
The presence of delirium did not correlate with any of the typical COVID-19 symptoms in particular; Kennedy noted that only 56% of patients in the cohort had a fever at presentation.
Delirium at presentation was significantly associated with a median hospital stay of more than 8 days (aRR, 1.14; 95% CI, .97 – 1.35) and a greater risk for discharge to a rehabilitation facility (aRR, 1.55; 95% CI, 1.07 – 2.26). Factors associated with delirium included age older than 75 years, residence in a nursing home or assisted-living facility, previous use of psychoactive medications, vision impairment, hearing impairment, stroke, and Parkinson’s disease.
Kennedy noted that the rate of delirium observed in this study is much higher than that generally reported in emergency department studies conducted before the COVID-19 pandemic. In those studies, the delirium rate ranged from 7% to 20%. The associated risk factors, however, are comparable.
“Mounting evidence supports the high occurrence of delirium and other neuropsychiatric manifestations with COVID-19, with previously reported rates of 22% to 33% among hospitalized patients,” Kennedy and associates write.
In Carpenter’s opinion, the development of incident delirium while receiving care in the emergency department, as opposed to delirium at the time of presentation, has been exacerbated by the no-visitor policies mandated by the pandemic, which have prevented visits even from personal caregivers of patients with moderate to severe dementia. “Although healthcare systems need to be cognizant of the risk of spread to uninfected caregivers, there’s a risk-benefit balance that must be found, because having one caregiver at the bedside can prevent delirium in cognitively impaired patients,” said Carpenter, who was not involved in the current study.
Among the barriers to improving the situation, Carpenter cited the lack of routine delirium screening and the absence of high-quality evidence to support emergency department interventions to mitigate delirium.
“Layer those challenges on top of COVID-19’s rapidly evolving diagnostic landscape, frequent atypical presentations, and asymptomatic carriers across all age groups and the negative impact of delirium is magnified,” Carpenter said.
Once elderly patients are hospitalized, Kennedy recommends the nonpharmacologic guidelines of the Hospital Elder Life Program for reducing delirium risk. Recommendations include the providing of adequate sleep, hydration, and nutrition, as well as function restoration, precipitant avoidance, and reorientation.
The study was supported in part by the National Institute on Aging and the Massachusetts Medical School. The authors, Carpenter, Hung, and Catic have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Delirium should be included on checklists of the presenting signs and symptoms of COVID-19, particularly in elderly adults, according to a multicenter study of seniors visiting emergency departments.
Overall, 28% of the 817 older adults who presented to the emergency department and were diagnosed with COVID-19 had delirium, according to a study published online November 19 in JAMA Network Open. Moreover, 16% of these patients had delirium that was not accompanied by typical symptoms or signs of SARS-CoV-2 infection.
Among patients with delirium, there was a greater probability of admission to the intensive care unit compared with patients who presented without delirium (adjusted relative risk [aRR], 1.67; 95% CI, 1.30 – 2.15), as well as a greater probability of death (aRR, 1.24; 95% CI, 1.00 – 1.55).
“These findings suggest the clinical importance of including delirium on checklists of presenting signs and symptoms of COVID-19 that guide screening, testing, and evaluation,” write Maura Kennedy, MD, MPH, and colleagues.
“I was absolutely seeing cases of delirium where there were no other symptoms of COVID-19, but we didn’t have lot of data on the frequency of this,” explained Kennedy, an emergency department physician at Massachusetts General Hospital and an assistant professor of emergency medicine at Harvard Medical School, Boston.
“And the rate was somewhat surprising compared with that seen in non-COVID studies of delirium, but then our study population was more at risk, coming from long-term care facilities and having prior stroke or dementia,” she said. The most common form of delirium was hypoactive sleepiness and nonresponsiveness, although hyperactivity and agitation were also seen.
Kennedy thinks the addition of delirium as a common presenting symptom to diagnostic checklists would prevent some cases from being missed and allow earlier identification and management of COVID-19 patients at high risk for poor outcomes. “We certainly don’t want to send them back undiagnosed to a long-term care facility or promote transmission within the hospital,” she told Medscape Medical News.
That step has already been implemented in some US centers. “Delirium is something we’ve been looking at since the early summer,” said geriatrician Angela Catic, MD, an assistant professor at Baylor College of Medicine’s Huffington Center on Aging and the Michael E. DeBakey VA Medical Center, Houston, Texas.
“If we see delirium, we’re looking for COVID-19,” said Catic, who was not involved in the study.
In Catic’s experience, it is “not at all atypical” to see patients whose only symptom of COVID-19 is delirium. As with other infections and diseases, “the aging brain is incredibly vulnerable,” she said.
According to William W. Hung, MD, MPH, an assistant professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, New York City, delirium is “generally a common sign of something seriously wrong” in older adults. “In the case of COVID-19, low oxygenation caused by the infection may play a role,” he told Medscape Medical News. Although he agreed that delirium should be included in the differential diagnosis of COVID-19, how frequently it is the only symptom at presentation would need to be determined in a considerably larger population, he said.
Joining the company of those observing this COVID-19 manifestation is Christopher R. Carpenter, MD, a professor of emergency medicine at Washington University in St. Louis, St. Louis, Missouri. He was not a participant in the current study.
“I have absolutely seen and documented delirium as the presenting complaint in older adult patients who were ultimately diagnosed with SARS-CoV-2, and since March, I contemplate SARS-CoV-2 each time I identify delirium,” Carpenter told Medscape Medical News. “Honestly, I ― and most of my colleagues ― are considering SARS-CoV-2 for a range of symptoms and complaints these days, because of the odd presentations we’ve all encountered.”
Study details
For the study, Kennedy and colleagues enrolled consecutive adults aged 65 years and older who were diagnosed with active COVID-19 and who presented to emergency departments at seven centers in Massachusetts, Maine, Connecticut, Michigan, and North Carolina on or after March 13, 2020. Active infection with SARS-CoV-2 was determined on the basis of results of nasal swab polymerase chain reaction tests (99% of cases) or the appearance and distribution of ground-glass opacities on chest radiography or CT (1%).
Of the 817 patients enrolled, 386 (47%) were men, 493 (62%) were White, 215 (27%) were Black, and 54 (7%) were Hispanic or Latinx. The mean age of patients was 77.7 years (standard deviation, 8.2). Their age placed them at risk for chronic comorbidities and cognitive problems; indeed, 15% had at least four chronic conditions, and 30% had existing cognitive impairment.
The authors note that among the 226 patients (28%) who had delirium at presentation, 60 (27%) had experienced delirium for a duration of 2 to 7 days.
Additionally, of the 226 patients who exhibited delirium as a primary symptom, 84 (37%) showed no typical COVID-19 symptoms or signs, such as cough, fever, or shortness of breath.
The presence of delirium did not correlate with any of the typical COVID-19 symptoms in particular; Kennedy noted that only 56% of patients in the cohort had a fever at presentation.
Delirium at presentation was significantly associated with a median hospital stay of more than 8 days (aRR, 1.14; 95% CI, .97 – 1.35) and a greater risk for discharge to a rehabilitation facility (aRR, 1.55; 95% CI, 1.07 – 2.26). Factors associated with delirium included age older than 75 years, residence in a nursing home or assisted-living facility, previous use of psychoactive medications, vision impairment, hearing impairment, stroke, and Parkinson’s disease.
Kennedy noted that the rate of delirium observed in this study is much higher than that generally reported in emergency department studies conducted before the COVID-19 pandemic. In those studies, the delirium rate ranged from 7% to 20%. The associated risk factors, however, are comparable.
“Mounting evidence supports the high occurrence of delirium and other neuropsychiatric manifestations with COVID-19, with previously reported rates of 22% to 33% among hospitalized patients,” Kennedy and associates write.
In Carpenter’s opinion, the development of incident delirium while receiving care in the emergency department, as opposed to delirium at the time of presentation, has been exacerbated by the no-visitor policies mandated by the pandemic, which have prevented visits even from personal caregivers of patients with moderate to severe dementia. “Although healthcare systems need to be cognizant of the risk of spread to uninfected caregivers, there’s a risk-benefit balance that must be found, because having one caregiver at the bedside can prevent delirium in cognitively impaired patients,” said Carpenter, who was not involved in the current study.
Among the barriers to improving the situation, Carpenter cited the lack of routine delirium screening and the absence of high-quality evidence to support emergency department interventions to mitigate delirium.
“Layer those challenges on top of COVID-19’s rapidly evolving diagnostic landscape, frequent atypical presentations, and asymptomatic carriers across all age groups and the negative impact of delirium is magnified,” Carpenter said.
Once elderly patients are hospitalized, Kennedy recommends the nonpharmacologic guidelines of the Hospital Elder Life Program for reducing delirium risk. Recommendations include the providing of adequate sleep, hydration, and nutrition, as well as function restoration, precipitant avoidance, and reorientation.
The study was supported in part by the National Institute on Aging and the Massachusetts Medical School. The authors, Carpenter, Hung, and Catic have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
COVID-19 cases in children continue to set records
As far as the pandemic is concerned, it seems like a pretty small thing. A difference of just 0.3%. Children now represent 11.8% of all COVID-19 cases that have occurred since the beginning of the pandemic, compared with 11.5% 1 week ago, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Hiding behind that 0.3%, however, is a much larger number: 144,145. That is the number of new child cases that occurred during the week that ended Nov. 19, and it’s the highest weekly figure yet, eclipsing the previous high of 111,946 from the week of Nov. 12, the AAP and the CHA said in their latest COVID-19 report. For the week ending Nov. 19, children represented 14.1% of all new cases, up from 14.0% the week before.
In the United States, more than 1.18 million children have been infected by the coronavirus since the beginning of the pandemic, with the total among all ages topping 10 million in 49 states (New York is not providing age distribution), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP/CHA data show. That works out to 11.8% of all cases.
The overall rate of child COVID-19 cases is now up to 1,573 per 100,000 children nationally, with considerable variation seen among the states. The lowest rates can be found in Vermont (344 per 100,000), Maine (452), and Hawaii (675), and the highest in North Dakota (5,589), South Dakota (3,993), and Wisconsin (3,727), the AAP and CHA said in the report.
Comparisons between states are somewhat problematic, though, because “each state makes different decisions about how to report the age distribution of COVID-19 cases, and as a result the age range for reported cases varies by state. … It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states at this time,” the two organizations noted.
Five more COVID-19–related deaths in children were reported during the week of Nov. 19, bringing the count to 138 and holding at just 0.06% of the total for all ages, based on data from 43 states and New York City. Children’s share of hospitalizations increased slightly in the last week, rising from 1.7% to 1.8% in the 24 states (and NYC) that are reporting such data. The total number of child hospitalizations in those jurisdictions is just over 6,700, the AAP and CHA said.
As far as the pandemic is concerned, it seems like a pretty small thing. A difference of just 0.3%. Children now represent 11.8% of all COVID-19 cases that have occurred since the beginning of the pandemic, compared with 11.5% 1 week ago, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Hiding behind that 0.3%, however, is a much larger number: 144,145. That is the number of new child cases that occurred during the week that ended Nov. 19, and it’s the highest weekly figure yet, eclipsing the previous high of 111,946 from the week of Nov. 12, the AAP and the CHA said in their latest COVID-19 report. For the week ending Nov. 19, children represented 14.1% of all new cases, up from 14.0% the week before.
In the United States, more than 1.18 million children have been infected by the coronavirus since the beginning of the pandemic, with the total among all ages topping 10 million in 49 states (New York is not providing age distribution), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP/CHA data show. That works out to 11.8% of all cases.
The overall rate of child COVID-19 cases is now up to 1,573 per 100,000 children nationally, with considerable variation seen among the states. The lowest rates can be found in Vermont (344 per 100,000), Maine (452), and Hawaii (675), and the highest in North Dakota (5,589), South Dakota (3,993), and Wisconsin (3,727), the AAP and CHA said in the report.
Comparisons between states are somewhat problematic, though, because “each state makes different decisions about how to report the age distribution of COVID-19 cases, and as a result the age range for reported cases varies by state. … It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states at this time,” the two organizations noted.
Five more COVID-19–related deaths in children were reported during the week of Nov. 19, bringing the count to 138 and holding at just 0.06% of the total for all ages, based on data from 43 states and New York City. Children’s share of hospitalizations increased slightly in the last week, rising from 1.7% to 1.8% in the 24 states (and NYC) that are reporting such data. The total number of child hospitalizations in those jurisdictions is just over 6,700, the AAP and CHA said.
As far as the pandemic is concerned, it seems like a pretty small thing. A difference of just 0.3%. Children now represent 11.8% of all COVID-19 cases that have occurred since the beginning of the pandemic, compared with 11.5% 1 week ago, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Hiding behind that 0.3%, however, is a much larger number: 144,145. That is the number of new child cases that occurred during the week that ended Nov. 19, and it’s the highest weekly figure yet, eclipsing the previous high of 111,946 from the week of Nov. 12, the AAP and the CHA said in their latest COVID-19 report. For the week ending Nov. 19, children represented 14.1% of all new cases, up from 14.0% the week before.
In the United States, more than 1.18 million children have been infected by the coronavirus since the beginning of the pandemic, with the total among all ages topping 10 million in 49 states (New York is not providing age distribution), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP/CHA data show. That works out to 11.8% of all cases.
The overall rate of child COVID-19 cases is now up to 1,573 per 100,000 children nationally, with considerable variation seen among the states. The lowest rates can be found in Vermont (344 per 100,000), Maine (452), and Hawaii (675), and the highest in North Dakota (5,589), South Dakota (3,993), and Wisconsin (3,727), the AAP and CHA said in the report.
Comparisons between states are somewhat problematic, though, because “each state makes different decisions about how to report the age distribution of COVID-19 cases, and as a result the age range for reported cases varies by state. … It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states at this time,” the two organizations noted.
Five more COVID-19–related deaths in children were reported during the week of Nov. 19, bringing the count to 138 and holding at just 0.06% of the total for all ages, based on data from 43 states and New York City. Children’s share of hospitalizations increased slightly in the last week, rising from 1.7% to 1.8% in the 24 states (and NYC) that are reporting such data. The total number of child hospitalizations in those jurisdictions is just over 6,700, the AAP and CHA said.
FDA authorizes baricitinib combo for COVID-19
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
FDA approves first at-home COVID-19 test kit
The FDA issued an emergency use authorization Tuesday for the first self-testing COVID-19 kit to use at home, which provides results in about 30 minutes.
The Lucira COVID-19 All-In-One Test-Kit is a single-use test that has a nasal swab to collect samples for people ages 14 and older. It’s available only by prescription, which can be given by a doctor who suspects a patient may have contracted the coronavirus.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn, MD, said in the statement.
The test kit can also be used in doctor’s offices, hospitals, urgent care centers, and emergency rooms for all ages, but samples must be collected by a health care professional if the patient is under age 14.
After using the nasal swab, the test works by swirling the sample in a vial and then placing it in the provided test unit, according to the FDA. Within 30 minutes, the results appear on the unit’s light-up display. People who receive a positive result should self-isolate and seek care from their doctor. Those who test negative but have COVID-like symptoms should follow up with their doctor, since a negative result doesn’t necessarily mean they don’t have the coronavirus.
Testing is still a key part of controlling the spread of the coronavirus, Reuters reports. The United States surpassed 11 million infections Sunday, only 8 days after passing 10 million cases.
With the at-home testing kit, public health officials still need to track and monitor results. As part of the emergency use authorization, the FDA requires doctors who prescribe the tests to report all results to public health authorities based on local, state, and federal requirements. Lucira Health, the test maker, also created box labeling and instructions to help doctors to report results.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in the statement.
This article first appeared on WebMD.com.
The FDA issued an emergency use authorization Tuesday for the first self-testing COVID-19 kit to use at home, which provides results in about 30 minutes.
The Lucira COVID-19 All-In-One Test-Kit is a single-use test that has a nasal swab to collect samples for people ages 14 and older. It’s available only by prescription, which can be given by a doctor who suspects a patient may have contracted the coronavirus.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn, MD, said in the statement.
The test kit can also be used in doctor’s offices, hospitals, urgent care centers, and emergency rooms for all ages, but samples must be collected by a health care professional if the patient is under age 14.
After using the nasal swab, the test works by swirling the sample in a vial and then placing it in the provided test unit, according to the FDA. Within 30 minutes, the results appear on the unit’s light-up display. People who receive a positive result should self-isolate and seek care from their doctor. Those who test negative but have COVID-like symptoms should follow up with their doctor, since a negative result doesn’t necessarily mean they don’t have the coronavirus.
Testing is still a key part of controlling the spread of the coronavirus, Reuters reports. The United States surpassed 11 million infections Sunday, only 8 days after passing 10 million cases.
With the at-home testing kit, public health officials still need to track and monitor results. As part of the emergency use authorization, the FDA requires doctors who prescribe the tests to report all results to public health authorities based on local, state, and federal requirements. Lucira Health, the test maker, also created box labeling and instructions to help doctors to report results.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in the statement.
This article first appeared on WebMD.com.
The FDA issued an emergency use authorization Tuesday for the first self-testing COVID-19 kit to use at home, which provides results in about 30 minutes.
The Lucira COVID-19 All-In-One Test-Kit is a single-use test that has a nasal swab to collect samples for people ages 14 and older. It’s available only by prescription, which can be given by a doctor who suspects a patient may have contracted the coronavirus.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn, MD, said in the statement.
The test kit can also be used in doctor’s offices, hospitals, urgent care centers, and emergency rooms for all ages, but samples must be collected by a health care professional if the patient is under age 14.
After using the nasal swab, the test works by swirling the sample in a vial and then placing it in the provided test unit, according to the FDA. Within 30 minutes, the results appear on the unit’s light-up display. People who receive a positive result should self-isolate and seek care from their doctor. Those who test negative but have COVID-like symptoms should follow up with their doctor, since a negative result doesn’t necessarily mean they don’t have the coronavirus.
Testing is still a key part of controlling the spread of the coronavirus, Reuters reports. The United States surpassed 11 million infections Sunday, only 8 days after passing 10 million cases.
With the at-home testing kit, public health officials still need to track and monitor results. As part of the emergency use authorization, the FDA requires doctors who prescribe the tests to report all results to public health authorities based on local, state, and federal requirements. Lucira Health, the test maker, also created box labeling and instructions to help doctors to report results.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” Jeff Shuren, MD, director of the FDA’s Center for Devices and Radiological Health, said in the statement.
This article first appeared on WebMD.com.
Telehealth finds acceptance among patients with CF, clinicians
(CF) and the physicians who treat them, according to three new studies. The surveys examined attitudes during the COVID-19 pandemic, which complicates interpretation of the survey, but the results nevertheless bode well for telehealth’s future in the management of CF.
“Patients could be responding positively just because they could have a visit during the pandemic,” said Andrew NeSmith, during a presentation of a survey of adults with CF at the virtual North American Cystic Fibrosis Conference. Mr. NeSmith is the clinical data coordinator at the University of Alabama at Birmingham Cystic Fibrosis Center.
Other posters at the conference examined attitudes among pediatric populations and treating physicians, with generally positive results, which has generated optimism that telehealth could become an important element of care after the pandemic fades. “This data suggests that telehealth could be integrated into routine follow-up care in the CF chronic care model,” said Mr. NeSmith.
His team collected responses from 119 individuals at the University of Alabama at Birmingham; Boston Children’s Hospital; Brigham and Women’s Hospital, Boston; Virginia Commonwealth University, Richmond; and West Virginia University, Morgantown. A total of 28% had conducted a prior telehealth visit before the study; 92% of visits were conducted with a medical doctor. Only 13% reported experiencing difficulties with their first telehealth visit. Eighty-five percent rated convenience, and 77% rated their satisfaction with telehealth as “high.” Most (92%) said they were able to see their desired disciplines, 95% felt all of their issues had been addressed, and 83% strongly agreed that telehealth visits were of adequate length.
Not everything was rosy. A total of 48% of participants expressed at least moderate concern over a lack of pulmonary function test or throat/sputum culture. There were much fewer concerns over missing vital signs or weight measurements.
The overall results weren’t surprising to Robert Giusti, MD, clinical professor of pediatrics at New York University and director of the Pediatric Cystic Fibrosis Center, New York, who was not involved in the study. “I was expecting that patients were going to like it. It makes their life easier,” he said in an interview.
A survey of families of pediatric individuals with CF at seven centers found similar levels of satisfaction. A total of 23% had used telehealth previously; 96% rated convenience, and 93% rated satisfaction as “high.” Almost all (99%) felt that all concerns were met, 98% said that sessions were adequately long, and 87% had no trouble connecting to the visit.
Some participants in this survey had concerns about what might be missing with a televisit. Half (52%) had at least a moderate concern over lack of pulmonary function tests, 45% over lack of vital signs, 29% about lack of weight measurements, and 64% about the need for throat/sputum culture. Despite those issues, 69% preferred that “some” and 22% preferred that “most” future visits be conducted by telehealth.
A survey of physicians who used telehealth with CF patients also found broad support. They reported some challenges, with 70% saying they experienced technical difficulty, and 77% saying it “took time” to resolve a visit with only 18% reporting that visits were “quickly resolved.” Most (86%) said they were satisfied with telehealth for care delivery, and 78% said it was appropriate for most patients. Most said telehealth improved the patient-physician relationship, and they believed visits were more efficient when conducted via telehealth than in person. A majority (81%) endorsed using telehealth for some visits, and 12% for most visits.
A key question will be how telehealth affects patient outcomes, according to Ryan Perkins, MD, who was a coauthor of the survey of physicians. “If they’re not doing as well from an outcomes perspective, that would be a huge limitation to our patients,” said Dr. Perkins, who is a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital.
Although the study examined only models of care that were entirely virtual, Dr. Perkins noted that hybrid in-person/virtual care models are also possible. “Do we have better outcomes doing it that way? Is there higher patient satisfaction? I’m sure that will be a hot topic moving forward.”
Dr. Perkins noted that patients expressed concern about not being able to get sputum cultures and spirometry recordings during telehealth sessions. “That’s not really surprising to me, but I think it raises the question as we’re imagining care models for the future – how can we implement those components into future care delivery?”
Another hurdle will be insurance coverage. “My fear is that insurance companies are going to cut down the amount of reimbursement for telehealth visits in the future and just going to make it more complicated,” said Dr. Giusti. “Certainly, though, I think telehealth is an important outreach that we’d like to continue with our patients.”
Mr. NeSmith, Dr. Giusti, and Dr. Perkins reported no relevant financial disclosures.
SOURCE: NeSmith A et al. NACFC 2020, Abstracts 797, 799, 810.
(CF) and the physicians who treat them, according to three new studies. The surveys examined attitudes during the COVID-19 pandemic, which complicates interpretation of the survey, but the results nevertheless bode well for telehealth’s future in the management of CF.
“Patients could be responding positively just because they could have a visit during the pandemic,” said Andrew NeSmith, during a presentation of a survey of adults with CF at the virtual North American Cystic Fibrosis Conference. Mr. NeSmith is the clinical data coordinator at the University of Alabama at Birmingham Cystic Fibrosis Center.
Other posters at the conference examined attitudes among pediatric populations and treating physicians, with generally positive results, which has generated optimism that telehealth could become an important element of care after the pandemic fades. “This data suggests that telehealth could be integrated into routine follow-up care in the CF chronic care model,” said Mr. NeSmith.
His team collected responses from 119 individuals at the University of Alabama at Birmingham; Boston Children’s Hospital; Brigham and Women’s Hospital, Boston; Virginia Commonwealth University, Richmond; and West Virginia University, Morgantown. A total of 28% had conducted a prior telehealth visit before the study; 92% of visits were conducted with a medical doctor. Only 13% reported experiencing difficulties with their first telehealth visit. Eighty-five percent rated convenience, and 77% rated their satisfaction with telehealth as “high.” Most (92%) said they were able to see their desired disciplines, 95% felt all of their issues had been addressed, and 83% strongly agreed that telehealth visits were of adequate length.
Not everything was rosy. A total of 48% of participants expressed at least moderate concern over a lack of pulmonary function test or throat/sputum culture. There were much fewer concerns over missing vital signs or weight measurements.
The overall results weren’t surprising to Robert Giusti, MD, clinical professor of pediatrics at New York University and director of the Pediatric Cystic Fibrosis Center, New York, who was not involved in the study. “I was expecting that patients were going to like it. It makes their life easier,” he said in an interview.
A survey of families of pediatric individuals with CF at seven centers found similar levels of satisfaction. A total of 23% had used telehealth previously; 96% rated convenience, and 93% rated satisfaction as “high.” Almost all (99%) felt that all concerns were met, 98% said that sessions were adequately long, and 87% had no trouble connecting to the visit.
Some participants in this survey had concerns about what might be missing with a televisit. Half (52%) had at least a moderate concern over lack of pulmonary function tests, 45% over lack of vital signs, 29% about lack of weight measurements, and 64% about the need for throat/sputum culture. Despite those issues, 69% preferred that “some” and 22% preferred that “most” future visits be conducted by telehealth.
A survey of physicians who used telehealth with CF patients also found broad support. They reported some challenges, with 70% saying they experienced technical difficulty, and 77% saying it “took time” to resolve a visit with only 18% reporting that visits were “quickly resolved.” Most (86%) said they were satisfied with telehealth for care delivery, and 78% said it was appropriate for most patients. Most said telehealth improved the patient-physician relationship, and they believed visits were more efficient when conducted via telehealth than in person. A majority (81%) endorsed using telehealth for some visits, and 12% for most visits.
A key question will be how telehealth affects patient outcomes, according to Ryan Perkins, MD, who was a coauthor of the survey of physicians. “If they’re not doing as well from an outcomes perspective, that would be a huge limitation to our patients,” said Dr. Perkins, who is a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital.
Although the study examined only models of care that were entirely virtual, Dr. Perkins noted that hybrid in-person/virtual care models are also possible. “Do we have better outcomes doing it that way? Is there higher patient satisfaction? I’m sure that will be a hot topic moving forward.”
Dr. Perkins noted that patients expressed concern about not being able to get sputum cultures and spirometry recordings during telehealth sessions. “That’s not really surprising to me, but I think it raises the question as we’re imagining care models for the future – how can we implement those components into future care delivery?”
Another hurdle will be insurance coverage. “My fear is that insurance companies are going to cut down the amount of reimbursement for telehealth visits in the future and just going to make it more complicated,” said Dr. Giusti. “Certainly, though, I think telehealth is an important outreach that we’d like to continue with our patients.”
Mr. NeSmith, Dr. Giusti, and Dr. Perkins reported no relevant financial disclosures.
SOURCE: NeSmith A et al. NACFC 2020, Abstracts 797, 799, 810.
(CF) and the physicians who treat them, according to three new studies. The surveys examined attitudes during the COVID-19 pandemic, which complicates interpretation of the survey, but the results nevertheless bode well for telehealth’s future in the management of CF.
“Patients could be responding positively just because they could have a visit during the pandemic,” said Andrew NeSmith, during a presentation of a survey of adults with CF at the virtual North American Cystic Fibrosis Conference. Mr. NeSmith is the clinical data coordinator at the University of Alabama at Birmingham Cystic Fibrosis Center.
Other posters at the conference examined attitudes among pediatric populations and treating physicians, with generally positive results, which has generated optimism that telehealth could become an important element of care after the pandemic fades. “This data suggests that telehealth could be integrated into routine follow-up care in the CF chronic care model,” said Mr. NeSmith.
His team collected responses from 119 individuals at the University of Alabama at Birmingham; Boston Children’s Hospital; Brigham and Women’s Hospital, Boston; Virginia Commonwealth University, Richmond; and West Virginia University, Morgantown. A total of 28% had conducted a prior telehealth visit before the study; 92% of visits were conducted with a medical doctor. Only 13% reported experiencing difficulties with their first telehealth visit. Eighty-five percent rated convenience, and 77% rated their satisfaction with telehealth as “high.” Most (92%) said they were able to see their desired disciplines, 95% felt all of their issues had been addressed, and 83% strongly agreed that telehealth visits were of adequate length.
Not everything was rosy. A total of 48% of participants expressed at least moderate concern over a lack of pulmonary function test or throat/sputum culture. There were much fewer concerns over missing vital signs or weight measurements.
The overall results weren’t surprising to Robert Giusti, MD, clinical professor of pediatrics at New York University and director of the Pediatric Cystic Fibrosis Center, New York, who was not involved in the study. “I was expecting that patients were going to like it. It makes their life easier,” he said in an interview.
A survey of families of pediatric individuals with CF at seven centers found similar levels of satisfaction. A total of 23% had used telehealth previously; 96% rated convenience, and 93% rated satisfaction as “high.” Almost all (99%) felt that all concerns were met, 98% said that sessions were adequately long, and 87% had no trouble connecting to the visit.
Some participants in this survey had concerns about what might be missing with a televisit. Half (52%) had at least a moderate concern over lack of pulmonary function tests, 45% over lack of vital signs, 29% about lack of weight measurements, and 64% about the need for throat/sputum culture. Despite those issues, 69% preferred that “some” and 22% preferred that “most” future visits be conducted by telehealth.
A survey of physicians who used telehealth with CF patients also found broad support. They reported some challenges, with 70% saying they experienced technical difficulty, and 77% saying it “took time” to resolve a visit with only 18% reporting that visits were “quickly resolved.” Most (86%) said they were satisfied with telehealth for care delivery, and 78% said it was appropriate for most patients. Most said telehealth improved the patient-physician relationship, and they believed visits were more efficient when conducted via telehealth than in person. A majority (81%) endorsed using telehealth for some visits, and 12% for most visits.
A key question will be how telehealth affects patient outcomes, according to Ryan Perkins, MD, who was a coauthor of the survey of physicians. “If they’re not doing as well from an outcomes perspective, that would be a huge limitation to our patients,” said Dr. Perkins, who is a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital.
Although the study examined only models of care that were entirely virtual, Dr. Perkins noted that hybrid in-person/virtual care models are also possible. “Do we have better outcomes doing it that way? Is there higher patient satisfaction? I’m sure that will be a hot topic moving forward.”
Dr. Perkins noted that patients expressed concern about not being able to get sputum cultures and spirometry recordings during telehealth sessions. “That’s not really surprising to me, but I think it raises the question as we’re imagining care models for the future – how can we implement those components into future care delivery?”
Another hurdle will be insurance coverage. “My fear is that insurance companies are going to cut down the amount of reimbursement for telehealth visits in the future and just going to make it more complicated,” said Dr. Giusti. “Certainly, though, I think telehealth is an important outreach that we’d like to continue with our patients.”
Mr. NeSmith, Dr. Giusti, and Dr. Perkins reported no relevant financial disclosures.
SOURCE: NeSmith A et al. NACFC 2020, Abstracts 797, 799, 810.
FROM NACFC 2020
Pfizer’s COVID-19 vaccine 95% effective in final phase 3 results
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
‘Hospital at home’ increases COVID capacity in large study
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
Myocarditis rare, macrophage infiltration common at COVID autopsy
An international autopsy study of 21 patients who died from COVID-19 has shown the presence of multifocal lymphocytic myocarditis in three patients (14%). In an additional six patients, focally increased interstitial T-lymphocytes within the myocardium were noted, with only focal or no myocyte injury.
However, increased interstitial macrophage infiltration, possibly related to cytokine infiltration, was seen in 86% of patients.
“One way to think about this is that, if these patients were having biopsies and not autopsies, there would be myocardial injury in the patients with myocarditis, even after they recovered. But with interstitial macrophages, there may or may not be any injury,” said cardiovascular pathologist James R. Stone, MD, PhD, Massachusetts General Hospital, Boston.
Dr. Stone and colleagues from Mass General, two hospitals in Italy, the University of Amsterdam, and the Mayo Clinic in Rochester, Minn., conducted the autopsies in March and April. The results were published in the October 14 issue of the European Heart Journal.
Their technique was rigorous: a median of 20 full-thickness blocks of myocardium were examined histologically (range, 5-29 blocks).
The presence of myocarditis, defined by the presence of multiple foci of inflammation with associated myocyte injury, was determined, and the inflammatory cell composition analyzed by immunohistochemistry.
“I think one of the take-homes from this study is that you have to do a thorough sampling of the heart in order to exclude myocardial injury. You cannot exclude myocarditis with just a biopsy or two,” said Dr. Stone in an interview.
“We looked at multiple different sections of tissue preserved in paraffin for every case and found only 14% had myocarditis. The vast majority of autopsies done on patients dying from COVID-19 have short-changed the autopsy and not been done in a way to exclude myocarditis,” he added.
For all patients, COVID-19 was the underlying cause of death, but the mechanisms of death were acute respiratory distress syndrome in 15, viral pneumonia in 4, cardiogenic shock in 1, and cardiac arrest in 1. Seven patients had a history of cardiovascular disease, including atrial fibrillation in four, coronary artery disease in three, left ventricular hypertrophy in one, and previous valve replacement in one. A total of 16 had hypertension, 7 had diabetes mellitus, and 1 had chronic obstructive pulmonary disease. In four cases, mild pericarditis was present. Acute myocyte injury in the right ventricle, most probably from strain or overload, was also present in four cases.
A nonsignificant trend was seen toward higher serum troponin levels in the patients with myocarditis compared with those without myocarditis. There were no reports of disrupted coronary artery plaques, coronary artery aneurysms, or large pulmonary emboli.
Macrophage infiltration rather than myocarditis, myocardial injury?
The study sheds more light on previous cardiac magnetic resonance (CMR) imaging findings that have suggested that many patients who recover from COVID-19 show signs suggestive of myocarditis. These earlier studies include a recent one in competitive athletes and the earlier Puntmann and colleagues study of relatively young COVID-19 patients, which showed ongoing myocardial involvement in a majority of patients.
“It would not surprise me if some or all of the cardiac MR changes seen in some of these recent imaging studies are due to the macrophages,” said Dr. Stone.
“What we saw was not a routine pathology by any means. It was a huge amount of macrophages, higher that what we saw in SARS and more similar to a study published in 2007 that looked at patients with bacterial sepsis,” said Dr. Stone.
In an older study of SARS patients, 35% had the virus detected in myocardial tissue by polymerase chain reaction. In that subset, the degree of myocardial macrophage infiltrate was comparable to that seen in 86% of the COVID-19 cases described in this series.
Another possibility is that the macrophage infiltration reflects underlying disease rather than COVID-19. All but one of the patients had known underlying medical conditions associated with cardiac remodeling, said Nikolaos G. Frangogiannis, MD, a cardiologist who studies the mechanisms of cardiac injury, repair, and remodeling.
Frangogiannis, from Albert Einstein College of Medicine, New York, wrote an editorial that accompanied the autopsy study.
“The problem with this finding of increased macrophage infiltration is that it’s very hard to interpret because as we age, and especially in a less healthy population, the numbers and the density of macrophages in the heart increase, so it’s impossible to interpret as an effect of the infection itself unless you have an appropriate control population that matches the same characteristics, which is almost impossible to ask for,” he said.
“I’ve observed since the beginning of the pandemic that there seemed to be some people who wanted every single case to be myocarditis and others who had a bias toward not wanting COVID-19 to be a cause of myocarditis. I think what we’re seeing is it’s not either/or for anything with this virus, it’s a bit of everything,” said Dr. Stone.
Dr. Stone and Dr. Frangogiannis reported no conflict of interest.
A version of this article originally appeared on Medscape.com.
An international autopsy study of 21 patients who died from COVID-19 has shown the presence of multifocal lymphocytic myocarditis in three patients (14%). In an additional six patients, focally increased interstitial T-lymphocytes within the myocardium were noted, with only focal or no myocyte injury.
However, increased interstitial macrophage infiltration, possibly related to cytokine infiltration, was seen in 86% of patients.
“One way to think about this is that, if these patients were having biopsies and not autopsies, there would be myocardial injury in the patients with myocarditis, even after they recovered. But with interstitial macrophages, there may or may not be any injury,” said cardiovascular pathologist James R. Stone, MD, PhD, Massachusetts General Hospital, Boston.
Dr. Stone and colleagues from Mass General, two hospitals in Italy, the University of Amsterdam, and the Mayo Clinic in Rochester, Minn., conducted the autopsies in March and April. The results were published in the October 14 issue of the European Heart Journal.
Their technique was rigorous: a median of 20 full-thickness blocks of myocardium were examined histologically (range, 5-29 blocks).
The presence of myocarditis, defined by the presence of multiple foci of inflammation with associated myocyte injury, was determined, and the inflammatory cell composition analyzed by immunohistochemistry.
“I think one of the take-homes from this study is that you have to do a thorough sampling of the heart in order to exclude myocardial injury. You cannot exclude myocarditis with just a biopsy or two,” said Dr. Stone in an interview.
“We looked at multiple different sections of tissue preserved in paraffin for every case and found only 14% had myocarditis. The vast majority of autopsies done on patients dying from COVID-19 have short-changed the autopsy and not been done in a way to exclude myocarditis,” he added.
For all patients, COVID-19 was the underlying cause of death, but the mechanisms of death were acute respiratory distress syndrome in 15, viral pneumonia in 4, cardiogenic shock in 1, and cardiac arrest in 1. Seven patients had a history of cardiovascular disease, including atrial fibrillation in four, coronary artery disease in three, left ventricular hypertrophy in one, and previous valve replacement in one. A total of 16 had hypertension, 7 had diabetes mellitus, and 1 had chronic obstructive pulmonary disease. In four cases, mild pericarditis was present. Acute myocyte injury in the right ventricle, most probably from strain or overload, was also present in four cases.
A nonsignificant trend was seen toward higher serum troponin levels in the patients with myocarditis compared with those without myocarditis. There were no reports of disrupted coronary artery plaques, coronary artery aneurysms, or large pulmonary emboli.
Macrophage infiltration rather than myocarditis, myocardial injury?
The study sheds more light on previous cardiac magnetic resonance (CMR) imaging findings that have suggested that many patients who recover from COVID-19 show signs suggestive of myocarditis. These earlier studies include a recent one in competitive athletes and the earlier Puntmann and colleagues study of relatively young COVID-19 patients, which showed ongoing myocardial involvement in a majority of patients.
“It would not surprise me if some or all of the cardiac MR changes seen in some of these recent imaging studies are due to the macrophages,” said Dr. Stone.
“What we saw was not a routine pathology by any means. It was a huge amount of macrophages, higher that what we saw in SARS and more similar to a study published in 2007 that looked at patients with bacterial sepsis,” said Dr. Stone.
In an older study of SARS patients, 35% had the virus detected in myocardial tissue by polymerase chain reaction. In that subset, the degree of myocardial macrophage infiltrate was comparable to that seen in 86% of the COVID-19 cases described in this series.
Another possibility is that the macrophage infiltration reflects underlying disease rather than COVID-19. All but one of the patients had known underlying medical conditions associated with cardiac remodeling, said Nikolaos G. Frangogiannis, MD, a cardiologist who studies the mechanisms of cardiac injury, repair, and remodeling.
Frangogiannis, from Albert Einstein College of Medicine, New York, wrote an editorial that accompanied the autopsy study.
“The problem with this finding of increased macrophage infiltration is that it’s very hard to interpret because as we age, and especially in a less healthy population, the numbers and the density of macrophages in the heart increase, so it’s impossible to interpret as an effect of the infection itself unless you have an appropriate control population that matches the same characteristics, which is almost impossible to ask for,” he said.
“I’ve observed since the beginning of the pandemic that there seemed to be some people who wanted every single case to be myocarditis and others who had a bias toward not wanting COVID-19 to be a cause of myocarditis. I think what we’re seeing is it’s not either/or for anything with this virus, it’s a bit of everything,” said Dr. Stone.
Dr. Stone and Dr. Frangogiannis reported no conflict of interest.
A version of this article originally appeared on Medscape.com.
An international autopsy study of 21 patients who died from COVID-19 has shown the presence of multifocal lymphocytic myocarditis in three patients (14%). In an additional six patients, focally increased interstitial T-lymphocytes within the myocardium were noted, with only focal or no myocyte injury.
However, increased interstitial macrophage infiltration, possibly related to cytokine infiltration, was seen in 86% of patients.
“One way to think about this is that, if these patients were having biopsies and not autopsies, there would be myocardial injury in the patients with myocarditis, even after they recovered. But with interstitial macrophages, there may or may not be any injury,” said cardiovascular pathologist James R. Stone, MD, PhD, Massachusetts General Hospital, Boston.
Dr. Stone and colleagues from Mass General, two hospitals in Italy, the University of Amsterdam, and the Mayo Clinic in Rochester, Minn., conducted the autopsies in March and April. The results were published in the October 14 issue of the European Heart Journal.
Their technique was rigorous: a median of 20 full-thickness blocks of myocardium were examined histologically (range, 5-29 blocks).
The presence of myocarditis, defined by the presence of multiple foci of inflammation with associated myocyte injury, was determined, and the inflammatory cell composition analyzed by immunohistochemistry.
“I think one of the take-homes from this study is that you have to do a thorough sampling of the heart in order to exclude myocardial injury. You cannot exclude myocarditis with just a biopsy or two,” said Dr. Stone in an interview.
“We looked at multiple different sections of tissue preserved in paraffin for every case and found only 14% had myocarditis. The vast majority of autopsies done on patients dying from COVID-19 have short-changed the autopsy and not been done in a way to exclude myocarditis,” he added.
For all patients, COVID-19 was the underlying cause of death, but the mechanisms of death were acute respiratory distress syndrome in 15, viral pneumonia in 4, cardiogenic shock in 1, and cardiac arrest in 1. Seven patients had a history of cardiovascular disease, including atrial fibrillation in four, coronary artery disease in three, left ventricular hypertrophy in one, and previous valve replacement in one. A total of 16 had hypertension, 7 had diabetes mellitus, and 1 had chronic obstructive pulmonary disease. In four cases, mild pericarditis was present. Acute myocyte injury in the right ventricle, most probably from strain or overload, was also present in four cases.
A nonsignificant trend was seen toward higher serum troponin levels in the patients with myocarditis compared with those without myocarditis. There were no reports of disrupted coronary artery plaques, coronary artery aneurysms, or large pulmonary emboli.
Macrophage infiltration rather than myocarditis, myocardial injury?
The study sheds more light on previous cardiac magnetic resonance (CMR) imaging findings that have suggested that many patients who recover from COVID-19 show signs suggestive of myocarditis. These earlier studies include a recent one in competitive athletes and the earlier Puntmann and colleagues study of relatively young COVID-19 patients, which showed ongoing myocardial involvement in a majority of patients.
“It would not surprise me if some or all of the cardiac MR changes seen in some of these recent imaging studies are due to the macrophages,” said Dr. Stone.
“What we saw was not a routine pathology by any means. It was a huge amount of macrophages, higher that what we saw in SARS and more similar to a study published in 2007 that looked at patients with bacterial sepsis,” said Dr. Stone.
In an older study of SARS patients, 35% had the virus detected in myocardial tissue by polymerase chain reaction. In that subset, the degree of myocardial macrophage infiltrate was comparable to that seen in 86% of the COVID-19 cases described in this series.
Another possibility is that the macrophage infiltration reflects underlying disease rather than COVID-19. All but one of the patients had known underlying medical conditions associated with cardiac remodeling, said Nikolaos G. Frangogiannis, MD, a cardiologist who studies the mechanisms of cardiac injury, repair, and remodeling.
Frangogiannis, from Albert Einstein College of Medicine, New York, wrote an editorial that accompanied the autopsy study.
“The problem with this finding of increased macrophage infiltration is that it’s very hard to interpret because as we age, and especially in a less healthy population, the numbers and the density of macrophages in the heart increase, so it’s impossible to interpret as an effect of the infection itself unless you have an appropriate control population that matches the same characteristics, which is almost impossible to ask for,” he said.
“I’ve observed since the beginning of the pandemic that there seemed to be some people who wanted every single case to be myocarditis and others who had a bias toward not wanting COVID-19 to be a cause of myocarditis. I think what we’re seeing is it’s not either/or for anything with this virus, it’s a bit of everything,” said Dr. Stone.
Dr. Stone and Dr. Frangogiannis reported no conflict of interest.
A version of this article originally appeared on Medscape.com.



