User login
When it’s not long, but medium COVID?
Symptom timelines surrounding COVID infection tend to center on either the immediate 5-day quarantine protocols for acute infection or the long-COVID symptoms that can last a month or potentially far longer.
People may return to work or daily routines, but something is off: What had been simple exercise regimens become onerous. Everyday tasks take more effort.
Does this ill-defined subset point to a “medium COVID?”
Farha Ikramuddin, MD, MHA, a physiatrist and rehabilitation specialist at the University of Minnesota and M Health Fairview in Minneapolis, points out there is no definition or diagnostic code or shared official understanding of a middle category for COVID.
“But am I seeing that? Absolutely,” she said in an interview.
“I have seen patients who are younger, healthier, [and] with not so many comorbidities have either persistence of symptoms or reappearance after the initial infection is done,” she said.
Some patients report they had very low infection or were nonsymptomatic and returned to their normal health fairly quickly after infection. Then a week later they began experiencing fatigue, lost appetite, loss of smell, and feeling full after a few bites, Dr. Ikramuddin said.
Part of the trouble in categorizing the space between returning to normal after a week and having symptoms for months is that organizations can’t agree on a timeline for when symptoms warrant a “long-COVID” label.
For instance, the Centers for Disease Control and Prevention defines it as 4 or more weeks after infection. The World Health Organization defines it as starting 3 months after COVID-19 symptom onset.
“I’m seeing ‘medium COVID’ – as one would call it – in younger and healthier patients. I’m also noticing that these symptoms are not severe enough to warrant stopping their job or changing their job schedules,” Dr. Ikramuddin said.
They go back to work, she said, but start noticing something is off.
“I am seeing that.”
“I discharge at least two patients a week from my clinic because they have moved on and no longer have symptoms,” Dr. Ikramuddin said.
In a story from Kaiser Health News published last month, WHYY health reporter Nina Feldman writes: “What I’ve come to think of as my ‘medium COVID’ affected my life. I couldn’t socialize much, drink, or stay up past 9:30 p.m. It took me 10 weeks to go for my first run – I’d been too afraid to try.”
She described a dinner with a friend after ending initial isolation protocols: “One glass of wine left me feeling like I’d had a whole bottle. I was bone-achingly exhausted but couldn’t sleep.”
Medical mystery
Dr. Ikramuddin notes the mechanism behind prolonged COVID-19 symptoms is still a medical mystery.
“In one scenario,” she said, “the question is being asked about whether the virus is staying dormant, similar to herpes zoster or HIV.”
“Right now, instead of getting more answers, we’re getting more questions,” Dr. Ikramuddin said.
Mouhib Naddour, MD, a pulmonary specialist with Sharp HealthCare in San Diego, said he’s seeing that it’s taking some patients who have had COVID longer to recover than it would for other viral infections.
Some patients fall between those recovering within 2-3 weeks and patients having long COVID. Those patients in the gap could be lumped into a middle-range COVID, he told this news organization.
“We try to put things into tables and boxes but it is hard with this disease,” Dr. Naddour said.
He agrees there’s no medical definition for “medium” COVID, but he said the idea should bring hope for patients to know that, if their symptoms are persisting they don’t necessarily have long COVID – and their symptoms may still disappear.
“This is an illness that may take longer to completely recover from,” he said. “The majority of patients we’re seeing in this group could be healthy young patients who get COVID, then 2-3 weeks after they test negative, still have lingering symptoms.”
Common symptoms
Some commonly reported symptoms of those with enduring illness, which often overlap with other stages of COVID, are difficulty breathing, chest tightness, dry cough, chest pain, muscle and joint pain, fatigue, difficulty sleeping, and mood swings, Dr. Naddour said.
“We need to do an extensive assessment to make sure there’s no other problem causing these symptoms,” he said.
Still, there is no set timeline for the medium-COVID range, he noted, so checking in with a primary care physician is important for people experiencing symptoms.
It’s a continuum, not a category
Fernando Carnavali, MD, coordinator for Mount Sinai’s Center for Post-COVID Care in New York, said he is not ready to recognize a separate category for a “medium” COVID.
He noted that science can’t even agree on a name for lasting post-COVID symptoms, whether it’s “long COVID” or “long-haul COVID,” “post-COVID syndrome” or “post-acute sequelae of COVID-19 (PASC ).” There’s no agreed-upon pathophysiology or biomarker.
“That creates these gaps of understanding on where we are,” Dr. Carnavali said in an interview.
He said he understands people’s need to categorize symptoms, but rather than a middle ground he sees a continuum.
It doesn’t mean what others may call COVID’s middle ground doesn’t exist, Dr. Carnavali said: “We are in the infancy of defining this. Trying to classify them may create more anxiety.”
The clinicians interviewed for this story report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Symptom timelines surrounding COVID infection tend to center on either the immediate 5-day quarantine protocols for acute infection or the long-COVID symptoms that can last a month or potentially far longer.
People may return to work or daily routines, but something is off: What had been simple exercise regimens become onerous. Everyday tasks take more effort.
Does this ill-defined subset point to a “medium COVID?”
Farha Ikramuddin, MD, MHA, a physiatrist and rehabilitation specialist at the University of Minnesota and M Health Fairview in Minneapolis, points out there is no definition or diagnostic code or shared official understanding of a middle category for COVID.
“But am I seeing that? Absolutely,” she said in an interview.
“I have seen patients who are younger, healthier, [and] with not so many comorbidities have either persistence of symptoms or reappearance after the initial infection is done,” she said.
Some patients report they had very low infection or were nonsymptomatic and returned to their normal health fairly quickly after infection. Then a week later they began experiencing fatigue, lost appetite, loss of smell, and feeling full after a few bites, Dr. Ikramuddin said.
Part of the trouble in categorizing the space between returning to normal after a week and having symptoms for months is that organizations can’t agree on a timeline for when symptoms warrant a “long-COVID” label.
For instance, the Centers for Disease Control and Prevention defines it as 4 or more weeks after infection. The World Health Organization defines it as starting 3 months after COVID-19 symptom onset.
“I’m seeing ‘medium COVID’ – as one would call it – in younger and healthier patients. I’m also noticing that these symptoms are not severe enough to warrant stopping their job or changing their job schedules,” Dr. Ikramuddin said.
They go back to work, she said, but start noticing something is off.
“I am seeing that.”
“I discharge at least two patients a week from my clinic because they have moved on and no longer have symptoms,” Dr. Ikramuddin said.
In a story from Kaiser Health News published last month, WHYY health reporter Nina Feldman writes: “What I’ve come to think of as my ‘medium COVID’ affected my life. I couldn’t socialize much, drink, or stay up past 9:30 p.m. It took me 10 weeks to go for my first run – I’d been too afraid to try.”
She described a dinner with a friend after ending initial isolation protocols: “One glass of wine left me feeling like I’d had a whole bottle. I was bone-achingly exhausted but couldn’t sleep.”
Medical mystery
Dr. Ikramuddin notes the mechanism behind prolonged COVID-19 symptoms is still a medical mystery.
“In one scenario,” she said, “the question is being asked about whether the virus is staying dormant, similar to herpes zoster or HIV.”
“Right now, instead of getting more answers, we’re getting more questions,” Dr. Ikramuddin said.
Mouhib Naddour, MD, a pulmonary specialist with Sharp HealthCare in San Diego, said he’s seeing that it’s taking some patients who have had COVID longer to recover than it would for other viral infections.
Some patients fall between those recovering within 2-3 weeks and patients having long COVID. Those patients in the gap could be lumped into a middle-range COVID, he told this news organization.
“We try to put things into tables and boxes but it is hard with this disease,” Dr. Naddour said.
He agrees there’s no medical definition for “medium” COVID, but he said the idea should bring hope for patients to know that, if their symptoms are persisting they don’t necessarily have long COVID – and their symptoms may still disappear.
“This is an illness that may take longer to completely recover from,” he said. “The majority of patients we’re seeing in this group could be healthy young patients who get COVID, then 2-3 weeks after they test negative, still have lingering symptoms.”
Common symptoms
Some commonly reported symptoms of those with enduring illness, which often overlap with other stages of COVID, are difficulty breathing, chest tightness, dry cough, chest pain, muscle and joint pain, fatigue, difficulty sleeping, and mood swings, Dr. Naddour said.
“We need to do an extensive assessment to make sure there’s no other problem causing these symptoms,” he said.
Still, there is no set timeline for the medium-COVID range, he noted, so checking in with a primary care physician is important for people experiencing symptoms.
It’s a continuum, not a category
Fernando Carnavali, MD, coordinator for Mount Sinai’s Center for Post-COVID Care in New York, said he is not ready to recognize a separate category for a “medium” COVID.
He noted that science can’t even agree on a name for lasting post-COVID symptoms, whether it’s “long COVID” or “long-haul COVID,” “post-COVID syndrome” or “post-acute sequelae of COVID-19 (PASC ).” There’s no agreed-upon pathophysiology or biomarker.
“That creates these gaps of understanding on where we are,” Dr. Carnavali said in an interview.
He said he understands people’s need to categorize symptoms, but rather than a middle ground he sees a continuum.
It doesn’t mean what others may call COVID’s middle ground doesn’t exist, Dr. Carnavali said: “We are in the infancy of defining this. Trying to classify them may create more anxiety.”
The clinicians interviewed for this story report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Symptom timelines surrounding COVID infection tend to center on either the immediate 5-day quarantine protocols for acute infection or the long-COVID symptoms that can last a month or potentially far longer.
People may return to work or daily routines, but something is off: What had been simple exercise regimens become onerous. Everyday tasks take more effort.
Does this ill-defined subset point to a “medium COVID?”
Farha Ikramuddin, MD, MHA, a physiatrist and rehabilitation specialist at the University of Minnesota and M Health Fairview in Minneapolis, points out there is no definition or diagnostic code or shared official understanding of a middle category for COVID.
“But am I seeing that? Absolutely,” she said in an interview.
“I have seen patients who are younger, healthier, [and] with not so many comorbidities have either persistence of symptoms or reappearance after the initial infection is done,” she said.
Some patients report they had very low infection or were nonsymptomatic and returned to their normal health fairly quickly after infection. Then a week later they began experiencing fatigue, lost appetite, loss of smell, and feeling full after a few bites, Dr. Ikramuddin said.
Part of the trouble in categorizing the space between returning to normal after a week and having symptoms for months is that organizations can’t agree on a timeline for when symptoms warrant a “long-COVID” label.
For instance, the Centers for Disease Control and Prevention defines it as 4 or more weeks after infection. The World Health Organization defines it as starting 3 months after COVID-19 symptom onset.
“I’m seeing ‘medium COVID’ – as one would call it – in younger and healthier patients. I’m also noticing that these symptoms are not severe enough to warrant stopping their job or changing their job schedules,” Dr. Ikramuddin said.
They go back to work, she said, but start noticing something is off.
“I am seeing that.”
“I discharge at least two patients a week from my clinic because they have moved on and no longer have symptoms,” Dr. Ikramuddin said.
In a story from Kaiser Health News published last month, WHYY health reporter Nina Feldman writes: “What I’ve come to think of as my ‘medium COVID’ affected my life. I couldn’t socialize much, drink, or stay up past 9:30 p.m. It took me 10 weeks to go for my first run – I’d been too afraid to try.”
She described a dinner with a friend after ending initial isolation protocols: “One glass of wine left me feeling like I’d had a whole bottle. I was bone-achingly exhausted but couldn’t sleep.”
Medical mystery
Dr. Ikramuddin notes the mechanism behind prolonged COVID-19 symptoms is still a medical mystery.
“In one scenario,” she said, “the question is being asked about whether the virus is staying dormant, similar to herpes zoster or HIV.”
“Right now, instead of getting more answers, we’re getting more questions,” Dr. Ikramuddin said.
Mouhib Naddour, MD, a pulmonary specialist with Sharp HealthCare in San Diego, said he’s seeing that it’s taking some patients who have had COVID longer to recover than it would for other viral infections.
Some patients fall between those recovering within 2-3 weeks and patients having long COVID. Those patients in the gap could be lumped into a middle-range COVID, he told this news organization.
“We try to put things into tables and boxes but it is hard with this disease,” Dr. Naddour said.
He agrees there’s no medical definition for “medium” COVID, but he said the idea should bring hope for patients to know that, if their symptoms are persisting they don’t necessarily have long COVID – and their symptoms may still disappear.
“This is an illness that may take longer to completely recover from,” he said. “The majority of patients we’re seeing in this group could be healthy young patients who get COVID, then 2-3 weeks after they test negative, still have lingering symptoms.”
Common symptoms
Some commonly reported symptoms of those with enduring illness, which often overlap with other stages of COVID, are difficulty breathing, chest tightness, dry cough, chest pain, muscle and joint pain, fatigue, difficulty sleeping, and mood swings, Dr. Naddour said.
“We need to do an extensive assessment to make sure there’s no other problem causing these symptoms,” he said.
Still, there is no set timeline for the medium-COVID range, he noted, so checking in with a primary care physician is important for people experiencing symptoms.
It’s a continuum, not a category
Fernando Carnavali, MD, coordinator for Mount Sinai’s Center for Post-COVID Care in New York, said he is not ready to recognize a separate category for a “medium” COVID.
He noted that science can’t even agree on a name for lasting post-COVID symptoms, whether it’s “long COVID” or “long-haul COVID,” “post-COVID syndrome” or “post-acute sequelae of COVID-19 (PASC ).” There’s no agreed-upon pathophysiology or biomarker.
“That creates these gaps of understanding on where we are,” Dr. Carnavali said in an interview.
He said he understands people’s need to categorize symptoms, but rather than a middle ground he sees a continuum.
It doesn’t mean what others may call COVID’s middle ground doesn’t exist, Dr. Carnavali said: “We are in the infancy of defining this. Trying to classify them may create more anxiety.”
The clinicians interviewed for this story report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New data confirm risk of Guillain-Barré with J&J COVID shot
The Janssen vaccine (Ad26.COV2.S) is a replication-incompetent adenoviral vector vaccine.
The data show no increased risk of GBS with the Pfizer (BNT162b2) or Moderna (mRNA-1273) shots – both mRNA vaccines.
“Our findings support the current guidance from U.S. health officials that preferentially recommend use of mRNA COVID-19 vaccines for primary and booster doses,” Nicola Klein, MD, PhD, with Kaiser Permanente Vaccine Study Center, Oakland, Calif., told this news organization.
“Individuals who choose to receive Janssen/J&J COVID-19 vaccine should be informed of the potential safety risks, including GBS,” Dr. Klein said.
The study was published online in JAMA Network Open.
Eleven cases
Between mid-December 2020 and mid-November 2021, roughly 15.1 million doses of COVID-19 vaccine were administered to nearly 7.9 million adults in the United States.
This includes roughly 483,000 doses of the Janssen vaccine, 8.8 million doses of the Pfizer vaccine, and 5.8 million doses of the Moderna vaccine.
The researchers confirmed 11 cases of GBS after the Janssen vaccine.
The unadjusted incidence of GBS (per 100,000 person-years) was 32.4 in the first 21 days after the Janssen vaccine – substantially higher than the expected background rate of 1 to 2 cases per 100,000 person-years.
There were 36 confirmed cases of GBS after mRNA vaccines. The unadjusted incidence in the first 21 days after mRNA vaccination was 1.3 per 100,000 person-years, similar to the overall expected background rate.
In an adjusted head-to-head comparison, GBS incidence during the 21 days after receipt of the Janssen vaccine was 20.6 times higher than the GBS incidence during the 21 days after the Pfizer or Moderna mRNA vaccines, amounting to 15.5 excess cases per million Janssen vaccine recipients.
Most cases of GBS after the Janssen vaccine occurred during the 1- to 21-day risk interval, with the period of greatest risk in the 1-14 days after vaccination.
The findings of this analysis of surveillance data of COVID-19 vaccines are “consistent with an elevated risk of GBS after primary Ad26.COV2.S vaccination,” the authors wrote.
Novel presentation?
The researchers note that nearly all individuals who developed GBS after the Janssen vaccine had facial weakness or paralysis, in addition to weakness and decreased reflexes in the limbs, suggesting that the presentation of GBS after COVID-19 adenoviral vector vaccine may be novel.
“More research is needed to determine if the presentation of GBS after adenoviral vector vaccine differs from GBS after other exposures such as Campylobacter jejuni, and to investigate the mechanism for how adenoviral vector vaccines may cause GBS,” Dr. Klein and colleagues said.
“The Vaccine Safety Datalink continues to conduct safety surveillance for all COVID-19 vaccines, including monitoring for GBS and other serious health outcomes after vaccination,” Dr. Klein said in an interview.
This study was supported by the Centers for Disease Control and Prevention. Dr. Klein reported receiving grants from Pfizer research support for a COVID vaccine clinical trial as well as other unrelated studies, grants from Merck, grants from GlaxoSmithKline, grants from Sanofi Pasteur, and grants from Protein Science (now Sanofi Pasteur) outside the submitted work.
A version of this article first appeared on Medscape.com.
The Janssen vaccine (Ad26.COV2.S) is a replication-incompetent adenoviral vector vaccine.
The data show no increased risk of GBS with the Pfizer (BNT162b2) or Moderna (mRNA-1273) shots – both mRNA vaccines.
“Our findings support the current guidance from U.S. health officials that preferentially recommend use of mRNA COVID-19 vaccines for primary and booster doses,” Nicola Klein, MD, PhD, with Kaiser Permanente Vaccine Study Center, Oakland, Calif., told this news organization.
“Individuals who choose to receive Janssen/J&J COVID-19 vaccine should be informed of the potential safety risks, including GBS,” Dr. Klein said.
The study was published online in JAMA Network Open.
Eleven cases
Between mid-December 2020 and mid-November 2021, roughly 15.1 million doses of COVID-19 vaccine were administered to nearly 7.9 million adults in the United States.
This includes roughly 483,000 doses of the Janssen vaccine, 8.8 million doses of the Pfizer vaccine, and 5.8 million doses of the Moderna vaccine.
The researchers confirmed 11 cases of GBS after the Janssen vaccine.
The unadjusted incidence of GBS (per 100,000 person-years) was 32.4 in the first 21 days after the Janssen vaccine – substantially higher than the expected background rate of 1 to 2 cases per 100,000 person-years.
There were 36 confirmed cases of GBS after mRNA vaccines. The unadjusted incidence in the first 21 days after mRNA vaccination was 1.3 per 100,000 person-years, similar to the overall expected background rate.
In an adjusted head-to-head comparison, GBS incidence during the 21 days after receipt of the Janssen vaccine was 20.6 times higher than the GBS incidence during the 21 days after the Pfizer or Moderna mRNA vaccines, amounting to 15.5 excess cases per million Janssen vaccine recipients.
Most cases of GBS after the Janssen vaccine occurred during the 1- to 21-day risk interval, with the period of greatest risk in the 1-14 days after vaccination.
The findings of this analysis of surveillance data of COVID-19 vaccines are “consistent with an elevated risk of GBS after primary Ad26.COV2.S vaccination,” the authors wrote.
Novel presentation?
The researchers note that nearly all individuals who developed GBS after the Janssen vaccine had facial weakness or paralysis, in addition to weakness and decreased reflexes in the limbs, suggesting that the presentation of GBS after COVID-19 adenoviral vector vaccine may be novel.
“More research is needed to determine if the presentation of GBS after adenoviral vector vaccine differs from GBS after other exposures such as Campylobacter jejuni, and to investigate the mechanism for how adenoviral vector vaccines may cause GBS,” Dr. Klein and colleagues said.
“The Vaccine Safety Datalink continues to conduct safety surveillance for all COVID-19 vaccines, including monitoring for GBS and other serious health outcomes after vaccination,” Dr. Klein said in an interview.
This study was supported by the Centers for Disease Control and Prevention. Dr. Klein reported receiving grants from Pfizer research support for a COVID vaccine clinical trial as well as other unrelated studies, grants from Merck, grants from GlaxoSmithKline, grants from Sanofi Pasteur, and grants from Protein Science (now Sanofi Pasteur) outside the submitted work.
A version of this article first appeared on Medscape.com.
The Janssen vaccine (Ad26.COV2.S) is a replication-incompetent adenoviral vector vaccine.
The data show no increased risk of GBS with the Pfizer (BNT162b2) or Moderna (mRNA-1273) shots – both mRNA vaccines.
“Our findings support the current guidance from U.S. health officials that preferentially recommend use of mRNA COVID-19 vaccines for primary and booster doses,” Nicola Klein, MD, PhD, with Kaiser Permanente Vaccine Study Center, Oakland, Calif., told this news organization.
“Individuals who choose to receive Janssen/J&J COVID-19 vaccine should be informed of the potential safety risks, including GBS,” Dr. Klein said.
The study was published online in JAMA Network Open.
Eleven cases
Between mid-December 2020 and mid-November 2021, roughly 15.1 million doses of COVID-19 vaccine were administered to nearly 7.9 million adults in the United States.
This includes roughly 483,000 doses of the Janssen vaccine, 8.8 million doses of the Pfizer vaccine, and 5.8 million doses of the Moderna vaccine.
The researchers confirmed 11 cases of GBS after the Janssen vaccine.
The unadjusted incidence of GBS (per 100,000 person-years) was 32.4 in the first 21 days after the Janssen vaccine – substantially higher than the expected background rate of 1 to 2 cases per 100,000 person-years.
There were 36 confirmed cases of GBS after mRNA vaccines. The unadjusted incidence in the first 21 days after mRNA vaccination was 1.3 per 100,000 person-years, similar to the overall expected background rate.
In an adjusted head-to-head comparison, GBS incidence during the 21 days after receipt of the Janssen vaccine was 20.6 times higher than the GBS incidence during the 21 days after the Pfizer or Moderna mRNA vaccines, amounting to 15.5 excess cases per million Janssen vaccine recipients.
Most cases of GBS after the Janssen vaccine occurred during the 1- to 21-day risk interval, with the period of greatest risk in the 1-14 days after vaccination.
The findings of this analysis of surveillance data of COVID-19 vaccines are “consistent with an elevated risk of GBS after primary Ad26.COV2.S vaccination,” the authors wrote.
Novel presentation?
The researchers note that nearly all individuals who developed GBS after the Janssen vaccine had facial weakness or paralysis, in addition to weakness and decreased reflexes in the limbs, suggesting that the presentation of GBS after COVID-19 adenoviral vector vaccine may be novel.
“More research is needed to determine if the presentation of GBS after adenoviral vector vaccine differs from GBS after other exposures such as Campylobacter jejuni, and to investigate the mechanism for how adenoviral vector vaccines may cause GBS,” Dr. Klein and colleagues said.
“The Vaccine Safety Datalink continues to conduct safety surveillance for all COVID-19 vaccines, including monitoring for GBS and other serious health outcomes after vaccination,” Dr. Klein said in an interview.
This study was supported by the Centers for Disease Control and Prevention. Dr. Klein reported receiving grants from Pfizer research support for a COVID vaccine clinical trial as well as other unrelated studies, grants from Merck, grants from GlaxoSmithKline, grants from Sanofi Pasteur, and grants from Protein Science (now Sanofi Pasteur) outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Children and COVID: New cases up for third straight week
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.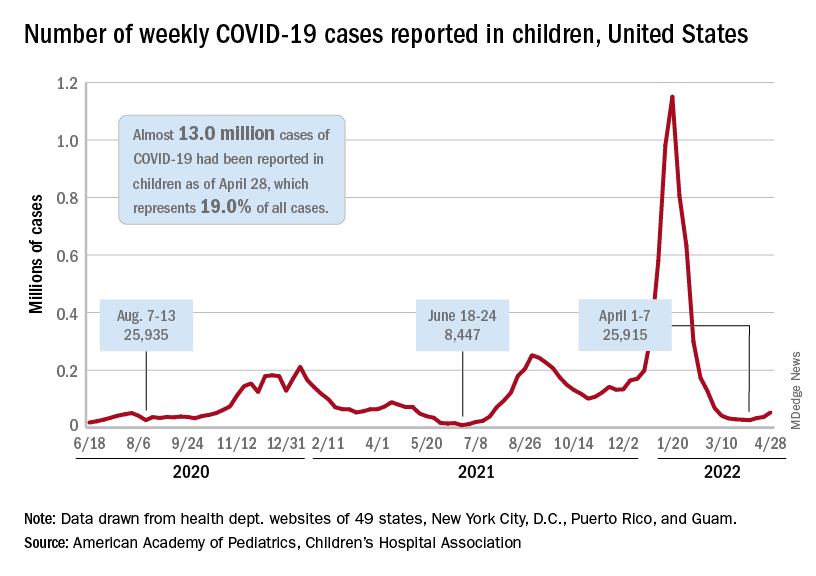
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”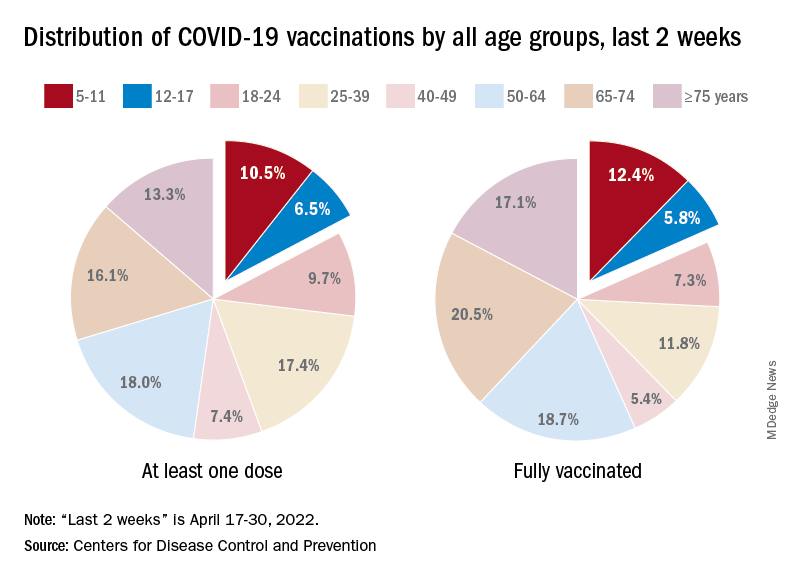
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
Paxlovid doesn’t prevent infection in households, Pfizer says
Paxlovid works as a treatment for COVID-19 but not as a preventive measure, particularly if you’ve been exposed to the coronavirus through a household member who is infected, according to a new announcement from Pfizer.
In a clinical trial, the oral antiviral tablets were tested for postexposure prophylactic use, or tested for how well they prevented a coronavirus infection in people exposed to the virus. Paxlovid somewhat reduced the risk of infection, but the results weren’t statistically significant.
“We designed the clinical development program for Paxlovid to be comprehensive and ambitious with the aim of being able to help combat COVID-19 in a very broad population of patients,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the announcement.
“While we are disappointed in the outcome of this particular study, these results do not impact the strong efficacy and safety data we’ve observed in our earlier trial for the treatment of COVID-19 patients at high risk of developing severe illness,” he said.
The trial included nearly 3,000 adults who were living with someone who recently tested positive for COVID-19 and had symptoms. The people in the trial, who tested negative and didn’t have symptoms, were given either Paxlovid twice daily for 5 or 10 days or a placebo. The study recruitment began in September 2021 and was completed during the peak of the Omicron wave.
Those who took the 5-day course of Paxlovid were found to be 32% less likely to become infected than the placebo group. Those who took the 10-day treatment had a 37% risk reduction. But the results weren’t statistically significant and may have been because of chance.
“Traditionally, it’s been difficult to use small-molecule antivirals for true prophylaxis because the biology of treating infection is different from the biology of preventing infection,” Daniel Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, told STAT News.
He also noted that the Omicron variant could have played a role.
“That hyperinfectiousness probably makes it more difficult to prevent infections,” Dr. Barouch said.
The safety data was consistent with that of previous studies, Pfizer said, which found that the treatment was about 90% effective at preventing hospitalization or death in COVID-19 patients with a high risk of severe illness if the pills were taken for 5 days soon after symptoms started.
Paxlovid is approved or authorized for conditional or emergency use in more than 60 countries to treat high-risk COVID-19 patients, Pfizer said. In the United States, the drug is authorized for emergency use for the treatment of mild to moderate COVID-19 in those aged 12 and older who face high risks for severe disease, hospitalization, or death.
The full study data will be released in coming months and submitted to a peer-reviewed publication, the company said. More details are on the ClinicalTrials.gov website (NCT05047601).
A version of this article first appeared on WebMD.com.
Paxlovid works as a treatment for COVID-19 but not as a preventive measure, particularly if you’ve been exposed to the coronavirus through a household member who is infected, according to a new announcement from Pfizer.
In a clinical trial, the oral antiviral tablets were tested for postexposure prophylactic use, or tested for how well they prevented a coronavirus infection in people exposed to the virus. Paxlovid somewhat reduced the risk of infection, but the results weren’t statistically significant.
“We designed the clinical development program for Paxlovid to be comprehensive and ambitious with the aim of being able to help combat COVID-19 in a very broad population of patients,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the announcement.
“While we are disappointed in the outcome of this particular study, these results do not impact the strong efficacy and safety data we’ve observed in our earlier trial for the treatment of COVID-19 patients at high risk of developing severe illness,” he said.
The trial included nearly 3,000 adults who were living with someone who recently tested positive for COVID-19 and had symptoms. The people in the trial, who tested negative and didn’t have symptoms, were given either Paxlovid twice daily for 5 or 10 days or a placebo. The study recruitment began in September 2021 and was completed during the peak of the Omicron wave.
Those who took the 5-day course of Paxlovid were found to be 32% less likely to become infected than the placebo group. Those who took the 10-day treatment had a 37% risk reduction. But the results weren’t statistically significant and may have been because of chance.
“Traditionally, it’s been difficult to use small-molecule antivirals for true prophylaxis because the biology of treating infection is different from the biology of preventing infection,” Daniel Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, told STAT News.
He also noted that the Omicron variant could have played a role.
“That hyperinfectiousness probably makes it more difficult to prevent infections,” Dr. Barouch said.
The safety data was consistent with that of previous studies, Pfizer said, which found that the treatment was about 90% effective at preventing hospitalization or death in COVID-19 patients with a high risk of severe illness if the pills were taken for 5 days soon after symptoms started.
Paxlovid is approved or authorized for conditional or emergency use in more than 60 countries to treat high-risk COVID-19 patients, Pfizer said. In the United States, the drug is authorized for emergency use for the treatment of mild to moderate COVID-19 in those aged 12 and older who face high risks for severe disease, hospitalization, or death.
The full study data will be released in coming months and submitted to a peer-reviewed publication, the company said. More details are on the ClinicalTrials.gov website (NCT05047601).
A version of this article first appeared on WebMD.com.
Paxlovid works as a treatment for COVID-19 but not as a preventive measure, particularly if you’ve been exposed to the coronavirus through a household member who is infected, according to a new announcement from Pfizer.
In a clinical trial, the oral antiviral tablets were tested for postexposure prophylactic use, or tested for how well they prevented a coronavirus infection in people exposed to the virus. Paxlovid somewhat reduced the risk of infection, but the results weren’t statistically significant.
“We designed the clinical development program for Paxlovid to be comprehensive and ambitious with the aim of being able to help combat COVID-19 in a very broad population of patients,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the announcement.
“While we are disappointed in the outcome of this particular study, these results do not impact the strong efficacy and safety data we’ve observed in our earlier trial for the treatment of COVID-19 patients at high risk of developing severe illness,” he said.
The trial included nearly 3,000 adults who were living with someone who recently tested positive for COVID-19 and had symptoms. The people in the trial, who tested negative and didn’t have symptoms, were given either Paxlovid twice daily for 5 or 10 days or a placebo. The study recruitment began in September 2021 and was completed during the peak of the Omicron wave.
Those who took the 5-day course of Paxlovid were found to be 32% less likely to become infected than the placebo group. Those who took the 10-day treatment had a 37% risk reduction. But the results weren’t statistically significant and may have been because of chance.
“Traditionally, it’s been difficult to use small-molecule antivirals for true prophylaxis because the biology of treating infection is different from the biology of preventing infection,” Daniel Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, told STAT News.
He also noted that the Omicron variant could have played a role.
“That hyperinfectiousness probably makes it more difficult to prevent infections,” Dr. Barouch said.
The safety data was consistent with that of previous studies, Pfizer said, which found that the treatment was about 90% effective at preventing hospitalization or death in COVID-19 patients with a high risk of severe illness if the pills were taken for 5 days soon after symptoms started.
Paxlovid is approved or authorized for conditional or emergency use in more than 60 countries to treat high-risk COVID-19 patients, Pfizer said. In the United States, the drug is authorized for emergency use for the treatment of mild to moderate COVID-19 in those aged 12 and older who face high risks for severe disease, hospitalization, or death.
The full study data will be released in coming months and submitted to a peer-reviewed publication, the company said. More details are on the ClinicalTrials.gov website (NCT05047601).
A version of this article first appeared on WebMD.com.
Inappropriate antibiotic use in U.S. hospitals increased during pandemic
LISBON – During the pandemic, critical and acute care hospitals with medium and high rates of antimicrobial resistance (AMR) showed significant increases in antibiotic prescriptions and longer durations of antibiotic treatment among all hospital admissions, and also in those patients who were bacterial culture negative, according to a large U.S.-based study.
The analysis across 271 U.S. hospitals also showed that AMR rates were significantly higher for pathogens during the pandemic period, compared with the prepandemic period in patients who were tested for SARS-CoV-2, and highest in SARS-CoV-2–positive patients.
More than a third of SARS-CoV-2–positive patients who were prescribed antibiotics were bacterial culture negative.
Findings of the study were presented by Vikas Gupta, PharmD, director of medical affairs at medical technology firm Becton Dickinson, at this year’s European Congress of Clinical Microbiology & Infectious Diseases. He conducted the study jointly with Karri Bauer, PharmD, from Merck Sharp & Dohme, Kenilworth, N.J., and colleagues.
“There are differences in AMR that go beyond COVID-positive admissions,” Dr. Gupta told this news organization. “There is opportunity for improvement especially with those hospitalized patients who had a negative culture result, or no culture collected.”
“We found a higher percentage of COVID-positive admissions that were prescribed antibacterial therapy even in those having [tested negative for bacteria] or no culture result,” said Dr. Gupta. “Our data also shows that the percentage of admissions with duration of antibacterial therapy over 3 days was significantly higher in COVID-positive but culture-negative/no culture patients, compared to other groups evaluated.”
Of all admissions prescribed antibiotics during the pandemic, 57.8% of SARS-CoV-2–positive patients were prescribed antibiotics whereas 88.1% of SARS-CoV-2–positive admissions were bacterial culture negative/no culture. Overall, prepandemic, 35% of admissions were prescribed antibiotics.
Duration of antibiotic therapy in the prepandemic era was an average of 3.5 days, compared with an average of 3.8 days overall in the pandemic and 5.7 days in patients who tested positive for SARS-CoV-2. Similarly, the percentage of patients who were bacterial culture negative or had no culture and received antibiotic therapy for more than 72 hours was 17.6% in the prepandemic era, compared with 19.2% overall in the pandemic era, and 41.1% in patients who tested positive for COVID-19.
Dr. Gupta and Dr. Bauer wanted to look at all patients admitted to hospitals segmented by SARS-CoV-2 positive, negative, and not tested, to get a sense of how much antibiotic use there was and how long patients were on antibiotics. “We ultimately want to optimize and not overuse antibiotics and prescribe them for right period of time,” said Dr. Gupta.
“To date, there has been no conclusive evidence about the suggestion that the pandemic has led to increased AMR rates, so we aimed to evaluate the pandemic’s impact on AMR and antibiotic use across U.S. hospitals,” he explained.
The multicenter, retrospective cohort analysis made use of BD’s infection surveillance platform (BD HealthSight Infection Advisor with MedMined Insights) and was conducted across 271 U.S. critical access/acute care facilities, representing approximately 10%-13% of U.S. hospital admissions. It included all hospitalized patients with more than 1 day of in-patient admission. Patients were considered SARS-CoV-2 positive by polymerase chain reaction test or antigen test either 7 days or less prior to or within 14 days of admission.
Patients were categorized as hospitalized during the “prepandemic” period (July 1, 2019 through February 29, 2020) and the “pandemic” period (March 1, 2020 through Oct. 30, 2021) and were stratified based on their SARS-CoV-2 result.
Investigators included all hospital admissions with an AMR event (first positive culture for select gram-negative or gram-positive pathogens that were reported as nonsusceptible across blood, urine, respiratory, intra-abdominal, skin/wound, and other sources).
The investigators calculated AMR rates at the patient-admission level and defined per 100 admissions. Also, they further evaluated AMR rates based on community onset (defined as culture collected ≤2 days from admission) or hospital onset (>2 days from admission). Finally, AMR rates were determined according to whether they related to prepandemic or pandemic periods.
Hospitals were also categorized according to their AMR rates as low (<25%), medium (25%-75%), and high (>75%).
Overall AMR rates were lower in the pandemic period, compared with the prepandemic period. However, reported Dr.Gupta, for hospital-onset pathogens specifically, AMR rates were significantly higher overall in the pandemic period and mostly driven by admissions tested for SARS-CoV-2 (whether positive or negative).
Hospitals with high AMR rates also tended to have more SARS-CoV-2 positive admissions (6.1% in high-AMR hospitals vs. 3% in low-AMR hospitals). The highest antibiotic-prescribing rates and highest duration of antibiotic use was also seen in those hospitals with highest AMR rates.
Of the SARS-CoV-2 patients who were bacterial culture negative/no culture and were prescribed antibiotics, 36.5% were in hospitals with a high AMR rate. “Roughly one-third of patients without culture evidence of a bacterial infection were prescribed antibiotics in hospitals with a high AMR rate,” said Dr. Gupta.
The researchers wanted to tease out whether hospitals with high, moderate, or low AMR rates look different with respect to antibiotic-prescribing patterns. During the pandemic period, they found that hospitals with high and medium AMR rates experienced significant increases in antibiotic prescriptions and longer durations. Prepandemic, the overall hospital-onset AMR rate was 0.8 per 100 admissions, whereas during the pandemic this rose to 1.4 per 100 admissions in high-AMR hospitals and dropped to 0.4 in low-AMR hospitals.
SARS-CoV-2–positive admission rates were higher in facilities with medium (5.6%) and high AMR (6.1%) rates than those with low (3%) AMR rates. “We found that those with medium and high AMR rates were more likely to have COVID-positive admissions than facilities with low AMR rates,” Dr. Gupta said. “It appears as if COVID is contributing to AMR in the facilities.”
Asked for independent comment, Jason C. Gallagher, PharmD, BCPS, clinical professor at Temple University School of Pharmacy in Philadelphia, said in an interview, “It is not surprising that there was more antimicrobial resistance in patients with COVID than those without. Even though antibiotics do not work for COVID, they are often prescribed, and antibiotic use is a major risk factor for antimicrobial resistance. This is likely because clinicians are sometimes concerned about coinfections with bacteria (which are rare) and because hospitalized patients with severe COVID can acquire other infections as they are treated.”
Antibiotic stewardship programs
Antibiotic stewardship programs have been highly stressed during the pandemic, so the researchers hope their data support the need for better antibiotic stewardship practices during pandemic surges when control is more challenging.
Dr. Gupta explained that they were seeing interesting associations that can inform antimicrobial stewardship programs and teams. “We are not trying to imply causality,” he stressed.
It is a common practice for stewardship teams to evaluate the need for continuation of antibiotic therapy at 3 days, especially in patients who are culture negative or did not have a culture collected.
“Antibiotic time-out at 3 days is a recommended practice to evaluate for continuing antibiotic therapy based on the patient’s condition and culture results,” he said. “This is what made our study unique because we wanted to look at what percentage of admissions were prescribed antibiotics beyond 3 days and compare to the prepandemic period.”
Session moderator Evangelos J. Giamarellos-Bourboulis, MD, PhD, an assistant professor of internal medicine and infectious diseases, University of Athens, Greece, thanked Dr. Gupta for his “eloquent presentation” and sought to clarify whether the data “refer to antimicrobial use that was empirical or whether use was in hospitals with high AMR rates, or whether the approach was driven through microbiology?”
Dr. Gupta replied that this was why they evaluated the negative-culture and no-culture patients. “We wanted to get a measure of antibacterial use in this population too,” he said. “Definitely, there is empirical therapy as well as definitive therapy, but I think the negative and no-culture group provide a reference point where we see similar signals and trends to that of the overall population.”
An audience member also addressed a question to Dr. Gupta: “Did you look at the patient population, because in many cases, during COVID, these patients may have been more severe than in the prepandemic period?”
Dr. Gupta replied: “In our manuscript we’ve done an analysis where we adjusted for patient-level facility and regional-level factors. There are definitely differences in the patient populations but overall, these are pretty sick patients when we look at the level of severity overall.”
Dr. Gupta is an employee of and a shareholder in Becton Dickinson. Dr. Bauer is an employee of and a shareholder in Merck. Dr. Gallagher consults for many pharmaceutical companies including Merck.
Dr. Giamarellos-Bourboulis disclosed honoraria (paid to the University of Athens) from Abbott CH, Brahms Thermo Fisher GMBH Germany, GlaxoSmithKline, and Sobi; serving as a consultant for Abbott CH, Fab’nTech, InflaRx GmbH, UCB, Sobi, and Xbiotech; research grants (paid to the Hellenic Institute for the Study of Sepsis) from Abbott CH, BioMerieux France, Johnson & Johnson, MSD, Sobi, Thermo Fisher Brahms GmbH; and EU research funding: Horizon 2020 ITN European Sepsis Academy (granted to the University of Athens); Horizon 2020 ImmunoSep and RISinCOVID (granted to the Hellenic Institute for the Study of Sepsis); Horizon Health EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis).
A version of this article first appeared on Medscape.com.
LISBON – During the pandemic, critical and acute care hospitals with medium and high rates of antimicrobial resistance (AMR) showed significant increases in antibiotic prescriptions and longer durations of antibiotic treatment among all hospital admissions, and also in those patients who were bacterial culture negative, according to a large U.S.-based study.
The analysis across 271 U.S. hospitals also showed that AMR rates were significantly higher for pathogens during the pandemic period, compared with the prepandemic period in patients who were tested for SARS-CoV-2, and highest in SARS-CoV-2–positive patients.
More than a third of SARS-CoV-2–positive patients who were prescribed antibiotics were bacterial culture negative.
Findings of the study were presented by Vikas Gupta, PharmD, director of medical affairs at medical technology firm Becton Dickinson, at this year’s European Congress of Clinical Microbiology & Infectious Diseases. He conducted the study jointly with Karri Bauer, PharmD, from Merck Sharp & Dohme, Kenilworth, N.J., and colleagues.
“There are differences in AMR that go beyond COVID-positive admissions,” Dr. Gupta told this news organization. “There is opportunity for improvement especially with those hospitalized patients who had a negative culture result, or no culture collected.”
“We found a higher percentage of COVID-positive admissions that were prescribed antibacterial therapy even in those having [tested negative for bacteria] or no culture result,” said Dr. Gupta. “Our data also shows that the percentage of admissions with duration of antibacterial therapy over 3 days was significantly higher in COVID-positive but culture-negative/no culture patients, compared to other groups evaluated.”
Of all admissions prescribed antibiotics during the pandemic, 57.8% of SARS-CoV-2–positive patients were prescribed antibiotics whereas 88.1% of SARS-CoV-2–positive admissions were bacterial culture negative/no culture. Overall, prepandemic, 35% of admissions were prescribed antibiotics.
Duration of antibiotic therapy in the prepandemic era was an average of 3.5 days, compared with an average of 3.8 days overall in the pandemic and 5.7 days in patients who tested positive for SARS-CoV-2. Similarly, the percentage of patients who were bacterial culture negative or had no culture and received antibiotic therapy for more than 72 hours was 17.6% in the prepandemic era, compared with 19.2% overall in the pandemic era, and 41.1% in patients who tested positive for COVID-19.
Dr. Gupta and Dr. Bauer wanted to look at all patients admitted to hospitals segmented by SARS-CoV-2 positive, negative, and not tested, to get a sense of how much antibiotic use there was and how long patients were on antibiotics. “We ultimately want to optimize and not overuse antibiotics and prescribe them for right period of time,” said Dr. Gupta.
“To date, there has been no conclusive evidence about the suggestion that the pandemic has led to increased AMR rates, so we aimed to evaluate the pandemic’s impact on AMR and antibiotic use across U.S. hospitals,” he explained.
The multicenter, retrospective cohort analysis made use of BD’s infection surveillance platform (BD HealthSight Infection Advisor with MedMined Insights) and was conducted across 271 U.S. critical access/acute care facilities, representing approximately 10%-13% of U.S. hospital admissions. It included all hospitalized patients with more than 1 day of in-patient admission. Patients were considered SARS-CoV-2 positive by polymerase chain reaction test or antigen test either 7 days or less prior to or within 14 days of admission.
Patients were categorized as hospitalized during the “prepandemic” period (July 1, 2019 through February 29, 2020) and the “pandemic” period (March 1, 2020 through Oct. 30, 2021) and were stratified based on their SARS-CoV-2 result.
Investigators included all hospital admissions with an AMR event (first positive culture for select gram-negative or gram-positive pathogens that were reported as nonsusceptible across blood, urine, respiratory, intra-abdominal, skin/wound, and other sources).
The investigators calculated AMR rates at the patient-admission level and defined per 100 admissions. Also, they further evaluated AMR rates based on community onset (defined as culture collected ≤2 days from admission) or hospital onset (>2 days from admission). Finally, AMR rates were determined according to whether they related to prepandemic or pandemic periods.
Hospitals were also categorized according to their AMR rates as low (<25%), medium (25%-75%), and high (>75%).
Overall AMR rates were lower in the pandemic period, compared with the prepandemic period. However, reported Dr.Gupta, for hospital-onset pathogens specifically, AMR rates were significantly higher overall in the pandemic period and mostly driven by admissions tested for SARS-CoV-2 (whether positive or negative).
Hospitals with high AMR rates also tended to have more SARS-CoV-2 positive admissions (6.1% in high-AMR hospitals vs. 3% in low-AMR hospitals). The highest antibiotic-prescribing rates and highest duration of antibiotic use was also seen in those hospitals with highest AMR rates.
Of the SARS-CoV-2 patients who were bacterial culture negative/no culture and were prescribed antibiotics, 36.5% were in hospitals with a high AMR rate. “Roughly one-third of patients without culture evidence of a bacterial infection were prescribed antibiotics in hospitals with a high AMR rate,” said Dr. Gupta.
The researchers wanted to tease out whether hospitals with high, moderate, or low AMR rates look different with respect to antibiotic-prescribing patterns. During the pandemic period, they found that hospitals with high and medium AMR rates experienced significant increases in antibiotic prescriptions and longer durations. Prepandemic, the overall hospital-onset AMR rate was 0.8 per 100 admissions, whereas during the pandemic this rose to 1.4 per 100 admissions in high-AMR hospitals and dropped to 0.4 in low-AMR hospitals.
SARS-CoV-2–positive admission rates were higher in facilities with medium (5.6%) and high AMR (6.1%) rates than those with low (3%) AMR rates. “We found that those with medium and high AMR rates were more likely to have COVID-positive admissions than facilities with low AMR rates,” Dr. Gupta said. “It appears as if COVID is contributing to AMR in the facilities.”
Asked for independent comment, Jason C. Gallagher, PharmD, BCPS, clinical professor at Temple University School of Pharmacy in Philadelphia, said in an interview, “It is not surprising that there was more antimicrobial resistance in patients with COVID than those without. Even though antibiotics do not work for COVID, they are often prescribed, and antibiotic use is a major risk factor for antimicrobial resistance. This is likely because clinicians are sometimes concerned about coinfections with bacteria (which are rare) and because hospitalized patients with severe COVID can acquire other infections as they are treated.”
Antibiotic stewardship programs
Antibiotic stewardship programs have been highly stressed during the pandemic, so the researchers hope their data support the need for better antibiotic stewardship practices during pandemic surges when control is more challenging.
Dr. Gupta explained that they were seeing interesting associations that can inform antimicrobial stewardship programs and teams. “We are not trying to imply causality,” he stressed.
It is a common practice for stewardship teams to evaluate the need for continuation of antibiotic therapy at 3 days, especially in patients who are culture negative or did not have a culture collected.
“Antibiotic time-out at 3 days is a recommended practice to evaluate for continuing antibiotic therapy based on the patient’s condition and culture results,” he said. “This is what made our study unique because we wanted to look at what percentage of admissions were prescribed antibiotics beyond 3 days and compare to the prepandemic period.”
Session moderator Evangelos J. Giamarellos-Bourboulis, MD, PhD, an assistant professor of internal medicine and infectious diseases, University of Athens, Greece, thanked Dr. Gupta for his “eloquent presentation” and sought to clarify whether the data “refer to antimicrobial use that was empirical or whether use was in hospitals with high AMR rates, or whether the approach was driven through microbiology?”
Dr. Gupta replied that this was why they evaluated the negative-culture and no-culture patients. “We wanted to get a measure of antibacterial use in this population too,” he said. “Definitely, there is empirical therapy as well as definitive therapy, but I think the negative and no-culture group provide a reference point where we see similar signals and trends to that of the overall population.”
An audience member also addressed a question to Dr. Gupta: “Did you look at the patient population, because in many cases, during COVID, these patients may have been more severe than in the prepandemic period?”
Dr. Gupta replied: “In our manuscript we’ve done an analysis where we adjusted for patient-level facility and regional-level factors. There are definitely differences in the patient populations but overall, these are pretty sick patients when we look at the level of severity overall.”
Dr. Gupta is an employee of and a shareholder in Becton Dickinson. Dr. Bauer is an employee of and a shareholder in Merck. Dr. Gallagher consults for many pharmaceutical companies including Merck.
Dr. Giamarellos-Bourboulis disclosed honoraria (paid to the University of Athens) from Abbott CH, Brahms Thermo Fisher GMBH Germany, GlaxoSmithKline, and Sobi; serving as a consultant for Abbott CH, Fab’nTech, InflaRx GmbH, UCB, Sobi, and Xbiotech; research grants (paid to the Hellenic Institute for the Study of Sepsis) from Abbott CH, BioMerieux France, Johnson & Johnson, MSD, Sobi, Thermo Fisher Brahms GmbH; and EU research funding: Horizon 2020 ITN European Sepsis Academy (granted to the University of Athens); Horizon 2020 ImmunoSep and RISinCOVID (granted to the Hellenic Institute for the Study of Sepsis); Horizon Health EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis).
A version of this article first appeared on Medscape.com.
LISBON – During the pandemic, critical and acute care hospitals with medium and high rates of antimicrobial resistance (AMR) showed significant increases in antibiotic prescriptions and longer durations of antibiotic treatment among all hospital admissions, and also in those patients who were bacterial culture negative, according to a large U.S.-based study.
The analysis across 271 U.S. hospitals also showed that AMR rates were significantly higher for pathogens during the pandemic period, compared with the prepandemic period in patients who were tested for SARS-CoV-2, and highest in SARS-CoV-2–positive patients.
More than a third of SARS-CoV-2–positive patients who were prescribed antibiotics were bacterial culture negative.
Findings of the study were presented by Vikas Gupta, PharmD, director of medical affairs at medical technology firm Becton Dickinson, at this year’s European Congress of Clinical Microbiology & Infectious Diseases. He conducted the study jointly with Karri Bauer, PharmD, from Merck Sharp & Dohme, Kenilworth, N.J., and colleagues.
“There are differences in AMR that go beyond COVID-positive admissions,” Dr. Gupta told this news organization. “There is opportunity for improvement especially with those hospitalized patients who had a negative culture result, or no culture collected.”
“We found a higher percentage of COVID-positive admissions that were prescribed antibacterial therapy even in those having [tested negative for bacteria] or no culture result,” said Dr. Gupta. “Our data also shows that the percentage of admissions with duration of antibacterial therapy over 3 days was significantly higher in COVID-positive but culture-negative/no culture patients, compared to other groups evaluated.”
Of all admissions prescribed antibiotics during the pandemic, 57.8% of SARS-CoV-2–positive patients were prescribed antibiotics whereas 88.1% of SARS-CoV-2–positive admissions were bacterial culture negative/no culture. Overall, prepandemic, 35% of admissions were prescribed antibiotics.
Duration of antibiotic therapy in the prepandemic era was an average of 3.5 days, compared with an average of 3.8 days overall in the pandemic and 5.7 days in patients who tested positive for SARS-CoV-2. Similarly, the percentage of patients who were bacterial culture negative or had no culture and received antibiotic therapy for more than 72 hours was 17.6% in the prepandemic era, compared with 19.2% overall in the pandemic era, and 41.1% in patients who tested positive for COVID-19.
Dr. Gupta and Dr. Bauer wanted to look at all patients admitted to hospitals segmented by SARS-CoV-2 positive, negative, and not tested, to get a sense of how much antibiotic use there was and how long patients were on antibiotics. “We ultimately want to optimize and not overuse antibiotics and prescribe them for right period of time,” said Dr. Gupta.
“To date, there has been no conclusive evidence about the suggestion that the pandemic has led to increased AMR rates, so we aimed to evaluate the pandemic’s impact on AMR and antibiotic use across U.S. hospitals,” he explained.
The multicenter, retrospective cohort analysis made use of BD’s infection surveillance platform (BD HealthSight Infection Advisor with MedMined Insights) and was conducted across 271 U.S. critical access/acute care facilities, representing approximately 10%-13% of U.S. hospital admissions. It included all hospitalized patients with more than 1 day of in-patient admission. Patients were considered SARS-CoV-2 positive by polymerase chain reaction test or antigen test either 7 days or less prior to or within 14 days of admission.
Patients were categorized as hospitalized during the “prepandemic” period (July 1, 2019 through February 29, 2020) and the “pandemic” period (March 1, 2020 through Oct. 30, 2021) and were stratified based on their SARS-CoV-2 result.
Investigators included all hospital admissions with an AMR event (first positive culture for select gram-negative or gram-positive pathogens that were reported as nonsusceptible across blood, urine, respiratory, intra-abdominal, skin/wound, and other sources).
The investigators calculated AMR rates at the patient-admission level and defined per 100 admissions. Also, they further evaluated AMR rates based on community onset (defined as culture collected ≤2 days from admission) or hospital onset (>2 days from admission). Finally, AMR rates were determined according to whether they related to prepandemic or pandemic periods.
Hospitals were also categorized according to their AMR rates as low (<25%), medium (25%-75%), and high (>75%).
Overall AMR rates were lower in the pandemic period, compared with the prepandemic period. However, reported Dr.Gupta, for hospital-onset pathogens specifically, AMR rates were significantly higher overall in the pandemic period and mostly driven by admissions tested for SARS-CoV-2 (whether positive or negative).
Hospitals with high AMR rates also tended to have more SARS-CoV-2 positive admissions (6.1% in high-AMR hospitals vs. 3% in low-AMR hospitals). The highest antibiotic-prescribing rates and highest duration of antibiotic use was also seen in those hospitals with highest AMR rates.
Of the SARS-CoV-2 patients who were bacterial culture negative/no culture and were prescribed antibiotics, 36.5% were in hospitals with a high AMR rate. “Roughly one-third of patients without culture evidence of a bacterial infection were prescribed antibiotics in hospitals with a high AMR rate,” said Dr. Gupta.
The researchers wanted to tease out whether hospitals with high, moderate, or low AMR rates look different with respect to antibiotic-prescribing patterns. During the pandemic period, they found that hospitals with high and medium AMR rates experienced significant increases in antibiotic prescriptions and longer durations. Prepandemic, the overall hospital-onset AMR rate was 0.8 per 100 admissions, whereas during the pandemic this rose to 1.4 per 100 admissions in high-AMR hospitals and dropped to 0.4 in low-AMR hospitals.
SARS-CoV-2–positive admission rates were higher in facilities with medium (5.6%) and high AMR (6.1%) rates than those with low (3%) AMR rates. “We found that those with medium and high AMR rates were more likely to have COVID-positive admissions than facilities with low AMR rates,” Dr. Gupta said. “It appears as if COVID is contributing to AMR in the facilities.”
Asked for independent comment, Jason C. Gallagher, PharmD, BCPS, clinical professor at Temple University School of Pharmacy in Philadelphia, said in an interview, “It is not surprising that there was more antimicrobial resistance in patients with COVID than those without. Even though antibiotics do not work for COVID, they are often prescribed, and antibiotic use is a major risk factor for antimicrobial resistance. This is likely because clinicians are sometimes concerned about coinfections with bacteria (which are rare) and because hospitalized patients with severe COVID can acquire other infections as they are treated.”
Antibiotic stewardship programs
Antibiotic stewardship programs have been highly stressed during the pandemic, so the researchers hope their data support the need for better antibiotic stewardship practices during pandemic surges when control is more challenging.
Dr. Gupta explained that they were seeing interesting associations that can inform antimicrobial stewardship programs and teams. “We are not trying to imply causality,” he stressed.
It is a common practice for stewardship teams to evaluate the need for continuation of antibiotic therapy at 3 days, especially in patients who are culture negative or did not have a culture collected.
“Antibiotic time-out at 3 days is a recommended practice to evaluate for continuing antibiotic therapy based on the patient’s condition and culture results,” he said. “This is what made our study unique because we wanted to look at what percentage of admissions were prescribed antibiotics beyond 3 days and compare to the prepandemic period.”
Session moderator Evangelos J. Giamarellos-Bourboulis, MD, PhD, an assistant professor of internal medicine and infectious diseases, University of Athens, Greece, thanked Dr. Gupta for his “eloquent presentation” and sought to clarify whether the data “refer to antimicrobial use that was empirical or whether use was in hospitals with high AMR rates, or whether the approach was driven through microbiology?”
Dr. Gupta replied that this was why they evaluated the negative-culture and no-culture patients. “We wanted to get a measure of antibacterial use in this population too,” he said. “Definitely, there is empirical therapy as well as definitive therapy, but I think the negative and no-culture group provide a reference point where we see similar signals and trends to that of the overall population.”
An audience member also addressed a question to Dr. Gupta: “Did you look at the patient population, because in many cases, during COVID, these patients may have been more severe than in the prepandemic period?”
Dr. Gupta replied: “In our manuscript we’ve done an analysis where we adjusted for patient-level facility and regional-level factors. There are definitely differences in the patient populations but overall, these are pretty sick patients when we look at the level of severity overall.”
Dr. Gupta is an employee of and a shareholder in Becton Dickinson. Dr. Bauer is an employee of and a shareholder in Merck. Dr. Gallagher consults for many pharmaceutical companies including Merck.
Dr. Giamarellos-Bourboulis disclosed honoraria (paid to the University of Athens) from Abbott CH, Brahms Thermo Fisher GMBH Germany, GlaxoSmithKline, and Sobi; serving as a consultant for Abbott CH, Fab’nTech, InflaRx GmbH, UCB, Sobi, and Xbiotech; research grants (paid to the Hellenic Institute for the Study of Sepsis) from Abbott CH, BioMerieux France, Johnson & Johnson, MSD, Sobi, Thermo Fisher Brahms GmbH; and EU research funding: Horizon 2020 ITN European Sepsis Academy (granted to the University of Athens); Horizon 2020 ImmunoSep and RISinCOVID (granted to the Hellenic Institute for the Study of Sepsis); Horizon Health EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis).
A version of this article first appeared on Medscape.com.
Long-COVID symptoms a serious challenge for older patients, physicians
Even mundane tasks such as making a meal can be exhausting for Louise Salant.
“I’m totally wiped out,” said the 71-year-old former private music instructor with asthma who lives in New York City and has been coping with debilitating symptoms of fatigue, shortness of breath, and gastrointestinal symptoms since recovering from a severe bout of COVID-19 2 years ago. “I just don’t have the energy.”
Ms. Salant is not alone. Many older people who contract COVID-19 experience prolonged symptoms of the disease. An analysis of Medicare Advantage claims data published in the BMJ found that about one-third of roughly 87,000 adults aged 65 in the database with a COVID-19 diagnosis sought care for persistent or new symptoms 21 or more days later.
That figure is about twice the rate of persistent COVID-19 related symptoms seen in a cohort of adults younger than age 65 with commercial insurance analyzed by the same group of researchers in a separate BMJ study. Compared with a 2020 comparator group of patients in this age cohort, these patients had a greater likelihood of respiratory failure, fatigue, hypertension, memory problems, kidney injury, mental health conditions, hypercoagulability, and cardiac rhythm disorders. When they compared post–COVID-19 symptoms to lasting symptoms of another serious viral disease – influenza – the researchers found that only respiratory failure, dementia, and post-viral fatigue were more common in the COVID-19 group.
“It became clear early in the pandemic that there is going to be a second pandemic related to all of the complications that we’ve seen related to COVID-19 infections,” said Ken Cohen, MD, executive director of translational research and national senior medical director for Optum Labs in Minnetonka, Minn., who coauthored the BMJ studies.
The results are among a growing body of evidence suggesting that older adults are at high risk of persistent post-COVID-19 symptoms.
Researchers in Rome, for example, found that 83% of 165 patients aged 65 or older who had been hospitalized for COVID-19 reported at least one lasting symptom – problems like fatigue, shortness of breath, joint pain, and coughing – in the months after hospitalization. One-third of those had two symptoms, and 46% had three or more.
A similar study in Norway found that two-thirds of patients aged 60 or older reported reduced health-related quality of life during follow-up visits 6 months after hospitalization for COVID-19. The most-reported impairments among those patients were the inability to perform the tasks of daily life, reduced mobility, and increased pain and discomfort.
Cognitive concerns
Mounting evidence indicates that COVID-19 may contribute to chronic cognitive impairment in older adults. A multisite U.S. study found that 28% of 817 adults presenting to emergency departments with COVID-19 had delirium and poorer outcomes. A Chinese case-control study that enrolled 1,438 individuals hospitalized in Wuhan for COVID-19, along with 438 of their uninfected spouses, found that 12% of COVID-19 survivors experienced cognitive impairment a year after discharge. Matteo Tosato, MD, PhD, head of the outpatient clinic for patients with long COVID symptoms at Gemelli Hospital in Rome, called those findings “very concerning.”
Jin Ho Han, MD, associate professor of emergency medicine at Vanderbilt University, Nashville, Tenn., said cognitive impairment is common after an acute illness, particularly in frail or vulnerable patients.
“Hospitalization and the acute illness itself accelerate cognitive decline,” said Dr. Han, and previous evidence links delirium with worsening cognition. He and his colleagues are studying the potential role of delirium in longer-term cognitive decline in older patients after COVID-19.
Dr. Han emphasized the importance of preventing COVID-19-related delirium through vaccines and other strategies to reduce exposure of older patients to the virus. “Once you have cognitive decline, there are no interventions to reverse it,” he said.
Alarm bells for long-term care
Experts expressed concern that the situation might be even worse for people living in long-term care facilities. Many already need assistance with tasks of daily living and could be particularly vulnerable to lasting effects of COVID-19, said Karl Steinberg, MD, president of the Society for Post-Acute and Long-Term Care Medicine. He estimated that roughly half of his patients who have had COVID-19, regardless of the severity of their symptoms, have endured some degree of functional decline.
“It’s common for long-term care facility residents to experience functional and cognitive decline, even after seemingly minor things, like a cold or a trip to the hospital,” Dr. Steinberg, who has been a medical director of long-term care facilities in San Diego County for more than 2 decades, told this news organization. “It makes it a little harder to determine whether the declines we’ve been seeing post COVID in these residents are attributable to post COVID versus just an accelerated step in their overall expected decline.”
The pandemic may have contributed to worse outcomes for people in long-term care facilities in several ways: the disease itself, its effects on health care delivery, and necessary preventive measures to protect long-term care residents from exposure to the virus.
“During the many months where family visits were prohibited, we saw people – whether they had COVID-19 or not – suffer major clinical, functional, cognitive declines or severe psychological symptoms,” Dr. Steinberg said.
He emphasized the importance of preventive measures such as vaccines and boosters in patients in long-term care facilities. He said the benefit of preventing lasting symptoms is often a strong motivator for family caregivers of people with dementia to get them vaccinated or boosted.
“It’s clear that vaccination and booster reduce the incidence of post-COVID symptoms,” he said. Almost all studies have been in younger cohorts, but he expects the benefits would also apply to older patients.
Easing symptoms and offering support
As with long COVID generally, many questions remain about the causes of lasting symptoms of COVID-19 in older patients, and how best to treat them. Dr. Tosato, who led the study of long-COVID patients in Rome, is focusing on inflammation as a critical factor in the condition. He and colleagues across Europe hope to answer some of them by launching a multicenter study of lasting COVID-19 symptoms.
In the meantime, Dr. Steinberg and Dr. Tosato said they are doing their best to evaluate and treat patients empirically.
“We pull from our armamentarium to treat system-specific symptoms,” Dr. Steinberg said. “We want to improve the quality of life and help each day be the best it can.”
Physicians in long-term care facilities might use medications such as antidepressants or nonpharmacologic approaches for patients experiencing depression symptoms. Families are also crucial in helping patients by bringing in home-cooked meals and encouraging loved ones who may be experiencing loss of taste or smell to eat, Dr. Steinberg said.
“We’ve seen with the return of families and loved ones visiting to some extent has alleviated some people’s symptoms, especially psychological ones,” he said.
Dr. Tosato said he and his colleagues start with an individualized, multidisciplinary assessment to determine what types of care may help. He noted that physicians might recommend medications or rehabilitative therapies depending on the patient’s needs.
“A personalized approach is key,” Dr. Tosato said. His study also found that the proportion of older patients experiencing symptoms declined over time – a glimmer of hope that many will recover.
Dr. Cohen emphasized the need for a multimodal rehabilitation, an evidence-based approach used to care for patients who survived hospitalization with severe COVID-19 – a group that has substantially higher rates of persistent symptoms. This approach includes cognitive rehabilitation, physical therapy, occupational therapy, and a graded exercise program.
Dr. Han and colleagues are studying potential therapies such as cognitive rehabilitation in adults who’ve experienced delirium. But until evidence-based treatments are available, they stress the role of support for patients with cognitive decline and their families.
“A lot of the work we do is teach patients and their families to compensate for newly acquired cognitive deficits from any illness, including COVID-19,” Dr. Han said.
Ms. Salant said she has experienced some improvement in her energy since her pulmonologist recommended a new inhaler based on her symptoms. Her sense of smell and taste, lost to the infection, returned after she received her first dose of a vaccine against COVID-19. She takes comfort in participating in Survivor Corps, a group of more than 170,000 COVID-19 survivors and their families who advocate for more scientific research on the disease.
She also expressed gratitude for the support she receives from her primary care physician, who she said has taken the time to learn more about the symptoms of long COVID, listens to her, and respects what she has to say.
“I have hope that I will keep getting better by baby steps,” Ms. Salant said.
Dr. Tosato, Dr. Steinberg, and Dr. Han have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even mundane tasks such as making a meal can be exhausting for Louise Salant.
“I’m totally wiped out,” said the 71-year-old former private music instructor with asthma who lives in New York City and has been coping with debilitating symptoms of fatigue, shortness of breath, and gastrointestinal symptoms since recovering from a severe bout of COVID-19 2 years ago. “I just don’t have the energy.”
Ms. Salant is not alone. Many older people who contract COVID-19 experience prolonged symptoms of the disease. An analysis of Medicare Advantage claims data published in the BMJ found that about one-third of roughly 87,000 adults aged 65 in the database with a COVID-19 diagnosis sought care for persistent or new symptoms 21 or more days later.
That figure is about twice the rate of persistent COVID-19 related symptoms seen in a cohort of adults younger than age 65 with commercial insurance analyzed by the same group of researchers in a separate BMJ study. Compared with a 2020 comparator group of patients in this age cohort, these patients had a greater likelihood of respiratory failure, fatigue, hypertension, memory problems, kidney injury, mental health conditions, hypercoagulability, and cardiac rhythm disorders. When they compared post–COVID-19 symptoms to lasting symptoms of another serious viral disease – influenza – the researchers found that only respiratory failure, dementia, and post-viral fatigue were more common in the COVID-19 group.
“It became clear early in the pandemic that there is going to be a second pandemic related to all of the complications that we’ve seen related to COVID-19 infections,” said Ken Cohen, MD, executive director of translational research and national senior medical director for Optum Labs in Minnetonka, Minn., who coauthored the BMJ studies.
The results are among a growing body of evidence suggesting that older adults are at high risk of persistent post-COVID-19 symptoms.
Researchers in Rome, for example, found that 83% of 165 patients aged 65 or older who had been hospitalized for COVID-19 reported at least one lasting symptom – problems like fatigue, shortness of breath, joint pain, and coughing – in the months after hospitalization. One-third of those had two symptoms, and 46% had three or more.
A similar study in Norway found that two-thirds of patients aged 60 or older reported reduced health-related quality of life during follow-up visits 6 months after hospitalization for COVID-19. The most-reported impairments among those patients were the inability to perform the tasks of daily life, reduced mobility, and increased pain and discomfort.
Cognitive concerns
Mounting evidence indicates that COVID-19 may contribute to chronic cognitive impairment in older adults. A multisite U.S. study found that 28% of 817 adults presenting to emergency departments with COVID-19 had delirium and poorer outcomes. A Chinese case-control study that enrolled 1,438 individuals hospitalized in Wuhan for COVID-19, along with 438 of their uninfected spouses, found that 12% of COVID-19 survivors experienced cognitive impairment a year after discharge. Matteo Tosato, MD, PhD, head of the outpatient clinic for patients with long COVID symptoms at Gemelli Hospital in Rome, called those findings “very concerning.”
Jin Ho Han, MD, associate professor of emergency medicine at Vanderbilt University, Nashville, Tenn., said cognitive impairment is common after an acute illness, particularly in frail or vulnerable patients.
“Hospitalization and the acute illness itself accelerate cognitive decline,” said Dr. Han, and previous evidence links delirium with worsening cognition. He and his colleagues are studying the potential role of delirium in longer-term cognitive decline in older patients after COVID-19.
Dr. Han emphasized the importance of preventing COVID-19-related delirium through vaccines and other strategies to reduce exposure of older patients to the virus. “Once you have cognitive decline, there are no interventions to reverse it,” he said.
Alarm bells for long-term care
Experts expressed concern that the situation might be even worse for people living in long-term care facilities. Many already need assistance with tasks of daily living and could be particularly vulnerable to lasting effects of COVID-19, said Karl Steinberg, MD, president of the Society for Post-Acute and Long-Term Care Medicine. He estimated that roughly half of his patients who have had COVID-19, regardless of the severity of their symptoms, have endured some degree of functional decline.
“It’s common for long-term care facility residents to experience functional and cognitive decline, even after seemingly minor things, like a cold or a trip to the hospital,” Dr. Steinberg, who has been a medical director of long-term care facilities in San Diego County for more than 2 decades, told this news organization. “It makes it a little harder to determine whether the declines we’ve been seeing post COVID in these residents are attributable to post COVID versus just an accelerated step in their overall expected decline.”
The pandemic may have contributed to worse outcomes for people in long-term care facilities in several ways: the disease itself, its effects on health care delivery, and necessary preventive measures to protect long-term care residents from exposure to the virus.
“During the many months where family visits were prohibited, we saw people – whether they had COVID-19 or not – suffer major clinical, functional, cognitive declines or severe psychological symptoms,” Dr. Steinberg said.
He emphasized the importance of preventive measures such as vaccines and boosters in patients in long-term care facilities. He said the benefit of preventing lasting symptoms is often a strong motivator for family caregivers of people with dementia to get them vaccinated or boosted.
“It’s clear that vaccination and booster reduce the incidence of post-COVID symptoms,” he said. Almost all studies have been in younger cohorts, but he expects the benefits would also apply to older patients.
Easing symptoms and offering support
As with long COVID generally, many questions remain about the causes of lasting symptoms of COVID-19 in older patients, and how best to treat them. Dr. Tosato, who led the study of long-COVID patients in Rome, is focusing on inflammation as a critical factor in the condition. He and colleagues across Europe hope to answer some of them by launching a multicenter study of lasting COVID-19 symptoms.
In the meantime, Dr. Steinberg and Dr. Tosato said they are doing their best to evaluate and treat patients empirically.
“We pull from our armamentarium to treat system-specific symptoms,” Dr. Steinberg said. “We want to improve the quality of life and help each day be the best it can.”
Physicians in long-term care facilities might use medications such as antidepressants or nonpharmacologic approaches for patients experiencing depression symptoms. Families are also crucial in helping patients by bringing in home-cooked meals and encouraging loved ones who may be experiencing loss of taste or smell to eat, Dr. Steinberg said.
“We’ve seen with the return of families and loved ones visiting to some extent has alleviated some people’s symptoms, especially psychological ones,” he said.
Dr. Tosato said he and his colleagues start with an individualized, multidisciplinary assessment to determine what types of care may help. He noted that physicians might recommend medications or rehabilitative therapies depending on the patient’s needs.
“A personalized approach is key,” Dr. Tosato said. His study also found that the proportion of older patients experiencing symptoms declined over time – a glimmer of hope that many will recover.
Dr. Cohen emphasized the need for a multimodal rehabilitation, an evidence-based approach used to care for patients who survived hospitalization with severe COVID-19 – a group that has substantially higher rates of persistent symptoms. This approach includes cognitive rehabilitation, physical therapy, occupational therapy, and a graded exercise program.
Dr. Han and colleagues are studying potential therapies such as cognitive rehabilitation in adults who’ve experienced delirium. But until evidence-based treatments are available, they stress the role of support for patients with cognitive decline and their families.
“A lot of the work we do is teach patients and their families to compensate for newly acquired cognitive deficits from any illness, including COVID-19,” Dr. Han said.
Ms. Salant said she has experienced some improvement in her energy since her pulmonologist recommended a new inhaler based on her symptoms. Her sense of smell and taste, lost to the infection, returned after she received her first dose of a vaccine against COVID-19. She takes comfort in participating in Survivor Corps, a group of more than 170,000 COVID-19 survivors and their families who advocate for more scientific research on the disease.
She also expressed gratitude for the support she receives from her primary care physician, who she said has taken the time to learn more about the symptoms of long COVID, listens to her, and respects what she has to say.
“I have hope that I will keep getting better by baby steps,” Ms. Salant said.
Dr. Tosato, Dr. Steinberg, and Dr. Han have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even mundane tasks such as making a meal can be exhausting for Louise Salant.
“I’m totally wiped out,” said the 71-year-old former private music instructor with asthma who lives in New York City and has been coping with debilitating symptoms of fatigue, shortness of breath, and gastrointestinal symptoms since recovering from a severe bout of COVID-19 2 years ago. “I just don’t have the energy.”
Ms. Salant is not alone. Many older people who contract COVID-19 experience prolonged symptoms of the disease. An analysis of Medicare Advantage claims data published in the BMJ found that about one-third of roughly 87,000 adults aged 65 in the database with a COVID-19 diagnosis sought care for persistent or new symptoms 21 or more days later.
That figure is about twice the rate of persistent COVID-19 related symptoms seen in a cohort of adults younger than age 65 with commercial insurance analyzed by the same group of researchers in a separate BMJ study. Compared with a 2020 comparator group of patients in this age cohort, these patients had a greater likelihood of respiratory failure, fatigue, hypertension, memory problems, kidney injury, mental health conditions, hypercoagulability, and cardiac rhythm disorders. When they compared post–COVID-19 symptoms to lasting symptoms of another serious viral disease – influenza – the researchers found that only respiratory failure, dementia, and post-viral fatigue were more common in the COVID-19 group.
“It became clear early in the pandemic that there is going to be a second pandemic related to all of the complications that we’ve seen related to COVID-19 infections,” said Ken Cohen, MD, executive director of translational research and national senior medical director for Optum Labs in Minnetonka, Minn., who coauthored the BMJ studies.
The results are among a growing body of evidence suggesting that older adults are at high risk of persistent post-COVID-19 symptoms.
Researchers in Rome, for example, found that 83% of 165 patients aged 65 or older who had been hospitalized for COVID-19 reported at least one lasting symptom – problems like fatigue, shortness of breath, joint pain, and coughing – in the months after hospitalization. One-third of those had two symptoms, and 46% had three or more.
A similar study in Norway found that two-thirds of patients aged 60 or older reported reduced health-related quality of life during follow-up visits 6 months after hospitalization for COVID-19. The most-reported impairments among those patients were the inability to perform the tasks of daily life, reduced mobility, and increased pain and discomfort.
Cognitive concerns
Mounting evidence indicates that COVID-19 may contribute to chronic cognitive impairment in older adults. A multisite U.S. study found that 28% of 817 adults presenting to emergency departments with COVID-19 had delirium and poorer outcomes. A Chinese case-control study that enrolled 1,438 individuals hospitalized in Wuhan for COVID-19, along with 438 of their uninfected spouses, found that 12% of COVID-19 survivors experienced cognitive impairment a year after discharge. Matteo Tosato, MD, PhD, head of the outpatient clinic for patients with long COVID symptoms at Gemelli Hospital in Rome, called those findings “very concerning.”
Jin Ho Han, MD, associate professor of emergency medicine at Vanderbilt University, Nashville, Tenn., said cognitive impairment is common after an acute illness, particularly in frail or vulnerable patients.
“Hospitalization and the acute illness itself accelerate cognitive decline,” said Dr. Han, and previous evidence links delirium with worsening cognition. He and his colleagues are studying the potential role of delirium in longer-term cognitive decline in older patients after COVID-19.
Dr. Han emphasized the importance of preventing COVID-19-related delirium through vaccines and other strategies to reduce exposure of older patients to the virus. “Once you have cognitive decline, there are no interventions to reverse it,” he said.
Alarm bells for long-term care
Experts expressed concern that the situation might be even worse for people living in long-term care facilities. Many already need assistance with tasks of daily living and could be particularly vulnerable to lasting effects of COVID-19, said Karl Steinberg, MD, president of the Society for Post-Acute and Long-Term Care Medicine. He estimated that roughly half of his patients who have had COVID-19, regardless of the severity of their symptoms, have endured some degree of functional decline.
“It’s common for long-term care facility residents to experience functional and cognitive decline, even after seemingly minor things, like a cold or a trip to the hospital,” Dr. Steinberg, who has been a medical director of long-term care facilities in San Diego County for more than 2 decades, told this news organization. “It makes it a little harder to determine whether the declines we’ve been seeing post COVID in these residents are attributable to post COVID versus just an accelerated step in their overall expected decline.”
The pandemic may have contributed to worse outcomes for people in long-term care facilities in several ways: the disease itself, its effects on health care delivery, and necessary preventive measures to protect long-term care residents from exposure to the virus.
“During the many months where family visits were prohibited, we saw people – whether they had COVID-19 or not – suffer major clinical, functional, cognitive declines or severe psychological symptoms,” Dr. Steinberg said.
He emphasized the importance of preventive measures such as vaccines and boosters in patients in long-term care facilities. He said the benefit of preventing lasting symptoms is often a strong motivator for family caregivers of people with dementia to get them vaccinated or boosted.
“It’s clear that vaccination and booster reduce the incidence of post-COVID symptoms,” he said. Almost all studies have been in younger cohorts, but he expects the benefits would also apply to older patients.
Easing symptoms and offering support
As with long COVID generally, many questions remain about the causes of lasting symptoms of COVID-19 in older patients, and how best to treat them. Dr. Tosato, who led the study of long-COVID patients in Rome, is focusing on inflammation as a critical factor in the condition. He and colleagues across Europe hope to answer some of them by launching a multicenter study of lasting COVID-19 symptoms.
In the meantime, Dr. Steinberg and Dr. Tosato said they are doing their best to evaluate and treat patients empirically.
“We pull from our armamentarium to treat system-specific symptoms,” Dr. Steinberg said. “We want to improve the quality of life and help each day be the best it can.”
Physicians in long-term care facilities might use medications such as antidepressants or nonpharmacologic approaches for patients experiencing depression symptoms. Families are also crucial in helping patients by bringing in home-cooked meals and encouraging loved ones who may be experiencing loss of taste or smell to eat, Dr. Steinberg said.
“We’ve seen with the return of families and loved ones visiting to some extent has alleviated some people’s symptoms, especially psychological ones,” he said.
Dr. Tosato said he and his colleagues start with an individualized, multidisciplinary assessment to determine what types of care may help. He noted that physicians might recommend medications or rehabilitative therapies depending on the patient’s needs.
“A personalized approach is key,” Dr. Tosato said. His study also found that the proportion of older patients experiencing symptoms declined over time – a glimmer of hope that many will recover.
Dr. Cohen emphasized the need for a multimodal rehabilitation, an evidence-based approach used to care for patients who survived hospitalization with severe COVID-19 – a group that has substantially higher rates of persistent symptoms. This approach includes cognitive rehabilitation, physical therapy, occupational therapy, and a graded exercise program.
Dr. Han and colleagues are studying potential therapies such as cognitive rehabilitation in adults who’ve experienced delirium. But until evidence-based treatments are available, they stress the role of support for patients with cognitive decline and their families.
“A lot of the work we do is teach patients and their families to compensate for newly acquired cognitive deficits from any illness, including COVID-19,” Dr. Han said.
Ms. Salant said she has experienced some improvement in her energy since her pulmonologist recommended a new inhaler based on her symptoms. Her sense of smell and taste, lost to the infection, returned after she received her first dose of a vaccine against COVID-19. She takes comfort in participating in Survivor Corps, a group of more than 170,000 COVID-19 survivors and their families who advocate for more scientific research on the disease.
She also expressed gratitude for the support she receives from her primary care physician, who she said has taken the time to learn more about the symptoms of long COVID, listens to her, and respects what she has to say.
“I have hope that I will keep getting better by baby steps,” Ms. Salant said.
Dr. Tosato, Dr. Steinberg, and Dr. Han have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fifth COVID shot recommended for patients with cancer
The National Comprehensive Cancer Network (NCCN) has recommended a fifth COVID-19 mRNA shot for people who are immunocompromised, including many with cancer or a history of cancer.
A fifth shot of an mRNA vaccine represents a second booster, the group explained, because the primary mRNA immunization series for immunocompromised individuals involves three doses of either the Pfizer or Moderna vaccine.
The update, issued today, comes from the NCCN’s Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis, which released its first vaccine guidelines for patients with cancer in January 2021. The NCCN has issued numerous updates since then as information about the virus and vaccines has evolved.
“We know a lot more about COVID-19 and the vaccines now, and we can use that knowledge to minimize the confusion and enhance the protection we can offer to our immunocompromised patients,” said advisory committee co-leader Lindsey Baden, MD, an infectious diseases specialist at the Dana-Farber Cancer Institute, Boston.
The latest iteration of the NCCN’s COVID guidelines includes an update for patients who initially received Johnson & Johnson’s single-shot vaccine, including a recommendation that patients receive an mRNA vaccine for both the first and second booster.
The group also updated dosing recommendations for pre-exposure prevention with tixagevimab plus cilgavimab (Evusheld, AstraZeneca), suggesting 300 mg of each monoclonal antibody instead of 150 mg, based on in vitro activity against Omicron variants.
The group noted that the Moderna and Pfizer shots can be used interchangeably for boosters.
“The NCCN Committee considers both homologous and heterologous boosters to be appropriate options,” the experts wrote.
A version of this article first appeared on Medscape.com.
The National Comprehensive Cancer Network (NCCN) has recommended a fifth COVID-19 mRNA shot for people who are immunocompromised, including many with cancer or a history of cancer.
A fifth shot of an mRNA vaccine represents a second booster, the group explained, because the primary mRNA immunization series for immunocompromised individuals involves three doses of either the Pfizer or Moderna vaccine.
The update, issued today, comes from the NCCN’s Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis, which released its first vaccine guidelines for patients with cancer in January 2021. The NCCN has issued numerous updates since then as information about the virus and vaccines has evolved.
“We know a lot more about COVID-19 and the vaccines now, and we can use that knowledge to minimize the confusion and enhance the protection we can offer to our immunocompromised patients,” said advisory committee co-leader Lindsey Baden, MD, an infectious diseases specialist at the Dana-Farber Cancer Institute, Boston.
The latest iteration of the NCCN’s COVID guidelines includes an update for patients who initially received Johnson & Johnson’s single-shot vaccine, including a recommendation that patients receive an mRNA vaccine for both the first and second booster.
The group also updated dosing recommendations for pre-exposure prevention with tixagevimab plus cilgavimab (Evusheld, AstraZeneca), suggesting 300 mg of each monoclonal antibody instead of 150 mg, based on in vitro activity against Omicron variants.
The group noted that the Moderna and Pfizer shots can be used interchangeably for boosters.
“The NCCN Committee considers both homologous and heterologous boosters to be appropriate options,” the experts wrote.
A version of this article first appeared on Medscape.com.
The National Comprehensive Cancer Network (NCCN) has recommended a fifth COVID-19 mRNA shot for people who are immunocompromised, including many with cancer or a history of cancer.
A fifth shot of an mRNA vaccine represents a second booster, the group explained, because the primary mRNA immunization series for immunocompromised individuals involves three doses of either the Pfizer or Moderna vaccine.
The update, issued today, comes from the NCCN’s Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis, which released its first vaccine guidelines for patients with cancer in January 2021. The NCCN has issued numerous updates since then as information about the virus and vaccines has evolved.
“We know a lot more about COVID-19 and the vaccines now, and we can use that knowledge to minimize the confusion and enhance the protection we can offer to our immunocompromised patients,” said advisory committee co-leader Lindsey Baden, MD, an infectious diseases specialist at the Dana-Farber Cancer Institute, Boston.
The latest iteration of the NCCN’s COVID guidelines includes an update for patients who initially received Johnson & Johnson’s single-shot vaccine, including a recommendation that patients receive an mRNA vaccine for both the first and second booster.
The group also updated dosing recommendations for pre-exposure prevention with tixagevimab plus cilgavimab (Evusheld, AstraZeneca), suggesting 300 mg of each monoclonal antibody instead of 150 mg, based on in vitro activity against Omicron variants.
The group noted that the Moderna and Pfizer shots can be used interchangeably for boosters.
“The NCCN Committee considers both homologous and heterologous boosters to be appropriate options,” the experts wrote.
A version of this article first appeared on Medscape.com.
Almost 60% of U.S. population has been infected by COVID-19: CDC
The percentage of Americans who have been infected with COVID-19 jumped from 34% in December 2021 to 58% in February 2022, a new study from the Centers for Disease Control and Prevention reveals.
This is the first time the seroprevalence of prior infection is more than 50% in the American population.
“I definitely expected that we were going to see an increase continue ... but I didn’t expect it to increase quite this much. But we follow the data ... and this is what the evidence is showing us,” lead study researcher Kristie E. N. Clarke, MD, said during a CDC media briefing April 26.
Researchers found that presence of antinucleocapsid (anti-N) antibodies from prior infection varied by age. The rate varied from as high as 75% in children and teenagers 17 years and younger to 33% in those 65 and older, for example.
The study showed that the anti-N antibodies were more common in age groups with the lowest vaccination numbers.
Combined with up-to-date CDC data on deaths, hospitalizations, and cases, the study provides a clearer picture of where we are now and where we might be headed in terms of the pandemic.
Vaccination still valuable
The fact that nearly 60% of Americans have antibodies from prior infection is not a reason to think people with a history of COVID-19 should skip vaccination, said CDC director Rochelle P. Walensky, MD.
“I can’t underscore enough that those with detectable antibodies from previous infection, we encourage them to still get vaccinated,” Dr. Walensky said.
“We do know that reinfections happen,” she said, “so that’s important in terms of thinking forward.”
The CDC continues to encourage all Americans to stay up to date with their COVID-19 vaccinations, said Dr. Clarke, colead for the CDC’s COVID-19 Epidemiology and Surveillance Taskforce Seroprevalence Team. “Having infection-induced antibodies does not necessarily mean you are protected against future infections.”
The study, published in the CDC’s Morbidity and Mortality Weekly Report (MMWR), did not evaluate antibody protection from COVID-19 vaccination.
It should also be noted that the study looked at presence or absence of anti-N antibodies, and not whether certain levels were linked to less or more protection.
Where are we now?
Dr. Walensky used the media briefing as an opportunity to share current COVID-19 numbers.
“Overall, we can continue to have some mixed trends. Deaths, fortunately, are continuing to trend downward with a 7-day average of about 300 per day, which represents an estimated 18% decline from the prior week,” she said.
Hospital admissions also remain low, at about 1,500 per day. “But we should note that for the second week in a row, they are slowly trending upwards,” Dr. Walensky said. There was an increase of about 9% at press time compared with the prior week.
Cases remain “comparatively low” to even where we were a month ago, at 44,000 per day,” Dr. Walensky said. “Although this too represents an increase of about 25% in the past week.”
Dr. Walensky noted that positive test numbers are not as reliable a metric as they were before the growth in use of rapid home tests. But it’s not the only measure. “We continue to believe that our PCR testing data, especially when we corroborate it with information from our other surveillance systems – like wastewater surveillance and emergency department surveillance – provide us a reliable picture of the trajectory of COVID-19 across our country.”
She recommended that people continue to consult the CDC’s COVID-19 county tracker to monitor local levels of COVID-19.
Dr. Walensky also shared recent findings from genomic sequencing that continue to show the predominance of the Omicron variant. “Essentially a hundred percent of what we’re finding now is Omicron,” she said. In terms of individual variants, the Omicron BA.1 variant is about 3% of circulating virus, the BA.2 variant is about 68%, and BA.2.12.1 makes up about 35%.
“We’re just starting to learn about the impact of BA2.121,” Dr. Walensky said. “It appears it might have a transmission advantage of about 25% over the BA2 subvariant.”
A version of this article first appeared on Medscape.com.
The percentage of Americans who have been infected with COVID-19 jumped from 34% in December 2021 to 58% in February 2022, a new study from the Centers for Disease Control and Prevention reveals.
This is the first time the seroprevalence of prior infection is more than 50% in the American population.
“I definitely expected that we were going to see an increase continue ... but I didn’t expect it to increase quite this much. But we follow the data ... and this is what the evidence is showing us,” lead study researcher Kristie E. N. Clarke, MD, said during a CDC media briefing April 26.
Researchers found that presence of antinucleocapsid (anti-N) antibodies from prior infection varied by age. The rate varied from as high as 75% in children and teenagers 17 years and younger to 33% in those 65 and older, for example.
The study showed that the anti-N antibodies were more common in age groups with the lowest vaccination numbers.
Combined with up-to-date CDC data on deaths, hospitalizations, and cases, the study provides a clearer picture of where we are now and where we might be headed in terms of the pandemic.
Vaccination still valuable
The fact that nearly 60% of Americans have antibodies from prior infection is not a reason to think people with a history of COVID-19 should skip vaccination, said CDC director Rochelle P. Walensky, MD.
“I can’t underscore enough that those with detectable antibodies from previous infection, we encourage them to still get vaccinated,” Dr. Walensky said.
“We do know that reinfections happen,” she said, “so that’s important in terms of thinking forward.”
The CDC continues to encourage all Americans to stay up to date with their COVID-19 vaccinations, said Dr. Clarke, colead for the CDC’s COVID-19 Epidemiology and Surveillance Taskforce Seroprevalence Team. “Having infection-induced antibodies does not necessarily mean you are protected against future infections.”
The study, published in the CDC’s Morbidity and Mortality Weekly Report (MMWR), did not evaluate antibody protection from COVID-19 vaccination.
It should also be noted that the study looked at presence or absence of anti-N antibodies, and not whether certain levels were linked to less or more protection.
Where are we now?
Dr. Walensky used the media briefing as an opportunity to share current COVID-19 numbers.
“Overall, we can continue to have some mixed trends. Deaths, fortunately, are continuing to trend downward with a 7-day average of about 300 per day, which represents an estimated 18% decline from the prior week,” she said.
Hospital admissions also remain low, at about 1,500 per day. “But we should note that for the second week in a row, they are slowly trending upwards,” Dr. Walensky said. There was an increase of about 9% at press time compared with the prior week.
Cases remain “comparatively low” to even where we were a month ago, at 44,000 per day,” Dr. Walensky said. “Although this too represents an increase of about 25% in the past week.”
Dr. Walensky noted that positive test numbers are not as reliable a metric as they were before the growth in use of rapid home tests. But it’s not the only measure. “We continue to believe that our PCR testing data, especially when we corroborate it with information from our other surveillance systems – like wastewater surveillance and emergency department surveillance – provide us a reliable picture of the trajectory of COVID-19 across our country.”
She recommended that people continue to consult the CDC’s COVID-19 county tracker to monitor local levels of COVID-19.
Dr. Walensky also shared recent findings from genomic sequencing that continue to show the predominance of the Omicron variant. “Essentially a hundred percent of what we’re finding now is Omicron,” she said. In terms of individual variants, the Omicron BA.1 variant is about 3% of circulating virus, the BA.2 variant is about 68%, and BA.2.12.1 makes up about 35%.
“We’re just starting to learn about the impact of BA2.121,” Dr. Walensky said. “It appears it might have a transmission advantage of about 25% over the BA2 subvariant.”
A version of this article first appeared on Medscape.com.
The percentage of Americans who have been infected with COVID-19 jumped from 34% in December 2021 to 58% in February 2022, a new study from the Centers for Disease Control and Prevention reveals.
This is the first time the seroprevalence of prior infection is more than 50% in the American population.
“I definitely expected that we were going to see an increase continue ... but I didn’t expect it to increase quite this much. But we follow the data ... and this is what the evidence is showing us,” lead study researcher Kristie E. N. Clarke, MD, said during a CDC media briefing April 26.
Researchers found that presence of antinucleocapsid (anti-N) antibodies from prior infection varied by age. The rate varied from as high as 75% in children and teenagers 17 years and younger to 33% in those 65 and older, for example.
The study showed that the anti-N antibodies were more common in age groups with the lowest vaccination numbers.
Combined with up-to-date CDC data on deaths, hospitalizations, and cases, the study provides a clearer picture of where we are now and where we might be headed in terms of the pandemic.
Vaccination still valuable
The fact that nearly 60% of Americans have antibodies from prior infection is not a reason to think people with a history of COVID-19 should skip vaccination, said CDC director Rochelle P. Walensky, MD.
“I can’t underscore enough that those with detectable antibodies from previous infection, we encourage them to still get vaccinated,” Dr. Walensky said.
“We do know that reinfections happen,” she said, “so that’s important in terms of thinking forward.”
The CDC continues to encourage all Americans to stay up to date with their COVID-19 vaccinations, said Dr. Clarke, colead for the CDC’s COVID-19 Epidemiology and Surveillance Taskforce Seroprevalence Team. “Having infection-induced antibodies does not necessarily mean you are protected against future infections.”
The study, published in the CDC’s Morbidity and Mortality Weekly Report (MMWR), did not evaluate antibody protection from COVID-19 vaccination.
It should also be noted that the study looked at presence or absence of anti-N antibodies, and not whether certain levels were linked to less or more protection.
Where are we now?
Dr. Walensky used the media briefing as an opportunity to share current COVID-19 numbers.
“Overall, we can continue to have some mixed trends. Deaths, fortunately, are continuing to trend downward with a 7-day average of about 300 per day, which represents an estimated 18% decline from the prior week,” she said.
Hospital admissions also remain low, at about 1,500 per day. “But we should note that for the second week in a row, they are slowly trending upwards,” Dr. Walensky said. There was an increase of about 9% at press time compared with the prior week.
Cases remain “comparatively low” to even where we were a month ago, at 44,000 per day,” Dr. Walensky said. “Although this too represents an increase of about 25% in the past week.”
Dr. Walensky noted that positive test numbers are not as reliable a metric as they were before the growth in use of rapid home tests. But it’s not the only measure. “We continue to believe that our PCR testing data, especially when we corroborate it with information from our other surveillance systems – like wastewater surveillance and emergency department surveillance – provide us a reliable picture of the trajectory of COVID-19 across our country.”
She recommended that people continue to consult the CDC’s COVID-19 county tracker to monitor local levels of COVID-19.
Dr. Walensky also shared recent findings from genomic sequencing that continue to show the predominance of the Omicron variant. “Essentially a hundred percent of what we’re finding now is Omicron,” she said. In terms of individual variants, the Omicron BA.1 variant is about 3% of circulating virus, the BA.2 variant is about 68%, and BA.2.12.1 makes up about 35%.
“We’re just starting to learn about the impact of BA2.121,” Dr. Walensky said. “It appears it might have a transmission advantage of about 25% over the BA2 subvariant.”
A version of this article first appeared on Medscape.com.
FROM MMWR
Shortage of ICU beds did not drive COVID-19 deaths
Contrary to popular belief, no association appeared between the number of intensive care unit beds and COVID-19 deaths, based on a review of data from all 50 states between March 1, 2020, and June 30, 2021.
One of the reasons for poor patient outcomes in the early months of the COVID-19 pandemic was the presumed scarcity of ICU beds, Omar Haider, MD, of Houston Methodist Hospital, and colleagues said. “We hypothesized that the states having a lower number of ICU beds had more COVID-related deaths when compared to the states that had a higher number of ICU beds,” they wrote in an abstract presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
According to the researchers, the total number of ICU beds in the United States is approximately 85,000. Hawaii has the highest number of beds per 10,000 persons, and the District of Columbia has the lowest (6.0 vs. 1.6).
The researchers collected data on ICU bed totals from the Kaiser Family Foundation. Statistics on COVID-19 deaths were obtained from The New York Times database, which provided real-time information collected from the Department of Health & Human Services, the Centers for Disease Control and Prevention, and the Census Bureau.
The researchers used the Pearson Correlation Coefficient to compare ICU beds and COVID deaths per 10,000 persons in each state. The R value was 0.29, which indicates no inverse correlation. “Our value of R2, the coefficient of determination, was 0.0858,” they added. They confirmed the results using the Spearman’s Rho, which yielded an rs of 0.3, also a sign of no inverse correlation. No correlation was found between low numbers of ICU beds and high numbers of COVID-19 deaths for any states.
The study findings were limited by several factors, including the lack of standardized reporting timelines across states, differences in state-based vaccination rates, the emergence of the Delta variant during the study period, and time-lag in contemporaneous database updates, the researchers noted.
However, the results suggest that physical ICU beds do not play a role in determining the number of COVID-related deaths. Instead, “other constraints such as less staffing, lack of medical supplies (ventilators and [personal protective equipment]) should be evaluated for potential implications on poor patients’ outcomes,” they concluded.
Pandemic challenges can inform future plans
“As the health care system emerges from the effects of the pandemic, it is important to understand the factors that contributed to adverse outcomes to better prepare for future challenges and improve the delivery of care,” Suman Pal, MBBS, of the University of New Mexico, Albuquerque, said in an interview.
“The findings are not surprising considering what is known about the multitude of factors that determine outcomes for our patients from medical comorbidities, and social determinants of health to upstream structural factors such as systemic inequities and generational trauma,” said Dr. Pal, who was not involved with the study. “Thus, a simple correlation of the number of ICU beds to COVID-19 outcomes is not likely to capture the interplay of all these factors.”
The challenges of the pandemic offer insights to inform future planning, said Dr. Pal.
“In my opinion, a key factor to understand and address would be employee wellness for health care workers,” he said. “The problem of burnout leading to health care workers leaving the workforce has exacerbated the already acute shortages in personnel in recent years.
“In the long term, it may be prudent to reconsider the approach to health by increasing support for preventative and primary care, addressing social factors such as education, nutrition, and housing, to mitigate preventable aspects of diseases.”
Further research is needed to examine the multitude of factors associated with the pandemic, and their interplay, said Dr. Pal. The goals of such research “would be needed to develop a deeper understanding of the factors that contributed to mortality in COVID-19 and the disparities with this across different subpopulations.”
The study received no outside funding. The researchers and Dr. Pal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contrary to popular belief, no association appeared between the number of intensive care unit beds and COVID-19 deaths, based on a review of data from all 50 states between March 1, 2020, and June 30, 2021.
One of the reasons for poor patient outcomes in the early months of the COVID-19 pandemic was the presumed scarcity of ICU beds, Omar Haider, MD, of Houston Methodist Hospital, and colleagues said. “We hypothesized that the states having a lower number of ICU beds had more COVID-related deaths when compared to the states that had a higher number of ICU beds,” they wrote in an abstract presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
According to the researchers, the total number of ICU beds in the United States is approximately 85,000. Hawaii has the highest number of beds per 10,000 persons, and the District of Columbia has the lowest (6.0 vs. 1.6).
The researchers collected data on ICU bed totals from the Kaiser Family Foundation. Statistics on COVID-19 deaths were obtained from The New York Times database, which provided real-time information collected from the Department of Health & Human Services, the Centers for Disease Control and Prevention, and the Census Bureau.
The researchers used the Pearson Correlation Coefficient to compare ICU beds and COVID deaths per 10,000 persons in each state. The R value was 0.29, which indicates no inverse correlation. “Our value of R2, the coefficient of determination, was 0.0858,” they added. They confirmed the results using the Spearman’s Rho, which yielded an rs of 0.3, also a sign of no inverse correlation. No correlation was found between low numbers of ICU beds and high numbers of COVID-19 deaths for any states.
The study findings were limited by several factors, including the lack of standardized reporting timelines across states, differences in state-based vaccination rates, the emergence of the Delta variant during the study period, and time-lag in contemporaneous database updates, the researchers noted.
However, the results suggest that physical ICU beds do not play a role in determining the number of COVID-related deaths. Instead, “other constraints such as less staffing, lack of medical supplies (ventilators and [personal protective equipment]) should be evaluated for potential implications on poor patients’ outcomes,” they concluded.
Pandemic challenges can inform future plans
“As the health care system emerges from the effects of the pandemic, it is important to understand the factors that contributed to adverse outcomes to better prepare for future challenges and improve the delivery of care,” Suman Pal, MBBS, of the University of New Mexico, Albuquerque, said in an interview.
“The findings are not surprising considering what is known about the multitude of factors that determine outcomes for our patients from medical comorbidities, and social determinants of health to upstream structural factors such as systemic inequities and generational trauma,” said Dr. Pal, who was not involved with the study. “Thus, a simple correlation of the number of ICU beds to COVID-19 outcomes is not likely to capture the interplay of all these factors.”
The challenges of the pandemic offer insights to inform future planning, said Dr. Pal.
“In my opinion, a key factor to understand and address would be employee wellness for health care workers,” he said. “The problem of burnout leading to health care workers leaving the workforce has exacerbated the already acute shortages in personnel in recent years.
“In the long term, it may be prudent to reconsider the approach to health by increasing support for preventative and primary care, addressing social factors such as education, nutrition, and housing, to mitigate preventable aspects of diseases.”
Further research is needed to examine the multitude of factors associated with the pandemic, and their interplay, said Dr. Pal. The goals of such research “would be needed to develop a deeper understanding of the factors that contributed to mortality in COVID-19 and the disparities with this across different subpopulations.”
The study received no outside funding. The researchers and Dr. Pal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Contrary to popular belief, no association appeared between the number of intensive care unit beds and COVID-19 deaths, based on a review of data from all 50 states between March 1, 2020, and June 30, 2021.
One of the reasons for poor patient outcomes in the early months of the COVID-19 pandemic was the presumed scarcity of ICU beds, Omar Haider, MD, of Houston Methodist Hospital, and colleagues said. “We hypothesized that the states having a lower number of ICU beds had more COVID-related deaths when compared to the states that had a higher number of ICU beds,” they wrote in an abstract presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
According to the researchers, the total number of ICU beds in the United States is approximately 85,000. Hawaii has the highest number of beds per 10,000 persons, and the District of Columbia has the lowest (6.0 vs. 1.6).
The researchers collected data on ICU bed totals from the Kaiser Family Foundation. Statistics on COVID-19 deaths were obtained from The New York Times database, which provided real-time information collected from the Department of Health & Human Services, the Centers for Disease Control and Prevention, and the Census Bureau.
The researchers used the Pearson Correlation Coefficient to compare ICU beds and COVID deaths per 10,000 persons in each state. The R value was 0.29, which indicates no inverse correlation. “Our value of R2, the coefficient of determination, was 0.0858,” they added. They confirmed the results using the Spearman’s Rho, which yielded an rs of 0.3, also a sign of no inverse correlation. No correlation was found between low numbers of ICU beds and high numbers of COVID-19 deaths for any states.
The study findings were limited by several factors, including the lack of standardized reporting timelines across states, differences in state-based vaccination rates, the emergence of the Delta variant during the study period, and time-lag in contemporaneous database updates, the researchers noted.
However, the results suggest that physical ICU beds do not play a role in determining the number of COVID-related deaths. Instead, “other constraints such as less staffing, lack of medical supplies (ventilators and [personal protective equipment]) should be evaluated for potential implications on poor patients’ outcomes,” they concluded.
Pandemic challenges can inform future plans
“As the health care system emerges from the effects of the pandemic, it is important to understand the factors that contributed to adverse outcomes to better prepare for future challenges and improve the delivery of care,” Suman Pal, MBBS, of the University of New Mexico, Albuquerque, said in an interview.
“The findings are not surprising considering what is known about the multitude of factors that determine outcomes for our patients from medical comorbidities, and social determinants of health to upstream structural factors such as systemic inequities and generational trauma,” said Dr. Pal, who was not involved with the study. “Thus, a simple correlation of the number of ICU beds to COVID-19 outcomes is not likely to capture the interplay of all these factors.”
The challenges of the pandemic offer insights to inform future planning, said Dr. Pal.
“In my opinion, a key factor to understand and address would be employee wellness for health care workers,” he said. “The problem of burnout leading to health care workers leaving the workforce has exacerbated the already acute shortages in personnel in recent years.
“In the long term, it may be prudent to reconsider the approach to health by increasing support for preventative and primary care, addressing social factors such as education, nutrition, and housing, to mitigate preventable aspects of diseases.”
Further research is needed to examine the multitude of factors associated with the pandemic, and their interplay, said Dr. Pal. The goals of such research “would be needed to develop a deeper understanding of the factors that contributed to mortality in COVID-19 and the disparities with this across different subpopulations.”
The study received no outside funding. The researchers and Dr. Pal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases rise again, but more slowly
New cases of COVID-19 in U.S. children went up for a second consecutive week, but the pace of increase slowed considerably, based on a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week’s count – about 33,000 new COVID cases for April 8-14 – was almost 30% higher than the week before and marked the first rise in incidence after 11 straight weeks of declines, the AAP and CHA said in their weekly COVID-19 report, which is based on data from state and territorial health departments.
The cumulative number of child COVID-19 cases since the start of the pandemic is now over 12.9 million, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention, which uses a different age range for children (0-17 years) than many states, reports corresponding figures of 12.4 million and 17.6%, along with 1,501 deaths.
ED visits show a similar rising trend over recent weeks, as the 7-day average of ED visits with confirmed COVID has crept up from 0.5% in late March/early April to 0.8% on April 22 for children aged 0-11 years, from 0.3% for 0.5% for those aged 12-15, and from 0.3% to 0.6% for 16- and 17-year-olds, based on CDC data.
The daily rate for new admissions for children with confirmed COVID has also moved up slightly, rising from 0.13 per 100,000 population as late as April 13 to 0.15 per 100,000 on April 23. For the number of actual admissions, the latest 7-day (April 17-23) average was 107 in children aged 0-17, compared with 102 for the week of April 10-16, the CDC reported.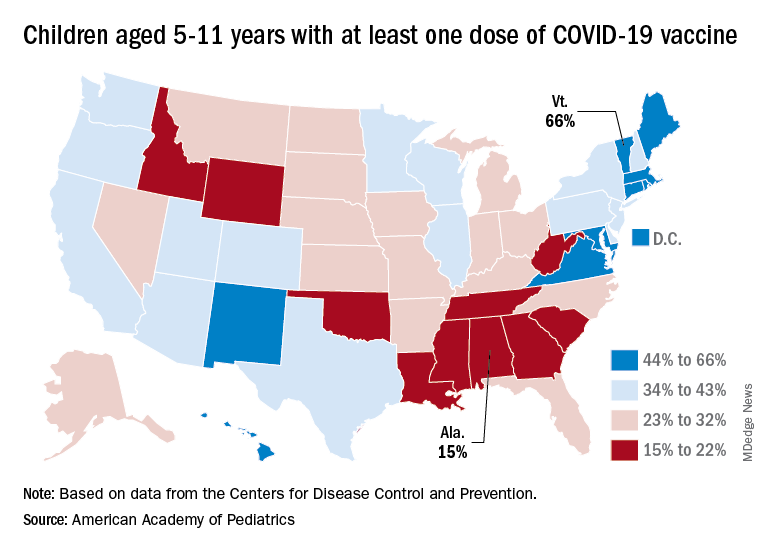
Uptake of the COVID vaccine, however, continued to slide since spiking in January. Initial vaccinations for the latest available week (April 14-20) were down to 48,000 from 59,000 the week before in children aged 5-11 years and 35,000 (vs. 47,000) for those aged 12-17. The weekly highs hit 500,000 and 331,000, respectively, during the Omicron surge, the AAP reported based on CDC data.
Among children aged 5-11, the CDC said that 35.0% had received at least one dose of COVID vaccine as of April 25 and that 28.3% are fully vaccinated, with corresponding figures of 68.8% and 58.8% for 12- to 17-year-olds on April 25.
Among the states, the highest vaccination rates generally are found in New England and the lowest in the Southeast. In Alabama, just 15% of children aged 5-11 have received an initial dose of the vaccine, compared with 66% in Vermont, while Wyoming is the lowest (41%) for children aged 12-17 and Massachusetts is the highest (96%), the AAP said in a separate report.
New cases of COVID-19 in U.S. children went up for a second consecutive week, but the pace of increase slowed considerably, based on a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week’s count – about 33,000 new COVID cases for April 8-14 – was almost 30% higher than the week before and marked the first rise in incidence after 11 straight weeks of declines, the AAP and CHA said in their weekly COVID-19 report, which is based on data from state and territorial health departments.
The cumulative number of child COVID-19 cases since the start of the pandemic is now over 12.9 million, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention, which uses a different age range for children (0-17 years) than many states, reports corresponding figures of 12.4 million and 17.6%, along with 1,501 deaths.
ED visits show a similar rising trend over recent weeks, as the 7-day average of ED visits with confirmed COVID has crept up from 0.5% in late March/early April to 0.8% on April 22 for children aged 0-11 years, from 0.3% for 0.5% for those aged 12-15, and from 0.3% to 0.6% for 16- and 17-year-olds, based on CDC data.
The daily rate for new admissions for children with confirmed COVID has also moved up slightly, rising from 0.13 per 100,000 population as late as April 13 to 0.15 per 100,000 on April 23. For the number of actual admissions, the latest 7-day (April 17-23) average was 107 in children aged 0-17, compared with 102 for the week of April 10-16, the CDC reported.
Uptake of the COVID vaccine, however, continued to slide since spiking in January. Initial vaccinations for the latest available week (April 14-20) were down to 48,000 from 59,000 the week before in children aged 5-11 years and 35,000 (vs. 47,000) for those aged 12-17. The weekly highs hit 500,000 and 331,000, respectively, during the Omicron surge, the AAP reported based on CDC data.
Among children aged 5-11, the CDC said that 35.0% had received at least one dose of COVID vaccine as of April 25 and that 28.3% are fully vaccinated, with corresponding figures of 68.8% and 58.8% for 12- to 17-year-olds on April 25.
Among the states, the highest vaccination rates generally are found in New England and the lowest in the Southeast. In Alabama, just 15% of children aged 5-11 have received an initial dose of the vaccine, compared with 66% in Vermont, while Wyoming is the lowest (41%) for children aged 12-17 and Massachusetts is the highest (96%), the AAP said in a separate report.
New cases of COVID-19 in U.S. children went up for a second consecutive week, but the pace of increase slowed considerably, based on a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week’s count – about 33,000 new COVID cases for April 8-14 – was almost 30% higher than the week before and marked the first rise in incidence after 11 straight weeks of declines, the AAP and CHA said in their weekly COVID-19 report, which is based on data from state and territorial health departments.
The cumulative number of child COVID-19 cases since the start of the pandemic is now over 12.9 million, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention, which uses a different age range for children (0-17 years) than many states, reports corresponding figures of 12.4 million and 17.6%, along with 1,501 deaths.
ED visits show a similar rising trend over recent weeks, as the 7-day average of ED visits with confirmed COVID has crept up from 0.5% in late March/early April to 0.8% on April 22 for children aged 0-11 years, from 0.3% for 0.5% for those aged 12-15, and from 0.3% to 0.6% for 16- and 17-year-olds, based on CDC data.
The daily rate for new admissions for children with confirmed COVID has also moved up slightly, rising from 0.13 per 100,000 population as late as April 13 to 0.15 per 100,000 on April 23. For the number of actual admissions, the latest 7-day (April 17-23) average was 107 in children aged 0-17, compared with 102 for the week of April 10-16, the CDC reported.
Uptake of the COVID vaccine, however, continued to slide since spiking in January. Initial vaccinations for the latest available week (April 14-20) were down to 48,000 from 59,000 the week before in children aged 5-11 years and 35,000 (vs. 47,000) for those aged 12-17. The weekly highs hit 500,000 and 331,000, respectively, during the Omicron surge, the AAP reported based on CDC data.
Among children aged 5-11, the CDC said that 35.0% had received at least one dose of COVID vaccine as of April 25 and that 28.3% are fully vaccinated, with corresponding figures of 68.8% and 58.8% for 12- to 17-year-olds on April 25.
Among the states, the highest vaccination rates generally are found in New England and the lowest in the Southeast. In Alabama, just 15% of children aged 5-11 have received an initial dose of the vaccine, compared with 66% in Vermont, while Wyoming is the lowest (41%) for children aged 12-17 and Massachusetts is the highest (96%), the AAP said in a separate report.