User login
Etanercept biosimilar SB4 a cost-effective alternative for psoriasis, PsA
The development of biosimilars such as etanercept SB4 offers a “significant opportunity to decrease medical care cost and increase treatment options,” Alessandro Giunta, MD, of the department of dermatology at the University of Rome Tor Vergata, and associates reported in a letter to the editor in the British Journal of Dermatology.
Dr. Giunta and his associates performed an observational, retrospective, single-center study to investigate etanercept biosimilar SB4 in patients being treated for plaque type psoriasis and psoriatic arthritis (PsA). They evaluated 40 patients – 21 men and 19 women – mean age 55, ranging from 19 to 79 years. The patients received the etanercept biosimilar SB4 between Oct. 21, 2016, and March 31, 2017, at University of Rome Tor Vergata’s department of dermatology. (The etanercept biosimilar SB4 was approved April 29 by the Food and Drug Administration under the brand name Eticovo [etanercept-ykro]. It is also approved in other countries under the names Benepali and Brenzys.)
Accounting for erythrocyte sedimentation rate as a variable, Dr. Giunta and colleagues calculated disease activity scores based on 28 joints; 14 patients (35%) had plaque psoriasis (mean Psoriasis Area Severity Index [PASI] of 9.61 at baseline), while 26 (65%) had psoriatic arthritis (mean PASI, 4.69). All patients reported prior treatment with systemic conventional and biologic treatments. A group of 10 patients (25%) who had been previously treated with etanercept originator underwent an intermittent treatment regimen of 24 weeks with etanercept biosimilar, which was interrupted once clinical resolution was achieved. No treatments were prescribed between etanercept originator and etanercept biosimilar. Mean exposure was 50.4 weeks, ranging from 24 to 96 weeks, with an average washout period of 12.1 weeks from originator to biosimilar (range 8-24).
A significant improvement in mean PASI score was observed in plaque type psoriasis patients as well as psoriatic arthritis patients at week 24 (P less than .0001 and P less than .001, respectively), noted Dr. Giunta and associates.
“All scores achieved a statistical significant improvement with the exception of [swollen joint count] that markedly improved but not significantly,” they added. One patient experienced injection site reaction, but no serious adverse events were observed.
Despite low sample size and limited follow-up time, the authors concluded that etanercept biosimilar achieved effectiveness as a treatment for psoriatic patients even in cases involving previous exposure to originator etanercept. Cost savings of 61.58% for 50-mg treatment and 62.55% for 25-mg treatment respectively guaranteed “the continuity of etanercept-treated patients’ care and gave us the opportunity to allocate patients in innovative but more expensive agents with marginal increase in our annual budget,” they noted.
The authors reported serving as consultants and speakers for AbbVie, Biogen, Eli Lilly, Janssen, Pfizer, and Novartis.
SOURCE: Giunta A et al. Br J Dermatol. 2019 May 3. doi: 10.1111/bjd.18090.
The development of biosimilars such as etanercept SB4 offers a “significant opportunity to decrease medical care cost and increase treatment options,” Alessandro Giunta, MD, of the department of dermatology at the University of Rome Tor Vergata, and associates reported in a letter to the editor in the British Journal of Dermatology.
Dr. Giunta and his associates performed an observational, retrospective, single-center study to investigate etanercept biosimilar SB4 in patients being treated for plaque type psoriasis and psoriatic arthritis (PsA). They evaluated 40 patients – 21 men and 19 women – mean age 55, ranging from 19 to 79 years. The patients received the etanercept biosimilar SB4 between Oct. 21, 2016, and March 31, 2017, at University of Rome Tor Vergata’s department of dermatology. (The etanercept biosimilar SB4 was approved April 29 by the Food and Drug Administration under the brand name Eticovo [etanercept-ykro]. It is also approved in other countries under the names Benepali and Brenzys.)
Accounting for erythrocyte sedimentation rate as a variable, Dr. Giunta and colleagues calculated disease activity scores based on 28 joints; 14 patients (35%) had plaque psoriasis (mean Psoriasis Area Severity Index [PASI] of 9.61 at baseline), while 26 (65%) had psoriatic arthritis (mean PASI, 4.69). All patients reported prior treatment with systemic conventional and biologic treatments. A group of 10 patients (25%) who had been previously treated with etanercept originator underwent an intermittent treatment regimen of 24 weeks with etanercept biosimilar, which was interrupted once clinical resolution was achieved. No treatments were prescribed between etanercept originator and etanercept biosimilar. Mean exposure was 50.4 weeks, ranging from 24 to 96 weeks, with an average washout period of 12.1 weeks from originator to biosimilar (range 8-24).
A significant improvement in mean PASI score was observed in plaque type psoriasis patients as well as psoriatic arthritis patients at week 24 (P less than .0001 and P less than .001, respectively), noted Dr. Giunta and associates.
“All scores achieved a statistical significant improvement with the exception of [swollen joint count] that markedly improved but not significantly,” they added. One patient experienced injection site reaction, but no serious adverse events were observed.
Despite low sample size and limited follow-up time, the authors concluded that etanercept biosimilar achieved effectiveness as a treatment for psoriatic patients even in cases involving previous exposure to originator etanercept. Cost savings of 61.58% for 50-mg treatment and 62.55% for 25-mg treatment respectively guaranteed “the continuity of etanercept-treated patients’ care and gave us the opportunity to allocate patients in innovative but more expensive agents with marginal increase in our annual budget,” they noted.
The authors reported serving as consultants and speakers for AbbVie, Biogen, Eli Lilly, Janssen, Pfizer, and Novartis.
SOURCE: Giunta A et al. Br J Dermatol. 2019 May 3. doi: 10.1111/bjd.18090.
The development of biosimilars such as etanercept SB4 offers a “significant opportunity to decrease medical care cost and increase treatment options,” Alessandro Giunta, MD, of the department of dermatology at the University of Rome Tor Vergata, and associates reported in a letter to the editor in the British Journal of Dermatology.
Dr. Giunta and his associates performed an observational, retrospective, single-center study to investigate etanercept biosimilar SB4 in patients being treated for plaque type psoriasis and psoriatic arthritis (PsA). They evaluated 40 patients – 21 men and 19 women – mean age 55, ranging from 19 to 79 years. The patients received the etanercept biosimilar SB4 between Oct. 21, 2016, and March 31, 2017, at University of Rome Tor Vergata’s department of dermatology. (The etanercept biosimilar SB4 was approved April 29 by the Food and Drug Administration under the brand name Eticovo [etanercept-ykro]. It is also approved in other countries under the names Benepali and Brenzys.)
Accounting for erythrocyte sedimentation rate as a variable, Dr. Giunta and colleagues calculated disease activity scores based on 28 joints; 14 patients (35%) had plaque psoriasis (mean Psoriasis Area Severity Index [PASI] of 9.61 at baseline), while 26 (65%) had psoriatic arthritis (mean PASI, 4.69). All patients reported prior treatment with systemic conventional and biologic treatments. A group of 10 patients (25%) who had been previously treated with etanercept originator underwent an intermittent treatment regimen of 24 weeks with etanercept biosimilar, which was interrupted once clinical resolution was achieved. No treatments were prescribed between etanercept originator and etanercept biosimilar. Mean exposure was 50.4 weeks, ranging from 24 to 96 weeks, with an average washout period of 12.1 weeks from originator to biosimilar (range 8-24).
A significant improvement in mean PASI score was observed in plaque type psoriasis patients as well as psoriatic arthritis patients at week 24 (P less than .0001 and P less than .001, respectively), noted Dr. Giunta and associates.
“All scores achieved a statistical significant improvement with the exception of [swollen joint count] that markedly improved but not significantly,” they added. One patient experienced injection site reaction, but no serious adverse events were observed.
Despite low sample size and limited follow-up time, the authors concluded that etanercept biosimilar achieved effectiveness as a treatment for psoriatic patients even in cases involving previous exposure to originator etanercept. Cost savings of 61.58% for 50-mg treatment and 62.55% for 25-mg treatment respectively guaranteed “the continuity of etanercept-treated patients’ care and gave us the opportunity to allocate patients in innovative but more expensive agents with marginal increase in our annual budget,” they noted.
The authors reported serving as consultants and speakers for AbbVie, Biogen, Eli Lilly, Janssen, Pfizer, and Novartis.
SOURCE: Giunta A et al. Br J Dermatol. 2019 May 3. doi: 10.1111/bjd.18090.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
PsA Fast Facts: Prevalence and incidence



Weight loss improves psoriatic arthritis
MAUI, HAWAII – Serious weight loss brings big improvement in psoriatic arthritis in obese patients, at least short term, according to a Swedish, single-arm, prospective, proof-of-concept study.
A dose-response effect was evident: the greater the lost poundage, the bigger the improvement across multiple dimensions of psoriatic arthritis.
The short-term efficacy was eye-catching, especially in view of the well-recognized increased prevalence of obesity in psoriatic arthritis patients. But the jury is still out as to the long-term impact of this nonpharmacologic therapy, Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He has spoken with the Swedish investigators and was happy to learn they’re continuing to follow study participants long term.
“That’s going to be the key, right? Because if you do this for 12 weeks, like every other fad crash diet, and then you let the weight go right back on again, you haven’t really accomplished anything. I think the key will be what happens at a year,” according to Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
The study included 46 obese psoriatic arthritis patients who signed on for a structured, medically supervised very-low-energy diet lasting 12-16 weeks, depending upon their baseline obesity level. The commercially available liquid diet (Cambridge Weight Plan Limited) is a type of therapy widely prescribed by Swedish physicians, clocking in at a mere 640 kcal/day.
“I don’t know about you, but I ate that at breakfast this morning,” quipped symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
Following completion of the strict very-low-energy diet, patients were gradually reintroduced to a less-draconian, solid-food, energy-restricted diet, to be followed through the 12-month mark. The full 12-month protocol was supervised by staff in the obesity unit at Sahlgrenska University Hospital in Gothenburg, Sweden. The 12-month results will be presented at the annual European Congress of Rheumatology in Madrid.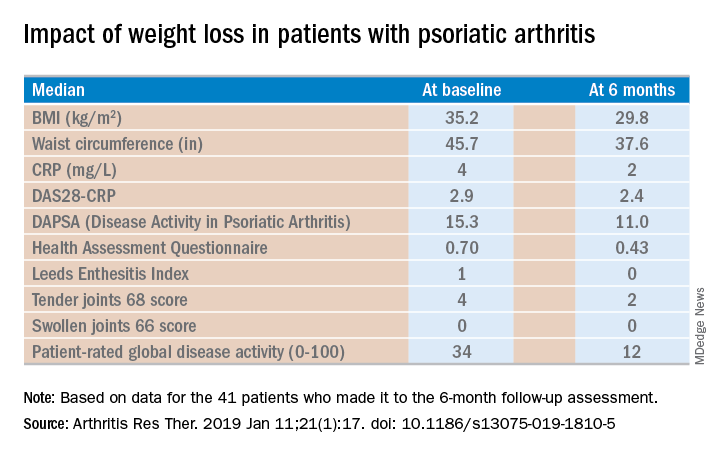
Of the 46 starters, 41 made it to the 6-month follow-up assessment. At that point they’d lost a median of 18.2 kg, or 18.6% of their baseline body weight. Their body mass index had dropped from an average of 35.2 to 29.8 kg/m2. And their psoriatic arthritis had improved significantly. For example, their median Disease Activity Score using 28 joint counts based upon C-reactive protein (DAS28-CRP) decreased from 2.9 at baseline to 2.4 at 6 months, with ACR 20, -50, and -70 responses of 51.2%, 34.1%, and 7.3% while disease-directed medications were held constant (Arthritis Res Ther. 2019 Jan 11;21[1]:17. doi: 10.1186/s13075-019-1810-5).
The investigators reported the very-low-energy diet phase was generally well tolerated. A total of 34 of the 41 patients deemed it “easier or much easier” than expected, prompting Dr. Ruderman to comment: “Because they thought it was going to be awful.”
Dr. Ruderman and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Serious weight loss brings big improvement in psoriatic arthritis in obese patients, at least short term, according to a Swedish, single-arm, prospective, proof-of-concept study.
A dose-response effect was evident: the greater the lost poundage, the bigger the improvement across multiple dimensions of psoriatic arthritis.
The short-term efficacy was eye-catching, especially in view of the well-recognized increased prevalence of obesity in psoriatic arthritis patients. But the jury is still out as to the long-term impact of this nonpharmacologic therapy, Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He has spoken with the Swedish investigators and was happy to learn they’re continuing to follow study participants long term.
“That’s going to be the key, right? Because if you do this for 12 weeks, like every other fad crash diet, and then you let the weight go right back on again, you haven’t really accomplished anything. I think the key will be what happens at a year,” according to Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
The study included 46 obese psoriatic arthritis patients who signed on for a structured, medically supervised very-low-energy diet lasting 12-16 weeks, depending upon their baseline obesity level. The commercially available liquid diet (Cambridge Weight Plan Limited) is a type of therapy widely prescribed by Swedish physicians, clocking in at a mere 640 kcal/day.
“I don’t know about you, but I ate that at breakfast this morning,” quipped symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
Following completion of the strict very-low-energy diet, patients were gradually reintroduced to a less-draconian, solid-food, energy-restricted diet, to be followed through the 12-month mark. The full 12-month protocol was supervised by staff in the obesity unit at Sahlgrenska University Hospital in Gothenburg, Sweden. The 12-month results will be presented at the annual European Congress of Rheumatology in Madrid.
Of the 46 starters, 41 made it to the 6-month follow-up assessment. At that point they’d lost a median of 18.2 kg, or 18.6% of their baseline body weight. Their body mass index had dropped from an average of 35.2 to 29.8 kg/m2. And their psoriatic arthritis had improved significantly. For example, their median Disease Activity Score using 28 joint counts based upon C-reactive protein (DAS28-CRP) decreased from 2.9 at baseline to 2.4 at 6 months, with ACR 20, -50, and -70 responses of 51.2%, 34.1%, and 7.3% while disease-directed medications were held constant (Arthritis Res Ther. 2019 Jan 11;21[1]:17. doi: 10.1186/s13075-019-1810-5).
The investigators reported the very-low-energy diet phase was generally well tolerated. A total of 34 of the 41 patients deemed it “easier or much easier” than expected, prompting Dr. Ruderman to comment: “Because they thought it was going to be awful.”
Dr. Ruderman and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – Serious weight loss brings big improvement in psoriatic arthritis in obese patients, at least short term, according to a Swedish, single-arm, prospective, proof-of-concept study.
A dose-response effect was evident: the greater the lost poundage, the bigger the improvement across multiple dimensions of psoriatic arthritis.
The short-term efficacy was eye-catching, especially in view of the well-recognized increased prevalence of obesity in psoriatic arthritis patients. But the jury is still out as to the long-term impact of this nonpharmacologic therapy, Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He has spoken with the Swedish investigators and was happy to learn they’re continuing to follow study participants long term.
“That’s going to be the key, right? Because if you do this for 12 weeks, like every other fad crash diet, and then you let the weight go right back on again, you haven’t really accomplished anything. I think the key will be what happens at a year,” according to Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University, Chicago.
The study included 46 obese psoriatic arthritis patients who signed on for a structured, medically supervised very-low-energy diet lasting 12-16 weeks, depending upon their baseline obesity level. The commercially available liquid diet (Cambridge Weight Plan Limited) is a type of therapy widely prescribed by Swedish physicians, clocking in at a mere 640 kcal/day.
“I don’t know about you, but I ate that at breakfast this morning,” quipped symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
Following completion of the strict very-low-energy diet, patients were gradually reintroduced to a less-draconian, solid-food, energy-restricted diet, to be followed through the 12-month mark. The full 12-month protocol was supervised by staff in the obesity unit at Sahlgrenska University Hospital in Gothenburg, Sweden. The 12-month results will be presented at the annual European Congress of Rheumatology in Madrid.
Of the 46 starters, 41 made it to the 6-month follow-up assessment. At that point they’d lost a median of 18.2 kg, or 18.6% of their baseline body weight. Their body mass index had dropped from an average of 35.2 to 29.8 kg/m2. And their psoriatic arthritis had improved significantly. For example, their median Disease Activity Score using 28 joint counts based upon C-reactive protein (DAS28-CRP) decreased from 2.9 at baseline to 2.4 at 6 months, with ACR 20, -50, and -70 responses of 51.2%, 34.1%, and 7.3% while disease-directed medications were held constant (Arthritis Res Ther. 2019 Jan 11;21[1]:17. doi: 10.1186/s13075-019-1810-5).
The investigators reported the very-low-energy diet phase was generally well tolerated. A total of 34 of the 41 patients deemed it “easier or much easier” than expected, prompting Dr. Ruderman to comment: “Because they thought it was going to be awful.”
Dr. Ruderman and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
PsA patients had durable responses after 1 year of IV golimumab treatment
, according to follow-up results of a randomized clinical trial.
The improvements in joint disease, skin disease, and health-related quality of life seen at 24 weeks in the phase 3 GO-VIBRANT study were maintained at this 52-week follow-up, according to M. Elaine Husni, MD, of the Cleveland Clinic, and coinvestigators.
Patients who crossed over to golimumab treatment after 24 weeks of placebo had similar rates of clinical response at 52 weeks, while patients receiving concomitant methotrexate had similar ACR response rates, compared with patients on golimumab monotherapy, Dr. Husni and colleagues reported.
Many patients who were not ACR20 responders at week 52 nevertheless had improvements in skin disease, enthesitis, and dactylitis, an exploratory analysis showed.
“These factors may have contributed to these patients remaining in the trial and continuing golimumab therapy despite not achieving an ACR20 response,” wrote Dr. Husni and coauthors. The report is in Arthritis Care & Research.
The Food and Drug Administration approved a once-monthly subcutaneous formulation of golimumab (Simponi) in 2009 for treatment of moderate to severe active psoriatic arthritis, rheumatoid arthritis, and active ankylosing spondylitis. The intravenous formulation of this TNF inhibitor (Simponi Aria) received a psoriatic arthritis indication in 2017 based on GO-VIBRANT data. Published results at the time showed that compared with placebo, intravenous golimumab given as a 2-mg/kg infusion at weeks 0, 4, and then every 8 weeks produced greater improvements in psoriatic arthritis signs and symptoms and less radiographic progression through week 24 of the study, and had adverse events consistent with other TNF inhibitors, according to investigators.
The follow-up report includes efficacy and safety data for golimumab-treated patients beyond 24 weeks, as well as data for patients on the placebo arm, who crossed over to receive golimumab at week 24, week 28, and then every 8 weeks thereafter.
The results show ACR response rates were maintained from week 24 to 52 in golimumab-treated patients, and were similar in the placebo crossover patients. The ACR20, ACR50, and ACR70 response rates in the golimumab group were 76.8%, 58.1%, and 38.6%, respectively, while in the crossover group, they were 77.0%, 53.6%, and 33.9%, respectively.
Radiographic progression was measured using van der Heijde-Sharp (vdH-S) score with modifications for psoriatic arthritis. The mean change in vdH-S score at 24 weeks was –0.4 and 2.0 in the golimumab and placebo groups, respectively; by week 52, the mean change was –0.5 and 0.8 for golimumab and placebo crossover.
Infection was the most common adverse event throughout 60 weeks of safety evaluation, occurring in 22.8% of all golimumab-treated patients, investigators said. Four infusion reactions occurred following golimumab administration, though none were considered serious or severe.
The GO-VIBRANT study, which comprised 480 adults, had limited follow-up and was not powered to identify rare safety events, investigators said.
“However, the totality of results through 1 year of the GO-VIBRANT study show a durable response to IV golimumab 2 mg/kg across several clinical efficacy, HRQoL, and radiographic endpoints with no new safety signals,” they concluded.
Study authors reported disclosures with AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Horizon, Janssen, Novartis, Pfizer, Sanofi, and UCB. Several study authors reported current or former employment with Janssen Research & Development and stock or stock options in Johnson & Johnson.
SOURCE: Husni ME et al. Arthritis Care Res. 2019 Apr 12. doi: 10.1002/acr.23905.
, according to follow-up results of a randomized clinical trial.
The improvements in joint disease, skin disease, and health-related quality of life seen at 24 weeks in the phase 3 GO-VIBRANT study were maintained at this 52-week follow-up, according to M. Elaine Husni, MD, of the Cleveland Clinic, and coinvestigators.
Patients who crossed over to golimumab treatment after 24 weeks of placebo had similar rates of clinical response at 52 weeks, while patients receiving concomitant methotrexate had similar ACR response rates, compared with patients on golimumab monotherapy, Dr. Husni and colleagues reported.
Many patients who were not ACR20 responders at week 52 nevertheless had improvements in skin disease, enthesitis, and dactylitis, an exploratory analysis showed.
“These factors may have contributed to these patients remaining in the trial and continuing golimumab therapy despite not achieving an ACR20 response,” wrote Dr. Husni and coauthors. The report is in Arthritis Care & Research.
The Food and Drug Administration approved a once-monthly subcutaneous formulation of golimumab (Simponi) in 2009 for treatment of moderate to severe active psoriatic arthritis, rheumatoid arthritis, and active ankylosing spondylitis. The intravenous formulation of this TNF inhibitor (Simponi Aria) received a psoriatic arthritis indication in 2017 based on GO-VIBRANT data. Published results at the time showed that compared with placebo, intravenous golimumab given as a 2-mg/kg infusion at weeks 0, 4, and then every 8 weeks produced greater improvements in psoriatic arthritis signs and symptoms and less radiographic progression through week 24 of the study, and had adverse events consistent with other TNF inhibitors, according to investigators.
The follow-up report includes efficacy and safety data for golimumab-treated patients beyond 24 weeks, as well as data for patients on the placebo arm, who crossed over to receive golimumab at week 24, week 28, and then every 8 weeks thereafter.
The results show ACR response rates were maintained from week 24 to 52 in golimumab-treated patients, and were similar in the placebo crossover patients. The ACR20, ACR50, and ACR70 response rates in the golimumab group were 76.8%, 58.1%, and 38.6%, respectively, while in the crossover group, they were 77.0%, 53.6%, and 33.9%, respectively.
Radiographic progression was measured using van der Heijde-Sharp (vdH-S) score with modifications for psoriatic arthritis. The mean change in vdH-S score at 24 weeks was –0.4 and 2.0 in the golimumab and placebo groups, respectively; by week 52, the mean change was –0.5 and 0.8 for golimumab and placebo crossover.
Infection was the most common adverse event throughout 60 weeks of safety evaluation, occurring in 22.8% of all golimumab-treated patients, investigators said. Four infusion reactions occurred following golimumab administration, though none were considered serious or severe.
The GO-VIBRANT study, which comprised 480 adults, had limited follow-up and was not powered to identify rare safety events, investigators said.
“However, the totality of results through 1 year of the GO-VIBRANT study show a durable response to IV golimumab 2 mg/kg across several clinical efficacy, HRQoL, and radiographic endpoints with no new safety signals,” they concluded.
Study authors reported disclosures with AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Horizon, Janssen, Novartis, Pfizer, Sanofi, and UCB. Several study authors reported current or former employment with Janssen Research & Development and stock or stock options in Johnson & Johnson.
SOURCE: Husni ME et al. Arthritis Care Res. 2019 Apr 12. doi: 10.1002/acr.23905.
, according to follow-up results of a randomized clinical trial.
The improvements in joint disease, skin disease, and health-related quality of life seen at 24 weeks in the phase 3 GO-VIBRANT study were maintained at this 52-week follow-up, according to M. Elaine Husni, MD, of the Cleveland Clinic, and coinvestigators.
Patients who crossed over to golimumab treatment after 24 weeks of placebo had similar rates of clinical response at 52 weeks, while patients receiving concomitant methotrexate had similar ACR response rates, compared with patients on golimumab monotherapy, Dr. Husni and colleagues reported.
Many patients who were not ACR20 responders at week 52 nevertheless had improvements in skin disease, enthesitis, and dactylitis, an exploratory analysis showed.
“These factors may have contributed to these patients remaining in the trial and continuing golimumab therapy despite not achieving an ACR20 response,” wrote Dr. Husni and coauthors. The report is in Arthritis Care & Research.
The Food and Drug Administration approved a once-monthly subcutaneous formulation of golimumab (Simponi) in 2009 for treatment of moderate to severe active psoriatic arthritis, rheumatoid arthritis, and active ankylosing spondylitis. The intravenous formulation of this TNF inhibitor (Simponi Aria) received a psoriatic arthritis indication in 2017 based on GO-VIBRANT data. Published results at the time showed that compared with placebo, intravenous golimumab given as a 2-mg/kg infusion at weeks 0, 4, and then every 8 weeks produced greater improvements in psoriatic arthritis signs and symptoms and less radiographic progression through week 24 of the study, and had adverse events consistent with other TNF inhibitors, according to investigators.
The follow-up report includes efficacy and safety data for golimumab-treated patients beyond 24 weeks, as well as data for patients on the placebo arm, who crossed over to receive golimumab at week 24, week 28, and then every 8 weeks thereafter.
The results show ACR response rates were maintained from week 24 to 52 in golimumab-treated patients, and were similar in the placebo crossover patients. The ACR20, ACR50, and ACR70 response rates in the golimumab group were 76.8%, 58.1%, and 38.6%, respectively, while in the crossover group, they were 77.0%, 53.6%, and 33.9%, respectively.
Radiographic progression was measured using van der Heijde-Sharp (vdH-S) score with modifications for psoriatic arthritis. The mean change in vdH-S score at 24 weeks was –0.4 and 2.0 in the golimumab and placebo groups, respectively; by week 52, the mean change was –0.5 and 0.8 for golimumab and placebo crossover.
Infection was the most common adverse event throughout 60 weeks of safety evaluation, occurring in 22.8% of all golimumab-treated patients, investigators said. Four infusion reactions occurred following golimumab administration, though none were considered serious or severe.
The GO-VIBRANT study, which comprised 480 adults, had limited follow-up and was not powered to identify rare safety events, investigators said.
“However, the totality of results through 1 year of the GO-VIBRANT study show a durable response to IV golimumab 2 mg/kg across several clinical efficacy, HRQoL, and radiographic endpoints with no new safety signals,” they concluded.
Study authors reported disclosures with AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Horizon, Janssen, Novartis, Pfizer, Sanofi, and UCB. Several study authors reported current or former employment with Janssen Research & Development and stock or stock options in Johnson & Johnson.
SOURCE: Husni ME et al. Arthritis Care Res. 2019 Apr 12. doi: 10.1002/acr.23905.
FROM ARTHRITIS CARE & RESEARCH
Surprise! MTX proves effective in psoriatic arthritis
MAUI, HAWAII – The first-ever, double-blind, randomized, controlled clinical trial evidence demonstrating that methotrexate indeed has therapeutic efficacy in psoriatic arthritis has come at an awkward time – on the heels of a basically negative Cochrane Collaboration systematic review as well as the latest American College of Rheumatology/National Psoriasis Foundation guidelines for treatment of psoriatic arthritis, which recommend anti–tumor necrosis factor therapy as first line, ahead of methotrexate.
The timing of the release of the SEAM-PsA randomized trial results was such that neither the Cochrane group nor the ACR/NPF guideline committee was able to consider the new, potentially game-changing study findings.
“I look at SEAM-PsA and have to say, methotrexate does seem to be an effective therapy. I think it calls into question the new guidelines, which were developed before the data were out. Now you look at this and have to ask, can you really say you should use a TNF inhibitor before methotrexate based on these results? I don’t know,” Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He also shared other problems he has with the new guidelines, which he considers seriously flawed.
The Cochrane Collaboration Systematic Review
The Cochrane group cast a net for all randomized, controlled clinical trials of methotrexate versus placebo or another disease-modifying antirheumatic drug (DMARD). They found eight, which they judged to be of poor quality. Their conclusion: “Low-quality evidence suggests that low-dose (15 mg or less) oral methotrexate might be slightly more effective than placebo when taken for 6 months; however, we are uncertain if it is more harmful” (Cochrane Database Syst Rev. 2019 Jan 18;1:CD012722. doi: 10.1002/14651858.CD012722.pub2).
“The new Cochrane Review concludes methotrexate doesn’t seem to work that well,” observed symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
“That’s because it’s based on published data, and there’s been very little of that,” said Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago.
“I think most people assume, based on clinical experience, that it does work well. It’s all we had for years and years and years,” he added.
That is, until SEAM-PsA.
SEAM-PsA
SEAM-PsA randomized 851 DMARD- and biologic-naive patients with a median 0.6-year duration of psoriatic arthritis to one of three treatment arms for 48 weeks: once-weekly etanercept at 50 mg plus oral methotrexate at 20 mg, etanercept plus oral placebo, or methotrexate plus injectable placebo.
This is a study that will reshape clinical practice for many rheumatologists, according to Dr. Kavanaugh. The hypothesis was that in psoriatic arthritis, just as has been shown to be the case in rheumatoid arthritis, the combination of a TNF inhibitor plus methotrexate would have greater efficacy than either agent alone. But the study brought a couple of major surprises.
“Methotrexate didn’t do so badly,” Dr. Kavanaugh observed. “And the combination did nothing. I would have bet that the combination would have shown methotrexate had a synergistic effect with the TNF inhibitor, especially for x-ray changes. But the combination didn’t do any better than etanercept alone.”
Make no mistake: Etanercept monotherapy significantly outperformed methotrexate monotherapy for the primary endpoint, the ACR 20 response at week 24, by a margin of 60.9% versus 50.7%. Dr. Ruderman deemed that methotrexate response rate to be quite respectable, although he bemoaned the absence of a double-placebo comparator arm. And the key secondary endpoint, the minimal disease activity response rate at week 24, was also significantly better with etanercept, at 35.9% compared with 22.9%. Moreover, both etanercept arms showed significantly less radiographic progression than with methotrexate alone.
However, that was it. There were no significant differences between etanercept and methotrexate in other secondary endpoints, including the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC), the Disease Activity in PSoriatic Arthritis (DAPSA) score, the Leeds Dactylitis Instrument (LDI), and quality of life as assessed by the 36-item Short Form Health Survey total score.
“Methotrexate showed generally good efficacy across multiple domains,” the investigators concluded (Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851).
“Another intriguing thing to come out of this study for me were the enthesitis and dactylitis results. My clinical experience suggested methotrexate wasn’t so great for that, but this study suggests that’s not true,” Dr. Ruderman said.
“There are a couple of key take-home points from this study,” according to Dr. Kavanaugh. “One is that the combination is not synergistic. When you start a rheumatoid arthritis patient on methotrexate, you try to keep him on methotrexate when you add a TNF inhibitor. This study would say there doesn’t seem like there’s a reason to do that in your psoriatic arthritis patient. And the second message is that methotrexate seems to work.”
New ACR/NPF psoriatic arthritis guidelines under fire
“The new guidelines are fuzzy, aren’t they?” Dr. Kavanaugh said in lobbing the topic over to Dr. Ruderman.
“Where do we start?” he replied, shaking his head. “These are evidence-based guidelines in an area in which there was virtually no evidence.”
Indeed, the guidelines committee proudly employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which forces committee members to issue “conditional” recommendations when there’s not enough evidence to make a “strong” recommendation.
“You’re not allowed to say, ‘We don’t know, there’s not enough evidence to make a choice,’ ” Dr. Ruderman said. “The problem with these guidelines is virtually everything in it is a conditional recommendation except ‘stop smoking,’ which was a strong recommendation.
“A conditional recommendation is pretty much a fancy term for expert opinion. It’s basically everybody in the room saying, ‘This is what we think.’ And that makes guidelines challenging because as a rheumatologist, you’re an expert. The people in the room have perhaps looked at the data more carefully than you’ve drilled down into the studies, but ultimately they’ve taken care of these patients and you’ve taken care of these patients, so why is their opinion better than your opinion, if it’s an informed opinion?”
His other critique of the 28-page guidelines is they don’t include the reasoning behind the conditional recommendations.
“If the conditional recommendation is, ‘In this situation, a TNF inhibitor is preferred over an IL-17 inhibitor,’ that would be great if they had also said, ‘This is why we thought that.’ But that’s not in the paper,” Dr. Ruderman said.
He and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – The first-ever, double-blind, randomized, controlled clinical trial evidence demonstrating that methotrexate indeed has therapeutic efficacy in psoriatic arthritis has come at an awkward time – on the heels of a basically negative Cochrane Collaboration systematic review as well as the latest American College of Rheumatology/National Psoriasis Foundation guidelines for treatment of psoriatic arthritis, which recommend anti–tumor necrosis factor therapy as first line, ahead of methotrexate.
The timing of the release of the SEAM-PsA randomized trial results was such that neither the Cochrane group nor the ACR/NPF guideline committee was able to consider the new, potentially game-changing study findings.
“I look at SEAM-PsA and have to say, methotrexate does seem to be an effective therapy. I think it calls into question the new guidelines, which were developed before the data were out. Now you look at this and have to ask, can you really say you should use a TNF inhibitor before methotrexate based on these results? I don’t know,” Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He also shared other problems he has with the new guidelines, which he considers seriously flawed.
The Cochrane Collaboration Systematic Review
The Cochrane group cast a net for all randomized, controlled clinical trials of methotrexate versus placebo or another disease-modifying antirheumatic drug (DMARD). They found eight, which they judged to be of poor quality. Their conclusion: “Low-quality evidence suggests that low-dose (15 mg or less) oral methotrexate might be slightly more effective than placebo when taken for 6 months; however, we are uncertain if it is more harmful” (Cochrane Database Syst Rev. 2019 Jan 18;1:CD012722. doi: 10.1002/14651858.CD012722.pub2).
“The new Cochrane Review concludes methotrexate doesn’t seem to work that well,” observed symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
“That’s because it’s based on published data, and there’s been very little of that,” said Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago.
“I think most people assume, based on clinical experience, that it does work well. It’s all we had for years and years and years,” he added.
That is, until SEAM-PsA.
SEAM-PsA
SEAM-PsA randomized 851 DMARD- and biologic-naive patients with a median 0.6-year duration of psoriatic arthritis to one of three treatment arms for 48 weeks: once-weekly etanercept at 50 mg plus oral methotrexate at 20 mg, etanercept plus oral placebo, or methotrexate plus injectable placebo.
This is a study that will reshape clinical practice for many rheumatologists, according to Dr. Kavanaugh. The hypothesis was that in psoriatic arthritis, just as has been shown to be the case in rheumatoid arthritis, the combination of a TNF inhibitor plus methotrexate would have greater efficacy than either agent alone. But the study brought a couple of major surprises.
“Methotrexate didn’t do so badly,” Dr. Kavanaugh observed. “And the combination did nothing. I would have bet that the combination would have shown methotrexate had a synergistic effect with the TNF inhibitor, especially for x-ray changes. But the combination didn’t do any better than etanercept alone.”
Make no mistake: Etanercept monotherapy significantly outperformed methotrexate monotherapy for the primary endpoint, the ACR 20 response at week 24, by a margin of 60.9% versus 50.7%. Dr. Ruderman deemed that methotrexate response rate to be quite respectable, although he bemoaned the absence of a double-placebo comparator arm. And the key secondary endpoint, the minimal disease activity response rate at week 24, was also significantly better with etanercept, at 35.9% compared with 22.9%. Moreover, both etanercept arms showed significantly less radiographic progression than with methotrexate alone.
However, that was it. There were no significant differences between etanercept and methotrexate in other secondary endpoints, including the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC), the Disease Activity in PSoriatic Arthritis (DAPSA) score, the Leeds Dactylitis Instrument (LDI), and quality of life as assessed by the 36-item Short Form Health Survey total score.
“Methotrexate showed generally good efficacy across multiple domains,” the investigators concluded (Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851).
“Another intriguing thing to come out of this study for me were the enthesitis and dactylitis results. My clinical experience suggested methotrexate wasn’t so great for that, but this study suggests that’s not true,” Dr. Ruderman said.
“There are a couple of key take-home points from this study,” according to Dr. Kavanaugh. “One is that the combination is not synergistic. When you start a rheumatoid arthritis patient on methotrexate, you try to keep him on methotrexate when you add a TNF inhibitor. This study would say there doesn’t seem like there’s a reason to do that in your psoriatic arthritis patient. And the second message is that methotrexate seems to work.”
New ACR/NPF psoriatic arthritis guidelines under fire
“The new guidelines are fuzzy, aren’t they?” Dr. Kavanaugh said in lobbing the topic over to Dr. Ruderman.
“Where do we start?” he replied, shaking his head. “These are evidence-based guidelines in an area in which there was virtually no evidence.”
Indeed, the guidelines committee proudly employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which forces committee members to issue “conditional” recommendations when there’s not enough evidence to make a “strong” recommendation.
“You’re not allowed to say, ‘We don’t know, there’s not enough evidence to make a choice,’ ” Dr. Ruderman said. “The problem with these guidelines is virtually everything in it is a conditional recommendation except ‘stop smoking,’ which was a strong recommendation.
“A conditional recommendation is pretty much a fancy term for expert opinion. It’s basically everybody in the room saying, ‘This is what we think.’ And that makes guidelines challenging because as a rheumatologist, you’re an expert. The people in the room have perhaps looked at the data more carefully than you’ve drilled down into the studies, but ultimately they’ve taken care of these patients and you’ve taken care of these patients, so why is their opinion better than your opinion, if it’s an informed opinion?”
His other critique of the 28-page guidelines is they don’t include the reasoning behind the conditional recommendations.
“If the conditional recommendation is, ‘In this situation, a TNF inhibitor is preferred over an IL-17 inhibitor,’ that would be great if they had also said, ‘This is why we thought that.’ But that’s not in the paper,” Dr. Ruderman said.
He and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – The first-ever, double-blind, randomized, controlled clinical trial evidence demonstrating that methotrexate indeed has therapeutic efficacy in psoriatic arthritis has come at an awkward time – on the heels of a basically negative Cochrane Collaboration systematic review as well as the latest American College of Rheumatology/National Psoriasis Foundation guidelines for treatment of psoriatic arthritis, which recommend anti–tumor necrosis factor therapy as first line, ahead of methotrexate.
The timing of the release of the SEAM-PsA randomized trial results was such that neither the Cochrane group nor the ACR/NPF guideline committee was able to consider the new, potentially game-changing study findings.
“I look at SEAM-PsA and have to say, methotrexate does seem to be an effective therapy. I think it calls into question the new guidelines, which were developed before the data were out. Now you look at this and have to ask, can you really say you should use a TNF inhibitor before methotrexate based on these results? I don’t know,” Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He also shared other problems he has with the new guidelines, which he considers seriously flawed.
The Cochrane Collaboration Systematic Review
The Cochrane group cast a net for all randomized, controlled clinical trials of methotrexate versus placebo or another disease-modifying antirheumatic drug (DMARD). They found eight, which they judged to be of poor quality. Their conclusion: “Low-quality evidence suggests that low-dose (15 mg or less) oral methotrexate might be slightly more effective than placebo when taken for 6 months; however, we are uncertain if it is more harmful” (Cochrane Database Syst Rev. 2019 Jan 18;1:CD012722. doi: 10.1002/14651858.CD012722.pub2).
“The new Cochrane Review concludes methotrexate doesn’t seem to work that well,” observed symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
“That’s because it’s based on published data, and there’s been very little of that,” said Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago.
“I think most people assume, based on clinical experience, that it does work well. It’s all we had for years and years and years,” he added.
That is, until SEAM-PsA.
SEAM-PsA
SEAM-PsA randomized 851 DMARD- and biologic-naive patients with a median 0.6-year duration of psoriatic arthritis to one of three treatment arms for 48 weeks: once-weekly etanercept at 50 mg plus oral methotrexate at 20 mg, etanercept plus oral placebo, or methotrexate plus injectable placebo.
This is a study that will reshape clinical practice for many rheumatologists, according to Dr. Kavanaugh. The hypothesis was that in psoriatic arthritis, just as has been shown to be the case in rheumatoid arthritis, the combination of a TNF inhibitor plus methotrexate would have greater efficacy than either agent alone. But the study brought a couple of major surprises.
“Methotrexate didn’t do so badly,” Dr. Kavanaugh observed. “And the combination did nothing. I would have bet that the combination would have shown methotrexate had a synergistic effect with the TNF inhibitor, especially for x-ray changes. But the combination didn’t do any better than etanercept alone.”
Make no mistake: Etanercept monotherapy significantly outperformed methotrexate monotherapy for the primary endpoint, the ACR 20 response at week 24, by a margin of 60.9% versus 50.7%. Dr. Ruderman deemed that methotrexate response rate to be quite respectable, although he bemoaned the absence of a double-placebo comparator arm. And the key secondary endpoint, the minimal disease activity response rate at week 24, was also significantly better with etanercept, at 35.9% compared with 22.9%. Moreover, both etanercept arms showed significantly less radiographic progression than with methotrexate alone.
However, that was it. There were no significant differences between etanercept and methotrexate in other secondary endpoints, including the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC), the Disease Activity in PSoriatic Arthritis (DAPSA) score, the Leeds Dactylitis Instrument (LDI), and quality of life as assessed by the 36-item Short Form Health Survey total score.
“Methotrexate showed generally good efficacy across multiple domains,” the investigators concluded (Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851).
“Another intriguing thing to come out of this study for me were the enthesitis and dactylitis results. My clinical experience suggested methotrexate wasn’t so great for that, but this study suggests that’s not true,” Dr. Ruderman said.
“There are a couple of key take-home points from this study,” according to Dr. Kavanaugh. “One is that the combination is not synergistic. When you start a rheumatoid arthritis patient on methotrexate, you try to keep him on methotrexate when you add a TNF inhibitor. This study would say there doesn’t seem like there’s a reason to do that in your psoriatic arthritis patient. And the second message is that methotrexate seems to work.”
New ACR/NPF psoriatic arthritis guidelines under fire
“The new guidelines are fuzzy, aren’t they?” Dr. Kavanaugh said in lobbing the topic over to Dr. Ruderman.
“Where do we start?” he replied, shaking his head. “These are evidence-based guidelines in an area in which there was virtually no evidence.”
Indeed, the guidelines committee proudly employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which forces committee members to issue “conditional” recommendations when there’s not enough evidence to make a “strong” recommendation.
“You’re not allowed to say, ‘We don’t know, there’s not enough evidence to make a choice,’ ” Dr. Ruderman said. “The problem with these guidelines is virtually everything in it is a conditional recommendation except ‘stop smoking,’ which was a strong recommendation.
“A conditional recommendation is pretty much a fancy term for expert opinion. It’s basically everybody in the room saying, ‘This is what we think.’ And that makes guidelines challenging because as a rheumatologist, you’re an expert. The people in the room have perhaps looked at the data more carefully than you’ve drilled down into the studies, but ultimately they’ve taken care of these patients and you’ve taken care of these patients, so why is their opinion better than your opinion, if it’s an informed opinion?”
His other critique of the 28-page guidelines is they don’t include the reasoning behind the conditional recommendations.
“If the conditional recommendation is, ‘In this situation, a TNF inhibitor is preferred over an IL-17 inhibitor,’ that would be great if they had also said, ‘This is why we thought that.’ But that’s not in the paper,” Dr. Ruderman said.
He and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
Positive psoriatic arthritis screens occur often in psoriasis patients
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Psoriatic Arthritis Journal Scan: April 2019
Systematic review of depression and anxiety in psoriatic arthritis.
Kamalaraj N, El-Haddad C, Hay P, Pile K. Int J Rheum Dis. 2019 Apr 26.
This is the first systematic review of point prevalence of depression and anxiety in patients with psoriatic arthritis. There is a moderate point prevalence of both depression and anxiety in patients with psoriatic arthritis, which is similar or slightly higher than the general population and comparable to that seen in other rheumatic diseases. The effects of treatment for psoriatic arthritis on comorbid depression and anxiety remain unclear.
Amplifying the concept of psoriatic arthritis: The role of autoimmunity in systemic psoriatic disease.
Chimenti MS, Caso F, Alivernini S, et al. Autoimmun Rev. 2019 Apr 5.
Recently, an autoimmune footprint of PsA pathogenesis has been demonstrated with the presence of autoantigens and related autoantibodies in PsA patients' sera. The purpose of this review is to describe the new pathogenetic mechanisms and the different clinical pictures of systemic psoriatic disease, with the ultimate goal of improving the knowledge of this heterogeneous chronic inflammatory condition.
Cardiac and cardiovascular morbidities in patients with psoriatic arthritis: a population-based case control study.
Kibari A, Cohen AD, Gazitt T, et al. Clin Rheumatol. 2019 Apr 1.
A retrospective case control study assessed the prevalence of risk factors associated with cardiovascular disease (CVD) and CVD-related morbidity in a large Middle-Eastern psoriatic arthritis (PsA) cohort. The results emphasize the importance of clinician awareness of the increased risk for CVD-related complications in PsA patients.
Exploring Dimensions of Stiffness in Rheumatoid and Psoriatic Arthritis: The ARAD and OMERACT Stiffness Special Interest Group collaboration.
Sinnathura P, Bartlett SJ, Halls S, et al. J Rheumatol. 2019 Apr 1.
The aims of this study were to: 1) compare stiffness in psoriatic arthritis (PsA) and rheumatoid arthritis (RA) using patient-reported outcomes, 2) explore how dimensions of stiffness are associated with each other and reflect the patient experience, 3) explore how different dimensions of stiffness are associated with physical function.
Acrodermatitis Continua of Hallopeau with Psoriatic Arthritis.
Khosravi-Hafshejani T, Zhou Y, Dutz JP. J Rheumatol. 2019 Apr;46(4):437-438.
Acrodermatitis continua of Hallopeau (ACH) is a form of localized pustular psoriasis that can be associated with psoriatic arthritis. This case report examines a 53-year-old male presented with a 1-year history of fingernail and toenail dystrophy and pustules on the distal toes.
Systematic review of depression and anxiety in psoriatic arthritis.
Kamalaraj N, El-Haddad C, Hay P, Pile K. Int J Rheum Dis. 2019 Apr 26.
This is the first systematic review of point prevalence of depression and anxiety in patients with psoriatic arthritis. There is a moderate point prevalence of both depression and anxiety in patients with psoriatic arthritis, which is similar or slightly higher than the general population and comparable to that seen in other rheumatic diseases. The effects of treatment for psoriatic arthritis on comorbid depression and anxiety remain unclear.
Amplifying the concept of psoriatic arthritis: The role of autoimmunity in systemic psoriatic disease.
Chimenti MS, Caso F, Alivernini S, et al. Autoimmun Rev. 2019 Apr 5.
Recently, an autoimmune footprint of PsA pathogenesis has been demonstrated with the presence of autoantigens and related autoantibodies in PsA patients' sera. The purpose of this review is to describe the new pathogenetic mechanisms and the different clinical pictures of systemic psoriatic disease, with the ultimate goal of improving the knowledge of this heterogeneous chronic inflammatory condition.
Cardiac and cardiovascular morbidities in patients with psoriatic arthritis: a population-based case control study.
Kibari A, Cohen AD, Gazitt T, et al. Clin Rheumatol. 2019 Apr 1.
A retrospective case control study assessed the prevalence of risk factors associated with cardiovascular disease (CVD) and CVD-related morbidity in a large Middle-Eastern psoriatic arthritis (PsA) cohort. The results emphasize the importance of clinician awareness of the increased risk for CVD-related complications in PsA patients.
Exploring Dimensions of Stiffness in Rheumatoid and Psoriatic Arthritis: The ARAD and OMERACT Stiffness Special Interest Group collaboration.
Sinnathura P, Bartlett SJ, Halls S, et al. J Rheumatol. 2019 Apr 1.
The aims of this study were to: 1) compare stiffness in psoriatic arthritis (PsA) and rheumatoid arthritis (RA) using patient-reported outcomes, 2) explore how dimensions of stiffness are associated with each other and reflect the patient experience, 3) explore how different dimensions of stiffness are associated with physical function.
Acrodermatitis Continua of Hallopeau with Psoriatic Arthritis.
Khosravi-Hafshejani T, Zhou Y, Dutz JP. J Rheumatol. 2019 Apr;46(4):437-438.
Acrodermatitis continua of Hallopeau (ACH) is a form of localized pustular psoriasis that can be associated with psoriatic arthritis. This case report examines a 53-year-old male presented with a 1-year history of fingernail and toenail dystrophy and pustules on the distal toes.
Systematic review of depression and anxiety in psoriatic arthritis.
Kamalaraj N, El-Haddad C, Hay P, Pile K. Int J Rheum Dis. 2019 Apr 26.
This is the first systematic review of point prevalence of depression and anxiety in patients with psoriatic arthritis. There is a moderate point prevalence of both depression and anxiety in patients with psoriatic arthritis, which is similar or slightly higher than the general population and comparable to that seen in other rheumatic diseases. The effects of treatment for psoriatic arthritis on comorbid depression and anxiety remain unclear.
Amplifying the concept of psoriatic arthritis: The role of autoimmunity in systemic psoriatic disease.
Chimenti MS, Caso F, Alivernini S, et al. Autoimmun Rev. 2019 Apr 5.
Recently, an autoimmune footprint of PsA pathogenesis has been demonstrated with the presence of autoantigens and related autoantibodies in PsA patients' sera. The purpose of this review is to describe the new pathogenetic mechanisms and the different clinical pictures of systemic psoriatic disease, with the ultimate goal of improving the knowledge of this heterogeneous chronic inflammatory condition.
Cardiac and cardiovascular morbidities in patients with psoriatic arthritis: a population-based case control study.
Kibari A, Cohen AD, Gazitt T, et al. Clin Rheumatol. 2019 Apr 1.
A retrospective case control study assessed the prevalence of risk factors associated with cardiovascular disease (CVD) and CVD-related morbidity in a large Middle-Eastern psoriatic arthritis (PsA) cohort. The results emphasize the importance of clinician awareness of the increased risk for CVD-related complications in PsA patients.
Exploring Dimensions of Stiffness in Rheumatoid and Psoriatic Arthritis: The ARAD and OMERACT Stiffness Special Interest Group collaboration.
Sinnathura P, Bartlett SJ, Halls S, et al. J Rheumatol. 2019 Apr 1.
The aims of this study were to: 1) compare stiffness in psoriatic arthritis (PsA) and rheumatoid arthritis (RA) using patient-reported outcomes, 2) explore how dimensions of stiffness are associated with each other and reflect the patient experience, 3) explore how different dimensions of stiffness are associated with physical function.
Acrodermatitis Continua of Hallopeau with Psoriatic Arthritis.
Khosravi-Hafshejani T, Zhou Y, Dutz JP. J Rheumatol. 2019 Apr;46(4):437-438.
Acrodermatitis continua of Hallopeau (ACH) is a form of localized pustular psoriasis that can be associated with psoriatic arthritis. This case report examines a 53-year-old male presented with a 1-year history of fingernail and toenail dystrophy and pustules on the distal toes.
FDA approves IL-23 inhibitor risankizumab for treating plaque psoriasis
Risankizumab, an interleukin-23 inhibitor, has been approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, the manufacturer announced on April 23.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4. It will be available in early May, according to an AbbVie press release announcing the approval.
The approval was based in part on data from two phase 3, 2-year studies, In UltIMMA-1 and UltIMMA-2, at 16 weeks, 75% of risankizumab patients in both studies achieved a Psoriasis Area and Severity Index (PASI 90), compared with 5% and 2% of those on placebo, respectively. These results were published in 2018 (Lancet. 2018 Aug 25;392[10148]:650-61).
At 1 year, 82% and 81% of those treated with risankizumab in the two studies achieved a PASI 90, and 56% and 60% achieved a PASI 100, respectively, according to the company.
Approval was also based on additional phase 3 studies, IMMhance and IMMvent.
Upper respiratory infections were among the most common adverse events associated with risankizumab in trials, reported in 13%, according to the company. Other adverse events associated with treatment included headache (3.5 %), fatigue (2.5 %), injection site reactions (1.5%) and tinea infections (1.1%). The AbbVie release states that candidates for treatment should be evaluated for tuberculosis before starting therapy, and patients should be instructed to report signs and symptoms of infection.
Risankizumab, which will be marketed as Skyrizi, was recently approved in Canada for the same indication, and in Japan, for plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults. It currently is under review in Europe.
AbbVie and Boehringer Ingelheim are collaborating on the development of risankizumab, according to an AbbVie press release. Studies of risankizumab for treatment of psoriatic arthritis and Crohn’s disease are underway.
Risankizumab, an interleukin-23 inhibitor, has been approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, the manufacturer announced on April 23.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4. It will be available in early May, according to an AbbVie press release announcing the approval.
The approval was based in part on data from two phase 3, 2-year studies, In UltIMMA-1 and UltIMMA-2, at 16 weeks, 75% of risankizumab patients in both studies achieved a Psoriasis Area and Severity Index (PASI 90), compared with 5% and 2% of those on placebo, respectively. These results were published in 2018 (Lancet. 2018 Aug 25;392[10148]:650-61).
At 1 year, 82% and 81% of those treated with risankizumab in the two studies achieved a PASI 90, and 56% and 60% achieved a PASI 100, respectively, according to the company.
Approval was also based on additional phase 3 studies, IMMhance and IMMvent.
Upper respiratory infections were among the most common adverse events associated with risankizumab in trials, reported in 13%, according to the company. Other adverse events associated with treatment included headache (3.5 %), fatigue (2.5 %), injection site reactions (1.5%) and tinea infections (1.1%). The AbbVie release states that candidates for treatment should be evaluated for tuberculosis before starting therapy, and patients should be instructed to report signs and symptoms of infection.
Risankizumab, which will be marketed as Skyrizi, was recently approved in Canada for the same indication, and in Japan, for plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults. It currently is under review in Europe.
AbbVie and Boehringer Ingelheim are collaborating on the development of risankizumab, according to an AbbVie press release. Studies of risankizumab for treatment of psoriatic arthritis and Crohn’s disease are underway.
Risankizumab, an interleukin-23 inhibitor, has been approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, the manufacturer announced on April 23.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4. It will be available in early May, according to an AbbVie press release announcing the approval.
The approval was based in part on data from two phase 3, 2-year studies, In UltIMMA-1 and UltIMMA-2, at 16 weeks, 75% of risankizumab patients in both studies achieved a Psoriasis Area and Severity Index (PASI 90), compared with 5% and 2% of those on placebo, respectively. These results were published in 2018 (Lancet. 2018 Aug 25;392[10148]:650-61).
At 1 year, 82% and 81% of those treated with risankizumab in the two studies achieved a PASI 90, and 56% and 60% achieved a PASI 100, respectively, according to the company.
Approval was also based on additional phase 3 studies, IMMhance and IMMvent.
Upper respiratory infections were among the most common adverse events associated with risankizumab in trials, reported in 13%, according to the company. Other adverse events associated with treatment included headache (3.5 %), fatigue (2.5 %), injection site reactions (1.5%) and tinea infections (1.1%). The AbbVie release states that candidates for treatment should be evaluated for tuberculosis before starting therapy, and patients should be instructed to report signs and symptoms of infection.
Risankizumab, which will be marketed as Skyrizi, was recently approved in Canada for the same indication, and in Japan, for plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults. It currently is under review in Europe.
AbbVie and Boehringer Ingelheim are collaborating on the development of risankizumab, according to an AbbVie press release. Studies of risankizumab for treatment of psoriatic arthritis and Crohn’s disease are underway.
TNF-alpha, adiponectin potential biomarkers for PsA, psoriasis differentiation
High plasma levels of tumor necrosis factor (TNF)–alpha and adiponectin can be used to differentiate patients with psoriasis and psoriatic arthritis, according to Wen-Qing Li, PhD, of Brown University, Providence, R.I., and his associates.
In a research letter published in the British Journal of Dermatology, the investigators detailed an analysis of 180 patients with psoriasis only and 143 patients with psoriatic arthritis (PsA) from the Psoriatic Arthritis and Psoriasis Follow-up Study. Patients in both groups had a mean age of 51 years. Plasma levels of interleukin-6, C-reactive protein, TNF-alpha, leptin, total adiponectin, and high-molecular-weight (HMW) adiponectin were assessed as potential biomarkers by ultrasensitive enzyme-linked immunosorbent assay or immunoturbidimetric assay.
Median TNF-alpha plasma levels were higher in patients with PsA, compared with those with psoriasis (3.27 vs. 1.32 pg/mL–1), while total and HMW adiponectin levels were lower in patients with PsA, compared with those with psoriasis (4.66 vs. 5.36 mcg/mL–1; 2.58 vs. 3.01 mcg/mL–1). After logistic regression, TNF-alpha (adjusted odds ratio, 2.25; 95% confidence interval, 1.41-3.61) and total adiponectin (aOR, 0.61; 95% CI, 0.39-0.96) remained significantly associated as biomarkers. HMW adiponectin maintained marginal significance (aOR, 0.64; 95% CI, 0.41-1.01).
“Further large-scale investigation in a prospective setting of patients with PsO [psoriasis] would be warranted, if a clinically useful screening test is to be developed for risk prediction of PsA based on circulating biomarkers,” the investigators concluded.
Two study authors reported consulting with or advising numerous pharmaceutical companies.
SOURCE: Li W-Q et al. Br J Dermatol. 2019 Jan 29. doi: 10.1111/bjd.17700.
High plasma levels of tumor necrosis factor (TNF)–alpha and adiponectin can be used to differentiate patients with psoriasis and psoriatic arthritis, according to Wen-Qing Li, PhD, of Brown University, Providence, R.I., and his associates.
In a research letter published in the British Journal of Dermatology, the investigators detailed an analysis of 180 patients with psoriasis only and 143 patients with psoriatic arthritis (PsA) from the Psoriatic Arthritis and Psoriasis Follow-up Study. Patients in both groups had a mean age of 51 years. Plasma levels of interleukin-6, C-reactive protein, TNF-alpha, leptin, total adiponectin, and high-molecular-weight (HMW) adiponectin were assessed as potential biomarkers by ultrasensitive enzyme-linked immunosorbent assay or immunoturbidimetric assay.
Median TNF-alpha plasma levels were higher in patients with PsA, compared with those with psoriasis (3.27 vs. 1.32 pg/mL–1), while total and HMW adiponectin levels were lower in patients with PsA, compared with those with psoriasis (4.66 vs. 5.36 mcg/mL–1; 2.58 vs. 3.01 mcg/mL–1). After logistic regression, TNF-alpha (adjusted odds ratio, 2.25; 95% confidence interval, 1.41-3.61) and total adiponectin (aOR, 0.61; 95% CI, 0.39-0.96) remained significantly associated as biomarkers. HMW adiponectin maintained marginal significance (aOR, 0.64; 95% CI, 0.41-1.01).
“Further large-scale investigation in a prospective setting of patients with PsO [psoriasis] would be warranted, if a clinically useful screening test is to be developed for risk prediction of PsA based on circulating biomarkers,” the investigators concluded.
Two study authors reported consulting with or advising numerous pharmaceutical companies.
SOURCE: Li W-Q et al. Br J Dermatol. 2019 Jan 29. doi: 10.1111/bjd.17700.
High plasma levels of tumor necrosis factor (TNF)–alpha and adiponectin can be used to differentiate patients with psoriasis and psoriatic arthritis, according to Wen-Qing Li, PhD, of Brown University, Providence, R.I., and his associates.
In a research letter published in the British Journal of Dermatology, the investigators detailed an analysis of 180 patients with psoriasis only and 143 patients with psoriatic arthritis (PsA) from the Psoriatic Arthritis and Psoriasis Follow-up Study. Patients in both groups had a mean age of 51 years. Plasma levels of interleukin-6, C-reactive protein, TNF-alpha, leptin, total adiponectin, and high-molecular-weight (HMW) adiponectin were assessed as potential biomarkers by ultrasensitive enzyme-linked immunosorbent assay or immunoturbidimetric assay.
Median TNF-alpha plasma levels were higher in patients with PsA, compared with those with psoriasis (3.27 vs. 1.32 pg/mL–1), while total and HMW adiponectin levels were lower in patients with PsA, compared with those with psoriasis (4.66 vs. 5.36 mcg/mL–1; 2.58 vs. 3.01 mcg/mL–1). After logistic regression, TNF-alpha (adjusted odds ratio, 2.25; 95% confidence interval, 1.41-3.61) and total adiponectin (aOR, 0.61; 95% CI, 0.39-0.96) remained significantly associated as biomarkers. HMW adiponectin maintained marginal significance (aOR, 0.64; 95% CI, 0.41-1.01).
“Further large-scale investigation in a prospective setting of patients with PsO [psoriasis] would be warranted, if a clinically useful screening test is to be developed for risk prediction of PsA based on circulating biomarkers,” the investigators concluded.
Two study authors reported consulting with or advising numerous pharmaceutical companies.
SOURCE: Li W-Q et al. Br J Dermatol. 2019 Jan 29. doi: 10.1111/bjd.17700.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Survey finds psoriasis patients seek relief with alternative therapies
Treatment (CAMs), despite limited documentation supporting their efficacy, reported Emily C. Murphy and her associates, in the department of dermatology, George Washington University, Washington.
They performed a survey-based statistical analysis to identify specific types of commonly used CAMs, and to explore reasons patients increasingly turn to alternative therapies. The survey was distributed in the National Psoriasis Foundation’s (NPF) October 2018 newsletter to its 100,927 members. Their results were published in a letter to the editor of the Journal of the American Academy of Dermatology.
Of the 6,101 NPF members who opened the newsletter, 324 clicked on the survey link. Of the 219 who completed the survey, almost 70% were women. The majority were white (84.1%), compared with Hispanic (6.2%), Asian (3.1%), and black (2.6%) participants. Most of the survey respondents had a dermatologist diagnosis of psoriasis, as well as access to health insurance to cover any prescribed medicines needed.
Of the 41% of respondents who reported using alternative therapies, usage was especially high among those who considered their psoriasis as severe (50% vs. 33.6% of those with nonsevere disease). Among the respondents, women were more likely than were men to use CAMs (45.6% vs. 26.5%, P = .002).
Only 4% cited access to care as a reason for choosing alternative therapies; the majority said they used CAMs because “traditional medications did not help or had side effects.”
While men were more likely than were women to use vitamins (24% vs. 18.9%, respectively), Dead Sea bath salts (17% vs. 7.8%), and cupping (3% vs. 0.8%), women were more likely to use herbals/botanicals (17% vs. 14%) and yoga (9.6% vs. 2%).
Patients with moderate psoriasis were significantly more likely than were those with mild or severe cases of the disease to recommend CAMs, regardless of insurance status (52.4% vs. 35% among those with mild disease and 40.4% for those with severe disease).
For some of the commonly used treatments, such as vitamins D and B12, there is insufficient evidence documenting their efficacy, although Dead Sea treatments have been shown to have therapeutic effects. And while there is efficacy evidence for indigo naturalis and meditation, these were not mentioned or were not commonly reported by respondents, the authors pointed out.
Although just 43% of patients said they would recommend a CAM to other people with psoriasis, its use remains widespread. For this reason, “educational initiatives that enable physicians to discuss evidence-based CAMs may improve patient satisfaction and outcomes,” observed Ms. Murphy, a research fellow, and her associates.
Previous studies have cited rates of CAM usage among patients with psoriasis as high as 62%, but researchers have failed to examine the reasons motivating usage. Not surprisingly, patients often use but misunderstand the benefits of alternative therapies.
“The onus is on us as physicians to not only ask our patients if they are using nonallopathic therapies for their psoriasis, but also to create an accepting environment that enables further discussion regarding said treatments to ensure patient safety and ultimately good outcomes,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, said in an interview.
The authors had no financial sources or conflicts of interest to disclose; there was no funding source.
SOURCE: Murphy E et al. J Am Acad Dermatol. 2019 Mar 29. pii: S0190-9622(19)30503-1. doi: 10.1016/j.jaad.2019.03.059.
Treatment (CAMs), despite limited documentation supporting their efficacy, reported Emily C. Murphy and her associates, in the department of dermatology, George Washington University, Washington.
They performed a survey-based statistical analysis to identify specific types of commonly used CAMs, and to explore reasons patients increasingly turn to alternative therapies. The survey was distributed in the National Psoriasis Foundation’s (NPF) October 2018 newsletter to its 100,927 members. Their results were published in a letter to the editor of the Journal of the American Academy of Dermatology.
Of the 6,101 NPF members who opened the newsletter, 324 clicked on the survey link. Of the 219 who completed the survey, almost 70% were women. The majority were white (84.1%), compared with Hispanic (6.2%), Asian (3.1%), and black (2.6%) participants. Most of the survey respondents had a dermatologist diagnosis of psoriasis, as well as access to health insurance to cover any prescribed medicines needed.
Of the 41% of respondents who reported using alternative therapies, usage was especially high among those who considered their psoriasis as severe (50% vs. 33.6% of those with nonsevere disease). Among the respondents, women were more likely than were men to use CAMs (45.6% vs. 26.5%, P = .002).
Only 4% cited access to care as a reason for choosing alternative therapies; the majority said they used CAMs because “traditional medications did not help or had side effects.”
While men were more likely than were women to use vitamins (24% vs. 18.9%, respectively), Dead Sea bath salts (17% vs. 7.8%), and cupping (3% vs. 0.8%), women were more likely to use herbals/botanicals (17% vs. 14%) and yoga (9.6% vs. 2%).
Patients with moderate psoriasis were significantly more likely than were those with mild or severe cases of the disease to recommend CAMs, regardless of insurance status (52.4% vs. 35% among those with mild disease and 40.4% for those with severe disease).
For some of the commonly used treatments, such as vitamins D and B12, there is insufficient evidence documenting their efficacy, although Dead Sea treatments have been shown to have therapeutic effects. And while there is efficacy evidence for indigo naturalis and meditation, these were not mentioned or were not commonly reported by respondents, the authors pointed out.
Although just 43% of patients said they would recommend a CAM to other people with psoriasis, its use remains widespread. For this reason, “educational initiatives that enable physicians to discuss evidence-based CAMs may improve patient satisfaction and outcomes,” observed Ms. Murphy, a research fellow, and her associates.
Previous studies have cited rates of CAM usage among patients with psoriasis as high as 62%, but researchers have failed to examine the reasons motivating usage. Not surprisingly, patients often use but misunderstand the benefits of alternative therapies.
“The onus is on us as physicians to not only ask our patients if they are using nonallopathic therapies for their psoriasis, but also to create an accepting environment that enables further discussion regarding said treatments to ensure patient safety and ultimately good outcomes,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, said in an interview.
The authors had no financial sources or conflicts of interest to disclose; there was no funding source.
SOURCE: Murphy E et al. J Am Acad Dermatol. 2019 Mar 29. pii: S0190-9622(19)30503-1. doi: 10.1016/j.jaad.2019.03.059.
Treatment (CAMs), despite limited documentation supporting their efficacy, reported Emily C. Murphy and her associates, in the department of dermatology, George Washington University, Washington.
They performed a survey-based statistical analysis to identify specific types of commonly used CAMs, and to explore reasons patients increasingly turn to alternative therapies. The survey was distributed in the National Psoriasis Foundation’s (NPF) October 2018 newsletter to its 100,927 members. Their results were published in a letter to the editor of the Journal of the American Academy of Dermatology.
Of the 6,101 NPF members who opened the newsletter, 324 clicked on the survey link. Of the 219 who completed the survey, almost 70% were women. The majority were white (84.1%), compared with Hispanic (6.2%), Asian (3.1%), and black (2.6%) participants. Most of the survey respondents had a dermatologist diagnosis of psoriasis, as well as access to health insurance to cover any prescribed medicines needed.
Of the 41% of respondents who reported using alternative therapies, usage was especially high among those who considered their psoriasis as severe (50% vs. 33.6% of those with nonsevere disease). Among the respondents, women were more likely than were men to use CAMs (45.6% vs. 26.5%, P = .002).
Only 4% cited access to care as a reason for choosing alternative therapies; the majority said they used CAMs because “traditional medications did not help or had side effects.”
While men were more likely than were women to use vitamins (24% vs. 18.9%, respectively), Dead Sea bath salts (17% vs. 7.8%), and cupping (3% vs. 0.8%), women were more likely to use herbals/botanicals (17% vs. 14%) and yoga (9.6% vs. 2%).
Patients with moderate psoriasis were significantly more likely than were those with mild or severe cases of the disease to recommend CAMs, regardless of insurance status (52.4% vs. 35% among those with mild disease and 40.4% for those with severe disease).
For some of the commonly used treatments, such as vitamins D and B12, there is insufficient evidence documenting their efficacy, although Dead Sea treatments have been shown to have therapeutic effects. And while there is efficacy evidence for indigo naturalis and meditation, these were not mentioned or were not commonly reported by respondents, the authors pointed out.
Although just 43% of patients said they would recommend a CAM to other people with psoriasis, its use remains widespread. For this reason, “educational initiatives that enable physicians to discuss evidence-based CAMs may improve patient satisfaction and outcomes,” observed Ms. Murphy, a research fellow, and her associates.
Previous studies have cited rates of CAM usage among patients with psoriasis as high as 62%, but researchers have failed to examine the reasons motivating usage. Not surprisingly, patients often use but misunderstand the benefits of alternative therapies.
“The onus is on us as physicians to not only ask our patients if they are using nonallopathic therapies for their psoriasis, but also to create an accepting environment that enables further discussion regarding said treatments to ensure patient safety and ultimately good outcomes,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, said in an interview.
The authors had no financial sources or conflicts of interest to disclose; there was no funding source.
SOURCE: Murphy E et al. J Am Acad Dermatol. 2019 Mar 29. pii: S0190-9622(19)30503-1. doi: 10.1016/j.jaad.2019.03.059.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY












