User login
Obesity might be targetable driver of psoriatic arthritis progression
MADRID – Two sets of data presented at the European Congress of Rheumatology support the potential for weight loss to be a valuable adjunctive strategy for improving outcomes in patients with psoriatic arthritis (PsA).
One set, drawn from the ongoing PsABio observational study, correlated increasing body mass index with greater disease activity and greater disability. Another, based on patients followed for 12 months, showed that a weight loss of about 15% is associated with a significant reduction in PsA activity.
“As clinicians, we largely focus on drugs in the treatment of PsA, but these data draw attention to obesity as a potential target for improving outcomes in PsA,” said Stefan Siebert, MD, a rheumatologist at the Institute of Infection, Immunity, and Inflammation at the University of Glasgow (Scotland).
Dr. Siebert cautioned that his data show association, not causation, but he said these data add to a growing body of evidence that provide compelling support for trials to test the premise that weight loss improves outcomes.
Although not a trial, a study by Eva Klingberg, MD, PhD, of the Sahlgrenska Academy at the University of Gothenburg (Sweden) and her associates tested this premise and showed weight loss was associated with improvement in multiple PsA activity parameters 6 and 12 months after a significant weight loss program.
“This is just one study, so we need more data, but we are already using weight loss to manage PsA in obese patients in Sweden,” said Dr. Klingberg, speaking about her work in advance of the presentation. Like Dr. Siebert, she agreed that weight loss is an important potential treatment strategy in PsA.
In the observational PsABio study, which is following patients with PsA at rheumatology centers in eight European countries, the goal of its analysis was to evaluate disease activity and outcomes in relationship to baseline weight for patients starting a biologic therapy as part of standard clinical practice. Of the 917 patients evaluated, 450 started ustekinumab (Stelara) and 467 started a tumor necrosis factor inhibitor (TNFi). The researchers had weight data for 827 of these patients.
At the time of enrollment, 40% were overweight as defined by a body mass index (BMI) ranging from 25 to 29 kg/m2, and 30.4% were obese as defined by a BMI greater than 30 kg/m2. The mean baseline BMI was 28.1 kg/m2. The mean age of the study population was 49.7 years. Slightly more than half were female.
Relative to a BMI of 30 kg/m2 or less, higher BMI at baseline is shown in multiple regression analysis to be independently and significantly linked to disease activity assessed by the clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA; P = .026), to patient perception of disease impact as measured by Psoriatic Arthritis Impact of Disease (PsAID-12; P less than .0001), and to greater disability as measured with Health Assessment Questionnaire Disability Index (HAQ-DI; P less than .0001).
“There are multiple sets of data that show obesity predicts who develops PsA. Our data further show that, of patients with PsA who are candidates for a biologic, those with obesity have greater disease activity,” Dr. Siebert said. “We are using all of these expensive drugs, but I think there is now a need to also focus on lifestyle interventions, in addition to drug therapy, to reduce disease activity and improve outcomes in PsA.”
The data to be presented by Dr. Klingberg provide a step in that direction. In this study, 46 PsA patients participated in a weight-loss treatment that restricted calorie intake to 640 kcal/day, and the researchers followed 39 of these patients for 1 year. The participants averaged 56 years old, and almost two-thirds were women. All enrolled patients had to have a BMI of at least 33 kg/m2, and the actual average BMI was 35 kg/m2. The median weight loss among the 39 patients followed for 1 year after the start of a 12- to 16-week weight-loss treatment was 16.1 kg, representing about 16% of their body weight at entry.
Dr. Klingberg showed that disease activity in those who achieved and maintained weight loss after the program was significant at 6 and 12 months when measured with the Psoriatic Arthritis Response Criteria (PsARC) or the American College of Rheumatology (ACR) 20, 50, and 70 criteria. In the 39 patients followed for 12 months, 36% fulfilled PsARC, and 54%, 36%, and 15% fulfilled the ACR 20, 50, and 70 responses, respectively.
“In Sweden, any obese individual can be referred for a weight loss program because of the multiple health benefits that are associated with weight reduction,” Dr. Klingberg explained. “We were able to look at patients with PsA and show that this substantially reduces the burden of their joint disease in addition to the other health advantages of losing weight.”
An improvement in symptoms is a logical expectation from reducing the mechanical strain imposed by obesity on inflamed joints, but Dr. Klingberg is more impressed by the potential for weight loss to reduce the proinflammatory signaling generated by adipose tissue. In PsA, there is evidence that weight loss reduces disease activity in the skin, as well as the joints, which supports this link.
“We need more data to document the benefits from weight loss in patients with PsA, but I think management of the comorbidities of PsA, including obesity, is something that should already be routinely discussed with patients,” Dr. Klingberg said.
Dr. Siebert has been a consultant to or speaker on behalf of AbbVie, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB, and he has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB. Dr. Klingberg has been an advisor to Novartis, a speaker on behalf of Lilly, and has receive research funding from Roche.
Mitchel L. Zoler contributed to this report.
SOURCE: Siebert S et al. Ann Rheum Dis. Jun 2019;78(suppl 2):69. Abstract OP0007. doi: 10.1136/annrheumdis-2019-eular.5841; Klingberg E et al. Ann Rheum Dis. Jun 2019;78(suppl 2):69-70. Abstract OP0008. doi: 10.1136/annrheumdis-2019-eular.5551.
MADRID – Two sets of data presented at the European Congress of Rheumatology support the potential for weight loss to be a valuable adjunctive strategy for improving outcomes in patients with psoriatic arthritis (PsA).
One set, drawn from the ongoing PsABio observational study, correlated increasing body mass index with greater disease activity and greater disability. Another, based on patients followed for 12 months, showed that a weight loss of about 15% is associated with a significant reduction in PsA activity.
“As clinicians, we largely focus on drugs in the treatment of PsA, but these data draw attention to obesity as a potential target for improving outcomes in PsA,” said Stefan Siebert, MD, a rheumatologist at the Institute of Infection, Immunity, and Inflammation at the University of Glasgow (Scotland).
Dr. Siebert cautioned that his data show association, not causation, but he said these data add to a growing body of evidence that provide compelling support for trials to test the premise that weight loss improves outcomes.
Although not a trial, a study by Eva Klingberg, MD, PhD, of the Sahlgrenska Academy at the University of Gothenburg (Sweden) and her associates tested this premise and showed weight loss was associated with improvement in multiple PsA activity parameters 6 and 12 months after a significant weight loss program.
“This is just one study, so we need more data, but we are already using weight loss to manage PsA in obese patients in Sweden,” said Dr. Klingberg, speaking about her work in advance of the presentation. Like Dr. Siebert, she agreed that weight loss is an important potential treatment strategy in PsA.
In the observational PsABio study, which is following patients with PsA at rheumatology centers in eight European countries, the goal of its analysis was to evaluate disease activity and outcomes in relationship to baseline weight for patients starting a biologic therapy as part of standard clinical practice. Of the 917 patients evaluated, 450 started ustekinumab (Stelara) and 467 started a tumor necrosis factor inhibitor (TNFi). The researchers had weight data for 827 of these patients.
At the time of enrollment, 40% were overweight as defined by a body mass index (BMI) ranging from 25 to 29 kg/m2, and 30.4% were obese as defined by a BMI greater than 30 kg/m2. The mean baseline BMI was 28.1 kg/m2. The mean age of the study population was 49.7 years. Slightly more than half were female.
Relative to a BMI of 30 kg/m2 or less, higher BMI at baseline is shown in multiple regression analysis to be independently and significantly linked to disease activity assessed by the clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA; P = .026), to patient perception of disease impact as measured by Psoriatic Arthritis Impact of Disease (PsAID-12; P less than .0001), and to greater disability as measured with Health Assessment Questionnaire Disability Index (HAQ-DI; P less than .0001).
“There are multiple sets of data that show obesity predicts who develops PsA. Our data further show that, of patients with PsA who are candidates for a biologic, those with obesity have greater disease activity,” Dr. Siebert said. “We are using all of these expensive drugs, but I think there is now a need to also focus on lifestyle interventions, in addition to drug therapy, to reduce disease activity and improve outcomes in PsA.”
The data to be presented by Dr. Klingberg provide a step in that direction. In this study, 46 PsA patients participated in a weight-loss treatment that restricted calorie intake to 640 kcal/day, and the researchers followed 39 of these patients for 1 year. The participants averaged 56 years old, and almost two-thirds were women. All enrolled patients had to have a BMI of at least 33 kg/m2, and the actual average BMI was 35 kg/m2. The median weight loss among the 39 patients followed for 1 year after the start of a 12- to 16-week weight-loss treatment was 16.1 kg, representing about 16% of their body weight at entry.
Dr. Klingberg showed that disease activity in those who achieved and maintained weight loss after the program was significant at 6 and 12 months when measured with the Psoriatic Arthritis Response Criteria (PsARC) or the American College of Rheumatology (ACR) 20, 50, and 70 criteria. In the 39 patients followed for 12 months, 36% fulfilled PsARC, and 54%, 36%, and 15% fulfilled the ACR 20, 50, and 70 responses, respectively.
“In Sweden, any obese individual can be referred for a weight loss program because of the multiple health benefits that are associated with weight reduction,” Dr. Klingberg explained. “We were able to look at patients with PsA and show that this substantially reduces the burden of their joint disease in addition to the other health advantages of losing weight.”
An improvement in symptoms is a logical expectation from reducing the mechanical strain imposed by obesity on inflamed joints, but Dr. Klingberg is more impressed by the potential for weight loss to reduce the proinflammatory signaling generated by adipose tissue. In PsA, there is evidence that weight loss reduces disease activity in the skin, as well as the joints, which supports this link.
“We need more data to document the benefits from weight loss in patients with PsA, but I think management of the comorbidities of PsA, including obesity, is something that should already be routinely discussed with patients,” Dr. Klingberg said.
Dr. Siebert has been a consultant to or speaker on behalf of AbbVie, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB, and he has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB. Dr. Klingberg has been an advisor to Novartis, a speaker on behalf of Lilly, and has receive research funding from Roche.
Mitchel L. Zoler contributed to this report.
SOURCE: Siebert S et al. Ann Rheum Dis. Jun 2019;78(suppl 2):69. Abstract OP0007. doi: 10.1136/annrheumdis-2019-eular.5841; Klingberg E et al. Ann Rheum Dis. Jun 2019;78(suppl 2):69-70. Abstract OP0008. doi: 10.1136/annrheumdis-2019-eular.5551.
MADRID – Two sets of data presented at the European Congress of Rheumatology support the potential for weight loss to be a valuable adjunctive strategy for improving outcomes in patients with psoriatic arthritis (PsA).
One set, drawn from the ongoing PsABio observational study, correlated increasing body mass index with greater disease activity and greater disability. Another, based on patients followed for 12 months, showed that a weight loss of about 15% is associated with a significant reduction in PsA activity.
“As clinicians, we largely focus on drugs in the treatment of PsA, but these data draw attention to obesity as a potential target for improving outcomes in PsA,” said Stefan Siebert, MD, a rheumatologist at the Institute of Infection, Immunity, and Inflammation at the University of Glasgow (Scotland).
Dr. Siebert cautioned that his data show association, not causation, but he said these data add to a growing body of evidence that provide compelling support for trials to test the premise that weight loss improves outcomes.
Although not a trial, a study by Eva Klingberg, MD, PhD, of the Sahlgrenska Academy at the University of Gothenburg (Sweden) and her associates tested this premise and showed weight loss was associated with improvement in multiple PsA activity parameters 6 and 12 months after a significant weight loss program.
“This is just one study, so we need more data, but we are already using weight loss to manage PsA in obese patients in Sweden,” said Dr. Klingberg, speaking about her work in advance of the presentation. Like Dr. Siebert, she agreed that weight loss is an important potential treatment strategy in PsA.
In the observational PsABio study, which is following patients with PsA at rheumatology centers in eight European countries, the goal of its analysis was to evaluate disease activity and outcomes in relationship to baseline weight for patients starting a biologic therapy as part of standard clinical practice. Of the 917 patients evaluated, 450 started ustekinumab (Stelara) and 467 started a tumor necrosis factor inhibitor (TNFi). The researchers had weight data for 827 of these patients.
At the time of enrollment, 40% were overweight as defined by a body mass index (BMI) ranging from 25 to 29 kg/m2, and 30.4% were obese as defined by a BMI greater than 30 kg/m2. The mean baseline BMI was 28.1 kg/m2. The mean age of the study population was 49.7 years. Slightly more than half were female.
Relative to a BMI of 30 kg/m2 or less, higher BMI at baseline is shown in multiple regression analysis to be independently and significantly linked to disease activity assessed by the clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA; P = .026), to patient perception of disease impact as measured by Psoriatic Arthritis Impact of Disease (PsAID-12; P less than .0001), and to greater disability as measured with Health Assessment Questionnaire Disability Index (HAQ-DI; P less than .0001).
“There are multiple sets of data that show obesity predicts who develops PsA. Our data further show that, of patients with PsA who are candidates for a biologic, those with obesity have greater disease activity,” Dr. Siebert said. “We are using all of these expensive drugs, but I think there is now a need to also focus on lifestyle interventions, in addition to drug therapy, to reduce disease activity and improve outcomes in PsA.”
The data to be presented by Dr. Klingberg provide a step in that direction. In this study, 46 PsA patients participated in a weight-loss treatment that restricted calorie intake to 640 kcal/day, and the researchers followed 39 of these patients for 1 year. The participants averaged 56 years old, and almost two-thirds were women. All enrolled patients had to have a BMI of at least 33 kg/m2, and the actual average BMI was 35 kg/m2. The median weight loss among the 39 patients followed for 1 year after the start of a 12- to 16-week weight-loss treatment was 16.1 kg, representing about 16% of their body weight at entry.
Dr. Klingberg showed that disease activity in those who achieved and maintained weight loss after the program was significant at 6 and 12 months when measured with the Psoriatic Arthritis Response Criteria (PsARC) or the American College of Rheumatology (ACR) 20, 50, and 70 criteria. In the 39 patients followed for 12 months, 36% fulfilled PsARC, and 54%, 36%, and 15% fulfilled the ACR 20, 50, and 70 responses, respectively.
“In Sweden, any obese individual can be referred for a weight loss program because of the multiple health benefits that are associated with weight reduction,” Dr. Klingberg explained. “We were able to look at patients with PsA and show that this substantially reduces the burden of their joint disease in addition to the other health advantages of losing weight.”
An improvement in symptoms is a logical expectation from reducing the mechanical strain imposed by obesity on inflamed joints, but Dr. Klingberg is more impressed by the potential for weight loss to reduce the proinflammatory signaling generated by adipose tissue. In PsA, there is evidence that weight loss reduces disease activity in the skin, as well as the joints, which supports this link.
“We need more data to document the benefits from weight loss in patients with PsA, but I think management of the comorbidities of PsA, including obesity, is something that should already be routinely discussed with patients,” Dr. Klingberg said.
Dr. Siebert has been a consultant to or speaker on behalf of AbbVie, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB, and he has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB. Dr. Klingberg has been an advisor to Novartis, a speaker on behalf of Lilly, and has receive research funding from Roche.
Mitchel L. Zoler contributed to this report.
SOURCE: Siebert S et al. Ann Rheum Dis. Jun 2019;78(suppl 2):69. Abstract OP0007. doi: 10.1136/annrheumdis-2019-eular.5841; Klingberg E et al. Ann Rheum Dis. Jun 2019;78(suppl 2):69-70. Abstract OP0008. doi: 10.1136/annrheumdis-2019-eular.5551.
REPORTING FROM EULAR 2019 CONGRESS
VIDEO: Dr. Lihi Eder on the diagnostic challenges of psoriatic arthritis

How strong are the links between psoriasis and psoriatic arthritis? What are the biggest challenges that keep clinicians from diagnosing PsA? And which approaches show promise in helping prevent PsA? In this expert analysis, Lihi Eder, MD, PhD, of Women’s College Research Institute, Toronto, and the University of Toronto offers answers to these questions and more.

How strong are the links between psoriasis and psoriatic arthritis? What are the biggest challenges that keep clinicians from diagnosing PsA? And which approaches show promise in helping prevent PsA? In this expert analysis, Lihi Eder, MD, PhD, of Women’s College Research Institute, Toronto, and the University of Toronto offers answers to these questions and more.

How strong are the links between psoriasis and psoriatic arthritis? What are the biggest challenges that keep clinicians from diagnosing PsA? And which approaches show promise in helping prevent PsA? In this expert analysis, Lihi Eder, MD, PhD, of Women’s College Research Institute, Toronto, and the University of Toronto offers answers to these questions and more.
Ixekizumab surpasses adalimumab in PsA head-to-head study
MADRID – The interleukin-17A inhibitor ixekizumab surpassed the tumor necrosis factor inhibitor adalimumab for treatment of patients with psoriatic arthritis in a multicenter, randomized study with 566 enrolled patients, the first reported results from a head-to-head comparison for this disease of two different classes of biological drugs.
The results showed that a standard, 24-week regimen with each of these agents, both of which already have regulatory approval for treating psoriatic arthritis (PsA), led to achievement of the primary endpoint in 36% of patients treated with ixekizumab (Taltz) and 28% of patients treated with adalimumab (Humira), a statistically significant difference, Philip J. Mease, MD, said at the European Congress of Rheumatology.
“Ixekizumab was superior to adalimumab for improving signs and symptoms of active PsA, as measured by simultaneous achievement of ACR50 [American College of Rheumatology] and PASI 100 [Psoriasis Area and Severity Index],” the study’s primary endpoint that combined a measure of joint disease activity with a measure of skin involvement, said Dr. Mease, a rheumatologist at Swedish Medical Center in Seattle.
This unconventional primary endpoint for testing drugs that treat PsA was called out during discussion of the report for having an inherent bias favoring ixekizumab by its inclusion of a skin outcome that received equal weight with an assessment tool that focused on joint responses. “This was a very unusual primary endpoint that favored ixekizumab,” Roy M. Fleischmann, MD, a Dallas rheumatologist, commented during the discussion.
Dr. Mease readily admitted that the study’s design stacked the deck in favor of ixekizumab, but he added that this decision reflected a desire by the researchers who ran the study to choose a primary endpoint that represented both of the prominent pathologies seen in patients with PsA.
The primary endpoint used in the study “looks at PsA more holistically,” Dr. Mease said in an interview. “It forced clinicians to look beyond just the joints,” in PsA patients. “That has been a limitation of prior PsA treatment assessments,” which until this study have uniformly used single primary outcomes that focus on joint responses, most commonly the ACR20 measure of joint disease activity.
The SPIRIT-H2H (A Study of Ixekizumab [LY2439821] Versus Adalimumab in Participants With Psoriatic Arthritis) study enrolled adults with active PsA who had never before received treatment with a biological drug. Enrolled patients had to have both active disease in their joints and active plaque psoriasis, with an inadequate response to at least one conventional synthetic disease-modifying antirheumatic drug. The 566 patients randomized in the study averaged about 48 years old, a bit more than half were men, and patients averaged about 6 years with diagnosed PsA and about 15 years diagnosed with psoriasis. Just over two-thirds of the patients were on concurrent treatment with a conventional synthetic agent, most often methotrexate.
The two components of the primary endpoint each showed the anticipated result. Among the 269 patients treated with adalimumab for the full 24 weeks, 47% had an ACR50 response, as did 51% of the 262 patients who completed their full course of ixekizumab, a between-group difference that was not statistically significant. In contrast, the PASI 100 measure of complete skin resolution occurred in 47% of the adalimumab-treated patients and 60% of those on ixekizumab, a statistically significant difference.
Dr. Mease reported results for several other efficacy measures, and what was notable was statistically significant superiority for ixekizumab in a measure of entheses disease activity, the SPARCC [Spondyloarthritis Research Consortium of Canada] Enthesitis Index, which fell to zero in 57% of the ixekizumab patients and 45% of those on adalimumab. “It makes you wonder whether there is something special about interleukin-17 in enthesitis,” Dr. Mease said.
The safety results of the study were consistent with the known adverse effect profiles of both drugs.
The impact of these findings on practice remains to be seen, and will likely depend on both cost considerations as well as clinicians trying to tailor drug choices to individual patients. The relatively new drug, ixekizumab, is consequentially more expensive than the older adalimumab, which has had its price depressed by the recent introduction of a biosimilar agent as well as long-standing competition from multiple TNF inhibitors.
“I think in the United States insurers will continue to steer patients toward whichever drug is cheapest among the highly-effective options,” but this result should lead to more use of interleukin-17 inhibitors as second-line agents for PsA, and it might push some clinicians to prescribe it as the first-line treatment, Dr. Mease said. He was confident that the efficacy profile shown by ixekizumab in SPIRIT-H2H was likely a class effect. Economics aside, the impetus to prescribe ixekizumab or another interleukin-17 inhibitor will be greatest when a patient has more extensive skin involvement, while for patients with little or no skin symptoms clinicians will likely stick with the more established TNF inhibitors as the first drug class to prescribe for PsA.
SPIRIT-H2H was sponsored by Eli Lilly, the company that markets ixekizumab (Taltz). Dr. Mease has been a consultant to, speaker for, and received research funding from Eli Lilly and from several other companies. Dr. Fleischmann has been a consultant to and has received research funding from several companies including Eli Lilly.
SOURCE: Mease PJ et al. Ann Rheum Dis. 2019 Jun. doi: 10.1136/annrheumdis-2019-eular.8709.
The results from SPIRIT-H2H confirm with rigorously-collected, prospective data what we had already seen during our routine use of ixekizumab for treating patients with psoriatic arthritis: it does a better job of resolving skin manifestations than any tumor necrosis factor (TNF) inhibitor. For joint symptoms, the two drugs are similar, but for skin ixekizumab has substantial superiority.
I had been hopeful that inhibition of interleukin-17 would surpass TNF inhibition for resolution of joint symptoms, but it looks like they are similar. That’s a little below expectations. But working well for improving skin symptoms is important because it’s something that many patients care about, especially those with more substantial skin symptoms. When matching the best drug to each PsA patient other considerations also exist, such as ease of use. These drugs are delivered by different devices, and ease of administration also matters to patients.
I think it would be premature to presume that the effects shown by ixekizumab extrapolate to all the other interleukin-17 inhibitors. Some of these drugs act via different mechanisms, and so the SPIRIT-H2H results may very well not reflect a class effect.
Thomas Dörner, MD, is professor of rheumatology at Charité University Hospital in Berlin. He has been a consultant to Eli Lilly, as well as to AbbVie, Celgene, Novartis, Pfizer, and Roche, he has been a speaker on behalf of Amgen, Biogen, and Celgene, and he has received research funding from Chugai, Janssen, Roche, and Sanofi. He made these comments in an interview.
The results from SPIRIT-H2H confirm with rigorously-collected, prospective data what we had already seen during our routine use of ixekizumab for treating patients with psoriatic arthritis: it does a better job of resolving skin manifestations than any tumor necrosis factor (TNF) inhibitor. For joint symptoms, the two drugs are similar, but for skin ixekizumab has substantial superiority.
I had been hopeful that inhibition of interleukin-17 would surpass TNF inhibition for resolution of joint symptoms, but it looks like they are similar. That’s a little below expectations. But working well for improving skin symptoms is important because it’s something that many patients care about, especially those with more substantial skin symptoms. When matching the best drug to each PsA patient other considerations also exist, such as ease of use. These drugs are delivered by different devices, and ease of administration also matters to patients.
I think it would be premature to presume that the effects shown by ixekizumab extrapolate to all the other interleukin-17 inhibitors. Some of these drugs act via different mechanisms, and so the SPIRIT-H2H results may very well not reflect a class effect.
Thomas Dörner, MD, is professor of rheumatology at Charité University Hospital in Berlin. He has been a consultant to Eli Lilly, as well as to AbbVie, Celgene, Novartis, Pfizer, and Roche, he has been a speaker on behalf of Amgen, Biogen, and Celgene, and he has received research funding from Chugai, Janssen, Roche, and Sanofi. He made these comments in an interview.
The results from SPIRIT-H2H confirm with rigorously-collected, prospective data what we had already seen during our routine use of ixekizumab for treating patients with psoriatic arthritis: it does a better job of resolving skin manifestations than any tumor necrosis factor (TNF) inhibitor. For joint symptoms, the two drugs are similar, but for skin ixekizumab has substantial superiority.
I had been hopeful that inhibition of interleukin-17 would surpass TNF inhibition for resolution of joint symptoms, but it looks like they are similar. That’s a little below expectations. But working well for improving skin symptoms is important because it’s something that many patients care about, especially those with more substantial skin symptoms. When matching the best drug to each PsA patient other considerations also exist, such as ease of use. These drugs are delivered by different devices, and ease of administration also matters to patients.
I think it would be premature to presume that the effects shown by ixekizumab extrapolate to all the other interleukin-17 inhibitors. Some of these drugs act via different mechanisms, and so the SPIRIT-H2H results may very well not reflect a class effect.
Thomas Dörner, MD, is professor of rheumatology at Charité University Hospital in Berlin. He has been a consultant to Eli Lilly, as well as to AbbVie, Celgene, Novartis, Pfizer, and Roche, he has been a speaker on behalf of Amgen, Biogen, and Celgene, and he has received research funding from Chugai, Janssen, Roche, and Sanofi. He made these comments in an interview.
MADRID – The interleukin-17A inhibitor ixekizumab surpassed the tumor necrosis factor inhibitor adalimumab for treatment of patients with psoriatic arthritis in a multicenter, randomized study with 566 enrolled patients, the first reported results from a head-to-head comparison for this disease of two different classes of biological drugs.
The results showed that a standard, 24-week regimen with each of these agents, both of which already have regulatory approval for treating psoriatic arthritis (PsA), led to achievement of the primary endpoint in 36% of patients treated with ixekizumab (Taltz) and 28% of patients treated with adalimumab (Humira), a statistically significant difference, Philip J. Mease, MD, said at the European Congress of Rheumatology.
“Ixekizumab was superior to adalimumab for improving signs and symptoms of active PsA, as measured by simultaneous achievement of ACR50 [American College of Rheumatology] and PASI 100 [Psoriasis Area and Severity Index],” the study’s primary endpoint that combined a measure of joint disease activity with a measure of skin involvement, said Dr. Mease, a rheumatologist at Swedish Medical Center in Seattle.
This unconventional primary endpoint for testing drugs that treat PsA was called out during discussion of the report for having an inherent bias favoring ixekizumab by its inclusion of a skin outcome that received equal weight with an assessment tool that focused on joint responses. “This was a very unusual primary endpoint that favored ixekizumab,” Roy M. Fleischmann, MD, a Dallas rheumatologist, commented during the discussion.
Dr. Mease readily admitted that the study’s design stacked the deck in favor of ixekizumab, but he added that this decision reflected a desire by the researchers who ran the study to choose a primary endpoint that represented both of the prominent pathologies seen in patients with PsA.
The primary endpoint used in the study “looks at PsA more holistically,” Dr. Mease said in an interview. “It forced clinicians to look beyond just the joints,” in PsA patients. “That has been a limitation of prior PsA treatment assessments,” which until this study have uniformly used single primary outcomes that focus on joint responses, most commonly the ACR20 measure of joint disease activity.
The SPIRIT-H2H (A Study of Ixekizumab [LY2439821] Versus Adalimumab in Participants With Psoriatic Arthritis) study enrolled adults with active PsA who had never before received treatment with a biological drug. Enrolled patients had to have both active disease in their joints and active plaque psoriasis, with an inadequate response to at least one conventional synthetic disease-modifying antirheumatic drug. The 566 patients randomized in the study averaged about 48 years old, a bit more than half were men, and patients averaged about 6 years with diagnosed PsA and about 15 years diagnosed with psoriasis. Just over two-thirds of the patients were on concurrent treatment with a conventional synthetic agent, most often methotrexate.
The two components of the primary endpoint each showed the anticipated result. Among the 269 patients treated with adalimumab for the full 24 weeks, 47% had an ACR50 response, as did 51% of the 262 patients who completed their full course of ixekizumab, a between-group difference that was not statistically significant. In contrast, the PASI 100 measure of complete skin resolution occurred in 47% of the adalimumab-treated patients and 60% of those on ixekizumab, a statistically significant difference.
Dr. Mease reported results for several other efficacy measures, and what was notable was statistically significant superiority for ixekizumab in a measure of entheses disease activity, the SPARCC [Spondyloarthritis Research Consortium of Canada] Enthesitis Index, which fell to zero in 57% of the ixekizumab patients and 45% of those on adalimumab. “It makes you wonder whether there is something special about interleukin-17 in enthesitis,” Dr. Mease said.
The safety results of the study were consistent with the known adverse effect profiles of both drugs.
The impact of these findings on practice remains to be seen, and will likely depend on both cost considerations as well as clinicians trying to tailor drug choices to individual patients. The relatively new drug, ixekizumab, is consequentially more expensive than the older adalimumab, which has had its price depressed by the recent introduction of a biosimilar agent as well as long-standing competition from multiple TNF inhibitors.
“I think in the United States insurers will continue to steer patients toward whichever drug is cheapest among the highly-effective options,” but this result should lead to more use of interleukin-17 inhibitors as second-line agents for PsA, and it might push some clinicians to prescribe it as the first-line treatment, Dr. Mease said. He was confident that the efficacy profile shown by ixekizumab in SPIRIT-H2H was likely a class effect. Economics aside, the impetus to prescribe ixekizumab or another interleukin-17 inhibitor will be greatest when a patient has more extensive skin involvement, while for patients with little or no skin symptoms clinicians will likely stick with the more established TNF inhibitors as the first drug class to prescribe for PsA.
SPIRIT-H2H was sponsored by Eli Lilly, the company that markets ixekizumab (Taltz). Dr. Mease has been a consultant to, speaker for, and received research funding from Eli Lilly and from several other companies. Dr. Fleischmann has been a consultant to and has received research funding from several companies including Eli Lilly.
SOURCE: Mease PJ et al. Ann Rheum Dis. 2019 Jun. doi: 10.1136/annrheumdis-2019-eular.8709.
MADRID – The interleukin-17A inhibitor ixekizumab surpassed the tumor necrosis factor inhibitor adalimumab for treatment of patients with psoriatic arthritis in a multicenter, randomized study with 566 enrolled patients, the first reported results from a head-to-head comparison for this disease of two different classes of biological drugs.
The results showed that a standard, 24-week regimen with each of these agents, both of which already have regulatory approval for treating psoriatic arthritis (PsA), led to achievement of the primary endpoint in 36% of patients treated with ixekizumab (Taltz) and 28% of patients treated with adalimumab (Humira), a statistically significant difference, Philip J. Mease, MD, said at the European Congress of Rheumatology.
“Ixekizumab was superior to adalimumab for improving signs and symptoms of active PsA, as measured by simultaneous achievement of ACR50 [American College of Rheumatology] and PASI 100 [Psoriasis Area and Severity Index],” the study’s primary endpoint that combined a measure of joint disease activity with a measure of skin involvement, said Dr. Mease, a rheumatologist at Swedish Medical Center in Seattle.
This unconventional primary endpoint for testing drugs that treat PsA was called out during discussion of the report for having an inherent bias favoring ixekizumab by its inclusion of a skin outcome that received equal weight with an assessment tool that focused on joint responses. “This was a very unusual primary endpoint that favored ixekizumab,” Roy M. Fleischmann, MD, a Dallas rheumatologist, commented during the discussion.
Dr. Mease readily admitted that the study’s design stacked the deck in favor of ixekizumab, but he added that this decision reflected a desire by the researchers who ran the study to choose a primary endpoint that represented both of the prominent pathologies seen in patients with PsA.
The primary endpoint used in the study “looks at PsA more holistically,” Dr. Mease said in an interview. “It forced clinicians to look beyond just the joints,” in PsA patients. “That has been a limitation of prior PsA treatment assessments,” which until this study have uniformly used single primary outcomes that focus on joint responses, most commonly the ACR20 measure of joint disease activity.
The SPIRIT-H2H (A Study of Ixekizumab [LY2439821] Versus Adalimumab in Participants With Psoriatic Arthritis) study enrolled adults with active PsA who had never before received treatment with a biological drug. Enrolled patients had to have both active disease in their joints and active plaque psoriasis, with an inadequate response to at least one conventional synthetic disease-modifying antirheumatic drug. The 566 patients randomized in the study averaged about 48 years old, a bit more than half were men, and patients averaged about 6 years with diagnosed PsA and about 15 years diagnosed with psoriasis. Just over two-thirds of the patients were on concurrent treatment with a conventional synthetic agent, most often methotrexate.
The two components of the primary endpoint each showed the anticipated result. Among the 269 patients treated with adalimumab for the full 24 weeks, 47% had an ACR50 response, as did 51% of the 262 patients who completed their full course of ixekizumab, a between-group difference that was not statistically significant. In contrast, the PASI 100 measure of complete skin resolution occurred in 47% of the adalimumab-treated patients and 60% of those on ixekizumab, a statistically significant difference.
Dr. Mease reported results for several other efficacy measures, and what was notable was statistically significant superiority for ixekizumab in a measure of entheses disease activity, the SPARCC [Spondyloarthritis Research Consortium of Canada] Enthesitis Index, which fell to zero in 57% of the ixekizumab patients and 45% of those on adalimumab. “It makes you wonder whether there is something special about interleukin-17 in enthesitis,” Dr. Mease said.
The safety results of the study were consistent with the known adverse effect profiles of both drugs.
The impact of these findings on practice remains to be seen, and will likely depend on both cost considerations as well as clinicians trying to tailor drug choices to individual patients. The relatively new drug, ixekizumab, is consequentially more expensive than the older adalimumab, which has had its price depressed by the recent introduction of a biosimilar agent as well as long-standing competition from multiple TNF inhibitors.
“I think in the United States insurers will continue to steer patients toward whichever drug is cheapest among the highly-effective options,” but this result should lead to more use of interleukin-17 inhibitors as second-line agents for PsA, and it might push some clinicians to prescribe it as the first-line treatment, Dr. Mease said. He was confident that the efficacy profile shown by ixekizumab in SPIRIT-H2H was likely a class effect. Economics aside, the impetus to prescribe ixekizumab or another interleukin-17 inhibitor will be greatest when a patient has more extensive skin involvement, while for patients with little or no skin symptoms clinicians will likely stick with the more established TNF inhibitors as the first drug class to prescribe for PsA.
SPIRIT-H2H was sponsored by Eli Lilly, the company that markets ixekizumab (Taltz). Dr. Mease has been a consultant to, speaker for, and received research funding from Eli Lilly and from several other companies. Dr. Fleischmann has been a consultant to and has received research funding from several companies including Eli Lilly.
SOURCE: Mease PJ et al. Ann Rheum Dis. 2019 Jun. doi: 10.1136/annrheumdis-2019-eular.8709.
REPORTING FROM THE EULAR 2019 CONGRESS
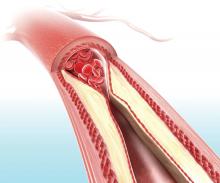
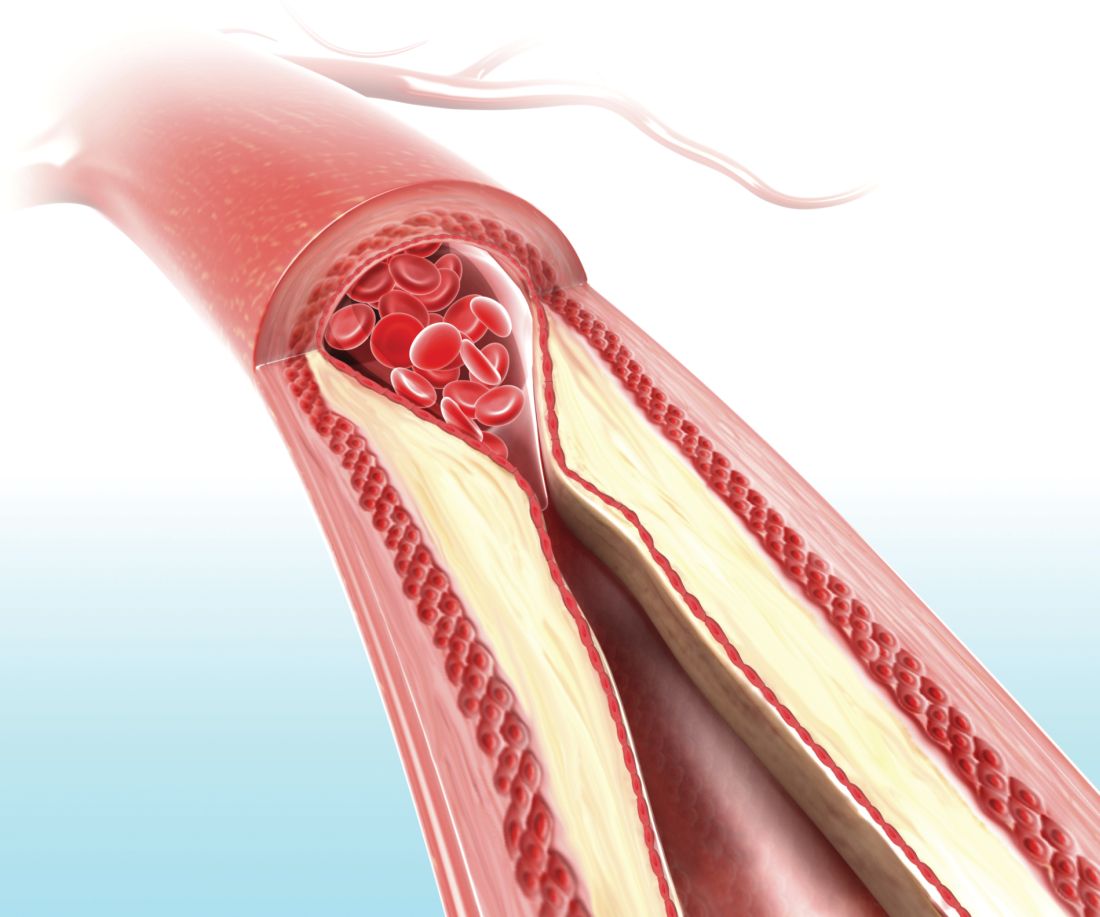
Carotid ultrasound may aid cardiovascular risk stratification of patients with psoriatic disease
according to findings from a retrospective study.
When added to the Framingham risk score, the measurement significantly improved its predictive ability, Curtis Sobchak, MD, and colleagues wrote in Arthritis & Rheumatology.
The findings indicate that carotid ultrasound could be a useful addition to cardiovascular risk stratification among these patients.
“Traditional algorithms do not consider other factors that may contribute to increased cardiovascular risk in rheumatic disease patients and tend to underestimate cardiovascular risk,” wrote Dr. Sobchak of the University of Toronto and coauthors.
“The advantage of ultrasound over other modalities for vascular imaging includes lack of radiation, low cost of the examination, and its widespread use in rheumatology for joint evaluation. Thus, this assessment could potentially be performed ‘at the bedside’ during consultation to provide immediate valuable information to complement clinical data from history, physical examination, and laboratory data,” they added.
The study retrospectively examined a prospective, observational cohort of 559 patients with psoriasis alone or psoriasis and psoriatic arthritis enrolled in the University of Toronto Psoriatic Disease Program. The investigators evaluated five ultrasound measures of atherosclerosis, including total plaque area (TPA), mean carotid intima-media thickness (cIMT), maximal cIMT, plaque category, and TPA category. Then they analyzed the risk relationship with major cardiovascular events (CVEs) classified as myocardial infarction, unstable angina, ischemic stroke, revascularization procedures, or cardiovascular-related death. Minor CVEs included stable angina, exacerbation of congestive heart failure, and transient ischemic attack over a mean follow-up close to 4 years.
The mean baseline TPA was 0.18 cm2 and mean cIMT was 639 mcm. Most patients had plaques, including 27.0% with unilateral and 31.5% with bilateral plaques.
The rate of a first CVE during the study period was 1.11 per 100 patient-years, and the rate of a first major CVE was 0.91 per 100 patient-years. The risk of each was significantly related to a higher baseline burden of atherosclerosis.
A multivariate analysis determined that increased TPA at baseline increased the risk of an event by nearly 200% (hazard ratio, 2.85). Mean cIMT was not an independent predictor in the final analysis, “suggesting that TPA is a stronger predictor for CVE than cIMT,” the authors wrote.
Finally, they examined the predictive value of atherosclerosis alone, as well as combined with the Framingham risk score. The 5-year model indicated that the bivariate model was slightly more accurate than the Framingham score alone (area under the curve, 0.84 vs. 0.81), although this was not a significant difference. The predictive value of the Framingham risk score plus maximal cIMT, mean cIMT, or TPA all significantly improved when they were calculated using only high-risk patients (those above the treatment threshold for dyslipidemia).
“To the best of our knowledge this is the first study to assess the utility of various measures of carotid atherosclerosis to predict CVE in patients with psoriasis and PsA [psoriatic arthritis]. ... Combining vascular imaging data with clinical and laboratory measures of traditional cardiovascular risk factors could improve accuracy of cardiovascular risk stratification in patients with psoriatic disease and facilitate earlier initiation of appropriate treatment to reduce CVE in this population,” the investigators wrote.
The study was supported in part by a Young Investigator Operating Grant from the Arthritis Society. Dr. Sobchak had no financial disclosures.
SOURCE: Sobchak C et al. Arthritis Rheumatol. 2019 Jun 5. doi: 10.1002/art.40925.
according to findings from a retrospective study.
When added to the Framingham risk score, the measurement significantly improved its predictive ability, Curtis Sobchak, MD, and colleagues wrote in Arthritis & Rheumatology.
The findings indicate that carotid ultrasound could be a useful addition to cardiovascular risk stratification among these patients.
“Traditional algorithms do not consider other factors that may contribute to increased cardiovascular risk in rheumatic disease patients and tend to underestimate cardiovascular risk,” wrote Dr. Sobchak of the University of Toronto and coauthors.
“The advantage of ultrasound over other modalities for vascular imaging includes lack of radiation, low cost of the examination, and its widespread use in rheumatology for joint evaluation. Thus, this assessment could potentially be performed ‘at the bedside’ during consultation to provide immediate valuable information to complement clinical data from history, physical examination, and laboratory data,” they added.
The study retrospectively examined a prospective, observational cohort of 559 patients with psoriasis alone or psoriasis and psoriatic arthritis enrolled in the University of Toronto Psoriatic Disease Program. The investigators evaluated five ultrasound measures of atherosclerosis, including total plaque area (TPA), mean carotid intima-media thickness (cIMT), maximal cIMT, plaque category, and TPA category. Then they analyzed the risk relationship with major cardiovascular events (CVEs) classified as myocardial infarction, unstable angina, ischemic stroke, revascularization procedures, or cardiovascular-related death. Minor CVEs included stable angina, exacerbation of congestive heart failure, and transient ischemic attack over a mean follow-up close to 4 years.
The mean baseline TPA was 0.18 cm2 and mean cIMT was 639 mcm. Most patients had plaques, including 27.0% with unilateral and 31.5% with bilateral plaques.
The rate of a first CVE during the study period was 1.11 per 100 patient-years, and the rate of a first major CVE was 0.91 per 100 patient-years. The risk of each was significantly related to a higher baseline burden of atherosclerosis.
A multivariate analysis determined that increased TPA at baseline increased the risk of an event by nearly 200% (hazard ratio, 2.85). Mean cIMT was not an independent predictor in the final analysis, “suggesting that TPA is a stronger predictor for CVE than cIMT,” the authors wrote.
Finally, they examined the predictive value of atherosclerosis alone, as well as combined with the Framingham risk score. The 5-year model indicated that the bivariate model was slightly more accurate than the Framingham score alone (area under the curve, 0.84 vs. 0.81), although this was not a significant difference. The predictive value of the Framingham risk score plus maximal cIMT, mean cIMT, or TPA all significantly improved when they were calculated using only high-risk patients (those above the treatment threshold for dyslipidemia).
“To the best of our knowledge this is the first study to assess the utility of various measures of carotid atherosclerosis to predict CVE in patients with psoriasis and PsA [psoriatic arthritis]. ... Combining vascular imaging data with clinical and laboratory measures of traditional cardiovascular risk factors could improve accuracy of cardiovascular risk stratification in patients with psoriatic disease and facilitate earlier initiation of appropriate treatment to reduce CVE in this population,” the investigators wrote.
The study was supported in part by a Young Investigator Operating Grant from the Arthritis Society. Dr. Sobchak had no financial disclosures.
SOURCE: Sobchak C et al. Arthritis Rheumatol. 2019 Jun 5. doi: 10.1002/art.40925.
according to findings from a retrospective study.
When added to the Framingham risk score, the measurement significantly improved its predictive ability, Curtis Sobchak, MD, and colleagues wrote in Arthritis & Rheumatology.
The findings indicate that carotid ultrasound could be a useful addition to cardiovascular risk stratification among these patients.
“Traditional algorithms do not consider other factors that may contribute to increased cardiovascular risk in rheumatic disease patients and tend to underestimate cardiovascular risk,” wrote Dr. Sobchak of the University of Toronto and coauthors.
“The advantage of ultrasound over other modalities for vascular imaging includes lack of radiation, low cost of the examination, and its widespread use in rheumatology for joint evaluation. Thus, this assessment could potentially be performed ‘at the bedside’ during consultation to provide immediate valuable information to complement clinical data from history, physical examination, and laboratory data,” they added.
The study retrospectively examined a prospective, observational cohort of 559 patients with psoriasis alone or psoriasis and psoriatic arthritis enrolled in the University of Toronto Psoriatic Disease Program. The investigators evaluated five ultrasound measures of atherosclerosis, including total plaque area (TPA), mean carotid intima-media thickness (cIMT), maximal cIMT, plaque category, and TPA category. Then they analyzed the risk relationship with major cardiovascular events (CVEs) classified as myocardial infarction, unstable angina, ischemic stroke, revascularization procedures, or cardiovascular-related death. Minor CVEs included stable angina, exacerbation of congestive heart failure, and transient ischemic attack over a mean follow-up close to 4 years.
The mean baseline TPA was 0.18 cm2 and mean cIMT was 639 mcm. Most patients had plaques, including 27.0% with unilateral and 31.5% with bilateral plaques.
The rate of a first CVE during the study period was 1.11 per 100 patient-years, and the rate of a first major CVE was 0.91 per 100 patient-years. The risk of each was significantly related to a higher baseline burden of atherosclerosis.
A multivariate analysis determined that increased TPA at baseline increased the risk of an event by nearly 200% (hazard ratio, 2.85). Mean cIMT was not an independent predictor in the final analysis, “suggesting that TPA is a stronger predictor for CVE than cIMT,” the authors wrote.
Finally, they examined the predictive value of atherosclerosis alone, as well as combined with the Framingham risk score. The 5-year model indicated that the bivariate model was slightly more accurate than the Framingham score alone (area under the curve, 0.84 vs. 0.81), although this was not a significant difference. The predictive value of the Framingham risk score plus maximal cIMT, mean cIMT, or TPA all significantly improved when they were calculated using only high-risk patients (those above the treatment threshold for dyslipidemia).
“To the best of our knowledge this is the first study to assess the utility of various measures of carotid atherosclerosis to predict CVE in patients with psoriasis and PsA [psoriatic arthritis]. ... Combining vascular imaging data with clinical and laboratory measures of traditional cardiovascular risk factors could improve accuracy of cardiovascular risk stratification in patients with psoriatic disease and facilitate earlier initiation of appropriate treatment to reduce CVE in this population,” the investigators wrote.
The study was supported in part by a Young Investigator Operating Grant from the Arthritis Society. Dr. Sobchak had no financial disclosures.
SOURCE: Sobchak C et al. Arthritis Rheumatol. 2019 Jun 5. doi: 10.1002/art.40925.
FROM ARTHRITIS & RHEUMATOLOGY
Psoriatic Arthritis Journal Scan: May 2019
The contribution of joint and skin improvements to the health-related quality of life of patients with psoriatic arthritis: a post hoc analysis of two randomised controlled studies.
Kavanaugh A, Gottlieb A, Morita A, et al. Ann Rheum Dis. 2019 May 21.
An integrated analysis to determine the contribution of joint and skin improvements to health-related quality of life (HRQoL) in patients with psoriatic arthritis (PsA). Optimal improvements in patients' HRQoL were dependent on successful treatment of both joint and skin symptoms.
Content validity and psychometric evaluation of Functional Assessment of Chronic Illness Therapy-Fatigue in patients with psoriatic arthritis.
Cella D, Wilson H, Shalhoub H, et al. J Patient Rep Outcomes. 2019 May 20;3(1):30.
One-on-one semi-structured qualitative interviews with adult patients evaluated the measurement properties (e.g., content validity, reliability, and ability to detect change) of the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale in patients with active psoriatic arthritis (PsA). Fatigue was confirmed to be an important symptom to patients with PsA, and FACIT-Fatigue was found to be a reliable and valid measure in this population.
Birth Outcomes and Disease Activity during Pregnancy in a Prospective Cohort of Women with Psoriatic Arthritis and Ankylosing Spondylitis.
Smith CJF, Bandoli G, Kavanaugh A, Chambers CD. Arthritis Care Res (Hoboken). 2019 May 10.
Women with PsA and AS have increased risk for selected adverse pregnancy outcomes. Compared to healthy controls (n=717), PsA (n = 117) was associated with increased risk for moderate preterm delivery (32-36 weeks' gestation), oligohydramnios, and Caesarean delivery. Active disease and corticosteroid use may increase the risk for some adverse pregnancy outcomes in women with these conditions.
Differential synovial tissue biomarkers among psoriatic arthritis and rheumatoid factor/anti-citrulline antibody-negative rheumatoid arthritis.
Alivernini S, Bruno D, Tolusso B, et al. Arthritis Res Ther. 2019 May 9;21(1):116.
The aim of the study was to identify synovial tissue (ST) biomarkers differentially expressed in PsA and seronegative rheumatoid arthritis and test their predictive value of therapeutic response. Histological analysis of ST may help to solve the clinical overlap between the two diseases and provides prognostic data about the therapy success.
Psoriatic arthritis - new perspectives.
Krakowski P, Gerkowicz A, Piertrzak A, et al. Arch Med Sci. 2019 May;15(3):580-589.
The management of PsA requires the care of a multidisciplinary team, which should include dermatologists, rheumatologists, physiotherapists, and orthopedic surgeons. PsA should be diagnosed as early as possible to slow down joint damage and progression of disability.
The contribution of joint and skin improvements to the health-related quality of life of patients with psoriatic arthritis: a post hoc analysis of two randomised controlled studies.
Kavanaugh A, Gottlieb A, Morita A, et al. Ann Rheum Dis. 2019 May 21.
An integrated analysis to determine the contribution of joint and skin improvements to health-related quality of life (HRQoL) in patients with psoriatic arthritis (PsA). Optimal improvements in patients' HRQoL were dependent on successful treatment of both joint and skin symptoms.
Content validity and psychometric evaluation of Functional Assessment of Chronic Illness Therapy-Fatigue in patients with psoriatic arthritis.
Cella D, Wilson H, Shalhoub H, et al. J Patient Rep Outcomes. 2019 May 20;3(1):30.
One-on-one semi-structured qualitative interviews with adult patients evaluated the measurement properties (e.g., content validity, reliability, and ability to detect change) of the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale in patients with active psoriatic arthritis (PsA). Fatigue was confirmed to be an important symptom to patients with PsA, and FACIT-Fatigue was found to be a reliable and valid measure in this population.
Birth Outcomes and Disease Activity during Pregnancy in a Prospective Cohort of Women with Psoriatic Arthritis and Ankylosing Spondylitis.
Smith CJF, Bandoli G, Kavanaugh A, Chambers CD. Arthritis Care Res (Hoboken). 2019 May 10.
Women with PsA and AS have increased risk for selected adverse pregnancy outcomes. Compared to healthy controls (n=717), PsA (n = 117) was associated with increased risk for moderate preterm delivery (32-36 weeks' gestation), oligohydramnios, and Caesarean delivery. Active disease and corticosteroid use may increase the risk for some adverse pregnancy outcomes in women with these conditions.
Differential synovial tissue biomarkers among psoriatic arthritis and rheumatoid factor/anti-citrulline antibody-negative rheumatoid arthritis.
Alivernini S, Bruno D, Tolusso B, et al. Arthritis Res Ther. 2019 May 9;21(1):116.
The aim of the study was to identify synovial tissue (ST) biomarkers differentially expressed in PsA and seronegative rheumatoid arthritis and test their predictive value of therapeutic response. Histological analysis of ST may help to solve the clinical overlap between the two diseases and provides prognostic data about the therapy success.
Psoriatic arthritis - new perspectives.
Krakowski P, Gerkowicz A, Piertrzak A, et al. Arch Med Sci. 2019 May;15(3):580-589.
The management of PsA requires the care of a multidisciplinary team, which should include dermatologists, rheumatologists, physiotherapists, and orthopedic surgeons. PsA should be diagnosed as early as possible to slow down joint damage and progression of disability.
The contribution of joint and skin improvements to the health-related quality of life of patients with psoriatic arthritis: a post hoc analysis of two randomised controlled studies.
Kavanaugh A, Gottlieb A, Morita A, et al. Ann Rheum Dis. 2019 May 21.
An integrated analysis to determine the contribution of joint and skin improvements to health-related quality of life (HRQoL) in patients with psoriatic arthritis (PsA). Optimal improvements in patients' HRQoL were dependent on successful treatment of both joint and skin symptoms.
Content validity and psychometric evaluation of Functional Assessment of Chronic Illness Therapy-Fatigue in patients with psoriatic arthritis.
Cella D, Wilson H, Shalhoub H, et al. J Patient Rep Outcomes. 2019 May 20;3(1):30.
One-on-one semi-structured qualitative interviews with adult patients evaluated the measurement properties (e.g., content validity, reliability, and ability to detect change) of the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale in patients with active psoriatic arthritis (PsA). Fatigue was confirmed to be an important symptom to patients with PsA, and FACIT-Fatigue was found to be a reliable and valid measure in this population.
Birth Outcomes and Disease Activity during Pregnancy in a Prospective Cohort of Women with Psoriatic Arthritis and Ankylosing Spondylitis.
Smith CJF, Bandoli G, Kavanaugh A, Chambers CD. Arthritis Care Res (Hoboken). 2019 May 10.
Women with PsA and AS have increased risk for selected adverse pregnancy outcomes. Compared to healthy controls (n=717), PsA (n = 117) was associated with increased risk for moderate preterm delivery (32-36 weeks' gestation), oligohydramnios, and Caesarean delivery. Active disease and corticosteroid use may increase the risk for some adverse pregnancy outcomes in women with these conditions.
Differential synovial tissue biomarkers among psoriatic arthritis and rheumatoid factor/anti-citrulline antibody-negative rheumatoid arthritis.
Alivernini S, Bruno D, Tolusso B, et al. Arthritis Res Ther. 2019 May 9;21(1):116.
The aim of the study was to identify synovial tissue (ST) biomarkers differentially expressed in PsA and seronegative rheumatoid arthritis and test their predictive value of therapeutic response. Histological analysis of ST may help to solve the clinical overlap between the two diseases and provides prognostic data about the therapy success.
Psoriatic arthritis - new perspectives.
Krakowski P, Gerkowicz A, Piertrzak A, et al. Arch Med Sci. 2019 May;15(3):580-589.
The management of PsA requires the care of a multidisciplinary team, which should include dermatologists, rheumatologists, physiotherapists, and orthopedic surgeons. PsA should be diagnosed as early as possible to slow down joint damage and progression of disability.
Active psoriatic arthritis, ankylosing spondylitis linked to increase in adverse pregnancy outcomes
Women with psoriatic arthritis and ankylosing spondylitis generally have favorable pregnancy outcomes, but high disease activity during pregnancy could increase the risk of adverse labor and delivery outcomes, according to 2004-2018 data from the Organization of Teratology Information Specialists (OTIS) Autoimmune Disease Project.
Corticosteroid use further increased risk for preterm delivery among women with ankylosing spondylitis.
While more research is needed, these findings suggest that better obstetric outcomes might be achieved via better disease control and minimal use of corticosteroids, according to Chelsey J. F. Smith, MD, of the University of California, San Diego, and colleagues.
“Future studies are needed to confirm the novel findings seen in our study, as well as to continue to analyze the effect of different disease activity measures and medication use on pregnancy outcomes in these two chronic conditions,” Dr. Smith and coauthors said in a report on the study in Arthritis Care and Research.
Many women affected by psoriatic arthritis and ankylosing spondylitis are of child-bearing age and consider planning a family, according to the researchers. Data on pregnancy outcomes are lacking, they said, “often making it difficult for rheumatologists and obstetricians to counsel their patients effectively.”
The study from Dr. Smith and coinvestigators comprised 963 women who enrolled in the OTIS prospective cohort study within 20 weeks of gestation and delivered at least one live-born infant. Of that cohort, 129 had ankylosing spondylitis, 117 had psoriatic arthritis, and the remaining 717 served as a control group.
Psoriatic arthritis conferred an 81% increased risk for moderate preterm delivery at 32-36 weeks gestation, compared with healthier women, 13.7% and 7.7% respectively. Risk was increased among women with psoriatic arthritis for preterm labor, 16.2% and 8.4% (adjusted risk ratio, 2.05, 95% confidence interval, 1.21-3.48), caesarean delivery, 48.7% and 26.2% (aRR, 1.63, 95% CI, 1.26-2.12), and oligohydramnios, 25% and 11% (aRR, 3.79, 95% CI, 1.34-10.74). Women with psoriatic arthritis were 2 years older on average and their average body mass index was 27 kg/m2 vs. 24.5 kg/m2 in the control group.
In women with ankylosing spondylitis, risk of infant hospitalization in the neonatal intensive care unit was increased by 67%, 17.2% vs. 11.9% in the control group.
Active disease measured by the Health Assessment Questionnaire (HAQ) or Routine Assessment of Patient Index Data 3 (RAPID3) was linked to increased risk of adverse obstetric outcomes in some cases, the investigators said.
For example, risk of preterm delivery was increased in women with psoriatic arthritis who had active disease at 32 weeks as measured by HAQ (27 women) and RAPID3 (28 women) scores, while in ankylosing spondylitis, active disease measured at intake by RAPID3 (46 women) was associated with increased risk of caesarean delivery.
Medication use in women with psoriatic arthritis was not associated with increased preterm delivery risk. However, women with ankylosing spondylitis who used corticosteroids in the second trimester had an increased risk of preterm delivery.
The rate of corticosteroid use was “surprisingly high” at 38% among the women with ankylosing spondylitis, Dr. Smith and coinvestigators said.
“The 2016 American College of Rheumatology guidelines in fact recommend against the use of systemic corticosteroids for the treatment of ankylosing spondylitis, with the exception of short-term treatment with rapid tapering in circumstances such as flares during pregnancy, flares of concomitant inflammatory bowel disease, or flare of peripheral arthritis,” they said in their report.
Dr. Smith and coauthors reported no conflicts of interest. The OTIS Collaborative Research Group has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Pfizer, and others.
SOURCE: Smith CJF et al. Arthritis Care Res. 2019 May 10. doi: 10.1002/acr.23924.
Women with psoriatic arthritis and ankylosing spondylitis generally have favorable pregnancy outcomes, but high disease activity during pregnancy could increase the risk of adverse labor and delivery outcomes, according to 2004-2018 data from the Organization of Teratology Information Specialists (OTIS) Autoimmune Disease Project.
Corticosteroid use further increased risk for preterm delivery among women with ankylosing spondylitis.
While more research is needed, these findings suggest that better obstetric outcomes might be achieved via better disease control and minimal use of corticosteroids, according to Chelsey J. F. Smith, MD, of the University of California, San Diego, and colleagues.
“Future studies are needed to confirm the novel findings seen in our study, as well as to continue to analyze the effect of different disease activity measures and medication use on pregnancy outcomes in these two chronic conditions,” Dr. Smith and coauthors said in a report on the study in Arthritis Care and Research.
Many women affected by psoriatic arthritis and ankylosing spondylitis are of child-bearing age and consider planning a family, according to the researchers. Data on pregnancy outcomes are lacking, they said, “often making it difficult for rheumatologists and obstetricians to counsel their patients effectively.”
The study from Dr. Smith and coinvestigators comprised 963 women who enrolled in the OTIS prospective cohort study within 20 weeks of gestation and delivered at least one live-born infant. Of that cohort, 129 had ankylosing spondylitis, 117 had psoriatic arthritis, and the remaining 717 served as a control group.
Psoriatic arthritis conferred an 81% increased risk for moderate preterm delivery at 32-36 weeks gestation, compared with healthier women, 13.7% and 7.7% respectively. Risk was increased among women with psoriatic arthritis for preterm labor, 16.2% and 8.4% (adjusted risk ratio, 2.05, 95% confidence interval, 1.21-3.48), caesarean delivery, 48.7% and 26.2% (aRR, 1.63, 95% CI, 1.26-2.12), and oligohydramnios, 25% and 11% (aRR, 3.79, 95% CI, 1.34-10.74). Women with psoriatic arthritis were 2 years older on average and their average body mass index was 27 kg/m2 vs. 24.5 kg/m2 in the control group.
In women with ankylosing spondylitis, risk of infant hospitalization in the neonatal intensive care unit was increased by 67%, 17.2% vs. 11.9% in the control group.
Active disease measured by the Health Assessment Questionnaire (HAQ) or Routine Assessment of Patient Index Data 3 (RAPID3) was linked to increased risk of adverse obstetric outcomes in some cases, the investigators said.
For example, risk of preterm delivery was increased in women with psoriatic arthritis who had active disease at 32 weeks as measured by HAQ (27 women) and RAPID3 (28 women) scores, while in ankylosing spondylitis, active disease measured at intake by RAPID3 (46 women) was associated with increased risk of caesarean delivery.
Medication use in women with psoriatic arthritis was not associated with increased preterm delivery risk. However, women with ankylosing spondylitis who used corticosteroids in the second trimester had an increased risk of preterm delivery.
The rate of corticosteroid use was “surprisingly high” at 38% among the women with ankylosing spondylitis, Dr. Smith and coinvestigators said.
“The 2016 American College of Rheumatology guidelines in fact recommend against the use of systemic corticosteroids for the treatment of ankylosing spondylitis, with the exception of short-term treatment with rapid tapering in circumstances such as flares during pregnancy, flares of concomitant inflammatory bowel disease, or flare of peripheral arthritis,” they said in their report.
Dr. Smith and coauthors reported no conflicts of interest. The OTIS Collaborative Research Group has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Pfizer, and others.
SOURCE: Smith CJF et al. Arthritis Care Res. 2019 May 10. doi: 10.1002/acr.23924.
Women with psoriatic arthritis and ankylosing spondylitis generally have favorable pregnancy outcomes, but high disease activity during pregnancy could increase the risk of adverse labor and delivery outcomes, according to 2004-2018 data from the Organization of Teratology Information Specialists (OTIS) Autoimmune Disease Project.
Corticosteroid use further increased risk for preterm delivery among women with ankylosing spondylitis.
While more research is needed, these findings suggest that better obstetric outcomes might be achieved via better disease control and minimal use of corticosteroids, according to Chelsey J. F. Smith, MD, of the University of California, San Diego, and colleagues.
“Future studies are needed to confirm the novel findings seen in our study, as well as to continue to analyze the effect of different disease activity measures and medication use on pregnancy outcomes in these two chronic conditions,” Dr. Smith and coauthors said in a report on the study in Arthritis Care and Research.
Many women affected by psoriatic arthritis and ankylosing spondylitis are of child-bearing age and consider planning a family, according to the researchers. Data on pregnancy outcomes are lacking, they said, “often making it difficult for rheumatologists and obstetricians to counsel their patients effectively.”
The study from Dr. Smith and coinvestigators comprised 963 women who enrolled in the OTIS prospective cohort study within 20 weeks of gestation and delivered at least one live-born infant. Of that cohort, 129 had ankylosing spondylitis, 117 had psoriatic arthritis, and the remaining 717 served as a control group.
Psoriatic arthritis conferred an 81% increased risk for moderate preterm delivery at 32-36 weeks gestation, compared with healthier women, 13.7% and 7.7% respectively. Risk was increased among women with psoriatic arthritis for preterm labor, 16.2% and 8.4% (adjusted risk ratio, 2.05, 95% confidence interval, 1.21-3.48), caesarean delivery, 48.7% and 26.2% (aRR, 1.63, 95% CI, 1.26-2.12), and oligohydramnios, 25% and 11% (aRR, 3.79, 95% CI, 1.34-10.74). Women with psoriatic arthritis were 2 years older on average and their average body mass index was 27 kg/m2 vs. 24.5 kg/m2 in the control group.
In women with ankylosing spondylitis, risk of infant hospitalization in the neonatal intensive care unit was increased by 67%, 17.2% vs. 11.9% in the control group.
Active disease measured by the Health Assessment Questionnaire (HAQ) or Routine Assessment of Patient Index Data 3 (RAPID3) was linked to increased risk of adverse obstetric outcomes in some cases, the investigators said.
For example, risk of preterm delivery was increased in women with psoriatic arthritis who had active disease at 32 weeks as measured by HAQ (27 women) and RAPID3 (28 women) scores, while in ankylosing spondylitis, active disease measured at intake by RAPID3 (46 women) was associated with increased risk of caesarean delivery.
Medication use in women with psoriatic arthritis was not associated with increased preterm delivery risk. However, women with ankylosing spondylitis who used corticosteroids in the second trimester had an increased risk of preterm delivery.
The rate of corticosteroid use was “surprisingly high” at 38% among the women with ankylosing spondylitis, Dr. Smith and coinvestigators said.
“The 2016 American College of Rheumatology guidelines in fact recommend against the use of systemic corticosteroids for the treatment of ankylosing spondylitis, with the exception of short-term treatment with rapid tapering in circumstances such as flares during pregnancy, flares of concomitant inflammatory bowel disease, or flare of peripheral arthritis,” they said in their report.
Dr. Smith and coauthors reported no conflicts of interest. The OTIS Collaborative Research Group has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Pfizer, and others.
SOURCE: Smith CJF et al. Arthritis Care Res. 2019 May 10. doi: 10.1002/acr.23924.
FROM ARTHRITIS CARE & RESEARCH
Th17-associated cytokines, CRP fail as biomarkers for ustekinumab success in PsA
Neither baseline Th17-associated cytokines nor C-reactive protein levels were predictive of response to ustekinumab in patients with psoriatic arthritis, despite significant reductions following treatment, according to Stefan Siebert, PhD, of the University of Glasgow (Scotland) and associates.
In a study published in Arthritis & Rheumatology, the authors retrospectively analyzed serum samples collected from 927 patients with psoriatic arthritis who participated in the phase 3 PSUMMIT trials. Patients received ustekinumab (Stelara) or placebo, and samples were collected at baseline, 4 weeks, and 24 weeks.
At baseline, interleukin-17A, -17F, and -23 levels were associated with skin disease scores, but neither these nor C-reactive protein (CRP) were associated with joint disease scores. While IL-17A, IL-17F, and CRP were reduced in patients who responded to ustekinumab, baseline levels of IL-17A, IL-17F, IL-23, and CRP were not associated with ustekinumab response in either skin or joints.
In patients who achieved a 75% reduction in their Psoriasis Area and Severity Index scores or a 20% reduction in their American College of Rheumatology response score after 24 weeks, CRP levels were significantly lower than in patients who did not achieve these scores (51%-58% vs. 32%-33%; P less than .05). However, IL-17A and IL-17F levels were not significantly different in these patients.
“While the biomarkers studied in the PSUMMIT program did not translate into therapeutic utility, it is important that relevant biomarker studies associated with phase 3 clinical trial programs are published in order to increase our understanding of this complex disease and further dissect the role of the IL-23/IL-17 pathway,” the investigators concluded.
The study was funded by Janssen; four coauthors reported being employed with Janssen.
SOURCE: Siebert S et al. Arthritis Rheumatol. 2019 May 9. doi: 10.1002/art.40921.
Neither baseline Th17-associated cytokines nor C-reactive protein levels were predictive of response to ustekinumab in patients with psoriatic arthritis, despite significant reductions following treatment, according to Stefan Siebert, PhD, of the University of Glasgow (Scotland) and associates.
In a study published in Arthritis & Rheumatology, the authors retrospectively analyzed serum samples collected from 927 patients with psoriatic arthritis who participated in the phase 3 PSUMMIT trials. Patients received ustekinumab (Stelara) or placebo, and samples were collected at baseline, 4 weeks, and 24 weeks.
At baseline, interleukin-17A, -17F, and -23 levels were associated with skin disease scores, but neither these nor C-reactive protein (CRP) were associated with joint disease scores. While IL-17A, IL-17F, and CRP were reduced in patients who responded to ustekinumab, baseline levels of IL-17A, IL-17F, IL-23, and CRP were not associated with ustekinumab response in either skin or joints.
In patients who achieved a 75% reduction in their Psoriasis Area and Severity Index scores or a 20% reduction in their American College of Rheumatology response score after 24 weeks, CRP levels were significantly lower than in patients who did not achieve these scores (51%-58% vs. 32%-33%; P less than .05). However, IL-17A and IL-17F levels were not significantly different in these patients.
“While the biomarkers studied in the PSUMMIT program did not translate into therapeutic utility, it is important that relevant biomarker studies associated with phase 3 clinical trial programs are published in order to increase our understanding of this complex disease and further dissect the role of the IL-23/IL-17 pathway,” the investigators concluded.
The study was funded by Janssen; four coauthors reported being employed with Janssen.
SOURCE: Siebert S et al. Arthritis Rheumatol. 2019 May 9. doi: 10.1002/art.40921.
Neither baseline Th17-associated cytokines nor C-reactive protein levels were predictive of response to ustekinumab in patients with psoriatic arthritis, despite significant reductions following treatment, according to Stefan Siebert, PhD, of the University of Glasgow (Scotland) and associates.
In a study published in Arthritis & Rheumatology, the authors retrospectively analyzed serum samples collected from 927 patients with psoriatic arthritis who participated in the phase 3 PSUMMIT trials. Patients received ustekinumab (Stelara) or placebo, and samples were collected at baseline, 4 weeks, and 24 weeks.
At baseline, interleukin-17A, -17F, and -23 levels were associated with skin disease scores, but neither these nor C-reactive protein (CRP) were associated with joint disease scores. While IL-17A, IL-17F, and CRP were reduced in patients who responded to ustekinumab, baseline levels of IL-17A, IL-17F, IL-23, and CRP were not associated with ustekinumab response in either skin or joints.
In patients who achieved a 75% reduction in their Psoriasis Area and Severity Index scores or a 20% reduction in their American College of Rheumatology response score after 24 weeks, CRP levels were significantly lower than in patients who did not achieve these scores (51%-58% vs. 32%-33%; P less than .05). However, IL-17A and IL-17F levels were not significantly different in these patients.
“While the biomarkers studied in the PSUMMIT program did not translate into therapeutic utility, it is important that relevant biomarker studies associated with phase 3 clinical trial programs are published in order to increase our understanding of this complex disease and further dissect the role of the IL-23/IL-17 pathway,” the investigators concluded.
The study was funded by Janssen; four coauthors reported being employed with Janssen.
SOURCE: Siebert S et al. Arthritis Rheumatol. 2019 May 9. doi: 10.1002/art.40921.
FROM ARTHRITIS & RHEUMATOLOGY
EMA: Stop high-dose Xeljanz in certain patients
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency has recommended that patients at high risk of blood clots in the lungs should not be prescribed the 10-mg, twice-daily dose of tofacitinib (Xeljanz).
The PRAC recommendation is based on results from an ongoing study of patients with rheumatoid arthritis (RA), which has shown that patients receiving the 10-mg, twice-daily dose – twice the approved dose for RA – are at an increased risk of blood clots in the lungs and death.
Patients at risk include those with heart failure, cancer, inherited blood clotting disorders, or a history of blood clots, as well as patients who take combined hormonal contraceptives, are receiving hormone replacement therapy, or are undergoing major surgery. In addition, age, obesity, smoking, or immobilization should also be considered as risk factors.
In Europe, tofacitinib is indicated for the treatment of RA, psoriatic arthritis, and severe ulcerative colitis. Because the 10-mg dose is the only indicated treatment for ulcerative colitis, patients with the disease and who are at high risk should not be started on tofacitinib, and patients who are already taking the drug should be switched to a different treatment.
“The new recommendations are temporary and follow previous PRAC advice not to exceed the recommended 5-mg, twice-daily dose when treating rheumatoid arthritis. The PRAC will now carry out a review of all available evidence, and updated guidance will be provided to patients and healthcare professionals once the review is concluded,” according to the European Medicines Agency.
Find the full press release on the European Medicines Agency website.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency has recommended that patients at high risk of blood clots in the lungs should not be prescribed the 10-mg, twice-daily dose of tofacitinib (Xeljanz).
The PRAC recommendation is based on results from an ongoing study of patients with rheumatoid arthritis (RA), which has shown that patients receiving the 10-mg, twice-daily dose – twice the approved dose for RA – are at an increased risk of blood clots in the lungs and death.
Patients at risk include those with heart failure, cancer, inherited blood clotting disorders, or a history of blood clots, as well as patients who take combined hormonal contraceptives, are receiving hormone replacement therapy, or are undergoing major surgery. In addition, age, obesity, smoking, or immobilization should also be considered as risk factors.
In Europe, tofacitinib is indicated for the treatment of RA, psoriatic arthritis, and severe ulcerative colitis. Because the 10-mg dose is the only indicated treatment for ulcerative colitis, patients with the disease and who are at high risk should not be started on tofacitinib, and patients who are already taking the drug should be switched to a different treatment.
“The new recommendations are temporary and follow previous PRAC advice not to exceed the recommended 5-mg, twice-daily dose when treating rheumatoid arthritis. The PRAC will now carry out a review of all available evidence, and updated guidance will be provided to patients and healthcare professionals once the review is concluded,” according to the European Medicines Agency.
Find the full press release on the European Medicines Agency website.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency has recommended that patients at high risk of blood clots in the lungs should not be prescribed the 10-mg, twice-daily dose of tofacitinib (Xeljanz).
The PRAC recommendation is based on results from an ongoing study of patients with rheumatoid arthritis (RA), which has shown that patients receiving the 10-mg, twice-daily dose – twice the approved dose for RA – are at an increased risk of blood clots in the lungs and death.
Patients at risk include those with heart failure, cancer, inherited blood clotting disorders, or a history of blood clots, as well as patients who take combined hormonal contraceptives, are receiving hormone replacement therapy, or are undergoing major surgery. In addition, age, obesity, smoking, or immobilization should also be considered as risk factors.
In Europe, tofacitinib is indicated for the treatment of RA, psoriatic arthritis, and severe ulcerative colitis. Because the 10-mg dose is the only indicated treatment for ulcerative colitis, patients with the disease and who are at high risk should not be started on tofacitinib, and patients who are already taking the drug should be switched to a different treatment.
“The new recommendations are temporary and follow previous PRAC advice not to exceed the recommended 5-mg, twice-daily dose when treating rheumatoid arthritis. The PRAC will now carry out a review of all available evidence, and updated guidance will be provided to patients and healthcare professionals once the review is concluded,” according to the European Medicines Agency.
Find the full press release on the European Medicines Agency website.
PsA Fast Facts: Symptoms



VA system lags in getting DMARDs to veterans with inflammatory arthritis
MADISON, WISC. – Only half of United States veterans with inflammatory arthritis received disease-modifying medication within 90 days of diagnosis if they received care within the Veterans Health Administration, according to a study presented at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Over the study period, 58.2% of all inflammatory arthritis patients began a disease-modifying antirheumatic drug (DMARD) within 12 months of diagnosis. Rates of DMARD initiation were similar for patients with rheumatoid arthritis (RA, 57.7%) and psoriatic arthritis (PsA, 64.3%), said the first author of the poster presentation, Sogol S. Amjadi, DO, a resident physician at Bingham Memorial Hospital, Blackfoot, Idaho.
However, at 12 months after diagnosis, only 29.6% of ankylosing spondylitis (AS) patients had not been started on a DMARD. “The ankylosing spondylitis group really had the lowest DMARD initiation over time,” said Dr. Amjadi in an interview.
The study used diagnosis codes and natural language processing to look for incident cases of the three inflammatory arthritides (IAs) among patients receiving care within the Veterans Health Administration from 2007 through 2015.
In all, 12,118 patients with incident IA were identified. Of these, 9,711 had RA, 1,472 had PsA, and 935 had AS. Patients were mostly (91.3%) male, with a mean age of 63.7 years.
Over the study period, 41.2% of IA patients were dispensed a DMARD within 30 days of diagnosis, and 50% received a DMARD within 90 days of diagnosis. Patients with PsA or RA had similar rates of DMARD prescription within 30 days of diagnosis (about 42% and 43%, respectively).
The investigators discovered in their analysis that another factor in prompt treatment was access to specialty care.“Timely access to a rheumatology provider is likely important for early DMARD treatment,” wrote Dr. Amjadi and her coauthors in the poster accompanying the presentation. Of patients who did receive a DMARD, 82.7% had received rheumatology specialty care before nonbiologic DMARD dispensing, as had 90.0% of patients receiving biologic DMARDs. Over the entire study period, about 10% of all IA patients had biologic DMARD exposure.
There was a trend over time for increased DMARD dispensing, said the investigators. “The percentage of IA patients with DMARD exposure during the 12-month follow-up period increased from 48.8% in 2008 to 66.4% in 2015.”
For AS patients, early DMARD prescribing rates rose from about 20% in 2007 to nearly 30% in 2015. “DMARD treatment rates during the initial 12 months after diagnosis increased between 2007 and 2015, but nontreatment remained common, particularly in patients with AS,” wrote the investigators. “Delays in treatment for inflammatory arthritis are associated with unfavorable outcomes, including impaired quality of life, irreversible joint damage, and disability.”
The authors reported no conflicts of interest and no outside sources of funding.
MADISON, WISC. – Only half of United States veterans with inflammatory arthritis received disease-modifying medication within 90 days of diagnosis if they received care within the Veterans Health Administration, according to a study presented at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Over the study period, 58.2% of all inflammatory arthritis patients began a disease-modifying antirheumatic drug (DMARD) within 12 months of diagnosis. Rates of DMARD initiation were similar for patients with rheumatoid arthritis (RA, 57.7%) and psoriatic arthritis (PsA, 64.3%), said the first author of the poster presentation, Sogol S. Amjadi, DO, a resident physician at Bingham Memorial Hospital, Blackfoot, Idaho.
However, at 12 months after diagnosis, only 29.6% of ankylosing spondylitis (AS) patients had not been started on a DMARD. “The ankylosing spondylitis group really had the lowest DMARD initiation over time,” said Dr. Amjadi in an interview.
The study used diagnosis codes and natural language processing to look for incident cases of the three inflammatory arthritides (IAs) among patients receiving care within the Veterans Health Administration from 2007 through 2015.
In all, 12,118 patients with incident IA were identified. Of these, 9,711 had RA, 1,472 had PsA, and 935 had AS. Patients were mostly (91.3%) male, with a mean age of 63.7 years.
Over the study period, 41.2% of IA patients were dispensed a DMARD within 30 days of diagnosis, and 50% received a DMARD within 90 days of diagnosis. Patients with PsA or RA had similar rates of DMARD prescription within 30 days of diagnosis (about 42% and 43%, respectively).
The investigators discovered in their analysis that another factor in prompt treatment was access to specialty care.“Timely access to a rheumatology provider is likely important for early DMARD treatment,” wrote Dr. Amjadi and her coauthors in the poster accompanying the presentation. Of patients who did receive a DMARD, 82.7% had received rheumatology specialty care before nonbiologic DMARD dispensing, as had 90.0% of patients receiving biologic DMARDs. Over the entire study period, about 10% of all IA patients had biologic DMARD exposure.
There was a trend over time for increased DMARD dispensing, said the investigators. “The percentage of IA patients with DMARD exposure during the 12-month follow-up period increased from 48.8% in 2008 to 66.4% in 2015.”
For AS patients, early DMARD prescribing rates rose from about 20% in 2007 to nearly 30% in 2015. “DMARD treatment rates during the initial 12 months after diagnosis increased between 2007 and 2015, but nontreatment remained common, particularly in patients with AS,” wrote the investigators. “Delays in treatment for inflammatory arthritis are associated with unfavorable outcomes, including impaired quality of life, irreversible joint damage, and disability.”
The authors reported no conflicts of interest and no outside sources of funding.
MADISON, WISC. – Only half of United States veterans with inflammatory arthritis received disease-modifying medication within 90 days of diagnosis if they received care within the Veterans Health Administration, according to a study presented at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Over the study period, 58.2% of all inflammatory arthritis patients began a disease-modifying antirheumatic drug (DMARD) within 12 months of diagnosis. Rates of DMARD initiation were similar for patients with rheumatoid arthritis (RA, 57.7%) and psoriatic arthritis (PsA, 64.3%), said the first author of the poster presentation, Sogol S. Amjadi, DO, a resident physician at Bingham Memorial Hospital, Blackfoot, Idaho.
However, at 12 months after diagnosis, only 29.6% of ankylosing spondylitis (AS) patients had not been started on a DMARD. “The ankylosing spondylitis group really had the lowest DMARD initiation over time,” said Dr. Amjadi in an interview.
The study used diagnosis codes and natural language processing to look for incident cases of the three inflammatory arthritides (IAs) among patients receiving care within the Veterans Health Administration from 2007 through 2015.
In all, 12,118 patients with incident IA were identified. Of these, 9,711 had RA, 1,472 had PsA, and 935 had AS. Patients were mostly (91.3%) male, with a mean age of 63.7 years.
Over the study period, 41.2% of IA patients were dispensed a DMARD within 30 days of diagnosis, and 50% received a DMARD within 90 days of diagnosis. Patients with PsA or RA had similar rates of DMARD prescription within 30 days of diagnosis (about 42% and 43%, respectively).
The investigators discovered in their analysis that another factor in prompt treatment was access to specialty care.“Timely access to a rheumatology provider is likely important for early DMARD treatment,” wrote Dr. Amjadi and her coauthors in the poster accompanying the presentation. Of patients who did receive a DMARD, 82.7% had received rheumatology specialty care before nonbiologic DMARD dispensing, as had 90.0% of patients receiving biologic DMARDs. Over the entire study period, about 10% of all IA patients had biologic DMARD exposure.
There was a trend over time for increased DMARD dispensing, said the investigators. “The percentage of IA patients with DMARD exposure during the 12-month follow-up period increased from 48.8% in 2008 to 66.4% in 2015.”
For AS patients, early DMARD prescribing rates rose from about 20% in 2007 to nearly 30% in 2015. “DMARD treatment rates during the initial 12 months after diagnosis increased between 2007 and 2015, but nontreatment remained common, particularly in patients with AS,” wrote the investigators. “Delays in treatment for inflammatory arthritis are associated with unfavorable outcomes, including impaired quality of life, irreversible joint damage, and disability.”
The authors reported no conflicts of interest and no outside sources of funding.
REPORTING FROM SPARTAN 2019
Key clinical point:
Major finding: Overall, 58.2% of inflammatory arthritis patients received a DMARD within the first year of diagnosis.
Study details: Retrospective review of 12,118 incident cases of inflammatory arthritis in the Veterans Health Administration during the period from 2007 through 2015.
Disclosures: The authors reported no conflicts of interest and no outside sources of funding.
Source: Amjadi SS et al. SPARTAN 2019.