User login
Home-use products show progress
DANA POINT, CALIF. – Nonablative fractional photothermolysis technology is a well-suited model for over-the-counter product development, especially within the 1430 nm to 1450 nm range, Dr. Brian S. Biesman said at a meeting sponsored by SkinCare Physicians and Northwestern University.
"I want to dispel the myth that there’s nothing in the home-use realm that works," said Dr. Biesman, director of the Nashville (Tenn.) Center for Laser and Facial Surgery. "There is a lot of money in the investment community tied up in the home-use realm, and there are some real significant devices in this area."
Considerations for adoption of intense pulsed light and laser devices for home use should involve "the exact same standards that we apply to the devices that we use in the office," Dr. Biesman said. These include the safety of core technology, in both use and misuse settings: tolerability, predictable efficacy, ease of use, affordable cost, robust premarket evidence, and alignment between claims and reality.
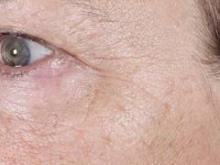
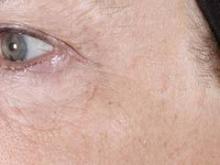
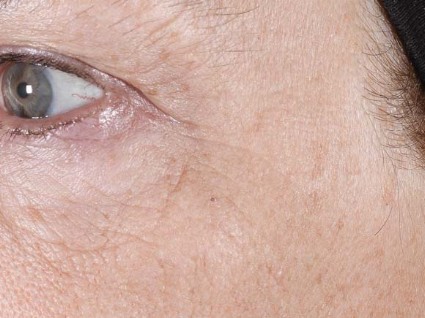
Three nonablative fractional laser options exist for home-based treatment of photodamaged skin: the 1435-nm Palomar PaloVia, the 1410-nm Solta RéAura (not yet FDA cleared), and the 1410-nm Tria Beauty SRL, which is also pending FDA clearance.
Dr. Biesman discussed the Tria SRL, a 1440-nm fractional nonablative laser device that can deliver energy up to 260 microns in depth with an adjustable energy range of 5-12 millijoules/pulse. "At first, given the parameters within which this device operated, I didn’t expect it to be clinically useful," Dr. Biesman noted.
In a safety, efficacy, and tolerability study sponsored by Tria, 90 patients aged 32-70 years old underwent treatment for dyschromia, periorbital wrinkles, and textural irregularities on the face. Of the 90 patients, 87 were women, 87 were white, and 62% had Fitzpatrick skin types II or III.
Patients underwent full face treatment 5 days/week for 12 weeks. They were then followed at 1 day, 2 weeks, 4 weeks, 8 weeks, and 12 weeks after the final treatment. Standard and polarized photos were taken on a VISIA CR system by Canfield Scientific, at baseline, every 2 weeks during treatment, and at each follow-up visit. Blinded investigators used a validated nine-point scale to evaluate each indication.
Dr. Biesman, who was not an investigator in the study, reported that investigator scoring showed statistically significant and clinically meaningful improvements in texture, periorbital wrinkles, and discoloration at 4 weeks and 12 weeks post treatment (all with a P value of less than.001). Common side effects included erythema, stinging/prickling sensations, and warm sensations. All side effects were reported to be mild and self-resolving, and no serious adverse events were reported.
Self-reported patient satisfaction ranged from 80%-90%, "which are similar numbers if you look at the subject satisfaction for the office-based nonablative devices," Dr. Biesman said.
Dr. Biesman advises clinicians to think of home-use laser devices for the treatment of photoaging, acne, and hair reduction "as prescriptives, much as we would retinoids. They’re not going to replace what we do in the office," he said. "But if someone has made a substantial investment for an office-based treatment plan, why not recommend something they can use at home that will help them maintain that outcome?"
In his opinion, nonablative resurfacing is "the next area of great opportunity" in home devices. "But I think the area of greatest opportunity is using these nonablative devices with other over-the-counter or prescriptive topical agents for laser-enhanced drug delivery," he noted.
"Using this approach, I believe we can accomplish some unique and very interesting objectives. This is an area that is only just beginning to be explored, but which holds tremendous potential. I look forward to the future of these devices as stand-alone treatments and to enhance drug delivery to facilitate reaching challenging therapeutic and aesthetic endpoints," he added.
Dr. Biesman disclosed that he is a consultant for and has received travel funds from Tria Beauty.
*Correction, 9/26/2013: An earlier version of this story included incorrect image order and captions.
DANA POINT, CALIF. – Nonablative fractional photothermolysis technology is a well-suited model for over-the-counter product development, especially within the 1430 nm to 1450 nm range, Dr. Brian S. Biesman said at a meeting sponsored by SkinCare Physicians and Northwestern University.
"I want to dispel the myth that there’s nothing in the home-use realm that works," said Dr. Biesman, director of the Nashville (Tenn.) Center for Laser and Facial Surgery. "There is a lot of money in the investment community tied up in the home-use realm, and there are some real significant devices in this area."
Considerations for adoption of intense pulsed light and laser devices for home use should involve "the exact same standards that we apply to the devices that we use in the office," Dr. Biesman said. These include the safety of core technology, in both use and misuse settings: tolerability, predictable efficacy, ease of use, affordable cost, robust premarket evidence, and alignment between claims and reality.
Three nonablative fractional laser options exist for home-based treatment of photodamaged skin: the 1435-nm Palomar PaloVia, the 1410-nm Solta RéAura (not yet FDA cleared), and the 1410-nm Tria Beauty SRL, which is also pending FDA clearance.
Dr. Biesman discussed the Tria SRL, a 1440-nm fractional nonablative laser device that can deliver energy up to 260 microns in depth with an adjustable energy range of 5-12 millijoules/pulse. "At first, given the parameters within which this device operated, I didn’t expect it to be clinically useful," Dr. Biesman noted.
In a safety, efficacy, and tolerability study sponsored by Tria, 90 patients aged 32-70 years old underwent treatment for dyschromia, periorbital wrinkles, and textural irregularities on the face. Of the 90 patients, 87 were women, 87 were white, and 62% had Fitzpatrick skin types II or III.
Patients underwent full face treatment 5 days/week for 12 weeks. They were then followed at 1 day, 2 weeks, 4 weeks, 8 weeks, and 12 weeks after the final treatment. Standard and polarized photos were taken on a VISIA CR system by Canfield Scientific, at baseline, every 2 weeks during treatment, and at each follow-up visit. Blinded investigators used a validated nine-point scale to evaluate each indication.
Dr. Biesman, who was not an investigator in the study, reported that investigator scoring showed statistically significant and clinically meaningful improvements in texture, periorbital wrinkles, and discoloration at 4 weeks and 12 weeks post treatment (all with a P value of less than.001). Common side effects included erythema, stinging/prickling sensations, and warm sensations. All side effects were reported to be mild and self-resolving, and no serious adverse events were reported.
Self-reported patient satisfaction ranged from 80%-90%, "which are similar numbers if you look at the subject satisfaction for the office-based nonablative devices," Dr. Biesman said.
Dr. Biesman advises clinicians to think of home-use laser devices for the treatment of photoaging, acne, and hair reduction "as prescriptives, much as we would retinoids. They’re not going to replace what we do in the office," he said. "But if someone has made a substantial investment for an office-based treatment plan, why not recommend something they can use at home that will help them maintain that outcome?"
In his opinion, nonablative resurfacing is "the next area of great opportunity" in home devices. "But I think the area of greatest opportunity is using these nonablative devices with other over-the-counter or prescriptive topical agents for laser-enhanced drug delivery," he noted.
"Using this approach, I believe we can accomplish some unique and very interesting objectives. This is an area that is only just beginning to be explored, but which holds tremendous potential. I look forward to the future of these devices as stand-alone treatments and to enhance drug delivery to facilitate reaching challenging therapeutic and aesthetic endpoints," he added.
Dr. Biesman disclosed that he is a consultant for and has received travel funds from Tria Beauty.
*Correction, 9/26/2013: An earlier version of this story included incorrect image order and captions.
DANA POINT, CALIF. – Nonablative fractional photothermolysis technology is a well-suited model for over-the-counter product development, especially within the 1430 nm to 1450 nm range, Dr. Brian S. Biesman said at a meeting sponsored by SkinCare Physicians and Northwestern University.
"I want to dispel the myth that there’s nothing in the home-use realm that works," said Dr. Biesman, director of the Nashville (Tenn.) Center for Laser and Facial Surgery. "There is a lot of money in the investment community tied up in the home-use realm, and there are some real significant devices in this area."
Considerations for adoption of intense pulsed light and laser devices for home use should involve "the exact same standards that we apply to the devices that we use in the office," Dr. Biesman said. These include the safety of core technology, in both use and misuse settings: tolerability, predictable efficacy, ease of use, affordable cost, robust premarket evidence, and alignment between claims and reality.
Three nonablative fractional laser options exist for home-based treatment of photodamaged skin: the 1435-nm Palomar PaloVia, the 1410-nm Solta RéAura (not yet FDA cleared), and the 1410-nm Tria Beauty SRL, which is also pending FDA clearance.
Dr. Biesman discussed the Tria SRL, a 1440-nm fractional nonablative laser device that can deliver energy up to 260 microns in depth with an adjustable energy range of 5-12 millijoules/pulse. "At first, given the parameters within which this device operated, I didn’t expect it to be clinically useful," Dr. Biesman noted.
In a safety, efficacy, and tolerability study sponsored by Tria, 90 patients aged 32-70 years old underwent treatment for dyschromia, periorbital wrinkles, and textural irregularities on the face. Of the 90 patients, 87 were women, 87 were white, and 62% had Fitzpatrick skin types II or III.
Patients underwent full face treatment 5 days/week for 12 weeks. They were then followed at 1 day, 2 weeks, 4 weeks, 8 weeks, and 12 weeks after the final treatment. Standard and polarized photos were taken on a VISIA CR system by Canfield Scientific, at baseline, every 2 weeks during treatment, and at each follow-up visit. Blinded investigators used a validated nine-point scale to evaluate each indication.
Dr. Biesman, who was not an investigator in the study, reported that investigator scoring showed statistically significant and clinically meaningful improvements in texture, periorbital wrinkles, and discoloration at 4 weeks and 12 weeks post treatment (all with a P value of less than.001). Common side effects included erythema, stinging/prickling sensations, and warm sensations. All side effects were reported to be mild and self-resolving, and no serious adverse events were reported.
Self-reported patient satisfaction ranged from 80%-90%, "which are similar numbers if you look at the subject satisfaction for the office-based nonablative devices," Dr. Biesman said.
Dr. Biesman advises clinicians to think of home-use laser devices for the treatment of photoaging, acne, and hair reduction "as prescriptives, much as we would retinoids. They’re not going to replace what we do in the office," he said. "But if someone has made a substantial investment for an office-based treatment plan, why not recommend something they can use at home that will help them maintain that outcome?"
In his opinion, nonablative resurfacing is "the next area of great opportunity" in home devices. "But I think the area of greatest opportunity is using these nonablative devices with other over-the-counter or prescriptive topical agents for laser-enhanced drug delivery," he noted.
"Using this approach, I believe we can accomplish some unique and very interesting objectives. This is an area that is only just beginning to be explored, but which holds tremendous potential. I look forward to the future of these devices as stand-alone treatments and to enhance drug delivery to facilitate reaching challenging therapeutic and aesthetic endpoints," he added.
Dr. Biesman disclosed that he is a consultant for and has received travel funds from Tria Beauty.
*Correction, 9/26/2013: An earlier version of this story included incorrect image order and captions.
EXPERT ANALYSIS FROM CONTROVERSIES AND CONVERSATIONS IN LASER AND COSMETIC SURGERY
Perioral dermatitis and diet
Perioral dermatitis is a common and frustrating skin condition that is often treatment resistant and recurs when treatment stops. Perioral dermatitis is classified in the rosacea family of skin diseases, and it is often associated with fair skin, light eyes, and marked actinic damage.
Although it is common in white skin, perioral dermatitis is underdiagnosed and an increasing problem in skin of color as well. The condition often begins as a papular, erythematous rash around the mouth. In darker skin types, however, it is often misdiagnosed because the erythema is masked by the skin pigmentation, so it appears as reddish-brown or even hyperpigmented papules around the mouth or eyes.
Popular treatments for perioral dermatitis include oral doxycycline, topical metronidazole, and topical tacrolimus. Often patients self-treat with topical corticosteroids for quick relief, which can initially improve the condition. However, corticosteroid use can result in exacerbation of the disease once the steroid is stopped, and often leads to recalcitrant cases. In skin of color patients, topical steroids used around the mouth and eyes also cause hypopigmentation of the skin, which further masks the clinical presentation of the disease and contributes to underdiagnosis and improper management.
In my practice, I have seen a consistent link between perioral dermatitis in skin of color patients and diet. Often patients who develop the rash have gluten sensitivity or mild, undiagnosed gluten intolerance. When these patients are switched to a gluten-free diet, their skin condition improves. Similarly, patients with no clinically diagnosed gluten sensitivity but who adopt a carbohydrate-free/low-glycemic-index and high-protein diet have shown dramatic improvement with minimal oral or topical treatments and less recurrence.
Although there are no well-controlled studies – or even case reports – linking carbohydrate or gluten intake to perioral dermatitis, studies have shown a strong link between diet and rosacea. Erythematotelangiectatic and papulopustular rosacea are known to be exacerbated by alcohol, hot or spicy foods, and chocolate. However, the common ingredient in these foods has never been identified as a link to the exacerbation of the disease. As all of the aforementioned foods often contain carbohydrates, could the common link simply be carbs or processed sugar?
Carbohydrates are the most common nutrients in the American diet. Often, emigrants to the United States develop perioral dermatitis or other inflammatory skin conditions such as acne and rosacea that they did not have in their home countries. Perhaps the Paleo or Mediterranean diets that have become popular for weight loss help control both bowel and skin inflammation. More studies are needed to better define the complex relationship and causality between diet and perioral dermatitis. In the meantime, I have been recommending carb-free diets in addition to topical tacrolimus or metronidazole for my skin of color patients with perioral dermatitis to prevent recurrences, and I have seen excellent results.
Dr. Talakoub is in private practice in McLean, Va.
Do you have questions about treating patients with dark skin? If so, send them to [email protected].
Perioral dermatitis is a common and frustrating skin condition that is often treatment resistant and recurs when treatment stops. Perioral dermatitis is classified in the rosacea family of skin diseases, and it is often associated with fair skin, light eyes, and marked actinic damage.
Although it is common in white skin, perioral dermatitis is underdiagnosed and an increasing problem in skin of color as well. The condition often begins as a papular, erythematous rash around the mouth. In darker skin types, however, it is often misdiagnosed because the erythema is masked by the skin pigmentation, so it appears as reddish-brown or even hyperpigmented papules around the mouth or eyes.
Popular treatments for perioral dermatitis include oral doxycycline, topical metronidazole, and topical tacrolimus. Often patients self-treat with topical corticosteroids for quick relief, which can initially improve the condition. However, corticosteroid use can result in exacerbation of the disease once the steroid is stopped, and often leads to recalcitrant cases. In skin of color patients, topical steroids used around the mouth and eyes also cause hypopigmentation of the skin, which further masks the clinical presentation of the disease and contributes to underdiagnosis and improper management.
In my practice, I have seen a consistent link between perioral dermatitis in skin of color patients and diet. Often patients who develop the rash have gluten sensitivity or mild, undiagnosed gluten intolerance. When these patients are switched to a gluten-free diet, their skin condition improves. Similarly, patients with no clinically diagnosed gluten sensitivity but who adopt a carbohydrate-free/low-glycemic-index and high-protein diet have shown dramatic improvement with minimal oral or topical treatments and less recurrence.
Although there are no well-controlled studies – or even case reports – linking carbohydrate or gluten intake to perioral dermatitis, studies have shown a strong link between diet and rosacea. Erythematotelangiectatic and papulopustular rosacea are known to be exacerbated by alcohol, hot or spicy foods, and chocolate. However, the common ingredient in these foods has never been identified as a link to the exacerbation of the disease. As all of the aforementioned foods often contain carbohydrates, could the common link simply be carbs or processed sugar?
Carbohydrates are the most common nutrients in the American diet. Often, emigrants to the United States develop perioral dermatitis or other inflammatory skin conditions such as acne and rosacea that they did not have in their home countries. Perhaps the Paleo or Mediterranean diets that have become popular for weight loss help control both bowel and skin inflammation. More studies are needed to better define the complex relationship and causality between diet and perioral dermatitis. In the meantime, I have been recommending carb-free diets in addition to topical tacrolimus or metronidazole for my skin of color patients with perioral dermatitis to prevent recurrences, and I have seen excellent results.
Dr. Talakoub is in private practice in McLean, Va.
Do you have questions about treating patients with dark skin? If so, send them to [email protected].
Perioral dermatitis is a common and frustrating skin condition that is often treatment resistant and recurs when treatment stops. Perioral dermatitis is classified in the rosacea family of skin diseases, and it is often associated with fair skin, light eyes, and marked actinic damage.
Although it is common in white skin, perioral dermatitis is underdiagnosed and an increasing problem in skin of color as well. The condition often begins as a papular, erythematous rash around the mouth. In darker skin types, however, it is often misdiagnosed because the erythema is masked by the skin pigmentation, so it appears as reddish-brown or even hyperpigmented papules around the mouth or eyes.
Popular treatments for perioral dermatitis include oral doxycycline, topical metronidazole, and topical tacrolimus. Often patients self-treat with topical corticosteroids for quick relief, which can initially improve the condition. However, corticosteroid use can result in exacerbation of the disease once the steroid is stopped, and often leads to recalcitrant cases. In skin of color patients, topical steroids used around the mouth and eyes also cause hypopigmentation of the skin, which further masks the clinical presentation of the disease and contributes to underdiagnosis and improper management.
In my practice, I have seen a consistent link between perioral dermatitis in skin of color patients and diet. Often patients who develop the rash have gluten sensitivity or mild, undiagnosed gluten intolerance. When these patients are switched to a gluten-free diet, their skin condition improves. Similarly, patients with no clinically diagnosed gluten sensitivity but who adopt a carbohydrate-free/low-glycemic-index and high-protein diet have shown dramatic improvement with minimal oral or topical treatments and less recurrence.
Although there are no well-controlled studies – or even case reports – linking carbohydrate or gluten intake to perioral dermatitis, studies have shown a strong link between diet and rosacea. Erythematotelangiectatic and papulopustular rosacea are known to be exacerbated by alcohol, hot or spicy foods, and chocolate. However, the common ingredient in these foods has never been identified as a link to the exacerbation of the disease. As all of the aforementioned foods often contain carbohydrates, could the common link simply be carbs or processed sugar?
Carbohydrates are the most common nutrients in the American diet. Often, emigrants to the United States develop perioral dermatitis or other inflammatory skin conditions such as acne and rosacea that they did not have in their home countries. Perhaps the Paleo or Mediterranean diets that have become popular for weight loss help control both bowel and skin inflammation. More studies are needed to better define the complex relationship and causality between diet and perioral dermatitis. In the meantime, I have been recommending carb-free diets in addition to topical tacrolimus or metronidazole for my skin of color patients with perioral dermatitis to prevent recurrences, and I have seen excellent results.
Dr. Talakoub is in private practice in McLean, Va.
Do you have questions about treating patients with dark skin? If so, send them to [email protected].
1,927-nm laser unveils improvements for melasma patients
DANA POINT, CALIF. – Patients with melasma who underwent treatment with a new low-energy and low-density nonablative fractional 1,927-nm diode laser experienced significant reduction of hyperpigmentation with limited side effects, a single-center study demonstrated.
At a meeting sponsored by SkinCare Physicians and Northwestern University, Dr. Roy G. Geronemus discussed his experience treating patients with the FDA-cleared technology, which is known as the Clear + Brilliant Permea.
Dr. Geronemus of the Laser and Skin Surgery Center of New York noted that existing laser treatments have so far failed to yield a consistent and long-term reduction in pigmentation, especially in patients with darker skin types. "Many current [laser] treatments for melasma make the condition worse," he said "What we ideally need is something that will be helpful, will not make it worse, and that can be repeated, because [melasma] probably will recur over time."
In an ongoing prospective study, Dr. Geronemus and his associates evaluated the 1,927-nm diode laser in melasma patients with a hunch that it would improve pigmentation and appearance with an improved safety profile and overall treatment outcomes. They enrolled patients aged 18-65 years with Fitzpatrick skin types I-VI who had clinical evidence of melasma or postinflammatory hyperpigmentation. They excluded patients who were either pregnant, breast-feeding, contemplating pregnancy, or not using effective means of birth control, as well as those known to be hypersensitive to light exposure and those with a history of melanoma or nonmelanoma skin cancer, keloidal scarring, immunosuppression, or immune deficiency disorder. Images were taken at baseline, prior to each treatment, and at follow-up using the Canfield VISIA complexion analysis system.
Up to six treatments were performed every 2 weeks, Dr. Geronemus said, with spot sizes of 100-180 mcm, energy of 5 mJ, 5-7.5% treatment coverage, and an average of 4-12 passes.
The researchers asked patients to rate their pain after each treatment, as well as their overall improvement in pigmentation. Pain was assessed on an 11-point scale, with 0 being none and 10 being "intolerable." Pigmentation improvement was measured on a 5-point scale, with 0 being none, 1 being mild (1-25%), 2 being moderate (26-50%), 3 being marked (51-75%), and 4 being very significant (76-100%). At the final 3-month visit, patients were asked to rate their overall satisfaction with the treatment on a 5-point scale ranging from very dissatisfied (1) to very satisfied (5).
Dr. Geronemus presented results from 14 patients who had completed 3-month follow-up visits. These 14 women included 10 with melasma and 4 with postinflammatory hyperpigmentation. The mean age of the patients was 42 years, and 9 had Fitzpatrick skin types I-III.
The patients rated their pain as 3.25 out of 10, their overall pigment improvement as 3 out of 4, and their overall satisfaction with the procedure as a 4.33 out of 5, Dr. Geronemus reported. "This is often technique sensitive," he said of the procedure. "I’ll have physicians call me up and say, ‘I’m not getting any results.’ I think you need 10-12 passes to get the [optimal] results."
The most common side effect was erythema, which typically resolved within 1 day. Though the device is effective as monotherapy, Dr. Geronemus pointed out that it is "ideally suited for combination therapy with a mild hydroquinone."
Dr. Geronemus disclosed that he serves on the medical advisory boards for Zeltiq, Syneron/Candela, and Cynosure. He also serves as an investigator for numerous device and pharmaceutical companies, and he holds stock in Zeltiq and OnLight Sciences.
DANA POINT, CALIF. – Patients with melasma who underwent treatment with a new low-energy and low-density nonablative fractional 1,927-nm diode laser experienced significant reduction of hyperpigmentation with limited side effects, a single-center study demonstrated.
At a meeting sponsored by SkinCare Physicians and Northwestern University, Dr. Roy G. Geronemus discussed his experience treating patients with the FDA-cleared technology, which is known as the Clear + Brilliant Permea.
Dr. Geronemus of the Laser and Skin Surgery Center of New York noted that existing laser treatments have so far failed to yield a consistent and long-term reduction in pigmentation, especially in patients with darker skin types. "Many current [laser] treatments for melasma make the condition worse," he said "What we ideally need is something that will be helpful, will not make it worse, and that can be repeated, because [melasma] probably will recur over time."
In an ongoing prospective study, Dr. Geronemus and his associates evaluated the 1,927-nm diode laser in melasma patients with a hunch that it would improve pigmentation and appearance with an improved safety profile and overall treatment outcomes. They enrolled patients aged 18-65 years with Fitzpatrick skin types I-VI who had clinical evidence of melasma or postinflammatory hyperpigmentation. They excluded patients who were either pregnant, breast-feeding, contemplating pregnancy, or not using effective means of birth control, as well as those known to be hypersensitive to light exposure and those with a history of melanoma or nonmelanoma skin cancer, keloidal scarring, immunosuppression, or immune deficiency disorder. Images were taken at baseline, prior to each treatment, and at follow-up using the Canfield VISIA complexion analysis system.
Up to six treatments were performed every 2 weeks, Dr. Geronemus said, with spot sizes of 100-180 mcm, energy of 5 mJ, 5-7.5% treatment coverage, and an average of 4-12 passes.
The researchers asked patients to rate their pain after each treatment, as well as their overall improvement in pigmentation. Pain was assessed on an 11-point scale, with 0 being none and 10 being "intolerable." Pigmentation improvement was measured on a 5-point scale, with 0 being none, 1 being mild (1-25%), 2 being moderate (26-50%), 3 being marked (51-75%), and 4 being very significant (76-100%). At the final 3-month visit, patients were asked to rate their overall satisfaction with the treatment on a 5-point scale ranging from very dissatisfied (1) to very satisfied (5).
Dr. Geronemus presented results from 14 patients who had completed 3-month follow-up visits. These 14 women included 10 with melasma and 4 with postinflammatory hyperpigmentation. The mean age of the patients was 42 years, and 9 had Fitzpatrick skin types I-III.
The patients rated their pain as 3.25 out of 10, their overall pigment improvement as 3 out of 4, and their overall satisfaction with the procedure as a 4.33 out of 5, Dr. Geronemus reported. "This is often technique sensitive," he said of the procedure. "I’ll have physicians call me up and say, ‘I’m not getting any results.’ I think you need 10-12 passes to get the [optimal] results."
The most common side effect was erythema, which typically resolved within 1 day. Though the device is effective as monotherapy, Dr. Geronemus pointed out that it is "ideally suited for combination therapy with a mild hydroquinone."
Dr. Geronemus disclosed that he serves on the medical advisory boards for Zeltiq, Syneron/Candela, and Cynosure. He also serves as an investigator for numerous device and pharmaceutical companies, and he holds stock in Zeltiq and OnLight Sciences.
DANA POINT, CALIF. – Patients with melasma who underwent treatment with a new low-energy and low-density nonablative fractional 1,927-nm diode laser experienced significant reduction of hyperpigmentation with limited side effects, a single-center study demonstrated.
At a meeting sponsored by SkinCare Physicians and Northwestern University, Dr. Roy G. Geronemus discussed his experience treating patients with the FDA-cleared technology, which is known as the Clear + Brilliant Permea.
Dr. Geronemus of the Laser and Skin Surgery Center of New York noted that existing laser treatments have so far failed to yield a consistent and long-term reduction in pigmentation, especially in patients with darker skin types. "Many current [laser] treatments for melasma make the condition worse," he said "What we ideally need is something that will be helpful, will not make it worse, and that can be repeated, because [melasma] probably will recur over time."
In an ongoing prospective study, Dr. Geronemus and his associates evaluated the 1,927-nm diode laser in melasma patients with a hunch that it would improve pigmentation and appearance with an improved safety profile and overall treatment outcomes. They enrolled patients aged 18-65 years with Fitzpatrick skin types I-VI who had clinical evidence of melasma or postinflammatory hyperpigmentation. They excluded patients who were either pregnant, breast-feeding, contemplating pregnancy, or not using effective means of birth control, as well as those known to be hypersensitive to light exposure and those with a history of melanoma or nonmelanoma skin cancer, keloidal scarring, immunosuppression, or immune deficiency disorder. Images were taken at baseline, prior to each treatment, and at follow-up using the Canfield VISIA complexion analysis system.
Up to six treatments were performed every 2 weeks, Dr. Geronemus said, with spot sizes of 100-180 mcm, energy of 5 mJ, 5-7.5% treatment coverage, and an average of 4-12 passes.
The researchers asked patients to rate their pain after each treatment, as well as their overall improvement in pigmentation. Pain was assessed on an 11-point scale, with 0 being none and 10 being "intolerable." Pigmentation improvement was measured on a 5-point scale, with 0 being none, 1 being mild (1-25%), 2 being moderate (26-50%), 3 being marked (51-75%), and 4 being very significant (76-100%). At the final 3-month visit, patients were asked to rate their overall satisfaction with the treatment on a 5-point scale ranging from very dissatisfied (1) to very satisfied (5).
Dr. Geronemus presented results from 14 patients who had completed 3-month follow-up visits. These 14 women included 10 with melasma and 4 with postinflammatory hyperpigmentation. The mean age of the patients was 42 years, and 9 had Fitzpatrick skin types I-III.
The patients rated their pain as 3.25 out of 10, their overall pigment improvement as 3 out of 4, and their overall satisfaction with the procedure as a 4.33 out of 5, Dr. Geronemus reported. "This is often technique sensitive," he said of the procedure. "I’ll have physicians call me up and say, ‘I’m not getting any results.’ I think you need 10-12 passes to get the [optimal] results."
The most common side effect was erythema, which typically resolved within 1 day. Though the device is effective as monotherapy, Dr. Geronemus pointed out that it is "ideally suited for combination therapy with a mild hydroquinone."
Dr. Geronemus disclosed that he serves on the medical advisory boards for Zeltiq, Syneron/Candela, and Cynosure. He also serves as an investigator for numerous device and pharmaceutical companies, and he holds stock in Zeltiq and OnLight Sciences.
EXPERT ANALYSIS FROM CONTROVERSIES AND CONVERSATIONS IN LASER AND COSMETIC SURGERY
FDA expands Botox approval to treat crow’s feet
The approval of onabotulinumtoxinA has been expanded to include the cosmetic treatment of the lateral canthal lines known as crow’s feet, the Food and Drug Administration announced on Sept. 11.
The product has been approved for "the temporary improvement in the appearance of moderate to severe lateral canthal lines" in adults, the FDA said in a statement. The product, marketed as Botox Cosmetic, is an acetylcholine release inhibitor and a neuromuscular blocking agent, and it was approved in 2002 for the temporary improvement of glabellar lines.
The new indication "will provide people with a new FDA-approved treatment option for those seeking a smoother appearance by temporarily minimizing the appearance of crow’s feet at the sides of the eyes," Dr. Susan Walker, director of the Division of Dermatology and Dental Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. Treatment for the glabellar lines (between the eyebrows) and the canthal lines can be administered at the same time, she said.
Approval was based on two studies of 833 adults with moderate to severe lateral canthal lines, randomized to treatment or placebo. Those treated with onabotulinumtoxinA had "greater improvement compared to placebo in the appearance of lateral canthal lines," the FDA statement said. Eyelid edema was the most common adverse reaction associated with the treatment.
The product has been used off label for canthal lines.
OnabotulinumtoxinA is also marketed as Botox, and is approved for the treatment of chronic migraine, severe axillary hyperhidrosis, blepharospasm associated with dystonia, strabismus, and several other indications. The label includes a boxed warning about the possibility that all botulinum toxin products may spread from the site of injection to other parts of the body, causing botulism-like symptoms. The warning states that such symptoms have been reported hours to week after the injection, and have included life-threatening cases of swallowing and breathing difficulties, and reports of deaths. The warning also states that the risk is "probably greatest in children treated for spasticity, but symptoms can also occur in adults, particularly in those patients who have an underlying condition that would predispose them to these symptoms."
However, "there has not been a confirmed serious case of toxin spread when Botox or Botox Cosmetic has been used at the recommended dose for the approved indications," according to the FDA’s statement announcing the approval.
Botox and Botox Cosmetic are marketed by Allergan Inc.
Adverse events associated with onabotulinumtoxinA should be reported to the FDA at 800-332-1088 or at MedWatch.
The approval of onabotulinumtoxinA has been expanded to include the cosmetic treatment of the lateral canthal lines known as crow’s feet, the Food and Drug Administration announced on Sept. 11.
The product has been approved for "the temporary improvement in the appearance of moderate to severe lateral canthal lines" in adults, the FDA said in a statement. The product, marketed as Botox Cosmetic, is an acetylcholine release inhibitor and a neuromuscular blocking agent, and it was approved in 2002 for the temporary improvement of glabellar lines.
The new indication "will provide people with a new FDA-approved treatment option for those seeking a smoother appearance by temporarily minimizing the appearance of crow’s feet at the sides of the eyes," Dr. Susan Walker, director of the Division of Dermatology and Dental Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. Treatment for the glabellar lines (between the eyebrows) and the canthal lines can be administered at the same time, she said.
Approval was based on two studies of 833 adults with moderate to severe lateral canthal lines, randomized to treatment or placebo. Those treated with onabotulinumtoxinA had "greater improvement compared to placebo in the appearance of lateral canthal lines," the FDA statement said. Eyelid edema was the most common adverse reaction associated with the treatment.
The product has been used off label for canthal lines.
OnabotulinumtoxinA is also marketed as Botox, and is approved for the treatment of chronic migraine, severe axillary hyperhidrosis, blepharospasm associated with dystonia, strabismus, and several other indications. The label includes a boxed warning about the possibility that all botulinum toxin products may spread from the site of injection to other parts of the body, causing botulism-like symptoms. The warning states that such symptoms have been reported hours to week after the injection, and have included life-threatening cases of swallowing and breathing difficulties, and reports of deaths. The warning also states that the risk is "probably greatest in children treated for spasticity, but symptoms can also occur in adults, particularly in those patients who have an underlying condition that would predispose them to these symptoms."
However, "there has not been a confirmed serious case of toxin spread when Botox or Botox Cosmetic has been used at the recommended dose for the approved indications," according to the FDA’s statement announcing the approval.
Botox and Botox Cosmetic are marketed by Allergan Inc.
Adverse events associated with onabotulinumtoxinA should be reported to the FDA at 800-332-1088 or at MedWatch.
The approval of onabotulinumtoxinA has been expanded to include the cosmetic treatment of the lateral canthal lines known as crow’s feet, the Food and Drug Administration announced on Sept. 11.
The product has been approved for "the temporary improvement in the appearance of moderate to severe lateral canthal lines" in adults, the FDA said in a statement. The product, marketed as Botox Cosmetic, is an acetylcholine release inhibitor and a neuromuscular blocking agent, and it was approved in 2002 for the temporary improvement of glabellar lines.
The new indication "will provide people with a new FDA-approved treatment option for those seeking a smoother appearance by temporarily minimizing the appearance of crow’s feet at the sides of the eyes," Dr. Susan Walker, director of the Division of Dermatology and Dental Products in the FDA’s Center for Drug Evaluation and Research, said in the statement. Treatment for the glabellar lines (between the eyebrows) and the canthal lines can be administered at the same time, she said.
Approval was based on two studies of 833 adults with moderate to severe lateral canthal lines, randomized to treatment or placebo. Those treated with onabotulinumtoxinA had "greater improvement compared to placebo in the appearance of lateral canthal lines," the FDA statement said. Eyelid edema was the most common adverse reaction associated with the treatment.
The product has been used off label for canthal lines.
OnabotulinumtoxinA is also marketed as Botox, and is approved for the treatment of chronic migraine, severe axillary hyperhidrosis, blepharospasm associated with dystonia, strabismus, and several other indications. The label includes a boxed warning about the possibility that all botulinum toxin products may spread from the site of injection to other parts of the body, causing botulism-like symptoms. The warning states that such symptoms have been reported hours to week after the injection, and have included life-threatening cases of swallowing and breathing difficulties, and reports of deaths. The warning also states that the risk is "probably greatest in children treated for spasticity, but symptoms can also occur in adults, particularly in those patients who have an underlying condition that would predispose them to these symptoms."
However, "there has not been a confirmed serious case of toxin spread when Botox or Botox Cosmetic has been used at the recommended dose for the approved indications," according to the FDA’s statement announcing the approval.
Botox and Botox Cosmetic are marketed by Allergan Inc.
Adverse events associated with onabotulinumtoxinA should be reported to the FDA at 800-332-1088 or at MedWatch.
Multiple same-day laser treatment may be effective for unwanted tattoos
DANA POINT, CALIF. – When it comes to removing unwanted tattoos with lasers, repetitive treatments on the same day appear to expedite tattoo clearance compared with a single treatment, a small study has shown.
Dr. Suzanne L. Kilmer discussed results from a study that she conducted with her associate, Dr. Omar Ibrahimi, that examined the effect of repetitive laser treatments on 17 patients who had a total of 26 tattoos among them. Of the 26 tattoos, 15 were divided into a grid that received one, two, three, or four laser treatments on the same day, while the remaining 17 were bisected and received one or four treatments in the same day, she said at a meeting sponsored by SkinCare Physicians and Northwestern University.
The researchers performed the treatments with a 755-nm Q-switched alexandrite laser or with a 532-nm and 1,064-nm Nd:YAG laser. Patients who underwent multiple treatments waited a minimum of 20 minutes between treatment sessions.
Dr. Kilmer, who heads a laser and skin surgery group practice in Sacramento, reported that lesion clearance on tattoos that had undergone multiple treatments on the same day "was clearly better" than on those receiving a single treatment on the same day. For example, at 1-month follow-up, the clearance rate jumped from 36% with one treatment to 50% for two same-day treatments, but the improvement was less pronounced with same-day treatments four and five (55%, and 59%, respectively).
Patients noted increased swelling with multiple same-day treatments "but there was no increase in pain," said Dr. Kilmer. "In fact, most felt less pain with subsequent treatments. There was no difference in post-inflammatory hyperpigmentation, and no scarring was noted. All patients preferred more rapid clearance of tattoos."
Dr. Kilmer said that the clearance of Cynosure’s PicoSure 750-nm picosecond aesthetic laser in 2012 represented an important advance in tattoo treatment. Its shorter pulse duration "shatters" the target ink into tiny particles, she said. In her clinical experience the PicoSure is especially effective for resistant tattoos – often with as few as two treatments though it makes less of a dent in red ink tattoos. "Colors matter," Dr. Kilmer noted. "We thought that the PicoSure would make color indifferent, but it doesn’t. We still need red light for green ink and green light for red ink."
Dr. Kilmer disclosed that she is a member of the medical advisory board for Candela-Syneron, Living Proof, Lumenis, Miramar, Ulthera, and Zeltiq. She also has received research support from Allergan and from numerous device companies.
DANA POINT, CALIF. – When it comes to removing unwanted tattoos with lasers, repetitive treatments on the same day appear to expedite tattoo clearance compared with a single treatment, a small study has shown.
Dr. Suzanne L. Kilmer discussed results from a study that she conducted with her associate, Dr. Omar Ibrahimi, that examined the effect of repetitive laser treatments on 17 patients who had a total of 26 tattoos among them. Of the 26 tattoos, 15 were divided into a grid that received one, two, three, or four laser treatments on the same day, while the remaining 17 were bisected and received one or four treatments in the same day, she said at a meeting sponsored by SkinCare Physicians and Northwestern University.
The researchers performed the treatments with a 755-nm Q-switched alexandrite laser or with a 532-nm and 1,064-nm Nd:YAG laser. Patients who underwent multiple treatments waited a minimum of 20 minutes between treatment sessions.
Dr. Kilmer, who heads a laser and skin surgery group practice in Sacramento, reported that lesion clearance on tattoos that had undergone multiple treatments on the same day "was clearly better" than on those receiving a single treatment on the same day. For example, at 1-month follow-up, the clearance rate jumped from 36% with one treatment to 50% for two same-day treatments, but the improvement was less pronounced with same-day treatments four and five (55%, and 59%, respectively).
Patients noted increased swelling with multiple same-day treatments "but there was no increase in pain," said Dr. Kilmer. "In fact, most felt less pain with subsequent treatments. There was no difference in post-inflammatory hyperpigmentation, and no scarring was noted. All patients preferred more rapid clearance of tattoos."
Dr. Kilmer said that the clearance of Cynosure’s PicoSure 750-nm picosecond aesthetic laser in 2012 represented an important advance in tattoo treatment. Its shorter pulse duration "shatters" the target ink into tiny particles, she said. In her clinical experience the PicoSure is especially effective for resistant tattoos – often with as few as two treatments though it makes less of a dent in red ink tattoos. "Colors matter," Dr. Kilmer noted. "We thought that the PicoSure would make color indifferent, but it doesn’t. We still need red light for green ink and green light for red ink."
Dr. Kilmer disclosed that she is a member of the medical advisory board for Candela-Syneron, Living Proof, Lumenis, Miramar, Ulthera, and Zeltiq. She also has received research support from Allergan and from numerous device companies.
DANA POINT, CALIF. – When it comes to removing unwanted tattoos with lasers, repetitive treatments on the same day appear to expedite tattoo clearance compared with a single treatment, a small study has shown.
Dr. Suzanne L. Kilmer discussed results from a study that she conducted with her associate, Dr. Omar Ibrahimi, that examined the effect of repetitive laser treatments on 17 patients who had a total of 26 tattoos among them. Of the 26 tattoos, 15 were divided into a grid that received one, two, three, or four laser treatments on the same day, while the remaining 17 were bisected and received one or four treatments in the same day, she said at a meeting sponsored by SkinCare Physicians and Northwestern University.
The researchers performed the treatments with a 755-nm Q-switched alexandrite laser or with a 532-nm and 1,064-nm Nd:YAG laser. Patients who underwent multiple treatments waited a minimum of 20 minutes between treatment sessions.
Dr. Kilmer, who heads a laser and skin surgery group practice in Sacramento, reported that lesion clearance on tattoos that had undergone multiple treatments on the same day "was clearly better" than on those receiving a single treatment on the same day. For example, at 1-month follow-up, the clearance rate jumped from 36% with one treatment to 50% for two same-day treatments, but the improvement was less pronounced with same-day treatments four and five (55%, and 59%, respectively).
Patients noted increased swelling with multiple same-day treatments "but there was no increase in pain," said Dr. Kilmer. "In fact, most felt less pain with subsequent treatments. There was no difference in post-inflammatory hyperpigmentation, and no scarring was noted. All patients preferred more rapid clearance of tattoos."
Dr. Kilmer said that the clearance of Cynosure’s PicoSure 750-nm picosecond aesthetic laser in 2012 represented an important advance in tattoo treatment. Its shorter pulse duration "shatters" the target ink into tiny particles, she said. In her clinical experience the PicoSure is especially effective for resistant tattoos – often with as few as two treatments though it makes less of a dent in red ink tattoos. "Colors matter," Dr. Kilmer noted. "We thought that the PicoSure would make color indifferent, but it doesn’t. We still need red light for green ink and green light for red ink."
Dr. Kilmer disclosed that she is a member of the medical advisory board for Candela-Syneron, Living Proof, Lumenis, Miramar, Ulthera, and Zeltiq. She also has received research support from Allergan and from numerous device companies.
AT CONTROVERSIES AND CONVERSATIONS IN LASER AND COSMETIC SURGERY
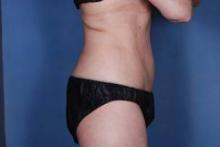
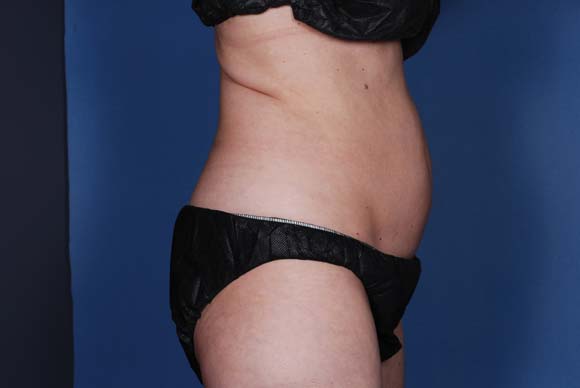
Body contouring procedures find their groove
DANA POINT, CALIF. – The market for nonsurgical body contouring has "settled into a rhythm," and increasing numbers of patients accept the fact that these technologies work, in the opinion of Dr. Michael S. Kaminer.
However, the evolution of technology in the realm of nonsurgical body contouring has slowed. "The absence of technology evolution will allow low-cost providers to gain market share," Dr. Kaminer said at a meeting sponsored by SkinCare Physicians and Northwestern University.
"It’s essential for us to brand these procedures as requiring skillful assessment and contouring expertise. In the short term, it may be more important for us to focus on superior results with current technology rather than trying to win the technology arms race. Advances in technology will help, but are they coming? Maybe it’s time to focus on getting better results with what we already own."
According to the results of a 2011 survey of American Society for Dermatologic Surgery members, noninvasive treatment of fat and cellulite was the most common body sculpting procedure, with 74,000 procedures noted. Cryolipolysis accounted for 12% of body sculpting treatments with a total of 55,500 procedures, followed by tumescent liposuction with 18,500 procedures.
"The bottom line is that our noninvasive procedures are far outstripping liposuction," said Dr. Kaminer, a managing partner at SkinCare Physicians, Chestnut Hill, Mass. "Because of this, patients and physicians are shifting their focus to noninvasive methods for fat removal. But they are also starting to expect more. I think that’s where our opportunity is. If we don’t advance the technology, it has the potential to go the route of laser hair removal, with competition based on price and convenience rather than on science and outcomes."
He went on to discuss ways to optimize outcomes with the existing technology cleared for body contouring. With cryolipolysis (CoolSculpting), for example, combining massage with the procedure has been shown to improve outcomes by as much as 60%, compared with patients who did not undergo concomitant massage.
Dr. Kaminer’s additional tips for cryolipolysis included performing multiple cycles over time for more complete fat removal and shape change, and learning how to use and optimize different applicators. "There are many of them, which is frustrating, but if you learn how to use the different applicators effectively you will get better body sculpting results," said Dr. Kaminer, who also serves as an associate clinical professor of dermatology at Yale University, New Haven, Conn. To that end, consider treating the inner and outer thighs with different applicators. Vertical placement of the flat applicator works well on inner thighs, he said, while the curved applicator works well on outer thighs.
When using ultrasound (Liposonix) for body sculpting, stacked pulses at lower fluences yield results similar to those of nonstacked pulses at high fluences, "but improve comfort for the patient," he said.
In Dr. Kaminer’s clinical experience, patients are satisfied with 40%-50% fat removal after 1-2 treatments with CoolSculpting and Liposonix. Workflow, staffing, patient pain, and time per treatment differ for each device. "Multiple procedures have the potential to give liposuction-like results," he noted. "To me, that’s where we should focus as a specialty. Also, should we focus more on the final shape rather than on how much fat we’re removing? This will differentiate us from spalike providers."
Dr. Kaminer disclosed that he is a consultant for and has received research funding from Zeltiq and Solta Medical.
DANA POINT, CALIF. – The market for nonsurgical body contouring has "settled into a rhythm," and increasing numbers of patients accept the fact that these technologies work, in the opinion of Dr. Michael S. Kaminer.
However, the evolution of technology in the realm of nonsurgical body contouring has slowed. "The absence of technology evolution will allow low-cost providers to gain market share," Dr. Kaminer said at a meeting sponsored by SkinCare Physicians and Northwestern University.
"It’s essential for us to brand these procedures as requiring skillful assessment and contouring expertise. In the short term, it may be more important for us to focus on superior results with current technology rather than trying to win the technology arms race. Advances in technology will help, but are they coming? Maybe it’s time to focus on getting better results with what we already own."
According to the results of a 2011 survey of American Society for Dermatologic Surgery members, noninvasive treatment of fat and cellulite was the most common body sculpting procedure, with 74,000 procedures noted. Cryolipolysis accounted for 12% of body sculpting treatments with a total of 55,500 procedures, followed by tumescent liposuction with 18,500 procedures.
"The bottom line is that our noninvasive procedures are far outstripping liposuction," said Dr. Kaminer, a managing partner at SkinCare Physicians, Chestnut Hill, Mass. "Because of this, patients and physicians are shifting their focus to noninvasive methods for fat removal. But they are also starting to expect more. I think that’s where our opportunity is. If we don’t advance the technology, it has the potential to go the route of laser hair removal, with competition based on price and convenience rather than on science and outcomes."
He went on to discuss ways to optimize outcomes with the existing technology cleared for body contouring. With cryolipolysis (CoolSculpting), for example, combining massage with the procedure has been shown to improve outcomes by as much as 60%, compared with patients who did not undergo concomitant massage.
Dr. Kaminer’s additional tips for cryolipolysis included performing multiple cycles over time for more complete fat removal and shape change, and learning how to use and optimize different applicators. "There are many of them, which is frustrating, but if you learn how to use the different applicators effectively you will get better body sculpting results," said Dr. Kaminer, who also serves as an associate clinical professor of dermatology at Yale University, New Haven, Conn. To that end, consider treating the inner and outer thighs with different applicators. Vertical placement of the flat applicator works well on inner thighs, he said, while the curved applicator works well on outer thighs.
When using ultrasound (Liposonix) for body sculpting, stacked pulses at lower fluences yield results similar to those of nonstacked pulses at high fluences, "but improve comfort for the patient," he said.
In Dr. Kaminer’s clinical experience, patients are satisfied with 40%-50% fat removal after 1-2 treatments with CoolSculpting and Liposonix. Workflow, staffing, patient pain, and time per treatment differ for each device. "Multiple procedures have the potential to give liposuction-like results," he noted. "To me, that’s where we should focus as a specialty. Also, should we focus more on the final shape rather than on how much fat we’re removing? This will differentiate us from spalike providers."
Dr. Kaminer disclosed that he is a consultant for and has received research funding from Zeltiq and Solta Medical.
DANA POINT, CALIF. – The market for nonsurgical body contouring has "settled into a rhythm," and increasing numbers of patients accept the fact that these technologies work, in the opinion of Dr. Michael S. Kaminer.
However, the evolution of technology in the realm of nonsurgical body contouring has slowed. "The absence of technology evolution will allow low-cost providers to gain market share," Dr. Kaminer said at a meeting sponsored by SkinCare Physicians and Northwestern University.
"It’s essential for us to brand these procedures as requiring skillful assessment and contouring expertise. In the short term, it may be more important for us to focus on superior results with current technology rather than trying to win the technology arms race. Advances in technology will help, but are they coming? Maybe it’s time to focus on getting better results with what we already own."
According to the results of a 2011 survey of American Society for Dermatologic Surgery members, noninvasive treatment of fat and cellulite was the most common body sculpting procedure, with 74,000 procedures noted. Cryolipolysis accounted for 12% of body sculpting treatments with a total of 55,500 procedures, followed by tumescent liposuction with 18,500 procedures.
"The bottom line is that our noninvasive procedures are far outstripping liposuction," said Dr. Kaminer, a managing partner at SkinCare Physicians, Chestnut Hill, Mass. "Because of this, patients and physicians are shifting their focus to noninvasive methods for fat removal. But they are also starting to expect more. I think that’s where our opportunity is. If we don’t advance the technology, it has the potential to go the route of laser hair removal, with competition based on price and convenience rather than on science and outcomes."
He went on to discuss ways to optimize outcomes with the existing technology cleared for body contouring. With cryolipolysis (CoolSculpting), for example, combining massage with the procedure has been shown to improve outcomes by as much as 60%, compared with patients who did not undergo concomitant massage.
Dr. Kaminer’s additional tips for cryolipolysis included performing multiple cycles over time for more complete fat removal and shape change, and learning how to use and optimize different applicators. "There are many of them, which is frustrating, but if you learn how to use the different applicators effectively you will get better body sculpting results," said Dr. Kaminer, who also serves as an associate clinical professor of dermatology at Yale University, New Haven, Conn. To that end, consider treating the inner and outer thighs with different applicators. Vertical placement of the flat applicator works well on inner thighs, he said, while the curved applicator works well on outer thighs.
When using ultrasound (Liposonix) for body sculpting, stacked pulses at lower fluences yield results similar to those of nonstacked pulses at high fluences, "but improve comfort for the patient," he said.
In Dr. Kaminer’s clinical experience, patients are satisfied with 40%-50% fat removal after 1-2 treatments with CoolSculpting and Liposonix. Workflow, staffing, patient pain, and time per treatment differ for each device. "Multiple procedures have the potential to give liposuction-like results," he noted. "To me, that’s where we should focus as a specialty. Also, should we focus more on the final shape rather than on how much fat we’re removing? This will differentiate us from spalike providers."
Dr. Kaminer disclosed that he is a consultant for and has received research funding from Zeltiq and Solta Medical.
EXPERT ANALYSIS FROM CONTROVERSIES AND CONVERSATIONS IN LASER AND COSMETIC SURGERY
Liposonix is not for all-comers
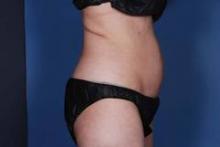
DANA POINT, CALIF. – Dr. Mark Jewell shared his clinical experience with Liposonix (Solta Medical), a high-intensity, focused ultrasound device that was cleared by the Food and Drug Administration in 2011 for noninvasive waist circumference reduction, at a meeting sponsored by Skincare Physicians and Northwestern University.
The device is unique, Dr. Jewell said, because the energy it delivers produces fat ablation and contraction and thickening of collagen in the midlamellar matrix – tissue depths of 13-23 mm beneath the skin surface – with minimal collateral damage.
"We’re hitting the collagen-rich layer, which I think is beneficial in terms of body contouring," said Dr. Jewell, associate clinical professor of plastic surgery at Oregon Health and Science University, Portland.
The treated area "gets warm enough to denature collagen, but also to ablate fat. It produces a cosmetic effect both in terms of volume reduction and tightening of collagen in the midlamellar matrix."
Patient selection is important "because we want to be able to deliver the ultrasound where it needs to go," he said. As a rule of thumb, Dr. Jewell does not use the Liposonix in patients with a body mass index of greater than 30 kg/m2 or in those who have more than 3 cm of superficial fat around the waist.
"Too much superficial fat will get bruised, but it will not tighten up," explained Dr. Jewell, who is a past president of the American Society for Aesthetic Plastic Surgery. "For this type of patient you might recommend that they hire a personal trainer for weight loss before you treat them."
Ideal candidates generally have 1.0 cm of fat tissue beyond the focal depth or pass the "pinch an inch" of fat test around their waist. Dr. Jewell uses high-resolution diagnostic ultrasound devices such as the SonoSite in an effort to determine the precise amount of superficial abdominal fat. This strategy "improves accuracy and the quality of our outcomes," he said.
For optimal effect, he strives for a total dosimetry threshold of 150-180 J with the Liposonix. "The device is software driven so it can be focused and patterned in a variety of ways," Dr. Jewell said. While the Liposonix can be operated by a properly trained subordinate in your office, "do the first 20 cases yourself so that you’re familiar with the technology and what’s involved," he advised.
Dr. Jewell disclosed that he is a consultant for Allergan, Medicis, Excaliard-Pfizer, Keller Medical, Solta Medical, and New Beauty Magazine. He is also an investigator for Allergan, Medicis, Excaliard-Pfizer, and Solta Medical.
DANA POINT, CALIF. – Dr. Mark Jewell shared his clinical experience with Liposonix (Solta Medical), a high-intensity, focused ultrasound device that was cleared by the Food and Drug Administration in 2011 for noninvasive waist circumference reduction, at a meeting sponsored by Skincare Physicians and Northwestern University.
The device is unique, Dr. Jewell said, because the energy it delivers produces fat ablation and contraction and thickening of collagen in the midlamellar matrix – tissue depths of 13-23 mm beneath the skin surface – with minimal collateral damage.
"We’re hitting the collagen-rich layer, which I think is beneficial in terms of body contouring," said Dr. Jewell, associate clinical professor of plastic surgery at Oregon Health and Science University, Portland.
The treated area "gets warm enough to denature collagen, but also to ablate fat. It produces a cosmetic effect both in terms of volume reduction and tightening of collagen in the midlamellar matrix."
Patient selection is important "because we want to be able to deliver the ultrasound where it needs to go," he said. As a rule of thumb, Dr. Jewell does not use the Liposonix in patients with a body mass index of greater than 30 kg/m2 or in those who have more than 3 cm of superficial fat around the waist.
"Too much superficial fat will get bruised, but it will not tighten up," explained Dr. Jewell, who is a past president of the American Society for Aesthetic Plastic Surgery. "For this type of patient you might recommend that they hire a personal trainer for weight loss before you treat them."
Ideal candidates generally have 1.0 cm of fat tissue beyond the focal depth or pass the "pinch an inch" of fat test around their waist. Dr. Jewell uses high-resolution diagnostic ultrasound devices such as the SonoSite in an effort to determine the precise amount of superficial abdominal fat. This strategy "improves accuracy and the quality of our outcomes," he said.
For optimal effect, he strives for a total dosimetry threshold of 150-180 J with the Liposonix. "The device is software driven so it can be focused and patterned in a variety of ways," Dr. Jewell said. While the Liposonix can be operated by a properly trained subordinate in your office, "do the first 20 cases yourself so that you’re familiar with the technology and what’s involved," he advised.
Dr. Jewell disclosed that he is a consultant for Allergan, Medicis, Excaliard-Pfizer, Keller Medical, Solta Medical, and New Beauty Magazine. He is also an investigator for Allergan, Medicis, Excaliard-Pfizer, and Solta Medical.
DANA POINT, CALIF. – Dr. Mark Jewell shared his clinical experience with Liposonix (Solta Medical), a high-intensity, focused ultrasound device that was cleared by the Food and Drug Administration in 2011 for noninvasive waist circumference reduction, at a meeting sponsored by Skincare Physicians and Northwestern University.
The device is unique, Dr. Jewell said, because the energy it delivers produces fat ablation and contraction and thickening of collagen in the midlamellar matrix – tissue depths of 13-23 mm beneath the skin surface – with minimal collateral damage.
"We’re hitting the collagen-rich layer, which I think is beneficial in terms of body contouring," said Dr. Jewell, associate clinical professor of plastic surgery at Oregon Health and Science University, Portland.
The treated area "gets warm enough to denature collagen, but also to ablate fat. It produces a cosmetic effect both in terms of volume reduction and tightening of collagen in the midlamellar matrix."
Patient selection is important "because we want to be able to deliver the ultrasound where it needs to go," he said. As a rule of thumb, Dr. Jewell does not use the Liposonix in patients with a body mass index of greater than 30 kg/m2 or in those who have more than 3 cm of superficial fat around the waist.
"Too much superficial fat will get bruised, but it will not tighten up," explained Dr. Jewell, who is a past president of the American Society for Aesthetic Plastic Surgery. "For this type of patient you might recommend that they hire a personal trainer for weight loss before you treat them."
Ideal candidates generally have 1.0 cm of fat tissue beyond the focal depth or pass the "pinch an inch" of fat test around their waist. Dr. Jewell uses high-resolution diagnostic ultrasound devices such as the SonoSite in an effort to determine the precise amount of superficial abdominal fat. This strategy "improves accuracy and the quality of our outcomes," he said.
For optimal effect, he strives for a total dosimetry threshold of 150-180 J with the Liposonix. "The device is software driven so it can be focused and patterned in a variety of ways," Dr. Jewell said. While the Liposonix can be operated by a properly trained subordinate in your office, "do the first 20 cases yourself so that you’re familiar with the technology and what’s involved," he advised.
Dr. Jewell disclosed that he is a consultant for Allergan, Medicis, Excaliard-Pfizer, Keller Medical, Solta Medical, and New Beauty Magazine. He is also an investigator for Allergan, Medicis, Excaliard-Pfizer, and Solta Medical.
EXPERT ANALYSIS FROM CONTROVERSIES AND CONVERSATIONS IN LASER AND COSMETIC SURGERY
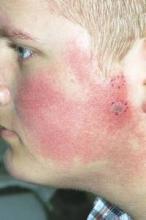
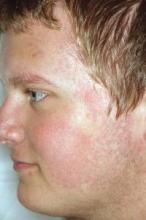
Pulsed dye laser targets keratosis pilaris rubra
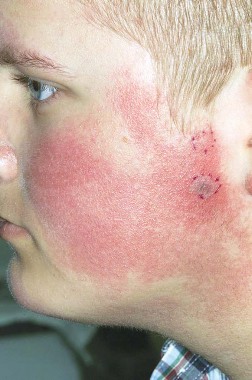
MILWAUKEE – Pulsed dye laser therapy reduced the redness associated with chronic keratosis pilaris rubra in as little as one session in a case series of seven adolescents.
The problem, however, is convincing insurers to pay for the procedure.
"That’s part of why we brought this to a forum like this meeting," Dr. Jennifer J. Schoch said at the annual meeting of the Society for Pediatric Dermatology. "These kids are so significantly affected by this and embarrassed [by the condition], that if we can do something in just one treatment and have a good response, it makes sense. But these kids required a lot of letters to the insurance companies, and a lot of them paid out of pocket."
Several attendees at the meeting echoed these comments, and some observed that pulse dye laser (PDL) therapy is not as effective in patients with higher Fitzpatrick skin types or that it was out of reach for their patients at a price tag of $200 or more per session.
"It seems to make a lot of sense, but I don’t think it would be worth the cost for my patients," Dr. Aimee Smidt, University of New Mexico, Albuquerque, said in an interview.
Keratosis pilaris rubra is often viewed as a benign condition, but one patient found it so socially disturbing that he flew from Alabama to Minnesota for treatment, said Dr. Schoch, a dermatologist at the Mayo Clinic in Rochester, Minn.
Dr. Schoch noted that there are few data in the literature on PDL treatment of keratosis pilaris rubra, and the current series is occurring over a 13-year-period at the clinic. In this series, adolescents aged 14-17 years received one to four sessions of PDL at a wavelength of 585 nm or 595 nm, for erythema and hyperkeratotic follicular papules on both cheeks. Two patients also received treatment to the chin, forehead and/or neck.
All patients had Fitzpatrick skin type I or II, five were male, and two also had ulerythema ophryogenes. Some patients had been misdiagnosed with severe acne, and all had failed a range of treatments including emollients, lactic acid, topical retinoids, urea, sulfacetamide lotion, and weak topical corticosteroids, as well as laser therapy with intense pulsed light, Fraxel, and Nd:YAG lasers, she said.
PDL treatment was physician-dependent. Starting fluences ranged from 4-9 J/cm2, with the goal of achieving a mild, bruiselike response. The spot size was predominantly 7 mm, and pulse duration was 1.5, 3, or 10 msec.
"I hypothesize that people are probably undertreating because it’s a benign condition, and you don’t want to cause problems, but to be effective, it seems you have to go for a little more aggressive response," Dr. Schoch said in an interview.
All patients experienced significant improvement after one to four treatments, based on patient report or provider assessment. Bruising resolved in 1-2 weeks. Resolution of erythema was observed, but not specifically measured. Patients also experienced some transient purpura, which was not well documented, said Dr. Schoch.
After 1-19 months follow-up, most patients remained satisfied with the results, although some flushing returned in two patients, she said. Blanching has not been a significant problem, and overlapping the treated areas reduced the risk of a honeycomb pattern developing on the skin.
The investigators are considering a prospective study to more objectively monitor responses. Treatment parameters will depend on patient’s response to test spots, Dr. Schoch said.
Dr. Schoch and her coauthors reported having no financial disclosures.
MILWAUKEE – Pulsed dye laser therapy reduced the redness associated with chronic keratosis pilaris rubra in as little as one session in a case series of seven adolescents.
The problem, however, is convincing insurers to pay for the procedure.
"That’s part of why we brought this to a forum like this meeting," Dr. Jennifer J. Schoch said at the annual meeting of the Society for Pediatric Dermatology. "These kids are so significantly affected by this and embarrassed [by the condition], that if we can do something in just one treatment and have a good response, it makes sense. But these kids required a lot of letters to the insurance companies, and a lot of them paid out of pocket."
Several attendees at the meeting echoed these comments, and some observed that pulse dye laser (PDL) therapy is not as effective in patients with higher Fitzpatrick skin types or that it was out of reach for their patients at a price tag of $200 or more per session.
"It seems to make a lot of sense, but I don’t think it would be worth the cost for my patients," Dr. Aimee Smidt, University of New Mexico, Albuquerque, said in an interview.
Keratosis pilaris rubra is often viewed as a benign condition, but one patient found it so socially disturbing that he flew from Alabama to Minnesota for treatment, said Dr. Schoch, a dermatologist at the Mayo Clinic in Rochester, Minn.
Dr. Schoch noted that there are few data in the literature on PDL treatment of keratosis pilaris rubra, and the current series is occurring over a 13-year-period at the clinic. In this series, adolescents aged 14-17 years received one to four sessions of PDL at a wavelength of 585 nm or 595 nm, for erythema and hyperkeratotic follicular papules on both cheeks. Two patients also received treatment to the chin, forehead and/or neck.
All patients had Fitzpatrick skin type I or II, five were male, and two also had ulerythema ophryogenes. Some patients had been misdiagnosed with severe acne, and all had failed a range of treatments including emollients, lactic acid, topical retinoids, urea, sulfacetamide lotion, and weak topical corticosteroids, as well as laser therapy with intense pulsed light, Fraxel, and Nd:YAG lasers, she said.
PDL treatment was physician-dependent. Starting fluences ranged from 4-9 J/cm2, with the goal of achieving a mild, bruiselike response. The spot size was predominantly 7 mm, and pulse duration was 1.5, 3, or 10 msec.
"I hypothesize that people are probably undertreating because it’s a benign condition, and you don’t want to cause problems, but to be effective, it seems you have to go for a little more aggressive response," Dr. Schoch said in an interview.
All patients experienced significant improvement after one to four treatments, based on patient report or provider assessment. Bruising resolved in 1-2 weeks. Resolution of erythema was observed, but not specifically measured. Patients also experienced some transient purpura, which was not well documented, said Dr. Schoch.
After 1-19 months follow-up, most patients remained satisfied with the results, although some flushing returned in two patients, she said. Blanching has not been a significant problem, and overlapping the treated areas reduced the risk of a honeycomb pattern developing on the skin.
The investigators are considering a prospective study to more objectively monitor responses. Treatment parameters will depend on patient’s response to test spots, Dr. Schoch said.
Dr. Schoch and her coauthors reported having no financial disclosures.
MILWAUKEE – Pulsed dye laser therapy reduced the redness associated with chronic keratosis pilaris rubra in as little as one session in a case series of seven adolescents.
The problem, however, is convincing insurers to pay for the procedure.
"That’s part of why we brought this to a forum like this meeting," Dr. Jennifer J. Schoch said at the annual meeting of the Society for Pediatric Dermatology. "These kids are so significantly affected by this and embarrassed [by the condition], that if we can do something in just one treatment and have a good response, it makes sense. But these kids required a lot of letters to the insurance companies, and a lot of them paid out of pocket."
Several attendees at the meeting echoed these comments, and some observed that pulse dye laser (PDL) therapy is not as effective in patients with higher Fitzpatrick skin types or that it was out of reach for their patients at a price tag of $200 or more per session.
"It seems to make a lot of sense, but I don’t think it would be worth the cost for my patients," Dr. Aimee Smidt, University of New Mexico, Albuquerque, said in an interview.
Keratosis pilaris rubra is often viewed as a benign condition, but one patient found it so socially disturbing that he flew from Alabama to Minnesota for treatment, said Dr. Schoch, a dermatologist at the Mayo Clinic in Rochester, Minn.
Dr. Schoch noted that there are few data in the literature on PDL treatment of keratosis pilaris rubra, and the current series is occurring over a 13-year-period at the clinic. In this series, adolescents aged 14-17 years received one to four sessions of PDL at a wavelength of 585 nm or 595 nm, for erythema and hyperkeratotic follicular papules on both cheeks. Two patients also received treatment to the chin, forehead and/or neck.
All patients had Fitzpatrick skin type I or II, five were male, and two also had ulerythema ophryogenes. Some patients had been misdiagnosed with severe acne, and all had failed a range of treatments including emollients, lactic acid, topical retinoids, urea, sulfacetamide lotion, and weak topical corticosteroids, as well as laser therapy with intense pulsed light, Fraxel, and Nd:YAG lasers, she said.
PDL treatment was physician-dependent. Starting fluences ranged from 4-9 J/cm2, with the goal of achieving a mild, bruiselike response. The spot size was predominantly 7 mm, and pulse duration was 1.5, 3, or 10 msec.
"I hypothesize that people are probably undertreating because it’s a benign condition, and you don’t want to cause problems, but to be effective, it seems you have to go for a little more aggressive response," Dr. Schoch said in an interview.
All patients experienced significant improvement after one to four treatments, based on patient report or provider assessment. Bruising resolved in 1-2 weeks. Resolution of erythema was observed, but not specifically measured. Patients also experienced some transient purpura, which was not well documented, said Dr. Schoch.
After 1-19 months follow-up, most patients remained satisfied with the results, although some flushing returned in two patients, she said. Blanching has not been a significant problem, and overlapping the treated areas reduced the risk of a honeycomb pattern developing on the skin.
The investigators are considering a prospective study to more objectively monitor responses. Treatment parameters will depend on patient’s response to test spots, Dr. Schoch said.
Dr. Schoch and her coauthors reported having no financial disclosures.
AT THE SPD ANNUAL MEETING
Difficult to treat hyperpigmentation – eyelids, axillae, and neck
Persons with Fitzpatrick skin types III-VI and those of certain ethnic groups tend to have a higher risk of darker pigmentation on certain parts of the body. Melasma, postinflammatory hyperpigmentation, and lentigines often respond to treatment with topical antipigment agents, chemical peels, and lasers. Darker pigment on the elbows and knees, if bothersome also can be treated with topical antipigment creams, plus or minus topicals that promote exfoliation (such as urea-based topicals). If the skin is acanthotic on elbows or knees, a topical steroid can be used first to thin the area before applying a lightening agent.
But what about pigmentation of the eyelids, axillae, and neck? At these thinner, more sensitive areas of skin, the cause of darker pigment could be multifactorial. Treatment can be difficult because the same methods we use to treat pigmentation in other areas can be too aggressive for these locations. A recent study by Saedi and Ganesan (J. Drugs Dermatol. 2013;12:563-7) surveyed practicing dermatologists’ methods of treating hyperpigmentation of the eyelids, axillae, and neck. Fifty dermatologists completed the survey, and 46 (92%) reported treating patients with darker skin. The ethnic groups treated included Hispanic (97.8%), black (97.8%), Middle Eastern (77.6%), and Asian (88.9%). Thirty-six survey respondents reported treating patients with hyperpigmentation under the eyes, and 22 (61.1%) thought the hyperpigmentation was a result of idiopathic increase in melanin deposition. Forty-two responded to treating hyperpigmentation in the axilla, most of whom thought it was related to acanthosis nigricans (69.0%) or contact dermatitis (59.5%). Forty responded to treating hyperpigmentation on the neck, most of whom treated the condition with hydroquinone (66%). Treatments for these three areas were not found to be effective.
Pigment in these areas could be normal and purely genetic, such as variations in skin pigment because of embryonic pigment demarcation areas, versus an underlying pathology.
For the eyelids, that pathology could include increased pigment from inflammatory conditions (eczema, allergies, allergic or irritant contact dermatitis, photodermatitis), autoimmune conditions (dermatomyositis, lupus), medications (bimatoprost, among others), heavy metal poisoning (colloidal silver, lead, mercury), or increased vascularity. Treating these underlying conditions may help improve the appearance of darker eyelids. Hyperpigmentation treatment options include a series of light chemical peels, topical lightening agents such as kojic acid, and resurfacing lasers, but caution must be taken to avoid additional postinflammatory pigmentation from these procedures. Long-term sun protection and sunscreen use is imperative in any area after treatment.
Tear trough deformity because of volume loss under the eyes also can cause the appearance of darker lower eyelids. However, hyperpigmentation of the skin is not the primary issue in these cases, and the appearance can often improve with placement of dermal fillers.
For the axillae and neck, conditions that could promote hyperpigmentation include postinflammatory pigmentation (caused by irritant or allergic contact dermatitis, infection, waxing, or friction), UV exposure, acanthosis nigricans, and photodermatitis, especially from photosensitizing medications. All of these conditions may also respond to topical antipigment ingredients and attention to the underlying condition, but unfortunately, not always to the patient’s greatest satisfaction.
What are your strategies for hyperpigmentation on the tough-to-treat areas of the eyelids, neck, or axillae?
Dr. Wesley practices dermatology in Beverly Hills, Calif.
Do you have questions about treating patients with dark skin? If so, send them to [email protected].
Persons with Fitzpatrick skin types III-VI and those of certain ethnic groups tend to have a higher risk of darker pigmentation on certain parts of the body. Melasma, postinflammatory hyperpigmentation, and lentigines often respond to treatment with topical antipigment agents, chemical peels, and lasers. Darker pigment on the elbows and knees, if bothersome also can be treated with topical antipigment creams, plus or minus topicals that promote exfoliation (such as urea-based topicals). If the skin is acanthotic on elbows or knees, a topical steroid can be used first to thin the area before applying a lightening agent.
But what about pigmentation of the eyelids, axillae, and neck? At these thinner, more sensitive areas of skin, the cause of darker pigment could be multifactorial. Treatment can be difficult because the same methods we use to treat pigmentation in other areas can be too aggressive for these locations. A recent study by Saedi and Ganesan (J. Drugs Dermatol. 2013;12:563-7) surveyed practicing dermatologists’ methods of treating hyperpigmentation of the eyelids, axillae, and neck. Fifty dermatologists completed the survey, and 46 (92%) reported treating patients with darker skin. The ethnic groups treated included Hispanic (97.8%), black (97.8%), Middle Eastern (77.6%), and Asian (88.9%). Thirty-six survey respondents reported treating patients with hyperpigmentation under the eyes, and 22 (61.1%) thought the hyperpigmentation was a result of idiopathic increase in melanin deposition. Forty-two responded to treating hyperpigmentation in the axilla, most of whom thought it was related to acanthosis nigricans (69.0%) or contact dermatitis (59.5%). Forty responded to treating hyperpigmentation on the neck, most of whom treated the condition with hydroquinone (66%). Treatments for these three areas were not found to be effective.
Pigment in these areas could be normal and purely genetic, such as variations in skin pigment because of embryonic pigment demarcation areas, versus an underlying pathology.
For the eyelids, that pathology could include increased pigment from inflammatory conditions (eczema, allergies, allergic or irritant contact dermatitis, photodermatitis), autoimmune conditions (dermatomyositis, lupus), medications (bimatoprost, among others), heavy metal poisoning (colloidal silver, lead, mercury), or increased vascularity. Treating these underlying conditions may help improve the appearance of darker eyelids. Hyperpigmentation treatment options include a series of light chemical peels, topical lightening agents such as kojic acid, and resurfacing lasers, but caution must be taken to avoid additional postinflammatory pigmentation from these procedures. Long-term sun protection and sunscreen use is imperative in any area after treatment.
Tear trough deformity because of volume loss under the eyes also can cause the appearance of darker lower eyelids. However, hyperpigmentation of the skin is not the primary issue in these cases, and the appearance can often improve with placement of dermal fillers.
For the axillae and neck, conditions that could promote hyperpigmentation include postinflammatory pigmentation (caused by irritant or allergic contact dermatitis, infection, waxing, or friction), UV exposure, acanthosis nigricans, and photodermatitis, especially from photosensitizing medications. All of these conditions may also respond to topical antipigment ingredients and attention to the underlying condition, but unfortunately, not always to the patient’s greatest satisfaction.
What are your strategies for hyperpigmentation on the tough-to-treat areas of the eyelids, neck, or axillae?
Dr. Wesley practices dermatology in Beverly Hills, Calif.
Do you have questions about treating patients with dark skin? If so, send them to [email protected].
Persons with Fitzpatrick skin types III-VI and those of certain ethnic groups tend to have a higher risk of darker pigmentation on certain parts of the body. Melasma, postinflammatory hyperpigmentation, and lentigines often respond to treatment with topical antipigment agents, chemical peels, and lasers. Darker pigment on the elbows and knees, if bothersome also can be treated with topical antipigment creams, plus or minus topicals that promote exfoliation (such as urea-based topicals). If the skin is acanthotic on elbows or knees, a topical steroid can be used first to thin the area before applying a lightening agent.
But what about pigmentation of the eyelids, axillae, and neck? At these thinner, more sensitive areas of skin, the cause of darker pigment could be multifactorial. Treatment can be difficult because the same methods we use to treat pigmentation in other areas can be too aggressive for these locations. A recent study by Saedi and Ganesan (J. Drugs Dermatol. 2013;12:563-7) surveyed practicing dermatologists’ methods of treating hyperpigmentation of the eyelids, axillae, and neck. Fifty dermatologists completed the survey, and 46 (92%) reported treating patients with darker skin. The ethnic groups treated included Hispanic (97.8%), black (97.8%), Middle Eastern (77.6%), and Asian (88.9%). Thirty-six survey respondents reported treating patients with hyperpigmentation under the eyes, and 22 (61.1%) thought the hyperpigmentation was a result of idiopathic increase in melanin deposition. Forty-two responded to treating hyperpigmentation in the axilla, most of whom thought it was related to acanthosis nigricans (69.0%) or contact dermatitis (59.5%). Forty responded to treating hyperpigmentation on the neck, most of whom treated the condition with hydroquinone (66%). Treatments for these three areas were not found to be effective.
Pigment in these areas could be normal and purely genetic, such as variations in skin pigment because of embryonic pigment demarcation areas, versus an underlying pathology.
For the eyelids, that pathology could include increased pigment from inflammatory conditions (eczema, allergies, allergic or irritant contact dermatitis, photodermatitis), autoimmune conditions (dermatomyositis, lupus), medications (bimatoprost, among others), heavy metal poisoning (colloidal silver, lead, mercury), or increased vascularity. Treating these underlying conditions may help improve the appearance of darker eyelids. Hyperpigmentation treatment options include a series of light chemical peels, topical lightening agents such as kojic acid, and resurfacing lasers, but caution must be taken to avoid additional postinflammatory pigmentation from these procedures. Long-term sun protection and sunscreen use is imperative in any area after treatment.
Tear trough deformity because of volume loss under the eyes also can cause the appearance of darker lower eyelids. However, hyperpigmentation of the skin is not the primary issue in these cases, and the appearance can often improve with placement of dermal fillers.
For the axillae and neck, conditions that could promote hyperpigmentation include postinflammatory pigmentation (caused by irritant or allergic contact dermatitis, infection, waxing, or friction), UV exposure, acanthosis nigricans, and photodermatitis, especially from photosensitizing medications. All of these conditions may also respond to topical antipigment ingredients and attention to the underlying condition, but unfortunately, not always to the patient’s greatest satisfaction.
What are your strategies for hyperpigmentation on the tough-to-treat areas of the eyelids, neck, or axillae?
Dr. Wesley practices dermatology in Beverly Hills, Calif.
Do you have questions about treating patients with dark skin? If so, send them to [email protected].
Summer hair care
Daily and weekly hair care for black women may include styling, relaxing, perming, coloring, braiding, or hot ironing. Moisture compromises the integrity of the hairstyles, which may lead some women to avoid contact with water, humidity, and sweat, all ingredients of summer for many people.
In my clinic, many black women are seen for scalp disorders such as scarring alopecia, folliculitis, hair breakage, and seborrhea. However, I also make a point of asking each of these patients about her hair care practices. If concerns for hair make them less inclined to swim or sweat, I remind them that proper hair care practices can minimize scalp diseases and hair loss, and that they need not avoid outdoor exercise when the weather heats up.
Summer hair care guidelines that I recommend to skin of color patients are mainly the same as at other times of the year, including proper moisturizing of the hair before and after treatments, minimizing time between chemical treatments, not coloring and chemically relaxing the hair at the same time, avoiding hot combs and hot styling tools, and treating any scalp inflammation early and aggressively.
However, summer is a great time to cut and style the hair with natural curls if possible, because beating the heat and humidity with harsh styling practices can break the hair and lead to irreversible hair shaft damage.
Other tips to help your patients manage their hair in the summer months include recommending sulfate-free shampoos in place of regular shampoos. Between washes, a dry shampoo is best used at the root of the hair, and is a great alternative to hair washing after the beach or after exercise. Dry shampoos are widely available and when used daily absorb dirt, oil, and hair products applied to the hair. After washing, I advise patients to apply a leave-in conditioner to damp or dry hair. These conditioners help protect the hair shaft from styling damage.
Prior to workouts, styling hair into a ponytail or French twist can help protect the hair from sweat. If patients report that their hair is starting to break, advise them to minimize the relaxing and heat styling, and to choose loose twists and gentle, loose braids. Finally, applying a serum or "smoothing lotion" with ingredients such as argan oil or mineral oil to the hair before styling can help to smooth strands, mask fly-aways, and prevent summer frizz.
Dr. Talakoub is in private practice in McLean, Va.
Daily and weekly hair care for black women may include styling, relaxing, perming, coloring, braiding, or hot ironing. Moisture compromises the integrity of the hairstyles, which may lead some women to avoid contact with water, humidity, and sweat, all ingredients of summer for many people.
In my clinic, many black women are seen for scalp disorders such as scarring alopecia, folliculitis, hair breakage, and seborrhea. However, I also make a point of asking each of these patients about her hair care practices. If concerns for hair make them less inclined to swim or sweat, I remind them that proper hair care practices can minimize scalp diseases and hair loss, and that they need not avoid outdoor exercise when the weather heats up.
Summer hair care guidelines that I recommend to skin of color patients are mainly the same as at other times of the year, including proper moisturizing of the hair before and after treatments, minimizing time between chemical treatments, not coloring and chemically relaxing the hair at the same time, avoiding hot combs and hot styling tools, and treating any scalp inflammation early and aggressively.
However, summer is a great time to cut and style the hair with natural curls if possible, because beating the heat and humidity with harsh styling practices can break the hair and lead to irreversible hair shaft damage.
Other tips to help your patients manage their hair in the summer months include recommending sulfate-free shampoos in place of regular shampoos. Between washes, a dry shampoo is best used at the root of the hair, and is a great alternative to hair washing after the beach or after exercise. Dry shampoos are widely available and when used daily absorb dirt, oil, and hair products applied to the hair. After washing, I advise patients to apply a leave-in conditioner to damp or dry hair. These conditioners help protect the hair shaft from styling damage.
Prior to workouts, styling hair into a ponytail or French twist can help protect the hair from sweat. If patients report that their hair is starting to break, advise them to minimize the relaxing and heat styling, and to choose loose twists and gentle, loose braids. Finally, applying a serum or "smoothing lotion" with ingredients such as argan oil or mineral oil to the hair before styling can help to smooth strands, mask fly-aways, and prevent summer frizz.
Dr. Talakoub is in private practice in McLean, Va.
Daily and weekly hair care for black women may include styling, relaxing, perming, coloring, braiding, or hot ironing. Moisture compromises the integrity of the hairstyles, which may lead some women to avoid contact with water, humidity, and sweat, all ingredients of summer for many people.
In my clinic, many black women are seen for scalp disorders such as scarring alopecia, folliculitis, hair breakage, and seborrhea. However, I also make a point of asking each of these patients about her hair care practices. If concerns for hair make them less inclined to swim or sweat, I remind them that proper hair care practices can minimize scalp diseases and hair loss, and that they need not avoid outdoor exercise when the weather heats up.
Summer hair care guidelines that I recommend to skin of color patients are mainly the same as at other times of the year, including proper moisturizing of the hair before and after treatments, minimizing time between chemical treatments, not coloring and chemically relaxing the hair at the same time, avoiding hot combs and hot styling tools, and treating any scalp inflammation early and aggressively.
However, summer is a great time to cut and style the hair with natural curls if possible, because beating the heat and humidity with harsh styling practices can break the hair and lead to irreversible hair shaft damage.
Other tips to help your patients manage their hair in the summer months include recommending sulfate-free shampoos in place of regular shampoos. Between washes, a dry shampoo is best used at the root of the hair, and is a great alternative to hair washing after the beach or after exercise. Dry shampoos are widely available and when used daily absorb dirt, oil, and hair products applied to the hair. After washing, I advise patients to apply a leave-in conditioner to damp or dry hair. These conditioners help protect the hair shaft from styling damage.
Prior to workouts, styling hair into a ponytail or French twist can help protect the hair from sweat. If patients report that their hair is starting to break, advise them to minimize the relaxing and heat styling, and to choose loose twists and gentle, loose braids. Finally, applying a serum or "smoothing lotion" with ingredients such as argan oil or mineral oil to the hair before styling can help to smooth strands, mask fly-aways, and prevent summer frizz.
Dr. Talakoub is in private practice in McLean, Va.
Major finding: Key numerical finding (e.g., number needed to treat to prevent one death/event; number lived or died as result of intervention). Maximum 10 words/1 sentence.
Data source: Include type of study (e.g., randomized, placebo controlled trial; retrospective case-control study). Include number in the study.
Disclosures: Sponsor of study, funding source, relevant disclosures. If author has no relevant disclosures, "Dr. X reported having no financial disclosures." If necessary, "Meeting Y did not require reports of financial disclosures." Check meeting website because many list disclosures. Written in sentence form.