User login
Judge dismisses doctors’ lawsuit against ABIM
A district court has dismissed a lawsuit levied by a group of physicians against the American Board of Internal Medicine (ABIM) over its maintenance of certification (MOC) program, calling the legal challenge “flawed.”
In a Sept. 26 decision, U.S. District Court Judge for the Eastern District of Pennsylvania Robert F. Kelly Sr. said the plaintiffs failed to demonstrate sufficient evidence for their antitrust and unjust enrichment claims against ABIM. The doctors also did not establish any showing of anticompetitive conduct by ABIM to support a monopolization claim, the judge ruled.
“We disagree with plaintiffs and find that ABIM’s initial certification and MOC products are part of a single product and do not occupy distinct markets,” Judge Kelly wrote in his decision. “Not only are we unconvinced by plaintiffs’ arguments, we find that plaintiffs’ entire framing of the ABIM certification to be flawed. In essence, plaintiffs are arguing that, in order to purchase ABIM’s initial certification, internists are forced to purchase MOC products as well. However, this is not the case. ... Nowhere in the amended complaint do plaintiffs allege that they were forced to buy MOC products in order to purchase the initial certification.”
The judge dismissed the suit, but allowed the plaintiffs 14 days to submit an amended complaint reoutlining their claims of illegal monopolization and racketeering against the board. If the amended complaint passes legal muster, the judge could revive those claims.
ABIM President Richard J. Baron, MD, expressed satisfaction that the court granted the board’s motion to dismiss the case for failure to state a valid claim.
“ABIM is pleased that the United States District Court for the Eastern District of Pennsylvania dismissed in its entirety a lawsuit that alleged physicians were harmed by the requirements for maintaining ABIM board certification,” Dr. Baron said in a statement.
C. Philip Curley, a Chicago-based attorney for the physician plaintiffs, said the case is far from over.
“The four internists who brought the lawsuit were invited to file amended claims, which is certainly being considered,” Mr. Curley said in an interview. “If necessary, all available appeals will also be pursued to the fullest. No one was under the impression that the fight to bring MOC to an end would be quick or easy.”
The original lawsuit, filed Dec. 6, 2018, in a Pennsylvania district court, claims that ABIM is charging inflated monopoly prices for maintaining certification, that the organization is forcing physicians to purchase MOC, and that ABIM is inducing employers and others to require ABIM certification. On Jan. 23 of this year the legal challenge was amended to include racketeering and unjust enrichment claims.
The four plaintiff-physicians want the court to find ABIM in violation of federal antitrust law and to bar the board from continuing its MOC process. The suit is filed as a class action on behalf of all internists and subspecialists required by ABIM to purchase MOC to maintain their ABIM certifications.
Two other lawsuits challenging MOC, one against the American Board of Psychiatry and Neurology and another against the American Board of Radiology, are ongoing. A fourth lawsuit against the American Board of Medical Specialties, the American Board of Emergency Medicine, and the American Board of Anesthesiology was filed in February.
Chicago-based cardiologist Wes Fisher, MD, and fellow physicians with the Practicing Physicians of America are funding the plaintiffs’ legal efforts through a fundraising campaign that has raised more than $300,000.
In an interview, Dr. Fisher called the legal fight against ABIM “a David versus Goliath effort” and said the battle will continue.
“The ABIM may have won this first round, but ... they have only dodged the antitrust tying claim and unjust enrichment claims,” Dr. Fisher said. “The monopoly claim and racketeering claims are still very much open. Plaintiffs have 14 days to amend their compliant.”
A district court has dismissed a lawsuit levied by a group of physicians against the American Board of Internal Medicine (ABIM) over its maintenance of certification (MOC) program, calling the legal challenge “flawed.”
In a Sept. 26 decision, U.S. District Court Judge for the Eastern District of Pennsylvania Robert F. Kelly Sr. said the plaintiffs failed to demonstrate sufficient evidence for their antitrust and unjust enrichment claims against ABIM. The doctors also did not establish any showing of anticompetitive conduct by ABIM to support a monopolization claim, the judge ruled.
“We disagree with plaintiffs and find that ABIM’s initial certification and MOC products are part of a single product and do not occupy distinct markets,” Judge Kelly wrote in his decision. “Not only are we unconvinced by plaintiffs’ arguments, we find that plaintiffs’ entire framing of the ABIM certification to be flawed. In essence, plaintiffs are arguing that, in order to purchase ABIM’s initial certification, internists are forced to purchase MOC products as well. However, this is not the case. ... Nowhere in the amended complaint do plaintiffs allege that they were forced to buy MOC products in order to purchase the initial certification.”
The judge dismissed the suit, but allowed the plaintiffs 14 days to submit an amended complaint reoutlining their claims of illegal monopolization and racketeering against the board. If the amended complaint passes legal muster, the judge could revive those claims.
ABIM President Richard J. Baron, MD, expressed satisfaction that the court granted the board’s motion to dismiss the case for failure to state a valid claim.
“ABIM is pleased that the United States District Court for the Eastern District of Pennsylvania dismissed in its entirety a lawsuit that alleged physicians were harmed by the requirements for maintaining ABIM board certification,” Dr. Baron said in a statement.
C. Philip Curley, a Chicago-based attorney for the physician plaintiffs, said the case is far from over.
“The four internists who brought the lawsuit were invited to file amended claims, which is certainly being considered,” Mr. Curley said in an interview. “If necessary, all available appeals will also be pursued to the fullest. No one was under the impression that the fight to bring MOC to an end would be quick or easy.”
The original lawsuit, filed Dec. 6, 2018, in a Pennsylvania district court, claims that ABIM is charging inflated monopoly prices for maintaining certification, that the organization is forcing physicians to purchase MOC, and that ABIM is inducing employers and others to require ABIM certification. On Jan. 23 of this year the legal challenge was amended to include racketeering and unjust enrichment claims.
The four plaintiff-physicians want the court to find ABIM in violation of federal antitrust law and to bar the board from continuing its MOC process. The suit is filed as a class action on behalf of all internists and subspecialists required by ABIM to purchase MOC to maintain their ABIM certifications.
Two other lawsuits challenging MOC, one against the American Board of Psychiatry and Neurology and another against the American Board of Radiology, are ongoing. A fourth lawsuit against the American Board of Medical Specialties, the American Board of Emergency Medicine, and the American Board of Anesthesiology was filed in February.
Chicago-based cardiologist Wes Fisher, MD, and fellow physicians with the Practicing Physicians of America are funding the plaintiffs’ legal efforts through a fundraising campaign that has raised more than $300,000.
In an interview, Dr. Fisher called the legal fight against ABIM “a David versus Goliath effort” and said the battle will continue.
“The ABIM may have won this first round, but ... they have only dodged the antitrust tying claim and unjust enrichment claims,” Dr. Fisher said. “The monopoly claim and racketeering claims are still very much open. Plaintiffs have 14 days to amend their compliant.”
A district court has dismissed a lawsuit levied by a group of physicians against the American Board of Internal Medicine (ABIM) over its maintenance of certification (MOC) program, calling the legal challenge “flawed.”
In a Sept. 26 decision, U.S. District Court Judge for the Eastern District of Pennsylvania Robert F. Kelly Sr. said the plaintiffs failed to demonstrate sufficient evidence for their antitrust and unjust enrichment claims against ABIM. The doctors also did not establish any showing of anticompetitive conduct by ABIM to support a monopolization claim, the judge ruled.
“We disagree with plaintiffs and find that ABIM’s initial certification and MOC products are part of a single product and do not occupy distinct markets,” Judge Kelly wrote in his decision. “Not only are we unconvinced by plaintiffs’ arguments, we find that plaintiffs’ entire framing of the ABIM certification to be flawed. In essence, plaintiffs are arguing that, in order to purchase ABIM’s initial certification, internists are forced to purchase MOC products as well. However, this is not the case. ... Nowhere in the amended complaint do plaintiffs allege that they were forced to buy MOC products in order to purchase the initial certification.”
The judge dismissed the suit, but allowed the plaintiffs 14 days to submit an amended complaint reoutlining their claims of illegal monopolization and racketeering against the board. If the amended complaint passes legal muster, the judge could revive those claims.
ABIM President Richard J. Baron, MD, expressed satisfaction that the court granted the board’s motion to dismiss the case for failure to state a valid claim.
“ABIM is pleased that the United States District Court for the Eastern District of Pennsylvania dismissed in its entirety a lawsuit that alleged physicians were harmed by the requirements for maintaining ABIM board certification,” Dr. Baron said in a statement.
C. Philip Curley, a Chicago-based attorney for the physician plaintiffs, said the case is far from over.
“The four internists who brought the lawsuit were invited to file amended claims, which is certainly being considered,” Mr. Curley said in an interview. “If necessary, all available appeals will also be pursued to the fullest. No one was under the impression that the fight to bring MOC to an end would be quick or easy.”
The original lawsuit, filed Dec. 6, 2018, in a Pennsylvania district court, claims that ABIM is charging inflated monopoly prices for maintaining certification, that the organization is forcing physicians to purchase MOC, and that ABIM is inducing employers and others to require ABIM certification. On Jan. 23 of this year the legal challenge was amended to include racketeering and unjust enrichment claims.
The four plaintiff-physicians want the court to find ABIM in violation of federal antitrust law and to bar the board from continuing its MOC process. The suit is filed as a class action on behalf of all internists and subspecialists required by ABIM to purchase MOC to maintain their ABIM certifications.
Two other lawsuits challenging MOC, one against the American Board of Psychiatry and Neurology and another against the American Board of Radiology, are ongoing. A fourth lawsuit against the American Board of Medical Specialties, the American Board of Emergency Medicine, and the American Board of Anesthesiology was filed in February.
Chicago-based cardiologist Wes Fisher, MD, and fellow physicians with the Practicing Physicians of America are funding the plaintiffs’ legal efforts through a fundraising campaign that has raised more than $300,000.
In an interview, Dr. Fisher called the legal fight against ABIM “a David versus Goliath effort” and said the battle will continue.
“The ABIM may have won this first round, but ... they have only dodged the antitrust tying claim and unjust enrichment claims,” Dr. Fisher said. “The monopoly claim and racketeering claims are still very much open. Plaintiffs have 14 days to amend their compliant.”
Palliative care programs continue growth in U.S. hospitals
Growth continues among palliative care programs in the United States, although access often depends “more upon accidents of geography than it does upon the needs of patients,” according to the Center to Advance Palliative Care and the National Palliative Care Research Center.
“As is true for many aspects of health care, geography is destiny. Where you live determines your access to the best quality of life and highest quality of care during a serious illness,” said Diane E. Meier, MD, director of the Center to Advance Palliative Care, in a written statement.
the two organizations said in their 2019 report card on palliative care access. What hasn’t changed since 2015, however, is the country’s overall grade, which remains a B.
Delaware, New Hampshire, Rhode Island, and Vermont have a palliative care program in all of their hospitals with 50 or more beds and each earned a grade of A (palliative care rate of greater than 80%), along with 17 other states. The lowest-performing states – Alabama, Mississippi, New Mexico, Oklahoma, and Wyoming – all received Ds for having a rate below 40%, the CAPC said.
The urban/rural divide also is prominent in palliative care: “90% of hospitals with palliative care are in urban areas. Only 17% of rural hospitals with fifty or more beds report palliative care programs,” the report said.
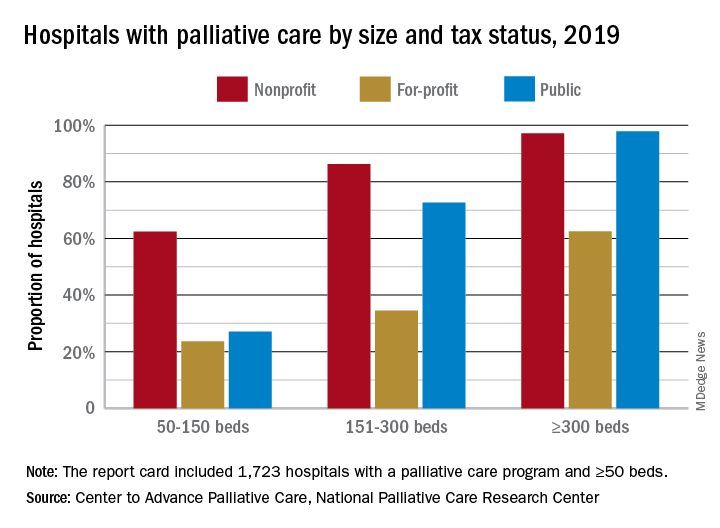
Hospital type is another source of disparity. Small, nonprofit hospitals are much more likely to offer access to palliative care than either for-profit or public facilities of the same size, but the gap closes as size increases, at least between nonprofit and public hospitals. For the largest institutions, the public hospitals pull into the lead, 98% versus 97%, over the nonprofits, with the for-profit facilities well behind at 63%.
“High quality palliative care has been shown to improve patient and family quality of life, improve patients’ and families’ health care experiences, and in certain diseases, prolong life. Palliative care has also been shown to improve hospital efficiency and reduce unnecessary spending,” said R. Sean Morrison, MD, director of the National Palliative Care Research Center.
The report card is based on data from the American Hospital Association’s Annual Survey Database, with additional data from the National Palliative Care Registry and Center to Advance Palliative Care’s Mapping Community Palliative Care initiative. The final sample included 2,409 hospitals with 50 or more beds.
Growth continues among palliative care programs in the United States, although access often depends “more upon accidents of geography than it does upon the needs of patients,” according to the Center to Advance Palliative Care and the National Palliative Care Research Center.

“As is true for many aspects of health care, geography is destiny. Where you live determines your access to the best quality of life and highest quality of care during a serious illness,” said Diane E. Meier, MD, director of the Center to Advance Palliative Care, in a written statement.
the two organizations said in their 2019 report card on palliative care access. What hasn’t changed since 2015, however, is the country’s overall grade, which remains a B.
Delaware, New Hampshire, Rhode Island, and Vermont have a palliative care program in all of their hospitals with 50 or more beds and each earned a grade of A (palliative care rate of greater than 80%), along with 17 other states. The lowest-performing states – Alabama, Mississippi, New Mexico, Oklahoma, and Wyoming – all received Ds for having a rate below 40%, the CAPC said.
The urban/rural divide also is prominent in palliative care: “90% of hospitals with palliative care are in urban areas. Only 17% of rural hospitals with fifty or more beds report palliative care programs,” the report said.
Hospital type is another source of disparity. Small, nonprofit hospitals are much more likely to offer access to palliative care than either for-profit or public facilities of the same size, but the gap closes as size increases, at least between nonprofit and public hospitals. For the largest institutions, the public hospitals pull into the lead, 98% versus 97%, over the nonprofits, with the for-profit facilities well behind at 63%.
“High quality palliative care has been shown to improve patient and family quality of life, improve patients’ and families’ health care experiences, and in certain diseases, prolong life. Palliative care has also been shown to improve hospital efficiency and reduce unnecessary spending,” said R. Sean Morrison, MD, director of the National Palliative Care Research Center.
The report card is based on data from the American Hospital Association’s Annual Survey Database, with additional data from the National Palliative Care Registry and Center to Advance Palliative Care’s Mapping Community Palliative Care initiative. The final sample included 2,409 hospitals with 50 or more beds.
Growth continues among palliative care programs in the United States, although access often depends “more upon accidents of geography than it does upon the needs of patients,” according to the Center to Advance Palliative Care and the National Palliative Care Research Center.

“As is true for many aspects of health care, geography is destiny. Where you live determines your access to the best quality of life and highest quality of care during a serious illness,” said Diane E. Meier, MD, director of the Center to Advance Palliative Care, in a written statement.
the two organizations said in their 2019 report card on palliative care access. What hasn’t changed since 2015, however, is the country’s overall grade, which remains a B.
Delaware, New Hampshire, Rhode Island, and Vermont have a palliative care program in all of their hospitals with 50 or more beds and each earned a grade of A (palliative care rate of greater than 80%), along with 17 other states. The lowest-performing states – Alabama, Mississippi, New Mexico, Oklahoma, and Wyoming – all received Ds for having a rate below 40%, the CAPC said.
The urban/rural divide also is prominent in palliative care: “90% of hospitals with palliative care are in urban areas. Only 17% of rural hospitals with fifty or more beds report palliative care programs,” the report said.
Hospital type is another source of disparity. Small, nonprofit hospitals are much more likely to offer access to palliative care than either for-profit or public facilities of the same size, but the gap closes as size increases, at least between nonprofit and public hospitals. For the largest institutions, the public hospitals pull into the lead, 98% versus 97%, over the nonprofits, with the for-profit facilities well behind at 63%.
“High quality palliative care has been shown to improve patient and family quality of life, improve patients’ and families’ health care experiences, and in certain diseases, prolong life. Palliative care has also been shown to improve hospital efficiency and reduce unnecessary spending,” said R. Sean Morrison, MD, director of the National Palliative Care Research Center.
The report card is based on data from the American Hospital Association’s Annual Survey Database, with additional data from the National Palliative Care Registry and Center to Advance Palliative Care’s Mapping Community Palliative Care initiative. The final sample included 2,409 hospitals with 50 or more beds.
Apixaban prevents clots with cancer
Background: Active cancer places patients at increased risk for VTE. Ambulatory patients can be risk stratified using the validated Khorana score to assess risk for VTE, a complication resulting in significant morbidity, mortality, and health care costs.
Study design: Randomized, placebo-controlled, double-blind clinical trial.
Setting: Ambulatory; Canada.
Synopsis: A total of 1,809 patients were assessed for eligibility, 1,235 were excluded, and 574 with a Khorana score of 2 or higher were randomized to apixaban 2.5 mg twice daily or placebo. Treatment or placebo was given within 24 hours after the initiation of chemotherapy and continued for 180 days. The primary efficacy outcome – first episode of major VTE within 180 days of randomization – occurred in 4.2% of the apixaban group and in 10.2% of the placebo group (hazard ratio, 0.41; 95% confidence interval, 0.26-0.65; P less than .001). Major bleeding in the modified intention-to-treat analysis occurred in 3.5% in the apixaban group and 1.8% in the placebo group (HR, 2.00; 95% CI, 1.01-3.95; P = .046).
There was no significant difference in overall survival, with 87% of deaths were related to cancer or cancer progression.
Bottom line: VTE is significantly lower with the use of apixaban, compared with placebo, in intermediate- to high-risk ambulatory patients with active cancer who are initiating chemotherapy.
Citation: Carrier M et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: Active cancer places patients at increased risk for VTE. Ambulatory patients can be risk stratified using the validated Khorana score to assess risk for VTE, a complication resulting in significant morbidity, mortality, and health care costs.
Study design: Randomized, placebo-controlled, double-blind clinical trial.
Setting: Ambulatory; Canada.
Synopsis: A total of 1,809 patients were assessed for eligibility, 1,235 were excluded, and 574 with a Khorana score of 2 or higher were randomized to apixaban 2.5 mg twice daily or placebo. Treatment or placebo was given within 24 hours after the initiation of chemotherapy and continued for 180 days. The primary efficacy outcome – first episode of major VTE within 180 days of randomization – occurred in 4.2% of the apixaban group and in 10.2% of the placebo group (hazard ratio, 0.41; 95% confidence interval, 0.26-0.65; P less than .001). Major bleeding in the modified intention-to-treat analysis occurred in 3.5% in the apixaban group and 1.8% in the placebo group (HR, 2.00; 95% CI, 1.01-3.95; P = .046).
There was no significant difference in overall survival, with 87% of deaths were related to cancer or cancer progression.
Bottom line: VTE is significantly lower with the use of apixaban, compared with placebo, in intermediate- to high-risk ambulatory patients with active cancer who are initiating chemotherapy.
Citation: Carrier M et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: Active cancer places patients at increased risk for VTE. Ambulatory patients can be risk stratified using the validated Khorana score to assess risk for VTE, a complication resulting in significant morbidity, mortality, and health care costs.
Study design: Randomized, placebo-controlled, double-blind clinical trial.
Setting: Ambulatory; Canada.
Synopsis: A total of 1,809 patients were assessed for eligibility, 1,235 were excluded, and 574 with a Khorana score of 2 or higher were randomized to apixaban 2.5 mg twice daily or placebo. Treatment or placebo was given within 24 hours after the initiation of chemotherapy and continued for 180 days. The primary efficacy outcome – first episode of major VTE within 180 days of randomization – occurred in 4.2% of the apixaban group and in 10.2% of the placebo group (hazard ratio, 0.41; 95% confidence interval, 0.26-0.65; P less than .001). Major bleeding in the modified intention-to-treat analysis occurred in 3.5% in the apixaban group and 1.8% in the placebo group (HR, 2.00; 95% CI, 1.01-3.95; P = .046).
There was no significant difference in overall survival, with 87% of deaths were related to cancer or cancer progression.
Bottom line: VTE is significantly lower with the use of apixaban, compared with placebo, in intermediate- to high-risk ambulatory patients with active cancer who are initiating chemotherapy.
Citation: Carrier M et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
SHM and Jefferson College of Population Health partner to provide vital education for hospitalists
Both the Society of Hospital Medicine and Jefferson College of Population Health, of Thomas Jefferson University in Philadelphia, share a goal to educate physicians to be effective leaders and managers in the pursuit of health care quality, safety, and population health, and they have entered into a partnership with this in mind.
Alexis Skoufalos, EdD, associate dean, strategic development, for Jefferson College of Population Health, recently spoke with The Hospitalist to discuss the importance of population health to hospital medicine professionals, the health care landscape as a whole, and the benefits of this new partnership with SHM.
Can you explain the importance of population health in the current health care landscape?
Many people confuse population health with public health. While they are related, they are different disciplines. Public health focuses on prevention and health promotion (clean water, vaccines, exercise, using seat belts, and so on), but it stops there.
Population health builds on the foundation of public health and goes a step further, working to connect health and health care delivery. It takes a more holistic approach, looking at what we need to do inside and outside the delivery system to help people to get and stay healthy, as well as take better care of them when they do get sick.
We work to identify and understand the health impact of social and environmental factors, while also looking for ways to make health care delivery safer, better, and more affordable and accessible.
This can get complicated. It involves sorting through lots of information to uncover the best way to meet the needs of a specific group, whether that is a community, a neighborhood, or a patient with a particular condition.
It’s about taking the time to really look at things from different vantage points. You won’t see the same view if you are looking at something through a telescope as you would looking through a microscope. That information can help you to adjust your perspective to identify the best course of action.
In order to be successful in improving population health, providers need to understand how to work with the other stakeholders in the health care ecosystem. Collaboration and coordination are the best ways to optimize the resources available.
It is important for delivery systems to establish good working relationships with community nonprofit and service organizations, faith-based organizations, social service providers, school systems, and federal, state, and local government.
At Jefferson, we thought it was important to create a college and programs that would prepare professionals across the workforce for this new challenge.
How did this partnership between SHM and Jefferson College of Population Health come to fruition?
Hospitalists are an important link with a person’s primary care team. The work they do to prepare a person and their family for successful discharge to the community after a hospital stay can make all the difference in a person’s recovery, condition management, and preventing readmission to the hospital.
Because both of our organizations are based in Philadelphia, we have had longstanding connections with SHM leadership. It was only natural for us to talk with SHM about how we can build upon the society’s excellent continuing education offerings and work together to provide members with additional content that can equip them to advance their careers.
How did SHM and Jefferson College of Population Health identify the mutually beneficial educational offerings in each institution that are included in this partnership?
Members of our respective leadership teams got together to complete a detailed review of the offerings from each organization. SHM’s Leadership Academy and JCPH’s Population Health Academy are rigorous continuing education programs that can provide physicians with excellent just-in-time information they can put to use right away.
After a careful examination of the curriculum, JCPH determined that SHM members can apply the credits they earn from completing two qualified sessions from the Leadership Academy to satisfy the elective course requirement for a Master’s degree. (Note: This does not apply to the Population Health Intelligence Program, which does not include an elective course.)
How will this partnership benefit Jefferson College of Population Health?
Our mission is to prepare health care leaders with the skills and tools they need to be effective in improving population health. Clinicians who work in a hospital setting have a key role to play.
We are also dedicated to making a difference right here in Philadelphia. The more students we have in our programs, the more of an impact we (and they) will have in improving outcomes in our own community.
We need to move the needle and get Philadelphia County out of the basement in terms of health rankings. We have a responsibility to do what we can to make a difference, and we appreciate the partnership with SHM to make it happen.
What other components of the partnership are especially noteworthy to highlight?
In addition to what I’ve already discussed, the following are some of the significant benefits that SHM members are entitled to as a result of the partnership with JCPH:
- 15% discount on tuition for any JCPH online graduate degree program.
- Registration discount for JCPH’s Population Health Academy in Philadelphia.
- Special registration rate for Annual Population Health Colloquium.
For more information about this partnership, visit hospitalmedicine.org/jefferson.
Both the Society of Hospital Medicine and Jefferson College of Population Health, of Thomas Jefferson University in Philadelphia, share a goal to educate physicians to be effective leaders and managers in the pursuit of health care quality, safety, and population health, and they have entered into a partnership with this in mind.
Alexis Skoufalos, EdD, associate dean, strategic development, for Jefferson College of Population Health, recently spoke with The Hospitalist to discuss the importance of population health to hospital medicine professionals, the health care landscape as a whole, and the benefits of this new partnership with SHM.
Can you explain the importance of population health in the current health care landscape?
Many people confuse population health with public health. While they are related, they are different disciplines. Public health focuses on prevention and health promotion (clean water, vaccines, exercise, using seat belts, and so on), but it stops there.
Population health builds on the foundation of public health and goes a step further, working to connect health and health care delivery. It takes a more holistic approach, looking at what we need to do inside and outside the delivery system to help people to get and stay healthy, as well as take better care of them when they do get sick.
We work to identify and understand the health impact of social and environmental factors, while also looking for ways to make health care delivery safer, better, and more affordable and accessible.
This can get complicated. It involves sorting through lots of information to uncover the best way to meet the needs of a specific group, whether that is a community, a neighborhood, or a patient with a particular condition.
It’s about taking the time to really look at things from different vantage points. You won’t see the same view if you are looking at something through a telescope as you would looking through a microscope. That information can help you to adjust your perspective to identify the best course of action.
In order to be successful in improving population health, providers need to understand how to work with the other stakeholders in the health care ecosystem. Collaboration and coordination are the best ways to optimize the resources available.
It is important for delivery systems to establish good working relationships with community nonprofit and service organizations, faith-based organizations, social service providers, school systems, and federal, state, and local government.
At Jefferson, we thought it was important to create a college and programs that would prepare professionals across the workforce for this new challenge.
How did this partnership between SHM and Jefferson College of Population Health come to fruition?
Hospitalists are an important link with a person’s primary care team. The work they do to prepare a person and their family for successful discharge to the community after a hospital stay can make all the difference in a person’s recovery, condition management, and preventing readmission to the hospital.
Because both of our organizations are based in Philadelphia, we have had longstanding connections with SHM leadership. It was only natural for us to talk with SHM about how we can build upon the society’s excellent continuing education offerings and work together to provide members with additional content that can equip them to advance their careers.
How did SHM and Jefferson College of Population Health identify the mutually beneficial educational offerings in each institution that are included in this partnership?
Members of our respective leadership teams got together to complete a detailed review of the offerings from each organization. SHM’s Leadership Academy and JCPH’s Population Health Academy are rigorous continuing education programs that can provide physicians with excellent just-in-time information they can put to use right away.
After a careful examination of the curriculum, JCPH determined that SHM members can apply the credits they earn from completing two qualified sessions from the Leadership Academy to satisfy the elective course requirement for a Master’s degree. (Note: This does not apply to the Population Health Intelligence Program, which does not include an elective course.)
How will this partnership benefit Jefferson College of Population Health?
Our mission is to prepare health care leaders with the skills and tools they need to be effective in improving population health. Clinicians who work in a hospital setting have a key role to play.
We are also dedicated to making a difference right here in Philadelphia. The more students we have in our programs, the more of an impact we (and they) will have in improving outcomes in our own community.
We need to move the needle and get Philadelphia County out of the basement in terms of health rankings. We have a responsibility to do what we can to make a difference, and we appreciate the partnership with SHM to make it happen.
What other components of the partnership are especially noteworthy to highlight?
In addition to what I’ve already discussed, the following are some of the significant benefits that SHM members are entitled to as a result of the partnership with JCPH:
- 15% discount on tuition for any JCPH online graduate degree program.
- Registration discount for JCPH’s Population Health Academy in Philadelphia.
- Special registration rate for Annual Population Health Colloquium.
For more information about this partnership, visit hospitalmedicine.org/jefferson.
Both the Society of Hospital Medicine and Jefferson College of Population Health, of Thomas Jefferson University in Philadelphia, share a goal to educate physicians to be effective leaders and managers in the pursuit of health care quality, safety, and population health, and they have entered into a partnership with this in mind.
Alexis Skoufalos, EdD, associate dean, strategic development, for Jefferson College of Population Health, recently spoke with The Hospitalist to discuss the importance of population health to hospital medicine professionals, the health care landscape as a whole, and the benefits of this new partnership with SHM.
Can you explain the importance of population health in the current health care landscape?
Many people confuse population health with public health. While they are related, they are different disciplines. Public health focuses on prevention and health promotion (clean water, vaccines, exercise, using seat belts, and so on), but it stops there.
Population health builds on the foundation of public health and goes a step further, working to connect health and health care delivery. It takes a more holistic approach, looking at what we need to do inside and outside the delivery system to help people to get and stay healthy, as well as take better care of them when they do get sick.
We work to identify and understand the health impact of social and environmental factors, while also looking for ways to make health care delivery safer, better, and more affordable and accessible.
This can get complicated. It involves sorting through lots of information to uncover the best way to meet the needs of a specific group, whether that is a community, a neighborhood, or a patient with a particular condition.
It’s about taking the time to really look at things from different vantage points. You won’t see the same view if you are looking at something through a telescope as you would looking through a microscope. That information can help you to adjust your perspective to identify the best course of action.
In order to be successful in improving population health, providers need to understand how to work with the other stakeholders in the health care ecosystem. Collaboration and coordination are the best ways to optimize the resources available.
It is important for delivery systems to establish good working relationships with community nonprofit and service organizations, faith-based organizations, social service providers, school systems, and federal, state, and local government.
At Jefferson, we thought it was important to create a college and programs that would prepare professionals across the workforce for this new challenge.
How did this partnership between SHM and Jefferson College of Population Health come to fruition?
Hospitalists are an important link with a person’s primary care team. The work they do to prepare a person and their family for successful discharge to the community after a hospital stay can make all the difference in a person’s recovery, condition management, and preventing readmission to the hospital.
Because both of our organizations are based in Philadelphia, we have had longstanding connections with SHM leadership. It was only natural for us to talk with SHM about how we can build upon the society’s excellent continuing education offerings and work together to provide members with additional content that can equip them to advance their careers.
How did SHM and Jefferson College of Population Health identify the mutually beneficial educational offerings in each institution that are included in this partnership?
Members of our respective leadership teams got together to complete a detailed review of the offerings from each organization. SHM’s Leadership Academy and JCPH’s Population Health Academy are rigorous continuing education programs that can provide physicians with excellent just-in-time information they can put to use right away.
After a careful examination of the curriculum, JCPH determined that SHM members can apply the credits they earn from completing two qualified sessions from the Leadership Academy to satisfy the elective course requirement for a Master’s degree. (Note: This does not apply to the Population Health Intelligence Program, which does not include an elective course.)
How will this partnership benefit Jefferson College of Population Health?
Our mission is to prepare health care leaders with the skills and tools they need to be effective in improving population health. Clinicians who work in a hospital setting have a key role to play.
We are also dedicated to making a difference right here in Philadelphia. The more students we have in our programs, the more of an impact we (and they) will have in improving outcomes in our own community.
We need to move the needle and get Philadelphia County out of the basement in terms of health rankings. We have a responsibility to do what we can to make a difference, and we appreciate the partnership with SHM to make it happen.
What other components of the partnership are especially noteworthy to highlight?
In addition to what I’ve already discussed, the following are some of the significant benefits that SHM members are entitled to as a result of the partnership with JCPH:
- 15% discount on tuition for any JCPH online graduate degree program.
- Registration discount for JCPH’s Population Health Academy in Philadelphia.
- Special registration rate for Annual Population Health Colloquium.
For more information about this partnership, visit hospitalmedicine.org/jefferson.
Dual therapy best for AFib with ACS no matter the treatment strategy
SAN FRANCISCO – Anticoagulation with apixaban and a P2Y12 inhibitor without aspirin provides superior safety and similar efficacy in patients with atrial fibrillation who have an acute coronary syndrome, compared with regimens that include vitamin K antagonists, aspirin, or both.
The findings come from a prespecified analysis of data from the AUGUSTUS trial presented by Stephan Windecker, MD, at the Transcatheter Cardiovascular Therapeutics annual meeting.
“This study adds very important information [to the notion] that triple therapy in the setting of atrial fibrillation and PCI [percutaneous coronary intervention] is really not the way to go,” Ori Ben-Yehuda, MD, FACC, executive director of the Cardiovascular Research Foundation’s Clinical Trials Center, said during a media briefing.
In the recent multicenter AUGUSTUS trial, Dr. Windecker, of the department of cardiology at Bern University Hospital, Switzerland, and colleagues found that among 4,614 patients with atrial fibrillation and a recent acute coronary syndrome or PCI treated with a P2Y12 inhibitor, apixaban without aspirin resulted in less bleeding, fewer hospitalizations, and no significant differences in ischemic events compared with regimens that included a vitamin K antagonist (VKA), aspirin, or both (N Engl J Med. 2019;380:1509-24). For this prespecified analysis, the researchers used a 2×2 factorial design to compare apixaban with VKA and aspirin with placebo in the AUGUSTUS trial participants with ACS treated medically (group 1; 1,097 patients, or 24%), those with ACS treated with PCI (group 2; 1,714 patients, or 37%), and those undergoing elective PCI (group 3; 1,784 patients, or 39%). The outcomes of interest were bleeding, death, and hospitalization as well as death and ischemic events by antithrombotic strategy in the study participants. This marks the only trial in the field that included patients with ACS managed medically, Dr. Windecker said.
At baseline, the median age of patients was 71 years, 30% were female, 36% had diabetes, and 45% had heart failure. Patients managed medically were younger (a median age of 70) and more frequently female; 57% presented with heart failure. The groups had identical CHA2DS2VASc scores (4), and very similar HAS-BLED scores (2 in groups 1 and 2, and 3 in group 3).
Apixaban compared with VKA showed lower International Society on Thrombosis and Haemostasis–defined major or clinically relevant nonmajor bleeding among patients in group 1 (HR, 0.44), group 2 (HR, 0.68), and group 3 (HR, 0.82) (P for interaction = .052). Apixaban compared with VKA reduced death or hospitalization among patients in group 1 (HR, 0.71), group 2 (HR 0.88), and group 3 (HR, 0.87) (P for interaction = .345). Compared with VKA, apixaban resulted in a similar effect on death and ischemic events among patients in all three treatment groups (P for interaction = .356).
Compared with placebo, aspirin had a higher rate of bleeding among patients in group 1 (HR, 1.49), group 2 (HR, 2.02) and group 3 (HR, 1.91) (P for interaction = .479). For the same comparison, there was no difference in outcomes among the three groups for the composite of death or hospitalization and death and ischemic events.
“The overall results of the AUGUSTUS trial are consistent across the three clinically important subgroups,” Dr. Windecker said. The reasons why patients received medical therapy remain unclear, “because it was at the physician’s discretion as to whether they were treated medically or underwent PCI,” he said. “The proportion very much reflects our clinical practice, where 20%-25% of patients are treated medically. What was surprising for me is that I would have anticipated there would be more elderly patients with comorbidities, but I did anticipate that there would be more female patients (in this subgroup).”
Robert A. Harrington, MD, an interventional cardiologist at Stanford (Calif.) University who served on the Data Safety and Monitoring Board for the trial, noted that the patients with atrial fibrillation represent 7%-10% of all ACS patients, “so it’s a big population,” he said. “What’s been disappointing is that none of the trials have been big enough to uncouple the bleeding vs. ischemic issue. We don’t know the answer for how long do you need the triple therapy versus when you can switch to the double therapy.”
Dr. Windecker said that the optimal duration of short-term aspirin remains unclear in this patient population. “Whether there is a benefit of giving aspirin for 2 weeks or 4 weeks remains unanswered,” he said. “Triple therapy is not the way to go, but we need to fine-tune, and probably individualize, which patients may benefit from a certain duration of aspirin.”
The study results were published online at the time of presentation (Circulation 2019 Sep 26. doi: 10.1161/CIRCULATIONAHA.119.043308.
AUGUSTUS was funded by Bristol-Myers Squibb and Pfizer Inc. Dr. Windecker reported having received institutional research and educational grants to Bern University Hospital from Abbott, Amgen, Bayer, BMS, CSL Behring, Boston Scientific, Biotronik, Edwards Lifesciences, Medtronic, Polares, and Sinomed. His coauthors reported having numerous financial ties to the pharmaceutical and device industries.
SOURCE: Windecker S. TCT 2019, Late-Breaking Trials 1 session.
SAN FRANCISCO – Anticoagulation with apixaban and a P2Y12 inhibitor without aspirin provides superior safety and similar efficacy in patients with atrial fibrillation who have an acute coronary syndrome, compared with regimens that include vitamin K antagonists, aspirin, or both.
The findings come from a prespecified analysis of data from the AUGUSTUS trial presented by Stephan Windecker, MD, at the Transcatheter Cardiovascular Therapeutics annual meeting.
“This study adds very important information [to the notion] that triple therapy in the setting of atrial fibrillation and PCI [percutaneous coronary intervention] is really not the way to go,” Ori Ben-Yehuda, MD, FACC, executive director of the Cardiovascular Research Foundation’s Clinical Trials Center, said during a media briefing.
In the recent multicenter AUGUSTUS trial, Dr. Windecker, of the department of cardiology at Bern University Hospital, Switzerland, and colleagues found that among 4,614 patients with atrial fibrillation and a recent acute coronary syndrome or PCI treated with a P2Y12 inhibitor, apixaban without aspirin resulted in less bleeding, fewer hospitalizations, and no significant differences in ischemic events compared with regimens that included a vitamin K antagonist (VKA), aspirin, or both (N Engl J Med. 2019;380:1509-24). For this prespecified analysis, the researchers used a 2×2 factorial design to compare apixaban with VKA and aspirin with placebo in the AUGUSTUS trial participants with ACS treated medically (group 1; 1,097 patients, or 24%), those with ACS treated with PCI (group 2; 1,714 patients, or 37%), and those undergoing elective PCI (group 3; 1,784 patients, or 39%). The outcomes of interest were bleeding, death, and hospitalization as well as death and ischemic events by antithrombotic strategy in the study participants. This marks the only trial in the field that included patients with ACS managed medically, Dr. Windecker said.
At baseline, the median age of patients was 71 years, 30% were female, 36% had diabetes, and 45% had heart failure. Patients managed medically were younger (a median age of 70) and more frequently female; 57% presented with heart failure. The groups had identical CHA2DS2VASc scores (4), and very similar HAS-BLED scores (2 in groups 1 and 2, and 3 in group 3).
Apixaban compared with VKA showed lower International Society on Thrombosis and Haemostasis–defined major or clinically relevant nonmajor bleeding among patients in group 1 (HR, 0.44), group 2 (HR, 0.68), and group 3 (HR, 0.82) (P for interaction = .052). Apixaban compared with VKA reduced death or hospitalization among patients in group 1 (HR, 0.71), group 2 (HR 0.88), and group 3 (HR, 0.87) (P for interaction = .345). Compared with VKA, apixaban resulted in a similar effect on death and ischemic events among patients in all three treatment groups (P for interaction = .356).
Compared with placebo, aspirin had a higher rate of bleeding among patients in group 1 (HR, 1.49), group 2 (HR, 2.02) and group 3 (HR, 1.91) (P for interaction = .479). For the same comparison, there was no difference in outcomes among the three groups for the composite of death or hospitalization and death and ischemic events.
“The overall results of the AUGUSTUS trial are consistent across the three clinically important subgroups,” Dr. Windecker said. The reasons why patients received medical therapy remain unclear, “because it was at the physician’s discretion as to whether they were treated medically or underwent PCI,” he said. “The proportion very much reflects our clinical practice, where 20%-25% of patients are treated medically. What was surprising for me is that I would have anticipated there would be more elderly patients with comorbidities, but I did anticipate that there would be more female patients (in this subgroup).”
Robert A. Harrington, MD, an interventional cardiologist at Stanford (Calif.) University who served on the Data Safety and Monitoring Board for the trial, noted that the patients with atrial fibrillation represent 7%-10% of all ACS patients, “so it’s a big population,” he said. “What’s been disappointing is that none of the trials have been big enough to uncouple the bleeding vs. ischemic issue. We don’t know the answer for how long do you need the triple therapy versus when you can switch to the double therapy.”
Dr. Windecker said that the optimal duration of short-term aspirin remains unclear in this patient population. “Whether there is a benefit of giving aspirin for 2 weeks or 4 weeks remains unanswered,” he said. “Triple therapy is not the way to go, but we need to fine-tune, and probably individualize, which patients may benefit from a certain duration of aspirin.”
The study results were published online at the time of presentation (Circulation 2019 Sep 26. doi: 10.1161/CIRCULATIONAHA.119.043308.
AUGUSTUS was funded by Bristol-Myers Squibb and Pfizer Inc. Dr. Windecker reported having received institutional research and educational grants to Bern University Hospital from Abbott, Amgen, Bayer, BMS, CSL Behring, Boston Scientific, Biotronik, Edwards Lifesciences, Medtronic, Polares, and Sinomed. His coauthors reported having numerous financial ties to the pharmaceutical and device industries.
SOURCE: Windecker S. TCT 2019, Late-Breaking Trials 1 session.
SAN FRANCISCO – Anticoagulation with apixaban and a P2Y12 inhibitor without aspirin provides superior safety and similar efficacy in patients with atrial fibrillation who have an acute coronary syndrome, compared with regimens that include vitamin K antagonists, aspirin, or both.
The findings come from a prespecified analysis of data from the AUGUSTUS trial presented by Stephan Windecker, MD, at the Transcatheter Cardiovascular Therapeutics annual meeting.
“This study adds very important information [to the notion] that triple therapy in the setting of atrial fibrillation and PCI [percutaneous coronary intervention] is really not the way to go,” Ori Ben-Yehuda, MD, FACC, executive director of the Cardiovascular Research Foundation’s Clinical Trials Center, said during a media briefing.
In the recent multicenter AUGUSTUS trial, Dr. Windecker, of the department of cardiology at Bern University Hospital, Switzerland, and colleagues found that among 4,614 patients with atrial fibrillation and a recent acute coronary syndrome or PCI treated with a P2Y12 inhibitor, apixaban without aspirin resulted in less bleeding, fewer hospitalizations, and no significant differences in ischemic events compared with regimens that included a vitamin K antagonist (VKA), aspirin, or both (N Engl J Med. 2019;380:1509-24). For this prespecified analysis, the researchers used a 2×2 factorial design to compare apixaban with VKA and aspirin with placebo in the AUGUSTUS trial participants with ACS treated medically (group 1; 1,097 patients, or 24%), those with ACS treated with PCI (group 2; 1,714 patients, or 37%), and those undergoing elective PCI (group 3; 1,784 patients, or 39%). The outcomes of interest were bleeding, death, and hospitalization as well as death and ischemic events by antithrombotic strategy in the study participants. This marks the only trial in the field that included patients with ACS managed medically, Dr. Windecker said.
At baseline, the median age of patients was 71 years, 30% were female, 36% had diabetes, and 45% had heart failure. Patients managed medically were younger (a median age of 70) and more frequently female; 57% presented with heart failure. The groups had identical CHA2DS2VASc scores (4), and very similar HAS-BLED scores (2 in groups 1 and 2, and 3 in group 3).
Apixaban compared with VKA showed lower International Society on Thrombosis and Haemostasis–defined major or clinically relevant nonmajor bleeding among patients in group 1 (HR, 0.44), group 2 (HR, 0.68), and group 3 (HR, 0.82) (P for interaction = .052). Apixaban compared with VKA reduced death or hospitalization among patients in group 1 (HR, 0.71), group 2 (HR 0.88), and group 3 (HR, 0.87) (P for interaction = .345). Compared with VKA, apixaban resulted in a similar effect on death and ischemic events among patients in all three treatment groups (P for interaction = .356).
Compared with placebo, aspirin had a higher rate of bleeding among patients in group 1 (HR, 1.49), group 2 (HR, 2.02) and group 3 (HR, 1.91) (P for interaction = .479). For the same comparison, there was no difference in outcomes among the three groups for the composite of death or hospitalization and death and ischemic events.
“The overall results of the AUGUSTUS trial are consistent across the three clinically important subgroups,” Dr. Windecker said. The reasons why patients received medical therapy remain unclear, “because it was at the physician’s discretion as to whether they were treated medically or underwent PCI,” he said. “The proportion very much reflects our clinical practice, where 20%-25% of patients are treated medically. What was surprising for me is that I would have anticipated there would be more elderly patients with comorbidities, but I did anticipate that there would be more female patients (in this subgroup).”
Robert A. Harrington, MD, an interventional cardiologist at Stanford (Calif.) University who served on the Data Safety and Monitoring Board for the trial, noted that the patients with atrial fibrillation represent 7%-10% of all ACS patients, “so it’s a big population,” he said. “What’s been disappointing is that none of the trials have been big enough to uncouple the bleeding vs. ischemic issue. We don’t know the answer for how long do you need the triple therapy versus when you can switch to the double therapy.”
Dr. Windecker said that the optimal duration of short-term aspirin remains unclear in this patient population. “Whether there is a benefit of giving aspirin for 2 weeks or 4 weeks remains unanswered,” he said. “Triple therapy is not the way to go, but we need to fine-tune, and probably individualize, which patients may benefit from a certain duration of aspirin.”
The study results were published online at the time of presentation (Circulation 2019 Sep 26. doi: 10.1161/CIRCULATIONAHA.119.043308.
AUGUSTUS was funded by Bristol-Myers Squibb and Pfizer Inc. Dr. Windecker reported having received institutional research and educational grants to Bern University Hospital from Abbott, Amgen, Bayer, BMS, CSL Behring, Boston Scientific, Biotronik, Edwards Lifesciences, Medtronic, Polares, and Sinomed. His coauthors reported having numerous financial ties to the pharmaceutical and device industries.
SOURCE: Windecker S. TCT 2019, Late-Breaking Trials 1 session.
AT TCT 2019
POCUS for hospitalists: The SHM position statement
Background: POCUS is becoming more prevalent in the daily practice of hospitalists; however, there are currently no established standards or guidelines for the use of POCUS for hospitalists.
Study design: Position statement.
Setting: SHM Executive Committee and Multi-Institutional POCUS faculty meeting through the Society of Hospital Medicine 2018 Annual Conference reviewed and approved this statement.
Synopsis: In contrast to the comprehensive ultrasound exam, POCUS is used by hospitalists to answer focused questions, by the same clinician who is generating the clinical question, to evaluate multiple body systems, or to serially investigate changes clinical status or evaluate responses to therapy.
This position statement provides guidance on the use of POCUS by hospitalists and the administrators who oversee it by outlining POCUS in terms of common diagnostic and procedural applications; training; assessments by the categories of basic knowledge, image acquisition, interpretation, clinical integration, and certification and maintenance of skills; and program management.
Bottom line: This position statement by the SHM provides guidance for hospitalists and administrators on the use and oversight of POCUS.
Citation: Soni NJ et al. Point-of-care ultrasound for hospitalists: A position statement of the Society of Hospital Medicine. J Hosp Med. 2019 Jan 2;14:E1-E6.
Dr. Wang is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: POCUS is becoming more prevalent in the daily practice of hospitalists; however, there are currently no established standards or guidelines for the use of POCUS for hospitalists.
Study design: Position statement.
Setting: SHM Executive Committee and Multi-Institutional POCUS faculty meeting through the Society of Hospital Medicine 2018 Annual Conference reviewed and approved this statement.
Synopsis: In contrast to the comprehensive ultrasound exam, POCUS is used by hospitalists to answer focused questions, by the same clinician who is generating the clinical question, to evaluate multiple body systems, or to serially investigate changes clinical status or evaluate responses to therapy.
This position statement provides guidance on the use of POCUS by hospitalists and the administrators who oversee it by outlining POCUS in terms of common diagnostic and procedural applications; training; assessments by the categories of basic knowledge, image acquisition, interpretation, clinical integration, and certification and maintenance of skills; and program management.
Bottom line: This position statement by the SHM provides guidance for hospitalists and administrators on the use and oversight of POCUS.
Citation: Soni NJ et al. Point-of-care ultrasound for hospitalists: A position statement of the Society of Hospital Medicine. J Hosp Med. 2019 Jan 2;14:E1-E6.
Dr. Wang is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: POCUS is becoming more prevalent in the daily practice of hospitalists; however, there are currently no established standards or guidelines for the use of POCUS for hospitalists.
Study design: Position statement.
Setting: SHM Executive Committee and Multi-Institutional POCUS faculty meeting through the Society of Hospital Medicine 2018 Annual Conference reviewed and approved this statement.
Synopsis: In contrast to the comprehensive ultrasound exam, POCUS is used by hospitalists to answer focused questions, by the same clinician who is generating the clinical question, to evaluate multiple body systems, or to serially investigate changes clinical status or evaluate responses to therapy.
This position statement provides guidance on the use of POCUS by hospitalists and the administrators who oversee it by outlining POCUS in terms of common diagnostic and procedural applications; training; assessments by the categories of basic knowledge, image acquisition, interpretation, clinical integration, and certification and maintenance of skills; and program management.
Bottom line: This position statement by the SHM provides guidance for hospitalists and administrators on the use and oversight of POCUS.
Citation: Soni NJ et al. Point-of-care ultrasound for hospitalists: A position statement of the Society of Hospital Medicine. J Hosp Med. 2019 Jan 2;14:E1-E6.
Dr. Wang is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Effects of hospitalization on readmission rate
Background: There is increasing concern that the patient experience in the hospital may be associated with post-hospital adverse outcomes, including new or recurrent illnesses after discharge or unplanned return to the hospital or readmission.
Study design: Prospective cohort that included 207 patients.
Setting: Two academic hospitals in Toronto.
Synopsis: These patients had been admitted to the internal medicine ward for more than 48 hours and were interviewed at discharge using a standardized questionnaire to assess four domains of the trauma of hospitalization defined as the cumulative effects of patient-reported sleep disturbance, mobility, nutrition, and mood. Among these patients, 64.3% experienced disturbance in more than one domain, and patients who experienced disturbance in three to four domains had a 15.8% greater absolute risk of 30-day readmission or ED visit.
Because this is an observational study, causal inferences were not possible; however, hospitalists should keep in mind the possible association of the patient experience and the link to clinical outcomes.
Bottom line: Trauma of hospitalization is common and may be associated with an increased 30-day risk of readmission or ED visit.
Citation: Rawal J et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179(1):38-45.
Dr. Wang is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: There is increasing concern that the patient experience in the hospital may be associated with post-hospital adverse outcomes, including new or recurrent illnesses after discharge or unplanned return to the hospital or readmission.
Study design: Prospective cohort that included 207 patients.
Setting: Two academic hospitals in Toronto.
Synopsis: These patients had been admitted to the internal medicine ward for more than 48 hours and were interviewed at discharge using a standardized questionnaire to assess four domains of the trauma of hospitalization defined as the cumulative effects of patient-reported sleep disturbance, mobility, nutrition, and mood. Among these patients, 64.3% experienced disturbance in more than one domain, and patients who experienced disturbance in three to four domains had a 15.8% greater absolute risk of 30-day readmission or ED visit.
Because this is an observational study, causal inferences were not possible; however, hospitalists should keep in mind the possible association of the patient experience and the link to clinical outcomes.
Bottom line: Trauma of hospitalization is common and may be associated with an increased 30-day risk of readmission or ED visit.
Citation: Rawal J et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179(1):38-45.
Dr. Wang is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: There is increasing concern that the patient experience in the hospital may be associated with post-hospital adverse outcomes, including new or recurrent illnesses after discharge or unplanned return to the hospital or readmission.
Study design: Prospective cohort that included 207 patients.
Setting: Two academic hospitals in Toronto.
Synopsis: These patients had been admitted to the internal medicine ward for more than 48 hours and were interviewed at discharge using a standardized questionnaire to assess four domains of the trauma of hospitalization defined as the cumulative effects of patient-reported sleep disturbance, mobility, nutrition, and mood. Among these patients, 64.3% experienced disturbance in more than one domain, and patients who experienced disturbance in three to four domains had a 15.8% greater absolute risk of 30-day readmission or ED visit.
Because this is an observational study, causal inferences were not possible; however, hospitalists should keep in mind the possible association of the patient experience and the link to clinical outcomes.
Bottom line: Trauma of hospitalization is common and may be associated with an increased 30-day risk of readmission or ED visit.
Citation: Rawal J et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179(1):38-45.
Dr. Wang is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Skip supplemental O2 in nonhypoxic ACS
PARIS – A massive randomized trial that included all New Zealanders with a suspected acute coronary syndrome during a 2-year period has provided definitive evidence that giving high-flow supplemental oxygen to those who are nonhypoxemic is of no clinical benefit, although it wasn’t harmful, either.

“Patients who have a normal blood oxygen saturation level are very unlikely to benefit from supplemental oxygen,” Ralph Stewart, MbChB, said in presenting the results of the NZOTACS (New Zealand Oxygen Therapy in Acute Coronary Syndromes) trial at the annual congress of the European Society of Cardiology.
“It’s amazing that oxygen has been used in patients with suspected heart attack for over 50 years, and during that time there’s never been definite evidence that it improves outcomes. And more recently some have even suggested giving high-level oxygen might actually cause harm,” observed Dr. Stewart, a cardiologist at Auckland City Hospital and the University of Auckland (New Zealand).
The primary outcome in NZOTACS was 30-day all-cause mortality. In the overall study population, the rate was 3.0% in the group assigned to the routine high-flow oxygen protocol and closely similar at 3.1% in those randomized to the conservative oxygen strategy. And there was reassuringly no signal that the liberal oxygen protocol caused any harm.
To conduct this cluster randomized crossover trial, Dr. Stewart and his coinvestigators divided New Zealand into quadrants and, taking advantage of the coordinated health care systems operative in the nation of 4.8 million, they arranged for all ambulances, emergency departments, and hospitals in each geographic region to utilize each supplemental oxygen strategy for a total of 12 months.
In the liberal oxygen strategy, patients with suspected ACS on the basis of ischemic chest pain or ECG changes received high-flow oxygen by face mask at 6-8 L/min regardless of their blood oxygen saturation (SaO2) level. The oxygen was stopped only upon clinical resolution of myocardial ischemia. In contrast, in the low-oxygen protocol, supplemental oxygen was reserved for patients with an initial SaO2 below 90%, with a target SaO2 of 90%-94%.
Roughly 90% of the nearly 41,000 study participants had a normal SaO2 of 90% or more. Their 30-day mortality was 2.1% with the high-oxygen protocol and similar at 1.9% with the conservative oxygen protocol.
In contrast, there was a suggestion of benefit for the routine liberal oxygen strategy in the subgroup of patients with ST-elevation MI. Their 30-day mortality was 8.8% with high-flow oxygen and 10.6% with the conservative oxygen protocol. The resultant 19% relative risk reduction barely missed statistical significance. There was also a trend for possible benefit of routine high-flow oxygen in the roughly 12% of NZOTACS participants with an SaO2 below 95%, a lower bar than the 90% SaO2 that defines hypoxemia. Their death rate at 30 days was 10.1% if they got supplemental oxygen and 11.1% if they only received oxygen in the event their SaO2 was below 90%. But these exploratory findings must be viewed as hypothesis-generating, and a large confirmatory study would be required, Dr. Stewart noted.
Discussant Robin Hofmann, MD, PhD, commented that, based on the NZOTACS results, he believes a couple of changes to the current ESC guidelines on management of ACS are in order. The guidelines now state that oxygen is indicated in patients with suspected ACS and hypoxemia as defined by an SaO2 below 90%, giving that recommendation a Class I Level of Evidence C. That should now be upgraded to the strongest-possible Class I A recommendation, according to Dr. Hofmann, a cardiologist at the Karolinska Institute in Stockholm.
The ESC guidelines also state that oxygen isn’t routinely recommended in patients with an SaO2 of 90% or more, rating that guidance Class III B. On the basis of NZOTACS coupled with earlier far smaller studies, that should be changed to a Class III A recommendation, meaning simply don’t do it. The hint provided by NZOTACS of a possible small benefit for oxygen in patients with an SaO2 below 95% isn’t strong enough evidence to carry the day, in Dr. Hofmann’s view.
Dr. Stewart and Dr. Hofmann reported having no financial conflicts of interest. The NZOTACS trial was funded by the National Heart Foundation of New Zealand.
SOURCE: Stewart R. ESC 2019, Hotline Session 2.
PARIS – A massive randomized trial that included all New Zealanders with a suspected acute coronary syndrome during a 2-year period has provided definitive evidence that giving high-flow supplemental oxygen to those who are nonhypoxemic is of no clinical benefit, although it wasn’t harmful, either.

“Patients who have a normal blood oxygen saturation level are very unlikely to benefit from supplemental oxygen,” Ralph Stewart, MbChB, said in presenting the results of the NZOTACS (New Zealand Oxygen Therapy in Acute Coronary Syndromes) trial at the annual congress of the European Society of Cardiology.
“It’s amazing that oxygen has been used in patients with suspected heart attack for over 50 years, and during that time there’s never been definite evidence that it improves outcomes. And more recently some have even suggested giving high-level oxygen might actually cause harm,” observed Dr. Stewart, a cardiologist at Auckland City Hospital and the University of Auckland (New Zealand).
The primary outcome in NZOTACS was 30-day all-cause mortality. In the overall study population, the rate was 3.0% in the group assigned to the routine high-flow oxygen protocol and closely similar at 3.1% in those randomized to the conservative oxygen strategy. And there was reassuringly no signal that the liberal oxygen protocol caused any harm.
To conduct this cluster randomized crossover trial, Dr. Stewart and his coinvestigators divided New Zealand into quadrants and, taking advantage of the coordinated health care systems operative in the nation of 4.8 million, they arranged for all ambulances, emergency departments, and hospitals in each geographic region to utilize each supplemental oxygen strategy for a total of 12 months.
In the liberal oxygen strategy, patients with suspected ACS on the basis of ischemic chest pain or ECG changes received high-flow oxygen by face mask at 6-8 L/min regardless of their blood oxygen saturation (SaO2) level. The oxygen was stopped only upon clinical resolution of myocardial ischemia. In contrast, in the low-oxygen protocol, supplemental oxygen was reserved for patients with an initial SaO2 below 90%, with a target SaO2 of 90%-94%.
Roughly 90% of the nearly 41,000 study participants had a normal SaO2 of 90% or more. Their 30-day mortality was 2.1% with the high-oxygen protocol and similar at 1.9% with the conservative oxygen protocol.
In contrast, there was a suggestion of benefit for the routine liberal oxygen strategy in the subgroup of patients with ST-elevation MI. Their 30-day mortality was 8.8% with high-flow oxygen and 10.6% with the conservative oxygen protocol. The resultant 19% relative risk reduction barely missed statistical significance. There was also a trend for possible benefit of routine high-flow oxygen in the roughly 12% of NZOTACS participants with an SaO2 below 95%, a lower bar than the 90% SaO2 that defines hypoxemia. Their death rate at 30 days was 10.1% if they got supplemental oxygen and 11.1% if they only received oxygen in the event their SaO2 was below 90%. But these exploratory findings must be viewed as hypothesis-generating, and a large confirmatory study would be required, Dr. Stewart noted.
Discussant Robin Hofmann, MD, PhD, commented that, based on the NZOTACS results, he believes a couple of changes to the current ESC guidelines on management of ACS are in order. The guidelines now state that oxygen is indicated in patients with suspected ACS and hypoxemia as defined by an SaO2 below 90%, giving that recommendation a Class I Level of Evidence C. That should now be upgraded to the strongest-possible Class I A recommendation, according to Dr. Hofmann, a cardiologist at the Karolinska Institute in Stockholm.
The ESC guidelines also state that oxygen isn’t routinely recommended in patients with an SaO2 of 90% or more, rating that guidance Class III B. On the basis of NZOTACS coupled with earlier far smaller studies, that should be changed to a Class III A recommendation, meaning simply don’t do it. The hint provided by NZOTACS of a possible small benefit for oxygen in patients with an SaO2 below 95% isn’t strong enough evidence to carry the day, in Dr. Hofmann’s view.
Dr. Stewart and Dr. Hofmann reported having no financial conflicts of interest. The NZOTACS trial was funded by the National Heart Foundation of New Zealand.
SOURCE: Stewart R. ESC 2019, Hotline Session 2.
PARIS – A massive randomized trial that included all New Zealanders with a suspected acute coronary syndrome during a 2-year period has provided definitive evidence that giving high-flow supplemental oxygen to those who are nonhypoxemic is of no clinical benefit, although it wasn’t harmful, either.

“Patients who have a normal blood oxygen saturation level are very unlikely to benefit from supplemental oxygen,” Ralph Stewart, MbChB, said in presenting the results of the NZOTACS (New Zealand Oxygen Therapy in Acute Coronary Syndromes) trial at the annual congress of the European Society of Cardiology.
“It’s amazing that oxygen has been used in patients with suspected heart attack for over 50 years, and during that time there’s never been definite evidence that it improves outcomes. And more recently some have even suggested giving high-level oxygen might actually cause harm,” observed Dr. Stewart, a cardiologist at Auckland City Hospital and the University of Auckland (New Zealand).
The primary outcome in NZOTACS was 30-day all-cause mortality. In the overall study population, the rate was 3.0% in the group assigned to the routine high-flow oxygen protocol and closely similar at 3.1% in those randomized to the conservative oxygen strategy. And there was reassuringly no signal that the liberal oxygen protocol caused any harm.
To conduct this cluster randomized crossover trial, Dr. Stewart and his coinvestigators divided New Zealand into quadrants and, taking advantage of the coordinated health care systems operative in the nation of 4.8 million, they arranged for all ambulances, emergency departments, and hospitals in each geographic region to utilize each supplemental oxygen strategy for a total of 12 months.
In the liberal oxygen strategy, patients with suspected ACS on the basis of ischemic chest pain or ECG changes received high-flow oxygen by face mask at 6-8 L/min regardless of their blood oxygen saturation (SaO2) level. The oxygen was stopped only upon clinical resolution of myocardial ischemia. In contrast, in the low-oxygen protocol, supplemental oxygen was reserved for patients with an initial SaO2 below 90%, with a target SaO2 of 90%-94%.
Roughly 90% of the nearly 41,000 study participants had a normal SaO2 of 90% or more. Their 30-day mortality was 2.1% with the high-oxygen protocol and similar at 1.9% with the conservative oxygen protocol.
In contrast, there was a suggestion of benefit for the routine liberal oxygen strategy in the subgroup of patients with ST-elevation MI. Their 30-day mortality was 8.8% with high-flow oxygen and 10.6% with the conservative oxygen protocol. The resultant 19% relative risk reduction barely missed statistical significance. There was also a trend for possible benefit of routine high-flow oxygen in the roughly 12% of NZOTACS participants with an SaO2 below 95%, a lower bar than the 90% SaO2 that defines hypoxemia. Their death rate at 30 days was 10.1% if they got supplemental oxygen and 11.1% if they only received oxygen in the event their SaO2 was below 90%. But these exploratory findings must be viewed as hypothesis-generating, and a large confirmatory study would be required, Dr. Stewart noted.
Discussant Robin Hofmann, MD, PhD, commented that, based on the NZOTACS results, he believes a couple of changes to the current ESC guidelines on management of ACS are in order. The guidelines now state that oxygen is indicated in patients with suspected ACS and hypoxemia as defined by an SaO2 below 90%, giving that recommendation a Class I Level of Evidence C. That should now be upgraded to the strongest-possible Class I A recommendation, according to Dr. Hofmann, a cardiologist at the Karolinska Institute in Stockholm.
The ESC guidelines also state that oxygen isn’t routinely recommended in patients with an SaO2 of 90% or more, rating that guidance Class III B. On the basis of NZOTACS coupled with earlier far smaller studies, that should be changed to a Class III A recommendation, meaning simply don’t do it. The hint provided by NZOTACS of a possible small benefit for oxygen in patients with an SaO2 below 95% isn’t strong enough evidence to carry the day, in Dr. Hofmann’s view.
Dr. Stewart and Dr. Hofmann reported having no financial conflicts of interest. The NZOTACS trial was funded by the National Heart Foundation of New Zealand.
SOURCE: Stewart R. ESC 2019, Hotline Session 2.
REPORTING FROM THE ESC CONGRESS 2019
Using ultrasound guidance for adult abdominal paracentesis
Background: Abdominal paracentesis is a commonly performed procedure, and with appropriate training, hospitalists can deliver similar outcomes when compared to interventional radiologists.
Study design: Position statement.
Setting: The Society of Hospital Medicine Point-of-Care Ultrasound (POCUS) Task Force developed these guidelines after reviewing available literature and voted on the appropriateness and consensus of a recommendation.
Synopsis: A total of 794 articles were screened, and 91 articles were included and incorporated into the recommendations. The 12 recommendations fall into three categories (clinical outcomes, technique, and training), and all 12 recommendations achieved consensus as strong recommendations.
To improve clinical outcomes, the authors recommended ultrasound guidance in performing paracentesis to reduce the risk of serious complications, to avoid attempting paracentesis with insufficient fluid, and to improve overall procedure success.
The authors advocated for several technique recommendations, including using the ultrasound to assess volume and location of intraperitoneal fluid, to identify the needle insertion site and confirm in multiple planes, to use color flow Doppler to identify abdominal wall vessels, to mark the insertion site immediately prior to the procedure, and to consider real-time ultrasound guidance.
When health care professionals are learning ultrasound-guided paracentesis, the authors recommended use of dedicated training sessions with simulation if available and that competency should be demonstrated before independently attempting the procedure.
Bottom line: These recommendations from SHM POCUS Task Force provides consensus guidelines on the use of ultrasound guidance when performing or learning abdominal paracentesis.
Citation: Cho J et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: A position statement of the Society of Hospital Medicine. 2019 Jan 2. doi: 10.12788/jhm.3095.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Background: Abdominal paracentesis is a commonly performed procedure, and with appropriate training, hospitalists can deliver similar outcomes when compared to interventional radiologists.
Study design: Position statement.
Setting: The Society of Hospital Medicine Point-of-Care Ultrasound (POCUS) Task Force developed these guidelines after reviewing available literature and voted on the appropriateness and consensus of a recommendation.
Synopsis: A total of 794 articles were screened, and 91 articles were included and incorporated into the recommendations. The 12 recommendations fall into three categories (clinical outcomes, technique, and training), and all 12 recommendations achieved consensus as strong recommendations.
To improve clinical outcomes, the authors recommended ultrasound guidance in performing paracentesis to reduce the risk of serious complications, to avoid attempting paracentesis with insufficient fluid, and to improve overall procedure success.
The authors advocated for several technique recommendations, including using the ultrasound to assess volume and location of intraperitoneal fluid, to identify the needle insertion site and confirm in multiple planes, to use color flow Doppler to identify abdominal wall vessels, to mark the insertion site immediately prior to the procedure, and to consider real-time ultrasound guidance.
When health care professionals are learning ultrasound-guided paracentesis, the authors recommended use of dedicated training sessions with simulation if available and that competency should be demonstrated before independently attempting the procedure.
Bottom line: These recommendations from SHM POCUS Task Force provides consensus guidelines on the use of ultrasound guidance when performing or learning abdominal paracentesis.
Citation: Cho J et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: A position statement of the Society of Hospital Medicine. 2019 Jan 2. doi: 10.12788/jhm.3095.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Background: Abdominal paracentesis is a commonly performed procedure, and with appropriate training, hospitalists can deliver similar outcomes when compared to interventional radiologists.
Study design: Position statement.
Setting: The Society of Hospital Medicine Point-of-Care Ultrasound (POCUS) Task Force developed these guidelines after reviewing available literature and voted on the appropriateness and consensus of a recommendation.
Synopsis: A total of 794 articles were screened, and 91 articles were included and incorporated into the recommendations. The 12 recommendations fall into three categories (clinical outcomes, technique, and training), and all 12 recommendations achieved consensus as strong recommendations.
To improve clinical outcomes, the authors recommended ultrasound guidance in performing paracentesis to reduce the risk of serious complications, to avoid attempting paracentesis with insufficient fluid, and to improve overall procedure success.
The authors advocated for several technique recommendations, including using the ultrasound to assess volume and location of intraperitoneal fluid, to identify the needle insertion site and confirm in multiple planes, to use color flow Doppler to identify abdominal wall vessels, to mark the insertion site immediately prior to the procedure, and to consider real-time ultrasound guidance.
When health care professionals are learning ultrasound-guided paracentesis, the authors recommended use of dedicated training sessions with simulation if available and that competency should be demonstrated before independently attempting the procedure.
Bottom line: These recommendations from SHM POCUS Task Force provides consensus guidelines on the use of ultrasound guidance when performing or learning abdominal paracentesis.
Citation: Cho J et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: A position statement of the Society of Hospital Medicine. 2019 Jan 2. doi: 10.12788/jhm.3095.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
A quarter of ICU admissions caused by substance abuse
according to results from a retrospective chart review.
The abuse of illicit drugs topped substance abuse–related ICU stays, accounting for 13% of total admissions, which represented 11% of total charges.
“We conducted a study to provide updated data on ICU utilization and costs related to licit and illicit abuse at a large county hospital,” wrote Donald Westerhausen, MD, of Indiana University, Indianapolis, and colleagues. The findings were reported in Chest .
The single-center study comprised 594 patients who were admitted for prescription, alcohol, or illicit drug use between May 2017 and October 2017. The team used laboratory data, in addition to medical history, to define substance abuse–related admissions.
A total of 611 admissions occurred during the 6-month study period. The researchers collected information on patient demographics, hospital charges, insurance coverage, and other clinical parameters.
After analysis, they found that patients admitted for substance abuse were generally younger than were those admitted for other reasons (44 years vs. 59 years; P less than .001). In addition, patients were more often male (64% vs. 48%), had greater mortality (14%), and experienced longer hospital stays (median, 6 days).
In total, 25.7% of ICU admissions were related to substance abuse, which comprised 23.1% of charges. In particular, 9.5% and 2.9% of admissions were related to alcohol use and prescription drugs, which represented 7.6% and 4.2% of total charges, respectively.
“Polysubstance abuse was the most frequent subcategory of illicit and prescription drug admissions,” the researchers wrote.
They acknowledged two limitations of the study: the short duration and single-center design. Future studies should account for seasonal differences in ICU admissions, they noted.
“Identifying and accurately describing the landscape of this current health crisis will help us take appropriate action in the future,” they concluded.
No funding sources were reported. The authors did not disclose any conflicts of interest.
SOURCE: Westerhausen D et al. Chest. 2019 Sep 5. doi: 10.1016/j.chest.2019.08.2180.
according to results from a retrospective chart review.
The abuse of illicit drugs topped substance abuse–related ICU stays, accounting for 13% of total admissions, which represented 11% of total charges.
“We conducted a study to provide updated data on ICU utilization and costs related to licit and illicit abuse at a large county hospital,” wrote Donald Westerhausen, MD, of Indiana University, Indianapolis, and colleagues. The findings were reported in Chest .
The single-center study comprised 594 patients who were admitted for prescription, alcohol, or illicit drug use between May 2017 and October 2017. The team used laboratory data, in addition to medical history, to define substance abuse–related admissions.
A total of 611 admissions occurred during the 6-month study period. The researchers collected information on patient demographics, hospital charges, insurance coverage, and other clinical parameters.
After analysis, they found that patients admitted for substance abuse were generally younger than were those admitted for other reasons (44 years vs. 59 years; P less than .001). In addition, patients were more often male (64% vs. 48%), had greater mortality (14%), and experienced longer hospital stays (median, 6 days).
In total, 25.7% of ICU admissions were related to substance abuse, which comprised 23.1% of charges. In particular, 9.5% and 2.9% of admissions were related to alcohol use and prescription drugs, which represented 7.6% and 4.2% of total charges, respectively.
“Polysubstance abuse was the most frequent subcategory of illicit and prescription drug admissions,” the researchers wrote.
They acknowledged two limitations of the study: the short duration and single-center design. Future studies should account for seasonal differences in ICU admissions, they noted.
“Identifying and accurately describing the landscape of this current health crisis will help us take appropriate action in the future,” they concluded.
No funding sources were reported. The authors did not disclose any conflicts of interest.
SOURCE: Westerhausen D et al. Chest. 2019 Sep 5. doi: 10.1016/j.chest.2019.08.2180.
according to results from a retrospective chart review.
The abuse of illicit drugs topped substance abuse–related ICU stays, accounting for 13% of total admissions, which represented 11% of total charges.
“We conducted a study to provide updated data on ICU utilization and costs related to licit and illicit abuse at a large county hospital,” wrote Donald Westerhausen, MD, of Indiana University, Indianapolis, and colleagues. The findings were reported in Chest .
The single-center study comprised 594 patients who were admitted for prescription, alcohol, or illicit drug use between May 2017 and October 2017. The team used laboratory data, in addition to medical history, to define substance abuse–related admissions.
A total of 611 admissions occurred during the 6-month study period. The researchers collected information on patient demographics, hospital charges, insurance coverage, and other clinical parameters.
After analysis, they found that patients admitted for substance abuse were generally younger than were those admitted for other reasons (44 years vs. 59 years; P less than .001). In addition, patients were more often male (64% vs. 48%), had greater mortality (14%), and experienced longer hospital stays (median, 6 days).
In total, 25.7% of ICU admissions were related to substance abuse, which comprised 23.1% of charges. In particular, 9.5% and 2.9% of admissions were related to alcohol use and prescription drugs, which represented 7.6% and 4.2% of total charges, respectively.
“Polysubstance abuse was the most frequent subcategory of illicit and prescription drug admissions,” the researchers wrote.
They acknowledged two limitations of the study: the short duration and single-center design. Future studies should account for seasonal differences in ICU admissions, they noted.
“Identifying and accurately describing the landscape of this current health crisis will help us take appropriate action in the future,” they concluded.
No funding sources were reported. The authors did not disclose any conflicts of interest.
SOURCE: Westerhausen D et al. Chest. 2019 Sep 5. doi: 10.1016/j.chest.2019.08.2180.
FROM CHEST














