User login
Is it safe to discharge patients with anemia?
Background: Anemia is common in hospitalized patients and is associated with short- and long-term morbidity and mortality. Current evidence shows that reduced red blood cell (RBC) use and more restrictive transfusion practices do not increase short-term mortality; however, few data exist on the long-term outcomes of anemia.
Study design: Retrospective cohort study.
Setting: Integrated health care system (Kaiser Permanente) with 21 hospitals located in Northern California.
Synopsis: From 2010 to 2014, there were 801,261 hospitalizations among 445,371 patients who survived to discharge. The prevalence of moderate anemia (hemoglobin between 7 and 10 g/dL) at hospital discharge increased from 20% to 25% (P less than .001) while RBC transfusions decreased by 28% (P less than .001). Resolution of moderate anemia within 6 months of hospital discharge decreased from 42% to 34% (P less than .001). RBC transfusion and rehospitalization rates at 6 months decreased as well. During the study period, adjusted 6-month mortality decreased from 16.1% to 15.6% (P = .04) in patients with moderate anemia.
Given the retrospective design of this study, data must be interpreted with caution in determining a causal relationship. The authors also acknowledge that there may be unmeasured confounding variables not accounted for in the study results.
Bottom line: Despite higher rates of moderate anemia at discharge, there was not an associated rise in subsequent RBC transfusions, readmissions, or mortality in the 6 months after hospital discharge.
Citation: Roubinian NH et al. Long-term outcomes among patients discharged from the hospital with moderate anemia: A retrospective cohort study. Ann Intern Med. 2019 Jan 14. doi: 10.7326/M17-3253.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Background: Anemia is common in hospitalized patients and is associated with short- and long-term morbidity and mortality. Current evidence shows that reduced red blood cell (RBC) use and more restrictive transfusion practices do not increase short-term mortality; however, few data exist on the long-term outcomes of anemia.
Study design: Retrospective cohort study.
Setting: Integrated health care system (Kaiser Permanente) with 21 hospitals located in Northern California.
Synopsis: From 2010 to 2014, there were 801,261 hospitalizations among 445,371 patients who survived to discharge. The prevalence of moderate anemia (hemoglobin between 7 and 10 g/dL) at hospital discharge increased from 20% to 25% (P less than .001) while RBC transfusions decreased by 28% (P less than .001). Resolution of moderate anemia within 6 months of hospital discharge decreased from 42% to 34% (P less than .001). RBC transfusion and rehospitalization rates at 6 months decreased as well. During the study period, adjusted 6-month mortality decreased from 16.1% to 15.6% (P = .04) in patients with moderate anemia.
Given the retrospective design of this study, data must be interpreted with caution in determining a causal relationship. The authors also acknowledge that there may be unmeasured confounding variables not accounted for in the study results.
Bottom line: Despite higher rates of moderate anemia at discharge, there was not an associated rise in subsequent RBC transfusions, readmissions, or mortality in the 6 months after hospital discharge.
Citation: Roubinian NH et al. Long-term outcomes among patients discharged from the hospital with moderate anemia: A retrospective cohort study. Ann Intern Med. 2019 Jan 14. doi: 10.7326/M17-3253.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Background: Anemia is common in hospitalized patients and is associated with short- and long-term morbidity and mortality. Current evidence shows that reduced red blood cell (RBC) use and more restrictive transfusion practices do not increase short-term mortality; however, few data exist on the long-term outcomes of anemia.
Study design: Retrospective cohort study.
Setting: Integrated health care system (Kaiser Permanente) with 21 hospitals located in Northern California.
Synopsis: From 2010 to 2014, there were 801,261 hospitalizations among 445,371 patients who survived to discharge. The prevalence of moderate anemia (hemoglobin between 7 and 10 g/dL) at hospital discharge increased from 20% to 25% (P less than .001) while RBC transfusions decreased by 28% (P less than .001). Resolution of moderate anemia within 6 months of hospital discharge decreased from 42% to 34% (P less than .001). RBC transfusion and rehospitalization rates at 6 months decreased as well. During the study period, adjusted 6-month mortality decreased from 16.1% to 15.6% (P = .04) in patients with moderate anemia.
Given the retrospective design of this study, data must be interpreted with caution in determining a causal relationship. The authors also acknowledge that there may be unmeasured confounding variables not accounted for in the study results.
Bottom line: Despite higher rates of moderate anemia at discharge, there was not an associated rise in subsequent RBC transfusions, readmissions, or mortality in the 6 months after hospital discharge.
Citation: Roubinian NH et al. Long-term outcomes among patients discharged from the hospital with moderate anemia: A retrospective cohort study. Ann Intern Med. 2019 Jan 14. doi: 10.7326/M17-3253.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Beta-blockers effective, safe for HFrEF with renal dysfunction
PARIS – Beta-blocking drugs were as effective for improving survival in patients with moderately severe renal dysfunction as they were in patients with normal renal function in a meta-analysis of more than 13,000 patients, a finding that seemed to solidify the role for this drug class for essentially all similar heart failure patients, regardless of their renal function.
This evidence could reshape usual care because “renal impairment is often considered a barrier in clinical practice” for starting a beta-blocker drug in patients with heart failure with reduced ejection fraction (HFrEF), Dipak Kotecha, MBChB, said at the annual congress of the European Society of Cardiology.
“We have shown with sufficient sample size that beta-blockers are effective in reducing mortality in patient with HFrEF and in sinus rhythm, even in those with an eGFR [estimated glomerular filtration rate] of 30-44 mL/min per 1.73 m2,” said Dr. Kotecha, a cardiologist at the University of Birmingham (England). “The results suggest that renal impairment should not obstruct the prescription and maintenance of beta-blockers in patients with HFrEF.”
“This important study was a novel attempt to look at [HFrEF] patients with renal insufficiency to see whether they received the same benefit from beta-blockers as other patients, and they did. So renal insufficiency is not a reason to withhold beta-blockers” from these patients, commented Mariell Jessup, MD, a heart failure physician and chief science and medical officer for the American Heart Association in Dallas. “The onus is on clinicians to find a reason not to give a beta-blocker to a patient with HFrEF because they are generally well tolerated and they can have enormous benefit, as we saw in this study,” she said in a video interview.
The analysis run by Dr. Kotecha and associates used data collected in 11 of the pivotal randomized, controlled trial run for beta-blockers during the 1990s and early 2000s, with each study comparing bucindolol, bisoprolol, carvedilol, metoprolol XL, or nebivolol against placebo. The studies collectively enrolled 18,637 patients, which the investigators whittled down in their analysis to 17,433 after excluding patients with a left ventricular ejection fraction below 50% or who were undocumented. The subgroup with HFrEF included 13,861 patient in sinus rhythm at entry, 2,879 with atrial fibrillation, and 693 with an unknown atrial status. The main analysis ran in the 13,861 patients with HFrEF and in sinus rhythm; 14% of this cohort had an eGFR of 30-44 mL/min per 1.73 m2 and 27% had an eGFR of 45-59 mL/min per 1.73 m2. The median age of all patients in the main analysis was 65 years, 23% were women, and their median left ventricular ejection fraction was 27%.
During follow-up of about 3 years, the impact of beta-blocker treatment on survival, compared with placebo, was “substantial” for all strata of patients by renal function, except for those with eGFRs below 30 mL/min per 1.73 m2. (Survival was similar regardless of beta-blocker treatment in the small number of patients with severe renal dysfunction.) The number needed to treat to prevent 1 death in patients with an eGFR of 30-44 mL/min per 1.73 m2 was 21, the same as among patients with an eGFR of 90 mL/min per 1.73 m2 or more, Dr. Kotecha said.
Among the subgroup of patients with atrial fibrillation, beta-blockers appeared to exert no survival benefit, compared with placebo. The investigators did not assess the survival benefits exerted by any individual beta-blocker, compared with the others, and Dr. Kotecha stressed that “my belief is that this is a class effect” and is roughly similar across all the beta-blockers used in the studies.
The analysis also showed good safety and tolerability of the beta-blockers in patients with renal dysfunction. The incidence of adverse events leading to treatment termination was very similar in the beta-blocker and placebo arms, and more than three-quarters of patients in each of the two subgroups with renal dysfunction were maintained on more than 50% of their target beta-blocker dosage.
Dr. Kotecha has been an advisor to Bayer, a speaker on behalf of Atricure, and has received research funding from GlaxoSmithKline and Menarini. Dr. Jessup had no disclosures.
This analysis of individual patient data is very important and extends our knowledge. The results confirm that beta-blocker treatment reduces mortality in patients with heart failure with reduced ejection fraction (HFrEF) and in sinus rhythm who also have moderately severe renal dysfunction with an estimated glomerular filtration rate as low as 30-44 mL/min per 1.73 m2. This is good news for patients with HFrEF and kidney disease. Clinicians often use comorbidities as a reason not to prescribe or up-titrate beta-blockers. These results show that beta-blockers can be used at guideline-directed dosages, even in patients with renal dysfunction. The findings highlight the importance of not looking for excuses to not treat patients with a beta-blocker. Do not worry about renal function.
Theresa A. McDonagh, MD, professor of cardiology at King’s College, London, made these comments as designated discussant for Dr. Kotecha’s report. She had no disclosures.
This analysis of individual patient data is very important and extends our knowledge. The results confirm that beta-blocker treatment reduces mortality in patients with heart failure with reduced ejection fraction (HFrEF) and in sinus rhythm who also have moderately severe renal dysfunction with an estimated glomerular filtration rate as low as 30-44 mL/min per 1.73 m2. This is good news for patients with HFrEF and kidney disease. Clinicians often use comorbidities as a reason not to prescribe or up-titrate beta-blockers. These results show that beta-blockers can be used at guideline-directed dosages, even in patients with renal dysfunction. The findings highlight the importance of not looking for excuses to not treat patients with a beta-blocker. Do not worry about renal function.
Theresa A. McDonagh, MD, professor of cardiology at King’s College, London, made these comments as designated discussant for Dr. Kotecha’s report. She had no disclosures.
This analysis of individual patient data is very important and extends our knowledge. The results confirm that beta-blocker treatment reduces mortality in patients with heart failure with reduced ejection fraction (HFrEF) and in sinus rhythm who also have moderately severe renal dysfunction with an estimated glomerular filtration rate as low as 30-44 mL/min per 1.73 m2. This is good news for patients with HFrEF and kidney disease. Clinicians often use comorbidities as a reason not to prescribe or up-titrate beta-blockers. These results show that beta-blockers can be used at guideline-directed dosages, even in patients with renal dysfunction. The findings highlight the importance of not looking for excuses to not treat patients with a beta-blocker. Do not worry about renal function.
Theresa A. McDonagh, MD, professor of cardiology at King’s College, London, made these comments as designated discussant for Dr. Kotecha’s report. She had no disclosures.
PARIS – Beta-blocking drugs were as effective for improving survival in patients with moderately severe renal dysfunction as they were in patients with normal renal function in a meta-analysis of more than 13,000 patients, a finding that seemed to solidify the role for this drug class for essentially all similar heart failure patients, regardless of their renal function.
This evidence could reshape usual care because “renal impairment is often considered a barrier in clinical practice” for starting a beta-blocker drug in patients with heart failure with reduced ejection fraction (HFrEF), Dipak Kotecha, MBChB, said at the annual congress of the European Society of Cardiology.
“We have shown with sufficient sample size that beta-blockers are effective in reducing mortality in patient with HFrEF and in sinus rhythm, even in those with an eGFR [estimated glomerular filtration rate] of 30-44 mL/min per 1.73 m2,” said Dr. Kotecha, a cardiologist at the University of Birmingham (England). “The results suggest that renal impairment should not obstruct the prescription and maintenance of beta-blockers in patients with HFrEF.”
“This important study was a novel attempt to look at [HFrEF] patients with renal insufficiency to see whether they received the same benefit from beta-blockers as other patients, and they did. So renal insufficiency is not a reason to withhold beta-blockers” from these patients, commented Mariell Jessup, MD, a heart failure physician and chief science and medical officer for the American Heart Association in Dallas. “The onus is on clinicians to find a reason not to give a beta-blocker to a patient with HFrEF because they are generally well tolerated and they can have enormous benefit, as we saw in this study,” she said in a video interview.
The analysis run by Dr. Kotecha and associates used data collected in 11 of the pivotal randomized, controlled trial run for beta-blockers during the 1990s and early 2000s, with each study comparing bucindolol, bisoprolol, carvedilol, metoprolol XL, or nebivolol against placebo. The studies collectively enrolled 18,637 patients, which the investigators whittled down in their analysis to 17,433 after excluding patients with a left ventricular ejection fraction below 50% or who were undocumented. The subgroup with HFrEF included 13,861 patient in sinus rhythm at entry, 2,879 with atrial fibrillation, and 693 with an unknown atrial status. The main analysis ran in the 13,861 patients with HFrEF and in sinus rhythm; 14% of this cohort had an eGFR of 30-44 mL/min per 1.73 m2 and 27% had an eGFR of 45-59 mL/min per 1.73 m2. The median age of all patients in the main analysis was 65 years, 23% were women, and their median left ventricular ejection fraction was 27%.
During follow-up of about 3 years, the impact of beta-blocker treatment on survival, compared with placebo, was “substantial” for all strata of patients by renal function, except for those with eGFRs below 30 mL/min per 1.73 m2. (Survival was similar regardless of beta-blocker treatment in the small number of patients with severe renal dysfunction.) The number needed to treat to prevent 1 death in patients with an eGFR of 30-44 mL/min per 1.73 m2 was 21, the same as among patients with an eGFR of 90 mL/min per 1.73 m2 or more, Dr. Kotecha said.
Among the subgroup of patients with atrial fibrillation, beta-blockers appeared to exert no survival benefit, compared with placebo. The investigators did not assess the survival benefits exerted by any individual beta-blocker, compared with the others, and Dr. Kotecha stressed that “my belief is that this is a class effect” and is roughly similar across all the beta-blockers used in the studies.
The analysis also showed good safety and tolerability of the beta-blockers in patients with renal dysfunction. The incidence of adverse events leading to treatment termination was very similar in the beta-blocker and placebo arms, and more than three-quarters of patients in each of the two subgroups with renal dysfunction were maintained on more than 50% of their target beta-blocker dosage.
Dr. Kotecha has been an advisor to Bayer, a speaker on behalf of Atricure, and has received research funding from GlaxoSmithKline and Menarini. Dr. Jessup had no disclosures.
PARIS – Beta-blocking drugs were as effective for improving survival in patients with moderately severe renal dysfunction as they were in patients with normal renal function in a meta-analysis of more than 13,000 patients, a finding that seemed to solidify the role for this drug class for essentially all similar heart failure patients, regardless of their renal function.
This evidence could reshape usual care because “renal impairment is often considered a barrier in clinical practice” for starting a beta-blocker drug in patients with heart failure with reduced ejection fraction (HFrEF), Dipak Kotecha, MBChB, said at the annual congress of the European Society of Cardiology.
“We have shown with sufficient sample size that beta-blockers are effective in reducing mortality in patient with HFrEF and in sinus rhythm, even in those with an eGFR [estimated glomerular filtration rate] of 30-44 mL/min per 1.73 m2,” said Dr. Kotecha, a cardiologist at the University of Birmingham (England). “The results suggest that renal impairment should not obstruct the prescription and maintenance of beta-blockers in patients with HFrEF.”
“This important study was a novel attempt to look at [HFrEF] patients with renal insufficiency to see whether they received the same benefit from beta-blockers as other patients, and they did. So renal insufficiency is not a reason to withhold beta-blockers” from these patients, commented Mariell Jessup, MD, a heart failure physician and chief science and medical officer for the American Heart Association in Dallas. “The onus is on clinicians to find a reason not to give a beta-blocker to a patient with HFrEF because they are generally well tolerated and they can have enormous benefit, as we saw in this study,” she said in a video interview.
The analysis run by Dr. Kotecha and associates used data collected in 11 of the pivotal randomized, controlled trial run for beta-blockers during the 1990s and early 2000s, with each study comparing bucindolol, bisoprolol, carvedilol, metoprolol XL, or nebivolol against placebo. The studies collectively enrolled 18,637 patients, which the investigators whittled down in their analysis to 17,433 after excluding patients with a left ventricular ejection fraction below 50% or who were undocumented. The subgroup with HFrEF included 13,861 patient in sinus rhythm at entry, 2,879 with atrial fibrillation, and 693 with an unknown atrial status. The main analysis ran in the 13,861 patients with HFrEF and in sinus rhythm; 14% of this cohort had an eGFR of 30-44 mL/min per 1.73 m2 and 27% had an eGFR of 45-59 mL/min per 1.73 m2. The median age of all patients in the main analysis was 65 years, 23% were women, and their median left ventricular ejection fraction was 27%.
During follow-up of about 3 years, the impact of beta-blocker treatment on survival, compared with placebo, was “substantial” for all strata of patients by renal function, except for those with eGFRs below 30 mL/min per 1.73 m2. (Survival was similar regardless of beta-blocker treatment in the small number of patients with severe renal dysfunction.) The number needed to treat to prevent 1 death in patients with an eGFR of 30-44 mL/min per 1.73 m2 was 21, the same as among patients with an eGFR of 90 mL/min per 1.73 m2 or more, Dr. Kotecha said.
Among the subgroup of patients with atrial fibrillation, beta-blockers appeared to exert no survival benefit, compared with placebo. The investigators did not assess the survival benefits exerted by any individual beta-blocker, compared with the others, and Dr. Kotecha stressed that “my belief is that this is a class effect” and is roughly similar across all the beta-blockers used in the studies.
The analysis also showed good safety and tolerability of the beta-blockers in patients with renal dysfunction. The incidence of adverse events leading to treatment termination was very similar in the beta-blocker and placebo arms, and more than three-quarters of patients in each of the two subgroups with renal dysfunction were maintained on more than 50% of their target beta-blocker dosage.
Dr. Kotecha has been an advisor to Bayer, a speaker on behalf of Atricure, and has received research funding from GlaxoSmithKline and Menarini. Dr. Jessup had no disclosures.
REPORTING FROM THE ESC CONGRESS 2019
Women’s residency and subspecialty choices diverging
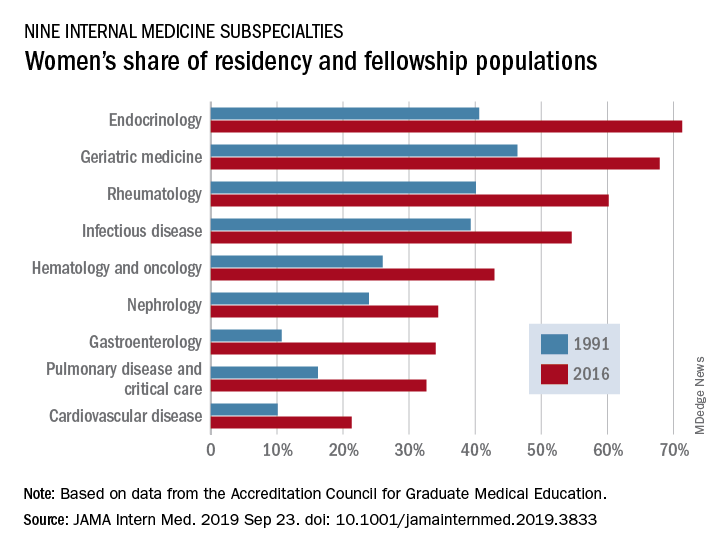
Women made up 43.2% of the internal medicine resident population in 2016, compared with 30.2% in 1991. Over that same time, however, the percentage of women in subspecialty fellowships dropped from 33.3% to 23.6%, Anna T. Stone, MD, and associates wrote in a research letter published in JAMA Internal Medicine.
“Many factors are associated with the decisions of medical students in choosing an internal medicine residency, including their sex, educational experience, views of patient care, and lifestyle perceptions. Similar considerations apply to subspecialty training,” wrote Dr. Stone of the department of cardiology at St. Vincent Hospital and Heart Center, Indianapolis, and associates.
When the investigators focused on a subset of nine internal medicine subspecialties, they saw growth: “The percentage of women entering each of the fields [residents plus fellows] increased over time, with variations between specialty and some year-to-year variations within a specialty.”
Although none of the nine subspecialties had been majority women in 1991, by 2016 women made up more than half of the residents and fellows in four: endocrinology (71.3%), geriatric medicine (67.9%), rheumatology (60.2%), and infectious disease (54.6%), according to data from the Accreditation Council for Graduate Medical Education.
And then there’s cardiology. Its low rate of participation among women – the only one of the nine subspecialties under 35% – “is an important issue that the cardiology profession should continue to address,” they wrote.
In a survey of internal medicine residents conducted by other researchers, women were more likely than men to report that they had never considered cardiology as a career choice, Dr. Stone and associates noted, and women in the survey “had different perceptions of cardiology than men.”
SOURCE: Stone AT et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3833.

Women made up 43.2% of the internal medicine resident population in 2016, compared with 30.2% in 1991. Over that same time, however, the percentage of women in subspecialty fellowships dropped from 33.3% to 23.6%, Anna T. Stone, MD, and associates wrote in a research letter published in JAMA Internal Medicine.
“Many factors are associated with the decisions of medical students in choosing an internal medicine residency, including their sex, educational experience, views of patient care, and lifestyle perceptions. Similar considerations apply to subspecialty training,” wrote Dr. Stone of the department of cardiology at St. Vincent Hospital and Heart Center, Indianapolis, and associates.
When the investigators focused on a subset of nine internal medicine subspecialties, they saw growth: “The percentage of women entering each of the fields [residents plus fellows] increased over time, with variations between specialty and some year-to-year variations within a specialty.”
Although none of the nine subspecialties had been majority women in 1991, by 2016 women made up more than half of the residents and fellows in four: endocrinology (71.3%), geriatric medicine (67.9%), rheumatology (60.2%), and infectious disease (54.6%), according to data from the Accreditation Council for Graduate Medical Education.
And then there’s cardiology. Its low rate of participation among women – the only one of the nine subspecialties under 35% – “is an important issue that the cardiology profession should continue to address,” they wrote.
In a survey of internal medicine residents conducted by other researchers, women were more likely than men to report that they had never considered cardiology as a career choice, Dr. Stone and associates noted, and women in the survey “had different perceptions of cardiology than men.”
SOURCE: Stone AT et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3833.

Women made up 43.2% of the internal medicine resident population in 2016, compared with 30.2% in 1991. Over that same time, however, the percentage of women in subspecialty fellowships dropped from 33.3% to 23.6%, Anna T. Stone, MD, and associates wrote in a research letter published in JAMA Internal Medicine.
“Many factors are associated with the decisions of medical students in choosing an internal medicine residency, including their sex, educational experience, views of patient care, and lifestyle perceptions. Similar considerations apply to subspecialty training,” wrote Dr. Stone of the department of cardiology at St. Vincent Hospital and Heart Center, Indianapolis, and associates.
When the investigators focused on a subset of nine internal medicine subspecialties, they saw growth: “The percentage of women entering each of the fields [residents plus fellows] increased over time, with variations between specialty and some year-to-year variations within a specialty.”
Although none of the nine subspecialties had been majority women in 1991, by 2016 women made up more than half of the residents and fellows in four: endocrinology (71.3%), geriatric medicine (67.9%), rheumatology (60.2%), and infectious disease (54.6%), according to data from the Accreditation Council for Graduate Medical Education.
And then there’s cardiology. Its low rate of participation among women – the only one of the nine subspecialties under 35% – “is an important issue that the cardiology profession should continue to address,” they wrote.
In a survey of internal medicine residents conducted by other researchers, women were more likely than men to report that they had never considered cardiology as a career choice, Dr. Stone and associates noted, and women in the survey “had different perceptions of cardiology than men.”
SOURCE: Stone AT et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3833.
FROM JAMA INTERNAL MEDICINE
Machine learning–derived risk score predicts heart failure risk in diabetes patients
PHILADELPHIA – For patients with high-risk diabetes, a novel, machine learning–derived risk score based on 10 common clinical variables can identify those facing a heart failure risk of up to nearly 20% over the ensuing 5 years, an investigator said at the annual meeting of the Heart Failure Society of America.
The risk score, dubbed WATCH-DM, has greater accuracy in predicting incident heart failure than traditional risk-based models, and requires no specific cardiovascular biomarkers or imaging, according to Muthiah Vaduganathan, MD, MPH, a cardiologist at Brigham and Women’s Hospital and faculty at Harvard Medical School in Boston.
The tool may help inform risk-based monitoring and introduction of sodium-glucose transporter 2 (SGLT2) inhibitors, which have been shown in multiple clinical trials to prevent heart failure in at-risk patients with type 2 diabetes mellitus (T2DM), Dr. Vaduganathan said.
“Patients identified at high risk based on WATCH-DM should be strongly considered for initiation of SGLT2 inhibitors in clinical practice,” Dr. Vaduganathan said in an interview.
WATCH-DM is available online at cvriskscores.com. Work is underway to integrate the tool into electronic health record systems at Brigham and Women’s Hospital and at the University of Texas Southwestern Medical Center in Dallas. “I expect that to be launched in the next year,” he said.
The WATCH-DM score was developed based on data from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial, including 8,756 T2DM patients with inadequate glycemic control at high cardiovascular risk and no heart failure at baseline.
Starting with 147 variables, the investigators used a decision-tree machine learning approach to identify predictors of heart failure.
“What machine learning does is automate the variable selection process, as a form of artificial intelligence,” Dr. Vaduganathan said.
The WATCH-DM risk score was based on the 10 best-performing predictors as selected by machine learning, including body mass index, age, systolic blood pressure, diastolic blood pressure, fasting plasma glucose, serum creatinine, high-density lipoprotein cholesterol, QRS duration, prior myocardial infarction, and prior coronary artery bypass grafting.
The 5-year risk of heart failure was just 1.1% for patients with WATCH-DM scores in the lowest quintile, increasing in a graded fashion to nearly 20% (17.4%) in the highest quintile, study results show.
Findings of the study were simultaneously published in the journal Diabetes Care.
Dr. Vaduganathan said he is supported by an award from Harvard Catalyst. He provided disclosures related to Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim (advisory boards), and with Novartis and the National Institutes of Health (participation on clinical endpoint committees).
SOURCE: HFSA 2019; Segar MW, Vaduganathan M et al. Diabetes Care. doi: 10.2337/dc19-0587.
PHILADELPHIA – For patients with high-risk diabetes, a novel, machine learning–derived risk score based on 10 common clinical variables can identify those facing a heart failure risk of up to nearly 20% over the ensuing 5 years, an investigator said at the annual meeting of the Heart Failure Society of America.
The risk score, dubbed WATCH-DM, has greater accuracy in predicting incident heart failure than traditional risk-based models, and requires no specific cardiovascular biomarkers or imaging, according to Muthiah Vaduganathan, MD, MPH, a cardiologist at Brigham and Women’s Hospital and faculty at Harvard Medical School in Boston.
The tool may help inform risk-based monitoring and introduction of sodium-glucose transporter 2 (SGLT2) inhibitors, which have been shown in multiple clinical trials to prevent heart failure in at-risk patients with type 2 diabetes mellitus (T2DM), Dr. Vaduganathan said.
“Patients identified at high risk based on WATCH-DM should be strongly considered for initiation of SGLT2 inhibitors in clinical practice,” Dr. Vaduganathan said in an interview.
WATCH-DM is available online at cvriskscores.com. Work is underway to integrate the tool into electronic health record systems at Brigham and Women’s Hospital and at the University of Texas Southwestern Medical Center in Dallas. “I expect that to be launched in the next year,” he said.
The WATCH-DM score was developed based on data from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial, including 8,756 T2DM patients with inadequate glycemic control at high cardiovascular risk and no heart failure at baseline.
Starting with 147 variables, the investigators used a decision-tree machine learning approach to identify predictors of heart failure.
“What machine learning does is automate the variable selection process, as a form of artificial intelligence,” Dr. Vaduganathan said.
The WATCH-DM risk score was based on the 10 best-performing predictors as selected by machine learning, including body mass index, age, systolic blood pressure, diastolic blood pressure, fasting plasma glucose, serum creatinine, high-density lipoprotein cholesterol, QRS duration, prior myocardial infarction, and prior coronary artery bypass grafting.
The 5-year risk of heart failure was just 1.1% for patients with WATCH-DM scores in the lowest quintile, increasing in a graded fashion to nearly 20% (17.4%) in the highest quintile, study results show.
Findings of the study were simultaneously published in the journal Diabetes Care.
Dr. Vaduganathan said he is supported by an award from Harvard Catalyst. He provided disclosures related to Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim (advisory boards), and with Novartis and the National Institutes of Health (participation on clinical endpoint committees).
SOURCE: HFSA 2019; Segar MW, Vaduganathan M et al. Diabetes Care. doi: 10.2337/dc19-0587.
PHILADELPHIA – For patients with high-risk diabetes, a novel, machine learning–derived risk score based on 10 common clinical variables can identify those facing a heart failure risk of up to nearly 20% over the ensuing 5 years, an investigator said at the annual meeting of the Heart Failure Society of America.
The risk score, dubbed WATCH-DM, has greater accuracy in predicting incident heart failure than traditional risk-based models, and requires no specific cardiovascular biomarkers or imaging, according to Muthiah Vaduganathan, MD, MPH, a cardiologist at Brigham and Women’s Hospital and faculty at Harvard Medical School in Boston.
The tool may help inform risk-based monitoring and introduction of sodium-glucose transporter 2 (SGLT2) inhibitors, which have been shown in multiple clinical trials to prevent heart failure in at-risk patients with type 2 diabetes mellitus (T2DM), Dr. Vaduganathan said.
“Patients identified at high risk based on WATCH-DM should be strongly considered for initiation of SGLT2 inhibitors in clinical practice,” Dr. Vaduganathan said in an interview.
WATCH-DM is available online at cvriskscores.com. Work is underway to integrate the tool into electronic health record systems at Brigham and Women’s Hospital and at the University of Texas Southwestern Medical Center in Dallas. “I expect that to be launched in the next year,” he said.
The WATCH-DM score was developed based on data from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial, including 8,756 T2DM patients with inadequate glycemic control at high cardiovascular risk and no heart failure at baseline.
Starting with 147 variables, the investigators used a decision-tree machine learning approach to identify predictors of heart failure.
“What machine learning does is automate the variable selection process, as a form of artificial intelligence,” Dr. Vaduganathan said.
The WATCH-DM risk score was based on the 10 best-performing predictors as selected by machine learning, including body mass index, age, systolic blood pressure, diastolic blood pressure, fasting plasma glucose, serum creatinine, high-density lipoprotein cholesterol, QRS duration, prior myocardial infarction, and prior coronary artery bypass grafting.
The 5-year risk of heart failure was just 1.1% for patients with WATCH-DM scores in the lowest quintile, increasing in a graded fashion to nearly 20% (17.4%) in the highest quintile, study results show.
Findings of the study were simultaneously published in the journal Diabetes Care.
Dr. Vaduganathan said he is supported by an award from Harvard Catalyst. He provided disclosures related to Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim (advisory boards), and with Novartis and the National Institutes of Health (participation on clinical endpoint committees).
SOURCE: HFSA 2019; Segar MW, Vaduganathan M et al. Diabetes Care. doi: 10.2337/dc19-0587.
REPORTING FROM HFSA 2019
Reversal agents for direct-acting oral anticoagulants
Summary of guidelines published in the Journal of Hospital Medicine
When on call for admissions, a hospitalist receives a request from a colleague to admit an octogenarian man with an acute uncomplicated deep vein thrombosis to start heparin, bridging to warfarin. The patient has no evidence of postphlebitic syndrome, pulmonary embolism, or right-sided heart strain. The hospitalist asks her colleague if he had considered treating the patient in the ambulatory setting using a direct-acting oral anticoagulant (DOAC). After all, this would save the patient an unnecessary hospitalization, weekly international normalized ratio checks, and other important lifestyle changes. In response, the colleague voices concern that the “new drugs don’t have antidotes.”
DOACs have several benefits over vitamin K antagonists (VKAs) and heparins. DOACs have quicker onset of action, can be taken by mouth, in general do not require dosage adjustment, and have fewer dietary and lifestyle modifications, compared with VKAs and heparins. In atrial fibrillation, DOACs have been shown to have lower all-cause and bleeding-related mortality than warfarin (see Table 1).1 Observational studies also suggest less risk of major bleeding with DOACs over warfarin but no difference in overall mortality when used to treat venous thromboembolism (see Table 2).2 Because of these combined advantages, DOACs are increasingly prescribed, accounting for approximately half of all oral anticoagulant prescriptions in 2014.3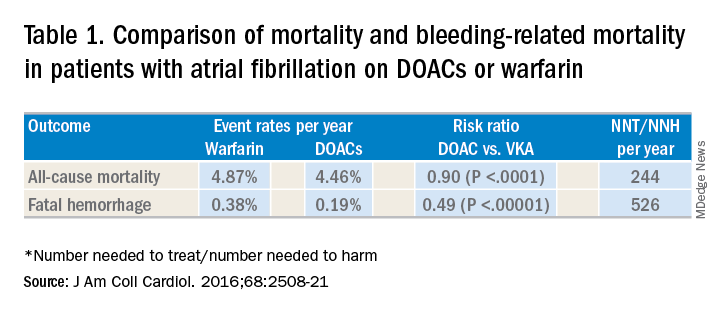
Although DOACs have been shown to be as good if not superior to VKAs and heparins in these circumstances, there are situations where a DOAC should not be used. There is limited data on the safety of DOACs in patients with mechanical heart valves, liver failure, and chronic kidney disease with a creatinine clearance less than 30 mL/min.4 Therefore, warfarin is still the preferred agent in these settings. There is some data that apixaban may be safe in patients with a creatinine clearance of greater than 10 mL/min, but long-term safety studies have not been performed in patients with end-stage renal disease on hemodialysis.5 Finally, in patients requiring concomitant inducers or inhibitors of the P-glycoprotein or cytochrome P450 enzymes like antiepileptics and protease inhibitors, VKAs and heparins are favored.4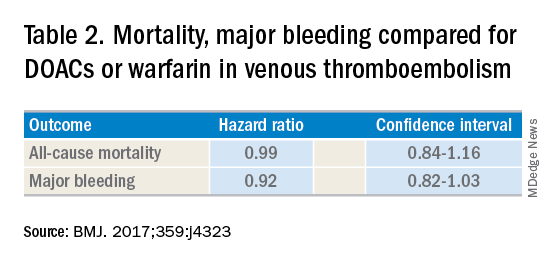
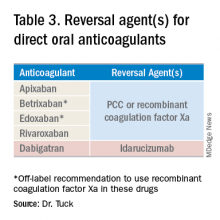
Notwithstanding their advantages, when DOACs first hit the market there were concerns that reversal agents were not available. In the August issue of the Journal of Hospital Medicine’s Clinical Guideline Highlights for the Hospitalist, Emily Gottenborg, MD, and Gregory Misky, MD, summarized guideline recommendations for reversal of the newer agents.6 This includes use of idarucizumab for patients on dabigatran and use of prothrombin complex concentrate (PCC) or recombinant coagulation factor Xa (andexanet alfa) for patients on apixaban or rivaroxaban for the treatment of life-threatening bleeding.
Idarucizumab is a monoclonal antibody developed to reverse the effects of dabigatran, the only DOAC that directly inhibits thrombin. In 2017, researchers reported on a cohort of subjects receiving idarucizumab for uncontrolled bleeding or who were on dabigatran and about to undergo an urgent procedure.7 Of those with uncontrolled bleeding, two-thirds had confirmed bleeding cessation within 24 hours. Periprocedural hemostasis was achieved in 93.4% of patients undergoing urgent procedures. However, it should be noted that use of idarucizumab conferred an increase risk (6.3%) of thrombosis within 90 days. Based on these findings, guidelines recommend use of idarucizumab in patients experiencing life-threatening bleeding, balanced against the risk of thrombosis.8
In 2018, the Food and Drug Administration approved recombinant coagulation factor Xa for treatment of life-threatening or uncontrolled bleeding in patients on apixaban or rivaroxaban.9 The approval came after a study by the ANNEXA-4 investigators showed that recombinant coagulation factor Xa quickly and effectively achieved hemostasis.10 Full study results were published in April 2019, demonstrating 82% of patients receiving the drug attained clinical hemostasis.11 However, as with idarucizumab, up to 10% of patients had a thrombotic event in the follow-up period. Use of recombinant coagulation factor Xa for treatment of life-threatening bleeding related to betrixaban and edoxaban is considered off label but is recommended by guidelines.8 Studies on investigational reversal agents for betrixaban and edoxaban are ongoing.
Both unactivated and activated PCC contain clotting factor X. Their use to control bleeding related to DOAC use is based on observational studies. In a systematic review of the nonrandomized studies, the efficacy of PCC to stem major bleeding was 69% and the risk for thromboembolism was 4%.12 There are no head-to-head studies comparing use of recombinant coagulation factor Xa and PCC. Therefore, guidelines are to use either recombinant factor Xa or PCC for the treatment of life-threatening bleeding related to DOAC use.7
As thrombosis risk heightens after use of any reversal agent, the recommendations are to resume anticoagulation within 90 days if the patient is at moderate or high risk for recurrent thromboembolism.8
After discussion with the hospitalist about the new agents available to reverse anticoagulation, the colleague decided to place the patient on a DOAC and keep the patient in his nursing home. Thankfully, the patient did not thereafter experience sustained bleeding necessitating use of these reversal agents. More importantly for the patient, he was able to stay in the comfort of his home.
Dr. Tuck is associate section chief for hospital medicine at the Veterans Affairs Medical Center in Washington, D.C.
References
1. Gómez-Outes A et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508-21.
2. Jun M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population-based, observational study. BMJ. 2017;359:j4323.
3. Barnes GD et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:(1300-5).e2.
4. Reddy P et al. Practical approach to VTE management in hospitalized patients. Am J Ther. 2017;24(4):e442-67.
5. Kimachi M et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017 Nov 6;11:CD011373.
6. Gottenborg E et al. Clinical guideline highlights for the hospitalist: The management of anticoagulation in the hospitalized adult. J Hosp Med. 2019; 14(8):499-500.
7. Pollack CV Jr et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377(5):431-41.
8. Witt DM et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-91.
9. Malarky M et al. FDA accelerated approval letter. Retrieved July 15, 2019. https://www.fda.gov/media/113285/download
10. Connolly SJ et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-41.
11. Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
12. Piran S et al. Management of direct factor Xa inhibitor–related major bleeding with prothrombin complex concentrate: A meta-analysis. Blood Adv. 2019;3(2):158-67.
Summary of guidelines published in the Journal of Hospital Medicine
Summary of guidelines published in the Journal of Hospital Medicine
When on call for admissions, a hospitalist receives a request from a colleague to admit an octogenarian man with an acute uncomplicated deep vein thrombosis to start heparin, bridging to warfarin. The patient has no evidence of postphlebitic syndrome, pulmonary embolism, or right-sided heart strain. The hospitalist asks her colleague if he had considered treating the patient in the ambulatory setting using a direct-acting oral anticoagulant (DOAC). After all, this would save the patient an unnecessary hospitalization, weekly international normalized ratio checks, and other important lifestyle changes. In response, the colleague voices concern that the “new drugs don’t have antidotes.”
DOACs have several benefits over vitamin K antagonists (VKAs) and heparins. DOACs have quicker onset of action, can be taken by mouth, in general do not require dosage adjustment, and have fewer dietary and lifestyle modifications, compared with VKAs and heparins. In atrial fibrillation, DOACs have been shown to have lower all-cause and bleeding-related mortality than warfarin (see Table 1).1 Observational studies also suggest less risk of major bleeding with DOACs over warfarin but no difference in overall mortality when used to treat venous thromboembolism (see Table 2).2 Because of these combined advantages, DOACs are increasingly prescribed, accounting for approximately half of all oral anticoagulant prescriptions in 2014.3
Although DOACs have been shown to be as good if not superior to VKAs and heparins in these circumstances, there are situations where a DOAC should not be used. There is limited data on the safety of DOACs in patients with mechanical heart valves, liver failure, and chronic kidney disease with a creatinine clearance less than 30 mL/min.4 Therefore, warfarin is still the preferred agent in these settings. There is some data that apixaban may be safe in patients with a creatinine clearance of greater than 10 mL/min, but long-term safety studies have not been performed in patients with end-stage renal disease on hemodialysis.5 Finally, in patients requiring concomitant inducers or inhibitors of the P-glycoprotein or cytochrome P450 enzymes like antiepileptics and protease inhibitors, VKAs and heparins are favored.4
Notwithstanding their advantages, when DOACs first hit the market there were concerns that reversal agents were not available. In the August issue of the Journal of Hospital Medicine’s Clinical Guideline Highlights for the Hospitalist, Emily Gottenborg, MD, and Gregory Misky, MD, summarized guideline recommendations for reversal of the newer agents.6 This includes use of idarucizumab for patients on dabigatran and use of prothrombin complex concentrate (PCC) or recombinant coagulation factor Xa (andexanet alfa) for patients on apixaban or rivaroxaban for the treatment of life-threatening bleeding.
Idarucizumab is a monoclonal antibody developed to reverse the effects of dabigatran, the only DOAC that directly inhibits thrombin. In 2017, researchers reported on a cohort of subjects receiving idarucizumab for uncontrolled bleeding or who were on dabigatran and about to undergo an urgent procedure.7 Of those with uncontrolled bleeding, two-thirds had confirmed bleeding cessation within 24 hours. Periprocedural hemostasis was achieved in 93.4% of patients undergoing urgent procedures. However, it should be noted that use of idarucizumab conferred an increase risk (6.3%) of thrombosis within 90 days. Based on these findings, guidelines recommend use of idarucizumab in patients experiencing life-threatening bleeding, balanced against the risk of thrombosis.8
In 2018, the Food and Drug Administration approved recombinant coagulation factor Xa for treatment of life-threatening or uncontrolled bleeding in patients on apixaban or rivaroxaban.9 The approval came after a study by the ANNEXA-4 investigators showed that recombinant coagulation factor Xa quickly and effectively achieved hemostasis.10 Full study results were published in April 2019, demonstrating 82% of patients receiving the drug attained clinical hemostasis.11 However, as with idarucizumab, up to 10% of patients had a thrombotic event in the follow-up period. Use of recombinant coagulation factor Xa for treatment of life-threatening bleeding related to betrixaban and edoxaban is considered off label but is recommended by guidelines.8 Studies on investigational reversal agents for betrixaban and edoxaban are ongoing.
Both unactivated and activated PCC contain clotting factor X. Their use to control bleeding related to DOAC use is based on observational studies. In a systematic review of the nonrandomized studies, the efficacy of PCC to stem major bleeding was 69% and the risk for thromboembolism was 4%.12 There are no head-to-head studies comparing use of recombinant coagulation factor Xa and PCC. Therefore, guidelines are to use either recombinant factor Xa or PCC for the treatment of life-threatening bleeding related to DOAC use.7
As thrombosis risk heightens after use of any reversal agent, the recommendations are to resume anticoagulation within 90 days if the patient is at moderate or high risk for recurrent thromboembolism.8
After discussion with the hospitalist about the new agents available to reverse anticoagulation, the colleague decided to place the patient on a DOAC and keep the patient in his nursing home. Thankfully, the patient did not thereafter experience sustained bleeding necessitating use of these reversal agents. More importantly for the patient, he was able to stay in the comfort of his home.
Dr. Tuck is associate section chief for hospital medicine at the Veterans Affairs Medical Center in Washington, D.C.
References
1. Gómez-Outes A et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508-21.
2. Jun M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population-based, observational study. BMJ. 2017;359:j4323.
3. Barnes GD et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:(1300-5).e2.
4. Reddy P et al. Practical approach to VTE management in hospitalized patients. Am J Ther. 2017;24(4):e442-67.
5. Kimachi M et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017 Nov 6;11:CD011373.
6. Gottenborg E et al. Clinical guideline highlights for the hospitalist: The management of anticoagulation in the hospitalized adult. J Hosp Med. 2019; 14(8):499-500.
7. Pollack CV Jr et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377(5):431-41.
8. Witt DM et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-91.
9. Malarky M et al. FDA accelerated approval letter. Retrieved July 15, 2019. https://www.fda.gov/media/113285/download
10. Connolly SJ et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-41.
11. Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
12. Piran S et al. Management of direct factor Xa inhibitor–related major bleeding with prothrombin complex concentrate: A meta-analysis. Blood Adv. 2019;3(2):158-67.
When on call for admissions, a hospitalist receives a request from a colleague to admit an octogenarian man with an acute uncomplicated deep vein thrombosis to start heparin, bridging to warfarin. The patient has no evidence of postphlebitic syndrome, pulmonary embolism, or right-sided heart strain. The hospitalist asks her colleague if he had considered treating the patient in the ambulatory setting using a direct-acting oral anticoagulant (DOAC). After all, this would save the patient an unnecessary hospitalization, weekly international normalized ratio checks, and other important lifestyle changes. In response, the colleague voices concern that the “new drugs don’t have antidotes.”
DOACs have several benefits over vitamin K antagonists (VKAs) and heparins. DOACs have quicker onset of action, can be taken by mouth, in general do not require dosage adjustment, and have fewer dietary and lifestyle modifications, compared with VKAs and heparins. In atrial fibrillation, DOACs have been shown to have lower all-cause and bleeding-related mortality than warfarin (see Table 1).1 Observational studies also suggest less risk of major bleeding with DOACs over warfarin but no difference in overall mortality when used to treat venous thromboembolism (see Table 2).2 Because of these combined advantages, DOACs are increasingly prescribed, accounting for approximately half of all oral anticoagulant prescriptions in 2014.3
Although DOACs have been shown to be as good if not superior to VKAs and heparins in these circumstances, there are situations where a DOAC should not be used. There is limited data on the safety of DOACs in patients with mechanical heart valves, liver failure, and chronic kidney disease with a creatinine clearance less than 30 mL/min.4 Therefore, warfarin is still the preferred agent in these settings. There is some data that apixaban may be safe in patients with a creatinine clearance of greater than 10 mL/min, but long-term safety studies have not been performed in patients with end-stage renal disease on hemodialysis.5 Finally, in patients requiring concomitant inducers or inhibitors of the P-glycoprotein or cytochrome P450 enzymes like antiepileptics and protease inhibitors, VKAs and heparins are favored.4
Notwithstanding their advantages, when DOACs first hit the market there were concerns that reversal agents were not available. In the August issue of the Journal of Hospital Medicine’s Clinical Guideline Highlights for the Hospitalist, Emily Gottenborg, MD, and Gregory Misky, MD, summarized guideline recommendations for reversal of the newer agents.6 This includes use of idarucizumab for patients on dabigatran and use of prothrombin complex concentrate (PCC) or recombinant coagulation factor Xa (andexanet alfa) for patients on apixaban or rivaroxaban for the treatment of life-threatening bleeding.
Idarucizumab is a monoclonal antibody developed to reverse the effects of dabigatran, the only DOAC that directly inhibits thrombin. In 2017, researchers reported on a cohort of subjects receiving idarucizumab for uncontrolled bleeding or who were on dabigatran and about to undergo an urgent procedure.7 Of those with uncontrolled bleeding, two-thirds had confirmed bleeding cessation within 24 hours. Periprocedural hemostasis was achieved in 93.4% of patients undergoing urgent procedures. However, it should be noted that use of idarucizumab conferred an increase risk (6.3%) of thrombosis within 90 days. Based on these findings, guidelines recommend use of idarucizumab in patients experiencing life-threatening bleeding, balanced against the risk of thrombosis.8
In 2018, the Food and Drug Administration approved recombinant coagulation factor Xa for treatment of life-threatening or uncontrolled bleeding in patients on apixaban or rivaroxaban.9 The approval came after a study by the ANNEXA-4 investigators showed that recombinant coagulation factor Xa quickly and effectively achieved hemostasis.10 Full study results were published in April 2019, demonstrating 82% of patients receiving the drug attained clinical hemostasis.11 However, as with idarucizumab, up to 10% of patients had a thrombotic event in the follow-up period. Use of recombinant coagulation factor Xa for treatment of life-threatening bleeding related to betrixaban and edoxaban is considered off label but is recommended by guidelines.8 Studies on investigational reversal agents for betrixaban and edoxaban are ongoing.
Both unactivated and activated PCC contain clotting factor X. Their use to control bleeding related to DOAC use is based on observational studies. In a systematic review of the nonrandomized studies, the efficacy of PCC to stem major bleeding was 69% and the risk for thromboembolism was 4%.12 There are no head-to-head studies comparing use of recombinant coagulation factor Xa and PCC. Therefore, guidelines are to use either recombinant factor Xa or PCC for the treatment of life-threatening bleeding related to DOAC use.7
As thrombosis risk heightens after use of any reversal agent, the recommendations are to resume anticoagulation within 90 days if the patient is at moderate or high risk for recurrent thromboembolism.8
After discussion with the hospitalist about the new agents available to reverse anticoagulation, the colleague decided to place the patient on a DOAC and keep the patient in his nursing home. Thankfully, the patient did not thereafter experience sustained bleeding necessitating use of these reversal agents. More importantly for the patient, he was able to stay in the comfort of his home.
Dr. Tuck is associate section chief for hospital medicine at the Veterans Affairs Medical Center in Washington, D.C.
References
1. Gómez-Outes A et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508-21.
2. Jun M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population-based, observational study. BMJ. 2017;359:j4323.
3. Barnes GD et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:(1300-5).e2.
4. Reddy P et al. Practical approach to VTE management in hospitalized patients. Am J Ther. 2017;24(4):e442-67.
5. Kimachi M et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017 Nov 6;11:CD011373.
6. Gottenborg E et al. Clinical guideline highlights for the hospitalist: The management of anticoagulation in the hospitalized adult. J Hosp Med. 2019; 14(8):499-500.
7. Pollack CV Jr et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377(5):431-41.
8. Witt DM et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-91.
9. Malarky M et al. FDA accelerated approval letter. Retrieved July 15, 2019. https://www.fda.gov/media/113285/download
10. Connolly SJ et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-41.
11. Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
12. Piran S et al. Management of direct factor Xa inhibitor–related major bleeding with prothrombin complex concentrate: A meta-analysis. Blood Adv. 2019;3(2):158-67.
Hospitalist movers and shakers – September 2019
Mark Williams, MD, MHM, FACP, recently was appointed chief quality and transformation officer for the University of Kentucky’s UK HealthCare (Lexington). Dr. Williams, a tenured professor in the division of hospital medicine at the UK College of Medicine, will serve as chair of UK HealthCare’s Executive Quality Committee. Dr. Williams will lead integration of quality improvement, safety, and quality reporting with data analytics.
Dr. Williams established the first hospitalist program at a public hospital (Grady Memorial Hospital) and academic hospitalist programs at Emory University, Northwestern University, and UK HealthCare. An inaugural member of SHM, he is a past president, was the founding editor-in-chief of the Journal of Hospital Medicine and led SHM’s Project BOOST.
Also at UK HealthCare, Romil Chadha, MD, MPH, SFHM, FACP, has been named interim chief of the division of hospital medicine and medical director of Physician Information Technology Services. Previously, he was associate chief of the division of hospital medicine, and he also serves as medical director of telemetry.
Dr. Chadha is the founder of the Kentucky chapter of SHM, where he is the immediate past president. He is also the codirector of the Heartland Hospital Medicine Conference.
Amit Vashist, MD, MBA, CPE, FHM, FACP, FAPA, has been named chief clinical officer at Ballad Health, a 21-hospital health system in Northeast Tennessee, Southwest Virginia, Northwest North Carolina, and Southeast Kentucky.
In his new role, he will focus on clinical quality, value-based initiatives to improve quality while reducing cost of care, performance improvement, oversight of the clinical delivery of care and will be the liaison to the Ballad Health Clinical Council. Dr. Vashist is a member of The Hospitalist’s editorial advisory board.
Nagendra Gupta, MD, FACP, CPE, has been appointed to the American Board of Internal Medicine’s Internal Medicine Specialty Board. ABIM Specialty Boards are responsible for the broad definition of the discipline across Certification and Maintenance of Certification (MOC). Specialty Board members work with physicians and medical societies to develop Certification and MOC credentials to recognize physicians for their specialized knowledge and commitment to staying current in their field.
Dr. Gupta is a full-time practicing hospitalist with Apogee Physicians and currently serves as the director of the hospitalist program at Texas Health Arlington (Tex.) Memorial Hospital. He also serves as vice president for SHM’s North Central Texas Chapter.
T. Steen Trawick Jr., MD, was named the CEO of Christus Shreveport-Bossier Health System in Shreveport, La., in August 2019.
Dr. Trawick has worked for Christus as a pediatric hospitalist since 2005 and most recently has served concurrently as associate chief medical officer for Sound Physicians. Through Sound Physicians, Dr. Trawick oversees the hospitalist and emergency medical programs for Christus and other hospitals – 14 in total – in Texas and Louisiana. He has worked in that role for the past 6 years.
Scott Shepherd, DO, FACP, has been selected chief medical officer of the health data enrichment and integration technology company Verinovum in Tulsa, Okla. Dr. Shepherd is the medical director for hospitalist medicine and a practicing hospitalist with St. John Health System in Tulsa, and also medical director of the Center for Health Systems Innovation at his alma mater, Oklahoma State University in Stillwater.
Amanda Logue, MD, has been elevated to chief medical officer at Lafayette (La.) General Hospital. Dr. Logue assumed her role in May 2019, which includes the title of senior vice president.
Dr. Logue has worked at Lafayette General since 2009. A hospitalist/internist, her duties at the facility have included department chair of medicine, physician champion for electronic medical record implementation, medical director of the hospitalist program, and most recently chief medical information officer.
Rina Bansal, MD, MBA, recently was appointed full-time president of Inova Alexandria (Va.) Hospital, taking the reins officially after serving as acting president since November 2018. Dr. Bansal has been at Inova since 2008, when she started as a hospitalist at Inova Fairfax (Va.).
Dr. Bansal created and led Inova’s Clinical Nurse Services Hospitalist program through its department of neurosciences and has done stints as Inova Fairfax’s associate chief medical officer, medical director of Inova Telemedicine, and chief medical officer at Inova Alexandria.
James Napoli, MD, has been named chief medical officer for Blue Cross and Blue Shield of Arizona (BCBSAZ). He has manned the CMO position in an interim role since March, taking those duties on top of his role as BCBSAZ’s enterprise medical director for health care ventures and innovation.
Dr. Napoli came to BCBSAZ in 2013 after more than a decade at Abrazo Arrowhead Campus (Glendale, Ariz.) At Abrazo, he was director of hospitalist services and vice-chief of staff, on top of his efforts as a practicing hospital medicine clinician.
Dr. Napoli was previously medical director at OptumHealth, working specifically in the medical management and quality improvement areas for the health management solutions organization’s Medicare Advantage clients.
Mercy Hospital Fort Smith (Ark.) has partnered with the Ob Hospitalist Group (Greenville, S.C.) to launch an obstetric hospitalist program. OB hospitalists deliver babies when a patient’s physician cannot be present, provide emergency care, and provide support to high-risk pregnancy patients, among other duties within the hospital.
The partnership has allowed Mercy Fort Smith to create a dedicated, four-room obstetric emergency department in its Mercy Childbirth Center. Eight OB hospitalists have been hired and will provide care 24 hours a day, 7 days a week.
Mark Williams, MD, MHM, FACP, recently was appointed chief quality and transformation officer for the University of Kentucky’s UK HealthCare (Lexington). Dr. Williams, a tenured professor in the division of hospital medicine at the UK College of Medicine, will serve as chair of UK HealthCare’s Executive Quality Committee. Dr. Williams will lead integration of quality improvement, safety, and quality reporting with data analytics.
Dr. Williams established the first hospitalist program at a public hospital (Grady Memorial Hospital) and academic hospitalist programs at Emory University, Northwestern University, and UK HealthCare. An inaugural member of SHM, he is a past president, was the founding editor-in-chief of the Journal of Hospital Medicine and led SHM’s Project BOOST.
Also at UK HealthCare, Romil Chadha, MD, MPH, SFHM, FACP, has been named interim chief of the division of hospital medicine and medical director of Physician Information Technology Services. Previously, he was associate chief of the division of hospital medicine, and he also serves as medical director of telemetry.
Dr. Chadha is the founder of the Kentucky chapter of SHM, where he is the immediate past president. He is also the codirector of the Heartland Hospital Medicine Conference.
Amit Vashist, MD, MBA, CPE, FHM, FACP, FAPA, has been named chief clinical officer at Ballad Health, a 21-hospital health system in Northeast Tennessee, Southwest Virginia, Northwest North Carolina, and Southeast Kentucky.
In his new role, he will focus on clinical quality, value-based initiatives to improve quality while reducing cost of care, performance improvement, oversight of the clinical delivery of care and will be the liaison to the Ballad Health Clinical Council. Dr. Vashist is a member of The Hospitalist’s editorial advisory board.
Nagendra Gupta, MD, FACP, CPE, has been appointed to the American Board of Internal Medicine’s Internal Medicine Specialty Board. ABIM Specialty Boards are responsible for the broad definition of the discipline across Certification and Maintenance of Certification (MOC). Specialty Board members work with physicians and medical societies to develop Certification and MOC credentials to recognize physicians for their specialized knowledge and commitment to staying current in their field.
Dr. Gupta is a full-time practicing hospitalist with Apogee Physicians and currently serves as the director of the hospitalist program at Texas Health Arlington (Tex.) Memorial Hospital. He also serves as vice president for SHM’s North Central Texas Chapter.
T. Steen Trawick Jr., MD, was named the CEO of Christus Shreveport-Bossier Health System in Shreveport, La., in August 2019.
Dr. Trawick has worked for Christus as a pediatric hospitalist since 2005 and most recently has served concurrently as associate chief medical officer for Sound Physicians. Through Sound Physicians, Dr. Trawick oversees the hospitalist and emergency medical programs for Christus and other hospitals – 14 in total – in Texas and Louisiana. He has worked in that role for the past 6 years.
Scott Shepherd, DO, FACP, has been selected chief medical officer of the health data enrichment and integration technology company Verinovum in Tulsa, Okla. Dr. Shepherd is the medical director for hospitalist medicine and a practicing hospitalist with St. John Health System in Tulsa, and also medical director of the Center for Health Systems Innovation at his alma mater, Oklahoma State University in Stillwater.
Amanda Logue, MD, has been elevated to chief medical officer at Lafayette (La.) General Hospital. Dr. Logue assumed her role in May 2019, which includes the title of senior vice president.
Dr. Logue has worked at Lafayette General since 2009. A hospitalist/internist, her duties at the facility have included department chair of medicine, physician champion for electronic medical record implementation, medical director of the hospitalist program, and most recently chief medical information officer.
Rina Bansal, MD, MBA, recently was appointed full-time president of Inova Alexandria (Va.) Hospital, taking the reins officially after serving as acting president since November 2018. Dr. Bansal has been at Inova since 2008, when she started as a hospitalist at Inova Fairfax (Va.).
Dr. Bansal created and led Inova’s Clinical Nurse Services Hospitalist program through its department of neurosciences and has done stints as Inova Fairfax’s associate chief medical officer, medical director of Inova Telemedicine, and chief medical officer at Inova Alexandria.
James Napoli, MD, has been named chief medical officer for Blue Cross and Blue Shield of Arizona (BCBSAZ). He has manned the CMO position in an interim role since March, taking those duties on top of his role as BCBSAZ’s enterprise medical director for health care ventures and innovation.
Dr. Napoli came to BCBSAZ in 2013 after more than a decade at Abrazo Arrowhead Campus (Glendale, Ariz.) At Abrazo, he was director of hospitalist services and vice-chief of staff, on top of his efforts as a practicing hospital medicine clinician.
Dr. Napoli was previously medical director at OptumHealth, working specifically in the medical management and quality improvement areas for the health management solutions organization’s Medicare Advantage clients.
Mercy Hospital Fort Smith (Ark.) has partnered with the Ob Hospitalist Group (Greenville, S.C.) to launch an obstetric hospitalist program. OB hospitalists deliver babies when a patient’s physician cannot be present, provide emergency care, and provide support to high-risk pregnancy patients, among other duties within the hospital.
The partnership has allowed Mercy Fort Smith to create a dedicated, four-room obstetric emergency department in its Mercy Childbirth Center. Eight OB hospitalists have been hired and will provide care 24 hours a day, 7 days a week.
Mark Williams, MD, MHM, FACP, recently was appointed chief quality and transformation officer for the University of Kentucky’s UK HealthCare (Lexington). Dr. Williams, a tenured professor in the division of hospital medicine at the UK College of Medicine, will serve as chair of UK HealthCare’s Executive Quality Committee. Dr. Williams will lead integration of quality improvement, safety, and quality reporting with data analytics.
Dr. Williams established the first hospitalist program at a public hospital (Grady Memorial Hospital) and academic hospitalist programs at Emory University, Northwestern University, and UK HealthCare. An inaugural member of SHM, he is a past president, was the founding editor-in-chief of the Journal of Hospital Medicine and led SHM’s Project BOOST.
Also at UK HealthCare, Romil Chadha, MD, MPH, SFHM, FACP, has been named interim chief of the division of hospital medicine and medical director of Physician Information Technology Services. Previously, he was associate chief of the division of hospital medicine, and he also serves as medical director of telemetry.
Dr. Chadha is the founder of the Kentucky chapter of SHM, where he is the immediate past president. He is also the codirector of the Heartland Hospital Medicine Conference.
Amit Vashist, MD, MBA, CPE, FHM, FACP, FAPA, has been named chief clinical officer at Ballad Health, a 21-hospital health system in Northeast Tennessee, Southwest Virginia, Northwest North Carolina, and Southeast Kentucky.
In his new role, he will focus on clinical quality, value-based initiatives to improve quality while reducing cost of care, performance improvement, oversight of the clinical delivery of care and will be the liaison to the Ballad Health Clinical Council. Dr. Vashist is a member of The Hospitalist’s editorial advisory board.
Nagendra Gupta, MD, FACP, CPE, has been appointed to the American Board of Internal Medicine’s Internal Medicine Specialty Board. ABIM Specialty Boards are responsible for the broad definition of the discipline across Certification and Maintenance of Certification (MOC). Specialty Board members work with physicians and medical societies to develop Certification and MOC credentials to recognize physicians for their specialized knowledge and commitment to staying current in their field.
Dr. Gupta is a full-time practicing hospitalist with Apogee Physicians and currently serves as the director of the hospitalist program at Texas Health Arlington (Tex.) Memorial Hospital. He also serves as vice president for SHM’s North Central Texas Chapter.
T. Steen Trawick Jr., MD, was named the CEO of Christus Shreveport-Bossier Health System in Shreveport, La., in August 2019.
Dr. Trawick has worked for Christus as a pediatric hospitalist since 2005 and most recently has served concurrently as associate chief medical officer for Sound Physicians. Through Sound Physicians, Dr. Trawick oversees the hospitalist and emergency medical programs for Christus and other hospitals – 14 in total – in Texas and Louisiana. He has worked in that role for the past 6 years.
Scott Shepherd, DO, FACP, has been selected chief medical officer of the health data enrichment and integration technology company Verinovum in Tulsa, Okla. Dr. Shepherd is the medical director for hospitalist medicine and a practicing hospitalist with St. John Health System in Tulsa, and also medical director of the Center for Health Systems Innovation at his alma mater, Oklahoma State University in Stillwater.
Amanda Logue, MD, has been elevated to chief medical officer at Lafayette (La.) General Hospital. Dr. Logue assumed her role in May 2019, which includes the title of senior vice president.
Dr. Logue has worked at Lafayette General since 2009. A hospitalist/internist, her duties at the facility have included department chair of medicine, physician champion for electronic medical record implementation, medical director of the hospitalist program, and most recently chief medical information officer.
Rina Bansal, MD, MBA, recently was appointed full-time president of Inova Alexandria (Va.) Hospital, taking the reins officially after serving as acting president since November 2018. Dr. Bansal has been at Inova since 2008, when she started as a hospitalist at Inova Fairfax (Va.).
Dr. Bansal created and led Inova’s Clinical Nurse Services Hospitalist program through its department of neurosciences and has done stints as Inova Fairfax’s associate chief medical officer, medical director of Inova Telemedicine, and chief medical officer at Inova Alexandria.
James Napoli, MD, has been named chief medical officer for Blue Cross and Blue Shield of Arizona (BCBSAZ). He has manned the CMO position in an interim role since March, taking those duties on top of his role as BCBSAZ’s enterprise medical director for health care ventures and innovation.
Dr. Napoli came to BCBSAZ in 2013 after more than a decade at Abrazo Arrowhead Campus (Glendale, Ariz.) At Abrazo, he was director of hospitalist services and vice-chief of staff, on top of his efforts as a practicing hospital medicine clinician.
Dr. Napoli was previously medical director at OptumHealth, working specifically in the medical management and quality improvement areas for the health management solutions organization’s Medicare Advantage clients.
Mercy Hospital Fort Smith (Ark.) has partnered with the Ob Hospitalist Group (Greenville, S.C.) to launch an obstetric hospitalist program. OB hospitalists deliver babies when a patient’s physician cannot be present, provide emergency care, and provide support to high-risk pregnancy patients, among other duties within the hospital.
The partnership has allowed Mercy Fort Smith to create a dedicated, four-room obstetric emergency department in its Mercy Childbirth Center. Eight OB hospitalists have been hired and will provide care 24 hours a day, 7 days a week.
Drug abuse–linked infective endocarditis spiking in U.S.
Hospitalizations for infective endocarditis associated with drug abuse doubled in the United States from 2002 to 2016, in a trend investigators call “alarming,” and link to a concurrent rise in opioid abuse.
Patients tend to be younger, poorer white males, according to findings published online in the Journal of the American Heart Association.
For their research, Amer N. Kadri, MD, of the Cleveland Clinic and colleagues looked at records for nearly a million hospitalizations for infective endocarditis (IE) in the National Inpatient Sample registry. All U.S. regions saw increases in drug abuse–linked cases of IE as a share of IE hospitalizations. Incidence of drug abuse–associated IC rose from 48 cases/100,000 population in 2002 to 79/100,000 in 2016. The Midwest saw the highest rate of change, with an annual percent increase of 4.9%.
While most IE hospitalizations in the study cohort were of white men (including 68% for drug-linked cases), the drug abuse–related cases were younger (median age, 38 vs. 70 years for nondrug-related IE), and more likely male (55.5% vs. 50%). About 45% of the drug-related cases were in people receiving Medicaid, and 42% were in the lowest quartile of median household income.
The drug abuse cases had fewer renal and cardiovascular comorbidities, compared with the nondrug cases, but were significantly more likely to present with HIV, hepatitis C, alcohol abuse, and liver disease. Inpatient mortality was lower among the drug-linked cases – 6% vs. 9% – but the drug cases saw significantly more cardiac or valve surgeries, longer hospital stays, and higher costs.
“Hospitalizations for IE have been increasing side by side with the opioid epidemic,” the investigators wrote in their analysis. “The opioid crisis has reached epidemic levels, and now drug overdoses have been the leading cause of injury-related death in the U.S. Heroin deaths had remained relatively low from 1999 until 2010 whereas it then increased threefold from 2010-2015.” The analysis showed a rise in drug abuse–associated IE “that corresponds to this general period.” The findings argue, the investigators said, for better treatment for opioid addiction after hospitalization and greater efforts to make drug rehabilitation available after discharge. The researchers described as a limitation of their study the use of billing codes that changed late in the study period, increasing detection of drug abuse cases after 2015. They reported no outside funding or conflicts of interest.
SOURCE: Kadri AN et al. J Am Heart Assoc. 2019 Sep 18.
Hospitalizations for infective endocarditis associated with drug abuse doubled in the United States from 2002 to 2016, in a trend investigators call “alarming,” and link to a concurrent rise in opioid abuse.
Patients tend to be younger, poorer white males, according to findings published online in the Journal of the American Heart Association.
For their research, Amer N. Kadri, MD, of the Cleveland Clinic and colleagues looked at records for nearly a million hospitalizations for infective endocarditis (IE) in the National Inpatient Sample registry. All U.S. regions saw increases in drug abuse–linked cases of IE as a share of IE hospitalizations. Incidence of drug abuse–associated IC rose from 48 cases/100,000 population in 2002 to 79/100,000 in 2016. The Midwest saw the highest rate of change, with an annual percent increase of 4.9%.
While most IE hospitalizations in the study cohort were of white men (including 68% for drug-linked cases), the drug abuse–related cases were younger (median age, 38 vs. 70 years for nondrug-related IE), and more likely male (55.5% vs. 50%). About 45% of the drug-related cases were in people receiving Medicaid, and 42% were in the lowest quartile of median household income.
The drug abuse cases had fewer renal and cardiovascular comorbidities, compared with the nondrug cases, but were significantly more likely to present with HIV, hepatitis C, alcohol abuse, and liver disease. Inpatient mortality was lower among the drug-linked cases – 6% vs. 9% – but the drug cases saw significantly more cardiac or valve surgeries, longer hospital stays, and higher costs.
“Hospitalizations for IE have been increasing side by side with the opioid epidemic,” the investigators wrote in their analysis. “The opioid crisis has reached epidemic levels, and now drug overdoses have been the leading cause of injury-related death in the U.S. Heroin deaths had remained relatively low from 1999 until 2010 whereas it then increased threefold from 2010-2015.” The analysis showed a rise in drug abuse–associated IE “that corresponds to this general period.” The findings argue, the investigators said, for better treatment for opioid addiction after hospitalization and greater efforts to make drug rehabilitation available after discharge. The researchers described as a limitation of their study the use of billing codes that changed late in the study period, increasing detection of drug abuse cases after 2015. They reported no outside funding or conflicts of interest.
SOURCE: Kadri AN et al. J Am Heart Assoc. 2019 Sep 18.
Hospitalizations for infective endocarditis associated with drug abuse doubled in the United States from 2002 to 2016, in a trend investigators call “alarming,” and link to a concurrent rise in opioid abuse.
Patients tend to be younger, poorer white males, according to findings published online in the Journal of the American Heart Association.
For their research, Amer N. Kadri, MD, of the Cleveland Clinic and colleagues looked at records for nearly a million hospitalizations for infective endocarditis (IE) in the National Inpatient Sample registry. All U.S. regions saw increases in drug abuse–linked cases of IE as a share of IE hospitalizations. Incidence of drug abuse–associated IC rose from 48 cases/100,000 population in 2002 to 79/100,000 in 2016. The Midwest saw the highest rate of change, with an annual percent increase of 4.9%.
While most IE hospitalizations in the study cohort were of white men (including 68% for drug-linked cases), the drug abuse–related cases were younger (median age, 38 vs. 70 years for nondrug-related IE), and more likely male (55.5% vs. 50%). About 45% of the drug-related cases were in people receiving Medicaid, and 42% were in the lowest quartile of median household income.
The drug abuse cases had fewer renal and cardiovascular comorbidities, compared with the nondrug cases, but were significantly more likely to present with HIV, hepatitis C, alcohol abuse, and liver disease. Inpatient mortality was lower among the drug-linked cases – 6% vs. 9% – but the drug cases saw significantly more cardiac or valve surgeries, longer hospital stays, and higher costs.
“Hospitalizations for IE have been increasing side by side with the opioid epidemic,” the investigators wrote in their analysis. “The opioid crisis has reached epidemic levels, and now drug overdoses have been the leading cause of injury-related death in the U.S. Heroin deaths had remained relatively low from 1999 until 2010 whereas it then increased threefold from 2010-2015.” The analysis showed a rise in drug abuse–associated IE “that corresponds to this general period.” The findings argue, the investigators said, for better treatment for opioid addiction after hospitalization and greater efforts to make drug rehabilitation available after discharge. The researchers described as a limitation of their study the use of billing codes that changed late in the study period, increasing detection of drug abuse cases after 2015. They reported no outside funding or conflicts of interest.
SOURCE: Kadri AN et al. J Am Heart Assoc. 2019 Sep 18.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point:
Major finding: Incidence of drug abuse–associated IC increased from 48 cases/100,000 in 2002 to 79/100,000 in 2016.
Study details: A retrospective cohort study identifying about a more than 950,000 cases of IC from the National Inpatient Sample registry.
Disclosures: None.
Source: Kadri AN et al. J Am Heart Assoc. 2019 Sep 18.
Observation versus inpatient status
A dilemma for hospitalists and patients
A federal effort to reduce health care expenditures has left many older Medicare recipients experiencing the sticker shock of “observation status.” Patients who are not sick enough to meet inpatient admission criteria, however, still require hospitalization, and may be placed under Medicare observation care.
Seniors can get frustrated, confused, and anxious as their status can be changed while they are in the hospital, and they may receive large medical bills after they are discharged. The Centers for Medicare & Medicaid Services’ “3-day rule” mandates that Medicare will not pay for skilled nursing facility care unless the patient is admitted as an “inpatient” for at least 3 days. Observation days do not count towards this 3-day hospital stay.
There has been an increase in outpatient services over the years since 2006. The 2018 State of Hospital Medicine Report (SoHM) highlights the percentage of discharges based on hospitalists’ billed Current Procedural Terminology codes. Codes 99217 (observation discharge) and 99238-99239 (inpatient discharge) were used to calculate the percentages. 80.7% of adult medicine hospitalist discharges were coded using inpatient discharge codes, while 19.3% of patients were discharged with observation discharge codes.
In the 2016 SoHM report, the ratio was 76.0% inpatient and 21.1% observation codes and in the 2014 report we saw 80.3% inpatient and 16.1% observation discharges (see table 1). But in both of those surveys, same-day admission/discharge codes were also separately reported, which did not occur in 2018. That makes year-over-year comparison of the data challenging.
Interestingly, the 2017 CMS data on Evaluation and Management Codes by Specialty for the first time included separate data for hospitalists, based on hospitalists who credentialed with Medicare using the new C6 specialty code. Based on that data, when looking only at inpatient (99238-99239) and observation (99217) codes, 83% of the discharges were inpatient and 17% were observation.
Physicians feel the pressure of strained patient-physician relationships as a consequence of patients feeling the brunt of the financing gap related to observation status. Patients often feel they were not warned adequately about the financial ramifications of observation status. Even if Medicare beneficiaries have received the Medicare Outpatient Observation Notice, outlined by the Notice of Observation Treatment and Implication for Care Eligibility Act, they have no rights to appeal.
Currently Medicare beneficiaries admitted as inpatients only incur a Part A deductible; they are not liable for tests, procedures, and nursing care. On the other hand, in observation status all services are billed separately. For Medicare Part B services (which covers observation care) patients must pay 20% of services after the Part B deductible, which could result in a huge financial burden. Costs for skilled nursing facilities, when they are not covered by Medicare Part A, because of the 3-day rule, can easily go up to $20,000 or more. Medicare beneficiaries have no cap on costs for an observation stay. In some cases, hospitals have to apply a condition code 44 and retroactively change the stay to observation status.
I attended the 2019 Society of Hospital Medicine Annual Conference in Washington. Hospitalists from all parts of the country advocated on Capitol Hill against the “observation bill,” and “meet and greets” with congressional representatives increased their opposition to the bill. These efforts may work in favor of protecting patients from surprise medical bills. Hospital medicine physicians are on the front lines for providing health care in the hospital setting; they have demanded a fix to this legislative loophole which brings high out of pocket costs to our nation’s most vulnerable seniors. The observation status “2-midnight rule” utilized by CMS has increased financial barriers and decreased access to postacute care, affecting the provision of high-quality care for patients.
My hospital has a utilization review committee which reviews all cases to determine the appropriateness of an inpatient versus an observation designation. (An interesting question is whether the financial resources used to support this additional staff could be better assigned to provide high-quality care.) Distribution of these patients is determined on very specific criteria as outlined by Medicare. Observation is basically considered a billing method implemented by payers to decrease dollars paid to acute care hospitals for inpatient care. It pertains to admission status, not to the level of care provided in the hospital. Unfortunately, it is felt that no two payers define observation the same way. A few examples of common observation diagnoses are chest pain, abdominal pain, syncope, and migraine headache; in other words, patients with diagnoses where it is suspected that a less than 24-hour stay in the hospital could be sufficient.
Observation care is increasing and can sometimes contribute to work flow impediments and frustrations in hospitalists; thus, hospitalists are demanding reform. It has been proposed that observation could be eliminated altogether by creating a payment blend of inpatient/outpatient rates. Another option could be to assign lower Diagnosis Related Group coding to lower acuity disease processes, instead of separate observation reimbursement.
Patients and doctors lament that “Once you are in the hospital, you are admitted!” I don’t know the right answer that would solve the observation versus inpatient dilemma, but it is intriguing to consider changes in policy that might focus on the complete elimination of observation status.
Dr. Puri is a hospitalist at Lahey Hospital and Medical Center in Burlington, Mass.
A dilemma for hospitalists and patients
A dilemma for hospitalists and patients
A federal effort to reduce health care expenditures has left many older Medicare recipients experiencing the sticker shock of “observation status.” Patients who are not sick enough to meet inpatient admission criteria, however, still require hospitalization, and may be placed under Medicare observation care.
Seniors can get frustrated, confused, and anxious as their status can be changed while they are in the hospital, and they may receive large medical bills after they are discharged. The Centers for Medicare & Medicaid Services’ “3-day rule” mandates that Medicare will not pay for skilled nursing facility care unless the patient is admitted as an “inpatient” for at least 3 days. Observation days do not count towards this 3-day hospital stay.
There has been an increase in outpatient services over the years since 2006. The 2018 State of Hospital Medicine Report (SoHM) highlights the percentage of discharges based on hospitalists’ billed Current Procedural Terminology codes. Codes 99217 (observation discharge) and 99238-99239 (inpatient discharge) were used to calculate the percentages. 80.7% of adult medicine hospitalist discharges were coded using inpatient discharge codes, while 19.3% of patients were discharged with observation discharge codes.
In the 2016 SoHM report, the ratio was 76.0% inpatient and 21.1% observation codes and in the 2014 report we saw 80.3% inpatient and 16.1% observation discharges (see table 1). But in both of those surveys, same-day admission/discharge codes were also separately reported, which did not occur in 2018. That makes year-over-year comparison of the data challenging.
Interestingly, the 2017 CMS data on Evaluation and Management Codes by Specialty for the first time included separate data for hospitalists, based on hospitalists who credentialed with Medicare using the new C6 specialty code. Based on that data, when looking only at inpatient (99238-99239) and observation (99217) codes, 83% of the discharges were inpatient and 17% were observation.
Physicians feel the pressure of strained patient-physician relationships as a consequence of patients feeling the brunt of the financing gap related to observation status. Patients often feel they were not warned adequately about the financial ramifications of observation status. Even if Medicare beneficiaries have received the Medicare Outpatient Observation Notice, outlined by the Notice of Observation Treatment and Implication for Care Eligibility Act, they have no rights to appeal.
Currently Medicare beneficiaries admitted as inpatients only incur a Part A deductible; they are not liable for tests, procedures, and nursing care. On the other hand, in observation status all services are billed separately. For Medicare Part B services (which covers observation care) patients must pay 20% of services after the Part B deductible, which could result in a huge financial burden. Costs for skilled nursing facilities, when they are not covered by Medicare Part A, because of the 3-day rule, can easily go up to $20,000 or more. Medicare beneficiaries have no cap on costs for an observation stay. In some cases, hospitals have to apply a condition code 44 and retroactively change the stay to observation status.
I attended the 2019 Society of Hospital Medicine Annual Conference in Washington. Hospitalists from all parts of the country advocated on Capitol Hill against the “observation bill,” and “meet and greets” with congressional representatives increased their opposition to the bill. These efforts may work in favor of protecting patients from surprise medical bills. Hospital medicine physicians are on the front lines for providing health care in the hospital setting; they have demanded a fix to this legislative loophole which brings high out of pocket costs to our nation’s most vulnerable seniors. The observation status “2-midnight rule” utilized by CMS has increased financial barriers and decreased access to postacute care, affecting the provision of high-quality care for patients.
My hospital has a utilization review committee which reviews all cases to determine the appropriateness of an inpatient versus an observation designation. (An interesting question is whether the financial resources used to support this additional staff could be better assigned to provide high-quality care.) Distribution of these patients is determined on very specific criteria as outlined by Medicare. Observation is basically considered a billing method implemented by payers to decrease dollars paid to acute care hospitals for inpatient care. It pertains to admission status, not to the level of care provided in the hospital. Unfortunately, it is felt that no two payers define observation the same way. A few examples of common observation diagnoses are chest pain, abdominal pain, syncope, and migraine headache; in other words, patients with diagnoses where it is suspected that a less than 24-hour stay in the hospital could be sufficient.
Observation care is increasing and can sometimes contribute to work flow impediments and frustrations in hospitalists; thus, hospitalists are demanding reform. It has been proposed that observation could be eliminated altogether by creating a payment blend of inpatient/outpatient rates. Another option could be to assign lower Diagnosis Related Group coding to lower acuity disease processes, instead of separate observation reimbursement.
Patients and doctors lament that “Once you are in the hospital, you are admitted!” I don’t know the right answer that would solve the observation versus inpatient dilemma, but it is intriguing to consider changes in policy that might focus on the complete elimination of observation status.
Dr. Puri is a hospitalist at Lahey Hospital and Medical Center in Burlington, Mass.
A federal effort to reduce health care expenditures has left many older Medicare recipients experiencing the sticker shock of “observation status.” Patients who are not sick enough to meet inpatient admission criteria, however, still require hospitalization, and may be placed under Medicare observation care.
Seniors can get frustrated, confused, and anxious as their status can be changed while they are in the hospital, and they may receive large medical bills after they are discharged. The Centers for Medicare & Medicaid Services’ “3-day rule” mandates that Medicare will not pay for skilled nursing facility care unless the patient is admitted as an “inpatient” for at least 3 days. Observation days do not count towards this 3-day hospital stay.
There has been an increase in outpatient services over the years since 2006. The 2018 State of Hospital Medicine Report (SoHM) highlights the percentage of discharges based on hospitalists’ billed Current Procedural Terminology codes. Codes 99217 (observation discharge) and 99238-99239 (inpatient discharge) were used to calculate the percentages. 80.7% of adult medicine hospitalist discharges were coded using inpatient discharge codes, while 19.3% of patients were discharged with observation discharge codes.
In the 2016 SoHM report, the ratio was 76.0% inpatient and 21.1% observation codes and in the 2014 report we saw 80.3% inpatient and 16.1% observation discharges (see table 1). But in both of those surveys, same-day admission/discharge codes were also separately reported, which did not occur in 2018. That makes year-over-year comparison of the data challenging.
Interestingly, the 2017 CMS data on Evaluation and Management Codes by Specialty for the first time included separate data for hospitalists, based on hospitalists who credentialed with Medicare using the new C6 specialty code. Based on that data, when looking only at inpatient (99238-99239) and observation (99217) codes, 83% of the discharges were inpatient and 17% were observation.
Physicians feel the pressure of strained patient-physician relationships as a consequence of patients feeling the brunt of the financing gap related to observation status. Patients often feel they were not warned adequately about the financial ramifications of observation status. Even if Medicare beneficiaries have received the Medicare Outpatient Observation Notice, outlined by the Notice of Observation Treatment and Implication for Care Eligibility Act, they have no rights to appeal.
Currently Medicare beneficiaries admitted as inpatients only incur a Part A deductible; they are not liable for tests, procedures, and nursing care. On the other hand, in observation status all services are billed separately. For Medicare Part B services (which covers observation care) patients must pay 20% of services after the Part B deductible, which could result in a huge financial burden. Costs for skilled nursing facilities, when they are not covered by Medicare Part A, because of the 3-day rule, can easily go up to $20,000 or more. Medicare beneficiaries have no cap on costs for an observation stay. In some cases, hospitals have to apply a condition code 44 and retroactively change the stay to observation status.
I attended the 2019 Society of Hospital Medicine Annual Conference in Washington. Hospitalists from all parts of the country advocated on Capitol Hill against the “observation bill,” and “meet and greets” with congressional representatives increased their opposition to the bill. These efforts may work in favor of protecting patients from surprise medical bills. Hospital medicine physicians are on the front lines for providing health care in the hospital setting; they have demanded a fix to this legislative loophole which brings high out of pocket costs to our nation’s most vulnerable seniors. The observation status “2-midnight rule” utilized by CMS has increased financial barriers and decreased access to postacute care, affecting the provision of high-quality care for patients.
My hospital has a utilization review committee which reviews all cases to determine the appropriateness of an inpatient versus an observation designation. (An interesting question is whether the financial resources used to support this additional staff could be better assigned to provide high-quality care.) Distribution of these patients is determined on very specific criteria as outlined by Medicare. Observation is basically considered a billing method implemented by payers to decrease dollars paid to acute care hospitals for inpatient care. It pertains to admission status, not to the level of care provided in the hospital. Unfortunately, it is felt that no two payers define observation the same way. A few examples of common observation diagnoses are chest pain, abdominal pain, syncope, and migraine headache; in other words, patients with diagnoses where it is suspected that a less than 24-hour stay in the hospital could be sufficient.
Observation care is increasing and can sometimes contribute to work flow impediments and frustrations in hospitalists; thus, hospitalists are demanding reform. It has been proposed that observation could be eliminated altogether by creating a payment blend of inpatient/outpatient rates. Another option could be to assign lower Diagnosis Related Group coding to lower acuity disease processes, instead of separate observation reimbursement.
Patients and doctors lament that “Once you are in the hospital, you are admitted!” I don’t know the right answer that would solve the observation versus inpatient dilemma, but it is intriguing to consider changes in policy that might focus on the complete elimination of observation status.
Dr. Puri is a hospitalist at Lahey Hospital and Medical Center in Burlington, Mass.
Managing alcohol withdrawal in the hospitalized patient
Symptom-triggered therapy has multiple benefits
Case
A 57-year-old man with a history of alcohol abuse (no history of seizures) presents to the ED “feeling awful.” He claims his last drink was 1 day prior. Initial vital signs are: T = 99.1°F, HR 102 bpm, BP 162/85 mm Hg, respirations 18/minute, and 99% oxygen saturation. He is tremulous, diaphoretic, and has an unsteady gait. What is the best way to manage his symptoms while hospitalized?
Brief overview of the issue
With over 15 million people with alcohol use disorder (AUD) in the United States alone, alcohol dependence and misuse remain significant issues among hospitalized patients.1 It is estimated that over 20% of admitted patients meet DSM-5 criteria for AUD and that over 2 million will withdraw each year.2,3 Acute withdrawal includes a spectrum of symptoms ranging from mild anxiety and diaphoresis to hallucinations, seizures, and delirium tremens. Onset of these symptoms ranges from 24 hours up to 5 days.
Severe alcohol withdrawal syndrome (SAWS) attributable to abrupt discontinuation of alcohol leads to increased morbidity and mortality; therefore, early detection and prevention in the acute care setting is critical. Several factors can help predict who may withdraw, and once detected, pharmacological treatment is necessary.4 Thorough evaluation and treatment can help reduce mortality from the most severe forms of alcohol withdrawal including delirium tremens, which has up to 40% mortality if left untreated.5
Overview of the data
How do we use benzodiazepines to treat alcohol withdrawal?
Benzodiazepines are the mainstay of alcohol withdrawal treatment. Benzodiazepines work by stimulating the gamma-aminobutyric acid (GABA) receptor resulting in a reduction of neuronal activity. This leads to a sedative effect and thus slows the progression of withdrawal symptoms.
Long-acting benzodiazepines, such as chlordiazepoxide and diazepam, are the preferred choices for most patients. Their active metabolites have a rapid onset of action and their long half-lives allow for a lower incidence of breakthrough symptoms and rebound phenomena such as seizures.6 Benzodiazepines with shorter half-lives, such as lorazepam and oxazepam, are preferred in patients with liver dysfunction and those prone to respiratory depression.
Intravenous administration has a rapid onset of action and is the standard administration route of choice in patients with acute severe withdrawal, delirium tremens, and seizure activity. In patients with mild withdrawal symptoms or those in the outpatient setting, oral administration is generally effective.6
The Clinical Institute Withdrawal Assessment (CIWA) is one commonly used titration model that requires calculation of a symptom-based withdrawal score. Data have consistently demonstrated that a symptom-triggered method results in administration of less total benzodiazepines over a significantly shorter duration, thereby reducing cost and duration of treatment and minimizing side effects. This regimen may also reduce the risk of undermedicating or overmedicating a patient since the dosing is based upon an individual’s symptoms.7,8
The efficacy of symptom-triggered regimens however, depends on the reliability and accuracy of the patient assessment. A fixed-interval benzodiazepine-dosing approach where benzodiazepines are administered regardless of symptoms is useful when frequent monitoring and reassessment are not feasible or are unreliable.
What about phenobarbital?
Phenobarbital has similar pharmacokinetics to the benzodiazepines frequently used for alcohol withdrawal, including simultaneous effects on gamma-aminobutyric acid (GABA) and N-methyl-D-aspartate (NMDA) receptors, and has been proposed as a treatment option for delirium tremens.
In 2019, as reported in the American Journal of Emergency Medicine, Nelson et al. found that incorporating phenobarbital into a benzodiazepine-based protocol or as sole agent led to similar rates of ICU admission, length of stay, and need for mechanical ventilation in patients treated for alcohol withdrawal in the emergency department.9 The authors concluded that “phenobarbital (was) a safe and effective treatment alternative for alcohol withdrawal.” The systematic review by Hammond et al. in 2017 found that phenobarbital, either as monotherapy or in conjunction with benzodiazepines, could have comparable or superior results in comparison to other treatments, including benzodiazepines monotherapy.10 Further studies are needed to determine dosing and the most effective way to incorporate the use of phenobarbital in treatment of Alcohol Withdrawal Syndrome (AWS).
Should gabapentin or any other medications be added to his treatment regimen?
Chronic alcohol use induces a reduction in GABA activity (the major inhibitory neurotransmitter in the brain) and alcohol cessation results in decreased inhibitory tone. This physiologic imbalance contributes to the syndrome of alcohol withdrawal. As such, gabapentin has emerged as a promising treatment option in AWS and may help reduce the need for benzodiazepines.
Gabapentin has few drug-drug interactions and is safe for use in patients with impaired liver function; however, dosage adjustment is required for renal dysfunction (CrCl less than 60 mL/min). Gabapentin’s neuroprotective effects may also help decrease the neurotoxic effects associated with AWS. Common side effects of gabapentin include dizziness, drowsiness, ataxia, diarrhea, nausea, and vomiting. The potential for misuse has been reported.
In several small studies, gabapentin monotherapy was found to be comparable to benzodiazepines in the treatment of mild to moderate AWS. Gabapentin is efficacious in reducing cravings as well as improving mood, anxiety, and sleep, and showed an advantage over benzodiazepines in preventing relapse with no difference in length of hospital stay.6,11 Given the small sample sizes of these studies and the differing methods, settings, and inclusion/ exclusion criteria used, the generalizability of these findings to patients with significant medical and/or psychiatric comorbidities remains limited. Additional studies are needed to standardize dosing protocols and treatment strategies for both inpatients and outpatients.
Alternative agents such as antipsychotics (e.g., haloperidol), centrally acting alpha-2 agonists (e.g., clonidine), beta-blockers, and an agonist of the GABA-B receptor (e.g., baclofen) may also attenuate the symptoms of withdrawal. Since these all have limited evidence of their efficacy and have potential for harm, such as masking symptoms of progressive withdrawal and lowering seizure threshold, these agents are not routinely recommended for use. Valproic acid/divalproex, levetiracetam, topiramate, and zonisamide have also showed some efficacy in reducing symptoms of alcohol withdrawal in limited studies. The data on prevention of withdrawal seizures or delirium tremens when used as monotherapy is less robust.12
A daily multivitamin and folate are ordered. What about thiamine? Does the route matter?
Alarmingly, 80% of people who chronically abuse alcohol are thiamine deficient.13 This deficiency is attributable to several factors including inadequate oral intake, malabsorption, and decreased cellular utilization. Thiamine is a crucial factor in multiple enzymatic and metabolic pathways. Its deficiency can lead to free radical production, neurotoxicity, impaired glucose metabolism, and ultimately, cell death.14 A clinical concern stemming from thiamine deficiency is the development of Wernicke’s encephalopathy (WE), which is potentially reversible with prompt recognition and treatment, in comparison to its irreversible amnestic sequela, Korsakoff’s syndrome.
Wernicke’s encephalopathy had been defined as a triad of ataxia, ophthalmoplegia, and global confusion. However, Harper et al. discovered that only 16% of patients presented with the classic triad and 19% had none of these signs.15 Diagnosis is clinical since thiamine serology results do not accurately represent brain storage.
Currently, there are no consistent guidelines regarding repletion of thiamine administration in the treatment or prevention of WE attributable to alcohol overuse. Thiamine has a safe toxicity profile as excess thiamine is excreted in the urine. Outside of rare reports of anaphylactoid reactions involving large parenteral doses, there is no concern for overtreatment. As Wernicke-Korsakoff syndrome is associated with significant morbidity and mortality, high doses such as 200 to 500 mg are recommended to ensure blood-brain barrier passage. The intravenous route is optimal over oral administration to bypass concerns of gastrointestinal malabsorption. Thiamine 100 mg by mouth daily for ongoing supplementation can be considered for patients who are at risk for WE. It is also important to recognize that magnesium and thiamine are intertwined in several key enzymatic pathways. To optimize the responsiveness of thiamine repletion, magnesium levels should be tested and repleted if low.
Application of the data to our patient
Nurses are able to frequently monitor the patient so he is started on symptom-triggered treatment with chlordiazepoxide using the CIWA protocol. This strategy will help limit the amount of benzodiazepines he receives and shorten his treatment duration. Given the ataxia, the patient is also started on high-dose IV thiamine three times a day to treat possible Wernicke’s encephalopathy. Gabapentin is added to his regimen to help manage his moderate alcohol withdrawal syndrome.
Bottom line
Long-acting benzodiazepines using symptom-triggered administration when feasible are the mainstay of treating alcohol withdrawal. Other medications such as gabapentin, carbamazepine, and phenobarbital can be considered as adjunctive agents. Given the high rate of thiamine deficiency and the low risk of overreplacement, intravenous thiamine can be considered for inpatients with AWS.
Dr. Agrawal, Dr. Chernyavsky, Dr. Dharapak, Dr. Grabscheid, Dr. Merrill, Dr. Pillay, and Dr. Rizk are hospitalists at Mount Sinai Beth Israel in New York.
References
1. CDC - Fact Sheets: “Alcohol Use And Health – Alcohol.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 3 Jan. 2018.
2. Rawlani V et al. Treatment of the hospitalized alcohol-dependent patient with alcohol withdrawal syndrome. Internet J Intern Med. 2008;8(1).
3. Grant BF et al. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757.
4. Wood E et al. Will this hospitalized patient develop severe alcohol withdrawal syndrome?: The Rational Clinical Examination Systematic Review. JAMA. 2018;320:825.
5. Sarkar S et al. Risk factors for the development of delirium in alcohol dependence syndrome: Clinical and neurobiological implications. Indian J Psychiatry. 2017 Jul-Sep;59(3):300-5.
6. Sachdeva A et al. Alcohol withdrawal syndrome: Benzodiazepines and beyond. J Clinical Diagn Res. 2015 Sep 9(9).
7. Sullivan JT et al. Benzodiazepine requirements during alcohol withdrawal syndrome: Clinical implications of using a standardized withdrawal scale. J Clin Psychopharmacol. 1991;11:291-5.
8. Saitz R et al. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA. 1994;272(7):519.
9. Nelson AC et al. Benzodiazepines vs. barbiturates for alcohol withdrawal: Analysis of 3 different treatment protocols. Am J Emerg Med. 2019 Jan 3.
10. Hammond DA et al. Patient outcomes associated with phenobarbital use with or without benzodiazepines for alcohol withdrawal syndrome: A systematic review. Hosp Pharm. 2017 Oct;52(9):607-16.
11. Mo Y et al. Current practice patterns in the management of alcohol withdrawal syndrome. P T. 2018 Mar;43(3):158-62.
12. Leung JG et al. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015 Aug;49(8):897-906.
13. Martin P et al. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003:27(2):134-42.
14. Flannery A et al. Unpeeling the evidence for the banana bag: Evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016 Aug:44(8):1545-52.
15. Harper CG et al. Clinical signs in Wernicke Korsakoff complex: A retrospective analysis of 131 cases diagnosed at autopsy. J Neurol Neurosurg Psychiatry. 1986;49(4):341-5.
Key points
- Alcohol use disorder and alcohol withdrawal are significant problems in hospitalized patients; early detection and treatment are crucial in preventing high morbidity and mortality.
- Long acting benzodiazepines with active metabolites such as chlordiazepoxide and diazepam are the preferred treatment for alcohol withdrawal except for patients with advanced liver disease or those prone to respiratory depression.
- Symptom-triggered therapy decreases the amount of medication, shortens treatment duration, and decreases inpatient length of stay, compared with fixed schedule dosing.
- Gabapentin may be effective in the treatment of mild to moderate AWS but cannot yet be routinely recommended as monotherapy in severe withdrawal, in patients with seizure history, or in patients who are at high risk for progression to delirium tremens.
- Thiamine deficiency is common in chronic alcohol use disorders; thiamine repletion should be considered for patients at risk or when Wernicke’s encephalopathy and Korsakoff’s syndrome are suspected.
Additional reading
1. Perry EC. Inpatient management of acute alcohol withdrawal syndrome. CNS Drugs. 2014;28(5):401-10.
2. Mayo-Smith MF. Pharmacological management of alcohol withdrawal: A meta-analysis and evidence-based practice guideline. JAMA. 1997;278(2):144-51.
3. Michael F. Mayo-Smith, MD, MPH et al. for the Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium: An evidence-based practice guideline. Arch Intern Med. 2004;164(13):1405-12.
Quiz
A 51-year-old female with a history of hypertension and continuous alcohol abuse presents to the hospital with fever and cough. She is found to have community-acquired pneumonia and is admitted for treatment. How else would you manage this patient?
A. Start scheduled benzodiazepines and oral thiamine.
B. Start CIWA protocol using a long-acting benzodiazepine and oral thiamine.
C. Start scheduled benzodiazepines and IV thiamine.
D. Start CIWA protocol using a long-acting benzodiazepine and consider IV or oral thiamine.
Answer: D. Symptom-triggered benzodiazepine therapy is favored as is consideration for thiamine repletion in the treatment of AWS.
Symptom-triggered therapy has multiple benefits
Symptom-triggered therapy has multiple benefits
Case
A 57-year-old man with a history of alcohol abuse (no history of seizures) presents to the ED “feeling awful.” He claims his last drink was 1 day prior. Initial vital signs are: T = 99.1°F, HR 102 bpm, BP 162/85 mm Hg, respirations 18/minute, and 99% oxygen saturation. He is tremulous, diaphoretic, and has an unsteady gait. What is the best way to manage his symptoms while hospitalized?
Brief overview of the issue
With over 15 million people with alcohol use disorder (AUD) in the United States alone, alcohol dependence and misuse remain significant issues among hospitalized patients.1 It is estimated that over 20% of admitted patients meet DSM-5 criteria for AUD and that over 2 million will withdraw each year.2,3 Acute withdrawal includes a spectrum of symptoms ranging from mild anxiety and diaphoresis to hallucinations, seizures, and delirium tremens. Onset of these symptoms ranges from 24 hours up to 5 days.
Severe alcohol withdrawal syndrome (SAWS) attributable to abrupt discontinuation of alcohol leads to increased morbidity and mortality; therefore, early detection and prevention in the acute care setting is critical. Several factors can help predict who may withdraw, and once detected, pharmacological treatment is necessary.4 Thorough evaluation and treatment can help reduce mortality from the most severe forms of alcohol withdrawal including delirium tremens, which has up to 40% mortality if left untreated.5
Overview of the data
How do we use benzodiazepines to treat alcohol withdrawal?
Benzodiazepines are the mainstay of alcohol withdrawal treatment. Benzodiazepines work by stimulating the gamma-aminobutyric acid (GABA) receptor resulting in a reduction of neuronal activity. This leads to a sedative effect and thus slows the progression of withdrawal symptoms.
Long-acting benzodiazepines, such as chlordiazepoxide and diazepam, are the preferred choices for most patients. Their active metabolites have a rapid onset of action and their long half-lives allow for a lower incidence of breakthrough symptoms and rebound phenomena such as seizures.6 Benzodiazepines with shorter half-lives, such as lorazepam and oxazepam, are preferred in patients with liver dysfunction and those prone to respiratory depression.
Intravenous administration has a rapid onset of action and is the standard administration route of choice in patients with acute severe withdrawal, delirium tremens, and seizure activity. In patients with mild withdrawal symptoms or those in the outpatient setting, oral administration is generally effective.6
The Clinical Institute Withdrawal Assessment (CIWA) is one commonly used titration model that requires calculation of a symptom-based withdrawal score. Data have consistently demonstrated that a symptom-triggered method results in administration of less total benzodiazepines over a significantly shorter duration, thereby reducing cost and duration of treatment and minimizing side effects. This regimen may also reduce the risk of undermedicating or overmedicating a patient since the dosing is based upon an individual’s symptoms.7,8
The efficacy of symptom-triggered regimens however, depends on the reliability and accuracy of the patient assessment. A fixed-interval benzodiazepine-dosing approach where benzodiazepines are administered regardless of symptoms is useful when frequent monitoring and reassessment are not feasible or are unreliable.
What about phenobarbital?
Phenobarbital has similar pharmacokinetics to the benzodiazepines frequently used for alcohol withdrawal, including simultaneous effects on gamma-aminobutyric acid (GABA) and N-methyl-D-aspartate (NMDA) receptors, and has been proposed as a treatment option for delirium tremens.
In 2019, as reported in the American Journal of Emergency Medicine, Nelson et al. found that incorporating phenobarbital into a benzodiazepine-based protocol or as sole agent led to similar rates of ICU admission, length of stay, and need for mechanical ventilation in patients treated for alcohol withdrawal in the emergency department.9 The authors concluded that “phenobarbital (was) a safe and effective treatment alternative for alcohol withdrawal.” The systematic review by Hammond et al. in 2017 found that phenobarbital, either as monotherapy or in conjunction with benzodiazepines, could have comparable or superior results in comparison to other treatments, including benzodiazepines monotherapy.10 Further studies are needed to determine dosing and the most effective way to incorporate the use of phenobarbital in treatment of Alcohol Withdrawal Syndrome (AWS).
Should gabapentin or any other medications be added to his treatment regimen?
Chronic alcohol use induces a reduction in GABA activity (the major inhibitory neurotransmitter in the brain) and alcohol cessation results in decreased inhibitory tone. This physiologic imbalance contributes to the syndrome of alcohol withdrawal. As such, gabapentin has emerged as a promising treatment option in AWS and may help reduce the need for benzodiazepines.
Gabapentin has few drug-drug interactions and is safe for use in patients with impaired liver function; however, dosage adjustment is required for renal dysfunction (CrCl less than 60 mL/min). Gabapentin’s neuroprotective effects may also help decrease the neurotoxic effects associated with AWS. Common side effects of gabapentin include dizziness, drowsiness, ataxia, diarrhea, nausea, and vomiting. The potential for misuse has been reported.
In several small studies, gabapentin monotherapy was found to be comparable to benzodiazepines in the treatment of mild to moderate AWS. Gabapentin is efficacious in reducing cravings as well as improving mood, anxiety, and sleep, and showed an advantage over benzodiazepines in preventing relapse with no difference in length of hospital stay.6,11 Given the small sample sizes of these studies and the differing methods, settings, and inclusion/ exclusion criteria used, the generalizability of these findings to patients with significant medical and/or psychiatric comorbidities remains limited. Additional studies are needed to standardize dosing protocols and treatment strategies for both inpatients and outpatients.
Alternative agents such as antipsychotics (e.g., haloperidol), centrally acting alpha-2 agonists (e.g., clonidine), beta-blockers, and an agonist of the GABA-B receptor (e.g., baclofen) may also attenuate the symptoms of withdrawal. Since these all have limited evidence of their efficacy and have potential for harm, such as masking symptoms of progressive withdrawal and lowering seizure threshold, these agents are not routinely recommended for use. Valproic acid/divalproex, levetiracetam, topiramate, and zonisamide have also showed some efficacy in reducing symptoms of alcohol withdrawal in limited studies. The data on prevention of withdrawal seizures or delirium tremens when used as monotherapy is less robust.12
A daily multivitamin and folate are ordered. What about thiamine? Does the route matter?
Alarmingly, 80% of people who chronically abuse alcohol are thiamine deficient.13 This deficiency is attributable to several factors including inadequate oral intake, malabsorption, and decreased cellular utilization. Thiamine is a crucial factor in multiple enzymatic and metabolic pathways. Its deficiency can lead to free radical production, neurotoxicity, impaired glucose metabolism, and ultimately, cell death.14 A clinical concern stemming from thiamine deficiency is the development of Wernicke’s encephalopathy (WE), which is potentially reversible with prompt recognition and treatment, in comparison to its irreversible amnestic sequela, Korsakoff’s syndrome.
Wernicke’s encephalopathy had been defined as a triad of ataxia, ophthalmoplegia, and global confusion. However, Harper et al. discovered that only 16% of patients presented with the classic triad and 19% had none of these signs.15 Diagnosis is clinical since thiamine serology results do not accurately represent brain storage.
Currently, there are no consistent guidelines regarding repletion of thiamine administration in the treatment or prevention of WE attributable to alcohol overuse. Thiamine has a safe toxicity profile as excess thiamine is excreted in the urine. Outside of rare reports of anaphylactoid reactions involving large parenteral doses, there is no concern for overtreatment. As Wernicke-Korsakoff syndrome is associated with significant morbidity and mortality, high doses such as 200 to 500 mg are recommended to ensure blood-brain barrier passage. The intravenous route is optimal over oral administration to bypass concerns of gastrointestinal malabsorption. Thiamine 100 mg by mouth daily for ongoing supplementation can be considered for patients who are at risk for WE. It is also important to recognize that magnesium and thiamine are intertwined in several key enzymatic pathways. To optimize the responsiveness of thiamine repletion, magnesium levels should be tested and repleted if low.
Application of the data to our patient
Nurses are able to frequently monitor the patient so he is started on symptom-triggered treatment with chlordiazepoxide using the CIWA protocol. This strategy will help limit the amount of benzodiazepines he receives and shorten his treatment duration. Given the ataxia, the patient is also started on high-dose IV thiamine three times a day to treat possible Wernicke’s encephalopathy. Gabapentin is added to his regimen to help manage his moderate alcohol withdrawal syndrome.
Bottom line
Long-acting benzodiazepines using symptom-triggered administration when feasible are the mainstay of treating alcohol withdrawal. Other medications such as gabapentin, carbamazepine, and phenobarbital can be considered as adjunctive agents. Given the high rate of thiamine deficiency and the low risk of overreplacement, intravenous thiamine can be considered for inpatients with AWS.
Dr. Agrawal, Dr. Chernyavsky, Dr. Dharapak, Dr. Grabscheid, Dr. Merrill, Dr. Pillay, and Dr. Rizk are hospitalists at Mount Sinai Beth Israel in New York.
References
1. CDC - Fact Sheets: “Alcohol Use And Health – Alcohol.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 3 Jan. 2018.
2. Rawlani V et al. Treatment of the hospitalized alcohol-dependent patient with alcohol withdrawal syndrome. Internet J Intern Med. 2008;8(1).
3. Grant BF et al. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757.
4. Wood E et al. Will this hospitalized patient develop severe alcohol withdrawal syndrome?: The Rational Clinical Examination Systematic Review. JAMA. 2018;320:825.
5. Sarkar S et al. Risk factors for the development of delirium in alcohol dependence syndrome: Clinical and neurobiological implications. Indian J Psychiatry. 2017 Jul-Sep;59(3):300-5.
6. Sachdeva A et al. Alcohol withdrawal syndrome: Benzodiazepines and beyond. J Clinical Diagn Res. 2015 Sep 9(9).
7. Sullivan JT et al. Benzodiazepine requirements during alcohol withdrawal syndrome: Clinical implications of using a standardized withdrawal scale. J Clin Psychopharmacol. 1991;11:291-5.
8. Saitz R et al. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA. 1994;272(7):519.
9. Nelson AC et al. Benzodiazepines vs. barbiturates for alcohol withdrawal: Analysis of 3 different treatment protocols. Am J Emerg Med. 2019 Jan 3.
10. Hammond DA et al. Patient outcomes associated with phenobarbital use with or without benzodiazepines for alcohol withdrawal syndrome: A systematic review. Hosp Pharm. 2017 Oct;52(9):607-16.
11. Mo Y et al. Current practice patterns in the management of alcohol withdrawal syndrome. P T. 2018 Mar;43(3):158-62.
12. Leung JG et al. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015 Aug;49(8):897-906.
13. Martin P et al. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003:27(2):134-42.
14. Flannery A et al. Unpeeling the evidence for the banana bag: Evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016 Aug:44(8):1545-52.
15. Harper CG et al. Clinical signs in Wernicke Korsakoff complex: A retrospective analysis of 131 cases diagnosed at autopsy. J Neurol Neurosurg Psychiatry. 1986;49(4):341-5.
Key points
- Alcohol use disorder and alcohol withdrawal are significant problems in hospitalized patients; early detection and treatment are crucial in preventing high morbidity and mortality.
- Long acting benzodiazepines with active metabolites such as chlordiazepoxide and diazepam are the preferred treatment for alcohol withdrawal except for patients with advanced liver disease or those prone to respiratory depression.
- Symptom-triggered therapy decreases the amount of medication, shortens treatment duration, and decreases inpatient length of stay, compared with fixed schedule dosing.
- Gabapentin may be effective in the treatment of mild to moderate AWS but cannot yet be routinely recommended as monotherapy in severe withdrawal, in patients with seizure history, or in patients who are at high risk for progression to delirium tremens.
- Thiamine deficiency is common in chronic alcohol use disorders; thiamine repletion should be considered for patients at risk or when Wernicke’s encephalopathy and Korsakoff’s syndrome are suspected.
Additional reading
1. Perry EC. Inpatient management of acute alcohol withdrawal syndrome. CNS Drugs. 2014;28(5):401-10.
2. Mayo-Smith MF. Pharmacological management of alcohol withdrawal: A meta-analysis and evidence-based practice guideline. JAMA. 1997;278(2):144-51.
3. Michael F. Mayo-Smith, MD, MPH et al. for the Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium: An evidence-based practice guideline. Arch Intern Med. 2004;164(13):1405-12.
Quiz
A 51-year-old female with a history of hypertension and continuous alcohol abuse presents to the hospital with fever and cough. She is found to have community-acquired pneumonia and is admitted for treatment. How else would you manage this patient?
A. Start scheduled benzodiazepines and oral thiamine.
B. Start CIWA protocol using a long-acting benzodiazepine and oral thiamine.
C. Start scheduled benzodiazepines and IV thiamine.
D. Start CIWA protocol using a long-acting benzodiazepine and consider IV or oral thiamine.
Answer: D. Symptom-triggered benzodiazepine therapy is favored as is consideration for thiamine repletion in the treatment of AWS.
Case
A 57-year-old man with a history of alcohol abuse (no history of seizures) presents to the ED “feeling awful.” He claims his last drink was 1 day prior. Initial vital signs are: T = 99.1°F, HR 102 bpm, BP 162/85 mm Hg, respirations 18/minute, and 99% oxygen saturation. He is tremulous, diaphoretic, and has an unsteady gait. What is the best way to manage his symptoms while hospitalized?
Brief overview of the issue
With over 15 million people with alcohol use disorder (AUD) in the United States alone, alcohol dependence and misuse remain significant issues among hospitalized patients.1 It is estimated that over 20% of admitted patients meet DSM-5 criteria for AUD and that over 2 million will withdraw each year.2,3 Acute withdrawal includes a spectrum of symptoms ranging from mild anxiety and diaphoresis to hallucinations, seizures, and delirium tremens. Onset of these symptoms ranges from 24 hours up to 5 days.
Severe alcohol withdrawal syndrome (SAWS) attributable to abrupt discontinuation of alcohol leads to increased morbidity and mortality; therefore, early detection and prevention in the acute care setting is critical. Several factors can help predict who may withdraw, and once detected, pharmacological treatment is necessary.4 Thorough evaluation and treatment can help reduce mortality from the most severe forms of alcohol withdrawal including delirium tremens, which has up to 40% mortality if left untreated.5
Overview of the data
How do we use benzodiazepines to treat alcohol withdrawal?
Benzodiazepines are the mainstay of alcohol withdrawal treatment. Benzodiazepines work by stimulating the gamma-aminobutyric acid (GABA) receptor resulting in a reduction of neuronal activity. This leads to a sedative effect and thus slows the progression of withdrawal symptoms.
Long-acting benzodiazepines, such as chlordiazepoxide and diazepam, are the preferred choices for most patients. Their active metabolites have a rapid onset of action and their long half-lives allow for a lower incidence of breakthrough symptoms and rebound phenomena such as seizures.6 Benzodiazepines with shorter half-lives, such as lorazepam and oxazepam, are preferred in patients with liver dysfunction and those prone to respiratory depression.
Intravenous administration has a rapid onset of action and is the standard administration route of choice in patients with acute severe withdrawal, delirium tremens, and seizure activity. In patients with mild withdrawal symptoms or those in the outpatient setting, oral administration is generally effective.6
The Clinical Institute Withdrawal Assessment (CIWA) is one commonly used titration model that requires calculation of a symptom-based withdrawal score. Data have consistently demonstrated that a symptom-triggered method results in administration of less total benzodiazepines over a significantly shorter duration, thereby reducing cost and duration of treatment and minimizing side effects. This regimen may also reduce the risk of undermedicating or overmedicating a patient since the dosing is based upon an individual’s symptoms.7,8
The efficacy of symptom-triggered regimens however, depends on the reliability and accuracy of the patient assessment. A fixed-interval benzodiazepine-dosing approach where benzodiazepines are administered regardless of symptoms is useful when frequent monitoring and reassessment are not feasible or are unreliable.
What about phenobarbital?
Phenobarbital has similar pharmacokinetics to the benzodiazepines frequently used for alcohol withdrawal, including simultaneous effects on gamma-aminobutyric acid (GABA) and N-methyl-D-aspartate (NMDA) receptors, and has been proposed as a treatment option for delirium tremens.
In 2019, as reported in the American Journal of Emergency Medicine, Nelson et al. found that incorporating phenobarbital into a benzodiazepine-based protocol or as sole agent led to similar rates of ICU admission, length of stay, and need for mechanical ventilation in patients treated for alcohol withdrawal in the emergency department.9 The authors concluded that “phenobarbital (was) a safe and effective treatment alternative for alcohol withdrawal.” The systematic review by Hammond et al. in 2017 found that phenobarbital, either as monotherapy or in conjunction with benzodiazepines, could have comparable or superior results in comparison to other treatments, including benzodiazepines monotherapy.10 Further studies are needed to determine dosing and the most effective way to incorporate the use of phenobarbital in treatment of Alcohol Withdrawal Syndrome (AWS).
Should gabapentin or any other medications be added to his treatment regimen?
Chronic alcohol use induces a reduction in GABA activity (the major inhibitory neurotransmitter in the brain) and alcohol cessation results in decreased inhibitory tone. This physiologic imbalance contributes to the syndrome of alcohol withdrawal. As such, gabapentin has emerged as a promising treatment option in AWS and may help reduce the need for benzodiazepines.
Gabapentin has few drug-drug interactions and is safe for use in patients with impaired liver function; however, dosage adjustment is required for renal dysfunction (CrCl less than 60 mL/min). Gabapentin’s neuroprotective effects may also help decrease the neurotoxic effects associated with AWS. Common side effects of gabapentin include dizziness, drowsiness, ataxia, diarrhea, nausea, and vomiting. The potential for misuse has been reported.
In several small studies, gabapentin monotherapy was found to be comparable to benzodiazepines in the treatment of mild to moderate AWS. Gabapentin is efficacious in reducing cravings as well as improving mood, anxiety, and sleep, and showed an advantage over benzodiazepines in preventing relapse with no difference in length of hospital stay.6,11 Given the small sample sizes of these studies and the differing methods, settings, and inclusion/ exclusion criteria used, the generalizability of these findings to patients with significant medical and/or psychiatric comorbidities remains limited. Additional studies are needed to standardize dosing protocols and treatment strategies for both inpatients and outpatients.
Alternative agents such as antipsychotics (e.g., haloperidol), centrally acting alpha-2 agonists (e.g., clonidine), beta-blockers, and an agonist of the GABA-B receptor (e.g., baclofen) may also attenuate the symptoms of withdrawal. Since these all have limited evidence of their efficacy and have potential for harm, such as masking symptoms of progressive withdrawal and lowering seizure threshold, these agents are not routinely recommended for use. Valproic acid/divalproex, levetiracetam, topiramate, and zonisamide have also showed some efficacy in reducing symptoms of alcohol withdrawal in limited studies. The data on prevention of withdrawal seizures or delirium tremens when used as monotherapy is less robust.12
A daily multivitamin and folate are ordered. What about thiamine? Does the route matter?
Alarmingly, 80% of people who chronically abuse alcohol are thiamine deficient.13 This deficiency is attributable to several factors including inadequate oral intake, malabsorption, and decreased cellular utilization. Thiamine is a crucial factor in multiple enzymatic and metabolic pathways. Its deficiency can lead to free radical production, neurotoxicity, impaired glucose metabolism, and ultimately, cell death.14 A clinical concern stemming from thiamine deficiency is the development of Wernicke’s encephalopathy (WE), which is potentially reversible with prompt recognition and treatment, in comparison to its irreversible amnestic sequela, Korsakoff’s syndrome.
Wernicke’s encephalopathy had been defined as a triad of ataxia, ophthalmoplegia, and global confusion. However, Harper et al. discovered that only 16% of patients presented with the classic triad and 19% had none of these signs.15 Diagnosis is clinical since thiamine serology results do not accurately represent brain storage.
Currently, there are no consistent guidelines regarding repletion of thiamine administration in the treatment or prevention of WE attributable to alcohol overuse. Thiamine has a safe toxicity profile as excess thiamine is excreted in the urine. Outside of rare reports of anaphylactoid reactions involving large parenteral doses, there is no concern for overtreatment. As Wernicke-Korsakoff syndrome is associated with significant morbidity and mortality, high doses such as 200 to 500 mg are recommended to ensure blood-brain barrier passage. The intravenous route is optimal over oral administration to bypass concerns of gastrointestinal malabsorption. Thiamine 100 mg by mouth daily for ongoing supplementation can be considered for patients who are at risk for WE. It is also important to recognize that magnesium and thiamine are intertwined in several key enzymatic pathways. To optimize the responsiveness of thiamine repletion, magnesium levels should be tested and repleted if low.
Application of the data to our patient
Nurses are able to frequently monitor the patient so he is started on symptom-triggered treatment with chlordiazepoxide using the CIWA protocol. This strategy will help limit the amount of benzodiazepines he receives and shorten his treatment duration. Given the ataxia, the patient is also started on high-dose IV thiamine three times a day to treat possible Wernicke’s encephalopathy. Gabapentin is added to his regimen to help manage his moderate alcohol withdrawal syndrome.
Bottom line
Long-acting benzodiazepines using symptom-triggered administration when feasible are the mainstay of treating alcohol withdrawal. Other medications such as gabapentin, carbamazepine, and phenobarbital can be considered as adjunctive agents. Given the high rate of thiamine deficiency and the low risk of overreplacement, intravenous thiamine can be considered for inpatients with AWS.
Dr. Agrawal, Dr. Chernyavsky, Dr. Dharapak, Dr. Grabscheid, Dr. Merrill, Dr. Pillay, and Dr. Rizk are hospitalists at Mount Sinai Beth Israel in New York.
References
1. CDC - Fact Sheets: “Alcohol Use And Health – Alcohol.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 3 Jan. 2018.
2. Rawlani V et al. Treatment of the hospitalized alcohol-dependent patient with alcohol withdrawal syndrome. Internet J Intern Med. 2008;8(1).
3. Grant BF et al. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757.
4. Wood E et al. Will this hospitalized patient develop severe alcohol withdrawal syndrome?: The Rational Clinical Examination Systematic Review. JAMA. 2018;320:825.
5. Sarkar S et al. Risk factors for the development of delirium in alcohol dependence syndrome: Clinical and neurobiological implications. Indian J Psychiatry. 2017 Jul-Sep;59(3):300-5.
6. Sachdeva A et al. Alcohol withdrawal syndrome: Benzodiazepines and beyond. J Clinical Diagn Res. 2015 Sep 9(9).
7. Sullivan JT et al. Benzodiazepine requirements during alcohol withdrawal syndrome: Clinical implications of using a standardized withdrawal scale. J Clin Psychopharmacol. 1991;11:291-5.
8. Saitz R et al. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA. 1994;272(7):519.
9. Nelson AC et al. Benzodiazepines vs. barbiturates for alcohol withdrawal: Analysis of 3 different treatment protocols. Am J Emerg Med. 2019 Jan 3.
10. Hammond DA et al. Patient outcomes associated with phenobarbital use with or without benzodiazepines for alcohol withdrawal syndrome: A systematic review. Hosp Pharm. 2017 Oct;52(9):607-16.
11. Mo Y et al. Current practice patterns in the management of alcohol withdrawal syndrome. P T. 2018 Mar;43(3):158-62.
12. Leung JG et al. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015 Aug;49(8):897-906.
13. Martin P et al. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003:27(2):134-42.
14. Flannery A et al. Unpeeling the evidence for the banana bag: Evidence-based recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU. Crit Care Med. 2016 Aug:44(8):1545-52.
15. Harper CG et al. Clinical signs in Wernicke Korsakoff complex: A retrospective analysis of 131 cases diagnosed at autopsy. J Neurol Neurosurg Psychiatry. 1986;49(4):341-5.
Key points
- Alcohol use disorder and alcohol withdrawal are significant problems in hospitalized patients; early detection and treatment are crucial in preventing high morbidity and mortality.
- Long acting benzodiazepines with active metabolites such as chlordiazepoxide and diazepam are the preferred treatment for alcohol withdrawal except for patients with advanced liver disease or those prone to respiratory depression.
- Symptom-triggered therapy decreases the amount of medication, shortens treatment duration, and decreases inpatient length of stay, compared with fixed schedule dosing.
- Gabapentin may be effective in the treatment of mild to moderate AWS but cannot yet be routinely recommended as monotherapy in severe withdrawal, in patients with seizure history, or in patients who are at high risk for progression to delirium tremens.
- Thiamine deficiency is common in chronic alcohol use disorders; thiamine repletion should be considered for patients at risk or when Wernicke’s encephalopathy and Korsakoff’s syndrome are suspected.
Additional reading
1. Perry EC. Inpatient management of acute alcohol withdrawal syndrome. CNS Drugs. 2014;28(5):401-10.
2. Mayo-Smith MF. Pharmacological management of alcohol withdrawal: A meta-analysis and evidence-based practice guideline. JAMA. 1997;278(2):144-51.
3. Michael F. Mayo-Smith, MD, MPH et al. for the Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium: An evidence-based practice guideline. Arch Intern Med. 2004;164(13):1405-12.
Quiz
A 51-year-old female with a history of hypertension and continuous alcohol abuse presents to the hospital with fever and cough. She is found to have community-acquired pneumonia and is admitted for treatment. How else would you manage this patient?
A. Start scheduled benzodiazepines and oral thiamine.
B. Start CIWA protocol using a long-acting benzodiazepine and oral thiamine.
C. Start scheduled benzodiazepines and IV thiamine.
D. Start CIWA protocol using a long-acting benzodiazepine and consider IV or oral thiamine.
Answer: D. Symptom-triggered benzodiazepine therapy is favored as is consideration for thiamine repletion in the treatment of AWS.
Hospital-acquired C. diff. tied to four ‘high-risk’ antibiotic classes
The use of four antibiotic classes designated “high risk” was found to be an independent predictor of hospital-acquired Clostridioides difficile (CDI), based upon an analysis of microbiologic and pharmacy data from 171 hospitals in the United States.
The high-risk antibiotic classes were second-, third-, and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and lincosamides, according to a report by Ying P. Tabak, PhD, of Becton Dickinson in Franklin Lakes, N.J., and colleagues published in Infection Control & Hospital Epidemiology.
Of the 171 study sites studied, 66 (39%) were teaching hospitals and 105 (61%) were nonteaching hospitals. The high-risk antibiotics most frequently used were cephalosporins (47.9%), fluoroquinolones (31.6%), carbapenems (13.0%), and lincosamides (7.6%). The sites were distributed across various regions of the United States. The hospital-level antibiotic use was measured as days of therapy (DOT) per 1,000 days present (DP).
The study was not able to determine specific links to individual antibiotic classes but to the use of high-risk antibiotics as a whole, except for cephalosporins, which were significantly correlated with hospital-acquired CDI (r = 0.23; P less than .01).
The overall correlation of high-risk antibiotic use and hospital-acquired CDI was 0.22 (P = .003). Higher correlation was observed in teaching hospitals (r = 0.38; P = .002) versus nonteaching hospitals (r = 0.19; P = .055), according to the researchers. The authors attributed this to the possibility of teaching hospitals dealing with more elderly and sicker patients.
After adjusting for significant confounders, the use of high-risk antibiotics was still independently associated with significant risk for hospital-acquired CDI. “For every 100-day increase of DOT per 1,000 DP in high-risk antibiotic use, there was a 12% increase in [hospital-acquired] CDI (RR, 1.12; 95% [confidence interval], 1.04-1.21; P = .002),” according to the authors. This translated to four additional hospital-acquired CDI cases with every 100 DOT increase per 1,000 DP.
“Using a large and current dataset, we found an independent impact of hospital-level high-risk antibiotic use on [hospital-acquired] CDI even after adjusting for confounding factors such as community CDI pressure, proportion of patients aged 65 years or older, average length of stay, and hospital teaching status,” the researchers concluded.
Funding was provided by Nabriva Therapeutics, an antibiotic development company. Four of the authors are full-time employees of Becton Dickinson, which sells diagnostics for infectious diseases, including CDI, and one author was an employee of Nabriva Therapeutics.
SOURCE: Tabak YP et al. Infect Control Hosp Epidemiol. 2019 Sep 16. doi: 10.1017/ice.2019.236.
The use of four antibiotic classes designated “high risk” was found to be an independent predictor of hospital-acquired Clostridioides difficile (CDI), based upon an analysis of microbiologic and pharmacy data from 171 hospitals in the United States.
The high-risk antibiotic classes were second-, third-, and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and lincosamides, according to a report by Ying P. Tabak, PhD, of Becton Dickinson in Franklin Lakes, N.J., and colleagues published in Infection Control & Hospital Epidemiology.
Of the 171 study sites studied, 66 (39%) were teaching hospitals and 105 (61%) were nonteaching hospitals. The high-risk antibiotics most frequently used were cephalosporins (47.9%), fluoroquinolones (31.6%), carbapenems (13.0%), and lincosamides (7.6%). The sites were distributed across various regions of the United States. The hospital-level antibiotic use was measured as days of therapy (DOT) per 1,000 days present (DP).
The study was not able to determine specific links to individual antibiotic classes but to the use of high-risk antibiotics as a whole, except for cephalosporins, which were significantly correlated with hospital-acquired CDI (r = 0.23; P less than .01).
The overall correlation of high-risk antibiotic use and hospital-acquired CDI was 0.22 (P = .003). Higher correlation was observed in teaching hospitals (r = 0.38; P = .002) versus nonteaching hospitals (r = 0.19; P = .055), according to the researchers. The authors attributed this to the possibility of teaching hospitals dealing with more elderly and sicker patients.
After adjusting for significant confounders, the use of high-risk antibiotics was still independently associated with significant risk for hospital-acquired CDI. “For every 100-day increase of DOT per 1,000 DP in high-risk antibiotic use, there was a 12% increase in [hospital-acquired] CDI (RR, 1.12; 95% [confidence interval], 1.04-1.21; P = .002),” according to the authors. This translated to four additional hospital-acquired CDI cases with every 100 DOT increase per 1,000 DP.
“Using a large and current dataset, we found an independent impact of hospital-level high-risk antibiotic use on [hospital-acquired] CDI even after adjusting for confounding factors such as community CDI pressure, proportion of patients aged 65 years or older, average length of stay, and hospital teaching status,” the researchers concluded.
Funding was provided by Nabriva Therapeutics, an antibiotic development company. Four of the authors are full-time employees of Becton Dickinson, which sells diagnostics for infectious diseases, including CDI, and one author was an employee of Nabriva Therapeutics.
SOURCE: Tabak YP et al. Infect Control Hosp Epidemiol. 2019 Sep 16. doi: 10.1017/ice.2019.236.
The use of four antibiotic classes designated “high risk” was found to be an independent predictor of hospital-acquired Clostridioides difficile (CDI), based upon an analysis of microbiologic and pharmacy data from 171 hospitals in the United States.
The high-risk antibiotic classes were second-, third-, and fourth-generation cephalosporins, fluoroquinolones, carbapenems, and lincosamides, according to a report by Ying P. Tabak, PhD, of Becton Dickinson in Franklin Lakes, N.J., and colleagues published in Infection Control & Hospital Epidemiology.
Of the 171 study sites studied, 66 (39%) were teaching hospitals and 105 (61%) were nonteaching hospitals. The high-risk antibiotics most frequently used were cephalosporins (47.9%), fluoroquinolones (31.6%), carbapenems (13.0%), and lincosamides (7.6%). The sites were distributed across various regions of the United States. The hospital-level antibiotic use was measured as days of therapy (DOT) per 1,000 days present (DP).
The study was not able to determine specific links to individual antibiotic classes but to the use of high-risk antibiotics as a whole, except for cephalosporins, which were significantly correlated with hospital-acquired CDI (r = 0.23; P less than .01).
The overall correlation of high-risk antibiotic use and hospital-acquired CDI was 0.22 (P = .003). Higher correlation was observed in teaching hospitals (r = 0.38; P = .002) versus nonteaching hospitals (r = 0.19; P = .055), according to the researchers. The authors attributed this to the possibility of teaching hospitals dealing with more elderly and sicker patients.
After adjusting for significant confounders, the use of high-risk antibiotics was still independently associated with significant risk for hospital-acquired CDI. “For every 100-day increase of DOT per 1,000 DP in high-risk antibiotic use, there was a 12% increase in [hospital-acquired] CDI (RR, 1.12; 95% [confidence interval], 1.04-1.21; P = .002),” according to the authors. This translated to four additional hospital-acquired CDI cases with every 100 DOT increase per 1,000 DP.
“Using a large and current dataset, we found an independent impact of hospital-level high-risk antibiotic use on [hospital-acquired] CDI even after adjusting for confounding factors such as community CDI pressure, proportion of patients aged 65 years or older, average length of stay, and hospital teaching status,” the researchers concluded.
Funding was provided by Nabriva Therapeutics, an antibiotic development company. Four of the authors are full-time employees of Becton Dickinson, which sells diagnostics for infectious diseases, including CDI, and one author was an employee of Nabriva Therapeutics.
SOURCE: Tabak YP et al. Infect Control Hosp Epidemiol. 2019 Sep 16. doi: 10.1017/ice.2019.236.
FROM INFECTION CONTROL & HOSPITAL EPIDEMIOLOGY
Key clinical point:
Major finding: For every 100-day increase in high-risk antibiotic therapy, there was a 12% increase in hospital-acquired C. difficile.
Study details: Microbiological and pharmacy data from 171 hospitals comparing hospitalwide use of four antibiotics classes on hospital-acquired C. difficile.
Disclosures: Funding was provided Nabriva Therapeutics, an antibiotic development company. Four of the authors are full-time employees of Becton Dickinson, which sells diagnostics for infectious diseases, including C. difficile, and one author was an employee of Nabriva Therapeutics.
Source: Tabak YP et al. Infect Control Hosp Epidemiol. 2019 Sep 16. doi: 10.1017/ice.2019.236.