User login
Rivaroxaban no help for heart failure outcomes
MUNICH – For patients with heart disease, coronary artery disease, and normal sinus rhythm, giving , investigators in the COMMANDER trial said.
Rivaroxaban did not improve rehospitalization rates either, reported lead author Faiez Zannad, MD, PhD, from the University of Henri Poincaré in Nancy, France, and his co-investigators.
“After an episode of worsening chronic heart failure, rates of readmission to the hospital and of death are high, especially in the first few months,” they said in a presentation at the annual congress of the European Society of Cardiology. The report of the research was published simultaneously in the New England Journal of Medicine.
Findings from previous research have suggested that, for patients with coronary artery disease, a combination of antiplatelet agents and low-dose rivaroxaban (2.5 mg twice daily) reduced incidence of death, myocardial infarction, and stroke. The authors designed the COMMANDER trial to test a similar regimen in patients with chronic heart failure and coronary heart disease without an arrhythmia. Results were published simultaneously in the New England Journal of Medicine.
The COMMANDER trial involved 5,022 patients with coronary artery disease, reduced left ventricular ejection fraction (less than or equal to 40%), worsening chronic heart failure (index event within past 21 days), and normal splasma concentration of brain natriuretic peptide (BNP) of at least 200 ng per liter or N-terminal pro-brain natriuretic peptide (NT-proBNP) of at least 800 ng per liter.
Patients were randomly assigned to receive rivaroxaban 2.5 mg twice daily (n = 2,507) or placebo (n = 2,515). Treatment was given in addition to standard care for coronary disease or heart failure (single or dual antiplatelet therapy was allowed). Patients were assessed at week 4 and week 12, then every 12 weeks.
The primary efficacy outcome was a composite of stroke, myocardial infarction, or death from any cause. Secondary efficacy outcomes included death from cardiovascular disease, rehospitalization for heart failure, a composite of either, or rehospitalization for cardiovascular events. The principal safety outcome was a composite of bleeding into a critical space with potential for permanent disability or fatal bleeding.
Death, myocardial failure, or stroke occurred in 626 patients (25%) in the rivaroxaban group compared with 658 patients (26.2%) in the placebo group (P = .27). Secondary efficacy outcomes were also highly similar between groups, differing at most by 0.9%. The principal safety outcome (fatal bleeding or bleeding into a critical space) occurred in 18 patients (0.7%) in the rivaroxaban group and 23 patients (0.9%) in the placebo group (P = .25). Again, no significant difference was found between groups.
These results suggest that while low-dose rivaroxaban may be safe, it also offers no treatment benefit. “The most likely reason for the failure … is that thrombin-mediated events are not the major driver of heart failure-related events in patients with recent hospitalization for heart failure,” the authors wrote.
“Whether a higher dose of rivaroxaban could have led to a more favorable outcome remains unknown,” they concluded.
The COMMANDER trial was funded by Janssen Research and Development. Authors reported compensation from Bayer, Servier, Novartis, Impulse Dynamics, and others.
SOURCE: Zannad F et al. NEJM/ESC.
.
MUNICH – For patients with heart disease, coronary artery disease, and normal sinus rhythm, giving , investigators in the COMMANDER trial said.
Rivaroxaban did not improve rehospitalization rates either, reported lead author Faiez Zannad, MD, PhD, from the University of Henri Poincaré in Nancy, France, and his co-investigators.
“After an episode of worsening chronic heart failure, rates of readmission to the hospital and of death are high, especially in the first few months,” they said in a presentation at the annual congress of the European Society of Cardiology. The report of the research was published simultaneously in the New England Journal of Medicine.
Findings from previous research have suggested that, for patients with coronary artery disease, a combination of antiplatelet agents and low-dose rivaroxaban (2.5 mg twice daily) reduced incidence of death, myocardial infarction, and stroke. The authors designed the COMMANDER trial to test a similar regimen in patients with chronic heart failure and coronary heart disease without an arrhythmia. Results were published simultaneously in the New England Journal of Medicine.
The COMMANDER trial involved 5,022 patients with coronary artery disease, reduced left ventricular ejection fraction (less than or equal to 40%), worsening chronic heart failure (index event within past 21 days), and normal splasma concentration of brain natriuretic peptide (BNP) of at least 200 ng per liter or N-terminal pro-brain natriuretic peptide (NT-proBNP) of at least 800 ng per liter.
Patients were randomly assigned to receive rivaroxaban 2.5 mg twice daily (n = 2,507) or placebo (n = 2,515). Treatment was given in addition to standard care for coronary disease or heart failure (single or dual antiplatelet therapy was allowed). Patients were assessed at week 4 and week 12, then every 12 weeks.
The primary efficacy outcome was a composite of stroke, myocardial infarction, or death from any cause. Secondary efficacy outcomes included death from cardiovascular disease, rehospitalization for heart failure, a composite of either, or rehospitalization for cardiovascular events. The principal safety outcome was a composite of bleeding into a critical space with potential for permanent disability or fatal bleeding.
Death, myocardial failure, or stroke occurred in 626 patients (25%) in the rivaroxaban group compared with 658 patients (26.2%) in the placebo group (P = .27). Secondary efficacy outcomes were also highly similar between groups, differing at most by 0.9%. The principal safety outcome (fatal bleeding or bleeding into a critical space) occurred in 18 patients (0.7%) in the rivaroxaban group and 23 patients (0.9%) in the placebo group (P = .25). Again, no significant difference was found between groups.
These results suggest that while low-dose rivaroxaban may be safe, it also offers no treatment benefit. “The most likely reason for the failure … is that thrombin-mediated events are not the major driver of heart failure-related events in patients with recent hospitalization for heart failure,” the authors wrote.
“Whether a higher dose of rivaroxaban could have led to a more favorable outcome remains unknown,” they concluded.
The COMMANDER trial was funded by Janssen Research and Development. Authors reported compensation from Bayer, Servier, Novartis, Impulse Dynamics, and others.
SOURCE: Zannad F et al. NEJM/ESC.
.
MUNICH – For patients with heart disease, coronary artery disease, and normal sinus rhythm, giving , investigators in the COMMANDER trial said.
Rivaroxaban did not improve rehospitalization rates either, reported lead author Faiez Zannad, MD, PhD, from the University of Henri Poincaré in Nancy, France, and his co-investigators.
“After an episode of worsening chronic heart failure, rates of readmission to the hospital and of death are high, especially in the first few months,” they said in a presentation at the annual congress of the European Society of Cardiology. The report of the research was published simultaneously in the New England Journal of Medicine.
Findings from previous research have suggested that, for patients with coronary artery disease, a combination of antiplatelet agents and low-dose rivaroxaban (2.5 mg twice daily) reduced incidence of death, myocardial infarction, and stroke. The authors designed the COMMANDER trial to test a similar regimen in patients with chronic heart failure and coronary heart disease without an arrhythmia. Results were published simultaneously in the New England Journal of Medicine.
The COMMANDER trial involved 5,022 patients with coronary artery disease, reduced left ventricular ejection fraction (less than or equal to 40%), worsening chronic heart failure (index event within past 21 days), and normal splasma concentration of brain natriuretic peptide (BNP) of at least 200 ng per liter or N-terminal pro-brain natriuretic peptide (NT-proBNP) of at least 800 ng per liter.
Patients were randomly assigned to receive rivaroxaban 2.5 mg twice daily (n = 2,507) or placebo (n = 2,515). Treatment was given in addition to standard care for coronary disease or heart failure (single or dual antiplatelet therapy was allowed). Patients were assessed at week 4 and week 12, then every 12 weeks.
The primary efficacy outcome was a composite of stroke, myocardial infarction, or death from any cause. Secondary efficacy outcomes included death from cardiovascular disease, rehospitalization for heart failure, a composite of either, or rehospitalization for cardiovascular events. The principal safety outcome was a composite of bleeding into a critical space with potential for permanent disability or fatal bleeding.
Death, myocardial failure, or stroke occurred in 626 patients (25%) in the rivaroxaban group compared with 658 patients (26.2%) in the placebo group (P = .27). Secondary efficacy outcomes were also highly similar between groups, differing at most by 0.9%. The principal safety outcome (fatal bleeding or bleeding into a critical space) occurred in 18 patients (0.7%) in the rivaroxaban group and 23 patients (0.9%) in the placebo group (P = .25). Again, no significant difference was found between groups.
These results suggest that while low-dose rivaroxaban may be safe, it also offers no treatment benefit. “The most likely reason for the failure … is that thrombin-mediated events are not the major driver of heart failure-related events in patients with recent hospitalization for heart failure,” the authors wrote.
“Whether a higher dose of rivaroxaban could have led to a more favorable outcome remains unknown,” they concluded.
The COMMANDER trial was funded by Janssen Research and Development. Authors reported compensation from Bayer, Servier, Novartis, Impulse Dynamics, and others.
SOURCE: Zannad F et al. NEJM/ESC.
.
Key clinical point: For patients with heart failure and coronary artery disease, rivaroxaban does not significantly reduce the risk of death, myocardial infarction, or stroke.
Major finding: Death, myocardial failure, or stroke occurred in 25.0% of patients in the rivaroxaban group compared with 26.2% of patients in the placebo group (P = .27).
Study details: The COMMANDER study was a double-blind, randomized trial involving 5,022 patients. Patients had heart failure, normal sinus rhythm, and coronary artery disease.
Disclosures: Funding was provided by Janssen Research and Development. Authors reported compensation from Bayer, Servier, Novartis, Impulse Dynamics, and others.
Source: Zannad F et al. NEJM/ESC.
VTE risk unchanged by rivaroxaban after discharge
For patients hospitalized for medical illness, giving rivaroxaban after discharge does not significantly reduce the risk of venous thromboembolism, investigators reported.
Previous research suggested that the risk of major bleeding from rivaroxaban outweighed its benefits; however, major bleeding was uncommon in the MARINER trial, reported lead author Alex C. Spyropoulos, MD, of Hofstra University in Hempstead, N.Y., and his colleagues.
“Patients who are hospitalized for acute medical illnesses, such as heart failure, respiratory insufficiency, stroke, and infectious or inflammatory diseases, are at increased risk for venous thromboembolism,” they wrote in the New England Journal of Medicine. The results were also presented at the annual congress of the European Society of Cardiology.
Although the increased risk of thromboembolism continues for at least 6 weeks after hospitalization, postdischarge anticoagulants, such as rivaroxaban, are controversial.
“Studies of extended thromboprophylaxis have shown either excess major bleeding or a benefit that is based mainly on reducing the risk of asymptomatic deep-vein thrombosis,” the investigators wrote.
The researchers aimed to clarify the benefits of rivaroxaban after hospitalization while modifying previous study regimens to limit major bleeding risk.
The double-blind MARINER study involved 12,019 patients who were hospitalized for medical illness and had an increased risk of venous thromboembolism. Hospitalization lasted 3-10 consecutive days. Sufficient risk of thromboembolism was defined by a modified International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) score of 4 or higher (range, 0-10), or an IMPROVE score of 2 or 3 with a plasma D-dimer measurement more than double the upper normal limit.
Patients were randomized to receive either 10 mg of rivaroxaban daily (n = 6,007) or placebo (n = 6,012) for 45 days after discharge. Patients with renal impairment had a reduced dose of 7.5 mg rivaroxaban.
A composite of symptomatic or fatal venous thromboembolism was the primary efficacy outcome. Major bleeding was the safety benchmark.
Efficacy was similar in both groups. Symptomatic or fatal venous thromboembolism occurred in 50 patients (0.83%) in the rivaroxaban group, compared with 66 patients (1.10%) in the placebo group (P = .14). These findings suggest that rivaroxaban provides a minor and insignificant benefit.
Although major bleeding was slightly more common in patients receiving rivaroxaban, compared with patients receiving placebo (0.28% vs. 0.15%), the researchers suggested that, in large populations, the marginal benefit of rivaroxaban might outweigh the increased bleeding risk. Still, the authors noted that “the usefulness of extended thromboprophylaxis remains uncertain.”
“Future studies should more accurately identify deaths caused by thrombotic mechanisms and focus on the patients who are at highest risk and who may benefit from anticoagulant prophylaxis,” the researchers wrote.
Funding was provided by Janssen Research and Development. Most of the study authors reported fees or grants from Janssen during the study, and relationships with other companies outside of the submitted work.
SOURCE : Spyropoulos AC et al. N Engl J Med. 2018 Aug 26. doi: 10.1056/NEJMoa1805090.
For patients hospitalized for medical illness, giving rivaroxaban after discharge does not significantly reduce the risk of venous thromboembolism, investigators reported.
Previous research suggested that the risk of major bleeding from rivaroxaban outweighed its benefits; however, major bleeding was uncommon in the MARINER trial, reported lead author Alex C. Spyropoulos, MD, of Hofstra University in Hempstead, N.Y., and his colleagues.
“Patients who are hospitalized for acute medical illnesses, such as heart failure, respiratory insufficiency, stroke, and infectious or inflammatory diseases, are at increased risk for venous thromboembolism,” they wrote in the New England Journal of Medicine. The results were also presented at the annual congress of the European Society of Cardiology.
Although the increased risk of thromboembolism continues for at least 6 weeks after hospitalization, postdischarge anticoagulants, such as rivaroxaban, are controversial.
“Studies of extended thromboprophylaxis have shown either excess major bleeding or a benefit that is based mainly on reducing the risk of asymptomatic deep-vein thrombosis,” the investigators wrote.
The researchers aimed to clarify the benefits of rivaroxaban after hospitalization while modifying previous study regimens to limit major bleeding risk.
The double-blind MARINER study involved 12,019 patients who were hospitalized for medical illness and had an increased risk of venous thromboembolism. Hospitalization lasted 3-10 consecutive days. Sufficient risk of thromboembolism was defined by a modified International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) score of 4 or higher (range, 0-10), or an IMPROVE score of 2 or 3 with a plasma D-dimer measurement more than double the upper normal limit.
Patients were randomized to receive either 10 mg of rivaroxaban daily (n = 6,007) or placebo (n = 6,012) for 45 days after discharge. Patients with renal impairment had a reduced dose of 7.5 mg rivaroxaban.
A composite of symptomatic or fatal venous thromboembolism was the primary efficacy outcome. Major bleeding was the safety benchmark.
Efficacy was similar in both groups. Symptomatic or fatal venous thromboembolism occurred in 50 patients (0.83%) in the rivaroxaban group, compared with 66 patients (1.10%) in the placebo group (P = .14). These findings suggest that rivaroxaban provides a minor and insignificant benefit.
Although major bleeding was slightly more common in patients receiving rivaroxaban, compared with patients receiving placebo (0.28% vs. 0.15%), the researchers suggested that, in large populations, the marginal benefit of rivaroxaban might outweigh the increased bleeding risk. Still, the authors noted that “the usefulness of extended thromboprophylaxis remains uncertain.”
“Future studies should more accurately identify deaths caused by thrombotic mechanisms and focus on the patients who are at highest risk and who may benefit from anticoagulant prophylaxis,” the researchers wrote.
Funding was provided by Janssen Research and Development. Most of the study authors reported fees or grants from Janssen during the study, and relationships with other companies outside of the submitted work.
SOURCE : Spyropoulos AC et al. N Engl J Med. 2018 Aug 26. doi: 10.1056/NEJMoa1805090.
For patients hospitalized for medical illness, giving rivaroxaban after discharge does not significantly reduce the risk of venous thromboembolism, investigators reported.
Previous research suggested that the risk of major bleeding from rivaroxaban outweighed its benefits; however, major bleeding was uncommon in the MARINER trial, reported lead author Alex C. Spyropoulos, MD, of Hofstra University in Hempstead, N.Y., and his colleagues.
“Patients who are hospitalized for acute medical illnesses, such as heart failure, respiratory insufficiency, stroke, and infectious or inflammatory diseases, are at increased risk for venous thromboembolism,” they wrote in the New England Journal of Medicine. The results were also presented at the annual congress of the European Society of Cardiology.
Although the increased risk of thromboembolism continues for at least 6 weeks after hospitalization, postdischarge anticoagulants, such as rivaroxaban, are controversial.
“Studies of extended thromboprophylaxis have shown either excess major bleeding or a benefit that is based mainly on reducing the risk of asymptomatic deep-vein thrombosis,” the investigators wrote.
The researchers aimed to clarify the benefits of rivaroxaban after hospitalization while modifying previous study regimens to limit major bleeding risk.
The double-blind MARINER study involved 12,019 patients who were hospitalized for medical illness and had an increased risk of venous thromboembolism. Hospitalization lasted 3-10 consecutive days. Sufficient risk of thromboembolism was defined by a modified International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) score of 4 or higher (range, 0-10), or an IMPROVE score of 2 or 3 with a plasma D-dimer measurement more than double the upper normal limit.
Patients were randomized to receive either 10 mg of rivaroxaban daily (n = 6,007) or placebo (n = 6,012) for 45 days after discharge. Patients with renal impairment had a reduced dose of 7.5 mg rivaroxaban.
A composite of symptomatic or fatal venous thromboembolism was the primary efficacy outcome. Major bleeding was the safety benchmark.
Efficacy was similar in both groups. Symptomatic or fatal venous thromboembolism occurred in 50 patients (0.83%) in the rivaroxaban group, compared with 66 patients (1.10%) in the placebo group (P = .14). These findings suggest that rivaroxaban provides a minor and insignificant benefit.
Although major bleeding was slightly more common in patients receiving rivaroxaban, compared with patients receiving placebo (0.28% vs. 0.15%), the researchers suggested that, in large populations, the marginal benefit of rivaroxaban might outweigh the increased bleeding risk. Still, the authors noted that “the usefulness of extended thromboprophylaxis remains uncertain.”
“Future studies should more accurately identify deaths caused by thrombotic mechanisms and focus on the patients who are at highest risk and who may benefit from anticoagulant prophylaxis,” the researchers wrote.
Funding was provided by Janssen Research and Development. Most of the study authors reported fees or grants from Janssen during the study, and relationships with other companies outside of the submitted work.
SOURCE : Spyropoulos AC et al. N Engl J Med. 2018 Aug 26. doi: 10.1056/NEJMoa1805090.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point:
Major finding: Symptomatic or fatal venous thromboembolism occurred in 0.83% of patients given rivaroxaban, compared with 1.10% of patients given placebo (P = .14).
Study details: The MARINER study was a double-blind, randomized trial involving 12,019 patients. Patients were recently hospitalized for medical illness and had an increased risk of venous thromboembolism.
Disclosures: Funding was provided by Janssen Research and Development. Most of the study authors reported fees or grants from Janssen during the study, and relationships with other companies outside of the submitted work.
Source: Spyropoulos AC et al. N Engl J Med. 2018 Aug 26. doi: 10.1056/NEJMoa1805090.
Variety is the spice of hospital medicine
Dr. Raj Sehgal enjoys a variety of roles
Unlike some children who wanted be firefighters or astronauts when they grew up, ever since Raj Sehgal, MD, FHM, was a boy, he dreamed of being a doctor.
Since earning his medical degree, Dr. Sehgal has kept himself involved in a wide variety of projects, driven by the desire to diversify his expertise.
Currently a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System, San Antonio, and University of Texas Health San Antonio, Dr. Sehgal has found his place as an educator as well as a clinician, earning the Division of Hospital Medicine Teaching Award in 2016.
As a member of the The Hospitalist’s volunteer editorial advisory board, Dr. Sehgal enjoys helping to educate and inform fellow hospitalists. He spoke with The Hospitalist to tell us more about himself.
How did you get into medicine?
I don’t know how old I was when I decided I was going to be a doctor, but it was at a very young age and I never really wavered in that desire. I guess I also would have wanted to be a baseball player or a musician, but I never had the talents for those, so it was doctor. That’s always what I was thinking of doing, straight through high school and college, and then after college I took a year off and joined AmeriCorps. I spent a year there and then went to medical school in Dallas at UT Southwestern. After medical school, I thought I should go somewhere as different from Dallas as possible, so I went to Portland, Ore., for my residency and then a fellowship in general internal medicine.
How did you end up in hospital medicine?
When I was doing my residency, I always enjoyed being a generalist. A lot of different areas of medicine interested me, but I like the breadth of things you encounter as a generalist, so I could never picture myself being a subspecialist, doing the same things every day, seeing the same things. I knew I wanted to keep practicing general internal medicine, so I took a fellowship where I was working both inpatient and outpatient, and when I was looking for a job, I sought out things that involved some inpatient and some outpatient work. It turned out hospital medicine was the best fit.
What would you say is your favorite part of hospital medicine?
My favorite part of the job is getting to teach, working with medical students and residents. I also like the variety of what I do as a hospitalist, so I’m about 50% clinical and the rest of the time I perform a variety of tasks, both administrative and educational.
What about your least favorite part of hospitalist work?
Sometimes, particularly if you’re doing clinical, educational, and administrative work, it can be a little overwhelming to try and do a little bit of everything. I think generally that’s a good thing, but sometimes it can feel like a little too much.
What is some of the best advice you have received regarding how to handle the stresses of hospital medicine?
Feel free to say no to things. When hospitalists are starting their careers, and particularly when they are new to a job and trying to express their desire to get involved, sometimes they can have too much thrown at them at once. People can get overloaded very quickly, so I think feeling like you’re able to say no to some requests, or to take some time to think before you accept an additional role. The other piece of advice I remember from my fellowship, is that, when you do something, make it count twice. For example, if you’re involved in a project, you get the practical clinical or educational benefits of whatever the project was. But also think about how you might write about your experience for research purposes, such as for a poster, article, or other presentation.
What is the worst advice you have been given?
I think it’s not necessarily bad advice, but I guess it’s advice that I haven’t really followed. Since I work in academic medicine, I’ve found that the people in academics fall into one of two categories: There are the people who find their niche and remain on that path, and they’re very clear about it and don’t really stray from it; and there are people who don’t find that niche right away. I think the advice I received when starting out was to try to find that niche, and if you’re building an academic career it is very helpful to have these things in which you have become the expert. But I’ve just tried to go where the job takes me. I don’t necessarily have a single academic niche or something that I spend all my time doing, but I do have my hand in a lot of different things. To me, that’s a lot more interesting because it adds to the variety of what you’re doing. Every day is a little different.
What else do you do professionally outside of hospital medicine?
I actually practice a little outpatient medicine. When I first started here, I wanted to keep some outpatient experience, and so I actually created my own clinic. It’s a procedure clinic where I do paracentesis on people who have cirrhosis. Then on the educational side, I sit on the admission committees for the medical school here, so I get to look through the applicants and choose who we interview, and then once we interview candidates, I help choose how we rank students for admission.
Where do you see yourself in the next 10 years?
I’ve never been one who looks at a particular job and says ‘Okay, I want to be the dean or have this position.’ I guess I just hope I’m better at the things I’m currently doing. I hope in 10 years that I’m a better teacher, that I’ll have learned more strategies to help more people, and that I have a better handle on the administrative side of the work. I hope I’ve progressed to a point in my career where I’m doing an even better job than I am now.
What are your goals as a member of the editorial board?
I have an interest not only in medicine but also in writing; I’ve gotten to do some writing both medical and nonmedical in the past. I’ve published a few articles in The Hospitalist, and hopefully, I can do more of that because writing is just another part of education.
Dr. Raj Sehgal enjoys a variety of roles
Dr. Raj Sehgal enjoys a variety of roles
Unlike some children who wanted be firefighters or astronauts when they grew up, ever since Raj Sehgal, MD, FHM, was a boy, he dreamed of being a doctor.
Since earning his medical degree, Dr. Sehgal has kept himself involved in a wide variety of projects, driven by the desire to diversify his expertise.
Currently a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System, San Antonio, and University of Texas Health San Antonio, Dr. Sehgal has found his place as an educator as well as a clinician, earning the Division of Hospital Medicine Teaching Award in 2016.
As a member of the The Hospitalist’s volunteer editorial advisory board, Dr. Sehgal enjoys helping to educate and inform fellow hospitalists. He spoke with The Hospitalist to tell us more about himself.
How did you get into medicine?
I don’t know how old I was when I decided I was going to be a doctor, but it was at a very young age and I never really wavered in that desire. I guess I also would have wanted to be a baseball player or a musician, but I never had the talents for those, so it was doctor. That’s always what I was thinking of doing, straight through high school and college, and then after college I took a year off and joined AmeriCorps. I spent a year there and then went to medical school in Dallas at UT Southwestern. After medical school, I thought I should go somewhere as different from Dallas as possible, so I went to Portland, Ore., for my residency and then a fellowship in general internal medicine.
How did you end up in hospital medicine?
When I was doing my residency, I always enjoyed being a generalist. A lot of different areas of medicine interested me, but I like the breadth of things you encounter as a generalist, so I could never picture myself being a subspecialist, doing the same things every day, seeing the same things. I knew I wanted to keep practicing general internal medicine, so I took a fellowship where I was working both inpatient and outpatient, and when I was looking for a job, I sought out things that involved some inpatient and some outpatient work. It turned out hospital medicine was the best fit.
What would you say is your favorite part of hospital medicine?
My favorite part of the job is getting to teach, working with medical students and residents. I also like the variety of what I do as a hospitalist, so I’m about 50% clinical and the rest of the time I perform a variety of tasks, both administrative and educational.
What about your least favorite part of hospitalist work?
Sometimes, particularly if you’re doing clinical, educational, and administrative work, it can be a little overwhelming to try and do a little bit of everything. I think generally that’s a good thing, but sometimes it can feel like a little too much.
What is some of the best advice you have received regarding how to handle the stresses of hospital medicine?
Feel free to say no to things. When hospitalists are starting their careers, and particularly when they are new to a job and trying to express their desire to get involved, sometimes they can have too much thrown at them at once. People can get overloaded very quickly, so I think feeling like you’re able to say no to some requests, or to take some time to think before you accept an additional role. The other piece of advice I remember from my fellowship, is that, when you do something, make it count twice. For example, if you’re involved in a project, you get the practical clinical or educational benefits of whatever the project was. But also think about how you might write about your experience for research purposes, such as for a poster, article, or other presentation.
What is the worst advice you have been given?
I think it’s not necessarily bad advice, but I guess it’s advice that I haven’t really followed. Since I work in academic medicine, I’ve found that the people in academics fall into one of two categories: There are the people who find their niche and remain on that path, and they’re very clear about it and don’t really stray from it; and there are people who don’t find that niche right away. I think the advice I received when starting out was to try to find that niche, and if you’re building an academic career it is very helpful to have these things in which you have become the expert. But I’ve just tried to go where the job takes me. I don’t necessarily have a single academic niche or something that I spend all my time doing, but I do have my hand in a lot of different things. To me, that’s a lot more interesting because it adds to the variety of what you’re doing. Every day is a little different.
What else do you do professionally outside of hospital medicine?
I actually practice a little outpatient medicine. When I first started here, I wanted to keep some outpatient experience, and so I actually created my own clinic. It’s a procedure clinic where I do paracentesis on people who have cirrhosis. Then on the educational side, I sit on the admission committees for the medical school here, so I get to look through the applicants and choose who we interview, and then once we interview candidates, I help choose how we rank students for admission.
Where do you see yourself in the next 10 years?
I’ve never been one who looks at a particular job and says ‘Okay, I want to be the dean or have this position.’ I guess I just hope I’m better at the things I’m currently doing. I hope in 10 years that I’m a better teacher, that I’ll have learned more strategies to help more people, and that I have a better handle on the administrative side of the work. I hope I’ve progressed to a point in my career where I’m doing an even better job than I am now.
What are your goals as a member of the editorial board?
I have an interest not only in medicine but also in writing; I’ve gotten to do some writing both medical and nonmedical in the past. I’ve published a few articles in The Hospitalist, and hopefully, I can do more of that because writing is just another part of education.
Unlike some children who wanted be firefighters or astronauts when they grew up, ever since Raj Sehgal, MD, FHM, was a boy, he dreamed of being a doctor.
Since earning his medical degree, Dr. Sehgal has kept himself involved in a wide variety of projects, driven by the desire to diversify his expertise.
Currently a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System, San Antonio, and University of Texas Health San Antonio, Dr. Sehgal has found his place as an educator as well as a clinician, earning the Division of Hospital Medicine Teaching Award in 2016.
As a member of the The Hospitalist’s volunteer editorial advisory board, Dr. Sehgal enjoys helping to educate and inform fellow hospitalists. He spoke with The Hospitalist to tell us more about himself.
How did you get into medicine?
I don’t know how old I was when I decided I was going to be a doctor, but it was at a very young age and I never really wavered in that desire. I guess I also would have wanted to be a baseball player or a musician, but I never had the talents for those, so it was doctor. That’s always what I was thinking of doing, straight through high school and college, and then after college I took a year off and joined AmeriCorps. I spent a year there and then went to medical school in Dallas at UT Southwestern. After medical school, I thought I should go somewhere as different from Dallas as possible, so I went to Portland, Ore., for my residency and then a fellowship in general internal medicine.
How did you end up in hospital medicine?
When I was doing my residency, I always enjoyed being a generalist. A lot of different areas of medicine interested me, but I like the breadth of things you encounter as a generalist, so I could never picture myself being a subspecialist, doing the same things every day, seeing the same things. I knew I wanted to keep practicing general internal medicine, so I took a fellowship where I was working both inpatient and outpatient, and when I was looking for a job, I sought out things that involved some inpatient and some outpatient work. It turned out hospital medicine was the best fit.
What would you say is your favorite part of hospital medicine?
My favorite part of the job is getting to teach, working with medical students and residents. I also like the variety of what I do as a hospitalist, so I’m about 50% clinical and the rest of the time I perform a variety of tasks, both administrative and educational.
What about your least favorite part of hospitalist work?
Sometimes, particularly if you’re doing clinical, educational, and administrative work, it can be a little overwhelming to try and do a little bit of everything. I think generally that’s a good thing, but sometimes it can feel like a little too much.
What is some of the best advice you have received regarding how to handle the stresses of hospital medicine?
Feel free to say no to things. When hospitalists are starting their careers, and particularly when they are new to a job and trying to express their desire to get involved, sometimes they can have too much thrown at them at once. People can get overloaded very quickly, so I think feeling like you’re able to say no to some requests, or to take some time to think before you accept an additional role. The other piece of advice I remember from my fellowship, is that, when you do something, make it count twice. For example, if you’re involved in a project, you get the practical clinical or educational benefits of whatever the project was. But also think about how you might write about your experience for research purposes, such as for a poster, article, or other presentation.
What is the worst advice you have been given?
I think it’s not necessarily bad advice, but I guess it’s advice that I haven’t really followed. Since I work in academic medicine, I’ve found that the people in academics fall into one of two categories: There are the people who find their niche and remain on that path, and they’re very clear about it and don’t really stray from it; and there are people who don’t find that niche right away. I think the advice I received when starting out was to try to find that niche, and if you’re building an academic career it is very helpful to have these things in which you have become the expert. But I’ve just tried to go where the job takes me. I don’t necessarily have a single academic niche or something that I spend all my time doing, but I do have my hand in a lot of different things. To me, that’s a lot more interesting because it adds to the variety of what you’re doing. Every day is a little different.
What else do you do professionally outside of hospital medicine?
I actually practice a little outpatient medicine. When I first started here, I wanted to keep some outpatient experience, and so I actually created my own clinic. It’s a procedure clinic where I do paracentesis on people who have cirrhosis. Then on the educational side, I sit on the admission committees for the medical school here, so I get to look through the applicants and choose who we interview, and then once we interview candidates, I help choose how we rank students for admission.
Where do you see yourself in the next 10 years?
I’ve never been one who looks at a particular job and says ‘Okay, I want to be the dean or have this position.’ I guess I just hope I’m better at the things I’m currently doing. I hope in 10 years that I’m a better teacher, that I’ll have learned more strategies to help more people, and that I have a better handle on the administrative side of the work. I hope I’ve progressed to a point in my career where I’m doing an even better job than I am now.
What are your goals as a member of the editorial board?
I have an interest not only in medicine but also in writing; I’ve gotten to do some writing both medical and nonmedical in the past. I’ve published a few articles in The Hospitalist, and hopefully, I can do more of that because writing is just another part of education.
Dr. Eric Howell joins SHM as chief operating officer
Veteran hospitalist will help define organizational goals
The Society of Hospital Medicine has announced the appointment of Eric Howell, MD, MHM, to the position of chief operating officer (COO).
“Having been involved with SHM in many capacities since first joining, I am honored to now transition to chief operating officer,” Dr. Howell said. “I always tell everyone that my goal is to make the world a better place, and I know that SHM’s staff will be able to do just that through the development and deployment of a variety of products, tools, and services to help hospitalists improve patient care.”
In his new role as COO at SHM, Dr. Howell will lead senior management’s strategic planning as well as define organizational goals to drive extensive, sustainable growth. In addition to serving as SHM’s COO, Dr. Howell will continue his role as director of the hospital medicine division of Johns Hopkins Bayview Medical Center in Baltimore and professor of medicine in the department of medicine at Johns Hopkins University, also in Baltimore. Dr. Howell joined the Johns Hopkins Bayview hospitalist program in 2000, began the Howard County (Md.) General Hospital hospitalist program in 2010, and now oversees more than 200 physicians and clinical staff providing patient care in three hospitals.
“Eric has the perfect background to take SHM, its staff, and its membership to the next level,” said Laurence Wellikson, MD, MHM, chief executive officer of SHM. “His foundational leadership in the hospital medicine movement makes him the ideal person to lead SHM forward in its quest to provide hospitalists with the tools necessary to make a noteworthy difference in their institutions and in the lives of their patients.”
Dr. Howell is also a past president of SHM, the course director for the SHM Leadership Academies, and most recently, served as the senior physician advisor to SHM’s Center for Quality Improvement, which conducts quality improvement programs for hospitalist teams. He received his electrical engineering degree from the University of Maryland, which he said has served as an instrumental piece of his background for managing and implementing change in the hospital. His research has focused on the relationship between the emergency department and medicine floors, improving communication, throughput, and patient outcomes.
Veteran hospitalist will help define organizational goals
Veteran hospitalist will help define organizational goals
The Society of Hospital Medicine has announced the appointment of Eric Howell, MD, MHM, to the position of chief operating officer (COO).
“Having been involved with SHM in many capacities since first joining, I am honored to now transition to chief operating officer,” Dr. Howell said. “I always tell everyone that my goal is to make the world a better place, and I know that SHM’s staff will be able to do just that through the development and deployment of a variety of products, tools, and services to help hospitalists improve patient care.”
In his new role as COO at SHM, Dr. Howell will lead senior management’s strategic planning as well as define organizational goals to drive extensive, sustainable growth. In addition to serving as SHM’s COO, Dr. Howell will continue his role as director of the hospital medicine division of Johns Hopkins Bayview Medical Center in Baltimore and professor of medicine in the department of medicine at Johns Hopkins University, also in Baltimore. Dr. Howell joined the Johns Hopkins Bayview hospitalist program in 2000, began the Howard County (Md.) General Hospital hospitalist program in 2010, and now oversees more than 200 physicians and clinical staff providing patient care in three hospitals.
“Eric has the perfect background to take SHM, its staff, and its membership to the next level,” said Laurence Wellikson, MD, MHM, chief executive officer of SHM. “His foundational leadership in the hospital medicine movement makes him the ideal person to lead SHM forward in its quest to provide hospitalists with the tools necessary to make a noteworthy difference in their institutions and in the lives of their patients.”
Dr. Howell is also a past president of SHM, the course director for the SHM Leadership Academies, and most recently, served as the senior physician advisor to SHM’s Center for Quality Improvement, which conducts quality improvement programs for hospitalist teams. He received his electrical engineering degree from the University of Maryland, which he said has served as an instrumental piece of his background for managing and implementing change in the hospital. His research has focused on the relationship between the emergency department and medicine floors, improving communication, throughput, and patient outcomes.
The Society of Hospital Medicine has announced the appointment of Eric Howell, MD, MHM, to the position of chief operating officer (COO).
“Having been involved with SHM in many capacities since first joining, I am honored to now transition to chief operating officer,” Dr. Howell said. “I always tell everyone that my goal is to make the world a better place, and I know that SHM’s staff will be able to do just that through the development and deployment of a variety of products, tools, and services to help hospitalists improve patient care.”
In his new role as COO at SHM, Dr. Howell will lead senior management’s strategic planning as well as define organizational goals to drive extensive, sustainable growth. In addition to serving as SHM’s COO, Dr. Howell will continue his role as director of the hospital medicine division of Johns Hopkins Bayview Medical Center in Baltimore and professor of medicine in the department of medicine at Johns Hopkins University, also in Baltimore. Dr. Howell joined the Johns Hopkins Bayview hospitalist program in 2000, began the Howard County (Md.) General Hospital hospitalist program in 2010, and now oversees more than 200 physicians and clinical staff providing patient care in three hospitals.
“Eric has the perfect background to take SHM, its staff, and its membership to the next level,” said Laurence Wellikson, MD, MHM, chief executive officer of SHM. “His foundational leadership in the hospital medicine movement makes him the ideal person to lead SHM forward in its quest to provide hospitalists with the tools necessary to make a noteworthy difference in their institutions and in the lives of their patients.”
Dr. Howell is also a past president of SHM, the course director for the SHM Leadership Academies, and most recently, served as the senior physician advisor to SHM’s Center for Quality Improvement, which conducts quality improvement programs for hospitalist teams. He received his electrical engineering degree from the University of Maryland, which he said has served as an instrumental piece of his background for managing and implementing change in the hospital. His research has focused on the relationship between the emergency department and medicine floors, improving communication, throughput, and patient outcomes.
Replacing warfarin with a NOAC in patients on chronic anticoagulation therapy
Hospitalists must consider clinical factors and patient preferences
Case
A 70-year old woman with hypertension, diabetes, nonischemic stroke, moderate renal insufficiency (creatinine clearance [CrCl] 45 mL/min), heart failure, and nonvalvular atrial fibrillation (AF) on warfarin is admitted because of a very supratherapeutic INR. She reports labile INR values despite strict adherence to her medication regimen. Her cancer screening tests had previously been unremarkable. She inquires about the risks and benefits of switching to a novel oral anticoagulant (NOAC) as advertised on television. Should you consider it while she is still in the hospital?
Brief overview of the issue
Lifelong anticoagulation therapy is common among patients with AF or recurrent venous thromboembolism (VTE). Until the advent of NOACs, a great majority of patients were prescribed warfarin, the oral vitamin K antagonist that requires regular blood tests for monitoring of the INR. In contrast to warfarin, NOACs are direct-acting agents (hence also known as “direct oral anticoagulants” or DOACs) that are selective for one specific coagulation factor, either thrombin (e.g., dabigatran) or factor Xa (e.g., rivaroxaban, apixaban, and edoxaban, all with an “X” in their names).
NOACS have been studied and approved by the Food and Drug Administration for nonvalvular AF, i.e., patients without rheumatic mitral stenosis, mechanical or bioprosthetic heart valve, or prior mitral valve repair. Compared to warfarin, NOACS have fewer drug or food interactions, have more predictable pharmacokinetics, and may be associated with reduced risk of major bleeding depending on the agent. The latter is a particularly attractive feature of NOAC therapy, especially when its use is considered among older patients at risk of intracranial hemorrhage (ICH), such as those with previous strokes, ICH, or reduced renal function. Unfortunately, data on the efficacy and safety of the use of NOACs in certain patient populations (e.g., those with severe renal insufficiency, active malignancy, the elderly, patients with suboptimal medication adherence) are generally lacking.
Overview of the data
There are no randomized controlled trials (RCTs) addressing the clinical benefits of switching from warfarin to NOAC therapy. However, based on a number of RCTs comparing warfarin to individual NOACs and their related meta-analyses, the following conclusions may be made about their attributes:
1. Noninferiority to warfarin in reducing the risk of ischemic stroke in AF.
2. Association with a lower rate of major bleeds (statistically significant or trend) and a lower rate of ICH and hemorrhagic strokes compared to warfarin.
3. Association with a higher rate of gastrointestinal bleeding compared to warfarin (except for apixaban, low-dose dabigatran, and edoxaban1).
4. Association with a decreased rate of all stroke and thromboembolism events compared to warfarin.
5. Association with a slightly decreased all-cause mortality in AF compared to warfarin in many studies,2-8 but not all.1,9
6. Noninferiority to warfarin in all-cause mortality in patients with VTE and for its secondary prevention.1,4
NOACS should be used with caution or avoided altogether in patients with severe liver disease or renal insufficiency (see Table 1). 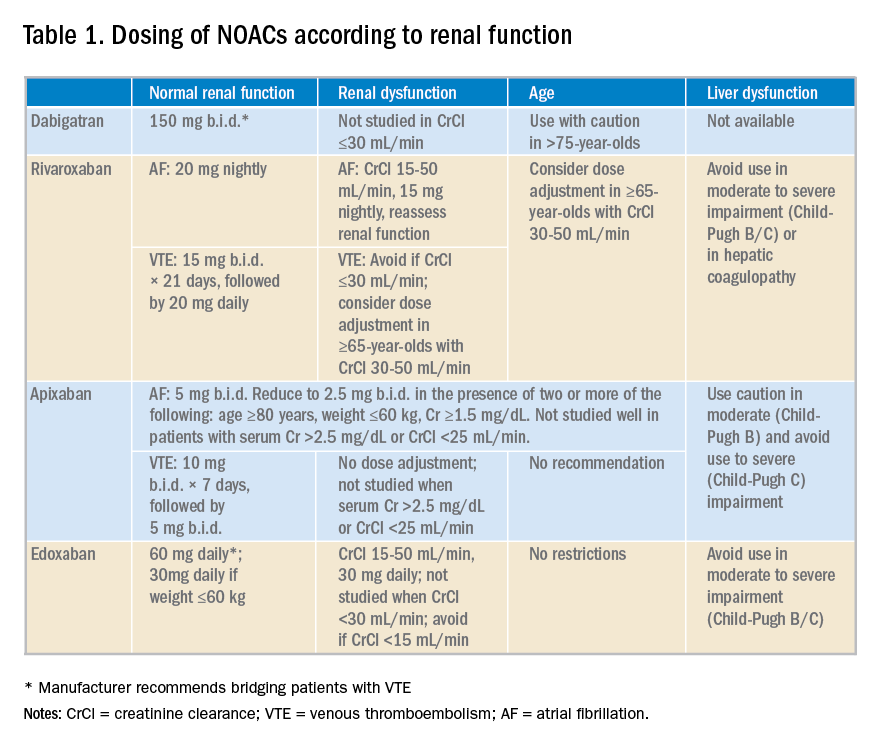
Potential advantages and disadvantages of NOAC therapy are listed in Table 2.
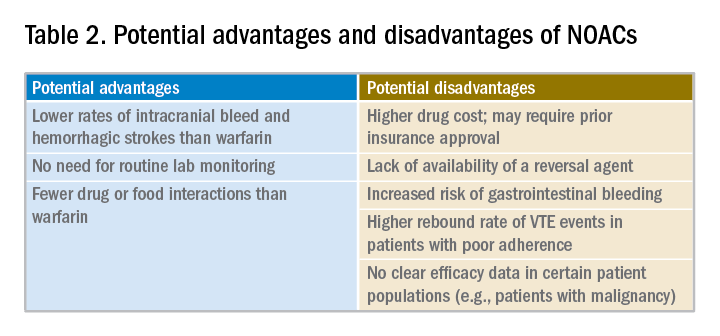
It should be emphasized that in patients with cancer or hypercoagulable state, no clear efficacy or safety data are currently available for the use of NOACs.
The 2016 CHEST guideline on antithrombotic therapy for VTE recommends NOACs over warfarin.10 The 2012 European Society of Cardiology AF guidelines also recommend NOACs over warfarin.11 However, the 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines on AF state that it is not necessary to change to a NOAC when patients are “stable, easily controlled, and satisfied with warfarin therapy.”12
Data from a relatively small, short-term study examining the safety of switching patients from warfarin to a NOAC suggest that although bleeding events are relatively common (12%) following such a switch, major bleeding and cardiac or cerebrovascular events are rare.10
Application of the data to our original case
Given a high calculated CHADS2VASC score of 8 in our patient, she has a clear indication for anticoagulation for AF. Her history of labile INRs, ischemic stroke, and moderate renal insufficiency place her at high risk for ICH.
A NOAC may reduce this risk but possibly at the expense of an increased risk for a gastrointestinal bleed. More importantly, however, she may be a good candidate for a switch to a NOAC because of her labile INRs despite good medication adherence. Her warfarin can be held while hospitalized and a NOAC may be initiated when the INR falls below 2.
Prior to discharge, potential cost of the drug to the patient should be explored and discussed. It is also important to involve the primary care physician in the decision-making process. Ultimately, selection of an appropriate NOAC should be based on a careful review of its risks and benefits, clinical factors, patient preference, and shared decision making.
Bottom line
Hospitalists are in a great position to discuss a switch to a NOAC in selected patients with history of good medication adherence and labile INRs or ICH risk factors.
Dr. Geisler, Dr. Liao, and Dr. Manian are hospitalists at Massachusetts General Hospital in Boston.
References
1. Sharma M et al. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: Systematic review and meta-analysis. Circulation. 2015;132(3):194-204.
2. Ruff CT et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet. 2014;383(9921):955-62.
3. Dentali F et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: A systematic review and meta-analysis of the literature. Circulation. 2012;126(20):2381-91.
4. Adam SS et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: A systematic review. Ann Intern Med. 2012;157(11):796-807.
5. Bruins Slot KM and Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev. 2013(8):CD008980.
6. Gomez-Outes A et al. Dabigatran, rivaroxaban, or apixaban versus warfarin in patients with nonvalvular atrial fibrillation: A systematic review and meta-analysis of subgroups. Thrombosis. 2013;2013:640723.
7. Miller CS et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110(3):453-60.
8. Baker WL and Phung OJ. Systematic review and adjusted indirect comparison meta-analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5(5):711-19.
9. Ntaios G et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: A systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43(12):3298-304.
10. Kearon C et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-52.
11. Camm AJ et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation – developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14(10):1385-413.
12. January CT et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-267.
Quiz
When considering a switch from warfarin to a NOAC, all the following factors should be considered a potential advantage, except:
A. No need for routing lab monitoring.
B. Lower risk of gastrointestinal bleeding.
C. Fewer drug interactions.
D. Lower rates of intracranial bleed and hemorrhagic stroke.
The correct answer is B. NOACs have been associated with lower risk of intracranial bleed and hemorrhagic stroke but not gastrointestinal bleed. Routine lab monitoring is not necessary during their use and they are associated with fewer drug interactions compared to warfarin.
Key Points
- NOACs represent a clear advancement in our anticoagulation armamentarium.
- Potential advantages of their use include lower rates of intracranial bleed and hemorrhagic strokes, fewer drug or food interactions, and lack of need for routing lab monitoring.
- Potential disadvantages of their use include increased rates of gastrointestinal bleed with some agents, general lack of availability of reversal agents, higher drug cost, unsuitability in patients with poor medication compliance, and lack of efficacy data in certain patient populations.
- Decision to switch from warfarin to a NOAC should thoroughly consider its pros and cons, clinical factors, and patient preferences.
Hospitalists must consider clinical factors and patient preferences
Hospitalists must consider clinical factors and patient preferences
Case
A 70-year old woman with hypertension, diabetes, nonischemic stroke, moderate renal insufficiency (creatinine clearance [CrCl] 45 mL/min), heart failure, and nonvalvular atrial fibrillation (AF) on warfarin is admitted because of a very supratherapeutic INR. She reports labile INR values despite strict adherence to her medication regimen. Her cancer screening tests had previously been unremarkable. She inquires about the risks and benefits of switching to a novel oral anticoagulant (NOAC) as advertised on television. Should you consider it while she is still in the hospital?
Brief overview of the issue
Lifelong anticoagulation therapy is common among patients with AF or recurrent venous thromboembolism (VTE). Until the advent of NOACs, a great majority of patients were prescribed warfarin, the oral vitamin K antagonist that requires regular blood tests for monitoring of the INR. In contrast to warfarin, NOACs are direct-acting agents (hence also known as “direct oral anticoagulants” or DOACs) that are selective for one specific coagulation factor, either thrombin (e.g., dabigatran) or factor Xa (e.g., rivaroxaban, apixaban, and edoxaban, all with an “X” in their names).
NOACS have been studied and approved by the Food and Drug Administration for nonvalvular AF, i.e., patients without rheumatic mitral stenosis, mechanical or bioprosthetic heart valve, or prior mitral valve repair. Compared to warfarin, NOACS have fewer drug or food interactions, have more predictable pharmacokinetics, and may be associated with reduced risk of major bleeding depending on the agent. The latter is a particularly attractive feature of NOAC therapy, especially when its use is considered among older patients at risk of intracranial hemorrhage (ICH), such as those with previous strokes, ICH, or reduced renal function. Unfortunately, data on the efficacy and safety of the use of NOACs in certain patient populations (e.g., those with severe renal insufficiency, active malignancy, the elderly, patients with suboptimal medication adherence) are generally lacking.
Overview of the data
There are no randomized controlled trials (RCTs) addressing the clinical benefits of switching from warfarin to NOAC therapy. However, based on a number of RCTs comparing warfarin to individual NOACs and their related meta-analyses, the following conclusions may be made about their attributes:
1. Noninferiority to warfarin in reducing the risk of ischemic stroke in AF.
2. Association with a lower rate of major bleeds (statistically significant or trend) and a lower rate of ICH and hemorrhagic strokes compared to warfarin.
3. Association with a higher rate of gastrointestinal bleeding compared to warfarin (except for apixaban, low-dose dabigatran, and edoxaban1).
4. Association with a decreased rate of all stroke and thromboembolism events compared to warfarin.
5. Association with a slightly decreased all-cause mortality in AF compared to warfarin in many studies,2-8 but not all.1,9
6. Noninferiority to warfarin in all-cause mortality in patients with VTE and for its secondary prevention.1,4
NOACS should be used with caution or avoided altogether in patients with severe liver disease or renal insufficiency (see Table 1). 
Potential advantages and disadvantages of NOAC therapy are listed in Table 2.

It should be emphasized that in patients with cancer or hypercoagulable state, no clear efficacy or safety data are currently available for the use of NOACs.
The 2016 CHEST guideline on antithrombotic therapy for VTE recommends NOACs over warfarin.10 The 2012 European Society of Cardiology AF guidelines also recommend NOACs over warfarin.11 However, the 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines on AF state that it is not necessary to change to a NOAC when patients are “stable, easily controlled, and satisfied with warfarin therapy.”12
Data from a relatively small, short-term study examining the safety of switching patients from warfarin to a NOAC suggest that although bleeding events are relatively common (12%) following such a switch, major bleeding and cardiac or cerebrovascular events are rare.10
Application of the data to our original case
Given a high calculated CHADS2VASC score of 8 in our patient, she has a clear indication for anticoagulation for AF. Her history of labile INRs, ischemic stroke, and moderate renal insufficiency place her at high risk for ICH.
A NOAC may reduce this risk but possibly at the expense of an increased risk for a gastrointestinal bleed. More importantly, however, she may be a good candidate for a switch to a NOAC because of her labile INRs despite good medication adherence. Her warfarin can be held while hospitalized and a NOAC may be initiated when the INR falls below 2.
Prior to discharge, potential cost of the drug to the patient should be explored and discussed. It is also important to involve the primary care physician in the decision-making process. Ultimately, selection of an appropriate NOAC should be based on a careful review of its risks and benefits, clinical factors, patient preference, and shared decision making.
Bottom line
Hospitalists are in a great position to discuss a switch to a NOAC in selected patients with history of good medication adherence and labile INRs or ICH risk factors.
Dr. Geisler, Dr. Liao, and Dr. Manian are hospitalists at Massachusetts General Hospital in Boston.
References
1. Sharma M et al. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: Systematic review and meta-analysis. Circulation. 2015;132(3):194-204.
2. Ruff CT et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet. 2014;383(9921):955-62.
3. Dentali F et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: A systematic review and meta-analysis of the literature. Circulation. 2012;126(20):2381-91.
4. Adam SS et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: A systematic review. Ann Intern Med. 2012;157(11):796-807.
5. Bruins Slot KM and Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev. 2013(8):CD008980.
6. Gomez-Outes A et al. Dabigatran, rivaroxaban, or apixaban versus warfarin in patients with nonvalvular atrial fibrillation: A systematic review and meta-analysis of subgroups. Thrombosis. 2013;2013:640723.
7. Miller CS et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110(3):453-60.
8. Baker WL and Phung OJ. Systematic review and adjusted indirect comparison meta-analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5(5):711-19.
9. Ntaios G et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: A systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43(12):3298-304.
10. Kearon C et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-52.
11. Camm AJ et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation – developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14(10):1385-413.
12. January CT et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-267.
Quiz
When considering a switch from warfarin to a NOAC, all the following factors should be considered a potential advantage, except:
A. No need for routing lab monitoring.
B. Lower risk of gastrointestinal bleeding.
C. Fewer drug interactions.
D. Lower rates of intracranial bleed and hemorrhagic stroke.
The correct answer is B. NOACs have been associated with lower risk of intracranial bleed and hemorrhagic stroke but not gastrointestinal bleed. Routine lab monitoring is not necessary during their use and they are associated with fewer drug interactions compared to warfarin.
Key Points
- NOACs represent a clear advancement in our anticoagulation armamentarium.
- Potential advantages of their use include lower rates of intracranial bleed and hemorrhagic strokes, fewer drug or food interactions, and lack of need for routing lab monitoring.
- Potential disadvantages of their use include increased rates of gastrointestinal bleed with some agents, general lack of availability of reversal agents, higher drug cost, unsuitability in patients with poor medication compliance, and lack of efficacy data in certain patient populations.
- Decision to switch from warfarin to a NOAC should thoroughly consider its pros and cons, clinical factors, and patient preferences.
Case
A 70-year old woman with hypertension, diabetes, nonischemic stroke, moderate renal insufficiency (creatinine clearance [CrCl] 45 mL/min), heart failure, and nonvalvular atrial fibrillation (AF) on warfarin is admitted because of a very supratherapeutic INR. She reports labile INR values despite strict adherence to her medication regimen. Her cancer screening tests had previously been unremarkable. She inquires about the risks and benefits of switching to a novel oral anticoagulant (NOAC) as advertised on television. Should you consider it while she is still in the hospital?
Brief overview of the issue
Lifelong anticoagulation therapy is common among patients with AF or recurrent venous thromboembolism (VTE). Until the advent of NOACs, a great majority of patients were prescribed warfarin, the oral vitamin K antagonist that requires regular blood tests for monitoring of the INR. In contrast to warfarin, NOACs are direct-acting agents (hence also known as “direct oral anticoagulants” or DOACs) that are selective for one specific coagulation factor, either thrombin (e.g., dabigatran) or factor Xa (e.g., rivaroxaban, apixaban, and edoxaban, all with an “X” in their names).
NOACS have been studied and approved by the Food and Drug Administration for nonvalvular AF, i.e., patients without rheumatic mitral stenosis, mechanical or bioprosthetic heart valve, or prior mitral valve repair. Compared to warfarin, NOACS have fewer drug or food interactions, have more predictable pharmacokinetics, and may be associated with reduced risk of major bleeding depending on the agent. The latter is a particularly attractive feature of NOAC therapy, especially when its use is considered among older patients at risk of intracranial hemorrhage (ICH), such as those with previous strokes, ICH, or reduced renal function. Unfortunately, data on the efficacy and safety of the use of NOACs in certain patient populations (e.g., those with severe renal insufficiency, active malignancy, the elderly, patients with suboptimal medication adherence) are generally lacking.
Overview of the data
There are no randomized controlled trials (RCTs) addressing the clinical benefits of switching from warfarin to NOAC therapy. However, based on a number of RCTs comparing warfarin to individual NOACs and their related meta-analyses, the following conclusions may be made about their attributes:
1. Noninferiority to warfarin in reducing the risk of ischemic stroke in AF.
2. Association with a lower rate of major bleeds (statistically significant or trend) and a lower rate of ICH and hemorrhagic strokes compared to warfarin.
3. Association with a higher rate of gastrointestinal bleeding compared to warfarin (except for apixaban, low-dose dabigatran, and edoxaban1).
4. Association with a decreased rate of all stroke and thromboembolism events compared to warfarin.
5. Association with a slightly decreased all-cause mortality in AF compared to warfarin in many studies,2-8 but not all.1,9
6. Noninferiority to warfarin in all-cause mortality in patients with VTE and for its secondary prevention.1,4
NOACS should be used with caution or avoided altogether in patients with severe liver disease or renal insufficiency (see Table 1). 
Potential advantages and disadvantages of NOAC therapy are listed in Table 2.

It should be emphasized that in patients with cancer or hypercoagulable state, no clear efficacy or safety data are currently available for the use of NOACs.
The 2016 CHEST guideline on antithrombotic therapy for VTE recommends NOACs over warfarin.10 The 2012 European Society of Cardiology AF guidelines also recommend NOACs over warfarin.11 However, the 2014 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines on AF state that it is not necessary to change to a NOAC when patients are “stable, easily controlled, and satisfied with warfarin therapy.”12
Data from a relatively small, short-term study examining the safety of switching patients from warfarin to a NOAC suggest that although bleeding events are relatively common (12%) following such a switch, major bleeding and cardiac or cerebrovascular events are rare.10
Application of the data to our original case
Given a high calculated CHADS2VASC score of 8 in our patient, she has a clear indication for anticoagulation for AF. Her history of labile INRs, ischemic stroke, and moderate renal insufficiency place her at high risk for ICH.
A NOAC may reduce this risk but possibly at the expense of an increased risk for a gastrointestinal bleed. More importantly, however, she may be a good candidate for a switch to a NOAC because of her labile INRs despite good medication adherence. Her warfarin can be held while hospitalized and a NOAC may be initiated when the INR falls below 2.
Prior to discharge, potential cost of the drug to the patient should be explored and discussed. It is also important to involve the primary care physician in the decision-making process. Ultimately, selection of an appropriate NOAC should be based on a careful review of its risks and benefits, clinical factors, patient preference, and shared decision making.
Bottom line
Hospitalists are in a great position to discuss a switch to a NOAC in selected patients with history of good medication adherence and labile INRs or ICH risk factors.
Dr. Geisler, Dr. Liao, and Dr. Manian are hospitalists at Massachusetts General Hospital in Boston.
References
1. Sharma M et al. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: Systematic review and meta-analysis. Circulation. 2015;132(3):194-204.
2. Ruff CT et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet. 2014;383(9921):955-62.
3. Dentali F et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: A systematic review and meta-analysis of the literature. Circulation. 2012;126(20):2381-91.
4. Adam SS et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: A systematic review. Ann Intern Med. 2012;157(11):796-807.
5. Bruins Slot KM and Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev. 2013(8):CD008980.
6. Gomez-Outes A et al. Dabigatran, rivaroxaban, or apixaban versus warfarin in patients with nonvalvular atrial fibrillation: A systematic review and meta-analysis of subgroups. Thrombosis. 2013;2013:640723.
7. Miller CS et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110(3):453-60.
8. Baker WL and Phung OJ. Systematic review and adjusted indirect comparison meta-analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5(5):711-19.
9. Ntaios G et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: A systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43(12):3298-304.
10. Kearon C et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-52.
11. Camm AJ et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation – developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14(10):1385-413.
12. January CT et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-267.
Quiz
When considering a switch from warfarin to a NOAC, all the following factors should be considered a potential advantage, except:
A. No need for routing lab monitoring.
B. Lower risk of gastrointestinal bleeding.
C. Fewer drug interactions.
D. Lower rates of intracranial bleed and hemorrhagic stroke.
The correct answer is B. NOACs have been associated with lower risk of intracranial bleed and hemorrhagic stroke but not gastrointestinal bleed. Routine lab monitoring is not necessary during their use and they are associated with fewer drug interactions compared to warfarin.
Key Points
- NOACs represent a clear advancement in our anticoagulation armamentarium.
- Potential advantages of their use include lower rates of intracranial bleed and hemorrhagic strokes, fewer drug or food interactions, and lack of need for routing lab monitoring.
- Potential disadvantages of their use include increased rates of gastrointestinal bleed with some agents, general lack of availability of reversal agents, higher drug cost, unsuitability in patients with poor medication compliance, and lack of efficacy data in certain patient populations.
- Decision to switch from warfarin to a NOAC should thoroughly consider its pros and cons, clinical factors, and patient preferences.
New Valsalva maneuver for SVT beats all others
NEW ORLEANS – , according to Jeet Mehta, MD, a resident in the combined medicine/pediatrics program at the University of Kansas, Wichita.
The 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommended vagal maneuvers as first-line treatment of supraventricular tachycardia, but added that there was no gold standard method. Since then, the situation has changed. Two well-conducted randomized clinical trials have been published that bring clarity as to the vagal maneuver of choice, Dr. Mehta reported at the annual meeting of the American College of Physicians.
He and his coinvestigators performed a meta-analysis of the three pre-2000 randomized controlled trials that compared the standard Valsalva maneuver to carotid sinus massage plus the two newer studies, both of which systematically compared a modified Valsalva maneuver with the standard version.
The clear winner in terms of efficacy was the modified Valsalva maneuver, in which patients with supraventricular tachycardia (SVT) performed a standardized strain while in a semirecumbent position, then immediately laid flat and had their legs raised to 45 degrees for 15 seconds before returning to the semirecumbent position. The purpose of this postural modification is to boost relaxation phase venous return and vagal stimulation.
In the 433-patient multicenter REVERT trial in the United Kingdom, 43% of those assigned to the modified Valsalva maneuver returned to sinus rhythm 1 minute after completing the task, compared with 17% of those randomized to the standard semirecumbent Valsalva maneuver. This resulted in significantly less need for adenosine and other treatments. Although REVERT investigators had the patients blow into a manometer at 40 mm Hg for 15 seconds, they noted that the same intensity of strain can be achieved more practically by blowing into a 10-mL syringe sufficient to just move the plunger (Lancet. 2015 Oct 31;386[10005]:1747-53).
The REVERT findings were confirmed by a second trial conducted by Turkish investigators, in which the modified Valsalva maneuver was successful in 43% of patients, compared with 11% in the standard Valsalva maneuver group (Am J Emerg Med. 2017 Nov;35[11]:1662-5).
Extrapolating from the published evidence, including a Cochrane Collaboration review (Cochrane Database Syst Rev. 2015 Feb 18;[2]:CD009502. doi: 10.1002/14651858.CD009502.pub3), Dr. Mehta and his coinvestigators ranked the likelihood of successful conversion of SVT to sinus rhythm from a high of 48% for the modified Valsalva maneuver, descending to 43% for a supine Valsalva maneuver, 36% for a standard semirecumbent Valsalva, 21% for a seated Valsalva, 19% for a standing one, and just 11% for carotid sinus massage.
“Based on evidence of high quality, we encourage that the modified Valsalva maneuver be done due its safety and low cost,” Dr. Mehta concluded.
He reported having no financial conflicts regarding his study, conducted free of commercial support.
NEW ORLEANS – , according to Jeet Mehta, MD, a resident in the combined medicine/pediatrics program at the University of Kansas, Wichita.
The 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommended vagal maneuvers as first-line treatment of supraventricular tachycardia, but added that there was no gold standard method. Since then, the situation has changed. Two well-conducted randomized clinical trials have been published that bring clarity as to the vagal maneuver of choice, Dr. Mehta reported at the annual meeting of the American College of Physicians.
He and his coinvestigators performed a meta-analysis of the three pre-2000 randomized controlled trials that compared the standard Valsalva maneuver to carotid sinus massage plus the two newer studies, both of which systematically compared a modified Valsalva maneuver with the standard version.
The clear winner in terms of efficacy was the modified Valsalva maneuver, in which patients with supraventricular tachycardia (SVT) performed a standardized strain while in a semirecumbent position, then immediately laid flat and had their legs raised to 45 degrees for 15 seconds before returning to the semirecumbent position. The purpose of this postural modification is to boost relaxation phase venous return and vagal stimulation.
In the 433-patient multicenter REVERT trial in the United Kingdom, 43% of those assigned to the modified Valsalva maneuver returned to sinus rhythm 1 minute after completing the task, compared with 17% of those randomized to the standard semirecumbent Valsalva maneuver. This resulted in significantly less need for adenosine and other treatments. Although REVERT investigators had the patients blow into a manometer at 40 mm Hg for 15 seconds, they noted that the same intensity of strain can be achieved more practically by blowing into a 10-mL syringe sufficient to just move the plunger (Lancet. 2015 Oct 31;386[10005]:1747-53).
The REVERT findings were confirmed by a second trial conducted by Turkish investigators, in which the modified Valsalva maneuver was successful in 43% of patients, compared with 11% in the standard Valsalva maneuver group (Am J Emerg Med. 2017 Nov;35[11]:1662-5).
Extrapolating from the published evidence, including a Cochrane Collaboration review (Cochrane Database Syst Rev. 2015 Feb 18;[2]:CD009502. doi: 10.1002/14651858.CD009502.pub3), Dr. Mehta and his coinvestigators ranked the likelihood of successful conversion of SVT to sinus rhythm from a high of 48% for the modified Valsalva maneuver, descending to 43% for a supine Valsalva maneuver, 36% for a standard semirecumbent Valsalva, 21% for a seated Valsalva, 19% for a standing one, and just 11% for carotid sinus massage.
“Based on evidence of high quality, we encourage that the modified Valsalva maneuver be done due its safety and low cost,” Dr. Mehta concluded.
He reported having no financial conflicts regarding his study, conducted free of commercial support.
NEW ORLEANS – , according to Jeet Mehta, MD, a resident in the combined medicine/pediatrics program at the University of Kansas, Wichita.
The 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommended vagal maneuvers as first-line treatment of supraventricular tachycardia, but added that there was no gold standard method. Since then, the situation has changed. Two well-conducted randomized clinical trials have been published that bring clarity as to the vagal maneuver of choice, Dr. Mehta reported at the annual meeting of the American College of Physicians.
He and his coinvestigators performed a meta-analysis of the three pre-2000 randomized controlled trials that compared the standard Valsalva maneuver to carotid sinus massage plus the two newer studies, both of which systematically compared a modified Valsalva maneuver with the standard version.
The clear winner in terms of efficacy was the modified Valsalva maneuver, in which patients with supraventricular tachycardia (SVT) performed a standardized strain while in a semirecumbent position, then immediately laid flat and had their legs raised to 45 degrees for 15 seconds before returning to the semirecumbent position. The purpose of this postural modification is to boost relaxation phase venous return and vagal stimulation.
In the 433-patient multicenter REVERT trial in the United Kingdom, 43% of those assigned to the modified Valsalva maneuver returned to sinus rhythm 1 minute after completing the task, compared with 17% of those randomized to the standard semirecumbent Valsalva maneuver. This resulted in significantly less need for adenosine and other treatments. Although REVERT investigators had the patients blow into a manometer at 40 mm Hg for 15 seconds, they noted that the same intensity of strain can be achieved more practically by blowing into a 10-mL syringe sufficient to just move the plunger (Lancet. 2015 Oct 31;386[10005]:1747-53).
The REVERT findings were confirmed by a second trial conducted by Turkish investigators, in which the modified Valsalva maneuver was successful in 43% of patients, compared with 11% in the standard Valsalva maneuver group (Am J Emerg Med. 2017 Nov;35[11]:1662-5).
Extrapolating from the published evidence, including a Cochrane Collaboration review (Cochrane Database Syst Rev. 2015 Feb 18;[2]:CD009502. doi: 10.1002/14651858.CD009502.pub3), Dr. Mehta and his coinvestigators ranked the likelihood of successful conversion of SVT to sinus rhythm from a high of 48% for the modified Valsalva maneuver, descending to 43% for a supine Valsalva maneuver, 36% for a standard semirecumbent Valsalva, 21% for a seated Valsalva, 19% for a standing one, and just 11% for carotid sinus massage.
“Based on evidence of high quality, we encourage that the modified Valsalva maneuver be done due its safety and low cost,” Dr. Mehta concluded.
He reported having no financial conflicts regarding his study, conducted free of commercial support.
REPORTING FROM ACP INTERNAL MEDICINE
Key clinical point: A simple postural modification to the standard Valsalva maneuver boosts conversion rate.
Major finding: Nearly half of patients in SVT converted to sinus rhythm in response to a modified Valsalva maneuver.
Study details: This was a meta-analysis of five randomized clinical trials of vagal maneuvers for conversion of supraventricular tachycardia to sinus rhythm.
Disclosures: The presenter reported having no financial conflicts regarding the study, conducted free of commercial support.
A systems-based charter on physician well-being
Don’t blame the burned-out clinician
“You can teach a canary in a coal mine to meditate, but it is still going to die.”
I have seen the canary sentiment above – used as a metaphor for health care and burnout – pop up a few times on Twitter, attributed to a few different thoughtful doctors, including Jenny Ramsey, MD, of the Cleveland Clinic (at Hospital Medicine 2018); Lucy Kalanithi, MD, a clinical assistant professor of medicine at Stanford (Calif.) University and widow of Paul Kalanithi, MD, of “When Breath Becomes Air” fame; and Stuart Slavin, MD, associate dean for curriculum and a professor of pediatrics at Saint Louis University.
To be honest, I am rather burned out on reading about physician burnout at this point. Nevertheless, I love the canary idea; it is such a perfect visual of the current problem facing physicians.
I was thinking about the meditating canary when I read the new “Charter on Physician Well-Being,” published in JAMA and already endorsed by most major medical organizations/acronyms, including SHM, ACP, SGIM, AMA, AAMC, AAIM, ABIM, ACCME, APA, and the IHI. This physician well-being charter was created by the Collaborative for Healing and Renewal in Medicine, a group that includes leading medical centers and organizations.
What makes this different from previous attempts at addressing burnout? The charter takes a systems-based approach to physician well-being. Aha, of course! As the patient safety movement realized more than 2 decades ago, real progress would only be made when we stopped focusing our attention, blame, and interventions on individuals and instead looked at systems; now, the physician well-being movement has officially made the same bold proclamation.
It is not the fault of the burned-out physician who apparently just needs to be hammered over the head with better coping skills – just as the majority of medical errors would not be fixed by continuing to tell physicians that they screwed up and should figure out how not to do that again!
We need to make real changes to the system. For example, one of the charter’s authors, Colin P. West, MD, PhD, highlighted why it is important that organizations commit to optimizing highly functioning interprofessional teams: “Can you imagine @KingJames [LeBron James] or @Oprah applying their unique skills AND personally seating the crowd, collecting stats, assessing satisfaction, etc.? So why do we?”
The authors also call for organizations to commit to reducing time spent on documentation and administration. Hallelujah!
Now the question is whether this charter will actually have any teeth or whether it will have the same fate as our canary, slowly fading away, never to be heard from again?
Read the full post at hospitalleader.org.
Dr. Moriates is the assistant dean for health care value and an associate professor of internal medicine at the University of Texas, Austin.
Also in The Hospital Leader
“What’s a Cost, Charge, and Price?” by Brad Flansbaum, DO, MPH, MHM
“There Is a ‘You’ in Team,” by Tracy Cardin, ACNP-BC, SFHM
“‘Harper’s Index’ of Hospital Medicine 2018,” by Jordan Messler, MD, SFHM
Don’t blame the burned-out clinician
Don’t blame the burned-out clinician
“You can teach a canary in a coal mine to meditate, but it is still going to die.”
I have seen the canary sentiment above – used as a metaphor for health care and burnout – pop up a few times on Twitter, attributed to a few different thoughtful doctors, including Jenny Ramsey, MD, of the Cleveland Clinic (at Hospital Medicine 2018); Lucy Kalanithi, MD, a clinical assistant professor of medicine at Stanford (Calif.) University and widow of Paul Kalanithi, MD, of “When Breath Becomes Air” fame; and Stuart Slavin, MD, associate dean for curriculum and a professor of pediatrics at Saint Louis University.
To be honest, I am rather burned out on reading about physician burnout at this point. Nevertheless, I love the canary idea; it is such a perfect visual of the current problem facing physicians.
I was thinking about the meditating canary when I read the new “Charter on Physician Well-Being,” published in JAMA and already endorsed by most major medical organizations/acronyms, including SHM, ACP, SGIM, AMA, AAMC, AAIM, ABIM, ACCME, APA, and the IHI. This physician well-being charter was created by the Collaborative for Healing and Renewal in Medicine, a group that includes leading medical centers and organizations.
What makes this different from previous attempts at addressing burnout? The charter takes a systems-based approach to physician well-being. Aha, of course! As the patient safety movement realized more than 2 decades ago, real progress would only be made when we stopped focusing our attention, blame, and interventions on individuals and instead looked at systems; now, the physician well-being movement has officially made the same bold proclamation.
It is not the fault of the burned-out physician who apparently just needs to be hammered over the head with better coping skills – just as the majority of medical errors would not be fixed by continuing to tell physicians that they screwed up and should figure out how not to do that again!
We need to make real changes to the system. For example, one of the charter’s authors, Colin P. West, MD, PhD, highlighted why it is important that organizations commit to optimizing highly functioning interprofessional teams: “Can you imagine @KingJames [LeBron James] or @Oprah applying their unique skills AND personally seating the crowd, collecting stats, assessing satisfaction, etc.? So why do we?”
The authors also call for organizations to commit to reducing time spent on documentation and administration. Hallelujah!
Now the question is whether this charter will actually have any teeth or whether it will have the same fate as our canary, slowly fading away, never to be heard from again?
Read the full post at hospitalleader.org.
Dr. Moriates is the assistant dean for health care value and an associate professor of internal medicine at the University of Texas, Austin.
Also in The Hospital Leader
“What’s a Cost, Charge, and Price?” by Brad Flansbaum, DO, MPH, MHM
“There Is a ‘You’ in Team,” by Tracy Cardin, ACNP-BC, SFHM
“‘Harper’s Index’ of Hospital Medicine 2018,” by Jordan Messler, MD, SFHM
“You can teach a canary in a coal mine to meditate, but it is still going to die.”
I have seen the canary sentiment above – used as a metaphor for health care and burnout – pop up a few times on Twitter, attributed to a few different thoughtful doctors, including Jenny Ramsey, MD, of the Cleveland Clinic (at Hospital Medicine 2018); Lucy Kalanithi, MD, a clinical assistant professor of medicine at Stanford (Calif.) University and widow of Paul Kalanithi, MD, of “When Breath Becomes Air” fame; and Stuart Slavin, MD, associate dean for curriculum and a professor of pediatrics at Saint Louis University.
To be honest, I am rather burned out on reading about physician burnout at this point. Nevertheless, I love the canary idea; it is such a perfect visual of the current problem facing physicians.
I was thinking about the meditating canary when I read the new “Charter on Physician Well-Being,” published in JAMA and already endorsed by most major medical organizations/acronyms, including SHM, ACP, SGIM, AMA, AAMC, AAIM, ABIM, ACCME, APA, and the IHI. This physician well-being charter was created by the Collaborative for Healing and Renewal in Medicine, a group that includes leading medical centers and organizations.
What makes this different from previous attempts at addressing burnout? The charter takes a systems-based approach to physician well-being. Aha, of course! As the patient safety movement realized more than 2 decades ago, real progress would only be made when we stopped focusing our attention, blame, and interventions on individuals and instead looked at systems; now, the physician well-being movement has officially made the same bold proclamation.
It is not the fault of the burned-out physician who apparently just needs to be hammered over the head with better coping skills – just as the majority of medical errors would not be fixed by continuing to tell physicians that they screwed up and should figure out how not to do that again!
We need to make real changes to the system. For example, one of the charter’s authors, Colin P. West, MD, PhD, highlighted why it is important that organizations commit to optimizing highly functioning interprofessional teams: “Can you imagine @KingJames [LeBron James] or @Oprah applying their unique skills AND personally seating the crowd, collecting stats, assessing satisfaction, etc.? So why do we?”
The authors also call for organizations to commit to reducing time spent on documentation and administration. Hallelujah!
Now the question is whether this charter will actually have any teeth or whether it will have the same fate as our canary, slowly fading away, never to be heard from again?
Read the full post at hospitalleader.org.
Dr. Moriates is the assistant dean for health care value and an associate professor of internal medicine at the University of Texas, Austin.
Also in The Hospital Leader
“What’s a Cost, Charge, and Price?” by Brad Flansbaum, DO, MPH, MHM
“There Is a ‘You’ in Team,” by Tracy Cardin, ACNP-BC, SFHM
“‘Harper’s Index’ of Hospital Medicine 2018,” by Jordan Messler, MD, SFHM
Some PE patients don’t require hospitalization
A new study suggests that
Researchers tested outpatient anticoagulant therapy in 200 patients with PE with a low mortality risk. At 90 days of follow-up, there were no deaths or recurrences of venous thromboembolism (VTE), but one patient experienced major bleeding after a traumatic injury.
A majority of patients said they were satisfied with outpatient care.
Joseph R. Bledsoe, MD, of Intermountain Medical Center in Salt Lake City, and his colleagues reported these results in Chest.
The researchers tracked patients who were treated for acute PE in five Intermountain Healthcare emergency departments (EDs) from 2013 to 2016. The patients had to have a low mortality risk according to the Pulmonary Embolism Severity Index (score less than 86), echocardiography (no signs of right heart strain), and whole-leg compression ultrasound. Patients could not have deep vein thrombosis proximal to the popliteal vein, hypoxia, hypotension, hepatic failure, or renal failure. They had to be eligible for therapeutic anticoagulation and could not have any condition requiring hospitalization.
With these criteria, the researchers selected 200 patients. They were observed in the ED or hospital for 12-24 hours and then discharged with anticoagulant therapy. Patients received rivaroxaban (n = 149), enoxaparin transitioned to warfarin (n = 26), apixaban (n = 24), or enoxaparin alone (n = 1).
Results
The study’s primary outcome was the 90-day composite rate of all-cause mortality, recurrent symptomatic VTE, and major bleeding. There were no deaths and no cases of recurrent VTE, but one patient did experience major bleeding at day 61 because of a traumatic thigh injury.
Within 7 days of study enrollment, there were 19 patients (9.5%) who returned to the ED and 2 patients (1%) who were admitted to the hospital. One patient with pulmonary infarct was admitted for pain control (day 2); the other was admitted for an elective coronary intervention (day 7) because of a positive cardiac stress test.
Within 30 days, 32 patients (16%) returned to the ED, and 5 (3%) were admitted to the hospital for events unrelated to their PE.
The study also showed that patients were largely satisfied with outpatient care. Of the 146 patients who completed a satisfaction survey at 90 days, 89% said they would choose outpatient management if they had another PE in the future.
“We found a large subset of patients with blood clots who’d do well at home; in fact, who probably did better at home,” Dr. Bledsoe said. “When patients are sent home versus staying in the hospital, they’re at lower risk of getting another infection. It’s a lot less expensive, too.”
Currently, the standard of care in the United States for acute PE is hospitalization for all patients. That’s recommended, in part, because their overall mortality rate is 17%. However, the lower mortality rate among some appropriately risk-stratified patients suggests that at-home care, which has become the norm in some European countries, leads to better outcomes for those patients overall and less chance of a hospital-introduced infection, according to Dr. Bledsoe. “Our findings show that if you appropriately risk-stratify patients, there are a lot of people with blood clots who are safe to go home.”
He added that similar research should be conducted outside of the Intermountain Healthcare system to confirm the results of this study and that a larger group of patients should be studied.
The investigators reported no conflicts related to this study.
SOURCE: Bledsoe JR et al. Chest. 2018 Aug;154(2):249-56.
A new study suggests that
Researchers tested outpatient anticoagulant therapy in 200 patients with PE with a low mortality risk. At 90 days of follow-up, there were no deaths or recurrences of venous thromboembolism (VTE), but one patient experienced major bleeding after a traumatic injury.
A majority of patients said they were satisfied with outpatient care.
Joseph R. Bledsoe, MD, of Intermountain Medical Center in Salt Lake City, and his colleagues reported these results in Chest.
The researchers tracked patients who were treated for acute PE in five Intermountain Healthcare emergency departments (EDs) from 2013 to 2016. The patients had to have a low mortality risk according to the Pulmonary Embolism Severity Index (score less than 86), echocardiography (no signs of right heart strain), and whole-leg compression ultrasound. Patients could not have deep vein thrombosis proximal to the popliteal vein, hypoxia, hypotension, hepatic failure, or renal failure. They had to be eligible for therapeutic anticoagulation and could not have any condition requiring hospitalization.
With these criteria, the researchers selected 200 patients. They were observed in the ED or hospital for 12-24 hours and then discharged with anticoagulant therapy. Patients received rivaroxaban (n = 149), enoxaparin transitioned to warfarin (n = 26), apixaban (n = 24), or enoxaparin alone (n = 1).
Results
The study’s primary outcome was the 90-day composite rate of all-cause mortality, recurrent symptomatic VTE, and major bleeding. There were no deaths and no cases of recurrent VTE, but one patient did experience major bleeding at day 61 because of a traumatic thigh injury.
Within 7 days of study enrollment, there were 19 patients (9.5%) who returned to the ED and 2 patients (1%) who were admitted to the hospital. One patient with pulmonary infarct was admitted for pain control (day 2); the other was admitted for an elective coronary intervention (day 7) because of a positive cardiac stress test.
Within 30 days, 32 patients (16%) returned to the ED, and 5 (3%) were admitted to the hospital for events unrelated to their PE.
The study also showed that patients were largely satisfied with outpatient care. Of the 146 patients who completed a satisfaction survey at 90 days, 89% said they would choose outpatient management if they had another PE in the future.
“We found a large subset of patients with blood clots who’d do well at home; in fact, who probably did better at home,” Dr. Bledsoe said. “When patients are sent home versus staying in the hospital, they’re at lower risk of getting another infection. It’s a lot less expensive, too.”
Currently, the standard of care in the United States for acute PE is hospitalization for all patients. That’s recommended, in part, because their overall mortality rate is 17%. However, the lower mortality rate among some appropriately risk-stratified patients suggests that at-home care, which has become the norm in some European countries, leads to better outcomes for those patients overall and less chance of a hospital-introduced infection, according to Dr. Bledsoe. “Our findings show that if you appropriately risk-stratify patients, there are a lot of people with blood clots who are safe to go home.”
He added that similar research should be conducted outside of the Intermountain Healthcare system to confirm the results of this study and that a larger group of patients should be studied.
The investigators reported no conflicts related to this study.
SOURCE: Bledsoe JR et al. Chest. 2018 Aug;154(2):249-56.
A new study suggests that
Researchers tested outpatient anticoagulant therapy in 200 patients with PE with a low mortality risk. At 90 days of follow-up, there were no deaths or recurrences of venous thromboembolism (VTE), but one patient experienced major bleeding after a traumatic injury.
A majority of patients said they were satisfied with outpatient care.
Joseph R. Bledsoe, MD, of Intermountain Medical Center in Salt Lake City, and his colleagues reported these results in Chest.
The researchers tracked patients who were treated for acute PE in five Intermountain Healthcare emergency departments (EDs) from 2013 to 2016. The patients had to have a low mortality risk according to the Pulmonary Embolism Severity Index (score less than 86), echocardiography (no signs of right heart strain), and whole-leg compression ultrasound. Patients could not have deep vein thrombosis proximal to the popliteal vein, hypoxia, hypotension, hepatic failure, or renal failure. They had to be eligible for therapeutic anticoagulation and could not have any condition requiring hospitalization.
With these criteria, the researchers selected 200 patients. They were observed in the ED or hospital for 12-24 hours and then discharged with anticoagulant therapy. Patients received rivaroxaban (n = 149), enoxaparin transitioned to warfarin (n = 26), apixaban (n = 24), or enoxaparin alone (n = 1).
Results
The study’s primary outcome was the 90-day composite rate of all-cause mortality, recurrent symptomatic VTE, and major bleeding. There were no deaths and no cases of recurrent VTE, but one patient did experience major bleeding at day 61 because of a traumatic thigh injury.
Within 7 days of study enrollment, there were 19 patients (9.5%) who returned to the ED and 2 patients (1%) who were admitted to the hospital. One patient with pulmonary infarct was admitted for pain control (day 2); the other was admitted for an elective coronary intervention (day 7) because of a positive cardiac stress test.
Within 30 days, 32 patients (16%) returned to the ED, and 5 (3%) were admitted to the hospital for events unrelated to their PE.
The study also showed that patients were largely satisfied with outpatient care. Of the 146 patients who completed a satisfaction survey at 90 days, 89% said they would choose outpatient management if they had another PE in the future.
“We found a large subset of patients with blood clots who’d do well at home; in fact, who probably did better at home,” Dr. Bledsoe said. “When patients are sent home versus staying in the hospital, they’re at lower risk of getting another infection. It’s a lot less expensive, too.”
Currently, the standard of care in the United States for acute PE is hospitalization for all patients. That’s recommended, in part, because their overall mortality rate is 17%. However, the lower mortality rate among some appropriately risk-stratified patients suggests that at-home care, which has become the norm in some European countries, leads to better outcomes for those patients overall and less chance of a hospital-introduced infection, according to Dr. Bledsoe. “Our findings show that if you appropriately risk-stratify patients, there are a lot of people with blood clots who are safe to go home.”
He added that similar research should be conducted outside of the Intermountain Healthcare system to confirm the results of this study and that a larger group of patients should be studied.
The investigators reported no conflicts related to this study.
SOURCE: Bledsoe JR et al. Chest. 2018 Aug;154(2):249-56.
FROM CHEST
Key clinical point: There were no deaths or recurrences of pulmonary embolism at 90 days in a group of patients stratified by criteria for low risk.
Major finding: At 90 days of follow-up, there were no deaths or recurrences of venous thromboembolism.
Study details: Researchers tested outpatient anticoagulant therapy in 200 patients with pulmonary embolism with a low mortality risk.
Disclosures: The investigators reported no conflicts related to this study.
Source: Bledsoe JR et al. Chest. 2018 Aug;154(2):249-56.
Comparison of analgesia methods for neonatal circumcision
Multiple pain management interventions exist
Clinical question
What is the optimal way to manage analgesia during neonatal circumcision?
Background
Neonatal circumcision is one of the most commonly performed surgical procedures. The American Academy of Pediatrics in 2012 noted that the health benefits outweigh the minor risks of the procedure, but that parents should make the decision to circumcise based on their own cultural, ethical, and religious beliefs.
One of the primary risks of neonatal circumcision is pain during and after the procedure. Multiple methods for managing analgesia exist, but it is unknown what combination of methods is optimal. Usual analgesia techniques include: local anesthetic cream composed of lidocaine and prilocaine (EMLA) applied to the skin prior to the procedure; oral sucrose solution given throughout the procedure; dorsal penile nerve block (DPNB); and penile ring block (RB).
Study design
Single-center, double-blinded, randomized, controlled trial.
Setting
Multispecialty freestanding hospital.
Synopsis
Parents of infant boys born at 36-41 weeks’ gestation who chose to have their children circumcised were offered participation in the study. Of 83 eligible participants, 70 were randomized, with 10 in the control group (EMLA only) and 20 in each intervention (EMLA + sucrose, EMLA + sucrose + RB, EMLA + sucrose + DPNB). A single pediatric urologist performed all circumcisions using the Gomco clamp technique.
A video camera recorded the infant’s face and upper torso during the procedure. Two researchers, who were blinded to the analgesia plan, scored these videos using a modified Neonatal Infant Pain Scale (NIPS). The NIPS used ranged from 0 to 6, with 6 considered severe pain. For rating purposes, the procedure was divided into 6 stages with a NIPS score assigned at each stage. There were no significant differences in baseline characteristics among the groups; no significant differences in the duration of the procedure by intervention; and there were no complications. Interrater reliability for the NIPS was good (kappa, 0.84). All interventions were superior to EMLA alone, with significantly decreased NIPS for all stages of the procedure. No significant differences in NIPS were found among the following:
EMLA + sucrose.
EMLA + sucrose + RB.
EMLA + sucrose + DPNB (for any stage of the procedure).
The one exception was that following lysis of foreskin adhesions, EMLA + sucrose + RB was superior (NIPS 2.25 for EMLA + sucrose + RB vs. NIPS 4.4 for EMLA + sucrose + DPNB vs. NIPS 4.3 for EMLA + sucrose vs. NIPS 5.8 for EMLA alone). In terms of crying time during the procedure, all interventions were significantly superior to EMLA alone. Of the interventions, crying time was statistically and clinically significantly shorter with EMLA + sucrose + RB (5.78 seconds vs. 11.5 for EMLA + sucrose + DPNB vs. 16.5 for EMLA + sucrose vs. 45.4 for EMLA alone). This was a single-center study and the procedures were performed by a pediatric urologist rather than by a general pediatrician, which potentially limits applicability.
Bottom line
All tested analgesia modalities for neonatal circumcision were superior to EMLA alone. The most effective analgesia of those tested was EMLA + sucrose + penile ring block.
Citation
Sharara-Chami R et al. Combination analgesia for neonatal circumcision: a randomized controlled trial. Pediatrics. 2017. doi: 10.1542/peds.2017-1935.
Dr. Stubblefield is a pediatric hospitalist at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a clinical assistant professor of pediatrics at Sidney Kimmel Medical College at Thomas Jefferson University in Philadelphia.
Multiple pain management interventions exist
Multiple pain management interventions exist
Clinical question
What is the optimal way to manage analgesia during neonatal circumcision?
Background
Neonatal circumcision is one of the most commonly performed surgical procedures. The American Academy of Pediatrics in 2012 noted that the health benefits outweigh the minor risks of the procedure, but that parents should make the decision to circumcise based on their own cultural, ethical, and religious beliefs.
One of the primary risks of neonatal circumcision is pain during and after the procedure. Multiple methods for managing analgesia exist, but it is unknown what combination of methods is optimal. Usual analgesia techniques include: local anesthetic cream composed of lidocaine and prilocaine (EMLA) applied to the skin prior to the procedure; oral sucrose solution given throughout the procedure; dorsal penile nerve block (DPNB); and penile ring block (RB).
Study design
Single-center, double-blinded, randomized, controlled trial.
Setting
Multispecialty freestanding hospital.
Synopsis
Parents of infant boys born at 36-41 weeks’ gestation who chose to have their children circumcised were offered participation in the study. Of 83 eligible participants, 70 were randomized, with 10 in the control group (EMLA only) and 20 in each intervention (EMLA + sucrose, EMLA + sucrose + RB, EMLA + sucrose + DPNB). A single pediatric urologist performed all circumcisions using the Gomco clamp technique.
A video camera recorded the infant’s face and upper torso during the procedure. Two researchers, who were blinded to the analgesia plan, scored these videos using a modified Neonatal Infant Pain Scale (NIPS). The NIPS used ranged from 0 to 6, with 6 considered severe pain. For rating purposes, the procedure was divided into 6 stages with a NIPS score assigned at each stage. There were no significant differences in baseline characteristics among the groups; no significant differences in the duration of the procedure by intervention; and there were no complications. Interrater reliability for the NIPS was good (kappa, 0.84). All interventions were superior to EMLA alone, with significantly decreased NIPS for all stages of the procedure. No significant differences in NIPS were found among the following:
EMLA + sucrose.
EMLA + sucrose + RB.
EMLA + sucrose + DPNB (for any stage of the procedure).
The one exception was that following lysis of foreskin adhesions, EMLA + sucrose + RB was superior (NIPS 2.25 for EMLA + sucrose + RB vs. NIPS 4.4 for EMLA + sucrose + DPNB vs. NIPS 4.3 for EMLA + sucrose vs. NIPS 5.8 for EMLA alone). In terms of crying time during the procedure, all interventions were significantly superior to EMLA alone. Of the interventions, crying time was statistically and clinically significantly shorter with EMLA + sucrose + RB (5.78 seconds vs. 11.5 for EMLA + sucrose + DPNB vs. 16.5 for EMLA + sucrose vs. 45.4 for EMLA alone). This was a single-center study and the procedures were performed by a pediatric urologist rather than by a general pediatrician, which potentially limits applicability.
Bottom line
All tested analgesia modalities for neonatal circumcision were superior to EMLA alone. The most effective analgesia of those tested was EMLA + sucrose + penile ring block.
Citation
Sharara-Chami R et al. Combination analgesia for neonatal circumcision: a randomized controlled trial. Pediatrics. 2017. doi: 10.1542/peds.2017-1935.
Dr. Stubblefield is a pediatric hospitalist at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a clinical assistant professor of pediatrics at Sidney Kimmel Medical College at Thomas Jefferson University in Philadelphia.
Clinical question
What is the optimal way to manage analgesia during neonatal circumcision?
Background
Neonatal circumcision is one of the most commonly performed surgical procedures. The American Academy of Pediatrics in 2012 noted that the health benefits outweigh the minor risks of the procedure, but that parents should make the decision to circumcise based on their own cultural, ethical, and religious beliefs.
One of the primary risks of neonatal circumcision is pain during and after the procedure. Multiple methods for managing analgesia exist, but it is unknown what combination of methods is optimal. Usual analgesia techniques include: local anesthetic cream composed of lidocaine and prilocaine (EMLA) applied to the skin prior to the procedure; oral sucrose solution given throughout the procedure; dorsal penile nerve block (DPNB); and penile ring block (RB).
Study design
Single-center, double-blinded, randomized, controlled trial.
Setting
Multispecialty freestanding hospital.
Synopsis
Parents of infant boys born at 36-41 weeks’ gestation who chose to have their children circumcised were offered participation in the study. Of 83 eligible participants, 70 were randomized, with 10 in the control group (EMLA only) and 20 in each intervention (EMLA + sucrose, EMLA + sucrose + RB, EMLA + sucrose + DPNB). A single pediatric urologist performed all circumcisions using the Gomco clamp technique.
A video camera recorded the infant’s face and upper torso during the procedure. Two researchers, who were blinded to the analgesia plan, scored these videos using a modified Neonatal Infant Pain Scale (NIPS). The NIPS used ranged from 0 to 6, with 6 considered severe pain. For rating purposes, the procedure was divided into 6 stages with a NIPS score assigned at each stage. There were no significant differences in baseline characteristics among the groups; no significant differences in the duration of the procedure by intervention; and there were no complications. Interrater reliability for the NIPS was good (kappa, 0.84). All interventions were superior to EMLA alone, with significantly decreased NIPS for all stages of the procedure. No significant differences in NIPS were found among the following:
EMLA + sucrose.
EMLA + sucrose + RB.
EMLA + sucrose + DPNB (for any stage of the procedure).
The one exception was that following lysis of foreskin adhesions, EMLA + sucrose + RB was superior (NIPS 2.25 for EMLA + sucrose + RB vs. NIPS 4.4 for EMLA + sucrose + DPNB vs. NIPS 4.3 for EMLA + sucrose vs. NIPS 5.8 for EMLA alone). In terms of crying time during the procedure, all interventions were significantly superior to EMLA alone. Of the interventions, crying time was statistically and clinically significantly shorter with EMLA + sucrose + RB (5.78 seconds vs. 11.5 for EMLA + sucrose + DPNB vs. 16.5 for EMLA + sucrose vs. 45.4 for EMLA alone). This was a single-center study and the procedures were performed by a pediatric urologist rather than by a general pediatrician, which potentially limits applicability.
Bottom line
All tested analgesia modalities for neonatal circumcision were superior to EMLA alone. The most effective analgesia of those tested was EMLA + sucrose + penile ring block.
Citation
Sharara-Chami R et al. Combination analgesia for neonatal circumcision: a randomized controlled trial. Pediatrics. 2017. doi: 10.1542/peds.2017-1935.
Dr. Stubblefield is a pediatric hospitalist at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a clinical assistant professor of pediatrics at Sidney Kimmel Medical College at Thomas Jefferson University in Philadelphia.
A new, simple, inexpensive DVT diagnostic aid
NEW ORLEANS – Both the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio proved to be better predictors of the presence or absence of deep vein thrombosis than the ubiquitous D-dimer test in a retrospective study, Jason Mouabbi, MD, reported at the annual meeting of the American College of Physicians.
What’s more, both the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) can be readily calculated from the readout of a complete blood count (CBC) with differential. A CBC costs an average of $16, and everybody that comes through a hospital emergency department gets one. In contrast, the average charge for a D-dimer test is about $231 nationwide, and depending upon the specific test used the results can take up to a couple of hours to come back, noted Dr. Mouabbi of St. John Hospital and Medical Center in Detroit.
“The NLR and PLR ratios offer a new, powerful, affordable, simple, and readily available tool in the hands of clinicians to help them in the diagnosis of DVT,” he said. “The NLR can be useful to rule out DVT when it’s negative, whereas PLR can be useful in ruling DVT when positive.”
Investigators in a variety of fields are looking at the NLR and PLR as emerging practical, easily obtainable biomarkers for systemic inflammation. And DVT is thought to be an inflammatory process, he explained.
Dr. Mouabbi presented a single-center retrospective study of 102 matched patients who presented with lower extremity swelling and had a CBC drawn, as well as a D-dimer test, on the same day they underwent a lower extremity Doppler ultrasound evaluation. In 51 patients, the ultrasound revealed the presence of DVT and anticoagulation was started. In the other 51 patients, the ultrasound exam was negative and they weren’t anticoagulated. Since the study purpose was to assess the implications of a primary elevation of NLR and/or PLR, patients with rheumatic diseases, inflammatory bowel disease, recent surgery, chronic renal or liver disease, inherited thrombophilia, infection, or other possible secondary causes of altered ratios were excluded from the study.
A positive NLR was considered 3.4 or higher, a positive PLR was a ratio of 230 or more, and a positive D-dimer level was 500 ng/mL or greater. The NLR and PLR collectively outperformed the D-dimer test in terms of sensitivity, specificity, positive predictive value, and negative predictive value.
In addition, 89% of the DVT group were classified as “double-positive,” meaning they were both NLR and PLR positive. That combination provided the best diagnostic value of all, since none of the controls were double-positive and only 2% were PLR positive.
While the results are encouraging, before NLR and PLR can supplant D-dimer in patients with suspected DVT in clinical practice a confirmatory prospective study should be carried out, according to Dr. Mouabbi. Ideally it should include the use of the Wells score, which is part of most diagnostic algorithms as a preliminary means of categorizing DVT probability as low, moderate, or high. However, the popularity of the Wells score has fallen off in the face of reports that the results are subjective and variable. Indeed, the Wells score was included in the electronic medical record of so few participants in Dr. Mouabbi’s study that he couldn’t evaluate its utility.
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
NEW ORLEANS – Both the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio proved to be better predictors of the presence or absence of deep vein thrombosis than the ubiquitous D-dimer test in a retrospective study, Jason Mouabbi, MD, reported at the annual meeting of the American College of Physicians.
What’s more, both the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) can be readily calculated from the readout of a complete blood count (CBC) with differential. A CBC costs an average of $16, and everybody that comes through a hospital emergency department gets one. In contrast, the average charge for a D-dimer test is about $231 nationwide, and depending upon the specific test used the results can take up to a couple of hours to come back, noted Dr. Mouabbi of St. John Hospital and Medical Center in Detroit.
“The NLR and PLR ratios offer a new, powerful, affordable, simple, and readily available tool in the hands of clinicians to help them in the diagnosis of DVT,” he said. “The NLR can be useful to rule out DVT when it’s negative, whereas PLR can be useful in ruling DVT when positive.”
Investigators in a variety of fields are looking at the NLR and PLR as emerging practical, easily obtainable biomarkers for systemic inflammation. And DVT is thought to be an inflammatory process, he explained.
Dr. Mouabbi presented a single-center retrospective study of 102 matched patients who presented with lower extremity swelling and had a CBC drawn, as well as a D-dimer test, on the same day they underwent a lower extremity Doppler ultrasound evaluation. In 51 patients, the ultrasound revealed the presence of DVT and anticoagulation was started. In the other 51 patients, the ultrasound exam was negative and they weren’t anticoagulated. Since the study purpose was to assess the implications of a primary elevation of NLR and/or PLR, patients with rheumatic diseases, inflammatory bowel disease, recent surgery, chronic renal or liver disease, inherited thrombophilia, infection, or other possible secondary causes of altered ratios were excluded from the study.
A positive NLR was considered 3.4 or higher, a positive PLR was a ratio of 230 or more, and a positive D-dimer level was 500 ng/mL or greater. The NLR and PLR collectively outperformed the D-dimer test in terms of sensitivity, specificity, positive predictive value, and negative predictive value.
In addition, 89% of the DVT group were classified as “double-positive,” meaning they were both NLR and PLR positive. That combination provided the best diagnostic value of all, since none of the controls were double-positive and only 2% were PLR positive.
While the results are encouraging, before NLR and PLR can supplant D-dimer in patients with suspected DVT in clinical practice a confirmatory prospective study should be carried out, according to Dr. Mouabbi. Ideally it should include the use of the Wells score, which is part of most diagnostic algorithms as a preliminary means of categorizing DVT probability as low, moderate, or high. However, the popularity of the Wells score has fallen off in the face of reports that the results are subjective and variable. Indeed, the Wells score was included in the electronic medical record of so few participants in Dr. Mouabbi’s study that he couldn’t evaluate its utility.
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
NEW ORLEANS – Both the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio proved to be better predictors of the presence or absence of deep vein thrombosis than the ubiquitous D-dimer test in a retrospective study, Jason Mouabbi, MD, reported at the annual meeting of the American College of Physicians.
What’s more, both the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) can be readily calculated from the readout of a complete blood count (CBC) with differential. A CBC costs an average of $16, and everybody that comes through a hospital emergency department gets one. In contrast, the average charge for a D-dimer test is about $231 nationwide, and depending upon the specific test used the results can take up to a couple of hours to come back, noted Dr. Mouabbi of St. John Hospital and Medical Center in Detroit.
“The NLR and PLR ratios offer a new, powerful, affordable, simple, and readily available tool in the hands of clinicians to help them in the diagnosis of DVT,” he said. “The NLR can be useful to rule out DVT when it’s negative, whereas PLR can be useful in ruling DVT when positive.”
Investigators in a variety of fields are looking at the NLR and PLR as emerging practical, easily obtainable biomarkers for systemic inflammation. And DVT is thought to be an inflammatory process, he explained.
Dr. Mouabbi presented a single-center retrospective study of 102 matched patients who presented with lower extremity swelling and had a CBC drawn, as well as a D-dimer test, on the same day they underwent a lower extremity Doppler ultrasound evaluation. In 51 patients, the ultrasound revealed the presence of DVT and anticoagulation was started. In the other 51 patients, the ultrasound exam was negative and they weren’t anticoagulated. Since the study purpose was to assess the implications of a primary elevation of NLR and/or PLR, patients with rheumatic diseases, inflammatory bowel disease, recent surgery, chronic renal or liver disease, inherited thrombophilia, infection, or other possible secondary causes of altered ratios were excluded from the study.
A positive NLR was considered 3.4 or higher, a positive PLR was a ratio of 230 or more, and a positive D-dimer level was 500 ng/mL or greater. The NLR and PLR collectively outperformed the D-dimer test in terms of sensitivity, specificity, positive predictive value, and negative predictive value.
In addition, 89% of the DVT group were classified as “double-positive,” meaning they were both NLR and PLR positive. That combination provided the best diagnostic value of all, since none of the controls were double-positive and only 2% were PLR positive.
While the results are encouraging, before NLR and PLR can supplant D-dimer in patients with suspected DVT in clinical practice a confirmatory prospective study should be carried out, according to Dr. Mouabbi. Ideally it should include the use of the Wells score, which is part of most diagnostic algorithms as a preliminary means of categorizing DVT probability as low, moderate, or high. However, the popularity of the Wells score has fallen off in the face of reports that the results are subjective and variable. Indeed, the Wells score was included in the electronic medical record of so few participants in Dr. Mouabbi’s study that he couldn’t evaluate its utility.
He reported having no financial conflicts regarding his study, which was conducted free of commercial support.
REPORTING FROM ACP INTERNAL MEDICINE
Key clinical point:
Major finding: The neutrophil-to-lymphocyte ratio was better than the D-dimer test at helping to rule out DVT, while the platelet-to-lymphocyte ratio bested the D-dimer at ruling in DVT.
Study details: A retrospective study of 102 patients with suspected DVT.
Disclosures: Dr. Mouabbi reported no financial conflicts regarding his study, which was conducted free of commercial support.








