User login
Latest rise in child COVID-19 cases is relatively small
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
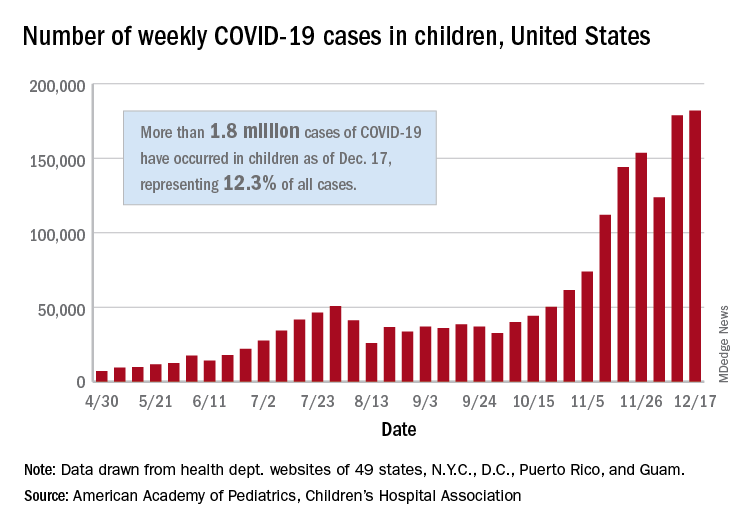
There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
Strategies for tracking SARS-CoV-2 could help detect next pandemic
Two recently published studies indicate that COVID-19 infections were already circulating in the United States in December 2019. The question is whether these methodologies that could be applied to track the next pandemic.
One study evaluating blood donations found antibodies on the West coast as early as Dec. 13, 2019, and in blood donated on the East Coast by early January 2020 (Clin Infect Dis. 2020; Nov 30. doi: 10.1093/cid/ciaa1785). Both preceded the first documented COVID-19 infection in the United States, which has been widely reported as occurring on Jan. 19, 2020, in a traveler returning from China.
The other study, utilizing electronic medical record (EMR) analytics, demonstrated a spike in visits or hospitalizations for cough, a trend that persisted from Dec. 22, 2019, onward, exceeding norms for seasonal flu ( J Med Internet Res. 2020;22:e21562). This spike was interpreted as evidence that the SARS-CoV-2 pandemic was already underway before the first case was established.
While the ongoing serologic testing of blood donations for viral antibodies “will advance understanding of the epidemiology” for SARS-CoV-2 and “inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality,” it might also be a strategy for disease surveillance in the next pandemic, according to a team led by investigators at the Centers for Disease Control and Prevention.
Blood donation surveillance is not now used routinely to monitor for population-based health threats, but it is not a new idea, according to the lead author of the study, Sridhar V. Basavaraju, MD, of Emory University and director of the CDC’s Office of Blood, Organ, and Other Tissue Safety, Atlanta, and his coinvestigators. Most recently, blood donation surveillance was used in the United States to track the penetration of the Zika virus.
For early detection of respiratory infections, blood donations might have unique advantages over alternatives, such as surveillance of respiratory specimens from symptomatic patients. Not least, blood donation surveillance captures individuals who are not seeking medical care, according to the investigators.
EMR surveillance might also have unique advantages for population-based monitoring of health threats. For one, aggregate data from large EMR systems have the potential to reveal symptom patterns before they become apparent at level of clinical care, according to a team of collaborating investigators from the University of California, Los Angeles, and the University of Washington, Seattle.
Emphasizing an urgent need for “agile healthcare analytics” to enable “disease surveillance in real time,” the first author of the EMR study, Joann G. Elmore, MD, professor in the department of health policy and management at the University of California, Los Angeles, expressed the hope that the approach will “lead to better preparation and the ability to quickly provide warnings and track the next pandemic.”
In the blood donation surveillance study, the goal was simply to determine whether SARS-CoV-2 reactive antibodies could be found in blood donations before the first case was identified. Of the 7,389 archived blood samples tested between Dec. 13, 2019, and Jan. 17, 2020, 106 (1.4%) were reactive.
These were not true positives, acknowledged the investigators. True positives would require reactive antibodies in the context of a positive molecular diagnostic test or paired acute convalescent sera with rising titers. The investigators also cautioned that false positives could not be completely ruled out, particularly in light of cross-reactivity that has been reported with other human coronaviruses.
Nevertheless, the monitoring of blood donations offers substantial promise for “understanding the dynamics of SARS-CoV-2 pandemic from early introduction,” and the CDC is now collaborating on ongoing surveillance with the goal of contributing information that could be applied “to mitigate morbidity and mortality.”
Lessons learned from this pandemic are potentially relevant to the next.
The EMR study simply looked at whether the word “cough” was included more often in the notes from visits or hospitalizations between December 2019 and February 2020 relative to the preceding 5 years. The investigators drew on data from three hospitals and more than 180 clinics.
From Dec. 22, 2019, onward, cough was noted above the 95% prediction interval for all 10 weeks of the study. The excess was seen in the outpatient setting and among hospitalized patients. There was also significant excess in the number of patients hospitalized with acute respiratory failure during the study period.
“Our approach to analyzing electronic records could be helpful in the future as we included consideration of data from the outpatient clinics in addition to the emergency departments and inpatient settings,” Dr. Elmore reported.
Surveillance of influenza and influenza-like infections has been undertaken in the United States for more than 20 years, but Dr. Elmore contends that EMR data, particularly data from outpatient clinics are “usually a harbinger of what is to come” for emergency department visits and, ultimately, hospitalizations. She thinks that this is a resource not yet fully exploited.
“There are always opportunities to better harness EMR data,” Dr. Elmore said.
These are intriguing studies and “useful” for reconsidering when SARS-CoV-2 was introduced in the United States, according to Janet G. Basemen, PhD, a professor of epidemiology and the associate dean of the University of Washington School of Public Health, Seattle. However, she noted that the task of translating data like these into actionable public health strategies has proven difficult in the past.
Symptom-based surveillance systems “have mostly served as situational awareness rather than early detection tools,” Dr. Baseman said. The problem is timely interpretation of a given signal.
Not that she doubts such tools “would be an incredible resource for humanity” if the current limitations can be resolved or that technological advances will lead to better methods of detecting and monitoring pandemics “at some point.” Rather, “we’re just not there yet,” she said.
SOURCE: Basavaraju SV et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1785); Elmore JG et al. J Med Internet Res. 2020;22:e21562).
Two recently published studies indicate that COVID-19 infections were already circulating in the United States in December 2019. The question is whether these methodologies that could be applied to track the next pandemic.
One study evaluating blood donations found antibodies on the West coast as early as Dec. 13, 2019, and in blood donated on the East Coast by early January 2020 (Clin Infect Dis. 2020; Nov 30. doi: 10.1093/cid/ciaa1785). Both preceded the first documented COVID-19 infection in the United States, which has been widely reported as occurring on Jan. 19, 2020, in a traveler returning from China.
The other study, utilizing electronic medical record (EMR) analytics, demonstrated a spike in visits or hospitalizations for cough, a trend that persisted from Dec. 22, 2019, onward, exceeding norms for seasonal flu ( J Med Internet Res. 2020;22:e21562). This spike was interpreted as evidence that the SARS-CoV-2 pandemic was already underway before the first case was established.
While the ongoing serologic testing of blood donations for viral antibodies “will advance understanding of the epidemiology” for SARS-CoV-2 and “inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality,” it might also be a strategy for disease surveillance in the next pandemic, according to a team led by investigators at the Centers for Disease Control and Prevention.
Blood donation surveillance is not now used routinely to monitor for population-based health threats, but it is not a new idea, according to the lead author of the study, Sridhar V. Basavaraju, MD, of Emory University and director of the CDC’s Office of Blood, Organ, and Other Tissue Safety, Atlanta, and his coinvestigators. Most recently, blood donation surveillance was used in the United States to track the penetration of the Zika virus.
For early detection of respiratory infections, blood donations might have unique advantages over alternatives, such as surveillance of respiratory specimens from symptomatic patients. Not least, blood donation surveillance captures individuals who are not seeking medical care, according to the investigators.
EMR surveillance might also have unique advantages for population-based monitoring of health threats. For one, aggregate data from large EMR systems have the potential to reveal symptom patterns before they become apparent at level of clinical care, according to a team of collaborating investigators from the University of California, Los Angeles, and the University of Washington, Seattle.
Emphasizing an urgent need for “agile healthcare analytics” to enable “disease surveillance in real time,” the first author of the EMR study, Joann G. Elmore, MD, professor in the department of health policy and management at the University of California, Los Angeles, expressed the hope that the approach will “lead to better preparation and the ability to quickly provide warnings and track the next pandemic.”
In the blood donation surveillance study, the goal was simply to determine whether SARS-CoV-2 reactive antibodies could be found in blood donations before the first case was identified. Of the 7,389 archived blood samples tested between Dec. 13, 2019, and Jan. 17, 2020, 106 (1.4%) were reactive.
These were not true positives, acknowledged the investigators. True positives would require reactive antibodies in the context of a positive molecular diagnostic test or paired acute convalescent sera with rising titers. The investigators also cautioned that false positives could not be completely ruled out, particularly in light of cross-reactivity that has been reported with other human coronaviruses.
Nevertheless, the monitoring of blood donations offers substantial promise for “understanding the dynamics of SARS-CoV-2 pandemic from early introduction,” and the CDC is now collaborating on ongoing surveillance with the goal of contributing information that could be applied “to mitigate morbidity and mortality.”
Lessons learned from this pandemic are potentially relevant to the next.
The EMR study simply looked at whether the word “cough” was included more often in the notes from visits or hospitalizations between December 2019 and February 2020 relative to the preceding 5 years. The investigators drew on data from three hospitals and more than 180 clinics.
From Dec. 22, 2019, onward, cough was noted above the 95% prediction interval for all 10 weeks of the study. The excess was seen in the outpatient setting and among hospitalized patients. There was also significant excess in the number of patients hospitalized with acute respiratory failure during the study period.
“Our approach to analyzing electronic records could be helpful in the future as we included consideration of data from the outpatient clinics in addition to the emergency departments and inpatient settings,” Dr. Elmore reported.
Surveillance of influenza and influenza-like infections has been undertaken in the United States for more than 20 years, but Dr. Elmore contends that EMR data, particularly data from outpatient clinics are “usually a harbinger of what is to come” for emergency department visits and, ultimately, hospitalizations. She thinks that this is a resource not yet fully exploited.
“There are always opportunities to better harness EMR data,” Dr. Elmore said.
These are intriguing studies and “useful” for reconsidering when SARS-CoV-2 was introduced in the United States, according to Janet G. Basemen, PhD, a professor of epidemiology and the associate dean of the University of Washington School of Public Health, Seattle. However, she noted that the task of translating data like these into actionable public health strategies has proven difficult in the past.
Symptom-based surveillance systems “have mostly served as situational awareness rather than early detection tools,” Dr. Baseman said. The problem is timely interpretation of a given signal.
Not that she doubts such tools “would be an incredible resource for humanity” if the current limitations can be resolved or that technological advances will lead to better methods of detecting and monitoring pandemics “at some point.” Rather, “we’re just not there yet,” she said.
SOURCE: Basavaraju SV et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1785); Elmore JG et al. J Med Internet Res. 2020;22:e21562).
Two recently published studies indicate that COVID-19 infections were already circulating in the United States in December 2019. The question is whether these methodologies that could be applied to track the next pandemic.
One study evaluating blood donations found antibodies on the West coast as early as Dec. 13, 2019, and in blood donated on the East Coast by early January 2020 (Clin Infect Dis. 2020; Nov 30. doi: 10.1093/cid/ciaa1785). Both preceded the first documented COVID-19 infection in the United States, which has been widely reported as occurring on Jan. 19, 2020, in a traveler returning from China.
The other study, utilizing electronic medical record (EMR) analytics, demonstrated a spike in visits or hospitalizations for cough, a trend that persisted from Dec. 22, 2019, onward, exceeding norms for seasonal flu ( J Med Internet Res. 2020;22:e21562). This spike was interpreted as evidence that the SARS-CoV-2 pandemic was already underway before the first case was established.
While the ongoing serologic testing of blood donations for viral antibodies “will advance understanding of the epidemiology” for SARS-CoV-2 and “inform allocation of resources and public health prevention interventions to mitigate morbidity and mortality,” it might also be a strategy for disease surveillance in the next pandemic, according to a team led by investigators at the Centers for Disease Control and Prevention.
Blood donation surveillance is not now used routinely to monitor for population-based health threats, but it is not a new idea, according to the lead author of the study, Sridhar V. Basavaraju, MD, of Emory University and director of the CDC’s Office of Blood, Organ, and Other Tissue Safety, Atlanta, and his coinvestigators. Most recently, blood donation surveillance was used in the United States to track the penetration of the Zika virus.
For early detection of respiratory infections, blood donations might have unique advantages over alternatives, such as surveillance of respiratory specimens from symptomatic patients. Not least, blood donation surveillance captures individuals who are not seeking medical care, according to the investigators.
EMR surveillance might also have unique advantages for population-based monitoring of health threats. For one, aggregate data from large EMR systems have the potential to reveal symptom patterns before they become apparent at level of clinical care, according to a team of collaborating investigators from the University of California, Los Angeles, and the University of Washington, Seattle.
Emphasizing an urgent need for “agile healthcare analytics” to enable “disease surveillance in real time,” the first author of the EMR study, Joann G. Elmore, MD, professor in the department of health policy and management at the University of California, Los Angeles, expressed the hope that the approach will “lead to better preparation and the ability to quickly provide warnings and track the next pandemic.”
In the blood donation surveillance study, the goal was simply to determine whether SARS-CoV-2 reactive antibodies could be found in blood donations before the first case was identified. Of the 7,389 archived blood samples tested between Dec. 13, 2019, and Jan. 17, 2020, 106 (1.4%) were reactive.
These were not true positives, acknowledged the investigators. True positives would require reactive antibodies in the context of a positive molecular diagnostic test or paired acute convalescent sera with rising titers. The investigators also cautioned that false positives could not be completely ruled out, particularly in light of cross-reactivity that has been reported with other human coronaviruses.
Nevertheless, the monitoring of blood donations offers substantial promise for “understanding the dynamics of SARS-CoV-2 pandemic from early introduction,” and the CDC is now collaborating on ongoing surveillance with the goal of contributing information that could be applied “to mitigate morbidity and mortality.”
Lessons learned from this pandemic are potentially relevant to the next.
The EMR study simply looked at whether the word “cough” was included more often in the notes from visits or hospitalizations between December 2019 and February 2020 relative to the preceding 5 years. The investigators drew on data from three hospitals and more than 180 clinics.
From Dec. 22, 2019, onward, cough was noted above the 95% prediction interval for all 10 weeks of the study. The excess was seen in the outpatient setting and among hospitalized patients. There was also significant excess in the number of patients hospitalized with acute respiratory failure during the study period.
“Our approach to analyzing electronic records could be helpful in the future as we included consideration of data from the outpatient clinics in addition to the emergency departments and inpatient settings,” Dr. Elmore reported.
Surveillance of influenza and influenza-like infections has been undertaken in the United States for more than 20 years, but Dr. Elmore contends that EMR data, particularly data from outpatient clinics are “usually a harbinger of what is to come” for emergency department visits and, ultimately, hospitalizations. She thinks that this is a resource not yet fully exploited.
“There are always opportunities to better harness EMR data,” Dr. Elmore said.
These are intriguing studies and “useful” for reconsidering when SARS-CoV-2 was introduced in the United States, according to Janet G. Basemen, PhD, a professor of epidemiology and the associate dean of the University of Washington School of Public Health, Seattle. However, she noted that the task of translating data like these into actionable public health strategies has proven difficult in the past.
Symptom-based surveillance systems “have mostly served as situational awareness rather than early detection tools,” Dr. Baseman said. The problem is timely interpretation of a given signal.
Not that she doubts such tools “would be an incredible resource for humanity” if the current limitations can be resolved or that technological advances will lead to better methods of detecting and monitoring pandemics “at some point.” Rather, “we’re just not there yet,” she said.
SOURCE: Basavaraju SV et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1785); Elmore JG et al. J Med Internet Res. 2020;22:e21562).
Doctors publish paper on COVID-19 protocol; Experts unconvinced
Physicians who developed a protocol for treating hospitalized patients with COVID-19 they call MATH+ have now published a literature review with observational mortality rates in the Journal of Intensive Care Medicine (JICM) that they say supports the protocol’s use.
The physicians have been promoting their MATH+ protocol as a way to improve survival from severe COVID-19 since the spring, and this is the first time their protocol and any results have been published in a peer-reviewed journal. But because the paper contains only hospital-level mortality rates compared with previously published observational data and clinical trials (not data from a randomized controlled trial testing the protocol), experts remain unconvinced the protocol benefits patients.
“This is not a study by any stretch of the imagination,” Hugh Cassiere, MD, director of critical care medicine at North Shore University Hospital in Manhasset, New York, told Medscape Medical News via email. “It is comparative data which should never be used to make conclusions of one therapy over another.”
“It’s food for thought for those clinicians [treating COVID-19] and it gives them some options,” said Pierre Kory, MD, MPA, a pulmonary critical care specialist in Wisconsin and one of the protocol developers. “What we really emphasize for this disease is it has to be a combination therapy protocol.”
As Medscape previously reported, MATH+ stands for methylprednisolone, ascorbic acid, thiamine, and heparin. The “+” includes additional therapies like vitamin D, zinc, melatonin, statins, and famotidine. The protocol originated as a variation of the “HAT therapy,” a combination of hydrocortisone, ascorbic acid, and thiamine, which critical care specialist Paul Marik, MD, created for treating critically ill patients with sepsis.
The protocol evolved over a few weeks this spring as Marik, chief of the division of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk, emailed with a small group of colleagues about treatments and their observations of SARS-CoV-2 in action. In March, when Marik and his colleagues formalized the MATH+ protocol, healthcare organizations like the World Health Organization (WHO) were advising against steroids for COVID-19 patients.
Determined to spread a different message, the MATH+ physicians began publicizing the protocol with a website and a small communications team. They tried to get their protocol in front of leading healthcare organizations, like the WHO, and Kory testified remotely in front of the Senate Homeland Security Committee in early May. (Kory testified in front of the committee again earlier this month about the use of ivermectin as a COVID-19 treatment. He told Medscape the MATH+ protocol has been updated to include ivermectin since the submission to JICM.)
The physicians have continued promoting the protocol in the summer and fall, even after the RECOVERY trial showed dexamethasone treatment decreased mortality in hospitalized patients with severe COVID-19 and the WHO and other organizations started recommending the drug.
In the newly published JICM article, the researchers describe a mix of randomized controlled trials, observational studies, and basic science research that inform each of the individual pieces of the MATH+ protocol. Some of the cited research pertains specifically to the treatment of COVID-19.
Other studies the authors use to support the protocol are based on data from other viral outbreaks, like H1N1 and SARS-CoV, as well as other medical conditions, like nonviral acute respiratory distress syndrome and sepsis. The researchers did not conduct a randomized controlled trial of MATH+ for patients with COVID-19 because, as they write in the article, they did not believe they had the clinical equipoise required for such a study.
“With respect to each of the individual ‘core’ therapies of MATH+, all authors felt the therapies either superior to any placebo or possessed evidence of minimal risk and cost compared to potential benefit,” they wrote in the paper.
“With a new disease, it is totally reasonable to take your best guess at a therapy,” wrote F. Perry Wilson, MD, MSCE, director of the Clinical and Translational Research Accelerator at Yale University School of Medicine, in an email to Medscape. “When there is limited information, you go with what you have. What I take issue with here is the authors’ implication that that’s where the scientific process stops. In my mind, it’s actually just the beginning.” Every investigator believes his or her intervention is beneficial but is not sure — that’s why they conduct a randomized controlled trial, Wilson said.
“Without robust trials, we are left with too many options on the table and no way to know what helps — leading to this ‘throw the book at them’ approach, where you just pick your favorite molecule and give it,” said Wilson.
Sam Parnia, MD, PhD, associate professor of medicine and director of critical care and resuscitation research at NYU Langone, echoed this sentiment: “Many of the individual components could be expected to provide benefit and combining therapies is something physicians often do,” Parnia said in an email to Medscape. “I think this is a promising approach; however, this ultimately needs to be studied.”
: United Memorial Hospital in Houston, Texas and Norfolk General Hospital in Norfolk, Virginia. At United Memorial, MATH+ was “systematically” followed for patients admitted to the hospital, and at Norfolk General it was followed for patients admitted to the ICU. The two hospitals treated 140 and 191 COVID-19 patients with MATH+, respectively, as of July 20.
The average observed hospital or 28-day mortality rate at United Memorial was 4.4% and at Norfolk General was 6.1%, for a combined mortality rate of 5.1%. The researchers compared this rate with reported outcomes from 10 studies of more than 400 hospitals in the United States (72 hospitals), the United Kingdom (386), and China (3). The mortality rate for COVID-19 patients at these hospitals ranged from 15.6% to 32%, for an average mortality rate of 22.9%.
The difference in average mortality rates represents a “more than 75% absolute risk reduction in mortality” with MATH+, according to the authors. The data from other hospitals were reported from January to early June, representative of death rates early in the pandemic and before the announcement of the RECOVERY trial results spurred increased use of dexamethasone.
The new numbers may not be convincing to other physicians.
“The comparison of the outcomes in the two hospitals where this protocol is implemented vs mortality rates in other published studies is quite a stretch,” Wilson told Medscape. “Hospitals with robust research programs that publish large cohorts tend to be tertiary care centers where sick patients get referred. Without data on the baseline characteristics of the patients in these studies, it’s really not appropriate to draw apples-to-apples comparisons.”
“There are many factors that lead to different mortality rates [between hospitals] and it often reflects the quality of general ICU care,” said Parnia. For example, many ICUs were overwhelmed and stretched during the pandemic, while others were not.
“This protocol remains a hypothesis in need of a prospective clinical trial,” said Daniel Kaul, MD, professor of infectious diseases at the University of Michigan, Ann Arbor. “Comparing gross mortality rates from different centers at different times with different case mixes is at most hypothesis generating.”
“The use of comparative data is useless information…not based on true comparison of groups,” said Cassiere of the average mortality rates. Only a randomized, placebo-controlled trial can prove if a treatment is effective. “This protocol should be abandoned.”
“The MATH+ is based on negative evidence,” Cassiere told Medscape, pointing to trials that showed no effect for vitamin C (ascorbic acid) and thiamine in critical illnesses. And, given the “overwhelming positive data’’ for dexamethasone to treat patients with severe COVID-19, its exclusion from MATH+ in favor of a steroid that has not been extensively studied for COVID-19 is “reckless and irresponsible,” he said.
Kory pushed back strongly against this assertion, pointing to the decades of research on methylprednisolone as a treatment for lung disease and ARDS outlined in the article. “It has far more evidence than dexamethasone,” he told Medscape over the phone.
“Our recommendation is based on a clear understanding of the pharmacological principle to guide prolonged glucocorticoid administration in ARDS and COVID-19,” wrote G. Umberto Meduri, MD, a MATH+ coauthor and professor in the Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Tennessee Health Science Center in Memphis.
A version of this article first appeared on Medscape.com.
Physicians who developed a protocol for treating hospitalized patients with COVID-19 they call MATH+ have now published a literature review with observational mortality rates in the Journal of Intensive Care Medicine (JICM) that they say supports the protocol’s use.
The physicians have been promoting their MATH+ protocol as a way to improve survival from severe COVID-19 since the spring, and this is the first time their protocol and any results have been published in a peer-reviewed journal. But because the paper contains only hospital-level mortality rates compared with previously published observational data and clinical trials (not data from a randomized controlled trial testing the protocol), experts remain unconvinced the protocol benefits patients.
“This is not a study by any stretch of the imagination,” Hugh Cassiere, MD, director of critical care medicine at North Shore University Hospital in Manhasset, New York, told Medscape Medical News via email. “It is comparative data which should never be used to make conclusions of one therapy over another.”
“It’s food for thought for those clinicians [treating COVID-19] and it gives them some options,” said Pierre Kory, MD, MPA, a pulmonary critical care specialist in Wisconsin and one of the protocol developers. “What we really emphasize for this disease is it has to be a combination therapy protocol.”
As Medscape previously reported, MATH+ stands for methylprednisolone, ascorbic acid, thiamine, and heparin. The “+” includes additional therapies like vitamin D, zinc, melatonin, statins, and famotidine. The protocol originated as a variation of the “HAT therapy,” a combination of hydrocortisone, ascorbic acid, and thiamine, which critical care specialist Paul Marik, MD, created for treating critically ill patients with sepsis.
The protocol evolved over a few weeks this spring as Marik, chief of the division of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk, emailed with a small group of colleagues about treatments and their observations of SARS-CoV-2 in action. In March, when Marik and his colleagues formalized the MATH+ protocol, healthcare organizations like the World Health Organization (WHO) were advising against steroids for COVID-19 patients.
Determined to spread a different message, the MATH+ physicians began publicizing the protocol with a website and a small communications team. They tried to get their protocol in front of leading healthcare organizations, like the WHO, and Kory testified remotely in front of the Senate Homeland Security Committee in early May. (Kory testified in front of the committee again earlier this month about the use of ivermectin as a COVID-19 treatment. He told Medscape the MATH+ protocol has been updated to include ivermectin since the submission to JICM.)
The physicians have continued promoting the protocol in the summer and fall, even after the RECOVERY trial showed dexamethasone treatment decreased mortality in hospitalized patients with severe COVID-19 and the WHO and other organizations started recommending the drug.
In the newly published JICM article, the researchers describe a mix of randomized controlled trials, observational studies, and basic science research that inform each of the individual pieces of the MATH+ protocol. Some of the cited research pertains specifically to the treatment of COVID-19.
Other studies the authors use to support the protocol are based on data from other viral outbreaks, like H1N1 and SARS-CoV, as well as other medical conditions, like nonviral acute respiratory distress syndrome and sepsis. The researchers did not conduct a randomized controlled trial of MATH+ for patients with COVID-19 because, as they write in the article, they did not believe they had the clinical equipoise required for such a study.
“With respect to each of the individual ‘core’ therapies of MATH+, all authors felt the therapies either superior to any placebo or possessed evidence of minimal risk and cost compared to potential benefit,” they wrote in the paper.
“With a new disease, it is totally reasonable to take your best guess at a therapy,” wrote F. Perry Wilson, MD, MSCE, director of the Clinical and Translational Research Accelerator at Yale University School of Medicine, in an email to Medscape. “When there is limited information, you go with what you have. What I take issue with here is the authors’ implication that that’s where the scientific process stops. In my mind, it’s actually just the beginning.” Every investigator believes his or her intervention is beneficial but is not sure — that’s why they conduct a randomized controlled trial, Wilson said.
“Without robust trials, we are left with too many options on the table and no way to know what helps — leading to this ‘throw the book at them’ approach, where you just pick your favorite molecule and give it,” said Wilson.
Sam Parnia, MD, PhD, associate professor of medicine and director of critical care and resuscitation research at NYU Langone, echoed this sentiment: “Many of the individual components could be expected to provide benefit and combining therapies is something physicians often do,” Parnia said in an email to Medscape. “I think this is a promising approach; however, this ultimately needs to be studied.”
: United Memorial Hospital in Houston, Texas and Norfolk General Hospital in Norfolk, Virginia. At United Memorial, MATH+ was “systematically” followed for patients admitted to the hospital, and at Norfolk General it was followed for patients admitted to the ICU. The two hospitals treated 140 and 191 COVID-19 patients with MATH+, respectively, as of July 20.
The average observed hospital or 28-day mortality rate at United Memorial was 4.4% and at Norfolk General was 6.1%, for a combined mortality rate of 5.1%. The researchers compared this rate with reported outcomes from 10 studies of more than 400 hospitals in the United States (72 hospitals), the United Kingdom (386), and China (3). The mortality rate for COVID-19 patients at these hospitals ranged from 15.6% to 32%, for an average mortality rate of 22.9%.
The difference in average mortality rates represents a “more than 75% absolute risk reduction in mortality” with MATH+, according to the authors. The data from other hospitals were reported from January to early June, representative of death rates early in the pandemic and before the announcement of the RECOVERY trial results spurred increased use of dexamethasone.
The new numbers may not be convincing to other physicians.
“The comparison of the outcomes in the two hospitals where this protocol is implemented vs mortality rates in other published studies is quite a stretch,” Wilson told Medscape. “Hospitals with robust research programs that publish large cohorts tend to be tertiary care centers where sick patients get referred. Without data on the baseline characteristics of the patients in these studies, it’s really not appropriate to draw apples-to-apples comparisons.”
“There are many factors that lead to different mortality rates [between hospitals] and it often reflects the quality of general ICU care,” said Parnia. For example, many ICUs were overwhelmed and stretched during the pandemic, while others were not.
“This protocol remains a hypothesis in need of a prospective clinical trial,” said Daniel Kaul, MD, professor of infectious diseases at the University of Michigan, Ann Arbor. “Comparing gross mortality rates from different centers at different times with different case mixes is at most hypothesis generating.”
“The use of comparative data is useless information…not based on true comparison of groups,” said Cassiere of the average mortality rates. Only a randomized, placebo-controlled trial can prove if a treatment is effective. “This protocol should be abandoned.”
“The MATH+ is based on negative evidence,” Cassiere told Medscape, pointing to trials that showed no effect for vitamin C (ascorbic acid) and thiamine in critical illnesses. And, given the “overwhelming positive data’’ for dexamethasone to treat patients with severe COVID-19, its exclusion from MATH+ in favor of a steroid that has not been extensively studied for COVID-19 is “reckless and irresponsible,” he said.
Kory pushed back strongly against this assertion, pointing to the decades of research on methylprednisolone as a treatment for lung disease and ARDS outlined in the article. “It has far more evidence than dexamethasone,” he told Medscape over the phone.
“Our recommendation is based on a clear understanding of the pharmacological principle to guide prolonged glucocorticoid administration in ARDS and COVID-19,” wrote G. Umberto Meduri, MD, a MATH+ coauthor and professor in the Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Tennessee Health Science Center in Memphis.
A version of this article first appeared on Medscape.com.
Physicians who developed a protocol for treating hospitalized patients with COVID-19 they call MATH+ have now published a literature review with observational mortality rates in the Journal of Intensive Care Medicine (JICM) that they say supports the protocol’s use.
The physicians have been promoting their MATH+ protocol as a way to improve survival from severe COVID-19 since the spring, and this is the first time their protocol and any results have been published in a peer-reviewed journal. But because the paper contains only hospital-level mortality rates compared with previously published observational data and clinical trials (not data from a randomized controlled trial testing the protocol), experts remain unconvinced the protocol benefits patients.
“This is not a study by any stretch of the imagination,” Hugh Cassiere, MD, director of critical care medicine at North Shore University Hospital in Manhasset, New York, told Medscape Medical News via email. “It is comparative data which should never be used to make conclusions of one therapy over another.”
“It’s food for thought for those clinicians [treating COVID-19] and it gives them some options,” said Pierre Kory, MD, MPA, a pulmonary critical care specialist in Wisconsin and one of the protocol developers. “What we really emphasize for this disease is it has to be a combination therapy protocol.”
As Medscape previously reported, MATH+ stands for methylprednisolone, ascorbic acid, thiamine, and heparin. The “+” includes additional therapies like vitamin D, zinc, melatonin, statins, and famotidine. The protocol originated as a variation of the “HAT therapy,” a combination of hydrocortisone, ascorbic acid, and thiamine, which critical care specialist Paul Marik, MD, created for treating critically ill patients with sepsis.
The protocol evolved over a few weeks this spring as Marik, chief of the division of pulmonary and critical care medicine at Eastern Virginia Medical School in Norfolk, emailed with a small group of colleagues about treatments and their observations of SARS-CoV-2 in action. In March, when Marik and his colleagues formalized the MATH+ protocol, healthcare organizations like the World Health Organization (WHO) were advising against steroids for COVID-19 patients.
Determined to spread a different message, the MATH+ physicians began publicizing the protocol with a website and a small communications team. They tried to get their protocol in front of leading healthcare organizations, like the WHO, and Kory testified remotely in front of the Senate Homeland Security Committee in early May. (Kory testified in front of the committee again earlier this month about the use of ivermectin as a COVID-19 treatment. He told Medscape the MATH+ protocol has been updated to include ivermectin since the submission to JICM.)
The physicians have continued promoting the protocol in the summer and fall, even after the RECOVERY trial showed dexamethasone treatment decreased mortality in hospitalized patients with severe COVID-19 and the WHO and other organizations started recommending the drug.
In the newly published JICM article, the researchers describe a mix of randomized controlled trials, observational studies, and basic science research that inform each of the individual pieces of the MATH+ protocol. Some of the cited research pertains specifically to the treatment of COVID-19.
Other studies the authors use to support the protocol are based on data from other viral outbreaks, like H1N1 and SARS-CoV, as well as other medical conditions, like nonviral acute respiratory distress syndrome and sepsis. The researchers did not conduct a randomized controlled trial of MATH+ for patients with COVID-19 because, as they write in the article, they did not believe they had the clinical equipoise required for such a study.
“With respect to each of the individual ‘core’ therapies of MATH+, all authors felt the therapies either superior to any placebo or possessed evidence of minimal risk and cost compared to potential benefit,” they wrote in the paper.
“With a new disease, it is totally reasonable to take your best guess at a therapy,” wrote F. Perry Wilson, MD, MSCE, director of the Clinical and Translational Research Accelerator at Yale University School of Medicine, in an email to Medscape. “When there is limited information, you go with what you have. What I take issue with here is the authors’ implication that that’s where the scientific process stops. In my mind, it’s actually just the beginning.” Every investigator believes his or her intervention is beneficial but is not sure — that’s why they conduct a randomized controlled trial, Wilson said.
“Without robust trials, we are left with too many options on the table and no way to know what helps — leading to this ‘throw the book at them’ approach, where you just pick your favorite molecule and give it,” said Wilson.
Sam Parnia, MD, PhD, associate professor of medicine and director of critical care and resuscitation research at NYU Langone, echoed this sentiment: “Many of the individual components could be expected to provide benefit and combining therapies is something physicians often do,” Parnia said in an email to Medscape. “I think this is a promising approach; however, this ultimately needs to be studied.”
: United Memorial Hospital in Houston, Texas and Norfolk General Hospital in Norfolk, Virginia. At United Memorial, MATH+ was “systematically” followed for patients admitted to the hospital, and at Norfolk General it was followed for patients admitted to the ICU. The two hospitals treated 140 and 191 COVID-19 patients with MATH+, respectively, as of July 20.
The average observed hospital or 28-day mortality rate at United Memorial was 4.4% and at Norfolk General was 6.1%, for a combined mortality rate of 5.1%. The researchers compared this rate with reported outcomes from 10 studies of more than 400 hospitals in the United States (72 hospitals), the United Kingdom (386), and China (3). The mortality rate for COVID-19 patients at these hospitals ranged from 15.6% to 32%, for an average mortality rate of 22.9%.
The difference in average mortality rates represents a “more than 75% absolute risk reduction in mortality” with MATH+, according to the authors. The data from other hospitals were reported from January to early June, representative of death rates early in the pandemic and before the announcement of the RECOVERY trial results spurred increased use of dexamethasone.
The new numbers may not be convincing to other physicians.
“The comparison of the outcomes in the two hospitals where this protocol is implemented vs mortality rates in other published studies is quite a stretch,” Wilson told Medscape. “Hospitals with robust research programs that publish large cohorts tend to be tertiary care centers where sick patients get referred. Without data on the baseline characteristics of the patients in these studies, it’s really not appropriate to draw apples-to-apples comparisons.”
“There are many factors that lead to different mortality rates [between hospitals] and it often reflects the quality of general ICU care,” said Parnia. For example, many ICUs were overwhelmed and stretched during the pandemic, while others were not.
“This protocol remains a hypothesis in need of a prospective clinical trial,” said Daniel Kaul, MD, professor of infectious diseases at the University of Michigan, Ann Arbor. “Comparing gross mortality rates from different centers at different times with different case mixes is at most hypothesis generating.”
“The use of comparative data is useless information…not based on true comparison of groups,” said Cassiere of the average mortality rates. Only a randomized, placebo-controlled trial can prove if a treatment is effective. “This protocol should be abandoned.”
“The MATH+ is based on negative evidence,” Cassiere told Medscape, pointing to trials that showed no effect for vitamin C (ascorbic acid) and thiamine in critical illnesses. And, given the “overwhelming positive data’’ for dexamethasone to treat patients with severe COVID-19, its exclusion from MATH+ in favor of a steroid that has not been extensively studied for COVID-19 is “reckless and irresponsible,” he said.
Kory pushed back strongly against this assertion, pointing to the decades of research on methylprednisolone as a treatment for lung disease and ARDS outlined in the article. “It has far more evidence than dexamethasone,” he told Medscape over the phone.
“Our recommendation is based on a clear understanding of the pharmacological principle to guide prolonged glucocorticoid administration in ARDS and COVID-19,” wrote G. Umberto Meduri, MD, a MATH+ coauthor and professor in the Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Tennessee Health Science Center in Memphis.
A version of this article first appeared on Medscape.com.
The top pediatric articles of 2019
Updates in pediatric hospital medicine
The expansion of the field of pediatric hospital medicine in the past 30 years has resulted in improved health care outcomes for hospitalized children1,2 and has been accompanied by a robust increase in the amount of scholarly work related to the field.3 We performed a review of the literature published in 2019 to identify the 10 articles that had the most impact on pediatric hospital medicine, and presented the findings at HM20 Virtual, the 2020 annual conference of the Society of Hospital Medicine. Five of the selected articles are highlighted here.
STUDY 1
Wechsler ME et al. Step-up therapy in black children and adults with poorly controlled asthma. N Engl J Med. 2019 Sep 26;381(13):1227-39.
Background
Current pediatric asthma guidelines suggest adding a long-acting beta-agonist (LABA) to inhaled corticosteroid (ICS) therapy, rather than increasing the ICS dose, for children with poorly controlled asthma. However, these data are based on trials with disproportionately few Black subjects. This study aimed to determine the best step-up therapy for Black patients whose asthma was poorly controlled on ICS monotherapy.
Study overview and results
The authors reported two parallel double-blind, randomized, controlled trials, one in children and one in adolescents and adults. The study of children included 280 subjects ranging in age from 5 to 11, with at least one Black grandparent, and with poorly controlled asthma on low-dose ICS therapy. It used a four-way crossover design in which each subject was treated with four different 14-week treatment regimens: either double (medium-dose) or quintuple (high-dose) their baseline ICS dose, with or without the addition of a LABA. A superior response was defined by the composite outcome of at least one fewer asthma exacerbation, more asthma-control days, or a 5–percentage point difference in predicted FEV1. Forty-six percent of children had improved asthma outcomes when the ICS dose was increased rather than with the addition of a LABA. In contrast, Black adolescents and Black adults had superior responses to the addition of a LABA. There was no significant interaction between the percentage of African ancestry as determined by DNA genotyping and the primary composite outcome. High-dose ICS was associated with a decrease in the ratio of urinary cortisol to creatinine in children younger than 8 years.
Limitations
Approximately 25% of children dropped out of the study, with disproportionately more children dropping out while on a high-dose ICS regimen. Additionally, the difference in the composite outcome was primarily driven by differences in FEV1, with few subjects demonstrating a difference in asthma exacerbations or asthma-control days. Although a decrease in urinary cortisol to creatinine ratio was noted in children under 8 on high-dose ICS, the study period was not long enough to determine the clinical implications of this finding.
Important findings and implications
While studies with a majority of white children have suggested a superior response from adding a LABA compared to increasing the dose of an ICS, almost half of Black children showed a superior response when the dose of an ICS was increased rather than adding a LABA. It is important to note that current guidelines are based on studies with a disproportionate majority of white subjects and may not accurately reflect optimal care for patients in other racial groups. This study underscores the need to include a diverse patient population in research studies.
STUDY 2
Chang PW, Newman TB. A simpler prediction rule for rebound hyperbilirubinemia. Pediatrics. 2019 Jul;144(1):e20183712.
Background
Hyperbilirubinemia (jaundice) is estimated to affect 50%-60% of all newborns. Rebound hyperbilirubinemia – a rise in bilirubin after cessation of phototherapy – is common and can lead to recently discharged infants being readmitted for additional therapy. Lack of clear guidelines regarding when to discharge infants with hyperbilirubinemia has likely contributed to practice variation and some trepidation regarding whether a bilirubin level is “low enough” to discontinue therapy.
Study overview and results
The authors had previously proposed a three-factor hyperbilirubinemia risk model and sought to simplify their rule further.4 They examined a retrospective cohort of 7,048 infants greater than or equal to 35 weeks’ gestation using a random split sample. The authors derived a two-factor model using the same methods and compared its performance to the three-factor model. The two-factor formula was shown to be a good fit as a logistic regression model (Hosmer-Lemeshow test 9.21; P = .33), and the AUROC (area under the receiver operating characteristic) curves for the derivation and validation cohorts were similar between the two-factor (0.877 and 0.876, respectively) and three-factor risk models (0.887 and 0.881, respectively).
Limitations
These data are limited to infants receiving their first treatment of phototherapy and have not been externally validated. An important variable, serum bilirubin at phototherapy termination, was estimated in most subjects, which may have affected the accuracy of the prediction rule. Whether infants received home phototherapy was based only on equipment orders, and some infants may have received phototherapy unbeknownst to investigators. Last, infants with rebound hyperbilirubinemia at less than 72 hours after phototherapy discontinuation may have been missed.
Important findings and implications
This prediction model provides evidence-based, concrete data that can be used in making joint decisions with families regarding discharge timing of infants with hyperbilirubinemia. It also could be beneficial when deciding appropriate follow-up time after discharge.
STUDY 3
Ramgopal S et al. Risk of serious bacterial infection in infants aged ≤60 days presenting to emergency departments with a history of fever only. J Pediatr. 2019 Jan;204:191-195. doi: 10.1016/j.jpeds.2018.08.043.
Background
Febrile infants aged 60 days and younger are at risk for serious bacterial infections (SBI) including urinary tract infections (UTI), bacteremia, and meningitis. As physical exam is a poor discriminator of SBI in this age group, providers frequently rely on laboratory values and risk factors to guide management. Infants presenting with documented fevers by caregivers but found to have no fever in the emergency department are a challenge, and there are limited data regarding SBI frequency in this population.
Study overview and results
The authors performed a secondary analysis of a prospectively gathered cohort of infants aged 60 days and younger within the Pediatric Emergency Care Applied Research Network (PECARN) who had blood, urine, and CSF data available. Notable exclusions included infants who were premature, had a focal infection, were clinically ill, had recent antibiotic use, did not have blood, urine, and CSF data available, or were lost to telephone follow-up at 7 days to ensure wellness. The study cohort included 6,014 infants, 1,233 (32%) who were febrile by history alone. Rates of overall SBI were lower in the afebrile group (8.8% vs. 12.8%). For infants 0-28 days, rates of UTI were lower for the afebrile group (9.5% vs. 14.5%), but there was no difference in the rates of bacteremia or meningitis. For infants 29-60 days, rates of UTI (6.6% vs. 9.3%) and bacteremia (.5% vs. 1.7%) were lower in the afebrile group.
Limitations
Neither the use of home antipyretics nor the method of temperature taking at home were studied. Also, as this was a secondary analysis, it is possible that not all infants who presented with history of fever only were captured, as work-up was dictated by individual treating providers who may have chosen not to work up certain afebrile infants.
Important findings and implications
Nearly one-third of infants presenting for fever evaluation are afebrile on arrival. Although overall rates of SBI were lower in the group with fever by history only, this difference is largely accounted for by differing rates of UTI. Rates of bacteremia and meningitis remained substantial between groups, particularly for infants aged 0-28 days. Because of the significant morbidity associated with these infections, it is reasonable to suggest that absence of fever on presentation alone should not alter clinical or laboratory work-up, particularly in infants 0-28 days.
STUDY 4
Humphrey-Murto S et al. The influence of prior performance information on ratings of current performance and implications for learner handover: A scoping review. Acad Med. 2019 Jul;94(7):1050-7.
Background
Learner Handover (LH) or “forward feeding” occurs when information about trainees is shared between faculty supervisors. Although this can be helpful to tailor educational experiences and build upon previous assessments, it risks stigmatizing trainees and adding bias to future feedback and assessments as the trainee never really has a “clean slate.” In this study, the authors sought to uncover the key concepts of how prior performance information (PPI) influences assessments and any implications for medical education.
Study overview and results
The authors performed a cross-disciplinary scoping review looking at over 17,000 articles published between 1980 and 2017 across the domains of psychology, sports, business, and education. Seven themes were identified with the following notable findings. Raters exposed to positive PPI scored a learner’s performance higher, and vice versa. There was a dose-response relationship with more positive and more negative PPI resulting in higher and lower assessments, respectively. General standards, such as a direction to complete all work in a timely manner, caused an assimilation effect, while specific standards, such as a direction to complete a certain task by a certain day, did not. More motivated and more experienced raters are less affected by PPI, and those who believe that people can change (incremental theorists) are less affected by PPI while those who believe personal attributes are fixed (entity theorists) are more affected.
Limitations
The heterogeneity of the studies and the fact that they were largely conducted in experimental settings may limit generalizability to medical education. Slightly less than half of the studies included a control arm. Last, most of the studies looked at the ratings of only one target performance, not multiple performances over time.
Important findings and implications
Ratings of current performance displace toward PPI direction, with negative PPI more influential than positive PPI. In a formative setting, PPI may help the assessor focus on areas of possible weakness. In contrast, for a summative assessment, PPI may be prejudicial and have an impact on the rating given to the student. Clinicians should be mindful of the information they share with future raters about learners and the potential bias on future assessments that can manifest as a result.
STUDY 5
McCann ME et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): An international, multicentre, randomised, controlled equivalence trial. The Lancet. 2019 Feb;393:664-77.
Background
Animal models and observational studies have suggested a link between early anesthesia exposure and adverse neurocognitive outcomes; however, findings have been mixed and studies are prone to confounding. This study is the first randomized controlled trial to compare neurocognitive outcomes for infants exposed to general anesthesia versus awake-regional anesthesia.
Study overview and results
In this international, multicenter, assessor-masked trial, 722 infants undergoing inguinal hernia repair were randomized to awake-regional anesthesia or single-agent sevoflurane-based general anesthesia. Infants born at greater than 26 weeks’ gestational age were eligible, while those with prior anesthesia exposure or risks for neurocognitive delay were excluded. The primary outcome was full-scale intelligence quotient (FSIQ) testing at 5 years of age on the Wechsler Preschool and Primary Scale of Intelligence, third edition (WPPSI-III). Seven additional neurodevelopmental assessments and parental questionnaires regarding behavior were administered as secondary outcomes. Average anesthesia exposure was 54 minutes and no infant had exposure greater than 120 minutes. There was no significant difference in mean scores on WPPSI-III FSIQ testing, and no difference in the additional neurocognitive assessments or parent-reported outcomes used as secondary outcomes.
Limitations
This study was limited to single, short periods of single-agent anesthesia exposure in children with no additional neurologic risk factors, so caution should be used in extrapolating these data to children with medical complexity and children undergoing multiple procedures, longer surgeries, or multidrug anesthetic regimens. The study population was majority male because of the surgical pathology selected and included only children in the narrow range of postmenstrual age 60 weeks or less. While this population represents a suspected a period of high cerebral vulnerability based on animal models, the implications of anesthesia exposure at other ages are unclear.
Important findings and implications
An estimated 10% of children from developed countries are exposed to general anesthesia during the first 3 years of life. While hospitalists do not typically select the route of anesthesia, they frequently care for patients undergoing procedures and must address parental concerns regarding the safety of anesthesia exposure. Given the rigorous study methods and long-term follow up in the current study, these data should provide reassurance that, for healthy infants undergoing short, single-agent anesthetic exposure, there is no evidence of future adverse neurologic outcomes.
Dr. Russo is director of pediatrics, medical director for quality and innovation, at WellSpan Health, York, Pa. Dr. Money is a pediatric hospitalist at Primary Children’s Hospital, University of Utah School of Medicine, Salt Lake City. Dr. Steed is instructor of hospital medicine, Northwestern Memorial Hospital and Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University School of Medicine, Chicago. The authors would like to thank Dr. Klint M. Schwenk and the Society for Hospital Medicine Pediatric Special Interest Group Executive Council.
References
1. Roberts KB, Fisher ER, and Rauch DA. The history of pediatric hospital medicine in the United States, 1996-2019. J Hosp Med. 2020 Jul;15(7):424-7.
2. Mussman GM and Conway PH. Pediatric hospitalist systems versus traditional models of care: Effect on quality and cost outcomes. J Hosp Med. 2012 Apr;7(4):350-7.
3. Wang ME, Shaughnessy EE, and Leyenaar JK. The future of pediatric hospital medicine: Challenges and opportunities. J Hosp Med. 2020 Jul;15(7):428-30.
4. Chang PW et al. A clinical prediction rule for rebound hyperbilirubinemia following inpatient phototherapy. Pediatrics. 2017;139 Mar;139(3):e20162896.
Updates in pediatric hospital medicine
Updates in pediatric hospital medicine
The expansion of the field of pediatric hospital medicine in the past 30 years has resulted in improved health care outcomes for hospitalized children1,2 and has been accompanied by a robust increase in the amount of scholarly work related to the field.3 We performed a review of the literature published in 2019 to identify the 10 articles that had the most impact on pediatric hospital medicine, and presented the findings at HM20 Virtual, the 2020 annual conference of the Society of Hospital Medicine. Five of the selected articles are highlighted here.
STUDY 1
Wechsler ME et al. Step-up therapy in black children and adults with poorly controlled asthma. N Engl J Med. 2019 Sep 26;381(13):1227-39.
Background
Current pediatric asthma guidelines suggest adding a long-acting beta-agonist (LABA) to inhaled corticosteroid (ICS) therapy, rather than increasing the ICS dose, for children with poorly controlled asthma. However, these data are based on trials with disproportionately few Black subjects. This study aimed to determine the best step-up therapy for Black patients whose asthma was poorly controlled on ICS monotherapy.
Study overview and results
The authors reported two parallel double-blind, randomized, controlled trials, one in children and one in adolescents and adults. The study of children included 280 subjects ranging in age from 5 to 11, with at least one Black grandparent, and with poorly controlled asthma on low-dose ICS therapy. It used a four-way crossover design in which each subject was treated with four different 14-week treatment regimens: either double (medium-dose) or quintuple (high-dose) their baseline ICS dose, with or without the addition of a LABA. A superior response was defined by the composite outcome of at least one fewer asthma exacerbation, more asthma-control days, or a 5–percentage point difference in predicted FEV1. Forty-six percent of children had improved asthma outcomes when the ICS dose was increased rather than with the addition of a LABA. In contrast, Black adolescents and Black adults had superior responses to the addition of a LABA. There was no significant interaction between the percentage of African ancestry as determined by DNA genotyping and the primary composite outcome. High-dose ICS was associated with a decrease in the ratio of urinary cortisol to creatinine in children younger than 8 years.
Limitations
Approximately 25% of children dropped out of the study, with disproportionately more children dropping out while on a high-dose ICS regimen. Additionally, the difference in the composite outcome was primarily driven by differences in FEV1, with few subjects demonstrating a difference in asthma exacerbations or asthma-control days. Although a decrease in urinary cortisol to creatinine ratio was noted in children under 8 on high-dose ICS, the study period was not long enough to determine the clinical implications of this finding.
Important findings and implications
While studies with a majority of white children have suggested a superior response from adding a LABA compared to increasing the dose of an ICS, almost half of Black children showed a superior response when the dose of an ICS was increased rather than adding a LABA. It is important to note that current guidelines are based on studies with a disproportionate majority of white subjects and may not accurately reflect optimal care for patients in other racial groups. This study underscores the need to include a diverse patient population in research studies.
STUDY 2
Chang PW, Newman TB. A simpler prediction rule for rebound hyperbilirubinemia. Pediatrics. 2019 Jul;144(1):e20183712.
Background
Hyperbilirubinemia (jaundice) is estimated to affect 50%-60% of all newborns. Rebound hyperbilirubinemia – a rise in bilirubin after cessation of phototherapy – is common and can lead to recently discharged infants being readmitted for additional therapy. Lack of clear guidelines regarding when to discharge infants with hyperbilirubinemia has likely contributed to practice variation and some trepidation regarding whether a bilirubin level is “low enough” to discontinue therapy.
Study overview and results
The authors had previously proposed a three-factor hyperbilirubinemia risk model and sought to simplify their rule further.4 They examined a retrospective cohort of 7,048 infants greater than or equal to 35 weeks’ gestation using a random split sample. The authors derived a two-factor model using the same methods and compared its performance to the three-factor model. The two-factor formula was shown to be a good fit as a logistic regression model (Hosmer-Lemeshow test 9.21; P = .33), and the AUROC (area under the receiver operating characteristic) curves for the derivation and validation cohorts were similar between the two-factor (0.877 and 0.876, respectively) and three-factor risk models (0.887 and 0.881, respectively).
Limitations
These data are limited to infants receiving their first treatment of phototherapy and have not been externally validated. An important variable, serum bilirubin at phototherapy termination, was estimated in most subjects, which may have affected the accuracy of the prediction rule. Whether infants received home phototherapy was based only on equipment orders, and some infants may have received phototherapy unbeknownst to investigators. Last, infants with rebound hyperbilirubinemia at less than 72 hours after phototherapy discontinuation may have been missed.
Important findings and implications
This prediction model provides evidence-based, concrete data that can be used in making joint decisions with families regarding discharge timing of infants with hyperbilirubinemia. It also could be beneficial when deciding appropriate follow-up time after discharge.
STUDY 3
Ramgopal S et al. Risk of serious bacterial infection in infants aged ≤60 days presenting to emergency departments with a history of fever only. J Pediatr. 2019 Jan;204:191-195. doi: 10.1016/j.jpeds.2018.08.043.
Background
Febrile infants aged 60 days and younger are at risk for serious bacterial infections (SBI) including urinary tract infections (UTI), bacteremia, and meningitis. As physical exam is a poor discriminator of SBI in this age group, providers frequently rely on laboratory values and risk factors to guide management. Infants presenting with documented fevers by caregivers but found to have no fever in the emergency department are a challenge, and there are limited data regarding SBI frequency in this population.
Study overview and results
The authors performed a secondary analysis of a prospectively gathered cohort of infants aged 60 days and younger within the Pediatric Emergency Care Applied Research Network (PECARN) who had blood, urine, and CSF data available. Notable exclusions included infants who were premature, had a focal infection, were clinically ill, had recent antibiotic use, did not have blood, urine, and CSF data available, or were lost to telephone follow-up at 7 days to ensure wellness. The study cohort included 6,014 infants, 1,233 (32%) who were febrile by history alone. Rates of overall SBI were lower in the afebrile group (8.8% vs. 12.8%). For infants 0-28 days, rates of UTI were lower for the afebrile group (9.5% vs. 14.5%), but there was no difference in the rates of bacteremia or meningitis. For infants 29-60 days, rates of UTI (6.6% vs. 9.3%) and bacteremia (.5% vs. 1.7%) were lower in the afebrile group.
Limitations
Neither the use of home antipyretics nor the method of temperature taking at home were studied. Also, as this was a secondary analysis, it is possible that not all infants who presented with history of fever only were captured, as work-up was dictated by individual treating providers who may have chosen not to work up certain afebrile infants.
Important findings and implications
Nearly one-third of infants presenting for fever evaluation are afebrile on arrival. Although overall rates of SBI were lower in the group with fever by history only, this difference is largely accounted for by differing rates of UTI. Rates of bacteremia and meningitis remained substantial between groups, particularly for infants aged 0-28 days. Because of the significant morbidity associated with these infections, it is reasonable to suggest that absence of fever on presentation alone should not alter clinical or laboratory work-up, particularly in infants 0-28 days.
STUDY 4
Humphrey-Murto S et al. The influence of prior performance information on ratings of current performance and implications for learner handover: A scoping review. Acad Med. 2019 Jul;94(7):1050-7.
Background
Learner Handover (LH) or “forward feeding” occurs when information about trainees is shared between faculty supervisors. Although this can be helpful to tailor educational experiences and build upon previous assessments, it risks stigmatizing trainees and adding bias to future feedback and assessments as the trainee never really has a “clean slate.” In this study, the authors sought to uncover the key concepts of how prior performance information (PPI) influences assessments and any implications for medical education.
Study overview and results
The authors performed a cross-disciplinary scoping review looking at over 17,000 articles published between 1980 and 2017 across the domains of psychology, sports, business, and education. Seven themes were identified with the following notable findings. Raters exposed to positive PPI scored a learner’s performance higher, and vice versa. There was a dose-response relationship with more positive and more negative PPI resulting in higher and lower assessments, respectively. General standards, such as a direction to complete all work in a timely manner, caused an assimilation effect, while specific standards, such as a direction to complete a certain task by a certain day, did not. More motivated and more experienced raters are less affected by PPI, and those who believe that people can change (incremental theorists) are less affected by PPI while those who believe personal attributes are fixed (entity theorists) are more affected.
Limitations
The heterogeneity of the studies and the fact that they were largely conducted in experimental settings may limit generalizability to medical education. Slightly less than half of the studies included a control arm. Last, most of the studies looked at the ratings of only one target performance, not multiple performances over time.
Important findings and implications
Ratings of current performance displace toward PPI direction, with negative PPI more influential than positive PPI. In a formative setting, PPI may help the assessor focus on areas of possible weakness. In contrast, for a summative assessment, PPI may be prejudicial and have an impact on the rating given to the student. Clinicians should be mindful of the information they share with future raters about learners and the potential bias on future assessments that can manifest as a result.
STUDY 5
McCann ME et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): An international, multicentre, randomised, controlled equivalence trial. The Lancet. 2019 Feb;393:664-77.
Background
Animal models and observational studies have suggested a link between early anesthesia exposure and adverse neurocognitive outcomes; however, findings have been mixed and studies are prone to confounding. This study is the first randomized controlled trial to compare neurocognitive outcomes for infants exposed to general anesthesia versus awake-regional anesthesia.
Study overview and results
In this international, multicenter, assessor-masked trial, 722 infants undergoing inguinal hernia repair were randomized to awake-regional anesthesia or single-agent sevoflurane-based general anesthesia. Infants born at greater than 26 weeks’ gestational age were eligible, while those with prior anesthesia exposure or risks for neurocognitive delay were excluded. The primary outcome was full-scale intelligence quotient (FSIQ) testing at 5 years of age on the Wechsler Preschool and Primary Scale of Intelligence, third edition (WPPSI-III). Seven additional neurodevelopmental assessments and parental questionnaires regarding behavior were administered as secondary outcomes. Average anesthesia exposure was 54 minutes and no infant had exposure greater than 120 minutes. There was no significant difference in mean scores on WPPSI-III FSIQ testing, and no difference in the additional neurocognitive assessments or parent-reported outcomes used as secondary outcomes.
Limitations
This study was limited to single, short periods of single-agent anesthesia exposure in children with no additional neurologic risk factors, so caution should be used in extrapolating these data to children with medical complexity and children undergoing multiple procedures, longer surgeries, or multidrug anesthetic regimens. The study population was majority male because of the surgical pathology selected and included only children in the narrow range of postmenstrual age 60 weeks or less. While this population represents a suspected a period of high cerebral vulnerability based on animal models, the implications of anesthesia exposure at other ages are unclear.
Important findings and implications
An estimated 10% of children from developed countries are exposed to general anesthesia during the first 3 years of life. While hospitalists do not typically select the route of anesthesia, they frequently care for patients undergoing procedures and must address parental concerns regarding the safety of anesthesia exposure. Given the rigorous study methods and long-term follow up in the current study, these data should provide reassurance that, for healthy infants undergoing short, single-agent anesthetic exposure, there is no evidence of future adverse neurologic outcomes.
Dr. Russo is director of pediatrics, medical director for quality and innovation, at WellSpan Health, York, Pa. Dr. Money is a pediatric hospitalist at Primary Children’s Hospital, University of Utah School of Medicine, Salt Lake City. Dr. Steed is instructor of hospital medicine, Northwestern Memorial Hospital and Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University School of Medicine, Chicago. The authors would like to thank Dr. Klint M. Schwenk and the Society for Hospital Medicine Pediatric Special Interest Group Executive Council.
References
1. Roberts KB, Fisher ER, and Rauch DA. The history of pediatric hospital medicine in the United States, 1996-2019. J Hosp Med. 2020 Jul;15(7):424-7.
2. Mussman GM and Conway PH. Pediatric hospitalist systems versus traditional models of care: Effect on quality and cost outcomes. J Hosp Med. 2012 Apr;7(4):350-7.
3. Wang ME, Shaughnessy EE, and Leyenaar JK. The future of pediatric hospital medicine: Challenges and opportunities. J Hosp Med. 2020 Jul;15(7):428-30.
4. Chang PW et al. A clinical prediction rule for rebound hyperbilirubinemia following inpatient phototherapy. Pediatrics. 2017;139 Mar;139(3):e20162896.
The expansion of the field of pediatric hospital medicine in the past 30 years has resulted in improved health care outcomes for hospitalized children1,2 and has been accompanied by a robust increase in the amount of scholarly work related to the field.3 We performed a review of the literature published in 2019 to identify the 10 articles that had the most impact on pediatric hospital medicine, and presented the findings at HM20 Virtual, the 2020 annual conference of the Society of Hospital Medicine. Five of the selected articles are highlighted here.
STUDY 1
Wechsler ME et al. Step-up therapy in black children and adults with poorly controlled asthma. N Engl J Med. 2019 Sep 26;381(13):1227-39.
Background
Current pediatric asthma guidelines suggest adding a long-acting beta-agonist (LABA) to inhaled corticosteroid (ICS) therapy, rather than increasing the ICS dose, for children with poorly controlled asthma. However, these data are based on trials with disproportionately few Black subjects. This study aimed to determine the best step-up therapy for Black patients whose asthma was poorly controlled on ICS monotherapy.
Study overview and results
The authors reported two parallel double-blind, randomized, controlled trials, one in children and one in adolescents and adults. The study of children included 280 subjects ranging in age from 5 to 11, with at least one Black grandparent, and with poorly controlled asthma on low-dose ICS therapy. It used a four-way crossover design in which each subject was treated with four different 14-week treatment regimens: either double (medium-dose) or quintuple (high-dose) their baseline ICS dose, with or without the addition of a LABA. A superior response was defined by the composite outcome of at least one fewer asthma exacerbation, more asthma-control days, or a 5–percentage point difference in predicted FEV1. Forty-six percent of children had improved asthma outcomes when the ICS dose was increased rather than with the addition of a LABA. In contrast, Black adolescents and Black adults had superior responses to the addition of a LABA. There was no significant interaction between the percentage of African ancestry as determined by DNA genotyping and the primary composite outcome. High-dose ICS was associated with a decrease in the ratio of urinary cortisol to creatinine in children younger than 8 years.
Limitations
Approximately 25% of children dropped out of the study, with disproportionately more children dropping out while on a high-dose ICS regimen. Additionally, the difference in the composite outcome was primarily driven by differences in FEV1, with few subjects demonstrating a difference in asthma exacerbations or asthma-control days. Although a decrease in urinary cortisol to creatinine ratio was noted in children under 8 on high-dose ICS, the study period was not long enough to determine the clinical implications of this finding.
Important findings and implications
While studies with a majority of white children have suggested a superior response from adding a LABA compared to increasing the dose of an ICS, almost half of Black children showed a superior response when the dose of an ICS was increased rather than adding a LABA. It is important to note that current guidelines are based on studies with a disproportionate majority of white subjects and may not accurately reflect optimal care for patients in other racial groups. This study underscores the need to include a diverse patient population in research studies.
STUDY 2
Chang PW, Newman TB. A simpler prediction rule for rebound hyperbilirubinemia. Pediatrics. 2019 Jul;144(1):e20183712.
Background
Hyperbilirubinemia (jaundice) is estimated to affect 50%-60% of all newborns. Rebound hyperbilirubinemia – a rise in bilirubin after cessation of phototherapy – is common and can lead to recently discharged infants being readmitted for additional therapy. Lack of clear guidelines regarding when to discharge infants with hyperbilirubinemia has likely contributed to practice variation and some trepidation regarding whether a bilirubin level is “low enough” to discontinue therapy.
Study overview and results
The authors had previously proposed a three-factor hyperbilirubinemia risk model and sought to simplify their rule further.4 They examined a retrospective cohort of 7,048 infants greater than or equal to 35 weeks’ gestation using a random split sample. The authors derived a two-factor model using the same methods and compared its performance to the three-factor model. The two-factor formula was shown to be a good fit as a logistic regression model (Hosmer-Lemeshow test 9.21; P = .33), and the AUROC (area under the receiver operating characteristic) curves for the derivation and validation cohorts were similar between the two-factor (0.877 and 0.876, respectively) and three-factor risk models (0.887 and 0.881, respectively).
Limitations
These data are limited to infants receiving their first treatment of phototherapy and have not been externally validated. An important variable, serum bilirubin at phototherapy termination, was estimated in most subjects, which may have affected the accuracy of the prediction rule. Whether infants received home phototherapy was based only on equipment orders, and some infants may have received phototherapy unbeknownst to investigators. Last, infants with rebound hyperbilirubinemia at less than 72 hours after phototherapy discontinuation may have been missed.
Important findings and implications
This prediction model provides evidence-based, concrete data that can be used in making joint decisions with families regarding discharge timing of infants with hyperbilirubinemia. It also could be beneficial when deciding appropriate follow-up time after discharge.
STUDY 3
Ramgopal S et al. Risk of serious bacterial infection in infants aged ≤60 days presenting to emergency departments with a history of fever only. J Pediatr. 2019 Jan;204:191-195. doi: 10.1016/j.jpeds.2018.08.043.
Background
Febrile infants aged 60 days and younger are at risk for serious bacterial infections (SBI) including urinary tract infections (UTI), bacteremia, and meningitis. As physical exam is a poor discriminator of SBI in this age group, providers frequently rely on laboratory values and risk factors to guide management. Infants presenting with documented fevers by caregivers but found to have no fever in the emergency department are a challenge, and there are limited data regarding SBI frequency in this population.
Study overview and results
The authors performed a secondary analysis of a prospectively gathered cohort of infants aged 60 days and younger within the Pediatric Emergency Care Applied Research Network (PECARN) who had blood, urine, and CSF data available. Notable exclusions included infants who were premature, had a focal infection, were clinically ill, had recent antibiotic use, did not have blood, urine, and CSF data available, or were lost to telephone follow-up at 7 days to ensure wellness. The study cohort included 6,014 infants, 1,233 (32%) who were febrile by history alone. Rates of overall SBI were lower in the afebrile group (8.8% vs. 12.8%). For infants 0-28 days, rates of UTI were lower for the afebrile group (9.5% vs. 14.5%), but there was no difference in the rates of bacteremia or meningitis. For infants 29-60 days, rates of UTI (6.6% vs. 9.3%) and bacteremia (.5% vs. 1.7%) were lower in the afebrile group.
Limitations
Neither the use of home antipyretics nor the method of temperature taking at home were studied. Also, as this was a secondary analysis, it is possible that not all infants who presented with history of fever only were captured, as work-up was dictated by individual treating providers who may have chosen not to work up certain afebrile infants.
Important findings and implications
Nearly one-third of infants presenting for fever evaluation are afebrile on arrival. Although overall rates of SBI were lower in the group with fever by history only, this difference is largely accounted for by differing rates of UTI. Rates of bacteremia and meningitis remained substantial between groups, particularly for infants aged 0-28 days. Because of the significant morbidity associated with these infections, it is reasonable to suggest that absence of fever on presentation alone should not alter clinical or laboratory work-up, particularly in infants 0-28 days.
STUDY 4
Humphrey-Murto S et al. The influence of prior performance information on ratings of current performance and implications for learner handover: A scoping review. Acad Med. 2019 Jul;94(7):1050-7.
Background
Learner Handover (LH) or “forward feeding” occurs when information about trainees is shared between faculty supervisors. Although this can be helpful to tailor educational experiences and build upon previous assessments, it risks stigmatizing trainees and adding bias to future feedback and assessments as the trainee never really has a “clean slate.” In this study, the authors sought to uncover the key concepts of how prior performance information (PPI) influences assessments and any implications for medical education.
Study overview and results
The authors performed a cross-disciplinary scoping review looking at over 17,000 articles published between 1980 and 2017 across the domains of psychology, sports, business, and education. Seven themes were identified with the following notable findings. Raters exposed to positive PPI scored a learner’s performance higher, and vice versa. There was a dose-response relationship with more positive and more negative PPI resulting in higher and lower assessments, respectively. General standards, such as a direction to complete all work in a timely manner, caused an assimilation effect, while specific standards, such as a direction to complete a certain task by a certain day, did not. More motivated and more experienced raters are less affected by PPI, and those who believe that people can change (incremental theorists) are less affected by PPI while those who believe personal attributes are fixed (entity theorists) are more affected.
Limitations
The heterogeneity of the studies and the fact that they were largely conducted in experimental settings may limit generalizability to medical education. Slightly less than half of the studies included a control arm. Last, most of the studies looked at the ratings of only one target performance, not multiple performances over time.
Important findings and implications
Ratings of current performance displace toward PPI direction, with negative PPI more influential than positive PPI. In a formative setting, PPI may help the assessor focus on areas of possible weakness. In contrast, for a summative assessment, PPI may be prejudicial and have an impact on the rating given to the student. Clinicians should be mindful of the information they share with future raters about learners and the potential bias on future assessments that can manifest as a result.
STUDY 5
McCann ME et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): An international, multicentre, randomised, controlled equivalence trial. The Lancet. 2019 Feb;393:664-77.
Background
Animal models and observational studies have suggested a link between early anesthesia exposure and adverse neurocognitive outcomes; however, findings have been mixed and studies are prone to confounding. This study is the first randomized controlled trial to compare neurocognitive outcomes for infants exposed to general anesthesia versus awake-regional anesthesia.
Study overview and results
In this international, multicenter, assessor-masked trial, 722 infants undergoing inguinal hernia repair were randomized to awake-regional anesthesia or single-agent sevoflurane-based general anesthesia. Infants born at greater than 26 weeks’ gestational age were eligible, while those with prior anesthesia exposure or risks for neurocognitive delay were excluded. The primary outcome was full-scale intelligence quotient (FSIQ) testing at 5 years of age on the Wechsler Preschool and Primary Scale of Intelligence, third edition (WPPSI-III). Seven additional neurodevelopmental assessments and parental questionnaires regarding behavior were administered as secondary outcomes. Average anesthesia exposure was 54 minutes and no infant had exposure greater than 120 minutes. There was no significant difference in mean scores on WPPSI-III FSIQ testing, and no difference in the additional neurocognitive assessments or parent-reported outcomes used as secondary outcomes.
Limitations
This study was limited to single, short periods of single-agent anesthesia exposure in children with no additional neurologic risk factors, so caution should be used in extrapolating these data to children with medical complexity and children undergoing multiple procedures, longer surgeries, or multidrug anesthetic regimens. The study population was majority male because of the surgical pathology selected and included only children in the narrow range of postmenstrual age 60 weeks or less. While this population represents a suspected a period of high cerebral vulnerability based on animal models, the implications of anesthesia exposure at other ages are unclear.
Important findings and implications
An estimated 10% of children from developed countries are exposed to general anesthesia during the first 3 years of life. While hospitalists do not typically select the route of anesthesia, they frequently care for patients undergoing procedures and must address parental concerns regarding the safety of anesthesia exposure. Given the rigorous study methods and long-term follow up in the current study, these data should provide reassurance that, for healthy infants undergoing short, single-agent anesthetic exposure, there is no evidence of future adverse neurologic outcomes.
Dr. Russo is director of pediatrics, medical director for quality and innovation, at WellSpan Health, York, Pa. Dr. Money is a pediatric hospitalist at Primary Children’s Hospital, University of Utah School of Medicine, Salt Lake City. Dr. Steed is instructor of hospital medicine, Northwestern Memorial Hospital and Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University School of Medicine, Chicago. The authors would like to thank Dr. Klint M. Schwenk and the Society for Hospital Medicine Pediatric Special Interest Group Executive Council.
References
1. Roberts KB, Fisher ER, and Rauch DA. The history of pediatric hospital medicine in the United States, 1996-2019. J Hosp Med. 2020 Jul;15(7):424-7.
2. Mussman GM and Conway PH. Pediatric hospitalist systems versus traditional models of care: Effect on quality and cost outcomes. J Hosp Med. 2012 Apr;7(4):350-7.
3. Wang ME, Shaughnessy EE, and Leyenaar JK. The future of pediatric hospital medicine: Challenges and opportunities. J Hosp Med. 2020 Jul;15(7):428-30.
4. Chang PW et al. A clinical prediction rule for rebound hyperbilirubinemia following inpatient phototherapy. Pediatrics. 2017;139 Mar;139(3):e20162896.
Tier 4 lockdown in England as virus variant spreading fast
Mr. Johnson told a Downing Street briefing: “The new variant could increase the R by 0.4 or more, and although there’s considerable uncertainty, it may be up to 70% more transmissible than the old variant, the original version of the disease.”
England’s Tier 4 is the equivalent of the old national lockdown restrictions and began Dec. 20.
It prevents Christmas relaxation for gatherings in Tier 4, aside from support bubbles.
Non-essential shops, gyms, and hairdressers will also close. People shouldn’t enter or leave Tier 4.
In the rest of England, special Christmas measures are reduced to 1 day down from the previous 5.
Canceling Christmas
Mr. Johnson had previously said it would be “inhuman” to cancel Christmas.
“When the science changes, we must change our response,” Mr. Johnson said. “When the virus changes its method of attack, we must change our method of defence. And as your Prime Minister I sincerely believe there is no alternative open to me.”
He added: “We’re sacrificing the chance to see our loved ones this Christmas so we have a better chance of protecting their lives so that we can see them at future Christmases.”
He denied he’d been slow to react to rising cases and evidence around the virus variant.
Rest of the UK
The PM’s announcement for England followed calls with the cabinet, and with the leaders of Scotland, Wales, and Northern Ireland.
Wales has brought forward its planned national lockdown to start at midnight with rules eased on Christmas Day. First Minister Mark Drakeford said: “The situation is incredibly serious. I cannot overstate this.”
Seventeen new variant cases have already been identified in Scotland. First Minister Nicola Sturgeon said: “We do now face a very serious situation. It is, in fact, probably the most serious and potentially dangerous juncture we have faced since the start of the COVID pandemic in February, and March.”
Although she said the situation in Scotland is not as severe as other parts of the UK, preventative measures were needed.
Restrictions will now only be lifted on Christmas day itself, and there’s a ban on non-essential travel to and from the rest of the UK.
Level 4 measures will be applied to all of mainland Scotland for 3 weeks from Boxing Day.
Ms. Sturgeon said making the announcement about Christmas made her want to cry.
New variant
The variant was identified through Public Health England genomic surveillance. Chief Medical Adviser Professor Chris Whitty issued a statement saying: “As a result of the rapid spread of the new variant, preliminary modelling data and rapidly rising incidence rates in the South East, the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) now consider that the new strain can spread more quickly.
“We have alerted the World Health Organisation and are continuing to analyse the available data to improve our understanding.
“There is no current evidence to suggest the new strain causes a higher mortality rate or that it affects vaccines and treatments although urgent work is underway to confirm this.”
He told the news briefing: “In the South East, 43% of the virus is now this new variant, in Eastern England it’s 59%, and in London 62%.”
Rates of hospitalisation were higher where the new variant was more prevalent.
‘Cause for concern’
Chief Scientific Adviser Sir Patrick Vallance said: “The new variant contains 23 different changes, many of them associated with changes in the protein that the virus makes. This is an unusually large number of variants. It’s also got variants in areas of the virus that are known to be associated with how the virus binds to cells and enters cells. So there are some changes, which cause concern in terms of how the virus looks.”
He added: “This virus transmits and spreads fast.”
The variant may have originated in the UK, Sir Patrick said: “There’s a large outbreak in the UK, it may have started here, we don’t know for sure.”
Earlier, SAGE member, and Director of the Wellcome Trust, Sir Jeremy Farrar tweeted: “The new strain of COVID-19 is worrying & real cause for concern & extra caution. Research is ongoing to understand more, but acting urgently now is critical. There is no part of the UK & globally that should not be concerned. As in many countries, the situation is fragile.”
Dr. Samantha Batt-Rawden, president of Doctors’ Association UK and a senior intensive care registrar in the South East of England commented: “We realise how disappointing the new restrictions will be for many today, especially those in Tier 4 areas. However, doctors across the UK, but especially those in the South East are telling us that the surge in cases is already putting hospitals and critical care units under enormous strain.”
Vaccines
Mr. Johnson said 350,000 people across the UK have now had the first dose of the Pfizer/BioNTech vaccine.
On Dec. 18, the US FDA granted emergency use of Moderna’s messenger RNA COVID-19 vaccine, the country’s second after the Pfizer/BioNTech product.
The Moderna vaccine, and the Oxford/AstraZeneca jab, are still being assessed by the UK’s MHRA.
Daily data
In Dec. 19’s daily data another 27,052 UK positive tests were reported and 534 deaths.
The total number of deaths within 28 days of a positive test now stands at 67,075.
There are 18,771 COVID-19 patients in hospital and 1,364 ventilator beds are in use.
A version of this article first appeared on Medscape.com.
Mr. Johnson told a Downing Street briefing: “The new variant could increase the R by 0.4 or more, and although there’s considerable uncertainty, it may be up to 70% more transmissible than the old variant, the original version of the disease.”
England’s Tier 4 is the equivalent of the old national lockdown restrictions and began Dec. 20.
It prevents Christmas relaxation for gatherings in Tier 4, aside from support bubbles.
Non-essential shops, gyms, and hairdressers will also close. People shouldn’t enter or leave Tier 4.
In the rest of England, special Christmas measures are reduced to 1 day down from the previous 5.
Canceling Christmas
Mr. Johnson had previously said it would be “inhuman” to cancel Christmas.
“When the science changes, we must change our response,” Mr. Johnson said. “When the virus changes its method of attack, we must change our method of defence. And as your Prime Minister I sincerely believe there is no alternative open to me.”
He added: “We’re sacrificing the chance to see our loved ones this Christmas so we have a better chance of protecting their lives so that we can see them at future Christmases.”
He denied he’d been slow to react to rising cases and evidence around the virus variant.
Rest of the UK
The PM’s announcement for England followed calls with the cabinet, and with the leaders of Scotland, Wales, and Northern Ireland.
Wales has brought forward its planned national lockdown to start at midnight with rules eased on Christmas Day. First Minister Mark Drakeford said: “The situation is incredibly serious. I cannot overstate this.”
Seventeen new variant cases have already been identified in Scotland. First Minister Nicola Sturgeon said: “We do now face a very serious situation. It is, in fact, probably the most serious and potentially dangerous juncture we have faced since the start of the COVID pandemic in February, and March.”
Although she said the situation in Scotland is not as severe as other parts of the UK, preventative measures were needed.
Restrictions will now only be lifted on Christmas day itself, and there’s a ban on non-essential travel to and from the rest of the UK.
Level 4 measures will be applied to all of mainland Scotland for 3 weeks from Boxing Day.
Ms. Sturgeon said making the announcement about Christmas made her want to cry.
New variant
The variant was identified through Public Health England genomic surveillance. Chief Medical Adviser Professor Chris Whitty issued a statement saying: “As a result of the rapid spread of the new variant, preliminary modelling data and rapidly rising incidence rates in the South East, the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) now consider that the new strain can spread more quickly.
“We have alerted the World Health Organisation and are continuing to analyse the available data to improve our understanding.
“There is no current evidence to suggest the new strain causes a higher mortality rate or that it affects vaccines and treatments although urgent work is underway to confirm this.”
He told the news briefing: “In the South East, 43% of the virus is now this new variant, in Eastern England it’s 59%, and in London 62%.”
Rates of hospitalisation were higher where the new variant was more prevalent.
‘Cause for concern’
Chief Scientific Adviser Sir Patrick Vallance said: “The new variant contains 23 different changes, many of them associated with changes in the protein that the virus makes. This is an unusually large number of variants. It’s also got variants in areas of the virus that are known to be associated with how the virus binds to cells and enters cells. So there are some changes, which cause concern in terms of how the virus looks.”
He added: “This virus transmits and spreads fast.”
The variant may have originated in the UK, Sir Patrick said: “There’s a large outbreak in the UK, it may have started here, we don’t know for sure.”
Earlier, SAGE member, and Director of the Wellcome Trust, Sir Jeremy Farrar tweeted: “The new strain of COVID-19 is worrying & real cause for concern & extra caution. Research is ongoing to understand more, but acting urgently now is critical. There is no part of the UK & globally that should not be concerned. As in many countries, the situation is fragile.”
Dr. Samantha Batt-Rawden, president of Doctors’ Association UK and a senior intensive care registrar in the South East of England commented: “We realise how disappointing the new restrictions will be for many today, especially those in Tier 4 areas. However, doctors across the UK, but especially those in the South East are telling us that the surge in cases is already putting hospitals and critical care units under enormous strain.”
Vaccines
Mr. Johnson said 350,000 people across the UK have now had the first dose of the Pfizer/BioNTech vaccine.
On Dec. 18, the US FDA granted emergency use of Moderna’s messenger RNA COVID-19 vaccine, the country’s second after the Pfizer/BioNTech product.
The Moderna vaccine, and the Oxford/AstraZeneca jab, are still being assessed by the UK’s MHRA.
Daily data
In Dec. 19’s daily data another 27,052 UK positive tests were reported and 534 deaths.
The total number of deaths within 28 days of a positive test now stands at 67,075.
There are 18,771 COVID-19 patients in hospital and 1,364 ventilator beds are in use.
A version of this article first appeared on Medscape.com.
Mr. Johnson told a Downing Street briefing: “The new variant could increase the R by 0.4 or more, and although there’s considerable uncertainty, it may be up to 70% more transmissible than the old variant, the original version of the disease.”
England’s Tier 4 is the equivalent of the old national lockdown restrictions and began Dec. 20.
It prevents Christmas relaxation for gatherings in Tier 4, aside from support bubbles.
Non-essential shops, gyms, and hairdressers will also close. People shouldn’t enter or leave Tier 4.
In the rest of England, special Christmas measures are reduced to 1 day down from the previous 5.
Canceling Christmas
Mr. Johnson had previously said it would be “inhuman” to cancel Christmas.
“When the science changes, we must change our response,” Mr. Johnson said. “When the virus changes its method of attack, we must change our method of defence. And as your Prime Minister I sincerely believe there is no alternative open to me.”
He added: “We’re sacrificing the chance to see our loved ones this Christmas so we have a better chance of protecting their lives so that we can see them at future Christmases.”
He denied he’d been slow to react to rising cases and evidence around the virus variant.
Rest of the UK
The PM’s announcement for England followed calls with the cabinet, and with the leaders of Scotland, Wales, and Northern Ireland.
Wales has brought forward its planned national lockdown to start at midnight with rules eased on Christmas Day. First Minister Mark Drakeford said: “The situation is incredibly serious. I cannot overstate this.”
Seventeen new variant cases have already been identified in Scotland. First Minister Nicola Sturgeon said: “We do now face a very serious situation. It is, in fact, probably the most serious and potentially dangerous juncture we have faced since the start of the COVID pandemic in February, and March.”
Although she said the situation in Scotland is not as severe as other parts of the UK, preventative measures were needed.
Restrictions will now only be lifted on Christmas day itself, and there’s a ban on non-essential travel to and from the rest of the UK.
Level 4 measures will be applied to all of mainland Scotland for 3 weeks from Boxing Day.
Ms. Sturgeon said making the announcement about Christmas made her want to cry.
New variant
The variant was identified through Public Health England genomic surveillance. Chief Medical Adviser Professor Chris Whitty issued a statement saying: “As a result of the rapid spread of the new variant, preliminary modelling data and rapidly rising incidence rates in the South East, the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) now consider that the new strain can spread more quickly.
“We have alerted the World Health Organisation and are continuing to analyse the available data to improve our understanding.
“There is no current evidence to suggest the new strain causes a higher mortality rate or that it affects vaccines and treatments although urgent work is underway to confirm this.”
He told the news briefing: “In the South East, 43% of the virus is now this new variant, in Eastern England it’s 59%, and in London 62%.”
Rates of hospitalisation were higher where the new variant was more prevalent.
‘Cause for concern’
Chief Scientific Adviser Sir Patrick Vallance said: “The new variant contains 23 different changes, many of them associated with changes in the protein that the virus makes. This is an unusually large number of variants. It’s also got variants in areas of the virus that are known to be associated with how the virus binds to cells and enters cells. So there are some changes, which cause concern in terms of how the virus looks.”
He added: “This virus transmits and spreads fast.”
The variant may have originated in the UK, Sir Patrick said: “There’s a large outbreak in the UK, it may have started here, we don’t know for sure.”
Earlier, SAGE member, and Director of the Wellcome Trust, Sir Jeremy Farrar tweeted: “The new strain of COVID-19 is worrying & real cause for concern & extra caution. Research is ongoing to understand more, but acting urgently now is critical. There is no part of the UK & globally that should not be concerned. As in many countries, the situation is fragile.”
Dr. Samantha Batt-Rawden, president of Doctors’ Association UK and a senior intensive care registrar in the South East of England commented: “We realise how disappointing the new restrictions will be for many today, especially those in Tier 4 areas. However, doctors across the UK, but especially those in the South East are telling us that the surge in cases is already putting hospitals and critical care units under enormous strain.”
Vaccines
Mr. Johnson said 350,000 people across the UK have now had the first dose of the Pfizer/BioNTech vaccine.
On Dec. 18, the US FDA granted emergency use of Moderna’s messenger RNA COVID-19 vaccine, the country’s second after the Pfizer/BioNTech product.
The Moderna vaccine, and the Oxford/AstraZeneca jab, are still being assessed by the UK’s MHRA.
Daily data
In Dec. 19’s daily data another 27,052 UK positive tests were reported and 534 deaths.
The total number of deaths within 28 days of a positive test now stands at 67,075.
There are 18,771 COVID-19 patients in hospital and 1,364 ventilator beds are in use.
A version of this article first appeared on Medscape.com.
CDC identifies next priority groups for COVID-19 vaccine
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention voted 13-1 for the recommendation. This builds on ACIP’s initial recommendation about which groups should be in the first wave of vaccinations, described as Phase 1a.
ACIP earlier recommended that Phase 1a include U.S. health care workers, a group of about 21 million people, and residents of long-term care facilities, a group of about 3 million.
On Dec. 20, ACIP said the next priority group, Phase 1b, should consist of what it called frontline essential workers, a group of about 30 million, and adults aged 75 years and older, a group of about 21 million. When overlap between the groups is taken into account, Phase 1b covers about 49 million people, according to the CDC.
Phase 1c then would include adults aged 65-74 years (a group of about 32 million), adults aged 16-64 years with high-risk medical conditions (a group of about 110 million), and essential workers who did not qualify for inclusion in Phase 1b (a group of about 57 million). With the overlap, Phase 1c would cover about 129 million.
The Food and Drug Administration recently granted emergency use authorizations for two COVID-19 vaccines, one developed by Pfizer-BioNTech and another from Moderna. Other companies, including Johnson & Johnson, have advanced their potential rival COVID-19 vaccines into late-stages of testing. To date, about 2.83 million doses of Pfizer’s COVID-19 vaccine have been distributed and 556,208 doses have been administered, according to the CDC.
But there will likely still be a period of months when competition for limited doses of COVID-19 vaccine will trigger difficult decisions. Current estimates indicate there will be enough supply to provide COVID-19 vaccines for 20 million people in December, 30 million people in January, and 50 million people in February, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases.
State governments and health systems will take ACIP’s recommendations into account as they roll out the initial supplies of COVID-19 vaccines.
There’s clearly wide latitude in these decisions. Recently, for example, many members of Congress tweeted photos of themselves getting COVID-19 vaccines, despite not falling into ACIP’s description of the Phase 1 group.
Difficult choices
All ACIP members described the Dec. 20 vote as a difficult decision. It forced them to choose among segments of the U.S. population that could benefit from early access to the limited supply of COVID-19 vaccines.
“For every group we add, it means we subtract a group. For every group we subtract, it means they don’t get the vaccine” for some months, said ACIP member Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn. “It’s incredibly humbling and heartbreaking.”
ACIP member Henry Bernstein, DO, who cast the lone dissenting vote, said he agreed with most of the panel’s recommendation. He said he fully supported the inclusion of adults aged 75 years and older and essential frontline workers in the second wave, Phase 1b. But he voted no because the data on COVID-19 morbidity and mortality for adults aged 65-74 years is similar enough to the older group to warrant their inclusion in the first wave.
“Therefore, inclusion of the 65- to 74-year-old group in Phase 1b made more sense to me,” said Dr. Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in New York.
As defined by the CDC, frontline essential workers included in phase 1b will be those commonly called “first responders,” such as firefighters and police officers. Also in this group are teachers, support staff, daycare providers, and those employed in grocery and agriculture industries. Others in this group would include U.S. Postal Service employees and transit workers.
ACIP panelists noted the difficulties that will emerge as government officials and leaders of health care organizations move to apply their guidance to real-world decisions about distributing a limited supply of COVID-19 vaccine. There’s a potential to worsen existing disparities in access to health care, as people with more income may find it easier to obtain proof that they qualify as having a high-risk condition, said José Romero, MD, the chair of ACIP.
Many people “don’t have access to medical care and can’t come up with a doctor’s note that says, ‘I have diabetes,’ ” he said.
ACIP panelists also noted in their deliberations that people may technically qualify for a priority group but have little risk, such as someone with a chronic medical condition who works from home.
And the risk for COVID-19 remains serious even for those who will ultimately fall into the phase 2 for vaccination. Young adults have suffered serious complications following COVID-19, such as stroke, that may alter their lives dramatically, ACIP member Dr. Talbot said, adding that she is reminded of this in her work.
“We need to be very cautious about saying, ‘Young adults will be fine,’ ” she said. “I spent the past week on back-up clinical call and have read these charts and have cried every day.”
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines. The other panel members have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention voted 13-1 for the recommendation. This builds on ACIP’s initial recommendation about which groups should be in the first wave of vaccinations, described as Phase 1a.
ACIP earlier recommended that Phase 1a include U.S. health care workers, a group of about 21 million people, and residents of long-term care facilities, a group of about 3 million.
On Dec. 20, ACIP said the next priority group, Phase 1b, should consist of what it called frontline essential workers, a group of about 30 million, and adults aged 75 years and older, a group of about 21 million. When overlap between the groups is taken into account, Phase 1b covers about 49 million people, according to the CDC.
Phase 1c then would include adults aged 65-74 years (a group of about 32 million), adults aged 16-64 years with high-risk medical conditions (a group of about 110 million), and essential workers who did not qualify for inclusion in Phase 1b (a group of about 57 million). With the overlap, Phase 1c would cover about 129 million.
The Food and Drug Administration recently granted emergency use authorizations for two COVID-19 vaccines, one developed by Pfizer-BioNTech and another from Moderna. Other companies, including Johnson & Johnson, have advanced their potential rival COVID-19 vaccines into late-stages of testing. To date, about 2.83 million doses of Pfizer’s COVID-19 vaccine have been distributed and 556,208 doses have been administered, according to the CDC.
But there will likely still be a period of months when competition for limited doses of COVID-19 vaccine will trigger difficult decisions. Current estimates indicate there will be enough supply to provide COVID-19 vaccines for 20 million people in December, 30 million people in January, and 50 million people in February, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases.
State governments and health systems will take ACIP’s recommendations into account as they roll out the initial supplies of COVID-19 vaccines.
There’s clearly wide latitude in these decisions. Recently, for example, many members of Congress tweeted photos of themselves getting COVID-19 vaccines, despite not falling into ACIP’s description of the Phase 1 group.
Difficult choices
All ACIP members described the Dec. 20 vote as a difficult decision. It forced them to choose among segments of the U.S. population that could benefit from early access to the limited supply of COVID-19 vaccines.
“For every group we add, it means we subtract a group. For every group we subtract, it means they don’t get the vaccine” for some months, said ACIP member Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn. “It’s incredibly humbling and heartbreaking.”
ACIP member Henry Bernstein, DO, who cast the lone dissenting vote, said he agreed with most of the panel’s recommendation. He said he fully supported the inclusion of adults aged 75 years and older and essential frontline workers in the second wave, Phase 1b. But he voted no because the data on COVID-19 morbidity and mortality for adults aged 65-74 years is similar enough to the older group to warrant their inclusion in the first wave.
“Therefore, inclusion of the 65- to 74-year-old group in Phase 1b made more sense to me,” said Dr. Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in New York.
As defined by the CDC, frontline essential workers included in phase 1b will be those commonly called “first responders,” such as firefighters and police officers. Also in this group are teachers, support staff, daycare providers, and those employed in grocery and agriculture industries. Others in this group would include U.S. Postal Service employees and transit workers.
ACIP panelists noted the difficulties that will emerge as government officials and leaders of health care organizations move to apply their guidance to real-world decisions about distributing a limited supply of COVID-19 vaccine. There’s a potential to worsen existing disparities in access to health care, as people with more income may find it easier to obtain proof that they qualify as having a high-risk condition, said José Romero, MD, the chair of ACIP.
Many people “don’t have access to medical care and can’t come up with a doctor’s note that says, ‘I have diabetes,’ ” he said.
ACIP panelists also noted in their deliberations that people may technically qualify for a priority group but have little risk, such as someone with a chronic medical condition who works from home.
And the risk for COVID-19 remains serious even for those who will ultimately fall into the phase 2 for vaccination. Young adults have suffered serious complications following COVID-19, such as stroke, that may alter their lives dramatically, ACIP member Dr. Talbot said, adding that she is reminded of this in her work.
“We need to be very cautious about saying, ‘Young adults will be fine,’ ” she said. “I spent the past week on back-up clinical call and have read these charts and have cried every day.”
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines. The other panel members have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention voted 13-1 for the recommendation. This builds on ACIP’s initial recommendation about which groups should be in the first wave of vaccinations, described as Phase 1a.
ACIP earlier recommended that Phase 1a include U.S. health care workers, a group of about 21 million people, and residents of long-term care facilities, a group of about 3 million.
On Dec. 20, ACIP said the next priority group, Phase 1b, should consist of what it called frontline essential workers, a group of about 30 million, and adults aged 75 years and older, a group of about 21 million. When overlap between the groups is taken into account, Phase 1b covers about 49 million people, according to the CDC.
Phase 1c then would include adults aged 65-74 years (a group of about 32 million), adults aged 16-64 years with high-risk medical conditions (a group of about 110 million), and essential workers who did not qualify for inclusion in Phase 1b (a group of about 57 million). With the overlap, Phase 1c would cover about 129 million.
The Food and Drug Administration recently granted emergency use authorizations for two COVID-19 vaccines, one developed by Pfizer-BioNTech and another from Moderna. Other companies, including Johnson & Johnson, have advanced their potential rival COVID-19 vaccines into late-stages of testing. To date, about 2.83 million doses of Pfizer’s COVID-19 vaccine have been distributed and 556,208 doses have been administered, according to the CDC.
But there will likely still be a period of months when competition for limited doses of COVID-19 vaccine will trigger difficult decisions. Current estimates indicate there will be enough supply to provide COVID-19 vaccines for 20 million people in December, 30 million people in January, and 50 million people in February, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases.
State governments and health systems will take ACIP’s recommendations into account as they roll out the initial supplies of COVID-19 vaccines.
There’s clearly wide latitude in these decisions. Recently, for example, many members of Congress tweeted photos of themselves getting COVID-19 vaccines, despite not falling into ACIP’s description of the Phase 1 group.
Difficult choices
All ACIP members described the Dec. 20 vote as a difficult decision. It forced them to choose among segments of the U.S. population that could benefit from early access to the limited supply of COVID-19 vaccines.
“For every group we add, it means we subtract a group. For every group we subtract, it means they don’t get the vaccine” for some months, said ACIP member Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn. “It’s incredibly humbling and heartbreaking.”
ACIP member Henry Bernstein, DO, who cast the lone dissenting vote, said he agreed with most of the panel’s recommendation. He said he fully supported the inclusion of adults aged 75 years and older and essential frontline workers in the second wave, Phase 1b. But he voted no because the data on COVID-19 morbidity and mortality for adults aged 65-74 years is similar enough to the older group to warrant their inclusion in the first wave.
“Therefore, inclusion of the 65- to 74-year-old group in Phase 1b made more sense to me,” said Dr. Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in New York.
As defined by the CDC, frontline essential workers included in phase 1b will be those commonly called “first responders,” such as firefighters and police officers. Also in this group are teachers, support staff, daycare providers, and those employed in grocery and agriculture industries. Others in this group would include U.S. Postal Service employees and transit workers.
ACIP panelists noted the difficulties that will emerge as government officials and leaders of health care organizations move to apply their guidance to real-world decisions about distributing a limited supply of COVID-19 vaccine. There’s a potential to worsen existing disparities in access to health care, as people with more income may find it easier to obtain proof that they qualify as having a high-risk condition, said José Romero, MD, the chair of ACIP.
Many people “don’t have access to medical care and can’t come up with a doctor’s note that says, ‘I have diabetes,’ ” he said.
ACIP panelists also noted in their deliberations that people may technically qualify for a priority group but have little risk, such as someone with a chronic medical condition who works from home.
And the risk for COVID-19 remains serious even for those who will ultimately fall into the phase 2 for vaccination. Young adults have suffered serious complications following COVID-19, such as stroke, that may alter their lives dramatically, ACIP member Dr. Talbot said, adding that she is reminded of this in her work.
“We need to be very cautious about saying, ‘Young adults will be fine,’ ” she said. “I spent the past week on back-up clinical call and have read these charts and have cried every day.”
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines. The other panel members have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 ‘far more serious’ than flu, inpatient data confirm
About twice as many patients were admitted to hospitals in France for COVID-19 during a 2-month period than were admitted for seasonal influenza during a 3-month period the previous year, according to a study published online in The Lancet Respiratory Medicine.
In-hospital mortality was nearly three times higher for COVID-19 than for seasonal influenza, researchers found. In addition, patients with COVID-19 were more likely to require invasive mechanical ventilation (9.7% vs. 4%) and had longer average ICU stays (15 days vs. 8 days).
“SARS-CoV-2 appears to have a higher potential for respiratory pathogenicity, leading to more respiratory complications in patients with fewer comorbidities, and it is associated with a higher risk of mortality, particularly in adolescents, although any conclusions for this age group must be treated with caution considering the small number of deaths,” wrote Lionel Piroth, MD, PhD, of the infectious diseases department, Dijon (France) University Hospital, and colleagues.
The study “is the largest to date to compare the two diseases and confirms that COVID-19 is far more serious than the flu,” study author Catherine Quantin, MD, PhD, said in a news release. “The finding that the COVID-19 death rate was three times higher than for seasonal influenza is particularly striking when reminded that the 2018/2019 flu season had been the worst in the past five years in France in terms of number of deaths,” continued Dr. Quantin, who jointly led the research. She is affiliated with the University Hospital of Dijon and Inserm.
The investigators analyzed data from a national database and compared 89,530 COVID-19 hospital admissions between March 1 and April 30, 2020, with 45,819 seasonal flu hospital admissions between Dec. 1, 2018, and Feb. 28, 2019.
The death rate was 16.9% among patients hospitalized with COVID-19, compared with 5.8% among patients hospitalized with influenza.
Fewer patients younger 18 years were hospitalized with COVID-19 than with seasonal influenza (1.4% vs. 19.5%; 1,227 vs. 8,942), but a larger proportion of those younger than 5 years required intensive care for COVID-19 (2.9% vs. 0.9%). The fatality rates in children younger than 5 years were similar for both groups (0.5% vs. 0.2%).
Among patients aged 11-17 years, 5 of 548 (1.1%) patients with COVID-19 died, compared with 1 of 804 (0.1%) patients with flu.
Testing practices for influenza likely varied across hospitals, whereas testing for COVID-19 may have been more standardized. This could be a limitation of the study, the researchers noted. In addition, flu seasons vary year to year, and influenza cases may depend on vaccination coverage and residual population immunity.
“The large sample size is an important strength of the study and it is assumed that the indication for hospital admission in the two periods was the same and thus does not bias the results,” Eskild Petersen, MD, DMsc, wrote in a comment accompanying the study. “The results ... clearly show that COVID-19 is more serious than seasonal influenza.”
Furthermore, this study and prior research show that “COVID-19 is not an innocent infection in children and adolescents,” said Dr. Petersen, who is affiliated with the University of Aarhus in Denmark and the European Society for Clinical Microbiology and Infectious Diseases Emerging Infections Task Force.
The study was funded by the French National Research Agency. Two authors have various financial ties to several pharmaceutical companies, details of which are available in the journal article. Dr. Petersen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
About twice as many patients were admitted to hospitals in France for COVID-19 during a 2-month period than were admitted for seasonal influenza during a 3-month period the previous year, according to a study published online in The Lancet Respiratory Medicine.
In-hospital mortality was nearly three times higher for COVID-19 than for seasonal influenza, researchers found. In addition, patients with COVID-19 were more likely to require invasive mechanical ventilation (9.7% vs. 4%) and had longer average ICU stays (15 days vs. 8 days).
“SARS-CoV-2 appears to have a higher potential for respiratory pathogenicity, leading to more respiratory complications in patients with fewer comorbidities, and it is associated with a higher risk of mortality, particularly in adolescents, although any conclusions for this age group must be treated with caution considering the small number of deaths,” wrote Lionel Piroth, MD, PhD, of the infectious diseases department, Dijon (France) University Hospital, and colleagues.
The study “is the largest to date to compare the two diseases and confirms that COVID-19 is far more serious than the flu,” study author Catherine Quantin, MD, PhD, said in a news release. “The finding that the COVID-19 death rate was three times higher than for seasonal influenza is particularly striking when reminded that the 2018/2019 flu season had been the worst in the past five years in France in terms of number of deaths,” continued Dr. Quantin, who jointly led the research. She is affiliated with the University Hospital of Dijon and Inserm.
The investigators analyzed data from a national database and compared 89,530 COVID-19 hospital admissions between March 1 and April 30, 2020, with 45,819 seasonal flu hospital admissions between Dec. 1, 2018, and Feb. 28, 2019.
The death rate was 16.9% among patients hospitalized with COVID-19, compared with 5.8% among patients hospitalized with influenza.
Fewer patients younger 18 years were hospitalized with COVID-19 than with seasonal influenza (1.4% vs. 19.5%; 1,227 vs. 8,942), but a larger proportion of those younger than 5 years required intensive care for COVID-19 (2.9% vs. 0.9%). The fatality rates in children younger than 5 years were similar for both groups (0.5% vs. 0.2%).
Among patients aged 11-17 years, 5 of 548 (1.1%) patients with COVID-19 died, compared with 1 of 804 (0.1%) patients with flu.
Testing practices for influenza likely varied across hospitals, whereas testing for COVID-19 may have been more standardized. This could be a limitation of the study, the researchers noted. In addition, flu seasons vary year to year, and influenza cases may depend on vaccination coverage and residual population immunity.
“The large sample size is an important strength of the study and it is assumed that the indication for hospital admission in the two periods was the same and thus does not bias the results,” Eskild Petersen, MD, DMsc, wrote in a comment accompanying the study. “The results ... clearly show that COVID-19 is more serious than seasonal influenza.”
Furthermore, this study and prior research show that “COVID-19 is not an innocent infection in children and adolescents,” said Dr. Petersen, who is affiliated with the University of Aarhus in Denmark and the European Society for Clinical Microbiology and Infectious Diseases Emerging Infections Task Force.
The study was funded by the French National Research Agency. Two authors have various financial ties to several pharmaceutical companies, details of which are available in the journal article. Dr. Petersen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
About twice as many patients were admitted to hospitals in France for COVID-19 during a 2-month period than were admitted for seasonal influenza during a 3-month period the previous year, according to a study published online in The Lancet Respiratory Medicine.
In-hospital mortality was nearly three times higher for COVID-19 than for seasonal influenza, researchers found. In addition, patients with COVID-19 were more likely to require invasive mechanical ventilation (9.7% vs. 4%) and had longer average ICU stays (15 days vs. 8 days).
“SARS-CoV-2 appears to have a higher potential for respiratory pathogenicity, leading to more respiratory complications in patients with fewer comorbidities, and it is associated with a higher risk of mortality, particularly in adolescents, although any conclusions for this age group must be treated with caution considering the small number of deaths,” wrote Lionel Piroth, MD, PhD, of the infectious diseases department, Dijon (France) University Hospital, and colleagues.
The study “is the largest to date to compare the two diseases and confirms that COVID-19 is far more serious than the flu,” study author Catherine Quantin, MD, PhD, said in a news release. “The finding that the COVID-19 death rate was three times higher than for seasonal influenza is particularly striking when reminded that the 2018/2019 flu season had been the worst in the past five years in France in terms of number of deaths,” continued Dr. Quantin, who jointly led the research. She is affiliated with the University Hospital of Dijon and Inserm.
The investigators analyzed data from a national database and compared 89,530 COVID-19 hospital admissions between March 1 and April 30, 2020, with 45,819 seasonal flu hospital admissions between Dec. 1, 2018, and Feb. 28, 2019.
The death rate was 16.9% among patients hospitalized with COVID-19, compared with 5.8% among patients hospitalized with influenza.
Fewer patients younger 18 years were hospitalized with COVID-19 than with seasonal influenza (1.4% vs. 19.5%; 1,227 vs. 8,942), but a larger proportion of those younger than 5 years required intensive care for COVID-19 (2.9% vs. 0.9%). The fatality rates in children younger than 5 years were similar for both groups (0.5% vs. 0.2%).
Among patients aged 11-17 years, 5 of 548 (1.1%) patients with COVID-19 died, compared with 1 of 804 (0.1%) patients with flu.
Testing practices for influenza likely varied across hospitals, whereas testing for COVID-19 may have been more standardized. This could be a limitation of the study, the researchers noted. In addition, flu seasons vary year to year, and influenza cases may depend on vaccination coverage and residual population immunity.
“The large sample size is an important strength of the study and it is assumed that the indication for hospital admission in the two periods was the same and thus does not bias the results,” Eskild Petersen, MD, DMsc, wrote in a comment accompanying the study. “The results ... clearly show that COVID-19 is more serious than seasonal influenza.”
Furthermore, this study and prior research show that “COVID-19 is not an innocent infection in children and adolescents,” said Dr. Petersen, who is affiliated with the University of Aarhus in Denmark and the European Society for Clinical Microbiology and Infectious Diseases Emerging Infections Task Force.
The study was funded by the French National Research Agency. Two authors have various financial ties to several pharmaceutical companies, details of which are available in the journal article. Dr. Petersen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Second COVID-19 vaccine ready for use, CDC panel says
The panel voted 11-0, with three recusals, to recommend use of Moderna’s vaccine for people aged 18 years and older, while seeking more information on risk for anaphylaxis. This vote followed the December 18th decision by the US Food and Drug Administration (FDA) to grant emergency use authorization (EUA) for the vaccine, known as mRNA-1273.
On December 11, the FDA granted the first US emergency clearance for a COVID-19 vaccine to the Pfizer-BioNTech product. ACIP met the following day and voted to endorse the use of that vaccine, with a vote of 11-0 and three recusals. The Pfizer-BioNTech COVID-19 vaccine is recommended for use in people aged 16 years and older.
Moderna’s vaccine is expected to help curb the pandemic, with clinical trial data showing a 94.1% efficacy rate. But there’s also concerns about side effects noted in testing of both vaccines and in the early rollout of the Pfizer vaccine, particularly anaphylaxis.
“There are likely going to be lots of bumps in the road” with the introduction of the COVID-19 vaccines, but these are being disclosed to the public in a way that is “fair and transparent,” said ACIP member Beth P. Bell, MD, MPH.
“Our systems so far appear to be doing what they are supposed to do” in terms of determining risks from the COVID-19 vaccine, added Bell, who is a clinical professor in the department of global health at the University of Washington’s School of Public Health in Seattle. The Moderna EUA “represents progress towards ending this horrific pandemic,” she said.
In a new forecast released this week, the CDC projects that the number of newly reported COVID-19 deaths will likely increase over the next 4 weeks, with 15,800 to 27,700 new deaths likely to be reported in the week ending January 9, 2021. That could bring the total number of COVID-19 deaths in the United States to between 357,000 and 391,000 by this date, according to the agency.
ACIP panelist Lynn Bahta, RN, MPH, CPH, said she had been “eager” to have the panel proceed with its endorsement of the Moderna vaccine, “especially in light of the fact that we are seeing an average 2600 deaths a day.”
Having two COVID-19 vaccines available might help slow down the pandemic, “despite the fact that we still have a lot to learn both about the disease and the vaccine,” said Bahta, who is an immunization consultant with the Minnesota Department of Health in Saint Paul.
ACIP members encouraged Moderna officials who presented at the meeting to continue studies for potential complications associated with the vaccine when given to women who are pregnant or breastfeeding.
Panelists also pressed for more data on the risk for Bell’s palsy, which the FDA staff also had noted in the agency’s review of Moderna’s vaccine. Moderna has reported four cases from a pivotal study, one in the placebo group and three among study participants who received the company’s vaccine. These cases occurred between 15 and 33 days after vaccination, and are all resolved or resolving, according to Moderna.
There was also a question raised about how many doses of vaccine might be squeezed out of a vial. CDC will explore this topic further at its meeting on COVID-19 vaccines December 20, said Nancy Messonnier, MD, director of the agency’s National Center for Immunization and Respiratory Diseases, at the Saturday meeting.
“In this time of public health crisis, none of us would want to squander a single dose of a vaccine that’s potentially lifesaving,” CDC’s Messonnier said. “We’re going to plan to have a short discussion of that issue tomorrow.”
Messonnier also responded to a comment made during the meeting about cases where people who received COVID-19 vaccine were unaware of the CDC’s V-safe tool.
V-safe is a smartphone-based tool that uses text messaging and web surveys to help people keep in touch with the medical community after getting the COVID-19 vaccine and is seen as a way to help spot side effects. Messonnier asked that people listening to the webcast of the ACIP meeting help spread the word about the CDC’s V-safe tool.
“Our perception, based on the number of people who have enrolled in V-safe, is that the message is getting out to many places, but even one site that doesn’t have this information is something that we want to try to correct,” she said.
Anaphylaxis concerns
The chief concern for ACIP members and CDC staff about COVID-19 vaccines appeared to be reports of allergic reactions. Thomas Clark, MD, MPH, a CDC staff member, told the ACIP panel that, as of December 18, the agency had identified six cases of anaphylaxis following administration of the Pfizer-BioNTech vaccine that met a certain standard, known as the Brighton Collaboration criteria.
Additional case reports have been reviewed and determined not to be anaphylaxis, Clark said. All suspect cases were identified through processes such as the federal Vaccine Adverse Event Reporting System (VAERS), he said.
People who experience anaphylaxis following COVID-19 vaccination should not receive additional doses of the shot, Clark said in his presentation to ACIP. Members of the panel asked Clark whether there have been any discernible patterns to these cases, such as geographic clusters.
Clark replied that it was “early” in the process to make reports, with investigations still ongoing. He did note that the people who had anaphylaxis following vaccination had received their doses from more than one production lot, with multiple lots having been distributed.
“You folks may have seen in the news a couple of cases from Alaska, but we’ve had reports from other jurisdictions so there’s no obvious clustering geographically,” Clark said.
Another CDC staff member, Sarah Mbaeyi, MD, MPH, noted in her presentation that there should be an observation period of 30 minutes following COVID-19 vaccination for anyone with a history of anaphylaxis for any reason, and of at least 15 minutes for other recipients.
Disclosure of ingredients used in the COVID-19 vaccines might help people with an allergy assess these products, the representative for the American Medical Association, Sandra Fryhofer, MD, told ACIP. As such, she thanked CDC’s Mbaeyi for including a breakout of ingredients in her presentation to the panel. Fryhofer encouraged Moderna officials to be as transparent as possible in disclosing the ingredients of the company’s COVID-19 vaccine.
“That might be important because I think it’s very essential that we figure out what might be triggering these anaphylactic reactions, because that is definitely going to affect the vaccine implementation,” Fryhofer said.
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said at the Saturday meeting he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said at the Saturday meeting that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines.
The other panel members have reported no relevant financial relationships.
This article first appeared on Medscape.com.
The panel voted 11-0, with three recusals, to recommend use of Moderna’s vaccine for people aged 18 years and older, while seeking more information on risk for anaphylaxis. This vote followed the December 18th decision by the US Food and Drug Administration (FDA) to grant emergency use authorization (EUA) for the vaccine, known as mRNA-1273.
On December 11, the FDA granted the first US emergency clearance for a COVID-19 vaccine to the Pfizer-BioNTech product. ACIP met the following day and voted to endorse the use of that vaccine, with a vote of 11-0 and three recusals. The Pfizer-BioNTech COVID-19 vaccine is recommended for use in people aged 16 years and older.
Moderna’s vaccine is expected to help curb the pandemic, with clinical trial data showing a 94.1% efficacy rate. But there’s also concerns about side effects noted in testing of both vaccines and in the early rollout of the Pfizer vaccine, particularly anaphylaxis.
“There are likely going to be lots of bumps in the road” with the introduction of the COVID-19 vaccines, but these are being disclosed to the public in a way that is “fair and transparent,” said ACIP member Beth P. Bell, MD, MPH.
“Our systems so far appear to be doing what they are supposed to do” in terms of determining risks from the COVID-19 vaccine, added Bell, who is a clinical professor in the department of global health at the University of Washington’s School of Public Health in Seattle. The Moderna EUA “represents progress towards ending this horrific pandemic,” she said.
In a new forecast released this week, the CDC projects that the number of newly reported COVID-19 deaths will likely increase over the next 4 weeks, with 15,800 to 27,700 new deaths likely to be reported in the week ending January 9, 2021. That could bring the total number of COVID-19 deaths in the United States to between 357,000 and 391,000 by this date, according to the agency.
ACIP panelist Lynn Bahta, RN, MPH, CPH, said she had been “eager” to have the panel proceed with its endorsement of the Moderna vaccine, “especially in light of the fact that we are seeing an average 2600 deaths a day.”
Having two COVID-19 vaccines available might help slow down the pandemic, “despite the fact that we still have a lot to learn both about the disease and the vaccine,” said Bahta, who is an immunization consultant with the Minnesota Department of Health in Saint Paul.
ACIP members encouraged Moderna officials who presented at the meeting to continue studies for potential complications associated with the vaccine when given to women who are pregnant or breastfeeding.
Panelists also pressed for more data on the risk for Bell’s palsy, which the FDA staff also had noted in the agency’s review of Moderna’s vaccine. Moderna has reported four cases from a pivotal study, one in the placebo group and three among study participants who received the company’s vaccine. These cases occurred between 15 and 33 days after vaccination, and are all resolved or resolving, according to Moderna.
There was also a question raised about how many doses of vaccine might be squeezed out of a vial. CDC will explore this topic further at its meeting on COVID-19 vaccines December 20, said Nancy Messonnier, MD, director of the agency’s National Center for Immunization and Respiratory Diseases, at the Saturday meeting.
“In this time of public health crisis, none of us would want to squander a single dose of a vaccine that’s potentially lifesaving,” CDC’s Messonnier said. “We’re going to plan to have a short discussion of that issue tomorrow.”
Messonnier also responded to a comment made during the meeting about cases where people who received COVID-19 vaccine were unaware of the CDC’s V-safe tool.
V-safe is a smartphone-based tool that uses text messaging and web surveys to help people keep in touch with the medical community after getting the COVID-19 vaccine and is seen as a way to help spot side effects. Messonnier asked that people listening to the webcast of the ACIP meeting help spread the word about the CDC’s V-safe tool.
“Our perception, based on the number of people who have enrolled in V-safe, is that the message is getting out to many places, but even one site that doesn’t have this information is something that we want to try to correct,” she said.
Anaphylaxis concerns
The chief concern for ACIP members and CDC staff about COVID-19 vaccines appeared to be reports of allergic reactions. Thomas Clark, MD, MPH, a CDC staff member, told the ACIP panel that, as of December 18, the agency had identified six cases of anaphylaxis following administration of the Pfizer-BioNTech vaccine that met a certain standard, known as the Brighton Collaboration criteria.
Additional case reports have been reviewed and determined not to be anaphylaxis, Clark said. All suspect cases were identified through processes such as the federal Vaccine Adverse Event Reporting System (VAERS), he said.
People who experience anaphylaxis following COVID-19 vaccination should not receive additional doses of the shot, Clark said in his presentation to ACIP. Members of the panel asked Clark whether there have been any discernible patterns to these cases, such as geographic clusters.
Clark replied that it was “early” in the process to make reports, with investigations still ongoing. He did note that the people who had anaphylaxis following vaccination had received their doses from more than one production lot, with multiple lots having been distributed.
“You folks may have seen in the news a couple of cases from Alaska, but we’ve had reports from other jurisdictions so there’s no obvious clustering geographically,” Clark said.
Another CDC staff member, Sarah Mbaeyi, MD, MPH, noted in her presentation that there should be an observation period of 30 minutes following COVID-19 vaccination for anyone with a history of anaphylaxis for any reason, and of at least 15 minutes for other recipients.
Disclosure of ingredients used in the COVID-19 vaccines might help people with an allergy assess these products, the representative for the American Medical Association, Sandra Fryhofer, MD, told ACIP. As such, she thanked CDC’s Mbaeyi for including a breakout of ingredients in her presentation to the panel. Fryhofer encouraged Moderna officials to be as transparent as possible in disclosing the ingredients of the company’s COVID-19 vaccine.
“That might be important because I think it’s very essential that we figure out what might be triggering these anaphylactic reactions, because that is definitely going to affect the vaccine implementation,” Fryhofer said.
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said at the Saturday meeting he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said at the Saturday meeting that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines.
The other panel members have reported no relevant financial relationships.
This article first appeared on Medscape.com.
The panel voted 11-0, with three recusals, to recommend use of Moderna’s vaccine for people aged 18 years and older, while seeking more information on risk for anaphylaxis. This vote followed the December 18th decision by the US Food and Drug Administration (FDA) to grant emergency use authorization (EUA) for the vaccine, known as mRNA-1273.
On December 11, the FDA granted the first US emergency clearance for a COVID-19 vaccine to the Pfizer-BioNTech product. ACIP met the following day and voted to endorse the use of that vaccine, with a vote of 11-0 and three recusals. The Pfizer-BioNTech COVID-19 vaccine is recommended for use in people aged 16 years and older.
Moderna’s vaccine is expected to help curb the pandemic, with clinical trial data showing a 94.1% efficacy rate. But there’s also concerns about side effects noted in testing of both vaccines and in the early rollout of the Pfizer vaccine, particularly anaphylaxis.
“There are likely going to be lots of bumps in the road” with the introduction of the COVID-19 vaccines, but these are being disclosed to the public in a way that is “fair and transparent,” said ACIP member Beth P. Bell, MD, MPH.
“Our systems so far appear to be doing what they are supposed to do” in terms of determining risks from the COVID-19 vaccine, added Bell, who is a clinical professor in the department of global health at the University of Washington’s School of Public Health in Seattle. The Moderna EUA “represents progress towards ending this horrific pandemic,” she said.
In a new forecast released this week, the CDC projects that the number of newly reported COVID-19 deaths will likely increase over the next 4 weeks, with 15,800 to 27,700 new deaths likely to be reported in the week ending January 9, 2021. That could bring the total number of COVID-19 deaths in the United States to between 357,000 and 391,000 by this date, according to the agency.
ACIP panelist Lynn Bahta, RN, MPH, CPH, said she had been “eager” to have the panel proceed with its endorsement of the Moderna vaccine, “especially in light of the fact that we are seeing an average 2600 deaths a day.”
Having two COVID-19 vaccines available might help slow down the pandemic, “despite the fact that we still have a lot to learn both about the disease and the vaccine,” said Bahta, who is an immunization consultant with the Minnesota Department of Health in Saint Paul.
ACIP members encouraged Moderna officials who presented at the meeting to continue studies for potential complications associated with the vaccine when given to women who are pregnant or breastfeeding.
Panelists also pressed for more data on the risk for Bell’s palsy, which the FDA staff also had noted in the agency’s review of Moderna’s vaccine. Moderna has reported four cases from a pivotal study, one in the placebo group and three among study participants who received the company’s vaccine. These cases occurred between 15 and 33 days after vaccination, and are all resolved or resolving, according to Moderna.
There was also a question raised about how many doses of vaccine might be squeezed out of a vial. CDC will explore this topic further at its meeting on COVID-19 vaccines December 20, said Nancy Messonnier, MD, director of the agency’s National Center for Immunization and Respiratory Diseases, at the Saturday meeting.
“In this time of public health crisis, none of us would want to squander a single dose of a vaccine that’s potentially lifesaving,” CDC’s Messonnier said. “We’re going to plan to have a short discussion of that issue tomorrow.”
Messonnier also responded to a comment made during the meeting about cases where people who received COVID-19 vaccine were unaware of the CDC’s V-safe tool.
V-safe is a smartphone-based tool that uses text messaging and web surveys to help people keep in touch with the medical community after getting the COVID-19 vaccine and is seen as a way to help spot side effects. Messonnier asked that people listening to the webcast of the ACIP meeting help spread the word about the CDC’s V-safe tool.
“Our perception, based on the number of people who have enrolled in V-safe, is that the message is getting out to many places, but even one site that doesn’t have this information is something that we want to try to correct,” she said.
Anaphylaxis concerns
The chief concern for ACIP members and CDC staff about COVID-19 vaccines appeared to be reports of allergic reactions. Thomas Clark, MD, MPH, a CDC staff member, told the ACIP panel that, as of December 18, the agency had identified six cases of anaphylaxis following administration of the Pfizer-BioNTech vaccine that met a certain standard, known as the Brighton Collaboration criteria.
Additional case reports have been reviewed and determined not to be anaphylaxis, Clark said. All suspect cases were identified through processes such as the federal Vaccine Adverse Event Reporting System (VAERS), he said.
People who experience anaphylaxis following COVID-19 vaccination should not receive additional doses of the shot, Clark said in his presentation to ACIP. Members of the panel asked Clark whether there have been any discernible patterns to these cases, such as geographic clusters.
Clark replied that it was “early” in the process to make reports, with investigations still ongoing. He did note that the people who had anaphylaxis following vaccination had received their doses from more than one production lot, with multiple lots having been distributed.
“You folks may have seen in the news a couple of cases from Alaska, but we’ve had reports from other jurisdictions so there’s no obvious clustering geographically,” Clark said.
Another CDC staff member, Sarah Mbaeyi, MD, MPH, noted in her presentation that there should be an observation period of 30 minutes following COVID-19 vaccination for anyone with a history of anaphylaxis for any reason, and of at least 15 minutes for other recipients.
Disclosure of ingredients used in the COVID-19 vaccines might help people with an allergy assess these products, the representative for the American Medical Association, Sandra Fryhofer, MD, told ACIP. As such, she thanked CDC’s Mbaeyi for including a breakout of ingredients in her presentation to the panel. Fryhofer encouraged Moderna officials to be as transparent as possible in disclosing the ingredients of the company’s COVID-19 vaccine.
“That might be important because I think it’s very essential that we figure out what might be triggering these anaphylactic reactions, because that is definitely going to affect the vaccine implementation,” Fryhofer said.
The three ACIP members who had conflicts that prevented their voting were Robert L. Atmar, MD, who said at the Saturday meeting he had participated in COVID-19 trials, including research on the Moderna vaccine; Sharon E. Frey, MD, who said at the Saturday meeting that she had been involved with research on COVID-19 vaccines, including Moderna’s; and Paul Hunter, MD, who said he has received a grant from Pfizer for pneumococcal vaccines.
The other panel members have reported no relevant financial relationships.
This article first appeared on Medscape.com.
FDA grants emergency use for Moderna COVID-19 vaccine
As expected, the US Food and Drug Administration granted Moderna an emergency use authorization (EUA) for its messenger RNA COVID-19 vaccine December 18.
There is one final step — the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices will need to recommend its use, as it did 2 days after the Pfizer/BioNTech mRNA vaccine received its EUA on December 10.
The EUA for the Moderna vaccine is “a major milestone in trying to contain this pandemic,” Hana Mohammed El Sahly, MD, told Medscape Medical News.
Scaling up distribution of the two vaccine products will come next. She notes that even under less emergent conditions, making sure people who need a vaccine receive it can be hard. “I hope the media attention around this will make more people aware that there are vaccines that might help them,” said El Sahly, chair of the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC).
The EUA for the Moderna vaccine follows a review by the independent VRBPAC members on December 17, which voted 20-0 with one abstention to recommend the EUA. The vaccine is authorized for use in people 18 and older.
Emergency approval of a second COVID-19 vaccine “is great — we need all the tools we can to fight this pandemic,” Stephen Schrantz, MD, infectious disease specialist and assistant professor of medicine at the University of Chicago, told Medscape Medical News. “The early data coming from Moderna looks good, and I agree with the FDA that an EUA is indicated.
“It’s incumbent upon all us healthcare professionals to put ourselves out there as supporting this vaccine and supporting people getting it,” Schrantz continued. “We want to make sure people who are on the fence understand this is a safe vaccine that has been vetted appropriately through the FDA and through phase 3 clinical trials.”
“I know the critical role physicians play as vaccine influencers,” AMA President Susan Bailey, MD, said during a December 14 webinar for journalists reporting on COVID-19 vaccines. “We have to continue to do what physicians have always done: review the evidence and trust the science. Lives are at stake.” The webinar was cosponsored by the AMA and the Poynter Institute.
Ramping up healthcare provider immunizations
“I am very excited to see the FDA’s positive review of the Moderna vaccine. We have been waiting to have another vaccine we can use for healthcare workers and staff, and now we have it,” Aneesh Mehta, MD, of Emory University School of Medicine in Atlanta, Georgia, told Medscape Medical News.
“We had been hoping for a vaccine with a 70% or 80% efficacy, and to see two vaccines now with greater than 90% efficacy is remarkable,” he added.
The efficacy levels associated with both mRNA vaccines “did exceed expectations for sure — this is not what we built the studies around. It was surprising in the good sense of the word,” said El Sahly, who is also associate professor of molecular virology and microbiology at Baylor College of Medicine in Houston, Texas.
Unanswered questions remain
Schrantz likewise said the high efficacy rate was important but not all that is needed. “[W]hat we know about this vaccine is it is very effective at preventing disease. We don’t have any understanding at this time whether or not these vaccines prevent infection and transmissibility.”
Bailey said, “The jury is still out on whether or not you can still transmit the virus after you’ve had the vaccine. Hopefully not, but we don’t really know that for sure.”
“It’s risky to think that once you get the shot in your arm everything goes back to normal. It doesn’t,” Bailey added.
Another unknown is the duration of protection following immunization. The Pfizer and Moderna products “have similar constructs, seem to have a reasonable safety profile, and excellent short-term efficacy,” El Sahly said. She cautioned, however, that long-term efficacy still needs to be determined.
Whether any rare adverse events will emerge in the long run is another question. Answers could come over time from the ongoing phase 3 trials, as well as from post-EUA surveillance among vaccine recipients.
“Our work is not done after issuing an EUA,” FDA Commissioner Stephen Hahn, MD, said in a JAMA webinar on December 14. The FDA is closely monitoring for any adverse event rates above the normal background incidence. “We are going to be transparent about it if we are seeing anything that is not at base level.”
“The key is to be humble, keep your eyes open and know that once the vaccine is out there, there may be things we learn that we don’t know now. That is true for virtually any medical innovation,” Paul Offit, MD, director of The Vaccine Education Center at Children’s Hospital of Philadelphia and a member of the FDA VRBPAC, said during the AMA/Poynter Institute webinar.
During the same webinar, an attendee asked about prioritizing immunization for spouses and family members of healthcare workers. “My husband wants to know that too,” replied Patricia A. Stinchfield, APRN, CNP, pediatric nurse practitioner in infectious diseases at Children’s Minnesota, St. Paul.
“It is true we should be thinking about our healthcare workers’ family members. But at this point in time we just don’t have the supplies to address it that way,” said Stinchfield, who is also the president-elect of the National Foundation for Infectious Diseases.
Advantages beyond the numbers?
“The major advantage of having two vaccines is sheer volume,” Mehta said. An additional advantage of more than one product is the potential to offer an option when a specific vaccine is contraindicated. “We could offer someone a different vaccine…similar to what we do with the influenza vaccine.”
“The more the merrier in terms of having more vaccine products,” Schrantz said. Despite differences in shipping, storage, minimum age requirements, and dosing intervals, the Pfizer and Moderna vaccines are very similar, he said. “Really the only difference between these two vaccines is the proprietary lipid nanoparticle — the delivery vehicle if you will.”
Both vaccines “appear very similar in their capacity to protect against disease, to protect [people in] various racial and ethnic backgrounds, and in their capacity to protect against severe disease,” Offit said.
In terms of vaccines in the development pipeline, “We don’t know but we might start to see a difference with the Johnson & Johnson vaccine or the Janssen vaccine, which are single dose. They might confer some advantages, but we are waiting on the safety and efficacy data,” Schrantz said.
As a two-dose vaccine, the AstraZeneca product does not offer an advantage on the dosing strategy, “but it is easier to transport than the mRNA vaccines,” he said. Some concerns with the initial data on the AstraZeneca vaccine will likely need to be addressed before the company applies for an EUA, Schrantz added.
“That is an important question,” El Sahly said. The ongoing studies should provide more data from participants of all ages and ethnic backgrounds that “will allow us to make a determination as to whether there is any difference between these two vaccines.
She added that the Pfizer and Moderna vaccines seem comparable from the early data. “We’ll see if this stands in the long run.”
Future outlook
Now that the FDA approved emergency use of two COVID-19 vaccines, “we need each state to quickly implement their plans to get the vaccines into the hands of providers who need to give the vaccines,” Mehta said. “We are seeing very effective rollout in multiple regions of the country. And we hope to see that continue as we get more vaccines from manufacturers over the coming months.”
“Within a year of identifying the sequence of this virus we have two large clinical vaccine trials that show efficacy,” Offit said. “That was an amazing technologic accomplishment, but now comes the hard part. Mass producing this vaccine, getting it out there, making sure everybody who most benefits gets it, is going to be really, really hard.”
“But I’m optimistic,” Offit said. “If we can do this by next Thanksgiving, we’re going to see a dramatic drop in the number of cases, hospitalizations and deaths, and we can get our lives back together again.”
“My greatest hope is that a year from now we look back and realize we did something really amazing together,” Bailey said, “and we have a feeling of accomplishment and appreciation for all the hard work that has been done.”
Mehta shared the important message he shares when walking around the hospital: “While these vaccines are coming and they are very promising, we need to continue to remember the 3 Ws: wearing a mask, washing your hands, and watching your distance,” he said.
“With the combination of those 3Ws and those vaccines, we will hopefully come through this COVID pandemic.”
El Sahly receives funding through the NIH for her research, including her role as co-chair of the Moderna vaccine phase 3 clinical trial. Schrantz is a site investigator for the Moderna and Janssen vaccine trials. Mehta also receives funding through the NIH. None of these experts had any relevant financial disclosures.
This article first appeared on Medscape.com.
As expected, the US Food and Drug Administration granted Moderna an emergency use authorization (EUA) for its messenger RNA COVID-19 vaccine December 18.
There is one final step — the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices will need to recommend its use, as it did 2 days after the Pfizer/BioNTech mRNA vaccine received its EUA on December 10.
The EUA for the Moderna vaccine is “a major milestone in trying to contain this pandemic,” Hana Mohammed El Sahly, MD, told Medscape Medical News.
Scaling up distribution of the two vaccine products will come next. She notes that even under less emergent conditions, making sure people who need a vaccine receive it can be hard. “I hope the media attention around this will make more people aware that there are vaccines that might help them,” said El Sahly, chair of the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC).
The EUA for the Moderna vaccine follows a review by the independent VRBPAC members on December 17, which voted 20-0 with one abstention to recommend the EUA. The vaccine is authorized for use in people 18 and older.
Emergency approval of a second COVID-19 vaccine “is great — we need all the tools we can to fight this pandemic,” Stephen Schrantz, MD, infectious disease specialist and assistant professor of medicine at the University of Chicago, told Medscape Medical News. “The early data coming from Moderna looks good, and I agree with the FDA that an EUA is indicated.
“It’s incumbent upon all us healthcare professionals to put ourselves out there as supporting this vaccine and supporting people getting it,” Schrantz continued. “We want to make sure people who are on the fence understand this is a safe vaccine that has been vetted appropriately through the FDA and through phase 3 clinical trials.”
“I know the critical role physicians play as vaccine influencers,” AMA President Susan Bailey, MD, said during a December 14 webinar for journalists reporting on COVID-19 vaccines. “We have to continue to do what physicians have always done: review the evidence and trust the science. Lives are at stake.” The webinar was cosponsored by the AMA and the Poynter Institute.
Ramping up healthcare provider immunizations
“I am very excited to see the FDA’s positive review of the Moderna vaccine. We have been waiting to have another vaccine we can use for healthcare workers and staff, and now we have it,” Aneesh Mehta, MD, of Emory University School of Medicine in Atlanta, Georgia, told Medscape Medical News.
“We had been hoping for a vaccine with a 70% or 80% efficacy, and to see two vaccines now with greater than 90% efficacy is remarkable,” he added.
The efficacy levels associated with both mRNA vaccines “did exceed expectations for sure — this is not what we built the studies around. It was surprising in the good sense of the word,” said El Sahly, who is also associate professor of molecular virology and microbiology at Baylor College of Medicine in Houston, Texas.
Unanswered questions remain
Schrantz likewise said the high efficacy rate was important but not all that is needed. “[W]hat we know about this vaccine is it is very effective at preventing disease. We don’t have any understanding at this time whether or not these vaccines prevent infection and transmissibility.”
Bailey said, “The jury is still out on whether or not you can still transmit the virus after you’ve had the vaccine. Hopefully not, but we don’t really know that for sure.”
“It’s risky to think that once you get the shot in your arm everything goes back to normal. It doesn’t,” Bailey added.
Another unknown is the duration of protection following immunization. The Pfizer and Moderna products “have similar constructs, seem to have a reasonable safety profile, and excellent short-term efficacy,” El Sahly said. She cautioned, however, that long-term efficacy still needs to be determined.
Whether any rare adverse events will emerge in the long run is another question. Answers could come over time from the ongoing phase 3 trials, as well as from post-EUA surveillance among vaccine recipients.
“Our work is not done after issuing an EUA,” FDA Commissioner Stephen Hahn, MD, said in a JAMA webinar on December 14. The FDA is closely monitoring for any adverse event rates above the normal background incidence. “We are going to be transparent about it if we are seeing anything that is not at base level.”
“The key is to be humble, keep your eyes open and know that once the vaccine is out there, there may be things we learn that we don’t know now. That is true for virtually any medical innovation,” Paul Offit, MD, director of The Vaccine Education Center at Children’s Hospital of Philadelphia and a member of the FDA VRBPAC, said during the AMA/Poynter Institute webinar.
During the same webinar, an attendee asked about prioritizing immunization for spouses and family members of healthcare workers. “My husband wants to know that too,” replied Patricia A. Stinchfield, APRN, CNP, pediatric nurse practitioner in infectious diseases at Children’s Minnesota, St. Paul.
“It is true we should be thinking about our healthcare workers’ family members. But at this point in time we just don’t have the supplies to address it that way,” said Stinchfield, who is also the president-elect of the National Foundation for Infectious Diseases.
Advantages beyond the numbers?
“The major advantage of having two vaccines is sheer volume,” Mehta said. An additional advantage of more than one product is the potential to offer an option when a specific vaccine is contraindicated. “We could offer someone a different vaccine…similar to what we do with the influenza vaccine.”
“The more the merrier in terms of having more vaccine products,” Schrantz said. Despite differences in shipping, storage, minimum age requirements, and dosing intervals, the Pfizer and Moderna vaccines are very similar, he said. “Really the only difference between these two vaccines is the proprietary lipid nanoparticle — the delivery vehicle if you will.”
Both vaccines “appear very similar in their capacity to protect against disease, to protect [people in] various racial and ethnic backgrounds, and in their capacity to protect against severe disease,” Offit said.
In terms of vaccines in the development pipeline, “We don’t know but we might start to see a difference with the Johnson & Johnson vaccine or the Janssen vaccine, which are single dose. They might confer some advantages, but we are waiting on the safety and efficacy data,” Schrantz said.
As a two-dose vaccine, the AstraZeneca product does not offer an advantage on the dosing strategy, “but it is easier to transport than the mRNA vaccines,” he said. Some concerns with the initial data on the AstraZeneca vaccine will likely need to be addressed before the company applies for an EUA, Schrantz added.
“That is an important question,” El Sahly said. The ongoing studies should provide more data from participants of all ages and ethnic backgrounds that “will allow us to make a determination as to whether there is any difference between these two vaccines.
She added that the Pfizer and Moderna vaccines seem comparable from the early data. “We’ll see if this stands in the long run.”
Future outlook
Now that the FDA approved emergency use of two COVID-19 vaccines, “we need each state to quickly implement their plans to get the vaccines into the hands of providers who need to give the vaccines,” Mehta said. “We are seeing very effective rollout in multiple regions of the country. And we hope to see that continue as we get more vaccines from manufacturers over the coming months.”
“Within a year of identifying the sequence of this virus we have two large clinical vaccine trials that show efficacy,” Offit said. “That was an amazing technologic accomplishment, but now comes the hard part. Mass producing this vaccine, getting it out there, making sure everybody who most benefits gets it, is going to be really, really hard.”
“But I’m optimistic,” Offit said. “If we can do this by next Thanksgiving, we’re going to see a dramatic drop in the number of cases, hospitalizations and deaths, and we can get our lives back together again.”
“My greatest hope is that a year from now we look back and realize we did something really amazing together,” Bailey said, “and we have a feeling of accomplishment and appreciation for all the hard work that has been done.”
Mehta shared the important message he shares when walking around the hospital: “While these vaccines are coming and they are very promising, we need to continue to remember the 3 Ws: wearing a mask, washing your hands, and watching your distance,” he said.
“With the combination of those 3Ws and those vaccines, we will hopefully come through this COVID pandemic.”
El Sahly receives funding through the NIH for her research, including her role as co-chair of the Moderna vaccine phase 3 clinical trial. Schrantz is a site investigator for the Moderna and Janssen vaccine trials. Mehta also receives funding through the NIH. None of these experts had any relevant financial disclosures.
This article first appeared on Medscape.com.
As expected, the US Food and Drug Administration granted Moderna an emergency use authorization (EUA) for its messenger RNA COVID-19 vaccine December 18.
There is one final step — the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices will need to recommend its use, as it did 2 days after the Pfizer/BioNTech mRNA vaccine received its EUA on December 10.
The EUA for the Moderna vaccine is “a major milestone in trying to contain this pandemic,” Hana Mohammed El Sahly, MD, told Medscape Medical News.
Scaling up distribution of the two vaccine products will come next. She notes that even under less emergent conditions, making sure people who need a vaccine receive it can be hard. “I hope the media attention around this will make more people aware that there are vaccines that might help them,” said El Sahly, chair of the FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC).
The EUA for the Moderna vaccine follows a review by the independent VRBPAC members on December 17, which voted 20-0 with one abstention to recommend the EUA. The vaccine is authorized for use in people 18 and older.
Emergency approval of a second COVID-19 vaccine “is great — we need all the tools we can to fight this pandemic,” Stephen Schrantz, MD, infectious disease specialist and assistant professor of medicine at the University of Chicago, told Medscape Medical News. “The early data coming from Moderna looks good, and I agree with the FDA that an EUA is indicated.
“It’s incumbent upon all us healthcare professionals to put ourselves out there as supporting this vaccine and supporting people getting it,” Schrantz continued. “We want to make sure people who are on the fence understand this is a safe vaccine that has been vetted appropriately through the FDA and through phase 3 clinical trials.”
“I know the critical role physicians play as vaccine influencers,” AMA President Susan Bailey, MD, said during a December 14 webinar for journalists reporting on COVID-19 vaccines. “We have to continue to do what physicians have always done: review the evidence and trust the science. Lives are at stake.” The webinar was cosponsored by the AMA and the Poynter Institute.
Ramping up healthcare provider immunizations
“I am very excited to see the FDA’s positive review of the Moderna vaccine. We have been waiting to have another vaccine we can use for healthcare workers and staff, and now we have it,” Aneesh Mehta, MD, of Emory University School of Medicine in Atlanta, Georgia, told Medscape Medical News.
“We had been hoping for a vaccine with a 70% or 80% efficacy, and to see two vaccines now with greater than 90% efficacy is remarkable,” he added.
The efficacy levels associated with both mRNA vaccines “did exceed expectations for sure — this is not what we built the studies around. It was surprising in the good sense of the word,” said El Sahly, who is also associate professor of molecular virology and microbiology at Baylor College of Medicine in Houston, Texas.
Unanswered questions remain
Schrantz likewise said the high efficacy rate was important but not all that is needed. “[W]hat we know about this vaccine is it is very effective at preventing disease. We don’t have any understanding at this time whether or not these vaccines prevent infection and transmissibility.”
Bailey said, “The jury is still out on whether or not you can still transmit the virus after you’ve had the vaccine. Hopefully not, but we don’t really know that for sure.”
“It’s risky to think that once you get the shot in your arm everything goes back to normal. It doesn’t,” Bailey added.
Another unknown is the duration of protection following immunization. The Pfizer and Moderna products “have similar constructs, seem to have a reasonable safety profile, and excellent short-term efficacy,” El Sahly said. She cautioned, however, that long-term efficacy still needs to be determined.
Whether any rare adverse events will emerge in the long run is another question. Answers could come over time from the ongoing phase 3 trials, as well as from post-EUA surveillance among vaccine recipients.
“Our work is not done after issuing an EUA,” FDA Commissioner Stephen Hahn, MD, said in a JAMA webinar on December 14. The FDA is closely monitoring for any adverse event rates above the normal background incidence. “We are going to be transparent about it if we are seeing anything that is not at base level.”
“The key is to be humble, keep your eyes open and know that once the vaccine is out there, there may be things we learn that we don’t know now. That is true for virtually any medical innovation,” Paul Offit, MD, director of The Vaccine Education Center at Children’s Hospital of Philadelphia and a member of the FDA VRBPAC, said during the AMA/Poynter Institute webinar.
During the same webinar, an attendee asked about prioritizing immunization for spouses and family members of healthcare workers. “My husband wants to know that too,” replied Patricia A. Stinchfield, APRN, CNP, pediatric nurse practitioner in infectious diseases at Children’s Minnesota, St. Paul.
“It is true we should be thinking about our healthcare workers’ family members. But at this point in time we just don’t have the supplies to address it that way,” said Stinchfield, who is also the president-elect of the National Foundation for Infectious Diseases.
Advantages beyond the numbers?
“The major advantage of having two vaccines is sheer volume,” Mehta said. An additional advantage of more than one product is the potential to offer an option when a specific vaccine is contraindicated. “We could offer someone a different vaccine…similar to what we do with the influenza vaccine.”
“The more the merrier in terms of having more vaccine products,” Schrantz said. Despite differences in shipping, storage, minimum age requirements, and dosing intervals, the Pfizer and Moderna vaccines are very similar, he said. “Really the only difference between these two vaccines is the proprietary lipid nanoparticle — the delivery vehicle if you will.”
Both vaccines “appear very similar in their capacity to protect against disease, to protect [people in] various racial and ethnic backgrounds, and in their capacity to protect against severe disease,” Offit said.
In terms of vaccines in the development pipeline, “We don’t know but we might start to see a difference with the Johnson & Johnson vaccine or the Janssen vaccine, which are single dose. They might confer some advantages, but we are waiting on the safety and efficacy data,” Schrantz said.
As a two-dose vaccine, the AstraZeneca product does not offer an advantage on the dosing strategy, “but it is easier to transport than the mRNA vaccines,” he said. Some concerns with the initial data on the AstraZeneca vaccine will likely need to be addressed before the company applies for an EUA, Schrantz added.
“That is an important question,” El Sahly said. The ongoing studies should provide more data from participants of all ages and ethnic backgrounds that “will allow us to make a determination as to whether there is any difference between these two vaccines.
She added that the Pfizer and Moderna vaccines seem comparable from the early data. “We’ll see if this stands in the long run.”
Future outlook
Now that the FDA approved emergency use of two COVID-19 vaccines, “we need each state to quickly implement their plans to get the vaccines into the hands of providers who need to give the vaccines,” Mehta said. “We are seeing very effective rollout in multiple regions of the country. And we hope to see that continue as we get more vaccines from manufacturers over the coming months.”
“Within a year of identifying the sequence of this virus we have two large clinical vaccine trials that show efficacy,” Offit said. “That was an amazing technologic accomplishment, but now comes the hard part. Mass producing this vaccine, getting it out there, making sure everybody who most benefits gets it, is going to be really, really hard.”
“But I’m optimistic,” Offit said. “If we can do this by next Thanksgiving, we’re going to see a dramatic drop in the number of cases, hospitalizations and deaths, and we can get our lives back together again.”
“My greatest hope is that a year from now we look back and realize we did something really amazing together,” Bailey said, “and we have a feeling of accomplishment and appreciation for all the hard work that has been done.”
Mehta shared the important message he shares when walking around the hospital: “While these vaccines are coming and they are very promising, we need to continue to remember the 3 Ws: wearing a mask, washing your hands, and watching your distance,” he said.
“With the combination of those 3Ws and those vaccines, we will hopefully come through this COVID pandemic.”
El Sahly receives funding through the NIH for her research, including her role as co-chair of the Moderna vaccine phase 3 clinical trial. Schrantz is a site investigator for the Moderna and Janssen vaccine trials. Mehta also receives funding through the NIH. None of these experts had any relevant financial disclosures.
This article first appeared on Medscape.com.
Contact tracing in hospitals falls off as COVID-19 cases rise
Like most health care workers at his hospital in Lafayette, Ind., Ramesh Adhikari, MD, FHM, occasionally gets an email noting that a patient he saw later tested positive for COVID-19. He’s reminded to self-monitor for symptoms. But 10 months into the pandemic, it has become increasingly unlikely for contact tracing investigations to result in clinicians quarantining.
The very act of working in the hospital, Dr. Adhikari said, means being likely to see COVID-19 every day, whether in a known patient or an asymptomatic person who tests positive later. If hospitalists had to quarantine after every interaction with a COVID-positive person, there wouldn’t be anyone left to do their jobs.
“It’s really hard to do [contact tracing] in health care workers thoroughly because of the way we work,” Dr. Adhikari said. “It’s impossible to do it absolutely.”
In a recently updated guidance, the Centers for Disease Control and Prevention extended more leeway in contact tracing when community rates of COVID-19 surge, even allowing that contact tracing “may not be possible” in certain situations. And by defining an exposure more narrowly – health care workers are only considered “exposed” if their contact was more than 15 minutes or lacking in some form of PPE – the guidelines suggest that hospitals can rely more on universal PPE and screening protocols, as Dr. Adhikari’s hospital does, and less on extensive contact tracing to curtail viral spread.
Accordingly, while contact tracing has gotten more lax, doctors say, universal precautions – including full PPE and screening of symptoms for patients and health care workers – have become more stringent.
It’s a shift from the beginning of the pandemic. At first, CDC recommended wearing masks only during aerosol-producing procedures. Exposures were frequently reported and health care workers sent home. With more evidence in favor of stricter PPE requirements, hospitals including the one where Shyam Odeti, MD, FHM, works in Johnson City, Tenn., have adopted a universal precaution strategy – requiring masks everywhere and a gown, face shield, gloves, and N95 to enter a COVID-positive patient’s room. Thus, most exposures fall into that low-risk category.
“If I get it and am asymptomatic, I don’t think my colleagues would be exposed by any means because of these stringent policies being enforced,” said Dr. Odeti, a hospitalist who often wears a surgical mask on top of his N95 all day. “And U.S. health care is not in a state that can afford to quarantine health care workers for 14 days.”
Can universal PPE precautions supplant contact tracing?
The extent of contact tracing varies by hospital. Larger university and community hospitals often have infection control and occupational health teams that can do their own contact tracing, while smaller institutions can’t always spare staff. And some state health departments get involved with contact tracing of health care workers while others do not.
“I would venture to say that most hospitals are doing something in terms of contact tracing,” said Pam Falk, MPH, CIC, a member of the Association for Professionals in Infection Control and Epidemiology’s COVID-19 task force and an infection control consultant. “It kind of depends on their bandwidth.”
But there’s no longer a norm. Outside of a pandemic, with ample staffing and far fewer instances that need to be investigated, standards for contact tracing are higher, Dr. Falk said: When a patient is found to have an airborne disease such as tuberculosis, measles, mumps, or chickenpox, a hospital’s infection prevention team should investigate, confirm the diagnosis and identify everyone who was exposed. The hospital’s occupational health team assists in deciding who will likely need prophylactic treatment and if employees should be furloughed. The thoroughness of such measures has always depended on a hospital’s bandwidth.
Because PPE seems to be able to contain COVID-19 better than some of the older diseases targeted by contact tracing, universal protections may be a reasonable alternative in current circumstances, doctors said – if PPE is available.
“At the end of the day, universal source control with surgical masks – and ideally eye protection for clinicians as well – should prevent most transmissions,” said Aaron Richterman, MD, from the division of infectious diseases at the Hospital of the University of Pennsylvania, Philadelphia, who coauthored a JAMA commentary on decreased transmission rates in hospitals.
Contact tracing is still useful, though, to identify weaknesses in universal protection measures, he said.
“I don’t think it’s worth abandoning. It’s like a tool in the toolbox. All are imperfect, and none work 100% of the time,” Dr. Richterman said, but using all of them can achieve a fairly high measure of safety. Of the tools, universal masking likely works the best, he contends, so it should be the top pick for hospitals without resources to use all of the tools.
A recent incident at Brigham and Women’s Hospital in Boston is a case study in how contact tracing can work together with universal protections to identify cracks in the system, said Dr. Richterman, who worked at the hospital earlier in the pandemic.
Mass General Brigham adopted a universal masking policy for staff and patients in March 2020. Then, when the system experienced an outbreak in September, the hospital did “a very detailed public evaluation that included contact tracing and universal testing,” Dr. Richterman said. Testing even included genetic analysis of the virus to confirm which cases were hospital acquired. In the end, the hospital identified weaknesses in infection control that could be rectified, such as clinicians eating too close together.
“The approach is not to point fingers, but to say: ‘What’s wrong with the system and how do we improve?’ ” Dr. Richterman said. “To ask, why did that maskless transmission happen? Is there not enough space to eat? Are people working too many hours? It’s useful for systems to understand where transmissions are happening.”
Amith Skandhan, MD, SFHM, a hospitalist in Dothan, Ala., is comfortable without much contact tracing as long as there is universal PPE use. His hospital informs clinicians of exposures, but “basically we’re trained to treat every patient as if they had COVID,” he said, so “I feel more secure in the hospital than in the community.” Masks have become so habitual they’re like part of your regular clothing, he said – you feel incomplete if you don’t have one.
While ad hoc approaches to contact tracing may be useful in the current stage of the pandemic, they are likely to be short-lived: Once a community’s positivity rate falls, the CDC’s guidance suggests how hospitals can return to full contact tracing.
A version of this article first appeared on Medscape.com.
Like most health care workers at his hospital in Lafayette, Ind., Ramesh Adhikari, MD, FHM, occasionally gets an email noting that a patient he saw later tested positive for COVID-19. He’s reminded to self-monitor for symptoms. But 10 months into the pandemic, it has become increasingly unlikely for contact tracing investigations to result in clinicians quarantining.
The very act of working in the hospital, Dr. Adhikari said, means being likely to see COVID-19 every day, whether in a known patient or an asymptomatic person who tests positive later. If hospitalists had to quarantine after every interaction with a COVID-positive person, there wouldn’t be anyone left to do their jobs.
“It’s really hard to do [contact tracing] in health care workers thoroughly because of the way we work,” Dr. Adhikari said. “It’s impossible to do it absolutely.”
In a recently updated guidance, the Centers for Disease Control and Prevention extended more leeway in contact tracing when community rates of COVID-19 surge, even allowing that contact tracing “may not be possible” in certain situations. And by defining an exposure more narrowly – health care workers are only considered “exposed” if their contact was more than 15 minutes or lacking in some form of PPE – the guidelines suggest that hospitals can rely more on universal PPE and screening protocols, as Dr. Adhikari’s hospital does, and less on extensive contact tracing to curtail viral spread.
Accordingly, while contact tracing has gotten more lax, doctors say, universal precautions – including full PPE and screening of symptoms for patients and health care workers – have become more stringent.
It’s a shift from the beginning of the pandemic. At first, CDC recommended wearing masks only during aerosol-producing procedures. Exposures were frequently reported and health care workers sent home. With more evidence in favor of stricter PPE requirements, hospitals including the one where Shyam Odeti, MD, FHM, works in Johnson City, Tenn., have adopted a universal precaution strategy – requiring masks everywhere and a gown, face shield, gloves, and N95 to enter a COVID-positive patient’s room. Thus, most exposures fall into that low-risk category.
“If I get it and am asymptomatic, I don’t think my colleagues would be exposed by any means because of these stringent policies being enforced,” said Dr. Odeti, a hospitalist who often wears a surgical mask on top of his N95 all day. “And U.S. health care is not in a state that can afford to quarantine health care workers for 14 days.”
Can universal PPE precautions supplant contact tracing?
The extent of contact tracing varies by hospital. Larger university and community hospitals often have infection control and occupational health teams that can do their own contact tracing, while smaller institutions can’t always spare staff. And some state health departments get involved with contact tracing of health care workers while others do not.
“I would venture to say that most hospitals are doing something in terms of contact tracing,” said Pam Falk, MPH, CIC, a member of the Association for Professionals in Infection Control and Epidemiology’s COVID-19 task force and an infection control consultant. “It kind of depends on their bandwidth.”
But there’s no longer a norm. Outside of a pandemic, with ample staffing and far fewer instances that need to be investigated, standards for contact tracing are higher, Dr. Falk said: When a patient is found to have an airborne disease such as tuberculosis, measles, mumps, or chickenpox, a hospital’s infection prevention team should investigate, confirm the diagnosis and identify everyone who was exposed. The hospital’s occupational health team assists in deciding who will likely need prophylactic treatment and if employees should be furloughed. The thoroughness of such measures has always depended on a hospital’s bandwidth.
Because PPE seems to be able to contain COVID-19 better than some of the older diseases targeted by contact tracing, universal protections may be a reasonable alternative in current circumstances, doctors said – if PPE is available.
“At the end of the day, universal source control with surgical masks – and ideally eye protection for clinicians as well – should prevent most transmissions,” said Aaron Richterman, MD, from the division of infectious diseases at the Hospital of the University of Pennsylvania, Philadelphia, who coauthored a JAMA commentary on decreased transmission rates in hospitals.
Contact tracing is still useful, though, to identify weaknesses in universal protection measures, he said.
“I don’t think it’s worth abandoning. It’s like a tool in the toolbox. All are imperfect, and none work 100% of the time,” Dr. Richterman said, but using all of them can achieve a fairly high measure of safety. Of the tools, universal masking likely works the best, he contends, so it should be the top pick for hospitals without resources to use all of the tools.
A recent incident at Brigham and Women’s Hospital in Boston is a case study in how contact tracing can work together with universal protections to identify cracks in the system, said Dr. Richterman, who worked at the hospital earlier in the pandemic.
Mass General Brigham adopted a universal masking policy for staff and patients in March 2020. Then, when the system experienced an outbreak in September, the hospital did “a very detailed public evaluation that included contact tracing and universal testing,” Dr. Richterman said. Testing even included genetic analysis of the virus to confirm which cases were hospital acquired. In the end, the hospital identified weaknesses in infection control that could be rectified, such as clinicians eating too close together.
“The approach is not to point fingers, but to say: ‘What’s wrong with the system and how do we improve?’ ” Dr. Richterman said. “To ask, why did that maskless transmission happen? Is there not enough space to eat? Are people working too many hours? It’s useful for systems to understand where transmissions are happening.”
Amith Skandhan, MD, SFHM, a hospitalist in Dothan, Ala., is comfortable without much contact tracing as long as there is universal PPE use. His hospital informs clinicians of exposures, but “basically we’re trained to treat every patient as if they had COVID,” he said, so “I feel more secure in the hospital than in the community.” Masks have become so habitual they’re like part of your regular clothing, he said – you feel incomplete if you don’t have one.
While ad hoc approaches to contact tracing may be useful in the current stage of the pandemic, they are likely to be short-lived: Once a community’s positivity rate falls, the CDC’s guidance suggests how hospitals can return to full contact tracing.
A version of this article first appeared on Medscape.com.
Like most health care workers at his hospital in Lafayette, Ind., Ramesh Adhikari, MD, FHM, occasionally gets an email noting that a patient he saw later tested positive for COVID-19. He’s reminded to self-monitor for symptoms. But 10 months into the pandemic, it has become increasingly unlikely for contact tracing investigations to result in clinicians quarantining.
The very act of working in the hospital, Dr. Adhikari said, means being likely to see COVID-19 every day, whether in a known patient or an asymptomatic person who tests positive later. If hospitalists had to quarantine after every interaction with a COVID-positive person, there wouldn’t be anyone left to do their jobs.
“It’s really hard to do [contact tracing] in health care workers thoroughly because of the way we work,” Dr. Adhikari said. “It’s impossible to do it absolutely.”
In a recently updated guidance, the Centers for Disease Control and Prevention extended more leeway in contact tracing when community rates of COVID-19 surge, even allowing that contact tracing “may not be possible” in certain situations. And by defining an exposure more narrowly – health care workers are only considered “exposed” if their contact was more than 15 minutes or lacking in some form of PPE – the guidelines suggest that hospitals can rely more on universal PPE and screening protocols, as Dr. Adhikari’s hospital does, and less on extensive contact tracing to curtail viral spread.
Accordingly, while contact tracing has gotten more lax, doctors say, universal precautions – including full PPE and screening of symptoms for patients and health care workers – have become more stringent.
It’s a shift from the beginning of the pandemic. At first, CDC recommended wearing masks only during aerosol-producing procedures. Exposures were frequently reported and health care workers sent home. With more evidence in favor of stricter PPE requirements, hospitals including the one where Shyam Odeti, MD, FHM, works in Johnson City, Tenn., have adopted a universal precaution strategy – requiring masks everywhere and a gown, face shield, gloves, and N95 to enter a COVID-positive patient’s room. Thus, most exposures fall into that low-risk category.
“If I get it and am asymptomatic, I don’t think my colleagues would be exposed by any means because of these stringent policies being enforced,” said Dr. Odeti, a hospitalist who often wears a surgical mask on top of his N95 all day. “And U.S. health care is not in a state that can afford to quarantine health care workers for 14 days.”
Can universal PPE precautions supplant contact tracing?
The extent of contact tracing varies by hospital. Larger university and community hospitals often have infection control and occupational health teams that can do their own contact tracing, while smaller institutions can’t always spare staff. And some state health departments get involved with contact tracing of health care workers while others do not.
“I would venture to say that most hospitals are doing something in terms of contact tracing,” said Pam Falk, MPH, CIC, a member of the Association for Professionals in Infection Control and Epidemiology’s COVID-19 task force and an infection control consultant. “It kind of depends on their bandwidth.”
But there’s no longer a norm. Outside of a pandemic, with ample staffing and far fewer instances that need to be investigated, standards for contact tracing are higher, Dr. Falk said: When a patient is found to have an airborne disease such as tuberculosis, measles, mumps, or chickenpox, a hospital’s infection prevention team should investigate, confirm the diagnosis and identify everyone who was exposed. The hospital’s occupational health team assists in deciding who will likely need prophylactic treatment and if employees should be furloughed. The thoroughness of such measures has always depended on a hospital’s bandwidth.
Because PPE seems to be able to contain COVID-19 better than some of the older diseases targeted by contact tracing, universal protections may be a reasonable alternative in current circumstances, doctors said – if PPE is available.
“At the end of the day, universal source control with surgical masks – and ideally eye protection for clinicians as well – should prevent most transmissions,” said Aaron Richterman, MD, from the division of infectious diseases at the Hospital of the University of Pennsylvania, Philadelphia, who coauthored a JAMA commentary on decreased transmission rates in hospitals.
Contact tracing is still useful, though, to identify weaknesses in universal protection measures, he said.
“I don’t think it’s worth abandoning. It’s like a tool in the toolbox. All are imperfect, and none work 100% of the time,” Dr. Richterman said, but using all of them can achieve a fairly high measure of safety. Of the tools, universal masking likely works the best, he contends, so it should be the top pick for hospitals without resources to use all of the tools.
A recent incident at Brigham and Women’s Hospital in Boston is a case study in how contact tracing can work together with universal protections to identify cracks in the system, said Dr. Richterman, who worked at the hospital earlier in the pandemic.
Mass General Brigham adopted a universal masking policy for staff and patients in March 2020. Then, when the system experienced an outbreak in September, the hospital did “a very detailed public evaluation that included contact tracing and universal testing,” Dr. Richterman said. Testing even included genetic analysis of the virus to confirm which cases were hospital acquired. In the end, the hospital identified weaknesses in infection control that could be rectified, such as clinicians eating too close together.
“The approach is not to point fingers, but to say: ‘What’s wrong with the system and how do we improve?’ ” Dr. Richterman said. “To ask, why did that maskless transmission happen? Is there not enough space to eat? Are people working too many hours? It’s useful for systems to understand where transmissions are happening.”
Amith Skandhan, MD, SFHM, a hospitalist in Dothan, Ala., is comfortable without much contact tracing as long as there is universal PPE use. His hospital informs clinicians of exposures, but “basically we’re trained to treat every patient as if they had COVID,” he said, so “I feel more secure in the hospital than in the community.” Masks have become so habitual they’re like part of your regular clothing, he said – you feel incomplete if you don’t have one.
While ad hoc approaches to contact tracing may be useful in the current stage of the pandemic, they are likely to be short-lived: Once a community’s positivity rate falls, the CDC’s guidance suggests how hospitals can return to full contact tracing.
A version of this article first appeared on Medscape.com.








