User login
Food insecurity: How to recognize & address it
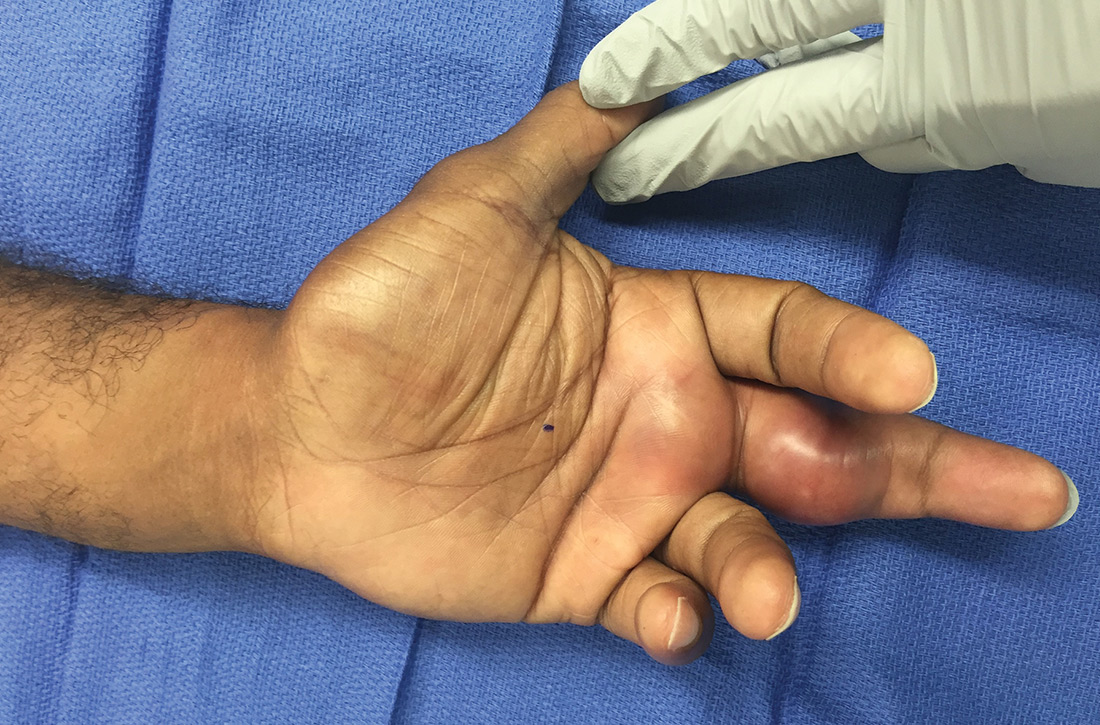
CASE Alice D is 20 years old and has type 1 diabetes, as well as retinopathy, hypertension, bipolar I disorder, and hyperlipidemia. She is a new patient at your clinic and reports that she is “occasionally homeless” and has difficulty affording food.
You renew Ms. D’s prescriptions during the visit, discuss nutrition with her, and

According to a 2016 report from the US Department of Agriculture (USDA) Economic Research Service, an estimated 12.3% of households in the United States are “food insecure.”1 To ascertain what food security and insecurity are, the USDA measured numerous variables, including household structure, race and ethnicity, geography, and income. The report of the Economic Research Service stands as one of the largest domestic sources of information about food insecurity.
Food insecurity is defined as “food intake of household members [that is] reduced and normal eating patterns that are disrupted.”1 It is often measured “per household,” but those data must be interpreted carefully because food insecurity affects household members differently. Some members, such as children, might be affected only mildly; adults, on the other hand, might be more severely affected. (Adults may, for instance, disrupt or skip their meals to maintain normal diets and meal patterns for their children.) In some households, food insecurity affects only a single member—such as an older adult—because of conditions unique to the people living in the home.
In this article, we review variables that can give rise to food insecurity in children, adults, and the elderly, and offer strategies to the family physician for identifying and alleviating the burden of food insecurity in these populations.
Food insecurity threatens children’s health, development
In 2016, households with children faced a higher level of food insecurity (16.5%) than the national average (12.3%).1 In a study of more than 280,000 households, food insecurity was sometimes so severe that children skipped meals or did not eat for the whole day.1 Although income strongly correlates with food insecurity, evidence shows that families above and below the poverty line suffer from food insecurity.2,3
According to the USDA, the rate of food insecurity is higher than the national average of 12.3% in several subgroups of children1:
- households with children < 6 years of age (16.6%)
- households with children headed by a single woman (31.6%) or single man (21.7%)
- households headed by a black non-Hispanic (22.5%) or Hispanic (18.5%) person
- low-income households in which the annual income is < 185% of the poverty threshold (31.6%).
Continue to: Evidence suggests that...
Evidence suggests that children in food-insecure homes experience poor diet, impaired cognitive development, an increased risk of chronic illness in adulthood, and emotional and behavioral problems.4-7 For caregivers in food-insecure homes, purchase price is the most influential factor when making food purchasing decisions. Thus, caregivers often purchase cheaper, more calorie-dense foods, rather than more expensive, nutrient-rich foods—leading to childhood obesity.8
Relief eludes many. Federal programs, such as the National School Lunch Program, School Breakfast Program, the Summer Food Service Program, and the Child and Adult Care Food Program, provide free or reduced-price meals for school-age children. Although these programs reduce food insecurity in households that participate, program policy has established that participation is based on household income.9 This is problematic: According to the literature,2 the income of 50% of households that are food-insecure is above the federal poverty level.
It would be more effective to have these programs target families based on geography, not income, because programs would then benefit those who are food-insecure but who live above the poverty line. Location is a significant factor in identifying food-insecure populations: Households outside metropolitan areas are disproportionately affected.1 If these programs were to privilege geography over income, they would include (for example): families in school districts with a low number of grocery stores; families with poor access to public transportation; and families that live in a “food desert”—ie, where fresh, low-cost food options are overshadowed by fast food.
One such program closely applied the model of privilege based on geography: In 2010, the Healthy, Hunger-Free Kids Act was passed, with a Community Eligibility Provision (CEP) that funded school districts in which ≥ 40% of students lived below the poverty line, so that students in those districts received a free school lunch.10 Although eligibility for CEP is still based on income, benefits go to all students who live in the district, including food-insecure students who live in a household above the poverty line. If eligibility criteria were expanded with CEP so that more school districts could participate, it might solve many obstacles faced by other existing programs.
Programs that provide nutrition for households with an infant or young child—eg, the Women, Infants and Children Special Supplemental Nutrition Program (WIC) and the Supplemental Nutrition Assistance Program (SNAP)—reduce food insecurity in households by 20%. However, several unstudied factors can affect food insecurity in families beyond these programs11; some assumptions about food insecurity, for example, strongly point to the influence of maternal mental health.12
Continue to: Data from the...
Data from the Early Childhood Longitudinal Study, Birth Cohort showed that mothers (with an infant) who were suffering from depression had a significantly increased risk of food insecurity.13 To better identify infants at risk of food insecurity, it would be beneficial to identify women suffering from depression during pregnancy or postpartum.13 These patients could then be referred to WIC and for SNAP benefits.
What you can do. The American Academy of Pediatrics recommends that physicians identify families that are food-insecure by conducting a validated14 2-question survey about food insecurity at every health-maintenance visit, as long as the child is a patient in the practice (TABLE 1).15 Physicians can then refer families that screen positively to local WIC and SNAP centers.

Ideally, physicians should be prepared to facilitate more active engagement by providing patients with the contact information of staff members working in such local programs. Staffing the practice with a patient-care manager can be an efficient way to navigate this process.
CASE Over the 6 weeks following Ms. D’s visit with you, she is admitted 5 times to the hospital in diabetic ketoacidosis, always with a significantly elevated blood glucose level. At each admission, she admits to “sometimes forgetting” to take insulin. Hospital staff members do not ask about her food intake. During each hospitalization, Ms. D is treated with insulin and intravenous fluids and discharged to home on her prior insulin regimen.
During a follow-up appointment with you and the clinic’s nurse–care manager, she talks about missing doses of insulin. She tells you that she has been getting food from the local food pantry, where available stocks are typically carbohydrate-based, including bread, rice, and cereal. She admits that she cannot afford other kinds of food—specifically, those that contain protein and monosaturated and polyunsaturated fats.
Continue to: Adults...
Adults: Poor financial health correlates with insecurity
The correlation between food insecurity and income is strong—evidenced by the spike in the number of adults who reported food insecurity during the 2008-2011 recession in the United States, to a high of 14.9%.1 As noted, households with children are more likely to report food insecurity. In addition, studies show that limited resources, race and ethnicity, underemployment or unemployment, and high housing costs are also strongly associated with food insecurity.16 Even subtle economic fluctuations—for example, an increase in the price of gasoline, natural gas, or electricity—contribute to food insecurity.17 Debt and coping mechanisms influence whether a household living below the poverty line is food-secure or food-insecure. Additional factors contributing to food insecurity include participation in SNAP, education, and severe depression.
Food insecurity in adults reduces the quality of food and nutritional intake, and is associated with chronic morbidity, such as type 2 diabetes, hypertension, and obesity.5-7 Adults in food-insecure homes are more likely to purchase cheap, calorie-dense, nutritionally poor foods (or refrain from purchasing food altogether, to pay other debts).17,18 The literature further suggests that food insecurity is associated with diseases that limit function and lead to disability, such as arthritis, stroke, and coronary artery disease, in adults and older adults (> 65 years of age; see the next section).5,6,19 These studies are weak, however, in their ability to show directionality: Does food insecurity cause disability or does disability cause food insecurity?
Patchwork of programs. Programs such as WIC are available for women who are pregnant or have children < 5 years of age. Federal programs for adults who do not have children are scarce, however, and the burden of food insecurity for this population is typically addressed by local programs, such as food banks and food kitchens. Evidence shows that (1) combining the efforts of federal and local food programs is the most effective method of stymieing food insecurity in adults and (2) it would benefit food-insecure adults to have access to such programs. Regrettably, many food programs are underutilized because of barriers that include poor outreach, ineffectual application, and ineligibility.
What you can do. Although it might not be an official, professional society guideline to include questions about food security in a patient wellness survey, physicians should consider creating one for their practice that they (or the office staff) can administer. Furthermore, physicians (or, again, the office staff) should familiarize themselves with programs in the community, such as SNAP or a food bank, to which they can refer patients, as needed.
CASE You ask the nurse–care manager to consult with staff of the food bank and request that, based on your evaluation and recommendation, Ms. D be given more protein-based foods, including peanut butter and beans, when she visits the food bank. The nurse–care manager also makes arrangements to procure an insulin pump for Ms. D.
Continue to: In a short time...
In a short time, Ms. D’s blood glucose level normalizes. She has no further admissions for diabetic ketoacidosis.
Older adults: Interplay of risk factors takes a toll
The USDA Economic Research Report on food insecurity1 states that older adults (≥ 65 years of age) report a lower rate of food insecurity—ie, 7.8% of households with an older adult and 8.9% of households in which the older adult lives alone—compared with the national average.1 The report is limited, however, in its ability to extrapolate data from older adults on food insecurity because its focus is on factors specific to adults and children.
Factors that contribute to food insecurity in the elderly include race and ethnicity, education, income, being a SNAP recipient, and severe depression.1,2,17,20,21 Older adult subgroups more likely to be food-insecure are Hispanic and black non-Hispanic—both significantly associated with being food-insecure, with Hispanic populations reporting the highest rates of food insecurity.20,21 This is a particularly interesting observation: Many traditional Hispanic homes are multigenerational and maintain a culture in which older adults are cared for by their children; that value system might be an indication of why many Hispanic households are disproportionately affected by food insecurity.
Other problems directly caused or exacerbated by food insecurity in the older population include a higher risk of malnutrition from periodontal disease, more frequent hospital admissions with longer length of stay, and an increased rate of falls and fractures. Polypharmacy, which can cause food–drug interactions that inhibit uptake of vitamins or create a higher demand for certain vitamins, is a noteworthy problem associated with food insecurity.
Problems with functionality might prevent older adults from performing physical tasks, such as shopping and preparing foods.21,22 Older adults who reported functional impairment in performing activities of daily living are more likely to report food insecurity.21,22 Last,older adults who live alone are more likely to have diminished nutritional intake than those who live with a spouse or partner.2,22,23
Continue to: Legislation enacted in 2010...
Legislation enacted in 2010 under the existing Older Americans Act provided home-delivered meals, nutritional screening, and education counseling to Americans > 60 years of age. That provision was not based on an income test, however, and served only 18% of the older population.23 (Other programs, such as SNAP, are utilized to a greater degree: 30% of eligible older adults participate, 75% of whom live alone.23) Possible reasons for underutilization include restricted funding, lower education level, lack of outreach, a confusing application process, and the impression that the process is intrusive.24-26
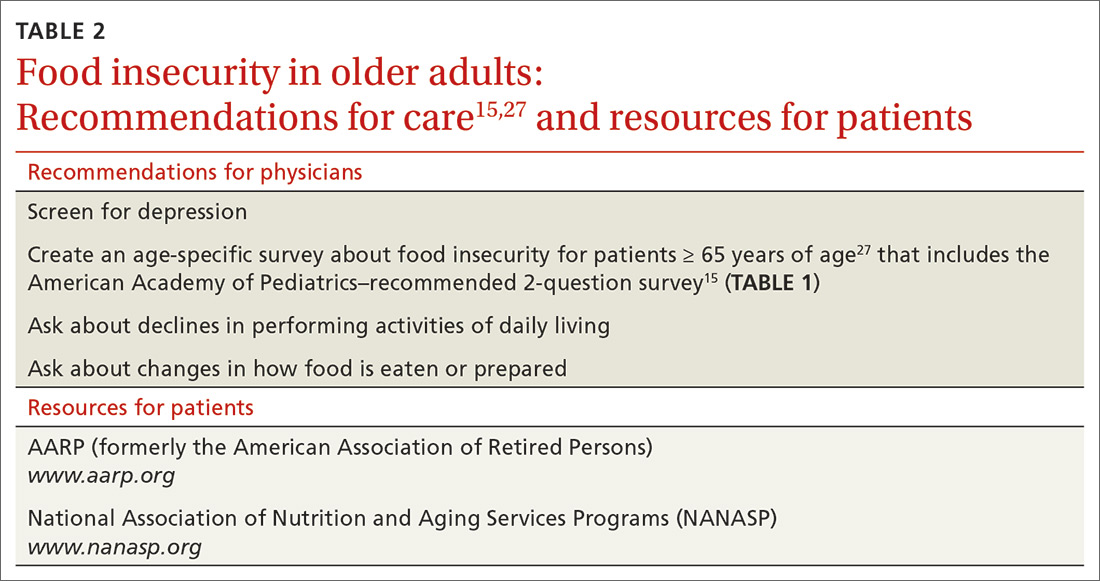
What you can do. To improve the nutritional intake of older adults, reconcile the patient’s medications at each visit to ensure that polypharmacy does not play a role in causing or exacerbating underlying conditions that can lead to poor nutritional intake. AARP (formerly the American Association of Retired Persons) recommends devising and conducting a survey of food insecurity with older adults that includes the 2-question American Academy of Pediatrics survey described earlier27 (TABLE 215,27).Such a survey, which can be administered by office staff, should also include a screen for depression, financial stability, ability to perform activities of daily living (eg, shopping and driving), and changes in diet that are a result of periodontal disease. The survey should also inquire about the effects of current or chronic disability on day-to-day life.
For all patients: Refer to community resources
The problems of food insecurity presented here only broadly address what each of these 3 groups face. Although the overall trend in food insecurity has been downward since 2011, deeper issues of food insecurity need to be studied more within each population. This is particularly true among the geriatric population, whose numbers are increasing, and among ethnic minorities, including black non-Hispanics, and Hispanics, who face additional daily stressors because of implicit biases in society.
More study is needed to decrease the rate of food insecurity across all populations in the United States. In the interim, family physicians should take advantage of their role in the care of families, children, and older people to address the problem of food insecurity in their patient population by applying the interventions we’ve outlined, with an emphasis on referral to resources in the community.
CORRESPONDENCE
Lillian Amèzquita, BS, The Warren Alpert Medical School, Brown University, Box G-9999, 222 Richmond Street, Providence, RI; [email protected].
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, et al. Household Food Security in the United States in 2016, ERR-237. Washington, DC: US Department of Agriculture, Economic Research Service; September 2017. www.ers.usda.gov/webdocs/publications/84973/err-237.pdf?v=0. Accessed January 10, 2019.
2. Rose D. Economic determinants and dietary consequences of food insecurity in the United States. J Nutr. 1999;129:517S-520S.
3. Gundersen C. Dynamic determinants of food insecurity. In: Andrews MS, Prell MA, eds. Second Food Security Measurement and Research Conference, Volume II: Papers. [Food Assistance and Nutrition Research Report 11-2.] Washington, DC: US Department of Agriculture, Economic Research Service; August 24, 2001:92-110.
4. Kaiser LL, Townsend MS. Food insecurity among US children: implications for nutrition and health. Top Clin Nutr. 2005;20:313-320.
5. Nguyen BT, Shuval K, Bertmann F, et al. The Supplemental Nutrition Assistance Program, food insecurity, dietary quality, and obesity among US adults. Am J Public Health. 2015;105:1453-1459.
6. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140:304-310.
7. Laraia BA. Food insecurity and chronic disease. Adv Nutr. 2013;4:203-212.
8. Nackers LM, Appelhans BM. Food insecurity is linked to a food environment promoting obesity in households with children. J Nutr Educ Behav. 2013;45:780-784.
9. Ralston K, Treen K, Coleman-Jensen A, et al. Children’s food security and USDA child nutrition programs. Economic Information Bulletin 174. US Department of Agriculture, Economic Research Service. June 2017. www.ers.usda.gov/webdocs/publications/84003/eib-174.pdf?v=0. Accessed January 10, 2020.
10. US Department of Agriculture, Food and Nutrition Service. National School Lunch Program: community eligibility provision. April 19, 2019. www.fns.usda.gov/school-meals/community-eligibility-provision. Accessed January 10, 2020.
11. Kreider B, Pepper JV, Roy M. Identifying the effects of WIC on food insecurity among infants and children. South Econ J. 2016;82:1106-1122.
12. Garg A, Toy S, Tripodis Y, et al. Influence of maternal depression on household food insecurity for low-income families. Acad Pediatr. 2015;15:305-310.
13. Noonan K, Corman H, Reichman NE. Effects of maternal depression on family food insecurity. Econ Hum Biol. 2016;22:201-215.
14. Hager ER, Quigg AM, Black MM, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126:e26-e32.
15. American Academy of Pediatrics Council on Community Pediatrics and Committee on Nutrition. Promoting food security for all children. Pediatrics. 2015;136:e1431-e1438.
16. Hamelin AM, Habicht JP, Beaudry M. Food insecurity: consequences for the household and broader social implications. J Nutr. 1999;129:525S-528S.
17. Gundersen C, Engelhard E, Hake M. The determinants of food insecurity among food bank clients in the United States. J Consum Aff. 2017;51:501-518.
18. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363:6-9.
19. Venci BJ, Lee S-Y. Functional limitation and chronic diseases are associated with food insecurity among U.S. adults. Ann Epidemiol. 2018;28:182-188.
20. Goldberg S, Mawn B. Predictors of food insecurity among older adults in the United States. Public Health Nurs. 2015;32:397-407.
21. Lee JS, Frongillo EA. Factors associated with food insecurity among U.S. elderly persons: importance of functional impairments. J Gerontol. 2001;56B:S94-S99.
22. Chang Y, Hickman H. Food insecurity and perceived diet quality among low-income older Americans with functional limitations. J Nutr Educ Behav. 2018;50:476-484.
23. Kamp B, Wellman N, Russell C. Position of the American Dietetic Association, American Society for Nutrition, and Society for Nutrition Education: Food and nutrition programs for community-residing older adults. J Nutr Educ Behav. 2010;42:72-82.
24. Cody S, Ohls JC. Evaluation of the US Department of Agriculture Elderly Nutrition Demonstration: Volume I, Evaluation Findings. Contractor and Cooperator Report No. 9-1. Washington, DC: US Department of Agriculture; July 2005.
25. US Department of Agriculture, Food and Nutrition Service; Office of Analysis, Nutrition, and Evaluation. Food stamp participation rates and benefits: an analysis of variation within demographic groups. May 2003. https://fns-prod.azureedge.net/sites/default/files/PartDemoGroup.pdf. Accessed January 10, 2020.
26. Russell JC, Flood VM, Yeatman H, et al. Food insecurity and poor diet quality are associated with reduced quality of life in older adults. Nutr Diet. 2016;73:50-58.
27. Pooler J, Levin M, Hoffman V, et al; AARP Foundation and IMPAQ International. Implementing food security screening and referral for older patients in primary care: a resource guide and toolkit. November 2016. www.aarp.org/content/dam/aarp/aarp_foundation/2016-pdfs/FoodSecurityScreening.pdf. Accessed January 10, 2020.
CASE Alice D is 20 years old and has type 1 diabetes, as well as retinopathy, hypertension, bipolar I disorder, and hyperlipidemia. She is a new patient at your clinic and reports that she is “occasionally homeless” and has difficulty affording food.
You renew Ms. D’s prescriptions during the visit, discuss nutrition with her, and

According to a 2016 report from the US Department of Agriculture (USDA) Economic Research Service, an estimated 12.3% of households in the United States are “food insecure.”1 To ascertain what food security and insecurity are, the USDA measured numerous variables, including household structure, race and ethnicity, geography, and income. The report of the Economic Research Service stands as one of the largest domestic sources of information about food insecurity.
Food insecurity is defined as “food intake of household members [that is] reduced and normal eating patterns that are disrupted.”1 It is often measured “per household,” but those data must be interpreted carefully because food insecurity affects household members differently. Some members, such as children, might be affected only mildly; adults, on the other hand, might be more severely affected. (Adults may, for instance, disrupt or skip their meals to maintain normal diets and meal patterns for their children.) In some households, food insecurity affects only a single member—such as an older adult—because of conditions unique to the people living in the home.
In this article, we review variables that can give rise to food insecurity in children, adults, and the elderly, and offer strategies to the family physician for identifying and alleviating the burden of food insecurity in these populations.
Food insecurity threatens children’s health, development
In 2016, households with children faced a higher level of food insecurity (16.5%) than the national average (12.3%).1 In a study of more than 280,000 households, food insecurity was sometimes so severe that children skipped meals or did not eat for the whole day.1 Although income strongly correlates with food insecurity, evidence shows that families above and below the poverty line suffer from food insecurity.2,3
According to the USDA, the rate of food insecurity is higher than the national average of 12.3% in several subgroups of children1:
- households with children < 6 years of age (16.6%)
- households with children headed by a single woman (31.6%) or single man (21.7%)
- households headed by a black non-Hispanic (22.5%) or Hispanic (18.5%) person
- low-income households in which the annual income is < 185% of the poverty threshold (31.6%).
Continue to: Evidence suggests that...
Evidence suggests that children in food-insecure homes experience poor diet, impaired cognitive development, an increased risk of chronic illness in adulthood, and emotional and behavioral problems.4-7 For caregivers in food-insecure homes, purchase price is the most influential factor when making food purchasing decisions. Thus, caregivers often purchase cheaper, more calorie-dense foods, rather than more expensive, nutrient-rich foods—leading to childhood obesity.8
Relief eludes many. Federal programs, such as the National School Lunch Program, School Breakfast Program, the Summer Food Service Program, and the Child and Adult Care Food Program, provide free or reduced-price meals for school-age children. Although these programs reduce food insecurity in households that participate, program policy has established that participation is based on household income.9 This is problematic: According to the literature,2 the income of 50% of households that are food-insecure is above the federal poverty level.
It would be more effective to have these programs target families based on geography, not income, because programs would then benefit those who are food-insecure but who live above the poverty line. Location is a significant factor in identifying food-insecure populations: Households outside metropolitan areas are disproportionately affected.1 If these programs were to privilege geography over income, they would include (for example): families in school districts with a low number of grocery stores; families with poor access to public transportation; and families that live in a “food desert”—ie, where fresh, low-cost food options are overshadowed by fast food.
One such program closely applied the model of privilege based on geography: In 2010, the Healthy, Hunger-Free Kids Act was passed, with a Community Eligibility Provision (CEP) that funded school districts in which ≥ 40% of students lived below the poverty line, so that students in those districts received a free school lunch.10 Although eligibility for CEP is still based on income, benefits go to all students who live in the district, including food-insecure students who live in a household above the poverty line. If eligibility criteria were expanded with CEP so that more school districts could participate, it might solve many obstacles faced by other existing programs.
Programs that provide nutrition for households with an infant or young child—eg, the Women, Infants and Children Special Supplemental Nutrition Program (WIC) and the Supplemental Nutrition Assistance Program (SNAP)—reduce food insecurity in households by 20%. However, several unstudied factors can affect food insecurity in families beyond these programs11; some assumptions about food insecurity, for example, strongly point to the influence of maternal mental health.12
Continue to: Data from the...
Data from the Early Childhood Longitudinal Study, Birth Cohort showed that mothers (with an infant) who were suffering from depression had a significantly increased risk of food insecurity.13 To better identify infants at risk of food insecurity, it would be beneficial to identify women suffering from depression during pregnancy or postpartum.13 These patients could then be referred to WIC and for SNAP benefits.
What you can do. The American Academy of Pediatrics recommends that physicians identify families that are food-insecure by conducting a validated14 2-question survey about food insecurity at every health-maintenance visit, as long as the child is a patient in the practice (TABLE 1).15 Physicians can then refer families that screen positively to local WIC and SNAP centers.

Ideally, physicians should be prepared to facilitate more active engagement by providing patients with the contact information of staff members working in such local programs. Staffing the practice with a patient-care manager can be an efficient way to navigate this process.
CASE Over the 6 weeks following Ms. D’s visit with you, she is admitted 5 times to the hospital in diabetic ketoacidosis, always with a significantly elevated blood glucose level. At each admission, she admits to “sometimes forgetting” to take insulin. Hospital staff members do not ask about her food intake. During each hospitalization, Ms. D is treated with insulin and intravenous fluids and discharged to home on her prior insulin regimen.
During a follow-up appointment with you and the clinic’s nurse–care manager, she talks about missing doses of insulin. She tells you that she has been getting food from the local food pantry, where available stocks are typically carbohydrate-based, including bread, rice, and cereal. She admits that she cannot afford other kinds of food—specifically, those that contain protein and monosaturated and polyunsaturated fats.
Continue to: Adults...
Adults: Poor financial health correlates with insecurity
The correlation between food insecurity and income is strong—evidenced by the spike in the number of adults who reported food insecurity during the 2008-2011 recession in the United States, to a high of 14.9%.1 As noted, households with children are more likely to report food insecurity. In addition, studies show that limited resources, race and ethnicity, underemployment or unemployment, and high housing costs are also strongly associated with food insecurity.16 Even subtle economic fluctuations—for example, an increase in the price of gasoline, natural gas, or electricity—contribute to food insecurity.17 Debt and coping mechanisms influence whether a household living below the poverty line is food-secure or food-insecure. Additional factors contributing to food insecurity include participation in SNAP, education, and severe depression.
Food insecurity in adults reduces the quality of food and nutritional intake, and is associated with chronic morbidity, such as type 2 diabetes, hypertension, and obesity.5-7 Adults in food-insecure homes are more likely to purchase cheap, calorie-dense, nutritionally poor foods (or refrain from purchasing food altogether, to pay other debts).17,18 The literature further suggests that food insecurity is associated with diseases that limit function and lead to disability, such as arthritis, stroke, and coronary artery disease, in adults and older adults (> 65 years of age; see the next section).5,6,19 These studies are weak, however, in their ability to show directionality: Does food insecurity cause disability or does disability cause food insecurity?
Patchwork of programs. Programs such as WIC are available for women who are pregnant or have children < 5 years of age. Federal programs for adults who do not have children are scarce, however, and the burden of food insecurity for this population is typically addressed by local programs, such as food banks and food kitchens. Evidence shows that (1) combining the efforts of federal and local food programs is the most effective method of stymieing food insecurity in adults and (2) it would benefit food-insecure adults to have access to such programs. Regrettably, many food programs are underutilized because of barriers that include poor outreach, ineffectual application, and ineligibility.
What you can do. Although it might not be an official, professional society guideline to include questions about food security in a patient wellness survey, physicians should consider creating one for their practice that they (or the office staff) can administer. Furthermore, physicians (or, again, the office staff) should familiarize themselves with programs in the community, such as SNAP or a food bank, to which they can refer patients, as needed.
CASE You ask the nurse–care manager to consult with staff of the food bank and request that, based on your evaluation and recommendation, Ms. D be given more protein-based foods, including peanut butter and beans, when she visits the food bank. The nurse–care manager also makes arrangements to procure an insulin pump for Ms. D.
Continue to: In a short time...
In a short time, Ms. D’s blood glucose level normalizes. She has no further admissions for diabetic ketoacidosis.
Older adults: Interplay of risk factors takes a toll
The USDA Economic Research Report on food insecurity1 states that older adults (≥ 65 years of age) report a lower rate of food insecurity—ie, 7.8% of households with an older adult and 8.9% of households in which the older adult lives alone—compared with the national average.1 The report is limited, however, in its ability to extrapolate data from older adults on food insecurity because its focus is on factors specific to adults and children.
Factors that contribute to food insecurity in the elderly include race and ethnicity, education, income, being a SNAP recipient, and severe depression.1,2,17,20,21 Older adult subgroups more likely to be food-insecure are Hispanic and black non-Hispanic—both significantly associated with being food-insecure, with Hispanic populations reporting the highest rates of food insecurity.20,21 This is a particularly interesting observation: Many traditional Hispanic homes are multigenerational and maintain a culture in which older adults are cared for by their children; that value system might be an indication of why many Hispanic households are disproportionately affected by food insecurity.
Other problems directly caused or exacerbated by food insecurity in the older population include a higher risk of malnutrition from periodontal disease, more frequent hospital admissions with longer length of stay, and an increased rate of falls and fractures. Polypharmacy, which can cause food–drug interactions that inhibit uptake of vitamins or create a higher demand for certain vitamins, is a noteworthy problem associated with food insecurity.
Problems with functionality might prevent older adults from performing physical tasks, such as shopping and preparing foods.21,22 Older adults who reported functional impairment in performing activities of daily living are more likely to report food insecurity.21,22 Last,older adults who live alone are more likely to have diminished nutritional intake than those who live with a spouse or partner.2,22,23
Continue to: Legislation enacted in 2010...
Legislation enacted in 2010 under the existing Older Americans Act provided home-delivered meals, nutritional screening, and education counseling to Americans > 60 years of age. That provision was not based on an income test, however, and served only 18% of the older population.23 (Other programs, such as SNAP, are utilized to a greater degree: 30% of eligible older adults participate, 75% of whom live alone.23) Possible reasons for underutilization include restricted funding, lower education level, lack of outreach, a confusing application process, and the impression that the process is intrusive.24-26
What you can do. To improve the nutritional intake of older adults, reconcile the patient’s medications at each visit to ensure that polypharmacy does not play a role in causing or exacerbating underlying conditions that can lead to poor nutritional intake. AARP (formerly the American Association of Retired Persons) recommends devising and conducting a survey of food insecurity with older adults that includes the 2-question American Academy of Pediatrics survey described earlier27 (TABLE 215,27).Such a survey, which can be administered by office staff, should also include a screen for depression, financial stability, ability to perform activities of daily living (eg, shopping and driving), and changes in diet that are a result of periodontal disease. The survey should also inquire about the effects of current or chronic disability on day-to-day life.

For all patients: Refer to community resources
The problems of food insecurity presented here only broadly address what each of these 3 groups face. Although the overall trend in food insecurity has been downward since 2011, deeper issues of food insecurity need to be studied more within each population. This is particularly true among the geriatric population, whose numbers are increasing, and among ethnic minorities, including black non-Hispanics, and Hispanics, who face additional daily stressors because of implicit biases in society.
More study is needed to decrease the rate of food insecurity across all populations in the United States. In the interim, family physicians should take advantage of their role in the care of families, children, and older people to address the problem of food insecurity in their patient population by applying the interventions we’ve outlined, with an emphasis on referral to resources in the community.
CORRESPONDENCE
Lillian Amèzquita, BS, The Warren Alpert Medical School, Brown University, Box G-9999, 222 Richmond Street, Providence, RI; [email protected].
CASE Alice D is 20 years old and has type 1 diabetes, as well as retinopathy, hypertension, bipolar I disorder, and hyperlipidemia. She is a new patient at your clinic and reports that she is “occasionally homeless” and has difficulty affording food.
You renew Ms. D’s prescriptions during the visit, discuss nutrition with her, and

According to a 2016 report from the US Department of Agriculture (USDA) Economic Research Service, an estimated 12.3% of households in the United States are “food insecure.”1 To ascertain what food security and insecurity are, the USDA measured numerous variables, including household structure, race and ethnicity, geography, and income. The report of the Economic Research Service stands as one of the largest domestic sources of information about food insecurity.
Food insecurity is defined as “food intake of household members [that is] reduced and normal eating patterns that are disrupted.”1 It is often measured “per household,” but those data must be interpreted carefully because food insecurity affects household members differently. Some members, such as children, might be affected only mildly; adults, on the other hand, might be more severely affected. (Adults may, for instance, disrupt or skip their meals to maintain normal diets and meal patterns for their children.) In some households, food insecurity affects only a single member—such as an older adult—because of conditions unique to the people living in the home.
In this article, we review variables that can give rise to food insecurity in children, adults, and the elderly, and offer strategies to the family physician for identifying and alleviating the burden of food insecurity in these populations.
Food insecurity threatens children’s health, development
In 2016, households with children faced a higher level of food insecurity (16.5%) than the national average (12.3%).1 In a study of more than 280,000 households, food insecurity was sometimes so severe that children skipped meals or did not eat for the whole day.1 Although income strongly correlates with food insecurity, evidence shows that families above and below the poverty line suffer from food insecurity.2,3
According to the USDA, the rate of food insecurity is higher than the national average of 12.3% in several subgroups of children1:
- households with children < 6 years of age (16.6%)
- households with children headed by a single woman (31.6%) or single man (21.7%)
- households headed by a black non-Hispanic (22.5%) or Hispanic (18.5%) person
- low-income households in which the annual income is < 185% of the poverty threshold (31.6%).
Continue to: Evidence suggests that...
Evidence suggests that children in food-insecure homes experience poor diet, impaired cognitive development, an increased risk of chronic illness in adulthood, and emotional and behavioral problems.4-7 For caregivers in food-insecure homes, purchase price is the most influential factor when making food purchasing decisions. Thus, caregivers often purchase cheaper, more calorie-dense foods, rather than more expensive, nutrient-rich foods—leading to childhood obesity.8
Relief eludes many. Federal programs, such as the National School Lunch Program, School Breakfast Program, the Summer Food Service Program, and the Child and Adult Care Food Program, provide free or reduced-price meals for school-age children. Although these programs reduce food insecurity in households that participate, program policy has established that participation is based on household income.9 This is problematic: According to the literature,2 the income of 50% of households that are food-insecure is above the federal poverty level.
It would be more effective to have these programs target families based on geography, not income, because programs would then benefit those who are food-insecure but who live above the poverty line. Location is a significant factor in identifying food-insecure populations: Households outside metropolitan areas are disproportionately affected.1 If these programs were to privilege geography over income, they would include (for example): families in school districts with a low number of grocery stores; families with poor access to public transportation; and families that live in a “food desert”—ie, where fresh, low-cost food options are overshadowed by fast food.
One such program closely applied the model of privilege based on geography: In 2010, the Healthy, Hunger-Free Kids Act was passed, with a Community Eligibility Provision (CEP) that funded school districts in which ≥ 40% of students lived below the poverty line, so that students in those districts received a free school lunch.10 Although eligibility for CEP is still based on income, benefits go to all students who live in the district, including food-insecure students who live in a household above the poverty line. If eligibility criteria were expanded with CEP so that more school districts could participate, it might solve many obstacles faced by other existing programs.
Programs that provide nutrition for households with an infant or young child—eg, the Women, Infants and Children Special Supplemental Nutrition Program (WIC) and the Supplemental Nutrition Assistance Program (SNAP)—reduce food insecurity in households by 20%. However, several unstudied factors can affect food insecurity in families beyond these programs11; some assumptions about food insecurity, for example, strongly point to the influence of maternal mental health.12
Continue to: Data from the...
Data from the Early Childhood Longitudinal Study, Birth Cohort showed that mothers (with an infant) who were suffering from depression had a significantly increased risk of food insecurity.13 To better identify infants at risk of food insecurity, it would be beneficial to identify women suffering from depression during pregnancy or postpartum.13 These patients could then be referred to WIC and for SNAP benefits.
What you can do. The American Academy of Pediatrics recommends that physicians identify families that are food-insecure by conducting a validated14 2-question survey about food insecurity at every health-maintenance visit, as long as the child is a patient in the practice (TABLE 1).15 Physicians can then refer families that screen positively to local WIC and SNAP centers.

Ideally, physicians should be prepared to facilitate more active engagement by providing patients with the contact information of staff members working in such local programs. Staffing the practice with a patient-care manager can be an efficient way to navigate this process.
CASE Over the 6 weeks following Ms. D’s visit with you, she is admitted 5 times to the hospital in diabetic ketoacidosis, always with a significantly elevated blood glucose level. At each admission, she admits to “sometimes forgetting” to take insulin. Hospital staff members do not ask about her food intake. During each hospitalization, Ms. D is treated with insulin and intravenous fluids and discharged to home on her prior insulin regimen.
During a follow-up appointment with you and the clinic’s nurse–care manager, she talks about missing doses of insulin. She tells you that she has been getting food from the local food pantry, where available stocks are typically carbohydrate-based, including bread, rice, and cereal. She admits that she cannot afford other kinds of food—specifically, those that contain protein and monosaturated and polyunsaturated fats.
Continue to: Adults...
Adults: Poor financial health correlates with insecurity
The correlation between food insecurity and income is strong—evidenced by the spike in the number of adults who reported food insecurity during the 2008-2011 recession in the United States, to a high of 14.9%.1 As noted, households with children are more likely to report food insecurity. In addition, studies show that limited resources, race and ethnicity, underemployment or unemployment, and high housing costs are also strongly associated with food insecurity.16 Even subtle economic fluctuations—for example, an increase in the price of gasoline, natural gas, or electricity—contribute to food insecurity.17 Debt and coping mechanisms influence whether a household living below the poverty line is food-secure or food-insecure. Additional factors contributing to food insecurity include participation in SNAP, education, and severe depression.
Food insecurity in adults reduces the quality of food and nutritional intake, and is associated with chronic morbidity, such as type 2 diabetes, hypertension, and obesity.5-7 Adults in food-insecure homes are more likely to purchase cheap, calorie-dense, nutritionally poor foods (or refrain from purchasing food altogether, to pay other debts).17,18 The literature further suggests that food insecurity is associated with diseases that limit function and lead to disability, such as arthritis, stroke, and coronary artery disease, in adults and older adults (> 65 years of age; see the next section).5,6,19 These studies are weak, however, in their ability to show directionality: Does food insecurity cause disability or does disability cause food insecurity?
Patchwork of programs. Programs such as WIC are available for women who are pregnant or have children < 5 years of age. Federal programs for adults who do not have children are scarce, however, and the burden of food insecurity for this population is typically addressed by local programs, such as food banks and food kitchens. Evidence shows that (1) combining the efforts of federal and local food programs is the most effective method of stymieing food insecurity in adults and (2) it would benefit food-insecure adults to have access to such programs. Regrettably, many food programs are underutilized because of barriers that include poor outreach, ineffectual application, and ineligibility.
What you can do. Although it might not be an official, professional society guideline to include questions about food security in a patient wellness survey, physicians should consider creating one for their practice that they (or the office staff) can administer. Furthermore, physicians (or, again, the office staff) should familiarize themselves with programs in the community, such as SNAP or a food bank, to which they can refer patients, as needed.
CASE You ask the nurse–care manager to consult with staff of the food bank and request that, based on your evaluation and recommendation, Ms. D be given more protein-based foods, including peanut butter and beans, when she visits the food bank. The nurse–care manager also makes arrangements to procure an insulin pump for Ms. D.
Continue to: In a short time...
In a short time, Ms. D’s blood glucose level normalizes. She has no further admissions for diabetic ketoacidosis.
Older adults: Interplay of risk factors takes a toll
The USDA Economic Research Report on food insecurity1 states that older adults (≥ 65 years of age) report a lower rate of food insecurity—ie, 7.8% of households with an older adult and 8.9% of households in which the older adult lives alone—compared with the national average.1 The report is limited, however, in its ability to extrapolate data from older adults on food insecurity because its focus is on factors specific to adults and children.
Factors that contribute to food insecurity in the elderly include race and ethnicity, education, income, being a SNAP recipient, and severe depression.1,2,17,20,21 Older adult subgroups more likely to be food-insecure are Hispanic and black non-Hispanic—both significantly associated with being food-insecure, with Hispanic populations reporting the highest rates of food insecurity.20,21 This is a particularly interesting observation: Many traditional Hispanic homes are multigenerational and maintain a culture in which older adults are cared for by their children; that value system might be an indication of why many Hispanic households are disproportionately affected by food insecurity.
Other problems directly caused or exacerbated by food insecurity in the older population include a higher risk of malnutrition from periodontal disease, more frequent hospital admissions with longer length of stay, and an increased rate of falls and fractures. Polypharmacy, which can cause food–drug interactions that inhibit uptake of vitamins or create a higher demand for certain vitamins, is a noteworthy problem associated with food insecurity.
Problems with functionality might prevent older adults from performing physical tasks, such as shopping and preparing foods.21,22 Older adults who reported functional impairment in performing activities of daily living are more likely to report food insecurity.21,22 Last,older adults who live alone are more likely to have diminished nutritional intake than those who live with a spouse or partner.2,22,23
Continue to: Legislation enacted in 2010...
Legislation enacted in 2010 under the existing Older Americans Act provided home-delivered meals, nutritional screening, and education counseling to Americans > 60 years of age. That provision was not based on an income test, however, and served only 18% of the older population.23 (Other programs, such as SNAP, are utilized to a greater degree: 30% of eligible older adults participate, 75% of whom live alone.23) Possible reasons for underutilization include restricted funding, lower education level, lack of outreach, a confusing application process, and the impression that the process is intrusive.24-26
What you can do. To improve the nutritional intake of older adults, reconcile the patient’s medications at each visit to ensure that polypharmacy does not play a role in causing or exacerbating underlying conditions that can lead to poor nutritional intake. AARP (formerly the American Association of Retired Persons) recommends devising and conducting a survey of food insecurity with older adults that includes the 2-question American Academy of Pediatrics survey described earlier27 (TABLE 215,27).Such a survey, which can be administered by office staff, should also include a screen for depression, financial stability, ability to perform activities of daily living (eg, shopping and driving), and changes in diet that are a result of periodontal disease. The survey should also inquire about the effects of current or chronic disability on day-to-day life.

For all patients: Refer to community resources
The problems of food insecurity presented here only broadly address what each of these 3 groups face. Although the overall trend in food insecurity has been downward since 2011, deeper issues of food insecurity need to be studied more within each population. This is particularly true among the geriatric population, whose numbers are increasing, and among ethnic minorities, including black non-Hispanics, and Hispanics, who face additional daily stressors because of implicit biases in society.
More study is needed to decrease the rate of food insecurity across all populations in the United States. In the interim, family physicians should take advantage of their role in the care of families, children, and older people to address the problem of food insecurity in their patient population by applying the interventions we’ve outlined, with an emphasis on referral to resources in the community.
CORRESPONDENCE
Lillian Amèzquita, BS, The Warren Alpert Medical School, Brown University, Box G-9999, 222 Richmond Street, Providence, RI; [email protected].
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, et al. Household Food Security in the United States in 2016, ERR-237. Washington, DC: US Department of Agriculture, Economic Research Service; September 2017. www.ers.usda.gov/webdocs/publications/84973/err-237.pdf?v=0. Accessed January 10, 2019.
2. Rose D. Economic determinants and dietary consequences of food insecurity in the United States. J Nutr. 1999;129:517S-520S.
3. Gundersen C. Dynamic determinants of food insecurity. In: Andrews MS, Prell MA, eds. Second Food Security Measurement and Research Conference, Volume II: Papers. [Food Assistance and Nutrition Research Report 11-2.] Washington, DC: US Department of Agriculture, Economic Research Service; August 24, 2001:92-110.
4. Kaiser LL, Townsend MS. Food insecurity among US children: implications for nutrition and health. Top Clin Nutr. 2005;20:313-320.
5. Nguyen BT, Shuval K, Bertmann F, et al. The Supplemental Nutrition Assistance Program, food insecurity, dietary quality, and obesity among US adults. Am J Public Health. 2015;105:1453-1459.
6. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140:304-310.
7. Laraia BA. Food insecurity and chronic disease. Adv Nutr. 2013;4:203-212.
8. Nackers LM, Appelhans BM. Food insecurity is linked to a food environment promoting obesity in households with children. J Nutr Educ Behav. 2013;45:780-784.
9. Ralston K, Treen K, Coleman-Jensen A, et al. Children’s food security and USDA child nutrition programs. Economic Information Bulletin 174. US Department of Agriculture, Economic Research Service. June 2017. www.ers.usda.gov/webdocs/publications/84003/eib-174.pdf?v=0. Accessed January 10, 2020.
10. US Department of Agriculture, Food and Nutrition Service. National School Lunch Program: community eligibility provision. April 19, 2019. www.fns.usda.gov/school-meals/community-eligibility-provision. Accessed January 10, 2020.
11. Kreider B, Pepper JV, Roy M. Identifying the effects of WIC on food insecurity among infants and children. South Econ J. 2016;82:1106-1122.
12. Garg A, Toy S, Tripodis Y, et al. Influence of maternal depression on household food insecurity for low-income families. Acad Pediatr. 2015;15:305-310.
13. Noonan K, Corman H, Reichman NE. Effects of maternal depression on family food insecurity. Econ Hum Biol. 2016;22:201-215.
14. Hager ER, Quigg AM, Black MM, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126:e26-e32.
15. American Academy of Pediatrics Council on Community Pediatrics and Committee on Nutrition. Promoting food security for all children. Pediatrics. 2015;136:e1431-e1438.
16. Hamelin AM, Habicht JP, Beaudry M. Food insecurity: consequences for the household and broader social implications. J Nutr. 1999;129:525S-528S.
17. Gundersen C, Engelhard E, Hake M. The determinants of food insecurity among food bank clients in the United States. J Consum Aff. 2017;51:501-518.
18. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363:6-9.
19. Venci BJ, Lee S-Y. Functional limitation and chronic diseases are associated with food insecurity among U.S. adults. Ann Epidemiol. 2018;28:182-188.
20. Goldberg S, Mawn B. Predictors of food insecurity among older adults in the United States. Public Health Nurs. 2015;32:397-407.
21. Lee JS, Frongillo EA. Factors associated with food insecurity among U.S. elderly persons: importance of functional impairments. J Gerontol. 2001;56B:S94-S99.
22. Chang Y, Hickman H. Food insecurity and perceived diet quality among low-income older Americans with functional limitations. J Nutr Educ Behav. 2018;50:476-484.
23. Kamp B, Wellman N, Russell C. Position of the American Dietetic Association, American Society for Nutrition, and Society for Nutrition Education: Food and nutrition programs for community-residing older adults. J Nutr Educ Behav. 2010;42:72-82.
24. Cody S, Ohls JC. Evaluation of the US Department of Agriculture Elderly Nutrition Demonstration: Volume I, Evaluation Findings. Contractor and Cooperator Report No. 9-1. Washington, DC: US Department of Agriculture; July 2005.
25. US Department of Agriculture, Food and Nutrition Service; Office of Analysis, Nutrition, and Evaluation. Food stamp participation rates and benefits: an analysis of variation within demographic groups. May 2003. https://fns-prod.azureedge.net/sites/default/files/PartDemoGroup.pdf. Accessed January 10, 2020.
26. Russell JC, Flood VM, Yeatman H, et al. Food insecurity and poor diet quality are associated with reduced quality of life in older adults. Nutr Diet. 2016;73:50-58.
27. Pooler J, Levin M, Hoffman V, et al; AARP Foundation and IMPAQ International. Implementing food security screening and referral for older patients in primary care: a resource guide and toolkit. November 2016. www.aarp.org/content/dam/aarp/aarp_foundation/2016-pdfs/FoodSecurityScreening.pdf. Accessed January 10, 2020.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, et al. Household Food Security in the United States in 2016, ERR-237. Washington, DC: US Department of Agriculture, Economic Research Service; September 2017. www.ers.usda.gov/webdocs/publications/84973/err-237.pdf?v=0. Accessed January 10, 2019.
2. Rose D. Economic determinants and dietary consequences of food insecurity in the United States. J Nutr. 1999;129:517S-520S.
3. Gundersen C. Dynamic determinants of food insecurity. In: Andrews MS, Prell MA, eds. Second Food Security Measurement and Research Conference, Volume II: Papers. [Food Assistance and Nutrition Research Report 11-2.] Washington, DC: US Department of Agriculture, Economic Research Service; August 24, 2001:92-110.
4. Kaiser LL, Townsend MS. Food insecurity among US children: implications for nutrition and health. Top Clin Nutr. 2005;20:313-320.
5. Nguyen BT, Shuval K, Bertmann F, et al. The Supplemental Nutrition Assistance Program, food insecurity, dietary quality, and obesity among US adults. Am J Public Health. 2015;105:1453-1459.
6. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140:304-310.
7. Laraia BA. Food insecurity and chronic disease. Adv Nutr. 2013;4:203-212.
8. Nackers LM, Appelhans BM. Food insecurity is linked to a food environment promoting obesity in households with children. J Nutr Educ Behav. 2013;45:780-784.
9. Ralston K, Treen K, Coleman-Jensen A, et al. Children’s food security and USDA child nutrition programs. Economic Information Bulletin 174. US Department of Agriculture, Economic Research Service. June 2017. www.ers.usda.gov/webdocs/publications/84003/eib-174.pdf?v=0. Accessed January 10, 2020.
10. US Department of Agriculture, Food and Nutrition Service. National School Lunch Program: community eligibility provision. April 19, 2019. www.fns.usda.gov/school-meals/community-eligibility-provision. Accessed January 10, 2020.
11. Kreider B, Pepper JV, Roy M. Identifying the effects of WIC on food insecurity among infants and children. South Econ J. 2016;82:1106-1122.
12. Garg A, Toy S, Tripodis Y, et al. Influence of maternal depression on household food insecurity for low-income families. Acad Pediatr. 2015;15:305-310.
13. Noonan K, Corman H, Reichman NE. Effects of maternal depression on family food insecurity. Econ Hum Biol. 2016;22:201-215.
14. Hager ER, Quigg AM, Black MM, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126:e26-e32.
15. American Academy of Pediatrics Council on Community Pediatrics and Committee on Nutrition. Promoting food security for all children. Pediatrics. 2015;136:e1431-e1438.
16. Hamelin AM, Habicht JP, Beaudry M. Food insecurity: consequences for the household and broader social implications. J Nutr. 1999;129:525S-528S.
17. Gundersen C, Engelhard E, Hake M. The determinants of food insecurity among food bank clients in the United States. J Consum Aff. 2017;51:501-518.
18. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363:6-9.
19. Venci BJ, Lee S-Y. Functional limitation and chronic diseases are associated with food insecurity among U.S. adults. Ann Epidemiol. 2018;28:182-188.
20. Goldberg S, Mawn B. Predictors of food insecurity among older adults in the United States. Public Health Nurs. 2015;32:397-407.
21. Lee JS, Frongillo EA. Factors associated with food insecurity among U.S. elderly persons: importance of functional impairments. J Gerontol. 2001;56B:S94-S99.
22. Chang Y, Hickman H. Food insecurity and perceived diet quality among low-income older Americans with functional limitations. J Nutr Educ Behav. 2018;50:476-484.
23. Kamp B, Wellman N, Russell C. Position of the American Dietetic Association, American Society for Nutrition, and Society for Nutrition Education: Food and nutrition programs for community-residing older adults. J Nutr Educ Behav. 2010;42:72-82.
24. Cody S, Ohls JC. Evaluation of the US Department of Agriculture Elderly Nutrition Demonstration: Volume I, Evaluation Findings. Contractor and Cooperator Report No. 9-1. Washington, DC: US Department of Agriculture; July 2005.
25. US Department of Agriculture, Food and Nutrition Service; Office of Analysis, Nutrition, and Evaluation. Food stamp participation rates and benefits: an analysis of variation within demographic groups. May 2003. https://fns-prod.azureedge.net/sites/default/files/PartDemoGroup.pdf. Accessed January 10, 2020.
26. Russell JC, Flood VM, Yeatman H, et al. Food insecurity and poor diet quality are associated with reduced quality of life in older adults. Nutr Diet. 2016;73:50-58.
27. Pooler J, Levin M, Hoffman V, et al; AARP Foundation and IMPAQ International. Implementing food security screening and referral for older patients in primary care: a resource guide and toolkit. November 2016. www.aarp.org/content/dam/aarp/aarp_foundation/2016-pdfs/FoodSecurityScreening.pdf. Accessed January 10, 2020.
PRACTICE RECOMMENDATIONS
› Consistently use the American Academy of Pediatrics 2-question survey to screen for food insecurity (all populations). A
› Identify and treat maternal depression during pregnancy and the postpartum period, and beyond A and screen for depression in older adults A because depression can reduce motivation to accomplish daily activities, such as obtaining and preparing food.
› Ask older adults about declines in performing activities of daily living C and how food is eaten or prepared . C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Evidence-based tools for premenstrual disorders
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
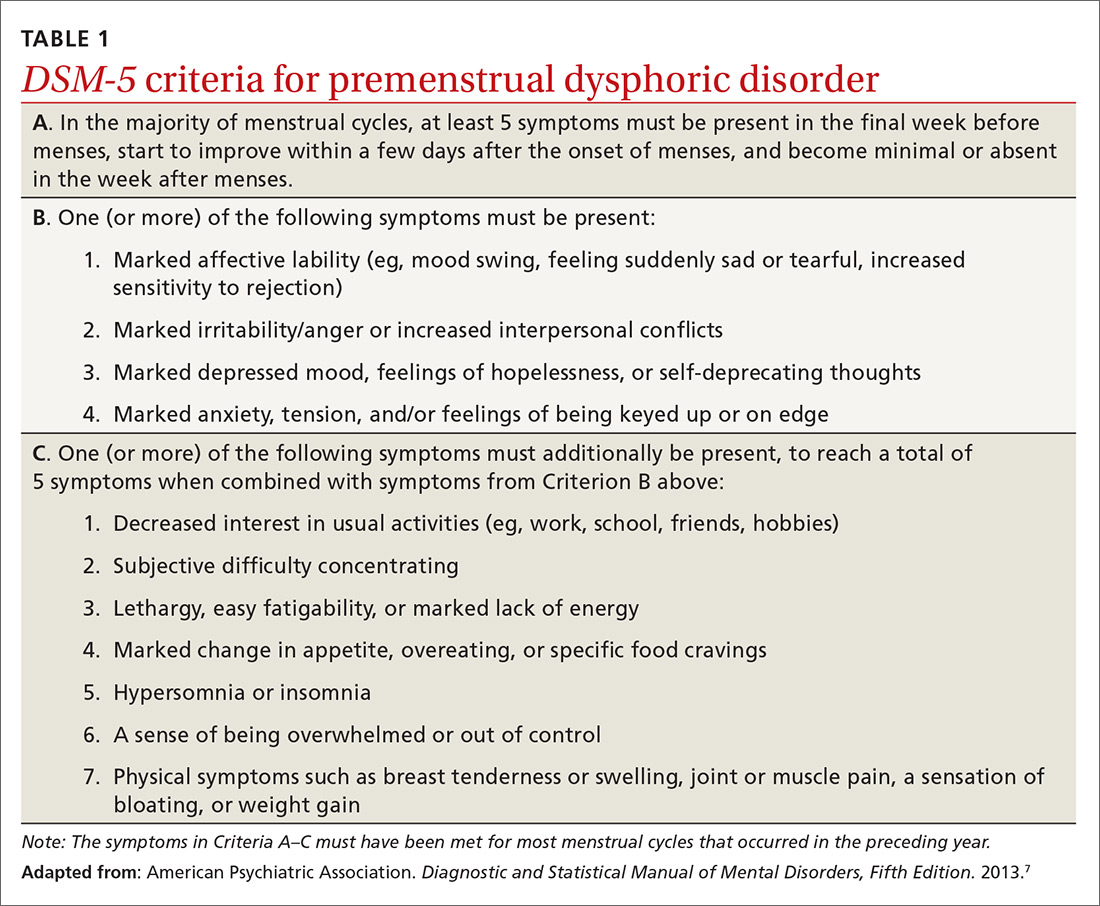
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7
Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
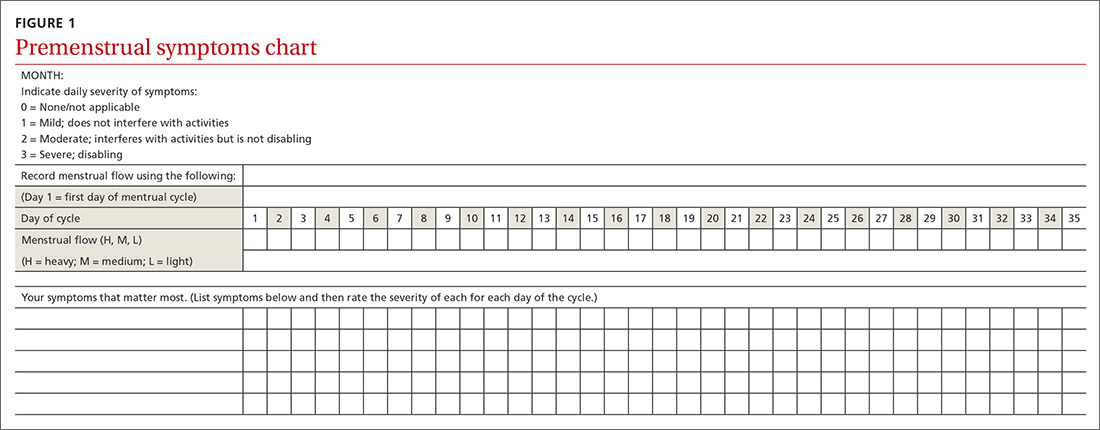
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.
Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
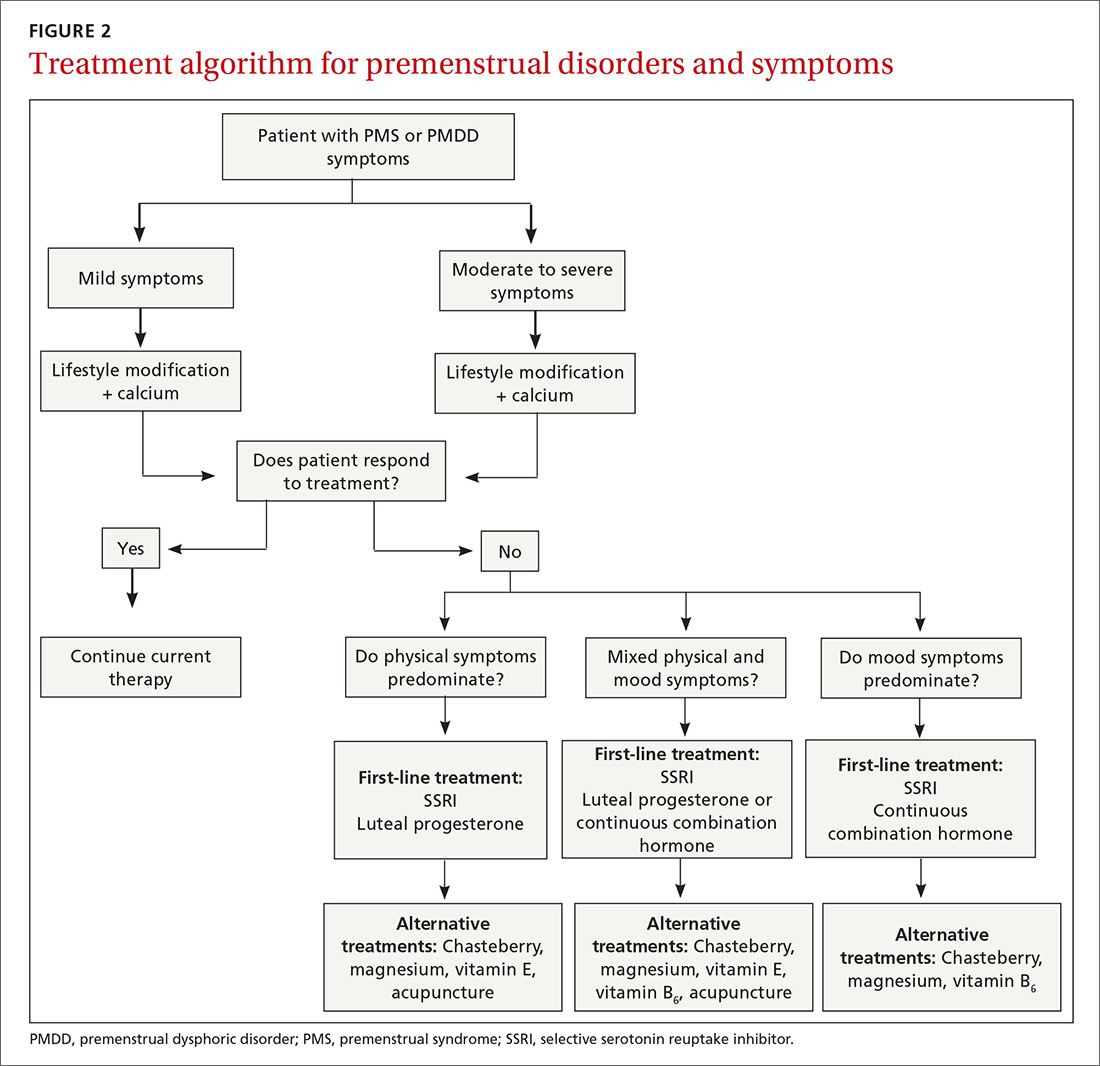
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness
Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25
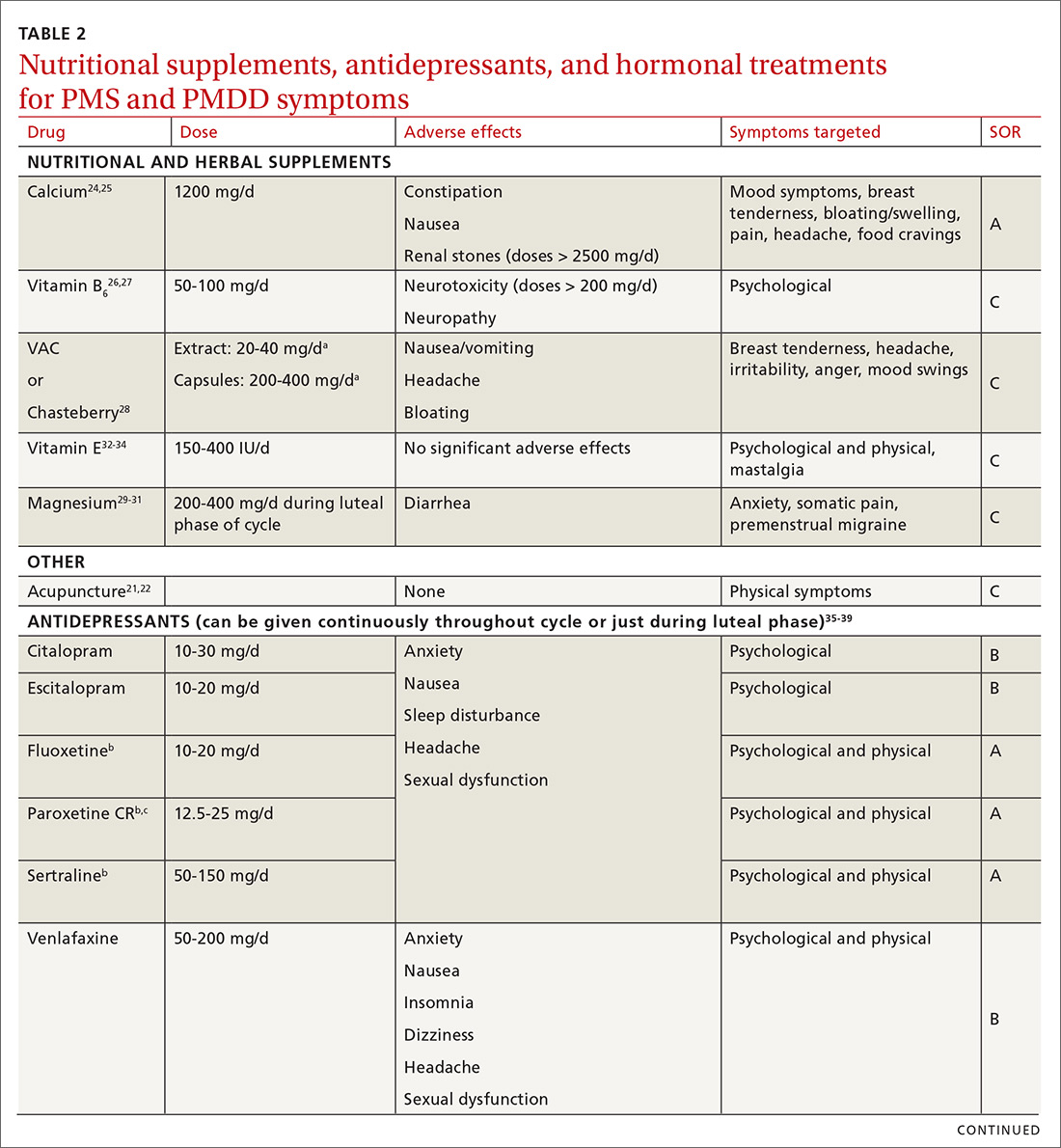
Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27
Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
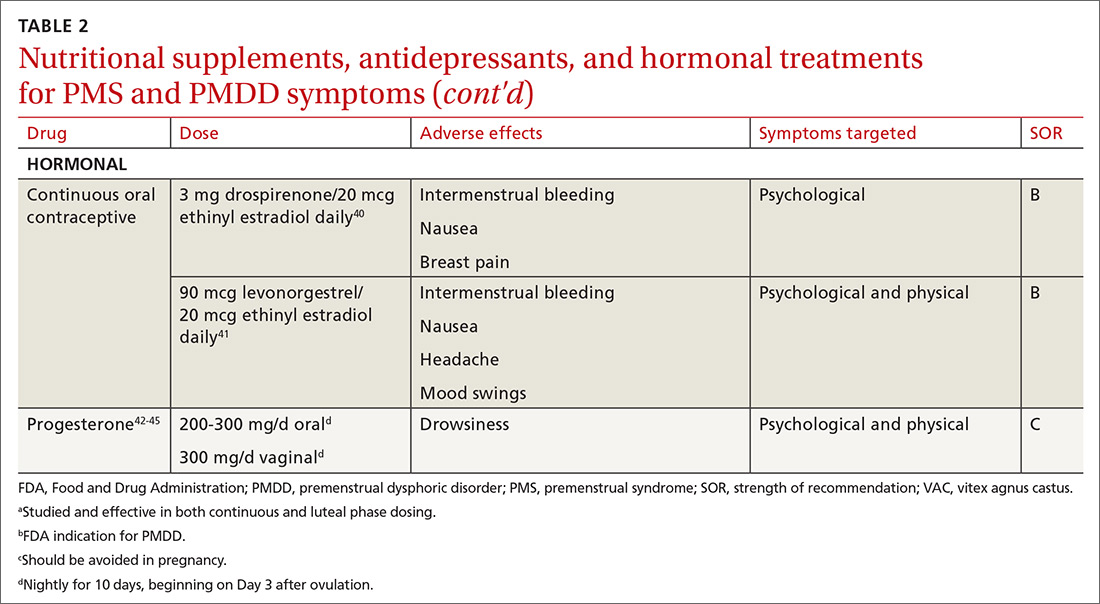
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; [email protected].
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7

Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.

Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness

Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25

Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27

Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; [email protected].
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7

Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.

Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness

Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25

Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27

Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; [email protected].
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
PRACTICE RECOMMENDATIONS
› Start calcium supplementation in all patients who report significant premenstrual symptoms. A
› Add a selective serotonin reuptake inhibitor (SSRI) to calcium supplementationfor patients who have more severe premenstrual psychological symptoms. A
› Consider hormonal treatment options for patients who require treatment beyond calcium and an SSRI. B
› Provide nutrition and exercise information to all patients who report significant premenstrual symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How best to approach these acute hand infections
Hand infections, if not treated properly, can cause severe chronic morbidity. The conditions I review here range from superficial to deep seated: herpetic whitlow located in the epidermis; felon in subcutaneous tissue; pyogenic flexor tenosynovitis (FTS) in the tendon sheath; and human bite infection at any level including possibly synovium and bone.
Superficial infections usually respond to nonsurgical management. However, antimicrobial therapy is not straightforward. There is no single regimen that covers all possible pathogens. Combination therapy must usually be started and then tailored once an organism and its susceptibility are known. Subcutaneous, tendon sheath, synovial, and bone infections frequently require surgical management.
Herpetic whitlow
Herpes simplex virus type 1 (HSV-1) is common, with as much as 90% of the population exposed by 60 years of age.1 Initial infection usually occurs in the oropharynx and is known as a fever blister or cold sore. However, HSV-1 also can cause herpetic whitlow, a primary infection in the fingertip in which the virus penetrates the subcutaneous tissue, usually after a breakdown in the skin barrier either from infected saliva or the lips of an infected individual.1
What you’ll see. The lesion is characterized by pain, swelling, erythema, and nonpurulent vesicle formation (FIGURE 1). The condition is usually self-limiting, with the inflammation resolving spontaneously, leaving normal healthy skin within 1 to 2 weeks. Herpetic whitlow is an occupational hazard for medical, nursing, paramedical, and dental personnel, and standard precautions should be used when handling secretions (Strength of Recommendation [SOR]: A).2
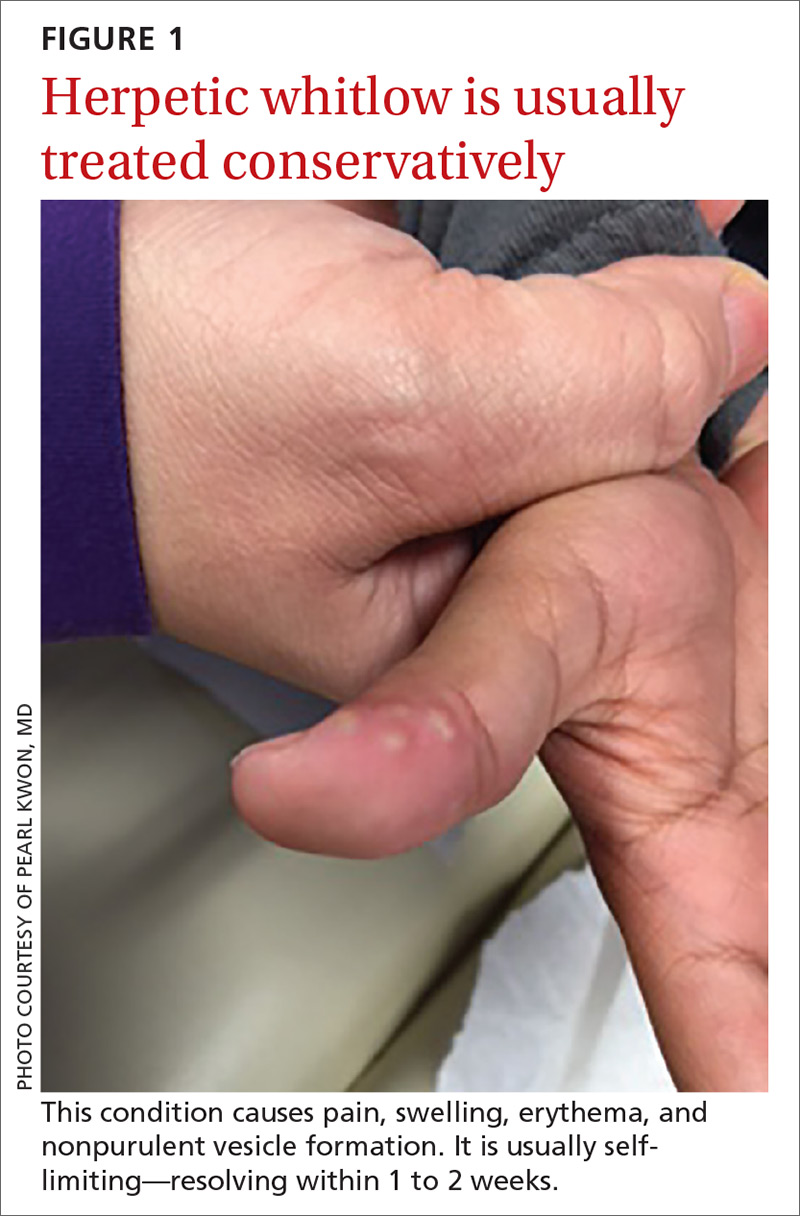
Reactivation of HSV-1 (and HSV-2) is common, with a prodrome of a “tingling sensation” and subsequent blister formation in the same location as the previous infection. It can cause pain and discomfort and may render the individual unable to perform usual activities.1,3 This lesion is often confused with bacterial (pyogenic) infections of the pulp of the finger or thumb (felon).1 Herpetic whitlow can be distinguished from a felon by its formation of vesicles, lack of a tense pulp space, and serous, rather than purulent, drainage. Scarring is not associated with herpes infection because penetration is limited to the epidermal area. Superimposed bacterial infection can be mistaken for an abscess (felon) and lead to unnecessary incision and drainage, causing associated morbidity and potential scarring in the affected area.1
How it’s treated. Treatment of herpetic whitlow is usually conservative, and topical application of acyclovir 5% appears to be beneficial (SOR: C).4 Two studies also suggest that oral acyclovir is beneficial for herpetic whitlow and may reduce the frequency of recurrence.5,6 Controlled studies in the use of acyclovir for herpetic whitlow have not been conducted. Despite a lack of direct evidence, acyclovir, famciclovir, and valacyclovir are accepted therapies for herpetic whitlow (TABLE 17) (SOR: B).6,8
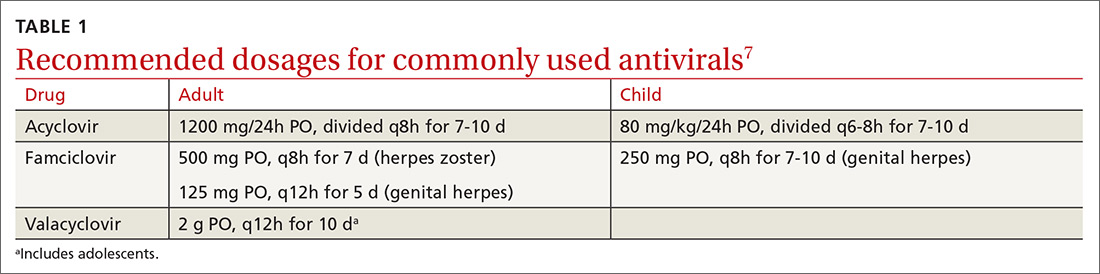
Felon
A felon is a closed-space infection affecting the pulp of the fingers or thumb. The anatomy of the finger is unique in that there are multiple septa attaching the periosteum to the skin, thereby creating several closed spaces prone to develop pockets of infection.
Continue to: What you'll see
What you’ll see. Clinical signs and symptoms include pain in the pulp of the finger with tenderness and swelling. Bacteria are usually introduced into fingertip space (fat pad) by a penetrating object. Some reported cases have involved individuals with diabetes who regularly check their blood sugar (FIGURE 2).9 A defining characteristic is that the infection usually does not extend past the interphalangeal joint. Radiologic evaluation may be necessary to detect the presence of foreign bodies or to assess bone involvement (osteomyelitis of the distal phalanx).10 The differential diagnosis includes paronychia, in which the infection starts in the nail area and pain is not as intense as in a felon infection.11
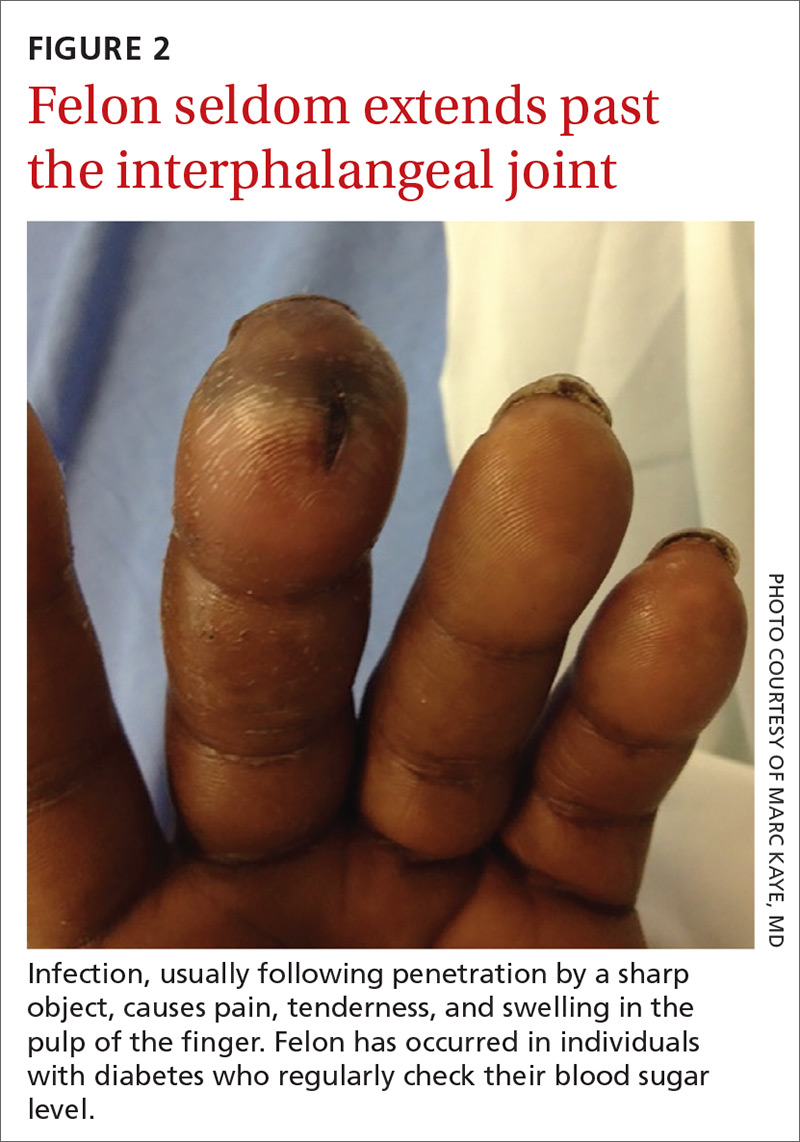
How it’s treated. Surgical treatment of felon is controversial. There is no doubt that pus should be drained; how the incision is best performed, however, has been debated.12 Before surgical debridement, obtain a sample of pus for Gram stain and for cultures of aerobic and anaerobic organisms, acid-fast bacilli (AFB), and fungi (SOR: A).13,14 Several surgical techniques and their pitfalls are described in the literature.
Lateral and tip incisions may help avoid painful scars. However, multiple reports of this procedure describe injury to neurovascular bundles, leading to ischemia and anesthesia.12 The “fish-mouth” incision and the “hockey stick” or “J” incision, as well as the transverse palmar incision, are no longer recommended due to painful sequelae, sensorial alterations, and risk of cutting the digital nerves.15 The preferred surgical procedure at this time is to make a very short incision over the area of maximum tenderness, then open and drain the abscess. Avoid placing packing in the affected area. Post-surgical management includes elevation, immobilization with an appropriate splint, and application of compresses until the wound has healed.12,15,16
Since Staphylococcus aureus and Streptococcus sp are the most common bacteria causing felon, start
Acute pyogenic flexor tenosynovitis
FTS is an aggressive closed-space bacterial infection that involves the flexor tendon synovial sheath. FTS accounts for up to 10% of acute hand infections and requires prompt medical attention with wound lavage, surgical management, and antimicrobial therapy to minimize serious consequences to the digit.18
Continue to: What you'll see
What you’ll see. FTS is diagnosed using clinical criteria19,20: fusiform swelling of the finger; exquisite tenderness over the entire course of the flexor tendon sheath; pain on passive extension; and flexed posture of the digit (FIGURE 3). Patients usually recall some type of trauma or puncture wound to the affected area, but hematogenous spread of Neisseria gonorrhoeae also has been reported.21 The most common bacterial pathogens are Staphylococcus sp or Streptococcus sp. However, obtain a sample for Gram stain and culture for aerobic, anaerobic, AFB, and fungal agents before irrigating the wound with copious fluids and initiating empirical antibiotic therapy.14 Once a pathogen has been isolated, tailor antimicrobial therapy based on identified sensitivities and local antibiogram.13

How it’s treated. Early treatment of FTS is of utmost importance to avoid adverse outcomes. If FTS is diagnosed early, manage conservatively with elevation of the hand, splinting in a neutral position, and intravenous (IV) antibiotics. The use of adjunct antibiotics has improved range-of-motion outcomes compared with elevation and splinting alone (54% excellent vs 14% excellent) (SOR: A).22
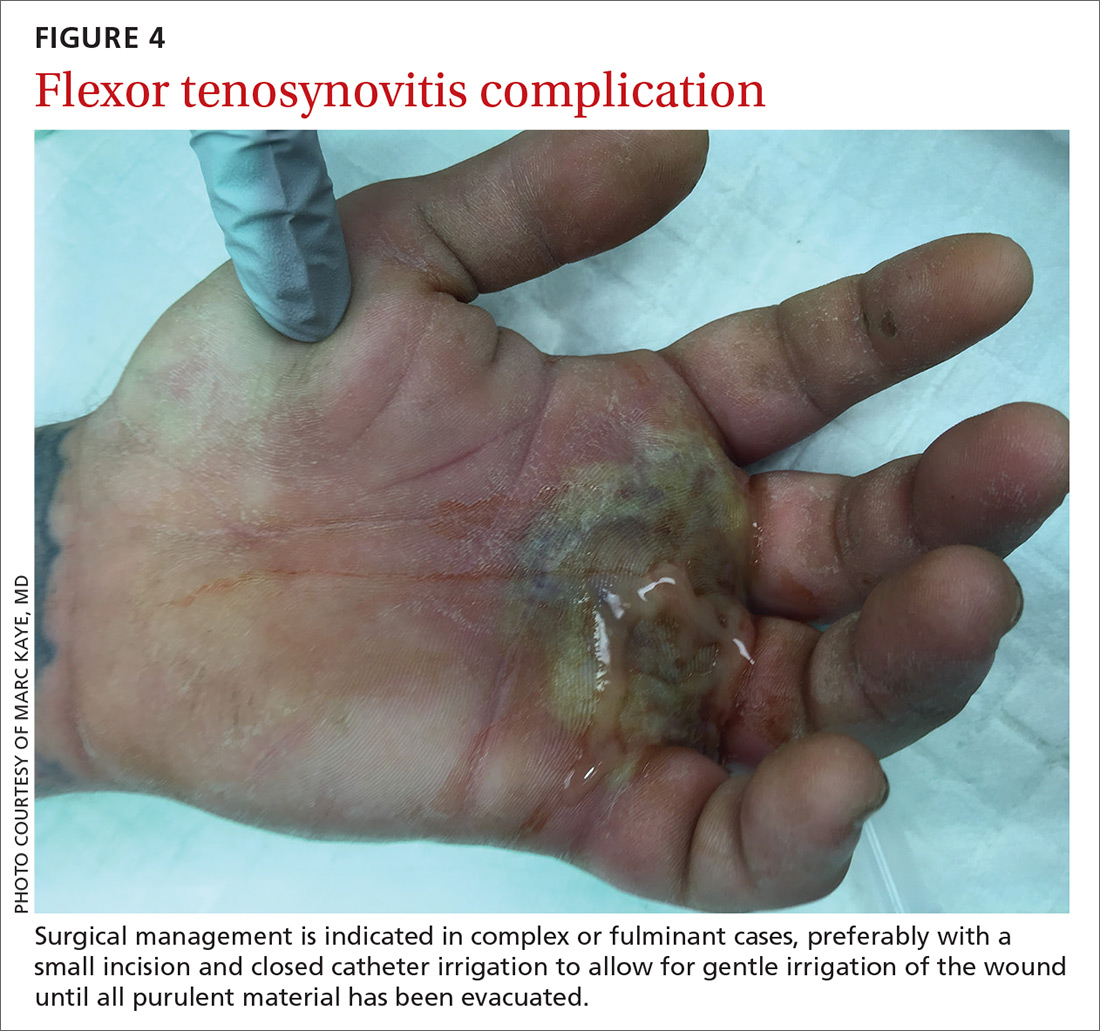
Surgical management of FTS has involved either an open or closed method. The open approach consists of open incision and drainage with exposure of the flexor tendon sheath, followed by large-volume sheath irrigation and closure of incision, in some cases over a drain. The closed approach with irrigation, rather than open washout, has been associated with improved outcomes (71% excellent vs 26% excellent).22 As a result, the procedure of choice is the closed approach, which uses closed catheter irrigation. An incision and placement of an angiocatheter allows for gentle irrigation of the wound until all purulent material has been evacuated (SOR: C).22,23
Human bite injuries
A human bite injury occurs in 1 of 2 ways, and each has a distinct pattern.
What you’ll see
Closed-fist injury occurs when a clenched fist strikes the teeth of another person. The resulting lesion can easily fool a clinician by appearing to show very little damage. If not appropriately evaluated and treated, the lesion can cause considerable morbidity (FIGURE 5). Injury to the extensor mechanism and joint capsule can also damage the articular cartilage and bone, allowing bacteria to grow in a closed environment. This usually affects the metacarpophalangeal joint (MCP) due to its prominence when a hand is clenched. Initially the lesion seems to be minor, with a small laceration of 3 to 5 mm on the overlying skin, thus inoculating mouth flora deep in the hand tissue. Once the hand is relaxed, the broken skin retracts proximally, covering the wound and making it look innocuous.24
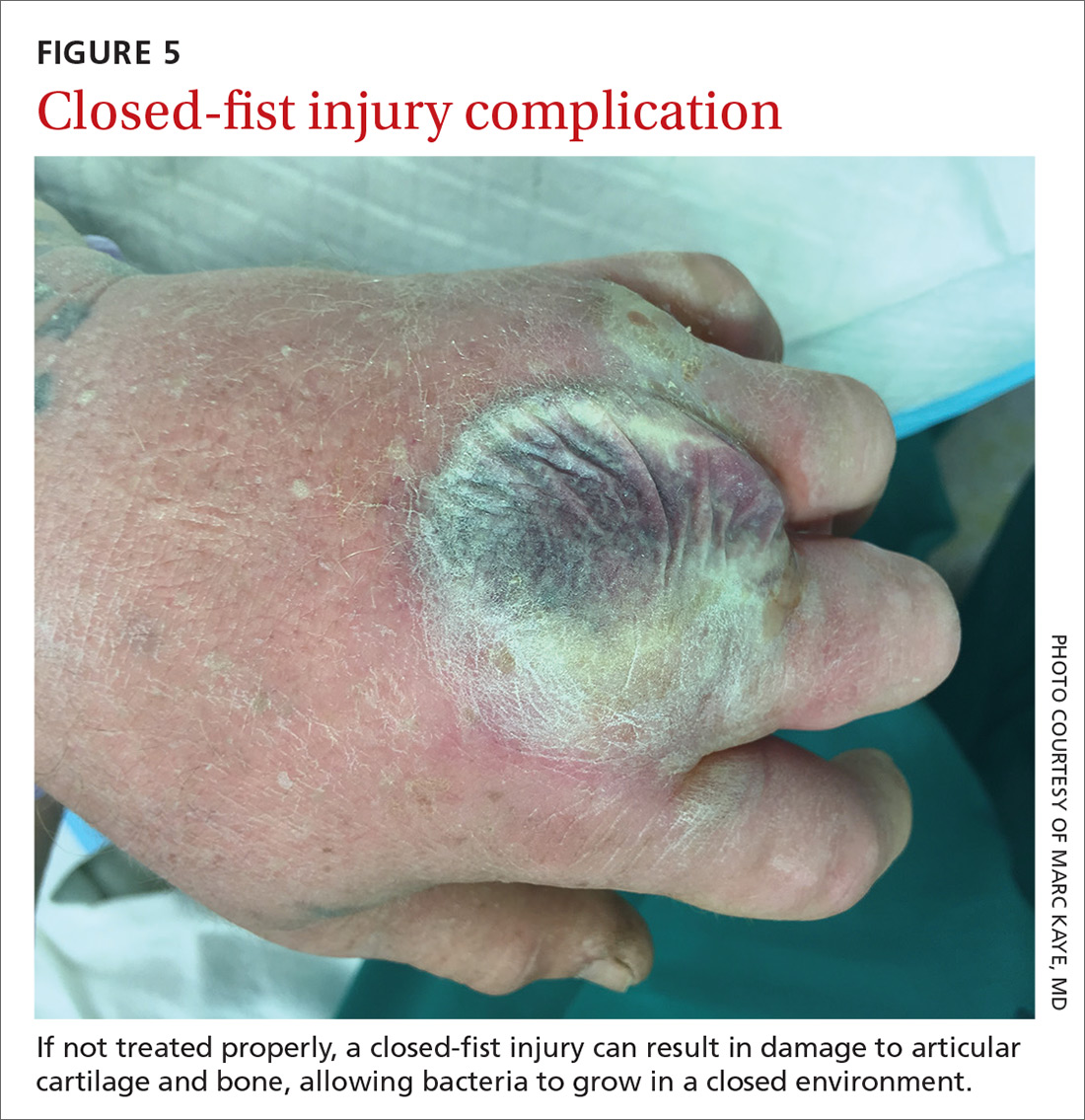
Continue to: Occlusive bite injury...
Occlusive bite injury occurs when one individual forcibly bites another. Such wounds tend to be less penetrating than clenched-fist injuries. However, they can vary from superficial lacerations to wounds with tissue loss, including traumatic finger amputation.24,25
One randomized prospective study compared mechanical wound care alone with combined mechanical wound care and oral or IV antibiotics and found that 47% of patients receiving wound care alone became infected vs no infection among those given oral or parenteral antibiotics (SOR: C).26 Experts in the field advise examining the wound after administering a local anesthetic, thereby allowing better visualization of possible tendon damage, joint penetration, fracture, or deep-tissue infection. The procedure should be performed by a physician experienced in treating hand wounds, whether in the emergency department (ED) or in an operating suite.
How it’s treated. There is controversy regarding whether an affected patient can be adequately treated as an outpatient. Most traumatic bite lesions occur in men, and in those abusing drugs or alcohol.25 In the latter case, individuals may be less likely to return for subsequent care or to finish the antibiotic course as prescribed. It is therefore strongly suggested that those individuals be admitted to receive IV antibiotics and physical therapy to expedite healing and avoid morbidity and sequelae of the lesions (SOR: C).25,27
Obtain cultures from the wound after giving analgesia but before starting the procedure. The sample should be sent to the Microbiology Department or outpatient reference lab for aerobic, anaerobic, AFB, and fungal cultures. Recommend that the laboratory use 10% CO2-enriched media for Eikenella corrodens isolation (SOR: A).28,29
Among oral human flora are large concentrations of anaerobic bacteria such as Bacteroides sp (including B fragilis), Prevotella sp, Peptostreptococcus sp, Fusobacterium sp, Veillonella sp, Enterobateriaceae, and Clostridum. B fragilis accounts for up to 41% of isolates in some studies.30 Most of them are beta-lactamase producers. The most common aerobic bacteria are alpha- and beta-hemolytic Streptococci, S aureus, Staphylococcus epidermis, Corynebacterium, and E corrodens. E corrodens accounts for up to 25% of bacteria isolated in clenched-fist injuries.27,29
Continue to: HSV-1 and HSV-2...
HSV-1 and HSV-2, as well as hepatitis B and C and human immunodeficiency virus (HIV) can be isolated in saliva of infected individuals and can be transmitted when contaminated blood is exposed to an open wound. Still, the presence of HIV in saliva is unlikely to result in disease transmission, due to salivary inhibitors rendering the virus non-infective in most cases.25 Obtain HIV and hepatitis B and C serology at baseline and at 3 and 6 months.25 If HIV infection is known or suspected, or if there was exposure to blood in the wound, the Centers for Disease Control and Prevention recommends postexposure prophylaxis with a 28-day course of anti-retroviral medication (SOR: A).24,31
Hepatitis B virus has an infectivity 100-fold greater than HIV.27 If possible, the 2 people involved in the altercation should be tested for hepatitis B surface antigen. If the result is positive, the individual with the skin wound should receive hepatitis B immune globulin (0.06 mL/kg/dose),32 and the vaccination schedule started if not done previously (SOR: A).33
Most experts recommend early antibiotic therapy given over 3 to 5 days for fresh, superficial wounds and specifically for wounds affecting hands, feet, joint, and genital area.11 For treatment of cellulitis or abscess, 10 to 14 days is sufficient; tenosynovitis requires 2 to 3 weeks; osteomyelitis requires 4 to 6 weeks.24 Wound care associated with daily dressing changes and antimicrobial therapy was superior to wound care alone (0% vs 47%).30 Assess tetanus status in all cases (SOR: A).17,33,34
Antimicrobial therapy for contamination with oral secretion is not straightforward. No one medication covers all possible pathogens. Use a combination therapy initially and then narrow coverage once the microorganism has been identified and susceptibilities are known. Empirical oral therapy with amoxicillin-clavulanate would be reasonable.
If IV therapy is needed, consider using ampicillin-sulbactam, cefazolin, or clindamycin. These antibiotics usually cover S aureus, Streptococcus sp, E corrodens and some anaerobes. Dicloxacillin will cover S aureus but provides poor coverage for E corrodens. First-generation cephalosporins cover S aureus, but E corrodens resistance is common. For penicillin-allergic individuals, use trimethoprim-sulfamethoxazole to cover E corrodens. Doxycycline can be used in children older than 8 years and in adults; avoid it in pregnant women.
Continue to: General principles guiding wound care, microbiology, and antibiotic management
General principles guiding wound care, microbiology, and antibiotic management
Elapsed time between injury and seeking medical attention is characterized as an early (24 hours), late (1-7 days), or delayed (> 7 days) presentation. The timing of an individual’s presentation and a determination of whether the wound is “clean” or “dirty” are both factors in the risk of infection and in associated morbidity and long-term sequelae.
General principles of surgery are important. The cornerstones of treatment are the use of topical anesthesia to provide pain control, which allows for better examination of the wound, debridement of devitalized tissue, collection of wound cultures, and irrigation of the wound with large volume of fluids to mechanically remove dirt, foreign bodies, and bacteria.
Surgical knowledge of hand anatomy increases the likelihood of favorable outcomes in morbidity and functionality. Depending on the circumstances and location, this procedure may be performed by an experienced hand surgeon or an ED physician. (Diversity in settings and available resources may explain why there is so much variability in the composition of patient populations and outcomes found in the medical literature.)
To maximize appropriate bacteriologic success, the Microbiology Department or lab needs to be informed of the type of samples that have been collected. Cultures should be sent for aerobic, anaerobic, AFB, and fungal cultures. Enrichment of aerobic culture with 10% CO2 increases the likelihood of isolating E corrodens (fastidious bacterium), which has been identified in approximately 25% of clenched-fist wounds.
Populations at heightened risk for human bite injuries include alcohol and drug abusers and those of poor socioeconomic status who may not have the resources to visit a medical facility early enough to obtain appropriate medical care. These patients are at risk for being lost to follow-up as well as medication noncompliance, so inpatient admission may diminish the possibilities of incomplete medical treatment, complications, and adverse outcomes such as loss of functionality of the affected extremity.
Continue to: Antimicrobial therapy is not easy
Antimicrobial therapy is not easy. No single regimen covers all possibilities. Start antimicrobial treatment empirically with wide-spectrum coverage, and tailor the regimen, as needed, based on microbiology results.
In clean surgical procedures, S aureus is the most common pathogen. It is acceptable to start empirical treatment with an antistaphylococcal penicillin, first-generation cephalosporin, or clindamycin. In contaminated wounds, gram-negative bacteria, anaerobes, fungal organisms, and mixed infections are more commonly seen.35-37
First-generation cephalosporin provides good coverage for gram-positive and gram-negative bacteria in clean wounds. However, in contaminated wounds with devitalized tissue, a more aggressive scheme is recommended: start with a penicillin and aminoglycoside.35-37 In some cases, monotherapy with either ampicillin/sulbactam, imipenem, meropenem, piperacillin/tazobactam, or tigecylline may be sufficient until culture results are available; at that point, antibiotic coverage can be narrowed as indicated (TABLE 27).35,36
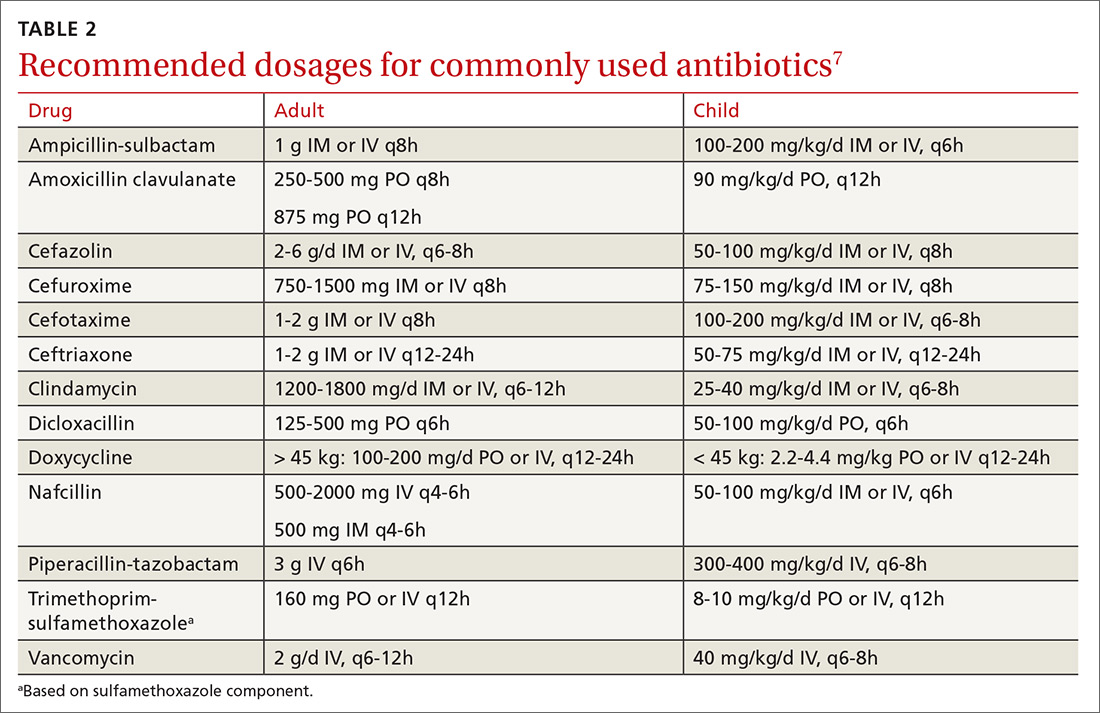
CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; [email protected].
1. Lewis MA. Herpes simplex virus: an occupational hazard in dentistry. Int Dent J. 2004;54:103-111.
2. CDC. Recommended infection-control practices for dentistry. MMWR Morb Mortal Wkly Rep. 1993;42:1-16.
3. Merchant VA, Molinari JA, Sabes WR. Herpetic whitlow: report of a case with multiple recurrences. Oral Surg. 1983;555:568-571.
4. Richards DM, Carmine AA, Brogden RN, et al. Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1983;26:378-438.
5. Laskin OL. Acyclovir and suppression of frequently recurring herpetic whitlow. Ann Int Med. 1985;102:494-495.
6. Schwandt NW, Mjos DP, Lubow RM. Acyclovir and the treatment of herpetic whitlow. Oral Surg Oral Med Oral Pathol. 1987;64:255-258.
7. Robertson J, Shilkofsk N, eds. The Harriet Lane Handbook. 17th Edition. Maryland Heights, MO; Elsevier Mosby; 2005:679-1009.
8. Usatine PR, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
9. Newfield RS, Vargas I, Huma Z. Eikenella corrodens infections. Case report in two adolescent females with IDDM. Diabetes Care. 1996;19:1011-1013.
10. Patel DB, Emmanuel NB, Stevanovic MV, et al. Hand infections: anatomy, types and spread of infection, imaging findings, and treatment options. Radiographics. 2014; 34:1968-1986.
11. Blumberg G, Long B, Koyfman A. Clinical mimics: an emergency medicine-focused review of cellulitis mimics. J Emerg Med. 2017;53:474-484.
12. Kilgore ES Jr, Brown LG, Newmeyer WL, et al. Treatment of felons. Am J Surgery. 1975;130:194-198.
13. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52.
14. Baron EJ, Miller M, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013;57:e22–e121.
15. Clark DC. Common acute hand infections. Am Fam Physician. 2003;68:2167-2176.
16. Swope BM. Panonychiae and felons. Op Tech Gen Surgery. 2002;4:270-273.
17. Yen C, Murray E, Zipprich J, et al. Missed opportunities for tetanus postexposure prophylaxis — California, January 2008-March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:243-246.
18. Barry RL, Adams NS, Martin MD. Pyogenic (suppurative) flexor tenosynovitis: assessment and management. Eplasty. 2016;16:ic7.
19. The symptoms, signs, and diagnosis of tenosynovitis and major facial space abscess. In: Kanavel AB, ed. Infections of the Hand. Philadelphia, PA: Lea & Febiger; 1912:201-226.
20. Kennedy CD, Huang JI, Hanel DP. In brief: Kanavel’s signs and pyogenic flexor tenosynovitis. Clin Ortho Relat Res. 2016;474;280-284.
21. Krieger LE, Schnall SB, Holtom PD, et al. Acute gonococcal flexor tenosynovitis. Orthopedics. 1997;20:649-650.
22. Giladi AM, Malay S, Chung KC. A systematic review of the management of acute pyogenic flexor tenosynovitis. J Hand Surg Eur Vol. 2015;40:720-728.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plastic Surgery. 2002;49:350-354.
24. Kennedy SA, Stoll LE, Lauder AS. Human and other mammalian bite injuries of the hand: evaluation and management. J Am Acad Orthop Surg. 2015;23:47-57.
25. Henry FP, Purcell EM, Eadie PA. The human bite injury: a clinical audit and discussion regarding the management of this alcohol fueled phenomenon. Emer Med J. 2007;24:455-458.
26. Zubowicz VN, Gravier M. Management of early human bites of the hand: a prospective randomized study. Plas Reconstr Surg. 1991;88:111-114.
27. Kelly IP, Cunney RJ, Smyth EG, et al. The management of human bite injuries of the hand. Injury. 1996;27:481-484.
28. Udaka T, Hiraki N, Shiomori T, et al. Eikenella corrodens in head and neck infections. J Infect. 2007;54:343-348.
29. Decker MD. Eikenella corrodens. Infect Control. 1986;7:36-41.
30. Griego RD, Rosen T, Orengo IF, et al. Dog, cats, and human bites: a review. J Am Acad Dermatol. 1995;33:1019-1029.
31. Panlilio AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1:17.
32. American Academy of Pediatrics. Hepatitis B. In: Kimberlin DW, Brady MT, Jackson MA, Long SS. Eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL. American Academy of Pediatrics; 2018:415.
33. Chapman LE, Sullivent EE, Grohskopf LA, et al. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events—United States, 2008: recommendations of the Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2008;57:1-21.
34. Harrison M. A 4-year review of human bite injuries presenting to emergency medicine and proposed evidence-based guidelines. Injury. 2009;40:826-830.
35. Taplitz RA. Managing bite wounds. Currently recommended antibiotics for treatment and prophylaxis. Postgrad Med. 2004;116:49-52.
36. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705-710.
37. Shapiro DB. Postoperative infection in hand surgery. Cause, prevention, and treatment. Hands Clinic. 1998;14:669-681.
Hand infections, if not treated properly, can cause severe chronic morbidity. The conditions I review here range from superficial to deep seated: herpetic whitlow located in the epidermis; felon in subcutaneous tissue; pyogenic flexor tenosynovitis (FTS) in the tendon sheath; and human bite infection at any level including possibly synovium and bone.
Superficial infections usually respond to nonsurgical management. However, antimicrobial therapy is not straightforward. There is no single regimen that covers all possible pathogens. Combination therapy must usually be started and then tailored once an organism and its susceptibility are known. Subcutaneous, tendon sheath, synovial, and bone infections frequently require surgical management.
Herpetic whitlow
Herpes simplex virus type 1 (HSV-1) is common, with as much as 90% of the population exposed by 60 years of age.1 Initial infection usually occurs in the oropharynx and is known as a fever blister or cold sore. However, HSV-1 also can cause herpetic whitlow, a primary infection in the fingertip in which the virus penetrates the subcutaneous tissue, usually after a breakdown in the skin barrier either from infected saliva or the lips of an infected individual.1
What you’ll see. The lesion is characterized by pain, swelling, erythema, and nonpurulent vesicle formation (FIGURE 1). The condition is usually self-limiting, with the inflammation resolving spontaneously, leaving normal healthy skin within 1 to 2 weeks. Herpetic whitlow is an occupational hazard for medical, nursing, paramedical, and dental personnel, and standard precautions should be used when handling secretions (Strength of Recommendation [SOR]: A).2

Reactivation of HSV-1 (and HSV-2) is common, with a prodrome of a “tingling sensation” and subsequent blister formation in the same location as the previous infection. It can cause pain and discomfort and may render the individual unable to perform usual activities.1,3 This lesion is often confused with bacterial (pyogenic) infections of the pulp of the finger or thumb (felon).1 Herpetic whitlow can be distinguished from a felon by its formation of vesicles, lack of a tense pulp space, and serous, rather than purulent, drainage. Scarring is not associated with herpes infection because penetration is limited to the epidermal area. Superimposed bacterial infection can be mistaken for an abscess (felon) and lead to unnecessary incision and drainage, causing associated morbidity and potential scarring in the affected area.1
How it’s treated. Treatment of herpetic whitlow is usually conservative, and topical application of acyclovir 5% appears to be beneficial (SOR: C).4 Two studies also suggest that oral acyclovir is beneficial for herpetic whitlow and may reduce the frequency of recurrence.5,6 Controlled studies in the use of acyclovir for herpetic whitlow have not been conducted. Despite a lack of direct evidence, acyclovir, famciclovir, and valacyclovir are accepted therapies for herpetic whitlow (TABLE 17) (SOR: B).6,8

Felon
A felon is a closed-space infection affecting the pulp of the fingers or thumb. The anatomy of the finger is unique in that there are multiple septa attaching the periosteum to the skin, thereby creating several closed spaces prone to develop pockets of infection.
Continue to: What you'll see
What you’ll see. Clinical signs and symptoms include pain in the pulp of the finger with tenderness and swelling. Bacteria are usually introduced into fingertip space (fat pad) by a penetrating object. Some reported cases have involved individuals with diabetes who regularly check their blood sugar (FIGURE 2).9 A defining characteristic is that the infection usually does not extend past the interphalangeal joint. Radiologic evaluation may be necessary to detect the presence of foreign bodies or to assess bone involvement (osteomyelitis of the distal phalanx).10 The differential diagnosis includes paronychia, in which the infection starts in the nail area and pain is not as intense as in a felon infection.11

How it’s treated. Surgical treatment of felon is controversial. There is no doubt that pus should be drained; how the incision is best performed, however, has been debated.12 Before surgical debridement, obtain a sample of pus for Gram stain and for cultures of aerobic and anaerobic organisms, acid-fast bacilli (AFB), and fungi (SOR: A).13,14 Several surgical techniques and their pitfalls are described in the literature.
Lateral and tip incisions may help avoid painful scars. However, multiple reports of this procedure describe injury to neurovascular bundles, leading to ischemia and anesthesia.12 The “fish-mouth” incision and the “hockey stick” or “J” incision, as well as the transverse palmar incision, are no longer recommended due to painful sequelae, sensorial alterations, and risk of cutting the digital nerves.15 The preferred surgical procedure at this time is to make a very short incision over the area of maximum tenderness, then open and drain the abscess. Avoid placing packing in the affected area. Post-surgical management includes elevation, immobilization with an appropriate splint, and application of compresses until the wound has healed.12,15,16
Since Staphylococcus aureus and Streptococcus sp are the most common bacteria causing felon, start
Acute pyogenic flexor tenosynovitis
FTS is an aggressive closed-space bacterial infection that involves the flexor tendon synovial sheath. FTS accounts for up to 10% of acute hand infections and requires prompt medical attention with wound lavage, surgical management, and antimicrobial therapy to minimize serious consequences to the digit.18
Continue to: What you'll see
What you’ll see. FTS is diagnosed using clinical criteria19,20: fusiform swelling of the finger; exquisite tenderness over the entire course of the flexor tendon sheath; pain on passive extension; and flexed posture of the digit (FIGURE 3). Patients usually recall some type of trauma or puncture wound to the affected area, but hematogenous spread of Neisseria gonorrhoeae also has been reported.21 The most common bacterial pathogens are Staphylococcus sp or Streptococcus sp. However, obtain a sample for Gram stain and culture for aerobic, anaerobic, AFB, and fungal agents before irrigating the wound with copious fluids and initiating empirical antibiotic therapy.14 Once a pathogen has been isolated, tailor antimicrobial therapy based on identified sensitivities and local antibiogram.13

How it’s treated. Early treatment of FTS is of utmost importance to avoid adverse outcomes. If FTS is diagnosed early, manage conservatively with elevation of the hand, splinting in a neutral position, and intravenous (IV) antibiotics. The use of adjunct antibiotics has improved range-of-motion outcomes compared with elevation and splinting alone (54% excellent vs 14% excellent) (SOR: A).22

Surgical management of FTS has involved either an open or closed method. The open approach consists of open incision and drainage with exposure of the flexor tendon sheath, followed by large-volume sheath irrigation and closure of incision, in some cases over a drain. The closed approach with irrigation, rather than open washout, has been associated with improved outcomes (71% excellent vs 26% excellent).22 As a result, the procedure of choice is the closed approach, which uses closed catheter irrigation. An incision and placement of an angiocatheter allows for gentle irrigation of the wound until all purulent material has been evacuated (SOR: C).22,23
Human bite injuries
A human bite injury occurs in 1 of 2 ways, and each has a distinct pattern.
What you’ll see
Closed-fist injury occurs when a clenched fist strikes the teeth of another person. The resulting lesion can easily fool a clinician by appearing to show very little damage. If not appropriately evaluated and treated, the lesion can cause considerable morbidity (FIGURE 5). Injury to the extensor mechanism and joint capsule can also damage the articular cartilage and bone, allowing bacteria to grow in a closed environment. This usually affects the metacarpophalangeal joint (MCP) due to its prominence when a hand is clenched. Initially the lesion seems to be minor, with a small laceration of 3 to 5 mm on the overlying skin, thus inoculating mouth flora deep in the hand tissue. Once the hand is relaxed, the broken skin retracts proximally, covering the wound and making it look innocuous.24

Continue to: Occlusive bite injury...
Occlusive bite injury occurs when one individual forcibly bites another. Such wounds tend to be less penetrating than clenched-fist injuries. However, they can vary from superficial lacerations to wounds with tissue loss, including traumatic finger amputation.24,25
One randomized prospective study compared mechanical wound care alone with combined mechanical wound care and oral or IV antibiotics and found that 47% of patients receiving wound care alone became infected vs no infection among those given oral or parenteral antibiotics (SOR: C).26 Experts in the field advise examining the wound after administering a local anesthetic, thereby allowing better visualization of possible tendon damage, joint penetration, fracture, or deep-tissue infection. The procedure should be performed by a physician experienced in treating hand wounds, whether in the emergency department (ED) or in an operating suite.
How it’s treated. There is controversy regarding whether an affected patient can be adequately treated as an outpatient. Most traumatic bite lesions occur in men, and in those abusing drugs or alcohol.25 In the latter case, individuals may be less likely to return for subsequent care or to finish the antibiotic course as prescribed. It is therefore strongly suggested that those individuals be admitted to receive IV antibiotics and physical therapy to expedite healing and avoid morbidity and sequelae of the lesions (SOR: C).25,27
Obtain cultures from the wound after giving analgesia but before starting the procedure. The sample should be sent to the Microbiology Department or outpatient reference lab for aerobic, anaerobic, AFB, and fungal cultures. Recommend that the laboratory use 10% CO2-enriched media for Eikenella corrodens isolation (SOR: A).28,29
Among oral human flora are large concentrations of anaerobic bacteria such as Bacteroides sp (including B fragilis), Prevotella sp, Peptostreptococcus sp, Fusobacterium sp, Veillonella sp, Enterobateriaceae, and Clostridum. B fragilis accounts for up to 41% of isolates in some studies.30 Most of them are beta-lactamase producers. The most common aerobic bacteria are alpha- and beta-hemolytic Streptococci, S aureus, Staphylococcus epidermis, Corynebacterium, and E corrodens. E corrodens accounts for up to 25% of bacteria isolated in clenched-fist injuries.27,29
Continue to: HSV-1 and HSV-2...
HSV-1 and HSV-2, as well as hepatitis B and C and human immunodeficiency virus (HIV) can be isolated in saliva of infected individuals and can be transmitted when contaminated blood is exposed to an open wound. Still, the presence of HIV in saliva is unlikely to result in disease transmission, due to salivary inhibitors rendering the virus non-infective in most cases.25 Obtain HIV and hepatitis B and C serology at baseline and at 3 and 6 months.25 If HIV infection is known or suspected, or if there was exposure to blood in the wound, the Centers for Disease Control and Prevention recommends postexposure prophylaxis with a 28-day course of anti-retroviral medication (SOR: A).24,31
Hepatitis B virus has an infectivity 100-fold greater than HIV.27 If possible, the 2 people involved in the altercation should be tested for hepatitis B surface antigen. If the result is positive, the individual with the skin wound should receive hepatitis B immune globulin (0.06 mL/kg/dose),32 and the vaccination schedule started if not done previously (SOR: A).33
Most experts recommend early antibiotic therapy given over 3 to 5 days for fresh, superficial wounds and specifically for wounds affecting hands, feet, joint, and genital area.11 For treatment of cellulitis or abscess, 10 to 14 days is sufficient; tenosynovitis requires 2 to 3 weeks; osteomyelitis requires 4 to 6 weeks.24 Wound care associated with daily dressing changes and antimicrobial therapy was superior to wound care alone (0% vs 47%).30 Assess tetanus status in all cases (SOR: A).17,33,34
Antimicrobial therapy for contamination with oral secretion is not straightforward. No one medication covers all possible pathogens. Use a combination therapy initially and then narrow coverage once the microorganism has been identified and susceptibilities are known. Empirical oral therapy with amoxicillin-clavulanate would be reasonable.
If IV therapy is needed, consider using ampicillin-sulbactam, cefazolin, or clindamycin. These antibiotics usually cover S aureus, Streptococcus sp, E corrodens and some anaerobes. Dicloxacillin will cover S aureus but provides poor coverage for E corrodens. First-generation cephalosporins cover S aureus, but E corrodens resistance is common. For penicillin-allergic individuals, use trimethoprim-sulfamethoxazole to cover E corrodens. Doxycycline can be used in children older than 8 years and in adults; avoid it in pregnant women.
Continue to: General principles guiding wound care, microbiology, and antibiotic management
General principles guiding wound care, microbiology, and antibiotic management
Elapsed time between injury and seeking medical attention is characterized as an early (24 hours), late (1-7 days), or delayed (> 7 days) presentation. The timing of an individual’s presentation and a determination of whether the wound is “clean” or “dirty” are both factors in the risk of infection and in associated morbidity and long-term sequelae.
General principles of surgery are important. The cornerstones of treatment are the use of topical anesthesia to provide pain control, which allows for better examination of the wound, debridement of devitalized tissue, collection of wound cultures, and irrigation of the wound with large volume of fluids to mechanically remove dirt, foreign bodies, and bacteria.
Surgical knowledge of hand anatomy increases the likelihood of favorable outcomes in morbidity and functionality. Depending on the circumstances and location, this procedure may be performed by an experienced hand surgeon or an ED physician. (Diversity in settings and available resources may explain why there is so much variability in the composition of patient populations and outcomes found in the medical literature.)
To maximize appropriate bacteriologic success, the Microbiology Department or lab needs to be informed of the type of samples that have been collected. Cultures should be sent for aerobic, anaerobic, AFB, and fungal cultures. Enrichment of aerobic culture with 10% CO2 increases the likelihood of isolating E corrodens (fastidious bacterium), which has been identified in approximately 25% of clenched-fist wounds.
Populations at heightened risk for human bite injuries include alcohol and drug abusers and those of poor socioeconomic status who may not have the resources to visit a medical facility early enough to obtain appropriate medical care. These patients are at risk for being lost to follow-up as well as medication noncompliance, so inpatient admission may diminish the possibilities of incomplete medical treatment, complications, and adverse outcomes such as loss of functionality of the affected extremity.
Continue to: Antimicrobial therapy is not easy
Antimicrobial therapy is not easy. No single regimen covers all possibilities. Start antimicrobial treatment empirically with wide-spectrum coverage, and tailor the regimen, as needed, based on microbiology results.
In clean surgical procedures, S aureus is the most common pathogen. It is acceptable to start empirical treatment with an antistaphylococcal penicillin, first-generation cephalosporin, or clindamycin. In contaminated wounds, gram-negative bacteria, anaerobes, fungal organisms, and mixed infections are more commonly seen.35-37
First-generation cephalosporin provides good coverage for gram-positive and gram-negative bacteria in clean wounds. However, in contaminated wounds with devitalized tissue, a more aggressive scheme is recommended: start with a penicillin and aminoglycoside.35-37 In some cases, monotherapy with either ampicillin/sulbactam, imipenem, meropenem, piperacillin/tazobactam, or tigecylline may be sufficient until culture results are available; at that point, antibiotic coverage can be narrowed as indicated (TABLE 27).35,36

CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; [email protected].
Hand infections, if not treated properly, can cause severe chronic morbidity. The conditions I review here range from superficial to deep seated: herpetic whitlow located in the epidermis; felon in subcutaneous tissue; pyogenic flexor tenosynovitis (FTS) in the tendon sheath; and human bite infection at any level including possibly synovium and bone.
Superficial infections usually respond to nonsurgical management. However, antimicrobial therapy is not straightforward. There is no single regimen that covers all possible pathogens. Combination therapy must usually be started and then tailored once an organism and its susceptibility are known. Subcutaneous, tendon sheath, synovial, and bone infections frequently require surgical management.
Herpetic whitlow
Herpes simplex virus type 1 (HSV-1) is common, with as much as 90% of the population exposed by 60 years of age.1 Initial infection usually occurs in the oropharynx and is known as a fever blister or cold sore. However, HSV-1 also can cause herpetic whitlow, a primary infection in the fingertip in which the virus penetrates the subcutaneous tissue, usually after a breakdown in the skin barrier either from infected saliva or the lips of an infected individual.1
What you’ll see. The lesion is characterized by pain, swelling, erythema, and nonpurulent vesicle formation (FIGURE 1). The condition is usually self-limiting, with the inflammation resolving spontaneously, leaving normal healthy skin within 1 to 2 weeks. Herpetic whitlow is an occupational hazard for medical, nursing, paramedical, and dental personnel, and standard precautions should be used when handling secretions (Strength of Recommendation [SOR]: A).2

Reactivation of HSV-1 (and HSV-2) is common, with a prodrome of a “tingling sensation” and subsequent blister formation in the same location as the previous infection. It can cause pain and discomfort and may render the individual unable to perform usual activities.1,3 This lesion is often confused with bacterial (pyogenic) infections of the pulp of the finger or thumb (felon).1 Herpetic whitlow can be distinguished from a felon by its formation of vesicles, lack of a tense pulp space, and serous, rather than purulent, drainage. Scarring is not associated with herpes infection because penetration is limited to the epidermal area. Superimposed bacterial infection can be mistaken for an abscess (felon) and lead to unnecessary incision and drainage, causing associated morbidity and potential scarring in the affected area.1
How it’s treated. Treatment of herpetic whitlow is usually conservative, and topical application of acyclovir 5% appears to be beneficial (SOR: C).4 Two studies also suggest that oral acyclovir is beneficial for herpetic whitlow and may reduce the frequency of recurrence.5,6 Controlled studies in the use of acyclovir for herpetic whitlow have not been conducted. Despite a lack of direct evidence, acyclovir, famciclovir, and valacyclovir are accepted therapies for herpetic whitlow (TABLE 17) (SOR: B).6,8

Felon
A felon is a closed-space infection affecting the pulp of the fingers or thumb. The anatomy of the finger is unique in that there are multiple septa attaching the periosteum to the skin, thereby creating several closed spaces prone to develop pockets of infection.
Continue to: What you'll see
What you’ll see. Clinical signs and symptoms include pain in the pulp of the finger with tenderness and swelling. Bacteria are usually introduced into fingertip space (fat pad) by a penetrating object. Some reported cases have involved individuals with diabetes who regularly check their blood sugar (FIGURE 2).9 A defining characteristic is that the infection usually does not extend past the interphalangeal joint. Radiologic evaluation may be necessary to detect the presence of foreign bodies or to assess bone involvement (osteomyelitis of the distal phalanx).10 The differential diagnosis includes paronychia, in which the infection starts in the nail area and pain is not as intense as in a felon infection.11

How it’s treated. Surgical treatment of felon is controversial. There is no doubt that pus should be drained; how the incision is best performed, however, has been debated.12 Before surgical debridement, obtain a sample of pus for Gram stain and for cultures of aerobic and anaerobic organisms, acid-fast bacilli (AFB), and fungi (SOR: A).13,14 Several surgical techniques and their pitfalls are described in the literature.
Lateral and tip incisions may help avoid painful scars. However, multiple reports of this procedure describe injury to neurovascular bundles, leading to ischemia and anesthesia.12 The “fish-mouth” incision and the “hockey stick” or “J” incision, as well as the transverse palmar incision, are no longer recommended due to painful sequelae, sensorial alterations, and risk of cutting the digital nerves.15 The preferred surgical procedure at this time is to make a very short incision over the area of maximum tenderness, then open and drain the abscess. Avoid placing packing in the affected area. Post-surgical management includes elevation, immobilization with an appropriate splint, and application of compresses until the wound has healed.12,15,16
Since Staphylococcus aureus and Streptococcus sp are the most common bacteria causing felon, start
Acute pyogenic flexor tenosynovitis
FTS is an aggressive closed-space bacterial infection that involves the flexor tendon synovial sheath. FTS accounts for up to 10% of acute hand infections and requires prompt medical attention with wound lavage, surgical management, and antimicrobial therapy to minimize serious consequences to the digit.18
Continue to: What you'll see
What you’ll see. FTS is diagnosed using clinical criteria19,20: fusiform swelling of the finger; exquisite tenderness over the entire course of the flexor tendon sheath; pain on passive extension; and flexed posture of the digit (FIGURE 3). Patients usually recall some type of trauma or puncture wound to the affected area, but hematogenous spread of Neisseria gonorrhoeae also has been reported.21 The most common bacterial pathogens are Staphylococcus sp or Streptococcus sp. However, obtain a sample for Gram stain and culture for aerobic, anaerobic, AFB, and fungal agents before irrigating the wound with copious fluids and initiating empirical antibiotic therapy.14 Once a pathogen has been isolated, tailor antimicrobial therapy based on identified sensitivities and local antibiogram.13

How it’s treated. Early treatment of FTS is of utmost importance to avoid adverse outcomes. If FTS is diagnosed early, manage conservatively with elevation of the hand, splinting in a neutral position, and intravenous (IV) antibiotics. The use of adjunct antibiotics has improved range-of-motion outcomes compared with elevation and splinting alone (54% excellent vs 14% excellent) (SOR: A).22

Surgical management of FTS has involved either an open or closed method. The open approach consists of open incision and drainage with exposure of the flexor tendon sheath, followed by large-volume sheath irrigation and closure of incision, in some cases over a drain. The closed approach with irrigation, rather than open washout, has been associated with improved outcomes (71% excellent vs 26% excellent).22 As a result, the procedure of choice is the closed approach, which uses closed catheter irrigation. An incision and placement of an angiocatheter allows for gentle irrigation of the wound until all purulent material has been evacuated (SOR: C).22,23
Human bite injuries
A human bite injury occurs in 1 of 2 ways, and each has a distinct pattern.
What you’ll see
Closed-fist injury occurs when a clenched fist strikes the teeth of another person. The resulting lesion can easily fool a clinician by appearing to show very little damage. If not appropriately evaluated and treated, the lesion can cause considerable morbidity (FIGURE 5). Injury to the extensor mechanism and joint capsule can also damage the articular cartilage and bone, allowing bacteria to grow in a closed environment. This usually affects the metacarpophalangeal joint (MCP) due to its prominence when a hand is clenched. Initially the lesion seems to be minor, with a small laceration of 3 to 5 mm on the overlying skin, thus inoculating mouth flora deep in the hand tissue. Once the hand is relaxed, the broken skin retracts proximally, covering the wound and making it look innocuous.24

Continue to: Occlusive bite injury...
Occlusive bite injury occurs when one individual forcibly bites another. Such wounds tend to be less penetrating than clenched-fist injuries. However, they can vary from superficial lacerations to wounds with tissue loss, including traumatic finger amputation.24,25
One randomized prospective study compared mechanical wound care alone with combined mechanical wound care and oral or IV antibiotics and found that 47% of patients receiving wound care alone became infected vs no infection among those given oral or parenteral antibiotics (SOR: C).26 Experts in the field advise examining the wound after administering a local anesthetic, thereby allowing better visualization of possible tendon damage, joint penetration, fracture, or deep-tissue infection. The procedure should be performed by a physician experienced in treating hand wounds, whether in the emergency department (ED) or in an operating suite.
How it’s treated. There is controversy regarding whether an affected patient can be adequately treated as an outpatient. Most traumatic bite lesions occur in men, and in those abusing drugs or alcohol.25 In the latter case, individuals may be less likely to return for subsequent care or to finish the antibiotic course as prescribed. It is therefore strongly suggested that those individuals be admitted to receive IV antibiotics and physical therapy to expedite healing and avoid morbidity and sequelae of the lesions (SOR: C).25,27
Obtain cultures from the wound after giving analgesia but before starting the procedure. The sample should be sent to the Microbiology Department or outpatient reference lab for aerobic, anaerobic, AFB, and fungal cultures. Recommend that the laboratory use 10% CO2-enriched media for Eikenella corrodens isolation (SOR: A).28,29
Among oral human flora are large concentrations of anaerobic bacteria such as Bacteroides sp (including B fragilis), Prevotella sp, Peptostreptococcus sp, Fusobacterium sp, Veillonella sp, Enterobateriaceae, and Clostridum. B fragilis accounts for up to 41% of isolates in some studies.30 Most of them are beta-lactamase producers. The most common aerobic bacteria are alpha- and beta-hemolytic Streptococci, S aureus, Staphylococcus epidermis, Corynebacterium, and E corrodens. E corrodens accounts for up to 25% of bacteria isolated in clenched-fist injuries.27,29
Continue to: HSV-1 and HSV-2...
HSV-1 and HSV-2, as well as hepatitis B and C and human immunodeficiency virus (HIV) can be isolated in saliva of infected individuals and can be transmitted when contaminated blood is exposed to an open wound. Still, the presence of HIV in saliva is unlikely to result in disease transmission, due to salivary inhibitors rendering the virus non-infective in most cases.25 Obtain HIV and hepatitis B and C serology at baseline and at 3 and 6 months.25 If HIV infection is known or suspected, or if there was exposure to blood in the wound, the Centers for Disease Control and Prevention recommends postexposure prophylaxis with a 28-day course of anti-retroviral medication (SOR: A).24,31
Hepatitis B virus has an infectivity 100-fold greater than HIV.27 If possible, the 2 people involved in the altercation should be tested for hepatitis B surface antigen. If the result is positive, the individual with the skin wound should receive hepatitis B immune globulin (0.06 mL/kg/dose),32 and the vaccination schedule started if not done previously (SOR: A).33
Most experts recommend early antibiotic therapy given over 3 to 5 days for fresh, superficial wounds and specifically for wounds affecting hands, feet, joint, and genital area.11 For treatment of cellulitis or abscess, 10 to 14 days is sufficient; tenosynovitis requires 2 to 3 weeks; osteomyelitis requires 4 to 6 weeks.24 Wound care associated with daily dressing changes and antimicrobial therapy was superior to wound care alone (0% vs 47%).30 Assess tetanus status in all cases (SOR: A).17,33,34
Antimicrobial therapy for contamination with oral secretion is not straightforward. No one medication covers all possible pathogens. Use a combination therapy initially and then narrow coverage once the microorganism has been identified and susceptibilities are known. Empirical oral therapy with amoxicillin-clavulanate would be reasonable.
If IV therapy is needed, consider using ampicillin-sulbactam, cefazolin, or clindamycin. These antibiotics usually cover S aureus, Streptococcus sp, E corrodens and some anaerobes. Dicloxacillin will cover S aureus but provides poor coverage for E corrodens. First-generation cephalosporins cover S aureus, but E corrodens resistance is common. For penicillin-allergic individuals, use trimethoprim-sulfamethoxazole to cover E corrodens. Doxycycline can be used in children older than 8 years and in adults; avoid it in pregnant women.
Continue to: General principles guiding wound care, microbiology, and antibiotic management
General principles guiding wound care, microbiology, and antibiotic management
Elapsed time between injury and seeking medical attention is characterized as an early (24 hours), late (1-7 days), or delayed (> 7 days) presentation. The timing of an individual’s presentation and a determination of whether the wound is “clean” or “dirty” are both factors in the risk of infection and in associated morbidity and long-term sequelae.
General principles of surgery are important. The cornerstones of treatment are the use of topical anesthesia to provide pain control, which allows for better examination of the wound, debridement of devitalized tissue, collection of wound cultures, and irrigation of the wound with large volume of fluids to mechanically remove dirt, foreign bodies, and bacteria.
Surgical knowledge of hand anatomy increases the likelihood of favorable outcomes in morbidity and functionality. Depending on the circumstances and location, this procedure may be performed by an experienced hand surgeon or an ED physician. (Diversity in settings and available resources may explain why there is so much variability in the composition of patient populations and outcomes found in the medical literature.)
To maximize appropriate bacteriologic success, the Microbiology Department or lab needs to be informed of the type of samples that have been collected. Cultures should be sent for aerobic, anaerobic, AFB, and fungal cultures. Enrichment of aerobic culture with 10% CO2 increases the likelihood of isolating E corrodens (fastidious bacterium), which has been identified in approximately 25% of clenched-fist wounds.
Populations at heightened risk for human bite injuries include alcohol and drug abusers and those of poor socioeconomic status who may not have the resources to visit a medical facility early enough to obtain appropriate medical care. These patients are at risk for being lost to follow-up as well as medication noncompliance, so inpatient admission may diminish the possibilities of incomplete medical treatment, complications, and adverse outcomes such as loss of functionality of the affected extremity.
Continue to: Antimicrobial therapy is not easy
Antimicrobial therapy is not easy. No single regimen covers all possibilities. Start antimicrobial treatment empirically with wide-spectrum coverage, and tailor the regimen, as needed, based on microbiology results.
In clean surgical procedures, S aureus is the most common pathogen. It is acceptable to start empirical treatment with an antistaphylococcal penicillin, first-generation cephalosporin, or clindamycin. In contaminated wounds, gram-negative bacteria, anaerobes, fungal organisms, and mixed infections are more commonly seen.35-37
First-generation cephalosporin provides good coverage for gram-positive and gram-negative bacteria in clean wounds. However, in contaminated wounds with devitalized tissue, a more aggressive scheme is recommended: start with a penicillin and aminoglycoside.35-37 In some cases, monotherapy with either ampicillin/sulbactam, imipenem, meropenem, piperacillin/tazobactam, or tigecylline may be sufficient until culture results are available; at that point, antibiotic coverage can be narrowed as indicated (TABLE 27).35,36

CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; [email protected].
1. Lewis MA. Herpes simplex virus: an occupational hazard in dentistry. Int Dent J. 2004;54:103-111.
2. CDC. Recommended infection-control practices for dentistry. MMWR Morb Mortal Wkly Rep. 1993;42:1-16.
3. Merchant VA, Molinari JA, Sabes WR. Herpetic whitlow: report of a case with multiple recurrences. Oral Surg. 1983;555:568-571.
4. Richards DM, Carmine AA, Brogden RN, et al. Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1983;26:378-438.
5. Laskin OL. Acyclovir and suppression of frequently recurring herpetic whitlow. Ann Int Med. 1985;102:494-495.
6. Schwandt NW, Mjos DP, Lubow RM. Acyclovir and the treatment of herpetic whitlow. Oral Surg Oral Med Oral Pathol. 1987;64:255-258.
7. Robertson J, Shilkofsk N, eds. The Harriet Lane Handbook. 17th Edition. Maryland Heights, MO; Elsevier Mosby; 2005:679-1009.
8. Usatine PR, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
9. Newfield RS, Vargas I, Huma Z. Eikenella corrodens infections. Case report in two adolescent females with IDDM. Diabetes Care. 1996;19:1011-1013.
10. Patel DB, Emmanuel NB, Stevanovic MV, et al. Hand infections: anatomy, types and spread of infection, imaging findings, and treatment options. Radiographics. 2014; 34:1968-1986.
11. Blumberg G, Long B, Koyfman A. Clinical mimics: an emergency medicine-focused review of cellulitis mimics. J Emerg Med. 2017;53:474-484.
12. Kilgore ES Jr, Brown LG, Newmeyer WL, et al. Treatment of felons. Am J Surgery. 1975;130:194-198.
13. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52.
14. Baron EJ, Miller M, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013;57:e22–e121.
15. Clark DC. Common acute hand infections. Am Fam Physician. 2003;68:2167-2176.
16. Swope BM. Panonychiae and felons. Op Tech Gen Surgery. 2002;4:270-273.
17. Yen C, Murray E, Zipprich J, et al. Missed opportunities for tetanus postexposure prophylaxis — California, January 2008-March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:243-246.
18. Barry RL, Adams NS, Martin MD. Pyogenic (suppurative) flexor tenosynovitis: assessment and management. Eplasty. 2016;16:ic7.
19. The symptoms, signs, and diagnosis of tenosynovitis and major facial space abscess. In: Kanavel AB, ed. Infections of the Hand. Philadelphia, PA: Lea & Febiger; 1912:201-226.
20. Kennedy CD, Huang JI, Hanel DP. In brief: Kanavel’s signs and pyogenic flexor tenosynovitis. Clin Ortho Relat Res. 2016;474;280-284.
21. Krieger LE, Schnall SB, Holtom PD, et al. Acute gonococcal flexor tenosynovitis. Orthopedics. 1997;20:649-650.
22. Giladi AM, Malay S, Chung KC. A systematic review of the management of acute pyogenic flexor tenosynovitis. J Hand Surg Eur Vol. 2015;40:720-728.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plastic Surgery. 2002;49:350-354.
24. Kennedy SA, Stoll LE, Lauder AS. Human and other mammalian bite injuries of the hand: evaluation and management. J Am Acad Orthop Surg. 2015;23:47-57.
25. Henry FP, Purcell EM, Eadie PA. The human bite injury: a clinical audit and discussion regarding the management of this alcohol fueled phenomenon. Emer Med J. 2007;24:455-458.
26. Zubowicz VN, Gravier M. Management of early human bites of the hand: a prospective randomized study. Plas Reconstr Surg. 1991;88:111-114.
27. Kelly IP, Cunney RJ, Smyth EG, et al. The management of human bite injuries of the hand. Injury. 1996;27:481-484.
28. Udaka T, Hiraki N, Shiomori T, et al. Eikenella corrodens in head and neck infections. J Infect. 2007;54:343-348.
29. Decker MD. Eikenella corrodens. Infect Control. 1986;7:36-41.
30. Griego RD, Rosen T, Orengo IF, et al. Dog, cats, and human bites: a review. J Am Acad Dermatol. 1995;33:1019-1029.
31. Panlilio AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1:17.
32. American Academy of Pediatrics. Hepatitis B. In: Kimberlin DW, Brady MT, Jackson MA, Long SS. Eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL. American Academy of Pediatrics; 2018:415.
33. Chapman LE, Sullivent EE, Grohskopf LA, et al. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events—United States, 2008: recommendations of the Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2008;57:1-21.
34. Harrison M. A 4-year review of human bite injuries presenting to emergency medicine and proposed evidence-based guidelines. Injury. 2009;40:826-830.
35. Taplitz RA. Managing bite wounds. Currently recommended antibiotics for treatment and prophylaxis. Postgrad Med. 2004;116:49-52.
36. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705-710.
37. Shapiro DB. Postoperative infection in hand surgery. Cause, prevention, and treatment. Hands Clinic. 1998;14:669-681.
1. Lewis MA. Herpes simplex virus: an occupational hazard in dentistry. Int Dent J. 2004;54:103-111.
2. CDC. Recommended infection-control practices for dentistry. MMWR Morb Mortal Wkly Rep. 1993;42:1-16.
3. Merchant VA, Molinari JA, Sabes WR. Herpetic whitlow: report of a case with multiple recurrences. Oral Surg. 1983;555:568-571.
4. Richards DM, Carmine AA, Brogden RN, et al. Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1983;26:378-438.
5. Laskin OL. Acyclovir and suppression of frequently recurring herpetic whitlow. Ann Int Med. 1985;102:494-495.
6. Schwandt NW, Mjos DP, Lubow RM. Acyclovir and the treatment of herpetic whitlow. Oral Surg Oral Med Oral Pathol. 1987;64:255-258.
7. Robertson J, Shilkofsk N, eds. The Harriet Lane Handbook. 17th Edition. Maryland Heights, MO; Elsevier Mosby; 2005:679-1009.
8. Usatine PR, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
9. Newfield RS, Vargas I, Huma Z. Eikenella corrodens infections. Case report in two adolescent females with IDDM. Diabetes Care. 1996;19:1011-1013.
10. Patel DB, Emmanuel NB, Stevanovic MV, et al. Hand infections: anatomy, types and spread of infection, imaging findings, and treatment options. Radiographics. 2014; 34:1968-1986.
11. Blumberg G, Long B, Koyfman A. Clinical mimics: an emergency medicine-focused review of cellulitis mimics. J Emerg Med. 2017;53:474-484.
12. Kilgore ES Jr, Brown LG, Newmeyer WL, et al. Treatment of felons. Am J Surgery. 1975;130:194-198.
13. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52.
14. Baron EJ, Miller M, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013;57:e22–e121.
15. Clark DC. Common acute hand infections. Am Fam Physician. 2003;68:2167-2176.
16. Swope BM. Panonychiae and felons. Op Tech Gen Surgery. 2002;4:270-273.
17. Yen C, Murray E, Zipprich J, et al. Missed opportunities for tetanus postexposure prophylaxis — California, January 2008-March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:243-246.
18. Barry RL, Adams NS, Martin MD. Pyogenic (suppurative) flexor tenosynovitis: assessment and management. Eplasty. 2016;16:ic7.
19. The symptoms, signs, and diagnosis of tenosynovitis and major facial space abscess. In: Kanavel AB, ed. Infections of the Hand. Philadelphia, PA: Lea & Febiger; 1912:201-226.
20. Kennedy CD, Huang JI, Hanel DP. In brief: Kanavel’s signs and pyogenic flexor tenosynovitis. Clin Ortho Relat Res. 2016;474;280-284.
21. Krieger LE, Schnall SB, Holtom PD, et al. Acute gonococcal flexor tenosynovitis. Orthopedics. 1997;20:649-650.
22. Giladi AM, Malay S, Chung KC. A systematic review of the management of acute pyogenic flexor tenosynovitis. J Hand Surg Eur Vol. 2015;40:720-728.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plastic Surgery. 2002;49:350-354.
24. Kennedy SA, Stoll LE, Lauder AS. Human and other mammalian bite injuries of the hand: evaluation and management. J Am Acad Orthop Surg. 2015;23:47-57.
25. Henry FP, Purcell EM, Eadie PA. The human bite injury: a clinical audit and discussion regarding the management of this alcohol fueled phenomenon. Emer Med J. 2007;24:455-458.
26. Zubowicz VN, Gravier M. Management of early human bites of the hand: a prospective randomized study. Plas Reconstr Surg. 1991;88:111-114.
27. Kelly IP, Cunney RJ, Smyth EG, et al. The management of human bite injuries of the hand. Injury. 1996;27:481-484.
28. Udaka T, Hiraki N, Shiomori T, et al. Eikenella corrodens in head and neck infections. J Infect. 2007;54:343-348.
29. Decker MD. Eikenella corrodens. Infect Control. 1986;7:36-41.
30. Griego RD, Rosen T, Orengo IF, et al. Dog, cats, and human bites: a review. J Am Acad Dermatol. 1995;33:1019-1029.
31. Panlilio AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1:17.
32. American Academy of Pediatrics. Hepatitis B. In: Kimberlin DW, Brady MT, Jackson MA, Long SS. Eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL. American Academy of Pediatrics; 2018:415.
33. Chapman LE, Sullivent EE, Grohskopf LA, et al. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events—United States, 2008: recommendations of the Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2008;57:1-21.
34. Harrison M. A 4-year review of human bite injuries presenting to emergency medicine and proposed evidence-based guidelines. Injury. 2009;40:826-830.
35. Taplitz RA. Managing bite wounds. Currently recommended antibiotics for treatment and prophylaxis. Postgrad Med. 2004;116:49-52.
36. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705-710.
37. Shapiro DB. Postoperative infection in hand surgery. Cause, prevention, and treatment. Hands Clinic. 1998;14:669-681.
PRACTICE RECOMMENDATIONS
› Obtain a sample of pus for Gram stain and for cultures of aerobic and anaerobic organisms, acid-fast bacilli, and fungi. A
› Use antibiotics as an adjunct to elevation and splinting in flexor tenosynovitis to improve range-of-motion outcomes. A
› Notify your microbiology lab to enrich cultures with 10% CO2 to isolate Eikenella corrodens. A
› Consider prescribing acyclovir, famciclovir, or valacyclovir for herpetic whitlow. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
PSA cancer screening: A case for shared decision-making
Prostate cancer is the most frequently diagnosed cancer in men and the third leading cause of cancer death in men worldwide.1 An estimated 174,650 new cases are diagnosed each year in the United States; 31,620 American men die annually from the disease.2 Although prostate cancer can be a serious disease, many men do not die from it. In fact, 2.9 million men who were diagnosed with prostate cancer at some point are alive today.3
Risk factors. Prostate cancer develops mainly in men ages ≥ 65 years and rarely occurs before age 40. In addition to age, family history and African American ethnicity are the major nonmodifiable risk factors for prostate cancer.4 From the 1970s to the most recent statistical analysis of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, African American men have continued to have significantly higher incidence of, and mortality rates from, prostate cancer than their European American counterparts. African American men are also more likely than men of European ancestry to have aggressive prostate cancers.5 Other risk factors include geographic location (higher risk in Northern Europe, North America, and Australia; lower risk in Asia, Africa, and South and Central America), mutations in the BRCA2 gene, and hereditary non-polyposis colon cancer syndrome.4
Prostate-specific antigen (PSA) was first used as a screening tool for prostate cancer in 1991.6 Prostate cancer incidence, especially organ-confined disease, has dramatically increased since then.7 PSA testing has a low sensitivity and specificity for the detection of prostate cancer, and there is no clear threshold at which biopsy can or should be offered. The most commonly used cutoff value of 4 ng/mL has a false-positive rate of about 70%.8
Benign prostatic conditions such as hypertrophy and infection can elevate PSA levels. In addition, the PSA test does not distinguish between aggressive and slow-growing cancers, and about 15% of patients with prostate cancer have a normal PSA level.9
A word about the digital rectal exam. While PSA testing has been the mainstay of prostate cancer screening, a few studies have included digital rectal exam (DRE) in their protocols. Data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that DRE captured an additional 2% of men with prostate cancer in the setting of a normal PSA test result.10 In the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial, the overall detection rate for prostate cancer was found to be better when DRE was combined with PSA and prostate biopsy than when DRE was used alone (4.5% vs 2.5%).11 Nevertheless, generally speaking, DRE can be omitted in the era of PSA screening.
Screening guidelines vary
Recommendations for prostate cancer screening vary by organization and are summarized in TABLE 1.9,12-14 In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer (Category D).15 In 2018, USPSTF provided an update with a new recommendation that clinicians inform men ages 55 to 69 years about the potential benefits and harms of PSA-based screening (Category C).14 The USPSTF continues to recommend against PSA-based screening for men ages ≥ 70 years (Category D).14
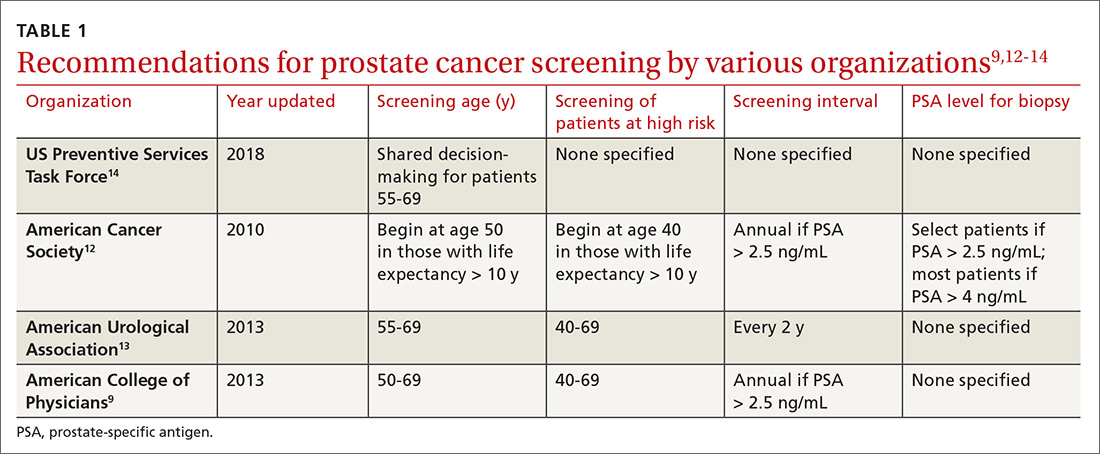
Does PSA-based screening improve patient-centered outcomes?
Several randomized controlled trials (RCTs) such as the Quebec Prospective Randomized Controlled Trial,16 the Norrköping Sweden Study,17 ERSPC,11 and PLCO10 have been conducted to assess the benefits of PSA testing. PLCO and ERSPC have contributed significantly to our understanding of prostate cancer screening even though their 13-year follow-up results are conflicting (TABLE 2).10,11,18
Continue to: In the ERSPC 13-year follow-up publication...
In the ERSPC 13-year follow-up publication, the authors concluded that a substantial reduction in prostate cancer mortality is attributable to testing with PSA.18 Despite limitations in the study design (eg, France entered after 2 years, screening intervals varied between 2 and 4 years, biopsy indications varied, and screening was discontinued at different times), PSA screening detected more prostate cancer than was detected in the control arm (10.2% vs 6.8%).
In the initial 11 years of follow-up, the study group experienced a 21% reduction in prostate cancer mortality, even though the absolute decrease ranged from only 0.6% (545 per 89,352) to 0.5% (355 per 72,891). The updated absolute risk reduction of death from prostate cancer at 13 years of follow-up showed a larger benefit: 0.11 per 1000 person-years or 1.28 per 1000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 (95% confidence interval [CI], 490-1929) men invited for screening, or 1 per 27 (17-66) additional prostate cancers detected.
The PLCO trial did not show any significant difference in prostate cancer detection (11.1% screened vs 9.9% control), and there was no improvement in prostate cancer mortality (3.7 vs 3.4 death per 10,000 person-years).10 However, the PLCO trial suffered from issues of contamination, which may have influenced the overall results. About 52% of men in the control (usual care) group received a PSA test at some point during the study. And more than two-thirds of the men who had a prostate biopsy because of a positive PSA test did not have prostate cancer.
Community standards for the PSA threshold for biopsy were applied in various centers (> 4 ng/ml in general) in PLCO, whereas in ERSPC, a cut-off PSA value ≥ 3 ng/mL was used for biopsy. Because of the lower PSA threshold, ERSPC may have identified cancers that would have had good outcomes without any intervention.
The harms of PSA screening
While it is unclear whether PSA screening results in any improvement in patient-centered outcomes, it does lead to downstream intervention due to overdiagnosis, which precipitates unnecessary anxiety, biopsies, and overtreatment (eg, excess radiation, overuse of androgen deprivation therapy).19 Biopsies carry the risk of hematuria (22.6%), hematospermia (50.4%), and urinary tract infection.20 Data from SEER-Medicare showed that prostate biopsy was associated with a 2.65-fold increased risk of hospitalization within 30 days of the procedure compared to a control population.21
Continue to: Overdiagnosis leads to overtreatment...
Overdiagnosis leads to overtreatment of low-risk prostate cancer. Both traditional treatment options for prostate cancer—radical prostatectomy and radiotherapy—are associated with urinary incontinence, erectile dysfunction, and issues with bowel function.22,23
The Prostate Cancer Intervention vs Observation Trial (PIVOT),24 the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4),25 and the Prostate Testing for Cancer and Treatment (ProtecT) trial,22,23 are the major RCTs that looked at the outcomes of treatment modalities for localized prostate cancer in the modern era of PSA testing.
PIVOT compared passive observation with radical prostatectomy.24 After 20 years of follow-up on 731 patients, the researchers concluded that radical prostatectomy did not reduce all-cause or prostate cancer–related mortality (TABLE 3).24
SPCG-4 showed survival benefits for men who underwent radical prostatectomy compared with men in a watchful waiting group, but only 5% of the study cohort had cancer detected by PSA screening (TABLE 4).25 The rest had either palpable tumors or symptoms of a tumor.
ProtecT, which followed patients with localized prostate cancer for more than 10 years,compared the outcomes and adverse effects of active surveillance, radical prostatectomy, and radiotherapy.23 Prostate cancer–specific mortality was low irrespective of the treatment,23 and there was no significant difference in all-cause mortality or prostate cancer–specific mortality between the 3 treatment groups.23 The active surveillance group had considerably fewer adverse events.22,23 The incidence rates of erectile dysfunction and urinary incontinence at the 1- and 6-year follow-up marks are outlined in TABLE 5.22
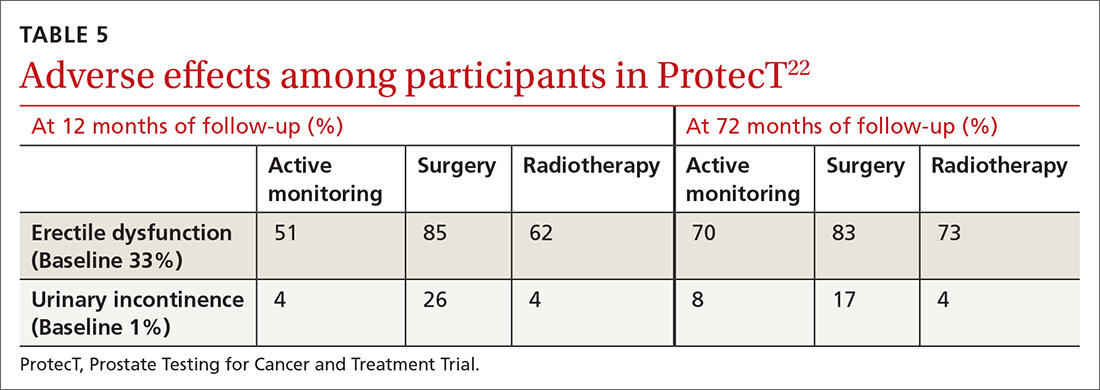
Continue to: The purpose of active monitoring...
The purpose of active monitoring is to minimize overtreatment by avoiding immediate radical intervention. Radical treatments with curative intent can be undertaken at any point while patients are being actively monitored. It is important to note that the active monitoring that took place in ProtecT23 was very different from the passive surveillance of PIVOT24 and SPCG-4.25 In ProtecT, once an elevated serum PSA level was noted, PSA levels were monitored every 3 months in the first year and every 6 to 12 months thereafter.23 Triggers to reassess patients and consider a change in clinical management were based largely on changes in PSA levels. Participants with an increase of at least 50% in PSA level during the previous 12 months were offered either continued monitoring or treatment after further testing.
Making individualized decisions about prostate cancer screening
Traditionally, the goal of cancer screening has been to maximize the number of people screened. Generally, the information provided to patients about cancer screening emphasizes the benefits and minimizes the harms. Recently, however, there has been a shift in communication about cancer screening with the emphasis now being placed on informed decision-making and encouraging patients to make individual decisions about screening participation.26
The treatment option of active surveillance, with its lower incidence of adverse outcomes, is an important reason for patients to make individualized decisions about prostate cancer screening.
Another reason relates to 5-alpha-reductase inhibitors. Although their role in the management of prostate cancer is currently not well defined, a reduction of almost 25% in the risk of prostate cancer and improvement in the performance of PSA has been reported.27
And yet another reason is that there are alternate strategies to manage the majority of patients who have been diagnosed with low-risk disease through transrectal ultrasound biopsy. The ERSPC study mentions multiparametric magnetic resonance imaging combined with targeted biopsy to identify high-grade disease.28,29 Genetic and epigenetic assays of the biopsied tissue can help grade disease based on aggressiveness.30 Transperineal mapping biopsy using a mapping software program can identify specific disease sites within the prostate gland, so that patients can be offered the option of targeted therapy.30
Continue to: Applying shared decision-making to prostate cancer screening
Applying shared decision-making to prostate cancer screening
Balancing errors of omission with errors of commission is challenging. Shared decision-making (SDM) is an approach whereby clinicians and patients share the best available evidence when faced with the task of medical decision-making and in which patients are supported while they consider their options and achieve their preferences.31 SDM is well supported by evidence from a number of RCTs and results in increased knowledge, involvement, and confidence on the part of patients.32 An individualized approach using the schematic diagram (FIGURE 13,18) may be helpful.
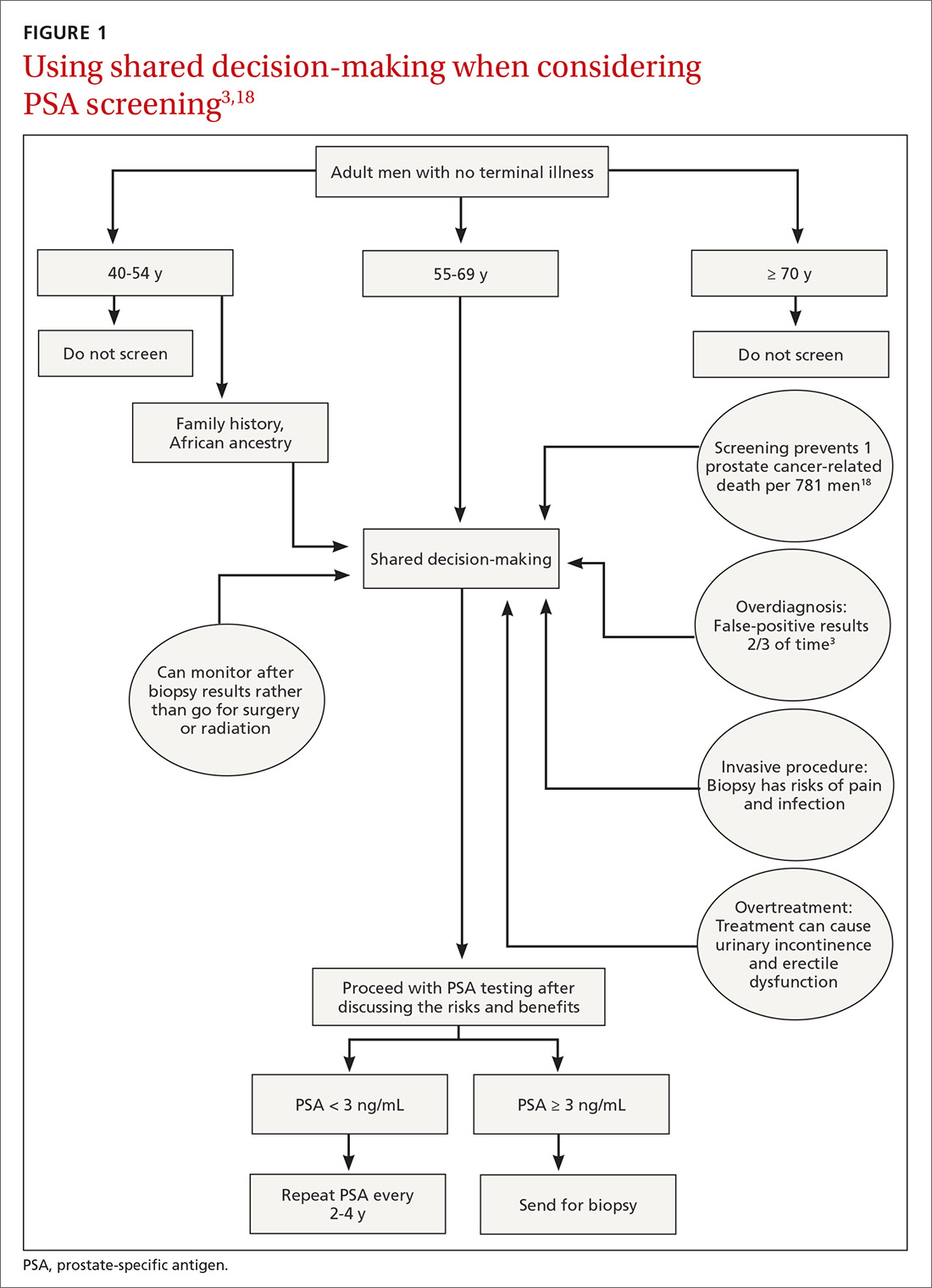
Barriers to SDM success. Many factors can interfere with the success of SDM including limited or poor communication; lack of time during busy office visits; and patients’ cultural, informational, and/or emotional needs. To improve patient-centered communication, we can: (1) make information understandable and available to patients and families; (2) prioritize training in communication; (3) use decision aid tools to facilitate communication; and (4) work to improve the payment model to incentivize patient-centered communication. Tools that facilitate SDM include videotapes, patient group discussions, brief scripts read to patients, and informational pamphlets. One such tool is the American Society for Clinical Oncology’s decision aid tool for PSA testing.33
Limited knowledge among patients. Decisions regarding treatment among men diagnosed with localized prostate cancer can be difficult because there are several treatment options with similar prognoses, but there are differences in adverse effects. One population-based cohort study of men with newly diagnosed localized prostate cancer found that most men had significant knowledge deficits regarding the survival benefits of the 2 major treatment options—surgery and radiation.34 In a large population-based study, 38% of men with localized prostate cancer reported receiving help from their primary care providers in the decision-making process for treatment.35
Learning to employ SDM. Elwyn et al proposed a 3-step model to incorporate SDM into clinical practice.31 They described key steps that include: choice talk (making sure patients are informed about the reasonable options), option talk (providing more detailed information about the options), and decision talk (supporting the work of patients considering their preferences and deciding what is best). Properly employing these methods requires training using simulations.31
The bottom line
Although current guidelines regarding PSA screening differ by organization, generally speaking PSA screening should be offered only to men with a life expectancy > 10 years. The PSA test has low sensitivity and specificity and lacks a clear cut-off value that warrants prostate biopsy. Men who choose to have PSA testing increase their chances of detecting prostate cancer, but most prostate cancers are slow growing and do not cause death. The decision to undergo PSA screening should be made by both the provider and the patient, after a discussion of the limited benefits and associated harms. The interval of follow-up screening may vary from 2 to 4 years depending on patient age, level of PSA, and whether a patient is taking medications such as 5-alpha-reductase inhibitors.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 Metro Health Medical Drive, Cleveland, Ohio 44109; [email protected].
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed January 16, 2020.
3. American Cancer Society. Key statistics for prostate cancer. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed January 16, 2020.
4. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152-156.
5. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444-449.
6. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-1161.
7. Jacobsen SJ, Katusic SK, Bergstraih EJ. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA. 1995;274:1445-1449.
8. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95-101.
9. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Int Med. 2013;158:761-769.
10. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
11. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
12. American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed January 16, 2020.
13. American Urologic Association. Early detection of prostate cancer (2018). Reviewed 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed January 16, 2020.
14. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
15 Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2012;157:120-134.
16. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83-91.
17. Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20-year follow-up. BMJ. 2011;342:d1539.
18. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027-2035.
19. McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719-725.
20 Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826-830.
21. Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830-1834.
22. Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-1437.
23. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
24. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132-142.
25. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2018;379:2319-2329.
26. Hersch JK, Nickel BL, Ghanouni A, et al. Improving communication about cancer screening: moving towards informed decision making. Public Health Res Pract. 2017;27(2).
27. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484-e492.
28. Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285.
29. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380-1386.
30. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778-787.
31. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367.
32. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
33. ASCO. Decision aid tool: prostate cancer screening with PSA testing. https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf. Accessed January 16, 2020.
34. Daum LM, Reamer EN, Ruterbusch JJ, et al. Patient knowledge and qualities of treatment decisions for localized prostate cancer. J Am Board Fam Med. 2017;30:288-297.
35. Radhakrishnan A, Grande D, Ross M, et al. When primary care providers (PCPs) help patients choose prostate cancer treatment. J Am Board Fam Med. 2017;30:298-307.
Prostate cancer is the most frequently diagnosed cancer in men and the third leading cause of cancer death in men worldwide.1 An estimated 174,650 new cases are diagnosed each year in the United States; 31,620 American men die annually from the disease.2 Although prostate cancer can be a serious disease, many men do not die from it. In fact, 2.9 million men who were diagnosed with prostate cancer at some point are alive today.3
Risk factors. Prostate cancer develops mainly in men ages ≥ 65 years and rarely occurs before age 40. In addition to age, family history and African American ethnicity are the major nonmodifiable risk factors for prostate cancer.4 From the 1970s to the most recent statistical analysis of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, African American men have continued to have significantly higher incidence of, and mortality rates from, prostate cancer than their European American counterparts. African American men are also more likely than men of European ancestry to have aggressive prostate cancers.5 Other risk factors include geographic location (higher risk in Northern Europe, North America, and Australia; lower risk in Asia, Africa, and South and Central America), mutations in the BRCA2 gene, and hereditary non-polyposis colon cancer syndrome.4
Prostate-specific antigen (PSA) was first used as a screening tool for prostate cancer in 1991.6 Prostate cancer incidence, especially organ-confined disease, has dramatically increased since then.7 PSA testing has a low sensitivity and specificity for the detection of prostate cancer, and there is no clear threshold at which biopsy can or should be offered. The most commonly used cutoff value of 4 ng/mL has a false-positive rate of about 70%.8
Benign prostatic conditions such as hypertrophy and infection can elevate PSA levels. In addition, the PSA test does not distinguish between aggressive and slow-growing cancers, and about 15% of patients with prostate cancer have a normal PSA level.9
A word about the digital rectal exam. While PSA testing has been the mainstay of prostate cancer screening, a few studies have included digital rectal exam (DRE) in their protocols. Data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that DRE captured an additional 2% of men with prostate cancer in the setting of a normal PSA test result.10 In the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial, the overall detection rate for prostate cancer was found to be better when DRE was combined with PSA and prostate biopsy than when DRE was used alone (4.5% vs 2.5%).11 Nevertheless, generally speaking, DRE can be omitted in the era of PSA screening.
Screening guidelines vary
Recommendations for prostate cancer screening vary by organization and are summarized in TABLE 1.9,12-14 In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer (Category D).15 In 2018, USPSTF provided an update with a new recommendation that clinicians inform men ages 55 to 69 years about the potential benefits and harms of PSA-based screening (Category C).14 The USPSTF continues to recommend against PSA-based screening for men ages ≥ 70 years (Category D).14

Does PSA-based screening improve patient-centered outcomes?
Several randomized controlled trials (RCTs) such as the Quebec Prospective Randomized Controlled Trial,16 the Norrköping Sweden Study,17 ERSPC,11 and PLCO10 have been conducted to assess the benefits of PSA testing. PLCO and ERSPC have contributed significantly to our understanding of prostate cancer screening even though their 13-year follow-up results are conflicting (TABLE 2).10,11,18

Continue to: In the ERSPC 13-year follow-up publication...
In the ERSPC 13-year follow-up publication, the authors concluded that a substantial reduction in prostate cancer mortality is attributable to testing with PSA.18 Despite limitations in the study design (eg, France entered after 2 years, screening intervals varied between 2 and 4 years, biopsy indications varied, and screening was discontinued at different times), PSA screening detected more prostate cancer than was detected in the control arm (10.2% vs 6.8%).
In the initial 11 years of follow-up, the study group experienced a 21% reduction in prostate cancer mortality, even though the absolute decrease ranged from only 0.6% (545 per 89,352) to 0.5% (355 per 72,891). The updated absolute risk reduction of death from prostate cancer at 13 years of follow-up showed a larger benefit: 0.11 per 1000 person-years or 1.28 per 1000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 (95% confidence interval [CI], 490-1929) men invited for screening, or 1 per 27 (17-66) additional prostate cancers detected.
The PLCO trial did not show any significant difference in prostate cancer detection (11.1% screened vs 9.9% control), and there was no improvement in prostate cancer mortality (3.7 vs 3.4 death per 10,000 person-years).10 However, the PLCO trial suffered from issues of contamination, which may have influenced the overall results. About 52% of men in the control (usual care) group received a PSA test at some point during the study. And more than two-thirds of the men who had a prostate biopsy because of a positive PSA test did not have prostate cancer.
Community standards for the PSA threshold for biopsy were applied in various centers (> 4 ng/ml in general) in PLCO, whereas in ERSPC, a cut-off PSA value ≥ 3 ng/mL was used for biopsy. Because of the lower PSA threshold, ERSPC may have identified cancers that would have had good outcomes without any intervention.
The harms of PSA screening
While it is unclear whether PSA screening results in any improvement in patient-centered outcomes, it does lead to downstream intervention due to overdiagnosis, which precipitates unnecessary anxiety, biopsies, and overtreatment (eg, excess radiation, overuse of androgen deprivation therapy).19 Biopsies carry the risk of hematuria (22.6%), hematospermia (50.4%), and urinary tract infection.20 Data from SEER-Medicare showed that prostate biopsy was associated with a 2.65-fold increased risk of hospitalization within 30 days of the procedure compared to a control population.21
Continue to: Overdiagnosis leads to overtreatment...
Overdiagnosis leads to overtreatment of low-risk prostate cancer. Both traditional treatment options for prostate cancer—radical prostatectomy and radiotherapy—are associated with urinary incontinence, erectile dysfunction, and issues with bowel function.22,23
The Prostate Cancer Intervention vs Observation Trial (PIVOT),24 the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4),25 and the Prostate Testing for Cancer and Treatment (ProtecT) trial,22,23 are the major RCTs that looked at the outcomes of treatment modalities for localized prostate cancer in the modern era of PSA testing.
PIVOT compared passive observation with radical prostatectomy.24 After 20 years of follow-up on 731 patients, the researchers concluded that radical prostatectomy did not reduce all-cause or prostate cancer–related mortality (TABLE 3).24

SPCG-4 showed survival benefits for men who underwent radical prostatectomy compared with men in a watchful waiting group, but only 5% of the study cohort had cancer detected by PSA screening (TABLE 4).25 The rest had either palpable tumors or symptoms of a tumor.

ProtecT, which followed patients with localized prostate cancer for more than 10 years,compared the outcomes and adverse effects of active surveillance, radical prostatectomy, and radiotherapy.23 Prostate cancer–specific mortality was low irrespective of the treatment,23 and there was no significant difference in all-cause mortality or prostate cancer–specific mortality between the 3 treatment groups.23 The active surveillance group had considerably fewer adverse events.22,23 The incidence rates of erectile dysfunction and urinary incontinence at the 1- and 6-year follow-up marks are outlined in TABLE 5.22

Continue to: The purpose of active monitoring...
The purpose of active monitoring is to minimize overtreatment by avoiding immediate radical intervention. Radical treatments with curative intent can be undertaken at any point while patients are being actively monitored. It is important to note that the active monitoring that took place in ProtecT23 was very different from the passive surveillance of PIVOT24 and SPCG-4.25 In ProtecT, once an elevated serum PSA level was noted, PSA levels were monitored every 3 months in the first year and every 6 to 12 months thereafter.23 Triggers to reassess patients and consider a change in clinical management were based largely on changes in PSA levels. Participants with an increase of at least 50% in PSA level during the previous 12 months were offered either continued monitoring or treatment after further testing.
Making individualized decisions about prostate cancer screening
Traditionally, the goal of cancer screening has been to maximize the number of people screened. Generally, the information provided to patients about cancer screening emphasizes the benefits and minimizes the harms. Recently, however, there has been a shift in communication about cancer screening with the emphasis now being placed on informed decision-making and encouraging patients to make individual decisions about screening participation.26
The treatment option of active surveillance, with its lower incidence of adverse outcomes, is an important reason for patients to make individualized decisions about prostate cancer screening.
Another reason relates to 5-alpha-reductase inhibitors. Although their role in the management of prostate cancer is currently not well defined, a reduction of almost 25% in the risk of prostate cancer and improvement in the performance of PSA has been reported.27
And yet another reason is that there are alternate strategies to manage the majority of patients who have been diagnosed with low-risk disease through transrectal ultrasound biopsy. The ERSPC study mentions multiparametric magnetic resonance imaging combined with targeted biopsy to identify high-grade disease.28,29 Genetic and epigenetic assays of the biopsied tissue can help grade disease based on aggressiveness.30 Transperineal mapping biopsy using a mapping software program can identify specific disease sites within the prostate gland, so that patients can be offered the option of targeted therapy.30
Continue to: Applying shared decision-making to prostate cancer screening
Applying shared decision-making to prostate cancer screening
Balancing errors of omission with errors of commission is challenging. Shared decision-making (SDM) is an approach whereby clinicians and patients share the best available evidence when faced with the task of medical decision-making and in which patients are supported while they consider their options and achieve their preferences.31 SDM is well supported by evidence from a number of RCTs and results in increased knowledge, involvement, and confidence on the part of patients.32 An individualized approach using the schematic diagram (FIGURE 13,18) may be helpful.

Barriers to SDM success. Many factors can interfere with the success of SDM including limited or poor communication; lack of time during busy office visits; and patients’ cultural, informational, and/or emotional needs. To improve patient-centered communication, we can: (1) make information understandable and available to patients and families; (2) prioritize training in communication; (3) use decision aid tools to facilitate communication; and (4) work to improve the payment model to incentivize patient-centered communication. Tools that facilitate SDM include videotapes, patient group discussions, brief scripts read to patients, and informational pamphlets. One such tool is the American Society for Clinical Oncology’s decision aid tool for PSA testing.33
Limited knowledge among patients. Decisions regarding treatment among men diagnosed with localized prostate cancer can be difficult because there are several treatment options with similar prognoses, but there are differences in adverse effects. One population-based cohort study of men with newly diagnosed localized prostate cancer found that most men had significant knowledge deficits regarding the survival benefits of the 2 major treatment options—surgery and radiation.34 In a large population-based study, 38% of men with localized prostate cancer reported receiving help from their primary care providers in the decision-making process for treatment.35
Learning to employ SDM. Elwyn et al proposed a 3-step model to incorporate SDM into clinical practice.31 They described key steps that include: choice talk (making sure patients are informed about the reasonable options), option talk (providing more detailed information about the options), and decision talk (supporting the work of patients considering their preferences and deciding what is best). Properly employing these methods requires training using simulations.31
The bottom line
Although current guidelines regarding PSA screening differ by organization, generally speaking PSA screening should be offered only to men with a life expectancy > 10 years. The PSA test has low sensitivity and specificity and lacks a clear cut-off value that warrants prostate biopsy. Men who choose to have PSA testing increase their chances of detecting prostate cancer, but most prostate cancers are slow growing and do not cause death. The decision to undergo PSA screening should be made by both the provider and the patient, after a discussion of the limited benefits and associated harms. The interval of follow-up screening may vary from 2 to 4 years depending on patient age, level of PSA, and whether a patient is taking medications such as 5-alpha-reductase inhibitors.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 Metro Health Medical Drive, Cleveland, Ohio 44109; [email protected].
Prostate cancer is the most frequently diagnosed cancer in men and the third leading cause of cancer death in men worldwide.1 An estimated 174,650 new cases are diagnosed each year in the United States; 31,620 American men die annually from the disease.2 Although prostate cancer can be a serious disease, many men do not die from it. In fact, 2.9 million men who were diagnosed with prostate cancer at some point are alive today.3
Risk factors. Prostate cancer develops mainly in men ages ≥ 65 years and rarely occurs before age 40. In addition to age, family history and African American ethnicity are the major nonmodifiable risk factors for prostate cancer.4 From the 1970s to the most recent statistical analysis of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, African American men have continued to have significantly higher incidence of, and mortality rates from, prostate cancer than their European American counterparts. African American men are also more likely than men of European ancestry to have aggressive prostate cancers.5 Other risk factors include geographic location (higher risk in Northern Europe, North America, and Australia; lower risk in Asia, Africa, and South and Central America), mutations in the BRCA2 gene, and hereditary non-polyposis colon cancer syndrome.4
Prostate-specific antigen (PSA) was first used as a screening tool for prostate cancer in 1991.6 Prostate cancer incidence, especially organ-confined disease, has dramatically increased since then.7 PSA testing has a low sensitivity and specificity for the detection of prostate cancer, and there is no clear threshold at which biopsy can or should be offered. The most commonly used cutoff value of 4 ng/mL has a false-positive rate of about 70%.8
Benign prostatic conditions such as hypertrophy and infection can elevate PSA levels. In addition, the PSA test does not distinguish between aggressive and slow-growing cancers, and about 15% of patients with prostate cancer have a normal PSA level.9
A word about the digital rectal exam. While PSA testing has been the mainstay of prostate cancer screening, a few studies have included digital rectal exam (DRE) in their protocols. Data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that DRE captured an additional 2% of men with prostate cancer in the setting of a normal PSA test result.10 In the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial, the overall detection rate for prostate cancer was found to be better when DRE was combined with PSA and prostate biopsy than when DRE was used alone (4.5% vs 2.5%).11 Nevertheless, generally speaking, DRE can be omitted in the era of PSA screening.
Screening guidelines vary
Recommendations for prostate cancer screening vary by organization and are summarized in TABLE 1.9,12-14 In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer (Category D).15 In 2018, USPSTF provided an update with a new recommendation that clinicians inform men ages 55 to 69 years about the potential benefits and harms of PSA-based screening (Category C).14 The USPSTF continues to recommend against PSA-based screening for men ages ≥ 70 years (Category D).14

Does PSA-based screening improve patient-centered outcomes?
Several randomized controlled trials (RCTs) such as the Quebec Prospective Randomized Controlled Trial,16 the Norrköping Sweden Study,17 ERSPC,11 and PLCO10 have been conducted to assess the benefits of PSA testing. PLCO and ERSPC have contributed significantly to our understanding of prostate cancer screening even though their 13-year follow-up results are conflicting (TABLE 2).10,11,18

Continue to: In the ERSPC 13-year follow-up publication...
In the ERSPC 13-year follow-up publication, the authors concluded that a substantial reduction in prostate cancer mortality is attributable to testing with PSA.18 Despite limitations in the study design (eg, France entered after 2 years, screening intervals varied between 2 and 4 years, biopsy indications varied, and screening was discontinued at different times), PSA screening detected more prostate cancer than was detected in the control arm (10.2% vs 6.8%).
In the initial 11 years of follow-up, the study group experienced a 21% reduction in prostate cancer mortality, even though the absolute decrease ranged from only 0.6% (545 per 89,352) to 0.5% (355 per 72,891). The updated absolute risk reduction of death from prostate cancer at 13 years of follow-up showed a larger benefit: 0.11 per 1000 person-years or 1.28 per 1000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 (95% confidence interval [CI], 490-1929) men invited for screening, or 1 per 27 (17-66) additional prostate cancers detected.
The PLCO trial did not show any significant difference in prostate cancer detection (11.1% screened vs 9.9% control), and there was no improvement in prostate cancer mortality (3.7 vs 3.4 death per 10,000 person-years).10 However, the PLCO trial suffered from issues of contamination, which may have influenced the overall results. About 52% of men in the control (usual care) group received a PSA test at some point during the study. And more than two-thirds of the men who had a prostate biopsy because of a positive PSA test did not have prostate cancer.
Community standards for the PSA threshold for biopsy were applied in various centers (> 4 ng/ml in general) in PLCO, whereas in ERSPC, a cut-off PSA value ≥ 3 ng/mL was used for biopsy. Because of the lower PSA threshold, ERSPC may have identified cancers that would have had good outcomes without any intervention.
The harms of PSA screening
While it is unclear whether PSA screening results in any improvement in patient-centered outcomes, it does lead to downstream intervention due to overdiagnosis, which precipitates unnecessary anxiety, biopsies, and overtreatment (eg, excess radiation, overuse of androgen deprivation therapy).19 Biopsies carry the risk of hematuria (22.6%), hematospermia (50.4%), and urinary tract infection.20 Data from SEER-Medicare showed that prostate biopsy was associated with a 2.65-fold increased risk of hospitalization within 30 days of the procedure compared to a control population.21
Continue to: Overdiagnosis leads to overtreatment...
Overdiagnosis leads to overtreatment of low-risk prostate cancer. Both traditional treatment options for prostate cancer—radical prostatectomy and radiotherapy—are associated with urinary incontinence, erectile dysfunction, and issues with bowel function.22,23
The Prostate Cancer Intervention vs Observation Trial (PIVOT),24 the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4),25 and the Prostate Testing for Cancer and Treatment (ProtecT) trial,22,23 are the major RCTs that looked at the outcomes of treatment modalities for localized prostate cancer in the modern era of PSA testing.
PIVOT compared passive observation with radical prostatectomy.24 After 20 years of follow-up on 731 patients, the researchers concluded that radical prostatectomy did not reduce all-cause or prostate cancer–related mortality (TABLE 3).24

SPCG-4 showed survival benefits for men who underwent radical prostatectomy compared with men in a watchful waiting group, but only 5% of the study cohort had cancer detected by PSA screening (TABLE 4).25 The rest had either palpable tumors or symptoms of a tumor.

ProtecT, which followed patients with localized prostate cancer for more than 10 years,compared the outcomes and adverse effects of active surveillance, radical prostatectomy, and radiotherapy.23 Prostate cancer–specific mortality was low irrespective of the treatment,23 and there was no significant difference in all-cause mortality or prostate cancer–specific mortality between the 3 treatment groups.23 The active surveillance group had considerably fewer adverse events.22,23 The incidence rates of erectile dysfunction and urinary incontinence at the 1- and 6-year follow-up marks are outlined in TABLE 5.22

Continue to: The purpose of active monitoring...
The purpose of active monitoring is to minimize overtreatment by avoiding immediate radical intervention. Radical treatments with curative intent can be undertaken at any point while patients are being actively monitored. It is important to note that the active monitoring that took place in ProtecT23 was very different from the passive surveillance of PIVOT24 and SPCG-4.25 In ProtecT, once an elevated serum PSA level was noted, PSA levels were monitored every 3 months in the first year and every 6 to 12 months thereafter.23 Triggers to reassess patients and consider a change in clinical management were based largely on changes in PSA levels. Participants with an increase of at least 50% in PSA level during the previous 12 months were offered either continued monitoring or treatment after further testing.
Making individualized decisions about prostate cancer screening
Traditionally, the goal of cancer screening has been to maximize the number of people screened. Generally, the information provided to patients about cancer screening emphasizes the benefits and minimizes the harms. Recently, however, there has been a shift in communication about cancer screening with the emphasis now being placed on informed decision-making and encouraging patients to make individual decisions about screening participation.26
The treatment option of active surveillance, with its lower incidence of adverse outcomes, is an important reason for patients to make individualized decisions about prostate cancer screening.
Another reason relates to 5-alpha-reductase inhibitors. Although their role in the management of prostate cancer is currently not well defined, a reduction of almost 25% in the risk of prostate cancer and improvement in the performance of PSA has been reported.27
And yet another reason is that there are alternate strategies to manage the majority of patients who have been diagnosed with low-risk disease through transrectal ultrasound biopsy. The ERSPC study mentions multiparametric magnetic resonance imaging combined with targeted biopsy to identify high-grade disease.28,29 Genetic and epigenetic assays of the biopsied tissue can help grade disease based on aggressiveness.30 Transperineal mapping biopsy using a mapping software program can identify specific disease sites within the prostate gland, so that patients can be offered the option of targeted therapy.30
Continue to: Applying shared decision-making to prostate cancer screening
Applying shared decision-making to prostate cancer screening
Balancing errors of omission with errors of commission is challenging. Shared decision-making (SDM) is an approach whereby clinicians and patients share the best available evidence when faced with the task of medical decision-making and in which patients are supported while they consider their options and achieve their preferences.31 SDM is well supported by evidence from a number of RCTs and results in increased knowledge, involvement, and confidence on the part of patients.32 An individualized approach using the schematic diagram (FIGURE 13,18) may be helpful.

Barriers to SDM success. Many factors can interfere with the success of SDM including limited or poor communication; lack of time during busy office visits; and patients’ cultural, informational, and/or emotional needs. To improve patient-centered communication, we can: (1) make information understandable and available to patients and families; (2) prioritize training in communication; (3) use decision aid tools to facilitate communication; and (4) work to improve the payment model to incentivize patient-centered communication. Tools that facilitate SDM include videotapes, patient group discussions, brief scripts read to patients, and informational pamphlets. One such tool is the American Society for Clinical Oncology’s decision aid tool for PSA testing.33
Limited knowledge among patients. Decisions regarding treatment among men diagnosed with localized prostate cancer can be difficult because there are several treatment options with similar prognoses, but there are differences in adverse effects. One population-based cohort study of men with newly diagnosed localized prostate cancer found that most men had significant knowledge deficits regarding the survival benefits of the 2 major treatment options—surgery and radiation.34 In a large population-based study, 38% of men with localized prostate cancer reported receiving help from their primary care providers in the decision-making process for treatment.35
Learning to employ SDM. Elwyn et al proposed a 3-step model to incorporate SDM into clinical practice.31 They described key steps that include: choice talk (making sure patients are informed about the reasonable options), option talk (providing more detailed information about the options), and decision talk (supporting the work of patients considering their preferences and deciding what is best). Properly employing these methods requires training using simulations.31
The bottom line
Although current guidelines regarding PSA screening differ by organization, generally speaking PSA screening should be offered only to men with a life expectancy > 10 years. The PSA test has low sensitivity and specificity and lacks a clear cut-off value that warrants prostate biopsy. Men who choose to have PSA testing increase their chances of detecting prostate cancer, but most prostate cancers are slow growing and do not cause death. The decision to undergo PSA screening should be made by both the provider and the patient, after a discussion of the limited benefits and associated harms. The interval of follow-up screening may vary from 2 to 4 years depending on patient age, level of PSA, and whether a patient is taking medications such as 5-alpha-reductase inhibitors.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 Metro Health Medical Drive, Cleveland, Ohio 44109; [email protected].
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed January 16, 2020.
3. American Cancer Society. Key statistics for prostate cancer. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed January 16, 2020.
4. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152-156.
5. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444-449.
6. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-1161.
7. Jacobsen SJ, Katusic SK, Bergstraih EJ. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA. 1995;274:1445-1449.
8. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95-101.
9. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Int Med. 2013;158:761-769.
10. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
11. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
12. American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed January 16, 2020.
13. American Urologic Association. Early detection of prostate cancer (2018). Reviewed 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed January 16, 2020.
14. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
15 Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2012;157:120-134.
16. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83-91.
17. Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20-year follow-up. BMJ. 2011;342:d1539.
18. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027-2035.
19. McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719-725.
20 Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826-830.
21. Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830-1834.
22. Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-1437.
23. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
24. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132-142.
25. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2018;379:2319-2329.
26. Hersch JK, Nickel BL, Ghanouni A, et al. Improving communication about cancer screening: moving towards informed decision making. Public Health Res Pract. 2017;27(2).
27. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484-e492.
28. Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285.
29. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380-1386.
30. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778-787.
31. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367.
32. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
33. ASCO. Decision aid tool: prostate cancer screening with PSA testing. https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf. Accessed January 16, 2020.
34. Daum LM, Reamer EN, Ruterbusch JJ, et al. Patient knowledge and qualities of treatment decisions for localized prostate cancer. J Am Board Fam Med. 2017;30:288-297.
35. Radhakrishnan A, Grande D, Ross M, et al. When primary care providers (PCPs) help patients choose prostate cancer treatment. J Am Board Fam Med. 2017;30:298-307.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed January 16, 2020.
3. American Cancer Society. Key statistics for prostate cancer. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed January 16, 2020.
4. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152-156.
5. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444-449.
6. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-1161.
7. Jacobsen SJ, Katusic SK, Bergstraih EJ. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA. 1995;274:1445-1449.
8. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95-101.
9. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Int Med. 2013;158:761-769.
10. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
11. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
12. American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed January 16, 2020.
13. American Urologic Association. Early detection of prostate cancer (2018). Reviewed 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed January 16, 2020.
14. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
15 Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2012;157:120-134.
16. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83-91.
17. Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20-year follow-up. BMJ. 2011;342:d1539.
18. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027-2035.
19. McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719-725.
20 Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826-830.
21. Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830-1834.
22. Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-1437.
23. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
24. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132-142.
25. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2018;379:2319-2329.
26. Hersch JK, Nickel BL, Ghanouni A, et al. Improving communication about cancer screening: moving towards informed decision making. Public Health Res Pract. 2017;27(2).
27. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484-e492.
28. Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285.
29. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380-1386.
30. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778-787.
31. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367.
32. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
33. ASCO. Decision aid tool: prostate cancer screening with PSA testing. https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf. Accessed January 16, 2020.
34. Daum LM, Reamer EN, Ruterbusch JJ, et al. Patient knowledge and qualities of treatment decisions for localized prostate cancer. J Am Board Fam Med. 2017;30:288-297.
35. Radhakrishnan A, Grande D, Ross M, et al. When primary care providers (PCPs) help patients choose prostate cancer treatment. J Am Board Fam Med. 2017;30:298-307.
PRACTICE RECOMMENDATIONS
› Recommend individualized decision-making to men ages 55 to 69 years after discussing the potential benefits and risks of prostate-specific antigen (PSA)-based screening. B
› Do not use a PSA-based screening method for prostate cancer in men ages < 50 years or > 70 years or men with a life expectancy < 10 years. C
› Do not routinely recommend PSA-based screening to men with a family history of prostate cancer or to men who are African American. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Scabies: Refine your exam, avoid these diagnostic pitfalls
It is estimated that up to 45% of cases of scabies are misdiagnosed as another condition.1 This can occur when common clinical features are overlooked, a skin exam is rushed (and the rash is chalked up to dermatitis), or the wrong part of the pruritic lesion is scraped (the papule, rather than the burrow). There are also atypical presentations of scabies, which can confound even the most astute clinician.1 Misdiagnosis can increase health care costs due to repeat office visits or multiple referrals. In this article, we review the typical and atypical presentations of scabies and provide recommendations to aid physicians in its early recognition and correct diagnosis.
The scope of scabies infection, and its clinical stages
The prevalence of scabies, a common skin infection caused by the mite Sarcoptes scabiei, is estimated at 300 million cases worldwide annually, with the greatest incidence occurring in children and adolescents.1 In the developing world, its clinical burden is highest among the homeless, those of lower socioeconomic status, and those with poor hygiene. In the developed world, the clinical burden is highest among hospitalized patients and residents of long-term living facilities.
The S scabiei mite is an obligate parasite that elicits an adaptive immune response in susceptible hosts. The female mite lays 60 to 90 eggs that mature into adult mites after completing the mite life cycle in human hosts. In immunocompetent patients, roughly 10 to 15 surviving mites can be found at any given point in the disease process.2 In crusted or disseminated scabies, which often occur in immunocompromised patients, thousands of mites may be found at any given point in the disease process. 2
Scabies infection has 2 stages: the latent primary infection and the symptomatic secondary infection.
The primary infection starts with the initial mite invasion, typically with the transfer of impregnated females during person-to-person contact. Females deposit eggs as they burrow into the epidermis at the level of the stratum corneum with the use of proteolytic enzymes (creating the mite burrow). Surviving eggs hatch into larvae that then mature into nymphs and adult mites. After these adult mites mate, the impregnated females create new burrows and lay additional eggs.3 Patients may be asymptomatic during this initial stage and the infection may be transmitted from person to person through direct skin contact.
The second stage of infection is when patients experience severe pruritus with inflammatory papules seen on exam. The pruritus associated with scabies results from a delayed type IV hypersensitivity reaction to mite infestation. This requires host sensitization to the scabies mite. Clinically, there is a delayed onset (weeks) of numerous erythematous papules and, later, excoriated papules.
Conditions that scabies can mimic
The differential of typical scabies includes diagnoses manifesting with moderate to severe pruritus. In the immunocompetent adult, conditions to consider are atopic dermatitis, tinea corporis, papular urticaria, seborrheic dermatitis, poison ivy and other causes of contact dermatitis, drug eruptions, and irritant dermatitis. In immunocompetent infants, think of seborrheic dermatitis, atopic dermatitis, acropustulosis, and viral exanthems.
Continue to: Nodular scabies variants...
Nodular scabies variants can masquerade as pseudolymphoma, lymphoma, or leukemia cutis. In immunocompromised and elderly individuals, crusted scabies is often mistaken for psoriasis, atopic dermatitis, keratoderma, and lichen planus.2,4,5
Scabies’ classic presentation
Typically, scabies causes intensely pruritic erythematous papules. Areas commonly affected are the webs and sides of fingers (FIGURE 1A and 1B), proximal palm and wrist flexors, extensor aspects of the arms and legs, axillary folds, periumbilical areas, the peri-areolar region in women, buttocks and thigh creases, and, in males, the genitals. The head may also be affected in children (FIGURE 1C), but seldom in adults. Interestingly, the back is usually spared across all age groups, though not always (FIGURE 2).
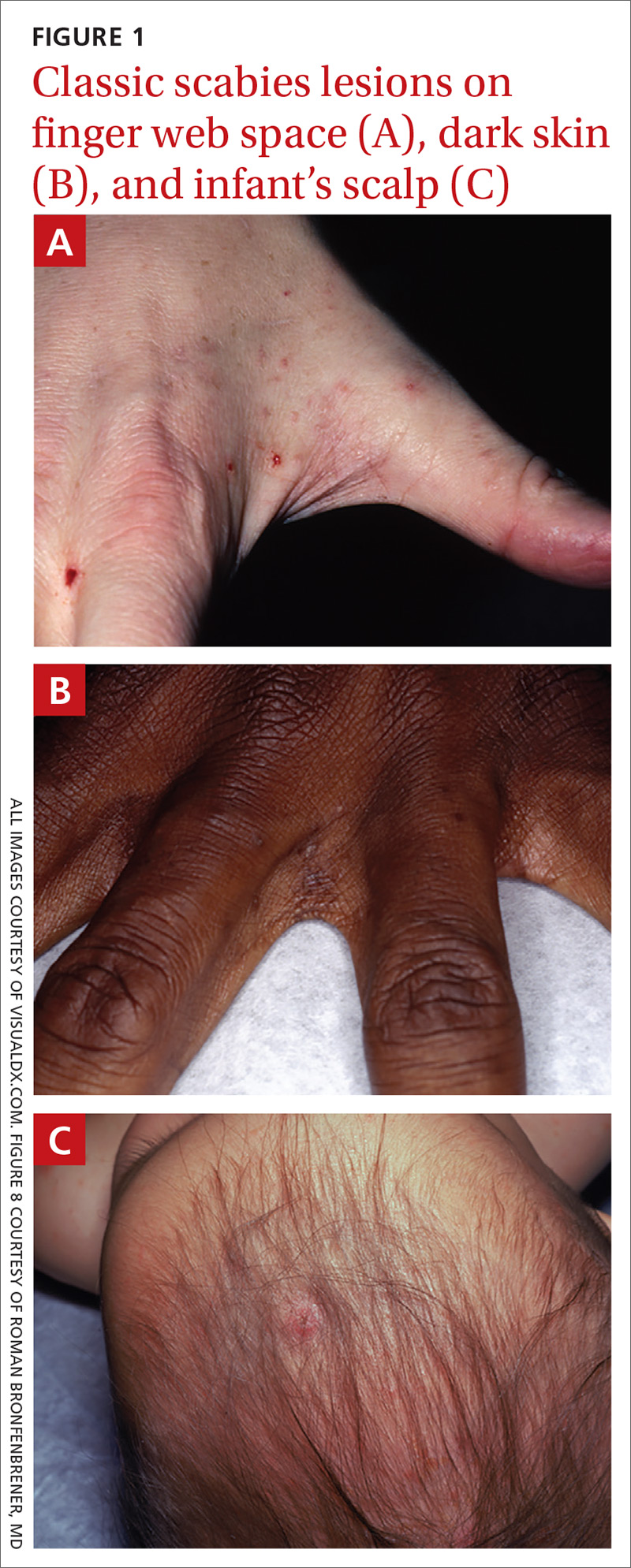
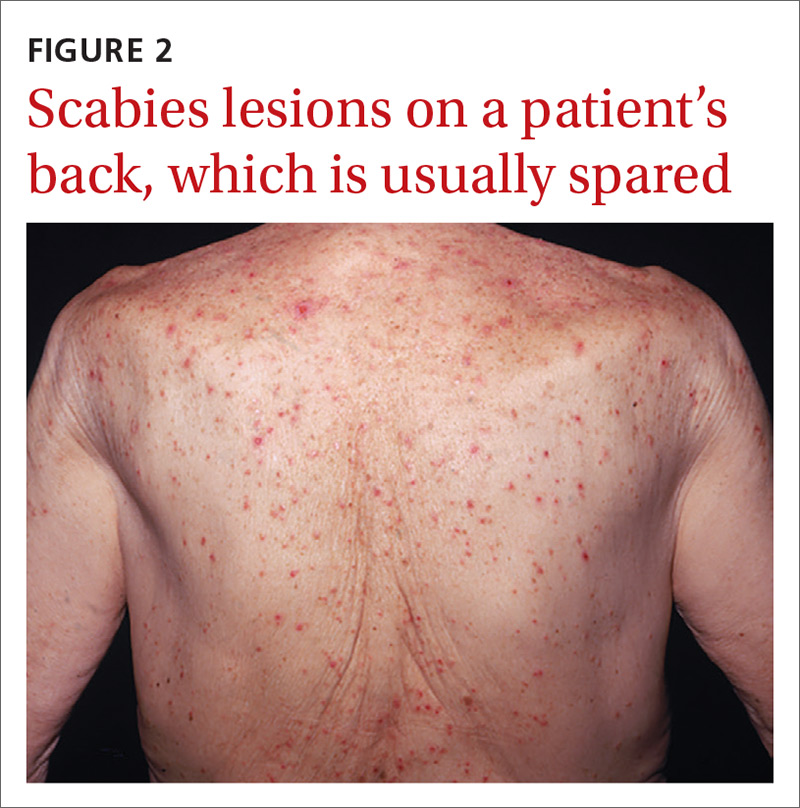
The classic presentation also varies across age groups and populations.2 In children, vesicles, pustules, and nodular pruritic lesions may coexist with eczema and impetigo. Among homeless individuals, coinfection with impetigo and eczema is common.
Scabies subtypes with varying presentations
Clinical manifestations of scabies subtypes may make it difficult to diagnose the disease. These subtypes include nodular, pustular, vesiculobullous, and crusted scabies (Norwegian scabies). Although rare, these subtypes merit acknowledgement, as atypical cases contribute to the high rate of misdiagnosis.
Nodular scabies is a clinical variant that accounts for about 7% of scabies cases.7 It can resist traditional scabies treatment (permethrin cream, ivermectin—which we’ll discuss in a bit) and often requires topical or intralesional corticosteroid management. Nodular scabies most commonly affects male genitalia. Patients may have multiple excoriated skin-colored erythematous papules and nodules in areas involving the classic distribution of scabies (web spaces of fingers, flexural areas [FIGURE 3], scrotum, and groin).8
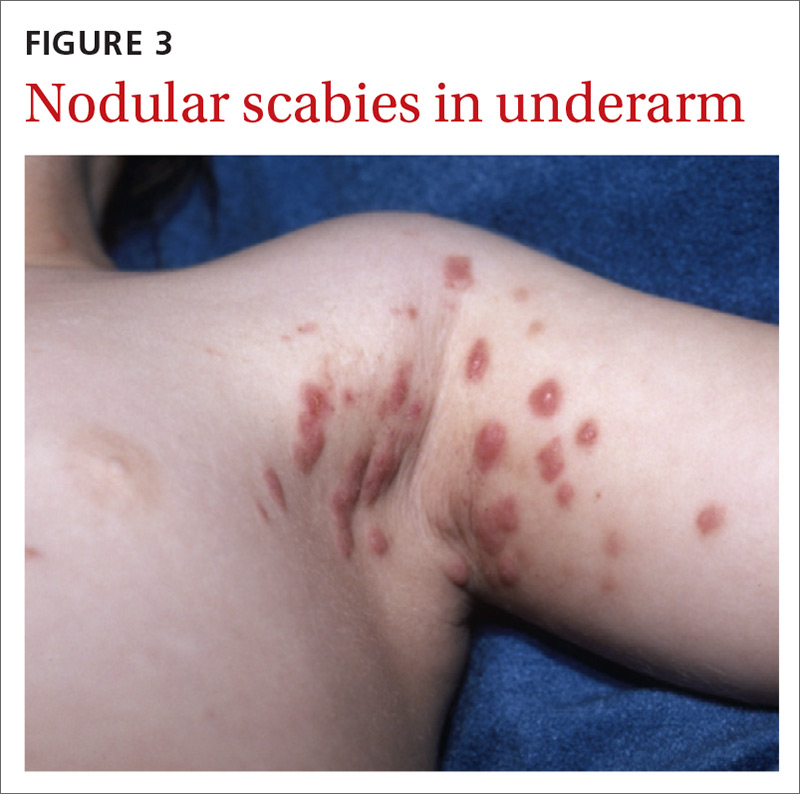
Continue to: Pustular scabies...
Pustular scabies is commonly seen in children and adolescents but can occur in all age groups. Patients may present with vesicular pustular lesions (FIGURE 4A and 4B).7 In some cases, topical corticosteroids have modified the classic clinical presentation into a pustular variant. However, scabies alone can cause pustular lesions.
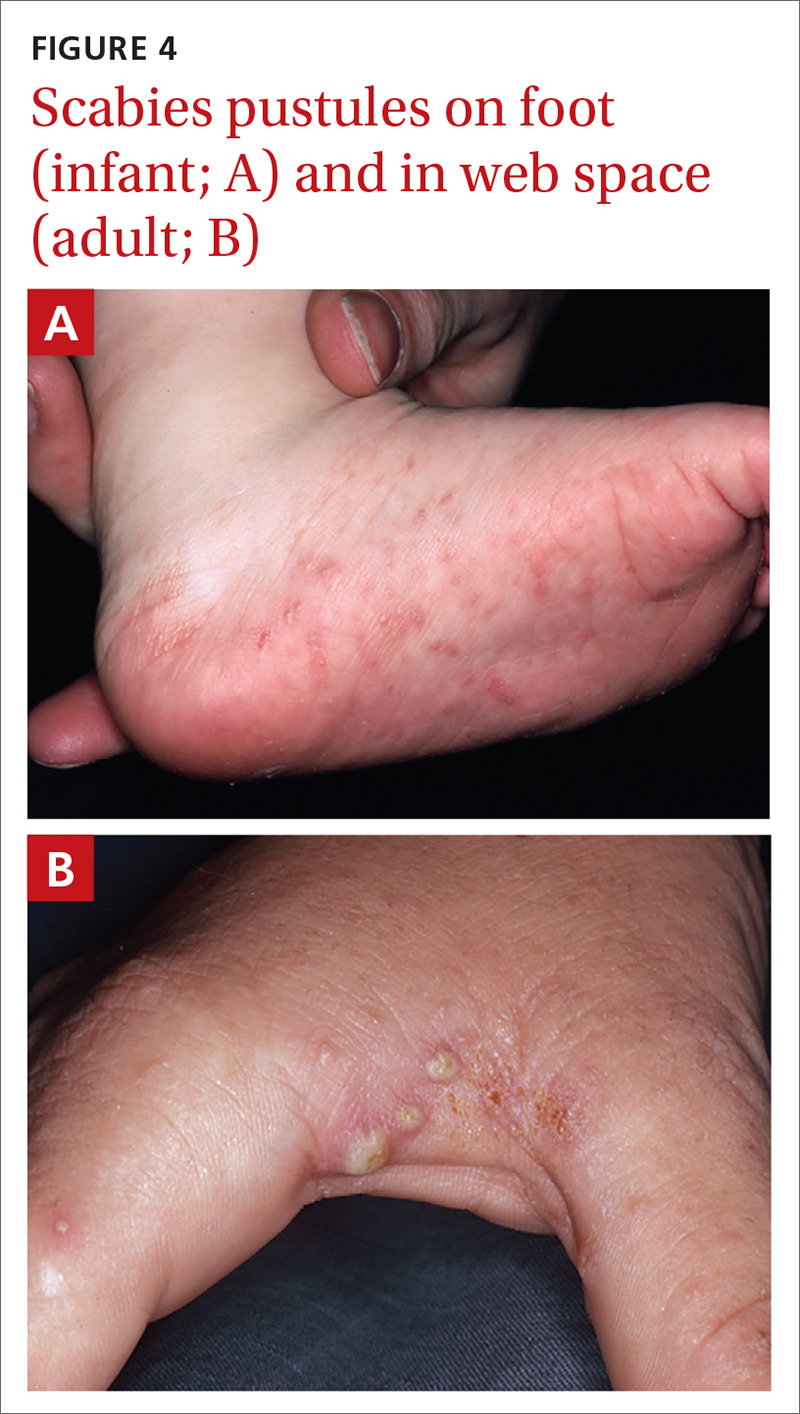
Vesiculobullous scabies (FIGURE 5A and 5B) is a clinical subtype that may be mistaken for pemphigus vulgaris9 or bullous pemphigoid10 because of its strikingly similar clinical presentation to those disorders.
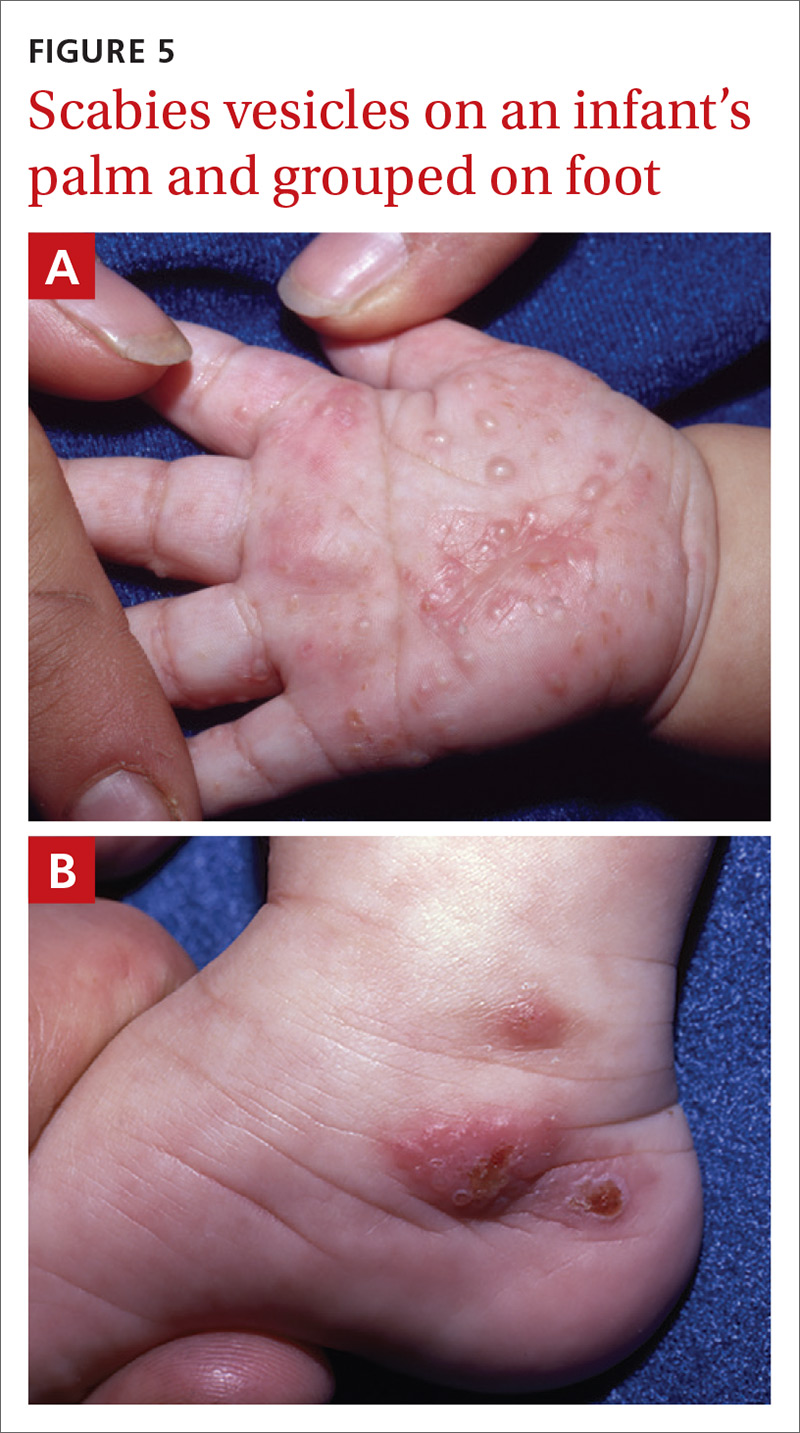
Crusted scabies (Norwegian scabies) is a severe, disseminated form of scabies that commonly affects immunocompromised patients, although cases are also seen in immunocompetent hosts. Afflicted immunocompetent patients often have a history of diabetes mellitus, liver cirrhosis, malnutrition, or alcohol abuse.11 Patients present with a thick powdery or crusted white or yellow scale involving the feet or hands (FIGURE 6) that sometimes extends onto the limbs. Severe cases can involve wide body surfaces. One unusual presentation also included a desquamating rash without pruritus.12
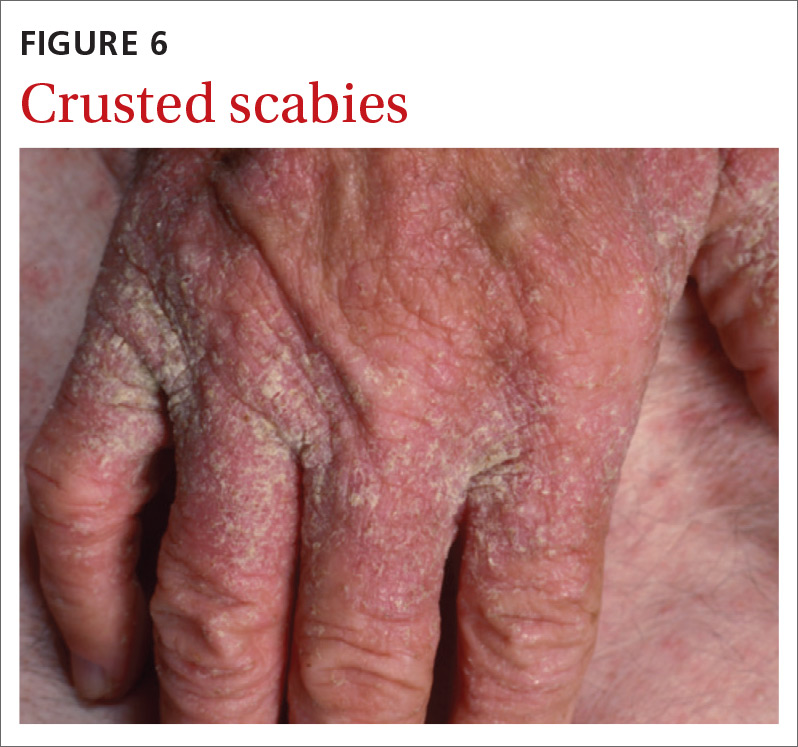
Highly atypical cases
Atypical presentations include lesions that appear outside of the classic distribution areas of scabies, lesions with uncharacteristic morphology, cases with coinfections, and instances in which patients are immunocompromised.13 Examples include scabies of the scalp coexisting with seborrheic dermatitis or dermatomyositis,14 scabies mimicking mastocytoma,15,16 and scabies with coinfections of impetigo or eczema.17 These coinfections and clinical variations can be particularly challenging. Other reports of atypical scabies leading to misdiagnosis include a case of crusted scabies mimicking erythrodermic psoriasis18 and a case initially attributed to suppurative folliculitis and prurigo nodularis.19
Decisive diagnostic measures
Identifying the mite’s burrow on clinical exam is essential to making the diagnosis of scabies. Microscopic examinations of burrow scrapings remain the diagnostic gold standard. Frequently, nondermatologists perform skin scrapings of the pruritic papules of scabies instead of the burrows. These papules are a hypersensitivity reaction to the mites, and no scabetic mites will be found there. Burrows appear on exam as small, linear, serpiginous lines (FIGURE 7A and 7B).
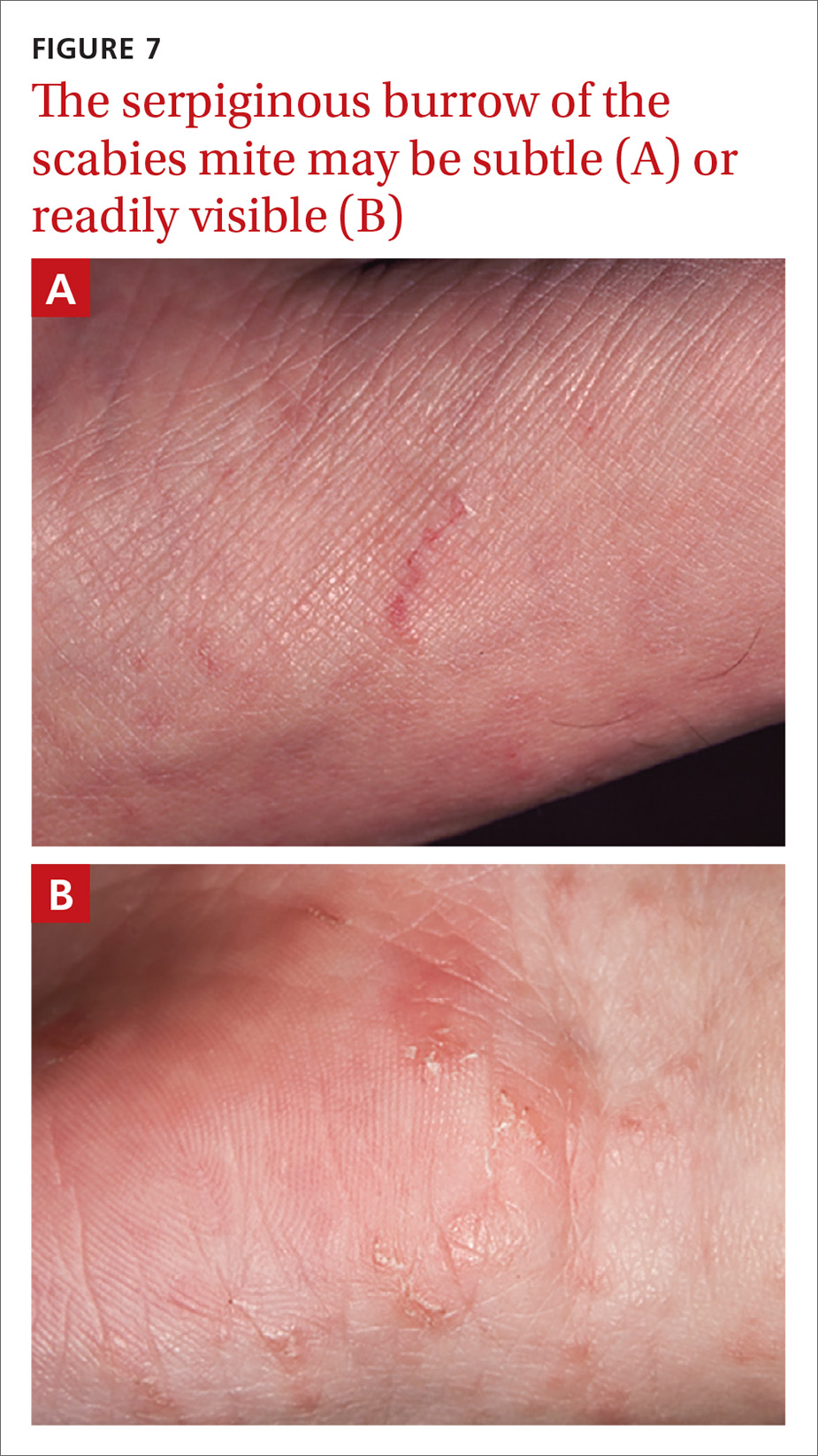
Continue to: Handheld illumination with a dermatoscope...
Handheld illumination with a dermatoscope will allow visualization of the mite (FIGURE 8). (See “How fast do scabies mites move? Dermoscopy video answers that question.”) Dermatoscope findings consistent with scabies include a “delta glider,” a dark triangular shape that is the mite’s head hidden in the burrow,20 or the classic “jet with contrail” (FIGURE 8).21 Scrape the burrow to discover the mature scabies mite (FIGURE 9) and confirm the diagnosis.
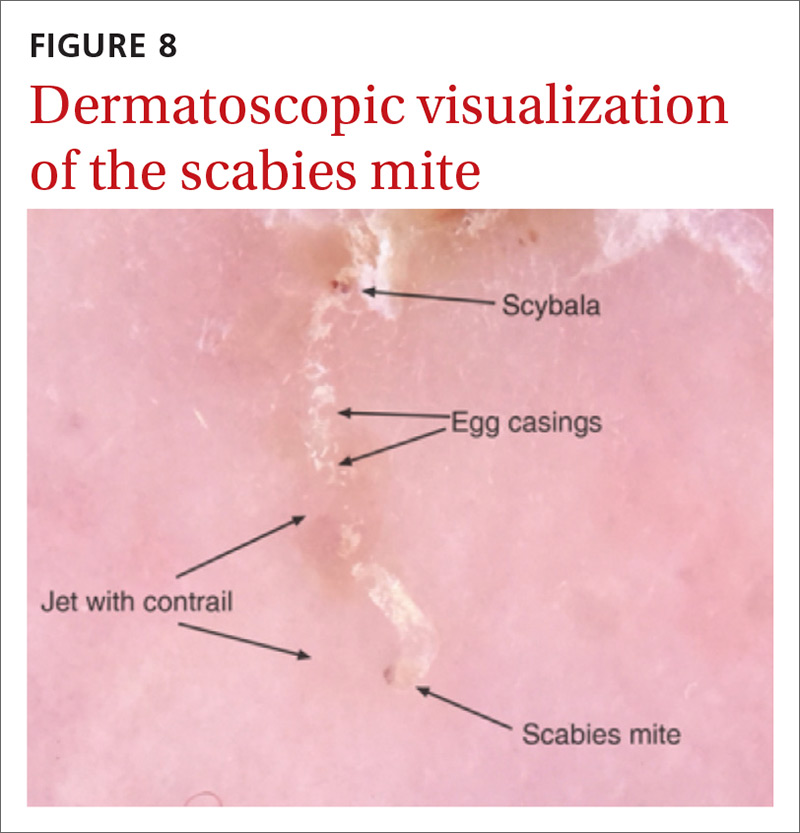
Microscopic examination of scrapings has a reported sensitivity and specificity of up to 90% and 100%, respectively, when collection of scrapings is performed accurately and contains ova, feces, or mites.14,20,22 Dermatologists increasingly use dermatoscopes to diagnose scabies. Dermoscopy's sensitivity is 91% and specificity is 85% to 86%,14 which is a reassuring frame of reference for physicians who do not routinely use dermatoscopes and instead rely on scrapings.
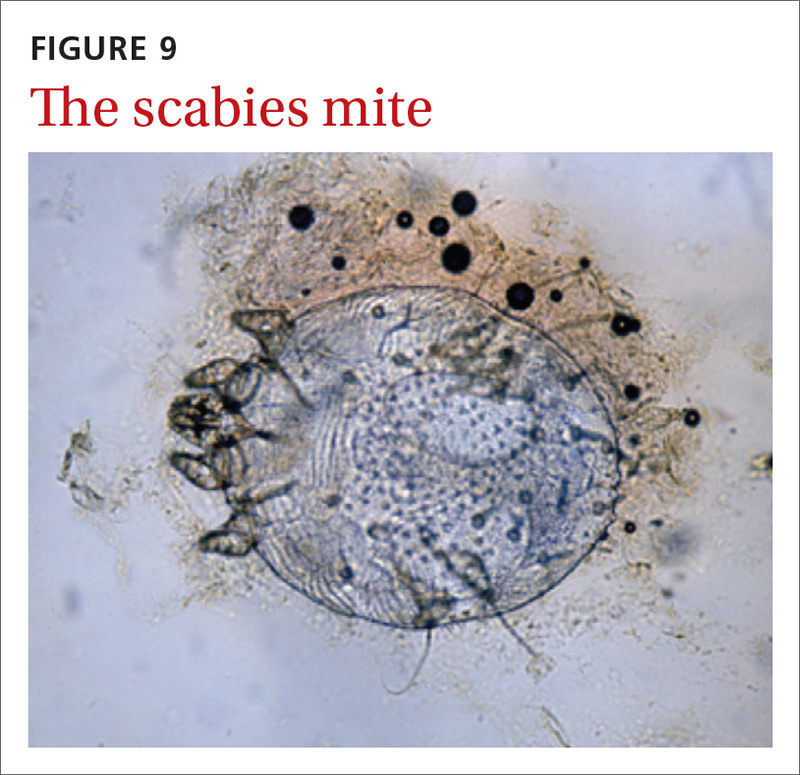
Continue to: How fast do scabies mites move? Dermoscopy video answers that question
SIDEBAR
How fast do scabies mites move? Dermoscopy video answers that question
Richard Usatine, MD; Ashfaq Marghoob, MD
University of Texas Health San Antonio (Dr. Usatine); Memorial Sloan Kettering Skin Cancer Center, Hauppauge, NY (Dr. Marghoob).
Dr. Marghoob reported that he has received honoraria for speaking for 3GEN. Dr. Usatine reported no potential conflict of interest relevant to this article.
A 22-year-old man infected with human immunodeficiency virus presented to a clinic with a 6-month history of intense pruritus. Physical examination revealed scale crust on the hands and wrists (FIGURE) and in the pubic region.
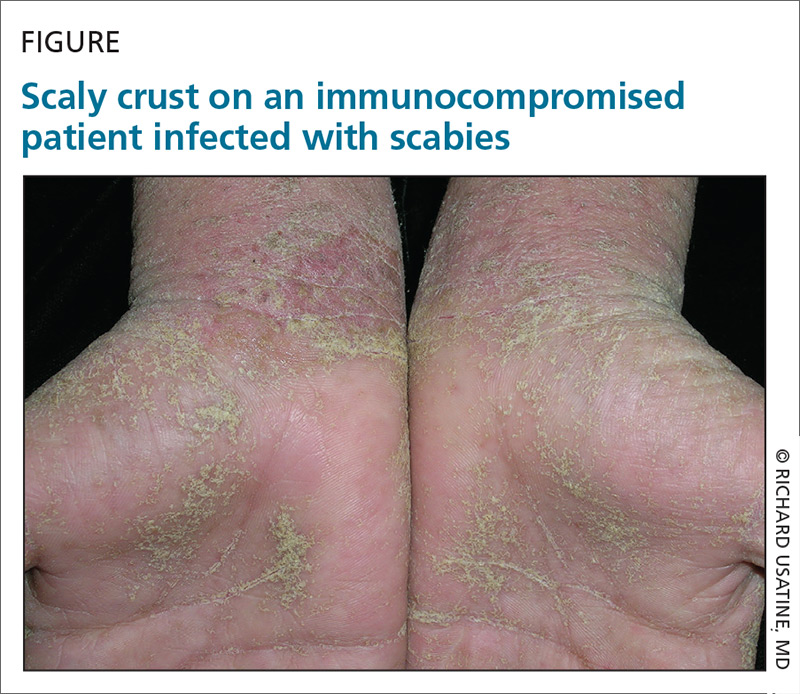
Dermoscopic examination of the rash on the wrist revealed scabies mites actively crawling on the surface of the skin. A superficial skin scraping revealed the Sarcoptes scabiei mite, several eggs, and scybala under the microscope. The extensive infestation in this patient with crusted scabies was related to his immunocompromised state. (The patient was successfully treated with oral ivermectin and topical permethrin.)
Unprecedented documentation of mite speed a clue to infectivity. The 36-second VIDEO captured an adult mite travelling a distance of 3 mm, translating to a speed of 5 mm/ min. Six younger adults/larvae were moving more rapidly with a maximum distance of 6 mm in 36 seconds (1 cm/min). The movement of a mite within the epidermis is slower, as the mite consumes keratin in creating burrows. The more rapid rate of movement on the skin surface may help to explain the contagious nature of scabies.

In this case, the mites and larvae were viewed on the screen of a smartphone to which a dermatoscope was attached magnetically. The mites were first visualized in the standard photo mode. Video mode was then used to capture the motion using the maximum zoom feature of the phone, to a magnification factor of 13.3×.
Literature to date has been silent on mites’ rate of motion. A Medline search yielded only 3 papers that addressed the issue of in vivo movement of scabies mites. None viewed the mites other than in their burrows and none calculated a rate of motion.
- In one study using videodermotoscopy, Micali stated that in most of the 16 cases identified, it was possible to observe the mites moving inside the burrows.1 No video images were published, and there was no mention of speed or characterization of the movement.1
- A second study used reflectance confocal microscopy (RCM) to examine a single patient with crusted scabies. The authors claim to have viewed the ectoparasite's motion within the human host but provided no details of that motion.2
- In the third study, videodermoscopy showed a slightly higher sensitivity for scabies detection than RCM (95% vs. 92%).3 The authors did not mention visualization of movement of mites in their work but did quote the Micali paper for its mention of movements of the mite.
Applying digital dermoscopy in practice. It appears that this is the first published video documenting the movement of scabies mites and larvae in vivo using dermoscopy. This should pave the way for additional observations of scabies movement on and below the skin using dermoscopy with video. We recommend using the maximum zoom capability of the device along with the dermatoscope to view this movement. What has been surmised before—that the mite must move above the skin to infect human contacts—has now been captured in vivo using the power of dermoscopy.
CORRESPONDENCE
Richard P. Usatine, MD, 903 West Martin Street, San Antonio, TX 78207; [email protected].
REFERENCES
1. Micali G, Lacarrubba F, Lo Guzzo G. Scraping versus videodermatoscopy for the diagnosis of scabies: a comparative study. Acta Derm Venereol. 1999; 79:396.
2. Gürel MS, Turgut Erdemir AV, Tekin B. A case report of real-time in vivo demonstration of Sarcoptes scabiei. Turkiye Parazitol Derg. 2017; 41:229-232.
3. Cinotti E, Labeille B, Cambazard F, et al. Videodermoscopy compared to reflectance confocal microscopy for the diagnosis of scabies. J Eur Acad Dermatol Venereol. 2016; 30:1573-1577.
Continue to: 3 diagnostic missteps to avoid
3 diagnostic missteps to avoid
Misdiagnosis is often due to an overreliance on the clinical history without performing an adequate physical exam. In such cases, the physician often diagnoses a form of dermatitis as the cause of pruritic rash. (Admittedly, diagnostic error can result in either false-positive or false-negative findings, and many patients are diagnosed with scabies when they have dermatitis.)
A second misstep? Scabies may be overlooked in a patient whose lesions are nonpruritic, such as someone with an immunocompromising condition.
And finally, crusted scabies is frequently mistaken for psoriasis or chronic dermatitis.8
Diagnostic errors are exceedingly troublesome for patients and caregivers. It is not unusual for a hospital or long-term care facility to lose significant employee work hours due to a scabies epidemic or fear of a scabies epidemic. In a 2003 outbreak of scabies in a Canadian long-term care facility, an estimated $200,000 was needed to control disease spread.23
A topical agent is a mainstay of treatment
Permethrin cream is usually the first-line treatment choice.24 Ivermectin, topical (cream) or systemic (pill), is the commonly used alternative for patients who do not respond to, or cannot tolerate, permethrin cream. A recent meta-analysis examined the effectiveness of 5% permethrin cream, 1% ivermectin cream, and oral ivermectin (200 mcg/kg single or double dose).24 Overall, findings suggested there was no difference in the efficacy or in adverse effects of permethrin cream compared with ivermectin (topical or systemic) among adults. One study reported that permethrin cream was slightly more effective than ivermectin (cream or oral) because of the more rapid treatment response (approximately 94% clearance within 2 weeks of treatment, compared with 90%).25
Continue to: Adjust treatment for special populations
Adjust treatment for special populations. Treatment of severe cases, such as crusted scabies, calls for combination therapy with oral ivermectin (200 mcg/kg) and 5% permethrin cream.26
Five percent permethrin cream is the preferred treatment for children weighing < 15 kg and pregnant women; oral ivermectin has not been studied for efficacy and safety in these populations.27
Effective response to treatment in these studies was measured by resolution of active scabies lesions and improvement in pruritus 1 to 2 weeks after treatment.
Anticipate these 3 clinical scenarios
The classic appearance of scabies usually triggers suspicion of its presence, leading to prompt identification of mite burrows and a correct diagnosis. Unfortunately, though, this is not always the case. And atypical presentations heighten the chance of diagnostic error, which overall occurs in nearly half of cases.1 Keep in mind the following common scenarios, to help improve diagnosis.
1. When a patient presents with a severe pruritic eruption, the clinician may be tempted to settle early on a form of dermatitis and not consider the possibility of scabies. When the patient is later seen by an expert, the burrows are easily identified. Solution: Whenever a patient complains of severe pruritus, use a dermatoscope to carefully examine the digits, web spaces, proximal palms, wrists, and ankles for burrows.
Continue to: A patient with distal white or yellow, thick, scaly, or crusted plaques
2. A patient with distal white or yellow, thick, scaly, or crusted plaques is often thought to have psoriasis or dermatitis. But scabies should be included in the differential diagnosis. In particular, worsening thick, scaly plaques in an immunocompromised patient should prompt consideration of scabies.
3. Smooth nodules of the genitals in males, or pruritic smooth papules and plaques in other locations, should lead to the consideration of scabies. These presentations can be mistaken for lichen planus, folliculitis, papular urticaria, insect bites, or atopic dermatitis.
Due to the limited amount of mite burrows early in the disease process of scabies, and the gross similarities to a patient with dermatitis with skin excoriations, a thorough exam is needed—one that goes beyond the traditional web spaces and includes hidden/atypical locations such as margins of the feet and hands, the scalp, and neck creases. Careful and deliberate inspection for burrows is critical before ruling out the diagnosis.
CORRESPONDENCE
Art Papier, MD, 400 Red Creek Drive, Suite 200, Rochester, NY 14623; [email protected].
ACKNOWLEDGEMENT
We thank Angela Delacenserie, MA, for reviewing the manuscript and providing editing suggestions.
1. Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017; 30:78-84.
2. Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006; 354:1718-1727.
3. Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017; 10:297.
4. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
5. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013; 310:2443-2450.
6. Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006; 6:769-779.
7. Reddy DR, Reddy PR. Nodular scabies: a classical case report in an adolescent boy. J Parasit Dis. 2015; 39:581-583.
8. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006; 367:1767-1774.
9. Karaca Ş, Kelekçi KH, Er O, et al. Scabies incognito presenting as a subcorneal pustular dermatosis-like eruption. Turkiye Parazitol Derg. 2015; 39:244-247.
10. Gutte RM. Bullous scabies in an adult: a case report with review of literature. Indian Dermatol Online J. 2013; 4:311-313.
11. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005; 50:375-381.
12. Ebrahim KC, Alves JB, Tomé LA, et al. Norwegian scabies—rare case of atypical manifestation. An Bras Dermatol. 2016; 91:826-828.
13. Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007; 20:268-279.
14. Dupuy E, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007; 56:53-62.
15. Phan A, Dalle S, Balme B, et al. Scabies with clinical features and positive Darier sign mimicking mastocytosis. Pediatr Dermatol. 2009; 26:363-364.
16. Salces IG, Alfaro J, Sáenz DE, et al. Scabies presenting as solitary mastocytoma-like eruption in an infant. Pediatr Dermatol. 2009; 26:486-488.
17. Tasani M, Tong SY, Andrews RM, et al. The importance of scabies coinfection in the treatment considerations for impetigo. Pediatr Infect Dis J. 2016; 35:374-378.
18. Fonseca V, Price HN, Jeffries M, et al. Crusted scabies misdiagnosed as erythrodermic psoriasis in a 3-year-old girl with Down syndrome. Pediatr Dermatol. 2014; 31:753-754.
19. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
20. Park JH, Kim CW, Kim SS. The diagnostic accuracy of dermoscopy for scabies. Ann Dermatol. 2012; 24:194-199.
21. Lallas A, Apalla Z, Lazaridou E, et al. Scabies escaping detection until dermoscopy was applied. Dermatol Pract Concept. 2017; 7:49-50.
22. Micali G, Lacarrubba F, Verzi AE, et al. Scabies: advances in noninvasive diagnosis. PLoS Negl Trop Dis. 2016; 10:e0004691.
23. de Beer G, Miller MA, Tremblay L, et al. An outbreak of scabies in a long-term care facility: the role of misdiagnosis and the costs associated with control. Infect Control Hosp Epidemiol. 2006; 27:517-518.
24. Dhana A. Yen H, Okhovat JP, et al. Ivermectin versus permethrin in the treatment of scabies: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2018; 78:194-198.
25. Sharma R, Singal A. Topical permethrin and oral ivermectin in the management of scabies: a prospective, randomized, double blind, controlled study. Indian J Dermatol Venereol Leprol. 2011; 77:581-586.
26. Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010; 362:717-725.
27. Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017; 31:1248-1253.
It is estimated that up to 45% of cases of scabies are misdiagnosed as another condition.1 This can occur when common clinical features are overlooked, a skin exam is rushed (and the rash is chalked up to dermatitis), or the wrong part of the pruritic lesion is scraped (the papule, rather than the burrow). There are also atypical presentations of scabies, which can confound even the most astute clinician.1 Misdiagnosis can increase health care costs due to repeat office visits or multiple referrals. In this article, we review the typical and atypical presentations of scabies and provide recommendations to aid physicians in its early recognition and correct diagnosis.
The scope of scabies infection, and its clinical stages
The prevalence of scabies, a common skin infection caused by the mite Sarcoptes scabiei, is estimated at 300 million cases worldwide annually, with the greatest incidence occurring in children and adolescents.1 In the developing world, its clinical burden is highest among the homeless, those of lower socioeconomic status, and those with poor hygiene. In the developed world, the clinical burden is highest among hospitalized patients and residents of long-term living facilities.
The S scabiei mite is an obligate parasite that elicits an adaptive immune response in susceptible hosts. The female mite lays 60 to 90 eggs that mature into adult mites after completing the mite life cycle in human hosts. In immunocompetent patients, roughly 10 to 15 surviving mites can be found at any given point in the disease process.2 In crusted or disseminated scabies, which often occur in immunocompromised patients, thousands of mites may be found at any given point in the disease process. 2
Scabies infection has 2 stages: the latent primary infection and the symptomatic secondary infection.
The primary infection starts with the initial mite invasion, typically with the transfer of impregnated females during person-to-person contact. Females deposit eggs as they burrow into the epidermis at the level of the stratum corneum with the use of proteolytic enzymes (creating the mite burrow). Surviving eggs hatch into larvae that then mature into nymphs and adult mites. After these adult mites mate, the impregnated females create new burrows and lay additional eggs.3 Patients may be asymptomatic during this initial stage and the infection may be transmitted from person to person through direct skin contact.
The second stage of infection is when patients experience severe pruritus with inflammatory papules seen on exam. The pruritus associated with scabies results from a delayed type IV hypersensitivity reaction to mite infestation. This requires host sensitization to the scabies mite. Clinically, there is a delayed onset (weeks) of numerous erythematous papules and, later, excoriated papules.
Conditions that scabies can mimic
The differential of typical scabies includes diagnoses manifesting with moderate to severe pruritus. In the immunocompetent adult, conditions to consider are atopic dermatitis, tinea corporis, papular urticaria, seborrheic dermatitis, poison ivy and other causes of contact dermatitis, drug eruptions, and irritant dermatitis. In immunocompetent infants, think of seborrheic dermatitis, atopic dermatitis, acropustulosis, and viral exanthems.
Continue to: Nodular scabies variants...
Nodular scabies variants can masquerade as pseudolymphoma, lymphoma, or leukemia cutis. In immunocompromised and elderly individuals, crusted scabies is often mistaken for psoriasis, atopic dermatitis, keratoderma, and lichen planus.2,4,5
Scabies’ classic presentation
Typically, scabies causes intensely pruritic erythematous papules. Areas commonly affected are the webs and sides of fingers (FIGURE 1A and 1B), proximal palm and wrist flexors, extensor aspects of the arms and legs, axillary folds, periumbilical areas, the peri-areolar region in women, buttocks and thigh creases, and, in males, the genitals. The head may also be affected in children (FIGURE 1C), but seldom in adults. Interestingly, the back is usually spared across all age groups, though not always (FIGURE 2).


The classic presentation also varies across age groups and populations.2 In children, vesicles, pustules, and nodular pruritic lesions may coexist with eczema and impetigo. Among homeless individuals, coinfection with impetigo and eczema is common.
Scabies subtypes with varying presentations
Clinical manifestations of scabies subtypes may make it difficult to diagnose the disease. These subtypes include nodular, pustular, vesiculobullous, and crusted scabies (Norwegian scabies). Although rare, these subtypes merit acknowledgement, as atypical cases contribute to the high rate of misdiagnosis.
Nodular scabies is a clinical variant that accounts for about 7% of scabies cases.7 It can resist traditional scabies treatment (permethrin cream, ivermectin—which we’ll discuss in a bit) and often requires topical or intralesional corticosteroid management. Nodular scabies most commonly affects male genitalia. Patients may have multiple excoriated skin-colored erythematous papules and nodules in areas involving the classic distribution of scabies (web spaces of fingers, flexural areas [FIGURE 3], scrotum, and groin).8

Continue to: Pustular scabies...
Pustular scabies is commonly seen in children and adolescents but can occur in all age groups. Patients may present with vesicular pustular lesions (FIGURE 4A and 4B).7 In some cases, topical corticosteroids have modified the classic clinical presentation into a pustular variant. However, scabies alone can cause pustular lesions.

Vesiculobullous scabies (FIGURE 5A and 5B) is a clinical subtype that may be mistaken for pemphigus vulgaris9 or bullous pemphigoid10 because of its strikingly similar clinical presentation to those disorders.

Crusted scabies (Norwegian scabies) is a severe, disseminated form of scabies that commonly affects immunocompromised patients, although cases are also seen in immunocompetent hosts. Afflicted immunocompetent patients often have a history of diabetes mellitus, liver cirrhosis, malnutrition, or alcohol abuse.11 Patients present with a thick powdery or crusted white or yellow scale involving the feet or hands (FIGURE 6) that sometimes extends onto the limbs. Severe cases can involve wide body surfaces. One unusual presentation also included a desquamating rash without pruritus.12

Highly atypical cases
Atypical presentations include lesions that appear outside of the classic distribution areas of scabies, lesions with uncharacteristic morphology, cases with coinfections, and instances in which patients are immunocompromised.13 Examples include scabies of the scalp coexisting with seborrheic dermatitis or dermatomyositis,14 scabies mimicking mastocytoma,15,16 and scabies with coinfections of impetigo or eczema.17 These coinfections and clinical variations can be particularly challenging. Other reports of atypical scabies leading to misdiagnosis include a case of crusted scabies mimicking erythrodermic psoriasis18 and a case initially attributed to suppurative folliculitis and prurigo nodularis.19
Decisive diagnostic measures
Identifying the mite’s burrow on clinical exam is essential to making the diagnosis of scabies. Microscopic examinations of burrow scrapings remain the diagnostic gold standard. Frequently, nondermatologists perform skin scrapings of the pruritic papules of scabies instead of the burrows. These papules are a hypersensitivity reaction to the mites, and no scabetic mites will be found there. Burrows appear on exam as small, linear, serpiginous lines (FIGURE 7A and 7B).

Continue to: Handheld illumination with a dermatoscope...
Handheld illumination with a dermatoscope will allow visualization of the mite (FIGURE 8). (See “How fast do scabies mites move? Dermoscopy video answers that question.”) Dermatoscope findings consistent with scabies include a “delta glider,” a dark triangular shape that is the mite’s head hidden in the burrow,20 or the classic “jet with contrail” (FIGURE 8).21 Scrape the burrow to discover the mature scabies mite (FIGURE 9) and confirm the diagnosis.

Microscopic examination of scrapings has a reported sensitivity and specificity of up to 90% and 100%, respectively, when collection of scrapings is performed accurately and contains ova, feces, or mites.14,20,22 Dermatologists increasingly use dermatoscopes to diagnose scabies. Dermoscopy's sensitivity is 91% and specificity is 85% to 86%,14 which is a reassuring frame of reference for physicians who do not routinely use dermatoscopes and instead rely on scrapings.

Continue to: How fast do scabies mites move? Dermoscopy video answers that question
SIDEBAR
How fast do scabies mites move? Dermoscopy video answers that question
Richard Usatine, MD; Ashfaq Marghoob, MD
University of Texas Health San Antonio (Dr. Usatine); Memorial Sloan Kettering Skin Cancer Center, Hauppauge, NY (Dr. Marghoob).
Dr. Marghoob reported that he has received honoraria for speaking for 3GEN. Dr. Usatine reported no potential conflict of interest relevant to this article.
A 22-year-old man infected with human immunodeficiency virus presented to a clinic with a 6-month history of intense pruritus. Physical examination revealed scale crust on the hands and wrists (FIGURE) and in the pubic region.

Dermoscopic examination of the rash on the wrist revealed scabies mites actively crawling on the surface of the skin. A superficial skin scraping revealed the Sarcoptes scabiei mite, several eggs, and scybala under the microscope. The extensive infestation in this patient with crusted scabies was related to his immunocompromised state. (The patient was successfully treated with oral ivermectin and topical permethrin.)
Unprecedented documentation of mite speed a clue to infectivity. The 36-second VIDEO captured an adult mite travelling a distance of 3 mm, translating to a speed of 5 mm/ min. Six younger adults/larvae were moving more rapidly with a maximum distance of 6 mm in 36 seconds (1 cm/min). The movement of a mite within the epidermis is slower, as the mite consumes keratin in creating burrows. The more rapid rate of movement on the skin surface may help to explain the contagious nature of scabies.

In this case, the mites and larvae were viewed on the screen of a smartphone to which a dermatoscope was attached magnetically. The mites were first visualized in the standard photo mode. Video mode was then used to capture the motion using the maximum zoom feature of the phone, to a magnification factor of 13.3×.
Literature to date has been silent on mites’ rate of motion. A Medline search yielded only 3 papers that addressed the issue of in vivo movement of scabies mites. None viewed the mites other than in their burrows and none calculated a rate of motion.
- In one study using videodermotoscopy, Micali stated that in most of the 16 cases identified, it was possible to observe the mites moving inside the burrows.1 No video images were published, and there was no mention of speed or characterization of the movement.1
- A second study used reflectance confocal microscopy (RCM) to examine a single patient with crusted scabies. The authors claim to have viewed the ectoparasite's motion within the human host but provided no details of that motion.2
- In the third study, videodermoscopy showed a slightly higher sensitivity for scabies detection than RCM (95% vs. 92%).3 The authors did not mention visualization of movement of mites in their work but did quote the Micali paper for its mention of movements of the mite.
Applying digital dermoscopy in practice. It appears that this is the first published video documenting the movement of scabies mites and larvae in vivo using dermoscopy. This should pave the way for additional observations of scabies movement on and below the skin using dermoscopy with video. We recommend using the maximum zoom capability of the device along with the dermatoscope to view this movement. What has been surmised before—that the mite must move above the skin to infect human contacts—has now been captured in vivo using the power of dermoscopy.
CORRESPONDENCE
Richard P. Usatine, MD, 903 West Martin Street, San Antonio, TX 78207; [email protected].
REFERENCES
1. Micali G, Lacarrubba F, Lo Guzzo G. Scraping versus videodermatoscopy for the diagnosis of scabies: a comparative study. Acta Derm Venereol. 1999; 79:396.
2. Gürel MS, Turgut Erdemir AV, Tekin B. A case report of real-time in vivo demonstration of Sarcoptes scabiei. Turkiye Parazitol Derg. 2017; 41:229-232.
3. Cinotti E, Labeille B, Cambazard F, et al. Videodermoscopy compared to reflectance confocal microscopy for the diagnosis of scabies. J Eur Acad Dermatol Venereol. 2016; 30:1573-1577.
Continue to: 3 diagnostic missteps to avoid
3 diagnostic missteps to avoid
Misdiagnosis is often due to an overreliance on the clinical history without performing an adequate physical exam. In such cases, the physician often diagnoses a form of dermatitis as the cause of pruritic rash. (Admittedly, diagnostic error can result in either false-positive or false-negative findings, and many patients are diagnosed with scabies when they have dermatitis.)
A second misstep? Scabies may be overlooked in a patient whose lesions are nonpruritic, such as someone with an immunocompromising condition.
And finally, crusted scabies is frequently mistaken for psoriasis or chronic dermatitis.8
Diagnostic errors are exceedingly troublesome for patients and caregivers. It is not unusual for a hospital or long-term care facility to lose significant employee work hours due to a scabies epidemic or fear of a scabies epidemic. In a 2003 outbreak of scabies in a Canadian long-term care facility, an estimated $200,000 was needed to control disease spread.23
A topical agent is a mainstay of treatment
Permethrin cream is usually the first-line treatment choice.24 Ivermectin, topical (cream) or systemic (pill), is the commonly used alternative for patients who do not respond to, or cannot tolerate, permethrin cream. A recent meta-analysis examined the effectiveness of 5% permethrin cream, 1% ivermectin cream, and oral ivermectin (200 mcg/kg single or double dose).24 Overall, findings suggested there was no difference in the efficacy or in adverse effects of permethrin cream compared with ivermectin (topical or systemic) among adults. One study reported that permethrin cream was slightly more effective than ivermectin (cream or oral) because of the more rapid treatment response (approximately 94% clearance within 2 weeks of treatment, compared with 90%).25
Continue to: Adjust treatment for special populations
Adjust treatment for special populations. Treatment of severe cases, such as crusted scabies, calls for combination therapy with oral ivermectin (200 mcg/kg) and 5% permethrin cream.26
Five percent permethrin cream is the preferred treatment for children weighing < 15 kg and pregnant women; oral ivermectin has not been studied for efficacy and safety in these populations.27
Effective response to treatment in these studies was measured by resolution of active scabies lesions and improvement in pruritus 1 to 2 weeks after treatment.
Anticipate these 3 clinical scenarios
The classic appearance of scabies usually triggers suspicion of its presence, leading to prompt identification of mite burrows and a correct diagnosis. Unfortunately, though, this is not always the case. And atypical presentations heighten the chance of diagnostic error, which overall occurs in nearly half of cases.1 Keep in mind the following common scenarios, to help improve diagnosis.
1. When a patient presents with a severe pruritic eruption, the clinician may be tempted to settle early on a form of dermatitis and not consider the possibility of scabies. When the patient is later seen by an expert, the burrows are easily identified. Solution: Whenever a patient complains of severe pruritus, use a dermatoscope to carefully examine the digits, web spaces, proximal palms, wrists, and ankles for burrows.
Continue to: A patient with distal white or yellow, thick, scaly, or crusted plaques
2. A patient with distal white or yellow, thick, scaly, or crusted plaques is often thought to have psoriasis or dermatitis. But scabies should be included in the differential diagnosis. In particular, worsening thick, scaly plaques in an immunocompromised patient should prompt consideration of scabies.
3. Smooth nodules of the genitals in males, or pruritic smooth papules and plaques in other locations, should lead to the consideration of scabies. These presentations can be mistaken for lichen planus, folliculitis, papular urticaria, insect bites, or atopic dermatitis.
Due to the limited amount of mite burrows early in the disease process of scabies, and the gross similarities to a patient with dermatitis with skin excoriations, a thorough exam is needed—one that goes beyond the traditional web spaces and includes hidden/atypical locations such as margins of the feet and hands, the scalp, and neck creases. Careful and deliberate inspection for burrows is critical before ruling out the diagnosis.
CORRESPONDENCE
Art Papier, MD, 400 Red Creek Drive, Suite 200, Rochester, NY 14623; [email protected].
ACKNOWLEDGEMENT
We thank Angela Delacenserie, MA, for reviewing the manuscript and providing editing suggestions.
It is estimated that up to 45% of cases of scabies are misdiagnosed as another condition.1 This can occur when common clinical features are overlooked, a skin exam is rushed (and the rash is chalked up to dermatitis), or the wrong part of the pruritic lesion is scraped (the papule, rather than the burrow). There are also atypical presentations of scabies, which can confound even the most astute clinician.1 Misdiagnosis can increase health care costs due to repeat office visits or multiple referrals. In this article, we review the typical and atypical presentations of scabies and provide recommendations to aid physicians in its early recognition and correct diagnosis.
The scope of scabies infection, and its clinical stages
The prevalence of scabies, a common skin infection caused by the mite Sarcoptes scabiei, is estimated at 300 million cases worldwide annually, with the greatest incidence occurring in children and adolescents.1 In the developing world, its clinical burden is highest among the homeless, those of lower socioeconomic status, and those with poor hygiene. In the developed world, the clinical burden is highest among hospitalized patients and residents of long-term living facilities.
The S scabiei mite is an obligate parasite that elicits an adaptive immune response in susceptible hosts. The female mite lays 60 to 90 eggs that mature into adult mites after completing the mite life cycle in human hosts. In immunocompetent patients, roughly 10 to 15 surviving mites can be found at any given point in the disease process.2 In crusted or disseminated scabies, which often occur in immunocompromised patients, thousands of mites may be found at any given point in the disease process. 2
Scabies infection has 2 stages: the latent primary infection and the symptomatic secondary infection.
The primary infection starts with the initial mite invasion, typically with the transfer of impregnated females during person-to-person contact. Females deposit eggs as they burrow into the epidermis at the level of the stratum corneum with the use of proteolytic enzymes (creating the mite burrow). Surviving eggs hatch into larvae that then mature into nymphs and adult mites. After these adult mites mate, the impregnated females create new burrows and lay additional eggs.3 Patients may be asymptomatic during this initial stage and the infection may be transmitted from person to person through direct skin contact.
The second stage of infection is when patients experience severe pruritus with inflammatory papules seen on exam. The pruritus associated with scabies results from a delayed type IV hypersensitivity reaction to mite infestation. This requires host sensitization to the scabies mite. Clinically, there is a delayed onset (weeks) of numerous erythematous papules and, later, excoriated papules.
Conditions that scabies can mimic
The differential of typical scabies includes diagnoses manifesting with moderate to severe pruritus. In the immunocompetent adult, conditions to consider are atopic dermatitis, tinea corporis, papular urticaria, seborrheic dermatitis, poison ivy and other causes of contact dermatitis, drug eruptions, and irritant dermatitis. In immunocompetent infants, think of seborrheic dermatitis, atopic dermatitis, acropustulosis, and viral exanthems.
Continue to: Nodular scabies variants...
Nodular scabies variants can masquerade as pseudolymphoma, lymphoma, or leukemia cutis. In immunocompromised and elderly individuals, crusted scabies is often mistaken for psoriasis, atopic dermatitis, keratoderma, and lichen planus.2,4,5
Scabies’ classic presentation
Typically, scabies causes intensely pruritic erythematous papules. Areas commonly affected are the webs and sides of fingers (FIGURE 1A and 1B), proximal palm and wrist flexors, extensor aspects of the arms and legs, axillary folds, periumbilical areas, the peri-areolar region in women, buttocks and thigh creases, and, in males, the genitals. The head may also be affected in children (FIGURE 1C), but seldom in adults. Interestingly, the back is usually spared across all age groups, though not always (FIGURE 2).


The classic presentation also varies across age groups and populations.2 In children, vesicles, pustules, and nodular pruritic lesions may coexist with eczema and impetigo. Among homeless individuals, coinfection with impetigo and eczema is common.
Scabies subtypes with varying presentations
Clinical manifestations of scabies subtypes may make it difficult to diagnose the disease. These subtypes include nodular, pustular, vesiculobullous, and crusted scabies (Norwegian scabies). Although rare, these subtypes merit acknowledgement, as atypical cases contribute to the high rate of misdiagnosis.
Nodular scabies is a clinical variant that accounts for about 7% of scabies cases.7 It can resist traditional scabies treatment (permethrin cream, ivermectin—which we’ll discuss in a bit) and often requires topical or intralesional corticosteroid management. Nodular scabies most commonly affects male genitalia. Patients may have multiple excoriated skin-colored erythematous papules and nodules in areas involving the classic distribution of scabies (web spaces of fingers, flexural areas [FIGURE 3], scrotum, and groin).8

Continue to: Pustular scabies...
Pustular scabies is commonly seen in children and adolescents but can occur in all age groups. Patients may present with vesicular pustular lesions (FIGURE 4A and 4B).7 In some cases, topical corticosteroids have modified the classic clinical presentation into a pustular variant. However, scabies alone can cause pustular lesions.

Vesiculobullous scabies (FIGURE 5A and 5B) is a clinical subtype that may be mistaken for pemphigus vulgaris9 or bullous pemphigoid10 because of its strikingly similar clinical presentation to those disorders.

Crusted scabies (Norwegian scabies) is a severe, disseminated form of scabies that commonly affects immunocompromised patients, although cases are also seen in immunocompetent hosts. Afflicted immunocompetent patients often have a history of diabetes mellitus, liver cirrhosis, malnutrition, or alcohol abuse.11 Patients present with a thick powdery or crusted white or yellow scale involving the feet or hands (FIGURE 6) that sometimes extends onto the limbs. Severe cases can involve wide body surfaces. One unusual presentation also included a desquamating rash without pruritus.12

Highly atypical cases
Atypical presentations include lesions that appear outside of the classic distribution areas of scabies, lesions with uncharacteristic morphology, cases with coinfections, and instances in which patients are immunocompromised.13 Examples include scabies of the scalp coexisting with seborrheic dermatitis or dermatomyositis,14 scabies mimicking mastocytoma,15,16 and scabies with coinfections of impetigo or eczema.17 These coinfections and clinical variations can be particularly challenging. Other reports of atypical scabies leading to misdiagnosis include a case of crusted scabies mimicking erythrodermic psoriasis18 and a case initially attributed to suppurative folliculitis and prurigo nodularis.19
Decisive diagnostic measures
Identifying the mite’s burrow on clinical exam is essential to making the diagnosis of scabies. Microscopic examinations of burrow scrapings remain the diagnostic gold standard. Frequently, nondermatologists perform skin scrapings of the pruritic papules of scabies instead of the burrows. These papules are a hypersensitivity reaction to the mites, and no scabetic mites will be found there. Burrows appear on exam as small, linear, serpiginous lines (FIGURE 7A and 7B).

Continue to: Handheld illumination with a dermatoscope...
Handheld illumination with a dermatoscope will allow visualization of the mite (FIGURE 8). (See “How fast do scabies mites move? Dermoscopy video answers that question.”) Dermatoscope findings consistent with scabies include a “delta glider,” a dark triangular shape that is the mite’s head hidden in the burrow,20 or the classic “jet with contrail” (FIGURE 8).21 Scrape the burrow to discover the mature scabies mite (FIGURE 9) and confirm the diagnosis.

Microscopic examination of scrapings has a reported sensitivity and specificity of up to 90% and 100%, respectively, when collection of scrapings is performed accurately and contains ova, feces, or mites.14,20,22 Dermatologists increasingly use dermatoscopes to diagnose scabies. Dermoscopy's sensitivity is 91% and specificity is 85% to 86%,14 which is a reassuring frame of reference for physicians who do not routinely use dermatoscopes and instead rely on scrapings.

Continue to: How fast do scabies mites move? Dermoscopy video answers that question
SIDEBAR
How fast do scabies mites move? Dermoscopy video answers that question
Richard Usatine, MD; Ashfaq Marghoob, MD
University of Texas Health San Antonio (Dr. Usatine); Memorial Sloan Kettering Skin Cancer Center, Hauppauge, NY (Dr. Marghoob).
Dr. Marghoob reported that he has received honoraria for speaking for 3GEN. Dr. Usatine reported no potential conflict of interest relevant to this article.
A 22-year-old man infected with human immunodeficiency virus presented to a clinic with a 6-month history of intense pruritus. Physical examination revealed scale crust on the hands and wrists (FIGURE) and in the pubic region.

Dermoscopic examination of the rash on the wrist revealed scabies mites actively crawling on the surface of the skin. A superficial skin scraping revealed the Sarcoptes scabiei mite, several eggs, and scybala under the microscope. The extensive infestation in this patient with crusted scabies was related to his immunocompromised state. (The patient was successfully treated with oral ivermectin and topical permethrin.)
Unprecedented documentation of mite speed a clue to infectivity. The 36-second VIDEO captured an adult mite travelling a distance of 3 mm, translating to a speed of 5 mm/ min. Six younger adults/larvae were moving more rapidly with a maximum distance of 6 mm in 36 seconds (1 cm/min). The movement of a mite within the epidermis is slower, as the mite consumes keratin in creating burrows. The more rapid rate of movement on the skin surface may help to explain the contagious nature of scabies.

In this case, the mites and larvae were viewed on the screen of a smartphone to which a dermatoscope was attached magnetically. The mites were first visualized in the standard photo mode. Video mode was then used to capture the motion using the maximum zoom feature of the phone, to a magnification factor of 13.3×.
Literature to date has been silent on mites’ rate of motion. A Medline search yielded only 3 papers that addressed the issue of in vivo movement of scabies mites. None viewed the mites other than in their burrows and none calculated a rate of motion.
- In one study using videodermotoscopy, Micali stated that in most of the 16 cases identified, it was possible to observe the mites moving inside the burrows.1 No video images were published, and there was no mention of speed or characterization of the movement.1
- A second study used reflectance confocal microscopy (RCM) to examine a single patient with crusted scabies. The authors claim to have viewed the ectoparasite's motion within the human host but provided no details of that motion.2
- In the third study, videodermoscopy showed a slightly higher sensitivity for scabies detection than RCM (95% vs. 92%).3 The authors did not mention visualization of movement of mites in their work but did quote the Micali paper for its mention of movements of the mite.
Applying digital dermoscopy in practice. It appears that this is the first published video documenting the movement of scabies mites and larvae in vivo using dermoscopy. This should pave the way for additional observations of scabies movement on and below the skin using dermoscopy with video. We recommend using the maximum zoom capability of the device along with the dermatoscope to view this movement. What has been surmised before—that the mite must move above the skin to infect human contacts—has now been captured in vivo using the power of dermoscopy.
CORRESPONDENCE
Richard P. Usatine, MD, 903 West Martin Street, San Antonio, TX 78207; [email protected].
REFERENCES
1. Micali G, Lacarrubba F, Lo Guzzo G. Scraping versus videodermatoscopy for the diagnosis of scabies: a comparative study. Acta Derm Venereol. 1999; 79:396.
2. Gürel MS, Turgut Erdemir AV, Tekin B. A case report of real-time in vivo demonstration of Sarcoptes scabiei. Turkiye Parazitol Derg. 2017; 41:229-232.
3. Cinotti E, Labeille B, Cambazard F, et al. Videodermoscopy compared to reflectance confocal microscopy for the diagnosis of scabies. J Eur Acad Dermatol Venereol. 2016; 30:1573-1577.
Continue to: 3 diagnostic missteps to avoid
3 diagnostic missteps to avoid
Misdiagnosis is often due to an overreliance on the clinical history without performing an adequate physical exam. In such cases, the physician often diagnoses a form of dermatitis as the cause of pruritic rash. (Admittedly, diagnostic error can result in either false-positive or false-negative findings, and many patients are diagnosed with scabies when they have dermatitis.)
A second misstep? Scabies may be overlooked in a patient whose lesions are nonpruritic, such as someone with an immunocompromising condition.
And finally, crusted scabies is frequently mistaken for psoriasis or chronic dermatitis.8
Diagnostic errors are exceedingly troublesome for patients and caregivers. It is not unusual for a hospital or long-term care facility to lose significant employee work hours due to a scabies epidemic or fear of a scabies epidemic. In a 2003 outbreak of scabies in a Canadian long-term care facility, an estimated $200,000 was needed to control disease spread.23
A topical agent is a mainstay of treatment
Permethrin cream is usually the first-line treatment choice.24 Ivermectin, topical (cream) or systemic (pill), is the commonly used alternative for patients who do not respond to, or cannot tolerate, permethrin cream. A recent meta-analysis examined the effectiveness of 5% permethrin cream, 1% ivermectin cream, and oral ivermectin (200 mcg/kg single or double dose).24 Overall, findings suggested there was no difference in the efficacy or in adverse effects of permethrin cream compared with ivermectin (topical or systemic) among adults. One study reported that permethrin cream was slightly more effective than ivermectin (cream or oral) because of the more rapid treatment response (approximately 94% clearance within 2 weeks of treatment, compared with 90%).25
Continue to: Adjust treatment for special populations
Adjust treatment for special populations. Treatment of severe cases, such as crusted scabies, calls for combination therapy with oral ivermectin (200 mcg/kg) and 5% permethrin cream.26
Five percent permethrin cream is the preferred treatment for children weighing < 15 kg and pregnant women; oral ivermectin has not been studied for efficacy and safety in these populations.27
Effective response to treatment in these studies was measured by resolution of active scabies lesions and improvement in pruritus 1 to 2 weeks after treatment.
Anticipate these 3 clinical scenarios
The classic appearance of scabies usually triggers suspicion of its presence, leading to prompt identification of mite burrows and a correct diagnosis. Unfortunately, though, this is not always the case. And atypical presentations heighten the chance of diagnostic error, which overall occurs in nearly half of cases.1 Keep in mind the following common scenarios, to help improve diagnosis.
1. When a patient presents with a severe pruritic eruption, the clinician may be tempted to settle early on a form of dermatitis and not consider the possibility of scabies. When the patient is later seen by an expert, the burrows are easily identified. Solution: Whenever a patient complains of severe pruritus, use a dermatoscope to carefully examine the digits, web spaces, proximal palms, wrists, and ankles for burrows.
Continue to: A patient with distal white or yellow, thick, scaly, or crusted plaques
2. A patient with distal white or yellow, thick, scaly, or crusted plaques is often thought to have psoriasis or dermatitis. But scabies should be included in the differential diagnosis. In particular, worsening thick, scaly plaques in an immunocompromised patient should prompt consideration of scabies.
3. Smooth nodules of the genitals in males, or pruritic smooth papules and plaques in other locations, should lead to the consideration of scabies. These presentations can be mistaken for lichen planus, folliculitis, papular urticaria, insect bites, or atopic dermatitis.
Due to the limited amount of mite burrows early in the disease process of scabies, and the gross similarities to a patient with dermatitis with skin excoriations, a thorough exam is needed—one that goes beyond the traditional web spaces and includes hidden/atypical locations such as margins of the feet and hands, the scalp, and neck creases. Careful and deliberate inspection for burrows is critical before ruling out the diagnosis.
CORRESPONDENCE
Art Papier, MD, 400 Red Creek Drive, Suite 200, Rochester, NY 14623; [email protected].
ACKNOWLEDGEMENT
We thank Angela Delacenserie, MA, for reviewing the manuscript and providing editing suggestions.
1. Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017; 30:78-84.
2. Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006; 354:1718-1727.
3. Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017; 10:297.
4. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
5. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013; 310:2443-2450.
6. Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006; 6:769-779.
7. Reddy DR, Reddy PR. Nodular scabies: a classical case report in an adolescent boy. J Parasit Dis. 2015; 39:581-583.
8. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006; 367:1767-1774.
9. Karaca Ş, Kelekçi KH, Er O, et al. Scabies incognito presenting as a subcorneal pustular dermatosis-like eruption. Turkiye Parazitol Derg. 2015; 39:244-247.
10. Gutte RM. Bullous scabies in an adult: a case report with review of literature. Indian Dermatol Online J. 2013; 4:311-313.
11. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005; 50:375-381.
12. Ebrahim KC, Alves JB, Tomé LA, et al. Norwegian scabies—rare case of atypical manifestation. An Bras Dermatol. 2016; 91:826-828.
13. Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007; 20:268-279.
14. Dupuy E, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007; 56:53-62.
15. Phan A, Dalle S, Balme B, et al. Scabies with clinical features and positive Darier sign mimicking mastocytosis. Pediatr Dermatol. 2009; 26:363-364.
16. Salces IG, Alfaro J, Sáenz DE, et al. Scabies presenting as solitary mastocytoma-like eruption in an infant. Pediatr Dermatol. 2009; 26:486-488.
17. Tasani M, Tong SY, Andrews RM, et al. The importance of scabies coinfection in the treatment considerations for impetigo. Pediatr Infect Dis J. 2016; 35:374-378.
18. Fonseca V, Price HN, Jeffries M, et al. Crusted scabies misdiagnosed as erythrodermic psoriasis in a 3-year-old girl with Down syndrome. Pediatr Dermatol. 2014; 31:753-754.
19. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
20. Park JH, Kim CW, Kim SS. The diagnostic accuracy of dermoscopy for scabies. Ann Dermatol. 2012; 24:194-199.
21. Lallas A, Apalla Z, Lazaridou E, et al. Scabies escaping detection until dermoscopy was applied. Dermatol Pract Concept. 2017; 7:49-50.
22. Micali G, Lacarrubba F, Verzi AE, et al. Scabies: advances in noninvasive diagnosis. PLoS Negl Trop Dis. 2016; 10:e0004691.
23. de Beer G, Miller MA, Tremblay L, et al. An outbreak of scabies in a long-term care facility: the role of misdiagnosis and the costs associated with control. Infect Control Hosp Epidemiol. 2006; 27:517-518.
24. Dhana A. Yen H, Okhovat JP, et al. Ivermectin versus permethrin in the treatment of scabies: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2018; 78:194-198.
25. Sharma R, Singal A. Topical permethrin and oral ivermectin in the management of scabies: a prospective, randomized, double blind, controlled study. Indian J Dermatol Venereol Leprol. 2011; 77:581-586.
26. Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010; 362:717-725.
27. Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017; 31:1248-1253.
1. Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017; 30:78-84.
2. Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006; 354:1718-1727.
3. Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017; 10:297.
4. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
5. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013; 310:2443-2450.
6. Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006; 6:769-779.
7. Reddy DR, Reddy PR. Nodular scabies: a classical case report in an adolescent boy. J Parasit Dis. 2015; 39:581-583.
8. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006; 367:1767-1774.
9. Karaca Ş, Kelekçi KH, Er O, et al. Scabies incognito presenting as a subcorneal pustular dermatosis-like eruption. Turkiye Parazitol Derg. 2015; 39:244-247.
10. Gutte RM. Bullous scabies in an adult: a case report with review of literature. Indian Dermatol Online J. 2013; 4:311-313.
11. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005; 50:375-381.
12. Ebrahim KC, Alves JB, Tomé LA, et al. Norwegian scabies—rare case of atypical manifestation. An Bras Dermatol. 2016; 91:826-828.
13. Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007; 20:268-279.
14. Dupuy E, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007; 56:53-62.
15. Phan A, Dalle S, Balme B, et al. Scabies with clinical features and positive Darier sign mimicking mastocytosis. Pediatr Dermatol. 2009; 26:363-364.
16. Salces IG, Alfaro J, Sáenz DE, et al. Scabies presenting as solitary mastocytoma-like eruption in an infant. Pediatr Dermatol. 2009; 26:486-488.
17. Tasani M, Tong SY, Andrews RM, et al. The importance of scabies coinfection in the treatment considerations for impetigo. Pediatr Infect Dis J. 2016; 35:374-378.
18. Fonseca V, Price HN, Jeffries M, et al. Crusted scabies misdiagnosed as erythrodermic psoriasis in a 3-year-old girl with Down syndrome. Pediatr Dermatol. 2014; 31:753-754.
19. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
20. Park JH, Kim CW, Kim SS. The diagnostic accuracy of dermoscopy for scabies. Ann Dermatol. 2012; 24:194-199.
21. Lallas A, Apalla Z, Lazaridou E, et al. Scabies escaping detection until dermoscopy was applied. Dermatol Pract Concept. 2017; 7:49-50.
22. Micali G, Lacarrubba F, Verzi AE, et al. Scabies: advances in noninvasive diagnosis. PLoS Negl Trop Dis. 2016; 10:e0004691.
23. de Beer G, Miller MA, Tremblay L, et al. An outbreak of scabies in a long-term care facility: the role of misdiagnosis and the costs associated with control. Infect Control Hosp Epidemiol. 2006; 27:517-518.
24. Dhana A. Yen H, Okhovat JP, et al. Ivermectin versus permethrin in the treatment of scabies: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2018; 78:194-198.
25. Sharma R, Singal A. Topical permethrin and oral ivermectin in the management of scabies: a prospective, randomized, double blind, controlled study. Indian J Dermatol Venereol Leprol. 2011; 77:581-586.
26. Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010; 362:717-725.
27. Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017; 31:1248-1253.
VIDEO shows scabies mite in motion
PRACTICE RECOMMENDATIONS
› Consider scabies with any severe pruritic eruption. Conduct a thorough physical exam, preferably with a dermatoscope, for burrows in the webs and sides of fingers, proximal palm, and wrists. A
› Consider scabies in all patients—especially the immunocompromised—who have distal white or yellow thick, scaly, or crusted plaques. C
› Include scabies in the differential when patients present with smooth nodules of the genitals or pruritic smooth papules and plaques in other locations. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
10 proven strategies to help patients maintain weight loss
New studies show that many people who lose weight can maintain that loss for longer than a few months by utilizing strategies that can be undertaken upon your recommendation and with your ongoing support. In this article, I review the evidence that supports the effectiveness of those interventions and activities for helping patients keep off the weight they’ve lost.
Prolonging the duration of weight maintenance
Until recently, most studies that focused on weight maintenance after weight loss followed subjects for only a few months or a year after the goal was achieved. With that limited window of follow-up, the belief arose in weight-loss medicine that most people gain back lost weight within 2 years. Findings that are emerging from recent studies with longer follow-up, however, suggest that weight loss can be maintained for as long as 8 years.1
The National Health and Nutrition Examination Survey2 and the Action in Health for Diabetes (Look AHEAD) trial3,4 reported that, among adults who lost 10% or more of body weight, approximately 60% maintained that weight loss at 1 year. Look AHEAD had a much longer duration: 42% of participants who lost at least 10% of body weight by the end of Year 1 maintained at least that 10% loss by the end of Year 4.5 In addition, Look AHEAD demonstrated that extended provision of maintenance interventions after weight loss can facilitate clinically meaningful weight loss for as long as 8 years—2 or 3 times longer than what was reported in earlier randomized trials.4
We have evidence-based guidance for achieving long-term weight maintenance and good reason to believe that success is achievable for patients. The 10 strategies that follow can help you to guide patients to become successful “maintainers.”

1. Emphasize more weight loss in the first 3 months of a program
Losing more weight initially seems to point to more success in relation to maintenance. This suggests that more intensive help, such as more frequent visits with a physician and a dietitian during the first 3 months might be an important step to help patients lose and maintain weight.
Much of our information on successful maintainers comes from the National Weight Control Registry (NWCR) at the Warren Alpert Medical School at Brown University.1 This research study has gathered information from more than 10,000 people who successfully lost ≥ 30 lb (average, 60 lb) and kept it off for at least 1 year. To challenge the widespread belief that only a few people who attempt weight loss succeed long term, the NWCR identifies and investigates the characteristics of individuals who have succeeded.
A new and encouraging finding is from a small study that showed that people can maintain weight loss brought about by either medical or surgical means: Those who lost > 15% of their starting weight and were followed closely by health care professionals maintained their weight loss at 1 year.5
Continue to: 2. Advise patients to consume fewer calories and eat more nonglycemic fruits and vegetables
2.
When a person loses weight, their basal metabolic rate drops; to maintain their new weight, they need to consume fewer calories. That person must continue to have a calorie deficit, which varies individually but is often about 500 kcal/d. There is no formula for this; at our clinic, when a patient achieves goal weight, we have them increase intake by 100 kcal/d/wk in nutritious food until they start to gain weight. When they start to gain weight, we have them decrease intake by 100 kcal/d until they do not gain any longer.
Many patients complain of hunger after they lose weight because of an increase in the body’s level of ghrelin, the hunger hormone, and a decrease in the level of leptin, which is associated with satiety. Many achieve a lower calorie count and fight hunger by increasing fiber intake.
In a 24-year study that looked at weight change, researchers noted a strong inverse association between increased intake of higher-fiber, lower-glycemic fruits and vegetables and weight change.6 Lower-glycemic vegetables include most vegetables (exceptions are corn, potatoes, and peas, which are associated with weight gain). Benefit was strongest with berries, apples, pears, tofu or soy, cauliflower, and cruciferous and green leafy vegetables.6 Adding 1 serving a day of nonstarchy fruits and vegetables was associated with less weight gain over time.
The order in which food is eaten might be important, as evidenced by a small study7 that focused on patients with diabetes. Investigators found that subjects who ate vegetables first, protein second, and grain third had fewer fluctuations in blood glucose level than those who ate carbohydrates first—suggesting that this order might be a good way for patients to eat at least some of their meals. The reduced insulin excursions observed in this experimental setting suggest that the vegetable–protein–grain meal pattern can improve insulin sensitivity and help with blood glucose control.
3.
In a small, randomized controlled study8 in 2019 at the National Institutes of Health, 20 inpatients were fed an ultraprocessed diet that was matched, in calories and macronutrients, in an unprocessed diet fed to controls. Subjects in the ultraprocessed food group ate, on average, 500 kcal/d more and gained 2 lbs in 2 weeks. An ultraprocessed breakfast might consist of a bagel with cream cheese and turkey bacon; the unprocessed breakfast was oatmeal with bananas, walnuts, and skim milk. Notably, the ultraprocessed diet was cheaper; nonprocessed foods cost 50% more.
Continue to: A retrospective review...
A retrospective review of a sample of US adults’ caloric and nutritional intake determined that eating at a full-service restaurant is not associated with consumption of fewer calories than eating at a fast food restaurant: Eating at either type of restaurant was associated with excess (approximately 200 kcal/meal) caloric intake.9
4. Emphasize the importance of eating breakfast and increasing protein intake
Increased protein throughout the day, particularly at breakfast, has been suggested to help with weight maintenance. In a large European study,10 even slightly increased protein intake (approximately 1.2 g/kg of body weight and of low glycemic index food) was associated with weight maintenance. In another review,11 researchers concluded that 25 to 30 g of protein at each meal can provide improvement in appetite and weight management, although they cautioned that further research is needed. A study that looked at increasing intake of protein at breakfast to 35 g in adolescent females resulted in less snacking later in the day.12
In the NWCR, successful maintainers had breakfast daily, a lower fat diet, and fewer calories (approximately 1500 kcal/d)— routines that were all associated with greater success.1 Therefore, eating protein at approximately 1.2 g/kg of body weight (possibly, even more [35 g] at breakfast) and ingesting less fat and fewer calories all contributed to successful maintenance. Eating nuts and legume-based proteins, such as beans and tofu, should be encouraged.
Only a few studies have looked at dairy protein intake and weight maintenance. In one study, consumption of dairy proteins was not associated with a change in body weight or other metabolic risk markers during weight maintenance.13 Yogurt, because of its probiotic content, might be good for weight maintenance, but this has not been studied well, and studies that have been conducted are inconclusive.14
Another study looked at consumption of protein supplements. It found no improvement in body composition over a 24-week period when protein intake was increased to 1.45 g/kg when compared to 1.16 g/kg in controls. Although subjects felt less hungry, this was not reflected in a reduction in caloric intake.15
Continue to: Most patients do need counseling...
Most patients do need counseling on whole grain intake: Explaining that a bagel is the same as 4 servings of toast and that a cup (ie, a fistful) of cooked pasta is 3 servings of grains is helpful. Patients should aim for 1 serving of grain at each meal; when shopping for grains, they should choose those that have the “whole” first on the list of ingredients because whole grain, rather than refined grain, intake is associated with less diabetes and colon cancer.16
5.
Self-monitoring is key to weight maintenance. This can mean weighing oneself or tracking one’s food intake (or both). Daily weighing is important: A study showed that patients who decrease how often they weigh themselves were likely to eat more and thus gain weight.17
Monitoring intake is also important. Recommended online calorie counters (eg, myfitnesspal.com, loseit.com), tools such as a Fitbit, or even keeping a food diary to help patients track intake. In a review of technology-based interventions to maintain weight loss, the use of apps was variable and effectiveness of devices was mixed. The authors recommended that physicians complement Web-based applications with personal contact.18
6. Encourage patients to spend more time exercising
After weight loss is achieved, maintaining a high level of activity is important. Recommendations focus on moving about 1 hr/d or 200 to 300 min/wk.19 A program of several daily “bouts,” or episodes of moderate-to-vigorous physical activity, is recommended in the new Centers for Disease Control and Prevention guidelines19 and might be preferable, or equivalent, to a concentrated expenditure of energy. Patients might consider, for example, a 10-minute session, 4 times a day, 5 days a week, instead of a single, 40-minute session, 5 days a week.19 Furthermore, to sustain weight loss, moderate exercise might be more effective than exercise of vigorous intensity or extended duration.19 Most patients in the NWCR report that walking is their principal form of activity.1
Resistance training, which improves muscle strength and endurance, with or without diet restriction, has not been shown to be effective for weight loss but might help with weight maintenance and might improve a patient’s lipid profile, insulin resistance, and blood pressure. In obese adolescents, resistance weight training led to positive changes in body composition, such as decreased waist circumference.20 Resistance training likely enhances weight maintenance and should be encouraged because of its effect on increasing lean muscle mass, the most important factor in determining basal metabolic rate.
Continue to: 7. Work with patients to ensure sound sleep hygiene
7. Work with patients to ensure sound sleep hygiene
Short sleep duration (< 6 hours a night) is associated with obesity. There are few studies on weight maintenance and sleep; a study that was reviewed by the NWCR found that people who are highly successful at both weight loss and long-term maintenance are more likely to (1) be categorized as a “morning-type” chronotype (ie, getting up early), and (2) report longer sleep duration and better sleep quality, compared to treatment-seeking overweight and obese subjects. Furthermore, these NWCR subjects were more likely to report shorter sleep latency (time required to fall asleep) and were less likely to report short sleep, defined as < 6 to 7 hours a night.21
Patients should strive for 7 to 8 hours of sleep a night; sleep apnea should be addressed as necessary.17 It is important for doctors to encourage patients to go to bed and get up at the same times every day (eg, 10 pm to 6 am daily).
8. Start a trial of medical therapy
Weight-loss medicines are beyond the scope of this article but worth discussing. In accordance with obesity guidelines, if a patient responds well to a weight-loss medication and loses ≥ 5% of body weight after 3 months, continue prescribing the medication. If the medication is ineffective or the patient experiences adverse effects, stop the prescription and consider an alternative medication or approach to maintenance.
The US Food and Drug Administration has approved 5 medications for long-term use in weight maintenance: the 2 combination formulations bupropion–naltrexone and phentermine–topiramate, as well as liraglutide, lorcaserin, and orlistat. A review of the use of these drugs over 1 year showed that they provide a modest favorable effect on cardiometabolic outcomes that vary by drug class.22 In particular, liraglutide has been shown to reduce the risk of cardiovascular disease outcomes in patients with diabetes who have a history of atherosclerotic disease or heart attack and stroke.23 Further research is needed to evaluate the long-term impact of these drugs on cardiovascular risk.
9.
Certain personal traits and behaviors appear to help people lose weight: In a study, maintainers were more likely to be characterized as being good problem-solvers, having hope, and having a more positive mood.24,25
Continue to: Addressing mental health issues...
Addressing mental health issues, especially depression, is paramount in patients with obesity. Treating patients with depression and hopelessness, as well as helping them with problem-solving, should be the focus of weight-management care.
Choosing an antidepressant not associated with the adverse effect of weight gain is important. Almost all selective serotonin reuptake inhibitors and tricyclic antidepressants are associated with weight gain; bupropion is weight neutral and should be first-line treatment for patients who are overweight and obese.26 If using an antidepressant associated with weight gain, initiate weight monitoring if the patient gains 3% of body weight in the first month of therapy. When caring for a patient who takes an antipsychotic, consider consulting with their mental health professional to determine the value of prescribing metformin, which has been shown to decrease weight gain associated with antipsychotics.27
Because depression, anxiety, and attention deficit-hyperactivity disorder are all associated with obesity, it is important to work with obese patients’ mental health care providers to design ways to improve their care.
10.
Focusing on establishing good habits can be helpful. In Great Britain, a program focused on forming new healthy habits and breaking old unhealthy habits by restructuring daily routines and increasing mindfulness was successful in keeping weight off in 65% of participants—an impressive degree of success.24 Successful maintainers were sent daily text messages requesting that they interrupt their routines, which shifted their attention away from eating. Some of these distracting tasks included driving to work by a different route or volunteering for a charity. Such small changes helped improve weight maintenance.
Intuitive eating and mindfulness can help. New concepts that focus on healthy eating without caloric restriction are also emerging; one such approach is intuitive eating, which promotes eating that is based not only on cues connected to hunger and fullness, but also on enjoyment of food, such as eating slowly and savoring every bite.28 Techniques such as sipping water or resting the fork between bites of food has been helpful with some patients. More research in the area of intuitive eating is needed.
Continue to: Recognizing true physiologic hunger...
Recognizing true physiologic hunger distinct from emotional hunger can improve with mindfulness training. This practice might provide a better way to help the so-called yo-yo dieter and binge eater to reach metabolic health. Interestingly, successful weight maintainers who allow themselves less restriction on weekends maintain weight better than those who try to restrict diet every day, according to a recent study.29
Recommend a support group. Accountability is important: Group therapy in obesity treatment may be more effective than individual treatment.30
The support of a group and the regular attendance at group meetings are connected to further significant weight loss in the weight-loss period and, later, during maintenance. Monthly meetings seem to help patients with weight maintenance but more research is needed to determine what interval of support-group meeting attendance is most effective.
CORRESPONDENCE
Marijane Hynes, MD, The George Washington University Medical Faculty Associates, Fifth Floor/Internal Medicine, 2150 Pennsylvania Avenue NW, Washington, DC 20037; [email protected].
1. Thomas JG, Bond DS, Phelan S, et al. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med. 2014;46:17-23.
2. Weiss EC, Galuska DA, Kettel Khan LK, et al. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34-40.
3. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374-1383.
4. Kulovitz MG, Kolkmeyer D, Conn CA, et al. Medical weight loss versus bariatric surgery: does method affect body composition and weight maintenance after 15% reduction in body weight? Nutrition. 2014;30:49-54.
5. Look AHEAD Research Group. Eight‐year weight losses with an intensive lifestyle intervention: The Look AHEAD study. Obesity (Silver Spring). 2014;22:5-13.
6. Bertoia ML, Mukamal KJ, Cahill LE, et al. Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: analysis from three prospective cohort studies. PLoS Med. 2015;12:e1001878.
7. Shukla AP, Iliescu RG, Thomas CE, et al. Food order has a significant impact on postprandial glucose and insulin levels. Diabetes Care. 2015;38:e98-e99.
8. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67-77.e3.
9. An R. Fast-food and full-service restaurant consumption and daily energy and nutrient intakes in US adults. Eur J Clin Nutr. 2016;70:97-103.
10. Larsen TM, Dalskov S-M, van Baak M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102-2113.
11. Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101:1320S-1329S.
12. Leidy HJ, Ortinau LC, Douglas SM, et al. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping,” late-adolescent girls. Am J Clin Nutr. 2013;97:677-688.
13. Bendtsen LQ, Lorenzen JK, Larsen TM, et al. Associations between dairy protein intake and body weight and risk markers of diabetes and CVD during weight maintenance. Br J Nutr. 2014;111:944-953.
14. Jacques PF, Wang H. Yogurt and weight management. Am J Clin Nutr. 2014;99(5 suppl):1229S-1234S.
15. Kjølbæk L, Sørensen LB, Søndertoft NB, et al. Protein supplements after weight loss do not improve weight maintenance compared with recommended dietary protein intake despite beneficial effects on appetite sensation and energy expenditure: a randomized, controlled, double-blinded trial. Am J Clin Nutr. 2017;106:684-697.
16. Reynolds A, Mann J, Cummings J, et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434-445.
17. Butryn ML, Phelan S, Hill JO, et al. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring, Md). 2007;15:3091-3096.
18. Lee S, Lindquist R. A review of technology-based interventions to maintain weight loss. Telemed J E Health. 2015;21:217-232.
19. 2018 Physical Activity Guidelines Advisory Committee, US Department of Health and Human Services. Physical activity guidelines for Americans. 2nd ed. Washington, DC: US Department of Health and Human Services; 2018. https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed January 13, 2020.
20. Shultz SP, Byrne NM, Dahiya MR, et al. Resistance weight training affects body composition in obese adolescents: a pilot study (Abstract 2932; Board #231). Med Sci Sports Exerc. 2011;43:834.
21. Ross KM, Graham Thomas JG, Wing RR. Successful weight loss maintenance associated with morning chronotype and better sleep quality. J Behav Med. 2016;39:465-471.
22. Khera R, Pandey A, Chandar AK, et al. Association of weight loss medications with cardiometabolic risk factors: systematic review and network meta-analysis. Circulation. 2016;134(suppl 1):A16413.
23. Verma RS, Poulter NR, Bhatt DL, et al. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without a history of myocardial infarction or stroke: post hoc analysis from the LEADER trial. Circulation. 2018;138:2884-2894.
24. Cleo G, Glasziou P, Beller E, et al. Habit-based interventions for weight loss maintenance in adults with overweight and obesity: a randomized controlled trial. Int J Obes (Lond). 2019;43:374-383.
25. Robertson S, Davies M, Winefield H. Positive psychological correlates of successful weight maintenance in Australia. Clinical Psychologist. 2017;21:236-244.
26. Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ. 2018;361:k1951.
27. Agarwal SM, Ahsan Z, Lockwood J, et al. A systematic review and meta-analysis of pharmacological interventions for reduction or prevention of weight gain in schizophrenia (poster). Schizophr Bull. 2018;44:S413.
28. Van Dyke N, Drinkwater Eric J. Relationships between intuitive eating and health indicators: literature review. Public Health Nutr. 2014;17:1757-1766.
29. Jorge R, Santos I, Teixeira VH, et al. Does diet strictness level during weekends and holiday periods influence 1-year follow-up weight loss maintenance? Evidence from the Portuguese Weight Control Registry. Nutr J. 2019;18:3.
30. Renjilian DA, Perri MG, Nezu AM, et al. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69:717-721.
New studies show that many people who lose weight can maintain that loss for longer than a few months by utilizing strategies that can be undertaken upon your recommendation and with your ongoing support. In this article, I review the evidence that supports the effectiveness of those interventions and activities for helping patients keep off the weight they’ve lost.
Prolonging the duration of weight maintenance
Until recently, most studies that focused on weight maintenance after weight loss followed subjects for only a few months or a year after the goal was achieved. With that limited window of follow-up, the belief arose in weight-loss medicine that most people gain back lost weight within 2 years. Findings that are emerging from recent studies with longer follow-up, however, suggest that weight loss can be maintained for as long as 8 years.1
The National Health and Nutrition Examination Survey2 and the Action in Health for Diabetes (Look AHEAD) trial3,4 reported that, among adults who lost 10% or more of body weight, approximately 60% maintained that weight loss at 1 year. Look AHEAD had a much longer duration: 42% of participants who lost at least 10% of body weight by the end of Year 1 maintained at least that 10% loss by the end of Year 4.5 In addition, Look AHEAD demonstrated that extended provision of maintenance interventions after weight loss can facilitate clinically meaningful weight loss for as long as 8 years—2 or 3 times longer than what was reported in earlier randomized trials.4
We have evidence-based guidance for achieving long-term weight maintenance and good reason to believe that success is achievable for patients. The 10 strategies that follow can help you to guide patients to become successful “maintainers.”

1. Emphasize more weight loss in the first 3 months of a program
Losing more weight initially seems to point to more success in relation to maintenance. This suggests that more intensive help, such as more frequent visits with a physician and a dietitian during the first 3 months might be an important step to help patients lose and maintain weight.
Much of our information on successful maintainers comes from the National Weight Control Registry (NWCR) at the Warren Alpert Medical School at Brown University.1 This research study has gathered information from more than 10,000 people who successfully lost ≥ 30 lb (average, 60 lb) and kept it off for at least 1 year. To challenge the widespread belief that only a few people who attempt weight loss succeed long term, the NWCR identifies and investigates the characteristics of individuals who have succeeded.
A new and encouraging finding is from a small study that showed that people can maintain weight loss brought about by either medical or surgical means: Those who lost > 15% of their starting weight and were followed closely by health care professionals maintained their weight loss at 1 year.5
Continue to: 2. Advise patients to consume fewer calories and eat more nonglycemic fruits and vegetables
2.
When a person loses weight, their basal metabolic rate drops; to maintain their new weight, they need to consume fewer calories. That person must continue to have a calorie deficit, which varies individually but is often about 500 kcal/d. There is no formula for this; at our clinic, when a patient achieves goal weight, we have them increase intake by 100 kcal/d/wk in nutritious food until they start to gain weight. When they start to gain weight, we have them decrease intake by 100 kcal/d until they do not gain any longer.
Many patients complain of hunger after they lose weight because of an increase in the body’s level of ghrelin, the hunger hormone, and a decrease in the level of leptin, which is associated with satiety. Many achieve a lower calorie count and fight hunger by increasing fiber intake.
In a 24-year study that looked at weight change, researchers noted a strong inverse association between increased intake of higher-fiber, lower-glycemic fruits and vegetables and weight change.6 Lower-glycemic vegetables include most vegetables (exceptions are corn, potatoes, and peas, which are associated with weight gain). Benefit was strongest with berries, apples, pears, tofu or soy, cauliflower, and cruciferous and green leafy vegetables.6 Adding 1 serving a day of nonstarchy fruits and vegetables was associated with less weight gain over time.
The order in which food is eaten might be important, as evidenced by a small study7 that focused on patients with diabetes. Investigators found that subjects who ate vegetables first, protein second, and grain third had fewer fluctuations in blood glucose level than those who ate carbohydrates first—suggesting that this order might be a good way for patients to eat at least some of their meals. The reduced insulin excursions observed in this experimental setting suggest that the vegetable–protein–grain meal pattern can improve insulin sensitivity and help with blood glucose control.
3.
In a small, randomized controlled study8 in 2019 at the National Institutes of Health, 20 inpatients were fed an ultraprocessed diet that was matched, in calories and macronutrients, in an unprocessed diet fed to controls. Subjects in the ultraprocessed food group ate, on average, 500 kcal/d more and gained 2 lbs in 2 weeks. An ultraprocessed breakfast might consist of a bagel with cream cheese and turkey bacon; the unprocessed breakfast was oatmeal with bananas, walnuts, and skim milk. Notably, the ultraprocessed diet was cheaper; nonprocessed foods cost 50% more.
Continue to: A retrospective review...
A retrospective review of a sample of US adults’ caloric and nutritional intake determined that eating at a full-service restaurant is not associated with consumption of fewer calories than eating at a fast food restaurant: Eating at either type of restaurant was associated with excess (approximately 200 kcal/meal) caloric intake.9
4. Emphasize the importance of eating breakfast and increasing protein intake
Increased protein throughout the day, particularly at breakfast, has been suggested to help with weight maintenance. In a large European study,10 even slightly increased protein intake (approximately 1.2 g/kg of body weight and of low glycemic index food) was associated with weight maintenance. In another review,11 researchers concluded that 25 to 30 g of protein at each meal can provide improvement in appetite and weight management, although they cautioned that further research is needed. A study that looked at increasing intake of protein at breakfast to 35 g in adolescent females resulted in less snacking later in the day.12
In the NWCR, successful maintainers had breakfast daily, a lower fat diet, and fewer calories (approximately 1500 kcal/d)— routines that were all associated with greater success.1 Therefore, eating protein at approximately 1.2 g/kg of body weight (possibly, even more [35 g] at breakfast) and ingesting less fat and fewer calories all contributed to successful maintenance. Eating nuts and legume-based proteins, such as beans and tofu, should be encouraged.
Only a few studies have looked at dairy protein intake and weight maintenance. In one study, consumption of dairy proteins was not associated with a change in body weight or other metabolic risk markers during weight maintenance.13 Yogurt, because of its probiotic content, might be good for weight maintenance, but this has not been studied well, and studies that have been conducted are inconclusive.14
Another study looked at consumption of protein supplements. It found no improvement in body composition over a 24-week period when protein intake was increased to 1.45 g/kg when compared to 1.16 g/kg in controls. Although subjects felt less hungry, this was not reflected in a reduction in caloric intake.15
Continue to: Most patients do need counseling...
Most patients do need counseling on whole grain intake: Explaining that a bagel is the same as 4 servings of toast and that a cup (ie, a fistful) of cooked pasta is 3 servings of grains is helpful. Patients should aim for 1 serving of grain at each meal; when shopping for grains, they should choose those that have the “whole” first on the list of ingredients because whole grain, rather than refined grain, intake is associated with less diabetes and colon cancer.16
5.
Self-monitoring is key to weight maintenance. This can mean weighing oneself or tracking one’s food intake (or both). Daily weighing is important: A study showed that patients who decrease how often they weigh themselves were likely to eat more and thus gain weight.17
Monitoring intake is also important. Recommended online calorie counters (eg, myfitnesspal.com, loseit.com), tools such as a Fitbit, or even keeping a food diary to help patients track intake. In a review of technology-based interventions to maintain weight loss, the use of apps was variable and effectiveness of devices was mixed. The authors recommended that physicians complement Web-based applications with personal contact.18
6. Encourage patients to spend more time exercising
After weight loss is achieved, maintaining a high level of activity is important. Recommendations focus on moving about 1 hr/d or 200 to 300 min/wk.19 A program of several daily “bouts,” or episodes of moderate-to-vigorous physical activity, is recommended in the new Centers for Disease Control and Prevention guidelines19 and might be preferable, or equivalent, to a concentrated expenditure of energy. Patients might consider, for example, a 10-minute session, 4 times a day, 5 days a week, instead of a single, 40-minute session, 5 days a week.19 Furthermore, to sustain weight loss, moderate exercise might be more effective than exercise of vigorous intensity or extended duration.19 Most patients in the NWCR report that walking is their principal form of activity.1
Resistance training, which improves muscle strength and endurance, with or without diet restriction, has not been shown to be effective for weight loss but might help with weight maintenance and might improve a patient’s lipid profile, insulin resistance, and blood pressure. In obese adolescents, resistance weight training led to positive changes in body composition, such as decreased waist circumference.20 Resistance training likely enhances weight maintenance and should be encouraged because of its effect on increasing lean muscle mass, the most important factor in determining basal metabolic rate.
Continue to: 7. Work with patients to ensure sound sleep hygiene
7. Work with patients to ensure sound sleep hygiene
Short sleep duration (< 6 hours a night) is associated with obesity. There are few studies on weight maintenance and sleep; a study that was reviewed by the NWCR found that people who are highly successful at both weight loss and long-term maintenance are more likely to (1) be categorized as a “morning-type” chronotype (ie, getting up early), and (2) report longer sleep duration and better sleep quality, compared to treatment-seeking overweight and obese subjects. Furthermore, these NWCR subjects were more likely to report shorter sleep latency (time required to fall asleep) and were less likely to report short sleep, defined as < 6 to 7 hours a night.21
Patients should strive for 7 to 8 hours of sleep a night; sleep apnea should be addressed as necessary.17 It is important for doctors to encourage patients to go to bed and get up at the same times every day (eg, 10 pm to 6 am daily).
8. Start a trial of medical therapy
Weight-loss medicines are beyond the scope of this article but worth discussing. In accordance with obesity guidelines, if a patient responds well to a weight-loss medication and loses ≥ 5% of body weight after 3 months, continue prescribing the medication. If the medication is ineffective or the patient experiences adverse effects, stop the prescription and consider an alternative medication or approach to maintenance.
The US Food and Drug Administration has approved 5 medications for long-term use in weight maintenance: the 2 combination formulations bupropion–naltrexone and phentermine–topiramate, as well as liraglutide, lorcaserin, and orlistat. A review of the use of these drugs over 1 year showed that they provide a modest favorable effect on cardiometabolic outcomes that vary by drug class.22 In particular, liraglutide has been shown to reduce the risk of cardiovascular disease outcomes in patients with diabetes who have a history of atherosclerotic disease or heart attack and stroke.23 Further research is needed to evaluate the long-term impact of these drugs on cardiovascular risk.
9.
Certain personal traits and behaviors appear to help people lose weight: In a study, maintainers were more likely to be characterized as being good problem-solvers, having hope, and having a more positive mood.24,25
Continue to: Addressing mental health issues...
Addressing mental health issues, especially depression, is paramount in patients with obesity. Treating patients with depression and hopelessness, as well as helping them with problem-solving, should be the focus of weight-management care.
Choosing an antidepressant not associated with the adverse effect of weight gain is important. Almost all selective serotonin reuptake inhibitors and tricyclic antidepressants are associated with weight gain; bupropion is weight neutral and should be first-line treatment for patients who are overweight and obese.26 If using an antidepressant associated with weight gain, initiate weight monitoring if the patient gains 3% of body weight in the first month of therapy. When caring for a patient who takes an antipsychotic, consider consulting with their mental health professional to determine the value of prescribing metformin, which has been shown to decrease weight gain associated with antipsychotics.27
Because depression, anxiety, and attention deficit-hyperactivity disorder are all associated with obesity, it is important to work with obese patients’ mental health care providers to design ways to improve their care.
10.
Focusing on establishing good habits can be helpful. In Great Britain, a program focused on forming new healthy habits and breaking old unhealthy habits by restructuring daily routines and increasing mindfulness was successful in keeping weight off in 65% of participants—an impressive degree of success.24 Successful maintainers were sent daily text messages requesting that they interrupt their routines, which shifted their attention away from eating. Some of these distracting tasks included driving to work by a different route or volunteering for a charity. Such small changes helped improve weight maintenance.
Intuitive eating and mindfulness can help. New concepts that focus on healthy eating without caloric restriction are also emerging; one such approach is intuitive eating, which promotes eating that is based not only on cues connected to hunger and fullness, but also on enjoyment of food, such as eating slowly and savoring every bite.28 Techniques such as sipping water or resting the fork between bites of food has been helpful with some patients. More research in the area of intuitive eating is needed.
Continue to: Recognizing true physiologic hunger...
Recognizing true physiologic hunger distinct from emotional hunger can improve with mindfulness training. This practice might provide a better way to help the so-called yo-yo dieter and binge eater to reach metabolic health. Interestingly, successful weight maintainers who allow themselves less restriction on weekends maintain weight better than those who try to restrict diet every day, according to a recent study.29
Recommend a support group. Accountability is important: Group therapy in obesity treatment may be more effective than individual treatment.30
The support of a group and the regular attendance at group meetings are connected to further significant weight loss in the weight-loss period and, later, during maintenance. Monthly meetings seem to help patients with weight maintenance but more research is needed to determine what interval of support-group meeting attendance is most effective.
CORRESPONDENCE
Marijane Hynes, MD, The George Washington University Medical Faculty Associates, Fifth Floor/Internal Medicine, 2150 Pennsylvania Avenue NW, Washington, DC 20037; [email protected].
New studies show that many people who lose weight can maintain that loss for longer than a few months by utilizing strategies that can be undertaken upon your recommendation and with your ongoing support. In this article, I review the evidence that supports the effectiveness of those interventions and activities for helping patients keep off the weight they’ve lost.
Prolonging the duration of weight maintenance
Until recently, most studies that focused on weight maintenance after weight loss followed subjects for only a few months or a year after the goal was achieved. With that limited window of follow-up, the belief arose in weight-loss medicine that most people gain back lost weight within 2 years. Findings that are emerging from recent studies with longer follow-up, however, suggest that weight loss can be maintained for as long as 8 years.1
The National Health and Nutrition Examination Survey2 and the Action in Health for Diabetes (Look AHEAD) trial3,4 reported that, among adults who lost 10% or more of body weight, approximately 60% maintained that weight loss at 1 year. Look AHEAD had a much longer duration: 42% of participants who lost at least 10% of body weight by the end of Year 1 maintained at least that 10% loss by the end of Year 4.5 In addition, Look AHEAD demonstrated that extended provision of maintenance interventions after weight loss can facilitate clinically meaningful weight loss for as long as 8 years—2 or 3 times longer than what was reported in earlier randomized trials.4
We have evidence-based guidance for achieving long-term weight maintenance and good reason to believe that success is achievable for patients. The 10 strategies that follow can help you to guide patients to become successful “maintainers.”

1. Emphasize more weight loss in the first 3 months of a program
Losing more weight initially seems to point to more success in relation to maintenance. This suggests that more intensive help, such as more frequent visits with a physician and a dietitian during the first 3 months might be an important step to help patients lose and maintain weight.
Much of our information on successful maintainers comes from the National Weight Control Registry (NWCR) at the Warren Alpert Medical School at Brown University.1 This research study has gathered information from more than 10,000 people who successfully lost ≥ 30 lb (average, 60 lb) and kept it off for at least 1 year. To challenge the widespread belief that only a few people who attempt weight loss succeed long term, the NWCR identifies and investigates the characteristics of individuals who have succeeded.
A new and encouraging finding is from a small study that showed that people can maintain weight loss brought about by either medical or surgical means: Those who lost > 15% of their starting weight and were followed closely by health care professionals maintained their weight loss at 1 year.5
Continue to: 2. Advise patients to consume fewer calories and eat more nonglycemic fruits and vegetables
2.
When a person loses weight, their basal metabolic rate drops; to maintain their new weight, they need to consume fewer calories. That person must continue to have a calorie deficit, which varies individually but is often about 500 kcal/d. There is no formula for this; at our clinic, when a patient achieves goal weight, we have them increase intake by 100 kcal/d/wk in nutritious food until they start to gain weight. When they start to gain weight, we have them decrease intake by 100 kcal/d until they do not gain any longer.
Many patients complain of hunger after they lose weight because of an increase in the body’s level of ghrelin, the hunger hormone, and a decrease in the level of leptin, which is associated with satiety. Many achieve a lower calorie count and fight hunger by increasing fiber intake.
In a 24-year study that looked at weight change, researchers noted a strong inverse association between increased intake of higher-fiber, lower-glycemic fruits and vegetables and weight change.6 Lower-glycemic vegetables include most vegetables (exceptions are corn, potatoes, and peas, which are associated with weight gain). Benefit was strongest with berries, apples, pears, tofu or soy, cauliflower, and cruciferous and green leafy vegetables.6 Adding 1 serving a day of nonstarchy fruits and vegetables was associated with less weight gain over time.
The order in which food is eaten might be important, as evidenced by a small study7 that focused on patients with diabetes. Investigators found that subjects who ate vegetables first, protein second, and grain third had fewer fluctuations in blood glucose level than those who ate carbohydrates first—suggesting that this order might be a good way for patients to eat at least some of their meals. The reduced insulin excursions observed in this experimental setting suggest that the vegetable–protein–grain meal pattern can improve insulin sensitivity and help with blood glucose control.
3.
In a small, randomized controlled study8 in 2019 at the National Institutes of Health, 20 inpatients were fed an ultraprocessed diet that was matched, in calories and macronutrients, in an unprocessed diet fed to controls. Subjects in the ultraprocessed food group ate, on average, 500 kcal/d more and gained 2 lbs in 2 weeks. An ultraprocessed breakfast might consist of a bagel with cream cheese and turkey bacon; the unprocessed breakfast was oatmeal with bananas, walnuts, and skim milk. Notably, the ultraprocessed diet was cheaper; nonprocessed foods cost 50% more.
Continue to: A retrospective review...
A retrospective review of a sample of US adults’ caloric and nutritional intake determined that eating at a full-service restaurant is not associated with consumption of fewer calories than eating at a fast food restaurant: Eating at either type of restaurant was associated with excess (approximately 200 kcal/meal) caloric intake.9
4. Emphasize the importance of eating breakfast and increasing protein intake
Increased protein throughout the day, particularly at breakfast, has been suggested to help with weight maintenance. In a large European study,10 even slightly increased protein intake (approximately 1.2 g/kg of body weight and of low glycemic index food) was associated with weight maintenance. In another review,11 researchers concluded that 25 to 30 g of protein at each meal can provide improvement in appetite and weight management, although they cautioned that further research is needed. A study that looked at increasing intake of protein at breakfast to 35 g in adolescent females resulted in less snacking later in the day.12
In the NWCR, successful maintainers had breakfast daily, a lower fat diet, and fewer calories (approximately 1500 kcal/d)— routines that were all associated with greater success.1 Therefore, eating protein at approximately 1.2 g/kg of body weight (possibly, even more [35 g] at breakfast) and ingesting less fat and fewer calories all contributed to successful maintenance. Eating nuts and legume-based proteins, such as beans and tofu, should be encouraged.
Only a few studies have looked at dairy protein intake and weight maintenance. In one study, consumption of dairy proteins was not associated with a change in body weight or other metabolic risk markers during weight maintenance.13 Yogurt, because of its probiotic content, might be good for weight maintenance, but this has not been studied well, and studies that have been conducted are inconclusive.14
Another study looked at consumption of protein supplements. It found no improvement in body composition over a 24-week period when protein intake was increased to 1.45 g/kg when compared to 1.16 g/kg in controls. Although subjects felt less hungry, this was not reflected in a reduction in caloric intake.15
Continue to: Most patients do need counseling...
Most patients do need counseling on whole grain intake: Explaining that a bagel is the same as 4 servings of toast and that a cup (ie, a fistful) of cooked pasta is 3 servings of grains is helpful. Patients should aim for 1 serving of grain at each meal; when shopping for grains, they should choose those that have the “whole” first on the list of ingredients because whole grain, rather than refined grain, intake is associated with less diabetes and colon cancer.16
5.
Self-monitoring is key to weight maintenance. This can mean weighing oneself or tracking one’s food intake (or both). Daily weighing is important: A study showed that patients who decrease how often they weigh themselves were likely to eat more and thus gain weight.17
Monitoring intake is also important. Recommended online calorie counters (eg, myfitnesspal.com, loseit.com), tools such as a Fitbit, or even keeping a food diary to help patients track intake. In a review of technology-based interventions to maintain weight loss, the use of apps was variable and effectiveness of devices was mixed. The authors recommended that physicians complement Web-based applications with personal contact.18
6. Encourage patients to spend more time exercising
After weight loss is achieved, maintaining a high level of activity is important. Recommendations focus on moving about 1 hr/d or 200 to 300 min/wk.19 A program of several daily “bouts,” or episodes of moderate-to-vigorous physical activity, is recommended in the new Centers for Disease Control and Prevention guidelines19 and might be preferable, or equivalent, to a concentrated expenditure of energy. Patients might consider, for example, a 10-minute session, 4 times a day, 5 days a week, instead of a single, 40-minute session, 5 days a week.19 Furthermore, to sustain weight loss, moderate exercise might be more effective than exercise of vigorous intensity or extended duration.19 Most patients in the NWCR report that walking is their principal form of activity.1
Resistance training, which improves muscle strength and endurance, with or without diet restriction, has not been shown to be effective for weight loss but might help with weight maintenance and might improve a patient’s lipid profile, insulin resistance, and blood pressure. In obese adolescents, resistance weight training led to positive changes in body composition, such as decreased waist circumference.20 Resistance training likely enhances weight maintenance and should be encouraged because of its effect on increasing lean muscle mass, the most important factor in determining basal metabolic rate.
Continue to: 7. Work with patients to ensure sound sleep hygiene
7. Work with patients to ensure sound sleep hygiene
Short sleep duration (< 6 hours a night) is associated with obesity. There are few studies on weight maintenance and sleep; a study that was reviewed by the NWCR found that people who are highly successful at both weight loss and long-term maintenance are more likely to (1) be categorized as a “morning-type” chronotype (ie, getting up early), and (2) report longer sleep duration and better sleep quality, compared to treatment-seeking overweight and obese subjects. Furthermore, these NWCR subjects were more likely to report shorter sleep latency (time required to fall asleep) and were less likely to report short sleep, defined as < 6 to 7 hours a night.21
Patients should strive for 7 to 8 hours of sleep a night; sleep apnea should be addressed as necessary.17 It is important for doctors to encourage patients to go to bed and get up at the same times every day (eg, 10 pm to 6 am daily).
8. Start a trial of medical therapy
Weight-loss medicines are beyond the scope of this article but worth discussing. In accordance with obesity guidelines, if a patient responds well to a weight-loss medication and loses ≥ 5% of body weight after 3 months, continue prescribing the medication. If the medication is ineffective or the patient experiences adverse effects, stop the prescription and consider an alternative medication or approach to maintenance.
The US Food and Drug Administration has approved 5 medications for long-term use in weight maintenance: the 2 combination formulations bupropion–naltrexone and phentermine–topiramate, as well as liraglutide, lorcaserin, and orlistat. A review of the use of these drugs over 1 year showed that they provide a modest favorable effect on cardiometabolic outcomes that vary by drug class.22 In particular, liraglutide has been shown to reduce the risk of cardiovascular disease outcomes in patients with diabetes who have a history of atherosclerotic disease or heart attack and stroke.23 Further research is needed to evaluate the long-term impact of these drugs on cardiovascular risk.
9.
Certain personal traits and behaviors appear to help people lose weight: In a study, maintainers were more likely to be characterized as being good problem-solvers, having hope, and having a more positive mood.24,25
Continue to: Addressing mental health issues...
Addressing mental health issues, especially depression, is paramount in patients with obesity. Treating patients with depression and hopelessness, as well as helping them with problem-solving, should be the focus of weight-management care.
Choosing an antidepressant not associated with the adverse effect of weight gain is important. Almost all selective serotonin reuptake inhibitors and tricyclic antidepressants are associated with weight gain; bupropion is weight neutral and should be first-line treatment for patients who are overweight and obese.26 If using an antidepressant associated with weight gain, initiate weight monitoring if the patient gains 3% of body weight in the first month of therapy. When caring for a patient who takes an antipsychotic, consider consulting with their mental health professional to determine the value of prescribing metformin, which has been shown to decrease weight gain associated with antipsychotics.27
Because depression, anxiety, and attention deficit-hyperactivity disorder are all associated with obesity, it is important to work with obese patients’ mental health care providers to design ways to improve their care.
10.
Focusing on establishing good habits can be helpful. In Great Britain, a program focused on forming new healthy habits and breaking old unhealthy habits by restructuring daily routines and increasing mindfulness was successful in keeping weight off in 65% of participants—an impressive degree of success.24 Successful maintainers were sent daily text messages requesting that they interrupt their routines, which shifted their attention away from eating. Some of these distracting tasks included driving to work by a different route or volunteering for a charity. Such small changes helped improve weight maintenance.
Intuitive eating and mindfulness can help. New concepts that focus on healthy eating without caloric restriction are also emerging; one such approach is intuitive eating, which promotes eating that is based not only on cues connected to hunger and fullness, but also on enjoyment of food, such as eating slowly and savoring every bite.28 Techniques such as sipping water or resting the fork between bites of food has been helpful with some patients. More research in the area of intuitive eating is needed.
Continue to: Recognizing true physiologic hunger...
Recognizing true physiologic hunger distinct from emotional hunger can improve with mindfulness training. This practice might provide a better way to help the so-called yo-yo dieter and binge eater to reach metabolic health. Interestingly, successful weight maintainers who allow themselves less restriction on weekends maintain weight better than those who try to restrict diet every day, according to a recent study.29
Recommend a support group. Accountability is important: Group therapy in obesity treatment may be more effective than individual treatment.30
The support of a group and the regular attendance at group meetings are connected to further significant weight loss in the weight-loss period and, later, during maintenance. Monthly meetings seem to help patients with weight maintenance but more research is needed to determine what interval of support-group meeting attendance is most effective.
CORRESPONDENCE
Marijane Hynes, MD, The George Washington University Medical Faculty Associates, Fifth Floor/Internal Medicine, 2150 Pennsylvania Avenue NW, Washington, DC 20037; [email protected].
1. Thomas JG, Bond DS, Phelan S, et al. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med. 2014;46:17-23.
2. Weiss EC, Galuska DA, Kettel Khan LK, et al. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34-40.
3. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374-1383.
4. Kulovitz MG, Kolkmeyer D, Conn CA, et al. Medical weight loss versus bariatric surgery: does method affect body composition and weight maintenance after 15% reduction in body weight? Nutrition. 2014;30:49-54.
5. Look AHEAD Research Group. Eight‐year weight losses with an intensive lifestyle intervention: The Look AHEAD study. Obesity (Silver Spring). 2014;22:5-13.
6. Bertoia ML, Mukamal KJ, Cahill LE, et al. Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: analysis from three prospective cohort studies. PLoS Med. 2015;12:e1001878.
7. Shukla AP, Iliescu RG, Thomas CE, et al. Food order has a significant impact on postprandial glucose and insulin levels. Diabetes Care. 2015;38:e98-e99.
8. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67-77.e3.
9. An R. Fast-food and full-service restaurant consumption and daily energy and nutrient intakes in US adults. Eur J Clin Nutr. 2016;70:97-103.
10. Larsen TM, Dalskov S-M, van Baak M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102-2113.
11. Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101:1320S-1329S.
12. Leidy HJ, Ortinau LC, Douglas SM, et al. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping,” late-adolescent girls. Am J Clin Nutr. 2013;97:677-688.
13. Bendtsen LQ, Lorenzen JK, Larsen TM, et al. Associations between dairy protein intake and body weight and risk markers of diabetes and CVD during weight maintenance. Br J Nutr. 2014;111:944-953.
14. Jacques PF, Wang H. Yogurt and weight management. Am J Clin Nutr. 2014;99(5 suppl):1229S-1234S.
15. Kjølbæk L, Sørensen LB, Søndertoft NB, et al. Protein supplements after weight loss do not improve weight maintenance compared with recommended dietary protein intake despite beneficial effects on appetite sensation and energy expenditure: a randomized, controlled, double-blinded trial. Am J Clin Nutr. 2017;106:684-697.
16. Reynolds A, Mann J, Cummings J, et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434-445.
17. Butryn ML, Phelan S, Hill JO, et al. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring, Md). 2007;15:3091-3096.
18. Lee S, Lindquist R. A review of technology-based interventions to maintain weight loss. Telemed J E Health. 2015;21:217-232.
19. 2018 Physical Activity Guidelines Advisory Committee, US Department of Health and Human Services. Physical activity guidelines for Americans. 2nd ed. Washington, DC: US Department of Health and Human Services; 2018. https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed January 13, 2020.
20. Shultz SP, Byrne NM, Dahiya MR, et al. Resistance weight training affects body composition in obese adolescents: a pilot study (Abstract 2932; Board #231). Med Sci Sports Exerc. 2011;43:834.
21. Ross KM, Graham Thomas JG, Wing RR. Successful weight loss maintenance associated with morning chronotype and better sleep quality. J Behav Med. 2016;39:465-471.
22. Khera R, Pandey A, Chandar AK, et al. Association of weight loss medications with cardiometabolic risk factors: systematic review and network meta-analysis. Circulation. 2016;134(suppl 1):A16413.
23. Verma RS, Poulter NR, Bhatt DL, et al. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without a history of myocardial infarction or stroke: post hoc analysis from the LEADER trial. Circulation. 2018;138:2884-2894.
24. Cleo G, Glasziou P, Beller E, et al. Habit-based interventions for weight loss maintenance in adults with overweight and obesity: a randomized controlled trial. Int J Obes (Lond). 2019;43:374-383.
25. Robertson S, Davies M, Winefield H. Positive psychological correlates of successful weight maintenance in Australia. Clinical Psychologist. 2017;21:236-244.
26. Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ. 2018;361:k1951.
27. Agarwal SM, Ahsan Z, Lockwood J, et al. A systematic review and meta-analysis of pharmacological interventions for reduction or prevention of weight gain in schizophrenia (poster). Schizophr Bull. 2018;44:S413.
28. Van Dyke N, Drinkwater Eric J. Relationships between intuitive eating and health indicators: literature review. Public Health Nutr. 2014;17:1757-1766.
29. Jorge R, Santos I, Teixeira VH, et al. Does diet strictness level during weekends and holiday periods influence 1-year follow-up weight loss maintenance? Evidence from the Portuguese Weight Control Registry. Nutr J. 2019;18:3.
30. Renjilian DA, Perri MG, Nezu AM, et al. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69:717-721.
1. Thomas JG, Bond DS, Phelan S, et al. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med. 2014;46:17-23.
2. Weiss EC, Galuska DA, Kettel Khan LK, et al. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34-40.
3. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374-1383.
4. Kulovitz MG, Kolkmeyer D, Conn CA, et al. Medical weight loss versus bariatric surgery: does method affect body composition and weight maintenance after 15% reduction in body weight? Nutrition. 2014;30:49-54.
5. Look AHEAD Research Group. Eight‐year weight losses with an intensive lifestyle intervention: The Look AHEAD study. Obesity (Silver Spring). 2014;22:5-13.
6. Bertoia ML, Mukamal KJ, Cahill LE, et al. Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: analysis from three prospective cohort studies. PLoS Med. 2015;12:e1001878.
7. Shukla AP, Iliescu RG, Thomas CE, et al. Food order has a significant impact on postprandial glucose and insulin levels. Diabetes Care. 2015;38:e98-e99.
8. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67-77.e3.
9. An R. Fast-food and full-service restaurant consumption and daily energy and nutrient intakes in US adults. Eur J Clin Nutr. 2016;70:97-103.
10. Larsen TM, Dalskov S-M, van Baak M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102-2113.
11. Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101:1320S-1329S.
12. Leidy HJ, Ortinau LC, Douglas SM, et al. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping,” late-adolescent girls. Am J Clin Nutr. 2013;97:677-688.
13. Bendtsen LQ, Lorenzen JK, Larsen TM, et al. Associations between dairy protein intake and body weight and risk markers of diabetes and CVD during weight maintenance. Br J Nutr. 2014;111:944-953.
14. Jacques PF, Wang H. Yogurt and weight management. Am J Clin Nutr. 2014;99(5 suppl):1229S-1234S.
15. Kjølbæk L, Sørensen LB, Søndertoft NB, et al. Protein supplements after weight loss do not improve weight maintenance compared with recommended dietary protein intake despite beneficial effects on appetite sensation and energy expenditure: a randomized, controlled, double-blinded trial. Am J Clin Nutr. 2017;106:684-697.
16. Reynolds A, Mann J, Cummings J, et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434-445.
17. Butryn ML, Phelan S, Hill JO, et al. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring, Md). 2007;15:3091-3096.
18. Lee S, Lindquist R. A review of technology-based interventions to maintain weight loss. Telemed J E Health. 2015;21:217-232.
19. 2018 Physical Activity Guidelines Advisory Committee, US Department of Health and Human Services. Physical activity guidelines for Americans. 2nd ed. Washington, DC: US Department of Health and Human Services; 2018. https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed January 13, 2020.
20. Shultz SP, Byrne NM, Dahiya MR, et al. Resistance weight training affects body composition in obese adolescents: a pilot study (Abstract 2932; Board #231). Med Sci Sports Exerc. 2011;43:834.
21. Ross KM, Graham Thomas JG, Wing RR. Successful weight loss maintenance associated with morning chronotype and better sleep quality. J Behav Med. 2016;39:465-471.
22. Khera R, Pandey A, Chandar AK, et al. Association of weight loss medications with cardiometabolic risk factors: systematic review and network meta-analysis. Circulation. 2016;134(suppl 1):A16413.
23. Verma RS, Poulter NR, Bhatt DL, et al. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without a history of myocardial infarction or stroke: post hoc analysis from the LEADER trial. Circulation. 2018;138:2884-2894.
24. Cleo G, Glasziou P, Beller E, et al. Habit-based interventions for weight loss maintenance in adults with overweight and obesity: a randomized controlled trial. Int J Obes (Lond). 2019;43:374-383.
25. Robertson S, Davies M, Winefield H. Positive psychological correlates of successful weight maintenance in Australia. Clinical Psychologist. 2017;21:236-244.
26. Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: population based cohort study. BMJ. 2018;361:k1951.
27. Agarwal SM, Ahsan Z, Lockwood J, et al. A systematic review and meta-analysis of pharmacological interventions for reduction or prevention of weight gain in schizophrenia (poster). Schizophr Bull. 2018;44:S413.
28. Van Dyke N, Drinkwater Eric J. Relationships between intuitive eating and health indicators: literature review. Public Health Nutr. 2014;17:1757-1766.
29. Jorge R, Santos I, Teixeira VH, et al. Does diet strictness level during weekends and holiday periods influence 1-year follow-up weight loss maintenance? Evidence from the Portuguese Weight Control Registry. Nutr J. 2019;18:3.
30. Renjilian DA, Perri MG, Nezu AM, et al. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69:717-721.
PRACTICE RECOMMENDATIONS
› Encourage patients to lose more weight early in their effort, which is predictive of successful long-term maintenance. B
› Support patients’ efforts to maintain weight loss by encouraging them to consume fewer calories and eat more nonglycemic fruits and vegetables A, eat at home and avoid processed foods B, work with you in addressing mental health concerns B, and increase time spent exercising A.
› Consider the potential value of prescribing a US Food and Drug Administration–indicated medication for weight maintenance. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Bringing the HPV vaccination rate into line with other adolescent immunizations
Overall adolescent vaccination coverage is improving in the United States.1 But for adolescents up to 15 years of age, there’s a large gap between the rate of vaccination for human papillomavirus (HPV) and the higher rates of coverage for tetanus, diphtheria, and acellular pertussis (Tdap) and meningococcal conjugate (MenACWY) vaccines.1 Adopting or refining practice customs reviewed in this article can increase HPV vaccination rates and continue to improve coverage of all vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) for adolescents between the ages of 11 and 12.
The evolution of ACIP’s HPV vaccine recommendations
Before December 2016, ACIP recommended a 3-dose HPV series for all adolescents between the ages of 11 and 12, given on a 0, 1-2, and 6-month schedule.2 The series could be started at 9 years of age. It could be administered to females as old as 26 years, and to males through 21 years (or ages 22-26 years for those who wish to be vaccinated, who have certain medical conditions, or who are included in special populations—ie, gay and bisexual men, men who have sex with men, immunocompromised men, men with human immunodeficiency virus [HIV], and transgender men).
In 2016, ACIP revised its recommendation for adolescents who initiate vaccination before their 15th birthday: a 2-dose schedule is adequate, with the second dose given 6 to 12 months after the first dose. For those who initiate vaccination on or after their 15th birthday, and for those with certain medical conditions, the recommendation remains 3 doses on a 0, 1-2, and 6-month schedule.3
As of August 2019,4 ACIP now recommends that all women and men receive catch-up HPV vaccination through age 26. For individuals 27 to 45 years of age who have not been adequately vaccinated, HPV vaccine may be given based on shared clinical decision making with their physician.
How are we doing?
Overall, adolescent vaccination coverage is improving in the United States (see “Vaccination goals from ACIP and Healthy People 2020”1,5,6), but the rate of improvement of HPV coverage is lower than that for Tdap and MenACWY coverage by age 15 years (although completion of the MenACWY vaccine series is low). From 2015 to 2016, coverage increased for 1 or more doses of Tdap, from 86.4% to 88% among 17-year olds (87.9% for 15-year olds), and coverage for 1 or more doses of MenACWY increased from 81.7% to 83.5% among 17-year olds (80.4% among 15-year olds).1 Both Tdap and MenACWY coverage rates have surpassed Healthy People 2020 goals of 80%, and the focus now is on maintenance of coverage. Data from the 2016 National Immunization Survey (NIS)-Teen show that completion of the HPV vaccine series (applying updated HPV vaccine recommendations retrospectively) increased to 45.4% for 15-year-olds,1 still far below the Healthy People 2020 goal of 80%. Completion rates for 2 or more doses of MenACWY also increased from 33.3% to 39.1%.1
SIDEBAR
Vaccination goals from ACIP and Healthy People 2020
The Advisory Committee on Immunization Practices (ACIP) recommends that adolescents routinely receive several vaccines between the ages of 11 and 12 years: an annual influenza vaccine, Tdap, the first dose of MenACWY, and initiation of the HPV series. ACIP also advises a booster dose of MenACWY at age 16 years, and teens and young adults (16-23 years) also may be vaccinated with a multidose serogroup B meningococcal vaccine, preferably before age 18. For those adolescents not up to date with their childhood vaccines, ACIP recommends the following catch-up vaccinations: measles, mumps, rubella (MMR, 2 doses); hepatitis B (HepB, 3 doses); and varicella (VAR, 2 doses).5
Healthy People 2020. In December 2010, the US Department of Health and Human Services released Healthy People 2020, a wide-ranging initiative on health promotion and disease prevention that includes 10-year objectives of increasing coverage with Tdap, at least one dose of MenACWY, and completion of the HPV series among 80% of those ages 13 to 15 years.6 This initiative reflects extensive feedback from more than 2000 organizations and authorities in public health and prevention at federal, state, and local levels—as well as from the public. Adolescent vaccination coverage is estimated by the Centers for Disease Control and Prevention using data from the National Immunization Survey (NIS)-Teen annual survey conducted among parents and guardians of adolescents ages 13 to 17 years.1
Common barriers to improved vaccine coverage
Barriers to improved vaccination rates include a lack of regular assessment of vaccine status; limited use of electronic records, tools, and immunization registries; lack of health care provider knowledge on current vaccine recommendations; vaccine costs; missed opportunities; and patient/parent refusals.7,8 The Community Preventive Services Task Force outlines several well-established evidence-based ways that administrators and physicians can counter these barriers:
- give a strong recommendation to vaccinate,9,10
- incorporate an audit/feedback mechanism for health care providers who vaccinate,9,11
- use electronic alerts to remind health care providers to vaccinate,9,12
- use your state’s electronic immunization information systems (IIS),7,13
- appoint a vaccine practice team/vaccine champion,9,14 and
- implement standing orders and reminder/recall systems.7,9,15
The passage of the Affordable Care Act (ACA)—which mandates that certain preventive services, including ACIP-recommended immunizations, be covered as part of basic care at no cost-sharing—reduces the once-common financial barrier to vaccine uptake.16 A key contributor to low uptake of HPV vaccination by adolescents is parental refusal.17
Continue to: The threats posed by HPV
The threats posed by HPV
HPV infections are the most commonly transmitted infections in the United States and nearly all men and women will be exposed to one or more types of HPV at some point in their lives. Current data show that 79 million Americans, most in their late teens and early 20s, are infected with HPV, and about 14 million people in the United States become newly infected each year.18 HPV is a DNA tumor virus that causes epithelial proliferation at cutaneous and mucosal surfaces.
There are more than 100 types of the virus,19 including more than 40 strains that infect the human genital tract. Of the latter 40 strains, there are oncogenic or high-risk types and non-oncogenic or low-risk types.20 HPV infection with high-risk types causes cervical, vaginal, and vulvar cancers in women; penile cancers in men; and oropharyngeal and anal cancers in both men and women. Low-risk HPV types cause genital warts in both men and women.21 The current available HPV vaccine in the United States is a 9-valent vaccine (9vHPV) that replaces the former 2- and 4-valent HPV vaccines and includes immunogenic coverage against high-risk HPV types 16, 18, 31, 33, 45, 52, and 58; and low-risk types 6 and 11.22
Centers for Disease Control and Prevention (CDC) data from 2010 to 2014 show that approximately 23,700 women and approximately 17,300 men in the United States developed HPV-associated cancer. Most common in women are cervical cancers and in men, oropharyngeal cancers (cancers of the back of the throat, base of the tongue, and tonsils). Using population-based data to genotype HPV types from cancer tissues, the CDC reports that HPV is responsible for about 90% of cervical and anal cancers, 70% of oropharyngeal, vaginal, and vulvar cancers, and 60% of penile cancers.23 A significant percentage of these cancers could potentially be prevented by receipt of 9vHPV.23,24
Make adolescent immunization a high priority
Anticipate opportunities to vaccinate and take steps to make your immunization and scheduling processes more prominent. With HPV specifically, you can strongly advocate for vaccination, address parental misgivings and educate them using clear communication styles, and acquire knowledge to answer concerns about potential vaccine adverse effects.
Every visit is an opportunity to vaccinate. The American Academy of Family Physicians and The American Academy of Pediatrics recommend that adolescents have annual preventive visits for screening, immunizations, and assessment and counseling for risky behaviors. However, many adolescents do not present annually for preventive visits, and fewer than half of adolescents receive regular preventive care.
Continue to: Missed opportunities for the HPV vaccine
Missed opportunities for the HPV vaccine. One study showed that at least 86% of unvaccinated adolescents had missed opportunities to receive HPV vaccine.29 A study of 14,588 adolescent girls from January 2010 through August 2015 showed that HPV vaccine was given at only 37.1% of visits in which MenACWY or Tdap vaccines were administered.30 The rate of HPV vaccination was just 26% during well adolescent visits, and 41.8% during all other primary care visits.30 Every adolescent health care visit—including visits for acute care, chronic care, follow-up, or office-based procedures—is an opportunity to review vaccination status.
Give vaccines concomitantly (simultaneously or same-day). ACIP counsels that minor illnesses, such as mild upper respiratory infections with or without low-grade fever, are not contraindications to routine vaccination.30 Also, the safety of simultaneous vaccine administration, often a concern of both parents and health care providers, has been well established. Each vaccine’s immunogenicity and safety profile are maintained when given concomitantly with other vaccines, and fewer visits are needed to complete an adolescent’s vaccination status.31,32
Immediately schedule follow up visits and use reminder/recall systems. Parents of adolescents who opt for HPV vaccination are not always aware of the timing of the 2- or 3-dose schedule and may not even be aware that more than 1 dose of vaccine is recommended.
A qualitative study of pediatric primary care providers and parents/guardians of adolescent patients showed that for HPV vaccination series completion, 65% of parents/guardians expected to be reminded of any needed doses, while 52% of the pediatric primary care providers relied on parents to schedule subsequent immunizations, and often the HPV series was not completed.33 Higher completion rates of the HPV vaccination series were achieved when follow-up appointments were scheduled at checkout for the 2nd or 3rd vaccine dose after initiation of HPV vaccination.33 The use of patient reminder/recall systems using telephone calls or mailings (phone usage is more effective than mailings) is also shown to improve vaccination completion rates.34
Recommend HPV vaccination clearly and resolutely
In a cross-sectional survey of 800 parents of adolescents ages 9 to 14 years, HPV vaccine was deemed the least likely vaccine to have been “very strongly” recommended by their health care provider, compared with the strength of recommendations for influenza, Tdap, and MenACWY vaccines.35 The strength of a health care provider’s recommendation to vaccinate is the single most influential factor in vaccine uptake.10,36,37 Most family physicians self-report “always recommending standard pediatric vaccines”; however, only a minority are following ACIP recommendations.38 A national study reported that only about two-thirds of parents who received HPV vaccine recommendations perceived a high level of health care provider endorsement.39 The takeaway point: Give a clear, unambiguous, strong recommendation to vaccinate with HPV to prevent infection; cervical, oropharyngeal, and other cancers; and genital warts.
Continue to: Tell parents why the timing is important
Tell parents why the timing is important. Inform parents that the HPV vaccine must be administered while their child is young (before the adolescent’s first sexual contact) to ensure the most robust immune response to the vaccine.40 Unsolicited explanations about sexual activity need not be offered when discussing HPV vaccination, as it is fair to assume that sexual contact is a reality for nearly all people in their adolescent or adult life; and by extension, most sexually active people will likely have exposure to HPV at some time in their lives. By adulthood, sexual activity is nearly universal: The National Longitudinal Study of Adolescent Health showed that only about 3% of participants tracked since adolescence reported no sexual experience by (average age) 28.5 years.41
How you say it matters. Many pediatricians and family physicians report recommending HPV vaccine inconsistently, behind schedule, or without urgency,42 sending mixed messages by failing to endorse HPV vaccination strongly, failing to differentiate it from other vaccines, and presenting it as an “optional” vaccine that could be delayed.43 Physicians and other health care providers who begin conversations about HPV vaccine by saying that the adolescent is “due” for the vaccine show higher vaccine recommendation quality scores than those who give unsolicited information about the vaccine, elicit questions before recommendation, or present the vaccine as an “option.”42 Parents who are “on the fence” may hesitate and decline HPV vaccination with a halfhearted recommendation.44
“Your child is due for his/her Tdap, HPV, influenza, and meningococcal vaccinations to prevent potentially devastating disease and several cancers. I highly recommend all 4 vaccinations today” is more persuasive than, “I recommend your child receive his/her Tdap, meningococcal, and influenza vaccines. And we can also discuss the HPV vaccine.”
Direct presumptive language that assumes vaccine delivery is associated with higher odds of HPV vaccine acceptance and same-day agreement to vaccination than is an open-ended participatory conversational style.45 Saying, “I believe in the importance of this cancer-preventing vaccine for your child” is more persuasive than saying, “What do you think about starting the HPV vaccination series today?”46
Don’t give up when parents initially refuse HPV vaccinations for their adolescents. Parents’ decisions about HPV vaccination may change over time. Repeated positive recommendations and counseling for HPV vaccination over multiple visits have been shown in a large multivariable analysis to increase parent acceptance of HPV vaccination: 45% of parents reported secondary acceptance of HPV vaccination, and an additional 24% intended to vaccinate in the next 12 months.47 Combining a presumptive communication style with motivational interviewing and a fact sheet has contributed to higher clinician-perceived levels of parental HPV vaccine acceptance and increased vaccination rates.48
Continue to: Know how to address parents' concerns about safety
Know how to address parents’ concerns about safety
Be prepared to discuss and answer parents’ questions or concerns regarding any vaccine, especially the HPV vaccine. Social networks are important in parents’ vaccination decision-making,49 and they may seek information from such sources as Twitter, Facebook, Google, and YouTube, where misinformation may be disseminated. A quantitative analysis of 560 YouTube videos relaying a false link between vaccines and autism or other serious adverse effects on children were uploaded between December 2007 and July 2017, with a peak of 224 videos uploaded in the first 7 months of 2017.50 Most were negative in tone and dispensed misinformation.50
The National Vaccine Information Center (NVIC) is an organization that takes a skeptical view of the US government and pharmaceutical companies. NVIC is widely criticized by scientists and leaders in vaccine science and public health as spreading false information on the risks of vaccines and, specifically, that HPV vaccination causes chronic disease. NVIC reports that receipt of HPV vaccine may increase the risk for cervical cancer and death.51 Pediatrician and vaccine researcher Dr. Paul Offit, interviewed by The Lancet in response to NVIC and other anti-vaccine groups’ messages, stated: “anti-vaccination organizations are unequivocally threatening public health.”52
Describe the robust safety-monitoring system. The CDC is aware of public concern about the safety of HPV vaccine. Ongoing monitoring of vaccine safety and studies conducted by the CDC, the Food and Drug Administration (FDA), and other organizations has documented a reassuring safety record since the vaccine’s introduction in 2006.53 Assure parents that the Vaccine Adverse Event Reporting System (VAERS) summary of 7244 reports following 9vHPV vaccination (December 1, 2014 – December 31, 2017) showed that most (97%) reports were nonserious: No new safety signals or unexpected patterns were observed, confirming consistency of the safety profile of 9vHPV with data from pre-licensure trials and post-licensure data on 4vHPV.54
Acknowledge the usually mild, transient potential risks of HPV vaccination as reported to VAERS: local injection site symptoms such as pain, redness, or swelling in the arm where the injection was given (most common adverse effect), dizziness, fainting, headache, nausea, and fever.53 Point out that fainting after vaccination is common in adolescents55 and that the CDC and ACIP recommend observation of adolescents for 15 minutes following HPV vaccination.56 Consider this 15-minute observation period after adolescent receipt of any vaccine to be part of standard practice in your vaccination setting.56
Contest unfounded views. Other common parental concerns about effects of HPV vaccine include supposed promotion of promiscuity, increased incidence of premature ovarian failure or insufficiency (POI), and increased risk of Guillain-Barré Syndrome (GBS), often propagated through published reports, media coverage, Web sites, and social media. Assure worried parents that many studies have shown that receipt of the vaccine is safe and does not lead to initiation of sexual activity or promiscuity, and, in fact, safer sexual health practices have been observed following vaccination.57-59
Continue to: A large longitudinal...
A large longitudinal adolescent health survey administered in British Columbia looked at sexual health behaviors and risk factors in adolescent girls before and after receipt of HPV vaccination (2003, 2008, 2013).59 Results showed no significant change in the reported number of sexual partners (2003-2013), increased reported use of contraception and condoms, and lower pregnancy rates.59 There is no evidence that HPV vaccines cause reproductive problems in women53; a review of VAERS reports from 2009 through 2015 did not detect any safety concerns for POI or other reproductive problems in females.60 A 2018 population-based study of nearly 200,000 women observed no increase of POI following receipt of HPV vaccination.61 In addition, several recent studies have shown no increased risk for GBS following receipt of HPV vaccine.62-64
CORRESPONDENCE
Pamela G. Rockwell, DO, FAAFP, 24 Frank Lloyd Wright Drive, SPC 5795, Room 2300, Lobby H, Ann Arbor, MI 48105; [email protected].
1. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:1-30.
3. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
5. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices (ACIP) Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 18 years or younger—United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66:134-135.
6. US Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020. www.healthypeople.gov/node/4654/data_details. Accessed December 4, 2019.
7. Rockwell PG. What you can do to improve adult immunization rates. J Fam Pract. 2015;64:625-633.
8. Kimmel Sr, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
9. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based guide to community preventive services-methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
10. Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the U.S. National Immunization Survey. Am J Public Health. 2013;103:164-169.
11. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2011;60:1-64.
12. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
13. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
14. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
15. Task Force on Community Preventive Services. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(suppl 1):92-96.
16. US Department of Health and Human Services. Preventive care. www.hhs.gov/healthcare/about-the-aca/preventive-care/index.html. Accessed December 4, 2019.
17. Gilkey MB, Calo WA, Marciniak, MW, et al. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Hum Vaccin Immunother. 2017;13:680-686.
18. CDC. Genital HPV infection—fact sheet. www.cdc.gov/std/hpv/stdfact-hpv.htm. Accessed December 4, 2019.
19. WHO. Human papillomavirus (HPV) and cervical cancer. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accessed December 4, 2019.
20. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-527.
21. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
22. CDC. Luxembourg A. Program summary and new 9-valent HPV vaccine trial data. Presented at the Advisory Committee on Immunization Practices (ACIP), October 30, 2014. Atlanta, Ga. 2014. www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-10.pdf. Accessed December 4, 2019.
23. CDC. HPV and cancer. www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed December 4, 2019.
24. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23.
25. Rand CM, Goldstein NPN. Patterns of primary care physician visits for US adolescents in 2014: implications for vaccination. Acad Pediatr. 2018;18:S72-S78.
26. Taylor JL, Aalsma MC, Gilbert AL, et al. Perspectives of family medicine physicians on the importance of adolescent preventive care: a multivariate analysis. BMC Fam Pract. 2016;17:4.
27. Harris SK, Aalsma MC, Weitzman ER, et al. Research on clinical preventive services for adolescents and young adults: Where are we and where do we need to go? J Adolesc Health. 2017;60:249-260.
28. Gilkey MB, Moss JL, McRee AL, et al. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30:5928-5934.
29. Espinosa CM, Marshall GS, Woods CR, et al. Missed opportunities for human papillomavirus vaccine initiation in an insured adolescent female population. J Pediatric Infect Dis Soc. 2017;6:360-365.
30. CDC. Update: Vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45:1-35.
31. Moss JL, Reiter PL, Brewer NT. Concomitant adolescent vaccination in the U.S., 2007-2012. Am J Prev Med. 2016;51:693-705.
32. Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine. 2014;32:2670-2674.
33. Perkins RB, Chigurupati NL, Apte G, et al. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother. 2016;12:1528-1535.
34. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
35. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18:S23-S27.
36. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
37. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother. 2013;9:2627-2633.
38. Bonville CA, Domachowske JB, Cibula DA, et al. Immunization attitudes and practices among family medicine providers. Hum Vaccin Immunother. 2017;13:2646-2653.
39. Wilson R, Brown DR, Boothe MA, et al. Knowledge and acceptability of the HPV vaccine among ethnically diverse black women. J Immigr Minor Health. 2013;15:747-757.
40. Iversen O, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–2421.
41. Haydon AA, Cheng MM, Herring AH, et al. Prevalence and predictors of sexual inexperience in adulthood. Arch Sex Behav. 2014;43:221-230.
42. Gilkey MB, Malo TL, Shah PD, et al. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev. 2015;24:1673-1679.
43. Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systemic review. Hum Vaccin Immunother. 2016;12:1454-1468.
44. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed December 4, 2019.
45. Sturm L, Donahue K, Kasting M, et al. Pediatrician-parent conversations about human papillomavirus vaccination: an analysis of audio recordings. J Adolesc Health. 2017;61:246-251.
46. Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
47. Kornides ML, McRee AL, Gilkey MB. Parents who decline HPV vaccination: Who later accepts and why? Acad Pediatr. 2018;18:S37-S43.
48. Reno JE, Thomas J, Pyrzanowski J, et al. Examining strategies for improving healthcare providers’ communication about adolescent HPV vaccination: evaluation of secondary outcomes in a randomized controlled trial. Hum Vaccin Immunother. 2018;15:1592-1598.
49. Brunson EK. The impact of social networks on parents’ vaccination decisions. Pediatrics. 2013;131:e1397-e1404.
50. Donzelli G, Palomba G, Federigi L, et al. Misinformation on vaccination: a quantitative analysis of YouTube videos. Hum Vaccin Immunother. 2018;14:1654-1659.
51. National Vaccine Information Center. Human papillomavirus (HPV) disease and vaccine information. www.nvic.org/Vaccines-and-Diseases/hpv.aspx. Accessed December 4, 2019.
52. Shetty P. Experts concerned about vaccination backlash. Lancet. 2010; 375:970-971.
53. CDC. Frequently asked questions about HPV vaccine safety. www.cdc.gov/vaccinesafety/vaccines/hpv/hpv-safety-faqs.html. Accessed December 4, 2019.
54. Arana J, Su J, Lewis P, et al. Post-licensure surveillance of 9-valent human papillomavirus vaccine (9vHPV) in the Vaccine Adverse Event Reporting System (VAERS), United States, 2014-2017. https://idsa.confex.com/idsa/2018/webprogram/Paper69618.html. Accessed December 4, 2019.
55. Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Ped Adolesc Med. 1997;151:255-259.
56. Kroger AT, Duchin J, Vázquez M. General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html. Accessed December 4, 2019.
57. Hansen BT. No evidence that HPV vaccination leads to sexual risk compensation. Hum Vaccin Immunother. 2016;12:1451-1453.
58. Smith LM, Kaufman JS, Strumpf EC, et al. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. 2015;187:E74-81.
59. Ogilvie GS, Phan F, Pederson HN, et al. Population-level sexual behaviours in adolescent girls before and after introduction of the human papillomavirus vaccine (2003-2013). CMAJ. 2018;190:E1221-E1226.
60. Arana JE, Harrington T, Cano M, et al. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015. Vaccine. 2018;36:1781-1788.
61. Naleway AL, Mittendorf KF, Irving SA, et al. Primary ovarian insufficiency and adolescent vaccination. Pediatrics. 2018;142. pii: e20190943.
62. Deceuninck G, Sauvageau C, Gilca V, et al. Absence of association between Guillain-Barré syndrome hospitalizations and HPV-vaccine. Expert Rev Vaccines. 2018;17:99-102.
63. Mouchet J, Salvo F, Raschi E, et al. Human papillomavirus vaccine and demyelinating diseases – a systematic review and meta-analysis. Pharmacol Res. 2018;132:108-118.
64. Gee J, Sukumaran L, Weinstraub E, et al. Risk of Guillain-Barre Syndrome following quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink. Vaccine. 2017;35:5756-5758.
Overall adolescent vaccination coverage is improving in the United States.1 But for adolescents up to 15 years of age, there’s a large gap between the rate of vaccination for human papillomavirus (HPV) and the higher rates of coverage for tetanus, diphtheria, and acellular pertussis (Tdap) and meningococcal conjugate (MenACWY) vaccines.1 Adopting or refining practice customs reviewed in this article can increase HPV vaccination rates and continue to improve coverage of all vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) for adolescents between the ages of 11 and 12.
The evolution of ACIP’s HPV vaccine recommendations
Before December 2016, ACIP recommended a 3-dose HPV series for all adolescents between the ages of 11 and 12, given on a 0, 1-2, and 6-month schedule.2 The series could be started at 9 years of age. It could be administered to females as old as 26 years, and to males through 21 years (or ages 22-26 years for those who wish to be vaccinated, who have certain medical conditions, or who are included in special populations—ie, gay and bisexual men, men who have sex with men, immunocompromised men, men with human immunodeficiency virus [HIV], and transgender men).
In 2016, ACIP revised its recommendation for adolescents who initiate vaccination before their 15th birthday: a 2-dose schedule is adequate, with the second dose given 6 to 12 months after the first dose. For those who initiate vaccination on or after their 15th birthday, and for those with certain medical conditions, the recommendation remains 3 doses on a 0, 1-2, and 6-month schedule.3
As of August 2019,4 ACIP now recommends that all women and men receive catch-up HPV vaccination through age 26. For individuals 27 to 45 years of age who have not been adequately vaccinated, HPV vaccine may be given based on shared clinical decision making with their physician.
How are we doing?
Overall, adolescent vaccination coverage is improving in the United States (see “Vaccination goals from ACIP and Healthy People 2020”1,5,6), but the rate of improvement of HPV coverage is lower than that for Tdap and MenACWY coverage by age 15 years (although completion of the MenACWY vaccine series is low). From 2015 to 2016, coverage increased for 1 or more doses of Tdap, from 86.4% to 88% among 17-year olds (87.9% for 15-year olds), and coverage for 1 or more doses of MenACWY increased from 81.7% to 83.5% among 17-year olds (80.4% among 15-year olds).1 Both Tdap and MenACWY coverage rates have surpassed Healthy People 2020 goals of 80%, and the focus now is on maintenance of coverage. Data from the 2016 National Immunization Survey (NIS)-Teen show that completion of the HPV vaccine series (applying updated HPV vaccine recommendations retrospectively) increased to 45.4% for 15-year-olds,1 still far below the Healthy People 2020 goal of 80%. Completion rates for 2 or more doses of MenACWY also increased from 33.3% to 39.1%.1
SIDEBAR
Vaccination goals from ACIP and Healthy People 2020
The Advisory Committee on Immunization Practices (ACIP) recommends that adolescents routinely receive several vaccines between the ages of 11 and 12 years: an annual influenza vaccine, Tdap, the first dose of MenACWY, and initiation of the HPV series. ACIP also advises a booster dose of MenACWY at age 16 years, and teens and young adults (16-23 years) also may be vaccinated with a multidose serogroup B meningococcal vaccine, preferably before age 18. For those adolescents not up to date with their childhood vaccines, ACIP recommends the following catch-up vaccinations: measles, mumps, rubella (MMR, 2 doses); hepatitis B (HepB, 3 doses); and varicella (VAR, 2 doses).5
Healthy People 2020. In December 2010, the US Department of Health and Human Services released Healthy People 2020, a wide-ranging initiative on health promotion and disease prevention that includes 10-year objectives of increasing coverage with Tdap, at least one dose of MenACWY, and completion of the HPV series among 80% of those ages 13 to 15 years.6 This initiative reflects extensive feedback from more than 2000 organizations and authorities in public health and prevention at federal, state, and local levels—as well as from the public. Adolescent vaccination coverage is estimated by the Centers for Disease Control and Prevention using data from the National Immunization Survey (NIS)-Teen annual survey conducted among parents and guardians of adolescents ages 13 to 17 years.1
Common barriers to improved vaccine coverage
Barriers to improved vaccination rates include a lack of regular assessment of vaccine status; limited use of electronic records, tools, and immunization registries; lack of health care provider knowledge on current vaccine recommendations; vaccine costs; missed opportunities; and patient/parent refusals.7,8 The Community Preventive Services Task Force outlines several well-established evidence-based ways that administrators and physicians can counter these barriers:
- give a strong recommendation to vaccinate,9,10
- incorporate an audit/feedback mechanism for health care providers who vaccinate,9,11
- use electronic alerts to remind health care providers to vaccinate,9,12
- use your state’s electronic immunization information systems (IIS),7,13
- appoint a vaccine practice team/vaccine champion,9,14 and
- implement standing orders and reminder/recall systems.7,9,15
The passage of the Affordable Care Act (ACA)—which mandates that certain preventive services, including ACIP-recommended immunizations, be covered as part of basic care at no cost-sharing—reduces the once-common financial barrier to vaccine uptake.16 A key contributor to low uptake of HPV vaccination by adolescents is parental refusal.17
Continue to: The threats posed by HPV
The threats posed by HPV
HPV infections are the most commonly transmitted infections in the United States and nearly all men and women will be exposed to one or more types of HPV at some point in their lives. Current data show that 79 million Americans, most in their late teens and early 20s, are infected with HPV, and about 14 million people in the United States become newly infected each year.18 HPV is a DNA tumor virus that causes epithelial proliferation at cutaneous and mucosal surfaces.
There are more than 100 types of the virus,19 including more than 40 strains that infect the human genital tract. Of the latter 40 strains, there are oncogenic or high-risk types and non-oncogenic or low-risk types.20 HPV infection with high-risk types causes cervical, vaginal, and vulvar cancers in women; penile cancers in men; and oropharyngeal and anal cancers in both men and women. Low-risk HPV types cause genital warts in both men and women.21 The current available HPV vaccine in the United States is a 9-valent vaccine (9vHPV) that replaces the former 2- and 4-valent HPV vaccines and includes immunogenic coverage against high-risk HPV types 16, 18, 31, 33, 45, 52, and 58; and low-risk types 6 and 11.22
Centers for Disease Control and Prevention (CDC) data from 2010 to 2014 show that approximately 23,700 women and approximately 17,300 men in the United States developed HPV-associated cancer. Most common in women are cervical cancers and in men, oropharyngeal cancers (cancers of the back of the throat, base of the tongue, and tonsils). Using population-based data to genotype HPV types from cancer tissues, the CDC reports that HPV is responsible for about 90% of cervical and anal cancers, 70% of oropharyngeal, vaginal, and vulvar cancers, and 60% of penile cancers.23 A significant percentage of these cancers could potentially be prevented by receipt of 9vHPV.23,24
Make adolescent immunization a high priority
Anticipate opportunities to vaccinate and take steps to make your immunization and scheduling processes more prominent. With HPV specifically, you can strongly advocate for vaccination, address parental misgivings and educate them using clear communication styles, and acquire knowledge to answer concerns about potential vaccine adverse effects.
Every visit is an opportunity to vaccinate. The American Academy of Family Physicians and The American Academy of Pediatrics recommend that adolescents have annual preventive visits for screening, immunizations, and assessment and counseling for risky behaviors. However, many adolescents do not present annually for preventive visits, and fewer than half of adolescents receive regular preventive care.
Continue to: Missed opportunities for the HPV vaccine
Missed opportunities for the HPV vaccine. One study showed that at least 86% of unvaccinated adolescents had missed opportunities to receive HPV vaccine.29 A study of 14,588 adolescent girls from January 2010 through August 2015 showed that HPV vaccine was given at only 37.1% of visits in which MenACWY or Tdap vaccines were administered.30 The rate of HPV vaccination was just 26% during well adolescent visits, and 41.8% during all other primary care visits.30 Every adolescent health care visit—including visits for acute care, chronic care, follow-up, or office-based procedures—is an opportunity to review vaccination status.
Give vaccines concomitantly (simultaneously or same-day). ACIP counsels that minor illnesses, such as mild upper respiratory infections with or without low-grade fever, are not contraindications to routine vaccination.30 Also, the safety of simultaneous vaccine administration, often a concern of both parents and health care providers, has been well established. Each vaccine’s immunogenicity and safety profile are maintained when given concomitantly with other vaccines, and fewer visits are needed to complete an adolescent’s vaccination status.31,32
Immediately schedule follow up visits and use reminder/recall systems. Parents of adolescents who opt for HPV vaccination are not always aware of the timing of the 2- or 3-dose schedule and may not even be aware that more than 1 dose of vaccine is recommended.
A qualitative study of pediatric primary care providers and parents/guardians of adolescent patients showed that for HPV vaccination series completion, 65% of parents/guardians expected to be reminded of any needed doses, while 52% of the pediatric primary care providers relied on parents to schedule subsequent immunizations, and often the HPV series was not completed.33 Higher completion rates of the HPV vaccination series were achieved when follow-up appointments were scheduled at checkout for the 2nd or 3rd vaccine dose after initiation of HPV vaccination.33 The use of patient reminder/recall systems using telephone calls or mailings (phone usage is more effective than mailings) is also shown to improve vaccination completion rates.34
Recommend HPV vaccination clearly and resolutely
In a cross-sectional survey of 800 parents of adolescents ages 9 to 14 years, HPV vaccine was deemed the least likely vaccine to have been “very strongly” recommended by their health care provider, compared with the strength of recommendations for influenza, Tdap, and MenACWY vaccines.35 The strength of a health care provider’s recommendation to vaccinate is the single most influential factor in vaccine uptake.10,36,37 Most family physicians self-report “always recommending standard pediatric vaccines”; however, only a minority are following ACIP recommendations.38 A national study reported that only about two-thirds of parents who received HPV vaccine recommendations perceived a high level of health care provider endorsement.39 The takeaway point: Give a clear, unambiguous, strong recommendation to vaccinate with HPV to prevent infection; cervical, oropharyngeal, and other cancers; and genital warts.
Continue to: Tell parents why the timing is important
Tell parents why the timing is important. Inform parents that the HPV vaccine must be administered while their child is young (before the adolescent’s first sexual contact) to ensure the most robust immune response to the vaccine.40 Unsolicited explanations about sexual activity need not be offered when discussing HPV vaccination, as it is fair to assume that sexual contact is a reality for nearly all people in their adolescent or adult life; and by extension, most sexually active people will likely have exposure to HPV at some time in their lives. By adulthood, sexual activity is nearly universal: The National Longitudinal Study of Adolescent Health showed that only about 3% of participants tracked since adolescence reported no sexual experience by (average age) 28.5 years.41
How you say it matters. Many pediatricians and family physicians report recommending HPV vaccine inconsistently, behind schedule, or without urgency,42 sending mixed messages by failing to endorse HPV vaccination strongly, failing to differentiate it from other vaccines, and presenting it as an “optional” vaccine that could be delayed.43 Physicians and other health care providers who begin conversations about HPV vaccine by saying that the adolescent is “due” for the vaccine show higher vaccine recommendation quality scores than those who give unsolicited information about the vaccine, elicit questions before recommendation, or present the vaccine as an “option.”42 Parents who are “on the fence” may hesitate and decline HPV vaccination with a halfhearted recommendation.44
“Your child is due for his/her Tdap, HPV, influenza, and meningococcal vaccinations to prevent potentially devastating disease and several cancers. I highly recommend all 4 vaccinations today” is more persuasive than, “I recommend your child receive his/her Tdap, meningococcal, and influenza vaccines. And we can also discuss the HPV vaccine.”
Direct presumptive language that assumes vaccine delivery is associated with higher odds of HPV vaccine acceptance and same-day agreement to vaccination than is an open-ended participatory conversational style.45 Saying, “I believe in the importance of this cancer-preventing vaccine for your child” is more persuasive than saying, “What do you think about starting the HPV vaccination series today?”46
Don’t give up when parents initially refuse HPV vaccinations for their adolescents. Parents’ decisions about HPV vaccination may change over time. Repeated positive recommendations and counseling for HPV vaccination over multiple visits have been shown in a large multivariable analysis to increase parent acceptance of HPV vaccination: 45% of parents reported secondary acceptance of HPV vaccination, and an additional 24% intended to vaccinate in the next 12 months.47 Combining a presumptive communication style with motivational interviewing and a fact sheet has contributed to higher clinician-perceived levels of parental HPV vaccine acceptance and increased vaccination rates.48
Continue to: Know how to address parents' concerns about safety
Know how to address parents’ concerns about safety
Be prepared to discuss and answer parents’ questions or concerns regarding any vaccine, especially the HPV vaccine. Social networks are important in parents’ vaccination decision-making,49 and they may seek information from such sources as Twitter, Facebook, Google, and YouTube, where misinformation may be disseminated. A quantitative analysis of 560 YouTube videos relaying a false link between vaccines and autism or other serious adverse effects on children were uploaded between December 2007 and July 2017, with a peak of 224 videos uploaded in the first 7 months of 2017.50 Most were negative in tone and dispensed misinformation.50
The National Vaccine Information Center (NVIC) is an organization that takes a skeptical view of the US government and pharmaceutical companies. NVIC is widely criticized by scientists and leaders in vaccine science and public health as spreading false information on the risks of vaccines and, specifically, that HPV vaccination causes chronic disease. NVIC reports that receipt of HPV vaccine may increase the risk for cervical cancer and death.51 Pediatrician and vaccine researcher Dr. Paul Offit, interviewed by The Lancet in response to NVIC and other anti-vaccine groups’ messages, stated: “anti-vaccination organizations are unequivocally threatening public health.”52
Describe the robust safety-monitoring system. The CDC is aware of public concern about the safety of HPV vaccine. Ongoing monitoring of vaccine safety and studies conducted by the CDC, the Food and Drug Administration (FDA), and other organizations has documented a reassuring safety record since the vaccine’s introduction in 2006.53 Assure parents that the Vaccine Adverse Event Reporting System (VAERS) summary of 7244 reports following 9vHPV vaccination (December 1, 2014 – December 31, 2017) showed that most (97%) reports were nonserious: No new safety signals or unexpected patterns were observed, confirming consistency of the safety profile of 9vHPV with data from pre-licensure trials and post-licensure data on 4vHPV.54
Acknowledge the usually mild, transient potential risks of HPV vaccination as reported to VAERS: local injection site symptoms such as pain, redness, or swelling in the arm where the injection was given (most common adverse effect), dizziness, fainting, headache, nausea, and fever.53 Point out that fainting after vaccination is common in adolescents55 and that the CDC and ACIP recommend observation of adolescents for 15 minutes following HPV vaccination.56 Consider this 15-minute observation period after adolescent receipt of any vaccine to be part of standard practice in your vaccination setting.56
Contest unfounded views. Other common parental concerns about effects of HPV vaccine include supposed promotion of promiscuity, increased incidence of premature ovarian failure or insufficiency (POI), and increased risk of Guillain-Barré Syndrome (GBS), often propagated through published reports, media coverage, Web sites, and social media. Assure worried parents that many studies have shown that receipt of the vaccine is safe and does not lead to initiation of sexual activity or promiscuity, and, in fact, safer sexual health practices have been observed following vaccination.57-59
Continue to: A large longitudinal...
A large longitudinal adolescent health survey administered in British Columbia looked at sexual health behaviors and risk factors in adolescent girls before and after receipt of HPV vaccination (2003, 2008, 2013).59 Results showed no significant change in the reported number of sexual partners (2003-2013), increased reported use of contraception and condoms, and lower pregnancy rates.59 There is no evidence that HPV vaccines cause reproductive problems in women53; a review of VAERS reports from 2009 through 2015 did not detect any safety concerns for POI or other reproductive problems in females.60 A 2018 population-based study of nearly 200,000 women observed no increase of POI following receipt of HPV vaccination.61 In addition, several recent studies have shown no increased risk for GBS following receipt of HPV vaccine.62-64
CORRESPONDENCE
Pamela G. Rockwell, DO, FAAFP, 24 Frank Lloyd Wright Drive, SPC 5795, Room 2300, Lobby H, Ann Arbor, MI 48105; [email protected].
Overall adolescent vaccination coverage is improving in the United States.1 But for adolescents up to 15 years of age, there’s a large gap between the rate of vaccination for human papillomavirus (HPV) and the higher rates of coverage for tetanus, diphtheria, and acellular pertussis (Tdap) and meningococcal conjugate (MenACWY) vaccines.1 Adopting or refining practice customs reviewed in this article can increase HPV vaccination rates and continue to improve coverage of all vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) for adolescents between the ages of 11 and 12.
The evolution of ACIP’s HPV vaccine recommendations
Before December 2016, ACIP recommended a 3-dose HPV series for all adolescents between the ages of 11 and 12, given on a 0, 1-2, and 6-month schedule.2 The series could be started at 9 years of age. It could be administered to females as old as 26 years, and to males through 21 years (or ages 22-26 years for those who wish to be vaccinated, who have certain medical conditions, or who are included in special populations—ie, gay and bisexual men, men who have sex with men, immunocompromised men, men with human immunodeficiency virus [HIV], and transgender men).
In 2016, ACIP revised its recommendation for adolescents who initiate vaccination before their 15th birthday: a 2-dose schedule is adequate, with the second dose given 6 to 12 months after the first dose. For those who initiate vaccination on or after their 15th birthday, and for those with certain medical conditions, the recommendation remains 3 doses on a 0, 1-2, and 6-month schedule.3
As of August 2019,4 ACIP now recommends that all women and men receive catch-up HPV vaccination through age 26. For individuals 27 to 45 years of age who have not been adequately vaccinated, HPV vaccine may be given based on shared clinical decision making with their physician.
How are we doing?
Overall, adolescent vaccination coverage is improving in the United States (see “Vaccination goals from ACIP and Healthy People 2020”1,5,6), but the rate of improvement of HPV coverage is lower than that for Tdap and MenACWY coverage by age 15 years (although completion of the MenACWY vaccine series is low). From 2015 to 2016, coverage increased for 1 or more doses of Tdap, from 86.4% to 88% among 17-year olds (87.9% for 15-year olds), and coverage for 1 or more doses of MenACWY increased from 81.7% to 83.5% among 17-year olds (80.4% among 15-year olds).1 Both Tdap and MenACWY coverage rates have surpassed Healthy People 2020 goals of 80%, and the focus now is on maintenance of coverage. Data from the 2016 National Immunization Survey (NIS)-Teen show that completion of the HPV vaccine series (applying updated HPV vaccine recommendations retrospectively) increased to 45.4% for 15-year-olds,1 still far below the Healthy People 2020 goal of 80%. Completion rates for 2 or more doses of MenACWY also increased from 33.3% to 39.1%.1
SIDEBAR
Vaccination goals from ACIP and Healthy People 2020
The Advisory Committee on Immunization Practices (ACIP) recommends that adolescents routinely receive several vaccines between the ages of 11 and 12 years: an annual influenza vaccine, Tdap, the first dose of MenACWY, and initiation of the HPV series. ACIP also advises a booster dose of MenACWY at age 16 years, and teens and young adults (16-23 years) also may be vaccinated with a multidose serogroup B meningococcal vaccine, preferably before age 18. For those adolescents not up to date with their childhood vaccines, ACIP recommends the following catch-up vaccinations: measles, mumps, rubella (MMR, 2 doses); hepatitis B (HepB, 3 doses); and varicella (VAR, 2 doses).5
Healthy People 2020. In December 2010, the US Department of Health and Human Services released Healthy People 2020, a wide-ranging initiative on health promotion and disease prevention that includes 10-year objectives of increasing coverage with Tdap, at least one dose of MenACWY, and completion of the HPV series among 80% of those ages 13 to 15 years.6 This initiative reflects extensive feedback from more than 2000 organizations and authorities in public health and prevention at federal, state, and local levels—as well as from the public. Adolescent vaccination coverage is estimated by the Centers for Disease Control and Prevention using data from the National Immunization Survey (NIS)-Teen annual survey conducted among parents and guardians of adolescents ages 13 to 17 years.1
Common barriers to improved vaccine coverage
Barriers to improved vaccination rates include a lack of regular assessment of vaccine status; limited use of electronic records, tools, and immunization registries; lack of health care provider knowledge on current vaccine recommendations; vaccine costs; missed opportunities; and patient/parent refusals.7,8 The Community Preventive Services Task Force outlines several well-established evidence-based ways that administrators and physicians can counter these barriers:
- give a strong recommendation to vaccinate,9,10
- incorporate an audit/feedback mechanism for health care providers who vaccinate,9,11
- use electronic alerts to remind health care providers to vaccinate,9,12
- use your state’s electronic immunization information systems (IIS),7,13
- appoint a vaccine practice team/vaccine champion,9,14 and
- implement standing orders and reminder/recall systems.7,9,15
The passage of the Affordable Care Act (ACA)—which mandates that certain preventive services, including ACIP-recommended immunizations, be covered as part of basic care at no cost-sharing—reduces the once-common financial barrier to vaccine uptake.16 A key contributor to low uptake of HPV vaccination by adolescents is parental refusal.17
Continue to: The threats posed by HPV
The threats posed by HPV
HPV infections are the most commonly transmitted infections in the United States and nearly all men and women will be exposed to one or more types of HPV at some point in their lives. Current data show that 79 million Americans, most in their late teens and early 20s, are infected with HPV, and about 14 million people in the United States become newly infected each year.18 HPV is a DNA tumor virus that causes epithelial proliferation at cutaneous and mucosal surfaces.
There are more than 100 types of the virus,19 including more than 40 strains that infect the human genital tract. Of the latter 40 strains, there are oncogenic or high-risk types and non-oncogenic or low-risk types.20 HPV infection with high-risk types causes cervical, vaginal, and vulvar cancers in women; penile cancers in men; and oropharyngeal and anal cancers in both men and women. Low-risk HPV types cause genital warts in both men and women.21 The current available HPV vaccine in the United States is a 9-valent vaccine (9vHPV) that replaces the former 2- and 4-valent HPV vaccines and includes immunogenic coverage against high-risk HPV types 16, 18, 31, 33, 45, 52, and 58; and low-risk types 6 and 11.22
Centers for Disease Control and Prevention (CDC) data from 2010 to 2014 show that approximately 23,700 women and approximately 17,300 men in the United States developed HPV-associated cancer. Most common in women are cervical cancers and in men, oropharyngeal cancers (cancers of the back of the throat, base of the tongue, and tonsils). Using population-based data to genotype HPV types from cancer tissues, the CDC reports that HPV is responsible for about 90% of cervical and anal cancers, 70% of oropharyngeal, vaginal, and vulvar cancers, and 60% of penile cancers.23 A significant percentage of these cancers could potentially be prevented by receipt of 9vHPV.23,24
Make adolescent immunization a high priority
Anticipate opportunities to vaccinate and take steps to make your immunization and scheduling processes more prominent. With HPV specifically, you can strongly advocate for vaccination, address parental misgivings and educate them using clear communication styles, and acquire knowledge to answer concerns about potential vaccine adverse effects.
Every visit is an opportunity to vaccinate. The American Academy of Family Physicians and The American Academy of Pediatrics recommend that adolescents have annual preventive visits for screening, immunizations, and assessment and counseling for risky behaviors. However, many adolescents do not present annually for preventive visits, and fewer than half of adolescents receive regular preventive care.
Continue to: Missed opportunities for the HPV vaccine
Missed opportunities for the HPV vaccine. One study showed that at least 86% of unvaccinated adolescents had missed opportunities to receive HPV vaccine.29 A study of 14,588 adolescent girls from January 2010 through August 2015 showed that HPV vaccine was given at only 37.1% of visits in which MenACWY or Tdap vaccines were administered.30 The rate of HPV vaccination was just 26% during well adolescent visits, and 41.8% during all other primary care visits.30 Every adolescent health care visit—including visits for acute care, chronic care, follow-up, or office-based procedures—is an opportunity to review vaccination status.
Give vaccines concomitantly (simultaneously or same-day). ACIP counsels that minor illnesses, such as mild upper respiratory infections with or without low-grade fever, are not contraindications to routine vaccination.30 Also, the safety of simultaneous vaccine administration, often a concern of both parents and health care providers, has been well established. Each vaccine’s immunogenicity and safety profile are maintained when given concomitantly with other vaccines, and fewer visits are needed to complete an adolescent’s vaccination status.31,32
Immediately schedule follow up visits and use reminder/recall systems. Parents of adolescents who opt for HPV vaccination are not always aware of the timing of the 2- or 3-dose schedule and may not even be aware that more than 1 dose of vaccine is recommended.
A qualitative study of pediatric primary care providers and parents/guardians of adolescent patients showed that for HPV vaccination series completion, 65% of parents/guardians expected to be reminded of any needed doses, while 52% of the pediatric primary care providers relied on parents to schedule subsequent immunizations, and often the HPV series was not completed.33 Higher completion rates of the HPV vaccination series were achieved when follow-up appointments were scheduled at checkout for the 2nd or 3rd vaccine dose after initiation of HPV vaccination.33 The use of patient reminder/recall systems using telephone calls or mailings (phone usage is more effective than mailings) is also shown to improve vaccination completion rates.34
Recommend HPV vaccination clearly and resolutely
In a cross-sectional survey of 800 parents of adolescents ages 9 to 14 years, HPV vaccine was deemed the least likely vaccine to have been “very strongly” recommended by their health care provider, compared with the strength of recommendations for influenza, Tdap, and MenACWY vaccines.35 The strength of a health care provider’s recommendation to vaccinate is the single most influential factor in vaccine uptake.10,36,37 Most family physicians self-report “always recommending standard pediatric vaccines”; however, only a minority are following ACIP recommendations.38 A national study reported that only about two-thirds of parents who received HPV vaccine recommendations perceived a high level of health care provider endorsement.39 The takeaway point: Give a clear, unambiguous, strong recommendation to vaccinate with HPV to prevent infection; cervical, oropharyngeal, and other cancers; and genital warts.
Continue to: Tell parents why the timing is important
Tell parents why the timing is important. Inform parents that the HPV vaccine must be administered while their child is young (before the adolescent’s first sexual contact) to ensure the most robust immune response to the vaccine.40 Unsolicited explanations about sexual activity need not be offered when discussing HPV vaccination, as it is fair to assume that sexual contact is a reality for nearly all people in their adolescent or adult life; and by extension, most sexually active people will likely have exposure to HPV at some time in their lives. By adulthood, sexual activity is nearly universal: The National Longitudinal Study of Adolescent Health showed that only about 3% of participants tracked since adolescence reported no sexual experience by (average age) 28.5 years.41
How you say it matters. Many pediatricians and family physicians report recommending HPV vaccine inconsistently, behind schedule, or without urgency,42 sending mixed messages by failing to endorse HPV vaccination strongly, failing to differentiate it from other vaccines, and presenting it as an “optional” vaccine that could be delayed.43 Physicians and other health care providers who begin conversations about HPV vaccine by saying that the adolescent is “due” for the vaccine show higher vaccine recommendation quality scores than those who give unsolicited information about the vaccine, elicit questions before recommendation, or present the vaccine as an “option.”42 Parents who are “on the fence” may hesitate and decline HPV vaccination with a halfhearted recommendation.44
“Your child is due for his/her Tdap, HPV, influenza, and meningococcal vaccinations to prevent potentially devastating disease and several cancers. I highly recommend all 4 vaccinations today” is more persuasive than, “I recommend your child receive his/her Tdap, meningococcal, and influenza vaccines. And we can also discuss the HPV vaccine.”
Direct presumptive language that assumes vaccine delivery is associated with higher odds of HPV vaccine acceptance and same-day agreement to vaccination than is an open-ended participatory conversational style.45 Saying, “I believe in the importance of this cancer-preventing vaccine for your child” is more persuasive than saying, “What do you think about starting the HPV vaccination series today?”46
Don’t give up when parents initially refuse HPV vaccinations for their adolescents. Parents’ decisions about HPV vaccination may change over time. Repeated positive recommendations and counseling for HPV vaccination over multiple visits have been shown in a large multivariable analysis to increase parent acceptance of HPV vaccination: 45% of parents reported secondary acceptance of HPV vaccination, and an additional 24% intended to vaccinate in the next 12 months.47 Combining a presumptive communication style with motivational interviewing and a fact sheet has contributed to higher clinician-perceived levels of parental HPV vaccine acceptance and increased vaccination rates.48
Continue to: Know how to address parents' concerns about safety
Know how to address parents’ concerns about safety
Be prepared to discuss and answer parents’ questions or concerns regarding any vaccine, especially the HPV vaccine. Social networks are important in parents’ vaccination decision-making,49 and they may seek information from such sources as Twitter, Facebook, Google, and YouTube, where misinformation may be disseminated. A quantitative analysis of 560 YouTube videos relaying a false link between vaccines and autism or other serious adverse effects on children were uploaded between December 2007 and July 2017, with a peak of 224 videos uploaded in the first 7 months of 2017.50 Most were negative in tone and dispensed misinformation.50
The National Vaccine Information Center (NVIC) is an organization that takes a skeptical view of the US government and pharmaceutical companies. NVIC is widely criticized by scientists and leaders in vaccine science and public health as spreading false information on the risks of vaccines and, specifically, that HPV vaccination causes chronic disease. NVIC reports that receipt of HPV vaccine may increase the risk for cervical cancer and death.51 Pediatrician and vaccine researcher Dr. Paul Offit, interviewed by The Lancet in response to NVIC and other anti-vaccine groups’ messages, stated: “anti-vaccination organizations are unequivocally threatening public health.”52
Describe the robust safety-monitoring system. The CDC is aware of public concern about the safety of HPV vaccine. Ongoing monitoring of vaccine safety and studies conducted by the CDC, the Food and Drug Administration (FDA), and other organizations has documented a reassuring safety record since the vaccine’s introduction in 2006.53 Assure parents that the Vaccine Adverse Event Reporting System (VAERS) summary of 7244 reports following 9vHPV vaccination (December 1, 2014 – December 31, 2017) showed that most (97%) reports were nonserious: No new safety signals or unexpected patterns were observed, confirming consistency of the safety profile of 9vHPV with data from pre-licensure trials and post-licensure data on 4vHPV.54
Acknowledge the usually mild, transient potential risks of HPV vaccination as reported to VAERS: local injection site symptoms such as pain, redness, or swelling in the arm where the injection was given (most common adverse effect), dizziness, fainting, headache, nausea, and fever.53 Point out that fainting after vaccination is common in adolescents55 and that the CDC and ACIP recommend observation of adolescents for 15 minutes following HPV vaccination.56 Consider this 15-minute observation period after adolescent receipt of any vaccine to be part of standard practice in your vaccination setting.56
Contest unfounded views. Other common parental concerns about effects of HPV vaccine include supposed promotion of promiscuity, increased incidence of premature ovarian failure or insufficiency (POI), and increased risk of Guillain-Barré Syndrome (GBS), often propagated through published reports, media coverage, Web sites, and social media. Assure worried parents that many studies have shown that receipt of the vaccine is safe and does not lead to initiation of sexual activity or promiscuity, and, in fact, safer sexual health practices have been observed following vaccination.57-59
Continue to: A large longitudinal...
A large longitudinal adolescent health survey administered in British Columbia looked at sexual health behaviors and risk factors in adolescent girls before and after receipt of HPV vaccination (2003, 2008, 2013).59 Results showed no significant change in the reported number of sexual partners (2003-2013), increased reported use of contraception and condoms, and lower pregnancy rates.59 There is no evidence that HPV vaccines cause reproductive problems in women53; a review of VAERS reports from 2009 through 2015 did not detect any safety concerns for POI or other reproductive problems in females.60 A 2018 population-based study of nearly 200,000 women observed no increase of POI following receipt of HPV vaccination.61 In addition, several recent studies have shown no increased risk for GBS following receipt of HPV vaccine.62-64
CORRESPONDENCE
Pamela G. Rockwell, DO, FAAFP, 24 Frank Lloyd Wright Drive, SPC 5795, Room 2300, Lobby H, Ann Arbor, MI 48105; [email protected].
1. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:1-30.
3. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
5. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices (ACIP) Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 18 years or younger—United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66:134-135.
6. US Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020. www.healthypeople.gov/node/4654/data_details. Accessed December 4, 2019.
7. Rockwell PG. What you can do to improve adult immunization rates. J Fam Pract. 2015;64:625-633.
8. Kimmel Sr, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
9. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based guide to community preventive services-methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
10. Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the U.S. National Immunization Survey. Am J Public Health. 2013;103:164-169.
11. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2011;60:1-64.
12. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
13. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
14. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
15. Task Force on Community Preventive Services. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(suppl 1):92-96.
16. US Department of Health and Human Services. Preventive care. www.hhs.gov/healthcare/about-the-aca/preventive-care/index.html. Accessed December 4, 2019.
17. Gilkey MB, Calo WA, Marciniak, MW, et al. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Hum Vaccin Immunother. 2017;13:680-686.
18. CDC. Genital HPV infection—fact sheet. www.cdc.gov/std/hpv/stdfact-hpv.htm. Accessed December 4, 2019.
19. WHO. Human papillomavirus (HPV) and cervical cancer. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accessed December 4, 2019.
20. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-527.
21. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
22. CDC. Luxembourg A. Program summary and new 9-valent HPV vaccine trial data. Presented at the Advisory Committee on Immunization Practices (ACIP), October 30, 2014. Atlanta, Ga. 2014. www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-10.pdf. Accessed December 4, 2019.
23. CDC. HPV and cancer. www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed December 4, 2019.
24. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23.
25. Rand CM, Goldstein NPN. Patterns of primary care physician visits for US adolescents in 2014: implications for vaccination. Acad Pediatr. 2018;18:S72-S78.
26. Taylor JL, Aalsma MC, Gilbert AL, et al. Perspectives of family medicine physicians on the importance of adolescent preventive care: a multivariate analysis. BMC Fam Pract. 2016;17:4.
27. Harris SK, Aalsma MC, Weitzman ER, et al. Research on clinical preventive services for adolescents and young adults: Where are we and where do we need to go? J Adolesc Health. 2017;60:249-260.
28. Gilkey MB, Moss JL, McRee AL, et al. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30:5928-5934.
29. Espinosa CM, Marshall GS, Woods CR, et al. Missed opportunities for human papillomavirus vaccine initiation in an insured adolescent female population. J Pediatric Infect Dis Soc. 2017;6:360-365.
30. CDC. Update: Vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45:1-35.
31. Moss JL, Reiter PL, Brewer NT. Concomitant adolescent vaccination in the U.S., 2007-2012. Am J Prev Med. 2016;51:693-705.
32. Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine. 2014;32:2670-2674.
33. Perkins RB, Chigurupati NL, Apte G, et al. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother. 2016;12:1528-1535.
34. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
35. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18:S23-S27.
36. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
37. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother. 2013;9:2627-2633.
38. Bonville CA, Domachowske JB, Cibula DA, et al. Immunization attitudes and practices among family medicine providers. Hum Vaccin Immunother. 2017;13:2646-2653.
39. Wilson R, Brown DR, Boothe MA, et al. Knowledge and acceptability of the HPV vaccine among ethnically diverse black women. J Immigr Minor Health. 2013;15:747-757.
40. Iversen O, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–2421.
41. Haydon AA, Cheng MM, Herring AH, et al. Prevalence and predictors of sexual inexperience in adulthood. Arch Sex Behav. 2014;43:221-230.
42. Gilkey MB, Malo TL, Shah PD, et al. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev. 2015;24:1673-1679.
43. Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systemic review. Hum Vaccin Immunother. 2016;12:1454-1468.
44. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed December 4, 2019.
45. Sturm L, Donahue K, Kasting M, et al. Pediatrician-parent conversations about human papillomavirus vaccination: an analysis of audio recordings. J Adolesc Health. 2017;61:246-251.
46. Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
47. Kornides ML, McRee AL, Gilkey MB. Parents who decline HPV vaccination: Who later accepts and why? Acad Pediatr. 2018;18:S37-S43.
48. Reno JE, Thomas J, Pyrzanowski J, et al. Examining strategies for improving healthcare providers’ communication about adolescent HPV vaccination: evaluation of secondary outcomes in a randomized controlled trial. Hum Vaccin Immunother. 2018;15:1592-1598.
49. Brunson EK. The impact of social networks on parents’ vaccination decisions. Pediatrics. 2013;131:e1397-e1404.
50. Donzelli G, Palomba G, Federigi L, et al. Misinformation on vaccination: a quantitative analysis of YouTube videos. Hum Vaccin Immunother. 2018;14:1654-1659.
51. National Vaccine Information Center. Human papillomavirus (HPV) disease and vaccine information. www.nvic.org/Vaccines-and-Diseases/hpv.aspx. Accessed December 4, 2019.
52. Shetty P. Experts concerned about vaccination backlash. Lancet. 2010; 375:970-971.
53. CDC. Frequently asked questions about HPV vaccine safety. www.cdc.gov/vaccinesafety/vaccines/hpv/hpv-safety-faqs.html. Accessed December 4, 2019.
54. Arana J, Su J, Lewis P, et al. Post-licensure surveillance of 9-valent human papillomavirus vaccine (9vHPV) in the Vaccine Adverse Event Reporting System (VAERS), United States, 2014-2017. https://idsa.confex.com/idsa/2018/webprogram/Paper69618.html. Accessed December 4, 2019.
55. Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Ped Adolesc Med. 1997;151:255-259.
56. Kroger AT, Duchin J, Vázquez M. General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html. Accessed December 4, 2019.
57. Hansen BT. No evidence that HPV vaccination leads to sexual risk compensation. Hum Vaccin Immunother. 2016;12:1451-1453.
58. Smith LM, Kaufman JS, Strumpf EC, et al. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. 2015;187:E74-81.
59. Ogilvie GS, Phan F, Pederson HN, et al. Population-level sexual behaviours in adolescent girls before and after introduction of the human papillomavirus vaccine (2003-2013). CMAJ. 2018;190:E1221-E1226.
60. Arana JE, Harrington T, Cano M, et al. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015. Vaccine. 2018;36:1781-1788.
61. Naleway AL, Mittendorf KF, Irving SA, et al. Primary ovarian insufficiency and adolescent vaccination. Pediatrics. 2018;142. pii: e20190943.
62. Deceuninck G, Sauvageau C, Gilca V, et al. Absence of association between Guillain-Barré syndrome hospitalizations and HPV-vaccine. Expert Rev Vaccines. 2018;17:99-102.
63. Mouchet J, Salvo F, Raschi E, et al. Human papillomavirus vaccine and demyelinating diseases – a systematic review and meta-analysis. Pharmacol Res. 2018;132:108-118.
64. Gee J, Sukumaran L, Weinstraub E, et al. Risk of Guillain-Barre Syndrome following quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink. Vaccine. 2017;35:5756-5758.
1. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:1-30.
3. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
5. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices (ACIP) Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 18 years or younger—United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66:134-135.
6. US Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020. www.healthypeople.gov/node/4654/data_details. Accessed December 4, 2019.
7. Rockwell PG. What you can do to improve adult immunization rates. J Fam Pract. 2015;64:625-633.
8. Kimmel Sr, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
9. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based guide to community preventive services-methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
10. Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the U.S. National Immunization Survey. Am J Public Health. 2013;103:164-169.
11. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2011;60:1-64.
12. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
13. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
14. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
15. Task Force on Community Preventive Services. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(suppl 1):92-96.
16. US Department of Health and Human Services. Preventive care. www.hhs.gov/healthcare/about-the-aca/preventive-care/index.html. Accessed December 4, 2019.
17. Gilkey MB, Calo WA, Marciniak, MW, et al. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Hum Vaccin Immunother. 2017;13:680-686.
18. CDC. Genital HPV infection—fact sheet. www.cdc.gov/std/hpv/stdfact-hpv.htm. Accessed December 4, 2019.
19. WHO. Human papillomavirus (HPV) and cervical cancer. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accessed December 4, 2019.
20. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-527.
21. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
22. CDC. Luxembourg A. Program summary and new 9-valent HPV vaccine trial data. Presented at the Advisory Committee on Immunization Practices (ACIP), October 30, 2014. Atlanta, Ga. 2014. www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-10.pdf. Accessed December 4, 2019.
23. CDC. HPV and cancer. www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed December 4, 2019.
24. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23.
25. Rand CM, Goldstein NPN. Patterns of primary care physician visits for US adolescents in 2014: implications for vaccination. Acad Pediatr. 2018;18:S72-S78.
26. Taylor JL, Aalsma MC, Gilbert AL, et al. Perspectives of family medicine physicians on the importance of adolescent preventive care: a multivariate analysis. BMC Fam Pract. 2016;17:4.
27. Harris SK, Aalsma MC, Weitzman ER, et al. Research on clinical preventive services for adolescents and young adults: Where are we and where do we need to go? J Adolesc Health. 2017;60:249-260.
28. Gilkey MB, Moss JL, McRee AL, et al. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30:5928-5934.
29. Espinosa CM, Marshall GS, Woods CR, et al. Missed opportunities for human papillomavirus vaccine initiation in an insured adolescent female population. J Pediatric Infect Dis Soc. 2017;6:360-365.
30. CDC. Update: Vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45:1-35.
31. Moss JL, Reiter PL, Brewer NT. Concomitant adolescent vaccination in the U.S., 2007-2012. Am J Prev Med. 2016;51:693-705.
32. Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine. 2014;32:2670-2674.
33. Perkins RB, Chigurupati NL, Apte G, et al. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother. 2016;12:1528-1535.
34. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
35. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18:S23-S27.
36. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
37. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother. 2013;9:2627-2633.
38. Bonville CA, Domachowske JB, Cibula DA, et al. Immunization attitudes and practices among family medicine providers. Hum Vaccin Immunother. 2017;13:2646-2653.
39. Wilson R, Brown DR, Boothe MA, et al. Knowledge and acceptability of the HPV vaccine among ethnically diverse black women. J Immigr Minor Health. 2013;15:747-757.
40. Iversen O, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–2421.
41. Haydon AA, Cheng MM, Herring AH, et al. Prevalence and predictors of sexual inexperience in adulthood. Arch Sex Behav. 2014;43:221-230.
42. Gilkey MB, Malo TL, Shah PD, et al. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev. 2015;24:1673-1679.
43. Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systemic review. Hum Vaccin Immunother. 2016;12:1454-1468.
44. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed December 4, 2019.
45. Sturm L, Donahue K, Kasting M, et al. Pediatrician-parent conversations about human papillomavirus vaccination: an analysis of audio recordings. J Adolesc Health. 2017;61:246-251.
46. Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
47. Kornides ML, McRee AL, Gilkey MB. Parents who decline HPV vaccination: Who later accepts and why? Acad Pediatr. 2018;18:S37-S43.
48. Reno JE, Thomas J, Pyrzanowski J, et al. Examining strategies for improving healthcare providers’ communication about adolescent HPV vaccination: evaluation of secondary outcomes in a randomized controlled trial. Hum Vaccin Immunother. 2018;15:1592-1598.
49. Brunson EK. The impact of social networks on parents’ vaccination decisions. Pediatrics. 2013;131:e1397-e1404.
50. Donzelli G, Palomba G, Federigi L, et al. Misinformation on vaccination: a quantitative analysis of YouTube videos. Hum Vaccin Immunother. 2018;14:1654-1659.
51. National Vaccine Information Center. Human papillomavirus (HPV) disease and vaccine information. www.nvic.org/Vaccines-and-Diseases/hpv.aspx. Accessed December 4, 2019.
52. Shetty P. Experts concerned about vaccination backlash. Lancet. 2010; 375:970-971.
53. CDC. Frequently asked questions about HPV vaccine safety. www.cdc.gov/vaccinesafety/vaccines/hpv/hpv-safety-faqs.html. Accessed December 4, 2019.
54. Arana J, Su J, Lewis P, et al. Post-licensure surveillance of 9-valent human papillomavirus vaccine (9vHPV) in the Vaccine Adverse Event Reporting System (VAERS), United States, 2014-2017. https://idsa.confex.com/idsa/2018/webprogram/Paper69618.html. Accessed December 4, 2019.
55. Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Ped Adolesc Med. 1997;151:255-259.
56. Kroger AT, Duchin J, Vázquez M. General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html. Accessed December 4, 2019.
57. Hansen BT. No evidence that HPV vaccination leads to sexual risk compensation. Hum Vaccin Immunother. 2016;12:1451-1453.
58. Smith LM, Kaufman JS, Strumpf EC, et al. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. 2015;187:E74-81.
59. Ogilvie GS, Phan F, Pederson HN, et al. Population-level sexual behaviours in adolescent girls before and after introduction of the human papillomavirus vaccine (2003-2013). CMAJ. 2018;190:E1221-E1226.
60. Arana JE, Harrington T, Cano M, et al. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015. Vaccine. 2018;36:1781-1788.
61. Naleway AL, Mittendorf KF, Irving SA, et al. Primary ovarian insufficiency and adolescent vaccination. Pediatrics. 2018;142. pii: e20190943.
62. Deceuninck G, Sauvageau C, Gilca V, et al. Absence of association between Guillain-Barré syndrome hospitalizations and HPV-vaccine. Expert Rev Vaccines. 2018;17:99-102.
63. Mouchet J, Salvo F, Raschi E, et al. Human papillomavirus vaccine and demyelinating diseases – a systematic review and meta-analysis. Pharmacol Res. 2018;132:108-118.
64. Gee J, Sukumaran L, Weinstraub E, et al. Risk of Guillain-Barre Syndrome following quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink. Vaccine. 2017;35:5756-5758.
From The Journal of Family Practice | 2019;68(10):E1-E7.
PRACTICE RECOMMENDATIONS
› Review vaccination status at every adolescent health care visit. C
› Give a clear, unambiguous, strong recommendation to vaccinate with human papillomavirus (HPV) to prevent infection; cervical, oropharyngeal, and other cancers; and genital warts. A
› Schedule follow-up appointments at checkout following initiation of HPV vaccination to help ensure completion of the series. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Treatment of OSA: What (else) can it accomplish?
Obstructive sleep apnea (OSA) is a common cause of daytime sleepiness, and severe OSA is a risk factor for hypertension, cardiovascular events, atrial fibrillation (AF), insulin resistance, cognitive impairment, motor vehicle crashes, adverse pregnancy outcomes, and overall mortality.1-8 The hazard ratio for mortality for patients with severe OSA may be as high as 3.8.5
OSA is diagnosed by the apnea-hypopnea index (AHI), defined as the number of apnea or hypopnea events per hour as determined by polysomnography. An AHI score ≤ 5 is considered normal; > 5 to ≤ 15 is mild; > 15 to < 30 is moderate; and ≥ 30 is severe. Most studies of OSA treatment use reduction of AHI as the measure of treatment effectiveness, and several types of treatment improve AHI.
In family medicine, we generally want to know whether treatment of OSA will improve outcomes of significance to patients. A recent systematic review of evidence for the US Preventive Services Task Force found that it was unclear whether OSA treatment improved most health outcomes, including mortality, cardiovascular events, or motor vehicle crashes.6 Several other organizations have published guidelines regarding OSA treatment; these guidelines are reviewed in the TABLE.9-13
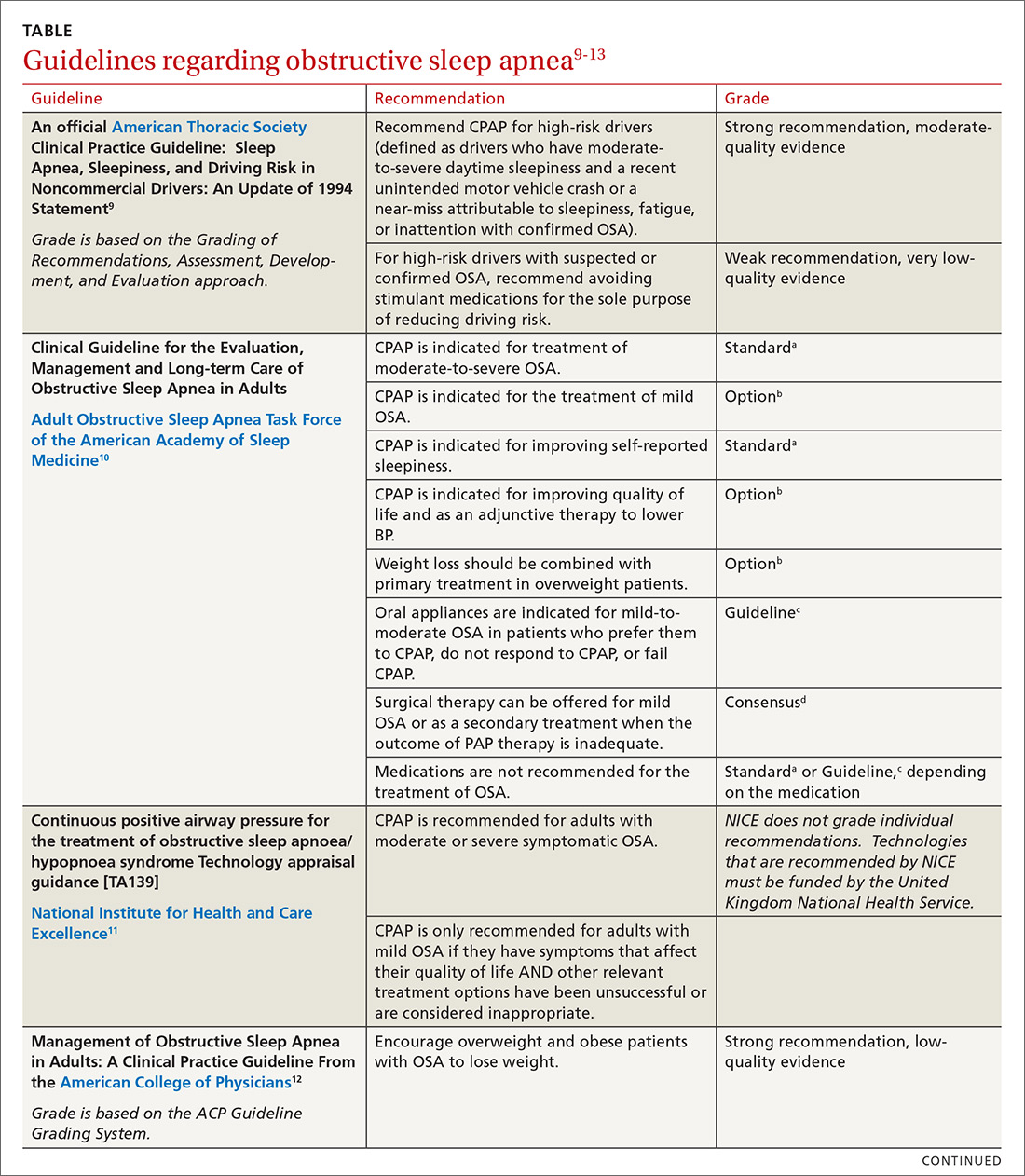
This article summarizes the current evidence surrounding the effect of treatment of OSA on outcomes of significance to patients. While multiple treatments have been advocated for patients with OSA, positive airway pressure (PAP) is the most widely used and studied and is recommended as standard treatment by most guidelines.9-13 Most available evidence about patient-oriented outcomes involves treatment with PAP; where there is evidence about the effect of other OSA treatments on a particular outcome, that evidence is also summarized.
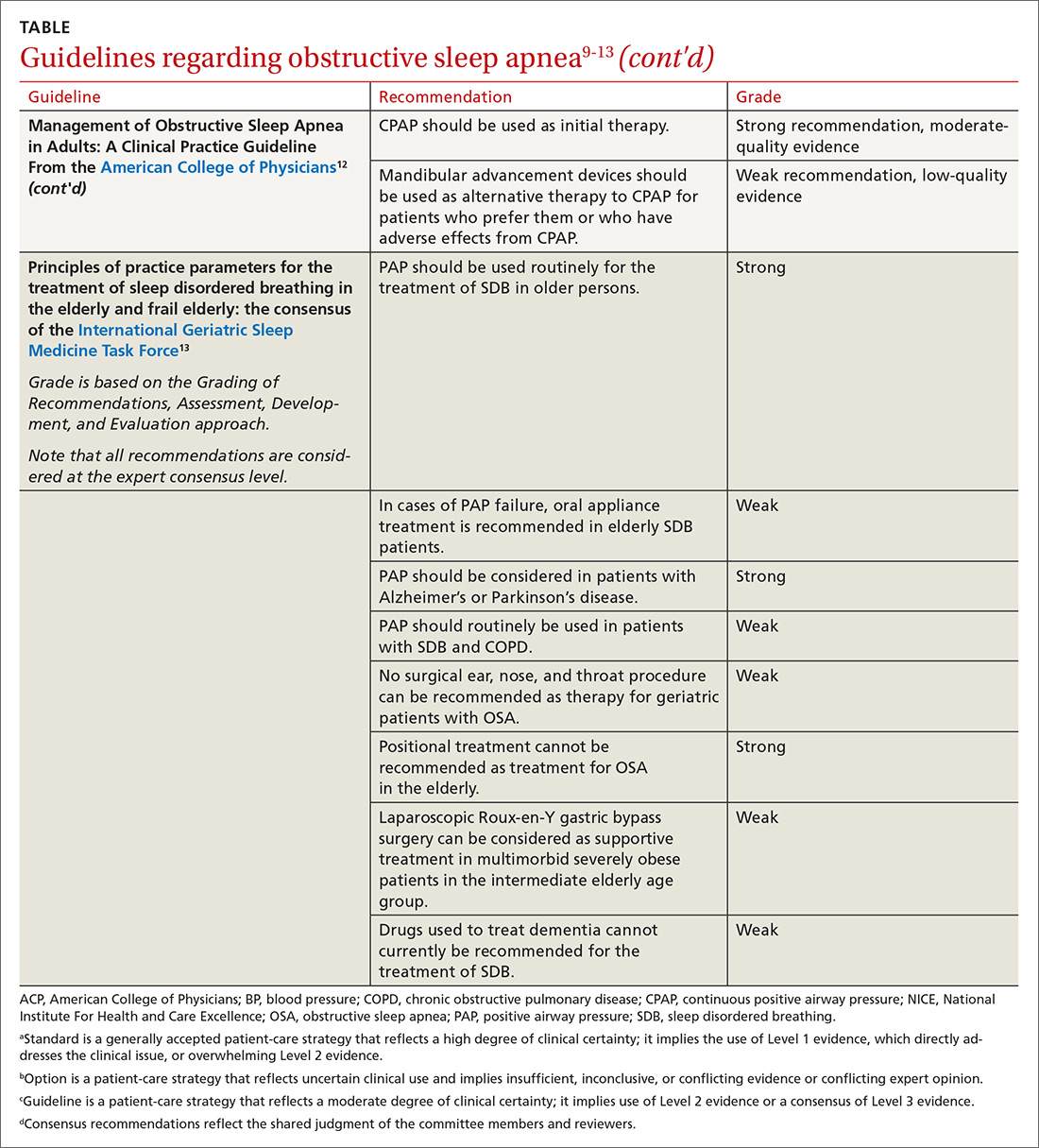
Benefits of OSA treatment
Patients with OSA who have excessive daytime sleepiness can gain substantial symptomatic benefit from treatment of their OSA with PAP or oral appliances (OAs), and might benefit from hypoglossal nerve stimulation or other surgical treatment. PAP is probably more effective than OAs in patients who use it ≥ 4 hours/night, but it is more difficult to comply with PAP.14
Evidence that treatment of asymptomatic OSA benefits other medical conditions is often conflicting. Given the low risk of treatment, it is reasonable to consider offering a trial of treatment, preferably with PAP, to asymptomatic patients with moderate-to-severe OSA and certain comorbidities, including obesity, resistant hypertension, high cardiovascular risk, congestive heart failure (CHF), AF, diabetes that is difficult to control, and pregnancy. Such patients should be strongly encouraged to use PAP ≥ 4 hours/night, and should be advised that benefits may not be immediately apparent.
Treatment of OSA improves daytime sleepiness
Daytime sleepiness is typically measured with the Epworth Sleepiness Scale (ESS), a self-administered questionnaire assessing a person’s level of drowsiness and propensity to fall asleep in 8 different daytime situations. Each situation is scored between 0 (would never doze) and 3 (high chance of dozing), with the scores then totaled to provide an overall score between 0 and 24. A score > 10 is considered abnormal.
Continue to: Treament of OSA...
Treatment of OSA with either PAP or OAs significantly improves ESS scores, with PAP being more effective.13 The difference appears to widen in patients with greater daytime sleepiness; in other words, patients with greater daytime sleepiness will gain even greater benefit from PAP, both overall and when compared with OAs.15
One randomized trial of an intensive lifestyle modification program for patients with OSA failed to show improvement in the ESS in the intention-to-treat analysis, but did demonstrate a 2.4-point greater reduction in ESS scores in those patients who successfully followed the program (achieving weight loss).16 Surgical treatments for OSA, such as uvulopalatopharyngoplasty or maxillary advancement, have been shown in some (but not all) studies to improve ESS scores; the different types of surgical treatment and the heterogeneity of studies prevents estimation of effect size.17 A meta-analysis of case series studies of hypoglossal nerve stimulation reported a mean improvement of 4.5 points on the ESS;18 comparison with other interventions is lacking.
Improved quality of life
Both PAP and OAs have been shown to improve sleep-related quality of life in patients with OSA. However, while the improvement is statistically significant, the effect size is small.14
That could be said of a study by Lewis et al.19 These researchers randomized patients with moderate-to-severe OSA and known coronary artery disease (CAD) or at least 3 risk factors for CAD to receive PAP, nocturnal oxygen, or lifestyle education.19 The patients randomized to receive PAP improved vitality scores by only 3.6 points on a 100-point scale; this was significantly better statistically than the improvement achieved by those randomized to lifestyle education. Smaller improvements were noted in depression, social function, and general health. Patients who had more daytime sleepiness at baseline had greater improvements in function.19
Cognitive function findings are mixed
In a systematic review published in 2004, Aloia et al4 found measurable impairments on neuropsychological tests of global cognitive functioning, attention/vigilance, executive functioning, memory, psychomotor function, and constructional abilities in patients with OSA. The results of treatment studies (all but 1 using PAP) were mixed. No studies showed improvement in psychomotor speed or language, and studies disagreed on whether treatment produced benefits in global cognition, attention, or executive functions.4
Continue to: Findings of more recent studies...
Findings of more recent studies remain mixed. A 3-month Spanish trial of PAP in older adults with severe OSA showed improvement in 2 of 4 neuropsychological tests of cognitive function; this was a secondary outcome measure.20 The PREDICT trial in the United Kingdom demonstrated a reduction in daytime sleepiness but no improvement in cognitive function in PAP-treated older adults with OSA but without dementia over a 1-year period.21
In contrast, a French long-term study of adults ages ≥ 65 years with severe (but not necessarily symptomatic) OSA showed better maintenance of memory performance; these results must be interpreted with caution, however, because the study was not randomized, controlled, or blinded, and the results were not adjusted for potential confounders.22 The severity of OSA may influence the impact of PAP treatment on cognitive function.
The prevalence of OSA in patients with dementia is high, and more severe dementia is associated with more severe OSA.23 Although it is intuitive that disrupted sleep may worsen cognitive function, and that treatment could improve it, minimal benefit on cognitive function was shown by neuropsychological testing in patients with Alzheimer’s disease and OSA treated with continuous positive airway pressure (CPAP) vs sham CPAP in 1 small short-term randomized trial.23
In another study of patients with Alzheimer’s disease, this time an observational (nonrandomized, non-controlled, single-blind) study of patients who also had severe symptomatic OSA, researchers followed the patients for 3 years and found a significant delay in median annual cognitive decline of 1.5 points per year on the Mini-Mental Status Examination in patients treated with PAP compared with those who did not receive PAP treatment.24
Hypertension: Small but positive results
A meta-analysis of PAP use in patients with OSA and resistant hypertension (defined as inadequate control while taking at least 3 antihypertensive agents or control requiring at least 4 agents) documented significant blood pressure (BP) lowering, with a pooled estimate of -7.21 mm Hg systolic and -4.99 mm Hg diastolic.25 The decrease in BP was demonstrated in both sleepy and non-sleepy subjects.
Continue to: Multiple studies have...
Multiple studies have shown a small reduction in BP readings (generally about 2 mm Hg) with PAP treatment in nonresistant hypertensive patients with OSA who are sleepy.26 Conversely, the literature is mixed on whether treatment of non-sleepy patients with OSA reduces BP. One long-term study demonstrated a small (1.89 mm Hg systolic, 2.19 mm Hg diastolic) BP reduction effect of PAP in non-sleepy subjects with OSA.27 Similarly, research has shown mandibular advancement devices to lower BP in patients with OSA, in a range similar to that achieved with PAP.28 Whether very small reductions in BP improve important clinical outcomes such as stroke or heart disease is unknown.
CV risk: Again, findings are mixed
The SAVE study is the largest randomized investigation of the effect of treatment of OSA with PAP for secondary prevention of cardiovascular events.29 The trial involved 2717 adults with cardiovascular disease, moderate-to-severe OSA, and minimal sleepiness, and had as its primary composite endpoint death from cardiovascular causes, myocardial infarction (MI), stroke, hospitalization for unstable angina, heart failure, or transient ischemic attack. Patients with severe daytime sleepiness or severe hypoxemia were excluded. The study found no difference between PAP and usual care in the primary outcome, despite a significant reduction in the AHI from a mean of 29 at baseline to 3.7 with PAP treatment.
Similarly, a randomized controlled trial (RCT) of 725 patients with non-sleepy OSA failed to show a reduction in cardiovascular events or in the development of hypertension.30 Peker et al31 randomized 244 adults with recently revascularized coronary artery disease and OSA without daytime sleepiness to auto-titrating CPAP or usual care and did not find a statistically significant difference in revascularization, MI, stroke, or cardiovascular mortality; however, those patients who were compliant with CPAP for ≥ 4 hours/night did have a statistically significant reduction in the combined endpoint.
In contrast, a trial of patients with first-ever stroke and moderate-to-severe OSA who were randomized to early nasal CPAP or usual care demonstrated better 5-year cardiovascular survival for the patients in the CPAP group, and a trend toward better cardiovascular event-free survival.32 Degree of daytime sleepiness was not stated in this study.
A recent meta-analysis of RCTs failed to find a reduction in major adverse cardiovascular events (MACE) in patients with moderate-to-severe OSA treated with PAP.33 In this study, subgroup analysis documented benefit in patients who were adherent with PAP for ≥ 4 hours/night. A larger meta-analysis, however, did not find a reduction in MACE even in the adherent subgroup.34
Continue to: AF and OSA
AF and OSA: An interesting relationship
OSA is an independent risk factor for AF, approximately doubling the risk.35 A review of 10,132 patients with AF (1841 with OSA) in a large observational study demonstrated no difference in outcomes of all-cause mortality, first hospitalization, major bleeding, or major cardiovascular events in OSA patients who were or were not treated with PAP. The PAP-treated patients did have a slightly lower (16% vs 18%) risk of worsening of AF over 2 years.36 Overall, AF patients with OSA had more symptoms and higher admission rates, but no difference in overall mortality or MACE. Observational studies have suggested that PAP treatment of OSA facilitates maintenance of normal sinus rhythm after cardioversion and after ablation.37
CHF: Results look promising
In one small study, 24 patients with heart failure with reduced ejection fraction who were optimally medically treated were randomized to receive PAP or sham PAP for 1 month.38 The treatment group demonstrated reduced systolic BP, reduced end systolic dimension, and significant improvement in ejection fraction from 25 ± 2.8% to 33.8 ± 2.4%.
OSA Tx improves insulin sensitivity
OSA is associated with impaired glucose tolerance, and PAP treatment of OSA has been documented to improve insulin sensitivity.39,40 An efficacy study utilizing PAP in a laboratory setting for 8 hours/night demonstrated significant reduction in fasting blood sugar and a reduction in the dawn phenomenon (an increase in early morning fasting glucose as a result of rebound from hypoglycemia during sleep).39 A 2015 meta-analysis of short-term studies also showed improvement in insulin sensitivity in OSA patients treated with PAP, but failed to find any reduction in A1C or in body mass index.40
All-cause mortality: Difference in findings between short- and long-term studies
Yu et al’s34 meta-analysis of 10 RCTs involving 7266 participants found no difference in mortality in treated (vs no treatment or sham treatment) OSA patients. This was true even in the more adherent subgroup. These studies were relatively short-term, with the longest mean follow-up being 68 months.
However, several longer-term population-based studies have suggested that OSA treatment improves all-cause mortality. An 18-year follow-up of a Wisconsin cohort documented dramatically increased mortality in patients with severe sleep apnea; mortality was even higher when patients treated with PAP were removed from the analysis, suggesting that PAP treatment was protective, mainly for cardiovascular death.5
Continue to: A Danish registry...
A Danish registry documented that patients treated with CPAP had higher rates of comorbidities before and during treatment; when these comorbidities were controlled, men ages ≥ 60 years had improved survival when treated with CPAP. There was no survival benefit in women.41
A recent analysis—the Sleep Heart Health Study—followed patients with obesity and severe OSA for a mean of 11.1 years and calculated a hazard ratio for all-cause mortality associated with prescribed PAP therapy of 0.58 (95% confidence interval [CI], 0.35-0.96) after propensity matching.42 The difference in mortality appeared 6 to 7 years after PAP therapy was prescribed. This delay may explain the failure of shorter-term studies to demonstrate evidence of benefit.
OSA Tx reduces motor vehicle crashes
Drowsy driving is widely accepted as a risk for motor vehicle crashes. Successful treatment of OSA with PAP has been shown to improve driving performance on a driving simulator.43 An analysis of 15 studies similarly demonstrated a significant reduction in driving accidents (incident rate ratio [IRR] = 0.45) and in near-miss accidents (IRR = 0.23) in patients with OSA treated with CPAP.44
Pulmonary hypertension: OSA Tx lowers pulmonary arterial pressure
Patients with OSA have higher than expected rates of pulmonary arterial hypertension—as high as 22%—documented by pulmonary artery catheterization findings.45 A meta-analysis of studies that examined the effect of PAP in patients with OSA and coexisting pulmonary hypertension but without other overt pulmonary or cardiac disease found significant reductions in pulmonary artery pressure.46 Whether this finding translates into improved patient-oriented outcomes is unknown.
OSA and pregnancy outcomes
A national cohort study demonstrated that OSA is an independent risk factor for multiple adverse pregnancy outcomes, including gestational diabetes, hypertensive disorders in pregnancy, intrauterine growth retardation, and stillbirth.7 OSA was also associated with the rare serious adverse outcomes of congestive heart failure, cardiomyopathy, and pulmonary embolism.7 There is little evidence to date with which to determine whether treatment of OSA improves outcomes, but PAP treatment is documented to be safe in pregnant women.8
CORRESPONDENCE
Stephen C. Sorsby, MD, MHA, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 530, Little Rock, AR 72205; [email protected].
1. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384.
2. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
3. Iftikhar IH, Hoyos CM, Phillips CL, et al. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med. 2015;11:475-485.
4. Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772-785.
5. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071-1078.
6. Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:415-433.
7. Bourjeily G, Danilack VA, Bublitz MA, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;35:50-57.
8. Booth JM, Tonidandel AM. Peripartum management of obstructive sleep apnea. Clin Obstet Gyn. 2017;60:405-417.
9. Strohl KP, Brown DB, Collop N, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med. 2013;187:1259-1266.
10. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
11. National Institute for Health and Care Excellence. Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. Technology appraisal guidance [TA139]. https://www.nice.org.uk/guidance/ta139. Revised February 2012. Accessed October 28, 2019.
12. Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471-483.
13. Netzer NC, Ancoli-Israel S, Bliwise DL, et al. Principles of practice parameters for the treatment of sleep disordered breathing in the elderly and frail elderly: the consensus of the International Geriatric Sleep Medicine Task Force. Eur Respir J. 2016;48:992-1018.
14. Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879-887.
15. Bratton DJ, Gaisl T, Schlatzer C, et al. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3:869-878.
16. Ng SSS, Chan RSM, Woo J, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest. 2015;148:1193-1203.
17. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;4:CD001004.
18. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015; 125:1254-1264.
19. Lewis EF, Rui W, Punjabi N, et al. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: A Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am Heart J. 2017;189:59-67.
20. Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46:142-151.
21. McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804-812.
22. Crawford-Achour E, Dauphinot V, Martin MS, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF study. J Clin Sleep Med. 2015;11:519-524.
23. Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076-2081.
24. Troussière AC, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1405-1408.
25. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757-764.
26. Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587-596.
27. Barbé F, Durán-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718-726.
28. Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280-2293.
29. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919-931.
30. Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161-2168.
31. Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613-620.
32. Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24:47-53.
33. Abuzaid AS, Al Ashray HS, Elbadaway A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure in patients with obstructive sleep apnea. Am J Card. 2017;120:693-699.
34. Yu J, Zhou Z, McEvoy D, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318:156-166.
35. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the incident risk of atrial fibrillation. J Amer Coll of Card. 2007;49:565-571.
36. Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169:647-654.e2.
37. Nalliah CJ, Sanders P, Kalman JM. Obstructive sleep apnea treatment and atrial fibrillation: a need for definitive evidence. J Cardiovasc Electrophysiol. 2016;27:1001-1010.
38. Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233-1241
39. Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes: a randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96-105.
40. Feng Y, Zhang Z, Dong ZZ. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15005.
41. Jennum P, Tonnesen P, Ibsen R, et al. Obstructive sleep apnea: effect of comorbidities and positive airway pressure on all-cause mortality. Sleep Med. 2017;36:62-66.
42. Lisan Q, Van Sloten T, Marques Vidal P, et al. Association of positive airway pressure prescription with mortality in patients with obesity and severe obstructive sleep apnea: the sleep heart health study. JAMA Otolaryngol Head Neck Surg. 2019;145:509-515.
43. Mazza S, Pépin JL, Naëgelé B, et al. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J. 2006;28:1020-1028.
44. Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, et al. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev. 2011;15:301-310.
45. Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2009;104:1300-1306.
46. Imran TF, Ghazipura M, Liu S, et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev. 2016;21:591-598.
Obstructive sleep apnea (OSA) is a common cause of daytime sleepiness, and severe OSA is a risk factor for hypertension, cardiovascular events, atrial fibrillation (AF), insulin resistance, cognitive impairment, motor vehicle crashes, adverse pregnancy outcomes, and overall mortality.1-8 The hazard ratio for mortality for patients with severe OSA may be as high as 3.8.5
OSA is diagnosed by the apnea-hypopnea index (AHI), defined as the number of apnea or hypopnea events per hour as determined by polysomnography. An AHI score ≤ 5 is considered normal; > 5 to ≤ 15 is mild; > 15 to < 30 is moderate; and ≥ 30 is severe. Most studies of OSA treatment use reduction of AHI as the measure of treatment effectiveness, and several types of treatment improve AHI.
In family medicine, we generally want to know whether treatment of OSA will improve outcomes of significance to patients. A recent systematic review of evidence for the US Preventive Services Task Force found that it was unclear whether OSA treatment improved most health outcomes, including mortality, cardiovascular events, or motor vehicle crashes.6 Several other organizations have published guidelines regarding OSA treatment; these guidelines are reviewed in the TABLE.9-13

This article summarizes the current evidence surrounding the effect of treatment of OSA on outcomes of significance to patients. While multiple treatments have been advocated for patients with OSA, positive airway pressure (PAP) is the most widely used and studied and is recommended as standard treatment by most guidelines.9-13 Most available evidence about patient-oriented outcomes involves treatment with PAP; where there is evidence about the effect of other OSA treatments on a particular outcome, that evidence is also summarized.

Benefits of OSA treatment
Patients with OSA who have excessive daytime sleepiness can gain substantial symptomatic benefit from treatment of their OSA with PAP or oral appliances (OAs), and might benefit from hypoglossal nerve stimulation or other surgical treatment. PAP is probably more effective than OAs in patients who use it ≥ 4 hours/night, but it is more difficult to comply with PAP.14
Evidence that treatment of asymptomatic OSA benefits other medical conditions is often conflicting. Given the low risk of treatment, it is reasonable to consider offering a trial of treatment, preferably with PAP, to asymptomatic patients with moderate-to-severe OSA and certain comorbidities, including obesity, resistant hypertension, high cardiovascular risk, congestive heart failure (CHF), AF, diabetes that is difficult to control, and pregnancy. Such patients should be strongly encouraged to use PAP ≥ 4 hours/night, and should be advised that benefits may not be immediately apparent.
Treatment of OSA improves daytime sleepiness
Daytime sleepiness is typically measured with the Epworth Sleepiness Scale (ESS), a self-administered questionnaire assessing a person’s level of drowsiness and propensity to fall asleep in 8 different daytime situations. Each situation is scored between 0 (would never doze) and 3 (high chance of dozing), with the scores then totaled to provide an overall score between 0 and 24. A score > 10 is considered abnormal.
Continue to: Treament of OSA...
Treatment of OSA with either PAP or OAs significantly improves ESS scores, with PAP being more effective.13 The difference appears to widen in patients with greater daytime sleepiness; in other words, patients with greater daytime sleepiness will gain even greater benefit from PAP, both overall and when compared with OAs.15
One randomized trial of an intensive lifestyle modification program for patients with OSA failed to show improvement in the ESS in the intention-to-treat analysis, but did demonstrate a 2.4-point greater reduction in ESS scores in those patients who successfully followed the program (achieving weight loss).16 Surgical treatments for OSA, such as uvulopalatopharyngoplasty or maxillary advancement, have been shown in some (but not all) studies to improve ESS scores; the different types of surgical treatment and the heterogeneity of studies prevents estimation of effect size.17 A meta-analysis of case series studies of hypoglossal nerve stimulation reported a mean improvement of 4.5 points on the ESS;18 comparison with other interventions is lacking.
Improved quality of life
Both PAP and OAs have been shown to improve sleep-related quality of life in patients with OSA. However, while the improvement is statistically significant, the effect size is small.14
That could be said of a study by Lewis et al.19 These researchers randomized patients with moderate-to-severe OSA and known coronary artery disease (CAD) or at least 3 risk factors for CAD to receive PAP, nocturnal oxygen, or lifestyle education.19 The patients randomized to receive PAP improved vitality scores by only 3.6 points on a 100-point scale; this was significantly better statistically than the improvement achieved by those randomized to lifestyle education. Smaller improvements were noted in depression, social function, and general health. Patients who had more daytime sleepiness at baseline had greater improvements in function.19
Cognitive function findings are mixed
In a systematic review published in 2004, Aloia et al4 found measurable impairments on neuropsychological tests of global cognitive functioning, attention/vigilance, executive functioning, memory, psychomotor function, and constructional abilities in patients with OSA. The results of treatment studies (all but 1 using PAP) were mixed. No studies showed improvement in psychomotor speed or language, and studies disagreed on whether treatment produced benefits in global cognition, attention, or executive functions.4
Continue to: Findings of more recent studies...
Findings of more recent studies remain mixed. A 3-month Spanish trial of PAP in older adults with severe OSA showed improvement in 2 of 4 neuropsychological tests of cognitive function; this was a secondary outcome measure.20 The PREDICT trial in the United Kingdom demonstrated a reduction in daytime sleepiness but no improvement in cognitive function in PAP-treated older adults with OSA but without dementia over a 1-year period.21
In contrast, a French long-term study of adults ages ≥ 65 years with severe (but not necessarily symptomatic) OSA showed better maintenance of memory performance; these results must be interpreted with caution, however, because the study was not randomized, controlled, or blinded, and the results were not adjusted for potential confounders.22 The severity of OSA may influence the impact of PAP treatment on cognitive function.
The prevalence of OSA in patients with dementia is high, and more severe dementia is associated with more severe OSA.23 Although it is intuitive that disrupted sleep may worsen cognitive function, and that treatment could improve it, minimal benefit on cognitive function was shown by neuropsychological testing in patients with Alzheimer’s disease and OSA treated with continuous positive airway pressure (CPAP) vs sham CPAP in 1 small short-term randomized trial.23
In another study of patients with Alzheimer’s disease, this time an observational (nonrandomized, non-controlled, single-blind) study of patients who also had severe symptomatic OSA, researchers followed the patients for 3 years and found a significant delay in median annual cognitive decline of 1.5 points per year on the Mini-Mental Status Examination in patients treated with PAP compared with those who did not receive PAP treatment.24
Hypertension: Small but positive results
A meta-analysis of PAP use in patients with OSA and resistant hypertension (defined as inadequate control while taking at least 3 antihypertensive agents or control requiring at least 4 agents) documented significant blood pressure (BP) lowering, with a pooled estimate of -7.21 mm Hg systolic and -4.99 mm Hg diastolic.25 The decrease in BP was demonstrated in both sleepy and non-sleepy subjects.
Continue to: Multiple studies have...
Multiple studies have shown a small reduction in BP readings (generally about 2 mm Hg) with PAP treatment in nonresistant hypertensive patients with OSA who are sleepy.26 Conversely, the literature is mixed on whether treatment of non-sleepy patients with OSA reduces BP. One long-term study demonstrated a small (1.89 mm Hg systolic, 2.19 mm Hg diastolic) BP reduction effect of PAP in non-sleepy subjects with OSA.27 Similarly, research has shown mandibular advancement devices to lower BP in patients with OSA, in a range similar to that achieved with PAP.28 Whether very small reductions in BP improve important clinical outcomes such as stroke or heart disease is unknown.
CV risk: Again, findings are mixed
The SAVE study is the largest randomized investigation of the effect of treatment of OSA with PAP for secondary prevention of cardiovascular events.29 The trial involved 2717 adults with cardiovascular disease, moderate-to-severe OSA, and minimal sleepiness, and had as its primary composite endpoint death from cardiovascular causes, myocardial infarction (MI), stroke, hospitalization for unstable angina, heart failure, or transient ischemic attack. Patients with severe daytime sleepiness or severe hypoxemia were excluded. The study found no difference between PAP and usual care in the primary outcome, despite a significant reduction in the AHI from a mean of 29 at baseline to 3.7 with PAP treatment.
Similarly, a randomized controlled trial (RCT) of 725 patients with non-sleepy OSA failed to show a reduction in cardiovascular events or in the development of hypertension.30 Peker et al31 randomized 244 adults with recently revascularized coronary artery disease and OSA without daytime sleepiness to auto-titrating CPAP or usual care and did not find a statistically significant difference in revascularization, MI, stroke, or cardiovascular mortality; however, those patients who were compliant with CPAP for ≥ 4 hours/night did have a statistically significant reduction in the combined endpoint.
In contrast, a trial of patients with first-ever stroke and moderate-to-severe OSA who were randomized to early nasal CPAP or usual care demonstrated better 5-year cardiovascular survival for the patients in the CPAP group, and a trend toward better cardiovascular event-free survival.32 Degree of daytime sleepiness was not stated in this study.
A recent meta-analysis of RCTs failed to find a reduction in major adverse cardiovascular events (MACE) in patients with moderate-to-severe OSA treated with PAP.33 In this study, subgroup analysis documented benefit in patients who were adherent with PAP for ≥ 4 hours/night. A larger meta-analysis, however, did not find a reduction in MACE even in the adherent subgroup.34
Continue to: AF and OSA
AF and OSA: An interesting relationship
OSA is an independent risk factor for AF, approximately doubling the risk.35 A review of 10,132 patients with AF (1841 with OSA) in a large observational study demonstrated no difference in outcomes of all-cause mortality, first hospitalization, major bleeding, or major cardiovascular events in OSA patients who were or were not treated with PAP. The PAP-treated patients did have a slightly lower (16% vs 18%) risk of worsening of AF over 2 years.36 Overall, AF patients with OSA had more symptoms and higher admission rates, but no difference in overall mortality or MACE. Observational studies have suggested that PAP treatment of OSA facilitates maintenance of normal sinus rhythm after cardioversion and after ablation.37
CHF: Results look promising
In one small study, 24 patients with heart failure with reduced ejection fraction who were optimally medically treated were randomized to receive PAP or sham PAP for 1 month.38 The treatment group demonstrated reduced systolic BP, reduced end systolic dimension, and significant improvement in ejection fraction from 25 ± 2.8% to 33.8 ± 2.4%.
OSA Tx improves insulin sensitivity
OSA is associated with impaired glucose tolerance, and PAP treatment of OSA has been documented to improve insulin sensitivity.39,40 An efficacy study utilizing PAP in a laboratory setting for 8 hours/night demonstrated significant reduction in fasting blood sugar and a reduction in the dawn phenomenon (an increase in early morning fasting glucose as a result of rebound from hypoglycemia during sleep).39 A 2015 meta-analysis of short-term studies also showed improvement in insulin sensitivity in OSA patients treated with PAP, but failed to find any reduction in A1C or in body mass index.40
All-cause mortality: Difference in findings between short- and long-term studies
Yu et al’s34 meta-analysis of 10 RCTs involving 7266 participants found no difference in mortality in treated (vs no treatment or sham treatment) OSA patients. This was true even in the more adherent subgroup. These studies were relatively short-term, with the longest mean follow-up being 68 months.
However, several longer-term population-based studies have suggested that OSA treatment improves all-cause mortality. An 18-year follow-up of a Wisconsin cohort documented dramatically increased mortality in patients with severe sleep apnea; mortality was even higher when patients treated with PAP were removed from the analysis, suggesting that PAP treatment was protective, mainly for cardiovascular death.5
Continue to: A Danish registry...
A Danish registry documented that patients treated with CPAP had higher rates of comorbidities before and during treatment; when these comorbidities were controlled, men ages ≥ 60 years had improved survival when treated with CPAP. There was no survival benefit in women.41
A recent analysis—the Sleep Heart Health Study—followed patients with obesity and severe OSA for a mean of 11.1 years and calculated a hazard ratio for all-cause mortality associated with prescribed PAP therapy of 0.58 (95% confidence interval [CI], 0.35-0.96) after propensity matching.42 The difference in mortality appeared 6 to 7 years after PAP therapy was prescribed. This delay may explain the failure of shorter-term studies to demonstrate evidence of benefit.
OSA Tx reduces motor vehicle crashes
Drowsy driving is widely accepted as a risk for motor vehicle crashes. Successful treatment of OSA with PAP has been shown to improve driving performance on a driving simulator.43 An analysis of 15 studies similarly demonstrated a significant reduction in driving accidents (incident rate ratio [IRR] = 0.45) and in near-miss accidents (IRR = 0.23) in patients with OSA treated with CPAP.44
Pulmonary hypertension: OSA Tx lowers pulmonary arterial pressure
Patients with OSA have higher than expected rates of pulmonary arterial hypertension—as high as 22%—documented by pulmonary artery catheterization findings.45 A meta-analysis of studies that examined the effect of PAP in patients with OSA and coexisting pulmonary hypertension but without other overt pulmonary or cardiac disease found significant reductions in pulmonary artery pressure.46 Whether this finding translates into improved patient-oriented outcomes is unknown.
OSA and pregnancy outcomes
A national cohort study demonstrated that OSA is an independent risk factor for multiple adverse pregnancy outcomes, including gestational diabetes, hypertensive disorders in pregnancy, intrauterine growth retardation, and stillbirth.7 OSA was also associated with the rare serious adverse outcomes of congestive heart failure, cardiomyopathy, and pulmonary embolism.7 There is little evidence to date with which to determine whether treatment of OSA improves outcomes, but PAP treatment is documented to be safe in pregnant women.8
CORRESPONDENCE
Stephen C. Sorsby, MD, MHA, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 530, Little Rock, AR 72205; [email protected].
Obstructive sleep apnea (OSA) is a common cause of daytime sleepiness, and severe OSA is a risk factor for hypertension, cardiovascular events, atrial fibrillation (AF), insulin resistance, cognitive impairment, motor vehicle crashes, adverse pregnancy outcomes, and overall mortality.1-8 The hazard ratio for mortality for patients with severe OSA may be as high as 3.8.5
OSA is diagnosed by the apnea-hypopnea index (AHI), defined as the number of apnea or hypopnea events per hour as determined by polysomnography. An AHI score ≤ 5 is considered normal; > 5 to ≤ 15 is mild; > 15 to < 30 is moderate; and ≥ 30 is severe. Most studies of OSA treatment use reduction of AHI as the measure of treatment effectiveness, and several types of treatment improve AHI.
In family medicine, we generally want to know whether treatment of OSA will improve outcomes of significance to patients. A recent systematic review of evidence for the US Preventive Services Task Force found that it was unclear whether OSA treatment improved most health outcomes, including mortality, cardiovascular events, or motor vehicle crashes.6 Several other organizations have published guidelines regarding OSA treatment; these guidelines are reviewed in the TABLE.9-13

This article summarizes the current evidence surrounding the effect of treatment of OSA on outcomes of significance to patients. While multiple treatments have been advocated for patients with OSA, positive airway pressure (PAP) is the most widely used and studied and is recommended as standard treatment by most guidelines.9-13 Most available evidence about patient-oriented outcomes involves treatment with PAP; where there is evidence about the effect of other OSA treatments on a particular outcome, that evidence is also summarized.

Benefits of OSA treatment
Patients with OSA who have excessive daytime sleepiness can gain substantial symptomatic benefit from treatment of their OSA with PAP or oral appliances (OAs), and might benefit from hypoglossal nerve stimulation or other surgical treatment. PAP is probably more effective than OAs in patients who use it ≥ 4 hours/night, but it is more difficult to comply with PAP.14
Evidence that treatment of asymptomatic OSA benefits other medical conditions is often conflicting. Given the low risk of treatment, it is reasonable to consider offering a trial of treatment, preferably with PAP, to asymptomatic patients with moderate-to-severe OSA and certain comorbidities, including obesity, resistant hypertension, high cardiovascular risk, congestive heart failure (CHF), AF, diabetes that is difficult to control, and pregnancy. Such patients should be strongly encouraged to use PAP ≥ 4 hours/night, and should be advised that benefits may not be immediately apparent.
Treatment of OSA improves daytime sleepiness
Daytime sleepiness is typically measured with the Epworth Sleepiness Scale (ESS), a self-administered questionnaire assessing a person’s level of drowsiness and propensity to fall asleep in 8 different daytime situations. Each situation is scored between 0 (would never doze) and 3 (high chance of dozing), with the scores then totaled to provide an overall score between 0 and 24. A score > 10 is considered abnormal.
Continue to: Treament of OSA...
Treatment of OSA with either PAP or OAs significantly improves ESS scores, with PAP being more effective.13 The difference appears to widen in patients with greater daytime sleepiness; in other words, patients with greater daytime sleepiness will gain even greater benefit from PAP, both overall and when compared with OAs.15
One randomized trial of an intensive lifestyle modification program for patients with OSA failed to show improvement in the ESS in the intention-to-treat analysis, but did demonstrate a 2.4-point greater reduction in ESS scores in those patients who successfully followed the program (achieving weight loss).16 Surgical treatments for OSA, such as uvulopalatopharyngoplasty or maxillary advancement, have been shown in some (but not all) studies to improve ESS scores; the different types of surgical treatment and the heterogeneity of studies prevents estimation of effect size.17 A meta-analysis of case series studies of hypoglossal nerve stimulation reported a mean improvement of 4.5 points on the ESS;18 comparison with other interventions is lacking.
Improved quality of life
Both PAP and OAs have been shown to improve sleep-related quality of life in patients with OSA. However, while the improvement is statistically significant, the effect size is small.14
That could be said of a study by Lewis et al.19 These researchers randomized patients with moderate-to-severe OSA and known coronary artery disease (CAD) or at least 3 risk factors for CAD to receive PAP, nocturnal oxygen, or lifestyle education.19 The patients randomized to receive PAP improved vitality scores by only 3.6 points on a 100-point scale; this was significantly better statistically than the improvement achieved by those randomized to lifestyle education. Smaller improvements were noted in depression, social function, and general health. Patients who had more daytime sleepiness at baseline had greater improvements in function.19
Cognitive function findings are mixed
In a systematic review published in 2004, Aloia et al4 found measurable impairments on neuropsychological tests of global cognitive functioning, attention/vigilance, executive functioning, memory, psychomotor function, and constructional abilities in patients with OSA. The results of treatment studies (all but 1 using PAP) were mixed. No studies showed improvement in psychomotor speed or language, and studies disagreed on whether treatment produced benefits in global cognition, attention, or executive functions.4
Continue to: Findings of more recent studies...
Findings of more recent studies remain mixed. A 3-month Spanish trial of PAP in older adults with severe OSA showed improvement in 2 of 4 neuropsychological tests of cognitive function; this was a secondary outcome measure.20 The PREDICT trial in the United Kingdom demonstrated a reduction in daytime sleepiness but no improvement in cognitive function in PAP-treated older adults with OSA but without dementia over a 1-year period.21
In contrast, a French long-term study of adults ages ≥ 65 years with severe (but not necessarily symptomatic) OSA showed better maintenance of memory performance; these results must be interpreted with caution, however, because the study was not randomized, controlled, or blinded, and the results were not adjusted for potential confounders.22 The severity of OSA may influence the impact of PAP treatment on cognitive function.
The prevalence of OSA in patients with dementia is high, and more severe dementia is associated with more severe OSA.23 Although it is intuitive that disrupted sleep may worsen cognitive function, and that treatment could improve it, minimal benefit on cognitive function was shown by neuropsychological testing in patients with Alzheimer’s disease and OSA treated with continuous positive airway pressure (CPAP) vs sham CPAP in 1 small short-term randomized trial.23
In another study of patients with Alzheimer’s disease, this time an observational (nonrandomized, non-controlled, single-blind) study of patients who also had severe symptomatic OSA, researchers followed the patients for 3 years and found a significant delay in median annual cognitive decline of 1.5 points per year on the Mini-Mental Status Examination in patients treated with PAP compared with those who did not receive PAP treatment.24
Hypertension: Small but positive results
A meta-analysis of PAP use in patients with OSA and resistant hypertension (defined as inadequate control while taking at least 3 antihypertensive agents or control requiring at least 4 agents) documented significant blood pressure (BP) lowering, with a pooled estimate of -7.21 mm Hg systolic and -4.99 mm Hg diastolic.25 The decrease in BP was demonstrated in both sleepy and non-sleepy subjects.
Continue to: Multiple studies have...
Multiple studies have shown a small reduction in BP readings (generally about 2 mm Hg) with PAP treatment in nonresistant hypertensive patients with OSA who are sleepy.26 Conversely, the literature is mixed on whether treatment of non-sleepy patients with OSA reduces BP. One long-term study demonstrated a small (1.89 mm Hg systolic, 2.19 mm Hg diastolic) BP reduction effect of PAP in non-sleepy subjects with OSA.27 Similarly, research has shown mandibular advancement devices to lower BP in patients with OSA, in a range similar to that achieved with PAP.28 Whether very small reductions in BP improve important clinical outcomes such as stroke or heart disease is unknown.
CV risk: Again, findings are mixed
The SAVE study is the largest randomized investigation of the effect of treatment of OSA with PAP for secondary prevention of cardiovascular events.29 The trial involved 2717 adults with cardiovascular disease, moderate-to-severe OSA, and minimal sleepiness, and had as its primary composite endpoint death from cardiovascular causes, myocardial infarction (MI), stroke, hospitalization for unstable angina, heart failure, or transient ischemic attack. Patients with severe daytime sleepiness or severe hypoxemia were excluded. The study found no difference between PAP and usual care in the primary outcome, despite a significant reduction in the AHI from a mean of 29 at baseline to 3.7 with PAP treatment.
Similarly, a randomized controlled trial (RCT) of 725 patients with non-sleepy OSA failed to show a reduction in cardiovascular events or in the development of hypertension.30 Peker et al31 randomized 244 adults with recently revascularized coronary artery disease and OSA without daytime sleepiness to auto-titrating CPAP or usual care and did not find a statistically significant difference in revascularization, MI, stroke, or cardiovascular mortality; however, those patients who were compliant with CPAP for ≥ 4 hours/night did have a statistically significant reduction in the combined endpoint.
In contrast, a trial of patients with first-ever stroke and moderate-to-severe OSA who were randomized to early nasal CPAP or usual care demonstrated better 5-year cardiovascular survival for the patients in the CPAP group, and a trend toward better cardiovascular event-free survival.32 Degree of daytime sleepiness was not stated in this study.
A recent meta-analysis of RCTs failed to find a reduction in major adverse cardiovascular events (MACE) in patients with moderate-to-severe OSA treated with PAP.33 In this study, subgroup analysis documented benefit in patients who were adherent with PAP for ≥ 4 hours/night. A larger meta-analysis, however, did not find a reduction in MACE even in the adherent subgroup.34
Continue to: AF and OSA
AF and OSA: An interesting relationship
OSA is an independent risk factor for AF, approximately doubling the risk.35 A review of 10,132 patients with AF (1841 with OSA) in a large observational study demonstrated no difference in outcomes of all-cause mortality, first hospitalization, major bleeding, or major cardiovascular events in OSA patients who were or were not treated with PAP. The PAP-treated patients did have a slightly lower (16% vs 18%) risk of worsening of AF over 2 years.36 Overall, AF patients with OSA had more symptoms and higher admission rates, but no difference in overall mortality or MACE. Observational studies have suggested that PAP treatment of OSA facilitates maintenance of normal sinus rhythm after cardioversion and after ablation.37
CHF: Results look promising
In one small study, 24 patients with heart failure with reduced ejection fraction who were optimally medically treated were randomized to receive PAP or sham PAP for 1 month.38 The treatment group demonstrated reduced systolic BP, reduced end systolic dimension, and significant improvement in ejection fraction from 25 ± 2.8% to 33.8 ± 2.4%.
OSA Tx improves insulin sensitivity
OSA is associated with impaired glucose tolerance, and PAP treatment of OSA has been documented to improve insulin sensitivity.39,40 An efficacy study utilizing PAP in a laboratory setting for 8 hours/night demonstrated significant reduction in fasting blood sugar and a reduction in the dawn phenomenon (an increase in early morning fasting glucose as a result of rebound from hypoglycemia during sleep).39 A 2015 meta-analysis of short-term studies also showed improvement in insulin sensitivity in OSA patients treated with PAP, but failed to find any reduction in A1C or in body mass index.40
All-cause mortality: Difference in findings between short- and long-term studies
Yu et al’s34 meta-analysis of 10 RCTs involving 7266 participants found no difference in mortality in treated (vs no treatment or sham treatment) OSA patients. This was true even in the more adherent subgroup. These studies were relatively short-term, with the longest mean follow-up being 68 months.
However, several longer-term population-based studies have suggested that OSA treatment improves all-cause mortality. An 18-year follow-up of a Wisconsin cohort documented dramatically increased mortality in patients with severe sleep apnea; mortality was even higher when patients treated with PAP were removed from the analysis, suggesting that PAP treatment was protective, mainly for cardiovascular death.5
Continue to: A Danish registry...
A Danish registry documented that patients treated with CPAP had higher rates of comorbidities before and during treatment; when these comorbidities were controlled, men ages ≥ 60 years had improved survival when treated with CPAP. There was no survival benefit in women.41
A recent analysis—the Sleep Heart Health Study—followed patients with obesity and severe OSA for a mean of 11.1 years and calculated a hazard ratio for all-cause mortality associated with prescribed PAP therapy of 0.58 (95% confidence interval [CI], 0.35-0.96) after propensity matching.42 The difference in mortality appeared 6 to 7 years after PAP therapy was prescribed. This delay may explain the failure of shorter-term studies to demonstrate evidence of benefit.
OSA Tx reduces motor vehicle crashes
Drowsy driving is widely accepted as a risk for motor vehicle crashes. Successful treatment of OSA with PAP has been shown to improve driving performance on a driving simulator.43 An analysis of 15 studies similarly demonstrated a significant reduction in driving accidents (incident rate ratio [IRR] = 0.45) and in near-miss accidents (IRR = 0.23) in patients with OSA treated with CPAP.44
Pulmonary hypertension: OSA Tx lowers pulmonary arterial pressure
Patients with OSA have higher than expected rates of pulmonary arterial hypertension—as high as 22%—documented by pulmonary artery catheterization findings.45 A meta-analysis of studies that examined the effect of PAP in patients with OSA and coexisting pulmonary hypertension but without other overt pulmonary or cardiac disease found significant reductions in pulmonary artery pressure.46 Whether this finding translates into improved patient-oriented outcomes is unknown.
OSA and pregnancy outcomes
A national cohort study demonstrated that OSA is an independent risk factor for multiple adverse pregnancy outcomes, including gestational diabetes, hypertensive disorders in pregnancy, intrauterine growth retardation, and stillbirth.7 OSA was also associated with the rare serious adverse outcomes of congestive heart failure, cardiomyopathy, and pulmonary embolism.7 There is little evidence to date with which to determine whether treatment of OSA improves outcomes, but PAP treatment is documented to be safe in pregnant women.8
CORRESPONDENCE
Stephen C. Sorsby, MD, MHA, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 530, Little Rock, AR 72205; [email protected].
1. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384.
2. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
3. Iftikhar IH, Hoyos CM, Phillips CL, et al. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med. 2015;11:475-485.
4. Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772-785.
5. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071-1078.
6. Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:415-433.
7. Bourjeily G, Danilack VA, Bublitz MA, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;35:50-57.
8. Booth JM, Tonidandel AM. Peripartum management of obstructive sleep apnea. Clin Obstet Gyn. 2017;60:405-417.
9. Strohl KP, Brown DB, Collop N, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med. 2013;187:1259-1266.
10. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
11. National Institute for Health and Care Excellence. Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. Technology appraisal guidance [TA139]. https://www.nice.org.uk/guidance/ta139. Revised February 2012. Accessed October 28, 2019.
12. Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471-483.
13. Netzer NC, Ancoli-Israel S, Bliwise DL, et al. Principles of practice parameters for the treatment of sleep disordered breathing in the elderly and frail elderly: the consensus of the International Geriatric Sleep Medicine Task Force. Eur Respir J. 2016;48:992-1018.
14. Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879-887.
15. Bratton DJ, Gaisl T, Schlatzer C, et al. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3:869-878.
16. Ng SSS, Chan RSM, Woo J, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest. 2015;148:1193-1203.
17. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;4:CD001004.
18. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015; 125:1254-1264.
19. Lewis EF, Rui W, Punjabi N, et al. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: A Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am Heart J. 2017;189:59-67.
20. Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46:142-151.
21. McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804-812.
22. Crawford-Achour E, Dauphinot V, Martin MS, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF study. J Clin Sleep Med. 2015;11:519-524.
23. Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076-2081.
24. Troussière AC, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1405-1408.
25. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757-764.
26. Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587-596.
27. Barbé F, Durán-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718-726.
28. Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280-2293.
29. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919-931.
30. Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161-2168.
31. Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613-620.
32. Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24:47-53.
33. Abuzaid AS, Al Ashray HS, Elbadaway A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure in patients with obstructive sleep apnea. Am J Card. 2017;120:693-699.
34. Yu J, Zhou Z, McEvoy D, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318:156-166.
35. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the incident risk of atrial fibrillation. J Amer Coll of Card. 2007;49:565-571.
36. Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169:647-654.e2.
37. Nalliah CJ, Sanders P, Kalman JM. Obstructive sleep apnea treatment and atrial fibrillation: a need for definitive evidence. J Cardiovasc Electrophysiol. 2016;27:1001-1010.
38. Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233-1241
39. Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes: a randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96-105.
40. Feng Y, Zhang Z, Dong ZZ. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15005.
41. Jennum P, Tonnesen P, Ibsen R, et al. Obstructive sleep apnea: effect of comorbidities and positive airway pressure on all-cause mortality. Sleep Med. 2017;36:62-66.
42. Lisan Q, Van Sloten T, Marques Vidal P, et al. Association of positive airway pressure prescription with mortality in patients with obesity and severe obstructive sleep apnea: the sleep heart health study. JAMA Otolaryngol Head Neck Surg. 2019;145:509-515.
43. Mazza S, Pépin JL, Naëgelé B, et al. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J. 2006;28:1020-1028.
44. Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, et al. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev. 2011;15:301-310.
45. Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2009;104:1300-1306.
46. Imran TF, Ghazipura M, Liu S, et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev. 2016;21:591-598.
1. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384.
2. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
3. Iftikhar IH, Hoyos CM, Phillips CL, et al. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med. 2015;11:475-485.
4. Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772-785.
5. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071-1078.
6. Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:415-433.
7. Bourjeily G, Danilack VA, Bublitz MA, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;35:50-57.
8. Booth JM, Tonidandel AM. Peripartum management of obstructive sleep apnea. Clin Obstet Gyn. 2017;60:405-417.
9. Strohl KP, Brown DB, Collop N, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med. 2013;187:1259-1266.
10. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
11. National Institute for Health and Care Excellence. Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. Technology appraisal guidance [TA139]. https://www.nice.org.uk/guidance/ta139. Revised February 2012. Accessed October 28, 2019.
12. Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471-483.
13. Netzer NC, Ancoli-Israel S, Bliwise DL, et al. Principles of practice parameters for the treatment of sleep disordered breathing in the elderly and frail elderly: the consensus of the International Geriatric Sleep Medicine Task Force. Eur Respir J. 2016;48:992-1018.
14. Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879-887.
15. Bratton DJ, Gaisl T, Schlatzer C, et al. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3:869-878.
16. Ng SSS, Chan RSM, Woo J, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest. 2015;148:1193-1203.
17. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;4:CD001004.
18. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015; 125:1254-1264.
19. Lewis EF, Rui W, Punjabi N, et al. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: A Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am Heart J. 2017;189:59-67.
20. Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46:142-151.
21. McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804-812.
22. Crawford-Achour E, Dauphinot V, Martin MS, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF study. J Clin Sleep Med. 2015;11:519-524.
23. Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076-2081.
24. Troussière AC, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1405-1408.
25. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757-764.
26. Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587-596.
27. Barbé F, Durán-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718-726.
28. Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280-2293.
29. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919-931.
30. Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161-2168.
31. Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613-620.
32. Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24:47-53.
33. Abuzaid AS, Al Ashray HS, Elbadaway A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure in patients with obstructive sleep apnea. Am J Card. 2017;120:693-699.
34. Yu J, Zhou Z, McEvoy D, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318:156-166.
35. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the incident risk of atrial fibrillation. J Amer Coll of Card. 2007;49:565-571.
36. Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169:647-654.e2.
37. Nalliah CJ, Sanders P, Kalman JM. Obstructive sleep apnea treatment and atrial fibrillation: a need for definitive evidence. J Cardiovasc Electrophysiol. 2016;27:1001-1010.
38. Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233-1241
39. Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes: a randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96-105.
40. Feng Y, Zhang Z, Dong ZZ. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15005.
41. Jennum P, Tonnesen P, Ibsen R, et al. Obstructive sleep apnea: effect of comorbidities and positive airway pressure on all-cause mortality. Sleep Med. 2017;36:62-66.
42. Lisan Q, Van Sloten T, Marques Vidal P, et al. Association of positive airway pressure prescription with mortality in patients with obesity and severe obstructive sleep apnea: the sleep heart health study. JAMA Otolaryngol Head Neck Surg. 2019;145:509-515.
43. Mazza S, Pépin JL, Naëgelé B, et al. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J. 2006;28:1020-1028.
44. Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, et al. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev. 2011;15:301-310.
45. Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2009;104:1300-1306.
46. Imran TF, Ghazipura M, Liu S, et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev. 2016;21:591-598.
PRACTICE RECOMMENDATIONS
› Treat patients with symptomatic obstructive sleep apnea (OSA) with positive airway pressure (PAP) or oral appliances to reduce daytime sleepiness, improve quality-of-life scores, and modestly reduce blood pressure in patients with hypertension. A
› Consider recommending at least 4 hours of PAP every night for asymptomatic patients (those without daytime sleepiness) with severe OSA and other conditions, including resistant hypertension, atrial fibrillation, congestive heart failure, cognitive impairment, obesity, and stroke. B
› Do not screen asymptomatic patients for OSA. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series