User login
Lumbar spinal stenosis: Can positional therapy alleviate pain?
- Positional therapy with a wheeled walker may help patients with spinal stenosis to walk, as well as ease their pain. This conservative approach has minimum risks—and minimum costs.
Methods: We analyzed a retrospective case series of 52 patients with spinal stenosis confirmed by spinal imaging and walking limitations treated with a wheeled walker set to induce lumbosacral flexion.
Results: Of the 52 patients, improvement in ambulation was classified as excellent for 30 (58%), good for 7 (13%), moderate for 8 (16%), and poor for 7 (13%). Among 48 patients with neurogenic pain, pain relief was classified as excellent for 22 (46%), good for 11 (23%), moderate for 7 (14.5%), and poor for 8 (16.5%).
Conclusions: These retrospective data from a case series support the hypothesis that positional therapy with a wheeled walker set to induce lumbosacral flexion relieves lower extremity symptoms of spinal stenosis. However, an adequate test of this hypothesis will require randomized trials of sufficient size and duration that include objective clinical endpoints such as quality-of-life measures, immobility complications and need for drugs, physical therapy, procedures including epidural injections, and spinal surgery.
In the meantime, this conservative strategy is an option for patients following the recommendations of the North American Spine Society, or for those who have contraindications (or aversions) to surgery or epidural injections, or who have found these options ineffective. Positional therapy with a wheeled walker offers the possibility of short-term benefits for ambulation and pain, with minimal risks and costs.
When shoppers at the grocery store are leaning forward on their carts, many of them could be trying to relieve the pain of lumbar spinal stenosis. This way of finding temporary relief is one we replicated with a wheeled walker for prolonged periods in a retrospective case series to see what further benefits might be gained.
Symptoms are affected by body position and activity level. For patients with lumbar spinal stenosis, lower extremity symptoms can be debilitating and include loss of sensation, paresthesias, burning, pain, weakness, claudication, difficulty standing or walking, or nocturnal neuropathic pain in the feet, legs, or thighs. Axial loading1 (as occurs during walking) and spinal extension2 (as occurs in an erect position) both decrease the diameter of the central spinal canal and lateral recesses, and may cause nerve compression and lower extremity symptoms. In contrast, lumbosacral flexion—facilitated, for example, by leaning forward on a grocery cart3—opens the spine and may reduce nerve compression and related symptoms.
Exhaust all medical options before turning to surgery. The North American Spine Society (NASS) has issued clinical guidelines for spinal stenosis that make recommendations regarding the value of pharmacologic interventions, manipulative techniques, behavioral therapies, and other conservative measures (www. guideline.gov).4 For patients with severe or unremitting symptoms requiring specialized care by spine specialists, NASS further outlines 3 phases of gradually intensifying medical therapy before turning to surgery, which is associated with increased morbidity and costs.5
A previously untested medical approach. Most patients may return to productivity within 2 to 4 months after starting conservative treatment, but some will still require treatment recommended for greater levels of severity.6 For these latter patients, no randomized trials have evaluated the efficacy of medical management with a wheeled walker. This new intervention, if effective, could avoid or delay the expense and side effects of surgery.7 In addition, a wheeled walker may decrease pain from spinal stenosis.8
To explore whether positional therapy with a wheeled walker relieves lower extremity symptoms of lumbar spinal stenosis, we conducted a retrospective case series of 52 patients with spinal imaging confirmed lumbar spinal stenosis and walking limitations.9
Methods
These observations were based on retrospective chart reviews of all patients in a podiatric private practice (SMG) over 1 year to identify those with lower extremity symptoms of lumbar spinal stenosis who were evaluated with positional testing.
Identifying possible stenosis by positional history
Patients were suspected of having spinal stenosis contributing to, or entirely responsible for, lower extremity neuropathic or claudication symptoms based on a positive positional history, including any of the following patterns:
- walking limitation in which the patient needed to sit or lean forward to get relief
- significant improvement in ambulation when pushing a grocery cart, walker, or baby stroller, or when on a treadmill that induced lumbosacral flexion
- constant, frequent, or occasional lower extremity symptoms of a neuropathic nature with an unclear cause that was exacerbated by walking or standing
- nocturnal exacerbation of neuropathic symptoms affected by sleep position.
Symptoms linked to the cause radiologically. Spinal stenosis was confirmed by spinal imaging (magnetic resonance imaging or computed tomography scan) showing stenosis in areas corresponding to symptoms in the lower extremities. Patients without confirmatory spinal imaging were excluded from our study.
Peripheral neuropathy was diagnosed by changes in nerve conduction studies interpreted as being consistent with axonal or demyelinating peripheral neuropathy. Using these criteria, we assembled a case series of 52 patients with imaging-confirmed lumbar spinal stenosis and walking limitations. Of the 52 patients, 33 had received a previous diagnosis of spinal stenosis confirmed by spinal imaging, but only 10 considered that to be the cause of their lower extremity symptoms, with the remainder presenting with a primary diagnosis of peripheral neuropathy—with or without arterial claudication.
Using positional testing to confirm suitability of rollator walker
Patients with lower extremity symptoms of lumbar spinal stenosis underwent a therapeutic trial of “positional testing” involving full-time use of a 3- or 4-wheeled rollator walker (usually provided as a loan) set to induce lumbosacral flexion for 3 days. For patients no taller than 4’ 9” to 5’ 2”, a reduced-height walker (29” to 32”) was usually necessary; patients shorter than 4’ 9” usually needed a modified pediatric walker.
Patients returned for adjustment of the walker if it was uncomfortable or unhelpful. We recommended they also use a shower stool and kitchen stool to minimize erect posture. If they experienced nocturnal exacerbation of neuropathic symptoms, we encouraged them to try sleeping in a recliner. If patients with neuropathic symptoms wanted to continue sleeping in bed, we encouraged them to try sleeping with a pillow beneath their thighs (if sleeping on their back), or sleeping in a fetal position with a pillow between their thighs (if sleeping on their side).10
We usually reevaluated patients in 3 to 5 days, comparing current pain severity and walking capability with previous levels. Patients reporting improvement were encouraged to maintain this full-time positional testing for a total of 10 days. During the subsequent “positional therapy” phase, they gradually reduced their use of the walker, if possible, to an amount just needed to maintain improvement. The therapy phase lasted for 3 months, bringing the total time that patients used a walker to nearly 14 weeks.
Criteria for successful treatment
We gauged treatment success according to self-reported walking capabilities and subjective descriptions of uncomfortable symptoms, using criteria previously described.10
Walking distance. Patients reported uninterrupted walking distance before using the walker and after they had begun using the walker. We classified improvement in walking distance as excellent (over 400% increase), good (250%– 399%), moderate (100%–249%), or poor (≤99%). (The distance a patient can walk—before pain sets in—may vary from day to day. We therefore gauged improvement in this distance by contrasting consistent walking distances achieved and maintained with positional management to the shortest usual walking distance before the intervention.)
Pain reduction. To define a decrease in discomfort reported during the positional testing phase and maintained with positional therapy, we used a verbal analog pain scale (1–3 out of 10=mild pain; 4–7=moderate pain; 8–10=severe pain). We classified reduction in discomfort stemming from spinal stenosis as excellent (75%–100%), good (50%–74%), moderate (25%–49%), or poor (≤24%).
Results
Rapid and dramatic improvement for most patients
The 52 patients in our case series ranged in age from 67 to 90 years; 19 were men. Of the 52, improvement in ambulation was excellent for 30 (58%), good for 7 (13%), moderate for 8 (16%), and poor for 7 (13%) after 3 to 5 days.
Of 48 patients with neurogenic pain, grading with the verbal analog pain scale showed relief was excellent for 22 (46%), good for 11 (23%), moderate for 7 (14.5%), and poor for 8 (16.5%) after 3 to 5 days.
Of the 37 patients with excellent or good improvement in ambulation, 11 needed to keep using the walker extensively, 22 frequently, and 4 occasionally or not at all. Of the 6 patients who had undergone spinal stenosis surgery, improvement was excellent for 3, good for 1, and poor for 2.
A subgroup of 36 patients in our study had diabetes. Of these, 25 had concomitant peripheral neuropathy; 18 reported good to excellent improvement of ambulation or reduction of pain.
Conclusion
Patients deserve a trial of positional therapy with the wheeled walker
These descriptive data support the hypothesis that positional therapy with a wheeled walker set to induce lumbosacral flexion alleviates lower extremity symptoms of spinal stenosis. Limitations of this case series:
- the lack of any comparison group
- improvements in ambulation are based on subjective criteria
- findings can be generalized only to older patients potentially eligible for surgery,11 those who have not benefited from surgery, or those who are undergoing medical therapies recommended by the North American Spine Society4
- patients seen in a podiatry office who present for lower extremity symptoms of spinal stenosis may differ from those seen in a family practitioner’s office who present with low back pain.
Nonetheless, this conservative strategy may be applicable to the evaluation and management of lower extremity symptoms of spinal stenosis regardless of presenting symptoms or source of medical care.
Walking limitations and lower extremity pain caused by spinal stenosis are physically and psychologically disabling. Relief can dramatically improve a person’s quality of life. Improved ambulation may also aid in the management of concurrent medical conditions, such as diabetes and cardiovascular disease.
Our hypothesis requires direct testing in randomized trials of sufficient size and duration. Such trials should include longer term and more objective clinical endpoints, such as quality of life measures, complications due to immobility and need for drugs, physical therapy, procedures such as epidural injections, or spinal surgery. Validation of this hypothesis would substantially reduce morbidity and costs, as well as increase the quality of life of patients with lower extremity symptoms of lumbar spinal stenosis. Until such studies are conducted, this conservative strategy may increase ambulation and decrease pain over the short term, with minimal risks and costs. It may also be helpful for those with contraindications (or aversions) to surgery or epidural injections or those who have found these approaches ineffective.
Correspondence
Charles H. Hennekens, MD, Sir Richard Doll Research Professor, College of Biomedical Science, Department of Clinical Science and Medical Education and Center of Excellence, Florida Atlantic University, Building 10–Administration, Division of Research, Room 244D, 777 Glades Road, Boca Raton, FL 33432; [email protected]
1. Willen J, Danielson B, Gaulitz A, Niklason T, Schönström N, Hansson T. Dynamic effect on the lumbar spinal canal: axial loaded CT-myelography and MRI in patients with sciatica and/or neurogenic claudication. Spine. 1997;22:2968-2976.
2. Harrison DE, Calliet R, Harrison DD, Troyanovich SJ, Harrison SO. A review of biomechanics of the central nervous system–part 1: spinal canal deformations resulting from changes in posture. J Manipulative Physiol Ther. 1999;22:227-234.
3. Goldsmith ME, Wiesel S. Spinal stenosis: a straightforward approach to a complex problem. J Clin Rheumatol. 1998;4:92.-
4. Diagnosis and treatment of degenerative lumbar spinal stenosis. National Guideline Clearinghouse Web site. Available at: www.guidelines.gov. Accessed March 6, 2008.
5. Wong DA, Mayer TG, Spivak JM, et al. North American Spine Society (NASS). Phase III clinical guide-lines for multidisciplinary spine care specialists. Spinal stenosis version 1.0. LaGrange, IL: North American Spine Society; 2002.
6. Snyder DL, Doggett D, Turkelson C. Treatment of degenerative lumbar spinal stenosis. Am Fam Physician. 2004;70:517-520.
7. Malmivaara A, Slätis P, Heliövaara M, et al. Finnish Lumbar Spinal Research Group. Surgical or non-operative treatment of lumbar spinal stenosis. Spine. 2007;32:1-8.
8. Hoenig H. Assistive technology and mobility aids for the older patient with disability. Ann Long Term Care. 2004;12:12-19.
9. Hennekens CH, Buring JE. Epidemiology in Medicine. Boston, MA: Little, Brown; 1987.
10. Goldman SM. Neuropathic pain at night in diabetic patients may be caused by spinal stenosis. Diabetic Med. 2005;22:1763-1765.
11. Tong HC, Haig AJ, Geisser ME. Comparing pain severity and functional status of older adults without spinal symptoms, with lumbar spinal stenosis, and with axial low back pain. Gerontology. 2007;53:111-115.
- Positional therapy with a wheeled walker may help patients with spinal stenosis to walk, as well as ease their pain. This conservative approach has minimum risks—and minimum costs.
Methods: We analyzed a retrospective case series of 52 patients with spinal stenosis confirmed by spinal imaging and walking limitations treated with a wheeled walker set to induce lumbosacral flexion.
Results: Of the 52 patients, improvement in ambulation was classified as excellent for 30 (58%), good for 7 (13%), moderate for 8 (16%), and poor for 7 (13%). Among 48 patients with neurogenic pain, pain relief was classified as excellent for 22 (46%), good for 11 (23%), moderate for 7 (14.5%), and poor for 8 (16.5%).
Conclusions: These retrospective data from a case series support the hypothesis that positional therapy with a wheeled walker set to induce lumbosacral flexion relieves lower extremity symptoms of spinal stenosis. However, an adequate test of this hypothesis will require randomized trials of sufficient size and duration that include objective clinical endpoints such as quality-of-life measures, immobility complications and need for drugs, physical therapy, procedures including epidural injections, and spinal surgery.
In the meantime, this conservative strategy is an option for patients following the recommendations of the North American Spine Society, or for those who have contraindications (or aversions) to surgery or epidural injections, or who have found these options ineffective. Positional therapy with a wheeled walker offers the possibility of short-term benefits for ambulation and pain, with minimal risks and costs.
When shoppers at the grocery store are leaning forward on their carts, many of them could be trying to relieve the pain of lumbar spinal stenosis. This way of finding temporary relief is one we replicated with a wheeled walker for prolonged periods in a retrospective case series to see what further benefits might be gained.
Symptoms are affected by body position and activity level. For patients with lumbar spinal stenosis, lower extremity symptoms can be debilitating and include loss of sensation, paresthesias, burning, pain, weakness, claudication, difficulty standing or walking, or nocturnal neuropathic pain in the feet, legs, or thighs. Axial loading1 (as occurs during walking) and spinal extension2 (as occurs in an erect position) both decrease the diameter of the central spinal canal and lateral recesses, and may cause nerve compression and lower extremity symptoms. In contrast, lumbosacral flexion—facilitated, for example, by leaning forward on a grocery cart3—opens the spine and may reduce nerve compression and related symptoms.
Exhaust all medical options before turning to surgery. The North American Spine Society (NASS) has issued clinical guidelines for spinal stenosis that make recommendations regarding the value of pharmacologic interventions, manipulative techniques, behavioral therapies, and other conservative measures (www. guideline.gov).4 For patients with severe or unremitting symptoms requiring specialized care by spine specialists, NASS further outlines 3 phases of gradually intensifying medical therapy before turning to surgery, which is associated with increased morbidity and costs.5
A previously untested medical approach. Most patients may return to productivity within 2 to 4 months after starting conservative treatment, but some will still require treatment recommended for greater levels of severity.6 For these latter patients, no randomized trials have evaluated the efficacy of medical management with a wheeled walker. This new intervention, if effective, could avoid or delay the expense and side effects of surgery.7 In addition, a wheeled walker may decrease pain from spinal stenosis.8
To explore whether positional therapy with a wheeled walker relieves lower extremity symptoms of lumbar spinal stenosis, we conducted a retrospective case series of 52 patients with spinal imaging confirmed lumbar spinal stenosis and walking limitations.9
Methods
These observations were based on retrospective chart reviews of all patients in a podiatric private practice (SMG) over 1 year to identify those with lower extremity symptoms of lumbar spinal stenosis who were evaluated with positional testing.
Identifying possible stenosis by positional history
Patients were suspected of having spinal stenosis contributing to, or entirely responsible for, lower extremity neuropathic or claudication symptoms based on a positive positional history, including any of the following patterns:
- walking limitation in which the patient needed to sit or lean forward to get relief
- significant improvement in ambulation when pushing a grocery cart, walker, or baby stroller, or when on a treadmill that induced lumbosacral flexion
- constant, frequent, or occasional lower extremity symptoms of a neuropathic nature with an unclear cause that was exacerbated by walking or standing
- nocturnal exacerbation of neuropathic symptoms affected by sleep position.
Symptoms linked to the cause radiologically. Spinal stenosis was confirmed by spinal imaging (magnetic resonance imaging or computed tomography scan) showing stenosis in areas corresponding to symptoms in the lower extremities. Patients without confirmatory spinal imaging were excluded from our study.
Peripheral neuropathy was diagnosed by changes in nerve conduction studies interpreted as being consistent with axonal or demyelinating peripheral neuropathy. Using these criteria, we assembled a case series of 52 patients with imaging-confirmed lumbar spinal stenosis and walking limitations. Of the 52 patients, 33 had received a previous diagnosis of spinal stenosis confirmed by spinal imaging, but only 10 considered that to be the cause of their lower extremity symptoms, with the remainder presenting with a primary diagnosis of peripheral neuropathy—with or without arterial claudication.
Using positional testing to confirm suitability of rollator walker
Patients with lower extremity symptoms of lumbar spinal stenosis underwent a therapeutic trial of “positional testing” involving full-time use of a 3- or 4-wheeled rollator walker (usually provided as a loan) set to induce lumbosacral flexion for 3 days. For patients no taller than 4’ 9” to 5’ 2”, a reduced-height walker (29” to 32”) was usually necessary; patients shorter than 4’ 9” usually needed a modified pediatric walker.
Patients returned for adjustment of the walker if it was uncomfortable or unhelpful. We recommended they also use a shower stool and kitchen stool to minimize erect posture. If they experienced nocturnal exacerbation of neuropathic symptoms, we encouraged them to try sleeping in a recliner. If patients with neuropathic symptoms wanted to continue sleeping in bed, we encouraged them to try sleeping with a pillow beneath their thighs (if sleeping on their back), or sleeping in a fetal position with a pillow between their thighs (if sleeping on their side).10
We usually reevaluated patients in 3 to 5 days, comparing current pain severity and walking capability with previous levels. Patients reporting improvement were encouraged to maintain this full-time positional testing for a total of 10 days. During the subsequent “positional therapy” phase, they gradually reduced their use of the walker, if possible, to an amount just needed to maintain improvement. The therapy phase lasted for 3 months, bringing the total time that patients used a walker to nearly 14 weeks.
Criteria for successful treatment
We gauged treatment success according to self-reported walking capabilities and subjective descriptions of uncomfortable symptoms, using criteria previously described.10
Walking distance. Patients reported uninterrupted walking distance before using the walker and after they had begun using the walker. We classified improvement in walking distance as excellent (over 400% increase), good (250%– 399%), moderate (100%–249%), or poor (≤99%). (The distance a patient can walk—before pain sets in—may vary from day to day. We therefore gauged improvement in this distance by contrasting consistent walking distances achieved and maintained with positional management to the shortest usual walking distance before the intervention.)
Pain reduction. To define a decrease in discomfort reported during the positional testing phase and maintained with positional therapy, we used a verbal analog pain scale (1–3 out of 10=mild pain; 4–7=moderate pain; 8–10=severe pain). We classified reduction in discomfort stemming from spinal stenosis as excellent (75%–100%), good (50%–74%), moderate (25%–49%), or poor (≤24%).
Results
Rapid and dramatic improvement for most patients
The 52 patients in our case series ranged in age from 67 to 90 years; 19 were men. Of the 52, improvement in ambulation was excellent for 30 (58%), good for 7 (13%), moderate for 8 (16%), and poor for 7 (13%) after 3 to 5 days.
Of 48 patients with neurogenic pain, grading with the verbal analog pain scale showed relief was excellent for 22 (46%), good for 11 (23%), moderate for 7 (14.5%), and poor for 8 (16.5%) after 3 to 5 days.
Of the 37 patients with excellent or good improvement in ambulation, 11 needed to keep using the walker extensively, 22 frequently, and 4 occasionally or not at all. Of the 6 patients who had undergone spinal stenosis surgery, improvement was excellent for 3, good for 1, and poor for 2.
A subgroup of 36 patients in our study had diabetes. Of these, 25 had concomitant peripheral neuropathy; 18 reported good to excellent improvement of ambulation or reduction of pain.
Conclusion
Patients deserve a trial of positional therapy with the wheeled walker
These descriptive data support the hypothesis that positional therapy with a wheeled walker set to induce lumbosacral flexion alleviates lower extremity symptoms of spinal stenosis. Limitations of this case series:
- the lack of any comparison group
- improvements in ambulation are based on subjective criteria
- findings can be generalized only to older patients potentially eligible for surgery,11 those who have not benefited from surgery, or those who are undergoing medical therapies recommended by the North American Spine Society4
- patients seen in a podiatry office who present for lower extremity symptoms of spinal stenosis may differ from those seen in a family practitioner’s office who present with low back pain.
Nonetheless, this conservative strategy may be applicable to the evaluation and management of lower extremity symptoms of spinal stenosis regardless of presenting symptoms or source of medical care.
Walking limitations and lower extremity pain caused by spinal stenosis are physically and psychologically disabling. Relief can dramatically improve a person’s quality of life. Improved ambulation may also aid in the management of concurrent medical conditions, such as diabetes and cardiovascular disease.
Our hypothesis requires direct testing in randomized trials of sufficient size and duration. Such trials should include longer term and more objective clinical endpoints, such as quality of life measures, complications due to immobility and need for drugs, physical therapy, procedures such as epidural injections, or spinal surgery. Validation of this hypothesis would substantially reduce morbidity and costs, as well as increase the quality of life of patients with lower extremity symptoms of lumbar spinal stenosis. Until such studies are conducted, this conservative strategy may increase ambulation and decrease pain over the short term, with minimal risks and costs. It may also be helpful for those with contraindications (or aversions) to surgery or epidural injections or those who have found these approaches ineffective.
Correspondence
Charles H. Hennekens, MD, Sir Richard Doll Research Professor, College of Biomedical Science, Department of Clinical Science and Medical Education and Center of Excellence, Florida Atlantic University, Building 10–Administration, Division of Research, Room 244D, 777 Glades Road, Boca Raton, FL 33432; [email protected]
- Positional therapy with a wheeled walker may help patients with spinal stenosis to walk, as well as ease their pain. This conservative approach has minimum risks—and minimum costs.
Methods: We analyzed a retrospective case series of 52 patients with spinal stenosis confirmed by spinal imaging and walking limitations treated with a wheeled walker set to induce lumbosacral flexion.
Results: Of the 52 patients, improvement in ambulation was classified as excellent for 30 (58%), good for 7 (13%), moderate for 8 (16%), and poor for 7 (13%). Among 48 patients with neurogenic pain, pain relief was classified as excellent for 22 (46%), good for 11 (23%), moderate for 7 (14.5%), and poor for 8 (16.5%).
Conclusions: These retrospective data from a case series support the hypothesis that positional therapy with a wheeled walker set to induce lumbosacral flexion relieves lower extremity symptoms of spinal stenosis. However, an adequate test of this hypothesis will require randomized trials of sufficient size and duration that include objective clinical endpoints such as quality-of-life measures, immobility complications and need for drugs, physical therapy, procedures including epidural injections, and spinal surgery.
In the meantime, this conservative strategy is an option for patients following the recommendations of the North American Spine Society, or for those who have contraindications (or aversions) to surgery or epidural injections, or who have found these options ineffective. Positional therapy with a wheeled walker offers the possibility of short-term benefits for ambulation and pain, with minimal risks and costs.
When shoppers at the grocery store are leaning forward on their carts, many of them could be trying to relieve the pain of lumbar spinal stenosis. This way of finding temporary relief is one we replicated with a wheeled walker for prolonged periods in a retrospective case series to see what further benefits might be gained.
Symptoms are affected by body position and activity level. For patients with lumbar spinal stenosis, lower extremity symptoms can be debilitating and include loss of sensation, paresthesias, burning, pain, weakness, claudication, difficulty standing or walking, or nocturnal neuropathic pain in the feet, legs, or thighs. Axial loading1 (as occurs during walking) and spinal extension2 (as occurs in an erect position) both decrease the diameter of the central spinal canal and lateral recesses, and may cause nerve compression and lower extremity symptoms. In contrast, lumbosacral flexion—facilitated, for example, by leaning forward on a grocery cart3—opens the spine and may reduce nerve compression and related symptoms.
Exhaust all medical options before turning to surgery. The North American Spine Society (NASS) has issued clinical guidelines for spinal stenosis that make recommendations regarding the value of pharmacologic interventions, manipulative techniques, behavioral therapies, and other conservative measures (www. guideline.gov).4 For patients with severe or unremitting symptoms requiring specialized care by spine specialists, NASS further outlines 3 phases of gradually intensifying medical therapy before turning to surgery, which is associated with increased morbidity and costs.5
A previously untested medical approach. Most patients may return to productivity within 2 to 4 months after starting conservative treatment, but some will still require treatment recommended for greater levels of severity.6 For these latter patients, no randomized trials have evaluated the efficacy of medical management with a wheeled walker. This new intervention, if effective, could avoid or delay the expense and side effects of surgery.7 In addition, a wheeled walker may decrease pain from spinal stenosis.8
To explore whether positional therapy with a wheeled walker relieves lower extremity symptoms of lumbar spinal stenosis, we conducted a retrospective case series of 52 patients with spinal imaging confirmed lumbar spinal stenosis and walking limitations.9
Methods
These observations were based on retrospective chart reviews of all patients in a podiatric private practice (SMG) over 1 year to identify those with lower extremity symptoms of lumbar spinal stenosis who were evaluated with positional testing.
Identifying possible stenosis by positional history
Patients were suspected of having spinal stenosis contributing to, or entirely responsible for, lower extremity neuropathic or claudication symptoms based on a positive positional history, including any of the following patterns:
- walking limitation in which the patient needed to sit or lean forward to get relief
- significant improvement in ambulation when pushing a grocery cart, walker, or baby stroller, or when on a treadmill that induced lumbosacral flexion
- constant, frequent, or occasional lower extremity symptoms of a neuropathic nature with an unclear cause that was exacerbated by walking or standing
- nocturnal exacerbation of neuropathic symptoms affected by sleep position.
Symptoms linked to the cause radiologically. Spinal stenosis was confirmed by spinal imaging (magnetic resonance imaging or computed tomography scan) showing stenosis in areas corresponding to symptoms in the lower extremities. Patients without confirmatory spinal imaging were excluded from our study.
Peripheral neuropathy was diagnosed by changes in nerve conduction studies interpreted as being consistent with axonal or demyelinating peripheral neuropathy. Using these criteria, we assembled a case series of 52 patients with imaging-confirmed lumbar spinal stenosis and walking limitations. Of the 52 patients, 33 had received a previous diagnosis of spinal stenosis confirmed by spinal imaging, but only 10 considered that to be the cause of their lower extremity symptoms, with the remainder presenting with a primary diagnosis of peripheral neuropathy—with or without arterial claudication.
Using positional testing to confirm suitability of rollator walker
Patients with lower extremity symptoms of lumbar spinal stenosis underwent a therapeutic trial of “positional testing” involving full-time use of a 3- or 4-wheeled rollator walker (usually provided as a loan) set to induce lumbosacral flexion for 3 days. For patients no taller than 4’ 9” to 5’ 2”, a reduced-height walker (29” to 32”) was usually necessary; patients shorter than 4’ 9” usually needed a modified pediatric walker.
Patients returned for adjustment of the walker if it was uncomfortable or unhelpful. We recommended they also use a shower stool and kitchen stool to minimize erect posture. If they experienced nocturnal exacerbation of neuropathic symptoms, we encouraged them to try sleeping in a recliner. If patients with neuropathic symptoms wanted to continue sleeping in bed, we encouraged them to try sleeping with a pillow beneath their thighs (if sleeping on their back), or sleeping in a fetal position with a pillow between their thighs (if sleeping on their side).10
We usually reevaluated patients in 3 to 5 days, comparing current pain severity and walking capability with previous levels. Patients reporting improvement were encouraged to maintain this full-time positional testing for a total of 10 days. During the subsequent “positional therapy” phase, they gradually reduced their use of the walker, if possible, to an amount just needed to maintain improvement. The therapy phase lasted for 3 months, bringing the total time that patients used a walker to nearly 14 weeks.
Criteria for successful treatment
We gauged treatment success according to self-reported walking capabilities and subjective descriptions of uncomfortable symptoms, using criteria previously described.10
Walking distance. Patients reported uninterrupted walking distance before using the walker and after they had begun using the walker. We classified improvement in walking distance as excellent (over 400% increase), good (250%– 399%), moderate (100%–249%), or poor (≤99%). (The distance a patient can walk—before pain sets in—may vary from day to day. We therefore gauged improvement in this distance by contrasting consistent walking distances achieved and maintained with positional management to the shortest usual walking distance before the intervention.)
Pain reduction. To define a decrease in discomfort reported during the positional testing phase and maintained with positional therapy, we used a verbal analog pain scale (1–3 out of 10=mild pain; 4–7=moderate pain; 8–10=severe pain). We classified reduction in discomfort stemming from spinal stenosis as excellent (75%–100%), good (50%–74%), moderate (25%–49%), or poor (≤24%).
Results
Rapid and dramatic improvement for most patients
The 52 patients in our case series ranged in age from 67 to 90 years; 19 were men. Of the 52, improvement in ambulation was excellent for 30 (58%), good for 7 (13%), moderate for 8 (16%), and poor for 7 (13%) after 3 to 5 days.
Of 48 patients with neurogenic pain, grading with the verbal analog pain scale showed relief was excellent for 22 (46%), good for 11 (23%), moderate for 7 (14.5%), and poor for 8 (16.5%) after 3 to 5 days.
Of the 37 patients with excellent or good improvement in ambulation, 11 needed to keep using the walker extensively, 22 frequently, and 4 occasionally or not at all. Of the 6 patients who had undergone spinal stenosis surgery, improvement was excellent for 3, good for 1, and poor for 2.
A subgroup of 36 patients in our study had diabetes. Of these, 25 had concomitant peripheral neuropathy; 18 reported good to excellent improvement of ambulation or reduction of pain.
Conclusion
Patients deserve a trial of positional therapy with the wheeled walker
These descriptive data support the hypothesis that positional therapy with a wheeled walker set to induce lumbosacral flexion alleviates lower extremity symptoms of spinal stenosis. Limitations of this case series:
- the lack of any comparison group
- improvements in ambulation are based on subjective criteria
- findings can be generalized only to older patients potentially eligible for surgery,11 those who have not benefited from surgery, or those who are undergoing medical therapies recommended by the North American Spine Society4
- patients seen in a podiatry office who present for lower extremity symptoms of spinal stenosis may differ from those seen in a family practitioner’s office who present with low back pain.
Nonetheless, this conservative strategy may be applicable to the evaluation and management of lower extremity symptoms of spinal stenosis regardless of presenting symptoms or source of medical care.
Walking limitations and lower extremity pain caused by spinal stenosis are physically and psychologically disabling. Relief can dramatically improve a person’s quality of life. Improved ambulation may also aid in the management of concurrent medical conditions, such as diabetes and cardiovascular disease.
Our hypothesis requires direct testing in randomized trials of sufficient size and duration. Such trials should include longer term and more objective clinical endpoints, such as quality of life measures, complications due to immobility and need for drugs, physical therapy, procedures such as epidural injections, or spinal surgery. Validation of this hypothesis would substantially reduce morbidity and costs, as well as increase the quality of life of patients with lower extremity symptoms of lumbar spinal stenosis. Until such studies are conducted, this conservative strategy may increase ambulation and decrease pain over the short term, with minimal risks and costs. It may also be helpful for those with contraindications (or aversions) to surgery or epidural injections or those who have found these approaches ineffective.
Correspondence
Charles H. Hennekens, MD, Sir Richard Doll Research Professor, College of Biomedical Science, Department of Clinical Science and Medical Education and Center of Excellence, Florida Atlantic University, Building 10–Administration, Division of Research, Room 244D, 777 Glades Road, Boca Raton, FL 33432; [email protected]
1. Willen J, Danielson B, Gaulitz A, Niklason T, Schönström N, Hansson T. Dynamic effect on the lumbar spinal canal: axial loaded CT-myelography and MRI in patients with sciatica and/or neurogenic claudication. Spine. 1997;22:2968-2976.
2. Harrison DE, Calliet R, Harrison DD, Troyanovich SJ, Harrison SO. A review of biomechanics of the central nervous system–part 1: spinal canal deformations resulting from changes in posture. J Manipulative Physiol Ther. 1999;22:227-234.
3. Goldsmith ME, Wiesel S. Spinal stenosis: a straightforward approach to a complex problem. J Clin Rheumatol. 1998;4:92.-
4. Diagnosis and treatment of degenerative lumbar spinal stenosis. National Guideline Clearinghouse Web site. Available at: www.guidelines.gov. Accessed March 6, 2008.
5. Wong DA, Mayer TG, Spivak JM, et al. North American Spine Society (NASS). Phase III clinical guide-lines for multidisciplinary spine care specialists. Spinal stenosis version 1.0. LaGrange, IL: North American Spine Society; 2002.
6. Snyder DL, Doggett D, Turkelson C. Treatment of degenerative lumbar spinal stenosis. Am Fam Physician. 2004;70:517-520.
7. Malmivaara A, Slätis P, Heliövaara M, et al. Finnish Lumbar Spinal Research Group. Surgical or non-operative treatment of lumbar spinal stenosis. Spine. 2007;32:1-8.
8. Hoenig H. Assistive technology and mobility aids for the older patient with disability. Ann Long Term Care. 2004;12:12-19.
9. Hennekens CH, Buring JE. Epidemiology in Medicine. Boston, MA: Little, Brown; 1987.
10. Goldman SM. Neuropathic pain at night in diabetic patients may be caused by spinal stenosis. Diabetic Med. 2005;22:1763-1765.
11. Tong HC, Haig AJ, Geisser ME. Comparing pain severity and functional status of older adults without spinal symptoms, with lumbar spinal stenosis, and with axial low back pain. Gerontology. 2007;53:111-115.
1. Willen J, Danielson B, Gaulitz A, Niklason T, Schönström N, Hansson T. Dynamic effect on the lumbar spinal canal: axial loaded CT-myelography and MRI in patients with sciatica and/or neurogenic claudication. Spine. 1997;22:2968-2976.
2. Harrison DE, Calliet R, Harrison DD, Troyanovich SJ, Harrison SO. A review of biomechanics of the central nervous system–part 1: spinal canal deformations resulting from changes in posture. J Manipulative Physiol Ther. 1999;22:227-234.
3. Goldsmith ME, Wiesel S. Spinal stenosis: a straightforward approach to a complex problem. J Clin Rheumatol. 1998;4:92.-
4. Diagnosis and treatment of degenerative lumbar spinal stenosis. National Guideline Clearinghouse Web site. Available at: www.guidelines.gov. Accessed March 6, 2008.
5. Wong DA, Mayer TG, Spivak JM, et al. North American Spine Society (NASS). Phase III clinical guide-lines for multidisciplinary spine care specialists. Spinal stenosis version 1.0. LaGrange, IL: North American Spine Society; 2002.
6. Snyder DL, Doggett D, Turkelson C. Treatment of degenerative lumbar spinal stenosis. Am Fam Physician. 2004;70:517-520.
7. Malmivaara A, Slätis P, Heliövaara M, et al. Finnish Lumbar Spinal Research Group. Surgical or non-operative treatment of lumbar spinal stenosis. Spine. 2007;32:1-8.
8. Hoenig H. Assistive technology and mobility aids for the older patient with disability. Ann Long Term Care. 2004;12:12-19.
9. Hennekens CH, Buring JE. Epidemiology in Medicine. Boston, MA: Little, Brown; 1987.
10. Goldman SM. Neuropathic pain at night in diabetic patients may be caused by spinal stenosis. Diabetic Med. 2005;22:1763-1765.
11. Tong HC, Haig AJ, Geisser ME. Comparing pain severity and functional status of older adults without spinal symptoms, with lumbar spinal stenosis, and with axial low back pain. Gerontology. 2007;53:111-115.
MEASLES HITS HOME: Sobering lessons from 2 travel-related outbreaks
Inform concerned parents about the safety and effectiveness of vaccines.
2 doses of measles-containing vaccine are 99% effective.
Those exposed who are not immune should be vaccinated or offered immune globulin if the vaccine is contraindicated.
Contraindications
- Primary immune deficiency diseases of T-cell functions
- Acquired immune deficiency from leukemia, lymphoma, or generalized malignancy
- Therapy with corticosteroids: 2 mg/kg prednisone >2 weeks
- Previous anaphylactic reaction to measles vaccine, gelatin, or neomycins
- Pregnancy
Measles is still a threat. Endemic transmission of measles no longer occurs in the United States (or any of the Americas), yet this highly infectious disease is still a threat from importation by visitors from other countries and from US residents who have traveled abroad. Two recent outbreaks (described at left) illustrate these risks.
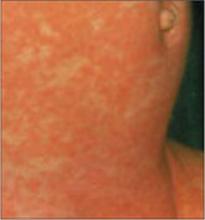
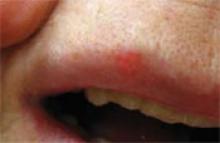
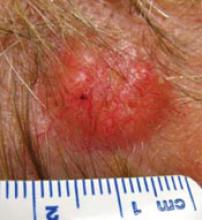
3 infants too young to be vaccinated contracted measles in their doctor’s office in San Diego, in January 2008. (An infant with measles rash [above] is for illustration only, and does not depict any of the 3.)
What the CDC discovered
The 2 outbreaks of import-linked measles brought home—literally—the sobering facts about vulnerability among US residents. The CDC report 1,2 of its investigation observed:
US travelers can be exposed almost anywhere, developed countries included. The California outbreak started with a visit to Switzerland.
Measles spreads rapidly in susceptible subgroups, unless effective control strategies are used. In California, on 2 consecutive days, 5 school children and 4 children in a doctor’s office were infected; all were unvaccinated.
People not considered at risk can contract measles. Although 2 doses of vaccine are 99% effective, vaccinated individuals, such as the college students, can contract measles. Likewise, people born before 1957 may not be immune, in contrast to the general definition of immunity (see Measles Basics. Case in point: the airline passenger, born in 1954.
Disease can be severe. The 40-year-old salesperson (no documented vaccination) was hospitalized with seizure, 105ºF fever, and pneumonia. One of the infants was hospitalized due to dehydration.
People in routine contact with travelers entering the United States can be exposed to measles—like the airline worker.
CALIFORNIA - A February 22 early-release CDC report1 linked 12 measles cases in California to an unvaccinated 7-year-old boy infected while traveling in Europe with his family in January. He was taken to his pediatrician after onset of rash, and to the emergency department the next day, because of high fever and generalized rash. No isolation precautions were used in the office or hospital.
The boy’s 2 siblings, 5 children at his school, and 4 children at the doctor’s office while he was there contracted measles (3 of whom were infants <12 months of age).
Nearly 10% of the children at the index case’s school were unvaccinated because of personal belief exemptions.
PENNSYLVANIA, MICHIGAN, TEXAS - A young boy from Japan participated in an international sporting event and attended a related sales event in Pennsylvania last August. He was infectious when he left Japan and as he traveled in the United States.
The CDC2 linked a total of 6 additional cases of measles in US-born residents to the index case: another young person from Japan who watched the sporting event; a 53-year-old airline passenger and a 25-year-old airline worker in Michigan; and a corporate sales representative who had met the index patient at the sales event and subsequently made sales visits to Houston-area colleges, where 2 college roommates became infected.
Viral genotyping supported a single chain of transmission, and genetic sequencing linked 6 of the 7 cases.
Take-home lessons for family physicians
Include measles in the differential diagnosis of patients who have fever and rash, especially if they have traveled to another country within the past 3 to 4 weeks. Any patient who meets the definition of measles (fever 101ºF or higher; rash; and at least 1 of the 3 Cs—cough, coryza, conjunctivitis) should be immediately reported to the local health department. The health department will provide instructions for collecting laboratory samples for confirmation; instructions on patient isolation; and assistance with notification and disease control measures for exposed individuals.
Immunize patients and staff. These recurring cases of imported measles underscore the importance of maintaining a high level of immunity. Outbreaks can happen even where immunity is 90% to 95%. When vaccination rates dip below 90%, sustained outbreaks can occur.6
Ensure that staff and patients are all immunized against vaccine-preventable diseases, and inform concerned parents about the safety and effectiveness of vaccines. Parents who refuse to have their children vaccinated place their children at risk and contribute to higher community risk. Communities that have higher rates of non-adherence to vaccine recommendations are more likely to have outbreaks.7,8
Use strict infection control in the office. The recent outbreak in California where 4 children were infected in their physician’s office reinforces the need for strict infection-control practices. Do not allow patients with rash and fever to remain in a common waiting area. Move them to an examination room, preferably an airborne infection isolation room. Keep the door to the examination room closed, and be sure that all health care personnel who come in contact with such patients are immune. Do not use triage rooms for 2 hours after the patient suspected of having measles leaves. Do not send these patients to other health care facilities, such as laboratories, unless infection control measures can be adhered to at those locations. Guidelines on infection control practices in health care settings are available.9,10
Quick response
Quick control of these outbreaks shows the value of the public health infrastructure. Disease surveillance and outbreak response is vital to the public health system, and its value is frequently under-appreciated by physicians and the public.
Fewer than 100 cases of measles occur in the United States each year, and virtually all are linked to imported cases.3 Before vaccine was introduced in 1963, 3 to 4 million cases per year occurred, and caused, on average, 450 deaths, 1000 chronic disabilities, and 28,000 hospitalizations.1 Success in controlling measles is due largely to high levels of coverage with 2 doses of measles-containing vaccine and public health surveillance and disease control.
Measles virus is highly infectious and is spread by airborne droplets and direct contact with nose and throat secretions. The incubation is 7 to 18 days.
Measles begins with fever, cough, coryza, conjunctivitis, and whitish spots on the buccal mucosa (Koplick spots).4 Rash appears on the 3rd to 7th day and lasts 4 to 7 days. It begins on the face but soon becomes generalized. An infected person is contagious from 5 days before the rash until 4 days after the rash appears. The diagnosis of measles can be confirrmed by serum measles IGM, which occurs within 3 days of rash, or a rise in measles IGG between acute and 2-week convalescent serum titers.
Complications: pneumonia (5%), otitis media (10%), and encephalitis 1/1000). Death rates: 1 to 2/1000, varying greatly based on age and nutrition; more severe in the very young and the malnourished. Worldwide, about 500,000 children die from measles each year.5
Immunity is defined as:
- 2 vaccine doses at least 1 month apart, both given after the 1st birthday,
- born before 1957,
- serological evidence, or
- history of physician-diagnosed measles.
1. CDC. Outbreak of measles—San Diego, California, January-February 2008. MMWR. 2008;57:Early Release February 22, 2008.-
2. CDC. Multistate measles outbreak associated with an international youth sporting event—Pennsylvania, Michigan, and Texas, August-September 2007. MMWR. 2008;57:169-173.
3. CDC. Measles—United States, 2005. MMWR. 2006;55:1348-1351.
4. Measles. In: Heyman DL. Control of Communicable Diseases Manual. 18th ed. Washington, DC: American Public Health Association.
5. CDC. Parents’ guide to childhood immunizations. Available at: http://www.cdc.gov/vaccines/vpd-vac/measles/downloads/pg_why_vacc_measles.pdf. Accessed March 17, 2008.
6. Richard JL, Masserey-Spicher V, Santibanez S, Mankertz A. Measles outbreak in Switzerland. Available at: http://www.eurosurveillance.org/edition/v13n08/080221_1.asp. Accessed March 17. 2008.
7. Salmon DA, Haber M, Gangarosa EJ, et al. Health consequences of religious and philosophical exemptions from immunization laws; individual and societal risk of measles. JAMA. 1999;282:47-53
8. Feikin DR, Lezotte DC, Hamman RF, et al. Individual and community risks of measles and pertussis associated with personal exemptions to immunization. JAMA. 2008;284:3145-3150.
9. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health care infection control practices advisory committee, 2007.Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(suppl 2):S65-164.
10. Campos-Outcalt D. Infection control in outpatient settings. J Fam Pract. 2004;53:485-488.
Inform concerned parents about the safety and effectiveness of vaccines.
2 doses of measles-containing vaccine are 99% effective.
Those exposed who are not immune should be vaccinated or offered immune globulin if the vaccine is contraindicated.
Contraindications
- Primary immune deficiency diseases of T-cell functions
- Acquired immune deficiency from leukemia, lymphoma, or generalized malignancy
- Therapy with corticosteroids: 2 mg/kg prednisone >2 weeks
- Previous anaphylactic reaction to measles vaccine, gelatin, or neomycins
- Pregnancy
Measles is still a threat. Endemic transmission of measles no longer occurs in the United States (or any of the Americas), yet this highly infectious disease is still a threat from importation by visitors from other countries and from US residents who have traveled abroad. Two recent outbreaks (described at left) illustrate these risks.
3 infants too young to be vaccinated contracted measles in their doctor’s office in San Diego, in January 2008. (An infant with measles rash [above] is for illustration only, and does not depict any of the 3.)
What the CDC discovered
The 2 outbreaks of import-linked measles brought home—literally—the sobering facts about vulnerability among US residents. The CDC report 1,2 of its investigation observed:
US travelers can be exposed almost anywhere, developed countries included. The California outbreak started with a visit to Switzerland.
Measles spreads rapidly in susceptible subgroups, unless effective control strategies are used. In California, on 2 consecutive days, 5 school children and 4 children in a doctor’s office were infected; all were unvaccinated.
People not considered at risk can contract measles. Although 2 doses of vaccine are 99% effective, vaccinated individuals, such as the college students, can contract measles. Likewise, people born before 1957 may not be immune, in contrast to the general definition of immunity (see Measles Basics. Case in point: the airline passenger, born in 1954.
Disease can be severe. The 40-year-old salesperson (no documented vaccination) was hospitalized with seizure, 105ºF fever, and pneumonia. One of the infants was hospitalized due to dehydration.
People in routine contact with travelers entering the United States can be exposed to measles—like the airline worker.
CALIFORNIA - A February 22 early-release CDC report1 linked 12 measles cases in California to an unvaccinated 7-year-old boy infected while traveling in Europe with his family in January. He was taken to his pediatrician after onset of rash, and to the emergency department the next day, because of high fever and generalized rash. No isolation precautions were used in the office or hospital.
The boy’s 2 siblings, 5 children at his school, and 4 children at the doctor’s office while he was there contracted measles (3 of whom were infants <12 months of age).
Nearly 10% of the children at the index case’s school were unvaccinated because of personal belief exemptions.
PENNSYLVANIA, MICHIGAN, TEXAS - A young boy from Japan participated in an international sporting event and attended a related sales event in Pennsylvania last August. He was infectious when he left Japan and as he traveled in the United States.
The CDC2 linked a total of 6 additional cases of measles in US-born residents to the index case: another young person from Japan who watched the sporting event; a 53-year-old airline passenger and a 25-year-old airline worker in Michigan; and a corporate sales representative who had met the index patient at the sales event and subsequently made sales visits to Houston-area colleges, where 2 college roommates became infected.
Viral genotyping supported a single chain of transmission, and genetic sequencing linked 6 of the 7 cases.
Take-home lessons for family physicians
Include measles in the differential diagnosis of patients who have fever and rash, especially if they have traveled to another country within the past 3 to 4 weeks. Any patient who meets the definition of measles (fever 101ºF or higher; rash; and at least 1 of the 3 Cs—cough, coryza, conjunctivitis) should be immediately reported to the local health department. The health department will provide instructions for collecting laboratory samples for confirmation; instructions on patient isolation; and assistance with notification and disease control measures for exposed individuals.
Immunize patients and staff. These recurring cases of imported measles underscore the importance of maintaining a high level of immunity. Outbreaks can happen even where immunity is 90% to 95%. When vaccination rates dip below 90%, sustained outbreaks can occur.6
Ensure that staff and patients are all immunized against vaccine-preventable diseases, and inform concerned parents about the safety and effectiveness of vaccines. Parents who refuse to have their children vaccinated place their children at risk and contribute to higher community risk. Communities that have higher rates of non-adherence to vaccine recommendations are more likely to have outbreaks.7,8
Use strict infection control in the office. The recent outbreak in California where 4 children were infected in their physician’s office reinforces the need for strict infection-control practices. Do not allow patients with rash and fever to remain in a common waiting area. Move them to an examination room, preferably an airborne infection isolation room. Keep the door to the examination room closed, and be sure that all health care personnel who come in contact with such patients are immune. Do not use triage rooms for 2 hours after the patient suspected of having measles leaves. Do not send these patients to other health care facilities, such as laboratories, unless infection control measures can be adhered to at those locations. Guidelines on infection control practices in health care settings are available.9,10
Quick response
Quick control of these outbreaks shows the value of the public health infrastructure. Disease surveillance and outbreak response is vital to the public health system, and its value is frequently under-appreciated by physicians and the public.
Fewer than 100 cases of measles occur in the United States each year, and virtually all are linked to imported cases.3 Before vaccine was introduced in 1963, 3 to 4 million cases per year occurred, and caused, on average, 450 deaths, 1000 chronic disabilities, and 28,000 hospitalizations.1 Success in controlling measles is due largely to high levels of coverage with 2 doses of measles-containing vaccine and public health surveillance and disease control.
Measles virus is highly infectious and is spread by airborne droplets and direct contact with nose and throat secretions. The incubation is 7 to 18 days.
Measles begins with fever, cough, coryza, conjunctivitis, and whitish spots on the buccal mucosa (Koplick spots).4 Rash appears on the 3rd to 7th day and lasts 4 to 7 days. It begins on the face but soon becomes generalized. An infected person is contagious from 5 days before the rash until 4 days after the rash appears. The diagnosis of measles can be confirrmed by serum measles IGM, which occurs within 3 days of rash, or a rise in measles IGG between acute and 2-week convalescent serum titers.
Complications: pneumonia (5%), otitis media (10%), and encephalitis 1/1000). Death rates: 1 to 2/1000, varying greatly based on age and nutrition; more severe in the very young and the malnourished. Worldwide, about 500,000 children die from measles each year.5
Immunity is defined as:
- 2 vaccine doses at least 1 month apart, both given after the 1st birthday,
- born before 1957,
- serological evidence, or
- history of physician-diagnosed measles.
Inform concerned parents about the safety and effectiveness of vaccines.
2 doses of measles-containing vaccine are 99% effective.
Those exposed who are not immune should be vaccinated or offered immune globulin if the vaccine is contraindicated.
Contraindications
- Primary immune deficiency diseases of T-cell functions
- Acquired immune deficiency from leukemia, lymphoma, or generalized malignancy
- Therapy with corticosteroids: 2 mg/kg prednisone >2 weeks
- Previous anaphylactic reaction to measles vaccine, gelatin, or neomycins
- Pregnancy
Measles is still a threat. Endemic transmission of measles no longer occurs in the United States (or any of the Americas), yet this highly infectious disease is still a threat from importation by visitors from other countries and from US residents who have traveled abroad. Two recent outbreaks (described at left) illustrate these risks.
3 infants too young to be vaccinated contracted measles in their doctor’s office in San Diego, in January 2008. (An infant with measles rash [above] is for illustration only, and does not depict any of the 3.)
What the CDC discovered
The 2 outbreaks of import-linked measles brought home—literally—the sobering facts about vulnerability among US residents. The CDC report 1,2 of its investigation observed:
US travelers can be exposed almost anywhere, developed countries included. The California outbreak started with a visit to Switzerland.
Measles spreads rapidly in susceptible subgroups, unless effective control strategies are used. In California, on 2 consecutive days, 5 school children and 4 children in a doctor’s office were infected; all were unvaccinated.
People not considered at risk can contract measles. Although 2 doses of vaccine are 99% effective, vaccinated individuals, such as the college students, can contract measles. Likewise, people born before 1957 may not be immune, in contrast to the general definition of immunity (see Measles Basics. Case in point: the airline passenger, born in 1954.
Disease can be severe. The 40-year-old salesperson (no documented vaccination) was hospitalized with seizure, 105ºF fever, and pneumonia. One of the infants was hospitalized due to dehydration.
People in routine contact with travelers entering the United States can be exposed to measles—like the airline worker.
CALIFORNIA - A February 22 early-release CDC report1 linked 12 measles cases in California to an unvaccinated 7-year-old boy infected while traveling in Europe with his family in January. He was taken to his pediatrician after onset of rash, and to the emergency department the next day, because of high fever and generalized rash. No isolation precautions were used in the office or hospital.
The boy’s 2 siblings, 5 children at his school, and 4 children at the doctor’s office while he was there contracted measles (3 of whom were infants <12 months of age).
Nearly 10% of the children at the index case’s school were unvaccinated because of personal belief exemptions.
PENNSYLVANIA, MICHIGAN, TEXAS - A young boy from Japan participated in an international sporting event and attended a related sales event in Pennsylvania last August. He was infectious when he left Japan and as he traveled in the United States.
The CDC2 linked a total of 6 additional cases of measles in US-born residents to the index case: another young person from Japan who watched the sporting event; a 53-year-old airline passenger and a 25-year-old airline worker in Michigan; and a corporate sales representative who had met the index patient at the sales event and subsequently made sales visits to Houston-area colleges, where 2 college roommates became infected.
Viral genotyping supported a single chain of transmission, and genetic sequencing linked 6 of the 7 cases.
Take-home lessons for family physicians
Include measles in the differential diagnosis of patients who have fever and rash, especially if they have traveled to another country within the past 3 to 4 weeks. Any patient who meets the definition of measles (fever 101ºF or higher; rash; and at least 1 of the 3 Cs—cough, coryza, conjunctivitis) should be immediately reported to the local health department. The health department will provide instructions for collecting laboratory samples for confirmation; instructions on patient isolation; and assistance with notification and disease control measures for exposed individuals.
Immunize patients and staff. These recurring cases of imported measles underscore the importance of maintaining a high level of immunity. Outbreaks can happen even where immunity is 90% to 95%. When vaccination rates dip below 90%, sustained outbreaks can occur.6
Ensure that staff and patients are all immunized against vaccine-preventable diseases, and inform concerned parents about the safety and effectiveness of vaccines. Parents who refuse to have their children vaccinated place their children at risk and contribute to higher community risk. Communities that have higher rates of non-adherence to vaccine recommendations are more likely to have outbreaks.7,8
Use strict infection control in the office. The recent outbreak in California where 4 children were infected in their physician’s office reinforces the need for strict infection-control practices. Do not allow patients with rash and fever to remain in a common waiting area. Move them to an examination room, preferably an airborne infection isolation room. Keep the door to the examination room closed, and be sure that all health care personnel who come in contact with such patients are immune. Do not use triage rooms for 2 hours after the patient suspected of having measles leaves. Do not send these patients to other health care facilities, such as laboratories, unless infection control measures can be adhered to at those locations. Guidelines on infection control practices in health care settings are available.9,10
Quick response
Quick control of these outbreaks shows the value of the public health infrastructure. Disease surveillance and outbreak response is vital to the public health system, and its value is frequently under-appreciated by physicians and the public.
Fewer than 100 cases of measles occur in the United States each year, and virtually all are linked to imported cases.3 Before vaccine was introduced in 1963, 3 to 4 million cases per year occurred, and caused, on average, 450 deaths, 1000 chronic disabilities, and 28,000 hospitalizations.1 Success in controlling measles is due largely to high levels of coverage with 2 doses of measles-containing vaccine and public health surveillance and disease control.
Measles virus is highly infectious and is spread by airborne droplets and direct contact with nose and throat secretions. The incubation is 7 to 18 days.
Measles begins with fever, cough, coryza, conjunctivitis, and whitish spots on the buccal mucosa (Koplick spots).4 Rash appears on the 3rd to 7th day and lasts 4 to 7 days. It begins on the face but soon becomes generalized. An infected person is contagious from 5 days before the rash until 4 days after the rash appears. The diagnosis of measles can be confirrmed by serum measles IGM, which occurs within 3 days of rash, or a rise in measles IGG between acute and 2-week convalescent serum titers.
Complications: pneumonia (5%), otitis media (10%), and encephalitis 1/1000). Death rates: 1 to 2/1000, varying greatly based on age and nutrition; more severe in the very young and the malnourished. Worldwide, about 500,000 children die from measles each year.5
Immunity is defined as:
- 2 vaccine doses at least 1 month apart, both given after the 1st birthday,
- born before 1957,
- serological evidence, or
- history of physician-diagnosed measles.
1. CDC. Outbreak of measles—San Diego, California, January-February 2008. MMWR. 2008;57:Early Release February 22, 2008.-
2. CDC. Multistate measles outbreak associated with an international youth sporting event—Pennsylvania, Michigan, and Texas, August-September 2007. MMWR. 2008;57:169-173.
3. CDC. Measles—United States, 2005. MMWR. 2006;55:1348-1351.
4. Measles. In: Heyman DL. Control of Communicable Diseases Manual. 18th ed. Washington, DC: American Public Health Association.
5. CDC. Parents’ guide to childhood immunizations. Available at: http://www.cdc.gov/vaccines/vpd-vac/measles/downloads/pg_why_vacc_measles.pdf. Accessed March 17, 2008.
6. Richard JL, Masserey-Spicher V, Santibanez S, Mankertz A. Measles outbreak in Switzerland. Available at: http://www.eurosurveillance.org/edition/v13n08/080221_1.asp. Accessed March 17. 2008.
7. Salmon DA, Haber M, Gangarosa EJ, et al. Health consequences of religious and philosophical exemptions from immunization laws; individual and societal risk of measles. JAMA. 1999;282:47-53
8. Feikin DR, Lezotte DC, Hamman RF, et al. Individual and community risks of measles and pertussis associated with personal exemptions to immunization. JAMA. 2008;284:3145-3150.
9. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health care infection control practices advisory committee, 2007.Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(suppl 2):S65-164.
10. Campos-Outcalt D. Infection control in outpatient settings. J Fam Pract. 2004;53:485-488.
1. CDC. Outbreak of measles—San Diego, California, January-February 2008. MMWR. 2008;57:Early Release February 22, 2008.-
2. CDC. Multistate measles outbreak associated with an international youth sporting event—Pennsylvania, Michigan, and Texas, August-September 2007. MMWR. 2008;57:169-173.
3. CDC. Measles—United States, 2005. MMWR. 2006;55:1348-1351.
4. Measles. In: Heyman DL. Control of Communicable Diseases Manual. 18th ed. Washington, DC: American Public Health Association.
5. CDC. Parents’ guide to childhood immunizations. Available at: http://www.cdc.gov/vaccines/vpd-vac/measles/downloads/pg_why_vacc_measles.pdf. Accessed March 17, 2008.
6. Richard JL, Masserey-Spicher V, Santibanez S, Mankertz A. Measles outbreak in Switzerland. Available at: http://www.eurosurveillance.org/edition/v13n08/080221_1.asp. Accessed March 17. 2008.
7. Salmon DA, Haber M, Gangarosa EJ, et al. Health consequences of religious and philosophical exemptions from immunization laws; individual and societal risk of measles. JAMA. 1999;282:47-53
8. Feikin DR, Lezotte DC, Hamman RF, et al. Individual and community risks of measles and pertussis associated with personal exemptions to immunization. JAMA. 2008;284:3145-3150.
9. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health care infection control practices advisory committee, 2007.Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(suppl 2):S65-164.
10. Campos-Outcalt D. Infection control in outpatient settings. J Fam Pract. 2004;53:485-488.
Helping patients kick the "other" habit
- Nicotine replacement therapy may be useful for short-term treatment of cravings, but may not improve cessation rates among patients who use smokeless tobacco (B).
- Patients who use >3 cans of smokeless tobacco a day may need higher than normal doses (42 mg/day) of nicotine replacement therapy (B).
- Evidence is insufficient to support the routine use of bupropion (Zyban) for smokeless tobacco cessation. It should be initiated at the physician’s discretion (B).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Through with Chew Week” and the “Great American Spitout.” Do they ring a bell?
If you answered no, you’re not alone.
“Through with Chew Week” was established by the American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) in 1989, but it hasn’t quite garnered the same kind of recognition as the American Cancer Society’s Great American Smokeout.
When it comes to tobacco use—and more importantly, cessation—smokeless tobacco just doesn’t generate the kind of attention that cigarette smoking does. In fact, smokeless tobacco’s low profile extends beyond talk of “smokeouts” and “spitouts” to research on effective ways to quit.
We found this out first-hand when we conducted a literature search of Medline, PubMed, and a number of other databases to learn which cessation methods have proven efficacy. What we learned is that not only is research on the subject of smokeless tobacco cessation limited, but there is no recommended medication therapy to help these patients quit. Specifically:
- Nicotine-replacement therapies have failed to demonstrate a clear benefit in smokeless tobacco cessation, but under-dosing may be a factor for some patients.
- Bupropion’s (Zyban) usefulness in smokeless tobacco cessation is unclear. Data, thus far, have been inconclusive.
- Varenicline’s (Chantix) usefulness in smokeless tobacco cessation is unknown. There are no published case reports or clinical trials on the subject.
Millions of “chew” users are at risk
Tobacco use is the leading preventable cause of premature death in the United States, with more than 440,000 Americans dying of tobacco-related disease each year.1 Cigarette smoking is by far the most common form of tobacco used; however, smokeless tobacco, also known as chew, spit tobacco, or snuff, is used by 8.2 million Americans.2 More men than women use tobacco products overall, and smokeless tobacco, in particular.2
Health risks include MI. Specific health risks associated with smokeless tobacco include cancer of the oral cavity and pharynx, oral and periodontal disease, tooth decay, and pregnancy-related problems.1 (See “Smokeless tobacco was to blame”)
In addition, an international, case-control study evaluating the risk of myocardial infarction associated with various forms of tobacco use found an increased risk of myocardial infarction associated with smokeless tobacco use compared to non-tobacco users (odds ratio [OR]=2.23; 95% confidence interval [CI], 1.41-3.52).3
Notably, smokeless tobacco users in the study who also smoked cigarettes had the highest risk of all tobacco users when compared to non-users (OR=4.09; 95% CI, 2.98-5.61). These findings demonstrate that nicotine dependence is detrimental to health—regardless of the form of tobacco used. Even more worrisome is the notion that risk may actually be increased when multiple forms of tobacco are used by the same patient.
Patients want medication to help them quit
Current guidelines for tobacco cessation recommend that patients using smokeless tobacco should be identified, urged to quit, and treated with counseling interventions.4 Despite this recommendation, many patients are interested in using a medication to aid them in their quit effort, and many physicians would like to prescribe medication to help patients succeed.
To that end, it seemed logical to us that the same treatments used for smoking cessation would also be effective for smokeless tobacco cessation, given that the underlying problem—despite the form of tobacco used—is nicotine dependence. So we did a literature review to determine the optimal treatment for smokeless tobacco cessation. Our search included: Medline (1950-2007), PubMed (1966-2006), International Pharmaceutical Abstracts (1970-2006), Science Direct, CINAHL, PsycArticles, and Dissertation Abstracts. We used the following search terms: smokeless tobacco, spit tobacco, chew tobacco, cessation, bupropion, nicotine, and nicotine replacement. (Searches in Medline for nicotine replacement therapy in smokeless tobacco cessation were limited to clinical trials.) What we found were a limited number of studies, which we’ve summarized here.
Nicotine patch is useful, but to what degree?
We reviewed four studies involving the use of a nicotine patch for smokeless tobacco cessation (TABLE 1). In chronological order:
Study #1: 15-mg patch. The first study to evaluate the nicotine patch was a randomized, double-blind, placebo controlled trial published in 1999. A 15-mg nicotine patch was used by approximately 420 patients.5 Patients included in the trial were at least 18 years of age, nonsmokers, and used at least 1 can of smokeless tobacco per week. The main outcome of this trial was cessation at 6 months.
A significant difference was found in abstinence rates between patients treated with active therapy compared to those treated with placebo early in the study; however, by study end, there were no significant differences between groups. The medication was well tolerated, though patients receiving active therapy did report an increase in adverse effects related to patch use, such as rash and itching.
TABLE 1
Conflicting findings on the nicotine patch for smokeless tobacco cessation
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Howard-Pitney5 (1999) | 420 patients • ≥18 years, nonsmokers • ≥1 can/week | • Nicotine patch (15 mg) • Placebo patch | • Written materials • Telephone contact | 6 months | No significant difference in cessation rates | N/A |
| Hatsukami6 (2000) | 400 patients • 1 can/week | • Nicotine patch (began on 22 mg and tapered to 7 mg by study end) + mint snuff • Nicotine patch (same tapering as above) + no mint snuff • Placebo patch + mint snuff • Placebo patch + no mint snuff | • Written materials • 10 minute behavioral counseling | 10 weeks | Patients receiving active NRT had significantly higher quit rates at 10 and 15 weeks (P=0.002 and 0.016, respectively) | Patients with mint snuff: • 10 weeks: 4.3 • 15 weeks: 5.5 Patients w/o mint snuff: • 10 weeks: 14.3 • 15 weeks: 20 |
| Stotts7 (2003) | 300 patients • 14- to 19- year-old males • ST use ≥5 days/week | • Nicotine patch • Placebo patch • Usual care | • Patch: Six, 50- minute behavioral counseling sessions • Usual care: one, 5- to 10-minute counseling session with one follow-up telephone contact | 1 year | No significant difference in cessation rates at 1 year | N/A |
| Ebbert8 (2007) | 42 patients • ≥18 years, in good health • ≥3 cans or pouches/day | • Nicotine patch (21, 42, or 63 mg/day) • Placebo patch | • None | 3 days | NRT with 42 mg/day provided similar levels of nicotine to active ST users | N/A |
| NNT, number needed to treat; NRT, nicotine replacement therapy; ST, smokeless tobacco. | ||||||
Study #2: Nicotine plus mint snuff. The second trial to evaluate nicotine patches randomized approximately 400 patients to 1 of 4 groups: active nicotine patch plus mint snuff (non-nicotine mint-leaf product), active nicotine patch and no mint snuff, placebo plus mint snuff, or placebo patch and no mint snuff.6 To be enrolled in the study, patients had to use 1 can of smokeless tobacco per week and express a desire to quit. Patients given the active nicotine patch were begun on 22 mg and tapered to 7 mg by the end of 10 weeks. At 10 weeks, continuous abstinence and abstinence since the last clinic visit were evaluated. (Researchers tracked the patients for a total of 62 weeks.)
Patients who received active nicotine patches had significantly higher cessation rates compared to placebo at 10 (P=0.002) and 15 (P=0.016) weeks, but not at any other time during the study. No information regarding adverse effects was reported.
Study #3: Patch in adolescents. In a third, randomized, placebo-controlled trial, the effect of nicotine replacement in adolescents was evaluated. Patients were randomized to an active patch, placebo patch, or usual care (1 counseling session with a follow-up phone call).7
Patients who received an active patch also received 6 weeks of behavior counseling sessions. Smokeless tobacco users were further stratified according to light/moderate or heavy use according to saliva cotinine levels: light/moderate users were started on 14 mg and heavy users on 21 mg of nicotine. All patients were tapered to 7 mg over a 6-week period. Approximately 300 males between the ages of 14 and 19 who used smokeless tobacco at least 5 days a week were enrolled in the study.
Cessation rates at 1 year were not statistically significant between the placebo and active patch (P=0.22). The nicotine patch was well tolerated, with the exception of 5 patients who experienced skin irritation or headache; however, 3 patients were removed from patch therapy due to skin hyper-reaction or headache.
Study #4: Dosing. A fourth study, by Ebbert et al,8 addressed potential under-dosing of nicotine patches. This study was a randomized, double-blind, placebo controlled trial that evaluated 21, 42, or 63 mg/day of nicotine delivered by patch or placebo in 42 patients. (The 21 mg/day dose is the highest recommended starting dose for smokers.) The patients had to be 18 years of age or older, in good health, and use 3 or more cans/pouches of smokeless tobacco per day.
The study took place in 3 phases: outpatient preadmission, inpatient research phase, and an outpatient follow-up phase. The inpatient phase measured nicotine and cotinine concentrations during active smokeless tobacco use and while receiving treatment or placebo. Patients treated with the 42 mg/day patch had levels closer to those measured during active smokeless tobacco use, while patients treated with 63 mg/day tended to be “over-replaced” and experienced more severe adverse effects, such as nausea, vomiting, and headache. Overall, nicotine replacement therapy was well tolerated throughout the trial.
Nicotine gum: No lasting benefit
The earliest trial to evaluate nicotine gum for smokeless tobacco cessation was conducted by Boyle9 and was only available in abstract form (TABLE 2). This randomized, double-blind, placebo-controlled trial evaluated 2 mg of nicotine gum vs placebo in 100 patients. The main outcome was quit rates at the end of 6 weeks. The quit rate was similar for each group, and did not reach statistical significance (no P value provided). No information regarding adverse effects was reported.
A second trial10 to evaluate nicotine gum was also a randomized placebo-controlled trial that enrolled just over 200 adult patients that used ≥1 tin of smokeless tobacco per week (TABLE 2). Patients were randomized to 2 mg of nicotine gum or placebo and group therapy sessions or minimal behavioral intervention contact for a 2-month treatment period.
This trial did show a significant difference at week 4 (P<0.01) in point prevalence abstinence—that is, the number of people reporting to be abstinent at the 4-week mark, regardless of whether their abstinence was continuous or not. Significance, however, was lost by week 8. Cessation rates were also significant at 1- and 6-month follow-up exams (P<0.05), but were not maintained at the 12-month follow-up.
TABLE 2
Nicotine gum for smokeless tobacco cessation: Benefit doesn’t last
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Boyle9 (1992) | 100 patients | • Nicotine gum (2 mg) • Placebo gum | • Weekly group meetings | 6 weeks | No significant difference at 6 weeks | N/A |
| Hatsukami10 (1996) | 200 adult patients • ≥1 tin/week | • Nicotine gum (2 mg) • Placebo gum | • 8 group therapy sessions or • Minimal behavioral intervention | 12 months (8 weeks of treatment) | Point prevalence abstinence* significantly different at 4 weeks (P<0.01); no significant difference at 8 weeks | • Patients in group therapy at 4 weeks: 42 • Patients with minimal intervention at 4 weeks: 7.8 |
| NNT, number needed to treat. | ||||||
| * Point prevalence abstinence is the number of patients reporting to be abstinent on a given day. | ||||||
Continuous abstinence rates were similar for all groups except the 2 mg nicotine and minimal intervention group. Patients in this group had significantly lower abstinence rates from all other groups (P<0.003). No information regarding adverse effects was reported.
Case report suggests bupropion has potential
Bupropion is a non-nicotine smoking cessation therapy that inhibits the uptake of norepinephrine and dopamine. It is unclear how bupropion aids in smoking cessation, though its ability to do so appears to be related to its dopaminergic properties.11
Given its utility in treatment for smoking cessation, it seems plausible that the drug may serve as an effective treatment for smokeless tobacco, as well. However, the literature regarding the use of bupropion for smokeless tobacco cessation is even more limited than that for nicotine replacement therapy.
Berigan and Deagle first described the use of bupropion for smokeless tobacco cessation in 1999 (TABLE 3).12 Their case report focused on a 31-year-old man who reported using 1 can of smokeless tobacco per day for 11 years. The patient reported having tried nicotine patches and attempting to quit “cold turkey,” with limited success.
The patient was started on a trial of bupropion along with approximately 1 month of behavioral counseling. After 1 week of medication, the patient reported a reduction in cravings. By 5 weeks, the patient reported no use of smokeless tobacco. A total of 10 weeks of medication was given to the patient and he experienced no withdrawal effects after cessation. At 8 months, the patient was still tobacco free. The patient also reported few adverse effects from bupropion.
2 bupropion clinical trials are less encouraging
We found 2 clinical trials evaluating sustained-release bupropion 300 mg/day (TABLE 3). The first was a randomized, placebo-controlled trial in which patients received 7 weeks of active or placebo medication, and were then followed (with minimal counseling) for an additional 5 weeks.13 A total of 70 men, 18 years of age and older, were enrolled in the study; all had expressed a desire to quit and used at least one-half can of smokeless tobacco a day in the past year.
The primary endpoint was smokeless tobacco abstinence during weeks 4 through 7 of the trial. At the end of week 7 (end of treatment), significantly more patients in the treatment group were free of smokeless tobacco, compared to the placebo group (OR=2.73; 95% CI, 1.07-7.72; P=0.04). However, significance was lost by week 12 (OR=1.93; 95% CI, 0.71-5.47; P=0.2). Bupropion was well tolerated throughout this trial and no serious adverse effects were reported.
TABLE 3
Usefulness of bupropion for smokeless tobacco cessation is unclear
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Berigan12 (1999) | 1 can/day | • Bupropion | • Behavioral counseling | 10 weeks | Patient in case report remained ST free at 8 months | N/A |
| Glover13 (2002) | 70 patients • ≥18 year-old- men • ≥½ can/day | • Bupropion SR • Placebo | • Telephone sessions (weeks 9-11) | 3 months | Patients receiving active NRT had significantly higher quit rates by week 7 (P=0.04) | 4.3 |
| Dale14 (2002) | 68 patients • ≥18 years • Regularly used ST | • Bupropion SR • Placebo | • 10 minute behavioral counseling | 24 weeks | No significant difference | N/A |
| NNT, number needed to treat; NRT, nicotine replacement therapy; SR, sustained release; ST, smokeless tobacco. | ||||||
The second trial14 evaluating the efficacy of bupropion for smokeless tobacco cessation was also a randomized, placebo-controlled trial. Enrollees were randomly assigned to bupropion SR 300 mg/day or matching placebo for 12 weeks. In addition, minimal behavioral intervention was provided until week 24. Researchers enrolled 68 patients; all but one were men. Participants in this study were 18 years of age or older who regularly used smokeless tobacco. The efficacy of bupropion SR for nicotine dependence was the primary endpoint evaluated.
At the completion of the treatment phase, more patients in the bupropion group reported cessation (44%) compared to placebo (26%). The difference between the 2 groups was nonsignificant and remained so until the end of the study. By study end, each group had a reported cessation rate of 29%.
One patient experienced a diffuse skin rash that resulted in discontinuation of the study medication; otherwise buproprion was well tolerated.
A product that shows promise for smoking cessation is varenicline, which was approved by the FDA in May 2006.15 Varenicline acts as an agonist at the α4β2 neuronal nicotinic acetylcholine receptors. Its action at this receptor subtype blocks the ability of nicotine to bind to the receptor and is therefore thought to blunt the feeling of reward experienced by smokers.16 In addition, the medication may possess some nicotine-like action at the nicotinic receptor that may decrease the amount of withdrawal symptoms experienced by patients.15
In theory, this mechanism of action may help smokeless tobacco users to succeed in their quit attempts. Unfortunately, though, there have been no published case reports or clinical trials to date regarding the use of varenicline in the treatment of smokeless tobacco cessation.
Helping patients despite the information gap
Our knowledge of the effectiveness of various tools in smokeless tobacco cessation is hampered on a number of fronts.
Nicotine replacement therapy. The conflicting evidence regarding nicotine replacement therapy may be the result of a number of factors. First, although many of the trials used a randomized, placebo-controlled design, there were a number of variations in the patient populations studied, inconsistencies in the amount and type of counseling used, and differences in the amount of smokeless tobacco used by the patients.
These 2 cases illustrate the damage that chew can do
Oral leukoplakia
Gum recession
This 24-year-old patient sought treatment for an asymptomatic oral lesion that he noticed while brushing his teeth. The patient, who did not smoke tobacco and drank alcohol socially on weekends, told us that he had started chewing tobacco while playing baseball in high school 8 years earlier. He said that over the past 3 years, he had increased the habit.
On examination, we noted a well-defined, macular, white lesion on his left buccal mucosa. The lesion was not painful with palpation and remained unchanged when scraped with a tongue depressor.
Our patient had an oral leukoplakia, which can result from the chronic use of chewing tobacco. Cessation of the habit typically results in the resolution of the lesion in approximately 4 weeks. That was the case with our patient: He quit the habit and in 4 weeks, 80% of the lesion resolved. He was closely monitored, and at 3 months the lesion was undetectable.
If, however, a patient discontinues the habit and there is no change in the lesion after 4 weeks, biopsy is indicated. The most serious consequence of a malignant transformation of leukoplakia is oral squamous cell carcinoma.
A 30-year-old man was referred to us for evaluation of gum recession that had worsened over the past year. The patient complained that his teeth were sensitive to hot and cold drinks, but had no other symptoms. He had a 5-year history of smokeless tobacco use and said he usually placed the tobacco along his inner vestibule. He said that he did not smoke tobacco, nor did he drink alcohol.
On examination, we noted that the patient had localized recession along the cervical area of his lower teeth. With manipulation, there was bleeding from the gingival surface. His teeth were otherwise in good shape and there were no other lesions within the oral mucosa.
Gingival recession is a common consequence of smokeless tobacco use. Treatment consists of a gingival graft, in which palatal connective tissue is removed and used to reestablish gingival attachment to the tooth. (Tissue harvesting surgery from the palate can be quite painful.) Left untreated, teeth can become loose and fall out.
We advised our patient to follow up for gingival grafting, but he did not return for his follow-up visit.
Denise Rizzolo, PA-C, PhD is a physician assistant at Care Station in Springfield, NJ and Thomas A. Chiodo, DDS, is an oral and maxillofacial surgeon in private practice in Somerville, NJ. Dr. Chiodo also teaches at UMDNJ Dental School in Newark, NJ; [email protected]
Additionally, recent evidence suggests it may be important to consider dosing nicotine replacement according to number of cans of smokeless tobacco the patient uses per week. Heavy smokeless tobacco users may require higher initial doses than routinely used for smoking cessation therapy (eg, 42 mg/day if >3 cans of smokeless tobacco/day used).8
Further clinical research is needed to validate this recommendation, as well as to give specific dosing recommendations.
Nicotine gum. Two issues come to mind when considering the research on nicotine gum. First, the studies had relatively small sample sizes and it’s possible that the studies were not properly designed to detect a difference between groups.6,7 Second, the concept of dosing nicotine gum based on the number of cans of smokeless tobacco used per day has not been explored.
Bupropion. Studies involving bupropion and smokeless tobacco cessation share several of the limitations we’ve just discussed. Differing results reported in the literature regarding bupropion’s effectiveness makes its potential benefit unclear. Use should be determined on a case-by-case basis with the understanding that it may—or may not—be useful in decreasing cravings or increasing abstinence rates.
Varenicline. There is currently no literature available to help us evaluate the usefulness of varenicline in smokeless tobacco cessation. The mechanism of action of varenicline is such that use in smokeless tobacco cessation is plausible. Again, consideration of patients on a case-by-case basis is warranted.
One size does not fit all
Although nicotine dependence is the underlying problem for patients who utilize smokeless tobacco, current literature does not support a “one-size-fits-all” approach to treatment of various forms of tobacco abuse. Further clinical investigations are needed to determine the true utility of bupropion and varenicline, as well as the appropriate dosing of nicotine replacement therapy when prescribed for smokeless tobacco cessation.
1. National Institutes of Health State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: Tobacco Use: Prevention, Cessation and Control. Ann Intern Med 2006;145:839-844.
2. Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings. Available at: www.oas.samhsa.gov/nsduh/2k6nsduh/2k6results.pdf. Accessed February 26, 2008.
3. Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647-658.
4. Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000.
5. Howard-Pitney B, Killen JD, Fortmann SP. Quitting Chew: Results from a Randomized Trial Using Nicotine Patches. Exp Clin Psychopharmacol. 1999;7:362-371.
6. Hatsukami DK, Grillo M, Boyle R, et al. Treatment of Spit Tobacco Users with Transdermal Nicotine System and Mint Snuff. J Consult Clin Psychol. l2000;68:241-249.
7. Stotts RC, Roberson PK, Hanna EY, Jones SK, Smith CK. A Randomised Clinical Trial of Nicotine Patches for Treatment of Spit Tobacco Addiction Among Adolescents. Tob Control. 2003;12(S4):iv11-15.
8. Ebbert JO, Post JA, Moyer, TP, et al. Nicotine percentage replacement among smokeless tobacco users with nicotine patch. Drug Alcohol Depend. 2007;89:223-226.
9. Boyle RG. Smokeless Tobacco Cessation with Nicotine Replacement: A Randomized Clinical Trial. Dissertation Abstracts International. 1992;54:825.-
10. Hatsukami D, Jensen J, Allen S, Grillo M, Bliss R. Effects of Behavioral and Pharmacological Treatment on Smokeless Tobacco Users. J Consult Clin Psychol. 1996;64:153-161.
11. Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; August 2007.
12. Berigan TR, Deagle EA. Treatment of Smokeless Tobacco Addiction with Bupropion and Behavior Modification. J Am Med Assoc. 1999;281:233.-
13. Glover ED, Glover PN, Sullivan CR, Cerullo CL, Hobbs G. A Comparison of Sustained-Release Bupropion and Placebo for Smokeless Tobacco Cessation. Am J Health Behav. 2002;26:389-393.
14. Dale LC, Ebbert JO, Schroeder DR, et al. Bupropion for the Treatment of Nicotine Dependence in Spit Tobacco Users: A Pilot Study. Nicotine Tob Res. 2002;4:267-274.
15. US Food and Drug Administration. FDA Approves Novel Medication for Smoking Cessation. Available at: www.fda.gov/bbs/topics/NEWS/2006/NEW01370.html. Accessed March 6, 2007.
16. Chantix [package insert]. New York, NY: Pfizer Labs; January 2008.
Correspondence Terri M. Wensel, PharmD, 316 North Main Street, Campus Box 3087, Wingate, NC 28174; [email protected].
- Nicotine replacement therapy may be useful for short-term treatment of cravings, but may not improve cessation rates among patients who use smokeless tobacco (B).
- Patients who use >3 cans of smokeless tobacco a day may need higher than normal doses (42 mg/day) of nicotine replacement therapy (B).
- Evidence is insufficient to support the routine use of bupropion (Zyban) for smokeless tobacco cessation. It should be initiated at the physician’s discretion (B).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Through with Chew Week” and the “Great American Spitout.” Do they ring a bell?
If you answered no, you’re not alone.
“Through with Chew Week” was established by the American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) in 1989, but it hasn’t quite garnered the same kind of recognition as the American Cancer Society’s Great American Smokeout.
When it comes to tobacco use—and more importantly, cessation—smokeless tobacco just doesn’t generate the kind of attention that cigarette smoking does. In fact, smokeless tobacco’s low profile extends beyond talk of “smokeouts” and “spitouts” to research on effective ways to quit.
We found this out first-hand when we conducted a literature search of Medline, PubMed, and a number of other databases to learn which cessation methods have proven efficacy. What we learned is that not only is research on the subject of smokeless tobacco cessation limited, but there is no recommended medication therapy to help these patients quit. Specifically:
- Nicotine-replacement therapies have failed to demonstrate a clear benefit in smokeless tobacco cessation, but under-dosing may be a factor for some patients.
- Bupropion’s (Zyban) usefulness in smokeless tobacco cessation is unclear. Data, thus far, have been inconclusive.
- Varenicline’s (Chantix) usefulness in smokeless tobacco cessation is unknown. There are no published case reports or clinical trials on the subject.
Millions of “chew” users are at risk
Tobacco use is the leading preventable cause of premature death in the United States, with more than 440,000 Americans dying of tobacco-related disease each year.1 Cigarette smoking is by far the most common form of tobacco used; however, smokeless tobacco, also known as chew, spit tobacco, or snuff, is used by 8.2 million Americans.2 More men than women use tobacco products overall, and smokeless tobacco, in particular.2
Health risks include MI. Specific health risks associated with smokeless tobacco include cancer of the oral cavity and pharynx, oral and periodontal disease, tooth decay, and pregnancy-related problems.1 (See “Smokeless tobacco was to blame”)
In addition, an international, case-control study evaluating the risk of myocardial infarction associated with various forms of tobacco use found an increased risk of myocardial infarction associated with smokeless tobacco use compared to non-tobacco users (odds ratio [OR]=2.23; 95% confidence interval [CI], 1.41-3.52).3
Notably, smokeless tobacco users in the study who also smoked cigarettes had the highest risk of all tobacco users when compared to non-users (OR=4.09; 95% CI, 2.98-5.61). These findings demonstrate that nicotine dependence is detrimental to health—regardless of the form of tobacco used. Even more worrisome is the notion that risk may actually be increased when multiple forms of tobacco are used by the same patient.
Patients want medication to help them quit
Current guidelines for tobacco cessation recommend that patients using smokeless tobacco should be identified, urged to quit, and treated with counseling interventions.4 Despite this recommendation, many patients are interested in using a medication to aid them in their quit effort, and many physicians would like to prescribe medication to help patients succeed.
To that end, it seemed logical to us that the same treatments used for smoking cessation would also be effective for smokeless tobacco cessation, given that the underlying problem—despite the form of tobacco used—is nicotine dependence. So we did a literature review to determine the optimal treatment for smokeless tobacco cessation. Our search included: Medline (1950-2007), PubMed (1966-2006), International Pharmaceutical Abstracts (1970-2006), Science Direct, CINAHL, PsycArticles, and Dissertation Abstracts. We used the following search terms: smokeless tobacco, spit tobacco, chew tobacco, cessation, bupropion, nicotine, and nicotine replacement. (Searches in Medline for nicotine replacement therapy in smokeless tobacco cessation were limited to clinical trials.) What we found were a limited number of studies, which we’ve summarized here.
Nicotine patch is useful, but to what degree?
We reviewed four studies involving the use of a nicotine patch for smokeless tobacco cessation (TABLE 1). In chronological order:
Study #1: 15-mg patch. The first study to evaluate the nicotine patch was a randomized, double-blind, placebo controlled trial published in 1999. A 15-mg nicotine patch was used by approximately 420 patients.5 Patients included in the trial were at least 18 years of age, nonsmokers, and used at least 1 can of smokeless tobacco per week. The main outcome of this trial was cessation at 6 months.
A significant difference was found in abstinence rates between patients treated with active therapy compared to those treated with placebo early in the study; however, by study end, there were no significant differences between groups. The medication was well tolerated, though patients receiving active therapy did report an increase in adverse effects related to patch use, such as rash and itching.
TABLE 1
Conflicting findings on the nicotine patch for smokeless tobacco cessation
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Howard-Pitney5 (1999) | 420 patients • ≥18 years, nonsmokers • ≥1 can/week | • Nicotine patch (15 mg) • Placebo patch | • Written materials • Telephone contact | 6 months | No significant difference in cessation rates | N/A |
| Hatsukami6 (2000) | 400 patients • 1 can/week | • Nicotine patch (began on 22 mg and tapered to 7 mg by study end) + mint snuff • Nicotine patch (same tapering as above) + no mint snuff • Placebo patch + mint snuff • Placebo patch + no mint snuff | • Written materials • 10 minute behavioral counseling | 10 weeks | Patients receiving active NRT had significantly higher quit rates at 10 and 15 weeks (P=0.002 and 0.016, respectively) | Patients with mint snuff: • 10 weeks: 4.3 • 15 weeks: 5.5 Patients w/o mint snuff: • 10 weeks: 14.3 • 15 weeks: 20 |
| Stotts7 (2003) | 300 patients • 14- to 19- year-old males • ST use ≥5 days/week | • Nicotine patch • Placebo patch • Usual care | • Patch: Six, 50- minute behavioral counseling sessions • Usual care: one, 5- to 10-minute counseling session with one follow-up telephone contact | 1 year | No significant difference in cessation rates at 1 year | N/A |
| Ebbert8 (2007) | 42 patients • ≥18 years, in good health • ≥3 cans or pouches/day | • Nicotine patch (21, 42, or 63 mg/day) • Placebo patch | • None | 3 days | NRT with 42 mg/day provided similar levels of nicotine to active ST users | N/A |
| NNT, number needed to treat; NRT, nicotine replacement therapy; ST, smokeless tobacco. | ||||||
Study #2: Nicotine plus mint snuff. The second trial to evaluate nicotine patches randomized approximately 400 patients to 1 of 4 groups: active nicotine patch plus mint snuff (non-nicotine mint-leaf product), active nicotine patch and no mint snuff, placebo plus mint snuff, or placebo patch and no mint snuff.6 To be enrolled in the study, patients had to use 1 can of smokeless tobacco per week and express a desire to quit. Patients given the active nicotine patch were begun on 22 mg and tapered to 7 mg by the end of 10 weeks. At 10 weeks, continuous abstinence and abstinence since the last clinic visit were evaluated. (Researchers tracked the patients for a total of 62 weeks.)
Patients who received active nicotine patches had significantly higher cessation rates compared to placebo at 10 (P=0.002) and 15 (P=0.016) weeks, but not at any other time during the study. No information regarding adverse effects was reported.
Study #3: Patch in adolescents. In a third, randomized, placebo-controlled trial, the effect of nicotine replacement in adolescents was evaluated. Patients were randomized to an active patch, placebo patch, or usual care (1 counseling session with a follow-up phone call).7
Patients who received an active patch also received 6 weeks of behavior counseling sessions. Smokeless tobacco users were further stratified according to light/moderate or heavy use according to saliva cotinine levels: light/moderate users were started on 14 mg and heavy users on 21 mg of nicotine. All patients were tapered to 7 mg over a 6-week period. Approximately 300 males between the ages of 14 and 19 who used smokeless tobacco at least 5 days a week were enrolled in the study.
Cessation rates at 1 year were not statistically significant between the placebo and active patch (P=0.22). The nicotine patch was well tolerated, with the exception of 5 patients who experienced skin irritation or headache; however, 3 patients were removed from patch therapy due to skin hyper-reaction or headache.
Study #4: Dosing. A fourth study, by Ebbert et al,8 addressed potential under-dosing of nicotine patches. This study was a randomized, double-blind, placebo controlled trial that evaluated 21, 42, or 63 mg/day of nicotine delivered by patch or placebo in 42 patients. (The 21 mg/day dose is the highest recommended starting dose for smokers.) The patients had to be 18 years of age or older, in good health, and use 3 or more cans/pouches of smokeless tobacco per day.
The study took place in 3 phases: outpatient preadmission, inpatient research phase, and an outpatient follow-up phase. The inpatient phase measured nicotine and cotinine concentrations during active smokeless tobacco use and while receiving treatment or placebo. Patients treated with the 42 mg/day patch had levels closer to those measured during active smokeless tobacco use, while patients treated with 63 mg/day tended to be “over-replaced” and experienced more severe adverse effects, such as nausea, vomiting, and headache. Overall, nicotine replacement therapy was well tolerated throughout the trial.
Nicotine gum: No lasting benefit
The earliest trial to evaluate nicotine gum for smokeless tobacco cessation was conducted by Boyle9 and was only available in abstract form (TABLE 2). This randomized, double-blind, placebo-controlled trial evaluated 2 mg of nicotine gum vs placebo in 100 patients. The main outcome was quit rates at the end of 6 weeks. The quit rate was similar for each group, and did not reach statistical significance (no P value provided). No information regarding adverse effects was reported.
A second trial10 to evaluate nicotine gum was also a randomized placebo-controlled trial that enrolled just over 200 adult patients that used ≥1 tin of smokeless tobacco per week (TABLE 2). Patients were randomized to 2 mg of nicotine gum or placebo and group therapy sessions or minimal behavioral intervention contact for a 2-month treatment period.
This trial did show a significant difference at week 4 (P<0.01) in point prevalence abstinence—that is, the number of people reporting to be abstinent at the 4-week mark, regardless of whether their abstinence was continuous or not. Significance, however, was lost by week 8. Cessation rates were also significant at 1- and 6-month follow-up exams (P<0.05), but were not maintained at the 12-month follow-up.
TABLE 2
Nicotine gum for smokeless tobacco cessation: Benefit doesn’t last
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Boyle9 (1992) | 100 patients | • Nicotine gum (2 mg) • Placebo gum | • Weekly group meetings | 6 weeks | No significant difference at 6 weeks | N/A |
| Hatsukami10 (1996) | 200 adult patients • ≥1 tin/week | • Nicotine gum (2 mg) • Placebo gum | • 8 group therapy sessions or • Minimal behavioral intervention | 12 months (8 weeks of treatment) | Point prevalence abstinence* significantly different at 4 weeks (P<0.01); no significant difference at 8 weeks | • Patients in group therapy at 4 weeks: 42 • Patients with minimal intervention at 4 weeks: 7.8 |
| NNT, number needed to treat. | ||||||
| * Point prevalence abstinence is the number of patients reporting to be abstinent on a given day. | ||||||
Continuous abstinence rates were similar for all groups except the 2 mg nicotine and minimal intervention group. Patients in this group had significantly lower abstinence rates from all other groups (P<0.003). No information regarding adverse effects was reported.
Case report suggests bupropion has potential
Bupropion is a non-nicotine smoking cessation therapy that inhibits the uptake of norepinephrine and dopamine. It is unclear how bupropion aids in smoking cessation, though its ability to do so appears to be related to its dopaminergic properties.11
Given its utility in treatment for smoking cessation, it seems plausible that the drug may serve as an effective treatment for smokeless tobacco, as well. However, the literature regarding the use of bupropion for smokeless tobacco cessation is even more limited than that for nicotine replacement therapy.
Berigan and Deagle first described the use of bupropion for smokeless tobacco cessation in 1999 (TABLE 3).12 Their case report focused on a 31-year-old man who reported using 1 can of smokeless tobacco per day for 11 years. The patient reported having tried nicotine patches and attempting to quit “cold turkey,” with limited success.
The patient was started on a trial of bupropion along with approximately 1 month of behavioral counseling. After 1 week of medication, the patient reported a reduction in cravings. By 5 weeks, the patient reported no use of smokeless tobacco. A total of 10 weeks of medication was given to the patient and he experienced no withdrawal effects after cessation. At 8 months, the patient was still tobacco free. The patient also reported few adverse effects from bupropion.
2 bupropion clinical trials are less encouraging
We found 2 clinical trials evaluating sustained-release bupropion 300 mg/day (TABLE 3). The first was a randomized, placebo-controlled trial in which patients received 7 weeks of active or placebo medication, and were then followed (with minimal counseling) for an additional 5 weeks.13 A total of 70 men, 18 years of age and older, were enrolled in the study; all had expressed a desire to quit and used at least one-half can of smokeless tobacco a day in the past year.
The primary endpoint was smokeless tobacco abstinence during weeks 4 through 7 of the trial. At the end of week 7 (end of treatment), significantly more patients in the treatment group were free of smokeless tobacco, compared to the placebo group (OR=2.73; 95% CI, 1.07-7.72; P=0.04). However, significance was lost by week 12 (OR=1.93; 95% CI, 0.71-5.47; P=0.2). Bupropion was well tolerated throughout this trial and no serious adverse effects were reported.
TABLE 3
Usefulness of bupropion for smokeless tobacco cessation is unclear
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Berigan12 (1999) | 1 can/day | • Bupropion | • Behavioral counseling | 10 weeks | Patient in case report remained ST free at 8 months | N/A |
| Glover13 (2002) | 70 patients • ≥18 year-old- men • ≥½ can/day | • Bupropion SR • Placebo | • Telephone sessions (weeks 9-11) | 3 months | Patients receiving active NRT had significantly higher quit rates by week 7 (P=0.04) | 4.3 |
| Dale14 (2002) | 68 patients • ≥18 years • Regularly used ST | • Bupropion SR • Placebo | • 10 minute behavioral counseling | 24 weeks | No significant difference | N/A |
| NNT, number needed to treat; NRT, nicotine replacement therapy; SR, sustained release; ST, smokeless tobacco. | ||||||
The second trial14 evaluating the efficacy of bupropion for smokeless tobacco cessation was also a randomized, placebo-controlled trial. Enrollees were randomly assigned to bupropion SR 300 mg/day or matching placebo for 12 weeks. In addition, minimal behavioral intervention was provided until week 24. Researchers enrolled 68 patients; all but one were men. Participants in this study were 18 years of age or older who regularly used smokeless tobacco. The efficacy of bupropion SR for nicotine dependence was the primary endpoint evaluated.
At the completion of the treatment phase, more patients in the bupropion group reported cessation (44%) compared to placebo (26%). The difference between the 2 groups was nonsignificant and remained so until the end of the study. By study end, each group had a reported cessation rate of 29%.
One patient experienced a diffuse skin rash that resulted in discontinuation of the study medication; otherwise buproprion was well tolerated.
A product that shows promise for smoking cessation is varenicline, which was approved by the FDA in May 2006.15 Varenicline acts as an agonist at the α4β2 neuronal nicotinic acetylcholine receptors. Its action at this receptor subtype blocks the ability of nicotine to bind to the receptor and is therefore thought to blunt the feeling of reward experienced by smokers.16 In addition, the medication may possess some nicotine-like action at the nicotinic receptor that may decrease the amount of withdrawal symptoms experienced by patients.15
In theory, this mechanism of action may help smokeless tobacco users to succeed in their quit attempts. Unfortunately, though, there have been no published case reports or clinical trials to date regarding the use of varenicline in the treatment of smokeless tobacco cessation.
Helping patients despite the information gap
Our knowledge of the effectiveness of various tools in smokeless tobacco cessation is hampered on a number of fronts.
Nicotine replacement therapy. The conflicting evidence regarding nicotine replacement therapy may be the result of a number of factors. First, although many of the trials used a randomized, placebo-controlled design, there were a number of variations in the patient populations studied, inconsistencies in the amount and type of counseling used, and differences in the amount of smokeless tobacco used by the patients.
These 2 cases illustrate the damage that chew can do
Oral leukoplakia
Gum recession
This 24-year-old patient sought treatment for an asymptomatic oral lesion that he noticed while brushing his teeth. The patient, who did not smoke tobacco and drank alcohol socially on weekends, told us that he had started chewing tobacco while playing baseball in high school 8 years earlier. He said that over the past 3 years, he had increased the habit.
On examination, we noted a well-defined, macular, white lesion on his left buccal mucosa. The lesion was not painful with palpation and remained unchanged when scraped with a tongue depressor.
Our patient had an oral leukoplakia, which can result from the chronic use of chewing tobacco. Cessation of the habit typically results in the resolution of the lesion in approximately 4 weeks. That was the case with our patient: He quit the habit and in 4 weeks, 80% of the lesion resolved. He was closely monitored, and at 3 months the lesion was undetectable.
If, however, a patient discontinues the habit and there is no change in the lesion after 4 weeks, biopsy is indicated. The most serious consequence of a malignant transformation of leukoplakia is oral squamous cell carcinoma.
A 30-year-old man was referred to us for evaluation of gum recession that had worsened over the past year. The patient complained that his teeth were sensitive to hot and cold drinks, but had no other symptoms. He had a 5-year history of smokeless tobacco use and said he usually placed the tobacco along his inner vestibule. He said that he did not smoke tobacco, nor did he drink alcohol.
On examination, we noted that the patient had localized recession along the cervical area of his lower teeth. With manipulation, there was bleeding from the gingival surface. His teeth were otherwise in good shape and there were no other lesions within the oral mucosa.
Gingival recession is a common consequence of smokeless tobacco use. Treatment consists of a gingival graft, in which palatal connective tissue is removed and used to reestablish gingival attachment to the tooth. (Tissue harvesting surgery from the palate can be quite painful.) Left untreated, teeth can become loose and fall out.
We advised our patient to follow up for gingival grafting, but he did not return for his follow-up visit.
Denise Rizzolo, PA-C, PhD is a physician assistant at Care Station in Springfield, NJ and Thomas A. Chiodo, DDS, is an oral and maxillofacial surgeon in private practice in Somerville, NJ. Dr. Chiodo also teaches at UMDNJ Dental School in Newark, NJ; [email protected]
Additionally, recent evidence suggests it may be important to consider dosing nicotine replacement according to number of cans of smokeless tobacco the patient uses per week. Heavy smokeless tobacco users may require higher initial doses than routinely used for smoking cessation therapy (eg, 42 mg/day if >3 cans of smokeless tobacco/day used).8
Further clinical research is needed to validate this recommendation, as well as to give specific dosing recommendations.
Nicotine gum. Two issues come to mind when considering the research on nicotine gum. First, the studies had relatively small sample sizes and it’s possible that the studies were not properly designed to detect a difference between groups.6,7 Second, the concept of dosing nicotine gum based on the number of cans of smokeless tobacco used per day has not been explored.
Bupropion. Studies involving bupropion and smokeless tobacco cessation share several of the limitations we’ve just discussed. Differing results reported in the literature regarding bupropion’s effectiveness makes its potential benefit unclear. Use should be determined on a case-by-case basis with the understanding that it may—or may not—be useful in decreasing cravings or increasing abstinence rates.
Varenicline. There is currently no literature available to help us evaluate the usefulness of varenicline in smokeless tobacco cessation. The mechanism of action of varenicline is such that use in smokeless tobacco cessation is plausible. Again, consideration of patients on a case-by-case basis is warranted.
One size does not fit all
Although nicotine dependence is the underlying problem for patients who utilize smokeless tobacco, current literature does not support a “one-size-fits-all” approach to treatment of various forms of tobacco abuse. Further clinical investigations are needed to determine the true utility of bupropion and varenicline, as well as the appropriate dosing of nicotine replacement therapy when prescribed for smokeless tobacco cessation.
- Nicotine replacement therapy may be useful for short-term treatment of cravings, but may not improve cessation rates among patients who use smokeless tobacco (B).
- Patients who use >3 cans of smokeless tobacco a day may need higher than normal doses (42 mg/day) of nicotine replacement therapy (B).
- Evidence is insufficient to support the routine use of bupropion (Zyban) for smokeless tobacco cessation. It should be initiated at the physician’s discretion (B).
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Through with Chew Week” and the “Great American Spitout.” Do they ring a bell?
If you answered no, you’re not alone.
“Through with Chew Week” was established by the American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) in 1989, but it hasn’t quite garnered the same kind of recognition as the American Cancer Society’s Great American Smokeout.
When it comes to tobacco use—and more importantly, cessation—smokeless tobacco just doesn’t generate the kind of attention that cigarette smoking does. In fact, smokeless tobacco’s low profile extends beyond talk of “smokeouts” and “spitouts” to research on effective ways to quit.
We found this out first-hand when we conducted a literature search of Medline, PubMed, and a number of other databases to learn which cessation methods have proven efficacy. What we learned is that not only is research on the subject of smokeless tobacco cessation limited, but there is no recommended medication therapy to help these patients quit. Specifically:
- Nicotine-replacement therapies have failed to demonstrate a clear benefit in smokeless tobacco cessation, but under-dosing may be a factor for some patients.
- Bupropion’s (Zyban) usefulness in smokeless tobacco cessation is unclear. Data, thus far, have been inconclusive.
- Varenicline’s (Chantix) usefulness in smokeless tobacco cessation is unknown. There are no published case reports or clinical trials on the subject.
Millions of “chew” users are at risk
Tobacco use is the leading preventable cause of premature death in the United States, with more than 440,000 Americans dying of tobacco-related disease each year.1 Cigarette smoking is by far the most common form of tobacco used; however, smokeless tobacco, also known as chew, spit tobacco, or snuff, is used by 8.2 million Americans.2 More men than women use tobacco products overall, and smokeless tobacco, in particular.2
Health risks include MI. Specific health risks associated with smokeless tobacco include cancer of the oral cavity and pharynx, oral and periodontal disease, tooth decay, and pregnancy-related problems.1 (See “Smokeless tobacco was to blame”)
In addition, an international, case-control study evaluating the risk of myocardial infarction associated with various forms of tobacco use found an increased risk of myocardial infarction associated with smokeless tobacco use compared to non-tobacco users (odds ratio [OR]=2.23; 95% confidence interval [CI], 1.41-3.52).3
Notably, smokeless tobacco users in the study who also smoked cigarettes had the highest risk of all tobacco users when compared to non-users (OR=4.09; 95% CI, 2.98-5.61). These findings demonstrate that nicotine dependence is detrimental to health—regardless of the form of tobacco used. Even more worrisome is the notion that risk may actually be increased when multiple forms of tobacco are used by the same patient.
Patients want medication to help them quit
Current guidelines for tobacco cessation recommend that patients using smokeless tobacco should be identified, urged to quit, and treated with counseling interventions.4 Despite this recommendation, many patients are interested in using a medication to aid them in their quit effort, and many physicians would like to prescribe medication to help patients succeed.
To that end, it seemed logical to us that the same treatments used for smoking cessation would also be effective for smokeless tobacco cessation, given that the underlying problem—despite the form of tobacco used—is nicotine dependence. So we did a literature review to determine the optimal treatment for smokeless tobacco cessation. Our search included: Medline (1950-2007), PubMed (1966-2006), International Pharmaceutical Abstracts (1970-2006), Science Direct, CINAHL, PsycArticles, and Dissertation Abstracts. We used the following search terms: smokeless tobacco, spit tobacco, chew tobacco, cessation, bupropion, nicotine, and nicotine replacement. (Searches in Medline for nicotine replacement therapy in smokeless tobacco cessation were limited to clinical trials.) What we found were a limited number of studies, which we’ve summarized here.
Nicotine patch is useful, but to what degree?
We reviewed four studies involving the use of a nicotine patch for smokeless tobacco cessation (TABLE 1). In chronological order:
Study #1: 15-mg patch. The first study to evaluate the nicotine patch was a randomized, double-blind, placebo controlled trial published in 1999. A 15-mg nicotine patch was used by approximately 420 patients.5 Patients included in the trial were at least 18 years of age, nonsmokers, and used at least 1 can of smokeless tobacco per week. The main outcome of this trial was cessation at 6 months.
A significant difference was found in abstinence rates between patients treated with active therapy compared to those treated with placebo early in the study; however, by study end, there were no significant differences between groups. The medication was well tolerated, though patients receiving active therapy did report an increase in adverse effects related to patch use, such as rash and itching.
TABLE 1
Conflicting findings on the nicotine patch for smokeless tobacco cessation
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Howard-Pitney5 (1999) | 420 patients • ≥18 years, nonsmokers • ≥1 can/week | • Nicotine patch (15 mg) • Placebo patch | • Written materials • Telephone contact | 6 months | No significant difference in cessation rates | N/A |
| Hatsukami6 (2000) | 400 patients • 1 can/week | • Nicotine patch (began on 22 mg and tapered to 7 mg by study end) + mint snuff • Nicotine patch (same tapering as above) + no mint snuff • Placebo patch + mint snuff • Placebo patch + no mint snuff | • Written materials • 10 minute behavioral counseling | 10 weeks | Patients receiving active NRT had significantly higher quit rates at 10 and 15 weeks (P=0.002 and 0.016, respectively) | Patients with mint snuff: • 10 weeks: 4.3 • 15 weeks: 5.5 Patients w/o mint snuff: • 10 weeks: 14.3 • 15 weeks: 20 |
| Stotts7 (2003) | 300 patients • 14- to 19- year-old males • ST use ≥5 days/week | • Nicotine patch • Placebo patch • Usual care | • Patch: Six, 50- minute behavioral counseling sessions • Usual care: one, 5- to 10-minute counseling session with one follow-up telephone contact | 1 year | No significant difference in cessation rates at 1 year | N/A |
| Ebbert8 (2007) | 42 patients • ≥18 years, in good health • ≥3 cans or pouches/day | • Nicotine patch (21, 42, or 63 mg/day) • Placebo patch | • None | 3 days | NRT with 42 mg/day provided similar levels of nicotine to active ST users | N/A |
| NNT, number needed to treat; NRT, nicotine replacement therapy; ST, smokeless tobacco. | ||||||
Study #2: Nicotine plus mint snuff. The second trial to evaluate nicotine patches randomized approximately 400 patients to 1 of 4 groups: active nicotine patch plus mint snuff (non-nicotine mint-leaf product), active nicotine patch and no mint snuff, placebo plus mint snuff, or placebo patch and no mint snuff.6 To be enrolled in the study, patients had to use 1 can of smokeless tobacco per week and express a desire to quit. Patients given the active nicotine patch were begun on 22 mg and tapered to 7 mg by the end of 10 weeks. At 10 weeks, continuous abstinence and abstinence since the last clinic visit were evaluated. (Researchers tracked the patients for a total of 62 weeks.)
Patients who received active nicotine patches had significantly higher cessation rates compared to placebo at 10 (P=0.002) and 15 (P=0.016) weeks, but not at any other time during the study. No information regarding adverse effects was reported.
Study #3: Patch in adolescents. In a third, randomized, placebo-controlled trial, the effect of nicotine replacement in adolescents was evaluated. Patients were randomized to an active patch, placebo patch, or usual care (1 counseling session with a follow-up phone call).7
Patients who received an active patch also received 6 weeks of behavior counseling sessions. Smokeless tobacco users were further stratified according to light/moderate or heavy use according to saliva cotinine levels: light/moderate users were started on 14 mg and heavy users on 21 mg of nicotine. All patients were tapered to 7 mg over a 6-week period. Approximately 300 males between the ages of 14 and 19 who used smokeless tobacco at least 5 days a week were enrolled in the study.
Cessation rates at 1 year were not statistically significant between the placebo and active patch (P=0.22). The nicotine patch was well tolerated, with the exception of 5 patients who experienced skin irritation or headache; however, 3 patients were removed from patch therapy due to skin hyper-reaction or headache.
Study #4: Dosing. A fourth study, by Ebbert et al,8 addressed potential under-dosing of nicotine patches. This study was a randomized, double-blind, placebo controlled trial that evaluated 21, 42, or 63 mg/day of nicotine delivered by patch or placebo in 42 patients. (The 21 mg/day dose is the highest recommended starting dose for smokers.) The patients had to be 18 years of age or older, in good health, and use 3 or more cans/pouches of smokeless tobacco per day.
The study took place in 3 phases: outpatient preadmission, inpatient research phase, and an outpatient follow-up phase. The inpatient phase measured nicotine and cotinine concentrations during active smokeless tobacco use and while receiving treatment or placebo. Patients treated with the 42 mg/day patch had levels closer to those measured during active smokeless tobacco use, while patients treated with 63 mg/day tended to be “over-replaced” and experienced more severe adverse effects, such as nausea, vomiting, and headache. Overall, nicotine replacement therapy was well tolerated throughout the trial.
Nicotine gum: No lasting benefit
The earliest trial to evaluate nicotine gum for smokeless tobacco cessation was conducted by Boyle9 and was only available in abstract form (TABLE 2). This randomized, double-blind, placebo-controlled trial evaluated 2 mg of nicotine gum vs placebo in 100 patients. The main outcome was quit rates at the end of 6 weeks. The quit rate was similar for each group, and did not reach statistical significance (no P value provided). No information regarding adverse effects was reported.
A second trial10 to evaluate nicotine gum was also a randomized placebo-controlled trial that enrolled just over 200 adult patients that used ≥1 tin of smokeless tobacco per week (TABLE 2). Patients were randomized to 2 mg of nicotine gum or placebo and group therapy sessions or minimal behavioral intervention contact for a 2-month treatment period.
This trial did show a significant difference at week 4 (P<0.01) in point prevalence abstinence—that is, the number of people reporting to be abstinent at the 4-week mark, regardless of whether their abstinence was continuous or not. Significance, however, was lost by week 8. Cessation rates were also significant at 1- and 6-month follow-up exams (P<0.05), but were not maintained at the 12-month follow-up.
TABLE 2
Nicotine gum for smokeless tobacco cessation: Benefit doesn’t last
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Boyle9 (1992) | 100 patients | • Nicotine gum (2 mg) • Placebo gum | • Weekly group meetings | 6 weeks | No significant difference at 6 weeks | N/A |
| Hatsukami10 (1996) | 200 adult patients • ≥1 tin/week | • Nicotine gum (2 mg) • Placebo gum | • 8 group therapy sessions or • Minimal behavioral intervention | 12 months (8 weeks of treatment) | Point prevalence abstinence* significantly different at 4 weeks (P<0.01); no significant difference at 8 weeks | • Patients in group therapy at 4 weeks: 42 • Patients with minimal intervention at 4 weeks: 7.8 |
| NNT, number needed to treat. | ||||||
| * Point prevalence abstinence is the number of patients reporting to be abstinent on a given day. | ||||||
Continuous abstinence rates were similar for all groups except the 2 mg nicotine and minimal intervention group. Patients in this group had significantly lower abstinence rates from all other groups (P<0.003). No information regarding adverse effects was reported.
Case report suggests bupropion has potential
Bupropion is a non-nicotine smoking cessation therapy that inhibits the uptake of norepinephrine and dopamine. It is unclear how bupropion aids in smoking cessation, though its ability to do so appears to be related to its dopaminergic properties.11
Given its utility in treatment for smoking cessation, it seems plausible that the drug may serve as an effective treatment for smokeless tobacco, as well. However, the literature regarding the use of bupropion for smokeless tobacco cessation is even more limited than that for nicotine replacement therapy.
Berigan and Deagle first described the use of bupropion for smokeless tobacco cessation in 1999 (TABLE 3).12 Their case report focused on a 31-year-old man who reported using 1 can of smokeless tobacco per day for 11 years. The patient reported having tried nicotine patches and attempting to quit “cold turkey,” with limited success.
The patient was started on a trial of bupropion along with approximately 1 month of behavioral counseling. After 1 week of medication, the patient reported a reduction in cravings. By 5 weeks, the patient reported no use of smokeless tobacco. A total of 10 weeks of medication was given to the patient and he experienced no withdrawal effects after cessation. At 8 months, the patient was still tobacco free. The patient also reported few adverse effects from bupropion.
2 bupropion clinical trials are less encouraging
We found 2 clinical trials evaluating sustained-release bupropion 300 mg/day (TABLE 3). The first was a randomized, placebo-controlled trial in which patients received 7 weeks of active or placebo medication, and were then followed (with minimal counseling) for an additional 5 weeks.13 A total of 70 men, 18 years of age and older, were enrolled in the study; all had expressed a desire to quit and used at least one-half can of smokeless tobacco a day in the past year.
The primary endpoint was smokeless tobacco abstinence during weeks 4 through 7 of the trial. At the end of week 7 (end of treatment), significantly more patients in the treatment group were free of smokeless tobacco, compared to the placebo group (OR=2.73; 95% CI, 1.07-7.72; P=0.04). However, significance was lost by week 12 (OR=1.93; 95% CI, 0.71-5.47; P=0.2). Bupropion was well tolerated throughout this trial and no serious adverse effects were reported.
TABLE 3
Usefulness of bupropion for smokeless tobacco cessation is unclear
| STUDY (YEAR) | POPULATION | INTERVENTION | COUNSELING | DURATION | RESULTS | NNT |
|---|---|---|---|---|---|---|
| Berigan12 (1999) | 1 can/day | • Bupropion | • Behavioral counseling | 10 weeks | Patient in case report remained ST free at 8 months | N/A |
| Glover13 (2002) | 70 patients • ≥18 year-old- men • ≥½ can/day | • Bupropion SR • Placebo | • Telephone sessions (weeks 9-11) | 3 months | Patients receiving active NRT had significantly higher quit rates by week 7 (P=0.04) | 4.3 |
| Dale14 (2002) | 68 patients • ≥18 years • Regularly used ST | • Bupropion SR • Placebo | • 10 minute behavioral counseling | 24 weeks | No significant difference | N/A |
| NNT, number needed to treat; NRT, nicotine replacement therapy; SR, sustained release; ST, smokeless tobacco. | ||||||
The second trial14 evaluating the efficacy of bupropion for smokeless tobacco cessation was also a randomized, placebo-controlled trial. Enrollees were randomly assigned to bupropion SR 300 mg/day or matching placebo for 12 weeks. In addition, minimal behavioral intervention was provided until week 24. Researchers enrolled 68 patients; all but one were men. Participants in this study were 18 years of age or older who regularly used smokeless tobacco. The efficacy of bupropion SR for nicotine dependence was the primary endpoint evaluated.
At the completion of the treatment phase, more patients in the bupropion group reported cessation (44%) compared to placebo (26%). The difference between the 2 groups was nonsignificant and remained so until the end of the study. By study end, each group had a reported cessation rate of 29%.
One patient experienced a diffuse skin rash that resulted in discontinuation of the study medication; otherwise buproprion was well tolerated.
A product that shows promise for smoking cessation is varenicline, which was approved by the FDA in May 2006.15 Varenicline acts as an agonist at the α4β2 neuronal nicotinic acetylcholine receptors. Its action at this receptor subtype blocks the ability of nicotine to bind to the receptor and is therefore thought to blunt the feeling of reward experienced by smokers.16 In addition, the medication may possess some nicotine-like action at the nicotinic receptor that may decrease the amount of withdrawal symptoms experienced by patients.15
In theory, this mechanism of action may help smokeless tobacco users to succeed in their quit attempts. Unfortunately, though, there have been no published case reports or clinical trials to date regarding the use of varenicline in the treatment of smokeless tobacco cessation.
Helping patients despite the information gap
Our knowledge of the effectiveness of various tools in smokeless tobacco cessation is hampered on a number of fronts.
Nicotine replacement therapy. The conflicting evidence regarding nicotine replacement therapy may be the result of a number of factors. First, although many of the trials used a randomized, placebo-controlled design, there were a number of variations in the patient populations studied, inconsistencies in the amount and type of counseling used, and differences in the amount of smokeless tobacco used by the patients.
These 2 cases illustrate the damage that chew can do
Oral leukoplakia
Gum recession
This 24-year-old patient sought treatment for an asymptomatic oral lesion that he noticed while brushing his teeth. The patient, who did not smoke tobacco and drank alcohol socially on weekends, told us that he had started chewing tobacco while playing baseball in high school 8 years earlier. He said that over the past 3 years, he had increased the habit.
On examination, we noted a well-defined, macular, white lesion on his left buccal mucosa. The lesion was not painful with palpation and remained unchanged when scraped with a tongue depressor.
Our patient had an oral leukoplakia, which can result from the chronic use of chewing tobacco. Cessation of the habit typically results in the resolution of the lesion in approximately 4 weeks. That was the case with our patient: He quit the habit and in 4 weeks, 80% of the lesion resolved. He was closely monitored, and at 3 months the lesion was undetectable.
If, however, a patient discontinues the habit and there is no change in the lesion after 4 weeks, biopsy is indicated. The most serious consequence of a malignant transformation of leukoplakia is oral squamous cell carcinoma.
A 30-year-old man was referred to us for evaluation of gum recession that had worsened over the past year. The patient complained that his teeth were sensitive to hot and cold drinks, but had no other symptoms. He had a 5-year history of smokeless tobacco use and said he usually placed the tobacco along his inner vestibule. He said that he did not smoke tobacco, nor did he drink alcohol.
On examination, we noted that the patient had localized recession along the cervical area of his lower teeth. With manipulation, there was bleeding from the gingival surface. His teeth were otherwise in good shape and there were no other lesions within the oral mucosa.
Gingival recession is a common consequence of smokeless tobacco use. Treatment consists of a gingival graft, in which palatal connective tissue is removed and used to reestablish gingival attachment to the tooth. (Tissue harvesting surgery from the palate can be quite painful.) Left untreated, teeth can become loose and fall out.
We advised our patient to follow up for gingival grafting, but he did not return for his follow-up visit.
Denise Rizzolo, PA-C, PhD is a physician assistant at Care Station in Springfield, NJ and Thomas A. Chiodo, DDS, is an oral and maxillofacial surgeon in private practice in Somerville, NJ. Dr. Chiodo also teaches at UMDNJ Dental School in Newark, NJ; [email protected]
Additionally, recent evidence suggests it may be important to consider dosing nicotine replacement according to number of cans of smokeless tobacco the patient uses per week. Heavy smokeless tobacco users may require higher initial doses than routinely used for smoking cessation therapy (eg, 42 mg/day if >3 cans of smokeless tobacco/day used).8
Further clinical research is needed to validate this recommendation, as well as to give specific dosing recommendations.
Nicotine gum. Two issues come to mind when considering the research on nicotine gum. First, the studies had relatively small sample sizes and it’s possible that the studies were not properly designed to detect a difference between groups.6,7 Second, the concept of dosing nicotine gum based on the number of cans of smokeless tobacco used per day has not been explored.
Bupropion. Studies involving bupropion and smokeless tobacco cessation share several of the limitations we’ve just discussed. Differing results reported in the literature regarding bupropion’s effectiveness makes its potential benefit unclear. Use should be determined on a case-by-case basis with the understanding that it may—or may not—be useful in decreasing cravings or increasing abstinence rates.
Varenicline. There is currently no literature available to help us evaluate the usefulness of varenicline in smokeless tobacco cessation. The mechanism of action of varenicline is such that use in smokeless tobacco cessation is plausible. Again, consideration of patients on a case-by-case basis is warranted.
One size does not fit all
Although nicotine dependence is the underlying problem for patients who utilize smokeless tobacco, current literature does not support a “one-size-fits-all” approach to treatment of various forms of tobacco abuse. Further clinical investigations are needed to determine the true utility of bupropion and varenicline, as well as the appropriate dosing of nicotine replacement therapy when prescribed for smokeless tobacco cessation.
1. National Institutes of Health State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: Tobacco Use: Prevention, Cessation and Control. Ann Intern Med 2006;145:839-844.
2. Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings. Available at: www.oas.samhsa.gov/nsduh/2k6nsduh/2k6results.pdf. Accessed February 26, 2008.
3. Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647-658.
4. Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000.
5. Howard-Pitney B, Killen JD, Fortmann SP. Quitting Chew: Results from a Randomized Trial Using Nicotine Patches. Exp Clin Psychopharmacol. 1999;7:362-371.
6. Hatsukami DK, Grillo M, Boyle R, et al. Treatment of Spit Tobacco Users with Transdermal Nicotine System and Mint Snuff. J Consult Clin Psychol. l2000;68:241-249.
7. Stotts RC, Roberson PK, Hanna EY, Jones SK, Smith CK. A Randomised Clinical Trial of Nicotine Patches for Treatment of Spit Tobacco Addiction Among Adolescents. Tob Control. 2003;12(S4):iv11-15.
8. Ebbert JO, Post JA, Moyer, TP, et al. Nicotine percentage replacement among smokeless tobacco users with nicotine patch. Drug Alcohol Depend. 2007;89:223-226.
9. Boyle RG. Smokeless Tobacco Cessation with Nicotine Replacement: A Randomized Clinical Trial. Dissertation Abstracts International. 1992;54:825.-
10. Hatsukami D, Jensen J, Allen S, Grillo M, Bliss R. Effects of Behavioral and Pharmacological Treatment on Smokeless Tobacco Users. J Consult Clin Psychol. 1996;64:153-161.
11. Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; August 2007.
12. Berigan TR, Deagle EA. Treatment of Smokeless Tobacco Addiction with Bupropion and Behavior Modification. J Am Med Assoc. 1999;281:233.-
13. Glover ED, Glover PN, Sullivan CR, Cerullo CL, Hobbs G. A Comparison of Sustained-Release Bupropion and Placebo for Smokeless Tobacco Cessation. Am J Health Behav. 2002;26:389-393.
14. Dale LC, Ebbert JO, Schroeder DR, et al. Bupropion for the Treatment of Nicotine Dependence in Spit Tobacco Users: A Pilot Study. Nicotine Tob Res. 2002;4:267-274.
15. US Food and Drug Administration. FDA Approves Novel Medication for Smoking Cessation. Available at: www.fda.gov/bbs/topics/NEWS/2006/NEW01370.html. Accessed March 6, 2007.
16. Chantix [package insert]. New York, NY: Pfizer Labs; January 2008.
Correspondence Terri M. Wensel, PharmD, 316 North Main Street, Campus Box 3087, Wingate, NC 28174; [email protected].
1. National Institutes of Health State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: Tobacco Use: Prevention, Cessation and Control. Ann Intern Med 2006;145:839-844.
2. Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings. Available at: www.oas.samhsa.gov/nsduh/2k6nsduh/2k6results.pdf. Accessed February 26, 2008.
3. Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647-658.
4. Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000.
5. Howard-Pitney B, Killen JD, Fortmann SP. Quitting Chew: Results from a Randomized Trial Using Nicotine Patches. Exp Clin Psychopharmacol. 1999;7:362-371.
6. Hatsukami DK, Grillo M, Boyle R, et al. Treatment of Spit Tobacco Users with Transdermal Nicotine System and Mint Snuff. J Consult Clin Psychol. l2000;68:241-249.
7. Stotts RC, Roberson PK, Hanna EY, Jones SK, Smith CK. A Randomised Clinical Trial of Nicotine Patches for Treatment of Spit Tobacco Addiction Among Adolescents. Tob Control. 2003;12(S4):iv11-15.
8. Ebbert JO, Post JA, Moyer, TP, et al. Nicotine percentage replacement among smokeless tobacco users with nicotine patch. Drug Alcohol Depend. 2007;89:223-226.
9. Boyle RG. Smokeless Tobacco Cessation with Nicotine Replacement: A Randomized Clinical Trial. Dissertation Abstracts International. 1992;54:825.-
10. Hatsukami D, Jensen J, Allen S, Grillo M, Bliss R. Effects of Behavioral and Pharmacological Treatment on Smokeless Tobacco Users. J Consult Clin Psychol. 1996;64:153-161.
11. Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; August 2007.
12. Berigan TR, Deagle EA. Treatment of Smokeless Tobacco Addiction with Bupropion and Behavior Modification. J Am Med Assoc. 1999;281:233.-
13. Glover ED, Glover PN, Sullivan CR, Cerullo CL, Hobbs G. A Comparison of Sustained-Release Bupropion and Placebo for Smokeless Tobacco Cessation. Am J Health Behav. 2002;26:389-393.
14. Dale LC, Ebbert JO, Schroeder DR, et al. Bupropion for the Treatment of Nicotine Dependence in Spit Tobacco Users: A Pilot Study. Nicotine Tob Res. 2002;4:267-274.
15. US Food and Drug Administration. FDA Approves Novel Medication for Smoking Cessation. Available at: www.fda.gov/bbs/topics/NEWS/2006/NEW01370.html. Accessed March 6, 2007.
16. Chantix [package insert]. New York, NY: Pfizer Labs; January 2008.
Correspondence Terri M. Wensel, PharmD, 316 North Main Street, Campus Box 3087, Wingate, NC 28174; [email protected].
Picking a PPI: It comes down to cost
Treatment strategy
- Choose the least expensive product available to the patient.
- Differences in drug interactions, efficacy, and adverse events are not significant. (A)
Dose and duration
- Use PPIs at the lowest effective dose for the minimum duration. (C)
- Since many indications for PPI therapy are for a finite duration, attempt to “step down” therapy to a lower dose or try switching to an H2 receptor antagonist periodically, to achieve the goal of minimum effective dose. (C)
- If stress ulcer prophylaxis was started in the hospital, re-evaluate the need for continued use of PPIs after hospital discharge. (C)
Safety
- PPIs have been on the market for more than 15 years and have a good adverse-event profile, which is comparable to placebo. (A)
- Limited data which attempt to link PPIs to several rare adverse events do not establish a cause-effect relationship. (B)
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A 33-year-old man returns to see you about his bothersome heartburn, which tends to occur after lunch and dinner. He says the trouble began about 2 weeks ago. Chewing Tums tablets after meals relieves his symptoms, but he does not like the chalky taste or frequent dosing. He tried OTC Pepcid, but it relieved his symptoms only occasionally. He says he has not lost weight or had nausea, vomiting, bleeding, or dysphagia. Since you saw him the previous month, he has been trying to exercise and watch his diet, to help manage his hypertension, as you advised. His blood pressure has improved, but his heartburn has not.
THE DILEMMA
You decide to start a PPI, but wonder whether there are significant differences in PPIs, and whether there are long-term health consequences.
The last time you wrote a prescription for a proton pump inhibitor, what came to mind? Did you write a prescription for the PPI you always use? Did you prescribe Prilosec because it is available in generic form? Did you decide on Prevacid because the patient had responded well to it before? Did you consider cost, even if the patient was insured? Was your main concern side effects?
Nexium, Prevacid, and Protonix were among the 25 most commonly dispensed medications in 2006.1 With multiple indications, high clinical efficacy, low incidence of adverse events, convenient dosing, and few drug interactions, it is understandable why PPIs are popular with physicians and patients. Use of prescription PPIs has continued to increase even after Prilosec OTC became available.
TABLES 1–3 give recommended dosing for PPI indications, formulations, drug interactions, cost, and adverse event profiles.
Drug action and efficacy. PPIs have a pharmacokinetic elimination half-life of 30 minutes to 2 hours, but once-daily dosing is possible due to long-lasting inactivation of the proton pump.2
Genetic polymorphism. Up to 6% of Caucasian and 23% of Asian patients are CYP P450 2C19 poor metabolizers.2,3 This is the common hepatic clearance pathway for currently available PPIs. However, due to PPI efficacy, low potential for drug interactions, and infrequent adverse events, this genetic polymorphism is not likely to affect the vast majority of patients on PPI therapy.
Are differences clinically significant? Many trials have compared PPIs using a variety of surrogate endpoints. A new PPI, S-tenatoprazole [not available in the US] has shown greater acid suppression than esomeprazole.4 However, no single agent has proven more efficacious than other PPIs at equipotent doses.5,6 Even though lansoprazole and omeprazole have the most FDA-approved indications, this should not discourage you from using any PPI for a variety of gastrointestinal indications. For example, lansoprazole and esomeprazole are the only PPIs approved for healing or risk reduction of NSAID-related gastric ulcers. Yet other PPIs have been used for this indication without any reason to suspect lack of effectiveness. A meta-analysis of 41 randomized, double-blind studies evaluated head-to-head clinical trials for many conditions, including Helicobacter pylori, gastroesophageal reflux disease (GERD) and peptic ulcer disease.5 No clinically important differences were noted when equipotent doses were used.5-7
Only 1 difference in the treatment of GERD was noted between esomeprazole 40 mg and omeprazole 20 mg in the pooled results (relative risk 1.18; 95% confidence interval 1.14-1.23). This is not surprising because the 2 medications were not given at equipotent doses.
All PPIs are well tolerated. The most common adverse effects are headache, diarrhea, and abdominal pain.8-12 The side-effect profile is consistent among PPIs. An exception is dose-related increases in the frequency of diarrhea reported with lansoprazole (ranging from 1.4% to 7.4%) and higher dose omeprazole therapy.2,8
Although considered relatively safe, several considerations have arisen from clinical trials. Some (occurrence of rebound acid hypersecretion after PPI discontinuation) are better documented than others.13 Additional data are needed to determine whether there is any significant association with PPI-induced elevated serum gastrin levels and neoplastic changes or an increased risk of hip fracture, community-acquired pneumonia, Clostridium difficile infection, vitamin B12 anemia, iron malabsorption, and atrophic gastritis (TABLE 3).13-27
It is promising that data which demonstrate safe and effective use of these agents for longer than 10 years are available.2,28
TABLE 1
Similarities and differences among proton pump inhibitors2,8-12,25,34,35
| PANTOPRAZOLE PROTONIX | LANSOPRAZOLE PREVACID | ESOMEPRAZOLE NEXIUM | OMEPRAZOLE PRILOSEC, ZEGERID | RABEPRAZOLE ACIPHEX | NOTABLE SIMILARITIES AND DIFFERENCES | |
|---|---|---|---|---|---|---|
| Available strengths | 20 mg, 40 mg tabs 40 mg/vial 40 mg granules for suspension | 15 mg, 30 mg caps Packets for suspension, SoluTabs | 20 mg, 40 mg caps, per vial, packet for suspension | 10 mg, 20 mg, 40 mg caps 20 mg, 40 mg Zegerid packet 20 mg tab (OTC) | 20 mg tabs | Injectable options: Pantoprazole, esomeprazole Generic Prilosec and Protonix are available |
| Renal dose adjustment | No dosage adjustment needed | |||||
| Hepatic dose adjustment | > 40 mg/d not studied in hepatic impairment | Consider lower dose in severe hepatic impairment | Max 20 mg/d in severe hepatic impairment | Consider lower dose in hepatic impairment | Consider lower dose in severe hepatic impairment | No differences Consider decreased dose in hepatic impairment |
| Pregnancy category | B | B | B | C | B | All are pregnancy category B except omeprazole, which is C |
| Pediatric use | Safety and efficacy not established for patients < 18 yo | FDA approved for pediatric use except patients ≤ 2 yo | Safety and efficacy not established for patients ≤ 12 yo | FDA approved for pediatric use except patients ≤ 2 yo | Safety and efficacy not established for patients < 18 yo | Lansoprazole and omeprazole are FDA approved for pediatrics No PPI is approved for use in patients ≤ 2 yo |
| Major drug interactions** | ||||||
| Formulation stability | Do not break, crush, or chew tablets | Capsules can be opened and granules sprinkled onto soft food/beverage* or swallowed intact SoluTab and oral suspension packets can be dissolved with water | Capsules can be opened and granules sprinkled onto apple-sauce or swallowed intact Oral suspension packets can be dissolved with water | Capsules can be opened and granules sprinkled onto apple-sauce or swallowed intact Zegerid capsules should not be opened | Do not break, crush, or chew tablets | Suitable for NG administration: esomeprazole, omeprazole (Zegerid), lansoprazole Lansoprazole, omeprazole, and pantoprazole can be compounded into oral suspensions at retail pharmacies if the patient has difficulty swallowing tablets/capsules |
| Cost *** | $128 brand $100 generic | $122-$161 | $150-$165 | $115-$216 brand $64 (20 mg generic) $22 (#28 OTC) $15 (#28 OTC generic) No generic for 40 mg | $160 | Prilosec is the only PPI available OTC Prices at retail pharmacies may differ |
| Applicable soft foods and beverages are applesauce, cottage cheese, Ensure pudding, yogurt, strained pears, apple juice, tomato juice, or orange juice. | ||||||
| ** In addition to cytochrome P450 enzyme interactions, H2receptor antagonists and proton pump inhibitors can affect drugs that rely on acid for absorption (i.e., can decrease the absorption of bases like ketoconazole, iron, or cefpodoxime and increase the absorption of acids like diazepam). There have been case reports of elevated INRs with warfarin and the use of proton pump inhibitors. Omeprazole can raise levels of diazepam, warfarin, and phenytoin. | ||||||
| ***Cost information accessed at http://www.drugstore.com or http://www.cvs.com on March 10, 2008 and is for #30 unless otherwise specified. | ||||||
TABLE 2
Cost comparison of proton pump inhibitor regimens* for 8 conditions8-12,25,29
| PANTOPRAZOLE PROTONIX | LANSOPRAZOLE PREVACID | ESOMEPRAZOLE NEXIUM | OMEPRAZOLE PRILOSEC, ZEGERID | RABEPRAZOLE ACIPHEX | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DOSAGE | PRICE | DOSAGE | PRICE | DOSAGE | PRICE | DOSAGE | PRICE | DOSAGE | PRICE | |
| GERD | 20 mg QD 4-8 wk | $128-$256 $110-$220 (G) | 15 mg QD up to 8 wk | $322 | 20 mg QD 4-8 wk | $165-$330 | 20 mg QD 4 wk | $142 $15 (OTC, G) | 20 mg QD 4-8 wk | $160-$320 |
| Erosive esophagitis associated with GERD | 40 mg QD 8-16 wk **40 mg QD IV 7-10 d | $256-$512 $220-$440 (G) | 30 mg QD 8-16 wk | $308-$616 | 20-40 mg QD 4-16 wk **20-40 mg QD IV up to 10 d | $150-$660 | 20 mg QD 4-8 wk | $142-$284 $15-$30 (OTC, G) | 20 mg QD 4-16 wk | $160-$640 |
| Maintenance of healing of erosive esophagitis | 40 mg QD up to 12 mo | $1536 or $1320 (G) /12 mo | 15 mg QD up to 12 mo | $1932/12 mo | 20 mg QD up to 6 mo | $990/6 mo | 20 mg QD up to 12 mo | $1704 or $180 (OTC, G)/12 mo | 20 mg QD up to 12 mo | $1920/12 mo |
| Hypersecretory conditions*** | 40 mg BID 80 mg BID IV 7-10 d | $256 $220 (G) | 60 mg QD | $309 | 40 mg BID | $300 | 60 mg QD | $426 or $45 (OTC, G) | 60 mg QD | $480 |
| Active duodenal ulcer | 40 mg QD 2-4 wk | $64-$128 $55-$110 (G) | 15 mg QD 4 wk | $161 | — | — | 20 mg QD 4-8 wk | $142-$284 $15-$30 (OTC, G) | 20 mg QD 4 wk | $160 |
| Active gastric ulcer | 40 mg QD up to 8 wk | $256 or $220 (G) | 30 mg QD up to 8 wk | $309 | — | — | 40 mg QD 4-8 wk | $216-$432$30-$60 (OTC, G) | 20 mg QD 3-6 wk | $112-$224 |
| Reduce risk of NSAID-associated gastric ulcer | 40 mg QD | $128 or $110(G)/mo | 15 mg QD up to 12 wk | $161/mo | 20-40 mg QD up to 6 mo | $150-$165/mo | 20 mg QD | $142 or $15 (OTC, G)/mo | — | — |
| Helicobacter pylori (Always part of a 3-drug combination) | 40 mg BID 10-14 d | $85-$120 $73-$103 (G) | 30 mg BID 10-14 d | $103-$144 | 40 mg QD 10-14 d | $50-$75 | 20 mg BID 10 d | $95 $15 (OTC, G) | 20 mg BID 7 d | $80 |
| * Doses in italics are from Ref. 29. | ||||||||||
| ** For GERD in patients with a history of erosive esophagitis. | ||||||||||
| *** Starting doses listed. Several years of therapy may be necessary. | ||||||||||
TABLE 3
Support vs refutation: Adverse events and long-term PPI therapy2,13-23,26,27
| POTENTIAL ADVERSE EVENT | PROPOSED MECHANISM | SUPPORT OR REFUTATION |
|---|---|---|
| Elevated serum gastrin levels and increased enterochromaffin-like (ECL) cells | PPIs elevate serum gastrin levels by interfering with negative feedback mechanism on gastrin release | Gastrin levels can be increased but typically remain within the normal range.2 Due to potential for gastrin to affect growth of tumors outside the gastrointestinal tract, further study of a potential relationship with stimulating tumor growth is needed.13 An increase in ECL cells has been seen in humans; neoplastic findings have not been seen in humans.2,13-15 |
| Vitamin B12 deficiency | Vitamin B12 depends on gastric acid to be released and absorbed from food. In the absence of gastric acid, there is potential for deficiency16 | There is no association between chronic PPI therapy and substantial reduction of vitamin B12.16 Consideration should be given to monitoring vitamin B12 levels periodically in patients who may be at higher risk for vitamin B12 deficiency.13 A small case-control study showed an association between vitamin B12deficiency and PPI/H2 antagonist therapy (OR=4.45, 95% CI 1.47-13.34). Other causes were not explored and data were not separated from the H2 antagonist group.16 |
| Risk of hip fracture | PPIs may inhibit bone resorption and reduce calcium absorption by inducing hypochlorhydria17 | In 2006, a nested, observational case-control study associating high-dose chronic PPI use with a higher incidence of hip fractures showed an adjusted OR=1.44 (95% CI 1.30-1.59) in patients on PPI therapy > 1 year.17 |
| Risk of community-acquired pneumonia (CAP) | Prolonged hypochlorhydria could lead to bacterial overgrowth | A case-control study had an adjusted OR=1.5 (95% CI 1.3-1.7) and the strongest association (OR=5; 95% CI 2.1-11.7) between PPI use and CAP was in patients who had started a PPI within the past 7 days.18 A higher percentage of case patients were on corticosteroid therapy and had concurrent chronic obstructive lung disease, diabetes mellitus, and heart disease. A small study of critically ill trauma patients did not find an increased risk of nosocomial infections in patients taking PPIs.19 A pediatric prospective study found 2 cases of pneumonia in follow-up of the control group of 95 patients, and 11 cases of pneumonia in 91 patients in the combined gastric acid suppression group (P=0.03).20 This trial did not differentiate between ranitidine and omeprazole and excluded patients with diseases identified as confounding variables in previous studies. |
| Risk of Clostridium difficile infection | Prolonged hypochlorhydria could lead to bacterial overgrowth | A case-control study found that the OR for a PPI exposure within the past 3 months and C difficile infection was 3.5 (95% CI 2.3-5.2).21 Previous antibiotic exposure within the same period was associated with an OR of 8.2 (95% CI 6.1-11.0). Notably, in this study, inflammatory bowel disease, renal failure, MRSA, cancer, pernicious anemia, and recent antibiotic use were all associated with a higher adjusted OR for increased risk of C difficile infection than PPI exposure. A 2nd cohort study found an unadjusted OR of 3.1 (95% CI 1.7-5.6) for PPIs and C difficile infection. Of note, higher unadjusted ORs were seen with both renal failure and MRSA.22 Another case-control study did not establish an association between PPI use and C difficile hospitalizations.23 |
| Atrophic gastritis | Unknown | One cohort study found atrophic gastritis can occur with chronic omeprazole therapy,26 but it should be noted that of 20 who patients developed atrophy, 18 were infected with H pylori (P < 0.001) and 2 were not infected (P=0.62). In a separate study, omeprazole was not associated with gastric atrophy after 3 years.27 |
Drug interactions
Two main types of drug interactions may occur with PPIs: those at the cytochrome P450 level, and those due to decreased absorption caused by acid suppression.
PPIs are metabolized by both CYP 2C19 and CYP 3A4. Theoretically, any medication that can induce or inhibit either of these enzymes could affect PPI blood concentration levels.
Evidence suggests omeprazole and its enantiomer esomeprazole have the most potential of the PPIs for drug interactions, as they have been shown to increase blood concentration levels of phenytoin, benzodiazepines, carbamazepine, cilostazol, clarithomycin, and R-warfarin by inhibiting CYP 2C19.2,3,29 These interactions do not typically require a dose adjustment. However, more frequent monitoring may be appropriate.2,6
As a class, PPIs may decrease or increase the bioavailability of drugs whose absorption is dependent on gastric pH. Common medications that are pH dependent for absorption are the azole antifungals, ampicillin, cefpodoxime, indinavir, delavirdine, cyanocobalamin, iron, and digoxin.2,3,6 Digoxin absorption may be increased, resulting in elevated digoxin levels. Enteric-coated medications, which typically dissolve at a higher pH, may also be affected by PPI therapy.2
There may be a decrease in PPI efficacy if given concurrently with other antisecretory medications such as histamine 2 (H2) antagonists or misoprostol. This interaction is apparently due to the capability of PPIs to exert their effects only on actively secreting proton pumps.2,29
Step-up and step-down therapy
Step therapy was a much larger issue prior to the availability of generic omeprazole and pantoprazole. The relative novelty of these agents combined with higher cost of therapy and unknown long-term safety information as well as the availability of effective alternatives justified the argument for step-up therapy.
Health care systems and insurance providers encouraged short-term use of PPIs, either prior to H2 receptor antagonists (step-down therapy) or only after failure of H2 receptor antagonists (step-up therapy). Those who favored step-up therapy took into account the cost advantage of H2 receptor antagonists. Those who favored step-down therapy believed patients on H2 receptor antagonists may experience worsening control of the disease and would therefore need to utilize more health care resources, offsetting the lower costs of H2 receptor antagonists. Further, for indications like the treatment of H. pylori, compliance to therapy and corresponding efficacy may be compromised by the use of an H2 receptor antagonist in place of a PPI. Of the 2 approaches, cost effectiveness studies favor the step-down approach.30-33
Switch to an H2 blocker? Proponents of step-down therapy reasoned that many patients are placed on PPIs and continued indefinitely without re-evaluation. The only way to see if a PPI is still needed is to decrease the dose, switch to an H2 receptor antagonist, or completely discontinue acid-suppression therapy after symptom resolution.
At this time, omeprazole is the only PPI available OTC; it typically retails at $22 for 28 capsules. A generic version of the OTC omeprazole product has been approved. The cost is generally $13 to $15 for 28 capsules. Each H2 receptor antagonist is available as a generic, and OTC at half prescription strength. Of note, famotidine 20-mg tablets and ranitidine 150-mg and 300-mg tablets are available on many of the $4 prescription plans at retail pharmacies.
Your patient reports he has been taking omeprazole 20 mg daily, as you prescribed, for the past 2 weeks. His heart-burn symptoms have improved and he no longer needs to use Tums for breakthrough symptoms, as he did when he was taking Pepcid.
You considered pantoprazole and omeprazole because they are both available in generic form. Since there are no significant differences in efficacy of the 2 drugs for the treatment of GERD, you decided to prescribe pantoprazole because it will cost less for the patient, who has no prescription insurance. The pharmacy calls you, asking if generic omeprazole could be used instead due to the cost difference.
According to http://www.drugstore.com, generic pantoprazole would cost the patient $110 per month and generic omeprazole would cost the patient $63 per month.
Even though costs at local pharmacies vary and are often higher than online costs, the savings to the patient seemed significant enough to change
Equal efficacy
The first PPI, Prilosec, was approved in 1989, and 4 additional agents have become available. All are equally efficacious at equipotent doses.
Long-term safety
Although there has been concern about potential for several different adverse events with long-term PPI use, it is important to remember that the majority of these data are either conflicting or derived from case-control studies. The latter cannot prove cause and effect, but can describe potential associations that merit further investigation.
Step therapy
Whether to lower the PPI dose or switch to an H2 receptor antagonist should be considered in all patients and attempted in most patients who have been on PPIs long enough for adequate ulcer healing or who have been symptom-free.
Consider the cost to the patient
Given the similarities in interaction potential, adverse events, and efficacy, select the PPI on the basis of cost to the patient.
1. Top 200 Prescription Drugs of 2006. http://www.pharmacytimes.com/Article.cfm?Menu=1&ID=4629. Accessed August 28, 2007.
2. Berardi RR. A critical evaluation of proton pump inhibitors in the treatment of gastroesophageal reflux disease. Am J Manage Care. 2000;6(suppl):S491-505.
3. Guimaraes EV, Marguet C, Camargos PAM. Treatment of gastroesophageal reflux disease. J Pediatr (Rio J). 2006;82(5 Suppl):S133-145.
4. Hunt RH, Armstrong D, Yaghoobi M, et al. S-tenatoprazole-Na 30 mg, 60 mg and 90 mg versus esomeprazole (ESO) 40 mg in healthy male volunteers (hmv). Gastroenterology. 2007;132:A-488.
5. Klok RM, Postma MJ, Van Hout BA, Brouwers JRBJ. Meta-analysis: comparing the efficacy of proton pump inhibitors in short-term use. Aliment Pharmacol Ther. 2003;17:1237-1245.
6. McDonagh MS, Carson S. Drug Class Review on Proton Pump Inhibitors. 2006. http://www.ohsu.edu/drugeffectiveness/reports/final.cfm. Accessed December 18, 2007.
7. Dachs R, Darby-Stewart A, Graber M. Choosing one PPI treatment over another. Am Fam Phys. 2007;76:1273-1280.
8. Prilosec (package insert). Wilmington, DE: Astra-Zeneca LP; Sep 2006.
9. Prevacid (package insert). Lake Forest, IL: TAP Pharmaceuticals, Inc; Jun 2007.
10. Protonix (package insert). Philadelphia, PA: Wyeth Laboratories; Jun 2007.
11. Aciphex (package insert). Teaneck, NJ: Eisai, Inc; Aug 2003.
12. Nexium (package insert). Wilmington, DE: Astra-Zeneca LP; Apr 2007.
13. Jensen RT. Consequences of long-term proton pump blockade: insights from studies of patients with gastrinomas. Basic Clin Pharmacol Toxicol. 2006;98:4-19.
14. Laine L, Ahnen D, McClain C, Solcia E, Walsh JH. Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:651-668.
15. Robinson M. Review article: current perspectives on hypergastrinaemia and enterochromaffin-like cell hyperplasia. Aliment Pharmacol Ther. 1999;13 (Suppl 5):5-10.
16. Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of B12 deficiency in older adults. J Clin Epidemiol. 2004;57:422-428.
17. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-2953.
18. Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia. Arch Intern Med. 2007;167:950-955.
19. Mallow S, Rebuck JA, Osler T, Ahern J, Healey MA, Rogers FB. Do proton pump inhibitors increase the incidence of nosocomial pneumonia and related infectious complications when compared with histamine-2 receptor antagonists in critically ill trauma patients? Curr Surg. 2004;61:452-458.
20. Canani RB, Cirillo P, Roggero P, Romano C, Malamisura B, Terrin G, Passariello A, Manguso F, Morelli L, Guarino A. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117:e817-e820.
21. Dial S, Delaney C, Schneider V, Suissa S. Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease defined by prescription for oral vancomycin therapy. CMAJ. 2006;175:745-748.
22. Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ 2004;171(1):33-38.
23. Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis. 2006;43:1272-1276.
24. Health Canada reviewing new safety information on cardiac events in patients taking Losec (omeprazole) or Nexium (esomeprazole). http://www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/2007/2007_95_e.html. Accessed on August 28, 2007.
25. Vanderhoff BT, Tahboub RM. Proton pump inhibitors: an update. Am Fam Phys. 2002;66:273-280.
26. Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018-1022.
27. Lundell L, Miettinen P, Myrvold HE, et al. Lack of effect of acid suppression therapy on gastric atrophy. Gastroenterology. 1999;117:319-326.
28. Hassall E, Kerr W, El-Serag HB. Characteristics of children receiving proton pump inhibitors continuously for up to 11 years duration. J Pediatr. 2007;150:262-267.
29. Clinical Pharmacology. http://cpip.gsm.com. Accessed on August 17, 2007.
30. Inadomi JM, et al. Step-down management of gastroesophageal disease. Gastroenterology. 2001;121:1095-1100.
31. Tytgat GNJ. Review article: management of mild and severe gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2003;17(Suppl 2):52-56.
32. Ofman JJ, Dorn GH, Fennerty MB, Fass R. The clinical and economic impact of competing management strategies for gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2002;16:261-273.
33. Gerson LB, Robbins AS, Garber A, Hornberger J, Tridafilopoulos G. A cost-effectiveness analysis of prescribing strategies in the management of gastroesophageal reflux disease. Am J Gastroenterol. 2000;95:395-407.
34. Zimmermann AE, Walters JK, Katona BG, Souney PF, Levine D. Review of omeprazole use in the treatment of acid-related disorders in children. Clin Ther. 2001;23:660-679.
35. Humphries TJ, Merritt GJ. Review article: drug interactions with agents used to treat acid-related diseases. Aliment Pharmacol Ther. 1999;23(Suppl 3):18-26.
Treatment strategy
- Choose the least expensive product available to the patient.
- Differences in drug interactions, efficacy, and adverse events are not significant. (A)
Dose and duration
- Use PPIs at the lowest effective dose for the minimum duration. (C)
- Since many indications for PPI therapy are for a finite duration, attempt to “step down” therapy to a lower dose or try switching to an H2 receptor antagonist periodically, to achieve the goal of minimum effective dose. (C)
- If stress ulcer prophylaxis was started in the hospital, re-evaluate the need for continued use of PPIs after hospital discharge. (C)
Safety
- PPIs have been on the market for more than 15 years and have a good adverse-event profile, which is comparable to placebo. (A)
- Limited data which attempt to link PPIs to several rare adverse events do not establish a cause-effect relationship. (B)
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A 33-year-old man returns to see you about his bothersome heartburn, which tends to occur after lunch and dinner. He says the trouble began about 2 weeks ago. Chewing Tums tablets after meals relieves his symptoms, but he does not like the chalky taste or frequent dosing. He tried OTC Pepcid, but it relieved his symptoms only occasionally. He says he has not lost weight or had nausea, vomiting, bleeding, or dysphagia. Since you saw him the previous month, he has been trying to exercise and watch his diet, to help manage his hypertension, as you advised. His blood pressure has improved, but his heartburn has not.
THE DILEMMA
You decide to start a PPI, but wonder whether there are significant differences in PPIs, and whether there are long-term health consequences.
The last time you wrote a prescription for a proton pump inhibitor, what came to mind? Did you write a prescription for the PPI you always use? Did you prescribe Prilosec because it is available in generic form? Did you decide on Prevacid because the patient had responded well to it before? Did you consider cost, even if the patient was insured? Was your main concern side effects?
Nexium, Prevacid, and Protonix were among the 25 most commonly dispensed medications in 2006.1 With multiple indications, high clinical efficacy, low incidence of adverse events, convenient dosing, and few drug interactions, it is understandable why PPIs are popular with physicians and patients. Use of prescription PPIs has continued to increase even after Prilosec OTC became available.
TABLES 1–3 give recommended dosing for PPI indications, formulations, drug interactions, cost, and adverse event profiles.
Drug action and efficacy. PPIs have a pharmacokinetic elimination half-life of 30 minutes to 2 hours, but once-daily dosing is possible due to long-lasting inactivation of the proton pump.2
Genetic polymorphism. Up to 6% of Caucasian and 23% of Asian patients are CYP P450 2C19 poor metabolizers.2,3 This is the common hepatic clearance pathway for currently available PPIs. However, due to PPI efficacy, low potential for drug interactions, and infrequent adverse events, this genetic polymorphism is not likely to affect the vast majority of patients on PPI therapy.
Are differences clinically significant? Many trials have compared PPIs using a variety of surrogate endpoints. A new PPI, S-tenatoprazole [not available in the US] has shown greater acid suppression than esomeprazole.4 However, no single agent has proven more efficacious than other PPIs at equipotent doses.5,6 Even though lansoprazole and omeprazole have the most FDA-approved indications, this should not discourage you from using any PPI for a variety of gastrointestinal indications. For example, lansoprazole and esomeprazole are the only PPIs approved for healing or risk reduction of NSAID-related gastric ulcers. Yet other PPIs have been used for this indication without any reason to suspect lack of effectiveness. A meta-analysis of 41 randomized, double-blind studies evaluated head-to-head clinical trials for many conditions, including Helicobacter pylori, gastroesophageal reflux disease (GERD) and peptic ulcer disease.5 No clinically important differences were noted when equipotent doses were used.5-7
Only 1 difference in the treatment of GERD was noted between esomeprazole 40 mg and omeprazole 20 mg in the pooled results (relative risk 1.18; 95% confidence interval 1.14-1.23). This is not surprising because the 2 medications were not given at equipotent doses.
All PPIs are well tolerated. The most common adverse effects are headache, diarrhea, and abdominal pain.8-12 The side-effect profile is consistent among PPIs. An exception is dose-related increases in the frequency of diarrhea reported with lansoprazole (ranging from 1.4% to 7.4%) and higher dose omeprazole therapy.2,8
Although considered relatively safe, several considerations have arisen from clinical trials. Some (occurrence of rebound acid hypersecretion after PPI discontinuation) are better documented than others.13 Additional data are needed to determine whether there is any significant association with PPI-induced elevated serum gastrin levels and neoplastic changes or an increased risk of hip fracture, community-acquired pneumonia, Clostridium difficile infection, vitamin B12 anemia, iron malabsorption, and atrophic gastritis (TABLE 3).13-27
It is promising that data which demonstrate safe and effective use of these agents for longer than 10 years are available.2,28
TABLE 1
Similarities and differences among proton pump inhibitors2,8-12,25,34,35
| PANTOPRAZOLE PROTONIX | LANSOPRAZOLE PREVACID | ESOMEPRAZOLE NEXIUM | OMEPRAZOLE PRILOSEC, ZEGERID | RABEPRAZOLE ACIPHEX | NOTABLE SIMILARITIES AND DIFFERENCES | |
|---|---|---|---|---|---|---|
| Available strengths | 20 mg, 40 mg tabs 40 mg/vial 40 mg granules for suspension | 15 mg, 30 mg caps Packets for suspension, SoluTabs | 20 mg, 40 mg caps, per vial, packet for suspension | 10 mg, 20 mg, 40 mg caps 20 mg, 40 mg Zegerid packet 20 mg tab (OTC) | 20 mg tabs | Injectable options: Pantoprazole, esomeprazole Generic Prilosec and Protonix are available |
| Renal dose adjustment | No dosage adjustment needed | |||||
| Hepatic dose adjustment | > 40 mg/d not studied in hepatic impairment | Consider lower dose in severe hepatic impairment | Max 20 mg/d in severe hepatic impairment | Consider lower dose in hepatic impairment | Consider lower dose in severe hepatic impairment | No differences Consider decreased dose in hepatic impairment |
| Pregnancy category | B | B | B | C | B | All are pregnancy category B except omeprazole, which is C |
| Pediatric use | Safety and efficacy not established for patients < 18 yo | FDA approved for pediatric use except patients ≤ 2 yo | Safety and efficacy not established for patients ≤ 12 yo | FDA approved for pediatric use except patients ≤ 2 yo | Safety and efficacy not established for patients < 18 yo | Lansoprazole and omeprazole are FDA approved for pediatrics No PPI is approved for use in patients ≤ 2 yo |
| Major drug interactions** | ||||||
| Formulation stability | Do not break, crush, or chew tablets | Capsules can be opened and granules sprinkled onto soft food/beverage* or swallowed intact SoluTab and oral suspension packets can be dissolved with water | Capsules can be opened and granules sprinkled onto apple-sauce or swallowed intact Oral suspension packets can be dissolved with water | Capsules can be opened and granules sprinkled onto apple-sauce or swallowed intact Zegerid capsules should not be opened | Do not break, crush, or chew tablets | Suitable for NG administration: esomeprazole, omeprazole (Zegerid), lansoprazole Lansoprazole, omeprazole, and pantoprazole can be compounded into oral suspensions at retail pharmacies if the patient has difficulty swallowing tablets/capsules |
| Cost *** | $128 brand $100 generic | $122-$161 | $150-$165 | $115-$216 brand $64 (20 mg generic) $22 (#28 OTC) $15 (#28 OTC generic) No generic for 40 mg | $160 | Prilosec is the only PPI available OTC Prices at retail pharmacies may differ |
| Applicable soft foods and beverages are applesauce, cottage cheese, Ensure pudding, yogurt, strained pears, apple juice, tomato juice, or orange juice. | ||||||
| ** In addition to cytochrome P450 enzyme interactions, H2receptor antagonists and proton pump inhibitors can affect drugs that rely on acid for absorption (i.e., can decrease the absorption of bases like ketoconazole, iron, or cefpodoxime and increase the absorption of acids like diazepam). There have been case reports of elevated INRs with warfarin and the use of proton pump inhibitors. Omeprazole can raise levels of diazepam, warfarin, and phenytoin. | ||||||
| ***Cost information accessed at http://www.drugstore.com or http://www.cvs.com on March 10, 2008 and is for #30 unless otherwise specified. | ||||||
TABLE 2
Cost comparison of proton pump inhibitor regimens* for 8 conditions8-12,25,29
| PANTOPRAZOLE PROTONIX | LANSOPRAZOLE PREVACID | ESOMEPRAZOLE NEXIUM | OMEPRAZOLE PRILOSEC, ZEGERID | RABEPRAZOLE ACIPHEX | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DOSAGE | PRICE | DOSAGE | PRICE | DOSAGE | PRICE | DOSAGE | PRICE | DOSAGE | PRICE | |
| GERD | 20 mg QD 4-8 wk | $128-$256 $110-$220 (G) | 15 mg QD up to 8 wk | $322 | 20 mg QD 4-8 wk | $165-$330 | 20 mg QD 4 wk | $142 $15 (OTC, G) | 20 mg QD 4-8 wk | $160-$320 |
| Erosive esophagitis associated with GERD | 40 mg QD 8-16 wk **40 mg QD IV 7-10 d | $256-$512 $220-$440 (G) | 30 mg QD 8-16 wk | $308-$616 | 20-40 mg QD 4-16 wk **20-40 mg QD IV up to 10 d | $150-$660 | 20 mg QD 4-8 wk | $142-$284 $15-$30 (OTC, G) | 20 mg QD 4-16 wk | $160-$640 |
| Maintenance of healing of erosive esophagitis | 40 mg QD up to 12 mo | $1536 or $1320 (G) /12 mo | 15 mg QD up to 12 mo | $1932/12 mo | 20 mg QD up to 6 mo | $990/6 mo | 20 mg QD up to 12 mo | $1704 or $180 (OTC, G)/12 mo | 20 mg QD up to 12 mo | $1920/12 mo |
| Hypersecretory conditions*** | 40 mg BID 80 mg BID IV 7-10 d | $256 $220 (G) | 60 mg QD | $309 | 40 mg BID | $300 | 60 mg QD | $426 or $45 (OTC, G) | 60 mg QD | $480 |
| Active duodenal ulcer | 40 mg QD 2-4 wk | $64-$128 $55-$110 (G) | 15 mg QD 4 wk | $161 | — | — | 20 mg QD 4-8 wk | $142-$284 $15-$30 (OTC, G) | 20 mg QD 4 wk | $160 |
| Active gastric ulcer | 40 mg QD up to 8 wk | $256 or $220 (G) | 30 mg QD up to 8 wk | $309 | — | — | 40 mg QD 4-8 wk | $216-$432$30-$60 (OTC, G) | 20 mg QD 3-6 wk | $112-$224 |
| Reduce risk of NSAID-associated gastric ulcer | 40 mg QD | $128 or $110(G)/mo | 15 mg QD up to 12 wk | $161/mo | 20-40 mg QD up to 6 mo | $150-$165/mo | 20 mg QD | $142 or $15 (OTC, G)/mo | — | — |
| Helicobacter pylori (Always part of a 3-drug combination) | 40 mg BID 10-14 d | $85-$120 $73-$103 (G) | 30 mg BID 10-14 d | $103-$144 | 40 mg QD 10-14 d | $50-$75 | 20 mg BID 10 d | $95 $15 (OTC, G) | 20 mg BID 7 d | $80 |
| * Doses in italics are from Ref. 29. | ||||||||||
| ** For GERD in patients with a history of erosive esophagitis. | ||||||||||
| *** Starting doses listed. Several years of therapy may be necessary. | ||||||||||
TABLE 3
Support vs refutation: Adverse events and long-term PPI therapy2,13-23,26,27
| POTENTIAL ADVERSE EVENT | PROPOSED MECHANISM | SUPPORT OR REFUTATION |
|---|---|---|
| Elevated serum gastrin levels and increased enterochromaffin-like (ECL) cells | PPIs elevate serum gastrin levels by interfering with negative feedback mechanism on gastrin release | Gastrin levels can be increased but typically remain within the normal range.2 Due to potential for gastrin to affect growth of tumors outside the gastrointestinal tract, further study of a potential relationship with stimulating tumor growth is needed.13 An increase in ECL cells has been seen in humans; neoplastic findings have not been seen in humans.2,13-15 |
| Vitamin B12 deficiency | Vitamin B12 depends on gastric acid to be released and absorbed from food. In the absence of gastric acid, there is potential for deficiency16 | There is no association between chronic PPI therapy and substantial reduction of vitamin B12.16 Consideration should be given to monitoring vitamin B12 levels periodically in patients who may be at higher risk for vitamin B12 deficiency.13 A small case-control study showed an association between vitamin B12deficiency and PPI/H2 antagonist therapy (OR=4.45, 95% CI 1.47-13.34). Other causes were not explored and data were not separated from the H2 antagonist group.16 |
| Risk of hip fracture | PPIs may inhibit bone resorption and reduce calcium absorption by inducing hypochlorhydria17 | In 2006, a nested, observational case-control study associating high-dose chronic PPI use with a higher incidence of hip fractures showed an adjusted OR=1.44 (95% CI 1.30-1.59) in patients on PPI therapy > 1 year.17 |
| Risk of community-acquired pneumonia (CAP) | Prolonged hypochlorhydria could lead to bacterial overgrowth | A case-control study had an adjusted OR=1.5 (95% CI 1.3-1.7) and the strongest association (OR=5; 95% CI 2.1-11.7) between PPI use and CAP was in patients who had started a PPI within the past 7 days.18 A higher percentage of case patients were on corticosteroid therapy and had concurrent chronic obstructive lung disease, diabetes mellitus, and heart disease. A small study of critically ill trauma patients did not find an increased risk of nosocomial infections in patients taking PPIs.19 A pediatric prospective study found 2 cases of pneumonia in follow-up of the control group of 95 patients, and 11 cases of pneumonia in 91 patients in the combined gastric acid suppression group (P=0.03).20 This trial did not differentiate between ranitidine and omeprazole and excluded patients with diseases identified as confounding variables in previous studies. |
| Risk of Clostridium difficile infection | Prolonged hypochlorhydria could lead to bacterial overgrowth | A case-control study found that the OR for a PPI exposure within the past 3 months and C difficile infection was 3.5 (95% CI 2.3-5.2).21 Previous antibiotic exposure within the same period was associated with an OR of 8.2 (95% CI 6.1-11.0). Notably, in this study, inflammatory bowel disease, renal failure, MRSA, cancer, pernicious anemia, and recent antibiotic use were all associated with a higher adjusted OR for increased risk of C difficile infection than PPI exposure. A 2nd cohort study found an unadjusted OR of 3.1 (95% CI 1.7-5.6) for PPIs and C difficile infection. Of note, higher unadjusted ORs were seen with both renal failure and MRSA.22 Another case-control study did not establish an association between PPI use and C difficile hospitalizations.23 |
| Atrophic gastritis | Unknown | One cohort study found atrophic gastritis can occur with chronic omeprazole therapy,26 but it should be noted that of 20 who patients developed atrophy, 18 were infected with H pylori (P < 0.001) and 2 were not infected (P=0.62). In a separate study, omeprazole was not associated with gastric atrophy after 3 years.27 |
Drug interactions
Two main types of drug interactions may occur with PPIs: those at the cytochrome P450 level, and those due to decreased absorption caused by acid suppression.
PPIs are metabolized by both CYP 2C19 and CYP 3A4. Theoretically, any medication that can induce or inhibit either of these enzymes could affect PPI blood concentration levels.
Evidence suggests omeprazole and its enantiomer esomeprazole have the most potential of the PPIs for drug interactions, as they have been shown to increase blood concentration levels of phenytoin, benzodiazepines, carbamazepine, cilostazol, clarithomycin, and R-warfarin by inhibiting CYP 2C19.2,3,29 These interactions do not typically require a dose adjustment. However, more frequent monitoring may be appropriate.2,6
As a class, PPIs may decrease or increase the bioavailability of drugs whose absorption is dependent on gastric pH. Common medications that are pH dependent for absorption are the azole antifungals, ampicillin, cefpodoxime, indinavir, delavirdine, cyanocobalamin, iron, and digoxin.2,3,6 Digoxin absorption may be increased, resulting in elevated digoxin levels. Enteric-coated medications, which typically dissolve at a higher pH, may also be affected by PPI therapy.2
There may be a decrease in PPI efficacy if given concurrently with other antisecretory medications such as histamine 2 (H2) antagonists or misoprostol. This interaction is apparently due to the capability of PPIs to exert their effects only on actively secreting proton pumps.2,29
Step-up and step-down therapy
Step therapy was a much larger issue prior to the availability of generic omeprazole and pantoprazole. The relative novelty of these agents combined with higher cost of therapy and unknown long-term safety information as well as the availability of effective alternatives justified the argument for step-up therapy.
Health care systems and insurance providers encouraged short-term use of PPIs, either prior to H2 receptor antagonists (step-down therapy) or only after failure of H2 receptor antagonists (step-up therapy). Those who favored step-up therapy took into account the cost advantage of H2 receptor antagonists. Those who favored step-down therapy believed patients on H2 receptor antagonists may experience worsening control of the disease and would therefore need to utilize more health care resources, offsetting the lower costs of H2 receptor antagonists. Further, for indications like the treatment of H. pylori, compliance to therapy and corresponding efficacy may be compromised by the use of an H2 receptor antagonist in place of a PPI. Of the 2 approaches, cost effectiveness studies favor the step-down approach.30-33
Switch to an H2 blocker? Proponents of step-down therapy reasoned that many patients are placed on PPIs and continued indefinitely without re-evaluation. The only way to see if a PPI is still needed is to decrease the dose, switch to an H2 receptor antagonist, or completely discontinue acid-suppression therapy after symptom resolution.
At this time, omeprazole is the only PPI available OTC; it typically retails at $22 for 28 capsules. A generic version of the OTC omeprazole product has been approved. The cost is generally $13 to $15 for 28 capsules. Each H2 receptor antagonist is available as a generic, and OTC at half prescription strength. Of note, famotidine 20-mg tablets and ranitidine 150-mg and 300-mg tablets are available on many of the $4 prescription plans at retail pharmacies.
Your patient reports he has been taking omeprazole 20 mg daily, as you prescribed, for the past 2 weeks. His heart-burn symptoms have improved and he no longer needs to use Tums for breakthrough symptoms, as he did when he was taking Pepcid.
You considered pantoprazole and omeprazole because they are both available in generic form. Since there are no significant differences in efficacy of the 2 drugs for the treatment of GERD, you decided to prescribe pantoprazole because it will cost less for the patient, who has no prescription insurance. The pharmacy calls you, asking if generic omeprazole could be used instead due to the cost difference.
According to http://www.drugstore.com, generic pantoprazole would cost the patient $110 per month and generic omeprazole would cost the patient $63 per month.
Even though costs at local pharmacies vary and are often higher than online costs, the savings to the patient seemed significant enough to change
Equal efficacy
The first PPI, Prilosec, was approved in 1989, and 4 additional agents have become available. All are equally efficacious at equipotent doses.
Long-term safety
Although there has been concern about potential for several different adverse events with long-term PPI use, it is important to remember that the majority of these data are either conflicting or derived from case-control studies. The latter cannot prove cause and effect, but can describe potential associations that merit further investigation.
Step therapy
Whether to lower the PPI dose or switch to an H2 receptor antagonist should be considered in all patients and attempted in most patients who have been on PPIs long enough for adequate ulcer healing or who have been symptom-free.
Consider the cost to the patient
Given the similarities in interaction potential, adverse events, and efficacy, select the PPI on the basis of cost to the patient.
Treatment strategy
- Choose the least expensive product available to the patient.
- Differences in drug interactions, efficacy, and adverse events are not significant. (A)
Dose and duration
- Use PPIs at the lowest effective dose for the minimum duration. (C)
- Since many indications for PPI therapy are for a finite duration, attempt to “step down” therapy to a lower dose or try switching to an H2 receptor antagonist periodically, to achieve the goal of minimum effective dose. (C)
- If stress ulcer prophylaxis was started in the hospital, re-evaluate the need for continued use of PPIs after hospital discharge. (C)
Safety
- PPIs have been on the market for more than 15 years and have a good adverse-event profile, which is comparable to placebo. (A)
- Limited data which attempt to link PPIs to several rare adverse events do not establish a cause-effect relationship. (B)
Strength of recommendation (SOR)
- Good quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A 33-year-old man returns to see you about his bothersome heartburn, which tends to occur after lunch and dinner. He says the trouble began about 2 weeks ago. Chewing Tums tablets after meals relieves his symptoms, but he does not like the chalky taste or frequent dosing. He tried OTC Pepcid, but it relieved his symptoms only occasionally. He says he has not lost weight or had nausea, vomiting, bleeding, or dysphagia. Since you saw him the previous month, he has been trying to exercise and watch his diet, to help manage his hypertension, as you advised. His blood pressure has improved, but his heartburn has not.
THE DILEMMA
You decide to start a PPI, but wonder whether there are significant differences in PPIs, and whether there are long-term health consequences.
The last time you wrote a prescription for a proton pump inhibitor, what came to mind? Did you write a prescription for the PPI you always use? Did you prescribe Prilosec because it is available in generic form? Did you decide on Prevacid because the patient had responded well to it before? Did you consider cost, even if the patient was insured? Was your main concern side effects?
Nexium, Prevacid, and Protonix were among the 25 most commonly dispensed medications in 2006.1 With multiple indications, high clinical efficacy, low incidence of adverse events, convenient dosing, and few drug interactions, it is understandable why PPIs are popular with physicians and patients. Use of prescription PPIs has continued to increase even after Prilosec OTC became available.
TABLES 1–3 give recommended dosing for PPI indications, formulations, drug interactions, cost, and adverse event profiles.
Drug action and efficacy. PPIs have a pharmacokinetic elimination half-life of 30 minutes to 2 hours, but once-daily dosing is possible due to long-lasting inactivation of the proton pump.2
Genetic polymorphism. Up to 6% of Caucasian and 23% of Asian patients are CYP P450 2C19 poor metabolizers.2,3 This is the common hepatic clearance pathway for currently available PPIs. However, due to PPI efficacy, low potential for drug interactions, and infrequent adverse events, this genetic polymorphism is not likely to affect the vast majority of patients on PPI therapy.
Are differences clinically significant? Many trials have compared PPIs using a variety of surrogate endpoints. A new PPI, S-tenatoprazole [not available in the US] has shown greater acid suppression than esomeprazole.4 However, no single agent has proven more efficacious than other PPIs at equipotent doses.5,6 Even though lansoprazole and omeprazole have the most FDA-approved indications, this should not discourage you from using any PPI for a variety of gastrointestinal indications. For example, lansoprazole and esomeprazole are the only PPIs approved for healing or risk reduction of NSAID-related gastric ulcers. Yet other PPIs have been used for this indication without any reason to suspect lack of effectiveness. A meta-analysis of 41 randomized, double-blind studies evaluated head-to-head clinical trials for many conditions, including Helicobacter pylori, gastroesophageal reflux disease (GERD) and peptic ulcer disease.5 No clinically important differences were noted when equipotent doses were used.5-7
Only 1 difference in the treatment of GERD was noted between esomeprazole 40 mg and omeprazole 20 mg in the pooled results (relative risk 1.18; 95% confidence interval 1.14-1.23). This is not surprising because the 2 medications were not given at equipotent doses.
All PPIs are well tolerated. The most common adverse effects are headache, diarrhea, and abdominal pain.8-12 The side-effect profile is consistent among PPIs. An exception is dose-related increases in the frequency of diarrhea reported with lansoprazole (ranging from 1.4% to 7.4%) and higher dose omeprazole therapy.2,8
Although considered relatively safe, several considerations have arisen from clinical trials. Some (occurrence of rebound acid hypersecretion after PPI discontinuation) are better documented than others.13 Additional data are needed to determine whether there is any significant association with PPI-induced elevated serum gastrin levels and neoplastic changes or an increased risk of hip fracture, community-acquired pneumonia, Clostridium difficile infection, vitamin B12 anemia, iron malabsorption, and atrophic gastritis (TABLE 3).13-27
It is promising that data which demonstrate safe and effective use of these agents for longer than 10 years are available.2,28
TABLE 1
Similarities and differences among proton pump inhibitors2,8-12,25,34,35
| PANTOPRAZOLE PROTONIX | LANSOPRAZOLE PREVACID | ESOMEPRAZOLE NEXIUM | OMEPRAZOLE PRILOSEC, ZEGERID | RABEPRAZOLE ACIPHEX | NOTABLE SIMILARITIES AND DIFFERENCES | |
|---|---|---|---|---|---|---|
| Available strengths | 20 mg, 40 mg tabs 40 mg/vial 40 mg granules for suspension | 15 mg, 30 mg caps Packets for suspension, SoluTabs | 20 mg, 40 mg caps, per vial, packet for suspension | 10 mg, 20 mg, 40 mg caps 20 mg, 40 mg Zegerid packet 20 mg tab (OTC) | 20 mg tabs | Injectable options: Pantoprazole, esomeprazole Generic Prilosec and Protonix are available |
| Renal dose adjustment | No dosage adjustment needed | |||||
| Hepatic dose adjustment | > 40 mg/d not studied in hepatic impairment | Consider lower dose in severe hepatic impairment | Max 20 mg/d in severe hepatic impairment | Consider lower dose in hepatic impairment | Consider lower dose in severe hepatic impairment | No differences Consider decreased dose in hepatic impairment |
| Pregnancy category | B | B | B | C | B | All are pregnancy category B except omeprazole, which is C |
| Pediatric use | Safety and efficacy not established for patients < 18 yo | FDA approved for pediatric use except patients ≤ 2 yo | Safety and efficacy not established for patients ≤ 12 yo | FDA approved for pediatric use except patients ≤ 2 yo | Safety and efficacy not established for patients < 18 yo | Lansoprazole and omeprazole are FDA approved for pediatrics No PPI is approved for use in patients ≤ 2 yo |
| Major drug interactions** | ||||||
| Formulation stability | Do not break, crush, or chew tablets | Capsules can be opened and granules sprinkled onto soft food/beverage* or swallowed intact SoluTab and oral suspension packets can be dissolved with water | Capsules can be opened and granules sprinkled onto apple-sauce or swallowed intact Oral suspension packets can be dissolved with water | Capsules can be opened and granules sprinkled onto apple-sauce or swallowed intact Zegerid capsules should not be opened | Do not break, crush, or chew tablets | Suitable for NG administration: esomeprazole, omeprazole (Zegerid), lansoprazole Lansoprazole, omeprazole, and pantoprazole can be compounded into oral suspensions at retail pharmacies if the patient has difficulty swallowing tablets/capsules |
| Cost *** | $128 brand $100 generic | $122-$161 | $150-$165 | $115-$216 brand $64 (20 mg generic) $22 (#28 OTC) $15 (#28 OTC generic) No generic for 40 mg | $160 | Prilosec is the only PPI available OTC Prices at retail pharmacies may differ |
| Applicable soft foods and beverages are applesauce, cottage cheese, Ensure pudding, yogurt, strained pears, apple juice, tomato juice, or orange juice. | ||||||
| ** In addition to cytochrome P450 enzyme interactions, H2receptor antagonists and proton pump inhibitors can affect drugs that rely on acid for absorption (i.e., can decrease the absorption of bases like ketoconazole, iron, or cefpodoxime and increase the absorption of acids like diazepam). There have been case reports of elevated INRs with warfarin and the use of proton pump inhibitors. Omeprazole can raise levels of diazepam, warfarin, and phenytoin. | ||||||
| ***Cost information accessed at http://www.drugstore.com or http://www.cvs.com on March 10, 2008 and is for #30 unless otherwise specified. | ||||||
TABLE 2
Cost comparison of proton pump inhibitor regimens* for 8 conditions8-12,25,29
| PANTOPRAZOLE PROTONIX | LANSOPRAZOLE PREVACID | ESOMEPRAZOLE NEXIUM | OMEPRAZOLE PRILOSEC, ZEGERID | RABEPRAZOLE ACIPHEX | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DOSAGE | PRICE | DOSAGE | PRICE | DOSAGE | PRICE | DOSAGE | PRICE | DOSAGE | PRICE | |
| GERD | 20 mg QD 4-8 wk | $128-$256 $110-$220 (G) | 15 mg QD up to 8 wk | $322 | 20 mg QD 4-8 wk | $165-$330 | 20 mg QD 4 wk | $142 $15 (OTC, G) | 20 mg QD 4-8 wk | $160-$320 |
| Erosive esophagitis associated with GERD | 40 mg QD 8-16 wk **40 mg QD IV 7-10 d | $256-$512 $220-$440 (G) | 30 mg QD 8-16 wk | $308-$616 | 20-40 mg QD 4-16 wk **20-40 mg QD IV up to 10 d | $150-$660 | 20 mg QD 4-8 wk | $142-$284 $15-$30 (OTC, G) | 20 mg QD 4-16 wk | $160-$640 |
| Maintenance of healing of erosive esophagitis | 40 mg QD up to 12 mo | $1536 or $1320 (G) /12 mo | 15 mg QD up to 12 mo | $1932/12 mo | 20 mg QD up to 6 mo | $990/6 mo | 20 mg QD up to 12 mo | $1704 or $180 (OTC, G)/12 mo | 20 mg QD up to 12 mo | $1920/12 mo |
| Hypersecretory conditions*** | 40 mg BID 80 mg BID IV 7-10 d | $256 $220 (G) | 60 mg QD | $309 | 40 mg BID | $300 | 60 mg QD | $426 or $45 (OTC, G) | 60 mg QD | $480 |
| Active duodenal ulcer | 40 mg QD 2-4 wk | $64-$128 $55-$110 (G) | 15 mg QD 4 wk | $161 | — | — | 20 mg QD 4-8 wk | $142-$284 $15-$30 (OTC, G) | 20 mg QD 4 wk | $160 |
| Active gastric ulcer | 40 mg QD up to 8 wk | $256 or $220 (G) | 30 mg QD up to 8 wk | $309 | — | — | 40 mg QD 4-8 wk | $216-$432$30-$60 (OTC, G) | 20 mg QD 3-6 wk | $112-$224 |
| Reduce risk of NSAID-associated gastric ulcer | 40 mg QD | $128 or $110(G)/mo | 15 mg QD up to 12 wk | $161/mo | 20-40 mg QD up to 6 mo | $150-$165/mo | 20 mg QD | $142 or $15 (OTC, G)/mo | — | — |
| Helicobacter pylori (Always part of a 3-drug combination) | 40 mg BID 10-14 d | $85-$120 $73-$103 (G) | 30 mg BID 10-14 d | $103-$144 | 40 mg QD 10-14 d | $50-$75 | 20 mg BID 10 d | $95 $15 (OTC, G) | 20 mg BID 7 d | $80 |
| * Doses in italics are from Ref. 29. | ||||||||||
| ** For GERD in patients with a history of erosive esophagitis. | ||||||||||
| *** Starting doses listed. Several years of therapy may be necessary. | ||||||||||
TABLE 3
Support vs refutation: Adverse events and long-term PPI therapy2,13-23,26,27
| POTENTIAL ADVERSE EVENT | PROPOSED MECHANISM | SUPPORT OR REFUTATION |
|---|---|---|
| Elevated serum gastrin levels and increased enterochromaffin-like (ECL) cells | PPIs elevate serum gastrin levels by interfering with negative feedback mechanism on gastrin release | Gastrin levels can be increased but typically remain within the normal range.2 Due to potential for gastrin to affect growth of tumors outside the gastrointestinal tract, further study of a potential relationship with stimulating tumor growth is needed.13 An increase in ECL cells has been seen in humans; neoplastic findings have not been seen in humans.2,13-15 |
| Vitamin B12 deficiency | Vitamin B12 depends on gastric acid to be released and absorbed from food. In the absence of gastric acid, there is potential for deficiency16 | There is no association between chronic PPI therapy and substantial reduction of vitamin B12.16 Consideration should be given to monitoring vitamin B12 levels periodically in patients who may be at higher risk for vitamin B12 deficiency.13 A small case-control study showed an association between vitamin B12deficiency and PPI/H2 antagonist therapy (OR=4.45, 95% CI 1.47-13.34). Other causes were not explored and data were not separated from the H2 antagonist group.16 |
| Risk of hip fracture | PPIs may inhibit bone resorption and reduce calcium absorption by inducing hypochlorhydria17 | In 2006, a nested, observational case-control study associating high-dose chronic PPI use with a higher incidence of hip fractures showed an adjusted OR=1.44 (95% CI 1.30-1.59) in patients on PPI therapy > 1 year.17 |
| Risk of community-acquired pneumonia (CAP) | Prolonged hypochlorhydria could lead to bacterial overgrowth | A case-control study had an adjusted OR=1.5 (95% CI 1.3-1.7) and the strongest association (OR=5; 95% CI 2.1-11.7) between PPI use and CAP was in patients who had started a PPI within the past 7 days.18 A higher percentage of case patients were on corticosteroid therapy and had concurrent chronic obstructive lung disease, diabetes mellitus, and heart disease. A small study of critically ill trauma patients did not find an increased risk of nosocomial infections in patients taking PPIs.19 A pediatric prospective study found 2 cases of pneumonia in follow-up of the control group of 95 patients, and 11 cases of pneumonia in 91 patients in the combined gastric acid suppression group (P=0.03).20 This trial did not differentiate between ranitidine and omeprazole and excluded patients with diseases identified as confounding variables in previous studies. |
| Risk of Clostridium difficile infection | Prolonged hypochlorhydria could lead to bacterial overgrowth | A case-control study found that the OR for a PPI exposure within the past 3 months and C difficile infection was 3.5 (95% CI 2.3-5.2).21 Previous antibiotic exposure within the same period was associated with an OR of 8.2 (95% CI 6.1-11.0). Notably, in this study, inflammatory bowel disease, renal failure, MRSA, cancer, pernicious anemia, and recent antibiotic use were all associated with a higher adjusted OR for increased risk of C difficile infection than PPI exposure. A 2nd cohort study found an unadjusted OR of 3.1 (95% CI 1.7-5.6) for PPIs and C difficile infection. Of note, higher unadjusted ORs were seen with both renal failure and MRSA.22 Another case-control study did not establish an association between PPI use and C difficile hospitalizations.23 |
| Atrophic gastritis | Unknown | One cohort study found atrophic gastritis can occur with chronic omeprazole therapy,26 but it should be noted that of 20 who patients developed atrophy, 18 were infected with H pylori (P < 0.001) and 2 were not infected (P=0.62). In a separate study, omeprazole was not associated with gastric atrophy after 3 years.27 |
Drug interactions
Two main types of drug interactions may occur with PPIs: those at the cytochrome P450 level, and those due to decreased absorption caused by acid suppression.
PPIs are metabolized by both CYP 2C19 and CYP 3A4. Theoretically, any medication that can induce or inhibit either of these enzymes could affect PPI blood concentration levels.
Evidence suggests omeprazole and its enantiomer esomeprazole have the most potential of the PPIs for drug interactions, as they have been shown to increase blood concentration levels of phenytoin, benzodiazepines, carbamazepine, cilostazol, clarithomycin, and R-warfarin by inhibiting CYP 2C19.2,3,29 These interactions do not typically require a dose adjustment. However, more frequent monitoring may be appropriate.2,6
As a class, PPIs may decrease or increase the bioavailability of drugs whose absorption is dependent on gastric pH. Common medications that are pH dependent for absorption are the azole antifungals, ampicillin, cefpodoxime, indinavir, delavirdine, cyanocobalamin, iron, and digoxin.2,3,6 Digoxin absorption may be increased, resulting in elevated digoxin levels. Enteric-coated medications, which typically dissolve at a higher pH, may also be affected by PPI therapy.2
There may be a decrease in PPI efficacy if given concurrently with other antisecretory medications such as histamine 2 (H2) antagonists or misoprostol. This interaction is apparently due to the capability of PPIs to exert their effects only on actively secreting proton pumps.2,29
Step-up and step-down therapy
Step therapy was a much larger issue prior to the availability of generic omeprazole and pantoprazole. The relative novelty of these agents combined with higher cost of therapy and unknown long-term safety information as well as the availability of effective alternatives justified the argument for step-up therapy.
Health care systems and insurance providers encouraged short-term use of PPIs, either prior to H2 receptor antagonists (step-down therapy) or only after failure of H2 receptor antagonists (step-up therapy). Those who favored step-up therapy took into account the cost advantage of H2 receptor antagonists. Those who favored step-down therapy believed patients on H2 receptor antagonists may experience worsening control of the disease and would therefore need to utilize more health care resources, offsetting the lower costs of H2 receptor antagonists. Further, for indications like the treatment of H. pylori, compliance to therapy and corresponding efficacy may be compromised by the use of an H2 receptor antagonist in place of a PPI. Of the 2 approaches, cost effectiveness studies favor the step-down approach.30-33
Switch to an H2 blocker? Proponents of step-down therapy reasoned that many patients are placed on PPIs and continued indefinitely without re-evaluation. The only way to see if a PPI is still needed is to decrease the dose, switch to an H2 receptor antagonist, or completely discontinue acid-suppression therapy after symptom resolution.
At this time, omeprazole is the only PPI available OTC; it typically retails at $22 for 28 capsules. A generic version of the OTC omeprazole product has been approved. The cost is generally $13 to $15 for 28 capsules. Each H2 receptor antagonist is available as a generic, and OTC at half prescription strength. Of note, famotidine 20-mg tablets and ranitidine 150-mg and 300-mg tablets are available on many of the $4 prescription plans at retail pharmacies.
Your patient reports he has been taking omeprazole 20 mg daily, as you prescribed, for the past 2 weeks. His heart-burn symptoms have improved and he no longer needs to use Tums for breakthrough symptoms, as he did when he was taking Pepcid.
You considered pantoprazole and omeprazole because they are both available in generic form. Since there are no significant differences in efficacy of the 2 drugs for the treatment of GERD, you decided to prescribe pantoprazole because it will cost less for the patient, who has no prescription insurance. The pharmacy calls you, asking if generic omeprazole could be used instead due to the cost difference.
According to http://www.drugstore.com, generic pantoprazole would cost the patient $110 per month and generic omeprazole would cost the patient $63 per month.
Even though costs at local pharmacies vary and are often higher than online costs, the savings to the patient seemed significant enough to change
Equal efficacy
The first PPI, Prilosec, was approved in 1989, and 4 additional agents have become available. All are equally efficacious at equipotent doses.
Long-term safety
Although there has been concern about potential for several different adverse events with long-term PPI use, it is important to remember that the majority of these data are either conflicting or derived from case-control studies. The latter cannot prove cause and effect, but can describe potential associations that merit further investigation.
Step therapy
Whether to lower the PPI dose or switch to an H2 receptor antagonist should be considered in all patients and attempted in most patients who have been on PPIs long enough for adequate ulcer healing or who have been symptom-free.
Consider the cost to the patient
Given the similarities in interaction potential, adverse events, and efficacy, select the PPI on the basis of cost to the patient.
1. Top 200 Prescription Drugs of 2006. http://www.pharmacytimes.com/Article.cfm?Menu=1&ID=4629. Accessed August 28, 2007.
2. Berardi RR. A critical evaluation of proton pump inhibitors in the treatment of gastroesophageal reflux disease. Am J Manage Care. 2000;6(suppl):S491-505.
3. Guimaraes EV, Marguet C, Camargos PAM. Treatment of gastroesophageal reflux disease. J Pediatr (Rio J). 2006;82(5 Suppl):S133-145.
4. Hunt RH, Armstrong D, Yaghoobi M, et al. S-tenatoprazole-Na 30 mg, 60 mg and 90 mg versus esomeprazole (ESO) 40 mg in healthy male volunteers (hmv). Gastroenterology. 2007;132:A-488.
5. Klok RM, Postma MJ, Van Hout BA, Brouwers JRBJ. Meta-analysis: comparing the efficacy of proton pump inhibitors in short-term use. Aliment Pharmacol Ther. 2003;17:1237-1245.
6. McDonagh MS, Carson S. Drug Class Review on Proton Pump Inhibitors. 2006. http://www.ohsu.edu/drugeffectiveness/reports/final.cfm. Accessed December 18, 2007.
7. Dachs R, Darby-Stewart A, Graber M. Choosing one PPI treatment over another. Am Fam Phys. 2007;76:1273-1280.
8. Prilosec (package insert). Wilmington, DE: Astra-Zeneca LP; Sep 2006.
9. Prevacid (package insert). Lake Forest, IL: TAP Pharmaceuticals, Inc; Jun 2007.
10. Protonix (package insert). Philadelphia, PA: Wyeth Laboratories; Jun 2007.
11. Aciphex (package insert). Teaneck, NJ: Eisai, Inc; Aug 2003.
12. Nexium (package insert). Wilmington, DE: Astra-Zeneca LP; Apr 2007.
13. Jensen RT. Consequences of long-term proton pump blockade: insights from studies of patients with gastrinomas. Basic Clin Pharmacol Toxicol. 2006;98:4-19.
14. Laine L, Ahnen D, McClain C, Solcia E, Walsh JH. Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:651-668.
15. Robinson M. Review article: current perspectives on hypergastrinaemia and enterochromaffin-like cell hyperplasia. Aliment Pharmacol Ther. 1999;13 (Suppl 5):5-10.
16. Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of B12 deficiency in older adults. J Clin Epidemiol. 2004;57:422-428.
17. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-2953.
18. Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia. Arch Intern Med. 2007;167:950-955.
19. Mallow S, Rebuck JA, Osler T, Ahern J, Healey MA, Rogers FB. Do proton pump inhibitors increase the incidence of nosocomial pneumonia and related infectious complications when compared with histamine-2 receptor antagonists in critically ill trauma patients? Curr Surg. 2004;61:452-458.
20. Canani RB, Cirillo P, Roggero P, Romano C, Malamisura B, Terrin G, Passariello A, Manguso F, Morelli L, Guarino A. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117:e817-e820.
21. Dial S, Delaney C, Schneider V, Suissa S. Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease defined by prescription for oral vancomycin therapy. CMAJ. 2006;175:745-748.
22. Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ 2004;171(1):33-38.
23. Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis. 2006;43:1272-1276.
24. Health Canada reviewing new safety information on cardiac events in patients taking Losec (omeprazole) or Nexium (esomeprazole). http://www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/2007/2007_95_e.html. Accessed on August 28, 2007.
25. Vanderhoff BT, Tahboub RM. Proton pump inhibitors: an update. Am Fam Phys. 2002;66:273-280.
26. Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018-1022.
27. Lundell L, Miettinen P, Myrvold HE, et al. Lack of effect of acid suppression therapy on gastric atrophy. Gastroenterology. 1999;117:319-326.
28. Hassall E, Kerr W, El-Serag HB. Characteristics of children receiving proton pump inhibitors continuously for up to 11 years duration. J Pediatr. 2007;150:262-267.
29. Clinical Pharmacology. http://cpip.gsm.com. Accessed on August 17, 2007.
30. Inadomi JM, et al. Step-down management of gastroesophageal disease. Gastroenterology. 2001;121:1095-1100.
31. Tytgat GNJ. Review article: management of mild and severe gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2003;17(Suppl 2):52-56.
32. Ofman JJ, Dorn GH, Fennerty MB, Fass R. The clinical and economic impact of competing management strategies for gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2002;16:261-273.
33. Gerson LB, Robbins AS, Garber A, Hornberger J, Tridafilopoulos G. A cost-effectiveness analysis of prescribing strategies in the management of gastroesophageal reflux disease. Am J Gastroenterol. 2000;95:395-407.
34. Zimmermann AE, Walters JK, Katona BG, Souney PF, Levine D. Review of omeprazole use in the treatment of acid-related disorders in children. Clin Ther. 2001;23:660-679.
35. Humphries TJ, Merritt GJ. Review article: drug interactions with agents used to treat acid-related diseases. Aliment Pharmacol Ther. 1999;23(Suppl 3):18-26.
1. Top 200 Prescription Drugs of 2006. http://www.pharmacytimes.com/Article.cfm?Menu=1&ID=4629. Accessed August 28, 2007.
2. Berardi RR. A critical evaluation of proton pump inhibitors in the treatment of gastroesophageal reflux disease. Am J Manage Care. 2000;6(suppl):S491-505.
3. Guimaraes EV, Marguet C, Camargos PAM. Treatment of gastroesophageal reflux disease. J Pediatr (Rio J). 2006;82(5 Suppl):S133-145.
4. Hunt RH, Armstrong D, Yaghoobi M, et al. S-tenatoprazole-Na 30 mg, 60 mg and 90 mg versus esomeprazole (ESO) 40 mg in healthy male volunteers (hmv). Gastroenterology. 2007;132:A-488.
5. Klok RM, Postma MJ, Van Hout BA, Brouwers JRBJ. Meta-analysis: comparing the efficacy of proton pump inhibitors in short-term use. Aliment Pharmacol Ther. 2003;17:1237-1245.
6. McDonagh MS, Carson S. Drug Class Review on Proton Pump Inhibitors. 2006. http://www.ohsu.edu/drugeffectiveness/reports/final.cfm. Accessed December 18, 2007.
7. Dachs R, Darby-Stewart A, Graber M. Choosing one PPI treatment over another. Am Fam Phys. 2007;76:1273-1280.
8. Prilosec (package insert). Wilmington, DE: Astra-Zeneca LP; Sep 2006.
9. Prevacid (package insert). Lake Forest, IL: TAP Pharmaceuticals, Inc; Jun 2007.
10. Protonix (package insert). Philadelphia, PA: Wyeth Laboratories; Jun 2007.
11. Aciphex (package insert). Teaneck, NJ: Eisai, Inc; Aug 2003.
12. Nexium (package insert). Wilmington, DE: Astra-Zeneca LP; Apr 2007.
13. Jensen RT. Consequences of long-term proton pump blockade: insights from studies of patients with gastrinomas. Basic Clin Pharmacol Toxicol. 2006;98:4-19.
14. Laine L, Ahnen D, McClain C, Solcia E, Walsh JH. Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:651-668.
15. Robinson M. Review article: current perspectives on hypergastrinaemia and enterochromaffin-like cell hyperplasia. Aliment Pharmacol Ther. 1999;13 (Suppl 5):5-10.
16. Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of B12 deficiency in older adults. J Clin Epidemiol. 2004;57:422-428.
17. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947-2953.
18. Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia. Arch Intern Med. 2007;167:950-955.
19. Mallow S, Rebuck JA, Osler T, Ahern J, Healey MA, Rogers FB. Do proton pump inhibitors increase the incidence of nosocomial pneumonia and related infectious complications when compared with histamine-2 receptor antagonists in critically ill trauma patients? Curr Surg. 2004;61:452-458.
20. Canani RB, Cirillo P, Roggero P, Romano C, Malamisura B, Terrin G, Passariello A, Manguso F, Morelli L, Guarino A. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117:e817-e820.
21. Dial S, Delaney C, Schneider V, Suissa S. Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease defined by prescription for oral vancomycin therapy. CMAJ. 2006;175:745-748.
22. Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ 2004;171(1):33-38.
23. Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis. 2006;43:1272-1276.
24. Health Canada reviewing new safety information on cardiac events in patients taking Losec (omeprazole) or Nexium (esomeprazole). http://www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/2007/2007_95_e.html. Accessed on August 28, 2007.
25. Vanderhoff BT, Tahboub RM. Proton pump inhibitors: an update. Am Fam Phys. 2002;66:273-280.
26. Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018-1022.
27. Lundell L, Miettinen P, Myrvold HE, et al. Lack of effect of acid suppression therapy on gastric atrophy. Gastroenterology. 1999;117:319-326.
28. Hassall E, Kerr W, El-Serag HB. Characteristics of children receiving proton pump inhibitors continuously for up to 11 years duration. J Pediatr. 2007;150:262-267.
29. Clinical Pharmacology. http://cpip.gsm.com. Accessed on August 17, 2007.
30. Inadomi JM, et al. Step-down management of gastroesophageal disease. Gastroenterology. 2001;121:1095-1100.
31. Tytgat GNJ. Review article: management of mild and severe gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2003;17(Suppl 2):52-56.
32. Ofman JJ, Dorn GH, Fennerty MB, Fass R. The clinical and economic impact of competing management strategies for gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2002;16:261-273.
33. Gerson LB, Robbins AS, Garber A, Hornberger J, Tridafilopoulos G. A cost-effectiveness analysis of prescribing strategies in the management of gastroesophageal reflux disease. Am J Gastroenterol. 2000;95:395-407.
34. Zimmermann AE, Walters JK, Katona BG, Souney PF, Levine D. Review of omeprazole use in the treatment of acid-related disorders in children. Clin Ther. 2001;23:660-679.
35. Humphries TJ, Merritt GJ. Review article: drug interactions with agents used to treat acid-related diseases. Aliment Pharmacol Ther. 1999;23(Suppl 3):18-26.
10 derm mistakes you don’t want to make
I’ve seen my share of missed diagnoses over the years while caring for patients who, through physician- or self-referral, have made their way to our multi-specialty group practice where I focus solely on skin.
There was the 93-year-old patient whose “sun spot” had been evaluated and treated by 2 different dermatologists in the past and turned out to be a lentigo maligna melanoma (FIGURE 1).
There was the patient who had a lesion on her lip for “at least 10—maybe 20—years” that neither caught the attention of her physician, nor her dentist (FIGURE 2). Histology showed she had an infiltrative basal cell carcinoma.
And then there was the wife of a healthcare professional who decided she wanted a “second opinion” for the asymptomatic lesion on her leg that her husband assured her was benign. Her diagnosis was not so simple: She had a MELanocytic Tumor of Unknown Malignant Potential (MELTUMP) that required careful follow-up (FIGURE 3).
Early detection, as we all know, is the name of the game when it comes to skin malignancies. Yet every day, opportunities to catch small, early lesions are missed.
During the past couple of years, I’ve had thousands of patient visits for skin problems and diagnosed more than 1000 skin malignancies. The majority of patients have had some treatment for their presenting dermatosis prior to arrival. Based on my experiences with these patients, I’ve developed a list of common dermatology “mistakes.” Here they are, with some tips for avoiding them.
FIGURE 1
Melanoma missed by 2 dermatologists
This “sun spot” was of no concern to the 93-year-old patient because she had been evaluated and treated by 2 different dermatologists. The darkest areas are dermoscopically-directed pen markings for incisional biopsy. (The size of the lesion and proximity to the eye precluded primary excisional biopsy.) This “sun spot” turned out to be lentigo maligna melanoma.
FIGURE 2
Lesion on lip for “10—maybe 20—years”
This patient had a lesion that had been on her lip for “at least 10—maybe 20—years.” The patient said it had never bled or crusted. Telangiectasia, pearliness, and some infiltration were present. Histologic diagnosis: infiltrative basal cell carcinoma.
FIGURE 3
A case of MELTUMP
Two dermatopathologists read the biopsy as Spitzoid malignant melanoma, Clark Level III, and Breslow thickness 0.9 mm. Two independent dermatopathologists read the same original slides in consultation as atypical Spitz nevus. The 4 could not reach agreement. Final clinical diagnosis: MEL anocytic Tumor of Unknown Malignant Potential (MELTUMP).
Mistake #1: Not looking (and not biopsying)
I had a woman come into the office to have a lesion assessed. She had seen a dermatologist a couple of weeks earlier and even had a number of lesions removed during that visit. The patient told me that she’d repeatedly tried to show the dermatologist one specific lesion—the one of greatest concern to her—just below her underpants line, but the physician was in and out so fast each time, she never had the chance to point out this one melanocytic, changing lesion.
The lesion turned out to be a dysplastic nevus with severe architectural and cytologic atypia. This type of lesion requires histology to differentiate it from melanoma, and could just as easily have been a melanoma.
Almost daily I treat patients who are being seen by their primary care physicians regularly, and have obvious basal cell carcinomas (BCC) or squamous cell carcinomas. I have even found skin malignancies on physicians, their spouses, and their family members.1 These lesions can be easily missed—if you don’t look carefully.
Consider, the following:
- FIGURE 4 illustrates a superficial BCC on the forearm of a physician who was totally unaware of it. (It was detected on “routine” skin examination.)
- FIGURE 5 illustrates a BCC on a patient’s central forehead that, by her history, had been there for many years, and was not of any concern to her. The patient was referred to our office for evaluation of itchy skin on her legs.
- FIGURE 2 illustrates a lesion that, according to the patient, had been on her lip for at least 10—and perhaps even 20—years and was of no concern to her. (It was not the reason for the visit.) Over the years, this patient certainly had numerous primary care and dental visits, but no one “saw” the lesion. Histology confirmed the clinical impression of BCC.
FIGURE 4
Superficial BCC on physician’s forearm
A physician asked for a skin examination, but was totally unaware of this asymptomatic lesion on his dorsal forearm. Once it was identified, he could provide no history about its duration. The lesion had never bled or crusted. Pathology confirmed that it was a superficial basal cell carcinoma.
FIGURE 5
“Mole” on forehead for many years
This patient was referred for itchy legs. She was unconcerned about a prominent “mole” noted on examination of her forehead, one that she said had been there, unchanged, for many years. Histology confirmed nodular basal cell carcinoma.
- Look, look, and look again, especially at sun-exposed areas (faces, ears, scalps [as hair thins], and dorsal forearms).
- Make sure patients are appropriately gowned no matter what the reason for their visit. Listening to heart and lung sounds and palpating abdomens through overcoats may work for some physicians, but not those interested in finding asymptomatic basal cell carcinomas, actinic keratoses, dysplastic nevi, and melanomas. Melanoma in men is most common on the back; in women, it’s most common on the legs. Seeing these areas requires that they be accessible.
- Biopsy when you’re in doubt. If you see a lesion and it is not recognizable as benign (eg, cherry angioma, seborrheic keratosis, nevus), biopsy it. Let the pathologist determine if it’s benign or malignant. If the lesion is questionable and is in an area that you are uncomfortable biopsying, refer the patient for evaluation and potential biopsy.
Mistake #2: Using insufficient light
As a former family practice academic, I used to preach to residents that they needed to use “light, light, and more light” for evaluation of skin lesions. Despite this, I was often asked to evaluate a patient with a resident who hadn’t even bothered to turn on a goose-neck lamp for illumination.
Even with my current “double bank” daylight fluorescent examination room lighting, it often takes additional (surgical-type) lighting to see the diagnostic features of skin lesions. Without such intense light, it is often impossible to see whether there is pearliness, rolled edges, or fine telangiectasia.
- Use whatever intense source of light you have, whether it’s a goose-neck lamp, a halogen, or a surgical light. If you don’t have at least a portable source of bright light, you are under-equipped for a good skin exam.
Mistake #3: Using insufficient (or no) magnification
I was once examining a patient in a hospital room that had inadequate light, despite my best efforts. So I took out my digital camera with flash and autofocus feature and photographed the lesion. I then looked at the lesion on the camera’s LCD monitor and determined that it was a BCC.
The benefit of the camera was 2-fold: Not only did the flash serve as an instant source of light, but the macro feature also provided magnification. Viewing a magnified digital image on a computer screen also allows unhurried, self “second opinions,” where details can often be ascertained that were not immediately apparent in “real time,” such as rolled edges, telangiectasia, dots, streaks, and subtleties of color.
- Use a hand-held magnifying lens (5X to 10X) routinely during skin exams. They’re not expensive and should be part of every primary care physician’s armamentarium—just like stethoscopes, ophthalmoscopes, and reflex hammers.
- Use a digital camera to provide the magnification needed to see details that can be missed clinically. I’m convinced that using a digital camera has made me a better observer—and clinician. Additionally, digital photographs can be stored and compared with histologic results as “self-education.” When purchasing a camera for this purpose, make sure it has a good close-up (macro) feature.
Mistake #4: Assuming that pathology is a perfect science
Most physicians assume that dermatopathologists have a high rate of interrater concordance with diagnoses such as melanoma. Unfortunately, that is not always the case. Consider the following:
- As part of a National Institutes of Health consensus conference on melanoma, 8 dermatopathologists considered experts in melanoma were asked to provide 5 slides each. The slides were relabeled and sent to the same 8 dermatopathologists. (Three slides were eliminated.) Their findings: At the extremes, 1 pathologist called 21 cases melanoma and 16 benign, whereas another called 10 melanoma, 26 benign, and one indeterminate.2 (Remember: These were all experts in melanoma.)
- In a study of 30 melanocytic lesion specimens (including Spitzoid lesions), 10 Harvard dermatopathologists evaluated each sample independently of each other. Given 5 diagnostic categories to choose from, in only one case did as many as 6 of the 10 agree on a diagnosis. In all of these cases, there was long-term clinical follow-up, so the biologic behavior of these lesions was known. Some lesions that proved fatal were categorized by most observers as benign (eg, Spitz nevi or atypical Spitz tumors). The converse, reporting benign lesions as melanoma, also occurred.3
So consider this: If this degree of discordance occurs among dermatopathologists, what results could we expect from non-dermatopathologists?
I have personally seen instances where reports of melanoma from non-dermatopathologists did not even report Clark and Breslow staging information (although one could determine Clark staging from reading the body of the report), and reports of dysplastic nevi that were accompanied by recommendations for re-excision with 1 cm margins.
When I have a report from a general pathologist suggesting a potentially worrisome lesion (melanoma, severely dysplastic nevus, [atypical] Spitz nevus), I always suggest to my patients that we get a dermatopathologic second opinion. (I send all my dermatopathology specimens to dermatopathologists, so this applies to patients referred in with prior pathology in hand.)
Sometimes, even the dermatopathologists do not agree on the nature of the lesion. In such cases, I have my “MELTUMP discussion” with patients. That is, I tell patients that we don’t know for sure what it is, and that ultimately only the final lab test—time—will tell us the true nature of the lesion (FIGURE 3).4
- Send all “skin” to a dermatopathologist. You owe it to your patients.
- Send pictures (electronic or hard copy) to the dermatopathologist when the pathology report and clinical picture do not appear to match. While pictures of skin lesions and dermoscopic photographs would most likely be meaningless to general pathologists, they are useful to dermatopathologists. Research has shown that pathologists in various areas of medicine may alter their diagnosis or differential diagnosis when presented with additional clinical information.5
Mistake #5: Freezing neoplasms without a definitive Dx
“We’ll freeze it and if it doesn’t go away, then…”
This approach poses a significant risk to you (medicolegally) and your patient.
While most of the time what I see has been inappropriately frozen first by first-line providers, that is not always the case. Dermatologists also fall into this trap. FIGURE 6 shows a lesion just behind the hairline on the frontoparietal scalp that was frozen by a very good, and reputable, dermatologist. The patient came to me for a second opinion with an “obvious” BCC.
Some clinicians are “thrown off” when a lesion (like the one on this patient) has hair. Some sources6 indicate that BCCs never have hair, but this is patently untrue.
FIGURE 6
This shouldn’t have been frozen
A dermatologist froze this lesion on a patient’s scalp, believing that it was seborrheic keratosis, based on the patient’s history. The patient sought a second opinion, and the lesion (which had hair) was histologically identified as basal cell carcinoma.
- Don’t freeze a lesion when you are unsure; biopsy it. This is especially critical when you consider that cryotherapy is not considered a first-line treatment for BCC, the most common human malignancy. It is better to biopsy, assure the diagnosis, and then provide the appropriate therapy.
Mistake #6: Treating psoriasis with systemic corticosteroids
Plaque psoriasis can, albeit uncommonly, be transformed to pustular psoriasis after the administration of oral or injectable systemic corticosteroids.7 Although this rarely occurs, most experts consider this poor practice and not worth the risk. In addition, some experts note that systemic glucocorticoids are a drug trigger for inducing or exacerbating psoriasis.8
- Avoid systemic corticosteroids in psoriasis, since psoriasis is generally a long-term disease and systemic corticosteroids are a short-term fix. if there is widespread psoriasis and you are not familiar with systemic treatments, refer the patient. If there is localized disease, consider topical treatment options—such as various strengths of corticosteroids, calcipotriene, and tazarotene (individually or in combination)—depending on location and plaque thickness.
Mistake #7: Doing shave biopsies on melanocytic lesions
For a melanoma, not only can shaving part way through the vertical dimension of the lesion interfere with staging, it can also hinder the pathologist’s ability to arrive at the correct diagnosis.9
- Do a full-thickness, narrow-margin, fully excisional biopsy when you suspect melanoma. Certainly, there are individuals who are expert at saucerization (deep shave biopsies, often with scalloped, sloping edges that go to the deep reticular dermis) and who can perform biopsies of melanocytic lesions while still obtaining reasonable pathologic staging information.
Mistake #8: Using corticosteroid/antifungal combination products
Corticosteroid/antifungal combination products are generally shunned by dermatologists, although they are used extensively by nondermatologists.9 The difficulty with a preparation like Lotrisone, which contains a class III corticosteroid (betamethasone dipropionate [Diprosone]) and the antifungal clotrimazole, is that it is often used long-term for presumed fungal infection on thin-skinned areas. Unfortunately, though, chronic use in these areas can lead to atrophy and striae (FIGURE 7).
Additionally, corticosteroid is essentially “fungus food.” Majocchi’s granulomas can form because the corticosteroid interferes with clotrimazole’s antifungal effect. Note also that these combination products can suppress fungus sufficiently to render cultures and KOH preparations falsely negative and alter the clinical appearance of psoriasiform dermatitis, interfering with subsequent, accurate diagnosis.
FIGURE 7
Striae after corticosteroid combination therapy
This patient developed striae and atrophy after using a combination high-potency topical corticosteroid/antifungal preparation in a thin-skinned area (proximal medial thigh). It was unclear what the prescriber was treating.
- Consider compounding with miconazole powder and hydrocortisone powder, if a corticosteroid/antifungal combination is necessary (which it rarely is). For example, hydrocortisone 1% or 0.5% ointment can be compounded with miconazole powder for short-term, careful external application, in cases of angular cheilitis.
- Limit your use of a topical corticosteroid for a fungal eruption (if one must be used) to the first few days of treatment. The corticosteroid class should be appropriate to the site of application.9
- Counsel your patients about the risks of using topical corticosteroids on thinskinned areas for more than a few days (few weeks, maximum). On the face, corticosteroids can cause rosacea and perioral dermatitis, as well as “rebound” vasodilation. Thus, topical corticosteroids should be used with great caution—and generally as a last resort—for chronic facial dermatoses.
Mistake #9: Corticosteroid underdosing and undercounseling
It’s not uncommon during the warm weather months for me to see patients who are near finishing a Medrol Dosepak that was prescribed by their primary care physicians for a case of contact dermatitis (eg, poison ivy). They come to me because the eruption has returned and it is “as bad as ever.”
Underdosing. Medrol Dosepaks are generally underpowered (too low a dosage) and too short a course.7,10,11
Undercounseling. Patients don’t always realize that contact dermatitis may actually last for 3 weeks or longer. They may also mistakenly believe that systemic treatment will get them through the whole episode (rather than the worst part).
- Design your own prednisone taper for when such tapers are needed. You might even have the prescription, with taper, prefilled on paper for signature. for significant allergic contact dermatitis, the taper may last 2 weeks.
- Dispel myths. Assure patients that by taking a thorough soap shower (and laundering the clothing they were wearing), they will remove all of the oil responsible for the disease.
- Limit your use of injectable corticosteroids. There is little need for injectable corticosteroids in cases of contact dermatitis. Oral corticosteroids work just as well, can be more easily titered based on response, and pose no injection risk of tissue atrophy or abscess formation.9
Mistake #10: Requiring red flags in both history and exam
Skin diagnosis is an “or” game—not an “and” game. By that I mean: If either the history (eg, rapid change, bleeding, crusting, nonhealing ulcer) or the examination is worrisome, biopsy. Even dermoscopy can be completely reassuring with biopsy yielding a melanoma.12 Note, too, our earlier examples of patients with suspect examinations who gave reassuring histories of lesions that had been present for many years. Either a worrisome history or a suspect examination is sufficient for concern. Remember, in general, the worst-case scenario from a biopsy is a scar; from a missed melanoma, an autopsy report.
- Get back to basics. Look carefully at your patient’s skin—even if it’s not the reason for the visit. Take a moment to ask your patient: Do you have any changing lesions or is there anything on your skin that is scaly, bleeding, or crusting? Doing so will cut down on the number of patients who ultimately learn that the lesion that’s “always been there,” and that “didn’t worry the other doctors” is actually a skin cancer.
Correspondence
Gary N. Fox, MD, 1400 E 2nd St, Defiance, OH 43512; [email protected]
1. Fox GN, Mehregan DR. A new papule and “age spots.” J Fam Pract 2007;56:278-282.
2. Farmer ER, Gonin R, Hanna MP. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol 1996;27:528-531.
3. Barnhill RL, Argenyi ZB, From L, et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol 1999;30:513-520.
4. Elder DE, Xu X. The approach to the patient with a difficult melanocytic lesion. Pathology 2004;36:428-434.
5. McBroom HM, Ramsay AD. The clinicopathological meeting. A means of auditing diagnostic performance. Am J Surg Pathol 1993;17:75-80.
6. Johr R, Soyer HP, Argenziano G, Hofmann-Wellenhof R, Scalvenzi M. Dermoscopy: The Essentials. New York, NY: Mosby; 2004.
7. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York, NY: Mosby; 2004.
8. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York, NY: McGraw-Hill; 2005.
9. Edwards L. Dermatology in Emergency Care. New York, NY: Churchill Livingstone; 1997.
10. Brodell RT, Williams L. Taking the itch out of poison ivy. Postgrad Med 1999;106:69-70.
11. Hall JC. Dermatologic allergy. In: Hall JC, ed. Sauer’s Manual of Skin Diseases. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:82.
12. Braun RP, Gaide O, Skaria AM, Kopf AW, Saurat JH, Marghoob AA. Exclusively benign dermoscopic pattern in a patient with acral melanoma. Arch Dermatol 2007;143:1213-1215.
I’ve seen my share of missed diagnoses over the years while caring for patients who, through physician- or self-referral, have made their way to our multi-specialty group practice where I focus solely on skin.
There was the 93-year-old patient whose “sun spot” had been evaluated and treated by 2 different dermatologists in the past and turned out to be a lentigo maligna melanoma (FIGURE 1).
There was the patient who had a lesion on her lip for “at least 10—maybe 20—years” that neither caught the attention of her physician, nor her dentist (FIGURE 2). Histology showed she had an infiltrative basal cell carcinoma.
And then there was the wife of a healthcare professional who decided she wanted a “second opinion” for the asymptomatic lesion on her leg that her husband assured her was benign. Her diagnosis was not so simple: She had a MELanocytic Tumor of Unknown Malignant Potential (MELTUMP) that required careful follow-up (FIGURE 3).
Early detection, as we all know, is the name of the game when it comes to skin malignancies. Yet every day, opportunities to catch small, early lesions are missed.
During the past couple of years, I’ve had thousands of patient visits for skin problems and diagnosed more than 1000 skin malignancies. The majority of patients have had some treatment for their presenting dermatosis prior to arrival. Based on my experiences with these patients, I’ve developed a list of common dermatology “mistakes.” Here they are, with some tips for avoiding them.
FIGURE 1
Melanoma missed by 2 dermatologists
This “sun spot” was of no concern to the 93-year-old patient because she had been evaluated and treated by 2 different dermatologists. The darkest areas are dermoscopically-directed pen markings for incisional biopsy. (The size of the lesion and proximity to the eye precluded primary excisional biopsy.) This “sun spot” turned out to be lentigo maligna melanoma.
FIGURE 2
Lesion on lip for “10—maybe 20—years”
This patient had a lesion that had been on her lip for “at least 10—maybe 20—years.” The patient said it had never bled or crusted. Telangiectasia, pearliness, and some infiltration were present. Histologic diagnosis: infiltrative basal cell carcinoma.
FIGURE 3
A case of MELTUMP
Two dermatopathologists read the biopsy as Spitzoid malignant melanoma, Clark Level III, and Breslow thickness 0.9 mm. Two independent dermatopathologists read the same original slides in consultation as atypical Spitz nevus. The 4 could not reach agreement. Final clinical diagnosis: MEL anocytic Tumor of Unknown Malignant Potential (MELTUMP).
Mistake #1: Not looking (and not biopsying)
I had a woman come into the office to have a lesion assessed. She had seen a dermatologist a couple of weeks earlier and even had a number of lesions removed during that visit. The patient told me that she’d repeatedly tried to show the dermatologist one specific lesion—the one of greatest concern to her—just below her underpants line, but the physician was in and out so fast each time, she never had the chance to point out this one melanocytic, changing lesion.
The lesion turned out to be a dysplastic nevus with severe architectural and cytologic atypia. This type of lesion requires histology to differentiate it from melanoma, and could just as easily have been a melanoma.
Almost daily I treat patients who are being seen by their primary care physicians regularly, and have obvious basal cell carcinomas (BCC) or squamous cell carcinomas. I have even found skin malignancies on physicians, their spouses, and their family members.1 These lesions can be easily missed—if you don’t look carefully.
Consider, the following:
- FIGURE 4 illustrates a superficial BCC on the forearm of a physician who was totally unaware of it. (It was detected on “routine” skin examination.)
- FIGURE 5 illustrates a BCC on a patient’s central forehead that, by her history, had been there for many years, and was not of any concern to her. The patient was referred to our office for evaluation of itchy skin on her legs.
- FIGURE 2 illustrates a lesion that, according to the patient, had been on her lip for at least 10—and perhaps even 20—years and was of no concern to her. (It was not the reason for the visit.) Over the years, this patient certainly had numerous primary care and dental visits, but no one “saw” the lesion. Histology confirmed the clinical impression of BCC.
FIGURE 4
Superficial BCC on physician’s forearm
A physician asked for a skin examination, but was totally unaware of this asymptomatic lesion on his dorsal forearm. Once it was identified, he could provide no history about its duration. The lesion had never bled or crusted. Pathology confirmed that it was a superficial basal cell carcinoma.
FIGURE 5
“Mole” on forehead for many years
This patient was referred for itchy legs. She was unconcerned about a prominent “mole” noted on examination of her forehead, one that she said had been there, unchanged, for many years. Histology confirmed nodular basal cell carcinoma.
- Look, look, and look again, especially at sun-exposed areas (faces, ears, scalps [as hair thins], and dorsal forearms).
- Make sure patients are appropriately gowned no matter what the reason for their visit. Listening to heart and lung sounds and palpating abdomens through overcoats may work for some physicians, but not those interested in finding asymptomatic basal cell carcinomas, actinic keratoses, dysplastic nevi, and melanomas. Melanoma in men is most common on the back; in women, it’s most common on the legs. Seeing these areas requires that they be accessible.
- Biopsy when you’re in doubt. If you see a lesion and it is not recognizable as benign (eg, cherry angioma, seborrheic keratosis, nevus), biopsy it. Let the pathologist determine if it’s benign or malignant. If the lesion is questionable and is in an area that you are uncomfortable biopsying, refer the patient for evaluation and potential biopsy.
Mistake #2: Using insufficient light
As a former family practice academic, I used to preach to residents that they needed to use “light, light, and more light” for evaluation of skin lesions. Despite this, I was often asked to evaluate a patient with a resident who hadn’t even bothered to turn on a goose-neck lamp for illumination.
Even with my current “double bank” daylight fluorescent examination room lighting, it often takes additional (surgical-type) lighting to see the diagnostic features of skin lesions. Without such intense light, it is often impossible to see whether there is pearliness, rolled edges, or fine telangiectasia.
- Use whatever intense source of light you have, whether it’s a goose-neck lamp, a halogen, or a surgical light. If you don’t have at least a portable source of bright light, you are under-equipped for a good skin exam.
Mistake #3: Using insufficient (or no) magnification
I was once examining a patient in a hospital room that had inadequate light, despite my best efforts. So I took out my digital camera with flash and autofocus feature and photographed the lesion. I then looked at the lesion on the camera’s LCD monitor and determined that it was a BCC.
The benefit of the camera was 2-fold: Not only did the flash serve as an instant source of light, but the macro feature also provided magnification. Viewing a magnified digital image on a computer screen also allows unhurried, self “second opinions,” where details can often be ascertained that were not immediately apparent in “real time,” such as rolled edges, telangiectasia, dots, streaks, and subtleties of color.
- Use a hand-held magnifying lens (5X to 10X) routinely during skin exams. They’re not expensive and should be part of every primary care physician’s armamentarium—just like stethoscopes, ophthalmoscopes, and reflex hammers.
- Use a digital camera to provide the magnification needed to see details that can be missed clinically. I’m convinced that using a digital camera has made me a better observer—and clinician. Additionally, digital photographs can be stored and compared with histologic results as “self-education.” When purchasing a camera for this purpose, make sure it has a good close-up (macro) feature.
Mistake #4: Assuming that pathology is a perfect science
Most physicians assume that dermatopathologists have a high rate of interrater concordance with diagnoses such as melanoma. Unfortunately, that is not always the case. Consider the following:
- As part of a National Institutes of Health consensus conference on melanoma, 8 dermatopathologists considered experts in melanoma were asked to provide 5 slides each. The slides were relabeled and sent to the same 8 dermatopathologists. (Three slides were eliminated.) Their findings: At the extremes, 1 pathologist called 21 cases melanoma and 16 benign, whereas another called 10 melanoma, 26 benign, and one indeterminate.2 (Remember: These were all experts in melanoma.)
- In a study of 30 melanocytic lesion specimens (including Spitzoid lesions), 10 Harvard dermatopathologists evaluated each sample independently of each other. Given 5 diagnostic categories to choose from, in only one case did as many as 6 of the 10 agree on a diagnosis. In all of these cases, there was long-term clinical follow-up, so the biologic behavior of these lesions was known. Some lesions that proved fatal were categorized by most observers as benign (eg, Spitz nevi or atypical Spitz tumors). The converse, reporting benign lesions as melanoma, also occurred.3
So consider this: If this degree of discordance occurs among dermatopathologists, what results could we expect from non-dermatopathologists?
I have personally seen instances where reports of melanoma from non-dermatopathologists did not even report Clark and Breslow staging information (although one could determine Clark staging from reading the body of the report), and reports of dysplastic nevi that were accompanied by recommendations for re-excision with 1 cm margins.
When I have a report from a general pathologist suggesting a potentially worrisome lesion (melanoma, severely dysplastic nevus, [atypical] Spitz nevus), I always suggest to my patients that we get a dermatopathologic second opinion. (I send all my dermatopathology specimens to dermatopathologists, so this applies to patients referred in with prior pathology in hand.)
Sometimes, even the dermatopathologists do not agree on the nature of the lesion. In such cases, I have my “MELTUMP discussion” with patients. That is, I tell patients that we don’t know for sure what it is, and that ultimately only the final lab test—time—will tell us the true nature of the lesion (FIGURE 3).4
- Send all “skin” to a dermatopathologist. You owe it to your patients.
- Send pictures (electronic or hard copy) to the dermatopathologist when the pathology report and clinical picture do not appear to match. While pictures of skin lesions and dermoscopic photographs would most likely be meaningless to general pathologists, they are useful to dermatopathologists. Research has shown that pathologists in various areas of medicine may alter their diagnosis or differential diagnosis when presented with additional clinical information.5
Mistake #5: Freezing neoplasms without a definitive Dx
“We’ll freeze it and if it doesn’t go away, then…”
This approach poses a significant risk to you (medicolegally) and your patient.
While most of the time what I see has been inappropriately frozen first by first-line providers, that is not always the case. Dermatologists also fall into this trap. FIGURE 6 shows a lesion just behind the hairline on the frontoparietal scalp that was frozen by a very good, and reputable, dermatologist. The patient came to me for a second opinion with an “obvious” BCC.
Some clinicians are “thrown off” when a lesion (like the one on this patient) has hair. Some sources6 indicate that BCCs never have hair, but this is patently untrue.
FIGURE 6
This shouldn’t have been frozen
A dermatologist froze this lesion on a patient’s scalp, believing that it was seborrheic keratosis, based on the patient’s history. The patient sought a second opinion, and the lesion (which had hair) was histologically identified as basal cell carcinoma.
- Don’t freeze a lesion when you are unsure; biopsy it. This is especially critical when you consider that cryotherapy is not considered a first-line treatment for BCC, the most common human malignancy. It is better to biopsy, assure the diagnosis, and then provide the appropriate therapy.
Mistake #6: Treating psoriasis with systemic corticosteroids
Plaque psoriasis can, albeit uncommonly, be transformed to pustular psoriasis after the administration of oral or injectable systemic corticosteroids.7 Although this rarely occurs, most experts consider this poor practice and not worth the risk. In addition, some experts note that systemic glucocorticoids are a drug trigger for inducing or exacerbating psoriasis.8
- Avoid systemic corticosteroids in psoriasis, since psoriasis is generally a long-term disease and systemic corticosteroids are a short-term fix. if there is widespread psoriasis and you are not familiar with systemic treatments, refer the patient. If there is localized disease, consider topical treatment options—such as various strengths of corticosteroids, calcipotriene, and tazarotene (individually or in combination)—depending on location and plaque thickness.
Mistake #7: Doing shave biopsies on melanocytic lesions
For a melanoma, not only can shaving part way through the vertical dimension of the lesion interfere with staging, it can also hinder the pathologist’s ability to arrive at the correct diagnosis.9
- Do a full-thickness, narrow-margin, fully excisional biopsy when you suspect melanoma. Certainly, there are individuals who are expert at saucerization (deep shave biopsies, often with scalloped, sloping edges that go to the deep reticular dermis) and who can perform biopsies of melanocytic lesions while still obtaining reasonable pathologic staging information.
Mistake #8: Using corticosteroid/antifungal combination products
Corticosteroid/antifungal combination products are generally shunned by dermatologists, although they are used extensively by nondermatologists.9 The difficulty with a preparation like Lotrisone, which contains a class III corticosteroid (betamethasone dipropionate [Diprosone]) and the antifungal clotrimazole, is that it is often used long-term for presumed fungal infection on thin-skinned areas. Unfortunately, though, chronic use in these areas can lead to atrophy and striae (FIGURE 7).
Additionally, corticosteroid is essentially “fungus food.” Majocchi’s granulomas can form because the corticosteroid interferes with clotrimazole’s antifungal effect. Note also that these combination products can suppress fungus sufficiently to render cultures and KOH preparations falsely negative and alter the clinical appearance of psoriasiform dermatitis, interfering with subsequent, accurate diagnosis.
FIGURE 7
Striae after corticosteroid combination therapy
This patient developed striae and atrophy after using a combination high-potency topical corticosteroid/antifungal preparation in a thin-skinned area (proximal medial thigh). It was unclear what the prescriber was treating.
- Consider compounding with miconazole powder and hydrocortisone powder, if a corticosteroid/antifungal combination is necessary (which it rarely is). For example, hydrocortisone 1% or 0.5% ointment can be compounded with miconazole powder for short-term, careful external application, in cases of angular cheilitis.
- Limit your use of a topical corticosteroid for a fungal eruption (if one must be used) to the first few days of treatment. The corticosteroid class should be appropriate to the site of application.9
- Counsel your patients about the risks of using topical corticosteroids on thinskinned areas for more than a few days (few weeks, maximum). On the face, corticosteroids can cause rosacea and perioral dermatitis, as well as “rebound” vasodilation. Thus, topical corticosteroids should be used with great caution—and generally as a last resort—for chronic facial dermatoses.
Mistake #9: Corticosteroid underdosing and undercounseling
It’s not uncommon during the warm weather months for me to see patients who are near finishing a Medrol Dosepak that was prescribed by their primary care physicians for a case of contact dermatitis (eg, poison ivy). They come to me because the eruption has returned and it is “as bad as ever.”
Underdosing. Medrol Dosepaks are generally underpowered (too low a dosage) and too short a course.7,10,11
Undercounseling. Patients don’t always realize that contact dermatitis may actually last for 3 weeks or longer. They may also mistakenly believe that systemic treatment will get them through the whole episode (rather than the worst part).
- Design your own prednisone taper for when such tapers are needed. You might even have the prescription, with taper, prefilled on paper for signature. for significant allergic contact dermatitis, the taper may last 2 weeks.
- Dispel myths. Assure patients that by taking a thorough soap shower (and laundering the clothing they were wearing), they will remove all of the oil responsible for the disease.
- Limit your use of injectable corticosteroids. There is little need for injectable corticosteroids in cases of contact dermatitis. Oral corticosteroids work just as well, can be more easily titered based on response, and pose no injection risk of tissue atrophy or abscess formation.9
Mistake #10: Requiring red flags in both history and exam
Skin diagnosis is an “or” game—not an “and” game. By that I mean: If either the history (eg, rapid change, bleeding, crusting, nonhealing ulcer) or the examination is worrisome, biopsy. Even dermoscopy can be completely reassuring with biopsy yielding a melanoma.12 Note, too, our earlier examples of patients with suspect examinations who gave reassuring histories of lesions that had been present for many years. Either a worrisome history or a suspect examination is sufficient for concern. Remember, in general, the worst-case scenario from a biopsy is a scar; from a missed melanoma, an autopsy report.
- Get back to basics. Look carefully at your patient’s skin—even if it’s not the reason for the visit. Take a moment to ask your patient: Do you have any changing lesions or is there anything on your skin that is scaly, bleeding, or crusting? Doing so will cut down on the number of patients who ultimately learn that the lesion that’s “always been there,” and that “didn’t worry the other doctors” is actually a skin cancer.
Correspondence
Gary N. Fox, MD, 1400 E 2nd St, Defiance, OH 43512; [email protected]
I’ve seen my share of missed diagnoses over the years while caring for patients who, through physician- or self-referral, have made their way to our multi-specialty group practice where I focus solely on skin.
There was the 93-year-old patient whose “sun spot” had been evaluated and treated by 2 different dermatologists in the past and turned out to be a lentigo maligna melanoma (FIGURE 1).
There was the patient who had a lesion on her lip for “at least 10—maybe 20—years” that neither caught the attention of her physician, nor her dentist (FIGURE 2). Histology showed she had an infiltrative basal cell carcinoma.
And then there was the wife of a healthcare professional who decided she wanted a “second opinion” for the asymptomatic lesion on her leg that her husband assured her was benign. Her diagnosis was not so simple: She had a MELanocytic Tumor of Unknown Malignant Potential (MELTUMP) that required careful follow-up (FIGURE 3).
Early detection, as we all know, is the name of the game when it comes to skin malignancies. Yet every day, opportunities to catch small, early lesions are missed.
During the past couple of years, I’ve had thousands of patient visits for skin problems and diagnosed more than 1000 skin malignancies. The majority of patients have had some treatment for their presenting dermatosis prior to arrival. Based on my experiences with these patients, I’ve developed a list of common dermatology “mistakes.” Here they are, with some tips for avoiding them.
FIGURE 1
Melanoma missed by 2 dermatologists
This “sun spot” was of no concern to the 93-year-old patient because she had been evaluated and treated by 2 different dermatologists. The darkest areas are dermoscopically-directed pen markings for incisional biopsy. (The size of the lesion and proximity to the eye precluded primary excisional biopsy.) This “sun spot” turned out to be lentigo maligna melanoma.
FIGURE 2
Lesion on lip for “10—maybe 20—years”
This patient had a lesion that had been on her lip for “at least 10—maybe 20—years.” The patient said it had never bled or crusted. Telangiectasia, pearliness, and some infiltration were present. Histologic diagnosis: infiltrative basal cell carcinoma.
FIGURE 3
A case of MELTUMP
Two dermatopathologists read the biopsy as Spitzoid malignant melanoma, Clark Level III, and Breslow thickness 0.9 mm. Two independent dermatopathologists read the same original slides in consultation as atypical Spitz nevus. The 4 could not reach agreement. Final clinical diagnosis: MEL anocytic Tumor of Unknown Malignant Potential (MELTUMP).
Mistake #1: Not looking (and not biopsying)
I had a woman come into the office to have a lesion assessed. She had seen a dermatologist a couple of weeks earlier and even had a number of lesions removed during that visit. The patient told me that she’d repeatedly tried to show the dermatologist one specific lesion—the one of greatest concern to her—just below her underpants line, but the physician was in and out so fast each time, she never had the chance to point out this one melanocytic, changing lesion.
The lesion turned out to be a dysplastic nevus with severe architectural and cytologic atypia. This type of lesion requires histology to differentiate it from melanoma, and could just as easily have been a melanoma.
Almost daily I treat patients who are being seen by their primary care physicians regularly, and have obvious basal cell carcinomas (BCC) or squamous cell carcinomas. I have even found skin malignancies on physicians, their spouses, and their family members.1 These lesions can be easily missed—if you don’t look carefully.
Consider, the following:
- FIGURE 4 illustrates a superficial BCC on the forearm of a physician who was totally unaware of it. (It was detected on “routine” skin examination.)
- FIGURE 5 illustrates a BCC on a patient’s central forehead that, by her history, had been there for many years, and was not of any concern to her. The patient was referred to our office for evaluation of itchy skin on her legs.
- FIGURE 2 illustrates a lesion that, according to the patient, had been on her lip for at least 10—and perhaps even 20—years and was of no concern to her. (It was not the reason for the visit.) Over the years, this patient certainly had numerous primary care and dental visits, but no one “saw” the lesion. Histology confirmed the clinical impression of BCC.
FIGURE 4
Superficial BCC on physician’s forearm
A physician asked for a skin examination, but was totally unaware of this asymptomatic lesion on his dorsal forearm. Once it was identified, he could provide no history about its duration. The lesion had never bled or crusted. Pathology confirmed that it was a superficial basal cell carcinoma.
FIGURE 5
“Mole” on forehead for many years
This patient was referred for itchy legs. She was unconcerned about a prominent “mole” noted on examination of her forehead, one that she said had been there, unchanged, for many years. Histology confirmed nodular basal cell carcinoma.
- Look, look, and look again, especially at sun-exposed areas (faces, ears, scalps [as hair thins], and dorsal forearms).
- Make sure patients are appropriately gowned no matter what the reason for their visit. Listening to heart and lung sounds and palpating abdomens through overcoats may work for some physicians, but not those interested in finding asymptomatic basal cell carcinomas, actinic keratoses, dysplastic nevi, and melanomas. Melanoma in men is most common on the back; in women, it’s most common on the legs. Seeing these areas requires that they be accessible.
- Biopsy when you’re in doubt. If you see a lesion and it is not recognizable as benign (eg, cherry angioma, seborrheic keratosis, nevus), biopsy it. Let the pathologist determine if it’s benign or malignant. If the lesion is questionable and is in an area that you are uncomfortable biopsying, refer the patient for evaluation and potential biopsy.
Mistake #2: Using insufficient light
As a former family practice academic, I used to preach to residents that they needed to use “light, light, and more light” for evaluation of skin lesions. Despite this, I was often asked to evaluate a patient with a resident who hadn’t even bothered to turn on a goose-neck lamp for illumination.
Even with my current “double bank” daylight fluorescent examination room lighting, it often takes additional (surgical-type) lighting to see the diagnostic features of skin lesions. Without such intense light, it is often impossible to see whether there is pearliness, rolled edges, or fine telangiectasia.
- Use whatever intense source of light you have, whether it’s a goose-neck lamp, a halogen, or a surgical light. If you don’t have at least a portable source of bright light, you are under-equipped for a good skin exam.
Mistake #3: Using insufficient (or no) magnification
I was once examining a patient in a hospital room that had inadequate light, despite my best efforts. So I took out my digital camera with flash and autofocus feature and photographed the lesion. I then looked at the lesion on the camera’s LCD monitor and determined that it was a BCC.
The benefit of the camera was 2-fold: Not only did the flash serve as an instant source of light, but the macro feature also provided magnification. Viewing a magnified digital image on a computer screen also allows unhurried, self “second opinions,” where details can often be ascertained that were not immediately apparent in “real time,” such as rolled edges, telangiectasia, dots, streaks, and subtleties of color.
- Use a hand-held magnifying lens (5X to 10X) routinely during skin exams. They’re not expensive and should be part of every primary care physician’s armamentarium—just like stethoscopes, ophthalmoscopes, and reflex hammers.
- Use a digital camera to provide the magnification needed to see details that can be missed clinically. I’m convinced that using a digital camera has made me a better observer—and clinician. Additionally, digital photographs can be stored and compared with histologic results as “self-education.” When purchasing a camera for this purpose, make sure it has a good close-up (macro) feature.
Mistake #4: Assuming that pathology is a perfect science
Most physicians assume that dermatopathologists have a high rate of interrater concordance with diagnoses such as melanoma. Unfortunately, that is not always the case. Consider the following:
- As part of a National Institutes of Health consensus conference on melanoma, 8 dermatopathologists considered experts in melanoma were asked to provide 5 slides each. The slides were relabeled and sent to the same 8 dermatopathologists. (Three slides were eliminated.) Their findings: At the extremes, 1 pathologist called 21 cases melanoma and 16 benign, whereas another called 10 melanoma, 26 benign, and one indeterminate.2 (Remember: These were all experts in melanoma.)
- In a study of 30 melanocytic lesion specimens (including Spitzoid lesions), 10 Harvard dermatopathologists evaluated each sample independently of each other. Given 5 diagnostic categories to choose from, in only one case did as many as 6 of the 10 agree on a diagnosis. In all of these cases, there was long-term clinical follow-up, so the biologic behavior of these lesions was known. Some lesions that proved fatal were categorized by most observers as benign (eg, Spitz nevi or atypical Spitz tumors). The converse, reporting benign lesions as melanoma, also occurred.3
So consider this: If this degree of discordance occurs among dermatopathologists, what results could we expect from non-dermatopathologists?
I have personally seen instances where reports of melanoma from non-dermatopathologists did not even report Clark and Breslow staging information (although one could determine Clark staging from reading the body of the report), and reports of dysplastic nevi that were accompanied by recommendations for re-excision with 1 cm margins.
When I have a report from a general pathologist suggesting a potentially worrisome lesion (melanoma, severely dysplastic nevus, [atypical] Spitz nevus), I always suggest to my patients that we get a dermatopathologic second opinion. (I send all my dermatopathology specimens to dermatopathologists, so this applies to patients referred in with prior pathology in hand.)
Sometimes, even the dermatopathologists do not agree on the nature of the lesion. In such cases, I have my “MELTUMP discussion” with patients. That is, I tell patients that we don’t know for sure what it is, and that ultimately only the final lab test—time—will tell us the true nature of the lesion (FIGURE 3).4
- Send all “skin” to a dermatopathologist. You owe it to your patients.
- Send pictures (electronic or hard copy) to the dermatopathologist when the pathology report and clinical picture do not appear to match. While pictures of skin lesions and dermoscopic photographs would most likely be meaningless to general pathologists, they are useful to dermatopathologists. Research has shown that pathologists in various areas of medicine may alter their diagnosis or differential diagnosis when presented with additional clinical information.5
Mistake #5: Freezing neoplasms without a definitive Dx
“We’ll freeze it and if it doesn’t go away, then…”
This approach poses a significant risk to you (medicolegally) and your patient.
While most of the time what I see has been inappropriately frozen first by first-line providers, that is not always the case. Dermatologists also fall into this trap. FIGURE 6 shows a lesion just behind the hairline on the frontoparietal scalp that was frozen by a very good, and reputable, dermatologist. The patient came to me for a second opinion with an “obvious” BCC.
Some clinicians are “thrown off” when a lesion (like the one on this patient) has hair. Some sources6 indicate that BCCs never have hair, but this is patently untrue.
FIGURE 6
This shouldn’t have been frozen
A dermatologist froze this lesion on a patient’s scalp, believing that it was seborrheic keratosis, based on the patient’s history. The patient sought a second opinion, and the lesion (which had hair) was histologically identified as basal cell carcinoma.
- Don’t freeze a lesion when you are unsure; biopsy it. This is especially critical when you consider that cryotherapy is not considered a first-line treatment for BCC, the most common human malignancy. It is better to biopsy, assure the diagnosis, and then provide the appropriate therapy.
Mistake #6: Treating psoriasis with systemic corticosteroids
Plaque psoriasis can, albeit uncommonly, be transformed to pustular psoriasis after the administration of oral or injectable systemic corticosteroids.7 Although this rarely occurs, most experts consider this poor practice and not worth the risk. In addition, some experts note that systemic glucocorticoids are a drug trigger for inducing or exacerbating psoriasis.8
- Avoid systemic corticosteroids in psoriasis, since psoriasis is generally a long-term disease and systemic corticosteroids are a short-term fix. if there is widespread psoriasis and you are not familiar with systemic treatments, refer the patient. If there is localized disease, consider topical treatment options—such as various strengths of corticosteroids, calcipotriene, and tazarotene (individually or in combination)—depending on location and plaque thickness.
Mistake #7: Doing shave biopsies on melanocytic lesions
For a melanoma, not only can shaving part way through the vertical dimension of the lesion interfere with staging, it can also hinder the pathologist’s ability to arrive at the correct diagnosis.9
- Do a full-thickness, narrow-margin, fully excisional biopsy when you suspect melanoma. Certainly, there are individuals who are expert at saucerization (deep shave biopsies, often with scalloped, sloping edges that go to the deep reticular dermis) and who can perform biopsies of melanocytic lesions while still obtaining reasonable pathologic staging information.
Mistake #8: Using corticosteroid/antifungal combination products
Corticosteroid/antifungal combination products are generally shunned by dermatologists, although they are used extensively by nondermatologists.9 The difficulty with a preparation like Lotrisone, which contains a class III corticosteroid (betamethasone dipropionate [Diprosone]) and the antifungal clotrimazole, is that it is often used long-term for presumed fungal infection on thin-skinned areas. Unfortunately, though, chronic use in these areas can lead to atrophy and striae (FIGURE 7).
Additionally, corticosteroid is essentially “fungus food.” Majocchi’s granulomas can form because the corticosteroid interferes with clotrimazole’s antifungal effect. Note also that these combination products can suppress fungus sufficiently to render cultures and KOH preparations falsely negative and alter the clinical appearance of psoriasiform dermatitis, interfering with subsequent, accurate diagnosis.
FIGURE 7
Striae after corticosteroid combination therapy
This patient developed striae and atrophy after using a combination high-potency topical corticosteroid/antifungal preparation in a thin-skinned area (proximal medial thigh). It was unclear what the prescriber was treating.
- Consider compounding with miconazole powder and hydrocortisone powder, if a corticosteroid/antifungal combination is necessary (which it rarely is). For example, hydrocortisone 1% or 0.5% ointment can be compounded with miconazole powder for short-term, careful external application, in cases of angular cheilitis.
- Limit your use of a topical corticosteroid for a fungal eruption (if one must be used) to the first few days of treatment. The corticosteroid class should be appropriate to the site of application.9
- Counsel your patients about the risks of using topical corticosteroids on thinskinned areas for more than a few days (few weeks, maximum). On the face, corticosteroids can cause rosacea and perioral dermatitis, as well as “rebound” vasodilation. Thus, topical corticosteroids should be used with great caution—and generally as a last resort—for chronic facial dermatoses.
Mistake #9: Corticosteroid underdosing and undercounseling
It’s not uncommon during the warm weather months for me to see patients who are near finishing a Medrol Dosepak that was prescribed by their primary care physicians for a case of contact dermatitis (eg, poison ivy). They come to me because the eruption has returned and it is “as bad as ever.”
Underdosing. Medrol Dosepaks are generally underpowered (too low a dosage) and too short a course.7,10,11
Undercounseling. Patients don’t always realize that contact dermatitis may actually last for 3 weeks or longer. They may also mistakenly believe that systemic treatment will get them through the whole episode (rather than the worst part).
- Design your own prednisone taper for when such tapers are needed. You might even have the prescription, with taper, prefilled on paper for signature. for significant allergic contact dermatitis, the taper may last 2 weeks.
- Dispel myths. Assure patients that by taking a thorough soap shower (and laundering the clothing they were wearing), they will remove all of the oil responsible for the disease.
- Limit your use of injectable corticosteroids. There is little need for injectable corticosteroids in cases of contact dermatitis. Oral corticosteroids work just as well, can be more easily titered based on response, and pose no injection risk of tissue atrophy or abscess formation.9
Mistake #10: Requiring red flags in both history and exam
Skin diagnosis is an “or” game—not an “and” game. By that I mean: If either the history (eg, rapid change, bleeding, crusting, nonhealing ulcer) or the examination is worrisome, biopsy. Even dermoscopy can be completely reassuring with biopsy yielding a melanoma.12 Note, too, our earlier examples of patients with suspect examinations who gave reassuring histories of lesions that had been present for many years. Either a worrisome history or a suspect examination is sufficient for concern. Remember, in general, the worst-case scenario from a biopsy is a scar; from a missed melanoma, an autopsy report.
- Get back to basics. Look carefully at your patient’s skin—even if it’s not the reason for the visit. Take a moment to ask your patient: Do you have any changing lesions or is there anything on your skin that is scaly, bleeding, or crusting? Doing so will cut down on the number of patients who ultimately learn that the lesion that’s “always been there,” and that “didn’t worry the other doctors” is actually a skin cancer.
Correspondence
Gary N. Fox, MD, 1400 E 2nd St, Defiance, OH 43512; [email protected]
1. Fox GN, Mehregan DR. A new papule and “age spots.” J Fam Pract 2007;56:278-282.
2. Farmer ER, Gonin R, Hanna MP. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol 1996;27:528-531.
3. Barnhill RL, Argenyi ZB, From L, et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol 1999;30:513-520.
4. Elder DE, Xu X. The approach to the patient with a difficult melanocytic lesion. Pathology 2004;36:428-434.
5. McBroom HM, Ramsay AD. The clinicopathological meeting. A means of auditing diagnostic performance. Am J Surg Pathol 1993;17:75-80.
6. Johr R, Soyer HP, Argenziano G, Hofmann-Wellenhof R, Scalvenzi M. Dermoscopy: The Essentials. New York, NY: Mosby; 2004.
7. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York, NY: Mosby; 2004.
8. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York, NY: McGraw-Hill; 2005.
9. Edwards L. Dermatology in Emergency Care. New York, NY: Churchill Livingstone; 1997.
10. Brodell RT, Williams L. Taking the itch out of poison ivy. Postgrad Med 1999;106:69-70.
11. Hall JC. Dermatologic allergy. In: Hall JC, ed. Sauer’s Manual of Skin Diseases. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:82.
12. Braun RP, Gaide O, Skaria AM, Kopf AW, Saurat JH, Marghoob AA. Exclusively benign dermoscopic pattern in a patient with acral melanoma. Arch Dermatol 2007;143:1213-1215.
1. Fox GN, Mehregan DR. A new papule and “age spots.” J Fam Pract 2007;56:278-282.
2. Farmer ER, Gonin R, Hanna MP. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol 1996;27:528-531.
3. Barnhill RL, Argenyi ZB, From L, et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol 1999;30:513-520.
4. Elder DE, Xu X. The approach to the patient with a difficult melanocytic lesion. Pathology 2004;36:428-434.
5. McBroom HM, Ramsay AD. The clinicopathological meeting. A means of auditing diagnostic performance. Am J Surg Pathol 1993;17:75-80.
6. Johr R, Soyer HP, Argenziano G, Hofmann-Wellenhof R, Scalvenzi M. Dermoscopy: The Essentials. New York, NY: Mosby; 2004.
7. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York, NY: Mosby; 2004.
8. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 5th ed. New York, NY: McGraw-Hill; 2005.
9. Edwards L. Dermatology in Emergency Care. New York, NY: Churchill Livingstone; 1997.
10. Brodell RT, Williams L. Taking the itch out of poison ivy. Postgrad Med 1999;106:69-70.
11. Hall JC. Dermatologic allergy. In: Hall JC, ed. Sauer’s Manual of Skin Diseases. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:82.
12. Braun RP, Gaide O, Skaria AM, Kopf AW, Saurat JH, Marghoob AA. Exclusively benign dermoscopic pattern in a patient with acral melanoma. Arch Dermatol 2007;143:1213-1215.
How do you spell relief for irritable bowel syndrome?
- Little or no diagnostic testing is required to make an accurate diagnosis of irritable bowel syndrome (IBS) in patients younger than 50 without alarm symptoms (C).
- IBS can develop and persist as a consequence of an episode of gastroenteritis (B).
- Tell patients with IBS that it has a physiologic basis and that psychosocial stressors aggravate the already painful and dysfunctional bowel, but do not cause the chronic dysfunction (B).
- Alosetron and tegaserod have proven efficacy, but are available only through limited access programs (A).
- Promising newer therapies for IBS include probiotics and a chloridechannel opener, as well as locally acting, non-absorbable antibiotics for small intestinal bacterial overgrowth-associated IBS (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
“I’ve always had some stomach pain,” says Mary Jane, a 36-year-old patient, whom you are seeing for the first time. “But this is becoming unmanageable.”
You see from Mary Jane’s chart that she has been to your group practice twice over the last few years with complaints of diarrhea that were diagnosed as gastroenteritis. She tells you that after each visit, it got “a little better,” but it “never really went away.”
She also tells you that her stomach bothers her a few days every month, but that it feels a little better after she defecates. She says that she thinks she may be sensitive to certain foods.
“Things are getting worse,” she tells you. “The bloating, pain, and diarrhea have gotten to the point that I can’t go anywhere without worrying where the nearest bathroom is.”
“I’ve already had my gallbladder and appendix removed,” she says, “but I still feel lousy.”
On exam, Mary Jane appears to be in good health. She is afebrile and has a normal abdominal exam, except for very mild diffuse tenderness. She tells you that she has not traveled to any locations where access to clean food or water is suspect. Her stool is heme negative. Urine dip is negative and she is not pregnant.
“Do you know what’s the matter with me?” she asks you. “Or do I need to see a specialist?”
No need for a specialist
Your patient meets the criteria for irritable bowel syndrome (IBS) set by Rome III, an international panel of experts in the field of functional gastrointestinal disorders. She’s had recurrent abdominal pain/discomfort for at least 6 months, and she’s had symptoms at least 3 days a month for the last 3 months (TABLE 1).1
Mary Jane tells you that she is relieved to finally get a diagnosis, having struggled for some time with stomach pain that never really went away. Her experience is not unusual: The wide range of concomitant gastrointestinal and extraintestinal symptoms in IBS patients make the initial diagnosis difficult.2,3 She’s also relieved to learn from you that contrary to popular belief, IBS has a physiologic basis and that psychosocial stressors merely aggravate an already painful and dysfunctional bowel. (See and Irritable bowel syndrome: Not just a functional disorder4-7)
We have a number of treatment options to offer patients like Mary Jane, including alosetron, tegaserod, lubiprostone, selective serotonin reuptake inhibitors (SSRIs), and tricyclic antidepressants, as well as complementary therapies (such as probiotics) and behavioral therapy. But before we review the evidence behind the different options, let’s take a look at the factors that may be at work in IBS, and the things you’ll want to pay special attention to during your assessment.
TABLE 1
Is it IBS? Rome III criteria provide guidance1
|
|
Infection, bacterial overgrowth may play a role
Between 4% and 26% of patients contract IBS for the first time after gastroenteritis.8 Tissue from patients with post-infectious IBS shows chronic mucosal lymphocytosis9,10 associated with enterochromaffin cell hyperplasia.11 Spiller et al12 noted these changes, as well as an increase in gut permeability, for more than 1 year after the resolution of Campylobacter enteritis. Using prednisolone for early intervention suppressed T-cell lymphocyte counts but not IBS symptoms.13
The role of bacterial overgrowth in IBS is controversial. Pimentel et al14 reported that 78% of 202 IBS patients had small intestinal bacterial overgrowth. After treatment, almost half no longer met the Rome criteria for IBS and showed a statistically significant improvement in diarrhea and abdominal pain but not in straining, urgency, or bloating.
A second study from the same group demonstrated that 84% of 111 consecutive IBS patients had small intestinal bacterial overgrowth compared with 20% of healthy controls. Thirty-five percent of the IBS patients treated with neomycin had improved composite scores compared with 11.4% of those receiving placebo.15
Correlation between small intestinal bacterial overgrowth and Rome criteria for IBS has not been replicated by other centers, and other investigators who have looked into this relationship have suggested that small intestinal bacterial overgrowth is not associated with IBS.16,17
Genetics and the environment may also be at work
Familial clustering of IBS is commonly seen.3,18 Several studies have suggested a genetic role but a recently published comprehensive review suggests that the evidence of genetic susceptibility to IBS is modest, if present at all.19
The discovery of physiological differences between IBS patients and control groups suggests that IBS entails significant underlying neuroenteric dysfunction wherein both function and physiology are altered.4,5 The endogenous release of serotonin (5-HT) initiates sensory, secretory, and motor signals within the enteric nervous system by binding to a variety of serotonergic receptors as well as stimulating afferent signals to the spinal cord and brain.4,6,7
Studies by multiple groups using human subjects or human tissue demonstrate physiologic differences between IBS patients and controls which include:5,7
- increased enterochromaffin cell numbers, specifically in post-infectious IBS
- a decrease in mRNA for TPH-1, an enzyme that synthesizes 5-HT
- significant differences in the 5-HT content of enterochromaffin cells
- decreased expression of the serotonin reuptake transporter protein that helps regulate serotonin signaling.
Significant disruption of 5-HT signaling is found in IBS patients compared with controls and more work is underway to better understand the relationship between physiologic dysfunction and symptoms.
Research tells us that the environment also plays a role; specifically, psychological stress is related to IBS symptoms. In one GI referral practice, women with diagnoses of functional disorders had experienced a high frequency of abuse.20
Abuse history is also associated with more severe symptoms, worse daily function, greater psychological distress, and poor health outcome.21 Removal of these stressors provides significant relief to the patient, but IBS still exists in the absence of significant psychosocial pressures.
Few tests (if any) are needed for diagnosis
IBS historically has been a diagnosis of exclusion, but this is no longer the case. You can make the diagnosis with few—if any—diagnostic tests, as long as there are no “red flag” findings or alarm symptoms (TABLE 21,22,23). In particular, surgery is not required to make a diagnosis, nor will it improve a patient’s condition, yet the incidence of abdominal and pelvic surgery in IBS patients is 87% greater, and cholecystectomy three-fold higher, than in the general population.24
While taking a history, you will of course ask about the patient’s altered bowel habits. In addition, though, you will need to:
Ask about personal and family history of inflammatory bowel disease (IBD), colon cancer, celiac disease, pregnancy, recent overseas travel or camping exposure to parasites or contaminated food and water, and history of recent gastroenteritis.25
Ask about alarm symptoms and red flags. Red flags on your examination include any focal positive physical findings, such as peritoneal signs, heme-positive stool, abdominal mass, or pelvic findings. Alarm symptoms that the patient may talk about include things like significant weight loss.
Red flags are based on observational data but have become the accepted standard of practice.1,22,23,26 Extensive testing, including the routine use of blood tests, stool studies, and imaging, however, is not required.22,25 If you have any doubt about history or findings, your diagnostic testing should focus on the issue in question.
When warranted by the presence of alarm symptoms or family history, you may need to schedule a colonoscopy to rule out IBD, tumors, or melanosis coli, which can be caused by excessive use of laxatives. Screening colonoscopy is the standard of care for all patients older than 50, regardless of symptoms.
Key in on food concerns. If specific foods aggravate symptoms, further investigation or a dietary exclusion trial may be helpful; however, even patients with proven lactase deficiency experience little or no bloating after drinking 240 mL milk.1,27 You may want to test for celiac disease if indicated by clinical features such as diarrhea, local prevalence, or family history. Routine testing of celiac disease, however, is not supported by the evidence.28,29
TABLE 2
These red flags and alarm symptoms should prompt further evaluation1,22,23
| PATIENT PRESENTATION |
| Age of onset >50 years Fever Nocturnal symptoms Blood in stools Anemia Weight loss >10% body weight Profuse or large volume of diarrhea Family history of inflammatory bowel disease or cancer |
| PHYSICAL EXAMINATION |
| Fever Fecal blood organomegaly Jaundice Positive physical findings such as peritoneal signs or focal abdominal tenderness |
Many treatment options, limited quality research
Few pharmaceutical compounds have been tested and proven effective in reproducible, high-quality, double-blind, placebo-controlled trials (TABLE 3).1,29-43 In addition, no alternative or complementary therapies have been proven in quality clinical trials to date (TABLE 4).39,44-46,50 Behavioral therapy has shown benefit, but lacks double-blind, placebo-controlled trial data required for a level A strength of recommendation (SOR) (TABLE 5).1,47,48 That said, there are various options that can help patients with IBS—specifically, those who have IBS with diarrhea, IBS with constipation, IBS with mixed bowel habit (where stools are reported as >25% hard or lumpy and >25% loose or watery1), or IBS with unspecified bowel habit.
TABLE 3
Pharmacologic options for IBS
| TREATMENT | CLINICAL EFFICACY* | SOR | COMMENTS |
|---|---|---|---|
| 5-HT3 receptor antagonist (alosetron)1,29,30 | IBS with diarrhea Global symptom relief, improvement in individual symptom measures, improved quality of life scores (NNT=7) | A | Drug available through limited access program |
| 5-HT4 receptor agonist (tegaserod)1,29,31 | IBS with constipation Global symptom relief, improvement in individual symptom measures, improved quality of life scores (NNT=14) | A | Marketing suspended on March 30, 2007;32 limited temporary access program makes individual symptom measures, improved drug available through FDA33 |
| Antibiotics (neomycin, rifaximin)34 | IBS/IBS with diarrhea Global symptom relief, improvement in bowel habits for IBS with diarrhea | B | May benefit a subset of IBS patients with small intestinal bacterial overgrowth. Other research groups have not replicated results to date in IBS patients. Rifaximin produces most durable results |
| Anticholinergics/Calcium channel blockers (hyoscyamine, dicyclomine, mebeverine, otilonium bromide, pinaverium bromide)35,36 | IBS Improvement in abdominal pain (NNT=4–15) | B | Long history of use in IBS patients; however, little credible evidence to support use or efficacy in relief of global IBS symptoms. Best evidence with calcium channel blockers and anticholinergics comes with agents not available in US: mebeverine, otilonium bromide, and pinaverium bromide |
| Bulking agents (ispaghula husk, polycarbophil)35,36 | IBS with constipation Improvement in bowel habits (NNT=2.2–3.5) | B | Improvement in bowel habits, but no significant difference vs placebo for other measures; may aggravate bloating and abdominal pain |
| Chloride-Channel receptor agonist, ClC2 (lubiprostone)37 | IBS with constipation Global symptom relief, improvement in individual symptom measures | B | Indicated for chronic constipation; pending FDA review of data for IBS with constipation indication |
| Loperamide1,38 | IBS with diarrhea Improvement in bowel habits (NNT=3–5) | B | Reduction in diarrhea, but no better than placebo in global relief of symptoms or pain |
| Osmotic Laxatives (polyethylene glycol, lactulose)1,39,40 | IBS with constipation Improvement in bowel habits (NNT=2–4) | B | Lacks randomized controlled trials in IBS. Improvement in bowel habits, but no significant difference vs placebo for other measures; may aggravate bloating and abdominal pain |
| SSRIs (citalopram, fluoxetine, paroxetine)35,41-43 | IBS/IBS with constipation Improvement in abdominal pain and quality of life scores (NNT=3–6) | B | Limited and inconclusive data; diarrhea common side effect on beginning therapy. Start with low dose, increase as needed; may be useful for IBS with constipation patients, but it’s uncertain if effect is on the enteric nervous system, central nervous system, or both |
| Tricyclic antidepressants (TCAs)35,41 | IBS/IBS with diarrhea Improvement in abdominal pain (NNT=3) | B | May increase constipation, does not produce global relief of symptoms, and patients experience poor tolerability (effect on gut function occurs predominantly with lower doses). Hypothesized to modify central- enteric nervous system communication |
| * We did not calculate NNT endpoints on studies of poor quality, those with a small number of patients, or in cases where results were not reproducible in a consistent fashion or were not published in manuscript form. | |||
| IBS, irritable bowel syndrome; SOR, strength of recommendation; NNT, number needed to treat; FDA, Food and Drug Administration; SSRI, selective serotonin reuptake inhibitor. | |||
TABLE 4
Complementary and alternative therapies for IBS
| TREATMENT | CLINICAL EFFICACY | SOR | COMMENTS |
|---|---|---|---|
| Acupuncture44 | No proven benefit | C | Poor-quality trial with heterogeneous interventions, controls, and outcomes measured |
| Chinese herbal therapy45 | Reported improvement in global symptoms and pain | C | Very few high-quality clinical trials. Mixtures vary, content unknown, and some mixtures can be toxic. Unknown which herbs might produce benefit |
| Herbal Therapy (Curcuma xanthorrhiza, Fumaria officinalis)45 | No proven benefit | C | Tested in quality clinical trial; no efficacy over placebo |
| Peppermint Oil (colpermin)39, 50 | Improvement in abdominal pain | B | Lacks data on global symptom improvement; mechanism of action is similar to that of calcium channel blockers |
| Probiotics (Bifidobacterium infantis, Lactobacillus, B animalis)46 | Global symptom relief and improvement in individual symptom measures | B | Preliminary trials encouraging, but differences in trial design, probiotic dose, strain, as well as unpredictable symptom response, has not yielded consistent evidence. No quality of life improvement seen in early studies |
| SOR, strength of recommendation | |||
TABLE 5
Hypnotherapy and psychotherapy for IBS
| TREATMENT | CLINICAL EFFICACY | SOR | COMMENTS |
|---|---|---|---|
| Hypnotherapy47 | Global symptom relief, improvement in individual symptom scores | B | Trials, though many, were of poor quality, but did show significant benefit. A large, randomized placebo-controlled trial is needed to demonstrate benefit |
| Psychotherapy (Cognitive behavioral therapy, biofeedback)1,48 | Global symptom relief, improvement in individual symptom scores (NNT=1–2) | B | Significant reduction in symptoms, but did not eliminate them. To maximize the likelihood of success, biofeedback techniques should be administered by a trained professional. Recommended as part of an overall treatment plan |
| SOR, strength of recommendation; NNT, number needed to treat. | |||
IBS with diarrhea
Options all have “but” clauses
Alosetron, the 5-HT3 receptor antagonist, has demonstrated efficacy in women (specifically) with IBS with diarrhea and has a grade A treatment recommendation from the American College of Gastroenterology.29,30 Given the concerns about severe constipation and ischemic colitis, alosetron was withdrawn from the market in November 2000 but was reintroduced in June 2002 under a limited-use program for patients with severe IBS with diarrhea, in whom standard therapy has failed (SOR: A). Research has since shown that IBS patients have a greater risk for ischemic colitis than the general population.49
Loperamide, a μ-opioid receptor agonist, which does not cross the blood-brain barrier, can be started at bedtime or in the morning at 2 mg and slowly titrated daily to effect. The effect, however, is limited to the bowel habits (SOR: B).38
Anticholinergics can be used for abdominal pain or discomfort, particularly in patients with IBS with diarrhea. Despite their lengthy history and broad use in IBS populations, limited evidence supports their efficacy, and many are not available in the US (SOR: B).35,36
Tricyclic antidepressants (TCAs) exert their effects by blocking the muscarinic receptors. TCAs can provide relief to patients with severe or refractory pain but perceived social stigma associated with taking antidepressants can be a barrier to this therapeutic approach (SOR: B).36,41
IBS with constipation
Laxatives, lubiprostone are options
Osmotic laxatives such as polyethylene glycol or lactulose can improve constipation. Despite widespread over-the-counter and prescription use, however, evidence is lacking for their efficacy and tolerability in IBS (SOR: B).39
Lubiprostone, a ClC2 chloride-channel opener, improved individual symptoms scores in 50 patients with IBS with constipation compared with placebo in a small phase II dose-ranging study (SOR: B).37
Tegaserod, a partial 5-HT4 receptor agonist, facilitates neurotransmission in the gut that is involved in motility and secretion and produces global symptom relief.31 The American College of Gastroenterology gave tegaserod a grade A recommendation for the treatment of women with IBS with constipation.29
On March 30, 2007, Novartis Pharmaceuticals agreed to stop selling tegaserod maleate (Zelnorm) because a safety analysis found a higher chance of heart attack, stroke, and worsening chest pain that could become a heart attack in patients treated with Zelnorm, compared with those receiving placebo.32 In July 2007, the FDA announced that it would permit restricted use of the drug in patients who meet strict criteria, have no known or pre-existing heart problems, and who are in critical need of the drug.33
Polycarbophil and ispaghula husk are the only fibers to have demonstrated any significant benefit in clinical trials for constipation,35 but may make pain and bloating worse (SOR: B).
SSRIS and low-dose benzodiazepines are an option for patients who have coexisting psychological illness.41 There is limited evidence for SSRI treatment (citalopram, paroxetine), either alone or in combination with additional treatments (SOR: B).35,41-43
IBS with mixed/unspecified bowel habit
Consider therapy
Cognitive behavioral therapy or other standard psychotherapy may be beneficial in many IBS patients (SOR: B).1,48
Peppermint oil for IBS is efficacious in recurrent abdominal pain compared with placebo and anticholinergic agents (SOR: B).39,50
Hypnosis has had a therapeutic impact on patients with IBS, even those whose conditions were refractory to other forms of therapy (SOR: B).47
Certain probiotic therapies have shown improvement in global symptoms and may prove to be promising therapeutic agents (SOR: B).46
Acupuncture has shown no proven efficacy in IBS (SOR: C).44
Some Chinese herbal medicines may improve the symptoms of IBS. Positive findings from trials should be interpreted with caution, though, due to inadequate methodology, small sample sizes, and lack of confirming data. In addition, herbal remedies may contain unknown substances that can pose serious risk for adverse events and drug interactions (SOR: C).45
Antibiotics for IBS patients with small intestinal bacterial overgrowth can provide some relief, according to Pimentel and colleagues,14 but other groups have not been able to duplicate these findings. Rifaximin in IBS and IBS-like symptoms has shown a sustained benefit (SOR: B).34
Exercise or dietary therapies have no proven benefit in otherwise healthy IBS patients (SOR: C).1
Set realistic goals, address patient fears
When caring for a patient with IBS, it’s important to set and discuss realistic goals. Be sure to address patient concerns, as well. IBS patients are frequently frustrated by a lack of diagnostic findings and may worry about being labeled as having a psychological disorder rather than having a true GI abnormality. This concern, especially if the patient feels that the physician is not adequately addressing her (or his) symptoms, may exacerbate the already troublesome IBS symptoms.
Assure your patient that comorbid psychological conditions do not cause symptoms, but can contribute to pain and bowel dysfunction. Consider the possibility of behavioral therapy, if indicated by patient history.
Advise the patient that the initial approach you are taking to alleviate her (or his) symptoms may provide significant improvement. Manage expectations appropriately and be open to discussion about what the patient may need to alleviate both physiologic and psychologic stressors that perpetuate symptoms. Family practitioners, not specialists, are ideally suited to address the patients’ needs and expectations as they are the ones who know the patients’ histories, personalities, and families best.1 Tell the patient that rather than hoping for a cure, the goal for the both the physician and patient should be to achieve symptom relief.
Mary Jane finally gets some relief
After speaking with Mary Jane and doing a thorough exam, you reassure her that her symptoms meet the criteria for the diagnosis of IBS, and that research indicates that she can safely be treated for the disorder.
You tell your patient that you’d like to begin treatment by putting her on loperamide—2 capsules at onset of diarrhea and 1 capsule after each diarrheal episode to a maximum of 8 capsules a day and a low-dose amitriptyline (25 mg at bed-time). You explain that the tricyclic should provide her with some relief by modifying the way the nervous system of the intestine communicates with the brain.
Two months later, during a follow-up visit, Mary Jane tells you that the medications are providing her with relief, but when she feels particularly stressed at home, she notices that her symptoms flare up. You and she discuss the benefits of cognitive behavioral therapy, and you provide her with the names of some therapists in the area. You suggest that she consult her health plan and make some phone calls to identify a provider that she feels comfortable with.
You advise her to schedule another follow-up visit in 3 months so that you can see how the therapy is working. You also tell her to call any time she experiences significant pain or any symptoms that are persistent or worrisome to her.
Correspondence
Neil T. Moynihan, MD, Johnson Memorial Hospital, 201 Chestnut Hill Road, Stafford Springs, CT 06076; [email protected]
1. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480-1491.
2. Drossman DA, Li Z, Andruzzi E, et al. US householder survey of functional gastrointestinal disorders: prevalence, sociodemography, and health impact. Dig Dis Sci 1993;38:1569-1580.
3. Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut 1986;27:37-40.
4. Gershon MD. Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 2004;20(suppl):3-14.
5. Mawe GM, Coates M, Moses PL. Review article: intestinal serotonin signaling in irritable bowel syndrome. Alimentary Pharmacol Ther 2006;23:1067-1076.
6. Tonini M. 5-Hydroxytryptamine effects in the gut: the 3, 4, and 7 receptors. Neurogastroenterol Motil 2005;17:637-642.
7. Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol 2005;39(suppl):184-193.
8. Parry S, Forgacs I. Intestinal infection and irritable bowel syndrome. Eur J Gastroenterol Hepatol 2005;17:5-9.
9. Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut 1999;44:400-406.
10. Wang L-H, Fang X-C, Pan G-Z. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut 2004;53:1096-1101.
11. Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol 2003;98:1578-1583.
12. Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000;47:804-811.
13. Dunlop SP, Jenkins D, Neal KR, Naesdal J, Borgaonker M, Collins SM. Randomized, double-blind, placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Aliment Pharmacol Ther 2003;18:77-84.
14. Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 2000;95:3503-3506.
15. Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: a double blind, randomized controlled study. Am J Gastroenterol 2003;98:412-419.
16. Posserud I, Stotzer ES, Bjornsson H, Abrahamsson M, Simren M. Altered counts of small bowel bacteria in patients with irritable bowel syndrome (IBS). Gastroenterology 2006;130:A739.-
17. Ruff KC, Saito-Loftus YA, Llocke GR, Harmsen WS, Zinsmeister AS, Talley NJ. Failure to detect association with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO). Am J Gastroenterol 2006;101(suppl 2):S488.-
18. Bellentani S, Baldoni P, Petrella S, et al. A simple score for the identification of patients at high risk of organic diseases of the colon in the family doctor consulting room: the local IBS Study Group. Fam Pract 1990;7:307-312.
19. Saito YA, Petersen GM, Locke R, III, Talley NJ. The genetics of irritable bowel syndrome. Clin Gastroenterol Hepatol 2005;3:1057-1065.
20. Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med 1990;113:828-833.
21. Drossman DA, Li Z, Leserman J, Toomey TC, Hu Y. Health status by gastrointestinal diagnosis and abuse history. Gastroenterology 1996;110:999-1007.
22. Malagelada JR. A symptom based approach to making a positive diagnosis of irritable bowel syndrome with constipation. Int J Clin Pract 2006;60:57-63.
23. Locke GR, 3rd. Natural history of irritable bowel syndrome and durability of diagnosis. Rev Gastroenterol Disord 2003;3(suppl):S12-S17.
24. Cole JA, Yeaw JM, Cutone JA, et al. The incidence of abdominal and pelvic surgery among patients with irritable bowel syndrome. Dig Dis Sci 2005;50:2268-2275.
25. Cash BD, Chey WD. Diagnosis of irritable bowel syndrome. Gastroenterol Clin N Am 2005;34:205-220.
26. Owens DM, Nelson DK, Talley NJ. The irritable bowel syndrome: long-term prognosis and the physician-patient interaction. Ann Intern Med 1995;122:107-112.
27. Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactosehydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med 1995;333:1-4.
28. Spiegel BM, Derosa VP, Gralnek IM, Wang V, Dulai GS. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: a cost-effective analysis. Gastroenterology 2004;126:1721-1732.
29. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol 2002;97(suppl 11):S1-S5.
30. Cremonini F, Delgado A, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil 2003;15:79-86.
31. Evans BW, Clark WK, Moore DJ, Whorwell PJ. Tegaserod for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2004;(1):CD003960.-
32. US Food and Drug Administration FDA Public Health Advisory: Tegaserod maleate (marketed as Zelnorm). March 30, 2007. Available at: www.fda.gov/CDER/Drug/advisory/tegaserod.htm. Accessed January 2, 2008.
33. US Food and Drug Administration FDA Permits restricted use of Zelnorm for qualifying patients. July 27, 2007. Available at: www.fda.gov/bbs/topics/NEWS/2007/NEW01673.html. Accessed January 2, 2008.
34. Frissora CL, Cash BD. Review article: the role of antibiotics vs. conventional pharmacotherapy in treating symptoms of irritable bowel syndrome. Aliment Pharmacol Ther 2007;25:1271-1281.
35. Quartero AO, Meineche-Schmidt V, Muris J, Rubin G, de Wit N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2005;(2):CD003460.-
36. Jailwala J, Impereiale T, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized controlled trials. Ann Intern Med 2000;133:136-147.
37. Johanson JF, Panas R, Holland P, Ueno R. A dose ranging, double-blind, placebo-controlled study of lubiprostone in subjects with irritable bowel syndrome and constipation (c-IBS). Gastroenterology 2006;130(suppl 2):A25.-
38. Amery W, Duyck F, Polak J, van den Bouwhuysen G. A multicentre double-blind study in acute diarrhoea comparing loperamide (R 18553) with two common antidiarrhoeal agents and a placebo. Curr Ther Res Clin Exp 1975;17:263-270.
39. Tack J, Fried M, Houghton LA, Spicak J, Fisher G. Systematic review: the efficacy of treatments for irritable bowel syndrome—a European perspective. Aliment Pharmacol Ther 2006;24:183-205.
40. Bleser SD. Chronic constipation: let symptom and severity direct treatment. J Fam Pract 2006;55:587-593.
41. Wald A. Psychotropic agents in irritable bowel syndrome. J Clin Gastroenterol 2002;35(suppl):S53-S57.
42. Tack J, Broekaert D, Fischler B, van Oudenhove , L, Gevers A, Janssens J. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006;55:1095-1103.
43. Tabas G, Beaves M, Wang J, Friday P, Mardini H, Arnold G. Paroxetine to treat irritable bowel syndrome not responding to high-fiber diet: a double-blind, placebo-controlled trial. Am J Gastroenterol 2004 May;99:914-920.
44. Lim B, Manheimer E, Lao L, Ziea E, Wisniewski J, Liu J, Berman B. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2006;(4):CD005111.-
45. Liu JP, Yang M, Liu YX, Wei ML, Grimsgaard S. Herbal medicines for treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2006;(1):CD004116.-
46. Quigley EMM, Flourie B. Probiotics and irritable bowel syndrome: a rational for their use and an assessment of the evidence to date. Neurogastroenterol Motil 2007;19:166-172.
47. Wilson S, Maddison T, Robers L, Greenfield S, Singh S. On behalf of the Birmingham IBS research group. Systematic review: The effectiveness of hypnotherapy in the management of irritable bowel syndrome. Aliment Pharmacol Ther 2006;24:769-780.
48. Lackner JM, Mesmer C, Morley S, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol 2004;72:1100-1113.
49. Cole JA, Cook SF, Sands BE, Ajene AN, Miller DP, Walker AM. Occurrence of colon ischemia in relation to irritable bowel syndrome. Am J Gastroenterol 2004;99:486-491.
50. Grigoleit HG, Grigoleit P. Peppermint oil in irritable bowel syndrome. Phytomedicine 2005;12:601-606.
- Little or no diagnostic testing is required to make an accurate diagnosis of irritable bowel syndrome (IBS) in patients younger than 50 without alarm symptoms (C).
- IBS can develop and persist as a consequence of an episode of gastroenteritis (B).
- Tell patients with IBS that it has a physiologic basis and that psychosocial stressors aggravate the already painful and dysfunctional bowel, but do not cause the chronic dysfunction (B).
- Alosetron and tegaserod have proven efficacy, but are available only through limited access programs (A).
- Promising newer therapies for IBS include probiotics and a chloridechannel opener, as well as locally acting, non-absorbable antibiotics for small intestinal bacterial overgrowth-associated IBS (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
“I’ve always had some stomach pain,” says Mary Jane, a 36-year-old patient, whom you are seeing for the first time. “But this is becoming unmanageable.”
You see from Mary Jane’s chart that she has been to your group practice twice over the last few years with complaints of diarrhea that were diagnosed as gastroenteritis. She tells you that after each visit, it got “a little better,” but it “never really went away.”
She also tells you that her stomach bothers her a few days every month, but that it feels a little better after she defecates. She says that she thinks she may be sensitive to certain foods.
“Things are getting worse,” she tells you. “The bloating, pain, and diarrhea have gotten to the point that I can’t go anywhere without worrying where the nearest bathroom is.”
“I’ve already had my gallbladder and appendix removed,” she says, “but I still feel lousy.”
On exam, Mary Jane appears to be in good health. She is afebrile and has a normal abdominal exam, except for very mild diffuse tenderness. She tells you that she has not traveled to any locations where access to clean food or water is suspect. Her stool is heme negative. Urine dip is negative and she is not pregnant.
“Do you know what’s the matter with me?” she asks you. “Or do I need to see a specialist?”
No need for a specialist
Your patient meets the criteria for irritable bowel syndrome (IBS) set by Rome III, an international panel of experts in the field of functional gastrointestinal disorders. She’s had recurrent abdominal pain/discomfort for at least 6 months, and she’s had symptoms at least 3 days a month for the last 3 months (TABLE 1).1
Mary Jane tells you that she is relieved to finally get a diagnosis, having struggled for some time with stomach pain that never really went away. Her experience is not unusual: The wide range of concomitant gastrointestinal and extraintestinal symptoms in IBS patients make the initial diagnosis difficult.2,3 She’s also relieved to learn from you that contrary to popular belief, IBS has a physiologic basis and that psychosocial stressors merely aggravate an already painful and dysfunctional bowel. (See and Irritable bowel syndrome: Not just a functional disorder4-7)
We have a number of treatment options to offer patients like Mary Jane, including alosetron, tegaserod, lubiprostone, selective serotonin reuptake inhibitors (SSRIs), and tricyclic antidepressants, as well as complementary therapies (such as probiotics) and behavioral therapy. But before we review the evidence behind the different options, let’s take a look at the factors that may be at work in IBS, and the things you’ll want to pay special attention to during your assessment.
TABLE 1
Is it IBS? Rome III criteria provide guidance1
|
|
Infection, bacterial overgrowth may play a role
Between 4% and 26% of patients contract IBS for the first time after gastroenteritis.8 Tissue from patients with post-infectious IBS shows chronic mucosal lymphocytosis9,10 associated with enterochromaffin cell hyperplasia.11 Spiller et al12 noted these changes, as well as an increase in gut permeability, for more than 1 year after the resolution of Campylobacter enteritis. Using prednisolone for early intervention suppressed T-cell lymphocyte counts but not IBS symptoms.13
The role of bacterial overgrowth in IBS is controversial. Pimentel et al14 reported that 78% of 202 IBS patients had small intestinal bacterial overgrowth. After treatment, almost half no longer met the Rome criteria for IBS and showed a statistically significant improvement in diarrhea and abdominal pain but not in straining, urgency, or bloating.
A second study from the same group demonstrated that 84% of 111 consecutive IBS patients had small intestinal bacterial overgrowth compared with 20% of healthy controls. Thirty-five percent of the IBS patients treated with neomycin had improved composite scores compared with 11.4% of those receiving placebo.15
Correlation between small intestinal bacterial overgrowth and Rome criteria for IBS has not been replicated by other centers, and other investigators who have looked into this relationship have suggested that small intestinal bacterial overgrowth is not associated with IBS.16,17
Genetics and the environment may also be at work
Familial clustering of IBS is commonly seen.3,18 Several studies have suggested a genetic role but a recently published comprehensive review suggests that the evidence of genetic susceptibility to IBS is modest, if present at all.19
The discovery of physiological differences between IBS patients and control groups suggests that IBS entails significant underlying neuroenteric dysfunction wherein both function and physiology are altered.4,5 The endogenous release of serotonin (5-HT) initiates sensory, secretory, and motor signals within the enteric nervous system by binding to a variety of serotonergic receptors as well as stimulating afferent signals to the spinal cord and brain.4,6,7
Studies by multiple groups using human subjects or human tissue demonstrate physiologic differences between IBS patients and controls which include:5,7
- increased enterochromaffin cell numbers, specifically in post-infectious IBS
- a decrease in mRNA for TPH-1, an enzyme that synthesizes 5-HT
- significant differences in the 5-HT content of enterochromaffin cells
- decreased expression of the serotonin reuptake transporter protein that helps regulate serotonin signaling.
Significant disruption of 5-HT signaling is found in IBS patients compared with controls and more work is underway to better understand the relationship between physiologic dysfunction and symptoms.
Research tells us that the environment also plays a role; specifically, psychological stress is related to IBS symptoms. In one GI referral practice, women with diagnoses of functional disorders had experienced a high frequency of abuse.20
Abuse history is also associated with more severe symptoms, worse daily function, greater psychological distress, and poor health outcome.21 Removal of these stressors provides significant relief to the patient, but IBS still exists in the absence of significant psychosocial pressures.
Few tests (if any) are needed for diagnosis
IBS historically has been a diagnosis of exclusion, but this is no longer the case. You can make the diagnosis with few—if any—diagnostic tests, as long as there are no “red flag” findings or alarm symptoms (TABLE 21,22,23). In particular, surgery is not required to make a diagnosis, nor will it improve a patient’s condition, yet the incidence of abdominal and pelvic surgery in IBS patients is 87% greater, and cholecystectomy three-fold higher, than in the general population.24
While taking a history, you will of course ask about the patient’s altered bowel habits. In addition, though, you will need to:
Ask about personal and family history of inflammatory bowel disease (IBD), colon cancer, celiac disease, pregnancy, recent overseas travel or camping exposure to parasites or contaminated food and water, and history of recent gastroenteritis.25
Ask about alarm symptoms and red flags. Red flags on your examination include any focal positive physical findings, such as peritoneal signs, heme-positive stool, abdominal mass, or pelvic findings. Alarm symptoms that the patient may talk about include things like significant weight loss.
Red flags are based on observational data but have become the accepted standard of practice.1,22,23,26 Extensive testing, including the routine use of blood tests, stool studies, and imaging, however, is not required.22,25 If you have any doubt about history or findings, your diagnostic testing should focus on the issue in question.
When warranted by the presence of alarm symptoms or family history, you may need to schedule a colonoscopy to rule out IBD, tumors, or melanosis coli, which can be caused by excessive use of laxatives. Screening colonoscopy is the standard of care for all patients older than 50, regardless of symptoms.
Key in on food concerns. If specific foods aggravate symptoms, further investigation or a dietary exclusion trial may be helpful; however, even patients with proven lactase deficiency experience little or no bloating after drinking 240 mL milk.1,27 You may want to test for celiac disease if indicated by clinical features such as diarrhea, local prevalence, or family history. Routine testing of celiac disease, however, is not supported by the evidence.28,29
TABLE 2
These red flags and alarm symptoms should prompt further evaluation1,22,23
| PATIENT PRESENTATION |
| Age of onset >50 years Fever Nocturnal symptoms Blood in stools Anemia Weight loss >10% body weight Profuse or large volume of diarrhea Family history of inflammatory bowel disease or cancer |
| PHYSICAL EXAMINATION |
| Fever Fecal blood organomegaly Jaundice Positive physical findings such as peritoneal signs or focal abdominal tenderness |
Many treatment options, limited quality research
Few pharmaceutical compounds have been tested and proven effective in reproducible, high-quality, double-blind, placebo-controlled trials (TABLE 3).1,29-43 In addition, no alternative or complementary therapies have been proven in quality clinical trials to date (TABLE 4).39,44-46,50 Behavioral therapy has shown benefit, but lacks double-blind, placebo-controlled trial data required for a level A strength of recommendation (SOR) (TABLE 5).1,47,48 That said, there are various options that can help patients with IBS—specifically, those who have IBS with diarrhea, IBS with constipation, IBS with mixed bowel habit (where stools are reported as >25% hard or lumpy and >25% loose or watery1), or IBS with unspecified bowel habit.
TABLE 3
Pharmacologic options for IBS
| TREATMENT | CLINICAL EFFICACY* | SOR | COMMENTS |
|---|---|---|---|
| 5-HT3 receptor antagonist (alosetron)1,29,30 | IBS with diarrhea Global symptom relief, improvement in individual symptom measures, improved quality of life scores (NNT=7) | A | Drug available through limited access program |
| 5-HT4 receptor agonist (tegaserod)1,29,31 | IBS with constipation Global symptom relief, improvement in individual symptom measures, improved quality of life scores (NNT=14) | A | Marketing suspended on March 30, 2007;32 limited temporary access program makes individual symptom measures, improved drug available through FDA33 |
| Antibiotics (neomycin, rifaximin)34 | IBS/IBS with diarrhea Global symptom relief, improvement in bowel habits for IBS with diarrhea | B | May benefit a subset of IBS patients with small intestinal bacterial overgrowth. Other research groups have not replicated results to date in IBS patients. Rifaximin produces most durable results |
| Anticholinergics/Calcium channel blockers (hyoscyamine, dicyclomine, mebeverine, otilonium bromide, pinaverium bromide)35,36 | IBS Improvement in abdominal pain (NNT=4–15) | B | Long history of use in IBS patients; however, little credible evidence to support use or efficacy in relief of global IBS symptoms. Best evidence with calcium channel blockers and anticholinergics comes with agents not available in US: mebeverine, otilonium bromide, and pinaverium bromide |
| Bulking agents (ispaghula husk, polycarbophil)35,36 | IBS with constipation Improvement in bowel habits (NNT=2.2–3.5) | B | Improvement in bowel habits, but no significant difference vs placebo for other measures; may aggravate bloating and abdominal pain |
| Chloride-Channel receptor agonist, ClC2 (lubiprostone)37 | IBS with constipation Global symptom relief, improvement in individual symptom measures | B | Indicated for chronic constipation; pending FDA review of data for IBS with constipation indication |
| Loperamide1,38 | IBS with diarrhea Improvement in bowel habits (NNT=3–5) | B | Reduction in diarrhea, but no better than placebo in global relief of symptoms or pain |
| Osmotic Laxatives (polyethylene glycol, lactulose)1,39,40 | IBS with constipation Improvement in bowel habits (NNT=2–4) | B | Lacks randomized controlled trials in IBS. Improvement in bowel habits, but no significant difference vs placebo for other measures; may aggravate bloating and abdominal pain |
| SSRIs (citalopram, fluoxetine, paroxetine)35,41-43 | IBS/IBS with constipation Improvement in abdominal pain and quality of life scores (NNT=3–6) | B | Limited and inconclusive data; diarrhea common side effect on beginning therapy. Start with low dose, increase as needed; may be useful for IBS with constipation patients, but it’s uncertain if effect is on the enteric nervous system, central nervous system, or both |
| Tricyclic antidepressants (TCAs)35,41 | IBS/IBS with diarrhea Improvement in abdominal pain (NNT=3) | B | May increase constipation, does not produce global relief of symptoms, and patients experience poor tolerability (effect on gut function occurs predominantly with lower doses). Hypothesized to modify central- enteric nervous system communication |
| * We did not calculate NNT endpoints on studies of poor quality, those with a small number of patients, or in cases where results were not reproducible in a consistent fashion or were not published in manuscript form. | |||
| IBS, irritable bowel syndrome; SOR, strength of recommendation; NNT, number needed to treat; FDA, Food and Drug Administration; SSRI, selective serotonin reuptake inhibitor. | |||
TABLE 4
Complementary and alternative therapies for IBS
| TREATMENT | CLINICAL EFFICACY | SOR | COMMENTS |
|---|---|---|---|
| Acupuncture44 | No proven benefit | C | Poor-quality trial with heterogeneous interventions, controls, and outcomes measured |
| Chinese herbal therapy45 | Reported improvement in global symptoms and pain | C | Very few high-quality clinical trials. Mixtures vary, content unknown, and some mixtures can be toxic. Unknown which herbs might produce benefit |
| Herbal Therapy (Curcuma xanthorrhiza, Fumaria officinalis)45 | No proven benefit | C | Tested in quality clinical trial; no efficacy over placebo |
| Peppermint Oil (colpermin)39, 50 | Improvement in abdominal pain | B | Lacks data on global symptom improvement; mechanism of action is similar to that of calcium channel blockers |
| Probiotics (Bifidobacterium infantis, Lactobacillus, B animalis)46 | Global symptom relief and improvement in individual symptom measures | B | Preliminary trials encouraging, but differences in trial design, probiotic dose, strain, as well as unpredictable symptom response, has not yielded consistent evidence. No quality of life improvement seen in early studies |
| SOR, strength of recommendation | |||
TABLE 5
Hypnotherapy and psychotherapy for IBS
| TREATMENT | CLINICAL EFFICACY | SOR | COMMENTS |
|---|---|---|---|
| Hypnotherapy47 | Global symptom relief, improvement in individual symptom scores | B | Trials, though many, were of poor quality, but did show significant benefit. A large, randomized placebo-controlled trial is needed to demonstrate benefit |
| Psychotherapy (Cognitive behavioral therapy, biofeedback)1,48 | Global symptom relief, improvement in individual symptom scores (NNT=1–2) | B | Significant reduction in symptoms, but did not eliminate them. To maximize the likelihood of success, biofeedback techniques should be administered by a trained professional. Recommended as part of an overall treatment plan |
| SOR, strength of recommendation; NNT, number needed to treat. | |||
IBS with diarrhea
Options all have “but” clauses
Alosetron, the 5-HT3 receptor antagonist, has demonstrated efficacy in women (specifically) with IBS with diarrhea and has a grade A treatment recommendation from the American College of Gastroenterology.29,30 Given the concerns about severe constipation and ischemic colitis, alosetron was withdrawn from the market in November 2000 but was reintroduced in June 2002 under a limited-use program for patients with severe IBS with diarrhea, in whom standard therapy has failed (SOR: A). Research has since shown that IBS patients have a greater risk for ischemic colitis than the general population.49
Loperamide, a μ-opioid receptor agonist, which does not cross the blood-brain barrier, can be started at bedtime or in the morning at 2 mg and slowly titrated daily to effect. The effect, however, is limited to the bowel habits (SOR: B).38
Anticholinergics can be used for abdominal pain or discomfort, particularly in patients with IBS with diarrhea. Despite their lengthy history and broad use in IBS populations, limited evidence supports their efficacy, and many are not available in the US (SOR: B).35,36
Tricyclic antidepressants (TCAs) exert their effects by blocking the muscarinic receptors. TCAs can provide relief to patients with severe or refractory pain but perceived social stigma associated with taking antidepressants can be a barrier to this therapeutic approach (SOR: B).36,41
IBS with constipation
Laxatives, lubiprostone are options
Osmotic laxatives such as polyethylene glycol or lactulose can improve constipation. Despite widespread over-the-counter and prescription use, however, evidence is lacking for their efficacy and tolerability in IBS (SOR: B).39
Lubiprostone, a ClC2 chloride-channel opener, improved individual symptoms scores in 50 patients with IBS with constipation compared with placebo in a small phase II dose-ranging study (SOR: B).37
Tegaserod, a partial 5-HT4 receptor agonist, facilitates neurotransmission in the gut that is involved in motility and secretion and produces global symptom relief.31 The American College of Gastroenterology gave tegaserod a grade A recommendation for the treatment of women with IBS with constipation.29
On March 30, 2007, Novartis Pharmaceuticals agreed to stop selling tegaserod maleate (Zelnorm) because a safety analysis found a higher chance of heart attack, stroke, and worsening chest pain that could become a heart attack in patients treated with Zelnorm, compared with those receiving placebo.32 In July 2007, the FDA announced that it would permit restricted use of the drug in patients who meet strict criteria, have no known or pre-existing heart problems, and who are in critical need of the drug.33
Polycarbophil and ispaghula husk are the only fibers to have demonstrated any significant benefit in clinical trials for constipation,35 but may make pain and bloating worse (SOR: B).
SSRIS and low-dose benzodiazepines are an option for patients who have coexisting psychological illness.41 There is limited evidence for SSRI treatment (citalopram, paroxetine), either alone or in combination with additional treatments (SOR: B).35,41-43
IBS with mixed/unspecified bowel habit
Consider therapy
Cognitive behavioral therapy or other standard psychotherapy may be beneficial in many IBS patients (SOR: B).1,48
Peppermint oil for IBS is efficacious in recurrent abdominal pain compared with placebo and anticholinergic agents (SOR: B).39,50
Hypnosis has had a therapeutic impact on patients with IBS, even those whose conditions were refractory to other forms of therapy (SOR: B).47
Certain probiotic therapies have shown improvement in global symptoms and may prove to be promising therapeutic agents (SOR: B).46
Acupuncture has shown no proven efficacy in IBS (SOR: C).44
Some Chinese herbal medicines may improve the symptoms of IBS. Positive findings from trials should be interpreted with caution, though, due to inadequate methodology, small sample sizes, and lack of confirming data. In addition, herbal remedies may contain unknown substances that can pose serious risk for adverse events and drug interactions (SOR: C).45
Antibiotics for IBS patients with small intestinal bacterial overgrowth can provide some relief, according to Pimentel and colleagues,14 but other groups have not been able to duplicate these findings. Rifaximin in IBS and IBS-like symptoms has shown a sustained benefit (SOR: B).34
Exercise or dietary therapies have no proven benefit in otherwise healthy IBS patients (SOR: C).1
Set realistic goals, address patient fears
When caring for a patient with IBS, it’s important to set and discuss realistic goals. Be sure to address patient concerns, as well. IBS patients are frequently frustrated by a lack of diagnostic findings and may worry about being labeled as having a psychological disorder rather than having a true GI abnormality. This concern, especially if the patient feels that the physician is not adequately addressing her (or his) symptoms, may exacerbate the already troublesome IBS symptoms.
Assure your patient that comorbid psychological conditions do not cause symptoms, but can contribute to pain and bowel dysfunction. Consider the possibility of behavioral therapy, if indicated by patient history.
Advise the patient that the initial approach you are taking to alleviate her (or his) symptoms may provide significant improvement. Manage expectations appropriately and be open to discussion about what the patient may need to alleviate both physiologic and psychologic stressors that perpetuate symptoms. Family practitioners, not specialists, are ideally suited to address the patients’ needs and expectations as they are the ones who know the patients’ histories, personalities, and families best.1 Tell the patient that rather than hoping for a cure, the goal for the both the physician and patient should be to achieve symptom relief.
Mary Jane finally gets some relief
After speaking with Mary Jane and doing a thorough exam, you reassure her that her symptoms meet the criteria for the diagnosis of IBS, and that research indicates that she can safely be treated for the disorder.
You tell your patient that you’d like to begin treatment by putting her on loperamide—2 capsules at onset of diarrhea and 1 capsule after each diarrheal episode to a maximum of 8 capsules a day and a low-dose amitriptyline (25 mg at bed-time). You explain that the tricyclic should provide her with some relief by modifying the way the nervous system of the intestine communicates with the brain.
Two months later, during a follow-up visit, Mary Jane tells you that the medications are providing her with relief, but when she feels particularly stressed at home, she notices that her symptoms flare up. You and she discuss the benefits of cognitive behavioral therapy, and you provide her with the names of some therapists in the area. You suggest that she consult her health plan and make some phone calls to identify a provider that she feels comfortable with.
You advise her to schedule another follow-up visit in 3 months so that you can see how the therapy is working. You also tell her to call any time she experiences significant pain or any symptoms that are persistent or worrisome to her.
Correspondence
Neil T. Moynihan, MD, Johnson Memorial Hospital, 201 Chestnut Hill Road, Stafford Springs, CT 06076; [email protected]
- Little or no diagnostic testing is required to make an accurate diagnosis of irritable bowel syndrome (IBS) in patients younger than 50 without alarm symptoms (C).
- IBS can develop and persist as a consequence of an episode of gastroenteritis (B).
- Tell patients with IBS that it has a physiologic basis and that psychosocial stressors aggravate the already painful and dysfunctional bowel, but do not cause the chronic dysfunction (B).
- Alosetron and tegaserod have proven efficacy, but are available only through limited access programs (A).
- Promising newer therapies for IBS include probiotics and a chloridechannel opener, as well as locally acting, non-absorbable antibiotics for small intestinal bacterial overgrowth-associated IBS (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
“I’ve always had some stomach pain,” says Mary Jane, a 36-year-old patient, whom you are seeing for the first time. “But this is becoming unmanageable.”
You see from Mary Jane’s chart that she has been to your group practice twice over the last few years with complaints of diarrhea that were diagnosed as gastroenteritis. She tells you that after each visit, it got “a little better,” but it “never really went away.”
She also tells you that her stomach bothers her a few days every month, but that it feels a little better after she defecates. She says that she thinks she may be sensitive to certain foods.
“Things are getting worse,” she tells you. “The bloating, pain, and diarrhea have gotten to the point that I can’t go anywhere without worrying where the nearest bathroom is.”
“I’ve already had my gallbladder and appendix removed,” she says, “but I still feel lousy.”
On exam, Mary Jane appears to be in good health. She is afebrile and has a normal abdominal exam, except for very mild diffuse tenderness. She tells you that she has not traveled to any locations where access to clean food or water is suspect. Her stool is heme negative. Urine dip is negative and she is not pregnant.
“Do you know what’s the matter with me?” she asks you. “Or do I need to see a specialist?”
No need for a specialist
Your patient meets the criteria for irritable bowel syndrome (IBS) set by Rome III, an international panel of experts in the field of functional gastrointestinal disorders. She’s had recurrent abdominal pain/discomfort for at least 6 months, and she’s had symptoms at least 3 days a month for the last 3 months (TABLE 1).1
Mary Jane tells you that she is relieved to finally get a diagnosis, having struggled for some time with stomach pain that never really went away. Her experience is not unusual: The wide range of concomitant gastrointestinal and extraintestinal symptoms in IBS patients make the initial diagnosis difficult.2,3 She’s also relieved to learn from you that contrary to popular belief, IBS has a physiologic basis and that psychosocial stressors merely aggravate an already painful and dysfunctional bowel. (See and Irritable bowel syndrome: Not just a functional disorder4-7)
We have a number of treatment options to offer patients like Mary Jane, including alosetron, tegaserod, lubiprostone, selective serotonin reuptake inhibitors (SSRIs), and tricyclic antidepressants, as well as complementary therapies (such as probiotics) and behavioral therapy. But before we review the evidence behind the different options, let’s take a look at the factors that may be at work in IBS, and the things you’ll want to pay special attention to during your assessment.
TABLE 1
Is it IBS? Rome III criteria provide guidance1
|
|
Infection, bacterial overgrowth may play a role
Between 4% and 26% of patients contract IBS for the first time after gastroenteritis.8 Tissue from patients with post-infectious IBS shows chronic mucosal lymphocytosis9,10 associated with enterochromaffin cell hyperplasia.11 Spiller et al12 noted these changes, as well as an increase in gut permeability, for more than 1 year after the resolution of Campylobacter enteritis. Using prednisolone for early intervention suppressed T-cell lymphocyte counts but not IBS symptoms.13
The role of bacterial overgrowth in IBS is controversial. Pimentel et al14 reported that 78% of 202 IBS patients had small intestinal bacterial overgrowth. After treatment, almost half no longer met the Rome criteria for IBS and showed a statistically significant improvement in diarrhea and abdominal pain but not in straining, urgency, or bloating.
A second study from the same group demonstrated that 84% of 111 consecutive IBS patients had small intestinal bacterial overgrowth compared with 20% of healthy controls. Thirty-five percent of the IBS patients treated with neomycin had improved composite scores compared with 11.4% of those receiving placebo.15
Correlation between small intestinal bacterial overgrowth and Rome criteria for IBS has not been replicated by other centers, and other investigators who have looked into this relationship have suggested that small intestinal bacterial overgrowth is not associated with IBS.16,17
Genetics and the environment may also be at work
Familial clustering of IBS is commonly seen.3,18 Several studies have suggested a genetic role but a recently published comprehensive review suggests that the evidence of genetic susceptibility to IBS is modest, if present at all.19
The discovery of physiological differences between IBS patients and control groups suggests that IBS entails significant underlying neuroenteric dysfunction wherein both function and physiology are altered.4,5 The endogenous release of serotonin (5-HT) initiates sensory, secretory, and motor signals within the enteric nervous system by binding to a variety of serotonergic receptors as well as stimulating afferent signals to the spinal cord and brain.4,6,7
Studies by multiple groups using human subjects or human tissue demonstrate physiologic differences between IBS patients and controls which include:5,7
- increased enterochromaffin cell numbers, specifically in post-infectious IBS
- a decrease in mRNA for TPH-1, an enzyme that synthesizes 5-HT
- significant differences in the 5-HT content of enterochromaffin cells
- decreased expression of the serotonin reuptake transporter protein that helps regulate serotonin signaling.
Significant disruption of 5-HT signaling is found in IBS patients compared with controls and more work is underway to better understand the relationship between physiologic dysfunction and symptoms.
Research tells us that the environment also plays a role; specifically, psychological stress is related to IBS symptoms. In one GI referral practice, women with diagnoses of functional disorders had experienced a high frequency of abuse.20
Abuse history is also associated with more severe symptoms, worse daily function, greater psychological distress, and poor health outcome.21 Removal of these stressors provides significant relief to the patient, but IBS still exists in the absence of significant psychosocial pressures.
Few tests (if any) are needed for diagnosis
IBS historically has been a diagnosis of exclusion, but this is no longer the case. You can make the diagnosis with few—if any—diagnostic tests, as long as there are no “red flag” findings or alarm symptoms (TABLE 21,22,23). In particular, surgery is not required to make a diagnosis, nor will it improve a patient’s condition, yet the incidence of abdominal and pelvic surgery in IBS patients is 87% greater, and cholecystectomy three-fold higher, than in the general population.24
While taking a history, you will of course ask about the patient’s altered bowel habits. In addition, though, you will need to:
Ask about personal and family history of inflammatory bowel disease (IBD), colon cancer, celiac disease, pregnancy, recent overseas travel or camping exposure to parasites or contaminated food and water, and history of recent gastroenteritis.25
Ask about alarm symptoms and red flags. Red flags on your examination include any focal positive physical findings, such as peritoneal signs, heme-positive stool, abdominal mass, or pelvic findings. Alarm symptoms that the patient may talk about include things like significant weight loss.
Red flags are based on observational data but have become the accepted standard of practice.1,22,23,26 Extensive testing, including the routine use of blood tests, stool studies, and imaging, however, is not required.22,25 If you have any doubt about history or findings, your diagnostic testing should focus on the issue in question.
When warranted by the presence of alarm symptoms or family history, you may need to schedule a colonoscopy to rule out IBD, tumors, or melanosis coli, which can be caused by excessive use of laxatives. Screening colonoscopy is the standard of care for all patients older than 50, regardless of symptoms.
Key in on food concerns. If specific foods aggravate symptoms, further investigation or a dietary exclusion trial may be helpful; however, even patients with proven lactase deficiency experience little or no bloating after drinking 240 mL milk.1,27 You may want to test for celiac disease if indicated by clinical features such as diarrhea, local prevalence, or family history. Routine testing of celiac disease, however, is not supported by the evidence.28,29
TABLE 2
These red flags and alarm symptoms should prompt further evaluation1,22,23
| PATIENT PRESENTATION |
| Age of onset >50 years Fever Nocturnal symptoms Blood in stools Anemia Weight loss >10% body weight Profuse or large volume of diarrhea Family history of inflammatory bowel disease or cancer |
| PHYSICAL EXAMINATION |
| Fever Fecal blood organomegaly Jaundice Positive physical findings such as peritoneal signs or focal abdominal tenderness |
Many treatment options, limited quality research
Few pharmaceutical compounds have been tested and proven effective in reproducible, high-quality, double-blind, placebo-controlled trials (TABLE 3).1,29-43 In addition, no alternative or complementary therapies have been proven in quality clinical trials to date (TABLE 4).39,44-46,50 Behavioral therapy has shown benefit, but lacks double-blind, placebo-controlled trial data required for a level A strength of recommendation (SOR) (TABLE 5).1,47,48 That said, there are various options that can help patients with IBS—specifically, those who have IBS with diarrhea, IBS with constipation, IBS with mixed bowel habit (where stools are reported as >25% hard or lumpy and >25% loose or watery1), or IBS with unspecified bowel habit.
TABLE 3
Pharmacologic options for IBS
| TREATMENT | CLINICAL EFFICACY* | SOR | COMMENTS |
|---|---|---|---|
| 5-HT3 receptor antagonist (alosetron)1,29,30 | IBS with diarrhea Global symptom relief, improvement in individual symptom measures, improved quality of life scores (NNT=7) | A | Drug available through limited access program |
| 5-HT4 receptor agonist (tegaserod)1,29,31 | IBS with constipation Global symptom relief, improvement in individual symptom measures, improved quality of life scores (NNT=14) | A | Marketing suspended on March 30, 2007;32 limited temporary access program makes individual symptom measures, improved drug available through FDA33 |
| Antibiotics (neomycin, rifaximin)34 | IBS/IBS with diarrhea Global symptom relief, improvement in bowel habits for IBS with diarrhea | B | May benefit a subset of IBS patients with small intestinal bacterial overgrowth. Other research groups have not replicated results to date in IBS patients. Rifaximin produces most durable results |
| Anticholinergics/Calcium channel blockers (hyoscyamine, dicyclomine, mebeverine, otilonium bromide, pinaverium bromide)35,36 | IBS Improvement in abdominal pain (NNT=4–15) | B | Long history of use in IBS patients; however, little credible evidence to support use or efficacy in relief of global IBS symptoms. Best evidence with calcium channel blockers and anticholinergics comes with agents not available in US: mebeverine, otilonium bromide, and pinaverium bromide |
| Bulking agents (ispaghula husk, polycarbophil)35,36 | IBS with constipation Improvement in bowel habits (NNT=2.2–3.5) | B | Improvement in bowel habits, but no significant difference vs placebo for other measures; may aggravate bloating and abdominal pain |
| Chloride-Channel receptor agonist, ClC2 (lubiprostone)37 | IBS with constipation Global symptom relief, improvement in individual symptom measures | B | Indicated for chronic constipation; pending FDA review of data for IBS with constipation indication |
| Loperamide1,38 | IBS with diarrhea Improvement in bowel habits (NNT=3–5) | B | Reduction in diarrhea, but no better than placebo in global relief of symptoms or pain |
| Osmotic Laxatives (polyethylene glycol, lactulose)1,39,40 | IBS with constipation Improvement in bowel habits (NNT=2–4) | B | Lacks randomized controlled trials in IBS. Improvement in bowel habits, but no significant difference vs placebo for other measures; may aggravate bloating and abdominal pain |
| SSRIs (citalopram, fluoxetine, paroxetine)35,41-43 | IBS/IBS with constipation Improvement in abdominal pain and quality of life scores (NNT=3–6) | B | Limited and inconclusive data; diarrhea common side effect on beginning therapy. Start with low dose, increase as needed; may be useful for IBS with constipation patients, but it’s uncertain if effect is on the enteric nervous system, central nervous system, or both |
| Tricyclic antidepressants (TCAs)35,41 | IBS/IBS with diarrhea Improvement in abdominal pain (NNT=3) | B | May increase constipation, does not produce global relief of symptoms, and patients experience poor tolerability (effect on gut function occurs predominantly with lower doses). Hypothesized to modify central- enteric nervous system communication |
| * We did not calculate NNT endpoints on studies of poor quality, those with a small number of patients, or in cases where results were not reproducible in a consistent fashion or were not published in manuscript form. | |||
| IBS, irritable bowel syndrome; SOR, strength of recommendation; NNT, number needed to treat; FDA, Food and Drug Administration; SSRI, selective serotonin reuptake inhibitor. | |||
TABLE 4
Complementary and alternative therapies for IBS
| TREATMENT | CLINICAL EFFICACY | SOR | COMMENTS |
|---|---|---|---|
| Acupuncture44 | No proven benefit | C | Poor-quality trial with heterogeneous interventions, controls, and outcomes measured |
| Chinese herbal therapy45 | Reported improvement in global symptoms and pain | C | Very few high-quality clinical trials. Mixtures vary, content unknown, and some mixtures can be toxic. Unknown which herbs might produce benefit |
| Herbal Therapy (Curcuma xanthorrhiza, Fumaria officinalis)45 | No proven benefit | C | Tested in quality clinical trial; no efficacy over placebo |
| Peppermint Oil (colpermin)39, 50 | Improvement in abdominal pain | B | Lacks data on global symptom improvement; mechanism of action is similar to that of calcium channel blockers |
| Probiotics (Bifidobacterium infantis, Lactobacillus, B animalis)46 | Global symptom relief and improvement in individual symptom measures | B | Preliminary trials encouraging, but differences in trial design, probiotic dose, strain, as well as unpredictable symptom response, has not yielded consistent evidence. No quality of life improvement seen in early studies |
| SOR, strength of recommendation | |||
TABLE 5
Hypnotherapy and psychotherapy for IBS
| TREATMENT | CLINICAL EFFICACY | SOR | COMMENTS |
|---|---|---|---|
| Hypnotherapy47 | Global symptom relief, improvement in individual symptom scores | B | Trials, though many, were of poor quality, but did show significant benefit. A large, randomized placebo-controlled trial is needed to demonstrate benefit |
| Psychotherapy (Cognitive behavioral therapy, biofeedback)1,48 | Global symptom relief, improvement in individual symptom scores (NNT=1–2) | B | Significant reduction in symptoms, but did not eliminate them. To maximize the likelihood of success, biofeedback techniques should be administered by a trained professional. Recommended as part of an overall treatment plan |
| SOR, strength of recommendation; NNT, number needed to treat. | |||
IBS with diarrhea
Options all have “but” clauses
Alosetron, the 5-HT3 receptor antagonist, has demonstrated efficacy in women (specifically) with IBS with diarrhea and has a grade A treatment recommendation from the American College of Gastroenterology.29,30 Given the concerns about severe constipation and ischemic colitis, alosetron was withdrawn from the market in November 2000 but was reintroduced in June 2002 under a limited-use program for patients with severe IBS with diarrhea, in whom standard therapy has failed (SOR: A). Research has since shown that IBS patients have a greater risk for ischemic colitis than the general population.49
Loperamide, a μ-opioid receptor agonist, which does not cross the blood-brain barrier, can be started at bedtime or in the morning at 2 mg and slowly titrated daily to effect. The effect, however, is limited to the bowel habits (SOR: B).38
Anticholinergics can be used for abdominal pain or discomfort, particularly in patients with IBS with diarrhea. Despite their lengthy history and broad use in IBS populations, limited evidence supports their efficacy, and many are not available in the US (SOR: B).35,36
Tricyclic antidepressants (TCAs) exert their effects by blocking the muscarinic receptors. TCAs can provide relief to patients with severe or refractory pain but perceived social stigma associated with taking antidepressants can be a barrier to this therapeutic approach (SOR: B).36,41
IBS with constipation
Laxatives, lubiprostone are options
Osmotic laxatives such as polyethylene glycol or lactulose can improve constipation. Despite widespread over-the-counter and prescription use, however, evidence is lacking for their efficacy and tolerability in IBS (SOR: B).39
Lubiprostone, a ClC2 chloride-channel opener, improved individual symptoms scores in 50 patients with IBS with constipation compared with placebo in a small phase II dose-ranging study (SOR: B).37
Tegaserod, a partial 5-HT4 receptor agonist, facilitates neurotransmission in the gut that is involved in motility and secretion and produces global symptom relief.31 The American College of Gastroenterology gave tegaserod a grade A recommendation for the treatment of women with IBS with constipation.29
On March 30, 2007, Novartis Pharmaceuticals agreed to stop selling tegaserod maleate (Zelnorm) because a safety analysis found a higher chance of heart attack, stroke, and worsening chest pain that could become a heart attack in patients treated with Zelnorm, compared with those receiving placebo.32 In July 2007, the FDA announced that it would permit restricted use of the drug in patients who meet strict criteria, have no known or pre-existing heart problems, and who are in critical need of the drug.33
Polycarbophil and ispaghula husk are the only fibers to have demonstrated any significant benefit in clinical trials for constipation,35 but may make pain and bloating worse (SOR: B).
SSRIS and low-dose benzodiazepines are an option for patients who have coexisting psychological illness.41 There is limited evidence for SSRI treatment (citalopram, paroxetine), either alone or in combination with additional treatments (SOR: B).35,41-43
IBS with mixed/unspecified bowel habit
Consider therapy
Cognitive behavioral therapy or other standard psychotherapy may be beneficial in many IBS patients (SOR: B).1,48
Peppermint oil for IBS is efficacious in recurrent abdominal pain compared with placebo and anticholinergic agents (SOR: B).39,50
Hypnosis has had a therapeutic impact on patients with IBS, even those whose conditions were refractory to other forms of therapy (SOR: B).47
Certain probiotic therapies have shown improvement in global symptoms and may prove to be promising therapeutic agents (SOR: B).46
Acupuncture has shown no proven efficacy in IBS (SOR: C).44
Some Chinese herbal medicines may improve the symptoms of IBS. Positive findings from trials should be interpreted with caution, though, due to inadequate methodology, small sample sizes, and lack of confirming data. In addition, herbal remedies may contain unknown substances that can pose serious risk for adverse events and drug interactions (SOR: C).45
Antibiotics for IBS patients with small intestinal bacterial overgrowth can provide some relief, according to Pimentel and colleagues,14 but other groups have not been able to duplicate these findings. Rifaximin in IBS and IBS-like symptoms has shown a sustained benefit (SOR: B).34
Exercise or dietary therapies have no proven benefit in otherwise healthy IBS patients (SOR: C).1
Set realistic goals, address patient fears
When caring for a patient with IBS, it’s important to set and discuss realistic goals. Be sure to address patient concerns, as well. IBS patients are frequently frustrated by a lack of diagnostic findings and may worry about being labeled as having a psychological disorder rather than having a true GI abnormality. This concern, especially if the patient feels that the physician is not adequately addressing her (or his) symptoms, may exacerbate the already troublesome IBS symptoms.
Assure your patient that comorbid psychological conditions do not cause symptoms, but can contribute to pain and bowel dysfunction. Consider the possibility of behavioral therapy, if indicated by patient history.
Advise the patient that the initial approach you are taking to alleviate her (or his) symptoms may provide significant improvement. Manage expectations appropriately and be open to discussion about what the patient may need to alleviate both physiologic and psychologic stressors that perpetuate symptoms. Family practitioners, not specialists, are ideally suited to address the patients’ needs and expectations as they are the ones who know the patients’ histories, personalities, and families best.1 Tell the patient that rather than hoping for a cure, the goal for the both the physician and patient should be to achieve symptom relief.
Mary Jane finally gets some relief
After speaking with Mary Jane and doing a thorough exam, you reassure her that her symptoms meet the criteria for the diagnosis of IBS, and that research indicates that she can safely be treated for the disorder.
You tell your patient that you’d like to begin treatment by putting her on loperamide—2 capsules at onset of diarrhea and 1 capsule after each diarrheal episode to a maximum of 8 capsules a day and a low-dose amitriptyline (25 mg at bed-time). You explain that the tricyclic should provide her with some relief by modifying the way the nervous system of the intestine communicates with the brain.
Two months later, during a follow-up visit, Mary Jane tells you that the medications are providing her with relief, but when she feels particularly stressed at home, she notices that her symptoms flare up. You and she discuss the benefits of cognitive behavioral therapy, and you provide her with the names of some therapists in the area. You suggest that she consult her health plan and make some phone calls to identify a provider that she feels comfortable with.
You advise her to schedule another follow-up visit in 3 months so that you can see how the therapy is working. You also tell her to call any time she experiences significant pain or any symptoms that are persistent or worrisome to her.
Correspondence
Neil T. Moynihan, MD, Johnson Memorial Hospital, 201 Chestnut Hill Road, Stafford Springs, CT 06076; [email protected]
1. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480-1491.
2. Drossman DA, Li Z, Andruzzi E, et al. US householder survey of functional gastrointestinal disorders: prevalence, sociodemography, and health impact. Dig Dis Sci 1993;38:1569-1580.
3. Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut 1986;27:37-40.
4. Gershon MD. Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 2004;20(suppl):3-14.
5. Mawe GM, Coates M, Moses PL. Review article: intestinal serotonin signaling in irritable bowel syndrome. Alimentary Pharmacol Ther 2006;23:1067-1076.
6. Tonini M. 5-Hydroxytryptamine effects in the gut: the 3, 4, and 7 receptors. Neurogastroenterol Motil 2005;17:637-642.
7. Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol 2005;39(suppl):184-193.
8. Parry S, Forgacs I. Intestinal infection and irritable bowel syndrome. Eur J Gastroenterol Hepatol 2005;17:5-9.
9. Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut 1999;44:400-406.
10. Wang L-H, Fang X-C, Pan G-Z. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut 2004;53:1096-1101.
11. Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol 2003;98:1578-1583.
12. Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000;47:804-811.
13. Dunlop SP, Jenkins D, Neal KR, Naesdal J, Borgaonker M, Collins SM. Randomized, double-blind, placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Aliment Pharmacol Ther 2003;18:77-84.
14. Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 2000;95:3503-3506.
15. Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: a double blind, randomized controlled study. Am J Gastroenterol 2003;98:412-419.
16. Posserud I, Stotzer ES, Bjornsson H, Abrahamsson M, Simren M. Altered counts of small bowel bacteria in patients with irritable bowel syndrome (IBS). Gastroenterology 2006;130:A739.-
17. Ruff KC, Saito-Loftus YA, Llocke GR, Harmsen WS, Zinsmeister AS, Talley NJ. Failure to detect association with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO). Am J Gastroenterol 2006;101(suppl 2):S488.-
18. Bellentani S, Baldoni P, Petrella S, et al. A simple score for the identification of patients at high risk of organic diseases of the colon in the family doctor consulting room: the local IBS Study Group. Fam Pract 1990;7:307-312.
19. Saito YA, Petersen GM, Locke R, III, Talley NJ. The genetics of irritable bowel syndrome. Clin Gastroenterol Hepatol 2005;3:1057-1065.
20. Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med 1990;113:828-833.
21. Drossman DA, Li Z, Leserman J, Toomey TC, Hu Y. Health status by gastrointestinal diagnosis and abuse history. Gastroenterology 1996;110:999-1007.
22. Malagelada JR. A symptom based approach to making a positive diagnosis of irritable bowel syndrome with constipation. Int J Clin Pract 2006;60:57-63.
23. Locke GR, 3rd. Natural history of irritable bowel syndrome and durability of diagnosis. Rev Gastroenterol Disord 2003;3(suppl):S12-S17.
24. Cole JA, Yeaw JM, Cutone JA, et al. The incidence of abdominal and pelvic surgery among patients with irritable bowel syndrome. Dig Dis Sci 2005;50:2268-2275.
25. Cash BD, Chey WD. Diagnosis of irritable bowel syndrome. Gastroenterol Clin N Am 2005;34:205-220.
26. Owens DM, Nelson DK, Talley NJ. The irritable bowel syndrome: long-term prognosis and the physician-patient interaction. Ann Intern Med 1995;122:107-112.
27. Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactosehydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med 1995;333:1-4.
28. Spiegel BM, Derosa VP, Gralnek IM, Wang V, Dulai GS. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: a cost-effective analysis. Gastroenterology 2004;126:1721-1732.
29. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol 2002;97(suppl 11):S1-S5.
30. Cremonini F, Delgado A, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil 2003;15:79-86.
31. Evans BW, Clark WK, Moore DJ, Whorwell PJ. Tegaserod for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2004;(1):CD003960.-
32. US Food and Drug Administration FDA Public Health Advisory: Tegaserod maleate (marketed as Zelnorm). March 30, 2007. Available at: www.fda.gov/CDER/Drug/advisory/tegaserod.htm. Accessed January 2, 2008.
33. US Food and Drug Administration FDA Permits restricted use of Zelnorm for qualifying patients. July 27, 2007. Available at: www.fda.gov/bbs/topics/NEWS/2007/NEW01673.html. Accessed January 2, 2008.
34. Frissora CL, Cash BD. Review article: the role of antibiotics vs. conventional pharmacotherapy in treating symptoms of irritable bowel syndrome. Aliment Pharmacol Ther 2007;25:1271-1281.
35. Quartero AO, Meineche-Schmidt V, Muris J, Rubin G, de Wit N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2005;(2):CD003460.-
36. Jailwala J, Impereiale T, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized controlled trials. Ann Intern Med 2000;133:136-147.
37. Johanson JF, Panas R, Holland P, Ueno R. A dose ranging, double-blind, placebo-controlled study of lubiprostone in subjects with irritable bowel syndrome and constipation (c-IBS). Gastroenterology 2006;130(suppl 2):A25.-
38. Amery W, Duyck F, Polak J, van den Bouwhuysen G. A multicentre double-blind study in acute diarrhoea comparing loperamide (R 18553) with two common antidiarrhoeal agents and a placebo. Curr Ther Res Clin Exp 1975;17:263-270.
39. Tack J, Fried M, Houghton LA, Spicak J, Fisher G. Systematic review: the efficacy of treatments for irritable bowel syndrome—a European perspective. Aliment Pharmacol Ther 2006;24:183-205.
40. Bleser SD. Chronic constipation: let symptom and severity direct treatment. J Fam Pract 2006;55:587-593.
41. Wald A. Psychotropic agents in irritable bowel syndrome. J Clin Gastroenterol 2002;35(suppl):S53-S57.
42. Tack J, Broekaert D, Fischler B, van Oudenhove , L, Gevers A, Janssens J. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006;55:1095-1103.
43. Tabas G, Beaves M, Wang J, Friday P, Mardini H, Arnold G. Paroxetine to treat irritable bowel syndrome not responding to high-fiber diet: a double-blind, placebo-controlled trial. Am J Gastroenterol 2004 May;99:914-920.
44. Lim B, Manheimer E, Lao L, Ziea E, Wisniewski J, Liu J, Berman B. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2006;(4):CD005111.-
45. Liu JP, Yang M, Liu YX, Wei ML, Grimsgaard S. Herbal medicines for treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2006;(1):CD004116.-
46. Quigley EMM, Flourie B. Probiotics and irritable bowel syndrome: a rational for their use and an assessment of the evidence to date. Neurogastroenterol Motil 2007;19:166-172.
47. Wilson S, Maddison T, Robers L, Greenfield S, Singh S. On behalf of the Birmingham IBS research group. Systematic review: The effectiveness of hypnotherapy in the management of irritable bowel syndrome. Aliment Pharmacol Ther 2006;24:769-780.
48. Lackner JM, Mesmer C, Morley S, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol 2004;72:1100-1113.
49. Cole JA, Cook SF, Sands BE, Ajene AN, Miller DP, Walker AM. Occurrence of colon ischemia in relation to irritable bowel syndrome. Am J Gastroenterol 2004;99:486-491.
50. Grigoleit HG, Grigoleit P. Peppermint oil in irritable bowel syndrome. Phytomedicine 2005;12:601-606.
1. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480-1491.
2. Drossman DA, Li Z, Andruzzi E, et al. US householder survey of functional gastrointestinal disorders: prevalence, sociodemography, and health impact. Dig Dis Sci 1993;38:1569-1580.
3. Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut 1986;27:37-40.
4. Gershon MD. Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 2004;20(suppl):3-14.
5. Mawe GM, Coates M, Moses PL. Review article: intestinal serotonin signaling in irritable bowel syndrome. Alimentary Pharmacol Ther 2006;23:1067-1076.
6. Tonini M. 5-Hydroxytryptamine effects in the gut: the 3, 4, and 7 receptors. Neurogastroenterol Motil 2005;17:637-642.
7. Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol 2005;39(suppl):184-193.
8. Parry S, Forgacs I. Intestinal infection and irritable bowel syndrome. Eur J Gastroenterol Hepatol 2005;17:5-9.
9. Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut 1999;44:400-406.
10. Wang L-H, Fang X-C, Pan G-Z. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut 2004;53:1096-1101.
11. Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol 2003;98:1578-1583.
12. Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000;47:804-811.
13. Dunlop SP, Jenkins D, Neal KR, Naesdal J, Borgaonker M, Collins SM. Randomized, double-blind, placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Aliment Pharmacol Ther 2003;18:77-84.
14. Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 2000;95:3503-3506.
15. Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: a double blind, randomized controlled study. Am J Gastroenterol 2003;98:412-419.
16. Posserud I, Stotzer ES, Bjornsson H, Abrahamsson M, Simren M. Altered counts of small bowel bacteria in patients with irritable bowel syndrome (IBS). Gastroenterology 2006;130:A739.-
17. Ruff KC, Saito-Loftus YA, Llocke GR, Harmsen WS, Zinsmeister AS, Talley NJ. Failure to detect association with irritable bowel syndrome (IBS) and small intestinal bacterial overgrowth (SIBO). Am J Gastroenterol 2006;101(suppl 2):S488.-
18. Bellentani S, Baldoni P, Petrella S, et al. A simple score for the identification of patients at high risk of organic diseases of the colon in the family doctor consulting room: the local IBS Study Group. Fam Pract 1990;7:307-312.
19. Saito YA, Petersen GM, Locke R, III, Talley NJ. The genetics of irritable bowel syndrome. Clin Gastroenterol Hepatol 2005;3:1057-1065.
20. Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med 1990;113:828-833.
21. Drossman DA, Li Z, Leserman J, Toomey TC, Hu Y. Health status by gastrointestinal diagnosis and abuse history. Gastroenterology 1996;110:999-1007.
22. Malagelada JR. A symptom based approach to making a positive diagnosis of irritable bowel syndrome with constipation. Int J Clin Pract 2006;60:57-63.
23. Locke GR, 3rd. Natural history of irritable bowel syndrome and durability of diagnosis. Rev Gastroenterol Disord 2003;3(suppl):S12-S17.
24. Cole JA, Yeaw JM, Cutone JA, et al. The incidence of abdominal and pelvic surgery among patients with irritable bowel syndrome. Dig Dis Sci 2005;50:2268-2275.
25. Cash BD, Chey WD. Diagnosis of irritable bowel syndrome. Gastroenterol Clin N Am 2005;34:205-220.
26. Owens DM, Nelson DK, Talley NJ. The irritable bowel syndrome: long-term prognosis and the physician-patient interaction. Ann Intern Med 1995;122:107-112.
27. Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactosehydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med 1995;333:1-4.
28. Spiegel BM, Derosa VP, Gralnek IM, Wang V, Dulai GS. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: a cost-effective analysis. Gastroenterology 2004;126:1721-1732.
29. American College of Gastroenterology Functional Gastrointestinal Disorders Task Force. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol 2002;97(suppl 11):S1-S5.
30. Cremonini F, Delgado A, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil 2003;15:79-86.
31. Evans BW, Clark WK, Moore DJ, Whorwell PJ. Tegaserod for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2004;(1):CD003960.-
32. US Food and Drug Administration FDA Public Health Advisory: Tegaserod maleate (marketed as Zelnorm). March 30, 2007. Available at: www.fda.gov/CDER/Drug/advisory/tegaserod.htm. Accessed January 2, 2008.
33. US Food and Drug Administration FDA Permits restricted use of Zelnorm for qualifying patients. July 27, 2007. Available at: www.fda.gov/bbs/topics/NEWS/2007/NEW01673.html. Accessed January 2, 2008.
34. Frissora CL, Cash BD. Review article: the role of antibiotics vs. conventional pharmacotherapy in treating symptoms of irritable bowel syndrome. Aliment Pharmacol Ther 2007;25:1271-1281.
35. Quartero AO, Meineche-Schmidt V, Muris J, Rubin G, de Wit N. Bulking agents, antispasmodic and antidepressant medication for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2005;(2):CD003460.-
36. Jailwala J, Impereiale T, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized controlled trials. Ann Intern Med 2000;133:136-147.
37. Johanson JF, Panas R, Holland P, Ueno R. A dose ranging, double-blind, placebo-controlled study of lubiprostone in subjects with irritable bowel syndrome and constipation (c-IBS). Gastroenterology 2006;130(suppl 2):A25.-
38. Amery W, Duyck F, Polak J, van den Bouwhuysen G. A multicentre double-blind study in acute diarrhoea comparing loperamide (R 18553) with two common antidiarrhoeal agents and a placebo. Curr Ther Res Clin Exp 1975;17:263-270.
39. Tack J, Fried M, Houghton LA, Spicak J, Fisher G. Systematic review: the efficacy of treatments for irritable bowel syndrome—a European perspective. Aliment Pharmacol Ther 2006;24:183-205.
40. Bleser SD. Chronic constipation: let symptom and severity direct treatment. J Fam Pract 2006;55:587-593.
41. Wald A. Psychotropic agents in irritable bowel syndrome. J Clin Gastroenterol 2002;35(suppl):S53-S57.
42. Tack J, Broekaert D, Fischler B, van Oudenhove , L, Gevers A, Janssens J. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006;55:1095-1103.
43. Tabas G, Beaves M, Wang J, Friday P, Mardini H, Arnold G. Paroxetine to treat irritable bowel syndrome not responding to high-fiber diet: a double-blind, placebo-controlled trial. Am J Gastroenterol 2004 May;99:914-920.
44. Lim B, Manheimer E, Lao L, Ziea E, Wisniewski J, Liu J, Berman B. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2006;(4):CD005111.-
45. Liu JP, Yang M, Liu YX, Wei ML, Grimsgaard S. Herbal medicines for treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2006;(1):CD004116.-
46. Quigley EMM, Flourie B. Probiotics and irritable bowel syndrome: a rational for their use and an assessment of the evidence to date. Neurogastroenterol Motil 2007;19:166-172.
47. Wilson S, Maddison T, Robers L, Greenfield S, Singh S. On behalf of the Birmingham IBS research group. Systematic review: The effectiveness of hypnotherapy in the management of irritable bowel syndrome. Aliment Pharmacol Ther 2006;24:769-780.
48. Lackner JM, Mesmer C, Morley S, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol 2004;72:1100-1113.
49. Cole JA, Cook SF, Sands BE, Ajene AN, Miller DP, Walker AM. Occurrence of colon ischemia in relation to irritable bowel syndrome. Am J Gastroenterol 2004;99:486-491.
50. Grigoleit HG, Grigoleit P. Peppermint oil in irritable bowel syndrome. Phytomedicine 2005;12:601-606.
Aspirin + clopidogrel therapy: How does your care compare to the evidence?
- Patients with drug-eluting stents should receive dual therapy (aspirin + clopidogrel) for at least 12 months (B).
- For patients who have had an ischemic stroke or transient ischemic attack, adding aspirin to clopidogrel increases the risk of hemorrhage and is not routinely recommended (A).
- Adding clopidogrel to aspirin is not more effective than aspirin alone in the primary prevention of coronary artery disease in patients with multiple risk factors. In fact, it may actually cause harm in patients without established cardiovascular disease (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Jessica is 72 years old and a new patient of yours, having recently moved to the area. She has a history of non-ST elevation myocardial infarction (NSTEMI) 8 months ago, hypertension, and hyperlipidemia. Her NSTEMI did not require stent placement. Upon reviewing her medications, you see she is taking aspirin 81 mg, clopidogrel 75 mg, simvastatin 80 mg, and metoprolol XL 100 mg. The patient isn’t sure how long she has been on clopidogrel and wants to know if she still needs to take it because she has a high co-pay.
What would you tell her?
John is a 60-year-old patient of yours with a history of coronary artery disease, who had an elective catheterization 18 months ago at which time a sirolimus drug-eluting stent was placed. Also at that time, he was placed on aspirin 325 mg daily and clopidogrel 75 mg daily. The patient says he has a friend at work who also has a coronary stent, but his friend stopped taking clopidogrel 12 months after his stent was placed. The patient wants to know why he is still taking clopidogrel and when he can stop it.
What would you tell him?
Anna is a 68-year-old patient of yours who has uncontrolled type 2 diabetes. She still smokes a pack a day, as she has done for the last 53 years, her BMI is 43, and her LDL is 103 on 80 mg of simvastatin. Her additional meds include lisinopril, 70/30 insulin, and a daily aspirin. Despite her inability to quit smoking or lose weight, she is compliant with her medications. You are both very concerned about her future risk of cardiovascular disease. You consider whether she would benefit from adding clopidogrel to aspirin in order to prevent future coronary events.
Do you write a prescription for clopidogrel, 75 mg/day?
In your conversation with Jessica, you should have told her that she needs to take the clopidogrel for another 4 months (making it 1 year of therapy following her NSTEMI). 2007 guidelines from the American Heart Association and the American College of Cardiology support this approach.1
In your discussion with John, you should have told him that for drugeluting stents, recent studies have shown an additional benefit to continuing dual therapy longer than 1 year, and that consideration should be given to continuing clopidogrel indefinitely.1,2
In the case of Anna, your patient with multiple atherothrombotic risk factors, you should have opted against writing her a prescription for clopidogrel. The evidence suggests that putting her on clopidogrel might actually increase her risk of cardiovascular death.3
These 3 cases demonstrate how much our understanding of dual antiplatelet therapy (aspirin + clopidogrel) has evolved over the past few years—and even months. Given the volume of information we must sift through daily, it is easy to occasionally miss a study or update. The result? You may be underutilizing combination antiplatelet therapy when treatment is indicated. Or, you may be combining aspirin and clopidogrel in situations where there is insufficient data to support its use—and possibly, in situations where it is associated with an increased incidence of adverse events.
This review can help.
Here we have summarized the latest trials and recommendations on dual antiplatelet therapy as they pertain to:
- acute coronary syndrome (ACS)
- coronary stents
- stroke
- the primary prevention and secondary prevention of distant cardiovascular events.
Should you need a quick reference tool for your office, we have also included an at-a-glance summary of current recommendations (TABLE).
TABLE
Dual antiplatelet therapy: The recommendations at a glance
| Unstable angina/non-ST elevation MI/acute coronary syndrome (without stenting) | American Heart Association/American College of Cardiology Low dose aspirin (75 to 162 mg per day) indefinitely (Strength of Recommendation [SOR]: A), and clopidogrel 75 mg per day for at least 1 month (SOR: A) and ideally up to 1 year (SOR: B)1,7 |
| Cardiac stent placement | American Heart Association/American College of Cardiology Bare metal stent
Drug-eluting stent (DES) |
| Stroke | American Heart Association/American Stroke Association
|
| Primary prevention/secondary prevention of distant cardiovascular events |
|
Dual therapy reduces future MI risk in ACS
The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial in 2001 was one of the first combination trials published; it looked at combination antiplatelet therapy in ACS.4 CURE enrolled patients with NSTEMI and randomized them to receive aspirin plus clopidogrel or aspirin plus placebo for 3 to 12 months. Out of the total number of patients, 9.3% of the dual therapy group reached the endpoint of cardiovascular death, MI, or stroke, compared with 11.4% of the aspirin-only group (relative risk [RR]=0.80 for dual therapy). Further, the rate of each component of the composite outcome tended to be lower in the combination-therapy group.
Colored scanning electron micrograph of a blood clot.In particular, there was an RR of 0.77 for MI and, more specifically, an RR of 0.60 for Q-wave MI in the dual therapy group. There was, however, a 1.38 RR for major bleeding (defined as bleeding resulting in disability, vision loss, or transfusion of at least 2 units of blood) in the combination-therapy group vs the aspirin-only group (3.7% to 2.7%). However, neither rates of life-threatening bleeding nor hemorrhagic stroke were significantly different between the 2 groups.
Overall, the CURE trial showed that combining clopidogrel and aspirin was superior to aspirin alone in preventing repeat ischemic events in patients with NSTEMI for up to 12 months, and this conclusion was supported by a 2007 Cochrane review.5
The Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) trial studied the short-term (30 days) effect of clopidogrel combined with aspirin and fibrinolytic therapy in patients with ST elevation MI (STEMI).6 The study showed that the addition of clopidogrel improved the patency rate of infarctrelated arteries and reduced short-term ischemic complications.
There are, however, no trials looking at combination antiplatelet therapy for STEMI patients beyond 30 days.
Unstable angina/non-ST elevation MI/acute coronary syndrome
The American Heart Association/American College of Cardiology Guidelines recommend that patients with unstable angina (UA)/NSTEMI or ACS receive both clopidogrel 75 mg and aspirin (75 to 162 mg/day) for at least a month, and preferably, up to 12 months (TABLE).1,7
Prolonged therapy needed with drug-eluting stents
The Clopidogrel for the Reduction of Events During Observation (CREDO) trial, published in 2002, enrolled patients referred for percutaneous coronary intervention (PCI), about 89% of whom received at least 1 cardiac stent, and followed them for 12 months.8 All patients received aspirin and clopidogrel up to day 28. From day 29 to 1 year, half the patients continued dual antiplatelet therapy and the other half received aspirin plus placebo.
The primary outcome measure was death, MI, or stroke. The combination therapy group showed a relative risk reduction of 37.4% (RR=0.73) in the combined endpoint, compared with the aspirin/placebo group. CREDO did not show a significant difference in risk for major bleeding between the 2 groups.
Further data from the CURE trial was released in 2004. Researchers conducted a subgroup analysis to see if there was a benefit to combination antiplatelet therapy for patients with NSTEMI who underwent PCI or coronary artery bypass grafting (CABG). This further analysis revealed an RR of 0.72 for the outcome of CV death, MI, or stroke with the addition of long-term (3 to 12 months) clopidogrel to aspirin in the PCI group and an RR of 0.89 for the CABG group with combination therapy. These findings led researchers to conclude that combination antiplatelet therapy is beneficial for high-risk patients undergoing revascularization for NSTEMI regardless of the type of revascularization.9
A 2007 observational study conducted at the Duke Heart Center investigated the addition of long-term clopidogrel to aspirin for both drug-eluting stents (DES) and bare-metal stents (BMS).2 The study population was split into 4 groups:
- combination antiplatelet therapy in patients with DES,
- aspirin alone with DES,
- combination therapy with BMS, and
- aspirin alone with BMS.
Follow-up was conducted at 6, 12, and 24 months. For patients with DES, continuing clopidogrel with aspirin therapy was associated with a significant decrease in death or MI at 24 months (3.1% vs 7.2%, RR=0.43). For patients with BMS, researchers found no significant benefit for those who continued dual antiplatelet therapy longer than 6 months compared with aspirin alone.
The data from this study led the authors to speculate that drug-eluting stents may require protracted—and possibly indefinite—dual antiplatelet therapy with clopidogrel and aspirin. Additional research with a large clinical trial is necessary to assess the appropriate length of treatment with combination antiplatelet therapy after DES, as it may extend even longer than current recommendations suggest.
An analysis of the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER) registry illustrates the catastrophic consequences of premature discontinuation of dual antiplatelet therapy.10 This study of 500 patients with acute MI treated with DES revealed a striking increase in mortality rate related to discontinuation of dual antiplatelet therapy within 3 months of their initial MI. Patients who prematurely stopped dual therapy had a mortality rate of 7.5% over the next 11 months compared to 0.7% in patients who continued dual antiplatelet therapy for a minimum of 3 months.
Of note: Researchers say that patients who self-discontinued the dual therapy were less likely to have been given instructions about their medication at discharge. In addition, these patients were less likely to have completed high school and more likely to avoid health care because of cost.
Finally, a recent study that evaluated the safety of off-label use of drug-eluting stents revealed further data in support of prolonged use of dual antiplatelet therapy in this group.11 (Off-label use included, among other things, using sirolimus- and paclitaxel eluting stents on lesions that were longer than specified in the stent manufacturer’s “information for use.”) Forty-seven percent of patients who received DES were for off-label indications.
Among the off-label patients, the 1-year rate of death, MI, or stent thrombosis was 7.7% for standard dual therapy duration (3 months for sirolimus-eluting stents and 6 months for paclitaxel-eluting stents) compared with 6.0% (RR=0.78) for prolonged duration (defined as beyond standard duration).
Cardiac stent placement
Current American Heart Association/American College of Cardiology recommendations state that full-dose aspirin (162–325 mg/day) should be used for 1 month after BMS implantation, at least 3 months after sirolimus DES implantation, and 6 months after paclitaxel DES implantation, then continued indefinitely at low dose (75 to 162 mg/day).1
Clopidogrel 75 mg/day should be added to aspirin for a minimum of 1 month, and ideally up to a year for BMS; and for a minimum of 12 months for all DES regardless of type.1 In addition to the duration and type of therapy, the American Heart Association states it is not appropriate to withhold dual therapy in stent patients for minor surgery or dental procedures within their recommended treatment period.12
Of note: The American Heart Association and American College of Cardiology guidelines, cited above, are an August 2007 revision to the groups’ 2002 guidelines.1 Although specifically written for UA /NSTEMI, the revision states that clopidogrel should be added to aspirin for at least 12 months in “all post-PCI patients receiving DES.” A revised AHA/ACC PCI guideline, however, has not yet been published to reflect these new data and recommendations.
Bleeding rates offset stroke prevention benefits
The Management of ATherothrombosis with Clopidogrel in High-risk patients (MATCH) trial is the only major trial that has evaluated combination clopidogrel and aspirin therapy for secondary prevention of stroke.13 The MATCH trial, first published in 2004, differed from the cardiovascular antiplatelet trials in that it used clopidogrel in its control group instead of aspirin.
Patients who’d had a recent ischemic stroke or transient ischemic attack (TIA) and at least 1 additional vascular risk factor were randomized to receive clopidogrel plus aspirin therapy or clopidogrel plus placebo for 18 months. Primary outcome measure was a composite of ischemic stroke, MI, vascular death, or rehospitalization for acute ischemia. Although researchers observed a consistent reduction of vascular events in the combination therapy group, the differences did not reach statistical significance. In addition, higher bleeding rates “offset any beneficial effect” of combination therapy.
Overall, treatment with aspirin and clopidogrel compared with aspirin alone might prevent 10 ischemic events per 1000 treated (not statistically significant) at a cost of 13 life-threatening hemorrhages per 1000 treated. The researchers concluded that there was no demonstrable benefit of adding aspirin to clopidogrel for secondary prevention of stroke.
A major limitation of this study was that the majority of patients enrolled had lacunar strokes, a stroke subtype that is not believed to be of pure atherothrombotic origin and that has the lowest recurrence rates.13 Future studies on secondary stroke prevention will address issues of stroke subtype and location.14
Other trials that did not specifically look at clopidogrel + aspirin therapy for noncardioembolic stroke but examined relevant issues also warrant mention:
- The Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events—W (ACTIVE W) trial compared an aspirin + clopidogrel combination to warfarin in patients with atrial fibrillation.15 ACTIVE W showed warfarin to be superior to combination antiplatelet therapy for prevention of vascular events including stroke, non-central nervous system emboli, MI, or vascular death (RR=1.44 for combination therapy).
- The Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial investigated combination antiplatelet therapy in patients with recently symptomatic carotid artery stenosis.16 Researchers in this small study used doppler ultrasound to see if combination therapy would lead to a reduction in asymptomatic microembolism from the carotid plaques. Patients received clopidogrel plus aspirin or aspirin alone for 7 days. The patients in the combination group had a 40% relative reduction in microembolism.
Clearly, the CARESS trial opens the door for further study into clinically important outcomes for carotid artery stenosis and dual antiplatelet therapy.
Stroke
The current American Heart Association/American Stroke Association guidelines recommend aspirin plus extended-release dipyridamole or clopidogrel alone for the secondary prevention of noncardioembolic stroke. The guidelines state that the “addition of aspirin to clopidogrel increases risk of hemorrhage and is not routinely recommended.” However, aspirin + clopidogrel combination therapy may be appropriate in stroke patients with recent ACS or vascular stents.17
When prevention efforts can harm patients
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial studied the value of using aspirin + clopidogrel combination therapy for primary or secondary prevention of distant coronary events (longer than 12 months).3 CHARISMA researchers studied patients with either multiple cardiac risk factors (termed “asymptomatic”) or clinically evident cardiovascular disease (termed “symptomatic”) who did not have a current indication for dual antiplatelet therapy (ie, recent MI or coronary stent).
The patients were randomized to receive aspirin plus clopidogrel or aspirin plus placebo and were followed for a median length of 28 months. The primary outcome measure was a combination of MI, stroke, or cardiovascular death. Overall, there was no significant difference in the primary endpoint, which occurred in 6.8% of the combination group and in 7.3% of the aspirin-only group (RR=0.93).
Subgroup analysis revealed that asymptomatic patients actually had a 20% increase in primary events with the addition of clopidogrel to aspirin (6.6% vs 5.5%, RR=1.20), and an increase in death (3.9% vs 2.2%, RR=1.77). In the symptomatic group, however, there was a “marginally significant” reduction in primary events, with 6.9% of patients in the combination group and 7.9% of patients in the aspirin-only group experiencing primary events (RR=0.88). The rate of severe bleeding in the combination group was 1.7% compared with 1.3% in the placebo group (RR=1.25).
The authors of CHARISMA propose a hypothesis to explain the seemingly paradoxical effect of an increase of primary endpoints in the asymptomatic group. They suggest that established vascular disease could be a proxy for hyperactive platelets. Thereby, the action of combination therapy may be more effective in patients with established vascular disease and less effective in patients with normal platelets.
Primary prevention/secondary prevention of distant cardiovascular events
No evidence or recommendations currently exist to support using dual antiplatelet therapy for primary prevention or secondary prevention of cardiovascular events in patients not covered under current American Heart Association/American College of Cardiology guidelines for recent ACS or stent/PCI. Adding clopidogrel to aspirin may cause harm in patients without established cardiovascular disease.3
Ongoing studies explore stroke subtypes, CABG
Because of insufficient or conflicting data, it is unclear whether dual therapy is advisable for a number of disease states, including specific stroke subtypes, peripheral artery disease, and CABG.18 The following ongoing dual therapy studies may provide some answers:14
- The Secondary Prevention of Small Subcortical Strokes (SPS3) trial is investigating secondary prevention of subcortical stroke.
- The Fast Assessment of Stroke and Transient ischemic attack to prevent Early Recurrence (FASTER) trial is also investigating secondary prevention of stroke.
- The Aortic arch Related Cerebral Hazard (ARCH) trial is investigating aortic plaques and stroke.
- The Effects of Physical Training, ASA and Clopidogrel on the Walking Capacity of Patients with Stage II PAD trial is investigating walking capacity in patients with peripheral artery disease.
Correspondence
B. Brent Simmons, MD, 150 E. Wynnewood Rd., Apt. 18 J, Wynnewood, PA 19096; [email protected].
1. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction-executive summary. J Am Coll Cardiol 2007;50:652-726.
2. Eisentstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA 2007;297:159-168.
3. Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706-1717.
4. CURE Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502.
5. Keller TT, Squizzato A, Middeldorp S. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular disease (review). Cochrane Database Syst Rev 2007;(3):CD005158.-
6. Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005;352:1179-1189.
7. Smith SC, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation 2006;113:2363-2372.
8. Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention. JAMA 2002;288:2411-2420.
9. Fox KAA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST elevation acute coronary syndrome. Circulation 2004;110:1202-1208.
10. Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement. Circulation 2006;113:2803-2809.
11. Beohar N, Davidson CJ, Kip KE, et al. Outcomes and complications associated with off-label and untested use of drug-eluting stents. JAMA 2007;297:1992-2000.
12. Grines CL, Bonow RO, Casey DE, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents. Circulation 2007;115:813-818.
13. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH). Lancet 2004;364:331-337.
14. US National Institutes of Health. ClinicalTrials.gov [Web site]. Available at: www.clinicaltrials.gov. Accessed on March 4, 2007.
15. ACTIVE Writing Group. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the atrial fibrillation clopidogrel trial with irbesartan for the prevention of vascular events. Lancet 2006;367:1903-1912.
16. Markus HS, Droste DV, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid artery stenosis evaluated using doppler embolic signal detection: CARESS trial. Circulation 2005;111:2233-2240.
17. Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Stroke 2006;37:577-617.
18. Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA guideline update for coronary artery bypass graft surgery. Circulation 2004;110:e340-e437.Available at: www.acc.org/qualityandscience/clinical/guidelines/cabg/index.pdf. Accessed November 11, 2007.
- Patients with drug-eluting stents should receive dual therapy (aspirin + clopidogrel) for at least 12 months (B).
- For patients who have had an ischemic stroke or transient ischemic attack, adding aspirin to clopidogrel increases the risk of hemorrhage and is not routinely recommended (A).
- Adding clopidogrel to aspirin is not more effective than aspirin alone in the primary prevention of coronary artery disease in patients with multiple risk factors. In fact, it may actually cause harm in patients without established cardiovascular disease (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Jessica is 72 years old and a new patient of yours, having recently moved to the area. She has a history of non-ST elevation myocardial infarction (NSTEMI) 8 months ago, hypertension, and hyperlipidemia. Her NSTEMI did not require stent placement. Upon reviewing her medications, you see she is taking aspirin 81 mg, clopidogrel 75 mg, simvastatin 80 mg, and metoprolol XL 100 mg. The patient isn’t sure how long she has been on clopidogrel and wants to know if she still needs to take it because she has a high co-pay.
What would you tell her?
John is a 60-year-old patient of yours with a history of coronary artery disease, who had an elective catheterization 18 months ago at which time a sirolimus drug-eluting stent was placed. Also at that time, he was placed on aspirin 325 mg daily and clopidogrel 75 mg daily. The patient says he has a friend at work who also has a coronary stent, but his friend stopped taking clopidogrel 12 months after his stent was placed. The patient wants to know why he is still taking clopidogrel and when he can stop it.
What would you tell him?
Anna is a 68-year-old patient of yours who has uncontrolled type 2 diabetes. She still smokes a pack a day, as she has done for the last 53 years, her BMI is 43, and her LDL is 103 on 80 mg of simvastatin. Her additional meds include lisinopril, 70/30 insulin, and a daily aspirin. Despite her inability to quit smoking or lose weight, she is compliant with her medications. You are both very concerned about her future risk of cardiovascular disease. You consider whether she would benefit from adding clopidogrel to aspirin in order to prevent future coronary events.
Do you write a prescription for clopidogrel, 75 mg/day?
In your conversation with Jessica, you should have told her that she needs to take the clopidogrel for another 4 months (making it 1 year of therapy following her NSTEMI). 2007 guidelines from the American Heart Association and the American College of Cardiology support this approach.1
In your discussion with John, you should have told him that for drugeluting stents, recent studies have shown an additional benefit to continuing dual therapy longer than 1 year, and that consideration should be given to continuing clopidogrel indefinitely.1,2
In the case of Anna, your patient with multiple atherothrombotic risk factors, you should have opted against writing her a prescription for clopidogrel. The evidence suggests that putting her on clopidogrel might actually increase her risk of cardiovascular death.3
These 3 cases demonstrate how much our understanding of dual antiplatelet therapy (aspirin + clopidogrel) has evolved over the past few years—and even months. Given the volume of information we must sift through daily, it is easy to occasionally miss a study or update. The result? You may be underutilizing combination antiplatelet therapy when treatment is indicated. Or, you may be combining aspirin and clopidogrel in situations where there is insufficient data to support its use—and possibly, in situations where it is associated with an increased incidence of adverse events.
This review can help.
Here we have summarized the latest trials and recommendations on dual antiplatelet therapy as they pertain to:
- acute coronary syndrome (ACS)
- coronary stents
- stroke
- the primary prevention and secondary prevention of distant cardiovascular events.
Should you need a quick reference tool for your office, we have also included an at-a-glance summary of current recommendations (TABLE).
TABLE
Dual antiplatelet therapy: The recommendations at a glance
| Unstable angina/non-ST elevation MI/acute coronary syndrome (without stenting) | American Heart Association/American College of Cardiology Low dose aspirin (75 to 162 mg per day) indefinitely (Strength of Recommendation [SOR]: A), and clopidogrel 75 mg per day for at least 1 month (SOR: A) and ideally up to 1 year (SOR: B)1,7 |
| Cardiac stent placement | American Heart Association/American College of Cardiology Bare metal stent
Drug-eluting stent (DES) |
| Stroke | American Heart Association/American Stroke Association
|
| Primary prevention/secondary prevention of distant cardiovascular events |
|
Dual therapy reduces future MI risk in ACS
The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial in 2001 was one of the first combination trials published; it looked at combination antiplatelet therapy in ACS.4 CURE enrolled patients with NSTEMI and randomized them to receive aspirin plus clopidogrel or aspirin plus placebo for 3 to 12 months. Out of the total number of patients, 9.3% of the dual therapy group reached the endpoint of cardiovascular death, MI, or stroke, compared with 11.4% of the aspirin-only group (relative risk [RR]=0.80 for dual therapy). Further, the rate of each component of the composite outcome tended to be lower in the combination-therapy group.
Colored scanning electron micrograph of a blood clot.In particular, there was an RR of 0.77 for MI and, more specifically, an RR of 0.60 for Q-wave MI in the dual therapy group. There was, however, a 1.38 RR for major bleeding (defined as bleeding resulting in disability, vision loss, or transfusion of at least 2 units of blood) in the combination-therapy group vs the aspirin-only group (3.7% to 2.7%). However, neither rates of life-threatening bleeding nor hemorrhagic stroke were significantly different between the 2 groups.
Overall, the CURE trial showed that combining clopidogrel and aspirin was superior to aspirin alone in preventing repeat ischemic events in patients with NSTEMI for up to 12 months, and this conclusion was supported by a 2007 Cochrane review.5
The Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) trial studied the short-term (30 days) effect of clopidogrel combined with aspirin and fibrinolytic therapy in patients with ST elevation MI (STEMI).6 The study showed that the addition of clopidogrel improved the patency rate of infarctrelated arteries and reduced short-term ischemic complications.
There are, however, no trials looking at combination antiplatelet therapy for STEMI patients beyond 30 days.
Unstable angina/non-ST elevation MI/acute coronary syndrome
The American Heart Association/American College of Cardiology Guidelines recommend that patients with unstable angina (UA)/NSTEMI or ACS receive both clopidogrel 75 mg and aspirin (75 to 162 mg/day) for at least a month, and preferably, up to 12 months (TABLE).1,7
Prolonged therapy needed with drug-eluting stents
The Clopidogrel for the Reduction of Events During Observation (CREDO) trial, published in 2002, enrolled patients referred for percutaneous coronary intervention (PCI), about 89% of whom received at least 1 cardiac stent, and followed them for 12 months.8 All patients received aspirin and clopidogrel up to day 28. From day 29 to 1 year, half the patients continued dual antiplatelet therapy and the other half received aspirin plus placebo.
The primary outcome measure was death, MI, or stroke. The combination therapy group showed a relative risk reduction of 37.4% (RR=0.73) in the combined endpoint, compared with the aspirin/placebo group. CREDO did not show a significant difference in risk for major bleeding between the 2 groups.
Further data from the CURE trial was released in 2004. Researchers conducted a subgroup analysis to see if there was a benefit to combination antiplatelet therapy for patients with NSTEMI who underwent PCI or coronary artery bypass grafting (CABG). This further analysis revealed an RR of 0.72 for the outcome of CV death, MI, or stroke with the addition of long-term (3 to 12 months) clopidogrel to aspirin in the PCI group and an RR of 0.89 for the CABG group with combination therapy. These findings led researchers to conclude that combination antiplatelet therapy is beneficial for high-risk patients undergoing revascularization for NSTEMI regardless of the type of revascularization.9
A 2007 observational study conducted at the Duke Heart Center investigated the addition of long-term clopidogrel to aspirin for both drug-eluting stents (DES) and bare-metal stents (BMS).2 The study population was split into 4 groups:
- combination antiplatelet therapy in patients with DES,
- aspirin alone with DES,
- combination therapy with BMS, and
- aspirin alone with BMS.
Follow-up was conducted at 6, 12, and 24 months. For patients with DES, continuing clopidogrel with aspirin therapy was associated with a significant decrease in death or MI at 24 months (3.1% vs 7.2%, RR=0.43). For patients with BMS, researchers found no significant benefit for those who continued dual antiplatelet therapy longer than 6 months compared with aspirin alone.
The data from this study led the authors to speculate that drug-eluting stents may require protracted—and possibly indefinite—dual antiplatelet therapy with clopidogrel and aspirin. Additional research with a large clinical trial is necessary to assess the appropriate length of treatment with combination antiplatelet therapy after DES, as it may extend even longer than current recommendations suggest.
An analysis of the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER) registry illustrates the catastrophic consequences of premature discontinuation of dual antiplatelet therapy.10 This study of 500 patients with acute MI treated with DES revealed a striking increase in mortality rate related to discontinuation of dual antiplatelet therapy within 3 months of their initial MI. Patients who prematurely stopped dual therapy had a mortality rate of 7.5% over the next 11 months compared to 0.7% in patients who continued dual antiplatelet therapy for a minimum of 3 months.
Of note: Researchers say that patients who self-discontinued the dual therapy were less likely to have been given instructions about their medication at discharge. In addition, these patients were less likely to have completed high school and more likely to avoid health care because of cost.
Finally, a recent study that evaluated the safety of off-label use of drug-eluting stents revealed further data in support of prolonged use of dual antiplatelet therapy in this group.11 (Off-label use included, among other things, using sirolimus- and paclitaxel eluting stents on lesions that were longer than specified in the stent manufacturer’s “information for use.”) Forty-seven percent of patients who received DES were for off-label indications.
Among the off-label patients, the 1-year rate of death, MI, or stent thrombosis was 7.7% for standard dual therapy duration (3 months for sirolimus-eluting stents and 6 months for paclitaxel-eluting stents) compared with 6.0% (RR=0.78) for prolonged duration (defined as beyond standard duration).
Cardiac stent placement
Current American Heart Association/American College of Cardiology recommendations state that full-dose aspirin (162–325 mg/day) should be used for 1 month after BMS implantation, at least 3 months after sirolimus DES implantation, and 6 months after paclitaxel DES implantation, then continued indefinitely at low dose (75 to 162 mg/day).1
Clopidogrel 75 mg/day should be added to aspirin for a minimum of 1 month, and ideally up to a year for BMS; and for a minimum of 12 months for all DES regardless of type.1 In addition to the duration and type of therapy, the American Heart Association states it is not appropriate to withhold dual therapy in stent patients for minor surgery or dental procedures within their recommended treatment period.12
Of note: The American Heart Association and American College of Cardiology guidelines, cited above, are an August 2007 revision to the groups’ 2002 guidelines.1 Although specifically written for UA /NSTEMI, the revision states that clopidogrel should be added to aspirin for at least 12 months in “all post-PCI patients receiving DES.” A revised AHA/ACC PCI guideline, however, has not yet been published to reflect these new data and recommendations.
Bleeding rates offset stroke prevention benefits
The Management of ATherothrombosis with Clopidogrel in High-risk patients (MATCH) trial is the only major trial that has evaluated combination clopidogrel and aspirin therapy for secondary prevention of stroke.13 The MATCH trial, first published in 2004, differed from the cardiovascular antiplatelet trials in that it used clopidogrel in its control group instead of aspirin.
Patients who’d had a recent ischemic stroke or transient ischemic attack (TIA) and at least 1 additional vascular risk factor were randomized to receive clopidogrel plus aspirin therapy or clopidogrel plus placebo for 18 months. Primary outcome measure was a composite of ischemic stroke, MI, vascular death, or rehospitalization for acute ischemia. Although researchers observed a consistent reduction of vascular events in the combination therapy group, the differences did not reach statistical significance. In addition, higher bleeding rates “offset any beneficial effect” of combination therapy.
Overall, treatment with aspirin and clopidogrel compared with aspirin alone might prevent 10 ischemic events per 1000 treated (not statistically significant) at a cost of 13 life-threatening hemorrhages per 1000 treated. The researchers concluded that there was no demonstrable benefit of adding aspirin to clopidogrel for secondary prevention of stroke.
A major limitation of this study was that the majority of patients enrolled had lacunar strokes, a stroke subtype that is not believed to be of pure atherothrombotic origin and that has the lowest recurrence rates.13 Future studies on secondary stroke prevention will address issues of stroke subtype and location.14
Other trials that did not specifically look at clopidogrel + aspirin therapy for noncardioembolic stroke but examined relevant issues also warrant mention:
- The Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events—W (ACTIVE W) trial compared an aspirin + clopidogrel combination to warfarin in patients with atrial fibrillation.15 ACTIVE W showed warfarin to be superior to combination antiplatelet therapy for prevention of vascular events including stroke, non-central nervous system emboli, MI, or vascular death (RR=1.44 for combination therapy).
- The Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial investigated combination antiplatelet therapy in patients with recently symptomatic carotid artery stenosis.16 Researchers in this small study used doppler ultrasound to see if combination therapy would lead to a reduction in asymptomatic microembolism from the carotid plaques. Patients received clopidogrel plus aspirin or aspirin alone for 7 days. The patients in the combination group had a 40% relative reduction in microembolism.
Clearly, the CARESS trial opens the door for further study into clinically important outcomes for carotid artery stenosis and dual antiplatelet therapy.
Stroke
The current American Heart Association/American Stroke Association guidelines recommend aspirin plus extended-release dipyridamole or clopidogrel alone for the secondary prevention of noncardioembolic stroke. The guidelines state that the “addition of aspirin to clopidogrel increases risk of hemorrhage and is not routinely recommended.” However, aspirin + clopidogrel combination therapy may be appropriate in stroke patients with recent ACS or vascular stents.17
When prevention efforts can harm patients
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial studied the value of using aspirin + clopidogrel combination therapy for primary or secondary prevention of distant coronary events (longer than 12 months).3 CHARISMA researchers studied patients with either multiple cardiac risk factors (termed “asymptomatic”) or clinically evident cardiovascular disease (termed “symptomatic”) who did not have a current indication for dual antiplatelet therapy (ie, recent MI or coronary stent).
The patients were randomized to receive aspirin plus clopidogrel or aspirin plus placebo and were followed for a median length of 28 months. The primary outcome measure was a combination of MI, stroke, or cardiovascular death. Overall, there was no significant difference in the primary endpoint, which occurred in 6.8% of the combination group and in 7.3% of the aspirin-only group (RR=0.93).
Subgroup analysis revealed that asymptomatic patients actually had a 20% increase in primary events with the addition of clopidogrel to aspirin (6.6% vs 5.5%, RR=1.20), and an increase in death (3.9% vs 2.2%, RR=1.77). In the symptomatic group, however, there was a “marginally significant” reduction in primary events, with 6.9% of patients in the combination group and 7.9% of patients in the aspirin-only group experiencing primary events (RR=0.88). The rate of severe bleeding in the combination group was 1.7% compared with 1.3% in the placebo group (RR=1.25).
The authors of CHARISMA propose a hypothesis to explain the seemingly paradoxical effect of an increase of primary endpoints in the asymptomatic group. They suggest that established vascular disease could be a proxy for hyperactive platelets. Thereby, the action of combination therapy may be more effective in patients with established vascular disease and less effective in patients with normal platelets.
Primary prevention/secondary prevention of distant cardiovascular events
No evidence or recommendations currently exist to support using dual antiplatelet therapy for primary prevention or secondary prevention of cardiovascular events in patients not covered under current American Heart Association/American College of Cardiology guidelines for recent ACS or stent/PCI. Adding clopidogrel to aspirin may cause harm in patients without established cardiovascular disease.3
Ongoing studies explore stroke subtypes, CABG
Because of insufficient or conflicting data, it is unclear whether dual therapy is advisable for a number of disease states, including specific stroke subtypes, peripheral artery disease, and CABG.18 The following ongoing dual therapy studies may provide some answers:14
- The Secondary Prevention of Small Subcortical Strokes (SPS3) trial is investigating secondary prevention of subcortical stroke.
- The Fast Assessment of Stroke and Transient ischemic attack to prevent Early Recurrence (FASTER) trial is also investigating secondary prevention of stroke.
- The Aortic arch Related Cerebral Hazard (ARCH) trial is investigating aortic plaques and stroke.
- The Effects of Physical Training, ASA and Clopidogrel on the Walking Capacity of Patients with Stage II PAD trial is investigating walking capacity in patients with peripheral artery disease.
Correspondence
B. Brent Simmons, MD, 150 E. Wynnewood Rd., Apt. 18 J, Wynnewood, PA 19096; [email protected].
- Patients with drug-eluting stents should receive dual therapy (aspirin + clopidogrel) for at least 12 months (B).
- For patients who have had an ischemic stroke or transient ischemic attack, adding aspirin to clopidogrel increases the risk of hemorrhage and is not routinely recommended (A).
- Adding clopidogrel to aspirin is not more effective than aspirin alone in the primary prevention of coronary artery disease in patients with multiple risk factors. In fact, it may actually cause harm in patients without established cardiovascular disease (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Jessica is 72 years old and a new patient of yours, having recently moved to the area. She has a history of non-ST elevation myocardial infarction (NSTEMI) 8 months ago, hypertension, and hyperlipidemia. Her NSTEMI did not require stent placement. Upon reviewing her medications, you see she is taking aspirin 81 mg, clopidogrel 75 mg, simvastatin 80 mg, and metoprolol XL 100 mg. The patient isn’t sure how long she has been on clopidogrel and wants to know if she still needs to take it because she has a high co-pay.
What would you tell her?
John is a 60-year-old patient of yours with a history of coronary artery disease, who had an elective catheterization 18 months ago at which time a sirolimus drug-eluting stent was placed. Also at that time, he was placed on aspirin 325 mg daily and clopidogrel 75 mg daily. The patient says he has a friend at work who also has a coronary stent, but his friend stopped taking clopidogrel 12 months after his stent was placed. The patient wants to know why he is still taking clopidogrel and when he can stop it.
What would you tell him?
Anna is a 68-year-old patient of yours who has uncontrolled type 2 diabetes. She still smokes a pack a day, as she has done for the last 53 years, her BMI is 43, and her LDL is 103 on 80 mg of simvastatin. Her additional meds include lisinopril, 70/30 insulin, and a daily aspirin. Despite her inability to quit smoking or lose weight, she is compliant with her medications. You are both very concerned about her future risk of cardiovascular disease. You consider whether she would benefit from adding clopidogrel to aspirin in order to prevent future coronary events.
Do you write a prescription for clopidogrel, 75 mg/day?
In your conversation with Jessica, you should have told her that she needs to take the clopidogrel for another 4 months (making it 1 year of therapy following her NSTEMI). 2007 guidelines from the American Heart Association and the American College of Cardiology support this approach.1
In your discussion with John, you should have told him that for drugeluting stents, recent studies have shown an additional benefit to continuing dual therapy longer than 1 year, and that consideration should be given to continuing clopidogrel indefinitely.1,2
In the case of Anna, your patient with multiple atherothrombotic risk factors, you should have opted against writing her a prescription for clopidogrel. The evidence suggests that putting her on clopidogrel might actually increase her risk of cardiovascular death.3
These 3 cases demonstrate how much our understanding of dual antiplatelet therapy (aspirin + clopidogrel) has evolved over the past few years—and even months. Given the volume of information we must sift through daily, it is easy to occasionally miss a study or update. The result? You may be underutilizing combination antiplatelet therapy when treatment is indicated. Or, you may be combining aspirin and clopidogrel in situations where there is insufficient data to support its use—and possibly, in situations where it is associated with an increased incidence of adverse events.
This review can help.
Here we have summarized the latest trials and recommendations on dual antiplatelet therapy as they pertain to:
- acute coronary syndrome (ACS)
- coronary stents
- stroke
- the primary prevention and secondary prevention of distant cardiovascular events.
Should you need a quick reference tool for your office, we have also included an at-a-glance summary of current recommendations (TABLE).
TABLE
Dual antiplatelet therapy: The recommendations at a glance
| Unstable angina/non-ST elevation MI/acute coronary syndrome (without stenting) | American Heart Association/American College of Cardiology Low dose aspirin (75 to 162 mg per day) indefinitely (Strength of Recommendation [SOR]: A), and clopidogrel 75 mg per day for at least 1 month (SOR: A) and ideally up to 1 year (SOR: B)1,7 |
| Cardiac stent placement | American Heart Association/American College of Cardiology Bare metal stent
Drug-eluting stent (DES) |
| Stroke | American Heart Association/American Stroke Association
|
| Primary prevention/secondary prevention of distant cardiovascular events |
|
Dual therapy reduces future MI risk in ACS
The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial in 2001 was one of the first combination trials published; it looked at combination antiplatelet therapy in ACS.4 CURE enrolled patients with NSTEMI and randomized them to receive aspirin plus clopidogrel or aspirin plus placebo for 3 to 12 months. Out of the total number of patients, 9.3% of the dual therapy group reached the endpoint of cardiovascular death, MI, or stroke, compared with 11.4% of the aspirin-only group (relative risk [RR]=0.80 for dual therapy). Further, the rate of each component of the composite outcome tended to be lower in the combination-therapy group.
Colored scanning electron micrograph of a blood clot.In particular, there was an RR of 0.77 for MI and, more specifically, an RR of 0.60 for Q-wave MI in the dual therapy group. There was, however, a 1.38 RR for major bleeding (defined as bleeding resulting in disability, vision loss, or transfusion of at least 2 units of blood) in the combination-therapy group vs the aspirin-only group (3.7% to 2.7%). However, neither rates of life-threatening bleeding nor hemorrhagic stroke were significantly different between the 2 groups.
Overall, the CURE trial showed that combining clopidogrel and aspirin was superior to aspirin alone in preventing repeat ischemic events in patients with NSTEMI for up to 12 months, and this conclusion was supported by a 2007 Cochrane review.5
The Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) trial studied the short-term (30 days) effect of clopidogrel combined with aspirin and fibrinolytic therapy in patients with ST elevation MI (STEMI).6 The study showed that the addition of clopidogrel improved the patency rate of infarctrelated arteries and reduced short-term ischemic complications.
There are, however, no trials looking at combination antiplatelet therapy for STEMI patients beyond 30 days.
Unstable angina/non-ST elevation MI/acute coronary syndrome
The American Heart Association/American College of Cardiology Guidelines recommend that patients with unstable angina (UA)/NSTEMI or ACS receive both clopidogrel 75 mg and aspirin (75 to 162 mg/day) for at least a month, and preferably, up to 12 months (TABLE).1,7
Prolonged therapy needed with drug-eluting stents
The Clopidogrel for the Reduction of Events During Observation (CREDO) trial, published in 2002, enrolled patients referred for percutaneous coronary intervention (PCI), about 89% of whom received at least 1 cardiac stent, and followed them for 12 months.8 All patients received aspirin and clopidogrel up to day 28. From day 29 to 1 year, half the patients continued dual antiplatelet therapy and the other half received aspirin plus placebo.
The primary outcome measure was death, MI, or stroke. The combination therapy group showed a relative risk reduction of 37.4% (RR=0.73) in the combined endpoint, compared with the aspirin/placebo group. CREDO did not show a significant difference in risk for major bleeding between the 2 groups.
Further data from the CURE trial was released in 2004. Researchers conducted a subgroup analysis to see if there was a benefit to combination antiplatelet therapy for patients with NSTEMI who underwent PCI or coronary artery bypass grafting (CABG). This further analysis revealed an RR of 0.72 for the outcome of CV death, MI, or stroke with the addition of long-term (3 to 12 months) clopidogrel to aspirin in the PCI group and an RR of 0.89 for the CABG group with combination therapy. These findings led researchers to conclude that combination antiplatelet therapy is beneficial for high-risk patients undergoing revascularization for NSTEMI regardless of the type of revascularization.9
A 2007 observational study conducted at the Duke Heart Center investigated the addition of long-term clopidogrel to aspirin for both drug-eluting stents (DES) and bare-metal stents (BMS).2 The study population was split into 4 groups:
- combination antiplatelet therapy in patients with DES,
- aspirin alone with DES,
- combination therapy with BMS, and
- aspirin alone with BMS.
Follow-up was conducted at 6, 12, and 24 months. For patients with DES, continuing clopidogrel with aspirin therapy was associated with a significant decrease in death or MI at 24 months (3.1% vs 7.2%, RR=0.43). For patients with BMS, researchers found no significant benefit for those who continued dual antiplatelet therapy longer than 6 months compared with aspirin alone.
The data from this study led the authors to speculate that drug-eluting stents may require protracted—and possibly indefinite—dual antiplatelet therapy with clopidogrel and aspirin. Additional research with a large clinical trial is necessary to assess the appropriate length of treatment with combination antiplatelet therapy after DES, as it may extend even longer than current recommendations suggest.
An analysis of the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER) registry illustrates the catastrophic consequences of premature discontinuation of dual antiplatelet therapy.10 This study of 500 patients with acute MI treated with DES revealed a striking increase in mortality rate related to discontinuation of dual antiplatelet therapy within 3 months of their initial MI. Patients who prematurely stopped dual therapy had a mortality rate of 7.5% over the next 11 months compared to 0.7% in patients who continued dual antiplatelet therapy for a minimum of 3 months.
Of note: Researchers say that patients who self-discontinued the dual therapy were less likely to have been given instructions about their medication at discharge. In addition, these patients were less likely to have completed high school and more likely to avoid health care because of cost.
Finally, a recent study that evaluated the safety of off-label use of drug-eluting stents revealed further data in support of prolonged use of dual antiplatelet therapy in this group.11 (Off-label use included, among other things, using sirolimus- and paclitaxel eluting stents on lesions that were longer than specified in the stent manufacturer’s “information for use.”) Forty-seven percent of patients who received DES were for off-label indications.
Among the off-label patients, the 1-year rate of death, MI, or stent thrombosis was 7.7% for standard dual therapy duration (3 months for sirolimus-eluting stents and 6 months for paclitaxel-eluting stents) compared with 6.0% (RR=0.78) for prolonged duration (defined as beyond standard duration).
Cardiac stent placement
Current American Heart Association/American College of Cardiology recommendations state that full-dose aspirin (162–325 mg/day) should be used for 1 month after BMS implantation, at least 3 months after sirolimus DES implantation, and 6 months after paclitaxel DES implantation, then continued indefinitely at low dose (75 to 162 mg/day).1
Clopidogrel 75 mg/day should be added to aspirin for a minimum of 1 month, and ideally up to a year for BMS; and for a minimum of 12 months for all DES regardless of type.1 In addition to the duration and type of therapy, the American Heart Association states it is not appropriate to withhold dual therapy in stent patients for minor surgery or dental procedures within their recommended treatment period.12
Of note: The American Heart Association and American College of Cardiology guidelines, cited above, are an August 2007 revision to the groups’ 2002 guidelines.1 Although specifically written for UA /NSTEMI, the revision states that clopidogrel should be added to aspirin for at least 12 months in “all post-PCI patients receiving DES.” A revised AHA/ACC PCI guideline, however, has not yet been published to reflect these new data and recommendations.
Bleeding rates offset stroke prevention benefits
The Management of ATherothrombosis with Clopidogrel in High-risk patients (MATCH) trial is the only major trial that has evaluated combination clopidogrel and aspirin therapy for secondary prevention of stroke.13 The MATCH trial, first published in 2004, differed from the cardiovascular antiplatelet trials in that it used clopidogrel in its control group instead of aspirin.
Patients who’d had a recent ischemic stroke or transient ischemic attack (TIA) and at least 1 additional vascular risk factor were randomized to receive clopidogrel plus aspirin therapy or clopidogrel plus placebo for 18 months. Primary outcome measure was a composite of ischemic stroke, MI, vascular death, or rehospitalization for acute ischemia. Although researchers observed a consistent reduction of vascular events in the combination therapy group, the differences did not reach statistical significance. In addition, higher bleeding rates “offset any beneficial effect” of combination therapy.
Overall, treatment with aspirin and clopidogrel compared with aspirin alone might prevent 10 ischemic events per 1000 treated (not statistically significant) at a cost of 13 life-threatening hemorrhages per 1000 treated. The researchers concluded that there was no demonstrable benefit of adding aspirin to clopidogrel for secondary prevention of stroke.
A major limitation of this study was that the majority of patients enrolled had lacunar strokes, a stroke subtype that is not believed to be of pure atherothrombotic origin and that has the lowest recurrence rates.13 Future studies on secondary stroke prevention will address issues of stroke subtype and location.14
Other trials that did not specifically look at clopidogrel + aspirin therapy for noncardioembolic stroke but examined relevant issues also warrant mention:
- The Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events—W (ACTIVE W) trial compared an aspirin + clopidogrel combination to warfarin in patients with atrial fibrillation.15 ACTIVE W showed warfarin to be superior to combination antiplatelet therapy for prevention of vascular events including stroke, non-central nervous system emboli, MI, or vascular death (RR=1.44 for combination therapy).
- The Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial investigated combination antiplatelet therapy in patients with recently symptomatic carotid artery stenosis.16 Researchers in this small study used doppler ultrasound to see if combination therapy would lead to a reduction in asymptomatic microembolism from the carotid plaques. Patients received clopidogrel plus aspirin or aspirin alone for 7 days. The patients in the combination group had a 40% relative reduction in microembolism.
Clearly, the CARESS trial opens the door for further study into clinically important outcomes for carotid artery stenosis and dual antiplatelet therapy.
Stroke
The current American Heart Association/American Stroke Association guidelines recommend aspirin plus extended-release dipyridamole or clopidogrel alone for the secondary prevention of noncardioembolic stroke. The guidelines state that the “addition of aspirin to clopidogrel increases risk of hemorrhage and is not routinely recommended.” However, aspirin + clopidogrel combination therapy may be appropriate in stroke patients with recent ACS or vascular stents.17
When prevention efforts can harm patients
The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial studied the value of using aspirin + clopidogrel combination therapy for primary or secondary prevention of distant coronary events (longer than 12 months).3 CHARISMA researchers studied patients with either multiple cardiac risk factors (termed “asymptomatic”) or clinically evident cardiovascular disease (termed “symptomatic”) who did not have a current indication for dual antiplatelet therapy (ie, recent MI or coronary stent).
The patients were randomized to receive aspirin plus clopidogrel or aspirin plus placebo and were followed for a median length of 28 months. The primary outcome measure was a combination of MI, stroke, or cardiovascular death. Overall, there was no significant difference in the primary endpoint, which occurred in 6.8% of the combination group and in 7.3% of the aspirin-only group (RR=0.93).
Subgroup analysis revealed that asymptomatic patients actually had a 20% increase in primary events with the addition of clopidogrel to aspirin (6.6% vs 5.5%, RR=1.20), and an increase in death (3.9% vs 2.2%, RR=1.77). In the symptomatic group, however, there was a “marginally significant” reduction in primary events, with 6.9% of patients in the combination group and 7.9% of patients in the aspirin-only group experiencing primary events (RR=0.88). The rate of severe bleeding in the combination group was 1.7% compared with 1.3% in the placebo group (RR=1.25).
The authors of CHARISMA propose a hypothesis to explain the seemingly paradoxical effect of an increase of primary endpoints in the asymptomatic group. They suggest that established vascular disease could be a proxy for hyperactive platelets. Thereby, the action of combination therapy may be more effective in patients with established vascular disease and less effective in patients with normal platelets.
Primary prevention/secondary prevention of distant cardiovascular events
No evidence or recommendations currently exist to support using dual antiplatelet therapy for primary prevention or secondary prevention of cardiovascular events in patients not covered under current American Heart Association/American College of Cardiology guidelines for recent ACS or stent/PCI. Adding clopidogrel to aspirin may cause harm in patients without established cardiovascular disease.3
Ongoing studies explore stroke subtypes, CABG
Because of insufficient or conflicting data, it is unclear whether dual therapy is advisable for a number of disease states, including specific stroke subtypes, peripheral artery disease, and CABG.18 The following ongoing dual therapy studies may provide some answers:14
- The Secondary Prevention of Small Subcortical Strokes (SPS3) trial is investigating secondary prevention of subcortical stroke.
- The Fast Assessment of Stroke and Transient ischemic attack to prevent Early Recurrence (FASTER) trial is also investigating secondary prevention of stroke.
- The Aortic arch Related Cerebral Hazard (ARCH) trial is investigating aortic plaques and stroke.
- The Effects of Physical Training, ASA and Clopidogrel on the Walking Capacity of Patients with Stage II PAD trial is investigating walking capacity in patients with peripheral artery disease.
Correspondence
B. Brent Simmons, MD, 150 E. Wynnewood Rd., Apt. 18 J, Wynnewood, PA 19096; [email protected].
1. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction-executive summary. J Am Coll Cardiol 2007;50:652-726.
2. Eisentstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA 2007;297:159-168.
3. Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706-1717.
4. CURE Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502.
5. Keller TT, Squizzato A, Middeldorp S. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular disease (review). Cochrane Database Syst Rev 2007;(3):CD005158.-
6. Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005;352:1179-1189.
7. Smith SC, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation 2006;113:2363-2372.
8. Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention. JAMA 2002;288:2411-2420.
9. Fox KAA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST elevation acute coronary syndrome. Circulation 2004;110:1202-1208.
10. Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement. Circulation 2006;113:2803-2809.
11. Beohar N, Davidson CJ, Kip KE, et al. Outcomes and complications associated with off-label and untested use of drug-eluting stents. JAMA 2007;297:1992-2000.
12. Grines CL, Bonow RO, Casey DE, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents. Circulation 2007;115:813-818.
13. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH). Lancet 2004;364:331-337.
14. US National Institutes of Health. ClinicalTrials.gov [Web site]. Available at: www.clinicaltrials.gov. Accessed on March 4, 2007.
15. ACTIVE Writing Group. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the atrial fibrillation clopidogrel trial with irbesartan for the prevention of vascular events. Lancet 2006;367:1903-1912.
16. Markus HS, Droste DV, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid artery stenosis evaluated using doppler embolic signal detection: CARESS trial. Circulation 2005;111:2233-2240.
17. Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Stroke 2006;37:577-617.
18. Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA guideline update for coronary artery bypass graft surgery. Circulation 2004;110:e340-e437.Available at: www.acc.org/qualityandscience/clinical/guidelines/cabg/index.pdf. Accessed November 11, 2007.
1. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction-executive summary. J Am Coll Cardiol 2007;50:652-726.
2. Eisentstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA 2007;297:159-168.
3. Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706-1717.
4. CURE Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502.
5. Keller TT, Squizzato A, Middeldorp S. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular disease (review). Cochrane Database Syst Rev 2007;(3):CD005158.-
6. Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005;352:1179-1189.
7. Smith SC, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation 2006;113:2363-2372.
8. Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention. JAMA 2002;288:2411-2420.
9. Fox KAA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST elevation acute coronary syndrome. Circulation 2004;110:1202-1208.
10. Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement. Circulation 2006;113:2803-2809.
11. Beohar N, Davidson CJ, Kip KE, et al. Outcomes and complications associated with off-label and untested use of drug-eluting stents. JAMA 2007;297:1992-2000.
12. Grines CL, Bonow RO, Casey DE, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents. Circulation 2007;115:813-818.
13. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH). Lancet 2004;364:331-337.
14. US National Institutes of Health. ClinicalTrials.gov [Web site]. Available at: www.clinicaltrials.gov. Accessed on March 4, 2007.
15. ACTIVE Writing Group. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the atrial fibrillation clopidogrel trial with irbesartan for the prevention of vascular events. Lancet 2006;367:1903-1912.
16. Markus HS, Droste DV, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid artery stenosis evaluated using doppler embolic signal detection: CARESS trial. Circulation 2005;111:2233-2240.
17. Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Stroke 2006;37:577-617.
18. Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA guideline update for coronary artery bypass graft surgery. Circulation 2004;110:e340-e437.Available at: www.acc.org/qualityandscience/clinical/guidelines/cabg/index.pdf. Accessed November 11, 2007.
An unusual case of chest pain
- Sharp chest pain radiating down the right arm
- Syncope
- Temp 97.3°F, pulse 78, respirations 21, BP 162/96 mm Hg, O2 saturation 98% on room air
- ECG: normal sinus rhythm, rate 70, and no ST-T wave changes
A 47-year-old African American woman came into our emergency department with right-sided chest pain that she described as sharp, and radiating down her right arm. She said she’d lost consciousness for a few seconds with only minimal exertion. She told us that she was being treated for hypertension by her primary care physician, and indicated that she’d never had a fainting spell before.
Our patient’s vital signs were:
- temperature 97.3°F
- pulse 78 beats per minute, respirations 21
- blood pressure 162/96 mm Hg
- O2 saturation 98% on room air.
She had a regular heart rate and rhythm, no murmurs, no rubs, no gal lops, and her pulses were 2+ bilateral and equal.
Her lung exam was clear to auscultation bilaterally; she had no tachypnea, wheezes, or crackles. The remainder of her physical exam was unremarkable. She was taking verapamil once a day and Excedrin as needed, but no other medications.
Our patient’s electrocardiogram (ECG) showed a normal sinus rhythm, rate 70, and no ST-T wave changes. We admitted her for further evaluation.
Following serial cardiac enzymes and ECGs, we ruled out a myocardial infarction. We ordered an exercise stress test, but it had to be stopped when she developed light-headedness and hypotension.
What do you suspect is causing this woman’s chest pain and fainting spells?
Echo unlocks the mystery
We ordered a transesophageal echo- cardiogram, which revealed a spherical 2 cm x 1.5 cm mass attached to the lateral wall of the right atrium just above the tricuspid valve (FIGURE, TOP LEFT). Differential diagnoses for this mass were atrial myxoma, intracardiac thrombus, lipomatous infiltrations, and calcified mitral annulus.1 A positron-emission tomography (PET) scan was nondiagnostic.
The mass was surgically excised. Pathology showed right atrial papillary myxoma, which was negative for signs of malignancy (FIGURE, BOTTOM LEFT).
A rare cardiac mass
While rare, papillary myxomas are the most common primary cardiac mass. On histologic exam, they are gelatinous structures that consist of myxoma cells embedded in a stroma rich in glycosamino- glycans.2,3 On macroscopic exam, myxomas are pedunculated and have a variable surface. These masses produce vascular endothelial growth factor, which could contribute to the malignant potential of histologically benign myxomas.4,5
Listen for “tumor plop”
Symptoms of myxomas depend on the location of the tumor. Myxomas are frequently accompanied by constitutional symptoms such as fever and weight loss (although our patient had neither). Embolization is also associated with such lesions.
Left-sided lesions present with symptoms of mitral valve obstruction, along with electrocardiographic changes of left atrial hypertrophy.2 Ventricular myxomas may cause outflow obstruction.3
The signs and symptoms of myxoma may have a sudden onset or be positional in nature, reflecting changes in tumor position due to gravity. An auscultatory finding, called “tumor plop,” is a characteristic low-pitched sound that may be audible during early or mid-diastole,6 though we heard no such sound during our evaluation of our patient.
Our patient was unusual
Diagnosis is made by echocardiography, and most (80%) of myxomas are found in the left atrium. Atrial myxoma typically appears as a well-circumscribed mobile mass that is attached to the atrial septum. Our patient, however, had a myxoma located in the right atrium that was attached to the lateral wall. Definitive diagnosis is made by surgical excision.
Treatment: Prompt resection
Due to the risk of embolization and cardiovascular compromise, prompt resection is recommended and is generally curative, as it was for our patient.3,7,8 Postoperative mortality rate after resection is under 5%. For multiple recurrent tumors, cardiac transplantation should be considered.1,9
Correspondence
Family medicine Department, John Peter Smith Hospital, 1500 South main St, Fort Worth, TX 76104; hrana@ jpshealth.org
1. Michler RE, Goldstein DJ. Treatment of cardiac tumors by orthotopic cardiac transplantation. Semin Oncol 1997;24:53-539.
2. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Balt) 2001;80:159-172.
3. Nishimura R, Gibbons R, Glockner J, Tajik A. Noninvasive cardiac imaging: echocardiography, nuclear cardiology, and mrI/cT imaging. In: Kasper Dl, Braunwald E, Hauser S, longo D, Jameson JL, Fauci AS (eds), Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: mcGraw-Hill; 2004:chap 211
4. Kono T, Koide N, Hama Y, et al. expression of vascular endothelial growth factor and angiogenesis in cardiac myxoma: A study of fifteen patients. J Thorac Cardiovasc Surg 2000;119:101-107.
5. Sakamoto H, Sakamaki T, Kanda T, et al. Vascular endothelial growth factor is an autocrine growth factor for cardiac myxoma cells. Circ J 2004;68:488-493.
6. Colucci W, Price D. Cardiac tumors, cardiac manifestations of systemic diseases, and traumatic cardiac injury. In: Kasper DL, Braunwald E, Hauser S, Longo D, Jameson JL, Fauci AS (eds), Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: mcGraw-Hill; 2004:chap 223
7. Keeling IM, Oberwalder P, Anelli-monti M, et al. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardiothorac Surg 2002;22:971-977.
8. Selkane C, Amahzoune B, Chavanis N, et al. Changing management of cardiac myxoma based on a series of 40 cases with long-term follow-up. Ann Thorac Surg 2003;76:1935-1938.
9. Goldstein DJ, Oz MC, Rose EA, Fisher P, Michler RE. Experience with heart transplantation for cardiac tumors. J Heart Lung Transplant 1995;14:382-386.
- Sharp chest pain radiating down the right arm
- Syncope
- Temp 97.3°F, pulse 78, respirations 21, BP 162/96 mm Hg, O2 saturation 98% on room air
- ECG: normal sinus rhythm, rate 70, and no ST-T wave changes
A 47-year-old African American woman came into our emergency department with right-sided chest pain that she described as sharp, and radiating down her right arm. She said she’d lost consciousness for a few seconds with only minimal exertion. She told us that she was being treated for hypertension by her primary care physician, and indicated that she’d never had a fainting spell before.
Our patient’s vital signs were:
- temperature 97.3°F
- pulse 78 beats per minute, respirations 21
- blood pressure 162/96 mm Hg
- O2 saturation 98% on room air.
She had a regular heart rate and rhythm, no murmurs, no rubs, no gal lops, and her pulses were 2+ bilateral and equal.
Her lung exam was clear to auscultation bilaterally; she had no tachypnea, wheezes, or crackles. The remainder of her physical exam was unremarkable. She was taking verapamil once a day and Excedrin as needed, but no other medications.
Our patient’s electrocardiogram (ECG) showed a normal sinus rhythm, rate 70, and no ST-T wave changes. We admitted her for further evaluation.
Following serial cardiac enzymes and ECGs, we ruled out a myocardial infarction. We ordered an exercise stress test, but it had to be stopped when she developed light-headedness and hypotension.
What do you suspect is causing this woman’s chest pain and fainting spells?
Echo unlocks the mystery
We ordered a transesophageal echo- cardiogram, which revealed a spherical 2 cm x 1.5 cm mass attached to the lateral wall of the right atrium just above the tricuspid valve (FIGURE, TOP LEFT). Differential diagnoses for this mass were atrial myxoma, intracardiac thrombus, lipomatous infiltrations, and calcified mitral annulus.1 A positron-emission tomography (PET) scan was nondiagnostic.
The mass was surgically excised. Pathology showed right atrial papillary myxoma, which was negative for signs of malignancy (FIGURE, BOTTOM LEFT).
A rare cardiac mass
While rare, papillary myxomas are the most common primary cardiac mass. On histologic exam, they are gelatinous structures that consist of myxoma cells embedded in a stroma rich in glycosamino- glycans.2,3 On macroscopic exam, myxomas are pedunculated and have a variable surface. These masses produce vascular endothelial growth factor, which could contribute to the malignant potential of histologically benign myxomas.4,5
Listen for “tumor plop”
Symptoms of myxomas depend on the location of the tumor. Myxomas are frequently accompanied by constitutional symptoms such as fever and weight loss (although our patient had neither). Embolization is also associated with such lesions.
Left-sided lesions present with symptoms of mitral valve obstruction, along with electrocardiographic changes of left atrial hypertrophy.2 Ventricular myxomas may cause outflow obstruction.3
The signs and symptoms of myxoma may have a sudden onset or be positional in nature, reflecting changes in tumor position due to gravity. An auscultatory finding, called “tumor plop,” is a characteristic low-pitched sound that may be audible during early or mid-diastole,6 though we heard no such sound during our evaluation of our patient.
Our patient was unusual
Diagnosis is made by echocardiography, and most (80%) of myxomas are found in the left atrium. Atrial myxoma typically appears as a well-circumscribed mobile mass that is attached to the atrial septum. Our patient, however, had a myxoma located in the right atrium that was attached to the lateral wall. Definitive diagnosis is made by surgical excision.
Treatment: Prompt resection
Due to the risk of embolization and cardiovascular compromise, prompt resection is recommended and is generally curative, as it was for our patient.3,7,8 Postoperative mortality rate after resection is under 5%. For multiple recurrent tumors, cardiac transplantation should be considered.1,9
Correspondence
Family medicine Department, John Peter Smith Hospital, 1500 South main St, Fort Worth, TX 76104; hrana@ jpshealth.org
- Sharp chest pain radiating down the right arm
- Syncope
- Temp 97.3°F, pulse 78, respirations 21, BP 162/96 mm Hg, O2 saturation 98% on room air
- ECG: normal sinus rhythm, rate 70, and no ST-T wave changes
A 47-year-old African American woman came into our emergency department with right-sided chest pain that she described as sharp, and radiating down her right arm. She said she’d lost consciousness for a few seconds with only minimal exertion. She told us that she was being treated for hypertension by her primary care physician, and indicated that she’d never had a fainting spell before.
Our patient’s vital signs were:
- temperature 97.3°F
- pulse 78 beats per minute, respirations 21
- blood pressure 162/96 mm Hg
- O2 saturation 98% on room air.
She had a regular heart rate and rhythm, no murmurs, no rubs, no gal lops, and her pulses were 2+ bilateral and equal.
Her lung exam was clear to auscultation bilaterally; she had no tachypnea, wheezes, or crackles. The remainder of her physical exam was unremarkable. She was taking verapamil once a day and Excedrin as needed, but no other medications.
Our patient’s electrocardiogram (ECG) showed a normal sinus rhythm, rate 70, and no ST-T wave changes. We admitted her for further evaluation.
Following serial cardiac enzymes and ECGs, we ruled out a myocardial infarction. We ordered an exercise stress test, but it had to be stopped when she developed light-headedness and hypotension.
What do you suspect is causing this woman’s chest pain and fainting spells?
Echo unlocks the mystery
We ordered a transesophageal echo- cardiogram, which revealed a spherical 2 cm x 1.5 cm mass attached to the lateral wall of the right atrium just above the tricuspid valve (FIGURE, TOP LEFT). Differential diagnoses for this mass were atrial myxoma, intracardiac thrombus, lipomatous infiltrations, and calcified mitral annulus.1 A positron-emission tomography (PET) scan was nondiagnostic.
The mass was surgically excised. Pathology showed right atrial papillary myxoma, which was negative for signs of malignancy (FIGURE, BOTTOM LEFT).
A rare cardiac mass
While rare, papillary myxomas are the most common primary cardiac mass. On histologic exam, they are gelatinous structures that consist of myxoma cells embedded in a stroma rich in glycosamino- glycans.2,3 On macroscopic exam, myxomas are pedunculated and have a variable surface. These masses produce vascular endothelial growth factor, which could contribute to the malignant potential of histologically benign myxomas.4,5
Listen for “tumor plop”
Symptoms of myxomas depend on the location of the tumor. Myxomas are frequently accompanied by constitutional symptoms such as fever and weight loss (although our patient had neither). Embolization is also associated with such lesions.
Left-sided lesions present with symptoms of mitral valve obstruction, along with electrocardiographic changes of left atrial hypertrophy.2 Ventricular myxomas may cause outflow obstruction.3
The signs and symptoms of myxoma may have a sudden onset or be positional in nature, reflecting changes in tumor position due to gravity. An auscultatory finding, called “tumor plop,” is a characteristic low-pitched sound that may be audible during early or mid-diastole,6 though we heard no such sound during our evaluation of our patient.
Our patient was unusual
Diagnosis is made by echocardiography, and most (80%) of myxomas are found in the left atrium. Atrial myxoma typically appears as a well-circumscribed mobile mass that is attached to the atrial septum. Our patient, however, had a myxoma located in the right atrium that was attached to the lateral wall. Definitive diagnosis is made by surgical excision.
Treatment: Prompt resection
Due to the risk of embolization and cardiovascular compromise, prompt resection is recommended and is generally curative, as it was for our patient.3,7,8 Postoperative mortality rate after resection is under 5%. For multiple recurrent tumors, cardiac transplantation should be considered.1,9
Correspondence
Family medicine Department, John Peter Smith Hospital, 1500 South main St, Fort Worth, TX 76104; hrana@ jpshealth.org
1. Michler RE, Goldstein DJ. Treatment of cardiac tumors by orthotopic cardiac transplantation. Semin Oncol 1997;24:53-539.
2. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Balt) 2001;80:159-172.
3. Nishimura R, Gibbons R, Glockner J, Tajik A. Noninvasive cardiac imaging: echocardiography, nuclear cardiology, and mrI/cT imaging. In: Kasper Dl, Braunwald E, Hauser S, longo D, Jameson JL, Fauci AS (eds), Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: mcGraw-Hill; 2004:chap 211
4. Kono T, Koide N, Hama Y, et al. expression of vascular endothelial growth factor and angiogenesis in cardiac myxoma: A study of fifteen patients. J Thorac Cardiovasc Surg 2000;119:101-107.
5. Sakamoto H, Sakamaki T, Kanda T, et al. Vascular endothelial growth factor is an autocrine growth factor for cardiac myxoma cells. Circ J 2004;68:488-493.
6. Colucci W, Price D. Cardiac tumors, cardiac manifestations of systemic diseases, and traumatic cardiac injury. In: Kasper DL, Braunwald E, Hauser S, Longo D, Jameson JL, Fauci AS (eds), Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: mcGraw-Hill; 2004:chap 223
7. Keeling IM, Oberwalder P, Anelli-monti M, et al. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardiothorac Surg 2002;22:971-977.
8. Selkane C, Amahzoune B, Chavanis N, et al. Changing management of cardiac myxoma based on a series of 40 cases with long-term follow-up. Ann Thorac Surg 2003;76:1935-1938.
9. Goldstein DJ, Oz MC, Rose EA, Fisher P, Michler RE. Experience with heart transplantation for cardiac tumors. J Heart Lung Transplant 1995;14:382-386.
1. Michler RE, Goldstein DJ. Treatment of cardiac tumors by orthotopic cardiac transplantation. Semin Oncol 1997;24:53-539.
2. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Balt) 2001;80:159-172.
3. Nishimura R, Gibbons R, Glockner J, Tajik A. Noninvasive cardiac imaging: echocardiography, nuclear cardiology, and mrI/cT imaging. In: Kasper Dl, Braunwald E, Hauser S, longo D, Jameson JL, Fauci AS (eds), Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: mcGraw-Hill; 2004:chap 211
4. Kono T, Koide N, Hama Y, et al. expression of vascular endothelial growth factor and angiogenesis in cardiac myxoma: A study of fifteen patients. J Thorac Cardiovasc Surg 2000;119:101-107.
5. Sakamoto H, Sakamaki T, Kanda T, et al. Vascular endothelial growth factor is an autocrine growth factor for cardiac myxoma cells. Circ J 2004;68:488-493.
6. Colucci W, Price D. Cardiac tumors, cardiac manifestations of systemic diseases, and traumatic cardiac injury. In: Kasper DL, Braunwald E, Hauser S, Longo D, Jameson JL, Fauci AS (eds), Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: mcGraw-Hill; 2004:chap 223
7. Keeling IM, Oberwalder P, Anelli-monti M, et al. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardiothorac Surg 2002;22:971-977.
8. Selkane C, Amahzoune B, Chavanis N, et al. Changing management of cardiac myxoma based on a series of 40 cases with long-term follow-up. Ann Thorac Surg 2003;76:1935-1938.
9. Goldstein DJ, Oz MC, Rose EA, Fisher P, Michler RE. Experience with heart transplantation for cardiac tumors. J Heart Lung Transplant 1995;14:382-386.
Hooked from the first cigarette
- Teach adolescents that one cigarette is often all it takes to get hooked (C).
- The “Hooked On Nicotine Checklist” is a self-assessment tool that may help motivate some adolescent smokers to quit (C).
- Even adolescents who smoke only a few cigarettes per week may need your help with quitting (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A 14-year-old girl comes into your office for a routine sports physical. She has been your patient since birth, and has seen you every year or two for the usual childhood illnesses and health exams. Her history is unremarkable, except that when you ask her whether she’s ever smoked, she confides in you that she occasionally smokes a cigarette with her girlfriends—something, she says, her parents know nothing about.
She tells you that she began smoking 2 months ago, but doesn’t smoke much—only about 3 cigarettes a week. She denies using any other form of tobacco (eg, smokeless tobacco) and tells you that she has not experimented with drugs.
When you ask her whether she’s tried to stop smoking, she tells you that she has, but that she’s already failed at several attempts to quit. You question her further and uncover some signs and symptoms of nicotine addiction, including cravings and a feeling of irritability when she isn’t able to smoke.
“Am I addicted to nicotine?” she asks you. But before you get a chance to respond, she continues: “And how is that possible? I don’t even smoke that much!”
Hooked from the first cigarette?
You bet.
Very soon after that first cigarette, adolescents can experience a loss of autonomy over tobacco, and recent research indicates that this loss of autonomy may play a key role in nicotine addiction.1
The challenge we face, though, is that many young patients think there’s no harm in trying a cigarette once. After all, what could be the problem with that? But there is a big risk to smoking just once and family physicians need to drive this message home to adolescent patients. A 10-point checklist can help.
10 questions that can open an adolescent’s eyes
The “Hooked On Nicotine Checklist” (PATIENT HANDOUT) is an objective measure of a patient’s loss of autonomy and it can be a real eye opener for adolescents. Simply give it to your adolescent patients and ask them to answer the 10 questions with a Yes or No answer.
A Yes response to any of the 10 questions indicates a loss of autonomy. The number of Yes responses gives an indication of the severity of the dependence, and may help to motivate adolescent smokers to quit. (Seven is the mean for adult smokers.) A smoker loses full autonomy when the sequelae of tobacco use present an obstacle to quitting—that is, when quitting requires an effort or results in discomfort.
Studies find that one cigarette is all it takes
Studies on a cohort of 7th graders found that every symptom on this validated checklist1 had been experienced by at least one young person within weeks of starting to smoke, sometimes after the first cigarette.2,3 These results have been replicated many times.4-7
Three New Zealand national surveys involving 25,722 adolescent smokers who used this checklist revealed a loss of autonomy in 25% to 30% of young people who had smoked their one and only cigarette during the preceding month.6
In another study using Diagnostic and Statistical Manual of Mental Disorders-IV criteria, 35% of young people who had symptoms of dependence had been smoking for one month or less when the first symptom appeared,5 challenging the assumption that addiction requires years of smoking. These studies also challenge the belief that repetition is the force that causes addiction, as at least one quarter had symptoms after one cigarette.
Expert opinion has also held that people who smoke fewer than 5 cigarettes daily are rarely addicted. Research data, however, indicate that 50% of young people were hooked on tobacco prior to smoking at a rate of 2 cigarettes per week.3,7 These results have also been replicated.4-7 Loss of autonomy has been reported even prior to the onset of smoking once per month.4,8
Which adolescents are most vulnerable?
About one quarter of young people experience the FIRE (First-Inhalation-Relaxation-Experience), a sensation of relaxation the first time they inhale from a cigarette, and this sensation predicts continued smoking.9 FIRE is the strongest predictor of the progression to the loss of autonomy and a diagnosis of tobacco dependence.10 One study demonstrated that an alarming 91% of those with the FIRE subsequently lost autonomy.10 FIRE appears to be a symptom of the neurological events that trigger addiction with the first cigarette.10
What happens in the brain after just one smoke?
As you would expect, long-term smokers have higher concentrations of nicotine receptors in the brain, according to autopsy studies,11 but what happens to the brain after, say, just one cigarette? A study by Dr Abreu-Villaca and colleagues revealed that there is an increase in nicotine receptors in the brain the day after the first dose of nicotine. The take-home message: It only takes a day for the brain to remodel itself in response to one dose of nicotine.12
It’s time to revisit our beliefs about withdrawal
Just as we have been taught that people who smoke just a few cigarettes daily are rarely addicted, so too, have we been taught that occasional smokers are unlikely to experience withdrawal symptoms. Our understanding has been that smokers who experience withdrawal must smoke frequently enough to maintain nicotine in the blood throughout their waking hours. With nicotine’s two hour half-life, this typically requires 5 cigarettes per day. In fact, some years ago, The New England Journal of Medicine published a proposal that cigarettes could be rendered practically non-addictive if their nicotine content was lowered to the point where smokers would not be able to obtain as much nicotine as was delivered by 5 ordinary cigarettes.13
Hooked On Nicotine Checklist
Are you skeptical that addiction can begin so quickly, after just a few cigarettes? Then complete this checklist.
A Yes response to any of the questions means you are already addicted to cigarettes. The number of Yes responses indicates how dependent you are on them.
| Yes | No | ||
|---|---|---|---|
| 1. | Have you ever tried to quit smoking, but couldn’t? | ||
| 2. | Do you smoke now because it is really hard to quit? | ||
| 3. | Have you ever felt like you were addicted to tobacco? | ||
| 4. | Do you ever have strong cravings to smoke? | ||
| 5. | Have you ever felt like you really needed a cigarette? | ||
| 6. | Is it hard to keep from smoking in places where you are not supposed to, like school? |
When you tried to stop smoking (or, when you haven’t used tobacco for a while):
| 7. | Did you find it hard to concentrate because you couldn’t smoke? | ||
| 8. | Did you feel more irritable because you couldn’t smoke? | ||
| 9. | Did you feel a strong need or urge to smoke? | ||
| 10. | Did you feel nervous, restless, or anxious because you couldn’t smoke? |
But a growing body of literature paints a different picture. We now know that tobacco withdrawal does, indeed, occur in those who do not smoke daily or who smoke fewer than 5 cigarettes per day.6-8,14-20
In fact, a survey of adult smokers found that adults who smoked only a few cigarettes weekly found quitting to be difficult; they experienced withdrawal symptoms, which some rated as unbearable.21 Most of these self-described “social smokers” were addicted to tobacco.
Timing of withdrawal is different for novice smokers
Although the Diagnostic and Statistical Manual of Mental Disorders-IV-TR defines nicotine withdrawal as beginning within 24 hours of the last cigarette, the timing of withdrawal had been studied only in heavy smokers. It was surprising to find in an as yet unpublished survey that my colleagues and I conducted that some high school smokers experienced withdrawal symptoms that did not appear until a week after their last cigarette. This period of time is defined as the latency to withdrawal, or the time from the last cigarette to the onset of withdrawal.22 When novice smokers first experience withdrawal symptoms, the latency to withdrawal is very long, allowing them to remain comfortable for a week or more between cigarettes.
How could a single cigarette keep withdrawal symptoms at bay for far longer than the 12 hours it takes to eliminate nicotine from the body? We don’t know what the answer is for humans, but we do know that in rats, the first dose of nicotine increases noradrenaline synthesis in the hippocampus for at least 30 days after the nicotine is gone.23 Given this information, it is quite plausible, then, that a few puffs from a cigarette could suppress withdrawal for many days, and perhaps even several weeks.
Research I conducted—and which I’ll describe in greater detail, in a bit—supports the notion that the latency to withdrawal period may shrink over time. The realization that the latency to withdrawal changes over time fundamentally alters our understanding of addiction.
Revisiting long-held beliefs of addiction and tolerance
The shortening of the latency to withdrawal is called dependence-related tolerance.24 One can think of dependence-related tolerance as either a diminution in the duration of withdrawal relief afforded by one cigarette, or conversely, a requirement to smoke more frequently to maintain comfort. The latency to withdrawal cannot shorten if there is no withdrawal. Therefore, dependence-related tolerance develops only after withdrawal symptoms are present.
Although we were all taught in medical school that tolerance precedes and causes addiction, addiction must be present before dependence-related tolerance can develop.
Nicotine leaves an indelible mark
To test this dependence-related tolerance hypothesis, my colleagues and I surveyed 2000 people who were smoking in public places, including the areas outside of our hospital’s entrance. We asked about their longest period of abstinence, how much they smoked before quitting, and how much they smoked at various times after resuming smoking. By plotting these data on a graph, we were able to see that dependence-related tolerance has 2 components, one reversible and the other permanent (irreversible).24,25
The permanent component can cut the duration of a cigarette’s effect to half its original length, then to a quarter, to an eighth, a sixteenth, a thirty-second, a sixty-fourth and so on.
The reversible component reduces what is left by about half again.
So if we consider only the permanent component, it would cut the duration of effect from, say, four weeks to two weeks (one “cut”), then from two weeks to one week (second cut), from one week to 3.5 days (third cut), and so on. On top of this, the reversible component halves it again.
After 3 months of abstinence, then, the reversible component of tolerance disappears (temporarily) and a relapsed pack a day smoker will be happy—for a time—smoking a half-pack (10 cigarettes) per day. But then the reversible component kicks in and they’re back to smoking a pack a day.
The take-home message? Long-term smokers’ brains will never return to the days when their addiction could be satisfied with one cigarette per month because of the permanent component of tolerance.
How to help light smokers like your young patient
The fact that loss of autonomy over tobacco can occur after 1 cigarette helps explain the clinical observation that it is difficult for even non-daily smokers to stop smoking completely.
Helping a 14-year-old patient like the one in the opener to quit smoking requires that you not underestimate your patient’s need for advice about cessation. Unfortunately, none of the medications for smoking cessation, such as varenicline or bupropion, have been approved for this purpose in patients under the age of 18.
As a result, you’ll need to help young patients formulate a quit plan, just as you would with an adult heavy smoker. Give them the “Hooked On Nicotine Checklist.” The answers will help you both to tailor the quit plan to the obstacles they face. You’ll also need to provide young patients with anticipatory guidance about what they can expect from nicotine withdrawal.
Don’t wait for adolescents to broach the subject
Early intervention requires that you ask patients who are 10 years of age or older about smoking (TABLE 2). Be sure to take advantage of teachable moments and tell young patients just how addictive smoking can be—even if they’ve only smoked 1 or 2 cigarettes.
TABLE 2
The 5 A’s of smoking cessation26
|
Your conversation might go like this:
The first cigarette you smoke changes your brain and may cause you to develop a craving for nicotine that could be hard to resist. The only sure way to avoid addiction is to never smoke that first cigarette.
If you are already smoking and you have a craving for a cigarette every once in a while, that is a sure sign of addiction. The sooner you quit, the better your chances of succeeding. Now let’s work on a quit plan that will help you do that.
Correspondence
Joseph R. DiFranza, MD, Department of Family Medicine and Community Health, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655; [email protected].
1. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med 2002;156:397-403.
2. DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescents. Tob Control 2000;9:313-319.
3. DiFranza JR, Savageau JA, Fletcher K, et al. Development of symptoms of tobacco dependence in youths: 30 month follow-up data from the DANDY study. Tob Control 2002;11:228-235.
4. Gervais A, O’Loughlin J, Meshefedjian G, Bancej C, Tremblay M. Milestones in the natural course of cigarette use onset in adolescents. Can Med Assoc J 2006;175:255-261.
5. Kandel DB, Hu MC, Griesler PC, Schaffran C. The timing of the experience of symptoms of nicotine dependence. Paper 12-4. Society for Research on Nicotine and Tobacco; February 15-18, 2006; Orlando, Fla.
6. Scragg R. Report of 1999–2005 National Year 10 Smoking Surveys: prepared for ASH. 2006. Available at: www.ash.org.nz/index.php?pa_id=45&top_parent_id=45&curr_level=0. Accessed on November 6, 2007.
7. DiFranza JR, Savageau JA, Fletcher K, et al. Symptoms of tobacco dependence after brief intermittent use. The Development and Assessment of Nicotine Dependence in Youth-2. Arch Pediatr Adolesc Med 2007;161:704-710.
8. O’Loughlin J, DiFranza J, Tyndale RF, et al. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. Am J Prev Med 2003;25:219-225.
9. Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never smokers. Addiction 1998;93:595-599.
10. DiFranza J, Savageau JA, Fletcher K, et al. Susceptibility to nicotine dependence: The Development and Assessment of Nicotine Dependence in Youth-2 Study. Pediatrics 2007;120:e974-e983.
11. Benwell M, Balfour D, Anderson J. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J Neurochem 1988;50:1243-1247.
12. Abreu-Villaca YA, Seidler FJ, Qiao D, et al. Shortterm adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology 2003;28:1935-1949.
13. Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. N Engl J Med 1994;331:123-125.
14. McNeill A, West R, Jarvis M, Jackson P, Bryant A. Cigarette withdrawal symptoms in adolescent smokers. Psychopharmacology 1986;90:533-536.
15. Goddard E. Why Children Start Smoking. London: Her Majesty’s Stationery Office (HMSO), the Social Survey Division of the Office of Population Censuses and Surveys (OPCS), on behalf of the Department of Health; 1990.
16. Barker D. Reasons for tobacco use and symptoms of nicotine withdrawal among adolescent and young adult tobacco users—United States, 1993. MMWR Morb Mortal Wkly Rep 1994;43:745-750.
17. Riedel B, Robinson L, Klesges R, McLain-Allen B. Ethnic differences in smoking withdrawal effects among adolescents. Addict Behav 2003;28:129-140.
18. Strong D, Kahler C, Ramsey S, Abrantes A, Brown R. Nicotine withdrawal among adolescents with acute psychopathology: An item response analysis. Nicotine Tob Res 2004;6:547-557.
19. An L, Lein E, Bliss R, et al. Loss of autonomy over nicotine use among college social smokers. Paper presented at: 10th Annual Meeting of the Society for Research on Nicotine and Tobacco; February 21-24, 2004; Austin, Tex.
20. Panday S, Reddy S, Ruiter R, Bergstrom E, de Vries H. Nicotine dependence and withdrawal symptoms among occasional smokers. J Adolesc Health 2007;40:144-150.
21. Wellman R, DiFranza J, Wood C. Tobacco chippers report diminished autonomy over tobacco use. Addict Behav 2006;31:717-721.
22. Fernando WWSA, Wellman RJ, DiFranza JR. The relationship between level of cigarette consumption and latency to the onset of retrospectively reported withdrawal symptoms. Psychopharmacology 2006;188:335-342.
23. Smith KM, Mitchell SN, Joseph MJ. Effects of chronic and subchronic nicotine on tyrosine hydroxylase activity in noradrenergic and dopaminergic neurones in the rat brain. J Neurochem 1991;57:1750-1756.
24. DiFranza JR, Wellman RJ. A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: Integrating the clinical and basic science literature. Nicotine Tob Res 2005;7:9-26.
25. Wellman RJ, DiFranza JR, Savageau JA, et al. The effect of abstinence on cigarette consumption upon the resumption of smoking. Addict Behav 2006;31:711-716.
26. Agency for Healthcare Research and Quality. Clinical Practice Guideline. Treating Tobacco Use and Dependence. Rockville, Md: US Department of Health and Human Services Public Health Service; 2000. Available at: www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat2.chapter.7644. Accessed November 1, 2007.
- Teach adolescents that one cigarette is often all it takes to get hooked (C).
- The “Hooked On Nicotine Checklist” is a self-assessment tool that may help motivate some adolescent smokers to quit (C).
- Even adolescents who smoke only a few cigarettes per week may need your help with quitting (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A 14-year-old girl comes into your office for a routine sports physical. She has been your patient since birth, and has seen you every year or two for the usual childhood illnesses and health exams. Her history is unremarkable, except that when you ask her whether she’s ever smoked, she confides in you that she occasionally smokes a cigarette with her girlfriends—something, she says, her parents know nothing about.
She tells you that she began smoking 2 months ago, but doesn’t smoke much—only about 3 cigarettes a week. She denies using any other form of tobacco (eg, smokeless tobacco) and tells you that she has not experimented with drugs.
When you ask her whether she’s tried to stop smoking, she tells you that she has, but that she’s already failed at several attempts to quit. You question her further and uncover some signs and symptoms of nicotine addiction, including cravings and a feeling of irritability when she isn’t able to smoke.
“Am I addicted to nicotine?” she asks you. But before you get a chance to respond, she continues: “And how is that possible? I don’t even smoke that much!”
Hooked from the first cigarette?
You bet.
Very soon after that first cigarette, adolescents can experience a loss of autonomy over tobacco, and recent research indicates that this loss of autonomy may play a key role in nicotine addiction.1
The challenge we face, though, is that many young patients think there’s no harm in trying a cigarette once. After all, what could be the problem with that? But there is a big risk to smoking just once and family physicians need to drive this message home to adolescent patients. A 10-point checklist can help.
10 questions that can open an adolescent’s eyes
The “Hooked On Nicotine Checklist” (PATIENT HANDOUT) is an objective measure of a patient’s loss of autonomy and it can be a real eye opener for adolescents. Simply give it to your adolescent patients and ask them to answer the 10 questions with a Yes or No answer.
A Yes response to any of the 10 questions indicates a loss of autonomy. The number of Yes responses gives an indication of the severity of the dependence, and may help to motivate adolescent smokers to quit. (Seven is the mean for adult smokers.) A smoker loses full autonomy when the sequelae of tobacco use present an obstacle to quitting—that is, when quitting requires an effort or results in discomfort.
Studies find that one cigarette is all it takes
Studies on a cohort of 7th graders found that every symptom on this validated checklist1 had been experienced by at least one young person within weeks of starting to smoke, sometimes after the first cigarette.2,3 These results have been replicated many times.4-7
Three New Zealand national surveys involving 25,722 adolescent smokers who used this checklist revealed a loss of autonomy in 25% to 30% of young people who had smoked their one and only cigarette during the preceding month.6
In another study using Diagnostic and Statistical Manual of Mental Disorders-IV criteria, 35% of young people who had symptoms of dependence had been smoking for one month or less when the first symptom appeared,5 challenging the assumption that addiction requires years of smoking. These studies also challenge the belief that repetition is the force that causes addiction, as at least one quarter had symptoms after one cigarette.
Expert opinion has also held that people who smoke fewer than 5 cigarettes daily are rarely addicted. Research data, however, indicate that 50% of young people were hooked on tobacco prior to smoking at a rate of 2 cigarettes per week.3,7 These results have also been replicated.4-7 Loss of autonomy has been reported even prior to the onset of smoking once per month.4,8
Which adolescents are most vulnerable?
About one quarter of young people experience the FIRE (First-Inhalation-Relaxation-Experience), a sensation of relaxation the first time they inhale from a cigarette, and this sensation predicts continued smoking.9 FIRE is the strongest predictor of the progression to the loss of autonomy and a diagnosis of tobacco dependence.10 One study demonstrated that an alarming 91% of those with the FIRE subsequently lost autonomy.10 FIRE appears to be a symptom of the neurological events that trigger addiction with the first cigarette.10
What happens in the brain after just one smoke?
As you would expect, long-term smokers have higher concentrations of nicotine receptors in the brain, according to autopsy studies,11 but what happens to the brain after, say, just one cigarette? A study by Dr Abreu-Villaca and colleagues revealed that there is an increase in nicotine receptors in the brain the day after the first dose of nicotine. The take-home message: It only takes a day for the brain to remodel itself in response to one dose of nicotine.12
It’s time to revisit our beliefs about withdrawal
Just as we have been taught that people who smoke just a few cigarettes daily are rarely addicted, so too, have we been taught that occasional smokers are unlikely to experience withdrawal symptoms. Our understanding has been that smokers who experience withdrawal must smoke frequently enough to maintain nicotine in the blood throughout their waking hours. With nicotine’s two hour half-life, this typically requires 5 cigarettes per day. In fact, some years ago, The New England Journal of Medicine published a proposal that cigarettes could be rendered practically non-addictive if their nicotine content was lowered to the point where smokers would not be able to obtain as much nicotine as was delivered by 5 ordinary cigarettes.13
Hooked On Nicotine Checklist
Are you skeptical that addiction can begin so quickly, after just a few cigarettes? Then complete this checklist.
A Yes response to any of the questions means you are already addicted to cigarettes. The number of Yes responses indicates how dependent you are on them.
| Yes | No | ||
|---|---|---|---|
| 1. | Have you ever tried to quit smoking, but couldn’t? | ||
| 2. | Do you smoke now because it is really hard to quit? | ||
| 3. | Have you ever felt like you were addicted to tobacco? | ||
| 4. | Do you ever have strong cravings to smoke? | ||
| 5. | Have you ever felt like you really needed a cigarette? | ||
| 6. | Is it hard to keep from smoking in places where you are not supposed to, like school? |
When you tried to stop smoking (or, when you haven’t used tobacco for a while):
| 7. | Did you find it hard to concentrate because you couldn’t smoke? | ||
| 8. | Did you feel more irritable because you couldn’t smoke? | ||
| 9. | Did you feel a strong need or urge to smoke? | ||
| 10. | Did you feel nervous, restless, or anxious because you couldn’t smoke? |
But a growing body of literature paints a different picture. We now know that tobacco withdrawal does, indeed, occur in those who do not smoke daily or who smoke fewer than 5 cigarettes per day.6-8,14-20
In fact, a survey of adult smokers found that adults who smoked only a few cigarettes weekly found quitting to be difficult; they experienced withdrawal symptoms, which some rated as unbearable.21 Most of these self-described “social smokers” were addicted to tobacco.
Timing of withdrawal is different for novice smokers
Although the Diagnostic and Statistical Manual of Mental Disorders-IV-TR defines nicotine withdrawal as beginning within 24 hours of the last cigarette, the timing of withdrawal had been studied only in heavy smokers. It was surprising to find in an as yet unpublished survey that my colleagues and I conducted that some high school smokers experienced withdrawal symptoms that did not appear until a week after their last cigarette. This period of time is defined as the latency to withdrawal, or the time from the last cigarette to the onset of withdrawal.22 When novice smokers first experience withdrawal symptoms, the latency to withdrawal is very long, allowing them to remain comfortable for a week or more between cigarettes.
How could a single cigarette keep withdrawal symptoms at bay for far longer than the 12 hours it takes to eliminate nicotine from the body? We don’t know what the answer is for humans, but we do know that in rats, the first dose of nicotine increases noradrenaline synthesis in the hippocampus for at least 30 days after the nicotine is gone.23 Given this information, it is quite plausible, then, that a few puffs from a cigarette could suppress withdrawal for many days, and perhaps even several weeks.
Research I conducted—and which I’ll describe in greater detail, in a bit—supports the notion that the latency to withdrawal period may shrink over time. The realization that the latency to withdrawal changes over time fundamentally alters our understanding of addiction.
Revisiting long-held beliefs of addiction and tolerance
The shortening of the latency to withdrawal is called dependence-related tolerance.24 One can think of dependence-related tolerance as either a diminution in the duration of withdrawal relief afforded by one cigarette, or conversely, a requirement to smoke more frequently to maintain comfort. The latency to withdrawal cannot shorten if there is no withdrawal. Therefore, dependence-related tolerance develops only after withdrawal symptoms are present.
Although we were all taught in medical school that tolerance precedes and causes addiction, addiction must be present before dependence-related tolerance can develop.
Nicotine leaves an indelible mark
To test this dependence-related tolerance hypothesis, my colleagues and I surveyed 2000 people who were smoking in public places, including the areas outside of our hospital’s entrance. We asked about their longest period of abstinence, how much they smoked before quitting, and how much they smoked at various times after resuming smoking. By plotting these data on a graph, we were able to see that dependence-related tolerance has 2 components, one reversible and the other permanent (irreversible).24,25
The permanent component can cut the duration of a cigarette’s effect to half its original length, then to a quarter, to an eighth, a sixteenth, a thirty-second, a sixty-fourth and so on.
The reversible component reduces what is left by about half again.
So if we consider only the permanent component, it would cut the duration of effect from, say, four weeks to two weeks (one “cut”), then from two weeks to one week (second cut), from one week to 3.5 days (third cut), and so on. On top of this, the reversible component halves it again.
After 3 months of abstinence, then, the reversible component of tolerance disappears (temporarily) and a relapsed pack a day smoker will be happy—for a time—smoking a half-pack (10 cigarettes) per day. But then the reversible component kicks in and they’re back to smoking a pack a day.
The take-home message? Long-term smokers’ brains will never return to the days when their addiction could be satisfied with one cigarette per month because of the permanent component of tolerance.
How to help light smokers like your young patient
The fact that loss of autonomy over tobacco can occur after 1 cigarette helps explain the clinical observation that it is difficult for even non-daily smokers to stop smoking completely.
Helping a 14-year-old patient like the one in the opener to quit smoking requires that you not underestimate your patient’s need for advice about cessation. Unfortunately, none of the medications for smoking cessation, such as varenicline or bupropion, have been approved for this purpose in patients under the age of 18.
As a result, you’ll need to help young patients formulate a quit plan, just as you would with an adult heavy smoker. Give them the “Hooked On Nicotine Checklist.” The answers will help you both to tailor the quit plan to the obstacles they face. You’ll also need to provide young patients with anticipatory guidance about what they can expect from nicotine withdrawal.
Don’t wait for adolescents to broach the subject
Early intervention requires that you ask patients who are 10 years of age or older about smoking (TABLE 2). Be sure to take advantage of teachable moments and tell young patients just how addictive smoking can be—even if they’ve only smoked 1 or 2 cigarettes.
TABLE 2
The 5 A’s of smoking cessation26
|
Your conversation might go like this:
The first cigarette you smoke changes your brain and may cause you to develop a craving for nicotine that could be hard to resist. The only sure way to avoid addiction is to never smoke that first cigarette.
If you are already smoking and you have a craving for a cigarette every once in a while, that is a sure sign of addiction. The sooner you quit, the better your chances of succeeding. Now let’s work on a quit plan that will help you do that.
Correspondence
Joseph R. DiFranza, MD, Department of Family Medicine and Community Health, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655; [email protected].
- Teach adolescents that one cigarette is often all it takes to get hooked (C).
- The “Hooked On Nicotine Checklist” is a self-assessment tool that may help motivate some adolescent smokers to quit (C).
- Even adolescents who smoke only a few cigarettes per week may need your help with quitting (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A 14-year-old girl comes into your office for a routine sports physical. She has been your patient since birth, and has seen you every year or two for the usual childhood illnesses and health exams. Her history is unremarkable, except that when you ask her whether she’s ever smoked, she confides in you that she occasionally smokes a cigarette with her girlfriends—something, she says, her parents know nothing about.
She tells you that she began smoking 2 months ago, but doesn’t smoke much—only about 3 cigarettes a week. She denies using any other form of tobacco (eg, smokeless tobacco) and tells you that she has not experimented with drugs.
When you ask her whether she’s tried to stop smoking, she tells you that she has, but that she’s already failed at several attempts to quit. You question her further and uncover some signs and symptoms of nicotine addiction, including cravings and a feeling of irritability when she isn’t able to smoke.
“Am I addicted to nicotine?” she asks you. But before you get a chance to respond, she continues: “And how is that possible? I don’t even smoke that much!”
Hooked from the first cigarette?
You bet.
Very soon after that first cigarette, adolescents can experience a loss of autonomy over tobacco, and recent research indicates that this loss of autonomy may play a key role in nicotine addiction.1
The challenge we face, though, is that many young patients think there’s no harm in trying a cigarette once. After all, what could be the problem with that? But there is a big risk to smoking just once and family physicians need to drive this message home to adolescent patients. A 10-point checklist can help.
10 questions that can open an adolescent’s eyes
The “Hooked On Nicotine Checklist” (PATIENT HANDOUT) is an objective measure of a patient’s loss of autonomy and it can be a real eye opener for adolescents. Simply give it to your adolescent patients and ask them to answer the 10 questions with a Yes or No answer.
A Yes response to any of the 10 questions indicates a loss of autonomy. The number of Yes responses gives an indication of the severity of the dependence, and may help to motivate adolescent smokers to quit. (Seven is the mean for adult smokers.) A smoker loses full autonomy when the sequelae of tobacco use present an obstacle to quitting—that is, when quitting requires an effort or results in discomfort.
Studies find that one cigarette is all it takes
Studies on a cohort of 7th graders found that every symptom on this validated checklist1 had been experienced by at least one young person within weeks of starting to smoke, sometimes after the first cigarette.2,3 These results have been replicated many times.4-7
Three New Zealand national surveys involving 25,722 adolescent smokers who used this checklist revealed a loss of autonomy in 25% to 30% of young people who had smoked their one and only cigarette during the preceding month.6
In another study using Diagnostic and Statistical Manual of Mental Disorders-IV criteria, 35% of young people who had symptoms of dependence had been smoking for one month or less when the first symptom appeared,5 challenging the assumption that addiction requires years of smoking. These studies also challenge the belief that repetition is the force that causes addiction, as at least one quarter had symptoms after one cigarette.
Expert opinion has also held that people who smoke fewer than 5 cigarettes daily are rarely addicted. Research data, however, indicate that 50% of young people were hooked on tobacco prior to smoking at a rate of 2 cigarettes per week.3,7 These results have also been replicated.4-7 Loss of autonomy has been reported even prior to the onset of smoking once per month.4,8
Which adolescents are most vulnerable?
About one quarter of young people experience the FIRE (First-Inhalation-Relaxation-Experience), a sensation of relaxation the first time they inhale from a cigarette, and this sensation predicts continued smoking.9 FIRE is the strongest predictor of the progression to the loss of autonomy and a diagnosis of tobacco dependence.10 One study demonstrated that an alarming 91% of those with the FIRE subsequently lost autonomy.10 FIRE appears to be a symptom of the neurological events that trigger addiction with the first cigarette.10
What happens in the brain after just one smoke?
As you would expect, long-term smokers have higher concentrations of nicotine receptors in the brain, according to autopsy studies,11 but what happens to the brain after, say, just one cigarette? A study by Dr Abreu-Villaca and colleagues revealed that there is an increase in nicotine receptors in the brain the day after the first dose of nicotine. The take-home message: It only takes a day for the brain to remodel itself in response to one dose of nicotine.12
It’s time to revisit our beliefs about withdrawal
Just as we have been taught that people who smoke just a few cigarettes daily are rarely addicted, so too, have we been taught that occasional smokers are unlikely to experience withdrawal symptoms. Our understanding has been that smokers who experience withdrawal must smoke frequently enough to maintain nicotine in the blood throughout their waking hours. With nicotine’s two hour half-life, this typically requires 5 cigarettes per day. In fact, some years ago, The New England Journal of Medicine published a proposal that cigarettes could be rendered practically non-addictive if their nicotine content was lowered to the point where smokers would not be able to obtain as much nicotine as was delivered by 5 ordinary cigarettes.13
Hooked On Nicotine Checklist
Are you skeptical that addiction can begin so quickly, after just a few cigarettes? Then complete this checklist.
A Yes response to any of the questions means you are already addicted to cigarettes. The number of Yes responses indicates how dependent you are on them.
| Yes | No | ||
|---|---|---|---|
| 1. | Have you ever tried to quit smoking, but couldn’t? | ||
| 2. | Do you smoke now because it is really hard to quit? | ||
| 3. | Have you ever felt like you were addicted to tobacco? | ||
| 4. | Do you ever have strong cravings to smoke? | ||
| 5. | Have you ever felt like you really needed a cigarette? | ||
| 6. | Is it hard to keep from smoking in places where you are not supposed to, like school? |
When you tried to stop smoking (or, when you haven’t used tobacco for a while):
| 7. | Did you find it hard to concentrate because you couldn’t smoke? | ||
| 8. | Did you feel more irritable because you couldn’t smoke? | ||
| 9. | Did you feel a strong need or urge to smoke? | ||
| 10. | Did you feel nervous, restless, or anxious because you couldn’t smoke? |
But a growing body of literature paints a different picture. We now know that tobacco withdrawal does, indeed, occur in those who do not smoke daily or who smoke fewer than 5 cigarettes per day.6-8,14-20
In fact, a survey of adult smokers found that adults who smoked only a few cigarettes weekly found quitting to be difficult; they experienced withdrawal symptoms, which some rated as unbearable.21 Most of these self-described “social smokers” were addicted to tobacco.
Timing of withdrawal is different for novice smokers
Although the Diagnostic and Statistical Manual of Mental Disorders-IV-TR defines nicotine withdrawal as beginning within 24 hours of the last cigarette, the timing of withdrawal had been studied only in heavy smokers. It was surprising to find in an as yet unpublished survey that my colleagues and I conducted that some high school smokers experienced withdrawal symptoms that did not appear until a week after their last cigarette. This period of time is defined as the latency to withdrawal, or the time from the last cigarette to the onset of withdrawal.22 When novice smokers first experience withdrawal symptoms, the latency to withdrawal is very long, allowing them to remain comfortable for a week or more between cigarettes.
How could a single cigarette keep withdrawal symptoms at bay for far longer than the 12 hours it takes to eliminate nicotine from the body? We don’t know what the answer is for humans, but we do know that in rats, the first dose of nicotine increases noradrenaline synthesis in the hippocampus for at least 30 days after the nicotine is gone.23 Given this information, it is quite plausible, then, that a few puffs from a cigarette could suppress withdrawal for many days, and perhaps even several weeks.
Research I conducted—and which I’ll describe in greater detail, in a bit—supports the notion that the latency to withdrawal period may shrink over time. The realization that the latency to withdrawal changes over time fundamentally alters our understanding of addiction.
Revisiting long-held beliefs of addiction and tolerance
The shortening of the latency to withdrawal is called dependence-related tolerance.24 One can think of dependence-related tolerance as either a diminution in the duration of withdrawal relief afforded by one cigarette, or conversely, a requirement to smoke more frequently to maintain comfort. The latency to withdrawal cannot shorten if there is no withdrawal. Therefore, dependence-related tolerance develops only after withdrawal symptoms are present.
Although we were all taught in medical school that tolerance precedes and causes addiction, addiction must be present before dependence-related tolerance can develop.
Nicotine leaves an indelible mark
To test this dependence-related tolerance hypothesis, my colleagues and I surveyed 2000 people who were smoking in public places, including the areas outside of our hospital’s entrance. We asked about their longest period of abstinence, how much they smoked before quitting, and how much they smoked at various times after resuming smoking. By plotting these data on a graph, we were able to see that dependence-related tolerance has 2 components, one reversible and the other permanent (irreversible).24,25
The permanent component can cut the duration of a cigarette’s effect to half its original length, then to a quarter, to an eighth, a sixteenth, a thirty-second, a sixty-fourth and so on.
The reversible component reduces what is left by about half again.
So if we consider only the permanent component, it would cut the duration of effect from, say, four weeks to two weeks (one “cut”), then from two weeks to one week (second cut), from one week to 3.5 days (third cut), and so on. On top of this, the reversible component halves it again.
After 3 months of abstinence, then, the reversible component of tolerance disappears (temporarily) and a relapsed pack a day smoker will be happy—for a time—smoking a half-pack (10 cigarettes) per day. But then the reversible component kicks in and they’re back to smoking a pack a day.
The take-home message? Long-term smokers’ brains will never return to the days when their addiction could be satisfied with one cigarette per month because of the permanent component of tolerance.
How to help light smokers like your young patient
The fact that loss of autonomy over tobacco can occur after 1 cigarette helps explain the clinical observation that it is difficult for even non-daily smokers to stop smoking completely.
Helping a 14-year-old patient like the one in the opener to quit smoking requires that you not underestimate your patient’s need for advice about cessation. Unfortunately, none of the medications for smoking cessation, such as varenicline or bupropion, have been approved for this purpose in patients under the age of 18.
As a result, you’ll need to help young patients formulate a quit plan, just as you would with an adult heavy smoker. Give them the “Hooked On Nicotine Checklist.” The answers will help you both to tailor the quit plan to the obstacles they face. You’ll also need to provide young patients with anticipatory guidance about what they can expect from nicotine withdrawal.
Don’t wait for adolescents to broach the subject
Early intervention requires that you ask patients who are 10 years of age or older about smoking (TABLE 2). Be sure to take advantage of teachable moments and tell young patients just how addictive smoking can be—even if they’ve only smoked 1 or 2 cigarettes.
TABLE 2
The 5 A’s of smoking cessation26
|
Your conversation might go like this:
The first cigarette you smoke changes your brain and may cause you to develop a craving for nicotine that could be hard to resist. The only sure way to avoid addiction is to never smoke that first cigarette.
If you are already smoking and you have a craving for a cigarette every once in a while, that is a sure sign of addiction. The sooner you quit, the better your chances of succeeding. Now let’s work on a quit plan that will help you do that.
Correspondence
Joseph R. DiFranza, MD, Department of Family Medicine and Community Health, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655; [email protected].
1. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med 2002;156:397-403.
2. DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescents. Tob Control 2000;9:313-319.
3. DiFranza JR, Savageau JA, Fletcher K, et al. Development of symptoms of tobacco dependence in youths: 30 month follow-up data from the DANDY study. Tob Control 2002;11:228-235.
4. Gervais A, O’Loughlin J, Meshefedjian G, Bancej C, Tremblay M. Milestones in the natural course of cigarette use onset in adolescents. Can Med Assoc J 2006;175:255-261.
5. Kandel DB, Hu MC, Griesler PC, Schaffran C. The timing of the experience of symptoms of nicotine dependence. Paper 12-4. Society for Research on Nicotine and Tobacco; February 15-18, 2006; Orlando, Fla.
6. Scragg R. Report of 1999–2005 National Year 10 Smoking Surveys: prepared for ASH. 2006. Available at: www.ash.org.nz/index.php?pa_id=45&top_parent_id=45&curr_level=0. Accessed on November 6, 2007.
7. DiFranza JR, Savageau JA, Fletcher K, et al. Symptoms of tobacco dependence after brief intermittent use. The Development and Assessment of Nicotine Dependence in Youth-2. Arch Pediatr Adolesc Med 2007;161:704-710.
8. O’Loughlin J, DiFranza J, Tyndale RF, et al. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. Am J Prev Med 2003;25:219-225.
9. Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never smokers. Addiction 1998;93:595-599.
10. DiFranza J, Savageau JA, Fletcher K, et al. Susceptibility to nicotine dependence: The Development and Assessment of Nicotine Dependence in Youth-2 Study. Pediatrics 2007;120:e974-e983.
11. Benwell M, Balfour D, Anderson J. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J Neurochem 1988;50:1243-1247.
12. Abreu-Villaca YA, Seidler FJ, Qiao D, et al. Shortterm adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology 2003;28:1935-1949.
13. Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. N Engl J Med 1994;331:123-125.
14. McNeill A, West R, Jarvis M, Jackson P, Bryant A. Cigarette withdrawal symptoms in adolescent smokers. Psychopharmacology 1986;90:533-536.
15. Goddard E. Why Children Start Smoking. London: Her Majesty’s Stationery Office (HMSO), the Social Survey Division of the Office of Population Censuses and Surveys (OPCS), on behalf of the Department of Health; 1990.
16. Barker D. Reasons for tobacco use and symptoms of nicotine withdrawal among adolescent and young adult tobacco users—United States, 1993. MMWR Morb Mortal Wkly Rep 1994;43:745-750.
17. Riedel B, Robinson L, Klesges R, McLain-Allen B. Ethnic differences in smoking withdrawal effects among adolescents. Addict Behav 2003;28:129-140.
18. Strong D, Kahler C, Ramsey S, Abrantes A, Brown R. Nicotine withdrawal among adolescents with acute psychopathology: An item response analysis. Nicotine Tob Res 2004;6:547-557.
19. An L, Lein E, Bliss R, et al. Loss of autonomy over nicotine use among college social smokers. Paper presented at: 10th Annual Meeting of the Society for Research on Nicotine and Tobacco; February 21-24, 2004; Austin, Tex.
20. Panday S, Reddy S, Ruiter R, Bergstrom E, de Vries H. Nicotine dependence and withdrawal symptoms among occasional smokers. J Adolesc Health 2007;40:144-150.
21. Wellman R, DiFranza J, Wood C. Tobacco chippers report diminished autonomy over tobacco use. Addict Behav 2006;31:717-721.
22. Fernando WWSA, Wellman RJ, DiFranza JR. The relationship between level of cigarette consumption and latency to the onset of retrospectively reported withdrawal symptoms. Psychopharmacology 2006;188:335-342.
23. Smith KM, Mitchell SN, Joseph MJ. Effects of chronic and subchronic nicotine on tyrosine hydroxylase activity in noradrenergic and dopaminergic neurones in the rat brain. J Neurochem 1991;57:1750-1756.
24. DiFranza JR, Wellman RJ. A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: Integrating the clinical and basic science literature. Nicotine Tob Res 2005;7:9-26.
25. Wellman RJ, DiFranza JR, Savageau JA, et al. The effect of abstinence on cigarette consumption upon the resumption of smoking. Addict Behav 2006;31:711-716.
26. Agency for Healthcare Research and Quality. Clinical Practice Guideline. Treating Tobacco Use and Dependence. Rockville, Md: US Department of Health and Human Services Public Health Service; 2000. Available at: www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat2.chapter.7644. Accessed November 1, 2007.
1. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med 2002;156:397-403.
2. DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescents. Tob Control 2000;9:313-319.
3. DiFranza JR, Savageau JA, Fletcher K, et al. Development of symptoms of tobacco dependence in youths: 30 month follow-up data from the DANDY study. Tob Control 2002;11:228-235.
4. Gervais A, O’Loughlin J, Meshefedjian G, Bancej C, Tremblay M. Milestones in the natural course of cigarette use onset in adolescents. Can Med Assoc J 2006;175:255-261.
5. Kandel DB, Hu MC, Griesler PC, Schaffran C. The timing of the experience of symptoms of nicotine dependence. Paper 12-4. Society for Research on Nicotine and Tobacco; February 15-18, 2006; Orlando, Fla.
6. Scragg R. Report of 1999–2005 National Year 10 Smoking Surveys: prepared for ASH. 2006. Available at: www.ash.org.nz/index.php?pa_id=45&top_parent_id=45&curr_level=0. Accessed on November 6, 2007.
7. DiFranza JR, Savageau JA, Fletcher K, et al. Symptoms of tobacco dependence after brief intermittent use. The Development and Assessment of Nicotine Dependence in Youth-2. Arch Pediatr Adolesc Med 2007;161:704-710.
8. O’Loughlin J, DiFranza J, Tyndale RF, et al. Nicotine-dependence symptoms are associated with smoking frequency in adolescents. Am J Prev Med 2003;25:219-225.
9. Pomerleau OF, Pomerleau CS, Namenek RJ. Early experiences with tobacco among women smokers, ex-smokers, and never smokers. Addiction 1998;93:595-599.
10. DiFranza J, Savageau JA, Fletcher K, et al. Susceptibility to nicotine dependence: The Development and Assessment of Nicotine Dependence in Youth-2 Study. Pediatrics 2007;120:e974-e983.
11. Benwell M, Balfour D, Anderson J. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J Neurochem 1988;50:1243-1247.
12. Abreu-Villaca YA, Seidler FJ, Qiao D, et al. Shortterm adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology 2003;28:1935-1949.
13. Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. N Engl J Med 1994;331:123-125.
14. McNeill A, West R, Jarvis M, Jackson P, Bryant A. Cigarette withdrawal symptoms in adolescent smokers. Psychopharmacology 1986;90:533-536.
15. Goddard E. Why Children Start Smoking. London: Her Majesty’s Stationery Office (HMSO), the Social Survey Division of the Office of Population Censuses and Surveys (OPCS), on behalf of the Department of Health; 1990.
16. Barker D. Reasons for tobacco use and symptoms of nicotine withdrawal among adolescent and young adult tobacco users—United States, 1993. MMWR Morb Mortal Wkly Rep 1994;43:745-750.
17. Riedel B, Robinson L, Klesges R, McLain-Allen B. Ethnic differences in smoking withdrawal effects among adolescents. Addict Behav 2003;28:129-140.
18. Strong D, Kahler C, Ramsey S, Abrantes A, Brown R. Nicotine withdrawal among adolescents with acute psychopathology: An item response analysis. Nicotine Tob Res 2004;6:547-557.
19. An L, Lein E, Bliss R, et al. Loss of autonomy over nicotine use among college social smokers. Paper presented at: 10th Annual Meeting of the Society for Research on Nicotine and Tobacco; February 21-24, 2004; Austin, Tex.
20. Panday S, Reddy S, Ruiter R, Bergstrom E, de Vries H. Nicotine dependence and withdrawal symptoms among occasional smokers. J Adolesc Health 2007;40:144-150.
21. Wellman R, DiFranza J, Wood C. Tobacco chippers report diminished autonomy over tobacco use. Addict Behav 2006;31:717-721.
22. Fernando WWSA, Wellman RJ, DiFranza JR. The relationship between level of cigarette consumption and latency to the onset of retrospectively reported withdrawal symptoms. Psychopharmacology 2006;188:335-342.
23. Smith KM, Mitchell SN, Joseph MJ. Effects of chronic and subchronic nicotine on tyrosine hydroxylase activity in noradrenergic and dopaminergic neurones in the rat brain. J Neurochem 1991;57:1750-1756.
24. DiFranza JR, Wellman RJ. A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: Integrating the clinical and basic science literature. Nicotine Tob Res 2005;7:9-26.
25. Wellman RJ, DiFranza JR, Savageau JA, et al. The effect of abstinence on cigarette consumption upon the resumption of smoking. Addict Behav 2006;31:711-716.
26. Agency for Healthcare Research and Quality. Clinical Practice Guideline. Treating Tobacco Use and Dependence. Rockville, Md: US Department of Health and Human Services Public Health Service; 2000. Available at: www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat2.chapter.7644. Accessed November 1, 2007.
It’s 5 pm Friday; the caller thinks he has strep—Do you write that script?
Should you treat a symptomatic patient by phone when his child has confirmed strep throat?1 A recent Clinical Inquiry to The Journal of Family Practice posed this common question. The respondent answered by insisting on having the patient come into the office.
While we agree that a thorough examination is preferred over telephone management, we also believe that physicians need a strategy to apply when the adult patient cannot come to the office. Specifically, what do you do when the call comes in on a Friday evening, and the office is closed on Saturdays? What do you do when the patient is currently out of town? What do you do when the patient will not agree to an office visit?
Consider this tool for that late Friday call
If an adult patient caller has a son or daughter who currently has strep, the prior probability of strep causing the parent’s sore throat increases dramatically. While we know of no studies that document this precise situation, we would estimate that the prior probability would increase to about 50%. (The authors of the Clinical Inquiry assumed a population prevalence of 10%.1)
In such a situation, you may want to consider a tool that helps to estimate the probability of strep based on taking a history.2 Using this scoring system, you would give a score of 0 to 3 (absent, mild, moderate, severe) for each of 3 symptoms—fever, difficulty swallowing, and cough. You would add the scores for difficulty swallowing and fever and then subtract the cough score. We recommend a score of +2 or greater as a reasonable cutoff for telephone management in this situation (sensitivity = 85%, specificity = 42%) (TABLE).2
This scoring system, while less well known than our examination based score,3 performed quite well. The ROC curve areas did not significantly differ from the areas of the scoring rule, which includes physical examination.
TABLE
Should you write that prescription? Adult sore throat telephone scoring system helps you decide2
| Add the scores for fever and difficulty swallowing. Then subtract the cough score. Consider writing a prescription for scores of +2 or greater. | ||||
|---|---|---|---|---|
| SEVERITY | ||||
| VARIABLES | ABSENT | MILD | MODERATE | SEVERE |
| Fever | 0 | +1 | +2 | +3 |
| Difficulty in swallowing | 0 | +1 | +2 | +3 |
| Cough | 0 | +1 | +2 | +3 |
Unpublished data explain why this tool works
Sore throat patients cluster their signs and symptoms into 3 groupings: fever, viral symptoms (cough and coryza), and inflammatory signs and symptoms (exudates, adenopathy, and difficulty swallowing). Our unpublished data indicate that the severity of difficulty swallowing correlates with the severity of tonsillar exudates. Thus, the “telephone score” also correlates highly with the examination based score.
Keep in mind these 2 important caveats
If you recommend initial management for a sore throat patient, you (or someone on the nursing staff) should explain to the patient that if symptoms worsen, he should return for further evaluation. Even with antibiotic treatment, some patients develop peritonsillar abscess or Lemierre’s syndrome.
In addition, this telephone scoring tool is restricted to adult patients. Adult pharyngitis and pediatric pharyngitis, while similar, have significant differences. We developed the telephone score using adult data, and we have no assurance that it would work for children.
That said, we submit that family physicians should use this telephone score when an office visit is not feasible. We further suggest that you can use the telephone score to reassure patients that it’s unlikely that they have strep throat. While we prefer seeing patients with sore throat, we need a rational strategy to apply to adults who cannot, or will not, come to the office.
ANSWER:
A) Prevent nonsuppurative complications.
B) Prevent suppurative complications.
C) Decrease the duration of symptoms.
D) Prevent transmission to others.
A, B, C, and D are, of course, all reasons why we treat strep throat. The evidence in support of each of them, however, varies greatly. Consider the following:
- Of the nonsuppurative complications, we only have data that we can decrease the probability of rheumatic fever. Rheumatic fever in the US occurs rarely, and thus no longer has a major influence on our decision-making process. A recent review estimated the number needed to treat (NNT) for benefit as 3000 to 4000.4
- While we believe that early treatment decreases suppurative complications, there is no good data on the impact of early treatment on decreasing suppurative complications. The most recent estimate that we can find for NNT to prevent suppurative complications is 27.4 While uncommon, suppurative complications cause great pain, high health-care costs, and occasionally, death.
- Antibiotics clearly decrease symptom duration for strep throat.5 In the Zwart study, symptoms resolved 2 days sooner when patients were treated with penicillin for 7 days. Since patients call us because they feel bad, decreasing symptom duration is the most important reason to start narrow spectrum antibiotics promptly.
- We do not have great data on the preventive benefit to close contacts. We do know that strep infections have high infectivity.
Correspondence
Robert M Centor, MD, FOT720, 1530 3rd Ave S, Birmingham, AL 35294-3407; [email protected].
1. Sheridan E, Ludwig J, Helmen J. Should you treat a symptomatic patient by phone when his child has confirmed strep throat? J Fam Pract 2007;56:234-235.
2. Clancy CM, Centor RM, Campbell MS, Dalton HP. Rational decision making based on history: adult sore throats. J Gen Intern Med 1988;3:213-217.
3. Centor RM, Witherspoon JM, Dalton HP, Brody CE. The diagnosis of strep throat in adults in the emergency room. Med Decis Making 1981;1:239-246.
4. Graham TAD. Diagnosis and treatment of pharyngitis in adults. CJEM 2002;4:429-430.
5. Zwart S, Sachs A, Ruijs GJ, Gubbels JW, Hoes AW, Melker RA. Penicillin for acute sore throat: randomised double blind trial of seven days versus three days treatment of placebo in adults. BMJ 2000;320:150-154.
Should you treat a symptomatic patient by phone when his child has confirmed strep throat?1 A recent Clinical Inquiry to The Journal of Family Practice posed this common question. The respondent answered by insisting on having the patient come into the office.
While we agree that a thorough examination is preferred over telephone management, we also believe that physicians need a strategy to apply when the adult patient cannot come to the office. Specifically, what do you do when the call comes in on a Friday evening, and the office is closed on Saturdays? What do you do when the patient is currently out of town? What do you do when the patient will not agree to an office visit?
Consider this tool for that late Friday call
If an adult patient caller has a son or daughter who currently has strep, the prior probability of strep causing the parent’s sore throat increases dramatically. While we know of no studies that document this precise situation, we would estimate that the prior probability would increase to about 50%. (The authors of the Clinical Inquiry assumed a population prevalence of 10%.1)
In such a situation, you may want to consider a tool that helps to estimate the probability of strep based on taking a history.2 Using this scoring system, you would give a score of 0 to 3 (absent, mild, moderate, severe) for each of 3 symptoms—fever, difficulty swallowing, and cough. You would add the scores for difficulty swallowing and fever and then subtract the cough score. We recommend a score of +2 or greater as a reasonable cutoff for telephone management in this situation (sensitivity = 85%, specificity = 42%) (TABLE).2
This scoring system, while less well known than our examination based score,3 performed quite well. The ROC curve areas did not significantly differ from the areas of the scoring rule, which includes physical examination.
TABLE
Should you write that prescription? Adult sore throat telephone scoring system helps you decide2
| Add the scores for fever and difficulty swallowing. Then subtract the cough score. Consider writing a prescription for scores of +2 or greater. | ||||
|---|---|---|---|---|
| SEVERITY | ||||
| VARIABLES | ABSENT | MILD | MODERATE | SEVERE |
| Fever | 0 | +1 | +2 | +3 |
| Difficulty in swallowing | 0 | +1 | +2 | +3 |
| Cough | 0 | +1 | +2 | +3 |
Unpublished data explain why this tool works
Sore throat patients cluster their signs and symptoms into 3 groupings: fever, viral symptoms (cough and coryza), and inflammatory signs and symptoms (exudates, adenopathy, and difficulty swallowing). Our unpublished data indicate that the severity of difficulty swallowing correlates with the severity of tonsillar exudates. Thus, the “telephone score” also correlates highly with the examination based score.
Keep in mind these 2 important caveats
If you recommend initial management for a sore throat patient, you (or someone on the nursing staff) should explain to the patient that if symptoms worsen, he should return for further evaluation. Even with antibiotic treatment, some patients develop peritonsillar abscess or Lemierre’s syndrome.
In addition, this telephone scoring tool is restricted to adult patients. Adult pharyngitis and pediatric pharyngitis, while similar, have significant differences. We developed the telephone score using adult data, and we have no assurance that it would work for children.
That said, we submit that family physicians should use this telephone score when an office visit is not feasible. We further suggest that you can use the telephone score to reassure patients that it’s unlikely that they have strep throat. While we prefer seeing patients with sore throat, we need a rational strategy to apply to adults who cannot, or will not, come to the office.
ANSWER:
A) Prevent nonsuppurative complications.
B) Prevent suppurative complications.
C) Decrease the duration of symptoms.
D) Prevent transmission to others.
A, B, C, and D are, of course, all reasons why we treat strep throat. The evidence in support of each of them, however, varies greatly. Consider the following:
- Of the nonsuppurative complications, we only have data that we can decrease the probability of rheumatic fever. Rheumatic fever in the US occurs rarely, and thus no longer has a major influence on our decision-making process. A recent review estimated the number needed to treat (NNT) for benefit as 3000 to 4000.4
- While we believe that early treatment decreases suppurative complications, there is no good data on the impact of early treatment on decreasing suppurative complications. The most recent estimate that we can find for NNT to prevent suppurative complications is 27.4 While uncommon, suppurative complications cause great pain, high health-care costs, and occasionally, death.
- Antibiotics clearly decrease symptom duration for strep throat.5 In the Zwart study, symptoms resolved 2 days sooner when patients were treated with penicillin for 7 days. Since patients call us because they feel bad, decreasing symptom duration is the most important reason to start narrow spectrum antibiotics promptly.
- We do not have great data on the preventive benefit to close contacts. We do know that strep infections have high infectivity.
Correspondence
Robert M Centor, MD, FOT720, 1530 3rd Ave S, Birmingham, AL 35294-3407; [email protected].
Should you treat a symptomatic patient by phone when his child has confirmed strep throat?1 A recent Clinical Inquiry to The Journal of Family Practice posed this common question. The respondent answered by insisting on having the patient come into the office.
While we agree that a thorough examination is preferred over telephone management, we also believe that physicians need a strategy to apply when the adult patient cannot come to the office. Specifically, what do you do when the call comes in on a Friday evening, and the office is closed on Saturdays? What do you do when the patient is currently out of town? What do you do when the patient will not agree to an office visit?
Consider this tool for that late Friday call
If an adult patient caller has a son or daughter who currently has strep, the prior probability of strep causing the parent’s sore throat increases dramatically. While we know of no studies that document this precise situation, we would estimate that the prior probability would increase to about 50%. (The authors of the Clinical Inquiry assumed a population prevalence of 10%.1)
In such a situation, you may want to consider a tool that helps to estimate the probability of strep based on taking a history.2 Using this scoring system, you would give a score of 0 to 3 (absent, mild, moderate, severe) for each of 3 symptoms—fever, difficulty swallowing, and cough. You would add the scores for difficulty swallowing and fever and then subtract the cough score. We recommend a score of +2 or greater as a reasonable cutoff for telephone management in this situation (sensitivity = 85%, specificity = 42%) (TABLE).2
This scoring system, while less well known than our examination based score,3 performed quite well. The ROC curve areas did not significantly differ from the areas of the scoring rule, which includes physical examination.
TABLE
Should you write that prescription? Adult sore throat telephone scoring system helps you decide2
| Add the scores for fever and difficulty swallowing. Then subtract the cough score. Consider writing a prescription for scores of +2 or greater. | ||||
|---|---|---|---|---|
| SEVERITY | ||||
| VARIABLES | ABSENT | MILD | MODERATE | SEVERE |
| Fever | 0 | +1 | +2 | +3 |
| Difficulty in swallowing | 0 | +1 | +2 | +3 |
| Cough | 0 | +1 | +2 | +3 |
Unpublished data explain why this tool works
Sore throat patients cluster their signs and symptoms into 3 groupings: fever, viral symptoms (cough and coryza), and inflammatory signs and symptoms (exudates, adenopathy, and difficulty swallowing). Our unpublished data indicate that the severity of difficulty swallowing correlates with the severity of tonsillar exudates. Thus, the “telephone score” also correlates highly with the examination based score.
Keep in mind these 2 important caveats
If you recommend initial management for a sore throat patient, you (or someone on the nursing staff) should explain to the patient that if symptoms worsen, he should return for further evaluation. Even with antibiotic treatment, some patients develop peritonsillar abscess or Lemierre’s syndrome.
In addition, this telephone scoring tool is restricted to adult patients. Adult pharyngitis and pediatric pharyngitis, while similar, have significant differences. We developed the telephone score using adult data, and we have no assurance that it would work for children.
That said, we submit that family physicians should use this telephone score when an office visit is not feasible. We further suggest that you can use the telephone score to reassure patients that it’s unlikely that they have strep throat. While we prefer seeing patients with sore throat, we need a rational strategy to apply to adults who cannot, or will not, come to the office.
ANSWER:
A) Prevent nonsuppurative complications.
B) Prevent suppurative complications.
C) Decrease the duration of symptoms.
D) Prevent transmission to others.
A, B, C, and D are, of course, all reasons why we treat strep throat. The evidence in support of each of them, however, varies greatly. Consider the following:
- Of the nonsuppurative complications, we only have data that we can decrease the probability of rheumatic fever. Rheumatic fever in the US occurs rarely, and thus no longer has a major influence on our decision-making process. A recent review estimated the number needed to treat (NNT) for benefit as 3000 to 4000.4
- While we believe that early treatment decreases suppurative complications, there is no good data on the impact of early treatment on decreasing suppurative complications. The most recent estimate that we can find for NNT to prevent suppurative complications is 27.4 While uncommon, suppurative complications cause great pain, high health-care costs, and occasionally, death.
- Antibiotics clearly decrease symptom duration for strep throat.5 In the Zwart study, symptoms resolved 2 days sooner when patients were treated with penicillin for 7 days. Since patients call us because they feel bad, decreasing symptom duration is the most important reason to start narrow spectrum antibiotics promptly.
- We do not have great data on the preventive benefit to close contacts. We do know that strep infections have high infectivity.
Correspondence
Robert M Centor, MD, FOT720, 1530 3rd Ave S, Birmingham, AL 35294-3407; [email protected].
1. Sheridan E, Ludwig J, Helmen J. Should you treat a symptomatic patient by phone when his child has confirmed strep throat? J Fam Pract 2007;56:234-235.
2. Clancy CM, Centor RM, Campbell MS, Dalton HP. Rational decision making based on history: adult sore throats. J Gen Intern Med 1988;3:213-217.
3. Centor RM, Witherspoon JM, Dalton HP, Brody CE. The diagnosis of strep throat in adults in the emergency room. Med Decis Making 1981;1:239-246.
4. Graham TAD. Diagnosis and treatment of pharyngitis in adults. CJEM 2002;4:429-430.
5. Zwart S, Sachs A, Ruijs GJ, Gubbels JW, Hoes AW, Melker RA. Penicillin for acute sore throat: randomised double blind trial of seven days versus three days treatment of placebo in adults. BMJ 2000;320:150-154.
1. Sheridan E, Ludwig J, Helmen J. Should you treat a symptomatic patient by phone when his child has confirmed strep throat? J Fam Pract 2007;56:234-235.
2. Clancy CM, Centor RM, Campbell MS, Dalton HP. Rational decision making based on history: adult sore throats. J Gen Intern Med 1988;3:213-217.
3. Centor RM, Witherspoon JM, Dalton HP, Brody CE. The diagnosis of strep throat in adults in the emergency room. Med Decis Making 1981;1:239-246.
4. Graham TAD. Diagnosis and treatment of pharyngitis in adults. CJEM 2002;4:429-430.
5. Zwart S, Sachs A, Ruijs GJ, Gubbels JW, Hoes AW, Melker RA. Penicillin for acute sore throat: randomised double blind trial of seven days versus three days treatment of placebo in adults. BMJ 2000;320:150-154.